
CDC Tuberculosis Surveillance Data Training
Report of Verified Case
Report of Verified CaseReport of Verified Case
Report of Verified Case
of Tuberculosis (RVCT)
of Tuberculosis (RVCT) of Tuberculosis (RVCT)
of Tuberculosis (RVCT)
Self-Study Modules
Participant Manual
This manual is designed to help health care staff learn how to accurately complete the
RVCT. Included are the instructions for how to complete each item on the RVCT and
exercises that will help you apply the instructions to life-like situations.
June, 2009
U.S. Department of Health and Human Services
Centers for Disease Control and Prevention
National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention
Division of Tuberculosis Elimination
Mycobacterium tuberculosis

This document was prepared by
The Report of a Verified Case of Tuberculosis (RVCT) Instructions and Self-Study Modules were
prepared by the following branches within the Centers for Disease Control and Prevention (CDC),
Division of Tuberculosis Elimination:
Surveillance, Epidemiology, and Outbreak Investigations Branch
Elvin Magee, MPH, MS
Lilia P. Manangan, RN, MPH
Carla Winston, PhD
Valerie Robison, DDS, MPH, PhD
Thomas R. Navin, MD
Communications, Education, and Behavioral Studies Branch
Cheryl Tryon, MS
Peri Hopkins, MPH
Trang Nguyen, MPH
Sarah Segerlind, MPH
Teresa Goss
Sherry Brown
Blen Mekuria, BA
Field Services and Evaluation Branch
Alstead Forbes
Bruce Heath
Others contributing to the production of this publication:
CDC Reviewers
Phil LoBue, MD, FACP, FCCP
John Jereb, MD
Sundari Mase, MD, MPH
Wanda Walton, PhD, MEd
Kashef Ijaz, MD, MPH
Amera Khan, MPH
Ann Lanner, BA
Marie S. Morgan, BA, ELS
Robert Pratt, BS
Lori Armstrong, PhD
Carla Jeffries, MPH
Jose Becerra, MD, MPH
Allison Maiuri, MPH
Ijeoma Agulefo, MPH
Glenda T. Newell, CSA
National Center for Health Marketing, Division of Creative Services
Sarah Cote
Howard Hall
RVCT Revision Work Group
Members, 2001-2007
RVCT
Reviewers
Janice Boutotte, PhD, RN Cecilia Teresa T. Arciaga
N. Alex Bowler, MPH, FACHE Sherri Austin
James Cobb Angelito Bravo, MD
Theresa Crisp, MPH Gayle L. Canfield, RN
Mayleen Jack Ekiek, MD Smita G. Chatterjee, MS
Kimberly Field, RN, PHN, MSN Christine A. Feaster, M(ASCP)
CM
, M(NRM)
Michael E Fleenor, MD, MPH Kitty B. Herrin, MA, PHD
Lorena Jeske, RN, MN Pat F. Infield, RN
Stephen E. Hughes, PhD Harvey L. Marx, Jr.
Scott Jones Sandra A. Morris, MPH
Yvonne Luster-Harvey, MPH Kathleen Moser, MD, MPH
Debbie Merz, MS Ellen Murray, RN, BSN
Masahiro Narita, MD Rebbie M. Ortega
Mary Naughton, MD, MPH Lillian T. Pirog, RN, BS, PNP
Lynelle Phillips, RN, MPH Vicki Randle, MPH, RN
Shameer Poonja, MPH Paul Regan, PHA
Carol Pozsik, RN, MPH Kristina Schaller
Randall Reves, MD, MSc Gladys Simon
Maria G. Rodriguez Barbara Simpkins
Diana Schneider, DrPH, MA Mary K. Sisk, RN, CIC
Barbara Seaworth, MD Sarah Macinski Sperry, MS
Sharon Sharnprapai, MS Richard A. Stevens, DrPH, MPH, MSHSA, MS
Muriel Silin, MPH Barbara L. Stone, MSPH
Wendy Mills Sutherland, MPH Jason Stout, MD
Jacinthe Thomas, MPH Sharon J. Thompson, RN
Janice Westenhouse, MPH Marie P. Villa, RN
Terri Wilson
Josephine L. Yumul, MSc
Edward Zuroweste, MD
CDC would also like to thank all of the
state and local health departments
whose staff participated in the field tests.
Table of Contents
i
Contents
ContentsContents
Contents
Page
Introduction
IntroductionIntroduction
Introduction
1
Introduction 3
Overview of the RVCT Form 6
What Is New in the RVCT 11
Overview of the RVCT Instructions 12
Overview of the RVCT Self-Study Modules 13
Continuing Education 15
To View or Order Materials 16
* Status of Item
New Revised No Change
Page
Module A
Module A Module A
Module A –
––
– RVCT (page 1)
RVCT (page 1) RVCT (page 1)
RVCT (page 1)
19
1 – Date Reported R 20
2 – Date Submitted R 25
3 – Case Numbers R 27
4 – Reporting Address for Case Counting NC 34
5 – Count Status N 40
6 – Date Counted R 48
7 – Previous Diagnosis of TB Disease NC 51
8 – Date of Birth NC 54
9 – Sex at Birth NC 56
10 – Ethnicity NC 58
11 – Race NC 60
12 – Country of Birth R 63
13 – Month-Year Arrived in U.S. NC 68
14 – Pediatric TB Patients (<15 years old) N 70
15 – Status at TB Diagnosis R 75
16 – Site of TB Disease R 78
Module B
Module B Module B
Module B –
––
– RVCT (page 2)
RVCT (page 2) RVCT (page 2)
RVCT (page 2)
81
17 – Sputum Smear R 82
18 – Sputum Culture R 85
19 – Smear/Pathology/Cytology of Tissue and Other Body Fluids R 89
20 – Culture of Tissue and Other Body Fluids R 94
21 – Nucleic Acid Amplification Test Result N 99
22A – Initial Chest Radiograph R 103
22B – Initial Chest CT Scan or Other Chest Imaging Study N 106
23 – Tuberculin (Mantoux) Skin Test at Diagnosis R 109
24 – Interferon Gamma Release Assay for Mycobacterium
Tuberculosis at Diagnosis
N 113
25 – Primary Reason Evaluated for TB Disease N 116
* Status of item refers to whether the items in the revised 2009 RVCT form are new, revised, or have no change.
Highlighted items = more complicated
Table of Contents
ii
Table of Contents
iii
Status of Item
New Revised No Change
Page
Module C
Module C Module C
Module C –
––
– RVCT (page 3)
RVCT (page 3) RVCT (page 3)
RVCT (page 3)
121
26 – HIV Status at Time of Diagnosis R 122
27 – Homeless Within Past Year NC 125
28 – Resident of Correctional Facility at Time of Diagnosis R 128
29 – Resident of Long-Term Care Facility at Time of Diagnosis NC 131
30 – Primary Occupation Within Past Year R 135
31 – Injecting Drug Use Within Past Year NC 138
32 – Non-Injecting Drug Use Within Past Year NC 140
33 – Excess Alcohol Use Within Past Year NC 143
34 – Additional TB Risk Factors N 145
35 – Immigration Status at First Entry to the U.S. N 149
36 – Date Therapy Started NC 153
37 – Initial Drug Regimen R 155
Mod
ModMod
Module D
ule D ule D
ule D –
––
– Initial Drug Susceptibility Report
Initial Drug Susceptibility Report Initial Drug Susceptibility Report
Initial Drug Susceptibility Report
Follow Up Report
Follow Up ReportFollow Up Report
Follow Up Report–
––
–1
11
1
157
38 – Genotyping Accession Number N 158
39 – Initial Drug Susceptibility Testing R 161
40 – Initial Drug Susceptibility Results R 164
Module E
Module E Module E
Module E –
––
– Case Completion Report
Case Completion Report Case Completion Report
Case Completion Report
Follow Up Rep
Follow Up RepFollow Up Rep
Follow Up Report
ortort
ort–
––
–2
22
2
169
41 – Sputum Culture Conversion Documented R 171
42 – Moved N 174
43 – Date Therapy Stopped NC 179
44 – Reason Therapy Stopped or Never Started R 182
45 – Reason Therapy Extended > 12 Months N 186
46 – Type of Outpatient Health Care Provider R 188
47 – Directly Observed Therapy (DOT) R 191
48 – Final Drug Susceptibility Testing R 196
49 – Final Drug Susceptibility Results R 199
Appendices
AppendicesAppendices
Appendices
Appendix A – Tuberculosis Case Definition for Public Health Surveillance 203
Appendix B – Recommendations for Reporting and Counting Tuberculosis Cases 205
Appendix C – Anatomic Codes 215
Appendix D – Reporting Area Codes 217
Appendix E – Country Codes 219
Appendix F – Glossary 225
Appendix G – Answer Key for Exercises 231
Note: Use of trade names in this publication is for identification purposes only and does not imply
endorsement by the Centers for Disease Control and Prevention.
Table of Contents
iv

1
Introduction
IntroductionIntroduction
Introduction
This section provides an introduction to the Report of Verified Case of Tuberculosis and an overview of
the form, the instructions, and the RVCT Self-Study Modules, as well as information on continuing
education and how to order materials.
2
Introduction
IntroductionIntroduction
Introduction
Topics
TopicsTopics
Topics
Section Page
Introduction
3
Background
3
Tuberculosis Surveillance Data
3
Impact of RVCT Data
3
Quality Assurance
4
Purpose of the RVCT Self-Study Modules
4
Target Audience
5
Course Objectives
5
Materials
5
How the Modules Can Be Used
5
Overview of the RVCT Form
6
Required and Recommended Uses of the RVCT
6
RVCT Form
7
RVCT Items
9
Unknown Dates
9
Pending vs. Unknown Information
9
Updating of Forms
9
Additional Reporting Forms
10
Data Entry and Security
10
Patient Confidentiality
10
What Is New in the RVCT
11
New and Updated Variables
11
Recurrences of TB
12
Overview of the RVCT Instructions
12
Overview of the RVCT Self-Study Modules
13
How to Work Through the Modules
13
Estimated Completion Time for Working Through the Modules
13
Materials Needed for Working Through the Modules
14
Continuing Education
15
Continuing Education Units
15
Continuing Education Registration and Test
15
Online Registration and Test
15
Disclosure Statement
16
To View or Order Materials
16
List of RVCT Training Materials
17
3
Introduction
IntroductionIntroduction
Introduction
Background
Tuberculosis (TB) is a nationally notifiable disease, in that reporting is mandated in all states. In 1953, a
national surveillance system was established to collect information on new cases of active TB. Since
1985, all states have been reporting TB cases to the Centers for Disease Control and Prevention (CDC)
using the Report of Verified Case of Tuberculosis (RVCT), the national TB surveillance form. Data are
collected by state and local TB programs and submitted electronically to CDC, Division of Tuberculosis
Elimination (DTBE). These data are used to monitor national TB trends, identify priority needs, and
create the DTBE annual surveillance report, Reported Tuberculosis in the United States.
To control and eventually eliminate TB, state and local TB control programs must be able to monitor
trends in TB disease in high-risk populations, as well as identify new patterns of disease and possible
outbreaks. The last major revision of the RVCT was completed in 1993. Since 2001, members of a
DTBE-sponsored work group consisting of nearly 30 public health professionals from 15 TB control
programs, DTBE, and the National TB Controllers Association (NTCA) have been working to revise the
RVCT. Modifications to the RVCT data collection now accommodate the changing epidemiology of TB
in terms of risk factors, new drug treatments, and enhanced laboratory capacity for diagnostic tests.
Note: A case of TB is defined as an episode of TB disease in a person meeting the laboratory or clinical
criteria for TB as defined in Appendix A – Tuberculosis Case Definition for Public Health Surveillance.
Tuberculosis Surveillance Data
Some states may use a modified version of the RVCT or a data collection tool that is unique to their
jurisdiction. These forms are used to collect the same data contained in the RVCT. However, just as the
actual RVCT form is not sent to CDC, neither are these locally defined variables or additional data. CDC
should never receive names of persons with TB. Names are retained by the state or local health
department. Locally assigned numbers and characters are used for case identification and are included in
Case Numbers (item 3) for use by CDC. See Case Numbers (item 3) for more information.
Impact of RVCT Data
The revised RVCT will assist TB control programs in gathering accurate and useful data. The additions
and changes made to the variables of the RVCT will enable programs to capture data that are more
inclusive of a variety of risk factors. These additional data will be essential to efficient and effective TB
program management. The following table illustrates how the revised RVCT data can improve TB
programs, and the consequences of having inaccurate or incomplete data.

4
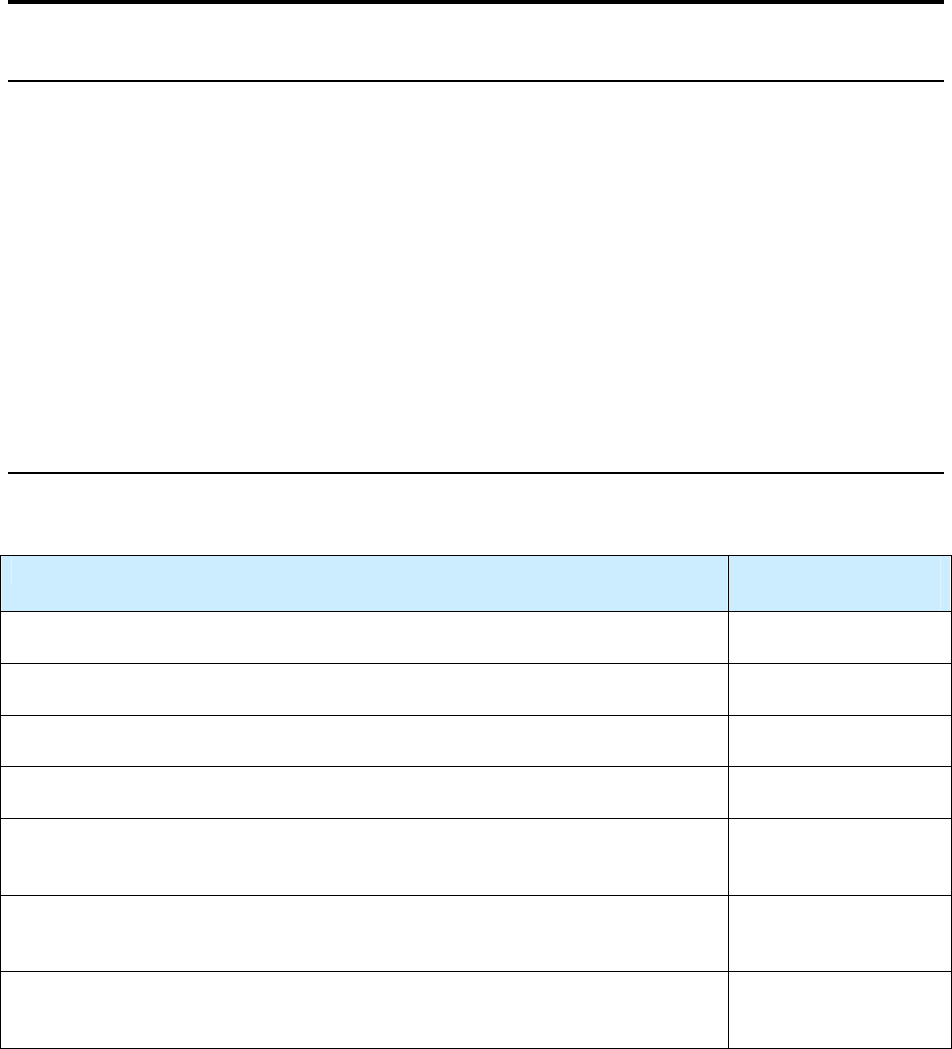
Impact of Revised RVCT Data
Benefits of RVCT Data Consequences of Inaccurate,
Incomplete, or Unknown RVCT Data
• Increased ability to assess program
performance, completeness of data
collection, and accuracy of reporting
• Improved data for program planning and
policy development (e.g., personnel,
resources, funding)
• Facilitation of patient services (e.g.,
quality of care, continuity of care,
sharing of accurate information with
patient and health facilities)
• Inaccurate follow-up of services to patients
• Inadequate resources (e.g., funding, staff,
facilities, drugs, and supplies)
• Inaccurate evaluation and policy development
• Misrepresentation of the public health burden
of TB
• Inability to measure TB program indicators that
are based on surveillance data
Quality Assurance
Assuring data completeness and quality is encouraged for all case reporting. Each reporting area should
develop its own policy or procedure for reviewing and updating incomplete or incorrect data. These
procedures should ensure that the data are collected and entered in the surveillance system accurately.
Although health departments share TB surveillance data with CDC, the responsibility and authority for
TB surveillance rests with the health department. States vary in the structure and organization of their
surveillance systems, and often in the completeness or quality assurance of their case reporting. As with
any reportable disease, the completeness of TB reporting reflects how actively health departments solicit
case report information. Historically, disease surveillance systems have been categorized as passive or
active.
• Passive surveillance
Health departments passively receive case reports from health care providers, depending on the
health care providers to know and comply with reporting requirements.
• Active surveillance
Health departments actively contact and interact with health care facilities or individual providers
to stimulate disease reporting, sometimes directly assuming the primary responsibility of
reporting cases from large or high-volume institutions.
CDC provides funding and technical assistance to health departments to actively stimulate TB case
reporting, and has encouraged them to take an active rather than passive approach to TB surveillance.
Health departments are encouraged to identify local or private health care facilities that serve TB patients.
Health departments are also encouraged to use other data sources to identify TB cases, including death
certificates and laboratory reports.
Purpose of the RVCT Self-Study Modules
The purpose of these self-study modules is to help participants learn how to accurately complete the
revised RVCT form. The modules can be used either as self-study materials or in a facilitated course.

5
Target Audience
The target audience includes health care workers who
• Collect the data from patients
• Complete the RVCT form (or local tuberculosis case reporting form specified by the reporting
jurisdiction)
• Enter data from the RVCT into the reporting system
• Monitor TB program data collection accuracy
• Analyze data from the RVCT
Course Objectives
After working through these modules, participants will be able to
• Distinguish between the three reports included in the RVCT form
• Recognize the items on the RVCT form
• Use the RVCT instructions to determine how to complete the RVCT form
• Accurately complete the RVCT form
Materials
You will need the following materials when you work through this course.
• Report of Verified Case of Tuberculosis (RVCT form)
• Report of Verified Case of Tuberculosis Self-Study Modules (these modules)
• Appendices: Tuberculosis Case Definition for Public Health Surveillance, Recommendations for
Reporting and Counting Tuberculosis Cases, Anatomic Codes, Reporting Area Codes, Country
Codes, Glossary, and Answer Key for Exercises
Note: Because some states use their own form (rather than the RVCT), the exercises and posttest are
available in Microsoft Word format so they can be adapted for training purposes.
How the Modules Can Be Used
The Report of Verified Case of Tuberculosis Self-Study Modules can be used in the following ways:
1. For individuals learning through self-study format
Health care workers can use the modules according to their needs
• Working through them at their own pace
o Completing the whole set of modules without interruption
o Completing one module at a time (e.g., one module per day)
• Using them as a reference
2. As part of a facilitator-led training course
The self-study modules can be used as part of a training course that is led by a facilitator.
• Participants work through the modules
• Facilitators lead group discussions about the instructions and the exercises and engage the
participants in learning how to use the RVCT
A facilitator’s guide is available that includes information on the best way to teach this course to
others.
6
Overview of the
Overview of theOverview of the
Overview of the RVCT
RVCT RVCT
RVCT Form
Form Form
Form
The RVCT form is designed for the collection of information on cases of TB. The expanded RVCT was
approved by the Office of Management and Budget (OMB) in 2008 to become effective January 2009.
Note:
On the RVCT form and throughout this document, the term state is used to refer to the reporting
jurisdiction (or count authority), though not all jurisdictions are states.
Required and Recommended Uses of the RVCT
The following table indicates the required and recommended uses of the RVCT.
Required Use
of the RVCT
Additional Recommended Uses
of the RVCT
Possible Use of the RVCT for
a Suspected Case of TB
The RVCT
must
be
completed for
all
verified cases of TB
that are to be included
in the reporting area’s
annual morbidity
count.
CDC
recommends
the use of RVCT
forms for the collection of data on the
following:
•
Transfer TB cases (e.g., TB cases
counted in another state or country)
•
TB cases that recur
within
12 months
after the completion of therapy
Reporting areas
may
also use the
RVCT forms for the collection of
data on a suspected case of TB or
on a patient with latent TB
infection (LTBI).
For the purposes of surveillance, a case of TB is defined on the basis of laboratory and/or clinical
evidence of active disease due to M. tuberculosis complex. For more information on the case definition of
M. tuberculosis complex, see Appendix A – Tuberculosis Case Definition for Public Health Surveillance.
Note:
The instructions contained in this document do
not
apply to suspected cases of TB or
to
patients with latent TB infection (LTBI).

7
RVCT Form
The expanded RVCT form comprises three data collection reports, which are printed in triplicate on
carbonless paper:
1. Report of Verified Case of Tuberculosis: Complete this form for all patients with a verified case of
TB.
2. Initial Drug Susceptibility Report (Follow-Up Report 1): Complete this form for all patients who
had a culture that was positive for M. tuberculosis complex.
3. Case Completion Report (Follow-Up Report 2): Complete this form for all patients who were
alive when TB was diagnosed.
The two follow-up reports supplement the Report of Verified Case of Tuberculosis.
The three reports in the RVCT form are
• Not necessarily completed for all patients
• Not completed all at one time.
The following table provides a description of each report, for whom it is completed, and when it is
completed.
Note:
It is strongly recommended that the hard copy of the RVCT form be completed by a health care
provider and maintained in the TB patient’s medical record in a secured (locked) area.

8
The Three Reports Comprising the RVCT Form
Report of Verified Case of Tuberculosis
• Includes data about patient demographics, laboratory results, and risk associated with TB
• Complete for all patients with a verified case of TB disease
• Complete over time (evaluation process and treatment) as the information from the patient, the
laboratory reports, and medical records become available
Page 1
(Items 1 – 16)
Page 2
(Items 17 – 25)
Page 3
(Items 26 – 37)
Initial Drug Susceptibility Report
(Follow Up Report - 1)
Case Completion Report
(Follow Up Report - 2)
• Includes genotyping information and
drug susceptibility testing results
• Complete for all patients who had a
positive culture result for
Mycobacterium tuberculosis complex
• Do not complete for patients with
negative culture or no results for
culture
• Complete after susceptibility test
results are received
• Includes treatment outcomes collected
• Complete for all patients who were alive when TB
was diagnosed
• Complete after treatment ends; the case completion
report is due no later than 2 years after the initial
RVCT
Page 1
(Items 38 – 40)
Page 1
(Items 41 – 46)
Page 2
(Items 47 – 49)

9
RVCT Items
The revised RVCT form includes 49 items. The characteristics are varied; for example,
• Some items include one variable response
• Some items include more than one response (e.g., Items 3 and 4)
• Each item is delineated in its own box
• Some boxes are grouped together in larger boxes to visually and logically organize the space
Items are not necessarily listed in the order in which you might receive the information
Data are entered on the RVCT form in several ways:
1. Writing in dates and other numbers (e.g., Items 1, 2, and 3)
2. Checking boxes (e.g., Items 9, 10, and 11)
a. Select one
b. Select all that apply
3. Writing in specific information (e.g., Items 12, 14)
4. Writing in comments (e.g., page 3, Follow Up Report–1, or Follow Up Report–2)
Unknown Dates
There are several items that include dates. When entering dates on the form, use “99” for an unknown
month or day, and “9999” for an unknown year. This may vary from what will be entered into a computer
software program.
• 03 99 2009 – for March, unknown day, in 2009
• 99 99 2009 – for unknown month and day in 2009
• 01 02 9999 – for January 2, in a year that is unknown
Note:
For each item that includes dates, read the instructions carefully about entering month, day, and
year. Some items (e.g.,
Date Reported
, Item 1) require that the actual month and year
always
need to be
entered. For those items, the actual month (not 99) should be entered, and the actual year (not 9999)
should be entered.
Pending vs. Unknown Information
Leave the item blank if the information requested is pending (or missing). If a valid value cannot be
determined and there is no check-box labeled Unknown, write the word Unknown inside the box that
encloses the numbered item. This unknown notation will help the person entering the data in the software
to know that the person who completed the form attempted to collect the information but was not able to
do so. The data entry person will thus be better able to distinguish between data that are unknown and
data that are pending (missing). CDC encourages active surveillance or collection of all applicable
information. Therefore “unknown” information should be rare.
Updating of Forms
It may be necessary to update RVCT forms if a case is reopened (e.g., a patient who had been lost to
follow-up is found) or if previously unavailable information is obtained. CDC recommends highlighting
such changes on the hard copy to facilitate data entry into the software system designated by your
jurisdiction. When updated data are entered in an electronic record, the new data will automatically
overwrite the old data.
10
Additional Reporting Forms
If the reporting area has its own TB case reporting form and uses it to complete the RVCT variables, the
staff should carefully review the RVCT variables and the instructions in this document to ensure that
variables on the reporting area’s form match those on the RVCT form.
Data Entry and Security
Data obtained from RVCT forms are entered in the software system designated by your jurisdiction and
then transmitted electronically to CDC.
Data security is the responsibility of the state or local health department.
Completed RVCT forms
should never be sent to CDC.
Access to the RVCT forms and data entry software should be restricted to individuals authorized to
perform TB surveillance activities. Hard copies should be stored in a secured (locked) area. Access to the
approved data entry software and local databases should be controlled through the use of a local user
identification (user ID) and password. All other electronic surveillance files should also be protected with
passwords known only to designated surveillance staff.
Patient Confidentiality
Case numbers must not include personal identifiers.
Do not use names, initials, Social Security
numbers, addresses, telephone numbers, or other information that could identify a patient.
Because of the sensitive nature of some of the data collected, CDC has provided an Assurance of
Confidentiality for the expanded surveillance system. Information on the RVCT forms and in the TB
surveillance databases that would permit identification of any individual will be held in confidence and
will not be released without the consent of the individual, in accordance with sections 306 and 308(d) of
the Public Health Services Act (42 U.S.C. 242k and 242m).
Local patient identifier information, although collected by state and local health departments, is not
reported to CDC. Surveillance information reported to CDC is used for statistical and analytic summaries
in which no individual can be identified and for special investigations of the natural history and
epidemiology of TB.
11
What
What What
What I
II
Is New in the RVCT
s New in the RVCTs New in the RVCT
s New in the RVCT
The RVCT form has items that are either new or revised from the previous RVCT that was published in
1993. To help orient previous RVCT users to the new items, the table of contents (at the beginning of this
document) indicates which items are new, revised, or unchanged.
The RVCT
State Case Number
(item 3), also known as the RVCT number, has been standardized by
adding a 4-digit code for year and a 2-character (alpha) code for state (or jurisdictional code for
jurisdictions that are not states) to the 9-character alphanumeric local identifier, so that each state case
number is unique for year and state. The additions to the State Case Number will help when trying to
identify a TB patient who has been transferred from one health jurisdiction (e.g., state) to another, and
when trying to link TB cases (e.g., recurrences, contact investigations).
New and Updated Variables
Eleven new variables were added to improve data collection. These variables (items) are indicated in the
table below.
New Variables in the Revised RVCT
Item # New Variables
5 Count status
14 Pediatric TB patients
21 Nucleic acid amplification test
22B Initial chest CT scan or other chest imaging study
24 Interferon gamma release assay
25 Primary reason evaluated for TB disease
34 Additional TB risk factors
35 Immigration status
38 Genotyping accession number
42 Moved
45 Reason therapy was extended for more than 12 months
A new variable called
Count Status
(item 5) was added to separate counted and noncountable TB cases.
Data can now be collected on noncountable TB cases to help identify specific cases for analysis and help
measure TB morbidity and case management burden. Noncountable cases are verified TB cases that
cannot be counted because they do
not
meet the surveillance definition of a countable case.
12
Additional new variables include TB risk factors, such as diabetes, end-stage renal disease,
immunosuppressive therapy, and the use of tumor necrosis factor-alpha antagonists.
Other variables have been updated to reflect the changing field of TB epidemiology and to collect more
accurate data on TB cases. Modified variables include the addition of dates of tuberculin skin testing
(item 23) and of specimen collection for other diagnostic tests, along with result dates by laboratory type
(items 17–21 for smear and culture results).
Recurrences of TB
The new variable,
Count Status
(item 5), allows data collection on the recurrence (more than one separate
and distinct episode) of TB. Most recurrences occur within 6–12 months after the completion of therapy.
For surveillance purposes, a description of how this is counted is illustrated in the following table.
Counting Reported TB Cases
A patient may have more than 1 discrete (separate and distinct) episode of TB disease
TB Disease Recurs
Within a Consecutive 12-month Period
After the Patient Completed Therapy
TB Disease Recurs
More Than 12 Months
After the Patient Completed Therapy
Recurrence is considered the same episode (count
only 1 episode as a case for that year; within a 12-
month period,
not
calendar year).
Recurrence is considered a separate episode.
Do
not
count as a new case.
Count as a new case.
More information about recurrences of TB is provided in
Case Number
(item 3).
Overview of the RVCT Instruction
Overview of the RVCT InstructionOverview of the RVCT Instruction
Overview of the RVCT Instructions
ss
s
The RVCT instructions provide information on how to complete the 49 items on the RVCT form. The
instructions provide details about each item, explain the nuances of how to answer the items, and also
provide examples to illustrate how to apply the instructions for entering data for a TB case. The
instructions are available in two formats.
• The Report of Verified Case of Tuberculosis Self-Study Modules (these modules).
In the modules, the instructions are integrated with exercises (study questions and case studies).
This provides an opportunity to practice applying the instructions to life-like situations.
• The Report of Verified Case of Tuberculosis Instruction Manual.
This document includes only the instructions (i.e., the exercises are not included) for each item on
the RVCT. It can be used as a reference tool by those who complete the RVCT. For downloading
the Instruction Manual from the internet, see the section below on “To View or Order the
Materials.”
13
Overview of th
Overview of thOverview of th
Overview of the RVCT Self
e RVCT Selfe RVCT Self
e RVCT Self-
--
-Study
Study Study
Study Module
ModuleModule
Modules
ss
s
How to Work Through the Modules (This is IMPORTANT – Be sure to read this)
Please follow these steps to work through the modules:
1. Work through the self-study modules
• Review an item on the RVCT form
• Read how to complete the item in the RVCT instructions
• Complete the exercises (study questions and/or case studies) for the item
• Use the Appendices as needed
2. Check your answers
• Check your answer(s) in Appendix G - Answer Key for Exercises
If something is not clear, then for each item that you answered incorrectly, re-read the RVCT
instructions and try to complete the exercises again.
Estimated Completion Time for Working through the Modules
The Report of Verified Case of Tuberculosis Self-Study Modules comprise a comprehensive curriculum.
The sections of the modules are listed below as well as the estimated completion time for each section.
Sections Estimated
Completion Time
Introduction (this module)
25 minutes
Module A – RVCT (page 1) Items 1 – 16
100 minutes
Module B – RVCT (page 2) Items 17 – 25
45 minutes
Module C – RVCT (page 3) Items 26 – 37
55 minutes
Module D – The Initial Drug Susceptibility Report (Follow Up Report – 1)
Items 38 – 40
20 minutes
Module E – The Case Completion Report (Follow Up Report – 2)
Items 41 – 49
55 minutes
TOTAL Approximate Time
300 minutes
(about 5 hours)

14
Materials Needed for Working Through the Modules
The following materials are needed to work through the RVCT Self-Study Modules.
• The Report of Verified Case of Tuberculosis form
This is the 49-item form.
• RVCT Self-Study Modules
The modules consist of the following components:
o Instructions for how to complete each item on the RVCT
Each item in the RVCT has detailed instructions that explain how to complete the item. It is
very important to read the instructions for an item before answering the study
questions. The instructions provide information on how to interpret the items and options,
and provide examples that illustrate how to answer in specified situations.
o Exercises
The instructions for each item are followed by exercises that will help you apply the
instructions to life-like situations and practice completing the RVCT.
• Each item on the RVCT has at least one exercise
• Types of exercises include
• Study questions
• Case studies
• Answer choices include
• Multiple choice
• Matching
• Select the ONE BEST ANSWER for ALL questions
• Some items are more complex; those items have several study questions and/or case
studies.
• Because several items are linked to each other (e.g., items 3 and 5 are linked), some
case studies include more than one item. These are designed to help you understand
how the items are linked.
• Appendices
The following appendices provide information and codes that are used to complete the RVCT:
• Appendix A – Tuberculosis Case Definition for Public Health Surveillance
• Appendix B – Recommendations for Reporting and Counting Tuberculosis Cases
• Appendix C – Anatomic Codes
• Appendix D – Reporting Area Codes
• Appendix E – Country Codes
• Appendix F – Glossary
• Appendix G – Answer Key for Exercises
This appendix provides answers to each of the exercises. Answer the questions first and then
check your answers with the key. Explanations for some of the difficult questions are
provided to help you understand the correct answer.
Note: For the purposes of the RVCT Self-Study Modules, use the codes listed in the appendices.
Some software programs used to enter data on the RVCT may NOT use the codes listed in the
appendices. For example, the Anatomic Codes may be a drop-down item where you choose the actual
site rather than enter a code. For more information, see instructions for the software you use.

15
Continuing Education
Continuing EducationContinuing Education
Continuing Education
Continuing Education Units
The following continuing education units are available free of charge after June 1, 2009, for the RVCT
Self-Study Modules:
• Continuing education units (CEUs)
The Centers for Disease Control and Prevention has been approved as an Authorized Provider by
the International Association for Continuing Education and Training (IACET), 8405 Greensboro
Drive, Suite 800, McLean, VA 22102. CDC is authorized by IACET to offer 0.5 CEUs for this
program.
• Continuing medical education (CME)
CDC is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to
provide continuing medical education for physicians.
CDC designates this educational activity for a maximum of 5.0 AMA PRA Category 1 Credits.
Physicians should only claim credit commensurate with the extent of their participation in the
activity.
• Continuing nursing education (CNEs)
CDC is accredited as a provider of Continuing Nursing Education by the American Nurses
Credentialing Center’s Commission on Accreditation.
This activity provides 5.0 contact hours.
• Continuing education contact hours (CECH)
CDC is a designated provider of continuing education contact hours (CECH) in health education
by the National Commission for Health Education Credentialing, Inc. This program is a
designated event for the CHES to receive 5.0 Category 1 contact hours in health education, CDC
provider number GA0082.
Continuing Education Registration and Test
You can register after June 1, 2009, and take the test for continuing education credits online for the RVCT
Self-Study Modules.
Online Registration and Test
To receive continuing education, you must go to CDC’s Training and Continuing Education (TCE)
Online system to register for this specific course and submit an evaluation.
•
Go to http://www2a.cdc.gov/TCEonline.
•
Login as a participant (Note: If you are a first-time user of this online system, you will need to
login as a new participant and create a participant profile.)
o
When you receive your reset password by email, log in as a participant and change the
password.
•
At Participant Services, click on Search and Register, type a keyword from the course title into
the keyword search, and click View. You can also find the course by typing in the course number.
The course number for this activity is SS1502.

16
•
Click on the title of your course, select the type of credit/contact hours you wish to receive at the
bottom, and click Submit.
•
Verify the demographic information and click Submit at the bottom.
•
Complete the course evaluation.
•
Complete the course post test (if applicable).
•
At Participant Services, click on Certificates and Transcripts and print your continuing education
certificate.
For assistance with the online system, call 1(800)-41-TRAIN Monday through Friday from 8:00 AM
to 4:00 PM Eastern Standard Time or email [email protected].
Disclosure Statement
CDC, our planners, and our presenters, wish to disclose they have no financial interests or other
relationships with the manufacturers of commercial products, suppliers of commercial services, or
commercial supporters.
Presentations will not include any discussion of the unlabeled use of a product or a product under
investigational use.
There was no commercial support received for this activity.
To View or Order the RVCT Materials
To View or Order the RVCT MaterialsTo View or Order the RVCT Materials
To View or Order the RVCT Materials
The chart on the next page describes the materials in detail and indicates how they can be used, lists the
available file formats, and describes how the materials can be ordered and downloaded. There are no
charges for ordering the materials from CDC.
17
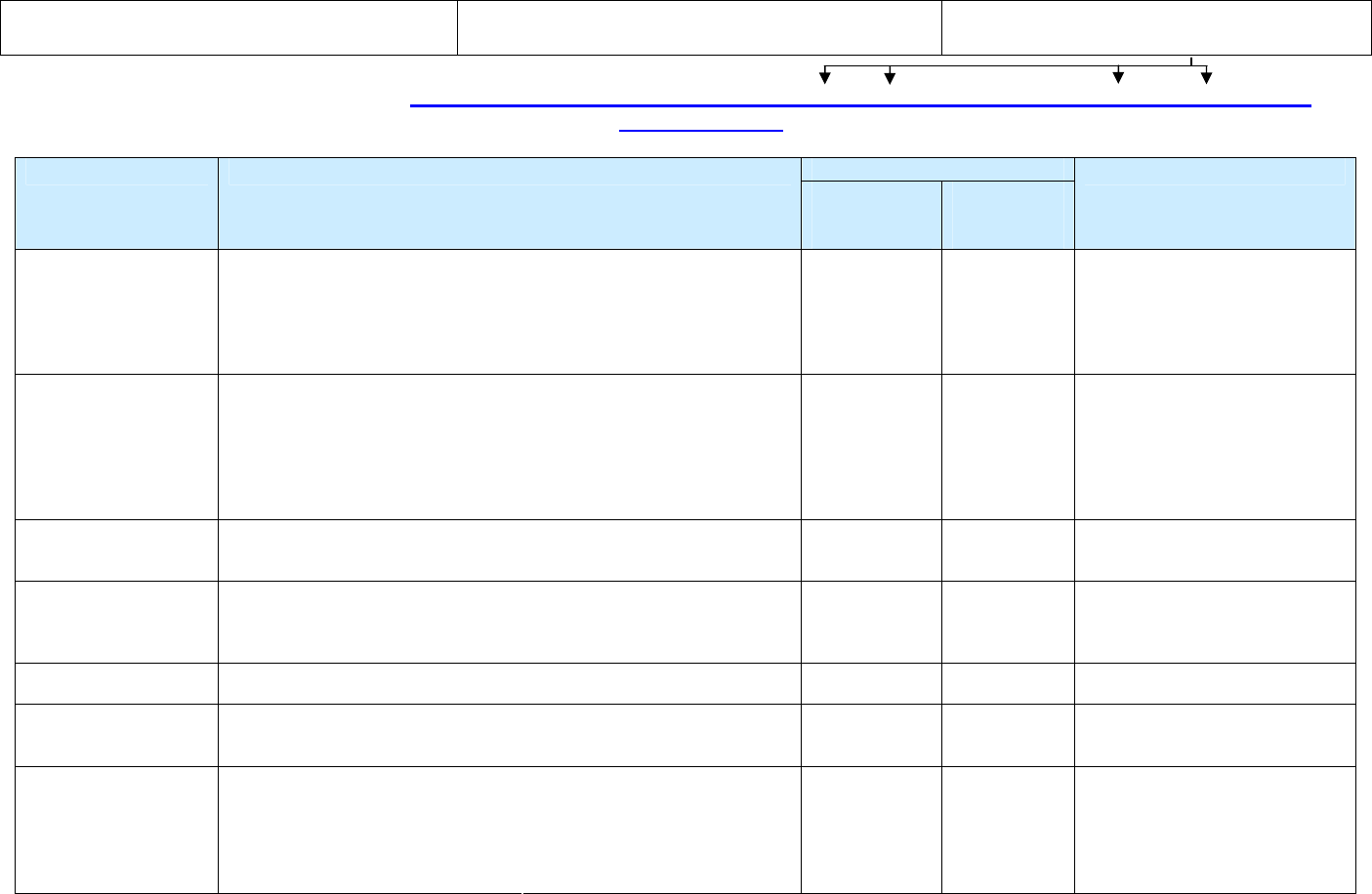
List of RVCT Training Materials
(There are no charges for these materials)
Note: the spaces in the FTP URL
FTP site to download RVCT materials: ftp://ftp.cdc.gov/pub/Software/TIMS/2009 RVCT Documentation/RVCT Training Materials/
CDC/DTBE web site to view and download RVCT materials: www.cdc.gov/tb
File Formats Available
Materials Description
On
FTP site and
CD ROM
On
CDC/DTBE
web site
How to Order
RVCT Self-Study
Modules
Participant Manual
(with CD ROM)
Print-based modules to help health care staff learn how to accurately complete
the RVCT. Includes
• Instructions for how to complete each item on the RVCT
• Exercises that will help participants apply the instructions to life-like
situations
Can be used as self-study or part of a training course.
PDF PDF E-mail or Fax RVCT Materials
Order Form (see form for
instructions)
RVCT Self-Study
Modules
Facilitator Manual
(with CD ROM)
Print-based modules for facilitators who will teach health care staff how to
complete the RVCT. Contains the same content as the RVCT Self-Study
Modules Participant Manual plus training materials for facilitators.
• Instructions for how to complete each item on the RVCT
• Exercises that will help participants apply the instructions to life-like
situations
• Facilitator guide, answers to exercises, and other training documents
PDF of manual.
Various formats
for other
training
documents
E-mail or Fax RVCT Materials
Order Form (see form for
instructions)
RVCT Instruction
Manual
Print-based document includes instructions for how to complete each item on
the RVCT. Can be used as a reference guide when completing the RVCT.
(Does
not
include the exercises from the Self-Study Modules.)
PDF PDF
RVCT Self-Study
Modules Exercises
Print-based document includes only the exercises (with answers) used in the
Self-Study Modules Participant Manual. (Does not include the instructions for
how to complete each item on the RVCT.) Exercises can be used and adapted
by local jurisdictions.
Microsoft Word (Available only on the FTP site or
CD ROM)
RVCT Materials
Description
Description of the RVCT materials PDF HTML
RVCT Materials Order
Form
Form used to order the RVCT materials from CDC. Microsoft Word
E-mail or Fax RVCT Materials
Order Form (see form for
instructions)
For those who want to order the CD ROM only. Includes the electronic files of
the following documents:
RVCT Materials
CD ROM
• RVCT Participant Manual
• RVCT Facilitator Manual and
training materials
• RVCT Instruction Manual
• RVCT Self-Study Module
Exercises
• RVCT Materials Description
• RVCT Materials Order Form
Various formats E-mail or Fax RVCT Materials
Order Form (see form for
instructions)
18

