1
ANNEX I
SUMMARY OF PRODUCT CHARACTERISTICS

2
1. NAME OF THE MEDICINAL PRODUCT
Baraclude 0.5 mg film-coated tablets
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
Each tablet contains 0.5 mg entecavir (as monohydrate).
Excipients with known effect
: each tablet contains 120.5 mg lactose.
For the full list of excipients, see section 6.1.
3. PHARMACEUTICAL FORM
Film-coated tablet (tablet)
White to off-white and triangular-shaped tablet with “BMS” debossed on one side and “1611” on the
other.
4. CLINICAL PARTICULARS
4.1 Therapeutic indications
Baraclude is indicated for the treatment of chronic hepatitis B virus (HBV) infection (see section 5.1)
in adults with:
compensated liver disease and evidence of active viral replication, persistently elevated serum
alanine aminotransferase (ALT) levels and histological evidence of active inflammation and/or
fibrosis.
decompensated liver disease (see section 4.4)
For both compensated and decompensated liver disease, this indication is based on clinical trial data in
nucleoside naive patients with HBeAg positive and HBeAg negative HBV infection. With respect to
patients with lamivudine-refractory hepatitis B, see sections 4.2, 4.4 and 5.1.
Baraclude is also indicated for the treatment of chronic HBV infection in nucleoside naive paediatric
patients from 2 to < 18 years of age with compensated liver disease who have evidence of active viral
replication and persistently elevated serum ALT levels, or histological evidence of moderate to severe
inflammation and/or fibrosis. With respect to the decision to initiate treatment in paediatric patients,
see sections 4.2, 4.4, and 5.1.
4.2 Posology and method of administration
Therapy should be initiated by a physician experienced in the management of chronic hepatitis B
infection.
Posology
Compensated liver disease
Nucleoside naïve patients: the recommended dose in adults is 0.5 mg once daily, with or without food.
Lamivudine-refractory patients (i.e. with evidence of viraemia while on lamivudine or the presence of
lamivudine resistance [LVDr] mutations) (see sections 4.4 and 5.1): the recommended dose in adults is
1 mg once daily, which must be taken on an empty stomach (more than 2 hours before and more than
3
2 hours after a meal) (see section 5.2). In the presence of LVDr mutations, combination use of
entecavir plus a second antiviral agent (which does not share cross-resistance with either lamivudine
or entecavir) should be considered in preference to entecavir monotherapy (see section 4.4.).
Decompensated liver disease
The recommended dose for adult patients with decompensated liver disease is 1 mg once daily, which
must be taken on an empty stomach (more than 2 hours before and more than 2 hours after a meal)
(see section 5.2). For patients with lamivudine-refractory hepatitis B, see sections 4.4 and 5.1.
Duration of therapy
The optimal duration of treatment is unknown. Treatment discontinuation may be considered as
follows:
In HBeAg positive adult patients, treatment should be administered at least until 12 months after
achieving HBe seroconversion (HBeAg loss and HBV DNA loss with anti-HBe detection on
two consecutive serum samples at least 3-6 months apart) or until HBs seroconversion or there
is loss of efficacy (see section 4.4).
In HBeAg negative adult patients, treatment should be administered at least until HBs
seroconversion or there is evidence of loss of efficacy. With prolonged treatment for more than
2 years, regular reassessment is recommended to confirm that continuing the selected therapy
remains appropriate for the patient.
In patients with decompensated liver disease or cirrhosis, treatment cessation is not recommended.
Paediatric population
The decision to treat paediatric patients should be based on careful consideration of individual patient
needs and with reference to current paediatric treatment guidelines including the value of baseline
histological information. The benefits of long-term virologic suppression with continued therapy must
be weighed against the risk of prolonged treatment, including the emergence of resistant hepatitis B
virus.
Serum ALT should be persistently elevated for at least 6 months prior to treatment of paediatric
patients with compensated liver disease due to HBeAg positive chronic hepatitis B; and for at least
12 months in patients with HBeAg negative disease.
Paediatric patients with body weight of at least 32.6 kg, should be administered a daily dose of one
0.5 mg tablet or 10 ml (0.5 mg) of the oral solution, with or without food. The oral solution should be
used for patients with body weight less than 32.6 kg.
Duration of therapy for paediatric patients
The optimal duration of treatment is unknown. In accordance with current paediatric practice
guidelines, treatment discontinuation may be considered as follows:
In HBeAg positive paediatric patients, treatment should be administered for at least 12 months
after achieving undetectable HBV DNA and HBeAg seroconversion (HBeAg loss and anti-HBe
detection on two consecutive serum samples at least 3-6 months apart) or until HBs
seroconversion or there is loss of efficacy. Serum ALT and HBV DNA levels should be
followed regularly after treatment discontinuation (see section 4.4).
In HBeAg negative paediatric patients, treatment should be administered until HBs
seroconversion or there is evidence of loss of efficacy.
Pharmacokinetics in paediatric patients with renal or hepatic impairment have not been studied.
Elderly: no dosage adjustment based on age is required. The dose should be adjusted according to the
patient’s renal function (see dosage recommendations in renal impairment and section 5.2).
Gender and race: no dosage adjustment based on gender or race is required.
4
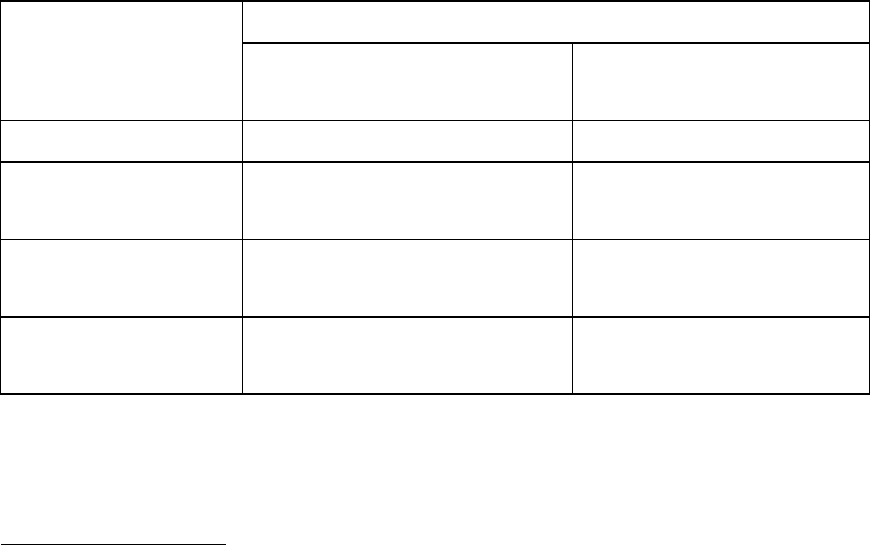
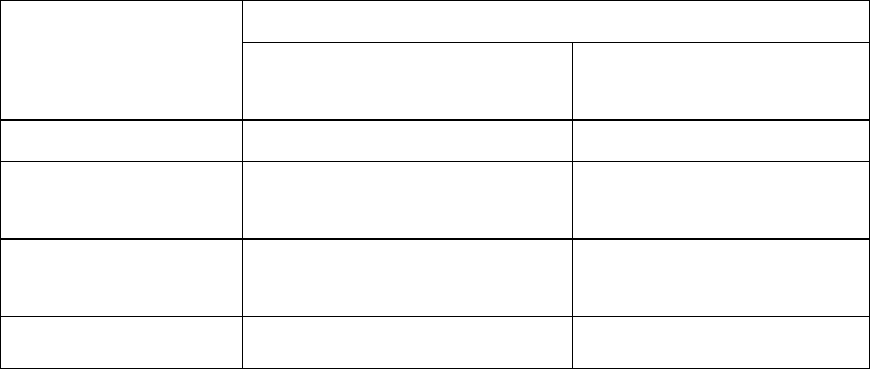
Renal impairment: the clearance of entecavir decreases with decreasing creatinine clearance (see
section 5.2). Dose adjustment is recommended for patients with creatinine clearance < 50 ml/min,
including those on haemodialysis or continuous ambulatory peritoneal dialysis (CAPD). A reduction
of the daily dose using Baraclude oral solution, as detailed in the table, is recommended. As an
alternative, in case the oral solution is not available, the dose can be adjusted by increasing the dosage
interval, also shown in the table. The proposed dose modifications are based on extrapolation of
limited data, and their safety and effectiveness have not been clinically evaluated. Therefore,
virological response should be closely monitored.
Baraclude dosage*
Creatinine clearance
(ml/min)
Nucleoside naïve patients
Lamivudine-refractory or
decompensated liver disease
≥ 50 0.5 mg once daily 1 mg once daily
30 - 49 0.25 mg once daily*
OR
0.5 mg every 48 hours
0.5 mg once daily
10 - 29 0.15 mg once daily*
OR
0.5 mg every 72 hours
0.3 mg once daily*
OR
0.5 mg every 48 hours
< 10
Haemodialysis or
CAPD**
0.05 mg once daily*
OR
0.5 mg every 5-7 days
0.1 mg once daily*
OR
0.5 mg every 72 hours
* for doses < 0.5 mg Baraclude oral solution is recommended.
** on haemodialysis days, administer entecavir after haemodialysis.
Hepatic impairment: no dose adjustment is required in patients with hepatic impairment.
Method of administration
Baraclude should be taken orally.
4.3 Contraindications
Hypersensitivity to the active substance or to any of the excipients listed in section 6.1.
4.4 Special warnings and precautions for use
Renal impairment: dosage adjustment is recommended for patients with renal impairment (see
section 4.2). The proposed dose modifications are based on extrapolation of limited data, and their
safety and effectiveness have not been clinically evaluated. Therefore, virological response should be
closely monitored.
Exacerbations of hepatitis: spontaneous exacerbations in chronic hepatitis B are relatively common
and are characterised by transient increases in serum ALT. After initiating antiviral therapy, serum
ALT may increase in some patients as serum HBV DNA levels decline (see section 4.8). Among
entecavir-treated patients on-treatment exacerbations had a median time of onset of 4-5 weeks. In
patients with compensated liver disease, these increases in serum ALT are generally not accompanied
by an increase in serum bilirubin concentrations or hepatic decompensation. Patients with advanced
liver disease or cirrhosis may be at a higher risk for hepatic decompensation following hepatitis
exacerbation, and therefore should be monitored closely during therapy.
Acute exacerbation of hepatitis has also been reported in patients who have discontinued hepatitis B
therapy (see section 4.2). Post-treatment exacerbations are usually associated with rising HBV DNA,
5
and the majority appears to be self-limited. However, severe exacerbations, including fatalities, have
been reported.
Among entecavir-treated nucleoside naive patients, post-treatment exacerbations had a median time to
onset of 23-24 weeks, and most were reported in HBeAg negative patients (see section 4.8). Hepatic
function should be monitored at repeated intervals with both clinical and laboratory follow-up for at
least 6 months after discontinuation of hepatitis B therapy. If appropriate, resumption of hepatitis B
therapy may be warranted.
Patients with decompensated liver disease: a higher rate of serious hepatic adverse events (regardless
of causality) has been observed in patients with decompensated liver disease, in particular in those
with Child-Turcotte-Pugh (CTP) class C disease, compared with rates in patients with compensated
liver function. Also, patients with decompensated liver disease may be at higher risk for lactic acidosis
and for specific renal adverse events such as hepatorenal syndrome. Therefore, clinical and laboratory
parameters should be closely monitored in this patient population (see also sections 4.8 and 5.1).
Lactic acidosis and severe hepatomegaly with steatosis: occurrences of lactic acidosis (in the absence
of hypoxaemia), sometimes fatal, usually associated with severe hepatomegaly and hepatic steatosis,
have been reported with the use of nucleoside analogues. As entecavir is a nucleoside analogue, this
risk cannot be excluded. Treatment with nucleoside analogues should be discontinued when rapidly
elevating aminotransferase levels, progressive hepatomegaly or metabolic/lactic acidosis of unknown
aetiology occur. Benign digestive symptoms, such as nausea, vomiting and abdominal pain, might be
indicative of lactic acidosis development. Severe cases, sometimes with fatal outcome, were associated
with pancreatitis, liver failure/hepatic steatosis, renal failure and higher levels of serum lactate.
Caution should be exercised when prescribing nucleoside analogues to any patient (particularly obese
women) with hepatomegaly, hepatitis or other known risk factors for liver disease. These patients
should be followed closely.
To differentiate between elevations in aminotransferases due to response to treatment and increases
potentially related to lactic acidosis, physicians should ensure that changes in ALT are associated with
improvements in other laboratory markers of chronic hepatitis B.
Resistance and specific precaution for lamivudine-refractory patients: mutations in the HBV
polymerase that encode lamivudine-resistance substitutions may lead to the subsequent emergence of
secondary substitutions, including those associated with entecavir associated resistance (ETVr). In a
small percentage of lamivudine-refractory patients, ETVr substitutions at residues rtT184, rtS202 or
rtM250 were present at baseline. Patients with lamivudine-resistant HBV are at higher risk of
developing subsequent entecavir resistance than patients without lamivudine resistance. The
cumulative probability of emerging genotypic entecavir resistance after 1, 2, 3, 4 and 5 years treatment
in the lamivudine-refractory studies was 6%, 15%, 36%, 47% and 51%, respectively. Virological
response should be frequently monitored in the lamivudine-refractory population and appropriate
resistance testing should be performed. In patients with a suboptimal virological response after 24
weeks of treatment with entecavir, a modification of treatment should be considered (see sections 4.5
and 5.1). When starting therapy in patients with a documented history of lamivudine-resistant HBV,
combination use of entecavir plus a second antiviral agent (which does not share cross-resistance with
either lamivudine or entecavir) should be considered in preference to entecavir monotherapy.
Pre-existing lamivudine-resistant HBV is associated with an increased risk for subsequent entecavir
resistance regardless of the degree of liver disease; in patients with decompensated liver disease,
virologic breakthrough may be associated with serious clinical complications of the underlying liver
disease. Therefore, in patients with both decompensated liver disease and lamivudine-resistant HBV,
combination use of entecavir plus a second antiviral agent (which does not share cross-resistance with
either lamivudine or entecavir) should be considered in preference to entecavir monotherapy.
Paediatric population: A lower rate of virologic response (HBV DNA < 50 IU/ml) was observed in
paediatric patients with baseline HBV DNA ≥ 8.0 log
10
IU/ml (see section 5.1). Entecavir should be
used in these patients only if the potential benefit justifies the potential risk to the child (e.g.
6
resistance). Since some paediatric patients may require long-term or even lifetime management of
chronic active hepatitis B, consideration should be given to the impact of entecavir on future treatment
options.
Liver transplant recipients: renal function should be carefully evaluated before and during entecavir
therapy in liver transplant recipients receiving cyclosporine or tacrolimus (see section 5.2).
Co-infection with hepatitis C or D: there are no data on the efficacy of entecavir in patients co-infected
with hepatitis C or D virus.
Human immunodeficiency virus (HIV)/HBV co-infected patients not receiving concomitant
antiretroviral therapy: entecavir has not been evaluated in HIV/HBV co-infected patients not
concurrently receiving effective HIV treatment. Emergence of HIV resistance has been observed when
entecavir was used to treat chronic hepatitis B infection in patients with HIV infection not receiving
highly active antiretroviral therapy (HAART) (see section 5.1). Therefore, therapy with entecavir
should not be used for HIV/HBV co-infected patients who are not receiving HAART. Entecavir has
not been studied as a treatment for HIV infection and is not recommended for this use.
HIV/HBV co-infected patients receiving concomitant antiretroviral therapy: entecavir has been studied
in 68 adults with HIV/HBV co-infection receiving a lamivudine-containing HAART regimen (see
section 5.1). No data are available on the efficacy of entecavir in HBeAg-negative patients co-infected
with HIV. There are limited data on patients co-infected with HIV who have low CD4 cell counts
(< 200 cells/mm
3
).
General: patients should be advised that therapy with entecavir has not been proven to reduce the risk
of transmission of HBV and therefore appropriate precautions should still be taken.
Lactose: this medicinal product contains 120.5 mg of lactose in each 0.5 mg daily dose.
Patients with rare hereditary problems of galactose intolerance, the Lapp lactase deficiency or glucose-
galactose malabsorption should not take this medicine. A lactose-free Baraclude oral solution is
available for these individuals.
4.5 Interaction with other medicinal products and other forms of interaction
Since entecavir is predominantly eliminated by the kidney (see section 5.2), coadministration with
medicinal products that reduce renal function or compete for active tubular secretion may increase
serum concentrations of either medicinal product. Apart from lamivudine, adefovir dipivoxil and
tenofovir disoproxil fumarate, the effects of coadministration of entecavir with medicinal products that
are excreted renally or affect renal function have not been evaluated. Patients should be monitored
closely for adverse reactions when entecavir is coadministered with such medicinal products.
No pharmacokinetic interactions between entecavir and lamivudine, adefovir or tenofovir were
observed.
Entecavir is not a substrate, an inducer or an inhibitor of cytochrome P450 (CYP450) enzymes (see
section 5.2). Therefore CYP450 mediated drug interactions are unlikely to occur with entecavir.
Paediatric population
Interaction studies have only been performed in adults.
4.6 Fertility, pregnancy and lactation
Women of childbearing potential: given that the potential risks to the developing foetus are unknown,
women of childbearing potential should use effective contraception.
Pregnancy: there are no adequate data from the use of entecavir in pregnant women. Studies in
animals have shown reproductive toxicity at high doses (see section 5.3). The potential risk for
7
humans is unknown. Baraclude should not be used during pregnancy unless clearly necessary. There
are no data on the effect of entecavir on transmission of HBV from mother to newborn infant.
Therefore, appropriate interventions should be used to prevent neonatal acquisition of HBV.
Breast-feeding: it is unknown whether entecavir is excreted in human milk. Available toxicological
data in animals have shown excretion of entecavir in milk (for details see section 5.3). A risk to the
infants cannot be excluded. Breast-feeding should be discontinued during treatment with Baraclude.
Fertility: toxicology studies in animals administered entecavir have shown no evidence of impaired
fertility (see section 5.3).
4.7 Effects on ability to drive and use machines
No studies on the effects on the ability to drive and use machines have been performed. Dizziness,
fatigue and somnolence are common side effects which may impair the ability to drive and use
machines.
4.8 Undesirable effects
a. Summary of the safety profile
In clinical studies in patients with compensated liver disease, the most common adverse reactions of
any severity with at least a possible relation to entecavir were headache (9%), fatigue (6%), dizziness
(4%) and nausea (3%). Exacerbations of hepatitis during and after discontinuation of entecavir therapy
have also been reported (see section 4.4 and c. Description of selected adverse reactions).
b. Tabulated list of adverse reactions
Assessment of adverse reactions is based on experience from postmarketing surveillance and four
clinical studies in which 1,720 patients with chronic hepatitis B infection and compensated liver
disease received double-blind treatment with entecavir (n = 862) or lamivudine (n = 858) for up to
107 weeks (see section 5.1). In these studies, the safety profiles, including laboratory abnormalities,
were comparable for entecavir 0.5 mg daily (679 nucleoside-naive HBeAg positive or negative
patients treated for a median of 53 weeks), entecavir 1 mg daily (183 lamivudine-refractory patients
treated for a median of 69 weeks), and lamivudine.
Adverse reactions considered at least possibly related to treatment with entecavir are listed by body
system organ class. Frequency is defined as very common (≥ 1/10); common (≥ 1/100 to < 1/10);
uncommon (≥ 1/1,000 to < 1/100); rare (≥ 1/10,000 to < 1/1,000). Within each frequency grouping,
undesirable effects are presented in order of decreasing seriousness.
Immune system disorders:
rare: anaphylactoid reaction
Psychiatric disorders:
common: insomnia
Nervous system disorders:
common: headache, dizziness, somnolence
Gastrointestinal disorders:
common: vomiting, diarrhoea, nausea, dyspepsia
Hepatobiliary disorders
common: increased transaminases
Skin and subcutaneous tissue disorders:
uncommon: rash, alopecia
General disorders and administration site
conditions:
common: fatigue
Cases of lactic acidosis have been reported, often in association with hepatic decompensation, other
serious medical conditions or drug exposures (see section 4.4).

8
Treatment beyond 48 weeks: continued treatment with entecavir for a median duration of 96 weeks did
not reveal any new safety signals.
c. Description of selected adverse reactions
Laboratory test abnormalities
: In clinical studies with nucleoside-naive patients, 5% had ALT
elevations > 3 times baseline, and < 1% had ALT elevations > 2 times baseline together with total
bilirubin > 2 times upper limit of normal (ULN) and > 2 times baseline. Albumin levels < 2.5 g/dl
occurred in < 1% of patients, amylase levels > 3 times baseline in 2%, lipase levels > 3 times baseline
in 11% and platelets < 50,000/mm
3
in < 1%.
In clinical studies with lamivudine-refractory patients, 4% had ALT elevations > 3 times baseline, and
< 1% had ALT elevations > 2 times baseline together with total bilirubin > 2 times ULN and > 2 times
baseline. Amylase levels > 3 times baseline occurred in 2% of patients, lipase levels > 3 times baseline
in 18% and platelets < 50,000/mm
3
in < 1%.
Exacerbations during treatment:
in studies with nucleoside naive patients, on treatment ALT elevations
> 10 times ULN and > 2 times baseline occurred in 2% of entecavir treated patients vs 4% of
lamivudine treated patients. In studies with lamivudine-refractory patients, on treatment ALT
elevations > 10 times ULN and > 2 times baseline occurred in 2% of entecavir treated patients vs 11%
of lamivudine treated patients. Among entecavir-treated patients, on-treatment ALT elevations had a
median time to onset of 4-5 weeks, generally resolved with continued treatment, and, in a majority of
cases, were associated with a ≥ 2 log
10
/ml reduction in viral load that preceded or coincided with the
ALT elevation. Periodic monitoring of hepatic function is recommended during treatment.
Exacerbations after discontinuation of treatment:
acute exacerbations of hepatitis have been reported
in patients who have discontinued anti-hepatitis B virus therapy, including therapy with entecavir (see
section 4.4). In studies in nucleoside-naive patients, 6% of entecavir-treated patients and 10% of
lamivudine-treated patients experienced ALT elevations (> 10 times ULN and > 2 times reference
[minimum of baseline or last end-of-dosing measurement]) during post-treatment follow-up. Among
entecavir-treated nucleoside-naive patients, ALT elevations had a median time to onset of 23-
24 weeks, and 86% (24/28) of ALT elevations occurred in HBeAg negative patients. In studies in
lamivudine-refractory patients, with only limited numbers of patients being followed up, 11% of
entecavir-treated patients and no lamivudine-treated patients developed ALT elevations during post-
treatment follow-up.
In the clinical trials entecavir treatment was discontinued if patients achieved a prespecified response.
If treatment is discontinued without regard to treatment response, the rate of post-treatment ALT flares
could be higher.
d. Paediatric Population
The safety of entecavir in paediatric patients from 2 to < 18 years of age is based on two ongoing
clinical trials in subjects with chronic HBV infection; one Phase 2 pharmacokinetic trial (study 028)
and one Phase 3 trial (study 189). These trials provide experience in 173 HBeAg-positive nucleoside-
treatment-naïve subjects treated with entecavir for a median duration of 60 weeks. The adverse
reactions observed in paediatric subjects who received treatment with entecavir were consistent with
those observed in clinical trials of entecavir in adults.(see a. Summary of the safety profile and
section 5.1)
e. Other special populations
Experience in patients with decompensated liver disease: the safety profile of entecavir in patients
with decompensated liver disease was assessed in a randomized open-label comparative study in
which patients received treatment with entecavir 1 mg/day (n = 102) or adefovir dipivoxil 10 mg/day
(n = 89) (study 048). Relative to the adverse reactions noted in section b. Tabulated list of adverse
reactions, one additional adverse reaction [decrease in blood bicarbonate (2%)] was observed in

