Official Air Force Aerospace Medicine Approved Medications
Effective: 13 June 2017
(Note: This list supersedes the medication list dated 01 January 2017)
This approved medication list shall be utilized for all aircrew and special operations duties including ATC/GBC. For MOD, see the Approved Missile
Operators Quick Reference List.
The approved medication list consists of drugs for acute and chronic conditions, listed by generic name under one of three categories, based on whether they may be self-prescribed
without flight surgeon consultation (see over the counter medication list), may be prescribed by the flight surgeon without higher approval, or require waiver. Drugs for acute
conditions generally fall under one of the first two categories, while medications for chronic conditions commonly fit into the last category. At the end of the document are listed a
number of drugs which are known to be unacceptable for all flying and special operational duty (SOD) classes. Waiver of such drugs is highly unlikely.
In general, for all 1042/2992 holders use of any medication whose known actions may affect alertness, judgment, cognition, special sensory function, mood, or coordination requires
DNIF/DNIC or appropriate duty restriction.
A large number of FDA-approved drugs are not listed under either section. If such drugs are used for acute conditions, it should be assumed that the drug is disqualifying for flying or
SOD duty, with the member returning to operational status after the condition has resolved, the medication has been discontinued, and its effects have dissipated, which usually entails
one additional day (the “24-hour rule”). For chronic conditions, most common conditions are treatable by one or more of the listed drugs, and use of these drugs is likely to receive
favorable consideration and a more expeditious result. If the member is intolerant of, or inadequately controlled by, a listed medication, but is successfully treated by a non-listed
drug, a waiver request for that drug may be submitted to AFMSA/SG3/5PF through the appropriate MAJCOM/SG (for rated officers and non-rated personnel). Such requests are not
delegated for initial or renewal waivers. The process for approval of such drugs is much more complicated because of the thorough review required. Note: Waivers for non-FDA
approved medications will not be considered. All medications and immunizations used by flying and SOD personnel must be FDA approved.
Note that while a specific drug may be acceptable without waiver, the treated condition may still require waiver.
Members pending waiver action must remain DNIF/DNIC until waiver has been granted. Verbal waivers are NOT authorized. Consult Aerospace Medicine Waiver Guide prior to
waiver submission.
For flying/SOD personnel, the following medications require ground testing, documented IAW AFI 48-123 paragraph 1.6., on the individual’s DD form 2766 under
“Medications” block on Page 1, IAW AF and MAJCOM guidance and restrictions (KX Operational/Flight Medicine
): Ciprofloxacin (mandatory ground test), Temazepam/
zolpidem/zaleplon (no-go pills) and dextroamphetamine/modafinil (go pills) must be ground tested (if member is eligible for use) OR declination of ground test must be documented.
Ground testing results (or declination) must also be updated in ASIMS. Once successfully ground tested, the operational use of go/no-go medications does not require DNIF/DNIC.
Clinical use of go/no-go medications DOES require DNIF/DNIC, despite prior ground testing. Only aircrew/SOD designated in current AF/SG, AF/A3O and MAJCOM guidance are
eligible for ground testing and operational use of hypnotics (no-go pills) or stimulants (go pills).
SUMMARY OF CHANGES:
Added tacrolimus and triptan medications. Both of these medications require a waiver, as does the underlying condition. See appropriate waiver guides for details. Minor spelling
and format updates.
Note: (1) Members pending waiver action must remain DNIF until waiver has been granted. 2
(2) Medications not on this list, singly or in combination, require review by AFMSA/SG3/5PF (rated officers) and MAJCOM/SG (non-rated personnel).
(3) Verbal waivers are NOT authorized.
(4) Waivers for non-FDA approved medications will not be considered.
Approved by AF/SG3/5P on 13 Jun 2017
7700 Arlington Blvd., Falls Church, VA 22042-5158
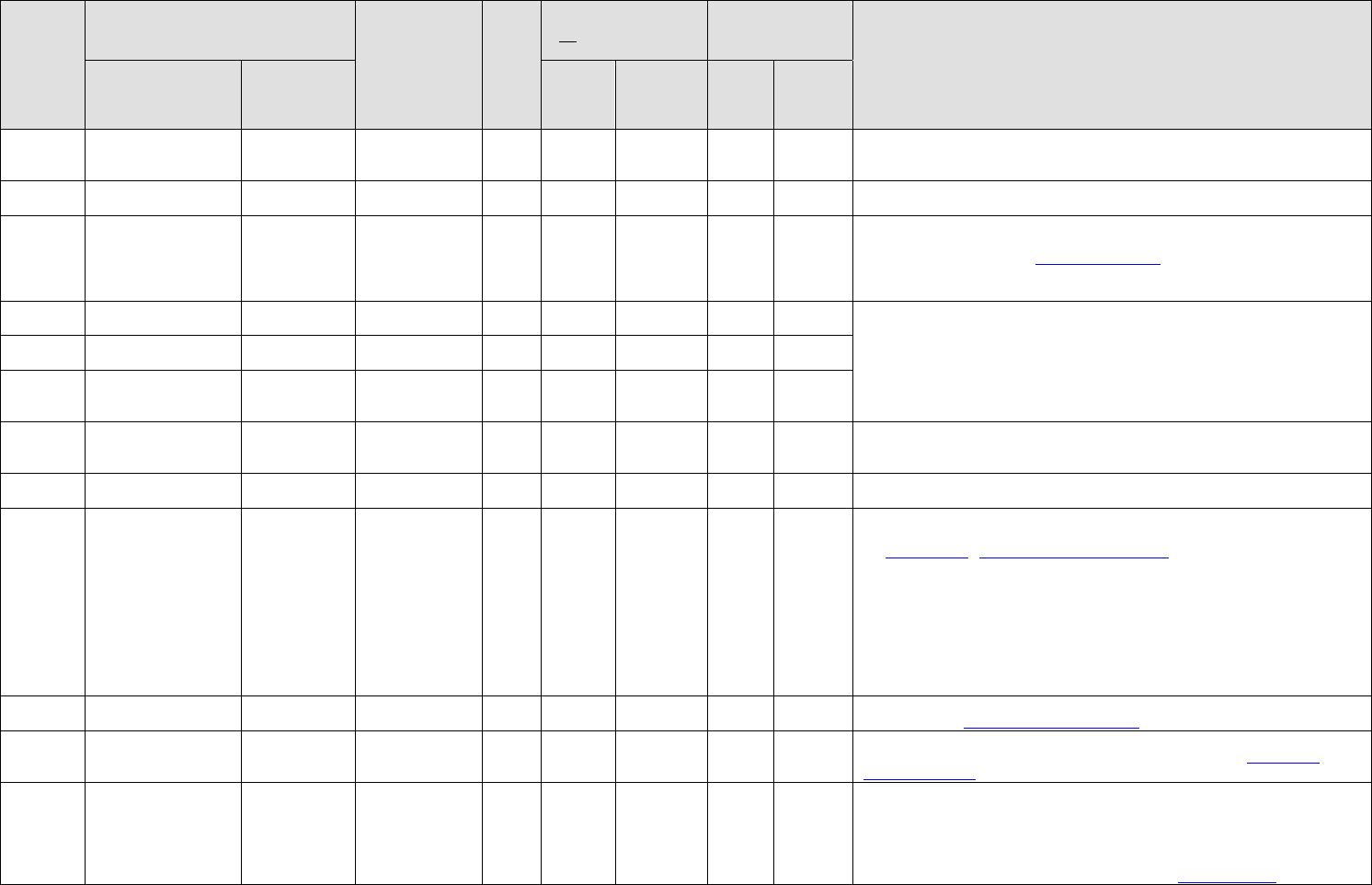
Category
Medication
Diagnosis
No
DNIF
(No Waiver Required)
DNIF
(Waiver Required)
Notes
Generic Name (Oral
Preparation Unless
Specified Otherwise)
Trade Name
(Not All
Inclusive)
or
Utilization
DNIF
For
Ground
Trial
Symptoms
Controlled
(No Side
Effect)
Flying
I/II/
RPA
Pilot
Flying
III/
GBC
Gen
Acetaminophen
Tylenol
Pain (acute
condition use)
X
DNIF until the underlying condition will not interfere with flying duties and there
are no adverse side effects. Usage is for acute conditions, less than 4 weeks, and
condition does not require waiver.
Gen
Acetaminophen
Tylenol
Pain (chronic
use)
X
X
Submit for waiver after potential idiosyncratic reaction has been ruled out and
control is maintained.
Gen
Acetazolamide
Diamox
Prevention of
acute altitude
sickness
X*
*Only if approved by MAJCOM protocol, for only those career fields noted by
AFPD 10-35 to be “Battlefield Airmen”. Dose approved 125-250 mg by mouth
two to three times a day (see
Acetazolamide Paper). Must ground test for three days
prior to operations. Do not take with aspirin containing products or if previous
hypersensitivity to sulfa-containing compounds.
Gen
Acupuncture
Seirin needle,
ASP needle
Pain (acute
condition use)
X
Minimum of 2 hours ground trial at initiation of therapy to ensure idiosyncratic
reaction is ruled out. After initial ground trial, no DNIF required unless underlying
condition interferes with flying duties.
Auricular ASP needles may be retained during duty performance for
RPA/GBC/ATC/MOD only.
No retained needles for aircrew for in-flight operations.
Gen
Acupuncture
Seirin needle,
ASP needle
Pain (chronic
use)
X
Gen
Acupuncture
Seirin needle,
ASP needle
Chronic medical
condition (i.e.
PTSD, OA)
X
Derm
Acyclovir
Zovirax
HSV (treatment
or suppression)
X
X
DNIF until the underlying condition will not interfere with flying duties and there
are no adverse side effects (minimum 72 hours). Note: For ≥10 recurrent episodes
per year, treat with acyclovir 400 mg Q12.
Derm
Acyclovir
(topical)
Zovirax
(topical)
HSV
X
DNIF not required unless condition or medication interferes with life support gear
or flying duties.
Gen
Adalimumab
Humira
Reactive
Arthritis/
Rheumatoid
Arthritis/
Psoriasis and
Psoriatic
Arthritis/
Ankylosing
Spondylitis/
Ulcerative
Colitis*, Crohns*
X
X
Submit for waiver after potential for idiosyncratic reaction has been ruled out and
control is maintained. FC IIC waiver by AFMSA/SGPA. Restricted Deployability,
see
Waiver Guide. Adalimumab Background Paper
*Consult Waiver Guide for use in IBD patients.
Derm
Adapalene
0.1% Gel (topical)
Differin
Acne vulgaris
X
DNIF not required unless condition or medication interferes with life support gear
or flying duties. Adapalene Background Paper
MS
Alendronate
Fosamax
Osteoporosis
(prophylaxis and
treatment)
X
X
Submit for waiver after potential for idiosyncratic reaction has been ruled out and
control is maintained. Take on non-flying days, if possible. See Alendronate
Background Paper.
GU
Alfuzosin
Uroxatral
BPH
X*
X
Max dose 10 mg daily.
*Not waiverable for FCI. Limited to FCIIA (restriction from high performance
aircraft and fly with another qualified pilot during critical phases of flight), FC III
and GBC. All alfuzosin waivers for FCII require AFMSA waiver, for all FCIII and
GBC the MAJCOM may disposition. Alfuzosin may be used with finasteride with
appropriate waiver authority noted for alfuzosin. See Alfuzosin Paper.
Note: (1) Members pending waiver action must remain DNIF until waiver has been granted. 3
(2) Medications not on this list, singly or in combination, require review by AFMSA/SG3/5PF (rated officers) and MAJCOM/SG (non-rated personnel).
(3) Verbal waivers are NOT authorized.
(4) Waivers for non-FDA approved medications will not be considered.
