Frontiers in Psychiatry 01 frontiersin.org
Omega-3 polyunsaturated fatty
acids and/or vitamin D in autism
spectrum disorders: a systematic
review
YuweiJiang
1
, WenjunDang
2
, HongNie
2
*, XiangyingKong
1
,
ZhimeiJiang
1
and JinGuo
1
1
College of Rehabilitation Medicine, Jiamusi University, Jiamusi, Heilongjiang, China,
2
Heilongjiang
University of Chinese Medicine, Harbin, Heilongjiang, China
This systematic review aims to oer an updated understanding of the relationship
between omega-3 supplementation and/or vitamin D and autism spectrum
disorders (ASD). The databases PubMed, Cochrane Library, Web of Science,
EMBASE, CINAHL, Vip, CNKI, Wanfang, China Biomedical Database databases
were searched using keywords, and relevant literature was hand-searched. Papers
(n = 1,151) were systematically screened and deemed eligible since 2002. Twenty
clinical controlled studies were included in the final review. The findings were
analyzed for intervention eects focusing on the core symptoms of ASD, included
social functioning, behavioral functioning, speech function and biomarkers
changes. The review found that the eects of omega-3 supplementation on
ASD were too weak to conclude that core symptoms were alleviated. Vitamin D
supplementation improved core symptoms, particularly behavioral functioning,
however, the results of the literatures included in this study were slightly mixed,
we cannot directly conclude that vitamin D supplementation has a beneficial
eect on a specific symptom of ASD, but the overall conclusion is that vitamin D
supplementation has a positive eect on behavioral functioning in ASD. Omega-3
and vitamin D combination supplementation has a good combined eect on
social and behavioral outcomes in patients with ASD.
KEYWORDS
autism spectrum disorders, omega-3, vitamin D, social functioning, behavioral
functioning, speech function, biomarkers changes
OPEN ACCESS
EDITED BY
Jerey C. Glennon,
University College Dublin, Ireland
REVIEWED BY
Wouter G. Staal,
Radboud University Medical Centre, Netherlands
Magdalena Budisteanu,
Prof. Dr. Alexandru Obregia Psychiatry Hospital,
Romania
Francesca Felicia Operto,
University of Salerno, Italy
*CORRESPONDENCE
Hong Nie
RECEIVED 12 June 2023
ACCEPTED 31 July 2023
PUBLISHED 16 August 2023
CITATION
Jiang Y, Dang W, Nie H, Kong X, Jiang Z and
Guo J (2023) Omega-3 polyunsaturated fatty
acids and/or vitamin D in autism spectrum
disorders: a systematic review.
Front. Psychiatry 14:1238973.
doi: 10.3389/fpsyt.2023.1238973
COPYRIGHT
© 2023 Jiang, Dang, Nie, Kong, Jiang and Guo.
This is an open-access article distributed under
the terms of the Creative Commons Attribution
License (CC BY). The use, distribution or
reproduction in other forums is permitted,
provided the original author(s) and the
copyright owner(s) are credited and that the
original publication in this journal is cited, in
accordance with accepted academic practice.
No use, distribution or reproduction is
permitted which does not comply with these
terms.
TYPE Systematic Review
PUBLISHED 16 August 2023
DOI 10.3389/fpsyt.2023.1238973
Highlights
What is known, then list 2–4 bullet points.
1. Studies supporting the importance of vitamin D and omega-3 LCPUFA for brain function
and structure, neurotransmitters and the glutamatergic system.
2. RCTs have investigated the eects of vitamin D and omega-3 LCPUFA each on core
symptoms and problem behavior in patients with ASD. However, ndings were mixed.
What is new, then list 2–4 bullet points.
1. is study analyses the eects of RCTs, focusing on the core symptoms of ASD, including
changes in social functioning, behavioral functioning, language functioning and
biomarkers, with research data from clinical that are more convincing.
Jiang et al. 10.3389/fpsyt.2023.1238973
Frontiers in Psychiatry 02 frontiersin.org
Introduction
Autism spectrum disorders (ASD) are neurodevelopmental
disorders that are characterized by impairments in social
communication, interaction and two core symptoms such as repetitive,
stereotyped behavior, narrow interests and activities (1). e
prevalence of ASD has reached as high as 1.5% in developed countries
(2, 3) and 0.7% among children aged 6–12 years in China (4). e
exact etiology and pathogenesis of ASD remains unclear, and
multisystem involvement and multiple co-morbidities oen lead to an
exacerbation of core symptoms in patients.
Given the unique disease manifestations and co-morbid
conditions of ASD, the emergence of adaptive behavioral problems,
language and communication problems, and emotional regulation
problems will bring a strong stress shock to the parents of ASD, and
the parents of ASD need to bear greater parenting pressure, life
pressure, and economic treatment pressure than the parents of normal
developmental children (5). e long-term accumulation of stress
causes parents of ASD to have higher levels of depression and higher
feelings of shame, fatigue, and powerlessness than parents of children
with other developmental disorders (intellectual disability, attention
decit hyperactivity disorder) (6). Parents of ASD oen show higher
levels of psychological stress, caregiver stress, and parenting stress, and
parents are prone to emotional changes such as anxiety and depression
(7), physical changes such as sleep disorders, and even can lead to
problems such as parental marital relationship breakdown,
intergenerational conict, and social isolation (8). Based on this,
wedesperately want to nd a practical and eective method or drug
that can improve the symptoms of ASD and reduce family stress. At
the same time, wealso try to bein the study of ASD mild patients,
aer treatment to restore social functioning how to better enter the
regular school, whether a period of pre-training is needed before
regular enrolment.
Essential fatty acids are a group of polyunsaturated fatty acids
(PUFA) that can not synthesized by humans, including omega-3 and
omega-6 and their derivatives. Omega-3 is an important component
of phospholipids include linoleic acid (LA), alpha-linolenic acid
(ALA), arachidonic acid (AA), eicosapentaenoic acid (EPA) and
docosahexaenoic acid (DHA). Omega-3 plays an important role in the
structure and function of cell membranes (9, 10). ALA is a precursor
to omega-3 and can beconverted to EPA and DHA. EPA and DHA
are found in natural foods and are mainly supplemented through diet
or deep-sea sh oil (11). DHA plays a role in cognitive function,
neurotransmission, neuronal survival and attenuating
neurodegeneration (12). e balance of essential fatty acids is essential
for brain development and considered as a possible biomarker for
ASD (13). Up to 60% of patients with ASD have some degree of
immune dysfunction, suggesting a link between PUFA and
inammatory homeostasis (14). Reduced levels of omega-3in the
blood of ASD patients can lead to overproduction of the
pro-inammatory cytokine omega-6 (15). Low levels of omega-3 and
omega-6 intake in ASD patients due to the fussy dietary behavior of
individuals lead to increased levels of autoantibodies to neuronal and
glial molecules and consequently to an omega-3/omega-6 ratio
disorders (16). Lower omega-3 levels, or a disturbed omega-3/
omega-6 ratio, increase inammatory cytokines and oxidative stress,
which in turn is associated with ASD symptoms (17).
Vitamin D is a neuroactive steroid that aects neuronal
dierentiation, axonal connections, brain structure and function.
Exogenous and self-synthesized VitD3 undergoes two hydroxylation
processes in the body before it exert biological eects.
25-hydroxyvitamin D3 [25 (OH)D3] is produced by the action of
25-hydroxylase (CYP2RA) in the microsomes and mitochondria of
hepatocytes. 25(OH)D3 is released from the liver into the bloodstream
and is the main stable form of vitamin D in the human blood
circulation, so serum 25(OH)D3 levels are a marker of Vitamin D
nutritional status. Studies have identied Vitamin D responsive
elements in the promoter regions of numerous genes that regulate cell
proliferation and dierentiation, so-called 1,25 (OH) 2D3 target
genes, and some genes can bedirectly aected by 1,25 (OH) 2D3,
including p21 and p27. Studies have shown that 57% of children with
ASD have vitamin D deciency and another 30% have vitamin D
insuciency (18). Studies have shown that vitamin D supplementation
has a benecial eect on ASD symptoms (19, 20). In clinical studies,
vitamin D supplementation has shown positive eects on autistic
behavior, the eects of which may bedue to vitamin D enhancing
immune system function and reducing inammation (21). Vitamin D
plays an important role in the regulation of central and blood
serotonin concentrations (22, 23). Mostafa and Al-Ayadhi study
showed that vitamin D deciency may beinvolved in the production
of autoantibodies in autistic patients (24). Patrick and Ames showed
that vitamin D deciency may have a signicant eect on serotonin,
oxytocin and vasopressin concentrations in the brain (22).
Some randomized controlled studies have been conducted on the
eects of vitamin D and omega-3 on the relief of core symptoms of
ASD, with supporting the importance of vitamin D and omega-3
LCPUFA (EPA and DHA) for brain function and structure,
neurotransmitters and the glutamatergic system, both of which have
immunomodulatory, anti-inammatory and antioxidant (25–27).
Randomized controlled trials have investigated the eects of vitamin
D and omega-3 LCPUFA each on core symptoms and problem
behavior in patients with ASD. However, ndings were mixed.
Systematic evaluation itself can contain up-to-date knowledge and
information with good reproducibility, economically, and can
maximize the improvement of clinical medical practice and guide the
direction of clinical research. e aim of this study was to explore the
role of omega-3 and/or vitamin D on clinical symptoms in patients
with ASD in an integrated manner by means of a systematic evaluation.
2. Study found that the eects of omega-3 supplementation on ASD were too weak to
conclude that core symptoms were alleviated. Vitamin D supplementation has a positive
eect on behavioral functioning in ASD. Omega-3 and vitamin D combination
supplementation had good combined eects in patients with ASD, with signicant
improvements in social and behavioral outcomes.
Jiang et al. 10.3389/fpsyt.2023.1238973
Frontiers in Psychiatry 03 frontiersin.org
Materials and methods
Search criteria
Searching PubMed, Cochrane Library, Web of Science, EMBASE,
CINAHL, Vip, CNKI, Wanfang, China Biomedical Database and
other databases. e subject terms and search combinations are:
omega-3 (“omega-3” OR “omega-3 PUFA” OR “ω-3”) AND vitamin
D (“vitamin D” OR “1,25 dihydroxyvitamin d3” OR “d3,1,25
dihydroxyvitamin” OR “25 hydroxyvitamin d3”) AND/OR autism
(“autism” OR “autism spectrum disorder” OR “ASD”). In addition to
the database search, references to the identied studies were checked
manually. Two subject members (Yu Jiang and Wenjun Dang)
independently checked the title and abstract of each paper and ltered
out irrelevant papers. Publication dates 2002–2022. A total of 1,002
papers were searched in English and 149in Chinese.
Search procedures
Literature screening criteria were set according to the PICO
methodology: the participants (P), the interventions or exposure (I),
the comparison (C), the outcome (O), the study design (S). P: Patients
with ASD, diagnostic criteria: DSM-IV or DSM-5, or ADOS or
ADI-R. I: Clinical studies of omega-3 or vitamin D or
omega-3 + vitamin D supplementation in patients with ASD. C:
omega-3 deciency, or vitamin D deciency. Outcome: ASD-related
symptoms have improved. Study design: randomized controlled trial.
e trial was divided into omega-3 control and observation
groups that were well balanced and comparable between groups; or
using their own pre- and post-control. Observations included at least
one of the following items: stereotyped behavior, speech, social
interaction, communication. Exclusion criteria: ①duplicate published
literature; ②interventions inuenced by other foods or medications so
that the nal treatment eect could not bejudged. Eventually included
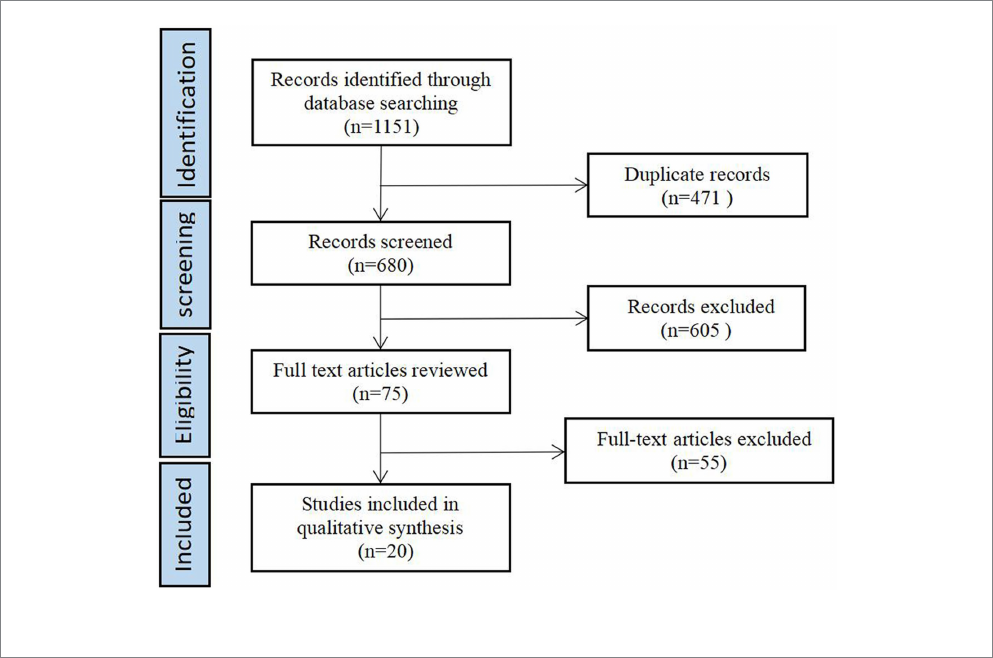
20 publications. For a ow chart of the literature screening, presented
in Figure1.
Data analysis
Data were extracted independently by two reviewers from eligible
studies and included the rst author name, year, country, study design,
sample size, age of participants, diagnostic criteria, vitamin D or omega-3
concentrations, intervention duration, and intervention outcomes.