9
entecavir-treated patients through week 48. The on-study cumulative death rate was 23% (23/102), and
causes of death were generally liver-related, as expected in this population. The on-study cumulative
rate of hepatocellular carcinoma (HCC) was 12% (12/102). Serious adverse events were generally
liver-related, with an on-study cumulative frequency of 69%. Patients with high baseline CTP score
were at higher risk of developing serious adverse events (see section 4.4).
Laboratory test abnormalities: through week 48 among entecavir-treated patients with decompensated
liver disease, none had ALT elevations both > 10 times ULN and > 2 times baseline, and 1% of
patients had ALT elevations > 2 times baseline together with total bilirubin > 2 times ULN and > 2
times baseline. Albumin levels < 2.5 g/dl occurred in 30% of patients, lipase levels > 3 times baseline
in 10% and platelets < 50,000/mm
3
in 20%.
Experience in patients co-infected with HIV:
the safety profile of entecavir in a limited number of
HIV/HBV co-infected patients on lamivudine-containing HAART (highly active antiretroviral
therapy) regimens was similar to the safety profile in monoinfected HBV patients (see section 4.4).
Gender/age:
there was no apparent difference in the safety profile of entecavir with respect to gender
(≈ 25% women in the clinical trials) or age (≈ 5% of patients > 65 years of age).
Reporting of suspected adverse reactions:
Reporting suspected adverse reactions after authorisation of
the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the
medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the
national reporting system listed in Appendix V.
4.9 Overdose
There is limited experience of entecavir overdose reported in patients. Healthy subjects who received
up to 20 mg/day for up to 14 days, and single doses up to 40 mg had no unexpected adverse reactions.
If overdose occurs, the patient must be monitored for evidence of toxicity and given standard
supportive treatment as necessary.
5. PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: antivirals for systemic use, nucleoside and nucleotide reverse
transcriptase inhibitors
ATC code: J05AF10
Mechanism of action: entecavir, a guanosine nucleoside analogue with activity against HBV
polymerase, is efficiently phosphorylated to the active triphosphate (TP) form, which has an
intracellular half-life of 15 hours. By competing with the natural substrate deoxyguanosine TP,
entecavir-TP functionally inhibits the 3 activities of the viral polymerase: (1) priming of the HBV
polymerase, (2) reverse transcription of the negative strand DNA from the pregenomic messenger
RNA, and (3) synthesis of the positive strand HBV DNA. The entecavir-TP K
i
for HBV DNA
polymerase is 0.0012 μM. Entecavir-TP is a weak inhibitor of cellular DNA polymerases α, β, and δ
with K
i
values of 18 to 40 µM. In addition, high exposures of entecavir had no relevant adverse effects
on γ polymerase or mitochondrial DNA synthesis in HepG2 cells (K
i
> 160 µM).
Antiviral activity: entecavir inhibited HBV DNA synthesis (50% reduction, EC
50
) at a concentration
of 0.004 µM in human HepG2 cells transfected with wild-type HBV. The median EC
50
value for
entecavir against LVDr HBV (rtL180M and rtM204V) was 0.026 µM (range 0.010-0.059 µM).
Recombinant viruses encoding adefovir-resistant substitutions at either rtN236T or rtA181V remained
fully susceptible to entecavir.
10
An analysis of the inhibitory activity of entecavir against a panel of laboratory and clinical HIV-1
isolates using a variety of cells and assay conditions yielded EC
50
values ranging from 0.026 to
> 10 µM; the lower EC
50
values were observed when decreased levels of virus were used in the assay.
In cell culture, entecavir selected for an M184I substitution at micromolar concentrations, confirming
inhibitory pressure at high entecavir concentrations. HIV variants containing the M184V substitution
showed loss of susceptibility to entecavir (see section 4.4).
In HBV combination assays in cell culture, abacavir, didanosine, lamivudine, stavudine, tenofovir or
zidovudine were not antagonistic to the anti-HBV activity of entecavir over a wide range of
concentrations. In HIV antiviral assays, entecavir at micromolar concentrations was not antagonistic to
the anti-HIV activity in cell culture of these six NRTIs or emtricitabine.
Resistance in cell culture: relative to wild-type HBV, LVDr viruses containing rtM204V and
rtL180M substitutions within the reverse transcriptase exhibit 8-fold decreased susceptibility to
entecavir. Incorporation of additional ETVr amino acid changes rtT184, rtS202 or rtM250 decreases
entecavir susceptibility in cell culture. Substitutions observed in clinical isolates (rtT184A, C, F, G, I,
L, M or S; rtS202 C, G or I; and/or rtM250I, L or V) further decreased entecavir susceptibility 16- to
741-fold relative to wild-type virus. The ETVr substitutions at residues rtT184, rtS202 and rtM250
alone have only a modest effect on entecavir susceptibility, and have not been observed in the absence
of LVDr substitutions in more than 1000 patient samples sequenced. Resistance is mediated by
reduced inhibitor binding to the altered HBV reverse transcriptase, and resistant HBV exhibits reduced
replication capacity in cell culture.
Clinical experience: the demonstration of benefit is based on histological, virological, biochemical,
and serological responses after 48 weeks of treatment in active-controlled clinical trials of 1,633 adults
with chronic hepatitis B infection, evidence of viral replication and compensated liver disease. The
safety and efficacy of entecavir were also evaluated in an active-controlled clinical trial of 191 HBV-
infected patients with decompensated liver disease and in a clinical trial of 68 patients co-infected with
HBV and HIV.
In studies in patients with compensated liver disease, histological improvement was defined as a ≥ 2-
point decrease in Knodell necro-inflammatory score from baseline with no worsening of the Knodell
fibrosis score. Responses for patients with baseline Knodell Fibrosis Scores of 4 (cirrhosis) were
comparable to overall responses on all efficacy outcome measures (all patients had compensated liver
disease). High baseline Knodell necroinflammatory scores (> 10) were associated with greater
histological improvement in nucleoside-naive patients. Baseline ALT levels ≥ 2 times ULN and
baseline HBV DNA ≤ 9.0 log
10
copies/ml were both associated with higher rates of virologic response
(Week 48 HBV DNA < 400 copies/ml) in nucleoside-naive HBeAg-positive patients. Regardless of
baseline characteristics, the majority of patients showed histological and virological responses to
treatment.
11
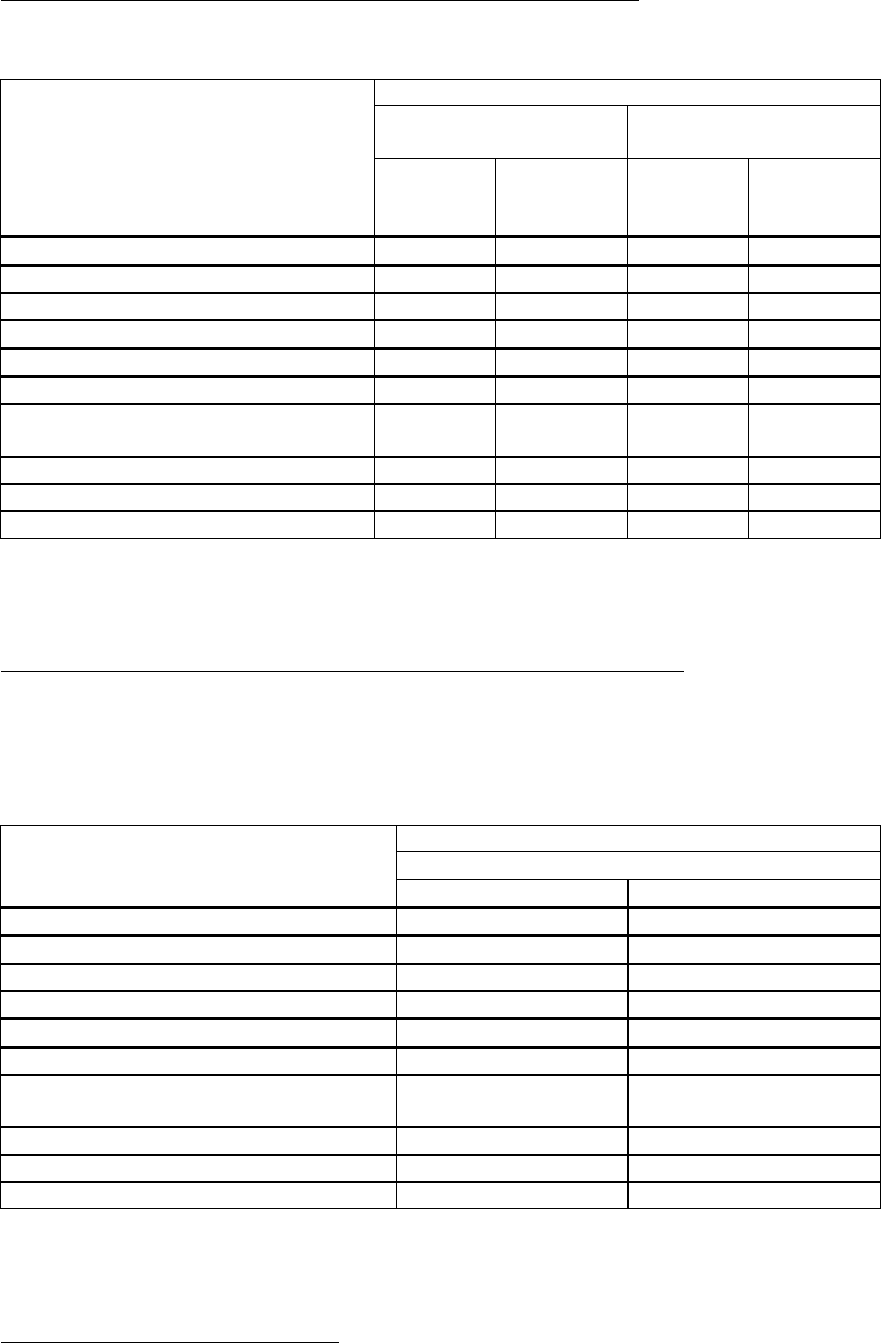
Experience in nucleoside-naive patients with compensated liver disease:
Results at 48 weeks of randomised, double blind studies comparing entecavir (ETV) to lamivudine
(LVD) in HBeAg positive (022) and HBeAg negative (027) patients are presented in the table.
Nucleoside Naive
HBeAg Positive
(study 022)
HBeAg Negative
(study 027)
ETV
0.5 mg
once daily
LVD
100 mg
once daily
ETV
0.5 mg
once daily
LVD
100 mg
once daily
n 314
a
314
a
296
a
287
a
Histological improvement
b
72%* 62% 70%* 61%
Ishak fibrosis score improvement 39% 35% 36% 38%
Ishak fibrosis score worsening 8% 10% 12% 15%
n 354 355 325 313
Viral load reduction (log
10
copies/ml)
c
-6.86* -5.39 -5.04* -4.53
HBV DNA undetectable
(< 300 copies/ml by PCR)
c
67%* 36% 90%* 72%
ALT normalisation (≤ 1 times ULN) 68%* 60% 78%* 71%
HBeAg Seroconversion 21% 18%
*p value vs lamivudine < 0.05
a
patients with evaluable baseline histology (baseline Knodell Necroinflammatory Score ≥ 2)
b
a primary endpoint
c
Roche Cobas Amplicor PCR assay (LLOQ = 300 copies/ml)
Experience in lamivudine-refractory patients with compensated liver disease:
In a randomised, double-blind study in HBeAg positive lamivudine-refractory patients (026), with
85% of patients presenting LVDr mutations at baseline, patients receiving lamivudine at study entry
either switched to entecavir 1 mg once daily, with neither a washout nor an overlap period (n = 141),
or continued on lamivudine 100 mg once daily (n = 145). Results at 48 weeks are presented in the
table.
Lamivudine-refractory
HBeAg positive (study 026)
ETV 1.0 mg once daily LVD 100 mg once daily
n 124
a
116
a
Histological improvement
b
55%* 28%
Ishak fibrosis score improvement 34%* 16%
Ishak fibrosis score worsening 11% 26%
n 141 145
Viral load reduction (log
10
copies/ml)
c
-5.11* -0.48
HBV DNA undetectable (< 300 copies/ml
by PCR)
c
19%* 1%
ALT normalisation (≤ 1 times ULN) 61%* 15%
HBeAg Seroconversion 8% 3%
*p value vs lamivudine < 0.05
a
patients with evaluable baseline histology (baseline Knodell Necroinflammatory Score ≥ 2)
b
a primary endpoint.
c
Roche Cobas Amplicor PCR assay (LLOQ = 300 copies/ml)
Results beyond 48 weeks of treatment:
Treatment was discontinued when prespecified response criteria were met either at 48 weeks or during
the second year of treatment. Response criteria were HBV virological suppression (HBV DNA
< 0.7 MEq/ml by bDNA) and loss of HBeAg (in HBeAg positive patients) or ALT < 1.25 times ULN
12
(in HBeAg negative patients). Patients in response were followed for an additional 24 weeks off-
treatment. Patients who met virologic but not serologic or biochemical response criteria continued
blinded treatment. Patients who did not have a virologic response were offered alternative treatment.
Nucleoside-naive:
HBeAg positive (study 022): treatment with entecavir for up to 96 weeks (n = 354) resulted in
cumulative response rates of 80% for HBV DNA < 300 copies/ml by PCR, 87% for ALT
normalisation, 31% for HBeAg seroconversion and 2% for HBsAg seroconversion (5% for HBsAg
loss). For lamivudine (n = 355), cumulative response rates were 39% for HBV DNA < 300 copies/ml
by PCR, 79% for ALT normalisation, 26% for HBeAg seroconversion, and 2% for HBsAg
seroconversion (3% for HBsAg loss).
At end of dosing, among patients who continued treatment beyond 52 weeks (median of 96 weeks),
81% of 243 entecavir-treated and 39% of 164 lamivudine-treated patients had HBV DNA
< 300 copies/ml by PCR while ALT normalisation (≤ 1 times ULN) occurred in 79% of entecavir-
treated and 68% of lamivudine-treated patients.
HBeAg negative (study 027): treatment with entecavir up to 96 weeks (n = 325) resulted in cumulative
response rates of 94% for HBV DNA < 300 copies/ml by PCR and 89% for ALT normalisation versus
77% for HBV DNA < 300 copies/ml by PCR and 84% for ALT normalisation for lamivudine-treated
patients (n = 313).
For 26 entecavir-treated and 28 lamivudine-treated patients who continued treatment beyond 52 weeks
(median 96 weeks), 96% of entecavir-treated and 64% of lamivudine-treated patients had HBV DNA
< 300 copies/ml by PCR at end of dosing. ALT normalisation (≤ 1 times ULN) occurred in 27% of
entecavir-treated and 21% of lamivudine-treated patients at end of dosing.
For patients who met protocol-defined response criteria, response was sustained throughout the 24-
week post-treatment follow-up in 75% (83/111) of entecavir responders vs 73% (68/93) for
lamivudine responders in study 022 and 46% (131/286) of entecavir responders vs 31% (79/253) for
lamivudine responders in study 027. By 48 weeks of post-treatment follow-up, a substantial number of
HBeAg negative patients lost response.
Liver biopsy results: 57 patients from the pivotal nucleoside-naive studies 022 (HBeAg positive) and
027 (HBeAg negative) who enrolled in a long-term rollover study were evaluated for long-term liver
histology outcomes. The entecavir dosage was 0.5 mg daily in the pivotal studies (mean exposure 85
weeks) and 1 mg daily in the rollover study (mean exposure 177 weeks), and 51 patients in the
rollover study initially also received lamivudine (median duration 29 weeks). Of these patients, 55/57
(96%) had histological improvement as previously defined (see above), and 50/57 (88%) had a ≥ 1-
point decrease in Ishak fibrosis score. For patients with baseline Ishak fibrosis score ≥ 2, 25/43 (58%)
had a ≥ 2-point decrease. All (10/10) patients with advanced fibrosis or cirrhosis at baseline (Ishak
fibrosis score of 4, 5 or 6) had a ≥ 1 point decrease (median decrease from baseline was 1.5 points). At
the time of the long-term biopsy, all patients had HBV DNA < 300 copies/ml and 49/57 (86%) had
serum ALT ≤ 1 times ULN. All 57 patients remained positive for HBsAg.
Lamivudine-refractory:
HBeAg positive (study 026): treatment with entecavir for up to 96 weeks (n = 141) resulted in
cumulative response rates of 30% for HBV DNA < 300 copies/ml by PCR, 85% for ALT
normalisation and 17% for HBeAg seroconversion.
For the 77 patients who continued entecavir treatment beyond 52 weeks (median 96 weeks), 40% of
patients had HBV DNA < 300 copies/ml by PCR and 81% had ALT normalisation (≤ 1 times ULN) at
end of dosing.
Age/gender:
There was no apparent difference in efficacy for entecavir based on gender (≈ 25% women in the
clinical trials) or age (≈ 5% of patients > 65 years of age).
13
Special populations
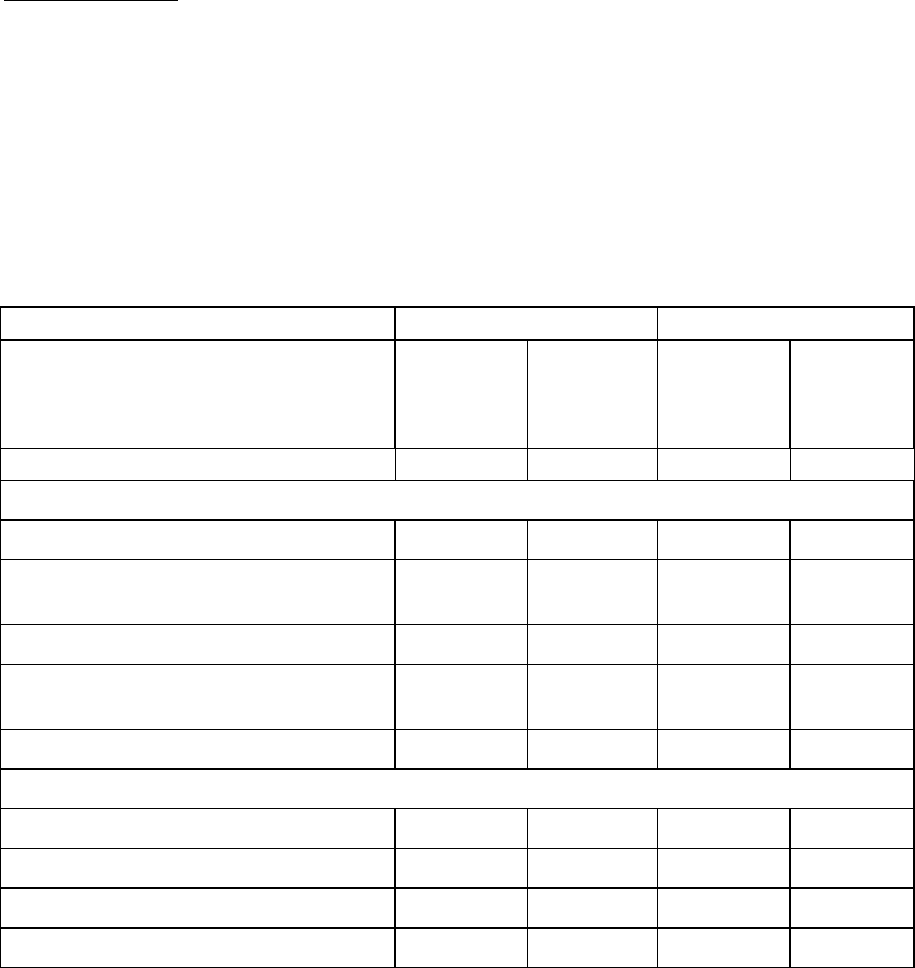
Patients with decompensated liver disease: in study 048, 191 patients with HBeAg positive or
negative chronic HBV infection and evidence of hepatic decompensation, defined as a CTP score of 7
or higher, received entecavir 1 mg once daily or adefovir dipivoxil 10 mg once daily. Patients were
either HBV-treatment-naïve or pretreated (excluding pretreatment with entecavir, adefovir dipivoxil,
or tenofovir disoproxil fumarate). At baseline, patients had a mean CTP score of 8.59 and 26% of
patients were CTP class C. The mean baseline Model for End Stage Liver Disease (MELD) score was
16.23. Mean serum HBV DNA by PCR was 7.83 log
10
copies/ml and mean serum ALT was 100 U/l;
54% of patients were HBeAg positive, and 35% of patients had LVDr substitutions at baseline.
Entecavir was superior to adefovir dipivoxil on the primary efficacy endpoint of mean change from
baseline in serum HBV DNA by PCR at week 24. Results for selected study endpoints at weeks 24
and 48 are shown in the table.
Week 24 Week 48
ETV
1 mg
once daily
Adefovir
Dipivoxil
10 mg
once daily
ETV
1 mg
once daily
Adefovir
Dipivoxil
10 mg
once daily
n 100 91 100 91
HBV DNA
a
Proportion undetectable (<300 copies/ml)
b
49%* 16% 57%* 20%
Mean change from baseline
(log
10
copies/ml)
c
-4.48* -3.40 -4.66 -3.90
Stable or improved CTP score
b
,
d
66% 71% 61% 67%
MELD score
Mean change from baseline
c,e
-2.0
-0.9
-2.6
-1.7
HBsAg loss
b
1% 0 5% 0
Normalization of:
f
ALT (≤1 X ULN)
b
46/78 (59%)* 28/71 (39%) 49/78 (63%)* 33/71 (46%)
Albumin (≥1 X LLN)
b
20/82 (24%) 14/69 (20%) 32/82 (39%) 20/69 (29%)
Bilirubin (≤1 X ULN)
b
12/75 (16%) 10/65 (15%) 15/75 (20%) 18/65 (28%)
Prothrombin time (≤1 X ULN)
b
9/95 (9%) 6/82 (7%) 8/95 (8%) 7/82 (9%)
a
Roche COBAS Amplicor PCR assay (LLOQ = 300 copies/ml).
b
NC=F (noncompleter=failure), meaning treatment discontinuations before the analysis week, including reasons such as
death, lack of efficacy, adverse event, noncompliance/loss-to-follow-up, are counted as failures (e.g., HBV
DNA ≥ 300 copies/ml)
c
NC=M (noncompleters=missing)
d
Defined as decrease or no change from baseline in CTP score.
e
Baseline mean MELD score was 17.1 for ETV and 15.3 for adefovir dipivoxil.
f
Denominator is patients with abnormal values at baseline.
*p<0.05
ULN=upper limit of normal, LLN=lower limit of normal.
The time to onset of HCC or death (whichever occurred first) was comparable in the two treatment
groups; on-study cumulative death rates were 23% (23/102) and 33% (29/89) for patients treated with
entecavir and adefovir dipivoxil, respectively, and on-study cumulative rates of HCC were 12%
(12/102) and 20% (18/89) for entecavir and adefovir dipivoxil, respectively.
For patients with LVDr substitutions at baseline, the percentage of patients with HBV DNA
<300 copies/ml was 44% for entecavir and 20% for adefovir at week 24 and 50% for entecavir and
17% for adefovir at week 48.
14
HIV/HBV co-infected patients receiving concomitant HAART: study 038 included 67 HBeAg positive
and 1 HBeAg negative patients co-infected with HIV. Patients had stable controlled HIV (HIV RNA
< 400 copies/ml) with recurrence of HBV viraemia on a lamivudine-containing HAART regimen.
HAART regimens did not include emtricitabine or tenofovir disoproxil fumarate. At baseline
entecavir-treated patients had a median duration of prior lamivudine therapy of 4.8 years and median
CD4 count of 494 cells/mm
3
(with only 5 subjects having CD4 count < 200 cells/mm
3
). Patients
continued their lamivudine-regimen and were assigned to add either entecavir 1 mg once daily
(n = 51) or placebo (n = 17) for 24 weeks followed by an additional 24 weeks where all received
entecavir. At 24 weeks the reduction in HBV viral load was significantly greater with entecavir (-3.65
vs an increase of 0.11 log
10
copies/ml). For patients originally assigned to entecavir treatment, the
reduction in HBV DNA at 48 weeks was -4.20 log
10
copies/ml, ALT normalisation had occurred in
37% of patients with abnormal baseline ALT and none achieved HBeAg seroconversion.
HIV/HBV co-infected patients not receiving concomitant HAART: entecavir has not been evaluated in
HIV/HBV co-infected patients not concurrently receiving effective HIV treatment. Reductions in HIV
RNA have been reported in HIV/HBV co-infected patients receiving entecavir monotherapy without
HAART. In some cases, selection of HIV variant M184V has been observed, which has implications
for the selection of HAART regimens that the patient may take in the future. Therefore, entecavir
should not be used in this setting due to the potential for development of HIV resistance (see section
4.4).
Liver transplant recipients: the safety and efficacy of entecavir 1 mg once daily were assessed in a
single-arm study in 65 patients who received a liver transplant for complications of chronic HBV
infection and had HBV DNA <172 IU/ml (approximately 1000 copies/ml) at the time of transplant.
The study population was 82% male, 39% Caucasian, and 37% Asian, with a mean age of 49 years;
89% of patients had HBeAg-negative disease at the time of transplant. Of the 61 patients who were
evaluable for efficacy (received entecavir for at least 1 month), 60 also received hepatitis B immune
globulin (HBIg) as part of the post-transplant prophylaxis regimen. Of these 60 patients, 49 received
more than 6 months of HBIg therapy. At Week 72 post-transplant, none of 55 observed cases had
virologic recurrence of HBV [defined as HBV DNA ≥50 IU/ml (approximately 300 copies/ml)], and
there was no reported virologic recurrence at time of censoring for the remaining 6 patients. All 61
patients had HBsAg loss post-transplantation, and 2 of these later became HBsAg positive despite
maintaining undetectable HBV DNA (<6 IU/ml). The frequency and nature of adverse events in this
study were consistent with those expected in patients who have received a liver transplant and the
known safety profile of entecavir.
Paediatric population: Study 189 is an ongoing study of the efficacy and safety of entecavir among
180 nucleoside-treatment-naïve children and adolescents from 2 to < 18 years of age with HBeAg-
positive chronic hepatitis B infection, compensated liver disease, and elevated ALT. Subjects were
randomized (2:1) to receive blinded treatment with entecavir 0.015 mg/kg up to 0.5 mg/day (N = 120)
or placebo (N = 60). The randomization was stratified by age group (2 to 6 years; > 6 to 12 years; and
> 12 to < 18 years). Baseline demographics and HBV disease characteristics were comparable
between the 2 treatment arms and across age cohorts. At study entry, the mean HBV DNA was
8.0 log
10
IU/ml and mean ALT was 105 U/l for the primary cohort (the first 123 treated subjects). The
primary efficacy endpoint was a composite of HBeAg seroconversion and serum HBV DNA
< 50 IU/ml (approximately 300 copies/ml) at Week 48 for the primary cohort.
Twenty-four percent (20/82) of subjects in the entecavir-treated group and 2% (1/41) of subjects in the
placebo-treated group met the primary endpoint. Forty-six percent (38/82) of entecavir-treated subjects
and 2% (1/41) of placebo-treated subjects achieved HBV DNA < 50 IU/ml at Week 48. When
assessed by baseline HBV DNA, 77% (26/34) of entecavir-treated subjects with HBV
DNA < 8 log
10
IU/ml at baseline and 25% (12/48) with HBV DNA ≥ 8 log
10
IU/ml achieved HBV
DNA < 50 IU/ml. ALT normalization occurred in 67% (55/82) of entecavir-treated subjects and 22%
(9/41) of placebo-treated subjects; 24% (20/82) of entecavir-treated subjects and 12% (5/41) of
placebo-treated subjects had HBeAg seroconversion.
15
In 2 paediatric studies (Studies 028 and 189), 110 patients who received entecavir for up to 48 weeks
were monitored for resistance. Genotypic evaluations were performed on all patients who had
virologic breakthrough, or HBV DNA ≥ 50 IU/ml at Week 48 or discontinued early. No amino acid
substitutions associated with resistance to entecavir were identified.
Clinical resistance: patients in clinical trials initially treated with entecavir 0.5 mg (nucleoside-naive)
or 1.0 mg (lamivudine-refractory) and with an on-therapy PCR HBV DNA measurement at or after
Week 24 were monitored for resistance.
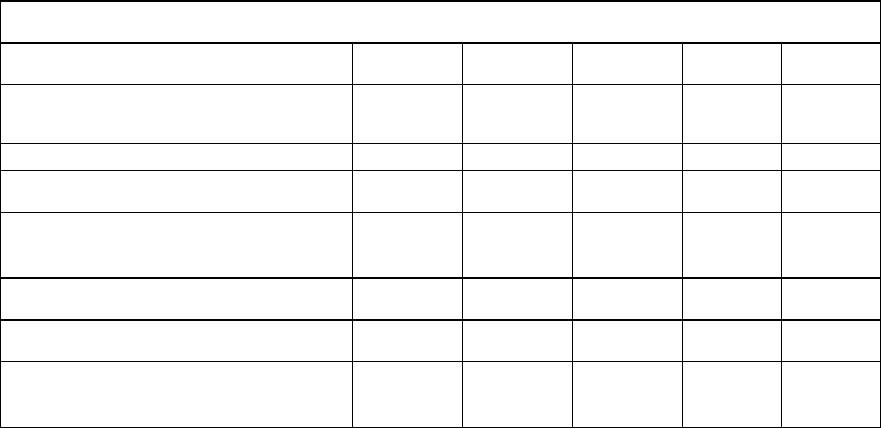
Through Week 240 in nucleoside-naive studies, genotypic evidence of ETVr substitutions at rtT184,
rtS202, or rtM250 was identified in 3 patients treated with entecavir, 2 of whom experienced virologic
breakthrough (see table). These substitutions were observed only in the presence of LVDr
substitutions (rtM204V and rtL180M).
Emerging Genotypic Entecavir Resistance Through Year 5, Nucleoside-Naive Studies
Year 1 Year 2
Year 3
a
Year 4
a
Year 5
a
Patients treated and monitored for
resistance
b
663 278 149 121 108
Patients in specific year with:
- emerging genotypic ETVr
c
1 1 1 0 0
- genotypic ETVr
c
with virologic
breakthrough
d
1 0 1 0 0
Cumulative probability of:
- emerging genotypic ETVr
c
0.2% 0.5% 1.2% 1.2% 1.2%
- genotypic ETVr
c
with virologic
breakthrough
d
0.2% 0.2% 0.8% 0.8% 0.8%
a
Results reflect use of a 1-mg dose of entecavir for 147 of 149 patients in Year 3 and all patients in Years 4 and 5
and of combination entecavir-lamivudine therapy (followed by long-term entecavir therapy) for a median of 20
weeks for 130 of 149 patients in Year 3 and for 1 week for 1 of 121 patients in Year 4 in a rollover study.
b
Includes patients with at least one on-therapy HBV DNA measurement by PCR at or after week 24 through week 58
(Year 1), after week 58 through week 102 (Year 2), after week 102 through week 156 (Year 3), after week 156
through week 204 (Year 4), or after week 204 through week 252 (Year 5).
c
Patients also have LVDr substitutions.
d
≥ 1 log
10
increase above nadir in HBV DNA by PCR, confirmed with successive measurements or at the end of the
windowed time point.
ETVr substitutions (in addition to LVDr substitutions rtM204V/I ± rtL180M) were observed at
baseline in isolates from 10/187 (5%) lamivudine-refractory patients treated with entecavir and
monitored for resistance, indicating that prior lamivudine treatment can select these resistance
substitutions and that they can exist at a low frequency before entecavir treatment. Through Week 240,
3 of the 10 patients experienced virologic breakthrough (≥ 1 log
10
increase above nadir). Emerging
entecavir resistance in lamivudine-refractory studies through Week 240 is summarized in the table.

16
Genotypic Entecavir Resistance Through Year 5, Lamivudine-Refractory Studies
Year 1 Year 2 Year 3
a
Year 4
a
Year 5
a
Patients treated and monitored for
resistance
b
187 146 80 52 33
Patients in specific year with:
- emerging genotypic ETVr
c
11 12 16 6 2
- genotypic ETVr
c
with virologic
breakthrough
d
2
e
14
e
13
e
9
e
1
e
Cumulative probability of:
- emerging genotypic ETVr
c
6.2% 15% 36.3% 46.6% 51.45%
- genotypic ETVr
c
with virologic
breakthrough
d
1.1%
e
10.7%
e
27%
e
41.3%
e
43.6%
e
a
Results reflect use of combination entecavir-lamivudine therapy (followed by long-term entecavir therapy) for a
median of 13 weeks for 48 of 80 patients in Year 3, a median of 38 weeks for 10 of 52 patients in Year 4, and for 16
weeks for 1 of 33 patients in Year 5 in a rollover study.
b
Includes patients with at least one on-therapy HBV DNA measurement by PCR at or after week 24 through week 58
(Year 1), after week 58 through week 102 (Year 2), after week 102 through week 156 (Year 3), after week 156 through
week 204 (Year 4), or after week 204 through week 252 (Year 5).
c
Patients also have LVDr substitutions.
d
≥ 1 log
10
increase above nadir in HBV DNA by PCR, confirmed with successive measurements or at the end of the
windowed time point.
e
ETVr occurring in any year; virologic breakthrough in specified year.
Among lamivudine-refractory patients with baseline HBV DNA < 10
7
log
10
copies/ml, 64% (9/14)
achieved HBV DNA < 300 copies/ml at Week 48. These 14 patients had a lower rate of genotypic
entecavir resistance (cumulative probability 18.8% through 5 years of follow-up) than the overall
study population (see table). Also, lamivudine-refractory patients who achieved HBV DNA < 10
4
log
10
copies/ml by PCR at Week 24 had a lower rate of resistance than those who did not (5-year cumulative
probability 17.6% [n= 50] versus 60.5% [n= 135], respectively).
5.2 Pharmacokinetic properties
Absorption: entecavir is rapidly absorbed with peak plasma concentrations occurring between 0.5-
1.5 hours. The absolute bioavailability has not been determined. Based on urinary excretion of
unchanged drug, the bioavailability has been estimated to be at least 70%. There is a dose-
proportionate increase in C
max
and AUC values following multiple doses ranging from 0.1-1 mg.
Steady-state is achieved between 6-10 days after once daily dosing with ≈ 2 times accumulation. C
max
and C
min
at steady-state are 4.2 and 0.3 ng/ml, respectively, for a dose of 0.5 mg, and 8.2 and
0.5 ng/ml, respectively, for 1 mg. The tablet and oral solution were bioequivalent in healthy subjects;
therefore, both forms may be used interchangeably.
Administration of 0.5 mg entecavir with a standard high-fat meal (945 kcal, 54.6 g fat) or a light meal
(379 kcal, 8.2 g fat) resulted in a minimal delay in absorption (1-1.5 hour fed vs. 0.75 hour fasted), a
decrease in C
max
of 44-46%, and a decrease in AUC of 18-20%. The lower C
max
and AUC when taken
with food is not considered to be of clinical relevance in nucleoside-naive patients but could affect
efficacy in lamivudine-refractory patients (see section 4.2).
Distribution: the estimated volume of distribution for entecavir is in excess of total body water.
Protein binding to human serum protein in vitro is ≈ 13%.
Biotransformation: entecavir is not a substrate, inhibitor or inducer of the CYP450 enzyme system.
Following administration of
14
C-entecavir, no oxidative or acetylated metabolites and minor amounts
of the phase II metabolites, glucuronide and sulfate conjugates, were observed.

17
Elimination: entecavir is predominantly eliminated by the kidney with urinary recovery of unchanged
drug at steady-state of about 75% of the dose. Renal clearance is independent of dose and ranges
between 360-471 ml/min suggesting that entecavir undergoes both glomerular filtration and net
tubular secretion. After reaching peak levels, entecavir plasma concentrations decreased in a bi-
exponential manner with a terminal elimination half-life of ≈ 128-149 hours. The observed drug
accumulation index is ≈ 2 times with once daily dosing, suggesting an effective accumulation half-life
of about 24 hours.
Hepatic impairment: pharmacokinetic parameters in patients with moderate or severe hepatic
impairment were similar to those in patients with normal hepatic function.
Renal impairment: entecavir clearance decreases with decreasing creatinine clearance. A 4 hour period
of haemodialysis removed ≈ 13% of the dose, and 0.3% was removed by CAPD. The
pharmacokinetics of entecavir following a single 1 mg dose in patients (without chronic hepatitis B
infection) are shown in the table below:
Baseline Creatinine Clearance (ml/min)
Unimpaired
> 80
(n = 6)
Mild
> 50;
≤ 80
(n = 6)
Moderate
30-50
(n = 6)
Severe
20-
< 30
(n = 6)
Severe
Managed with
Haemodialysis
(n = 6)
Severe
Managed
with CAPD
(n = 4)
C
max
(ng/ml)
(CV%)
8.1
(30.7)
10.4
(37.2)
10.5
(22.7)
15.3
(33.8)
15.4
(56.4)
16.6
(29.7)
AUC
(0-T)
(ng·h /ml)
(CV)
27.9
(25.6)
51.5
(22.8)
69.5
(22.7)
145.7
(31.5)
233.9
(28.4)
221.8
(11.6)
CLR (ml/min)
(SD)
383.2
(101.8)
197.9
(78.1)
135.6
(31.6)
40.3
(10.1)
NA NA
CLT/F (ml/min)
(SD)
588.1
(153.7)
309.2
(62.6)
226.3
(60.1)
100.6
(29.1)
50.6
(16.5)
35.7
(19.6)
Post-Liver transplant: entecavir exposure in HBV-infected liver transplant recipients on a stable dose
of cyclosporine A or tacrolimus (n = 9) was ≈ 2 times the exposure in healthy subjects with normal
renal function. Altered renal function contributed to the increase in entecavir exposure in these patients
(see section 4.4).
Gender: AUC was 14% higher in women than in men, due to differences in renal function and weight.
After adjusting for differences in creatinine clearance and body weight there was no difference in
exposure between male and female subjects.
Elderly: the effect of age on the pharmacokinetics of entecavir was evaluated comparing elderly
subjects in the age range 65-83 years (mean age females 69 years, males 74 years) with young subjects
in the age range 20-40 years (mean age females 29 years, males 25 years). AUC was 29% higher in
elderly than in young subjects, mainly due to differences in renal function and weight. After adjusting
for differences in creatinine clearance and body weight, elderly subjects had a 12.5% higher AUC than
young subjects.The population pharmacokinetic analysis covering patients in the age range 16-
75 years did not identify age as significantly influencing entecavir pharmacokinetics.
Race: the population pharmacokinetic analysis did not identify race as significantly influencing
entecavir pharmacokinetics. However, conclusions can only be drawn for the Caucasian and Asian
groups as there were too few subjects in the other categories.

