
SPECIAL REPORT: NUTRACEUTICALS
Formulations, Recommendations, and Resources for the Pharmacist
PRACTICAL INFORMATION FOR TODAY’S PHARMACIST®
®
The Role of Nutraceuticals: What
Pharmacists Need to Know in 2020
Melatonin: Considerations for Use
in Patients With Sleep Disorders
To D or Not to D:
That Is the Question
Omega-3 Recommendations:
Counseling Points for Pharmacists
Prenatal and Postnatal
Supplementation: What Do
Pharmacists Need to Know?
Identification and Communication
Approaches to Drug and Dietary
Supplement Interactions
APRIL 2020
VITAMINS & SUPPLEMENTS
GUIDE FOR PHARMACISTS

Sign up today!
www.PharmacyTimes.com
Access resources through multiple communication platforms
Receive Current
Pharmacy News and
Continuing Education
Updates Daily!
l
Market Trends
l
Clinical Information
l
Product Reports
l Expert Videos
®

VITAMINS & SUPPLEMENTS
GUIDE FOR PHARMACISTS
Special Report: Formulations, Recommendations, and Resources
APRIL 2020
SPECIAL REPORT: NUTRACEUTICALS
Formulations, Recommendations, and Resources for the Pharmacist
PRACTICAL INFORMATION FOR TODAY’S PHARMACIST®
®
COVER STORY
The Role of Nutraceuticals: What Pharmacists Need to Know
in 2020
LUMA MUNJY, PHARMD
FEATURES
Melatonin: Considerations for Use in Patients With
Sleep Disorders
RASHI C. WAGHEL, PHARMD, BCACP; AND JENNIFER A. WILSON, PHARMD, BCACP
To D or Not to D: That Is the Question
CHELSEA RENFRO, PHARMD, CHSE; AND ALEX STANLEY, PHARMD CANDIDATE
Omega-3 Recommendations: Counseling Points for Pharmacists
BRADY COLE, RPH
Prenatal and Postnatal Supplementation: What Do Pharmacists
Need to Know?
CORTNEY MOSPAN, PHARMD, BCACP, BCGP
Identification and Communication Approaches to Drug and
Dietary Supplement Interactions
JAY HIGHLAND, PHARMD
2
6
9
11
13
16
Opinions expressed by authors, contributors, and advertisers are their own and not necessarily those of Pharmacy & Healthcare Communications, LLC, the
editorial staff, or any member of the editorial advisory board. Pharmacy & Healthcare Communications, LLC, is not responsible for accuracy of dosages
given in articles printed herein. The appearance of advertisements in this journal is not a warranty, endorsement, or approval of the products or services
advertised or of their effectiveness, quality, or safety. Pharmacy & Healthcare Communications, LLC, disclaims responsibility for any injury to persons or
property resulting from any ideas or products referred to in the articles or advertisements.
EDITOR-IN-CHIEF Troy Trygstad, PharmD, PhD, MBA
EDITORIAL & PRODUCTION
EXECUTIVE EDITORIAL DIRECTOR Colleen Hall
ASSOCIATE EDITORIAL DIRECTOR Davy James
MANAGING EDITOR Caitlin Mollison
MANAGING EDITOR Kristen Crossley, MA
ASSISTANT EDITOR Jill Murphy
ASSISTANT EDITOR Aislinn Antrim
SENIOR VICE PRESIDENT Jeff Prescott, PharmD, RPh
ASSISTANT DIRECTOR, CONTENT SERVICES
Angelia Szwed
SCIENTIFIC DIRECTORS Darria Zangari, PharmD, BCPS,
BCGP, Danielle Jamison, PharmD, MS
MEDICAL WRITERS Amber Schilling, Valerie Sjoberg
SENIOR CLINICAL PROJECT MANAGERS
Ida Delmendo, Danielle Mroz, MA
CLINICAL PROJECT MANAGERS Ted Pigeon,
Lauren Burawski
ASSOCIATE EDITORS Jillian Pastor, Hayley Fahey,
Amanda Thomas
COPY CHIEF Jennifer Potash
COPY SUPERVISOR Paul Silverman
MEDICAL & SCIENTIFIC QUALITY REVIEW EDITOR
Stacey Abels, PhD
COPY EDITORS Rachelle Laliberte, Kirsty Mackay,
Amy Oravec, and Holly Poulos
CREATIVE DIRECTOR, PUBLISHING Melissa Feinen
SENIOR ART DIRECTOR Marie Maresco
PHARMACY TIMES
CONTINUING EDUCATION™ STAFF
PRESIDENT Jim Palatine, RPh, MBA
EXECUTIVE VICE PRESIDENT, PHARMACY
ADVOCACY Ed Cohen, PharmD, FAPhA
PUBLISHING
VICE PRESIDENT, PHARMACY & HEALTHCARE
COMMUNICATIONS John Hydrusko
ASSOCIATE DIRECTOR,
PARTNERSHIPS & PROGRAMS Grace Rhee
SALES & MARKETING COORDINATOR Marissa Silvestri
ADVERTISING REPRESENTATIVES
Anthony Costella; [email protected]
Madeline Garrigan; mgarrigan@pharmacytimes.com
Molly Higgins; mhiggins@pharmacytimes.com
Matt Miniconzi; mminiconzi@pharmacytimes.com
Kelly Walsh; kw[email protected]
MAIN NUMBER: 609-716-7777
OPERATIONS & FINANCE
CIRCULATION DIRECTOR
Jon Severn; [email protected]
VICE PRESIDENT, FINANCE Leah Babitz, CPA
CONTROLLER Katherine Wyckoff
CORPORATE
CHAIRMAN & FOUNDER Mike Hennessy Sr
VICE CHAIRMAN Jack Lepping
PRESIDENT & CEO Mike Hennessy Jr
CHIEF FINANCIAL OFFICER Neil Glasser, CPA/CFE
EXECUTIVE VICE PRESIDENT, OPERATIONS Tom Tolvé
SENIOR VICE PRESIDENT, CONTENT Silas Inman
SENIOR VICE PRESIDENT, INFORMATION TECHNOLOGY
OFFICER John Moricone
SENIOR VICE PRESIDENT, AUDIENCE GENERATION &
PRODUCT FULFILLMENT Joy Puzzo
VICE PRESIDENT, HUMAN RESOURCES AND
ADMINISTRATION Shari Lundenberg
VICE PRESIDENT, BUSINESS INTELLIGENCE Chris Hennessy
VICE PRESIDENT, MARKETING Amy Erdman
EXECUTIVE CREATIVE DIRECTOR Jeff Brown
PUBLISHING STAFF

SPECIAL REPORT: NUTRACEUTICALS
APRIL
PHARMACYTIMES.COM
2
WHAT ARE NUTRACEUTICALS?
Nutraceuticals are commonly defined as any
substance that is a food or part of a food which
provides medicinal or health benefits, including
the prevention and treatment of disease.
1
This
term includes a broad array of agents such as
dietary supplements, isolated nutrients, herbal
supplements, and specific food products.
2
It is
estimated that 77% of Americans use dietary sup-
plements, including more than 70% of adults who
are aged more than 60 years.
3,4
With an increase
in use and variety of nutraceuticals, it is essential
that pharmacists are made aware of the potential
benefits and risks of the products that are available
for consumer use.
MONITORING OF NUTRACEUTICAL
PRODUCTS IN THE UNITED STATES
Monitoring of nutraceutical products differs from
that of prescription drugs. Nutraceuticals are
broadly regulated under the Federal Food, Drug
and Cosmetic Act, with more specific regulation
for dietary supplements, vitamins, and minerals,
falling under the Dietary Supplement Health and
Education Act of 1994 (DSHEA). Although the
FDA oversees the manufacturing and distributing
process of supplements, rigorous clinical trials
and investigations of safety and efficacy are not
required to market such products. Nutraceuticals
are not intended, according to FDA standards, to
prevent, treat, or cure disease.
5-7
According to the DSHEA, manufacturers and
distributors of dietary and herbal supplements must
ensure the safety and accurate labeling of their
products, to guarantee that they are not adulterated
or misbranded.
7
If adulteration or misbranding
is identified, the FDA is responsible for taking
action to ensure safety and remove products from
consumer use. For example, in March 2019, the
FDA took action against foreign and domestic
companies stating false claims for more than
50 supplement products alleging to prevent, cure,
or treat Alzheimer disease.
8,9
To learn about the
latest warnings and alerts regarding the safety of
such products, pharmacists can refer to the FDA
Dietary Supplement and Advisory List available on
the FDA’s website.
10
In addition to FDA oversight, the official
United States Pharmacopeia (USP) and the official
National Formulary are considered national com-
pendia in the United States, accepted as sources
to provide official guidance. The USP sets qual-
ity standards for drug substances, drug products,
excipients, and dietary supplements under fed-
eral law in the United States, and USP standards
are considered binding under the Federal Food,
Drug, and Cosmetic Act for any manufacturer
claiming USP approval.
11-13
The 4 P’s of quali-
ty that the USP provides are: Positive identity,
Potency, Purity, and Performance of ingredients
in a product.
14
Positive identity ensures the listed
ingredients are present in the supplement and that
The Role of Nutraceuticals: What Pharmacists
Need to Know in 2020
BY LUMA MUNJY, PHARMD
LUMA MUNJY, PHARMD
COVER STORY
© IRYNA / ADOBE STOCK
PHARMACYTIMES.COM
APRIL
3
rigorous testing and auditing have been conducted for verifica-
tion. Assessment of potency guarantees the listed ingredients
are present in the stated amounts. Purity safeguards against
harmful excipients and/or contaminants such as pesticides, mold,
and active pharmaceutical agents, to name a few. Performance
ensures the formulation will break down and release the appro-
priate ingredients, allowing absorption via the labeled route
of administration.
14
USP also provides standards for food ingredients under the
umbrella of nutraceutical products. Pharmacists can refer to the
Food Chemicals Codex monographs for references regarding
assessment of food chemicals and additives.
12
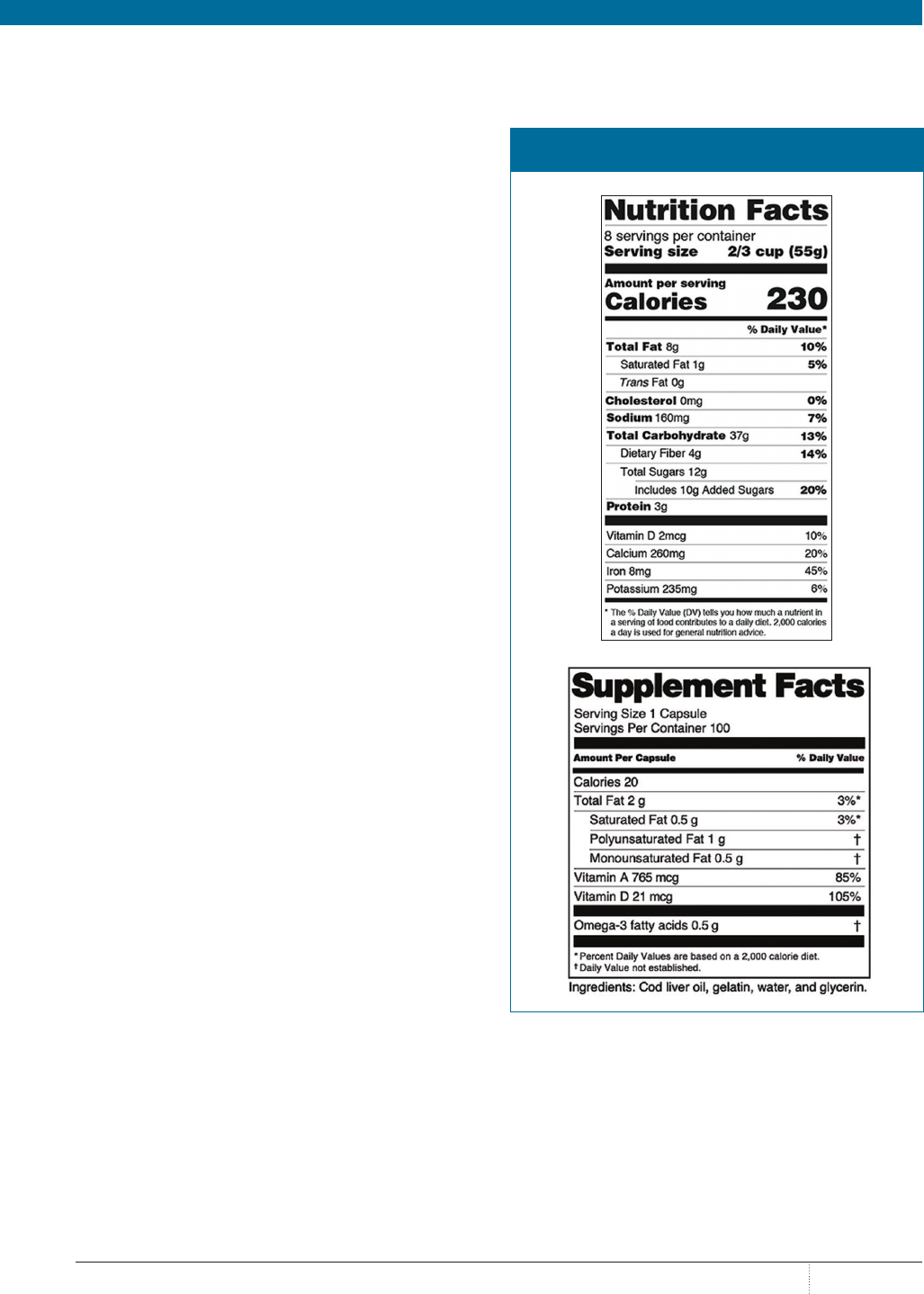
UNDERSTANDING NUTRACEUTICAL LABELS
Because the term nutraceuticals refers to both dietary supple-
ments and food products, understanding label information is
essential for providing appropriate consultations and preven-
tion of potential harm to patients. Supplement labels provide
information regarding suggested use, serving size, percent daily
value of the active ingredients, and a list of inactive ingredients,
as well as cautions and warnings. The manufacturer’s address,
lot number, and notice of potential allergens should also be
present. It is important to note that only the potency of the active
ingredients is listed on the product label. Inactive ingredients
are not tested for strength or potency in the supplement but are
verified only as being present in the product.
15
Food product labels that fall under the category of nutraceu-
ticals must abide by labeling requirements under the FDA’s
Nutrition Facts Labeling Guidance as well. These are also regu-
lated under the Federal Food, Drug and Cosmetic Act. Labeling
for food products requires Nutrition Facts labeling, whereas
dietary supplements require Supplement Facts labeling. A nota-
ble difference in Nutrition Facts compared with Supplement
Facts includes the requirement to list “zero” amounts of nutrients
in the Nutrition Facts label. Additionally, sources of dietary
ingredients and ingredients without a daily reference intake or
daily recommended value cannot be listed in the Nutrition Facts
panel for foods.
16
The images in the
figure depict the differences between a
Nutrition Facts and a Supplement Facts label.
16
USE OF NUTRACEUTICALS IN THE UNITED STATES
As previously stated, the Council on Responsible Nutrition
reported that dietary supplement usage has been at an all-time
high in recent years, with approximately 77% of Americans
reporting using supplements in 2017, and rates have been
steadily rising.
3
It is estimated that 9 of 10 Americans have some
form of nutritional deficiency and 8 of 10 physicians recom-
mend supplements for patient use.
3
Additionally, an increased
number of millennials adhere to specialized eating plans, such
as gluten-free, vegan, vegetarian, and dairy-free diets; this
makes their need for nutritional supplementation potentially
higher, to ensure that they consume essential nutrients.
14,17
Overall, nutraceuticals are used for numerous health purposes.
An overview of some common nutraceutical products and their
use follows.
FIGURE. NUTRITION FACTS VERSUS SUPPLEMENT
FACTS LABEL
Reprinted with permission from US Department of Health and Human Services, FDA,
Center for Food Safety and Applied Nutrition. fda.gov/media/134505/download.
Published January 2020. Accessed March 9, 2020.