19
Module
Module Module
Module A
AA
A
–
––
–
R
RR
RVCT
VCTVCT
VCT
(page 1
(page 1(page 1
(page 1 of 3
of 3 of 3
of 3)
))
)
Items 1
Items 1 Items 1
Items 1 –
––
– 16
16 16
16
The RVCT report includes the first three pages of the RVCT data collection form. Pages 1, 2, and 3 of the
RVCT report will be covered in Modules A, B, and C, respectively. Complete this report for all
patients with a verified case of TB disease.
Module A provides instructions and exercises for completing page 1 of the RVCT report. This page
includes data about patient demographics and site of disease.
20
1.
Date Reported
Primary Purpose: Case management. Data are used to determine when the health department or
counting authority was first notified that a person may have TB. This is important in contact
investigations.
Note:
Item 1 requires that the actual month and year
always
be entered. The actual month (not 99)
should be entered and the actual year (not 9999) should be entered.
Description Comment
Month, day, and year
(e.g., 01/17/2009)
Date that a health department (e.g.,
local, county, state) first suspected
that the patient may have TB.
or
Date the health department received
notification (verbal or written) from
a health care provider that a patient
was suspected of having TB.
If the day is unknown, enter 99 as the
default value (e.g., 01/99/2009).
In this item, the actual month and
year always need to be entered. Do
not use 99 for the month or 9999 for
the year.
Comment: Date Reported
If the patient had a previous diagnosis of tuberculosis,
Date Reported
applies to the current TB episode.
Note:
On the form and throughout this document, the term state is used to refer to the reporting
jurisdiction (or count authority), though all jurisdictions are
not
states.
21
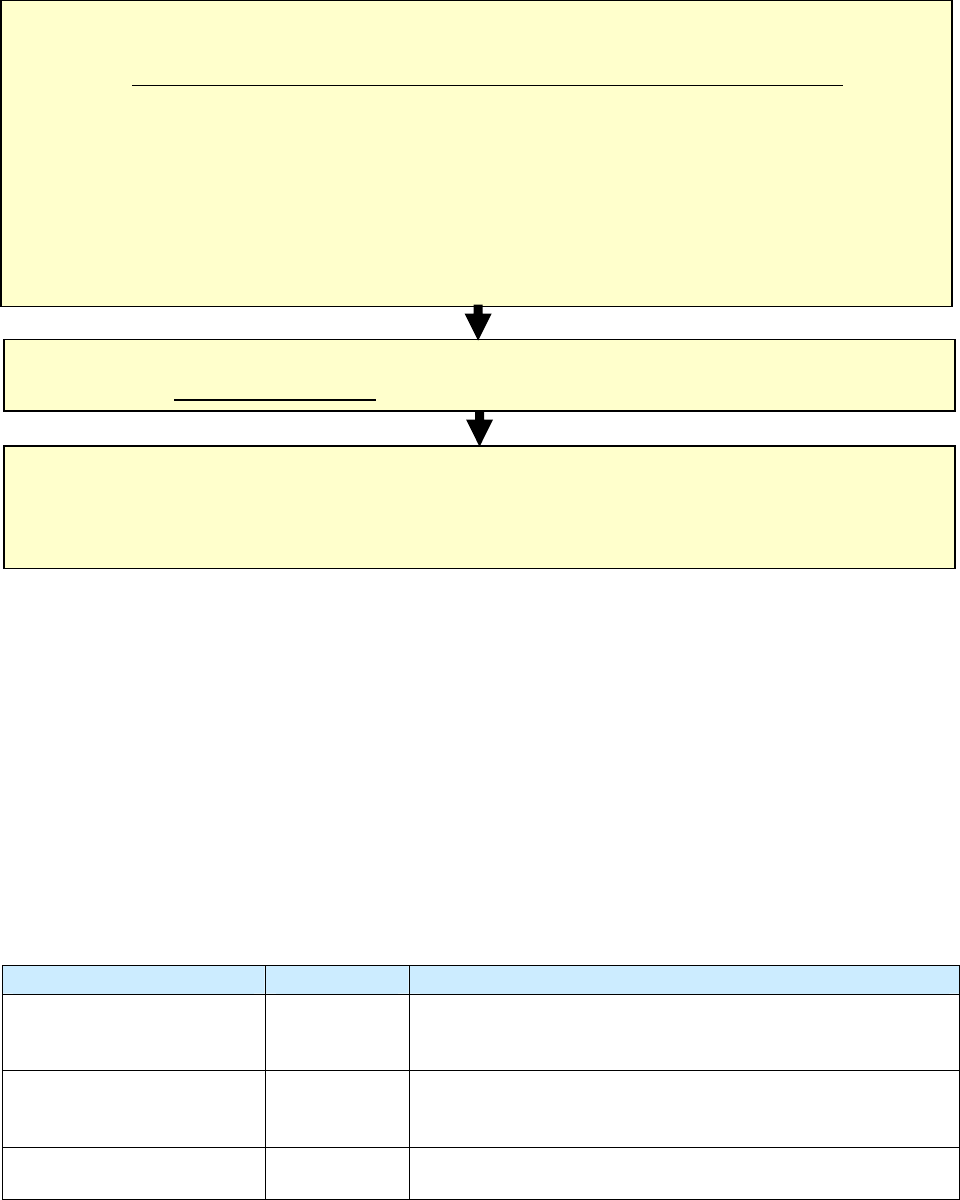
Comparison of
Date Reported (Item 1), Date Submitted (Item 2), and Date Counted (Item 6)
There is frequently a lot of confusion between
Date Reported
(it 1),
Date Submitted
(item 2)
,
and
Date
Counted
(item 6). The following information describes the differences in these three items.
Summary of Events for
Item 1. Date Reported, Item 2. Date Submitted, and Item 6. Date Counted
Comment: Sequence of dates
The Date Reported (item 1) usually occurs before the Date Submitted (item 2). But sometimes they can
occur on the same date. The Date Submitted usually occurs before the Date Counted (item 6). But all 3
dates could occur on the same date if the count authority determines that it is a case of TB on the same
day as the Date Reported and Date Submitted.
Comment: Who determines the dates
In most reporting areas (e.g., state), the state health department has count authority and reviews the
RVCT to determine whether to officially count the case (
Date Counted
). However, a few states have
granted local or county health departments count authority. In these states, the local or county health
departments determine the
Date Counted
(see
Date Submitted
[item 2] and
Date Counted
[item 6]).
Summary of Date Reported, Date Submitted, and Date Counted
Type of Date Who/What
Description of Action
Date Reported (item 1)
TB suspect
Reported to the health department (either by the health
department itself or another health care provider)
Date Submitted (item 2)
RVCT form
Submitted to the reporting area (e.g., state health
department)
Date Counted (item 6)
TB Case
Counted as a case of TB (by the count authority)
Da
te Reported
(
Item 1
)
• Date the health department (local, county, or state) first suspected that a patient has TB
or
• Date the health department receives notification (verbal or written) from a health care provider
that a patient is suspected of having TB
Often, an RVCT is created by a local or county health department because this is the level at which TB
is first suspected, and that also determines the Date Reported.
Date Submitted
(
Item 2
)
• Date that the RVCT was submitted to the reporting area (e.g., state health department)
Date Counted
(
Item 6
)
• Date that the count authority (usually the state health department, but may be another designated
authority) reviewed the RVCT and determined whether to officially count the case. The count
authority determines the Date Counted.
22
Comment: Date Reported
Often, an RVCT is created by a local or county health department because this is the level at which TB
is first suspected, and determines
Date Reported
(item 1). If a health care provider suspects that the
patient may have TB and then notifies the local or county health department, the
Date Reported
is the
date the health department received the report (verbal or written notification) from the health care
provider.
Example: Year Reported
A case reported in December may not be counted until the next year. For example, if a case is reported
in December 2008 but not counted until January 2009, the Year Reported for the
case number
would
be 2008.
23
Exercise
1.
Date Reported
-+
For all questions in the Self-Study Modules, choose the one best answer.
1.1
The Date Reported is the date that the health department …
(circle the one best answer)
A.
First suspects that the patient has TB
B.
Receives notification from a health care provider that the patient is
suspected of having TB
C.
Submits the RVCT to the reporting area
D.
A, B, and C are all correct
E.
Only A and B are correct
1.2
How would January 3, 2009, be entered as the date for Date Reported?
(circle the one best answer)
A. Month Day Year
J A 0 3 2 0 0 9
B. Month Day Year
0 1 0 3 2 0 0 9
N
ote
:
Date Reported
(item 1) is frequently confused with
Date Submitted
(item 2) and
Date
Counted (item 6). Read the instructions for each of the items to understand the differences. In
the exercises for item 6, there is a study question and case study that involves all three items to
help you learn the differences.
24
1.3
How would February 2009 be entered as the date for Date Reported? The exact
day of the month is not known.
(circle the one best answer)
A. Month Day Year
0 2 9 9 2 0 0 9
B. Month Day Year
0 2 0 0 2 0 0 9
Case Study – Rose
On January 6, 2009, Dr. Joseph, a private physician, calls to notify the county health
department about Rose, a patient who has signs and symptoms of TB. On January 10, the
county health department receives a faxed copy of the laboratory results indicating that
Rose’s sputum is AFB smear positive. Then on January 27, the RVCT form is completed
by the county health department and sent to the state TB program.
1.4
Which date would you enter as the Date Reported?
(circle the one best answer)
A.
January 6, 2009
B.
January 10, 2009
C.
January 27, 2009
25
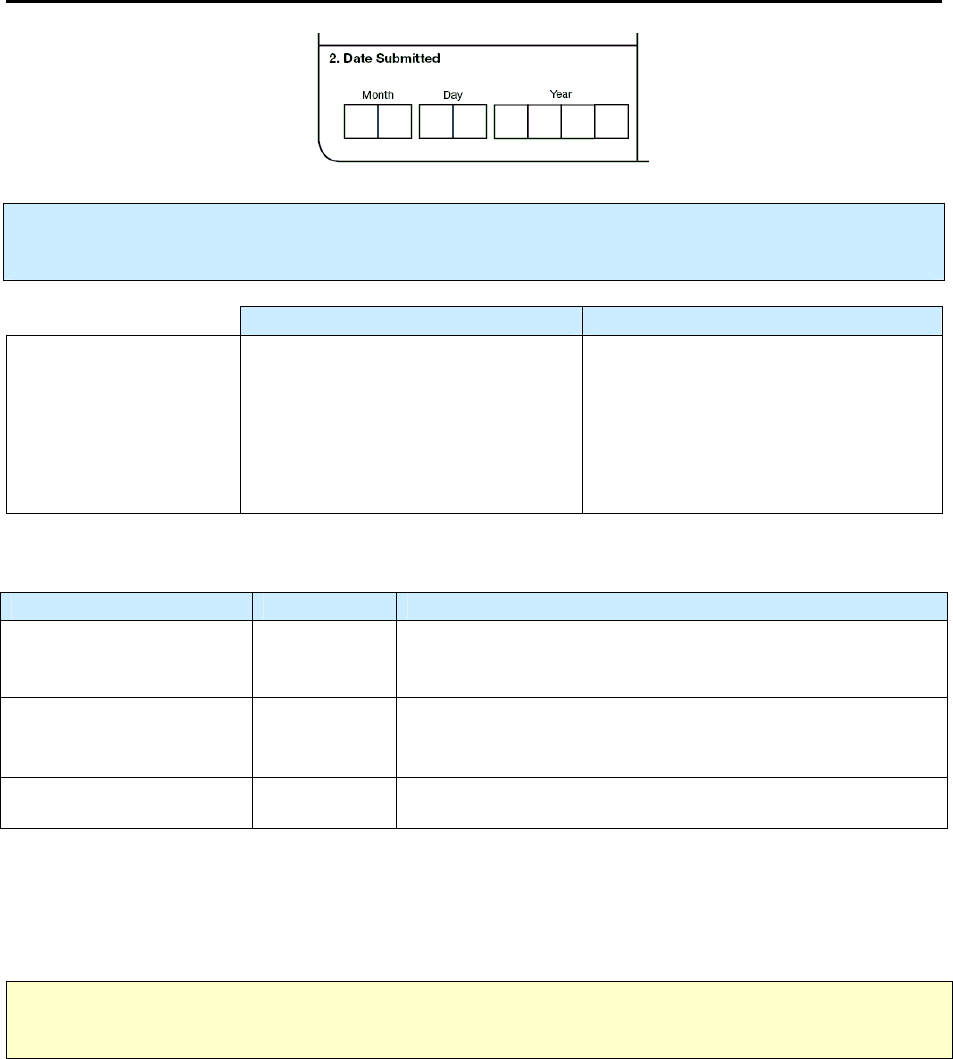
2.
Date Submitted
Primary Purpose: Programmatic function. Data are used to evaluate the time between case report and
submission to the health department or count authority.
Description Comment
Month, day, and year
(e.g., 01/17/2009)
Date the RVCT form was submitted
to the reporting area (e.g., state
health department).
If the day is unknown, enter 99 as the
default value (e.g., 01/99/2009).
(Note: this may vary from what will
be entered into a computer software
program)
Summary of Date Reported, Date Submitted, and Date Counted
Type of Date Who/What
Description of Action
Date Reported (item 1)
TB suspect
Reported to the health department (either by the health
department itself or another health care provider)
Date Submitted (item 2)
RVCT form
Submitted to the reporting area (e.g., state health
department)
Date Counted (item 6)
TB Case
Counted as a Case of TB (by the count authority)
Comment: Date Submitted
In most cases, the RVCT is completed by the health department (local or county) and submitted to the
reporting area (state health department). In some locations, the RVCT may be completed and the case
counted at the state level.
Note:
On the RVCT form and throughout this document, the term state is used to refer to the reporting
jurisdiction (or count authority), though not all jurisdictions are states.

26
Exercise
2.
Date Submitted
2.1
The Date Submitted is the date that the…
(circle the one best answer)
A.
Sputum sample is submitted to the laboratory
B.
RVCT is submitted to the reporting area
C.
Laboratory submits a confirmed diagnosis of TB to the health department
D.
A, B, and C are all correct
E.
Only A and B are correct
Case Study – Sue
On June 1, 2009, Sue, the health care worker at a county TB program, completes the
RVCT for a patient from the TB clinic. On June 30, Sue sends the RVCT to the state TB
program. On July 10, 2009, the state TB program determines that it is a case of TB.
2.2
Which date would you enter as Date Submitted?
(circle the one best answer)
A.
June 1, 2009
B.
June 30, 2009
C.
July 10, 2009
27
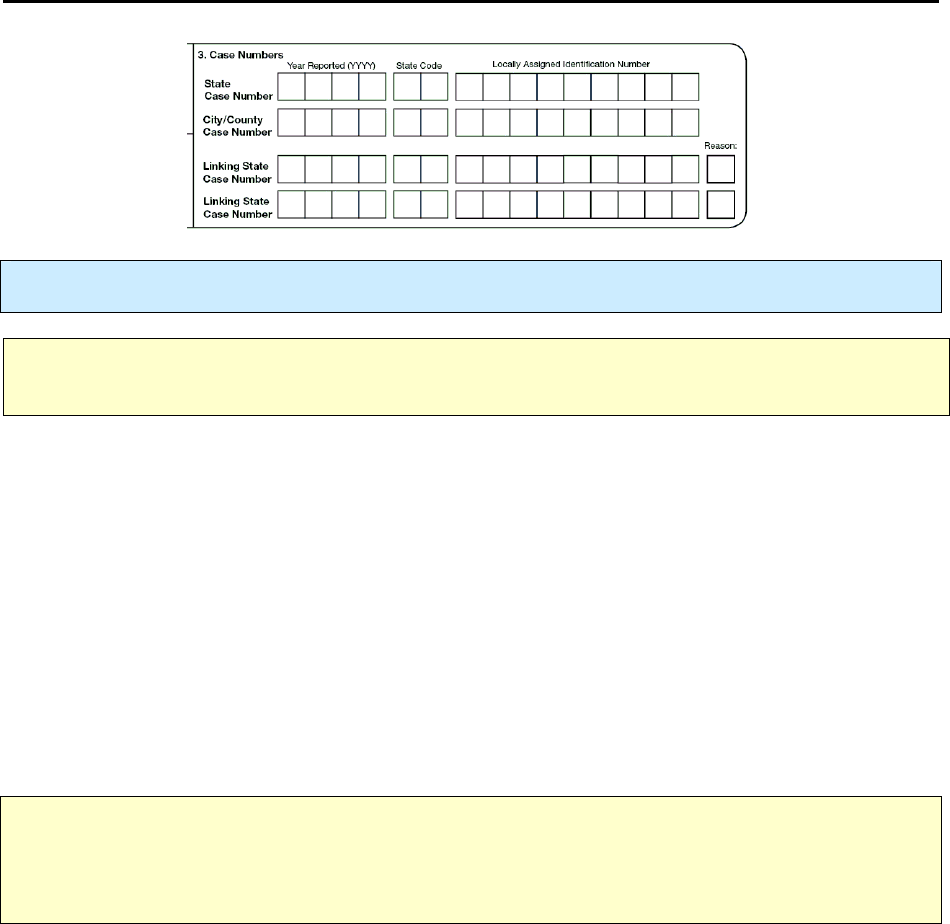
3.
Case Numbers
Primary Purpose: Surveillance. A unique number is assigned to each case without personal identifiers.
Note:
On the form and throughout this document, the term state is used to refer to the reporting
jurisdiction (or count authority), though not all jurisdictions are states.
State Case Number
The
State Case Number
is the
official identification number for the case
. If additional communication
about a record is required between CDC and a reporting area, this number is used to identify the record.
The
State Case Number
is commonly known as the RVCT number.
City/County Case Number
List the
City/County Case Number
. Every case reported, whether from a city/county or state
surveillance system, must have a unique case number for identification purposes.
Comment: Case Numbers
A single case may be assigned identical
City/County Case
and
State Case Numbers
. A
City/County
Case Number
may not be assigned to more than one case during a calendar year. Similarly, a
State Case
Number
may not be assigned to more than one case during a calendar year.
Note:
Case numbers must not include personal identifiers
. To maintain patient confidentiality, do
not
use names (either patient or provider), initials, Social Security numbers, addresses, telephone numbers,
or other information that could identify a patient. Case numbers are transmitted to CDC and therefore
must not include personal identifying information.
28
Assigning case numbers
Both the
State Case Number
and the
City/County Case Number
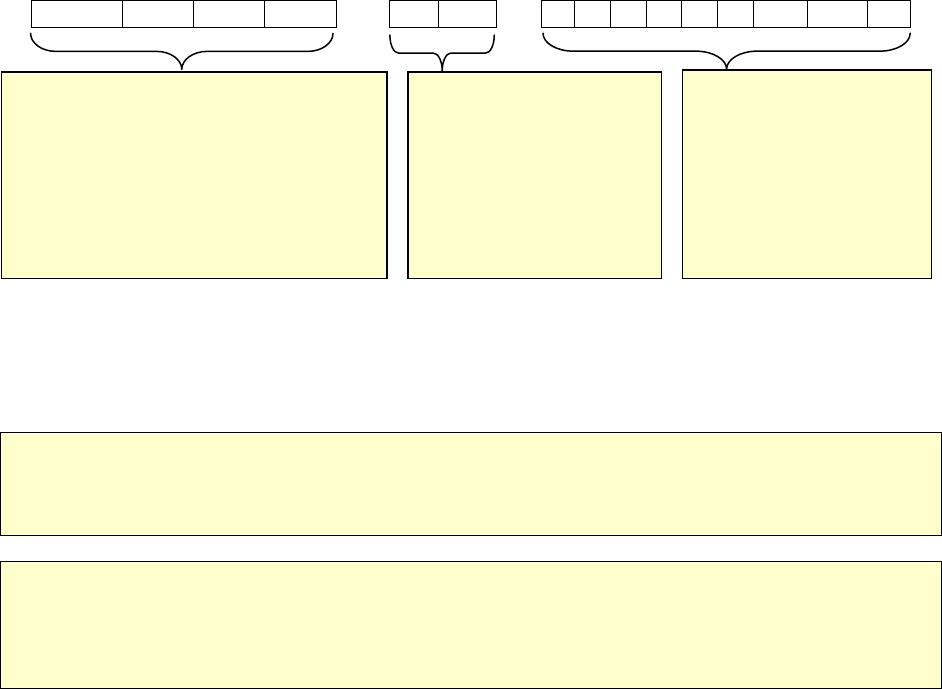
have 15 alphanumeric characters.
Year Reported (YYYY) State
Code
Locally Assigned Identification
Number
2 0 1 0 G A 0
0 0 1 2 3 4 5 6
Example: Year Reported
A case reported in December may not be counted until the next year. For example, if a case is reported in
December 2008 but not counted until January 2009, the Year Reported for the
case number
would be
2008.
Note:
All countable and noncountable TB cases should receive a unique case number. Documenting
noncountable cases provides evidence of increased workload or burden to programs when cases are
not
countable.
Note: For the purposes of the RVCT Materials, use the codes listed in the appendices. Some software
programs used to enter data on the RVCT may NOT use the codes listed in the appendices. For
example, the Anatomic Codes may be a drop-down item where you choose the actual site rather than
enter a code. For more information, see instructions for the software you use.
Linking State Case Numbers
For the purposes of linking RVCT forms, you
may enter as many as 2 RVCT
State Case Numbers
under
Linking State Case Number
.
Under
Reason for Linking Case
, explain the purpose of the link by entering one of the single-digit codes
indicated in the table below.
•
Year Reported indicates the year in
which the case is reported (e.g., 2010).
•
Year reported is used rather than the
year counted since there may be a lag
between when states or city/county
areas first suspected that the patient
might have TB and when diagnostic
criteria are verified.
State Code indicates the 2-
letter postal code of the
state reporting this case
(e.g., GA for Georgia) (see
Appendix D – Reporting
Area Codes). This
abbreviation is also known
as the state
code.
Locally Assigned
Identification Number
indicates the 9
numbers/characters that are
locally assigned to identify
this RVCT.
29
Rationale for Linking RVCT Forms
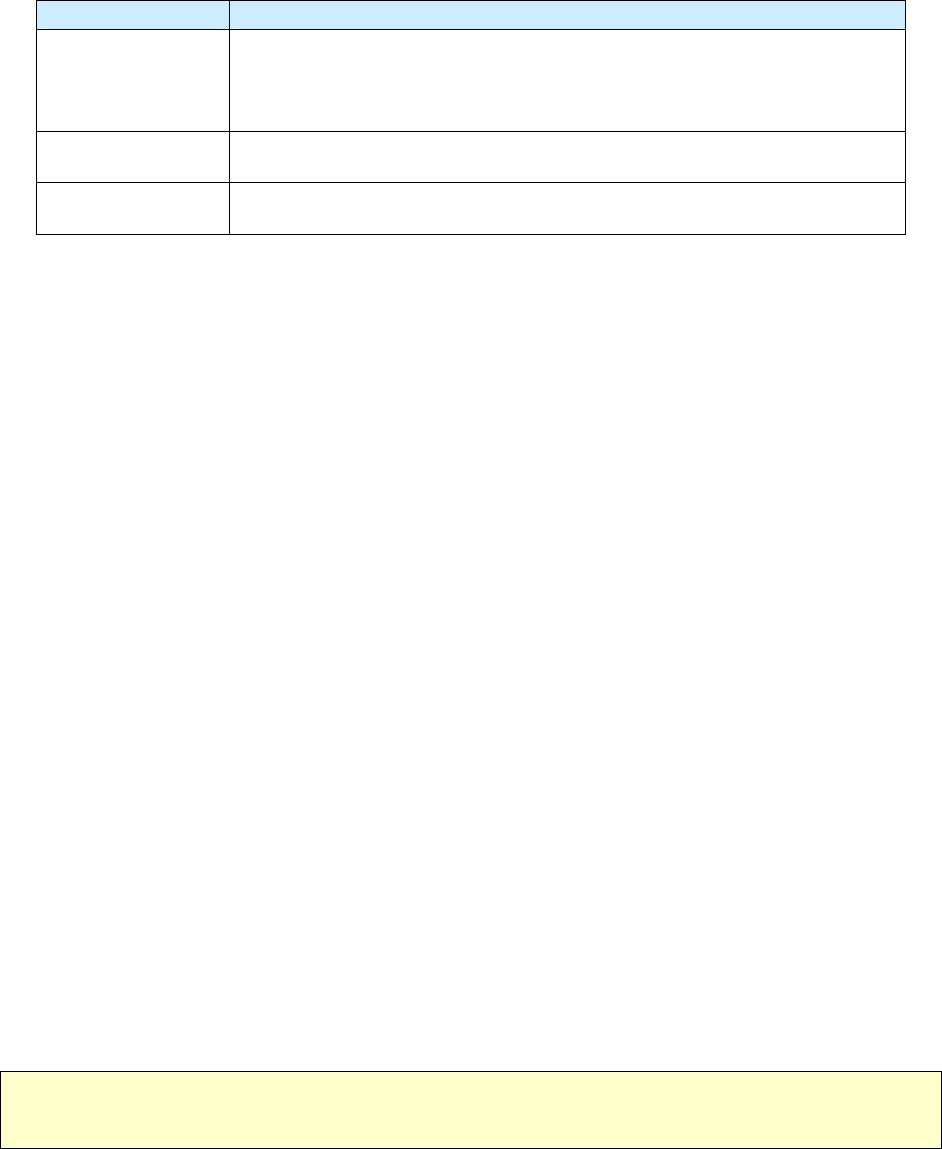
Reason Code Reason for Linking Case
1
Recurrence
or
Previous diagnosis of TB
2
Epidemiologically linked case, source case, or contact with another case
3
Case transferred from another area
Examples: Reasons for Linking Case
•
Reason 1 – Recurrence or previous diagnosis of TB*
If you are completing a “recurrence” RVCT for a diagnosis of TB disease in the same patient that
recurred
within
12 months after the completion of therapy, you must enter the RVCT
State Case
Number
of the original TB case under
Linking State Case Number
, and enter 1 as the
Reason
code.
A previous diagnosis of TB can have occurred any time in the past.
A patient is considered to have had a previous diagnosis of TB disease if
o
TB disease was verified in the past
or
o
The patient completed therapy (even if the case-to-case interval is within 12 months)*
or
o
The patient was lost to supervision for more than 12 months and now has verified disease
again.
If a patient had previous TB disease anytime in the past, enter 1 as the
Reason
code.
•
Reason 2 – Epidemiologically linked case, source case, or contact with another case
If you have identified the source case for the TB case for which you are completing the RVCT
and the RVCT
State Case Number
of the source case is available, enter the RVCT
State Case
Number
of the source case under
Linking State Case Number,
and enter 2 as the
Reason
code.
Another example of an
Epidemiologically linked case
is transmission of TB from one family
member to another.
•
Reason 3 – Case transferred from another area
If you are managing a TB case counted by another area, enter the RVCT
State Case Number
of
the case from the transferring jurisdiction under
Linking State Case Number,
and enter 3 as the
Reason
code. Transfer cases are linked when the patient is in therapy and transfers from another
reporting area. The patient could have moved or appeared at a health department in another area
after being lost to follow-up.
*Note: Recurrent cases within 12 months of completion of therapy should be considered noncountable,
regardless of whether the initial and the subsequent genotypes are the same or are different.
30
Comment: Recurrence of TB
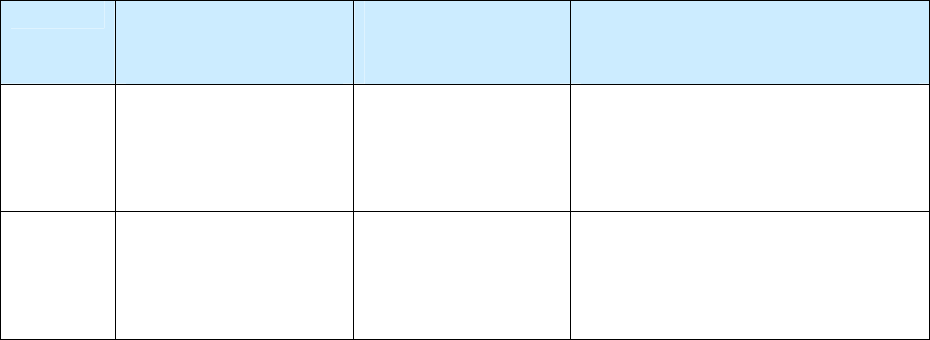
A recurrence (more than one separate and distinct episode) is defined as the return of TB disease in a
patient whose specimen result can be described by either of the options listed in the table below.
Specimen Results Required for Recurrence of TB Disease
Option Specimen Result
at Time of
Diagnosis
Specimen Result
While Receiving
Anti-TB therapy
Specimen Result
After Completion
of Therapy
Option 1 Culture positive
Becomes and
remains
culture negative
Becomes culture positive for M.
tuberculosis complex, or clinical or
radiologic evidence is consistent with
TB disease.
Option 2 Smear negative or
culture negative
(TB diagnosis is based
on clinical evidence)
Remains smear
negative or culture
negative
Becomes culture positive for M.
tuberculosis complex, or clinical or
radiologic evidence is consistent with
TB disease.
The process for reporting a recurrence of TB is illustrated in the table below.
31
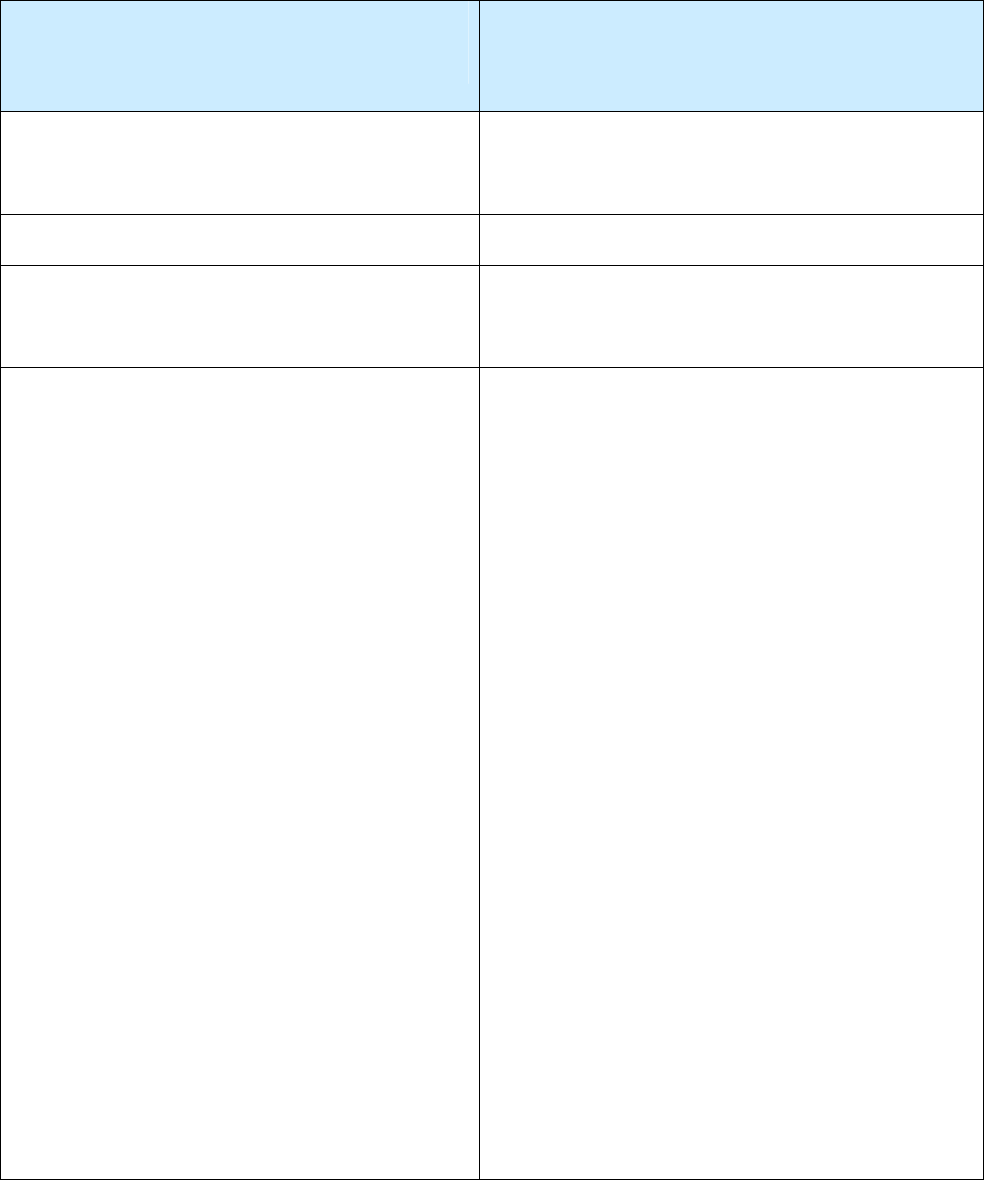
Process for Reporting a Recurrence of TB
A person may have more than 1 discrete (separate and distinct) episode of TB disease
TB Disease Recurs
Within a Consecutive 12-month Period
After the Patient Completed Therapy
TB Disease Recurs
More Than a Consecutive 12-month
Period
After the Patient Completed Therapy
Recurrence is considered the same TB episode
(count only 1 episode as a case for that year; within
a 12-month period,
not
calendar year).
Recurrence is considered a separate TB episode.
Do
not
count as a new case.
Count as a new case.
Count only one TB episode as a case for that year
(within a 12-month period,
not
calendar year).
No updates are needed for the initial RVCT form
because therapy was completed at least 12 months
before the recurrence was diagnosed.
Complete 2 RVCT Forms
(Only the initial TB episode is countable)
1) For the initial countable TB episode:
a) Ensure that
Date Therapy Stopped
(item
43) reflects a date of therapy completion
before TB recurrence.
b) Do not update any other variables on the
RVCT form.
2) For the noncountable TB episode:
a) Use a new RVCT
State Case Number
(item 3), that is, a number that is different
from the
State Case Number
on the
countable TB episode form.
b) Enter the countable TB episode
State Case
Number
under
Linking State Case
Number
and specify as
Reason 1 –
Recurrence or previous diagnosis of TB
so
that these 2 forms can be linked.
c) Check
Verified Case: Recurrent TB
within 12 months
for the variable
Count
Status
(item 5).
d) Complete the remainder of the RVCT form as
appropriate. This case will not be included in
the TB case count of the reporting area, but will
provide valuable information on recurrences
within
12 months after the completion of
therapy. This allows electronic linkage
between the countable TB episode and data
associated with the recurrence.
Complete 2 RVCT Forms
(Both TB episodes are countable)
1) For the initial countable TB episode:
a.)
Do not update any other variables on the
RVCT form.
2) For the second countable TB episode:
a.)
Enter a new RVCT
State Case Number
(item
3), different from the
State Case Number
on
the initial RVCT form.
b.)
Enter the initial RVCT
State Case Number
,
if available, under
Linking State Case
Numbers
and specify as
Reason 1 –
Recurrence or previous diagnosis of TB
so
that these 2 forms can be linked.
c.)
Check
Count as a TB Case
for
Count Status
(item 5). Do
not
check
Verified Case:
Recurrent TB ≤ 12 months
.
d.)
Complete the remainder of the RVCT form as
appropriate. This TB case will be counted
because, for surveillance purposes, it is
considered a separate TB episode. Also, it will
provide valuable information on recurrences
more than 12 months after the completion of
therapy. This allows electronic linkage
between the initial TB episode and the new
TB episode.
32
Exercise
3.
Case Numbers
Case Study – Henry
Henry is diagnosed with TB in June 2008 in Seattle, WA. His TB is counted as a new
case and his 2008 locally assigned identification number is 000080301. He successfully
completes directly observed therapy on December 12, 2008.
In August 2009, Henry presents to the Seattle hospital emergency room with a recent
history of fever, weight loss, and non-productive cough. His subsequent sputum smears
are positive. In 2009 he is reported as having TB disease, but it is NOT counted as a
new TB case (i.e., this is less than 12 months since he completed treatment for his prior
diagnosis of TB). His 2009 locally assigned identification number is 000090056.
3.1
What is Henry’s 2009 State Case Number?
(circle the one best answer)
A. Year Reported
(YYYY)
State
Code
Locally Assigned Identification Number
2 0 0 9 W A 0 0 0 0 9 0 0 5 6
B. Year Reported
(YYYY)
State
Code
Locally Assigned Identification Number
2 0 0 9 W A 0 0 0 0 8 0 3 0 1
3.2
What is his Linking State Case Number?
(circle the one best answer)
A. Year Reported
(YYYY)
State
Code
Locally Assigned Identification Number
2 0 0 8 W A 0 0 0 0 9 0 0 5 6
B. Year Reported
(YYYY)
State
Code
Locally Assigned Identification Number
2 0 0 8 W A 0 0 0 0 8 0 3 0 1
3.3
What is the Reason for the Linking State Case Number?
(circle the one best answer)
A.
Reason 1 – Recurrence or previous diagnosis of TB
B.
Reason 2 – Epidemiologically linked case, source case or contact with another case
C.
Reason 3 – Case transferred from another area
33
Case Study – Lisa
In May 2008, Lisa is part of a contact investigation for her brother, who has infectious
TB disease. Lisa is evaluated and diagnosed with TB disease also. She starts treatment for
TB in June 2008.
3.4
For Item 3 Case Number, what would you choose as the reason for the Linking
State Case Number for Lisa?
(circle the one best answer)
A.
Reason 1 – Recurrence or previous diagnosis of TB
B.
Reason 2 – Epidemiologically linked case, source case, or contact with another
case
C.
Reason 3 – Case transferred from another area
34
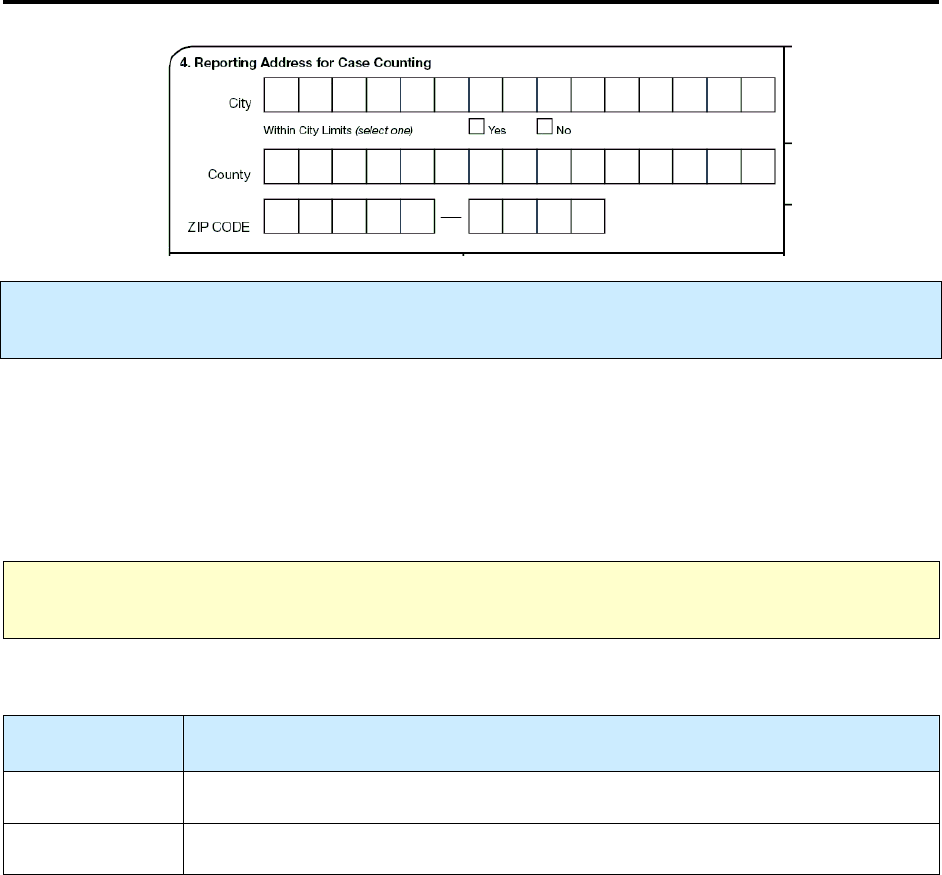
4.
Reporting Address for Case Counting
Primary Purpose:
Programmatic function. Data are used to document the patient’s address from the
state or jurisdiction that is counting the case.
The Reporting Address for Case Counting is usually the City, County, and ZIP Code of the patient’s
residence at the time of diagnosis. But there are exceptions to this, which are indicated in the Guidelines
to Determine Reporting Address for Case Counting table below. To the extent possible, the address for
case counting should represent the home address (whether permanent or temporary) of the patient.
Recommendations for counting reported TB cases are outlined in Appendix B – Recommendations for
Reporting and Counting Tuberculosis Cases.
Note:
For countable and noncountable cases, enter the TB patient’s address from the state or
jurisdiction that is reporting and documenting the case.
For
Within City Limits
select the best option.
Option
(select one)
Description
Yes
Patient lives within the city limits
No
Patient does not live within the city limits
35
Guidelines to Determine Reporting Address
Patient Scenarios How to Count Reporting
Address
Migrant, immigrant (i.e.,
resident alien living in the
United States), U.S. military
personnel, and other
transient persons
Count in the area in which he/she lived
at the time that the TB diagnostic
evaluation was performed or initiated
Enter city, county,
and ZIP Code
where he/she lives
at the time of
diagnosis
Homeless or does not have
a fixed residence
Count in area in which he/she was
living at the time that the TB diagnostic
evaluation was performed or initiated
(e.g., the locality of the shelter or area in
which the patient was living)
Enter city, county,
and ZIP Code of
that locality
Resident of correctional
facility at time of TB
diagnosis (e.g. local, state,
federal, military)
Count in area in which the correctional
facility is located at the time that the TB
diagnostic evaluation was performed or
initiated
Enter city, county,
and ZIP Code of
the correctional
facility
Specific Populations
(these groups supersede Specific Locations,
but not Other People Entering the United States)
Resident of long-term care
facility at time of TB
diagnosis
Count in area in which the long-term
care facility is located at the time that
the TB diagnostic evaluation was
performed or initiated
Enter city, county,
and ZIP Code of
the long-term
care facility
Receives a new TB
diagnosis in the community
that he/she considers home
Count in the morbidity count for that
area
Enter city, county,
and ZIP Code of
residence
Receives a new TB
diagnosis, but is an out-of-
area resident and will
return home for treatment
Count in morbidity count of their home
area
Enter city, county,
and ZIP Code of
his/her home area
Receives a new TB
diagnosis, but is an out-of-
area resident and
completes therapy where
he/she was diagnosed
Count in morbidity count where they
live at the time that the TB diagnostic
evaluation was performed or initiated
Enter city, county,
and ZIP Code
where he/she lives
at the time of
diagnosis
Specific Locations
Staying in a community
only for TB diagnosis and
hospitalization
Count in the morbidity count of his/her
area of residence, not the community
where diagnosed and hospitalized.
Communication between health
departments may be necessary to decide
which jurisdiction will count the case.
Enter city, county,
and ZIP Code of
his/her home area
36
Foreign visitor who receives a
TB diagnosis in the United
States, is receiving anti-TB
therapy, and has been, or plans
to remain, in the country for
90 days or more
Count in the area in which he/she lived
at the time that the TB diagnostic
evaluation was performed or initiated
Enter city, county,
and ZIP Code of
current residence
Foreign visitor who receives a
diagnosis of TB in the United
States, is receiving anti-TB
therapy, and has been, or plans
to remain, in the country for
less than 90 days
Should not be included in the count of
TB cases in the United States.
Enter city, county,
and ZIP Code of
current residence
Other People Entering the United States
Receives a diagnosis of TB
before arriving in the United
States
Should not be included in the count of
TB cases in the United States. Submit it
as a noncountable case because the case
is considered to have occurred in
another country, even if therapy is
continued or completed in the United
States.
Enter city, county,
and ZIP Code of
current residence
Comment: People Entering the United States
For additional information on immigrants, refugees, permanent resident aliens, border crossers, and
foreign visitors see Appendix B – Recommendations for Reporting and Counting Tuberculosis Cases.
Guidelines for classifying transfer cases
A total of 60 areas are responsible for reporting cases of TB to CDC. These reporting areas are the 50
states, the District of Columbia, New York City, Puerto Rico, American Samoa, the Federated States of
Micronesia, Guam, the Republic of the Marshall Islands, the Commonwealth of the Northern Mariana
Islands, the Republic of Palau, and the U.S. Virgin Islands. Because of the additional (follow-up)
reporting requirements for expanded surveillance, specific instructions are necessary for the completion
of forms for patients who move within a reporting area and for those who move from one reporting area
to another during treatment.
•
To minimize the number of TB patients who are lost to follow-up, update the patient’s street
address regularly during treatment.
•
Periodically, ask patients whether they anticipate moving so that arrangements can be made to
maintain continuity of care and ensure submission of follow-up RVCTs. Encourage patients who
anticipate moving to report their new address, so that necessary patient information can be
forwarded to health care providers, and to the TB control program in the area to which the patient
is moving. Health departments should use the National TB Controllers Association (NTCA)
Interjurisdictional Tuberculosis Notification and Follow-up forms to notify TB control program
staff in another reporting area that a TB patient is moving to their area.
Communication between TB control programs to ensure continuity of care and submission of
follow-up reports regarding a patient who is moving from one area to another should be
conducted as efficiently and securely as possible (e.g., telephone, e-mail, fax, express courier).
37
Example:
Moves within the reporting area
If a TB patient with an existing RVCT record moves within the reporting area that initially reported the
case (e.g., from county A to county B within a state), communication between county or local health
departments may be all that is necessary to maintain continuity of care and ensure submission of follow-
up reports for the RVCT. In this instance, the responsibility for following the case to closure and for
submitting follow-up reports to CDC remains with the initial reporting area (e.g., the state). To avoid
duplicate case reporting, the state may need to coordinate the submission of forms with counties A and B
so that only one counted case is submitted. County B can complete a noncountable RVCT to gather
surveillance data and demonstrate patient management.
Example:
Moves from one reporting area to another
If a TB patient with an RVCT record moves from one reporting area to another (e.g., from state A
[Louisiana] to state B [Georgia]), the responsibility for submitting follow-up reports to CDC remains with
the state or reporting area that initially reported the case to CDC and counted it (e.g., state A [Louisiana]).
This responsibility remains with the initial area only for surveillance purposes (i.e., to minimize
duplication of case reports and to simplify the reporting of the final disposition of the case). In other
words, state B will conduct case management and follow-up and will then share follow-up surveillance
information with state A, which will officially submit follow-up information to CDC. State B is
encouraged to complete an RVCT for a noncountable transfer case.
To facilitate this process, state A should send the NTCA Interjurisdictional Tuberculosis Notification and
Follow-up forms to state B and should inform state B that the case has been reported to CDC and
counted. State A should also inform state B of the surveillance information that has been reported to CDC
and the information that will need to be collected by state B and forwarded to state A for reporting to
CDC. State B should use the forms to inform state A when or if the TB patient has been located and to
inform state A of the final disposition of the case (e.g., patient completed therapy, patient died).
Comment: Definition for Migrant/seasonal worker
A migrant or seasonal worker is a person who is required to be absent from a permanent place of
residence for the purpose of seeking employment or who may vary their employment for the purpose of
remaining employed while maintaining a permanent place of residence.
Examples:
Migrant/seasonal worker
•
Migratory agricultural worker
•
Seasonal agricultural worker
•
Migrant factory worker
•
Migrant construction worker
•
Migrant service industry worker
•
Migrant sporting worker (e.g., horse racing, dog racing)

38
Comments: Definitions for Homeless
There are many definitions for homeless (National Coalition for the Homeless). A
homeless
person may be an individual who has
1.
No fixed, regular, and adequate nighttime residence
and
2.
A primary nighttime residence that is
a.
A supervised publicly or privately operated shelter designed to provide temporary living
accommodations, including welfare hotels, congregate shelters, and transitional housing for
the mentally ill
or
b.
An institution that provides a temporary residence for individuals intended to be
institutionalized
or
c.
A public or private place not designated for, or ordinarily used as, a regular sleeping
accommodation for human beings.
A
homeless
person may also be defined as a person who has no home (e.g., is not paying rent, does not
own a home, and is not steadily living with relatives or friends). Persons in unstable housing situations
(e.g., alternating between multiple residences for short stays of uncertain duration) may also be
considered homeless.
A
homeless
person may be a person who lacks customary and regular access to a conventional dwelling
or residence. Included as homeless are persons who live on streets or in nonresidential buildings. Also
included are residents of homeless shelters and shelters for battered women. Residents of welfare hotels,
and single room occupancy (SRO) hotels could also be considered homeless. In the rural setting, where
there are usually few shelters, a homeless person may live in non-residential structures, or substandard
housing, or with relatives. Homeless does not refer to a person who is imprisoned or in a correctional
facility.
Note:
The homeless category is limited to living conditions in the United States and does
not
apply to
living in refugee camps outside the United States.

