i
Medicare Communications and Marketing Guidelines (MCMG)
Date: September 5, 2018
Table of Contents
10 – Introduction ............................................................................................................................. 1
20 – Communications and Marketing Definitions ....................................................................... 2
20.1
– Factors for Activity and Material Determination ................................................................. 2
20.2
– Activity and Material Designation ....................................................................................... 3
30 – General Communication Requirements ............................................................................... 4
30.1
– Anti-Discrimination ............................................................................................................. 4
30.2
– Standardization of Plan Name Type ..................................................................................... 4
30.3
– Non-English Speaking Population ....................................................................................... 4
30.4
– Hours of Operation Requirements for Materials .................................................................. 5
30.5
– Use of TTY Numbers ........................................................................................................... 5
30.6
– Electronic Communication Policy ........................................................................................ 5
30.7
– Prohibited Terminology/Statements ..................................................................................... 6
30.8
– Product Endorsements/Testimonials .................................................................................... 6
30.9
– Co-branding .......................................................................................................................... 7
30.9.1
– Co-branding with Providers or Downstream Entities ........................................................ 7
30.9.2
– Plan’s/Part D Sponsor’s Relationships with State Pharmaceutical Assistance Programs
(SPAP) ................................................................................................................................... 7
40 – General Marketing Requirements ......................................................................................... 8
40.1
– Plan Comparisons ................................................................................................................. 8
40.2
– Marketing Through Unsolicited Contacts ............................................................................ 8
40.3
– Marketing Through Telephonic Contact .............................................................................. 9
40.4
– Nominal Gifts ..................................................................................................................... 10
40.5
– Exclusion of Meals as a Nominal Gift ............................................................................... 10
40.6
– Marketing Star Ratings ....................................................................................................... 10
40.6.1
– Marketing Plans/Part D Sponsors with an Overall 5-Star Rating ................................... 11
40.6.2
– Low Performing Icon Plans/Part D Sponsors .................................................................. 11
40.7
– Prohibition of Open Enrollment Period Marketing ............................................................ 12
40.8
– Marketing of Rewards and Incentives Programs ............................................................... 12
50 – Outreach Activities................................................................................................................ 13
ii
50.1
– Educational Events ............................................................................................................. 13
50.2
– Marketing/Sales Events ...................................................................................................... 13
50.3
– Personal/Individual Marketing Appointments ................................................................... 14
60 – Activities in a Healthcare Setting ......................................................................................... 14
60.1
– Provider-Initiated Activities ............................................................................................... 14
60.2
– Plan-Initiated Provider Activities in the Healthcare Setting .............................................. 14
60.3
– Contracted Provider Oversight Responsibilities ................................................................ 15
60.4
– Plan/Part D Sponsor Activities in the Healthcare Setting .................................................. 16
60.4.1 – Special Guidance for Institutional Special Needs Plans (I-SNPs) Serving Long- Term
Care Facility Residents ........................................................................................................ 16
60.5 – Provider Affiliation Announcements ................................................................................. 17
70 – Websites and Social/Electronic Media ................................................................................ 17
70.1
– Plan/Part D Sponsor Required Websites ............................................................................ 17
70.1.1
– General Website Requirements ....................................................................................... 17
70.1.2
– Documents to be Posted on Website ............................................................................... 18
70.1.3
– Required Content ............................................................................................................. 19
70.2
– Searchable Formularies and Directories ............................................................................ 20
70.3
– Social Media....................................................................................................................... 20
70.4
– Mobile Applications ........................................................................................................... 20
80 – Call Centers ........................................................................................................................... 21
80.1
– Customer Service Call Center Requirements and Standards ............................................. 21
80.2
– Customer Service Call Center Hours of Operations .......................................................... 21
80.3
– Informational Scripts .......................................................................................................... 22
80.4
– Telesales and Enrollment Scripts ....................................................................................... 22
80.5
– Pharmacy Technical Help Call Center Requirements and Standards ................................ 23
80.6
– Part D Sponsor Coverage Determinations and Appeals Call Center Requirements and
Standards ............................................................................................................................. 23
80.7
– Activities That Do Not Require the Use of State-Licensed Marketing Representatives ... 24
90 – Tracking, Submission, and Review Process ........................................................................ 24
90.1
– Material Identification........................................................................................................ 24
90.1.1 – Materials Subject to Submission .................................................................................... 25
90.2
– Material Replacement ........................................................................................................ 25
90.3
– Non-English Language and Alternate Format Materials ................................................... 26
90.4
– Submission of Websites and Webpages for Review .......................................................... 26
iii
90.5
– Submission of Multi-Plan Materials .................................................................................. 26
90.6
– Status of HPMS Material ................................................................................................... 28
90.7
– Resubmitting Previously Disapproved Pieces ................................................................... 29
90.8
– File & Use Process ............................................................................................................. 29
90.9
– File & Use Retrospective Monitoring Reviews ................................................................. 30
90.10
– Standardized Model Materials ......................................................................................... 30
90.11
– Non-Standardized Model Materials ................................................................................. 30
90.12
– Template Materials .......................................................................................................... 30
90.13
– Static Templates ............................................................................................................... 31
90.14
– Standard Templates .......................................................................................................... 31
100 – Required Materials ............................................................................................................. 32
100.1
– Mailings to Multiple Beneficiaries at One Household ..................................................... 32
100.2
– Electronic Delivery of Materials ...................................................................................... 32
100.2.1
- Notification of Availability of Electronic Materials ...................................................... 32
100.2.2
– Electronic Delivery of Required Materials.................................................................... 33
100.3
– Changes and Corrections to Existing Documents ............................................................. 33
100.4
– List of Required Materials ................................................................................................ 34
110 – Agent/Broker Activities, Oversight, and Compensation Requirements......................... 52
110.1
– Agent Requirements ......................................................................................................... 52
110.2
– Permitted Agent Activities ............................................................................................... 52
110.3
– Plan/Part D Sponsor Oversight ......................................................................................... 53
110.4
– Compensation Applicability and Definitions ................................................................... 53
110.5
– Plan/Part D Sponsor Compensation Reporting Requirements .......................................... 54
110.6
– Compensation ................................................................................................................... 54
110.6.1
– Initial Compensation ..................................................................................................... 54
110.6.2
– Renewal Compensation ................................................................................................. 54
110.6.3
– Referral/Finder’s Fees ................................................................................................... 54
110.6.4
– Paying Compensation .................................................................................................... 55
110.6.5
– Paying Initial Compensation ......................................................................................... 55
110.6.6
– Paying Renewal Compensation ..................................................................................... 55
110.6.7
– Other Compensation Scenarios ..................................................................................... 55
110.7
– Compensation Recovery Requirements (Charge-backs) .................................................. 56
110.7.1
– Rapid Disenrollment ...................................................................................................... 56
iv
110.7.2 – Other Compensation Recovery ..................................................................................... 57
110.8 – Payments other than Compensation ................................................................................. 57
120 – Use of Medicare Beneficiary Information Obtained from CMS .................................... 57
120.1 – Consent Requirements for Non-Health Related Mailings ............................................... 58
Appendix 1 – Definitions .............................................................................................................. 59
Appendix 2 – Disclaimers ............................................................................................................. 63
Appendix 3 – Pre-Enrollment Checklist ..................................................................................... 67
Appendix 4 – External Links ....................................................................................................... 69
Appendix 5 – Summary of Benefits Instructions ....................................................................... 71
Appendix 6 - Employer/Union Group Health Plans .................................................................. 74
Appendix 7 – Use of Medicare Mark for Part D Sponsors ....................................................... 76

1
10 – Introduction
The Medicare Communications and Marketing Guidelines (MCMG) interpret and provide
guidance on the marketing and communication rules for Medicare Advantage (MA-only, MA-
PD) plans (also referred to as “plans”), Medicare Prescription Drug plans (PDP) (also referred to
as “Part D sponsors”), and except where otherwise specified, Section 1876 cost plans (also
referred to as “plans”) and employer/union-sponsored group MA or Part D plans. These plans are
governed under Title 42 of the Code of Federal Regulations (CFR), Parts 422, 423, and 417.
These requirements also apply to Medicare-Medicaid Plans (MMPs), except as modified or
clarified in state-specific marketing guidance for each state’s demonstration. Such state-specific
guidance for MMPs is considered an addendum to the MCMG, and will be posted to:
https://www.cms.gov/Medicare-Medicaid-Coordination/Medicare-and-Medicaid-
Coordination/Medicare-Medicaid-Coordination-
Office/FinancialAlignmentInitiative/MMPInformationandGuidance/MMPMarketingInformation
andResources.html.
These requirements generally do not apply to Programs of All-Inclusive Care for the Elderly
(PACE) organizations or section 1833 Health Care Pre-payment plans unless otherwise noted in
the MCMG.
The term “marketing” is referenced at sections 1851(h) and 1860 D-4 of the Social Security Act
(the Act). “Communications” and “marketing” covered by the MCMG are defined at 42 CFR
sections 422.2260 and 423.2260.
Compliance
Plans/Part D sponsors are responsible for ensuring compliance with CMS’ current
communication and marketing regulations and guidance. This includes monitoring and
overseeing the activities of their subcontractors, downstream entities, and/or delegated entities. If
CMS finds that Plans/Part D sponsors have failed to comply with applicable rules and guidance,
CMS may take compliance and/or enforcement actions, including, but not limited to, intermediate
sanctions and/or civil money penalties.
Note: Plans/Part D sponsors may impose additional restrictions on their subcontractors,
downstream entities, and/or delegated entities, provided they do not conflict with the
requirements outlined in the MCMG.
The guidance set forth in this document is subject to change as policy, communications
technology, and industry marketing practices evolve. Any new rulemaking or interpretive
guidance (e.g., annual Call Letter guidance or Health Plan Management System (HPMS)
memoranda) may supersede the guidance provided in this document.

2
20 – Communications and Marketing Definitions
42 CFR §§ 422.2260, 423.2260
Communications means activities and use of materials to provide information to current and
prospective enrollees. This means that all activities and materials aimed at prospective and
current enrollees, including their caregivers and other decision makers associated with a
prospective or current enrollee, are “communications” within the scope of the regulations at 42
CFR Parts 417, 422, and 423.
Marketing is a subset of communications and includes activities and use of materials by the
Plan/Part D sponsor with the intent to draw a beneficiary's attention to a plan or plans and to
influence a beneficiary's decision-making process when selecting a plan for enrollment or
deciding to stay enrolled in a plan (that is, retention-based marketing).
Additionally, marketing contains information about the plan’s benefit structure, cost sharing,
measuring, or ranking standards.
However, CMS excludes materials that might meet the definition of marketing based on content,
but do not meet the intent requirements of marketing. Additionally, CMS excludes certain
required materials (as outlined under section 100), and reserves the ability to exclude additional
materials based on their use or purpose.
The MCMG discusses requirements applicable to all communication activities and materials, as
well as additional requirements only applicable to marketing activities and materials. All
marketing, unless otherwise noted, must adhere to the communication requirements.
20.1
– Factors for Activity and Material Determination
42 CFR §§ 422.2260, 422.2262, 422.2268, 423.2260, 423.2262, 423.2268
As outlined above, communication activities and materials are distinguished from marketing
activities and materials based on both intent and content.
Intent – the purpose of marketing activities and materials is to draw a prospective or current
enrollee’s attention to a plan or group of plans to influence a beneficiary’s decision when
selecting and enrolling in a plan or deciding to stay in a plan (retention-based marketing).
Content – based on the exclusions in the definition of marketing and marketing materials and the
type of information that would be intended to draw attention to a plan or influence a beneficiary’s
enrollment decision, marketing activities and materials include:
Information about benefits or benefit structure;
Information about premiums and cost sharing;
Comparisons to other Plan(s)/Part D sponsor(s);
Rankings or measurements in reference to other Plan(s)/Part D sponsor(s); or
Information about Star Ratings.
To identify marketing activities and materials, CMS will evaluate both the intent and content of
the activities and materials to determine if the definition of marketing is met.

3
Examples:
1.
A flyer reads “Swell Health is now offering Medicare Advantage coverage in Nowhere
County. Call us at 1-800-SWELL-ME for more information.”
Marketing or Communication? Communication. While the intent is to draw a
beneficiary’s attention to Swell Health, the information provided does not contain
any marketing content.
2.
A billboard reads “Swell Health Offers $0 Premium Plans in Nowhere County” Marketing
or Communication? Marketing. The advertisement includes both the intent to draw
the viewer’s attention to the plan and has content that mentions zero-dollar
premiums being available.
3.
A letter is sent to enrollees to remind them to get their flu shot. The body of the letter
says, “Swell Health enrollees can get their flu shot for $0 copay at a network
pharmacy…”
Marketing or Communication? Communication. While the letter mentions cost
sharing, the intent is not to steer the reader into making a plan selection or to stay
with the plan, but rather to encourage existing enrollees to get a flu shot. The letter
contains factual information about coverage and was provided only to current
enrollees.
20.2
– Activity and Material Designation
42 CFR §§ 422.2260, 423.2260
CMS designates as communication or marketing all required materials in Section 100 of this
document. Plans/Part D sponsors will need to review regulations at 42 CFR §§ 422.2260 and
423.2260 and these guidelines to determine if a plan-created material (i.e., something not listed as
a required material in Section 100) is considered a communication or marketing material. The
difference between communication and marketing activities and materials is based on the intent
and content of what is being conveyed. Plans/Part D sponsors are also encouraged to consult with
their Regional Office Account Manager or Marketing Reviewer should they have any marketing
or communication questions.
Materials are static in nature, whereas activities are more dynamic. Interactions with a beneficiary
could begin as a communication activity but become a marketing activity. In cases where a
communication activity has the potential to become a marketing activity, the Plan/Part D sponsor
or its downstream entities must adhere to all marketing requirements to ensure full compliance.
For example, an enrollee calls the Plan’s/Part D sponsor’s customer service number for questions
related to coverage under the plan in which in the beneficiary is currently enrolled; during the
call, the enrollee asks about other health plan options, moving the call from communications to
marketing.
4
30 – General Communication Requirements
The following guidance applies to all communications (including marketing). Items distributed by
the Plan/Part D sponsor and/or its first tier, downstream, and related entities must meet the
requirements in this section.
30.1
– Anti-Discrimination
42 CFR §§ 422.110, 422.2268(a)(12), 423.2268(a)(12), and 45 CFR Part 92
Plans/Part D sponsors may not discriminate based on race, ethnicity, national origin, religion,
gender, sex, age, mental or physical disability, health status, receipt of health care, claims
experience, medical history, genetic information, evidence of insurability, or geographic location.
Plans/Part D sponsors may not target potential enrollees from higher income areas, state or imply
that plans are only available to seniors rather than to all Medicare beneficiaries, or state or imply
that plans are only available to Medicaid beneficiaries unless the plan is a Dual Eligible Special
Needs Plan (D-SNP) or MMP. Only Special Needs Plans (SNPs) and MMPs may limit
enrollments to individuals meeting eligibility requirements based on health and/or other status;
such limitations must be consistent with the scope of their Medicare Advantage, Medicare
Improvement for Patients and Providers Act of 2008 (MIPPA) or three-way contracts with CMS.
Plans/Part D sponsors must comply with their obligations under other federal anti-discrimination
rules and requirements. This guidance focuses only on the anti-discrimination requirements in 42
C.F.R. Parts 422 and 423; Plans/Part D sponsors must be aware of and comply with their other
obligations under federal law that are not addressed here.
30.2
– Standardization of Plan Name Type
Sections 1851(h)(6) and 1860D-4(l)(3) of the Social Security Act; 42 CFR §§ 422.2268(a)(6),
423.2268(a)(6)
Plans/Part D sponsors must include the plan type in each plan’s name using standard terminology.
This must be placed at the end of each plan name. For instance, an HMO plan named “Golden
Medicare Plan” would appear as follows: “Golden Medicare Plan (HMO).” Plans/Part D sponsors
containing the plan type at the end of the plan name (e.g., Gold Plan Private Fee-For-Service) are
not required to repeat the plan type at the end of the plan name. Plans/Part D sponsors must
include the plan type on all communication or marketing materials when the plan name is
mentioned.
Plans/Part D sponsors must include the plan type on the front page or at the beginning of the
communication or marketing document. The plan type is not required throughout the document.
30.3
– Non-English Speaking Population
42 CFR §§ 422.111(h)(1), 422.112(a)(8), 423.128(d)(1)(iii), 422.2268(a)(7), 423.2268(a)(7)
Plan/Part D sponsor call centers receive calls from current and prospective enrollees. Call centers
must have interpreter services available to answer questions from non-English speaking or limited
English proficient (LEP) beneficiaries. This requirement is applicable regardless of the percentage
of non-English speaking or LEP beneficiaries in a plan benefit package (PBP) service area.

5
CMS has designated materials in section 100 that the agency considers vital and thus Plans/Part D
sponsors must make available in any language that is the primary language of at least five (5)
percent of a Plan’s/Part D sponsor’s PBP service area.
Note: The enrollee identification (ID) card is excluded from this requirement.
30.4
– Hours of Operation Requirements for Materials
42 CFR §§ 422.111(h), 422.2262(c), 423.128(d), 423.2262(c)
Plan’s/Part D sponsor’s hours and days of operation must be included when a customer service
number is provided on all marketing and communications materials in order to ensure that notice
of the customer service contact information is adequate and not confusing or misleading. This
does not apply to enrollee ID cards and the standardized Star Ratings document. In addition,
Plans/Part D sponsors must list the hours and days of operation for 1-800-MEDICARE on every
material where 1-800-MEDICARE or Medicare TTY appears (i.e., 24 hours a day/7 days a
week).
Note: CMS requires Plans/Part D sponsors to list the hours and days of operation only once
in conjunction with the customer service number and 1-800-MEDICARE listings.
30.5
– Use of TTY Numbers
Section 504 of the Rehabilitation Act
A toll-free TTY number must appear in conjunction with the customer service number in the
same font size as the other phone numbers, except as outlined below. Plans/Part D sponsors may
use their own TTY number, 711 for Telecommunications Relay Service, or state relay services, as
long as the number is accessible from TTY equipment.
Exceptions:
Outdoor advertising (ODA) or banner/banner-like ads
Radio advertisements and radio sponsorships (e.g., sponsoring an hour of public radio)
In television ads, the TTY number may be a different font size/style than other phone
numbers to limit possible confusion
30.6
– Electronic Communication Policy
42 CFR §§ 422.2268(b), 423.2268(b)
A Plan/Part D sponsor may initiate contact via email to prospective enrollees and to retain
enrollment for current enrollees. Plans/Part D sponsors must include an opt-out process on each
communication to elect to no longer receive emails.
Note: Text messaging and other forms of electronic direct messaging (e.g., social media
platforms) would fall under unsolicited contact and is not permitted.