SPECIAL REPORT: NUTRACEUTICALS
APRIL
PHARMACYTIMES.COM
4
DIETARY SUPPLEMENTS AND THE COMMON COLD
Zinc, Echinacea purpurea, nasal saline, honey (buckwheat),
geranium extract, and garlic have all been marketed as dietary
supplements used for the common cold. Meta-analyses assess-
ing the effectiveness of zinc for reducing symptoms of the
common cold have concluded that zinc lozenges shortened the
duration of nasal discharge, nasal congestion, sneezing, sore
throat, cough, and muscle aches, with minimal adverse effects
(AEs) noted.
18
Evidence has demonstrated that the use of buck-
wheat honey showed improvement over placebo for decreasing
the frequency of cough and improving the quality of sleep in
pediatric patients.
19
Echinacea purpurea, nasal saline, gerani-
um extract, and garlic have provided inconsistent results and
require improved trials to demonstrate their effectiveness for
use in the common cold.
20
DIETARY SUPPLEMENTS AND DEPRESSION
Marketing for the use of dietary supplements in the manage-
ment of depression is widespread; the most common supple-
ments include omega-3 fatty acids, St John’s wort, SAMe,
and inositol. Of these therapies, meta-analyses have provided
evidence that St John’s wort may have effectiveness in the
treatment of mild to moderate depression in comparison with
placebo; however, well-controlled trials are needed to con-
firm its place in therapy.
21
It should also be noted that several
drug–drug interactions exist with the use of St John’s wort,
and pharmacists should be diligent in assessing all medica-
tions for interactions before recommending use of the product.
Omega-3 fatty acids, SAMe, and inositol have inconclusive
evidence and require further assessment before recommenda-
tions can be made.
22
DIETARY SUPPLEMENTS AND SPECIAL POPULATIONS
Special populations—eg, those who are pregnant and/or nurs-
ing; older adults—may be at greater risk for AEs, and caution
should be taken when recommending nutraceutical products
in these populations. During pregnancy, for example, levels
of essential vitamins and minerals such as iron, calcium, and
folic acid may decline, but they are required for proper growth
and development of the fetus.
23
Although prenatal vitamins
are readily available without prescription, pharmacists should
recommend that patients who are pregnant be assessed by their
obstetrician prior to the use of supplements or nutritional prod-
ucts.
24
In geriatric populations, the use of nutraceuticals should
be monitored because of the increased risk for drug, supple-
ment, and food interactions that may lead to AEs.
4
The National
Institute on Aging recommends a balanced diet including a
variety of healthy foods and fortified food products to maintain
adequate nutrition in geriatric patients; however, individuals
with malabsorption of nutrients due to disease- or drug-induced
nutrient depletions should be assessed by a health care provider
to determine need for supplementation.
25
For further informa-
tion, pharmacists can access the US Department of Agriculture
Dietary Reference Intake calculator to assess specific nutrient
needs in various populations.
26
HERBAL SUPPLEMENTS
Herbal supplements are a subset of dietary supplements that
contain 1 or more herbs. They are also referred to as botanicals
and are made from plants, fungi and/or algae, or a combination
of these substances. Herbal products are often sold as teas,
extracts, tablets, capsules, or powders.
27
Common herbal sup-
plements include green tea, valerian root, cinnamon, Ginkgo
biloba, evening primrose oil, black cohosh, and chamomile,
to name a few. An ample number of herbal supplements exist,
and pharmacists can consult the National Institute of Health’s
National Center for Complementary and Integrative Health for
current research and recommendations regarding their use.
28
PROBIOTICS
Probiotics are also under the umbrella of nutraceutical prod-
ucts. Probiotics generally consist of live microorganisms that
can be placed in dietary supplements and fermented foods and
in topically applied products including cosmetics. Probiotics
may contain a variety of diverse bacteria; the most common
include Lactobacillus and Bifidobacterium Yeast, too, such
as Saccharomyces boulardii, may be included in probiotic
supplements. Probiotics have demonstrated some effectiveness
in specific health conditions, such as preventing antibiot-
ic-associated diarrhea, preventing necrotizing enterocolitis in
premature infants, treating periodontal disease, and supporting
remission of ulcerative colitis. Probiotic use shows promising
results; however, studies with consistent formulations and
amounts of each culture are needed to establish guidance
regarding products. Probiotics are generally safe but should
be used cautiously in patients who are immunocompromised
and/or critically ill to prevent new infections or worsen
current ones.
29
DRUG-INDUCED NUTRIENT DEPLETIONS
Drug-induced nutrient depletions pose an additional area for
pharmacist consultation regarding use of nutraceutical products.
Drug-induced depletions may be mild to moderate in nature and
can be corrected through use of nonprescription products. For
example, use of histamine-2 receptor blockers has been associated
with calcium depletion; therefore, calcium supplementation may
be needed, especially in older adults who are at a higher risk of
bone fractures and osteoporosis. More severe depletions require

PHARMACYTIMES.COM
APRIL
5
evaluation by a health care professional to establish replacement
needs, as in the case of the depletion of such electrolytes as potas-
sium and magnesium in the presence of thiazide and loop diuret-
ics. Pharmacists should be aware of common drug-induced
nutrient depletions and educate patients regarding the need for
nutrient replacement and/or referral for evaluation. The
table
highlights some common nutrient–drug interactions.
30
CONCLUSIONS
Nutraceutical use across the United States is increasing, and
this provides an opportunity for pharmacists to counsel patients
on the appropriate use of available products. As the number of
nutraceuticals increases, it is essential for pharmacists to remain
informed on the latest recommendations for their use and safety.
Pharmacists can refer to the FDA website for current informa-
tion regarding the purity, safety, efficacy, and use of nutraceuti-
cal products. Product selection should be based on verification
of authenticity through national compendia such as the official
USP. Only products with a USP label ensure the purity, poten-
cy, performance, and presence of the listed ingredients on the
label. The need for supplementation is highly patient-specific:
It ranges from broad use of multivitamins to specific replace-
ment of nutrients due to drug-induced nutrient depletions and
conditional replacement during pregnancy and lactation. A
detailed patient history, assessment of current medications, and
determination of risk and benefit should guide pharmacists’
recommendations of nutraceutical products. ®
REFERENCES
1. Andlauer W, Furst P. Nutraceuticals: a piece of history, present status and out-
look. Food Res Int. 2002;35(2-3):171-176. doi: 10.1016/s0963-9969(01)00179-x.
2. Food labeling & nutrition. FDA website. fda.gov/food/food-labeling-nutrition.
Updated February 20, 2020. Accessed March 9, 2020.
3. Dietary supplement use reaches all time high. Council on Responsible
Nutrition website. crnusa.org/newsroom/dietary-supplement-use-reaches-all-
time-high-available-purchase-consumer-survey-reaffirms. Published September
30, 2019. Accessed March 26, 2020.
4. Gahche JJ, Bailey RL, Potischman N, Dwyer JT. Dietary supplement use
was very high among older adults in the United States in 2011-2014. J Nutr.
2017;147(10):1968-1976. doi: 10.3945/jn.117.255984.
5. Dietary supplements. FDA website. fda.gov/food/dietary-supplements.
Updated August 16, 2018. Accessed March 9, 2020.
6. Dietary supplement products and ingredients. FDA website. fda.gov/food/
dietary-supplements/dietary-supplement-products-ingredients. Updated August
16, 2019. Accessed March 9, 2020.
7. Dietary supplements guidance documents & regulatory information. FDA web-
site. fda.gov/food/guidance-documents-regulatory-information-topic-food-and-di-
etary-supplements/dietary-supplements-guidance-documents-regulatory-informa-
tion#healthclaims. Updated September 5, 2019. Accessed March 9, 2020.
8. Watch out for false promises about so-called Alzheimer’s cures. FDA website.
fda.gov/consumers/consumer-updates/watch-out-false-promises-about-so-called-
alzheimers-cures-0. Updated March 28, 2019. Accessed March 9, 2020.
9. Unproven Alzheimer’s disease products. FDA website. fda.gov/consumers/
health-fraud-scams/unproven-alzheimers-disease-products. Updated December
22, 2018. Accessed March 9, 2020.
10. Dietary supplement ingredient advisory list. FDA website. fda.gov/food/
dietary-supplement-products-ingredients/dietary-supplement-ingredient-adviso-
ry-list. Updated December 16, 2019. Accessed March 9, 2020.
11. USP and FDA working together to protect public health. United States
Pharmacopeia website. usp.org/about/public-policy/usp-fda-roles. Accessed
March 9, 2020.
12. Legal recognition – standards categories. United States Pharmacopeia website.
usp.org/about/legal-recognition/standard-categories. Accessed March 9, 2020.
13. Dietary Supplements Verification Program. United States Pharmacopeia
website. usp.org/verification-services/dietary-supplements-verification-program.
Accessed March 9, 2020.
14. Choose a quality supplement. United States Pharmacopeia website. quality-
matters.usp.org/sites/default/files/user-uploaded-files/when-food-is-not-enough-
download_0.pdf. Accessed March 9, 2020.
15. How to read a supplement label. United States Pharmacopeia website.
qualitymatters.usp.org/how-read-supplement-label. Published August 26, 2016.
Accessed March 9, 2020.
16. Small Entity Compliance Guide: revision of the Nutrition Facts and Supplement
Facts Labels. FDA website. fda.gov/regulatory-information/search-fda-guid-
ance-documents/small-entity-compliance-guide-revision-nutrition-and-supple-
ment-facts-labels. Updated February 3, 2020. Accessed March 9, 2020.
17. Gahche JJ, Bailey RL, Potischman N, et al. Federal monitoring of dietary
TABLE. COMMON NUTRIENTDRUG INTERACTIONS
Nutrient
Depleted
Associated Drugs/Drug Classes
Calcium Corticosteroids, loop diuretics, H
2
RAs,
benzodiazepines, digoxin, SSRIs
Vitamin D Corticosteroids, bile acid sequestrants,
H
2
RAs, SSRIs
Folic acid Oral contraceptives, pancreatic
enzymes, hormone replacement
therapy
Vitamin B
12
Metformin, H
2
RAs, PPIs, hormone
replacement therapy
Vitamins A and K Bile acid sequestrants
Potassium Loop diuretics, thiazide diuretics,
corticosteroids, digoxin
Magnesium Oral contraceptives, loop diuretics,
thiazide diuretics, PPIs, H
2
RAs,
digoxin, hormone replacement therapy
H
2
RA indicates histamine-2 receptor antagonist; PPI, proton pump inhibitor; SSRI,
selective serotonin reuptake inhibitor.
page 18