39
Exercise
4.
Reporting Address for Case Counting
Case Study – Laverne
Laverne lives in Chicago, Illinois. On May 1, 2009, she visits her sister Shirley in
Milwaukee, Wisconsin, for a month. During the visit Laverne develops a bad cough and
fever. She goes to the Milwaukee health clinic and is diagnosed with TB disease. On
June 2 Laverne returns home to Chicago, where she completes treatment.
4.1
What reporting address should be entered on the 2009 RVCT for Laverne?
(circle the one best answer)
A.
Chicago, Illinois
B.
Milwaukee, Wisconsin
Case Study – Eduardo
Eduardo, a migrant worker, resides with his family in Sacramento, California. He travels
south to work the strawberry harvest every year beginning in February. During harvest
season, he lives in a boarding facility in the city of Watsonville, California. In May 2009,
Eduardo is diagnosed with TB as part of a Watsonville screening program for migrant
workers and begins treatment. After 2 months of treatment, he returns home to
Sacramento, where he completes treatment.
4.2
What reporting address should be entered on the 2009 RVCT?
(circle the one best answer)
A.
Sacramento
B.
Watsonville

40
5.
Count Status
Primary Purpose: Surveillance. Data are used to document the number of TB cases and disease trends
that occur in the United States; to determine the burden of TB disease within all areas; and to serve as a
basis for allocation of resources, including funding.
In addition to requiring the completion of an RVCT form for all counted TB cases, CDC recommends
that a reporting area complete an RVCT form for TB patients being managed in that area but counted by
another reporting area, even though the area providing case management cannot include such cases in its
annual morbidity count. This will help indicate the burden of disease within all areas. Moreover, CDC
recommends that a reporting area complete an RVCT form for TB recurrences which are within 12
months after the completion of therapy, which are also not included in the annual morbidity count. For
CDC guidelines on counting TB cases, see Appendix B – Recommendations for Reporting and Counting
Tuberculosis Cases.
Countable TB Case
Option Description
Officially counted as a TB case, by the jurisdiction with count authority
(usually state health department).
Count as a TB case
For a diagnosis to be counted as a TB case, it must meet the following criteria:
1. Is a verified case of TB (see Case Definition for Tuberculosis below)
2. Confirmed that it is NOT counted by another area
3. Meets surveillance definition and is NOT a recurrent case (within 12
months of completion of therapy) of TB
Note: A case of TB is defined as an episode of TB disease in a person meeting the laboratory or
clinical criteria for TB as defined in Appendix A – Tuberculosis Case Definition for Public Health
Surveillance for criteria.
Note:
Communication between TB control programs to ensure continuity of care and submission of
reports regarding a patient who is moving from one area to another should be conducted as securely
and efficiently as possible (e.g., telephone, e-mail, secure fax, express courier).
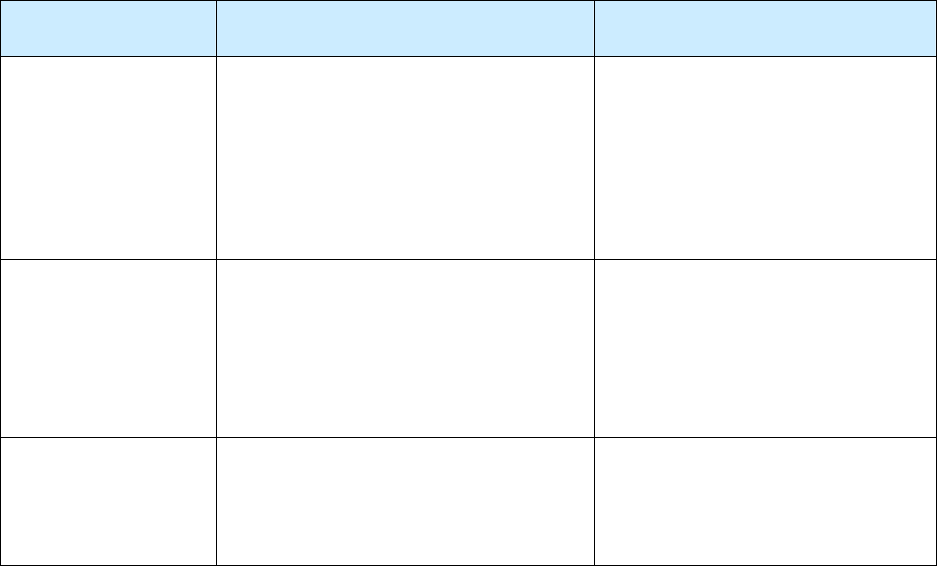
41
Noncountable TB Case
If the verified TB case was
not
counted by the jurisdiction with count authority, select one option to
indicate the reason the verified TB case was noncountable.
Option
(select one)
Description Comment
Counted by another
area (e.g., county,
state, or counting
authority)
TB case counted by another U.S. area
such as a state or other counting
authority (e.g., transfer in)
Typically, diagnostic workup has
been completed, and patient is
receiving anti-TB medications.
Count authority includes the U.S.
Territories, U.S. Island Areas, and
U.S. Outlying Areas.
TB treatment
initiated in another
country
TB case counted by another
country
Under Specify, enter the country where
TB treatment was initiated.
It may be difficult to verify whether
a case has been counted in another
country because typically,
diagnostic work-up may have been
completed and patient is receiving
anti-TB medications.
Recurrent TB
within 12 months
after completion of
therapy
Complete a new RVCT form because of
recurrence within 12 months after the
completion of therapy (not when
therapy was initiated)
Completing a new RVCT form
allows the RVCT forms to be linked
and information on the recurrence
to be collected.
Comment: 12 months
The term 12 months refers to 12 consecutive months, not a calendar year.
Comment: U.S. Territories, U.S. Island Areas, and U.S. Outlying Areas
Counted by another area or counting authority
includes the U.S. Territories, U.S. Island Areas, and U.S.
Outlying Areas (e.g., Puerto Rico, American Samoa, Federated States of Micronesia, Guam, Republic of
the Marshall Islands, Commonwealth of the Northern Mariana Islands, Republic of Palau, U.S. Virgin
Islands). These independent countries have Compacts of Free Association with the United States; under
these compacts, the countries are fully sovereign in domestic and foreign affairs, but give responsibility
for their health, education, defense, and other essential operations to the United States.
42
Counting Recurrent TB Cases
A person may have more than 1 discrete (separate and distinct) episode of TB disease
TB Disease Recurs
Within a Consecutive 12-month Period
After the Patient Completed Therapy
TB Disease Recurs
More Than 12 Months
After the Patient Completed Therapy
Recurrence is considered the same TB episode
(count only 1 episode as a case for that year; within
a 12-month period,
not
calendar year).
Recurrence is considered a separate TB episode.
Do
not
count as a new case.
Count as a new case.
Note: Recurrent cases within 12 months of completion of therapy should be considered noncountable,
regardless of whether the initial and the subsequent genotypes are the same or are different.
Comment: People Entering the United States
For additional information on immigrants, refugees, permanent resident aliens, border crossers and
foreign visitors see Appendix B – Recommendations for Reporting and Counting Tuberculosis Cases.

43
Exercise
5.
Count Status
Count Status (item 5) can be quite confusing, so there are 13 study questions and case studies
included to help you understand this item.
5.1
What information is needed to determine the Count Status?
(circle the one best answer)
A.
That it is a verified case of TB
B.
Whether the case is countable (meets surveillance definition)
C.
Confirmed that it is NOT counted by another US area
D.
A, B, and C are all correct
E.
Only A and B are both correct
Case Study – Carlos
Carlos is diagnosed with TB in May 2008 after being evaluated at a free clinic for
homeless persons in Springfield, Missouri. He successfully completes directly observed
therapy on December 10, 2008.
In July 2009, Carlos presents to Springfield Hospital, and an astute clinician recognizes
signs of TB disease. Carlos’s chest radiograph is consistent with TB disease. His sputum
smear is AFB-positive and he is started on anti-TB therapy. Later his culture result is
positive for M. tuberculosis complex.
5.2
Does Carlos have a countable case for 2009 national surveillance?
(circle the one best answer)
A.
Yes, because his recurrence of TB disease is more than 12 months after
completion of therapy
B.
Yes, because his recurrence of TB disease is more than 12 months after initial
diagnosis
C.
No, because his recurrence of TB disease is within 12 months of completion of
therapy
D.
No, because his recurrence of TB disease is more than 12 months after initial
diagnosis
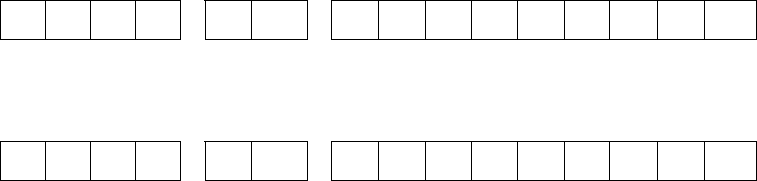
44
Case Study – Carlos (continued)
Instead of having a TB recurrence diagnosed in July 2009, Carlos presents to the hospital
on January 18, 2010. All clinical characteristics remain the same.
5.3
Does Carlos have a countable case for 2010 national surveillance?
(circle the one best answer)
A.
Yes, because his recurrence of TB disease is more than 12 months after
completion of therapy
B.
Yes, because his recurrence of TB disease is more than 12 months after initial
diagnosis
C.
No, because his recurrence of TB disease is within 12 months of completion of
therapy
D.
No, because his recurrence of TB disease is more than 12 months after initial
diagnosis
Case Study for Items 3 and 5 – Raul
Raul lives in El Paso, Texas, and is diagnosed with TB disease in September 2008 and
completes therapy on March 21, 2009. His Texas locally assigned identification number
is 200800121. In December 2009, Raul moves to Arizona. He presents to the Tucson
General Hospital with symptoms of TB on May 1, 2010, and is evaluated for TB. He is
diagnosed with TB disease on May 12, 2010. His Arizona locally assigned identification
number is 201000032.
5.4
What is the State Case Number for Item 3 on the 2010 RVCT?
(circle the one best answer)
A.
Year Reported
(YYYY)
State
Code
Locally Assigned Identification Number
2 0 0 8 T X 2 0 0 8 0 0 1 2 1
B. Year Reported
(YYYY)
State
Code
Locally Assigned Identification Number
2 0 1 0 A Z 2 0 1 0 0 0 0 3 2
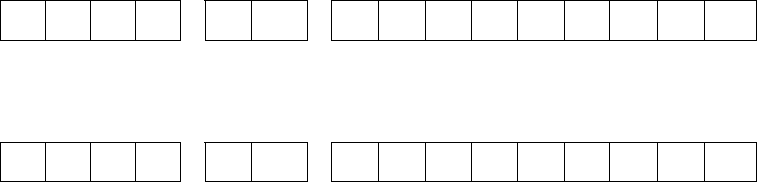
45
5.5
What is the Linking State Case Number for Item 3 on the 2010 RVCT?
(circle the one best answer)
A.
Year Reported
(YYYY)
State
Code
Locally Assigned Identification Number
2 0 0 8 T X 2 0 0 8 0 0 1 2 1
B. Year Reported
(YYYY)
State
Code
Locally Assigned Identification Number
2 0 1 0 A Z 2 0 1 0 0 0 0 3 2
5.6
What is the Reason for the Linking State Case Number for Raul?
(circle the one best answer)
A.
Reason 1 – Recurrence or previous diagnosis of TB
B.
Reason 2 – Epidemiologically linked case, source case, or contact with another
case
C.
Reason 3 – Case transferred from another area
5.7
How would you complete item 5, Count Status, on the 2010 RVCT?
(circle the one best answer)
A.
Count as a TB case
B.
Noncountable – Verified Case: Counted by another U.S. area
C.
Noncountable – Verified Case: TB treatment initiated in another country
D.
Noncountable – Verified Case: Recurrent TB within 12 months after completion of
therapy
46
Case Study for Items 3 and 5 – Elton
Elton lives in Cut and Shoot, Texas, and is diagnosed with TB disease in June 2009 and
immediately starts therapy. In December 2009, Elton moves to Truth or Consequences,
New Mexico, and continues DOT through the County Health Department.
5.8
What is the Reason for the Linking State Case Number for Elton?
(circle the one best answer)
A.
Reason 1 – Recurrence or previous diagnosis of TB
B.
Reason 2 – Epidemiologically linked case, source case, or contact with another
case
C.
Reason 3 – Case transferred from another area
Case Study for Items 3, 4, and 5 – John
The Tennessee TB program confirms that John is diagnosed with TB on November 1,
2008, and he starts on DOT. Tennessee counts his case as a 2008 case. John receives only
one week of anti-TB therapy while in Tennessee. He is then reported as lost.
In December 2008, John moves to Fulton County, Georgia. He goes to the Fulton County
TB Clinic on February 2, 2009, complaining of a prolonged cough and fever. During the
interview, John reveals that he was treated for TB in the past in Tennessee. The Fulton
County TB clinic evaluates him for TB disease. He is started on anti-TB therapy. The
Fulton County TB Clinic contacts the Tennessee TB program to confirm the information
and to inform the Tennessee program that John is now living in Georgia. On February
16, TB disease is confirmed based on a positive culture, and he completes treatment in
Georgia, in August 2009.
5.9
Which state should count the case?
(circle the one best answer)
A.
Tennessee
B.
Georgia
5.10 What reporting address should be entered on the 2008 RVCT?
(circle the one best answer)
A.
John’s address in Tennessee
B.
John’s address in Georgia
47
5.11 What is the Reason for the Linking State Case Number of the 2009 RVCT?
(circle the one best answer)
A.
Reason 1 – Recurrence or previous diagnosis of TB
B.
Reason 2 – Epidemiologically linked case, source case, or contact with another
case
C.
Reason 3 – Case transferred from another area
5.12 What reporting address should be entered on the 2009 RVCT?
(circle the one best answer)
A.
John’s address in Tennessee
B.
John’s address in Georgia
5.13 How would you complete item 5, Count Status, on the 2009 RVCT?
(circle the one best answer)
A.
Count as a TB case
B.
Noncountable – Verified Case: Counted by another U.S. area
C.
Noncountable – Verified Case: TB treatment initiated in another country
D.
Noncountable – Verified Case: Recurrent TB within 12 months
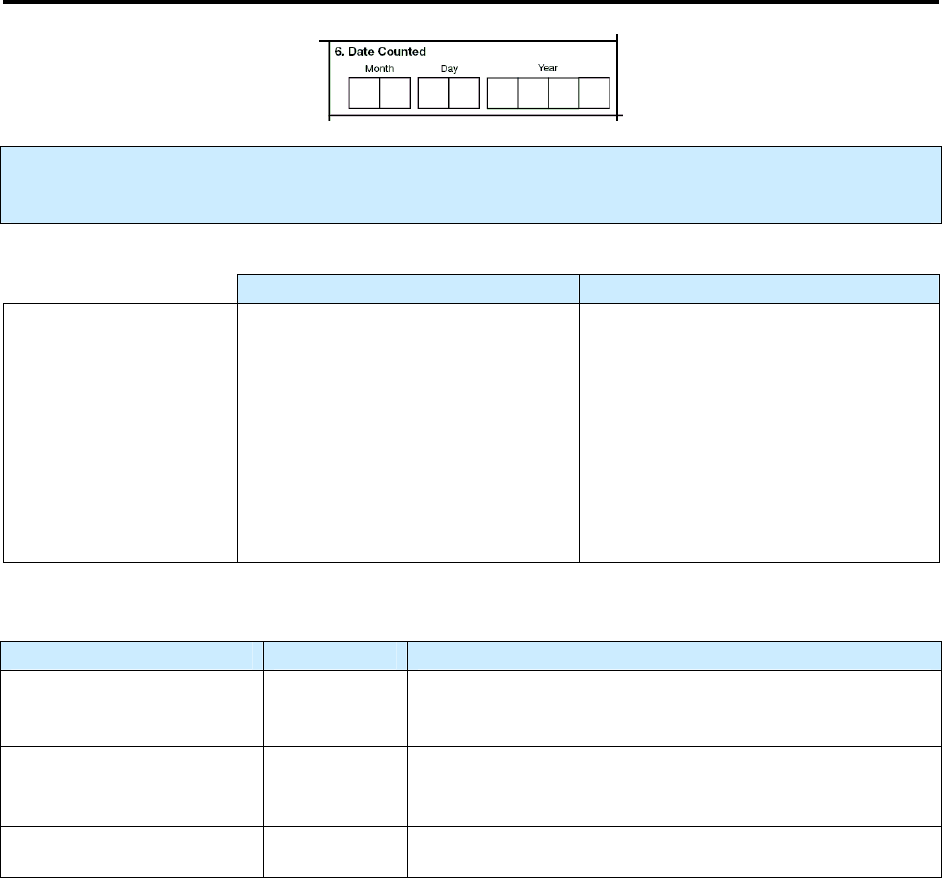
48
6.
Date Counted
Primary Purpose: Surveillance. Data are used by the count authority to tally the official TB case count
for the month, quarter, and year.
Description Comment
Month, day, and year
(e.g., 01/17/2009)
Date that the responsible count
authority (usually the state health
department, but might be another
designated authority)
• Reviewed the RVCT
• Verified the case as TB
and
• Included it in the official TB
case count
If the day is unknown, enter 99 as the
default value (e.g., 01/99/2009).
Summary of Date Reported, Date Submitted, and Date Counted
Type of Date Who/What
Description of Action
Date Reported (item 1)
TB suspect
Reported to the health department (either by the health
department itself or another health care provider)
Date Submitted (item 2)
RVCT form
Submitted to the reporting area (e.g., state health
department)
Date Counted (item 6)
TB case
Counted as a Case of TB (by the count authority)
Comment: Pending results
Suspected cases for which bacteriologic results are pending or for which verification of disease is
questioned for any reason should be counted only after they are determined to be verified TB cases. This
could mean that a case reported in one year may not be counted until the following year.
Example: Date Counted
If a case is reported to the health department in December 2008, but bacteriologic or clinical evidence of
TB is not available until January 2009, the case should be counted in January 2009 (when TB disease was
verified), not in December.
49
Exercise
6.
Date Counted
For questions 6.1 – 6.3
What is the description for the following items?
(Choose the one best answer by matching the description with the RVCT item. Write the letter
for the description on the line next to the question number.)
Item Description
___
6.1
Item 1, Date Reported
A.
Date the reporting area sends the RVCT to
the counting authority
___
6.2
Item 2, Date Submitted
B.
Date that the count authority verifies the
case as TB and it is included in the official
case count
___
6.3
Item 6, Date Counted
C.
Date that a health department first suspects
that the patient might have TB
Case Study – Richard
On September 3, 2009, Richard is diagnosed with culture-positive TB. It is confirmed
that he has never had TB disease before. The RVCT form is completed by the county
health department on September 12 and sent to the state TB program. On September 21
the state TB program determines that it is a new verified case of TB and reports it to
CDC.
6.4
Which date is the Date Counted?
(circle the one best answer)
A.
September 3, 2009
B.
September 12, 2009
C.
September 21, 2009
50
Case Study - Items 1, 2, and 6 – Reviewing the RVCT
You are reviewing an RVCT that your colleague Jim completed. You look at the
following dates that were entered for Items 1, 2, and 6. You notice that there is a problem
with the following dates.
Item 1 – Date Reported – May 18, 2009
Item 2 – Date Submitted – May 18, 2009
Item 6 – Date Counted – May 3, 2009
6.5
What is the problem with the dates?
(circle the one best answer)
A.
The Date Reported cannot be the same date as the Date Submitted because the
case cannot be submitted the same day that it was reported.
B.
The Date Counted cannot come before the Date Reported because the case has
to be reported and evaluated before it can be counted.
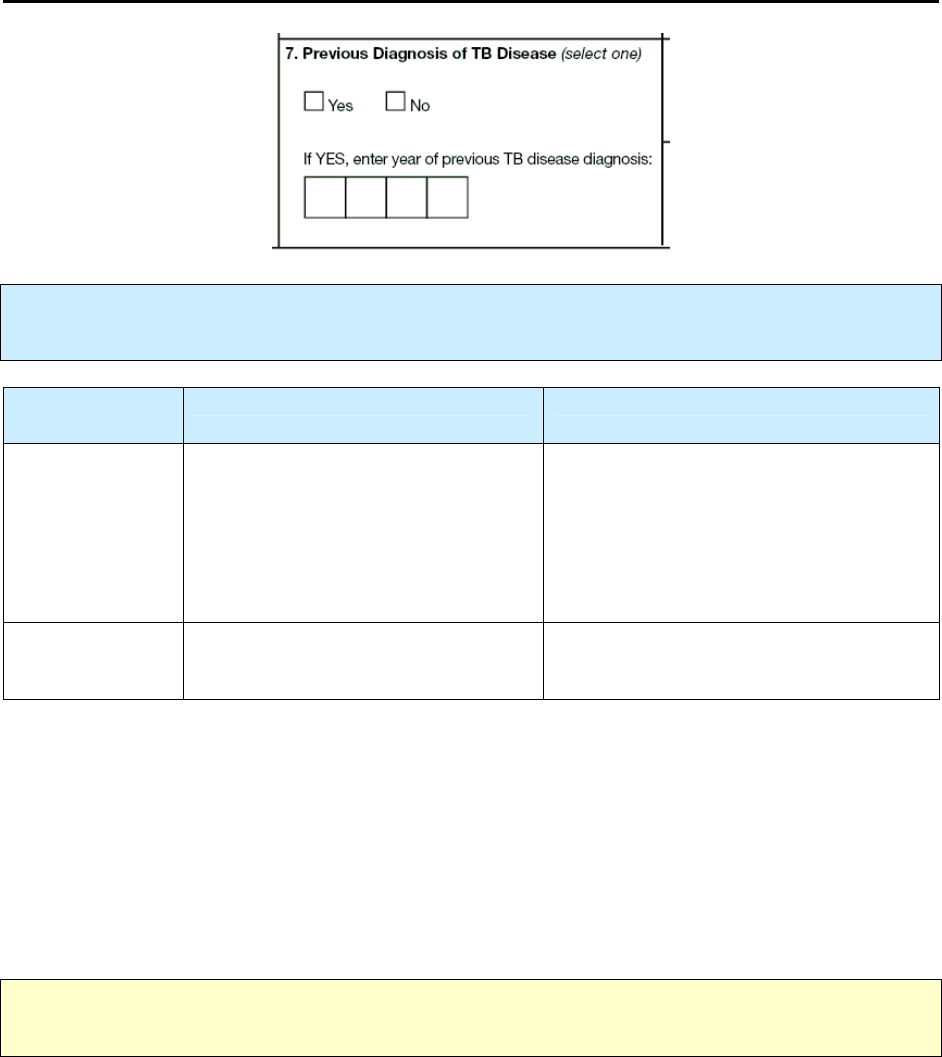
51
7.
Previous Diagnosis of TB Disease
Primary Purpose: Case management and surveillance. Data are used to evaluate the patient’s response
to treatment and to analyze drug resistance from a previous episode of TB disease.
Option
(select one)
Description Comment
Patient has received a previous
diagnosis of TB disease.
Do not enter a previous diagnosis of latent
TB infection (LTBI).
Yes
If you selected Yes, enter the year of
previous diagnosis of TB disease
(e.g., 1985).
If the patient had more than 1 previous
episode of TB disease, enter the year of
the most recent previous episode.
No
Patient has not received a previous
diagnosis of TB disease.
Comments: Yes
A patient is considered to have had a previous diagnosis of TB disease if
•
TB disease was verified in the past
or
•
The patient completed therapy for TB disease (even if the case-to-case interval is within 12
months)
or
•
The patient with TB disease was lost to supervision for more than 12 months and now has
verified TB disease again.
Note: Recurrent cases within 12 months of completion of therapy should be considered noncountable,
regardless of whether the initial and the subsequent genotypes are the same or are different.
52
If the patient had a previous episode of TB that was reported to U.S. surveillance, you should, for the
purposes of linking RVCT forms
•
Contact the state in which the case was counted to ask for the most recent previous diagnosis
•
Enter the most recent previous RVCT
State Case Number
for this case under
Linking State
Case Number
(item 3)
•
Enter the code for the
Reason
linking is desired (e.g., enter 1 for recurrence or previous diagnosis
of TB)
Documentation of Previous Diagnosis of TB Disease
Often, TB disease is confused with latent TB infection (LTBI), which should not be coded as previous TB
disease. Therefore, documentation of the previous episode of TB disease is important. Follow the priority
indicated below.
Oral Report
W
hen written documentation is
not
available, oral report of
a previous episode of TB disease is acceptable (e.g.,
medications taken, length of course of medication, results
of sputum smear examination).
Written Documentation
Written documentation of the previous episode of TB
disease is ideal. However, if the TB disease episode
occurred years ago or in another location (e.g., other
country), obtaining written documentation may be difficult.
1
st
Priority
Written Documentation
If written documentation is
not available use the 2
nd
priority
2
nd
Priority
Oral Report

53
Exercise
7.
Previous Diagnosis of TB Disease
Case Study – David
In January 2009, David has a positive tuberculin skin test (TST) result. His sputum result
is smear negative, with a normal chest radiograph, and he is treated for latent TB
infection (LTBI). He completes treatment July 15, 2009. He goes to Cape Town, South
Africa, for international training and returns home August 15, 2009. Four months later
David becomes severely ill and on December 17, 2009, his chest radiographs are
abnormal and consistent with TB. At that time, a sputum sample is sent to the laboratory.
His sputum results indicate both smear and culture positive for M. tuberculosis complex.
7.1
Is David considered to have a Previous Diagnosis of TB Disease?
(circle the one best answer)
A.
Yes, because he had a positive TST
B.
Yes, because he was treated for LTBI
C.
No, because he had LTBI and not TB disease
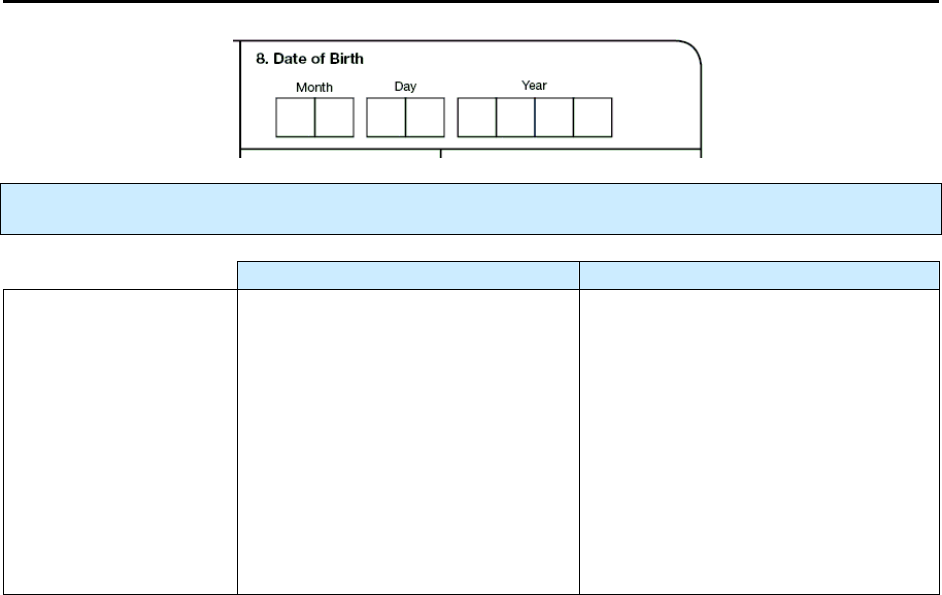
54
8.
Date of Birth
Primary Purpose: Surveillance. Data are used to document patient demographic information.
Description Comment
Month, day, and year
(e.g., 04/26/1968)
Patient’s complete date of birth
should be entered (i.e., values
should be entered for month, day,
and year).
Some societies or cultures throughout
the world do not document the day,
month, or even the year of birth.
If the day is unknown, or the month
and the day are unknown, enter 99 as
the default value (e.g., 04/99/1968 or
99/99/1968).
If the month, day, and year of birth
are unknown, enter 99/99/9999.

55
Exercise
8.
Date of Birth
Case Study – Edris
Your patient Edris is from Iran. When you ask him for his date of birth, he says that he is
born in 1947, but he has never seen his birth certificate and his parents could not
remember when he was born, so he isn’t sure of the month and day. He also mentions
that he sometimes uses March 21, 1947, as his birth date and sometimes February 23 as
the date.
8.1
What would be entered for Edris’ date of birth?
(circle the one best answer)
A. Month Day Year
0 3 2 1 1 9 4 7
B. Month Day Year
0 2 2 3 1 9 4 7
C. Month Day Year
9 9 9 9 1 9 4 7
56
9.
Sex at Birth
Primary Purpose: Surveillance. Data are used to document patient demographic information.
Option
(select one)
Description
Male
The biological sex of the TB patient was
Male
at birth.
Female
The biological sex of the TB patient was
Female
at birth.

57
Exercise
9.
Sex at Birth
Case Study – Dahlia
You are meeting with Dahlia and filling out the RVCT form. You ask Dahlia what was
her sex at birth. Dahlia insists that she is a woman. You are suspicious because Dahlia
looks and dresses like a woman, but she has a very deep voice, large hands and feet, and
there appears to be some facial hair. While showing respect for Dahlia and the sensitivity
of the situation, you stress confidentiality and mention that this form is for surveillance
purposes only. You ask her what sex is listed on her birth certificate. Dahlia mentions
that it is male, but she had an operation that brought out her feminine side.
9.1
What do you list as Dahlia’s sex at birth?
(circle the one best answer)
A.
Male
B.
Female
58
10.
Ethnicity
Primary Purpose: Surveillance. Data are used to detect high-risk groups for TB by ethnicity.
Option
(select one)
Description Comment
Hispanic or Latino
Patient considers himself or herself
Cuban, Mexican, Puerto Rican,
South or Central American, or of
other Spanish culture or origin,
regardless of race.
Some patients prefer the term
“Spanish origin” to Hispanic or
Latino.
Not Hispanic or
Latino
Patient does not consider himself or
herself Hispanic or Latino.
Comment: Self-identity or self-reporting
The response to this item should be based on the patient’s self-identity or self-reporting.
It should
not
be based on appearance or surname.
Example: Not Hispanic or Latino but has a Hispanic name
A patient may have a Hispanic name, but may not be Hispanic or Latino. For example, if a woman who is
not Hispanic marries a Hispanic man, she may self-report as “Not Hispanic or Latino.” Similarly, people
from the Philippines may have Hispanic names, but self-report as “Not Hispanic.”

59
Exercise
10.
Ethnicity
Case Study – Susy
Susy is a school teacher. She went to the Houston, Texas, Health Department for TB
testing because of an outbreak at her school. She was diagnosed with TB disease. You
ask her ethnicity and she says multi-racial because her father is an American Indian from
Arizona and her mother is Cuban. You explain the difference between race and ethnicity
and you ask her if she considers herself Hispanic or Latino, or not Hispanic or Latino.
Susy says that she is part Hispanic.
10.1 What do you check for Item 10 Ethnicity?
(circle the one best answer)
A.
Hispanic or Latino
B.
Not Hispanic or Latino
60
11.
Race
Primary Purpose: Surveillance. Data are used to detect high-risk groups for TB by race.
Option
(select one or more)
Description
American Indian or
Alaska Native
Patient has origins in any of the original peoples of North and South
America (including Central America).
Asian
Patient has origins in any of the original peoples of the Far East, Southeast
Asia, or the Indian subcontinent (e.g., including Bangladesh, Cambodia,
China, India, Indonesia, Japan, Korea, Malaysia, Nepal, Pakistan, the
Philippine Islands, Thailand, and Vietnam).
Black or African
American
Patient has origins in any of the black racial groups of Africa.
Native Hawaiian or
Other Pacific Islander
Patient has origins in any of the original peoples of Hawaii, Guam,
American Samoa, or other Pacific Islands.
White
Patient has origins in any of the original peoples of Europe, the Middle
East, or North Africa.
Comment: Self-identity or self-reporting
The response to this item should be based on the patient’s self-identity or self-reporting.
Therefore, patients should be offered the option of selecting more than one racial designation.
Comment: Asian
or Native Hawaiian or Other Pacific Islander
If you selected
Asian
or
Native Hawaiian or Other Pacific Islander
, use the National Electronic
Disease Surveillance System (NEDSS) Person Race Categories to complete
Specify
. The chart below
indicates who is considered Asian and who is considered Native Hawaiian or Other Pacific Islander.
61
National Electronic Disease Surveillance System (NEDSS)
Person Race Categories for Asian and for
Native Hawaiian or Other Pacific Islander*
Asian
Native Hawaiian or
Other Pacific Islander
Asian Indian
Bangladeshi
Bhutanese
Burmese
Cambodian
Chinese
Filipino
Hmong
Indonesian
Iwo Jiman
Japanese
Korean
Laotian
Madagascar
Malaysian
Maldivian
Nepalese
Okinawan
Pakistani
Singaporean
Sri Lankan
Taiwanese
Thai
Vietnamese
Carolinian
Chamorro
Chuukese
Fijian
Guamanian
Kiribati
Kosraean
Mariana Islander
Marshallese
Melanesian
Micronesian
Native Hawaiian
New Hebrides
Other Pacific Islander
Palauan
Papua New Guinean
Pohnpeian
Polynesian
Saipanese
Samoan
Solomon Islander
Tahitian
Tokelauan
Tongan
Yapese
*From NEDSS Logical Data Model Data Dictionary: Appendix B, 1. Standardized Vocabulary, 1.4 Person Race Categories and
Codes
(http://www.cdc.gov/nedss/DataModels/NEDSS_LDM_Dictionary_II.pdf;
last updated 11-19-2001)

62
Exercise
11.
Race
Case Study – Joaquin
You ask Joaquin how he defines his race. You state that there are several options for race
and he can select whatever he thinks is appropriate. Joaquin says his father is African
American and his mother is full-blooded Cherokee Indian, so he thinks he is both of those
races.
11.1 Which of the following do you check for Item 11 Race?
(circle the one best answer)
A.
American Indian or Alaska Native
B.
Asian
C.
Black or African American
D.
Native Hawaiian or Other Pacific Islander
E.
Both A and C
Case Study – Trang
The health care worker asks Trang what race she is. She states that she is Filipino and
Vietnamese.
11.2 Which of the following do you check for Item 11 Race?
(circle the one best answer)
A.
American Indian or Alaska Native
B.
Asian
C.
Black or African American
D.
Native Hawaiian or Other Pacific Islander
E.
Both A and C
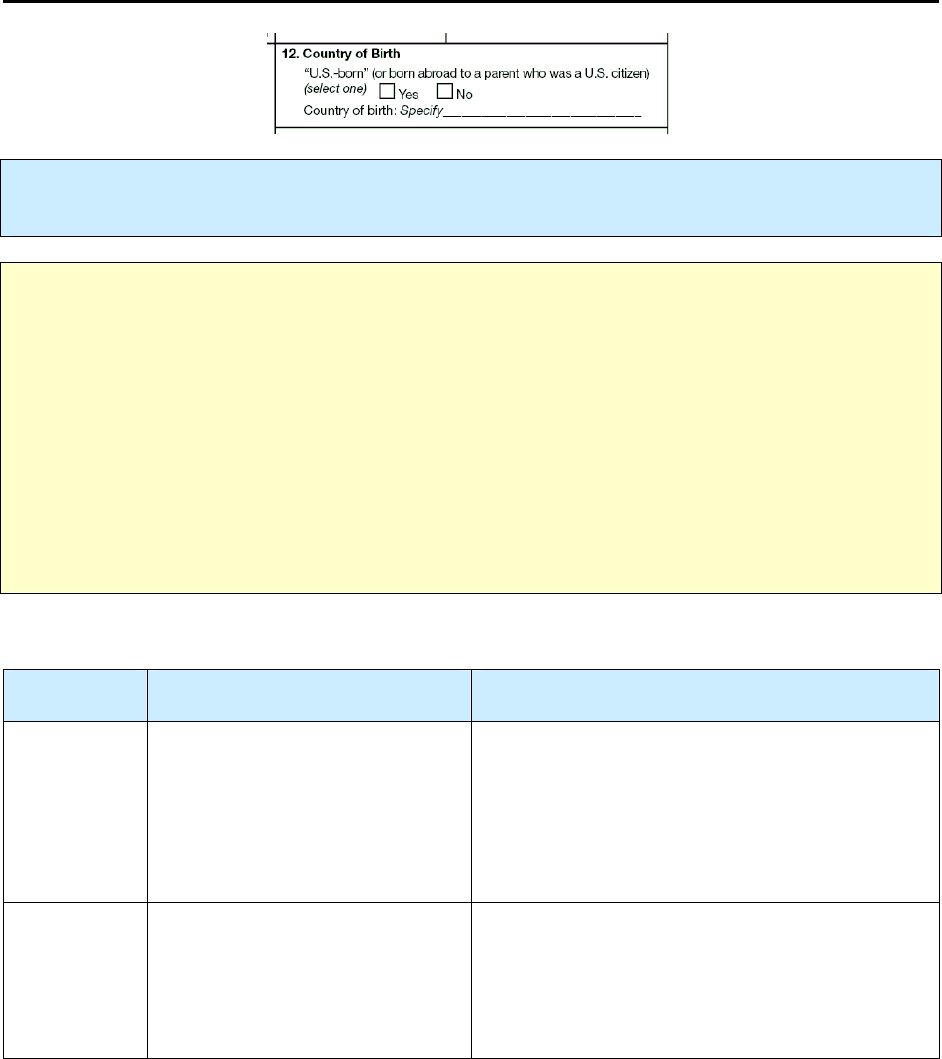
63
12.
Country of Birth
Primary Purpose: Surveillance. Data are used to determine the rate of TB among “U.S.-born” and
foreign-born persons and to identify persons from countries with a high rate of TB.
Note:
This portion of the RVCT asks 2 questions to help classify a person based on where the person
was born.
•
“U.S.-born.”
The U.S. Census Bureau defines a “U.S.-born” person to be someone born in 1 of the
50 states or the District of Columbia, or someone born outside the United States to at least one parent
who was a U.S. citizen. In order to be consistent with the U.S. Census Bureau and to be able to apply
census bureau population data to calculate TB rates, CDC uses the same definition for “U.S.-born.”
•
Country of birth.
In order to distinguish persons who were born in another country (whether or not
they had a parent who was a U.S. citizen) from those who were born in the United States, this
question simply asks to record the actual country of birth. Therefore, a patient who was born in
France and whose father was a U.S. citizen would be “U.S.-born” and their country of birth would be
France.
“U.S.-born” (or born abroad to a parent who was a U.S. citizen)
OPTION
(select one)
Description Comment
Yes
If the person was born
• In 1 of the 50 U.S. states or
the District of Columbia,
or
• Abroad to a parent who was a
U.S. citizen.
“U.S.-born” does not mean the same as U.S.
citizen, and it does not necessarily mean that the
person was born in the United States.
Not all U.S. citizens (e.g., naturalized citizens) are
“U.S.-born.”
No
If the person was born
• Abroad
and
• Neither parent was a U.S.
citizen.
Select for any country other than the United
States.
64
Country of birth
Description
Country of birth (specify)
(e.g., United States, Mexico,
China)
Enter the name of the country in which the person was actually born.
Fill this out for all patients (whether they were “U.S.-born” or not).
Examples of U.S.-Born
Patient Father Mother
U.S.-born U.S. citizen U.S. citizen
Yes No
Description
Yes No Yes No
Yes
Born in 1 of the 50 states or the District of
Columbia
Yes
Yes
Yes
Born in 1 of the 50 states or the District of
Columbia
No
No
Yes
Born in another country
Yes
Yes
Yes
Born in another country
Yes
No
Yes
Born in another country
No
Yes
No
Born in another country
No
No
Note:
People born in Puerto Rico, Guam, the U.S. Virgin Islands, or the Commonwealth of the
Northern Mariana Islands are U.S. citizens, but are
only considered “U.S.-born”
if they are born to a
parent who is a U.S. citizen.
Comment: “U.S.-born”
If the patient was born in 1 of the 50 states or the District of Columbia, or born abroad to a parent who
was a U.S. citizen (either the mother or father or both parents), the patient is considered “U.S.-born.”
Select Yes for “U.S.-born.” For country of birth, enter the name of the country where the person was
actually born.
Example: “U.S.-born” and actually born in 1 of the 50 states or the District of Columbia
If the person was actually born in 1 of the 50 states or the District of Columbia, enter
• “U.S.-born” – Yes
• Country of birth – United States
Example: “U.S.-born” and actually born in another country
If the patient is born in Haiti, his mother is Haitian, but his father is a U.S. citizen, enter
• “U.S.-born” – Yes
• Country of birth – Haiti
65
Example: “U.S.-born” and born to parents who were born in Puerto Rico, Guam, the U.S.
Virgin Islands, or the Commonwealth of the Mariana Islands (people born in these countries are
U.S. citizens)
If the patient was born in Puerto Rico and both parents were born in Puerto Rico (therefore U.S.
citizens), enter
• “U.S.-born” – Yes
• Country of birth – Puerto Rico
Comment: Not “U.S.-born” and born in any country other than the U.S.
If the patient was born in a country other than the United States to parents who were
not
“U.S. citizens,”
enter
•
“U.S.-born” – No
•
Country of birth – name of the country where the person was actually born
Example: Not “U.S.-born” but born in Puerto Rico, Guam, the U.S. Virgin Islands, or the
Commonwealth of the Mariana Islands (people born in these countries are U.S. citizens but not
necessarily U.S.-born)
If the patient was born in Puerto Rico and but neither parent was a U.S. citizen, enter
• “U.S.-born” - No
• Country of birth – Puerto Rico
Example: Not “U.S.-born” and born to parents who are not U.S. citizens
If the patient was born in Russia and both parents are Russian citizens, enter
• “U.S.-born” - No
• Country of birth – Russia