6
30.7
– Prohibited Terminology/Statements
42 CFR §§ 422.2264, 423.2264, 422.2268(a)(2), 423.2268(a)(2)
Plans/Sponsors are prohibited from distributing communications that are materially inaccurate,
misleading, or otherwise make misrepresentations or could confuse beneficiaries.
Plans/Part D sponsors may not:
Claim that they are recommended or endorsed by CMS, Medicare, or the Department of
Health & Human Services (DHHS);
Use unsubstantiated absolute or qualified superlatives or pejoratives;
Note: Unsubstantiated absolute and/or qualified superlatives may be used in
logos/taglines.
Market that they will not disenroll individuals due to failure to pay premiums; or,
Use the term “free” to describe a zero-dollar premium, reduction in premiums (including
Part B buy-down), reduction in deductibles or cost sharing, low-income subsidy (LIS),
cost sharing for individuals with dual eligibility.
Note: Medical Savings Account (MSA) plans may not imply that the plan operates as a
supplement to Medicare.
MA plans that are not D-SNPs may not:
Imply that their plan is designed for dual eligible individuals;
Claim that they have a relationship with the state Medicaid agency, unless the MA plan
(or its parent organization) has contracted with the state to coordinate Medicaid services,
and the contract is specific to that MA plan (not for a separate D-SNP or MMP); or
Target their marketing efforts exclusively to dual eligible individuals.
Plans/Part D sponsors may:
State that the Plan/Part D sponsor is approved to participate in Medicare programs and/or
is contracted to administer Medicare benefits;
Use the term “Medicare-approved” to describe their benefits and/or services within their
marketing materials; and,
Use the term “free” in conjunction with mandatory, supplemental, and preventive benefits
provided at a zero-dollar cost sharing for all enrollees.
30.8
– Product Endorsements/Testimonials
42 CFR §§ 422.2264, 423.2264, 422.2268, 423.2268
Product endorsements and testimonials must adhere to the following requirements:
The speaker must identify the Plan’s/Part D sponsor’s product or company by name;
Medicare beneficiaries endorsing or promoting a Plan/Part D sponsor must be enrolled in
the Plan/Part D sponsor at the time the endorsement or testimonial was created;
If an individual is paid or has been paid to endorse or promote the plan or product, the
advertisement must clearly state this (e.g., “paid endorsement”);
If an individual, such as an actor, is paid to portray a real or fictitious situation, the
advertisement must clearly state it is a “Paid Actor Portrayal”, and,

7
The Plan/Part D sponsor must be able to substantiate any claims made in the
endorsement/testimonial.
Note: Reuse of individual users’ content or comment from social media sites (e.g.,
Facebook, Twitter) that promotes a Plan’s/Part D sponsor’s product is considered a product
endorsement/testimonial and must adhere to the guidance in this section.
30.9
– Co-branding
42 CFR §§ 422.2262, 422.2264, 422.2268, 423.2262, 423.2264, 423.2268
Plans/Part D sponsors must enter in HPMS any co-branding relationships, including any changes
in or newly formed co-branding relationships, prior to marketing them. CMS does not review or
approve such relationships but needs the information to review associated marketing materials.
Plans/Part D sponsors should refer to the HPMS Bid Submission User Manual for instructions on
entering co-branding information.
30.9.1
– Co-branding with Providers or Downstream Entities
42 CFR §§ 422.2262, 422.2268(a)(5), 423.2262, 423.2268(a)(5)
Plans/Part D sponsors that choose to co-brand with providers/pharmacies may include the name
and/or logo of co-branded providers/pharmacies on materials other than enrollee ID cards as long
as the Plan/Part D sponsor makes it clear to beneficiaries that the providers/pharmacies are part of
the plan’s network. In addition, Plans/Part D sponsors must include the appropriate model
disclaimer on co-branded materials (refer to Appendix 2).
Neither the Plan/Part D sponsor nor its co-branding providers/pharmacies may imply that the co-
branding partner is endorsed by CMS, or that its products or services are Medicare-approved.
The Plan/Part D sponsor must submit the co-branded marketing materials to CMS.
Names and/or logos of co-branded providers on the plan’s enrollee ID card are prohibited, unless
the provider names and/or logos are in the name of the plan name and/or are related to an
enrollee's selection of a specific provider/provider organization, (e.g., physician, hospital, medical
group).
Part D sponsors are prohibited from displaying the names and/or logos of co-branded pharmacies
on the Part D sponsor’s enrollee ID card.
Note: Consistent with the National Council for Prescription Drug Program’s (NCPDP)
“Pharmacy and/or Combination ID Card” standard, the Pharmacy Benefit Manager (PBM)
name may be included on an enrollee ID card.
30.9.2
– Plan’s/Part D Sponsor’s Relationships with State Pharmaceutical Assistance
Programs (SPAP)
A Plan’s/Part D sponsor’s logo may be used in connection with the coverage of benefits provided
under an SPAP and may contain an emblem or symbol indicating such a relationship.
8
40 – General Marketing Requirements
42 CFR §§ 422.2262, 422.2264, 422.2268(a)(2), 423.2262, 423.2264, 423.2268(a)(2)
Plans/Part D sponsors cannot market for an upcoming plan year prior to October 1. Plans/Part D
sponsors are permitted to simultaneously market the current and prospective years starting on
October 1, provided marketing materials clearly indicate what plan year is being discussed.
Plans/Part D sponsors may only advertise in their defined service area, unless unavoidable (e.g.,
advertising in media with a national audience or with an audience that includes some individuals
outside of the service area, such as a Metropolitan Statistical Area that covers two regions).
Plans/Part D sponsors must clearly disclose their service area in the marketing materials.
40.1
– Plan Comparisons
42 CFR §§422.2260, 422.2268(a)(2), 423.2260, 423.2268(a)(2)
Plans/Part D sponsors may compare their plan to another Plan/Part D sponsor, provided the
Plans/Part D sponsors can support them (e.g., by studies or statistical data) and such comparisons
are factually based and not misleading.
40.2
– Marketing Through Unsolicited Contacts
42 CFR §§ 422.2268(b)(13), 423.2268(b)(13)
Plans/Part D Sponsors may make unsolicited direct contact with potential enrollees using the
following methods:
Conventional mail and other print media (e.g., advertisements, direct mail)
Email provided all emails contain an opt-out function
Plans/Part D sponsors may not:
Use door-to-door solicitation, including leaving information such as a leaflet or flyer at a
residence;
Approach potential enrollees in common areas (e.g., parking lots, hallways, lobbies,
sidewalks, etc.); or,
Use telephonic solicitation, including text messages and leaving electronic voicemail
messages.
Note: Agents/brokers who have a pre-scheduled appointment with a potential enrollee who
is a “no-show” may leave information at that potential enrollee’s residence. If a potential
enrollee provides permission to be contacted, the contact must be event- specific, and may
not be treated as open-ended permission for future contacts.
9
40.3
– Marketing through Telephonic Contact
42 CFR §§ 422.2268, 423.2268
Plans/Part D sponsors and their agents/brokers may not make unsolicited telephone calls to
prospective enrollees. However, they are permitted to contact their current enrollees to discuss
plan business, but cannot market prior to October 1 under the pretense of plan business.
Plans/Part D sponsors, and their agents/brokers, may conduct the following specific telephonic
activities:
Call current enrollees, including those in non-Medicare products, to discuss plan business
(examples of this include calls to enrollees aging into Medicare from commercial products
offered by the same organization, calls to an organization’s existing Medicaid/MMP plan
enrollees to talk about its Medicare products, and calls to current MA enrollees to promote
other Medicare plan types or to discuss plan benefits);
Call beneficiaries who submit enrollment applications to conduct business related to
enrollment;
Call former enrollees after the disenrollment effective date to conduct disenrollment
surveys for quality improvement purposes (disenrollment surveys conducted
telephonically, email or conventional mail may not include sales or marketing
information);
Under limited circumstances with approval from the CMS Account Manager, call LIS-
eligible enrollees that a plan is prospectively losing due to reassignment to encourage
them to remain enrolled in their current plan;
Call individuals who have given permission for a plan or sales agent to contact them
(examples of permission include filling out a business reply card, emailing the Plan/Part D
sponsor requesting a return call, or asking a customer service representative to have an
agent contact them); and,
Note: Permission applies only to the entity from which the individual requested
contact and for the duration and topic of that transaction.
Return phone calls or messages from individuals or enrollees, as these are not considered
unsolicited contacts.
Plans/Part D sponsors, and their agents/brokers, may not conduct telephonic activities that
include, but are not limited to, the following:
Unsolicited calls about other business as a means of generating leads for Medicare plans
(e.g., bait and switch strategies);
Calls based on referrals (if an individual would like to refer a friend or relative to an agent
or Plan/Part D sponsor, the agent or Plan/Part D sponsor may provide contact information
such as a business card that the individual could provide to a friend or relative);
Calls to market plans or products to former enrollees who have disenrolled, or to current
enrollees who are in the process of voluntarily disenrolling;
Calls to beneficiaries who attended a sales event, unless the beneficiary gave express
permission at the event for a follow-up call (there must be documentation of permission to
be contacted); or,

10
Calls to prospective enrollees to confirm receipt of mailed information.
40.4
– Nominal Gifts
42 CFR §§ 422.2268(b)(1),(2),(12), 423.2268(b)(1),(2),(12)
Plans/Part D sponsors may offer nominal gifts ($15 or less, $75 aggregate, per person, per year)
to beneficiaries for marketing purposes, provided the gift is given regardless of whether they
enroll, and without discrimination.
The following rules apply to nominal gifts:
If a nominal gift is a chance to receive one large gift or a communal experience (e.g., a
concert, raffle, drawing), the total fair market value must not exceed the nominal per
person value based on anticipated attendance. For example, if 10 people are expected to
attend an event, the nominal gift may not be worth more than $150 ($15 for each of the 10
anticipated attendees). Anticipated attendance must be based on venue size, response rate,
and/or advertisement circulation.
Nominal gifts may not be in the form of cash or other monetary rebates even if their worth
is $15 or less.
Note: Plans/Part D sponsors should refer to the Office of Inspector General’s website for
advisory opinions and guidance on gifts and gift cards.
40.5
– Exclusion of Meals as a Nominal Gift
42 CFR §§ 422.2268(b)(15), 423.2268(b)(15)
Plans/Part D sponsors may not provide or subsidize meals at sales/marketing events.
Refreshments and light snacks may be provided. Plans/Part D sponsors should ensure that items
provided could not be reasonably considered a meal and/or that multiple items are not being
“bundled” and provided as if a meal.
Meals may be provided at CMS-defined educational events and other events that would fall under
the definition of communications (refer to Appendix 1).
40.6
– Marketing Star Ratings
42 CFR §§ 422.2260, 422.2262, 422.2264, 422.2268(a)(1) and (2), 423.2260, 423.2262,
423.2264, 423.2268(a)(1) and (2)
Plans/Part D sponsors must provide Star Ratings information to beneficiaries through the
standardized Star Ratings information document available in HPMS. New Plans/Part D sponsors
that do not have any Star Ratings information are not required to provide this information until
their Star Ratings data are available.
The following rules apply to all Plans/Part D sponsors when referring to Star Ratings:
References to individual Star Ratings measure(s) must also include references to the
contract’s highest rating, overall rating (MA-PD), Part C summary rating (MA-only), or
Part D summary rating (PDPs), with equal or greater prominence;
Must not use an individual underlying category or measure to imply higher overall or
summary Star Ratings;
11
Any reference to a contract’s Star Rating must make it clear that the rating is “ out of
five (5) stars.” (letters, numbers, graphic representation, or any combination may be
used);
Must clearly identify which Star Ratings contract year applies;
May only market the Star Ratings in the service area in which the Star Rating is
applicable; and,
May direct beneficiaries to https://www.medicare.gov for more information on Star
Ratings.
40.6.1
– Marketing Plans/Part D Sponsors with an Overall 5-Star Rating
42 CFR §§ 422.2260, 422.2262, 422.2264, 422.2268(a)(1) and (2), 423.2260, 423.2262,
423.2264, 423.2268(a)(1) and (2)
The following marketing rights and rules apply to Plans/Part D Sponsors with an overall 5-star
rating:
May market their ability to enroll beneficiaries through the 5-star special enrollment
period (SEP).
May not specifically target enrollees in poor performing plans.
May include CMS’ gold star icon on marketing materials. Must be clear that the 5-star
rating is for the applicable contract. CMS’ Regional Offices will provide the gold star icon
each fall. Plans are not permitted to create their own gold star icon or any other icon of
distinction.
Plans with one or more contracts without 5-star ratings must not disseminate materials
that imply other contracts achieved this rating. Materials must list specific contracts with
overall 5-star ratings or be specific to a contract with an overall 5-star rating.
If a 5-star contract fails to receive a 5-star rating for the upcoming year, marketing for the
purpose of accepting enrollees under the 5-star SEP must be discontinued by November
30 of the current year.
40.6.2
– Low Performing Icon Plans/Part D Sponsors
42 CFR §§ 422.2260, 422.2262, 422.2264, 422.2268(a)(1), 423.2260, 423.2262, 423.2264,
423.2268(a)(1)
The following marketing rules apply to low performing Plans/Part D Sponsors:
Low Performing Icon (LPI) must be included in all marketing materials about or that
reference the specific contract’s Star Ratings (Note: changes to the icon are not
permitted);
Plans/Part D sponsors assigned an LPI may not attempt to discredit or refute their LPI
status by showcasing a higher overall Star Rating on another contract or specific measure
Star Ratings;
Must clearly indicate LPI status when referencing its Star Ratings - must state the LPI
means that the Plan/Part D sponsor received a summary rating of 2.5 stars or below in Part
C and/or Part D for the last three years;
If an LPI has an overall Star Rating of 3 or above on its marketing materials, the LPI must
also be clearly stated on its marketing materials; and,
May not inform beneficiaries that they may request an SEP and move to a higher rated

12
plan if they are dissatisfied with low performing plan.
40.7
– Prohibition of Open Enrollment Period Marketing
42 CFR §§ 422.2268(b)(10), 423.2268(b)(10)
Plans/Part D sponsors are prohibited from knowingly targeting or sending unsolicited marketing
materials to any MA enrollee or Part D enrollee during the continuous Open Enrollment Period
(OEP) (January 1 to March 31). “Knowingly” takes into account the intended recipient as well as
the content of the message.
During the OEP, Plans/Part D sponsors may:
Conduct marketing activities that focus on other enrollment opportunities, including but
not limited to:
o
Marketing to age-ins (who have not yet made an enrollment decision),
o
Marketing by 5-star plans regarding their continuous enrollment SEP, and
o
Marketing to dual-eligible and LIS beneficiaries who, in general, may make
changes once per calendar quarter during the first nine months of the year.
Send marketing materials when a beneficiary makes a proactive request;
At the beneficiary’s request, have one-on-one meetings with a sales agent; and
At the beneficiary’s request, provide information on the OEP through the call center
Note: The unintentional receipt of other marketing materials by beneficiaries who have
already made an enrollment decision is not be considered knowingly targeting. For
example, if a Plan sends mailers to a list of age-ins discussing the Initial Coverage Election
Period (ICEP), it is possible that some recipients may have already made an enrollment
decision; however, the content of the message to the intended audience of age-ins is not
prohibited OEP marketing.
During the OEP, Plans/Part D sponsors may not:
Send unsolicited materials advertising the ability/opportunity to make an additional
enrollment change or referencing the OEP;
Specifically target beneficiaries who are in the OEP because they made a choice during
Annual Enrollment Period (AEP) by purchase of mailing lists or other means of
identification;
Engage in or promote agent/broker activities that intend to target the OEP as an
opportunity to make further sales; or
Call or otherwise contact former enrollees who have selected a new plan during the AEP.
For more information on the OEP, please reference to Chapter 2 - Medicare Advantage
Enrollment and Disenrollment of the Medicare Managed Care Manual or Appendix 4 to access
the CMS Eligibility and Enrollment Guidance link.
40.8
– Marketing of Rewards and Incentives Programs
42 CFR §§ 422.134(c)(2)(ii), 422.2268
MA Plans may include information about rewards and incentives programs in marketing
materials for potential enrollees. Marketing of rewards and incentives programs must:
13
Not be used in exchange for enrollment;
Be provided to all potential enrollees without discrimination;
Be provided in conjunction with information about plan benefits; and
Include information about all rewards and incentives programs offered by the MA Plan,
and are not limited to a specific program, or a specific reward or incentive within a
program.
Note: For information regarding rewards and incentives program requirements, see Chapter
4 of the Medicare Managed Care Manual. Nominal gifts that are part of a promotional
activity are different from rewards and incentives.
Part D plans are not permitted by 42 CFR § 423.134 to develop or use rewards and incentives
programs; therefore, Part D sponsors may not market reward and incentive programs.
50 - Outreach Activities
50.1
– Educational Events
42 CFR §§ 422.2262, 422.2268(b)(7),(8), and (11),423.2262, 423.2268(b)(7),(8), and (11)
Educational events are designed to inform beneficiaries about Medicare Advantage, Prescription
Drug, or other Medicare programs. Educational events:
Must be explicitly advertised as educational;
May be hosted in a public venue by the Plan/Part D sponsor or an outside entity;
May include communication activities and distribution of communication materials;
May answer beneficiary initiated questions;
May set up a future marketing appointment, and distribute business cards and contact
information for beneficiaries to initiate contact (this includes completing and collecting a
Scope of Appointment (SOA) form);
Must not include marketing or sales activities or distribution of marketing materials or
enrollment forms; and
May not conduct a marketing/sales event immediately following an educational event in
the same general location (e.g., same hotel).
50.2
– Marketing/Sales Events
42 CFR §§ 422.2268(b)(1-5), 423.2268(b)(1-5)
Marketing/Sales Events are designed to steer or attempt to steer potential enrollees, or the
retention of current enrollees, toward a plan or limited set of plans. The following requirements
apply to all marketing/sales events:
Plans/Part D sponsors must submit scripts and presentations to CMS prior to use,
including those to be used by agents/brokers;
Sign in sheets must clearly be labeled as optional;
Health screenings or other activities that may be perceived as, or used for, “cherry
picking” are not permitted;
Plans/Part D sponsors may not require attendees to provide contact information as a
prerequisite for attending an event; and

14
Contact information provided for raffles or drawings may only be used for that purpose.
50.3
– Personal/Individual Marketing Appointments
42 CFR §§ 422.2268(b)(3-5),(11), 423.2268(b)(3-5) and (11)
SOA parameters (and documentation) are required for all one-on-one appointments, regardless of
venue (e.g., home, telephone). During these appointments, discussions may only concern
previously agreed upon plan products documented in the SOA, and may only market health-
related products, and not, for example, annuities or life insurance. Individuals may not
solicit/accept enrollment applications for a January 1 effective date until October 15 of the
preceding calendar year, unless the beneficiary is entitled under another enrollment period.
60 –Activities in a Healthcare Setting
Section 1851(j)(1)(D), 42 CFR §§ 422.2268(a)(1),(2), and (5); 422.2268(b)(7) and (9),
423.2268(a)(1),(2), and (5), 423.2268(b)(7) and (9)
To maintain appropriate beneficiary safeguards while not impeding the provider/patient
relationship, CMS distinguishes between provider-initiated activities and plan-initiated activities
in a healthcare setting.
60.1
– Provider-Initiated Activities
42 CFR §§ 422.2260, 423.2260
Provider-initiated activities are those conducted by a healthcare professional, including
pharmacists, at the request of the patient or as a matter of a course of treatment, when meeting
with the patient as part of the professional relationship between healthcare provider and patient.
Provider-initiated activities do not include those conducted at the request of the Plan/Part D
sponsor or pursuant to the network participation agreement between the Plan/Part D sponsor and
the provider. Provider-initiated activities fall outside of the definition of marketing as outlined in
§§422.2260 and 423.2260.
Permissible contracted provider-initiated activities include:
Distributing unaltered, printed materials created by CMS, such as reports from Medicare
Plan Finder, the “Medicare & You” handbook, or “Medicare Options Compare” (from
https://www.medicare.gov) including in areas where care is delivered;
Providing the names of Plans/Part D sponsors with which they contract and/or participate;
Answering questions or discussing the merits of a plan or plans, including cost sharing
and benefit information (these discussions may occur in areas where care is delivered);
Referring patients to other sources of information, such as State Health Insurance
Assistance Program (SHIP) representatives, plan marketing representatives, State
Medicaid Office, local Social Security Office, CMS’ website at
https://www.medicare.gov, or 1-800-MEDICARE;
Referring patients to Plan marketing materials available in common areas; and
Providing information and assistance in applying for the LIS.
60.2
– Plan-Initiated Provider Activities in the Healthcare Setting
42 CFR §§ 422.2260, 422.2268(b)(7), 423.2260, 423.2268(b)(7)
15
CMS defines plan-initiated activities as those activities where either a Plan/Part D sponsor
requests contracted providers to perform a task or the provider is acting -on behalf of the
Plan/Part D sponsor. For the purpose of plan-initiated activities, the Plan/Part D sponsor must
ensure compliance with requirements applicable to communication and marketing.
Plan/Part D sponsor requests for providers to discuss benefits and cost sharing would fall under
the definition of marketing and are hence prohibited from taking place where care is being
delivered.
Plans/Part D sponsors may not allow contracted providers to:
Accept/collect scope of appointment forms;
Accept Medicare enrollment applications;
Make phone calls or direct, urge, or attempt to persuade their patients to enroll in a
specific plan based on financial or any other interests of the provider;
Mail marketing materials on behalf of Plans/Part D sponsors;
Offer inducements to persuade their patients to enroll in a particular plan or organization;
Conduct health screenings as a marketing activity;
Distribute marketing materials/applications in areas where care is being delivered;
Offer anything of value to induce enrollees to select them as their provider; or
Accept compensation from the plan for any marketing or enrollment activities.
Plans/Part D sponsors may allow contracted providers to:
Make available, distribute, and display communication materials, including in areas where
care is being delivered; and
Provide or make available plan marketing materials and enrollment forms outside of the
areas where care is delivered (such as common entryways, vestibules, hospital or nursing
home cafeterias, and community, recreational, or conference rooms).
60.3
– Contracted Provider Oversight Responsibilities
42 CFR §§ 422.2268 and 423.2268
Plans/Part D sponsors must advise contracted providers, through their agreements with those
providers, of the need for providers to remain neutral when assisting beneficiaries with
enrollment decisions.
Plans/Part D sponsors that have agreements with providers in connection with plan marketing
activities should ensure that those agreements address marketing activities in a manner consistent
with Medicare regulations and guidelines.
Plans/Part D sponsors may use providers and/or facilities to distribute and/or make available
Plan/Part D sponsor marketing materials, as long as the provider and/or facility distributes or
makes available Plan/Part D sponsor marketing materials for all plans with which the provider
participates, if requested by other Plan/Part D sponsors. CMS does not expect providers to
proactively contact all participating Plans.