18
Paediatric population: the steady-state pharmacokinetics of entecavir were evaluated (study 028) in
24 nucleoside naïve and 19 lamivudine-experienced HBeAg-positive paediatric subjects from
2 to < 18 years of age with compensated liver disease. Entecavir exposure among nucleoside naïve
subjects receiving once daily doses of entecavir 0.015 mg/kg up to a maximum dose of 0.5 mg was
similar to the exposure achieved in adults receiving once daily doses of 0.5 mg. The Cmax, AUC(0-
24), and Cmin for these subjects was 6.31 ng/ml, 18.33 ng h/ml, and 0.28 ng/ml, respectively.
Entecavir exposure among lamivudine-experienced subjects receiving once daily doses of entecavir
0.030 mg/kg up to a maximum dose of 1.0 mg was similar to the exposure achieved in adults receiving
once daily doses of 1.0 mg. The Cmax, AUC(0-24), and Cmin for these subjects was 14.48 ng/ml,
38.58 ng·h/ml, and 0.47 ng/ml, respectively.
5.3 Preclinical safety data
In repeat-dose toxicology studies in dogs, reversible perivascular inflammation was observed in the
central nervous system, for which no-effect doses corresponded to exposures 19 and 10 times those in
humans (at 0.5 and 1 mg respectively). This finding was not observed in repeat-dose studies in other
species, including monkeys administered entecavir daily for 1 year at exposures ≥ 100 times those in
humans.
In reproductive toxicology studies in which animals were administered entecavir for up to 4 weeks, no
evidence of impaired fertility was seen in male or female rats at high exposures. Testicular changes
(seminiferous tubular degeneration) were evident in repeat-dose toxicology studies in rodents and dogs
at exposures ≥ 26 times those in humans. No testicular changes were evident in a 1-year study in
monkeys.
In pregnant rats and rabbits administered entecavir, no effect levels for embryotoxicity and maternal
toxicity corresponded to exposures ≥ 21 times those in humans. In rats, maternal toxicity, embryo-
foetal toxicity (resorptions), lower foetal body weights, tail and vertebral malformations, reduced
ossification (vertebrae, sternebrae, and phalanges), and extra lumbar vertebrae and ribs were observed
at high exposures. In rabbits, embryo-foetal toxicity (resorptions), reduced ossification (hyoid), and an
increased incidence of 13th rib were observed at high exposures. In a peri-postnatal study in rats, no
adverse effects on offspring were observed. In a separate study wherein entecavir was administered to
pregnant lactating rats at 10 mg/kg, both foetal exposure to entecavir and secretion of entecavir into
milk were demonstrated. In juvenile rats administered entecavir from postnatal days 4 to 80, a
moderately reduced acoustic startle response was noted during the recovery period (postnatal days
110 to 114) but not during the dosing period at AUC values ≥ 92 times those in humans at the 0.5 mg
dose or paediatric equivalent dose. Given the exposure margin, this finding is considered of unlikely
clinical significance.
No evidence of genotoxicity was observed in an Ames microbial mutagenicity assay, a mammalian-
cell gene mutation assay, and a transformation assay with Syrian hamster embryo cells. A
micronucleus study and a DNA repair study in rats were also negative. Entecavir was clastogenic to
human lymphocyte cultures at concentrations substantially higher than those achieved clinically.
Two-year carcinogenicity studies: in male mice, increases in the incidences of lung tumours were
observed at exposures ≥ 4 and ≥ 2 times that in humans at 0.5 mg and 1 mg respectively. Tumour
development was preceded by pneumocyte proliferation in the lung which was not observed in rats,
dogs, or monkeys, indicating that a key event in lung tumour development observed in mice likely was
species-
specific. Increased incidences of other tumours including brain gliomas in male and female
rats, liver carcinomas in male mice, benign vascular tumours in female mice, and liver adenomas and
carcinomas in female rats were seen only at high lifetime exposures. However, the no effect levels
could not be precisely established. The predictivity of the findings for humans is not known.
19
6. PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Tablet core:
Crospovidone
Lactose monohydrate
Magnesium stearate
Cellulose, Microcrystalline
Povidone
Tablet coating:
Titanium dioxide
Hypromellose
Macrogol 400
Polysorbate 80 (E433)
6.2 Incompatibilities
Not applicable.
6.3 Shelf life
2 years
6.4 Special precautions for storage
Blisters:
Do not store above 30°C. Store in the original carton.
Bottles:
Do not store above 25°C. Keep the bottle tightly closed.
6.5 Nature and contents of container
Each carton contains either:
30 x 1 film-coated tablet; 3 blister cards of 10 x 1 film-coated tablet each in Alu/Alu perforated
unit dose blisters, or
90 x 1 film-coated tablet; 9 blister cards of 10 x 1 film-coated tablet each in Alu/Alu perforated
unit dose blisters.
High-density polyethylene (HDPE) bottle with child resistant polypropylene closure containing
30 film-coated tablets. Each carton contains one bottle.
Not all pack sizes and container types may be marketed.
6.6 Special precautions for disposal
Any unused medicinal product or waste material should be disposed of in accordance with local
requirements.
7. MARKETING AUTHORISATION HOLDER
BRISTOL-MYERS SQUIBB PHARMA EEIG
Uxbridge Business Park
Sanderson Road
20
Uxbridge UB8 1DH
United Kingdom
8. MARKETING AUTHORISATION NUMBER(S)
Blister packs: EU/1/06/343/003
EU/1/06/343/006
Bottle packs: EU/1/06/343/001
9. DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
Date of first authorisation: 26 June 2006
Date of latest renewal: 26 June 2011
10. DATE OF REVISION OF THE TEXT
{MM/YYYY}
Detailed information on this medicinal product is available on the website of the European Medicines
Agency http://www.ema.europa.eu/.

21
1. NAME OF THE MEDICINAL PRODUCT
Baraclude 1 mg film-coated tablets
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
Each tablet contains 1 mg entecavir (as monohydrate).
Excipients with known effect
: each tablet contains 241 mg lactose.
For the full list of excipients, see section 6.1.
3. PHARMACEUTICAL FORM
Film-coated tablet (tablet)
Pink and triangular-shaped tablet with “BMS” debossed on one side and “1612” on the other.
4. CLINICAL PARTICULARS
4.1 Therapeutic indications
Baraclude is indicated for the treatment of chronic hepatitis B virus (HBV) infection (see section 5.1)
in adults with:
compensated liver disease and evidence of active viral replication, persistently elevated serum
alanine aminotransferase (ALT) levels and histological evidence of active inflammation and/or
fibrosis.
decompensated liver disease (see section 4.4)
For both compensated and decompensated liver disease, this indication is based on clinical trial data in
nucleoside naive patients with HBeAg positive and HBeAg negative HBV infection. With respect to
patients with lamivudine-refractory hepatitis B, see sections 4.2, 4.4 and 5.1.
Baraclude is also indicated for the treatment of chronic HBV infection in nucleoside naive paediatric
patients from 2 to < 18 years of age with compensated liver disease who have evidence of active viral
replication and persistently elevated serum ALT levels, or histological evidence of moderate to severe
inflammation and/or fibrosis. With respect to the decision to initiate treatment in paediatric patients,
see sections 4.2, 4.4, and 5.1.
4.2 Posology and method of administration
Therapy should be initiated by a physician experienced in the management of chronic hepatitis B
infection.
Posology
Compensated liver disease
Nucleoside naïve patients: the recommended dose in adults is 0.5 mg once daily, with or without food.
Lamivudine-refractory patients (i.e. with evidence of viraemia while on lamivudine or the presence of
lamivudine resistance [LVDr] mutations) (see sections 4.4 and 5.1): the recommended dose in adults is
1 mg once daily, which must be taken on an empty stomach (more than 2 hours before and more than
2 hours after a meal) (see section 5.2). In the presence of LVDr mutations, combination use of
22
entecavir plus a second antiviral agent (which does not share cross-resistance with either lamivudine
or entecavir) should be considered in preference to entecavir monotherapy (see section 4.4.).
Decompensated liver disease
The recommended dose for adult patients with decompensated liver disease is 1 mg once daily, which
must be taken on an empty stomach (more than 2 hours before and more than 2 hours after a meal)
(see section 5.2). For patients with lamivudine-refractory hepatitis B, see sections 4.4 and 5.1.
Duration of therapy
The optimal duration of treatment is unknown. Treatment discontinuation may be considered as
follows:
In HBeAg positive adult patients, treatment should be administered at least until 12 months after
achieving HBe seroconversion (HBeAg loss and HBV DNA loss with anti-HBe detection on
two consecutive serum samples at least 3-6 months apart) or until HBs seroconversion or there
is loss of efficacy (see section 4.4).
In HBeAg negative adult patients, treatment should be administered at least until HBs
seroconversion or there is evidence of loss of efficacy. With prolonged treatment for more than
2 years, regular reassessment is recommended to confirm that continuing the selected therapy
remains appropriate for the patient.
In patients with decompensated liver disease or cirrhosis, treatment cessation is not recommended.
Paediatric population
For appropriate dosing in the paediatric population, Baraclude oral solution or Baraclude 0.5 mg film-
coated tablets are available.
Elderly: no dosage adjustment based on age is required. The dose should be adjusted according to the
patient’s renal function (see dosage recommendations in renal impairment and section 5.2).
Gender and race: no dosage adjustment based on gender or race is required.
Renal impairment: the clearance of entecavir decreases with decreasing creatinine clearance (see
section 5.2). Dose adjustment is recommended for patients with creatinine clearance < 50 ml/min,
including those on haemodialysis or continuous ambulatory peritoneal dialysis (CAPD). A reduction
of the daily dose using Baraclude oral solution, as detailed in the table, is recommended. As an
alternative, in case the oral solution is not available, the dose can be adjusted by increasing the dosage
interval, also shown in the table. The proposed dose modifications are based on extrapolation of
limited data, and their safety and effectiveness have not been clinically evaluated. Therefore,
virological response should be closely monitored.
Baraclude dosage*
Creatinine clearance
(ml/min)
Nucleoside naïve patients
Lamivudine-refractory or
decompensated liver disease
≥ 50 0.5 mg once daily 1 mg once daily
30 - 49 0.25 mg once daily*
OR
0.5 mg every 48 hours
0.5 mg once daily
10 - 29 0.15 mg once daily*
OR
0.5 mg every 72 hours
0.3 mg once daily*
OR
0.5 mg every 48 hours
< 10
Haemodialysis or
0.05 mg once daily*
OR
0.1 mg once daily*
OR

23
CAPD** 0.5 mg every 5-7 days 0.5 mg every 72 hours
* for doses < 0.5 mg Baraclude oral solution is recommended.
** on haemodialysis days, administer entecavir after haemodialysis.
Hepatic impairment: no dose adjustment is required in patients with hepatic impairment.
Method of administration
Baraclude should be taken orally.
4.3 Contraindications
Hypersensitivity to the active substance or to any of the excipients listed in section 6.1.
4.4 Special warnings and precautions for use
Renal impairment: dosage adjustment is recommended for patients with renal impairment (see
section 4.2). The proposed dose modifications are based on extrapolation of limited data, and their
safety and effectiveness have not been clinically evaluated. Therefore, virological response should be
closely monitored.
Exacerbations of hepatitis: spontaneous exacerbations in chronic hepatitis B are relatively common
and are characterised by transient increases in serum ALT. After initiating antiviral therapy, serum
ALT may increase in some patients as serum HBV DNA levels decline (see section 4.8). Among
entecavir-treated patients on-treatment exacerbations had a median time of onset of 4-5 weeks. In
patients with compensated liver disease, these increases in serum ALT are generally not accompanied
by an increase in serum bilirubin concentrations or hepatic decompensation. Patients with advanced
liver disease or cirrhosis may be at a higher risk for hepatic decompensation following hepatitis
exacerbation, and therefore should be monitored closely during therapy.
Acute exacerbation of hepatitis has also been reported in patients who have discontinued hepatitis B
therapy (see section 4.2). Post-treatment exacerbations are usually associated with rising HBV DNA,
and the majority appears to be self-limited. However, severe exacerbations, including fatalities, have
been reported.
Among entecavir-treated nucleoside naive patients, post-treatment exacerbations had a median time to
onset of 23-24 weeks, and most were reported in HBeAg negative patients (see section 4.8). Hepatic
function should be monitored at repeated intervals with both clinical and laboratory follow-up for at
least 6 months after discontinuation of hepatitis B therapy. If appropriate, resumption of hepatitis B
therapy may be warranted.
Patients with decompensated liver disease: a higher rate of serious hepatic adverse events (regardless
of causality) has been observed in patients with decompensated liver disease, in particular in those
with Child-Turcotte-Pugh (CTP) class C disease, compared with rates in patients with compensated
liver function. Also, patients with decompensated liver disease may be at higher risk for lactic acidosis
and for specific renal adverse events such as hepatorenal syndrome. Therefore, clinical and laboratory
parameters should be closely monitored in this patient population (see also sections 4.8 and 5.1).
Lactic acidosis and severe hepatomegaly with steatosis: occurrences of lactic acidosis (in the absence
of hypoxaemia), sometimes fatal, usually associated with severe hepatomegaly and hepatic steatosis,
have been reported with the use of nucleoside analogues. As entecavir is a nucleoside analogue, this
risk cannot be excluded. Treatment with nucleoside analogues should be discontinued when rapidly
elevating aminotransferase levels, progressive hepatomegaly or metabolic/lactic acidosis of unknown
aetiology occur. Benign digestive symptoms, such as nausea, vomiting and abdominal pain, might be
indicative of lactic acidosis development. Severe cases, sometimes with fatal outcome, were associated
with pancreatitis, liver failure/hepatic steatosis, renal failure and higher levels of serum lactate.
24
Caution should be exercised when prescribing nucleoside analogues to any patient (particularly obese
women) with hepatomegaly, hepatitis or other known risk factors for liver disease. These patients
should be followed closely.
To differentiate between elevations in aminotransferases due to response to treatment and increases
potentially related to lactic acidosis, physicians should ensure that changes in ALT are associated with
improvements in other laboratory markers of chronic hepatitis B.
Resistance and specific precaution for lamivudine-refractory patients: mutations in the HBV
polymerase that encode lamivudine-resistance substitutions may lead to the subsequent emergence of
secondary substitutions, including those associated with entecavir associated resistance (ETVr). In a
small percentage of lamivudine-refractory patients, ETVr substitutions at residues rtT184, rtS202 or
rtM250 were present at baseline. Patients with lamivudine-resistant HBV are at higher risk of
developing subsequent entecavir resistance than patients without lamivudine resistance. The
cumulative probability of emerging genotypic entecavir resistance after 1, 2, 3, 4 and 5 years treatment
in the lamivudine-refractory studies was 6%, 15%, 36%, 47% and 51%, respectively. Virological
response should be frequently monitored in the lamivudine-refractory population and appropriate
resistance testing should be performed. In patients with a suboptimal virological response after 24
weeks of treatment with entecavir, a modification of treatment should be considered (see sections 4.5
and 5.1). When starting therapy in patients with a documented history of lamivudine-resistant HBV,
combination use of entecavir plus a second antiviral agent (which does not share cross-resistance with
either lamivudine or entecavir) should be considered in preference to entecavir monotherapy.
Pre-existing lamivudine-resistant HBV is associated with an increased risk for subsequent entecavir
resistance regardless of the degree of liver disease; in patients with decompensated liver disease,
virologic breakthrough may be associated with serious clinical complications of the underlying liver
disease. Therefore, in patients with both decompensated liver disease and lamivudine-resistant HBV,
combination use of entecavir plus a second antiviral agent (which does not share cross-resistance with
either lamivudine or entecavir) should be considered in preference to entecavir monotherapy.
Paediatric population: A lower rate of virologic response (HBV DNA < 50 IU/ml) was observed in
paediatric patients with baseline HBV DNA ≥ 8.0 log
10
IU/ml (see section 5.1). Entecavir should be
used in these patients only if the potential benefit justifies the potential risk to the child (e.g.
resistance). Since some paediatric patients may require long-term or even lifetime management of
chronic active hepatitis B, consideration should be given to the impact of entecavir on future treatment
options.
Liver transplant recipients: renal function should be carefully evaluated before and during entecavir
therapy in liver transplant recipients receiving cyclosporine or tacrolimus (see section 5.2).
Co-infection with hepatitis C or D: there are no data on the efficacy of entecavir in patients co-infected
with hepatitis C or D virus.
Human immunodeficiency virus (HIV)/HBV co-infected patients not receiving concomitant
antiretroviral therapy: entecavir has not been evaluated in HIV/HBV co-infected patients not
concurrently receiving effective HIV treatment. Emergence of HIV resistance has been observed when
entecavir was used to treat chronic hepatitis B infection in patients with HIV infection not receiving
highly active antiretroviral therapy (HAART) (see section 5.1). Therefore, therapy with entecavir
should not be used for HIV/HBV co-infected patients who are not receiving HAART. Entecavir has
not been studied as a treatment for HIV infection and is not recommended for this use.
HIV/HBV co-infected patients receiving concomitant antiretroviral therapy: entecavir has been studied
in 68 adults with HIV/HBV co-infection receiving a lamivudine-containing HAART regimen (see
section 5.1). No data are available on the efficacy of entecavir in HBeAg-negative patients co-infected
with HIV. There are limited data on patients co-infected with HIV who have low CD4 cell counts
(< 200 cells/mm
3
).
25
General: patients should be advised that therapy with entecavir has not been proven to reduce the risk
of transmission of HBV and therefore appropriate precautions should still be taken.
Lactose: this medicinal product contains 241 mg of lactose in each 1 mg daily dose.
Patients with rare hereditary problems of galactose intolerance, the Lapp lactase deficiency or glucose-
galactose malabsorption should not take this medicine. A lactose-free Baraclude oral solution is
available for these individuals.
4.5 Interaction with other medicinal products and other forms of interaction
Since entecavir is predominantly eliminated by the kidney (see section 5.2), coadministration with
medicinal products that reduce renal function or compete for active tubular secretion may increase
serum concentrations of either medicinal product. Apart from lamivudine, adefovir dipivoxil and
tenofovir disoproxil fumarate, the effects of coadministration of entecavir with medicinal products that
are excreted renally or affect renal function have not been evaluated. Patients should be monitored
closely for adverse reactions when entecavir is coadministered with such medicinal products.
No pharmacokinetic interactions between entecavir and lamivudine, adefovir or tenofovir were
observed.
Entecavir is not a substrate, an inducer or an inhibitor of cytochrome P450 (CYP450) enzymes (see
section 5.2). Therefore CYP450 mediated drug interactions are unlikely to occur with entecavir.
Paediatric population
Interaction studies have only been performed in adults.
4.6 Fertility, pregnancy and lactation
Women of childbearing potential: given that the potential risks to the developing foetus are unknown,
women of childbearing potential should use effective contraception.
Pregnancy: there are no adequate data from the use of entecavir in pregnant women. Studies in
animals have shown reproductive toxicity at high doses (see section 5.3). The potential risk for
humans is unknown. Baraclude should not be used during pregnancy unless clearly necessary. There
are no data on the effect of entecavir on transmission of HBV from mother to newborn infant.
Therefore, appropriate interventions should be used to prevent neonatal acquisition of HBV.
Breast-feeding: it is unknown whether entecavir is excreted in human milk. Available toxicological
data in animals have shown excretion of entecavir in milk (for details see section 5.3). A risk to the
infants cannot be excluded. Breast-feeding should be discontinued during treatment with Baraclude.
Fertility: toxicology studies in animals administered entecavir have shown no evidence of impaired
fertility (see section 5.3).
4.7 Effects on ability to drive and use machines
No studies on the effects on the ability to drive and use machines have been performed. Dizziness,
fatigue and somnolence are common side effects which may impair the ability to drive and use
machines.
4.8 Undesirable effects
a. Summary of the safety profile
In clinical studies in patients with compensated liver disease, the most common adverse reactions of
any severity with at least a possible relation to entecavir were headache (9%), fatigue (6%), dizziness
(4%) and nausea (3%). Exacerbations of hepatitis during and after discontinuation of entecavir therapy
have also been reported (see section 4.4 and c. Description of selected adverse reactions).
26
b. Tabulated list of adverse reactions
Assessment of adverse reactions is based on experience from postmarketing surveillance and four
clinical studies in which 1,720 patients with chronic hepatitis B infection and compensated liver
disease received double-blind treatment with entecavir (n = 862) or lamivudine (n = 858) for up to
107 weeks (see section 5.1). In these studies, the safety profiles, including laboratory abnormalities,
were comparable for entecavir 0.5 mg daily (679 nucleoside-naive HBeAg positive or negative
patients treated for a median of 53 weeks), entecavir 1 mg daily (183 lamivudine-refractory patients
treated for a median of 69 weeks), and lamivudine.
Adverse reactions considered at least possibly related to treatment with entecavir are listed by body
system organ class. Frequency is defined as very common (≥ 1/10); common (≥ 1/100 to < 1/10);
uncommon (≥ 1/1,000 to < 1/100); rare (≥ 1/10,000 to < 1/1,000). Within each frequency grouping,
undesirable effects are presented in order of decreasing seriousness.
Immune system disorders:
rare: anaphylactoid reaction
Psychiatric disorders:
common: insomnia
Nervous system disorders:
common: headache, dizziness, somnolence
Gastrointestinal disorders:
common: vomiting, diarrhoea, nausea, dyspepsia
Hepatobiliary disorders
common: increased transaminases
Skin and subcutaneous tissue disorders:
uncommon: rash, alopecia
General disorders and administration site
conditions:
common: fatigue
Cases of lactic acidosis have been reported, often in association with hepatic decompensation, other
serious medical conditions or drug exposures (see section 4.4).
Treatment beyond 48 weeks: continued treatment with entecavir for a median duration of 96 weeks did
not reveal any new safety signals.
c. Description of selected adverse reactions
Laboratory test abnormalities
: In clinical studies with nucleoside-naive patients, 5% had ALT
elevations > 3 times baseline, and < 1% had ALT elevations > 2 times baseline together with total
bilirubin > 2 times upper limit of normal (ULN) and > 2 times baseline. Albumin levels < 2.5 g/dl
occurred in < 1% of patients, amylase levels > 3 times baseline in 2%, lipase levels > 3 times baseline
in 11% and platelets < 50,000/mm
3
in < 1%.
In clinical studies with lamivudine-refractory patients, 4% had ALT elevations > 3 times baseline, and
< 1% had ALT elevations > 2 times baseline together with total bilirubin > 2 times ULN and > 2 times
baseline. Amylase levels > 3 times baseline occurred in 2% of patients, lipase levels > 3 times baseline
in 18% and platelets < 50,000/mm
3
in < 1%.
Exacerbations during treatment:
in studies with nucleoside naive patients, on treatment ALT elevations
> 10 times ULN and > 2 times baseline occurred in 2% of entecavir treated patients vs 4% of
lamivudine treated patients. In studies with lamivudine-refractory patients, on treatment ALT
elevations > 10 times ULN and > 2 times baseline occurred in 2% of entecavir treated patients vs 11%
of lamivudine treated patients. Among entecavir-treated patients, on-treatment ALT elevations had a
median time to onset of 4-5 weeks, generally resolved with continued treatment, and, in a majority of
cases, were associated with a ≥ 2 log
10
/ml reduction in viral load that preceded or coincided with the
ALT elevation. Periodic monitoring of hepatic function is recommended during treatment.