66
Exercise
12.
Country of Birth
Case Study – Wolfgang
You are completing an RVCT on Wolfgang and you ask him his country of birth. He says
he was born in Germany. His father was born in the United States and is a U.S. citizen.
His mother is a German citizen. They married when his father was stationed in Germany.
12.1 What do you specify for “U.S.-born”?
(circle the one best answer)
A.
Yes
B.
No
12.2 What do you specify for Country of Birth?
(circle the one best answer)
A.
United States
B.
Germany
Case Study – Bernard
Bernard was born in Tryon, North Carolina. Both of his parents were born in Hungary.
12.3 What do you specify for “U.S.-born”?
(circle the one best answer)
A.
Yes
B.
No
12.4 What do you specify for Country of Birth?
(circle the one best answer)
A.
United States
B.
Hungary
67
Case Study – Mayleen
Mayleen was born in Guam. Both of her parents were born in Palau. They had moved to
Guam for one year during the time when Mayleen was born.
12.5 What do you specify for “U.S.-born”?
(circle the one best answer)
A.
Yes
B.
No
12.6 What do you specify for Country of Birth?
(circle the one best answer)
A.
Palau
B.
Guam
C.
United States
Case Study – Jiguna
Jiguna was born in Kenya. Both his mother and father were Kenyan citizens. The family
moved to the United States when Jiguna was 2 months old.
12.7 What do you specify for “U.S.-born”?
(circle the one best answer)
A.
Yes
B.
No
12.8 What do you specify for Country of Birth?
(circle the one best answer)
A.
United States
B.
Kenya
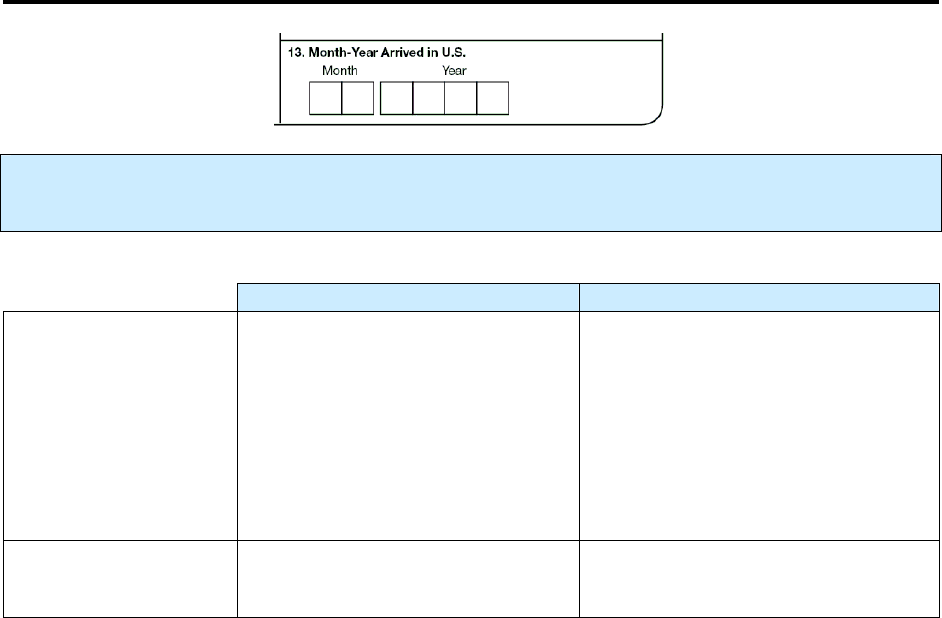
68
13.
Month-Year Arrived in U.S.
Primary Purpose: Programmatic function. Data are used to guide TB programs in developing
strategies for TB prevention and control for persons born outside the U.S.
Description Comment
Month and year
(e.g., 02/1975)
When the patient first arrived in the
United States (1 of the 50 states or
the District of Columbia).
Complete this item if the patient was
born in another country.
If month is unknown, enter 99 as the
default value (e.g., 99/1975).
If neither month nor year is known,
enter 99/9999.
Leave item blank
If patient was born in 1 of the 50
states or the District of Columbia.
Comment: If the patient was born abroad to a parent who was a U.S. citizen
If a patient was born abroad to a parent who was a U.S. citizen, enter the month and year that the patient
first arrived in the United States (1 of the 50 states or the District of Columbia).
Example: If the patient was born abroad to a parent who was a U.S. citizen
If a patient was born in Germany to a parent who was a U.S. citizen, enter the month and year that the
patient first arrived in the United States (1 of the 50 states or the District of Columbia).
Example: If the patient was born abroad to a parent who was a U.S. citizen
If a patient was born in Puerto Rico to a parent who was a U.S. citizen, enter the month and year that the
patient first arrived in the United States (1 of the 50 states or the District of Columbia).
Example: Date that a patient first arrived from another country who enters on student visa
If a patient is a citizen from another country and comes to the United States (1 of the 50 states or the
District of Columbia) on a student visa and returns home, and then later returns to the United States, the
date when the patient first arrived in the United States as a student would be the date that should be
entered, even if the patient doesn’t return for many years.
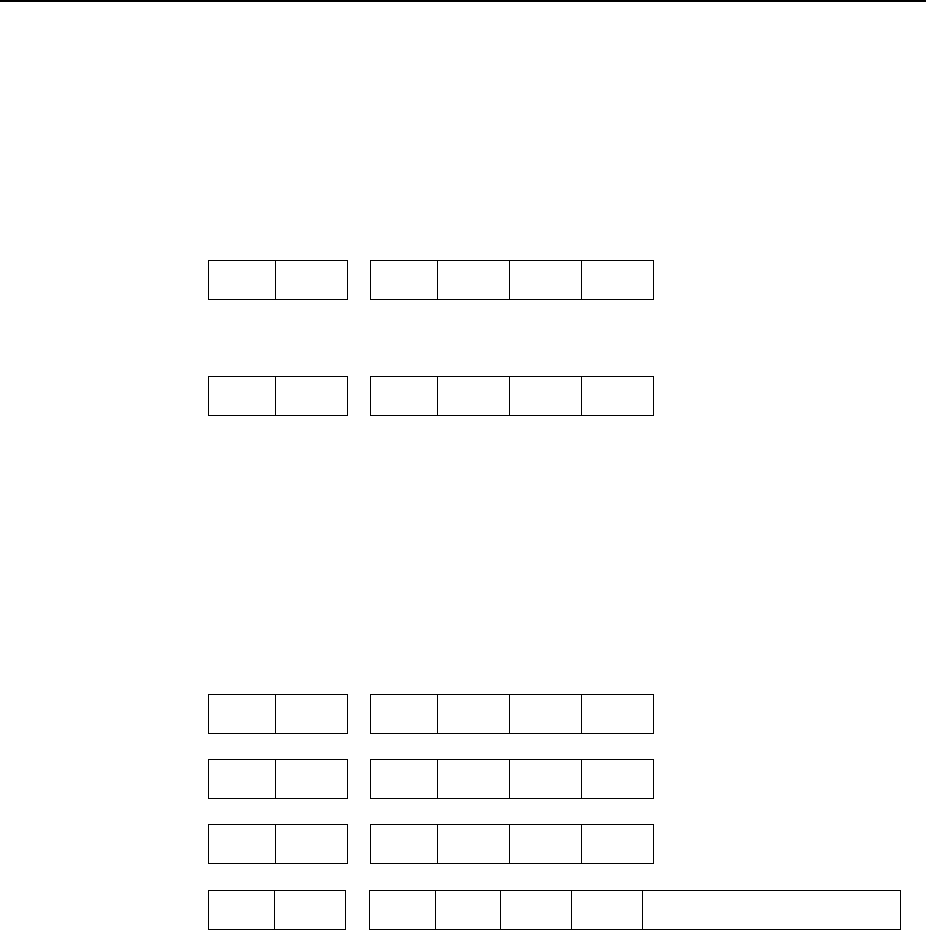
69
Exercise
13.
Month-Year Arrived in U.S.
Case Study – Julia
Julia is a citizen of El Salvador. In January 2008 she came to the U.S. on a student visa.
In June 2009 she returned home to El Salvador. In September 2010 she returned to the
United States on another student visa.
13.1 What is the month and year that Julia arrived in the United States?
(circle the one best answer)
A. Month Year
0 1 2 0 0 8
B. Month Year
0 9 2 0 1 0
Case Study – Ken
Ken was born March 16, 2000, in Majuro, Republic of the Marshall Islands (RMI). His
mother is a U.S. citizen and his father is a citizen of the RMI. On December 20, 2009, he
migrated with his family to Arkansas. He is diagnosed with TB on March 5, 2010.
13.2 What do you enter for Ken for Month-Year Arrived in the U.S.?
(circle the one best answer)
A. Month Year
0 3 2 0 0 0
B. Month Year
1 2 2 0 0 9
C. Month Year
0 3 2 0 1 0
D. Month Year
(Leave answer blank)
70
14.
Pediatric TB Patients (<15 years old)
Primary Purpose: Surveillance. Data are used to capture risk factors for guardians born in countries
that have a high burden of TB and when pediatric patients live in TB endemic countries.
To better capture important information about pediatric TB patients (<15 years old), this variable provides
information on country of birth for primary guardians (or primary care givers) of the pediatric patient and
whether the patient lived outside the United States (1 of the 50 states or the District of Columbia) for an
uninterrupted
period of more than 2 months.
Note: Pediatric TB Patients (item 14) should be completed for all pediatric patients. For all pediatric
patients, ask the country of birth for parents or primary guardians and whether the patient has lived
outside the United States for >2 months consecutively.
Complete this item for
all pediatric
TB patients (<15 years old).
Description
Country of birth for the
primary guardians (e.g.,
mother, father, adoptive or
foster parent, grandparent)
Enter the names of the countries where the primary guardians were
actually born.
Enter as many as 2 parents or primary guardians.
Complete this item for
all pediatric
TB patients (<15 years old).
Option
(select one)
Description Comment
Yes
Pediatric patient lived outside the United
States (1 of the 50 states or the District of
Columbia) for an uninterrupted period of
more than 2 months.
Although it may be difficult to
determine the exact amount of
uninterrupted time that a patient lived
outside the United States, check Yes
and enter the names of the countries if
the period is believed to be more than
8 consecutive weeks (2 months).
No
Pediatric patient did not live outside the
United States for an uninterrupted period
of more than 2 months.
71
Unknown
It is not known whether the pediatric patient
lived outside the United States for an
uninterrupted period of more than 2
months.
Comment: Lived outside the United States
Lived outside the United States refers to the place where a person stayed or slept most of the time, or the
place the person considered home during the stated period.
If you selected
Yes
, enter the following information.
Description Comment
Countries
(specify)
Enter the names of the countries where
the pediatric patient lived.
Enter as many as 3 non-U.S. countries in
which the patient most recently lived for a
total of more than 2 uninterrupted
months.
Example: Yes, Lived outside the United States in as many as 3 countries for a total of more than 2
uninterrupted months
From January 1 to March 15, the patient lived outside the United States
•
Lived in Zambia for 10 weeks, then
•
Returned to the United States
Example: Yes, Lived outside the United States in as many as 3 countries for a total of more than 2
uninterrupted months
From January 1 to March 15 the patient lived outside the United States
•
Lived in Zambia for 4 weeks, then
•
Lived in South Africa for 3 weeks, then
•
Lived in Botswana 3 weeks, then
•
Returned to the United States
Example: No, Lived outside the United States in as many as 3 countries for a total of more than 2
months, but travel was interrupted
From January 1 – March 15 the patient lived outside the United States
•
Lived in Zambia for 5 weeks, then
•
Returned to the United States for 2 weeks, then
•
Lived in South Africa for 5 weeks, then
•
Returned to the United States

72
Exercise
14.
Pediatric TB Patients (<15 years old)
Case Study – Pim
Pim, a pediatric TB patient, was born in Portland, Oregon, on March 6, 2000. Her father
was born in Thailand and immigrated to the U.S. in 1998. Her mother was born in
Burlington, Vermont. The family traveled to Thailand for 3 weeks in 2005, but have not
been out of the U.S. since that time. Pim’s parents were divorced in 2006 and her mother
remarried in 2008. Pim lives with her mother and stepfather who was also born in
Vermont. Her father and mother share joint custody, and Pim stays with her father every
other weekend.
14.1 What would you enter for Country of Birth for the primary guardians?
(circle the one best answer)
A.
Guardian 1 - United States (based on mother)
Guardian 2 - Thailand (based on father)
B.
Guardian 1 - United States (based on mother)
Guardian 2 - United States (based on stepfather)
14.2 What do you specify for Patient lived outside the U.S. for >2 months?
(circle the one best answer)
A.
Yes
B.
No
C.
Unknown
73
Case Study for Items 12 and 14 – Antonio
Antonio, a pediatric patient, was born in El Salvador. His father was born in the United
States and living in El Salvador when Antonio was born. His mother was born in El
Salvador and was a citizen of El Salvador. Antonio’s mother died shortly after his birth,
and Antonio was raised mostly by his father.
In January 2003, 8-year-old Antonio moved from El Salvador to Houston, Texas, when
his father could no longer care for him. At that time, his uncle and aunt, the Trujillos,
became his legal guardians and he has lived with them since that time. Both his uncle and
aunt were born in El Salvador, but have been living in the United States for over 30
years.
14.3 For Item 12, what do you select for “U.S.-born”?
(circle the one best answer)
A.
Yes
B.
No
14.4 For Item 12, what is the Country of Birth for Antonio?
(circle the one best answer)
A.
El Salvador
B.
United States
14.5 For Item 14, what is the Country of Birth for the primary guardians at time of
diagnosis for TB?
(circle the one best answer)
A.
Guardian 1 – US (based on father)
Guardian 2 – El Salvador (based on his uncle and/or aunt)
B.
Guardian 1 – El Salvador (based on uncle)
Guardian 2 – El Salvador (based on aunt)
74
Case Study for Item 14 – Antonio (cont.)
In 2007, Antonio visited his father in El Salvador during June 5 – September 30. He
returned to the United States on October 1.
14.6 For Item 14, did Antonio live outside the U.S. for >2 months?
(circle the one best answer)
A.
Yes
B.
No
C.
Unknown
Case Study – Regina
Regina, a pediatric TB patient, visited her grandmother in Russia during April 7 – June 1,
2008. She returned to the United States on June 1.
14.7 For Item 14, did Regina live outside the U.S. for >2 months?
(circle the one best answer)
A.
Yes
B.
No
C.
Unknown
Case Study – Lisa
Lisa, a pediatric TB patient, traveled with her parents to the Philippines September 1 –
November 1, 2009. They traveled to Taiwan and visited her grandmother November 1 –
November 15. Then they traveled to Vietnam November 15 – December 15. Lisa and her
parents returned to the United States on December 16.
14.8 For Item 14, did Lisa live outside the U.S. for >2 months?
(circle the one best answer)
A.
Yes
B.
No
C.
Unknown
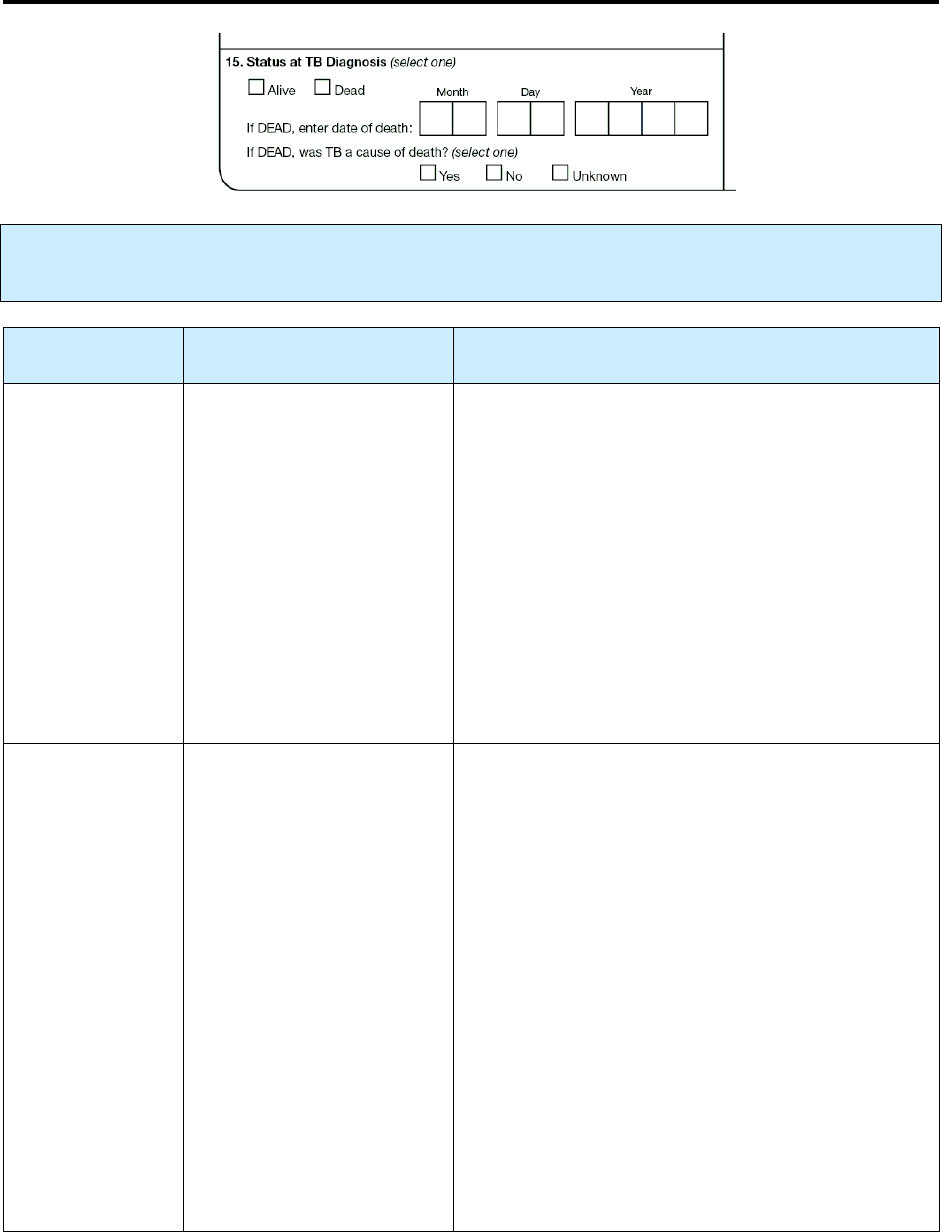
75
15.
Status at TB Diagnosis
Primary Purpose: Surveillance. Data are used to examine mortality and to determine if TB was a cause
of death.
Option
(select one)
Description Comment
Alive
Patient was alive at time
• Laboratory results
confirming a TB
diagnosis (e.g., positive
culture or nucleic acid
amplification [NAA]
test result consistent
with TB) were known
to the provider
or
• TB medications were
started
If the patient
• Was known to be culture or NAA test result
positive consistent with TB prior to the date of
death but did not start TB therapy per
ATS/CDC/IDSA guidelines, classify the patient
as alive at TB diagnosis
• Started empiric therapy for TB disease (per
ATS/CDC/IDSA guidelines), but TB was not
verified until after the patient’s death, classify
as alive at TB diagnosis
• Started TB therapy, regardless of laboratory or
clinical confirmation for TB diagnosis, classify
the patient as alive at TB diagnosis
Dead
Patient was deceased at the
time laboratory results
confirming a TB diagnosis
(e.g., positive culture or
NAA test result consistent
with TB) were known to
the provider
• If diagnostic specimens were collected for
evaluation of TB prior to death, but positive
results to make a diagnosis of TB were not
available until after death, and patient did not
start TB therapy, classify as dead at TB
diagnosis
• If TB diagnosis was made after death based on a
constellation of clinical and other findings (e.g.,
symptoms, TST, and imaging studies) in the
absence of laboratory confirmation, and the
patient did not start therapy, classify as dead at
TB diagnosis
• If patient was receiving treatment for latent TB
infection at death because active TB disease was
not suspected, and TB was diagnosed after
death, classify as dead at TB diagnosis
• If patient was diagnosed at autopsy, classify as
dead at TB diagnosis

76
Comment:
If a person dies while taking isoniazid as preventive therapy for latent TB infection, and this person is
found after death to have had TB disease, he/she should be classified as
Dead
at TB diagnosis.
If you selected
Dead
at TB diagnosis, enter
date of death
.
Description Comment
Date of death
(e.g., 01/17/2005)
Month, day, and year patient
died
If day is unknown, enter 99 as the default value
(e.g., 01/99/2005).
If you selected
Dead
at TB diagnosis,
was TB a
cause of death
?
Option
(select one)
Description Comment
Yes
(related to TB
disease)
TB was
• The immediate cause
or
• An underlying cause
or
• Another significant condition
contributing to death (even if
TB was not the main cause
of death)
Written documentation of the cause of death
(e.g., death certificate, autopsy report, medical
record) is recommended. However, oral
information from a reliable source (e.g., a health
care provider) will be accepted.
A death certificate is not necessarily required to
complete this field. In some cases deaths may be
certified before receipt of results of
• Positive M. tuberculosis culture
or
• Other findings consistent with TB
If the patient died as a result of a surgical
procedure that was indicated for TB, or TB
complicated a surgical procedure not related to
TB.
No
(unrelated to TB
disease)
TB was not
• The immediate cause
or
• An underlying cause
or
• Another significant condition
contributing to death
Unknown
Cause of death is not known.
Every effort should be made to determine if
death was related to TB disease before
classifying as unknown.
Note:
This should reflect current or active TB disease (not LTBI) whenever death certificate or death
documentation is used.

77
Exercise
15.
Status at TB Diagnosis
Case Study – Thomas
Thomas is admitted to the hospital on August 5, 2009. He is coughing up blood. He
reports having symptoms consistent with TB for the past 7 months and his chest
radiograph shows significant deterioration of both lung lobes. He is placed in a TB
isolation unit and begins isoniazid, ethambutol, rifampin, and pyrazinamide. He dies 1
week later on August 12, 2009.
15.1 What is the Status at TB Diagnosis?
(circle the one best answer)
A.
Alive
B.
Dead
Case Study – Ruth
Ruth comes to the emergency room on April 30, 2009. She is diagnosed with pneumonia,
given antibiotics, and discharged. She dies 2 weeks later on May 15, 2009. At autopsy,
the pathology shows granulomatous changes consistent with TB disease. A lung biopsy
culture is found to be positive for M. tuberculosis complex.
15.2 What is the Status at TB Diagnosis?
(circle the one best answer)
A.
Alive
B.
Dead
15.3 Was TB a cause of death for Ruth?
(circle the one best answer)
A.
Yes
B.
No
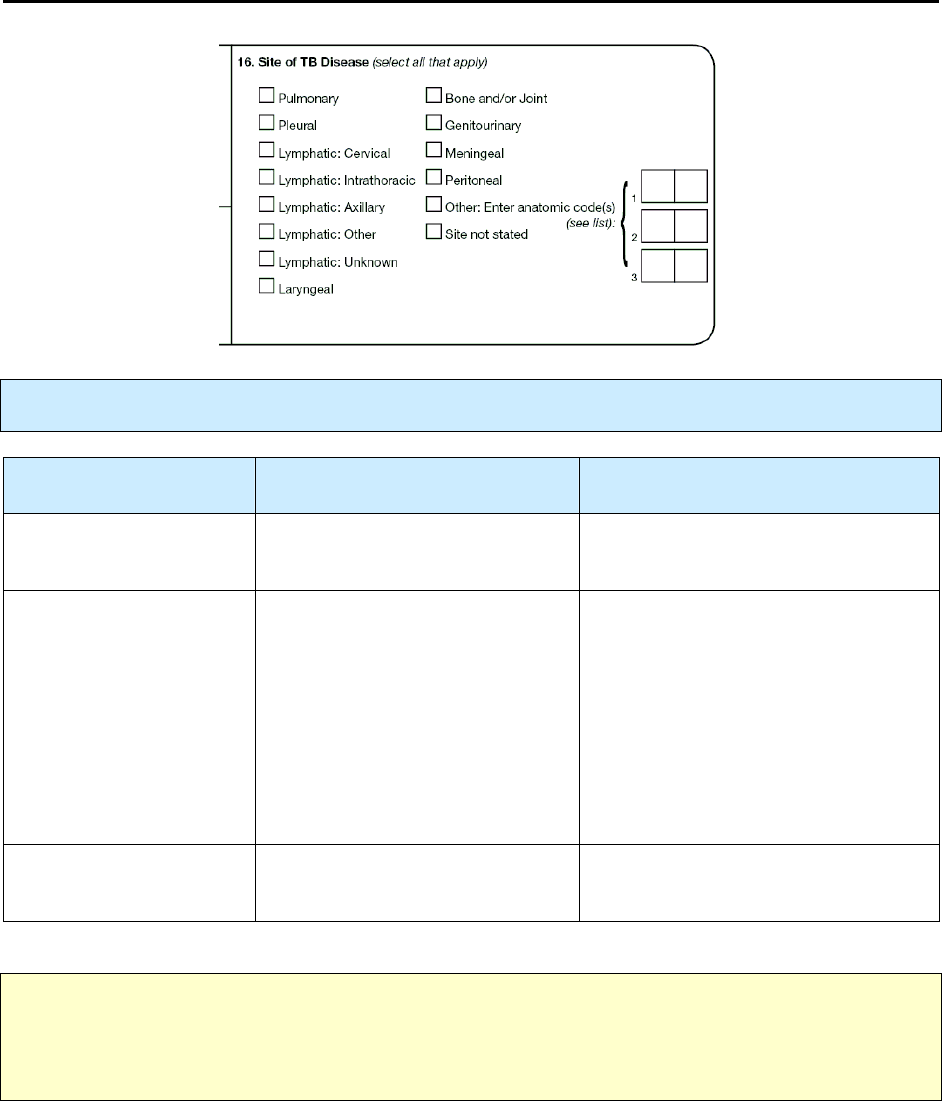
78
16.
Site of TB Disease
Primary Purpose: Surveillance. Data are used to document site of TB disease.
Option
(select all that apply)
Description Comment
Pulmonary, pleural,
lymphatic, etc.
Select boxes corresponding to the
site(s) of TB disease.
Other: enter
anatomic code(s)
If site of TB disease is a site
other than those listed, enter the
anatomic code(s) (see
Appendix C – Anatomic Codes).
You may enter as many as 3
Other anatomic codes.
Refer to the listings for site of TB
disease and anatomic codes. In
Appendix C – Anatomic Codes,
anatomic codes for Other are marked
with an asterisk (*). Select only from
sites marked with an asterisk (*).
Anatomic codes without an asterisk
are parts of organ systems
corresponding to Site of TB Disease.
Site not stated
If you selected Site not stated, do not
check any other box.
Note: For the purposes of the RVCT training materials, use the codes listed in the appendices. Some
software programs used to enter data on the RVCT may NOT use the codes listed in the appendices.
For example, the Anatomic Codes may be a drop-down item where you choose the actual site rather
than enter a code. For more information, see instructions for the software you use.
79
Comment: If more than 1 organ or disease site is involved
If there is evidence that more than 1 organ or disease site is involved, check all involved sites of disease.
Comment: Lymphatic intrathoracic
Lymphatic intrathoracic includes hilar, bronchial, mediastinal, peritracheal, and other lymph nodes within
the thorax.
Comment: Miliary TB
Unlike the previous RVCT form, the new form has no place to select miliary TB in
Site of Disease
(item
16). If the report of the initial chest radiograph or the initial chest CT scan indicates “miliary TB or a
miliary or bilateral micronodular pattern,” record this finding under
Initial Chest Radiograph
(item
22A) or
Initial Chest CT Scan or Other Chest Imaging Study
(item 22B), respectively. However,
pulmonary should be selected as
Site of Disease
(item 16) if the chest x-ray or CT scan shows evidence
of nodules consistent with miliary TB.
Miliary TB is a serious type of tuberculosis infection. It is a clinical or radiologic finding, rather than a
site of disease. Miliary TB is the result of a TB lung infection eroding into the bloodstream and from there
disseminating throughout the body to multiple organs. It appears on radiograph as a great number of small
(1- to 2-mm), well-defined nodules that look like millet seeds scattered throughout the lungs, hence the
name “miliary.”

80
Exercise
16.
Site of TB Disease
Case Study – June
June is 65 years old and has been diagnosed with TB disease. Her chest x-ray shows a
right pleural effusion, a right upper lobe infiltrate, and enlargement of the right
intrathoracic lymph nodes. The pleural biopsy showed granulomas that were AFB
positive and the sputum culture grew M. tuberculosis complex.
16.1 What is (are) the Site(s) of TB Disease?
(circle the one best answer)
A.
Pulmonary
B.
Pleural
C.
Lymphatic intrathoracic
D.
Peritoneal
E.
A, B, and C are all correct
Case Study – Leonard
Leonard is a 47-year-old HIV-positive male with a 3-month history of progressive fatigue
and shortness of breath. His doctor diagnosed severe anemia, and a bone marrow biopsy
showed extensive granulomatous inflammation teeming with AFB. Culture of blood and
bone marrow grew M. tuberculosis complex. His chest x-ray and chest CT scan were
normal.
16.2 What is (are) the Site(s) of TB Disease?
(circle the one best answer)
A.
Bone and/or joint
B.
Other, anatomic code 04
C.
Other, anatomic code 06
D.
A and B are both correct
E.
B and C are both correct

81
Module
Module Module
Module B
BB
B
-
--
- R
R R
RVCT
VCTVCT
VCT
(page 2
(page 2(page 2
(page 2 of 3
of 3 of 3
of 3)
))
)
Items 17
Items 17 Items 17
Items 17 –
––
– 25
25 25
25
Module B provides instructions and exercises for completing page 2 of the RVCT report. It includes data
about laboratory results and primary reason the patient was evaluated for TB disease.
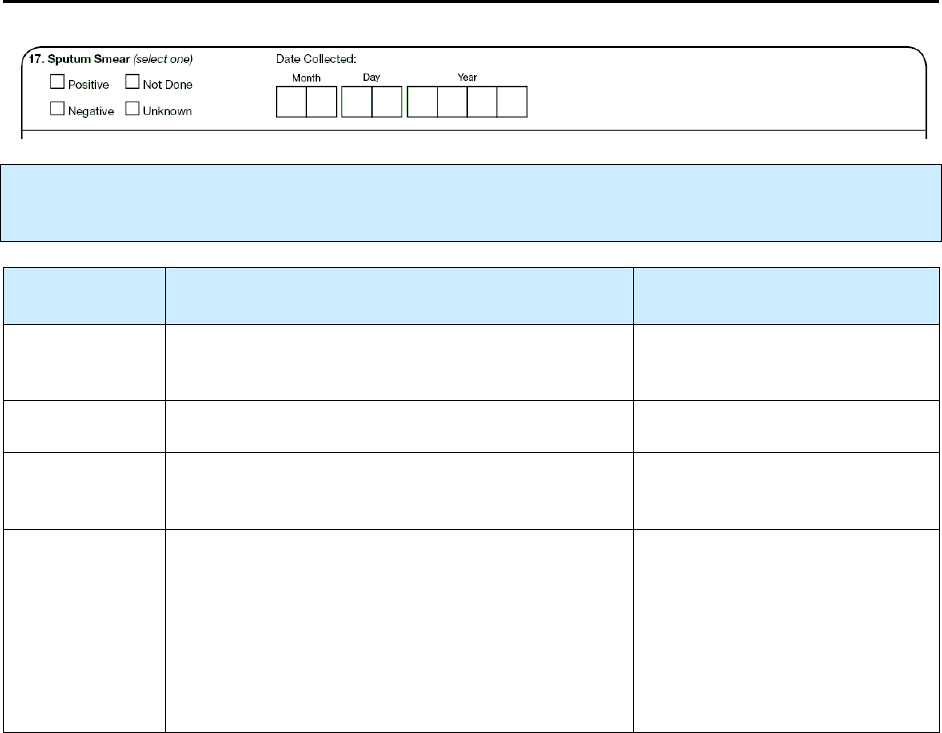
82
17.
Sputum Smear
Primary Purpose: Case management and surveillance. This result is one factor in determining whether
the patient’s disease meets the public health definition of TB.
Option
(select one)
Description Comment
Positive
The result of any sputum examination was
positive for acid-fast bacilli (AFB).
Negative
Results of all examinations were negative.
Not done
Sputum smear examination is known not to have
been done.
Unknown
It is not known whether a sputum smear
examination was performed.
or
Results are not known for a reason other than
pending results (e.g., result was lost or specimen
was contaminated, and no other specimen can be
obtained).
If an initial sputum specimen
was collected and results are
unknown, but results later
become known, update the
results.
Comments: Sputum
Sputum includes spontaneous and induced sputum. Do
not
include the results of microscopic examination
of pulmonary secretions obtained by tracheal suction, bronchoscopy procedures (e.g., bronchial washing
or lavage, scrapings, biopsies), or gastric aspiration. See
Smear/Pathology/Cytology of Tissue and
Other Body Fluids
(item 19).
Sputum should have been collected during the diagnostic evaluation or shortly thereafter. Do
not
record
specimens collected after the patient has received treatment for more than 2 weeks.
83
For
Positive
or
Negative
results of sputum smear examinations, enter the following information.
Description Comment
Month, day, and year the first
sputum specimen with a positive
result was collected (e.g.,
01/17/2009)
If several sputum examinations were done and
the results of 1 or more sputum examinations
were positive, enter the date the first sputum
specimen with a positive result was collected.
Date
collected
Month, day, and year the first
negative sputum specimen was
collected (e.g., 01/17/2009) if all
results were negative
If several sputum examinations were done and
all results were negative, enter the date the first
sputum specimen with a negative result was
collected.

84
Exercise
17.
Sputum Smear (revised)
Case Study for Items 17 and 18 – James
James is in the hospital from January 13 through January 28, 2009. During that time he is
diagnosed with TB. You subsequently receive laboratory reports from the hospital with
James’ sputum smear results. They are as follows:
• Specimen collected on January 13 - sputum smear result was positive
• Specimen collected on January 16 - sputum smear result was positive
• Specimen collected on January 22 - sputum smear result was negative
• Specimen collected on January 28 - sputum smear result was negative
17.1 What option should be selected for Sputum Smear?
(circle the one best answer)
A.
Positive
B.
Negative
C.
Not done
D.
Unknown
17.2 What is the date collected?
(circle the one best answer)
A.
January 13
B.
January 16
C.
January 22
D.
January 28
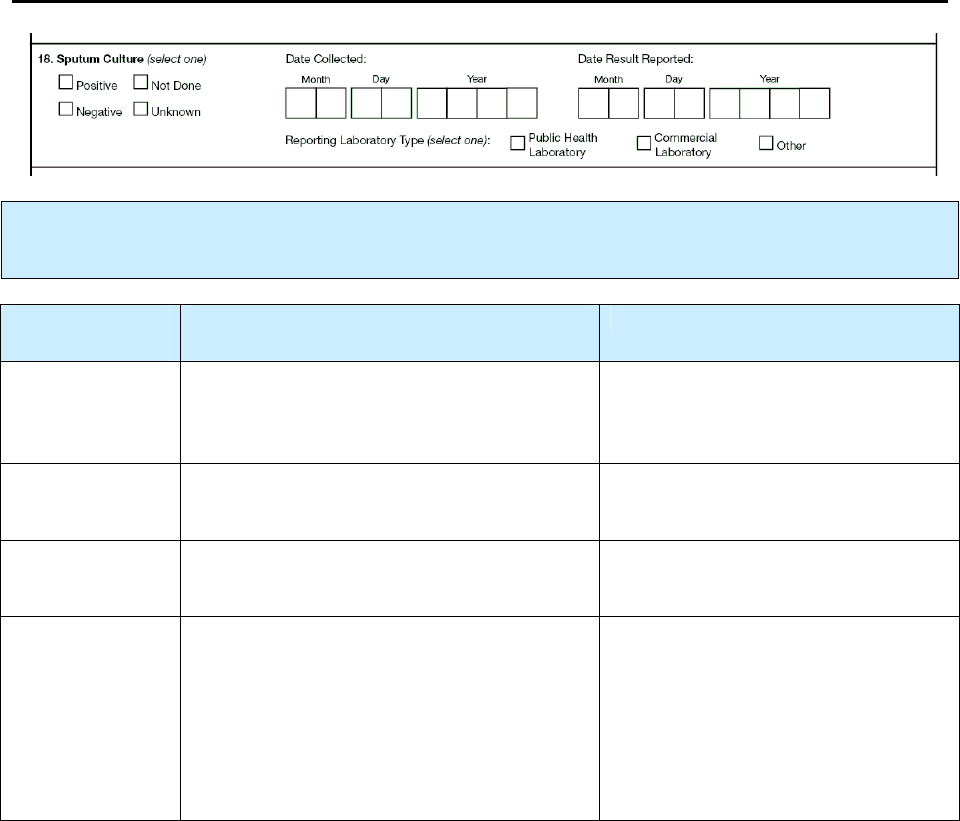
85
18.
Sputum Culture
Primary Purpose: Case management and surveillance. This result is a main factor in determining
whether the patient’s disease meets the public health definition of TB.
Option
(select one)
Description Comment
Positive
The result of any (or the only) sputum
culture was positive for M. tuberculosis
complex.
Negative
Results of all sputum cultures were
negative for M. tuberculosis complex.
Not done
It is known that the sputum culture was not
done.
Unknown
It is not known whether a sputum culture
was performed.
or
Results are not known for a reason other
than pending results (e.g., result was lost
or specimen was contaminated, and no other
specimen can be obtained).
If an initial sputum specimen was
collected and results are unknown,
but results later become known,
update the results.
Comment: Sputum
Sputum includes spontaneous and induced sputum.
Do not include
the culture results of pulmonary
secretions obtained by tracheal suction, bronchoscopy procedures (e.g., bronchial washing or lavage,
scrapings, biopsies), or gastric aspiration. For more information, see
Culture of Tissue and Other Body
Fluids (
item 20).
Sputum should have been collected during diagnostic work-up or shortly thereafter. Do
not
record
specimens collected after the patient has received treatment more than 2 weeks.
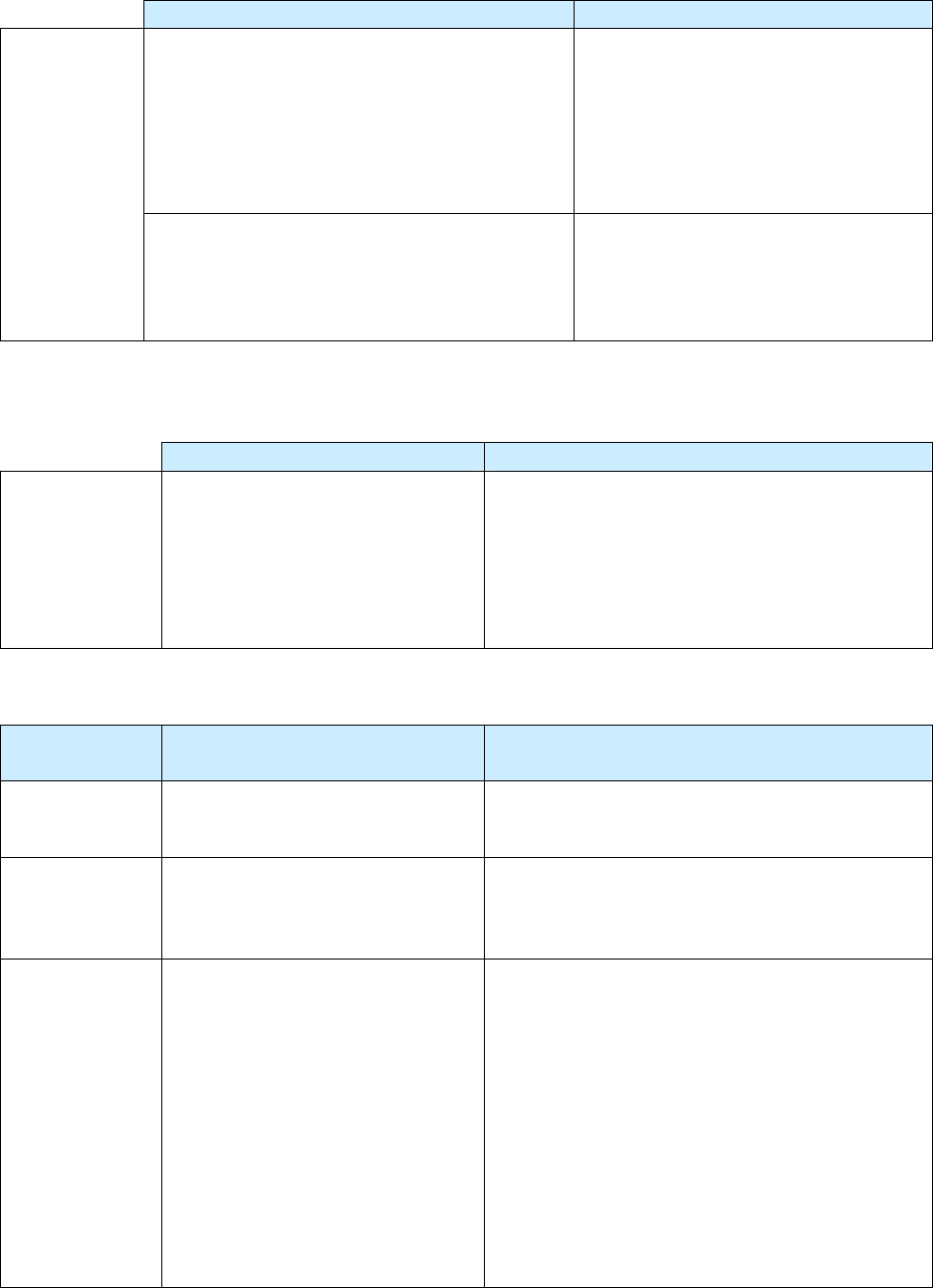
86
For
positive
or
negative
results of sputum cultures, enter the following information.
Description Comment
Month, day, and year the first sputum
specimen with a positive culture result was
collected (e.g., 01/17/2009)
If several sputum cultures were
performed and the results of 1 or
more were
positive
for M.
tuberculosis complex, enter the date
the
first
sputum culture with a positive
result was collected.
Date
collected
Month, day, and year the first sputum
specimen with a negative culture result was
collected (e.g., 01/17/2009) if all results were
negative
If several sputum cultures were done
and all results were negative, enter
the date the first sputum specimen
with a negative result was collected.
For the
first
sputum culture reported
positive
for M. tuberculosis complex, enter the following
information.
Description Comment
Date result
reported
Month, day, and year the
laboratory reported the result
(e.g., 01/17/2009)
This date can be found on the laboratory report
as the date the report is released or made
available.
If the day is unknown, enter 99 as the default
value (e.g., 01/99/2009).
For
positive
culture results, select the option that best describes the
reporting laboratory type
.
Option
(select one)
Description Comment
Public health
laboratory
Any laboratory associated with a
local or a state health department
Commercial
laboratory
Any laboratory that charges
a fee for each specimen
processed or test performed
Other
Any other laboratory that is
not considered a public health
laboratory or a commercial
laboratory
Hospital laboratories (e.g., National Jewish
Health hospital laboratory) or laboratories
associated with federal public health agencies
(e.g., Centers for Disease Control and
Prevention, Veterans Administration, Indian
Health Service [IHS], Tribal Health
Department, or Bureau of Prisons)
National Jewish Health hospital laboratory
sometimes charges for services, but for the
purposes of the RVCT it is categorized as
“Other.”