SPECIAL REPORT: NUTRACEUTICALS
APRIL
PHARMACYTIMES.COM
6
M
elatonin, an endogenous, or natural,
hormone mainly produced by the pineal
gland in the brain, regulates several
body processes, including circadian rhythm and
sleep patterns.
1,2
Darkness stimulates melatonin
release, whereas light, especially blue light emitted
by screens, suppresses release.
2
Secretion of mela-
tonin varies by age, with highest secretion in those
aged approximately 1 to 3 years and lowest secre-
tion in young infants (<1 year) and elderly adults
aged at least 65 years.
3,4
Melatonin can be given exogenously, such as in
the form of a synthetically produced supplement,
and has been investigated for various medical con-
ditions, most commonly sleep disorders, including
jet lag, insomnia, shift-work disorder, and other
circadian rhythm disorders (eg, delayed sleep phase
syndrome or non–24-hour sleep–wake disorder).
1,2
When given exogenously, melatonin is
proposed to improve sleep onset latency (time to
fall asleep) rather than cause drowsiness to induce
sleep.
2,3
Doses of 1 mg to 5 mg of melatonin taken
at night can produce 10 to 100 times higher
nighttime peaks within an hour when compared
with endogenous peaks.
3
Metabolism of melatonin
occurs in the liver, resulting in a relatively short
half-life of 30 minutes to 60 minutes.
3
Most exog-
enous melatonin is synthetically produced.
2
Less
often, it is produced from animal pineal gland;
bovine derivations should be avoided because of
possible bacterial contamination.
2
SAFETY OF MELATONIN SUPPLEMENTS
Melatonin appears to be safe for short-term use
in the general population as an 8-mg dose per day
for up to 6 months. Even with larger doses (up to
10 mg, which is safe to use for up to 2 months),
only mild adverse effects (AEs) such as dizziness,
headache, nausea, and drowsiness were general-
ly reported. Some patients may be able to take
melatonin safely for up to 2 years.
1
Long-term
studies of the use of melatonin for up to 2 years in
children have shown similar AEs as those in short-
term studies; however, because these studies are
limited, long-term use of melatonin should occur
under health care provider supervision, regardless
of age.
1,5
There is a lack of evidence in pregnant women
regarding safety, and so melatonin should be
avoided in this population. Higher doses (75-300
mg/day) have been associated with inhibition of
ovulation, and so patients desiring to become
pregnant should avoid high or frequent doses.
1,5
Few data are available regarding use in lactation,
and so women who are breastfeeding should be
counseled to avoid use.
1,5
In children, melatonin may be safe when used
short term in low doses. Dosing should be limited
to 3 mg daily for infants (aged >6 months) and
children. Melatonin use in adolescents may poten-
tially affect sexual hormones and development, so
doses should not exceed 5 mg daily if medically
needed. Otherwise, it should be avoided for healthy
children.
1,5
Elderly patients may be more suscepti-
ble to AEs such as daytime drowsiness because of
decreased clearance of the drug in this population.
5
Melatonin is specifically not recommended in
elderly patients with dementia who have irregular
sleep–wake rhythm disorder.
6
EFFICACY OF MELATONIN SUPPLEMENTS
Melatonin has been studied in a variety of sleep
disorders, but evidence is often weak or conflict-
ing because of smaller or lower-quality studies.
Most evidence supports its use in delayed sleep
phase syndrome, non–24-hour sleep–wake dis-
order in the blind, and primary insomnia.
1,6
It
is often used for occasional insomnia, although
evidence and dosage is less certain.
2
In chronic
insomnia, there is evidence of a slight reduction
Melatonin: Considerations for Use in Patients
With Sleep Disorders
BY RASHI C. WAGHEL, PHARMD, BCACP; AND JENNIFER A. WILSON, PHARMD, BCACP
FEATURE
RASHI C. WAGHEL,
PHARMD, BCACP
JENNIFER A. WILSON,
PHARMD, BCACP
PHARMACYTIMES.COM
APRIL
7
in sleep onset latency (about 7-12 minutes) compared with
placebo and a small improvement in subjective sleep quality.
1,7,8
However, the American Academy of Sleep Medicine and the
American College of Pharmacy both state that sufficient evi-
dence is lacking to recommend use of melatonin in the general
population for chronic insomnia.
7,8
Despite the lack of evidence,
guidelines recognize that an informed patient would be more
likely to use melatonin over no treatment.
8
An overview of mel-
atonin efficacy in insomnia, along with other sleep disorders,
can be found in the
table.
1,2,6-14
COUNSELING POINTS
Pharmacists can counsel patients on selecting products to ensure
that they choose the intended product strength (eg, melatonin
TABLE. MELATONIN USE FOR SLEEP DISORDERS SUMMARY
,,
Condition Studied
a
Doses Studied Results
DSPS - likely effective
1,5,6
0.3-5.0 mg daily up to 4 weeks Improves sleep onset latency; improves QOL
(eg, mental health, vitality, bodily pain) in
young adults with DSPS
Non–24-hour sleep–wake
disorder - likely effective
1,5,6
0.5-10 mg daily in adults and 0.5-4.0 mg daily
in children for up to 6 years
Improves circadian rhythm sleep disorders
in adults and children who are blind
Beta blocker–induced insomnia
- possibly effective
1
2.5-5.0 mg at night (eg, 1 hour before bed and
after taking the beta blocker)
May improve sleep latency, total wake time,
wakefulness after sleep onset, and/or
increase total sleep time to counter proposed
decrease in endogenous melatonin with beta
blocker use
Insomnia -
possibly effective
1,2,6,7,8,9
0.3-5.0 mg in adults nightly
typically for ≥21 days
5 mg in children for 28 days
(0.05-0.15 mg/kg for 7 days in 1 trial)
Short-term use may improve sleep-onset
latency, increase total sleep time, and improve
sleep quality; more benefit may be seen in
elderly individuals (due to melatonin defi-
ciency); more benefit seen with insomnia with
certain comorbidities (depression, schizophre-
nia, bipolar disorder, epilepsy, asthma, cystic
fibrosis, tuberous sclerosis, autism spectrum
disorders, and developmental disabilities),
whereas conflicting results have been seen
with other conditions (Alzheimer disease,
dementia, Parkinson disease, traumatic brain
injury, substance use disorder, and dialysis)
Jet lag - possibly effective
1,2,10,11
0.5-5.0 mg (preferably 2.0-3.0 mg)
at local bedtime on day of arrival and
for 2-5 nights thereafter
May improve alertness, jet lag, psychomotor
performance, daytime sleepiness, and fatigue;
may be most effective when traveling eastward
through >5 time zones
Preoperative anxiety and
sedation - possibly effective
1,12
3.0-14.0 mg orally or 0.05-0.2 mg/kg
sublingually in adults
0.05-0.4 mg/kg in children
Studies show conflicting efficacy; may
improve sedation and reduce preoperative
anxiety similar to taking midazolam, cloni-
dine, or gabapentin as a preanesthetic agent
Shift-work disorder -
possibly ineffective
1,2,11,13
1.0-10.0 mg after night shift Does not significantly improve sleep latency,
sleep efficiency, or adjustment to rotating shift
work; may slightly increase total sleep time
and/or overall sleep quality
REM sleep behavior disorder -
insufficient evidence
1,6
3.0 mg nightly for 4 weeks May increase likelihood of appropriate muscle
paralysis during REM sleep
DSPS indicates delayed sleep phase syndrome; QOL, quality of life; REM, rapid eye movement.
a
Ratings (likely effective, possibly effective, possibly ineffective, insufficient evidence) based on the Natural Medicines Comprehensive Database rating system.
1
SPECIAL REPORT: NUTRACEUTICALS
APRIL
PHARMACYTIMES.COM
8
can be dosed in both milligrams and micrograms: 0.3 mg or
equivalently, 300 mcg). A 300-mcg dose of exogenous mela-
tonin produces higher-than-normal physiologic concentrations,
and so high doses are not usually necessary.
2
Lower doses may
also minimize potential AEs such as dizziness, headache, nau-
sea, and sleepiness, but no study results support this.
5
Patients
should be counseled on appropriate timing of dose and length
of use depending on indication (eg, 30-60 minutes before
bedtime for insomnia).
2
Additional details can be found in the
Table.
1,2,6-14
Regardless of whether a patient chooses to use
melatonin for sleep disorders, patients should be counseled on
appropriate sleep hygiene, such as establishing a regular sleep
pattern, avoiding daytime naps, and avoiding use of electronics
before bed.
15
CONCLUSIONS
Strong evidence from long-term clinical trials evaluating the
use of melatonin for sleep disorders is lacking. Although some
evidence shows modest benefit in reducing sleep onset latency
and improving sleep quality, the overall clinical impact may be
limited. However, melatonin is generally regarded as safe in
the general population, with only mild AEs reported. As such,
despite the lack of strong evidence, patients may opt to try mel-
atonin to help with sleep disorders such as insomnia or jet lag. ®
REFERENCES
1. Melatonin. Natural Medicines website. naturalmedicines.therapeuticresearch.
com/databases/food,-herbs-supplements/professional.aspx?productid=940.
Updated February 19, 2020. Accessed February 28, 2020.
2. McQueen CE, Orr KK. Natural products. In: Krinsky DL, Ferreri SP,
Hemstreet BA, et al. Handbook of Nonprescription Drugs: An Interactive
Approach to Self-Care. 19th ed. Washington, DC: American Pharmacists
Association; 2017:957-994.
3. Wassmer E, Whitehouse WP. Melatonin and sleep in children with neurodevel-
opmental disabilities and sleep disorders. Curr Paediatrics. 2006;16(2):132-138.
doi: 10.1016/j.cupe.2006.01.001.
4. Mishima K, Okawa M, Shimizu T, Hishikawa Y. Diminished melatonin
secretion in the elderly caused by insufficient environmental illumination. J Clin
Endocrinol Metab. 2001;86(1):129-134. doi: 10.1210/jcem.86.1.7097.
5. Andersen LP, Gögenur I, Rosenberg J, Reiter RJ. The safety of melatonin
in humans. Clin Drug Investig. 2016;36(3):169-175. doi: 10.1007/s40261-015-
0368-5.
6. Auger RR, Burgess HJ, Emens JS, Deriy LV, Thomas SM, Sharkey KM.
Clinical practice guideline for the treatment of intrinsic circadian rhythm
sleep-wake disorders: advanced sleep-wake phase disorder (ASWPD), delayed
sleep-wake phase disorder (DSWPD), non-24-hour sleep-wake rhythm disorder
(N24SWD), and irregular sleep-wake rhythm disorder (ISWRD). an update for
2015: an American Academy of Sleep Medicine clinical practice guideline. J Clin
Sleep Med. 2015;11(10):1199-1236. doi: 10.5664/jcsm.5100.
7. Auld F, Maschauer EL, Morrison I, Skene DJ, Riha RL. Evidence for the
efficacy of melatonin in the treatment of primary adult sleep disorders. Sleep Med
Rev. 2017;34:10-22. doi: 10.1016/j.smrv.2016.06.005.
8. Qaseem A, Kansagara D, Forciea MA, Cooke M, Denberg TD; Clinical
Guidelines Committee of the American College of Physicians. Management
of chronic insomnia disorder in adults: a clinical practice guideline from the
American College of Physicians. Ann Intern Med. 2016;165(2):125-133. doi:
10.7326/M15-2175.
9. Sateia MJ, Buysse DJ, Krystal AD, Neubauer DN, Heald JL. Clinical practice
guideline for the pharmacologic treatment of chronic insomnia in adults: an
American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep
Med. 2017;13(2):307-349. doi: 10.5664/jcsm.6470.
10. Ferracioli-Oda E, Qawasmi A, Bloch MH. Meta-analysis: melatonin for the
treatment of primary sleep disorders. PLoS One. 2013;8(5):e63773. doi: 10.1371/
journal.pone.0063773.
11. Herxheimer A. Jet lag. BMJ Clin Evid. 2014;2014.
12. Costello RB, Lentino CV, Boyd CC, et al. The effectiveness of melatonin for
promoting healthy sleep: a rapid evidence assessment of the literature. Nutr J.
2014;13:106. doi: 10.1186/1475-2891-13-106.
13. Hansen MV, Halladin NL, Rosenberg J, Gögenur I, Møller AM. Melatonin
for pre- and postoperative anxiety in adults. Cochrane Database Syst Rev.
2015;(4):CD009861. doi: 10.1002/14651858.CD009861.pub2.
14. Liira J, Verbeek JH, Costa G, et al. Pharmacological interventions for sleep-
iness and sleep disturbances caused by shift work. Cochrane Database Syst Rev.
2014;(8):CD009776. doi: 10.1002/14651858.CD009776.pub2.
15. Melton ST, Kirkwood CK. Insomnia, drowsiness, and fatigue. In: Krinsky
DL, Ferreri SP, Hemstreet B, et al. Handbook of Nonprescription Drugs:
An Interactive Approach to Self-Care. 19th ed. Washington, DC: American
Pharmacists Association; 2017:855-871.
ABOUT THE AUTHORS
RASHI C. WAGHEL, PHARMD, BCACP, is an associate professor of pharmacy at
Wingate University in Charlotte, North Carolina.
JENNIFER A. WILSON, PHARMD, BCACP, is an associate professor of pharmacy at
Wingate University.

PHARMACYTIMES.COM
APRIL
9
SPECIAL REPORT: NUTRACEUTICALS
V
itamin D encompasses a group of fat-
soluble secosterols that are found in
certain foods and supplements and can
also be produced when synthesis of vitamin D
is triggered after the sun’s ultraviolet rays touch
skin. It is required for bone growth and
remodeling,and when paired with calcium, it
can help to prevent osteoporosis. Low levels of
vitamin D have been linked with poorer health
and with disease.
1
Although the importance of
vitamin D in regard to bone health has been
established, its importance in other areas, such
as skin pigmentation, pregnancy, cancer, and
immune function, is unclear and not supported
by clinical evidence.
1,2
WHO NEEDS VITAMIN D
Because few foods naturally contain vitamin D,
supplementation is commonly used to avoid risk
of vitamin D deficiency (
table).
1
Herein are
described 4 populations of patients who may
benefit from vitamin D supplementation.
Breastfed Infants
The American Academy of Pediatrics (AAP)
recommends that infants who are breastfed receive
vitamin D supplementation. Although most infant
formulas contain vitamin D, the AAP still rec-
ommends that infants who are receiving less than
1 L of formula per day also receive supplemen-
tation, in addition to those who are exclusively
breastfed.
3
The results of a 2010 study of data
from the Infant Feeding Practices Study II, in
which mothers of both breastfed and formula-fed
infants responded to mailed questionnaires on
a variety of topics, including dietary intake,
found that 44% to 58% of infants met the 2003
recommended amount of 5 mcg per day of vitamin
D. When the AAP recommendation increased to
10 mcg per day in 2008, less than a quarter
of infants would have met the recommenda-
tion.
4
Mothers who responded to the question
gave a variety of reasons for not supplementing
their infants with vitamin D, including lack of
knowledge about supplementation, misinforma-
tion about vitamins in formula and breast milk,
inconvenience of administering supplements, and
infant’s dislike of them. Common phrases repeat-
ed by the mothers were, “I didn’t know I should,”
“Baby formula has all that is needed and recom-
mended,” and “It causes [the baby] to spit up.”
5
Of the mothers who responded to the question-
naire who were breastfeeding, most (88.4%) pre-
ferred to take a vitamin D supplement themselves
rather than directly administer it to their infant. The
benefits of maternal supplementation with vitamin
D include ease of administration, both mother and
infant receiving vitamin D, and decreased risk
of infant toxicity due to dosing errors. Maternal
supplementation at 100 mcg to 162.5 mcg per day,
or a single monthly dose of 3750 mcg, can suffi-
ciently enrich breast milk with enough vitamin D
to meet an infant’s needs without causing toxicity.
If mothers choose to give their infants vitamin D
supplementation directly, the infant should receive
10 mcg per day through drops administered by
mouth or in the bottle.
5
Older Adults
The results of a study conducted between 2011
and 2014 found that vitamin D supplementation
use has increased in the United States, with 26%
of older adults (≥60 years) taking a vitamin D
supplement.
6
As people grow older, their skin
becomes thinner and they cannot absorb and
process vitamin D as efficiently.
1
Further, renal
decline can occur in older adults. This affects
vitamin D levels because the kidneys are needed
to convert vitamin D in the body.
1
When levels of vitamin D in the body are inad-
equate, bones can become thin, fragile, and mis-
shapen, and the risk of osteoporosis is increased.
2
Therefore, the National Osteoporosis Foundation
recommends that women and men aged under
50 years receive 10 mcg to 20 mcg of vitamin D
per day, and that those 50 years and older receive
To D or Not to D: That Is the Question
BY CHELSEA RENFRO, PHARMD, CHSE; AND ALEX STANLEY, PHARMD CANDIDATE
CHELSEA RENFRO, PHARMD, CHSE
FEATURE
ALEX STANLEY,
PHARMD CANDIDATE