27
Exacerbations after discontinuation of treatment:
acute exacerbations of hepatitis have been reported
in patients who have discontinued anti-hepatitis B virus therapy, including therapy with entecavir (see
section 4.4). In studies in nucleoside-naive patients, 6% of entecavir-treated patients and 10% of
lamivudine-treated patients experienced ALT elevations (> 10 times ULN and > 2 times reference
[minimum of baseline or last end-of-dosing measurement]) during post-treatment follow-up. Among
entecavir-treated nucleoside-naive patients, ALT elevations had a median time to onset of 23-
24 weeks, and 86% (24/28) of ALT elevations occurred in HBeAg negative patients. In studies in
lamivudine-refractory patients, with only limited numbers of patients being followed up, 11% of
entecavir-treated patients and no lamivudine-treated patients developed ALT elevations during post-
treatment follow-up.
In the clinical trials entecavir treatment was discontinued if patients achieved a prespecified response.
If treatment is discontinued without regard to treatment response, the rate of post-treatment ALT flares
could be higher.
d. Paediatric Population
The safety of entecavir in paediatric patients from 2 to < 18 years of age is based on two ongoing
clinical trials in subjects with chronic HBV infection; one Phase 2 pharmacokinetic trial (study 028)
and one Phase 3 trial (study 189). These trials provide experience in 173 HBeAg-positive nucleoside-
treatment-naïve subjects treated with entecavir for a median duration of 60 weeks. The adverse
reactions observed in paediatric subjects who received treatment with entecavir were consistent with
those observed in clinical trials of entecavir in adults.(see a. Summary of the safety profile and
section 5.1)
e. Other special populations
Experience in patients with decompensated liver disease: the safety profile of entecavir in patients
with decompensated liver disease was assessed in a randomized open-label comparative study in
which patients received treatment with entecavir 1 mg/day (n = 102) or adefovir dipivoxil 10 mg/day
(n = 89) (study 048). Relative to the adverse reactions noted in section b. Tabulated list of adverse
reactions, one additional adverse reaction [decrease in blood bicarbonate (2%)] was observed in
entecavir-treated patients through week 48. The on-study cumulative death rate was 23% (23/102), and
causes of death were generally liver-related, as expected in this population. The on-study cumulative
rate of hepatocellular carcinoma (HCC) was 12% (12/102). Serious adverse events were generally
liver-related, with an on-study cumulative frequency of 69%. Patients with high baseline CTP score
were at higher risk of developing serious adverse events (see section 4.4).
Laboratory test abnormalities: through week 48 among entecavir-treated patients with decompensated
liver disease, none had ALT elevations both > 10 times ULN and > 2 times baseline, and 1% of
patients had ALT elevations > 2 times baseline together with total bilirubin > 2 times ULN and > 2
times baseline. Albumin levels < 2.5 g/dl occurred in 30% of patients, lipase levels > 3 times baseline
in 10% and platelets < 50,000/mm
3
in 20%.
Experience in patients co-infected with HIV:
the safety profile of entecavir in a limited number of
HIV/HBV co-infected patients on lamivudine-containing HAART (highly active antiretroviral
therapy) regimens was similar to the safety profile in monoinfected HBV patients (see section 4.4).
Gender/age:
there was no apparent difference in the safety profile of entecavir with respect to gender
(≈ 25% women in the clinical trials) or age (≈ 5% of patients > 65 years of age).
Reporting of suspected adverse reactions:
Reporting suspected adverse reactions after authorisation of
the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the
medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the
national reporting system listed in Appendix V.
28
4.9 Overdose
There is limited experience of entecavir overdose reported in patients. Healthy subjects who received
up to 20 mg/day for up to 14 days, and single doses up to 40 mg had no unexpected adverse reactions.
If overdose occurs, the patient must be monitored for evidence of toxicity and given standard
supportive treatment as necessary.
5. PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: antivirals for systemic use, nucleoside and nucleotide reverse
transcriptase inhibitors
ATC code: J05AF10
Mechanism of action: entecavir, a guanosine nucleoside analogue with activity against HBV
polymerase, is efficiently phosphorylated to the active triphosphate (TP) form, which has an
intracellular half-life of 15 hours. By competing with the natural substrate deoxyguanosine TP,
entecavir-TP functionally inhibits the 3 activities of the viral polymerase: (1) priming of the HBV
polymerase, (2) reverse transcription of the negative strand DNA from the pregenomic messenger
RNA, and (3) synthesis of the positive strand HBV DNA. The entecavir-TP K
i
for HBV DNA
polymerase is 0.0012 μM. Entecavir-TP is a weak inhibitor of cellular DNA polymerases α, β, and δ
with K
i
values of 18 to 40 µM. In addition, high exposures of entecavir had no relevant adverse effects
on γ polymerase or mitochondrial DNA synthesis in HepG2 cells (K
i
> 160 µM).
Antiviral activity: entecavir inhibited HBV DNA synthesis (50% reduction, EC
50
) at a concentration
of 0.004 µM in human HepG2 cells transfected with wild-type HBV. The median EC
50
value for
entecavir against LVDr HBV (rtL180M and rtM204V) was 0.026 µM (range 0.010-0.059 µM).
Recombinant viruses encoding adefovir-resistant substitutions at either rtN236T or rtA181V remained
fully susceptible to entecavir.
An analysis of the inhibitory activity of entecavir against a panel of laboratory and clinical HIV-1
isolates using a variety of cells and assay conditions yielded EC
50
values ranging from 0.026 to
> 10 µM; the lower EC
50
values were observed when decreased levels of virus were used in the assay.
In cell culture, entecavir selected for an M184I substitution at micromolar concentrations, confirming
inhibitory pressure at high entecavir concentrations. HIV variants containing the M184V substitution
showed loss of susceptibility to entecavir (see section 4.4).
In HBV combination assays in cell culture, abacavir, didanosine, lamivudine, stavudine, tenofovir or
zidovudine were not antagonistic to the anti-HBV activity of entecavir over a wide range of
concentrations. In HIV antiviral assays, entecavir at micromolar concentrations was not antagonistic to
the anti-HIV activity in cell culture of these six NRTIs or emtricitabine.
Resistance in cell culture: relative to wild-type HBV, LVDr viruses containing rtM204V and
rtL180M substitutions within the reverse transcriptase exhibit 8-fold decreased susceptibility to
entecavir. Incorporation of additional ETVr amino acid changes rtT184, rtS202 or rtM250 decreases
entecavir susceptibility in cell culture. Substitutions observed in clinical isolates (rtT184A, C, F, G, I,
L, M or S; rtS202 C, G or I; and/or rtM250I, L or V) further decreased entecavir susceptibility 16- to
741-fold relative to wild-type virus. The ETVr substitutions at residues rtT184, rtS202 and rtM250
alone have only a modest effect on entecavir susceptibility, and have not been observed in the absence
of LVDr substitutions in more than 1000 patient samples sequenced. Resistance is mediated by
reduced inhibitor binding to the altered HBV reverse transcriptase, and resistant HBV exhibits reduced
replication capacity in cell culture.
Clinical experience: the demonstration of benefit is based on histological, virological, biochemical,
and serological responses after 48 weeks of treatment in active-controlled clinical trials of 1,633 adults
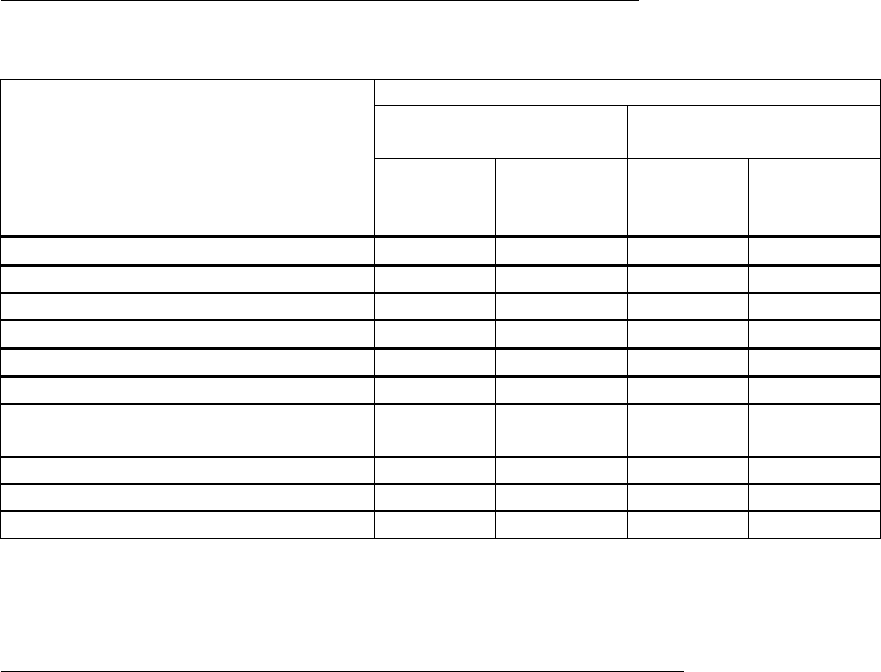
29
with chronic hepatitis B infection, evidence of viral replication and compensated liver disease. The
safety and efficacy of entecavir were also evaluated in an active-controlled clinical trial of 191 HBV-
infected patients with decompensated liver disease and in a clinical trial of 68 patients co-infected with
HBV and HIV.
In studies in patients with compensated liver disease, histological improvement was defined as a ≥ 2-
point decrease in Knodell necro-inflammatory score from baseline with no worsening of the Knodell
fibrosis score. Responses for patients with baseline Knodell Fibrosis Scores of 4 (cirrhosis) were
comparable to overall responses on all efficacy outcome measures (all patients had compensated liver
disease). High baseline Knodell necroinflammatory scores (> 10) were associated with greater
histological improvement in nucleoside-naive patients. Baseline ALT levels ≥ 2 times ULN and
baseline HBV DNA ≤ 9.0 log
10
copies/ml were both associated with higher rates of virologic response
(Week 48 HBV DNA < 400 copies/ml) in nucleoside-naive HBeAg-positive patients. Regardless of
baseline characteristics, the majority of patients showed histological and virological responses to
treatment.
Experience in nucleoside-naive patients with compensated liver disease:
Results at 48 weeks of randomised, double blind studies comparing entecavir (ETV) to lamivudine
(LVD) in HBeAg positive (022) and HBeAg negative (027) patients are presented in the table.
Nucleoside Naive
HBeAg Positive
(study 022)
HBeAg Negative
(study 027)
ETV
0.5 mg
once daily
LVD
100 mg
once daily
ETV
0.5 mg
once daily
LVD
100 mg
once daily
n 314
a
314
a
296
a
287
a
Histological improvement
b
72%* 62% 70%* 61%
Ishak fibrosis score improvement 39% 35% 36% 38%
Ishak fibrosis score worsening 8% 10% 12% 15%
n 354 355 325 313
Viral load reduction (log
10
copies/ml)
c
-6.86* -5.39 -5.04* -4.53
HBV DNA undetectable
(< 300 copies/ml by PCR)
c
67%* 36% 90%* 72%
ALT normalisation (≤ 1 times ULN) 68%* 60% 78%* 71%
HBeAg Seroconversion 21% 18%
*p value vs lamivudine < 0.05
a
patients with evaluable baseline histology (baseline Knodell Necroinflammatory Score ≥ 2)
b
a primary endpoint
c
Roche Cobas Amplicor PCR assay (LLOQ = 300 copies/ml)
Experience in lamivudine-refractory patients with compensated liver disease:
In a randomised, double-blind study in HBeAg positive lamivudine-refractory patients (026), with
85% of patients presenting LVDr mutations at baseline, patients receiving lamivudine at study entry
either switched to entecavir 1 mg once daily, with neither a washout nor an overlap period (n = 141),
or continued on lamivudine 100 mg once daily (n = 145). Results at 48 weeks are presented in the
table.
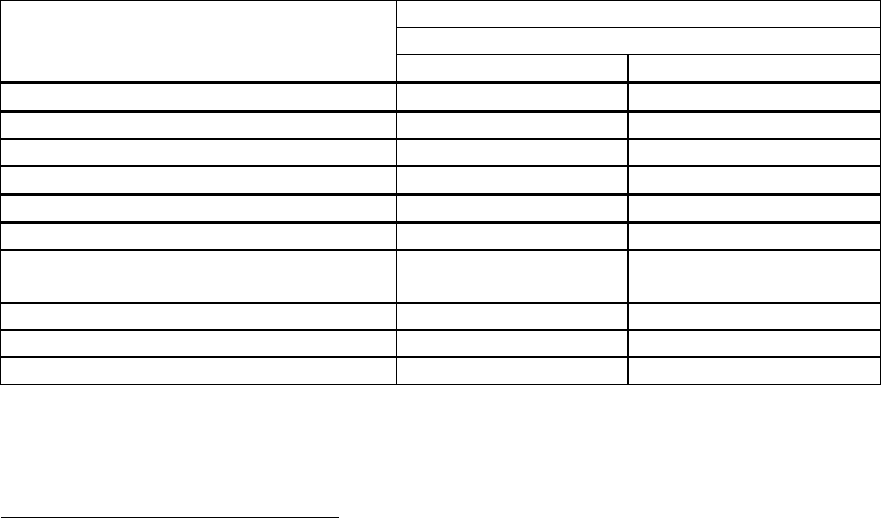
30
Lamivudine-refractory
HBeAg positive (study 026)
ETV 1.0 mg once daily LVD 100 mg once daily
n 124
a
116
a
Histological improvement
b
55%* 28%
Ishak fibrosis score improvement 34%* 16%
Ishak fibrosis score worsening 11% 26%
n 141 145
Viral load reduction (log
10
copies/ml)
c
-5.11* -0.48
HBV DNA undetectable (< 300 copies/ml
by PCR)
c
19%* 1%
ALT normalisation (≤ 1 times ULN) 61%* 15%
HBeAg Seroconversion 8% 3%
*p value vs lamivudine < 0.05
a
patients with evaluable baseline histology (baseline Knodell Necroinflammatory Score ≥ 2)
b
a primary endpoint.
c
Roche Cobas Amplicor PCR assay (LLOQ = 300 copies/ml)
Results beyond 48 weeks of treatment:
Treatment was discontinued when prespecified response criteria were met either at 48 weeks or during
the second year of treatment. Response criteria were HBV virological suppression (HBV DNA
< 0.7 MEq/ml by bDNA) and loss of HBeAg (in HBeAg positive patients) or ALT < 1.25 times ULN
(in HBeAg negative patients). Patients in response were followed for an additional 24 weeks off-
treatment. Patients who met virologic but not serologic or biochemical response criteria continued
blinded treatment. Patients who did not have a virologic response were offered alternative treatment.
Nucleoside-naive:
HBeAg positive (study 022): treatment with entecavir for up to 96 weeks (n = 354) resulted in
cumulative response rates of 80% for HBV DNA < 300 copies/ml by PCR, 87% for ALT
normalisation, 31% for HBeAg seroconversion and 2% for HBsAg seroconversion (5% for HBsAg
loss). For lamivudine (n = 355), cumulative response rates were 39% for HBV DNA < 300 copies/ml
by PCR, 79% for ALT normalisation, 26% for HBeAg seroconversion, and 2% for HBsAg
seroconversion (3% for HBsAg loss).
At end of dosing, among patients who continued treatment beyond 52 weeks (median of 96 weeks),
81% of 243 entecavir-treated and 39% of 164 lamivudine-treated patients had HBV DNA
< 300 copies/ml by PCR while ALT normalisation (≤ 1 times ULN) occurred in 79% of entecavir-
treated and 68% of lamivudine-treated patients.
HBeAg negative (study 027): treatment with entecavir up to 96 weeks (n = 325) resulted in cumulative
response rates of 94% for HBV DNA < 300 copies/ml by PCR and 89% for ALT normalisation versus
77% for HBV DNA < 300 copies/ml by PCR and 84% for ALT normalisation for lamivudine-treated
patients (n = 313).
For 26 entecavir-treated and 28 lamivudine-treated patients who continued treatment beyond 52 weeks
(median 96 weeks), 96% of entecavir-treated and 64% of lamivudine-treated patients had HBV DNA
< 300 copies/ml by PCR at end of dosing. ALT normalisation (≤ 1 times ULN) occurred in 27% of
entecavir-treated and 21% of lamivudine-treated patients at end of dosing.
For patients who met protocol-defined response criteria, response was sustained throughout the 24-
week post-treatment follow-up in 75% (83/111) of entecavir responders vs 73% (68/93) for
lamivudine responders in study 022 and 46% (131/286) of entecavir responders vs 31% (79/253) for
lamivudine responders in study 027. By 48 weeks of post-treatment follow-up, a substantial number of
HBeAg negative patients lost response.
Liver biopsy results: 57 patients from the pivotal nucleoside-naive studies 022 (HBeAg positive) and
027 (HBeAg negative) who enrolled in a long-term rollover study were evaluated for long-term liver

31
histology outcomes. The entecavir dosage was 0.5 mg daily in the pivotal studies (mean exposure 85
weeks) and 1 mg daily in the rollover study (mean exposure 177 weeks), and 51 patients in the
rollover study initially also received lamivudine (median duration 29 weeks). Of these patients, 55/57
(96%) had histological improvement as previously defined (see above), and 50/57 (88%) had a ≥ 1-
point decrease in Ishak fibrosis score. For patients with baseline Ishak fibrosis score ≥ 2, 25/43 (58%)
had a ≥ 2-point decrease. All (10/10) patients with advanced fibrosis or cirrhosis at baseline (Ishak
fibrosis score of 4, 5 or 6) had a ≥ 1 point decrease (median decrease from baseline was 1.5 points). At
the time of the long-term biopsy, all patients had HBV DNA < 300 copies/ml and 49/57 (86%) had
serum ALT ≤ 1 times ULN. All 57 patients remained positive for HBsAg.
Lamivudine-refractory:
HBeAg positive (study 026): treatment with entecavir for up to 96 weeks (n = 141) resulted in
cumulative response rates of 30% for HBV DNA < 300 copies/ml by PCR, 85% for ALT
normalisation and 17% for HBeAg seroconversion.
For the 77 patients who continued entecavir treatment beyond 52 weeks (median 96 weeks), 40% of
patients had HBV DNA < 300 copies/ml by PCR and 81% had ALT normalisation (≤ 1 times ULN) at
end of dosing.
Age/gender:
There was no apparent difference in efficacy for entecavir based on gender (≈ 25% women in the
clinical trials) or age (≈ 5% of patients > 65 years of age).
Special populations
Patients with decompensated liver disease: in study 048, 191 patients with HBeAg positive or
negative chronic HBV infection and evidence of hepatic decompensation, defined as a CTP score of 7
or higher, received entecavir 1 mg once daily or adefovir dipivoxil 10 mg once daily. Patients were
either HBV-treatment-naïve or pretreated (excluding pretreatment with entecavir, adefovir dipivoxil,
or tenofovir disoproxil fumarate). At baseline, patients had a mean CTP score of 8.59 and 26% of
patients were CTP class C. The mean baseline Model for End Stage Liver Disease (MELD) score was
16.23. Mean serum HBV DNA by PCR was 7.83 log
10
copies/ml and mean serum ALT was 100 U/l;
54% of patients were HBeAg positive, and 35% of patients had LVDr substitutions at baseline.
Entecavir was superior to adefovir dipivoxil on the primary efficacy endpoint of mean change from
baseline in serum HBV DNA by PCR at week 24. Results for selected study endpoints at weeks 24
and 48 are shown in the table.
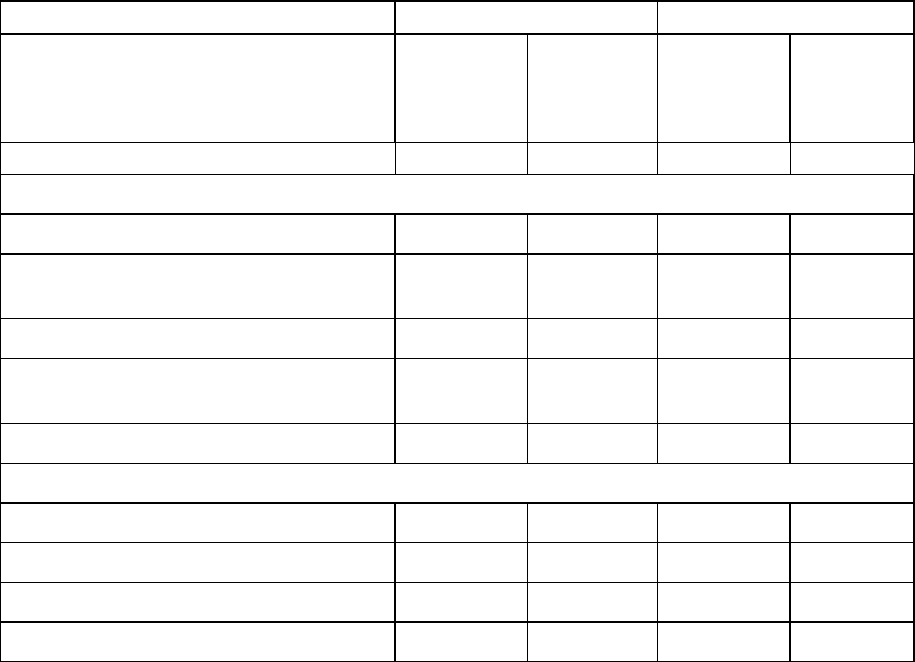
32
Week 24 Week 48
ETV
1 mg
once daily
Adefovir
Dipivoxil
10 mg
once daily
ETV
1 mg
once daily
Adefovir
Dipivoxil
10 mg
once daily
n 100 91 100 91
HBV DNA
a
Proportion undetectable (<300 copies/ml)
b
49%* 16% 57%* 20%
Mean change from baseline
(log
10
copies/ml)
c
-4.48* -3.40 -4.66 -3.90
Stable or improved CTP score
b
,
d
66% 71% 61% 67%
MELD score
Mean change from baseline
c,e
-2.0
-0.9
-2.6
-1.7
HBsAg loss
b
1% 0 5% 0
Normalization of:
f
ALT (≤1 X ULN)
b
46/78 (59%)* 28/71 (39%) 49/78 (63%)* 33/71 (46%)
Albumin (≥1 X LLN)
b
20/82 (24%) 14/69 (20%) 32/82 (39%) 20/69 (29%)
Bilirubin (≤1 X ULN)
b
12/75 (16%) 10/65 (15%) 15/75 (20%) 18/65 (28%)
Prothrombin time (≤1 X ULN)
b
9/95 (9%) 6/82 (7%) 8/95 (8%) 7/82 (9%)
a
Roche COBAS Amplicor PCR assay (LLOQ = 300 copies/ml).
b
NC=F (noncompleter=failure), meaning treatment discontinuations before the analysis week, including reasons such as
death, lack of efficacy, adverse event, noncompliance/loss-to-follow-up, are counted as failures (e.g., HBV
DNA ≥ 300 copies/ml)
c
NC=M (noncompleters=missing)
d
Defined as decrease or no change from baseline in CTP score.
e
Baseline mean MELD score was 17.1 for ETV and 15.3 for adefovir dipivoxil.
f
Denominator is patients with abnormal values at baseline.
*p<0.05
ULN=upper limit of normal, LLN=lower limit of normal.
The time to onset of HCC or death (whichever occurred first) was comparable in the two treatment
groups; on-study cumulative death rates were 23% (23/102) and 33% (29/89) for patients treated with
entecavir and adefovir dipivoxil, respectively, and on-study cumulative rates of HCC were 12%
(12/102) and 20% (18/89) for entecavir and adefovir dipivoxil, respectively.
For patients with LVDr substitutions at baseline, the percentage of patients with HBV DNA
<300 copies/ml was 44% for entecavir and 20% for adefovir at week 24 and 50% for entecavir and
17% for adefovir at week 48.
HIV/HBV co-infected patients receiving concomitant HAART: study 038 included 67 HBeAg positive
and 1 HBeAg negative patients co-infected with HIV. Patients had stable controlled HIV (HIV RNA
< 400 copies/ml) with recurrence of HBV viraemia on a lamivudine-containing HAART regimen.
HAART regimens did not include emtricitabine or tenofovir disoproxil fumarate. At baseline
entecavir-treated patients had a median duration of prior lamivudine therapy of 4.8 years and median
CD4 count of 494 cells/mm
3
(with only 5 subjects having CD4 count < 200 cells/mm
3
). Patients
continued their lamivudine-regimen and were assigned to add either entecavir 1 mg once daily
(n = 51) or placebo (n = 17) for 24 weeks followed by an additional 24 weeks where all received
entecavir. At 24 weeks the reduction in HBV viral load was significantly greater with entecavir (-3.65
vs an increase of 0.11 log
10
copies/ml). For patients originally assigned to entecavir treatment, the
reduction in HBV DNA at 48 weeks was -4.20 log
10
copies/ml, ALT normalisation had occurred in
37% of patients with abnormal baseline ALT and none achieved HBeAg seroconversion.
33
HIV/HBV co-infected patients not receiving concomitant HAART: entecavir has not been evaluated in
HIV/HBV co-infected patients not concurrently receiving effective HIV treatment. Reductions in HIV
RNA have been reported in HIV/HBV co-infected patients receiving entecavir monotherapy without
HAART. In some cases, selection of HIV variant M184V has been observed, which has implications
for the selection of HAART regimens that the patient may take in the future. Therefore, entecavir
should not be used in this setting due to the potential for development of HIV resistance (see section
4.4).
Liver transplant recipients: the safety and efficacy of entecavir 1 mg once daily were assessed in a
single-arm study in 65 patients who received a liver transplant for complications of chronic HBV
infection and had HBV DNA <172 IU/ml (approximately 1000 copies/ml) at the time of transplant.
The study population was 82% male, 39% Caucasian, and 37% Asian, with a mean age of 49 years;
89% of patients had HBeAg-negative disease at the time of transplant. Of the 61 patients who were
evaluable for efficacy (received entecavir for at least 1 month), 60 also received hepatitis B immune
globulin (HBIg) as part of the post-transplant prophylaxis regimen. Of these 60 patients, 49 received
more than 6 months of HBIg therapy. At Week 72 post-transplant, none of 55 observed cases had
virologic recurrence of HBV [defined as HBV DNA ≥50 IU/ml (approximately 300 copies/ml)], and
there was no reported virologic recurrence at time of censoring for the remaining 6 patients. All 61
patients had HBsAg loss post-transplantation, and 2 of these later became HBsAg positive despite
maintaining undetectable HBV DNA (<6 IU/ml). The frequency and nature of adverse events in this
study were consistent with those expected in patients who have received a liver transplant and the
known safety profile of entecavir.
Paediatric population: Study 189 is an ongoing study of the efficacy and safety of entecavir among
180 nucleoside-treatment-naïve children and adolescents from 2 to < 18 years of age with HBeAg-
positive chronic hepatitis B infection, compensated liver disease, and elevated ALT. Subjects were
randomized (2:1) to receive blinded treatment with entecavir 0.015 mg/kg up to 0.5 mg/day (N = 120)
or placebo (N = 60). The randomization was stratified by age group (2 to 6 years; > 6 to 12 years; and
> 12 to < 18 years). Baseline demographics and HBV disease characteristics were comparable
between the 2 treatment arms and across age cohorts. At study entry, the mean HBV DNA was
8.0 log
10
IU/ml and mean ALT was 105 U/l for the primary cohort (the first 123 treated subjects). The
primary efficacy endpoint was a composite of HBeAg seroconversion and serum HBV DNA
< 50 IU/ml (approximately 300 copies/ml) at Week 48 for the primary cohort.
Twenty-four percent (20/82) of subjects in the entecavir-treated group and 2% (1/41) of subjects in the
placebo-treated group met the primary endpoint. Forty-six percent (38/82) of entecavir-treated subjects
and 2% (1/41) of placebo-treated subjects achieved HBV DNA < 50 IU/ml at Week 48. When
assessed by baseline HBV DNA, 77% (26/34) of entecavir-treated subjects with HBV
DNA < 8 log
10
IU/ml at baseline and 25% (12/48) with HBV DNA ≥ 8 log
10
IU/ml achieved HBV
DNA < 50 IU/ml. ALT normalization occurred in 67% (55/82) of entecavir-treated subjects and 22%
(9/41) of placebo-treated subjects; 24% (20/82) of entecavir-treated subjects and 12% (5/41) of
placebo-treated subjects had HBeAg seroconversion.
In 2 paediatric studies (Studies 028 and 189), 110 patients who received entecavir for up to 48 weeks
were monitored for resistance. Genotypic evaluations were performed on all patients who had
virologic breakthrough, or HBV DNA ≥ 50 IU/ml at Week 48 or discontinued early. No amino acid
substitutions associated with resistance to entecavir were identified.
Clinical resistance: patients in clinical trials initially treated with entecavir 0.5 mg (nucleoside-naive)
or 1.0 mg (lamivudine-refractory) and with an on-therapy PCR HBV DNA measurement at or after
Week 24 were monitored for resistance.
Through Week 240 in nucleoside-naive studies, genotypic evidence of ETVr substitutions at rtT184,
rtS202, or rtM250 was identified in 3 patients treated with entecavir, 2 of whom experienced virologic
breakthrough (see table). These substitutions were observed only in the presence of LVDr
substitutions (rtM204V and rtL180M).
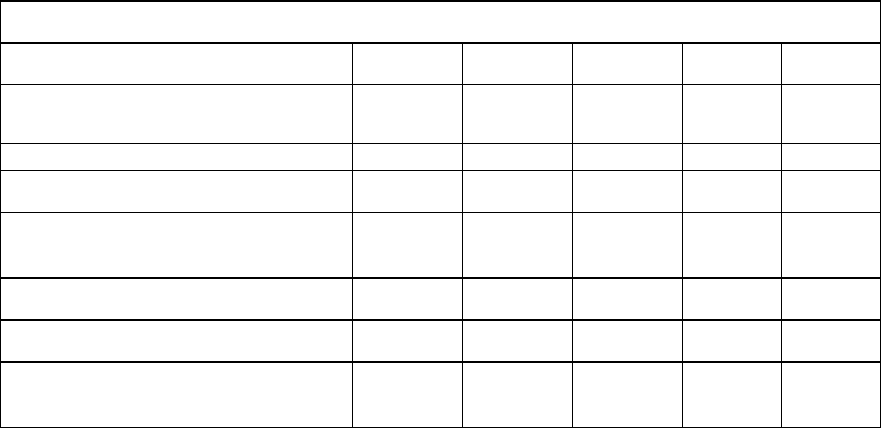
34
Emerging Genotypic Entecavir Resistance Through Year 5, Nucleoside-Naive Studies
Year 1 Year 2
Year 3
a
Year 4
a
Year 5
a
Patients treated and monitored for
resistance
b
663 278 149 121 108
Patients in specific year with:
- emerging genotypic ETVr
c
1 1 1 0 0
- genotypic ETVr
c
with virologic
breakthrough
d
1 0 1 0 0
Cumulative probability of:
- emerging genotypic ETVr
c
0.2% 0.5% 1.2% 1.2% 1.2%
- genotypic ETVr
c
with virologic
breakthrough
d
0.2% 0.2% 0.8% 0.8% 0.8%
a
Results reflect use of a 1-mg dose of entecavir for 147 of 149 patients in Year 3 and all patients in Years 4 and 5
and of combination entecavir-lamivudine therapy (followed by long-term entecavir therapy) for a median of 20
weeks for 130 of 149 patients in Year 3 and for 1 week for 1 of 121 patients in Year 4 in a rollover study.
b
Includes patients with at least one on-therapy HBV DNA measurement by PCR at or after week 24 through week 58
(Year 1), after week 58 through week 102 (Year 2), after week 102 through week 156 (Year 3), after week 156
through week 204 (Year 4), or after week 204 through week 252 (Year 5).
c
Patients also have LVDr substitutions.
d
≥ 1 log
10
increase above nadir in HBV DNA by PCR, confirmed with successive measurements or at the end of the
windowed time point.
ETVr substitutions (in addition to LVDr substitutions rtM204V/I ± rtL180M) were observed at
baseline in isolates from 10/187 (5%) lamivudine-refractory patients treated with entecavir and
monitored for resistance, indicating that prior lamivudine treatment can select these resistance
substitutions and that they can exist at a low frequency before entecavir treatment. Through Week 240,
3 of the 10 patients experienced virologic breakthrough (≥ 1 log
10
increase above nadir). Emerging
entecavir resistance in lamivudine-refractory studies through Week 240 is summarized in the table.
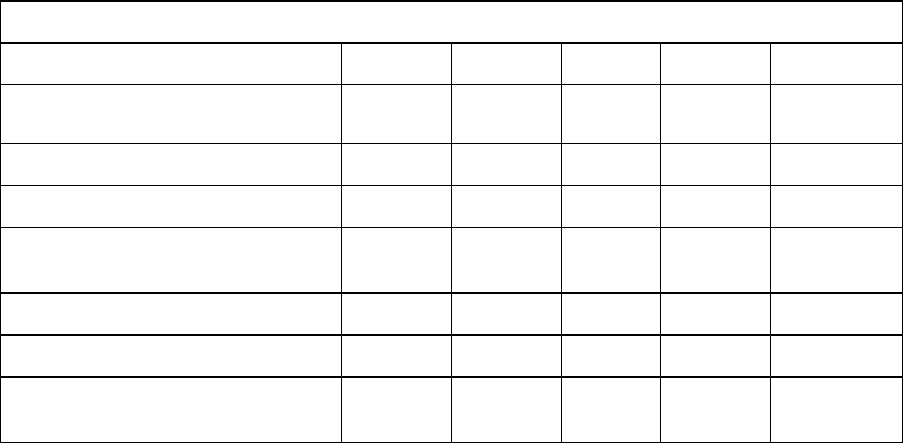
35
Genotypic Entecavir Resistance Through Year 5, Lamivudine-Refractory Studies
Year 1 Year 2 Year 3
a
Year 4
a
Year 5
a
Patients treated and monitored for
resistance
b
187 146 80 52 33
Patients in specific year with:
- emerging genotypic ETVr
c
11 12 16 6 2
- genotypic ETVr
c
with virologic
breakthrough
d
2
e
14
e
13
e
9
e
1
e
Cumulative probability of:
- emerging genotypic ETVr
c
6.2% 15% 36.3% 46.6% 51.45%
- genotypic ETVr
c
with virologic
breakthrough
d
1.1%
e
10.7%
e
27%
e
41.3%
e
43.6%
e
a
Results reflect use of combination entecavir-lamivudine therapy (followed by long-term entecavir therapy) for a
median of 13 weeks for 48 of 80 patients in Year 3, a median of 38 weeks for 10 of 52 patients in Year 4, and for 16
weeks for 1 of 33 patients in Year 5 in a rollover study.
b
Includes patients with at least one on-therapy HBV DNA measurement by PCR at or after week 24 through week 58
(Year 1), after week 58 through week 102 (Year 2), after week 102 through week 156 (Year 3), after week 156 through
week 204 (Year 4), or after week 204 through week 252 (Year 5).
c
Patients also have LVDr substitutions.
d
≥ 1 log
10
increase above nadir in HBV DNA by PCR, confirmed with successive measurements or at the end of the
windowed time point.
e
ETVr occurring in any year; virologic breakthrough in specified year.
Among lamivudine-refractory patients with baseline HBV DNA < 10
7
log
10
copies/ml, 64% (9/14)
achieved HBV DNA < 300 copies/ml at Week 48. These 14 patients had a lower rate of genotypic
entecavir resistance (cumulative probability 18.8% through 5 years of follow-up) than the overall
study population (see table). Also, lamivudine-refractory patients who achieved HBV DNA < 10
4
log
10
copies/ml by PCR at Week 24 had a lower rate of resistance than those who did not (5-year cumulative
probability 17.6% [n= 50] versus 60.5% [n= 135], respectively).
5.2 Pharmacokinetic properties
Absorption: entecavir is rapidly absorbed with peak plasma concentrations occurring between 0.5-
1.5 hours. The absolute bioavailability has not been determined. Based on urinary excretion of
unchanged drug, the bioavailability has been estimated to be at least 70%. There is a dose-
proportionate increase in C
max
and AUC values following multiple doses ranging from 0.1-1 mg.
Steady-state is achieved between 6-10 days after once daily dosing with ≈ 2 times accumulation. C
max
and C
min
at steady-state are 4.2 and 0.3 ng/ml, respectively, for a dose of 0.5 mg, and 8.2 and
0.5 ng/ml, respectively, for 1 mg. The tablet and oral solution were bioequivalent in healthy subjects;
therefore, both forms may be used interchangeably.
Administration of 0.5 mg entecavir with a standard high-fat meal (945 kcal, 54.6 g fat) or a light meal
(379 kcal, 8.2 g fat) resulted in a minimal delay in absorption (1-1.5 hour fed vs. 0.75 hour fasted), a
decrease in C
max
of 44-46%, and a decrease in AUC of 18-20%. The lower C
max
and AUC when taken
with food is not considered to be of clinical relevance in nucleoside-naive patients but could affect
efficacy in lamivudine-refractory patients (see section 4.2).
Distribution: the estimated volume of distribution for entecavir is in excess of total body water.
Protein binding to human serum protein in vitro is ≈ 13%.
Biotransformation: entecavir is not a substrate, inhibitor or inducer of the CYP450 enzyme system.
Following administration of
14
C-entecavir, no oxidative or acetylated metabolites and minor amounts
of the phase II metabolites, glucuronide and sulfate conjugates, were observed.

