
Washington State
Health Care Authority
Medicaid Provider Guide
A Guide to Prescription Drug Program
(Chapter 182-530 WAC)
January 1, 2014
A Billing Instruction
About this guide
This publication by the Health Care Authority (agency) supersedes all previous Prescription
Drug Program Medicaid Provider Guides published by the agency.
Note: The underlined words and phrases are links in this guide. Some are internal, taking you to
a different place within the document, and some are external to the guide, leading you to
information on other websites.
What has changed?
Reason for
Change
Effective
Date
Subject
Change
PN 13-107,
13-108, 13-
110, and
14-04
January 1,
2014
How can I verify a
patient’s eligibility?
Add update information about verifying
patient eligibility.
Does a provider
need agency
approval to bill for
splitting single dose
vials?
Clarify how to submit documentation for
agency approval to bill for splitting
single dose vials.
How are agency-
covered vaccines
and vaccine
administration fees
billed?
Add text to clarify how to bill for the
administration of vaccines for influenza,
pneumonia, and the administration of the
medication Zostavax®.
Billing for vaccine
administration
Add HCPCS code 90471 for the
Administration of Zostavax vaccine.
Update Maximum Allowable fees for the
administration of vaccines.
Alpha-Agonist
Age/Dose Limits
Add table for dose limits for clients 17
years of age and younger for combined
doses of alpha agonists.
Newer
Anticoagulants
Specify dosage limits for Xaralto®.
Oral, Transdermal,
and Intra-Vaginal
Contraceptives
Clarify the dispensing and supply
requirements for contraceptive products.
Cough/cold drug
coverage
Replace the covered cough/cold product
list table with a link to a web page.
Medicare Part B
crossover claims
Remove sentence regarding extending
the billing period for certain claims.
Reason for
Change
Effective
Date
Subject
Change
Does the agency
reimburse for
client’s
prescriptions when
enrolled in an
agency managed
care plan?
Clarify information regarding how clients
enrolled in a Managed Care Organization
(MCO) are to fill prescriptions for
selected classes of drugs.
Billing for managed
care clients
Update the information about how to bill
prescriptions fee-for-service.
Medicare Part D
Remove information about Medicare
copayments for prescriptions because the
agency no longer provides coverage for
Medicare Part D copayments.
How can I get agency provider documents?
To download and print agency provider notices and Medicaid provider guides, go to the agency’s
Provider Publications website.
Copyright disclosure
Current Procedural Terminology copyright 2012 American
Medical Association. All rights reserved. CPT is a registered
trademark of the American Medical Association.
Fee schedules, relative value units, conversion factors and/or
related components are not assigned by the AMA, are not part of
CPT, and the AMA is not recommending their use. The AMA does
not directly or indirectly practice medicine or dispense medical
services. The AMA assumes no liability for data contained or not
contained herein.

Prescription Drug Program
Alert! The page numbers in this table of contents are now “clickable”—do a “control + click” on a page
number to go directly to a spot. As an Adobe (.pdf) document, the guide also is easily navigated by
using bookmarks on the left side of the document. If you don’t immediately see the bookmarks,
right click on the gray area next to the document and select Page Display Preferences.
Click on the bookmark icon on the left.)
-i-
Table of Contents
Resources Available ......................................................................................................................... 1
Troubleshooting ............................................................................................................................... 2
Definitions ......................................................................................................................................... 5
About the Program ........................................................................................................................ 11
What is the purpose of the prescription drug program? ..............................................................11
What are the provider requirements? ..........................................................................................12
Important notes ...........................................................................................................................12
Client Eligibility ............................................................................................................................. 14
What types of identification prove eligibility? ...........................................................................14
How can I verify a patient’s eligibility? .....................................................................................15
What if a claim is denied by the point-of-sale (POS) system? ...................................................16
Are clients enrolled in an agency managed care plan eligible for pharmacy services? ..............16
Program Restrictions ..................................................................................................................... 17
How does the agency determine which drugs to cover? .............................................................17
What drugs, devices, and supplies are covered? .........................................................................17
What drugs, devices, and supplies are not covered? ...................................................................19
What are the exceptions to the prescription requirement? ..........................................................23
When does the agency pay for over-the-counter (OTC) nicotine replacement therapy
(NRT)? ..................................................................................................................................23
Compliance Packaging .................................................................................................................. 24
What is included in compliance packaging? ...............................................................................24
How is it determined that a client is eligible for compliance packaging? ..................................24
What is required when billing for compliance packaging? ........................................................25
Compounded Prescriptions ........................................................................................................... 27
What is compounding? ...............................................................................................................27
Which ingredients are not reimbursed in compounds? ...............................................................27
Is authorization required to compound prescriptions? ................................................................28
Billing for Compounded Prescriptions ........................................................................................ 29
Special Programs/Services ............................................................................................................ 30
Who is included in the agency's Smoking Cessation program? .................................................30

Prescription Drug Program
Alert! The page numbers in this table of contents are now “clickable”—do a “control + click” on a page
number to go directly to a spot. As an Adobe (.pdf) document, the guide also is easily navigated by
using bookmarks on the left side of the document. If you don’t immediately see the bookmarks,
right click on the gray area next to the document and select Page Display Preferences.
Click on the bookmark icon on the left.)
-ii-
How does a pharmacy bill the agency for Clozaril/Clozapine and related services? .................32
What is the Patient Review and Coordination (PRC) program? .................................................35
What is the pharmacy’s role in the PRC Program? ....................................................................37
What happens if a restricted client goes to a non-assigned pharmacy? ......................................38
How are agency-covered vaccines and vaccine administration fees billed? ..............................39
Which vaccines are covered and if they are available free from DOH? .....................................39
How must a pharmacy bill the agency for influenza, pneumonia, and Zostavax®
vaccine?.................................................................................................................................40
How does the agency reimburse for human papillomavirus (HPV) vaccine? ............................41
What form is used to bill for pre-filling syringes? ......................................................................41
What special drug initiatives, projects, and services are available? ...........................................42
ADHD (attention deficit hyperactivity disorder) drug initiatives ........................................ 42
Safety Edit – AGE ......................................................................................................................42
Alpha-Agonist Age/Dose Limits ......................................................................................... 45
Newer Anticoagulants .......................................................................................................... 46
Cough/cold drug coverage ................................................................................................... 50
Authorization for leukotriene modifiers .............................................................................. 52
Authorization for Proton Pump Inhibitors (PPIs) ................................................................ 53
Narcotic Review Project ................................................................................................................ 54
What does the Narcotic Review Project do for clients? .............................................................54
How were the opioid dosing guidelines developed? ...................................................................54
Does the agency cover Over-the-Counter (OTC) drugs? ...........................................................55
Sedative/hypnotic restrictions for children .......................................................................... 55
Step therapy for inhaled long-acting beta agonist/corticosteroid combination drugs .......... 55
What is the agency’s criteria Suboxone® (buprenorphine/naloxone) authorization? ................56
Where is information available for Synagis®? ...........................................................................57
What are the authorization rrequirements for Vivitrol® (naltrexone IM)? ................................57
What does emergency fill mean? ................................................................................................57
Does the agency pay for Hemophilia - and von Willebrand-related products for home
administration? ......................................................................................................................58
What are the criteria to become a Qualified Hemophilia Center of Excellence (COE)? ...........58
Why is there an annual documentation requirement? .................................................................59
Authorization.................................................................................................................................. 60
When does the agency require authorization? ............................................................................60
How do I obtain authorization? ...................................................................................................61
What information must a pharmacist have ready before calling the agency for an
authorization number? ..........................................................................................................61
Who determines authorization status for drugs in the agency’s drug file? .................................62
How is authorization status determined for drugs in the agency’s drug file? .............................62
What authorization status may be assigned to a drug? ...............................................................63

Prescription Drug Program
Alert! The page numbers in this table of contents are now “clickable”—do a “control + click” on a page
number to go directly to a spot. As an Adobe (.pdf) document, the guide also is easily navigated by
using bookmarks on the left side of the document. If you don’t immediately see the bookmarks,
right click on the gray area next to the document and select Page Display Preferences.
Click on the bookmark icon on the left.)
-iii-
How are drugs added to the agency’s drug file? .........................................................................63
Is there a list of drugs that do not require authorization? ...........................................................64
What criteria will justify early refills? ........................................................................................64
Can clients receive early refills or extended days' supply for travel? .........................................65
Is authorization required for brand name drugs? ........................................................................66
What is an exception to rule (ETR)?...........................................................................................66
What is expedited authorization (EA)? .......................................................................................67
Reimbursement .............................................................................................................................. 69
What in general does the agency need to process a reimbursement for services? ......................69
How does the point of sale system (POS) establish reimbursement rates? ................................70
How does the agency use the estimated acquisition cost (EAC)? ..............................................70
How are federal upper limits calculated? ....................................................................................71
How is the automated maximum allowable cost (A-MAC) calculated? ....................................71
When is the state maximum allowable cost (S-MAC) applied? .................................................71
How is tax computed? .................................................................................................................72
Does the agency pay dispensing fees for non-drug items? .........................................................72
How is the drug rebate program used? .......................................................................................73
Billing .............................................................................................................................................. 74
What are the general instructions for billing? .............................................................................74
When does the tamper-resistant prescription pad requirement apply? .......................................75
What is the requirement? ............................................................................................................75
How are clients enrolled in managed care affected? ..................................................................76
What about emergency dispensing? ............................................................................................76
What about Medicaid clients with retroactive certification? ......................................................76
What are the documentation and records retention requirements? .............................................77
What is needed for prescription transfers between pharmacies? ................................................77
What is the time limit for billing? ...............................................................................................77
What is the national provider identifier (NPI) requirement? ......................................................79
What is needed to bill for filling a newborn prescription? .........................................................80
When is a pharmacy allowed to bill a client? .............................................................................80
Who is eligible? ..........................................................................................................................81
What services are billed for hospice clients? ..............................................................................81
Does the agency reimburse for client’s prescriptions when enrolled in an agency
managed care plan? ...............................................................................................................82
Billing for managed care clients .......................................................................................... 83
What drugs may be prescribed for Family planning only and TAKE CHARGE clients? .........83
Does the agency reimburse for skilled nursing facility (SNF) clients? ......................................85
How are medications filled for SNF clients on leave? ...............................................................85
What is an emergency kit? ..........................................................................................................86
What unit dose delivery systems are recognized by the agency? ...............................................86

Prescription Drug Program
Alert! The page numbers in this table of contents are now “clickable”—do a “control + click” on a page
number to go directly to a spot. As an Adobe (.pdf) document, the guide also is easily navigated by
using bookmarks on the left side of the document. If you don’t immediately see the bookmarks,
right click on the gray area next to the document and select Page Display Preferences.
Click on the bookmark icon on the left.)
-iv-
How do pharmacies become eligible for a unit dose dispensing fee? ........................................86
How do pharmacies bill the agency under a unit dose delivery system? ....................................87
Who is responsible for the cost of repackaging client’s bulk medications? ...............................87
What records do SNF pharmacies need to keep? ........................................................................88
What needs to be submitted annually to the agency? .................................................................88
What additional records do pharmacies need to keep? ...............................................................89
Coordination of Benefits................................................................................................................ 91
How are client resources applied? ..............................................................................................91
Other coverage codes ..................................................................................................................... 92
Why are other coverage codes important? ..................................................................................92
When may providers use other coverage codes? ........................................................................92
How is authorization obtained for nonformulary or noncovered drugs? ....................................95
Coordination of Benefits Frequently Asked Questions (FAQ) .................................................. 96
How is prescription drug coverage verified and who processes the prescriptions? ...................96
What if a client’s insurance states there is no coverage or the insurance coverage has
ended? ...................................................................................................................................96
What if a client’s insurance plan cannot identify the client? ......................................................96
What is discount only or mail order only coverage? ..................................................................97
Why would a claim be paid at zero or denied by insurance? ......................................................97
What if the insurance states copay is 100% or claim is paid at zero? ........................................98
How are after hour services billed? ............................................................................................98
What is “meeting client’s immediate needs?” ............................................................................98
What is the service area? .............................................................................................................98
What if POS will not accept an Other Coverage Code, or a field is not provided to enter
Other Coverage Code? ..........................................................................................................98
Why does a claim get a rejection code DV (MISSING/INVALID OTHER PAYER
AMOUNT PAID) or E8 (MISSING/INVALID OTHER COVERAGE CODE)
when billing the balance to the agency? ...............................................................................99
If the claim does not go through, is entering $.01 in the insurance paid field allowed? ............99
When can Other Coverage Code 8 be used? ...............................................................................99
How is a claim submitted to the agency when the insurance allowed amount is less than
or equal to the copay amount? ............................................................................................100
What is a closed pharmacy network? ........................................................................................100
Does the agency require clients to use pharmacy providers that are contracted with the
client’s private insurance carrier? .......................................................................................101
What if a client’s insurance coverage requires paper billing and the pharmacy only bills
electronically? .....................................................................................................................101
How are paper bills submitted to the agency after the primary insurance has been
billed? ..................................................................................................................................101

Prescription Drug Program
Alert! The page numbers in this table of contents are now “clickable”—do a “control + click” on a page
number to go directly to a spot. As an Adobe (.pdf) document, the guide also is easily navigated by
using bookmarks on the left side of the document. If you don’t immediately see the bookmarks,
right click on the gray area next to the document and select Page Display Preferences.
Click on the bookmark icon on the left.)
-v-
If the client is enrolled in an agency-contracted managed care organization and private
insurance, is the MCO billed for the service or the private insurance? ..............................102
If I bill the insurance carrier and the denial reason is “plan limits exceeded,” can I bill
the agency with an Other Coverage Code? .........................................................................102
How do I bill if the insurance carrier requires authorization? ..................................................102
The insurance carrier requires authorization. The prescriber will not provide
information to the pharmacy or insurance carrier and authorization cannot be
obtained. Can the agency be billed directly? ......................................................................103
How long does documentation need to be kept? ......................................................................103
The client has insurance coverage through multiple carriers. Am I required to bill all
potential payers? .................................................................................................................103
How are clients billed who are eligible for both Medicare and Medicaid? ..............................105
Medicare Part B ................................................................................................................. 105
Part B—Medical Insurance ................................................................................................ 106
Medicare part B medications ............................................................................................. 106
Medicare part D ................................................................................................................. 107
What if Medicare denies a prescription as nonformulary? .......................................................108
Hardcopy Billing: Pharmacy Statement form HCA 13-714 ................................................... 109
What are the general instructions for billing? ...........................................................................109
Hardcopy Billing: CMS-1500 Claim Form............................................................................. 112
Electronic Billing .......................................................................................................................... 113
What is point-of-sale (POS)? ....................................................................................................113
What do the POS rejection codes mean? ..................................................................................113
What is the prospective drug use review (Pro-DUR) used for? ................................................117
What is the national drug code (NDC)? ....................................................................................117
NCPDP Version D.0 Claim Format ........................................................................................... 118
What transaction segments are supported? ...............................................................................118
Therapeutic Interchange Program ............................................................................................. 121
What is the therapeutic interchange program? ..........................................................................121
What is an endorsing practitioner? ...........................................................................................121
What does this mean to pharmacies? ........................................................................................121
When are substitutions not required? ........................................................................................122
What if a non-endorsing practitioner issues a prescription for a nonpreferred drug? ..............122
How does the pharmacy bill for a DAW prescription written by an endorsing
practitioner? ........................................................................................................................123
Washington Preferred Drug List ................................................................................................ 124

Prescription Drug Program
Alert! The page numbers in this table of contents are now “clickable”—do a “control + click” on a page
number to go directly to a spot. As an Adobe (.pdf) document, the guide also is easily navigated by
using bookmarks on the left side of the document. If you don’t immediately see the bookmarks,
right click on the gray area next to the document and select Page Display Preferences.
Click on the bookmark icon on the left.)
-vi-
What is the Washington preferred drug list? ............................................................................124
What is the process to obtain drugs on the Washington PDL? .................................................124
What are the authorization criteria that must be met to obtain a nonpreferred drug? ..............125
Where is the Washington Preferred Drug List? ........................................................................125
Prescription Drug Program
CPT® codes and descriptions only are copyright 2013 American Medical Association.
-1-
Resources Available
Topic
Resource Information
Becoming a provider or
submitting a change of
address or ownership.
See the agency’s Resources Available web page.
Finding out about payments,
denials, claims processing, or
agency-contracted managed
care organizations.
Electronic or paper billing.
Finding agency documents
(e.g., Medicaid provider
guides, provider notices, and
fee schedules).
Private insurance or third-
party liability, other than
agency-contracted managed
care.
Authorization
Website for pharmacy
information.
See the agency’s Pharmacy website.
Backup documentation
Backup documentation ONLY must be mailed or faxed to:
Pharmacy Authorization Section
Drug Use and Review
PO Box 45506
Olympia WA 98504-5506
Fax: 866-668-1214
Technical questions about
switch vendor issues or
system availability issues
Contact the switch vendor.
Where can I find Pharmacy
Document Submission Cover
Sheets?
See the agency’s Document Submission Cover Sheets.
Prescription Drug Program
-2-
Troubleshooting
If your situation is:
Then you must:
Claim rejection stating Prior
Authorization required or Call
800-562-3022
Use Pharmacy Information Authorization, form 13-835a.
Fax form to 866-668-1214 or
Call 800-562-3022
Claim rejection starting with
Pref or Preferred
Refill too soon or early refill
Call the Medical Assistance Customer Service Center
(MACSC) at 800-562-3022.
When you call, you must know:
When was the last fill for this client?
Was this a change in dose from the last fill?
Find out which drugs are on
the Washington Preferred
Drug List (PDL)
See the Washington Preferred Drug List (WPDL)
Any of the following return
messages:
Prior authorization required
Expedited code required and
does not meet criteria
Drug exceeds limits.
Use Pharmacy Authorization form, HCA 13-835a.
See the Pharmacy Information webpage for:
Expedited authorization criteria
List of Drugs with Limitations
Special programs in this provider guide
Fax form to 866-668-1214 or
Call 800-562-3022
Dispensed an emergency
supply to a client with an
emergency that could not wait.
Use Pharmacy Information Authorization form, HCA 13-835a
and fax to 866-668-1214; or
Call 800-562-3022
Dispense as Written (DAW)
Reimbursement less than cost for
a DAW prescription.
Reimbursement less than cost
Reimbursement less than cost for
a prescription that is substitution
Prescription Drug Program
-3-
If your situation is:
Then you must:
permitted.
Claim rejection stating “Client
is restricted to one pharmacy”
What pharmacy or doctor is this
client restricted to?
Call Medical Assistance Customer Service Center (MACSC)
at 800-562-3022 (option 2).
Claim rejection stating Client
is restricted to one pharmacy
How to get medically
necessary medications to a
client restricted to a different
pharmacy.
Where to report clients
abusing their medications.
Where to report suspected
fraudulent activity.
Call MACSC at 800-562-3022 (extension 51780).
Lost or stolen medications
Has the client reported a lost or
stolen prescription in the last 6
months?
Call MACSC at 800-562-3022.
Prescription Drug Program
-4-
If your situation is:
Then you must:
Expedited Authorization
criteria
See the agency’s Expedited Authorization List.
Other claim- or pharmacy-
related questions or situations:
Appropriate use of NCPDP
fields in response to claim
edits.
Is this client eligible?
What program is this client
on?
Where can clients or doctor’s
offices call for questions
about authorizations or
drugs?
What drugs are covered?
What is the TIP program?
How to become an endorsing
prescriber.
Where to find a list of over
the counter family planning
products.
Call MACSC at 800-562-3022 or visit the Pharmacy
Information web page.
Prescription Drug Program
-5-
Definitions
This list defines terms and abbreviations, including acronyms, used in this provider guide. See
the agency’s Medical Assistance Glossary for a more complete list of definitions.
Active Ingredient – The chemical
component of a drug responsible for a
drug’s prescribed/intended therapeutic
effect. The agency limits coverage of active
ingredients to those with a national drug
code (NDC) and those specifically
authorized by the agency.
Actual Acquisition Cost – The actual price
a provider paid for a drug marketed in the
package size of drug purchased, or sold by a
particular manufacturer or labeler. The
actual acquisition cost is calculated based on
factors including, but not limited to:
Invoice price, including other invoice-
based considerations, such as prompt
payment discounts.
Order quantity and periodic purchase
volume discount policies of suppliers
(wholesalers and/or manufacturers).
Membership/participation in purchasing
cooperatives.
Advertising and other promotion/display
allowances, free merchandise deals.
Transportation or freight allowances.
Administer – the direct application of a
prescription drug by injection, inhalation,
ingestion, or any other means, to the body of
a patient by a practitioner, or at the direction
of the practitioner.
Appointing Authority – For the evidence-
based prescription drug program of the
participating agencies in the state-operated
health care programs, the following persons
acting jointly: the Director of the Health
Care Authority (HCA or the agency), and
the director of the Department of Labor and
Industries (L&I).
Automated Maximum Allowable Cost
(A-MAC) – The rate established by the
Medicaid Purchasing Administration (MPA)
for a multiple-source drug that is not on the
maximum allowable cost (MAC) list and
that is designated by two or more products,
at least one of which must be under a federal
drug rebate contract.
Authorization Number – A number
assigned by the agency that identifies a
specific request for approval for services or
equipment.
Authorization Requirement - A condition
of coverage and reimbursement for specific
services or equipment, when required by
WAC or Medicaid provider guides.
Average Wholesale Price (AWP) - The
average price of a drug product that is
calculated from wholesale prices nationwide
at a point in time and reported to the agency
by the agency’s drug file contractor.
Brand Name - The proprietary or trade
name selected by the manufacturer and
placed upon a drug, its container, label, or
wrapping at the time of packaging.
Prescription Drug Program
-6-
Closed Pharmacy Network - An
arrangement made by an insurer which
restricts prescription coverage to an
exclusive list of pharmacies. This
arrangement prohibits the coverage and/or
payment of prescriptions provided by a
pharmacy that is not included on the
exclusive list. (WAC 182-530-7800)
Code of Federal Regulations (CFR) –
Rules adopted by the federal government.
Combination drug – A commercially
available drug including two or more active
ingredients.
Compliance packaging – Reusable or non-
reusable drug packaging containers (e.g.,
Mediset, bingo cards, blister packs).
Compounding - The act of combining two
or more active ingredients or adjusting
therapeutic strengths in the preparation of a
prescription.
Contract Drugs - Drugs manufactured or
distributed by manufacturers/labelers who
have signed a drug rebate agreement with
the federal Department of Health and
Human Services (DHHS).
Covered Outpatient Drug - A drug
approved for safety and effectiveness as a
prescription drug under the federal Food,
Drug, and Cosmetic Act, which is used for a
medically accepted indication.
Dispensing Fee – The fee the agency sets to
pay pharmacy providers for dispensing
agency-covered prescriptions. The fee is the
agency’s maximum payment for expenses
involved in the practice of pharmacy and is
in addition to the agency’s reimbursement
for the costs of covered ingredients.
Drug Enforcement Agency - (DEA) -
federal agency responsible for enforcing
laws and regulations governing narcotics
and controlled substances.
Drug File – A list of drug products, pricing,
and other information provided to the
agency’s drug database and maintained by a
drug file contractor.
Drug Rebates – Payments provided by
pharmaceutical manufacturers to state
Medicaid programs under the terms of the
manufacturers’ agreements with the
Department of Health and Human Services.
Drug-Related Supplies – Non-drug items
necessary for the administration, delivery, or
monitoring of a drug or drug regimen.
Drug Use Review (DUR) – A review of
covered outpatient drugs that assures
prescriptions are appropriate, medically
necessary, and not likely to result in adverse
medical outcomes.
Emergency kit - A set of limited
pharmaceuticals furnished to a nursing
facility by the pharmacy that provides
prescription dispensing services to that
facility. Each kit is specifically set up to
meet the needs of each nursing facility’s
client population and is for use during those
hours when pharmacy services are
unavailable.
Endorsing Practitioner - A provider who
has reviewed the Washington preferred drug
list (PDL) and has enrolled (see
www.rx.wa.gov) with the Health Care
Authority (HCA), agreeing to allow
therapeutic interchange (substitution) of a
preferred drug for any non-preferred drug in
a given therapeutic class on the Washington
PDL.
Prescription Drug Program
-7-
Estimated Acquisition Cost (EAC) – The
agency’s estimate of the price providers
generally and currently pay for a drug
marketed or sold by a particular
manufacturer or labeler.
Evidence-Based Practice Center – A
research organization that has been
designated by the agency for Healthcare
Research and Quality (AHRQ) of the U.S.
government to conduct systematic reviews
of all the evidence to produce evidence
tables and technology assessments to guide
health care decisions.
Federal upper limit (FUL) – The
maximum allowable payment set by the
Centers for Medicare and Medicaid Services
(CMS) for a multiple-source drug.
Federally approved hemophilia treatment
center - A hemophilia treatment center
(HTC) which:
(1) Receives funding from the U.S.
Department of Health and Human
Services, Maternal and Child Health
Bureau National Hemophilia
Program.
(2) Is qualified to participate in 340B
discount purchasing as an HTC.
(3) Has a U.S. Center for Disease
Control (CDC) and prevention
surveillance site identification
number and is listed in the HTC
directory on the CDC website.
(4) Is recognized by the Federal
Regional Hemophilia Network that
includes Washington State.
(5) Is a direct care provider offering
comprehensive hemophilia care
consistent with treatment
recommendations set by the Medical
and Scientific Advisory Council
(MASAC) of the National
Hemophilia Foundation in their
standards and criteria for the care of
persons with congenital bleeding
disorders.
Immediate Needs – An emergency situation
when pharmacists to use their professional
judgment in determining the quantity to
dispense to best meet the client’s needs in
the emergency.
Generic name – The official title of a drug
or drug ingredients published in the latest
edition of a nationally recognized
pharmacopoeia or formulary.
Less-than-effective drug or Drug Efficacy
Study Implementation (DESI) – Those
drugs that lack substantial evidence of
effectiveness as determined by the Food and
Drug Administration (FDA).
(WAC 182-530-1050)
Maximum Allowable - The maximum
dollar amount the agency will reimburse a
provider for a specific service, supply, or
piece of equipment.
Maximum Allowable Cost (MAC) - The
maximum amount that the agency
reimburses for a specific dosage form and
strength of a multiple-source drug product.
Prescription Drug Program
-8-
Medically Accepted Indication – Any use
for a covered outpatient drug:
(1) Which is approved under the federal
Food, Drug, and Cosmetic Act.
(2) The use of which is supported by one or
more citations included or approved for
inclusion in any of the following
compendia of drug information.
(a) The American Hospital Formulary
Service Drug Information
(b) The United States Pharmacopoeia
Drug Information
(c) DRUGDEX Information System
Medically Necessary – See WAC 182-500-
0005
Modified Unit Dose Delivery System (also
known as blister packs or “bingo/punch
cards”) - A method in which each patient's
medication is delivered to a nursing facility:
In individually sealed, single-dose
packages or "blisters".
In quantities for one month's supply,
unless the prescriber specifies a shorter
period of therapy.
Multiple-Source Drug - A drug marketed
or sold by:
Two or more manufacturers or labelers.
The same manufacturer or labeler:
Under two or more different
proprietary names.
-Or-
Under a proprietary name and a
generic name.
National Drug Code (NDC) - The 11-digit
number the manufacturer or labeler and
FDA assigns to a pharmaceutical product
and attaches to the product container at the
time of packaging. The NDC is composed of
digits in 5-4-2 groupings. The first five
digits comprise the labeler code assigned to
the manufacturer by the FDA. The second
grouping of four digits is assigned by the
manufacturer to describe the ingredients,
dose form, and strength. The last grouping
of two digits describes the package size.
Non-Contract Drugs - Drugs manufactured
or distributed by manufacturers/labelers who
have not signed a drug rebate agreement
with the federal Department of Health and
Human Services (DHHS).
Non-Formulary Drug – Medications that
are not on the primary insurance plan’s
formulary (preferred) drug list.
Non-Preferred Drug – A drug that has not
been selected as a preferred drug within the
therapeutic class (es) of drugs on the
preferred drug list.
Obsolete NDC – An NDC replaced or
discontinued by the manufacturer or labeler.
Other Coverage Code – A billing code that
indicates whether or not a client has other
insurance coverage. If the client has
coverage, use of the code identifies how the
claim was processed by the insurance
carrier.
Over-the-Counter (OTC) Drugs – Drugs
that do not require a prescription under
federal law before they can be sold or
dispensed.
Prescription Drug Program
-9-
Pharmacist - A person licensed in the
practice of pharmacy by the state in which
the prescription is filled.
Pharmacy - Every location licensed by the
State Board of Pharmacy in the state where
the practice of pharmacy is conducted.
Point-of-Sale (POS) - A pharmacy claims
processing system capable of receiving and
adjudicating claims on-line.
Poly-Prescribing – Multiple prescribers
duplicating drug therapy for the same client.
Practitioner – An individual who has met
the professional and legal requirements
necessary to provide a health care service,
such as a physician, nurse, dentist, physical
therapist, pharmacist or other person
authorized by state law as a practitioner.
Preferred Drug – Drug(s) of choice within
a selected therapeutic class that are selected
based on clinical evidence of safety,
efficacy, and effectiveness.
Prepay Plan – A type of insurance coverage
that requires the client to pay at the time of
service, and the insurance reimbursement is
made to the subscriber/client.
Privately Purchased HMO – Indicates a
client with a privately purchased HMO
insurance policy. ProviderOne indicates that
the client is enrolled in a managed health
care plan. These clients must comply with
the requirements of their plan and are
required to use the HMO facilities for their
pharmacy services.
Prescriber – A physician, osteopathic
physician/surgeon, dentist, nurse, physician
assistant, optometrist, pharmacist, or other
person authorized by law or rule to prescribe
drugs.
Prescription - An order for drugs or devices
issued by a practitioner authorized by state
law or rule to prescribe drugs or devices, in
the course of the practitioner’s professional
practice, for a legitimate medical purpose.
Prescription drugs - Drugs required by any
applicable federal or state law or regulation
to be dispensed by prescription only or that
are restricted to use by practitioners only.
Prospective Drug Use Review (Pro-DUR)
A process in which a request for a drug
product for a particular client is screened,
before the product is dispensed, for potential
drug therapy problems.
Reconstitution – The process of returning a
single active ingredient previously altered
for preservation and storage, to its
approximate original state. Reconstitution is
not compounding.
Retrospective Drug Utilization Review
(Retro-DUR) - The process in which
client’s drug utilization is reviewed on a
periodic basis to identify patterns of fraud,
abuse, gross overuse, or inappropriate or
unnecessary care.
Service Area – An area within 25 miles or
45 minutes from the client’s residential
address to the pharmacy.
Single Source Drug - A drug produced or
distributed under an original new drug
application approved by the FDA.
Prescription Drug Program
-10-
Skilled Nursing Facility (SNF) - An
institution or part of an institution which is
primarily engaged in providing:
Skilled nursing care and related services
for residents who require medical or
nursing care.
Rehabilitation services for injured,
disabled or sick clients.
Health-related care and services to
individuals who, because of their mental
or physical conditions, require care
which can only be provided through
institutional facilities and which is not
primarily for the care and treatment of
mental diseases. (See Section 1919(a) of
the Federal Social Security Act for
specific requirements.)
Systematic Review – A specific and
reproducible method to identify, select, and
appraise all the studies that meet minimum
quality standards and are relevant to a
particular question. The results of the
studies are then analyzed and summarized
into evidence tables to be used to guide
evidence-based decisions.
Terminated NDC – An NDC that is
discontinued by the manufacturer for any
reason. The NDC may be terminated
immediately due to health or safety issues or
it may be phased out based on the product’s
shelf life.
Therapeutic Alternative – A drug product
that contains a different chemical structure
than the drug prescribed, but is in the same
pharmacologic or therapeutic class and can
be expected to have a similar therapeutic
effect and adverse reaction profile when
administered to patients in a therapeutically
equivalent dosage.
Therapeutic Interchange – To dispense a
therapeutic alternative to a prescribed drug
when written by an endorsing practitioner
who has indicated that substitution is
permitted. See Therapeutic Interchange
Program (TIP).
Therapeutic Interchange Program (TIP)
– The process developed by participating
state agencies under RCW 69.41.190 and
70.14.050, to allow prescribers to endorse a
Washington preferred drug list, and in most
cases, to require pharmacists to
automatically substitute a preferred,
equivalent drug from the list.
Therapeutically Equivalent – Drug
products that contain different chemical
structures but have the same efficacy and
safety when administered to an individual,
as determined by:
Information from the Food and Drug
Administration (FDA).
Published and peer-reviewed scientific
data.
Randomized controlled clinical trials.
Other scientific evidence.
True Unit Dose Delivery - A method in
which each patient’s medication is delivered
to the nursing facility in quantities sufficient
only for the day’s required dosage.
Washington Preferred Drug List
(Washington PDL) – The list of drugs
selected by the appointing authority to be
used by applicable state agencies as the
basis for purchase of drugs in state-operated
health care programs.