Approved by AF/SG3/5P on 13 Jun 2017
7700 Arlington Blvd., Falls Church, VA 22042-5158
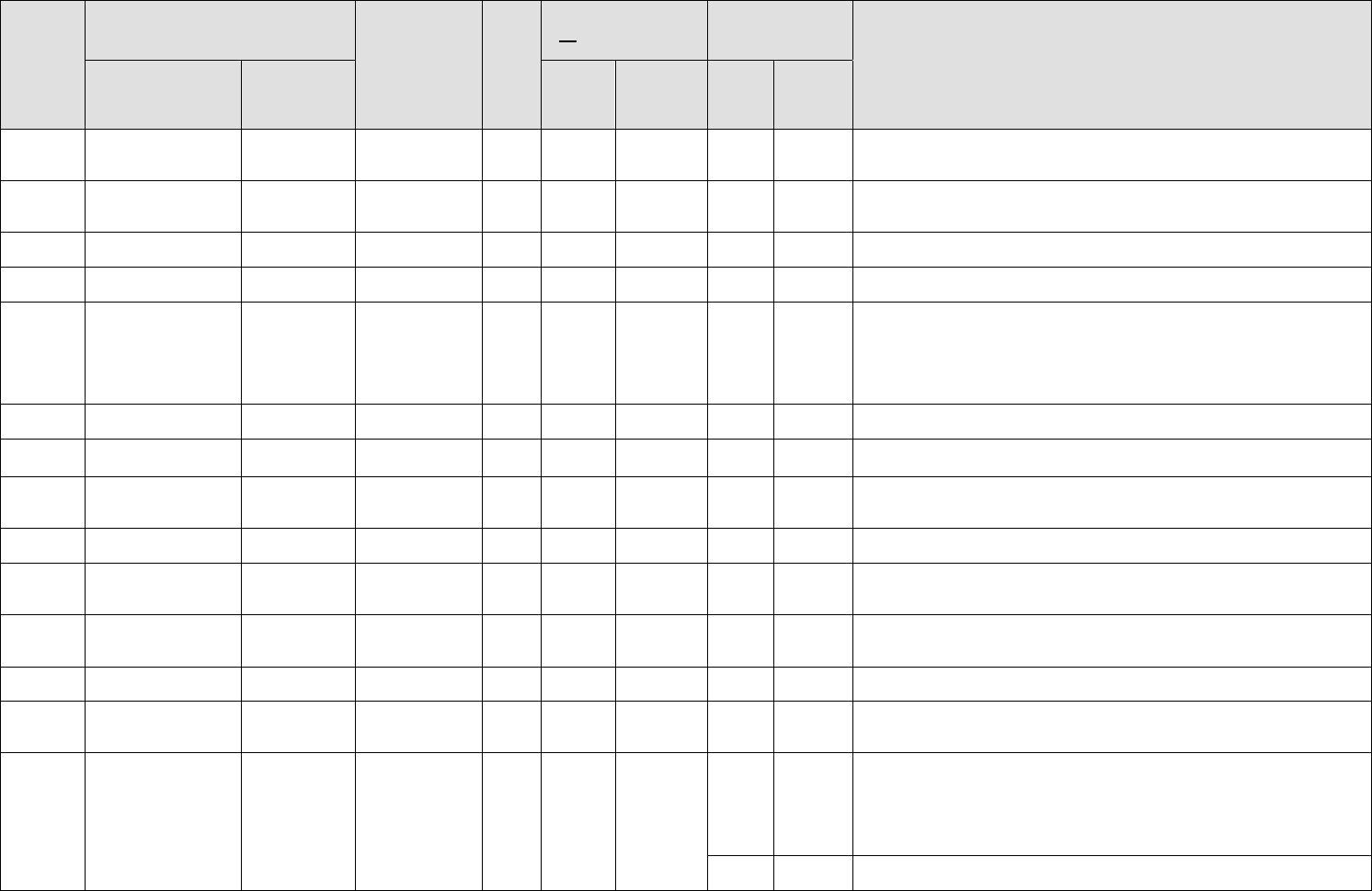
Category
Medication
Diagnosis
No
DNIF
(No Waiver Required)
DNIF
(Waiver Required)
Notes
Generic Name (Oral
Preparation Unless
Specified Otherwise)
Trade Name
(Not All
Inclusive)
or
Utilization
DNIF
For
Ground
Trial
Symptoms
Controlled
(No Side
Effect)
Flying
I/II/
RPA
Pilot
Flying
III/
GBC
MS
Allopurinol
Zyloprim
Gout and
urolithiasis
X
X
For urolithiasis either alone or in combination with thiazide (hydrochlorothiazide or
chlorothiazide). Submit for waiver after potential for idiosyncratic reaction has
been ruled out and control is maintained.
Gen
Amlodipine
Norvasc
Hypertension and
Raynaud’s
X
X
Submit for waiver after potential for idiosyncratic reaction has been ruled out and
control is maintained. Minimum 7-day observation after last dose adjustment.
Approved for FC IIA, RPA Pilot and FC III waivers.
Antibiotic
Amoxicillin
Amoxil
Acute infection
X
DNIF until potential for idiosyncratic reaction has been ruled out and acute
infectious process is asymptomatic.
Antibiotic
Ampicillin
Polycillin
Acute infection
X
DNIF until potential for idiosyncratic reaction has been ruled out and acute
infectious process is asymptomatic.
GU
Ampicillin
Polycillin
Suppressive
therapy for
chronic or
recurrent
prostatitis /
cystitis
X
X
Submit for waiver after potential for idiosyncratic reaction has been ruled out and
control is maintained.
Gen
Anesthetic Agents
(local or regional)
Surgical
procedures
X
Aircrew/SOD members cannot fly for at least 8 hours after receiving a local or
regional anesthetic agent.
Derm
Antibiotics (topical)
Acne
X
DNIF not required unless condition or medication interferes with life support gear
or flying duties.
Derm
Antifungals (topical)
Tinactin
Lamisil
Lotrimin
Tinea pedis
Tinea cruris
Tinea corporis
X
DNIF not required unless condition or medication interferes with life support gear
or flying duties.
Derm
Anti-infectives/
Antiseptics
Silvadene
Neosporin
Acute injury
(burns, abrasions)
X
DNIF not required unless condition or medication interferes with life support gear
or flying duties.
Gen
Aspirin
Bayer Aspirin
Cardiovascular
prophylaxis
X
Single ground trial is required for members who have never previously taken aspirin
- 81 mg or 325 mg once daily for prophylactic therapy as clinically indicated.
Underlying disqualifying condition (when present) continue to require waiver.
Gen
Aspirin
Bayer Aspirin,
Ecotrin
Pain, anti-
inflammatory
(acute use)
X
DNIF until the underlying condition will not interfere with flying duties and there
are no adverse side effects. Usage is for acute conditions, less than 4 weeks, and
condition does not require waiver.
Gen
Aspirin
Bayer Aspirin
Ecotrin
Pain (chronic
use)
X
X
Submit for waiver after potential for idiosyncratic reaction has been ruled out and
control is maintained.
Gen
Atenolol
Hypertension (2
nd
line), atrial
arrhythmia
X
X
Limited to a FC IIA or RPA Pilot waiver initially by AFMSA/SGP and renewals
may not be delegated down by MAJCOM/SGPA.
Gen
Atorvastatin
Lipitor
Hyperlipidemia
X
Waiver not required if on single approved statin medication for hyperlipidemia..
Approved medications include simvastatin, pravastatin, and lovastatin up to
40mg/day and atorvastatin up to 80 mg/day. Higher doses or combination of
medication requires waiver. Requires at least 5 day ground trail when starting
medication or for any adjustments to dosage to rule out idiosyncratic reactions.
Follow up of lipids and LFTs should conform with accepted practice standards.
X*
X*
Combination therapy with Gemfibrozil is limited to a FC IIA waiver by
MAJCOM/SGPA or RPA Pilot (AFMSA) and may not be further delegated.
Note: (1) Members pending waiver action must remain DNIF until waiver has been granted. 4
(2) Medications not on this list, singly or in combination, require review by AFMSA/SG3/5PF (rated officers) and MAJCOM/SG (non-rated personnel).
(3) Verbal waivers are NOT authorized.
(4) Waivers for non-FDA approved medications will not be considered.
Approved by AF/SG3/5P on 13 Jun 2017
7700 Arlington Blvd., Falls Church, VA 22042-5158
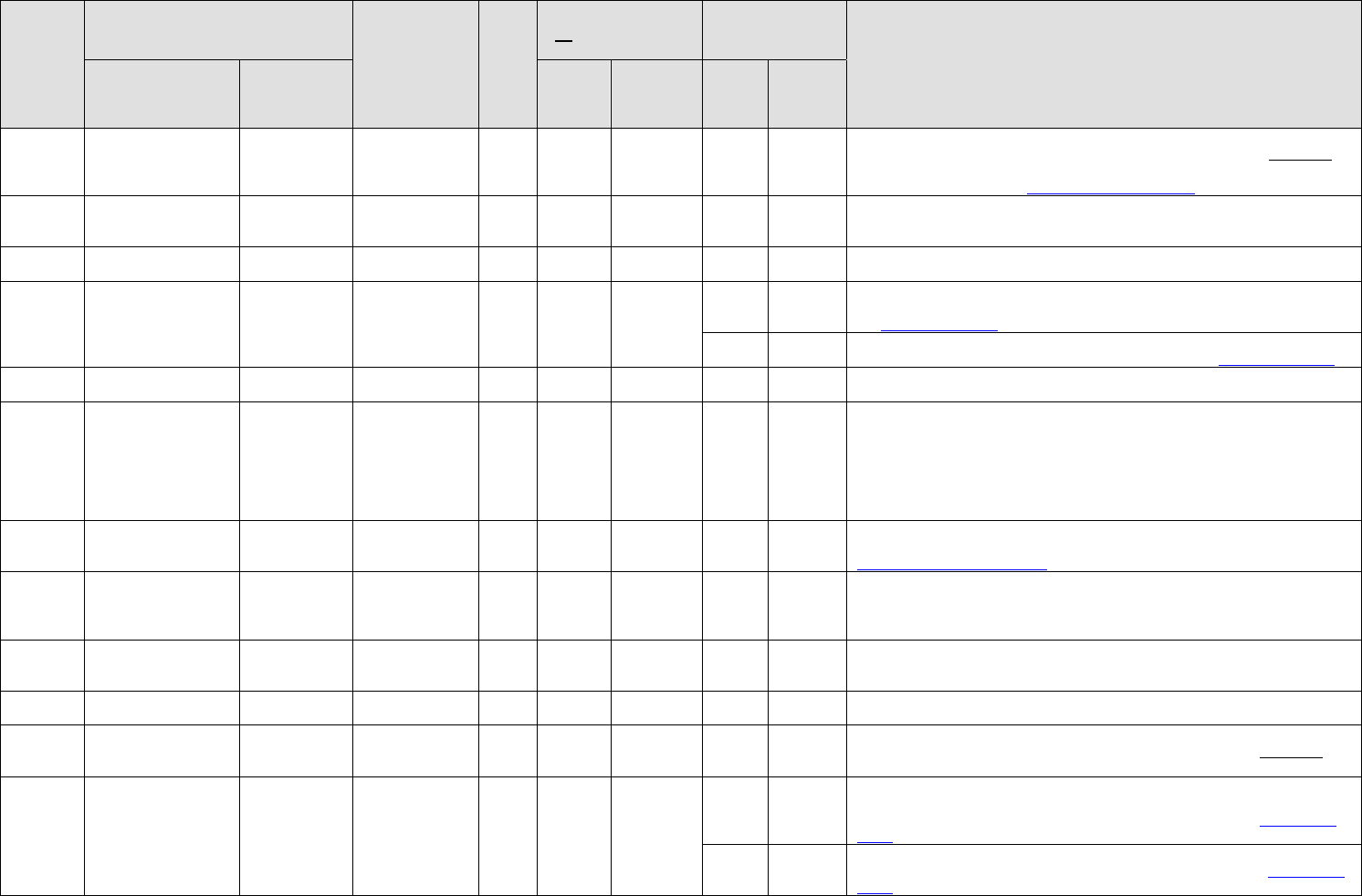
Category
Medication
Diagnosis
No
DNIF
(No Waiver Required)
DNIF
(Waiver Required)
Notes
Generic Name (Oral
Preparation Unless
Specified Otherwise)
Trade Name
(Not All
Inclusive)
or
Utilization
DNIF
For
Ground
Trial
Symptoms
Controlled
(No Side
Effect)
Flying
I/II/
RPA
Pilot
Flying
III/
GBC
Gen
Atovaquone/
Proguanil
(combination)
Malarone
Malaria
p
rophylaxis
X
Single dose ground trial required, Malarone (250 mg atovaquone/100 mg proguanil)
daily beginning 1-2 days prior to travel, ending 7 days after exposure (Reminder:
last 7 days of Malarone should be taken with primaquine followed by another 7
days of primaquine alone.) Malarone Background Paper
ENT
Azelastine
Astelin
Vasomotor
rhinitis
X
X
Minimum 72 hours ground trial at initiation of therapy and adequate control of
rhinitis is required.
