City
of
New
York
Administration for Children's Services
Policy and Procedure
2014/08
Medical
Consents
for
Children in Foster Care
ApprovedB~
arri6n,
Esq.
Social Services Law § 383-b;
Domestic Relations Law §
111(1);
Mental
Hygiene Law
Articles 31 and 80;
Mental
Hygiene Law § 33.21;
Education
Law§
6902(3)
Public Health Law Article 28;
Public Health
Law§§
2305(2),
2504(1)(3)(4), 4210, 4301;
General Obligations Law § 5-
15511; Family Court
Act§
355.4(2)
Supporting Regulations:
18
NYCRR
§§
441.17,
441.22(d); 428.3(b)(2)(ii);
14
NYCRR
§ 633.11
Date
Issued:
ACS
Divisions/Provider Agencies:
Office
of
the
Commissioner; Office
of
the
General Counsel; Divisions
of
Child Protection, Family Support
Services, Family Permanency
Services, Youth and Family Justice,
Family Court
Legal
Services, and
Policy, Planning and
Measurement; and
foster
care and
non-secure juvenile justice
placement provider agencies
Related Policies:
• Procedure 102A, Medical
Consents and Medical Referrals
in Suspected Child
Sexual/Physical Abuse
Cases
• Policy #2010/03, Guidelines
for
the
Provision
of
Emergency and
Inpatient Mental Health
Services
for
Children in
the
Foster
Care
and Child
Protective System
• Policy and Procedure
#2014/07,Consentto
Withhold
or
Withdraw
Life-
Sustaining
Treatment
• Confidentiality Policy
Memo,
dated February 20, 2004
• Sexual and Reproductive
Health
Care
for
Youth in Foster
Care
Number
of
Pages:
25
Number
of
Attachments:
3
Contact Office
/Unit:
Beatrice Aladin
Director
Health Policy and Planning
Office
of
Child & Family Health
Bulletins & Directives:
• 08-0CFS-INF-02 The
Use
of
Psychiatric Medications
for
Children and Youth in
Placement;
Authority
to
Consent
to
Medical Care
• 90-ADM-21 Foster Care:
Medical Services
for
Children
in Foster Care.
• 08-0CFS ADM-01 Changes
Associated
with
CONNECTIONS
(CNNX)
Build
18.9: Health, Education and
Permanency Hearing Report
Modules.
2
Supporting Case Law:
Carey v. Population Services
International, 431 U.S. 678
(1977)
Supporting Standards:
ACS Foster Care Quality Assurance
Standards, 2012
Supersedes:
This policy replaces Procedure
102/Bulletin 99-1 (Amended),
Guidelines for Providing Medical
Consents for Children in Foster
Care, dated October 18, 1999.
Related Forms:
FSS 007 - Medical Authorization For Routine Treatment or Emergency Care (Attachment A)
FSS 001 - Psychotropic Medications Unit (PMU) Override Consent Request (Attachment B)
FSS 010 - Consent Forms Psychotropic Medication (Attachment C)
Summary:
When children in the custody of the Administration for Children’s Services need medical treatment,
ACS and provider agency staff must act expeditiously to obtain consent for such treatment from the
appropriate individual(s). This policy sets forth conditions under which parents, provider agencies,
ACS, or youth may provide consent for medical, dental, and hospital services; and identifies special
situations or exceptions in which a provider agency may not provide consent.
Key Words:
medical consent, medical, consent, foster care, foster, care, authorization, capacity, affirmative
objection, informed consent

POLICY HIGHLIGHTS
In most circumstances, medical consent must first be sought from a child’s parent or
guardian, as long as parental rights have not been terminated or surrendered.
Parents whose rights have been terminated or surrendered must not be contacted for
consent and have no legal authority to give consent. In such cases, ACS acts as the parent
and can authorize all health care services for children in foster care.
The following youth in foster care may consent for their own medical, dental, and health
services:
Youth who are 18 years of age or older;
Youth who are parents, regardless of their age; or
Youth who are married.
Youth may consent for their own sexual and reproductive health care services regardless
of their age.
If a parent or guardian refuses to give consent, cannot be located, or cannot be contacted
after reasonable efforts, ACS has the authority to provide medical consent in some
instances and may delegate this authority to the foster care agency. This delegation of
authority does not extend to circumstances where the consent authority is solely the ACS
Commissioner’s to make.
Treatment may be provided without consent when, in a physician’s judgment: 1) an
emergency exists and the child is in immediate need of medical attention; and 2) an
attempt to secure consent would result in a delay of treatment which would increase the
risk to the child’s life or health.
Provider agencies must establish policies and procedures to guide their medical consent
process and submit them to the ACS Medical Consent Unit every two years.
Exceptions to regular consent procedures apply to the following children and youth:
Children or youth who are paroled or are on trial discharge;
Children or youth whose parents’ capacity to consent is questioned;
Parenting youth; and
Children or youth whose capacity to consent is questioned.
3
Table of Contents
I. INTRODUCTION ................................................................................................................................... 4
II. GENERAL POLICY GUIDELINES .............................................................................................................. 4
A. Youth in Foster Care Who May Consent for Themselves ....................................................................... 4
B. ACS’ Authority to Consent ....................................................................................................................... 5
C. Delegation of Authority to the Foster Care Provider Agencies ............................................................... 5
D. Treatment Without Consent ................................................................................................................... 5
E. Parents Whose Rights Have Been Terminated or Surrendered .............................................................. 6
F. When Neither ACS Nor the Provider Agency is Authorized to Consent ................................................. 6
G. Provider Staff Who May Consent on ACS’ Behalf ................................................................................... 6
H. Division of Child Protection Cases Involving Sexual/Physical Abuse ...................................................... 7
I. 24-Hour Access to Someone with Authority to Provide Consent ........................................................... 7
J. Medical Authorization for Routine Treatment or Emergency Care Form .............................................. 7
K. Parental Pre-Authorization for Routine and Emergency Care ................................................................ 7
L. Principles and Guidelines for Obtaining Non-Routine Treatment Consents .......................................... 8
M. Sharing Medication Information for Children in Child Welfare Placements ........................................ 10
N. Psychiatric Hospitalization Requirements............................................................................................. 10
O. Treatment of Non-Routine Acute Illnesses ........................................................................................... 10
P. Treatment of Chronic Illnesses ............................................................................................................. 11
Q. Parental Objection to Specific Treatments ........................................................................................... 11
R. Psychiatric Treatment and Psychotropic Medication ........................................................................... 12
S. Continuity of Active Psychotropic Medication Treatment During Transitions ..................................... 14
T. Psychotropic Treatment Requirements and Waivers of Requirements ............................................... 15
U. Interviews to Determine Youth Eligibility for Programs ....................................................................... 17
V. Quality Assurance Requirements .......................................................................................................... 17
III. GUIDELINES BY TYPE OF ENTRY INTO FOSTER CARE ............................................................................ 18
A. Children Remanded or Placed in Foster Care for Child Protective Reasons ....................................... 18
B. Youth Placed with ACS in Juvenile Justice Placements Pursuant to Article 3 ..................................... 20
C. Youth Placed Through Voluntary Placement Agreements .................................................................. 20
D. Youth Placed with ACS on PINS Petitions Pursuant to Article 7 .......................................................... 21
IV. EXCEPTIONS TO AGENCY OR ACS CONSENT ........................................................................................ 22
A. Subject Children Under Family Court Act Article 10 Cases Who are Not Physically in the Custody of
ACS ....................................................................................................................................................... 22
B. Children for Whom Parental Capacity to Consent is Questioned ....................................................... 22
C Parenting Youth Cases ......................................................................................................................... 23
D. Youth Whose Capacity to Consent is Questioned ............................................................................... 23
E. Children Who are Placed With an Agency Under the Auspices of a Governmental Agency Other
Than ACS .............................................................................................................................................. 24
F. Children Who are Placed as Destitute Children .................................................................................. 24
G. Children Who Are in Foster Care but Whose Legal Status Needs Confirmation ................................. 24
H. Consent for Sexual and Reproductive Health Services........................................................................ 24
I. Testing for HIV ..................................................................................................................................... 25
J. Consent for Autopsy or Organ Donation (Anatomical Gift) ............................................................... 25
K. Religious Objections to Treatment or Immunizations ......................................................................... 25
L. Do Not Resuscitate (DNR) or Withhold or Withdraw Life-Sustaining Treatment Orders ................... 25
V. ATTACHMENTS
A. FSS 007 Medical Authorization for Routine Treatment or Emergent Care (English and Spanish)
B. FSS 001 Psychotropic Medications Unit (PMU) Override Consent Request
C. FSS 010 Consent for Psychotropic Medication

