
1
S
ome decades ago, the Inuit people of Greenland were noted
to have a low rate of cardiovascular disease,
1
and this was
attributed to their high intake of oily fish. Early trials testing
this hypothesis found a beneficial effect of fish oil on mortal-
ity,
2,3
with subsequent recommendations developed for the use
of omega 3 fatty acids (ω-3 FA) for the primary and secondary
prevention of cardiovascular disease. More recent trials have,
however, failed to replicate these initial positive results, with
several large studies reporting null effects. Systematic reviews
of the accumulating data
4–8
done during the last decade have
also delivered variable findings. In part, this is because more
recent overviews have included new data from large neutral tri-
als,
9,10
and in part it is because different overviews have sought
to address particular questions for specific patient groups.
4,7,8
This is the first overview to include all recent trials, systemati-
cally address the effects on all-important outcomes, and fully
explore the potentially different effects achieved with particu-
lar interventions in major patient subgroups and in primary and
secondary prevention. With several large trials completed in
the past 18 months, we sought to more precisely and reliably
define the effects of ω-3 FA on a broad range of clinical out-
comes, overall and in major patient subsets.
Methods
Data Sources and Searches
The study was undertaken according to the PRISMA statement
11
for
overviews of intervention studies. Randomized, controlled trials were
identified without language restriction by searching Medline via Ovid
(from 1946 to March 2011), EMBASE (from 1966 through March
2011), and the Cochrane Central Register of Controlled Trials (until
March 2011). Reference lists of relevant trials and review articles
were also hand searched. The MeSH terms used were the following:
fish oils, omega fatty acids, omega 3, fatty acids, α linolenic acid,
docosahexaenoic acids, eicosapentaenoic acid, cardiovascular
disease, heart failure, cardiovascular death, myocardial infarction,
revascularization, stroke, coronary disease, arrhythmia, sudden
death, cardiovascular outcome, mortality, chronic kidney failure,
renal insufficiency, kidney failure, kidney disease, renal failure, renal
outcome, albuminuria, serum creatinine, exp creatinine, and cancer.
All terms were not used in every database, but all spellings of the
terms were used as needed. Subject headings were exploded and
truncated where necessary.
Background—Early trials evaluating the effect of omega 3 fatty acids (ω-3 FA) reported benefits for mortality and
cardiovascular events but recent larger studies trials have variable findings. We assessed the effects of ω-3 FA on
cardiovascular and other important clinical outcomes.
Methods and Results—We searched MEDLINE, EMBASE, and the Cochrane Central Register of Controlled Trials for
all randomized studies using dietary supplements, dietary interventions, or both. The primary outcome was a composite
of cardiovascular events (mostly myocardial infarction, stroke, and cardiovascular death). Secondary outcomes were
arrhythmia, cerebrovascular events, hemorrhagic stroke, ischemic stroke, coronary revascularization, heart failure,
total mortality, nonvascular mortality, and end-stage kidney disease. Twenty studies including 63 030 participants were
included. There was no overall effect of ω-3 FA on composite cardiovascular events (relative risk [RR]=0.96; 95%
confidence interval [CI], 0.90–1.03; P=0.24) or on total mortality (RR=0.95; 95% CI, 0.86–1.04; P=0.28). ω-3 FA did
protect against vascular death (RR=0.86; 95% CI, 0.75–0.99; P=0.03) but not coronary events (RR=0.86; 95% CI, 0.67–
1.11; P=0.24). There was no effect on arrhythmia (RR=0.99; 95% CI, 0.85–1.16; P=0.92) or cerebrovascular events
(RR=1.03; 95% CI, 0.92–1.16; P=0.59). Adverse events were more common in the treatment group than the placebo
group (RR=1.18, 95% CI, 1.02–1.37; P=0.03), predominantly because of an excess of gastrointestinal side effects.
Conclusions—ω-3 FA may protect against vascular disease, but the evidence is not clear-cut, and any benefits are almost
certainly not as great as previously believed. (Circ Cardiovasc Qual Outcomes. 2012;5:00-00.)
Key Words: cardiac outcomes
◼
cardiovascular disease
◼
fatty acids
◼
meta-analysis
◼
systematic review
© 2012 American Heart Association, Inc.
Circ Cardiovasc Qual Outcomes is available at http://circoutcomes.ahajournals.org DOI: 10.1161/CIRCOUTCOMES.112.966168
Received August 22, 2011; accepted September 18, 2012.
From the George Institute for Global Health, University of Sydney, Sydney, Australia (S.K., M.J., V.P., B.N.); and University of Sydney, Sydney,
Australia (D.S.).
The online-only Data Supplement is available at http://circoutcomes.ahajournals.org/lookup/suppl/doi: 10.1161/CIRCOUTCOMES.112.966168/-/
DC1.
Correspondence to Sradha Kotwal, BHB, MBChB, FRACP, the George Institute for Global Health, University of Sydney, PO Box M201, Missenden Rd,
Sydney, NSW 2050, Australia. E-mail [email protected]g.au
Omega 3 Fatty Acids and Cardiovascular Outcomes
Systematic Review and Meta-Analysis
Sradha Kotwal, BHB, MBChB, FRACP; Min Jun, BSc (Hons), MSc; David Sullivan, MBBS, FRACP, FRCPA;
Vlado Perkovic, MBBS, PhD, FRACP; Bruce Neal, MBChB, PhD, FRACP
Original Article

2 Circ Cardiovasc Qual Outcomes November 2012
Study Inclusion Criteria
Studies were included if they were done in adults, were randomized
or quasirandomized (trials using methods not completely random for
patient allocation, eg, sequential allocation, etc), reported effects on 1
or more of the primary or secondary outcomes, included a comparison
between ω-3 FA (delivered as either a dietary supplement or as dietary
modification) and control, and recorded ≥100 patient years or more of
follow-up per randomized group. Trials with a crossover design were
excluded, as were trials done in pregnant women or children.
Data Extraction and Quality Assessment
Two investigators (S.K. and M.J.) reviewed all abstracts independent-
ly for eligibility according to the prespecified inclusion criteria. A
third investigator (V.P.) resolved any discrepancies. For selected stud-
ies, the full text articles were reviewed and data were extracted from
each qualifying study into a standard form by 2 independent investi-
gators, with discrepancies resolved by reviewing the original data or
with the assistance of a third investigator as necessary. Data extracted
included the baseline characteristics of the trial participants (age, sex,
history of hypertension, history of diabetes mellitus, history of prior
cardiovascular disease, mean systolic and diastolic blood pressure
levels, baseline and end trial lipid levels, smoking status, body mass
index, and medication use), type of ω-3 FA supplementation used,
dose of ω-3 FA, nature of intervention (dietary change or supplemen-
tation), follow-up duration, outcome events, compliance, and adverse
events. The quality of the studies was assessed by the application of a
modified version of the Jadad criteria, which is a quality assessment
tool to assess the methodological quality of clinical trials
12
with ad-
ditional recording of the use of intention-to-treat analysis methods. A
higher Jadad score indicates higher methodological quality.