SPECIAL REPORT: NUTRACEUTICALS
APRIL
PHARMACYTIMES.COM
10
20 mcg to 25 mcg of vitamin D per day.
7
Increasing vitamin D levels is also a modifiable risk factor
to potentially reduce falls and fractures in older adults if taken
daily. Sanders and colleagues sought to determine whether a high
annual dose of vitamin D, instead of daily doses, would reduce
the risk of falls and fractures. They conducted a study in 2010
on community-dwelling women 70 years and older and found
that annual administration of 12,500 mcg of vitamin D increased
the risk of falls and fractures, with the highest risk being within
the first 3 months after administration.
8
Based on these findings,
daily dosing for older adults is preferred. It is recommended that
adults younger than 70 years receive 15 mcg of vitamin D per
day, and those 70 years and older receive 20 mcg per day.
2
Individuals Who Have Undergone Bariatric Surgery
Obesity, which is prevalent in the United States, poses many
health risks, and an increasing number of adults are undergo-
ing weight loss (bariatric) surgery.
9
Although bariatric surgery
decreases the risk of disease and other complications related to
obesity, it also decreases the body’s ability to absorb vitamins
and other nutrients.
10
According to the American Society for
Metabolic and Bariatric Surgery, vitamin D deficiency occurs in
up to 100% of patients who have undergone surgery for weight
loss. To this end, they recommend vitamin D
3
supplementation
for these patients at daily doses of 75 mcg.
11
Individuals With Diabetes
Vitamin D deficiency also has been linked with hypertension,
kidney disease, and diabetes.
12
Beta cells in the pancreas, where
insulin is secreted, have vitamin D receptors, and it is speculat-
ed that vitamin D may improve insulin sensitivity and secretion,
as well as glomerular filtration rate.
13
Study results related
to this effect have been inconclusive, however, and research
on the effect of vitamin D supplementation in patients with
diabetes continues.
14
Regardless, supplementing with 15 mcg of
vitamin D per day in people younger than 70 years, and 20 mcg
per day in those 70 years and older, to ensure that each patient
meets the recommended daily value, has many health benefits
and may later prove to be beneficial in patients with type 1 and
type 2 diabetes.
2
WHAT PHARMACISTS AND THEIR PATIENTS NEED
TO KNOW
Although in some instances quantities that are higher than the
recommended dietary allowance are indicated, overaggressive
supplementation of vitamin D, or any nutrient, may result
in adverse reactions.
1
Inform patients that evidence supports
the benefit of vitamin D to bone health but that its use is
unclear in areas previously discussed, such as skin pigmenta-
tion, pregnancy, cancer, and immune function. For the average,
healthy adult patient, the recommended dietary allowance
of 15 mcg per day—and 20 mcg per day for those aged
over 70 years—is appropriate. ®
REFERENCES
1. Institute of Medicine (US) Committee to Review Dietary Reference Intakes for
Vitamin D and Calcium; Ross AC, Taylor CL, Yaktine AL, Del Valle HB, eds.
Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: National
Academies Press; 2011.
2. Vitamin D: fact sheet for health professionals. NIH Office of Dietary Supplements
website. ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/#ref. Updated
August 7, 2019. Accessed March 7, 2020.
3. Vitamin D supplementation for infants. American Academy of Pediatrics web-
site. aap.org/en-us/about-the-aap/aap-press-room/pages/vitamin-d-supplementa-
tion-for-infants.aspx. Published March 22, 2010. Accessed March 6, 2020.
4. Perrine CG, Sharma AJ, Jefferds ME, Serdula MK, Scanlon KS. Adherence to
vitamin D recommendations among US infants. Pediatrics. 2010;125(4):627-632.
doi: 10.1542/peds.2009-2571.
5. Umaretiya PJ, Oberhelman SS, Cozine EW, Maxson JA, Quigg SM, Thacher
TD. Maternal preferences for vitamin D supplementation in breastfed infants. Ann
Fam Med. 2017;15(1):68-70. doi: 10.1370/afm.2016.
6. Gahche JJ, Bailey RL, Potischman N, Dwyer JT. Dietary supplement use
was very high among older adults in the United States in 2011-2014. J Nutri.
2017;174(10):1968-1976. doi: 10.3945/jn.117.255984.
7. Calcium and vitamin D. National Osteoporosis Foundation website. nof.org/
patients/treatment/calciumvitamin-d/. Updated February 26, 2018. Accessed
March 8, 2020.
8. Sanders KM, Stuart AL, Williamson EJ, et al. Annual high-dose oral vitamin
D and falls and fractures in older women: a randomized controlled trial. JAMA.
2010;303(18):1815-1822. doi: 10.1001/jama.2010.594.
9. Adult obesity facts. CDC website. cdc.gov/obesity/data/adult.html. Updated
February 27, 2020. Accessed March 13, 2020.
10. Lupoli R, Lembo E, Saldalamacchia G, Avola CK, Angrisani L, Capaldo
B. Bariatric surgery and long-term nutritional issues. World J Diabetes.
TABLE. RECOMMENDED DIETARY ALLOWANCES
OF VITAMIN D
a
Age Male Female Pregnancy Lactation
0-12 months 10 mcg 10 mcg - -
1-13 years 15 mcg 15 mcg - -
14-18 years 15 mcg 15 mcg 15 mcg 15 mcg
19-50 years 15 mcg 15 mcg 15 mcg 15 mcg
51-70 years 15 mcg 15 mcg - -
>70 years 20 mcg 20 mcg - -
a
Adequate intake.
Adapted with permission: Dietary Reference Intakes for Calcium and Vitamin D.
Washington, DC: National Academies Press; 2011.
page 18

PHARMACYTIMES.COM
APRIL
11
SPECIAL REPORT: NUTRACEUTICALS
O
mega-3 fatty acids (omega-3s) have
received much publicity and advertising
attention over the last few years stating
that they are an essential supplement many peo-
ple should consider taking. As a pharmacist, it is
important to know which patients may benefit from
omega-3 supplements the most, the supplements’
proper dosage, and the benefits that can be expect-
ed. It is also important to know which health claims
about omega-3s have the most validity.
OMEGA-3 COMPONENTS AND SOURCES
Omega-3 fatty acids have 2 main components
that are beneficial in humans: eicosapentaenoic
acid (EPA) and docosahexaenoic acid (DHA).
DHA levels are highest in the retina and brain.
Omega-3s can also be used to form eicosa-
noids, which have activity in the cardiovascular,
pulmonary, immune, and endocrine systems.
A third component of omega-3s, alpha-linolenic
acid (ALA), is not active in the body, but it can be
converted to EPA and DHA.
1,2
The primary vehicle for EPA and DHA to
enter the body is through the consumption of
fish and other seafood, so the American Heart
Association recommends consuming 2 servings
of fish per week, particularly fatty fish such as
tuna, salmon, herring, or sardines, which have
high levels of omega-3s.
1,3
For patients who do
not get enough omega-3s through their diet or who
require a higher level than what their diet pro-
vides, OTC and prescription dietary supplements of
omega-3s may help to meet their daily needs.
FISH OIL SUPPLEMENTS
Fish oil supplements are a common source of
DHA and EPA. For individuals who cannot tol-
erate fish oil, or do not wish to take it, omega-3s
are also contained in krill, cod liver, and algal
oil supplements.
Fish oil supplements come in various dos-
age forms or combinations. A target dose of
around 1 g of omega-3s is a good place to start.
4
When taking fish oil supplements, patients may
experience an unpleasant “fishy” taste; how-
ever, the use of higher-quality products with a
United States Pharmacopeia seal may alleviate this
problem, as these products may be less likely
to have the unpleasant taste or smell.
5
Patients
may also be advised to store the capsules in the
refrigerator or to take them at bedtime to avoid the
unpleasant taste.
Krill oil, sourced from tiny crustaceans called
krill, can be an alternative for patients who cannot
tolerate the fishy smell or taste that can be associat-
ed with fish oil supplements. Krill oil is more stable
than fish oil, which may mean it is absorbed better,
and because it is not sourced from fish, it may be
less likely to cause a fishy aftertaste. The use of
krill oil has not been studied as extensively as that
of fish oil, however, and probably should remain as
a secondary recommendation until further research
reinforces its safety and effectiveness. The rec-
ommended dosage from the manufacturer will be
included on the krill oil product that is selected.
6
For those patients who follow a vegetarian or
vegan diet, pharmacists may recommend an algal
oil supplement to add omega-3s to their diet. Algal
oil is derived from algae and may be a good source
of EPA and DHA; however, studies on algal oil
have not been extensive.
7,8
Recommendations of
these products may need to be limited to only
those patients who cannot tolerate fish oil or those
patients who do not consume any fish products
because of dietary preferences or needs.
Pharmaceutical-grade omega-3 products are also
available and are prescribed in dosages as high
as 4 g per day. These products are indicated
for patients with very high triglyceride levels.
9
Patients should be advised to not take dosages
BRADY COLE, RPH
FEATURE
Omega-3 Recommendations: Counseling
Points for Pharmacists
BY BRADY COLE, RPH

SPECIAL REPORT: NUTRACEUTICALS
APRIL
PHARMACYTIMES.COM
12
in this range through OTC products without the advice of
their physician.
BENEFITS OF OMEGA-3s
The efficacy of omega-3s in various conditions has been
researched extensively, sometimes with conflicting results.
Several trials have been conducted researching the link between
a diet rich in omega-3s and a decreased risk of cardiovascular
disease.
9
Although these data vary across studies, the FDA
states that there is supportive (but not conclusive) research
indicating that consumption of EPA and DHA may reduce the
risk of coronary heart disease.
9
DHA is important for fetal growth and is found in high concen-
trations in the cellular membranes of the brain and the retina, and
so many prenatal vitamins and infant formulas are fortified with
DHA. Omega-3s have anti-inflammatory properties, and their use
may provide some relief from mild inflammation or joint pain
as well as help to reduce patients’ reliance on nonsteroidal anti-
inflammatory drugs for inflammation.
10
Many other benefits claimed for omega-3s have been studied
but have been proved inconclusive. These include potential
benefits studied in patients with dementia, depression, and
attention deficit/hyperactivity disorder, as well as cancer
prevention.
9
Continued research is needed to try to uncover
additional benefits or to confirm the validity of other perceived
advantages of a diet rich in omega-3s.
RECOMMENDED DOSES
According to Dietary Guidelines for Americans 2015-2020,
the goal for most Americans should be to consume 8 oz of
seafood per week, which is about 250 mg of EPA and DHA per
day.
11
For those patients who are looking for more advanced
benefits from omega-3s, pharmacists may recommend a total
dose of 1 g per day via supplements.
4
Patients with very high
levels of triglycerides can be prescribed doses as high as
4 g per day while under supervision of a physician.
9
The Institute of Medicine published a guideline in 2005 for
intake of total omega-3s for infants and of ALA for children and
adults, which is still used by the National Institutes of Health
today (see
table).
2
CONCLUSIONS
The use of omega-3 supplements may be beneficial for some
patients; however, the most effective way to add omega-3s to
the diet is by consuming them through food. Pharmacists may
recommend 2 servings of fatty fish per week to patients as a
starting point, which will not only introduce the beneficial EPA
and DHA components into the diet but may also replace foods
or meals that are not as healthy. For patients who are unwilling
or unable to eat fish every week, other foods that are rich in
omega-3s, such as flaxseeds, walnuts, Brussels sprouts, soy-
beans, or seaweed, can be recommended. Omega-3 supplements
such as fish oil, krill oil, or algal oil are the next alternative
for patients who cannot consume enough omega-3s from their
diet. Pharmacists should be prepared to answer questions about
omega-3 supplementation and know which types of patients
could benefit from them the most. Educating patients on the
reasoning for a recommendation and encouraging them to dis-
cuss recommendations with their physician will go a long way
in ensuring positive outcomes. ®
REFERENCES
1. Harris WS. Omega-3 fatty acids. In: Coates PM, Betz JM, Blackman MR, et
al, eds. Encyclopedia of Dietary Supplements. 2nd ed. London and New York:
Informa Healthcare; 2010:577-586.
2. Institute of Medicine. Dietary Reference Intakes for Energy, Carbohydrate,
Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. Washington, DC:
National Academies Press; 2005. doi: 10.17226/10490.
3. Fish and omega-3 fatty acids. American Heart Association website. heart.
org/en/healthy-living/healthy-eating/eat-smart/fats/fish-and-omega-3-fatty-acids.
Updated March 23, 2017. Accessed March 4, 2020.
4. Should you be taking an omega-3 supplement? Harvard Health Publishing
website. health.harvard.edu/staying-healthy/should-you-be-taking-an-omega-3-
supplement. Published April 2019. Accessed March 2, 2020.
5. Fish oil. Mayo Clinic website. mayoclinic.org/drugs-supplements-fish-oil/art-
20364810. Published October 24, 2017. Accessed March 13, 2020.
6. Krill oil vs fish oil: what’s the difference between them?. Drugs.com website.
drugs.com/medical-answers/krill-oil-vs-fish-oil-difference-3040407. Updated
April 12, 2019. Accessed March 4, 2020.
7. Sasso S, Pohnert G, Lohr M, Mittag M, Hertweck C. Microalgae in the post-
TABLE. ADEQUATE INTAKES FOR OMEGAs
Age Male Female Pregnancy Lactation
Birth to 6 months
a
0.5 g 0.5 g - -
7-12 months
a
0.5 g 0.5 g - -
1-3 years
b
0.7 g 0.7 g - -
4-8 years
b
0.9 g 0.9 g - -
9-13 years
b
1.2 g 1.0 g - -
14-18 years
b
1.6 g 1.1 g 1.4 g 1.3 g
19-50 years
b
1.6 g 1.1 g 1.4 g 1.3 g
51+ years
b
1.6 g 1.1 g - -
a
As total omega-3s.
b
As alpha-linolenic acid.
Reprinted with permission from: Institute of Medicine. Dietary Reference Intakes for
Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids.
Washington, DC: The National Academies Press; 2005.
page 18