4
I. INTRODUCTION
A. In support of timely delivery of medical, dental, mental health, and hospital services for
children in the care and custody or custody and guardianship of the Commissioner of the
Administration for Children’s Services (ACS), ACS is revising its policy for the provision of
consent for medical treatment. The services for which consent may be necessary
include, but are not limited to, routine or emergency surgery, hospitalization,
medication, referrals to the Home and Community-Based Services Waiver Program
“Bridges to Health” (B2H), admissions to New York State Office for People with
Developmental Disabilities (OPWDD) programs, or admissions to New York State Office
of Mental Health (OMH) programs.
B. ACS’ Standard for Culturally Respectful Practice
ACS is committed to working with children, youth, and families in a manner that is
respectful of all cultural backgrounds. Accordingly, ACS and provider agency staff must
be sensitive to the beliefs and values of clients when discussing or providing information
about medical consent. Staff should never allow their own cultural values to interfere
with their responsibility to provide unbiased information and quality services.
II. GENERAL POLICY GUIDELINES
ACS’ policy is that, except in certain circumstances described in this document, medical
consent must always be sought first from a child’s parents/legal guardians, if parental rights
have not been terminated or surrendered.
1
A. Youth in Foster Care Who May Consent for Themselves
2
The following youth in foster care may provide consent
3
for their own medical, dental,
health, and hospital services
4
:
1. Regardless of age, youth may consent for their own sexual and reproductive health
care services (see section IV(H) below about consent for sexual and reproductive
health care services);
2. Youth who are 18 years of age or older;
3. Youth who are parents, regardless of their age; or
4. Youth who are married.
1
Parental consent includes consent to using the particular specified medical provider (e.g., hospital or doctor) for the
proposed treatment.
2
See also section IV(H) below about consent for sexual and reproductive health care services.
3
See Section IV(D) below regarding youth whose capacity to consent is questioned.
4
See Public Health Law § 2504(1).
5
In these circumstances, there may not be an override of the youths’ declining of
medical treatment.
5
B. ACS’ Authority to Consent
If the parents/guardians refuse to consent, cannot be located, or cannot be contacted
after reasonable efforts to locate them, contact them, and obtain consent, ACS has the
authority to provide medical consent in some cases. Such authority applies in the
following situations:
1. When a child has been placed in the custody of ACS under Family Court Act Article
10
6
;
2. When a child has been taken into or kept in protective custody with ACS or has
been removed by ACS from the place where he or she has been residing for child
protective reasons
7
;
3. When a child has been placed at disposition in the custody of ACS under Family
Court Act Article 3 and requires routine medical treatment
8
; and
4. When a child has been placed voluntarily in foster care
9
and requires routine
medical treatment except if otherwise specified in the voluntary placement
agreement.
C. Delegation of Authority to the Foster Care Provider Agencies
In cases where ACS has the legal authority to consent, as described and outlined in this
policy, ACS delegates this authority to consent to the Executive Directors (or their
designees) of the authorized foster care agencies responsible for the care and planning
of the foster child. This delegation of authority does not extend to circumstances
where the consent authority is solely the ACS Commissioner’s to make. See sections
II(G) and V(J) for more details.
D. Treatment Without Consent
Medical, dental, health, and hospital services may be rendered to children placed with
ACS without the consent of a parent/guardian when, in the physician’s judgment: 1) an
emergency exists and the child is in immediate need of medical attention; and 2) an
attempt to secure consent would result in a delay of treatment which would increase
5
Except in circumstances covered under section II(D) of this policy. See Public Health Law §2504 (4).
6
See Social Services Law § 383-b.
7
Ibid.
8
See Family Court Act § 355.4(2).
9
In all cases, a voluntary placement agreement must be reviewed to confirm that its contents do not conflict with this
policy. If a conflict exists, the voluntary placement agreement supersedes this policy.

6
the risk
10
to the child’s life or health.
11
In such cases the physician may render
treatment without seeking consent.
E. Parents Whose Rights Have Been Terminated or Surrendered
Parents whose rights have been terminated or surrendered must not be contacted for
consent and have no legal authority to give consent. When parents’ rights have been
terminated or surrendered, ACS acts as the parent and can authorize all health care
services for children in foster care.
F. When Neither ACS Nor the Provider Agency is Authorized to Consent
There are certain situations in which neither ACS nor provider agencies
12
are authorized
to consent to medical care and are required to seek a court order to obtain medical
treatment or services. These exceptions are set forth in Section IV, titled “Exceptions to
Agency or ACS Consent” below.
G. Provider Staff Who May Consent on ACS’ Behalf
Provider agency Executive Directors or their designees are able to provide direct
medical consent for children in their care and custody on behalf of ACS in the
circumstances described in this policy. The designee shall be a senior staff member,
director of social services, director of family foster care, or director of residential care.
For children who are in the care and custody of ACS where the Division of Child
Protection (DCP) is responsible for case management (e.g., children who are in the
hospital awaiting placement), the Deputy Director of the Borough Office in which the
case is assigned may provide consent. For children who are in the care and custody of
ACS where the Division of Family Permanency Services (FPS) is responsible for case
management (e.g., youth in special school placements), the Deputy Commissioner for
FPS or his or her designee may provide consent. Similarly, for youth in non-secure and
limited secure juvenile justice placement, the Deputy Commissioner for the Division of
Youth and Family Justice (DYFJ) or his or her designee may provide consent. Staff who
are not in these categories do not have the authority to provide medical consent for
children in foster care.
1. Authority to consent is contingent upon the child being in the legal custody of
ACS when medical services are required.
10
Risk is determined by the attending physician.
11
See Public Health Law § 2504(4).
12
For the purposes of this policy, “provider agencies” include foster care agencies and DYFJ/OYFD non-secure juvenile
justice placement provider agencies.

7
H. Division of Child Protection Cases Involving Sexual/Physical Abuse
For DCP cases involving physical or sexual abuse see Procedure 102A, Medical Consents
and Medical Referrals in Suspected Child Sexual/Physical Abuse Cases (May, 2002),
which addresses the investigative stage in such cases.
I. 24-Hour Access to Someone With Authority to Provide Consent
All provider agencies must verify that foster parents, approved relatives, child care
workers, and social service staff have access to a contact number providing 24-hour
access to someone with the authority to provide medical consent.
J. Medical Authorization for Routine Treatment or Emergency Care Form
ACS will continue to use Attachment A, Form FSS 007
13
(formerly CM-339), Medical
Authorization for Routine Treatment or Emergency Care, as its written consent form
when a physician or medical facility requests written consent for medical or surgical
treatment for children in foster care. Once agency consent is given, the provider
agency Executive Director or his or her designee is authorized to sign physician-specific
or hospital-specific consent forms if such forms are required by the treatment provider.
1. For children placed outside of New York State in accordance with the Interstate
Compact on the Placement of Children (ICPC), the policy and procedures for
obtaining medical consent shall be the same as for children placed within New York
State as described in this policy.
K. Parental Pre-Authorization for Routine
14
and Emergency Care
15
1. Although it is the policy of ACS to attempt to involve parents/guardians in all
medical consents on an individual treatment basis, when applicable, it is also
essential that parents/guardians be given the opportunity to pre-authorize ACS and
its provider agencies to provide routine medical and/or mental health screening,
immunizations, medical treatment, and emergency medical or surgical care in the
event that the parent/guardian cannot be located at the time such care becomes
necessary.
16
13
This is the only consent form that should be used by provider agencies.
14
Routine medical treatment is defined as medical, dental, mental health and hospital services which are customarily
given as part of preventive health care and /or for ordinary childhood diseases or illnesses (FSS 007).
15
Emergency medical, dental, health and hospital services or surgical care is defined as care that should be provided
immediately because delay of such care places the health of the child in serious jeopardy or in the case of a behavioral
condition places the health of each child or others in serious jeopardy.
16
For further information, see the OCFS Manual - Working Together: Health Services for Children in Foster Care. Links
to chapters and appendices can be found at http://www.ocfs.state.ny.us/main/sppd/health_services/manual.asp.

8
2. In the case of emergencies, such as life-threatening medical conditions or traumatic
injuries when, in the physician’s judgment:
a. An emergency exists and the child is in immediate need of medical attention;
and
b. An attempt to secure consent would result in a delay of treatment which
would increase the risk to the child’s life or health, medical, dental, health,
and hospital services may be rendered without the consent of a parent or
legal guardian.
17
It is the responsibility of the provider agency to inform the
parent/legal guardian of such emergency treatment as soon as possible.
3. Even if the parents/guardians of a court-placed child initially decline to sign such a
pre-authorization, the case planner should make ongoing efforts to explain its
benefit and encourage parents/guardians to sign the form. The pre-authorization,
when signed, must be kept in the medical section of the foster care record. If the
child is transferred to a foster home or facility under the supervision of another
agency, the case planner must forward the pre-authorization form to the new
planning agency. A pre-authorization is valid if signed by one parent whose
parental rights have not been terminated or surrendered.
4. In the event that the parents/legal guardians do not sign the FSS 007 form or
affirmatively object [see section III(A)(9) of this policy] to routine or emergency
care, the agency’s Executive Director or his or her designee has the authority to sign
the FSS 007 form as the temporary consenter for routine and emergency medical
care. However, see sections III(C) and III(D) below for information about voluntary
placement agreements and Article 7 PINS placements. There is no need to contact
Children’ Services for an override in this situation.
L. Principles and Guidelines for Obtaining Non-Routine Treatment Consents
1. Beginning with the first day of a child’s placement with a provider agency, that
agency becomes responsible for the management of the non-routine consent
process.
2. Non-routine treatment refers to any medical or mental health care component not
generally provided as part of primary health care. Any such non-routine treatment
requires specific informed consent by the child’s parent/guardian or authorized
representative, where appropriate, from ACS or the provider agency, including the
elements of informed consent as listed below [see section II(L)(5) below for
additional information on informed consent]. Non-routine treatment includes, but
is not limited to, psychiatric evaluations, psychotherapy, psychotropic medications,
17
See Public Health Law § 2504(4).