Requires FCIIC (with another qualified pilot) waiver by AFMSA.
Antibiotic
Azithromycin
Zithromax
Acute infection
X
DNIF until potential for idiosyncratic reaction has been ruled out and acute
infectious process is asymptomatic.
Gen
Benazepril
Lotensin
Hypertension
X
Waiver not required for monotherapy. Minimum 7-day DNIF observation period at
initial treatment and subsequent dose adjustments. Symptom control = BP <140/90.
See HTN Waiver Guide for treatment parameters.
X*
X*
*Combination therapy with HCTZ or other antihypertensive requires waiver.
Combo therapy requires categorical restriction for FCII - see HTN Waiver Guide.
Ophth
Betaxolol
(ophth drops)
Betoptic
Glaucoma
X
X
Submit for waiver after potential for idiosyncratic reaction has been ruled out and
control is maintained.
Psych
Buproprion
Wellbutrin
SR or XL
Depression or
other waiverable
diagnoses
X*
X
Max dose 450 mg/day. *Not waiverable for FCI. Limited to FCIIC (multicrew
aircraft, except for B-2), GBC, and FCIII. Waiver will not be considered until
member is on medication with stable dose and clinically asymptomatic for at least
six months. All FCII and FCIII listed (Boom Operator, Flight Engineer,
Loadmaster, Aerial Gunner, Combat Control) require ACS evaluation and AFMSA
waiver. All other FCIII AFSCs, ACS evaluation is encouraged and MAJCOM
dispositions waiver.
Derm
Calcipotriene
0.005% Ointment
(topical)
Dovonex
Psoriasis
X
X
Submit for waiver after potential for idiosyncratic reaction has been ruled out and
control is maintained. Doses limited to 100 gm of ointment per week.
Calcipotriene Background Paper
Gen
Celecoxib
Celebrex
Pain
(chronic use)
X
X
Approved for pain and inflammation with no waiver required as long as underlying
condition does not require waiver. Member will be DNIF/DNIC until
pain/inflammation control is achieved AND for seven days following the final
dosage adjustment.
Gen
Celecoxib
Celebrex
Pain
(acute condition
use)
X
DNIF until the underlying condition will not interfere with flying duties and there
are no adverse side effects. Usage is for acute conditions, less than 4 weeks, and
condition does not require waiver.
Antibiotic
Cephalexin
Keflex
Acute infection
X
DNIF until potential for idiosyncratic reaction has been ruled out and acute
infectious process is asymptomatic.
Gen
Chloroquine
Aralen
Malaria
prophylaxis
X
Single dose ground trial required. 500 mg tablet (300 mg base) once weekly
beginning 1-2 weeks prior to travel, ending 4 weeks after exposure. (Reminder:
last 2 weeks should be taken with primaquine.)
Gen
Chlorothiazide
Diuril
Hypertension
X
For hypertension: either alone or in combination with triamterene does not require
waiver. Minimum 7-day DNIF observation period at initial treatment and
subsequent dose adjustments. Symptom control = BP <140/90. See HTN Waiver
Guide for treatment parameters.
X*
X*
*Combination therapy with ACEi, ARB, and other antihypertensive requires
waiver. Combo therapy requires categorical restriction for FCII – see HTN Waiver
Guide.
Note: (1) Members pending waiver action must remain DNIF until waiver has been granted. 5
(2) Medications not on this list, singly or in combination, require review by AFMSA/SG3/5PF (rated officers) and MAJCOM/SG (non-rated personnel).
(3) Verbal waivers are NOT authorized.
(4) Waivers for non-FDA approved medications will not be considered.
Approved by AF/SG3/5P on 13 Jun 2017
7700 Arlington Blvd., Falls Church, VA 22042-5158
Category
Medication
Diagnosis
No
DNIF
(No Waiver Required)
DNIF
(Waiver Required)
Notes
Generic Name (Oral
Preparation Unless
Specified Otherwise)
Trade Name
(Not All
Inclusive)
or
Utilization
DNIF
For
Ground
Trial
Symptoms
Controlled
(No Side
Effect)
Flying
I/II/
RPA
Pilot
Flying
III/
GBC
Gen
Chlorothiazide
Diuril
Urolithiasis
X
X
For urolithiasis: either alone or in combination with allopurinol or oral potassium
supplements. Submit for waiver after potential for idiosyncratic reaction has been
ruled out and control is maintained.
Gen
Cholestyramine
Questran
Hyperlipidemia
X
DNIF until potential for idiosyncratic reaction has been ruled out.
Derm
Ciclopirox (topical)
Loprox
Seborrheic
dermatitis
X
DNIF not required unless condition or medication interferes with life support gear
or flying duties.
Antibiotic
Ciprofloxacin
Cipro
BW prophylaxis
only
X
Neurotoxicity risk precludes usage in non-BW environment. Ciprofloxacin may
be used operationally after monitored ground trial (500 mg every 12 hours for 2
doses with 48 hours DNIF documented in medical records) in event of BW incident
for post-exposure treatment and prophylaxis for inhalational anthrax only.
Cipro Policy Letter.
Psych
Citalopram
Celexa
Depression or
other waiverable
diagnoses
X*
X
Max dose 40 mg/day. *Not waiverable for FCI. Limited to FCIIC (multicrew
aircraft, except for B-2), GBC, and FCIII. Waiver will not be considered until
member is on medication with stable dose and clinically asymptomatic for at least
six months. All FCII and FCIII listed (Boom Operator, Flight Engineer,
Loadmaster, Aerial Gunner, Combat Control) require ACS evaluation and AFMSA
waiver. All other FCIII AFSCs, ACS evaluation is encouraged and MAJCOM
dispositions waiver.
Derm
Clindamycin (topical)
Cleocin T
Acne
X
DNIF not required unless condition or medication interferes with life support gear
or flying duties.
GU
Clomiphene
Clomid
Infertility
X
X
Submit for waiver after potential for idiosyncratic reaction has been ruled out.
Gen
Colestipol
Colestid
Hyperlipidemia
X
DNIF until potential for idiosyncratic reaction has been ruled out.
GU
Contraceptives (oral)
Contraception
X
Minimum of 7-days ground trial is required. Changes of dosage or preparation
requires an additional 7-day observation period.
GU
Contraceptives
(transdermal)
Contraception
X
Minimum of 7-days ground trial is required. Changes of dosage or preparation
requires an additional 7-day observation period.
GU
Contraceptives
(subdermal)
Implanon
Contraception
X
Minimum of 7-days ground trial is required.
ENT
Cromolyn (nasal)
Crolom
Mild allergic,
non-allergic, or
vasomotor
rhinitis
X
Length of DNIF dictated by time required for adequate control of underlying
symptoms.
Ophth
Cyclosporine
Restasis
Dry eye
X*
X*
*Waiverable for FCII/III (trained assets only) and based on severity as specified in
the waiver guide
Ops
Dextroamphetamine
Dexadrine
Fatigue
management (go
pill)
X
OPERATIONAL USE ONLY: NOTE: Only “Immediate Release” is approved for
operational use. (See AFI 48-149 Section 7.4, AFI 11-202V3 Section 2.8.1., and
MAJCOM Guidance.) Check with MAJCOM/SGP prior to prescribing. Ground
trial (10 mg every 4 hours for 2 doses, documented in the medical record) with
mandatory DNIF required prior to operational use. The normal dose for operational
use is 10 mg PO q 4 hours PRN, not to exceed 20 mg in 24 hours.
Dextroamphetamine is not authorized for routine clinical use in flyers/special duty
personnel.
Note: (1) Members pending waiver action must remain DNIF until waiver has been granted. 6
(2) Medications not on this list, singly or in combination, require review by AFMSA/SG3/5PF (rated officers) and MAJCOM/SG (non-rated personnel).
(3) Verbal waivers are NOT authorized.
(4) Waivers for non-FDA approved medications will not be considered.
Approved by AF/SG3/5P on 13 Jun 2017
7700 Arlington Blvd., Falls Church, VA 22042-5158
Category
Medication
Diagnosis
No
DNIF
(No Waiver Required)
DNIF
(Waiver Required)
Notes
Generic Name (Oral
Preparation Unless
Specified Otherwise)
Trade Name
(Not All
Inclusive)
or
Utilization
DNIF
For
Ground
Trial
Symptoms
Controlled
(No Side
Effect)
Flying
I/II/
RPA
Pilot
Flying
III/
GBC
Ops
Dextroamphetamine
Geldex,
Procentra
Fatigue
management
U-2S pilots only
OPERATIONAL USE ONLY: Only approved for U-2S pilots when conducting U-
2S operational sorties IAW applicable guidance. (See AFI 48-149 Section 7.4, AFI
11-202V3 Section 2.8.1., and MAJCOM Guidance.) Check with MAJCOM/SGP
prior to prescribing. Ground trial (10 mg every 4 hours for 2 doses, documented in
the medical record) with mandatory DNIF required prior to operational use.
Dextroamphetamine is not authorized for routine clinical use in flyers/special duty
personnel.
Gen
Dextroamphetamine/
Scopolamine
Dex/Scop
Airsickness
X
Alone or in combination with dextroamphetamine for airsickness in formal
training programs only. *Not authorized for solo flight (see AETCI 48-102).
Gen
Dietary/ Herbal/
Nutritional
Supplements
Wellness
X
Dietary, herbal, and nutritional supplements can only be used with the
approval of a flight surgeon. The flight surgeon should consider aeromedical
implications of the supplement. In general, the use of nutritional supplements is not
recommended.
Nutritional Supplement Policy Letter, Ephedra Policy Letter, SF
600 Overprint (optional tool for convenience) http://hprc-online.org/dietary-
supplements/dietary-supplement-classification-system-1
Antibiotic
Dicloxacillin
Dynapen
Acute infection
X
DNIF until potential for idiosyncratic reaction has been ruled out and acute
infectious process is asymptomatic.
Derm
Doxycycline
Vibramycin
Acne
X
DNIF until potential for idiosyncratic reaction has been ruled out and underlying
condition does not interfere with duties. If previous ground trial has been
accomplished and documented, no DNIF is required.