Quality appraisal and data extraction
Two investigators independently evaluated the quality of the
selected literature, judged the inclusion and exclusion of literature, and
ultimately obtained 20 literatures. e quality assessment of the
included literature was based on the risk assessment scale developed
by the Cochrane Collaboration Network for randomized controlled
trials. e assessment consisted of seven entries. If each entry was low
risk, the study was considered low risk and high quality; if one or more
entries were of unknown risk, the study was considered unknown risk
and moderate quality; if one entry was of high risk, the study was
considered high risk and low quality. Two researchers exchanged
checks with each other aer assessment, and in case of disagreement,
a third researcher was brought in to make a judgment. 20 RCTs were
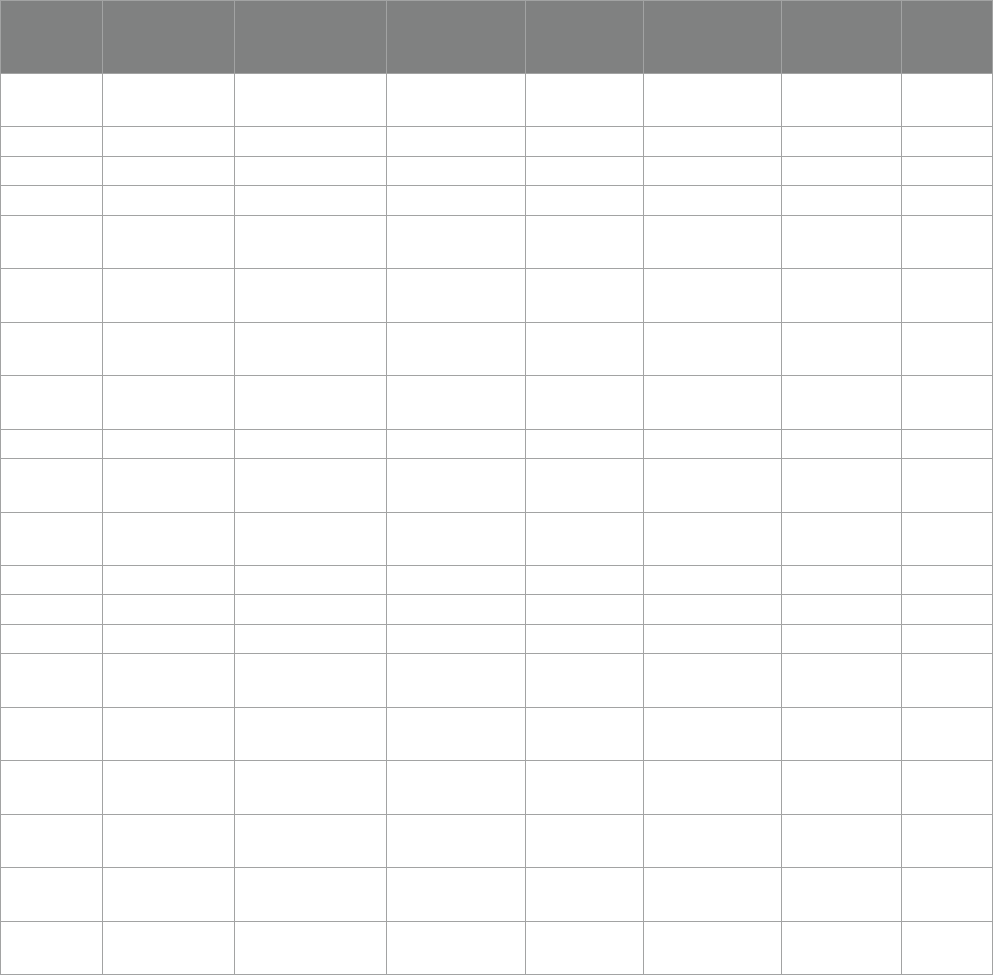
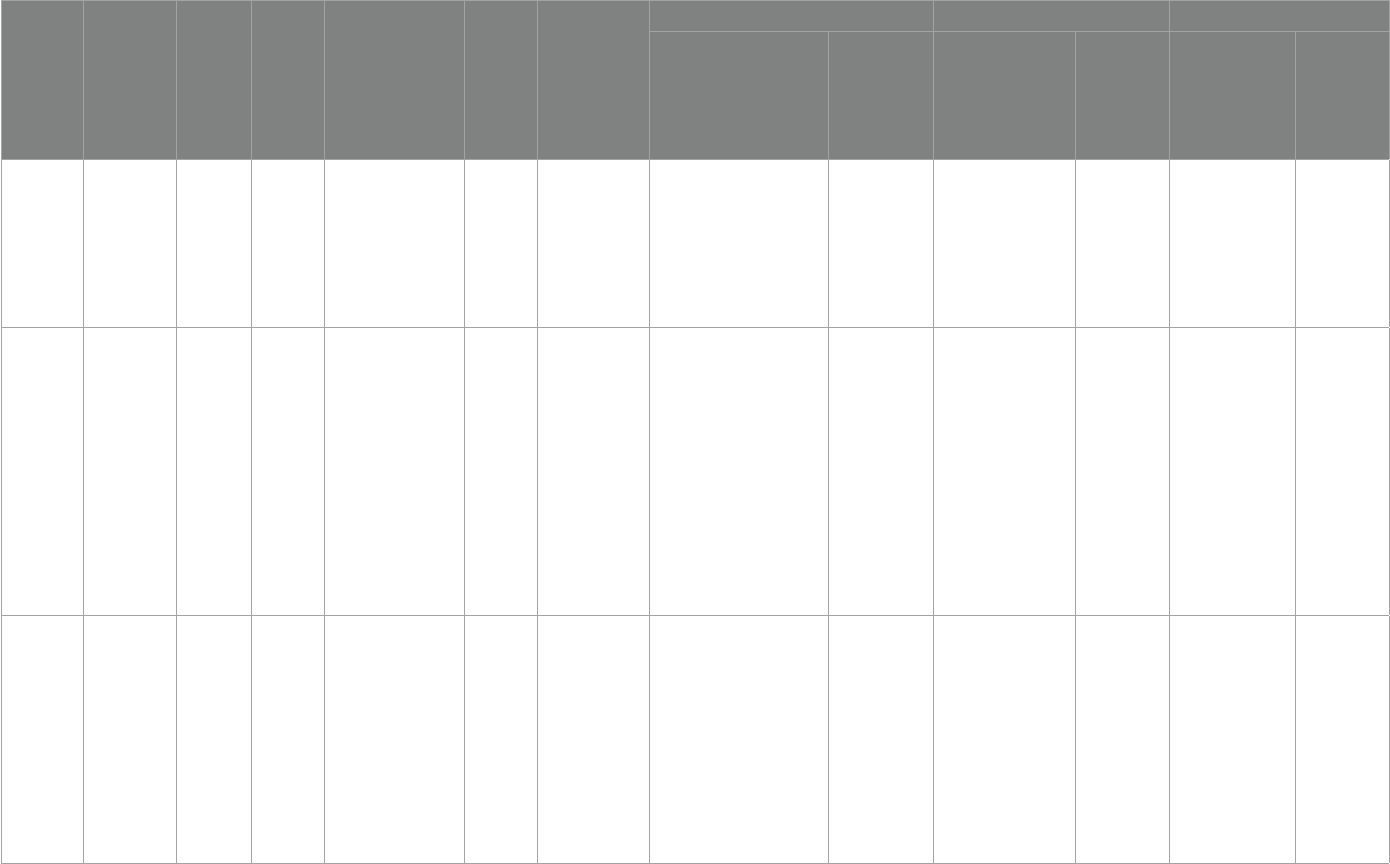
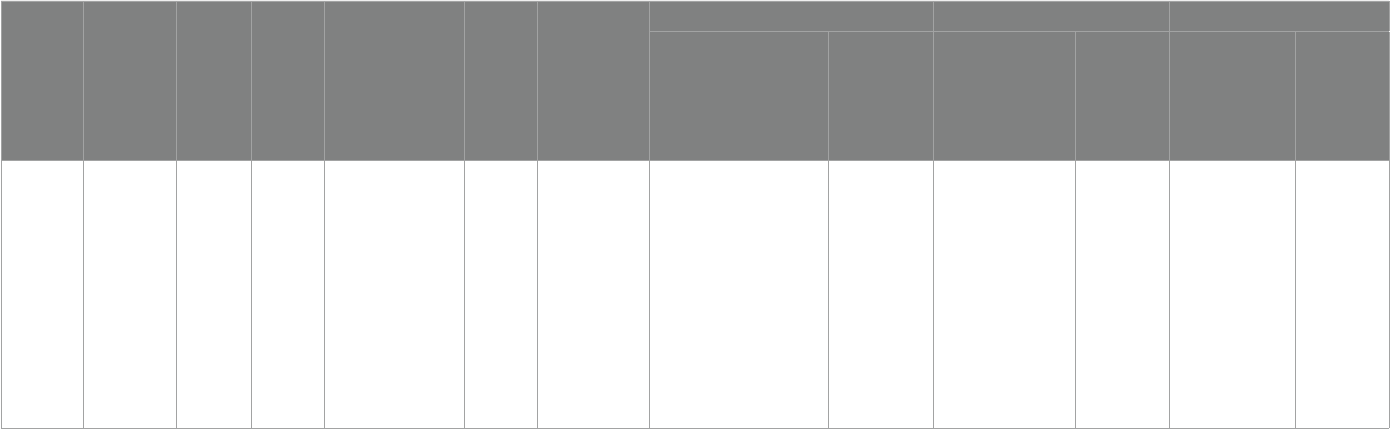
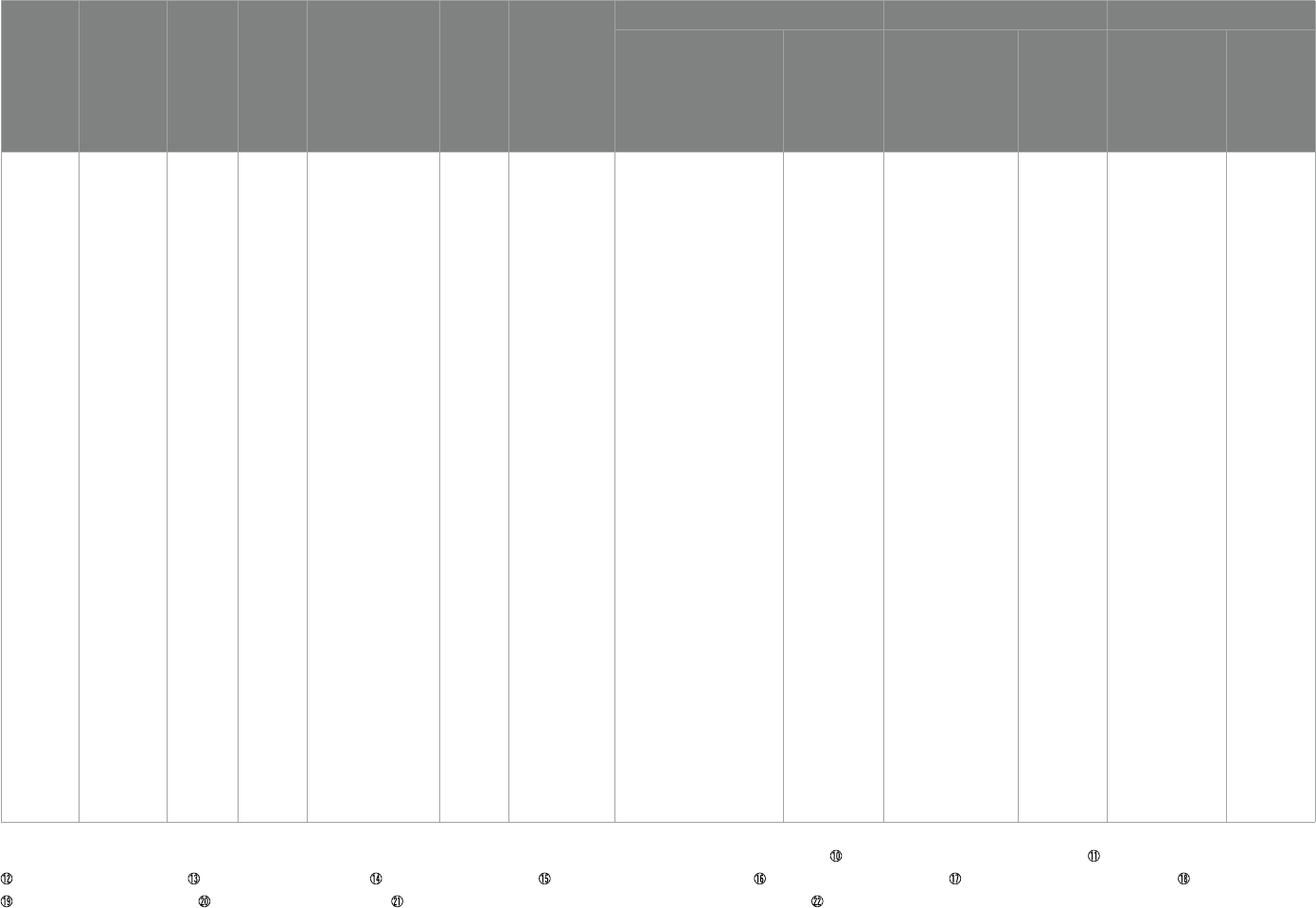
nally included, shown in Table1.
e data were extracted independently by 2 researchers and
exchanged for verication aer extraction. e extraction included:
basic information about each literature, including author, year,
country, age, sample size, diagnostic criteria, intervention time,
measurement tools, experimental reagents, and outcome indicators;
extraction of data on the social, behavioral, and verbal treatment
eects of patients with ASD in each literature.
Results
Summary of studies
A total of 20 papers were included all using randomized, double-
blind, open clinical trial studies, sample size of 13–109, a total of 991
study subjects. Study come from 12 countries: USA, NewZealand,
China, Egypt, Iran, Ireland, Austria, Canada, Japan, Switzerland,
Spain, Italy. ree studies from USA, three from NewZealand, three
from China, two from Egypt, two from Iran. All subjects were ASD
patients, aged 2–40 years, and the duration of the study ranged from
6 weeks to 12 months, with omega-3 interventions lasting from
6 weeks to 6 months, vitamin D interventions lasting from 3 to
6 months, but the combined omega-3 and vitamin D supplementation
trials all lasted 12 months. In the study design, the intervention studies
for both omega-3 and vitamin D were two-group controlled studies
and the combined omega-3 and vitamin D supplementation trial was
a four-group controlled study with a reasonable study design.
Most of the included studies used the DSM scale to conrm the
diagnosis of ASD patients (n = 19) and ADOS scale (n = 3), SCQ
questionnaire (n = 3), and Wechsler Intelligence Scale (n = 2). Studies
assessed social, behavioral and speech functioning in ASD, using the
ABC scale (n = 12), SRS scale (n = 8), GARS scale (n = 7), CGI scale
(n = 4) and BASC scale (n = 3). 12 studies reported changes in
laboratory indicators, mainly considering the levels of EPA, DHA,
25(OH)D3, and related biochemical tests in the subjects. Specic
results of the quality assessment of the literature are shown in Table2.
ASD core symptoms
All 20 papers examined the eects of omega-3 and/or vitamin D
on core symptoms of ASD, including social function, behavioral
function, and speech function.
Social functioning
Eight papers examined the eects of omega-3 on social function
in people with ASD, mainly using the SRS scale, including social
awareness, social cognition, social communicative functioning, social
motivation, autistic mannerism.
Four of the omega-3 intervention studies used the SRS scale. Ooi
(29) study showed that aer 12 weeks of omega-3 intervention, for
subjects assessed using the SRS scale, there were signicant
Jiang et al. 10.3389/fpsyt.2023.1238973
Frontiers in Psychiatry 04 frontiersin.org
improvements in social perception, social cognition, social
communication, social motivation and autistic behavioral styles, and
total scores. Yui (36) showed signicant improvements in social
communication aer 16 weeks of omega-3 supplementation. Parellada
(28) showed no improvement in overall social functioning aer
omega-3 supplementation, but it was also noted in the study that
Parellada used a crossover design and that there could bea sequential
eect during the treatment phase, which would confound the
treatment eect, Although the researchers designed an eect removal
time of 2 weeks to avoid sequential eects, the in vivo metabolic cycle
of omega-3 was not further claried to ensure that the elution period
was suciently long. Bent (31) and others showed no improvement
in social functioning aer 6 weeks of omega-3 supplementation.
Vitamin D intervention study had two studies using the SRS scale.
Saad (39) showed that aer 4 months of vitamin D supplementation,
the SRS-autistic mannerism, SRS-social cognition, and SRS-social
awareness statistical studies have signicantly improved. Kerley (41)
showed that aer 20 weeks of supplementation, there was no dierence
in the individual data in the SRS scale.
In contrast, in the combined omega-3 and vitamin D intervention
studies, two studies used the SRS scale and both were with the same
group of researchers. Mazahery (44, 45) showed that aer 12 months
of supplementation, the SRS-social awareness, SRS-social
communicative functioning, and SRS-total were statistically signicant
before and aer supplementation.
In a comprehensive analysis, ten supplementary studies involving
omega-3, four studies used the SRS scale but only two showed
statistical dierences, but of these (29) studies had two high risk and
two unknown risk, with low quality literature. In the other study (36),
only one social communicative functioning showed a statistical
dierence. Of the six supplementation studies involving Vitamin D,
two used the SRS scale, but only one showed statistical dierences,
which only three indicators also showed statistical dierences.
However, 4 studies with combined omega-3 and vitamin D
interventions, only two used the SRS scale, with all three indicators
showing a dierence.
Behavioral functioning
To evaluate behavioral functioning, the ABC scale, the BASC
system, the CBCL and GARS were used.
Aberrant behavior checklist
Twelve papers used the ABC scale, examined the eects of
omega-3 on abnormal behavior in people with ASD, which consists of
ve factors: irritability, hyperactivity, lethargy/social, inappropriate
speech, stereotypic behavior. Bent (31) showed that taking omega-3
for six weeks had a signicant improvement in lethargy, stereotypic
behavior in the parent-rated version of the ABC scale for subjects in
2014. Bent (30) showed no eect on abnormal behavior in subjects by
taking omega-3 for 12 weeks in 2011. Stephen Bent conducted two
controlled clinical studies of omega-3in patients with ASD in 2011
and 2014, respectively, and the observation group in the 2011 study
showed no dierence compared to the control group. ere was no
dierence in the ABC scale. e reasons for this analysis were
FIGURE1
Flow diagram of paper selection.
Jiang et al. 10.3389/fpsyt.2023.1238973
Frontiers in Psychiatry 05 frontiersin.org
considered to bepossibly due to the inclusion of too few patients
(n = 25) to determine ecacy. Yui (36) showed that aer 16 weeks of
omega-3 supplement, there was a signicant improvement in social
withdrawal in the observation group compared to the control group,
with a signicant dierence. Amminger (34) showed that aer six
weeks of omega-3 supplement, a repeated measures ANOVA showed
no signicant dierence between the observation group and the
control group, but there was a tendency for the observation group to
have remission of hyperactivity symptoms. Voigt (35) showed that
aer 6 months of omega-3 supplementation, there was no signicant
dierence between the observation group and control group were not
signicantly dierent.
Saad (39) study showed a signicant improvement in ABC scores
in the vitamin D supplementation group compared to the placebo
group. ere were signicant changes in irritability, hyperactivity,
social withdrawal, stereotypic behavior, and inappropriate speech
statistics. Furthermore, this study found a high correlation between
serum 25 (OH)D3 levels and ABC-total scores and ABC-language
subscale scores, with signicant reduction in ABC aer vitamin D
supplementation, and this treatment eect was more pronounced in
younger patients. Duan (40) showed a statistically signicant
reduction in the total ABC score and interaction, somatic motor
ability, speech and self-care individual scores for before and aer
3 months of treatment in the study compared to the pre-treatment
period. And further divided into early treatment group (age ≤ 3 years)
and late treatment group (age > 3 years), for the dierence in total ABC
score early treatment group was greater than late treatment group, the
dierence was statistically signicant. Moreover, this study also
showed that serum 25(OH)D3 levels were negatively correlated with
total ABC scores and individual ABC verbal ability scores. However,
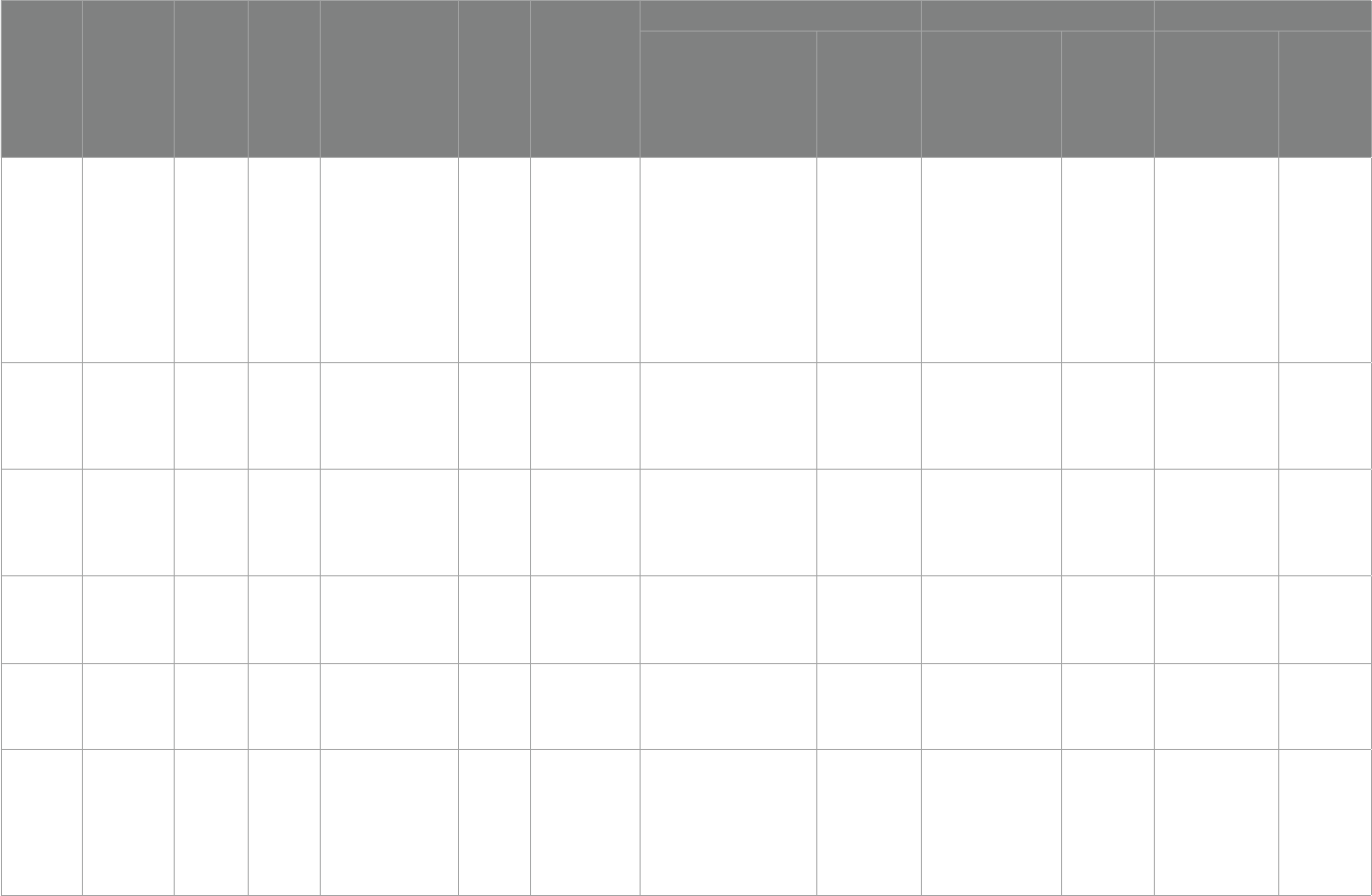
TABLE1 Quality assessment.