9
any hospitalization, any procedure that requires general anesthesia, any surgery, or
any invasive diagnostic procedure or treatment.
3. For non-routine treatment, the agency must confine each consent to a specific
procedure and/or treatment, or a specific medication and range of medication
dosages, to avoid providing “blanket consents.” This practice will help encourage
the medical professional to provide timely notification to the agency of any changes
in the child’s health/treatment status.
4. If emergency treatment is necessary, the agency must secure a written diagnosis
and recommendation for treatment prior to the child leaving an emergency
treatment setting, which must be dated and signed by an appropriate qualified
clinician.
5. “Informed consent” means that the person giving consent has had the opportunity
to discuss any questions or concerns about the treatment with a qualified individual
and has conveyed understanding of the following information wherever applicable:
a. Nature of the procedure/treatment;
b. Diagnosis and symptoms being treated;
c. How the procedure/therapy fits within the treatment plan;
d. Expected benefits;
e. Major risks and side effects;
f. Expected course and duration of treatment;
g. Alternative treatment choices along with their risks and benefits, including the
choice of no treatment;
h. Monitoring plan for complications and side effects;
i. For medications: whether or not a medication is U.S. Food and Drug
Administration (FDA) approved for the patient’s condition and major risks
including any FDA Black Box
18
warnings;
j. How to contact the clinical provider of the proposed procedure/treatment;
k. Location where the procedure/treatment will be performed;
l. Necessity, type, and risks of anesthesia, if any; and
m. Proposed length of hospitalization, if any.
6. Qualified agency personnel or a health professional at the provider agency shall
make best efforts to obtain informed consent by discussing the above elements in
detail and documenting this discussion on a signed consent form and/or in the
medical section of the patient’s foster care record.
18
Drugs that have special problems, particularly ones that may lead to death or serious injury, may have this warning
information displayed within a box in the prescribing information. This is often referred to as a "boxed" or "black box"
warning. See http://www.fda.gov/downloads/ForConsumers/ConsumerUpdates/ucm107976.pdf for drug safety
terms.
10
7. Although the consent of a child (under the age of 18) is not required for the
provision of medical treatment or medication, clinicians shall provide all children
and youth with a developmentally appropriate explanation of the treatment and
the opportunity to ask and receive explanations to any questions. Whenever
possible, and where a child has the capacity to do so, the child’s assent
19
to the
treatment should be sought. This is particularly important for adolescents, who
should be encouraged to be active participants in health-related decision-making.
When a youth does not assent to treatment, the provider must document it in the
case record and continue to explain the importance of the procedure or treatment
for the child’s safety and well-being. As noted above, youth who are 18 years of
age and older, youth who are parents, or youth who are married may consent for
their own medical and psychiatric care, and the consent of no other person shall be
necessary.
M. Sharing Medication Information for Children in Child Welfare Placements
Qualified provider agency personnel or a health professional at the provider agency
must share medication information including name, dosage, indication, and potential
side effects with the foster parent in order for the foster parent to provide information
to the prescribing physician regarding changes in the child’s medical condition and
response to the medication.
N. Psychiatric Hospitalization Requirements
For cases in which a child in foster care, including non-secure juvenile justice
placement, is psychiatrically hospitalized, provider agencies and DCP or DYFJ must
continue to adhere to the procedures set forth in the revised Policy # 2010/03 entitled
Guidelines for the Provision of Emergency and Inpatient Mental Health Services for
Children in the Foster Care and Child Protective System
20
.
O. Treatment of Non-Routine Acute Illnesses
Provider agencies seeking consent from parents/guardians or ACS for medications or
procedures to manage acute (new onset, not chronic) illnesses must document in the
child’s medical record and in the CONNECTIONS (CNNX) Health Narrative tab that the
child has received at least one physical examination [complete or focused on the organ
system(s) involved] within one month of entering care. Appropriate laboratory tests
(as they apply to the specific diagnosis or condition) shall accompany the request and
19
Assent applies to children under 18 years of age for whom someone else’s consent is needed to allow the child to
receive a procedure, medication, and/or medical/mental health treatment. Assent means the child’s affirmative
agreement and willingness to receive the procedure, medication, and/or medical/mental health treatment.
20
This document is available at the online ACS Policy Library, which can be accessed through this link:
http://www.nyc.gov/html/acs/html/home/policy_library.shtml.

11
must be conducted within accepted time frames for standards of care for that
diagnosis.
P. Treatment of Chronic Illnesses
For children who suffer from chronic illnesses or who require long-term medical,
dental, or hospital services, an agency may approve an entire treatment plan rather
than individual treatments. A “treatment plan” is defined as a detailed plan setting
forth the child’s medical condition; the manner in which the health care provider
intends to provide services for the condition; the benefits and consequences of such
services; and the alternative treatments, including their benefits and consequences,
which may be used if the initial treatment proves unsuccessful. The agency must
document that the child has received at least one physical examination [complete or
focused on the organ system(s) involved] within six (6) months of the request for
consent. Appropriate laboratory tests (as they apply to the specific diagnosis or
condition) shall accompany the request and must be conducted within accepted time
frames for the standards of care for that diagnosis.
Q. Parental Objection to Specific Treatments
1. For cases in which the parents/guardians are available but affirmatively object
21
to
the recommended medical treatment, the case planner or case planning team is
required to consult with the ACS Medical Consent Unit or Psychotropic Medications
Unit (PMU), depending on the nature of the treatment and/or diagnosis, in order to
request authorization to provide consent against parental objection. All requests
should be directed to the Medical Consent Unit, except those regarding
psychotropic medication or psychiatric hospitalization, which should be directed to
the PMU. The provider agency or relevant DCP, FPS, or DYFJ unit is responsible for
submitting the legal and clinical information necessary for the relevant unit to
review the consent request. Provider agency and ACS staff may request these
forms from the Medical Consent Unit. Please send all requests of this nature,
including the date by which a response is needed, via email to:
2. The reviewing unit will then send its recommendations to the Office of the General
Counsel’s (OGC) Legal Counsel Unit or the Division of Family Court Legal Services
(FCLS) Legal Compliance Unit.
3. The FCLS Legal Compliance Unit attorney or OGC Legal Counsel Unit attorney will
review the case for legal authority and compliance with ACS’ procedures and refer
the matter back to the PMU or the Medical Consent Unit. If the treatment plan
appears to be clinically appropriate and the Medical Consent Unit or PMU makes
21
See section III(A)(9) below.

12
the decision to override the parental objection, the decision will be forwarded to
DCP, FPS, DYFJ, or the requesting provider agency whose Executive Director or
designee will sign the consent form. As appropriate, the need for court
intervention will be determined on a case-by-case basis in consultation with the
FCLS Legal Compliance Unit and/or assigned attorney.
a. If one parent affirmatively objects to the treatment but the other parent (whose
rights have not been terminated or surrendered) consents, the consent is valid,
and no override is needed. In the event that both parents/guardians are
available but affirmatively object
22
to the recommended medical treatment,
refer to the guidelines in section II(Q) above, entitled “Parental Objection to
Specific Treatments.”
b. The case planner must document a parent’s affirmative objection to non-
routine and non-emergency medical care in CNNX.
R. Psychiatric Treatment and Psychotropic Medication
1. Psychotropic Medication Consent and Review
a. Consent for psychotropic medication must be sought first from
parents/guardians whose rights have not been terminated or surrendered. In
order to achieve informed consent and the appropriate use of psychotropic
medication, the person signing
23
the consent must understand the reason for
its use, the benefits it should provide, its unwanted effects or dangers, the
treatment alternatives, and the other information elements listed above in
section II(L)(5) of this policy. Persons authorized to sign treatment consents
have the right to have any questions or concerns addressed before giving
consent. Clinical providers must use Form FSS 010A or FSS 010B (Attachment
C) Consent for Psychotropic Medication
24
.
b. Psychotropic medication consent requests must be filled out and signed by the
clinician who prescribed the psychotropic medication. The clinician must be a
child and adolescent psychiatrist, a developmental behavioral pediatrician, or
another medical clinician who has been approved by the PMU or accredited by
OMH or the New York State Department of Health (DOH), or an equivalent out-
of-state agency to prescribe psychotropic medications to children as specified
22
See section III(A)(10).
23
The child’s parent/guardian, the child if able to consent (see section II(A) above), or an authorized representative
where appropriate from ACS or the provider agency.
24
There are different versions of this form for parents or legal guardians, foster care agency designees, and youth.
Each version of the form includes the elements of informed consent required by ACS.