36
Elimination: entecavir is predominantly eliminated by the kidney with urinary recovery of unchanged
drug at steady-state of about 75% of the dose. Renal clearance is independent of dose and ranges
between 360-471 ml/min suggesting that entecavir undergoes both glomerular filtration and net
tubular secretion. After reaching peak levels, entecavir plasma concentrations decreased in a bi-
exponential manner with a terminal elimination half-life of ≈ 128-149 hours. The observed drug
accumulation index is ≈ 2 times with once daily dosing, suggesting an effective accumulation half-life
of about 24 hours.
Hepatic impairment: pharmacokinetic parameters in patients with moderate or severe hepatic
impairment were similar to those in patients with normal hepatic function.
Renal impairment: entecavir clearance decreases with decreasing creatinine clearance. A 4 hour period
of haemodialysis removed ≈ 13% of the dose, and 0.3% was removed by CAPD. The
pharmacokinetics of entecavir following a single 1 mg dose in patients (without chronic hepatitis B
infection) are shown in the table below:
Baseline Creatinine Clearance (ml/min)
Unimpaired
> 80
(n = 6)
Mild
> 50;
≤ 80
(n = 6)
Moderate
30-50
(n = 6)
Severe
20-
< 30
(n = 6)
Severe
Managed with
Haemodialysis
(n = 6)
Severe
Managed
with CAPD
(n = 4)
C
max
(ng/ml)
(CV%)
8.1
(30.7)
10.4
(37.2)
10.5
(22.7)
15.3
(33.8)
15.4
(56.4)
16.6
(29.7)
AUC
(0-T)
(ng·h /ml)
(CV)
27.9
(25.6)
51.5
(22.8)
69.5
(22.7)
145.7
(31.5)
233.9
(28.4)
221.8
(11.6)
CLR (ml/min)
(SD)
383.2
(101.8)
197.9
(78.1)
135.6
(31.6)
40.3
(10.1)
NA NA
CLT/F (ml/min)
(SD)
588.1
(153.7)
309.2
(62.6)
226.3
(60.1)
100.6
(29.1)
50.6
(16.5)
35.7
(19.6)
Post-Liver transplant: entecavir exposure in HBV-infected liver transplant recipients on a stable dose
of cyclosporine A or tacrolimus (n = 9) was ≈ 2 times the exposure in healthy subjects with normal
renal function. Altered renal function contributed to the increase in entecavir exposure in these patients
(see section 4.4).
Gender: AUC was 14% higher in women than in men, due to differences in renal function and weight.
After adjusting for differences in creatinine clearance and body weight there was no difference in
exposure between male and female subjects.
Elderly: the effect of age on the pharmacokinetics of entecavir was evaluated comparing elderly
subjects in the age range 65-83 years (mean age females 69 years, males 74 years) with young subjects
in the age range 20-40 years (mean age females 29 years, males 25 years). AUC was 29% higher in
elderly than in young subjects, mainly due to differences in renal function and weight. After adjusting
for differences in creatinine clearance and body weight, elderly subjects had a 12.5% higher AUC than
young subjects.The population pharmacokinetic analysis covering patients in the age range 16-
75 years did not identify age as significantly influencing entecavir pharmacokinetics.
Race: the population pharmacokinetic analysis did not identify race as significantly influencing
entecavir pharmacokinetics. However, conclusions can only be drawn for the Caucasian and Asian
groups as there were too few subjects in the other categories.

37
Paediatric population: the steady-state pharmacokinetics of entecavir were evaluated (study 028) in
24 nucleoside naïve and 19 lamivudine-experienced HBeAg-positive paediatric subjects from
2 to < 18 years of age with compensated liver disease. Entecavir exposure among nucleoside naïve
subjects receiving once daily doses of entecavir 0.015 mg/kg up to a maximum dose of 0.5 mg was
similar to the exposure achieved in adults receiving once daily doses of 0.5 mg. The Cmax, AUC(0-
24), and Cmin for these subjects was 6.31 ng/ml, 18.33 ng h/ml, and 0.28 ng/ml, respectively.
Entecavir exposure among lamivudine-experienced subjects receiving once daily doses of entecavir
0.030 mg/kg up to a maximum dose of 1.0 mg was similar to the exposure achieved in adults receiving
once daily doses of 1.0 mg. The Cmax, AUC(0-24), and Cmin for these subjects was 14.48 ng/ml,
38.58 ng·h/ml, and 0.47 ng/ml, respectively.
5.3 Preclinical safety data
In repeat-dose toxicology studies in dogs, reversible perivascular inflammation was observed in the
central nervous system, for which no-effect doses corresponded to exposures 19 and 10 times those in
humans (at 0.5 and 1 mg respectively). This finding was not observed in repeat-dose studies in other
species, including monkeys administered entecavir daily for 1 year at exposures ≥ 100 times those in
humans.
In reproductive toxicology studies in which animals were administered entecavir for up to 4 weeks, no
evidence of impaired fertility was seen in male or female rats at high exposures. Testicular changes
(seminiferous tubular degeneration) were evident in repeat-dose toxicology studies in rodents and dogs
at exposures ≥ 26 times those in humans. No testicular changes were evident in a 1-year study in
monkeys.
In pregnant rats and rabbits administered entecavir, no effect levels for embryotoxicity and maternal
toxicity corresponded to exposures ≥ 21 times those in humans. In rats, maternal toxicity, embryo-
foetal toxicity (resorptions), lower foetal body weights, tail and vertebral malformations, reduced
ossification (vertebrae, sternebrae, and phalanges), and extra lumbar vertebrae and ribs were observed
at high exposures. In rabbits, embryo-foetal toxicity (resorptions), reduced ossification (hyoid), and an
increased incidence of 13th rib were observed at high exposures. In a peri-postnatal study in rats, no
adverse effects on offspring were observed. In a separate study wherein entecavir was administered to
pregnant lactating rats at 10 mg/kg, both foetal exposure to entecavir and secretion of entecavir into
milk were demonstrated. In juvenile rats administered entecavir from postnatal days 4 to 80, a
moderately reduced acoustic startle response was noted during the recovery period (postnatal days
110 to 114) but not during the dosing period at AUC values ≥ 92 times those in humans at the 0.5 mg
dose or paediatric equivalent dose. Given the exposure margin, this finding is considered of unlikely
clinical significance.
No evidence of genotoxicity was observed in an Ames microbial mutagenicity assay, a mammalian-
cell gene mutation assay, and a transformation assay with Syrian hamster embryo cells. A
micronucleus study and a DNA repair study in rats were also negative. Entecavir was clastogenic to
human lymphocyte cultures at concentrations substantially higher than those achieved clinically.
Two-year carcinogenicity studies: in male mice, increases in the incidences of lung tumours were
observed at exposures ≥ 4 and ≥ 2 times that in humans at 0.5 mg and 1 mg respectively. Tumour
development was preceded by pneumocyte proliferation in the lung which was not observed in rats,
dogs, or monkeys, indicating that a key event in lung tumour development observed in mice likely was
species-
specific. Increased incidences of other tumours including brain gliomas in male and female
rats, liver carcinomas in male mice, benign vascular tumours in female mice, and liver adenomas and
carcinomas in female rats were seen only at high lifetime exposures. However, the no effect levels
could not be precisely established. The predictivity of the findings for humans is not known.
38
6. PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Tablet core:
Crospovidone
Lactose monohydrate
Magnesium stearate
Cellulose, Microcrystalline
Povidone
Tablet coating:
Titanium dioxide
Hypromellose
Macrogol 400
Iron oxide red
6.2 Incompatibilities
Not applicable.
6.3 Shelf life
2 years
6.4 Special precautions for storage
Blisters:
Do not store above 30°C. Store in the original carton.
Bottles:
Do not store above 25°C. Keep the bottle tightly closed.
6.5 Nature and contents of container
Each carton contains either:
30 x 1 film-coated tablet; 3 blister cards of 10 x 1 film-coated tablet each in Alu/Alu perforated
unit dose blisters, or
90 x 1 film-coated tablet; 9 blister cards of 10 x 1 film-coated tablet each in Alu/Alu perforated
unit dose blisters.
High-density polyethylene (HDPE) bottle with child resistant polypropylene closure containing
30 film-coated tablets. Each carton contains one bottle.
Not all pack sizes and container types may be marketed.
6.6 Special precautions for disposal
Any unused medicinal product or waste material should be disposed of in accordance with local
requirements.
7. MARKETING AUTHORISATION HOLDER
BRISTOL-MYERS SQUIBB PHARMA EEIG
Uxbridge Business Park
Sanderson Road
39
Uxbridge UB8 1DH
United Kingdom
8. MARKETING AUTHORISATION NUMBER(S)
Blister packs: EU/1/06/343/004
EU/1/06/343/007
Bottle packs: EU/1/06/343/002
9. DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
Date of first authorisation: 26 June 2006
Date of latest renewal: 26 June 2011
10. DATE OF REVISION OF THE TEXT
{MM/YYYY}
Detailed information on this medicinal product is available on the website of the European Medicines
Agency http://www.ema.europa.eu/.

40
1. NAME OF THE MEDICINAL PRODUCT
Baraclude 0.05 mg/ml oral solution
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
Each ml oral solution contains 0.05 mg entecavir (as monohydrate).
Excipients with known effect
: 380 mg maltitol/ml
1.5 mg methylhydroxybenzoate/ml
0.18 mg propylhydroxybenzoate/ml
For the full list of excipients, see section 6.1.
3. PHARMACEUTICAL FORM
Oral solution
Clear, colourless to pale yellow solution
4. CLINICAL PARTICULARS
4.1 Therapeutic indications
Baraclude is indicated for the treatment of chronic hepatitis B virus (HBV) infection (see section 5.1)
in adults with:
compensated liver disease and evidence of active viral replication, persistently elevated serum
alanine aminotransferase (ALT) levels and histological evidence of active inflammation and/or
fibrosis.
decompensated liver disease (see section 4.4).
For both compensated and decompensated liver disease, this indication is based on clinical trial data in
nucleoside naive patients with HBeAg positive and HBeAg negative HBV infection. With respect to
patients with lamivudine-refractory hepatitis B, see sections 4.2, 4.4 and 5.1.
Baraclude is also indicated for the treatment of chronic HBV infection in nucleoside naive paediatric
patients from 2 to < 18 years of age with compensated liver disease who have evidence of active viral
replication and persistently elevated serum ALT levels, or histological evidence of moderate to severe
inflammation and/or fibrosis. With respect to the decision to initiate treatment in paediatric patients,
see sections 4.2, 4.4, and 5.1.
4.2 Posology and method of administration
Therapy should be initiated by a physician experienced in the management of chronic hepatitis B
infection.
It is recommended that the dosing spoon be rinsed with water after each daily dose.
Posology
Compensated liver disease
Nucleoside naïve patients: the recommended dose in adults is 0.5 mg once daily, with or without food.

41
Lamivudine-refractory patients (i.e. with evidence of viraemia while on lamivudine or the presence of
lamivudine resistance [LVDr] mutations) (see sections 4.4 and 5.1): the recommended dose in adults is
1 mg once daily, which must be taken on an empty stomach (more than 2 hours before and more than
2 hours after a meal) (see section 5.2). In the presence of LVDr mutations, combination use of
entecavir plus a second antiviral agent (which does not share cross-resistance with either lamivudine
or entecavir) should be considered in preference to entecavir monotherapy (see section 4.4.).
Decompensated liver disease
The recommended dose for adult patients with decompensated liver disease is 1 mg once daily, which
must be taken on an empty stomach (more than 2 hours before and more than 2 hours after a meal)
(see section 5.2). For patients with lamivudine-refractory hepatitis B, see sections 4.4 and 5.1.
Duration of therapy
The optimal duration of treatment is unknown. Treatment discontinuation may be considered as
follows:
In HBeAg positive adult patients, treatment should be administered at least until 12 months after
achieving HBe seroconversion (HBeAg loss and HBV DNA loss with anti-HBe detection on
two consecutive serum samples at least 3-6 months apart) or until HBs seroconversion or there
is loss of efficacy (see section 4.4).
In HBeAg negative adult patients, treatment should be administered at least until HBs
seroconversion or there is evidence of loss of efficacy. With prolonged treatment for more than
2 years, regular reassessment is recommended to confirm that continuing the selected therapy
remains appropriate for the patient.
In patients with decompensated liver disease or cirrhosis, treatment cessation is not recommended.
Paediatric population
The decision to treat paediatric patients should be based on careful consideration of individual patient
needs and with reference to current paediatric treatment guidelines including the value of baseline
histological information. The benefits of long-term virologic suppression with continued therapy must
be weighed against the risk of prolonged treatment, including the emergence of resistant hepatitis B
virus.
Serum ALT should be persistently elevated for at least 6 months prior to treatment of paediatric
patients with compensated liver disease due to HBeAg positive chronic hepatitis B; and for at least
12 months in patients with HBeAg negative disease.
The recommended once-daily dose in paediatric patients weighing at least 10 kg, is presented in the
table below. Patients may be dosed with or without food. The oral solution should be used for patients
with body weight less than 32.6 kg. Paediatric patients with body weight at least 32.6 kg, should be
administered 10 ml (0.5 mg) of the oral solution or one 0.5 mg tablet once daily.
Dosing for nucleoside naive paediatric patients aged 2 to < 18 years
Body Weight
a
Recommended Once Daily Dose of Oral
Solution
b
10.0 - 14.1 kg 4.0 ml
14.2 - 15.8 kg 4.5 ml
15.9 - 17.4 kg 5.0 ml
17.5 - 19.1 kg 5.5 ml
19.2 - 20.8 kg 6.0 ml
20.9 - 22.5 kg 6.5 ml
22.6 - 24.1 kg 7.0 ml
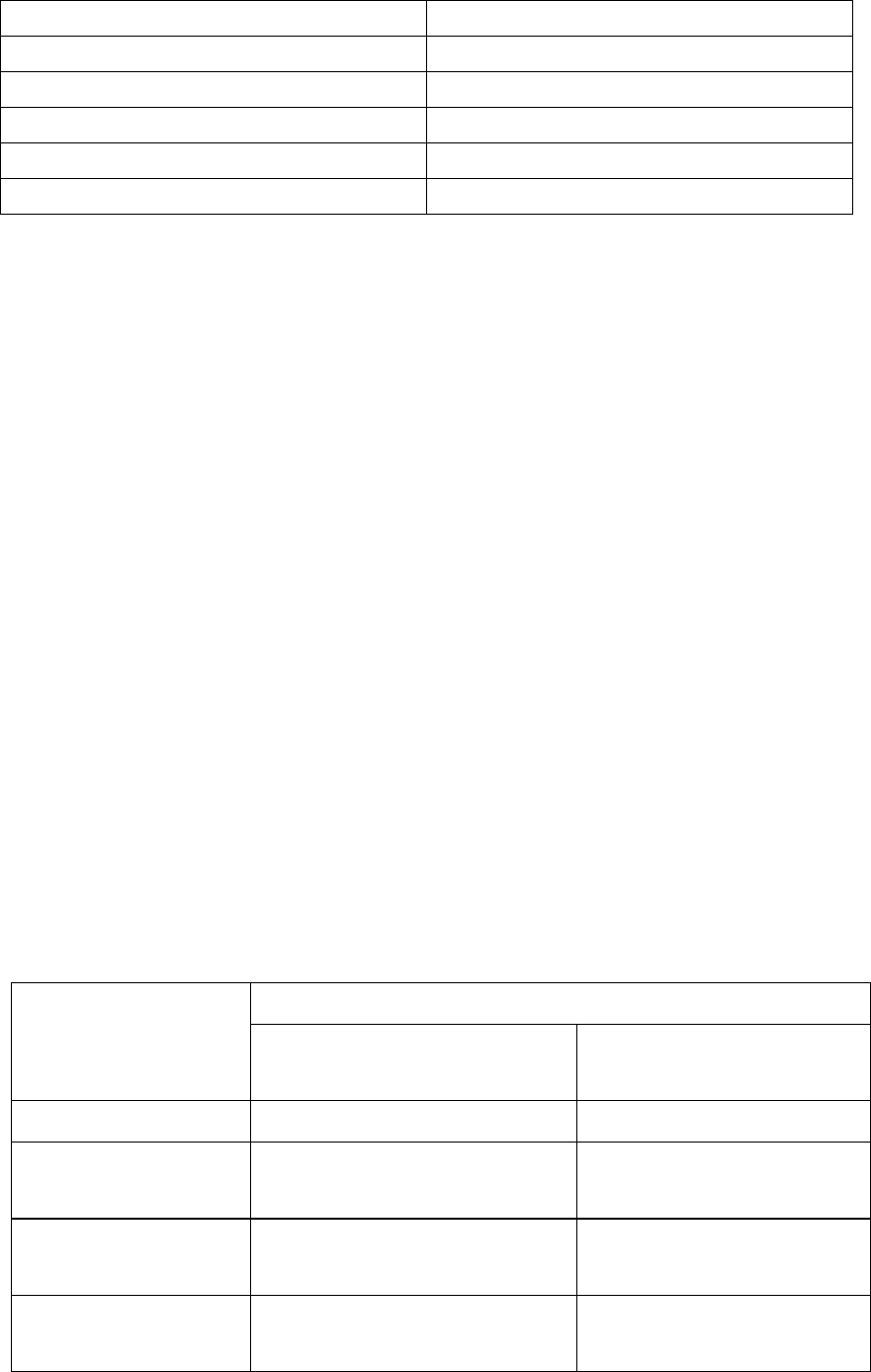
42
24.2 - 25.8 kg 7.5 ml
25.9 - 27.5 kg 8.0 ml
27.6 - 29.1 kg 8.5 ml
29.2 - 30.8 kg 9.0 ml
30.9 - 32.5 kg 9.5 ml
At least 32.6 kg
b
10.0 ml
a
Body weight should be rounded to the nearest 0.1 kg.
b
Children with body weight at least 32.6 kg should receive 10.0 ml (0.5 mg) of oral solution or one 0.5 mg tablet once daily.
Duration of therapy for paediatric patients
The optimal duration of treatment is unknown. In accordance with current paediatric practice
guidelines, treatment discontinuation may be considered as follows:
In HBeAg positive paediatric patients, treatment should be administered for at least 12 months
after achieving undetectable HBV DNA and HBeAg seroconversion (HBeAg loss and anti-HBe
detection on two consecutive serum samples at least 3-6 months apart) or until HBs
seroconversion or there is loss of efficacy. Serum ALT and HBV DNA levels should be
followed regularly after treatment discontinuation (see section 4.4).
In HBeAg negative paediatric patients, treatment should be administered until HBs seroconversion
or there is evidence of loss of efficacy.
Pharmacokinetics in paediatric patients with renal or hepatic impairment have not been studied.
Elderly: no dosage adjustment based on age is required. The dose should be adjusted according to the
patient’s renal function (see dosage recommendations in renal impairment and section 5.2).
Gender and race: no dosage adjustment based on gender or race is required.
Renal impairment: the clearance of entecavir decreases with decreasing creatinine clearance (see
section 5.2). Dose adjustment is recommended for patients with creatinine clearance < 50 ml/min,
including those on haemodialysis or continuous ambulatory peritoneal dialysis (CAPD). A reduction
of the daily dose using Baraclude oral solution, as detailed in the table, is recommended. As an
alternative, in case the oral solution is not available, the dose can be adjusted by increasing the dosage
interval, also shown in the table. The proposed dose modifications are based on extrapolation of
limited data, and their safety and effectiveness have not been clinically evaluated. Therefore,
virological response should be closely monitored.
Baraclude dosage
Creatinine clearance
(ml/min)
Nucleoside naïve patients
Lamivudine-refractory or
decompensated liver disease
≥ 50 0.5 mg once daily 1 mg once daily
30 - 49 0.25 mg once daily
OR
0.5 mg every 48 hours
0.5 mg once daily
10 - 29 0.15 mg once daily
OR
0.5 mg every 72 hours
0.3 mg once daily
OR
0.5 mg every 48 hours
< 10
Haemodialysis or
CAPD**
0.05 mg once daily
OR
0.5 mg every 5-7 days
0.1 mg once daily
OR
0.5 mg every 72 hours
**on haemodialysis days, administer entecavir after haemodialysis.

43
Hepatic impairment: no dose adjustment is required in patients with hepatic impairment.
Method of administration
Baraclude should be taken orally.
4.3 Contraindications
Hypersensitivity to the active substance or to any of the excipients listed in section 6.1.
4.4 Special warnings and precautions for use
Renal impairment: dosage adjustment is recommended for patients with renal impairment (see
section 4.2). The proposed dose modifications are based on extrapolation of limited data, and their
safety and effectiveness have not been clinically evaluated. Therefore, virological response should be
closely monitored.
Exacerbations of hepatitis: spontaneous exacerbations in chronic hepatitis B are relatively common
and are characterised by transient increases in serum ALT. After initiating antiviral therapy, serum
ALT may increase in some patients as serum HBV DNA levels decline (see section 4.8). Among
entecavir-treated patients on-treatment exacerbations had a median time of onset of 4-5 weeks. In
patients with compensated liver disease, these increases in serum ALT are generally not accompanied
by an increase in serum bilirubin concentrations or hepatic decompensation. Patients with advanced
liver disease or cirrhosis may be at a higher risk for hepatic decompensation following hepatitis
exacerbation, and therefore should be monitored closely during therapy.
Acute exacerbation of hepatitis has also been reported in patients who have discontinued hepatitis B
therapy (see section 4.2). Post-treatment exacerbations are usually associated with rising HBV DNA,
and the majority appears to be self-limited. However, severe exacerbations, including fatalities, have
been reported.
Among entecavir-treated nucleoside naive patients, post-treatment exacerbations had a median time to
onset of 23-24 weeks, and most were reported in HBeAg negative patients (see section 4.8). Hepatic
function should be monitored at repeated intervals with both clinical and laboratory follow-up for at
least 6 months after discontinuation of hepatitis B therapy. If appropriate, resumption of hepatitis B
therapy may be warranted.
Patients with decompensated liver disease: a higher rate of serious hepatic adverse events (regardless
of causality) has been observed in patients with decompensated liver disease, in particular in those
with Child-Turcotte-Pugh (CTP) class C disease, compared with rates in patients with compensated
liver function. Also, patients with decompensated liver disease may be at higher risk for lactic acidosis
and for specific renal adverse events such as hepatorenal syndrome. Therefore, clinical and laboratory
parameters should be closely monitored in this patient population (see also sections 4.8 and 5.1).
Lactic acidosis and severe hepatomegaly with steatosis: occurrences of lactic acidosis (in the absence
of hypoxaemia), sometimes fatal, usually associated with severe hepatomegaly and hepatic steatosis,
have been reported with the use of nucleoside analogues. As entecavir is a nucleoside analogue, this
risk cannot be excluded. Treatment with nucleoside analogues should be discontinued when rapidly
elevating aminotransferase levels, progressive hepatomegaly or metabolic/lactic acidosis of unknown
aetiology occur. Benign digestive symptoms, such as nausea, vomiting and abdominal pain, might be
indicative of lactic acidosis development. Severe cases, sometimes with fatal outcome, were associated
with pancreatitis, liver failure/hepatic steatosis, renal failure and higher levels of serum lactate.
Caution should be exercised when prescribing nucleoside analogues to any patient (particularly obese
women) with hepatomegaly, hepatitis or other known risk factors for liver disease. These patients
should be followed closely.
44
To differentiate between elevations in aminotransferases due to response to treatment and increases
potentially related to lactic acidosis, physicians should ensure that changes in ALT are associated with
improvements in other laboratory markers of chronic hepatitis B.
Resistance and specific precaution for lamivudine-refractory patients: mutations in the HBV
polymerase that encode lamivudine-resistance substitutions may lead to the subsequent emergence of
secondary substitutions, including those associated with entecavir associated resistance (ETVr). In a
small percentage of lamivudine-refractory patients, ETVr substitutions at residues rtT184, rtS202 or
rtM250 were present at baseline. Patients with lamivudine-resistant HBV are at higher risk of
developing subsequent entecavir resistance than patients without lamivudine-resistance. The
cumulative probability of emerging genotypic entecavir resistance after 1, 2, 3, 4 and 5 years treatment
in the lamivudine-refractory studies was 6%, 15%, 36%, 47% and 51%, respectively. Virological
response should be frequently monitored in the lamivudine-refractory population and appropriate
resistance testing should be performed. In patients with a suboptimal virological response
after 24
weeks of treatment with entecavir, a modification of treatment should be considered (see sections 4.5
and 5.1). When starting therapy in patients with a documented history of lamivudine-resistant HBV,
combination use of entecavir plus a second antiviral agent (which does not share cross-resistance with
either lamivudine or entecavir) should be considered in preference to entecavir monotherapy.
Pre-existing lamivudine-resistant HBV is associated with an increased risk for subsequent entecavir
resistance regardless of the degree of liver disease; in patients with decompensated liver disease,
virologic breakthrough may be associated with serious clinical complications of the underlying liver
disease. Therefore, in patients with both decompensated liver disease and lamivudine-resistant HBV,
combination use of entecavir plus a second antiviral agent (which does not share cross-resistance with
either lamivudine or entecavir) should be considered in preference to entecavir monotherapy.
Paediatric population: A lower rate of virologic response (HBV DNA < 50 IU/ml) was observed in
paediatric patients with baseline HBV DNA ≥ 8.0 log
10
IU/ml (see section 5.1). Entecavir should be
used in these patients only if the potential benefit justifies the potential risk to the child (e.g.
resistance). Since some paediatric patients may require long-term or even lifetime management of
chronic active hepatitis B, consideration should be given to the impact of entecavir on future treatment
options.
Liver transplant recipients: renal function should be carefully evaluated before and during entecavir
therapy in liver transplant recipients receiving cyclosporine or tacrolimus (see section 5.2).
Co-infection with hepatitis C or D: there are no data on the efficacy of entecavir in patients co-infected
with hepatitis C or D virus.
Human immunodeficiency virus (HIV)/HBV co-infected patients not receiving concomitant
antiretroviral therapy: entecavir has not been evaluated in HIV/HBV co-infected patients not
concurrently receiving effective HIV treatment. Emergence of HIV resistance has been observed when
entecavir was used to treat chronic hepatitis B infection in patients with HIV infection not receiving
highly active antiretroviral therapy (HAART) (see section 5.1). Therefore, therapy with entecavir
should not be used for HIV/HBV co-infected patients who are not receiving HAART. Entecavir has
not been studied as a treatment for HIV infection and is not recommended for this use.
HIV/HBV co-infected patients receiving concomitant antiretroviral therapy: entecavir has been studied
in 68 adults with HIV/HBV co-infection receiving a lamivudine-containing HAART regimen (see
section 5.1). No data are available on the efficacy of entecavir in HBeAg-negative patients co-infected
with HIV. There are limited data on patients co-infected with HIV who have low CD4 cell counts
(< 200 cells/mm
3
).
General: patients should be advised that therapy with entecavir has not been proven to reduce the risk
of transmission of HBV and therefore appropriate precautions should still be taken.
45
Maltitol: Baraclude oral solution contains maltitol. Patients with rare hereditary problems of fructose
intolerance should not take this medicine. Baraclude tablets do not contain maltitol and can be taken
by patients with fructose intolerance.
Parahydroxybenzoates: Baraclude oral solution contains the preservatives methylhydroxybenzoate
and propylhydroxybenzoate, that may cause allergic reactions (possibly delayed).
4.5 Interaction with other medicinal products and other forms of interaction
Since entecavir is predominantly eliminated by the kidney (see section 5.2), coadministration with
medicinal products that reduce renal function or compete for active tubular secretion may increase
serum concentrations of either medicinal product. Apart from lamivudine, adefovir dipivoxil and
tenofovir disoproxil fumarate, the effects of coadministration of entecavir with medicinal products that
are excreted renally or affect renal function have not been evaluated. Patients should be monitored
closely for adverse reactions when entecavir is coadministered with such medicinal products.
No pharmacokinetic interactions between entecavir and lamivudine, adefovir or tenofovir were
observed.
Entecavir is not a substrate, an inducer or an inhibitor of cytochrome P450 (CYP450) enzymes (see
section 5.2). Therefore CYP450 mediated drug interactions are unlikely to occur with entecavir.
Paediatric population
Interaction studies have only been performed in adults.
4.6 Fertility, pregnancy and lactation
Women of childbearing potential: given that the potential risks to the developing foetus are unknown,
women of childbearing potential should use effective contraception.
Pregnancy: there are no adequate data from the use of entecavir in pregnant women. Studies in
animals have shown reproductive toxicity at high doses (see section 5.3). The potential risk for
humans is unknown. Baraclude should not be used during pregnancy unless clearly necessary. There
are no data on the effect of entecavir on transmission of HBV from mother to newborn infant.
Therefore, appropriate interventions should be used to prevent neonatal acquisition of HBV.
Breast-feeding: it is unknown whether entecavir is excreted in human milk. Available toxicological
data in animals have shown excretion of entecavir in milk (for details see section 5.3). A risk to the
infants cannot be excluded. Breast-feeding should be discontinued during treatment with Baraclude.
Fertility: toxicology studies in animals administered entecavir have shown no evidence of impaired
fertility (see section 5.3).
4.7 Effects on ability to drive and use machines
No studies on the effects on the ability to drive and use machines have been performed. Dizziness,
fatigue and somnolence are common side effects which may impair the ability to drive and use
machines.
4.8 Undesirable effects
a. Summary of the safety profile
In clinical studies in patients with compensated liver disease, the most common adverse reactions of
any severity with at least a possible relation to entecavir were headache (9%), fatigue (6%), dizziness
(4%) and nausea (3%). Exacerbations of hepatitis during and after discontinuation of entecavir therapy
have also been reported (see section 4.4 and c. Description of selected adverse reactions).
46
b. Tabulated list of adverse reactions
Assessment of adverse reactions is based on experience from postmarketing surveillance and four
clinical studies in which 1,720 patients with chronic hepatitis B infection and compensated liver
disease received double-blind treatment with entecavir (n = 862) or lamivudine (n = 858) for up to
107 weeks (see section 5.1). In these studies, the safety profiles, including laboratory abnormalities,
were comparable for entecavir 0.5 mg daily (679 nucleoside-naive HBeAg positive or negative
patients treated for a median of 53 weeks), entecavir 1 mg daily (183 lamivudine-refractory patients
treated for a median of 69 weeks), and lamivudine.
Adverse reactions considered at least possibly related to treatment with entecavir are listed by body
system organ class. Frequency is defined as very common (≥ 1/10); common (≥ 1/100 to 1/10);
uncommon (≥ 1/1,000 to < 1/100); rare(≥ 1/10,000 to < 1/1,000). Within each frequency grouping,
undesirable effects are presented in order of decreasing seriousness.
Immune system disorders:
rare: anaphylactoid reaction
Psychiatric disorders:
common: insomnia
Nervous system disorders:
common: headache, dizziness, somnolence
Gastrointestinal disorders:
common: vomiting, diarrhoea, nausea, dyspepsia
Hepatobiliary disorders
common: increased transaminases
Skin and subcutaneous tissue disorders:
uncommon: rash, alopecia
General disorders and administration site
conditions:
common: fatigue
Cases of lactic acidosis have been reported, often in association with hepatic decompensation, other
serious medical conditions or drug exposures (see section 4.4).
Treatment beyond 48 weeks: continued treatment with entecavir for a median duration of 96 weeks did
not reveal any new safety signals.
c. Description of selected adverse reactions
Laboratory test abnormalities
: In clinical studies with nucleoside-naive patients, 5% had ALT
elevations > 3 times baseline, and < 1% had ALT elevations > 2 times baseline together with total
bilirubin > 2 times upper limit of normal (ULN) and > 2 times baseline. Albumin levels < 2.5 g/dl
occurred in < 1% of patients, amylase levels > 3 times baseline in 2%, lipase levels > 3 times baseline
in 11% and platelets < 50,000/mm
3
in < 1%.
In clinical studies with lamivudine-refractory patients, 4% had ALT elevations > 3 times baseline, and
< 1% had ALT elevations > 2 times baseline together with total bilirubin > 2 times ULN and > 2 times
baseline. Amylase levels > 3 times baseline occurred in 2% of patients, lipase levels > 3 times baseline
in 18% and platelets < 50,000/mm
3
in < 1%.
Exacerbations during treatment:
in studies with nucleoside naive patients, on treatment ALT elevations
> 10 times ULN and > 2 times baseline occurred in 2% of entecavir treated patients vs 4% of
lamivudine treated patients. In studies with lamivudine-refractory patients, on treatment ALT
elevations > 10 times ULN and > 2 times baseline occurred in 2% of entecavir treated patients vs 11%
of lamivudine treated patients. Among entecavir-treated patients, on-treatment ALT elevations had a
median time to onset of 4-5 weeks, generally resolved with continued treatment, and, in a majority of
cases, were associated with a ≥ 2 log
10
/ml reduction in viral load that preceded or coincided with the
ALT elevation. Periodic monitoring of hepatic function is recommended during treatment.