Antibiotic
Doxycycline
Vibramycin
Acute infection
X
Preventive
Doxycycline
Vibramycin
Acute mild
diarrhea
X
Preventive
Doxycycline
Vibramycin
BW prophylaxis
(2
nd
line)
X
Preventive
Doxycycline
Vibramycin
Malaria
prophylaxis
X
Preventive
Doxycycline
Vibramycin
Prophylaxis
against diarrhea
X
GU
Doxycycline
Vibramycin
Suppressive
yherapy for
chronic or
recurrent
prostatitis/
cystitis
X
X
Submit for waiver after potential for idiosyncratic reaction has been ruled out and
control is maintained.
Endo
Eplerenone
Inspra
Hyper-
aldosteronism
X
X
Submit for waiver after potential for idiosyncratic reaction has been ruled out and
control is maintained. FCIIA or RPA Pilot waiver only. Eplerenone and
Spironolactone Background Paper.
Derm
Erythromycin
E-mycin
Acne
X
X
Submit for waiver after potential for idiosyncratic reaction has been ruled out and
control is maintained.
Antibiotic
Erythromycin
E-mycin
Acute infection
X
DNIF until potential for idiosyncratic reaction has been ruled out and acute
infectious process is asymptomatic.
Derm
Erythromycin (topical)
T-Stat
Acne
X
DNIF not required unless condition or medication interferes with life support gear
or flying duties.
Note: (1) Members pending waiver action must remain DNIF until waiver has been granted. 7
(2) Medications not on this list, singly or in combination, require review by AFMSA/SG3/5PF (rated officers) and MAJCOM/SG (non-rated personnel).
(3) Verbal waivers are NOT authorized.
(4) Waivers for non-FDA approved medications will not be considered.
Approved by AF/SG3/5P on 13 Jun 2017
7700 Arlington Blvd., Falls Church, VA 22042-5158
Category
Medication
Diagnosis
No
DNIF
(No Waiver Required)
DNIF
(Waiver Required)
Notes
Generic Name (Oral
Preparation Unless
Specified Otherwise)
Trade Name
(Not All
Inclusive)
or
Utilization
DNIF
For
Ground
Trial
Symptoms
Controlled
(No Side
Effect)
Flying
I/II/
RPA
Pilot
Flying
III/
GBC
Psych
Escitalopram
Lexapro
Depression or
other waiverable
diagnoses
X*
X
Max dose 20 mg/day. * Not waiverable for FCI. Limited to FCIIC (multicrew
aircraft, except for B-2), GBC, and FCIII. Waiver will not be considered until
member is on medication with stable dose and clinically asymptomatic for at least
six months. All FCII and FCIII listed (Boom Operator, Flight Engineer,
Loadmaster, Aerial Gunner, Combat Control) require ACS evaluation and AFMSA
waiver. All other FCIII AFSCs, ACS evaluation is encouraged and MAJCOM
dispositions waiver.
Gen
Esomeprazole
Nexium
GERD
X
DNIF until potential for idiosyncratic reaction has been ruled out (minimum 3 days)
and symptom control is maintained.
Gen
Esomeprazole
Nexium
Peptic ulcer
disease
X
X
Submit for waiver after potential for idiosyncratic reaction has been ruled out and
control is maintained. Authorized under a single waiver with the other approved
proton pump inhibitors (PPIs) esomeprazole, omeprazole, rabeprazole, lansoprazole
and pantoprazole. Medication change between the approved PPIs at the base level,
while still requiring a mandatory 3-day ground observation period, does not
necessitate notification of the waiver authority. These changes should be
documented in AIMWTS at the time of waiver renewal.
Endo
Estrogen (alone or
with progestin or
testosterone)
Contraception,
Hormone
Replacement
Therapy
X
X
Minimum of 7-days ground trial is required. Changes of dosage or preparation
requires an additional 7-day observation period.
Endo
Estrogen (alone or
with progestin)
(topical)
Contraception,
Hormone
Replacement
Therapy
X
X
Minimum of 7-days ground trial is required. Changes of dosage or preparation
requires an additional 7-day observation period.
Gen
Etanercept
Enbrel
Reactive arthritis,
rheumatoid
arthritis,
psoriasis and
psoriatic arthritis,
ankylosing
spondyltits
X
X
Requires refrigeration at 36-46 degrees F. Submit for waiver after potential for
idiosyncratic reaction has been ruled out and control is maintained. FCIIC waiver
by AFMSA/SGPA. Restricted Deployability, see
Waiver Guide. Etanercept
Background Paper
Endo
Etonogestrel/Ethinyl
Estradiol (vaginal
ring)
NuvaRing
Contraception
X
Minimum of 7 days ground trial is required. Changes of dosage or preparation
requires an additional 7-day observation period.
Gen
Ezetimibe
Zetia
Hyperlipidemia
(2
nd
line)
X
X
Submit for waiver after potential for idiosyncratic reaction has been ruled out
(minimum 3 days) and control is maintained. Ezetimibe Background Paper.
Gen
Ezetimbe/Simva-statin
Vytorin
Hyperlipidemia
X
X
Submit for waiver after potential for idiosyncratic reaction has been ruled out
(minimum 5 days) and control is maintained. Ezetimibe Background Paper.
Gen
Fenofibrate
Tricor
Hyperlipidemia
X
X
Combination therapy with approved statin for hyperlipidemia is limited to FCIIA
waiver by MAJCOM/SGPA or RPA Pilot (AFMSA) and may not be further
delegated. See Fenofibrate Background paper.
Gen
Ferrous Sulfate
Iron deficiency
anemia
X
X
Submit for waiver after potential for idiosyncratic reaction has been ruled out.
ENT
Fexofenadine
Allegra
Mild allergic
rhinitis
X
Minimum 72 hours ground trial at initiation of therapy and adequate control of
rhinitis is required.
Note: (1) Members pending waiver action must remain DNIF until waiver has been granted. 8
(2) Medications not on this list, singly or in combination, require review by AFMSA/SG3/5PF (rated officers) and MAJCOM/SG (non-rated personnel).
(3) Verbal waivers are NOT authorized.
(4) Waivers for non-FDA approved medications will not be considered.
Approved by AF/SG3/5P on 13 Jun 2017
7700 Arlington Blvd., Falls Church, VA 22042-5158
Category
Medication
Diagnosis
No
DNIF
(No Waiver Required)
DNIF
(Waiver Required)
Notes
Generic Name (Oral
Preparation Unless
Specified Otherwise)
Trade Name
(Not All
Inclusive)
or
Utilization
DNIF
For
Ground
Trial
Symptoms
Controlled
(No Side
Effect)
Flying
I/II/
RPA
Pilot
Flying
III/
GBC
GU
Finasteride
Proscar
Benign Prostatic
Hyperplasia
X
X
Submit for waiver after potential for idiosyncratic reaction has been ruled out
(minimum 3 days) and condition does not interfere with flying duties. DoD policy
prohibits purchase of this drug for treatment hair loss using DoD funds (see
Finasteride Background Paper). If used in combination with silodosin, follow
silodosin requirement.
GU
Finasteride
(1 mg)
Propecia
Hair loss
X
DNIF until potential for idiosyncratic reaction has been ruled out (minimum 3
days).
DoD policy prohibits purchase of this drug for treatment hair loss using DoD funds.
GI
Folate
Sprue
X
X
Submit for waiver after potential for idiosyncratic reaction has been ruled out and
control is maintained.
Gen
Gemfibrozil
Lopid
Hyperlipidemia
X
X
Submit for waiver after potential for idiosyncratic reaction has been ruled out.
*Combination therapy of Gemfibrozil with an approved statin (lovastatin,
pravastatin, simvastatin, or atorvastatin) is limited to a FCIIA waiver by
MAJCOM/SGPA or RPA Pilot (AFMSA) and may not be further delegated.
GI
Hemorrhoidal
suppository
Hemorrhoids
X
DNIF is not required once symptoms relieved.
Gen
Hyaluronate
derivatives
Synvisc,
Synvisc-One,
Euflexxa,
Hyalgan,
Orthovisc
Osteoarthritis
pain
X
For intra-articular injection only. 48 hour post-injection DNIF required. Use of this
medication does not require waiver. However, depending on severity, underlying
condition MAY require waiver.
Gen
Hydrochlorothiazide
Hydrodiuril
Hypertension
X
For hypertension: either alone or in combination with triamterene does not require
waiver. Minimum 7-day DNIF observation period at initial treatment and
subsequent dose adjustments. Symptom control = BP <140/90. See HTN Waiver
Guide for treatment parameters.
X*
X*
*Combination therapy with ACEi, ARB, and other antihypertensive requires
waiver. Combo therapy requires categorical restriction for FCII – see HTN Waiver
Guide.
Gen
Hydrochlorothiazide
Hydrodiuril
Urolithiasis
X
X
For urolithiasis: either alone or in combination with allopurinol or oral potassium
supplements. Submit for waiver after potential for idiosyncratic reaction has been
ruled out and control is maintained.
Gen
Hydroxychloroquine
Plaquenil
Arthritis
X
X
Submit for waiver after potential for idiosyncratic reaction has been ruled out and
control is maintained.
Gen
Ibuprofen
Motrin
Pain
(chronic use)
X
X
Submit for waiver after potential for idiosyncratic reaction has been ruled out and
control is maintained.
Gen
Ibuprofen
Motrin
Pain
(acute condition
use)
X
DNIF until the underlying condition will not interfere with flying duties and there
are no adverse side effects. Usage is for acute conditions, less than 4 weeks, and
condition does not require waiver.
Derm
Imiquimod
(topical)
Aldara,
Zyclara
Warts, actinic
keratosis,
basal cell cancer
X
DNIF not required unless condition or medication interferes with life support gear
or flying duties. Localized inflammatory reactions at the site of application are
common, and should be considered prior to initiation of therapy.
Note: (1) Members pending waiver action must remain DNIF until waiver has been granted. 9
(2) Medications not on this list, singly or in combination, require review by AFMSA/SG3/5PF (rated officers) and MAJCOM/SG (non-rated personnel).
(3) Verbal waivers are NOT authorized.
(4) Waivers for non-FDA approved medications will not be considered.
Approved by AF/SG3/5P on 13 Jun 2017
7700 Arlington Blvd., Falls Church, VA 22042-5158
Category
Medication
Diagnosis
No
DNIF
(No Waiver Required)
DNIF
(Waiver Required)
Notes
Generic Name (Oral
Preparation Unless
Specified Otherwise)
Trade Name
(Not All
Inclusive)
or
Utilization
DNIF
For
Ground
Trial
Symptoms
Controlled
(No Side
Effect)
Flying
I/II/
RPA
Pilot
Flying
III/
GBC
Gen
Infliximab
Remicade
Ankylosing
spondylitis,
psoriatic arthritis,
psoriasis#,
ulcerative
colitis*, Crohns*
X*
X*
*No initial flying class waivers. Requires 6 months symptom control prior to
waiver submission.
#Psoriasis when other medications have failed.
Consult Waiver Guide for use in IBD patients.
Restricted deployability, see Waiver Guide.
See Infliximab (Remicade) background
paper.
Immuno
Immunization
Wellness
X
Adverse reactions are rare. Access to medical care on the ground is recommended
for a period of 4 hours for all personnel, unless operational needs dictate otherwise.
Recommend timing live immunizations such that side effects, if present, will have
minimal operational impact. This guidance also applies to JEV (IXIARO).
Immuno
Immunotherapy
Allergy
X
X
Submit for waiver after potential for idiosyncratic reaction has been ruled out and
control is maintained. Once waiver has been granted, a 4-hour verbal DNIF is
required for aircrew/SOD after each injection. DNIF not required for ground
operators. Aircrew/SOD will not deploy on immunotherapy.
Pulm
Isoniazid (INH)
Nydrazid
TB prophylaxis
X
For tuberculin converters who do not have active TB, minimum 72 hours ground
trial.
Antibiotic
INH-Rifapentine
Priftin
Latent TB
X
X
Directly Observed Therapy regimens only, IAW CDC/IDSA recommendations.