13
below.
25
At a minimum, any child who is being considered for psychotropic
medications must, prior to the initiation of drug treatment, be assessed by:
i. A board certified or board eligible child and adolescent psychiatrist; or
ii. A pediatrician who is board certified or board eligible in pediatric neurology,
neurodevelopmental pediatrics, or developmental behavioral pediatrics.
c. In addition to the diagnosis and medical recommendation for the drug(s), the
agency’s medical records must contain documentation of appropriate
monitoring of the child’s behavioral/physiological reaction, laboratory results,
and side effects to the drug(s) at least as frequently as recommended by the
prescribing clinician. ACS will conduct random reviews of case records,
including medical records of children who are receiving or who have received
psychotropic medication. When consenting to psychotropic medication, it is
appropriate to consent to either a specific dosage or to a therapeutic range as
prescribed by the treating clinician. Agencies must document the child’s
prescribed maintenance dosage.
d. Under no circumstances may psychotropic medications be prescribed or used
solely to control a child’s behavior, except as permitted pursuant to the New
York Codes, Rules and Regulations
26
, which addresses, among other forms of
restraints, “pharmacological restraint.”
27
As part of seeking consent for
psychotropic medication, the agency must document that the child has
received an initial physical examination [complete, or focused on the organ
system(s) involved or that may be affected by the medication] from a
pediatrician and appropriate laboratory tests within 12 weeks prior to the
administration of medications.
i. Thereafter, children on psychotropic medication must have a documented
physical examination and appropriate laboratory tests every six (6) months.
A pediatrician, psychiatrist, and/or certified family, pediatric, or psychiatric
nurse practitioner may perform the follow-up examination and laboratory
tests. Agencies must use the Alternative Medication Safety Exam form to
document the follow-up physical and laboratory findings and may request
the form by emailing: [email protected].
e. Any questions regarding psychotropic medications and the pharmacological
treatment of mental health-related conditions of children in foster care must
first be directed to the agency’s clinical, medical and/or mental health director
25
For more information on the process for approval of clinicians, please contact the PMU at [email protected]v.
26
See 18 NYCRR § 441.17.
27
Note: No agency may use any form of restraint, unless the New York State Office of Children and Family Services
has approved the agency’s restraint policy in writing.

14
or an individual serving in a corresponding capacity. In cases that involve
overriding a parent’s/guardian’s refusal to consent, the clinical or medical
director who is reviewing a case may contact the PMU for any needed
clarification of ACS treatment guidelines or for specialty consultation. Agencies
are responsible for verifying that ACS’ guidelines are followed and that any
PMU recommendations are carried out appropriately. Providers must consult
with the PMU in the following circumstances:
i. Whenever there is a request to override a parent’s/guardian’s affirmative
objection to psychiatric treatment for a child in foster care pursuant to
Family Court Act Article 10, including requests for psychotropic medication,
hospitalization and/or placement in a psychiatric treatment facility;
28
and
ii. When, after reviewing a psychiatric treatment plan, a provider agency’s
clinical or medical director questions the appropriateness of the plan, even
when the parent or guardian has given consent; in these situations, the
agency must request a review by the PMU within ten (10) working days of
the clinician’s proposal for the new medication or other treatment.
f. To request a consent review for override authorization, the referring agency
must submit the necessary legal and clinical information by email to:
i. Using Form FSS 001 PMU Override Consent Request (Attachment B). The
PMU will then review the case materials and make a recommendation
regarding the medical appropriateness of the treatment in question.
S. Continuity of Active Psychotropic Medication Treatment During Transitions
1. When a youth who is being treated with psychotropic medication is transitioned
into a new setting (e.g., from the hospital into foster care, including juvenile justice
placement), as with other medications, the youth must be able to continue the
treatment regimen prescribed by his or her previous medical provider until the
medication plan is reviewed by a new licensed medical provider. All medications
prescribed by the former mental health provider, for which consent had previously
been obtained, must be thoroughly reviewed by the new provider within one
month of the entry of the youth into the new setting, at which time a renewed
consent request must be initiated. If the agency has authority to provide the
consent, then the medication should not be stopped.
28
See ACS Policy #2010/03, Guidelines for Provision of Emergency and Inpatient Mental Health Services for Children in
the Foster Care and Child Protective System, revised November 3, 2010.

15
2. For youth placed pursuant to Article 3 of Family Court Act, the new medical
provider must determine whether the administration of medication for a particular
youth is part of that youth’s ongoing mental health plan. If it is part of a youth’s
ongoing mental health plan, the provider agency has the authority to continue the
psychiatric medication as part of the youth’s routine care.
29
Any revisions made to
the youth’s prescription must be based on an appropriate medical professional’s
clinical review of the youth’s progress and response to treatment.
T. Psychotropic Treatment Requirements and Waivers of Requirements
1. ACS requires that all psychotropic medications be:
a. Prescribed by a child and adolescent psychiatrist; and
b. Clinically reviewed by that psychiatrist every month.
c. Board certified specialists in pediatric neurology, neuro-developmental
pediatrics, or developmental-behavioral pediatrics may also prescribe
psychotropic medications.
2. All provider agencies must verify the credentials of the health care providers that
serve their population based on documentation of updated licenses and certificates
maintained by the health care institution under which the health care providers are
employed.
3. Clinicians affiliated with OMH or DOH certified clinics
30
do not need their
credentials verified by ACS. Similarly, clinicians in out-of-state facilities having their
own credentialing processes do not require verification by ACS.
4. On a case-by-case basis the PMU may grant a waiver to permit follow-up
appointments by clinicians or practitioners other than the aforementioned to
prescribe psychotropic medication. Such practitioners would include:
a. Board certified/eligible general pediatricians;
b. Board certified/eligible general (adult) psychiatrists; and
c. Advanced practice nurses (APNs)/nurse practitioners in psychiatry (PMHNPs)
certified in psychiatry/mental health by the New York State Department of
Education.
5. The psychiatric APN/PMHNP may prescribe psychotropic medication pursuant to a
joint protocol with a collaborating child and adolescent psychiatrist according to the
following criteria:
31
29
See Family Court Act § 355.4(3) (2013).
30
See Article 31 of the Mental Hygiene Law and Article 28 of the Public Health Law.
31
See New York Education Law § 6902(3).

16
a. The collaborating physician must be board certified or board eligible in child
and adolescent psychiatry;
b. The working relationship between the PMHNP and collaborating physician
32
must be meaningful and substantial;
c. The following specifications must be included in Section 3 of the New York
State Education Department Office of Professions Division of Professional
Licensing Services Nurse Practitioner Form 4NP: Verification of Collaborative
Agreement and Practice Protocol:
i. Prescribing of psychotropic medication to children in foster care will occur
according to the evolving prescribing guidelines supported by the New York
State Office of Child and Family Services (OCFS) and ACS.
ii. New York State law requires the Collaborating Agreement between a
physician and nurse practitioner to contain specific language to authorize
the nurse practitioner to prescribe medications. In addition, ACS requires
that the Collaborating Agreement must specify that direct discussion
between the nurse practitioner and the collaborating physician must occur
for specific cases as noted below in this policy. This discussion may include
diagnosis, drug selection, dosage change, drug strength, overall treatment
plan, and other medical concerns.
iii. The collaborating physician in this agreement shall review with the nurse
practitioner any patient under six (6) years of age who takes any
psychotropic medication, any child patient taking three (3) or more
psychotropic medications simultaneously, all children/patients taking
antipsychotic medications for more than a three (3) month period, and any
child taking psychotropic medication who is not receiving psychotherapy.
iv. The collaborating physician in this agreement must discuss clinical matters
with the nurse practitioner by phone or in person a minimum of twice per
month for approximately 60 minutes per discussion, with more time being
spent whenever determined by clinical need.
d. A copy of the Nurse Practitioner Form 4NP must be submitted to PMU along
with other necessary materials as part of the waiver request.
32
The Nurse Practitioners Modernization Act will become law on January 1, 2015. For more information, see
https://npagr.enpnetwork.com/nurse-practitioner-news/46031-nurse-practitioners-modernization-act-will-become-
law.
17
6. A pediatrician or family physician, board certified pediatric APN/NP, board certified
family APN/NP, or board certified psychiatric APN/NP may prescribe stimulant
medication to a child for uncomplicated attention deficit hyperactivity disorder
(ADHD). Clinicians providing such treatments do not require a waiver request
pertaining to other requirements. However, if the child is also being treated for
another psychiatric disorder by another specialist, the prescriber must coordinate
care with that professional.
7. Agencies may submit requests to the PMU for a waiver of the above mentioned
requirements (in 1-5 above), if appropriate, given the circumstances of the case.
Requests regarding waivers for non-child and adolescent psychiatrists or other
pediatric specialists as described above must be requested via:
8. Waiver requests may be made for either of two situations:
a. When the provider agency is interested in hiring a non-child and adolescent
psychiatrist or other pediatric specialists as described above to provide
medication prescription and/or oversight to multiple children in its care (i.e., a
clinician-specific waiver); or
b. When the provider agency is interested in having a community-based, non-child
and adolescent psychiatrist or other pediatric specialists as described above to
provide psychotropic medication to an individual child for a specific clinical
reason (i.e., a child-specific waiver).
U. Interviews to Determine Youth Eligibility for Programs
Parental consent is not required in order for a case planner to refer a child or bring a
child to a facility (such as an OMH residential treatment facility) for an eligibility
interview.
V. Quality Assurance Requirements
1. Provider agencies must establish internal policies and procedures to guide their
medical consent process. Procedures shall describe the level/title of staff eligible to
provide medical consents [see section II(G), (I) and (J) for the type of information
required to give informed consent]; the use of second opinions listed in section
III(A)(8)); the availability of medical and mental health staff to provide consultation
24 hours a day, seven days a week in section II(I); and internal prospective and
retrospective review of medical consents.
2. Note: Every two (2) years, provider agencies must submit their internal medical
consent policies via email to the Medical Consent Unit at:

18
III. GUIDELINES BY TYPE OF ENTRY INTO FOSTER CARE
33
Requirements for ACS and its provider agencies to consent for medical, dental, mental
health, and hospital services vary depending on whether a youth is placed in foster care for
child protective reasons; whether a youth is placed on a juvenile delinquency (JD) case
pursuant to Family Court Act Article 3; whether a youth is placed through a voluntary
placement agreement; whether a youth is placed on a Persons in Need of Supervision (PINS)
case pursuant to Family Court Act Article 7; and whether a youth is placed as a destitute
child. Parents whose rights have been terminated or surrendered must not be contacted
for consent and have no legal authority to give consent. ACS functions as a parent and can
authorize all health care services for children in foster care whose parents’ rights have been
terminated or surrendered.
A. Children Remanded or Placed in Foster Care for Child Protective Reasons
1. For children who have been taken into protective custody and/or have been
removed from the place where they have been residing for child protective reasons,
or who have been remanded or placed in foster care pursuant to Family Court Act
Article 10, the children’s parents/guardians are the primary persons from whom
consent must be sought.
2. Attempts to secure parental consent in these situations must be made for all
medical care except when, in the physician’s judgment, an emergency exists and
the child is in immediate need of medical attention and an attempt to secure
consent would result in a delay of treatment which would increase the risk to the
child’s life or health. In such cases the physician may render treatment without
seeking consent.
3. A provider agency Executive Director or his or her designees may provide
consent for this group of children when:
a. The parents’/guardians’ current whereabouts are unknown and reasonable
efforts have failed to locate them;
b. The parents’/guardians’ whereabouts are known but they cannot be contacted
despite reasonable efforts;
c. The parents/guardians have been contacted and decline to consent but do not
affirmatively object [see section III(A)(9) below for information on “affirmative
objection”] to medical treatment; or
33
See section II(E) of this policy for authorization of consent in situations where parental rights have been terminated
or surrendered.

19
d. The parents/guardians have been contacted and verbally consented but refuse
or are unwilling to sign the consent form.
4. For each of the above scenarios, the agency shall document its reasonable efforts to
secure parental consent, including all dates and methods used to secure the
consent, in the child’s medical record and in CNNX. Reasonable efforts must
include the following actions at minimum:
a. One telephone call if a phone number is known;
b. One personal visit to the parents’/guardians’ current or last known address; and
c. One letter to the parents’/guardians’ current or last known address.
5. The efforts to secure consent must be directed toward each parent/guardian,
regardless of whether the parents/guardians live together or separately from one
another. Reasonable efforts must be properly documented (dated and signed) in
the child’s records (i.e., CNNX and medical record).
6. Note that “parent” does not include an individual whose parental rights have been
terminated or surrendered. Consent from a father should be obtained only if he is
considered a “consent” father under the law.
34
This is true even if such an
individual is listed as a “father” or “respondent” on a child protective petition.
7. For purposes of authorizing treatment, one parent’s/guardian’s consent is
sufficient.
8. If a parent/guardian wishes to obtain a second opinion regarding a proposed
medical treatment, the provider agency must arrange one as appropriate and
feasible. ACS does not provide clinical second opinions.
9. “Affirmative objection to treatment” means any statement, orally or in writing,
indicating that the parent is opposed to the treatment. The case planner must
document such statements in CNNX and place a copy of the CNNX entry in the
medical record section of the foster care record to satisfy this requirement.
10. Parents’/guardians’ affirmative objection to treatment is addressed above in
Section II(Q) Parental Objection to Specific Treatments and Section II(R) Psychiatric
Treatment and Psychotropic Medication.
11. Children for Whom Parental Rights have been Terminated or Surrendered
When ACS and the foster care agency have custody and guardianship of a child
through a termination of parental rights or surrender (i.e., the child is freed for
34
As described in Domestic Relations Law § 111(1) and applicable case law.

20
adoption), ACS and/or the provider agency may consent for medical treatment.
Consents signed by individuals whose parental rights have been terminated or
surrendered, or former guardians, are neither necessary nor valid.
B. Youth Placed with ACS in Juvenile Justice Placements Pursuant to Article 3
35
1. Under the Close to Home legislation, ACS has been granted the authority to consent
to routine medical, dental, and mental health services and treatment to youth
placed with ACS on or after September 1, 2012
36
under Family Court Act Article 3 on
juvenile delinquency cases after a dispositional hearing. Non-routine services and
treatment require a parent’s consent. If consent cannot be obtained, then a court
order must be sought
37
. The case planner must call in a report to the Statewide
Central Register (SCR), or as appropriate, request any currently assigned DCP Child
Protective Specialist (CPS) to consider holding a Child Safety Conference if a
parent’s failure to consent is endangering the health or safety of the child.
2. In these situations, provider staff shall make attempts to secure parental consent
[see section III(A)(5) above] for all medical care except when, in the physician’s
judgment, an emergency exists and the child is in immediate need of medical
attention and an attempt to secure consent would result in a delay of treatment
which would increase the risk to the child’s life or health. In such cases the
physician may render treatment without seeking consent.
C. Youth Placed Through Voluntary Placement Agreements
1. Authority to provide medical consent for children who are voluntarily placed into
family foster care or residential care shall be in accordance with this policy unless it
conflicts with the terms set forth in the voluntary placement agreement (VPA).
Non-routine services and treatment require a parent’s consent unless otherwise
specified in the VPA. If consent cannot be obtained, then a court order may be
sought as appropriate.
2. The standard VPA used by ACS includes provisions in which the parent/guardian
authorizes the Commissioner or his or her designee to consent to regular medical
examinations, routine immunizations, and tests and treatments, including dental
treatments that are needed for a child’s well-being
38
. The VPA also provides that if
35
The term “placement” in this context does not include Article 3 detention.
36
For youth placed with ACS prior to September 1, 2012, staff must seek parental consent for any treatment, including
routine treatment.
37
FCLS has a designated attorney in each borough for Close to Home cases so that when a court order is needed for
medical consent, the case planner can contact the FCLS attorney.
38
DCP-006 Voluntary Placement Agreement by Parent/Guardian has the parental preauthorization for routine and
emergency care. The FSS 007 form is not signed in voluntary placement cases because the VPA already includes the
pre-authorization.

