
TECHNICAL REPORT
www.ecdc.europa.eu
Guide to revision of
national pandemic influenza
preparedness plans
Lessons learned from the 2009 A(H1N1) pandemic

ECDC TECHNICAL REPORT
Guide to revision of national pandemic
influenza preparedness plans
Lessons learned from the 2009 A(H1N1) pandemic
ii
This report of the European Centre for Disease Prevention and Control (ECDC) and the WHO Regional Office for
Europe was developed by experts in pandemic preparedness planning from 11 European Union Member States and
staff members of the following organisations: WHO Regional Office for Europe, the European Commission, the
WHO Collaborating Centre for Pandemic Influenza and Research (University of Nottingham), the US Centers for
Disease Control and Prevention, and ECDC.
It was coordinated and compiled by Caroline Brown (WHO), Massimo Ciotti (ECDC), Michala Hegermann-
Lindencrone (WHO), Pasi Penttinen (ECDC), René Snacken (ECDC) and Angus Nicoll (ECDC).
Contributing authors
Theodor Ziegler, Florence Allot, Joan O Donnell, Ehud Kaliner, André Jacobi, Radu Cojocaru, Kosim Kuirbonov,
Nicholas Phin, Jonathan Nguyen Van Tam (WHO Collaborating Centre for Pandemic Influenza and Research, Helmut
Walerius (European Commission) and Adrienne Rashford (WHO Regional Office for Europe).
We acknowledge the valuable input from public health experts from all EU Member States and all countries of the
WHO European Region.
Suggested citation: European Centre for Disease Prevention and Control. Guide to revision of national pandemic
influenza preparedness plans - Lessons learned from the 2009 A(H1N1) pandemic. Stockholm: ECDC; 2017.
Stockholm, November 2017
Print
ISBN 978-92-9498-090-8
doi: 10.2900/466319
TQ-04-17-829-EN-C
PDF
ISBN 978-92-9498-091-5
doi: 10.2900/096346
TQ-04-17-829-EN-N
Cover picture: © Government of Alberta, image licensed under a Creative Commons attribution 2.0 generic license
(CC BY-NC-ND 2.0)
© European Centre for Disease Prevention and Control, 2017
Reproduction is authorised, provided the source is acknowledged.
For any use or reproduction of photos or other material that is not under the EU copyright, permission must be
sought directly from the copyright holders.

TECHNICAL REPORT Guide to revision of national pandemic influenza preparedness plans
iii
Contents
Abbreviations ............................................................................................................................................... iv
Introduction .................................................................................................................................................. 1
Twelve key areas of pandemic preparedness .................................................................................................... 4
Key area 1: Framework for pandemic planning ............................................................................................ 4
Key area 2: Pandemic planning and response based on risk assessment ........................................................ 5
Key area 3: Coordination, command and control .......................................................................................... 7
Key area 4: Risk communication................................................................................................................. 8
Key area 5: Pandemic vaccines .................................................................................................................. 9
Key area 6: Antivirals and other essential medicines .................................................................................. 10
Key area 7: Healthcare services preparedness ........................................................................................... 11
Key area 8: Non-pharmaceutical public health measures ............................................................................ 13
Key area 9: Essential services and business continuity................................................................................ 14
Key area 10: Special groups and settings .................................................................................................. 15
Key area 11: After the pandemic – recovery and transition phase ............................................................... 15
Key area 12: International cooperation, coordination and interoperability..................................................... 16
References and resources ............................................................................................................................. 17
General references .................................................................................................................................. 17
Key area 1: Framework for pandemic planning .......................................................................................... 17
Key area 2: Pandemic planning and response based on risk assessment ...................................................... 17
Key area 3: Coordination, command and control ........................................................................................ 18
Key area 4: Risk communication............................................................................................................... 18
Key area 5: Pandemic vaccines ................................................................................................................ 18
Key area 6: Antivirals and other essential medicines .................................................................................. 19
Key area 7: Healthcare services preparedness ........................................................................................... 19
Key area 8: Non-pharmaceutical public health measures ............................................................................ 19
Key area 9: Essential services and business continuity................................................................................ 20
Key area 10: Special groups and settings .................................................................................................. 20
Key area 11: After the pandemic: Recovery and transition phase ................................................................ 20
Key area 12: International cooperation, coordination and interoperability..................................................... 20
Figures
Figure 1. Key elements of the pandemic preparedness planning cycle ................................................................. 2
Figure 2. The continuum of pandemic phases ................................................................................................... 6
Table
Table 1. Example of stakeholder-representation in the national pandemic preparedness planning committee ......... 5

Guide to revision of national pandemic influenza preparedness plans TECHNICAL REPORT
iv
Abbreviations
AEFI Adverse events after immunization
ARI Acute respiratory infection
AV Antiviral drugs
BCP Business continuity plan
CDC US Centres for Disease Control and Prevention
EC European Commission
ECDC European Centre for Disease Prevention and Control
ECMO Extracorporeal membrane oxygenation
EEA European Economic Area
EMA European Medicines Agency
EU European Union
EuroFlu WHO Regional Office for Europe, influenza surveillance bulletin
EWRS Early Warning and Response System
GISRS Global Influenza Surveillance and Response System
HCW Healthcare worker
ICU Intensive care unit
I-MOVE Influenza Monitoring Vaccine Effectiveness
NIC National influenza centre
NITAG National Immunisation Technical Advisory Group
NPI Non-pharmaceutical intervention
NRA National regulatory authority
SAGE Strategic Advisory Group of Experts on Immunisation
UN United Nations
VAESCO Vaccine Adverse Event Surveillance and Communication
WHO World Health Organization
WHOCC WHO Collaborating Centre for Reference and Research on Influenza
WHO/Europe WHO Regional Office for Europe
WISO ECDC Weekly Influenza Surveillance Overview