Outcomes
The primary outcome was a composite of cardiovascular events (myo-
cardial infarction, stroke, and cardiovascular death, or, as defined by
the authors of the contributing trials) (Table 1). Secondary outcomes
were vascular death (death from myocardial infarction, stroke, sud-
den death), myocardial infarction, cerebrovascular events (recorded
separately for hemorrhagic and ischemic stroke where reported),
coronary revascularization (percutaneous coronary interventions and
coronary artery bypass grafting), arrhythmia (atrial fibrillation, ven-
tricular fibrillation, or ventricular tachycardia), total mortality, non-
vascular mortality, and end-stage kidney disease. Wherever reported,
medication adherence rates and total adverse events (which mostly
comprised gastrointestinal side effects) were recorded.
Data Synthesis and Analysis
Overall estimates of effect were estimated using random effects
models to calculate relative risks (RR) with 95% confidence inter-
vals (CIs). In each case, the numerator was the number of patients
with an event and the denominator the total number of patients ran-
domized. Where there were no events recorded in 1 randomized
group in a trial, 0.5 was added to the numerator and denominator to
enable the trial to be included in the analysis.
13
The I
2
statistic was
used to quantify heterogeneity in the results of studies contributing
to each overview analysis, with sensitivity analyses excluding indi-
vidual trials done to explore the heterogeneity. Subgroup analyses
and univariate meta-regressions were used to explore the association
between the primary outcome and study characteristics, including
median age of patients, proportion with hypertension, proportion
with diabetes mellitus, mean baseline lipid levels (triglycerides, low-
density lipoprotein, high-density lipoprotein, and total cholesterol),
proportion using lipid lowering agents, dietary modification versus
supplementation, dose of free fatty acids (high versus low dose), me-
dian follow-up time, every 5-year increase in publication year, study
size, trial setting, and Jadad score. The presence of publication bias
was investigated and quantified using Egger test and Begg funnel
plots of the natural log of the RR versus its standard error
14
for com-
posite cardiovascular outcomes and all cause mortality. P<0.05 was
considered unlikely to have arisen by chance, and all analyses were
done using Stata version 11.1 (Stata, College Station, TX).
Results
Search Results
Initial search identified 2362 possibly eligible studies, of
which 201 were duplicates, leaving 2161 abstracts that were
reviewed by the 2 investigators. Two thousand, one hundred
sixteen were excluded on the basis that they did not evaluate
an intervention of interest, did not report an outcome of
interest, were nonrandomized studies, were trials done in a
pediatric or pregnant population, included <100 patient years
of follow-up per arm, were repeat publications from the same
trial or were crossover designs. Full text reports were obtained
for the 47 remaining studies, and on further review another 27
were excluded. One study was excluded subsequently on the
basis of strong suspicion of fraud.
15
The recent ORIGIN trial
16
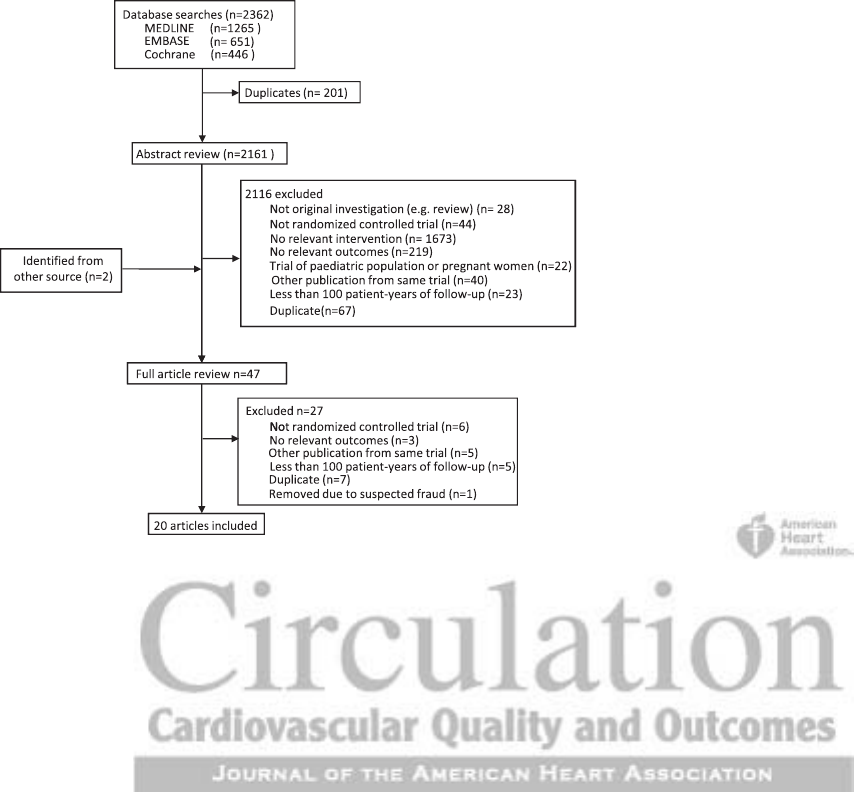
has also been included yielding a total of 20 studies being
included for the meta-analysis (Figure 1).
Characteristics of Included Studies
The 20 studies randomized a total of 62 851 patients with
31 456 assigned to active treatment and 31 395 to control
(Table 1). The total sample size of contributing trials ranged
from 106 to 18 645 participants, and the follow-up duration
from 6 months to 6 years. The median age of the participants
was 61 years, and 50% of the participants were male despite
several studies being conducted exclusively in men.
2,17,18
Sev-
enteen were multicenter trials,
2,3,9,10,16–28
and 2 were exclusively
studies of primary prevention.
18,19
The trials were variously
conducted in the United States, the United Kingdom, Europe,
and Japan.
Three trials assessed the effect of dietary advice
2,17,24
and the
remainder, the effects of fish oil supplements.
3,9,10,16,18–23,25–31
Fourteen used supplements comprising a combination of eicos-
apentaenoic acid and docosahexaenoic acid,
3,10,16,18,20–23,25,27–30
WHAT IS KNOWN
• On the basis of the positive results of early trials, var-
ious clinical guidelines recommend the use of omega
3 fatty acid supplements to reduce mortality and car-
diovascular risk.
• Several recent large trials have reported no benefit of
omega 3 acids on cardiovascular outcomes; however,
the recommendations for their use remain.
WHAT THIS ARTICLE ADDS
• This meta-analysis, which includes 20 trials and
>60 000 patients, summarizes the entire body of evi-
dence on this subject including all the recent trials.
• The results of this meta-analysis report that omega
3 fatty acids protect against vascular death, but there
is no clear effect on total mortality, sudden death,
stroke, or arrhythmia.
• The beneficial effects of omega 3 fatty acids are not
as large as previously implied and recommendations
for widespread use should be tempered.