21
a physician decides that the child has a medical emergency, and waiting to find the
parent/guardian to obtain consent would place the child’s life or health in danger,
emergency medical, mental health, dental, or hospital services and/or emergency
surgical care may be provided to the child without the parent’s/guardian’s consent.
3. When ACS or the foster care agency requests a parent’s/guardian’s consent for a
non-routine medical procedure or treatment for a child voluntarily placed in foster
care and the parent/guardian does not grant his or her consent, and the
Commissioner or his or her designee believes that the parent’s/guardian’s failure to
provide such consent endangers the life or health of the child, the case planner
must contact the assigned FCLS attorney immediately in order to discuss the
appropriateness and feasibility of obtaining a court order for the medical care. The
case planner must call in a report to the SCR, or as appropriate, request any
currently assigned DCP CPS to consider holding a Child Safety Conference if the
parent’s failure to consent is endangering the health or safety of the child.
4. When a person entrusted with a child’s care (as opposed to a parent or guardian) is
the individual who signs a voluntary placement agreement (known as an
“entrustment agreement” in this situation), that person retains no rights to consent
to medical care under this policy and must not be consulted. The Commissioner or
his or her designee must seek the consent of the parent or guardian even if this
requirement is not explicit in the agreement.
D. Youth Placed with ACS on PINS Petitions Pursuant to Article 7
1. Authority to provide medical consent rests with the parents of children who are
placed under Family Court Act Article 7 on Persons In Need of Supervision (PINS)
cases. Neither ACS nor the provider agencies have the authority to provide consent
for medical treatment for children who are remanded or placed on PINS cases in
absence of a court order.
2. Only parents/legal guardians may consent for medical treatment for PINS youth
remanded or placed with ACS pursuant to Article 7. The foster care agency must
request authorization in writing from the child’s parent/guardian for routine
medical and psychological assessments, immunizations, medical treatment, and
emergency medical and surgical care if the parent/guardian is unavailable when
such care becomes necessary.
39
The provider agency must make this request within
10 days after the child is taken into care. In the event that consent cannot be
obtained, a court order may be sought as appropriate.
39
Refer to 08-OCFS-INF-02 which indicates that, pursuant to 18 NYCRR § 441.22(d), local social services districts must
request authorization in writing from the child’s parent or guardian for routine medical and psychological
assessments, immunizations, medical treatment, and emergency medical or surgical care if the parent or guardian is
unavailable when such care becomes necessary.
22
3. If foster care agency staff or appropriate ACS staff are concerned about a
parent’s/guardian’s refusal to provide medical consent, they must consult with FCLS
to determine whether to seek a court order for treatment. The case planner must
call in a report to the SCR or request any currently assigned DCP CPS to consider
holding a Child Safety Conference if the parent’s/guardian’s failure to consent is
endangering the health or safety of the child.
IV. EXCEPTIONS TO AGENCY OR ACS CONSENT
As noted above, there are several populations of children in foster care for whom there are
exceptions to the regular consent procedures.
A. Subject Children Under Family Court Act Article 10 Cases Who are Not Physically in the
Custody of ACS
1. This group includes, but is not limited to, children who are “paroled” (i.e., in the
temporary custody of a parent or other person) or on trial discharge status. Only
parents/legal guardians may consent for medical treatment for children in this
category. If foster care agency staff or appropriate ACS staff are concerned about
the parent’s/guardian’s refusal or inability to provide consent for medical care to
children on parole or trial discharge status, the staff must immediately consult with
the FCLS attorney as to whether seeking a court order for treatment would be
appropriate and feasible. If a parent’s lack of consent is endangering the health or
safety of a child, the foster care agency must immediately take steps to resolve the
situation, or as appropriate, take the child into the physical custody of ACS and
contact the FCLS attorney. In the case of a parole, ACS must seek to modify the
order in court before removing the child unless there is imminent danger to the
child and insufficient time to seek a court order.
2. For cases still in the investigative stage that involve physical or sexual abuse, see
Procedure 102A, Medical Consents and Medical Referrals in Suspected Child
Sexual/Physical Abuse Cases, available at the online ACS Policy Library through the
following link: http://www.nyc.gov/html/acs/html/home/policy_library.shtml.
B. Children for Whom Parental Capacity to Consent is Questioned
1. For cases in which there is doubt or question about the capacity of the parent/legal
guardian (whose rights have not been terminated or surrendered) to provide
informed consent, the case planning agency must send an email inquiry
40
to:
MedicalConsentRequests@acs.nyc.gov.
40
See the ACS policy titled, Security of Confidential, Case Specific and/or Personally Identifiable Information, December
6, 2010. This document is available via search in the ACS Intranet Policy Library located at http://nycacs/lib-pl using
key word “confidential.”

23
2. The Medical Consent Unit will consult with the assigned attorney from FCLS
regarding the appropriateness and feasibility of seeking a court order. In the event
that the parent/guardian gains the ability to provide informed consent for
treatment, the case planning agency must obtain such consent from him or her as
described in this policy.
C. Parenting Youth Cases
Parenting youth, including youth who are under age 18 and are parents who are in
foster care, must consent to their own medical treatment.
41
Pregnant youth who are
minors may consent to medical, dental, mental health, and hospital services related to
prenatal care.
42
Youth who are minors and have given birth are also the sole persons
authorized to consent for their child’s medical treatment when the child is not in ACS’
custody (i.e., a minor parent in foster care who resides in the same foster care
placement as his or her child who is not in foster care).
43
D. Youth Whose Capacity to Consent is Questioned
1. For cases in which there is doubt or question about the capacity of a youth
otherwise legally authorized to consent to his or her own medical treatment to
provide informed consent for his or her own treatment, the case planning agency
must obtain an independent assessment of the mental capacity of the youth by a
mental health professional appropriately trained and qualified to make such an
assessment.
2. If the assessment shows that the youth is incompetent to provide consent or that
there is doubt about his or her capacity to consent, the case planning agency must
contact the Medical Consent Unit at: [email protected].
The Medical Consent Unit will consult with the assigned attorney from FCLS. If
appropriate, and if there is a legal basis to do so, the attorney will seek a court
order for treatment.
41
See Public Health Law § 2504(1) and Mental Hygiene Law § 33.21(a)(1).
42
See Public Health Law § 2504(3).
43
Any delegation of authority for consent of health care by the minor parent must be in compliance with General
Obligations Law § 5-1551, regarding the power of a parent to designate a person in parental relation. Otherwise, the
agency has no authority to consent for health care for the minor parent’s child (unless that child is also placed
pursuant to Article 10 of the Family Court Act). The law does not preclude an authorized agency from giving effective
consent if the minor parent agrees to this. If an authorized agency believes that a minor parent is not making prudent
decisions concerning his or her health care or the health care of his or her child, and there are safety concerns, the
agency should make a report to the SCR. The agency should alert the FCLS attorney to discuss informing the Court and
parties, as well as the appropriateness and feasibility of seeking a court order for treatment. Health-related decisions
that are made by the minor parent should be clearly documented in CNNX as contemporaneously as possible.

24
3. In the event that the youth gains the ability to provide informed consent for
treatment, the case planning agency must obtain such consent from him or her as
described above.
E. Children Who are Placed with an Agency Under the Auspices of a Governmental Agency
Other Than ACS
Authority to provide consent for medical care to children in the custody of and placed
in a facility operated by OCFS, OMH, OPWDD, or with their contractors rests with the
appropriate oversight agency and not with ACS. Children who are legally placed with
ACS but are physically placed in an OCFS, OMH, or OPWDD facility, however, remain
subject to the policies herein.
44
F. Children Who are Placed as Destitute Children
Typically these children’s parents are deceased, unknown, or unavailable. Case
planners shall consult with FCLS as necessary regarding medical consent involving these
children.
G. Children Who are in Foster Care but Whose Legal Status Needs Confirmation
Case planners who need to confirm the legal status of a child who needs medical
treatment shall consult with FCLS before taking action with respect to medical consent.
H. Consent for Sexual and Reproductive Health Services
45
1. In New York State, a youth, regardless of age, is authorized to consent to receive
reproductive health and family planning services,
46
sexually transmitted infection
testing and treatment,
47
and abortion services.
48
Consent for these services is not
required from the child’s parent/guardian, ACS, or an authorized agency.
2. When a minor (defined as a youth under the age of 18) consents to his or her
reproductive health care, that health care and information is confidential and must
not be disclosed, even to the minor’s parents, unless an appropriate written
consent has been obtained from the youth.
3. The case planner must ask a pregnant youth about whether she wants to notify her
parent/legal guardian about the pregnancy. No disclosure to the youth’s
44
See Mental Hygiene Law Article 80 and 14 NYCRR § 633.11 for information concerning medical consent for
individuals who are residents of facilities operated or certified by OPWDD or OMH.
45
See Procedure #2007/01, Policy Guidelines for Family Planning and Pregnancy Related Information and Service,
November 8, 2007 (in revision, to be renamed Sexual and Reproductive Health Care for Youth in Foster Care).
46
See United States Supreme Court decision in Carey v. Population Services International, 431 U.S. 678 (1977).
47
See Public Health Law § 2305(2).
48
See Public Health Law § 2504(1) and (3).

25
parent/legal guardian may occur unless the youth gives written consent. The case
planner must document discussions, including topic areas discussed, in the CNNX
Health Narrative field.
I. Testing for HIV
49
The parent/guardian must provide written consent before his/her child can be tested
for HIV unless the child has the capacity to give consent to such testing and has
consented for him/herself. No one other than the child can consent to an HIV test if
the child has the capacity to consent. If there is an urgent need for the child to be
tested for HIV, and the parent/guardian refuses to give consent, or ACS cannot find the
parent, or the parent is unable to give consent due to mental or physical illness, staff at
the case planning agency must contact FCLS to determine whether obtaining a court
order for the child to be tested is appropriate and feasible.
J. Consent for Autopsy or Organ Donation (Anatomical Gift)
Only a deceased child’s “next of kin,” a district attorney, a sheriff, the chief of a police
department of a city or county, the superintendent of state police, or coroner or
medical examiner may authorize an autopsy.
50
Neither the Commissioner of ACS nor
an authorized agency may consent for an autopsy under any circumstances.
For organ donation, there is a specific statutory priority list describing who may
provide consent.
51
Under no circumstances may staff at a foster care agency or ACS,
other than the Commissioner, provide consent for organ donation. If a request is
made, the provider must consult with the ACS Office of Child and Family Health (OCFH).
K. Religious Objections to Treatment or Immunizations
Parental objections to treatment that are based on religious beliefs, including routine
immunizations, shall be discussed on a case-by-case basis. If there is a concern, the
case planner must consider calling in a report to the SCR as necessary or seek to have
DCP convene a Child Safety Conference (if the case is active in DCP), and must also
consult with FCLS.
L. Do Not Resuscitate (DNR) or Withhold or Withdraw Life-Sustaining Treatment Orders
Other than the Commissioner of ACS, under no circumstances may any staff at ACS or a
foster care agency provide consent to a “Do Not Resuscitate” (DNR) order or an order
to withhold or withdraw life-sustaining treatment.
49
See 18 NYCRR § 441.22.
50
See Public Health Law § 4210.
51
See Public Health Law § 4301.