Prior to deployment ensure PH clearance for completion of DOT.
Gen
Ketamine
Ketalar
Anesthesia
X
Minimum 3-week DNIF required.
GI
Lansoprazole
Prevacid
GERD
X
DNIF until potential for idiosyncratic reaction has been ruled out (minimum 3 days)
and symptom control is maintained.
GI
Lansoprazole
Prevacid
Peptic Ulcer
Disease
X
X
DNIF until potential for idiosyncratic reaction has been ruled out (minimum 3 days
and symptom control is maintained. Authorized under a single waiver along with
the other approved proton pump inhibitors (PPIs) esomeprazole, omeprazole,
rabeprazole, lansoprazole and pantoprazole. Medication change between approved
PPIs at the base level, while still requiring a mandatory 3 day ground observation
period, does not necessitate notification of the waiver authority. These changes
should be documented in AIMWTS at the time of waiver renewal.
Ophth
Latanoprost
(ophth drops)
Xalatan
Glaucoma
X
X
Submit for waiver after potential for idiosyncratic reaction has been ruled out and
control is maintained.
Ophth
Levobunolol
(ophth drops)
Betagan
Glaucoma
X
X
Submit for waiver after potential for idiosyncratic reaction has been ruled out and
control is maintained.
Gen
Levothyroxine
Synthroid
Hypothyroidism
or thyroid
suppression
X
X
Submit for waiver after potential for idiosyncratic reaction has been ruled out and
control is maintained.
Gen
Lisinopril
Zestril
Hypertension
X
Waiver not required for monotherapy. Minimum 7-day DNIF observation period at
initial treatment and subsequent dose adjustments. Symptom control = BP <140/90.
See
HTN Waiver Guide for treatment parameters.
X*
X*
*Combination therapy with HCTZ or other antihypertensive requires waiver.
Combo therapy requires categorical restriction for FCII - see HTN Waiver Guide.
ENT
Loratadine
Claritin
Allergy
X
Minimum 72 hours ground trial at initiation of therapy and adequate control of
rhinitis is required. Maximum dosage is limited to 10 mg per day.
Gen
Losartan
Cozaar
Hypertension
X
Waiver not required for monotherapy. Minimum 7-day DNIF observation period at
initial treatment and subsequent dose adjustments. Symptom control = BP <140/90.
See
HTN Waiver Guide for treatment parameters.
Note: (1) Members pending waiver action must remain DNIF until waiver has been granted. 10
(2) Medications not on this list, singly or in combination, require review by AFMSA/SG3/5PF (rated officers) and MAJCOM/SG (non-rated personnel).
(3) Verbal waivers are NOT authorized.
(4) Waivers for non-FDA approved medications will not be considered.
Approved by AF/SG3/5P on 13 Jun 2017
7700 Arlington Blvd., Falls Church, VA 22042-5158
Category
Medication
Diagnosis
No
DNIF
(No Waiver Required)
DNIF
(Waiver Required)
Notes
Generic Name (Oral
Preparation Unless
Specified Otherwise)
Trade Name
(Not All
Inclusive)
or
Utilization
DNIF
For
Ground
Trial
Symptoms
Controlled
(No Side
Effect)
Flying
I/II/
RPA
Pilot
Flying
III/
GBC
X*
X*
*Combination therapy with HCTZ or other antihypertensive requires waiver.
Combo therapy requires categorical restriction for FCII - see HTN Waiver Guide.
Gen
Lovastatin
Mevacor
Hyperlipidemia
X
Waiver not required if on single approved statin medication for hyperlipidemia.
Approved medications include simvastatin, pravastatin, lovastatin and rosuvastatin
up to 40 mg/day and atorvastatin up to 80 mg/day. Higher doses or combination of
medication requires waiver. Requires at least 5 day ground trail when starting
medication or for any adjustments to dosage to rule out idiosyncratic reactions.
Follow up of lipids and LFTs should conform to accepted practice standards.
X*
X*
*Combination therapy with Gemfibrozil is limited to a FCIIA or RPA Pilot
(AFMSA for waiver and may not be further delegated).
Gen
Meloxicam
Mobic
Pain,
inflammation
(chronic use)
X
X
Approved for pain and inflammation up to a dose of 15 mg per day, no waiver
required. Member will be DNIF/DNIC until pain/inflammation control is achieved
AND for seven days following the final dosage adjustment.
Gen
Mesalamine
(complexed with
methyl/methacrylic
acid resin)
Asacol,
Delizicol
Inflammatory
Bowel Disorder
X
X
Submit for waiver after potential for idiosyncratic reaction has been ruled out and
control is maintained. See Waiver Guide.
Gen
Mesalamine (delayed
release via polymer)
Lialda
Inflammatory
Bowel Disorder
X
X
Submit for waiver after potential for idiosyncratic reaction has been ruled out and
control is maintained. See Waiver Guide.
Gen
Mesalamine
(complexed with ethyl
cellulose)
Pentasa
Inflammatory
Bowel Disorder
X
X
Submit for waiver after potential for idiosyncratic reaction has been ruled out and
control is maintained. See Waiver Guide.
Gen
Mesalamine
(enema/suppositories)
Rowasa
Inflammatory
Bowel Disorder
X
X
Submit for waiver after potential for idiosyncratic reaction has been ruled out and
control is maintained. See Waiver Guide.
Gen
Metformin
Glucophage
Diabetes
mellitus, pre-
diabetes (includes
impaired fasting
glucose)
X
X
Submit for waiver after patient has been on medication for at least 30 days and the
requirements for waiver submission (as defined by the Diabetes Waiver Guide) have
been met. Note: initial waiver for the diagnosis of Diabetes still resides at AFMSA.
GU
Metformin
Glucophage
Polycystic
Ovarian
Syndrome
X
X
Submit for waiver after patient has been on medication for at least 30 days and the
requirements for waiver submission (as defined by the PCOS Waiver Guide) have
been met.
Gen
Metoprolol
Toprol,
Lopressor
Hypertension
(2nd line), atrial
arrhythmia
X
X
Limited to a FC IIA or RPA Pilot waiver initially by AFMSA/SGP3F and renewals
may not be delegated down by MAJCOM.
Derm
Metronidazole
(topical)
Flagyl
Rosacea
X
DNIF not required unless condition or medication interferes with life support gear
or flying duties.
GU
Metronidazole
(topical)
Flagyl
Vaginitis
X
DNIF is not required unless condition is symptomatic.
Dental
Procedure
Minocycline
(microspheres)
Arestin
Adjunct to dental
scaling/root
planing
X
Used alone as one dose for dental procedure only does not require DNIF. DNIF is
indicated for use of associated anesthetics or any adverse effects of the procedure.
Note: (1) Members pending waiver action must remain DNIF until waiver has been granted. 11
(2) Medications not on this list, singly or in combination, require review by AFMSA/SG3/5PF (rated officers) and MAJCOM/SG (non-rated personnel).
(3) Verbal waivers are NOT authorized.
(4) Waivers for non-FDA approved medications will not be considered.
Approved by AF/SG3/5P on 13 Jun 2017
7700 Arlington Blvd., Falls Church, VA 22042-5158
Category
Medication
Diagnosis
No
DNIF
(No Waiver Required)
DNIF
(Waiver Required)
Notes
Generic Name (Oral
Preparation Unless
Specified Otherwise)
Trade Name
(Not All
Inclusive)
or
Utilization
DNIF
For
Ground
Trial
Symptoms
Controlled
(No Side
Effect)
Flying
I/II/
RPA
Pilot
Flying
III/
GBC
Ops
Modafinil
Provigil
Fatigue
management
(go pill)
X
OPERATIONAL USE ONLY: See AFI 48-149 Section 7.4, AFI 11-202V3 Section
2.8.1., and MAJCOM Guidance. Check with MAJCOM/SGP prior to prescribing.
Ground trial (200 mg every 8 hours for 2 doses) required.
See Modafinil Policy Letter.
Modafinil is not authorized for routine clinical use in flyers/special duty personnel.
ENT
Derm
Pulm
Montelukast
Singulair
Allergic rhinitis,
urticaria
asthma
X*
*While the medication itself does not require a waiver, the condition might. If
waiver is required, submit for waiver when symptom control is achieved.
Montelukast Background Paper.
Gen
Naproxen
Naprosyn
Pain
(acute use)
X
DNIF until underlying condition will not interfere with flying duties and there are
no adverse side effects. Usage is for acute conditions, less than 4 weeks, and
condition does not require waiver.
Gen
Naproxen
Naprosyn
Pain
(chronic use)
X
X
Submit for waiver after potential for idiosyncratic reaction has been ruled out and
control is maintained.
Gen
Nifedipine Coat Core
Nifedipine GITS
Adalat CC
Procardia XL
Hypertension and
Raynaud’s
X
X
Submit for waiver after potential for idiosyncratic reaction has been ruled out and
control is maintained. Minimum 7-day observation after last dose adjustment.
Approved for FCIIA, RPA Pilot and FCIII waivers. NOTE: NO OTHER
FORMULATIONS OF NIFEDIPINE ARE COVERED UNDER THIS
POLICY. Nifedipine Background Paper
Gen
Nicotine Inhaler
Nicotrol
Tobacco
addiction
X
Not for use while in flight.
Ophth
Olopatadine
Patanol
Allergic
conjunctivitis
X
Do not prescribe if member uses contact lenses. DNIF until potential for
idiosyncratic reaction has been ruled out (minimum 3 days) and symptom control is
maintained.
GI
Omeprazole
Prilosec
GERD
X
DNIF until potential for idiosyncratic reaction has been ruled out (minimum 3 days)
and symptom control is maintained.
Anti-
emetic
Ondansetron 8 mg
Zofran
Motion sickness
X*
*Only as approved by MAJCOM protocol. Specifically for prevention and
treatment for motion sickness on sea operations for pararescue, combat rescue
officers, special tactics officers and combat controllers. Must ground test for one
dose prior to operations.
Contraindicated in patients with a history of congenital QT
prolongation and caution must be exercised in patients with other underlying
cardiac disease.
Gen
Oseltamivir
Tamiflu
Influenza
prophylaxis
(2
nd
line)
X
For unvaccinated personnel during community outbreaks or mission essential
operations IAW MAJCOM policy. Requires 1-day ground trial. Oseltamivir
Background Paper.
Gen
Oseltamivir
Tamiflu
Influenza
treatment
X
DNIF until potential for idiosyncratic reaction has been ruled out and acute
infectious process is asymptomatic. Oseltamivir Background Paper.
Antibiotic
Oxacillin
Bactocill
Acute infection
X
DNIF until potential for idiosyncratic reaction has been ruled out and acute
infectious process is asymptomatic.
ENT
Oxymetazoline (nasal)
Afrin
Eustachian tube
dysfunction,
sinus block
X
May be used as a “get me down” for unexpected ear/sinus blocks during flight or
while in a critical phase of decompressive dive duties. Not for treatment of
symptoms existing prior to flight.
GI
Pantoprazole
Protonix
GERD
X
DNIF until potential for idiosyncratic reaction has been ruled out (minimum 3 days)
and symptom control is maintained.
Note: (1) Members pending waiver action must remain DNIF until waiver has been granted. 12
(2) Medications not on this list, singly or in combination, require review by AFMSA/SG3/5PF (rated officers) and MAJCOM/SG (non-rated personnel).
(3) Verbal waivers are NOT authorized.
(4) Waivers for non-FDA approved medications will not be considered.