TECHNICAL REPORT Guide to revision of national pandemic influenza preparedness plans
1
Introduction
During the past decade, the 53 Member States of the WHO European Region, 31 of which are part of the European
Union/European Economic Area, invested considerably in pandemic preparedness. This came in the wake of global
threats posed by (re-)emerging diseases such as avian influenza A(H5N1) and A(H7N9), the SARS outbreak of
2003, and the outbreak of MERS (Middle East respiratory syndrome) which began in 2012. Adequate preparedness
is also a national obligation under the International Health Regulations (2005) and the EU Decision on serious
cross-border threats to health (No 1082/2013/EU).
The first pandemic since 1968 occurred in 2009, caused by a new strain of influenza A(H1N1) of swine origin. The
virus spread rapidly around the globe and caused only mild disease in the majority of cases. However, severe
disease and deaths occurred in a significant number of people, mostly in the same groups that are at risk of
complications due to seasonal influenza infection, but also in other risk groups and even in previously healthy
individuals. It has been estimated that in the first year of the pandemic between 151 000 and 475 000 deaths
worldwide were attributable to influenza. Healthcare services, particularly critical care units, were often stretched
to their limits, and early recognition and treatment of severe disease could be life-saving.
The 2009 pandemic tested national plans, and in the aftermath many countries and international organisations
evaluated their preparedness and response activities. European countries, particularly in the western part of the
Region, were generally better prepared for the 2009 pandemic than most countries. But when confronted with a
milder pandemic than was expected, even the better prepared countries experienced gaps in their surveillance and
healthcare systems. Their planning assumptions were not flexible enough, they faced difficult communications and
logistics issues with respect to pandemic vaccines, and often failed to establish effective communication lines with
front-line healthcare responders.
An evaluation performed by the WHO Regional Office for Europe in collaboration with the WHO Collaborating
Centre for Pandemic Influenza and Research, University of Nottingham, United Kingdom, showed that pandemic
preparedness activities undertaken prior to the 2009 pandemic were useful in the response to the pandemic, and
guidance from WHO and ECDC was critical in the preparedness phase. However, a global review of the functioning
of the International Health Regulations and the response to the pandemic by both countries and WHO came to the
conclusion that the ‘world is ill-prepared to respond to a severe influenza pandemic or to any similarly global,
sustained and threatening public-health emergency.’ The recommendations of this review have been only partially
implemented, and the world has since been confronted with the failure to respond rapidly – and on the scale
needed – to prevent the largest outbreak of Ebola ever recorded. As a result, the 69th World Health Assembly
agreed to reform the WHO emergency response arrangements. It also agreed that the full implementation of the
IHR core capacities by all Member States must be accelerated. In 2016, the new WHO Health Emergencies
Programme was established (http://www.who.int/topics/emergencies/en/).
A future influenza pandemic is inevitable, although it cannot be predicted when it will happen nor how severe it will
be. Since the stress on the non-healthcare sectors was limited during the 2009 pandemic, only limited experience
has been gained in multisectoral coordination. Business continuity which will be crucial in a more severe pandemic.
Earlier findings from European assessments and exercises show that there are still weaknesses in those areas.
Since 2009, only thirteen countries in the WHO European Region have published revised pandemic preparedness
plans (as of July 2017). This document therefore takes into account:
the need for all countries to review and revise as necessary their pandemic plans based on the lessons
learned from the 2009 pandemic and WHO guidance on pandemic influenza risk management (see:
http://www.who.int/influenza/preparedness/pandemic/influenza_risk_management_update2017/en/);
the need for continuous integration of pandemic preparedness with preparedness for other public health
emergencies, in line with the International Health Regulations, Decision No. 1082/2013/EU, and in light of
shrinking resources;
the need to develop plans for different scenarios of severity with more emphasis on national risk
assessment to inform pandemic response; and
the need to revise the ‘WHO/Europe and ECDC Joint European Pandemic Preparedness Self-Assessment
Indicators’ and develop a planning document that is useful to all Member States.
Why is pandemic preparedness planning important?
Influenza pandemics, whether mild, moderate or severe, affect a large proportion of the population and require a
multisectoral response over several months or even years. For this reason, countries develop plans describing their
strategies for responding to a pandemic supported by operational plans at national and subnational levels.
Preparing for an influenza pandemic is a continuous process of planning, exercising, revising and translating into
action national and subnational pandemic preparedness and response plans. A pandemic plan is thus a living
Guide to revision of national pandemic influenza preparedness plans TECHNICAL REPORT
2
document which is reviewed at intervals and revised if there is a change in global guidance or evidence-base;
lessons learned from a pandemic, an exercise, or other relevant outbreak; or changes to national or international
legislation related to communicable disease prevention and control (Figure 1).
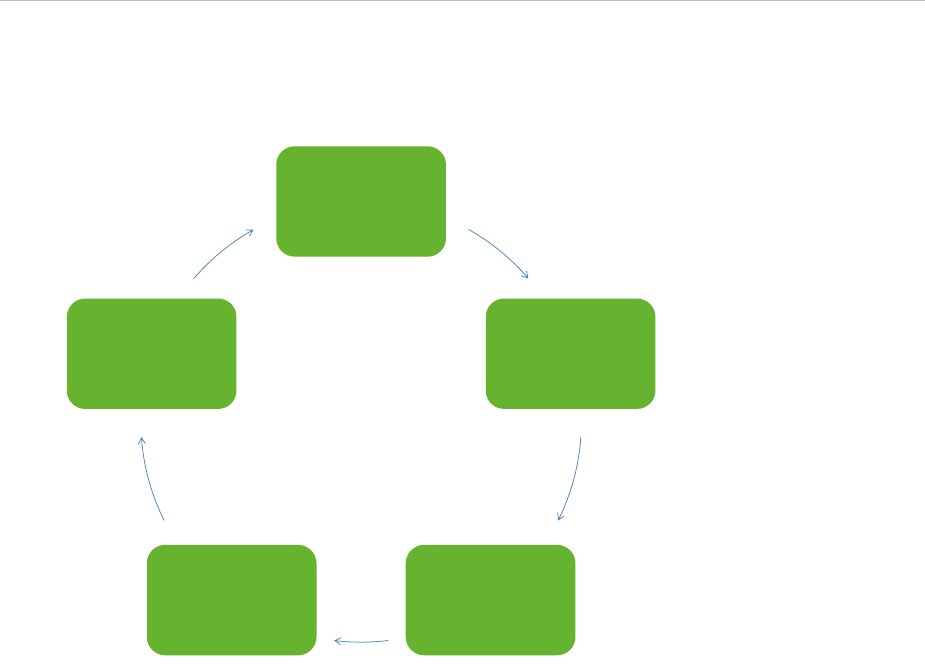
Figure 1. Key elements of the pandemic preparedness planning cycle
Strategic
planning
Operational
planning
Exercises,
reviews
Implementation
Evaluation
Pandemic preparedness is most effective if it is built on general principles that guide preparedness planning for any
acute threat to public health. This includes the following:
Pandemic preparedness, response and evaluation should be built on generic preparedness platforms,
structures, mechanisms and plans for crisis and emergency management.
To the extent possible, pandemic preparedness should aim to strengthen existing systems rather than
developing new ones, in particular components of national seasonal influenza prevention and control
programmes.
New systems that will be implemented during a pandemic should be tested during the inter-pandemic
period.
Adequate resources must be allocated for all aspects of pandemic preparedness and response.
The planning process, implementing what is planned, testing and revising the plan in order for key
stakeholders to familiarise themselves with the issues at hand, may be even more important than the
pandemic plan itself.
Pandemic response requires that business continuity plans and surge capacity plans be developed for the
health sector and all other sectors that could be affected by a pandemic to ensure sustained capacity during
a pandemic.
The response to a pandemic must be evidence-based where this is available and commensurate with the
threat, in accordance with the IHR. Planning should be based on pandemics of differing severity while the
response is based on the actual situation determined by national and global risk assessments.
Not all countries will be in a position to contribute to global risk assessment, nor conduct evaluations such
as pandemic vaccine effectiveness. They must all have the capacity to access and interpret data for risk
assessment provided by WHO, ECDC, and from other countries or sources.
Target audience, purpose and use of the guide
This guide is intended for use by those involved in pandemic preparedness planning, generic preparedness and
implementation of IHR core capacities in European countries. The document describes good practice for pandemic
preparedness planning based on lessons learned from the 2009 pandemic. It can be used to identify gaps in
pandemic preparedness, improve plans, and advocate for and prioritise resources to close those gaps. It can be
used to guide requests for support to ECDC, the WHO Regional Office for Europe, or other agencies and donors.

TECHNICAL REPORT Guide to revision of national pandemic influenza preparedness plans
3
Description of the guide
Pandemic planning can be divided into 12 key areas. For each key area, the rationale and a list of good practice
requirements for effective pandemic preparedness are provided.
For each key area, or requirement under a key area, countries may:
add requirements, indicators or outcomes for determining if a key area or requirement has been covered or
implemented, or if progress has been made;
indicate changes that have been made to their pandemic plans after the 2009 pandemic;
provide to the WHO Regional Office for Europe and ECDC examples of good practice which may be shared
with other countries; and
include questions to be addressed for each key area.

Guide to revision of national pandemic influenza preparedness plans TECHNICAL REPORT
4
Twelve key areas of pandemic preparedness
Key area 1: Framework for pandemic planning
Rationale
The purpose of developing national pandemic preparedness plans is to define, organise and coordinate the wide
range of activities that are necessary to respond to a pandemic. A national pandemic plan is a strategy or
framework that guides subnational and operational planning in the health and non-health sectors by identifying the
areas that need to be addressed in pandemic preparedness. National pandemic plans vary from being a legal act or
law in some countries to an internal planning document by the ministry of health in others.
Strategic planning at the national level is needed to identify and involve key stakeholders from all sectors and
administrative levels and to lay out key components of the national response to a pandemic, informed by a range
of realistic, risk-based planning assumptions which take into account that it is not possible beforehand to predict
the severity or impact of a future pandemic. In order to maximise the flexibility, national responses should be
based on national risk-assessments guided by global and European risk assessments which will allow different
response measures to be implemented in different parts of the country at different stages of a pandemic.
At the time of the 2009 pandemic, although there were well-advanced national pandemic plans in many countries,
operational plans were lacking at the subnational level or within different domains of the health sector, thus
slowing down and weakening the initial responses. Since much of the operational response to a pandemic in a
country takes place at the subnational level, well-developed pandemic preparedness plans at the subnational level
and local operational plans – down to the facility/organisation level – are crucial. Border regions may also benefit
from exchanging and coordinating pandemic plans.
Good practice for strategic planning
Political commitment and dedicated, sustained government funding for the development, endorsement and
publication of the pandemic plan and its subsequent implementation.
A national pandemic plan based on clear planning assumptions that serves as a strategic framework and
guidance for national and subnational operational planning.
A national planning committee representing relevant stakeholders from the health and non-health sectors
(Table 1 provides a sample list of stakeholders) established by the government which meets regularly to
develop and update the national pandemic preparedness plan, coordinates the strategic and operational
planning process and ensures there are working links between the pandemic preparedness plan and generic
preparedness or civil protection plans.
Realistic and coordinated national and subnational pandemic plans that were produced by involving
subnational representatives in national planning (and national representatives in subnational planning), and
a mechanism to maintain regular communication between national and subnational level planners.
National and subnational plans that define the roles and responsibilities of relevant stakeholders that would
be involved in the pandemic response (authorities, nongovernmental organisations, private sector
participants, experts to provide advice, etc.).
Pandemic plans that include planning assumptions based on a range of possible scenarios that consider
different response measures that can be tailored to the actual situation during a pandemic and that provide
triggers for the activation/de-escalation of specific measures.
Pandemic plans that include methodologies and protocols to evaluate the response to a pandemic
(assessment of the measures applied to mitigate the effects of the pandemic, coordination, etc).
There is a legal framework for the deployment of pandemic response measures, e.g. regulatory approval
and procurement of pandemic vaccines and other pharmaceutical countermeasures, social distancing
measures, emergency release of budget.
Pandemic plans that take into account ethical aspects, such as prioritisation of clinical treatment or vaccine,
provision of pandemic vaccine to healthcare workers, handling of conflicts of interest, possible infringement
of basic human rights in pandemic response, extent of medical professionals’ duty of care.
TECHNICAL REPORT Guide to revision of national pandemic influenza preparedness plans
5
Table 1. Example of stakeholder-representation in the national pandemic preparedness planning
committee
Healthcare sector
Other sectors
National and regional health authorities
(includes public health)
National influenza centre and its epidemiological
counterpart
National expert advisory group
National immunisation technical advisory group
National drug regulatory agency
Medical professional associations representing
doctors and nurses, e.g. ICU physicians,
paediatricians, gynaecologists, etc.
Representatives from the association of
healthcare workers and general practitioners
Pharmacists
Scientists
Pharmaceutical/vaccine manufactures
Subnational level representatives
Department or ministry responsible for civil emergency response
Social services administration
Ministry responsible for education
Representatives from water and power plant associations
Representatives from the transport sector
Media relations experts/risk communication specialists
Representatives from the business and financial sectors
Nongovernmental and community-based organisations, e.g. Red
Cross, Red Crescent, health charities
Ministry of agriculture and the veterinary sector
Local government bodies
Good practice for effective operational planning and implementation
of pandemic plans
Operational planning at subnational and local levels (operational plans) based on national strategies and
planning assumptions that serve as a framework, guidance and unifying basis.
Operational plans based on templates provided by the national level that include performance criteria
allowing peer reviews or audits of subnational plans against the national pandemic plan, especially to
ensure multisectoral and vertical interoperability of plans.
Operational plans that have a number of components, for example healthcare facility plans, business
continuity plans for the health and non-health sectors, communication strategies, vaccination plans, and
logistics plans.
Operational plans that clearly define responsibilities in pandemic response at subnational and local levels
and are developed with input from healthcare facilities and essential businesses in the geographical area
covered.
Operational plans that are coordinated with those of neighbouring regions or municipalities.
Operational plans whose implementation includes communicating the plan to responders and other
stakeholders at national and subnational levels, conducting pandemic simulation exercises to test strategic
and operational responses, also with neighbouring countries, training staff etc.
Communication channels between operational planners at national, subnational and local levels.
Key area 2: Pandemic planning and response based on risk
assessment
Rationale
Pandemic plans describe the actions and measures that would be implemented during a pandemic to reduce
morbidity and mortality. The data and information that are needed to determine which measures should be
implemented and when will be obtained by assessing the overall risk of the pandemic by assessing severity and
groups at risk of severe disease, as well as overall impact on the population, health services and other essential
services.
One of the main lessons learned from the 2009 pandemic was that the six global WHO phases were inadequate as
triggers for national response actions due to the different timing of the pandemic in different parts of the world and
different severity in different countries. In addition, many pandemic plans based the response on a single scenario
which was more severe than the 2009 pandemic, and response strategies had to be adjusted. In particular,
countries that changed their pandemic vaccine strategies (e.g. from total population coverage to vaccinating risk
groups only) encountered difficulties in rapidly implementing new deployment strategies. Pandemic plans that were
triggered in this way by the WHO phases were considered to be inflexible.
The 2017 WHO guide on pandemic influenza risk management takes this into account: there are four phases which
are not linked to specific actions at the country level (Figure 2), and countries are encouraged to conduct their own
risk assessment.