Study ID Random
sequence
generation
Allocation
concealment
Blinding of
participants
and personnel
Blinding of
outcome
data
Incomplete
outcome data
Selective
reporting
Other
bias
Parellada etal.
(28)
Low risk Low risk Low risk Low risk Low risk Low risk Unknow
Ooi etal. (29) High risk Unknow High risk Low risk Low risk Low risk Unknow
Bent etal. (30) Low risk Low risk Low risk Low risk Low risk Low risk Unknow
Bent etal. (31) Low risk Low risk Low risk Low risk Low risk Low risk Unknow
Mankad etal.
(32)
Low risk Low risk Unknow Low risk Low risk Low risk Unknow
Politi etal.
(33)
High risk High risk Low risk High risk High risk High risk Unknow
Amminger
etal. (34)
Unknow Unknow Low risk Low risk Low risk Low risk Unknow
Voigt etal.
(35)
Low risk Low risk Low risk Low risk Low risk Low risk Unknow
Yui etal. (36) Low risk Low risk Low risk Low risk Low risk Low risk Unknow
Doaei etal.
(37)
Low risk Low risk Low risk Low risk Low risk Low risk Unknow
Javadfar etal.
(38)
Low risk Low risk Low risk Low risk Low risk Low risk Unknow
Saad etal. (39) Low risk Low risk Low risk Low risk Low risk Low risk Unknow
Saad etal. (20) High risk High risk Low risk Low risk High risk Low risk Unknow
Duan (40) High risk High risk Low risk Low risk High risk High risk Unknow
Kerley etal.
(41)
Low risk Low risk Low risk Low risk Low risk Low risk Unknow
Feng etal.
(42)
High risk High risk Low risk Low risk High risk Low risk Unknow
Mazahery
etal. (43)
Low risk Low risk Low risk Low risk Low risk Low risk Unknow
Mazahery
etal. (44)
Low risk Low risk Low risk Low risk Low risk Low risk Unknow
Mazahery
etal. (45)
Low risk Low risk Low risk Low risk Low risk Low risk Unknow
Fang etal.
(46)
Low risk High risk Low risk Low risk High risk High risk Unknow
Jiang et al. 10.3389/fpsyt.2023.1238973
Frontiers in Psychiatry 06 frontiersin.org
(Continued)
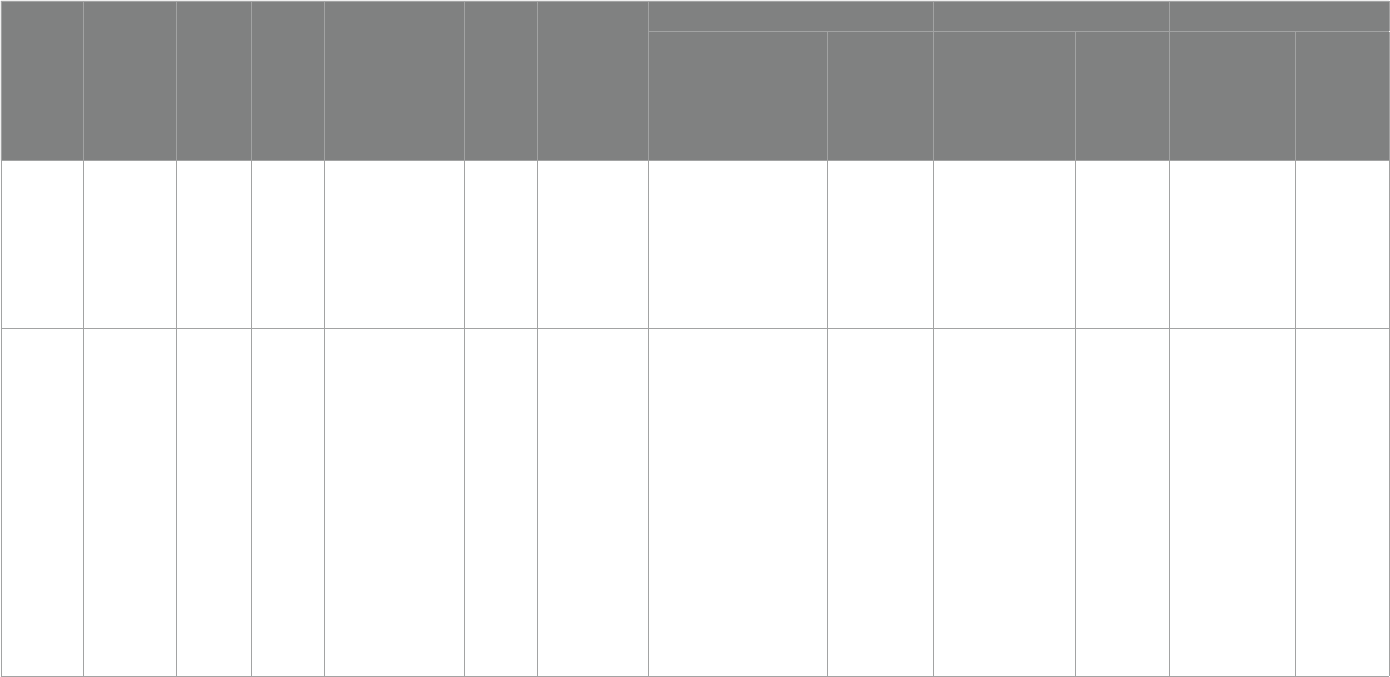
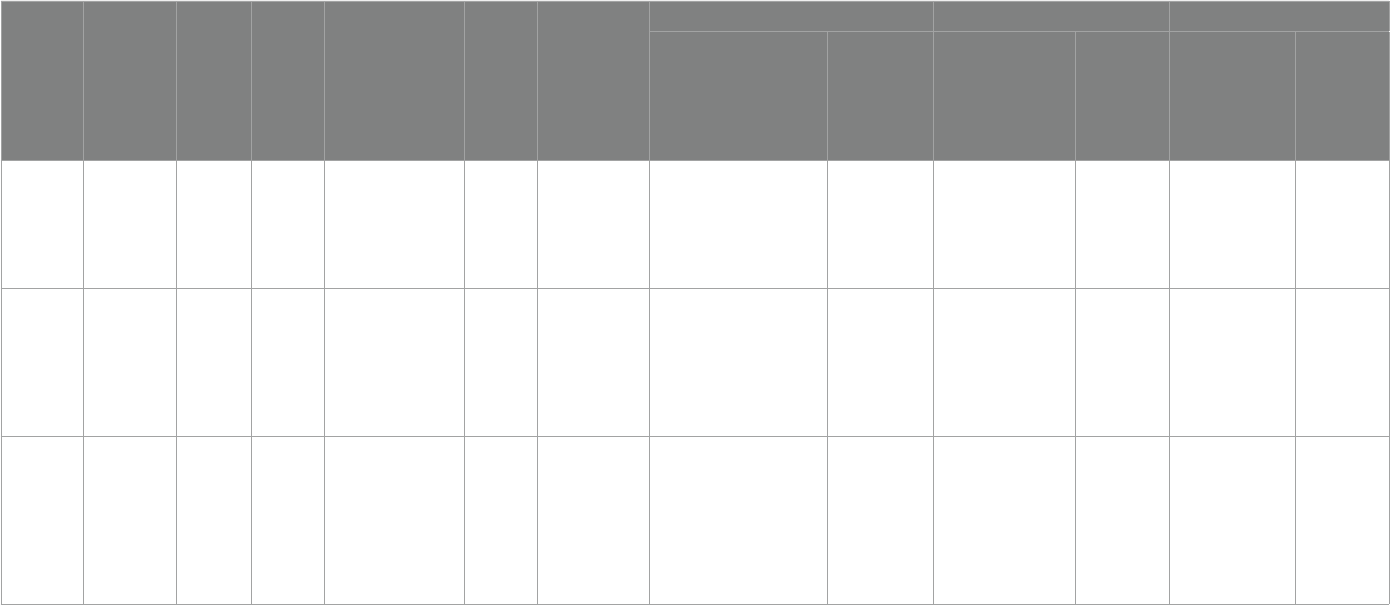
TABLE2 Summary of study details.
Intervention Type
Literature (country)
Age (years)
Sample size
Diagnostic criteria
intervention
time
Measurement tool
experimental supplement Biomarkers Scale score
experimental
group
Placebo
group
experimental
group
Placebo
group
experimental
group
Placebo
group
Omega-3
Parellada etal.
(28) Spain
5–17 67
Child psychiatrist,DSM-
IV diagnosis of Pervasive
Developmental Disorder
18 weeks
(phase 1:
8 weeks;
2 weeks
interva:eect
removal time
l;phase 2:
8 weeks)
①ω3 PUFAs
②TAS
③SRS
④CGI-S
Patients aged 5–11 years, 964.1 mg
(EPA 577.5 mg + DHA
385 mg + Vitamin E 1.6 mg);
Patients aged 12–17 years,
1157.01 mg (EPA 693 mg + DHA
462 mg + Vitamin E 2.01 mg)
Liquid paran and
vitamin E at the
same dose as the
observation group
①AA/DHA intervention
group(subject impact
F= 8.248, p< 0.001)
time×group
eect(F= 11.548, p< 0.001)
②ω3/ω6(time×group eect
F= 8.667,p< 0.001)
No improvement No improvement No improvement
Omega-3
Ooi etal. (29)
Switzerland
7–18 41
Child psychiatrist [autistic
symptoms rating at least
moderate severity(CGI)],
DSM-IV, WISC-IV, WPPSI
12 weeks
①SRS
②CBCL
③Blood status
15 mL liquid (Efamol Efalex) daily,
1 g/day of omega-3(DHA
840 mg,EPA 192 mg, pure evening
primrose oil 1,278 mg)
No mentioned
①Percentage of AA:EPA
(p= 0.0001)
②Percentage of omega-3
highly (p= 0.0001)
③Percentage of EPA
(p= 0.001)
④Percentage of DHA
(p= 0.0001)
No improvement
①SRS-Social awareness
(p= 0.01)
②SRS-Social cognition
(p= 0.0001)
③SRS-Social
communication
(p= 0.0001)
④SRS-Social motivation
(p= 0.01)
⑤SRS-Autistic
mannerisms (p= 0.0001)
⑥SRS—Total score
(p= 0.0001)
⑦CBCL-Total score
(p= 0.02)
⑧CBCL-Attention
problems (p= 0.03)
No improvement
Jiang et al. 10.3389/fpsyt.2023.1238973
Frontiers in Psychiatry 07 frontiersin.org
Intervention Type
Literature (country)
Age (years)
Sample size
Diagnostic criteria
intervention
time
Measurement tool
experimental supplement Biomarkers Scale score
experimental
group
Placebo
group
experimental
group
Placebo
group
experimental
group
Placebo
group
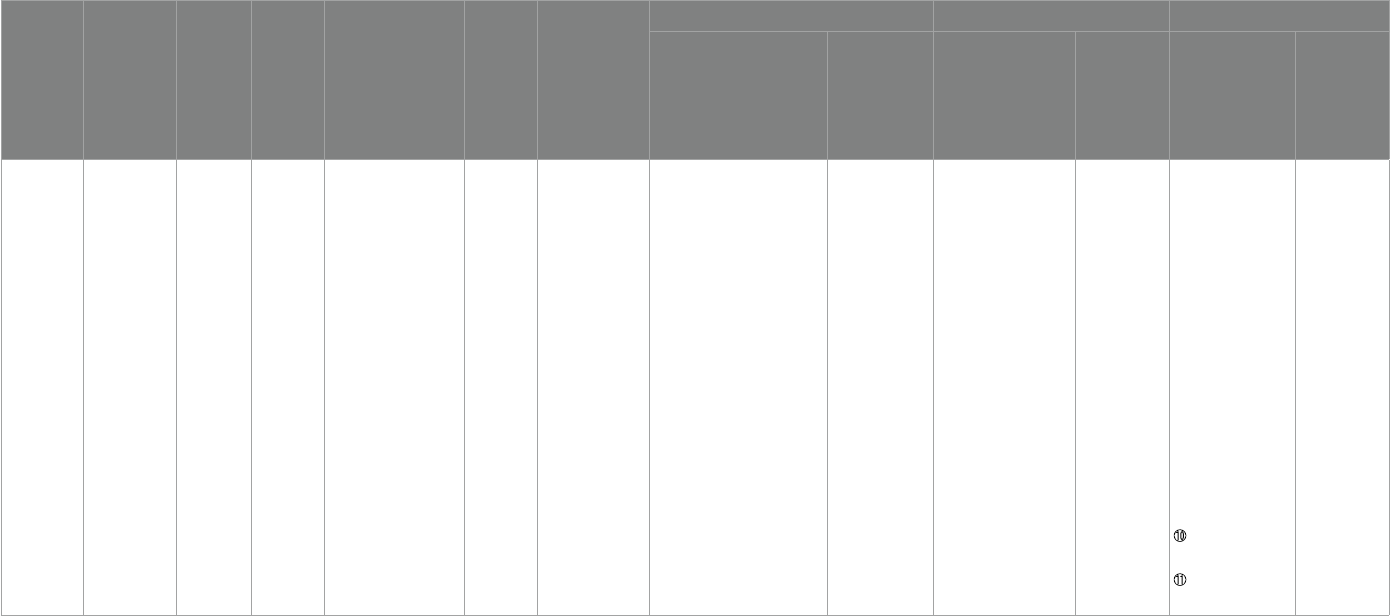
Omega-3
Bent etal. (30)
USA
3–8 25
Expert clinician,ADOS、
SCQ > 12、DSM-IV
12 weeks
①ABC
②BASC
③CGI-I
④Free fatty acid
changes
Orange-avored pudding packets
(EPA 350 mg and DHA 230 mg)
Orange-avored
pudding included
saower oil
①22:6n3 (DHA) (p= 0.02)
②20:5n3 (EPA) (p= 0.03)
③% Monounsaturated
(p= 0.007)
④% Polyunsaturated
(p= 0.04)
⑤% Omega-3 (p= 0.01)
⑥% Omega-9 (p= 0.01)
⑦cytokines TNF-α
(p= 0.023)
No improvement No improvement No improvement
Omega-3
Bent etal. (31)
USA
5–8 57
Professional
personnel,
ADOS,
ADI-R,
SCQ > 12
6 weeks
①ABC-H > 20
②SRS
③CGI-I
Orange-avored pudding packets
(EPA 350 mg and DHA 230 mg)
Orange-avored
pudding included
saower oil
- -
①ABC-Stereotypy
Parent Ratings (p= 0.05)
②ABC-lethargy Parent
Ratings (p= 0.01)
No improvement
Omega-3
Mankad etal.
(32) Canada
2–5 38
No mentioned
diagnostician,
DSM-IV
6 months
①PDDBI
②BASC-2
③PLS-4
④CGI-I
⑤omega-3
First 2 weeks, EPA + DHA 0.75 g
(1.875 mL once a day), aer
2 weeks, the dose was doubled to
1.5 g (3.5 mL)
Placebo contained
rened olive oil and
medium chain
triglycerides
No improvement No improvement No improvement No improvement
Omega-3
Politi etal. (33)
Italy
18–40 19
A doctor and a
psychologist,
DSM-IV、 WAIS
6 weeks
①e Rossago
Behavioral Checklist
(personal
communication)
Two gelatin capsules of sh oil
supplements containing 0.93 g of
EPA and DHA
No mentioned – – No improvement No improvement
Omega-3
Amminger etal.
(34) Austria
5–17 13
No mentioned
diagnostician,DSM-IV
6 weeks
①ABC
1.5 g/d omega-3(EPA 0.84 g/d
,DHA 0.7 g/d)
Coconut oil
1 g(contained
vitamin E
1 mg、sh oil 1 mg)
– – No improvement No improvement
Omega-3
Voigt etal. (35)
USA
3–10 48
An experienced clinician,
DSM-IV, CARS≥30
6 months
①ABC
②CDI
③BASC
④TESS
⑤CGI-I
500 mg triglyceride oil capsules
containing 200mg DHA daily
500 mg
daily(250 mg corn
oil and 250 mg
soybean oil)500 mg
daily(250 mg corn
oil and 250 mg
soybean oil)
– –
①BASC Parent—social
skills (p= 0.04)
②BASC Teacher—
functional
communication
(p= 0.02)
No improvement
(Continued)
TABLE2 (Continued)
Jiang et al. 10.3389/fpsyt.2023.1238973
Frontiers in Psychiatry 08 frontiersin.org
Intervention Type
Literature (country)
Age (years)
Sample size
Diagnostic criteria
intervention
time
Measurement tool
experimental supplement Biomarkers Scale score
experimental
group
Placebo
group
experimental
group
Placebo
group
experimental
group
Placebo
group
Omega-3
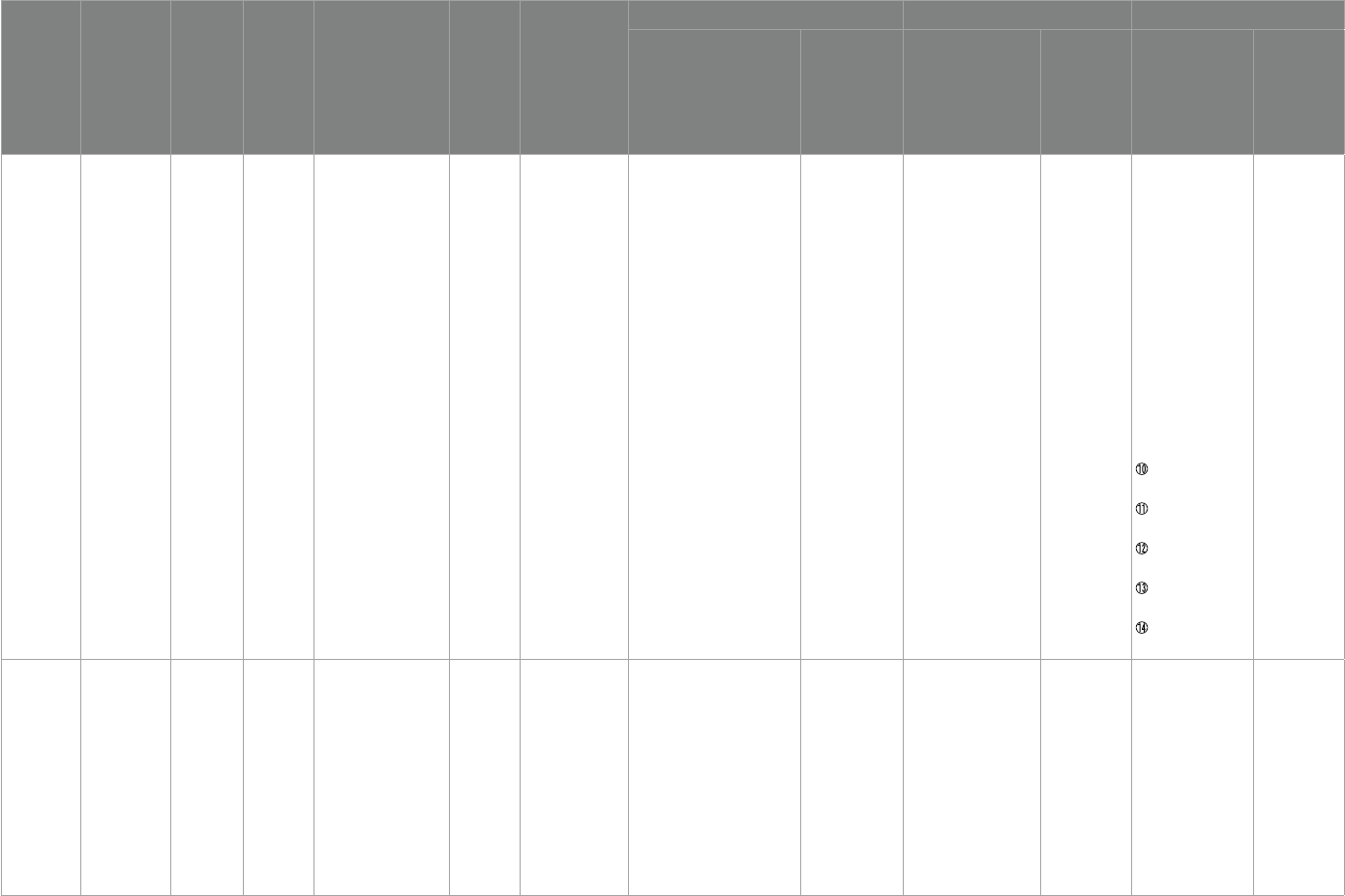
Yui etal. (36)
Japan
6–28 13
2 independent
psychiatrists,DSM-
IV、WISC-IV > 80
16 weeks
①ABC
②SS
③Biomarkers:
PUFAs
(DHA、ARA) ;
Transferrin ; SOD
6 capsules a day(240 mg)containing
ARA、DHA、0.16 mg astaxanthin
120 mg daily,
Aravita containing
olive oil capsule
Transferrin (p< 0.05) No improvement
①ABC-Social
withdrawal (P< 0.01)
②SRS-Communication
(p< 0.05)
No improvement
Omega-3
Doaei,et al. (37)
Iran
5–15 54
Clinician,ADOS、DSM-
IV
8 weeks
①BMI
②FFQ
③GARS
1 g/d (180 mg EPA + 120 mg DHA)
(Zahravi Company, Iran)
1 g/d (medium
chain triglyceride)
– –
①GARS-stereotyped
behaviors (p= 0.02)
②GARS-social
communication
(p= 0.02)
③GARS-total score
(P= 0.001)
No improvement
vitamin D
Javadfar etal.
