
Our exams
explained
GCSE science exams from
summer 2018
Version 2.0 September 2016
How we create exams that give students of all
abilities the best opportunity to get the results
they deserve.
• GCSE Combined Science: Trilogy
(double award)
• GCSE Combined Science: Synergy
(double award)
• GCSE Biology
• GCSE Chemistry
• GCSE Physics
See all our specimen question papers and mark
schemes at aqa.org.uk/gcse-science

Our exams explained – GCSE science exams from summer 2018
3
Contents
Exam structure
4
Introduction 4
Ramping and level of demand 5
Common questions between tiers 7
Working scientifically 7
Assessment objectives 8
Extended response and linked questions 8
Mathematical skills 8
Equations 9
Accessibility 9
Sample questions with commentaries
10
Question scaffolding 10
Multiple choice questions 12
Link box 16
Sentence completion 18
Short answer 19
Calculations 22
Labelling and drawing diagrams 32
Graphs 36
Chemical equations 44
Extended response 47
Linking of ideas 54
Practical skills 60

4
Exam structure
Introduction
Our GCSE Science papers have been carefully designed to engage students so that they can show what
they can do.
The papers are deliberately flexible in terms of the mixture of question types and number of marks within
each topic. This approach gives examiners the freedom to choose the best question style for the context
and science content being assessed. It is also in line with the regulatory requirements.
The topics we’re assessing are split across each paper so that you can prepare for examinations more
easily. Each question assesses an assessment objective and in some cases more than one
assessment objective.
We use a consistent range of question types:
• Closed − multiple-choice, link boxes, sentence completion, labelling diagrams.
• Open − labelling/drawing diagrams, short answer, calculations, extended response.
Our questions are structured:
• Within a whole question (eg question 01) there are several parts (eg 01.1, 01.2, 01.3 and 01.4) that
link to a common theme/topic.
• We’ll scaffold questions more in Foundation Tier papers. There will be a higher proportion of
multiple-choice and short answer questions in the Foundation Tier too.
• In Higher Tier papers there will be more marks for open and extended response questions.
Our exams explained – GCSE science exams from summer 2018
5
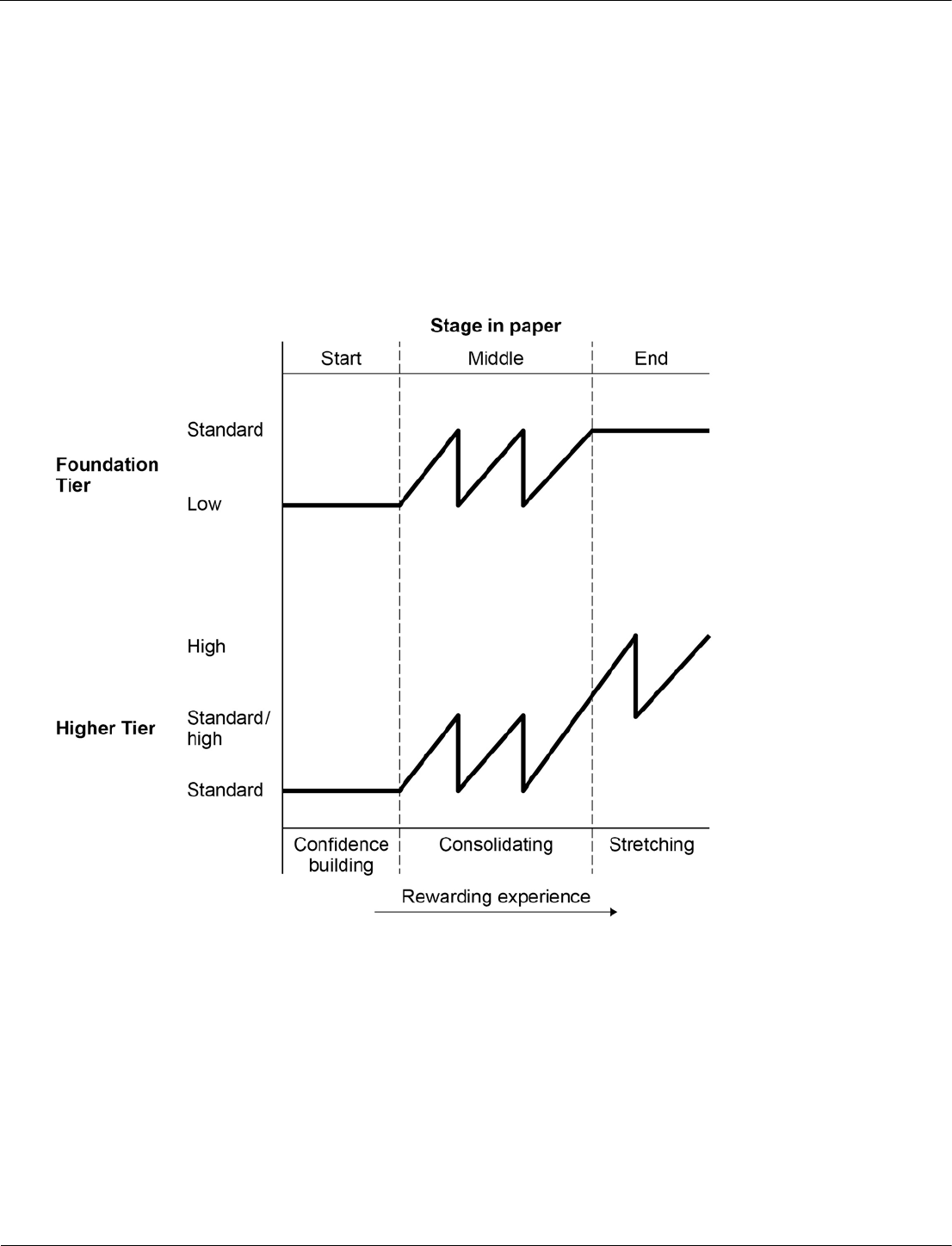
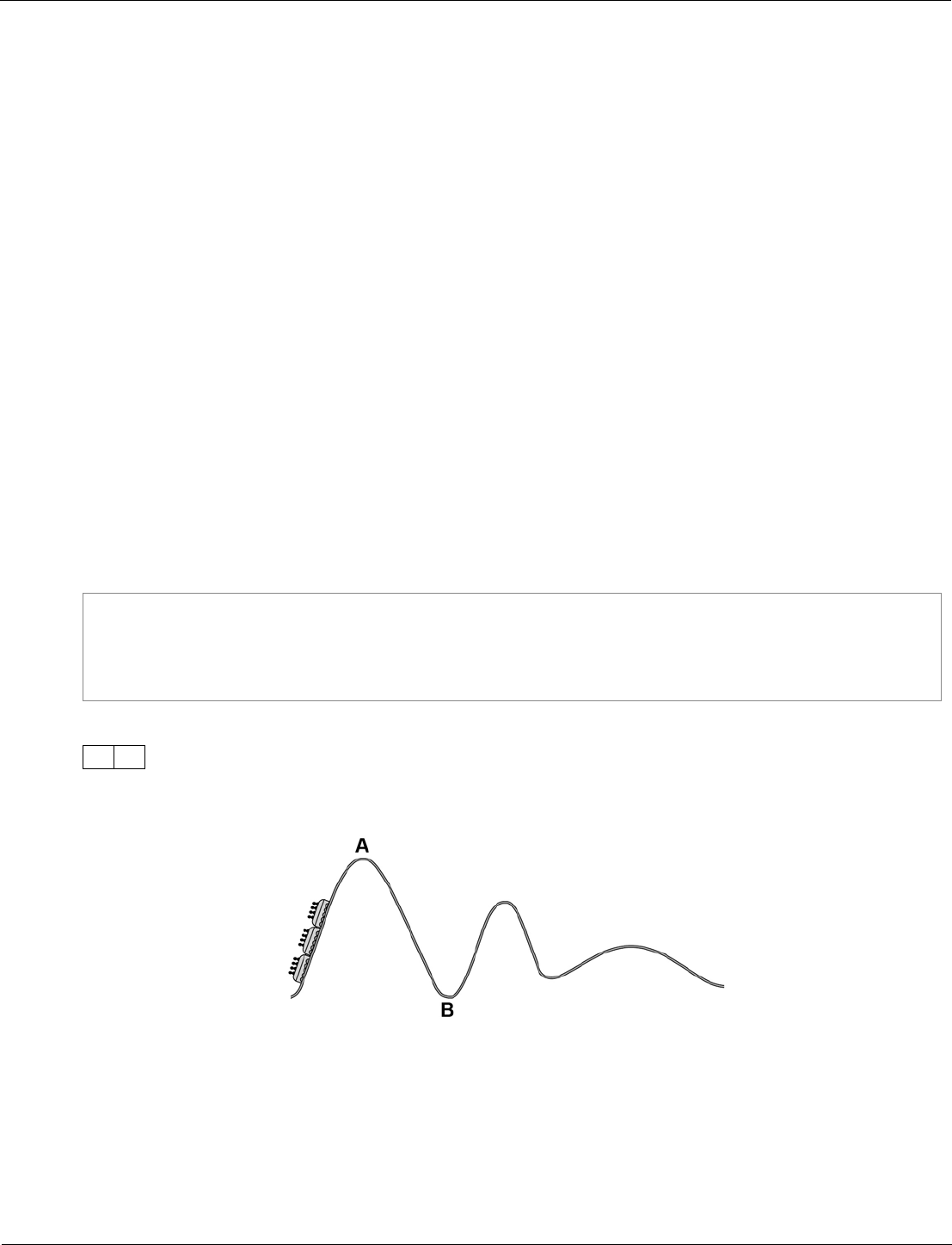
Ramping and level of demand
Ramping means that a question gets progressively more difficult as you work through it.
Questions for any topic area will be ramped in terms of demand within the question, as well as within the
paper. This allows all students a fair chance of gaining some marks on each topic area throughout
the paper.
Some questions will step up in demand gradually, others quite sharply. In addition the demand also
increases steadily throughout the paper.
We use the model above to structure the ramping of Foundation Tier and Higher Tier question papers.
• Both tiers start with confidence-building questions set at the lowest demand for the paper: ‘Low’ for
Foundation Tier; and ‘Standard’ for Higher Tier.
• The middle of each paper introduces ramping of each question up to the next level of demand. Within
each question the demand increases, then the following question starts again at a lower demand.
• The end of the paper is where the students’ ability is stretched the most. In the Foundation Tier this
means questions are set at standard demand (common with the Higher Tier). In the Higher Tier the
latter questions continue to ramp, but at a much higher level.
6
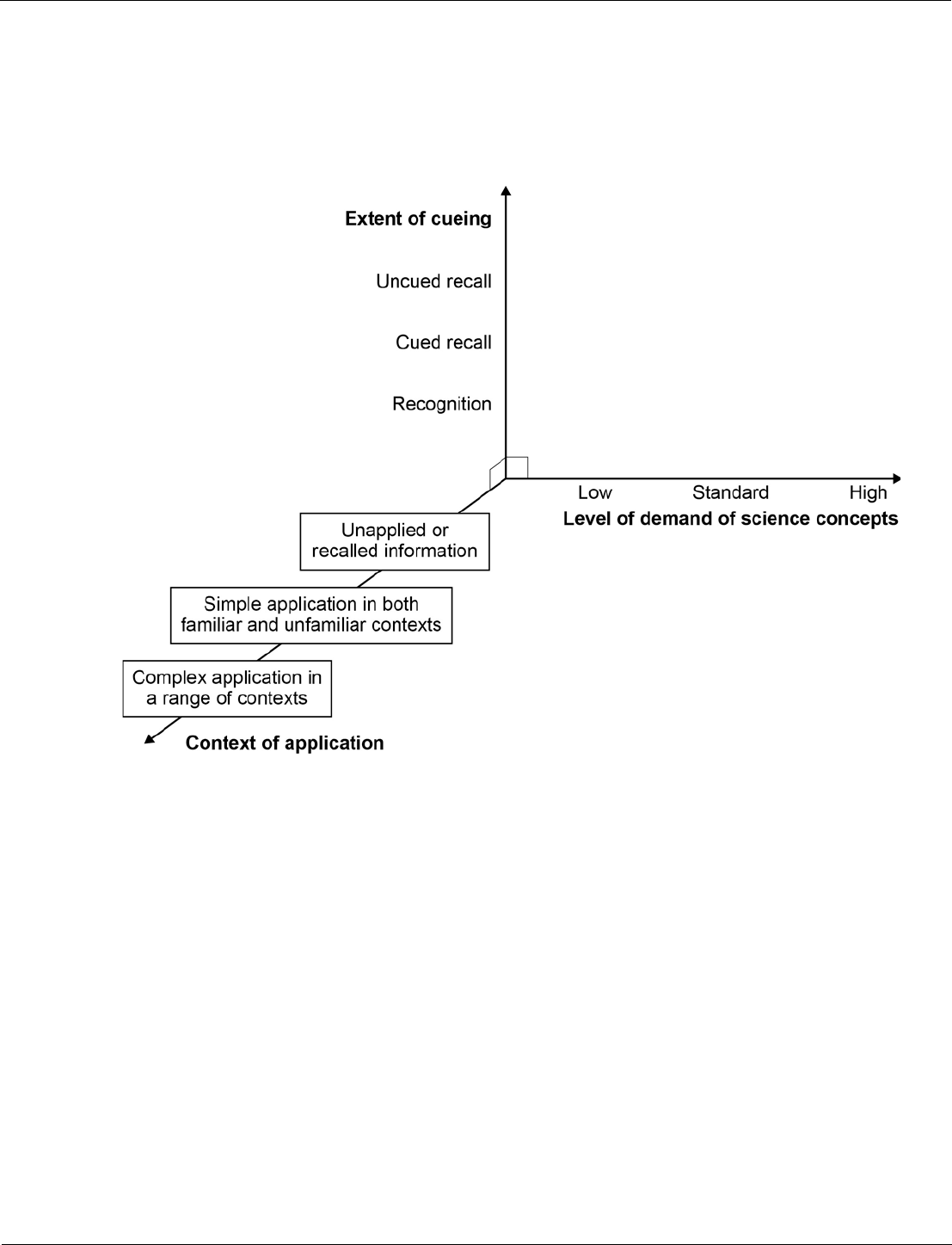
Level of demand is determined by factors such as: concept, context, the number of marks and the
command word(s) used.
The number of marks assigned to each level of demand within each paper is not precise (within a range)
due to the complexity of these factors.
Foundation Tier papers are made up of:
• low demand questions (aimed at grades 1‒3)
• standard demand questions (aimed at grades 4‒5).
A greater proportion of questions will be low demand.
Higher Tier papers are made up of:
• standard demand questions (aimed at grades 4‒5)
• standard/high demand questions (aimed at grades 6‒7)
• high demand questions (aimed at grades 8‒9).

Our exams explained – GCSE science exams from summer 2018
7
Common questions between tiers
We will have 30% of marks that are common between Foundation and Higher Tier papers. This allows
us to better align standards for grades 4‒5. Common questions will be standard demand.
Working scientifically
Working scientifically
†
is a fundamental part of learning science. It is the sum of all the activities that
scientists do and is a fundamental part of learning about and learning through science.
Students need to develop their working scientifically skills so that they can fully understand the
scientific process.
These skills fall broadly into four main strands:
1. the development of scientific thinking
2. experimental skills and strategies
3. analysis and evaluation
4. vocabulary, units, symbols and nomenclature.
Exams will include questions that assess all of these strands. So, students should expect to see
questions that:
• require knowledge of how scientists know what they know
• directly assess practical and enquiry skills.
†
indicates requirements set by the DfE and Ofqual.

8
Assessment objectives
The exams will measure how students have achieved the following assessment objectives
†
.
AO1: Demonstrate knowledge and understanding of:
40% 1) scientific ideas
2) scientific techniques and procedures.
AO2: Apply knowledge and understanding of:
40% 1) scientific ideas
2) scientific enquiry, techniques and procedures.
AO3: Analyse information and ideas to:
20% 1a) interpret
1b) evaluate
2a) make judgements
2b) draw conclusions
3a) develop experimental procedures
3b) improve experimental procedures.
Extended response and linked questions
†
Some questions will test students’ ability to link topics across different areas of the specification. These
questions are more likely to require an extended response
†
and are marked using a levels of response
mark scheme.
Mathematical skills
†
Mathematical skills will be tested at least to the standard of:
• Key Stage 3 Mathematics in Foundation Tier papers
• Level 1/Foundation Tier GCSE Mathematics in Higher Tier papers.
†
A minimum of 10% of marks will test mathematical skills in biology; 20% in chemistry; and 30% in
physics. For the combined sciences a minimum of 20% of marks will test mathematical skills (made up
of a minimum of 10% in biology; 20% in chemistry; and 30% in physics).

Our exams explained – GCSE science exams from summer 2018
9
Equations
†
There are two lists of physics equations:
1. Students must be able to recall and apply these equations.
2. Equations are given on a sheet inserted into the question papers. Students must be able to select
and apply these equations.
These equations are given in the specification as part of the subject content and in an appendix at
the back.
Accessibility
Clear language and layout of papers is important for all students. Our aim is to ensure students can
understand what the question is asking and assess the science, not comprehension. This will give
students the opportunity to gain the credit they deserve.
There are basic principles we work towards. We:
• put command words at the beginning of a sentence, and use one per sentence
• use standard wording/instructions
• use direct questioning (eg what, why, how) where suitable
• add ‘scaffolding’ to questions to act as a guide (appropriate to level of demand)
• ensure each sentence contains fewer than 20 words
• use bullet points to clearly display specific strands of information
• embolden key bits of information
• ensure diagrams are relevant and clear
• only use italics for binomial names eg S. mokarran, and equations eg E
p
= m g h
• explain unfamiliar terms
• reduce repeated information
• include plenty of white space to improve readability.
Our Making questions clear booklet gives more detail of what we’ve done to improve accessibility. This
booklet can be found on our website on each specification page under ‘assessment resources’:
aqa.org.uk/gcse-science
†
indicates requirements set by the DfE and Ofqual.