Guide to revision of national pandemic influenza preparedness plans TECHNICAL REPORT
6
Figure 2. The continuum of pandemic phases
Source: 2017 WHO guide on pandemic influenza risk management
The four phases are the interpandemic phase (the period between influenza pandemics), the alert phase (when
influenza caused by a new subtype has been identified in humans [see reference to WHO case definitions on page
18, last entry under key area 2], e.g. the emergence of avian influenza A(H7N9) in 2013), the pandemic phase (the
period of global spread of human influenza caused by a new subtype) and the transition phase (when the global
risk drops and reductions in response activities may be appropriate, see key area 11). Global risk assessments will
determine in which phase we are, based on virological, epidemiological and clinical data. Depending on the risk
assessment, WHO may convene an Emergency Committee under the IHR, which may issue temporary
recommendations to Member States. The Director General of WHO can declare a Public Health Emergency of
International Concern (PHEIC) under Article 12 of the IHR.
Good practice for an effective risk assessment-based approach to
pandemic planning
Countries conduct mapping of all (potential) threats in order to prioritise resources for pandemic
preparedness.
Pandemic plans based on planning assumptions, i.e. different scenarios of intensity, severity and impact
based on expected clinical attack rate, infectiousness, number of children and adults requiring primary,
hospital and higher-level (intensive) care per unit of population, death rate, and the peak percentage of the
workforce unavailable to work. WHO is developing a pandemic influenza severity assessment methodology
which can be used for this severity and impact assessment.
Pandemic plans that include options for response but allow for the planned response measures to be
implemented in a flexible way. Measures can be implemented at different times (depending on local
differences in the timing, spread and peak of the pandemic), can be adjusted, can be postponed, or can be
cancelled. Unplanned response measures can be implemented, depending on the actual severity and impact
of the pandemic.
Risk assessments are conducted throughout the pandemic and integrated with communication plans and
the decision-making process for the pandemic response.
Risk assessments consider – apart from the public health risks – the social and economic risks and weigh
the consequences of action versus non-action. This includes interpretation of data from the countries that
were affected first and that will inform the response as well as national data collection needs.
Risk assessments consider impact (on risk groups, healthcare capacities and essential public services) and
effectiveness of countermeasures, both medical (e.g. influenza-specific antivirals, antibiotics and pandemic
vaccines) and non-pharmaceutical public health measures (e.g. social distancing, personal protective
equipment, hygiene measures).
Mathematical modelling to determine the basic reproductive number R
0
, serial interval, estimated case
fatality rates and other parameters as needed in countries that have the capacity to do so.
Good practice for effective risk assessment and surveillance during a
pandemic
Investigating individual cases and clusters early in the response
Early warning systems and event-based surveillance (national epidemic intelligence capacity) to detect and
assess the risk of a pandemic coupled with the capacity to communicate this to experts and decision makers
in a timely fashion.
Outbreak response teams, procedures and laboratory capacities in place to swiftly conduct investigations.

TECHNICAL REPORT Guide to revision of national pandemic influenza preparedness plans
7
A system to rapidly implement the national notification of cases in order to detect the first cases in a
country.
Investigation of the first cases and their contacts, especially in the countries that are affected first;
investigation should focus on determining transmission characteristics, basic reproduction number, clinical
symptoms, severity, and characteristics of the virus. These factors differ from pandemic to pandemic and
determine operational decisions, e.g. the use of antiviral stockpiles among groups experiencing the most
transmission and the most severe disease.
Investigation includes seroepidemiology in countries that have this capacity.
National influenza centres share influenza viruses with WHO Collaborating Centres for Reference and
Research on Influenza throughout a pandemic, but particularly in the early stages (for risk assessment
purposes and timely vaccine development). This is part of their obligations to the Pandemic Influenza
Preparedness (PIP) framework and the WHO Global Influenza Surveillance and Response System (GISRS).
Surveillance when a pandemic becomes widespread
There are routine influenza surveillance systems based in primary healthcare facilities (acute respiratory
infections and influenza-like illness) and hospitals (severe acute respiratory infections, laboratory-confirmed
influenza cases) that provide information on trends and patterns of disease, namely the spread, affected
age groups, risk factors, virus characteristics, magnitude, and severity of the epidemic compared with
previous seasons pandemics.
Countries that do not have a surveillance system for severe/hospitalised cases have developed and tested a
plan for rapid implementation during a pandemic or rely on data from other countries.
Countries that will establish alternative systems, such as telephone or web-based surveillance, have tested
them beforehand.
Surveillance units have discussed beforehand with decision makers the information that should be provided
and have been allocated adequate resources and manpower to do so during a pandemic. Reporting of
surveillance data to WHO and ECDC is sustained throughout the pandemic.
Off-the-shelf research protocols with prior ethical and review board approvals implemented in order to
study severity of disease, transmission patterns, effectiveness of pharmaceutical and non-pharmaceutical
countermeasures, etc.
Laboratory testing
National influenza centres (NICs) have the capacity to rapidly implement assays for emerging influenza
viruses to be able to detect early cases in a pandemic and conduct risk assessments. The current gold
standard for influenza detection is RT-PCR.
The influenza laboratory network in a country may consist of public health and/or clinical laboratories that
can, supported by the NIC, quickly establish testing of the new virus in a pandemic.
A system to transport samples to the NIC in a timely and safe fashion.
Defined roles and responsibilities of the laboratory network for the different stages of a pandemic with
respect to testing for surveillance purposes versus clinical diagnosis of patients.
Once infections with the pandemic virus become widespread, laboratory surveillance focuses on testing of
sentinel samples, monitoring the antigenic and genetic properties of viruses, and antiviral susceptibility by
characterising a representative sample of viruses.
Countries may opt to confirm a subset of cases for surveillance purposes, e.g. all deaths due to pandemic
influenza, or a subset thereof.
Key area 3: Coordination, command and control
Rationale
An influenza pandemic is a complex emergency of uncertain severity and long duration. It involves health and non-
health sectors at all administrative levels, and it impacts the entire society and different actors at different stages.
For example, the animal health sector will be involved during the alert phase if a new influenza virus is detected in
animals but will have less of a role if the virus gains the ability to transmit efficiently among humans. Therefore, in
a pandemic as well as in other major health crises, it is important that a structure for coordination and chain of
command exists.
Good practice for effective coordination, command and control in a
pandemic
A robust and operational crisis management structure with adequate health emergency operations/crisis
management infrastructure including IT and telecommunications equipment and software.
A business continuity plan for the crisis management structure.
Standard operating procedures to ensure implementation of response actions.