87
Exercise
18.
Sputum Culture
Case Study – James (continued from Item 17)
In February you received faxed copies of the final sputum culture results for James from
Forbes Diagnostics Incorporated. These were for the sputum culture specimens collected
while James was in the hospital.
• Specimen collected on January 13 – sputum culture result was negative and result
was received on February 13
• Specimen collected on January 16 – sputum culture result was positive and result
was received on February 16
• Specimen collected on January 22 – sputum culture result was positive, and result
was received on February 22
• Specimen collected on January 28 – sputum culture result was positive, and result
was received on February 28
18.1 What option should be selected for the Sputum Culture?
(circle the one best answer)
A.
Positive
B.
Negative
C.
Not done
D.
Unknown
18.2 What is the date collected?
(circle the one best answer)
A.
January 13
B.
January 16
C.
January 22
D.
January 28
88
18.3 What is the date that the result is reported?
(circle the one best answer)
A.
February 13
B.
February 16
C.
February 22
D.
February 28
18.4 What reporting laboratory type should be selected?
(circle the one best answer)
A.
Public health laboratory
B.
Commercial laboratory
C.
Other
18.5 What Reporting Laboratory Type should be selected if the sputum sample had
been analyzed by National Jewish Health hospital laboratory? (circle the one best
answer)
A.
Public health laboratory
B.
Commercial laboratory
C.
Other
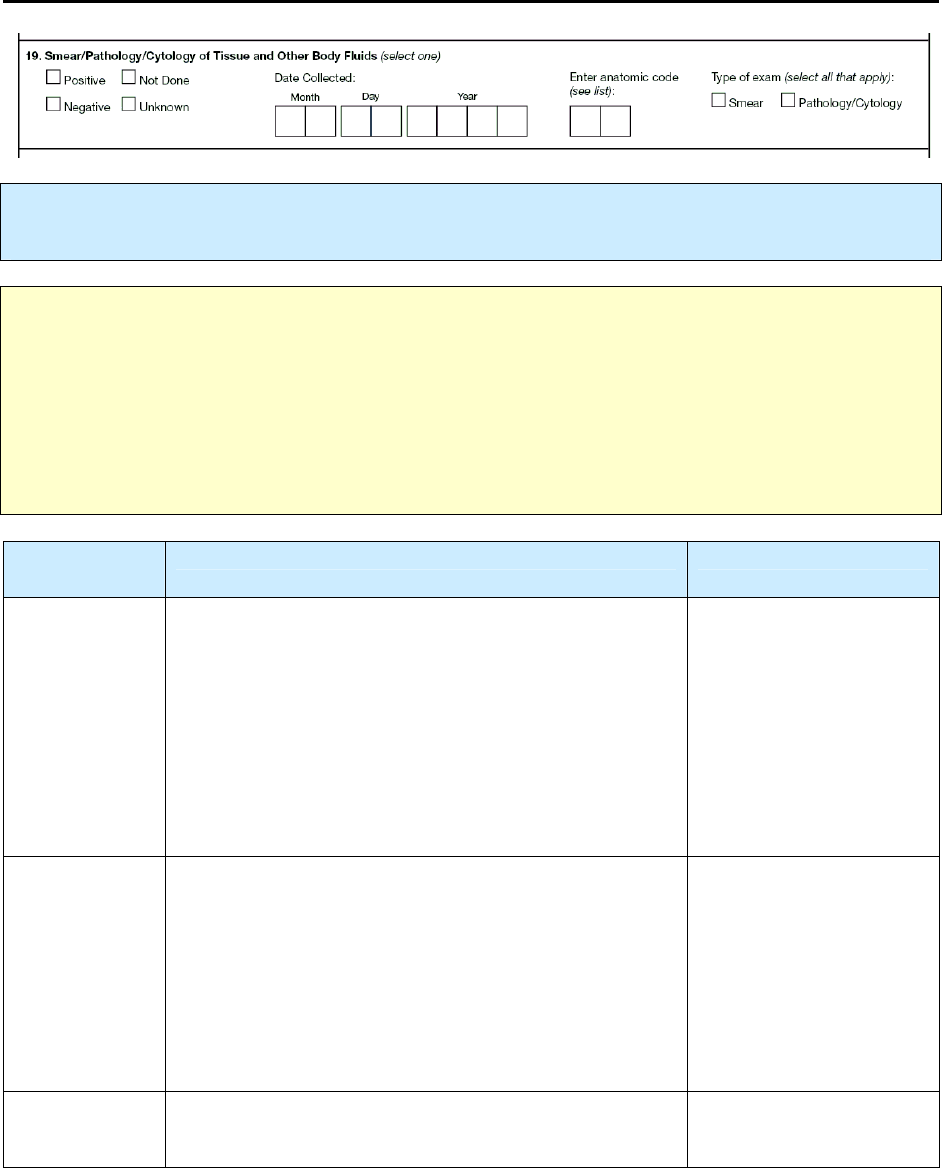
89
19.
Smear/Pathology/Cytology of Tissue and Other Body
Fluids
Primary Purpose: Case management and surveillance. This result is a factor in determining whether
the patient’s disease meets the public health definition of TB.
Note: This item is for recording results of a smear, or pathology, or cytology of tissue and/or other
body fluids.
In this
item, “tissue and other body fluids” does
not
include sputum. Examples of tissue
and other body fluids are tracheal aspirate, bronchial cells and fluid, urine, bone marrow, lymph node,
cerebral spinal fluid, lung tissue or fluid, and pleural fluid that are collected from various procedures
(e.g., bronchoscopy, bronchial washing or lavage, biopsy, gastric aspiration, pleural aspiration).
Results from sputum smear examinations and sputum cultures should be entered under
Sputum Smear
(item 17) and
Sputum Culture
(item 18).
Option
(select one)
Description Comment
Positive
Any tissue or body fluid other than sputum that (see
Note above)
• Tested positive by smear examination
or
• Showed granulomas, granulomatous inflammation,
or other pathologic or histologic findings consistent
with TB disease during a pathologic/cytologic
examination. (Such findings are listed on the
pathology or the cytology report.)
Any positive result
supersedes a negative
result.
Negative
All specimens of tissue or fluid that
• Tested negative by smear examination
or
• Showed no evidence of granulomas, granulomatous
inflammation, or other pathologic or histologic
findings consistent with TB disease during a
pathologic/cytologic examination. (Such findings
are listed on the pathology or the cytology report.)
Not done
Examinations of tissue or fluids are known not to have
been done.
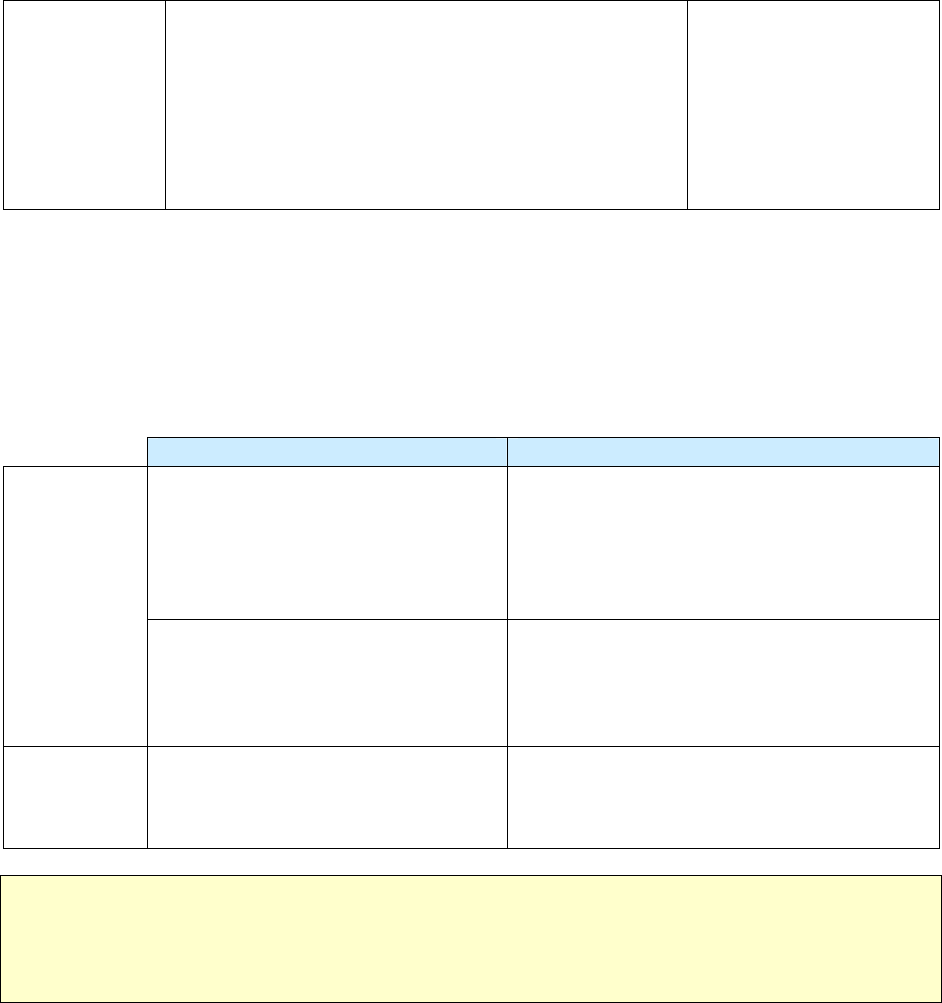
90
Unknown
It is not known whether tissue or fluids
• Were examined
or
• Results are not known for a reason other than
pending results (e.g., result was lost or specimen
was contaminated, and no other specimen can be
obtained).
If an initial specimen was
collected and results are
unknown, but results later
become known, update
the results.
Comment: When to collect specimen
A smear, or pathology, or cytology specimen should have been collected during diagnostic workup or
shortly thereafter. Do
not
record specimens collected after the patient has received treatment more than 2
weeks.
For
positive
or
negative
results of an examination for a smear or, pathology, or cytology of tissue and/or
other body fluids, enter the following information.
Description Comment
Month, day, and year the first
specimen with a positive result was
collected (e.g., 01/17/2009)
If several specimen (tissue or fluid)
examinations were done and the results of 1
or more examinations were positive, enter the
date the first specimen with a positive result
was collected.
Date
collected
Month, day, and year the first negative
specimen was collected (e.g.,
01/17/2009) if all results were negative
If several specimen examinations were done
and all results were negative, enter the date
the first specimen with a negative result was
collected.
Anatomic
code
Enter appropriate anatomic code (e.g.,
30 for pericardium) from Appendix
C – Anatomic Codes.
Note: For the purposes of the RVCT training materials, use the codes listed in the appendices. Some
software programs used to enter data on the RVCT may NOT use the codes listed in the appendices.
For example, the Anatomic Codes may be a drop-down item where you choose the actual site rather
than enter a code. For more information, see instructions for the software you use.
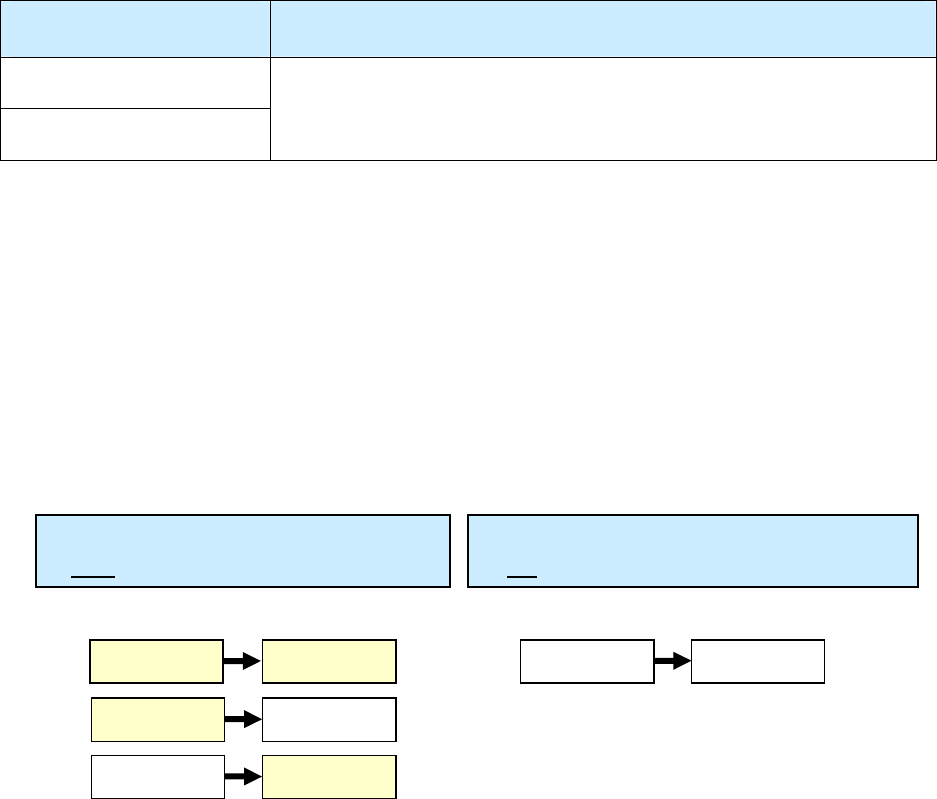
91
For
Type of Exam
, select both of the following if applicable.
Option
(select all that apply)
Comment
Smear
Pathology/cytology
Select the type(s) of exam that correspond(s) to the result selected in
item 19.
Comment: Any positive result supersedes a negative result in reporting TB diagnostic criteria
.
If the results are discrepant (smear negative, pathology positive), then Type of Exam should correspond to
the result captured as positive. If both smear and pathology had been positive, both smear and
pathology/cytology should be checked under Type of Exam. Likewise, if both smear and pathology had
been negative, then both smear and pathology/cytology should be checked under Type of Exam.
Example: Positive result superseding a negative result
If the smear results were negative and the pathology was positive, then Type of Exam selected should be
Pathology. In this case, smear would not be selected because the result was negative.
Reporting TB Diagnostic Criteria
(Positive Result Supersedes Negative Results)
Positive
Positive Diagnosis
(Any TB Test Result Is Positive)
Positive
Positive Negative
Negative Positive
1
st
Test
2
nd
Test
Negative Diagnosis
(All TB Test Results Are Negative)
Negative Negative
1
s
t
Test
2
nd
Test

92
Exercise
19.
Smear/Pathology/Cytology of Tissue and Other Body Fluids
Case Study – Tricia
Four-year-old Tricia has a gastric aspiration procedure done on admission to the hospital
on January 7, 2009. The laboratory report indicates that the gastric aspirate smear is
negative for acid fast bacilli (AFB).
19.1 What option should be selected for Smear/Pathology/Cytology of Tissue and
Other Body Fluids?
(circle the one best answer)
A.
Positive
B.
Negative
C.
Not done
D.
Unknown
19.2 What is the anatomic code?
(circle the one best answer)
A.
52 – gastric aspirate
B.
56 – gastric aspirate
C.
68 – gastric aspirate
19.3 What is the type of exam?
(circle the one best answer)
A.
Smear
B.
Pathology/Cytology
93
Case Study – Sam
Sam has fine needle aspiration to sample lung fluid followed by a lung biopsy. The
laboratory report indicates that the smear of the lung fluid is negative for AFB. However,
the lung tissue shows AFB, inflammation, and granulomas consistent with TB disease.
19.4 What entry should be selected for the results?
(circle the one best answer)
A.
Positive, because the lung tissue shows AFB organisms
B.
Negative, because the smear results of the fluid are negative
C.
Unknown, because there is a discrepancy between the two tests
19.5 What would be selected for the type of exam?
(circle the one best answer)
A.
Smear
B.
Pathology/Cytology
C.
Both A and B are correct
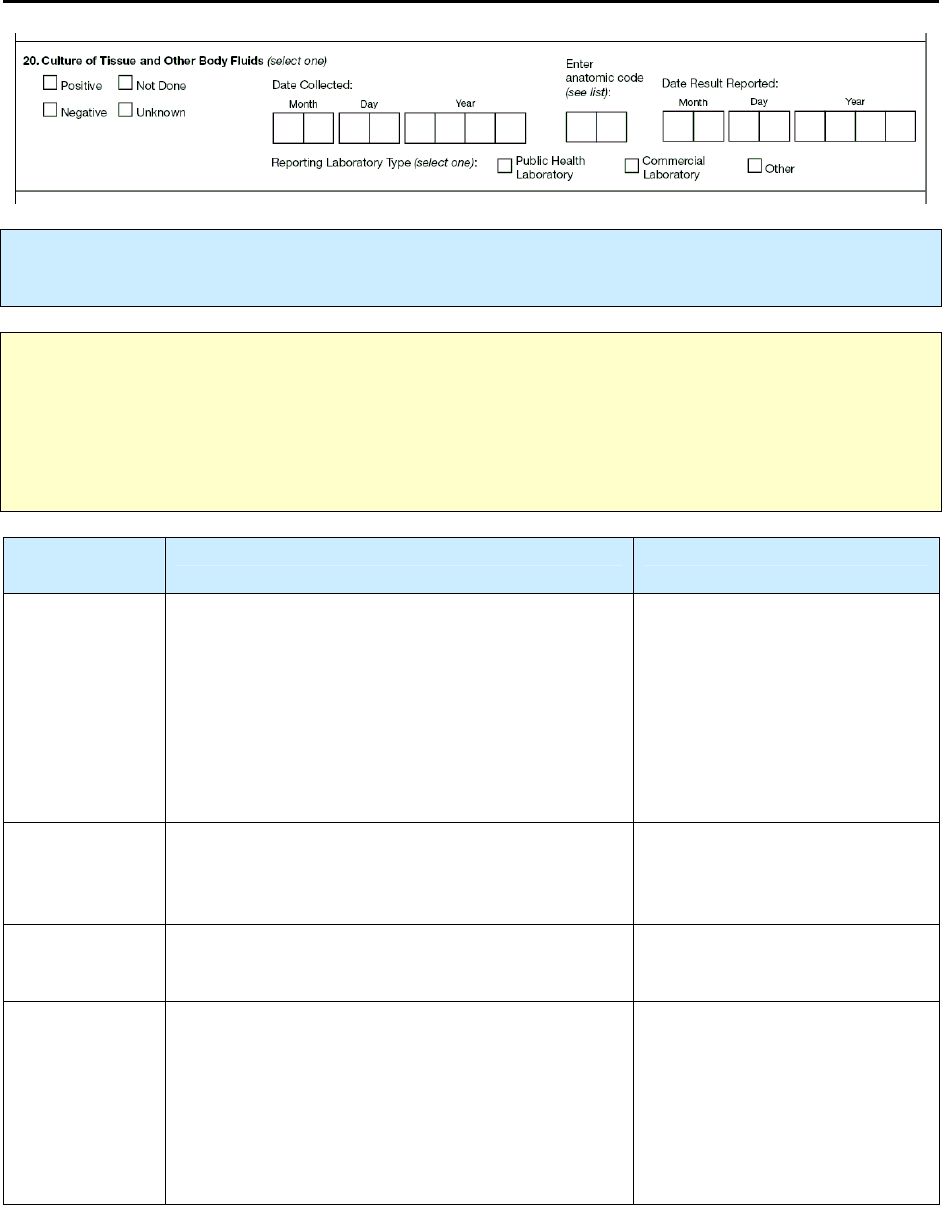
94
20.
Culture of Tissue and Other Body Fluids
Primary Purpose: Case management and surveillance. This result is a factor in determining whether
the patient’s disease meets the public health definition of TB.
Note:
The term “tissue and other body fluids”
does not include
sputum. Examples of tissue and other
body fluids are tracheal aspirate, bronchial cells and fluid, urine, bone marrow, lymph node, cerebral
spinal fluid, lung tissue or fluid, and pleural fluid collected from various procedures (e.g.,
bronchoscopy, bronchial washing or lavage, biopsy, gastric aspiration, pleural aspiration).
Results from sputum smear examinations and sputum cultures should be entered under
Sputum Smear
(item 17) and
Sputum Culture
(item 18).
Option
(select one)
Description Comment
Positive
The culture results for any tissue or fluid culture
other than sputum (see Note above) was positive
for M. tuberculosis complex.
If an initial specimen was
collected and results are
unknown, but results later
become known, update the
results.
Any positive result supersedes
a negative result.
Negative
The culture results for all tissue or fluid cultures,
other than sputum cultures, were negative for M.
tuberculosis complex.
Not done
It is known that tissue or fluid cultures were not
done.
Unknown
It is not known whether tissue or fluid cultures
were performed
or
Results are not known for a reason other than
pending results (e.g., result was lost or specimen
was contaminated, and no other specimen can be
obtained).
If an initial specimen was
collected and results are
unknown, but results later
become known, update the
results.
95
Comment: When to collect specimen
Specimens of tissue and other body fluids should have been collected during diagnostic workup or shortly
thereafter. Do
not
record specimens collected after the patient has received treatment for more
than 2 weeks.
For
positive
or
negative
result of tissue or fluid culture, enter the following information.
Description Comment
Month, day, and year the first
specimen with a positive result
was collected (e.g.,
01/17/2009)
If cultures were performed on several specimens
of tissue or fluid and the results of 1 or more were
positive
for M. tuberculosis complex, enter the
date the
first specimen with a positive
culture
result was collected.
Date
collected
Month, day, and year the first
specimen with a negative result
was collected (e.g., 1/17/2009)
if all results were negative
If several cultures were done and all results were
negative, enter the date the first specimen with a
negative result was collected.
Anatomic code
Enter appropriate anatomic
code (e.g., 30 for pericardium)
from Appendix C – Anatomic
Codes.
Note: For the purposes of the RVCT training materials, use the codes listed in the appendices. Some
software programs used to enter data on the RVCT may NOT use the codes listed in the appendices.
For example, the Anatomic Codes may be a drop-down item where you choose the actual site rather
than enter a code. For more information, see instructions for the software you use.
96
For the
first
tissue or fluid culture reported to be
positive
for M. tuberculosis complex, enter the
following information.
Description Comment
Date result
reported
Month, day, and year the result
was reported by the laboratory
(e.g., 01/17/2009)
This date can be found on the laboratory report as
the date the report is released or made available.
If the day is unknown, enter 99 as the
default value (e.g., 01/99/2009).
For
positive
results, select the option that best describes the
reporting laboratory type
.
Option
(select one)
Description Comment
Public health
laboratory
Any laboratory associated with
a local or a state health
department
Commercial
laboratory
Any laboratory that charges
a fee for each specimen
processed or test performed
Other
Any other laboratory that is
not considered a public health
laboratory or a commercial
laboratory
Hospital laboratories (e.g., National Jewish Health
hospital laboratory) and laboratories associated
with federal public health agencies (e.g., Centers
for Disease Control and Prevention, Veterans
Administration, Indian Health Service [IHS],
Tribal Health Department, and Bureau of Prisons)
National Jewish Health hospital laboratory
sometimes charges for services, but for the
purposes of the RVCT it is categorized as
“Other.”

97
Exercise
20.
Culture of Tissue and Other Body Fluids
Case Study – Kevin
Kevin is hospitalized for TB meningitis. On October 13, 2009, his cerebral spinal fluid is
collected. On October 20, the test result is returned and indicates that the sample had
been contaminated. On October 25, a second cerebral spinal fluid sample is taken and
sent for culture to the state health laboratory. On November 22, the culture result for the
second sample is reported to the hospital as positive for M. tuberculosis complex. That
same day the result is also reported to the health department.
20.1 What would be selected for Culture of Tissue and Other Body Fluids?
(circle the one best answer)
A.
Positive
B.
Negative
C.
Not done
D.
Unknown
20.2 What is the date collected?
(circle the one best answer)
A.
October 13, 2009
B.
October 16, 2009
C.
October 25, 2009
20.3 What should you enter for date result reported?
(circle the one best answer)
A.
October 16, 2009
B.
October 25, 2009
C.
November 22, 2009
98
20.4 What reporting laboratory type should be selected?
(circle the one best answer)
A.
Public health laboratory
B.
Commercial laboratory
C.
Other
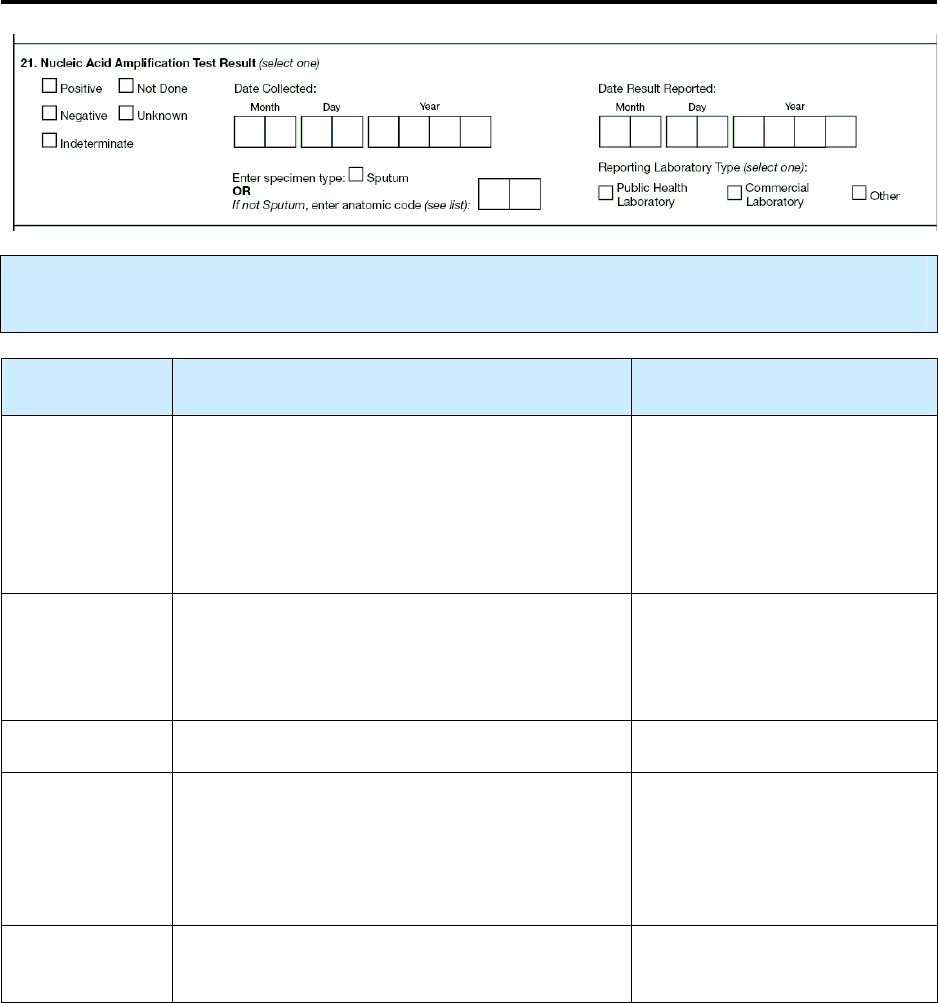
99
21.
Nucleic Acid Amplification Test Result
Primary Purpose: Case management and surveillance. This result is a factor in determining whether
the patient’s disease meets the public health definition of TB.
Option
(select one)
Description Comment
Positive
Any NAA test result was positive for M.
tuberculosis complex.
Any positive result supersedes
all other test results (e.g., 1
positive and 2 negatives =
positive; 1 indeterminate and 1
negative and 1 positive =
positive).
Negative
No NAA test results were positive for M.
tuberculosis complex and at least one result was
negative.
A negative result supersedes
indeterminate (e.g., 1 negative
and 1 indeterminate =
negative).
Not done
NAA test was not performed.
Unknown
It is not known whether an NAA test was
performed.
or
NAA test results are not known or result is not
known for a reason other than pending results.
If an initial specimen was
collected and results are
unknown, but results later
become known, update the
results.
Indeterminate
All NAA tests were indeterminate (e.g.,
inconclusive, inhibitory).
All tests are indeterminate.
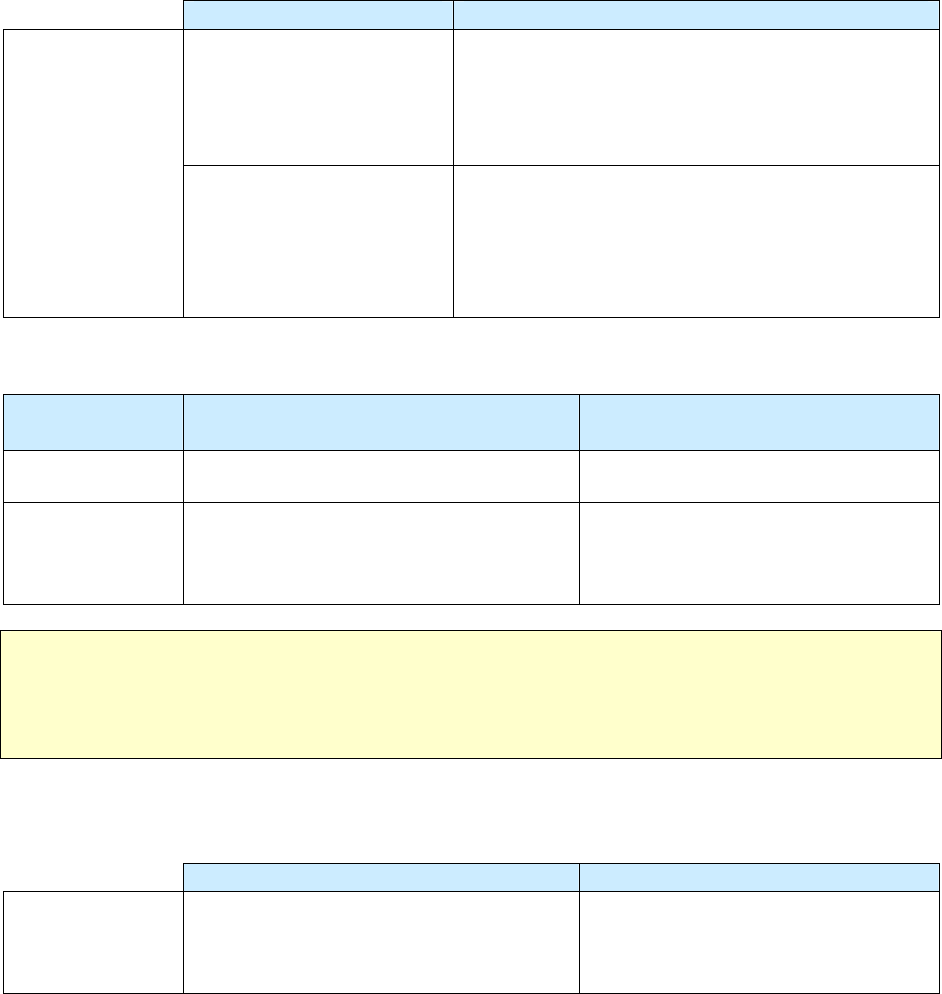
100
For
positive
or
negative
results of NAA testing, enter the following information.
Description Comment
Month, day, and year the
first sputum specimen with
a positive result was
collected (e.g., 01/17/2009)
If several specimens were collected and the NAA
test results of 1 or more were
positive
for M.
tuberculosis complex, enter the date the
first
specimen with a positive result was collected.
Date
collected
Month, day, and year the
first sputum specimen with
a negative result was
collected (e.g., 01/17/2009)
if all results were negative
If several specimens were collected and all NAA
test results were negative, enter the date the first
sputum specimen with a negative result was
collected.
Select the
Specimen Type
on which NAA testing was done.
Option
(select one)
Description Comment
Sputum
Not sputum
Enter appropriate anatomic code (e.g., 30
for pericardium) from Appendix C –
Anatomic Codes
Note: For the purposes of the RVCT training materials, use the codes listed in the appendices. Some
software programs used to enter data on the RVCT may NOT use the codes listed in the appendices.
For example, the Anatomic Codes may be a drop-down item where you choose the actual site rather
than enter a code. For more information, see instructions for the software you use.
For the
first
NAA test result reported
positive
for M. tuberculosis complex, enter the following
information.
Description Comment
Date result
reported
Month, day, and year the result was
reported by the laboratory (e.g.,
01/17/2009)
This date can be found on the
laboratory report as the date the report
is released or made available.
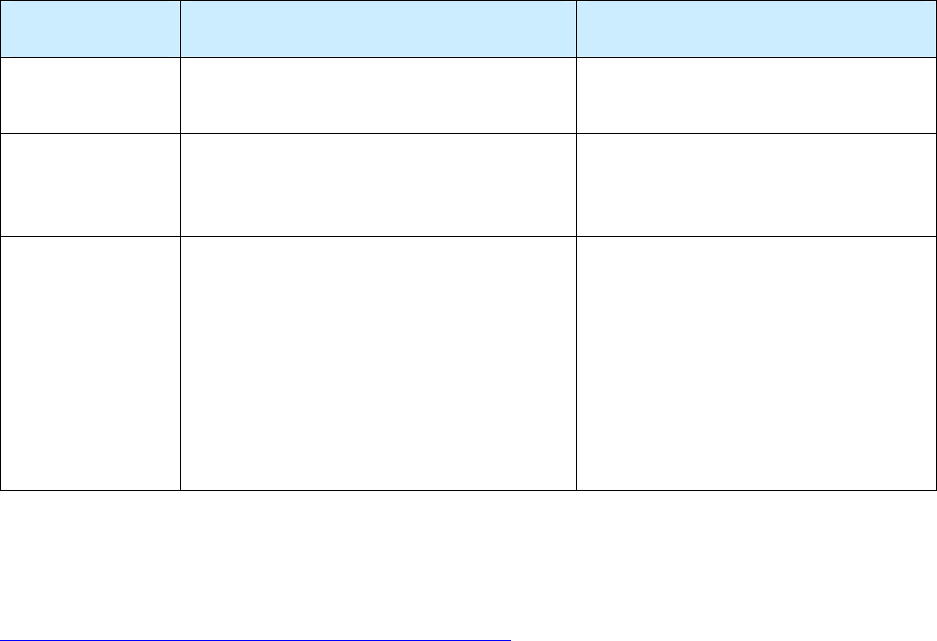
101
For
positive
NAA test results, select the option that best describes the
reporting laboratory type
.
Option
(select one)
Description Comment
Public health
laboratory
Any laboratory associated with a local or
a state health department
Commercial
laboratory
Any laboratory that charges
a fee for each specimen
processed or test performed
Other
Any other laboratory that is
not considered a public health
laboratory or a commercial laboratory
Hospital laboratories (e.g., National
Jewish Health hospital laboratory)
and laboratories associated with
federal public health agencies (e.g.,
Centers for Disease Control and
Prevention, Veterans Administration,
Indian Health Service [IHS], Tribal
Health Department, and Bureau of
Prisons)
Comment: Nucleic Acid Amplification Tests
The MMWR report, “Updated Guidelines for the Use of Nucleic Acid Amplification Tests in the
Diagnosis of Tuberculosis,” provides information on the NAA tests that have been approved by the Food
and Drug Administration for use with AFB smear-positive respiratory specimens. Accessible at
www.cdc.gov/mmwr/preview/mmwrhtml/mm5801a3.htm.

102
Exercise
21.
Nucleic Acid Amplification Test Result
Case Study – Newton
Newton is planning to go to England on vacation in one week. He is feeling sick and
presents at the Newnan County Health Department with symptoms of TB. His doctor
requests a nucleic acid amplification (NAA) test because he is a TB suspect. The test
can determine if Newton has TB disease before he leaves on vacation. The NAA test
result is inconclusive.
21.1 What NAA test result should be selected?
(circle the one best answer)
A.
Positive
B.
Negative
C.
Not done
D.
Unknown
E.
Indeterminate
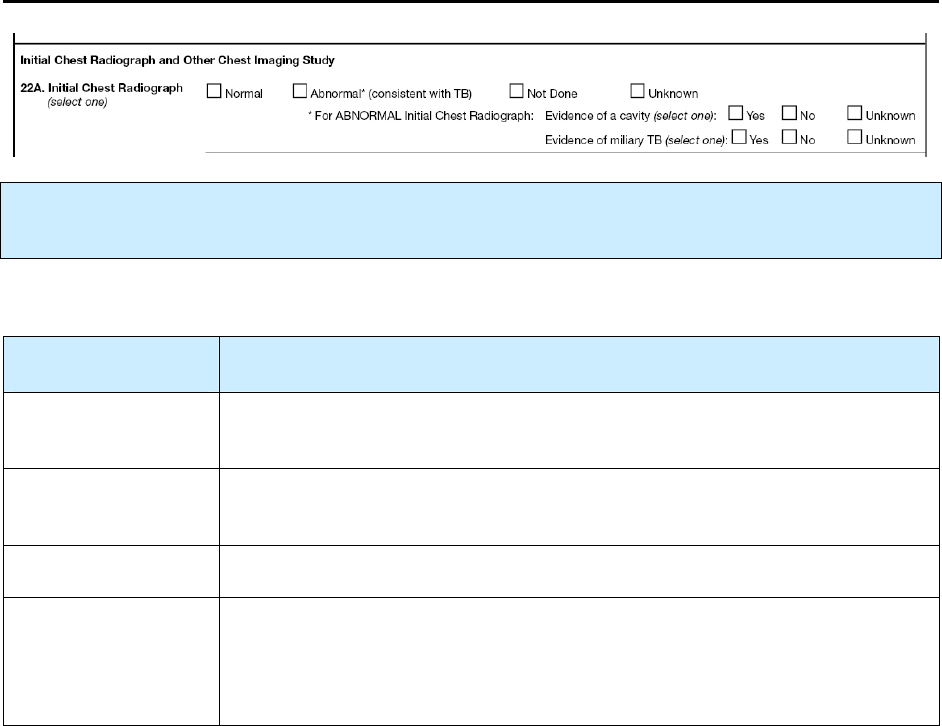
103
22A.
Initial Chest Radiograph
Primary Purpose: Case management and surveillance. This is part of a diagnostic evaluation used to
determine whether the patient’s disease meets the public health definition of TB.
Select the result of the
initial
chest radiograph(s) performed during the diagnostic evaluation for TB.
Option
(select one)
Description
Normal
Initial chest radiograph(s) showed no abnormalities consistent with TB. This
category includes any other abnormalities that are not consistent with TB.
Abnormal
(consistent with TB)
Any initial chest radiograph showing abnormalities (e.g., hilar adenopathy,
effusion, infiltrate[s], cavity, scarring) consistent with TB.
Not done
It is known that the initial chest radiograph was not done.
Unknown
It is not known whether an initial chest radiograph was done.
or
Result of initial chest radiograph is not known or result is not known for a
reason other than pending results.
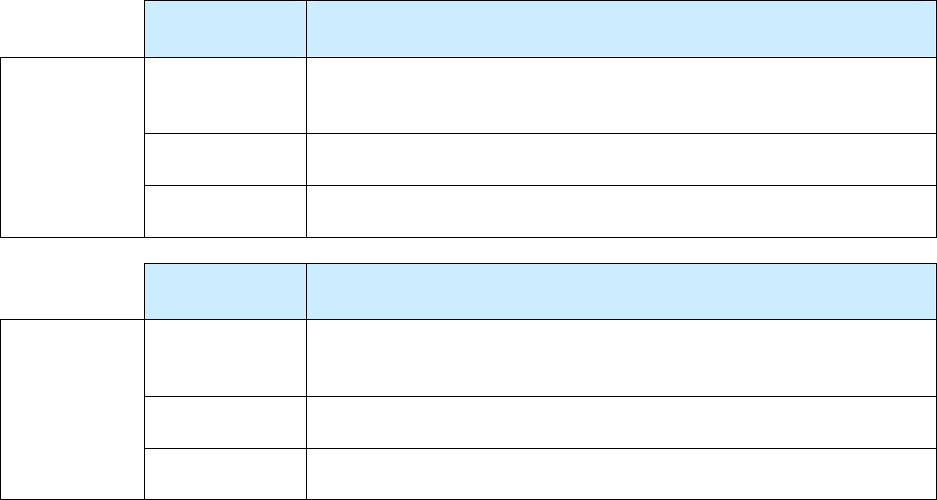
104
For
abnormal
results, select one option for each type of evidence.
Option
(select one)
Description
Yes
Any initial chest radiograph(s) showing evidence of 1 or more lung
cavities
No
Evidence of
a cavity
Unknown
Option
(select one)
Description
Yes
Any initial chest radiograph(s) showed evidence of miliary disease
(e.g., miliary TB, or miliary or bilateral micronodular pattern)
No
Evidence of
miliary TB
Unknown
Comment: Miliary TB
Unlike the previous RVCT form, the new form has no place to select miliary TB in
Site of Disease
(item
16). If the report of the initial chest radiograph or the initial chest CT scan indicates “miliary TB or a
miliary or bilateral micronodular pattern,” record this finding under
Initial Chest Radiograph
(item
22A) or
Initial Chest CT Scan or Other Chest Imaging Study
(item 22B), respectively. However,
pulmonary should be selected as
Site of Disease
(item 16) if the chest x-ray or CT scan shows evidence
of nodules consistent with miliary TB.
Miliary TB is a serious type of tuberculosis infection. It is a clinical or radiologic finding, rather than a
site of disease. Miliary TB is the result of a TB lung infection eroding into the bloodstream and from there
disseminating throughout the body to multiple organs. It appears on radiograph as a great number of small
(1- to 2-mm), well-defined nodules that look like millet seeds scattered throughout the lungs, hence the
name “miliary.”

105
Exercise
22 A.
Initial Chest Radiograph
What is the result of the Initial Chest Radiograph for the following patients?
(Choose the one best answer by matching the results of the initial chest radiograph with the
patient. Write in the letter for the result on the line next to the question number.)
Patient Initial Chest Radiograph
___
22 A.1
Emily is a 4-year old whose chest
radiograph shows tiny
(1- to 2-millimeters), well-defined
nodules.
___
22 A.2
Alice has a radiograph that shows no
evidence of TB.
___
22 A.3
Lawrence was evaluated for TB while
living in Arabia and placed on anti-TB
drugs. He remembers getting a chest
radiograph but is not sure of the result.
___
22 A.4
Roy has a chest radiograph that shows
cavities in the left upper lobe of his
lung.
___
22 A.5
Frank’s laboratory results are
consistent with TB disease. On his
way to the clinic to get a chest
radiograph, he dies in an automobile
crash.
A.
B.
C.
D.
E.
Normal
Abnormal with evidence of
cavitary lesion
Abnormal with evidence of
miliary TB
Not Done
Unknown
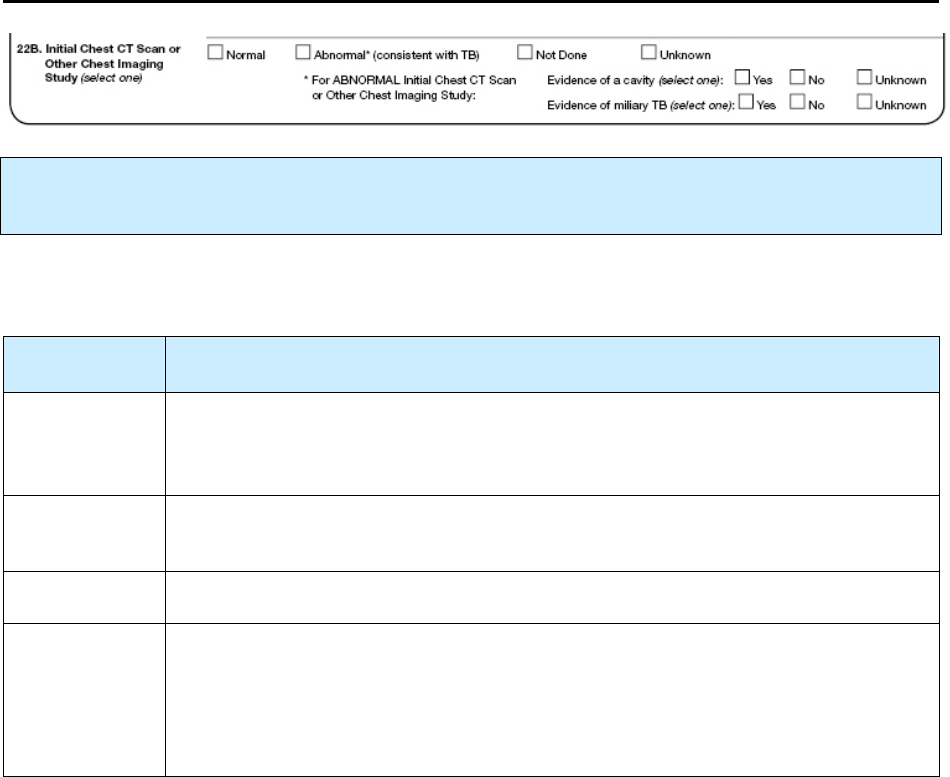
106
22B.
Initial Chest CT Scan or Other Chest Imaging Study
Primary Purpose: Case management. This is part of a diagnostic evaluation used to determine whether
the patient’s disease meets the public health definition of TB.
Select the result of the
initial
chest computerized tomography (CT) or other chest imaging study such as
magnetic resonance imaging (MRI), performed during the diagnostic evaluation for TB.
Option
(select one)
Description
Normal
Initial chest CT scan or other chest imaging study showed no abnormalities
consistent with TB. This category includes any other abnormalities that are not
consistent with TB.
Abnormal
(consistent
with TB)
Any initial chest CT scan or other chest imaging study showed abnormality
(e.g., hilar adenopathy, effusion, infiltrate[s], cavity, scarring) consistent with TB.
Not done
It is known that the initial chest CT scan or other chest imaging study was not done.
Unknown
It is not known whether an initial chest CT scan or other chest imaging study was
done.
or
Result of initial chest CT scan or other chest imaging study is not known or result is
not known for a reason other than pending results.
107
For
abnormal
chest CT scan or other chest imaging study results, select one option for each type of
evidence.
Option
(select one)
Description
Yes
Any initial chest CT scan or other chest imaging study showed
evidence of 1 or more cavities.
No
Evidence of a
cavity
Unknown
Option
(select one)
Description
Yes
Any initial chest CT scan or other chest imaging study showed
evidence of miliary disease (e.g., miliary TB, or miliary or
bilateral micronodular pattern).
No
Evidence of
miliary TB
Unknown
Comment:
Miliary TB
Unlike the previous RVCT form, the new form has no place to select miliary TB in
Site of Disease
(item
16). If the report of the initial chest radiograph or the initial chest CT scan indicates “miliary TB or a
miliary or bilateral micronodular pattern,” record this finding under
Initial Chest Radiograph
(item
22A) or
Initial Chest CT Scan or Other Chest Imaging Study
(item 22B), respectively. However,
pulmonary should be selected as
Site of Disease
(item 16) if the chest x-ray or CT scan shows evidence
of nodules consistent with miliary TB.
Miliary TB is a serious type of tuberculosis infection. It is a clinical or radiologic finding, rather than a
site of disease. Miliary TB is the result of a TB lung infection eroding into the bloodstream and from there
disseminating throughout the body to multiple organs. It appears on radiograph as a great number of small
(1- to 2-mm), well-defined nodules that look like millet seeds scattered throughout the lungs, hence the
name “miliary.”