Prescription Drug Program
-11-
About the Program
(WAC 182-530-1000)
What is the purpose of the prescription drug
program?
The purpose of the Prescription Drug Program is to pay providers for outpatient drugs, devices,
and drug-related supplies according to agency rules and subject to the limitations and
requirements specified in this guide.
The agency programs are governed by federal and state regulations. This guide is intended to
help providers comply with the rules and requirements of the program.
Basic things to know:
The agency reimburses for medically necessary drugs, devices, and supplies according to rules in
Washington Administrative Code (WAC) and Reimbursement.
The agency covers outpatient drugs, including over-the-counter drugs listed on the agency’s
Covered Over-the-Counter Drug list, when:
The manufacturer has a signed drug rebate agreement with the federal Department of
Health and Human Services (DHHS). (Exceptions to this rule are described in
Compounded Prescriptions.)
Approved by the Food and Drug Administration (FDA).
Prescribed by a provider within the scope of their prescribing authority, and has not had
his/her core provider agreement terminated or denied.
Prescribed for a medically accepted indication.
Prescribed for an eligible client.
Not excluded from coverage under WAC 182-501-0050, 182-530-2100, and the Program
Restriction’s section of this guide. (“What drugs, devices, and supplies are not
covered?”).
The agency does not cover:
Drugs used to treat sexual or erectile dysfunction, in accordance with section
1927(d)(2)(K) of the Social Security Act, unless such drugs are used to treat a condition
other than sexual or erectile dysfunction and these uses have been approved by the FDA.
A drug that is not approved by the FDA.
A drug prescribed for a non-medically accepted indication or dosing level.

Prescription Drug Program
-12-
A drug from a manufacturer without a federal rebate agreement.
Drugs and indications excluded from coverage by Washington Administrative Code
(WAC) such as drugs prescribed for:
Weight loss or gain.
Infertility, frigidity, or impotence.
Sexual or erectile dysfunction.
Cosmetic purposes or hair growth.
What are the provider requirements?
In order to be reimbursed by the agency, the pharmacy must:
Be properly licensed.
Have a signed core provider agreement (CPA).
Follow the guidelines in this guide and applicable WAC.
Retain documentation demonstrating that all other possible payers have been billed
appropriately.
The agency may require a pharmacy to:
Obtain authorization for a drug or product.
Determine and document that certain diagnosis requirements are met.
Meet other requirements for client safety and program management.
Important notes
The following practices constitute an abuse of the program and a misuse of taxpayer dollars:
Prescription splitting: Billing inappropriately to obtain additional dispensing fees. For
example:
Supplying medication in amounts less than necessary to cover the days
prescribed.
Supplying medications in strengths less than those prescribed to gain more than
one dispensing fee.
Excessive Filling: Excessive filling consists of billing for an amount of a drug or supply
greater than the prescribed quantity (except when the agency specifies a mandatory
minimum of an OTC drug).

Prescription Drug Program
-13-
Prescription shorting: Billing for a drug or supply greater than the quantity actually
dispensed.
Substitution to achieve a higher price: Billing for a higher priced drug than prescribed
even though the prescribed lower priced drug was available (except when the agency
identifies a higher-priced drug as preferred).

Prescription Drug Program
-14-
Client Eligibility
What types of identification prove eligibility?
Valid types of eligibility identification:
A copy of the benefit inquiry screen from ProviderOne.
A printout of a medical identification screen from the client's local Community Services
Office (CSO), Home and Community Service (HCS) office, or the agency.
An award letter from the CSO or HCS.
Medical eligibility verification (MEV) receipt provided by an authorized MEV vendor
with an “as of” date within the same month as the date of service.
Note: Providers enrolled with ProviderOne can check eligibility by accessing the
Provider Portal and choosing eligibility inquiry from the main menu. For
information on enrolling visit the New Provider Enrollment web page.
The computer printout or award letter may be used as valid identification since both list the
eligibility information that appears in ProviderOne.
The agency recommends that providers make a photocopy of valid identification when it is
presented, in order to have a copy for the file.
Check the identification for the following information:
Beginning and ending eligibility dates
The ProviderOne Client ID
Other specific information (e.g., Medicare, Medicare Part D, private insurance, or
managed care coverage, hospice, patient requiring regulation, etc.)
Retroactive or delayed certification eligibility dates, if any
Note: Do not accept any form of identification that appears to have been altered.
Request to see another form of identification.
Prescription Drug Program
-15-
How can I verify a patient’s eligibility?
Providers must verify that a patient has Washington Apple Health coverage for the date of
service, and that the client’s benefit package covers the applicable service. This helps prevent
delivering a service the agency will not pay for.
Verifying eligibility is a two-step process:
Step 1. Verify the patient’s eligibility for Washington Apple Health. For detailed
instructions on verifying a patient’s eligibility for Washington Apple Health, see the
Client Eligibility, Benefit Packages, and Coverage Limits section in the agency’s
current ProviderOne Billing and Resource Guide.
If the patient is eligible for Washington Apple Health, proceed to Step 2. If the patient
is not eligible, see the note box below.
Step 2. Verify service coverage under the Washington Apple Health client’s benefit
package. To determine if the requested service is a covered benefit under the
Washington Apple Health client’s benefit package, see the agency’s Health Care
Coverage—Program Benefit Packages and Scope of Service Categories web page.
Note: Patients who are not Washington Apple Health clients may submit an
application for health care coverage in one of the following ways:
1. By visiting the Washington Healthplanfinder’s website at:
www.wahealthplanfinder.org
2. By calling the Customer Support Center toll-free at: 855-WAFINDER
(855-923-4633) or 855-627-9604 (TTY)
3. By mailing the application to:
Washington Healthplanfinder
PO Box 946
Olympia, WA 98507
In-person application assistance is also available. To get information about in-
person application assistance available in their area, people may visit
www.wahealthplanfinder.org or call the Customer Support Center.

Prescription Drug Program
-16-
What if a claim is denied by the point-of-sale
(POS) system?
The POS system does not solve the problem of identifying clients who are not currently in the
agency’s eligibility file. For clients who show as eligible in ProviderOne, but the POS system
denies their claims for lack of eligibility, do one of the following:
FAX a copy of the client’s benefit inquiry screen in ProviderOne to 360-586-1403.
Mail in a completed paper claim with a photocopy of the client’s benefit inquiry screen
in ProviderOne attached.
The agency will update eligibility information from the copies of the client benefit inquiry screen
in ProviderOne within two working days so claims may be resubmitted.
Are clients enrolled in an agency managed care
plan eligible for pharmacy services?
Yes! Clients who are enrolled in an agency managed care plan are eligible for pharmacy
services under their designated plan. Managed care enrollment will be displayed on the client
benefit inquiry screen in ProviderOne.
See Billing for information regarding clients enrolled in an agency managed care plan.
Newborns of clients enrolled in an agency managed care plan are the responsibility of the
mother’s plan for the first 60 days of life. If the mother changes plans, the baby follows the
mother’s plan.
Note: To prevent billing denials, check the client’s eligibility prior to scheduling
services and at the time of the service and make sure proper authorization or referral
is obtained from the plan. See the agency’s ProviderOne Billing and Resource Guide
for instructions on how to verify a client’s eligibility.
Prescription Drug Program
-17-
Program Restrictions
(WAC 182-530-2000 (2))
How does the agency determine which drugs to
cover?
Coverage determinations for the agency are decided by:
The agency in consultation with federal guidelines
The Drug Use Review (DUR) Board.
The agency's medical consultants and pharmacist(s).
If a product is determined to be covered, it will be assigned an authorization status (see
Authorization.)
The agency evaluates a request for a drug that is listed as noncovered under the
provisions of WAC 182-501-0160 related to noncovered services. The request for
a noncovered drug is called a request for an exception to rule. See WAC 182-
501-0160 for information about exception to rule.
What drugs, devices, and supplies are covered?
(WAC 182-530-2000(1))
The agency covers:
Outpatient drugs, including over-the-counter drugs listed on the agency’s Covered Over-
the-Counter Drug list, as defined in WAC 182-530-1050, subject to the limitations and
requirements within this guide, when:
The drug is approved by the Food and Drug Administration (FDA).
The drug is for a medically accepted indication as defined in WAC 182-530-1050.
The drug is not excluded from coverage (see “What drugs, devices, and supplies
are not covered?”).

Prescription Drug Program
-18-
The manufacturer has a signed drug rebate agreement with the federal Department
of Health and Human Services (DHHS). Exceptions to the drug rebate
requirement are described in WAC 182-530-7500 which describes the drug rebate
program.
Family planning drugs, devices, and drug-related supplies per chapter 182-532 WAC
such as:
Over-the-counter (OTC) family planning drugs, devices, and drug-related supplies
without a prescription when the agency determines it necessary for client access
and safety.
Family planning drugs that do not meet the federal drug rebate requirement in
WAC 182-530-7500 on a case-by-case basis.
Contraceptive patches, contraceptive rings, and oral contraceptives, only when
dispensed in at least a three-month supply, unless otherwise directed by the
prescriber. There is no required minimum for how many cycles of emergency
contraception may be dispensed.
Prescription vitamins and mineral products, only as follows:
When prescribed for clinically documented deficiencies
Prenatal vitamins, when prescribed and dispensed to pregnant women
Fluoride varnish for children under the early and periodic screening, diagnosis,
and treatment (EPSDT) program
Drug-related devices and drug-related supplies as an outpatient pharmacy benefit when
they are :
Prescribed by a provider with prescribing authority.
Essential for the administration of a covered drug.
Not excluded from coverage under WAC 182-530-2100.
Determined by the agency, that a product covered under chapter 182-543 WAC
Durable medical equipment and supplies should be available at retail pharmacies.
Note: For exceptions to the prescription (prescriber’s order) requirement, see
Exceptions to the Prescription Requirement.
Preservatives, flavoring and/or coloring agents, only when used as a suspending agent in
a compound.

Prescription Drug Program
-19-
Over-the-counter drugs to promote smoking cessation, without a prescription, only when
the client is:
18 years of age and older.
Participating in an agency-approved smoking cessation program.
Prescription drugs to promote smoking cessation, only when the client is:
18 years of age and older.
Participating in an agency-approved smoking cessation program.
What drugs, devices, and supplies are not
covered?
(WAC 182-530-2100 and 182-530-7500)
The agency does not reimburse under the Prescription Drug Program for drugs and drug-related
supplies administered by health care professionals as a component of hospital services,
physician-related services, or billed in conjunction with home health services. Reimbursement
for drugs and drug-related supplies in these situations may be available when billed under the
rules of the related program.
The agency does not reimburse for any of the following under the Prescription Drug Program:
Nutritional supplements such as shakes, bars, puddings, powders, medical foods, etc.
These products may be reimbursable under the conditions of the Nondurable Medical
Supplies and Equipment (MSE) and/or Enteral Nutrition programs.
Drugs when the manufacturer has not signed a rebate agreement with the federal
Department of Health and Human Services.

Prescription Drug Program
-20-
Drugs considered less than effective and withdrawn by the Food and Drug Administration
(FDA) as a result of the Drug Efficacy Study Implementation (DESI) review.
Free pharmaceutical samples.
Drugs (prescription or over-the-counter (OTC)) and drug-related supplies:
Which have not been prescribed by a provider with prescriptive authority (with
the exception of OTC family planning products and OTC smoking cessation
products).
Which have been prescribed by a provider whose application for a Core Provider
Agreement (CPA) has been denied, or whose CPA has been terminated with
cause.
Drugs prescribed for:
Weight loss or gain.
Infertility, frigidity, or impotence.
Sexual or erectile dysfunction.
Cosmetic purposes or hair growth.
OTC drugs which are not listed on the agency’s Covered Over-the-Counter Drug list.
Drugs and drug-related supplies for multiple patient use.
Any drug regularly supplied as an integral part of program activity by other public
agencies (such as drugs, vaccines, or biological products available without charge to the
client from the Department of Health).
Products or items that do not have an 11-digit national drug code (NDC).
Drugs with NDCs which have been designated as obsolete for more than two years.
Drugs whose shelf life has expired prior to being dispensed.
Drugs which have been terminated or removed from the market.
More than a 34-day supply of any product except:
Drugs when the smallest package size exceeds a 34-day supply.
Drugs with special packaging instructions which would require dispense of a
quantity that exceeds a 34-day supply.
Prescription Drug Program
-21-
Contraceptive patches, contraceptive rings, and oral contraceptives not used for
emergency contraception. These products must be dispensed at a minimum of a
three-month supply, unless otherwise directed by the prescriber.
When the drug is specifically identified as exempt from the 34-day limit.
Any vitamin product other than:
Prenatal vitamins prescribed to pregnant women.
Vitamins determined by the agency to be the least costly therapeutic alternative
for the treatment of a client’s diagnosed condition.
When the agency agrees that the vitamin product is the least costly alternative in
treating documented vitamin deficiency which has been confirmed by laboratory
testing.
Fluoride preparations other than as prescribed for children under the Early and Periodic
Screening, Diagnosis, and Treatment (EPSDT) program.
Non-preferred drugs in drug classes in the Washington Preferred Drug List (PDL), except
as detailed in WPDL.
Drugs, biological products, insulin, supplies, appliances, and equipment included in other
reimbursement methods including, but not limited to:
Diagnosis-related group (DRG)
Ratio of costs-to-charges (RCC)
OTC products supplied to Skilled Nursing Facility (SNF) residents (unless
included in the Washington PDL)
Managed care capitation rates
Block grants
Drugs prescribed for clients who are in the agency’s Hospice program when the
drugs are related to the client’s terminal condition.