FSS-007(Face)
Rev. 03/12
Attachment A
MEDICAL AUTHORIZATION FOR ROUTINE TREATMENT OR EMERGENCY CARE
1. I/We (and) ,
parent(s)/guardian(s) of born on
,
a child in the legal custody of the New York City Commissioner of Social Services, authorize the Commissioner
or his representative to consent to any routine treatment that my/our child may need while placed in foster care
and for emergency medical or surgical care in the event that I/we cannot be contacted at the time that such care
becomes necessary, or when a physician determines that the time needed to secure my consent would endanger
my child’s immediate welfare.
Routine medical treatment is defined as medical, dental, mental health and
hospital services which are customarily given as part of preventive health care and/or care for
ordinary childhood diseases or illnesses.
Emergency medical, dental, health and hospital services or surgical care is defined as care that
should be provided immediately because delay of such care places the health of the child in
serious jeopardy or in the case of a behavioral condition places the health of such child or
others in serious jeopardy. (New York State Public Health Law § 4900(3).)
2. I/we understand that the Commissioner or his representative will keep me/us informed of my/our child’s
progress, development and health.
3. I/we understand that this authorization will remain in effect for the duration of my/our child’s stay in foster care,
without regard to the authorized agency or facility in which my/our child is placed, unless I/we expressly revoke
the terms of this authorization.
4. I/we understand that if I/we refuse to sign this consent, the Commissioner of Social Services or his representative
may provide consent (in lieu of my/our consent) where authorized under Section 383-b of the Social Services Law.
5. I/we understand that when I/we object to a medical procedure and the Commissioner or his representative
believes that my/our objection would endanger the life, health or safety of my/our child, a court proceeding may
be initiated to review the decision to provide such care.
Signature of
Parent/Guardian
D
a
te
Signature of
Parent/Guardian
D
a
te
Signature of Witness D
a
te
ACS Division/Authorized Agency:
Address: City State Zip

FSS-007(Face)
Rev. 03/12
Attachment A
AUTORIZATIÓN PARA TRATAMIENTO DE RUTINA O URGENCIA
1. Yo/Nosotros (y)
padre(s)/guardián(es) de nacido
Ciudad de Nueva York, autorizo(mos) al tante autorice cualqueir tratamiento de rutina o de urgencia (emergen
cia) que mi niño/a pueda necesitar mien tras este alojado en el sistema de hogares de crianza en caso de que tal
cuidado sea neces sario, o cuando un medico determine que el periodo necesario para tener mi consentimiento
arriesagaría el bien estar inmediato de mi niño(a).
El tratamiento medico rutinario es definido como el servicio medico
mental y dental, servicios salud y hospitales el cual no es
rutinariamente dado como parte de salud preventiva y/o cuidado por
enfermedades ordinarias de la niñez.
El tratamiento urgente (emergencia), es definido como el servicio medico (pediatrico y
siquiatrico), dental, servicios salud y hospitales el cual no es rutinariamente dado como parte de
salud preventiva y/o cuidado por enfermedades ordinarias del al niñez.
2. Yo entiendo que el Comisionado o su representante me mantendría informado del progreso del desarollo y salud
de mi niño(a).
3. Yo entiendo que esta autorización permanecera en efecto por la duración del alojamiento de mi niño en el sis
tema de hogares de crianza, sin importar la agencia autorizada o facilidad en la cual se encuentre mi niño, al
menos que yo expresamente revoque los términos de esta autorización.
4. Yo entiendo que si me niego a firmar esta autorización, el Comisionado de Servicios Soicales o su representante
proveerá consentimiento (en mi lugar) como lo autoriza la Sección 383-b de las Leyes de los Servicios Sociales.
5. Yo entiendo que si yo no consiento a un procedimiento medico y el Comisionado o su representante crean que
mi negativa al dar dicho consentimiento pondría en peligro la vida, salud o seguridad de mi niño, un proced
imiento del servicio protectivo podría ser iniciado ante la corte para el procedimiento medico.
Firma del
Padre/Guardián
F
ec
h
a
Firma del
Padre/Guardián
F
ec
h
a
Firma del Testigo
F
ec
h
a
División de ACS/Agencia Autorizado:
Dirección: Ciudad Estado Código Postal

Attachment B
PSYCHOTROPIC MEDICATIONS UNIT (PMU)
OVERRIDE CONSENT REQUEST
FSS 001
REV 12/11
ACS Psychotropic Medication Override Form 11-10-11
This form is used to request override for Psychotropic Medications and Other Mental Health Services for a child
in foster care when a parent or legal guardian has refused to provide consent.
Please complete Sections A, B & C for ALL requests; Sections D, E & F should only be completed as applicable.
This form must be submitted by email to Glen[email protected]ate.ny.us. Additional information must be
submitted by fax (send to 212-227-4010). For questions please call 212-341-3966.
Section A: Child’s Information (for all requests)
Name:
DOB:
Date of Request:
Case Name:
Case #:
CIN:
Foster Care Agency:
Case Planner:
Case Planner Email:
Case Planner Phone #:
Supervisor Email:
Supervisor Phone #:
Child’s Legal Status:
Article 10 Article 3 Article 7 Voluntary Placement Freed for Adoption
Current Foster Care Placement:
Foster Home TFBH Group Home RTC Other:
Current Location:
Foster Care Placement Emergency Room Psychiatric Hospital
Mental Health Treatment Setting:
Other:
Person Completing Form (Name & Title):
Commissioner’s Designee authorized to sign treatment consent for child:
Section B: Current Mental Health Treatment (for all requests)
Is this child currently hospitalized?
yes no
Current Diagnoses:
Axis I:
Axis II:
Axis III:
Axis IV:
Axis V:
Current mental health provider:
Phone #:
Treating psychiatrist:
Phone #:
Other mental health services:
Pediatrician:
Phone #:
Current Medication:
Name:
Dosage/Sched:
(attach additional
Name:
Dosage/Sched:
pages if necessary)
Name:
Dosage/Sched:
Name:
Dosage/Sched:
Section C: Informed Consent (for all requests)
What are the anticipated benefits of pursuing the proposed treatment?
What are the anticipated risks of pursuing the proposed treatment, if any?
What are the anticipated risks of not pursuing the proposed treatment?
Has all of the above been discussed with the parent?
yes no
By whom?
?
Please describe the parent’s concerns about the proposed treatment approach:
Attachment B
PSYCHOTROPIC MEDICATIONS UNIT (PMU)
OVERRIDE CONSENT REQUEST
FSS 001
REV 12/11
ACS Psychotropic Medication Override Form 11-10-11
Section D: Consent Requests for Psychotropic Medication
Type of consent request:
renewal of existing medication new medication type and/or dosage
Name/dosage/schedule of medication:
What is the indication for the new medication
(if applicable)?
What are the alternatives to the proposed treatment?
Has this child had a physical within the past 6 months?
yes (attached) no
Have appropriate lab tests been done for the requested
medication(s)?
yes (attached) no
Please attach a clinical note from the prescribing psychiatrist that has been written within the last 60 days and includes
1) a 5-Axis diagnosis, 2) the target symptoms for each medication, 3) responses to current medication and 4)
justification for any doses or combinations that exceed common recommendations. Please also attach a copy of the
(unsigned) consent form, with the prescriber’s signature/initials, listing the medication(s) being prescribed.
Section E: Consent Requests for Acute Psychiatric Hospitalization
1
Was the child already admitted to the hospital on an emergency basis?
yes (date: _______ ) no
If yes, did the foster care agency give consent for the emergency admission?
yes no
Has the evaluating psychiatrist given clinical justification for this hospitalization?
yes (clinical note attached)
no
Please specify the alternatives to
hospitalization that have been
attempted:
Has the child’s current outpatient
yes – recommendation:
mental health provider been
consulted about this Hospitalization?
no
I am aware that the Mental Health Coordination Unit must be notified @
acs.sm.mentalhealth@dfa.state.ny.us within 24 hours of this child’s admission to the Hospital.
Section F: Consent Requests for Other Mental Health Treatment (including transfer to an Intermediate-Level
Psychiatric Hospital, i.e., “State” hospital)
Proposed mental health treatment:
Outpatient Psychotherapy Case Management HCBI
Family-Based Treatment Community Residence RTF
Intermediate-level psychiatric hospital (i.e., “State” hospital)
Other:
What problems will this treatment address?
What lower-level treatment(s) have been tried and what was the outcome?
What alternate treatment(s) have been considered and why were they not pursued?
Has the outpatient treatment provider been consulted about this proposed treatment?
yes no
If yes, what was his/her recommendation?
Please attach/fax any additional and/or relevant documentation or information you have about this child.
1
Please note that foster care agencies are authorized to give consent to urgent psychiatric hospitalizations if an evaluating
psychiatrist has determined that a delay in admission would pose a risk to the life, health, or safety of the child or others.
Any such emergency psychiatric hospitalizations must be submitted for override review within 48 hours of the admission.