PHARMACYTIMES.COM
APRIL
13
SPECIAL REPORT: NUTRACEUTICALS
P
renatal vitamins are designed to support both
the health of the mother and the development
of the baby during pregnancy. Pregnancy is
difficult to predict; it may take a woman 1 month,
1 year, or longer of trying to conceive before
she becomes pregnant. Additionally, many crit-
ical fetal developments occur before a woman
even knows that she is pregnant.
1
The results of a
2016 study found that in 2011, nearly half (45%)
of pregnancies were unplanned, with a rate of
unintended pregnancy among women of
reproductive age of 4.5%.
2
WHEN SHOULD A PRENATAL VITAMIN
BE STARTED?
Because of the prevalence of unintended
pregnancy as well as the uncertainty of how
quickly or slowly conception will occur, pre-
natal vitamins should be started 3 months prior
to attempted conception.
1
This is to ensure that
any potential nutritional deficiencies have been
corrected, or increased needs supplied, prior to
conception.
1
If prenatal vitamins cannot be started
3 months in advance, folic acid supplementation
should be initiated at least 1 month before trying
to get pregnant. This is crucial because folic acid
aids in growth and development and because the
neural tube, which later develops into the baby’s
spinal cord, spine, brain, and skull, forms between
week 4 and week 6 of gestation, before most
women know they are pregnant. This can help
reduce the risk of neural tube defects.
3,4
Prenatal
vitamins should be continued throughout the
entire pregnancy.
4
The results of a 2017 survey by the March
of Dimes found that only 34% of women aged
18 to 45 years who took a prenatal vitamin during
their current or last pregnancy started the prenatal
vitamin before they knew that they were pregnant.
Although 97% took a prenatal vitamin, these may
have not been started by the optimal time to prevent
birth defects, which have an annual prevalence
in the United States of 120,000, or 3% of births
per year. Use of prenatal vitamins prior to the
knowledge of pregnancy was lower in minority
populations, with just 10% of African American
and 27% of Hispanic patients taking them before
they knew they were pregnant.
5
WHAT VITAMINS SHOULD PREGNANT
PATIENTS TAKE?
The American College of Obstetricians and
Gynecologists (ACOG) recommends that all
female patients of childbearing potential “be
screened regarding their diet and vitamin sup-
plements to ensure they are meeting recom-
mended daily allowances for calcium, iron, vita-
min A, vitamin B
6
[pyridoxine], vitamin B
12
[cobalamin], vitamin D, and other nutrients.”
6
Folic acid supplementation should be encouraged
for these patients as well regardless of dietary
intake of folic acid, to reduce the risk of neural
tube defects.
7
Despite being recommended in 1998 by the
National Academy of Medicine as an essential
nutrient,
8
the role of choline in maternal and
fetal development remains underrecognized. Of
the top 25 prenatal vitamins, none contained the
450-mg recommended daily allowance, often
providing only 0 mg to 55 mg per day.
9-11
Lack
of sufficient levels provided in prenatal vitamins
could be of consequence because only 25% of
women of childbearing potential from high-in-
come countries such as the United States obtain
enough choline from their diets.
10-13
Choline is
emerging as a nutrient of important consequence
during pregnancy because it plays an import-
ant role in neural tube development, memory
development, stem cell proliferation, and apop-
tosis.
9
Choline is thought to have an impact on
Prenatal and Postnatal Supplementation:
What Do Pharmacists Need to Know?
BY CORTNEY MOSPAN, PHARMD, BCACP, BCGP
CORTNEY MOSPAN,
PHARMD, BCACP, BCGP
FEATURE

SPECIAL REPORT: NUTRACEUTICALS
APRIL
PHARMACYTIMES.COM
14
the risk of development of neural tube defects independent of
folic acid intake.
The
table includes information from ACOG, CDC, FDA,
and the World Health Organization (WHO) regarding the
recommended vitamins and minerals a woman should take
during pregnancy.
7,14-19
WHEN CAN A PRENATAL VITAMIN BE STOPPED?
Patients who are pregnant may struggle with long-term adher-
ence to their prenatal vitamin because of undesirable effects
such as a fishy aftertaste
20
due to docosahexaenoic acid (DHA),
constipation from iron or calcium, or general nausea from tak-
ing the prenatal vitamin on an empty stomach. Thus, there is a
delicate balance between advising women of proper duration of
use for health benefits for the mother and baby and preventing
unnecessary supplementation due to adverse effects that can
affect patients’ quality of life.
21
Breastfeeding is well established as the best nutrition option
for infants if mothers are able to breastfeed. One of the values
of breastfeeding is provision of essential vitamins and nutrients
in breast milk. However, it is debated whether simply following
a well-balanced diet may be sufficient to provide these valuable
nutrients to infants.
22,23
The CDC recommends continuation of
nutrient supplementation in mothers who breastfeed only if they
follow restrictive diets (eg, vegetarian diets). They do state that
nutritional supplementation may also offer benefit in women who
breastfeed who consume balanced diets.
22,23
Supplementation
likely provides the greatest benefit to meet increased iodine
needs.
22
No leading organization provides any clear or specific
vitamin or nutrition supplement recommendations in lactation.
Most women will continue the same prenatal vitamin used
throughout pregnancy during lactation, but there are differ-
ent and unique nutritional needs during pregnancy.
23
ACOG
makes no definitive recommendation on how long prenatal
supplements should be continued during the postnatal period
or which vitamins should be supplemented and at what dose.
24
Supplementation with DHA, vitamin D, folic acid, or iodine
has been shown to improve the infant’s visual acuity, hand/eye
coordination, attention, problem solving, and information pro-
cessing.
25
The WHO recommends continuation of prenatal vita-
TABLE. RECOMMENDED DAILY INTAKE OF VITAMINS AND MINERALS DURING PREGNANCY
,
ACOG CDC FDA
a
WHO
Calcium (elemental)
>19 years: 1000 mg
14-18 years: 1300 mg
N/A 1300 mg 1500-2000 mg
b
Choline N/A N/A 550 mg N/A
DHA N/A N/A N/A N/A
Folic acid (vitamin B
3
) 400 mcg before
pregnancy
600 mcg during
pregnancy
400 mcg 600 mcg 400 mcg
Iodine 200 mcg 220 mcg 290 mcg N/A
Iron (elemental) 27 mg N/A 27 mg 30-60 mg
Vitamin A
>19 years: 770 mcg
14-18 years: 750 mcg
10,000 IU 1300 mcg Only recommended in areas with
severe vitamin A deficiency
Vitamin B
6
1.9 mg N/A 2 mg Not recommended
Vitamin B
12
2.6 mcg N/A 2.8 mcg N/A
Vitamin C
>19 years: 85 mg
14-18 years: 80 mg
N/A 120 mg Not recommended
Vitamin D 15 mcg N/A 15 mcg Not recommended
Vitamin E Not recommended
unless needed to
prevent deficiency
N/A 19 mg Not recommended
ACOG indicates American College of Obstetricians and Gynecologists; DHA, docosahexaenoic acid; N/A, no recommendation available; WHO, World Health Organization.
a
Recommended intake during pregnancy.
b
Recommended intake during pregnancy with low dietary intake of calcium.
Reprinted and updated with permission from Segal K, Cieri-Hutcherson NE, Lampkin S. Recommending prenatal vitamins: a pharmacist’s guide. Pharmacy Times® website.
pharmacytimes.com/resource-centers/omega-3/recommending-prenatal-vitamins-a-pharmacists-guide. Published October 4, 2018. Accessed March 20, 2020.

PHARMACYTIMES.COM
APRIL
15
mins for at least 3 months in the postpartum period in geographic
regions with a high incidence (> 40%) of anemia in pregnancy.
26
It is recommended to increase choline intake to 550 mg daily
during lactation.
12
Continuation of prenatal supplements until the
mother has completed breastfeeding may be worthwhile if the
supplement is tolerable and affordable for the mother in light of
these data.
KEY POINTS FOR PHARMACISTS
Pharmacists can play a key role in ensuring that patients are tak-
ing appropriate prenatal and postnatal supplements—including
ensuring that patients are taking formulations that include the
vitamins and nutrients recommended by leading organizations
at appropriate dosages. Pharmacists can screen both women
using contraception and women who are actively planning to try
to get pregnant for potential supplementation needs by asking,
“Are you planning to become pregnant in the next 12 months?”
This allows prepregnancy planning to occur to ensure that
patients can try to prevent adverse health outcomes associated
with pregnancy and potential birth defects before they occur. At
a minimum, all female patients of reproductive potential should
be advised to take folic acid, even if adherent to contraception,
to reduce the risk of neural tube defects.
Selecting a prenatal vitamin can be an overwhelming task
for patients, as nutrient contents vary greatly from one prenatal
vitamin to the next and especially because there are no nutrient
standards or requirements that must be adhered to for a product
to be labeled a prenatal vitamin. Prenatal vitamins that contain
appropriate appointments of folic acid, iron, and iodine should
be targeted, and these will often contain adequate amounts of
other important nutrients such as B vitamins, calcium, copper,
DHA, vitamin A, vitamin D, vitamin E, and zinc.
27
In their 2018
study, DeSalvo and colleagues found that of the 163 OTC and
88 prescription prenatal vitamins included in the study, more
than 80% were able to correct vitamin and mineral deficiencies
in the average pregnant woman who could not get those vita-
mins and minerals from dietary intake alone.
28
Generally, these
vitamins contained recommended daily allowances for most
vitamins and minerals; however, choline, magnesium, and vita-
min D were often not provided in sufficient levels.
28
Pharmacists
should pay attention to the selection of prenatal vitamins and
ensure that they include the recommended daily allowance for
these vitamins and minerals. Alternatively, they may need to
recommend supplementation with an additional supplement to
meet these levels. ®
REFERENCES
1. When should you start taking prenatal vitamins? consider the 3-month
rule. Ritual website. ritual.com/articles/1-when-to-start-taking-prenatal-vitamins.
Accessed March 5, 2020.
2. Finer LB, Zolna MR. Declines in unintended pregnancy in the United States,
2008-2011. N Engl J Med. 2016;374(9):843-852. doi: 10.1056/NEJMsa1506575.
3. Folic acid now. CDC website. cdc.gov/Images_-_Video_and_Audio/Images/
Folic_Acid/QandAfactfolic.pdf. Published June 2003. Accessed March 5, 2020.
4. What are prenatal vitamins? Planned Parenthood website. plannedparenthood.
org/learn/pregnancy/pre-pregnancy-health/what-are-prenatal-vitamins. Accessed
March 5, 2020.
5. Fewer than half of U.S. women take recommended vitamins prior to pregnan-
cy, according to March of Dimes new prenatal health & nutritional survey. March
of Dimes website. marchofdimes.org/news/fewer-than-half-of-u-s-women-take-
recommended-vitamins-prior-to-pregnancy-according-to-march-of-dimes-new-
prenatal-health-nutrition-survey.aspx#. Published September 19, 2017. Accessed
March 5, 2020.
6. ACOG Committee opinion no. 762: prepregnancy counseling. Obstet Gynecol.
2019;133(1):e78-e89. doi: 10.1097/AOG.0000000000003013.
7. Nutrition during pregnancy. American College of Obstetricians and
Gynecologists website. acog.org/patient-resources/faqs/pregnancy/nutri-
tion-during-pregnancy. Published February 2018. Accessed March 5, 2020.
8. Institute of Medicine (US) Standing Committee on the Scientific Evaluation
of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and
Choline. Dietary reference intakes for thiamin, riboflavin, niacin, vitamin B6,
folate, vitamin B12, pantothenic acid, biotin, and choline. The National Academies
Collection: Reports funded by National Institutes of Health. Washington, DC:
National Academies Press; 1998.
9. Berg S. AMA backs global health experts in calling infertility a disease.
American Medical Association website. ama-assn.org/delivering-care/pub-
lic-health/ama-backs-global-health-experts-calling-infertility-disease. Published
June 13, 2017. Accessed March 5, 2020.
10. Bell CC, Aujla J. Prenatal vitamins deficient in recommended choline intake
for pregnant women. J Fam Med Dis Prev. 2016;2(4):048.
11. Mun JG, Legett LL, Ikonte CJ, Mitmesser SH. Choline and DHA in maternal
and infant nutrition: synergistic implications in brain and eye health. Nutrients.
2019;11(5). pii: E1125. doi: 10.3390/nu11051125.
12. Zeisel SH, da Costa KA. Choline: an essential nutrient for public health. Nutr
Rev. 2009;67(11):615-623. doi: 10.1111/j.1753-4887.2009.00246.x.
13. Zeisel SH. Nutrition in pregnancy: the argument for including a source of
choline. Int J Womens Health. 2013;5:193-199. doi: 10.2147/IJWH.S36610.
14. American Academy of Pediatrics’ Committee on Fetus and Newborn (author);
American College of Obstetricians and Gynecologists’ Committee on Obstetric
Practice (editor). Guidelines for Perinatal Care. 7th ed. Elk Grove Village, IL, and
Washington, DC: American Academy of Pediatrics and The American College of
Obstetricians and Gynecologists; 2012.
15. Folic acid for the prevention of neural tube defects: preventive medication. US
Preventive Services Task Force website. uspreventiveservicestaskforce.org/Page/
Document/RecommendationStatementFinal/folic-acid-for-the-prevention-of-neu-
ral-tube-defects-preventive-medication. Updated January 10, 2017. Accessed
March 5, 2020.
16. Food labeling: revision of the Nutrition and Supplement Facts Labels:
guidance for industry – small entity compliance guide. FDA website. fda.gov/
media/134505/download. Updated February 3, 2020. Accessed March 23, 2020.
page 19