Prescription Drug Program
-22-
Drugs prescribed for an indication that is not evidence-based as determined by:
The agency in consultation with federal guidelines.
The Drug Use Review (DUR) Board.
Agency medical consultants and pharmacist(s).
Drugs that are:
Not approved by the Food and Drug Administration (FDA).
Prescribed for non-FDA approved indications or dosing, which is not otherwise
supported by quality evidence in the recognized compendia of drug information.
Unproven for efficacy or safety.
Outpatient drugs for which the manufacturer requires as a condition of sale that
associated tests or monitoring services be purchased exclusively from the manufacturer
or manufacturer’s designee.
Preservatives, flavoring, and/or coloring agents.
Prescriptions written on pre-signed prescription blanks completed by SNF operators or
pharmacists. The agency may terminate the CPA of pharmacies involved in this practice.
Drugs used to replace those taken from SNF emergency kits.
The cost differential between the least costly dosage form of a drug and a more expensive
dosage form within the same route of administration, unless the prescriber designated the
costlier dosage form as medically necessary.
Over-the-counter or prescription drugs to promote smoking cessation unless the client is
18 years of age and older and participating in an agency-approved smoking cessation
program.
Prescription Drug Program
-23-
What are the exceptions to the prescription
requirement?
(WAC 182-530-2000(4))
Over-the-counter family planning products
The agency reimburses specific OTC family planning drugs, devices, and supplies without a
prescription. The following OTC contraceptives may be dispensed without a prescription to any
agency client with a current Services Card:
Condoms (including female condom)
Vaginal spermicidal foam with applicator and refills
Vaginal jelly with applicator
Vaginal creams and gels
Vaginal suppositories
Emergency contraception (Plan B) is also available without a prescription for females 18 years of
age and older.
BILLING
Point-of-Sale billers must:
Bill the agency fee-for-service using the Product ID Qualifier of 03 in field 436-
E1, and the product-specific NDC number in field 407-D7. Use Prescriber ID
Qualifier (466-EZ) 01 and Prescriber ID (407-D7) of 5123456787. Regardless of
the contraceptive, bill the NDC as stated on the package.
Hardcopy billers must:
Enter 5123456787 in the Prescriber NPI field.
When does the agency pay for over-the-counter
(OTC) nicotine replacement therapy (NRT)?
The agency reimburses for specific OTC NRT products without a prescription when distributed
by an agency-approved smoking cessation program (see Smoking Cessation).
Prescription Drug Program
-24-
Compliance Packaging
The agency, the Home Care Association of Washington (HCAW), and the Washington State
Pharmacy Association (WSPA) developed the following guidelines in a cooperative effort to
improve drug therapy outcomes for the most at-risk segment of the medical assistance population.
What is included in compliance packaging?
(WAC 182-530-7400(2))
Compliance packaging includes both of the following:
Reusable, hard plastic containers of any type (e.g., Medisets, weekly minders, etc.).
Non-reusable compliance packaging (e.g., blister packs, bingo cards, bubble packs, etc.).
How is it determined that a client is eligible for
compliance packaging?
(WAC 182-530-7400(1))
Prescribers are encouraged to communicate to high-risk clients the need for compliance packaging
if, in their professional judgment, such packaging is appropriate.
Clients are considered high-risk and eligible to receive compliance packaging if they:
Do not reside in a skilled nursing facility or other inpatient facility.
Have one or more of the following representative disease conditions:
Alzheimer's disease
Blood clotting disorders
Cardiac arrhythmia
Congestive heart failure
Depression
Diabetes
Epilepsy
HIV/AIDS
Hypertension
Schizophrenia
Tuberculosis
-AND-
Prescription Drug Program
-25-
Concurrently consume two or more prescribed medications for chronic medical
conditions that are dosed at three or more intervals per day.
Demonstrate a pattern of noncompliance that is potentially harmful to the client’s health.
The client’s pattern of noncompliance with the prescribed drug regimen must be fully
documented in the provider’s file.
Prefilling a syringe is not considered compliance packaging. See Special Programs/Services
for Syringe Filling Guidelines.
What is required when billing for compliance
packaging?
To bill for compliance packaging:
1. Bill on an approved professional services claim form (e.g., paper CMS-1500 claim form;
electronic CMS-1500 claim form; or electronic 837-P claim form).
2. Include the NPI of the ordering practitioner in the ‘referring’ field. The ordering
practitioner is the prescriber or pharmacist who determined the client meets compliance
packaging criteria.
3. Bill your usual and customary charge. Reimbursement will be the billed charge or the
maximum allowable fee, whichever is less.
4. Use the following procedure codes in combination with the appropriate modifier. The
agency will deny claims for these procedure codes without the accompanying modifier.
Short Description
HCPCS
Code
Modifier
Maximum Allowable Fee
Reusable compliance
device or container
T1999*
UE
$6.00 maximum per device
(limit of 4 per client, per year).
Reusable compliance
device or container,
extra-large capacity
T1999*
SC
$16.91 maximum per device (limit of 4 per
client, per year).
Filling fee for a
reusable compliance
device or container
A9901
SC
$2.50 per fill (limit of 4 fills per client, per
month).
Non-reusable
compliance device or
container
T1999
NU
$3.00 (limit of 4 fills per client, per month.)
Includes reimbursement for materials and filling
time. Bill one unit each time non-reusable
compliance packages are filled.
* May be billed in combination but not to exceed a total of 4 per year.
The agency does not pay for compliance packaging in excess of the limits listed above. Requests
for limitation extensions will not be approved.

Prescription Drug Program
-26-
Does a provider need agency approval to bill for
splitting single dose vials?
Yes. Providers must obtain agency approval to bill for splitting single dose vials. To receive agency
approval, submit the following documentation by fax to the attention of the Pharmacy Administrator,
at 360-725-1328.
Documentation showing all requirements of the United States Pharmacopeia General Chapter
797, Pharmaceutical Compounding - Sterile Preparations regulations are met, including the
date of the last laminar flow hood inspection and through date of the certification,
The policy you have established regarding IV admixture preparations,
The policy you have established regarding when single dose vials are split and how the
remainder is to be used.
The billing NPI(s) of the requesting provider.
The agency will provide an approval or denial of your request within 10 business days.

Prescription Drug Program
-27-
Compounded Prescriptions
(WAC 182-530-7150)
What is compounding?
(WAC 182-530-7150(1)(3))
Compounding is the act of combining two or more active ingredients or the medically necessary
adjustment of therapeutic strengths and/or forms by a pharmacist for a single active ingredient.
The agency does not consider drug reconstitution to be compounding. The agency reimburses
pharmacists for compounding drugs only if the client’s drug therapy needs are unable to be met
by commercially available dosage strengths and/or forms of the medically necessary drug.
Note: All compound ingredients must be billed on one claim. Each ingredient must be
separately detailed using the National Council for Prescription Drug Programs (NCPDP)
Compound Segment. The agency’s Point-of-Sale (POS) system does not accept highest
cost ingredient compound billing.
Note: The pharmacist must document in the client’s file the need for the adjustment of
the drug’s therapeutic strength and/or form.
Which ingredients are not reimbursed in
compounds?
(See WAC 182-530-7150(2))
Coloring agents, preservatives, and flavoring agents used in compounded prescriptions
except when they are necessary as a complete vehicle for compounding (e.g., simple
syrup).
Any product which would not be reimbursable when used outside of a compound, except
as detailed on the following page.
Prescription Drug Program
-28-
What additional ingredients are reimbursable in compounds?
Bulk chemicals which are active ingredients and are considered non-drug items when
used outside of a compound.
Vehicles or suspending agents necessary for the completion of the compound.
The agency reimburses for compounding ingredients from the following chemical supply
companies who have not signed Federal Rebate agreements:
Labeler Code
Company
00395
Humco Labs
00802
Emerson Labs
10106
J T Baker
17317
Amend
49452
A-A Spectrum
Note: Other chemical suppliers’ products are reimbursable only if they have
been reported to the agency’s current drug file contractor with a valid 11-digit
national drug code (NDC) and the manufacturer has signed a Federal Rebate
agreement.
Is authorization required to compound
prescriptions?
(WAC 182-530-7150(5)(b) and (c))
No. The agency does not require authorization to compound prescriptions.
Individual ingredients requiring authorization still require authorization when used in a
compound, except as previously noted.
The need for authorization of any single ingredient within a compound will cause the entire
compound claim to be rejected until authorized, but only the individual ingredient actually
requires authorization.
Prescription Drug Program
-29-
Billing for Compounded
Prescriptions
(WAC 182-530-7150(4) and (5))
Pharmacies must bill each ingredient used in compounded prescriptions using the 11-
digit NDC for each ingredient.
Bill the appropriate quantity used for each ingredient on one claim. Do not bill the
combined total quantity.
The agency pays a dispensing fee for each payable ingredient. The agency does not pay
separate fees for compounding time or preparation fees.
Note: If a compound is rejected, pharmacies may elect to accept reimbursement
for any payable ingredient within the compound by entering an 8 in the
Submission Clarification Code field (420-DK).
BILLING ILLING
Hard copy billers must:
Complete the Pharmacy Statement (525-106) form, 13-714, using Section 1 for
information regarding the entire compound.
Enter National Drug Code of 00000-0000-00 in Section 1, and individual
ingredient NDCs in Section 3.
Enter “Compound Prescription” in the Justification/Comments field.
Do not complete Section 2. Only one compound may be billed per claim form.
Point-of-Sale billers must:
Enter a Compound Code (field 406-D6) of 2 in the Claim Segment;
Enter a Product/Service ID Qualifier (436-E1) of 03 in the Claim Segment;
Enter a Product/Service ID (407-D7) of 00000-0000-00 in the Claim Segment;
Enter the separate ingredient details using the Compound Segment.
Prescription Drug Program
-30-
Special Programs/Services
Who is included in the agency's Smoking
Cessation program?
Client eligibility
The agency’s Smoking Cessation Program includes eligible fee-for-service clients who meet the
coverage requirements. Smoking cessation is not a covered benefit for clients eligible under the
Family Planning Only, TAKE CHARGE, or Alien Emergency Medical programs.
Coverage requirements during pregnancy
For eligible fee-for-service clients who are pregnant, the agency will cover smoking cessation
products through the Free and Clear Program or through a pharmacy.
The agency pays for prescription and over-the-counter smoking cessation products through a
pharmacy for pregnant women when the client meets both of the following criteria:
Is pregnant with a verifiable estimated due date (EDD).
Is receiving smoking cessation counseling from the prescribing provider.
For pregnant clients receiving smoking cessation products through a pharmacy, treatment is
limited to two courses of therapy over a calendar year. For limits on specific smoking cessation
products see the list of Drugs with Limitations.
Pharmacists with a collaborative practice agreement may provide smoking cessation counseling
and prescribe for pregnant clients. For counseling requirements, limitations, billing information,
and resources see the Physician-Related Services/Health Care Professional Services Medicaid
Provider Guide.
Coverage requirements for all clients
The agency covers smoking cessation for all eligible fee-for-service clients through the Free and
Clear Program which includes:
Over-the-counter drugs, without a prescription, to promote smoking cessation only
when all of the following requirements are met:
The client is 18 years of age and older.

Prescription Drug Program
-31-
The client is participating in an agency-approved smoking cessation program.
The product is distributed by the Free and Clear Program.
Prescription drugs to promote smoking cessation, only when the client meets the
following requirements:
Is 18 years of age and older.
Participating in an agency-approved smoking cessation program.
The agency covers the following smoking cessation drugs:
Nicotine gum
Nicotine Transdermal Patches
Bupropion SR (Zyban®)
Chantix® (varenicline tartrate).
The agency does not allow combinations within or across smoking cessation drug types.
Coverage limitations and restrictions
The agency’s limitations and restrictions for smoking cessation drugs are as follows:
The required use of Free & Clear Inc. behavior modification for all smoking cessation
drug therapy. You may contact Free & Clear Inc. toll-free at: 800-QUIT NOW (800-784-
8669).
The limitation of all smoking cessation drugs to 12 weeks per year, per client.
The agency will authorize bupropion SR (Zyban®) only if a client does not have a
history of seizures or bipolar disorder.
The agency will authorize Chantix® (varenicline tartrate) only if the client does not have
a history of neuropsychiatric symptoms and dosage reductions are based on renal
clearance.
Nicotine replacement therapy (NRT)
The agency contracts with Free & Clear Inc. to provide the nicotine replacement therapy (NRT)
only as follows:
No combination within NRT delivery systems or in combination with prescription
smoking cessation drugs is allowed.
NRT coverage includes only transdermal patches and gum.
NRT must be in conjunction with behavioral modification.
NRT is limited to 12 weeks per year, per client.

Prescription Drug Program
-32-
How does a pharmacy bill the agency for
Clozaril/Clozapine and related services?
The agency reimburses pharmacies for Clozaril/Clozapine plus pays a dispensing fee. Bill
Clozaril/Clozapine using the appropriate national drug code (NDC) on either the Point-of-Sale
(POS) system or the Pharmacy Statement form, HCA 13-714.
Any licensed or registered pharmacy with clinical experience in monitoring patient mental and
health status may provide and bill for case coordination (medication management) for clients
receiving Clozaril/Clozapine.
Persons providing case coordination serve as a focal point for the client’s Clozaril/Clozapine
therapy. All services must be documented and are subject to quality assurance review. When
providing case coordination, providers must:
Coordinate a plan of care with the:
Client
Client’s caregiver
Prescriber
Pharmacy
Assure services are provided to the client as specified in the plan of care.
Assure blood samples are drawn according to the Food and Drug Administration (FDA)
labeling, blood counts are within normal range, and the client is compliant with the plan
of care.
Follow-up with the client on missed medical appointments.
Maintain detailed, individual client records to document the client's progress.
Provide feedback to the prescriber on the client’s progress, immediately report abnormal
blood counts, and client noncompliance.
Assure smooth transition to a new case coordinator, when necessary.
Prescription Drug Program
-33-
Use the following procedure codes to bill for Clozaril/Clozapine related services on an approved
professional services claim form (e.g., paper CMS-1500 claim form; electronic CMS-1500 claim
form; or electronic 837-P claim form):
Procedure
Code
Modifier
Description
Reimbursement
36415
Routine
Venipuncture
Per the Resource-Based Relative Value
Scale (RBRVS) fee schedule
99605
HE
Case coordination
for new clients.
Prescribed by RSN
must be entered in
the comments field
when services are
provided through an
RSN Community
Health Center.
$10 per week, per client
99606
HE
Case coordination
for returning clients.
Prescribed by RSN
must be entered in
the comments field
when services are
provided through an
RSN Community
Health Center.
$10 per week, per client
Note: Due to close monitoring requirements, the agency allows up to five (5) fills
per month.
Emergency contraceptive pills (ECP)
The agency reimburses for emergency contraceptive pills (ECP) through the POS system for
female clients in eligible programs as follows:
Clients age 17 years of age and younger must have a prescription for ECP.
Clients age 18 years of age and older do not need a prescription for ECP.
To receive reimbursement, pharmacies must bill the agency fee-for-service (FFS) using the
specific NDC and Prescriber ID number 5123456787. It is common practice to dispense two
packages at a time, especially for clients using barrier contraceptive methods. Pharmacies are
instructed to dispense the quantity requested by the client. Pharmacies that are members of, or
subcontract with, managed care plans and are serving a managed care client must bill the
prescription cost to the plan. The agency reimburses pharmacists for ECP plus pays a dispensing
fee. Bill for ECP using the appropriate NDC.
Prescription Drug Program
-34-
Emergency contraception (EC) counseling
When a pharmacist with an EC protocol approved by the Board of Pharmacy prescribes ECPs,
the pharmacy may bill the agency for the counseling portion.
Pharmacists performing EC counseling must ensure that a copy of the pharmacist’s current
approved protocol certificate from the Board of Pharmacy is on file at the pharmacy where the
service was performed. Performing EC Counseling without a currently approved protocol is
subject to sanction by the Board of Pharmacy. Billing the agency for EC Counseling without a
current, approved protocol on file is subject to recoupment of payment.
The counseling is a service-related item, not a drug, and must be billed on an approved
professional services claim form (e.g., paper CMS-1500 claim form, electronic CMS-1500 claim
form, or electronic 837-P claim form).
BBILLING ON A 1500 claim form ON A 1500 Claim Form
Enter the diagnosis code V25.09 (contraceptive management) in field 24E.
Use the following procedure code and modifier to bill for EC counseling:
Procedure Code
Modifier
Description
Maximum Allowable
Fee
99605
FP
EC Counseling
$13.50
Anti-emetics
Pharmacists with prescriptive authority for emergency contraceptive pills may prescribe and bill
for selected anti-emetics only when these drugs are dispensed in conjunction with ECPs. The
agency reimburses for the following only when they are prescribed and dispensed in the
strength/dose/form listed:
Meclizine hydrochloride 25 mg tablets
Diphenhydramine hydrochloride 25 mg tablets/capsules
Dimenhydrinate 50 mg tablets
Promethazine hydrochloride 25 mg tablets or 25 mg suppositories
Metoclopramide 5 mg, 10 mg tablets
Prochlorperazine 25 mg suppository
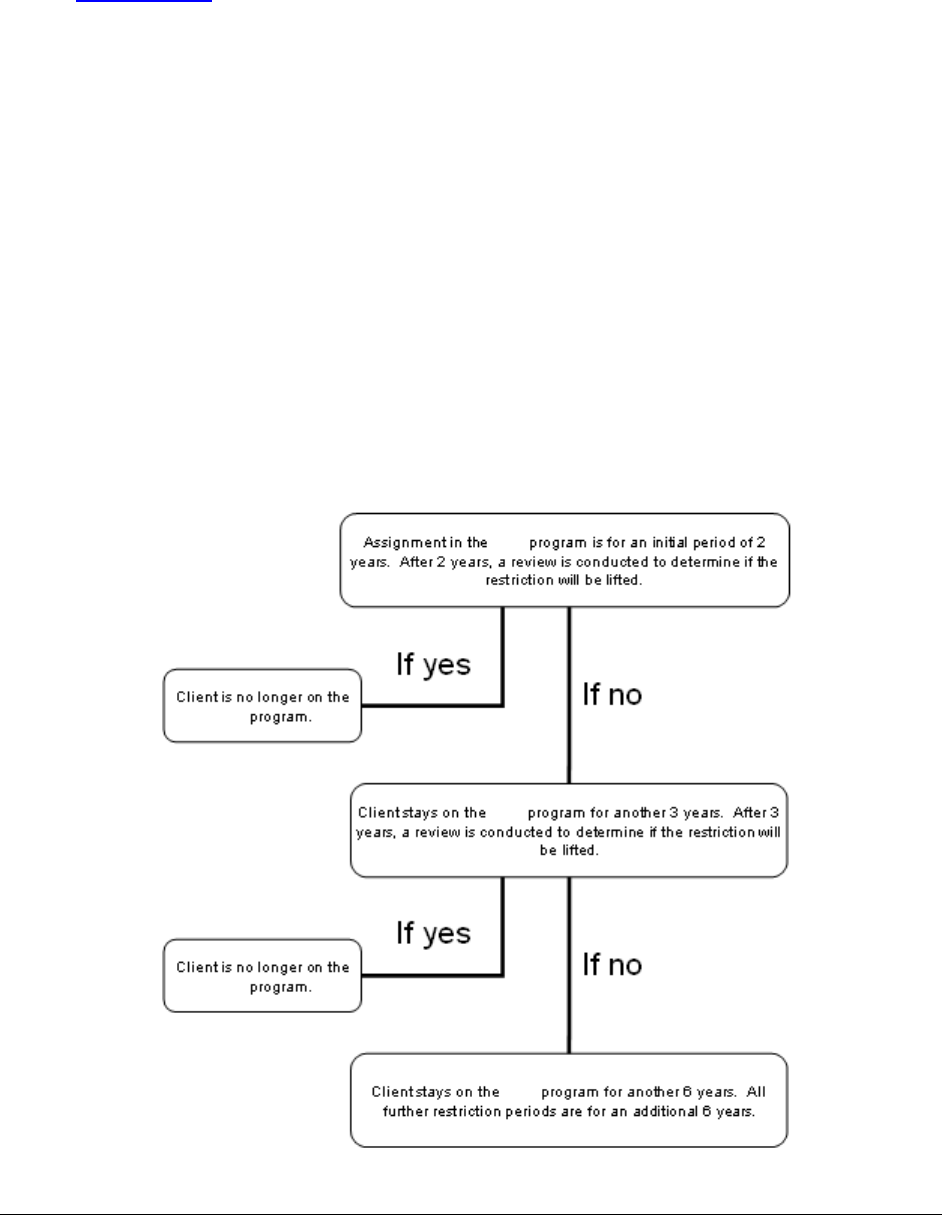
Prescription Drug Program
-35-
What is the Patient Review and Coordination
(PRC) program?
(WAC 182-501-0135(8)(a))
PRC is a health and safety program for fee-for-service (FFS) and managed care clients needing
help in the appropriate use of medical services.
Clients assigned to the PRC program are identified as such in ProviderOne.
When a client is initially placed in the PRC program, the agency or managed care organization
(MCO) places the client for no less than 24 months with one or more of the following types of
health care providers:
Primary care provider (PCP)
Pharmacy for all prescriptions
Prescriber of controlled substances
Hospital for nonemergency services unless referred by the assigned PCP or a specialist. A
client may receive covered emergency services from any hospital
Another qualified provider type, as determined by the agency or MCO staff on a case-by-
case basis
PRC
PRC
PRC
PRC
PRC

Prescription Drug Program
-36-
PRC criteria:
(WAC 182-501-0135(6)(a)-(d))
Agency or MCO staff use the following usage guidelines to initiate a review for PRC placement.
A client may be placed in the PRC program when either the client’s medical history or billing
history, or both, documents any of the following:
Any two or more of the following conditions occurred in a period of 90 consecutive
calendar days in the previous 12-months. The client:
Received services from four or more different providers, including physicians,
ARNPs, and PAs not located in the same clinic or practice.
Had prescriptions filled by four or more different pharmacies.
Received ten or more prescriptions.
Had prescriptions written by four or more different prescribers not located in the
same clinic or practice.
Received similar services on the same day not located in the same clinic or
practice.
Had ten or more office visits.
-OR-
Any one of the following occurred within a period of 90 consecutive calendar days in the
previous 12-months. The client:
Made two or more emergency agency visits.
Exhibits at-risk usage patterns.
Made repeated and documented efforts to seek health care services that are not
medically necessary.
Was counseled at least once by a health care provider, or an agency or an MCO
staff member with clinical oversight, about the appropriate use of health care
services.
-OR-
The client received prescriptions for controlled substances from two or more different
prescribers not located in the same clinic or practice in any one month within the 90-day
review period.

Prescription Drug Program
-37-
-OR-
The client has either a medical history or billing history, or both, that demonstrates a
pattern of the following at any time in the previous 12-months:
Using health care services in a manner that is duplicative, excessive, or
contraindicated.
Seeking conflicting health care services, drugs, or supplies that are not within
acceptable medical practice.
Being on substance abuse programs such as the alcohol and drug abuse treatment
and support act (ADATSA).
What is the pharmacy’s role in the PRC
Program?
The assigned pharmacy is a key player in managing the client’s prescriptions. The pharmacist
will be able to alert the client’s primary care physician (PCP), narcotic prescriber, or the
agency’s PRC staff of misuse or potential problems with the client’s prescriptions.
Since pharmaceuticals are an agency-covered service, do not accept cash from clients except for
drugs not covered by the agency per WAC 182-502-0160.
A major focus of the PRC Program is education. Educating the client on appropriate use of
prescriptions, drug interactions, the importance of maintaining one PCP and pharmacy to manage
and monitor one’s care are key elements in helping the client appropriately utilize services.
Clients who have been in the PRC program have shown a 33% decrease in emergency room use,
a 37% decrease in physician visits, and a 24% decrease in the number of prescriptions.