(38) Iran
3–13 52 Pediatrician,DSM-IV 15 weeks
①CARS
②ABC-C
③ATEC
④BMI
⑤Serum
25(OH)
D3,IL-6,
serotonin
300 IU/kg daily up to a maximum
of
6,000 IU/d vitamin D syrup
No mentioned 25(OH)D3 (p= 0.006) No improvement
①CARS total (p= 0.021)
②ATEC (p= 0.020)
No improvement
TABLE2 (Continued)
(Continued)
Jiang et al. 10.3389/fpsyt.2023.1238973
Frontiers in Psychiatry 09 frontiersin.org
Intervention Type
Literature (country)
Age (years)
Sample size
Diagnostic criteria
intervention
time
Measurement tool
experimental supplement Biomarkers Scale score
experimental
group
Placebo
group
experimental
group
Placebo
group
experimental
group
Placebo
group
vitamin D
Saad etal. (39)
Egypt
3–10 109
Two pediatricians, one
psychiatrist, and two
experienced psychologists;
DSM-IV
4
months
①25(OH)D3
②CARS
③ABC
④SRS
⑤ATEC
⑥Biomarkers:vitamin
D levels, calcium,
phosphorous,
magnesium, glucose,
potassium, alkaline
phosphate, lead,
blood urea nitrogen
(BUN), serum
creatinine, AST, ALT
300 IU/kg/d not to exceed 5,000 IU/
day (Egyptian Ministry of Health)
Polysorbate 20
①Vitamin D levels
(p< 0.001)
No improvement
①ABC—Irritability
(p< 0.01)
②ABC—Hyperactivity
(P= 0.014)
③ABC—Lethargy/social
withdrawal (p= 0.003)
④ABC—Inappropriate
speech (p< 0.01)
⑤ABC—Stereotypic
behavior (p< 0.01)
⑥ATEC—Cognitive
awareness (p < 0.05)
⑦ATEC—Behavior
(p< 0.05)
⑧SRS—Social awareness
(< 0.001)
⑨SRS—Social cognition
(p< 0.001)
SRS—Autistic
mannerism (p< 0.01)
Total CARS scores
(p= 0.02)
No improvement
TABLE2 (Continued)
(Continued)
Jiang et al. 10.3389/fpsyt.2023.1238973
Frontiers in Psychiatry 10 frontiersin.org
Intervention Type
Literature (country)
Age (years)
Sample size
Diagnostic criteria
intervention
time
Measurement tool
experimental supplement Biomarkers Scale score
experimental
group
Placebo
group
experimental
group
Placebo
group
experimental
group
Placebo
group
Vitamin D
Saad etal. (20)
Egypt
3–9 83
No mentioned
diagnostician,DSM-IV
3 months
①CARS
②ABC
300 IU/kg/d not to exceed 5,000 IU/
day (Egyptian Ministry of Health)
No mentioned – –
①ABC—irritability
(p= 0.021)
②ABC—lethargy/social
withdrawal (p= 0.028)
③ABC—hyperactivity
(p= 0.01)
④ABC—stereotypic
behavior (P= 0.04)
⑤CARS—Relating to
people (p< 0.001)
⑥CARS—Emotional
response (p< 0.001)
⑦CARS—Imitation
(p< 0.001)
⑧CARS—Body use
(p= 0.01)
⑨CARS—Object use
(p= 0.01)
CARS—Adaptation
to change (p= 0.004)
CARS—Listening
response (p= 0.01)
CARS—Visual
response (p= 0.003)
CARS—General
impression (p< 0.001)
CARS—Total CARS
score (p< 0.001)
No improvement
Vitamin D
Duan etal. (40)
China
3–6 36
No mentioned, ICD-10,
DSM-IV
3 months
①ABC
②CARS
③25(OH)D3
Alfacalcalcitol 400 IU/time, orally
once a day; Vitamin D3 injection
150,000 IU/time, once a month
intramuscular injection
No mentioned
①25(OH) D3 rised
(p≤ 0.001)
No improvement
①ABC—total score
(p= 0.000) ;
②ABC—interaction
(p= 0.002) ;
③ABC—somatic motor
ability (p= 0.000) ;
④ABC—speech
(p= 0.011) ;
⑤ABC—self-care
individual scores
(p= 0.000
⑥CARS total score
(p= 0.002)
No improvement
TABLE2 (Continued)
(Continued)
Jiang et al. 10.3389/fpsyt.2023.1238973
Frontiers in Psychiatry 11 frontiersin.org
Intervention Type
Literature (country)
Age (years)
Sample size
Diagnostic criteria
intervention
time
Measurement tool
experimental supplement Biomarkers Scale score
experimental
group
Placebo
group
experimental
group
Placebo
group
experimental
group
Placebo
group
Vitamin D
Kerley etal. (41)
Ireland
3–30 38
No mentioned, DSM-IV;
SCQ > 15; aged <18 years
20 weeks
①ABC
②SRS
③DD-CGAS
④Biomarkers:
complete blood
count, 25(OH)D3 、
C reactive protein
(CRP)
2000 IU/day No mentioned
①25(OH) D rised
(p= 0.0016)
No improvement
①DD-CGAS— self-care
(p= 0.02)
No improvement
Vitamin D
Feng etal. (42)
China
3–5 37 Pediatrician,DSM-IV 3 months
①ABC
②CARS
③ serum 25(OH)D3
150,000 IU per month, and orally
400 IU per day (in total 3 months)
No mentioned
①25(OH)D rised
(p= 0.000)
No improvement
①total ABC scores
(p< 0.05)
②ABC—sensory
subscale (p< 0.05)
③ABC—social skills
(p< 0.05)
④ABC—body and
object use (p< 0.05)
⑤ABC—speech subscale
(p< 0.05)
⑥ABC—social or
self-help (p< 0.05)
⑦total CARS scores
(p< 0.05)
No improvement
Omega-3
+ vitamin D
Mazahery etal.
(43)
NewZealand
2.5–8 73 Pediatrician,DSM-IV 12 months
①ABC
②Biomarkers: full
blood count,
erythrocyte fatty
acids, 25(OH)D3,
calcium, albumin,
iron studies (iron,
iron binding capacity,
ferritin, and
transferrin
saturation), vitamin
B12, folate
ree groups -- VID group:vitamin
D3(2000IU/day) ; OM
group:omega-3 LCPUFA(722
DHA/day) ; VIDOM group:vitamin
D3(2000IU/day),DHA(722 mg/
day)
No improvement No improvement
①Irritability(VID vs.
placebo (p= 0.01) OM
vs. placebo (p= 0.001)
VIDOM vs. placebo
(p= 0.09))
②Hyperactivity(VID vs.
placebo (p= 0.047))
③Lethargy(OM vs.
placebo (p= 0.02))
No improvement
TABLE2 (Continued)
(Continued)
Jiang et al. 10.3389/fpsyt.2023.1238973
Frontiers in Psychiatry 12 frontiersin.org
Intervention Type
Literature (country)
Age (years)
Sample size
Diagnostic criteria
intervention
time
Measurement tool
experimental supplement Biomarkers Scale score
experimental
group
Placebo
group
experimental
group
Placebo
group
experimental
group
Placebo
group
Omega-3 +
vitamin D
Mazahery etal.
(44)
NewZealand
2.5–8 73 Pediatrician,DSM-IV 12 months
①SRS
②SPM
③Biomarkers: full
blood count,
erythrocyte fatty
acids, and 25(OH)
D3, calcium,
albumin, iron studies
(iron, iron binding
capacity, ferritin, and
transferrin
saturation), vitamin
B12, folate
ree groups -- VID group:vitamin
D3(2000IU/day) ; OM
group:omega-3 LCPUFA(722
DHA/day) ; VIDOM group:vitamin
D3(2000IU/day),DHA(722 mg/
day)
No improvement No improvement
①SRS-social
awareness(OM and
VIDOM) (p= 0.03)
②SRS-social
communicative
functioning(VIDOM)
p < 0.1
③SRS-total(OM) p< 0.1
④SPM-taste/
smell(VIDOM) p < 0.1
⑤SPM-balance/motion
(OM) p < 0.1
No improvement
TABLE2 (Continued)
(Continued)
p

Jiang et al. 10.3389/fpsyt.2023.1238973
Frontiers in Psychiatry 13 frontiersin.org
Intervention Type
Literature (country)
Age (years)
Sample size
Diagnostic criteria
intervention
time
Measurement tool
experimental supplement Biomarkers Scale score
experimental
group
Placebo
group
experimental
group
Placebo
group
experimental
group
Placebo
group
Omega-3 +
vitamin D
Mazahery etal.
(45)
NewZealand
2.5–8 67 Pediatrician,DSM-IV 12 months
①SRS
②Biomarkers:
25(OH)D3、
RBC、IL-1β、
calcium、
albumin、iron、
vitamin B12
ree groups -- VID group:vitamin
D3(2000IU/day) ; OM
group:omega-3 LCPUFA(722
DHA/day) ; VIDOM group:vitamin
D3(2000IU/day),DHA(722 mg/day)
No improvement No improvement 1.All children
①SRS-awareness
(p= 0.01)——OM group
②SRS-awareness
(p= 0.01)——VIDOM
group
③SRS-social
communicative
functioning
(p= 0.05)——VIDOM
group
2.children with elevated
IL-1β at baseline
①SRS-awareness
(p= 0.01)——VID
group
②SRS-awareness
(p= 0.003)——OM
group
③SRS-total
(p= 0.01)——OM group
④SRS-social
communicative
functioning
(p= 0.03)——OM group
⑤SRS-motivation
(p= 0.05)——OM group
⑥SRS-awareness
(p= 0.01)——VIDOM
group
⑦SRS-social
communicative
functioning
(p= 0.05)——VIDOM
group
TABLE2 (Continued)
(Continued)
Jiang et al. 10.3389/fpsyt.2023.1238973
Frontiers in Psychiatry 14 frontiersin.org
Intervention Type
Literature (country)
Age (years)
Sample size
Diagnostic criteria
intervention
time
Measurement tool
experimental supplement Biomarkers Scale score
experimental
group
Placebo
group
experimental
group
Placebo
group
experimental
group
Placebo
group
Omega-
3 + vitamin D
Fang etal.
(46)
China
5–12 48 No mentioned,DSM-IV 12 months 1.CARS ree groups——VD group:VD
800 U/d ; ω3 group:ω3 900 mg/
d ; Combination group:VD
800 U/d + ω3 900 mg/d
– –
①VD group and
Combination
group(compared to
placebo
group):emotional
response, imitation,
relationship with
inanimate objects,
adaptation to
environmental change,
proximity sensory
response, visual
response, anxiety
response, and overall
impression score
decreased (p< 0.05)
②ω3 group and placebo
group:auditory
response, visual
response, anxiety
response score
decreased (p< 0.05)
③Combination group
and ω3 group:Emotional
response, imitation,
relationship with
inanimate objects,
adaptation to
environmental change,
anxiety response
decreased (p< 0.05)
④Combination group
and VD group:Visual
response, auditory
response (p< 0.05)
No improvement
①DSM-IV, Diagnostic and Statistical Manual of Mental Disorders ; ②TAS, Otal Antioxidant Status; ③SRS, Social Responsiveness Scale; ④CGI, Clinical Global Impression; ⑤WISC-IV, Wechsler Intelligence Scale for Children; ⑥WPPSI, e Wechsler Preschool and
Primary Scaleof Intelligence; ⑦CBCL, Child Behavior Check List; ⑧ADOS, e Autism Diagnostic Observation Schedule; ⑨SCQ, Social Communication Questionnaire; BASC, Behavior Assessment System for Children; ADI, Autism Diagnostic Interview;
ABC, Aberrant Behavior Checklist; PDDBI, PDD Behavior Inventory; PLS, Preschool Language Scale; WAIS, Wechsler Adult Intelligence Scale; CDI, Child Development Inventory; TESS, Treatment Emergent Symptom Scale; BMI, Body Mass Index;
FFQ, Food Frequency Questionnaire; GARS, Gilliam Autism Rating Scale; DD-CGAS, e Developmental Disabilities — Children’s Global Assessment Scale; SPM, Sensory Processing Measure.
TABLE2 (Continued)
Jiang et al. 10.3389/fpsyt.2023.1238973
Frontiers in Psychiatry 15 frontiersin.org
further statistical analysis showed that serum 25(OH)D3 levels did not
correlate with the gender. Considering further, serum 25(OH)D3
levels had a signicant negative correlation with anti-MAG, and
anti-MAG deciency may aect anti-MAG levels, suggesting that
anti-MAG is highly correlated with ASD, serum 25(OH)D3 levels are
correlated with the degree of behavioral abnormalities in ASD patients
(47). Feng (42) monthly intramuscular vitamin D3 injections
(150,000 IU) and daily oral vitamin D3 (400 IU) had statistically
signicant dierences in total ABC scores, social skills, body and
object use, speech, and social or self-help compared before and aer
treatment. eir study also showed that 25(OH)D3 levels were
negatively correlated with total ABC scores and speech scores on the
ABC. Saad (20) showed statistically signicant improvements in
irritability, lethargy/social withdrawal, hyperactivity and stereotypic
behavior on the ABC scale before and aer vitamin treatment. Kerley
(41) showed that there was no statistically signicant dierence in
ABC levels for patients aer 20 weeks of vitamin D supplementation,
but the original article also speculated that the lack of change in ABC
levels may berelated to the milder symptoms of the included ASD
patients. Javadfar (38) showed that aer 15 weeks of vitamin D
supplementation, there was no statistically signicant eect on the
pre- and post-ABC scale symptom statistics.
Mazahery (43) showed that combined omega-3 and vitamin D
supplementation showed statistical dierences in irritability,
hyperactivity, and lethargy in ABC.
In the comprehensive analysis, ve of the ten omega-3
supplementary studies used the ABC scale, but only two of them
showed statistical dierences for a total of three subscales, so the eect
was not signicant, vitamin D supplementation had a greater impact
on patients’ behavioral functioning. In six studies of Vitamin D
supplementation, all of which used the ABC scale to investigate the
impact on patients’ behavioral functioning, four studies showed that
patients’ behavioral functioning improved in various ways aer
supplementation, with statistically dierences. However, it should also
benoted that of the Vitamin D supplementation studies, Saad K’s (20)
study had three high risk and one unknown risk; Xiaoyan’s (40) study
had 4 high risk and 1 unknown risk; and Feng J’s (42) study had 3 high
risk and 1 unknown risk, with low quality literature. However, 4
studies with combined omega-3 and vitamin D interventions, only 1
used the ABC scale and showed statistical dierences in some of
the indicators.
Behavior assessment system for children
ree articles used the BASC system to evaluate children’s
behavior, all of which were also omega-3 supplementation studies. e
BASC system is divided into self-reported, parent-rated, teacher-rated,
and student-observed versions, with the aim of providing a
comprehensive assessment of children’s behavior using dierent
evaluators. Voigt (35) used teacher-rated and parent-rated, and aer
6 months of the omega-3 intervention, the observation group had
signicant dierences in the social function and the communication
function compared to the control group. Mankad (32) used the
parent-rated versions in their study aer 6 months, monitored at
baseline, week 12 and week 24, but there was no signicant dierence
between the observation and control groups. Bent (30) did not specify
which version of the BASC system was used, but there was no
signicant dierence before and aer the intervention.
Child behavior check list
e CBCL is used to assess children’s social skills and behavioral
problems in omega-3 supplemental study. Ooi’s (29) study used a
parent-reported version and showed a signicant dierence between
the results of the observation groups and control groups, improvement
in social and attention problems for patients.
Gilliam autism rating scale
Seven papers used the GARS scale, which is a standardized tool
for assessing autism spectrum disorders and other severe
behavioral disorders.
