
1
Package leaflet: Information for the user
Baraclude 0.05 mg/ml oral solution
Entecavir
Read all of this leaflet carefully before you start taking this medicine because it contains
important information for you.
▪ Keep this leaflet. You may need to read it again.
▪ If you have any further questions, ask your doctor or pharmacist.
▪ This medicine has been prescribed for you only. Do not pass it on to others. It may harm them,
even if their signs of illness are the same as yours.
▪ If you get any side effects, talk to your doctor or pharmacist. This includes any possible side
effects not listed in this leaflet. See section 4.
What is in this leaflet
1. What Baraclude is and what it is used for
2. What you need to know before you take Baraclude
3. How to take Baraclude
4. Possible side effects
5. How to store Baraclude
6. Contents of the pack and other information
1. What BARACLUDE is and what it is used for
Baraclude oral solution is an anti-viral medicine, used to treat chronic (long term) hepatitis B
virus (HBV) infection in adults. Baraclude can be used in people whose liver is damaged but still
functions properly (compensated liver disease) and in people whose liver is damaged and does not
function properly (decompensated liver disease).
Baraclude oral solution is also used to treat chronic (long term) HBV infection in children and
adolescents aged 2 years to less than 18 years. Baraclude can be used in children whose liver is
damaged but still functions properly (compensated liver disease).
Infection by the hepatitis B virus can lead to damage to the liver. Baraclude reduces the amount of
virus in your body, and improves the condition of the liver.
2. What you need to know before you take BARACLUDE
Do not take Baraclude
▪ if you are allergic (hypersensitive) to entecavir or any of the other ingredients of this medicine
(listed in section 6).
Warning and precautions
Talk to your doctor or pharmacist before taking Baraclude
▪ if you have ever had problems with your kidneys, tell your doctor. This is important because
Baraclude is eliminated from your body through the kidneys and your dose or dosing schedule
may need to be adjusted.
▪ do not stop taking Baraclude without your doctor’s advice since your hepatitis may worsen
after stopping treatment. When your treatment with Baraclude is stopped, your doctor will
continue to monitor you and take blood tests for several months.
▪ discuss with your doctor whether your liver functions properly and, if not, what the possible
effects on your Baraclude treatment may be.
Approved
1.0
v

2
▪ if you are also infected with HIV (human immunodeficiency virus) be sure to tell your doctor.
You should not take Baraclude to treat your hepatitis B infection unless you are taking
medicines for HIV at the same time, as the effectiveness of future HIV treatment may be
reduced. Baraclude will not control your HIV infection.
▪ taking Baraclude will not stop you from infecting other people with hepatitis B virus
(HBV) through sexual contact or body fluids (including blood contamination). So, it is
important to take appropriate precautions to prevent others from becoming infected with HBV.
A vaccine is available to protect those at risk from becoming infected with HBV.
▪ Baraclude belongs to a class of medicines that can cause lactic acidosis (excess of lactic acid
in your blood) and enlargement of the liver. Symptoms such as nausea, vomiting and stomach
pain might indicate the development of lactic acidosis. This rare but serious side effect has
occasionally been fatal. Lactic acidosis occurs more often in women, particularly if they are
very overweight. Your doctor will monitor you regularly while you are receiving Baraclude.
▪ if you have previously received treatment for chronic hepatits B, please inform your doctor.
Children and adolescents
Baraclude should not be used for children below 2 years of age or weighing less than 10 kg.
Other medicines and Baraclude
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other
medicines.
Baraclude with food and drink
In most cases you may take Baraclude with or without food. However, if you have had a previous
treatment with a medicine containing the active substance lamivudine you should consider the
following. If you were switched over to Baraclude because the treatment with lamivudine was not
successful, you should take Baraclude on an empty stomach once daily. If your liver disease is very
advanced, your doctor will also instruct you to take Baraclude on an empty stomach.Empty stomach
means at least 2 hours after a meal and at least 2 hours before your next meal.
Children and adolescents (from 2 to less than 18 years of age) can take Baraclude with or without
food.
Pregnancy, breast-feeding and fertility
Tell your doctor if you are pregnant or planning to become pregnant. It has not been demonstrated that
Baraclude is safe to use during pregnancy. Baraclude must not be used during pregnancy unless
specifically directed by your doctor. It is important that women of childbearing age receiving
treatment with Baraclude use an effective method of contraception to avoid becoming pregnant.
You should not breast-feed during treatment with Baraclude. Tell your doctor if you are breast-
feeding. It is not known whether entecavir, the active ingredient in Baraclude, is excreted in human
breast milk.
Driving and using machines
Dizziness, tiredness (fatigue) and sleepiness (somnolence) are common side effects which may impair
your ability to drive and use machines. If you have any concerns consult your doctor.
Baraclude contains maltitol, methylhydroxybenzoate (E218), propylhydroxybenzoate (E216)
and sodium
This medicinal product contains maltitol. If you have been told by your doctor that you have an
intolerance to some sugars, contact your doctor before taking this medicinal product.