Guide to revision of national pandemic influenza preparedness plans TECHNICAL REPORT
8
Committed representation of the relevant ministries and departments/agencies in the crisis management
structure which is linked to technical and decision-making levels.
Lines of command and control are based on existing structures and mechanisms and are described in the
pandemic plan. The plan anticipates how these may change during the response according to the actual
severity and impact of the pandemic.
All those that will be involved in the response are aware of their tasks and responsibilities, familiar with
each other and with the pandemic plan, and have received training where necessary.
The pandemic plan defines coordination between sectors and different administrative levels.
Lines of communication within the crisis management structure and with relevant outside stakeholders and
sectors have been practiced to ensure a coordinated response.
Information and data requirements for decision-making have been defined in advance, based on the data
that are expected to be available (see key area 2).
Mechanisms are in place for the regular monitoring of capacities in the healthcare sector (primary,
secondary and higher level care), stockpiles, use and distribution of medical countermeasures (antivirals,
vaccines, antibiotics, equipment for mechanical ventilation and oxygenation) and other supplies.
Two-way communication channels are in place from the national to local level and vice versa in order to
disseminate information effectively and for the crisis management structure to receive feedback which
resources are available and where support is needed.
Key area 4: Risk communication
Rationale
Risk communication is a key public health tool in pandemic planning and in the response to a pandemic because it
aims to establish public and professional confidence and trust. Lessons learned during the 2009 pandemic
highlighted difficulties that public health authorities had in communicating scientific uncertainty and special
attention needs. Risk communication is targeted to the public but also to key stakeholders and responders like
health workers, to continuously update them about the evolving situation as well as about interventions, in
particular the pandemic vaccine.
Good practice for effective risk communication in a pandemic
A communication strategy describing how communication will be conducted in a pandemic, i.e. how to
disseminate information rapidly during a pandemic and which information should be disseminated when and
by whom.
The communication strategy is tailored to different groups (stakeholders in different sectors and
administrative levels, the public, healthcare workers, media, etc.) and addresses the type of messages
needed at different stages of a pandemic. For example, after the first reports of an emerging pandemic
virus, the following may be communicated: a first assessment of severity, reports about the first cases in
the country, reports when infections become widespread, information on which groups are affected most,
reports on the first deaths in a country, or a declaration of a Public Health Emergency of International
Concern by WHO.
The communication strategy takes into account behavioural aspects of how people react and act on advice
and information received, both from authorities but also from other sources (mass and social media).
Risk communication during a pandemic is informed by evidence that is consistent from different authorities,
across sectors, and at different organisational levels.
The mass media are informed about the communication strategy and there are regular press conferences
planned during the response phase in order to channel the information disseminated by the media to the
general public.
A spokesperson within the ministry in charge of the response has been appointed to conduct regular press
conferences during a pandemic. An alternate spokesperson is available should the lead ministry change.
Communication materials and distribution channels that have been tested in the interpandemic period (e.g.
information about pandemic vs. seasonal influenza, personal protective measures, etc.).
Methods (such as population surveys, focus groups) for regularly monitoring the perception and opinions of
the public and healthcare workers during a pandemic in order to enhance interpretation and understanding
of the messages and to guide the risk communication efforts.
The communication cycle will include situational awareness, achieved with epidemic intelligence using
media monitoring techniques and targeted communications for public health professionals.

TECHNICAL REPORT Guide to revision of national pandemic influenza preparedness plans
9
Key area 5: Pandemic vaccines
Rationale
During a pandemic, countries will endeavour to vaccinate a large number of people within a short time period.
Using current technology for producing inactivated egg-based or culture-based influenza vaccines, it will take about
six months from the start of a pandemic to develop, manufacture and deliver the first batches of a vaccine, which
then has to be delivered and distributed to vaccination sites. These sites may be the same sites as for seasonal
influenza vaccination or sites set up for mass vaccination. How the pandemic vaccine is delivered will be partly
determined by whether countries decide to offer the vaccine to the whole population, to risk groups only (which
may differ from those for seasonal influenza), health workers and/or other essential workers, and how delivery to
these target groups is prioritised.
Good practice for effective use of pandemic vaccines
Pandemic vaccine deployment plan
Seasonal influenza vaccination programmes are a framework upon which pandemic vaccination strategies
are based. This includes the role of national regulatory authorities (NRA) and national immunisation
technical advisory groups (NITAGs) that determine risk groups, cost effectiveness and vaccine effectiveness,
regulatory issues, communication strategies, monitoring and reporting of adverse events.
A pandemic vaccine deployment plan that includes a strategy, or strategies, for procurement, distribution,
storage and policy for use of pandemic vaccines during a pandemic. This plan should have been rehearsed
through simulation exercises.
The strategy considers destruction of remaining pandemic vaccine stocks when the pandemic is over.
The strategy is flexible in that it considers different options for pandemic vaccination (whole population, risk
groups only, essential workers only) which will be implemented or adjusted according to the actual severity
of the pandemic. It also considers what to do if excess vaccine becomes available, e.g. donation to WHO
and/or countries in need.
The strategy takes into account ethical and equity issues: safety (risk–benefit), priority groups (vaccinating
non-health sector essential services staff versus healthcare workers versus risk groups), prioritisation within
priority groups when supplies are low, mandatory vaccination for healthcare workers, etc.
The actual number of doses, the communication strategies and the mode of delivery (mass vaccination
campaign versus using existing mechanisms for seasonal influenza vaccination) are determined by the
severity of the pandemic.
Authorities have considered how groups not normally vaccinated during seasonal influenza will be offered
the vaccine, e.g. universal vaccination of children and first responders.
The process of making decisions on the procurement and delivery of pandemic vaccines, using different
scenarios, has been rehearsed, e.g. by an exercise involving the ministry of health and other ministries,
departments and stakeholders such as the NRA, NITAG, vaccine manufacturers, and the authority
responsible for adverse event monitoring.
Countries that have advanced purchase agreements with vaccine manufacturers have considered the timing
of activation of the contract which will rely on the national as well as the global risk assessment.
Contracts may be developed as part of a coordinated approach involving multiple countries, e.g. the joint
procurement of medical countermeasures for EU countries described in Decision No 1082/2013/EU.
The pandemic vaccine strategy takes into account that the vaccine will probably be available in smaller
batches initially, and that prioritisation within the groups that are going to be targeted for vaccination –
based on a risk assessment – is necessary.
As part of the pandemic vaccination campaign and to generate confidence/acceptance in the newly
developed vaccine, risk communication will be carried out among target groups (in particular among those
groups that are not normally targeted for seasonal influenza vaccination).
Staff that will administer the vaccine have been identified and trained, taking into account different
scenarios, e.g. a mass vaccination campaign has different training requirements compared to targeting risk
groups, in which case it may be possible to employ the same mechanism used to vaccinate against seasonal
influenza.
Regulations and monitoring
Countries improve the process behind vaccination choices and decisions by strengthening NRAs and NITAGs
and ensure they have access to rapid scientific and expert advice for evidence-based strategic decisions.
Expert panels have been identified and members have public declarations of interest to avoid jeopardising
the integrity of the advisory bodies they are supporting.
Countries expecting to be eligible to receive vaccine donations (e.g. from another country or through WHO)
have considered the regulatory issues beforehand to not delay the donation.

Guide to revision of national pandemic influenza preparedness plans TECHNICAL REPORT
10
Countries are aware of the rapid authorisation pathway available in the EU, which is designed specifically for
pandemic vaccines to minimise the administrative delay of introducing a new vaccine, namely the pre-
approval by the European Medicines Agency (EMA) of vaccines based on a mock-up model of H5N1 vaccine
to be rapidly adapted to a newly emerging pandemic strain.
There is reciprocal approval between countries in an emergency, e.g. the national regulatory authority
(NRA) of a country outside the EU may adopt EMA approval, which is valid in all EU Member States, or the
NRA of a country may adopt approval bilaterally.
If vaccination is to be provided by staff that do not normally do so (e.g. nurses), the required competencies
have been defined and training has been provided.
A system for monitoring vaccine uptake and adverse events following immunisation (AEFIs), e.g. VAESCO.
Mechanisms for monitoring the effectiveness of pandemic vaccines. Currently, the I-MOVE project monitors
seasonal influenza vaccine effectiveness in Europe.
Communication and information
Communication strategies tailored to different target groups, e.g. professionals (healthcare workers) and
the public, that address questions about vaccine effectiveness and adverse events, some of which have
been predicted and prepared beforehand.
Attitudes and beliefs are monitored during a pandemic.
The use of social media to deliver messages related to pandemic vaccines.
Communication strategies have been tested by conducting an exercise based on an unforeseen crisis, e.g.
rehearse the communication aspects of a scenario whereby serious side effects are encountered.
Communication strategies utilise information from WHO (e.g. SAGE recommendations on risk groups for
vaccination), ECDC, the EMA (e.g. information on pharmacovigilance data), and other Member States
through the IHR, EWRS or other regional platforms.
Key area 6: Antivirals and other essential medicines
Rationale
During a pandemic, it is expected that the number of both severe and mild cases of influenza will increase
compared with seasonal influenza. This may lead to shortages of influenza-specific antiviral drugs (AV), specifically
neuraminidase inhibitors. The use of AV complements other medical and non-medical measures that may be
implemented and for which it is necessary to stockpile supplies. Examples include infection control materials,
antibiotics and other essential medicines. There is no set formula for the size of stockpile required as this depends
on the strategy adopted by the country and the market availability of supplies. This in turn depends production
capacity and the extent to which there is regular use of AV during seasonal influenza. During the 2009 pandemic,
antiviral manufacturers were able to increase production, and thus the necessity for using the AV stockpiles was
less critical.
Good practice for effective use of antivirals and other essential
medicines in a pandemic
Planning
A strategy for the procurement, storage, distribution and policy for the use (including paediatric use) of AV
during a pandemic.
The AV strategy is flexible in that it describes different options for use in accordance with different scenarios
of severity of a pandemic; it is understood by all stakeholders that the strategy may be adjusted during a
pandemic in accordance with a national risk assessment.
The strategy includes a provisional priority list for the choice of AV, or combination AV, and prioritisation
during different stages of a pandemic in accordance with different scenarios of severity.
The strategy considers the establishment of a stockpile of AV for use in a pandemic. The most effective
stockpile is a rolling stockpile, i.e. one that is used routinely during seasonal influenza and replenished. AV
use in a pandemic will be under government control, e.g. by prescription from general practitioners,
pharmacies, or at collection points.
The strategy includes how to deal with AV stockpiles that are unused after a pandemic, by describing use
during seasonal influenza, or possible extension of the expiry date and later destruction of unused
stockpiles.
The increased need for other medicines or techniques, notably antibiotics and ECMO, are included in the
plan (see also key area 7).
Countries that may be eligible to receive donations of AV and/or other essential medicines from other
countries or organisations have developed an essential medicines list and addressed regulatory issues in
order not to delay the donation.