Saeid Doaei (37) showed an improvement in GARS stereotypical
behavior, social communication, and total scores in the observation
group aer an 8-week omega-3 intervention, with
signicant dierences.
e GARS scale was used in all 6 vitamin D supplementation
studies. Javadfar (38) showed that the decrease in total GARS scores
before and aer the intervention, was signicantly greater than in the
placebo group, and the dierence was statistically signicant. Khaled
Saad (39) showed a signicant improvement in the total CARS score
in the vitamin D supplementation group compared to the placebo
group aer a study period of 4 months. Khaled Saad (20) study shown
that GARS scale (relating to people, emotional response, imitation,
body use, object use, adaptation to change, listening response visual
response, general impression, total CARS score), before and aer
treatment the dierence was statistically signicant. Serum 25(OH)
D3 levels were signicantly and negatively correlated with CARS
scores. Children with 25(OH)D3 levels of >40 ng/mL all had improved
CARS scores. Xiaoyan (40) showed a statistically signicant reduction
in total CARS score aer 3 months of treatment compared to
pre-treatment. Feng (42) showed a statistically signicant dierence
in the reduction in total CARS score between the early treatment
group and the late treatment group. However, in their study, CARS
scores did not correlate with 25(OH)D3 levels.
Fang’s study (46) showed that children in the VD group and the
combination group had signicantly lower (p < 0.05) scores for
emotional reactions, imitation (words and actions), relationship with
inanimate objects, adaptation to environmental changes, proximal
sensory reactions, visual reactions, anxiety reactions, and overall
impressions, suggesting that taking VD or VD combined with
omega-3 was eective in improving the above symptoms in children
with ASD. Compared to the placebo group, children in the omega-3
group had signicantly lower auditory response, visual response, and
anxiety response scores only (p < 0.05). Compared to the omega-3
group, children in the combination group showed signicantly lower
scores for emotional responses, imitation (words and actions),
relationship to inanimate objects, adaptation to environmental
changes, and anxiety responses (p < 0.05). e combined medication
group was more eective in improving the children’s visual and
auditory responses than the VD group (p < 0.05).
Jiang et al. 10.3389/fpsyt.2023.1238973
Frontiers in Psychiatry 16 frontiersin.org
In the comprehensive analysis, ten omega-3 supplementary
studies,only one used the GARS scale, but only three indicators
showed statistical dierences. Six vitamin D supplementation studies,
all of which used the GARS scale, there was a signicant increase in
the total GARS score in each of these studies, while a total of 10
indicators showed statistical dierences in the study (20). However,
four studies with combined omega-3 and vitamin D interventions,
only one used the GARS scale and showed statistical dierences in a
number of indicators.
Clinical global impression
Five articles used the CGI Scale, all of which were omega-3
supplemented studies, in which the CGI-I scale was considered to
bethe commonly used measure in all clinical trials of patients with
ASD to evaluate clinical outcomes, but all of their results showed no
signicant dierences before and aer the intervention.
Speech function
Mankad (32) used the PLS (Preschool Language Scale) to assess
the language skills at the beginning and at 24 weeks. However, the
results of the study showed that there was no signicant dierence
between the observation group and the control group. Mankad’s
language assessment was conducted at the beginning and at the end
of the study, using linear regression in the statistics, and lacked a
follow-up phase during the study.
Biomarkers changes
Specic blood fatty acid levels have been associated with
changes in the core symptoms of ASD (48). Related studies have
shown that red blood cells and brain tissue have dierent
polyunsaturated fatty acid compositions. Polyunsaturated fatty
acids are more readily available in erythrocyte membranes than in
brain tissue, and tests targeting polyunsaturated fatty acids in blood
can help to suggest the amount of polyunsaturated fatty acids in
brain tissue.
Parellada (28) examined the ratio of omega-3in erythrocyte
membranes (AA/DHA, AA/EPA, ω3/ω6) and plasma total
antioxidant status (TAS). e results of the study showed a
signicant time eect and a time group eect with signicant
dierences in the AA/DHA and ω3/ω6 ratios between the two
groups. Ooi (29) study considered the blood levels of patients and
tested AA/EPA, omega-3, EPA, and DHA levels. Aer the
intervention, there was a signicant decrease in the percentage of
AA/EPA and a signicant dierence in the dierence in omega-3,
EPA, and DHA changes, and the study showed that changes in
blood level indicators were associated with a decrease in the severity
of ASD behaviors. In the Bent (30) study, omega-3 percentages
increased in the intervention group and decreased slightly in the
control group. DHA and EPA levels increased in the intervention
group compared to the control group and the dierence was
signicant. Nine individual fatty acids (15:0, 22:0, 24:0, 18:1n9,
22:4n6, 22:5n6, 20:5n3 (EPA), 22:5n3, 22:6n3 (DHA)) and four of
the eight category percentages (% monounsaturated fatty acids, %
polyunsaturated fatty acids, % omega-3, omega-9) also showed
signicant dierences in change over the course of the study,
indicating that 12 weeks of omega-3 supplementation signicantly
aected fatty acid distribution. Stephen Bent also measured 29
cytokines, but only one (TNFa) showed a signicant dierence in
mean change over the course of the study between groups, with
TNFa increasing in the observation group and decreasing in the
control group. Yui (36) examined plasma levels of SOD, Transferrin
and PUFAs and there was a trend toward a signicant dierence in
the change in plasma Transferrin levels between the two groups
(p = 0.03) ,and a trend toward a signicant dierence in plasma
SOD levels (p = 0.08), and plasma DHA (p = 0.74) and ARA
(p = 0.86) levels were not statistically signicant. Transferrin is
involved in signal transduction, SOD plays a role in lipid signaling
in defense against oxidative stress, and the interrelationship
between SOD and transferrin mediates the signaling pathway.
Mankad (32) examined omega-3 fatty acid levels and cytokines in
his study, but the dierences were not signicant between groups.
In Javadfar’s (38) study, Serum 25(OH)D3, IL-6, serotonin levels
were measured in patients, but only serum 25(OH)D3 levels
increased signicantly aer vitamin D supplementation, with a
statistically signicant dierence (p = 0.006). IL-6 is an indicator of
chronic inammation and an independent and reliable predictor of
ASD, but no dierential change was found in this study. Aer the
study (39), serum 25(OH)D3 levels were signicantly higher in the
observation group, with a statistically signicant dierence
(p = <0.001), and no dierences in any other biomarkers. Serum
25(OH)D3 levels were signicantly higher in the observation group
before and aer treatment, and the dierence was statistically
signicant (p = 0.000) (40). Serum 25(OH)D3 levels were signicantly
higher before and aer supplementation (41), with a statistically
signicant dierence (p = 0.0016), while there were no dierences in
other biochemical indicators. Serum 25(OH)D3 levels were
signicantly higher in all patients aer vitamin D supplementation
(p = 0.000) (42).
Study (44) showed that analysis of serum 25(OH)D3 concentration
and omega-3 index showed a signicant (p < 0.01) interaction between
follow-up time and treatment time point and observation group. e
study (43) showed no statistically signicant dierence in changes in
serum 25(OH)D3 concentrations or omega-3 index before and aer
supplementation. Studies (45) have shown no statistical dierence in
changes in serum 25(OH)D3 concentrations or omega-3 index before
and aer supplementation.
In a comprehensive analysis, thirteen of the twenty papers dealt
with relevant biochemical indicators. Ten studies of omega-3
supplementation included in this study, ve considered biomarkers at
the time of testing, with changes in blood levels of fatty acids. Four of
these studies showed a signicant dierence in blood levels between
the observation group and the control group aer omega-3
supplementation, with signicant dierences. Of the 6 Vitamin D
supplementation studies, 5 considered biomarkers at the time of
testing and serum 25(OH)D3 concentrations were increased in ve of
these studies, with a statistically signicant dierence between before
and aer. However, of the four studies of combined omega-3 and
vitamin D interventions, three considered biomarkers and only one
(44) showed statistical dierences in omega-3, serum 25(OH)
D3 concentrations.
Jiang et al. 10.3389/fpsyt.2023.1238973
Frontiers in Psychiatry 17 frontiersin.org
Discussion
Given that there are no eective drugs for the treatment of ASD
(49), and considering the food selectivity of patients with ASD, some
studies are gradually looking at nutritional therapy as a way to
compensate for nutritional deciencies and alleviate core symptoms
(50), based on which fatty acid supplementation stands out among the
many nutritional therapies (51). Ten studies analyzing the eects of
omega-3 supplementation in people with ASD showed variation in the
results of the literature. Only some of the studies showed that
supplementation was eective in improving the core symptoms
associated with ASD, with the best study being that of Ooi. However,
the Ooi study was relatively low quality, with two high risk assessments
and two unknown risk assessments, and the quality of the literature
was low. Six studies analyzed the eects of vitamin D supplementation
in patients with ASD, vitamin D supplementation improved some of
the core symptoms of ASD in patients with ASD, mainly behavioral
functioning. However, the results of the literature included in this
study were slightly mixed. Four studies analyzed the eects of
combined omega-3 and vitamin D supplementation in patients with
ASD, and the combined eect was good, with signicant
improvements in social and behavioral outcomes.
Adherence to nutritional interventions is an important factor
inuencing the eectiveness of the study. Studies have shown that
high adherence with family interventions for ASD facilitates
treatment delivery, helps relieve patients’ symptoms and improves
their intelligence (52). Patient adherence control does have a direct
impact on the eectiveness of studies of omega-3 supplementation in
patients with ASD. e literature included in this study also varied in
the approach taken to adherence control. Parellada (28) monitored
patient adherence in a number of ways, with the team asking
participants to hand in all omega-3 capsules (empty or not), along
with a weekly calendar, and the researcher would also count the
number of medications. ere was also a researcher who conducted
telephone assessments every other week during the intervention to
check patient adherence and adverse events. Bent (30) was contacted
by telephone at week 2, week 8, a brief assessment at week 6 and a
nal visit at week 12 during. Bent (31) used an internet-based
random controlled trial approach in his 2014 trial, in which 863
registered members were invited via email upfront, and interested
parents were invited to complete an initial test via an embedded link
in an email to a screening questionnaire. A parent or carer or teacher
is also required who is willing to complete the baseline information
and assessment via email. Parents of children screened through
eligibility, online informed consent is completed in the form of an
electronic signature. And all participants in the study have the option
to speak to the researcher by telephone before signing the informed
consent form. Parents participating in the study then received weekly
follow-up emails reporting medication adherence, medical problems
and were also assessed by email at week 3 and week 6. e study
collected data in a manner consistent with the US Food and Drug
Administration (FDA) and regulations in the Health Insurance
Portability and Accountability Act. Once a subject has had an adverse
event and it is entered into the web-based platform, the two principal
investigators receive an email alert from the platform, will consult on
the adverse event and call the parents to record further information
about it. is internet-based experimental approach has shown the
advantages of low cost, rapid registration, high completion rate and
ease of participation, and facilitates the replication of more studies at
a later stage.
Patients with ASD face their own symptoms of stereotypical
behavior, communication disorders, social interaction disorders, and
the long duration of the disease, the high cost of treatment, the burden
on families and the psychological burden on carers. ese factors have
a signicant impact on adherence with ASD, and more ways to
increase patient compliance management can also help patients
recover their social functioning. It was also found during the study
that Bent (30) lost one patient in the 2011 study but none in the 2014
study. It can also be seen that an internet-based, multi-path
intervention approach can increase patient adherence. Current expert
consensus and guidelines for the management of patients with ASD
mention the need for long-term interventions for patients with ASD
(53–55), and adherence to treatment is an important indicator in
intervention studies and an important basis for ensuring the
eectiveness of long-term treatment for patients with ASD (56).
Interleukin-1β is frequently elevated in the plasma of children and
adults with ASD (57). Mutations and polymorphisms in IL-1β and its
receptor have been shown to beassociated with ASD and cognitive
performance (58, 59). e ndings suggest that participants with
higher levels of inammation are immune responders if the
intervention itself is immunomodulatory, whereas participants with
higher levels of inammation are not inammation-responsive if the
intervention has no immunomodulatory eect. Studies have shown
immune alterations in the cerebrospinal uid and peripheral blood of
patients with ASD (57), with associated elevations of pro-inammatory
cytokines such as IL-1α and β, IL-1Ra, IL-4, IL-6, IL-10, TNF-α, and
IFN-γ (60). On this basis it is possible to speculate that omega-3 and
vitamin D may improve the clinical symptoms of ASD through the
inammatory response (61). Both studies (44, 45) come from the
same group of researchers, but the study (45) discusses more
profoundly the alteration of pretreatment inammatory status on the
therapeutic eects of combined vitamin D and omega-3 interventions
(45). Baseline information on participants’ inammatory status
(IL-1ra, IL-6, and hs-CRP) was included prior to the study and
stratication of inammatory status was performed prior to the start
of the study, with results showing that participants with high
inammatory status showed more improvement in treatment
outcomes than the placebo group. Moreover, in the study (45), there
were no dierences in IL-1β statistics between the four groups at
baseline, but a trend toward greater improvement in SRS-total,
SRS-social communicative functioning, and SRS-RRB occurred when
IL-1β was elevated over the course of the study.
It should also benoted that omega-3 supplementation does have
associated adverse eects, with Parellada (28) showing that the only
signicant adverse event during the intervention was a small increase
in total cholesterol during the trial. In the Bent (30) study, ve subjects
in the observation group reported adverse events: 2 rashes, 1 upper
respiratory tract infection, 1 nosebleed and 1 exacerbation of
gastrointestinal symptoms. However, there were also 4 adverse events
in the control group, 3 increases in hyperactivity and 1 increase in
self-stimulatory behavior. e dierence between the observation and
control groups was not signicant in comparison. Amminger (34)
showed that in the observation group mild adverse events occurred as
fever, but the control group also had headache and insomnia. In
addition to this there were associated adverse events, all of which were
mild, but the dierence between the observation and control groups
Jiang et al. 10.3389/fpsyt.2023.1238973
Frontiers in Psychiatry 18 frontiersin.org
was not signicant. Of concern is the predominance of adverse events
such as gastrointestinal problems (diarrhea, vomiting) (30, 31, 44, 46).
Vitamin D supplementation studies have been associated with some
adverse reactions. In the study by Javadfar (38) there were 3 patients
who discontinued treatment: 2 due to rash and 1 due to diarrhea. Five
patients in Khaled Saad’s study (39) presented with rash, pruritus and
diarrhea. In the study by Mazahery (44), a rash, facial papules, and red
ears were seen.
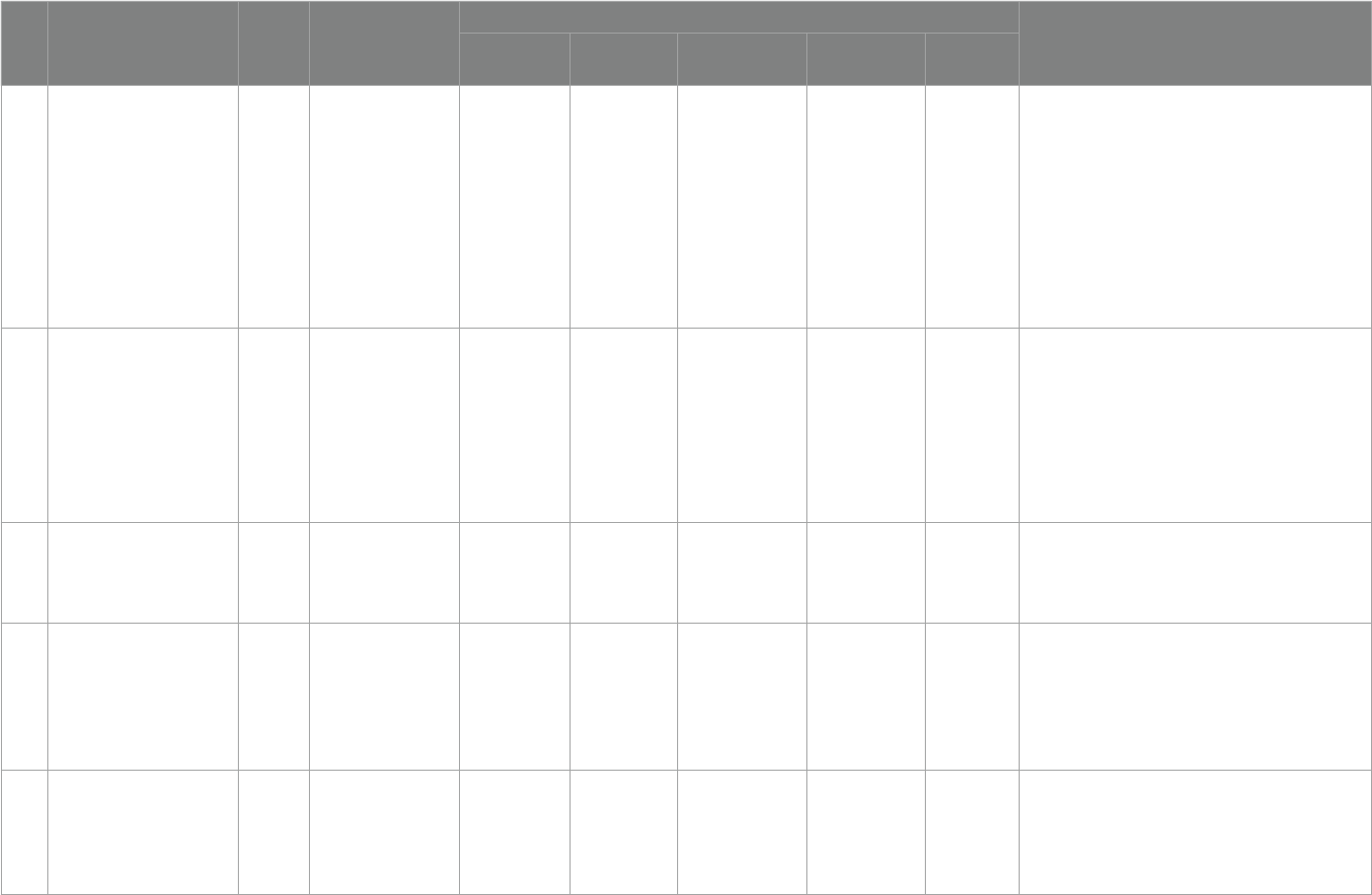
We sorted out the criteria for denition of vitamin D and
supplementation criteria by the society and related organizations
further in the last decade 2013–2023. According to Table3, it can
beseen that there are dierent denition criteria regarding vitamin
D. ere is no exact international standard denition and there are
no precise criteria for vitamin D supplementation, but further
analysis shows that the existing criteria for supplementation in
children tend to apply the criteria of 0-6 months: 400 IU/day,
6–12 months: 400–600 IU/day, but it is important to note that the
proposed criteria for supplementation are not In the context of ASD
disease, it is accurate to say that there is no precise international
standard for the amount of supplementation needed for children
with ASD. And with further reference to Table 2, there is no
complete harmonization of supplementation levels for children
with ASD.