This product contains methylhydroxybenzoate (E218) and propylhydroxybenzoate (E216) that may
cause allergic reactions (possibly delayed).
Approved
1.0
v
3
This medicine contains less than 1 mmol sodium (23 mg) per ml, that is to say essentially ‘sodium-
free’.
3. How to take BARACLUDE
Not all patients need to take the same dose of Baraclude.
Always take this medicine exactly as your doctor has told you. Check with your doctor or pharmacist
if you are not sure.
For adults the recommended dose is either 0.5 mg (10 ml) or 1 mg (20 ml) once daily orally (by
mouth).
Your dose will depend on:
▪ whether you have been treated for HBV infection before, and what medicine you received.
▪ whether you have kidney problems. Your doctor may prescribe a lower dose for you or instruct
you to take it less often than once a day.
▪ the condition of your liver.
For children and adolescents (from 2 to less than 18 years of age), your child's doctor will decide the
right dose based on your child's weight. The correct dose of Baraclude oral solution for children and
adolescents is calculated by body weight and is taken once daily orally (by mouth) as shown below:
Body Weight
Recommended Once Daily Dose of Oral
Solution
10.0 - 14.1 kg
4.0 ml
14.2 - 15.8 kg
4.5 ml
15.9 - 17.4 kg
5.0 ml
17.5 - 19.1 kg
5.5 ml
19.2 - 20.8 kg
6.0 ml
20.9 - 22.5 kg
6.5 ml
22.6 - 24.1 kg
7.0 ml
24.2 - 25.8 kg
7.5 ml
25.9 - 27.5 kg
8.0 ml
27.6 - 29.1 kg
8.5 ml
29.2 - 30.8 kg
9.0 ml
30.9 - 32.5 kg
9.5 ml
At least 32.6 kg
10.0 ml
There are no dosing recommendations for Baraclude in children less than 2 years of age or weighing
less than 10 kg.
Your doctor will advise you on the dose that is right for you. Always take the dose recommended by
your doctor to ensure that your medicine is fully effective and to reduce the development of resistance
to treatment. Take Baraclude as long as your doctor has told you. Your doctor will tell you if and when
you should stop the treatment.
Baraclude oral solution is designed as a ready-to-use product. Do not dilute or mix this solution with
water or anything else.
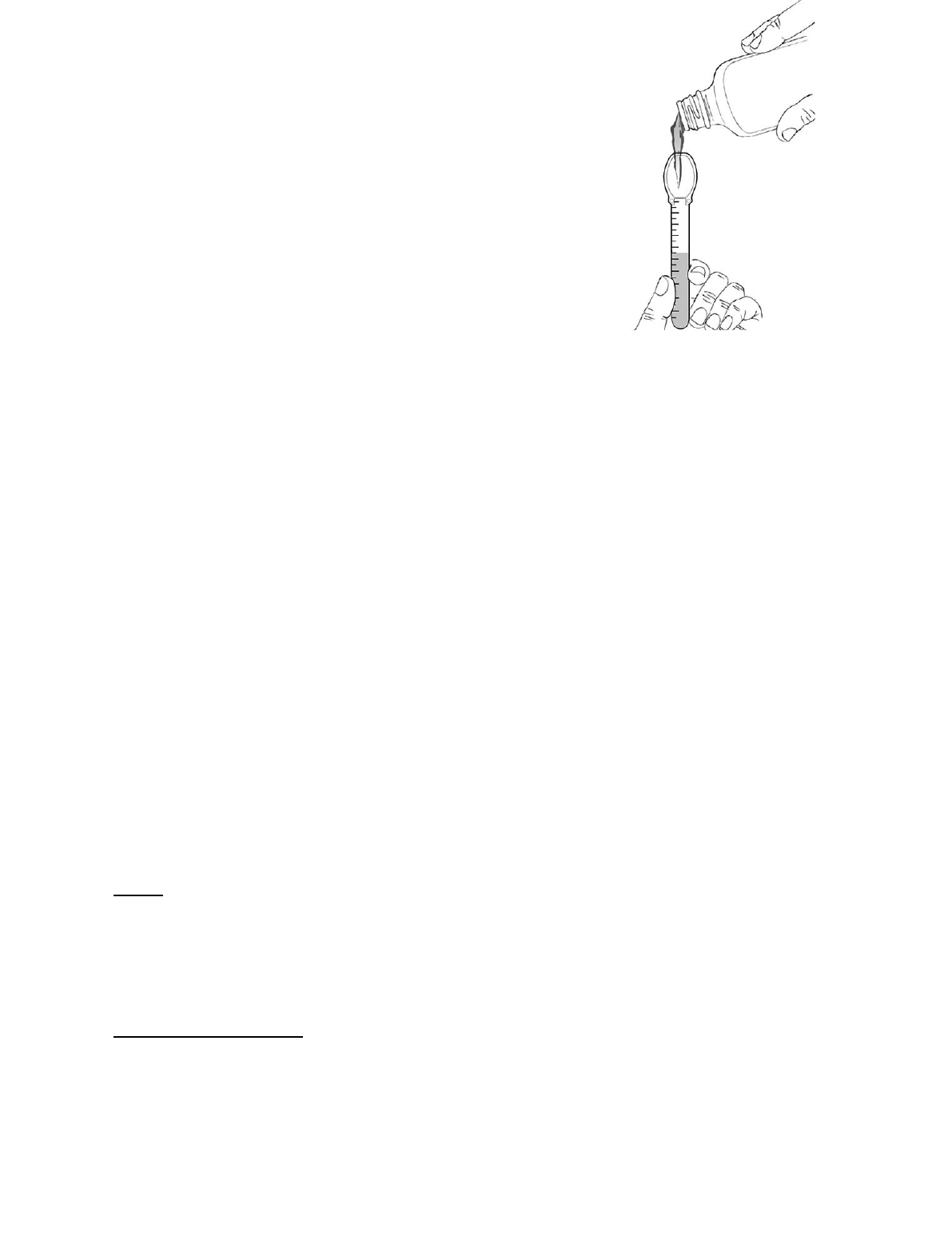
Baraclude oral solution comes with a measuring spoon with markings from 0.5 up to 10 milliliters.