SPECIAL REPORT: NUTRACEUTICALS
APRIL
PHARMACYTIMES.COM
16
D
ietary supplement use is common among
adults in the United States. According
to the results of the 2019 Council for
Responsible Nutrition Consumer Survey on
Dietary Supplements, 77% of Americans reported
consuming dietary supplements.
1
Data on pre-
scription drug use from the National Center for
Health Statistics (2013-2016) indicated that about
48% of Americans have used at least 1 prescription
medication in the past 30 days, and research has
shown that approximately one-third of American
adults have reported taking dietary supplements
while using prescription medications.
2,3
Although
drug interactions exist among many prescription
medications and dietary supplements, certain
nutrients may be beneficial for patients, particularly
while taking certain medications, and as frontline
providers, pharmacists are ideally positioned to
educate patients on which supplements to take with
their medications.
1,4
The following patient cases provide examples
of potential dietary supplement and medication
interactions along with counseling approaches
and suggested supplements that could be used in
such scenarios.
Case Study #1
An Adult Woman Picks Up Birth Control
Medication and Would Like to Purchase
a St John’s Wort Supplement
A 35-year-old woman arrived at the pharmacy
window to pick up her birth control medica-
tion (norethindrone acetate + ethinyl estradiol +
ferrous fumarate). She also brought a bottle of
St John’s wort to the window to add to her
purchase. After prompting from the pharmacist,
the patient stated she was taking the St John’s
wort for mild depression and stress support.
What concerns might exist regarding her current
birth control medication and this supplement?
PHARMACY PROCEDURES
The pharmacy technician who helped the woman
with her medication pickup knew to notify the
pharmacist of any other medications or supple-
ments the patient was taking. The pharmacist then
confirmed with the patient that she was not taking
any other dietary supplements. He then completed
an updated drug utilization review (DUR) with the
new information provided.
The pharmacist was then able to counsel the
patient on the potential for drug–supplement inter-
actions. He explained that St John’s wort can
interact with the birth control medication the
patient is currently prescribed, decreasing the birth
control medication’s effectiveness by increasing its
breakdown. The patient stated that she previously
received a diagnosis of mild depression but had
not followed up with her regular primary care
doctor after the initial diagnosis and had not
used any medications or other therapy.
The pharmacist then recommended that she not
initiate the St John’s wort until speaking with her
primary care provider or psychiatrist because of
the potential interaction.
5
The pharmacist advised
the patient that alternative prescription options,
as well as other therapies, could treat depression
while not decreasing the effectiveness of her birth
control. He offered to provide recommendations to
her health care provider if it was determined that a
medication option was warranted. The pharmacist
also recommended several supplements to ensure
that she receives the nutritive support she may need
while taking birth control therapy.
6
Identification and Communication Approaches
to Drug and Dietary Supplement Interactions
BY JAY HIGHLAND, PHARMD
JAY HIGHLAND, PHARMD
FEATURE
© JACKF / ADOBE STOCK

PHARMACYTIMES.COM
APRIL
17
After the pharmacist provided the information, the patient
appreciated that he took the time to alert her to the potential
interaction and offered to speak with her health care provider
about alternative options, if necessary. The patient was receptive
to following up with her primary care provider. The pharmacist
contacted the patient by phone a few days after their discussion;
she had seen her primary care provider, and they had determined
she would try counseling to help her cope with her mild depres-
sion and stress, without initiating prescription medication at this
time. Because of the recommendations made by her pharmacist
and the subsequent discussion with her prescriber, she also initi-
ated supplements to reach the total recommended daily value of
folic acid, magnesium, and vitamin B
6
, as these nutrients may be
depleted after chronic use of birth control.
7,8
Case Study #2
An Elderly Patient Prescribed Digoxin Inquires About
Hawthorn Supplements
A 75-year-old man approached the pharmacy drop-off window
with a prescription for digoxin and requested to speak with the
pharmacist about a hawthorn supplement he also brought
to the counter.
What concerns might exist regarding digoxin and this
supplement?
PHARMACY PROCEDURES
The patient explained to the pharmacist that his wife had read
an article online saying that hawthorn could be helpful for
patients with heart failure like himself. Because the pharmacist
was not familiar with the hawthorn supplement, she consulted
an online medication profile and drug interaction checker. She
also reviewed the patient’s comprehensive medication list in his
patient profile in the pharmacy fulfilment system (
table) and
confirmed with the patient that the list was current.
The online medication profile and drug interaction checker
confirmed that hawthorn may enhance the activity of digoxin,
which is a medication with an already-narrow therapeutic index.
The pharmacist explained to the patient that hawthorn use could
potentially cause digoxin to reach toxic levels in his body, and
so hawthorn would be emphatically not recommended. Further,
the pharmacist told him that his chronic digoxin therapy could
possibly deplete his nutritional stores of calcium, magnesium,
and potassium. She suggested supplement dosing to be sure he
got the total daily recommended values.
7,8
The patient was surprised by this information. He was unaware
that supplements could interact with his prescription medications,
and he thanked the pharmacist for alerting him. The pharmacist
advised the patient to always inform health care providers of any
herbal or other supplements he takes, or might be interested in
taking, to help prevent future drug interactions.
CASE STUDIES DISCUSSION
Pharmacists can meaningfully impact the prevention of drug–
supplement interactions and the complications that interactions
may cause. As frontline health care providers, pharmacists are
ideally positioned to provide guidance and education when
TABLE. CASE STUDY : PATIENT’S CURRENT MEDICATION LIST
Drug Dose Directions
Losartan 50 mg Take 1 tablet by mouth daily in the evening.
Metoprolol succinate 100 mg Take 1 tablet by mouth daily.
Spironolactone 25 mg Take 1 tablet by mouth daily.
Furosemide 20 mg Take 1 tablet by mouth daily in the morning.
Digoxin 0.125 mg Take 1 tablet by mouth daily.
Potassium chloride 20 mEq Take 1 tablet by mouth twice daily with food.
Atorvastatin 40 mg Take 1 tablet by mouth daily.
page 19
© IVAN / ADOBE STOCK

SPECIAL REPORT: NUTRACEUTICALS
APRIL
PHARMACYTIMES.COM
18
| page 5, from ‘The Role of Nutraceuticals: What Pharmacists Need to Know in 2020’
supplement use in the resident, civilian, noninstitutionalized US population,
National Health and Nutrition Examination Survey. J Nutr. 2018;148(suppl
2):1436S-1444S. doi: 10.1093/jn/nxy093.
18. Hemila H, Chalker. E. The effectiveness of high dose zinc acetate lozenges on
various common cold symptoms: a meta-analysis. BMC Fam Pract. 2015;16:24.
doi: 10.1186/s12875-015-0237-6.
19. Shadkam MN, Mozaffari-Khosravi H, Mozayan MR. A comparison of the
effect of honey, dextromethorphan, and diphenhydramine on nightly cough
and sleep quality in children and their parents. J Altern Complement Med.
2010;16(7):787-793. doi: 10.1089/acm.2009.0311.
20. The common cold and complementary health approaches: what the science
says. National Center for Complementary and Integrative Health website. nccih.
nih.gov/health/providers/digest/cold-science#heading1. Updated January 17,
2020. Accessed March 9, 2020.
21. Linde K, Kriston L, Rücker G, et al. Efficacy and acceptability of pharmaco-
logic treatments for depressive disorders in primary care: systematic review and
network meta-analysis. Ann Fam Med. 2015;13(1):69-79. doi: 10.1370/afm.1687.
22. Depression and complementary health approaches: what the science says.
National Center for Complementary and Integrative Health website. nccih.nih.
gov/health/providers/digest/depression-science#heading3. Updated October 17,
2019. Accessed March 9, 2020.
23. Picciano MF, McGuire MK. Use of dietary supplements by pregnant and
lactating women in North America. Am J Clin Nutr. 2009;89(2):663S-667S. doi:
10.3945/ajcn.2008.26811B.
24. Pregnancy nutrition. American Pregnancy Association website. american-
pregnancy.org/pregnancy-health/pregnancy-nutrition/. Accessed March 9, 2020.
25. Dietary supplements. National Institute on Aging website. nia.nih.gov/health/
dietary-supplements. Reviewed November 20, 2017. Accessed March 9, 2020.
26. DRI calculator for health professionals. US Department of Agriculture web-
site. www.nal.usda.gov/fnic/dri-calculator/. Accessed March 9, 2020.
27. Using dietary supplements wisely. National Center for Complementary and
Integrative Health website. nccih.nih.gov/health/supplements/wiseuse.htm#hed3.
Updated March 12, 2020. Accessed March 16, 2020.
28. Herbal medicine. US National Library of Medicine website. medlineplus.gov/
herbalmedicine.html. Updated March 2, 2020. Accessed March 9, 2020.
29. Probiotics: what you need to know. National Center for Complementary
and Integrative Health website. nccih.nih.gov/health/probiotics/introduction.htm.
Updated August 2019. Accessed March 9, 2020.
30. Mohn ES, Kern HJ, Saltzman E, Mitmesser SH, McKay DL. Evidence of
drug-nutrient interactions with chronic use of commonly prescribed medica-
tions: an update. Pharmaceutics. 2018;10(1). pii:E36. doi: 10.3390/pharmaceu-
tics10010036.
ABOUT THE AUTHOR
LUMA MUNJY, PHARMD, is an assistant professor of pharmacy practice, Chapman
University School of Pharmacy, Irvine, California.
| page 10, from ‘To D or Not to D: That Is the Question’
2017;8(11):464-474. doi: 10.4239/wjd.v8.i11.464.
11. Parrott J, Frank L, Rabena R, Craggs-Dino L, Isom KA, Greiman L. American
Society for Metabolic and Bariatric Surgery integrated health nutritional guide-
lines for the surgical weight loss patient 2016 update: micronutrients. Surg Obes
Relat Dis. 2017;13(5):727-741. doi: 10.1016/j.soard.2016.12.018.
12. Vaidya A, Williams JS. The relationship between vitamin D and the renin-an-
giotensin system in the pathophysiology of hypertension, kidney disease, and
diabetes. Metabolism. 2012;61(4):450-458. doi: 10.1016/j.metabol.2011.09.007.
13. de Boer IH, Zelnick LR, Ruzinski J, et al. Effect of vitamin D and omega-3 fatty
acid supplementation on kidney function in patients with type 2 diabetes: a random-
ized clinical trial. JAMA. 2019;322(19):1899-1909. doi: 10.1001/jama.2019.17380.
14. Angellotti E, D’Alessio D, Dawson-Hughes B, et al. Vitamin D supplementa-
tion in patients with type 2 diabetes: the Vitamin D for Established Type 2 Diabetes
(DDM2) study. J Endocr Soc. 2018;2(4):310-321. doi: 10.1210/js.2018-00015.
ABOUT THE AUTHORS
CHELSEA RENFRO, PHARMD, CHSE, is an assistant professor in the Department of
Clinical Pharmacy and Translational Science at The University of Tennessee Health Science
Center in Memphis, Tennessee.
ALEX STANLEY is a PharmD candidate at The University of Tennessee Health Science Center.
| page 12, from ‘Omega-3 Recommendations: Counseling Points for Pharmacists’
genomic era: a blooming reservoir for new natural products. FEMS Microbiol
Rev.2012;36(4):761-785. doi: 10.1111/j.1574-6976.2011.00304.x.
8. Lane K, Derbyshire E, Li W, Brennan C. Bioavailability and potential uses of
vegetarian sources of omega-3 fatty acids: a review of the literature. Crit Rev Food
Sci Nutr. 2014;54(5):572-579. doi: 10.1080/10408398.2011.596292.
9. Omega-3 fatty acids: fact sheet for health professionals. Office of Dietary
Supplements. National Institutes of Health website. ods.od.nih.gov/factsheets/
Omega3FattyAcids-HealthProfessional/#en78. Updated October 17, 2019.
Accessed March 5, 2020.
10. Goldberg RJ, Katz J. A meta-analysis of the analgesic effects of omega-3
polyunsaturated fatty acid supplementation for inflammatory joint pain. Pain.
2007;129(1-2):210-223. doi: 10.1016/j.pain.2007.01.020.
11. Dietary guidelines for Americans 2015-2020. Department of Health & Human
Services website. health.gov/sites/default/files/2019-09/2015-2020_Dietary_
Guidelines.pdf. Published December 2015. Accessed March 25, 2020.
ABOUT THE AUTHOR
BRADY COLE, RPH, is pharmacy manager at Tom Thumb Pharmacy in Plano, Texas, and an
active preceptor at Texas Tech University and the University of Houston. He is also the founder
of the website Helpful Pharmacist (helpfulpharmacist.com).

PHARMACYTIMES.COM
APRIL
19
| page 15, from ‘Prenatal and Postnatal Supplementation: What Do Pharmacists Need to Know?’
17. Second national report on biochemical indicators of diet and nutrition in the
U.S. population. CDC website. cdc.gov/nutritionreport/pdf/Nutrition_Book_com-
plete508_final. pdf. Published 2012. Accessed March 5, 2020.
18. WHO recommendations on antenatal care for a positive pregnancy expe-
rience. World Health Organization website. apps.who.int/iris/bitstream/han-
dle/10665/250800/WHO-RHR-16.12-eng.pdf?sequence=1. Published 2016.
Accessed March 5, 2020.
19. Segal K, Cieri-Hutcherson NE, Lampkin S. title. Recommending prenatal
vitamins: a pharmacist’s guide. Pharmacy Times
®
website. pharmacytimes.com/
resource-centers/omega-3/recommending-prenatal-vitamins-a-pharmacists-guide.
Published October 4, 2018. Accessed March 20, 2020.
20. Fish oil. Mayo Clinic website. mayoclinic.org/drugs-supplements-fish-oil/art-
20364810. Published October 24, 2017. Accessed March 13, 2020.
21. Nguyen P, Thomas M, Koren G. Predictors of prenatal multivitamin
adherence in pregnant women. J Clin Pharmacol. 2009;49(6):735-742. doi:
10.1177/0091270009333487.
22. Maternal diet: diet considerations for breastfeeding mothers. CDC website.
cdc.gov/breastfeeding/breastfeeding-special-circumstances/diet-and-micronutri-
ents/maternal-diet.html. Updated February 10, 2020. Accessed March 5, 2020.
23. Postnatal vitamins while breastfeeding. American Pregnancy Association
website. americanpregnancy.org/breastfeeding/postnatal-vitamins-while-breast-
feeding/. Updated October 13, 2019. Accessed March 5, 2020.
24. ACOG Committee Opinion No. 736: optimizing postpartum care. Obstet
Gynecol. 2018;131(5):e140-e150. doi: 10.1097/AOG.0000000000002633.
25. Morse NL. Benefits of docosahexaenoic acid, folic acid, vitamin D and
iodine on foetal and infant brain development and function following maternal
supplementation during pregnancy and lactation. Nutrients. 2012;4(7):799-840.
doi: 10.3390/nu4070799.
26. World Health Organization, Department of Making Pregnancy Safer and
Department of Reproductive Health and Research. Standards for maternal and
neonatal care: iron and folate supplementation. World Health Organization
website. who.int/reproductivehealth/publications/maternal_perinatal_health/iron_
folate_supplementation.pdf. Published 2006. Accessed March 5, 2020.
27. Haack V. Prenatal vitamins – topic of the month. Minnesota Department of
Health website. www.health.state.mn.us/docs/people/wic/localagency/wedupdate/
moyr/2017/01jan/18/vitamins.pdf. Published January 18, 2017. Accessed March
5, 2020.
28. DeSalvo K, Stamm CA, Borgelt LM. Evaluation of reported contents in pre-
scription and over-the-counter prenatal multivitamins. J Am Pharm Assoc (2003).
2018;58(3):258-267.e3. doi: 10.1016/j.japh.2018.02.006.
ABOUT THE AUTHOR
CORTNEY MOSPAN, PHARMD, BCACP, BCGP, is an assistant professor of
pharmacy at the Wingate University Levine College of Health Sciences in Wingate, North
Carolina, and a clinical pharmacist practitioner at the Novant Health Arboretum Family & Sports
Medicine/Internal Medicine in Charlotte, North Carolina.
situations like the ones described above occur. They can remind
patients and/or their caregivers about the importance of alerting
their health care providers to any supplements they take.
Proper education of the entire pharmacy staff to check
for missing information or missing medications in a patient
profile can help to provide comprehensive care and ensure the
best patient outcomes. This can be achieved through system-
atic approaches—for instance, during all medication pickups,
inquiring about allergies and about medications and herbal
supplements that may not be on file. Documenting this
information will make the patient’s profile more complete and
will make future DURs more efficient. ®
REFERENCES
1. Dietary supplement use reaches all time high. Council on Responsible
Nutrition website. crnusa.org/newsroom/dietary-supplement-use-reaches-all-
time-high-available-purchase-consumer-survey-reaffirms. Published September
30, 2019. Accessed March 26, 2020.
2. Therapeutic drug use. CDC website. cdc.gov/nchs/fastats/drug-use-therapeu-
tic.htm. Reviewed January 19, 2017. Accessed March 1, 2020.
3. Farina EK, Austin KG, Lieberman HR. Concomitant dietary supplement
and prescription medication use is prevalent among US adults with doctor-in-
formed medical conditions. J Acad Nutr Diet. 2014;114(11):1784-1790.e2. doi:
10.1016/j.jand.2014.01.016.
4. Meletis CD, Zabriskie N. Common nutrient depletions caused by pharmaceuti-
cals. Alt Comp Ther. 2007;13(1):10-17. doi: 10.1089/act.2006.13102.
5. Horn JR, Hansten PD. Oral contraceptives and St. John’s wort. Pharmacy
Times
®
website. pharmacytimes.com/publications/issue/2018/January2018/oral-con-
traceptives-and-st-johns-wort. Published February 2, 2018. Accessed March 2, 2020.
6. Prescott JD, Drake VJ, Stevens JF. Medications and micronutrients: identi-
fying clinically relevant interactions and addressing nutritional needs. J Pharm
Technol. 2018;34(5):216-230. doi: 10.1177/8755122518780742.
7. Weitzel KW, Goode J-VR. Constipation. In: Krinsky DL, Ferreri SP,
Hemstreet B, et al. Handbook of Nonprescription Drugs: An Interactive
Approach to Self-Care. 19th ed. New York, NY: Humana Press; 2017:chapter
15. doi: 10.21019/9781582122656.ch15.
8. Food labeling: revision of the Nutrition and Supplement Facts Labels:
guidance for industry – small entity compliance guide. FDA website. fda.gov/
media/134505/download. Updated February 3, 2020. Accessed March 23, 2020.
ABOUT THE AUTHOR
JAY HIGHLAND, PHARMD, is a patient care pharmacist and residency coordinator at
Jewel-Osco Pharmacy in Chicago, Illinois.
| page 17, from ‘Identification and Communication Approaches to Drug and Dietary Supplement Interactions’