10
Sample questions with commentaries
Questions are from the specimen assessment materials accredited by Ofqual.
Each example shows the:
• specification reference
• mathematical skill (MS)
• working scientifically (WS) reference
• assessment objective (AO).
Question scaffolding
Throughout this booklet we have illustrated many ways on how we ‘scaffold’ questions to help students
express their answer.
Answer line prompts are one type of scaffolding we often use. These make the question more
accessible by taking information already given in the question to help students structure their answer.
Biology ‒ paper 1F, question 03.5
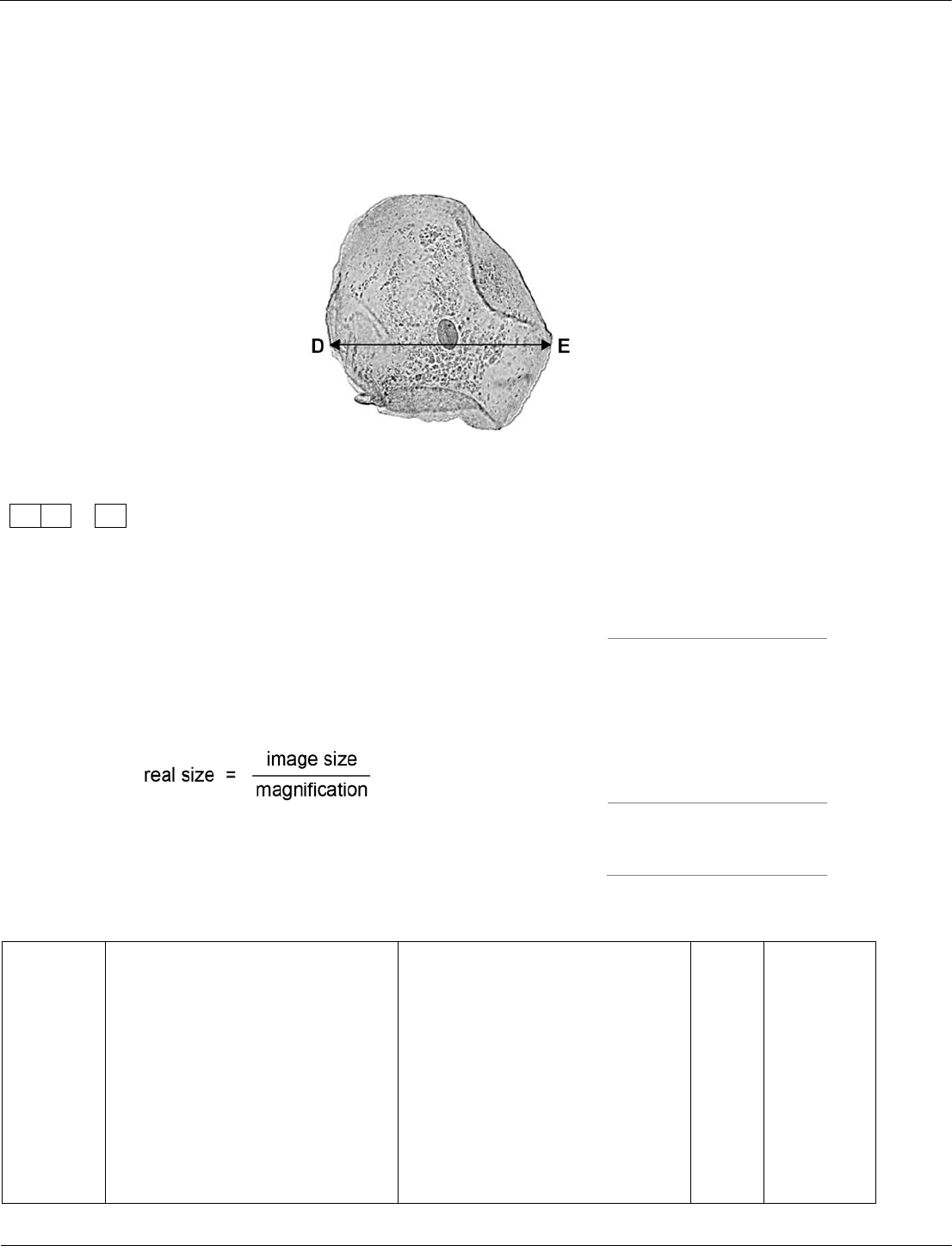
This question requires students to use a step-by-step process to estimate the size of a cheek cell.
Students should be able to carry out calculations involving magnification, real size and image size.
The question is scaffolded into three steps of increasing difficulty. This is to make the calculation
accessible to all students, particularly those who find calculations difficult.
The standard command words ‘calculate’ and ‘complete’ indicate the responses required. Each step
uses a specific instruction:
• ‘measure’ to direct students to use a ruler
• ‘use the equation to work out’ to lead them correctly through the calculation
• ‘convert’ to answer with the appropriate SI units.
This question is designed to differentiate across the entire Foundation Tier attainment range. The final
step is likely to be too difficult for some students.
Spec. ref.:
4.1.1.5
MS:
1c
2h
WS:
3.2
3.3
AO:
AO2/2
Demand:
Low‒
standard
Our exams explained – GCSE science exams from summer 2018
11
The cheek cell in Figure 6 is magnified 250 times.
The width of the cell is shown by the line D to E.
Figure 6
0
3
.
5
Calculate the width of the cheek cell in micrometres (µm).
Complete the following steps.
[3 marks]
Measure the width of the cell using a ruler
mm
Use the equation to work out the real width of the cell
in mm:
mm
Convert mm to µm
µm
03.5
45 (mm)
45 / 250 or 0.18 (mm)
180 (µm)
allow ecf
allow 180 (µm) with no working
shown for 3 marks
1
1
1
AO2/2
4.1.1.5
AO2/2
4.1.1.5
AO2/2
4.1.1.5
Cheek cell © Ed Reschke/Getty Images

12
Multiple-choice questions (MCQs)
MCQs will feature in all papers. Often MCQs will appear at the beginning of questions to give students
confidence. Including these questions allows us to test a greater breadth of content across our exams.
However, MCQs should not be considered as easy marks! Some feature in Higher Tier papers at higher
levels of demand.
MCQ types include:
• tick box
• linking boxes
• sentence completion.
Our exams explained – GCSE science exams from summer 2018
13
Combined Science: Trilogy ‒ chemistry paper 1F, question 07.1
This is an example of a standard tick box MCQ that tests recall of knowledge. The question is concise
and direct to avoid any ambiguity when choosing an answer.
Spec. ref.:
5.4.2.2
MS:
n/a
WS:
n/a
AO:
AO1/1
Demand:
Standard
0
7
.
1
What type of compound is calcium oxide?
[1 mark]
Tick one box.
An acid
A base
A carbonate
A salt
07.1
a base
1
AO1/1
5.4.2.2
14
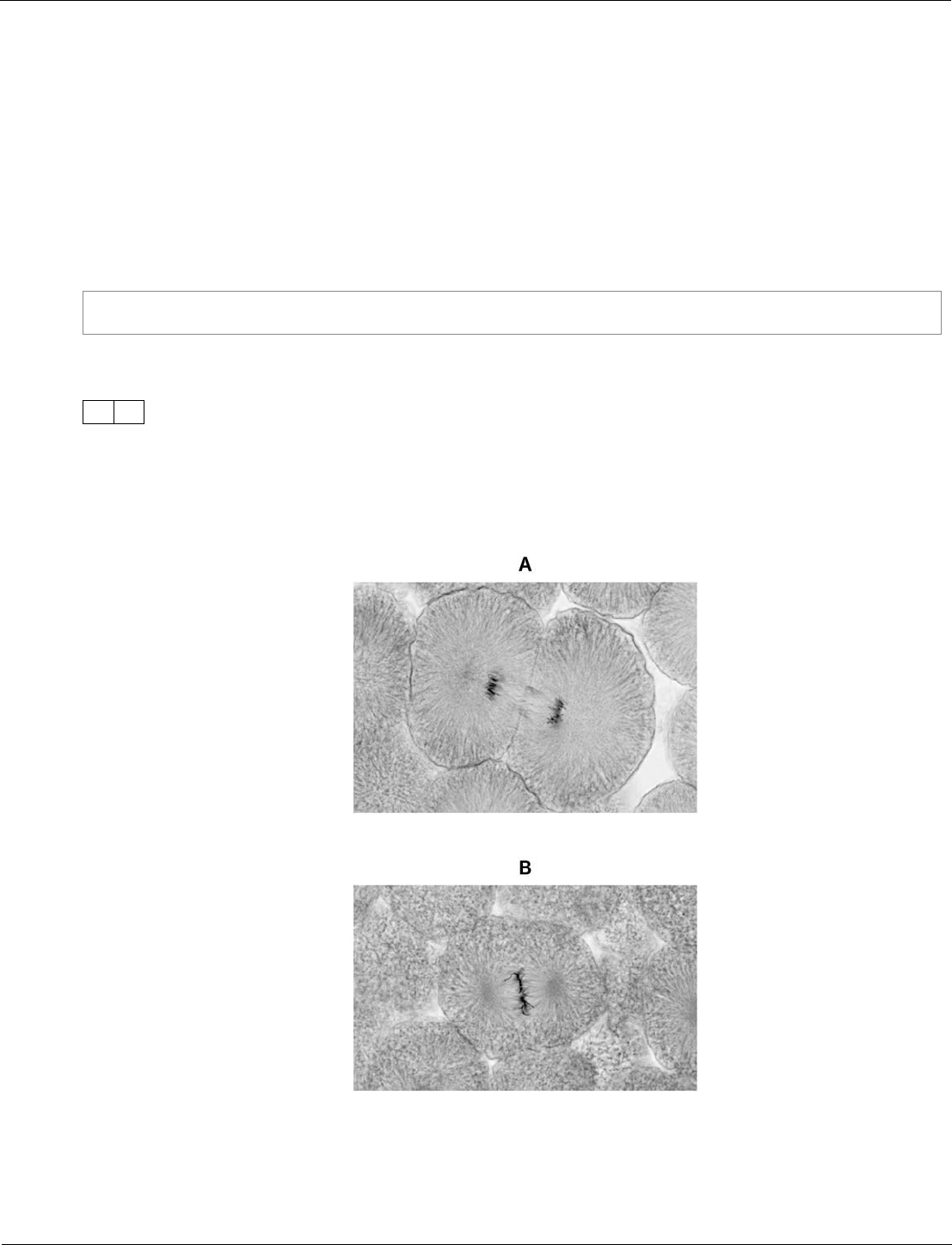
Biology ‒ paper 1H, question 09.1
This question is an example of where a MCQ can be used to assess knowledge at high demand. It is
expected that only higher attaining students will have studied cell division in sufficient detail to
answer correctly.
The question is worded simply to make it accessible and students are given three options from which to
choose the correct answer. We try not to use negative questions but when we do we make it clear to
students by emboldening the ‘not’. The photographs are presented at a size whereby it’s easy for
students to distinguish between the three.
Spec. ref.:
4.1.2.2
MS:
n/a
WS:
3.5
AO:
AO2/1
Demand:
High
0
9
Figure 6 shows photographs of some animal cells at different stages during the
cell cycle.
Figure 6

Our exams explained – GCSE science exams from summer 2018
15
0
9
.
1
Which photograph in Figure 6 shows a cell that is not going through mitosis?
[1 mark]
Tick one box.
A
B
C
09.1
C
1
AO2/1
4.1.2.2
Telophase © Ed Reschke/Getty Images
Metapahse © Ed Reschke/Getty Images
Interphase © Ed Reschke/Getty Images

16
Link box
This type of question is useful for asking about multiple things that are linked, often definitions.
They are good at discriminating within a level of demand.
In terms of accessibility link box questions:
• are clear and straightforward
• reduce the amount of text required for a question
• introduce white space.
For link box questions we use a set direct style that is familiar to students:
‘Draw x line(s) from each y to z’.
Our exams explained – GCSE science exams from summer 2018
17
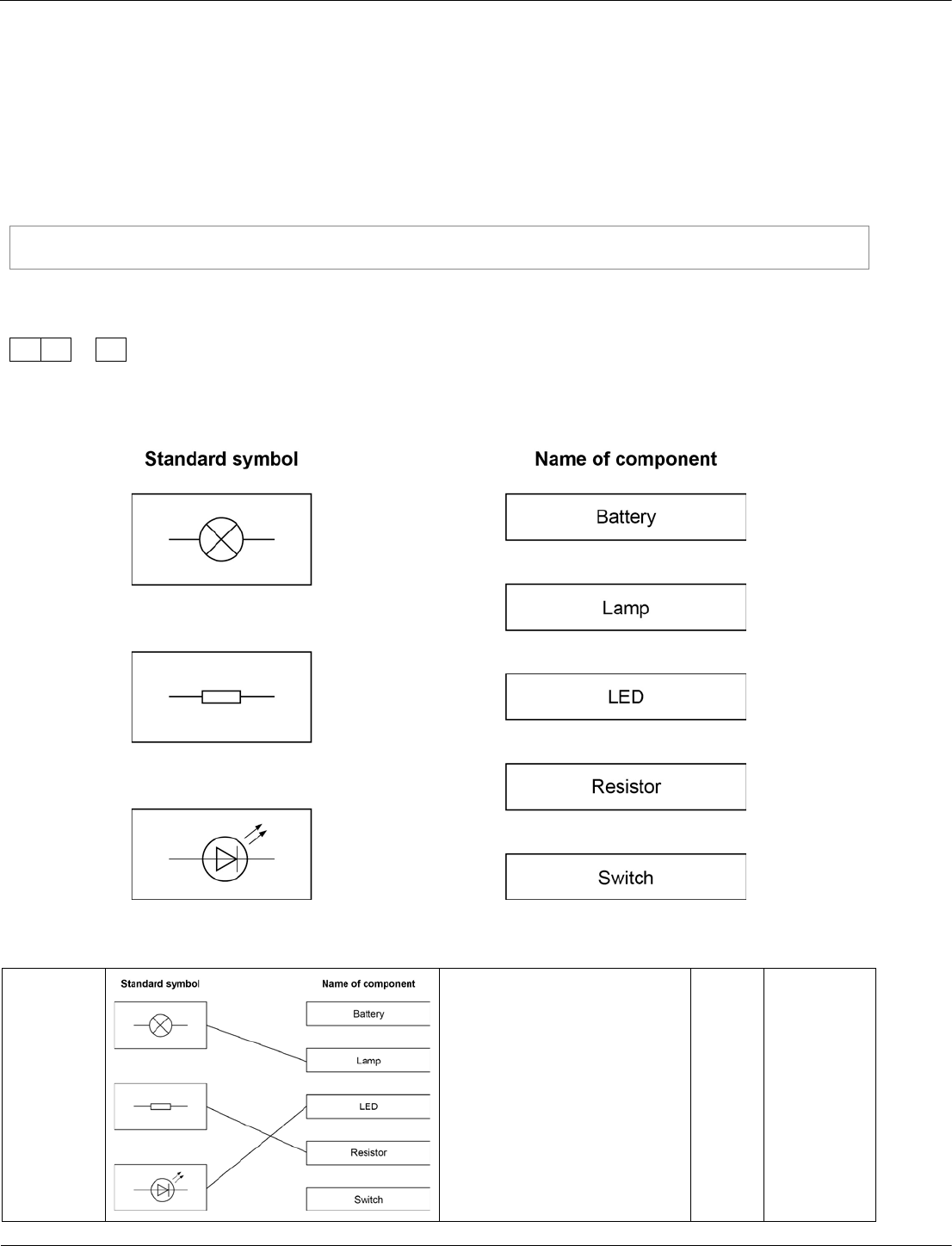
Combined Science: Synergy ‒ paper 3F, question 06.2
Students are required to recognise and use the standard symbols for common circuit elements.
Low attaining students can find drawing symbols and circuits daunting.
This is a straightforward way of allowing students to demonstrate their knowledge without taxing their
drawing skills.
Spec. ref.:
4.7.2.4
MS:
n/a
WS:
n/a
AO:
AO1/1
Demand:
Low
0
6
.
2
Draw one line from each symbol to the name of the component.
[3 marks]
06.2
extra lines from a symbol
negate the mark
3
AO1/1
4.7.2.4
18
Sentence completion
These questions require students to complete sentences by either using prompts (words in a box) or
without using prompts. As with link box questions, sentence completion questions are useful for asking
about multiple things that are linked.
The sentences are simple in terms of language construction. The gaps in the sentences are at the end
of each sentence so that students do not need to refer back.
Biology ‒ paper 2F, question 05.3
Students are expected to use their knowledge of deforestation to explain what effect this has upon the
gases in the atmosphere.
The earlier parts of the question lead students into this low demand closed question. Students need to
complete simple sentences using words from the box. There are six words to choose from for two
marks. This question will differentiate well. The options pick up on common misconceptions
encountered in this topic about the roles of photosynthesis and respiration.
Spec. ref.:
4.7.3.4
MS:
n/a
WS:
n/a
AO:
AO1/1
Demand:
Low
0
5
.
3
More forest was lost in 2012 than in 2009.
Use words from the box to complete the sentences.
[2 marks]
carbon dioxide excretion nitrogen
oxygen photosynthesis respiration
The increase in the area of forest lost has caused an increase
in the gas
.
The increase of this gas has been caused because less of the gas is being
absorbed by plants for the process of
.
05.3
carbon dioxide
photosynthesis
in this order only
1
1
AO1/1
4.7.3.4
AO1/1
4.7.3.4
Our exams explained – GCSE science exams from summer 2018
19
Short answer
Short answer questions will feature in all papers. Often they will be at the beginning of a group of related
questions to give students confidence. They are a good way to introduce students into an area of
specification content before building up to more demanding questions.
Combined Science: Trilogy ‒ chemistry paper 2F, question 02.6
Earlier on in this question a context is given about separating a mixture of liquids in a fuel. This question
requires students to use their knowledge and draw a comparison between this fuel and crude oil.
Spec. ref.:
5.7.1.1
MS:
n/a
WS:
n/a
AO:
AO1/1
Demand:
Low‒
standard
0
2
.
6
Describe how this fuel is different from crude oil.
[2 marks]
02.6
the fuel is a pure compound
and crude oil is a mixture
or
the fuel is made up of four
hydrocarbons
and crude oil could have many
more
allow crude oil contains a large
number of compounds and the
fuel contains four
1
1
AO1/1
5.7.1.1
AO1/1
5.7.1.1
20
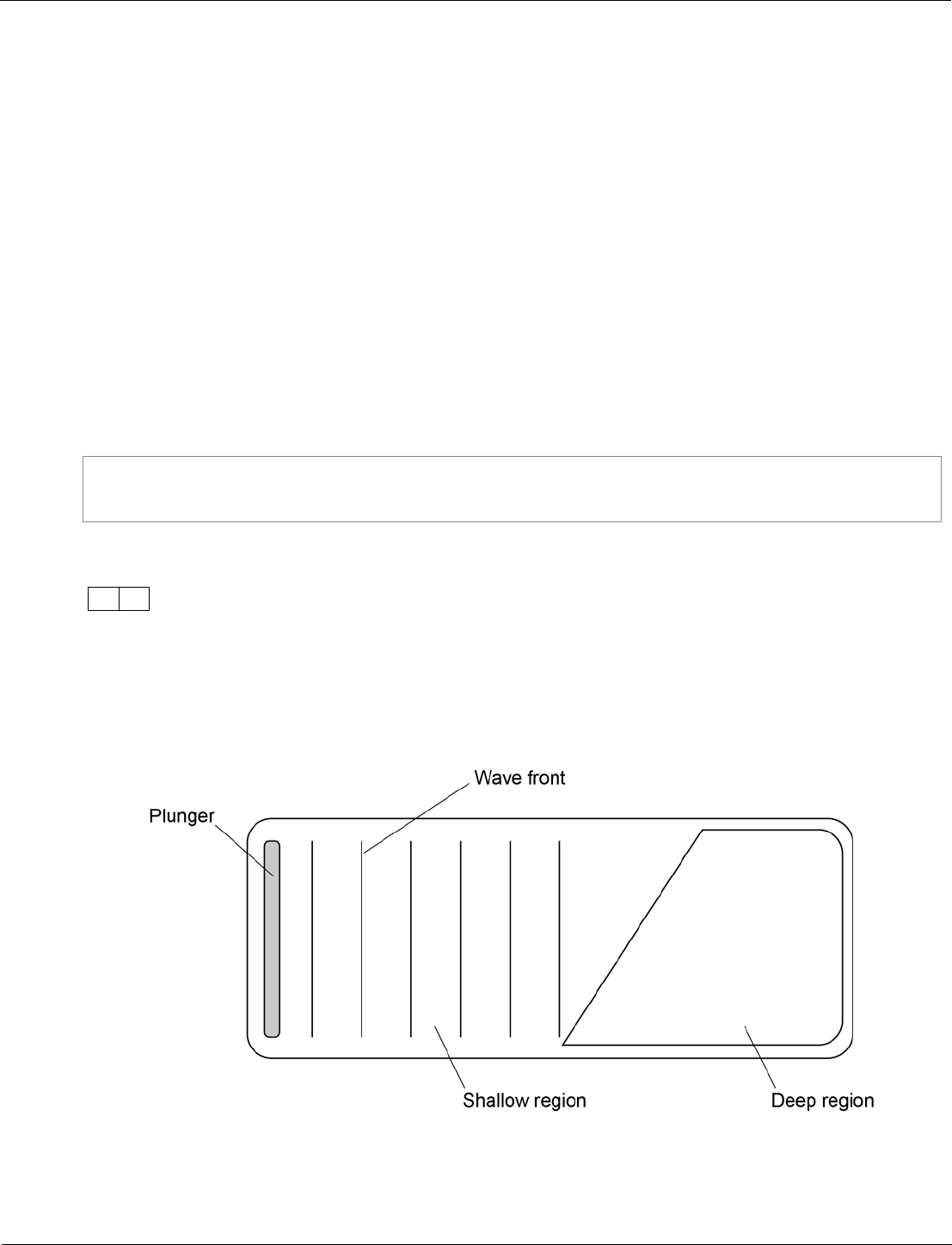
Combined Science: Synergy ‒ paper 1H, question 09.4
The specification requires students to be able to describe evidence that it is the wave and not the water
that moves. The question is based on a required practical situation, presenting two contrasting
statements and asking students to devise a way of working out which is correct.
The question is set at standard/high demand. This is because students need to take what they have
learned about this required practical and apply it in a complex scenario.
The command word ‘suggest’ recognises that this is a situation in which students may not be familiar
and indicates that they should apply their knowledge.
The question is made accessible by:
• referring students back to the diagram
• giving each statement on a separate line
• using bold lettering to identify each student.
Spec. ref.:
RPA5
MS:
n/a
WS:
2.2
AO:
AO2/2
Demand:
Standard/
high
0
9
Some students did an investigation to study the behaviour of waves.
Figure 9 shows a ripple tank that they used to model the behaviour of waves.
Figure 9
Our exams explained – GCSE science exams from summer 2018
21
0
9
.
4
Some students investigate the properties of the waves generated in Figure 9.
Student A says ‘the waves move water from one end of the tank to the other’.
Student B says ‘that’s wrong. Only the waves move, not the water’.
Suggest what the students could do to decide which of them is correct.
[2 marks]
09.4
place a floating object / plastic
duck on the surface of the water
it will stay in the same place
or
only bob up and down if the
water does not move
1
1
AO2/2
RPA5
AO2/2
RPA5