108
Exercise
22 B.
Initial Chest CT Scan or Other Chest Imaging Study
Case Study for Items 16, 22A, and 22B – Anna
Anna is a 3-year-old who was diagnosed with miliary TB disease. It appeared in both her
initial chest radiograph and her initial chest CT scan.
22. B. 1 In which item(s) would you record responses indicating that Anna has miliary
TB? (circle the one best answer)
A.
Item 16 – Site of Disease
B.
Item 22 A – Initial Chest Radiograph - Evidence of miliary TB
C.
Item 22 B – Initial Chest CT Scan or Other Chest Imaging Study – Evidence of
miliary TB
D.
A, B, and C are correct
E.
Only B and C are correct
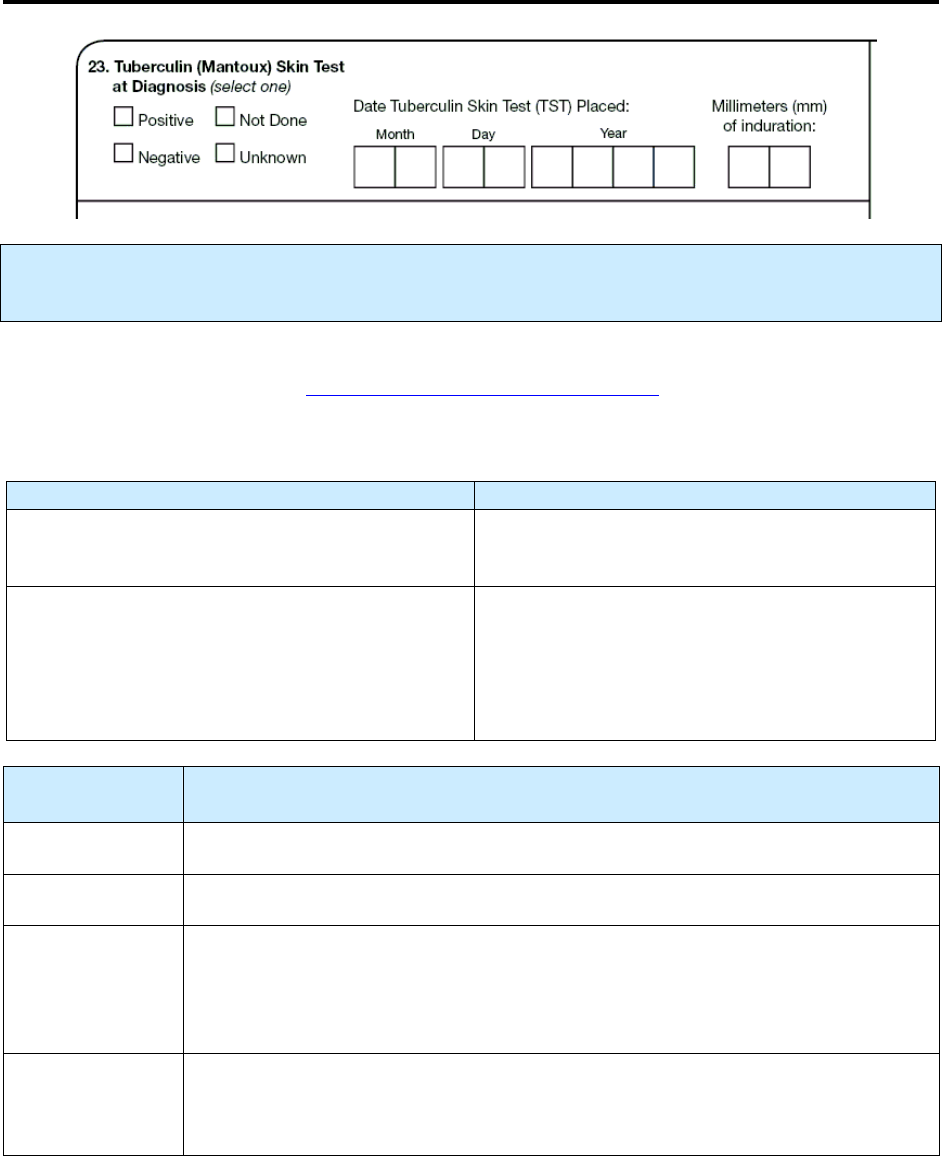
109
23.
Tuberculin (Mantoux) Skin Test at Diagnosis
Primary Purpose: Case management. This result helps guide clinicians in diagnosing TB infection and
is a factor in determining whether the patient’s disease meets the public health definition of TB.
Positive
or
negative
result of the tuberculin skin test (TST) should be interpreted according to Table 7 of
the currently accepted guidelines (www.cdc.gov/mmwr/PDF/rr/rr4906.pdf).
Guidelines for Entering TST Results
Enter Do Not Enter
Results from a TST performed during the current
diagnostic evaluation
A patient’s
undocumented
self-report of a
previous positive result is
not
acceptable
May enter previous positive test result
•
If patient had tested
positive
to a previous TST
and
•
The previous positive result
is documented
in
the medical record.
A previous
negative
TST result (reported by the
patient or documented or both) is also
not
acceptable
Option
(select one)
Description
Positive
Meets the criteria for a positive TST result.
Negative
Result of TST did not meet current criteria for a positive test result.
Not done
TST was not performed
or
Patient reports a positive result of an earlier TST, but it cannot be documented,
and now the patient refuses a TST.
Unknown
It is not known whether a TST was performed
or
result is not known for a reason other than pending results.
110
For
positive
or
negative
TST results, enter the following information.
Description Comment
Date TST placed
Month, day, and year the TST was
placed (e.g., 01/17/2009)
If the month or day is unknown, enter
99 as the default value (e.g.,
01/99/2009).
Year must be recorded. Do not use
9999 for the year.
Millimeters (mm)
of induration
Measurement (in millimeters, mm) of
the induration (e.g., 05 mm)
If the millimeters of the induration are
not expressed, enter 99 as the default
value.
111
Interpreting the TST Reaction
5 or more millimeters 10 or more millimeters 15 or more millimeters
An induration of 5 or more
millimeters is considered
positive for
• People living with HIV
• Recent contacts of persons
with infectious TB
• People who have previously
had TB disease
• Patients with organ
transplants and other
immunosuppressed patients
(including patients taking a
prolonged course of oral or
intravenous corticosteriods
or TNF-α antagonists)
An induration of 10 or more
millimeters is considered positive
for
• People who have come to the
U.S. within the last 5 years
from areas of the world where
TB is common (for example,
Asia, Africa, Eastern Europe,
Russia, or Latin America)
• People who inject illegal drugs
• Mycobacteriology lab workers
• People who live or work in
high-risk congregate settings
• People with certain medical
conditions that place them at
high risk for TB (silicosis,
diabetes mellitus, severe kidney
disease, certain types of cancer,
and certain intestinal
conditions)
• Children younger than 4 years
• Infants, children, and
adolescents exposed to adults in
high-risk categories
An induration of 15 or more
millimeters is considered
positive for
• People with no known
risk factors for TB
112
Exercise
23.
Tuberculin (Mantoux) Skin Test at Diagnosis
Case Study – Duc
Duc immigrated from Laos to the United States on May 5, 1995. At that time he was seen
at the health department, where he had a TST. The TST result was read 2 days later as an
induration of 10 millimeters. Since Duc is from Asia, a TST reaction that is 10 or more
millimeters is considered a positive reaction. He completed LTBI treatment in May 1996.
In March 2009 he is diagnosed with extrapulmonary TB of the left tibia.
23.1 What would you select for Tuberculin (Mantoux) Skin Test at Diagnosis?
(circle the one best answer)
A.
Positive
B.
Negative
C.
Not done
D.
Unknown
23.2 What is the date tuberculin skin test (TST) placed?
(circle the one best answer)
A. Month Day Year
0 5 0 5 1 9 9 5
B.
Month Day Year
0 5 9 9 1 9 9 5
C. Month Day Year
0 5 9 9 1 9 9 6
23.3 What would be entered for millimeters (mm) of induration?
(circle the one best answer)
A.
Millimeters (mm) of induration 9 9
B.
Millimeters (mm) of induration 1 0
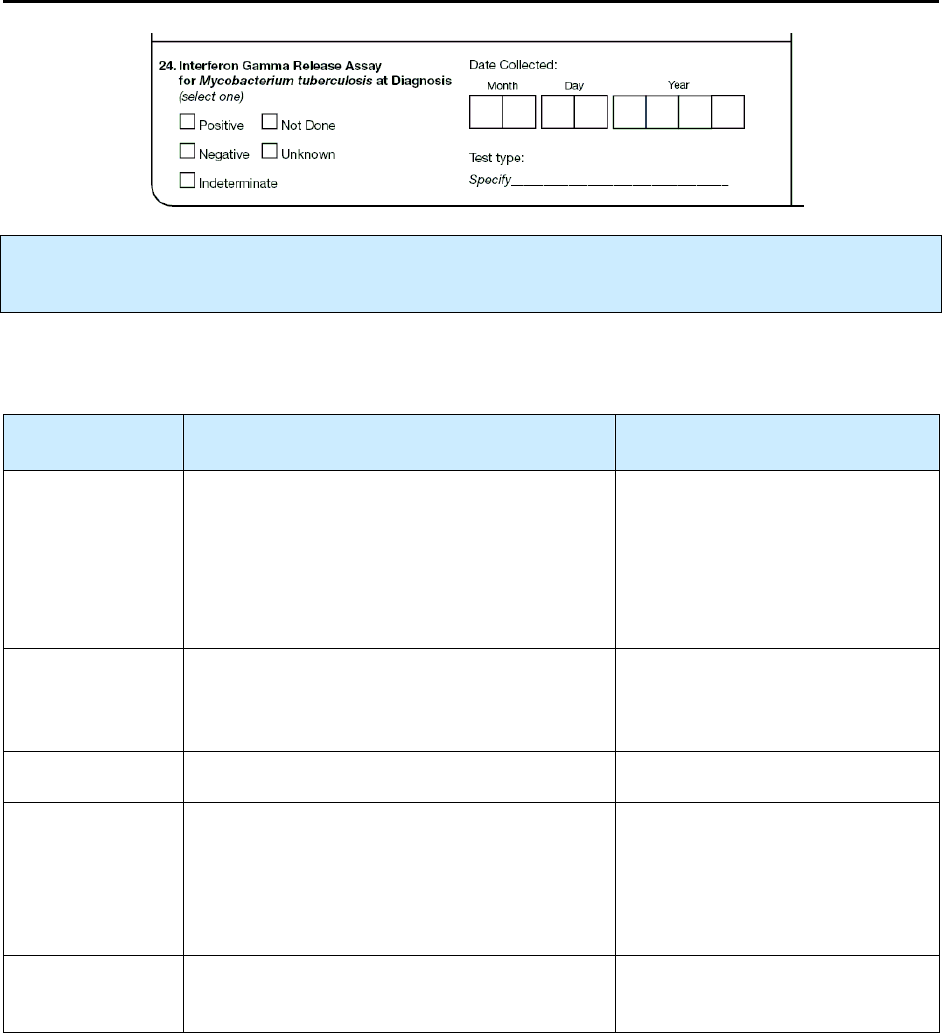
113
24.
Interferon Gamma Release Assay for
Mycobacterium
tuberculosis
at Diagnosis
Primary Purpose: Case management. This result helps guide clinicians in diagnosing TB infection and
is a factor in determining whether the patient’s disease meets the public health definition of TB.
Interferon gamma release assays (IGRAs) are blood tests for detecting M. tuberculosis infection.
This variable applies to an IGRA performed during the diagnostic evaluation.
Option
(select one)
Description Comment
Positive
Any IGRA result was interpreted as “M.
tuberculosis infection is likely.”
Any positive result supersedes
all other test results (e.g., 1
positive and 2 negatives =
positive; 1 indeterminate and 1
negative and 1 positive =
positive).
Negative
All IGRA results were interpreted as “M.
tuberculosis infection is unlikely.”
A negative result supersedes
indeterminate (e.g., 1 negative
and 1 indeterminate = negative).
Not done
IGRA was not performed.
Unknown
It is not known whether IGRA was
performed.
or
IGRA results are not known, or result is not
known for a reason other than pending results.
Indeterminate
IGRA results could not be determined to be
positive or negative.
114
For
positive
or
negative
results of IGRA, enter the following information.
Description Comment
Date collected
Month, day, and year the
blood sample was collected
(e.g., 01/17/2009)
If several blood tests were performed and the
results of one or more tests were positive for M.
tuberculosis complex, enter the date the first blood
test with a positive result was collected.
Test type
(specify)
Specify the blood test
performed [e.g.,
QuantiFERON
®
-TB Gold test
(QFT-G)]
If more than 1 test was performed, enter the name
of the test used for the specimen for which you
entered the result.

115
Exercise
24.
Interferon Gamma Release Assay for Mycobacterium tuberculosis at
Diagnosis
Case Study – Shepo
Shepo is a recent immigrant from Kenya. He works as a maintenance worker at the
Winston Animal Research Facility. The facility conducts annual employee screening for
TB to prevent exposing the primates to TB. Shepo is tested with a QuantiFERON
®
-TB
Gold test (QFT-G) because he had BCG vaccination as a child. The QFT-G test result is
positive. Upon further evaluation, Shepo is diagnosed with active TB disease and an
RVCT is initiated for him.
24.1 What would you select for Interferon Gamma Release Assay for Mycobacterium
tuberculosis at Diagnosis?
(circle the one best answer)
A.
Positive
B.
Negative
C.
Not done
D.
Unknown
E.
Indeterminate
24.2 What would you specify as the Test Type?
(circle the one best answer)
A.
Interferon gamma release assay
B.
Tuberculin skin test
C.
Blood test
D.
QuantiFERON
®
-TB Gold test (QFT-G)
E.
Unknown
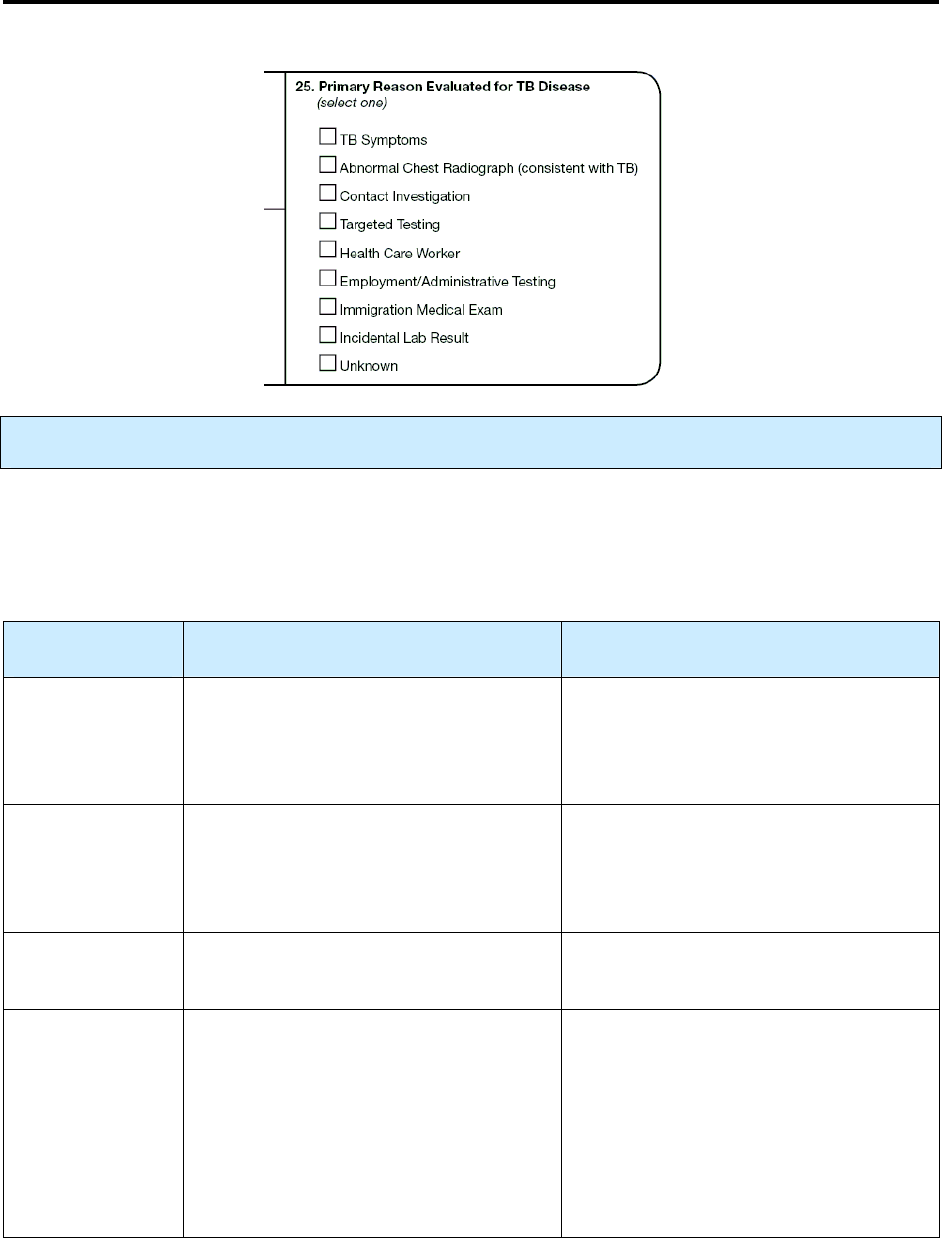
116
25.
Primary Reason Evaluated for TB Disease
Primary Purpose: Programmatic function. Data are helpful in assessing how a TB case was found.
Select the
single primary or initial reason
the patient was evaluated for TB disease. The definition of
“primary or initial reason” is the situation or reason that led to the initial suspicion that the patient might
have TB disease. If the patient was referred for evaluation, but the reason for the evaluation is unknown,
try to determine that reason.
Option
(select one)
Description Comment
TB symptoms
Signs and symptoms consistent with TB
(e.g., prolonged persistent cough, fever,
lymphadenopathy, night sweats, weight
loss)
Select if patient seeks medical attention
because of symptoms. Do not select if
symptoms discovered during a
screening program.
Abnormal chest
radiograph
Incidental chest radiograph consistent
with TB disease
Reason for the chest radiograph should
be independent of the other choices
listed and should not have been the
result of suspicion of TB disease.
Contact
investigation
Result of a contact investigation or
source case finding
Targeted testing
Positive result of tuberculin skin test
(TST) or interferon gamma release
assay (IGRA) administered because the
patient was specifically considered as
high risk for TB (e.g., persons from area
of the world with high rate of TB) or as
part of a testing program focused on
specific groups at risk for TB
Do not select if another reason (e.g.,
contact investigation, immigration
medical examination, employment/
administrative testing, or health care
worker status) is more appropriate
(see other choices).
117
Health care
worker
Positive result of TST or IGRA
administered because the patient was a
health care worker
Refers to all paid and unpaid persons
working in health care settings who
have the potential for exposure to M.
tuberculosis.
For health care workers being evaluated
for TB disease, health care worker
supersedes targeted testing and
employment/administrative testing.
Other situations (e.g., TB symptoms,
contact investigation) supersede health
care worker.
Employment/
administrative
testing
Results from routine physical
examination before or periodically
during employment, TST or IGRA
required by employer, or primary or
secondary school program for routine
TST
Reflects an administrative requirement
(e.g., a TST program applied to all 5th
graders in a school or to all job
applicants) rather than testing of a group
considered at high risk. If TST or IGRA
was performed because the person was
considered at high risk, select targeted
testing or a more appropriate category,
such as health care worker. If
employment was health care–related,
select health care worker rather than
employment/administrative testing.
Immigration
medical exam
Findings of a medical examination that
was part of the immigration application
process
A medical examination is mandatory for
specific categories of persons seeking
admission to the United States (e.g.,
immigrants, refugees, asylees). These
medical examinations may be
performed overseas or in the United
States depending on the situation. In
addition, a medical examination may be
required for some persons applying for
nonimmigrant visas or special status
(e.g., parolees) for temporary admission
to the United States.
Incidental lab
result
The clinical evaluation was for
something other than TB (e.g.,
bronchoscopy or autopsy). Specimens
were collected and submitted for
evaluation of TB and other diseases for
diagnostic completeness. TB was not
expected.
Unknown
Reason for evaluating the patient not
known
118
Example: TB Symptoms
If a TB patient seeks medical care because of TB symptoms, select
TB Symptoms
as the primary
reason for the evaluation. If, however, a TB patient was initially encountered via a contact investigation
and during that investigation was also noted to have TB symptoms, select
Contact Investigation
as the
primary reason for the evaluation.
Example: Abnormal Chest Radiograph
If the chest radiograph was performed during a workup for TB disease because of a positive TST result
obtained during targeted testing, select
Targeted Testing
. However, if a chest radiograph was
performed as part of preoperative testing (TB disease was not suspected), select
Abnormal Chest
Radiograph
.
Examples: Health Care Worker
Includes full time, part-time, temporary, or contract staff. Examples include:
•
Physicians
•
Nurses
•
Health aides
•
Dental workers
•
Health technicians
•
Staff in laboratories and morgues
•
Emergency medical personnel
•
Students enrolled in health care
programs
•
Persons who deliver health care in the community
(e.g., public health nurse, visiting nurse, outreach
worker)
•
Persons not involved directly in patient care, but
potentially at risk for occupational exposure
(e.g., correctional facility staff, volunteers; outreach
workers; dietary/nutritional, housekeeping,
maintenance, clerical, janitorial staff, administrative
staff and supervisors)

119
Exercise
25.
Primary Reason Evaluated for TB Disease
What is the Primary Reason Evaluated for TB for the following patients?
(Choose the one best answer by matching the primary reason evaluated with the patient. Write
the letter for the reason on the line next to the question number.)
Patient Primary Reason
Evaluated for TB
___
25.1
Alan is a nurse at New York City health
department and participated in the annual
workplace TST screening program.
___
25.2
During an autopsy performed on Ben, the
pathologist orders a complete analysis of
various tests to help determine cause of
death. TB is not suspected, but the AFB test
result indicates Ben had TB.
___
25.3
A health care worker is providing DOT to
Carla’s father. During one of the DOT visits
the health care worker evaluates Carla for
TB. Carla is then diagnosed with TB disease.
___
25.4
Drew is in an automobile accident and has a
chest radiograph taken for a possible broken
rib. The chest radiograph also shows a lung
cavity.
___
25.5
Ellen goes to the out patient clinic because
she has been coughing and recently lost
weight.
___
25.6
Tran recently immigrated from Vietnam and
was diagnosed with TB disease.
___
25.7
George is HIV infected. His doctor at the
HIV clinic has him tested for TB infection.
___
25.8
Harry is a new primate keeper at the
Magnolia Zoo where TSTs are routinely
performed on all new employees.
A.
B.
C.
D.
E.
F.
G.
H.
TB symptoms
Abnormal chest
radiograph
Contact investigation
Targeted testing
Health care worker
Employment/
Administrative
testing
Immigration medical
exam
Incidental laboratory
result
120
Case Study – Lanie
Lanie is a nurse at the Canton Health Department in Ohio. She is identified as a contact
during the contact investigation for her brother-in-law, who has TB disease. During the
evaluation, Lanie is diagnosed with TB disease also. She completes therapy on
September 12, 2009.
25.9 What is the Primary Reason Lanie was Evaluated for TB in Ohio?
(circle the one best answer)
A.
Health care worker
B.
Contact investigation
C.
TB symptoms
D.
Targeted testing
Case Study – Lanie (continued)
On October 1, 2009, Lanie accepts a nursing position at the Indianapolis Health
Department in Indiana. On December 14, 2009, Lanie goes to the clinic because she is
coughing up blood and is worried that TB has returned. Her laboratory results indicate
that her sputum is smear and culture positive.
25.10 What is the Primary Reason Lanie was Evaluated for TB in Indiana?
(circle the one best answer)
A.
Health care worker
B.
Contact investigation
C.
TB symptoms
D.
Targeted testing
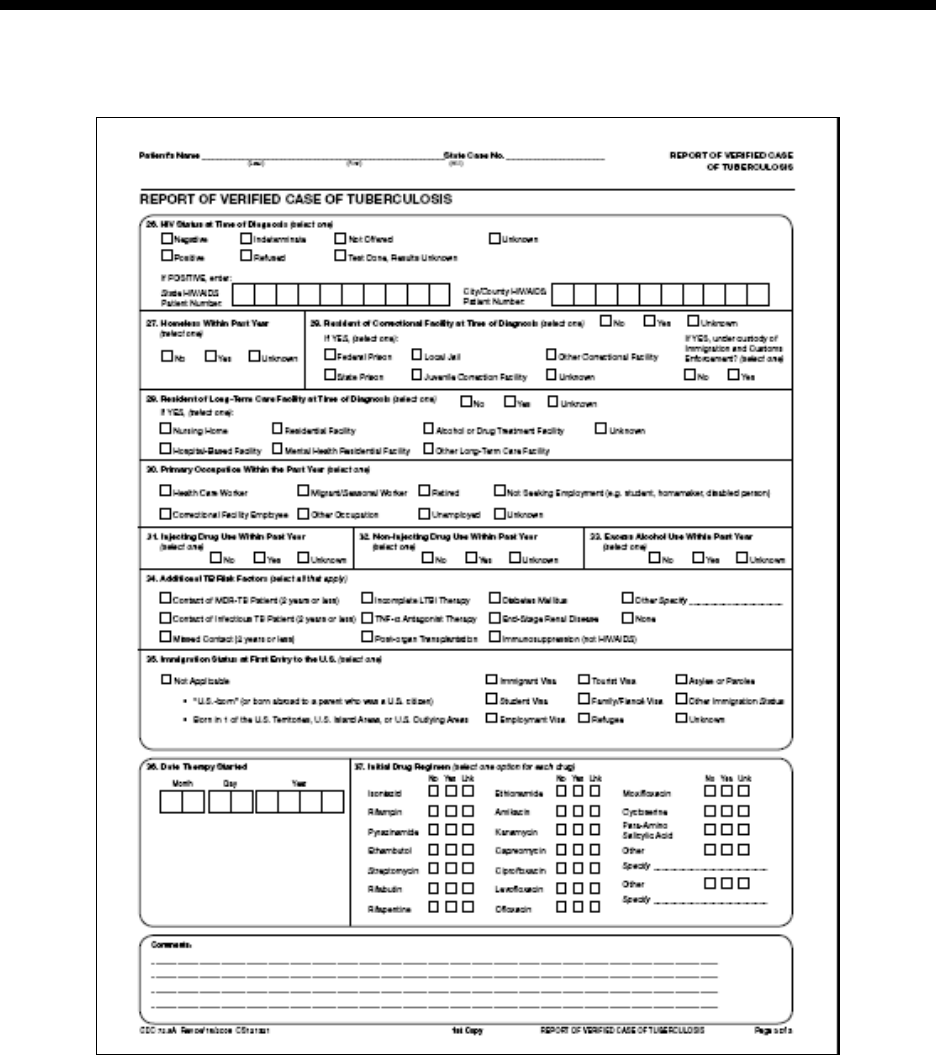
121
Module
Module Module
Module C
CC
C
-
--
- R
R R
RVCT
VCT VCT
VCT
(page 3
(page 3(page 3
(page 3 of 3
of 3 of 3
of 3)
))
)
Items 26
Items 26 Items 26
Items 26 –
––
– 37
37 37
37
Module C provides instructions and exercises for completing page 3 of the RVCT report. It includes data
about risks associated with TB, the date that therapy was started, and the initial drug regimen.
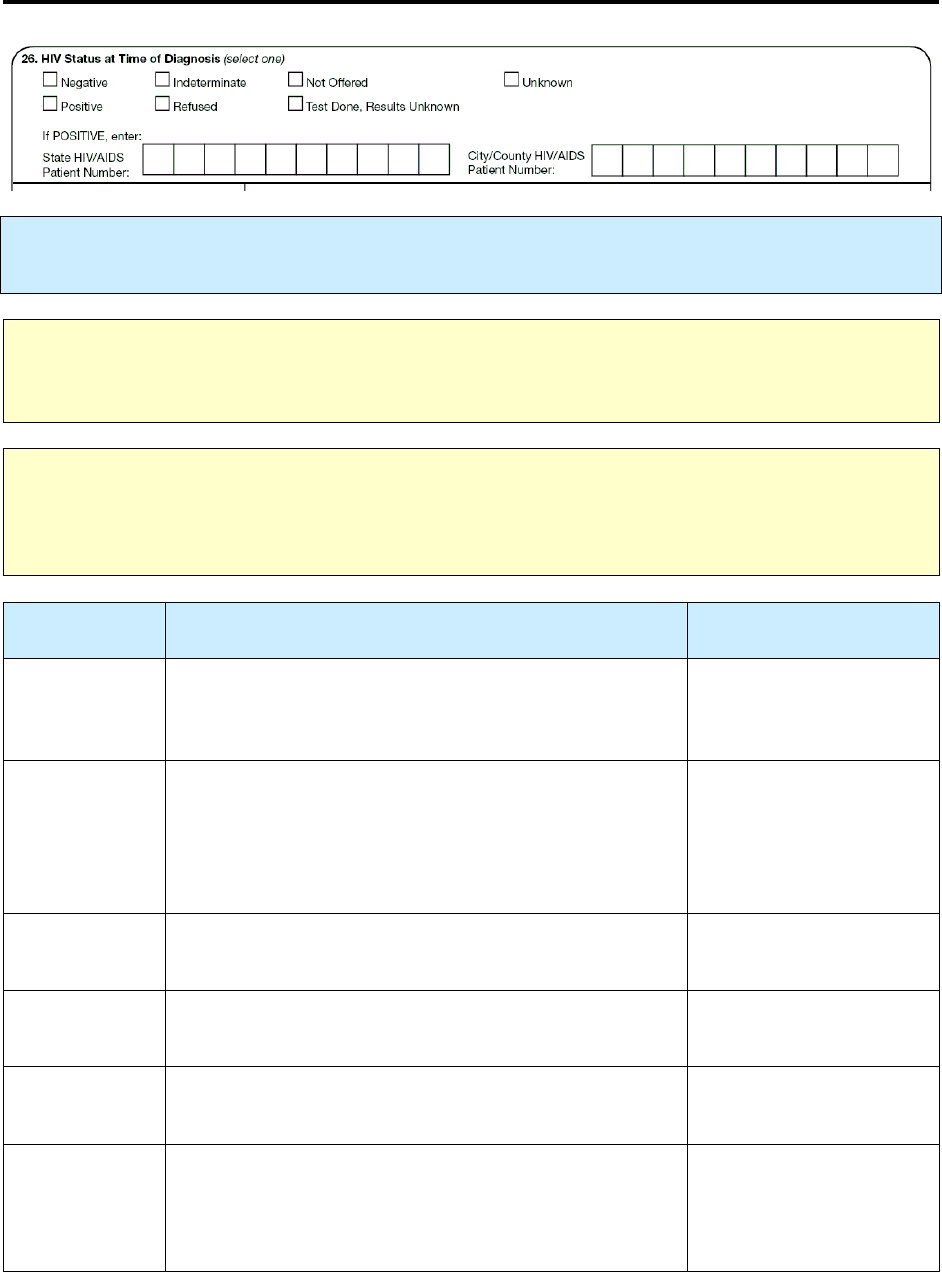
122
26.
HIV Status at Time of Diagnosis
Primary Purpose: Case management and surveillance. Data are used to determine TB/HIV co-
morbidity.
Note:
CDC recommends that
all
persons receive HIV testing at the time of TB diagnostic evaluation or
TB diagnosis. Refer to the CDC public health surveillance definition of HIV infection
(http://www.cdc.gov/mmwr/preview/mmwrhtml/rr4813a2.htm).
Note: Documentation of an HIV test result is needed. This documentation from a hospital, clinic, or
private provider, should be written evidence of the test result, or it can be notes in the medical record.
The test result should have been determined within the specified time indicated in the instructions
below.
Option
(select one)
Description Comment
Negative
Documented negative result of HIV test at the time of
TB diagnostic evaluation or at TB diagnosis or earlier,
but not exceeding 1 year
Undocumented report is
not acceptable.
Positive
Laboratory result interpreted as positive according to
published criteria
or
Documented positive result of an earlier HIV test or
documented earlier diagnosis of HIV infection or AIDS
Undocumented report is
not acceptable.
Indeterminate
Documented indeterminate result of an HIV test at the
time of TB diagnostic evaluation or TB diagnosis
Undocumented report is
not acceptable.
Refused
HIV testing offered but declined at the time of the TB
diagnostic evaluation or TB diagnosis
Not offered
HIV testing not offered at the time of the TB diagnostic
evaluation or TB diagnosis
Test done,
results
unknown
HIV test performed at the time of the TB diagnostic
evaluation or TB diagnosis, but the results not known to
the TB program, or result is not known for a reason
other than pending results.
123
Unknown
Not known whether the patient
• Has had an HIV test
• Was ever offered a test
• Was referred for HIV counseling and testing (e.g.
anonymous testing center, private testing center)
Comments: Negative HIV status
•
Undocumented patient report that an HIV test result was negative is
not
acceptable. Such patients
should be offered the opportunity to be tested for HIV.
•
If a patient has had a negative test result, regardless of when the HIV test was performed, the
patient should be offered HIV testing at the time of TB diagnostic evaluation or TB diagnosis.
•
If the patient had received HIV testing
less than 1 year before the TB diagnostic evaluation or
TB diagnosis
, the documented results were negative for HIV infection, and the patient reports no
risk factor for HIV, check
Negative
for this item.
•
A documented negative HIV test from 1 year ago or longer is
not
valid for checking
Negative
.
Note:
Update this item if additional information is obtained during the course of treatment.
For
Positive
HIV status at the time of TB diagnosis, enter the following information.
Description
State HIV/AIDS
patient number
Can be obtained from the state or local HIV/AIDS surveillance program
City/county HIV/AIDS
patient number
Can be obtained from the state or local HIV/AIDS surveillance program

124
Exercise
26.
HIV Status at Time of Diagnosis
Case Study – Harvey
Harvey is diagnosed with TB at the County Health Department in May 2009. He is
offered HIV testing. He states that he was tested earlier that year in January 2009 and the
results were negative. Harvey has no other risk factors for HIV. With Harvey’s
permission, the TB program confirms the negative HIV test result by reviewing his HIV
result listed in his medical records.
26.1 What would be selected for Harvey’s HIV Status at Time of Diagnosis?
(circle the one best answer)
A.
Negative
B.
Positive
C.
Indeterminate
D.
Refused
E.
Not offered
F.
Test Done, Results Unknown
G.
Unknown
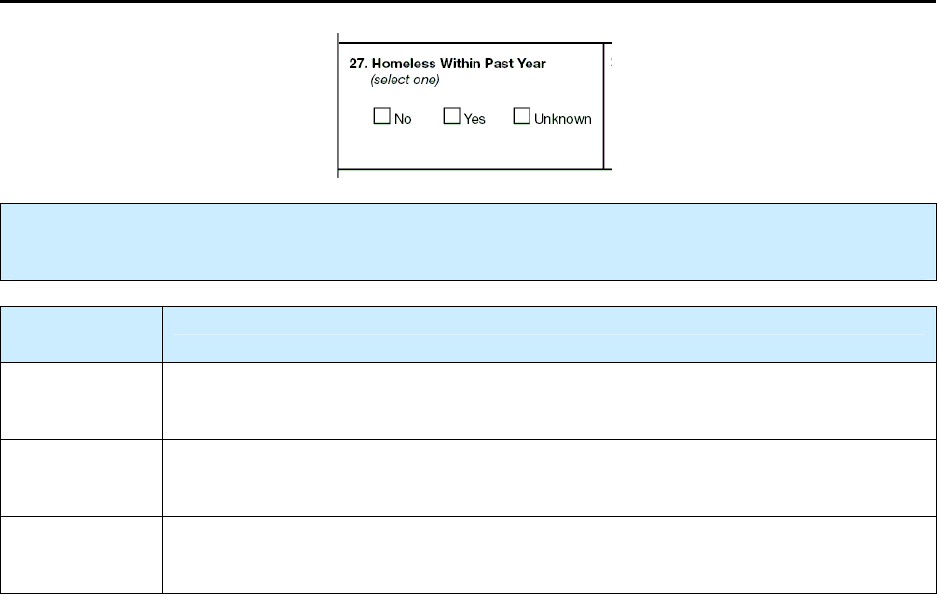
125
27.
Homeless Within Past Year
Primary Purpose: Surveillance. Data are used to determine the extent to which recent homelessness is
associated with TB disease.
Option
(select one)
Description
No
Not homeless during the 12 months before the TB diagnostic evaluation was
performed or initiated
Yes
Homeless at any time during the 12 months before the TB diagnostic
evaluation was performed or initiated
Unknown
Not known whether the patient was homeless during the 12 months
before the TB diagnostic evaluation was performed or initiated
Comments: Definitions for Homeless
There are many definitions for homeless (National Coalition for the Homeless). A
homeless
person may be an individual who has
1.
No fixed, regular, and adequate nighttime residence
and
2.
A primary nighttime residence that is
a.
A supervised publicly or privately operated shelter designed to provide temporary living
accommodations, including welfare hotels, congregate shelters, and transitional housing for
the mentally ill
or
b.
An institution that provides a temporary residence for individuals intended to be
institutionalized
or
c.
A public or private place not designated for, or ordinarily used as, a regular sleeping
accommodation for human beings.
A
homeless
person may also be defined as a person who has no home (e.g., is not paying rent, does not
own a home, and is not steadily living with relatives or friends). Persons in unstable housing situations
(e.g., alternating between multiple residences for short stays of uncertain duration) may also be
considered homeless.
126
A
homeless
person may be a person who lacks customary and regular access to a conventional dwelling
or residence. Included as homeless are persons who live on streets or in nonresidential buildings. Also
included are residents of homeless shelters and shelters for battered women. Residents of welfare hotels
and single room occupancy (SRO) hotels could also be considered homeless. In the rural setting, where
there are usually few shelters, a homeless person may live in non-residential structures, or substandard
housing, or with relatives. Homeless does not refer to a person who is imprisoned or in a correctional
facility.
Note:
The homeless category is limited to living conditions in the United States and does
not
apply to
living in refugee camps outside the United States.
Note:
Update this item if additional information is obtained during the course of treatment.

127
Exercise
27.
Homeless Within Past Year
Case Study – Oscar
Oscar lost his job in December 2008 and subsequently lost his apartment. He had no
place to live except when staying with friends or relatives for a few days at a time
throughout January 2009. Since then he has lived in homeless shelters and had meals at
soup kitchens operated by the American Red Cross. Oscar found a job in July 2009 and
has been living in his current apartment since then. He is diagnosed with TB at the
Carroll County Health Department in November 2009.
27.1 How would you fill out Homeless within the Past Year for Oscar?
(circle the one best answer.)
A.
No
B.
Yes
C.
Unknown
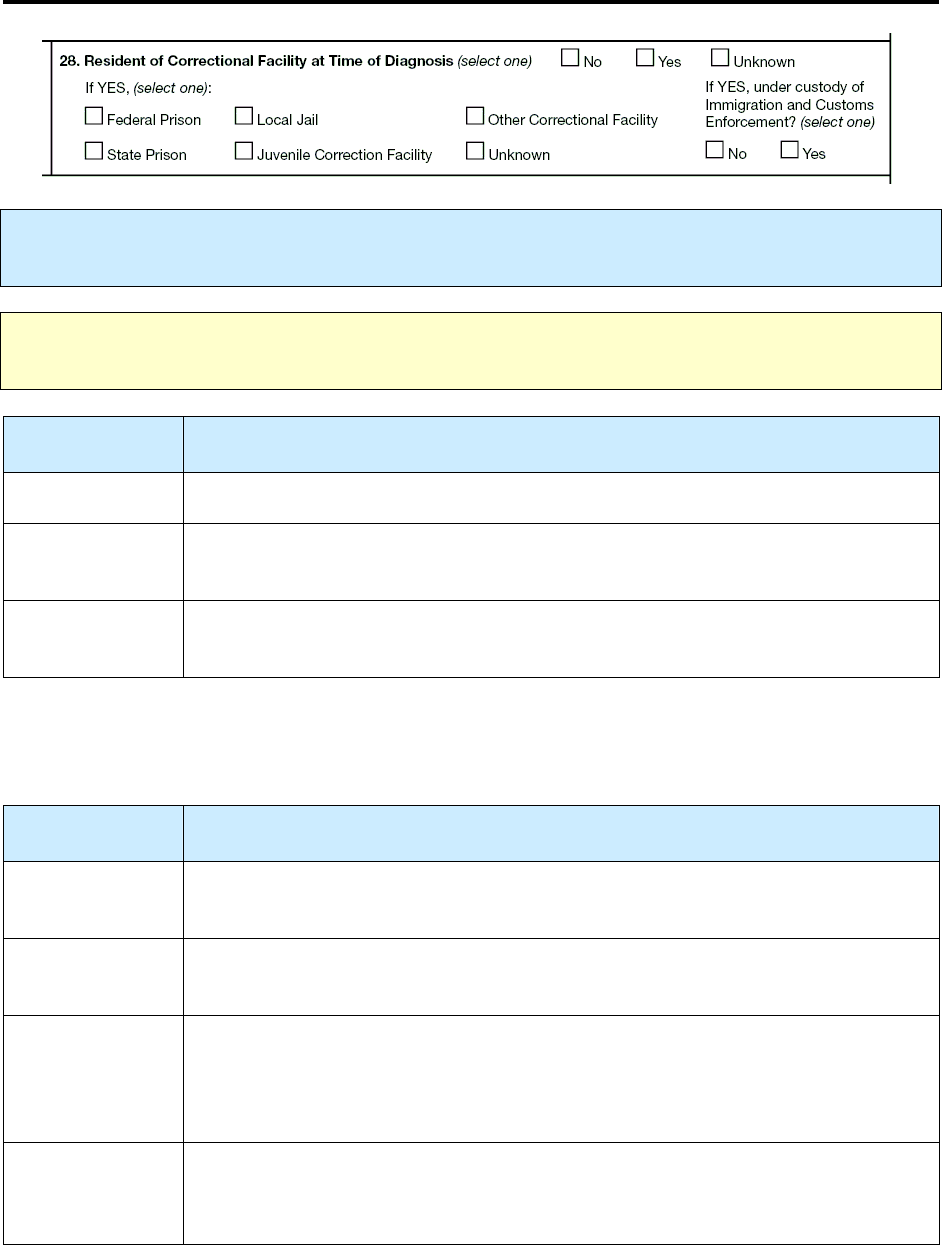
128
28.
Resident of Correctional Facility at Time of Diagnosis
Primary Purpose: Surveillance. Data are used to determine if residence in a correctional facility is
associated with TB disease.
Note:
Direct the questions regarding classification of a specific correctional facility (federal, state,
local, juvenile, or other) to the Department of Corrections in your state.
Option
(select one)
Description
No
Not an inmate when the TB diagnostic evaluation was performed or initiated
Yes
An inmate of a correctional facility when the TB diagnostic evaluation was
performed or initiated
Unknown
Not known whether the patient was an inmate when the TB diagnostic evaluation
was performed or initiated
If you selected
Yes,
indicate the type of correctional facility where the patient was an inmate. If the TB
patient was a resident of more than 1 facility during the diagnostic evaluation, select the facility where the
initial TB diagnostic evaluation was performed.
Option
(select one)
Description
Federal prison
Confinement facility administered by a federal agency; includes privately operated
federal correctional facilities
State prison
Confinement facility administered by a state agency; includes privately
operated state correctional facilities
Local jail
Confinement facility usually administered by a local law enforcement agency,
intended for adults but sometimes also containing juveniles; holds persons
detained pending adjudication and/or persons committed after adjudication,
typically for sentences of 1 year or less
Juvenile
correctional
facility
Public or private residential facility; includes juvenile detention centers, reception
and diagnostic centers, ranches, camps, farms, boot camps, residential treatment
centers, and halfway houses or group homes designated specifically for juveniles
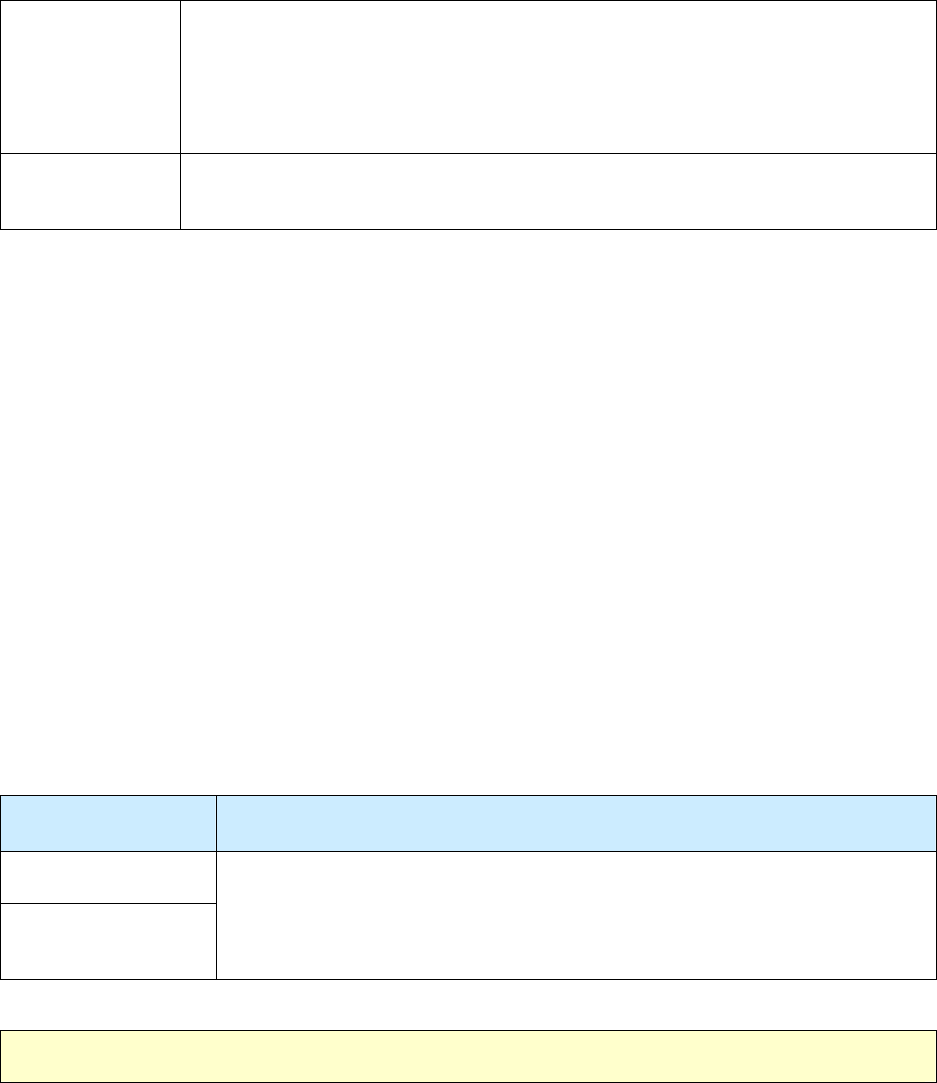
129
Other
correctional
facility
Includes Immigration and Customs Enforcement (ICE) detention centers, Indian
reservation facilities (e.g., tribal jails), military stockades and jails, federal park
police facilities, police lockups (temporary holding facilities for persons who have
not been formally charged in court), or other correctional facilities that are not
included in the other specific choices
Unknown
Inmate when the TB diagnostic evaluation was performed, but the type of
correctional facility is not known
Comment: Correctional facility at time of diagnosis
If a patient is an inmate at a correctional facility and goes to a hospital for TB diagnostic evaluation, you
would select
• Yes, an inmate of a correctional facility when the TB diagnostic evaluation was performed
• The type of correctional facility (rather than the hospital) where he/she resided at time of
diagnostic evaluation
Comment: Local Jail
Excludes temporary holding facilities, or lockups, that do not hold persons after they have been formally
charged in court. Includes city and county jails and privately operated local correctional facilities. Report
federal and state prisoners who are boarded at local jails as residents of the local jail.
Comment: Juvenile Correctional Facility
Includes
juveniles charged or adjudicated as delinquents, juveniles who are not delinquents or criminal
offenders (e.g., runaways, truants, incorrigibles, curfew violators), and juveniles committed or detained
for treatment of abuse, dependency, neglect, or other reasons. Report juveniles who are boarded at federal
or state prisons or local jails as residents of the facility at which they are boarded.
If you selected
Yes
, indicate whether this patient was
under custody of Immigration and Customs
Enforcement (ICE)
.
Option
(select one)
Comment
No
Yes
Response indicates whether the patient was under the custody of ICE at the
time of diagnosis. Persons in ICE custody can be housed in standalone ICE
detention centers, or other correctional facilities (e.g., federal or state prison,
local jail) when a standalone ICE detention center is not available.
Note:
Update this item if additional information is obtained during the course of treatment.