Use the spoon as follows:
Approved
1.0
v
4
1. Hold the spoon in a vertical (upright) position and fill it
gradually to the mark corresponding to the prescribed
dose. Holding the spoon with the volume marks facing
you, check that it has been filled to the proper mark.
2. Swallow the medicine directly from the measuring spoon.
3. After each use, rinse the spoon with water and allow it to
air dry.
Some patients must take Baraclude on an empty stomach (see Baraclude with food and drink in
Section 2). If your doctor instructs you to take Baraclude on an empty stomach, empty stomach means
at least 2 hours after a meal and at least 2 hours before your next meal.
If you take more Baraclude than you should
Contact your doctor at once.
If you forget to take Baraclude
It is important that you do not miss any doses. If you miss a dose of Baraclude, take it as soon as
possible, and then take your next scheduled dose at its regular time. If it is almost time for your next
dose, do not take the missed dose. Wait and take the next dose at the regular time. Do not take a
double dose to make up for a forgotten dose.
Do not stop Baraclude without your doctor’s advice
Some people get very serious hepatitis symptoms when they stop taking Baraclude. Tell your doctor
immediately about any changes in symptoms that you notice after stopping treatment.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Patients treated with Baraclude have reported the following side effects:
Adults
▪ common (at least 1 in 100 patients): headache, insomnia (inability to sleep), fatigue (extreme
tiredness), dizziness, somnolence (sleepiness), vomiting, diarrhoea, nausea, dyspepsia
(indigestion), and increased blood levels of liver enzymes.
▪ uncommon (at least 1 in 1,000 patients): rash, hair loss.
▪ rare (at least 1 in 10,000 patients): severe allergic reaction.
Children and adolescents
The side effects experienced in children and adolescents are similar to those experienced in adults as
described above with the following difference:
Very common (at least 1 in 10 patients): low levels of neutrophils (one type of white blood cells,
which are important in fighting infection).
Approved
1.0
v

5
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects
not listed in this leaflet.
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist or. This includes any possible side effects
not listed in this leaflet. You can also report side effects directly (see details below). By reporting side
effects you can help provide more information on the safety of this medicine.
Yellow Card Scheme
Website: at: www.mhra.gov.uk/yellowcard or search for MHRA Yellow Card in the Google Play or
Apple App Store
5. How to store BARACLUDE
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on bottle or carton after EXP. That
expiry date refers to the last day of that month.
Do not store above 30°C. Keep the bottle in the outer carton in order to protect from light.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to
throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information
What Baraclude contains
▪ The active substance is entecavir. Each ml of oral solution contains 0.05 mg entecavir.
▪ The other ingredients are: citric acid anhydrous, maltitol (E965), methylhydroxybenzoate
(E218), propylhydroxybenzoate (E216), orange flavour (acacia and natural flavours), sodium
citrate, sodium hydroxide, hydrochloric acid and purified water.
What Baraclude looks like and contents of the pack
The oral solution is a clear, colorless to pale yellow solution. Baraclude 0.05 mg/ml oral solution is
supplied in a bottle containing 210 ml oral solution. Each carton includes a measuring spoon
(polypropylene) with markings from 0.5 ml up to 10 ml
Marketing Authorisation Holder and Manufacturer
Marketing Authorisation Holder:
Bristol-Myers Squibb Pharma EEIG
Plaza 254
Blanchardstown Corporate Park 2
Dublin 15, D15 T867
Ireland
Manufacturer:
CATALENT ANAGNI S.R.L.
Loc. Fontana del Ceraso snc
Strada Provinciale 12 Casilina, 41
03012 Anagni (FR)
Italy
Swords Laboratories Unlimited Company T/A Bristol-Myers Squibb Pharmaceutical Operations,
External Manufacturing
Plaza 254
Approved
1.0
v

6
Blanchardstown Corporate Park 2
Dublin 15, D15 T867
Ireland
This leaflet was last revised in June 2022
Approved
1.0
v