22
Calculations
We have a set approach to the recall of equations and the level of demand of calculations. This
consistency is to ensure students are rewarded fairly, across all subjects, for calculation questions.
For calculation questions we use the ‘error carried forward’ principle so that students are only penalised
for a mathematical error once.
Low demand: equations from the ‘recall’ list will be given in the body of the question. This will allow
lower-attaining students to access AO2 calculation questions.
• Should only be ‘simple’ equations with substitution of two numbers that are easy to manipulate.
• No transformation.
• Identifying equations from a list (MCQ)
• Simple conversion eg minutes to seconds.
Standard demand: a prompt will be given with regard to the ‘recall’ equation required eg ‘Write down
the equation which links distance, speed and time.’
Questions will involve substitution with something ‘extra’ (or be a more complex equation). The ‘extra’
could be:
• a transformation
• change a quantity ie grams to kg
• obtaining data from somewhere eg from a graph or selecting appropriate data to use.
Standard/high demand: No prompt given. Questions involve a transformation and something ‘extra’.
High demand: use of one of the equations that students have difficulty with. Also could include a
transformation/something ’extra’ or a multi-step calculation with no lead in or guidance given.
For calculations from the ‘select’ list, students will be expected to be able to select the appropriate
equation from the Physics Equations sheet.
The nature of calculations means that there are often several strands of information and more than one
command. So, for students to be able to take all of this information in, we split strands of information on
separate lines. We also give prompts on the final answer line to reinforce what we’re looking for in
an answer.
Our exams explained – GCSE science exams from summer 2018
23
GCSE Combined Science: Trilogy ‒ physics paper 1F, question 05.4
This low demand calculation is set in the wider context of ice on a car’s rear window. The equation
required has been provided (the equation will be required recall at least once in the lifetime of
the specification).
The command word ‘calculate’ is a standard command word indicating the type of response required.
Also included is a prompt to the number of decimal places given.
The mark scheme allows students that show a correct method to gain credit. This is even though the
student may not be able to complete the arithmetic correctly. Two marks would be awarded if the correct
final answer is found without any working shown.
Spec. ref.:
6.1.2.2
MS:
1a
3c
3d
WS:
n/a
AO:
AO2/1
Demand:
Low
0
5
.
4
When the heater is supplied with 120 J of energy each second, the internal energy of
the ice increases by 45 J each second.
Use the following equation to calculate the efficiency of the heater.
efficiency =
output energy transfer
input energy transfer
Give your answer to two decimal places.
[2 marks]
Efficiency =
05.4
45 / 120 = 0.375
0.38
allow 0.38 with no working
shown for 2 marks
allow 0.375 with no working
shown for 1 mark
1
1
AO2/1
6.1.2.2
AO2/1
6.1.2.2
24
Physics ‒ paper 1F/1H, question 12.4/04.4
This question requires students to select and apply an equation from the Physics Equations Sheet to this
practical situation. They have to rearrange the specific heat capacity equation to calculate the mass of
water used.
Students get one mark for a correct substitution of the numbers into the equation. The second mark is
for the rearrangement of the equation. And the third mark is for giving the correct answer.
Spec. ref.:
4.1.1.3
MS:
3b
3c
3d
WS:
n/a
AO:
AO2/2
Demand:
Standard
1
2
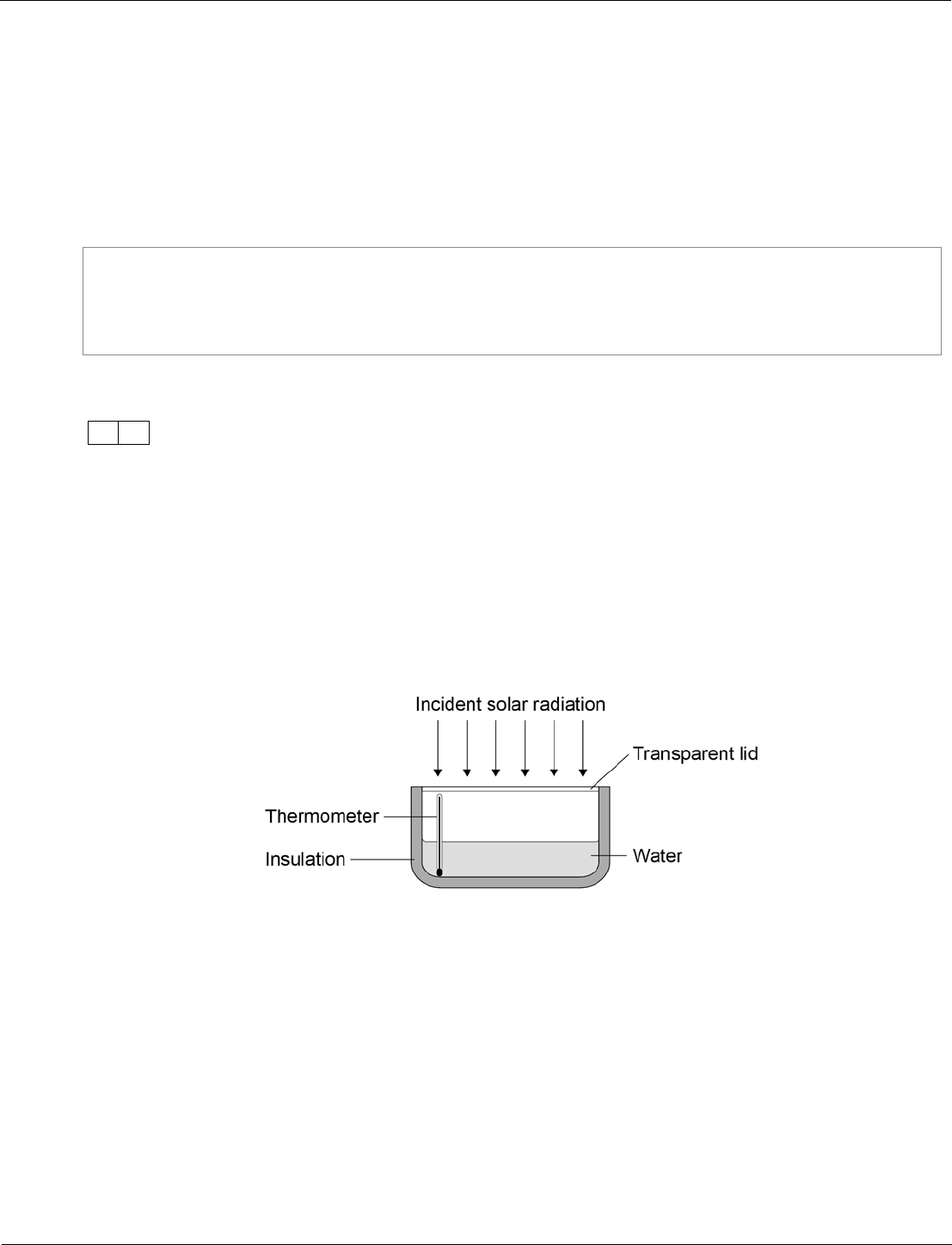
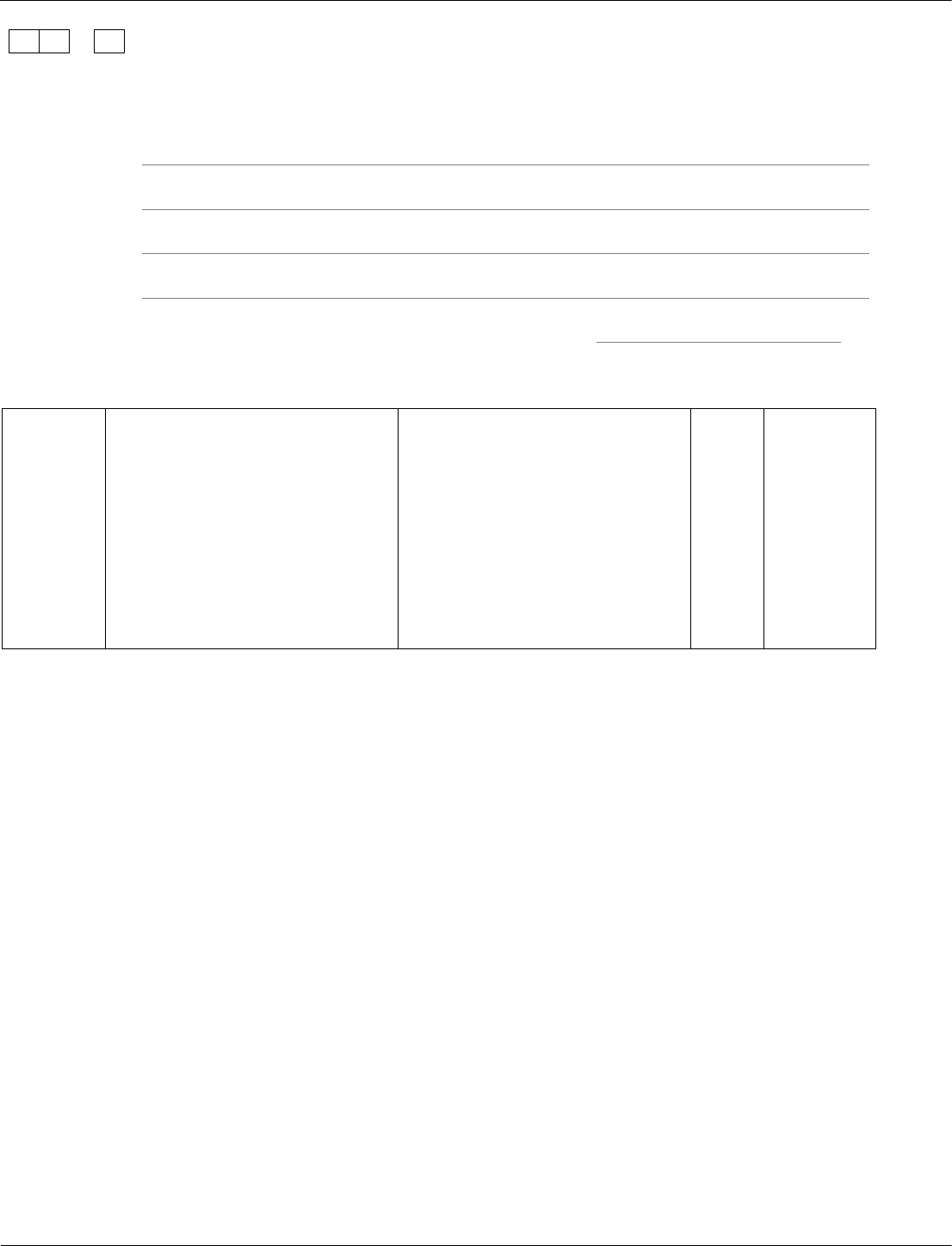
A student investigated how much energy from the Sun was incident on the Earth’s
surface at her location.
She put an insulated pan of water in direct sunlight and measured the time it took for
the temperature of the water to increase by 0.6 °C.
The apparatus she used is shown in Figure 14.
Figure 14
The energy transferred to the water was 1050 J.
The time taken for the water temperature to increase by 0.6 °C was 5 minutes.
The specific heat capacity of water is 4200 J/kg °C.
Our exams explained – GCSE science exams from summer 2018
25
1
2
.
4
Calculate the mass of water the student used in her investigation.
Use the correct equation from the Physics Equations Sheet.
[3 marks]
Mass =
kg
12.4
1050 = m × 4200 × 0.6
m = 1050 / (4200 × 0.6)
m = 0.417 (kg)
accept 0.417 (kg) with no
working shown for 3 marks
1
1
1
AO2/2
4.1.1.3
AO2/2
4.1.1.3
AO2/2
4.1.1.3
26
Combined Science: Trilogy ‒ chemistry paper 2H, question 04.3
Students are required to deduce the molecular formula of paracetamol from a given diagram.
The command word ‘calculate’ makes it clear what is required. Providing the relative atomic masses in
the question reduces errors in reading values from the periodic table.
Spec. ref.:
5.2.1.4
5.3.1.2
MS:
n/a
WS:
1.2
AO:
AO2/1
Demand:
Standard/
high
0
4
.
3
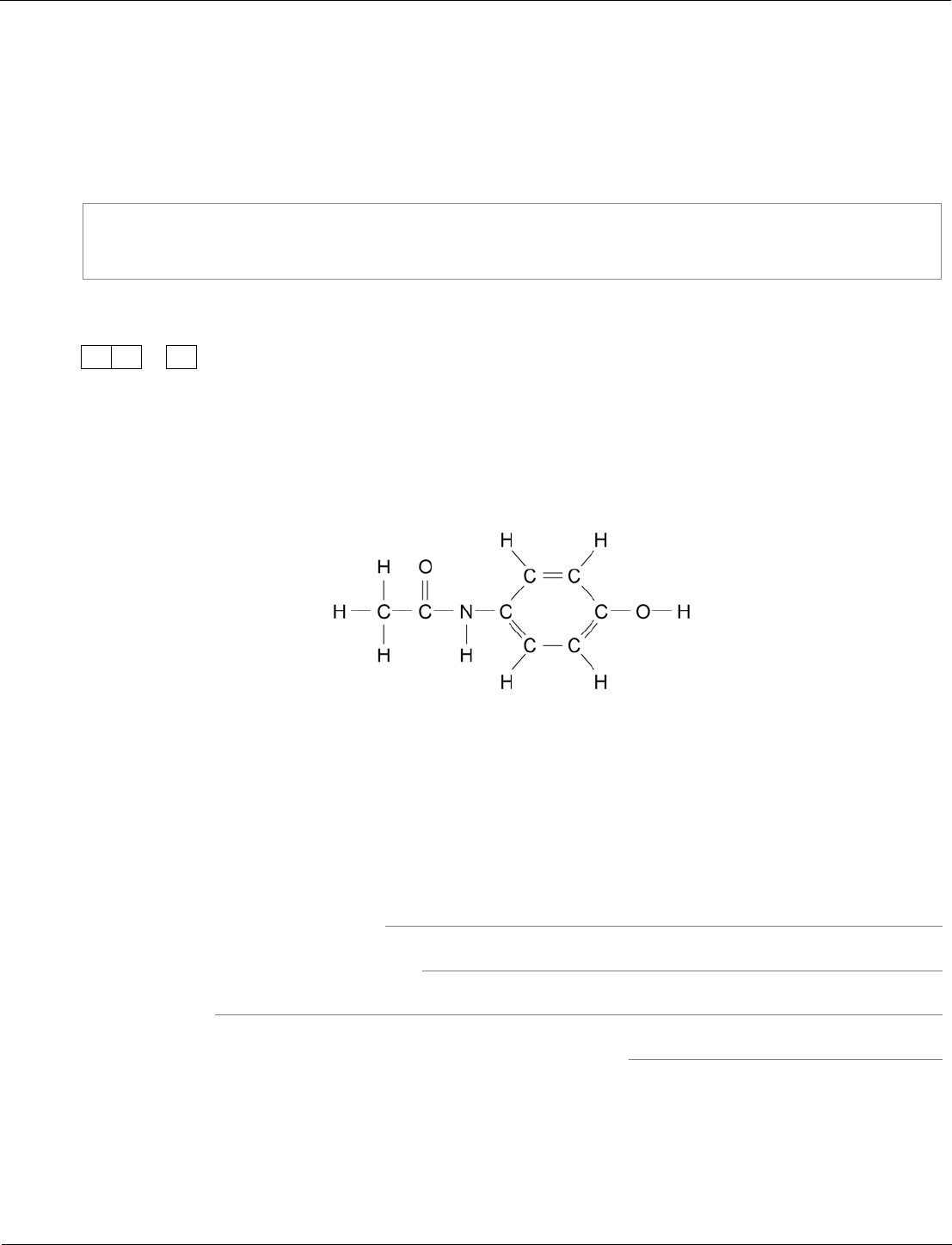
The main ingredient in Aqamed is a painkiller called paracetamol.
Figure 8 represents a molecule of paracetamol.
Figure 8
Give the molecular formula of paracetamol.
Calculate its relative formula mass (M
r
).
[2 marks]
Relative atomic masses (A
r
): H = 1; C = 12; N = 14; O = 16
Molecular formula
Relative formula mass
M
r
=
Our exams explained – GCSE science exams from summer 2018
27
04.3
C
8
H
9
NO
2
151
any order of elements
1
1
AO2/1
5.2.1.4
AO2/1
5.3.1.2
28
Combined Science: Trilogy ‒ physics paper 1H, question 06.4
Students are required to recall the equation: density = mass × volume. The other four marks require
students to apply this equation to the situation given to come up with an answer. They must then use
this answer, select the appropriate equation from the equation sheet, perform another substitution and
calculate the final answer.
The mark scheme clearly shows which mark is available for which part of the calculation. It also clarifies
that there is no mark available for the selection of the equation from the Physics Equations Sheet.
This is a very challenging multi-step calculation which will test the mathematics skills and physics
understanding of the most able. The logical and coherent response required means that it counts as an
extended response question.
The first three lines of 06.4 contain the strands of figures required to make the calculation. They’re
deliberately on separate lines to improve readability.
Spec. ref.:
6.3.2.3
MS:
1b
3b
3c
3d
WS:
n/a
AO:
AO2/1
Demand:
High
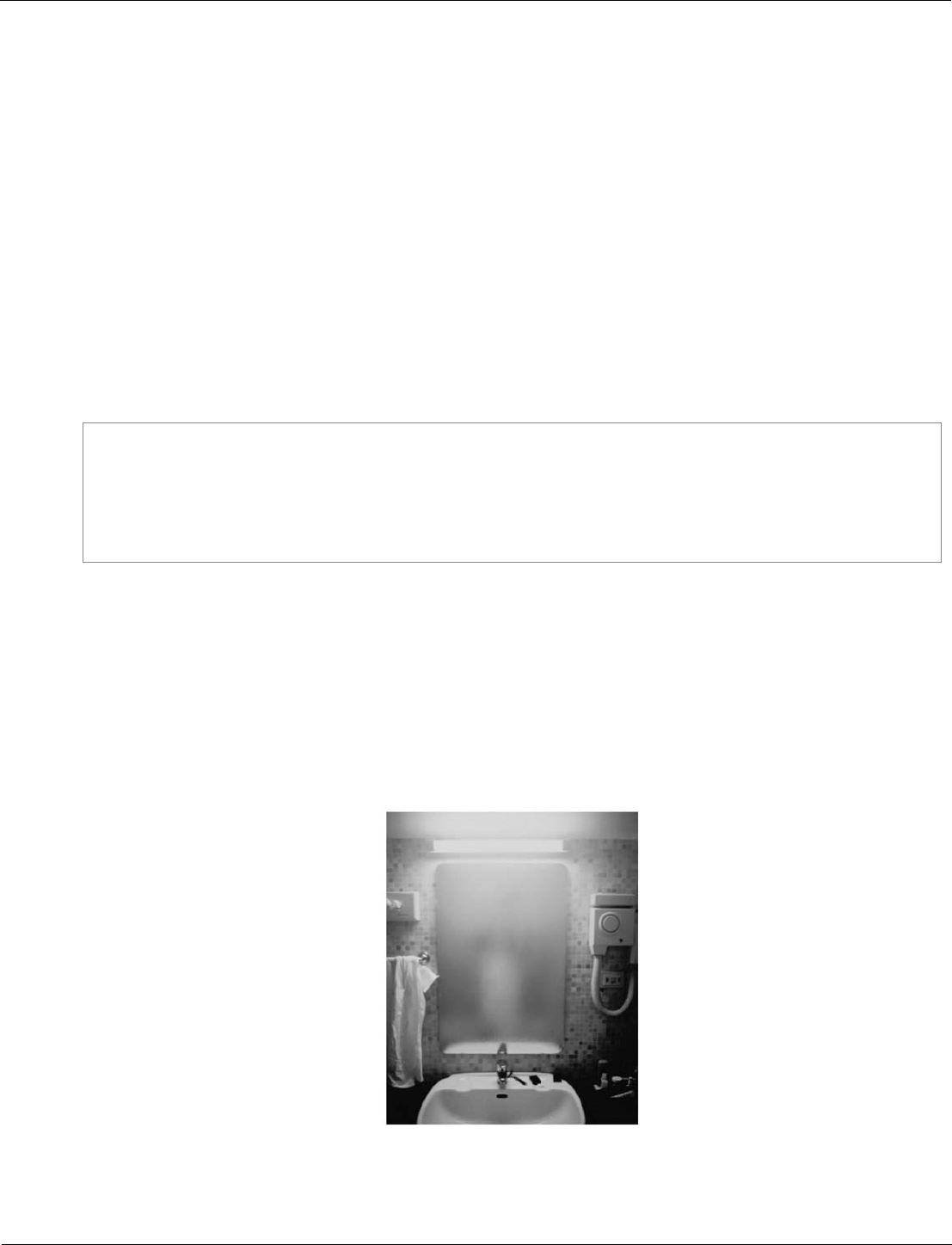
Water vapour is a gas. Gases change state when they cool.
Figure 8 shows condensation on a cold bathroom mirror.
Figure 8
Bathroom mirror condensation © Dwight Eschliman/Getty Images
Our exams explained – GCSE science exams from summer 2018
29
0
6
.
4
A volume of 2.5 × 10
–5
m
3
of condensation forms on the mirror.
Density of water = 1000 kg/m
3
Specific latent heat of vaporisation of water = 2.26 × 10
6
J/kg.
Calculate the energy released when the condensation forms.
[5 marks]
Energy released =
J
06.4
1000 = m / 2.5 × 10
−5
m = 2.5 × 10
−5
× 1000
m = 0.025 (kg)
E = 0.025 × 2 260 000
E = 56 500 (J)
allow 56 500 (J) without working
shown for 5 marks
0 marks awarded for E = m × L
1
1
1
1
1
AO2/1
6.3.2.3
AO2/1
6.3.2.3
AO2/1
6.3.2.3
AO2/1
6.3.2.3
AO2/1
6.3.2.3
30
Combined Science: Synergy ‒ paper 4H, question 09.2
This question is intended to stretch the most able students. They must recall the equations for
calculating gravitational potential and kinetic energy from the specification; then apply them to the given
situation. They are told to make an assumption that will help them in the calculation.
Students need to go through a number of steps to produce the correct answer. It also counts as an
extended response question.
Students must:
1. understand that in this situation the gravitational potential energy is equal to the kinetic energy
2. be able to recall and substitute into the equation to calculate the gravitational potential energy
3. recall the equation for calculating kinetic energy
4. rearrange the equation and substitute into it to calculate the correct answer (this is one of the
equations where marks are awarded for rearrangement).
Students should show their working in all calculations. If they simply give an answer and it is wrong they
will gain no marks. If they do give their working, then they may achieve marks for correct working even if
the final answer is incorrect. Students who do not show their working but give the correct answer will
receive full marks. This is because they’re assumed to have used the correct equations (although no
mark is awarded for recall at this level of demand).
Spec. ref.:
4.6.1.5
4.7.1.9
MS:
3b
3c
3d
WS:
3.3
AO:
AO2/1
Demand:
High
0
9
Figure 7 shows a rollercoaster.
Figure 7
The rollercoaster car is raised a vertical distance of 35 m to point A by a motor
in 45 seconds.
The mass of the rollercoaster is 600 kg.
The motor has a power rating of 8 000 W.