NOTES

NOTES
Page 1
©2019 Pharmavite LLC
Purpose: For educational use by healthcare professionals only.
Disclaimer: People taking prescription drugs may be more likely to have reduced levels of certain nutrients. Low nutrient levels may lead to other problems. Prescriptions are
important to the consumer’s health and will function without the recommended dietary supplements. The dietary supplements mentioned here are not intended to replace
prescription drugs. It is important to advise consumers to consult with their healthcare provider before beginning a dietary supplement regimen.
DND = Drug Nutrient Depletion
General Recommendation for all Categories: Daily Multivitamin
Common Drug Classes, Drug-Nutrient Depletions, & Drug-Nutrient Interactions
Pharmavite LLC
DRUG CATEGORY
Drug Category Brief Description Drug-Induced Nutrient Depletions
Additional Suggested Supplements
for Nutritional Support*
Dietary Supplements that
have Potential for Interactions
with Drug (or Drug Class)**
1. ACID-SUPPRESSING
DRUGS and ANTACIDS
1–5
Ex: Nexium
®
, Pepcid
®
, Prevacid
®
,
Prilosec
®
, Tagamet
®
and others
1. H2 antagonists block histamine (H2)
receptors on gastric mucosal cells and
decrease the production and secretion
of acid.
2. Proton-Pump Inhibitors block the acid
transporter pump on the luminal surface
preventing acid from entering the gastric
lumen.
3. Antacids directly neutralize existing acid
in the stomach.
DND:
H2 antagonists deplete calcium, folic
acid, iron, vitamin B
12
, and vitamin D.
Proton-pump inhibitors deplete
magnesium and vitamin B
12
.
RECOMMENDED SUPPLEMENTATION:
• H2 antagonists and proton-pump
inhibitors:
* Vitamin B
12
: 25–1000 mcg/day
* Magnesium: 250–400 mg/day
Calcium: 500 mg daily
Iron
a
: discuss with healthcare provider.
Vitamin D
b
: 25-50 mcg (1000-2000 IU) daily
Vitamin C - with H. pylori
C
: 250-500 mg/day
Zinc
d
: 15 mg daily
Goldenseal and Ginger:
These supplements may increase
stomach acid and thus might interfere
with antacids, H2 antagonists, and proton
pump inhibitors.
Green Tea:
Tagamet
®
(cimetidine) can inhibit the
metabolism of caffeine in green tea and
significantly reduce its clearance.
2. ANTIBIOTICS
1–4,6
Ex: Amoxil
®
, Bactrim
®
, Ceclor
®
,
Cipro
®
, Levaquin
®
and others
Antibiotics are used to treat bacterial
infections.
DND:
Antibiotics deplete calcium,
magnesium, potassium as well as
certain B vitamins (B
1
-thiamin, B
2
-
riboflavin, B
3
-niacin, B
5
-pantothenic
acid, B
6
, B
9
-folic acid, B
12
) and vitamin K.
RECOMMENDED SUPPLEMENTATION:
• Calcium: 500–1000 mg daily in
divided doses
• Magnesium: 250–400 mg daily
Calcium, Iron, Magnesium, and Zinc:
When taken concurrently with antibiotics,
absorption of both can be affected due to
formation of insoluble complexes.
Green Tea Catechins:
Certain antibiotics (fluoroquinolones)
reduce clearance of some green tea
constituents (caffeine and theophylline)
and may increase the risk of their side
effects: nervousness, palpitations, and
insomia.
St. John’s wort:
It causes photosensitivity and may
exacerbate the photosensitizing effects of
certain antibiotics.
3. ANTIDEPRESSANTS
1–3, 6–7
(continued page 2)
Ex: Cymbalta
®
, Lexapro
®
, Paxil
®
,
Prozac
®
, Zoloft
®
and others
This class of medications increases the
levels of one or more of the biogenic
amines (e.g. norepinephrine, serotonin,
dopamine) in the central nervous system.
Clinical improvement from antidepressant
therapy generally takes 3–6 weeks.
Calcium
e
: 500-100 mg/day
Vitamin D
e
: 25-50 mcg (1000-2000 IU) daily
Folic acid
f
: 240 mcg daily
Melatonin:
Melatonin may interact with medications
that inhibit serotonin reuptake including
a number of antidepressant medications.
Endogenous melatonin levels are reduced
by SSRI medications.
Page 2
©2019 Pharmavite LLC
DRUG CATEGORY
Drug Category Brief Description Drug-Induced Nutrient Depletions
Additional Suggested Supplements
for Nutritional Support*
Dietary Supplements that
have Potential for Interactions
with Drug (or Drug Class)**
3. ANTIDEPRESSANTS
1–3, 6–7
(continued from page 1)
SAM-e:
Studies suggest SAM-e may augment
the actions of anti-depressant drugs
in individuals who are refractory to,
or do not get full remission from their
anti-depressants.
St. John’s wort and 5-HTP:
St. John’s wort and other supplements
such as 5-HTP, in combination with drugs
that increase CNS serotonin levels, can
increase the risk of serotonergic side
effects, including serotonin syndrome.
4. ANTIEPILEPTICS
1–3
(Anticonvulsants)
Ex: Dilantin
®
, Lyrica
®
, Mysoline
®
,
Tegertol
®
, Trileptal
®
and others
These drugs work by decreasing the firing
of aberrant neurons in the brain and/or
decreasing the spread of abnormal activity
to the surrounding regions of the brain.
Calcium
g
: 500 mg daily
Vitamin D
g
: 25–50 mcg (1000–2000 IU) daily
Vitamin B
12
h
: 25–1000 mcg daily
Use caution with the following
supplements since they may interfere with
the effectiveness of antiepileptic drugs.
Folic acid
Gingko biloba
Niacin
St. John’s wort
5. ANTIPSYCHOTICS
1–3
(continued page 3)
Ex: Abilify
®
, Haldol
®
, Seroquel
®
,
Risperdal
®
, Zyprexa
®
and others
Antipsychotics block receptors for
neurotransmitters (i.e. dopamine,
serotonin). They can reduce the symptoms
of schizophrenia, decrease agitation
and/or aggression associated with other
psychiatric conditions and may stabilize
mood in bipolar disease.
DND:
Vitamin B
2
(Riboflavin)
RECOMMENDED SUPPLEMENTATION:
• Daily Multivitamin
• B Vitamins
Vitamin C
i
: 250–500 mg daily Echinacea:
Echinacea may inhibit the human drug
metabolizing enzyme CYP1A2 leading to
decreased clearance (increased blood
levels) of Zyprexa
®
, and this increases
potential for side effects.
Evening Primrose Oil:
Seizures have been reported in people
with schizophrenia treated concomitantly
with phenothiazine drugs and evening
primrose oil.
Ginkgo biloba:
Ginkgo has been report to cause seizures
or lower seizure threshold. Thus, in
combination with drugs that lower seizure
threshold (including antipsychotics), there
may be a significant increase in risk of
seizures.
Ginseng:
Ginseng may exacerbate some
psychiatric conditions including hysteria,
mania, and schizophrenia and thus
compromise the therapeutic benefit
of antipsychotics. It may also inhibit
some of the drug metabolizing enzymes
responsible for clearance of antipsychotic
drugs.
Page 3
©2019 Pharmavite LLC
DRUG CATEGORY
Drug Category Brief Description Drug-Induced Nutrient Depletions
Additional Suggested Supplements
for Nutritional Support*
Dietary Supplements that
have Potential for Interactions
with Drug (or Drug Class)**
5. ANTIPSYCHOTICS
1–3
(continued from 3)
Goldenseal:
Goldenseal can inhibit cytochrome
P450 2D6 (CYP2D6) and might affect
effectiveness of several antipsychotics as
well as impact potential for side effects.
St. John’s wort:
St. John’s wort in combination with
antipsychotic drugs may lead to
unpredictable effects. It is also known to
cause photosensitivity and this risk may
be increased in combination with certain
antipsychotics (phenothiazines), which
also can cause photosensitivity.
6. ANXIETY MEDICATION
1–3
(Benzodiapezines)
Ex: Ativan
®
, Prosom
®
, Restoril
®
Valium
®
, Xanax
®
and others
Benzodiazepines are a class of drugs
primarily used to treat anxiety.
DND:
Calcium
These medications decrease calcium
absorption by increasing metabolism of
vitamin D, which is needed for calcium
absorption.
RECOMMENDED SUPPLEMENTATION:
• Calcium: 500–1000 mg daily in
divided doses
Melatonin
j
: 1–3 mg daily Kava:
The combination of kava and
benzodiazepines is not recommended
due to their similar effects.
7. BIRTH CONTROL
1–3
(Oral Contraceptives)
Synthetic and semi-synthetic analogs of
estrogen and/or progesterone are used
to prevent pregnancy by (1) inhibiting
ovulation, (2) thickening cervical mucus
and/or (3) diminishing endometrial integrity.
DND:
Folic acid
Magnesium
Vitamin B
6
RECOMMENDED SUPPLEMENTATION:
• Folic acid: 240 mcg daily
• Magnesium: 250–400 mg daily
• Vitamin B
6
: 2-5 mg daily
Calcium
k
: 500 mg daily
Vitamin B12
l
: 25–1000 mcg/day
Copper and Iron:
Oral contraceptives may increase serum
copper and iron levels.
Garlic and St. John’s wort:
Garlic and St. John’s wort supplements
may decrease effectiveness of oral
contraceptives. St. John’s wort also
causes photosensitivity which may be
exacerbated by oral contraceptives.
Green Tea:
Use caution with green tea and oral
contraceptives. Oral contraceptives can
decrease caffeine clearance by 40–65%
and may increase adverse effects of
caffeine in green tea. Adjust dose or
discontinue if necessary.
Page 4
©2019 Pharmavite LLC
DRUG CATEGORY
Drug Category Brief Description Drug-Induced Nutrient Depletions
Additional Suggested Supplements
for Nutritional Support*
Dietary Supplements that
have Potential for Interactions
with Drug (or Drug Class)**
8. BLOOD PRESSURE
MEDICATION
1–3,8
(Anti-hypertensives)
Ex: ACE Inhibitors, Angiotensin
Receptor Blockers (ARBs), Beta
Blockers, Calcium Channel
Blockers.
The major classes of anti-hypertensive
drugs include: ACE inhibitors, ARBs, beta
blockers, and calcium channel blockets.
These drugs help reduce blood pressure
by either decreasing total peripheral
resistance, or cardiac output or both.
DND:
ACE inhibitors deplete zinc.
Calcium channel blockers deplete
potassium.
RECOMMENDED SUPPLEMENTATION:
• ACE inhibitors- Zinc: 15 mg daily
• Calcium channel blockers-Potassium:
≤ 100 mg daily
CoQ10
m
: 100–200 mg daily
Iron
n
: Take as directed by
healthcare provider
Calcium (with calcium channel blockers only):
Calcium supplements may interfere with
the blood pressure lowering activity of
these drugs.
CoQ10 and Fish Oil:
These supplements may decrease
blood pressure in combination with
anti-hypertensive drugs. Monitor blood
pressure regularly.
Garlic, Ginkgo biloba & St. John’s wort:
These supplements have the potential
to interfere with the cytochrome
P450 system and therefore affect the
metabolism and/or clearance of drugs.
Green Tea and Goldenseal:
These supplements may affect therapeutic
benefits of anti-hypertensive drugs.
Melatonin:
Melatonin may impair the efficacy of
some calcium channel blockers. Monitor
for changes in therapeutic efficacy and
adjust doses as necessary and/or avoid
use of melatonin with this drug class.
Potassium (with ACE inhibitors and ARBs only):
Taking these drugs along with potassium
supplements increases risk for
hyperkalemia due to a decrease in renal
potassium excretion.
Vitamin D:
Vitamin D supplements interfere with
the activity of a calcium channel blocker
(verapamil).
9. BLOOD THINNING
MEDICATION
1–3
(Anticoagulants/Antiplatelets)
(continued page 5)
Ex: Aspirin, Coumadin
®
(Warfarin),
Plavix
®
, Ticlid
®
and others.
1. Anticoagulants decrease the potential
for clotting via the Prothrombin-
Thrombin-Fibrinogen cascade.
2. Antiplatelets decrease potential for
clots as a result of impacting platelet
aggregation.
Use caution with the following
supplements as they may increase
effectiveness of medication and potentially
increased risk of bleeding.
Bilberry
Cod Liver Oil
Dong Qual
Evening Primrose Oil
Feverfew
Fish Oil
Flaxseed Oil
Garlic
Ginger Root
Ginkgo biloba
Ginseng
Glucosamine
Goldenseal
Grape Seed Extract
Green Tea
Horse Chestnut
Milk Thistle
Saw Palmetto
Vitamin C
Vitamin E
Page 5
©2019 Pharmavite LLC
DRUG CATEGORY
Drug Category Brief Description Drug-Induced Nutrient Depletions
Additional Suggested Supplements
for Nutritional Support*
Dietary Supplements that
have Potential for Interactions
with Drug (or Drug Class)**
9. BLOOD THINNING
MEDICATION
1–3
(Anticoagulants/Antiplatelets)
(continued from page 4)
Vitamin K:
People taking anticoagulant medications
should maintain consistent amount of
vitamin K from their diet and supplement
regimen, while avoiding fluctuations in
intake or large doses of vitamin K.
Coenzyme Q10 (CoQ10):
CoQ10 is structurally similar to vitamin K
and my interfere with effectiveness of
anticoagulants.
10. CHOLESTEROL
LOWERING MEDICATION
(Statins)
1–3
Ex: Crestor
®
, Lescol
®
, Lipitor
®
,
Mevacor
®
, Zocor
®
and others
Statins inhibit the HMG CoA reductase
enzyme–a key step in the hepatic synthesis
of cholesterol. The reduction of cholesterol
synthesis subsequently increases
the liver’s removal of circulating LDL
cholesterol.
Note: HMG CoA reductase is also a key
enzyme in the synthesis of coenzyme Q10
(CoQ10)
DND:
CoQ10
RECOMMENDED SUPPLEMENTATION:
• CoQ10: 100–200 mg/day
Vitamin D
o
: 25–50 mcg (1000-2000 IU) daily
Fish Oil
p
: 500–1000 mg EPA + DHA daily
Garlic (containing allicin) and
St. John’s wort:
These supplements may impact
cytochrome P450 metabolism of some
statins and affect their effectiveness.
Red Yeast Rice:
Red yeast rice contains lovastatin which
also lowers blood cholesterol levels. This
supplement should not be taken with
cholesterol-lowering drugs unless under
the supervision of healthcare professional.
Vitamin A:
Long term use of cholesterol lowering
drugs may increase vitamin A levels in the
blood. Vitamin A levels may need to be
monitored in some individuals.
11. CORTICOSTEROIDS
2–3
Ex: Prednisone
Corticosteroids are synthetic compounds
that mimic the effects of hormones
naturally produced in the body by adrenal
glands. They are known for relieving
inflammation, pain and discomfort resulting
from various health conditions
DND:
Calcium
Vitamin D
Magnesium
RECOMMENDED SUPPLEMENTATION:
• Calcium: 500 mg daily
• Vitamin D: 25–50 mcg (1000–2000 IU) daily
• Magnesium: 250–400 mg daily
Use caution with the following
supplements as they may interact with
and/or affect effectiveness of medication.
Herbal Supplements
Licorice
St. John’s wort
12. DIABETES MEDICATION
(Oral Hypoglycemics)
1–3,10–11
Ex: Avandia
®
, Diabeta
®
,
Glucophage
®
(Metformin),
Prandin
®
, and others
DND:
Folic acid
Vitamin B
12
RECOMMENDED SUPPLEMENTATION:
• Folic acid: 120–240 mcg daily
• Vitamin B
12
: 25–1000 mcg daily
Use caution with the following
supplements as they may interfere with
the effectiveness of oral hypoglycemic
drugs and/or cause additive blood glucose
lowering effects and increase risk of
hypoglycemia when used in combination.
Alfalfa
Aloe Vera
Alpha Lipoic Acid
Bilberry
CoQ10
Chromium
Garlic
Ginkgo biloba
Ginseng
Green Tea
Melatonin
Milk Thistle
Niacin
St. John’s wort
Vitamin K
1
Page 6
©2019 Pharmavite LLC
DRUG CATEGORY
Drug Category Brief Description Drug-Induced Nutrient Depletions
Additional Suggested Supplements
for Nutritional Support*
Dietary Supplements that
have Potential for Interactions
with Drug (or Drug Class)**
13. DIGIOXIN
1–3
Ex: Cardoxin
®
, Digitek
®
,
Lanoxicaps
®
, Lanoxin
®
and others
Digoxin is derived from the leaves of
the Digitalis lantata plant (a variety of
foxglove). It is used to treat heart failure
and atrial fibrillation.
DND:
Calcium
Magnesium
Phosphorus
Potassium
Vitamin B
1
(Thiamin)
RECOMMENDED SUPPLEMENTATION:
• Calcium: 500–1000 mg daily in
divided doses
• Magnesium: 250–400 mg daily
• Potassium: ≤ 100 mg daily
Calcium:
High levels of calcium increase the
likelihood of a toxic reaction to digoxin.
Low levels of calcium interfere with the
function of digoxin. Consistent intake
of calcium and monitoring of calcium
levels by a healthcare professional is
recommended.
Hawthorn:
The activity of digoxin may be enhanced
by hawthorn supplements.
St. John’s wort:
St. John’s wort supplements may reduce
serum levels of digoxin.
14. DIURETICS
1–3,9
Ex: Aldactone
®
, Diamox
®
, Lasix
®
,
Microzide
®
(HCTZ), Zaroxolyn
®
and
others
DND:
Loop diuretics (especially furosemide)
can increase calcium excretion and
decrease calcium status. Thiazide
diuretics deplete magnesium,
potassium, and zinc.
Potassium sparing diuretics deplete
folic acid.
RECOMMENDED SUPPLEMENTATION:
Loop Diuretics
Calcium: 500-1000 mg/day
and Thiazide Diuretics
Magnesium: 250-400 mg/daily
Potassium: ≤100 mg daily
Zinc: 15 mg daily
Potassium-Sparing Diuretics
Folic acid: 240 mcg daily
Calcium:
Thiazide diuretics reduce calcium
excretion by the kidneys and may
increase risk for hypercalcemia, metabolic
alkalosis, and possible renal failure.
CoQ10 and Fish Oil:
When taken together with diuretics, these
supplements may have additive blood
pressure lowering effects and increase
risk for hypotension.
Ginkgo biloba:
Ginkgo may reduce the effectiveness of
some diuretics.
15. HORMONE
REPLACEMENT THERAPY
(Estrogens)
3
Ex: Estrace
®
, Premarin
®
, Prempro
®
Hormone replacement therapy is used
to replace female hormones that are no
longer produced after menopause.
DND:
Folic acid
Magnesium
Vitamin B
6
Vitamin B
12
RECOMMENDED SUPPLEMENTATION:
• Folic acid: 240 mcg daily
• Magnesium: 250–400 mg daily
• Vitamin B
6
: 2-5 mg daily
• Vitamin B
12
: 25–1000 mcg daily
Caffeine:
The stimulating effects of caffeine may be
increased due to inhibition of metabolism
or clearance of caffeine by hormone
replacement therapy.
Calcium and Vitamin D:
Calcium and vitamin D may increase
absorption of hormone replacements.
These supplements are recommended
to improve bone mineral density during
estrogen therapy.
Red Clover Extract and Soy Isoflavones:
These supplements may interfere with
the activity or absorption of hormone
replacement therapy.
St. John’s wort:
St. John’s wort may alter hormone
metabolism including estrogen and
progesterone. This supplement is
not recommended during hormone
replacement therapy.
Zinc and Magnesium:
Excretion of these minerals is reduced by
hormone replacement therapy.