130
Exercise
28.
Resident of Correctional Facility at Time of Diagnosis
Case Study – Pedro
Pedro has been in the U.S. 6 months. He is incarcerated at the Lanner County Jail. He has
signs and symptoms of TB. He is evaluated by a health care provider at the jail. A sputum
sample is collected on April 25 and sent to the health department. On May 1 he is
transferred to the Immigration and Customs Enforcement (ICE) Detention Center in
Margaritaville, Alabama, where he waits to be deported. The test result is positive for TB
and becomes available on May 5.
28.1 What type of correctional facility should be selected at time of diagnosis
(diagnostic evaluation) for Pedro? (circle the one best answer)
A.
Federal Prison
B.
State Prison
C.
Local Jail
D.
Juvenile Correction Facility
E.
Other Correctional Facility
F.
Unknown
131
29.
Resident of Long-Term Care Facility at Time of
Diagnosis
Primary Purpose: Surveillance. Data are used to determine if residence in a long-term care facility is
associated with TB disease.
Note:
The state licensing agency for long-term care facilities can assist in determining the
category under which a facility is classified.
Option
(select one)
Description
No
Not a resident of a long-term care facility when the TB diagnostic evaluation was
performed
Yes
Resident of a long-term care facility when the TB diagnostic evaluation was performed
Unknown
Not
known whether the patient was a resident of a long-term care facility when the
TB diagnostic evaluation was performed
If you selected
Yes
, indicate the type of long-term care facility of which the patient was a resident. If the
TB patient was a resident of more than 1 facility during the diagnostic evaluation, select the facility where
most of the TB diagnostic evaluation was performed.
Option
(select one)
Description Comment
Nursing home
Freestanding facility with 3 or more beds
(i.e., is classified as a residential facility
or congregate residential setting) that
provides nursing care services (e.g.,
nursing or medical care and/or
supervision of medications that may be
self-administered)
Facilities may be certified by Medicare
or Medicaid or may be licensed by the
state as a nursing home (e.g., skilled
nursing facility, intermediate care
facility, nursing care unit of a retirement
center)
Hospital-
based facility
Distinct unit with 3 or more beds that is
physically attached to, or managed by, a
hospital
Facilities may be certified by Medicare
or Medicaid or may be licensed by the
state.
132
Residential
facility
Facility with 3 or more beds (i.e., is
classified as a residential facility or
congregate residential setting) and meets both
of the following criteria:
1. Not classified as a nursing home or
hospital-based facility, as described above
and
2. Provides personal care or supervision (not
nursing care services) to its residents, in
addition to room and board (e.g., help with
bathing, dressing, eating, walking, shopping)
This might be an assisted living facility.
Mental health
residential
facility
Facility that provides 24-hour care in a
hospital, residential treatment, or
supportive setting
Include state and local mental hospitals,
private psychiatric hospitals, general
hospitals, the Department of Veterans
Affairs (VA), residential treatment
centers for emotionally disturbed
children, and multiservice mental health
organizations with residential treatment
programs.
For other federal mental health
residential facilities, select “Other
long-term care facility.” Examples
include the Department of Defense,
Bureau of Prisons, Public Health
Service, Indian Health Service, and
Indian reservation facilities that are not
federal.
Alcohol or
drug
treatment
facility
Only long-term rehabilitation or
residential facilities designated for
treatment of 30 days or longer
Exclude all ambulatory or outpatient
facilities, detoxification units, and
facilities designated for fewer than 30
days of treatment. The state agency
responsible for alcohol and drug treatment
can assist in determining whether a
facility is considered residential.
Other long-
term care
facility
A facility not mentioned above that is
designated for treatment of 30 days or
longer and facility type is not Unknown
Unknown
Patient known to be a resident of a long-
term care facility, but the type of facility
is not known

133
Examples:
Residential Facility
•
Assisted living facilities
•
Homes for mentally retarded or developmentally disabled persons
•
Boarding and care homes (e.g., residential care homes, group homes, homes for the aged, family
care homes, adult foster care homes, personal care homes, adult congregate living facilities,
residential community care facilities, domiciliary care homes)
Examples:
Mental Health Residential Facility
•
State and local mental hospitals
•
Private psychiatric hospitals
•
General hospitals (not federal) with separate psychiatric services
•
Department of Veterans Affairs (VA) medical centers
•
Residential treatment centers for emotionally disturbed children
•
Multiservice mental health organizations with residential treatment programs
Note:
Update this item if additional information is obtained during the course of treatment.

134
Exercise
29.
Resident of Long-Term Care Facility at Time of Diagnosis
Case Study – Gladys
Gladys was a resident of the Brittany Nursing Home for 5 months from April 2009 until
September 2009 while she recovered from a stroke. She returned to her home; 2 months
later, in November 2009, she is evaluated and diagnosed with TB.
29.1 Was Gladys a Resident of Long-Term Care Facility at Time of Diagnosis?
(circle the one best answer)
A.
No
B.
Yes
C.
Unknown
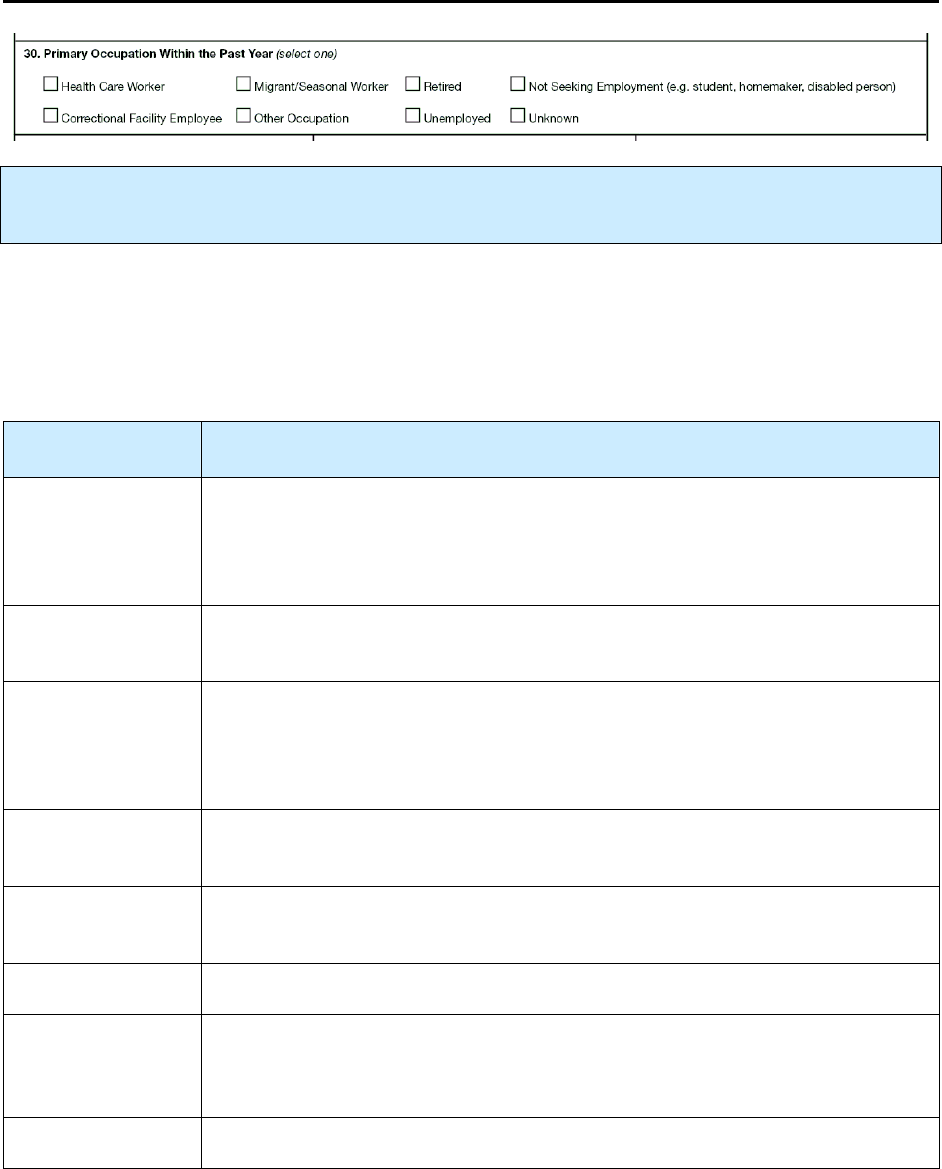
135
30.
Primary Occupation Within the Past Year
Primary Purpose: Surveillance. Data are used to determine if certain primary occupations are
associated with TB disease.
Select one option that best describes the patient’s occupation within the 12 months before the diagnostic TB
evaluation. If the patient held more than 1 occupation during that period, select the longest-held
occupation or the occupation to which the patient devoted more time (i.e., the patient’s
primary
occupation). For example, if the patient was a full-time health care worker and a student (e.g., taking
night classes), the patient’s primary occupation would be
Health Care Worker
.
Option
(select one)
Description
Health care
worker
Paid or unpaid person working in a health care setting, with potential for
exposure to M. tuberculosis. For health care workers being evaluated for TB
disease, health care worker supersedes correctional facility or other
occupations.
Correctional
facility employee
Person working in a correctional facility; not a health care worker
Migrant/seasonal
worker
Person who is required to be absent from a permanent place of residence for the
purpose of seeking employment, or who may vary their employment for the
purpose of remaining employed while maintaining a permanent place of
residence
Other occupation
Person employed for pay or income in any occupation that is not included in the
options listed above
Retired
Person who was retired during the 12 months before the TB diagnostic
evaluation
Unemployed
Person not employed during the 12 months before the TB diagnostic evaluation
Not seeking
employment
Person not seeking employment, such as infant, child, student, homemaker,
person receiving permanent disability benefits, or person who was
institutionalized
Unknown
Person whose employment status is not known

136
Examples: Health Care Worker
Includes full time, part-time, temporary, or contract staff. Examples include:
•
Physicians
•
Nurses
•
Health Aides
•
Dental workers
•
Health Technicians
•
Staff in laboratories and morgues
•
Emergency medical personnel
•
Students
•
Persons who deliver health care in the community
(e.g., public health nurse, visiting nurse, outreach
worker)
•
Persons not involved directly in patient care, but
potentially at risk for occupational exposure
(e.g., volunteers; outreach workers;
dietary/nutritional, housekeeping, maintenance,
clerical, janitorial, administrative and supervisory
staff)
Examples:
Correctional Facility Employee
•
Federal or state prison
•
Local jail
•
Juvenile correctional facility
•
Immigration and Customs Enforcement (ICE) detention center or other correctional facility
(See
Resident of Correctional Facility
[item 28].)
•
Paid or unpaid persons working in correctional facilities, with potential for exposure to M.
tuberculosis complex (e.g., volunteers; outreach workers; dietary/nutritional, housekeeping,
maintenance, clerical, and janitorial staff)
Examples:
Migrant/Seasonal Worker
•
Migratory agricultural worker
•
Seasonal agricultural worker
•
Migrant factory worker
•
Migrant construction worker
•
Migrant service industry worker
•
Migrant sporting worker (e.g., horse racing, dog racing)
Comment: Unemployed
Select
Unemployed
if a person not included in the preceding list was unemployed for most of the past 12
months. Do not select this option for a person who was unemployed for a short time (e.g., 1 week
during the past 12 months).
Note:
Update this item if additional information is obtained during the course of treatment.

137
Exercise
30.
Primary Occupation Within Past Year
What is the Primary Occupation Within the Past Year?
(Choose the one best answer by matching the Primary Occupation Within the Past Year with
the patient. Write the letter for the occupation on the line next to the question number.)
Patient Primary Occupation within
the Past Year
___
30.1
Vince completed 30 years of service with
the U.S. Army 15 months ago and now
spends all of his time working in his
garden.
___
30.2
Joe, a full time student, does not have a job
and is not looking for work.
___
30.3
Isabella refused to disclose where she
worked.
___
30.4
Roberto picks tomatoes at Lane Produce
Company during the summer season.
___
30.5
Florence is a nurse at the local correctional
jail.
___
30.6
Billy Bob is a truck driver for Texaco.
___
30.7
Marybelle is a cook at the juvenile
detention center.
___
30.8
Andy has been without a job for the past
year.
___
30.9
Debbie is a dietitian at Grady Hospital.
A.
B.
C.
D.
E.
F.
G.
H.
Health care worker
Correctional facility
employee
Migrant/seasonal
worker
Other occupation
Retired
Unemployed
Not seeking
employment
Unknown
138
31.
Injecting Drug Use Within Past Year
Primary Purpose: Surveillance. Data are used to determine the extent to which injecting drug use is
associated with TB.
Option
(select one)
Description
No
Patient has not injected drugs within the past 12 months.
Yes
Patient is known to have injected drugs within the past 12 months
Unknown
It is not known whether the patient injected drugs within the past 12 months
Comment: Injecting Drug Use
Medical documentation or other indications of enrollment in a drug treatment program (e.g., methadone
detoxification; methadone maintenance; outpatient, residential, or inpatient treatment, halfway house;
prison or jail treatment; Narcotics Anonymous, Cocaine Anonymous, or other self-help program),
medical or laboratory documentation of injection drug use (e.g., urine testing), or physical evidence (e.g.,
needle tracks) may be useful in answering this question. Because many patients respond negatively during
the interview, it may be necessary to ask the patient about drug use at multiple visits.
Comment: Definition of Injecting Drug Use
Injecting drug use involves the use of hypodermic needles and syringes for the injection of drugs not
prescribed by a health care provider. Route of administration may be intravenous, subcutaneous (e.g., skin
popping), or intramuscular.
Note:
Update this item if additional information is obtained during the course of treatment.
Examples: Commonly injected drugs
•
Heroin and other opiates (e.g.,
Demerol, Dilaudid, morphine, opium)
•
Cocaine (e.g., speedball)
•
Methamphetamines
•
Amphetamines
•
Other stimulants
•
Phencyclidine (PCP, also known as angel dust)
•
Other hallucinogens
•
Barbiturates
•
Steroids
•
Other hormones
•
Fentanyl

139
Exercise
31.
Injecting Drug Use Within Past Year
Case Study – Chaz
Chaz has visible needle marks on his arms and appears high, spaced-out, and incoherent.
He denies ever using injectable drugs. However, his medical record shows a previous use
of injectable drugs and that he was in a methadone detoxification program 11 months
ago.
31.1 How would you fill out Injecting Drug Use Within Past Year for Chaz?
(circle the one best answer)
A.
No
B.
Yes
C.
Unknown
140
32.
Non-Injecting Drug Use Within Past Year
Primary Purpose: Surveillance. Data are used to determine the extent to which non-injecting drug use
is associated with TB.
Option
(select one)
Description
No
Patient has no history of using non-injecting drugs within the past 12 months
Yes
It is known that the patient has used non-injecting drugs within the past 12 months.
Unknown
It is not known whether the patient used non-injecting drugs within the past 12
months.
Comment: Non-Injecting Drug Use
A history of enrollment in a drug treatment program (e.g., outpatient, residential, or inpatient treatment;
halfway house; prison or jail treatment; Cocaine Anonymous or other self-help program), as well as
medical or laboratory documentation of drug use (e.g., urine testing), may be useful in answering this
question. Because many patients respond negatively during the interview, it may be necessary to ask the
patient about drug use at multiple visits.
Comment: Definition of Non-Injecting Drug Use
Non-injecting drug use involves the use of licensed or prescription drugs or illegal drugs that were not
injected and were not prescribed for the patient by a health care provider. The drugs may be ingested,
inhaled, sniffed, or smoked.
Note:
Update this item if additional information is obtained during the course of treatment.

141
Examples: Non-injecting drugs
•
Heroin or other opiates (e.g., Demerol, Percocet, codeine, Dilaudid, MS Contin, nonprescription
methadone)
•
Cocaine (e.g., snorted) and crack (e.g., smoked)
•
Ingested amphetamines (e.g., speed, uppers, bennies)
•
Xanax, Ativan, Valium, or other benzodiazepams
•
Phencyclidine (PCP), ketamine, LSD, or other hallucinogens
•
Barbiturates
•
Marijuana (e.g., pot, weed, grass, reefers), hashish
•
Inhalants (e.g., nitrous [whippets] oxide, poppers, rush, huff, gasoline, spray paint, butane)
•
Steroids
Note:
Alcohol is
not
included as a non-injecting drug (see
Excess Alcohol Use within Past Year
[item 33]).

142
Exercise
32.
Non-Injecting Drug Use Within Past Year
Case Study – Spider
Spider is interviewed for the RVCT on December 13, 2009. He says he completed a drug
detoxification program about 14 months ago, on October 30, 2008, and has been drug-
free since that time.
32.1 How would you answer Non-Injecting Drug Use Within Past Year for Spider?
(circle the one best answer)
A.
No
B.
Yes
C.
Unknown
143
33.
Excess Alcohol Use Within Past Year
Primary Purpose: Surveillance. Data are used to determine the extent to which excess alcohol use is
associated with TB.
Option
(select one)
Description
No
Patient has not used alcohol to excess within the past 12 months.
Yes
Patient has used alcohol to excess within the past 12 months.
Unknown
It is
not
known whether the patient used alcohol to excess within the past
12 months.
Comment:
This information is collected because the patient is in a high risk group for TB. The patient’s response to
this question is sought as an indicator of recent excess alcohol use. Because many patients respond
negatively during the interview, it may be necessary to ask the patient, at multiple visits, about excess use.
Note:
Update this item if additional information is obtained during the course of treatment.
Definition of Excess Alcohol Use:
There is no standard definition. Excess alcohol use can be assessed by
various methods. Examples of reliable indicators of excess alcohol use include:
•
Participation in self-help programs (e.g., Alcoholics Anonymous) or alcohol treatment programs
•
Medical record documentation of excess alcohol use or hospitalization for alcohol-related
medical conditions (e.g., delirium tremens [DTs], pancreatitis, cirrhosis)
•
More than one arrest for intoxication or drunk and disorderly behavior. This can be found by
asking the patient, or contacting the local correctional facility regarding charges.
The National Household Survey on Drug Abuse defines heavy alcohol use as “five or more drinks on the
same occasion on each of 5 or more days in the past 30 days.” Numerous screening instruments (e.g.,
CAGE, AUDIT, MAST) can be helpful in identifying persons who may use alcohol to excess.
A standard drink in the United States is equal to 13.7 grams (0.6 ounces) of pure alcohol or
•
12 ounces of beer
•
8 ounces of malt liquor
•
5 ounces of wine
•
1.5 ounces or a “shot” of 80-proof distilled spirits or liquor (e.g., gin, rum, vodka, or whiskey)

144
Exercise
33.
Excess Alcohol Use Within Past Year
Case Study – Jack Daniel
Jack Daniel comes to the clinic for daily DOT. He often appears intoxicated and smelling
of alcohol. He denies using excess alcohol, but says that he might occasionally have 1 or
2 beers a day. His records indicate that he enrolled in an alcohol treatment program
within the past year, but he never completed the program.
33.1 How would you answer Excess Alcohol Use Within Past Year for Jack Daniel?
(circle the one best answer)
A.
No
B.
Yes
C.
Unknown

145
34.
Additional TB Risk Factors
Primary Purpose: Surveillance. Data are used to evaluate how these additional risk factors are
associated with TB disease.
Select
all
additional TB risk factors that the TB patient may have. Document additional TB risk factors
from the medical records or a reliable source (e.g., health care provider). Undocumented reporting (e.g.,
oral report from the patient or person other than a medical health care provider) is
not
acceptable.
Note:
Other specific TB risk factors (e.g., occupation, HIV infection) are collected through other items
of the RVCT.
Option
(select all that
apply)
Description
Contact of MDR TB
patient
(2 years or less)
Patient for whom the RVCT form is being completed is a contact of a
patient with multidrug-resistant (MDR) TB, within 2 years or less, regardless
of whether the patient with MDR TB was infectious.
Contact of infectious
TB patient
(2 years or less)
Patient for whom the RVCT form is being completed is a contact of an
infectious TB patient within 2 years or less.
Missed contact
(2 years or less)
Patient for whom the RVCT form is being completed is a contact of a known
TB patient, but was not evaluated or diagnosed with LTBI or TB at that time
(within 2 years or less of current diagnosis).
Incomplete LTBI
treatment
Patient had a previous diagnosis of latent TB infection (LTBI) and did not
complete treatment for LTBI.
Tumor necrosis
factor-alpha
(TNF-α)
antagonist therapy
Patient had recently received, or was receiving, TNF-α antagonist therapy at
the time of TB diagnosis.
Post-organ
transplantation
Patient has received a solid organ transplant (e.g., kidney, heart).
Diabetes mellitus
Patient has a diagnosis of diabetes mellitus (Type I or Type II) either before or
at the time of TB diagnosis.
End-stage renal
disease
Patient had end-stage renal disease or chronic renal failure at the time of
TB diagnosis.
146
Immunosuppression
Patient had immunosuppression due to either a medical condition or
medication, such as hematologic or reticuloendothelial malignancies
(e.g., leukemia, Hodgkin’s lymphoma, carcinoma of the head or neck),
or immunosuppressive therapy, such as prolonged use of high-dose
adrenocorticosteriods (e.g., prednisone).
Other
Patient had a risk factor not included in the preceding choices (e.g.,
undernutrition due to intestinal bypass surgery for obesity, gastrectomy,
jejunoileal bypass, chronic malabsorption syndromes; silicosis; travel to a TB-
endemic country).
None
No TB risk factors could be identified.
Comments: Contact of MDR TB Patient
•
MDR TB is defined as resistance to at least isoniazid and rifampin.
•
If a patient with MDR TB was the only known contact for the patient for whom you are
completing the RVCT, select
Contact of MDR TB Patient
and do
not
select
Contact of
Infectious TB Patient
. The association between the TB patients may have been found through
investigation (e.g., a formal contact investigation) or identified as an incidental finding.
•
The contact with the patient with MDR TB must have been within the last 2 years.
•
If the patient with MDR TB has an RVCT number, enter that number as the
Linking State Case
Number
(item 3), and enter reason 2 - Epidemiologically Linked Case.
Comments:
Contact of Infectious TB Patient
•
If the infectious TB patient is known to have had MDR TB, and the TB patient for whom the
RVCT form is being completed was not a contact of any other infectious TB patient, select only
Contact of MDR TB Patient
(do
not
select
Contact of Infectious TB Patient
).
•
The association between the TB patients may have been found through investigation (e.g., a
formal contact investigation) or as an incidental finding. The contact with an infectious TB
patient must have been within the last 2 years.
•
If the infectious TB patient has an RVCT number, enter that number as the
Linking State Case
Number
(item 3), and enter reason 2 - Epidemiologically Linked Case
Comment: Missed Contact
The contact must have been within the last 2 years. Do
not
select this option for TB patients identified as
having TB disease during, or as a result of, a contact investigation: such patients are
not
missed contacts.
Here, the intention is to record information about patients whose TB could have been prevented if they
had been identified before developing TB disease.
Comment:
Incomplete LTBI Treatment
The intention is to record information about a patient who started treatment for LTBI. However, the
patient did not complete a full course of treatment.
147
Comment: Tumor Necrosis Factor-alpha (TNF-α) Antagonist Therapy
The Food and Drug Administration (FDA) has approved TNF-α antagonist therapy for treatment of
rheumatoid arthritis and other selected autoimmune diseases. The FDA has also recently determined that
TB disease is a potential adverse reaction to treatment with TNF-α antagonists. The three TNF-α
antagonists currently approved by the FDA are infliximab (Remicade
®
), etanercept (Enbrel
®
), and
adalimumab (Humira
®
).
Comments: Immunosuppression
•
If the TB patient has diabetes mellitus or end-stage renal disease, check
Diabetes Mellitus
or
End-Stage Renal Disease
or both (do
not
select
Immunosuppression
unless the patient has
another immunosuppressive condition).
•
If the patient is infected with HIV, complete
HIV Status at Time of Diagnosis
(item 26) (do
not
select
Immunosuppressio
n unless the patient has another immunosuppressive condition).
Comments: Other
Do
not
include risk factors identified in items 27–33:
•
Being homeless within past year
(item 27)
•
Residence status at diagnosis
o
Correctional facility
(item 28)
o
Long-term care facility
(item 29)
•
Primary occupation within past year
(item 30)
•
Drug or excess alcohol use within past year
(items 31–33)
On the line labeled
Specify
, write comments regarding
Other
reasons.

148
Exercise
34.
Additional TB Risk Factors
What are the Additional Risk Factors for the following patients?
(Choose the one best answer by matching the additional risk factor with the patient. Write the
letter for the additional risk factor on the line next to the question number.)
Patient Additional Risk Factor
___
34.1
Ralph has rheumatoid arthritis and has
been receiving tumor necrosis factor-
alpha antagonist therapy.
___
34.2
Maddox works at the local market where
he is exposed to a co-worker who has
MDR TB. Two weeks later Maddox is
contacted by the health department, and
they determine that he has active TB.
___
34.3
Rema has a kidney transplant in January
2009 and is diagnosed with TB disease 2
months later in March.
___
34.4
Luella is diagnosed with TB disease in
2010 while being treated for leukemia.
___
34.5
Gloria starts LTBI therapy in November
2008, but stops taking the medication in
late January 2009.
___
34.6
Diego lives with his brother, who is
diagnosed with pulmonary TB disease in
March 2009. Three months later, Diego is
also diagnosed with TB disease.
___
34.7
In July 2009, Debbie, who has diabetes, is
diagnosed with TB disease during a
routine physical.
A.
B.
C.
D.
E.
F.
G.
H.
I.
J.
K.
Contact of MDR TB
patient
Contact of infectious
TB patient
Missed contact
Incomplete LTBI
therapy
TNF-α Antagonist
Therapy
Post-organ
transplantation
Diabetes mellitus
End-stage renal disease
Immunosuppression
(not HIV/AIDS)
Other
None
___
34.8
Marjorie works as a substitute teacher at
Dallas High School during the month of
April 2009. That same month, there is an
outbreak of TB at the high school, but
Marjorie does not know about that
situation, even though some of the
students who have TB are in her class. In
May she moves to Denver and is
diagnosed with TB disease in June.
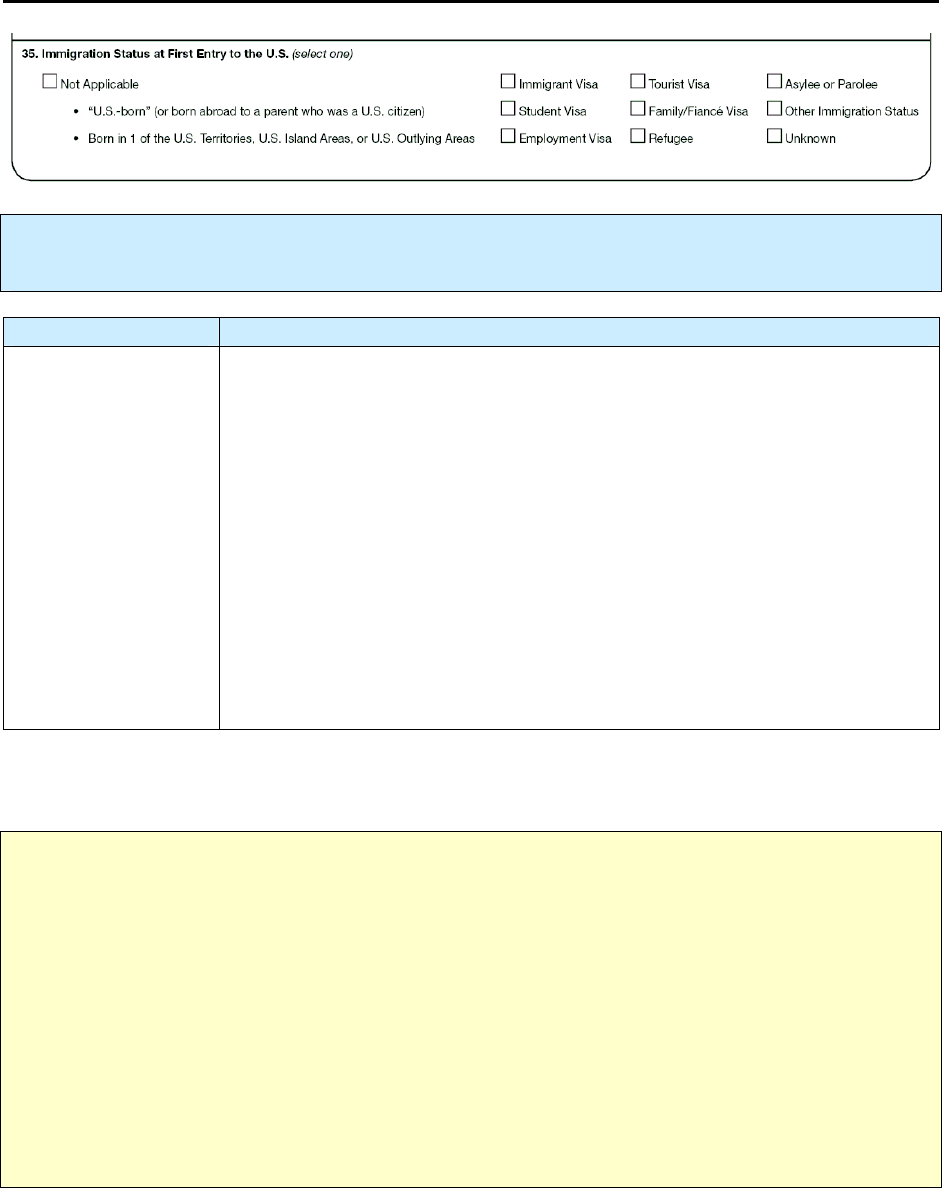
149
35.
Immigration Status at First Entry to the U.S.
Primary Purpose: Surveillance. Data are used to observe the association between immigration status
and TB.
Option Description
Not applicable Patient was
• “U.S.-born”
o Born in 1 of the 50 states or the District of Columbia
or
o Born abroad to a parent who was a U.S. citizen (e.g., born on a military
installation)
(See Item 12 for complete instructions on “U.S.-born”)
• Born in 1 of the U.S. Territories, U.S. Island Areas, or U.S. Outlying
Areas
o American Samoa, Federated States of Micronesia, Republic of the
Marshall Islands, Commonwealth of the Northern Mariana Islands,
Republic of Palau, Guam, Puerto Rico, or the U.S. Virgin Islands
If you did
not
select
Not Applicable,
select one option to indicate the patient’s immigration status at first
entry to the United States.
Note:
If the patient had a visa at first entry to the United States, specify the type of visa. Oral
information from a reliable source is acceptable.
There are 2 main types of legal immigration status: permanent (immigrants) and nonimmigrant
(persons with a visa issued for specific purpose and period).
1.
Permanent residents (immigrants) are issued an alien resident card (i.e., green card) and should
carry this card with them.
2.
Nonimmigrants with visas (e.g., student, tourist, employment, V visa, K visa) should be aware of
their visa type, which is stated in their passport (I-94 arrival document stapled in passport).
Refugees are separate from the 2 main categories above: they should have an arrival document
(I-94) showing their status as refugees and they should carry this card with them.
150
Option
(select one)
Description
Immigrant
visa
For foreign-born TB patients who first entered the United States with permanent
resident status (immigrants).
For foreign-born pediatric TB patients who are adopted by U.S. citizens, the patients
enter the U.S. on an immigrant visa.
Student visa
For foreign-born TB patients who first entered the United States with a student visa.
This is a nonimmigrant visa and is obtained by an alien coming to the United States
for a specific period to pursue a full course of study in an approved institution.
Employment
visa
For foreign-born patients who first entered the United States with a nonimmigrant
employment visa (an alien coming to the United States to work for a specific period).
There are many categories of nonimmigrant employment visas (category depends
upon the type of work).
Tourist visa
For foreign-born TB patients who first entered the United States for a specific
period for business or pleasure. This is a nonimmigrant visa.
Family/ fiancé
visa
For foreign-born TB patients who first entered the United States with a V visa or a
K visa.
Refugee
For foreign-born TB patients who first entered the United States as refugees.
Asylee or
parolee
For foreign-born patients who first entered the United States seeking asylum or who
are parolees.
Other
immigration
status
For foreign-born TB patients who first entered the United States with a status that is
not Immigrant, Refugee, Asylee, Parolee, Student, Tourist, Employment, with a V
visa or a K visa, and whose status is not Unknown. This includes foreign-born
persons who were not required to obtain a visa (e.g., foreign-born visitors from
specific countries, such as Canada, that are part of the U.S. visa waiver program and
thus are not required to obtain visas if visiting the United States for short periods
[e.g., <90 days]) or those who entered the United States with no official immigration
status (e.g., they were “undocumented”).
Unknown
For jurisdictions with directives or policies that forbid asking TB patients their
immigration status
• Foreign-born TB patients who
o Do not know their immigration status at first entry to the United States
o May have had a visa at entry to the United States, but the type of visa is
unknown
• Patients who refuse to respond
Note:
For jurisdictions with directives or policies that forbid asking TB patients their immigration
status, please check
Unknown.
151
Comments:
Family/Fiancé Visa
•
The V visa (in the nonimmigrant category) allows the spouse or child of a U.S. legal permanent
resident to live and work in the United States.
•
The K visa (in the nonimmigrant category) allows the fiancé of a U.S. citizen to enter the United
States for a specific period and specifically for the purpose of marriage.
Comment:
Refugee
A refugee is a foreign-born person who is in a country other than his or her country of nationality and
who is unable or unwilling to return to that country because of persecution or a well-founded fear of
persecution.
Comments:
Asylee and Parolee
An asylee is a foreign-born person in the United States who is unable or unwilling to return to his or her
country of nationality because of persecution or a well-founded fear of persecution. An asylee meets the
same criteria as those for a refugee; the only difference is the person’s location at the time of
application—the potential asylee is in the United States or applying for admission at a port of entry, and
the potential refugee is outside the United States.
A parolee is a foreign-born person allowed to enter the United States for urgent humanitarian reasons or
because entry is determined to be of significant public benefit.
Comment: Born in 1 of the U.S. Territories, U.S. Island Areas, or U.S. Outlying Areas (American
Samoa, Federated States of Micronesia, Republic of the Marshall Islands, Commonwealth of the Northern
Mariana Islands, Republic of Palau, Guam, Puerto Rico, or the U.S. Virgin Islands)
Example: For born in 1 of the U.S. Territories, U.S. Island Areas, or U.S. Outlying Areas select
not applicable for
• Entering the United States
or
• Entering one of the other U.S. Territories, U.S. Island Areas, or U.S. Outlying Areas

152
Exercise
35.
Immigration Status at First Entry to the U.S.
What is the Immigration Status at First Entry to the United States for the following patients?
(Choose the one best answer by matching the Immigration Status at First Entry into the U.S.
with the patient. Write the letter for the Immigration Status on the line next to the question
number.)
Patient Immigration Status
___
35.1
Katrina, a law student from the Ukraine, visited
Disney World and interviewed for a job at the
Thayer Law Firm.
___
35.2
Graciano, a Cuban doctor, escaped to Miami from
Havana and is applying for a U.S. visa. He will be
persecuted if he returns to Cuba.
___
35.3
Pratibha was born in India and is attending a PhD
program in Public Health Surveillance at Harvard.
___
35.4
Wong, a Chinese software designer, is visiting the
United States to work for Microsoft for 3 months.
___
35.5
Ali was born in Saudi Arabia and entered the United
States with permanent resident status.
___
35.6
Anshuman, a bilingual interpreter for the U.S.
military, is a refugee from Pakistan. He cannot
return to his native Pakistan because of fear of
persecution.
___
35.7
Svetlana was born in Moscow. Her father is a U.S.
citizen and her mother is a Russian citizen.
___
35.8
Sabeen, an Iraqi secretary, met her fiancé Jim while
he served in the Gulf War. She is coming to the
United States to meet his parents and marry him.
___
35.9
Boris refuses to define his status.
___
35.10
Jose, a Mexican migrant farmer, illegally entered
the United States through Big Bend, Texas.
A.
B.
C.
D.
E.
F.
G.
H.
I.
J.
Not applicable
(U.S.-born)
Immigrant visa
Student visa
Employment
visa
Tourist visa
Family/fiancé
visa
Refugee
Asylee or
parolee
Other
immigration
status
Unknown
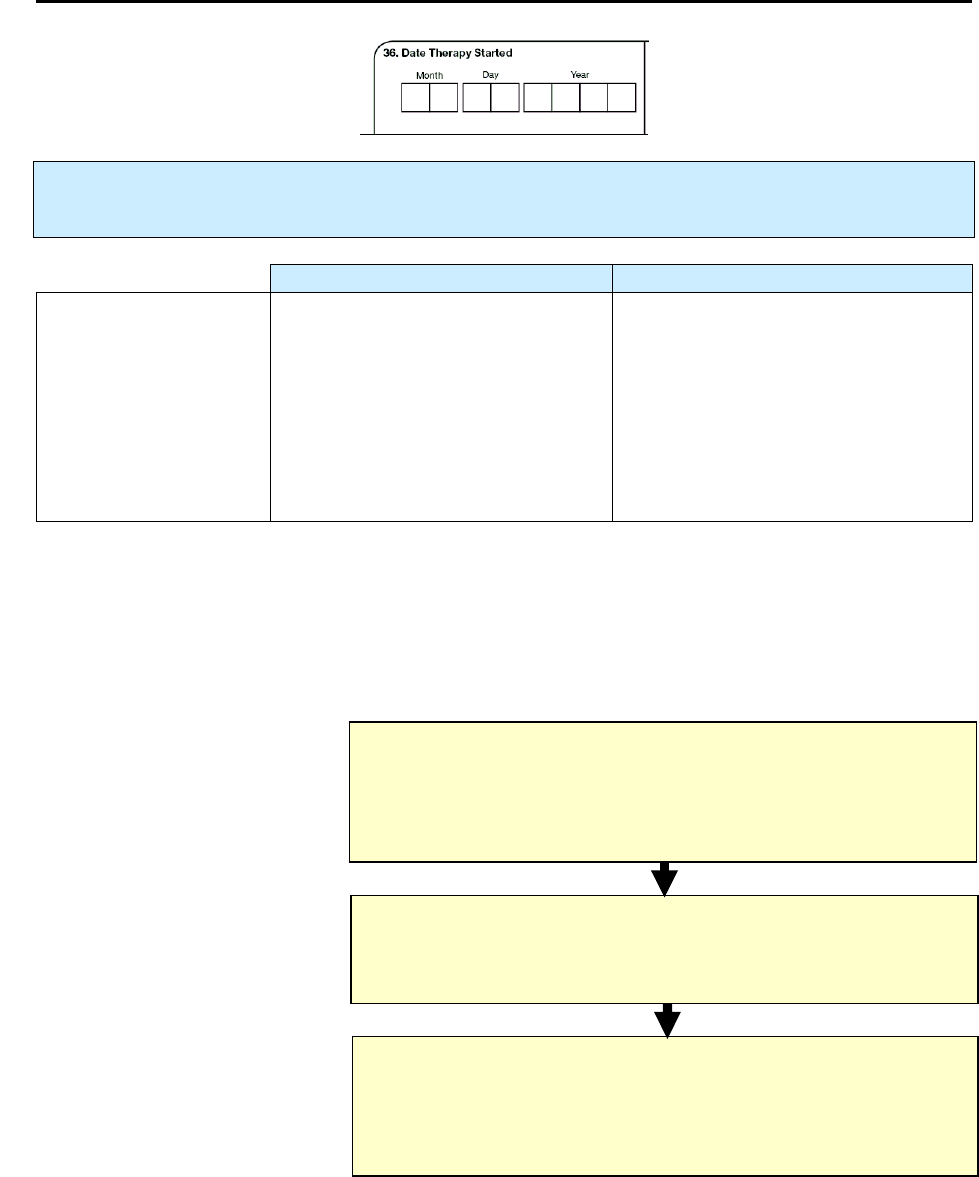
153
36.
Date Therapy Started
Primary Purpose: Programmatic function. Data are used for calculating program management
indicators.
Description Comment
Month, day, and year
(e.g., 01/17/2009)
Date the patient began multidrug
therapy for TB disease or
suspected TB disease
This may be one of several dates,
ideally, when the patient first ingested
medication if documented in a
medical record.
If the month or day is unknown, enter
99 as the default value (e.g.,
01/99/2009).
Date Therapy Started
is the month, day, and year the patient began multidrug therapy for TB disease or
suspected TB disease. Patient history without medical documentation is not acceptable and should be
entered as unknown. Enter a date according to the following chart:
Hierarchy of Determining Date Therapy Started
(Base decision on documented evidence)
Date
Medication was First Dispensed to the Patient
as
documented
by medical or pharmacy record
Date
Medication was First Prescribed to the Patient
by the Health Care Provider
as
documented
by medical or pharmacy record
Date
Patient First Ingested Medication
if
documented
in a medical record, such as hospital,
or
clinic,
or
directly observed therapy (DOT) record (preferred)
Preferred
Date Therapy Started
If this date is not known
choose the next alternative
Next Alternative
Date Therapy Started
If this date is not known
choose the last alternative
Last Alternative
Date Therapy Started

154
Exercise
36.
Date Therapy Started
Case Study – Thelma
On February 14, 2010, Thelma is diagnosed with TB. That same day her physician
prescribes the initial standard four-drug regimen of isoniazid, rifampin, pyrazinamide,
and ethambutol. On February 15, Thelma picks up the drugs from the pharmacy. Her
medical records indicate that Thelma is not sure when she started taking the drugs. She
thinks maybe it was on February 17, February 21, or even later.
36.1 Which date is the Date Therapy was Started?
(circle the one best answer)
A.
February 14, 2010
B.
February 15, 2010
C.
February 17, 2010
D.
February 21, 2010

155
37.
Initial Drug Regimen
Primary Purpose: Programmatic function. Data are used for calculating program management
indicators.
Select an option for
each
drug listed.
Option
(select one)
Description Comment
No
Drug is known to not be part of the initial regimen.
Yes
Drug is known to be part of the initial regimen.
Yes
indicates that the drug was
initially
prescribed
for treatment of TB disease and was taken for at
least 2 weeks. The 2-week requirement should
eliminate most of the record updates necessitated
by changes in regimen after treatment has begun.
If you cannot determine the
initial regimen of at least 2
weeks’ duration, select Yes
for the initial drugs known to
have been prescribed.
Unknown
It is not known whether the drug was part of the
initial regimen.
Comment:
Combination drugs
For combination drugs, select
Yes
for each drug that is a component of the combination drug.
For example
•
Rifamate is a combination of izoniazid and rifampin
•
Rifater is a combination of izoniazid, rifampin, and pyrazinamide
Example: Combination drugs
For Rifamate, select
Yes
for isoniazid and
Yes
for rifampin.
Note:
For
Other
, enter only anti-TB drugs (do
not
include pyridoxine, vitamin B6).