In our study we found that researchers validated study
preconceptions in multiple studies on the same research topic. Two
studies (30, 31) come from the same research team, experimental
studies conducted in 2011 and 2014, respectively. A pilot randomized
controlled trial (30) was conducted in 2011 to determine the feasibility,
initial safety and ecacy of omega-3 for the treatment of ADHD in
children with ASD. Aer 12 weeks of treatment, there was a correlation
between reduced levels of fatty acids and reduced levels of ADHD, and
the treatment was well accepted. However there was no statistically
signicant eect of omega-3 on core symptoms of ADHD or autism.
A new, internet-based clinical trial (31) design was conducted in 2014,
and the study suggests that internet-based randomized controlled
trials of treatments for children with ASD are feasible and may lead to
signicant reductions in the time and cost of completing a trial.
However, omega-3 fatty acids did not lead to a signicant reduction
in ADHD, but a trend toward a non-signicant benecial eect
was observed.
Two studies (20, 39) come from the same research team,
experimental studies were conducted in 2016 and 2015.A cross-
sectional study was rst conducted in 2015 to assess the vitamin D
status of individuals with ASD and the relationship between vitamin
D deciency and autism severity. An open trial of vitamin D
supplementation in children with ASD was also conducted. e
results of the study indicated that vitamin D may bebenecial for
individuals with ASD, there was a signicant negative correlation
between serum 25(OH)D levels and the severity of autism as assessed
by CARS scores. Subsequently in 2016, based on the results of the
2015 trial, the eect of vitamin D supplementation on core symptoms
of autism in children was further assessed. Using a double-blind,
random clinical trial methodology and a more carefully designed
study extending from 3 to 4 months in 2015, the results of the study
suggest that oral vitamin D supplementation can safely improve signs
and symptoms of ASD and can be recommended for children
with ASD.
ree studies (43–45) come from the same research team. Two
studies (43, 44) were experiments conducted in the same year. A
randomized, double-blind, placebo-controlled (43) design to test
whether vitamin D and omega-3 were eective in reducing irritability
and hyperactivity symptoms in children with ASD. e eect of
changes in biomarkers of vitamin D (serum 25(OH)D) or − 3 LCPUFA
(omega-3 index) on treatment response was also investigated. Studies
have shown that vitamin D and omega-3 can treat irritability
symptoms in children with autism, and that vitamin D has a signicant
benet on ADHD in these children. e study (44) focusing on,
Vitamin D and omega-3 for the treatment of core symptoms of autism
in children, the results of the study suggest that supplementation with
omega-3 alone or in combination with vitamin D may beeective in
treating core symptoms of ASD in children. e study (45) focusing
on the alleviation of inammatory factors in ASD following vitamin
D and omega-3 supplementation, ndings suggest that vitamin D and
omega-3 have the potential to enhance social and communicative
functioning in children with ASD, especially when based on known
pre-treatment inammatory conditions.
However, based on this, weare unable to conclude that vitamin D
and/or omega-3 supplementation is eective in alleviating ASD, and
the results of the omega-3 supplementation studies are individually
signicant, while the results of the other studies are not signicant.
Vitamin D supplementation was shown to beeective in improving
symptoms, but wewere unable to draw rm conclusions based on
several experiments, and weare inclined to conclude that vitamin D
supplementation improved some of the core symptoms of ASD in
patients with ASD, mainly behavioral functioning. However, regarding
the experiments on combined vitamin D and omega-3
supplementation, there was a positive eect on ASD, but it was not
possible to further identify the starting components. Werecommend
vitamin D supplementation to improve behavioral functioning in
clinical applications.
We need to take into account in our clinical practice the impact of
risks such as ethnicity, diet, associated diseases (Hepatic failure,
cholestasis, Chronic kidney disease), sun exposure, latitude and
longitude of residence on vitamin D levels. Currently, many vitamin
D guidelines do not require vitamin D monitoring as part of routine
screening, but for many diseases with a high risk of vitamin D, testing
is necessary during the disease assessment phase. However, for
patients with ASD, many studies have shown that there is a correlation
between vitamin D and the development of ASD, and that taking
vitamin D can alleviate some of the symptoms. Wesuggest that when
the correlation between vitamin D and the development of ASD is
further established (which is where our future research will
bedirected), vitamin D testing can beadded to the ASD screening
programme. And it is worth mentioning that in A Central and Eastern
European Expert Consensus Statement mentioned that assessing the
success of vitamin D treatment aer at least 6 to 12 weeks,which is
worth guiding us in designing the duration of monitoring in our
future studies.
Currently, the main circulating form of vitamin D is 25(OH)D,
which has a half-life of 2–3 weeks, and it is the best marker for
monitoring vitamin D status. For this reason, blood control for
monitoring vitamin D levels is a more idealized means of monitoring,
but in practice, frequent blood sampling for patients with ASD is not
relatively easy to achieve, and when it is done it is expensive, whether
Jiang et al. 10.3389/fpsyt.2023.1238973
Frontiers in Psychiatry 19 frontiersin.org
TABLE3 Definition of vitamin D status and supplementation criteria.
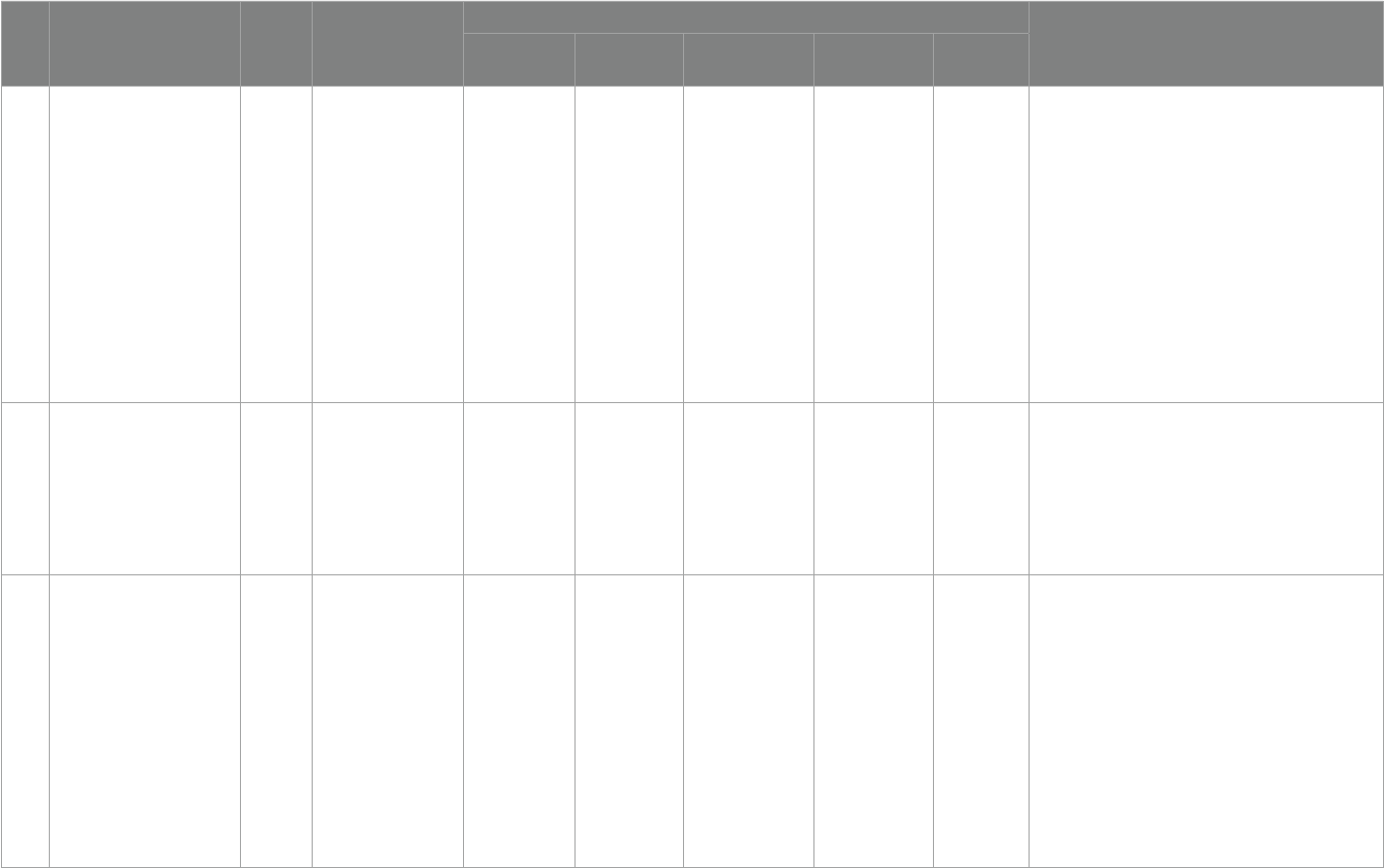
NO Title Year Society/
Organization
Diagnostic standards Supplementary standards
Severe
deficiency
Deficiency Insuciency Suciency toxicity
1
Guidelines for Preventing and
Treating Vitamin D Deciency:
A 2023 Update in Poland (62)
2023
Nutrition-related expert
groups
- <20 ng/mL 20–30 ng/mL 30-50 ng/mL -
[1] 0–6 months: 400 IU/day;
[2] 6–12 months: 400–600 IU/day;
[3] 1–3 years: 600 IU/day;
[4] 4–10 years: 600–1000 IU/day;
[5] Adolescents (11–18 Years): 1000–2000 IU/day;
[6] Adults (19–65 Years):1000–2000 IU/day;
[7] Younger Seniors (>65–75 Years): 1000–2000 IU/day;
[8] Older Seniors (>75–89 Years) and the Oldest Old Seniors
(90 Years and Older): 2000–4,000 IU/day;
[9] Pregnancy and Lactation: 2000 IU/day
2
Denition,Assessment, and
Management of Vitamin D
Inadequacy: Suggestions,
Recommendations, and
Warnings from the Italian
Society for Osteoporosis,
Mineral Metabolism and Bone
Diseases (63)
2022
Italian Society for
Osteoporosis, Mineral
Metabolism
and Bone Diseases
– <10 ng/mL <20 ng/mL 20-50 ng/mL - No mention
3
Vitamin D and calcium intakes
in general pediatric
populations: A French expert
consensus paper (64)
2022
Group of Experts in
Paediatric Related
Medicine
<10 ng/mL <20 ng/mL 20–29 ng/mL 30-60 ng/mL >80 ng/mL
[1] 0–18 years: 400 IU- 800 IU vitD/ day;
[2] 2–18 years: intermittent supplementation in the case of
non adherence, vitD3 with either 50,000 IU quarterly or
80,000–100,000 IU twice in fall and winter.
4
Clinical Practice in the
Prevention, Diagnosis and
Treatment of Vitamin D
Deciency: A Central and
Eastern European Expert
Consensus Statement (65)
2022
Group of Experts in
Paediatric Related
Medicine
- <20 ng/mL 20–30 ng/mL 30-50 ng/mL >100 ng/mL
[1] Healthy adults: 800–2000 IU/day;
[2] Elderly(>65 years): 800–2000 IU/day;
[3] Hospitalized/institutionalized individuals: 800–2000 IU/
day;
5
Indian Academy of Pediatrics
Revised (2021) Guidelines on
Prevention and Treatment of
Vitamin D Deciency and
Rickets (66)
2021
Indian Academy of
Pediatrics
- <12 ng/mL 12–20 ng/mL >20 ng/mL -
[1] Infancy:400 IU/day;
[2] Childhood:400 IU/day;
[3] Adolescents:600 IU/day;
(Continued)
Jiang et al. 10.3389/fpsyt.2023.1238973
Frontiers in Psychiatry 20 frontiersin.org
NO Title Year Society/
Organization
Diagnostic standards Supplementary standards
Severe
deficiency
Deficiency Insuciency Suciency toxicity
6 Vitamin D testing (67) 2019
Clinical Practice
Guidelines and Protocols
in British Columbia
- 12 ng/mL 12–20 ng/mL
≥20 ng/mL
>50 ng/mL
[1] Infants 0–6 months: 400 IU (10 μg)/D allowance;1,000 IU
(25 μg)/D tolerable upper intake level;
[2] Infants 7–12 months: 400 IU (10 μg)/D allowance;1,500 IU
(38 μg)/D tolerable upper intake level;
[3] Children 1–3 years: 600 IU (15 μg)/D allowance; 2,500 IU
(63 μg)/D tolerable upper intake level;
[4] Children 4–8 years: 600 IU (15 μg)/D allowance; 3,000 IU
(75 μg)/D tolerable upper intake level;
[5] Children and adults 9–70 years (including pregnant and
lactating women): 600 IU(15 μg)/D allowance;
4,000 IU(100 μg)/D tolerable upper intake level;
[6] Adults >70 years: 800 IU (20 μg)/D allowance; 4,000 IU
(100 μg)/D tolerable upper intake level
7
Vitamin D in pediatric age:
consensus of the Italian
Pediatric Society and the Italian
Society of Preventive and Social
Pediatrics,jointly with the
Italian Federation of
Pediatricians (68)
2018
Italian Pediatric Society
and the Italian
Society of Preventive
and Social Pediatrics
<10 ng/mL <20 ng/mL 20–29 ng/mL
≥30 ng/mL
–
[1] 0–12 months
(1) Infants without risk factors: 400 IU/day
(2) Infants with risk factors: 1000 IU/day
[2] 1–18 years
(1) 600–1,000 IU/day
8
Vitamin D Supplementation
Guidelines for General
Population and Groups at Risk
of Vitamin D Deciency in
Poland —Recommendations of
the Polish Society of Pediatric
Endocrinology and Diabetes
and the Expert Panel with
Participation of National
Specialist Consultants and
Representatives of Scientic
Societies—2018 Update (69)
2018
Polish Society of
Pediatric Endocrinology
and Diabetes and the
Expert Panel with
Participation of National
Specialist Consultants
and Representatives of
Scientic Societies
<10 ng/mL 10-20 ng/mL 20–30 ng/mL 30-50 ng/mL >100 ng/mL
[1] 0-6 months:400 IU/day;
[2] 6–12 months: 400–600 IU/day;
[3] 2–10 years: 600–1,000 IU/day;
[4] 11–18 years: 800–2000 IU/day;
[5] >18 years: 800–2000 IU/day;
[6] >75 years: 2000–4,000 IU/day;
[7] Pregnancy and lactation:2000 IU/day;
TABLE3 (Continued)
(Continued)
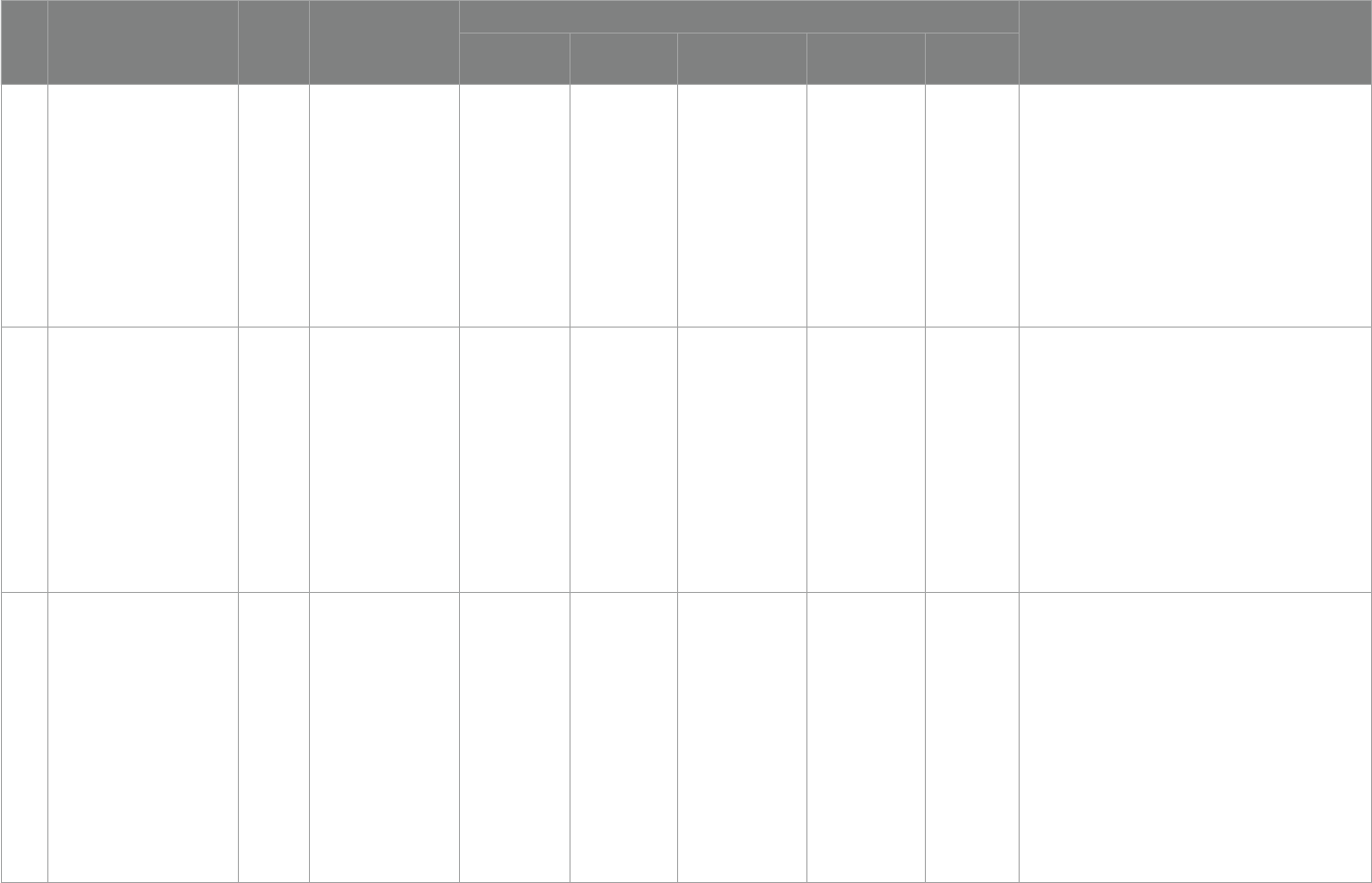
Jiang et al. 10.3389/fpsyt.2023.1238973
Frontiers in Psychiatry 21 frontiersin.org
NO Title Year Society/
Organization
Diagnostic standards Supplementary standards
Severe
deficiency
Deficiency Insuciency Suciency toxicity
9
Assessment criteria for vitamin
D deciency/ insuciency in
Japan—proposal by an expert
panel supported by Research
Program of Intractable
Diseases, Ministry of Health,
Labour and Welfare, Japan, e
Japanese Society for Bone and
Mineral Research and e
Japan Endocrine Society (70)
2017
An expert panel
supported by Research
Program of Intractable
Diseases, Ministry of
Health, Labour and
Welfare
- <20 ng/mL 20–30 ng/mL
≥30 ng/mL
- No mention
10
Assessment criteria for vitamin
D deciency/ insuciency in
Japan: proposal by an expert
panel supported by the
Research Program of
Intractable Diseases, Ministry
of Health, Labour and Welfare,
Japan, the Japanese Society for
Bone and Mineral Research and
the Japan Endocrine Society
(70)
2017
Japanese Society for
Bone and Mineral
Research, Japan
Endocrine Society
- <20 ng/mL 20–29 ng/mL
≥30 ng/mL
- No mention
11
Clinical practice guidelines for
vitamin D in the
UnitedArabEmirates (71)
2016 United Arab Emirates - <20 ng/mL 20–29 ng/mL
≥30 ng/mL
-
[1] Breastfed infants: 400 IU/day up to age 6 months, 400–
600 IU/day between 6 and 12 months;
[2] Children and adolescents of age 1–18 years:600–1,000 IU/
day;
[3] > 18 years: 1000–2000 IU/day;
[4] e elderly (over 65 years): 2000 IU/day;
[5] Pregnant and breast feed women: 2000 IU/day;
[6] Premature infants: 400–800 IU/day;
[7] Obese, individuals and those with metabolic syndrome:
2000 IU/day;
[8] Individuals with dark skin complexions and for night
workers: 1000–2000 IU/day;
TABLE3 (Continued)
(Continued)
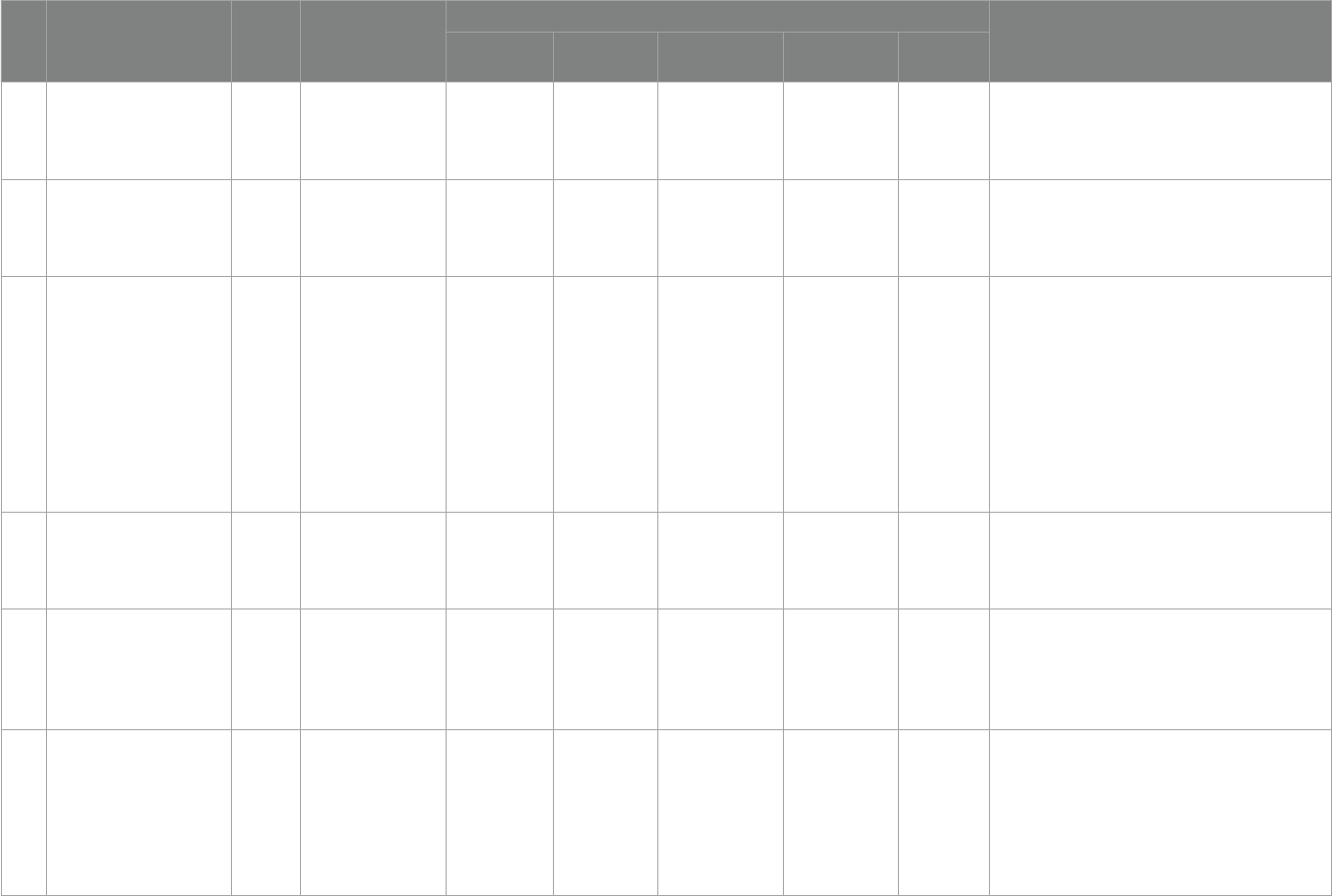
Jiang et al. 10.3389/fpsyt.2023.1238973
Frontiers in Psychiatry 22 frontiersin.org
NO Title Year Society/
Organization
Diagnostic standards Supplementary standards
Severe
deficiency
Deficiency Insuciency Suciency toxicity
12
Global Consensus
Recommendations on
Prevention and Management of
Nutritional Rickets (72)
2016
Global Consensus for
rickets
- <12 ng/mL 12–19 ng/mL
≥20 ng/mL