TECHNICAL REPORT Guide to revision of national pandemic influenza preparedness plans
11
AV may be purchased through contracts developed as part of a coordinated approach involving multiple
countries, e.g. the joint procurement of medical countermeasures for EU countries described in Decision No
1082/2013/EU.
An alternative therapeutic strategy in case of reduced susceptibility to neuraminidase inhibitors.
Prioritisation and use of AV
AV are stockpiled centrally or have been distributed to local stocks across a country before a pandemic.
Mechanisms are in place to distribute AV rapidly when a pandemic is declared, and this has been tested by
an exercise.
For countries whose AV supplies or stockpile are insufficient to treat all cases of pandemic influenza, the
first priority is to treat persons with severe disease.
Use of AV to delay spread or reduce transmission and peak sickness rates, or for long-term prophylaxis for
healthcare workers, has been considered if the pandemic is severe and/or during the early stages of a
pandemic when vaccines are not yet available.
AV guidelines recommend initiation of treatment as early as possible and preferably within 48 hours of
symptom onset, based on clinical judgement alone. There is no case for delaying treatment pending
laboratory confirmation. Initiation of treatment of severe cases is also considered after 48 hours from
symptom onset.
The use of AV does not replace other basic life-saving strategies, such as monitoring for vital signs and
supportive therapy, particularly oxygen.
Monitoring AV use and surveillance for AV resistance
A mechanism for monitoring the national AV stockpile.
Countries that plan to use AV in a pandemic have mechanisms to rapidly detect and monitor signals
indicating adverse events or side effects associated with their use.
Surveillance to rapidly detect and assess signals indicating the emergence and spread of antiviral resistant
pandemic influenza viruses, or rapid shipment of viruses to a Collaborating Centre for Reference and
Research on Influenza (WHO CC) is in place.
Key area 7: Healthcare services preparedness
Rationale
The aim of preparing the healthcare services for a pandemic is to ensure the continuation of regular and
emergency services while providing appropriate clinical care for cases of pandemic influenza, whether these
present to primary healthcare, are hospitalised or admitted to critical or intensive care units (ICUs). Appropriate
clinical treatment will reduce morbidity and mortality and thus mitigate the effects of the pandemic.
During a pandemic, primary and secondary healthcare facilities will experience a significant increase in the number
of respiratory patients (in addition to the usual number of ill persons) while healthcare workers will also become ill
and so be absent from work. There will be an excess demand for healthcare services with potentially fewer
healthcare workers to deliver these services. In addition to limited staff, other resources will be stretched, including
beds, medicines and mechanical ventilators, and this may last for several months. Hospitals may face a situation
where it is necessary to discharge non-critical patients (pandemic and non-pandemic) to free up resources for
severely ill patients and to cancel planned non-urgent treatments.
The 2009 pandemic showed that even during a relatively mild pandemic, healthcare services may easily be
overwhelmed. Hence, health services preparedness planning is vital regardless of the severity of the pandemic in
order to be able to continue services when capacities are exceeded.
Good practice for effective healthcare system response in a pandemic
Planning
Healthcare services planning should be based on national assumptions as to the estimated numbers of
deaths and the number of cases requiring primary, secondary and higher level care – in relation to local
demographics and in accordance with different scenarios of severity.
Plans that include provisions on how to increase critical care capacity/respiratory support.
Plans that take into account the need for cross-referral between healthcare facilities in the country or
region.
Local plans that encompass all health and social care services, both public and private, e.g. conventional,
long-term, acute care, and care by community-based organisations. This should ensure that there is
harmonised care and coordinated transfer of patients.
Healthcare services response planning that involves representatives of professional organisations (covering
family doctors, hospital and ICU physicians, occupational health, social care workers, etc.).

Guide to revision of national pandemic influenza preparedness plans TECHNICAL REPORT
12
Health system preparedness exercises performed at the geographical level, i.e. covering the area that a
health authority or facility serves, that address large numbers of people seeking care with potentially
increased numbers needing higher levels of care than normal.
All healthcare professionals are briefed with respect to pandemic preparedness arrangements in their
facility, their obligations, responsibilities and rights.
Business continuity plans are available for key healthcare providers and public health stakeholders.
Financial issues related to the referral of patients (ambulance, testing, care provided, etc.) have been
considered and the necessary arrangements made.
Surge capacity and monitoring of resources
As part of general healthcare services management, countries have an inventory of existing capacities,
including both public and private healthcare facilities, number of hospital beds, ICU beds, equipment and
medicines for the treatment of severe cases, staffing and options for surge capacity (e.g. retirees, medical
students, etc.).
Plans for surge capacity that estimate the capacities required to deal with pandemics of different severities
and thus numbers of severe cases. There are indicators for filling or exceeding existing capacity and for
triggering arrangements to increase capacity at the local/healthcare facility level.
Plans for surge capacity based on different scenarios of severity
and
local differences in the timing of the
pandemic which may peak at different times compared with the national level.
Plans that include the care of non-influenza acute/chronic patients and provisions for cancellation of elective
surgery.
Provisions to ensure that clinical diagnostic laboratory services (for haematology, biochemistry, immunology
etc.) are maintained for pandemic cases and other priority patients.
Adequate plans for handling larger numbers of deaths than usual.
In addition to healthcare authorities, individual healthcare facilities have plans to manage surges of patients
for a sustained period of time, taking into account the 2009 experience of some patients requiring longer
periods of care (for example patients with acute respiratory distress syndrome).
The legal and financial requirements to recruit a reserve workforce, e.g. retirees, students, nurses
performing additional tasks normally allocated to clinicians, and to pay existing staff overtime, have been
planned for.
There is a national (electronic) system to monitor in real-time healthcare capacities and resources. As a
minimum, health authorities have planned to have regular contact with the healthcare services to monitor
the situation and needs throughout a pandemic, e.g. by means of weekly teleconferences.
Clinical management of patients
Guidelines for the clinical management and infection control aspects of severe acute respiratory infections –
associated with influenza (seasonal, zoonotic, or pandemic) or other respiratory infections – developed by
health authorities in collaboration with relevant professional organisations.
There is a mechanism to review and adjust these guidelines during a pandemic, according to the
characteristics of the new influenza virus, severity of illness, and the groups at risk of severe disease. They
include triage, the use and prioritisation of AV in patients, and AV prophylaxis for healthcare workers in
different settings (e.g. ICUs).
The clinical management guidelines can be made rapidly available to all staff in all healthcare facilities
during a pandemic.
Treatment of patients is on the basis of clinical assessment alone rather than awaiting laboratory
confirmation for pandemic influenza. This is particularly important when considering the use of AV (see also
key area 6).
The administration of AV to patients is informed by surveillance that monitors for the emergence of AV
resistance in the pandemic virus.
Off-the-shelf research (including clinical trial) protocols with prior ethical and review board approvals
implemented in order to study severity of disease, transmission patterns, effectiveness of pharmaceutical
and non-pharmaceutical countermeasures, etc.
Protection of healthcare workers
Plans at healthcare facilities include modifications to standard infection prevention and control procedures
that may be required in a pandemic, according to the severity and transmission characteristics of the virus.
Plans at healthcare facilities include provisions for personal protective equipment, a plan for providing
healthcare workers (HCW) with AV, and prioritisation of HCW for receiving pandemic vaccine.
Healthcare facilities have policies and procedures in place should HCW get sick or need to care for sick
family members. HCW who are reluctant to come to work during a pandemic will be encouraged to do so by
providing proper protective measures, by making arrangements to allow them to care for sick family
members, etc. The ethical aspects of staff refusing to work will be discussed with HCW as part of pandemic
preparedness planning.

TECHNICAL REPORT Guide to revision of national pandemic influenza preparedness plans
13
The psychological well-being of HCW has been considered and the needs for support during a pandemic
have been planned for. HCW know beforehand where they can obtain information and support during a
pandemic.
Laboratory testing for clinical purposes
In the early stages of a pandemic it is desirable to test all cases for both risk assessment and clinical
management purposes.
When infections are widespread, testing of a subset of mild and severe cases should suffice for risk
assessment and surveillance purposes, but throughout the pandemic – depending on resources – countries
may strive to laboratory-confirm all fatal cases and possibly also severe cases, e.g. those requiring critical
care. The results of testing performed for clinical purposes can also contribute to risk assessment and
surveillance and should, where possible, be coordinated by the NIC.
The treatment of patients with symptoms of respiratory illness, whether mild or severe, is initiated based on
clinical judgement alone, without waiting for laboratory test results. This includes supportive treatment,
such as the administration of oxygen, as well as AV.
Clinicians request testing of all patients that do not react to AV to determine if the cause is a pathogen
other than pandemic influenza or if resistance to the AV has developed.
Testing is performed for infection control purposes if needed to ensure that infected patients are placed in
the correct section of a healthcare facility.
Testing for clinical management purposes and for secondary bacterial infections is performed in situ, i.e. by
the hospital laboratory. If this is not possible, arrangements are in place with another laboratory.
All laboratories that test clinical specimens from patients suspected of infection with the pandemic virus
have established appropriate biosafety procedures in accordance with their own risk assessment and refer
to national and WHO guidelines.
Communication with clinicians, information sharing among clinicians
During the preparedness phase, medical professional groups and HCW have been informed how information
will be provided in a pandemic, e.g. through a dedicated website, professional medical associations etc.
Mechanisms have been tested to rapidly inform medical professional groups and HCW about the situation,
risk groups for severe disease as soon as they are known, and up-to-date guidance and/or practice notes
for the clinical management of patients.
This communication channel includes information on decisions taken at national or subnational levels on
measures to be implemented and on any changes in the response, e.g. whether confirmed cases are
notifiable, where patients should seek care, updates to guidelines for clinical care, laboratory testing, etc.
Communication channels have been tested and a range of decisions has been exercised before a pandemic
to determine the feasibility of implementation of the proposed measures and increase the likelihood of
timely implementation of measures in an actual pandemic.
A network or platform for frequent and rapid exchange of information and experience among clinicians,
nurses, public health laboratories, public health authorities, and healthcare services authorities on the
clinical management of severe cases and on healthcare facility needs when resources are stretched. Such
networks can also be utilised during a severe seasonal influenza epidemic.
Key area 8: Non-pharmaceutical public health measures
Rationale
Non-pharmaceutical interventions (NPIs) may help reduce transmission of the pandemic virus and may be
especially important in the first months of a pandemic when there is no pandemic vaccine available and in
countries with limited antiviral stockpiles or critical care capacities. Implementation of any public health measure
requires planning and proper communication and often this involves multiple sectors. Countries should consider the
recommendations provided by WHO and EU authorities in the event of a pandemic and the measures implemented
should be commensurate with the IHR (2005), and, for EU Member States, coordinated under the provisions of
Decision No 1082/2013/EU, whether they pertain to international travel and trade, personal protection or social
distancing. It should be noted that the evidence-base for the effectiveness of many NPIs is weak. This, as well as
acceptability, feasibility and legal framework for implementation differs between countries and should be taken into
account in national planning.
Good practice for effective public health measures in a pandemic
A national list of NPIs based on evidence, international guidance and best practice – all likely to be effective
and feasible for the setting/country – are included in the pandemic plan. Their implementation and timing
depends on the actual situation and severity in a pandemic. The main goals of NPIs are to delay and reduce
the number of cases and severe or fatal outcomes.