FSS 010 Attachment C
pg. 1
Consent for Psychotropic Medication
FOSTER CARE AGENCY PROGRAM NAME
AGENCY CONTACT NAME TITLE CONTACT PHONE NUMBER CONTACT EMAIL ADDRESS
NAME OF CHILD DATE OF BIRTH
PRESENTING PROBLEM(S)
PRIMARY PSYCHIATRIC DIAGNOSES (DSM-5)
__________________________________________________________
__________________________________________________________
________________________________________________________________________
______________________________________________________________________
SOCIAL AND ENVIRONMENTAL FACTORS (V or Z Codes)
PROPOSED MEDICATION AND DOSE RANGE SPECIFIC TARGET SYMPTOMS
EXPECTED BENEFITS RISKS/POSSIBLE UNTOWARD EFFECTS
IS THERE A BLACK BOX WARNING ON THIS MEDICATION?
YES NO IF YES, SPECIFY: __________________________
IS THIS MEDICATION FDA-APPROVED FOR THE PATIENT’S CONDITION?
YES NO AND FOR THE PATIENT’S AGE? YES NO
ESTIMATED DURATION/LENGTH OF TREATMENT
Using this form, consent may be requested for one alternative medication to be prescribed if above medication is not effective.
Signature on this form does not indicate consent to prescribe both medications simultaneously beyond the period of cross-
titration.
PROPOSED ALTERNATE MEDICATION AND DOSE RANGE TARGET SYMPTOMS
EXPECTED BENEFITS RISKS/POSSIBLE UNTOWARD EFFECTS
IS THERE A BLACK BOX WARNING ON THIS MEDICATION?
NO YES (SPECIFY): _________________________________
IS THIS MEDICATION FDA-APPROVED FOR THE PATIENT’S CONDITION?
YES NO AND FOR THE PATIENT’S AGE? YES NO
CURRENT NON-PSYCHOPHARMACOLOGICAL TREATMENT MODALITIES
NON-PSYCHOPHARMACOLOGICAL TREATMENT MODALITIES BEING CONSIDERED
REQUEST SUBMITTED BY (printed name of psychiatrist) SIGNATURE OF PSYCHIATRIST (or authorized prescriber) DATE
The information contained in this form has been explained to me to my satisfaction. Permission is hereby granted to provide the
above-named psychotropic medication(s) to the above-named child as indicated. I understand this this consent can be withdrawn by
so advising the medication prescriber and foster care agency in writing.
PARENT OR LEGAL GUARDIAN (NAME) RELATIONSHIP TO CHILD PARENT OR LEGAL GUARDIAN (SIGNATURE) DATE
The information contained in this form has been explained to me to my satisfaction and I give my assent to the above-named foster
care agency to administer the above named psychotropic medication to me as indicated above.
PRINTED NAME OF CLIENT CLIENT SIGNATURE DATE
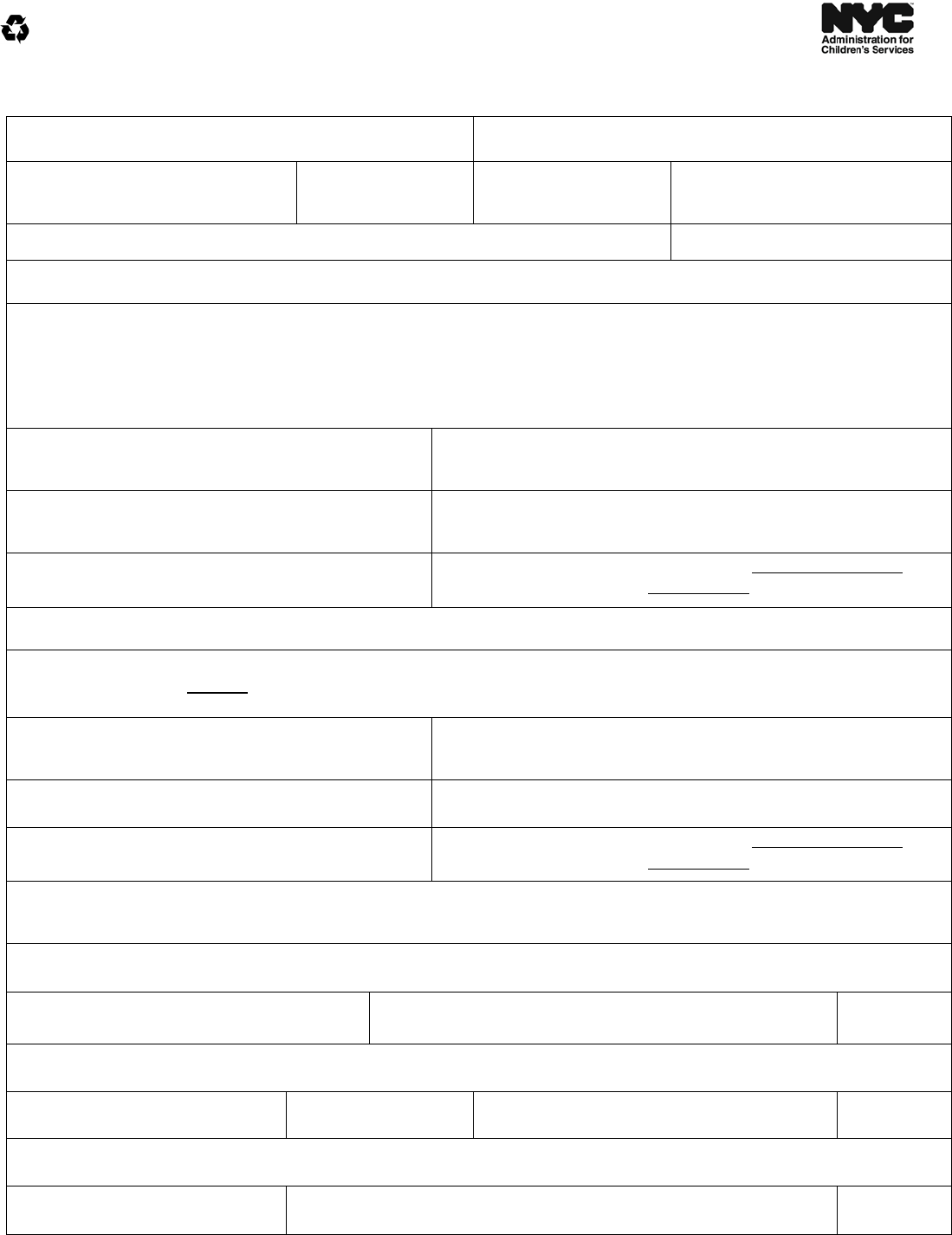
FSS 010 Attachment C
pg. 2
Foster Care Agency Designee Consent for Psychotropic Medication
FOSTER CARE AGENCY PROGRAM NAME
AGENCY CONTACT NAME TITLE CONTACT PHONE NUMBER CONTACT EMAIL ADDRESS
NAME OF CHILD DATE OF BIRTH
PRESENTING PROBLEM(S)
PRIMARY PSYCHIATRIC DIAGNOSES (DSM-5)
__________________________________________________________
__________________________________________________________
________________________________________________________________________
______________________________________________________________________
SOCIAL AND ENVIRONMENTAL FACTORS (V or Z Codes)
PROPOSED MEDICATION AND DOSE RANGE SPECIFIC TARGET SYMPTOMS
EXPECTED BENEFITS RISKS/POSSIBLE UNTOWARD EFFECTS
IS THERE A BLACK BOX WARNING ON THIS MEDICATION?
YES NO IF YES, SPECIFY: __________________________
IS THIS MEDICATION FDA-APPROVED FOR THE PATIENT’S CONDITION?
YES NO AND FOR THE PATIENT’S AGE? YES NO
ESTIMATED DURATION/LENGTH OF TREATMENT
Using this form, consent may be requested for one alternative medication to be prescribed if above medication is not effective.
Signature on this form does not indicate consent to prescribe both medications simultaneously beyond the period of cross-
titration.
PROPOSED ALTERNATE MEDICATION AND DOSE RANGE TARGET SYMPTOMS
EXPECTED BENEFITS RISKS/POSSIBLE UNTOWARD EFFECTS
IS THERE A BLACK BOX WARNING ON THIS MEDICATION?
NO YES (SPECIFY): _________________________________
IS THIS MEDICATION FDA-APPROVED FOR THE PATIENT’S CONDITION?
YES NO AND FOR THE PATIENT’S AGE? YES NO
CURRENT NON-PSYCHOPHARMACOLOGICAL TREATMENT MODALITIES
NON-PSYCHOPHARMACOLOGICAL TREATMENT MODALITIES BEING CONSIDERED
REQUEST SUBMITTED BY (printed name of psychiatrist) SIGNATURE OF PSYCHIATRIST (or authorized prescriber) DATE
The information contained in this form has been explained to me to my satisfaction. Permission is hereby granted to provide the
above-named psychotropic medication(s) to the above-named child as indicated.
EXECUTIVE DIRECTOR OR DESIGNEE TITLE EXECUTIVE DIRECTOR OR DESIGNEE SIGNATURE DATE
The information contained in this form has been explained to me to my satisfaction and I give my assent to the above-named foster
care agency to administer the above named psychotropic medication to me as indicated above.
PRINTED NAME OF CLIENT CLIENT SIGNATURE DATE