Prescription Drug Program
-38-
What happens if a restricted client goes to a non-
assigned pharmacy?
If a restricted client goes to a non-assigned pharmacy, the POS system will reject the claim. In
the case of a non-emergency situation, the client should be referred back to their assigned
pharmacy.
Washington State has the prudent layman’s law, in which clients can go to the emergency room
if they think they have a problem and must be seen by the emergency room staff. However,
emergency room prescriptions cannot be overridden in the POS system by a non-assigned PRC
pharmacy. In this situation, the pharmacist may:
Call the PRC referral line during regular business hours (Monday-Friday, 8 a.m. – 5 p.m.) at
360-725-1780 to request an override.
At their discretion in an emergency situation, the pharmacist may fill all medications except
scheduled drugs, unless verification is made with the prescriber that there is a legitimate medical
necessity. Justification for the emergency fill must be provided to the PRC Program the next
business day in order for an override to be completed.
For more information, or to report over-utilization of services, contact:
Patient Review and Coordination (PRC) Program
PO Box 45532
Olympia, Washington 98504-5532
Phone: 800-794-4360, ext. 51780 or 360-725-1780
FAX: 360-725-1969
Visit the agency’s Patient Review and Coordination (PRC) Program website

Prescription Drug Program
-39-
How are agency-covered vaccines and vaccine
administration fees billed?
All covered vaccines other than influenza, pneumonia and Zostavax® must be billed on
the CMS-1500 claim form.
Administration fees must be billed on the CMS-1500 claim form (including influenza,
pneumonia and Zostavax® for clients 18 years of age and younger). The POS does not
have the capability to reimburse for professional services other than dispensing fees.
The agency reimburses qualified pharmacists for the administration of all agency-covered
vaccines for clients on eligible programs.
The agency does not reimburse for any vaccine available free from the Department of
Health (DOH).
Influenza and pneumonia vaccines for adults (19 years of age and older) are reimbursed
through the POS system only.
Clients 18 years of age and younger
The agency pays only the administration fee for any vaccine available at no cost from the
Department of Health (DOH) through the Universal Vaccine Distribution program and the
Federal Vaccines for Children program.
Which vaccines are covered and if they are
available free from DOH?
To check which vaccines are free from the DOH refer to the Injectable Drug Fee Schedule.
Billing for the administration of a vaccine available free from DOH
Bill for the administration of these vaccine(s) with the appropriate procedure code for the
vaccine and use modifier SL (e.g., 90707 SL).
Prescription Drug Program
-40-
Billing for vaccines that are not free from DOH
Bill for the cost of the vaccine with the appropriate procedure code for the vaccine.
Bill for the vaccine administration using CPT® codes 90471 (first vaccination) and
90472 (additional vaccinations). The agency limits reimbursement to a maximum of one
unit of 90471 and one unit of 90472 per client, for the same date of service.
The administration codes must be billed on the same claim as the procedure code for the
vaccine.
DO NOT use modifier SL with these vaccines.
How must a pharmacy bill the agency for
influenza, pneumonia, and Zostavax® vaccine?
Pharmacists must bill for flu and pneumonia vaccines for clients 19 years of age and older with
national drug codes (NDCs) through the Point-of-Sale (POS) system, and for the administration
on the CMS-1500 claim form as follows:
Billing for vaccine administration
The agency pays pharmacists for administering influenza, pneumonia, and Zostavax® (shingles)
vaccinations only if they have an immunization collaborative practice protocol on file with the
Washington State Department of Health (DOH), State Board of Pharmacy. When billing for the
administration of an agency-covered vaccine, the pharmacist’s NPI must be entered in the
Prescriber ID field (411-DB).
Note: Pharmacies may not use their Pharmacy Location NPI as the Prescriber ID in field 411-DB, or
in the Referring/Performing Provider field on 837-P transactions or CMS-1500 claim forms.
Procedure Code
Description
Maximum Allowable Fee
G0008
Administration of influenza virus vaccine
$15.84
G0009
Administration of pneumococcal vaccine
$15.84
90471
Administration of Zostavax® vaccine
$15.84
The agency only pays pharmacies for procedure codes G0008, G0009 and 90471 (administration
codes) when billed with place of service 01 (pharmacy).
Bill the agency for the vaccine administration using only an approved professional services claim
form (e.g., paper CMS-1500 claim form; electronic 1500 claim form; or electronic 837-P claim
form). Vaccine administrations cannot be billed through the pharmacy POS system.
CPT® codes and descriptions only are copyright 2013 American Medical Association.
Prescription Drug Program
-41-
Note: When billing on the CMS-1500 claim form, use the NPI – do not use the
NCPDP number. Continue to bill the influenza, pneumonia or Zostavax® vaccine
itself through the POS system using the NDC.
How does the agency reimburse for human
papillomavirus (HPV) vaccine?
GARDASIL®
The agency reimburses for GARDASIL® (HPV (Types 6,11,16,18) Recombinant Vaccine). See
the Immunizations in Physician-Related Services/Healthcare Professional Services Medicaid
Provider Guide.
What form is used to bill for pre-filling syringes?
Fees for pre-filling syringes may be billed on an approved professional services claim form (e.g.,
paper CMS-1500 claim form; electronic CMS-1500 claim form; or electronic 837-P claim form).
These fees are not billable on POS.
Each unit billed must be for a two-week supply
The maximum number of units allowed per month is three
Use the following HCPCS code:
Note: If additional fills are necessary for dose adjustment, indicate the comment
Dose adjustment required in field 19 (Reserved for Local Use field) of the
CMS-1500 claim form.
If additional fills are necessary due to multiple prescriptions/types, indicate the
comment, Multiple prescriptions/types required in field 19 (Reserved for
Local Use field) of the CMS-1500 claim form.
If an emergency fill is necessary resulting in less than a two-week supply, indicate
the comment Emergency fill in field 19 of the CMS-1500 claim form.
Description
HCPCS
Code
Maximum
Allowable
Pharmacy compounding and dispensing
services (to be used for pre-filling
syringes)
S9430
$10.00 per unit

Prescription Drug Program
-42-
What special drug initiatives, projects, and
services are available?
The agency has developed targeted drug initiatives to:
Guide appropriate drug therapy.
Improve therapeutic outcomes.
Improve the quality of life for agency clients.
The agency has specific services that:
Help identify potentially dangerous drug therapy.
Reduce duplication of therapy.
Reduce poly-prescribing.
Assist providers in complex clinical decision-making.
Examples of these services are described below:
ADHD (attention deficit hyperactivity disorder) drug
initiatives
The agency promotes the safe and effective use of ADHD medication in children. Specific areas
include the use of medication in children younger than five years of age and appropriate dosing
limits in the prescribing of these medications. The agency’s ADHD program helps safeguard
clients receiving ADHD drugs when:
The clients are 5 years of age and younger.
The dose exceeds the recommended maximum dosage limits or when drug combinations
are prescribed outside the guidelines established by the statewide Mental Health
Stakeholders Workgroup in 2006.
Safety Edit – AGE
Five years of age and younger
ADHD medications prescribed for children five years of age and younger require authorization
and an agency-approved second opinion. When the patient is already taking the ADHD drug, the
agency will authorize continuation for 90-days while the second opinion is taking place.
Prescribers of new ADHD prescriptions for children five years of age and younger are required
to obtain the agency-approved second opinion prior to submitting an authorization request to the
agency. See the Second Opinion Network Provider List.
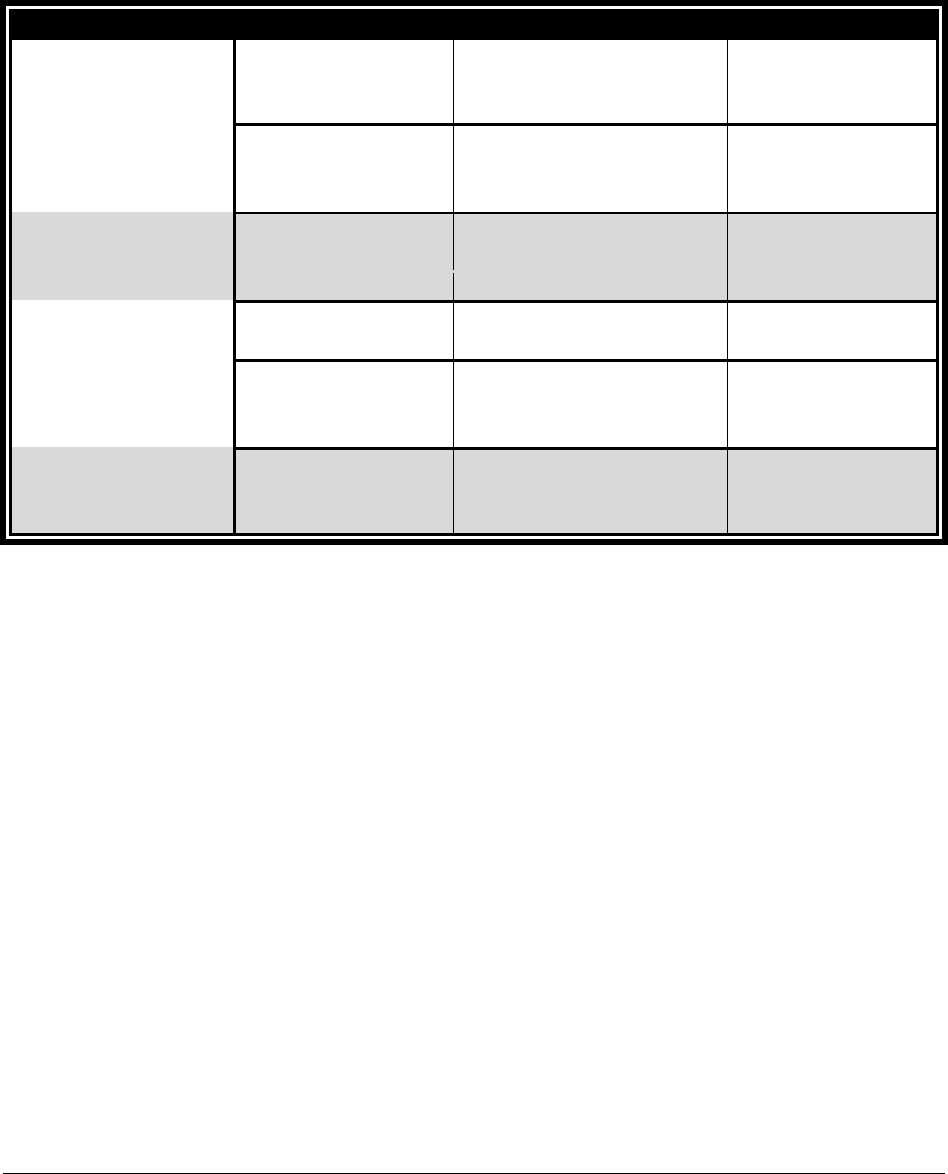
Prescription Drug Program
-43-
Safety Edit - DOSAGE
Dosing limits for clients five years of age and older:
Adult and child dosing greater than the following requires review:
Drug Class
Form
Dosage
Limitation
Methylphenidates:
Daytrana®
transdermal patch
30mg per day for clients
unable to take oral
medications.
See dosage.
Long acting forms
120mg per day as a single
daily dose
Total of 120mg per
day across long and
short acting form.
All other forms
120 mg per day.
Dexmethylphenidate:
Long acting forms
60mg per day as a single
daily dose
Total of 60mg per
day across long and
short acting forms.
All other forms
60mg per day.
Amphetamines:
Vyvanse®
70mg per day as a single
daily dose.
See dosage.
Long acting forms
60mg per day as a single
daily dose
Total of 60mg per
day across long and
short acting forms.
All other forms
60mg per day.
Atomoxetine:
All forms
120mg per day as a single
or twice daily divided
dose.
See dosage.
Children 18 years of age and younger require an agency-approved second opinion and
agency authorization. Adult doses exceeding the limits require authorization by the
Medical Director, or his designee, who will review clinical chart notes that must show:
Less risk than usual care.
Less cost to the state.
The next step in reasonable care, including tried and failed FDA dosing.
Refills above dose limits are authorized until the review is completed.
Initiation of therapy above these dose limits requires review prior to payment.
When the patient is already taking the medication and the authorization request is denied, the
agency will allow one additional refill (up to a 34-day supply) of medication for the purpose of
tapering the dose to fall within the accepted limits stated above. New prescriptions exceeding the
recommended doses for children 18 years of age and younger require the recommendations of an
agency-designated Mental Health Specialist from a Second Opinion Network Provider.
Prescription Drug Program
-44-
Safety Edit – ADHD DRUG COMBINATIONS
Combinations across drug types (e.g., methylphenidate with amphetamine) require
authorization.
Combinations of Strattera with stimulant ADHD drugs require authorization.
Continuation of a combination is authorized for a maximum of 30-days while tapering a
client off of a drug.
The chart below shows the drugs the Mental Health Stakeholders Workgroup has determined to
be duplicative. The squares marked with an X indicate the combinations that will require
authorization after 34-days of concurrent therapy.
Combinations of Medications in Two or More ADHD Categories
Methylphenidate
Dexmethylphendidate
Amphetamines
Strattera®
Methylphenidate
X
X
X
Dexmethylphendidate
X
X
X
Amphetamines
X
X
X
Strattera®
X
X
X
The agency reimburses combinations of short-acting and long-acting forms of the same ADHD
drug without authorization. The agency requires the pharmacy to request authorization for a
combination of ADHD medications across drug categories. When the pharmacy requests
authorization, the agency will contact the prescriber to obtain medical justification for the
combination therapy.
Alcohol and Substance Abuse Pilot Project
The agency has expanded its drug and alcohol assessment and treatment services. The agency
can better help address the very complex issue of abuse and addiction that some agency client’s
face.
Starting with a four-county pilot project in Yakima, Clark, Spokane, and Pierce Counties, the
agency will offer prescribers a set of tools that will help get the necessary care to agency clients
with a potential substance or alcohol abuse/dependency issue. The agency can provide a
comprehensive listing of services (ER, hospital services, medication profiles, and other services)
to medical professionals who have treated the individual clients in the past 12-months. To protect
client confidentiality, the information on some clients may be incomplete as the client profile
does not contain any mental health diagnosis or any prescriptions typically associated with
mental health treatment. Instructions on the last page of the Tool Kit detail how to obtain a
complete profile. For more important information, see the Tool Kit.
Prescription Drug Program
-45-
Alpha-Agonist Age/Dose Limits
Based on recommendations by the Pediatric Mental Health Workgroup, the Health Care
Authority (agency) requires authorization for alpha-agonists that exceed the agency’s dose for
clients 17 years of age and younger.
For dosing limits, see the List of Drugs with Limitations.
For combined doses of alpha-agonists that exceed the agency’s dose limit for clients 17 years of
age and younger authorization is required.
Age
Clonidine Equivalent Dose*
0 - 3 years of age
PA required
4 - 5 years of age
0.2mg
6 - 8 years of age
0.3mg
9 - 17 years of age
0.4mg
*Clonidine Equivalent Dose is 0.1mg clonidine = 1 mg guanfacine.
As part of the authorization process, prescribers are required to engage in a phone consultation
with an agency-designated mental health specialist from the Second Opinion Network (SON). To
receive payment for the phone consultation with SON, use procedure code 99441 on the claim.
Note: A SON representative will contact prescribers to schedule the required
phone consultation.
At the time of the authorization request, continuation of therapy will be approved until the SON
consultation process is complete. Agency authorization decisions will be based on the
recommendations to the agency by the SON mental health specialist.
Note: Dispensed As Written (DAW) by an endorsing prescriber does not override
the PA requirement for exceeding the alpha-agonist dose limits.
Prescription Drug Program
-46-
Newer Anticoagulants
The agency requires authorization for all newer anticoagulants. Newer anticoagulants will be
authorized only for the indications, age ranges, and doses currently approved by the Food and
Drug Administration (FDA) when first line therapies have proven inadequate to treat or control
the patient’s condition.
Xarelto® will be authorized without prior authorization for a one-time fill for the following
indications and dosages:
Hip replacement: 10mg once daily for 35 days.
Knee replacement: 10mg once daily for 12 days.
Treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE): 15mg twice
daily for 21 days.
Anticonvulsants, off-label use initiative
The agency requires authorization for off-label use of certain anticonvulsants (Neurontin
®
or
gabapentin, Topamax
®
, Keppra
®
, and Gabitril
®
). The anticonvulsants are all used for treatment
of seizures, and the agency has established expedited authorization (EA) codes to allow
immediate authorization for this use. Any other use outside of the FDA labeling requires the
pharmacy to call the agency’s Authorization toll-free telephone number 800-562-3022.
The agency does not require authorization for all first-line anticonvulsant drugs used for seizure
disorders, such as phenytoin and carbamazepine.
EA codes and criteria:
Drug
Code
Criteria
Gabitril
®
(tiagabine)
036
Treatment of seizures
Keppra
®
(levetiracetam)
036
Treatment of seizures
Neurontin
®
(gabapentin)
035
036
063
Treatment of post-herpetic neuralgia
Treatment of seizures
Treatment of diabetic peripheral neuropathy
Topamax
®
/Topamax
®
Sprinkle
(topiramate)
036
045
Treatment of seizures
Migraine Prophylaxis
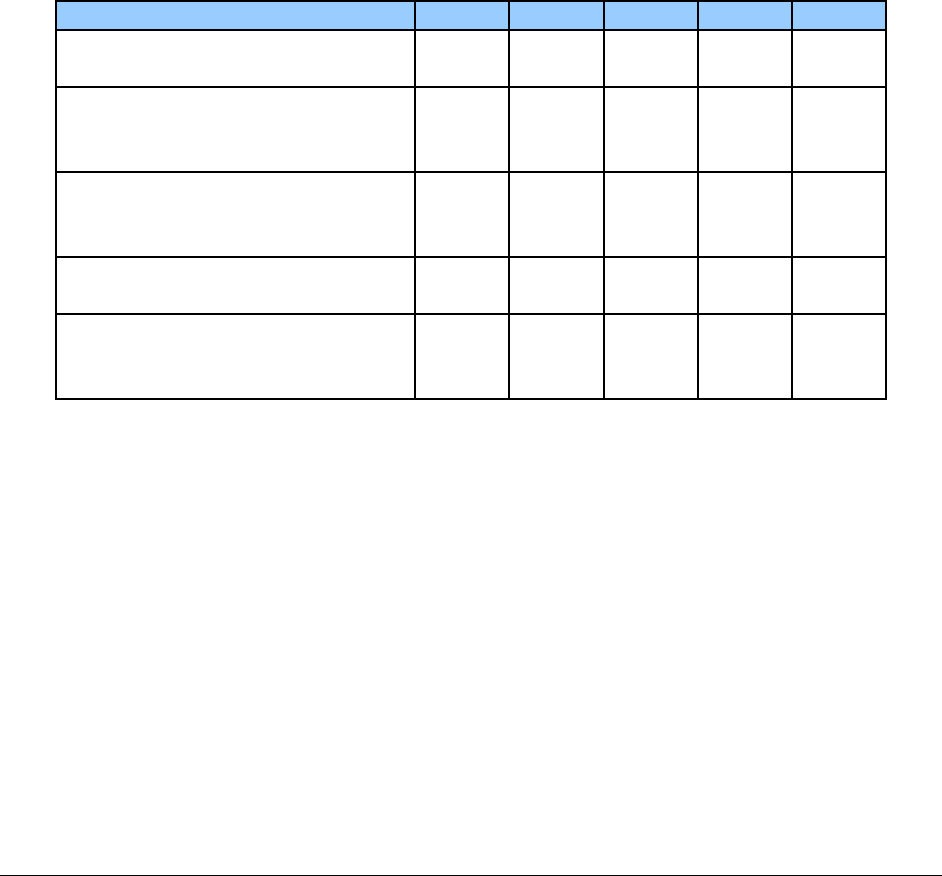
Prescription Drug Program
-47-
Antidepressants, therapeutic duplication
It is routine to have a client on more than one antidepressant drug when in the process of
changing antidepressants, in order to taper from one drug while starting another. This process
can take as long as two months, but after that, it is inadvisable to maintain a client on duplicative
therapies. Multiple antidepressants with same/similar mechanisms of action are likely to
cause increased side effects with little or no increase in efficacy. In fact, it is possible for the
drugs to compete, interfering with the efficacy of one or both drugs.
Based on the determination of a state-wide workgroup of mental health experts, the agency
requires authorization for duplication of therapy which has lasted longer than a two-month taper
period (68 days) for the classes listed in the chart below. The chart is presented as a cross
reference of drugs the workgroup has determined to be duplicative. The squares marked with an
X indicate the combinations that will require authorization after 68 days of concurrent therapy.
The blanks indicate appropriate combinations that may be reimbursed.
Class
SSRI
NaSSA
NDRI
SARI
SNRI
SSRI (Selective Serotonin
Reuptake Inhibitor)
X
X
X
NaSSA (Noradrenergic and
Specific Serotonergic
Antidepressant)
X
X
NDRI
(Norepinephrine/Dopamine
Reuptake Inhibitor)
X
SARI (Serotonin Antagonist
Reuptake Inhibitor)
X
X
X
SNRI (Serotonin
Norepinephrine Reuptake
Inhibitor)
X
X
SSRI – Celexa
®
(citalopram), Lexapro
®
(escitalopram), Luvox
®
(fluvoxamine), Paxil
®
(paroxetine HCl), Pexeva
®
(paroxetine mesylate), Prozac
®
(fluoxetine), and Zoloft
®
(sertraline)
NaSSA – Remeron
®
(mirtazapine)
NDRI – Wellbutrin
®
(bupropion)
SARI – Serzone
®
(nefazodone)
SNRI – Cymbalta (duloxetine), Effexor (venlafaxine), Pristiq (desvenlafaxine)
Duplication sometimes can occur when multiple prescribers are unaware that duplicative care for
the same client is taking place. In an effort to help prescribers coordinate care for clients, the
agency provides information to each prescriber involved when inappropriate duplication of
antidepressants is found. The agency requests that health care practitioners coordinate with each
other to establish a plan for the client’s care.

Prescription Drug Program
-48-
Antipsychotic Age/Dose Limits for children 17
years of age and younger
Based on recommendations by the Pediatric Mental Health Workgroup, the agency requires
authorization for atypical antipsychotics that exceed the agency’s dosing limitation for clients 17
years of age and younger. As part of the authorization process, prescribers are required to engage
in a phone consultation with an agency-designated mental health specialist from the Second
Opinion Network (SON). To receive payment for the phone consultation with SON, use
procedure code 99441 on the claim.
For dosing limits, see the List of Drugs with Limitations.
Antipsychotic therapeutic duplication for
children 17 years of age and younger
To reduce the therapeutic duplication of antipsychotic drugs prescribed for children 17 years of
age and younger the agency requires authorization after 60 days of continued use. As part of the
authorization process, prescribers are required to participate in a second opinion review process
with an agency-designated Mental Health Specialist from the Second Opinion Network (SON).
During the review process continuation of a combination may be authorized for up to 60 days, to
allow the prescriber to complete the SON review or to taper a client off a drug.
Buprenorphine for pregnant women
The agency covers buprenorphine for pregnant women when the client:
Is 16 years of age and older.
Is pregnant with a verifiable estimated due date (EDD).
Has a DSM-IV-TR diagnosis of opioid dependence.
Is psychiatrically stable, or is under the supervision of a mental health specialist.
Is not abusing alcohol, benzodiazepines, barbiturates, or other sedative-hypnotics.
Does not have a history of failing multiple previous opioid agonist treatments and
multiple relapses.

Prescription Drug Program
-49-
Does not have concomitant prescriptions of azole antifungal agents, macrolide
antibiotics, protease inhibitors, phenobarbital, carbamazepine, phenytoin, and rifampin,
unless the dosage adjusted appropriately.
Is enrolled in a state-certified chemical dependency treatment program. See WAC 388-
805-610.
Limitations:
No more than a seven-day supply may be prescribed at a time
Urine drug screens for benzodiazepines, amphetamine/ methamphetamine, cocaine,
methadone, opiates, and barbiturates must be done before each prescription is dispensed.
The prescriber must ensure that the drug screen has been completed prior to prescribing
the next seven-day supply
Liver function tests must be monitored periodically to guard against buprenorphine-
induced hepatic abnormalities
Payment for the medication is limited to the duration of pregnancy
A Buprenorphine Authorization for Pregnancy form, DSHS 13-901, must be completed and sent to
the Department of Social and Health Services, Division of Behavioral Health and Recovery (DBHR)
before prior authorization can be considered.
Oral, Transdermal, and Intra-Vaginal
Contraceptives
The agency requires oral and transdermal contraceptives to be dispensed in a 12-month (13
cycles) supply (See WAC 182-530-2000 (1)(b)(iii)). For the purposes of dispensing these
contraceptive products, 12-month (13 cycles) means a 365-day supply.
Any prescription currently written for a specific quantity less than a 12-month (13 cycles)
supply, regardless of how many refills are currently available, cannot be dispensed as a 12-
month (13 cycles) supply without confirming a change to the order with the prescriber. The
agency encourages pharmacies to request prescription changes. For prescriptions written with a
dispensing quantity less than a 12-month supply the agency encourages pharmacies to contact
the prescriber to request a change in the dispensing quantity. If the prescriber is unwilling to
change the dispensed quantity you may submit the claim using EA code 85000000364 to receive
payment for the shorter days’ supply as indicated on the prescription.
For prescriptions written with a dispensing quantity less than a 12-month supply and the client
does not want all dispensed at once you may submit the claim using EA code 85000000365 to
receive payment for the shorter days’ supply as indicated on the prescription.

Prescription Drug Program
-50-
For prescriptions written with a dispensing quantity less than a 12-month supply and the pharmacy is
unwilling to contact the prescriber and request a dispensing quantity change you may submit the
claim using EA code 85000000366 to receive payment for the shorter days’ supply as indicated on
the prescription.
Note: When submitting a claim with an EA code you must document on the
prescription the code that was used and the reason.
Cough/cold drug coverage
The agency restricts coverage of drugs used to treat cough and colds to those drugs listed on the
Covered Cough/Cold Product List. The agency bases its decision on which drugs to place on this
list using evidence of efficacy and safety and current best practices.
OTC drugs used to treat cough and colds which are not on the Covered Cough/Cold Product
List are noncovered and may be billed directly to the client as a nonprescribed OTC.
Prescription drugs used to treat cough and colds which are not on the Covered Cough/Cold
Product List may be purchased by the client with a signed waiver. (See Billing the Client,
WAC 182-502-0160 and Coordination of Benefits.)
Generics first (GF)
The agency requires that a preferred generic drug be used as a client’s first course of treatment
within specific drug classes on the Washington Preferred Drug List (PDL). Only clients who are
new to a drug class will be required to start on a preferred generic product. When a client has not
received a drug in one of these drug classes within 180-days prior to the date of the fill, the
agency’s POS system will reject claims for both nonpreferred and preferred brand name drugs as
well as nonpreferred generic drugs.
If the brand name drug has been prescribed by a non-endorsing practitioner, or by an endorsing
practitioner who has not indicated Dispense As Written (DAW), the brand will be noncovered by
the agency. If the prescriber is an endorsing practitioner, and Therapeutic Interchange is allowed
in the drug class the product should be switched to a preferred drug. Otherwise, when requested
to do so by POS return messaging, contact the prescriber to request a change of the prescription
to a preferred generic drug.
If the prescription is signed “DAW” by an endorsing practitioner for a drug within a GF drug
class, contact the agency to request authorization. The agency will provide the endorsing
practitioner with an opportunity to justify the medical necessity for starting the client on a brand
name drug or a nonpreferred generic as their first course of therapy.

