BARACLUDE- entecavir tablet, film coated
BARACLUDE- entecavir solution
E.R. Squibb & Sons, L.L.C.
----------
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use BARACLUDE safely and
effectively. See full prescribing information for BARACLUDE.
BARACLUDE (entecavir) tablets, for oral use
BARACLUDE (entecavir) oral solution
Initial U.S. Approval: 2005
WARNING: SEVERE ACUTE EXACERBATIONS OF HEPATITIS B, PATIENTS CO-INFECTED
WITH HIV AND HBV, and LACTIC ACIDOSIS AND HEPATOMEGALY
See full prescribing information for complete boxed warning.
•Severe acute exacerbations of hepatitis B have been reported in patients who have discontinued
anti-hepatitis B therapy, including entecavir. Hepatic function should be monitored closely for at least
several months after discontinuation. Initiation of anti-hepatitis B therapy may be warranted. (5.1)
•BARACLUDE is not recommended for patients co-infected with human immunodeficiency virus (HIV)
and hepatitis B virus (HBV) who are not also receiving highly active antiretroviral therapy (HAART),
because of the potential for the development of resistance to HIV nucleoside reverse transcriptase
inhibitors. (5.2)
•Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported
with the use of nucleoside analogue inhibitors. (5.3)
INDICATIONS AND USAGE
BARACLUDE is a hepatitis B virus nucleoside analogue reverse transcriptase inhibitor indicated for the
treatment of chronic hepatitis B virus infection in adults and children at least 2 years of age with evidence
of active viral replication and either evidence of persistent elevations in serum aminotransferases (ALT or
AST) or histologically active disease. (1)
DOSAGE AND ADMINISTRATION
•
•
•
•
•
•
DOSAGE FORMS AND STRENGTHS
•
•
CONTRAINDICATIONS
•
WARNINGS AND PRECAUTIONS
•
•
•
®
®
Nucleoside-inhibitor-treatment-naïve with compensated liver disease (greater than or equal to 16
years old): 0.5 mg once daily. (2.2)
Nucleoside-inhibitor-treatment-naïve and lamivudine-experienced pediatric patients at least 2 years
of age and weighing at least 10 kg: dosing is based on weight. (2.3)
Lamivudine-refractory or known lamivudine or telbivudine resistance substitutions (greater than or
equal to 16 years old): 1 mg once daily. (2.2)
Decompensated liver disease (adults): 1 mg once daily. (2.2)
Renal impairment: Dosage adjustment is recommended if creatinine clearance is less than 50
mL/min. (2.4)
BARACLUDE should be administered on an empty stomach. (2.1)
Tablets: 0.5 mg and 1 mg (3, 16)
Oral solution: 0.05 mg/mL (3, 16)
None. (4)
Severe acute exacerbations of hepatitis B virus infection after discontinuation: Monitor hepatic
function closely for at least several months. (5.1, 6.1)
Co-infection with HIV: BARACLUDE is not recommended unless the patient is also receiving HAART.
(5.2)
Lactic acidosis and severe hepatomegaly with steatosis: If suspected, treatment should be

•
ADVERSE REACTIONS
•
To report SUSPECTED ADVERSE REACTIONS, contact Bristol-Myers Squibb at 1-800-721-5072
or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
USE IN SPECIFIC POPULATIONS
•
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 11/2019
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: SEVERE ACUTE EXACERBATIONS OF HEPATITIS B, PATIENTS CO-
INFECTED WITH HIV AND HBV, and LACTIC ACIDOSIS AND HEPATOMEGALY
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Timing of Administration
2.2 Recommended Dosage in Adults
2.3 Recommended Dosage in Pediatric Patients
2.4 Renal Impairment
2.5 Hepatic Impairment
2.6 Duration of Therapy
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Severe Acute Exacerbations of Hepatitis B
5.2 Patients Co-infected with HIV and HBV
5.3 Lactic Acidosis and Severe Hepatomegaly with Steatosis
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Racial/Ethnic Groups
8.7 Renal Impairment
8.8 Liver Transplant Recipients
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Lactic acidosis and severe hepatomegaly with steatosis: If suspected, treatment should be
suspended. (5.3)
In adults, the most common adverse reactions (≥3%, all severity grades) are headache, fatigue,
dizziness, and nausea. The adverse reactions observed in pediatric patients were consistent with
those observed in adults. (6.1)
Liver transplant recipients: Limited data on safety and efficacy are available. (8.8)
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Outcomes in Adults
14.2 Outcomes in Pediatric Subjects
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
*
FULL PRESCRIBING INFORMATION
WARNING: SEVERE ACUTE EXACERBATIONS OF HEPATITIS B, PATIENTS
CO-INFECTED WITH HIV AND HBV, and LACTIC ACIDOSIS AND
HEPATOMEGALY
Severe acute exacerbations of hepatitis B have been reported in
patients who have discontinued anti-hepatitis B therapy, including
entecavir. Hepatic function should be monitored closely with both clinical
and laboratory follow-up for at least several months in patients who
discontinue anti-hepatitis B therapy. If appropriate, initiation of anti-
hepatitis B therapy may be warranted [see Warnings and Precautions
(5.1)].
Limited clinical experience suggests there is a potential for the
development of resistance to HIV (human immunodeficiency virus)
nucleoside reverse transcriptase inhibitors if BARACLUDE is used to
treat chronic hepatitis B virus (HBV) infection in patients with HIV
infection that is not being treated. Therapy with BARACLUDE is not
recommended for HIV/HBV co-infected patients who are not also
receiving highly active antiretroviral therapy (HAART) [see Warnings and
Precautions (5.2)].
Lactic acidosis and severe hepatomegaly with steatosis, including fatal
cases, have been reported with the use of nucleoside analogue
inhibitors alone or in combination with antiretrovirals [see Warnings and
Precautions (5.3)].
1 INDICATIONS AND USAGE
BARACLUDE (entecavir) is indicated for the treatment of chronic hepatitis B virus
infection in adults and pediatric patients 2 years of age and older with evidence of active
viral replication and either evidence of persistent elevations in serum aminotransferases
(ALT or AST) or histologically active disease.
Sections or subsections omitted from the full prescribing information are not listed.
®
2 DOSAGE AND ADMINISTRATION
2.1 Timing of Administration
BARACLUDE should be administered on an empty stomach (at least 2 hours after a meal
and 2 hours before the next meal).
2.2 Recommended Dosage in Adults
Compensated Liver Disease
The recommended dose of BARACLUDE for chronic hepatitis B virus infection in
nucleoside-inhibitor-treatment-naïve adults and adolescents 16 years of age and older is
0.5 mg once daily.
The recommended dose of BARACLUDE in adults and adolescents (at least 16 years of
age) with a history of hepatitis B viremia while receiving lamivudine or known lamivudine
or telbivudine resistance substitutions rtM204I/V with or without rtL180M, rtL80I/V, or
rtV173L is 1 mg once daily.
Decompensated Liver Disease
The recommended dose of BARACLUDE for chronic hepatitis B virus infection in adults
with decompensated liver disease is 1 mg once daily.
2.3 Recommended Dosage in Pediatric Patients
Table 1 describes the recommended dose of BARACLUDE for pediatric patients 2 years
of age or older and weighing at least 10 kg. The oral solution should be used for patients
with body weight up to 30 kg.
Table 1: Dosing Schedule for Pediatric Patients
Recommended Once-Daily Dose of Oral
Solution (mL)
Body Weight (kg) Treatment-Naïve
Patients
Lamivudine-Experienced
Patients
10 to 11 3 6
greater than 11 to 14 4 8
greater than 14 to 17 5 10
greater than 17 to 20 6 12
greater than 20 to 23 7 14
greater than 23 to 26 8 16
greater than 26 to 30 9 18
greater than 30 10 20
Children with body weight greater than 30 kg should receive 10 mL (0.5 mg) of oral
solution or one 0.5 mg tablet once daily.
Children with body weight greater than 30 kg should receive 20 mL (1 mg) of oral
solution or one 1 mg tablet once daily.
2.4 Renal Impairment
a b
a
b
In adult subjects with renal impairment, the apparent oral clearance of entecavir
decreased as creatinine clearance decreased [see Clinical Pharmacology (12.3)]. Dosage
adjustment is recommended for patients with creatinine clearance less than 50 mL/min,
including patients on hemodialysis or continuous ambulatory peritoneal dialysis (CAPD),
as shown in Table 2. The once-daily dosing regimens are preferred.
Table 2: Recommended Dosage of BARACLUDE in Adult Patients with Renal
Impairment
Creatinine
Clearance
(mL/min)
Usual Dose (0.5 mg) Lamivudine-Refractory or
Decompensated Liver Disease
(1 mg)
For doses less than 0.5 mg, BARACLUDE Oral Solution is recommended.
If administered on a hemodialysis day, administer BARACLUDE after the hemodialysis
session.
50 or greater 0.5 mg once daily 1 mg once daily
30 to less than 50 0.25 mg once daily
OR
0.5 mg every 48 hours
0.5 mg once daily
OR
1 mg every 48 hours
10 to less than 30 0.15 mg once daily
OR
0.5 mg every 72 hours
0.3 mg once daily
OR
1 mg every 72 hours
Less than 10
Hemodialysis or
CAPD
0.05 mg once daily
OR
0.5 mg every 7 days
0.1 mg once daily
OR
1 mg every 7 days
Although there are insufficient data to recommend a specific dose adjustment of
BARACLUDE in pediatric patients with renal impairment, a reduction in the dose or an
increase in the dosing interval similar to adjustments for adults should be considered.
2.5 Hepatic Impairment
No dosage adjustment is necessary for patients with hepatic impairment.
2.6 Duration of Therapy
The optimal duration of treatment with BARACLUDE for patients with chronic hepatitis B
virus infection and the relationship between treatment and long-term outcomes such as
cirrhosis and hepatocellular carcinoma are unknown.
3 DOSAGE FORMS AND STRENGTHS
•
•
•
a
b
a
a a
b
a a
BARACLUDE 0.5 mg film-coated tablets are white to off-white, triangular-shaped,
and debossed with “BMS” on one side and “1611” on the other side.
BARACLUDE 1 mg film-coated tablets are pink, triangular-shaped, and debossed
with “BMS” on one side and “1612” on the other side.
BARACLUDE oral solution, 0.05 mg/mL, is a ready-to-use, orange-flavored, clear,
colorless to pale yellow, aqueous solution. Ten milliliters of the oral solution provides
a 0.5 mg dose and 20 mL provides a 1 mg dose of entecavir.