16
60.4
– Plan/Part D Sponsor Activities in the Healthcare Setting
42 CFR §§ 422.2268(b)(7) and 423.2268(b)(7)
Plans/Part D sponsors may conduct sales activities, including sales presentations, the distribution
of marketing materials, and the distribution and collection of enrollment forms in common areas
of a healthcare setting. Common areas in a healthcare setting include, but are not limited to
common entryways, vestibules, waiting rooms, hospital or nursing home cafeterias, and
community, recreational, or conference rooms. Plans/Part D sponsors may not market in
restricted areas. Restricted areas generally include, but are not limited to: exam rooms, hospital
patient rooms, treatment areas where patients interact with a provider and his/her clinical team
and receive treatment (including dialysis treatment facilities), and pharmacy counter areas (where
patients interact with pharmacy providers and obtain medications).
Communication materials may be distributed and displayed in all areas of the healthcare setting.
60.4.1 – Special Guidance for Institutional Special Needs Plans (I-SNPs) Serving Long-
Term Care Facility Residents
42 CFR §§ 422.2260, 422.2268(b)(7), 423.2260, 423.2268(b)(7)
In addition to the guidance previously provided in this section, the following requirements apply
to marketing and communication by I-SNPs serving long-term care facility residents and by
contracted providers of I-SNPs.
As outlined in section 60.1, when contracted providers I-SNPs discuss health care options at the
request of residents of a long-term care facility or as a matter of course in treatment, it is
considered provider-initiated and does not fall under the requirements of marketing. Plans/Part D
sponsors may provide contracted long-term care facilities with materials for inclusion in
admission packets that announce the Plan/Part D sponsor’s contractual relationship. CMS
considers these communication materials.
CMS permits Plans/Part D sponsors to schedule appointments with residents of long-term care
facilities (e.g., nursing homes, assisted living facilities, board and care homes) upon a resident’s
request. If a resident did not request an appointment, any visit by an agent or broker is considered
unsolicited door-to-door marketing.
Depending on the context of a given situation, I-SNP long-term care facilities and staff can be
viewed as both provider and Plan. I-SNPs should put the necessary boundaries in place between
clinical and sales staff to mitigate conflicts of interest. I-SNPs may use staff operating in a social
worker capacity to provide information, including marketing materials, to residents. Such
information must not include an enrollment form and the social worker may not accept or collect
a scope of appointment or enrollment form on behalf of the I-SNP. The beneficiary or authorized
representative must initiate additional communication with the Plan/Part D sponsor following the
receipt of a business reply card, phone number or marketing materials.

17
60.5 – Provider Affiliation Announcements
42 CFR §§ 422.2262(a), 422.2268, 423.2262(a), 423.2268
Plans/Part D sponsors and/or contracted providers (including pharmacies) may announce new or
continuing affiliations with specific Plans/Part D sponsors once a contractual agreement between
the Plan/Part D sponsor and provider has been agreed upon by both parties. Provider affiliation
announcements may be made through direct mail, email, telephone, or advertisement. The
announcement must clearly state that the provider may also contract with other Plans/Part D
sponsors, if applicable. These announcements are considered communication materials.
Provider affiliation announcement materials that include additional information, such as
descriptions of plan benefits, premiums, or cost sharing are considered marketing materials.
Plans/Part D sponsors must submit these materials in HPMS. Plans/Part D sponsors must ensure
their network providers and pharmacies adhere to distribution and mailing guidance set forth in
these guidelines.
70 – Websites and Social/Electronic Media
70.1
– Plan/Part D Sponsor Required Websites
42 CFR §§ 422.111(h), 422.2264, 422.2268, 423.128(d), 423.2264, 423.2268
CMS requires all Plans/Part D sponsors to have a website that includes the specific documents
and content listed outlined in sections 70.1.1 and 70.1.2. Plans/Part D sponsors may include other
information, including both communications and marketing information on their website.
70.1.1
– General Website Requirements
42 CFR §§ 422.111(h), 422.2264, 422.2268, 423.128(d), 423.2264, 423.2268
Plan/Part D sponsor websites must:
Be clear and easy to navigate;
Maintain the current contract year content through December 31 of each year;
Notify users when they will leave the Plan’s/Part D sponsor’s Medicare information
webpage if there is a link that will take an individual to non-Medicare information
webpage or to a different website; and
Include applicable model text and disclaimers (refer to Appendix 2) on each webpage.
Plan/Part D sponsor websites may not:
Provide links to foreign drug sales, including links from advertisements on the Plan/Part
D sponsor’s website;
Require users to enter any information other than a zip code, county, and/or state for
access to non-beneficiary specific website content; or
State that the Plan/Part D sponsor is not responsible for the content of their social media
pages or the websites of any downstream entity that provides information on the
Plan’s/Part D sponsor’s behalf.
Plan’s/Part D sponsor’s must ensure that their website:
18
Maintains a separate and distinct section for their Medicare information covered by these
guidelines if the Plan/Part D sponsor markets other lines of business. Plans/Part D
sponsors may not include or market Medicare Supplement (Medigap) content in their
Medicare information section;
Is reviewed monthly and updated as needed (See Prescription Drug Benefit Manual,
Chapter 6 for information on updates and notice to beneficiaries regarding midyear
formulary changes);
Includes the date of the last update on each webpage;
Clearly labels all links; and
Complies with Section 508 of the Rehabilitation Act.
70.1.2
– Documents to be posted on Website
42 CFR §§ 422.111(b) and (h)(2), 423.128(b) and (d)(2)
Plans/Part D sponsors must post on their website all required documents outlined below and
ensure these documents are downloadable. This includes translated documents, as applicable.
“(M)” denotes a required marketing material, and “(C)” denotes a required communication
material. If a required document is found to have errors, or is updated, the updated document
must posted on the website as soon as possible.
Required Document
Required Posting Date for
Renewing Plans
Required Posting date
for New Plans
Summary of Benefits (M)*
By October 15
By October 15
Annual Notice of Change (M)*
By October 15
Not applicable
Evidence of Coverage (C)*
By October 15
By October 15
Provider Directory (C)
By October 15
By October 15
Pharmacy Directory (C)
By October 15
By October 15
Formulary (C)*
By October 15
By October 15
CMS Star Ratings document
(M)*
21 days after the release of Star
Ratings on Medicare Plan
Finder
21 days after the release
of Star Ratings on
Medicare Plan Finder
Privacy Notice under the HIPAA
Privacy Rule*
Posted all year (updates as
required)
By January 1
Exception Request Forms for
physicians*
Posted all year (updates as
required)
By January 1
Utilization Management Forms
for physicians and enrollees*
Posted all year (updates as
required)
By October 15
19
Prescription Drug Transition
Policy*
Posted all year (updates as
required)
By January 1
LIS Premium Summary Chart
Posted all year (updates as
required)
By October 15
Prior Authorization Forms for
Physicians and Enrollees*
Posted all year (updates as
required)
By January 1
Part D Model Coverage
Determination and
Redetermination Request Forms
Posted all year (updates as
required)
By January 1
* If the document is updated following the initial posting, the date of the new document must be indicated in the link
or near the link
70.1.3
– Required Content
42 CFR §§ 417.427, 422.111(a)(3), (b) and (h)(2), 423.128(a)(3), (b) and (d)(2)
In addition to required documents, the following content must be present on the website:
Toll-free customer service number, days and hours of operation, TTY number, and either
a physical or Post Office Box address;
Information on enrollees’ and Plans’/Part D sponsors’ rights and responsibilities upon
disenrollment;
Instructions on how to appoint a representative and a link to the downloadable version of
the CMS Appointment of Representative Form (CMS Form-1696);
A description of and information on how to file a grievance, an organizational/coverage
determination, and an appeal, including:
o
Procedures for filing;
o
A direct link on the grievance/coverage determination webpage to the
Medicare.gov complaint website at:
https://www.medicare.gov/MedicareComplaintForm/home.aspx, where an enrollee
can enter a complaint in lieu of calling 1-800-MEDICARE;
o
Phone number(s) for receiving oral requests;
o
Mailing address for written requests;
o
Fax number for written requests;
o
Links, if applicable, to any forms created by the Plan/Part D sponsor for appeals
and grievances;
o
Information on how to obtain an aggregate number of grievances, appeals, and
exceptions filed with the Plan/Part D sponsor; and
o
Contact numbers for enrollees and/or physicians to use for process or status
questions.
A link to the PFFS Terms and Conditions of Payment (for PFFS plans);
Immediate access to the coverage determination and redetermination processes through a
secure location (for Plans offering Part D);
Quality assurance policies and procedures;
Drug and/or utilization management information (for plans offering Part D);
For MSA plan websites only:

20
o
The statement: “You must file Form 1040, ‘US Individual Income Tax Return,’
along with Form 8853, ‘Archer MSA and Long-Term Care Insurance Contracts’
with the Internal Revenue Service (IRS) for any distributions made from your
Medicare MSA account to ensure you aren’t taxed on your MSA account
withdrawals. You must file these tax forms for any year in which an MSA account
withdrawal is made, even if you have no taxable income or other reason for filing a
Form 1040. MSA account withdrawals for qualified medical expenses are tax free,
while account withdrawals for non-medical expenses are subject to both income
tax and a fifty (50) percent tax penalty.”
o
The statement: “Tax publications are available on the IRS website at
http://www.irs.gov or from 1-800-TAX-FORM (1-800-829-3676).”
Enrollment instructions and forms; and
Medication Therapy Management (MTM) program requirements as found on
https://www.cms.gov/Medicare/Prescription-Drug-
Coverage/PrescriptionDrugCovContra/MTM.html.
70.2
– Searchable Formularies and Directories
42 CFR §§ 422.111(h)(2), 423.128(b)(4), (b)(5), (d)(2)
Plans/Part D sponsors must have provider/pharmacy directories and formularies posted on their
websites for use and download/printing. Plans/Part D sponsors are encouraged to have searchable,
machine-readable formularies, provider, and pharmacy directories. A searchable tool (e.g., search
engine/database) may be a substitute for downloadable directories and formularies (i.e., electronic
versions of hard copies) when the searchable tool complies with all instructions and contains all
required template information, including introductory language and disclaimers, in the model
documents.
70.3
– Social Media
Plans/Part D sponsors must submit social media posts (e.g., Facebook, Twitter, YouTube) that
meet the definition of marketing into HPMS, using code 4038.
70.4
– Mobile Applications
42 CFR §§ 422.111, 422.2260, 422.2262, 422.2268, 423.128, 423.2260, 423.2262, 423.2268
Plans/Part D sponsors that use mobile applications (apps) must:
Submit data that meets the definition of marketing into HPMS;
Provide CMS, upon request, access to the mobile app;
If limited plan benefit/cost sharing information is in the app, instruct beneficiaries where
to find complete information; and
Provide equal prominence to all in-network providers/pharmacies if the app contains this
data. Apps that limit data based on geographic location must indicate the limited
geographic area.

21
80 – Call Centers
80.1
– Customer Service Call Center Requirements and Standards
42 CFR §§ 422.111(h)(1), 422.564, 422.2268, 423.128(d)(1), 423.564, 423.2268
Plans/Part D sponsors must operate a toll-free customer service call center. All requirements in
this section also apply to call centers operated by downstream entities.
Customer service call centers must:
Provide information in response to inquiries outlined in sections 80.3 and 80.4;
Follow an explicitly defined process for handling customer complaints;
Provide interpreter services when requested and inform callers that interpreter services are
free. Interpreters should be available within eight (8) minutes of reaching the customer
service representative (CSR);
Provide TTY service. CSRs available through the TTY service should be available within
seven (7) minutes of the time of answer;
Limit average hold time to two (2) minutes. The average hold time is defined as the time
spent on hold by the caller following the interactive voice response (IVR) system, touch-
tone response system, or recorded greeting and before reaching a live person;
Answer 80 percent of incoming calls within 30 seconds;
Limit the disconnect rate of all incoming calls to five (5) percent (a disconnected call is
defined as a call that is unexpectedly dropped by the Plan/Part D sponsor);
Plans/Part D sponsors are prohibited from using hold time messages to sell non-health
related products.
80.2
– Customer Service Call Center Hours of Operations
42 CFR §§ 422.111(h)(1)(i), 423.128(d)(1)(i)
Plans/Part D sponsor customer service call centers must operate during usual business hours.
Customer service call center hours and days must be the same for all individuals regardless of
whether they speak English, a non-English language, or use assistive devices for communication.
In light of the scope and nature of services and benefits provided by Plans/Part D sponsors, CMS
interprets usual business hours as meaning at least the following:
October 1 – March 31:
• Live CSRs available seven days a week, from 8:00 a.m. to 8:00 p.m. in all
time zones for the regions in which they operate; and
• Interactive voice response system or similar technologies for Thanksgiving
and Christmas Day (messages must be returned within one (1) business day)
April 1 – September 30:
• Live CSRs available Monday through Friday, from 8:00 a.m. to 8:00 p.m. in
all time zones for the regions in which they operate; and
• Interactive voice response system or similar technologies for Saturdays,
Sundays and Federal Holidays (messages must be returned within one (1)
business day)

22
80.3
- Informational Scripts
42 CFR §§ 422.111(b) and (c), 422.2262, 422.2264, 423.128(b) and (c), 423.2262, 423.2264
Informational scripts are communications intended to educate and answer beneficiary questions.
Informational scripts must make it clear when a beneficiary is going to be transferred to a
sales/enrollment department (i.e., the conversation moving from a communication activity to a
marketing activity). Before making any transfer to a sales/enrollment (i.e., marketing) department,
CSRs must receive the beneficiaries consent, ideally with a yes/no question.
The following represents a sampling of topics for which Plans/Part D sponsors must have scripted
language to address enrollee questions:
Best Available Evidence policy (applicable for Part D);
Request for pre-enrollment information;
Benefit and cost sharing information, including out-of-network information;
Formulary information, including transition process;
Pharmacy information, including whether a beneficiary’s pharmacy is in the Part D
sponsor’s network;
Provider information, including whether a beneficiary’s physician is in the plan’s network
Claims submission, processing, and payment information Grievance,
organization/coverage determination (including exceptions) and appeals process
information;
Information on extra help, including how the beneficiary can obtain extra help;
Current true out-of-pocket status (applicable for Part D);
Information on how to obtain needed forms;
Information on replacing an enrollee’s identification card; and
Service area information.
80.4
– Telesales and Enrollment Scripts
42 CFR §§ 422.60(c), 422.2262, 422.2264, 422.2268, 423.32(b), 423.2262, 423.2264, 423.2268
Telesales and enrollment scripts are considered marketing and must be submitted to CMS as
outlined in Section 90. If a sales call progresses to a telephonic enrollment, the sales staff must
clearly inform the beneficiary that they are enrolling into the Plan (using the specific Plan
name/type).
In addition, enrollment scripts must:
Follow all requirements described in CMS Eligibility and Enrollment Guidance
(https://www.cms.gov/Medicare/Eligibility-and-
Enrollment/MedicareMangCareEligEnrol/index.html) (Chapter 2 of the Medicare
Managed Care Manual) and Chapter 17, Subchapter D, of the Medicare Managed Care
Manual, as well as Chapter 3 of the Medicare Prescription Drug Benefit Manual
(https://www.cms.gov/Medicare/Prescription-Drug-
Coverage/PrescriptionDrugCovContra/PartDManuals.html);
Provide confirmation of having accepted/completed the telephone enrollment request,
such as a confirmation tracking number or other tracking mechanism;
23
Provide a statement that the individual will receive a notice acknowledging receipt of the
enrollment (e.g., acknowledging request for additional information or denial of
enrollment); and
Provide contact information for questions including toll-free telephone and TTY numbers.
80.5
– Pharmacy Technical Help Call Center Requirements and Standards
42 CFR § 423.128(d)(1)
Part D sponsors must operate a toll-free pharmacy technical help call center or make available
call support to respond to inquiries from pharmacies and providers regarding the beneficiary’s
Medicare prescription drug benefit. Inquiries may pertain to operational areas such as claims
processing, benefit coverage, claims submission, and claims payment. This requirement can be
accommodated through the use of on-call staff pharmacists or by contracting with the
organization’s Pharmacy Benefit Manager (PBM) during non-business hours as long as the
individual answering the call is able to address the call at that time.
The call center technical assistance for pharmacies and providers must operate or be available
during usual business hours, which CMS interprets to mean during the entire period in which the
Part D sponsor’s network pharmacies in its Plans’ service areas are open (e.g., Part D sponsors
whose pharmacy networks include twenty-four (24) hour pharmacies must operate their
pharmacy technical help call centers twenty-four (24) hours a day as well).
The pharmacy technical help call center must operate within the following standards:
Average hold time not to exceed two (2) minutes (the average hold time is defined as the
time spent on hold by the caller following the interactive voice response (IVR) system,
touch-tone response system, or recorded greeting and before reaching a live person);
Eighty (80) percent of incoming calls answered within thirty (30) seconds; and
Disconnect rate of all incoming calls not to exceed five (5) percent.
80.6
– Part D Sponsor Coverage Determinations and Appeals Call Center Requirements
and Standards
42 CFR §§ 423.128(b)(7), 423.128(d)(1)(iv), 423.566(a)
All Part D sponsors must operate a toll-free call center with live customer service representatives
available to respond to providers or enrollees for information related to coverage determinations,
including exceptions, prior authorizations, and appeals. Sponsors must provide immediate access
to the coverage determination and redetermination processes via their toll-free call centers.
Sponsors must operate during normal business hours, 8:00 a.m. to 8:00 p.m., Monday through
Friday; in the time zones for the regions in which they operate. Part D sponsors are expected to
accept requests for coverage determinations/redeterminations outside of normal business hours,
but are not required to have live customer service representatives available to accept such
requests outside normal business hours. Additional details are available in Chapter 18 of the
Medicare Prescription Drug Benefit Manual (http://www.cms.gov/Medicare/Prescription-Drug-
Coverage/PrescriptionDrugCovContra/PartDManuals.html).
Voicemail may be used outside of normal business hours and the voicemail message should:
Indicate that the mailbox is secure;
24
List the information that must be provided so the case can be worked, (e.g., provider
identification, beneficiary identification, type of request (coverage determination or
appeal), physician support for an exception request, and whether the enrollee is making an
expedited or standard request);
For coverage determination calls (including exceptions requests), articulate the process for
resolution within twenty-four (24) hours of call for expedited requests and seventy- two
(72) hours for standard requests;
For appeals calls, articulate the process for resolution within seventy-two (72) hours for
expedited appeal requests and seven (7) calendar days for standard appeal requests; and
For plans that permit oral requests for payment appeals, articulate that different
timeframes apply to such appeal requests.
80.7
– Activities That Do Not Require the Use of State-Licensed Marketing Representatives
Certain activities conducted by Plan/Part D sponsor customer service representatives do not
require the use of state-licensed marketing representatives, unless required by state law. Such
activities include:
Providing factual information;
Fulfilling a request for materials; or
Taking demographic information in order to complete an enrollment application at the
initiative of the prospective enrollee.
Licensed agents/brokers (employed or contracted) that are customer service representatives
cannot act simultaneously as both a customer service representative and a sales/marketing
agent/broker. Such individuals must make it clear to the beneficiary when the individual’s role
changes to a marketing/sales role, subject to beneficiary request and concurrence.
90 – Tracking, Submission, and Review Process
90.1
– Material Identification
42 CFR §§ 422.2262, 423.2262, 422.2264, 423.2264
CMS requires Plans/Part D sponsors to use a standardized method of identification (material ID)
for oversight and tracking of materials beneficiaries receive. This material ID is required on all
materials except:
Membership ID card;
Envelopes, radio ads, outdoor advertisements, banner or banner-like ads, and social media
comments and posts;
OMB-approved forms/documents, except when otherwise specified by CMS; and
Agent-developed communication materials that are not marketing.
The material ID is made up of three parts:
Plan’s/Part D sponsor’s contract or MCE number, (i.e., “H” for MA or Section 1876 Cost Plans,