Page 7
©2019 Pharmavite LLC
RN 126418
†Additional references available upon request.
*Suggested supplements that may support overall health and are not at all intended to replace
any prescription medications.
**These supplements listed may have the potential to interact with the drug or drug classes. Use
caution or avoid these supplements unless approved by your physician or preferred healthcare
provider.
a. Iron may be affected H2 antagonists in those with elevated risk/pre-existing iron deficiency.
However, iron is not recommended to be routinely supplemented while taking H2 antagonists.
High levels of iron can cause unnecessary oxidative stress and other undesirable effects. Iron
supplementation is only recommended for those with the effects of iron depletion (i.e. anemia).
b. Vitamin D is important for calcium absorption.
c. PPI use may be associated with reduced serum /plasma levels of vitamin C in patients
with H. pylori infection.
d. Zinc may be affected by H2 affected by H2 antagonists. However, zinc supplementation
may not be recommended for all individuals. One should consult their health care provider
on the best option for supplementation and consider health status, health history, and current
medication use.
e. An association between SSRI use and risk for osteoporosis has been established. In
addition, SSRI’s may impact bone formation and resorption through serotonin receptors.
f. Observational data have shown lower folate status in patients with major depressive disorder
(MDD), compared to healthy controls. Discuss supplementation with your physician or preferred
health care professional, especially if on SSRI antidepressant therapy.
g. Dilantin, Phenobarbital, and Tegretol can increase the metabolism/clearance of vitamin D,
leading to a subsequent decrease of calcium absorption. Individuals taking these medications
for 6 months or more should consider calcium and vitamin D supplements.
h. Dilantin, Phenobarbital, and Mysoline have been reported to reduce vitamin B
12
absorption
as well as serum and cerebrospinal fluid vitamin B
12
levels in some individuals. Megaloblastic
anemia and neuropsychiatric side effects have been associated with these drugs.
i. Vitamin C taken in adjunct with atypical antipsychotics may reduce oxidative stress.
j. Endogenous melatonin is depleted by benzodiazepines.
k. Calcium supplementation may be warranted with oral contraceptive use to help support bone
health if dietary calcium intake is inadequate.
l. Serum levels of vitamin B
12
have shown to be lower in those using oral contraceptives
compared to non-users. Supplementation may be a consideration for individuals already at risk
for low vitamin B
12
status or a deficiency, such as vegetarians.
m. Beta blockers can deplete CoQ10.
n. Low dose ferrous sulfate supplements may help alleviate ACE inhibitor-related cough.
o. Consider supplementing with vitamin D. Fat soluble vitamins (vitamins A, D, E, K) may be
affected by medication use.
p. EPA and DHA omega-3 fatty acids help support heart health.
Sources:
†
1. Mohn ES, Kern HJ, Saltzman E, Mitmesser SH, and McKay DL. Evidence of Drug-
Nutrient Interactions with Chronic Use of Commonly Prescribed Medications: An update.
Pharmaceutics 2018; 10(36).
2. US National Library of Medicine. Drugs, Herbs and Supplements. Internet: https://www.nlm.
nih.gov/medlineplus/druginformation.html Accessed 28 April 2016.
3. Meletis CD & Zabriskie N. Common nutrient depletions caused by pharmaceuticals. Alt Comp
Ther 2007;13(1):10-17.
4. Hyla Cass, M.D. A Practical Guide to Avoiding Drug-Induced Nutrient Depletion: http://
nutritionreview.org/2013/04/practical-guide-avoiding-drug-induced-nutrient-depletion/
5. Clayton,JA, Rodgers S, Blakey J. Thiazide diuretic prescription and electrolyte abnormalities
in primary care. Br J Clin Pharmacol 2006 Jan;61:87-95.
6. Pak CY. Correction of thiazide-induced hypomagnesemia by potassium-magnesium citrate
from review of prior trials. Clin Nephrol 2000;54:271-275.
Page 8
©2019 Pharmavite LLC
RN 126418
ABOUT PHARMAVITE LLC
For 45 years, Pharmavite has been a trusted leader in the wellness industry, recognized for providing high-quality vitamin, mineral and herbal
supplements under its Nature Made
®
brand.
Nature Made
®
is the number one selling national vitamin and supplement brand in traditional retail scanning outlets.* Nature Made
®
adheres to strict
manufacturing standards and was the first national supplement brand to have a product verified by United States Pharmacopeia (USP), and it is the
national supplement brand with the most products carrying the USP Verified Mark-verification that products meet stringent quality criteria for purity
and potency.
Additionally, Pharmavite’s commitment to Good Manufacturing Practices (GMPs) and quality extends to every aspect of our production, from
purchasing high-quality raw materials, to the meticulous production and testing of every product. The dietary supplement industry is regulated by the
U.S. Food and Drug Administration and the Federal Trade Commission Act, as well as by respective government agencies in each of the 50 states.
Pharmavite’s emphasis on health and nutrition knowledge, emerging scientific research and new technology has enabled us to forge compelling
partnerships with many distinguished educational institutions. The end result is that Pharmavite stays on the leading edge of key scientific
advancements and innovations that make a difference in people’s lives. Based in West Hills, California, Pharmavite LLC operates as a subsidiary of
Otsuka Pharmaceutical Co., Ltd. For more information, please visit http://www.pharmavite.com.
*Nature Made
®
is the #1 selling national vitamin and supplement brand in traditional retail scanning outlets (based in part on data reported by Nielsen through its Scantrack
®
service for the Total Vitamins category for the 52-week period ending 12/31/2016 in US xAOC and US Food Drug Mass channels. ©2017 The Nielsen Company).

RECOGNIZE SOMEONE YOU
BELIEVE IS A NEXT-GENERATION
PHARMACIST
®
Nominations are now open through May 15, 2020
for the pharmacy industry’s leading national awards
program. Recognize an outstanding pharmacist,
industry advocate, technician and student pharmacist
who is elevating the practice of pharmacy.
2019 Winners
nextgenpharmacist.com
#NextGenPharm
2020 CATEGORIES:
• Civic Leader
• Entrepreneur
• Future Pharmacist
• Health-System Pharmacist
• Lifetime Leadership
• Patient Care Provider
• Rising Star
• Specialty Pharmacist
• Technician
• Technology Innovator
Submit your nominations at nextgenpharmacist.com.
2020 Sponsors