4 CONTRAINDICATIONS
None.
5 WARNINGS AND PRECAUTIONS
5.1 Severe Acute Exacerbations of Hepatitis B
Severe acute exacerbations of hepatitis B have been reported in patients who have
discontinued anti-hepatitis B therapy, including entecavir [see Adverse Reactions (6.1)].
Hepatic function should be monitored closely with both clinical and laboratory follow-up
for at least several months in patients who discontinue anti-hepatitis B therapy. If
appropriate, initiation of anti-hepatitis B therapy may be warranted.
5.2 Patients Co-infected with HIV and HBV
BARACLUDE has not been evaluated in HIV/HBV co-infected patients who were not
simultaneously receiving effective HIV treatment. Limited clinical experience suggests
there is a potential for the development of resistance to HIV nucleoside reverse
transcriptase inhibitors if BARACLUDE is used to treat chronic hepatitis B virus infection
in patients with HIV infection that is not being treated [see Microbiology (12.4)].
Therefore, therapy with BARACLUDE is not recommended for HIV/HBV co-infected
patients who are not also receiving HAART. Before initiating BARACLUDE therapy, HIV
antibody testing should be offered to all patients. BARACLUDE has not been studied as a
treatment for HIV infection and is not recommended for this use.
5.3 Lactic Acidosis and Severe Hepatomegaly with Steatosis
Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been
reported with the use of nucleoside analogue inhibitors, including BARACLUDE, alone or
in combination with antiretrovirals. A majority of these cases have been in women.
Obesity and prolonged nucleoside inhibitor exposure may be risk factors. Particular
caution should be exercised when administering nucleoside analogue inhibitors to any
patient with known risk factors for liver disease; however, cases have also been
reported in patients with no known risk factors.
Lactic acidosis with BARACLUDE use has been reported, often in association with
hepatic decompensation, other serious medical conditions, or drug exposures. Patients
with decompensated liver disease may be at higher risk for lactic acidosis. Treatment
with BARACLUDE should be suspended in any patient who develops clinical or laboratory
findings suggestive of lactic acidosis or pronounced hepatotoxicity (which may include
hepatomegaly and steatosis even in the absence of marked transaminase elevations).
6 ADVERSE REACTIONS
The following adverse reactions are discussed in other sections of the labeling:
•
•
Exacerbations of hepatitis after discontinuation of treatment [see Boxed Warning,
Warnings and Precautions (5.1)].
Lactic acidosis and severe hepatomegaly with steatosis [see Boxed Warning,
•
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction
rates observed in the clinical trials of a drug cannot be directly compared to rates in the
clinical trials of another drug and may not reflect the rates observed in practice.
Clinical Trial Experience in Adults
Compensated Liver Disease
Assessment of adverse reactions is based on four studies (AI463014, AI463022,
AI463026, and AI463027) in which 1720 subjects with chronic hepatitis B virus infection
and compensated liver disease received double-blind treatment with BARACLUDE 0.5
mg/day (n=679), BARACLUDE 1 mg/day (n=183), or lamivudine (n=858) for up to 2
years. Median duration of therapy was 69 weeks for BARACLUDE-treated subjects and
63 weeks for lamivudine-treated subjects in Studies AI463022 and AI463027 and 73
weeks for BARACLUDE-treated subjects and 51 weeks for lamivudine-treated subjects in
Studies AI463026 and AI463014. The safety profiles of BARACLUDE and lamivudine
were comparable in these studies.
The most common adverse reactions of any severity (≥3%) with at least a possible
relation to study drug for BARACLUDE-treated subjects were headache, fatigue,
dizziness, and nausea. The most common adverse reactions among lamivudine-treated
subjects were headache, fatigue, and dizziness. One percent of BARACLUDE-treated
subjects in these four studies compared with 4% of lamivudine-treated subjects
discontinued for adverse events or abnormal laboratory test results.
Clinical adverse reactions of moderate-severe intensity and considered at least possibly
related to treatment occurring during therapy in four clinical studies in which
BARACLUDE was compared with lamivudine are presented in Table 3.
Table 3: Clinical Adverse Reactionsa of Moderate-Severe Intensity (Grades
2–4) Reported in Four Entecavir Clinical Trials Through 2 Years
Nucleoside-Inhibitor-
Naïve
Lamivudine- Refractory
Body System/
Adverse Reaction
BARACLUDE
0.5 mg
n=679
Lamivudine
100 mg
n=668
BARACLUDE
1 mg
n=183
Lamivudine
100 mg
n=190
Any Grade 2–4 adverse
reaction
15% 18% 22% 23%
Gastrointestinal
Diarrhea <1% 0 1% 0
Dyspepsia <1% <1% 1% 0
Nausea <1% <1% <1% 2%
Vomiting <1% <1% <1% 0
General
Fatigue 1% 1% 3% 3%
Nervous System
Lactic acidosis and severe hepatomegaly with steatosis [see Boxed Warning,
Warnings and Precautions (5.3)].
b
c
a
Includes events of possible, probable, certain, or unknown relationship to treatment
regimen.
Studies AI463022 and AI463027.
Includes Study AI463026 and the BARACLUDE 1 mg and lamivudine treatment arms
of Study AI463014, a Phase 2 multinational, randomized, double-blind study of three
doses of BARACLUDE (0.1, 0.5, and 1 mg) once daily versus continued lamivudine 100
mg once daily for up to 52 weeks in subjects who experienced recurrent viremia on
lamivudine therapy.
Headache 2% 2% 4% 1%
Dizziness <1% <1% 0 1%
Somnolence <1% <1% 0 0
Psychiatric
Insomnia <1% <1% 0 <1%
Laboratory Abnormalities
Frequencies of selected treatment-emergent laboratory abnormalities reported during
therapy in four clinical trials of BARACLUDE compared with lamivudine are listed in Table
4.
Table 4: Selected Treatment-Emergent Laboratory Abnormalities Reported
in Four Entecavir Clinical Trials Through 2 Years
Nucleoside-Inhibitor-
Naïve
Lamivudine-Refractory
Test
BARACLUDE
0.5 mg
n=679
Lamivudine
100 mg
n=668
BARACLUDE
1 mg
n=183
Lamivudine
100 mg
n=190
On-treatment value worsened from baseline to Grade 3 or Grade 4 for all parameters
except albumin (any on-treatment value <2.5 g/dL), confirmed creatinine increase ≥0.5
mg/dL, and ALT >10 × ULN and >2 × baseline.
Studies AI463022 and AI463027.
Any Grade 3–4 laboratory
abnormality
35% 36% 37% 45%
ALT >10 × ULN and >2 ×
baseline
2% 4% 2% 11%
ALT >5 × ULN 11% 16% 12% 24%
Albumin <2.5 g/dL <1% <1% 0 2%
Total bilirubin >2.5 × ULN 2% 2% 3% 2%
Lipase ≥2.1 × ULN 7% 6% 7% 7%
Creatinine >3 × ULN 0 0 0 0
Confirmed creatinine increase
≥0.5 mg/dL
1% 1% 2% 1%
Hyperglycemia, fasting >250
mg/dL
2% 1% 3% 1%
Glycosuria 4% 3% 4% 6%
Hematuria 9% 10% 9% 6%
Platelets <50,000/mm <1% <1% <1% <1%
a
b
c
a
b
c
a
b
d
e
f
3
Studies AI463022 and AI463027.
Includes Study AI463026 and the BARACLUDE 1 mg and lamivudine treatment arms
of Study AI463014, a Phase 2 multinational, randomized, double-blind study of three
doses of BARACLUDE (0.1, 0.5, and 1 mg) once daily versus continued lamivudine 100
mg once daily for up to 52 weeks in subjects who experienced recurrent viremia on
lamivudine therapy.
Includes hematology, routine chemistries, renal and liver function tests, pancreatic
enzymes, and urinalysis.
Grade 3 = 3+, large, ≥500 mg/dL; Grade 4 = 4+, marked, severe.
Grade 3 = 3+, large; Grade 4 = ≥4+, marked, severe, many.
ULN=upper limit of normal.
Among BARACLUDE-treated subjects in these studies, on-treatment ALT elevations
greater than 10 times the upper limit of normal (ULN) and greater than 2 times baseline
generally resolved with continued treatment. A majority of these exacerbations were
associated with a ≥2 log /mL reduction in viral load that preceded or coincided with the
ALT elevation. Periodic monitoring of hepatic function is recommended during treatment.
Exacerbations of Hepatitis After Discontinuation of Treatment
An exacerbation of hepatitis or ALT flare was defined as ALT greater than 10 times ULN
and greater than 2 times the subject’s reference level (minimum of the baseline or last
measurement at end of dosing). For all subjects who discontinued treatment (regardless
of reason), Table 5 presents the proportion of subjects in each study who experienced
post-treatment ALT flares. In these studies, a subset of subjects was allowed to
discontinue treatment at or after 52 weeks if they achieved a protocol-defined response
to therapy. If BARACLUDE is discontinued without regard to treatment response, the
rate of post-treatment flares could be higher. [See Warnings and Precautions (5.1).]
Table 5: Exacerbations of Hepatitis During Off-Treatment Follow-up,
Subjects in Studies AI463022, AI463027, and AI463026
Subjects with ALT Elevations >10 × ULN and >2 ×
Reference
BARACLUDE Lamivudine
a Reference is the minimum of the baseline or last measurement at end of dosing.
Median time to off-treatment exacerbation was 23 weeks for BARACLUDE-treated
subjects and 10 weeks for lamivudine-treated subjects.
Nucleoside-inhibitor-naïve
HBeAg-positive 4/174 (2%) 13/147 (9%)
HBeAg-negative 24/302 (8%) 30/270 (11%)
Lamivudine-refractory 6/52 (12%) 0/16
Decompensated Liver Disease
Study AI463048 was a randomized, open-label study of BARACLUDE 1 mg once daily
versus adefovir dipivoxil 10 mg once daily given for up to 48 weeks in adult subjects with
chronic HBV infection and evidence of hepatic decompensation, defined as a Child-
Turcotte-Pugh (CTP) score of 7 or higher [see Clinical Studies (14.1)]. Among the 102
subjects receiving BARACLUDE, the most common treatment-emergent adverse events
c
d
e
f
10
a

of any severity, regardless of causality, occurring through Week 48 were peripheral
edema (16%), ascites (15%), pyrexia (14%), hepatic encephalopathy (10%), and upper
respiratory infection (10%). Clinical adverse reactions not listed in Table 3 that were
observed through Week 48 include blood bicarbonate decreased (2%) and renal failure
(<1%).
Eighteen of 102 (18%) subjects treated with BARACLUDE and 18/89 (20%) subjects
treated with adefovir dipivoxil died during the first 48 weeks of therapy. The majority of
deaths (11 in the BARACLUDE group and 16 in the adefovir dipivoxil group) were due to
liver-related causes such as hepatic failure, hepatic encephalopathy, hepatorenal
syndrome, and upper gastrointestinal hemorrhage. The rate of hepatocellular carcinoma
(HCC) through Week 48 was 6% (6/102) for subjects treated with BARACLUDE and 8%
(7/89) for subjects treated with adefovir dipivoxil. Five percent of subjects in either
treatment arm discontinued therapy due to an adverse event through Week 48.
No subject in either treatment arm experienced an on-treatment hepatic flare (ALT >2 ×
baseline and >10 × ULN) through Week 48. Eleven of 102 (11%) subjects treated with
BARACLUDE and 11/89 (13%) subjects treated with adefovir dipivoxil had a confirmed
increase in serum creatinine of 0.5 mg/dL through Week 48.
HIV/HBV Co-infected
The safety profile of BARACLUDE 1 mg (n=51) in HIV/HBV co-infected subjects enrolled
in Study AI463038 was similar to that of placebo (n=17) through 24 weeks of blinded
treatment and similar to that seen in non-HIV infected subjects [see Warnings and
Precautions (5.2)].
Liver Transplant Recipients
Among 65 subjects receiving BARACLUDE in an open-label, post-liver transplant trial [see
Use in Specific Populations (8.8)], the frequency and nature of adverse events were
consistent with those expected in patients who have received a liver transplant and the
known safety profile of BARACLUDE.
Clinical Trial Experience in Pediatric Subjects
The safety of BARACLUDE in pediatric subjects 2 to less than 18 years of age is based
on two clinical trials in subjects with chronic HBV infection (one Phase 2 pharmacokinetic
trial [AI463028] and one Phase 3 trial [AI463189]). These trials provided experience in
168 HBeAg-positive subjects treated with BARACLUDE for a median duration of 72
weeks. The adverse reactions observed in pediatric subjects who received treatment
with BARACLUDE were consistent with those observed in clinical trials of BARACLUDE in
adults. Adverse drug reactions reported in greater than 1% of pediatric subjects
included abdominal pain, rash events, poor palatability (“product taste abnormal”),
nausea, diarrhea, and vomiting.
6.2 Postmarketing Experience
Data from Long-Term Observational Study
Study AI463080 was a randomized, global, observational, open-label Phase 4 study to
assess long-term risks and benefits of BARACLUDE (0.5 mg/day or 1 mg/day) treatment
as compared to other standard-of-care HBV nucleos(t)ide analogues in subjects with
chronic HBV infection.
A total of 12,378 patients were treated with BARACLUDE (n=6,216) or other HBV
nucleos(t)ide treatment [non-entecavir (ETV)] (n=6,162). Patients were evaluated at
baseline and subsequently every 6 months for up to 10 years. The principal clinical
outcome events assessed during the study were overall malignant neoplasms, liver-
related HBV disease progression, HCC, non-HCC malignant neoplasms, and death. The
study showed that BARACLUDE was not significantly associated with an increased risk
of malignant neoplasms compared to other standard-of-care HBV nucleos(t)ides, as
assessed by either the composite endpoint of overall malignant neoplasms or the
individual endpoint of non-HCC malignant neoplasms. The most commonly reported
malignancy in both the BARACLUDE and non-ETV groups was HCC followed by
gastrointestinal malignancies. The data also showed that long-term BARACLUDE use was
not associated with a lower occurrence of HBV disease progression or a lower rate of
death overall compared to other HBV nucleos(t)ides. The principal clinical outcome event
assessments are shown in Table 6.
Table 6: Principal Analyses of Time to Adjudicated Events - Randomized
Treated Subjects
Number of Subjects with
Events
Endpoint
BARACLUDE
N=6,216
Non-ETV
N=6,162
Hazard Ratio
[BARACLUDE:Non-
ETV] (CI )
Primary Endpoints
Overall malignant neoplasm 331 337 0.93 (0.800, 1.084)
Liver-related HBV disease
progression
350 375 0.89 (0.769, 1.030)
Death 238 264 0.85 (0.713, 1.012)
Secondary Endpoints
Non-HCC malignant neoplasm 95 81 1.10 (0.817, 1.478)
HCC 240 263 0.87 (0.727, 1.032)
Analyses were stratified by geographic region and prior HBV nucleos(t)ide experience.
95.03% CI for overall malignant neoplasm, death, and liver-related HBV disease
progression; 95% CI for non-HCC malignant neoplasm and HCC.
One subject had a pre-treatment HCC event and was excluded from the analysis.
Overall malignant neoplasm is a composite event of HCC or non-HCC malignant
neoplasm. Liver-related HBV disease progression is a composite event of liver-related
death, HCC, or non-HCC HBV disease progression.
CI = confidence interval; N = total number of subjects.
Limitations of the study included population changes over the long-term follow-up period
and more frequent post-randomization treatment changes in the non-ETV group. In
addition, the study was underpowered to demonstrate a difference in the non-HCC
malignancy rate because of the lower than expected background rate.
Adverse Reactions from Postmarketing Spontaneous Reports
The following adverse reactions have been reported during postmarketing use of
c
a
b
a
b
c