>100 ng/mL
e supplemental quantities mentioned in the article are all
for nutritional rickets and osteomalacia
13
Vitamin D and bone health: a
practical clinical guideline for
management in children and
young people (73)
2015
National Osteoporosis
Society
- <10 ng/mL 10–20 ng/mL >20 ng/mL
[1] 1–6 months: 3,000 IU orally daily for 8–12 weeks;
[2] 6 months to 12 years: 6,000 IU orally daily for 8–12 weeks;
[3] 12–18 years: 10,000 IU orally daily for 8–12 weeks; a single
or divided oral dose totalling 300,000 units.
14
Pathogenesis and diagnostic
criteria for rickets and
osteomalacia—proposal by an
expert panel supported by the
Ministry of Health, Labour and
Welfare, Japan, the Japanese
Society for Bone and Mineral
Research, and the Japan
Endocrine
Society (74)
2015
Japanese Society for
Bone and Mineral
Research, Japan
Endocrine Society
- <20 ng/mL - - - No mention
15
Optimizing bone health in
children and adolescents (75)
2014
American Academy of
Pediatrics
- <20 ng/mL -
≥20 ng/mL
>80 ng/mL
[1] 0–18 years: minimum of 400 IU /day, maximum of 800 IU
/day;
[2] 2–18 years:50,000 IU quarterly or 80,000–100,000 IU twice
in fall and winter;
16
Vitamin D in the Healthy
European Paediatric Population
(76)
2013
European Society for
Paediatric
Gastroenterology
Hepatology and
Nutrition
<10 ng/mL <20 ng/mL -
≥20 ng/mL
>96 ng/mL
[1] Infants: 400 IU/day, <1,000 IU/day;
[2] 1–10 years: <2000 IU/day;
[3] 11–17 years: <4,000 IU/day
17
Practical guidelines for the
supplementation of vitamin D
and the treatment of decits in
Central Europe- recommended
vitamin D intakes in the general
population and groups at risk of
vitamin D deciency (77)
2013 Central Europe - <20 ng/mL 20–29 ng/mL
≥30 ng/mL
-
[1] 0-6 months:400 IU/day;
[2] 6–12 months: 400–600 IU/day;
[3] 2–18 years: 600–1,000 IU/day;
[4] > 18 years: 800–2000 IU/day;
[5] Pregnancy and lactation: 1500–2000 IU/day.
TABLE3 (Continued)
(Continued)

Jiang et al. 10.3389/fpsyt.2023.1238973
Frontiers in Psychiatry 23 frontiersin.org
NO Title Year Society/
Organization
Diagnostic standards Supplementary standards
Severe
deficiency
Deficiency Insuciency Suciency toxicity
18
Recommended Vitamin D
Intake and Management of Low
Vitamin D Status in
Adolescents: A Position
Statement of the Society for
Adolescent Health
and Medicine (78)
2013
Society for Adolescent
Health and Medicine
- <20 ng/mL 20–29 ng/mL
≥30 ng/mL
>200 ng/mL
[1] Healthy adolescents: 600 IU /day
[2] Deciency or insuciency adolescents: at least 1,000 IU /
day
19
Vitamin D and health in
pregnancy, infants, children and
adolescents in Australia and
NewZealand: a position
statement (79)
2013 Australia/New Zealand <5 ng/mL 5–11 ng/mL 12–19 ng/mL
≥20 ng/mL
>200 ng/mL
[1] Preterm
(1) Mild deciency:Treatment: 200–400 IU/day; Maintenance
and prevention:200–400 IU/day;
(2) Moderate or severe deciency: Treatment:800 IU/day;
Maintenance and prevention:200–400 IU/day;
[2] < 3 months old
(1) Mild deciency:Treatment: 400 IU/day for 3 months;
Maintenance and prevention:400 IU/day;
(2) Moderate or severe deciency: Treatment:1000 IU/day for
3 months; Maintenance and prevention:400 IU/day;
[3] 3–12 months old
(1) Mild deciency:Treatment: 400 IU/day for 3 months;
Maintenance and prevention:400 IU/day;
(2) Moderate or severe deciency: Treatment:1000 IU/day for
3 months, or 50,000 IU stat and review aer 1 month
(consider repeating dose); Maintenance and
prevention:400 IU/day;
[4] 1–18 years old
(1) Mild deciency:Treatment: 1000–2000 IU/day for
3 months, or 150,000 IU stat; Maintenance and
prevention:400 IU/day or 150,000 IU at start of Autumn;
(2) Moderate or severe deciency: Treatment:1000–2000 IU/
day for 6 months, or 3,000–4,000 IU/day for 3 months, or
150,000 IU stat and repeat 6 weeks later; Maintenance and
prevention:400 IU/day or 150,000 IU at start of Autumn;
TABLE3 (Continued)
Jiang et al. 10.3389/fpsyt.2023.1238973
Frontiers in Psychiatry 24 frontiersin.org
the monitoring is borne by the patient’s family or the hospital. What
is more important for us to explore before further blood control is the
metabolic pathways, the pathways of action of vitamin D in patients
with ASD, and to provide better evidence for the timing of monitoring.
However, this study has its limitations, rstly the small volume of
literature included in this study, the small number of subjects relative to
the observation group, the fact that the studies were not methodologically
consistent with each other, and the risk of assessment bias, despite the
fact that weselected multiple individuals with evidence-based experience
to judge each other independently when searching the evaluation
literature. ere is a lack of good quality research on omega-3in patients
with ASD, and there is a lack of standard studies on the timing and
dosage of omega-3 supplementation. Regarding the standard omega-3
supplementation dose for patients with ASD, the recommended
reference amount, based on evidence-based rationale, is EPA + DHA at
a combination of 1.3–1.5 g/day for 16–24 weeks to treat ASD (80). It is
recommended that future multicentre studies with large sample sizes
beconducted to validate the dose and method of supplementation to
promote symptom relief in patients with ASD.
Conclusion
is review systematically examined the current literature to
increase the relief of core symptoms of ASD by omega-3 and vitamin
D supplementation. e review found that omega-3 supplementation
was weakly eective in improving ASD and was not sucient to
conclude that core symptoms were alleviated. Vitamin D
supplementation improved some of the core symptoms of ASD in
patients with ASD, mainly behavioral functioning. However, the
results of the literature included in this study were slightly mixed.
erefore, we cannot directly conclude that vitamin D
supplementation has a benecial eect on a specic symptom of ASD,
but the overall conclusion is that vitamin D supplementation has a
positive eect on behavioral functioning in ASD. Omega-3 and
vitamin D combination supplementation had good combined eects
in patients with ASD, with signicant improvements in social and
behavioral outcomes. More in-depth studies are needed to explore the
mechanisms of action of omega-3 and vitamin D in patients with ASD.
Data availability statement
e original contributions presented in the study are included in
the article/supplementary material, further inquiries can bedirected
to the corresponding authors.
Author contributions
YJ and WD: conceptualization and investigation. YJ, WD, and XK:
methodology. YJ, WD, and HN: writing—original dra preparation.
XK and JG: writing—review and editing. ZJ: supervision. HN: project
administration. All authors have read and agreed to the published
version of the manuscript.
Funding
is research was funded by Doctoral Fund of Jiamusi
University—Construction and application of gastrointestinal risk
prediction model for autism spectrum disorder (JMSUBZ2020-07);
Heilongjiang Young Scientist Project (2022QNTJ-016).
Conflict of interest
e authors declare that the research was conducted in the
absence of any commercial or nancial relationships that could
beconstrued as a potential conict of interest.
Publisher’s note
All claims expressed in this article are solely those of the
authors and do not necessarily represent those of their affiliated
organizations, or those of the publisher, the editors and the
reviewers. Any product that may be evaluated in this article, or
claim that may be made by its manufacturer, is not guaranteed or
endorsed by the publisher.
References
1. Lord C, Elsabbagh M, Baird G, Veenstra-Vanderweele J. Autism spectrum disorder.
Lancet. (2018) 392:508–20. doi: 10.1016/S0140-6736(18)31129-2
2. Maenner MJ, Shaw KA, Baio J, EdS1Washington A, Patrick M, et al. Prevalence of
autism spectrum disorder among children aged 8 years-autism and developmental
disabilities monitoring network, 11sites, UnitedStates, 2016. MMWR Surveill Summ.
(2020) 69:1–12. doi: 10.15585/mmwr.ss6904a1
3. Chiarotti F, Venerosi A. Epidemiology of autism spectrum disorders: a review of
worldwide prevalence estimates since 2014. Brain Sci. (2020) 10:274. doi: 10.3390/
brainsci10050274
4. Zhou H, Xu X, Yan W, Zou X, Wu L, Luo X, et al. Prevalence of autism spectrum
disorder in China: a nationwide multi- center population-based study among
children aged 6 to 12 years. Neurosci Bull. (2020) 36:961–71. doi: 10.1007/
s12264-020-00530-6
5. Baker JK, Seltzer MM, Greenberg JS. Longitudinal eects of adaptability on
behavior problems and maternal depression in families of adolescents with autism. J
Fam Psychol. (2011) 25:601–9. doi: 10.1037/a0024409
6. Estes A, Munson J, Dawson G, Koehler E, Zhou XH, Abbott R. Parenting stress and
psychological functioning among mothers of pre-school children with autism and
developmental delay. Autism. (2009) 13:375–87. doi: 10.1177/1362361309105658
7. Hamlyn-Wright S, Draghi-Lorenz R, Ellis J. Locus of control fails to mediate
between stress and anxiety and depression in parents of children with a developmental
disorder. Autism. (2007) 11:489–501. doi: 10.1177/1362361307083258
8. Hayes SA, Watson SL. e impact of parenting stress:a meta-analysis of studies
comparing the experience of parenting stress in parents of children with and without
autism spectrum disorder. J Autism Dev Disord. (2013) 43:629–42. doi: 10.1007/
s10803-012-1604-y
9. Chiang N, Serha CN. Specialized pro-resolving mediator network: an update on
production and actions. Essays Biochem. (2020) 64:443–62. doi: 10.1042/EBC20200018
10. Cliord J, Kozil A. Dietary Fat and Cholesterol[EB/OL]. Available at: https://
extension.colostate.edu/topic-areas/nutrition-foodsafety-health/dietary-fat-and-
cholesterol-9-319 (Accessed April 14, 2021).
11. Rog J, Karakuła K, Próchnicka A, Karakula-Juchnowicz H. Nutritional assessment
of omega-3fatty acids intake in schizophrenia patients group. Curr Probl Psychiatr.
(2019) 20:138–48. doi: 10.2478/cpp-2019-0008
12. de la Torre-Aguilar MJ, Gomez-Fernandez A, Flores-Rojas K, Martin-Borreguero
P, Mesa MD, Perez-Navero JL, et al. Docosahexaenoic and eicosapentaenoic intervention
modies plasma and erythrocyte omega-3 fatty acid proles but not the clinical course
of children with autism spectrum disorder: a randomized control trial. Front Nutr.
(2022) 9:790250. doi: 10.3389/fnut.2022.790250
Jiang et al. 10.3389/fpsyt.2023.1238973
Frontiers in Psychiatry 25 frontiersin.org
13. Kittana M, Ahmadani A, al Marzooq F, Attlee A. Dietary fat eect on the gut
microbiome, and its role in the modulation of gastrointestinal disorders in children with
autism spectrum disorder. Nutrients. (2021) 13:3818. doi: 10.3390/nu13113818
14. Gesundheit B, Rosenzweig JP, Naor D, Lerer B, Zachor DA, Procházka V, et al.
Immunological and autoimmune considerations of autism spectrum disorders. J
Autoimmun. (2013) 44:1–7. doi: 10.1016/j.jaut.2013.05.005
15. Mazahery H, Stonehouse W, Delshad M, Kruger M, Conlon C, Beck K, et al.
Relationship between long chain n-3 polyunsaturated fatty acids and autism spectrum
disorder: systematic review and meta-analysis of case-control and randomised
controlled trials. Nutrients. (2017) 9:155. doi: 10.3390/nu9020155
16. Sivamaruthi BS, Suganthy N, Kesika P, Chaiyasut C. e role of microbiome,
dietary supplements, and probiotics in autism spectrum disorder. Int J Env Res Public
Health. (2020) 17:2647. doi: 10.3390/ijerph17082647
17. de Crescenzo F, D'Alò GL, Morgano GP, Minozzi S, Mitrova Z, Saulle R, et al.
Impact of polyunsaturated fatty acids on patient-important outcomes in children and
adolescents with autism spectrum disorder: a systematic review. Health Qual Life
Outcomes. (2020) 18:1–12. doi: 10.1186/s12955-020-01284-5
18. Saad K, Abdel-Rahman AA, Elserogy YM, Al-Atram AA, Cannell JJ, Bjørklund G,
et al. Vitamin D status in autism spectrum disorders and the ecacy of vitamin D
supplementation in autistic children. Nutr Neurosci. (2015) 19:346–51. doi:
10.1179/1476830515Y.0000000019
19. Jia F, Wang B, Shan L, Xu Z, Staal WG, Du L. Core symptoms of autism improved
aer vitamin D supplementation. Pediatrics. (2015) 135:e196–8. doi: 10.1542/
peds.2014-2121
20. Saad K, Abdel-rahman AA, Elserogy YM, Al-Atram AA, Cannell JJ, Bjørklund G,
et al. Vitamin D status in autism spectrum disorders and the ecacy of vitamin D
supplementation in autistic children. Nutr Neurosci. (2016) 19:346–51. doi:
10.1179/1476830515Y.0000000019
21. Cass WA, Smith MP, Peters LE. Calcitriol protects against the dopamine and
serotonin depleting eects of neurotoxic doses of methamphetamine. Ann N Y Acad Sci.