Prescription Drug Program
-51-
The agency will only cover preferred generic drugs as a client’s first course of therapy within the
following drug classes:
ACE Inhibitors
Atypical Antipsychotics
Attention Deficit Hyperactivity Disorder (ADHD) Drugs
Beta Blockers
Long-acting opioids
Nasal corticosteroids
Newer Antihistamines
Newer Sedative/Hypnotics
Nonsteroidal anti-inflammatory drugs (NSAIDS)
Proton Pump Inhibitors (PPI)
Second generation antidepressants
Skeletal Muscle Relaxants
Statin-type cholesterol-lowering agents.
Inhaled Corticosteroid (ICS)/Long-acting Beta
Agonist (LABA) combination drugs
The agency requires authorization for Advair, Dulera, and Symbicort to verify that a clinically
appropriate stepwise treatment plan has been followed which reflects an accurate assessment of
disease severity for the treatment of asthma or Chronic Obstructive Pulmonary Disease (COPD).
ICS/LABA products will only be approved for clients within age limits as indicated by FDA
labeling:
Advair Diskus for patients 4 years of age and older
Advair HFA, Dulera, or Symbicort for patients 12 years of age and older.
The agency may approve the use of ICS/LABA combination products in patients:
With asthma not adequately controlled by a trial with an ICS as monotherapy
With COPD not adequately controlled by a trial of a bronchodilator as monotherapy
With asthma or COPD whose disease severity warrants initiation of treatment with two
maintenance drugs.
Note: Advair 250/50 twice daily and Symbicort 160/4.5 two inhalations twice
daily are the only approved dosages for the treatment of COPD. Use of other
strengths or Dulera will not be approved by the agency for COPD.
Prescription Drug Program
-52-
Authorization for leukotriene modifiers
The agency requires authorization for all leukotriene modifiers. Leukotriene modifiers will be
authorized only for the indications, age ranges, and doses currently approved by the Food and
Drug Administration (FDA) when first line therapies have proven inadequate to treat or control
the patient’s condition. All leukotriene modifiers are approved by the FDA for the prophylaxis
and chronic treatment of asthma. In addition, Singulair® (montelukast) is also indicated for the
acute prevention of exercise-induced bronchoconstriction (EIB) and relief of symptoms of
allergic rhinitis (AR).
Clinical Guidelines
Asthma and EIB: A leukotriene modifier will be approved for persistent asthma in
patients who have tried and failed an inhaled corticosteroid (ICS) and are concurrently
treated with an ICS. For exercise-induced bronchoconstriction a trial of a short-acting
beta-agonist is required.
According to the National Heart, Lung, and Blood Institute (NHLBI) Guidelines for the
Diagnosis and Management of Asthma, inhaled corticosteroids (ICS) are the preferred
and most effective long-term control medication for treatment of persistent asthma.
Leukotriene receptor antagonists (LTRAs) such as montelukast and zafirkulast, are an
alternative, but are not preferred therapy for mild persistent asthma and can also be used
as adjunctive therapy with ICSs. The leukotriene modifier zileutin is another alternative
but less desirable option to the LTRAs due to more limited efficacy data and the need for
liver function monitoring. For preventing exercise-induced bronchoconstriction, the
Expert Panel recommended that short-acting beta-agonists (SABAs) are the drug of
choice.
Allergic Rhinitis: montelukast will be approved for patients who have tried and failed an
oral second generation antihistamine and a nasal corticosteroid.
According to the American Academy of Allergy, Asthma and Immunology (AAAAI)
practice parameters, intranasal corticosteroids are the most effective medication class for
controlling symptoms of allergic rhinitis, and in most studies, are more effective than the
combined use of an antihistamine and an LTRA for seasonal allergic rhinitis. There is
also no significant difference in efficacy between LTRA and antihistamines (with
loratadine as the usual comparator).
Prescription Drug Program
-53-
Mental Health Polypharmacy
Mental health experts participated in the agency’s Mental Health Drug Initiative Stakeholder
Workgroup to determine the threshold for clinical review and select which drugs are recognized
as mental health drugs (see the agency’s list of recognized mental health drugs).
The agency requires authorization for concurrent therapy with five or more mental health drugs
for clients who are 17 years of age and younger.
As part of the authorization process, prescribers are required to engage in a phone consultation
with an agency-designated mental health specialist (MHS) from the Second Opinion Network
(SON). Agency authorization decisions are based on the recommendations to the agency by the
SON MHS.
Note: A SON representative will contact prescribers to schedule the required
phone consultation.
At the time of the authorization request, continuation of the concurrent therapy will be approved
until the SON consultation process has been completed.
Authorization for Proton Pump Inhibitors (PPIs)
The agency requires authorization for the continued use of Proton Pump Inhibitors (PPIs) for
more than 90 consecutive days. Clients receiving a PPI for more than 90 days may be candidates
for “step-down” therapy.
Prescribers should:
Reevaluate therapy for clients diagnosed with Gastroesophageal Reflux Disease (GERD)
and negative findings on endoscopy
Consider discontinuing the PPI and/or step-down to ranitidine, a Histamine-2 Receptor
Antagonist (H2RA) medication.

Prescription Drug Program
-54-
Narcotic Review Project
What does the Narcotic Review Project do for
clients?
The agency’s Narcotic Review Project reduces misuse of narcotics in clients considered at “high
risk” of abuse/misuse of narcotic prescriptions. This program can assist providers in this complex
area of medicine. The key to the program is keeping prescribers informed about their patients’
narcotic utilization by faxing information to the prescriber who wrote the latest prescription.
Pharmacy Authorization staff fax the patient’s recent narcotic profile (12-months of all narcotic
prescriptions and prescriber names) to the prescriber who wrote the latest prescription. After the
prescriber reviews the profile and makes a decision whether to continue with the prescription or
not, the prescriber faxes the form back to Pharmacy Authorization, and the prescription is
authorized or not authorized, depending on the prescriber’s response.
If the prescriber believes that the patient’s pattern of narcotic utilization is medically necessary
and does not show abuse/misuse, the patient’s case is reviewed by the agency’s Drug Use
Review Team, and a decision is made whether to remove the patient from the authorization
requirement.
For more information about drug abuse prevention or treatment, contact the 24-Hour
Alcohol/Drug Helpline at 800-562-1240, or call the state Division of Alcohol and Substance
Abuse at 877-301-4557 and ask for the regional administrator for your county to help you access
public care. If you believe a patient may need help for drug abuse, refer these clients to the
Helpline.
How were the opioid dosing guidelines
developed?
These guidelines were developed by the Interagency Workgroup on Practice Guidelines (the
Department of Corrections, Department of Health, Department of Labor and Industries,
Department of Social and Health Services, and the Health Care Authority) in collaboration with
actively practicing physicians who specialize in pain management. The guidelines are to assist
the practitioner in prescribing opioids in a safe and effective manner. The guidelines do not apply
to the treatment of cancer pain or end-of-life (hospice) care.
For more information see the Washington State Agency Medical Directors’ Group (AMDG)
Opioid dosing guidelines.
Prescription Drug Program
-55-
Does the agency cover Over-the-Counter (OTC)
drugs?
The agency has reviewed and determined that the OTC drugs on the “Covered Over-the-Counter
Drug List” list are the least costly therapeutic alternatives for medically accepted indications.
(WAC 182-530-2000(1)(d))
Visit the Medicaid Coverage Lists for more information.
Note: OTC family planning products and OTC drugs used to treat cough and colds are governed
under different rules and have their own coverage lists.
OTC drugs not included on any agency Covered Drug List are noncovered and may be billed
directly to the client as a nonprescribed OTC.
Sedative/hypnotic restrictions for children
Sedatives and hypnotics in children 18 years of age and younger are limited to a one-time
authorization of less than five doses in a 30-day period.
Step therapy for inhaled long-acting beta
agonist/corticosteroid combination drugs
The agency requires authorization for inhaled long-acting beta agonist/corticosteroid
combination drugs when there is no record in the agency’s payment system that the client has
had a previous trial of an inhaled corticosteroid medication.
The agency requires documentation of one of the following:
The client tried and failed inhaled corticosteroid monotherapy.
Disease severity warranting initiation with two maintenance therapies.
The client is being treated for airflow obstruction associated with Chronic Obstructive
Pulmonary Disease (including emphysema and chronic bronchitis).
Black box warning for these combination drugs state that long-acting beta agonists may increase
the risk of asthma-related death. The FDA-approved prescribing information for the inhaled
long-acting beta agonist/corticosteroid combinations states that for the indication of asthma, the
combination should be used only for patients:

Prescription Drug Program
-56-
Whose disease is not adequately controlled on other asthma-controller medications (e.g.,
low- to medium- dose inhaled corticosteroids).
Whose disease severity clearly warrants initiation of treatment with two maintenance
therapies, as found in the Agency for Healthcare Research and Quality (AHRQ) report.
What is the agency’s criteria Suboxone®
(buprenorphine/naloxone) authorization?
Before authorization will be approved, the patient must meet all of the following criteria.
The patient:
Is 16 years of age and older.
Has a DSM-IV-TR diagnosis of opioid dependence.
Is psychiatrically stable or is under the supervision of a mental health specialist.
Is not abusing alcohol, benzodiazepines, barbiturates, or other sedative-hypnotics.
Is not pregnant or nursing.
Does not have a history of failing multiple previous opioid agonists treatments and multiple
relapses.
Does not have concomitant prescriptions of azole antifungal agents, macrolide antibiotics,
protease inhibitors, phenobarbital, carbamazepine, phenytoin, and rifampin, unless the
dosage is adjusted appropriately.
Is enrolled in a state-certified intensive outpatient chemical dependency treatment program.
See WAC 388-805-610.
Limitations:
No more than 14-day supply may be prescribed at a time
Urine drug screens for benzodiazepines, amphetamine/ methamphetamine, cocaine,
methadone, opiates, and barbiturates must be done before each prescription is dispensed. The
prescriber must ensure that the drug screen has been completed prior to prescribing the next
14-day supply
Liver function tests must be monitored periodically to guard against buprenorphine-induced
hepatic abnormalities
Clients may receive up to 6-months of buprenorphine treatment for detoxification and
stabilization.
A Suboxone® Authorization form, DSHS 13-720 must be completed and sent to DASA before
authorization is issued.

Prescription Drug Program
-57-
Where is information available for Synagis®?
See the EPSDT Screening Components Section and the Client Eligibility Section in the agency’s
current Early and Periodic Screening, Diagnosis and Treatment (EPSDT) Program Medicaid
Provider Guide for information on Synagis.
What are the authorization rrequirements for
Vivitrol® (naltrexone IM)?
To request prior authorization for Vivitrol®, complete a Vivitrol IM Physician Prior Authorization
form, DSHS 13-791.
Note: Upon completion of the DSHS 13-791 form, you must fax the form to the
Department of Social and Health Services, Division of Behavioral Health and
Recovery (DBHR) at 360-586-0343 for review.
What does emergency fill mean?
Emergency fill means that the dispensing pharmacist used their professional judgment to meet a
client’s urgent medical need and dispensed the medication to the client prior to receiving
reimbursement from the agency.
The agency guarantees claim payment for an emergency fill. If the agency rejects an electronic
claim for an emergency fill, contact the agency with the information that the claim is for an
emergency fill.
If the dispensing pharmacist decides that the client has an urgent medical need, the following
steps are required:
Determine the quantity necessary to meet the client’s urgent medical need
(up to a 34-day supply).
Dispense the medication to the client.
Request an emergency fill by using either of the following two options:
Calling Pharmacy Authorizations at 800-562-3022 ext. 15483.
Faxing a request for an emergency fill authorization to 866-668-1214.
Contact the agency within seven calendar days or before filling the medication again (whichever
is sooner). Medical necessity requirements will be applied to any future fills of the same
medication, but will be waived to ensure payment of the emergency fill.
Prescription Drug Program
-58-
Does the agency pay for Hemophilia - and von
Willebrand-related products for home
administration?
(WAC 182-531-1625)
The agency does not pay for hemophilia- and von Willebrand-related products for administration
in the home when dispensed through and billed by retail or specialty pharmacies. The agency
pays for hemophilia- and von Willebrand-related products shipped to fee-for-service clients only
when the products are provided through a qualified hemophilia treatment center of excellence
(COE).
Note: If a client has not yet established a care relationship with a qualified
hemophilia COE, but an initial appointment has been scheduled, specialty
pharmacy providers may contact the agency to request an authorization to
continue to dispense product to the client. The pharmacy must call the agency’s
Pharmacy Authorization Section at 800-562-3022, extension 15486.
What are the criteria to become a Qualified
Hemophilia Center of Excellence (COE)?
To become a qualified hemophilia COE, a hemophilia center must meet all of the following:
Have a current core provider agreement in accordance with WAC 182-502-0005.
Be a federally approved hemophilia treatment center (HTC) as defined in Definitions and
meet or exceed all Medical and Scientific Advisory Council (MASAC) standards of care
and delivery of services.
Participate in the public health service 340B provider drug discount program and be
listed in the Medicaid exclusion files maintained by the federal Health Resources and
Services Administration (HRSA) Office of Pharmacy Affairs (OPA).
Submit a written request to the agency to be a qualified hemophilia treatment center of
excellence and include proof of the following:
U.S. Centers for Disease Control (CDC) and prevention surveillance site
identification number.
Listing in the hemophilia treatment center (HTC) directory.

Prescription Drug Program
-59-
Receive written approval including the conditions of payment and billing
procedures from the agency.
To be recognized as a qualified hemophilia COE, submit a written request to:
Hemophilia Treatment COE
Health Care Authority – Health Care Services
PO Box 45506
Olympia WA 98504-5506
Why is there an annual documentation
requirement?
To remain a qualified hemophilia COE, the hemophilia COE must annually submit both of the
following to the agency:
Copies of grant documents and reports submitted to the Maternal and Child Health
Bureau/Human Resources and Services Administration/Department of Health and Human
Services or to their designated subcontractors.
Proof of continued federal funding by the National Hemophilia Program and listing with
the regional hemophilia network and the CDC.
To view the list of qualified Centers of Excellence (COE) for hemophilia treatment, see the
agency’s current Physician-Related Services/Health Care Professional Services Medicaid
Provider Guide.
Prescription Drug Program
-60-
Authorization
Authorization does not guarantee payment.
All administrative requirements (client eligibility, claim timeliness, etc.) must be
met before the agency reimburses.
When does the agency require authorization?
(WAC 182-530-3000(2))
Pharmacists are required to obtain authorization for some drugs and drug-related supplies before
providing them to the client. Other drugs require authorization only when specific limits on
dosage, quantity, utilization, or duration of use are exceeded. The agency may also require
situational authorization that is not directly related to the product being dispensed. These
situations include, but are not limited to:
Early refills
Therapeutic duplications
Client’s whose utilization patterns are under review.
More than four prescriptions or prescription refills per calendar month for the same
product in any of the following categories:
Antibiotics
Anti-asthmatics
Schedule II & III drugs
Anti-neoplastic agents
Topical preparations
Propoxyphene, propoxyphene napsylate, and all propoxyphene combinations.
More than two prescriptions or prescription refills per calendar month for any other
product.
The agency reviews authorization requests for medical necessity. The requested service or item
must be covered within the scope of the client's program.
Exception: In emergency situations, pharmacists may fill prescription drugs that require
authorization without receiving an authorization number prior to dispensing.
Note: To receive reimbursement, justification for the emergency fill must be
provided to the agency no later than 7 days after the fill date.

Prescription Drug Program
-61-
How do I obtain authorization?
To obtain authorization for drug products requiring authorization, providers may:
Fax a Pharmacy Information Authorization form, 13-835A, to the agency at 866-668-
1214.
Call the agency at 800-562-3022.
What information must a pharmacist have ready
before calling the agency for an authorization
number?
When calling for an authorization number, pharmacists must have the following information ready:
Previous authorization number, if available
Pharmacy NCPDP #
Pharmacy NPI#
Rx #
Quantity and days supply
Tried and failed
Client's ProviderOne Client ID
National Drug Code (NDC) being dispensed
Prescriber’s name and specialty (if known)
Prescriber’s phone and fax number
Date(s) of dispense
Justification for the requested service:
The medical need for the drug and/or dosing (sig)
The diagnosis or condition of the client
Other therapies that have been tried and failed in the treatment of the same
condition.
The agency may request additional information, depending on the drug product.
Prescription Drug Program
-62-
Who determines authorization status for drugs in
the agency’s drug file?
(WAC 182-530-3100(1))
For drugs in therapeutic classes included in the Washington Preferred Drug List (PDL),
authorization status is determined by its designation as preferred or non-preferred.
For drugs not in therapeutic classes included in the PDL, agency pharmacists, medical
consultants, and the Drug Use Review Team evaluates drugs to determine authorization status of
the drug file. The agency may consult with an evidence-based practice center, the Drug Use
Review (DUR) Board, and/or participating agency providers in this evaluation.
How is authorization status determined for drugs
in the agency’s drug file?
(WAC 182-530-3200(2) and (3))
Drug manufacturers who wish to facilitate the evaluation process for a drug product may send
the agency pharmacist(s) a written request and all of the following supporting documentation:
Background data about the drug
Product package information
Any pertinent clinical studies
Outcome and effectiveness data using the Academy of Managed Care Pharmacy’s drug
review submission process
Any additional information the manufacturer considers appropriate
The agency evaluates a drug based on, but not limited to, the following criteria:
Whether the manufacturer has signed a federal
drug rebate contract agreement.
Whether the drug falls into one of the
categories authorized by federal law to be
excluded from coverage.
Whether the drug is a less-than-effective drug.
The drug’s potential for abuse.
The drug’s risk/benefit ratio.
Whether outcome data demonstrate that the
drug is cost effective.
Whether like drugs are on the agency’s drug
file and a less costly therapeutic alternative
drug is available.

Prescription Drug Program
-63-
What authorization status may be assigned to a
drug?
The agency may determine that a covered drug is:
Covered without restriction.
Requires authorization.
Requires authorization when exceeding the agency-determined limitations.
Decisions regarding restrictions are based on, but are not limited to:
Client safety
FDA-approved indications
Quantity
Client age and/or gender
Cost
Note: Visit the agency’s current List of Drugs with Limitations for information
about drugs with limits. Physicians and pharmacists should monitor the use of
these drugs and counsel patients when they exceed the limit. Authorization is
required in order to exceed these limits.
How are drugs added to the agency’s drug file?
(WAC 182-530-3000(2) and (3))
The agency’s drug file is maintained by Medi-Span® (a drug file contractor). Manufacturers
must report their products to Medi-Span® for them to be included in the agency’s drug file for
potential coverage and reimbursement.
Prescription Drug Program
-64-
Is there a list of drugs that do not require
authorization?
See the agency’s current Expedited Authorization Codes and Criteria Table (EA) list for
drugs that do not require authorization.
Note: Products on the EA list are subject to all other coverage rules.
What criteria will justify early refills?
(WAC 182-530-3000(5)(b))
The following circumstances are justification for early refills:
If a client’s prescription is lost, stolen, or destroyed (only once every six months, per
medication).
If a client needs a refill sooner than originally scheduled due to a prescriber dosage
change. (The pharmacist must document the dosage change.)
If a client is suicidal, at-risk for potential drug abuse, or being monitored by the
prescriber.
If a client needs a take-home supply of medication for school or camp, or for skilled
nursing facility clients.
For any other circumstance, the provider must contact the agency's Pharmacy Authorization
Section to request approval and an authorization number (see Resources Available.)
Prescription Drug Program
-65-
Pharmacy providers have the right to ask clients for documentation relating to reported theft or
destruction, (e.g., fire, earthquake, etc.). If clients residing in a skilled nursing facility (SNF)
have their prescription lost or stolen, the replacement prescription is the responsibility of the
SNF. Clients who have trouble managing their drug therapy should be considered for the use of
compliance devices (e.g., Medisets).
BILLING
Hard copy billers must enter one of the following justification descriptions in the
Justification/Comments field on the Pharmacy Statement (525-106) form, 13-714.
Point-of-Sale billers must enter one of the following codes in the Claims Segment,
Prior Authorization Type Code (461-EU) field.
Justification Description Code
"Lost or Stolen Drug Replacement" 5
"School or Camp" 8
“Monitoring” 8
“Suicidal Risk (SR)" 8
“Take Home Supply (Skilled Nursing Facility Client)” 8
Can clients receive early refills or extended days'
supply for travel?
(WAC 182-530-3000(5)(b))
The agency will not approve early refills or prescriptions filled for over a 34-day supply for
clients who will be out of the area. Early refills for the purpose of travel are considered to be
services rendered for a future date. Clients may elect to self-pay for an early refill or a larger
days’ supply, as they are not considered Medicaid eligible for the future service at the time of
fill.
It is also possible to help clients who will be out of the area to receive refills covered by the
agency at a time they are due for a regular refill. Providers may assist clients with any of the
following options:
If clients will not be out of state, they may have their prescription filled at any agency-
contracted pharmacy throughout Washington or border areas of Idaho and Oregon.
Clients may arrange with you to refill their prescription at the appropriate time, and have
a relative or other designee pick up the prescription for them. The client’s designee may
then ship the medication to the client at their own expense.

Prescription Drug Program
-66-
You can make arrangements with the client to ship the medication from your pharmacy.
The cost of shipping is not billable to the agency and will not be covered. Shipping is at
your expense or the client’s expense if you retain a signed consent on file.
Some chain stores have the ability to “transfer stock”, billing the prescription from a local
Washington pharmacy, while having the medication dispensed from a store in another
part of the country.
Is authorization required for brand name drugs?
Prescribers and pharmacies should prescribe and dispense the generic form of a drug whenever
possible. Authorization may be required for reimbursement of brand name drugs at brand name
pricing when any generic therapeutic equivalent is available. If the brand name drug is
prescribed instead of a generic therapeutic equivalent, the prescriber must provide medical
justification for the use of the brand name drug to the pharmacist. Authorization is based on
medical need, such as clinically demonstrated, observed, and documented adverse reactions
which have occurred when generic therapeutic equivalents have been used.
Substitute generic drugs for listed brand name drugs when both of the following are true:
They are approved by the FDA as therapeutically equivalent drugs.
They are permitted by the prescribing physician under current state law.
To request authorization, call the agency at: 800-562-3022.
What is an exception to rule (ETR)?
The process used by the agency to consider the appropriateness of a noncovered item when that
service is specifically needed for that client because their clinical needs are so different than the
rest of the population.
Providers may request an ETR to request coverage for a noncovered service by contacting the
agency and providing the necessary information for the program to make a decision in each
client’s individual case.
For detailed requirements regarding ETR requests for a noncovered product (see WAC 182-501-
0160).
Prescription Drug Program
-67-
What is expedited authorization (EA)?
(WAC 182-530-3200(4))
The agency’s EA process is designed to eliminate the need to request authorization from the
agency. The intent is to establish authorization criteria and associate these criteria with specific
codes, enabling providers to create an “EA” number when appropriate.
How is an EA number created?
To bill the agency for drugs that meet the expedited authorization criteria on the following pages,
the pharmacist must create an 11-digit EA number. The first 8 digits of the EA number must be
85000000. The last 3 digits must be the code number of the diagnosis/condition that meets the
EA criteria.
BILLING
Hardcopy billers must enter the EA Number in the Authorization Number field on the
Pharmacy Statement (525-106) form, 13-714.
Point of Sale billers must enter the EA Number in the Claims Segment, Prior
Authorization Number Submitted field.
Example: The 11-digit EA number for Accutane (for the treatment of "severe,
recalcitrant acne rosacea in adults unresponsive to conventional therapy") would be
85000000002 (85000000 = first eight digits, 002 = diagnosis/condition code).
Reminder: EA numbers are only for drugs listed in the Expedited Authorization Code and
Criteria Table. EA numbers are not valid for any of the following:
Other drugs requiring authorization through the Prescription Drug Program.
Waiving the S-MAC or A-MAC price.
Authorizing the third or fifth fill in the month.
Note: Use of an EA number does not exempt claims from edits, such as per-
calendar-month prescription limits or early refills.