Approved by AF/SG3/5P on 13 Jun 2017
7700 Arlington Blvd., Falls Church, VA 22042-5158
Category
Medication
Diagnosis
No
DNIF
(No Waiver Required)
DNIF
(Waiver Required)
Notes
Generic Name (Oral
Preparation Unless
Specified Otherwise)
Trade Name
(Not All
Inclusive)
or
Utilization
DNIF
For
Ground
Trial
Symptoms
Controlled
(No Side
Effect)
Flying
I/II/
RPA
Pilot
Flying
III/
GBC
GI
Pantoprazole
Protonix
Peptic Ulcer
Disease
X
X
DNIF until potential for idiosyncratic reaction has been ruled out (minimum 3 days)
and symptom control is maintained. Authorized under a single waiver along with
the other approved proton pump inhibitors (PPIs) esomeprazole, omeprazole,
rabeprazole, lansoprazole and pantoprazole. Medication change between the
approved PPIs at the base level, while still requiring a mandatory 3-day ground
observation period, does not necessitate notification of the waiver authority. These
changes should be documented in AIMWTS at the time of waiver renewal.
Antibiotic
Penicillin
Pen-Vee-K
Acute infection
X
DNIF until potential for idiosyncratic reaction has been ruled out and acute
infectious process is asymptomatic.
ENT
Phenylephrine
(nasal)
Eustachian tube
dysfunction,
sinus block
X
May be used as a “get me down” for unexpected ear/sinus blocks during flight or
while in a critical phase of decompressive dive duties. Not for treatment of
symptoms existing prior to flight.
Derm
Pimecrolimus
1% Cream (topical)
Elidel
Atopic dermatitis
X
X
Submit for waiver after potential for idiosyncratic reaction has been ruled out and
control is maintained.
Derm
Podofilox
(topical)
Condylox
Warts
X
DNIF not required unless condition or medication interferes with life support gear
or flying duties.
GU
Potassium Citrate
Urocit-K
Urolithiasis
X
X
Submit for waiver after potential for idiosyncratic reaction has been ruled out and
control is maintained.
Gen
Pravastatin
Pravacor
Hyperlipidemia
X
Waiver not required if on single approved statin medication for hyperlipidemia.
Approved medications include simvastatin, pravastatin, lovastatin and rosuvastatin
up to 40 mg/day and atorvastatin up to 80 mg/day. Higher doses or combination of
medication requires waiver. Requires at least 5 day ground trail when starting
medication or for any adjustments to dosage to rule out idiosyncratic reactions.
Follow up of lipids and LFTs should conform to accepted practice standards.
X*
X*
*Combination therapy with Gemfibrozil is limited to a FCIIA waiver by
MAJCOM/SGPA or RPA Pilot (AFMSA) and may not be further delegated.
Gen
Primaquine
Primaquine
Malaria
prophylaxis
(terminal phase)
X
Single dose ground trial required. 30 mg (base) daily (recommendation for increase
from 15 mg to 30 mg by CDC) for terminal 14 days of post-exposure prophylaxis.
Contraindication: G-6-PD deficiency, pregnancy, and possibly lactation (if
infant has G-6-PD deficiency).
MS
Probenecid
Benemid
Gout
X
X
Submit for waiver after potential for idiosyncratic reaction has been ruled out and
control is maintained.
Gen
Potassium Iodide
Thyroshield,
ThyroSafe,
Iostat
Radiation
prophylaxis
X
8 hour ground trial prior to first expected use (as operations allow). Do not
prescribe for members with known iodine sensitivity, thyroiditis, goiter,
hyperkalemia, or pregnancy. Do not ground test unless use is anticipated/directed
by MAJCOM or COCOM. Document ground test in PIMR.
GU
Progestin (injectable)
Depo-Provera
Contraception
X
Minimum of 7 days ground trial is required. Changes of dosage or preparation
requires an additional 7 day observation period.
GU
Progestin (implantable
timed released)
Mirena
Contraception
X
Minimum of 7 days ground trial is required. Changes of dosage or preparation
requires an additional 7 day observation period.
Gen
Proguanil/
Atovaquone
(combination)
Malarone
Malaria
prophylaxis
(2
nd
line)
X
Single dose ground trial required. Malarone (250 mg atovaquone/100 mg
proguanil) daily beginning 1-2 days prior to travel, ending 7 days after exposure.
(Reminder: last 7 days of Malarone should be taken with primaquine followed
by another 7 days of primaquine alone.) Malarone Background Paper.
Neuro
Pyridostigmine
Mestinon
CW prophylaxis
X
DNIF until potential idiosyncratic reactions has been ruled out. Use IAW with
operational guidance, single dose ground trial advised.
Note: (1) Members pending waiver action must remain DNIF until waiver has been granted. 13
(2) Medications not on this list, singly or in combination, require review by AFMSA/SG3/5PF (rated officers) and MAJCOM/SG (non-rated personnel).
(3) Verbal waivers are NOT authorized.
(4) Waivers for non-FDA approved medications will not be considered.
Approved by AF/SG3/5P on 13 Jun 2017
7700 Arlington Blvd., Falls Church, VA 22042-5158
Category
Medication
Diagnosis
No
DNIF
(No Waiver Required)
DNIF
(Waiver Required)
Notes
Generic Name (Oral
Preparation Unless
Specified Otherwise)
Trade Name
(Not All
Inclusive)
or
Utilization
DNIF
For
Ground
Trial
Symptoms
Controlled
(No Side
Effect)
Flying
I/II/
RPA
Pilot
Flying
III/
GBC
GI
Rabeprazole
Aciphex
GERD
X
DNIF until potential for idiosyncratic reaction has been ruled out (minimum 3 days)
and symptom control is maintained.
GI
Rabeprazole
Aciphex
Peptic Ulcer
Disesae
X
X
DNIF until potential for idiosyncratic reaction has been ruled out (minimum 3 days)
and symptom control is maintained. Authorized under a single waiver along with
the other approved proton pump inhibitors (PPIs) esomeprazole, omeprazole,
rabeprazole, lansoprazole and pantoprazole. Medication change between the
approved PPIs at the base level, while still requiring a mandatory 3-day ground
observation period, does not necessitate notification of the waiver authority. These
changes should be documented in AIMWTS at the time of waiver renewal.
Onc
Raloxifene
Evista
Breast cancer
prophylaxis
X
X
Use for breast cancer chemoprophylaxis in coordination with a specialist
experienced in breast cancer chemoprophylaxis only. All other uses require review
on case-by-case basis. Submit for waiver after at least 1 month and stable on
therapy. See Raloxifene Paper.
Gen
Ramipril
Altace
Hypertension
(2
nd
line)
X
Waiver not required for monotherapy. Minimum 7-day DNIF observation period at
initial treatment and subsequent dose adjustments. Symptom control = BP <140/90.
See
HTN Waiver Guide for treatment parameters. Dosage restriction: 5 to 20 mg.
Ramipril Background Paper.
X*
X*
*Combination therapy with ACEi, ARB, and other antihypertensive requires
waiver. Combo therapy requires categorical restriction for FCII - see HTN Waiver
Guide.
GI
Ranitidine
Zantac
GERD
X
DNIF until potential for idiosyncratic reaction has been ruled out (minimum 3 days)
and symptom control is maintained,.
Gen
Resin Binding Agent
Hyperlipidemia
X
DNIF until potential for idiosyncratic reaction has been ruled out.
Pulm
Rifampin
TB prophylaxis
X
For tuberculin converters who do not have active TB, minimum 72 hours ground
trial.
Gen
Rosuvastatin
Crestor
Hyperlipidemia
X
Waiver not required if on single approved statin medication for hyperlipidemia.
Approved medications include simvastatin, pravastatin, lovastatin and rosuvastatin
up to 40 mg/day and atorvastatin up to 80 mg/day. Higher doses or combination of
medication requires waiver. Requires at least 5 day ground trail when starting
medication or for any adjustments to dosage to rule out idiosyncratic reactions.
Follow up of lipids and LFTs should conform to accepted practice standards.
X*
X*
*Combination therapy with Gemfibrozil is limited to a FCIIA or RPA Pilot waiver
by MAJCOM/AFMSA and may not be further delegated.
Psych
Sertraline
Zoloft
Depression or
other waiverable
diagnoses
X*
X
Max dose 200 mg/day. *Not waiverable for FCI. Limited to FCIIC (multicrew
aircraft, except for B-2), GBC, and FCIII. Waiver will not be considered until
member is on medication with stable dose and clinically asymptomatic for at least
six months. All FCII and FCIII listed (Boom Operator, Flight Engineer,
Loadmaster, Aerial Gunner, Combat Control) require ACS evaluation and AFMSA
waiver. All other FCIII AFSCs, ACS evaluation is encouraged and MAJCOM
dispositions waiver.
GU
Sildenafil
Viagra
Erectile
dysfunction
X*
*24 hours DNIF required after each dosage, verbal DNIF acceptable.
*Not authorized for daily use.
Note: (1) Members pending waiver action must remain DNIF until waiver has been granted. 14
(2) Medications not on this list, singly or in combination, require review by AFMSA/SG3/5PF (rated officers) and MAJCOM/SG (non-rated personnel).
(3) Verbal waivers are NOT authorized.
(4) Waivers for non-FDA approved medications will not be considered.
Approved by AF/SG3/5P on 13 Jun 2017
7700 Arlington Blvd., Falls Church, VA 22042-5158
Category
Medication
Diagnosis
No
DNIF
(No Waiver Required)
DNIF
(Waiver Required)
Notes
Generic Name (Oral
Preparation Unless
Specified Otherwise)
Trade Name
(Not All
Inclusive)
or
Utilization
DNIF
For
Ground
Trial
Symptoms
Controlled
(No Side
Effect)
Flying
I/II/
RPA
Pilot
Flying
III/
GBC
GU
Silodosin
Rapaflo
BPH
X*
X
Maximum dose 8 mg daily. *Not waiverable for FCI. Limited to FCIIA (restricted
to non-high performance aircraft), FCIII and GBC. All silodosin waivers for FCII
require AFMSA waiver, for all FCIII and GBC MAJCOM may disposition.
Silodosin may be used with finasteride with appropriate waiver authority noted for
silodosin. See Silodosin Paper.
Gen
Simvastatin
Zocor
Hyperlipidemia
X
Waiver not required if on single approved statin medication for hyperlipidemia.
Approved medications include simvastatin, pravastatin, lovastatin and rosuvastatin
up to 40 mg/day and atorvastatin up to 80 mg/day. Higher doses or combination of
medication requires waiver. Requires at least 5 day ground trail when starting
medication or for any adjustments to dosage to rule out idiosyncratic reactions.
Follow up of lipids and LFTs should conform to accepted practice standards.
X*
X*
*Combination therapy with Gemfibrozil is limited to a FCIIA or RPA Pilot waiver
by MAJCOM/AFMSA and may not be further delegated.
Endo
Sitagliptin
Januvia
Diabetes with
normal renal
function
X*
X
Max dose 100 mg daily. *Not waiverable for FCI. Only approved for FCIIC (no
single-seat aircraft). Submit for waiver after patient has been on medication for at
least 30 days and the requirements for waiver submission (as defined by the
Diabetes Waiver Guide) have been met. All FCII require AFMSA waiver. For all
FCIII and GBC MAJCOM may disposition the waiver. Note: initial waiver for the
diagnoses still resides at AFMSA. See sitagliptin paper.
Gen
Spironolactone
Aldactone
Hirsutism, hyper-
aldosteronism
(2
nd
line)
X
X
Submit for waiver after potential for idiosyncratic reaction has been ruled out and
control is maintained. FCIIA or RPA Pilot waiver only. Eplerenone and
Spironolactone Background Paper.
ENT
Steroids
(nasal)
Mild allergic,
non-allergic, or
vasomotor
rhinitis
X
Length of DNIF dictated by time required for adequate control of underlying
symptoms.
Derm
Steroids
(topical)
Rash or skin
disease
(acute usage)
X
DNIF until potential for idiosyncratic reaction has been ruled out and condition does
not interfere with flying duties. Usage is for acute conditions, less than 4 weeks,
and condition does not require waiver.