Our exams explained – GCSE science exams from summer 2018
31
0
9
.
2
The rollercoaster rolls from point A to point B, a drop of 35 m.
Calculate the speed of the rollercoaster at point B.
[6 marks]
Assume that the decrease in potential energy store is equal to the increase in kinetic
energy store.
Speed at point B =
m/s
09.2
gpe = 600 × 9.8 × 35
= 205 800
gpe = KE = ½ m v
2
v =
�
2 × KE
m
=
�
411 600
600
26.2 (m/s)
allow 26.2 with no working
shown for 6 marks
1
1
1
1
1
1
AO2/1
4.6.1.5
AO2/1
4.6.1.5
AO2/1
4.7.1.9
AO2/1
4.7.1.9
AO2/1
4.7.1.9
AO2/1
4.7.1.9
32
Labelling and drawing diagrams
There are many types of questions centred around work on diagrams. They can be something as simple
as using words from a box to label a diagram, all the way through to drawing and labelling a diagram as
part of an extended response.
Chemistry ‒ paper 2F/2H, question 10.3/02.3
This question is based on a required practical activity that students are expected to have carried out to
distil salt solution. It requires a basic knowledge of mixtures.
Students are required to recall information directly from the specification. They must use their
experience to complete and label a diagram to show how you would distil salt water.
Spec. ref.:
4.1.1.2
4.10.1.2
MS:
n/a
WS:
2.2
2.3
AO:
AO1/2
Demand:
Standard
1
0
.
3
Some countries make drinking water from seawater.
Complete Figure 11 to show how you can distil salt solution to produce and collect
pure water.
Label the following:
• pure water
•
salt solution.
[3 marks]
Figure 11
Our exams explained – GCSE science exams from summer 2018
33
10.3
(as part of glassware attached
to bung)
salt solution in (conical) flask
(at end of delivery tube)
pure water in test tube which
must not be sealed
heat source (to heat container
holding salt solution)
allow suitable alternative
equipment eg boiling tube
allow suitable alternative
equipment eg beaker,
condenser
if no other mark obtained allow
for 1 mark suitable equipment
drawn as part of glassware
attached to bung and at end of
delivery tube
1
1
1
AO1/2
4.1.1.2
4.10.1.2
AO1/2
4.1.1.2
4.10.1.2
AO1/2
4.1.1.2
4.10.1.2
34
Biology ‒ paper 2H, question 07.4
This is a five mark question with multiple steps of understanding needed to fully answer the question.
The unscaffolded nature of the question and the unfamiliar context means the demand is at
standard/high. This question has far less scaffolding than a similar question, 08.4, on the
Foundation Tier.
The question draws upon multiple skills, requiring students to apply their understanding of genetic
inheritance and genetic diagrams to draw a Punnett square. Then students need to analyse their
Punnett square to give the probability of inheriting syndrome H.
The information is in three distinct ‘chunks’ so students can easily find what they need. Symbols are
given to use for dominant and recessive alleles and there’s ample space for students to draw their
Punnett square.
Spec. ref.:
4.6.1.6
MS:
2e
WS:
3.2
AO:
AO2/2
AO3/2b
Demand:
Standard/
high
0
7
.
4
A recessive allele causes syndrome H.
A heterozygous woman and a homozygous recessive man want to have a child.
Draw a Punnett square diagram to find out the probability of the child having
syndrome H.
Identify any children with syndrome H.
[5 marks]
Use the following symbols:
A = dominant allele
a = recessive allele
Probability =
Our exams explained – GCSE science exams from summer 2018
35
07.4
mother / woman’s gametes
correct: A a
father / man’s gametes correct:
a a
correct derivation of offspring
identification of child with
syndrome H or genotype aa
0.5
ecf
ecf
allow 50% / 1/2 / 1 in 2 / 1:1
do not accept 1:2
1
1
1
1
1
AO2/2
4.6.1.6
AO2/2
4.6.1.6
AO2/2
4.6.1.6
AO2/2
4.6.1.6
AO3/2b
4.6.1.6
36
Graphs
We use a variety of graphs to test a range of skills covering all levels of demand.
We have focused on making our graphs clearer. For example, the amount of information displayed
should be concise and relevant to the level being tested (ie graphs with two scales on the y axes are
limited to more challenging questions). Also, where suitable we use simpler gridlines instead of the
standard 2 mm
2
grids.
We want to assess students’ ability to interpret information rather than bamboozling them by having to
‘go searching in detail’ for relevant information.
Combined Science: Trilogy ‒ chemistry paper 2F, question 03.3
This is a simple one mark question requiring students to read information from a table and transfer it
directly onto a bar graph.
Taking data and displaying it requires no manipulation or calculation. Therefore, this is low demand and
is positioned near the beginning of the Foundation Tier paper to help build student confidence.
Spec. ref.:
5.10.1.2
MS:
2c
WS:
3.2
AO:
AO2/2
Demand:
Low
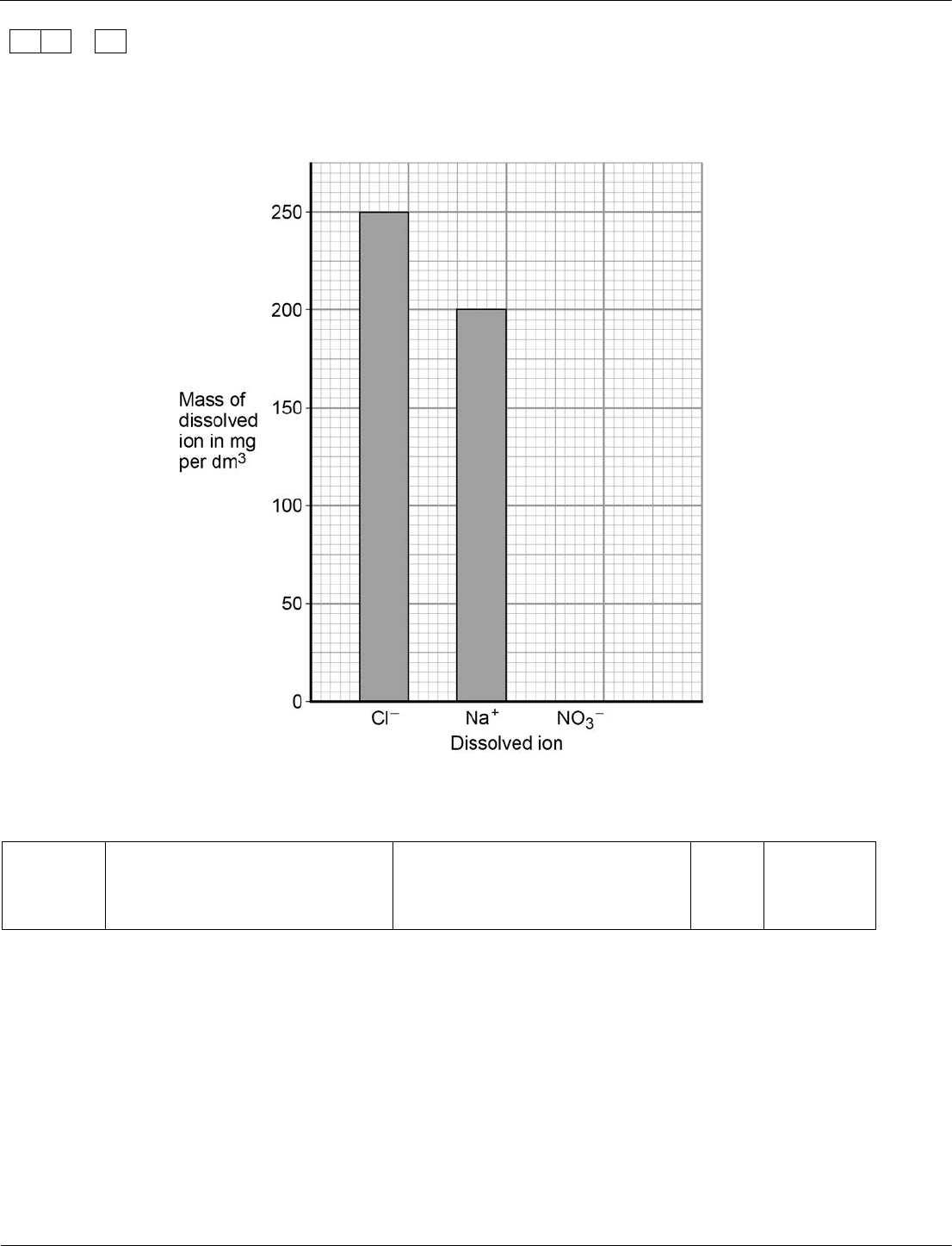
Table 3 shows the amounts of dissolved ions in a sample of drinking water.
Table 3
Dissolved ion
Mass in mg
per dm
3
Cl
−
250
Na
+
200
NO
3
–
40
Our exams explained – GCSE science exams from summer 2018
37
0
3
.
3
Use the information in Table 3 to complete the bar chart in Figure 2.
[1 mark]
Figure 2
03.3
correct bar for NO
3
−
1
AO2/2
5.10.1.2
38
Chemistry ‒ paper 2F, question 09.2
Students have to plot a graph from given data. They need to then make a judgement from the points
plotted on where to draw a line of best fit.
Students have been given the scale and labelled axes as scaffolding to the question. Also, the
instructions for completing the graph are made clear by using a bullet list.
Spec. ref.:
4.6.1.1
4.6.1.2
MS:
4a
4c
WS:
3.5
AO:
AO2/2
AO3/2a
Demand:
Low‒
standard
0
9
.
2
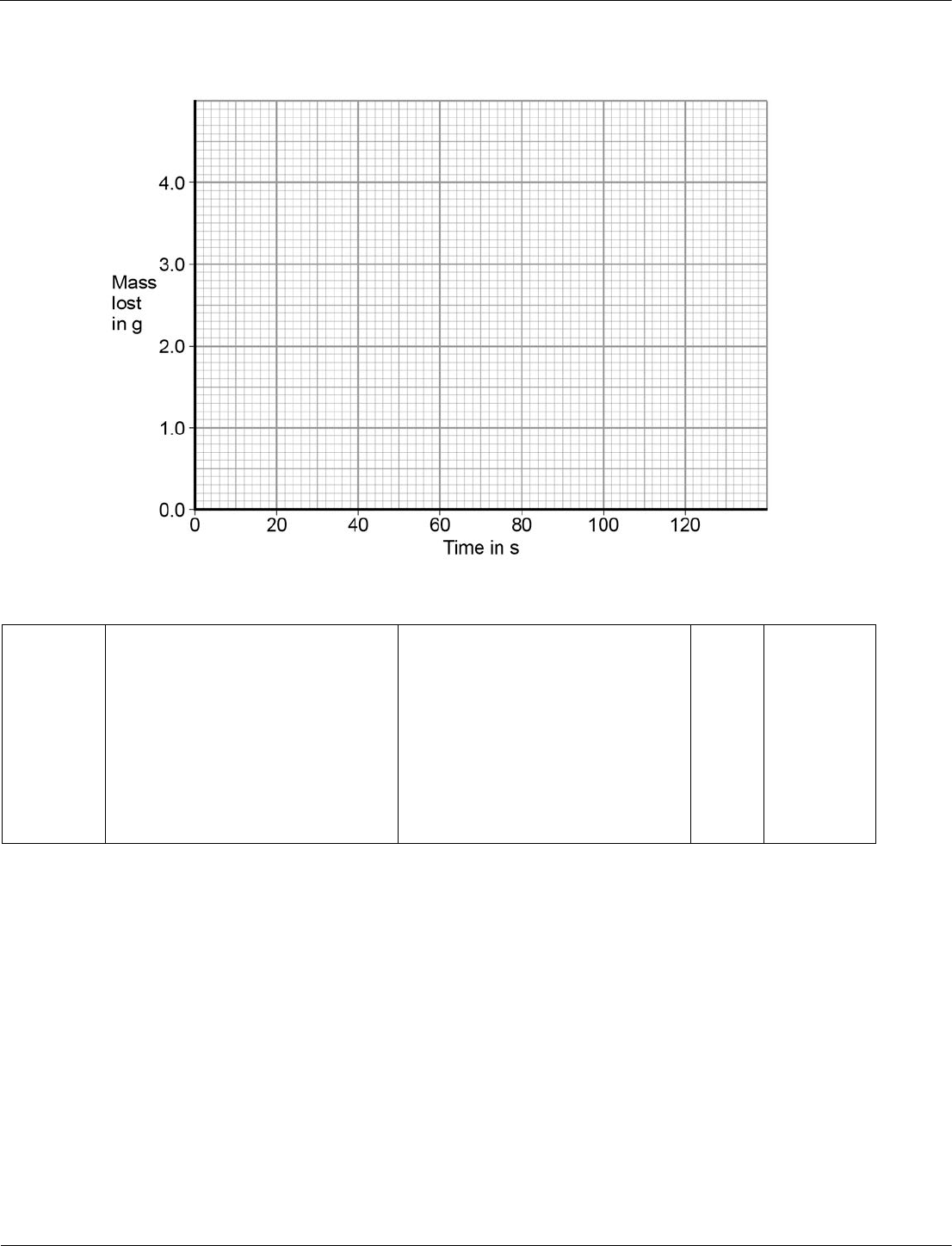
Table 4 shows the student’s results for one investigation.
Table 4
Time
in s
Mass lost
in g
0 0.0
20 1.6
40 2.6
60 2.9
80 3.7
100 4.0
120 4.0
On Figure 10:
• plot these results on the grid
•
draw a line of best fit.
[3 marks]
Our exams explained – GCSE science exams from summer 2018
39
Figure 10
09.2
all points correct
best fit line
± ½ small square
allow 1 mark if 5 or 6 of the
points are correct
must not deviate towards
anomalous point
2
1
AO2/2
4.6.1.1
4.6.1.2
AO3/2a
4.6.1.1
4.6.1.2
Plotting graphs can be much more demanding where you would expect only the highest attaining
students to gain credit. These skills align to those required in GCSE Mathematics.
For example, more challenging graph questions could include:
• negative numbers
• curved line of best fit
• four quadrant areas to plot data
• multiple y-axes
• multiple trend lines.
40
Combined Science: Trilogy ‒ chemistry paper 1H, question 06.1
This question requires students to recall information directly from the specification. The complex nature
of the topic being tested makes this more challenging. To help with making this content accessible the
instructions are made clear by using a bullet list.
This question falls into a category of graph work where we ask students to sketch a graph. The question
below is a very technical example that requires a precise drawing. However, other questions may ask
students to extrapolate information already given on a graph and draw a rough trend line.
Spec. ref.:
5.5.1.2
MS:
n/a
WS:
1.2
AO:
AO1/1
Demand:
Standard/
high
0
6
.
1
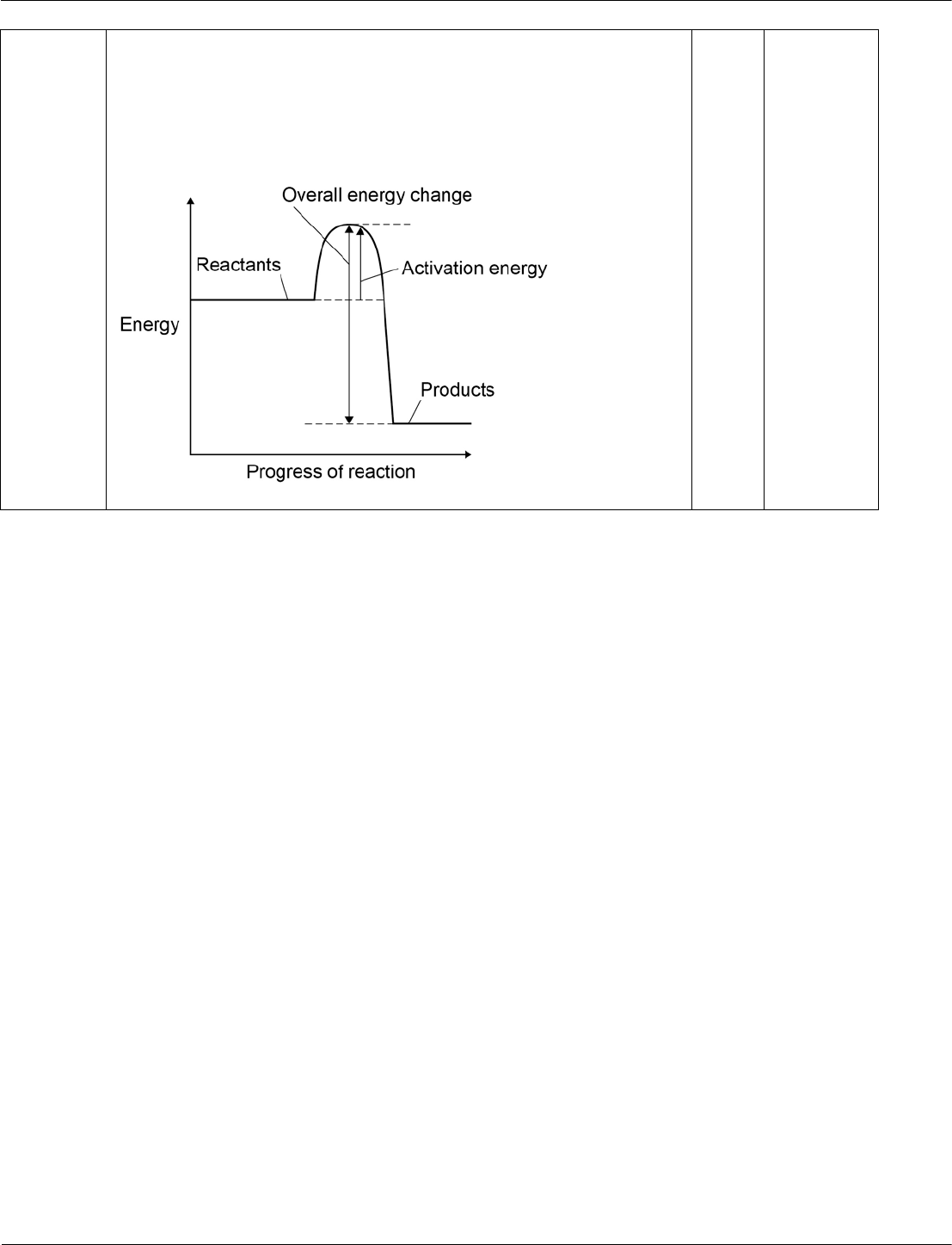
Draw a reaction profile for an exothermic reaction using the axes in Figure 5.
Show the:
• relative energies of the reactants and products
• activation energy and overall energy change.
[2 marks]
Figure 5
Our exams explained – GCSE science exams from summer 2018
41
06.1
the relative energies of the reactants, products and the overall
energy change
the activation energy
1
1
AO1/1
5.5.1.2
AO1/1
5.5.1.2
42
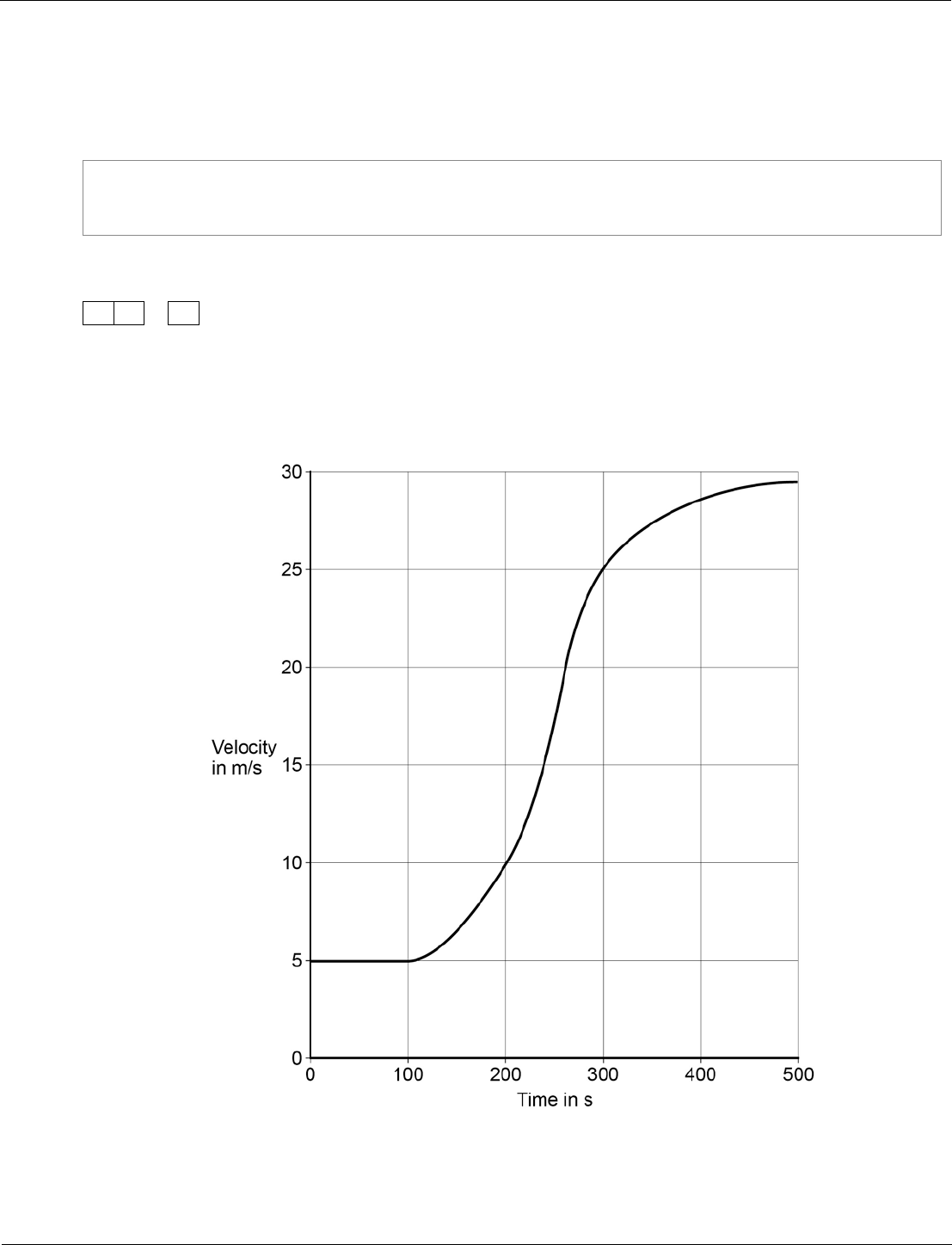
Physics ‒ paper 2H, question 08.4
In this question students are asked to estimate the area under a curve. This is conceptually challenging
and should differentiate across the upper attainment range.
Spec. ref.:
4.5.6.1.5
MS:
4a
4f
WS:
n/a
AO:
AO2/2
Demand:
Standard/
high
0
8
.
4
Figure 15 shows how the velocity of the train changes with time as the train travels
along a straight section of the journey.
Figure 15
Our exams explained – GCSE science exams from summer 2018
43
Estimate the distance travelled by the train along the section of the journey shown
in Figure 15.
To gain full marks you must show how you worked out your answer.
[3 marks]
Distance =
m
08.4
number of squares below line =
17
each square represents 500 m
distance = number of squares ×
value of each square correctly
calculated – 8500 m
accept any number between 16
and 18 inclusive
1
1
1
AO2/2
4.5.6.1.5
AO2/2
4.5.6.1.5
AO2/2
4.5.6.1.5
44
Chemical equations
It is expected that students are able to show their understanding of equations in all science disciplines.
There are various levels of equations we ask ranging from:
• adding state symbols to an equation
• completing a nuclear equation
• writing full balanced equations.
We give more scaffolding to questions on the Foundation Tier.
Chemistry ‒ paper 2F/2H, question 11.4/03.4
This is a straightforward equation on the oxidation of iron. All students are expected to know something
about this. Two marks are awarded for three correct reactants, while one mark is available for two
correct reactants.
Although the question asks for a word equation, if students give the correct symbol equation they gain
full marks.
Spec. ref.:
4.10.3.1
MS:
n/a
WS:
n/a
AO:
AO1/1
Demand:
Standard
1
1
.
4
Rust is hydrated iron(III) oxide.
Complete the word equation for the reaction.
[2 marks]
+
+
hydrated iron(III) oxide
11.4
iron + oxygen + water
all three needed for 2 marks
2 correct = 1 mark
ignore air
2
AO1/1
4.10.3.1
Our exams explained – GCSE science exams from summer 2018
45
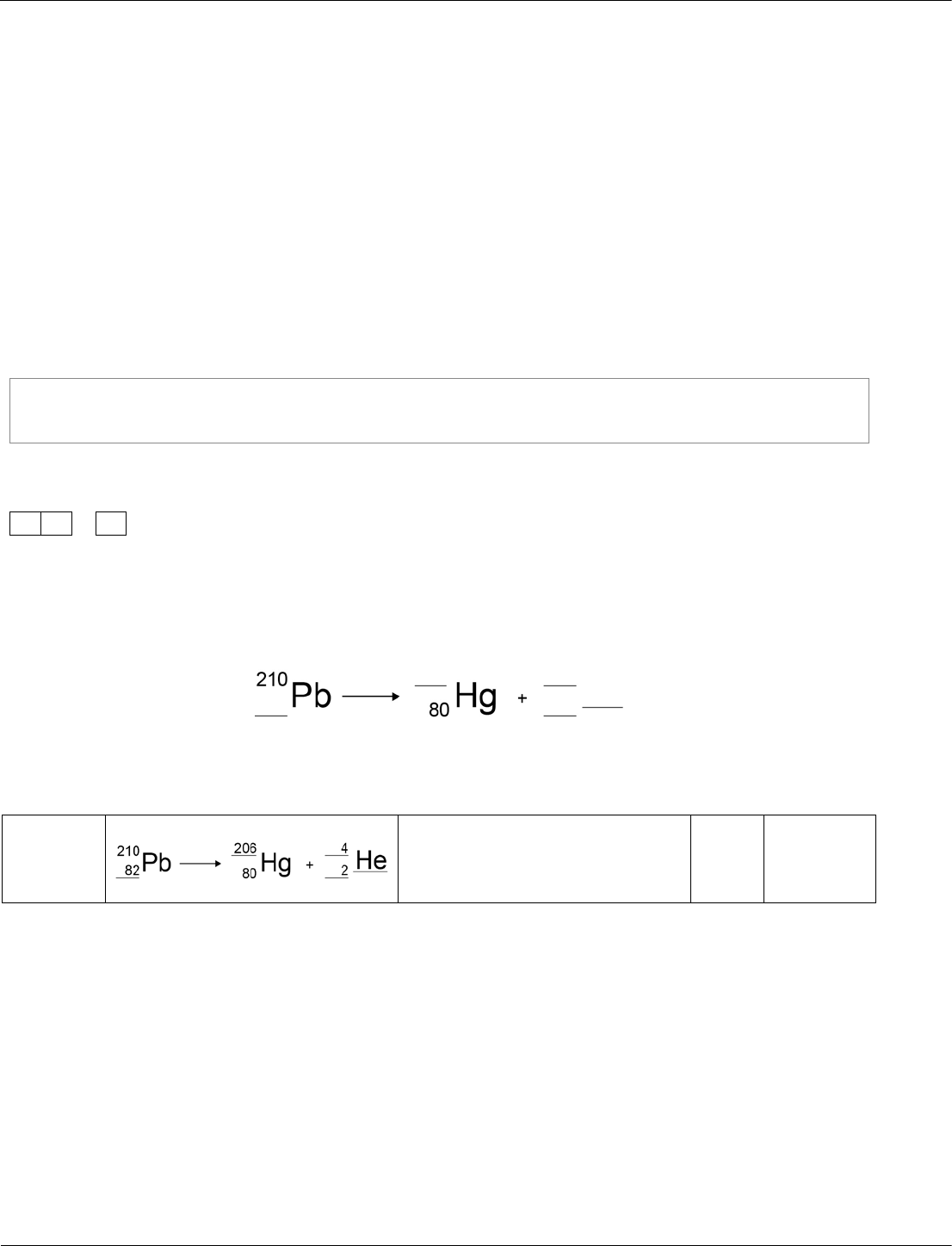
Combined Science: Synergy ‒ paper 1H, question 11.2
Students should be able to use the names and symbols of common nuclei and particles to write
balanced equations that show single alpha and beta decay. This is limited to balancing the atomic
numbers and mass numbers.
The question tells students that lead is undergoing alpha decay, so they should be able to recall the
missing product. They should then use this knowledge to deduce the rest of the numbers from the
information already given.
The numbers given are straightforward. However, the calculation by deduction from an alpha particle is
not straightforward. So, this question is set at standard/high demand.
For clarity, the equation text is enlarged to help students write their answer clearly.
Spec. ref.:
4.3.2.2
MS:
n/a
WS:
n/a
AO:
AO2/1
Demand:
Standard/
high
1
1
.
2
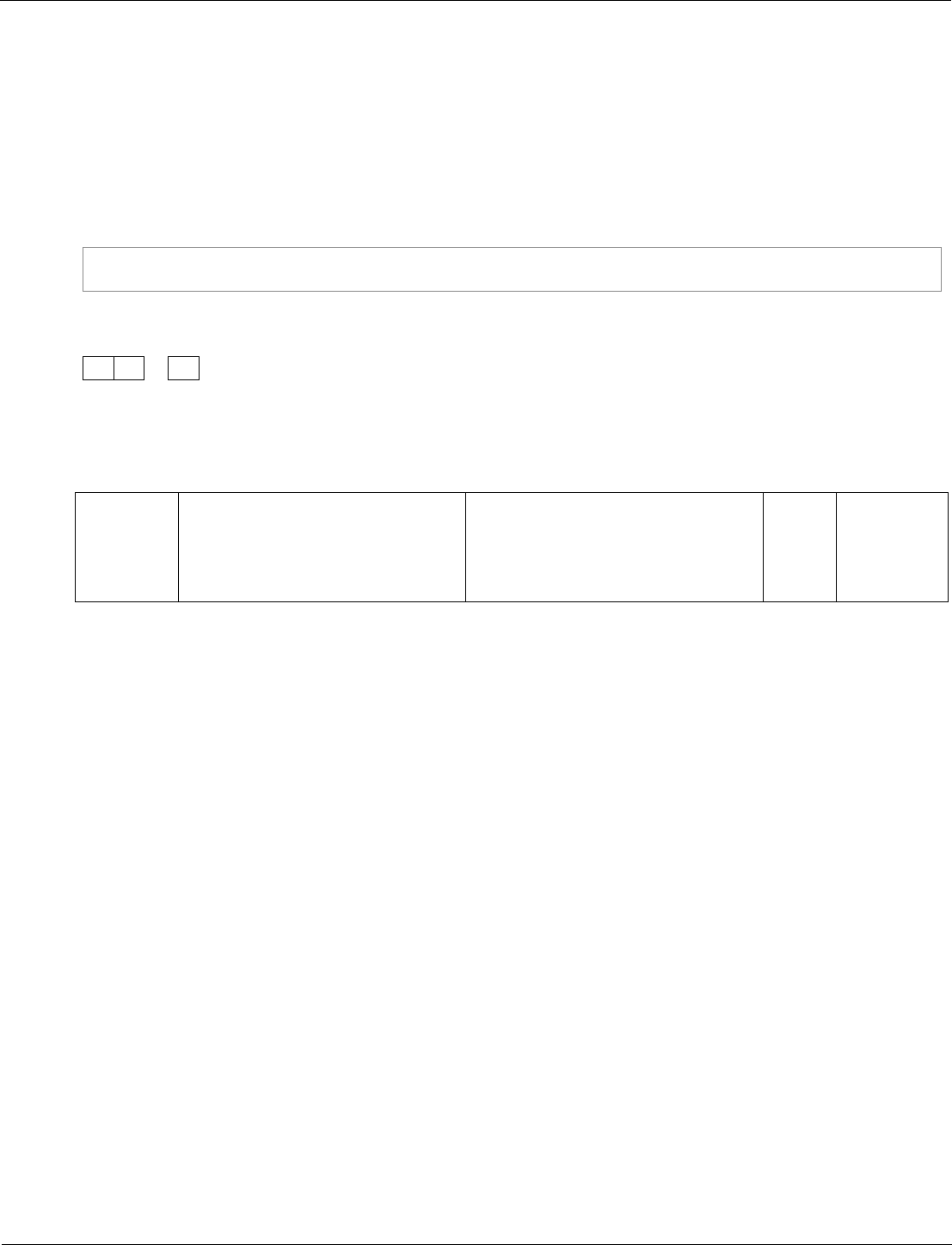
Lead-210 is a radioactive isotope that decays to an isotope of mercury
by alpha decay.
Complete the nuclear equation to show the alpha decay of lead-210.
[3 marks]
11.2
one mark for each correct
element in the equation
3
AO2/1
4.3.2.2
46
Chemistry ‒ paper 1H, question 06.5
This question requires recall of the half equations for the reactions that occur in a hydrogen fuel cell.
Higher Tier students are expected to be able to recall the equations for the reactions at the electrodes in
a hydrogen fuel cell.
Previously recall of these half equations was at A-level A2. Now it forms part of the GCSE course under
the new DfE criteria.
Spec. ref.:
4.5.2.2
MS:
n/a
WS:
1.4
AO:
AO1/1
Demand:
High
0
6
.
5
Write the two half equations for the reactions that occur at the electrodes in a
hydrogen fuel cell.
[2 marks]
06.5
H
2
→ 2H
+
+ 2e
−
O
2
+ 4H
+
+ 4e
−
→ 2H
2
O
1
1
AO1/1
4.5.2.2