(2006) 1074:261–71. doi: 10.1196/annals.1369.023
22. Patrick RP, Ames BN. Vitamin D hormone regulates serotonin synthesis. Part 1:
relevance for autism. FASEB J. (2014) 28:2398–413. doi: 10.1096/.13-246546
23. Sabir MS, Haussler MR, Mallick S, Kaneko I, Lucas DA, Haussler CA, et al.
Optimal vitamin D spurs serotonin: 1, 25-dihydroxyvitamin D represses serotonin
reuptake transport (SERT) and degradation (MAO-A) gene expression in cultured rat
serotonergic neuronal cell lines. Genes Nutr. (2018) 13:19. doi: 10.1186/
s12263-018-0605-7
24. Mostafa GA, Al-Ayadhi LY. Reduced serum concentrations of 25-hydroxy vitamin
D in children with autism: relation to autoimmunity. J Neuroinamm. (2012) 9:201. doi:
10.1186/1742-2094-9-201
25. Mazahery H, Camargo CA Jr, Conlon C, Beck KL, Kruger MC, von Hurst PR.
Vitamin D and autism spectrum disorder: a literature review. Nutrients. (2016a) 8:236.
doi: 10.3390/nu8040236
26. Mazahery H, Stonehouse W, Delshad M, Kruger MC, Conlon CA, Beck KL, et al.
Relationship between long chain n–3 polyunsaturated fatty acids and autism spectrum
disorder: systematic review and meta-analysis of case–control and randomised
controlled trials. Nutrients. (2017) 9:28. doi: 10.3390/NU9020155
27. Tang M, Zhang M, Cai H, Li H, Jiang P, Dang R, et al. Maternal diet of
polyunsaturated fatty acid altered the cell proliferation in the dentate gyrus of
hippocampus and inuenced glutamatergic and serotoninergic systems of neonatal
female rats. Lipids Health Dis. (2016) 15:71. doi: 10.1186/s12944-016-0236-1
28. Parellada M, Llorente C, Calvo R, Gutierrez S, Lázaro L, Graell M, et al.
Randomized trial of omega-3 for autism spectrum disorders: eect on cell membrane
composition and behavior. Eur Neuropsychopharmacol. (2017) 27:1319–30. doi:
10.1016/j.euroneuro.2017.08.426
29. Ooi YP, Weng SJ, Jang LY, Low L, Seah J, Teo S, et al. Omega-3 fatty acids in the
management of autism spectrum disorders: ndings from an open-label pilot study in
Singapore. Eur J Clin Nutr. (2015) 69:969–71. doi: 10.1038/ejcn.2015.28
30. Bent S, Bertoglio K, Ashwood P, Bostrom A, Hendren RL. A pilot randomized
controlled trial of omega-3 fatty acids for autism spectrum disorder. J Autism Dev
Disord. (2011) 41:545–54. doi: 10.1007/s10803-010-1078-8
31. Bent S, Hendren RL, Zandi T, Law K, Choi JE, Widjaja F, et al. Internet-based,
randomized, controlled trial of omega-3 fatty acids for hyperactivity in autism. J
AmAcad Child Adolesc Psychiatry. (2014) 53:658–66. doi: 10.1016/j.jaac.2014.01.018
32. Mankad D, Dupuis A, Smile S, Roberts W, Brian J, Lui T, et al. A randomized,
placebo controlled trial of omega-3 fatty acids in the treatment of young children with
autism. Mol Autism. (2015) 6:18. doi: 10.1186/s13229-015-0010-7
33. Politi P, Cena H, Comelli M, Marrone G, Allegri C, Emanuele E, et al. Behavioral
eects of omega-3 fatty acid supplementation in young adults with severe autism: an
open label study. Arch Med Res. (2008) 39:682–5. doi: 10.1016/j.arcmed.2008.06.005
34. Amminger GP, Berger GE, Schäfer MR, Klier C, Friedrich MH, Feucht M.
Omega-3 fatty acids supplementation in children with autism: a double-blind
randomized, placebo-controlled pilot study. Biol Psychiatry. (2007) 61:551–3. doi:
10.1016/j.biopsych.2006.05.007
35. Voigt RG, Mellon MW, Katusic SK, Weaver AL, Matern D, Mellon B, et al. Dietary
docosahexaenoic acid supplementation in children with autism. J Pediatr Gastroenterol
Nutr. (2014) 58:715–22. doi: 10.1097/MPG.0000000000000260
36. Yui K, Koshiba M, Nakamura S, Kobayashi Y. Eects of large doses of arachidonic
acid added to docosahexaenoic acid on social impairment in individuals with autism
spectrum disorders: a double-blind, placebo-controlled, randomized trial. J Clin
Psychopharmacol. (2012) 32:200–6. doi: 10.1097/JCP.0b013e3182485791
37. Doaei S, Bourbour F, Teymoori Z, Jafari F, Kalantari N, Abbas Torki S, et al. e
eect of omega-3 fatty acids supplementation on social and behavioral disorders of
children with autism: a randomized clinical trial. Pediatr Endocrinol Diabetes Metab.
(2021) 27:12–8. doi: 10.5114/pedm.2020.101806
38. Javadfar Z, Abdollahzad H, Moludi J, Rezaeian S, Amirian H, Foroughi AA, et al.
Eects of vitamin D supplementation on core symptoms, serum serotonin, and
interleukin-6in children with autism spectrum disorders: a randomized clinical trial.
Nutrition. (2020) 79-80:110986. doi: 10.1016/j.nut.2020.110986. PMID: 32966919
39. Saad K, Abdel-Rahman AA, Elserogy YM, Al-Atram AA, El-Houfey AA, Othman
HA, et al. Randomized controlled trial of vitamin D supplementation in children with
autism spectrum disorder. J Child Psychol Psychiatry. (2018) 59:20–9. doi: 10.1111/
jcpp.12652
40. Duan X. A preliminary study of vitamin D for autism spectrum disorders in children
Jilin University (2015).
41. Kerley CP, Power C, Gallagher L, Coghlan D. Lack of eect of vitamin D3
supplementation in autism: a 20-week, placebo-controlled RCT. Arch Dis Child. (2017)
102:1030–6. doi: 10.1136/archdischild-2017-312783
42. Feng J, Shan L, Du L, Wang B, Li H, Wang W, et al. Clinical improvement following
vitamin D3 supplementation in Autism Spectrum Disorder. Nutr Neurosci. (2017)
20:284–90. doi: 10.1080/1028415X.2015.1123847
43. Mazahery H, Conlon CA, Beck KL, Mugridge O, Kruger MC, Stonehouse W, et al.
A randomised controlled trial of vitamin D and omega-3 long chain polyunsaturated
fatty acids in the treatment of irritability and hyperactivity among children with autism
spectrum disorder. J Steroid Biochem Mol Biol. (2019) 187:9–16. doi: 10.1016/j.
jsbmb.2018.10.017
44. Mazahery H, Conlon CA, Beck KL, Mugridge O, Kruger MC, Stonehouse W, et al.
A randomised-controlled trial of vitamin D and Omega-3 long chain polyunsaturated
fatty acids in the treatment of core symptoms of autism spectrum disorder in children.
J Autism Dev Disord. (2019) 49:1778–94. doi: 10.1007/s10803-018-3860-y
45. Mazahery H, Conlon CA, Beck KL, Mugridge O, Kruger MC, Stonehouse W, et al.
Inammation (IL-1β) modies the eect of vitamin D and omega-3 long chain
polyunsaturated fatty acids on core symptoms of autism spectrum disorder-an
exploratory pilot study. Nutrients. (2020) 12:661. doi: 10.3390/nu12030661
46. Fang L, Jiang X, Huang Y, et al. Ecacy of vitamin D combined with omega-3 fatty
acids in the treatment of autism in children. Pharm Care Res. (2018) 18:347–50. doi:
10.5428/pcar20180508
47. Mostafa GA, AL-Ayadhi LY. Reduced serum concentrations of 25-hydroxy vitamin
D in children with autism: relation to autoimmunity. J Neuroinammation. (2012)
9:201–7. doi: 10.1186/1742-2094-9-201
48. Mazahery H, Conlon CA, Beck KL, Mugridge O, Kruger MC, Stonehouse W, et al.
Inammation (IL-1β) modies the eect of vitamin d and omega-3 long chain
polyunsaturated fatty acids on core symptoms of autism spectrum disorder. Nutrients.
(2020) 12:661. doi: 10.3390/nu12030661
49. McCracken JT, Anagnostou E, Arango C, Dawson G, Farchione T, Mantua V, et al.
Drug development for autism spectrum disorder (ASD): progress, challenges, and future
directions. Eur Neuropsychopharmacol. (2021) 48:3–31. doi: 10.1016/j.
euroneuro.2021.05.010
50. Adams JB, Audhya T, McDonough-Means S, Geis E, Gehn E, Fimbres V, et al.
Comprehensive nutritional and dietary intervention for autism spectrum disorder-a
randomized, controlled 12-month trial. Nutrients. (2018) 10:369. doi: 10.3390/
nu10030369
51. de Andrade Wobido K, de Sá Barreto da Cunha M, Miranda SS, da Mota Santana
J, da Silva DCG, Pereira M. Non-specic eect of omega-3 fatty acid supplementation
on autistic spectrum disorder: systematic review and meta-analysis. Nutr Neurosci.
(2022) 25:1995–2007. doi: 10.1080/1028415X.2021.1913950
52. Xie G, Yi Q, Zeng J, et al. Investigation and analysis of factors inuencing the
implementation eectiveness of family intervention treatment adherence for children
with autism. Nursing Practice & Research. (2019) 16:8–10.
53. Hyman SL, Levy SE, Myers SM, COUNCIL ON CHILDREN WITH
DISABILITIES, SECTION ON DEVELOPMENTAL AND BEHAVIORAL
PEDIATRICSKuo DZ, Apkon S, et al. Identication, evaluation, and management of
children with autism spectrum disorder. Pediatrics. (2020) 145:e20193447. doi: 10.1542/
peds.2019-3447,
54. Developmental Behaviour Group of the Paediatrics Branch of the Chinese Medical
Association, China Medical Doctor's Association, Paediatrics Branch, Child Health Care
Professional Committee. Expert group for the research project on techniques and
standards for the diagnosis and prevention of childhood autism. Expert consensus on
early identication screening and early intervention for children with autism spectrum
disorders. Chin. J Pediatr. (2017) 55:890–7. doi: 10.3760/cma.j.issn.0578-1310.2017.12.004
Jiang et al. 10.3389/fpsyt.2023.1238973
Frontiers in Psychiatry 26 frontiersin.org
55. Ministry of Health. Guidelines for the treatment and rehabilitation of children
with autism(〔2010〕123). Chin. J. Child Health. (2011) 19:289–94.
56. Wang J, Yang Q, Xiaoxue Y, et al. Development and reliability testing of the
treatment adherence scale for children with autism spectrum disorders (parent version).
Chin J Nurs. (2021) 56:1812–8.
57. Masi A, Breen EJ, Alvares GA, Glozier N, Hickie IB, Hunt A, et al. Cytokine levels
and associations with symptom severity in male and female children with autism
spectrum disorder. Mol Autism. (2017) 8:63–3. doi: 10.1186/s13229-017-0176-2
58. Handley MT, Lian LY, Haynes LP, Burgoyne RD. Structural and functional decits
in a neuronal calcium sensor-1 mutant identied in a case of autistic spectrum disorder.
PLoS One. (2010) 5:e10534. doi: 10.1371/journal.pone.0010534
59. Tsai SJ, Hong CJ, Liu ME, Hou SJ, Yen FC, Hsieh CH, et al. Interleukin-1 beta
(C-511T) genetic polymorphism is associated with cognitive performance in elderly
males without dementia. Neurobiol Aging. (2010) 31:1950–5. doi: 10.1016/j.
neurobiolaging.2008.10.002
60. Gladysz D, Krzywdzinska A, Hozyasz KK. Immune abnormalities in autism
spectrum disorder-could they hold promise for causative treatment? Mol Neurobiol.
(2018) 55:6387–435. doi: 10.1007/s12035-017-0822-x
61. Victoria R, Caroline Flora S, Youssef D, Jules Alexandre D. Prediction by
pharmacogenetics of safety and ecacy of non-steroidal anti- inammatory drugs: a review.
Curr Drug Metab. (2014) 15:326–43. doi: 10.2174/1389200215666140202214454
62. Płudowski P, Kos-Kudła B, Walczak M, Fal A, Zozulińska-Ziółkiewicz D,
Sieroszewski P, et al. Guidelines for preventing and treating vitamin D deciency: a 2023
update in Poland. Nutrients. 15:695. doi: 10.3390/nu15030695
63. Bertoldo F, Cianferotti L, di Monaco M, Falchetti A, Fassio A, Gatti D, et al.
Denition, assessment, and management of vitamin D inadequacy: suggestions,
recommendations, and warnings from the Italian society for osteoporosis, mineral
metabolism and bone diseases (SIOMMMS). Nutrients. (2022) 14:4148. doi: 10.3390/
nu14194148
64. Bacchetta J, Edouard T, Laverny G, Bernardor J, Bertholet-omas A, Castanet M,
et al. Vitamin D and calcium intakes in general pediatric populations: a French expert
consensus paper. Arch Pediatr. (2022) 29:312–25. doi: 10.1016/j.arcped.2022.02.008
65. Pludowski P, Takacs I, Boyanov M, Belaya Z, Diaconu CC, Mokhort T, et al.
Clinical practice in the prevention, diagnosis and treatment of vitamin D deciency: a
central and Eastern European expert consensus statement. Nutrients. (2022) 14:1483.
doi: 10.3390/nu14071483
66. Gupta P, Dabas A, Seth A, Bhatia VL, Khadgawat R, Kumar P, et al. Indian academy of
pediatrics revised (2021) guidelines on prevention and treatment of vitamin D deciency
and rickets. Indian Pediatr. (2022) 59:142–58. doi: 10.1007/s13312-022-2448-y
67. Dobson R, Cock HR, Brex P, Giovannoni G. Vitamin D supplementation. Pract
Neurol. (2018) 18:35–42. doi: 10.1136/practneurol-2017-001720, PMID: 28947637
68. Saggese G, Vierucci F, Prodam F, Cardinale F, Cetin I, Chiappini E, et al. Vitamin
D in pediatric age: consensus of the Italian Pediatric Society and the Italian Society of
Preventive and Social Pediatrics, jointly with the Italian Federation of Pediatricians. Ital
J Pediatr. (2018) 44:51. doi: 10.1186/s13052-018-0488-7
69. Rusińska A, Płudowski P, Walczak M, Borszewska-Kornacka MK, Bossowski A,
Chlebna-Sokół D, et al. Vitamin D supplementation guidelines for general population
and groups at risk of vitamin D deciency in Poland—recommendations of the polish
Society of Pediatric Endocrinology and Diabetes and the expert panel with participation
of National Specialist Consultants and representatives of scientic societies—2018
update. Front Endocrinol. (2018) 9:246. doi: 10.3389/fendo.2018.00246
70. Okazaki R, Ozono K, Fukumoto S, Inoue D, Yamauchi M, Minagawa M, et al.
Assessment criteria for vitamin D deciency/insuciency in Japan: proposal by an
expert panel supported by the Research Program of Intractable Diseases, Ministry of
Health, Labour and Welfare, Japan, the Japanese Society for Bone and Mineral Research
and the Japan Endocrine Society [Opinion]. J Bone Miner Metab. (2017) 35:1–5. doi:
10.1007/s00774-016-0805-4
71. Haq A, Wimalawansa SJ, Pludowski P, Anouti FA. Clinical practice guidelines for
vitamin D in the UnitedArabEmirates. J Steroid Biochem Mol Biol. (2016) 175:4–11.
doi: 10.1016/j.jsbmb.2016.09.021
72. Munns CF, Shaw N, Kiely M, Specker BL, acher TD, Ozono K, et al. Global
consensus recommendations on prevention and management of nutritional rickets. J
Clin Endocrinol Metab. (2016) 101:394–415. doi: 10.1210/jc.2015-2175
73. Arundel P, Shaw N. Royal Osteoporosis Society. Vitamin D and bone health: a
practical clinical guideline for management in children and young people. ROS (2018).
Available at: theros.org.uk/media/54vpzzaa/ros-vitamin-d-and-bone-health-in-
children-november-2018.pdf
74. Fukumoto S, Ozono K, Michigami T, Minagawa M, Okazaki R, Sugimoto T, et al.
Pathogenesis and diagnostic criteria for rickets and osteomalacia–proposal by an expert
panel supported by the Ministry of Health, Labour and Welfare, Japan, the Japanese
Society for Bone and Mineral Research,and the Japan Endocrine Society. J Bone Miner
Metab. (2015) 33:467–73. doi: 10.1007/s00774-015-0698-7
75. Golden NH, Abrams SA, Committee on Nutrition. Optimizing bone health in
children and adolescents. Pediatrics. (2014) 134:e1229–43. doi: 10.1542/peds.2014-2173
76. Braegger C, Campoy C, Colomb V, Decsi T, Domellof M, Fewtrell M, et al. Vitamin
D in the healthy European paediatric population. J Pediatr Gastroenterol Nutr. (2013)
56:692–701. doi: 10.1097/MPG.0b013e31828f3c05
77. Płudowski P, Karczmarewicz E, Bayer M, Carter G, Chlebna-Sokół D, Czech-
Kowalska J, et al. Practical guidelines for the supplementation of vitamin D and the
treatment of decits in Central Europe- recommended vitamin D intakes in the general
population and groups at risk of vitamin D deciency. Endokrynol Pol. (2013) 64:319–27.
doi: 10.5603/EP.2013.0012
78. Society for Adolescent Health and Medicine. Recommended vitamin D intake and
management of low vitamin D status in adolescents: a position statement of the society
for adolescent health and medicine. J Adolesc Health. (2013) 52:801–3. doi: 10.1016/j.
jadohealth.2013.03.022
79. Paxton GA, Teale GR, Nowson CA, Mason RS, McGrath JJ, ompson MJ, et al.
Vitamin D and health in pregnancy, infants, children and adolescents in Australia and
New Zealand: a position statement. Med J Aust. (2013) 198:142–3. doi: 10.5694/
mja11.11592
80. Chang JPC, Su KP. Nutritional neuroscience as mainstream of psychiatry: the
evidence-based treatment guidelines for using omega-3 fatty acids as a new treatment
for psychiatric disorders in children and adolescents. Clin Psychopharmacol Neurosci.
(2020) 18:469–83. doi: 10.9758/cpn.2020.18.4.469