Guide to revision of national pandemic influenza preparedness plans TECHNICAL REPORT
14
As a minimum, the public will be informed about the measures they can take to protect themselves and
others from getting ill, i.e. by applying universal hygiene measures, such as frequent handwashing and
cough etiquette. Such information is part of seasonal influenza campaigns and is re-emphasised in a
pandemic.
Social distancing measures, including closure of schools, pre-schools and other educational institutions,
banning of mass gatherings, adjusted working patterns, or advising contacts of cases to reduce their social
interactions, are considered.
Advice for travellers is given.
There are mechanisms for communicating with those who will implement the measures and those that will
be affected, e.g. parents and teachers by school closures, and both the mechanisms and messages have
been tested.
The benefit–cost ratio and feasibility of NPIs has been calculated and assessed in advance.
The legal and ethical ramifications and effects of NPIs have been taken into account in planning and
appropriate risk mitigation strategies have been considered.
The scientific evidence for the effects has been weighed against socio-political considerations and their
negative impact.
The pandemic plan identifies triggers that determine when a particular measure will be implemented and
terminated.
Mechanisms for monitoring the effects/effectiveness of NPIs have been identified.
Off-the-shelf research protocols with prior ethical and review board approvals implemented in order to
study the effectiveness and response to non-pharmaceutical countermeasures.
Key area 9: Essential services and business continuity
Rationale
A severe pandemic has the potential to affect all sectors due to a significant number of people in the population
falling ill or dying or simply being too worried to come to work. Even a pandemic of mild severity may affect other
sectors than health. High workplace absenteeism will affect the functioning of businesses and services. Some
businesses or services are essential for society (e.g. water supply, electricity production, waste management, law
enforcement), and it needs to be ensured that these services continue to function during a pandemic. Therefore,
relevant authorities need to ensure that essential services develop operational business continuity plans, which also
cover an influenza pandemic. This planning requires guidance and support from national and regional levels.
Business continuity planning is important for many emergencies and should not be seen in isolation from other
resilience planning, but pandemics differ from other hazards as they may last for many months and therefore
specific planning for business continuity during pandemics is necessary.
Good practice for effective business continuity planning in a pandemic
Business continuity management requires interministerial and multisectoral coordination, e.g. for workers’
rights and obligations, and has been considered by all sectors.
Business continuity is particularly important for the ministry of health, key public health agencies and major
health facilities, as well as other essential public and private services, public authorities and corporations,
private sector stakeholders and community-based organisations.
Business continuity planning is supported by including a list of essential public and private services in the
national pandemic preparedness plan that has been agreed across government. These services have been
prioritised through a sectoral risk and impact assessment, national ethics committees, and relevant
stakeholders.
Guidance and formal frameworks for business continuity plans (BCPs), evaluations and audits, have been
provided by the government. All BCPs are guided by the nationally adopted planning assumptions and
strategies (pharmaceutical and non-pharmaceutical interventions, etc.).
BCPs developed by the identified essential services include roles and coordination mechanisms among
sectors and have been tested. This will ensure that there is appropriate interoperability across public and
private sectors and services.
Business continuity planning for essential public and private services and private businesses occurs both at
local and international levels and BCPs interlink with national plans.
The identified essential services, and first and foremost the healthcare sector, are prioritised in the national
pandemic planning process to be among the first to benefit from medical interventions and NPIs in order to
protect the adequate functioning of these services during a pandemic.
BCPs prepare for a long-term crisis (especially for human resources), make provisions for staff to have time
off during the response, and make sure there is continuation of usual tasks by some staff.

TECHNICAL REPORT Guide to revision of national pandemic influenza preparedness plans
15
BCPs consider the legal frameworks and insurance issues surrounding business continuity, e.g. compulsory
measures to reduce infection.
BCPs consider the role of community-based groups in, for example, awareness raising and surge capacity
and identify methodologies for non-profit organisations to support vulnerable groups during a pandemic.
Key area 10: Special groups and settings
Rationale
There are some groups in society that require special attention and consideration in preparedness planning as they
have specific needs in terms of language or means of communication. These groups need to be identified and their
particular needs should be determined during the preparedness planning process. Groups, facilities and institutions
include:
Prisons
Schools for children with learning or other disabilities
Psychiatric patients
Residential homes
Migrants and persons in transit
Asylum seekers
Homeless people
Community-based organisations
Others as appropriate
Good practice for effective inclusion of special groups in pandemic
preparedness
Prioritisation and inclusions of special groups have been broadly discussed at the national level as part of
the planning process. These discussions should include ethical considerations.
Consider if special strategies or response measures need to be developed for certain groups in society, e.g.
if information material in a minority language needs to be developed.
Test the strategies or measures on the specific groups, as part of the pandemic planning cycle.
Key area 11: After the pandemic – recovery and transition
phase
Rationale
Pandemics do not end overnight. A pandemic virus may cause several waves of severe epidemics worldwide over
the following years before continuing to circulate as seasonal influenza. The first post-pandemic winter season may
be worse than the actual pandemic. This happened in some European countries in 1968–70 and 2009–2011. The
workload for responders and stakeholders can remain high after the first epidemic wave, due to evaluation of the
response, recovery, and scientific and administrative assessment procedures. The decision on when a pandemic is
over is likely to be just as complex as the decision to announce a pandemic.
Good practice for effective recovery and transition
The pandemic plan incorporates recovery and transition activities to facilitate the return to ‘normal’ seasonal
influenza activities after a pandemic.
Recovery and transition includes replenishment of stockpiles (medicines, equipment, etc.)
The effectiveness of all implemented response measures should be evaluated, as should the overall
response. Results from evaluations should be used to revise the national pandemic plan.
The need for human resources mobilised during the pandemic as included in BCPs should be assessed (in
terms of psychosocial impact and core functions) and its return to normal business appropriately timed.
Awareness of the pandemic is utilised to promote precautionary measures for seasonal influenza and other
respiratory infections.
Risk-based triggers to identify the end of the pandemic at national and possibly subnational levels are
included in the pandemic plan.
Operational plans include mechanisms to de-escalate response measures, e.g. triggers for retrieving and
storing pandemic vaccine stocks, returning to normal procedures in healthcare facilities, etc.

Guide to revision of national pandemic influenza preparedness plans TECHNICAL REPORT
16
Key area 12: International cooperation, coordination and
interoperability
Rationale
A pandemic, by definition, affects many regions and countries of the world. Global populations move freely across
borders, and this is particularly so for citizens within internal EU borders. Citizens often obtain healthcare across
borders, and in the case of the EU, this is an entitlement.
However, Member States are responsible for preparedness for their citizens and residents only within the sovereign
national borders. Therefore, in order to enhance coordination of pandemic planning and response, intercountry
cooperation during the pandemic planning process, plans based on commonly agreed scenarios of severity, and
plans that take into account cross-border issues (such as migrant workers, cross-border healthcare, etc.) will all
enhance the interoperability of pandemic preparedness and response. As it is provided by EU legislation (Decision
No 1082/2013/EU), exchange of national pandemic plans between countries and necessity for creating
interoperability of pandemic plans between countries offers confidence in a coherent and consistent response and
is highly valuable in terms of professional support during national planning processes.
A coherent and consistent response to a pandemic across countries can only be achieved if public health
authorities rapidly share information on pandemic developments. Particular focus should be on the experience of
the first affected countries in Europe to address the ‘known unknowns’ of pandemics. There is a tendency to think
internally in countries during a pandemic, especially as work pressure increases. It is important to think about
national findings and experiences that might be useful to other countries. Providing information to WHO and the
EU during a pandemic is required under the IHR and EU legislation but its utility will be enhanced if it happens
based on prior thought and planning.
Good practice for effective international cooperation, coordination
and interoperability
Planning
National pandemic plans based on WHO and EU pandemic guidance.
Coordinated UN agency approaches to support national pandemic preparedness in countries.
National and local pandemic plans consider interoperability aspects of plans of neighbouring countries –
including migrants and citizens of other countries, migrant workers – and access to cross-border healthcare.
Neighbouring countries conduct cross-border simulation exercises as a platform to exchange information on
national pandemic plans, test responses, and highlight areas for improvement.
Member States participate in international activities facilitated by WHO and ECDC; topics include pandemic
planning, exercises and information exchange.
National pandemic plans and good practice are shared among countries by translating plans or through
collaborative meetings between countries and are facilitated by WHO and ECDC.
Where appropriate, countries participate in joint procurement of pandemic vaccines, e.g. under the common
EU procedure adopted under EU Decision 1082/2013/EU.
Countries contribute to the global risk assessment during a pandemic through their contribution to the
Pandemic Influenza Preparedness (PIP) framework, the provision of surveillance data and by conducting
additional investigations in a pandemic.
Institutionally approved common research protocols agreed upon in order to implement multi-country
research on severity, transmission and effectiveness of countermeasures.
Communication
Staff responsible for international liaison during a pandemic has been appointed who ensure neighbouring
countries are informed of key communications beforehand.
Countries share among themselves and with international organisations approaches and strategies for
communications during a pandemic.
Communication channels between regions of neighbouring countries have been arranged beforehand.
Informal international networks of experts that include the first affected countries established by WHO to
inform the global risk assessment.