Kotwal et al Omega 3 Fatty Acids and Cardiovascular Outcomes 3
Table 1. Baseline Characteristics of Included Studies
Study Inclusion Criteria
Treatment
Group Placebo
Design/
Country of
Origin
Mean
Follow-Up,
y
No. of
Patients
Mean
Age, y
Male,
%
Hypertension,
%
Diabetes
mellitus,
%
Primary or
Secondary
Prevention
No. of
Composite
CV Events
No. of All
Cause
Mortality
No. of
Coronary
Events
DART study (1989)
2
Men<70 post acute AMI Dietary
advice
No dietary
advice
Randomized,
UK
2 2033 56.55 100 24 NR Secondary 276 224 NR
Lyon Diet heart
Study (1994)
24
Men and women <70 post
AMI within past 6 mo
Dietary
advice
No dietary
advice
Randomized,
France
1.25 605 53.5 91 NR NR Secondary NR 28 22
IgA nephropathy (1994)
20
Biopsy proven IgA nephropathy,
urinary protein excretion of
≥1 g/d, serum Cr≤3.0 mg/dL
and survival >2 y
EPA/DHA
1680/970
Placebo Randomized,
US
3 106 37 91 58 NR Secondary NR NR NR
CART study (1999)
29
Patients for elective coronary
angioplasty
EPA/DHA
900/780
Corn oil
as Placebo
Randomised,
Norway
0.5 500 59.7 77.6 34 8.6 Secondary NR 4 NR
GISSI-Prevenzione (1999)
3
Patients with recent AMI EPA/DHA
850/882
Placebo Randomized,
Italy
3.5 5664 59.4 85 35 15 Secondary 584 529 NR
Effect of Dietary Omega-3
fatty acids on coronary
atherosclerosis (1999)
31
Patients hospitalised for
coronary angiography
Fish oil,
1650–3300
mg
Placebo Randomized,
Germany
2 223 52.35 80 49 NR Secondary 9 NR 4
High dose n-3 fatty acids
introduced early after
AMI (2001)
30
Patients post AMI EPA/DHA
1700/1764
Corn oil
as Placebo
Randomized,
Norway
1.5 300 64 79 24 10 Secondary 78 46 NR
Dietary advice to men with
angina (2003)
17
Men<70 with angina Dietary
advice
Sensible
eating
Randomized,
UK
6 1528 61.1 100 49 12 Secondary NR 250 NR
Fish oil Supplementation
and risk of VT and VF
in patients with implantable
defibrillators (2005)
25
Patients receiving ICD for
VT/VF not because of AMI
Fish oil, EPA/
DHA 756/540
Placebo Randomized,
US
1.97 200 62.5 86 51 24 Secondary NR 14 4
FAATI (2005)
26
Patients with ICD at high risk
for fatal ventricular arrhythmias
EPA 2600 mg Placebo Randomized,
US
1 402 65.5 83 NR NR Secondary NR 25 NR
SOFA trial (2006)
27
Men and women with 1 true
spontaneous VT or VF in past
3 mo and either had or were
receiving ICD
Fish oil EPA/
DHA 464/335
Placebo Randomized,
Europe
0.9753 546 61.5 84 51 16 Secondary NR 22 4
OPACH study group
(2006)
21
Patients with CVD and
stablished on HD for 6 mo
EPA/DHA
765/638
Placebo Randomized,
Denmark
1.53 206 67 65 78 24 Secondary 121 64 17
JELIS trial (2007)
19
Patients with
hypercholesterolemia
EPA 1800 mg
and statin
Placebo
and statin
Randomized,
Japan
4.6
18645
61
31
35
16
Primary
and
secondary
586
551
145
(Continued)
AQ13

4 Circ Cardiovasc Qual Outcomes November 2012
Table 1. (Continued)
Study Inclusion Criteria
Treatment
Group Placebo
Design/
Country of
Origin
Mean
Follow-Up,
y
No. of
Patients
Mean
Age, y
Male,
%
Hypertension,
%
Diabetes
mellitus,
%
Primary or
Secondary
Prevention
No. of
Composite
CV Events
No. of All
Cause
Mortality
No. of
Coronary
Events
GISSI-Prevenzione HF
(2008)
22
Men and women >18 with
clinical evidence of heart
failure
EPA/DHA
850–882 mg
Placebo Randomized,
Italy
3.9 7046 67 78 54 28 Secondary 3322 1969 236
Omega (2010)
10
Men and women >18 with
acute STEMI or NSTEMI
EPA/DHA
460/380
Placebo Randomized,
Germany
1 3851 64 74 66 27 Secondary 331 158 NR
Efficacy and safety of
prescription N-3 FA for
the prevention of recurrent
symptomatic AF (2010)
28
Patients>18 with persistent
or paroxysmal AF
EPA/DHA
1860/1500
Placebo Randomized,
US
0.5 663 60.5 56 NR NR Secondary NR 2 NR
Alpha Omega (2010)
9
Men and women, 60–80 y
of age, with MI in past 10 y
EPA/DHA/ALA ALA and
Placebo
Randomized,
Netherlands
3.4 4837 69 78 90 21 Secondary 671 NR NR
SU.FOL.OM3 (2010)
23
Men and women aged
45–80 y who had an acute
coronary or cerebral ischemic
events within last 12 mo
EPA/DHA
1200/600
Placebo Randomized,
France
4.7 2501 60.9 79 NR NR Secondary 157 117 60
Diet and Omega 3
Intervention trial (2010)
18
Survivors from a population of
healthy men with
hypercholesterolemia from
the OSLO Diet & Antismoking
study
EPA/DHA
1176/840
Placebo Randomized,
Norway
3 563 70.1 100 28 14 Primary 68 38 NR
n-3 Fatty Acids and Cardio-
vascular Outcomes in Patients
with Dysglycemia (2012)
16
Patients with impaired fasting
glucose, impaired glucose
tolerance or diabetes mellitus
EPA/DHA
465/375
Olive oil as
placebo
Randomized,
International
6.2 12611 63.5 65 79 100 Primary
and
secondary
2051 1915 660
EPA indicates eicosapentaenoic acid, DHA, docosahexaenoic acid.
Kotwal et al Omega 3 Fatty Acids and Cardiovascular Outcomes 5
with the daily doses of eicosapentaenoic acid/docosahexae-
noic acid ranging between 464 to 1860 mg and 335 to 1500 mg,
respectively,
27,28
compared with recommended dietary intakes
of 250 to 2000 mg/d for each.
32
The placebo composition
varied and included control,
19,21–23,31
corn oil,
18,28–30
and olive
oil.
10,16,25,26
The reporting of trial methodology was variable (Table 2),
with the earlier studies reporting less information about their
methods of randomization, allocation concealment, and com-
pleteness of follow-up. Nine studies scored 4 on the Jadad
scale,
9,16,18,21,23,25,27,28,31
5 studies scored 3,
19,20,22,24,26
3 studies
scored 2,
3,10,30
1 study scored 1,
2
and 2 studies scored zero.