25
“R” for Regional PPO plans (RPPOs), “S” for PDPs, or “Y” for Multi-Contract Entity (MCE)
identifier) followed by an underscore;
Any series of alpha numeric characters (Plan/Part D sponsor discretion) followed by an
underscore; and
An uppercase “C” for communication materials or an uppercase “M” for marketing materials (for
example: H1234_abc123_C or H5678_efg456_M). The material ID for multi-plan marketing
materials must begin with the word “MULTI-PLAN” instead of the organization’s contract
number.
Note: All website pages (including plan-required and third-party) must have a Material ID,
but the ID does not have to include “M” or “C”.
Non-English and alternate format materials, based on previously created materials, may have the
same material ID as the material on which they are based. Refer to section 90.3 for additional
information about the submission of non-English and alternate format materials.
90.1.1 – Materials Subject to Submission
42 CFR §§ 422.2262, 422.111, 423.2262, 423.128
The HPMS Marketing Module is the system of record for the collection, review, and storage of
designated materials. Please refer to the HPMS User Guide for questions regarding the Marketing
Module and how to submit materials in HPMS.
Plans/Part D sponsors must submit all marketing materials to CMS using HPMS. Only those
required communication materials designated by CMS (e.g., EOC) require prospective submission
to HPMS. Please see section 100 on submission requirements for required materials.
HPMS indicates the review time based on the material type. Prospective review materials have a
review timeframe of 10 or 45 days, which begins on the date the material is submitted.
Note: Under certain circumstances, and with prior approval from CMS, Plans/Part D
sponsors may submit materials other than through HPMS.
90.2
– Material Replacement
42 CFR §§ 422.2262, 423.2262
If Plans/Part D sponsors change their current year documents, the Plan/Part D sponsor must
resubmit updated materials. For the specified materials below, HPMS now has a “material
replacement” functionality to allow updated materials to be resubmitted using the same material
ID. Plans/Part D sponsors must resubmit the following materials using the material replacement
function:
ANOC
SB
EOC
Star Ratings
Sales scripts and presentations
Enrollment scripts

26
Plans/Part D sponsors may make corrections to all other materials by marking the original material
as “no longer in use” and then resubmitting the replacement under a new material ID.
Note: Non-English and alternate format materials should accurately reflect any changes or
revisions that Plans/Part D sponsors make to the original English version.
90.3
– Non-English Language and Alternate Format Materials
42 CFR §§ 422.2262, 422.2268(a)(7), 423.2262, 423.2268(a)(7)
Plans/Part D sponsors are not required to submit non-English language materials that are based on
an English version. If a Plan/Part D sponsor creates a material to be used only in a non- English
language, the Plan/Part D sponsor must submit an English translation to HPMS.
Plans/Part D sponsors are not required to submit alternate format versions of a previously
submitted standard material.
Plans/Part D sponsors must submit multi-lingual marketing materials that include English and
another language (or languages). CMS requires Plans/Part D sponsors to include a note in the
comments field specifying that the material is multi-lingual.
Refer to Appendix 1 for the definition of alternate format materials.
90.4
– Submission of Websites and Webpages for Review
42 CFR §§ 422.2262, 422.2264, 423.2262, 423.2264
Websites containing any marketing content must be submitted to CMS via HPMS. Plans/Part D
sponsors must use code 4006 for required websites and code 4037 for other websites, including
those managed by contracted third parties. All websites are considered File & Use submissions.
An initial website must be submitted as a Word document containing the URL. Screenshots, test
sites, etc. are not needed. The website may go live five days after HPMS submission. Subsequent
submissions (e.g., for website updates) must include the website’s URL on a Word document and a
summary of the changes. The updates may go live five days after the HPMS submission.
Note: Plans/Part D sponsors are not required to take down their website while making
updates. However, the requested changes must not go live for five days following the
submission of the changes.
90.5
– Submission of Multi-Plan Materials
42 CFR §§ 422.504(l), 422.2262, 423.505(l), 423.2262
Plans/Part D sponsors are responsible for the content of multi-plan materials, including those
created by or the activities of third-party on behalf of several Plans/Part D sponsors. Plans/Part D
sponsors must ensure that all materials developed on their behalf remain compliant with the most
current CMS requirements.
27
Relevant terms for the multi-plan submission process include:
Primary Material - The base marketing material that serves as a model for submission
by Plans/Part D sponsors. Primary materials must first be submitted for review/acceptance
prior to all subsequent auxiliary submissions.
Auxiliary Material - The secondary versions of the CMS-approved Primary Material
used by other plans.
Coordinating Entity (CE) - The third-party entity that develops the Primary Material for
use by the Plans/Part D sponsors with which it contracts.
Lead Plan/Part D sponsor (LP) - Contracted Plan/Part D sponsor that submits the
Primary Material for CMS review.
Non-Lead Plan/Part D sponsor (NLP) - A Plan/Part D sponsor that produces and
submits the Auxiliary Material to CMS, based on the approved a Primary Material.
Steps for Multi-Plan Submission:
1.
The LP submits the Primary Material into HPMS (LP Region reviews) using the correct
material type under the standard submission. The LP must insert the following in the
comments field in HPMS:
a.
“MULTI-PLAN MARKETING MATERIAL PRIMARY” (standardized text) in
the first line.
b.
The name and role of the CE (e.g., ABC FMO or XYZ PBM) inserted in the
second line.
c.
A list of all Multi-Contract Entities (MCE) or Plan/Part D sponsor contracts for
which the material is applicable
d.
Any other information necessary for CMS review
2.
Once approved/accepted, the LP notifies the CE.
3.
The CE provides the NLPs with the primary material’s material ID number and marketing
code.
4.
The NLPs submit Auxiliary Materials. The Auxiliary Material ID must be identical to the
approved/accepted Primary Material. The NLPs must insert the following in the
comments field in HPMS:
a.
“MULTI-PLAN MATERIAL AUXILIARY” (standardized text) in the first line.
b.
The name and role of the CE
c.
Brief description of the material’s previous submission history (e.g., This Multi-
plan website was approved by CMS on Month/Day/Year. It was initially submitted
by ABC123 Health Care under XXXX material type).
For Multi-plan submission:
NLPs may not make changes to the previously approved/accepted piece, except to
variable fields, name/logo, or changes allowed by CMS.
NLP auxiliary materials qualify as File & Use and may be distributed following the five
(5) calendar day waiting period.
Communications regarding this process should be documented for tracking purposes.
The following guidelines apply when Plans/Part D sponsors wish to add an additional
Plan/Part D sponsor to a previously approved/accepted multi-plan material:
28
If a CE wishes to use a material for a Plan/Part D sponsor not identified in the original LP
submission (e.g., because a CE contracts with a new Plan/Part D sponsor) the new NLP
submits the material and provides an explanation in the comments of HPMS for why it
was not listed in the initial listing of contract numbers (e.g., the NLP was not contracted
with the CE during the initial submission). The Plan/Part D sponsor should also include
the name, phone, and email contact of the CE.
90.6
– Status of HPMS Material
42 CFR §§ 422.2262, 423.2262
Plans/Part D sponsors should refer to regulations, laws, the MCMG, and other relevant guidance
in developing materials to avoid disapproval by CMS. A disposition indicator will be provided
for all materials submitted in HPMS. This includes indicators such as: accepted, alternate format,
approved, deemed, disapproved, non-marketing, pending, populated template, SA/LIS, or
withdrawn, as defined below:
Note: If a Plan/Part D sponsor does not have an executed contract with CMS, all submitted
materials will be marked as conditional.
Accepted: Indicates that the marketing material, submitted as File & Use, may be used in the
submitted format. The Plan/Part D sponsor may distribute it five (5) calendar days after the
submission date.
Alternate Format: Indicates the material is submitted in a non-English format.
Approved: Indicates the material may be distributed by a Plan/Part D sponsor in the submitted
format.
Deemed: If CMS does not approve or disapprove materials within the specified review time
frame, the materials are deemed approved. The following conditions apply:
Materials subject to a forty five (45) day review period will be given the status of
“deemed” on day 46;
Materials subject to a ten (10) day review period will be given a status of “deemed” on
day 11; and
Materials from a Plan/Part D sponsor without an executed contract with CMS will receive
a conditional deemed approval. After the contract is awarded, the material disposition will
be changed to “deemed” and can be used by the Plan/Part D sponsor.
Disapproved: Indicates that the material does not comply with applicable standards. CMS will
provide a reason for the disapproval in HPMS.
Non-Marketing: Indicates that the material type is designated as “Non-Marketing.” Plans/Part D
sponsors should use the Marketing Code Lookup in HPMS to identify Non-Marketing review
materials.
Pending: Indicates that the material is currently under review.

29
Populated Template: Plans/Part D sponsors should submit populated standard templates in HPMS
under “Final Expedited Review” codes. These documents will receive a status of “Populated
Template (refer to section 90.14).”
SA/LIS: Applies to special categories of materials. Please see the HPMS User Guide for an
explanation of when this status is applicable (see section 90.2 for information on the material
submission process).
Withdrawn: Plans/Part D sponsors may request to withdraw a submission prior to use. CMS
requires the Plan/Part D sponsor to submit a written request to the Account Manager or Marketing
Reviewer stating the reason(s) for the withdrawal. Plans/Part D sponsors may not distribute
withdrawn materials.
Please see the HPMS User Guide for additional information.
Note: Materials that have been used, but are no longer used should be marked “no longer in
use.”
90.7
– Resubmitting Previously Disapproved Pieces
42 CFR §§ 422.2262, 423.2262
When a Plan/Part D sponsor resubmits a previously disapproved material, the Plan/Part D sponsor
should clearly indicate all changes/updates to the material by highlighting text changes or
inserting notes or identifying changes in the comments section.
Note: CMS may require a Plan/Part D sponsor to change previously submitted materials if
they are found to be inaccurate, misleading, altered, or otherwise non-compliant.
90.8
– File & Use Process
42 CFR §§ 422.2262(b), 423.2262(b)
Under the File & Use process, Plans/Part D sponsors must submit eligible marketing materials at
least five (5) calendar days prior to their distribution and certify that the materials comply with all
applicable standards. The Marketing Code Lookup in HPMS identifies those materials that
qualify for File & Use.
Plans/Part D sponsors without an executed contract may submit File & Use materials. However,
please note that once the contract is executed, CMS presumes that the officer of the Plan/Part D
sponsor has, by submission, attested that the material complies with the File & Use requirements.
Plans/Part D sponsors may be subject to compliance actions if they:
Submit or use materials that do not meet the applicable requirements; or
Fail to file material(s) at least five (5) calendar days prior to its distribution.

30
90.9
– File & Use Retrospective Monitoring Reviews
42 CFR §§ 422.2262(b), 422.2264, 423.2262(b), 423.2264
CMS may periodically conduct retrospective reviews of materials that Plans/Part D sponsors
submit under File & Use to ensure compliance with requirements.
90.10
– Standardized Model Materials
42 CFR §§ 422.2262(c), 423.2262(c)
CMS provides Plans/Part D sponsors with standardized model materials that must be used
without modification to the language, content, format, or order, except as noted below. Some of
these materials must be submitted in HPMS. If submission is required, as articulated in section
100, Plans/Part D sponsors must indicate the model/exhibit title and applicable CMS
chapter/manual or HPMS memorandum date within the comments section of HPMS.
CMS allows Plans/Part D sponsors to make the following changes to all standardized materials:
Populating variable fields;
Correcting grammatical errors;
Adding customer service phone numbers;
Adding plan name/logo;
Adding required disclaimers (refer to Appendix 2 for the appropriate disclaimers);
Adding plan type; and
Adding the CMS marketing material identification number.
90.11
– Non-Standardized Model Materials
42 CFR §§ 422.2262(c), 423.2262(c)
CMS provides non-standardized models that Plans/Part D sponsors may use at their discretion.
A Plan/Part D sponsor that chooses to modify the non-standardized model must ensure that the
non-model document the Plan/Part D sponsor creates contains all content that is in the model.
Plans/Part D sponsors must remove any reference to the words “exhibit,” “model,” or “appendix”
used in the title of the model document from the title of the non-model document.
90.12
– Template Materials
42 CFR §§ 422.2262, 423.2262
“Template materials” are marketing materials that include placeholders for variable data that a
Plan/Part D sponsor will populate at a later time. Template materials are created by Plan/Part D
sponsors and are classified as static or standard. Prior to submitting these materials in HPMS,
Plans/Part D sponsors should use the Marketing Code Lookup to verify that the material type
allows for the submission of templates.
Unpopulated templates must first be submitted with placeholders identifying the field or listing

31
all variables (e.g., “<date>”, “<$10.00 Copay/$15.00 Copay>”). If a Plan/Part D sponsor changes
the non-variable text, the Plan/Part D sponsor must resubmit the template in HPMS.
90.13
– Static Templates
42 CFR §§ 422.2262, 423.2262
Template materials are considered “static” when they include placeholders for the following
variable data fields ONLY:
Dates;
Events;
Addresses, phone or fax numbers;
Hours of operation;
Organization or company names;
Plan/Part D sponsor name;
Logos;
Agent/Agency;
Federal contracting statement/disclaimer (refer to Appendix 2);
Persons’ names and pronoun variations;
Enrollee specific variables (e.g., case numbers, drug specific references);
Co-branding information;
Photos;
Email and web addresses; and
Page number references.
CMS does not require Plans/Part D sponsors to submit populated static templates in HPMS.
90.14
– Standard Templates
42 CFR §§ 422.2262, 423.2262
Standard Templates are marketing materials that include placeholders for variable data not listed
in section 90.13, such as plan specific benefits, premiums, and cost sharing. These materials must
be submitted into HPMS.
Plans/Part D sponsors must submit final, populated versions of standard templates in HPMS using
the associated “Final Expedited Review” code within thirty (30) days of use. If the template does
not have a “Final Expedited Review” code, Plans/Part D sponsors may not submit the document
as a standard template. Plans/Part D sponsors must instead submit the document with all fields
populated, other than those fields listed in section 90.13. Refer to the HPMS Users’ Guide for
technical template submission instructions.

32
100 – Required Materials
42 CFR §§ 422.111, 422.2264(a), 423.128, 423.2264(a)
This section provides guidance specific to additional CMS required materials. It also outlines the
required materials that Plans/Part D sponsors must have ready to provide to potential and/or
existing enrollees. Required materials must be in 12-point Times New Roman font or equivalent.
Unless otherwise noted, the materials below designated as communications materials do not
require HPMS submission. Materials must be provided as outlined below and must also be
provided upon enrollee request. In addition, Plans/Part D sponsors must provide materials in
alternate formats upon request.
100.1
– Mailings to Multiple Beneficiaries at One Household
42 CFR §§ 422.111, 422.2264, 423.128, 423.2264
Plans/Part D sponsors may mail a single copy of the ANOC, EOC, Provider/Pharmacy Directory,
Formulary, and/or notification for electronic documents as described below to multiple members
of a household provided members are in the same plan, have the same address, including
apartment number (if applicable), and the Plan/Part D sponsor reasonably believes the members
are related. The documents (e.g., envelope, cover letter) must clearly notate each individual name.
Members in community residences (e.g., nursing facilities, group homes) must receive their own
document, regardless of whether they have the same address.
100.2
– Electronic Delivery of Materials
42 CFR §§ 422.64, 422.111, 423.48, 423.128
CMS permits the electronic delivery of materials. This section outlines two distinct processes for
electronic delivery. The first, as outlined in section 100.2.1, allows Plans/Part D sponsors to make
certain required materials available to beneficiaries without requiring the beneficiary to opt-in. The
second, as outlined in section 100.2.2, allows for a wider range of materials to be delivered
electronically, but also requires the beneficiary to first opt-in.
100.2.1
- Notification of Availability of Electronic Materials
42 CFR §§ 422.64, 422.111, 423.48, 423.128
Without prior beneficiary authorization, Plans/Part D sponsors may send existing (i.e., not
prospective) enrollees a notice informing enrollees how to access CMS designated required
materials electronically instead of mailing hard copies of the documents. The following required
materials may use this process:
EOC;
Provider/Pharmacy Directories; or
Formularies.
The notice may reference multiple required documents (e.g., EOC, Provider Directory), must
include the plan website to access these documents, the date the documents will be available, and,
at minimum, a phone number to request hardcopy documents (additional methods of requesting a
hardcopy may also be included with the phone number).