Our exams explained – GCSE science exams from summer 2018
47
Extended response
An extended response is defined by Ofqual as:
‘…evidence generated by a Learner which is of sufficient length to allow that
Learner to demonstrate the ability to construct and develop a sustained line of
reasoning which is coherent, relevant, substantiated and logically structured’.
So, what does this mean for our assessments?
• They require an extended answer (4‒6 marks) written in prose.
• Must require a coherent and relevant sustained line of reasoning. Therefore not all 4‒6 mark
questions will be classified as extended response.
• Typically have a command word such as: evaluate, explain, calculate and compare.
• Are questions that may require linking in terms of knowledge, understanding and skills from more than
one area of the specification.
• Also, can be a multi-step calculation (two or more steps are completed in the correct order).
• Marked using ‘levels of response’ mark schemes (unless multi-step calculation).
Levels of response mark schemes provide a framework in which students are rewarded according to the
level of skill that they demonstrate.
The level is determined by:
• looking at the overall quality of the answer
• taking into account the content descriptor for each level
• using a ‘best-fit’ approach.
Once the level has been decided, the mark within the level is determined by the quality of the response
at that level.
48
Combined Science: Synergy - paper 4F, question 06.4
This question asks students to evaluate information given and come up with a reasoned conclusion.
They need to develop a reasoned line of thinking through their response to come up with a clear and
coherent conclusion that is consistent with their reasoning.
This question is marked using three levels, each of which is worth two marks. This combination
performs well in differentiating between students of differing abilities where a complex and varied answer
can be given.
Spec. ref.:
4.8.2.4
4.8.2.8
4.8.2.9
MS:
n/a
WS:
1.4
3.5
3.6
AO:
AO3/1b
Demand:
Low‒
standard
0
6
.
4
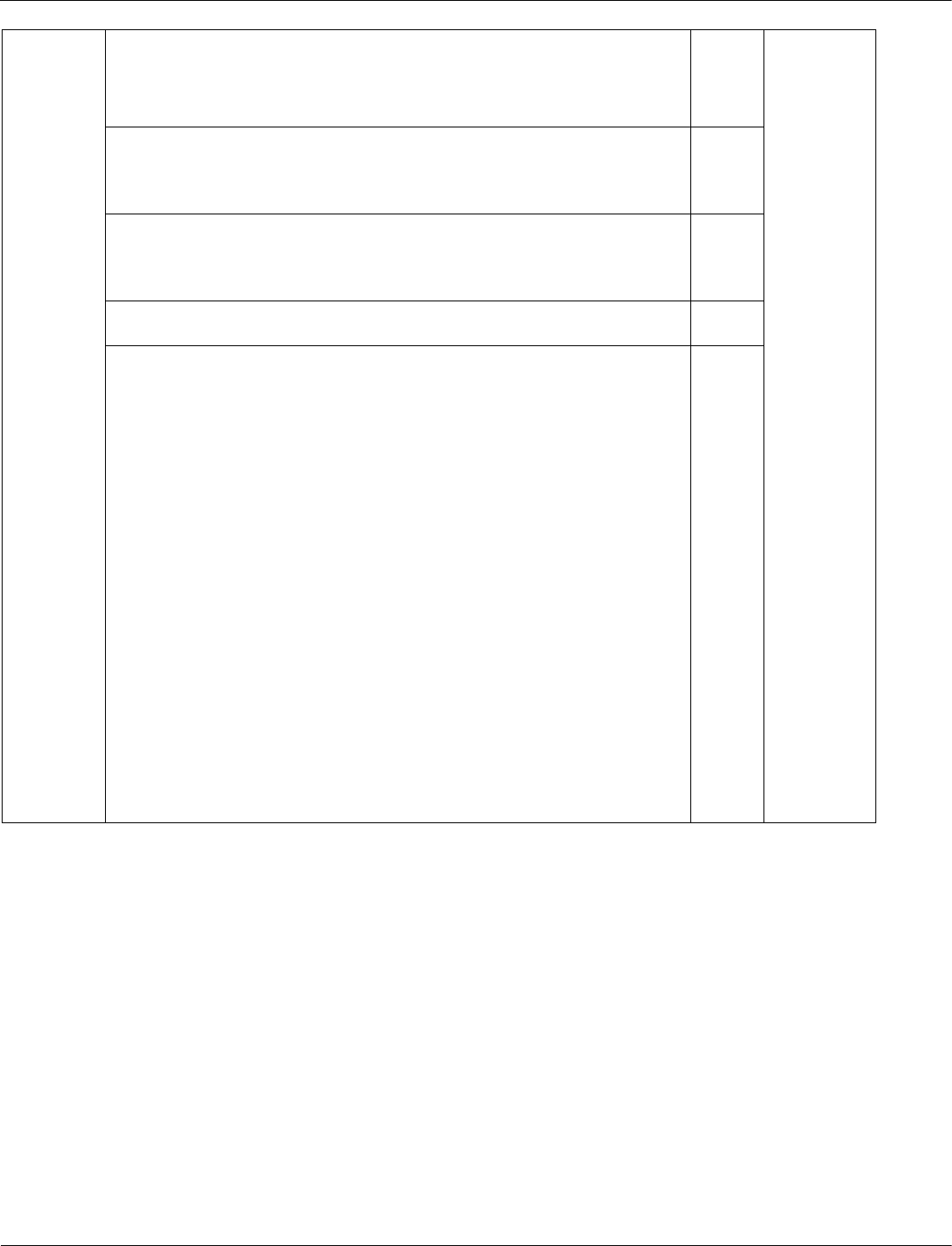
Poly(ethene) is a polymer made from ethene.
Poly(ethene) is used to make plastic bags.
Table 6 is from a life cycle assessment comparing paper bags and plastic bags.
Table 6
Paper bag Plastic bag
Raw material Wood (renewable) Oil or gas (non-renewable)
Energy used to make in MJ 1.7 1.5
Solid waste produced in g 50 14
Carbon dioxide produced in kg 0.23 0.53
Evaluate which type of bag is more environmentally friendly.
[6 marks]
Use data from Table 6 and your own knowledge to support your answer.
Our exams explained – GCSE science exams from summer 2018
49
06.4 Level 3: A detailed and coherent evaluation is provided that
considers a range of relevant points, quotes relevant data from the
table and comes to a conclusion consistent with the reasoning.
5–6 AO3/1b
4.8.2.4
4.8.2.8
4.8.2.9
4.8.2.4
Level 2: An attempt to describe relevant points which comes to a
conclusion. The logic and use of data may be inconsistent at times
but builds towards a coherent argument.
3–4
Level 1: Discrete, relevant points made. The logic may be unclear
and the conclusion, if present, may not be consistent with the
reasoning.
1–2
No relevant content. 0
Indicative content
conclusion as to which bag is more environmentally friendly
Points that may be used in argument:
• paper bags are made from a renewable resource (wood)
• paper bags more sustainable
• paper bags are biodegradable
• plastic bags are made from a finite resource (oil or gas)
• plastic bags not sustainable
• paper bags require more energy to manufacture (1.7 MJ
compared with 1.5 MJ)
• paper bags produce more waste (50 g compared with 14 g)
• paper bags create less CO
2
than plastic bags
• so manufacture of plastic bags has more effect on global
warming / climate change / environmental effects
• plastic bags can be recycled
• recycling reduces use of energy sources in manufacture
• justified
50
Biology - paper 2F/2H, question 12.3/03.3
Students are expected to know the process of selective breeding and apply it to the context of a specific
selectively bred cat for minimising allergic reactions.
The mark scheme for this question ensures that full marks can only be gained from applying the ideas to
the given example in a detailed and coherent way. A simple recount of selective breeding without
context will not gain full marks.
The information given is concise. It uses short sentences to ensure students can engage with the
information in order to achieve full marks.
Spec. ref.:
4.6.2.3
MS:
n/a
WS:
n/a
AO:
AO2/1
Demand:
Standard
1
2
.
3
Many people have breathing problems because they are allergic to cats.
The allergy is caused by a chemical called Fel D1.
Different cats produce different amounts of Fel D1.
A cat has been bred so that it does not produce Fel D1.
The cat does not cause an allergic reaction.
Explain how the cat has been produced using selective breeding.
[4 marks]
Our exams explained – GCSE science exams from summer 2018
51
12.3
Level 2: A detailed and coherent explanation is given, which
logically links the process of selective breeding with explanations of
how this produces cats that do not cause allergic reactions.
3–4
AO2/1
4.6.2.3
Level 1: Simple statements are made relating to process of
selective breeding, but no attempt to link to explanations.
1–2
No relevant content 0
Indicative content
process:
• parents with the desired characteristic are selected
• the parents are bred together to produce offspring
• offspring with the desired characteristics are selected and bred
• this is repeated over many generations.
explanations:
• parents who produce the least Fel D1 are initially selected
• in their offspring there will be individuals with differing amounts
of Fel D1 produced
• of these, in each generation, the lowest Fel D1 producing
individuals are chosen
• care is taken to ensure cats are healthy and avoid possible
problems associated with selective breeding
• over time the population of (selectively bred) cats will produce
less Fel D1
52
Physics ‒ paper 2H, question 11.3
This is a high demand extended response question where students need to link their knowledge of
waves and the electromagnetism. Students are required to provide a coherent explanation of how the
loudspeaker converts a current to sound waves.
Spec. ref.:
4.6.1.1
4.7.2.4
MS:
n/a
WS:
n/a
AO:
AO1/1
Demand:
High
1
1
.
3
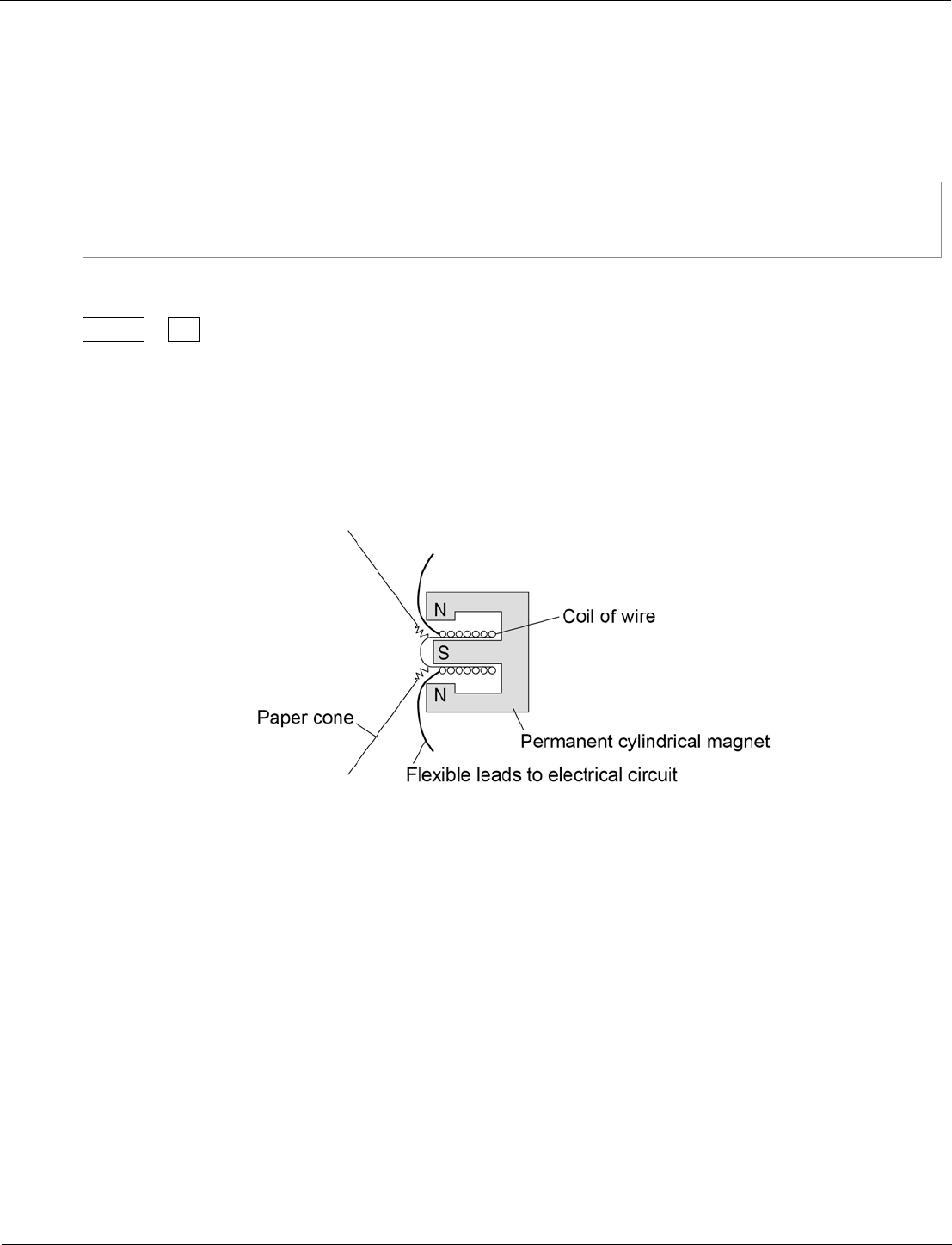
Figure 19 shows the parts of a moving-coil loudspeaker.
A coil of wire is positioned in the gap between the north and south poles of the
cylindrical magnet.
Figure 19
Explain how the loudspeaker converts current in an electrical circuit to a sound wave.
[6 marks]
Our exams explained – GCSE science exams from summer 2018
53
11.3
Level 3: A detailed explanation linking variations in current to the
pressure variations of a sound wave, with a logical sequence.
5–6
AO1/2
4.6.1.1
4.7.2.4
Level 2: A number of relevant points made, but not precisely. A
link between the loudspeaker and a sound wave is made.
3–4
Level 1: Some relevant points but fragmented with no logical
structure.
1–2
No relevant content 0
Indicative content
• the current in the electrical circuit is varying
• the current passes through the coil
• the coil experiences a force (inwards or outwards)
• reversing the current reverses the force
• the size of the current affects the size of the force
• the varying current causes the coil to vibrate
• the (vibrating) coil causes the cone to vibrate
• the vibrating cone causes the air molecules to move
• the movement of the air molecules produces the pressure
variations in the air needed for a sound wave
• the air molecules bunch together forming compressions and
spread apart forming rarefactions