TECHNICAL REPORT Guide to revision of national pandemic influenza preparedness plans
17
References and resources
General references (from the introduction)
World Health Organization. International Health Regulations (2005). Second edition. Geneva: WHO; 2008. Available
from: http://apps.who.int/iris/bitstream/10665/43883/1/9789241580410_eng.pdf
Decision No 1082/2013/EU of the European Parliament and of the Council of 22 October 2013 on serious cross-
border threats to health and repealing Decision No 2119/98/EC. Available from: http://eur-
lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2013:293:0001:0015:EN:PDF
Simonsen L, Spreeuwenberg P, Lustig R, Taylor RJ, Fleming DM, Kroneman M, Van Kerkhove MD, et al. Global
mortality estimates for the 2009 influenza pandemic from the GLaMOR project: a modeling study. PLoS Med. 2013
Nov;10(11):e1001558. Available from:
http://www.plosmedicine.org/article/info%3Adoi%2F10.1371%2Fjournal.pmed.1001558
Dawood FS, Iuliano AD, Reed C, Meltzer MI, Shay DK, Cheng PY, et al. Estimated global mortality associated with
the first 12 months of 2009 pandemic influenza A H1N1 virus circulation: a modeling study. Lancet Infect Dis. 2012
Sep;12(9):687-95. Available from: http://www.thelancet.com/journals/laninf/article/PIIS1473-3099(12)70121-
4/abstract
Sixty-Fourth World Health Assembly. Implementation of the International Health Regulations (2005), report of the
Review Committee on the functioning of the International Health Regulations (2005) in relation to pandemic
(H1N1) 2009. Report by the Director-General. Geneva: WHO; 2011. Available from:
http://apps.who.int/gb/ebwha/pdf_files/WHA64/A64_10-en.pdf
World Health Organization, Regional Office for Europe. Key changes to pandemic plans by Member States of the
WHO European Region based on lessons learned from the 2009 pandemic. Copenhagen: WHO Europe; 2012.
Available from: http://www.euro.who.int/__data/assets/pdf_file/0006/161664/ECDC_WHO_WHO Regional Office
for Europe_PiP-Workshops-Summary-Report_FINAL-26032012.pdf and
http://www.ecdc.europa.eu/en/publications/Publications/1203-MER-Joint_WHO_WHO Regional Office for
Europe_PiP%20Workshops%20Summary.pdf
World Health Organization. Pandemic influenza preparedness and response: a WHO guidance document. Geneva:
WHO; 2009. Available from: http://apps.who.int/iris/bitstream/10665/44123/1/9789241547680_eng.pdf
World Health Organization. Pandemic influenza risk management. A WHO guide to inform and harmonize national
and international pandemic preparedness and response. Geneva: WHO; 2017. Available from:
http://www.who.int/influenza/preparedness/pandemic/PIRM_withCoverPage_201709_FINAL.pdf
Key area 1: Framework for pandemic planning
Van Tam JN, Sellwood C, editors. Introduction to pandemic influenza (2nd edition). Wallingford, UK: CABI
Publishing; 2013.
World Health Organization. WHO checklist for influenza pandemic preparedness planning. Geneva: WHO; 2005.
Available from: http://www.who.int/influenza/resources/documents/FluCheck6web.pdf
European Centre for Disease Prevention and Control. Some suggested ‘acid tests’ for helping assess, strengthen
local preparedness for moderate or severe pandemics. Stockholm: ECDC; 2007. Available from:
https://ecdc.europa.eu/sites/portal/files/media/en/healthtopics/seasonal_influenza/Documents/Tool/0702_Local_As
sessment_Acid_Tests.pdf
World Health Organization. Considerations on exercises to validate pandemic preparedness plans. Geneva: WHO;
[no year]. Available from: http://www.who.int/entity/influenza/resources/documents/ExerciseConsiderations.pdf
World Health Organization. Ethical considerations in developing a public health response to pandemic influenza.
Geneva: WHO; 2007. Available from: http://www.who.int/ethics/influenza_project/en/index.html
Key area 2: Pandemic planning and response based on risk
assessment
World Health Organization, Regional Office for the Western Pacific. A guide to establishing event-based
surveillance. Manila: WHO Western Pacific; 2008. Available from:
http://www.wpro.who.int/emerging_diseases/documents/docs/eventbasedsurv.pdf

Guide to revision of national pandemic influenza preparedness plans TECHNICAL REPORT
18
World Health Organization. Rapid risk assessment of acute public health events. Geneva: WHO; 2012. Available
from: http://apps.who.int/iris/bitstream/10665/70810/1/WHO_HSE_GAR_ARO_2012.1_eng.pdf
World Health Organization, WHO Regional Office for Europe. Guidance for sentinel influenza surveillance in humans
2011. Copenhagen: WHO; 2011. Available from:
http://www.euro.who.int/__data/assets/pdf_file/0020/90443/E92738.pdf
Nicoll A, Ammon A, Amato Gauci A, Ciancio B, Zucs P, Devaux I, et al. Experience and lessons from surveillance
and studies of the 2009 pandemic in Europe. Public Health. 2010 Jan;124(1):14-23. Available from:
http://www.ncbi.nlm.nih.gov/pubmed/20141821
European Centre for Disease Prevention and Control. Known facts and known unknowns. Stockholm: ECDC; 2010.
Consortium for the Standardization of Influenza Seroepidemiology (CONSISE) [homepage on the internet].
[Accessed 2 Oct 2017]. Available from: http://consise.tghn.org/
World Health Organization. Global Influenza Surveillance and Response System (GISRS) [homepage on the
internet]. [Accessed 2 Oct 2017]. Available from: http://www.who.int/influenza/gisrs_laboratory/en/
World Health Organization. Pandemic Influenza Preparedness (PIP) framework [homepage on the internet].
[Accessed 2 Oct 2017]. Available from: http://www.who.int/influenza/pip/en/
World Health Organization. Case definitions for the four diseases requiring notification in all circumstances under
the International Health Regulations (2005). Geneva: World Health Organization [no year]. Available from:
http://www.who.int/ihr/Case_Definitions.pdf
Key area 3: Coordination, command and control
World Health Organization. Emergency Response Framework. Geneva: WHO; 2013. Available from:
http://www.who.int/hac/about/erf_.pdf
World Health Organization. Whole-of-society pandemic readiness: WHO guidelines for pandemic preparedness and
response in the non-health sector. Geneva: WHO; 2009. Available from:
http://www.who.int/influenza/preparedness/pandemic/2009-0808_wos_pandemic_readiness_final.pdf
Key area 4: Risk communication
World Health Organization. Outbreak Communication planning guide. Geneva: WHO; 2008. Available from:
http://www.who.int/ihr/elibrary/WHOOutbreakCommsPlanngGuide.pdf
Covello VT, Allen F. Seven cardinal rules of risk communication. Environmental Protection Agency, Office of Policy
Analysis: Washington, DC; 1988: Available from: http://odphp.osophs.dhhs.gov/pubs/prevrpt/archives/95fm1.htm
Key area 5: Pandemic vaccines
World Health Organization. Guidance on development and implementation of a national deployment and
vaccination plan for pandemic influenza vaccines. Geneva: WHO; 2012. Available from:
http://apps.who.int/iris/bitstream/10665/75246/1/9789241503990_eng.pdf
World Health Organization. Guidelines on the use of vaccines and antivirals during influenza pandemics. Geneva:
WHO; 2004. Available from:
http://www.who.int/influenza/resources/documents/WHO_CDS_CSR_RMD_2004_8/en/index.html
European Medicines Agency. Guidance documents relevant to the development and approval of pandemic-influenza
vaccines in the European Union [internet]. London: EMA; 2004–2014 [accessed 2 Oct 2017]. Available from:
http://www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/document_listing/document_listing_000246.jsp&
mid=WC0b01ac0580418608
European Centre for Disease Prevention and Control. Interim guidance: Use of specific pandemic influenza vaccines
during the H1N1 2009 pandemic. Stockholm: ECDC; 2009. Available from:
http://ecdc.europa.eu/en/publications/Publications/0908_GUI_Pandemic_Influenza_Vaccines_during_the_H1N1_20
09_Pandemic.pdf
European Centre for Disease Prevention and Control. Seasonal influenza vaccines [internet]. ECDC: Stockholm;
2017 [accessed 2 Oct 2017]. Available from: https://ecdc.europa.eu/en/seasonal-influenza/prevention-and-
control/seasonal-influenza-vaccines
Influenza – Monitoring Vaccine Effectiveness (I-MOVE). I-MOVE in Europe, external links [internet]. [Accessed
2 Oct 2017]. Available from: https://sites.google.com/site/epiflu/external-links