Derm
Steroids
(topical)
Rash or skin
diseases
(chronic usage)
X
X
Submit for waiver after potential for idiosyncratic reaction has been ruled out and
control is maintained.
Pulm
Steroids
(inhaled orally)
Asthma
X
X
All inhaled corticosteroids approved for use in asthma by the FDA as of 13 May
2012 may be used. Submit for waiver after potential for idiosyncratic reaction has
been ruled out and control is maintained.
GI
Steroids
(metered-dose inhaler)
Eosinophilic
Esophagitis
X
X
Topical corticosteroid therapy, administered via metered-dose inhaler (swallowed),
is approved for treatment of eosinophilic esophagitis. Submit for waiver after
potential for idiosyncratic reaction has been ruled out and control is maintained –
see EoE Waiver Guide.
GI
Sucralfate
Carafate
Prevention of
recurrent,
uncomplicated
duodenal ulcer
X
X
1 gram once daily. Submit for waiver after potential for idiosyncratic reaction has
been ruled out and control is maintained.
Note: (1) Members pending waiver action must remain DNIF until waiver has been granted. 15
(2) Medications not on this list, singly or in combination, require review by AFMSA/SG3/5PF (rated officers) and MAJCOM/SG (non-rated personnel).
(3) Verbal waivers are NOT authorized.
(4) Waivers for non-FDA approved medications will not be considered.
Approved by AF/SG3/5P on 13 Jun 2017
7700 Arlington Blvd., Falls Church, VA 22042-5158
Category
Medication
Diagnosis
No
DNIF
(No Waiver Required)
DNIF
(Waiver Required)
Notes
Generic Name (Oral
Preparation Unless
Specified Otherwise)
Trade Name
(Not All
Inclusive)
or
Utilization
DNIF
For
Ground
Trial
Symptoms
Controlled
(No Side
Effect)
Flying
I/II/
RPA
Pilot
Flying
III/
GBC
Rheum
Sulfasalazine
Azulfidine
Reactive arthritis,
rheumatoid
arthritis
X
X
Submit for waiver after potential for idiosyncratic reaction has been ruled out and
control is maintained. Only authorized for RA cases that show no progression of
disease (only 10% of cases). Mesalamine is better choice for inflammatory bowel
disease control.
Derm
Tacrolimus 0.1%
ointment
Protopic
Eczema, psoriasis
X
X
Topical formulations only.
Submit for waiver after potential for idiosyncratic reaction has been ruled out and
control is maintained. AFMSA retains waiver authority.
See Waiver Guide for additional details
Onc
Tamoxifen
Soltamox,
Nolvadex
Breast cancer
prophylaxis
X
X
Use for breast cancer chemoprophylaxis in coordination with a specialist
experienced in breast cancer chemoprophylaxis only. All other uses require review
on case-by-case basis. Submit for waiver after at least 1 month and stable on
therapy. See Tamoxifen Paper.
GU
Tamulosin
Flomax
BPH
X*
X
Max dose 0.4 mg daily, take 30 minutes after same meal daily. *Not waiverable
for FCI. Limited to FCIIA (restriction from high performance aircraft and fly with
another qualified pilot during critical phases of flight), FCIII and GBC. All
tamulosin waivers for FCII require AFMSA waiver. For all FCIII and GBC the
MAJCOM may disposition. Tamulosin may be used with finasteride with
appropriate waiver authority noted for tamulosin. See Tamulosin Paper.
Derm
Tazarotene
0.1% Gel (topical)
Tazorac
Acne vulgaris
X
DNIF not required unless condition or medication interferes with life support gear
or flying duties. Tazarotene Background Paper
Derm
Tazarotene 0.05% and
0.1% Gel (topical)
Tazorac
Psoriasis
X
X
Submit for waiver after potential for idiosyncratic reaction has been ruled out and
control is maintained. Tazarotene Background Paper
Gen
Telmisartan
Micardis
Hypertension
X
Waiver not required for monotherapy. Minimum 7-day DNIF observation period at
initial treatment and subsequent dose adjustments. Symptom control = BP <140/90.
See HTN Waiver Guide for treatment parameters.
X*
X*
*Combination therapy with HCTZ or other antihypertensive requires waiver.
Combo therapy requires categorical restriction for FCII – see HTN Waiver Guide.
Ops
Temazepam
Restoril
No-go pill
X
OPERATIONAL USE: For the safe performance of mission IAW AF and
MAJCOM policy. Requires ground trial (DNIF for 12 hours after a single dose up
to 30 mg) documented in medical records prior to operational use. Furthermore,
verbal DNIF for 12 hours before resumption of duties is required after each dosage.
Max 7 consecutive days, not to exceed 20 days/60 day period. No-Go Pill Policy
Letter. CLINICAL USE: Requires DNIF for treatment period.
Derm
Terbinafine
Lamisil
Fungal infection,
onychomycosis
X
For treatment of fungal culture or formal histopathologically confirmed fungal
infections only (positive KOH is not acceptable). DNIF for 72 hours ground trial
and obtain baseline LFTs. For pedal onychomycosis: 250 mg daily for 12 weeks.
Terbinafine Background Paper.
GU
Testosterone and
Estrogen
(combination)
Estratest
Hormone
Replacement
Therapy
(menopause)
X
X
Minimum of 7-days ground trial is required. Changes of dosage or preparation
requires an additional 7-day observation period.
GU
Testosterone
(injectable)
Hormone
Replacement
Therapy
X
X
Appropriate urological work-up is required prior to starting medication. Submit for
waiver after potential for idiosyncratic reaction has been ruled out and control is
maintained (minimum 7-day observation after last dose adjustment). A change of
dosage or preparation requires an additional 7-day observation period.
(Note: Testosterone has been classified as a Schedule 3 Controlled Drug).
Note: (1) Members pending waiver action must remain DNIF until waiver has been granted. 16
(2) Medications not on this list, singly or in combination, require review by AFMSA/SG3/5PF (rated officers) and MAJCOM/SG (non-rated personnel).
(3) Verbal waivers are NOT authorized.
(4) Waivers for non-FDA approved medications will not be considered.
Approved by AF/SG3/5P on 13 Jun 2017
7700 Arlington Blvd., Falls Church, VA 22042-5158
Category
Medication
Diagnosis
No
DNIF
(No Waiver Required)
DNIF
(Waiver Required)
Notes
Generic Name (Oral
Preparation Unless
Specified Otherwise)
Trade Name
(Not All
Inclusive)
or
Utilization
DNIF
For
Ground
Trial
Symptoms
Controlled
(No Side
Effect)
Flying
I/II/
RPA
Pilot
Flying
III/
GBC
GU
Testosterone
(transdermal)
Hormone
Replacement
Therapy
X
X
Appropriate urological work-up is required prior to starting medication. Submit for
waiver after potential for idiosyncratic reaction has been ruled out and control is
maintained (minimum 7-day observation after last dose adjustment). A change of
dosage or preparation requires an additional 7-day observation period.
(Note: Testosterone has been classified as a Schedule 3 Controlled Drug).
Derm
Tetracycline
Sumycin
Acne
X
X
Submit for waiver after potential for idiosyncratic reaction has been ruled out and
control is maintained.
Antibiotic
Tetracycline
Sumycin
Acute infection
X
DNIF until potential for idiosyncratic reaction has been ruled out and acute
infectious process is asymptomatic.
GU
Tetracycline
Sumycin
Suppressive
therapy for
chronic or
recurrent
prostatitis/
cystitis
X
X
Submit for waiver after potential for idiosyncratic reaction has been ruled out and
control is maintained.
Ophth
Timolol
(ophth drops)
Timoptic
Glaucoma
X
X
Submit for waiver after potential for idiosyncratic reaction has been ruled out and
control is maintained.
Derm
Tretinoin
(topical)
Retin-A
Acne
X
DNIF not required unless condition or medication interferes with life support gear
or flying duties.
Gen
Triamterene
Dyrenium
Hypertension
X
Monotherapy, or in combination with thiazide diuretic no longer requires waiver.
Minimum 7 – day DNIF observation period at initial treatment and subsequent dose
adjustments. Symptom control = BP < 140/90. See
HTN Waiver Guide for
treatment parameters.
X*
X*
*Combination therapy with ACEi, ARB, or other antihypertensive requires waiver .
Combo therapy requires categorical restriction for FCII – see HTN Waiver Guide.
Antibiotic
Trimethoprim-
Sulfamethoxazole
Bactrim
Acute infection
X
DNIF until potential for idiosyncratic reaction has been ruled out and acute
infectious process is asymptomatic.
Derm
Trimethoprim-
Sulfamethoxazole
Bactrim
Acne
X
X
Submit for waiver after potential for idiosyncratic reaction has been ruled out and
control is maintained.
GU
Trimethoprim-
Sulfamethoxazole
Bactrim
Suppressive
therapy for
chronic or
recurrent
prostatitis /
cystitis
X
X
Submit for waiver after potential for idiosyncratic reaction has been ruled out and
control is maintained.
Neuro
Triptan class of
medications
Imitrex
Zomig
Maxalt
Relpax
Migraine
headaches
X*
X
*Not considered for IFCI/IA, FCII requires a categorical waiver.
Non-injection formulations only. Submit for waiver after potential for idiosyncratic
reaction has been ruled out and control is maintained. Requires ACS Neurology
review on an individual basis. Efficacy and tolerance of triptan on at least 2
migraine episodes must be documented. 24 hour DNIF period after each use.
See Headache Waiver Guide for additional details.
GU
Vaginal Preparation
(creams and
suppositories)
Vaginitis
X
DNIF is not required for occasional OTC use to provide relief from minor self-
limiting conditions unless underlying condition is symptomatic.
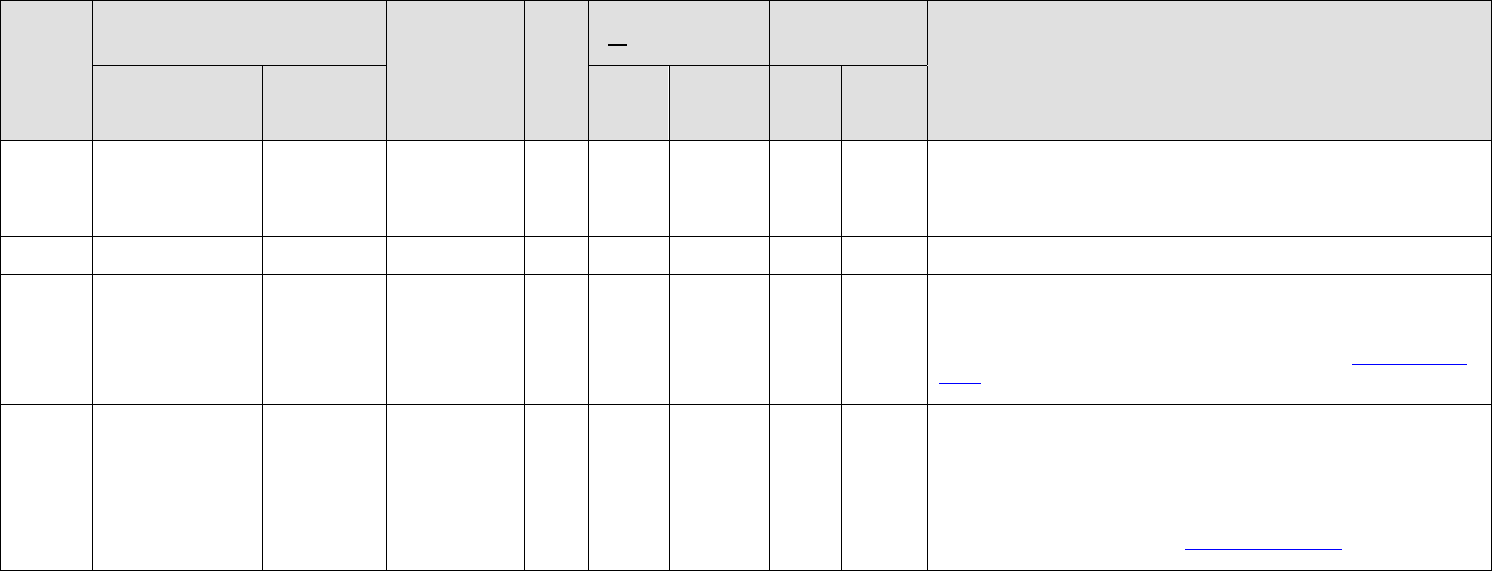
Note: (1) Members pending waiver action must remain DNIF until waiver has been granted. 17
(2) Medications not on this list, singly or in combination, require review by AFMSA/SG3/5PF (rated officers) and MAJCOM/SG (non-rated personnel).