54
Linking of ideas
Linking of ideas is defined by Ofqual as questions that allow students to:
‘…demonstrate their ability to draw together different areas of knowledge, skills
and/or understanding from across a full course of study for that qualification’.
Linking of key scientific ideas and skills are a fundamental part of learning of science.
Making links in science involves linking areas of subject content as well as the ‘Key ideas’ given in the
specification. For example:
• using more than one conceptual model or theory to make sense of an observation.
• linking aspects of the investigative process to specific concepts or models.
Such questions will enable students to demonstrate their ability to draw together different areas of
knowledge, skills and/or understanding from across the whole course of study.
In these questions students must make the links themselves. We cannot structure questions to give
prompts
†
eg for each area of the specification or idea.
Linking questions can range from 1‒6 marks.
• At the lower mark range: recognition of the links between knowledge and skills by bringing together
one area to explain another observation.
• At the higher mark range: explicit expression of these links is typically required in extended
response questions.
Again, these questions could also take the form of a multi-step calculation that requires students to use
equations from different parts of the specification.
Linking of scientific ideas can be demonstrated at different levels of demand.
†
indicates requirements set by the DfE and Ofqual.
Our exams explained – GCSE science exams from summer 2018
55
Combined Science: Trilogy ‒ biology paper 1H, question 08
Content from 4.1.3.3: Active transport; and 4.4.2.1: Aerobic and anaerobic respiration, needs to be linked
in this question. In this case, it requires some degree of lateral thinking to access full marks. Coupled
with the difficult concept of active transport and an unfamiliar context, this question is designed to stretch
the most able of students.
This is an ‘explain’ question, so all points in the mark scheme have to be made in order to gain
full marks.
• The first three marks are for recall about active transport.
• The last two are for application of this knowledge.
The wording is kept concise and direct to avoid any confusion about what we require students to do.
Spec. ref.:
4.1.3.3
4.4.2.1
MS:
n/a
WS:
3.6
AO:
AO1/1
AO2/1
Demand:
Standard/
high‒high
0
8
Plants need nitrate ions in order to make proteins.
A plant is growing in soil flooded with water.
Explain why the plant cannot absorb enough nitrate ions.
[5 marks]
08
(nitrate) ions are absorbed by
active transport
(active transport) is the
movement of ions against the
concentration gradient
(active transport) requires
energy from respiration
(respiration) requires oxygen
no / little oxygen / air in
water-logged soil
allow (active transport) is the
movement of ions from a dilute
to a more concentrated solution
1
1
1
1
1
AO1/1
4.1.3.3
AO1/1
4.1.3.3
AO1/1
4.1.3.3
AO2/1
4.4.2.1
AO2/1
4.4.2.1
56
Combined Science: Synergy ‒ paper 4H, question 10
This is a high demand ‘evaluate’ question. Students are required to interpret the information and
produce an objective and reasoned argument by applying their knowledge and understanding of:
• metal extraction
• use of energy resources
• the effects of these processes on the environment.
The question specifies the issues that students should consider in their answer.
The open response format allows students to link ideas and develop a coherent response,
demonstrating their scientific literacy. Also see that this is an extended response question.
Spec. ref.:
4.4.1.4
4.4.1.5
4.4.1.6
4.8.1.2
4.8.2.3
4.8.2.4
MS:
n/a
WS:
1.4
1.5
3.5
3.6
AO:
AO3/2b
Demand:
Standard/
high‒high
1
0
Read the information about production of copper.
• World demand for copper in 2014 was about 22 million tonnes.
• World reserves of copper are about 700 million tonnes.
• Most of the copper today is obtained from copper ores. The ores are mined.
• Copper ore is heated in a furnace to produce copper sulfide. The furnace is
heated by burning fossil fuels. Air is blown through the hot copper sulfide to
produce copper and sulfur dioxide.
• Some copper is extracted from low-grade ores by phytomining. Phytomining
uses plants to absorb copper compounds. The plants are burned and copper is
extracted from the ashes.
A scientist stated:
‘More copper should be extracted by phytomining.’
Use the information to justify the scientist’s statement.
[6 marks]

Our exams explained – GCSE science exams from summer 2018
57
10 Level 3: A detailed, coherent and logical justification of the
scientist’s statement, with relevant links made between statements
in the question, phytomining and the effects of other methods of
metal production on the environment.
5–6
AO3/2b
4.4.1.4
4.4.1.5
4.4.1.6
4.8.1.2
4.8.2.3
4.8.2.4
Level 2: An attempt to justify the scientist’s statement is made, with
some attempt at linking statements. The logic may be inconsistent
at times but builds towards a coherent argument.
3–4
Level 1: Discrete relevant points made. The logic may be unclear
and may not be consistent with the reasoning. Links are not made.
1–2
No relevant content 0
Indicative content
• phytomining conserves supplies of ores
• copper will be available for longer as at present rate of use
copper ores will run out in about 35 years
• phytomining conserves supplies of fossil fuels or energy
• less fuel used at a lower cost
• mining scars landscape or produces noise pollution
• mining destroys wildlife habitats
• with more phytomining less need to mine ores
• with phytomining less habitat destroyed or less scarring of
landscape
• with phytomining less need to use landfill for waste
• burning fossil fuels produces carbon dioxide / greenhouse gas
• burning fossil fuels causes global warming or climate change
• extraction from ores produces sulfur dioxide which causes acid
rain
58
Physics ‒ paper 2H, question 12.3
This conceptually challenging question requires students to use equations from different sections of the
specification. Students are also required to recall the unit of magnetic flux density.
Spec. ref.:
4.5.4
4.7.2.2
MS:
1b
3b
3c
WS:
n/a
AO:
AO1/1
AO2/1
Demand:
Standard/
high‒high
1
2
.
3
The wire X is 5 cm long and carries a current of 1.5 A.
The small weight causes a clockwise moment of 4.8 × 10
−4
Nm.
Calculate the magnetic flux density where the wire X is positioned
Give the unit.
[6 marks]
Magnetic flux density =
Unit
Our exams explained – GCSE science exams from summer 2018
59
12.3
4.8 × 10
−4
= F × 8 × 10
−2
F = 6 x 10
-3
(N)
6 × 10
−3
= B × 1.5 × 5 × 10
−2
B =
6 × 10
-3
7.5 × 10
-2
B = 8 × 10
−2
or 0.08
Tesla
allow 8 × 10
−2
or 0.08 with no
working shown for 5 marks
a correct method with correct
calculation using an incorrect
value of F gains 3 marks
accept T
do not accept t
1
1
1
1
1
1
AO2/1
4.7.2.2
4.5.4
AO2/1
4.7.2.2
4.5.4
AO2/1
4.7.2.2
4.5.4
AO2/1
4.7.2.2
4.5.4
AO2/1
4.7.2.2
4.5.4
AO1/1
4.7.2.2

60
Practical skills
Questions on practicals will feature across papers with at least two required practical activities per
subject featured in each series. The question types include all of those already contained within this
booklet. There will be a minimum of 15% of marks
†
allocated to questions on practicals.
The specifications list the apparatus and techniques
†
that the required practicals are designed to cover.
Questions will reflect this list and include working scientifically sections 2 and 3.
Some questions will be on theoretical aspects of experimentation. For non-required practical contexts,
all relevant information required to answer the questions will be given.
Questions based on practicals are very varied.
• Questions can be set in a practical context, but the question centres on the science, not the
practical work.
• Some questions require specific aspects of a practical procedure to be understood in order to answer
a question about the underlying science.
• Other questions require a thorough knowledge of the required practicals in order to answer the
questions. This could be for planning or improving a method.
The following pages give examples of types of practical questions we use. They demonstrate the skills
required through various stages of an investigation.
More detail, guidance, and specific examples on practicals is given at aqa.org.uk/gcse-science
†
indicates requirements set by the DfE and Ofqual.