17,29
Effects of ω-3 Fatty Acids on Clinical Outcomes
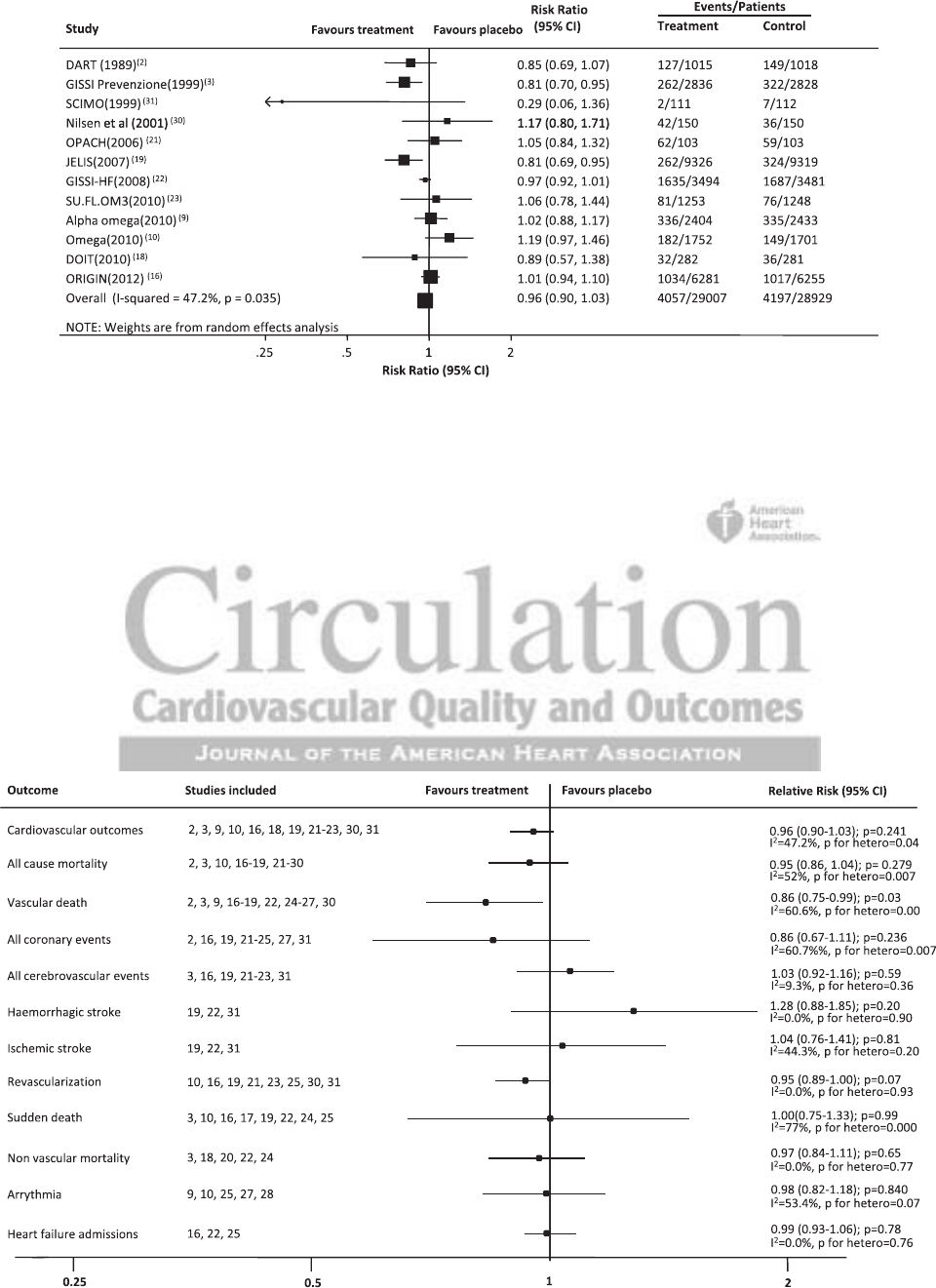
Composite Cardiovascular Outcome
For the primary composite cardiovascular outcome, 12 stud-
ies involving 57 936 participants
2,3,9,10,16,18,19,21–23,30,31
recorded
8254 events (Figure 2). There was no clear effect of ω-3 FA
(RR=0.96; 95% CI, 0.90–1.03; P=0.24) on this outcome.
There was, however, moderate heterogeneity in the effects
of treatment across the included studies (I
2
=47.2%; P=0.04).
Sequentially excluding individual studies as part of a sensitiv-
ity analysis did not identify a single trial responsible for the
heterogeneity. The definition of the composite cardiovascular
outcome differed somewhat between studies with the majority
comprising cardiovascular death, myocardial infarction, and
sudden death but not all including stroke outcomes.
Vascular Death and Sudden Death
Thirteen studies reported 3776 heart disease deaths, stroke
deaths, or sudden deaths that occurred among 54 834
randomized participants.
2,3,9,16–19,22,24–27,30
Of these stud-
ies, 8 separately reported 1496 sudden deaths among
49 971 participants.
3,10,16,17,19,22,24,25
Treatment with ω-3 FA
protected against vascular death (Figure 3; RR=0.86; 95% CI,
0.75–0.99; P=0.03) but not against sudden death (RR=1.00;
95% CI, 0.75–1.33; P=0.99). There was substantial heteroge-
neity (I
2
=60.7%, P=0.001) across the results of the 12 trials
contributing to the analysis of vascular death and across the 8
trials that evaluated sudden death (I
2
=77.0%, P<0.0001). No
individual trial was able to explain a substantial proportion of
the heterogeneity for the vascular death or the sudden death
outcome.
Total Mortality and Nonvascular Mortality
Seventeen studies done in 57 671 participants
2,3,10,16–19,21–30
reported 5956 deaths from any cause, and 5 studies reported
723 deaths of nonvascular origin that occurred among 13 913
individuals.
3,18,20,22,24
There was no evidence that ω-3 FA
reduced total mortality (Figure 3; RR=0.95; 95% CI, 0.86–
1.04; P=0.28) or nonvascular mortality (RR=0.97; 95% CI,
0.84–1.11; P=0.65). The heterogeneity across the individual
trial results for total mortality (I
2
=52.1%; P=0.007) was sig-
nificant but once again it was hard to identify specific trials
that caused this.
Coronary Events and Revascularization
One thousand, two hundred thirty-four coronary events were
reported by 10 studies among 44 470 participants
2,16,19,21–25,27,31
and 3537 occurrences of cardiac revascularization in 8 studies
and 38 429 participants.
10,16,19,21,23,25,30,31
There was no evidence
of benefit for coronary events (Figure 3; RR=0.86; 95% CI,
0.67–1.11, P=0.24) and no significant benefit for revascular-
ization (RR=0.95; 95% CI, 0.89–1.00; P=0.07). There was
moderate heterogeneity for the coronary outcome (I
2
=60.6%,
P=0.07).
Cerebrovascular Events
Only 7 studies done among 46 750 participants
3,16,19,21–23,31
reported on stroke outcomes. There were a total of 1369
Figure 1. Flow of papers.