BARACLUDE. Because these reactions were reported voluntarily from a population of
unknown size, it is not possible to reliably estimate their frequency or establish a causal
relationship to BARACLUDE exposure.
Immune system disorders: Anaphylactoid reaction.
Metabolism and nutrition disorders: Lactic acidosis.
Hepatobiliary disorders: Increased transaminases.
Skin and subcutaneous tissue disorders: Alopecia, rash.
7 DRUG INTERACTIONS
Since entecavir is primarily eliminated by the kidneys [see Clinical Pharmacology (12.3)],
coadministration of BARACLUDE with drugs that reduce renal function or compete for
active tubular secretion may increase serum concentrations of either entecavir or the
coadministered drug. Coadministration of entecavir with lamivudine, adefovir dipivoxil, or
tenofovir disoproxil fumarate did not result in significant drug interactions. The effects of
coadministration of BARACLUDE with other drugs that are renally eliminated or are
known to affect renal function have not been evaluated, and patients should be
monitored closely for adverse events when BARACLUDE is coadministered with such
drugs.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women
exposed to BARACLUDE during pregnancy. Healthcare providers are encouraged to
register patients by calling the Antiretroviral Pregnancy Registry (APR) at 1-800-258-
4263.
Risk Summary
Prospective pregnancy data from the APR are not sufficient to adequately assess the
risk of birth defects, miscarriage or adverse maternal or fetal outcomes. Entecavir use
during pregnancy has been evaluated in a limited number of individuals reported to the
APR and the number of exposures to entecavir is insufficient to make a risk assessment
compared to a reference population. The estimated background rate for major birth
defects is 2.7% in the U.S. reference population of the Metropolitan Atlanta Congenital
Defects Program (MACDP). The rate of miscarriage is not reported in the APR. All
pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In
the U.S. general population, the estimated background risk of miscarriage in clinically
recognized pregnancies is 15–20%.
In animal reproduction studies, no adverse developmental effects were observed with
entecavir at clinically relevant exposures. No developmental toxicities were observed at
systemic exposures (AUC) approximately 25 (rats) and 200 (rabbits) times the
exposure at the maximum recommended human dose (MRHD) of 1 mg/day (see Data).
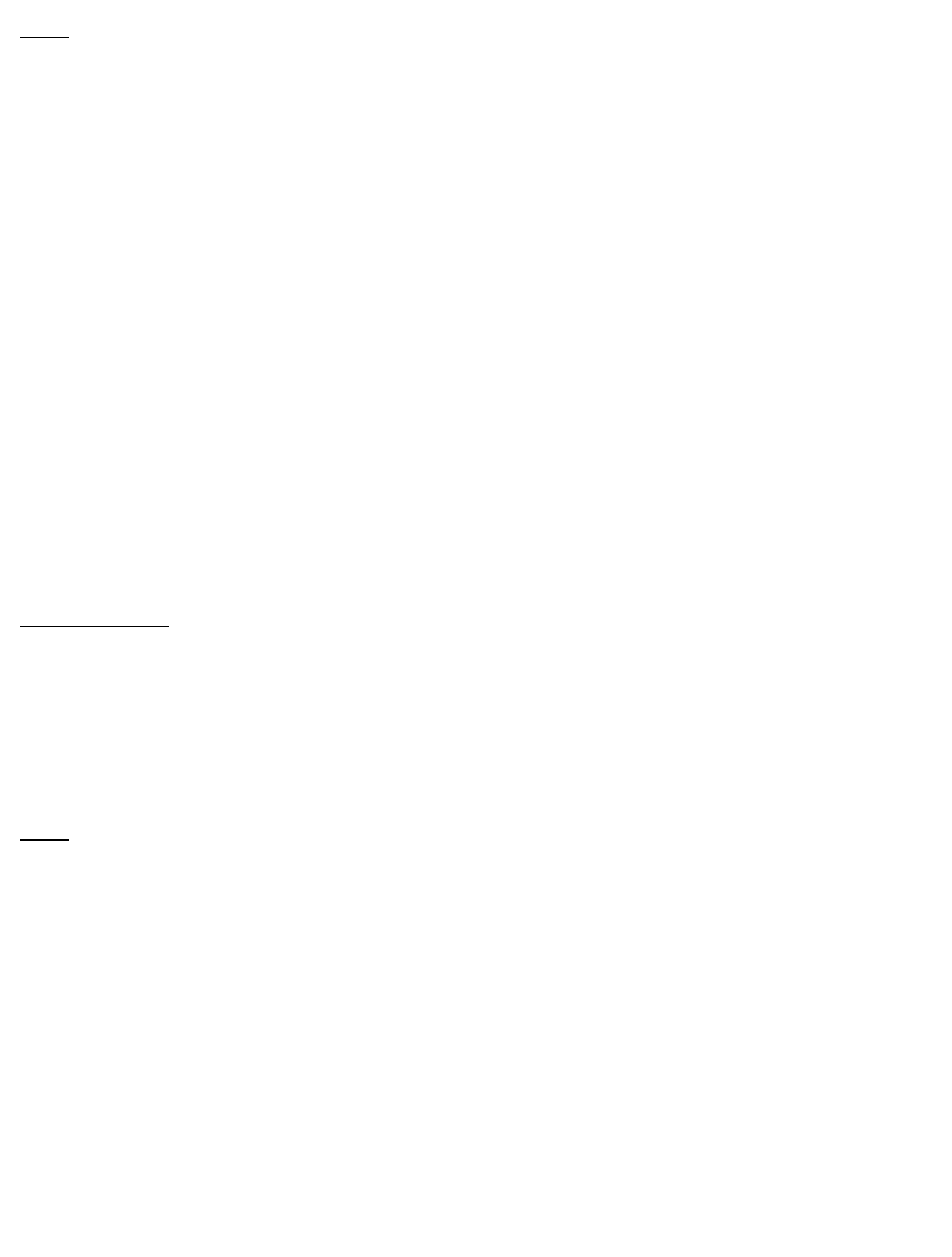
Data
Animal Data
Entecavir was administered orally to pregnant rats (at 2, 20, and 200 mg per kg per
day) and rabbits (at 1, 4, and 16 mg per kg per day) during organogenesis (on gestation
Days 6 through 15 [rat] and 6 through 18 [rabbit]). In rats, embryofetal toxicity
including post-implantation loss, resorptions, tail and vertebral malformations, skeletal
variations including reduced ossification (vertebrate, sternebrae, and phalanges) and
extra lumbar vertebrae and ribs, and lower fetal body weights were observed at
systemic exposures (AUC) 3,100 times those in humans at the MRHD. Maternal toxicity
was also observed at this dose level. In rabbits, embryofetal toxicity including post-
implantation loss, resorptions and skeletal variations, including reduced ossification
(hyoid) and increased incidence of 13 rib, were observed at systemic exposures (AUC)
883 times those in humans at the MRHD. There were no signs of embryofetal toxicity
when pregnant animals received oral entecavir at 28 (rat) and 212 (rabbit) times the
human exposure (AUC) at the MRHD. In a pre/postnatal development study, entecavir
was administered orally to pregnant rats at 0.3, 3, and 30 mg per kg per day from
gestation day 6 to lactation/post-partum day 20. No adverse effects on the offspring
occurred at up to the highest dose evaluated, resulting in exposures (AUC) greater than
94 times those in humans at the MRHD.
8.2 Lactation
Risk Summary
It is not known whether BARACLUDE is present in human breast milk, affects human
milk production, or has effects on the breastfed infant. When administered to lactating
rats, entecavir was present in milk (see Data). The developmental and health benefits of
breastfeeding should be considered along with the mother’s clinical need for
BARACLUDE and any potential adverse effects on the breastfed infant from
BARACLUDE or from the underlying maternal condition.
Data
Entecavir was excreted into the milk of lactating rats following a single oral dose of 10
mg per kg on lactation day 7. Entecavir in milk was approximately 25% that in maternal
plasma (based on AUC).
8.4 Pediatric Use
BARACLUDE was evaluated in two clinical trials of pediatric subjects 2 years of age and
older with HBeAg-positive chronic HBV infection and compensated liver disease. The
exposure of BARACLUDE in nucleoside-inhibitor-treatment-naïve and lamivudine-
experienced pediatric subjects 2 years of age and older with HBeAg-positive chronic
HBV infection and compensated liver disease receiving 0.015 mg/kg (up to 0.5 mg once
daily) or 0.03 mg/kg (up to 1 mg once daily), respectively, was evaluated in Study
AI463028. Safety and efficacy of the selected dose in treatment-naïve pediatric subjects
were confirmed in Study AI463189, a randomized, placebo-controlled treatment trial
[see Indications and Usage (1), Dosage and Administration (2.3), Adverse Reactions
(6.1), Clinical Pharmacology (12.3), and Clinical Studies (14.2)].
th

There are limited data available on the use of BARACLUDE in lamivudine-experienced
pediatric patients; BARACLUDE should be used in these patients only if the potential
benefit justifies the potential risk to the child. Since some pediatric patients may require
long-term or even lifetime management of chronic active hepatitis B, consideration
should be given to the impact of BARACLUDE on future treatment options [see
Microbiology (12.4)].
The efficacy and safety of BARACLUDE have not been established in patients less than 2
years of age. Use of BARACLUDE in this age group has not been evaluated because
treatment of HBV in this age group is rarely required.
8.5 Geriatric Use
Clinical studies of BARACLUDE did not include sufficient numbers of subjects aged 65
years and over to determine whether they respond differently from younger subjects.
Entecavir is substantially excreted by the kidney, and the risk of toxic reactions to this
drug may be greater in patients with impaired renal function. Because elderly patients
are more likely to have decreased renal function, care should be taken in dose selection,
and it may be useful to monitor renal function [see Dosage and Administration (2.4)].
8.6 Racial/Ethnic Groups
There are no significant racial differences in entecavir pharmacokinetics. The safety and
efficacy of BARACLUDE 0.5 mg once daily were assessed in a single-arm, open-label trial
of HBeAg-positive or -negative, nucleoside-inhibitor-naïve, Black/African American
(n=40) and Hispanic (n=6) subjects with chronic HBV infection. In this trial, 76% of
subjects were male, the mean age was 42 years, 57% were HBeAg-positive, the mean
baseline HBV DNA was 7.0 log IU/mL, and the mean baseline ALT was 162 U/L. At
Week 48 of treatment, 32 of 46 (70%) subjects had HBV DNA <50 IU/mL
(approximately 300 copies/mL), 31 of 46 (67%) subjects had ALT normalization (≤1 ×
ULN), and 12 of 26 (46%) HBeAg-positive subjects had HBe seroconversion. Safety data
were similar to those observed in the larger controlled clinical trials.
Because of low enrollment, safety and efficacy have not been established in the US
Hispanic population.
8.7 Renal Impairment
Dosage adjustment of BARACLUDE is recommended for patients with creatinine
clearance less than 50 mL/min, including patients on hemodialysis or CAPD [see Dosage
and Administration (2.4) and Clinical Pharmacology (12.3)].
8.8 Liver Transplant Recipients
The safety and efficacy of BARACLUDE were assessed in a single-arm, open-label trial in
65 subjects who received a liver transplant for complications of chronic HBV infection.
Eligible subjects who had HBV DNA less than 172 IU/mL (approximately 1000 copies/mL)
at the time of transplant were treated with BARACLUDE 1 mg once daily in addition to
usual post-transplantation management, including hepatitis B immune globulin. The trial
population was 82% male, 39% Caucasian, and 37% Asian, with a mean age of 49 years;
89% of subjects had HBeAg-negative disease at the time of transplant.
Four of the 65 subjects received 4 weeks or less of BARACLUDE (2 deaths, 1 re-
10
transplantation, and 1 protocol violation) and were not considered evaluable. Of the 61
subjects who received more than 4 weeks of BARACLUDE, 60 received hepatitis B
immune globulin post-transplant. Fifty-three subjects (82% of all 65 subjects treated)
completed the trial and had HBV DNA measurements at or after 72 weeks treatment
post-transplant. All 53 subjects had HBV DNA <50 IU/mL (approximately 300
copies/mL). Eight evaluable subjects did not have HBV DNA data available at 72 weeks,
including 3 subjects who died prior to study completion. No subjects had HBV DNA
values ≥50 IU/mL while receiving BARACLUDE (plus hepatitis B immune globulin). All 61
evaluable subjects lost HBsAg post-transplant; 2 of these subjects experienced
recurrence of measurable HBsAg without recurrence of HBV viremia. This trial was not
designed to determine whether addition of BARACLUDE to hepatitis B immune globulin
decreased the proportion of subjects with measurable HBV DNA post-transplant
compared to hepatitis B immune globulin alone.
If BARACLUDE treatment is determined to be necessary for a liver transplant recipient
who has received or is receiving an immunosuppressant that may affect renal function,
such as cyclosporine or tacrolimus, renal function must be carefully monitored both
before and during treatment with BARACLUDE [see Dosage and Administration (2.4) and
Clinical Pharmacology (12.3)].
10 OVERDOSAGE
There is limited experience of entecavir overdosage reported in patients. Healthy
subjects who received single entecavir doses up to 40 mg or multiple doses up to 20
mg/day for up to 14 days had no increase in or unexpected adverse events. If overdose
occurs, the patient must be monitored for evidence of toxicity, and standard supportive
treatment applied as necessary.
Following a single 1 mg dose of entecavir, a 4-hour hemodialysis session removed
approximately 13% of the entecavir dose.
11 DESCRIPTION
BARACLUDE is the tradename for entecavir, a guanosine nucleoside analogue with
selective activity against HBV. The chemical name for entecavir is 2-amino-1,9-dihydro-9-
[(1S,3R,4S)-4-hydroxy-3-(hydroxymethyl)-2-methylenecyclopentyl]-6H-purin-6-one,
monohydrate. Its molecular formula is C H N O ∙H O, which corresponds to a
molecular weight of 295.3. Entecavir has the following structural formula:
®
12 15 5 3 2