33
Note: It is acceptable to state “currently available” instead of a date if the documents are
already posted before the notice.
If a plan sends a notice that required documents are available online, CMS expects the notice to be
mailed no earlier than September 1. Such notes must be sent in time for an enrollee to receive the
documents by October 15.
Plans/Part D sponsors may also send a notice informing new members enrolling throughout the
year (e.g., June 1 effective date) how to access the EOC, Provider/Pharmacy Directories, and/or
Formularies. If an enrollee requests any of these documents in hardcopy, the Plan/Part D sponsor
must mail the hard copy within three (3) business days of the request. The hardcopy request
remains until the enrollee leaves the plan or requests that hard copies be discontinued
Note: Plans/Part D sponsors may inquire to the member whether the request for a hard
copy is a one-time request or is a request to receive the document in hard copy
permanently.
100.2.2
– Electronic Delivery of Required Materials
42 CFR §§ 422.64, 422.111, 423.48, 423.128
In addition to providing electronic access to the materials outlined in 100.2.1, with prior consent
from the enrollee, Plans/Part D sponsors can provide any materials in different media types (e.g.,
email, web portal, CD, DVD). Plans must:
Obtain prior consent from the enrollee;
Specify both the media type and the specific materials;
Provide an opt-out mechanism so enrollees can receive hard copies;
When applicable, provide instructions on how and when enrollees can access the
electronic documents (for example, if the Plan/Part D sponsor posts the information on a
website, they must also provide an email or hardcopy notice informing the enrollee where
and when the Plan/Part D sponsor will post the document);
Provide hard copies of all enrollee materials (excluding plan web pages) to enrollees upon
request;
Ensure enrollee contact information is current, communication materials are delivered and
received timely and appropriately, and materials are identified in a way that enrollees
understand their importance; and
Have a process for automatic mailing of hard copies when electronic versions or choice of
media types are undeliverable (e.g., an expired email account).
Documents delivered electronically will be considered to be received by the enrollee as of the date
the Plan/Part D sponsor sends it, not when the enrollee opens/accesses it.
100.3
– Changes and Corrections to Existing Documents
42 CFR §§ 422.2262, 423.2262
Plans/Part D sponsors must review all required documents for accuracy. If changes or corrections
to submitted materials (e.g., the benefit or cost-sharing information differs from that in the
34
approved bid) are required, the Plan/Part D sponsor must correct those materials and resubmit as
applicable. CMS encourages Plans/Part D sponsors to reprint the corrected version of the ANOC
and SB. Below are the mailing and timeframe requirements regarding corrected documents.
ANOC, EOC, and formulary erratas must be sent in hard copy within a reasonable
timeframe;
SB addenda or reprints must be sent only to existing enrollees if the Plan/Part D sponsor
mass mailed the SB; and
Both hardcopy and online provider/pharmacy directories must be updated within 30
calendar days of receipt of updated or corrected information from the provider/pharmacy
to ensure accurate information is provided to enrollees. Upon enrollee request, updated
hardcopy directories (reprint including addenda) must be mailed within three (3) business
days of the request.
100.4
– List of Required Materials
42 CFR Parts 417, 422, 423
Below is a list of required materials identifying each item as either “Marketing” or
“Communications.” In addition, high-level information is provided for each material. Guidance
(as noted) should be reviewed as applicable. Additionally, Plans/Part D sponsors should consult
the HPMS Marketing Code Lookup for specific codes for uploading required materials.
Note: Required materials (e.g., drug recall notices) not listed below are considered
communications.
Plans/Part D sponsors may enclose additional benefit/plan operation materials with required
materials, unless specifically prohibited in instructions (e.g., ANOC instructions) or prohibited as
noted below for each material. Additional materials must be distinct from required materials and
must be related to the plan in which the beneficiary enrolled.
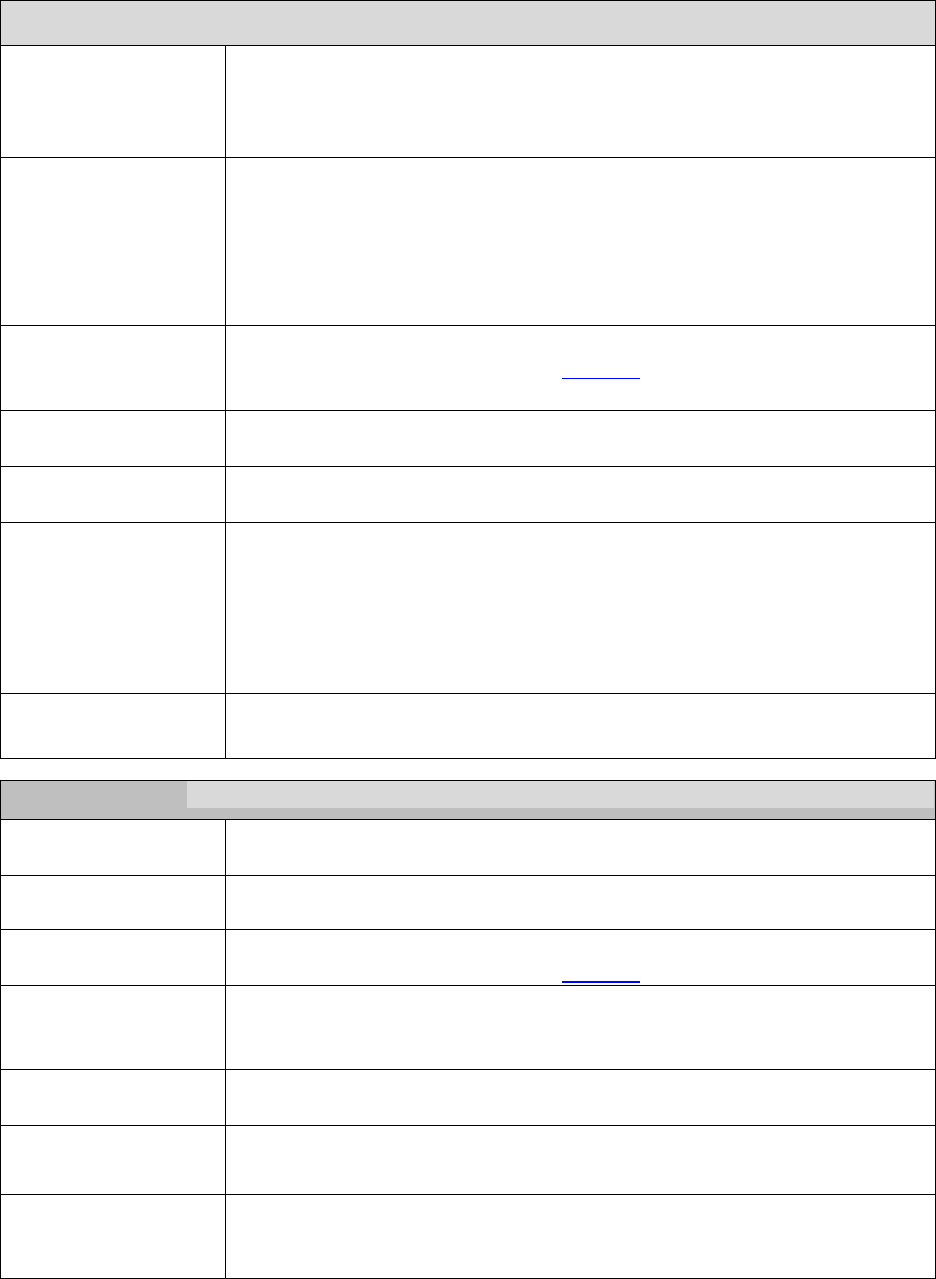
35
Annual Notice of Change (Marketing)
To Whom Required:
Must be provided to current enrollees of plan, including those with
October 1, November 1, and December 1 effective dates. October 1,
November 1, and December 1 enrollees must receive both the current
year EOC and the next calendar year EOC.
Timing:
All Plans/Part D sponsors and all 1876 cost plans must send for
enrollee receipt no later than September 30 of each year.
Note: ANOC must be posted on Plan/Part D website by 10/15.
October 1, November 1, and December 1 enrollees must receive
within ten (10) calendar days from receipt of CMS confirmation of
enrollment or by last day of month prior to effective date, whichever
is later.
Method of Delivery:
Hard copy, or electronically, if enrollee has opted into receiving
electronic version as permitted in 100.2.2.
HPMS Timing and
Submission:
Code 1140 File and Use. Must be submitted prior to mailing ANOCs.
May be used immediately following submission.
Format
Specification:
Model Required - Current Contract Year. Modifications permitted per
instructions.
Guidance and Other
Needed Information:
Actual Mail Dates (AMDs) and number of recipients (not the
number of ANOCs mailed) must be entered into HPMS within 15
days of mailing.
Plans may include the following with the ANOC:
o
Summary of Benefits
o
Notification of Electronic Documents
Translation Required
(5% Threshold):
Yes.
ANOC (Marketing) and EOC (Communications) Errata
To Whom Required:
Must be provided when errors are found in the ANOC or EOC and sent
to current enrollees.
Timing:
Must send to enrollees immediately following CMS approval.
Method of Delivery:
Hard copy, or electronically, if enrollee has opted into receiving
electronic version as permitted in 100.2.2.
HPMS Timing and
Submission:
Codes 1160 (ANOC Errata) and 1170 (EOC Errata). ANOC errata must
be submitted by October 15. EOC errata must be submitted by
November 15.
Format
Specification:
Model required.
Guidance and Other
Needed Information:
Refer to the annual “Issuance of Contract Year Model Materials” memo.
Translation Required
(5% Threshold):
Yes.
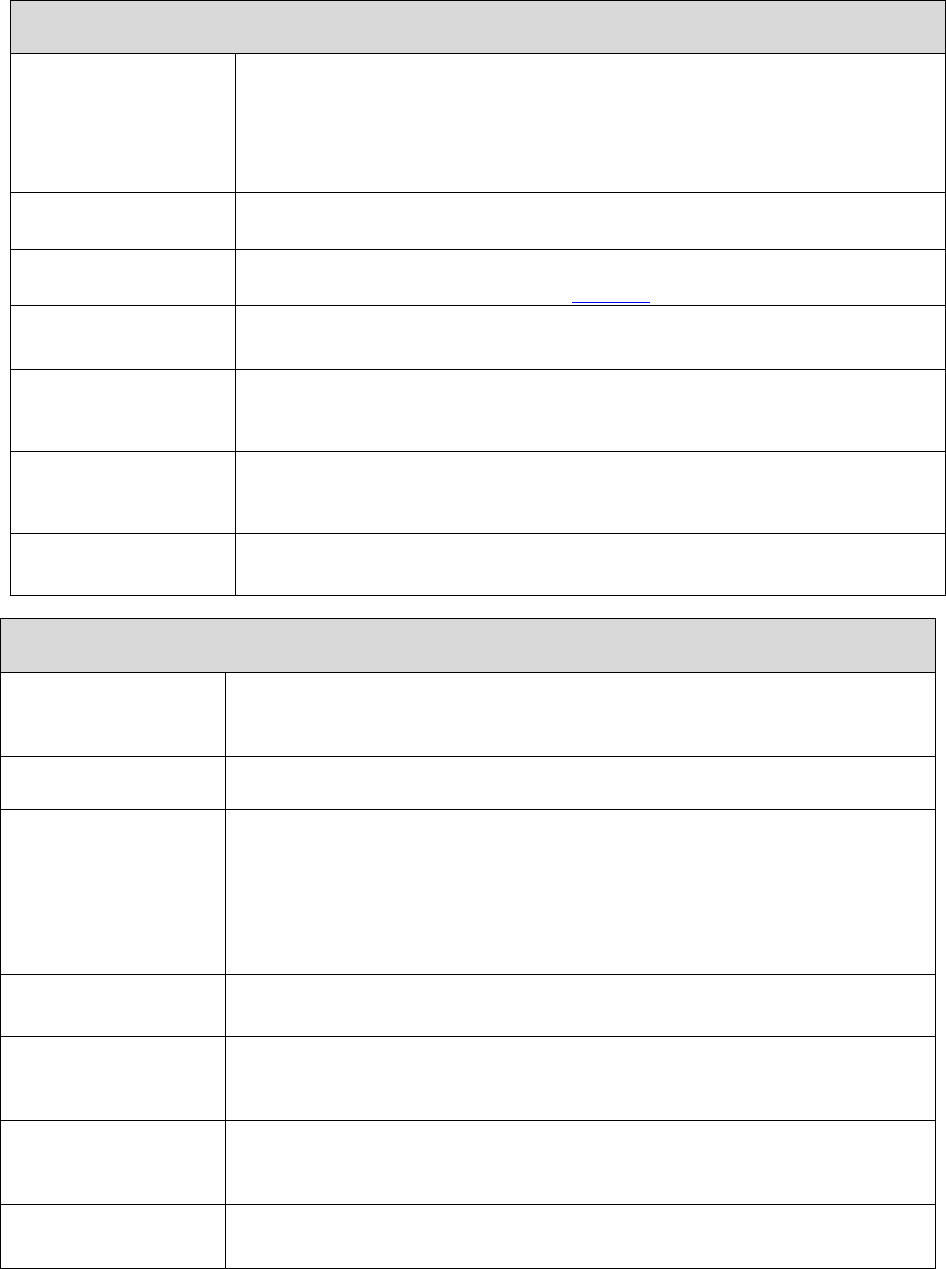
36
Coverage/Organization Determination, Discharge, Appeals and Grievance Notices
(Communications)
To Whom Required:
Must be provided to enrollees who have requested an appeal or have
had an appeal requested on their behalf. A grievance requested by an
enrollee in writing (or on the enrollee’s behalf) must be responded to in
writing. Grievances requested orally may be responded to orally or in
writing, unless the enrollee requests a written response.
Timing:
Provided to enrollees (generally by mail) on an ad hoc basis, based on
required timeframes in Parts 422 and 423, subpart M.
Method of Delivery:
Hard copy, or electronically, if enrollee has opted into receiving
electronic version as permitted in 100.2.2.
HPMS Timing and
Submission:
Not applicable.
Format
Specification:
Standardized OMB-approved denial notices for initial coverage denials;
model notices for plan level appeals.
Guidance and Other
Needed Information:
Chapter 13 of the Medicare Managed Care Manual and Chapter 18 of
the Medicare Prescription Drug Benefit Manual.
Translation Required
(5% Threshold):
Yes.
Enrollment Form/Request (Communications)
To Whom Required:
Upon request. All organizations/sponsors must have available a paper
enrollment form in addition to any other acceptable enrollment
mechanisms.
Timing:
Not applicable.
Method of Delivery:
Paper enrollment forms may be in hard copy or electronic format (e.g.,
PDF file) and must be provided via email, online portal for current
members, and upon request (e.g., if beneficiary does not want to enroll
telephonically or electronically). Any enrollment mechanism outlined in
enrollment guidance is acceptable for an enrollment request.
HPMS Timing and
Submission:
Submission required by statute. Code 1070 or 1072.
Format
Specification:
Model provided, modification permitted. Must follow requirements for
enrollment mechanisms and required data elements outlined in
enrollment guidance.
Guidance and Other
Needed Information:
Eligibility, Enrollment, and Disenrollment – Medicare Managed Care
Manual-Chapters 2 and 17D, and Medicare Prescription Drug Manual-
Chapter 3.
Translation Required
(5% Threshold):
Yes.
37
Enrollment and Disenrollment Notices (Communications)
To Whom Required:
Must be provided as outlined in enrollment guidance.
Timing:
Varies; must follow required timeframes as outlined in enrollment
guidance.
Method of Delivery:
Hard copy, or electronically, if enrollee has opted into receiving
electronic version as permitted in 100.2.2.
HPMS Timing and
Submission:
Not applicable.
Format
Specification:
Models provided, modification permitted. Must include elements
outlined in enrollment guidance.
Guidance and Other
Needed Information:
Includes all enrollment processing notices (acknowledgement,
confirmation, denial, rejection, request for more information,
attestation of creditable coverage, cancellation, reinstatement, etc.).
Includes all enrollment adjustment notices (700-series transaction
reply code notices, Part D late enrollment penalty notices, etc.).
Includes all disenrollment notices (advance warning, research of
possible ineligibility, disenrollment, cancellation, request for more
information, etc.).
Plans must maintain copies of required notices, as outlined in
enrollment guidance. Eligibility, Enrollment, and Disenrollment –
Medicare Managed Care Manual-Chapters 2 and 17D and Medicare
Prescription Drug Manual-Chapter 3.
Creditable Coverage Determinations and Late Enrollment Penalty
– Medicare Prescription Drug Manual – Chapter 4.
Non-renewal and Service Area Reduction Guidance.
Translation Required
(5% Threshold):
Yes.
38
Evidence of Coverage (Communications)
To Whom Required:
Must be provided to all enrollees of plan.
Timing:
Must be provided to current enrollees of plan by October 15 of each
year.
Must be provided to new enrollees within ten (10) calendars days
from receipt of CMS confirmation of enrollment or by last day of
month prior to effective date, whichever is later.
Method of Delivery:
Hard copy; made available electronically, as permitted in 100.2.1; or
electronically, if enrollee has opted into receiving electronic version as
permitted in 100.2.2.
HPMS Timing and
Submission:
Code 1150 File and Use. Submitted prior to October 15 of each year.
May be used immediately following submission.
Format
Specification:
Model required. Current Contract Year EOC.
Modifications permitted per instructions.
Guidance and Other
Needed Information:
No additional information.
Translation Required
(5% Threshold):
Yes.
Excluded Provider Letter (Communications)
To Whom Required:
When a sponsor has excluded a prescriber or pharmacy from
participating in the Medicare program based on an OIG exclusion.
Timing:
Provided to enrollees on an ad hoc basis.
Method of Delivery:
Hard copy, or electronically, if enrollee has opted into receiving
electronic version as permitted in 100.2.2
HPMS Timing and
Submission:
Not applicable.
Format
Specification:
Model required.
Guidance and Other
Needed Information:
https://oig.hhs.gov/fraud/exclusions.asp
Translation Required
(5% Threshold):
Yes.
39
Explanation of Benefits – Part C (Communications)
To Whom Required:
Must be provided anytime an enrollee utilizes a Part C benefit.
Timing:
Plan may send monthly or per claim with a quarterly summary.
Method of Delivery:
Hard copy, or electronically, if enrollee has opted into receiving
electronic version as permitted in 100.2.2.
HPMS Timing and
Submission:
Not applicable.
Format
Specification:
Model provided, modification permitted.
Guidance and Other
Needed Information:
Managed Care Manual Chapter 4, Section 190.
Translation Required
(5% Threshold):
Yes.
Explanation of Benefits – Part D (Communications)
To Whom Required:
Must be provided anytime an enrollee utilizes their prescription drug
benefit.
Timing:
Sent at the end of month following the month when benefit was utilized.
Method of Delivery:
Hard copy, or electronically, if enrollee has opted into receiving
electronic version as permitted in 100.2.2.
HPMS Timing and
Submission:
Not applicable.
Format
Specification:
Model required.
Guidance and Other
Needed Information:
Medicare Prescription Drug Manual Chapters 5 and 6.
Translation Required
(5% Threshold):
Yes.
40
Formulary (Communications)
To Whom Required:
Must be provided to all enrollees of plan.
Timing:
Must be provided to current enrollees of plan by October 15 of each
year.
Must also provide to new enrollees within ten (10) calendars days
from receipt of CMS confirmation of enrollment or by last day of
month prior to effective date, whichever is later.
Method of Delivery:
Hard copy; made available electronically, as permitted in 100.2.1; or
electronically, if enrollee has opted into receiving electronic version as
permitted in 100.2.2.
HPMS Timing and
Submission:
Not applicable.
Format
Specification:
Model required, modifications permitted.
Guidance and Other
Needed Information:
Refer to Part D Model Materials at:
https://www.cms.gov/Medicare/Prescription-Drug-
Coverage/PrescriptionDrugCovContra/Part-D-Model-Marketing-
Materials.html and Medicare Prescription Drug Manual Chapter 6.
Translation Required
(5% Threshold):
Yes.
Low Income Premium Subsidy (Communications)
To Whom Required:
Must be provided to potential enrollees of what their plan premium will
be once they are eligible for Extra Help and receive the low-income
subsidy.
Timing:
Must be provided prior to effective date of enrollment.
Method of Delivery:
Electronic (web posting)
HPMS Timing and
Submission:
Not applicable.
Format
Specification:
Model provided, modification permitted.
Guidance and Other
Needed Information:
Refer to Part D Model Materials at:
https://www.cms.gov/Medicare/Prescription-Drug-
Coverage/PrescriptionDrugCovContra/Part-D-Model-Marketing-
Materials.html.
Translation Required
(5% Threshold):
Yes.
41
Low Income Subsidy (LIS) Rider (Communications)
To Whom Required:
Must be provided to all current enrollees who qualify for Extra Help.
Enrollees will get an LIS rider from the Plan telling them how much
help they will receive in the benefit year toward their Part D premium,
deductible, and copayments.
Timing:
Must be provided at least once per year by September 30.
Must be sent to enrollees who qualify for Extra Help or have a
change in LIS levels within 30 days of receiving notification from
CMS.
Method of Delivery:
Hard copy.
HPMS Timing and
Submission:
Not applicable.
Format
Specification:
Model required.
Guidance and Other
Needed Information:
Medicare Prescription Drug Benefit Manual, Chapter 13, Section 70.2.
Translation Required
(5% Threshold):
Yes.
42
Membership ID Cards (Communications)
To Whom Required:
Must be provided to all plan enrollees.
Timing:
Must be provided to new enrollees within ten (10) calendars days from
receipt of CMS confirmation of enrollment or by last day of month
prior to effective date, whichever is later. Must also be provided to all
enrollees if information on existing card changes.
Method of Delivery:
Must be provided in hard copy. In addition to the hard copy, plans may
also provide a digital version (e.g., app).
HPMS Timing and
Submission:
Not applicable.
Format
Specification:
Model not available, must include required content. Combination health
and drug cards must follow the Workgroup for Electronic Data
Interchange (WEDI) standards. Standalone Part D cards must follow the
National Council for Prescription Drug Program (NCPDP) standards.
Guidance and Other
Needed Information:
Cards must include Plan/Part D sponsor’s website address, customer
service number, and contract/PBP number.
The front of the Part D sponsor card must include the Medicare
Prescription Drug Benefit Program Mark.
Combination health and drug plan cards must follow the WEDI
standard and must include the required information.
PPO and PFFS ID cards must include the phrase “Medicare limiting
charges apply.”
May not use social security number (SSN) or Health care Insurance
Claim number (HICN).
Translation Required
(5% Threshold):
No.
43
Mid-Year Change Notification to Enrollees (Communications)
To Whom Required:
Must be provided to all applicable enrollees when there is a mid-year
change in benefits, plan rules, formulary, provider network, or
pharmacy network.
Timing:
Ad hoc, based on specific requirements for each issue.
Method of Delivery:
Hard copy, or electronically, if enrollee has opted into receiving
electronic version as permitted in 100.2.2.
HPMS Timing and
Submission:
Not applicable.
Format
Specification:
Model not available, must include required content.
Guidance and Other
Needed Information:
Notices of changes in plan rules unless otherwise addressed in a
regulation must be provided 30 days in advance.
For changes in provider network and/or pharmacy network, see 42
CFR 422.111 and 422.128.
National Coverage Determination (NCD) changes announced or
finalized less than 30 days before effective date, notification
required as soon as possible.
Mid-year NCD or legislative changes must publish no later than 30
days after the NCD is announced. Plans may include change in next
plan mass mailing (e.g., newsletter), provided it is within 30 days
and must be reflected on Plan/Part D website.
Plan/Part D sponsor developed letter/communication. Required
elements must be included in letter/communication.
Medicare Managed Care Manual-Chapter 4.
Medicare Prescription Drug Benefit Manual- Chapter 6 and
forthcoming guidance effectuating 42 CFR 423.120(b)(5) on
formulary changes and required notice to beneficiaries and other
entities.
National Coverage Determination website.
Translation Required
(5% Threshold):
Yes.
44
Non-Renewal Notices (Communication)
To Whom Required:
Must be provided to each affected enrollee after the Plan/Part D sponsor
decides to non-renew or reduce their plan’s service area.
Timing:
At least 90 days before the end of the current contract period. Cost Plans
(without Part D) provide notice at least 60 days before the end of the
current contract period.
Method of Delivery:
Notices must be hard copy and sent via U.S. mail. First class postage is
recommended.
HPMS Timing and
Submission:
Not applicable.
Format
Specification:
Model required - Current contract year. Modifications permitted per
instructions.
Guidance and Other
Needed Information:
Information about non-renewals or service area reductions may not be
released to the public, including current enrollees, until notice is
received from CMS.
MAOs may elect to share Non-Renewal and Service Area Reduction
(NR/SAR) information only with first tier, downstream, and related
entities (FDRs) or anyone that the MAO does business with (i.e.,
contracted providers).
Additional NR/SAR notice information can be found in the annual
“Non-Renewal and Service Area Reduction Guidance and Enrollee
Notification Models” HPMS memo.
Translation Required
(5% Threshold):
Yes.
45
Outbound Enrollment Verification (Communications)
To Whom Required:
Must be provided for all agent/broker assisted enrollments.
Timing:
Must be conducted within 15 calendar days following the receipt of the
enrollment request.
Method of Delivery:
Hard copy, telephonic, email.
HPMS Timing and
Submission:
Not applicable.
Format
Specification:
Model not available, must include required content.
Guidance and Other
Needed Information:
Communication must address enrollment into Part C or Part D Plan
and provide customer service number for beneficiary questions
regarding costs, benefits, rules, or any other question about the Part
C/Part D Plan.
May be completed via phone call (including during welcome call) or
via email, if email is requested by an enrollee.
Must send a written communication if the Plan/Part D sponsor fails
to speak with the individual within 15 calendar days of enrollment
requests.
Agent/brokers are not permitted to be part of the enrollment
verification call.
Enrollment verification processes must stop if Plan/Part D sponsor is
notified that beneficiary is ineligible to enroll in plan or if
beneficiary has canceled the enrollment.
Method and timing of the enrollment verification must be
documented (date, time, and method of contact).
Translation Required
(5% Threshold):
Yes.
46
Part D Transition Letter (Communications)
To Whom Required:
Must be provided when a beneficiary receives a transition fill for a non-
formulary drug.
Timing:
Must be sent within three (3) days of adjudication of temporary
transition fill.
Method of Delivery:
Hard copy.
HPMS Timing and
Submission:
Not applicable.
Format
Specification:
Model required
Modifications permitted
Guidance and Other
Needed Information:
Chapter 6, Section 30.4.10.
Translation Required
(5% Threshold):
Yes.
47
Pharmacy Directory (Communications)
To Whom Required:
Must be provided to all current enrollees of the plan.
Timing:
Must be provided to current enrollees of plan by October 15 of each
year.
Must be provided to new enrollees within ten (10) calendars days
from receipt of CMS confirmation of enrollment or by last day of
month prior to effective date, whichever is later.
Must be provided to current enrollees upon request, within three (3)
business days of the request.
Must update directory information any time they become aware of
changes. All updates to the online provider directories are expected
to be completed within 30 days of receiving information. Updates to
hardcopy provider directories must be completed within 30 days,
however, hardcopy directories that include separate updates via
addenda are considered up-to-date.
Method of Delivery:
Hard copy; made available electronically, as permitted in 100.2.1; or
electronically, if enrollee has opted into receiving electronic version as
permitted in 100.2.2.
HPMS Timing and
Submission:
Not applicable.
Format
Specification:
Model provided, modifications permitted.
Guidance and Other
Needed Information:
For electronic and hard copy versions of a pharmacy directory, the
language and guidelines issued in the HPMS memo on 8/16/2016,
Pharmacy Directories and Disclaimers, for further information please
refer to the Part D Model Materials at:
https://www.cms.gov/Medicare/Prescription-Drug-
Coverage/PrescriptionDrugCovContra/Part-D-Model-Marketing-
Materials.html.
Translation Required
(5% Threshold):
Yes.
48
Pre-Enrollment Checklist (Communications)
To Whom Required:
Must be provided to potential enrollees with the Summary of Benefits
when the Summary of Benefits is accompanying an enrollment form.
Timing:
Prior to enrollment.
Method of Delivery:
In the same format the SB was provided.
HPMS Timing and
Submission:
Not applicable.
Format
Specification:
Model required. Modifications to disclaimer language is not permitted,
however, Plans/Part D sponsors may delete bullets that do not apply to a
specific plan type. When the pre-enrollment checklist is used for multiple
products, they are permitted to add a sentence or additional language
before or after the disclaimer to clarify or distinguish how a disclaimer
applies to different products.
Guidance and Other
Needed Information:
Must accompany the SB. Refer to Appendix 3.
Translation Required
(5% Threshold):
Yes.
Prescription Transfer Letter (Communications)
To Whom Required:
When a Part D sponsor requests permission from an enrollee to fill a
prescription at a different network pharmacy than the one currently being
used by enrollee.
Timing:
Ad hoc.
Method of Delivery:
Hard copy.
HPMS Timing and
Submission:
Not applicable.
Format
Specification:
Model required.
Guidance and Other
Needed Information:
Refer to the Part D Model Materials at:
https://www.cms.gov/Medicare/Prescription-Drug-
Coverage/PrescriptionDrugCovContra/Part-D-Model-Marketing-
Materials.html.
Translation Required
(5% Threshold):
Yes.
49
Provider Directory (Communications)
To Whom Required:
Must be provided to all current enrollees of the plan.
Timing:
Must be provided to current enrollees of Plan by October 15 of each
year.
Must be provided to new enrollees within ten (10) calendars days
from receipt of CMS confirmation of enrollment or by last day of
month prior to effective date, whichever is later.
Must be provided to current enrollees upon request, within three (3)
business days of the request.
Must update directory information any time they become aware of
changes. All updates to the online provider directories are expected
to be completed within 30 days of receiving information. Updates to
hardcopy provider directories must be completed within 30 days,
however, hardcopy directories that include separate updates via
addenda are considered up-to-date.
Method of Delivery:
Hard copy; made available electronically, as permitted in 100.2.1; or
electronically, if enrollee has opted into receiving electronic version as
permitted in 100.2.2.
HPMS Timing and
Submission:
Not applicable.
Format
Specification:
Model provided, modification permitted.
Guidance and Other
Needed Information:
Chapter 4 of the Medicare Managed Care Manual and Medicare
Advantage and Section 1876 Cost Plan Provider Directory Model.
Translation Required
(5% Threshold):
Yes.
50
Scope of Appointment (Communications)
To Whom Required:
Must be documented for all marketing activities, in-person,
telephonically, including walk-ins to Plan/Part D sponsor or agent
offices.
Timing:
Prior to the appointment.
Method of Delivery:
Beneficiary signed hard copy, telephonic recording, or electronically
signed.
HPMS Timing and
Submission:
Not applicable.
Format
Specification:
No model required, must include required content.
Guidance and Other
Needed Information:
The following requirements must be on the scope of appointment
form or on the recorded call:
o
Product types to be discussed
o
Date of appointment
o
Beneficiary and agent contact information
o
Statement stating, no obligation to enroll, current or
future Medicare enrollment status will not be impacted,
and automatic enrollment will not occur.
A new SOA is required if, during an appointment, the beneficiary
requests information regarding a different plan type than previously
agreed upon.
Translation Required
(5% Threshold):
Yes.
51
Star Ratings Document (Marketing)
To Whom Required:
Must be provided to all prospective enrollees when an enrollment form
is provided. For online enrollment, Star Ratings document and SB must
be made available electronically (e.g., via link) prior to the completion
and submission of enrollment request.
Timing:
Must be provided prior to enrollment.
Method of Delivery:
Hard copy.
HPMS Timing and
Submission:
Code 1090.
Must be uploaded within 21 calendar days of the release of the updated
information.
Format
Specification:
Model required. Standardized Star Ratings document is generated from
HPMS.
Guidance and Other
Needed Information:
All Plans/Part D sponsors must provide the Star Ratings document.
New Plans/Part D sponsors that have no Star Ratings are not
required to provide until the following contract year.
Updated Star Ratings must be used within 21 calendar days of
release of updated information on Medicare Plan Finder.
Updated Star Ratings must not be used until CMS releases Star
Ratings on Medicare Plan Finder.
Only the Plan logo may be added to the document (no other changes
or alterations are permitted).
Translation Required
(5% Threshold):
Yes.
Summary of Benefits (Marketing)
To Whom Required:
Must be provided to all prospective enrollees when an enrollment form
is provided.
Timing:
Must be available by October 15 of each year.
Method of Delivery:
Hardcopy or electronic, depending on the format of the enrollment
mechanism.
HPMS Timing and
Submission:
Code 1099 File & Use.
Submitted prior to October 15 of each year.
Format
Specification:
No model required, must include required content.
Guidance and Other
Needed Information:
Appendix 5 of the MCMG.
Translation Required
(5% Threshold):
Yes.
52
Termination Notices (Communication)
To Whom Required:
Notice must be provided to affected enrollees before the termination
effective date or the end of services for provider terminations.
Timing:
CMS and MAO initiated terminations and provider terminations require
enrollee notices be sent as specified in CFR Title 42.
Method of Delivery:
Notices must be hard copy and sent via U.S. mail. First class postage is
recommended.
Notice to the general public requires publishing in one or more
newspapers of general circulation.
HPMS Timing and
Submission:
Not applicable.
Format
Specification:
Model required - Current contract year.
Guidance and Other
Needed Information:
Relevant notice requirements are provided in 42 CFR §§ 422.111(e),
422. 512, 422.510, and 422. 508.
Translation Required
(5% Threshold):
Yes.
110 – Agent/Broker Activities, Oversight, and Compensation Requirements
110.1
– Agent Requirements
42 CFR §§ 422.2272(c), 422.2274, 423.2272(c), 423.2274
Agents selling Medicare Advantage-Prescription Drug plans (MA, MA-PD), Medicare
Prescription Drug plans (PDP) and Section 1876 cost plans must:
Be licensed and appointed (if applicable) per State law to sell Medicare products;
Be trained and tested annually;
Achieve an 85 percent or higher on agent testing; and
Secure and document an SOA prior to meeting with potential enrollees. (Refer to section
100.4 for specific requirements).
110.2
– Permitted Agent Activities
Permitted activities include, but are not limited to:
Conducting sales presentations;
Holding one-on-one sales appointments with potential enrollees;
Providing business reply cards at educational events;
Creating and distributing communication materials. As outlined in section 90.1, broker-
53
created communications do not require a material identification number;
Distributing marketing materials as long as CMS has accepted/approved those materials as
submitted through the Plan(s)/Part D sponsor(s) with whom the broker contracts; and
Using CMS-created materials provided the materials are not modified in any way.
110.3
– Plan/Part D Sponsor Oversight
42 CFR §§ 422.504(l), 422.2272(c)-(d), 422.2274, 423.505(l), 423.2272(c)-(d), 423.2274
Plans/Part D sponsors must oversee downstream entities to ensure agents/brokers abide by all
applicable state and federal laws, regulations, and requirements. Plans/Part D sponsors are
required to:
Ensure agents/brokers are properly licensed and appointed, per individual state
requirements;
Report to the state where agent is appointed the termination of an agent/broker, including
the reason for termination if state law requires;
Report to CMS Account Managers all enrollments made by unlicensed agent(s) and report
for-cause terminations of an agent/broker to CMS.
Provide training and testing that meets CMS’ requirements as found in the Agent and
Broker Training and Testing Guidelines;
Report annually to CMS whether the Plan/Part D sponsor intends to use employed,
captive, or independent agents or brokers in upcoming plan year and, if applicable, the
specific amount or range of amounts the agents/brokers will be paid;
Submit agent/broker marketing materials to CMS through HPMS prior to use;
Ensure beneficiaries are not charged a marketing fee by agents; and
Ensure SOAs are completed for all marketing appointments (including telephonic and
walk-ins).
110.4
– Compensation Applicability and Definitions
42 CFR §§ 422.2274, 423.2274
Compensation requirements, unless otherwise noted, only apply to independent agent/brokers.
Plans/Part D sponsors must ensure the following requirements are met in order to pay
compensation:
Plans/Part D sponsors may only pay agents/brokers who meet state licensure/appointment
requirements;
Agents/brokers who represent the Plan/Part D sponsor must be trained and tested to
receive compensation; representation includes:
o
Selling products;
o
Outreach to existing or potential beneficiaries; and
o
Answering or potentially answering questions from existing or potential
beneficiaries.
Unless otherwise noted, compensation is governed by terms of the contract between the
Plan/Part D sponsor and the agent/broker and must comply with regulations at 42 CFR
422.2274 and 423.2274. Contracts, including re-negotiating of contracts, is between the
Plan/Part D sponsor and the agent/broker.
54
110.5
– Plan/Part D Sponsor Compensation Reporting Requirements
42 CFR §§ 422.2274, 423.2274
Plans/Part D sponsors must notify CMS whether they are using employed, captive, or
independent agents, as well as the compensation payment rates or ranges and referral fees.
CMS will notify Plans/Part D sponsors of the deadline annually. Once the submission
deadline passes, Plans/Part D sponsors may not change their rates, ranges, or referral fees
until the next compensation year.
Full compensation structures for the next contract year must be in place by October 1 of
each year. The structure must include details on compensation dissemination, including
specifying payment amounts for initial and renewal compensation.
Plans/Part D sponsors are responsible for ensuring first tier, downstream, and related
entities that represent the plan adhere to all agent/broker and compensation requirements.
110.6
– Compensation
42 CFR §§ 422.2274(a)(1) and (2), 423.2274(a)(1) and (2)
Compensation includes monetary or non-monetary remuneration relating to the sale or renewal of
a policy including, but not limited to:
Commissions;
Bonuses;
Gifts, prizes, awards; and
Referral/finder’s fees.
Compensation DOES NOT include:
The payment of fees to comply with state appointment laws;
Costs associated with training/testing;
Certification costs; or
Reimbursement for actual costs (e.g., mileage to and from appointments with
beneficiaries, venue rent, snack, materials).
110.6.1
– Initial Compensation
42 CFR §§ 422.2274(b)(1)(i), 423.2274(b)(1)(i)
Plans/Part D sponsors may pay initial compensation at or below the fair market value (FMV) as
published annually by CMS.
110.6.2
– Renewal Compensation
42 CFR §§ 422.2274(b)(1)(ii), 423.2274(b)(1)(ii)
For each enrollment in Year 2 and beyond, Plans/Part D sponsors may pay renewal compensation
at an amount that is up to fifty (50) percent of the current FMV a published annually by CMS.
110.6.3
– Referral/Finder’s Fees
42 CFR §§ 422.2274(h), 423.2274(h)
55
Referral/Finder’s fees paid to independent, captive or employed agents/brokers may not exceed
the amount that CMS determines could reasonably be expected to provide financial incentive for
an agent or broker to recommend or enroll a beneficiary into a plan that is not the most
appropriate to meet his or her needs. Referral fees must be included in the total compensation not
to exceed the fair market value for that calendar year. For 2019, the amount is $100 for MA plans
and $25 for PDPs.
110.6.4
– Paying Compensation
42 CFR §§ 422.2274, 423.2274
The compensation year is January 1 through December 31. Payments must be calculated
and made during the January 1 through December 31 enrollment year (including AEP
enrollments).
Plans/Part D sponsors may determine their payment schedule (e.g., monthly, quarterly).
However, compensation must be paid during the enrollment year.
Compensation is based on the number of months a member is enrolled.
Compensation paid to third parties selling MA/Part D products must adhere to
compensation requirements.
110.6.5
– Paying Initial Compensation
42 CFR §§ 422.2274, 423.2274
Plans/Part D sponsors may pay either a full or pro-rated Initial Compensation when:
The beneficiary’s first year of enrollment in any plan, or
A beneficiary moves from an employer group plan to a non-employer group plan (either
within the same Parent Organization or between Parent Organizations).
Plans/Part D sponsors must pro-rate Initial Compensation when:
A beneficiary changes plans during their initial enrollment year, or
A beneficiary makes an “unlike plan change.” The new plan would only pay the months
that the beneficiary is enrolled. The previous plan would recoup the months that the
beneficiary was not in the plan.
110.6.6
– Paying Renewal Compensation
42 CFR §§ 422.2274, 423.2274
Plans/Part D sponsors may pay Renewal Compensation when:
In any year following the initial year compensation;
When a beneficiary enrolls in a new “like plan” within the same Parent Organization or
between two (2) different Parent Organizations; or
When a beneficiary who is a member of an MMP switches to an MA plan with a PDP or
an MA-PD plan (and vice versa), if applicable per state MMP policy.
110.6.7
– Other Compensation Scenarios
41 CFR §§ 422.2274, 423.2274
For MA-PD enrollees, Plans/Part D sponsors may pay only the MA compensation.
56
When a beneficiary enrolls in both a Section 1876 Cost Plan and a stand-alone PDP, the
Cost Plan must pay compensation for the Cost Plan enrollment and the Part D sponsor
must pay compensation for the Part D enrollment.
For enrollees in an MA only plan and a PDP plan, compensation for both the MA plan and
the PDP plan is paid.
A “like plan type” enrollment includes:
A PDP to another PDP;
An MA, MA-PD, or MMP to another MA, MA-PD, or MMP; or
A Section 1876 Cost Plan to another Section 1876 Cost Plan.
An “unlike plan type” enrollment includes:
An MA or MA-PD plan to a PDP or Section 1876 Cost Plan;
A PDP to a Section 1876 Cost Plan or an MA (or MA-PD) plan; or
A Section 1876 Cost Plan to an MA (or MA-PD) plan or PDP.
Note: For enrollees who change from two plans (e.g., MA and a stand-alone PDP) (dual
enrollments) to one plan (MA-PD), compensation is at the renewal rate for the MA-PD
product.
110.7
– Compensation Recovery Requirements (Charge-backs)
42 CFR §§ 422.2274, 423.2274
Compensation must be recovered when:
A beneficiary disenrolls from a plan within the first three (3) months of enrollment (rapid
disenrollment); or
Any other time a beneficiary is not enrolled in a plan, but the Plan/Part D sponsor paid
compensation for that time period.
CMS expects Plans/Part D sponsors to retroactively pay or recoup funds based on retroactive
beneficiary changes for the current and previous calendar years. Plans/Part D sponsors may
choose to recoup or pay compensation for years prior to the previous calendar year. However, if a
Plan/Part D sponsor chooses to recoup payments from a prior calendar year, it must also pay
funds retroactively that would have been due during that same year.
110.7.1
– Rapid Disenrollment
42 CFR §§ 422.2274, 423.2274
Rapid disenrollment applies when an enrollee makes any plan change (regardless of Parent
Organization) within the first three (3) months of enrollment. Rapid disenrollment compensation
recovery does not apply when a beneficiary enrolls effective October 1, November 1, or
December 1 and subsequently uses the Annual Election Period to change plans for an effective
date of January 1.
Exceptions to the requirement for a Plan/Part D sponsor to recover compensation because of a
rapid disenrollment may be granted when CMS determines that recoupment is not in the best
interests of the Medicare program. CMS has made that determination for changes in enrollment
57
made for the following reasons:
Other creditable coverage (e.g., employer plan);
Moving into or out of an institution;
Gains/drops employer/union sponsored coverage;
Plan termination, non-renewal, or CMS imposed sanction;
To coordinate with Part D enrollment periods or State Pharmaceutical Assistance
Program;
Becoming LIS or dual (Medicare and Medicaid) eligible;
Dual eligible beneficiaries moving from an MA-PD to MMP;
Qualifying for another plan based on special needs;
Due to an auto, facilitated, or passive enrollment;
Death;
Moves out of the service area;
Non-payment of premium;
Loss of entitlement or retroactive notice of entitlement; and
Moving to a 5-star plan or from an LPI plan into a plan with three or more stars.
110.7.2 – Other Compensation Recovery
42 CFR §§ 422.2274, 423.2274
Plans/Part D sponsors must recover a pro-rated amount of compensation (initial and renewal)
from an agent/broker when an enrollee disenrolls from a plan, equal to the number of months not
enrolled. If a full initial compensation is paid, regardless of effective date, and the enrollee
disenrolls mid-year, the total number of months not enrolled must be deducted from
compensation and recovered from the agent/broker. (e.g., Age-in effective April 1 and disenrolls
September 30 of the same year. Full initial was paid. Recovery is equal to 6/12ths of the initial
compensation (January through March and October through December)).
110.8 – Payments other than Compensation
42 CFR §§ 422.2274, 423.2274
Payments made to third parties for services other than enrollment of beneficiaries (e.g., training
customer service, or agent recruitment) must not exceed FMV or an amount that is commensurate
with the amounts paid to a third party for similar services during each of the previous two (2)
years. Administrative payments to third parties can be based on enrollment, provided payments
are at or below FMV.
120 – Use of Medicare Beneficiary Information Obtained from CMS
CMS provides Medicare beneficiary data to Plans/Part D sponsors for the purpose of enrolling,
disenrolling, and providing care to members in their plan. The permitted uses of data are outlined
in the data use agreement that Plans/Part D sponsors sign. Plans/Part D sponsors may not use
Medicare data to develop, market, or operate lines of business unrelated to their Medicare plan
operations.
Plans/Part D sponsors who received data from a previous commercial relationship with Medicare
58
beneficiaries (and employers offering Medicare plans) are not obligated to follow the Data Use
Agreement in connection with data previously received. Examples of previous commercial
relationships include membership in such products as:
Long-term care insurance;
Life-insurance policies;
Non-Medicare employer or retiree group health plans; or
Medigap policies.
Plans/Part D sponsors are reminded that other laws – such as the HIPAA privacy rules - may limit
the use of information gathered from other sources or in connection with other products offered
by the Plan/Part D sponsor. Nothing in this guidance creates an exemption or exception to other
applicable laws.
120.1 – Consent Requirements for Non-Health Related Mailings
Plans/Part D sponsors may seek and rely on consent from enrollees to use information about them
for purposes of marketing. Such authorization and consent must be compliant with 45 CFR
164.508. The consent requirements for non-health related mailings are separate from “opt-in”
requirements for plan materials.
59
Appendix 1 – Definitions
42 CFR §§ 422.111, 422.2260, 422.2268, 423.128, 423.2260, 423.2268
The following definitions apply for purposes of the MCMG only.
Advertisement
A read, watched, or heard call to attention. Advertisements can be considered communication or
marketing based on the content of the message.
Age-ins
An individual who is aging into Medicare eligibility, typically the seven month period consisting
of three months prior to the individual’s birth month, the individual’s birth month, and three
months following the individual’s birth month.
Alternate Formats
Alternate formats are used to convey information to individuals with visual, speech, physical,
hearing, and intellectual disabilities (e.g., braille, large print, audio).
Banner and Banner-Like Advertisements
Banner advertisements are typically used in television ads, and flash information quickly across a
screen for the sole purpose of enticing a prospective enrollee to contact the Plan/Part D sponsor to
enroll or obtain more information. A “banner-like” advertisement is usually in some media other
than television (e.g., outdoor advertising and internet banner ads). Banner advertisements are
intended to be brief and to entice someone to call the Plan/Part D sponsor or to alert someone that
information is forthcoming.
Co-Branding
Co-branding is defined as a relationship between two or more separate legal entities, one of which
is an organization that sponsors a Medicare Plan. Co-branding is when a Plan/Part D sponsor
displays the name(s) or brand(s) of the co-branding entity or entities on its materials to signify a
business arrangement. Co-branding arrangements allow a Plan/Part D sponsor and its co-branding
partner(s) to promote enrollment in the plan. Co-branding relationships are entered into
independent of the contract that the Plan/Part D sponsor has with CMS.
Communication
Communications means activities and use of materials to provide information to current and
prospective enrollees.
Direct Mail
Direct mail is information sent to an individual to attract his/her attention or interest and allow
him/her to request additional information.
Downstream Entity
Any party that enters into a written arrangement, acceptable to CMS, with persons or entities
involved with the MA or Part D benefit, below the level of the arrangement between an MA or
PDP organization (or applicant) and a first tier entity. These written arrangements continue down
to the level of the ultimate provider of both health and administrative services.