(3) Verbal waivers are NOT authorized.
(4) Waivers for non-FDA approved medications will not be considered.
Approved by AF/SG3/5P on 13 Jun 2017
7700 Arlington Blvd., Falls Church, VA 22042-5158
Category
Medication
Diagnosis
No
DNIF
(No Waiver Required)
DNIF
(Waiver Required)
Notes
Generic Name (Oral
Preparation Unless
Specified Otherwise)
Trade Name
(Not All
Inclusive)
or
Utilization
DNIF
For
Ground
Trial
Symptoms
Controlled
(No Side
Effect)
Flying
I/II/
RPA
Pilot
Flying
III/
GBC
Derm
Valacyclovir
Valtrex
HSV
(suppression)
X
DNIF until the underlying condition will not interfere with flying duties and there
are no adverse side effects (minimum 72 hours). For suppression of HSV
recurrence following regimens recommended: 1. For <10 recurrent episodes per
year – valacylcovir 500 mg q.d. 2. For ≥10 recurrent episodes per year valcyclovir
250 mg bid.
GU
Vardenafil
Levitra
Erectile
Dysfunction
X*
*24 hour DNIF after each dosage (verbal DNIF acceptable).
*Not authorized for daily use.
Ops
Zaleplon
Sonata
No-go pill
X
OPERATIONAL USE: For the safe performance of mission IAW AF and
MAJCOM policy. Requires ground trial (DNIF for 4 hours after a single dose up
to 10 mg) documented in medical records prior to operational use. Furthermore,
verbal DNIF for 4 hours before resumption of duties is required after each dosage.
Max 10 consecutive days, not to exceed 28 days/60 day period.
No-Go Pill Policy
Letter.
CLINICAL USE: Requires DNIF for treatment period.
Ops
Zolpidem
Ambien
No-go pill
X
OPERATIONAL USE: For the safe performance of mission IAW MAJCOM and
AF policy. Requires ground trial (DNIF for 6 hours after a single dose up to 10 mg
for males, 5 mg for females) documented in medical records prior to operational
use. If female aviator ground tested the 10 mg dose prior to 15 May 2013, aviator
may continue with verification of ground testing in medical record. Furthermore,
verbal DNIF for 6 hours before resumption of duties is required after each dosage.
Max 7 consecutive days, not to exceed 20 days/60 day period. Not authorized for
use during routine training missions.
No-Go Pill Policy Letter.
CLINICAL USE: Requires DNIF for treatment period.
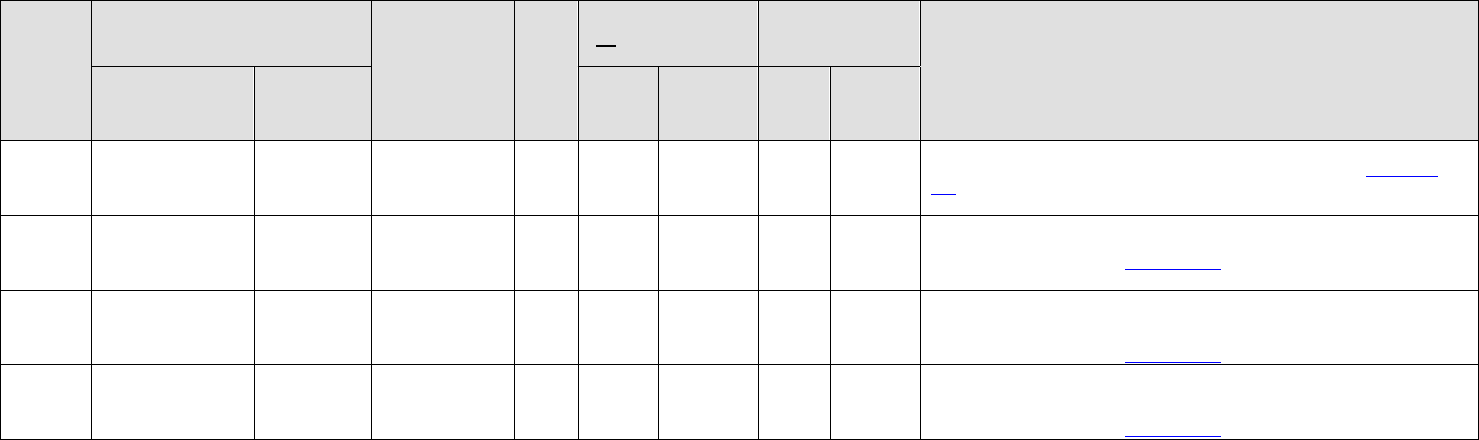
Note: (1) Members pending waiver action must remain DNIF until waiver has been granted. 18
(2) Medications not on this list, singly or in combination, require review by AFMSA/SG3/5PF (rated officers) and MAJCOM/SG (non-rated personnel).
(3) Verbal waivers are NOT authorized.
(4) Waivers for non-FDA approved medications will not be considered.
Approved by AF/SG3/5P on 13 Jun 2017
7700 Arlington Blvd., Falls Church, VA 22042-5158
Air Force Approved Air Sickness Management Program Medications
Over the Counter (OTC) Medications NOT Allowed Without Flight Surgeon Approval
The USAF Airsickness Management Program (AMP) is described in AETC Instruction 48-102. All medication must be taken in accordance with the directions on the
package. No other medications may be taken without consultation with a flight surgeon. These medications may be augmented by natural and non-pharmacologic
techniques in coordination with the flight surgeon. Medication use, efficacy, and side effects should be documented clearly in the medical record and in the AMP reporting
tools. Additionally, final outcome of each case should be documented and tracked for annual reporting to AETC/SGP.
MEDICATION FOR USE BY AIRCREW/SOD IN STUDENT STATUS ONLY, FOR THE TREATMENT OF AIRSICKNESS, AND
ONLY WHILE UNDER DIRECT SUPERVISION. THESE MEDS WILL NOT BE USED FOR TRAINED PERSONNEL.
Category
Medication
Diagnosis
No
DNIF
(No Waiver Required)
DNIF
(Waiver Required)
Notes
Generic Name (Oral
Preparation Unless
Specified
Otherwise)
Trade Name
(Not
Inclusive)
or
Utilization
DNIF
For
Ground
Trial
Symptoms
Controlled
(No Side
Effect)
Flying
I/II
Flying III
Anti-
emetic/
Stimulant
Scopolamine
Scopolamine
Airsickness
X
May be used in conjunction with non-pharmacologic interventions for airsickness
in formal training programs. *Not authorized for solo flight (see AETCI 48-
102). DNIF is not required for dual pilot training sorties. May not be used within 5
sorties of solo flight.
Anti-
emetic/
Stimulant
Scopolamine/
Dextroamphetamine
Transderm-
Scop,
Scopace,
Dexedrine
Airsickness
X
Alone or in combination with dextroamphetamine or in conjunction with non-
pharmacologic interventions for airsickness in formal training programs. *Not
authorized for solo flight (see
AETCI 48-102). DNIF is not required for dual pilot
training sorties. May not be used within 5 sorties of solo flight.
Anti-
emetic/
Stimulant
Promethazine 25mg/
dextroamphetamine 5
mg
Phenergan,
Dexedrine,
ProCentra
Airsickness
X
Specifically for airsickness in formal AETC aircrew training programs. DNIF is
not required for dual pilot training sorties. May not be used within 5 sorties of solo
flight. May be used in conjunction with non-pharmacologic interventions. *Not
authorized for solo flight (see AETCI 48-102).
Anti-
emetic/
Stimulant
Promethazine 25 mg/
Ephedrine 25 mg
Phenergan,
Ephedra, Herb
má huáng
Airsickness
X
Specifically for airsickness in formal AETC aircrew training programs. DNIF is
not required for dual pilot training sorties. May not be used within 5 sorties of solo
flight. May be used in conjunction with non-pharmacologic interventions. *Not
authorized for solo flight (see AETCI 48-102).
Note: (1) Members pending waiver action must remain DNIF until waiver has been granted. 19
(2) Medications not on this list, singly or in combination, require review by AFMSA/SG3/5PF (rated officers) and MAJCOM/SG (non-rated personnel).
(3) Verbal waivers are NOT authorized.
(4) Waivers for non-FDA approved medications will not be considered.
Approved by AF/SG3/5P on 13 Jun 2017
7700 Arlington Blvd., Falls Church, VA 22042-5158
Non-Waiverable Medications On This Page
Toxin
Botulinum
Toxin
Botox
Cosmetic
When used for facial cosmetic purposes, side effects may include visual blurring,
ptosis, corneal ulceration, diplopia. For treatment of non-cosmetic issues, ACS eval
is required.
Gen
Bupropion
Zyban
Smoking
cessation
Approved Jan 2012 for use with waiver in FCIII for depression. Use in smoking
cessation not approved.
Antibiotic
Ciprofloxacin
Cipro
Other than BW
prophylaxis
Unacceptable CNS excitability. DNIF during treatment. BW prophylaxis against
inhalational anthrax authorized only (risk/benefit compared with CNS excitability
must be considered at the operational level). Cipro Policy Letter
Gen
Depo-Medrol
Allergy
Condition requiring injectable steroid is reasons for grounding.
Derm
Isotretinoin
Accutane
Acne
Anxiety, irritability, anger, or depression.
Derm
Itraconazole
Sporanox
Fungal infection
Negative ionotropic effects. Aviators using this fungistatic medication must be
grounded for the duration of therapy plus 1 week for the wash out period due to its
long half life. Pulse therapy requires 2 week grounding per pulse (1 week during
treatment plus 1 week wash out period)
Gen
Mefloquine
Lariam
Malaria
prophylaxis
Adverse effects include but not limited to: optic neuritis, cataracts, decreased night
vision, blurred vision and photosensitivity, seudotumor cerebri, depression,
psychosis, and suicide.
Gen
Melatonin
Insomnia
Nightmares, headaches, morning grogginess, and mild depression.
Derm
Minocycline
Minocin
Acne
Unacceptable (up to 70%) incidence of vestibular side-effects.
Derm
Minoxidil
Rogaine
Hair Loss
Hypotension.
Gen
Niacin
Hyperlipidemia
Dizziness, headache, shortness of breath.
Gen
Steroid (systemic)
Inflammatory
diseases
DNIF for duration of therapy – any regimen in excess of three weeks requires
documentation of intact adrenal axis. See waiver guide for additional details.
Gen
Varenicline
Chantix
Smoking
cessation
While primarily thought to be a nicotine agonist, also has dopaminergic activity.
FDA issued and Early Communication (Nov 2007) about an ongoing safety review
after receiving reports of suicidal thoughts and aggressive and erratic behavior in
patients who had taken Chantix.
Non-Waiverable Medications On This Page
Not Waiverable