Entecavir is a white to off-white powder. It is slightly soluble in water (2.4 mg/mL), and
the pH of the saturated solution in water is 7.9 at 25° C ± 0.5° C.
BARACLUDE film-coated tablets are available for oral administration in strengths of 0.5
mg and 1 mg of entecavir. BARACLUDE 0.5 mg and 1 mg film-coated tablets contain the
following inactive ingredients: lactose monohydrate, microcrystalline cellulose,
crospovidone, povidone, and magnesium stearate. The tablet coating contains titanium
dioxide, hypromellose, polyethylene glycol 400, polysorbate 80 (0.5 mg tablet only), and
iron oxide red (1 mg tablet only). BARACLUDE Oral Solution is available for oral
administration as a ready-to-use solution containing 0.05 mg of entecavir per milliliter.
BARACLUDE Oral Solution contains the following inactive ingredients: maltitol, sodium
citrate, citric acid, methylparaben, propylparaben, and orange flavor.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Entecavir is an antiviral drug against hepatitis B virus [see Microbiology (12.4)].
12.3 Pharmacokinetics
The single- and multiple-dose pharmacokinetics of entecavir were evaluated in healthy
subjects and subjects with chronic hepatitis B virus infection.
Absorption
Following oral administration in healthy subjects, entecavir peak plasma concentrations
occurred between 0.5 and 1.5 hours. Following multiple daily doses ranging from 0.1 to
1 mg, C and area under the concentration-time curve (AUC) at steady state
increased in proportion to dose. Steady state was achieved after 6 to 10 days of once-
daily administration with approximately 2-fold accumulation. For a 0.5 mg oral dose,
C at steady state was 4.2 ng/mL and trough plasma concentration (C ) was 0.3
ng/mL. For a 1 mg oral dose, C was 8.2 ng/mL and C was 0.5 ng/mL.
In healthy subjects, the bioavailability of the tablet was 100% relative to the oral solution.
The oral solution and tablet may be used interchangeably.
Effects of food on oral absorption: Oral administration of 0.5 mg of entecavir with a
standard high-fat meal (945 kcal, 54.6 g fat) or a light meal (379 kcal, 8.2 g fat) resulted
in a delay in absorption (1.0–1.5 hours fed vs. 0.75 hours fasted), a decrease in C of
44%–46%, and a decrease in AUC of 18%–20% [see Dosage and Administration (2)].
Distribution
Based on the pharmacokinetic profile of entecavir after oral dosing, the estimated
apparent volume of distribution is in excess of total body water, suggesting that
entecavir is extensively distributed into tissues.
Binding of entecavir to human serum proteins in vitro was approximately 13%.
Metabolism and Elimination
Following administration of C-entecavir in humans and rats, no oxidative or acetylated
max
max trough
max trough
max
14
metabolites were observed. Minor amounts of phase II metabolites (glucuronide and
sulfate conjugates) were observed. Entecavir is not a substrate, inhibitor, or inducer of
the cytochrome P450 (CYP450) enzyme system. See Drug Interactions, below.
After reaching peak concentration, entecavir plasma concentrations decreased in a bi-
exponential manner with a terminal elimination half-life of approximately 128–149 hours.
The observed drug accumulation index is approximately 2-fold with once-daily dosing,
suggesting an effective accumulation half-life of approximately 24 hours.
Entecavir is predominantly eliminated by the kidney with urinary recovery of unchanged
drug at steady state ranging from 62% to 73% of the administered dose. Renal
clearance is independent of dose and ranges from 360 to 471 mL/min suggesting that
entecavir undergoes both glomerular filtration and net tubular secretion [see Drug
Interactions (7)].
Special Populations
Gender: There are no significant gender differences in entecavir pharmacokinetics.
Race: There are no significant racial differences in entecavir pharmacokinetics.
Elderly: The effect of age on the pharmacokinetics of entecavir was evaluated following
administration of a single 1 mg oral dose in healthy young and elderly volunteers.
Entecavir AUC was 29.3% greater in elderly subjects compared to young subjects. The
disparity in exposure between elderly and young subjects was most likely attributable to
differences in renal function. Dosage adjustment of BARACLUDE should be based on the
renal function of the patient, rather than age [see Dosage and Administration (2.4)].
Pediatrics: The steady-state pharmacokinetics of entecavir were evaluated in nucleoside-
inhibitor-naïve and lamivudine-experienced HBeAg-positive pediatric subjects 2 to less
than 18 years of age with compensated liver disease. Results are shown in Table 7.
Entecavir exposure among nucleoside-inhibitor-naïve subjects was similar to the
exposure achieved in adults receiving once-daily doses of 0.5 mg. Entecavir exposure
among lamivudine-experienced subjects was similar to the exposure achieved in adults
receiving once-daily doses of 1 mg.
Table 7: Pharmacokinetic Parameters in Pediatric Subjects
Subjects received once-daily doses of 0.015 mg/kg up to a maximum of 0.5 mg.
Subjects received once-daily doses of 0.030 mg/kg up to a maximum of 1 mg.
Nucleoside-Inhibitor-
Naïve
Lamivudine- Experienced
n=24 n=19
C (ng/mL)
(CV%)
6.31
(30)
14.48
(31)
AUC – (ng•h/mL)
(CV%)
18.33
(27)
38.58
(26)
C (ng/mL)
(CV%)
0.28
(22)
0.47
(23)
Renal impairment: The pharmacokinetics of entecavir following a single 1 mg dose were
studied in subjects (without chronic hepatitis B virus infection) with selected degrees of
a
b
a
b
max
(0 24)
min
renal impairment, including subjects whose renal impairment was managed by
hemodialysis or continuous ambulatory peritoneal dialysis (CAPD). Results are shown in
Table 8 [see Dosage and Administration (2.4)].
Table 8: Pharmacokinetic Parameters in Subjects with Selected Degrees of
Renal Function
Dosed immediately following hemodialysis.
CLR = renal clearance; CLT/F = apparent oral clearance.
Renal Function Group
Baseline Creatinine Clearance
(mL/min)
Unimpaired
>80
Mild
>50–≤80
Moderate
30–50
Severe
<30
Severe
Managed
with
Hemodialysis
Severe
Managed
with CAPD
n=6 n=6 n=6 n=6 n=6 n=4
C (ng/mL)
(CV%)
8.1
(30.7)
10.4
(37.2)
10.5
(22.7)
15.3
(33.8)
15.4
(56.4)
16.6
(29.7)
AUC –
(ng•h/mL)
(CV)
27.9
(25.6)
51.5
(22.8)
69.5
(22.7)
145.7
(31.5)
233.9
(28.4)
221.8
(11.6)
CLR (mL/min)
(SD)
383.2
(101.8)
197.9
(78.1)
135.6
(31.6)
40.3
(10.1)
NA NA
CLT/F (mL/min)
(SD)
588.1
(153.7)
309.2
(62.6)
226.3
(60.1)
100.6
(29.1)
50.6
(16.5)
35.7
(19.6)
Following a single 1 mg dose of entecavir administered 2 hours before the hemodialysis
session, hemodialysis removed approximately 13% of the entecavir dose over 4 hours.
CAPD removed approximately 0.3% of the dose over 7 days [see Dosage and
Administration (2.4)].
Hepatic impairment: The pharmacokinetics of entecavir following a single 1 mg dose
were studied in adult subjects (without chronic hepatitis B virus infection) with moderate
or severe hepatic impairment (Child-Turcotte-Pugh Class B or C). The pharmacokinetics
of entecavir were similar between hepatically impaired and healthy control subjects;
therefore, no dosage adjustment of BARACLUDE is recommended for patients with
hepatic impairment. The pharmacokinetics of entecavir have not been studied in
pediatric subjects with hepatic impairment.
Post-liver transplant: Limited data are available on the safety and efficacy of BARACLUDE
in liver transplant recipients. In a small pilot study of entecavir use in HBV-infected liver
transplant recipients on a stable dose of cyclosporine A (n=5) or tacrolimus (n=4),
entecavir exposure was approximately 2-fold the exposure in healthy subjects with
normal renal function. Altered renal function contributed to the increase in entecavir
exposure in these subjects. The potential for pharmacokinetic interactions between
entecavir and cyclosporine A or tacrolimus was not formally evaluated [see Use in
Specific Populations (8.8)].
a
a
max
(0 T)

Drug Interactions
The metabolism of entecavir was evaluated in in vitro and in vivo studies. Entecavir is not
a substrate, inhibitor, or inducer of the cytochrome P450 (CYP450) enzyme system. At
concentrations up to approximately 10,000-fold higher than those obtained in humans,
entecavir inhibited none of the major human CYP450 enzymes 1A2, 2C9, 2C19, 2D6,
3A4, 2B6, and 2E1. At concentrations up to approximately 340-fold higher than those
observed in humans, entecavir did not induce the human CYP450 enzymes 1A2, 2C9,
2C19, 3A4, 3A5, and 2B6. The pharmacokinetics of entecavir are unlikely to be affected
by coadministration with agents that are either metabolized by, inhibit, or induce the
CYP450 system. Likewise, the pharmacokinetics of known CYP substrates are unlikely to
be affected by coadministration of entecavir.
The steady-state pharmacokinetics of entecavir and coadministered drug were not
altered in interaction studies of entecavir with lamivudine, adefovir dipivoxil, and
tenofovir disoproxil fumarate [see Drug Interactions (7)].
12.4 Microbiology
Mechanism of Action
Entecavir, a deoxyguanosine nucleoside analogue with activity against HBV reverse
transcriptase (rt), is efficiently phosphorylated to the active triphosphate form, which
has an intracellular half-life of 15 hours. By competing with the natural substrate
deoxyguanosine triphosphate, entecavir triphosphate functionally inhibits all three
activities of the HBV reverse transcriptase: (1) base priming, (2) reverse transcription of
the negative strand from the pregenomic messenger RNA, and (3) synthesis of the
positive strand of HBV DNA. Entecavir triphosphate is a weak inhibitor of cellular DNA
polymerases α, β, and δ and mitochondrial DNA polymerase γ with K values ranging
from 18 to >160 μM.
Antiviral Activity
Entecavir inhibited HBV DNA synthesis (50% reduction, EC ) at a concentration of
0.004 μM in human HepG2 cells transfected with wild-type HBV. The median EC value
for entecavir against lamivudine-resistant HBV (rtL180M, rtM204V) was 0.026 μM (range
0.010–0.059 μM).
The coadministration of HIV nucleoside/nucleotide reverse transcriptase inhibitors
(NRTIs) with BARACLUDE is unlikely to reduce the antiviral efficacy of BARACLUDE
against HBV or of any of these agents against HIV. In HBV combination assays in cell
culture, abacavir, didanosine, lamivudine, stavudine, tenofovir, or zidovudine were not
antagonistic to the anti-HBV activity of entecavir over a wide range of concentrations. In
HIV antiviral assays, entecavir was not antagonistic to the cell culture anti-HIV activity of
these six NRTIs or emtricitabine at concentrations greater than 100 times the C of
entecavir using the 1 mg dose.
Antiviral Activity Against HIV
A comprehensive analysis of the inhibitory activity of entecavir against a panel of
laboratory and clinical HIV type 1 (HIV-1) isolates using a variety of cells and assay
conditions yielded EC values ranging from 0.026 to >10 μM; the lower EC values
were observed when decreased levels of virus were used in the assay. In cell culture,
i
50
50
max
50 50