Prescription Drug Program
-68-
EA guidelines:
Diagnoses - Diagnostic information may be obtained from the prescriber, client, client’s
caregiver, or family member to meet the conditions for EA. Drug claims submitted
without an appropriate diagnosis/condition code for the dispensed drug are denied.
Unlisted Diagnoses - If the drug is prescribed for a diagnosis/condition, or age that does
not appear on the EA list, additional justification is required. The pharmacist must
request authorization by either one of the following:
Phone 800-562-3022.
Fax 866-668-1214.
Documentation - Dispensing pharmacists must write both of the following on the
original prescription:
The full name of the person who provided the diagnostic information.
The diagnosis/condition and/or the criteria code from the attached table.

Prescription Drug Program
-69-
Reimbursement
What in general does the agency need to process
a reimbursement for services?
Remember the agency is a taxpayer-funded program and the payer of last resort -
meaning providers must pursue all other possible medical coverage first. See
Coordination of Benefits for more information.
The agency is required to be a prudent purchaser on behalf of the taxpayer. Drug
reimbursements are subject to federal upper limit (FUL) payment rules (See
Reimbursement), and the agency is permitted to pay for outpatient drugs only when the
manufacturer has a signed drug rebate contract with the federal Department of Health and
Human Services (DHHS). See Drug Rebate Program for more information.
Bill the agency the usual and customary charge using the complete 11-digit national drug
code (NDC) from the dispensing container.
Accurately report the quantity dispensed, using the appropriate metric or metric decimal
quantity for the product.
Delivery of a service or product does not guarantee payment. For example, the agency
does not reimburse when:
The request for payment is not presented within the 365 day billing limit (see
Billing.)
The service or product is not medically necessary or is not reimbursable by the
agency.
The client has third party coverage and the third party pays as much as, or more
than, the agency allows for the service or product.
The service or product is covered in the managed care capitation rate.
The service or product is included in the Nursing Home per diem rate.
The client is no longer eligible or isn’t eligible for the drug being dispensed.
A prescription has been used to meet a client’s financial obligation towards
spenddown.
Prescription Drug Program
-70-
How does the point of sale system (POS) establish
reimbursement rates?
(WAC 182-530-7000)
The agency’s reimbursement for a drug dispensed by a pharmacy is adjudicated by the
POS system. POS reimburses at the lowest of the appropriate rates for the product.
Depending upon the status of the drug, POS reimburses for the:
Estimated Acquisition Cost (EAC) plus a dispensing fee.
State Maximum Allowable Cost (S-MAC) plus a dispensing fee.
Federal Upper Limit (FUL) plus a dispensing fee.
Automated Maximum Allowable Cost (A-MAC) plus a dispensing fee.
Provider’s usual and customary charge to the non-Medicaid population.
Actual Acquisition Cost (AAC) plus a dispensing fee for drugs purchased under
section 340 B of the Public Health Services (PHS) Act and dispensed to medical
assistance clients.
Note:
If the pharmacy provider offers a discount, rebate, promotion or other
incentive that directly relates to the reduction of the price of a prescription
to the individual non-Medicaid customer, the provider must similarly
reduce its charge to the agency for the prescription. (Example: A $5.00
off coupon for purchases elsewhere in the store.)
Any drug or product provided free to the general public must also be
provided free to the Medicaid customer.
How does the agency use the estimated
acquisition cost (EAC)?
(WAC 182-530-8000)
The agency uses Estimated Acquisition Cost (EAC) for drugs not otherwise covered by FUL, S-
MAC, or A-MAC rates. The agency selects the source for reference pricing and determines the
calculation to be used for estimated acquisition (EAC) cost.
Currently, the EAC is calculated as a discount off the average wholesale price (AWP):
For single source drugs and multiple source drugs with fewer than five manufacturers/
labelers, the discount from AWP is 16%.
For multiple source drugs with five or more manufacturers/labelers, the discount from
AWP is 50%.
Prescription Drug Program
-71-
How are federal upper limits calculated?
(WAC 182-530-8050)
Federal Upper Limits (FUL) for multiple source drugs are calculated by DHHS, Centers for
Medicare and Medicaid (CMS). The agency is required to comply with the federal limits.
FUL rules are being revised in response to the federal Deficit Reduction Act and are currently in
draft circulation for comment.
Note: For more information see CMS Federal Upper Limits.
Drugs subject to FUL may also be subject to other agency pricing methodologies. The agency
reimburses the lower of EAC, S-MAC, A-MAC, FUL, or usual and customary charges.
How is the automated maximum allowable cost
(A-MAC) calculated?
(WAC 182-530-8150)
The agency’s POS payment system calculates an A-MAC price for all multiple source drugs not
currently on the State Maximum Allowable Cost (S-MAC) list.
A-MAC reimbursement for all products with the same ingredient, form and strength is at the
A-MAC determined for the second lowest priced product, or the A-MAC of the lowest priced
drug from a manufacturer with a current, signed federal rebate agreement.
For drugs with five or more manufacturers or labelers the A-MAC price is AWP minus 50%.
When is the state maximum allowable cost (S-
MAC) applied?
(WAC 182-530-8150)
The MAC may be applied to specific, equivalent multiple-source drugs. If applied, the agency
reimburses both the brand name and generic drugs at the MAC price.
The MAC may be waived for:
Preferred drugs
Some Dispense as Written (DAW) prescriptions
Limited other circumstances.
Visit the most up-to-date S-MAC list.
Prescription Drug Program
-72-
How is tax computed?
Tax is computed by the POS system for items that the Washington State Department of Revenue
determines to be taxable.
Does the agency pay dispensing fees for non-drug
items?
(WAC 182-530-7050)
The agency does not pay a dispensing fee for non-drug items, devices, or supplies unless the
agency determines that the drug file is not maintaining prices sufficient to cover product cost.
The agency uses a three-tier dispensing fee structure with an adjusted fee allowed for pharmacies
that participate in the Unit Dose programs.
Listed below are the agency’s dispensing fee allowances for pharmacies:
High-volume pharmacies (over 35,000 Rx/yr) ...............................$4.24/Rx
Mid-volume pharmacies (15,001-35,000 Rx/yr) ............................$4.56/Rx
Low volume pharmacies (15,000 Rx/yr and under) .......................$5.25/Rx
Unit dose systems ...........................................................................$5.25/Rx
A provider's dispensing fee is determined by the:
Total volume of prescriptions.
Pharmacy fills for agency clients.
General public.
Providers are required to respond to an annual prescription count survey.
Return the annual prescription count survey to:
Provider Enrollment Unit
PO Box 45562
Olympia, WA 98504-5562

Prescription Drug Program
-73-
How is the drug rebate program used?
The Omnibus Budget Reconciliation Act (OBRA) of 1990 mandates that states claim federal
financial participation (FFP) only for outpatient prescription drugs supplied by a drug
manufacturer who has entered into a drug rebate contract with the Department of Health and
Human Services.
Note: Providers must bill the actual and complete 11-digit NDC for the drug
dispensed and the actual quantity, using the appropriate unit of measure.
Using an incorrect NDC or inaccurate reporting of a drug quantity will cause the agency to report
false drug rebate calculations to manufacturers.
To download the agency’s version of the Federal List of Drug Manufacturers
Participating in the Centers for Medicare and Medicaid’s (CMS) visit the
agency’s Drug Rebate Program list.
Prescription Drug Program
-74-
Billing
What are the general instructions for billing?
Providers must follow the billing requirements found in the agency ProviderOne Billing
and Resource Guide.
Bill the agency your usual and customary charge using the complete 11-digit NDC from
the dispensing container.
Report the actual quantity dispensed using the appropriate metric or metric decimal
quantity for the product.
Remember that the agency is the payer of last resort. See “Coordination of Benefits”
later in this section. (Claims paid inappropriately when other coverage is available
may be recouped.)
Clients who are enrolled in an agency managed care plan are eligible for pharmacy
services under their designated plan. Bill the plan first.
Important Notes:
When another insurer or an agency managed care plan requires authorization for a
drug, perform all steps necessary to obtain the authorization.
Requiring authorization is not the same as a denial of coverage.

Prescription Drug Program
-75-
When does the tamper-resistant prescription pad
requirement apply?
The requirement for tamper resistant pads or paper applies whether Medicaid is the primary or
secondary payer. All written prescriptions for Medicaid clients in fee-for-service (FFS)
programs, including over-the-counter medications, must be on tamper-resistant pads or paper, in
compliance with federal regulations.
What is the requirement?
42 United States Code (USC) Section 1936b(i)(23) requires that the tamper-resistant paper must
prevent the prescription from being changed by having at least one of the following
characteristics:
No copying: For example, pantographs that reveal the word “VOID” when
copied.
No altering: For example, chemical stains or an altered background reveal
attempts at ink or toner removal.
No counterfeiting: For example, pads have a watermark and cannot be reproduced.
The prescription pads must have all three characteristics to be considered tamper-resistant.
Information about vendors who provide prescription pads that meet the federal requirement can
be found at Washington Medicaid. The Health Care Authority (agency) does not endorse any
specific vendor. The agency does not reimburse prescribers’ costs for tamper-resistant paper.
Prescriptions that are telephoned, faxed, or sent electronically to the pharmacy are exempt
from this federal requirement. Pharmacists receiving non-compliant, written prescriptions are
encouraged to verify the prescription with the prescriber.

Prescription Drug Program
-76-
How are clients enrolled in managed care
affected?
This requirement for tamper-resistant paper does not apply to prescriptions paid for by a
managed care plan for enrollees in one of the following:
Healthy Options (HO) Managed Care
Basic Health Plus (BHP+)
General Assistance Unemployable-Managed Care (GAU-MC)
Washington Medicaid Integration Partnership (WMIP)
Medicare/Medicaid Implementation Program (MMIP).
This requirement does apply when a managed care contract excludes a drug if the drug is
otherwise reimbursable under fee-for-service.
If a client has third-party liability (TPL) with a managed care plan for non-contracted
Medicaid services, then the requirement does apply.
What about emergency dispensing?
Pharmacists are allowed to dispense a prescription written on non-compliant paper as long as the
pharmacy receives verification from the prescriber by telephone, fax, or email within 72-hours of
filling the prescription. Federal controlled substance laws must continue to be met when
prescribing or dispensing Schedule II drugs.
What about Medicaid clients with retroactive
certification?
To submit a claim for a Medicaid client retroactively certified for Medicaid, the following
applies:
The prescription must meet the tamper-resistant paper requirement.
Refills that occur after the date on which the client is determined to be eligible require a
new, tamper-resistant prescription.
If the original order is not compliant with the tamper-resistant paper requirement, the
pharmacy may either obtain a verbal, faxed, or email confirmation of the prescription
from the prescriber.
The pharmacy must reimburse the client in accordance with WAC 182-502-0160.

Prescription Drug Program
-77-
What are the documentation and records
retention requirements?
The pharmacist must document that the prescriber was contacted by telephone, fax, or email to
verify that the legitimacy of the prescription written on non-compliant paper before it was
dispensed.
Prescription records, including documentation for non-compliant prescriptions, must be kept for
six years according to WAC 182-502-0020.
What is needed for prescription transfers
between pharmacies?
The pharmacy accepting a prescription transfer from another pharmacy only need to obtain a
telephone call or fax from the transferring pharmacy in order to confirm the authenticity of the
tamper-resistant prescription.
What is the time limit for billing?
(WAC 182-502-0150)
The agency requires providers to submit initial claims and adjust prior claims in a timely manner.
The following are the agency’s timeliness standards for initial claims, resubmitted claims, and
for claim adjustments in the Prescription Drug Program. For more information on timelines for
billing, refer to the agency ProviderOne Billing and Resource Guide.
Medicare Part B crossover claims: If Medicare Part B allows the claim, it is no
longer billable as a Prescription Drug Program claim through the Point-of-Sale (POS)
system. A claim allowed by Medicare Part B is billable as a crossover claim on a CMS-
1500 claim form within six months of the date that Medicare processes the claim. If
Medicare denies payment of the claim, the agency requires the provider to meet the
agency’s initial 365-day requirement for the initial claim. If you have billed Medicare but
have not received a response, you must still bill the agency within 365 days of the date of
service to establish timeliness.

Prescription Drug Program
-78-
Resubmitted claims and adjustments
The agency allows providers to resubmit, modify, or adjust any prescription drug claim
with a timely ICN within 15-months of the date the service was provided to the client.
Claims may be resubmitted, modified, or adjusted by the pharmacy electronically for 365
days from the date dispensed. Resubmissions, modifications, or adjustments between 12
and 15 months old must be submitted as a hardcopy claim on an appropriate agency form.
Hardcopy claims and adjustments over 365 days old must include the ICN of the original
timely transaction. After 15-months, the agency does not accept a prescription drug claim
for resubmission, modification, or adjustment.
Reversals
The agency allows pharmacies to reverse any prescription drug claim with a timely ICN
within 15-months of the date the service was provided to the client. Hardcopy paper
adjustment forms must be submitted if a pharmacy wishes to reverse a transaction that
can no longer be reversed electronically from their own system. This includes all three of
the following:
Reversals between 12 and 15 months from the dispensing date.
“Lost” transactions (paid claim not found in the pharmacy’s own system).
Claims older than the pharmacy’s own system will allow them to reverse.
Overpayments that must be refunded to the agency
The 15-month period allowed for resubmission of claims above does not apply to
overpayments that a prescription drug provider must refund to the agency. After 15-
months, a provider must refund overpayments by a negotiable financial instrument, such
as a bank check. Do not submit a claim adjustment. Questions regarding overpayments
may be directed to the MPA Cash Control at 360-725-1279.
Client's responsibility
The agency does not allow a provider or any provider’s agent to bill a client or a client’s
estate when the provider fails to meet the requirements, resulting in the claim not being
paid by the agency (see Billing a Client.)

Prescription Drug Program
-79-
What is the national provider identifier (NPI)
requirement?
Pharmacy providers are required to provide the pharmacy and prescriber National Provider
Identifiers (NPIs) on all prescription drug claims.
The agency requires a prescriber to provide its individual NPI (Type 1) with prescription drug
orders that are written, transmitted, called in, or faxed. This NPI requirement applies to all
providers who write prescription orders for drugs.
The prescriber NPI must be for an individual (Type 1) rather than an organization (Type 2). The
ProviderOne POS does not recognize Type 2 NPIs for organizations (such as hospitals) as valid
prescribers.
The following are examples of how to report the practitioner’s individual NPI (Type 1) with
prescription orders:
An emergency room practitioner must report his or her individual NPI (Type 1), not the
supervising practitioner’s NPI with a prescription order.
Each practitioner in a teaching hospital must report his or her individual NPI (Type 1)
with a prescription order that is submitted to the dispensing pharmacy.
BILLING
Hard copy billers must enter a valid Type 1 NPI in the Prescriber NPI field on the
Pharmacy Statement (525-106) form, 13-714.
Point-of-Sale billers:
Enter 01 in the Service Provider ID Qualifier field (202-B2).
Enter your NPI in the Service Provider ID field (201-B1).
Enter 01 in the Prescriber ID Qualifier field (466-EZ).
Enter the prescribers NPI in the Prescriber ID field (411-DB).

Prescription Drug Program
-80-
What is needed to bill for filling a newborn
prescription?
BILLING
Hard copy billers must indicate "Baby using mom’s ProviderOne Client ID” in the
Justification/Comments field on the Pharmacy Statement (525-106) form, HCA 13-714.
Point-of-Sale billers: Enter “2” in the Insurance Segment, Eligibility Clarification
Code field.
When is a pharmacy allowed to bill a client?
(WAC 182-502-0160)
A pharmacy may bill a fee-for-service client for a noncovered prescription if the client and
provider complete and sign an Agreement to Pay for Healthcare Services form, HCA 13-879.
The provider may NOT bill the client for any service which is potentially covered with prior
authorization, unless that authorization has been requested and denied.
The agency asks that pharmacists use their professional judgment when accepting cash for
noncovered services. If you believe that a prescription may not be medically necessary for the
client (such as a noncovered early refill for large volumes of narcotics), you have no obligation
to accept cash payment and may refuse service to the client.
REMINDER: A common billing complaint is the pharmacist misinterpreting a POS
message as a denial and charging the client instead of calling the agency for authorization.
Remember that it is the pharmacist's responsibility to call the agency for authorization
when the pharmacist receives an authorization message from the POS system.

Prescription Drug Program
-81-
Who is eligible?
The POS system does not solve the problem of identifying clients who are not currently in the
agency's eligibility file. It is not appropriate to charge a client cash if they are currently eligible
on the Benefit Inquiry Screen in ProviderOne. For a client who’s benefit inquiry screen in
ProviderOne shows that they are eligible, but their claims deny in the POS system for lack of
eligibility, take one the following steps:
FAX a copy of the client’s benefit screen from ProviderOne to Claims Entry at 866-668-
1214.
Mail in a completed paper claim with a photocopy of the benefit inquiry screen from
ProviderOne attached.
The benefit inquiry screen in ProviderOne will be updated within two working days in order for
claims to be resubmitted. Do not fax claims to this number.
See Billing the Client WAC 182-502-0160.
What services are billed for hospice clients?
Clients enrolled in the Hospice program waive services outside the Hospice program that are
directly related to their terminal illness. All services related to their terminal illness must be
coordinated by the designated hospice agency (be sure to call the hospice agency to find out what
must be billed under hospice) and attending physician only.
Services not related to their terminal illness may be provided to clients on an FFS basis. When
billing for hospice clients and the service is not related to the terminal illness (be sure to call the
hospice agency to find out what medications are not related to the hospice diagnosis or end-of-
life care needs), use the following billing procedures:
BILLING
Hard copy billers must enter “K” in the Justification/Comments field on the Pharmacy
Statement (525-106) form, 13-714.
Point-of-Sale billers must enter “11” in the Patient Segment, Patient Residence (384-
4X) field.
Do not use this procedure for dates the client is not on hospice services. Be sure to check with
the hospice agency before you use the “K” or “11”.

Prescription Drug Program
-82-
Does the agency reimburse for client’s
prescriptions when enrolled in an agency
managed care plan?
Eligible medical assistance clients enrolled in an agency contracted managed care organization
(MCO) must have their prescriptions filled at a pharmacy contracted with the MCO. If your
pharmacy is non-contract, the client must be referred to an MCO-contracted pharmacy.
The agency reimburses for drugs dispensed to clients enrolled in an agency managed care plan
only when a managed care contract excludes the drug or pharmaceutical supply and the product
is otherwise reimbursable fee-for-service (FFS). The following may be billed FFS to the agency:
Prescriptions written by dentists or related to dental health.
Chemical dependency treatment drugs when the client is enrolled in a state certified
chemical dependency program.
Antibiotics, anti-infectives, non-narcotic analgesics, and oxytocics prescribed following a
voluntary termination of pregnancy.
Hemophilia and von Willebrand-related products when used for administration in the
home.
Prescriptions written by practitioners working for a non-contracted Health Department, or
a non-contracted Family Planning agency with the therapeutic classifications listed
below:
Family planning agencies may prescribe contraceptives, drugs for sexually-transmitted diseases
(STD) (excluding HIV) when related to the client’s family planning method, and drugs related to
a sterilization procedure within the following therapeutic drug classes:
Analgesics
Antibiotics
Anti-emetics
Antifungals
Anti-infectives
Anti-inflammatories
Contraceptive drugs/devices
Prescription Drug Program
-83-
Health departments may prescribe drugs for STD (excluding HIV), tuberculosis, and
prescription contraceptives within the following therapeutic drug classes:
Antibiotics
Anti-emetics
Anti-infectives
Contraceptive drugs/devices
Tuberculosis drugs
Billing for managed care clients
BILLING
Hard copy billers must enter the following comment in the Justification/
Comments field on the Pharmacy Statement (525-106) form, HCA 13-714.
Managed Care Exclusion
Point-of-Sale billers must enter “2” in the Claim Segment, Prior Authorization Type
Code (461-EU) field.
Pharmacists must document on the original prescription the reason for billing FFS. All FFS rules
apply, including authorization requirements.
What drugs may be prescribed for Family
planning only and TAKE CHARGE clients?
Family planning agencies may prescribe the following drugs related to family planning and
contraceptives within the following therapeutic drug classes to Family Planning Only or TAKE
CHARGE clients:
Contraceptives and Drugs
Contraceptives, oral, including
emergency contraception
Macrolides
Contraceptives, injectables
Antibiotics, misc. other
Contraceptives, transdermal
Quinolones
Contraceptives, intravaginal
Cephalosporins – 1
st
generation
Contraceptives, intravaginal, systemic
Cephalosporins – 2nd generation
Contraceptives, implantable
Cephalosporins – 3rd generation
Vaginal lubricant preparations
Absorbable Sulfonamides
Condoms
Nitrofuran Derivatives
Prescription Drug Program
-84-
Diaphragms/cervical caps
Antifungal Antibiotics
Intrauterine devices
Antifungal Agents
Vaginal antifungals
Anaerobic antiprotozoal – antibacterial agents
Vaginal Sulfonamides
Vaginal Antibiotics
Tetracyclines
The agency covers anti-anxiety medications before a sterilization procedure and pain
medications after a sterilization procedure for Family Planning Only and TAKE CHARGE
clients as follows:
Anti-anxiety Medication – Before Sterilization Procedure
Medication
Maximum Number of Doses
Diazepam
2
Alprazolam
2
Pain Medication – After Sterilization Procedure
Medication
Maximum Number of Doses
Acetaminophen with Codeine #3
12
Oxycodone HCl/Acetaminophen
5/500
12
Hydrocodone
Bit/Acetaminophen
12
Oxycodone HCl/ Acetaminophen
12
BILLING
When billing for the covered preoperative anti-anxiety medications and postoperative
pain medications for TAKE CHARGE or Family Planning Only clients:
Hard copy billers must enter “Family planning sterilization medication” in the
Justification/Comments field on the Pharmacy Statement (525-106) form, HCA 13-714.
Point-of-Sale billers must enter “6” in the Claim Segment, Prior Authorization Type
Code (461-EU) field.

Prescription Drug Program
-85-
Does the agency reimburse for skilled nursing
facility (SNF) clients?
Over-the-counter (OTC) drugs
The agency does not reimburse for OTC drugs when the client resides in a SNF unless the drugs
are on the Washington Preferred Drug List (see Therapeutic Interchange Program (TIP)) or the
agency’s Covered Over-the-Counter Drug List. Reimbursement for OTC drugs is included in the
SNF per diem.
How are medications filled for SNF clients on
leave?
SNF clients on leave should have their additional maintenance prescriptions filled for the
duration of the leave. If the client leaves weekly, prescriptions should be filled for a one-month
supply.
SNFs should determine which one of the following methods will be followed when a SNF client
goes on leave:
The client may take the prescription medication home and keep it there for use during
SNF absences.
The client may return the prescription medication to the SNF following each leave so that
it may be stored for safekeeping. The prescription medication is the client’s personal
property.
Both of these practices are in accordance with state pharmaceutical regulations.
BILLING
Hard copy billers must indicate weekend pass or take home/leave supply in the
Justification/Comments field on the Pharmacy Statement (525-106) form, HCA
13-714.
Point-of-Sale billers: Enter 8 in the Claim Segment, Prior Authorization Type
Code (461-EU) field.