TECHNICAL REPORT Guide to revision of national pandemic influenza preparedness plans
19
Vaccine Adverse Event Surveillance & Communication (VAESCO). VAESCO [homepage on the internet]. Available
from: http://vaesco.net
European Commission, Directorate-General Health and Food Safety. Joint procurement of medical countermeasures
[internet]. Brussels: Directorate-General Health and Food Safety; 2010. Available from:
https://ec.europa.eu/health/preparedness_response/joint_procurement_en
World Health Organization, Regional Office for Europe. The guide to tailoring immunization programmes (TIP).
Copenhagen: WHO; 2013. Available from: http://www.euro.who.int/__data/assets/pdf_file/0003/187347/The-
Guide-to-Tailoring-Immunization-Programmes-TIP.pdf
Key area 6: Antivirals and other essential medicines
World Health Organization. WHO guidelines for pharmacological management of pandemic influenza A(H1N1) 2009
and other influenza viruses. Geneva: WHO; 2010. Available from:
http://www.who.int/csr/resources/publications/swineflu/h1n1_guidelines_pharmaceutical_mngt.pdf
World Health Organization. WHO guidelines on the use of vaccines and antivirals during influenza pandemics.
Geneva: WHO; 2004. Available from: http://www.who.int/influenza/resources/documents/11_29_01_A.pdf
European Centre for Disease Prevention and Control. ECDC Influenza Antivirals Guidance for Pandemics Available
from: http://ecdc.europa.eu/en/healthtopics/seasonal_influenza/antivirals/Pages/antivirals.aspx
Key area 7: Healthcare services preparedness
World Health Organization, Regional Office for Europe. Hospital emergency response checklist – An all-hazards tool
for hospital administrators and emergency managers. Copenhagen, WHO, 2011. Available from:
http://apps.who.int/iris/bitstream/10665/70094/1/WHO_HSE_GAR_BDP_2009.1a_eng.pdf
World Health Organization. Clinical management of influenza and other acute respiratory illness in resource-limited
settings: learning from the influenza pandemic (H1N1) 2009. Geneva: WHO; 2010. Available from:
http://www.who.int/influenza/patient_care/clinical/858-WHOGIPReport_A4_WEB_FA.pdf
World Health Organization, Regional Office for Europe. WHO critical care training short course. Available from:
http://www.euro.who.int/__data/assets/pdf_file/0010/214786/WHO-Critical-Care-Training-Short-Course.pdf and
http://www.euro.who.int/en/health-topics/communicable-diseases/influenza/publications/2013/who-clinical-care-
training-short-course
World Health Organization. Infection prevention and control of epidemic- and pandemic-prone acute respiratory
diseases in healthcare. Geneva: WHO; 2007. Available from:
http://www.who.int/csr/resources/publications/WHO_CDS_EPR_2007_6c.pdf
Walunj A, Thomson G, Gent N, Dunning J, Brett S. Global clinical networking and pandemic influenza. J Int Care
Soc. 2010;11(3):165-70.
Key area 8: Non-pharmaceutical public health measures
European Centre for Disease Prevention and Control. Guide to public health measures to reduce the impact of
influenza pandemics in Europe: ‘The ECDC Menu’. Stockholm: ECDC; 2009. Available from:
http://ecdc.europa.eu/en/publications/Publications/0906_TER_Public_Health_Measures_for_Influenza_Pandemics.pdf
World Health Organization. Reducing transmission of pandemic (H1N1) 2009 in school settings. Geneva: WHO;
2009. Available from: http://www.who.int/csr/resources/publications/reducing_transmission_h1n1_2009.pdf
World Health Organization. Public health measures during the influenza A(H1N1)2009 pandemic – meeting report.
Geneva: WHO; 2011. Available from:
http://apps.who.int/iris/bitstream/10665/70747/1/WHO_HSE_GIP_ITP_2011.3_eng.pdf
World Health Organization. Interim planning considerations for mass gatherings in the context of pandemic (H1N1)
2009 influenza. Geneva: WHO; 2009. Available from:
http://www.who.int/csr/disease/swineflu/guidance/pandemic_preparedness/en/index.html
European Centre for Disease Prevention and Control. Personal protective measures (non-pharmaceutical) for
reducing the risk of acquiring or transmitting human influenza [page on the internet]. Stockholm: ECDC; 2017
[accessed 3 Oct 2017]. Available from: https://ecdc.europa.eu/en/seasonal-influenza/prevention-and-
control/personal-protective-measures

Guide to revision of national pandemic influenza preparedness plans TECHNICAL REPORT
20
Hatchett RJ, Mecher CE, Lipsitch M. Public health interventions and epidemic intensity during the 1918 influenza
pandemic. Proc Natl Acad Sci USA. 2007 May 1;104(18):7582-7. Available from:
http://www.pnas.org/content/104/18/7582.full
Key area 9: Essential services and business continuity
Missouri Department of Health and Senior Services. Pandemic influenza business planning toolkit. Jefferson City,
MO: Missouri Department of Health and Senior Services; [no year]. Available from:
http://health.mo.gov/emergencies/panflu/pdf/panflubusinesstoolkit.pdf
United States Department of Labour, Occupational Safety and Health Administration. Guidance on preparing
workplaces for an influenza pandemic. Washington, DC: OSHA; 2009. Available from:
https://www.osha.gov/Publications/OSHA3327pandemic.pdf
World Health Organization. Whole-of-society pandemic readiness: WHO guidelines for pandemic preparedness and
response in the non-health sector. Geneva: WHO; 2009. Available from:
http://www.who.int/influenza/preparedness/pandemic/2009-0808_wos_pandemic_readiness_final.pdf
Key area 10: Special groups and settings
Hoffmann S. Preparing for disaster: protecting the most vulnerable in emergencies. UC Davis Law Rev; 42:1491.
2009. Available from: http://lawreview.law.ucdavis.edu/issues/42/5/articles/42-5_Hoffman.pdf
O’Sullivan T, Bourgoin M. Vulnerability in an influenza pandemic: looking beyond medical risk. Ottawa: Public Health
Agency of Canada; 2010. Available from: http://www.icid.com/files/Marg_Pop_Influenza/Lit_Review_-
_Vulnerability_in_Pandemic_EN.pdf
Office of the Assistant Secretary for Preparedness and Response; Office for At-Risk Individuals, Behavioral Health,
and Human Services Coordination. Report of the Interagency Workgroup on Pandemic Influenza and At-Risk
Individuals. Washington, DC: Department of Health and Human Services; 2009. Available from:
http://www.phe.gov/Preparedness/planning/abc/Documents/at-risk-panflu.pdf
Key area 11: After the pandemic: Recovery and transition
phase
European Centre for Disease Prevention and Control. Pandemic 2009 evaluations and lessons learnt [internet].
Stockholm: ECDC; 2012 [accessed 2 Oct 2017]. Available from: https://ecdc.europa.eu/en/seasonal-
influenza/2009-influenza-h1n1/pandemic-preparedness/evaluations
World Health Organization, Regional Office for Europe. Recommendations for good practice in pandemic
preparedness: identified through evaluation of the response to pandemic (H1N1) 2009. Copenhagen: WHO; 2010.
Available from: http://www.euro.who.int/__data/assets/pdf_file/0017/128060/e94534.pdf
Sixty-Fourth World Health Assembly. Implementation of the International Health Regulations (2005), report of the
Review Committee on the functioning of the International Health Regulations (2005) in relation to pandemic
(H1N1) 2009. Report by the Director-General. Geneva: WHO; 2011. Available from:
http://apps.who.int/gb/ebwha/pdf_files/WHA64/A64_10-en.pdf
Key area 12: International cooperation, coordination and
interoperability
World Health Organization. International Health Regulations (2005). Second edition. Geneva: WHO; 2008. Available
from: http://apps.who.int/iris/bitstream/10665/43883/1/9789241580410_eng.pdf
Decision No 1082/2013/EU of the European Parliament and of the Council of 22 October 2013 on serious cross-
border threats to health and repealing Decision No 2119/98/EC. Available from: http://eur-
lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2013:293:0001:0015:EN:PDF

ECDC is committed to ensuring the transparency
and independence of its work
In accordance with the Staff Regulations for Officials and Conditions of Employment of Other Servants of the European Union and the
ECDC Independence Policy, ECDC staff members shall not, in the performance of their duties, deal with a matter in which, directly or
indirectly, they have any personal interest such as to impair their independence. Declarations of interest must be received from any
prospective contractor(s) before any contract can be awarded.
www.ecdc.europa.eu/en/about-us/transparency
HOW TO OBTAIN EU PUBLICATIONS
Free publications:
• onecopy:
viaEUBookshop(http://bookshop.europa.eu);
• morethanonecopyorposters/maps:
fromtheEuropeanUnion’srepresentations(http://ec.europa.eu/represent_en.htm);
fromthedelegationsinnon-EUcountries(http://eeas.europa.eu/delegations/index_en.htm);
bycontactingtheEuropeDirectservice(http://europa.eu/europedirect/index_en.htm)or
calling 00 800 6 7 8 9 10 11 (freephone number from anywhere in the EU) (*).
(*)Theinformationgivenisfree,asaremostcalls(thoughsomeoperators,phoneboxesorhotelsmaychargeyou).
Priced publications:
• viaEUBookshop(http://bookshop.europa.eu).
European Centre for Disease
Prevention and Control (ECDC)
Postaladdress:
Granits väg 8, SE-171 65 Solna, Sweden
Visitingaddress:
Tomtebodavägen 11, SE-171 65 Solna, Sweden
Tel. +46 858601000
Fax+46858601001
www.ecdc.europa.eu
An agency of the European Union
www.europa.eu
Subscribe to our monthly email
www.ecdc.europa.eu/en/publications
Contact us
publications@ecdc.europa.eu
Follow us on Twitter
@
ECDC_EU
Like our Facebook page
www.facebook.com/ECDC.EU