entecavir selected for an M184I substitution in HIV reverse transcriptase at micromolar
concentrations, confirming inhibitory pressure at high entecavir concentrations. HIV
variants containing the M184V substitution showed loss of susceptibility to entecavir.
Resistance
In Cell Culture
In cell-based assays, 8- to 30-fold reductions in entecavir phenotypic susceptibility were
observed for lamivudine-resistant strains. Further reductions (>70-fold) in entecavir
phenotypic susceptibility required the presence of amino acid substitutions rtM204I/V
with or without rtL180M along with additional substitutions at residues rtT184, rtS202,
or rtM250, or a combination of these substitutions with or without an rtI169 substitution
in the HBV reverse transcriptase. Lamivudine-resistant strains harboring rtL180M plus
rtM204V in combination with the amino acid substitution rtA181C conferred 16- to 122-
fold reductions in entecavir phenotypic susceptibility.
Clinical Studies
Nucleoside-inhibitor-naïve subjects: Genotypic evaluations were performed on evaluable
samples (>300 copies/mL serum HBV DNA) from 562 subjects who were treated with
BARACLUDE for up to 96 weeks in nucleoside-inhibitor-naïve studies (AI463022,
AI463027, and rollover study AI463901). By Week 96, evidence of emerging amino acid
substitution rtS202G with rtL180M and rtM204V substitutions was detected in the HBV
of 2 subjects (2/562=<1%), and 1 of them experienced virologic rebound (≥1 log
increase above nadir). In addition, emerging amino acid substitutions at rtM204I/V and
rtL80I, rtV173L, or rtL180M, which conferred decreased phenotypic susceptibility to
entecavir in the absence of rtT184, rtS202, or rtM250 changes, were detected in the
HBV of 3 subjects (3/562=<1%) who experienced virologic rebound. For subjects who
continued treatment beyond 48 weeks, 75% (202/269) had HBV DNA <300 copies/mL
at end of dosing (up to 96 weeks).
HBeAg-positive (n=243) and -negative (n=39) treatment-naïve subjects who failed to
achieve the study-defined complete response by 96 weeks were offered continued
entecavir treatment in a rollover study. Complete response for HBeAg-positive was <0.7
MEq/mL (approximately 7 × 10 copies/mL) serum HBV DNA and HBeAg loss and, for
HBeAg-negative was <0.7 MEq/mL HBV DNA and ALT normalization. Subjects received 1
mg entecavir once daily for up to an additional 144 weeks. Of these 282 subjects, 141
HBeAg-positive and 8 HBeAg-negative subjects entered the long-term follow-up rollover
study and were evaluated for entecavir resistance. Of the 149 subjects entering the
rollover study, 88% (131/149), 92% (137/149), and 92% (137/149) attained serum HBV
DNA <300 copies/mL by Weeks 144, 192, and 240 (including end of dosing),
respectively. No novel entecavir resistance-associated substitutions were identified in a
comparison of the genotypes of evaluable isolates with their respective baseline isolates.
The cumulative probability of developing rtT184, rtS202, or rtM250 entecavir resistance-
associated substitutions (in the presence of rtL180M and rtM204V substitutions) at
Weeks 48, 96, 144, 192, and 240 was 0.2%, 0.5%, 1.2%, 1.2%, and 1.2%, respectively.
Lamivudine-refractory subjects: Genotypic evaluations were performed on evaluable
samples from 190 subjects treated with BARACLUDE for up to 96 weeks in studies of
lamivudine-refractory HBV (AI463026, AI463014, AI463015, and rollover study
AI463901). By Week 96, resistance-associated amino acid substitutions at rtT184,
10
5
rtS202, or rtM250, with or without rtI169 changes, in the presence of amino acid
substitutions rtM204I/V with or without rtL80V, rtV173L/M, or rtL180M emerged in the
HBV from 22 subjects (22/190=12%), 16 of whom experienced virologic rebound (≥1
log increase above nadir) and 4 of whom were never suppressed <300 copies/mL.
The HBV from 4 of these subjects had entecavir resistance substitutions at baseline and
acquired further changes on entecavir treatment. In addition to the 22 subjects, 3
subjects experienced virologic rebound with the emergence of rtM204I/V and rtL80V,
rtV173L/M, or rtL180M. For isolates from subjects who experienced virologic rebound
with the emergence of resistance-associated substitutions (n=19), the median fold-
change in entecavir EC values from reference was 19-fold at baseline and 106-fold at
the time of virologic rebound. For subjects who continued treatment beyond 48 weeks,
40% (31/77) had HBV DNA <300 copies/mL at end of dosing (up to 96 weeks).
Lamivudine-refractory subjects (n=157) who failed to achieve the study-defined
complete response by Week 96 were offered continued entecavir treatment. Subjects
received 1 mg entecavir once daily for up to an additional 144 weeks. Of these subjects,
80 subjects entered the long-term follow-up study and were evaluated for entecavir
resistance. By Weeks 144, 192, and 240 (including end of dosing), 34% (27/80), 35%
(28/80), and 36% (29/80), respectively, attained HBV DNA <300 copies/mL. The
cumulative probability of developing rtT184, rtS202, or rtM250 entecavir resistance-
associated substitutions (in the presence of rtM204I/V with or without rtL180M
substitutions) at Weeks 48, 96, 144, 192, and 240 was 6.2%, 15%, 36.3%, 46.6%, and
51.5%, respectively. The HBV of 6 subjects developed rtA181C/G/S/T amino acid
substitutions while receiving entecavir, and of these, 4 developed entecavir resistance-
associated substitutions at rtT184, rtS202, or rtM250 and 1 had an rtT184S substitution
at baseline. Of 7 subjects whose HBV had an rtA181 substitution at baseline, 2 also had
substitutions at rtT184, rtS202, or rtM250 at baseline and another 2 developed them
while on treatment with entecavir.
In a post-approval integrated analysis of entecavir resistance data from 17 Phase 2 and
3 clinical trials, an emergent entecavir resistance-associated substitution rtA181C was
detected in 5 out of 1461 (0.3%) subjects during treatment with entecavir. This
substitution was detected only in the presence of lamivudine resistance-associated
substitutions rtL180M plus rtM204V.
Cross-resistance
Cross-resistance has been observed among HBV nucleoside analogue inhibitors. In cell-
based assays, entecavir had 8- to 30-fold less inhibition of HBV DNA synthesis for HBV
containing lamivudine and telbivudine resistance-associated substitutions rtM204I/V with
or without rtL180M than for wild-type HBV. Substitutions rtM204I/V with or without
rtL80I/V, rtV173L, or rtL180M, which are associated with lamivudine and telbivudine
resistance, also confer decreased phenotypic susceptibility to entecavir. The efficacy of
entecavir against HBV harboring adefovir resistance-associated substitutions has not
been established in clinical trials. HBV isolates from lamivudine-refractory subjects failing
entecavir therapy were susceptible in cell culture to adefovir but remained resistant to
lamivudine. Recombinant HBV genomes encoding adefovir resistance-associated
substitutions at either rtA181V or rtN236T had 1.1- or 0.3-fold shifts in susceptibility to
entecavir in cell culture, respectively.
13 NONCLINICAL TOXICOLOGY
10
50

13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Long-term oral carcinogenicity studies of entecavir in mice and rats were carried out at
exposures up to approximately 42 times (mice) and 35 times (rats) those observed in
humans at the highest recommended dose of 1 mg/day. In mouse and rat studies,
entecavir was positive for carcinogenic findings. It is not known how predictive the
results of rodent carcinogenicity studies may be for humans [see Adverse Reactions
(6.2)].
In mice, lung adenomas were increased in males and females at exposures 3 and 40
times those in humans. Lung carcinomas in both male and female mice were increased
at exposures 40 times those in humans. Combined lung adenomas and carcinomas
were increased in male mice at exposures 3 times and in female mice at exposures 40
times those in humans. Tumor development was preceded by pneumocyte proliferation
in the lung, which was not observed in rats, dogs, or monkeys administered entecavir,
supporting the conclusion that lung tumors in mice may be a species-specific event.
Hepatocellular carcinomas were increased in males and combined liver adenomas and
carcinomas were also increased at exposures 42 times those in humans. Vascular
tumors in female mice (hemangiomas of ovaries and uterus and hemangiosarcomas of
spleen) were increased at exposures 40 times those in humans. In rats, hepatocellular
adenomas were increased in females at exposures 24 times those in humans; combined
adenomas and carcinomas were also increased in females at exposures 24 times those
in humans. Brain gliomas were induced in both males and females at exposures 35 and
24 times those in humans. Skin fibromas were induced in females at exposures 4 times
those in humans.
Mutagenesis
Entecavir was clastogenic to human lymphocyte cultures. Entecavir was not mutagenic
in the Ames bacterial reverse mutation assay using S. typhimurium and E. coli strains in
the presence or absence of metabolic activation, a mammalian-cell gene mutation assay,
and a transformation assay with Syrian hamster embryo cells. Entecavir was also
negative in an oral micronucleus study and an oral DNA repair study in rats.
Impairment of Fertility
In reproductive toxicology studies, in which animals were administered entecavir at up to
30 mg/kg for up to 4 weeks, no evidence of impaired fertility was seen in male or female
rats at systemic exposures greater than 90 times those achieved in humans at the
highest recommended dose of 1 mg/day. In rodent and dog toxicology studies,
seminiferous tubular degeneration was observed at exposures 35 times or greater than
those achieved in humans. No testicular changes were evident in monkeys.
14 CLINICAL STUDIES
14.1 Outcomes in Adults
At 48 Weeks