6 Circ Cardiovasc Qual Outcomes November 2012
Table 2. Quality Assessment of Trials Included in the Systematic Review and Meta-Analysis
Study
Randomization
Process
Described
Randomization
Process
Achieved
Allocation
Concealment
Described
Allocation
Concealment
Adequately
Achieved
Similarity
of Baseline
Characteristics
Eligibility
Criteria
Described
Double-
Blinding
Described
Completeness
of Follow-Up/
Loss to Follow-Up
Described
Intention-
To-Treat
Described
Completion Rate
(Treatment/
Placebo)
Jadad
Score
DART study (1989)
2
No Yes No NR Yes Yes No Yes Yes 6.8/6.9 1
Lyon Diet heart Study (1994)
24
No Yes No NR Yes Yes Yes Yes Yes NR 3
IgA nephropathy (1994)
20
No Yes No NR Yes Yes Yes Yes Yes 71% 3
CART study (1999)
29
Yes Yes Yes NR Yes Yes No Yes No 78% 0
GISSI-Prevenzione (1999)
3
Yes Yes No NR Yes Yes No No Yes Reported 2
Effect of Dietary Omega-3
fatty acids on coronary
atherosclerosis (1999)
31
Yes Yes Yes Yes Yes Yes Yes Yes Yes NR 4
High dose n-3 fatty acids introduced
early after AMI (2001)
30
No NR No NR Yes Yes Yes No Yes NR 2
Dietary advice to men with
angina (2003)
17
No No No NR No Yes No No Yes NR 0
Fish oil Supplementation
and risk of VT and VF in patients
with implantable defibrillators (2005)
25
Yes Yes No NR Yes Yes Yes Yes Yes 98%/94% 4
FAATI (2005)
26
Yes Yes No NR Yes Yes No Yes Yes 86% 3
SOFA trial (2006)
(27)
Yes Yes Yes Yes Yes Yes Yes Yes Yes 89%/91% 4
OPACH study group (2006)
21
Yes Yes Yes Yes Yes Yes Yes Yes Yes 74%/78% 4
JELIS trial (2007)
19
Yes Yes Yes NR Yes Yes No Yes Yes 71%/73% 3
GISSI-Prevenzione HF (2008)
22
Yes Yes Yes Yes Yes Yes Yes No No 99%/99% 3
Omega (2010)
10
No Yes Yes Yes Yes Yes Yes No No NR 2
Efficacy and safety of prescription
n-3 FA for the prevention of recurrent
symptomatic AF (2010)
28
Yes Yes Yes Yes Yes Yes Yes Yes No 88% 4
Alpha Omega (2010)
9
Yes Yes No NR Yes Yes Yes Yes Yes 90%/92% 4
SU.FOL.OM3 (2010)
23
Yes Yes Yes Yes Yes Yes Yes Yes Yes 90%/90% 4
Diet and Omega 3 Intervention
trial (2010)
18
Yes No Yes Yes No Yes Yes Yes Yes 94% 4
ORIGIN Trial
16
Yes Yes Yes Yes Yes Yes Yes Yes Yes 88% 4
Kotwal et al Omega 3 Fatty Acids and Cardiovascular Outcomes 7
events. Of these 6 studies, 3 provided a further subclassifi-
cation into strokes of ischemic and hemorrhagic origin.
19,22,31
Overall, there was no clear effect of ω-3 FA on all cerebrovas-
cular events (Figure 3; RR=1.03; 95% CI, 0.92–1.16; P=0.59),
although the point estimate of effect was just to the right side
of unity. The trend toward harm was stronger for hemorrhagic
stroke (RR=1.28; 95% CI, 0.88–1.85; P=0.20) than ischemic
stroke (RR=1.04; 95% CI, 0.76–1.41; P=0.80).
Arrhythmia
This outcome was reported in 5 studies involving 10 097 par-
ticipants
9,10,25,27,28
and recorded 758 events with no treatment
effect identified overall (Figure 3; RR=0.98; 95% CI, 0.82–
1.18; P=0.84) or for the subset of 3 trials that included patients
with a confirmed prior history of atrial fibrillation, ventricular
tachycardia, or ventricular fibrillation.
25,27,28
There was evidence
of moderate heterogeneity across the included trials (I
2
=53.5%;
P=0.07) but no 1 trial was clearly responsible for this.
Other Outcomes
Two thousand, six hundred fifty heart failure admissions were
reported by 3 studies of 19 711 participants.
16,22,25
The overall
estimate of effect was a RR of 0.99 (Figure 3; 95% CI, 0.93–
1.06; P= 0.78). Renal outcomes were reported by 1 study
20
of
106 participants with IgA nephropathy, in which the use of
fish oil protected renal function. A further longer-term follow-
up of these same patients confirmed this result,
33
although the
long-term data were not included in our meta-analysis.
Figure 2. Effect of ω-3 fatty acids on composite cardiovascular outcomes. CI indicates confidence interval.
Figure 3. Effect of ω-3 fatty acids on all outcomes. CI indicates confidence interval.
8 Circ Cardiovasc Qual Outcomes November 2012
Subgroup Analysis
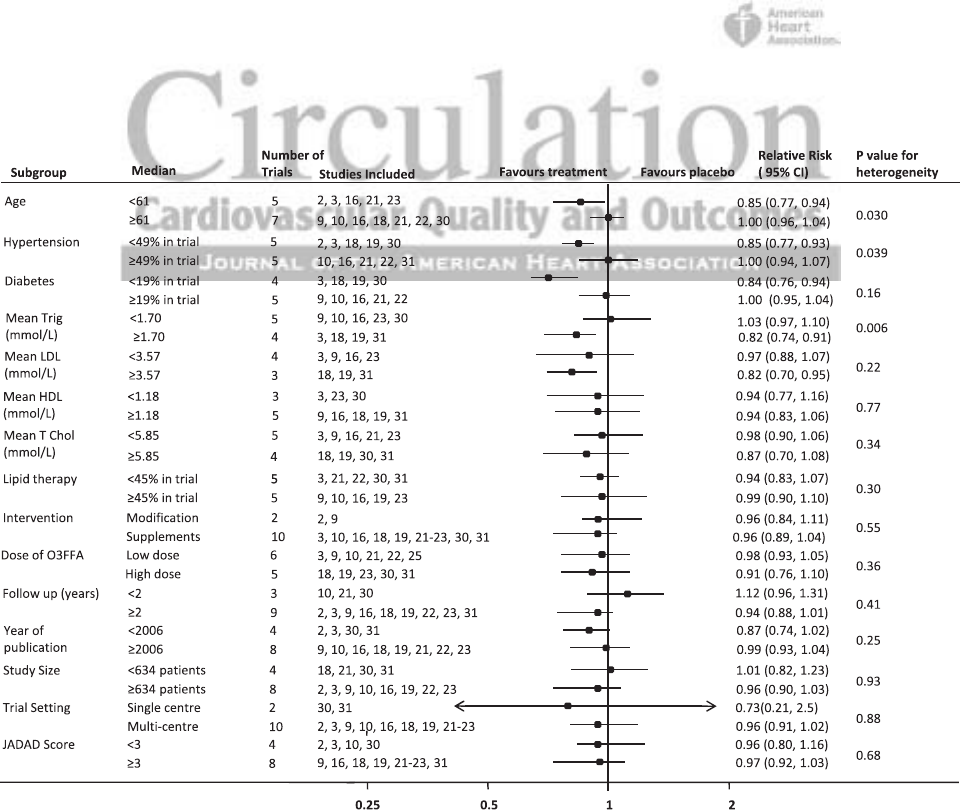
We undertook a large number of subgroup analyses accord-
ing to the baseline characteristics of patients and design fea-
tures of the trials (Figure 4). These analyses identified greater
protection against the composite cardiovascular outcomes in
trials of younger patients, in trials with fewer hypertensive
patients, and in trials in which patients had higher baseline
triglyceride levels. There was no difference in effect based on
era of publication, study size, or trial setting or when trials
were separated into those that used dietary advice compared
with those that used dietary supplementation. Univariate
meta-regression, hypertension, diabetes mellitus, achieved
cholesterol reduction, year of publication, and Jadad score
were associated with the likelihood of benefit from ω-3 FA
(Table 3) with these variables being well distributed to allow
us to estimate the change in proportional risk.
Side Effects
Thirteen studies reported adverse effects among a total of
52 213 participants.