Our exams explained – GCSE science exams from summer 2018
61
Constructing hypotheses or finding evidence for a hypothesis
Physics ‒ paper 2F, question 02.4
Students need to interpret and evaluate data in terms of a given hypothesis. Interpreting data is a skill
that students will have used in their practical experience. Students will also have experience of writing a
hypothesis then testing to establish if the hypothesis can be supported.
This question is worth one mark. Students should be able to evaluate the data to realise that the pattern
does not support the hypothesis. The pattern and hypothesis are not complex and are easy to interpret
and relate to each other.
Spec. ref.:
4.7.1
MS:
1d
2h
WS:
3.6
AO:
AO3/1b
Demand:
Low
0
2
.
4
Before starting the investigation the student wrote the following hypothesis:
‘The bigger the area of a fridge magnet the stronger the magnet will be.’
The area of each magnet is given in Table 1.
Table 1
Fridge
magnet
Area of
magnet
in mm
2
Number of
sheets of
paper held
A
40 20
B
110 16
C
250 6
D
340 8
E
1350 4
Give one reason why the evidence from the investigation does not support the
student’s hypothesis.
[1 mark]
02.4
because the magnet with the
biggest area was not the
strongest
accept any correct reason that
confirms the hypothesis is
wrong eg smallest magnet holds
more sheets than the largest
1
AO3/1b
4.7.1
62
Plan a method
Combined Science: Trilogy ‒ chemistry paper 1H, question 06.3
This question is based on recall of one of the required practical activities. By asking questions about the
required practical activities, the need to undertake all of the activities is underlined.
This question allows students to demonstrate their practical knowledge in the form of an
extended response.
Spec. ref.:
5.4.2.1
5.4.1.2
5.5.1.1
MS:
n/a
WS:
2.2
2.3
AO:
AO1/2
Demand:
Standard‒
high
Figure 5 shows the chemicals given to a student.
Figure 5
0
6
.
3
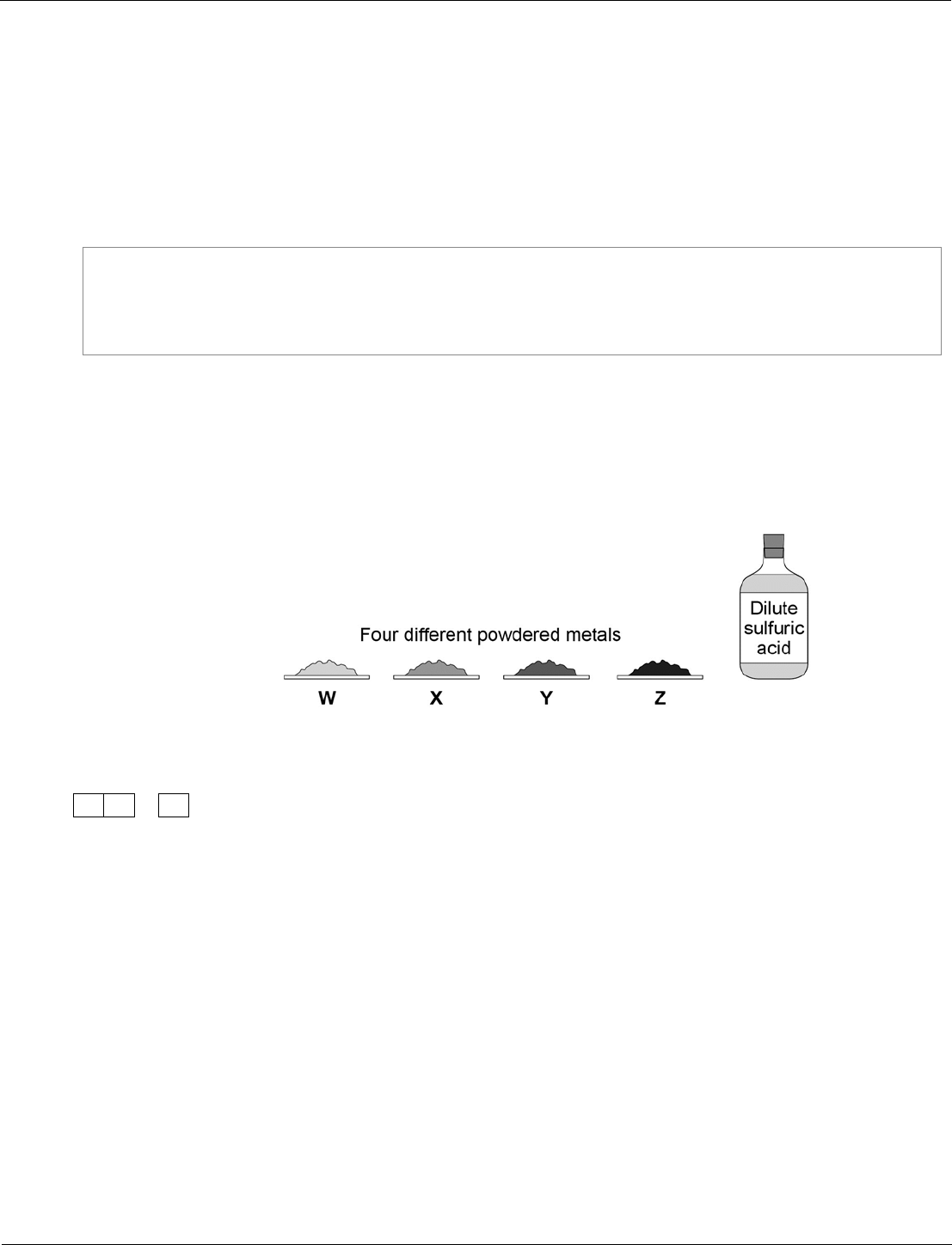
The student wants to investigate the reactivity of the four metals.
Outline a plan the student could use to investigate the relative reactivity of the four
metals, W, X, Y and Z.
[6 marks]
The plan should use the fact that all four metals react exothermically with dilute
sulfuric acid.
You should name the apparatus used and comment on the safe use of the chemicals.
Our exams explained – GCSE science exams from summer 2018
63
06.3
Level 3: A coherent method is described with relevant detail, which
demonstrates a broad understanding of the relevant scientific
techniques and procedures. The steps in the method are logically
ordered with the dependent and control variables correctly
identified. The method would lead to the production of valid results.
5–6
AO1/2
5.4.1.2
5.4.2.1
5.5.1.1
Level 2: The bulk of a method is described with mostly relevant
detail, which demonstrates a reasonable understanding of the
relevant scientific techniques and procedures. The method may
not be in a completely logical sequence and may be missing some
detail.
3–4
Level 1: Simple statements are made which demonstrate some
understanding of some of the relevant scientific techniques and
procedures. The response may lack a logical structure and would
not lead to the production of valid results.
1–2
No relevant content
0
Indicative content
Named apparatus
• thermometer
• measuring cylinder
• stirring rod
• spatula
• plastic cup (with lid) or beaker
• stopwatch
• filter paper or watch glass
• balance
Method
• weigh the same mass of each metal in each same state of
division eg powder
• measure a set volume of sulfuric acid into a plastic cup or
beaker
• measure and record the temperature of the sulfuric acid
• add metal W into the plastic cup or beaker
• stir and record the highest temperature or record the
temperature after a set time
• calculate the increase in temperature
• repeat the method for metals X, Y and Z
• repeat for each metal at least three times to calculate a mean
Safe use
• comment on safe use should include wearing safety glasses
64
Safety considerations
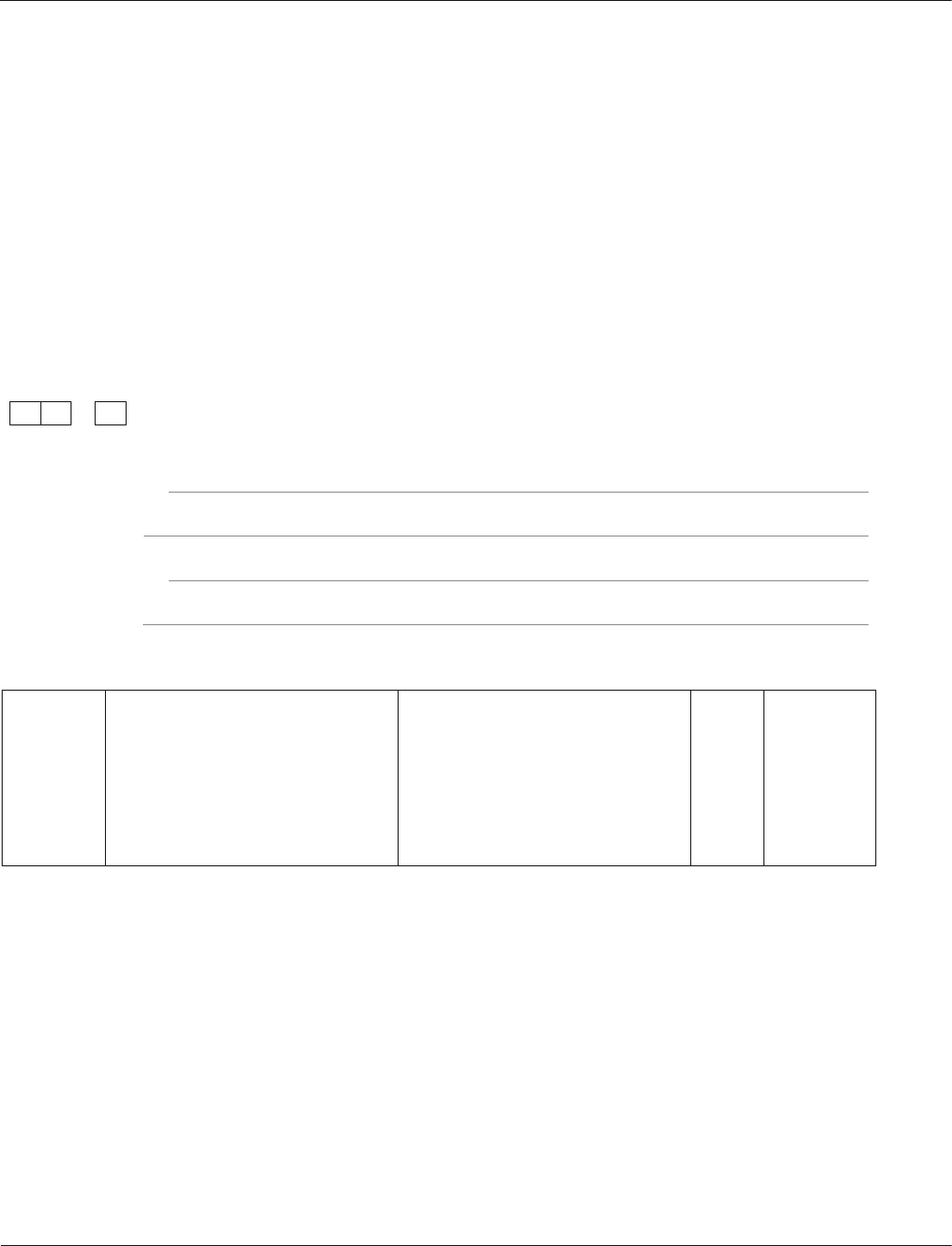
Combined Science: Trilogy physics ‒ paper 1F, question 04.3
Students need to be able to recall possible safety precautions from the required practical activities they
will have done during their study of the course.
This question also illustrates how we present detailed methods in question papers.
We use:
• direct wording to introduce the method: ‘This is the method used’
• a numbered list to clearly show a sequence of events.
Spec. ref.:
6.1.1.4
MS:
n/a
WS:
2.4
AO:
AO1/2
Demand:
Low
0
4
A student investigated the change in temperature when oils of different specific heat
capacities were heated.
She set up the apparatus shown in Figure 5.
Figure 5
Our exams explained – GCSE science exams from summer 2018
65
This is the method used.
1. Put 25 g of oil into a boiling tube.
2. Pour 100 ml of water into a beaker and heat it with a Bunsen burner.
3. When the water is boiling, put the boiling tube into the beaker.
4. When the temperature of the oil reaches 30 °C, heat for a further 30 seconds and
record the rise in temperature.
5. Repeat with different oils.
6. Repeat the whole investigation.
0
4
.
3
Give two safety precautions the student should have taken.
[2 marks]
1
2
04.3
wear safety goggles
oil not heated directly
accept any reasonable comment
about not handling hot
apparatus
1
1
AO1/2
6.1.1.4
AO1/2
6.1.1.4
66
Understanding variables