The safety and efficacy of BARACLUDE in adults were evaluated in three Phase 3 active-
controlled trials. These studies included 1633 subjects 16 years of age or older with
chronic hepatitis B virus infection (serum HBsAg-positive for at least 6 months)
accompanied by evidence of viral replication (detectable serum HBV DNA, as measured
by the bDNA hybridization or PCR assay). Subjects had persistently elevated ALT levels
at least 1.3 times ULN and chronic inflammation on liver biopsy compatible with a
diagnosis of chronic viral hepatitis. The safety and efficacy of BARACLUDE were also
evaluated in a study of 191 HBV-infected subjects with decompensated liver disease and
in a study of 68 subjects co-infected with HBV and HIV.
Nucleoside-inhibitor-naïve Subjects with Compensated Liver Disease
HBeAg-positive: Study AI463022 was a multinational, randomized, double-blind study of
BARACLUDE 0.5 mg once daily versus lamivudine 100 mg once daily for a minimum of
52 weeks in 709 (of 715 randomized) nucleoside-inhibitor-naïve subjects with chronic
hepatitis B virus infection, compensated liver disease, and detectable HBeAg. The mean
age of subjects was 35 years, 75% were male, 57% were Asian, 40% were Caucasian,
and 13% had previously received interferon-α. At baseline, subjects had a mean Knodell
Necroinflammatory Score of 7.8, mean serum HBV DNA as measured by Roche COBAS
Amplicor PCR assay was 9.66 log copies/mL, and mean serum ALT level was 143
U/L. Paired, adequate liver biopsy samples were available for 89% of subjects.
HBeAg-negative (anti-HBe-positive/HBV DNA-positive): Study AI463027 was a
multinational, randomized, double-blind study of BARACLUDE 0.5 mg once daily versus
lamivudine 100 mg once daily for a minimum of 52 weeks in 638 (of 648 randomized)
nucleoside-inhibitor-naïve subjects with HBeAg-negative (HBeAb-positive) chronic
hepatitis B virus infection and compensated liver disease. The mean age of subjects was
44 years, 76% were male, 39% were Asian, 58% were Caucasian, and 13% had
previously received interferon-α. At baseline, subjects had a mean Knodell
Necroinflammatory Score of 7.8, mean serum HBV DNA as measured by Roche COBAS
Amplicor PCR assay was 7.58 log copies/mL, and mean serum ALT level was 142 U/L.
Paired, adequate liver biopsy samples were available for 88% of subjects.
In Studies AI463022 and AI463027, BARACLUDE was superior to lamivudine on the
primary efficacy endpoint of Histologic Improvement, defined as a 2-point or greater
reduction in Knodell Necroinflammatory Score with no worsening in Knodell Fibrosis
Score at Week 48, and on the secondary efficacy measures of reduction in viral load
and ALT normalization. Histologic Improvement and change in Ishak Fibrosis Score are
shown in Table 9. Selected virologic, biochemical, and serologic outcome measures are
shown in Table 10.
Table 9: Histologic Improvement and Change in Ishak Fibrosis Score at
Week 48, Nucleoside-Inhibitor-Naïve Subjects in Studies AI463022 and
AI463027
Study AI463022 (HBeAg-
Positive)
Study AI463027 (HBeAg-
Negative)
BARACLUDE
0.5 mg
n=314
Lamivudine
100 mg
n=314
BARACLUDE
0.5 mg
n=296
Lamivudine
100 mg
n=287
Histologic Improvement
®
10
10
a a a a
Subjects with evaluable baseline histology (baseline Knodell Necroinflammatory Score
≥2).
≥2-point decrease in Knodell Necroinflammatory Score from baseline with no
worsening of the Knodell Fibrosis Score.
For Ishak Fibrosis Score, improvement = ≥1-point decrease from baseline and
worsening = ≥1-point increase from baseline.
(Knodell Scores)
Improvement 72% 62% 70% 61%
No improvement 21% 24% 19% 26%
Ishak Fibrosis Score
Improvement 39% 35% 36% 38%
No change 46% 40% 41% 34%
Worsening 8% 10% 12% 15%
Missing Week 48
biopsy
7% 14% 10% 13%
Table 10: Selected Virologic, Biochemical, and Serologic Endpoints at Week
48, Nucleoside-Inhibitor-Naïve Subjects in Studies AI463022 and AI463027
Study AI463022
(HBeAg-Positive)
Study AI463027
(HBeAg-Negative)
BARACLUDE
0.5 mg
n=354
Lamivudine
100 mg
n=355
BARACLUDE
0.5 mg
n=325
Lamivudine
100 mg
n=313
Roche COBAS Amplicor PCR assay [lower limit of quantification (LLOQ) = 300
copies/mL].
HBV DNA
Proportion undetectable
(<300 copies/mL)
67% 36% 90% 72%
Mean change
from baseline
(log copies/mL)
−6.86 −5.39 −5.04 −4.53
ALT normalization (≤1 ×
ULN)
68% 60% 78% 71%
HBeAg seroconversion 21% 18% NA NA
Histologic Improvement was independent of baseline levels of HBV DNA or ALT.
Lamivudine-refractory Subjects with Compensated Liver Disease
Study AI463026 was a multinational, randomized, double-blind study of BARACLUDE in
286 (of 293 randomized) subjects with lamivudine-refractory chronic hepatitis B virus
infection and compensated liver disease. Subjects receiving lamivudine at study entry
either switched to BARACLUDE 1 mg once daily (with neither a washout nor an overlap
period) or continued on lamivudine 100 mg for a minimum of 52 weeks. The mean age
of subjects was 39 years, 76% were male, 37% were Asian, 62% were Caucasian, and
52% had previously received interferon-α. The mean duration of prior lamivudine
therapy was 2.7 years, and 85% had lamivudine resistance substitutions at baseline by
a
b
c
b
c
c
a
a
10
an investigational line probe assay. At baseline, subjects had a mean Knodell
Necroinflammatory Score of 6.5, mean serum HBV DNA as measured by Roche COBAS
Amplicor PCR assay was 9.36 log copies/mL, and mean serum ALT level was 128 U/L.
Paired, adequate liver biopsy samples were available for 87% of subjects.
BARACLUDE was superior to lamivudine on a primary endpoint of Histologic
Improvement (using the Knodell Score at Week 48). These results and change in Ishak
Fibrosis Score are shown in Table 11. Table 12 shows selected virologic, biochemical,
and serologic endpoints.
Table 11: Histologic Improvement and Change in Ishak Fibrosis Score at
Week 48, Lamivudine-Refractory Subjects in Study AI463026
BARACLUDE
1 mg
n=124
Lamivudine
100 mg
n=116
Subjects with evaluable baseline histology (baseline Knodell Necroinflammatory Score
≥2).
≥2-point decrease in Knodell Necroinflammatory Score from baseline with no
worsening of the Knodell Fibrosis Score.
For Ishak Fibrosis Score, improvement = ≥1-point decrease from baseline and
worsening = ≥1-point increase from baseline.
Histologic Improvement (Knodell Scores)
Improvement 55% 28%
No improvement 34% 57%
Ishak Fibrosis Score
Improvement 34% 16%
No change 44% 42%
Worsening 11% 26%
Missing Week 48 biopsy 11% 16%
Table 12: Selected Virologic, Biochemical, and Serologic Endpoints at Week
48, Lamivudine-Refractory Subjects in Study AI463026
BARACLUDE
1 mg
n=141
Lamivudine
100 mg
n=145
Roche COBAS Amplicor PCR assay (LLOQ = 300 copies/mL).
HBV DNA
Proportion undetectable (<300
copies/mL)
19% 1%
Mean change from baseline
(log copies/mL)
−5.11 −0.48
ALT normalization (≤1 × ULN) 61% 15%
HBeAg seroconversion 8% 3%
Histologic Improvement was independent of baseline levels of HBV DNA or ALT.
10
a a
a
b
c
b
c
c
a
a
10
Subjects with Decompensated Liver Disease
Study AI463048 was a randomized, open-label study of BARACLUDE 1 mg once daily
versus adefovir dipivoxil 10 mg once daily in 191 (of 195 randomized) adult subjects
with HBeAg-positive or -negative chronic HBV infection and evidence of hepatic
decompensation, defined as a Child-Turcotte-Pugh (CTP) score of 7 or higher. Subjects
were either HBV-treatment-naïve or previously treated, predominantly with lamivudine or
interferon-α.
In Study AI463048, 100 subjects were randomized to treatment with BARACLUDE and
91 subjects to treatment with adefovir dipivoxil. Two subjects randomized to treatment
with adefovir dipivoxil actually received treatment with BARACLUDE for the duration of
the study. The mean age of subjects was 52 years, 74% were male, 54% were Asian,
33% were Caucasian, and 5% were Black/African American. At baseline, subjects had a
mean serum HBV DNA by PCR of 7.83 log copies/mL and mean ALT level of 100 U/L;
54% of subjects were HBeAg-positive; 35% had genotypic evidence of lamivudine
resistance. The baseline mean CTP score was 8.6. Results for selected study endpoints
at Week 48 are shown in Table 13.
Table 13: Selected Endpoints at Week 48, Subjects with Decompensated
Liver Disease, Study AI463048
BARACLUDE
1 mg
n=100
Adefovir Dipivoxil
10 mg
n=91
Endpoints were analyzed using intention-to-treat (ITT) method, treated subjects as
randomized.
Roche COBAS Amplicor PCR assay (LLOQ = 300 copies/mL).
Defined as decrease or no change from baseline in CTP score.
Denominator is subjects with abnormal values at baseline.
ULN=upper limit of normal.
HBV DNA
Proportion undetectable (<300
copies/mL)
57% 20%
Stable or improved CTP score 61% 67%
HBsAg loss 5% 0
Normalization of ALT (≤1 × ULN) 49/78 (63%) 33/71 (46%)
Subjects Co-infected with HIV and HBV
Study AI463038 was a randomized, double-blind, placebo-controlled study of
BARACLUDE versus placebo in 68 subjects co-infected with HIV and HBV who
experienced recurrence of HBV viremia while receiving a lamivudine-containing highly
active antiretroviral (HAART) regimen. Subjects continued their lamivudine-containing
HAART regimen (lamivudine dose 300 mg/day) and were assigned to add either
BARACLUDE 1 mg once daily (51 subjects) or placebo (17 subjects) for 24 weeks
followed by an open-label phase for an additional 24 weeks where all subjects received
BARACLUDE. At baseline, subjects had a mean serum HBV DNA level by PCR of 9.13
log copies/mL. Ninety-nine percent of subjects were HBeAg-positive at baseline, with a
mean baseline ALT level of 71.5 U/L. Median HIV RNA level remained stable at
10
a a
a
b
c
d
b
c
d
10
approximately 2 log copies/mL through 24 weeks of blinded therapy. Virologic and
biochemical endpoints at Week 24 are shown in Table 14. There are no data in patients
with HIV/HBV co-infection who have not received prior lamivudine therapy. BARACLUDE
has not been evaluated in HIV/HBV co-infected patients who were not simultaneously
receiving effective HIV treatment [see Warnings and Precautions (5.2)].
Table 14: Virologic and Biochemical Endpoints at Week 24, Study AI463038
BARACLUDE 1 mg
n=51
Placebo
n=17
All subjects also received a lamivudine-containing HAART regimen.
Roche COBAS Amplicor PCR assay (LLOQ = 300 copies/mL).
Percentage of subjects with abnormal ALT (>1 × ULN) at baseline who achieved ALT
normalization (n=35 for BARACLUDE and n=12 for placebo).
HBV DNA
Proportion undetectable (<300
copies/mL)
6% 0
Mean change from baseline (log
copies/mL)
−3.65 +0.11
ALT normalization (≤1 × ULN) 34% 8%
For subjects originally assigned to BARACLUDE, at the end of the open-label phase
(Week 48), 8% of subjects had HBV DNA <300 copies/mL by PCR, the mean change
from baseline HBV DNA by PCR was −4.20 log copies/mL, and 37% of subjects with
abnormal ALT at baseline had ALT normalization (≤1 × ULN).
Beyond 48 Weeks
The optimal duration of therapy with BARACLUDE is unknown. According to protocol-
mandated criteria in the Phase 3 clinical trials, subjects discontinued BARACLUDE or
lamivudine treatment after 52 weeks according to a definition of response based on HBV
virologic suppression (<0.7 MEq/mL by bDNA assay) and loss of HBeAg (in HBeAg-
positive subjects) or ALT <1.25 × ULN (in HBeAg-negative subjects) at Week 48.
Subjects who achieved virologic suppression but did not have serologic response
(HBeAg-positive) or did not achieve ALT <1.25 × ULN (HBeAg-negative) continued
blinded dosing through 96 weeks or until the response criteria were met. These
protocol-specified subject management guidelines are not intended as guidance for
clinical practice.
Nucleoside-inhibitor-naïve Subjects
Among nucleoside-inhibitor-naïve, HBeAg-positive subjects (Study AI463022), 243 (69%)
BARACLUDE-treated subjects and 164 (46%) lamivudine-treated subjects continued
blinded treatment for up to 96 weeks. Of those continuing blinded treatment in Year 2,
180 (74%) BARACLUDE subjects and 60 (37%) lamivudine subjects achieved HBV DNA
<300 copies/mL by PCR at the end of dosing (up to 96 weeks). 193 (79%) BARACLUDE
subjects achieved ALT ≤1 × ULN compared to 112 (68%) lamivudine subjects, and
HBeAg seroconversion occurred in 26 (11%) BARACLUDE subjects and 20 (12%)
lamivudine subjects.
10
a a
a
b
c
b
10
c c
10