9,10,16,18,19,21–23,25,27–29,31
The use of ω-3 sup-
plements compared with placebo showed an increased risk
of side effects (RR=1.18; 95% CI, 1.02–1.37; P=0.03). The
majority were gastrointestinal and comprised nausea, diar-
rhea, and other mild gastrointestinal disturbances. Eight stud-
ies
9,10,18,19,22,23,25,27
also reported rates of malignancies during
the study period with no significant association between the
use of ω-3 FA and the incidence of cancer detected (RR=1.10,
95% CI 0.98–1.23, P=0.10).
Publication Bias
We found evidence of publication bias (online only Data Sup-
plement Figures I and II) as our funnel plots show an asym-
metrical distribution of data. The majority of the studies are
located at the base of the plots.
Discussion
The findings of this large systematic review, that includes
data from 20 trials, >60 000 individuals and >6000 major
cardiovascular events raises important questions about
the use of fish oil for the prevention of cardiovascular
disease. Recommendations for the use of fish oil supple-
ments are included in a number of guidelines,
34,35
but the
neutral outcomes of recent large trials
9,10,16
have served to
weaken rather than strengthen the evidence base. Although
it remains possible that fish oil supplements will produce
health benefits through the prevention of vascular compli-
cations, the size of these gains are probably smaller than
previously believed, and both physician and patient expec-
tations may need to be reset.
A key strength of this overview is the attempt to extract
data on all commonly reported vascular outcomes from all
trials and to systematically report the summary estimates of
effect in each case. The impact of this approach has been
to move the focus of attention from the positive or nega-
tive findings for particular outcomes identified in individual
studies or overviews, to the overall estimates of effect across
Figure 4. Subgroup analysis for the effect of ω-3 free fatty acids on cardiovascular outcomes. CI indicates confidence interval.

Kotwal et al Omega 3 Fatty Acids and Cardiovascular Outcomes 9
the entire body of evidence. This attempt to apply greater
objectivity to the analysis of the data has not, however, been
without its challenges. In part because the reporting of out-
comes across studies is inconsistent and in part because
there is significant heterogeneity between the trials’ results
for several of the outcomes studied. This heterogeneity may
also contribute to the absence of positive findings in this
meta-analysis.
The heterogeneity in results between trials was large for
several analyses and is unlikely to simply reflect the play
of chance. Although not easy to explain in every case, the
analyses of trial subgroups suggest a number of explanations
based on plausible biological phenomena and correlations
in the data. Greater average effects on the primary outcome
were observed in the studies that used higher doses of ω-3
FA
3,18,19,22,23,30,31
and trials done in patients with higher base-
line levels of triglycerides.
3,19,31
These findings are similar to
those reported for fibrates, which is notable given that both
classes of agents have similar effects on lipid profiles, and
especially on triglyceride levels.
36
We also observed greater
average effects in those with younger average ages,
3,23,31
and
in trials with lower proportions of hypertensives
2,3,18,19,30
and
lower proportions of diabetes mellitus.
3,18,19,30
These differ-
ences between subgroups are difficult to explain on the basis
of physiology, although there are also animal data that sug-
gest a mechanism of action for lesser effects in patients with
diabetes mellitus.
37
The subgroup findings also seem to be
heavily influenced by the characteristics and results of a few
large trials. The GISSI-Prevenzione trial,
3
for example, stud-
ied mostly younger individuals who were nonhypertensive,
and was reported before the year 2000. As a major contributor
of events to all 3 of these subgroup analyses, it is easy to see
how the heterogeneity of effects by these trial characteristics
arises even if it is not entirely explained. The neutral find-
ings of the more recent trials might also be associated with
underlying levels of marine oil intake, but with background
consumption data reported by only 7 trials
3,9,10,17,24,29,30
that
used very different definitions this was difficult to robustly
investigate. Variation in the composition of the placebo com-
pound may also contribute to the neutrality of the findings,
although a subgroup analysis based on the use of an inactive
control compared with corn oil or olive oil identified signifi-
cant heterogeneity.
Vascular death was the only outcome for which a signifi-
cant benefit was observed. This result does not seem to have
been driven by an effect on sudden death or arrhythmia, both
of which had more moderate and nonsignificant estimates of
effect. The JELIS trial
19
as well as the GISSI-Prevenzione
trial
3
however, found a reduction in major cardiovascular
events. The absence of benefit in the more recent trials raises
the possibility that the effects of ω-3 FA are determined by
some as yet unquantified external factor.
The overview identified no effect of fish oil on the over-
all risk of stroke, although there was a trend toward harm for
intracerebral hemorrhage. This is an effect that might be antic-
ipated on the basis of the known effects of ω-3 FA on bleeding
time and platelet aggregation.
38–40
The conduct of the analyses on tabular data extracted from
the original trial reports was a limitation of our study design.
Access to the original trial datasets would likely uncover
additional outcome events from trials that did not publish data
on each of the outcomes we studied. This would increase the
power of the analyses by raising the number of events avail-
able as well as permitting analyses based on more directly
comparable definitions than has been possible here. Analyses
done on individual participant data would also allow for
much more sophisticated exploration of the effects in dif-
ferent patient subgroups, and provide a better understand-
ing of the sources of heterogeneity in the trial findings. A
notable deficit in the current data are the systematic reporting
Table 3. Univariate Meta-Regression
Variable Scale RR 95% CI No. of Studies
Age Every 5 y 1.08 0.90 1.26 12
Male, % Every 10% increase 0.99 0.92 1.06 12
HTN Every 10% increase 1.09 1.04 1.13 10
DM Every 10% increase 1.07 1.05 1.08 8
Mean baseline TRIG Every 1 mmol/L increase 0.50 −0.56 1.55 6
Mean baseline LDL Every 1 mmol/L increase 0.76 0.51 1.02 5
Mean baseline HDL Every 1 mmol/L increase 1.00 0.98 1.03 6
Mean baseline cholesterol Every 1 mmol/L increase 0.84 18.00 19.68 7
TRIG difference: TX1 vs TX2 Every 0.1 mmol/L difference 0.89 0.62 1.17 3
HDL difference: TX1 vs TX2 Every 0.02 mmol/L reduction 1.04 0.88 1.20 3
CHOL difference: TX1 vs TX2 Every 0.1 mmol/L increase 0.93 0.92 0.95 3
Drug dose (composite cv outcomes) Every 200 mg increase 0.97 0.92 1.02 11
Follow-up (y) Every 1 y 1.04 0.97 1.11 12
Year of publication Every 5 y 1.09 1.01 1.18 12
Study size Every 100 participants 1.00 1.00 1.00 12
Jadad score Every 1 point increase 1.12 1.02 1.23 12
HTN indicates hypertension; RR, risk ratio, CI, confidence interval, DM, diabetes mellitus; TRIG, triglycerides; LDL, low-density lipoprotein, HDL, high-density lipo-
protein; and CHOL, cholesterol.

10 Circ Cardiovasc Qual Outcomes November 2012
of side effects. Total adverse events, although mostly fairly
benign, were clearly higher with fish oil, and if the benefits
are smaller than previously believed the risk-benefit trade-off
will require more careful evaluation. Therapies that amelio-
rate cardiovascular risk, such as antihypertensives, statins,
and antiplatelet agents have become prevalent and potent
over time and, therefore, trials, such as the ORIGIN trial that
include patients with moderate cardiovascular risk at base-
line, are likely to comprise patients that are on these thera-
pies. This might further dilute the detectable effect of ω-3
FA. Finally, the generalizability of the overview findings may
be questioned, given that aside from a handful of trials,
18,19
the data derive entirely from the secondary prevention setting
and white populations.