60
Educational Event
Educational events are designed to inform Medicare beneficiaries about Medicare Advantage,
Prescription Drug or other Medicare programs and do not include marketing.
Enrollment Materials
Enrollment materials are materials used to enroll or disenroll a beneficiary from a plan, or
materials used to convey information specific to enrollment and disenrollment issues such as
enrollment and disenrollment notices.
First Tier Entity
Any party that enters into a written arrangement, acceptable to CMS, with an MA or PDP
organization or applicant to provide administrative services, health care, or prescription drug
services for a Medicare eligible individual under the MA or Part D program.
Health Plan Management System (HPMS) Marketing Module
The HPMS Marketing Module is an automated tool used to enter, track, and maintain materials
submitted to CMS for review and approval. Plans/Part D sponsors must submit marketing
materials and specified communication materials through the HPMS Marketing Module. The
HPMS Marketing Module User Guide provides extensive information on how to use HPMS.
Plans/Part D sponsors should refer to the User Guide for any questions regarding the Marketing
Module or how to submit materials in HPMS.
Joint Enterprise
A joint enterprise is a group of organizations that are state-licensed as risk-bearing entities that
jointly enter into a single contract with CMS to offer a Regional Preferred Provider Organization
(RPPO) Plan or PDP in a multi-state region. The participating organizations contract with each
other to create a single “joint enterprise” and are considered an “entity” for purposes of offering a
RPPO or PDP.
Marketing
Activities and use of materials that are conducted by the Plan/Part D sponsor with the intent to
draw a beneficiary's attention to a plan or plans and to influence a beneficiary's decision- making
process when selecting a plan for enrollment or deciding to stay enrolled in a plan (that is,
retention-based marketing). Additionally, marketing contains information about the plan’s benefit
structure, cost sharing, measuring or ranking standards.
Marketing/Sales Event
Marketing/sales events are events designed to steer, or attempt to steer, enrollees or potential
enrollees toward a plan or a limited set of plans.
61
Marketing Appointments
Marketing appointments are individual appointments designed to steer or, attempt to steer,
enrollees or potential enrollees toward a plan or limited number of plans. All individual
appointments between an agent and a beneficiary are considered marketing/sales appointments
regardless of the content discussed.
Medication Therapy Management (MTM) program materials
MTM program materials are:
Materials provided to enrollees enrolled in the MA or PDP plan who are eligible for the
plan’s MTM program;
Materials as a result of MTM services that address issues unique to individual enrollees;
and
The Part D MTM program comprehensive medication review summary in CMS’
standardized format that is provided to a beneficiary.
Note: MTM program materials must not include any marketing messages, or promotional
messages.
Model Document
Model documents are materials for which CMS has provided model language.
Multi–Contract Entities (MCE)
MCE is a designation available for Plans/Part D sponsors that have multiple MA/PDP contracts
with CMS. Being designated as an MCE allows a Plan/Part D sponsor to submit materials to CMS
that are representative of all or a selection of the Plan’s/Part D sponsor’s contracts.
Nominal Value
Nominal value as used here means minimal or trifling value; the standard is an individual
item/service worth $15 or less (based on the retail value of the item).
Outdoor Advertising (ODA)
Outdoor advertising is an outdoor material intended to capture the attention of a passing audience
(e.g., billboards, signs attached to transportation vehicles). ODA may be a communication or
marketing.
Related Entity
Any entity that is related to the MA or Part D organization by common ownership or control and
1. Performs some of the MA or Part D organization’s management functions under contract or
delegation;
2. Furnishes services to Medicare enrollees under an oral or written agreement; or
3. Leases real property or sells materials to the MA or Part D organization at a cost of more
than $2,500 during a contract period.
62
Scripts
Scripts are standardized text. Informational scripts are designed to respond to beneficiary questions
and requests and provide objective information about a plan or the Medicare program. Sales and
enrollment scripts are intended to steer a beneficiary towards a plan or limited number of plans, or
to enroll a beneficiary into a plan.
Standardized Language
Standardized language is language developed by CMS or another Federal agency that is mandatory
for use by the Plan/Part D sponsor and cannot be modified except as noted by CMS (e.g., ANOC,
EOC, Plan Ratings).
State Pharmaceutical Assistance Program (SPAP)
An SPAP is a state program which helps pay drug plan premiums and/or other drug costs for
people with Medicare.
Template Materials
Template materials are any materials that include placeholders for variable data to be populated at
a later time.
Third Party Marketing Organization (TMO)
Third-party marketing organizations are entities such as a Field Marketing Organization (FMO),
General Agent (GA), or similar type of organization that has been retained to sell or promote a
Plan’s/Part D sponsor’s Medicare products on the Plan’s/Part D sponsor’s behalf either directly or
through sales agents or a combination of both.
63
Appendix 2 – Disclaimers
General Disclaimers
(Applicable to Most Plan Types)
1
42 CFR Sections 422.2262(c); 423.2262(c) are applicable to all. Note: Disclaimers are not required on the following material types:
ID cards, call scripts, banners and banner-like ads, envelopes, outdoor advertising, text messages, and social media.
Disclaimer
42 CFR Section(s)
Example or Required Text
Applicable Documents and Notes
1.
Federal Contracting
Statement
(communications and
marketing)
422.2264(c),
423.2264(c)
Example Text: “[Plan’s/Part D sponsor’s
legal or marketing name] is a [plan type]
with a Medicare contract. Enrollment in
[Plan’s/Part D sponsor’s legal or marketing
name] depends on contract renewal.”
Required on all materials except those
specifically excluded by CMS
Required elements in statement:
Legal or marketing name
Type of plan (e.g., HMO, PPO,
PFFS, PDP)
Enrollment depends on contract
renewal
Plans should incorporate contract with
state/Medicaid Program when
appropriate.
2.
Benefit Disclaimer
(marketing materials
mentioning benefits)
422.111(a) and (b),
422.2264, 422.2268,
423.128(a) and (b),
423.2264, 423.2268
Required Text: “This information is not a
complete description of benefits. Call
[insert customer service phone
number/TTY] for more information.”
Only required on marketing pieces
listing 10 benefits or more.
64
Disclaimer
42 CFR Section(s)
Example or Required Text
Applicable Documents and Notes
3.
Availability of Non-
English Translations (some
communication and
marketing materials)
422.2268(a)(7),
423.2268(a)(7)
Required Text: “ATTENTION: If you
speak [insert language], language
assistance services, free of charge, are
available to you. Call 1-xxx-xxx-xxxx
(TTY: 1-xxx-xxx-xxxx)."
Must be placed in non-English
languages that meet the five (5) percent
threshold for language translation, as
highlighted in Section 100.4.
4.
Plan Online Enrollment
Center (marketing)
422.60(c),
422.2262(c),
423.32(b),
423.2262(c)
Required Text: “Medicare beneficiaries
may also enroll in <plan name> through
the CMS Medicare Online Enrollment
Center located at
http://www.medicare.gov.”
Required on the website page where
online enrollment requests are
accepted.
5.
Star Ratings (marketing)
422.2260,
422.2262(c),
423.2260, 423.2262(c)
Required Text: “Every year, Medicare
evaluates plans based on a 5-star rating
system.”
Required on any document that
references Star Ratings.
6.
Accommodations
Disclaimer
(communications and
marketing)
422.2268(b)(12)
423.2268(b)(12)
Required Text: “For accommodations of
persons with special needs at meetings call
<insert phone and TTY number>.
Required on all advertisements and
invitations to events (educational and
marketing).
7.
Mailing Statements
(communications and
marketing)
422.2268(a)(2),
423.2268(a)(2)
Plan information – Required Text:
“Important [Insert Plan Name]
information”
Health and wellness information –
Required Text: “Health and wellness or
prevention information”
These statements are intended for
materials sent to current members.
If the plan name is already on the
envelope, the plan name does not need
to be mentioned in the disclaimer.
Delegated or sub-contracted entities
and downstream entities that conduct
mailings on behalf of a Plan/Part D
sponsor must also comply with this
requirement.
65
Disclaimer
42 CFR Section(s)
Example or Required Text
Applicable Documents and Notes
8.
Promoting Drawings,
Prizes or Free Gifts
(marketing)
422.2268(b)(2),
423.2268(b)(2)
Example Text: “Eligible for a free
drawing, gift, or prizes with no obligation
to enroll.”
Example Text: “Free gift without
obligation to enroll.”
Required when promoting drawings,
prizes, or free gifts.
The statement must make it clear that
that there is no obligation to enroll in the
plan.
9.
Provider Co-branding
Materials (communications
and marketing)
422.2268(a)(5),
423.2268(a)(5)
Required Text: “Other
<Pharmacies/Physicians/Providers> are
available in our network.”
Required on documents that identify
co-branding relationships with network
providers/pharmacies.
10.
Out-of-Network/Non-
Contracted Providers
(marketing)
422.2262, 422.2264(d)
Required Text: “Out-of-network/non-
contracted providers are under no
obligation to treat <Plan> members, except
in emergency situations. Please call our
customer service number or see your
Evidence of Coverage for more
information, including the cost- sharing
that applies to out-of-network services.”
Required on all materials referencing
out–of-network/non-contracted
providers.
Does not apply to standalone PDP
plans.
11.
Materials Developed by a
Third Party (marketing)
422.2264, 423.2264
Required Text: “For a complete list of
available plans please contact 1-800-
MEDICARE (TTY users should call 1-
877-486-2048), 24 hours a day/7 days a
week or consult www.medicare.gov.”
Required on third party materials when
the material lists or markets a subset of
plans.
66
Disclaimer
42 CFR Section(s)
Example or Required Text
Applicable Documents and Notes
12.
Part D sponsors with
Limited Access to
Preferred Cost Sharing
Pharmacies
(communications and
marketing)
422.111(a) and (b),
422.2262, 423.128(a)
and (b), 423.2262
Required Text: “<insert organization/plan
name>’s pharmacy network includes
limited lower-cost, preferred pharmacies in
<insert geographic area type(s) and state(s)
for which plan is an outlier)>. The lower
costs advertised in our plan materials for
these pharmacies may not be available at
the pharmacy you use. For up-to-date
information about our network pharmacies,
including whether there are any lower-cost
preferred pharmacies in your area, please
call <insert Member Services phone
number and TTY> or consult the online
pharmacy directory at
<insert website>.”
Required on all materials mentioning
preferred pharmacies when there is
limited access to preferred pharmacies.
13.
NCQA SNP Approval
(communications and
marketing)
422.4(a)(1)(iv),
422.111(b)(2)(iii) ,
422.2262, 422.2264,
423.2262, 423.2264
Required Text: “[Insert Plan Name] has
been approved by the National Committee
for Quality Assurance (NCQA) to operate
as a Special Needs Plan (SNP) until [insert
last contract year of NCQA approval]
based on a review of [insert Plan Name’s]
Model of Care.”
Required on all documents that
reference NCQA SNP approval.
Plans/Part D sponsors may not discuss
numeric SNP approval scores in
marketing materials or press releases.
No other language related to the
NCQA SNP approval may be used.