Among nucleoside-inhibitor-naïve, HBeAg-positive subjects, 74 (21%) BARACLUDE
subjects and 67 (19%) lamivudine subjects met the definition of response at Week 48,
discontinued study drugs, and were followed off treatment for 24 weeks. Among
BARACLUDE responders, 26 (35%) subjects had HBV DNA <300 copies/mL, 55 (74%)
subjects had ALT ≤1 × ULN, and 56 (76%) subjects sustained HBeAg seroconversion at
the end of follow-up. Among lamivudine responders, 20 (30%) subjects had HBV DNA
<300 copies/mL, 41 (61%) subjects had ALT ≤1 × ULN, and 47 (70%) subjects
sustained HBeAg seroconversion at the end of follow-up.
Among nucleoside-inhibitor-naïve, HBeAg-negative subjects (Study AI463027), 26 (8%)
BARACLUDE-treated subjects and 28 (9%) lamivudine-treated subjects continued blinded
treatment for up to 96 weeks. In this small cohort continuing treatment in Year 2, 22
BARACLUDE and 16 lamivudine subjects had HBV DNA <300 copies/mL by PCR, and 7
and 6 subjects, respectively, had ALT ≤1 × ULN at the end of dosing (up to 96 weeks).
Among nucleoside-inhibitor-naïve, HBeAg-negative subjects, 275 (85%) BARACLUDE
subjects and 245 (78%) lamivudine subjects met the definition of response at Week 48,
discontinued study drugs, and were followed off treatment for 24 weeks. In this cohort,
very few subjects in each treatment arm had HBV DNA <300 copies/mL by PCR at the
end of follow-up. At the end of follow-up, 126 (46%) BARACLUDE subjects and 84 (34%)
lamivudine subjects had ALT ≤1 × ULN.
Lamivudine-refractory Subjects
Among lamivudine-refractory subjects (Study AI463026), 77 (55%) BARACLUDE-treated
subjects and 3 (2%) lamivudine subjects continued blinded treatment for up to 96
weeks. In this cohort of BARACLUDE subjects, 31 (40%) subjects achieved HBV DNA
<300 copies/mL, 62 (81%) subjects had ALT ≤1 × ULN, and 8 (10%) subjects
demonstrated HBeAg seroconversion at the end of dosing.
14.2 Outcomes in Pediatric Subjects
The pharmacokinetics, safety and antiviral activity of BARACLUDE in pediatric subjects
were initially assessed in Study AI463028. Twenty-four treatment-naïve and 19
lamivudine-experienced HBeAg-positive pediatric subjects 2 to less than 18 years of age
with compensated chronic hepatitis B virus infection and elevated ALT were treated with
BARACLUDE 0.015 mg/kg (up to 0.5 mg) or 0.03 mg/kg (up to 1 mg) once daily. Fifty-
eight percent (14/24) of treatment-naïve subjects and 47% (9/19) of lamivudine-
experienced subjects achieved HBV DNA <50 IU/mL at Week 48 and ALT normalized in
83% (20/24) of treatment-naïve and 95% (18/19) of lamivudine-experienced subjects.
Safety and antiviral efficacy were confirmed in Study AI463189, a study of BARACLUDE
among 180 nucleoside-inhibitor-treatment-naïve pediatric subjects 2 to less than 18
years of age with HBeAg-positive chronic hepatitis B infection, compensated liver
disease, and elevated ALT. Subjects were randomized 2:1 to receive blinded treatment
with BARACLUDE 0.015 mg/kg up to 0.5 mg/day (N=120) or placebo (N=60). The
randomization was stratified by age group (2 to 6 years; >6 to 12 years; and >12 to
<18 years). Baseline demographics and HBV disease characteristics were comparable
between the 2 treatment arms and across age cohorts. At study entry, the mean HBV
DNA was 8.1 log IU/mL and mean ALT was 103 U/L. The primary efficacy endpoint was
a composite of HBeAg seroconversion and serum HBV DNA <50 IU/mL at Week 48
assessed in the first 123 subjects reaching 48 weeks of blinded treatment. Twenty-four
10

percent (20/82) of subjects in the BARACLUDE-treated group and 2% (1/41) of subjects
in the placebo-treated group met the primary endpoint. Forty-six percent (38/82) of
BARACLUDE-treated subjects and 2% (1/41) of placebo-treated subjects achieved HBV
DNA <50 IU/mL at Week 48. ALT normalization occurred in 67% (55/82) of
BARACLUDE-treated subjects and 22% (9/41) of placebo-treated subjects; 24% (20/82)
of BARACLUDE-treated subjects and 12% (5/41) of placebo-treated subjects had HBeAg
seroconversion.
16 HOW SUPPLIED/STORAGE AND HANDLING
BARACLUDE (entecavir) Tablets and Oral Solution are available in the following
strengths and configurations of plastic bottles with child-resistant closures:
Product
Strength and
Dosage Form
Description Quantity NDC Number
0.5 mg film-
coated tablet
White to off-white, triangular-shaped
tablet, debossed with “BMS” on one
side and “1611” on the other side.
30 tablets 0003-1611-12
1 mg film-coated
tablet
Pink, triangular-shaped tablet,
debossed with “BMS” on one side and
“1612” on the other side.
30 tablets 0003-1612-12
0.05 mg/mL oral
solution
Ready-to-use, orange-flavored, clear,
colorless to pale yellow, aqueous
solution in a 260 mL bottle.
210 mL 0003-1614-12
BARACLUDE Oral Solution is a ready-to-use product; dilution or mixing with water or any
other solvent or liquid product is not recommended. Each bottle of the oral solution is
accompanied by a dosing spoon that is calibrated in 0.5 mL increments up to 10 mL.
Storage
BARACLUDE Tablets should be stored in a tightly closed container at 25°C (77°F);
excursions permitted between 15°C and 30°C (59°F and 86°F) [see USP Controlled
Room Temperature]. Store in the outer carton to protect from light.
BARACLUDE Oral Solution should be stored in the outer carton at 25°C (77°F);
excursions permitted between 15°C and 30°C (59°F and 86°F) [see USP Controlled
Room Temperature]. Protect from light. After opening, the oral solution can be used up
to the expiration date on the bottle. The bottle and its contents should be discarded
after the expiration date.
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (Patient Information).
Severe Acute Exacerbation of Hepatitis after Discontinuation of Treatment
Inform patients that discontinuation of anti-hepatitis B therapy, including BARACLUDE,
®

may result in severe acute exacerbations of hepatitis B. Advise the patient to not
discontinue BARACLUDE without first informing their healthcare provider [see Warnings
and Precautions (5.1)].
Risk of Development of HIV-1 Resistance in Patients with HIV-1 Coinfection
Inform patients that if they have or develop HIV infection and are not receiving effective
HIV treatment, BARACLUDE may increase the risk of development of resistance to HIV
medication [see Warnings and Precautions (5.2)].
Lactic Acidosis and Severe Hepatomegaly
Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been
reported with use of drugs similar to BARACLUDE. Advise patients to contact their
healthcare provider immediately and stop BARACLUDE if they develop clinical symptoms
suggestive of lactic acidosis or pronounced hepatotoxicity [see Warnings and
Precautions (5.3)].
Missed Dosage
Inform patients that it is important to take BARACLUDE on a regular dosing schedule on
an empty stomach (at least 2 hours after a meal and 2 hours before the next meal) and
to avoid missing doses as it can result in development of resistance [see Dosage and
Administration (2.1)].
Treatment Duration
Advise patients that in the treatment of chronic hepatitis B, the optimal duration of
treatment is unknown. The relationship between response and long-term prevention of
outcomes such as hepatocellular carcinoma is not known.
Instructions for Use
Inform patients using the oral solution to hold the dosing spoon in a vertical position and
fill it gradually to the mark corresponding to the prescribed dose. Rinsing of the dosing
spoon with water is recommended after each daily dose. Some patients may find it
difficult to accurately measure the prescribed dose using the provided dosing spoon;
therefore, patients/caregivers should refer to the steps in the Patient Information
section that demonstrate the correct technique of using the provided dosing spoon to
measure the prescribed BARACLUDE dose.
Pregnancy Registry
Advise patients that there is a pregnancy exposure registry that monitors pregnancy
outcomes in women exposed to BARACLUDE during pregnancy [see Use in Specific
Populations (8.1)].
Patient Information
BARACLUDE (BEAR ah klude)
(entecavir)
Tablets
®
®

BARACLUDE (BEAR ah klude)
(entecavir)
Oral Solution
Read this Patient Information before you start taking BARACLUDE and each time you get
a refill. There may be new information. This information does not take the place of talking
with your healthcare provider about your medical condition or treatment.
What is the most important information I should know about BARACLUDE?
1.
2.
BARACLUDE can cause serious side effects including:
3.
4.
®
Your hepatitis B virus (HBV) infection may get worse if you stop taking
BARACLUDE. This usually happens within 6 months after stopping BARACLUDE.
•
•
•
•
Take BARACLUDE exactly as prescribed.
Do not run out of BARACLUDE.
Do not stop BARACLUDE without talking to your healthcare provider.
Your healthcare provider should monitor your health and do regular blood
tests to check your liver if you stop taking BARACLUDE.
If you have or get HIV that is not being treated with medicines while
taking BARACLUDE, the HIV virus may develop resistance to certain HIV
medicines and become harder to treat. You should get an HIV test before you
start taking BARACLUDE and anytime after that when there is a chance you were
exposed to HIV.
Lactic acidosis (buildup of acid in the blood). Some people who have
taken BARACLUDE or medicines like BARACLUDE (a nucleoside analogue)
have developed a serious condition called lactic acidosis. Lactic acidosis is a
serious medical emergency that can cause death. Lactic acidosis must be treated in
the hospital. Reports of lactic acidosis with BARACLUDE generally involved patients
who were seriously ill due to their liver disease or other medical condition.
Call your healthcare provider right away if you get any of the following
signs or symptoms of lactic acidosis:
•
•
•
•
•
•
•
You feel very weak or tired.
You have unusual (not normal) muscle pain.
You have trouble breathing.
You have stomach pain with nausea and vomiting.
You feel cold, especially in your arms and legs.
You feel dizzy or light-headed.
You have a fast or irregular heartbeat.
Serious liver problems. Some people who have taken medicines like
BARACLUDE have developed serious liver problems called hepatotoxicity,
with liver enlargement (hepatomegaly) and fat in the liver (steatosis).
Hepatomegaly with steatosis is a serious medical emergency that can
cause death.
Call your healthcare provider right away if you get any of the following

You may be more likely to get lactic acidosis or serious liver problems if you are female,
very overweight, or have been taking nucleoside analogue medicines, like BARACLUDE,
for a long time.
What is BARACLUDE?
BARACLUDE is a prescription medicine used to treat chronic hepatitis B virus (HBV) in
adults and children 2 years of age and older who have active liver disease.
•
•
•
•
•
•
What should I tell my healthcare provider before taking BARACLUDE?
Before you take BARACLUDE, tell your healthcare provider if you:
•
•
•
•
•
Tell your healthcare provider about all the medicines you take, including
signs or symptoms of liver problems:
•
•
•
•
•
•
Your skin or the white part of your eyes turns yellow (jaundice).
Your urine turns dark.
Your bowel movements (stools) turn light in color.
You don’t feel like eating food for several days or longer.
You feel sick to your stomach (nausea).
You have lower stomach pain.
BARACLUDE will not cure HBV.
BARACLUDE may lower the amount of HBV in the body.
BARACLUDE may lower the ability of HBV to multiply and infect new liver cells.
BARACLUDE may improve the condition of your liver.
It is not known whether BARACLUDE will reduce your chances of getting liver
cancer or liver damage (cirrhosis), which may be caused by chronic HBV infection.
It is not known if BARACLUDE is safe and effective for use in children less than 2
years of age.
have kidney problems. Your BARACLUDE dose or schedule may need to be
changed.
have received medicine for HBV before. Some people, especially those who have
already been treated with certain other medicines for HBV infection, may develop
resistance to BARACLUDE. These people may have less benefit from treatment with
BARACLUDE and may have worsening of hepatitis after resistant virus appears.
Your healthcare provider will test the level of the hepatitis B virus in your blood
regularly.
have any other medical conditions.
are pregnant or plan to become pregnant. It is not known if BARACLUDE will harm
your unborn baby. Talk to your healthcare provider if you are pregnant or plan to
become pregnant.
Antiretroviral Pregnancy Registry. If you take BARACLUDE while you are
pregnant, talk to your healthcare provider about how you can take part in the
BARACLUDE Antiretroviral Pregnancy Registry. The purpose of the pregnancy
registry is to collect information about the health of you and your baby.
are breastfeeding or plan to breastfeed. It is not known if BARACLUDE can pass
into your breast milk. You and your healthcare provider should decide if you will
take BARACLUDE or breastfeed.
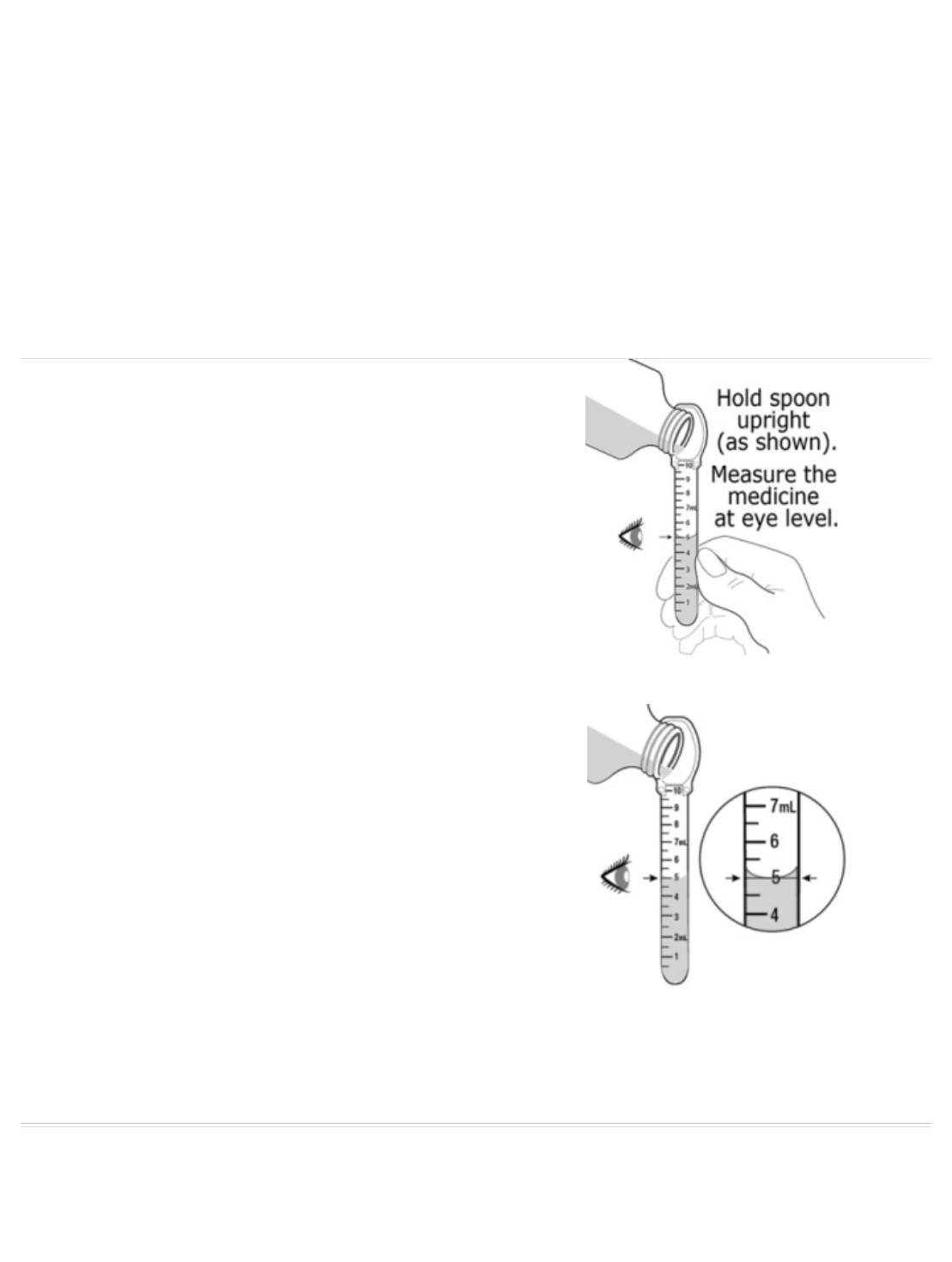
prescription and over-the-counter medicines, vitamins, and herbal supplements.
Especially tell your healthcare provider if you have taken a medicine to treat HBV in the
past.
Know the medicines you take. Keep a list of your medicines with you to show your
healthcare provider and pharmacist when you get a new medicine.
How should I take BARACLUDE?
•
•
•
•
•
•
Figure 1
Figure 2
Take BARACLUDE exactly as your healthcare provider tells you to.
Your healthcare provider will tell you how much BARACLUDE to take.
Your healthcare provider will tell you when and how often to take BARACLUDE.
Take BARACLUDE on an empty stomach, at least 2 hours after a meal and at
least 2 hours before the next meal.
If you are taking BARACLUDE Oral
Solution, or giving it to your child, carefully
measure the dose with the dosing spoon
provided, as follows:
Hold the dosing spoon in an upright
(vertical) position and slowly fill it to the
measurement line on the dosing spoon
that is the same as the prescribed dose.
Bring the dosing spoon to eye level to be
sure that the level of the BARACLUDE Oral
Solution is at the correct measurement line
(see Figure 1).
• With the dosing spoon at eye level,
holding it with the measurement lines
facing you, check that it has been
filled to the correct measurement line.
The top of the BARACLUDE Oral
Solution in the dosing spoon will look
curved, not flat. Measure the dose of
BARACLUDE Oral Solution at the
bottom of the curve. Your dose of
BARACLUDE Oral Solution is
measured correctly when the bottom
of the curve is lined up with the
measurement line of the prescribed
dose. As an example, Figure 2 shows
the right way to measure a 5 mL
dose of BARACLUDE (see Figure 2).
•
•
•
•
BARACLUDE Oral Solution should be swallowed directly from the dosing
spoon.
BARACLUDE Oral Solution should not be mixed with water or any other liquid.
After each use, rinse the dosing spoon with water and allow it to air dry.
If you lose the dosing spoon, call your pharmacist or healthcare provider for