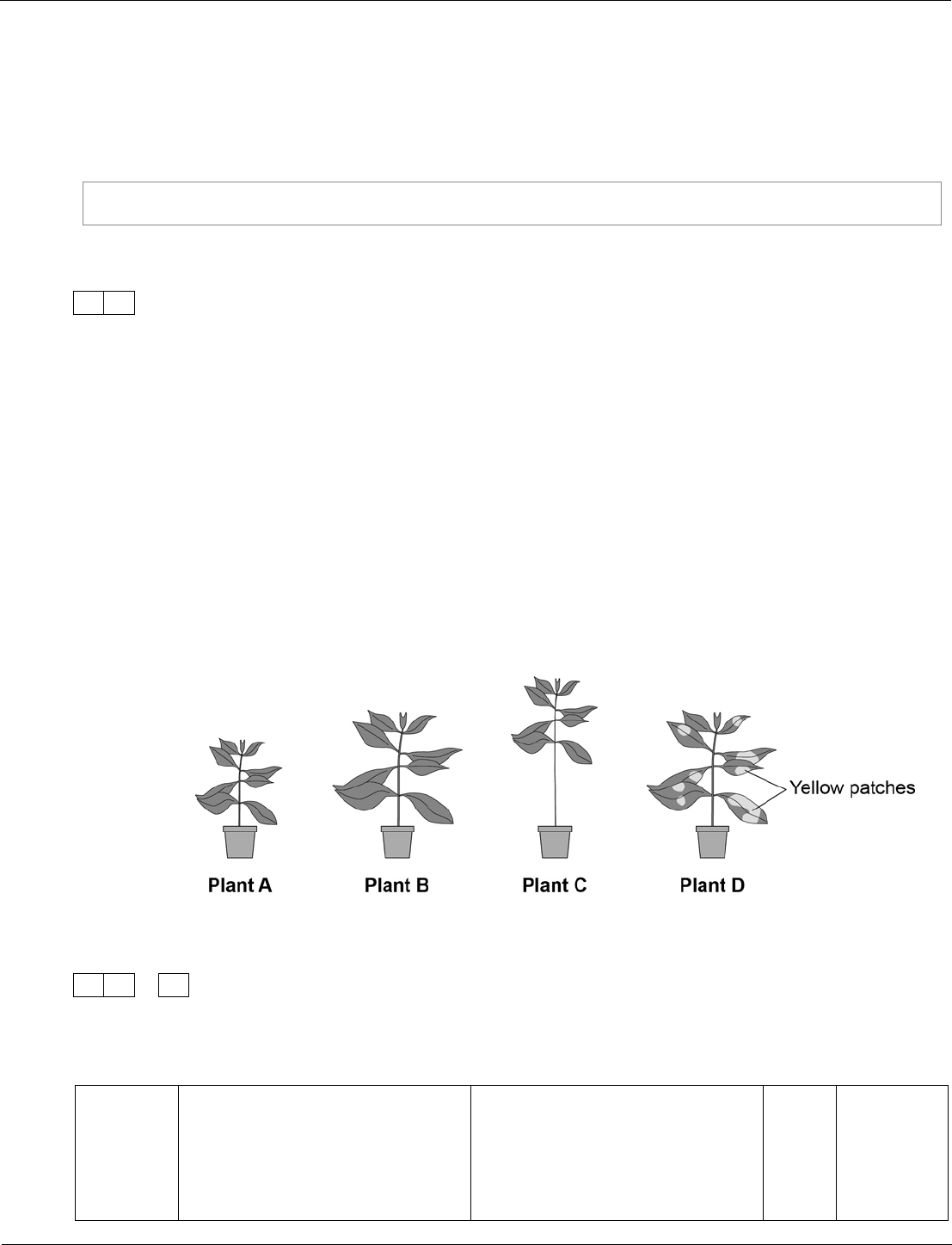
Biology ‒ paper 1F, question 08.3
In this question students need to understand a method and identify a required variable. In this case it’s
on an unfamiliar practical. However, questions on variables can also be asked on required practicals.
Spec. ref.:
4.3.3.1
MS:
n/a
WS:
2.2
AO:
AO2/2
Demand:
Standard
0
8
To be healthy, plants need the right amount of mineral ions from the soil.
Figure 12 shows four plants.
The plants were grown in four different growing conditions:
• sunny area, with nitrate and magnesium added to the soil
• sunny area, with magnesium but no nitrate added to the soil
• sunny area, with nitrate but no magnesium added to the soil
• dark area, with nitrate and magnesium added to the soil.
Figure 12
0
8
.
3
Give one variable that was kept constant in this experiment.
[1 mark]
08.3
use the same type of plant
or
give equal amount of water to
each plant
ignore size of pot
1
AO2/2
4.3.3.1
Our exams explained – GCSE science exams from summer 2018
67
Reading scales
Combined Science: Synergy ‒ paper 3F, question 01.2
Students need to take a reading from a newtonmeter. The use of a newtonmeter to measure weight is
specified in the specification, so students should be familiar with this apparatus.
In these types of question the diagram has a scale that is clear and easy to read. The diagram could be
enlarged or given in a tactile format for visually impaired students. This mirrors the standard methods
used for making reasonable adjustments in question papers.
Spec. ref.:
4.6.1.4
MS:
n/a
WS:
2.6
AO:
AO2/2
Demand:
Low
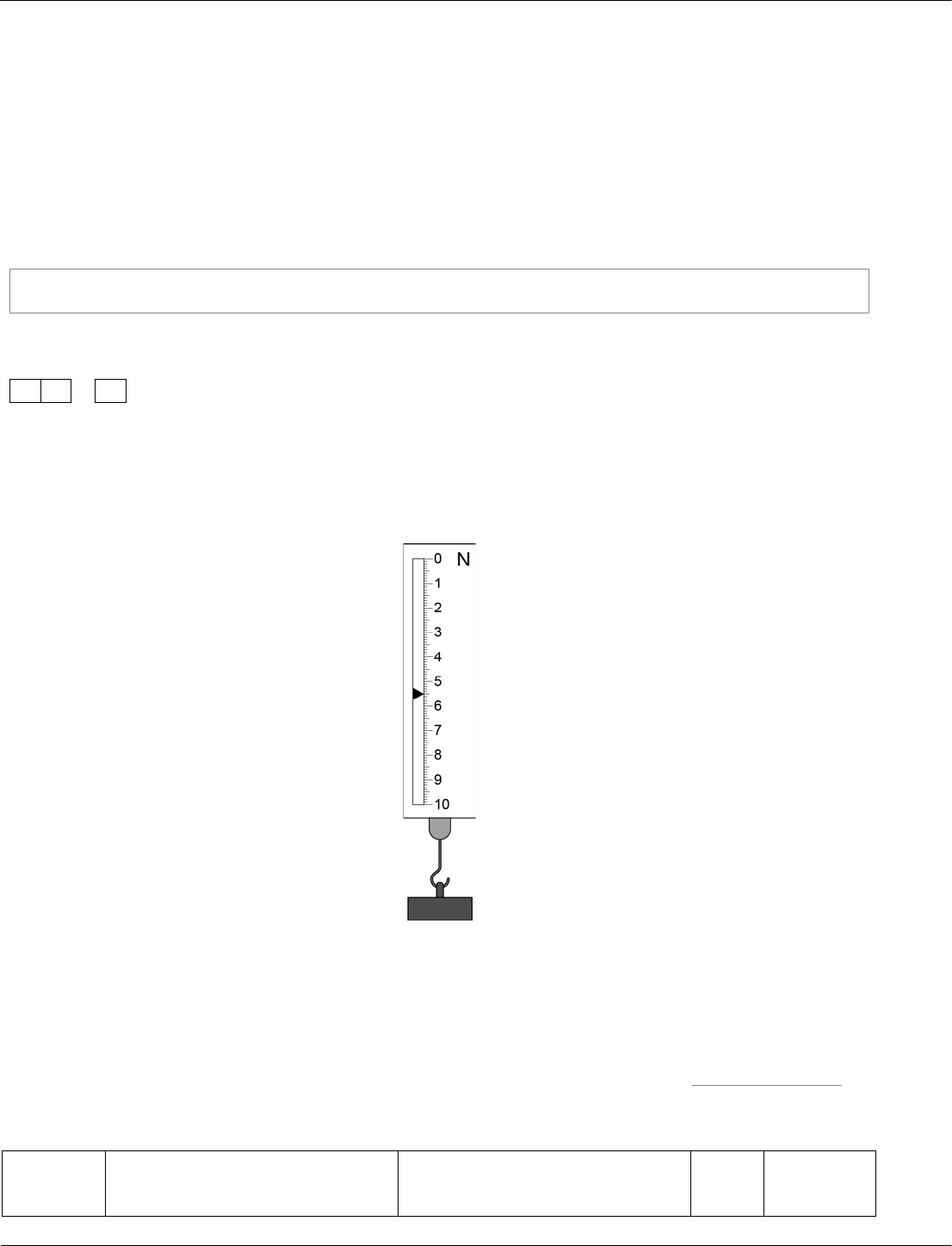
0
1
.
2
A newtonmeter measures the weight of an object.
Look at Figure 1.
Figure 1
What is the weight of the object in Figure 1?
[1 mark]
Weight =
N
01.2
5.5 1 AO2/2
4.6.1.4
68
Identifying errors
Combined Science: Synergy ‒ paper 2F/2H, question 09.5/02.5
Students need to identify common mistakes in a practical set up. They then need to explain what effect
the mistakes would have on the data obtained. The knowledge gained by doing this as one of the
required practical activities is underlined in this question.
Spec. ref.:
RPA9
MS:
n/a
WS:
2.7
AO:
AO3/3a
Demand:
Standard
0
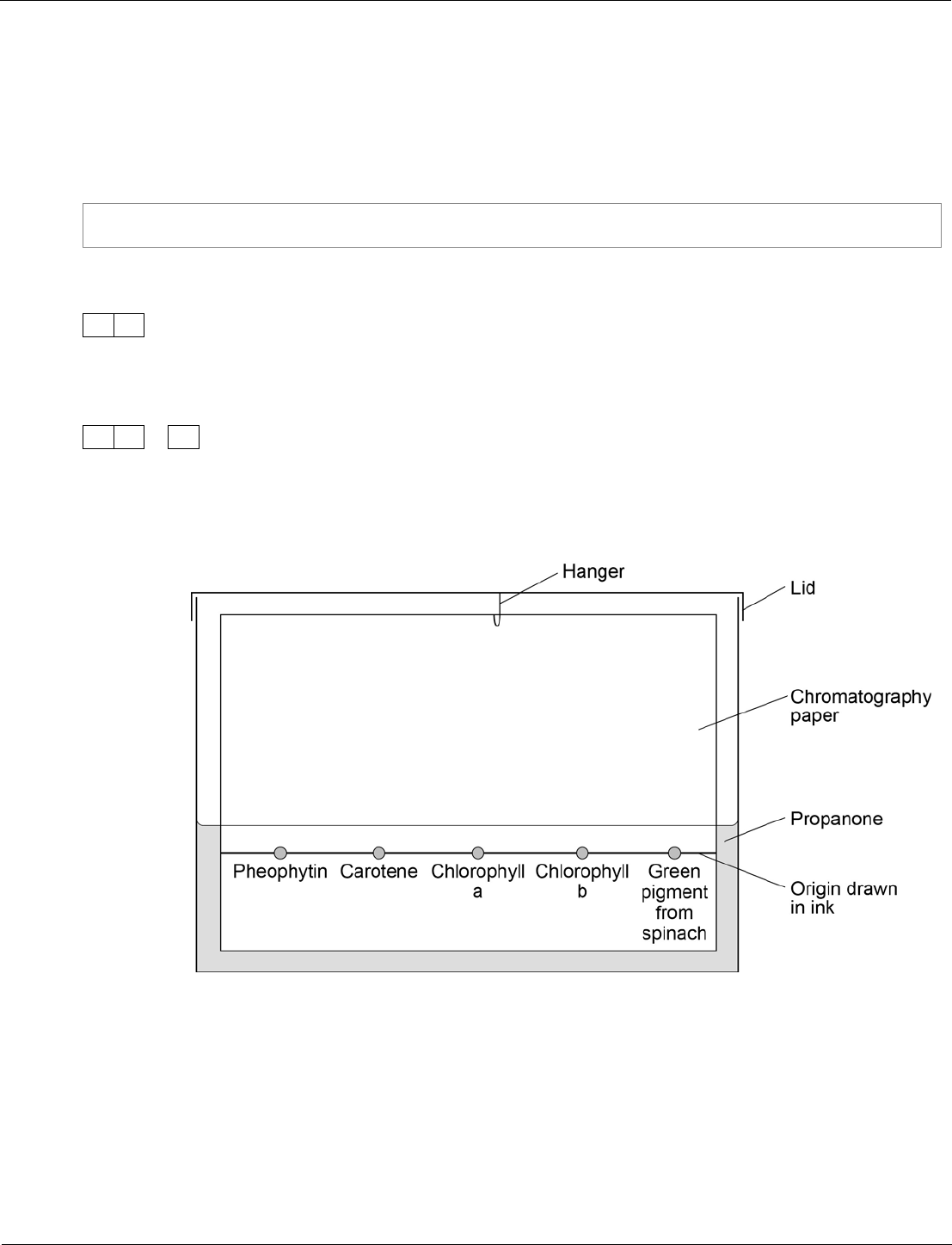
9
A student used paper chromatography to identify the pigments in spinach leaves.
She used propanone as a solvent.
0
9
.
5
Another student set up the apparatus shown in Figure 13.
Figure 13
This student did not set up the apparatus correctly.
Identify the errors the student made.
Explain how the errors she made would affect her results.
[4 marks]
Our exams explained – GCSE science exams from summer 2018
69
09.5
origin line drawn in ink
so it will run
or
dissolve in the solvent
or
split up
spots under solvent
or
solvent above spots / origin line
so they will mix with solvent
or
wash off paper
or
colour the solvent
or
dissolve in the solvent
1
1
1
1
AO3/3a
RPA9
AO3/3a
RPA9
AO3/3a
RPA9
AO3/3a
RPA9
70
Data analysis
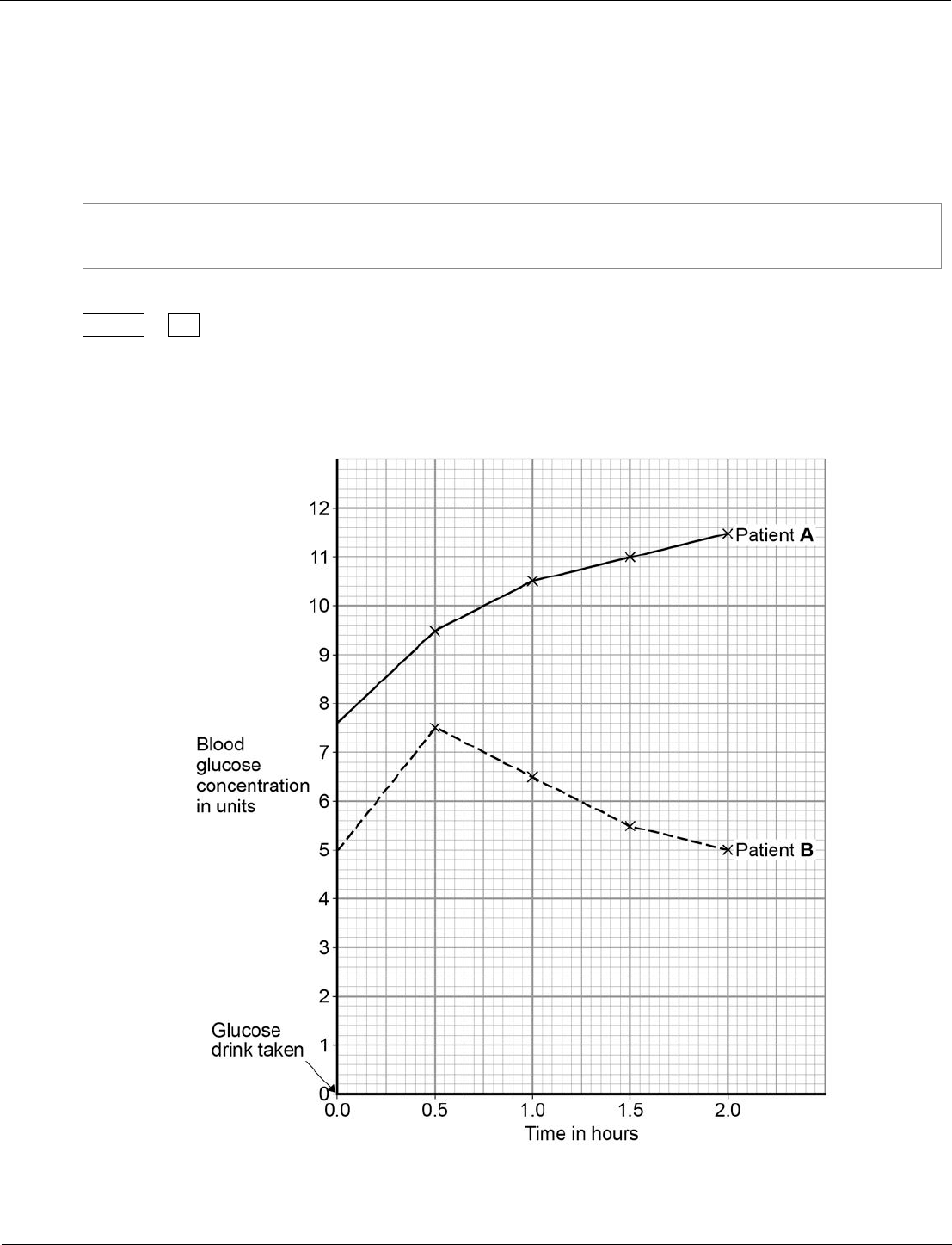
Biology ‒ paper 2H, question 09.6
Students are required to analyse the graphical data by applying their knowledge of blood glucose
concentration to identify which patient has diabetes. Also see how the question and answer space is
scaffolded to support their answer.
Spec. ref.:
4.5.3.2
MS:
n/a
WS:
n/a
AO:
AO3/2a
Demand:
Standard/
high
0
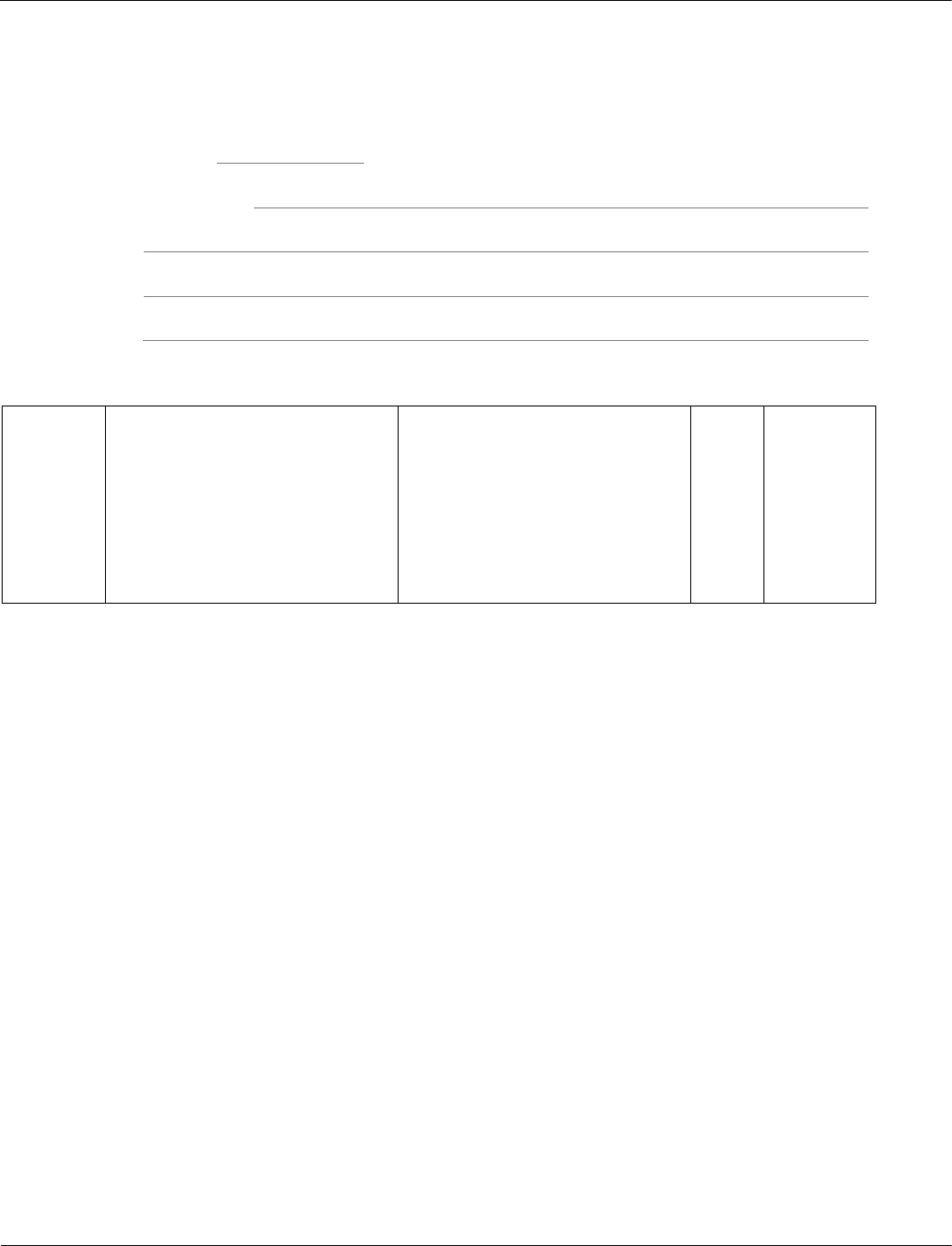
9
.
6
Figure 11 shows the results of a glucose tolerance test for two patients, A and B.
Figure 11
Our exams explained – GCSE science exams from summer 2018
71
Which patient has diabetes?
Justify your answer.
[2 marks]
Patient
Justification
09.6
(patient A)
glucose level much higher
(than B)
and remains high / does not fall
no mark for identifying A
1
1
AO3/2a
4.5.3.2
AO3/2a
4.5.3.2
72
Improvements
Combined Science: Trilogy ‒ physics paper 1F, question 04.4
Students need to analyse the method and equipment presented. They then need to use this analysis
and their practical experience to suggest an improvement.
Spec. ref.:
6.1.1.4
MS:
n/a
WS:
2.7
AO:
AO3/3b
Demand:
Low–
standard
(The stem for this question can be found on pages 64 & 65.)
0
4
.
4
Suggest one improvement to the student’s method.
[2 marks]
04.4
repeat the experiment
and calculate the mean
temperature rise
or
heat the oil for a longer period of
time (1)
to get a wider range of
temperatures (1)
1
1
AO3/3b
6.1.1.4
AO3/3b
6.1.1.4

Our exams explained – GCSE science exams from summer 2018
73

74
Notes
Copyright © 2016 AQA and its licensors. All rights reserved.
AQA Education (AQA) is a registered charity (registered charity number 1073334) and a company limited by guarantee registered in
England and Wales (company number 3644723). Registered address: AQA, Devas Street, Manchester M15 6EX.
September 2016
Visit our website to see our specications, question
papers, mark schemes and resources:
aqa.org.uk/gcse-science
You can talk directly to our science team
Telephone: 01483 477 756
Email: [email protected]g.uk
Our suite of seven KS4 science qualications gives you
a real choice for all abilities. Find out more at
aqa.org.uk/ks4-changes
The information in this document is based on the specimen question papers and mark schemes accredited by Ofqual in April 2016.
Find out more
Science for all
Want to keep up-to-date with the
latest news about our specications,
exams, resources and training?
Register at aqa.org.uk/ks4-updates