67
Appendix 3 – Pre-Enrollment Checklist
Instructions:
In addition to displaying disclaimers on the marketing materials listed in Appendix 2, Plans/Part D
sponsors must use the model pre-enrollment checklist, which consists of a list of important
disclaimers. The one-page document must be included with the SB.
Plans/Part D sponsors are permitted to remove parts or portions of the checklist that are not
applicable to a particular plan type or product. Plans/Part D sponsors are not permitted to alter the
disclaimer language, but when the pre-enrollment checklist is used for multiple products, they are
permitted to add a sentence or additional language before or after the disclaimer to clarify or
distinguish how a disclaimer applies to different products. For example: When selecting an HMO
product, remember that except in emergency or urgent situations, we do not cover services by out-
of-network providers (doctors who are not listed in the provider directory).
Pre-Enrollment Checklist
Before making an enrollment decision, it is important that you fully understand our benefits and
rules. If you have any questions, you can call and speak to a customer service representative at
[insert customer service phone number]
Understanding the Benefits
Review the full list of benefits found in the Evidence of Coverage (EOC), especially for
those services for which you routinely see a doctor. Visit [insert Plan website] or call
[insert plan phone number] to view a copy of the EOC.
Review the provider directory (or ask your doctor) to make sure the doctors you see now
are in the network. If they are not listed, it means you will likely have to select a new
doctor.
Review the pharmacy directory to make sure the pharmacy you use for any prescription
medicine is in the network. If the pharmacy is not listed, you will likely have to select a
new pharmacy for your prescriptions.
Understanding Important Rules
In addition to your monthly plan premium [plans may delete the monthly plan premium
portion for $0 premium plans], you must continue to pay your Medicare Part B premium.
This premium is normally taken out of your Social Security check each month.
[Note: Fully integrated dual SNPs may elect to remove this section or modify it to
convey that the Part B premium is already paid. Plans that have a Part B buy-down
may alter the language to convey the amount the plan pays and the beneficiary owes.]
Benefits, premiums and/or copayments/co-insurance may change on January 1, [insert

68
year].
Except in emergency or urgent situations, we do not cover services by out-of-network
providers (doctors who are not listed in the provider directory).
[For PPO, PFFS, and other plans that offer out of network coverage] Our plan allows you
to see providers outside of our network (non-contracted providers). However, while we
will pay for covered services [HMO-POS may insert “certain covered services”] provided
by a non-contracted provider, the provider must agree to treat you. Except in an emergency
or urgent situations, non-contracted providers may deny care. [If applicable, plans must add
the following language] In addition, you will pay a higher co-pay for services received by
non-contracted providers.
[For C-SNP plans] This plan is a chronic condition special needs plan (C-SNP). Your
ability to enroll will be based on verification that you have a qualifying specific severe or
disabling chronic condition.
[For D-SNP plans] This plan is a dual eligible special needs plan (D-SNP). Your ability to
enroll will be based on verification that you are entitled to both Medicare and medical
assistance from a state plan under Medicaid. [D-SNPs may provide additional information
if they impose restrictions to specific Medicaid eligibility category(ies)]
[For I-SNP plans] This plan is an institutional special needs plan (I-SNP). Your ability to
enroll will be based on verification that you, for 90 days or longer, have had or are
expected to need the level of services provided in a long-term care (LTC) skilled nursing
facility (SNF), a LTC nursing facility (NF), a SNF/NF, an intermediate care facility for
individuals with intellectual disabilities (ICF/IDD), or an inpatient psychiatric facility.
[For I-SNPs accepting members prior to having at least 90 days of institutional level of
care] - This plan is an institutional special needs plan (I-SNP). Your ability to enroll will be
based on verification that your condition makes it likely that either the length of stay or the
need for an institutional level of care would be at least 90 days.
[For MSAs] MSA Plans combine a high deductible Medicare Advantage Plan and a trust or
custodial savings account (as defined and/or approved by the IRS). The plan deposits
money from Medicare into the account. You can use this money to pay for your health care
costs, but only Medicare-covered expenses count toward your deductible. The amount
deposited is usually less than your deductible amount, so you generally have to pay money
out of pocket before your coverage begins.
Medicare MSA Plans do not cover prescription drugs. If you join a Medicare MSA Plan,
you can also join any separate Medicare Prescription Drug Plan.
There are additional restrictions to join an MSA plan, and enrollment is for a full calendar
year unless you meet certain exceptions. Those who disenroll during the calendar year will
owe a portion of the account deposit back to the plan. Contact the plan at [insert customer
service and TTY] for additional information.