•
•
•
•
What are the possible side effects of BARACLUDE?
BARACLUDE may cause serious side effects. See “What is the most important
information I should know about BARACLUDE?”
The most common side effects of BARACLUDE include:
•
•
•
•
Tell your healthcare provider if you have any side effect that bothers you or that does
not go away.
These are not all the possible side effects of BARACLUDE. For more information, ask
your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to the
FDA at 1-800-FDA-1088.
How should I store BARACLUDE?
•
•
•
•
Keep BARACLUDE and all medicines out of the reach of children.
General information about the safe and effective use of BARACLUDE
BARACLUDE does not stop you from spreading the hepatitis B virus (HBV) to others by
sex, sharing needles, or being exposed to your blood. Talk with your healthcare provider
about safe sexual practices that protect your partner. Never share needles. Do not
share personal items that can have blood or body fluids on them, like toothbrushes or
razor blades. A shot (vaccine) is available to protect people at risk from becoming
instructions.
Do not change your dose or stop taking BARACLUDE without talking to
your healthcare provider.
If you miss a dose of BARACLUDE, take it as soon as you remember and then
take your next dose at its regular time. If it is almost time for your next dose, skip
the missed dose. Do not take two doses at the same time. Call your healthcare
provider or pharmacist if you are not sure what to do.
When your supply of BARACLUDE starts to run low, call your healthcare provider
or pharmacy for a refill. Do not run out of BARACLUDE.
If you take too much BARACLUDE, call your healthcare provider or go to the
nearest emergency room right away.
headache
tiredness
dizziness
nausea
Store BARACLUDE Tablets or Oral Solution at room temperature, between 68°F and
77°F (20°C and 25°C).
Keep BARACLUDE Tablets in a tightly closed container.
Store BARACLUDE Tablets or BARACLUDE Oral Solution in the original carton, and
keep the carton out of the light.
Safely throw away BARACLUDE that is out of date or no longer needed. Dispose of
unused medicines through community take-back disposal programs when available
or place BARACLUDE in an unrecognizable closed container in the household trash.

infected with HBV.
Medicines are sometimes prescribed for purposes other than those listed in a Patient
Information leaflet. Do not use BARACLUDE for a condition for which it was not
prescribed. Do not give BARACLUDE to other people, even if they have the same
symptoms you have. It may harm them.
This Patient Information leaflet summarizes the most important information about
BARACLUDE. If you would like more information, talk with your healthcare provider. You
can ask your healthcare provider or pharmacist for information about BARACLUDE that
is written for health professionals.
For more information, go to www.Baraclude.com or call 1-800-321-1335.
What are the ingredients in BARACLUDE?
Active ingredient: entecavir
Inactive ingredients in BARACLUDE Tablets: lactose monohydrate, microcrystalline
cellulose, crospovidone, povidone, magnesium stearate.
Tablet film-coat: titanium dioxide, hypromellose, polyethylene glycol 400, polysorbate 80
(0.5 mg tablet only), and iron oxide red (1 mg tablet only).
Inactive ingredients in BARACLUDE Oral Solution: maltitol, sodium citrate, citric acid,
methylparaben, propylparaben, and orange flavor.
Distributed by:
Bristol-Myers Squibb Company
Princeton, NJ 08543 USA
This Patient Information has been approved by the U.S. Food and Drug Administration.
Revised: August 2015
BARACLUDE 0.5 mg Tablets Representative Packaging
See HOW SUPPLIED section for a complete list of available packages of BARACLUDE.
30 Tablets NDC 0003-1611-12
Baraclude
(entecavir)
Tablets
0.5 mg
Bristol-Myers Squibb
Rx only
®

BARACLUDE 1 mg Tablets Representative Packaging
30 Tablets NDC 0003-1612-12
Baraclude
(entecavir)
Tablets
1 mg
Bristol-Myers Squibb
Rx only
®

BARACLUDE 0.05 mg/mL Oral Solution Representative Packaging
210 mL NDC 0003-1614-12
Baraclude
(entecavir)
Oral Solution
0.05 mg/mL
Store Bottle in Carton to Protect from Light
Bristol-Myers Squibb
Rx only
®

BARACLUDE
entecavir tablet, film coated
Product Information
Product Type
HUMAN PRESCRIPTION DRUG
Item Code (Source)
NDC:0003-1611
Route of Administration
ORAL
Active Ingredient/Active Moiety
Ingredient Name Basis of Strength Strength
ENTECAVIR (UNII: 5968Y6H45M) (entecavir anhydrous - UNII:NNU2O4609D) ENTECAVIR 0.5 mg

Inactive Ingredients
Ingredient Name Strength
LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X)
MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U)
CROSPOVIDONE (15 MPA.S AT 5%) (UNII: 68401960MK)
POVIDONE, UNSPECIFIED (UNII: FZ989GH94E)
MAGNESIUM STEARATE (UNII: 70097M6I30)
TITANIUM DIOXIDE (UNII: 15FIX9V2JP)
HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO)
POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ)
POLYSORBATE 80 (UNII: 6OZ P39Z G8H)
Product Characteristics
Color
WHITE
Score
no s core
Shape
TRIANGLE
Size
8mm
Flavor Imprint Code
BMS;1611
Contains
Packaging
# Item Code Package Description
Marketing Start
Date
Marketing End
Date
1
NDC:0003-1611-
12
1 in 1 CARTON 03/29/2005
1
30 in 1 BOTTLE; Type 0: Not a Combination
Product
2
NDC:0003-1611-
13
1 in 1 CARTON 03/29/2005 11/30/2018
2
90 in 1 BOTTLE; Type 0: Not a Combination
Product
Marketing Information
Marketing
Category
Application Number or Monograph
Citation
Marketing Start
Date
Marketing End
Date
NDA NDA021797 03/29/2005
BARACLUDE
entecavir tablet, film coated
Product Information
Product Type
HUMAN PRESCRIPTION DRUG
Item Code (Source)
NDC:0003-1612
Route of Administration
ORAL
Active Ingredient/Active Moiety

Ingredient Name Basis of Strength Strength
ENTECAVIR (UNII: 5968Y6H45M) (entecavir anhydrous - UNII:NNU2O4609D) ENTECAVIR 1.0 mg
Inactive Ingredients
Ingredient Name Strength
LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X)
MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U)
CROSPOVIDONE (15 MPA.S AT 5%) (UNII: 68401960MK)
POVIDONE, UNSPECIFIED (UNII: FZ989GH94E)
MAGNESIUM STEARATE (UNII: 70097M6I30)
TITANIUM DIOXIDE (UNII: 15FIX9V2JP)
HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO)
POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ)
FERRIC OXIDE RED (UNII: 1K09F3G675)
Product Characteristics
Color
PINK
Score
no s core
Shape
TRIANGLE
Size
10mm
Flavor Imprint Code
BMS;1612
Contains
Packaging
# Item Code Package Description
Marketing Start
Date
Marketing End
Date
1
NDC:0003-1612-
12
1 in 1 CARTON 03/29/2005
1
30 in 1 BOTTLE; Type 0: Not a Combination
Product
Marketing Information
Marketing
Category
Application Number or Monograph
Citation
Marketing Start
Date
Marketing End
Date
NDA NDA021797 03/29/2005
BARACLUDE
entecavir solution
Product Information
Product Type
HUMAN PRESCRIPTION DRUG
Item Code (Source)
NDC:0003-1614
Route of Administration
ORAL
Active Ingredient/Active Moiety

E.R. Squibb & Sons, L.L.C.
Ingredient Name Basis of Strength Strength
ENTECAVIR (UNII: 5968Y6H45M) (entecavir anhydrous - UNII:NNU2O4609D) ENTECAVIR 0.05 mg in 1 mL
Inactive Ingredients
Ingredient Name Strength
MALTITOL (UNII: D65DG142WK)
SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR)
CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP)
METHYLPARABEN (UNII: A2I8C7HI9T)
PROPYLPARABEN (UNII: Z 8IX2SC1OH)
Packaging
# Item Code Package Description
Marketing Start
Date
Marketing End
Date
1
NDC:0003-1614-
12
1 in 1 CARTON 03/29/2005
1
210 mL in 1 BOTTLE; Type 0: Not a Combination
Product
Marketing Information
Marketing
Category
Application Number or Monograph
Citation
Marketing Start
Date
Marketing End
Date
NDA NDA021798 03/29/2005
Labeler - E.R. Squibb & Sons, L.L.C. (011550092)
Establishment
Name Address ID/FEI Business Operations
Patheon Inc. 240769596 manufacture(0003-1611, 0003-1612) , analysis (0003-1611, 0003-1612)
Establishment
Name Address ID/FEI Business Operations
Bristol-Myers S quibb Pharmaceuticals Limited 213617645 analysis (0003-1611, 0003-1612)
Revised: 5/2024