47
Exacerbations after discontinuation of treatment:
acute exacerbations of hepatitis have been reported
in patients who have discontinued anti-hepatitis B virus therapy, including therapy with entecavir (see
section 4.4). In studies in nucleoside-naive patients, 6% of entecavir-treated patients and 10% of
lamivudine-treated patients experienced ALT elevations (> 10 times ULN and > 2 times reference
[minimum of baseline or last end-of-dosing measurement]) during post-treatment follow-up. Among
entecavir-treated nucleoside-naive patients, ALT elevations had a median time to onset of 23-
24 weeks, and 86% (24/28) of ALT elevations occurred in HBeAg negative patients. In studies in
lamivudine-refractory patients, with only limited numbers of patients being followed up, 11% of
entecavir-treated patients and no lamivudine-treated patients developed ALT elevations during post-
treatment follow-up.
In the clinical trials entecavir treatment was discontinued if patients achieved a prespecified response.
If treatment is discontinued without regard to treatment response, the rate of post-treatment ALT flares
could be higher.
d. Paediatric Population
The safety of entecavir in paediatric patients from 2 to < 18 years of age is based on two ongoing
clinical trials in subjects with chronic HBV infection; one Phase 2 pharmacokinetic trial (study 028)
and one Phase 3 trial (study 189). These trials provide experience in 173 HBeAg-positive nucleoside-
treatment-naïve subjects treated with entecavir for a median duration of 60 weeks. The adverse
reactions observed in paediatric subjects who received treatment with entecavir were consistent with
those observed in clinical trials of entecavir in adults.(see a. Summary of the safety profile and
section 5.1)
e. Other special populations
Experience in patients with decompensated liver disease: the safety profile of entecavir in patients
with decompensated liver disease was assessed in a randomized open-label comparative study in
which patients received treatment with entecavir 1 mg/day (n = 102) or adefovir dipivoxil 10 mg/day
(n = 89) (study 048). Relative to the adverse reactions noted in section b. Tabulated list of adverse
reactions, one additional adverse reaction [decrease in blood bicarbonate (2%)] was observed in
entecavir-treated patients through week 48. The on-study cumulative death rate was 23% (23/102), and
causes of death were generally liver-related, as expected in this population. The on-study cumulative
rate of hepatocellular carcinoma (HCC) was 12% (12/102). Serious adverse events were generally
liver-related, with an on-study cumulative frequency of 69%. Patients with high baseline CTP score
were at higher risk of developing serious adverse events (see section 4.4).
Laboratory test abnormalities: through week 48 among entecavir-treated patients with decompensated
liver disease, none had ALT elevations both > 10 times ULN and > 2 times baseline, and 1% of
patients had ALT elevations > 2 times baseline together with total bilirubin > 2 times ULN and > 2
times baseline. Albumin levels < 2.5 g/dl occurred in 30% of patients, lipase levels > 3 times baseline
in 10% and platelets < 50,000/mm
3
in 20%.
Experience in patients co-infected with HIV:
the safety profile of entecavir in a limited number of
HIV/HBV co-infected patients on lamivudine-containing HAART (highly active antiretroviral
therapy) regimens was similar to the safety profile in monoinfected HBV patients (see section 4.4).
Gender/age:
there was no apparent difference in the safety profile of entecavir with respect to gender
(≈ 25% women in the clinical trials) or age (≈ 5% of patients > 65 years of age).
Reporting of suspected adverse reactions:
Reporting suspected adverse reactions after authorisation of
the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the
medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the
national reporting system listed in Appendix V.
48
4.9 Overdose
There is limited experience of entecavir overdose reported in patients. Healthy subjects who received
up to 20 mg/day for up to 14 days, and single doses up to 40 mg had no unexpected adverse reactions.
If overdose occurs, the patient must be monitored for evidence of toxicity and given standard
supportive treatment as necessary.
5. PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: antivirals for systemic use, nucleoside and nucleotide reverse
transcriptase inhibitors
ATC code: J05AF10
Mechanism of action: entecavir, a guanosine nucleoside analogue with activity against HBV
polymerase, is efficiently phosphorylated to the active triphosphate (TP) form, which has an
intracellular half-life of 15 hours. By competing with the natural substrate deoxyguanosine TP,
entecavir-TP functionally inhibits the 3 activities of the viral polymerase: (1) priming of the HBV
polymerase, (2) reverse transcription of the negative strand DNA from the pregenomic messenger
RNA, and (3) synthesis of the positive strand HBV DNA. The entecavir-TP K
i
for HBV DNA
polymerase is 0.0012 μM. Entecavir-TP is a weak inhibitor of cellular DNA polymerases α, β, and δ
with K
i
values of 18 to 40 µM. In addition, high exposures of entecavir had no relevant adverse effects
on γ polymerase or mitochondrial DNA synthesis in HepG2 cells (K
i
> 160 µM).
Antiviral activity: entecavir inhibited HBV DNA synthesis (50% reduction, EC
50
) at a concentration
of 0.004 µM in human HepG2 cells transfected with wild-type HBV. The median EC
50
value for
entecavir against LVDr HBV (rtL180M and rtM204V) was 0.026 µM (range 0.010-0.059 µM).
Recombinant viruses encoding adefovir-resistant substitutions at either rtN236T or rtA181V remained
fully susceptible to entecavir.
An analysis of the inhibitory activity of entecavir against a panel of laboratory and clinical HIV-1
isolates using a variety of cells and assay conditions yielded EC
50
values ranging from 0.026 to
> 10 µM; the lower EC
50
values were observed when decreased levels of virus were used in the assay.
In cell culture, entecavir selected for an M184I substitution at micromolar concentrations, confirming
inhibitory pressure at high entecavir concentrations. HIV variants containing the M184V substitution
showed loss of susceptibility to entecavir (see section 4.4).
In HBV combination assays in cell culture, abacavir, didanosine, lamivudine, stavudine, tenofovir or
zidovudine were not antagonistic to the anti-HBV activity of entecavir over a wide range of
concentrations. In HIV antiviral assays, entecavir at micromolar concentrations was not antagonistic to
the anti-HIV activity in cell culture of these six NRTIs or emtricitabine.
Resistance in cell culture: relative to wild-type HBV, LVDr viruses containing rtM204V and
rtL180M substitutions within the reverse transcriptase exhibit 8-fold decreased susceptibility to
entecavir. Incorporation of additional ETVr amino acid changes rtT184, rtS202 or rtM250 decreases
entecavir susceptibility in cell culture. Substitutions observed in clinical isolates (rtT184A, C, F, G, I,
L, M or S; rtS202 C, G or I; and/or rtM250I, L or V) further decreased entecavir susceptibility 16- to
741-fold relative to wild-type virus. The ETVr substitutions at residues rtT184, rtS202 and rtM250
alone have only a modest effect on entecavir susceptibility, and have not been observed in the absence
of LVDr substitutions in more than 1000 patient samples sequenced. Resistance is mediated by
reduced inhibitor binding to the altered HBV reverse transcriptase, and resistant HBV exhibits reduced
replication capacity in cell culture.
Clinical experience: the demonstration of benefit is based on histological, virological, biochemical,
and serological responses after 48 weeks of treatment in active-controlled clinical trials of 1,633 adults
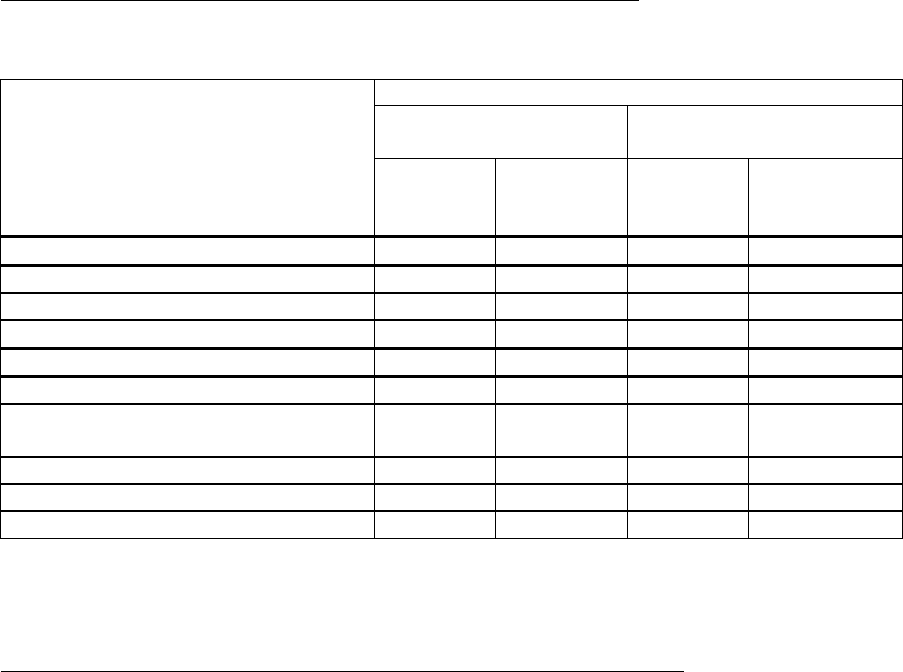
49
with chronic hepatitis B infection, evidence of viral replication and compensated liver disease. The
safety and efficacy of entecavir were also evaluated in an active-controlled clinical trial of 191 HBV-
infected patients with decompensated liver disease and in a clinical trial of 68 patients co-infected with
HBV and HIV.
In studies in patients with compensated liver disease, histological improvement was defined as a ≥ 2-
point decrease in Knodell necro-inflammatory score from baseline with no worsening of the Knodell
fibrosis score. Responses for patients with baseline Knodell Fibrosis Scores of 4 (cirrhosis) were
comparable to overall responses on all efficacy outcome measures (all patients had compensated liver
disease). High baseline Knodell necroinflammatory scores (> 10) were associated with greater
histological improvement in nucleoside-naive patients. Baseline ALT levels ≥ 2 times ULN and
baseline HBV DNA ≤ 9.0 log
10
copies/ml were both associated with higher rates of virologic response
(Week 48 HBV DNA < 400 copies/ml) in nucleoside-naive HBeAg-positive patients. Regardless of
baseline characteristics, the majority of patients showed histological and virological responses to
treatment.
Experience in nucleoside-naive patients with compensated liver disease:
Results at 48 weeks of randomised, double blind studies comparing entecavir (ETV) to lamivudine
(LVD) in HBeAg positive (022) and HBeAg negative (027) patients are presented in the table.
Nucleoside Naive
HBeAg Positive
(study 022)
HBeAg Negative
(study 027)
ETV
0.5 mg
once daily
LVD
100 mg
once daily
ETV
0.5 mg
once daily
LVD 100 mg
once daily
n 314
a
314
a
296
a
287
a
Histological improvement
b
72%* 62% 70%* 61%
Ishak fibrosis score improvement 39% 35% 36% 38%
Ishak fibrosis score worsening 8% 10% 12% 15%
n 354 355 325 313
Viral load reduction (log
10
copies/ml)
c
-6.86* -5.39 -5.04* -4.53
HBV DNA undetectable
(< 300 copies/ml by PCR)
c
67%* 36% 90%* 72%
ALT normalisation (≤ 1 times ULN) 68%* 60% 78%* 71%
HBeAg Seroconversion 21% 18%
*p value vs lamivudine < 0.05
a
patients with evaluable baseline histology (baseline Knodell Necroinflammatory Score ≥ 2)
b
a primary endpoint
c
Roche Cobas Amplicor PCR assay (LLOQ = 300 copies/ml)
Experience in lamivudine-refractory patients with compensated liver disease:
In a randomised, double-blind study in HBeAg positive lamivudine-refractory patients (026), with
85% of patients presenting LVDr mutations at baseline, patients receiving lamivudine at study entry
either switched to entecavir 1 mg once daily, with neither a washout nor an overlap period (n = 141),
or continued on lamivudine 100 mg once daily (n = 145). Results at 48 weeks are presented in the
table.
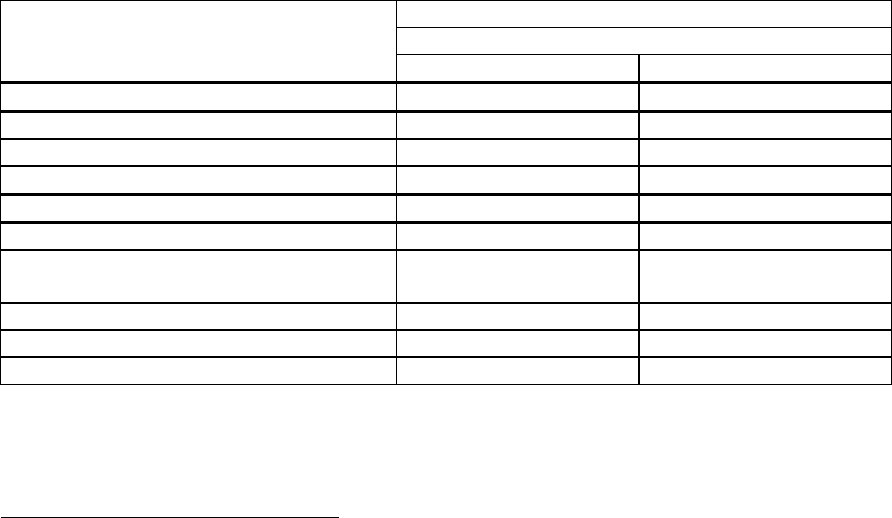
50
Lamivudine-refractory
HBeAg positive (study 026)
ETV 1.0 mg once daily LVD 100 mg once daily
n 124
a
116
a
Histological improvement
b
55%* 28%
Ishak fibrosis score improvement 34%* 16%
Ishak fibrosis score worsening 11% 26%
n 141 145
Viral load reduction (log
10
copies/ml)
c
-5.11* -0.48
HBV DNA undetectable (< 300 copies/ml
by PCR)
c
19%* 1%
ALT normalisation (≤ 1 times ULN) 61%* 15%
HBeAg Seroconversion 8% 3%
*p value vs lamivudine < 0.05
a
patients with evaluable baseline histology (baseline Knodell Necroinflammatory Score ≥ 2)
b
a primary endpoint.
c
Roche Cobas Amplicor PCR assay (LLOQ = 300 copies/ml)
Results beyond 48 weeks of treatment:
Treatment was discontinued when prespecified response criteria were met either at 48 weeks or during
the second year of treatment. Response criteria were HBV virological suppression (HBV DNA
< 0.7 MEq/ml by bDNA) and loss of HBeAg (in HBeAg positive patients) or ALT < 1.25 times ULN
(in HBeAg negative patients). Patients in response were followed for an additional 24 weeks off-
treatment. Patients who met virologic but not serologic or biochemical response criteria continued
blinded treatment. Patients who did not have a virologic response were offered alternative treatment.
Nucleoside-naive:
HBeAg positive (study 022): treatment with entecavir for up to 96 weeks (n = 354) resulted in
cumulative response rates of 80% for HBV DNA < 300 copies/ml by PCR, 87% for ALT
normalisation, 31% for HBeAg seroconversion and 2% for HBsAg seroconversion (5% for HBsAg
loss). For lamivudine (n = 355), cumulative response rates were 39% for HBV DNA < 300 copies/ml
by PCR, 79% for ALT normalisation, 26% for HBeAg seroconversion, and 2% for HBsAg
seroconversion (3% for HBsAg loss).
At end of dosing, among patients who continued treatment beyond 52 weeks (median of 96 weeks),
81% of 243 entecavir-treated and 39% of 164 lamivudine-treated patients had HBV DNA
< 300 copies/ml by PCR while ALT normalisation (≤ 1 times ULN) occurred in 79% of entecavir-
treated and 68% of lamivudine-treated patients.
HBeAg negative (study 027): treatment with entecavir up to 96 weeks (n = 325) resulted in cumulative
response rates of 94% for HBV DNA < 300 copies/ml by PCR and 89% for ALT normalisation versus
77% for HBV DNA < 300 copies/ml by PCR and 84% for ALT normalisation for lamivudine-treated
patients (n = 313).
For 26 entecavir-treated and 28 lamivudine-treated patients who continued treatment beyond 52 weeks
(median 96 weeks), 96% of entecavir-treated and 64% of lamivudine-treated patients had HBV DNA
< 300 copies/ml by PCR at end of dosing. ALT normalisation (≤ 1 times ULN) occurred in 27% of
entecavir-treated and 21% of lamivudine-treated patients at end of dosing.
For patients who met protocol-defined response criteria, response was sustained throughout the 24-
week post-treatment follow-up in 75% (83/111) of entecavir responders vs 73% (68/93) for
lamivudine responders in study 022 and 46% (131/286) of entecavir responders vs 31% (79/253) for
lamivudine responders in study 027. By 48 weeks of post-treatment follow-up, a substantial number of
HBeAg negative patients lost response.
Liver biopsy results: 57 patients from the pivotal nucleoside-naive studies 022 (HBeAg positive) and
027 (HBeAg negative) who enrolled in a long-term rollover study were evaluated for long-term liver

51
histology outcomes. The entecavir dosage was 0.5 mg daily in the pivotal studies (mean exposure 85
weeks) and 1 mg daily in the rollover study (mean exposure 177 weeks), and 51 patients in the
rollover study initially also received lamivudine (median duration 29 weeks). Of these patients, 55/57
(96%) had histological improvement as previously defined (see above), and 50/57 (88%) had a ≥ 1-
point decrease in Ishak fibrosis score. For patients with baseline Ishak fibrosis score ≥ 2, 25/43 (58%)
had a ≥ 2-point decrease. All (10/10) patients with advanced fibrosis or cirrhosis at baseline (Ishak
fibrosis score of 4, 5 or 6) had a ≥ 1 point decrease (median decrease from baseline was 1.5 points). At
the time of the long-term biopsy, all patients had HBV DNA < 300 copies/ml and 49/57 (86%) had
serum ALT ≤ 1 times ULN. All 57 patients remained positive for HBsAg.
Lamivudine-refractory:
HBeAg positive (study 026): treatment with entecavir for up to 96 weeks (n = 141) resulted in
cumulative response rates of 30% for HBV DNA < 300 copies/ml by PCR, 85% for ALT
normalisation and 17% for HBeAg seroconversion.
For the 77 patients who continued entecavir treatment beyond 52 weeks (median 96 weeks), 40% of
patients had HBV DNA < 300 copies/ml by PCR and 81% had ALT normalisation (≤ 1 times ULN) at
end of dosing.
Age/gender:
There was no apparent difference in efficacy for entecavir based on gender (≈ 25% women in the
clinical trials) or age (≈ 5% of patients > 65 years of age).
Special populations
Patients with decompensated liver disease: in study 048, 191 patients with HBeAg positive or
negative chronic HBV infection and evidence of hepatic decompensation, defined as a CTP score of 7
or higher, received entecavir 1 mg once daily or adefovir dipivoxil 10 mg once daily. Patients were
either HBV-treatment-naïve or pretreated (excluding pretreatment with entecavir, adefovir dipivoxil,
or tenofovir disoproxil fumarate). At baseline, patients had a mean CTP score of 8.59 and 26% of
patients were CTP class C. The mean baseline Model for End Stage Liver Disease (MELD) score was
16.23. Mean serum HBV DNA by PCR was 7.83 log
10
copies/ml and mean serum ALT was 100 U/l;
54% of patients were HBeAg positive, and 35% of patients had LVDr substitutions at baseline.
Entecavir was superior to adefovir dipivoxil on the primary efficacy endpoint of mean change from
baseline in serum HBV DNA by PCR at week 24. Results for selected study endpoints at weeks 24
and 48 are shown in the table.
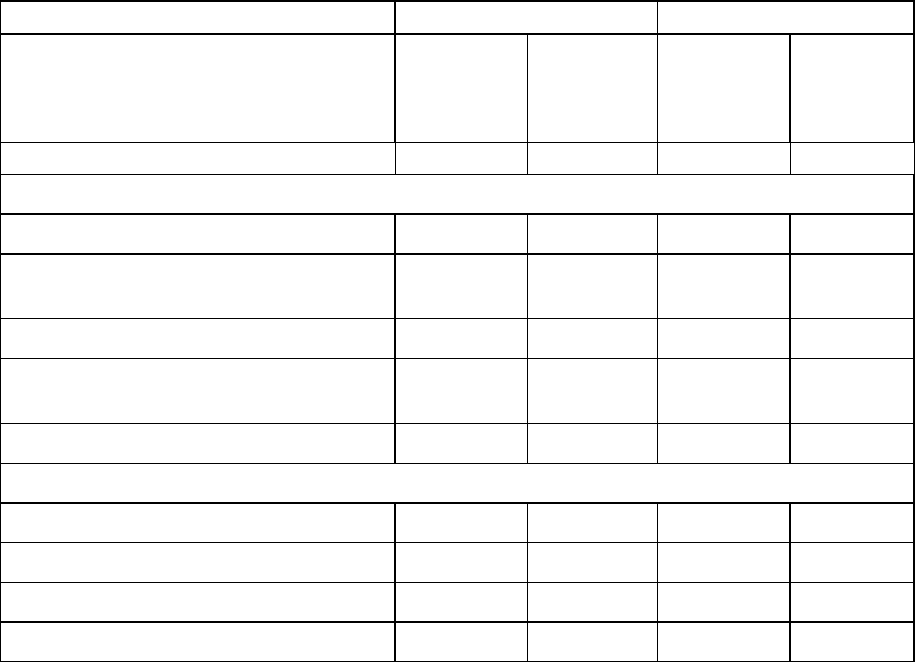
52
Week 24 Week 48
ETV
1 mg
once daily
Adefovir
Dipivoxil
10 mg
once daily
ETV
1 mg
once daily
Adefovir
Dipivoxil
10 mg
once daily
n 100 91 100 91
HBV DNA
a
Proportion undetectable (<300 copies/ml)
b
49%* 16% 57%* 20%
Mean change from baseline
(log
10
copies/ml)
c
-4.48* -3.40 -4.66 -3.90
Stable or improved CTP score
b
,
d
66% 71% 61% 67%
MELD score
Mean change from baseline
c,e
-2.0
-0.9
-2.6
-1.7
HBsAg loss
b
1% 0 5% 0
Normalization of:
f
ALT (≤1 X ULN)
b
46/78 (59%)* 28/71 (39%) 49/78 (63%)* 33/71 (46%)
Albumin (≥1 X LLN)
b
20/82 (24%) 14/69 (20%) 32/82 (39%) 20/69 (29%)
Bilirubin (≤1 X ULN)
b
12/75 (16%) 10/65 (15%) 15/75 (20%) 18/65 (28%)
Prothrombin time (≤1 X ULN)
b
9/95 (9%) 6/82 (7%) 8/95 (8%) 7/82 (9%)
a
Roche COBAS Amplicor PCR assay (LLOQ = 300 copies/ml).
b
NC=F (noncompleter=failure), meaning treatment discontinuations before the analysis week, including reasons such as
death, lack of efficacy, adverse event, noncompliance/loss-to-follow-up, are counted as failures (e.g., HBV
DNA ≥ 300 copies/ml)
c
NC=M (noncompleters=missing)
d
Defined as decrease or no change from baseline in CTP score.
e
Baseline mean MELD score was 17.1 for ETV and 15.3 for adefovir dipivoxil.
f
Denominator is patients with abnormal values at baseline.
*p<0.05
ULN=upper limit of normal, LLN=lower limit of normal.
The time to onset of HCC or death (whichever occurred first) was comparable in the two treatment
groups; on-study cumulative death rates were 23% (23/102) and 33% (29/89) for patients treated with
entecavir and adefovir dipivoxil, respectively, and on-study cumulative rates of HCC were 12%
(12/102) and 20% (18/89) for entecavir and adefovir dipivoxil, respectively.
For patients with LVDr substitutions at baseline, the percentage of patients with HBV DNA
<300 copies/ml was 44% for entecavir and 20% for adefovir at week 24 and 50% for entecavir and
17% for adefovir at week 48.
HIV/HBV co-infected patients receiving concomitant HAART: study 038 included 67 HBeAg positive
and 1 HBeAg negative patients co-infected with HIV. Patients had stable controlled HIV (HIV RNA
< 400 copies/ml) with recurrence of HBV viraemia on a lamivudine-containing HAART
regimen.HAART regimens did not include emtricitabine or tenofovir disoproxil fumarate. At baseline
entecavir-treated patients had a median duration of prior lamivudine therapy of 4.8 years and median
CD4 count of 494 cells/mm
3
(with only 5 subjects having CD4 count < 200 cells/mm
3
). Patients
continued their lamivudine-regimen and were assigned to add either entecavir 1 mg once daily
(n = 51) or placebo (n = 17) for 24 weeks followed by an additional 24 weeks where all received
entecavir. At 24 weeks the reduction in HBV viral load was significantly greater with entecavir (-3.65
vs an increase of 0.11 log
10
copies/ml). For patients originally assigned to entecavir treatment, the
reduction in HBV DNA at 48 weeks was -4.20 log
10
copies/ml, ALT normalisation had occurred in
37% of patients with abnormal baseline ALT and none achieved HBeAg seroconversion.
53
HIV/HBV co-infected patients not receiving concomitant HAART: entecavir has not been evaluated in
HIV/HBV co-infected patients not concurrently receiving effective HIV treatment. Reductions in HIV
RNA have been reported in HIV/HBV co-infected patients receiving entecavir monotherapy without
HAART. In some cases, selection of HIV variant M184V has been observed, which has implications
for the selection of HAART regimens that the patient may take in the future. Therefore, entecavir
should not be used in this setting due to the potential for development of HIV resistance (see section
4.4).
Liver transplant recipients: the safety and efficacy of entecavir 1 mg once daily were assessed in a
single-arm study in 65 patients who received a liver transplant for complications of chronic HBV
infection and had HBV DNA <172 IU/ml (approximately 1000 copies/ml) at the time of transplant.
The study population was 82% male, 39% Caucasian, and 37% Asian, with a mean age of 49 years;
89% of patients had HBeAg-negative disease at the time of transplant. Of the 61 patients who were
evaluable for efficacy (received entecavir for at least 1 month), 60 also received hepatitis B immune
globulin (HBIg) as part of the post-transplant prophylaxis regimen. Of these 60 patients, 49 received
more than 6 months of HBIg therapy. At Week 72 post-transplant, none of 55 observed cases had
virologic recurrence of HBV [defined as HBV DNA ≥50 IU/ml (approximately 300 copies/ml)], and
there was no reported virologic recurrence at time of censoring for the remaining 6 patients. All 61
patients had HBsAg loss post-transplantation, and 2 of these later became HBsAg positive despite
maintaining undetectable HBV DNA (<6 IU/ml). The frequency and nature of adverse events in this
study were consistent with those expected in patients who have received a liver transplant and the
known safety profile of entecavir.
Paediatric population: Study 189 is an ongoing study of the efficacy and safety of entecavir among
180 nucleoside-treatment-naïve children and adolescents from 2 to < 18 years of age with HBeAg-
positive chronic hepatitis B infection, compensated liver disease, and elevated ALT. Subjects were
randomized (2:1) to receive blinded treatment with entecavir 0.015 mg/kg up to 0.5 mg/day (N = 120)
or placebo (N = 60). The randomization was stratified by age group (2 to 6 years; > 6 to 12 years; and
> 12 to < 18 years). Baseline demographics and HBV disease characteristics were comparable
between the 2 treatment arms and across age cohorts. At study entry, the mean HBV DNA was
8.0 log
10
IU/ml and mean ALT was 105 U/l for the primary cohort (the first 123 treated subjects). The
primary efficacy endpoint was a composite of HBeAg seroconversion and serum HBV DNA
< 50 IU/ml (approximately 300 copies/ml) at Week 48 for the primary cohort.
Twenty-four percent (20/82) of subjects in the entecavir-treated group and 2% (1/41) of subjects in the
placebo-treated group met the primary endpoint. Forty-six percent (38/82) of entecavir-treated subjects
and 2% (1/41) of placebo-treated subjects achieved HBV DNA < 50 IU/ml at Week 48. When
assessed by baseline HBV DNA, 77% (26/34) of entecavir-treated subjects with HBV DNA
< 8 log
10
IU/ml at baseline and 25% (12/48) with HBV DNA ≥ 8 log
10
IU/ml achieved HBV DNA
< 50 IU/ml. ALT normalization occurred in 67% (55/82) of entecavir-treated subjects and 22% (9/41)
of placebo-treated subjects; 24% (20/82) of entecavir-treated subjects and 12% (5/41) of placebo-
treated subjects had HBeAg seroconversion.
In 2 paediatric studies (Studies 028 and 189), 110 patients who received entecavir for up to 48 weeks
were monitored for resistance. Genotypic evaluations were performed on all patients who had
virologic breakthrough, or HBV DNA ≥ 50 IU/ml at Week 48 or discontinued early. No amino acid
substitutions associated with resistance to entecavir were identified.
Clinical resistance: patients in clinical trials initially treated with entecavir 0.5 mg (nucleoside-naive)
or 1.0 mg (lamivudine-refractory) and with an on-therapy PCR HBV DNA measurement at or after
Week 24 were monitored for resistance.
Through Week 240 in nucleoside-naive studies, genotypic evidence of ETVr substitutions at rtT184,
rtS202, or rtM250 was identified in 3 patients treated with entecavir, 2 of whom experienced virologic
breakthrough (see table). These substitutions were observed only in the presence of LVDr
substitutions (rtM204Vand rtL180M).
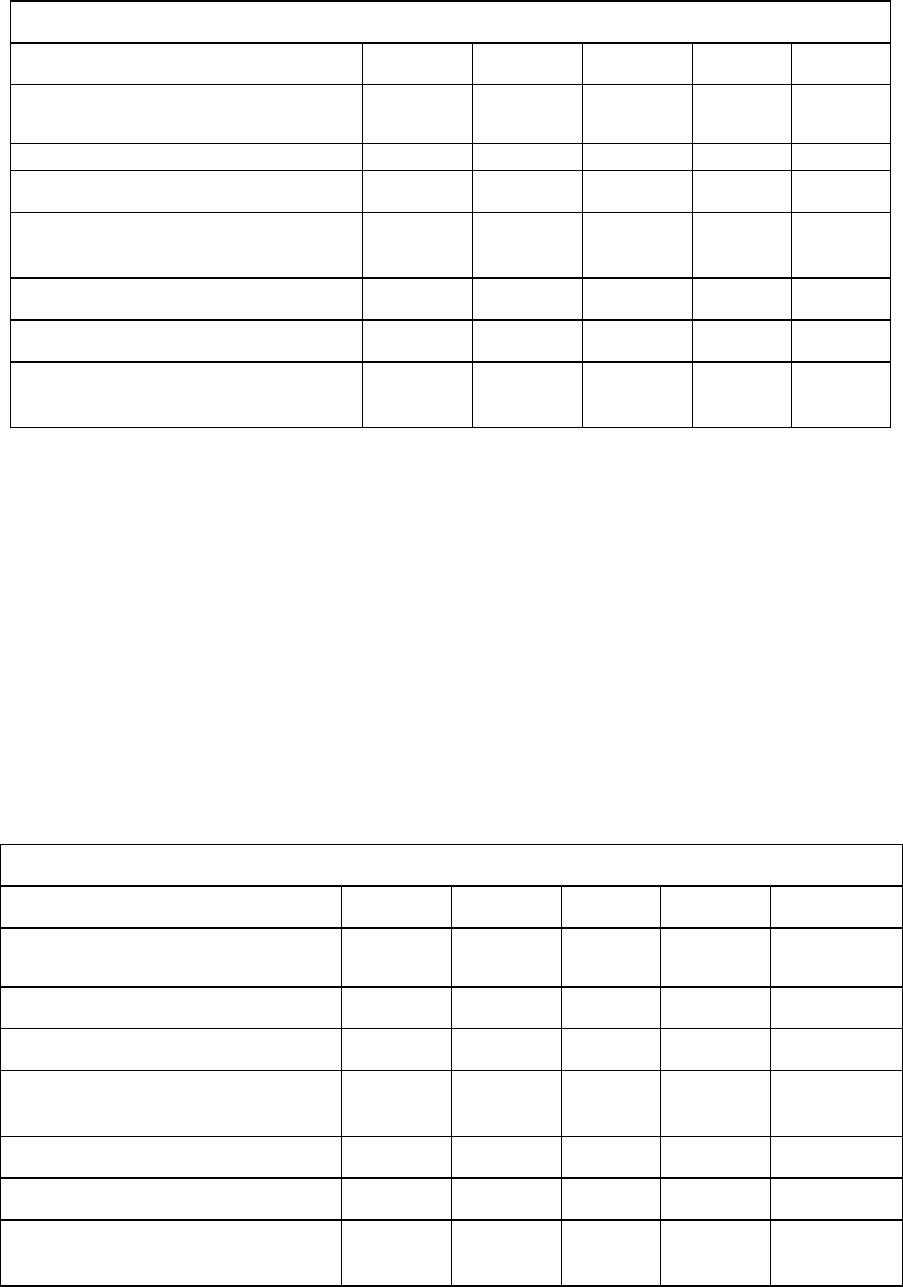
54
Emerging Genotypic Entecavir Resistance Through Year 5, Nucleoside-Naive Studies
Year 1 Year 2
Year 3
a
Year 4
a
Year 5
a
Patients treated and monitored for
resistance
b
663 278 149 121 108
Patients in specific year with:
- emerging genotypic ETVr
c
1 1 1 0 0
- genotypic ETVr
c
with virologic
breakthrough
d
1 0 1 0 0
Cumulative probability of:
- emerging genotypic ETVr
c
0.2% 0.5% 1.2% 1.2% 1.2%
- genotypic ETVr
c
with virologic
breakthrough
d
0.2% 0.2% 0.8% 0.8% 0.8%
a
Results reflect use of a 1-mg dose of entecavir for 147 of 149 patients in Year 3 and all patients in Years 4 and 5
and of combination entecavir-lamivudine therapy (followed by long-term entecavir therapy) for a median of 20
weeks for 130 of 149 patients in Year 3 and for 1 week for 1 of 121 patients in Year 4 in a rollover study.
b
Includes patients with at least one on-therapy HBV DNA measurement by PCR at or after week 24 through week 58
(Year 1), after week 58 through week 102 (Year 2), after week 102 through week 156 (Year 3), after week 156
through week 204 (Year 4), or after week 204 through week 252 (Year 5).
c
Patients also have LVDr substitutions.
d
≥1 log
10
increase above nadir in HBV DNA by PCR, confirmed with successive measurements or at the end of the
windowed time point.
ETVr substitutions (in addition to LVDr substitutions rtM204V/I ± rtL180M) were observed at
baseline in isolates from 10/187 (5%) lamivudine-refractory patients treated with entecavir and
monitored for resistance, indicating that prior lamivudine treatment can select these resistance
substitutions and that they can exist at a low frequency before entecavir treatment. Through Week 240,
3 of the 10 patients experienced virologic breakthrough (≥ 1 log
10
increase above nadir). Emerging
entecavir resistance in lamivudine-refractory studies through Week 240 is summarized in the table.
Genotypic Entecavir Resistance Through Year 5, Lamivudine-Refractory Studies
Year 1 Year 2 Year 3
a
Year 4
a
Year 5
a
Patients treated and monitored for
resistance
b
187 146 80 52 33
Patients in specific year with:
- emerging genotypic ETVr
c
11 12 16 6 2
- genotypic ETVr
c
with virologic
breakthrough
d
2
e
14
e
13
e
9
e
1
e
Cumulative probability of:
- emerging genotypic ETVr
c
6.2% 15% 36.3% 46.6% 51.45%
- genotypic ETVr
c
with virologic
breakthrough
d
1.1%
e
10.7%
e
27%
e
41.3%
e
43.6%
e
a
Results reflect use of combination entecavir-lamivudine therapy (followed by long-term entecavir therapy) for a
median of 13 weeks for 48 of 80 patients in Year 3, a median of 38 weeks for 10 of 52 patients in Year 4, and for 16
weeks for 1 of 33 patients in Year 5 in a rollover study.
b
Includes patients with at least one on-therapy HBV DNA measurement by PCR at or after week 24 through week 58
(Year 1), after week 58 through week 102 (Year 2), after week 102 through week 156 (Year 3), after week 156 through
week 204 (Year 4), or after week 204 through week 252 (Year 5).
c
Patients also have LVDr substitutions.
d
≥1 log
10
increase above nadir in HBV DNA by PCR, confirmed with successive measurements or at the end of the
windowed time point.
e
ETVr occurring in any year; virologic breakthrough in specified year.