Prescription Drug Program
-86-
What is an emergency kit?
The emergency kit is a set of limited pharmaceuticals furnished to a SNF by the pharmacy that
provides prescription dispensing services to that facility. Each kit is specifically set up to meet
the emergency needs of each SNF’s client population and is for use during those hours when
pharmacy services are unavailable.
Medications supplied from the emergency kit are the responsibility of the SNF.
What unit dose delivery systems are recognized
by the agency?
(WAC 182-530-7350)
The agency recognizes two types of Unit Dose Delivery Systems for SNFs:
True Unit Dose Delivery System
Modified Unit Dose Delivery System
Eligible unit dose providers receive the unit dose dispensing fee when dispensing in-house unit
dose prescriptions. The term in-house unit dose applies to bulk pharmaceutical products that are
packaged by the pharmacy for unit dose delivery. Providers receive the regular pharmacy
dispensing fee for drugs that are manufacturer packaged in unit dose form.
Refer to Reimbursement for agency Dispensing Fee Allowances for pharmacies.
How do pharmacies become eligible for a unit
dose dispensing fee?
(WAC 182-530-5100(1))
To be eligible for a unit dose dispensing fee from the agency, a pharmacy must:
1. Notify the agency in writing of its intent to provide unit dose service.
2. Specify the type of unit dose service to be provided.
3. Identify the SNF or facilities to be served.
4. Indicate the approximate date unit dose service to the facility or facilities will commence.
5. Sign an agreement to follow the agency requirements for unit dose reimbursement.
For information on becoming a unit dose provider, contact Provider Enrollment (see Resources
Available).
Prescription Drug Program
-87-
How do pharmacies bill the agency under a unit
dose delivery system?
(WAC 182-530-5100(2), (3), and (4))
Under a unit dose delivery system, a pharmacy must bill the agency only for the number of drug
units actually used by the agency client in the skilled nursing facility (SNF).
It is the unit dose pharmacy provider’s responsibility to coordinate with the SNFs to ensure that
the unused drugs the pharmacy dispensed to the facility for distribution to an agency client are
returned to the pharmacy for credit.
The pharmacy must submit an adjustment form or claims reversal of the charge to the agency for
the cost of all unused drugs returned to the pharmacy from the SNF on or before the 60th day
following the date the drug was dispensed. This adjustment must conform to the SNF’s monthly
log.
Exception:
Unit dose providers are not required to credit the agency for federally designated
schedule II drugs that are returned to the pharmacy. These returned drugs must be
disposed of according to federal regulations.
BILLING
Hard copy billers must indicate "In-house unit dose” in the Justification/Comments
field on the Pharmacy Statement (525-106) form, HCA 13-714.
Point-of-Sale billers: Enter “3” in the Claim Segment, Unit Dose Indicator (429-DT)
field.
Who is responsible for the cost of repackaging
client’s bulk medications?
(WAC 182-530-5100(5))
The cost of repackaging is the responsibility of the SNF when repackaging is done for either of
the following reasons:
To conform to the SNF’s delivery system
For the SNF’s convenience
Pharmacies may not charge clients or the agency a fee for repackaging a client’s bulk
medications in unit dose form.

Prescription Drug Program
-88-
What records do SNF pharmacies need to keep?
(WAC 182-530-5100(6) and (7))
The pharmacy must maintain detailed records of medications dispensed under unit dose delivery
systems. The pharmacy must keep a monthly log for each SNF served, including, but not limited
to the following information:
Facility name and address
Client’s name and ProviderOne Client ID
Drug name/strength
National Drug Code (NDC)
Quantity and date dispensed
Quantity and date returned
Value of returned drugs or amount credited
Explanation for no credit given or nonreusable returns
Prescription number
Upon request, the pharmacy must submit copies of these monthly logs to the agency. The agency
may request the pharmacy submit such logs on a monthly, quarterly, or annual basis.
What needs to be submitted annually to the
agency?
(WAC 182-530-5100(8))
When the pharmacy submits the completed annual prescription volume survey to the agency, it
must include an updated list of SNFs served under unit dose systems.

Prescription Drug Program
-89-
What additional records do pharmacies need to
keep?
(WAC 182-530-5000)
In addition to the record keeping requirements found in the agency ProviderOne Billing and
Resource Guide, pharmacies must comply with the following:
Provision of prescription drugs
Keep any specifically required documents for the provision of prescription drugs, including but
not limited to:
Authorizing an order (prescription).
Name of person performing the service (dispensing pharmacist).
Details of medications and/or supplies prescribed or provided including NDC, name,
strength, and manufacturer.
Drug Use Review (DUR), intervention, and outcome documentation.
Expedited authorization (EA) documentation.
Proof of fill.
Proof of delivery
When a provider delivers an item directly to the client or the client's authorized
representative, the provider must be able to furnish proof of delivery including signature,
client’s name, and a detailed description of the item(s) delivered.
When a provider mails an item to the client, the provider must be able to furnish proof of
delivery, including a mail log.
When a provider uses a delivery/shipping service to deliver items, the provider must be
able to furnish proof of delivery documentation of the following:
Include the delivery service tracking slip with the client's name or a reference to
the client's package(s); the delivery service package identification number; and
the delivery address.
Include the supplier's shipping invoice, with the client's name; the shipping
service package identification number; and a detailed description(s).

Prescription Drug Program
-90-
When a client or the client’s authorized representative picks up the prescription, the
provider must be able to furnish proof of delivery including signature, client’s name, and
a detailed description of the item(s) delivered.
Make proof of delivery records available to the agency, upon request.

Prescription Drug Program
- 91 - Coordination of Benefits
Coordination of Benefits
How are client resources applied?
The agency is required by federal regulation to determine the liability of third-party resources
available to agency clients. All resources available to the client that are applicable to the costs of
medical care must be used. Once the applicable resources are applied, the agency may make
reimbursement for the balance if the insurance payment is less than the agency’s allowed
amount.
It is the provider’s responsibility to bill the agency appropriately after pursuing any potentially
liable third-party resource when:
Health insurance is indicated in ProviderOne.
The Point-of-Sale (POS) system alerts the provider to a client’s insurance.
The provider believes insurance is available.
(See WAC 182-501-0200)
The Insurance Carrier List and carrier information is available on the agency website at
http://www.hca.wa.gov/medicaid/billing/documents/hcarrier.txt. The information can be
downloaded and printed or used as an online reference.
The agency’s billing time limit is 365-days, but an insurance carrier’s time limit to bill may be
different. It is the provider’s responsibility to meet the insurance carrier’s billing time limit prior
to receiving any payment by the agency. The provider should not bill the agency with an Other
Coverage Code if the claim was denied by the insurance carrier for late filings.
Prescription Drug Program
- 92 - Coordination of Benefits
Other coverage codes
Why are other coverage codes important?
The agency POS system alerts a provider when a client has other insurance. When a provider
submits a claim through the POS system, and the agency files indicate that a client has insurance,
the agency will deny the claim. Then the provider must bill the client’s insurance before
using the Other Coverage Codes.
The provider’s weekly Remittance and Status Report (RA) show that the claim is denied with the
Explanation of Benefits (EOB) 090. The EOB states “Bill your claim to the insurance
company as instructed. For questions call 800-562-6136.” The insurance carrier information is
printed on the RA for the provider's reference.
When may providers use other coverage codes?
The following chart lists situations in which other insurance is available, gives some direction to
the provider, and explains which Other Coverage Codes to enter. In all of the situations
described below, the pharmacy must bill the other insurance before using an Other Coverage
Code.
The chart also provides information about documentation. Pharmacy providers who submit their
claims through the POS system are not required to submit third-party documents. However, the
provider must have these documents available for audit purposes. Examples of the
documentation that would justify the provider’s use of an Other Coverage Code are listed
below in italics.
Contact the Coordination of Benefits (COB) Hotline 800-562-3022 for any situations that are not
listed below. See Point-of-Sale (POS) for values and definitions of the Other Coverage Codes.
A removable summary of the following table is available at the end of the COB Section.
Situations
Explanation/
Solution
Other
Coverage
Code
The insurance has made payment to the pharmacy.
(An EOB or electronic transmission from insurance
identifying the insurance paid amount.)
Bill balance to
the agency.
2
Prescription Drug Program
- 93 - Coordination of Benefits
Situations
Explanation/
Solution
Other
Coverage
Code
Insurance allowed amount of the prescription is less than or
equal to the copay.
(An EOB or an electronic transmission from insurance
identifying both the insurance allowed and co-pay
amounts.)
Bill the agency.
4
The prescription must be filled by mail order.
(Contract verification that the prescription must be filled
by mail order; or denial from insurance stating mail
order.)
Bill the agency.
3
The plan only covers a new prescription.
(An EOB or electronic transmission from insurance
showing only new prescriptions covered.)
Bill refills to the
agency.
3
The insurance carrier applied the claim charges to the
client’s deductible.
(An EOB or electronic transmission from insurance
identifying the claim amount was applied to the
deductible.)
Bill the agency.
4
The client’s insurance plan maximum annual benefit has
been met.
(An EOB or electronic transmission from insurance
identifying the annual benefit has been met.)
Bill the agency.
4
The insurance denied the medication as a noncovered drug.
Clarify if denial is for noncovered or nonformulary drugs. If
nonformulary, third-party payment procedures must be
followed.
(An EOB or electronic transmission identifying the drug
is noncovered or include a copy of the contract drug
exclusion list.)
Bill the agency.
3
The client has a discount card.
(Verification of discount card or denial from insurance
stating “discount card”.)
Bill the agency.
3
Capitated service agreement with insurance carrier.
(Group Health and Kaiser pharmacies only.)
Bill the agency.
8
Medicare Part D copay. The agency does not provide
coverage for Medicare Part D medication copayments.
Medicare Part D copayments is be the responsibility of the
client.
For questions, call the agency Customer Service Center
at 800-562-3022.)
Note: For questions on the use of Other Coverage Codes or acceptable documentation,
call Coordination of Benefits at 800-562-3022.

Prescription Drug Program
- 94 - Coordination of Benefits
If one of the previously listed situations occurs, providers may resubmit the claim entering an
Other Coverage Code into the POS system to bypass the edit for other insurance coverage.
Inappropriate use of Other Coverage Codes may result in an audit of your POS claims and
recoupment of improper payments.
Note: In instances where the primary insurance has made payment, the normal
34-day supply limit may be exceeded.
Clients with privately purchased HMO insurance
A client with privately purchased health maintenance organization (HMO) insurance will have
an HI, HO, or HM identifier on the client benefit inquiry screen in ProviderOne. The client is
required to use the HMO facilities for pharmacy services. If services are provided that are not
covered by the HMO plan, the claim may be submitted to the agency for processing.
Situations may occur when a client is out of the HMO service area or HMO coverage is not
accessible, a pharmacy provider may proceed to meet the client’s immediate needs.
Billing
Pharmacy providers who submit their claims through the POS system are not required to submit
insurance EOB documents. However, documentation must be retained and kept by the
provider for audit purposes. (See WAC 182-502-0020)
Primary insurance billing exceptions
Primary insurance billing exceptions listed below are examples of third-party situations and how
they are processed in the POS system. All amounts billed to the insurance and to the agency must
be usual and customary charges, except for capitated copayments.
What does the provider do if a third-party liability question arises and it is after COB
hours?
Situations may occur when a client is asking to fill a prescription, a question arises and it is
outside of COB’s regular business hours. After making reasonable attempts to access the primary
insurance coverage, proceed with filling what is necessary to meet the client’s immediate needs.
“Immediate needs” means pharmacists using their professional judgment to determine the
quantity to dispense to best meet the client’s needs in an emergency. The pharmacy must contact
COB within 7 days for billing assistance.

Prescription Drug Program
- 95 - Coordination of Benefits
Examples may include:
The agency indicates that the patient has insurance, but the coverage cannot be identified
and the patient does not provide it.
The patient has HMO private insurance or has a closed pharmacy network.
What does the provider do if the client’s coverage is prepay?
Contact COB for billing assistance if the client’s coverage is prepay. Prepay means the client’s
identified insurance coverage policy requires the client to pay at the time of service, and the
insurance reimbursement is made only to the subscriber. Do not bill the insurance and do not bill
the agency with an Other Coverage Code. Prepay is defined on a case-by-case basis.
How is authorization obtained for nonformulary
or noncovered drugs?
Pharmacists are required to obtain prior authorization from the insurance carrier for
nonformulary drugs before providing the drugs to the client. When the denial reason is related to
a nonformulary drug, the pharmacy may need to coordinate with the prescriber and/or the insurer
to authorize an alternative drug or get the insurer to cover the prescription as prescribed. Do not
use an Other Coverage Code. The pharmacy must meet all third-party billing requirements
prior to billing the agency.
Noncovered drugs are not to be confused with nonformulary drugs. It is the provider’s
responsibility to correctly determine if the drug is noncovered or nonformulary with the primary
insurance carrier. Noncovered drugs may be billed to the agency using the Other Coverage
Code 3.
Prescription Drug Program
- 96 - Coordination of Benefits
Coordination of Benefits
Frequently Asked Questions
(FAQ)
How is prescription drug coverage verified and
who processes the prescriptions?
Ask the client for an insurance card, and/or Services Card. If the client benefit inquiry screen
indicates the client has an insurance carrier and you do not know where to submit the claims,
contact the insurance carrier. Verify there is retail prescription coverage with the insurance
carrier and ask where to submit claims. When you submit a claim through the POS system and
no Other Coverage Code has been entered, you will be notified if the client has prescription
coverage.
To find insurance carrier contact information, visit insurance carrier contact information.
What if a client’s insurance states there is no
coverage or the insurance coverage has ended?
If there is no coverage or the coverage has ended, notify COB at 800-562-3022.
What if a client’s insurance plan cannot identify
the client?
If the insurance carrier cannot identify the client, contact COB at 800-562-3022 to verify the
cardholder identification and the plan being billed are the same as on file with COB. We will
assist you with verifying the client’s prescription coverage or update COB records if the client
does not have coverage.
Prescription Drug Program
- 97 - Coordination of Benefits
What is discount only or mail order only
coverage?
Discount only or mail order only coverage means insurance does not reimburse for any
prescriptions filled at retail pharmacies.
If a client has discount only or mail order only benefits, the agency does not consider
this a primary insurance. Bill the agency.
If you bill the agency and we deny the claim to bill the insurance carrier, and you believe
the client has discount only coverage, contact COB.
Note:
Some clients have mail order only on certain prescriptions, requiring
them to use mail order when they refill prescriptions on an ongoing or
regular basis.
Insurance carriers may refer to mail order as “maintenance”. For example,
some plans consider mail order to be maintenance when a certain
prescription is refilled more than two times. Bill the insurance carrier first.
If the claim is denied by insurance to use mail order, then bill the agency
with an Other Coverage Code 3.
Why would a claim be paid at zero or denied by
insurance?
If the reason the claim was paid at zero cannot be verified, contact the insurance carrier and find
out why the claim was paid at zero or denied. If there are questions about why the claim was
denied or paid at zero after contacting the insurance carrier, contact COB.

Prescription Drug Program
- 98 - Coordination of Benefits
What if the insurance states copay is 100% or
claim is paid at zero?
Contact the insurance carrier. Examples of when the insurance states copay is 100% are:
A prepay plan. Prepay means the client’s insurance coverage requires the client to pay at
the time of service, and the insurance reimbursement is made to the subscriber. In this
instance, reverse your billing to the primary insurance, and call COB for billing
assistance at 800-562-3022. Do not bill the insurance, and do not bill the agency with an
Other Coverage Code.
Less than copay, benefits are exhausted, or any other paid at zero response. Bill the
agency using Other Coverage Code 4.
How are after hour services billed?
After hours services means prescriptions filled outside of COB regular business hours. After
making reasonable attempts to meet the primary insurance carrier’s billing requirements, proceed
with filling what is necessary to meet the client’s immediate needs.
What is “meeting client’s immediate needs?”
Immediate needs means pharmacists are to use their professional judgment to determine the
quantity to dispense to best meet the client’s needs in an emergency. Contact COB within 7-days
for billing assistance. Examples may include:
The agency indicates the client has insurance, but you cannot identify the coverage; or
The client has HMO private insurance or has a closed pharmacy network.
What is the service area?
Service area means the nearest pharmacy that accepts the insurance within 25 miles or 45
minutes in one direction from the client’s address.
What if POS will not accept an Other Coverage
Code, or a field is not provided to enter Other
Coverage Code?
Contact your pharmacy software or switch vendor.

Prescription Drug Program
- 99 - Coordination of Benefits
Why does a claim get a rejection code DV
(MISSING/INVALID OTHER PAYER
AMOUNT PAID) or E8 (MISSING/INVALID
OTHER COVERAGE CODE) when billing the
balance to the agency?
If there is a rejection code DV, you have indicated that insurance made payment by entering 2 in
the Other Coverage Code field, but the payer amount was entered as 0.00.
If there is a rejection code E8, an insurance payment was entered, but a 2 in the Other Coverage
Code field was not.
Verify the insurance carrier has made payment, and enter the amount in the other payer amount
field. If there is no insurance payment, do not enter a 2 in the Other Coverage Code field;
contact the insurance carrier to find out why the payment was not made. If you have verified the
insurance amount paid and the payment amount is not displayed on the POS system, contact your
software or switch vendor.
If the claim does not go through, is entering $.01
in the insurance paid field allowed?
No. Enter an amount only if $.01 or another amount is the actual amount paid by the insurance.
Entering any amount paid by the insurance carrier other than the actual amount paid could be
considered fraudulent.
When can Other Coverage Code 8 be used?
For non-Medicare Part D billing, the agency allows only pharmacy providers that have a
capitated service agreement with an insurance carrier to use this Other Coverage Code. At this
time, only Group Health and Kaiser are known to have capitated service agreements.

Prescription Drug Program
- 100 - Coordination of Benefits
How is a claim submitted to the agency when the
insurance allowed amount is less than or equal to
the copay amount?
The copay is the amount that private insurance has determined the person with the private
insurance coverage is expected to pay per prescription.
Note: Eligible Medicaid clients with private insurance are not expected to pay a
copay. When the insurance allowed or payable amount is less than or equal to the
copay amount, the insurance non payment reason is less than the copay. Bill the
agency, after you bill the insurance. Use a 4 in the Other Coverage Code field.
What is a closed pharmacy network?
Closed pharmacy network means an insurer restricting prescription coverage to an exclusive
list of pharmacies. This arrangement prohibits the coverage and/or payment of prescriptions
provided by a pharmacy not included on the exclusive list (see WAC 182-502-0130 (3)(a)).
The agency may pay for the prescription without requiring the client to use a participating
network pharmacy ONLY in the following situations:
When the prescription is not covered by the policy.
If the client is out of the service area.
If you provided medications to meet a client’s immediate need for services.
If you are not a participating pharmacy, do not bill with an Other Coverage Code prior to
contacting COB.

Prescription Drug Program
- 101 - Coordination of Benefits
Does the agency require clients to use pharmacy
providers that are contracted with the client’s
private insurance carrier?
The agency requires clients to use pharmacy providers contracted with their private insurance
carrier. Clients with managed care private insurance will have an HM, HI, or HO identifier on
the client benefit inquiry screen in ProviderOne.
If the insurance carrier provides pre-pay plan coverage for noncontracted pharmacy providers,
contact COB for billing assistance.
If a pharmacy is not contracted and the coverage is not pre-pay, the agency may pay for the
prescription without requiring the client to use a contracted pharmacy ONLY in the following
situations:
When the prescription is not covered by the policy
If the client is out of the service area
If you provided medications to meet a client’s immediate need for services
Do not bill with an Other Coverage Code prior to contacting COB.
What if a client’s insurance coverage requires
paper billing and the pharmacy only bills
electronically?
The pharmacy must meet all third-party billing requirements prior to billing the agency.
If the insurance coverage is a pre-pay plan for paper billers, contact COB for billing assistance.
Do not bill with an Other Coverage Code prior to contacting COB.
How are paper bills submitted to the agency after
the primary insurance has been billed?
While POS billing is preferred, pharmacies who submit their claims to the agency on paper must
enter any amount paid by the primary insurance in the insurance paid amount field and the
primary insurance processing details in the justification/comments section on the Pharmacy
Statement (525-106) form, HCA 13-714.

Prescription Drug Program
- 102 - Coordination of Benefits
If the client is enrolled in an agency-contracted
managed care organization and private
insurance, is the MCO billed for the service or
the private insurance?
If a client is enrolled in an agency-contracted managed care organization (MCO) and also has
private insurance for the date of service, the pharmacy bills the MCO. Contact the MCO for
billing assistance and information about the primary coverage.
If I bill the insurance carrier and the denial
reason is “plan limits exceeded,” can I bill the
agency with an Other Coverage Code?
If the client has exceeded their insurance benefit, it is appropriate to bill the agency with an
Other Coverage Code 3.
The pharmacy must meet all third-party billing requirements prior to billing the agency.
How do I bill if the insurance carrier requires
authorization?
The primary insurance carrier requirements must be met. Contact the insurance carrier for
authorization review, and to determine if, and how the medication is covered by the insurance
plan. If the primary insurance carrier’s authorization process has been followed to completion
and authorization is denied, bill the agency with Other Coverage Code 3.

Prescription Drug Program
- 103 - Coordination of Benefits
The insurance carrier requires authorization.
The prescriber will not provide information to
the pharmacy or insurance carrier and
authorization cannot be obtained. Can the agency
be billed directly?
No. The insurance carrier requirements must be met. It is not appropriate to bill the agency with
an Other Coverage Code unless the billing conditions of the insurance carrier have been met.
How long does documentation need to be kept?
(WAC 182-502-0020)
Providers are required to make documentation available to the agency for six-years from the date
of service. Pharmacy providers who submit their claims through the POS system are not required
to submit third-party EOB documents. However, the provider must retain documentation for
audit purposes.
The client has insurance coverage through
multiple carriers. Am I required to bill all
potential payers?
(WAC 182-501-0150)
Yes. It is the provider’s responsibility to seek timely reimbursement from a third-party when a
client has available third-party resources.
.
- 104 -
(You may remove this page for ease of use.)
Agency other coverage code summary
Situations
Explanation/Solution
Other
Coverage
Code
The insurance has made payment to the pharmacy.
Bill balance to the
agency.
2
Insurance allowed amount of the prescription is less
than or equal to the copay.
Bill the agency.
4
The prescription must be filled by mail order.
Bill the agency.
3
The plan only covers a new prescription.
Bill refills to the agency.
3
The insurance carrier applied the claim charges to the
client’s deductible.
Bill the agency.
4
The client’s insurance plan maximum annual benefit
has been met.
Bill the agency.
4
The insurance denied the medication as a noncovered
drug. Clarify if denial is for noncovered or
nonformulary drugs. If nonformulary, third-party
payment procedures must be followed.
Bill the agency.
3
The client has a discount card.
Bill the agency.
3
Capitated service agreement with insurance carrier.
Bill the agency.
8
Medicare Part D copay.
Medicare Part D is not
covered
For questions on the use of Other Coverage Codes or acceptable
documentation, call the agency at: 800-562-3022.
Prescription Drug Program
- 105 -
How are clients billed who are eligible for both
Medicare and Medicaid?
Some Medicaid clients are also eligible for Medicare Part B or Part D benefits. Bill Medicare
first. The following instructions will assist in billing for dual eligible clients.
Medicare Part B
Some Medicaid clients are also eligible for Medicare benefits. Benefits under Part B Medicare
cover some drugs and drug-related supplies. When you have a client who is eligible for both
Medicaid and Medicare benefits, you should submit claims for that client to your Medicare
intermediary or carrier first. Medicare is the primary payer of claims.
The agency cannot make direct payments to clients to cover the deductible and/or coinsurance
amount of Part B Medicare. The agency can pay these costs to the provider on behalf of the
client when:
The provider accepts assignment.
The total combined reimbursement to the provider from Medicare and Medicaid does not
exceed Medicare's allowed amount.
The agency will pay up to Medicare's allowable, or the agency’s allowable, whichever is less.
ProviderOne will indicate whether or not the client is Medicare-eligible.
QMB with CNP or MNP (Qualified Medicare Beneficiaries with Categorically Needy
Program or Medically Needy Program)
(Clients covered under the CNP or MNP benefit service package as well as QMB)
If Medicare and Medicaid cover the service, the agency will pay only the deductible
and/or coinsurance up to Medicare or Medicaid’s allowed amount, whichever is less.
If only Medicare and not Medicaid covers the service, the agency will pay only the
deductible and/or coinsurance up to Medicare's allowed amount.
If Medicaid covers the service and Medicare does not cover the service, the agency will
reimburse for the service.

Prescription Drug Program
- 106 -
Part B—Medical Insurance
Medicare Part B covers a limited set of drugs. Medicare Part B covers injectable and infusible
drugs that are not usually self-administered and are furnished and administered as part of a
physician service. If the injection is usually self-administered (e.g., Imitrex) or is not furnished
and administered as part of a physician service, it may not be covered by Part B. Medicare Part B
also covers a limited number of other types of drugs. (Regional differences in Part B drug
coverage policies can occur in the absence of a national coverage decision.) For more
information visit Medicare coverage database website.
Medicare part B medications (that are not covered through Part D)
After Medicare Part B has processed the claim, and if Medicare has allowed the medication(s), in
most cases Medicare will forward the claim to the agency for any supplemental Medicaid
payment. When the words, "Claim information forwarded to Medicaid," appear on the
Medicare remittance notice, it means that the claim has been forwarded to the agency or a private
insurer.
If Medicare Part B has paid for a medication and the Medicare crossover claim does
not appear on the agency Remittance and Status Report within 30 days of the Medicare
statement date, bill the agency.
A claim must be submitted to the agency within 6-months of the Medicare
statement date. If Medicare prints remark code MA07 or the phrase “claim
information forwarded to Medicaid” on the EOMB, the agency will extend
the billing period for these claims to 12-months from the date of service.
If Medicare Part B has denied a medication, bill the agency through the POS system
using the appropriate DUR outcome code. Claims may also be billed on the Pharmacy
Statement form and must have the Medicare denial letter or Explanation of Benefits
(EOMB) attached.
Note: When Medicare denies a service that requires authorization, the agency waives the
prior requirement, but authorization is still required.
Bill Medicare’s coinsurance and deductible using the CMS-1500 claim form.