In conclusion, these results raise further uncertainty about
the net effects of ω-3 fish oil therapy and reinforce the
importance of the forthcoming ASCEND
41
and R&P
42
trials.
Although it is probably reasonable for patients with exist-
ing vascular disease who are currently using fish oil to con-
tinue to do so, better evidence is required to support the more
widespread promulgation of this strategy, particularly among
lower risk patients. Individuals with high triglyceride levels
or IgA nephropathy may be especially worthy of investiga-
tion, and higher rather than lower doses seem more likely to
produce benefit. Further research in the primary prevention
setting would be welcome given the potential implications of
evidence about fish oil intake to dietary advice about fish con-
sumption in the general population.
Acknowledgments
S. Kotwal, M. Jun, D. Sullivan, V. Perkovic, and B. Neal were respon-
sible for the design of the study. S. Kotwal and M. Jun were respon-
sible for data collection and analysis. All authors were responsible for
interpretation and manuscript preparation. All authors contributed to
data interpretation and critical revision of the publication. S. Kotwal
had full access to all data in the study and takes responsibility for the
integrity of the data and accuracy of the analysis.
Sources of Funding
M. Jun was supported by an Australian Postgraduate Award and
the Australasian Kidney Trials Network, Dr Perkovic by a New
South Wales Cardiovascular Research Network/Australian Heart
Foundation Career Development Award, and Dr Neal by an Australian
Research Council Future Fellowship.
Disclosures
D. Sullivan reports educational and advisory consultancy to
Pfizer, Merck Schering Plough, AstraZeneca, Abbott, Amgen, and
Roche, as well as research projects involving Pfizer (2007), Merck
Schering Plough (2010), Abbott (2005), Sanofi-Aventis (2010),
and AstraZeneca (2009). V. Perkovic reports his employer has re-
ceived grants for clinical trials from Baxter, Johnson and Johnson,
Novartis, Roche, and Servier; lecture fees from Abbott, AstraZeneca,
Roche, and Servier; serving on a grant review panel for Baxter
and on a steering Committee for Abbott. B. Neal reports receiv-
ing consulting fees from Pfizer, Roche, and Takeda; grant support
from Johnson and Johnson, Merck Schering Plough, Servier, and
United Healthcare Group; lecture fees and travel reimbursements
from Amgen, AstraZeneca, GlaxoSmithKline, Pfizer, Roche, Sanofi-
Aventis, Servier, and Tanabe; and being a member of advisory boards
for Pfizer and Roche. The other authors report no conflicts.
References
1. Dyerberg J, Bang HO, Hjorne N. Fatty acid composition of the plasma
lipids in Greenland Eskimos. Am J Clin Nutr. 1975;28:958–966.
2. Burr ML, Fehily AM, Gilbert JF, Rogers S, Holliday RM, Sweetnam
PM, Elwood PC, Deadman NM. Effects of changes in fat, fish, and fibre
intakes on death and myocardial reinfarction: diet and reinfarction trial
(DART). Lancet. 1989;2:757–761.
3. GISSI-Prevenzione Investigators. Dietary supplementation with n-3 poly-
unsaturated fatty acids and vitamin E after myocardial infarction: results
of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della So-
pravvivenza nell’Infarto miocardico. Lancet. 1999;354:447–455.
4. Filion KB, El Khoury F, Bielinski M, Schiller I, Dendukuri N, Brophy
JM. Omega-3 fatty acids in high-risk cardiovascular patients: a meta-
analysis of randomized controlled trials. BMC Cardiovasc Disord. 2010;
10:24.
5. Mozaffarian D, Rimm EB. Fish intake, contaminants, and human health:
evaluating the risks and the benefits. JAMA. 2006;296:1885–1899.
6. Wang C, Harris WS, Chung M, Lichtenstein AH, Balk EM, Kupelnick B,
Jordan HS, Lau J. n-3 Fatty acids from fish or fish-oil supplements, but
not alpha-linolenic acid, benefit cardiovascular disease outcomes in pri-
mary- and secondary-prevention studies: a systematic review. Am J Clin
Nutr. 2006;84:5–17.
7. León H, Shibata MC, Sivakumaran S, Dorgan M, Chatterley T, Tsuyuki
RT. Effect of fish oil on arrhythmias and mortality: systematic review.
BMJ. 2008;337:a2931.
8. Kwak SM, Myung SK, Lee YJ, Seo HG; Korean Meta-analysis Study
Group. Efficacy of omega-3 fatty acid supplements (eicosapentaenoic acid
and docosahexaenoic acid) in the secondary prevention of cardiovascular
disease: a meta-analysis of randomized, double-blind, placebo-controlled
trials. Arch Intern Med. 2012;172:686–694.
9. Kromhout D, Giltay EJ, Geleijnse JM; Alpha Omega Trial Group. n-3
fatty acids and cardiovascular events after myocardial infarction. N Engl J
Med. 2010;363:2015–2026.
10. Kwak SM, Myung SK, Lee YJ, Seo HG; Korean Meta-analysis Study
Group. Efficacy of omega-3 fatty acid supplements (eicosapentaenoic acid
and docosahexaenoic acid) in the secondary prevention of cardiovascular
disease: a meta-analysis of randomized, double-blind, placebo-controlled
trials. Arch Intern Med. 2012;172:686–694.
11. PRISMA. PRISMA: Transparent reporting of systematic reviews and meta-
analysis. 2009 [cited 2011]. Available at: http://www.prisma-statement.org/.
Accessed March 1, 2011.
12. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis
detected by a simple, graphical test. BMJ. 1997;315:629–634.
13. Woodward M. Epidemiology: Design and Data Analysis. 2nd ed . Boca
Raton, FL: Chapman and Hall/CRC Press; 2005.
14. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis
detected by a simple, graphical test. BMJ. 1997;315:629–634.
15. Singh RB, Niaz MA, Sharma JP, Kumar R, Rastogi V, Moshiri M. Ran-
domized, double-blind, placebo-controlled trial of fish oil and mus-
tard oil in patients with suspected acute myocardial infarction: the
Indian experiment of infarct survival–4. Cardiovasc Drugs Ther. 1997;11:
485–491.
16. The ORIGIN Trial Investigators. n-3 fatty acids and cardiovascular out-
comes in patients with dysglycemia. N Engl J Med. 2012;367:309–318.
17. Burr ML, Ashfield-Watt PA, Dunstan FD, Fehily AM, Breay P, Ashton
T, Zotos PC, Haboubi NA, Elwood PC. Lack of benefit of dietary ad-
vice to men with angina: results of a controlled trial. Eur J Clin Nutr.
2003;57:193–200.
18. Einvik G, Klemsdal TO, Sandvik L, Hjerkinn EM. A randomized clini-
cal trial on n-3 polyunsaturated fatty acids supplementation and all-cause
mortality in elderly men at high cardiovascular risk. Eur J Cardiovasc
Prev Rehabil. 2010;17:588–592.
19. Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa
Y, Oikawa S, Sasaki J, Hishida H, Itakura H, Kita T, Kitabatake A,
Nakaya N, Sakata T, Shimada K, Shirato K; Japan EPA lipid intervention
study (JELIS) Investigators. Effects of eicosapentaenoic acid on major
coronary events in hypercholesterolaemic patients (JELIS): a randomised
open-label, blinded endpoint analysis. Lancet. 2007;369:1090–1098.
20. Donadio JV Jr, Bergstralh EJ, Offord KP, Spencer DC, Holley KE. A con-
trolled trial of fish oil in IgA nephropathy. Mayo Nephrology Collabora-
tive Group. N Engl J Med. 1994;331:1194–1199.
21. Svensson M, Schmidt EB, Jørgensen KA, Christensen JH; OPACH Study
Group. N-3 fatty acids as secondary prevention against cardiovascular

Kotwal et al Omega 3 Fatty Acids and Cardiovascular Outcomes 11
events in patients who undergo chronic hemodialysis: a randomized,
placebo-controlled intervention trial. Clin J Am Soc Nephrol. 2006;1:
780–786.
22. Gissi HFI, Tavazzi L, Maggioni AP, Marchioli R, Barlera S, Franzosi MG,
Latini R, Lucci D, Nicolosi GL, Porcu M, Tognoni G. Effect of n-3 poly-
unsaturated fatty acids in patients with chronic heart failure (the GISSI-
HF trial): a randomised, double-blind, placebo-controlled trial. Lancet.
2008;372:1223–1230.
23. Galan P, Kesse-Guyot E, Czernichow S, Briancon S, Blacher J, Hercberg
S; SU.FOL.OM3 Collaborative Group. Effects of B vitamins and omega
3 fatty acids on cardiovascular diseases: a randomised placebo controlled
trial. BMJ. 2010;341:c6273.
24. de Lorgeril M, Renaud S, Mamelle N, Salen P, Martin JL, Monjaud I,
Guidollet J, Touboul P, Delaye J. Mediterranean alpha-linolenic acid-
rich diet in secondary prevention of coronary heart disease. Lancet.
1994;343:1454–1459.
25. Raitt MH, Connor WE, Morris C, Kron J, Halperin B, Chugh SS, Mc-
Clelland J, Cook J, MacMurdy K, Swenson R, Connor SL, Gerhard G,
Kraemer DF, Oseran D, Marchant C, Calhoun D, Shnider R, McAnulty J.
Fish oil supplementation and risk of ventricular tachycardia and ventricu-
lar fibrillation in patients with implantable defibrillators: a randomized
controlled trial. JAMA. 2005;293:2884–2891.
26. Leaf A, Albert CM, Josephson M, Steinhaus D, Kluger J, Kang JX, Cox
B, Zhang H, Schoenfeld D; Fatty Acid Antiarrhythmia Trial Investigators.
Prevention of fatal arrhythmias in high-risk subjects by fish oil n-3 fatty
acid intake. Circulation. 2005;112:2762–2768.
27. Brouwer IA, Zock PL, Camm AJ, Böcker D, Hauer RN, Wever EF, Dulle-
meijer C, Ronden JE, Katan MB, Lubinski A, Buschler H, Schouten EG;
SOFA Study Group. Effect of fish oil on ventricular tachyarrhythmia and
death in patients with implantable cardioverter defibrillators: the Study
on Omega-3 Fatty Acids and Ventricular Arrhythmia (SOFA) randomized
trial. JAMA. 2006;295:2613–2619.
28. Kowey PR, Reiffel JA, Ellenbogen KA, Naccarelli GV, Pratt CM. Efficacy
and safety of prescription omega-3 fatty acids for the prevention of recur-
rent symptomatic atrial fibrillation: a randomized controlled trial. JAMA.
2010;304:2363–2372.
29. Johansen O, Brekke M, Seljeflot I, Abdelnoor M, Arnesen H. N-3 fatty
acids do not prevent restenosis after coronary angioplasty: results from the
CART study. Coronary Angioplasty Restenosis Trial. J Am Coll Cardiol.
1999;33:1619–1626.
30. Nilsen DW, Albrektsen G, Landmark K, Moen S, Aarsland T, Woie L. Ef-
fects of a high-dose concentrate of n-3 fatty acids or corn oil introduced
early after an acute myocardial infarction on serum triacylglycerol and
HDL cholesterol. Am J Clin Nutr. 2001;74:50–56.
31. von Schacky C, Angerer P, Kothny W, Theisen K, Mudra H. The ef-
fect of dietary omega-3 fatty acids on coronary atherosclerosis. A ran-
domized, double-blind, placebo-controlled trial. Ann Intern Med.
1999;130:554–562.
32. FAO/WHO. Fats and fatty acids in human nutrition. Proceedings of the
Joint FAO/WHO Expert Consultation. November 10–14, 2008. Geneva,
Switzerland. Ann Nutr Metab. 2009;55:5–300.
33. Donadio JV Jr, Grande JP, Bergstralh EJ, Dart RA, Larson TS, Spencer
DC. The long-term outcome of patients with IgA nephropathy treated with
fish oil in a controlled trial. Mayo Nephrology Collaborative Group. J Am
Soc Nephrol. 1999;10:1772–1777.
34. Kris-Etherton PM, Harris WS, Appel LJ; AHA Nutrition Committee.
American Heart Association. Omega-3 fatty acids and cardiovascular dis-
ease: new recommendations from the American Heart Association. Arte-
rioscler Thromb Vasc Biol. 2003;23:151–152.
35. National Heart Foundation of Australia, Cardiac Society of Australia
and New Zealand. Lipid management guidelines--2008. Med J Aust.
2008;75:S57–S85.
36. Jun M, Foote C, Lv J, Neal B, Patel A, Nicholls SJ, Grobbee DE, Cass A,
Chalmers J, Perkovic V. Effects of fibrates on cardiovascular outcomes: a
systematic review and meta-analysis. Lancet. 2010;375:1875–1884.
37. Sullivan DR, Yue DK, Capogreco C, McLennan S, Nicks J, Cooney G,
Caterson I, Turtle JR, Hensley WJ. The effects of dietary n - 3 fatty acid
in animal models of type 1 and type 2 diabetes. Diabetes Res Clin Pract.
1990;9:225–230.
38. Agren JJ, Väisänen S, Hänninen O, Muller AD, Hornstra G. Hemostatic
factors and platelet aggregation after a fish-enriched diet or fish oil or doc-
osahexaenoic acid supplementation. Prostaglandins Leukot Essent Fatty
Acids. 1997;57:419–421.
39. Mori TA, Beilin LJ, Burke V, Morris J, Ritchie J. Interactions between di-
etary fat, fish, and fish oils and their effects on platelet function in men at risk
of cardiovascular disease. Arterioscler Thromb Vasc Biol. 1997;17:279–286.
40. De Caterina R. n-3 fatty acids in cardiovascular disease. N Engl J Med.
2011;364:2439–2450.
41. ASCEND : A Study of Cardiovascular Events iN Diabetes 2011. Available
at: http://www.ctsu.ox.ac.uk/ascend/. Accessed July 1, 2011.
42. Rischio and Prevenzione Investigators. Efficacy of n-3 polyunsaturated
fatty acids and feasibility of optimizing preventive strategies in patients
at high cardiovascular risk: rationale, design and baseline characteristics
of the Rischio and Prevenzione study, a large randomised trial in general
practice. Trials. 2010;11:68.