55
Among lamivudine-refractory patients with baseline HBV DNA <10
7
log
10
copies/ml, 64% (9/14)
achieved HBV DNA <300 copies/ml at Week 48. These 14 patients had a lower rate of genotypic
entecavir resistance (cumulative probability 18.8% through 5 years of follow-up) than the overall
study population (see table). Also, lamivudine-refractory patients who achieved HBV DNA <10
4
log
10
copies/ml by PCR at Week 24 had a lower rate of resistance than those who did not (5-year cumulative
probability 17.6% [n=50] versus 60.5% [n=135], respectively).
5.2 Pharmacokinetic properties
Absorption: entecavir is rapidly absorbed with peak plasma concentrations occurring between 0.5-
1.5 hours. The absolute bioavailability has not been determined. Based on urinary excretion of
unchanged drug, the bioavailability has been estimated to be at least 70%. There is a dose-
proportionate increase in C
max
and AUC values following multiple doses ranging from 0.1-1 mg.
Steady-state is achieved between 6-10 days after once daily dosing with ≈ 2 times accumulation. C
max
and C
min
at steady-state are 4.2 and 0.3 ng/ml, respectively, for a dose of 0.5 mg, and 8.2 and
0.5 ng/ml, respectively, for 1 mg. The tablet and oral solution were bioequivalent in healthy subjects;
therefore, both forms may be used interchangeably.
Administration of 0.5 mg entecavir with a standard high-fat meal (945 kcal, 54.6 g fat) or a light meal
(379 kcal, 8.2 g fat) resulted in a minimal delay in absorption (1-1.5 hour fed vs. 0.75 hour fasted), a
decrease in C
max
of 44-46%, and a decrease in AUC of 18-20%. The lower C
max
and AUC when taken
with food is not considered to be of clinical relevance in nucleoside-naive patients but could affect
efficacy in lamivudine-refractory patients (see section 4.2).
Distribution: the estimated volume of distribution for entecavir is in excess of total body water.
Protein binding to human serum protein in vitro is ≈ 13%.
Biotransformation: entecavir is not a substrate, inhibitor or inducer of the CYP450 enzyme system.
Following administration of
14
C-entecavir, no oxidative or acetylated metabolites and minor amounts
of the phase II metabolites, glucuronide and sulfate conjugates, were observed.
Elimination: entecavir is predominantly eliminated by the kidney with urinary recovery of unchanged
drug at steady-state of about 75% of the dose. Renal clearance is independent of dose and ranges
between 360-471 ml/min suggesting that entecavir undergoes both glomerular filtration and net
tubular secretion. After reaching peak levels, entecavir plasma concentrations decreased in a bi-
exponential manner with a terminal elimination half-life of ≈ 128-149 hours. The observed drug
accumulation index is ≈ 2 times with once daily dosing, suggesting an effective accumulation half-life
of about 24 hours.
Hepatic impairment: pharmacokinetic parameters in patients with moderate or severe hepatic
impairment were similar to those in patients with normal hepatic function.
Renal impairment: entecavir clearance decreases with decreasing creatinine clearance. A 4 hour period
of haemodialysis removed ≈ 13% of the dose, and 0.3% was removed by CAPD. The
pharmacokinetics of entecavir following a single 1 mg dose in patients (without chronic hepatitis B
infection) are shown in the table below:
Baseline Creatinine Clearance (ml/min)
Unimpaired
> 80
(n = 6)
Mild
> 50;
≤ 80
(n = 6)
Moderate
30-50
(n = 6)
Severe
20-
< 30
(n = 6)
Severe
Managed with
Haemodialysis
(n = 6)
Severe
Managed
with CAPD
(n = 4)
C
max
(ng/ml)
(CV%)
8.1
(30.7)
10.4
(37.2)
10.5
(22.7)
15.3
(33.8)
15.4
(56.4)
16.6
(29.7)

56
AUC
(0-T)
(ng·h /ml)
(CV)
27.9
(25.6)
51.5
(22.8)
69.5
(22.7)
145.7
(31.5)
233.9
(28.4)
221.8
(11.6)
CLR (ml/min)
(SD)
383.2
(101.8)
197.9
(78.1)
135.6
(31.6)
40.3
(10.1)
NA NA
CLT/F (ml/min)
(SD)
588.1
(153.7)
309.2
(62.6)
226.3
(60.1)
100.6
(29.1)
50.6
(16.5)
35.7
(19.6)
Post-Liver transplant: entecavir exposure in HBV-infected liver transplant recipients on a stable dose
of cyclosporine A or tacrolimus (n = 9) was ≈ 2 times the exposure in healthy subjects with normal
renal function. Altered renal function contributed to the increase in entecavir exposure in these patients
(see section 4.4).
Gender: AUC was 14% higher in women than in men, due to differences in renal function and weight.
After adjusting for differences in creatinine clearance and body weight there was no difference in
exposure between male and female subjects.
Elderly: the effect of age on the pharmacokinetics of entecavir was evaluated comparing elderly
subjects in the age range 65-83 years (mean age females 69 years, males 74 years) with young subjects
in the age range 20-40 years (mean age females 29 years, males 25 years). AUC was 29% higher in
elderly than in young subjects, mainly due to differences in renal function and weight. After adjusting
for differences in creatinine clearance and body weight, elderly subjects had a 12.5% higher AUC than
young subjects.The population pharmacokinetic analysis covering patients in the age range 16-
75 years did not identify age as significantly influencing entecavir pharmacokinetics.
Race: the population pharmacokinetic analysis did not identify race as significantly influencing
entecavir pharmacokinetics. However, conclusions can only be drawn for the Caucasian and Asian
groups as there were too few subjects in the other categories.
Paediatric population: the steady-state pharmacokinetics of entecavir were evaluated (study 028) in
24 nucleoside naïve and 19 lamivudine-experienced HBeAg-positive paediatric subjects from
2 to < 18 years of age with compensated liver disease. Entecavir exposure among nucleoside naïve
subjects receiving once daily doses of entecavir 0.015 mg/kg up to a maximum dose of 0.5 mg was
similar to the exposure achieved in adults receiving once daily doses of 0.5 mg. The Cmax, AUC(0-
24), and Cmin for these subjects was 6.31 ng/ml, 18.33 ng·h/ml, and 0.28 ng/ml, respectively.
Entecavir exposure among lamivudine-experienced subjects receiving once daily doses of entecavir
0.030 mg/kg up to a maximum dose of 1.0 mg was similar to the exposure achieved in adults receiving
once daily doses of 1.0 mg. The Cmax, AUC(0-24), and Cmin for these subjects was 14.48 ng/ml,
38.58 ng h/ml, and 0.47 ng/ml, respectively.
5.3 Preclinical safety data
In repeat-dose toxicology studies in dogs, reversible perivascular inflammation was observed in the
central nervous system, for which no-effect doses corresponded to exposures 19 and 10 times those in
humans (at 0.5 and 1 mg respectively). This finding was not observed in repeat-dose studies in other
species, including monkeys administered entecavir daily for 1 year at exposures ≥ 100 times those in
humans.
In reproductive toxicology studies in which animals were administered entecavir for up to 4 weeks, no
evidence of impaired fertility was seen in male or female rats at high exposures. Testicular changes
(seminiferous tubular degeneration) were evident in repeat-dose toxicology studies in rodents and dogs
at exposures ≥ 26 times those in humans. No testicular changes were evident in a 1-year study in
monkeys.

57
In pregnant rats and rabbits administered entecavir, no effect levels for embryotoxicity and maternal
toxicity corresponded to exposures ≥ 21 times those in humans. In rats, maternal toxicity, embryo-
foetal toxicity (resorptions), lower foetal body weights, tail and vertebral malformations, reduced
ossification (vertebrae, sternebrae, and phalanges), and extra lumbar vertebrae and ribs were observed
at high exposures. In rabbits, embryo-foetal toxicity (resorptions), reduced ossification (hyoid), and an
increased incidence of 13th rib were observed at high exposures. In a peri-postnatal study in rats, no
adverse effects on offspring were observed. In a separate study wherein entecavir was administered to
pregnant lactating rats at 10 mg/kg, both foetal exposure to entecavir and secretion of entecavir into
milk were demonstrated. In juvenile rats administered entecavir from postnatal days 4 to 80, a
moderately reduced acoustic startle response was noted during the recovery period (postnatal days
110 to 114) but not during the dosing period at AUC values ≥ 92 times those in humans at the 0.5 mg
dose or paediatric equivalent dose. Given the exposure margin, this finding is considered of unlikely
clinical significance.
No evidence of genotoxicity was observed in an Ames microbial mutagenicity assay, a mammalian-
cell gene mutation assay, and a transformation assay with Syrian hamster embryo cells. A
micronucleus study and a DNA repair study in rats were also negative. Entecavir was clastogenic to
human lymphocyte cultures at concentrations substantially higher than those achieved clinically.
Two-year carcinogenicity studies: in male mice, increases in the incidences of lung tumours were
observed at exposures ≥ 4 and ≥ 2 times that in humans at 0.5 mg and 1 mg respectively. Tumour
development was preceded by pneumocyte proliferation in the lung which was not observed in rats,
dogs, or monkeys, indicating that a key event in lung tumour development observed in mice likely was
species-
specific. Increased incidences of other tumours including brain gliomas in male and female
rats, liver carcinomas in male mice, benign vascular tumours in female mice, and liver adenomas and
carcinomas in female rats were seen only at high lifetime exposures. However, the no effect levels
could not be precisely established. The predictivity of the findings for humans is not known.
6. PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Maltitol (E965)
Sodium citrate
Citric acid, anhydrous
Methylhydroxybenzoate (E218)
Propylhydroxybenzoate (E216)
Orange flavour (acacia and natural flavours)
Sodium hydroxide to adjust pH to approximately 6
Hydrochloric acid to adjust pH to approximately 6
Purified water
6.2 Incompatibilities
This medicinal product must not be mixed with water, other solvents or other medicinal products.
6.3 Shelf life
2 years
After opening, the solution can be used up to the expiry date on the bottle.
6.4 Special precautions for storage
Do not store above 30°C. Keep the bottle in the outer carton in order to protect from light.
58
6.5 Nature and contents of container
210 ml oral solution in a HDPE bottles with child-resistant closures (polypropylene). Each carton
includes a measuring spoon (polypropylene) with markings from 0.5 ml up to 10 ml
6.6 Special precautions for disposal
Any unused medicinal product or waste material should be disposed of in accordance with local
requirements.
7. MARKETING AUTHORISATION HOLDER
BRISTOL-MYERS SQUIBB PHARMA EEIG
Uxbridge Business Park
Sanderson Road
Uxbridge UB8 1DH
United Kingdom
8. MARKETING AUTHORISATION NUMBER(S)
EU/1/06/343/005
9. DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
Date of first authorisation: 26 June 2006
Date of latest renewal: 26 June 2011
10. DATE OF REVISION OF THE TEXT
{MM/YYYY}
Detailed information on this medicinal product is available on the website of the European Medicines
Agency http://www.ema.europa.eu/.
59
ANNEX II
A. MANUFACTURER RESPONSIBLE FOR BATCH RELEASE
B. CONDITIONS OR RESTRICTIONS REGARDING SUPPLY AND USE
C. OTHER CONDITIONS AND REQUIREMENTS OF THE MARKETING
AUTHORISATION
D. CONDITIONS OR RESTRICTIONS WITH REGARD TO THE SAFE AND
EFFECTIVE USE OF THE MEDICINAL PRODUCT

60
A. MANUFACTURER RESPONSIBLE FOR BATCH RELEASE
Name and address of the manufacturer responsible for batch release
Bristol-Myers Squibb S.r.l., Contrada Fontana del Ceraso, 03012 Anagni (FR), Italy
B. CONDITIONS OR RESTRICTIONS REGARDING SUPPLY AND USE
Medicinal product subject to restricted medical prescription (see Annex I: Summary of Product
Characteristics, section 4.2).
C. OTHER CONDITIONS AND REQUIREMENTS OF THE MARKETING
AUTHORISATION
Periodic Safety Update Reports
The marketing authorisation holder shall submit periodic safety update reports for this product in
accordance with the requirements set out in the list of Union reference dates (EURD list) provided for
under Article 107c(7) of Directive 2001/83/EC and published on the European medicines web-portal.
D. CONDITIONS OR RESTRICTIONS WITH REGARD TO THE SAFE AND
EFFECTIVE USE OF THE MEDICINAL PRODUCT
Risk Management Plan (RMP)
The MAH shall perform the required pharmacovigilance activities and interventions detailed in the
agreed RMP presented in Module 1.8.2 of the Marketing Authorisation and any agreed subsequent
updates of the RMP.
An updated RMP should be submitted:
At the request of the European Medicines Agency;
Whenever the risk management system is modified, especially as the result of new information
being received that may lead to a significant change to the benefit/risk profile or as the result of
an important (pharmacovigilance or risk minimisation) milestone being reached.
If the dates for submission of a PSUR and the update of a RMP coincide, they can be submitted at the
same time.
61
ANNEX III
LABELLING AND PACKAGE LEAFLET
62
A. LABELLING

63
PARTICULARS TO APPEAR ON THE OUTER PACKAGING AND THE IMMEDIATE
PACKAGING
OUTER CARTON TEXT (BOTTLE AND BLISTER PRESENTATIONS) AND BOTTLE
LABEL TEXT
1. NAME OF THE MEDICINAL PRODUCT
Baraclude 0.5 mg film-coated tablets
entecavir
2. STATEMENT OF ACTIVE SUBSTANCE(S)
Each film-coated tablet contains 0.5 mg of entecavir.
3. LIST OF EXCIPIENTS
Also contains lactose monohydrate.
4. PHARMACEUTICAL FORM AND CONTENTS
Blister pack: 30 x 1 film-coated tablet
90 x 1 film-coated tablet
Bottle pack: 30 film-coated tablets
5. METHOD AND ROUTE(S) OF ADMINISTRATION
Read the package leaflet before use.
Oral use
6. SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE SIGHT AND REACH OF CHILDREN
Keep out of the sight and reach of children.
7. OTHER SPECIAL WARNING(S), IF NECESSARY
8. EXPIRY DATE
EXP
9. SPECIAL STORAGE CONDITIONS
Blister pack:
Do not store above 30°C.
Store in the original carton.
64
Bottle pack:
Do not store above 25°C.
Keep the bottle tightly closed.
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
BRISTOL-MYERS SQUIBB PHARMA EEIG
Uxbridge Business Park
Sanderson Road
Uxbridge UB8 1DH
United Kingdom
12. MARKETING AUTHORISATION NUMBER(S)
Blister pack : EU/1/06/343/003 30 x 1 film-coated tablet
EU/1/06/343/006 90 x 1 film-coated tablet
Bottle pack: EU/1/06/343/001 30 film-coated tablets
13. BATCH NUMBER
Lot
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription
15. INSTRUCTIONS ON USE
16. INFORMATION IN BRAILLE
Outer carton: Baraclude 0.5 mg

65
MINIMUM PARTICULARS TO APPEAR ON BLISTERS OR STRIPS
1. NAME OF THE MEDICINAL PRODUCT
Baraclude 0.5 mg tablets
entecavir
2. NAME OF THE MARKETING AUTHORISATION HOLDER
BRISTOL-MYERS SQUIBB PHARMA EEIG
3. EXPIRY DATE
EXP
4. BATCH NUMBER
Lot
5. OTHER

66
PARTICULARS TO APPEAR ON THE OUTER PACKAGING AND THE IMMEDIATE
PACKAGING
OUTER CARTON TEXT (BOTTLE AND BLISTER PRESENTATIONS) AND BOTTLE
LABEL TEXT
1. NAME OF THE MEDICINAL PRODUCT
Baraclude 1 mg film-coated tablets
entecavir
2. STATEMENT OF ACTIVE SUBSTANCE(S)
Each film-coated tablet contains 1 mg of entecavir.
3. LIST OF EXCIPIENTS
Also contains lactose monohydrate.
4. PHARMACEUTICAL FORM AND CONTENTS
Blister pack: 30 x 1 film-coated tablet
90 x 1 film-coated tablet
Bottle pack: 30 film-coated tablets
5. METHOD AND ROUTE(S) OF ADMINISTRATION
Read the package leaflet before use.
Oral use
6. SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE SIGHT AND REACH OF CHILDREN
Keep out of the sight and reach of children.
7. OTHER SPECIAL WARNING(S), IF NECESSARY
8. EXPIRY DATE
EXP
9. SPECIAL STORAGE CONDITIONS
Blister pack:
Do not store above 30°C.
Store in the original carton.
67
Bottle pack:
Do not store above 25°C.
Keep the bottle tightly closed.
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
BRISTOL-MYERS SQUIBB PHARMA EEIG
Uxbridge Business Park
Sanderson Road
Uxbridge UB8 1DH
United Kingdom
12. MARKETING AUTHORISATION NUMBER(S)
Blister pack : EU/1/06/343/004 30 x 1 film-coated tablet
EU/1/06/343/007 90 x 1 film-coated tablet
Bottle pack: EU/1/06/343/002 30 film-coated tablets
13. BATCH NUMBER
Lot
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription
15. INSTRUCTIONS ON USE
16. INFORMATION IN BRAILLE
Outer carton: Baraclude 1 mg

68
MINIMUM PARTICULARS TO APPEAR ON BLISTERS OR STRIPS
1. NAME OF THE MEDICINAL PRODUCT
Baraclude 1 mg tablets
entecavir
2. NAME OF THE MARKETING AUTHORISATION HOLDER
BRISTOL-MYERS SQUIBB PHARMA EEIG
3. EXPIRY DATE
EXP
4. BATCH NUMBER
Lot
5. OTHER
69
PARTICULARS TO APPEAR ON THE OUTER PACKAGING AND THE IMMEDIATE
PACKAGING
OUTER CARTON AND BOTTLE LABEL TEXT
1. NAME OF THE MEDICINAL PRODUCT
Baraclude 0.05 mg/ml oral solution
entecavir
2. STATEMENT OF ACTIVE SUBSTANCE(S)
Each ml contains 0.05 mg entecavir.
3. LIST OF EXCIPIENTS
Also contains: maltitol, preservatives E216, E218.
4. PHARMACEUTICAL FORM AND CONTENTS
210 ml oral solution with a measuring spoon.
5. METHOD AND ROUTE(S) OF ADMINISTRATION
Read the package leaflet before use.
Oral use
6. SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT
OF THE SIGHT AND REACH OF CHILDREN
Keep out of the sight and reach of children.
7. OTHER SPECIAL WARNING(S), IF NECESSARY
8. EXPIRY DATE
EXP
9. SPECIAL STORAGE CONDITIONS
Do not store above 30°C.
Keep the bottle in the outer carton in order to protect from light.
70
10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS
OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF
APPROPRIATE
11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER
BRISTOL-MYERS SQUIBB PHARMA EEIG
Uxbridge Business Park
Sanderson Road
Uxbridge UB8 1DH
United Kingdom
12. MARKETING AUTHORISATION NUMBER(S)
EU/1/06/343/005
13. BATCH NUMBER
Lot
14. GENERAL CLASSIFICATION FOR SUPPLY
Medicinal product subject to medical prescription
15. INSTRUCTIONS ON USE
16. INFORMATION IN BRAILLE
Outer carton: Baraclude 0.05 mg/ml
71
B. PACKAGE LEAFLET
72
Package leaflet: Information for the user
Baraclude 0.5 mg film-coated tablets
Entecavir
Read all of this leaflet carefully before you start taking this medicine because it contains
important information for you.
Keep this leaflet. You may need to read it again.
If you have any further questions, ask your doctor or pharmacist.
This medicine has been prescribed for you only. Do not pass it on to others. It may harm them,
even if their signs of illness are the same as yours.
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side
effects not listed in this leaflet. See section 4.
What is in this leaflet
1. What Baraclude is and what it is used for
2. What you need to know before you take Baraclude
3. How to take Baraclude
4. Possible side effects
5. How to store Baraclude
6. Contents of the pack and other information
1. What BARACLUDE is and what it is used for
Baraclude tablets are anti-viral medicines, used to treat chronic (long term) hepatitis B virus
(HBV) infection in adults. Baraclude can be used in people whose liver is damaged but still functions
properly (compensated liver disease) and in people whose liver is damaged and does not function
properly (decompensated liver disease).
Baraclude tablets are also used to treat chronic (long term) HBV infection in children and
adolescents aged 2 years to less than 18 years. Baraclude can be used in children whose liver is
damaged but still functions properly (compensated liver disease).
Infection by the hepatitis B virus can lead to damage to the liver. Baraclude reduces the amount of
virus in your body, and improves the condition of the liver.
2. What you need to know before you take BARACLUDE
Do not take Baraclude
if you are allergic (hypersensitive) to entecavir or any of the other ingredients of this medicine
(listed in section 6).
Warning and precautions
Talk to your doctor or pharmacist before taking Baraclude
if you have ever had problems with your kidneys, tell your doctor. This is important because
Baraclude is eliminated from your body through the kidneys and your dose or dosing schedule
may need to be adjusted.
do not stop taking Baraclude without your doctor’s advice since your hepatitis may worsen
after stopping treatment. When your treatment with Baraclude is stopped, your doctor will
continue to monitor you and take blood tests for several months.
discuss with your doctor whether your liver functions properly and, if not, what the possible
effects on your Baraclude treatment may be.
73
if you are also infected with HIV (human immunodeficiency virus) be sure to tell your doctor.
You should not take Baraclude to treat your hepatitis B infection unless you are taking
medicines for HIV at the same time, as the effectiveness of future HIV treatment may be
reduced. Baraclude will not control your HIV infection.
taking Baraclude will not stop you from infecting other people with hepatitis B virus
(HBV) through sexual contact or body fluids (including blood contamination). So, it is
important to take appropriate precautions to prevent others from becoming infected with HBV.
A vaccine is available to protect those at risk from becoming infected with HBV.
Baraclude belongs to a class of medicines that can cause lactic acidosis (excess of lactic acid
in your blood) and enlargement of the liver. Symptoms such as nausea, vomiting and stomach
pain might indicate the development of lactic acidosis. This rare but serious side effect has
occasionally been fatal. Lactic acidosis occurs more often in women, particularly if they are
very overweight. Your doctor will monitor you regularly while you are receiving Baraclude.
if you have previously received treatment for chronic hepatitis B, please inform your doctor.
Children and adolescents
Baraclude should not be used for children below 2 years of age or weighing less than 10 kg.
Other medicines and Baraclude
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other
medicines.
Baraclude with food and drink
In most cases you may take Baraclude with or without food. However, if you have had a previous
treatment with a medicine containing the active substance lamivudine you should consider the
following. If you were switched over to Baraclude because the treatment with lamivudine was not
successful, you should take Baraclude on an empty stomach once daily. If your liver disease is very
advanced, your doctor will also instruct you to take Baraclude on an empty stomach. Empty stomach
means at least 2 hours after a meal and at least 2 hours before your next meal.
Children and adolescents (from 2 to less than 18 years of age) can take Baraclude with or without
food.
Pregnancy, breast-feeding and fertility
Tell your doctor if you are pregnant or planning to become pregnant. It has not been demonstrated that
Baraclude is safe to use during pregnancy. Baraclude must not be used during pregnancy unless
specifically directed by your doctor. It is important that women of childbearing age receiving
treatment with Baraclude use an effective method of contraception to avoid becoming pregnant.
You should not breast-feed during treatment with Baraclude. Tell your doctor if you are breast-
feeding. It is not known whether entecavir, the active ingredient in Baraclude, is excreted in human
breast milk.
Driving and using machines
Dizziness, tiredness (fatigue) and sleepiness (somnolence) are common side effects which may impair
your ability to drive and use machines. If you have any concerns consult your doctor.
Baraclude contains lactose
This medicinal product contains lactose. If you have been told by your doctor that you have an
intolerance to some sugars, contact your doctor before taking this medicinal product.
3. How to take BARACLUDE
Not all patients need to take the same dose of Baraclude.
74
Always take this medicine exactly as your doctor has told you. Check with your doctor or pharmacist
if you are not sure.
For adults the recommended dose is either 0.5 mg or 1 mg once daily orally (by mouth).
Your dose will depend on:
whether you have been treated for HBV infection before, and what medicine you received.
whether you have kidney problems. Your doctor may prescribe a lower dose for you or instruct
you to take it less often than once a day.
the condition of your liver.
For children and adolescents (from 2 to less than 18 years of age), your child's doctor will decide the
right dose based on your child's weight. The Baraclude oral solution is recommended for patients
weighing from 10 kg to 32.5 kg.Children weighing at least 32.6 kg may take the oral solution or the
0.5 mg tablet. All dosing will be taken once daily orally (by mouth). There are no recommendations
for Baraclude in children less than 2 years of age or weighing less than 10 kg.
Your doctor will advise you on the dose that is right for you. Always take the dose recommended by
your doctor to ensure that your medicine is fully effective and to reduce the development of resistance
to treatment. Take Baraclude as long as your doctor has told you. Your doctor will tell you if and when
you should stop the treatment.
Some patients must take Baraclude on an empty stomach (see Baraclude with food and drink in
Section 2). If your doctor instructs you to take Baraclude on an empty stomach, empty stomach means
at least 2 hours after a meal and at least 2 hours before your next meal.
If you take more Baraclude than you should
Contact your doctor at once.
If you forget to take Baraclude
It is important that you do not miss any doses. If you miss a dose of Baraclude, take it as soon as
possible, and then take your next scheduled dose at its regular time. If it is almost time for your next
dose, do not take the missed dose. Wait and take the next dose at the regular time. Do not take a
double dose to make up for a forgotten dose.
Do not stop Baraclude without your doctor’s advice
Some people get very serious hepatitis symptoms when they stop taking Baraclude. Tell your doctor
immediately about any changes in symptoms that you notice after stopping treatment.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Patients treated with Baraclude have reported the following side effects:
common (at least 1 in 100 patients): headache, insomnia (inability to sleep), fatigue (extreme
tiredness), dizziness, somnolence (sleepiness), vomiting, diarrhoea, nausea, dyspepsia (indigestion),
and increased blood levels of liver enzymes.
uncommon (at least 1 in 1,000 patients): rash, hair loss.
rare (at least 1 in 10,000 patients): severe allergic reaction.
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects
not listed in this leaflet.
Reporting of side effects