Prescription Drug Program
- 107 -
Medicare part D
Copayments
The agency no longer provides coverage for Medicare Part D medication copayments. Medicare
Part D copayments are the responsibility of the client.
Prescription drug insurance
Medicare Part D-covered drugs are:
Biological products
Insulin and medical supplies associated with the injection of insulin (syringes, needles,
alcohol swabs, and gauze)
Vaccines
Drugs that are:
Available only by prescription.
Used and sold in the United States.
Used for a medically accepted indication.
Certain drugs or classes of drugs, or their medical uses, are excluded by law from Medicare Part
D coverage. Visit the Medicaid Covered Drugs for Part D Dual Eligibles for more information.
While these drugs or uses are excluded from basic Medicare Part D coverage, drug plans may
choose to include them as part of supplemental benefits, not covered by Medicare.

Prescription Drug Program
- 108 -
What if Medicare denies a prescription as
nonformulary?
When the client is covered by Medicare Part D, Medicaid does not pay for any prescriptions that
are the responsibility of Medicare Part D. Contact the prescription drug plan for authorization for
nonformulary drugs. Due process under the Medicaid appeal rules such as an administrative
hearing and Exception to Rule are not available to the client under this circumstance.
Helpful hyperlinks
List of medications that the agency will cover
Medicare Part D website
The agency’s Medicare website
SHIBA website:
http://www.insurance.wa.gov/about-oic/what-we-do/advocate-for-consumers/shiba/.
CMS website:
http://www.cms.gov/.
Prescription Drug Program
- 109 -
Hardcopy Billing:
Pharmacy Statement form
HCA 13-714
What are the general instructions for billing?
The boxes on the Pharmacy Statement (525-106) form, HCA 13-714, are referred to as fields.
Complete all of the fields on the form to ensure prompt processing of your claims. Fields left
blank may result in denial of payment or the return of your Pharmacy Statement as incomplete.
The following fields are required for your submitted form to be processed as a claim:
NPI Number
Patient Identification
Prescription Number
Refill Code
Date Filled
National Drug Code
Those fields that are required for billing are marked with an asterisk (*) on the following pages.
The agency will return incomplete forms submitted to the agency with one or more required
fields left blank to the billing pharmacy.
Hardcopy claim submission is required for rebilling a claim between 12 and 15
months beyond the date of dispense and any reversal that the pharmacy cannot
submit electronically via Point of Sale.

Prescription Drug Program
- 110 -
Provider Name and Address - Enter your
name and address as recorded by the agency.
*NPI Number - Enter your National
Provider Identifier. If this field is left blank,
the form will be returned unprocessed.
*Patient Identification –Copy the
ProviderOne Client ID as it appears on the
client’s Services Card. If this field is left
blank, the form will be returned unprocessed.
Patient Name And Address - Enter the
client's last name, first name and middle
initial. Enter the client's address.
*Prescription Number - Assign in sequence
with regular prescriptions filled by the
pharmacy. The original prescription number
may be used for refills, or a new number may
be assigned. If this field is left blank, the
form will be returned unprocessed.
*Refill Code – Enter the sequentially
assigned two-digit number where 0
represents the original dispense and 1 – 99
represent subsequent refills on the same
Prescription Number. If this field is left
blank, the form will be returned unprocessed.
Date Written - Enter the date on which the
prescriber wrote/ordered the prescription. If
this field is left blank, the claim will be
denied.
*Date Filled - Enter the date the prescription
was dispensed to the client. If this field is left
blank, the form will be returned unprocessed.
Quantity Filled - Enter quantity dispensed
to the client. If this field is left blank, the
claim will be denied.
Estimated Days Supply - Enter the
estimated days' supply for the quantity
dispensed at the prescribed Sig. If this field is
left blank, the claim will be denied.
*National Drug Code (NDC) - Enter the
manufacturer's complete 11-digit NDC from
the dispensing container. For compound
claims, enter the NDC of the primary active
ingredient.
All digits, including zeros, must be
entered. The three sections in the NDC field
must have numbers entered in the correct
section. The labeler code portion of the 11-
digit NDC will always consist of five
numeric characters; the product code
portion consists of four numeric characters;
and the package size will be two numeric
characters.
SAMPLE NDC NUMBER: 12345-6789-
10
12345 = labeler code
6789 = product code
10 = package size
For Compound prescriptions, enter NDC
00000-0000-00 in Section 1, and individual
ingredient NDCs in Section 3.
If this field is left blank, the form will be
returned unprocessed.
Drug Name - Enter the drug name and
strength.

Prescription Drug Program
- 111 -
Prescriber's NPI - Enter the 10-digit
National Prescriber Identifier of the
prescriber. If the prescriber does not have an
NPI number, enter the prescriber’s 9-digit
DEA number. Be sure to use the unique
individual provider identification number.
Do not complete with a group billing
number. If this field is left blank, the claim
will be denied.
Prescription (Directions for Use) - Enter
the Sig.
Authorization Number - Enter the 11-digit
authorization number or Expedited
Authorization number if applicable.
Generic - Enter an "X" under Yes if generic
substitution is permitted by the prescriber.
Enter an "X" under No if the prescriber has
indicated the order should be Dispensed As
Written.
Justification/Comments – Enter values or
descriptions using this guide. Enter any
other information applicable to this
prescription.
Total Charge - Enter your Usual and
Customary charge (U&C), including your
dispensing fee. Do not include tax.
Insurance Paid Amount - Enter any amount
paid by insurance. Do not enter the co -
payment amount here (see Coordination of
Benefits).
Balance Due - Enter the amount due after
deducting any insurance.
Section 3
For Compound claims:
Name – Enter the drug name and strength.
NDC – Enter the manufacturer's complete
11-digit NDC from the dispensing
container.
Quantity – Enter the quantity of the
individual ingredient used in the compound.
Measure the quantity used according to the
uncompounded unit of measure. Do not
measure the quantity by compounded
volume.
Cost – Enter your usual and customary
charge for the individual ingredient.

Prescription Drug Program
- 113 -
Electronic Billing
What is point-of-sale (POS)?
The agency's POS system is a real-time pharmacy claims processing system which uses the
National Council for Prescription Drug Programs (NCPDP) version D.0 format. Each claim
submission, reversal, or re-bill that you successfully transmit via your switch vendor is captured
and appears on your weekly Remittance and Status Report (RA). Track each transaction and
reconcile your RA completely before contacting the agency.
What do the POS rejection codes mean?
The agency's POS system uses NCPDP D.0 reject codes. Although these codes have meaning
within the NCPDP standard, the agency's POS system returns a message of explanation with any
claim rejection. As the complexity of prescription drug benefit management increases, it is
important for the agency to provide clear explanations of denial in real time. It is also important
for pharmacies to read these messages so they can take appropriate action when serving our
mutual clients. The agency returns reject messages up to 80 characters in length, viewable within
most POS applications. If you do not know how to access these reject messages, contact your
software vendor for assistance.
Agency providers cannot accept payment from our clients for any service potentially covered
under the client agency benefit. See "Billing a Client” in the Billing Section within this guide. It
is important for providers to understand that a claim rejection through the POS system is
not necessarily a denial of service. Some claim rejections represent a final denial by the
agency, while others may indicate additional steps are necessary to determine coverage for the
product or service.
The chart on the following page outlines categories of potential reasons for claim rejection,
rather than specific rejection messages.
Prescription Drug Program
- 114 -
Rejection
Message
Description
Reason
Required Action
Service Denial By
the Agency?
The message
starts with
‘TIP:’
Therapeutic Interchange Program is
required under Senate Bill 6088;
Chapter 29, Laws of 2003. See
Washington Preferred Drug List
Section in this guide.
The pharmacist must
substitute a preferred
drug for the
nonpreferred drug
prescribed, unless
the prescription is
ordered Dispense As
Written (DAW).
Not a denial of
service. If the
prescription is a
DAW resubmit
claim with Product
Selection Code of
1. If not DAW,
dispense a preferred
therapeutic
alternative within
the same drug class.
The message
starts with
“Preferred” or
“Pref”
Product prescribed is non-preferred
for agency clients.
See Washington
Preferred Drug List.
Consult prescriber to
determine whether a
preferred alternative
can be prescribed. If
the medication
cannot be changed to
a preferred
alternative, contact
Pharmacy
Authorization.
Not a denial of
service, unless
authorization is
requested and
denied in writing by
the agency.
Detail of
missing or
invalid codes.
(Any standard
NCPDP D.0
reject code
which states
“M/I”)
A required field has been left blank,
or an invalid value has been
submitted in a field that could
affect claim adjudication.
Correct claim and
resubmit.
Not a denial of
service. A claim
must be re-
submitted with
valid values to
determine coverage.
Prescription Drug Program
- 115 -
Rejection
Message
Description
Reason
Required Action
Service Denial By
the Agency?
Drug Use
Review
(DUR) edits
(NCPDP D.0
reject code
88)
Pro-DUR editing has found a
potential therapy problem.
If claim information
is correct, the
pharmacist should
use professional
judgment or confer
with the prescriber
to determine the
appropriateness of
therapy. If therapy
is appropriate,
NCPDP Pro-DUR
codes can be used to
indicate what
professional
intervention
occurred.
Dependent on result
of professional
services. If
appropriate DUR
codes have been
entered, and the
claim is still
rejected, call
Pharmacy
Authorizations at
800-562-3022 to
request assistance.
An agency
representative will
determine whether
authorization is
required, or if
service has been
denied.
“Labeler Has
No Federal
Rebate
Agreement”
The manufacturer has not chosen to
make its products available for
dispense to agency clients.
Dispense an
equivalent product
from a manufacturer
who participates in
the Federal Rebate
Program.
Not a denial of
service. An
equivalent product
must be substituted
and the claim
resubmitted with
the new NDC.
States
‘Maximum’
or ‘Minimum’
in relation to
quantity, days
supplied,
client age,
fills per
month, etc.
The agency has established
therapeutic parameters for the use
of the product. Claims may be
authorized outside of those
conditions.
See List of Drugs with Limitations.
Verify accuracy of
the submitted claims
information. If all
information is
accurate, contact
prescriber to
consider alternate
therapies within
FDA indications. If
prescriber still feels
that the product
should be dispensed
as prescribed,
contact Pharmacy
Authorization.
Not a denial of
service, unless
authorization is
requested and
denied in writing by
the agency.
Prescription Drug Program
- 116 -
Rejection
Message
Description
Reason
Required Action
Service Denial By
the Agency?
States that a
product is ‘not
billable
through POS’,
states ‘Bill as
a professional
service’; or
‘Refer to
DME/Non-
DME
Medicaid
provider
guide.
The product is a potentially covered
benefit, but not considered part of
the client’s prescription drug
benefit.
Consult appropriate
Medicaid provider
guide and bill as a
professional service
on a CMS-1500
claim form, or
comparable HIPAA
compliant electronic
claim format.
Not a denial of
service. Benefit
may be payable as
Durable Medical
Equipment, Enteral
Nutrition, or a
professional
service.
“Expedited
Authorization
Code
Required”
See Authorization. The product
has an expedited code available for
authorization if specific criteria are
met.
Consult Expedited
Authorization List in
Authorization. If
criteria are met,
resubmit claim with
appropriate EA code
in the Prior
Authorization
Number Submitted
field (462-EV). If
criteria are not met,
contact Pharmacy
Authorization.
Not a denial of
service, unless
authorization is
requested and
denied in writing by
the agency.
States that a
product or
situation is
NONCOVER
ED, and does
not provide a
toll free
number.
The product or situation is not a
covered benefit for the client.
Work with the
client's prescriber to
find an alternate
covered therapy
which meets the
client's medical
needs.
Yes. The requested
service is denied. If
originally
prescribed therapy
has not been
changed, POS
denial as
noncovered can be
considered final.
Contains a toll
free number,
or message is
not otherwise
addressed
above.
Product or situation requires
Authorization or other review by
the agency.
Pharmacy calls the
toll free number
indicated to request
authorization or
assistance.
Not a denial of
service, unless the
request for
authorization is
denied in writing by
the agency.
Prescription Drug Program
- 117 -
What is the prospective drug use review (Pro-
DUR) used for?
The agency provides Pro-DUR screening as a feature of the POS system. Early Refill, High
Dose, Low Dose, and Therapeutic Duplication edits post and claims are rejected when potential
drug therapy problems are identified. Once pharmacists have conducted their professional
review, the agency recognized NCPDP DUR Reason for Service, Professional Service, and
Result of Service codes can be used to respond to the Pro-DUR edits.
When appropriate, enter one of the NCPDP DUR codes from each of the categories in the
appropriate POS field. Entering DUR codes will not automatically bypass DUR screening. The
agency considers different codes to be appropriate for different situations. Only a combination of
codes appropriate to address the potential therapy problem will satisfy the DUR screening
process.
By placing the information on the claim, the provider is certifying that the indicated DUR code is
true and documentation is on file. POS claim coding is subject to review and audit by the agency.
The agency does not provide additional reimbursement for DUR services. DUR coding is
supported for the purpose of ensuring potential drug therapy problems are addressed by a health
care professional.
Hardcopy (paper) claims must note the appropriate DUR codes in the Justification/Comments
field on the Pharmacy Statement (525-106) form, HCA 13-714, if applicable.
What is the national drug code (NDC)?
The NDC is an 11-digit code assigned to all pharmaceutical products by the labeler or distributor
of the product under FDA regulations. (See WAC 182-530-1050)
Note: When submitting claims to the agency the provider must use the actual,
complete 11-digit NDC from the dispensing container.
(See WAC 182-530-5000(1)(b))
The agency accepts only the 5-4-2 NDC format. All 11 digits, including zeros, must be
entered. The three segments of the NDC are:
SAMPLE NDC: 12345-6789-10
12345 = labeler code
6789 = product code
10 = package size

Prescription Drug Program
- 118 -
NCPDP Version D.0 Claim
Format
In order to comply with the Health Insurance and Accountability Act (HIPAA) requirements, the
agency requires all pharmacy providers to use NCPDP Version D.0 claim format when
submitting Point-of-Sale (POS) claims. See the Payer Specification Sheet for more information.
General information
The NCPDP Version D.0 Claim Format:
Defines the record layout for real-time prescription claim transactions between providers
and processors
Is a variable format
Accepts up to four transactions per transmission (except when billing compounds, only
one transaction is allowed per transmission)
What transaction segments are supported?
Transaction header segment
The Transaction Header Segment is mandatory on all transactions and all fields within the
segment are mandatory.
The Transaction Header Segment tells the system where to send the claim, what type of
submission it is, how many transactions, who is submitting the claim, date of service, and the
vendor certification number.

Prescription Drug Program
- 119 -
Patient segment
The Patient Segment is mandatory for all transaction types. The NCPDP standard requires the
submission of Date of Birth (304-C4) and Patient Gender Code (305-C5) fields. The agency
requires submission of the Patient Residence (384-4X) field depending on the situation. When
appropriate, and necessary for claim adjudication, use the following values in the Patient
Residence field:
01 - To indicate the client resides at home, in an assisted living facility, group home, or
adult family home;
02 - To indicate the client resides in a skilled nursing facility;
11 - To indicate a hospice patient whose claim is unrelated to their terminal condition; or
12 - To indicate an ITA claim.
Insurance segment
The Insurance Segment is mandatory on all transactions except reversals (B2).
This segment contains data describing the ProviderOne Client ID. The client’s ProviderOne
Client ID is required in the Cardholder ID field, and Patient Relationship Code should be set to
1.
Claim segment
The Claim Segment is mandatory on all Billing (B1, B2, B3), and some Authorization (P1, P2)
transactions. This segment contains data relating to the dispensing of the actual prescription, or
when authorization is requested. Some fields are required only for billing transactions. The
Claim Segment is also used to identify a partially filled prescription, and some fields are required
only when submitting a partial fill.
Prescriber segment
The Prescriber Segment contains data describing the prescriber and is required on all
Authorization (P1, P2, P3, P4) or Billing transactions with the exception of Reversals (B1, B3).
The mandatory/required fields are the Segment Identification, Prescriber ID Qualifier, and
Prescriber ID. For Authorization transactions, Prescriber Last Name and Phone Number are also
required.
COB/other payment segment
This segment may be required in some situations when billing or rebilling if the pharmacist or
The agency indicates other coverage. The COB/Other Payments Segment contains information
indicating the presence of other payers or insurers.
Use the Other Coverage Code field in the Claim Segment to indicate insurance coverage
information. Refer to Other coverage codes.
DUR/PPS segment
The DUR/PPS Segment contains data for the resolution of DUR rejections.
Prescription Drug Program
- 120 -
Pricing segment
The Pricing Segment is required on all incoming billing and rebilling transactions (B1, B3). This
segment contains data describing how the product is to be priced. The mandatory fields are:
Segment Identification, Ingredient Cost Submitted, Usual and Customary Charge, and Gross
Amount Due.
Compound segment
This segment is required for the multi-line submission of compounds. The Compound Segment
may only be submitted on billing or rebilling. This segment is not sent on claim reversals.
Information describing the compound ingredients is included here. If the segment is submitted
the following fields are required: Segment Identification, Compound Dosage Form Description
Code, Compound Dispensing Unit Form Indicator, Compound Ingredient Component Count,
Compound Ingredient Drug Cost, and Compound Ingredient Basis of Cost Determination. The
following fields are also required, and may be repeated for multiple ingredients: Compound
Product ID Qualifier, Compound Product ID, and Compound Ingredient Quantity. The agency
will reimburse a dispensing fee for each payable ingredient. Each line will be adjudicated
separately and will be subject to all applicable edits, including authorization. Compounds may
not be submitted as a partial fill. If a pharmacy chooses to receive reimbursement only for the
payable ingredients within a compound, a value of 8 in the Submission Clarification Code field
from the claim segment must be entered.
Prior authorization segment
The Prior Authorization segment is situational, and only required on Authorization transactions
(P1, P2, P3, P4). When submitting an Authorization transaction, the following fields are
required: Segment Identification, Request Type, Request Period Date-Begin, Request Period
Date-End, and the Basis of Request. No other fields within this segment are captured or
supported.

Prescription Drug Program
- 121 -
Therapeutic Interchange
Program
(Senate Bill 6088; Chapter 29, Laws of 2003)
What is the therapeutic interchange program?
The therapeutic interchange program (TIP) is a process developed by the Department of Social
and Health Services, the Health Care Authority (HCA), and Labor and Industries (L&I) to allow
physicians and other prescribers to endorse the Washington Preferred Drug List (PDL). TIP is
intended to streamline administrative procedures and make prescription drugs more affordable to
Washington residents and state health care programs.
TIP applies only to drugs on the Washington PDL prescribed by an endorsing practitioner and
not to other drugs requiring authorization.
What is an endorsing practitioner?
An endorsing practitioner is a provider who has reviewed the Washington PDL, signed up as
an endorsing provider, and agrees to allow therapeutic interchange of a preferred drug for any
nonpreferred drug in a given therapeutic class. (See http://www.rx.wa.gov.)
What does this mean to pharmacies?
When an endorsing practitioner issues a prescription to a medical assistance client for a
nonpreferred drug on the Washington PDL, the filling pharmacist must dispense the preferred
drug in that therapeutic class in place of the nonpreferred drug. When this therapeutic
interchange is made, the pharmacist must notify the endorsing practitioner of the specific drug
and dose dispensed.

Prescription Drug Program
- 122 -
When are substitutions not required?
In some instances, the endorsing practitioner may determine that the nonpreferred drug is
medically necessary and instruct the dispensing pharmacist to dispense the nonpreferred drug as
written (DAW). When an endorsing practitioner indicates "DAW" on a prescription for a
nonpreferred drug, the agency will not require authorization, and the dispensing pharmacist will
dispense the nonpreferred drug as prescribed.
Exemptions from TIP
Senate Bill 6088 exempts the following drug classes from TIP when the drug classes are placed
on the Washington PDL:
Antipsychotic
Antidepressant
Chemotherapy
Antiretroviral
Immunosuppressive
Immunomodulator/antiviral drugs used to treat hepatitis C for which an established, fixed
duration of therapy is prescribed for 24-weeks but no more than 48 weeks. (See RCW
69.41.190)
Not all of these drug classes are on the Washington PDL, and unless the drug class is on the
Washington PDL, it is not eligible for the continuation of therapy privilege.
Continuation of therapy privilege for exempted drug classes
Pharmacists must not substitute a preferred drug if the prescription is for a refill or continuation
of therapy in any of the exempted drug classes on the Washington PDL.
What if a non-endorsing practitioner issues a
prescription for a nonpreferred drug?
When a non-endorsing practitioner issues a prescription for a nonpreferred drug, the agency
requires authorization, and the dispensing pharmacist must fax a completed Pharmacy
Information Authorization form, HCA 13-835A to 866-668-1214, or call the agency at 800-562-
3022 to request authorization by providing medical justification.
See Washington PDL or go to the agency’s Pharmacy Information website for further
information.

Prescription Drug Program
- 123 -
How does the pharmacy bill for a DAW
prescription written by an endorsing
practitioner?
Hard copy billers must enter “DAW” in the Justification/Comments field on the
Pharmacy Statement (525-106) form, HCA 13-714.
Point-of-Sale billers must enter “1” in the Dispense as Written (DAW)/Product
Selection Code field.

Prescription Drug Program
- 124 -
Washington Preferred Drug
List
What is the Washington preferred drug list?
(WAC 182-530-4100)
The Health Care Authority (agency) and Labor & Industries (L&I), have developed a list of
preferred drugs within a chosen therapeutic class that are selected based on clinical evidence of
safety, efficacy, and effectiveness. The drugs within a chosen therapeutic class are studied by an
evidence-based practice center (EPC). A written report on the comparative safety, efficacy, and
effectiveness from the EPC is evaluated by the Washington State Pharmacy and Therapeutic
Committee which makes recommendations to state agencies regarding the selection of the
preferred drugs on the Washington Preferred Drug List (PDL).
What is the process to obtain drugs on the
Washington PDL?
Preferred Drugs - Prescription claims for preferred drugs submitted to the agency are
reimbursed without authorization requirements unless the drug requires authorization for:
Safety criteria.
Special subpopulation criteria.
Limits based on age, gender, dose, or quantity.
Non-preferred Drugs - Prescription claims for non-preferred drugs submitted to the
agency are reimbursed without authorization requirements when written by an Endorsing
Practitioner who has indicated “DAW” on the prescription unless the drug requires
restrictions for safety (see WAC 182-530-4150.)
Prescription claims for non-preferred drugs submitted to the agency are reimbursed only
after authorizing criteria are met if written by a non-endorsing practitioner.
Pharmacies must call the agency for authorization when required. Call 866-668-1214.

Prescription Drug Program
- 125 -
What are the authorization criteria that must be
met to obtain a nonpreferred drug?
For most drug classes on the Washington PDL, the authorization criteria is that the client
must have tried and failed, or is intolerant to, at least one preferred drug. Drugs may have
criteria that go beyond these basic criteria for the reasons stated in #1 on the previous
page.
Drugs that are in drug classes on the Washington PDL that have not been studied by the
evidence-based practice center(s) and have not been reviewed by the P&T committee will
be treated as nonpreferred drugs and will require authorization.
The agency requires pharmacies to obtain authorization for nonpreferred drugs when a
therapeutic equivalent is on the Washington PDL. The following table shows the preferred and
nonpreferred drug in each therapeutic drug class on the Washington PDL.
Note: The agency changed the format for multiple drug listings. A slash ( / ) is
used to denote multiple forms of a drug. For example: “Cardizem
®
/CD/LA/SR”
represents immediate release Cardizem, as well as the CD, LA, and SR forms. A
hyphen ( - ) is used to indicate combination products. For example: “benazepril-
HCTZ” represents the combination product of benazepril and
hydrochlorothiazide, rather than benazepril AND the combination product.
Where is the Washington Preferred Drug List?
See the agency’s Washington Preferred Drug List web page.