69
Appendix 4 – External Links
CMS Eligibility and Enrollment Guidance https://www.cms.gov/Medicare/Eligibility-and-
Enrollment/MedicareMangCareEligEnrol/index.html
CMS Medicare Online Enrollment Center https://www.medicare.gov
CMS Plan Finder
https://www.medicare.gov or 1-877-486-2048) https://www.medicare.gov/find-a-
plan/questions/home.aspx
CMS Star Ratings https://www.medicare.gov
https://www.medicare.gov/find-a-plan/questions/home.aspx
HIPAA Privacy Rule and Disclosure Requirements https://www.hhs.gov/ocr/privacy/
HIPAA Privacy Rule and Security Requirements
https://www.hhs.gov/ocr/privacy/hipaa/understanding/coveredentities/privacyguidance.html
Internal Revenue Service (IRS) Tax publications https://www.irs.gov or 1-800-TAX-FORM (1-
800-829-3676)
Medicare.gov Complaint Website
https://www.medicare.gov/MedicareComplaintForm/home.aspx
Medicare Managed Care Manual
https://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/Internet-Only-Manuals-
IOMs-Items/CMS019326.html?DLPage=2&DLEntries=10&DLSort=0&DLSortDir=ascending
Medicare Part D Model Materials
https://www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovContra/Part-
D-Model-Marketing-Materials.html
Medicare Prescription Drug Benefit Manual https://www.cms.gov/Medicare/Prescription-Drug-
Coverage/PrescriptionDrugCovContra/PartDManuals.html
National Coverage Determinations (NCD) https://www.cms.gov/medicare-coverage-
database/overview-and-quick- search.aspx?list_type=nca
Section 508 of the Rehabilitation Act

70
https://www.section508.gov
State-specific marketing guidance for MMPs
https://www.cms.gov/Medicare-Medicaid-Coordination/Medicare-and-Medicaid-
Coordination/Medicare-Medicaid-Coordination-
Office/FinancialAlignmentInitiative/InformationandGuidanceforPlans.html.
WEDI Health Identification Card Implementation Guide https://www.wedi.org
71
Appendix 5 – Summary of Benefits Instructions
Plans must reflect Part C and Part D benefits and cost sharing (if applicable) in the SB. Part C
benefits and cost sharing must include and be in the following order:
Monthly Plan Premium, including Part C and Part D premium, when applicable;
Part B premium Buy-Down, if applicable;
Deductibles, including plan level and category level deductible;
Maximum Out-of-Pocket Responsibility (does not include prescription drugs);
Inpatient Hospital coverage;
Outpatient Hospital coverage;
Doctor Visits (Primary Care Providers and Specialists);
Preventive Care;
Emergency Care;
Urgently Needed Services;
Diagnostic Services/Labs/Imaging (include diagnostic tests and procedures, labs,
diagnostic radiology, and X-rays);
Hearing Services (Include mandatory and optional supplemental benefits (if offered));
Dental Services (Include mandatory and optional supplemental benefits (if offered));
Vision Services (Include mandatory and optional supplemental benefits (if offered));
Mental Health Services;
Skilled Nursing Facility;
Physical Therapy;
Ambulance;
Transportation; and
Medicare Part B Drugs.
Part D benefits must include:
Information about all phases of the benefit that apply to your Plan. At a minimum, Part D
sponsors must include information that cost-sharing may change when entering another
phase of the Part D benefit.
The levels of prescription medication with both the tier number/name/cost sharing (e.g.,
Tier 1, Tier 2, etc.) and the more general description (e.g., generic, preferred brand, etc.).
Information regarding cost sharing differences relative to the pharmacy’s status as
preferred or non-preferred, mail-order, Long Term Care (LTC) or home infusion, and 30
or 90 days’ supply.
To avoid beneficiary confusion when comparing plans, Plans/Part D sponsors must maintain the
above order of the data elements. The monthly premium, deductible and the maximum-out-of
pocket cost must always be displayed first. Plans/Part D sponsors may then decide whether to
display drug or health benefits next. If any of the benefits are not offered (e.g., transportation),
indicate them as “not covered.” Plans/Part D sponsors may remove certain benefits if they are not
applicable to a particular plan type (e.g., Part D only plan removes Part C benefits).
Additional benefits may be listed after all the required elements are provided in the SB.

72
Other required information in the SB:
The document must be labeled as “Summary of Benefits” noting the plan year;
The plan name and type must be clearly labeled for all Plans in the SB. For example,
<Plan name, HMO or PPO>;
Description of the plan and types (e.g., HMO, HMO-POS, PPO, MSA, PFFS, SNPs);
Service area and eligibility requirements, including the Medicaid eligibility criteria
applicable to Dual Eligible Special Needs Plans (D-SNPs);
Phone number, including TTY/TDD;
Days and hours of operation;
Website address;
In-network and out-of-network cost-sharing information for applicable plan types;
Applicable disclaimers as per Appendix 2;
Language stating that the complete list of services is found in the Evidence of Coverage
(EOC), as well as language directing readers how to access or order the EOC;
Language that directs readers how to access or order the "Medicare & You" handbook;
If the SB includes plans with and without Part D prescription drug coverage, the
distinction between plans must be clear;
Notate services that require a physician referral or prior authorization; and
If optional supplemental benefits are offered, Plans/Part D sponsors must include the
additional premium amount.
Medicare Medical Savings Account Plans (MSA) MSA plans must:
Insert the amount Medicare will deposit into the beneficiaries MSA account; and
Include language that the beneficiary will pay nothing once the deductible is met.
D-S
NPs
The Medicare Improvements for Patients and Providers Act of 2008 (MIPPA) requires Plans
provide the Medicaid benefits to prospective enrollees. This may be done by:
Including the Medicaid benefits in the SB or
Providing a separate document with the Medicaid benefits. If a separate document is
provided, it must also accompany the enrollment form.
D-S
NPs open to dually eligible enrollees with differing levels of cost clearly state how cost-
sharing and benefits differ depending on the level of Medicaid eligibility. D-SNPs must describe
the Medicaid benefits, if any, provided by the plan. Fully integrated dual eligible SNPs (FIDE
SNPs) and other D-SNPs that provide Medicaid benefits have the option to display integrated
benefits in the SB. We encourage FIDE SNPs to work with their contracted State Medicaid
agencies in developing an SB that displays integrated benefits.
Medicare Premium and Deductible
Plans that use Medicare premium, deductible, or cost sharing amounts (e.g., inpatient hospital)
must insert the prior year’s Medicare amounts. In addition, the benefit category must also note
that these amounts may change for the following year and the plan will provide updated rates as
soon as Medicare releases them.
73
Overall design and layout:
Plans may present multiple plan benefit packages (PBPs) in the same document by displaying the
benefits in separate columns. Plans using this option may include similar or different plan types
(e.g., HMO to HMO, or HMO to PFFS, or HMO to PPO). Plans may also:
Make use of colors to enhance the ability to navigate the document.
Incorporate various icons/graphics to help locate important information, such as how to
complete an application online or contact information (i.e., phone number) for customer
service help.
Note: SNPs must remain separate from non-SNP plans to avoid confusion for beneficiaries.
Recommendations:
The following recommendations are based on consumer testing:
Avoid the use of multiple folds and large charts as it may make it cumbersome and
difficult to use;
Include definitions and purpose of the document;
Avoid using dimensions that are too large as it could diminish the usefulness of the SB;
and,
Avoid the use of footnotes; if necessary to include footnoted information, visually
emphasize (e.g., larger or bold font) the inclusion of superscripts in coverage charts.
HPMS Submission Process:
The SB must be submitted in HPMS as one document under the File & Use process using code
1099. SBs may not be submitted as a template or with bracketed information (Refer to section 90
for information on the material submission process).

74
Appendix 6 - Employer/Union Group Health Plans
Sections 1857(i) and 1860D-22(b) of the Social Security Act, 42 CFR §§ 422.2276, 423.458,
423.2276
Plans/Part D sponsors offering employer group health plans including Employer Group Waiver
Plans (EGWPs) are not required to submit at time of use communication and marketing materials
specific only to those employer plans. However, as a condition of CMS providing particular
waivers or modifications to employer group plans, CMS may request and review any materials in
the event of beneficiary complaints or for any other reason it determines to ensure the information
accurately and adequately informs Medicare beneficiaries about their rights and obligations under
the plan.
CMS waivers to employer group plans are limited in scope to their stated parameters, and
employer group plans (including EGWPs) must follow all rules in these guidelines unless CMS
explicitly waives them. For specific guidance regarding these waivers, please refer to Chapter 9 of
the Medicare Managed Care Manual and Chapter 12 of the Medicare Prescription Drug Benefit
Manual (https://www.cms.gov/Medicare/Prescription-Drug-
Coverage/PrescriptionDrugCovContra/PartDManuals.html).
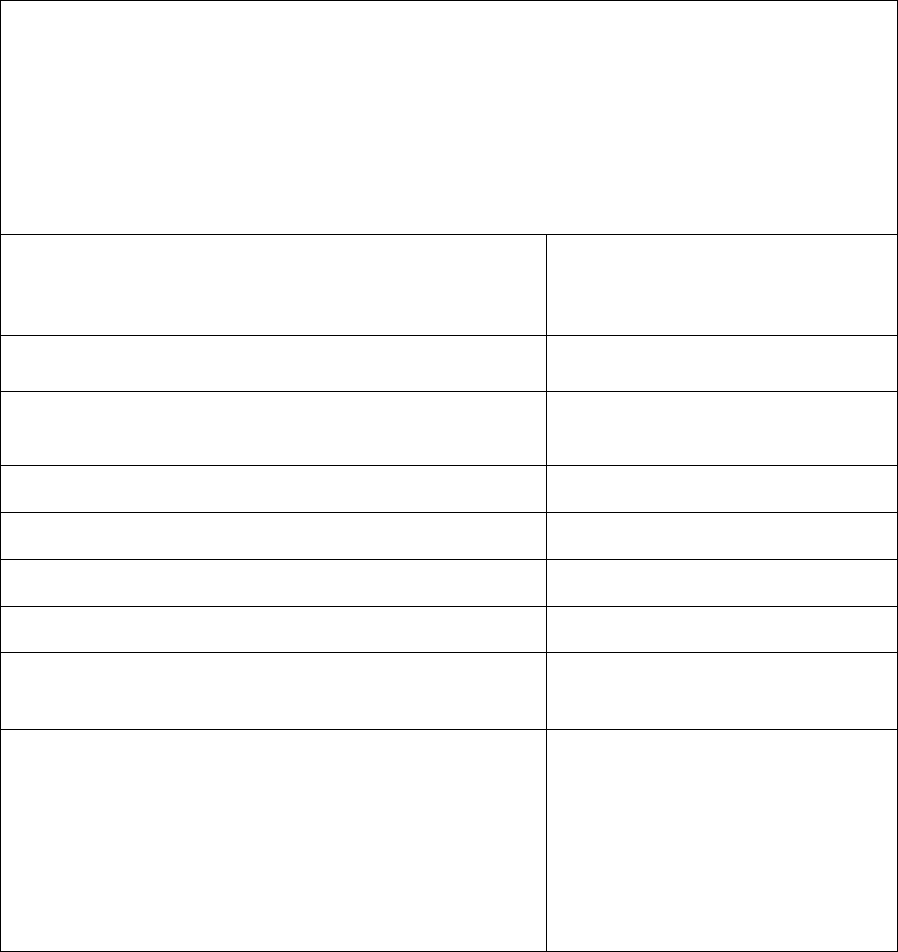
75
Marketing Provisions Table – Employer/Union Group Plans
Marketing Provisions that apply to Employer/Union Group Plans
These requirements are applicable to the transaction between the agent/broker selling the
plan to the employer/union. All activities conducted by the employer/union or its designees
to sign up individual employees to the plan(s) selected by the employer/union are excluded
from these provisions.
Note: This table contains a partial list of exclusions.
Applicable Provisions (Not Waived)
Not Applicable Provisions
(Waived)
Nominal Gifts
Unsolicited Contacts
Sales/Marketing in Health Care Settings
Cross-selling
Sales/Marketing at Educational Events
Scope of Appointments
Co-branding
Provision of Meals
Appointment of Agents/Brokers
Agent/Broker Compensation
State Licensure Requirements
Agent/Broker Testing
Reporting of Terminated Agents/Brokers
CMS Prior Review of
Marketing Materials
Agent/Broker Training
Agents must be thoroughly familiar with the products
they are selling, including the plan specific details and
the Medicare rules that apply to the specific products.
The organization/sponsor is responsible for ensuring
that the agents selling for them have sufficient
knowledge.
Pre-Enrollment Checklist

76
Appendix 7 – Use of Medicare Mark for Part D Sponsors
Section 1140 of the Social Security Act
All Part D sponsors may use the Medicare Prescription Drug Benefit Program Mark only after
electronically executing the Medicare Mark License Agreement in HPMS. In certain
circumstances, the Medicare Mark License Agreement may be signed in hard copy rather than
electronically. Only a CEO, CFO, or COO who is designated as an authorized signer in HPMS is
eligible to execute the Medicare Mark License Agreement. The license agreement is effective for
a single contract year and Part D sponsors must renew annually to continue using the Medicare
Mark. Unless otherwise approved, no individuals, organizations, and/or commercial firms may
distribute materials bearing the Medicare Prescription Drug Benefit Program Mark. Part D
sponsors may use the mark on communications and marketing materials consistent with this
chapter.
Use of Medicare Prescription Drug Benefit Program Mark on Items for Sale or Distribution
Section 1140 of the Social Security Act
All Part D sponsors may use the Medicare Prescription Drug Benefit Program Mark on items they
distribute, provided the item(s) follow(s) guidelines for nominal gifts, as provided in 40.4. Plans
cannot sell items with the Medicare Prescription Drug Benefit Program Mark for profit.
Approval to Use the Medicare Prescription Drug Benefit Program Mark
Plans/Part D sponsors must submit requests to distribute other items (materials that are not
included in this chapter) bearing the Medicare Prescription Drug Benefit Program Mark to CMS
at least thirty (30) days prior to the anticipated date of distribution. Plans/Part D sponsors should
send requests sent to:
Office of the Administrator/Office of Communications Visual & Multimedia Communications
Group
7500 Security Blvd.
Baltimore, MD 21244-1850
Once CMS has approved a request, the following will apply: 1) approval will be effective for a
period not to exceed one year; and 2) approval will be granted only for those items for which use
of the mark was requested in the request letter and for which CMS granted written approval.
Prohibition on Misuse of the Medicare Prescription Drug Benefit Program Mark Section
1140 of the Social Security Act
42 U.S.C. section 1320b-10 prohibits the misuse of the Medicare name and marks. In general, it
authorizes the Inspector General of DHHS to impose penalties on any person who misuses the
term Medicare or other names associated with DHHS in a manner which the person knows or
should know gives the false impression that DHHS has approved, endorsed, or authorized it.
Offenders are subject to fines of up to $5,000 per violation or in the case of a broadcast or telecast
violation, $25,000.

77
Mark Guidelines
Section 1140 of the Social Security Act
The Medicare Prescription Drug Benefit Program Mark is a logotype comprised of the words
Medicare Rx with the words Prescription Drug Coverage directly beneath.
Always use reproducible art available electronically. Do not attempt to recreate the Program Mark
or combine it with other elements to make a new graphic. Artwork will be supplied in
.EPS, .TIFF or .JPG format after notification of approval into the program.
Mark Guidelines - Negative Program Mark
Section 1140 of the Social Security Act
The Medicare Prescription Drug Benefit Program Mark may be reversed out in white. The entire
mark must be legible.
Mark Guidelines - Approved Colors
Section 1140 of the Social Security Act
The two (2)-color mark is the preferred version. It uses PMS 704 (burgundy) and sixty-five (65)
percent process black. It is recommended that if the CMS mark is used in conjunction with the
brand mark, that the black versions of those logos be used.
The 1-color version in grayscale is acceptable. The mark elements are one-hundred (100) percent
black except for the word “Medicare” which is fifty-five (55) percent black.

78
The 1-color version in one-hundred (100) percent black also is acceptable.
Mark Guidelines on Languages
Section 1140 of the Social Security Act
The Spanish version of the Medicare Prescription Drug Benefit Program Mark may be used in place
of the English language version on materials produced entirely in Spanish. The two (2)- color
version is preferred, but the grayscale, black and negative versions may be used.
Mark Guidelines on Size
Section 1140 of the Social Security Act
To maintain clear legibility of the Program Mark, never reproduce it at a size less than one (1)
inch wide. The entire mark must be legible.
Mark Guidelines on Clear Space Allocation
Section 1140 of the Social Security Act
The clear space around the Medicare Prescription Drug Benefit Program Mark prevents any
nearby text, image or illustration from interfering with the legibility and impact of the mark. The
measurement “x” can be defined as the height of the letter “x” in “Rx” in the Program Mark.
Any type or graphic elements must be at least “x” distance from the mark as shown by the
illustration.

79
Mark Guidelines on Bleed Edge Indicator
Section 1140 of the Social Security Act
The Program Mark may not bleed off any edge of the item. The mark should sit at least one--
eighth (1/8) inch inside any edges of the item.
Mark Guidelines on Incorrect Use
Section 1140 of the Social Security Act
Following are rules for preventing incorrect use of the Medicare Prescription Drug Benefit
Program Mark:
Do not alter the position of the mark elements;
Do not alter the aspect ratio of the certification mark;
Do not stretch or distort the mark;
Always use the mark only as provided in the CMS approval/license agreement;
Do not rotate the mark or any of its elements;
Do not alter or change the typeface of the mark;
Do not alter the color of any of the mark elements;
Do not position the mark near other items or images. Maintain the clear space allocation;
Do not position the mark to bleed off any edge. Maintain one-eighth (1/8) inch safety
from any edge;
Do not use any of the mark elements to create a new mark or graphic; and
Do not use the mark on background colors, images or other artwork that interfere with the
legibility of the mark.
Mark Guidelines for Part D Standard Pharmacy ID Card Design
Section 1140 of the Social Security Act
Use of the Medicare Prescription Drug Benefit Program Mark on an ID Card must be consistent
with guidance mentioned in this section.

80