75
If you get any side effects, talk to your doctor or pharmacist or. This includes any possible side effects
not listed in this leaflet. You can also report side effects directly via the national reporting system
listed in Appendix V*. By reporting side effects you can help provide more information on the safety
of this medicine.
5. How to store BARACLUDE
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the bottle, blister or carton after EXP.
That expiry date refers to the last day of that month.
Blister packs: do not store above 30°C. Store in the original carton.
Bottle packs: do not store above 25°C. Keep the bottle tightly closed.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to
throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information
What Baraclude contains
The active substance is entecavir. Each film-coated tablet contains 0.5 mg entecavir.
The other ingredients are:
Tablet core
: crospovidone, lactose monohydrate, magnesium stearate, cellulose microcrystalline
and povidone.
Tablet coating
: hypromellose, macrogol 400, titanium dioxide (E171), and polysorbate 80
(E433).
What Baraclude looks like and contents of the pack
The film-coated tablets (tablets) are white to off-white and triangular-shaped. They are marked with
“BMS” on one side and “1611” on the other. Baraclude 0.5 mg film-coated tablets are supplied in
cartons containing 30 x 1 or 90 x 1 film-coated tablet (in unit-dose blisters) and in bottles containing
30 film-coated tablets.
Not all pack sizes may be marketed in your country.
Marketing Authorisation Holder and Manufacturer
Marketing Authorisation Holder:
BRISTOL-MYERS SQUIBB PHARMA EEIG
Uxbridge Business Park
Sanderson Road
Uxbridge UB8 1DH
United Kingdom
Manufacturer:
Bristol-Myers Squibb S.r.l.
Contrada Fontana del Ceraso
03012 Anagni (FR)
Italy
For any information about this medicine, please contact the local representative of the Marketing
Authorisation Holder.
76
Belgique/België/Belgien
N
.V. Bristol-Myers Squibb Belgium S.A.
Tél/Tel: + 32 2 352 76 11
Lietuva
Bristol-Myers Squibb Gyógyszerkereskedelmi Kft.
Tel: + 370 5 2790 762
България
Bristol-Myers Squibb Gyógyszerkereskedelmi Kft.
Teл.: + 359 800 12 400
Luxembourg/Luxemburg
N
.V. Bristol-Myers Squibb Belgium S.A.
Tél/Tel: + 32 2 352 76 11
Česká republika
Bristol-Myers Squibb spol. s r.o.
Tel: + 420 221 016 111
Magyarország
Bristol-Myers Squibb Gyógyszerkereskedelmi Kft.
Tel.: + 36 1 301 9700
Danmark
Bristol-Myers Squibb
Tlf: + 45 45 93 05 06
Malta
B
RISTOL-MYERS SQUIBB S.R.L.
Tel: + 39 06 50 39 61
Deutschland
Bristol-Myers Squibb GmbH & Co. KGaA
Tel: + 49 89 121 42-0
Nederland
Bristol-Myers Squibb B.V.
Tel: + 31 (0)30 300 2222
Eesti
Bristol-Myers Squibb Gyógyszerkereskedelmi Kft.
Tel: + 372 6827 400
Norge
Bristol-Myers Squibb Norway Ltd
Tlf: + 47 67 55 53 50
Ελλάδα
B
RISTOL-MYERS SQUIBB A.E.
Τηλ: + 30 210 6074300
Österreich
Bristol-Myers Squibb GesmbH
Tel: + 43 1 60 14 30
España
B
RISTOL-MYERS SQUIBB, S.A.
Tel: + 34 91 456 53 00
Polska
B
RISTOL-MYERS SQUIBB POLSKA SP. Z O.O.
Tel.: + 48 22 5796666
France
Bristol-Myers Squibb SARL
Tél: + 33 (0)810 410 500
Portugal
Bristol-Myers Squibb Farmacêutica Portuguesa,
S.A.
Tel: + 351 21 440 70 00
Hrvatska
Bristol-Myers Squibb spol. s r.o.
T
EL: +385 (1) 6311-833
România
Bristol-Myers Squibb Gyógyszerkereskedelmi Kft.
Tel: + 40 (0)21 272 16 00
Ireland
Bristol-Myers Squibb Pharmaceuticals Ltd
Tel: + 353 (1 800) 749 749
Slovenija
Bristol-Myers Squibb spol. s r.o.
Tel: + 386 1 236 47 00
Ísland
Vistor hf.
Sími: + 354 535 7000
Slovenská republika
Bristol-Myers Squibb spol. s r.o.
Tel: + 421 2 59298411
Italia
B
RISTOL-MYERS SQUIBB S.R.L.
Tel: + 39 06 50 39 61
Suomi/Finland
Oy Bristol-Myers Squibb (Finland) Ab
Puh/Tel: + 358 9 251 21 230
Κύπρος
B
RISTOL-MYERS SQUIBB A.E.
Τηλ: + 357 800 92666
Sverige
Bristol-Myers Squibb AB
Tel: + 46 8 704 71 00
77
Latvija
Bristol-Myers Squibb Gyógyszerkereskedelmi Kft.
Tel: + 371 67 50 21 85
United Kingdom
Bristol-Myers Squibb Pharmaceuticals Ltd
Tel: + 44 (0800) 731 1736
This leaflet was last revised in
Other sources of information
Detailed information on this medicine is available on the European Medicines Agency web site:
http://www.ema.europa.eu/.
78
Package leaflet: Information for the user
Baraclude 1 mg film-coated tablets
Entecavir
Read all of this leaflet carefully before you start taking this medicine because it contains
important information for you.
Keep this leaflet. You may need to read it again.
If you have any further questions, ask your doctor or pharmacist.
This medicine has been prescribed for you only. Do not pass it on to others. It may harm them,
even if their signs of illness are the same as yours.
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side
effects not listed in this leaflet. See section 4.
What is in this leaflet
1. What Baraclude is and what it is used for
2. What you need to know before you take Baraclude
3. How to take Baraclude
4. Possible side effects
5. How to store Baraclude
6. Contents of the pack and other information
1. What BARACLUDE is and what it is used for
Baraclude tablets are anti-viral medicines, used to treat chronic (long term) hepatitis B virus
(HBV) infection in adults. Baraclude can be used in people whose liver is damaged but still functions
properly (compensated liver disease) and in people whose liver is damaged and does not function
properly (decompensated liver disease).
Baraclude tablets are also used to treat chronic (long term) HBV infection in children and
adolescents aged 2 years to less than 18 years. Baraclude can be used in children whose liver is
damaged but still functions properly (compensated liver disease).
Infection by the hepatitis B virus can lead to damage to the liver. Baraclude reduces the amount of
virus in your body, and improves the condition of the liver.
2. What you need to know before you take BARACLUDE
Do not take Baraclude
if you are allergic (hypersensitive) to entecavir or any of the other ingredients of this medicine
(listed in section 6).
Warning and precautions
Talk to your doctor or pharmacist before taking Baraclude
if you have ever had problems with your kidneys, tell your doctor. This is important because
Baraclude is eliminated from your body through the kidneys and your dose or dosing schedule
may need to be adjusted.
do not stop taking Baraclude without your doctor’s advice since your hepatitis may worsen
after stopping treatment. When your treatment with Baraclude is stopped, your doctor will
continue to monitor you and take blood tests for several months.
discuss with your doctor whether your liver functions properly and, if not, what the possible
effects on your Baraclude treatment may be.
79
if you are also infected with HIV (human immunodeficiency virus) be sure to tell your doctor.
You should not take Baraclude to treat your hepatitis B infection unless you are taking
medicines for HIV at the same time, as the effectiveness of future HIV treatment may be
reduced. Baraclude will not control your HIV infection.
taking Baraclude will not stop you from infecting other people with hepatitis B virus
(HBV) through sexual contact or body fluids (including blood contamination). So, it is
important to take appropriate precautions to prevent others from becoming infected with HBV.
A vaccine is available to protect those at risk from becoming infected with HBV.
Baraclude belongs to a class of medicines that can cause lactic acidosis (excess of lactic acid
in your blood) and enlargement of the liver. Symptoms such as nausea, vomiting and stomach
pain might indicate the development of lactic acidosis. This rare but serious side effect has
occasionally been fatal. Lactic acidosis occurs more often in women, particularly if they are
very overweight. Your doctor will monitor you regularly while you are receiving Baraclude.
if you have previously received treatment for chronic hepatitis B, please inform your doctor.
Children and adolescents
Baraclude should not be used for children below 2 years of age or weighing less than 10 kg.
Other medicines and Baraclude
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other
medicines.
Baraclude with food and drink
In most cases you may take Baraclude with or without food. However, if you have had a previous
treatment with a medicine containing the active substance lamivudine you should consider the
following. If you were switched over to Baraclude because the treatment with lamivudine was not
successful, you should take Baraclude on an empty stomach once daily. If your liver disease is very
advanced, your doctor will also instruct you to take Baraclude on an empty stomach. Empty stomach
means at least 2 hours after a meal and at least 2 hours before your next meal.
Pregnancy, breast-feeding and fertility
Tell your doctor if you are pregnant or planning to become pregnant. It has not been demonstrated that
Baraclude is safe to use during pregnancy. Baraclude must not be used during pregnancy unless
specifically directed by your doctor. It is important that women of childbearing age receiving
treatment with Baraclude use an effective method of contraception to avoid becoming pregnant.
You should not breast-feed during treatment with Baraclude. Tell your doctor if you are breast-
feeding. It is not known whether entecavir, the active ingredient in Baraclude, is excreted in human
breast milk.
Driving and using machines
Dizziness, tiredness (fatigue) and sleepiness (somnolence) are common side effects which may impair
your ability to drive and use machines. If you have any concerns consult your doctor.
Baraclude contains lactose
This medicinal product contains lactose. If you have been told by your doctor that you have an
intolerance to some sugars, contact your doctor before taking this medicinal product.
3. How to take BARACLUDE
Not all patients need to take the same dose of Baraclude.

80
Always take this medicine exactly as your doctor has told you. Check with your doctor or pharmacist
if you are not sure.
For adults the recommended dose is either 0.5 mg or 1 mg once daily orally (by mouth).
Your dose will depend on:
whether you have been treated for HBV infection before, and what medicine you received.
whether you have kidney problems. Your doctor may prescribe a lower dose for you or instruct
you to take it less often than once a day.
the condition of your liver.
For children and adolescents (from 2 to less than 18 years of age), Baraclude oral solution or
Baraclude 0.5 mg tablets are available
Your doctor will advise you on the dose that is right for you. Always take the dose recommended by
your doctor to ensure that your medicine is fully effective and to reduce the development of resistance
to treatment. Take Baraclude as long as your doctor has told you. Your doctor will tell you if and when
you should stop the treatment.
Some patients must take Baraclude on an empty stomach (see Baraclude with food and drink in
Section 2). If your doctor instructs you to take Baraclude on an empty stomach, empty stomach means
at least 2 hours after a meal and at least 2 hours before your next meal.
If you take more Baraclude than you should
Contact your doctor at once.
If you forget to take Baraclude
It is important that you do not miss any doses. If you miss a dose of Baraclude, take it as soon as
possible, and then take your next scheduled dose at its regular time. If it is almost time for your next
dose, do not take the missed dose. Wait and take the next dose at the regular time. Do not take a
double dose to make up for a forgotten dose.
Do not stop Baraclude without your doctor’s advice
Some people get very serious hepatitis symptoms when they stop taking Baraclude. Tell your doctor
immediately about any changes in symptoms that you notice after stopping treatment.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Patients treated with Baraclude have reported the following side effects:
common (at least 1 in 100 patients): headache, insomnia (inability to sleep), fatigue (extreme
tiredness), dizziness, somnolence (sleepiness), vomiting, diarrhoea, nausea, dyspepsia (indigestion),
and increased blood levels of liver enzymes.
uncommon (at least 1 in 1,000 patients): rash, hair loss.
rare (at least 1 in 10,000 patients): severe allergic reaction.
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects
not listed in this leaflet.
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist or. This includes any possible side effects
not listed in this leaflet. You can also report side effects directly via the national reporting system

81
listed in Appendix V*. By reporting side effects you can help provide more information on the safety
of this medicine.
5. How to store BARACLUDE
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the bottle, blister or carton after EXP.
That expiry date refers to the last day of that month.
Blister packs: do not store above 30°C. Store in the original carton.
Bottle packs: do not store above 25°C. Keep the bottle tightly closed.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to
throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information
What Baraclude contains
The active substance is entecavir. Each film-coated tablet contains 1 mg entecavir.
The other ingredients are:
Tablet core
: crospovidone, lactose monohydrate, magnesium stearate, cellulose microcrystalline
and povidone.
Tablet coating
: hypromellose, macrogol 400, titanium dioxide (E171), and iron oxide red.
What Baraclude looks like and contents of the pack
The film-coated tablets (tablets) are pink and triangular-shaped. They are marked with “BMS” on one
side and “1612” on the other. Baraclude 1 mg film-coated tablets are supplied in cartons containing
30 x 1 or 90 x 1 film-coated tablet (in unit-dose blisters) and in bottles containing 30 film-coated
tablets.
Not all pack sizes may be marketed in your country.
Marketing Authorisation Holder and Manufacturer
Marketing Authorisation Holder:
BRISTOL-MYERS SQUIBB PHARMA EEIG
Uxbridge Business Park
Sanderson Road
Uxbridge UB8 1DH
United Kingdom
Manufacturer:
Bristol-Myers Squibb S.r.l.
Contrada Fontana del Ceraso
03012 Anagni (FR)
Italy
For any information about this medicine, please contact the local representative of the Marketing
Authorisation Holder.
Belgique/België/Belgien
N
.V. Bristol-Myers Squibb Belgium S.A.
Tél/Tel: + 32 2 352 76 11
Lietuva
Bristol-Myers Squibb Gyógyszerkereskedelmi Kft.
Tel: + 370 5 2790 762
82
България
Bristol-Myers Squibb Gyógyszerkereskedelmi Kft.
Teл.: + 359 800 12 400
Luxembourg/Luxemburg
N
.V. Bristol-Myers Squibb Belgium S.A.
Tél/Tel: + 32 2 352 76 11
Česká republika
Bristol-Myers Squibb spol. s r.o.
Tel: + 420 221 016 111
Magyarország
Bristol-Myers Squibb Gyógyszerkereskedelmi Kft.
Tel.: + 36 1 301 9700
Danmark
Bristol-Myers Squibb
Tlf: + 45 45 93 05 06
Malta
B
RISTOL-MYERS SQUIBB S.R.L.
Tel: + 39 06 50 39 61
Deutschland
Bristol-Myers Squibb GmbH & Co. KGaA
Tel: + 49 89 121 42-0
Nederland
Bristol-Myers Squibb B.V.
Tel: + 31 (0)30 300 2222
Eesti
Bristol-Myers Squibb Gyógyszerkereskedelmi Kft.
Tel: + 372 6827 400
Norge
Bristol-Myers Squibb Norway Ltd
Tlf: + 47 67 55 53 50
Ελλάδα
B
RISTOL-MYERS SQUIBB A.E.
Τηλ: + 30 210 6074300
Österreich
Bristol-Myers Squibb GesmbH
Tel: + 43 1 60 14 30
España
B
RISTOL-MYERS SQUIBB, S.A.
Tel: + 34 91 456 53 00
Polska
BRISTOL-MYERS SQUIBB POLSKA SP. Z O.O.
Tel.: + 48 22 5796666
France
Bristol-Myers Squibb SARL
Tél: + 33 (0)810 410 500
Portugal
Bristol-Myers Squibb Farmacêutica Portuguesa,
S.A.
Tel: + 351 21 440 70 00
Hrvatska
Bristol-Myers Squibb spol. s r.o.
T
EL: +385 (1) 6311-833
România
Bristol-Myers Squibb Gyógyszerkereskedelmi Kft.
Tel: + 40 (0)21 272 16 00
Ireland
Bristol-Myers Squibb Pharmaceuticals Ltd
Tel: + 353 (1 800) 749 749
Slovenija
Bristol-Myers Squibb spol. s r.o.
Tel: + 386 1 236 47 00
Ísland
Vistor hf.
Sími: + 354 535 7000
Slovenská republika
Bristol-Myers Squibb spol. s r.o.
Tel: + 421 2 59298411
Italia
B
RISTOL-MYERS SQUIBB S.R.L.
Tel: + 39 06 50 39 61
Suomi/Finland
Oy Bristol-Myers Squibb (Finland) Ab
Puh/Tel: + 358 9 251 21 230
Κύπρος
B
RISTOL-MYERS SQUIBB A.E.
Τηλ: + 357 800 92666
Sverige
Bristol-Myers Squibb AB
Tel: + 46 8 704 71 00
83
Latvija
Bristol-Myers Squibb Gyógyszerkereskedelmi Kft.
Tel: + 371 67 50 21 85
United Kingdom
Bristol-Myers Squibb Pharmaceuticals Ltd
Tel: + 44 (0800) 731 1736
This leaflet was last revised in
Other sources of information
Detailed information on this medicine is available on the European Medicines Agency web site:
http://www.ema.europa.eu/.
84
Package leaflet: Information for the user
Baraclude 0.05 mg/ml oral solution
Entecavir
Read all of this leaflet carefully before you start taking this medicine because it contains
important information for you.
Keep this leaflet. You may need to read it again.
If you have any further questions, ask your doctor or pharmacist.
This medicine has been prescribed for you only. Do not pass it on to others. It may harm them,
even if their signs of illness are the same as yours.
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side
effects not listed in this leaflet. See section 4.
What is in this leaflet
1. What Baraclude is and what it is used for
2. What you need to know before you take Baraclude
3. How to take Baraclude
4. Possible side effects
5. How to store Baraclude
6. Contents of the pack and other information
1. What BARACLUDE is and what it is used for
Baraclude oral solution is an anti-viral medicine, used to treat chronic (long term) hepatitis B
virus (HBV) infection in adults. Baraclude can be used in people whose liver is damaged but still
functions properly (compensated liver disease) and in people whose liver is damaged and does not
function properly (decompensated liver disease).
Baraclude oral solution is also used to treat chronic (long term) HBV infection in children and
adolescents aged 2 years to less than 18 years. Baraclude can be used in children whose liver is
damaged but still functions properly (compensated liver disease).
Infection by the hepatitis B virus can lead to damage to the liver. Baraclude reduces the amount of
virus in your body, and improves the condition of the liver.
2. What you need to know before you take BARACLUDE
Do not take Baraclude
if you are allergic (hypersensitive) to entecavir or any of the other ingredients of this medicine
(listed in section 6).
Warning and precautions
Talk to your doctor or pharmacist before taking Baraclude
if you have ever had problems with your kidneys, tell your doctor. This is important because
Baraclude is eliminated from your body through the kidneys and your dose or dosing schedule
may need to be adjusted.
do not stop taking Baraclude without your doctor’s advice since your hepatitis may worsen
after stopping treatment. When your treatment with Baraclude is stopped, your doctor will
continue to monitor you and take blood tests for several months.
discuss with your doctor whether your liver functions properly and, if not, what the possible
effects on your Baraclude treatment may be.
85
if you are also infected with HIV (human immunodeficiency virus) be sure to tell your doctor.
You should not take Baraclude to treat your hepatitis B infection unless you are taking
medicines for HIV at the same time, as the effectiveness of future HIV treatment may be
reduced. Baraclude will not control your HIV infection.
taking Baraclude will not stop you from infecting other people with hepatitis B virus
(HBV) through sexual contact or body fluids (including blood contamination). So, it is
important to take appropriate precautions to prevent others from becoming infected with HBV.
A vaccine is available to protect those at risk from becoming infected with HBV.
Baraclude belongs to a class of medicines that can cause lactic acidosis (excess of lactic acid
in your blood) and enlargement of the liver. Symptoms such as nausea, vomiting and stomach
pain might indicate the development of lactic acidosis. This rare but serious side effect has
occasionally been fatal. Lactic acidosis occurs more often in women, particularly if they are
very overweight. Your doctor will monitor you regularly while you are receiving Baraclude.
if you have previously received treatment for chronic hepatits B, please inform your doctor.
Children and adolescents
Baraclude should not be used for children below 2 years of age or weighing less than 10 kg.
Other medicines and Baraclude
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other
medicines.
Baraclude with food and drink
In most cases you may take Baraclude with or without food. However, if you have had a previous
treatment with a medicine containing the active substance lamivudine you should consider the
following. If you were switched over to Baraclude because the treatment with lamivudine was not
successful, you should take Baraclude on an empty stomach once daily. If your liver disease is very
advanced, your doctor will also instruct you to take Baraclude on an empty stomach.Empty stomach
means at least 2 hours after a meal and at least 2 hours before your next meal.
Children and adolescents (from 2 to less than 18 years of age) can take Baraclude with or without
food.
Pregnancy, breast-feeding and fertility
Tell your doctor if you are pregnant or planning to become pregnant. It has not been demonstrated that
Baraclude is safe to use during pregnancy. Baraclude must not be used during pregnancy unless
specifically directed by your doctor. It is important that women of childbearing age receiving
treatment with Baraclude use an effective method of contraception to avoid becoming pregnant.
You should not breast-feed during treatment with Baraclude. Tell your doctor if you are breast-
feeding. It is not known whether entecavir, the active ingredient in Baraclude, is excreted in human
breast milk.
Driving and using machines
Dizziness, tiredness (fatigue) and sleepiness (somnolence) are common side effects which may impair
your ability to drive and use machines. If you have any concerns consult your doctor.
Baraclude contains maltitol, methylhydroxybenzoate (E218) and propylhydroxybenzoate (E216)
This medicinal product contains maltitol. If you have been told by your doctor that you have an
intolerance to some sugars, contact your doctor before taking this medicinal product.
This product contains methylhydroxybenzoate (E218) and propylhydroxybenzoate (E216) that may
cause allergic reactions (possibly delayed).
86
3. How to take BARACLUDE
Not all patients need to take the same dose of Baraclude.
Always take this medicine exactly as your doctor has told you. Check with your doctor or pharmacist
if you are not sure.
For adults the recommended dose is either 0.5 mg (10 ml) or 1 mg (20 ml) once daily orally (by
mouth).
Your dose will depend on:
whether you have been treated for HBV infection before, and what medicine you received.
whether you have kidney problems. Your doctor may prescribe a lower dose for you or instruct
you to take it less often than once a day.
the condition of your liver.
For children and adolescents (from 2 to less than 18 years of age), your child's doctor will decide the
right dose based on your child's weight. The correct dose of Baraclude oral solution for children and
adolescents is calculated by body weight and is taken once daily orally (by mouth) as shown below:
Body Weight
Recommended Once Daily Dose of Oral
Solution
10.0 - 14.1 kg 4.0 ml
14.2 - 15.8 kg 4.5 ml
15.9 - 17.4 kg 5.0 ml
17.5 - 19.1 kg 5.5 ml
19.2 - 20.8 kg 6.0 ml
20.9 - 22.5 kg 6.5 ml
22.6 - 24.1 kg 7.0 ml
24.2 - 25.8 kg 7.5 ml
25.9 - 27.5 kg 8.0 ml
27.6 - 29.1 kg 8.5 ml
29.2 - 30.8 kg 9.0 ml
30.9 - 32.5 kg 9.5 ml
At least 32.6 kg 10.0 ml
There are no dosing recommendations for Baraclude in children less than 2 years of age or weighing
less than 10 kg.
Your doctor will advise you on the dose that is right for you. Always take the dose recommended by
your doctor to ensure that your medicine is fully effective and to reduce the development of resistance
to treatment. Take Baraclude as long as your doctor has told you. Your doctor will tell you if and when
you should stop the treatment.
Baraclude oral solution is designed as a ready-to-use product. Do not dilute or mix this solution with
water or anything else.
Baraclude oral solution comes with a measuring spoon with markings from 0.5 up to 10 milliliters.
Use the spoon as follows:
87
1. Hold the spoon in a vertical (upright) position and fill it
gradually to the mark corresponding to the prescribed
dose. Holding the spoon with the volume marks facing
you, check that it has been filled to the proper mark.
2. Swallow the medicine directly from the measuring spoon.
3. After each use, rinse the spoon with water and allow it to
air dry.
Some patients must take Baraclude on an empty stomach (see Baraclude with food and drink in
Section 2). If your doctor instructs you to take Baraclude on an empty stomach, empty stomach means
at least 2 hours after a meal and at least 2 hours before your next meal.
If you take more Baraclude than you should
Contact your doctor at once.
If you forget to take Baraclude
It is important that you do not miss any doses. If you miss a dose of Baraclude, take it as soon as
possible, and then take your next scheduled dose at its regular time. If it is almost time for your next
dose, do not take the missed dose. Wait and take the next dose at the regular time. Do not take a
double dose to make up for a forgotten dose.
Do not stop Baraclude without your doctor’s advice
Some people get very serious hepatitis symptoms when they stop taking Baraclude. Tell your doctor
immediately about any changes in symptoms that you notice after stopping treatment.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Patients treated with Baraclude have reported the following side effects:
common (at least 1 in 100 patients): headache, insomnia (inability to sleep), fatigue (extreme
tiredness), dizziness, somnolence (sleepiness), vomiting, diarrhoea, nausea, dyspepsia (indigestion),
and increased blood levels of liver enzymes.
uncommon (at least 1 in 1,000 patients): rash, hair loss.
rare (at least 1 in 10,000 patients): severe allergic reaction.
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects
not listed in this leaflet.
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist or. This includes any possible side effects
not listed in this leaflet. You can also report side effects directly via the national reporting system
listed in Appendix V*. By reporting side effects you can help provide more information on the safety
of this medicine.
88
5. How to store BARACLUDE
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on bottle or carton after EXP. That
expiry date refers to the last day of that month.
Do not store above 30°C. Keep the bottle in the outer carton in order to protect from light.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to
throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information
What Baraclude contains
The active substance is entecavir. Each ml of oral solution contains 0.05 mg entecavir.
The other ingredients are: citric acid anhydrous, maltitol (E965), methylhydroxybenzoate
(E218), propylhydroxybenzoate (E216), orange flavour (acacia and natural flavours), sodium
citrate, sodium hydroxide, hydrochloric acid and purified water.
What Baraclude looks like and contents of the pack
The oral solution is a clear, colorless to pale yellow solution. Baraclude 0.05 mg/ml oral solution is
supplied in a bottle containing 210 ml oral solution. Each carton includes a measuring spoon
(polypropylene) with markings from 0.5 ml up to 10 ml
Marketing Authorisation Holder and Manufacturer
Marketing Authorisation Holder:
BRISTOL-MYERS SQUIBB PHARMA EEIG
Uxbridge Business Park
Sanderson Road
Uxbridge UB8 1DH
United Kingdom
Manufacturer:
Bristol-Myers Squibb S.r.l.
Contrada Fontana del Ceraso
03012 Anagni (FR)
Italy
For any information about this medicine, please contact the local representative of the Marketing
Authorisation Holder.
Belgique/België/Belgien
N
.V. Bristol-Myers Squibb Belgium S.A.
Tél/Tel: + 32 2 352 76 11
Lietuva
Bristol-Myers Squibb Gyógyszerkereskedelmi Kft.
Tel: + 370 5 2790 762
България
Bristol-Myers Squibb Gyógyszerkereskedelmi Kft.
Teл.: + 359 800 12 400
Luxembourg/Luxemburg
N
.V. Bristol-Myers Squibb Belgium S.A.
Tél/Tel: + 32 2 352 76 11
Česká republika
Bristol-Myers Squibb spol. s r.o.
Tel: + 420 221 016 111
Magyarország
Bristol-Myers Squibb Gyógyszerkereskedelmi Kft.
Tel.: + 36 1 301 9700
89
Danmark
Bristol-Myers Squibb
Tlf: + 45 45 93 05 06
Malta
B
RISTOL-MYERS SQUIBB S.R.L.
Tel: + 39 06 50 39 61
Deutschland
Bristol-Myers Squibb GmbH & Co. KGaA
Tel: + 49 89 121 42-0
Nederland
Bristol-Myers Squibb B.V.
Tel: + 31 (0)30 300 2222
Eesti
Bristol-Myers Squibb Gyógyszerkereskedelmi Kft.
Tel: + 372 6827 400
Norge
Bristol-Myers Squibb Norway Ltd
Tlf: + 47 67 55 53 50
Ελλάδα
B
RISTOL-MYERS SQUIBB A.E.
Τηλ: + 30 210 6074300
Österreich
Bristol-Myers Squibb GesmbH
Tel: + 43 1 60 14 30
España
B
RISTOL-MYERS SQUIBB, S.A.
Tel: + 34 91 456 53 00
Polska
B
RISTOL-MYERS SQUIBB POLSKA SP. Z O.O.
Tel.: + 48 22 5796666
France
Bristol-Myers Squibb SARL
Tél: + 33 (0)810 410 500
Portugal
Bristol-Myers Squibb Farmacêutica Portuguesa,
S.A.
Tel: + 351 21 440 70 00
Hrvatska
Bristol-Myers Squibb spol. s r.o.
T
EL: +385 (1) 6311-833
România
Bristol-Myers Squibb Gyógyszerkereskedelmi Kft.
Tel: + 40 (0)21 272 16 00
Ireland
Bristol-Myers Squibb Pharmaceuticals Ltd
Tel: + 353 (1 800) 749 749
Slovenija
Bristol-Myers Squibb spol. s r.o.
Tel: + 386 1 236 47 00
Ísland
Vistor hf.
Sími: + 354 535 7000
Slovenská republika
Bristol-Myers Squibb spol. s r.o.
Tel: + 421 2 59298411
Italia
B
RISTOL-MYERS SQUIBB S.R.L.
Tel: + 39 06 50 39 61
Suomi/Finland
Oy Bristol-Myers Squibb (Finland) Ab
Puh/Tel: + 358 9 251 21 230
Κύπρος
B
RISTOL-MYERS SQUIBB A.E.
Τηλ: + 357 800 92666
Sverige
Bristol-Myers Squibb AB
Tel: + 46 8 704 71 00
Latvija
Bristol-Myers Squibb Gyógyszerkereskedelmi Kft.
Tel: + 371 67 50 21 85
United Kingdom
Bristol-Myers Squibb Pharmaceuticals Ltd
Tel: + 44 (0800) 731 1736
The measuring spoon is manufactured by: Comar Plastics Division, One Comar Place, Buena, NJ
08310, USA.
90
Authorised Representative in the EEA for Comar Plastics: MDSS, Burckhardstrasse 1, 30163
Hannover, Germany
This leaflet was last revised in
Other sources of information
Detailed information on this medicine is available on the European Medicines Agency web site:
http://www.ema.europa.eu/.