156
Exercise
37.
Initial Drug Regimen
Case Study – Zelda
On March 30, 2010, Zelda is diagnosed with TB. That same day, her physician prescribes
the initial standard four-drug regimen of isoniazid, rifampin, pyrazinamide, and
ethambutol.
What is the Initial Drug Regimen that was prescribed?
(Choose the one best answer by checking the box indicating Initial Drug
Regimen for each drug.)
A.
No
B.
Yes
C.
Unknown
37.1
Isoniazid
□ □
□
37.2
Rifampin
□
□
□
37.3
Pyrazinamide
□
□
□
37.4
Ethambutol
□ □ □
37.5
Streptomycin
□ □
□
37.6
Rifabutin
□
□
□

157
Modu
ModuModu
Modul
ll
le
e e
e D
DD
D
-
--
-
Initial Drug Susceptibility Report (Follow Up Report
Initial Drug Susceptibility Report (Follow Up Report Initial Drug Susceptibility Report (Follow Up Report
Initial Drug Susceptibility Report (Follow Up Report -
--
- 1)
1) 1)
1)
(page 1 of 1)
(page 1 of 1)(page 1 of 1)
(page 1 of 1)
Items 38
Items 38 Items 38
Items 38 –
––
– 40
40 40
40
Module D provides instructions and exercises for completing the Initial Drug Susceptibility Report. This
page is a follow-up report to the RVCT and includes data about genotyping, as well as initial drug
susceptibility testing and results.
Complete this report only for cases with positive culture results for M. tuberculosis complex.
Complete and submit this report as soon as initial drug susceptibility results are available. Copy patient
name and address from page 1 of the RVCT. The patient name and address are retained at the local level
for identification purposes; they are not sent to CDC. Enter
Year Counted
,
State Case Number
, and
City/County Case Number
for data entry purposes.
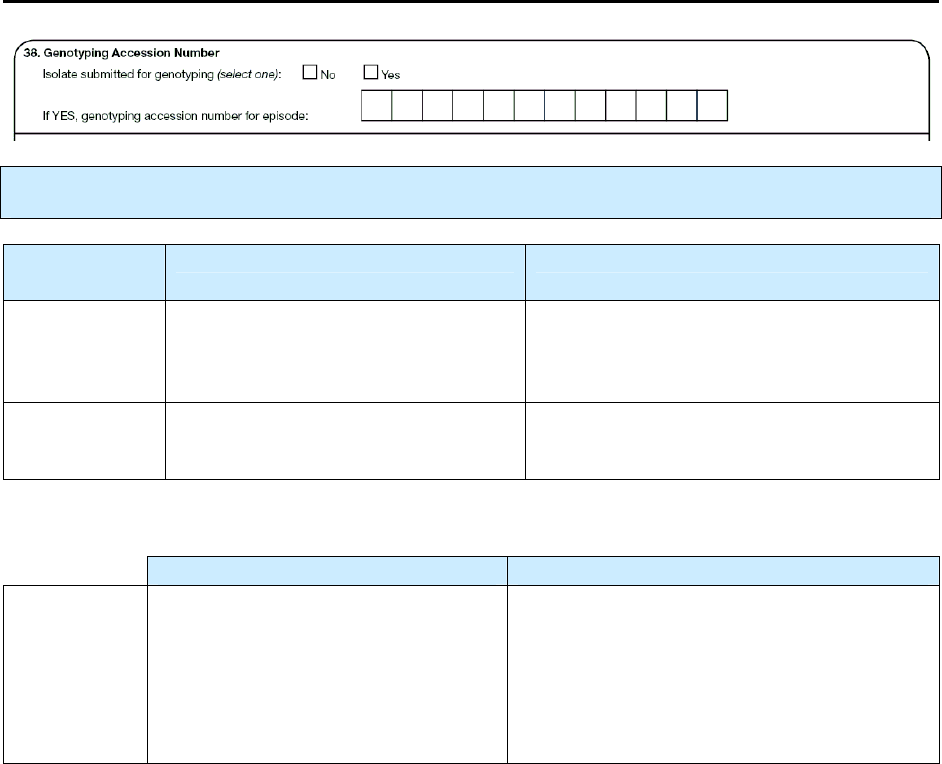
158
38.
Genotyping Accession Number
Primary Purpose: Surveillance. Data are used to link genotyping results with RVCT data.
Option
(select one)
Description Comment
No
No isolate was submitted for
genotyping.
No does not indicate that no results were
received or that “untypeable” results were
reported.
Yes
Isolate was submitted for genotyping,
regardless of genotyping results.
If you selected
Yes
, enter the following information.
Description Comment
Genotyping
accession
number
The genotyping accession number for
the current TB episode. This number
is assigned by the genotyping
reference laboratory.
If multiple isolates have been submitted for
one patient, please consult with your
laboratorian or genotyping surveillance
coordinator to determine the correct
genotyping accession number for the current
episode.
Comment:
Genotyping accession number
In 2004, CDC established the National Tuberculosis Genotyping Service (NTGS). The goal was to
genotype one M. tuberculosis isolate from every culture-confirmed TB case in the United States. The
genotyping accession number is the number assigned by the genotyping reference laboratory. Under
current contracts, the numbers are formatted in the following table.
159
Genotyping Accession Number
Sample
Laboratories
Performing
Genotyping Service
Format for
Genotyping Accession Number
Sample
California lab
YY (the 2-digit year), followed by L and 4 digits 05L1234
Michigan lab
YY (the 2-digit year), followed by RF and 4 digits 06RF5678
CDC lab
YY (the 2-digit year), followed by a hyphen and 6 digits
06-012345
When entering the genotyping accession number, begin at the first box and continue to fill to the right.
Include all hyphens and letters. Do not add zeros in the remaining boxes (beyond the number provided by
the reference lab).

160
Exercise
38.
Genotyping Accession Number
Case study - Jenna
Jenna is diagnosed with culture-positive TB in Hawaii in 2009. After the initial
diagnosis, her isolate is sent to the California reference laboratory as part of universal TB
strain genotyping. The Excel spreadsheet that came back from the laboratory looks like
this:
09L9564 09AF0254 777777777760731 222325153325 PCR00208
38.1 What should be entered for the Genotyping Accession Number?
(circle the one best answer)
A.
0 9 L 9 5 6 4
B.
0 9 A F 0 2 5 4
C.
P C R 0 0 2 0 8

161
39.
Initial Drug Susceptibility Testing
Primary Purpose: Programmatic function. Data are used to monitor the rate of susceptibility testing
and calculate indicators.
Option
(select one)
Description
No
Initial drug susceptibility testing was not performed.
Yes
An initial isolate was obtained, submitted for drug susceptibility testing, and
results are available.
Unknown
It is not known whether initial drug susceptibility testing was performed.
Comments:
If drug susceptibility testing was performed on multiple initial isolates, select one of the following (there is
no hierarchy for selecting these options):
•
The isolate associated with the primary, or major, site of disease
or
•
The initial isolate from the major site of disease that yields the best or most information concerning
drug susceptibility results
or
•
The initial culture-positive isolate.
Note:
If the answer is
No
or
Unknown
, do
not
complete the remainder of this form (Initial Drug
Susceptibility Report [Follow Up Report–1]).
162
If you selected
Yes
, enter the following information.
Description Comment
Date first specimen
for which drug
susceptibility testing
was done
Month, day, and year the first
specimen was collected
(e.g., 01/17/2009)
If the month or day is unknown, enter
99 as the default value (e.g.,
01/99/2009).
Select the
specimen type
on which initial drug susceptibility testing was performed.
Option
(select one)
Description
Sputum
Not sputum
Enter appropriate anatomic code (e.g., 30 for pericarditis) from the Anatomic
Code list (see Appendix C – Anatomic Codes).
Note: For the purposes of the RVCT training materials, use the codes listed in the appendices. Some
software programs used to enter data on the RVCT may NOT use the codes listed in the appendices.
For example, the Anatomic Codes may be a drop-down item, where you choose the actual site rather
than enter a code. For more information, see instructions for the software you use.

163
Exercise
39.
Initial Drug Susceptibility Testing
Case study – Esmeralda
On July 3, 2009, Esmeralda is suspected of having TB. That same day a urine specimen
is collected and she starts treatment. On July 29 the result of the urine specimen indicates
that it is culture positive for TB. Drug susceptibility results on the urine specimen are
received on August 8.
Three consecutive sputum specimens are collected from Esmeralda on July 5, 6, and 7.
On August 13, the results indicate that all three specimens are smear negative and culture
positive for TB. Drug susceptibility testing performed on the July 5 sputum specimen is
reported on August 21 and is identical to the drug susceptibility testing results from the
urine specimen.
39.1 What is the date of the first specimen on which drug susceptibility testing was
done?
(circle the one best answer)
A.
July 3, 2009
B.
July 5, 2009
C.
August 8, 2009
D.
August 13, 2009
E.
August 21, 2009
39.2 What should be entered for specimen type?
(circle the one best answer)
A.
Sputum
B.
Anatomic Code - Urine (69)
C.
Both A and B are correct
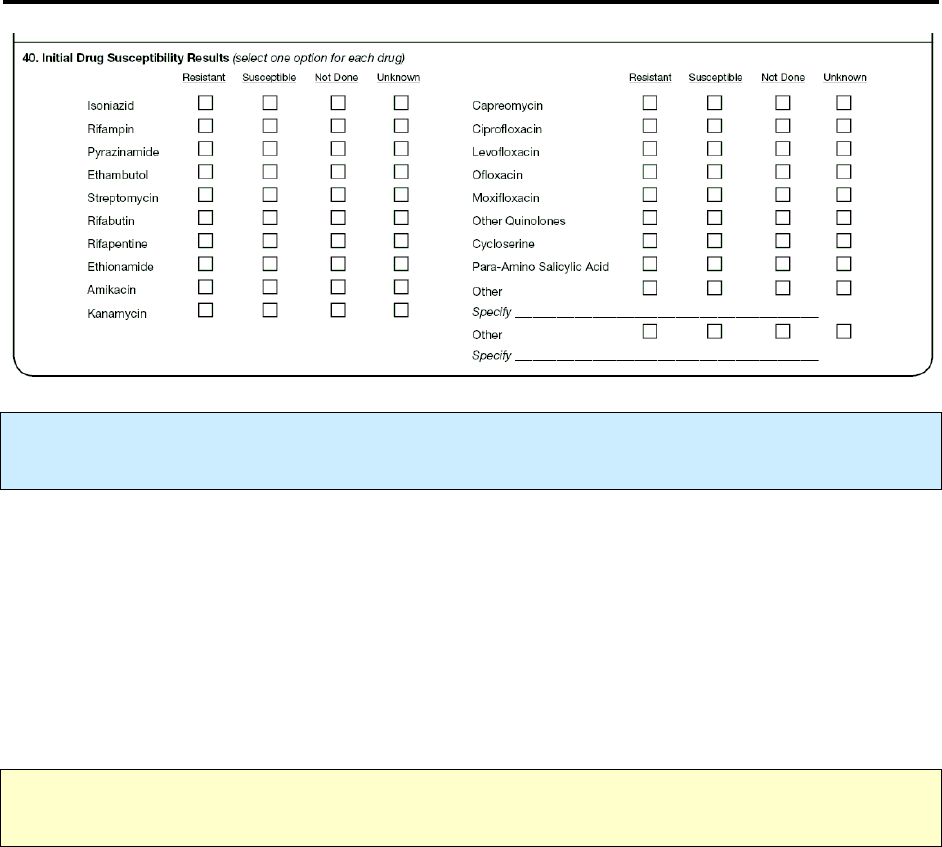
164
40.
Initial Drug Susceptibility Results
Primary Purpose: Programmatic function. Data are used to monitor trends in drug resistance and
calculate indicators.
Record the results of initial drug susceptibility testing on the first specimen on which drug susceptibility
testing was performed. If drug susceptibility testing was performed on multiple initial isolates, select one
of the following (there is no hierarchy for selecting these options):
•
The isolate associated with the primary, or major, site of disease
or
•
The initial isolate from the major site of disease that yields the best or most information
concerning drug susceptibility results
or
•
The initial culture-positive isolate.
Note:
Report results from conventional drug susceptibility tests (DST) only. Do
not
report rapid DST
test results (molecular beacon, molecular line probe assays, or other molecular tests).
165
First-line and Second-line Anti-TB Drugs
First-line Drugs Second-line Drugs
•
Isoniazid
•
Rifampin
•
Pyrazinamide
•
Ethambutol
•
Streptomycin
•
Rifabutin
•
Rifapentine
•
Ethionamide
•
Amikacin
•
Kanamycin
•
Capreomycin
•
Ciprofloxacin
•
Levofloxacin
•
Ofloxacin
•
Moxifloxacin
•
Other Quinolones
•
Cycloserine
•
Para-Amino
Salicylic Acid
•
Other
Comments:
•
If drug susceptibility testing for first-line anti-TB drugs was performed on a specific specimen
and resistance to one or more drugs was noted, thus prompting drug susceptibility testing for
second-line anti-TB drugs, this testing should be done on the same specimen. Enter both first- and
second-line testing results for this variable, even if the results are received at different times.
•
If the same specimen is used for drug susceptibility testing for second-line anti-TB drugs after
testing for first-line anti-TB drugs, update these variables when results become available.
•
If a second specimen is needed for drug susceptibility testing for second-line anti-TB drugs, the
second specimen should be collected as soon as possible after the first specimen was collected for
drug susceptibility testing for first-line anti-TB drugs (i.e., interval between specimen collections
should be less than 4 weeks). Update these variables when results become available.
For
each
drug listed, select one of the options listed below.
Option
(select one)
Description
Resistant
Drug has any degree of resistance (even partial resistance, resistance at a low
concentration of the drug, or a result other than completely susceptible).
Susceptible
Select only if completely susceptible.
Not done
Susceptibility testing was not done for this drug.
Unknown
It is not known whether the test was performed.
or
Results are not available or result is not known for a reason other than pending
results.
Note: Other Quinolones
excludes ciprofloxacin, levofloxacin, moxifloxacin, and ofloxacin
because they are listed on the form.
Use the space at the bottom of the form to write comments (e.g., name of the laboratory that
performed drug susceptibility testing) regarding the case of TB reported on this form (Initial Drug
Susceptibility Report).
If radiometric and conventional results on the same specimen differ (e.g., one is resistant, the other is
susceptible), discuss the results with your state TB laboratory director and complete the item
accordingly.

166
Comment:
Combination drugs
For combination drugs (e.g., Rifamate, Rifater), select
Yes
for each drug that is a component of the
combination drug.
For example
•
Rifamate is a combination of izoniazid and rifampin
•
Rifater is a combination of izoniazid, rifampin and pyrazinamide
Example: Rifamate
For Rifamate, select
Yes
for isoniazid and
Yes
for rifampin.
Note:
For
Other
, enter only anti-TB drugs (do
not
include pyridoxine, [vitamin B6]).
167
Exercise
40.
Initial Drug Susceptibility Results
Case study – Esmeralda (continued from Item 39)
The following first-line drug susceptibility test results for the isolate of M. tuberculosis
complex are received on August 21 from laboratory #1. The second-line laboratory
results are received on September 19 from laboratory #2. The laboratory results are
indicated in the table below.
Results from Laboratory #1 Results from Laboratory #2
• INH – Low-level resistance
• Rifampin – No resistance
• Pyrazinamide – No resistance
• Ethambutol – Resistance
• Streptomycin – Testing not done
• Rifabutin – Resistance
• Rifapentine – Testing not done
• Ethionamide – Not known if test was done
• Amikacin – Susceptible
• Kanamycin – Testing not done
What are the Initial Drug Susceptibility Results entered on the RVCT?
(Choose the one best answer by checking the box indicating Initial Drug
Susceptibility Results from the laboratory for each drug.)
A.
Resistant
B.
Susceptible
C.
Not Done
D.
Unknown
40.1
Isoniazid
□ □
□
□
40.2
Rifampin
□
□
□
□
40.3
Pyrazinamide
□
□
□
□
40.4
Ethambutol
□ □ □ □
40.5
Streptomycin
□ □
□
□
40.6
Rifabutin
□
□
□
□
40.7
Rifapentine
□
□
□
□
40.8
Ethionamide
□ □
□
□
40.9
Amikacin
□
□
□
□
40.10
Kanamycin
□
□
□
□
168

169
Module
Module Module
Module E
EE
E
-
--
-
Case Completion Report
Case Completion Report Case Completion Report
Case Completion Report (Follow
(Follow(Follow
(Follow U
U U
Up Report
p Report p Report
p Report –
––
–
2)
2)2)
2)
Items 41
Items 41 Items 41
Items 41 –
––
– 49
49 49
49
Module E provides instructions and exercises for completing the Case Completion Report. This 2 page
report includes data about treatment outcomes, provider status, and if the patient moved during treatment.
Complete this form for all patients who were alive at the time of TB diagnosis. Enter data as soon as
information becomes available during patient follow-up. This report should be completed when the case
is closed to supervision and is due no later than 2 years after the initial RVCT.
Copy patient name and address from page 1 of the RVCT form. Patient name and address are retained at
the local level for identification purposes; they are not sent to CDC. Enter
Year Counted
,
State Case
Number
, and
City/County Case Number
for data entry purposes.
(page 1 of 2)
(page 1 of 2)(page 1 of 2)
(page 1 of 2)

170
(page 2 of 2)
(page 2 of 2)(page 2 of 2)
(page 2 of 2)
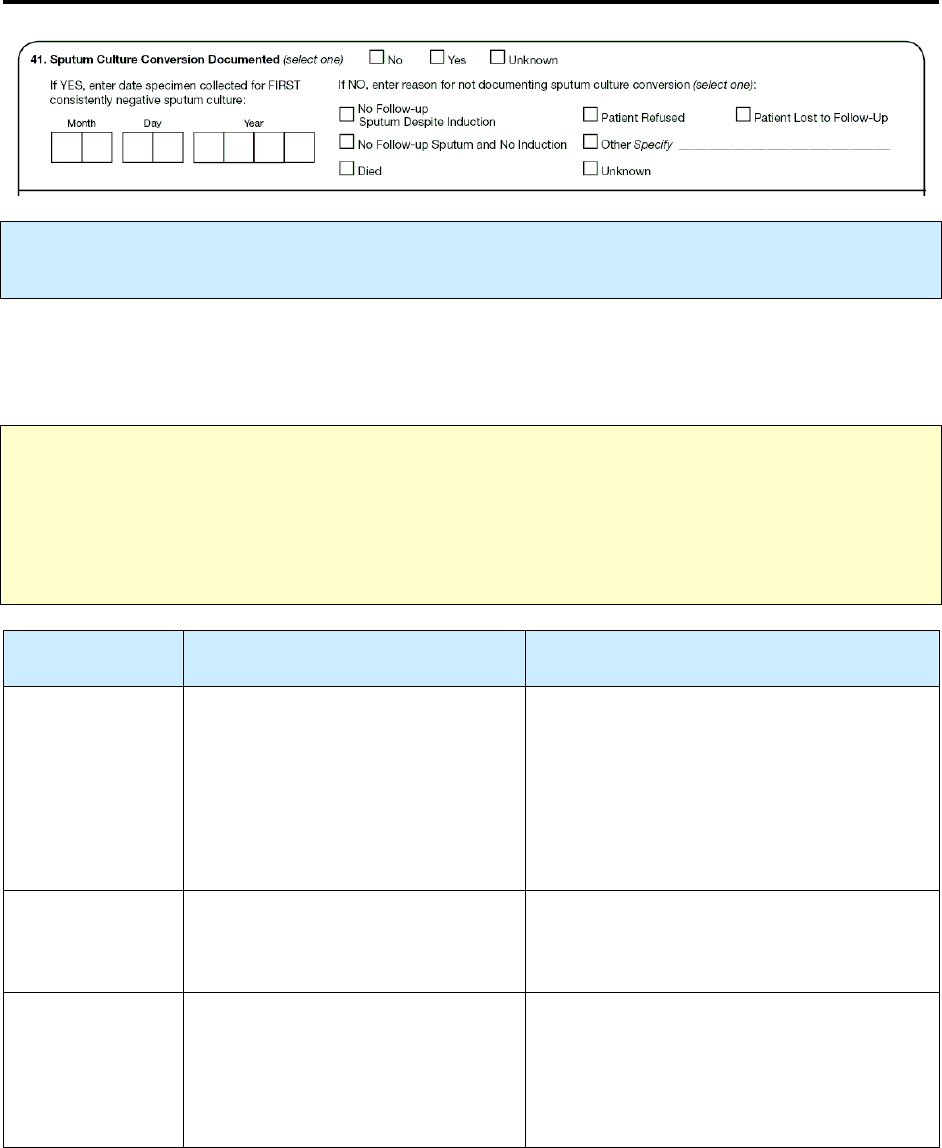
171
41.
Sputum Culture Conversion Documented
Primary Purpose: Programmatic function. Data are used to monitor the rate of sputum culture
conversion.
Provide information on sputum culture conversion only for patients with initially positive sputum
cultures
. Sources for documentation of sputum culture conversion include patient medical records and
laboratory reports.
Note: Do NOT complete this item for patients whose –
•
Sputum culture was
not
indicated as positive in
Sputum Culture
(item 18).
•
Initial sputum specimen did
not
test positive and whose other pulmonary specimens (e.g.,
bronchoscopy fluid) tested positive in
Culture of Tissue and Other Body Fluids
(item 20).
Option
(select one)
Description Comment
No
Initial sputum specimen was
culture-positive; no later specimens
were culture-negative (e.g., all
follow-up cultures were positive,
patient could not produce sputum
after therapy started, or no follow-
up sputum cultures were obtained).
Yes
Initial sputum specimen was
culture-positive, followed by at
least 1 negative sputum culture.
There should be no positive cultures after the
negative culture(s).
Unknown
Results of all follow-up cultures are
not known.
or
It is not known whether follow-up
cultures were done.
172
If you selected
Yes
, enter the following information.
Description Comment
Date specimen
collected for
FIRST
consistently
negative sputum
culture
Month, day, and year when the
first of the consistently negative
sputum specimens was
collected (e.g., 01/17/2009).
Complete only for patients who had 1 or more
positive sputum cultures and who
subsequently had at least 1 documented
negative culture.
This date should be at least 1 week after the
last positive culture result. There should be no
positive cultures after this date.
This information may be available from
medical records or laboratory reports.
If the month or day is unknown, enter 99 as
the default value (e.g., 01/99/2009).
If you selected
No,
select one reason for
not documenting sputum culture conversion
.
Option
(select one)
Description
No follow-up
sputum despite
induction
Repeat sputum collection was attempted (including induced sputum collection),
but because of clinical improvement, patient was not able to produce sputum.
No follow-up
sputum and no
induction
Induction was not attempted (e.g., the health care provider did not order a repeat
specimen, or there were no facilities or equipment for induction).
Died
Patient died before having an opportunity to submit sputum to document whether
the sputum culture had converted.
Patient lost to
follow-up
Patient was lost to follow-up before having an opportunity to submit a sputum to
document whether the sputum culture had converted.
Patient refused
Patient refused to provide a sputum specimen for a repeat culture.
Other
(specify)
A reason not included in the above choices (e.g., treatment failed, or the patient
moved outside the United States).
Unknown
It is not known why a repeat sputum culture was not obtained.
173
Exercise
41.
Sputum Culture Conversion Documented
How would you document Sputum Culture Conversion for the following patients?
(Choose the one best answer by matching the sputum culture conversion with the patient. Write
the letter for the sputum culture conversion on the line next to the question number.)
Patient Sputum Culture
Conversion
___
41.1
Alice has a positive sputum culture in
September. After that she does not allow
any more specimens to be taken because it
hurts when she coughs.
___
41.2
Gene has 3 positive sputum cultures in
May. His physician does not order any
other additional induction to collect
additional sputum samples.
___
41.3
Ivan has a terrible cough. On June 6 he
produces a positive sputum culture. He dies
on July 5 before any other specimen is
collected.
___
41.4
Joyce has a positive sputum culture in July.
Subsequent sputum specimens are
collected on September 21, 22, and 23. Her
medical records indicate that on November
8 all September sputum sample cultures are
negative.
___
41.5
Ollie has a positive sputum culture in
November, and by December his condition
improves. An induction machine is
unsuccessful in collecting additional
sputum specimens.
___
41.6
Roy has a positive sputum culture in July.
He is lost to follow-up and no more sputum
samples are collected.
A
B.
C.
D.
E.
F.
No, because no follow-
up sputum despite
induction
No, because no follow-
up sputum and no
induction
No, because patient
died
No, because patient
refused
No, because patient
was lost to follow-up
Yes
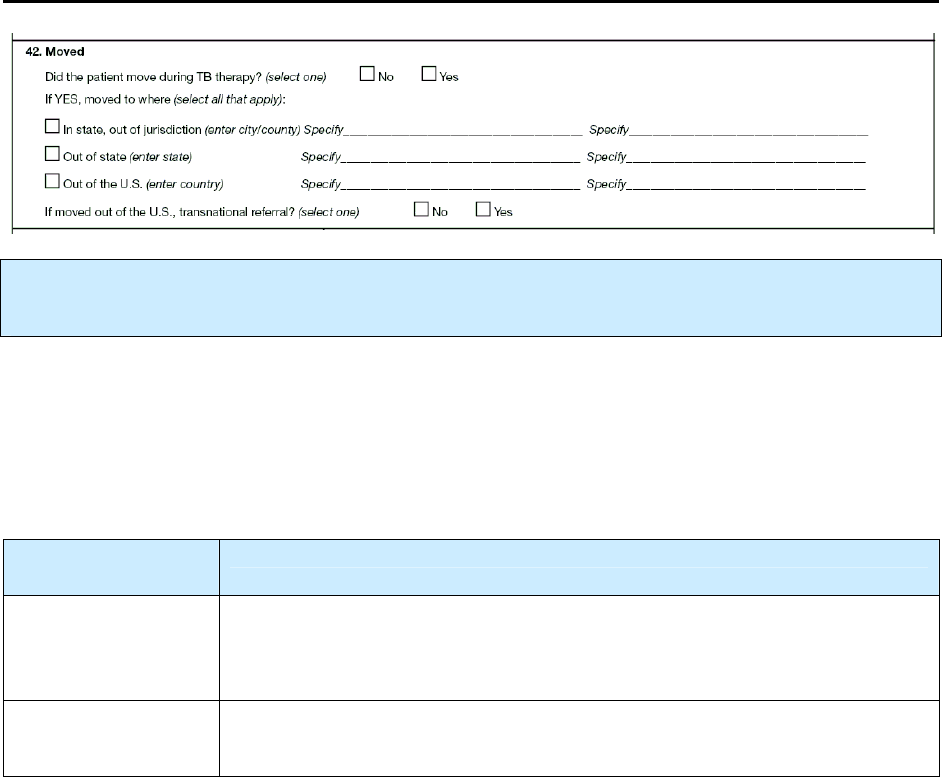
174
42.
Moved
Primary Purpose: Programmatic function. Data are used to facilitate efficient communication between
TB control programs in providing continuity of care for the patient.
This variable is used to record whether the patient moved during TB therapy. The responsibility for
follow-up reporting generally remains with the reporting area that initially reported the case to CDC and
counted it. (For a detailed description of the responsibility for submitting follow-up reports to CDC, see
the instructions for
Reporting Address for Case Counting
[item 4].)
Definition of Moved:
Relocated, the result of which is a change in local health department jurisdictions.
Option
(select one)
Description
No
Patient did not move.
or
Patient moved within the same local health department jurisdiction.
Yes
Patient moved to an area where another jurisdiction must now provide or
coordinate TB care.
175
If you selected
Yes
, select all the options that apply to the area
to which
the patient moved
.
Option
(select all that apply)
Description
COMMENT
In-state, out-of
jurisdiction
(specify)
Patient moved within the state, but
out of the local heath department
jurisdiction, such as moved to
different county or city.
Enter the city or county health
department jurisdiction to which the
patient moved.
If the patient moved more than
twice, enter the first 2 moves.
Patient moved from 1 of the 50 U.S.
states or the District of Columbia to
• Another state (e.g., moved from
Georgia to Alabama)
or
• A U.S. Territory, U.S. Island
Area, or U.S. Outlying Area
Out of state
(specify)
Patient moved from a U.S. Territory,
U.S. Island Area, or U.S. Outlying
Area to
• One of the 50 U.S. states or the
District of Columbia
or
• A U.S. Territory, U.S. Island
Area, or U.S. Outlying Area
Enter the name of the state or
reporting area to which the patient
moved.
If the patient moved more than
twice, enter the first 2 moves.
176
Patient moved from the United States
to
• Another country (other than a
U.S. Territory, U.S. Island Area,
or U.S. Outlying Area)
Out of the U.S.
(specify)
Moved from a U.S. Territory, U.S.
Island Area, or U.S. Outlying Area to
• Another country (other than the
United States or another U.S.
Territory, U.S. Island Area, or
U.S. Outlying Area)
Enter the name of the country to
which the patient moved.
If the patient moved more than
twice, enter the first 2 moves.
If patient moved
out of the U.S.
, select one option to indicate whether a
transnational referral
was
made.
Option
(select all
that apply)
Description Comment
No
Referral was not made to
a TB program or
physician outside the
United States.
Yes
Referral was made to a
TB program or physician
outside the United States.
Transnational referral includes participation in
programs such as
• TBNet
• CureTB
• Immigration and Customs Enforcement (ICE)
Communication between programs is important
• To help ensure case management after
deportation
• For completing a case management transfer
and obtaining information from TB programs
and/or physicians outside the United States for
case completion
For more information, visit the CDC/DTBE web
site on the Process for Notification of TB Cases at
www.cdc.gov/tb/pubs/international/default.htm
177
Example: Moved within a county, parish, or within a state
A move could be within a county, parish, or even within a state provided that the same health department
jurisdiction is primarily responsible for providing the TB case management, completing the RVCT, and
ensuring the completion of treatment.
Example: New York City
New York State and New York City (NYC) are separate TB reporting areas that report TB cases directly
to CDC. If a patient moves from New York State to NYC or vice versa, the move is considered “in-state,
out of jurisdiction.” Select In-state, out-of jurisdiction.
Example:
Reporting from one of the U.S. Territories, U.S. Island Areas, or U.S. Outlying Areas
If you are reporting from one of the U.S. Territories, U.S. Island Areas, or U.S. Outlying Areas
(American Samoa, Federated States of Micronesia, Guam, Republic of the Marshall Islands,
Commonwealth of the Northern Mariana Islands, Republic of Palau, Puerto Rico, or U.S. Virgin Islands),
select
Out of state
if a patient moves out of your reporting area to the United States or to another U.S.
Territory, U.S. Island Area, or U.S. Outlying Area.
However, if a patient moves from your jurisdiction to a country other than the United States or another
U.S. Territory, U.S. Island Area, or U.S. Outlying Area, select
Out of the U.S.
Examples of Moved
Moved
From To
Select
Dallas County, Texas Harris County, Texas In-state, out-of jurisdiction
Denali Borough, Alaska Bethel Borough, Alaska In-state, out-of jurisdiction
Orleans Parish, Louisiana Vernon Parish, Louisiana In-state, out-of jurisdiction
Chuuk, Federated States of
Micronesia (FSM)
Yap, FSM In-state, out-of jurisdiction
California Hawaii Out of state
Washington, D.C. Baltimore, Maryland Out of state
California Guam Out of state
Guam Palau Out of state
Guam Hawaii Out of state
Chuuk, FSM Guam Out of state
Chuuk, FSM California Out of state
Puerto Rico Florida Out of state
Guam China Out of the U.S.
California China Out of the U.S.

178
Exercise
42.
Moved
Case Study – Johann
Johann is diagnosed on August 30 with TB disease at the Bogalusa Medical Center in
Washington Parish, Louisiana. He receives DOT and adheres to treatment. On
September 30 his job requires him to relocate from his home in Bogalusa to Opelousas,
Louisiana, 170 miles away. He continues TB treatment at the St. Landry Parish Health
Clinic in Opelousas.
42.1 Which of the following statements is true about whether Johann moved?
(circle the one best answer)
A.
No, did not move during TB therapy
B.
Yes, moved in state, out of jurisdiction
C.
Yes, moved out of state
D.
Yes, moved out of the U.S. and indicate no for U.S. transnational referral
E.
Yes, moved out of the U.S. and indicate yes for U.S. transnational referral
179
43.
Date Therapy Stopped
Primary Purpose: Programmatic function. Data are used to monitor completion of therapy within a
specified time.
Description Comment
Month, day, and
year
(e.g., 01/17/2005)
Date the patient stopped taking
therapy for TB disease or
suspected TB disease
This may be one of several dates, ideally,
when the patient last ingested medication if
documented in a medical record.
If the month or day is unknown, enter 99 as
the default value (e.g., 01/99/2005).
Comment: Date Therapy Stopped
The interval between
Date Therapy Started
(item 36) and
Date Therapy Stopped
(item 43) is meant to
encompass the entire period (including interruptions in therapy) that the patient was receiving medication
to treat TB disease or suspected TB disease.
Patient self-report without medical documentation is not
acceptable.
Although there may be interruptions in anti-TB drug therapy, enter the final documented date
on which the patient last ingested medication for TB disease or suspected TB. For patients being treated
for TB disease or suspected TB disease, enter
Date Therapy Stopped
, according to the following chart:
Hierarchy for Determining Date Therapy Stopped
(for entire treatment period)
Date
Patient Last Ingested Medication
Date
Medication Dispensed to the Patient
Would Have Run Out if the Patient had Taken
All the
Medication
Date
Medication Prescribed to the Patient Would
Have Run Out if the Patient Had Taken All the
Medication from the Date of Prescription
(from the final prescription for the last bottle)
Preferred
Date Therapy Stopped
If this date is not known, choose
the next alternative date stopped
Next Alternative
Date Therapy Stopped
If this date is not known, choose
the last alternative date
Last Alternative
Date Therapy Stopped
180
Comment: Update the date that therapy was stopped
Update the date therapy was stopped only if a patient was lost to follow-up and then returns and
completes therapy.
Comment: Reopened case
If a case is reopened (e.g., patient who has been lost to follow-up is found, restarts therapy, and then
completes therapy), update this form (Case Completion Report [Follow Up – Report 2]) to reflect that the
patient completed therapy.
181
Exercise
43.
Date Therapy Stopped
Case Study – Hannibal
Hannibal, a convicted murderer, was diagnosed on October 31, 2009, with TB disease
while incarcerated at Folsom Prison. He also had HIV and liver disease. He received
DOT. On November 23, 2009, he complained of nausea, fever, and had yellowish skin.
On November 24, Hannibal received DOT in the morning. He became very ill and died
later that evening. The medical examiner determined that Hannibal’s death was likely due
to an adverse reaction to INH.
43.1 What is the Date for Therapy Stopped?
(circle the one best answer)
A. Month Day Year
1 1 2 3 2 0 0 9
B. Month Day Year
1 1 2 4 2 0 0 9
C. Month Day Year
1 1 9 9 2 0 0 9
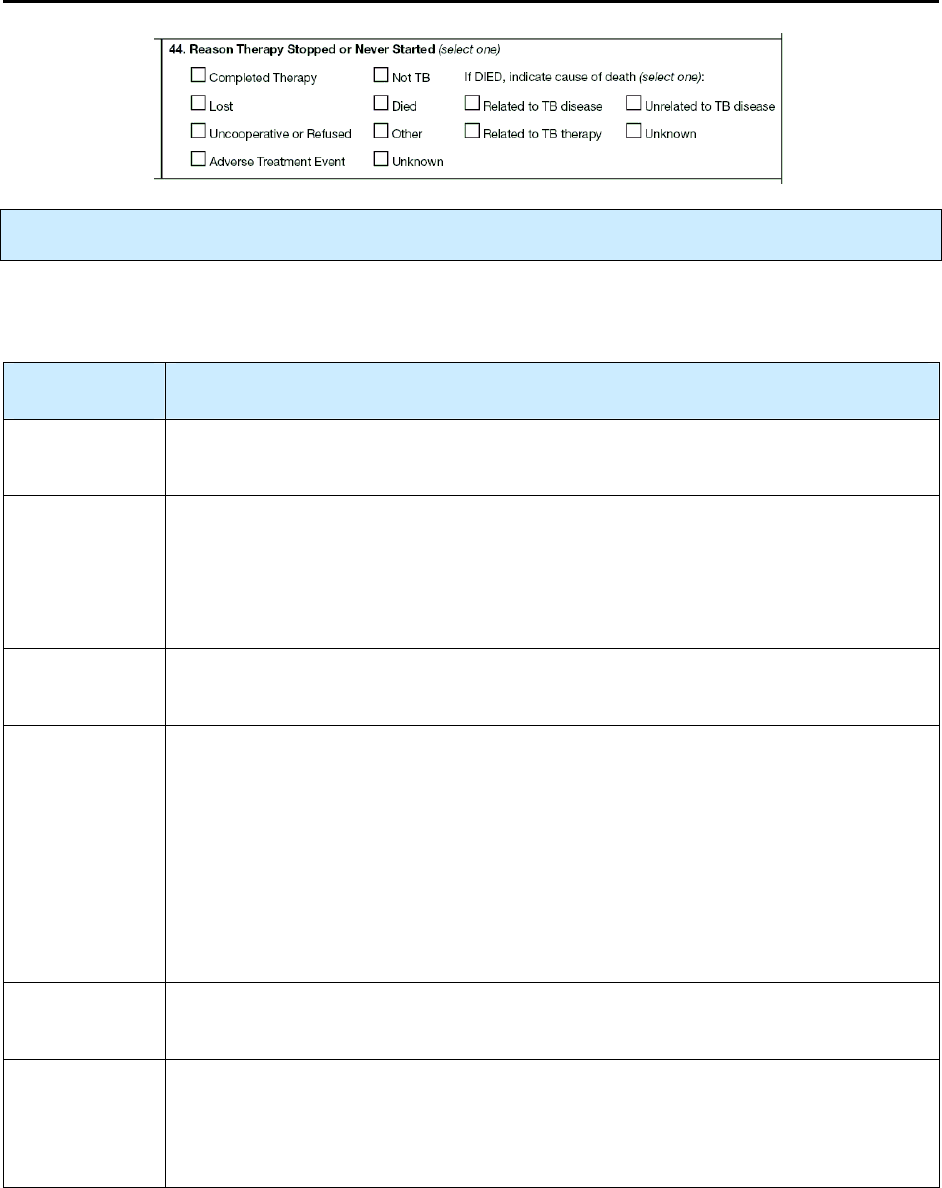
182
44.
Reason Therapy Stopped or Never Started
Primary Purpose: Programmatic function. Data are used to document treatment outcome.
Complete this item when the patient completes therapy or the case is closed. Select the primary reason
that TB therapy was ended and not resumed, or was never started.
Option
(select one)
Description
Completed
therapy
Patient completed the prescribed course of therapy per the medical record as
recorded by the clinician caring for the patient.
Lost
Patient could not be located before the start or the completion of treatment (e.g., the
patient moved to an unknown location, or the forwarding address is known but the
patient was not found at that address).
Code patients who move outside the United States and cannot be followed up as
Other.
Uncooperative
or refused
Patient refused to complete therapy (e.g., stopped taking drugs).
Adverse
treatment
event
Therapy was permanently stopped because of an adverse event due to anti-TB
medications. Select this option only if the patient lived.
If the patient died because of an adverse TB treatment event, select
• Died as the Reason Therapy Stopped
and then select
• Related to TB Therapy for Cause of Death even if the patient stopped TB
therapy prior to the death due to an adverse treatment event.
This is a determination that has to be made by the clinician.
Not TB
Completed diagnostic evaluation did not substantiate the diagnosis of TB (e.g., M.
avium was isolated from a clinical specimen).
Died
Patient was alive at diagnosis but died before the start or completion of treatment.
This also applies to a patient classified as alive for Status at TB Diagnosis (item 15)
if the patient was taking at least 2 anti-TB drugs before the day of death, even though
the TB case was not verified and counted until after death.
183
Other
Therapy was discontinued for a known reason not included in the above choices and
is not Unknown, (e.g., patient moved outside the United States, or patient moved
from state A to state B, and state A notified state B, but state B never followed up).
Unknown
Reason that therapy was stopped is not known.
Comment: Reopen a case
If a case is reopened (e.g., patient who has been lost to follow-up is found within 12 months of when the
patient was lost, restarts therapy, and then completes therapy), update this form (Case Completion Report
[Follow Up – Report 2]) to reflect that the patient completed therapy.
If you selected
Died
, indicate one
Cause of Death
.
Option
(select one)
Description Comment
Related to TB
disease
TB was
• The immediate cause
or
• An underlying cause
or
• Another significant condition
contributing to death (even if
TB was not the main cause of
death)
Written documentation of the cause of death
(e.g., death certificate, autopsy report, medical
records) is recommended. However, oral
information from a reliable source (e.g., a
health care provider) will be accepted.
A death certificate is not necessarily required
to complete this field. In some cases deaths
may be certified before receipt of results of
• Positive M. tuberculosis culture
or
• Other findings consistent with TB
Classify as related to TB disease if the patient
died as a result of a surgical procedure for
which
• The primary indication was the diagnosis
of TB
or
• TB complicated a surgical procedure not
related to TB (e.g., heart surgery)
Criteria for determining the cause of death
related to TB disease should be specified by
the clinician.
Related to TB
therapy
TB therapy (e.g., adverse
treatment event) was related to
the cause of death.
Criteria for determining the cause of death
related to TB therapy should be specified by
the clinician.
184
Unrelated to TB
disease
TB was not
• The immediate cause
or
• An underlying cause
or
• Another significant condition
contributing to death
Unknown
Cause of death is not known.
Every effort should be made to determine if
death was related to TB disease before
classifying as unknown.
Note:
Update this item if additional information is obtained.

185
Exercise
44.
Reason Therapy Stopped or Never Started
Case Study – Hannibal (continued from Item 43)
44.1 What is the Reason that Therapy was Stopped for Hannibal?
(circle the one best answer.)
A.
Completed Therapy
B.
Adverse Treatment Event
C.
Died
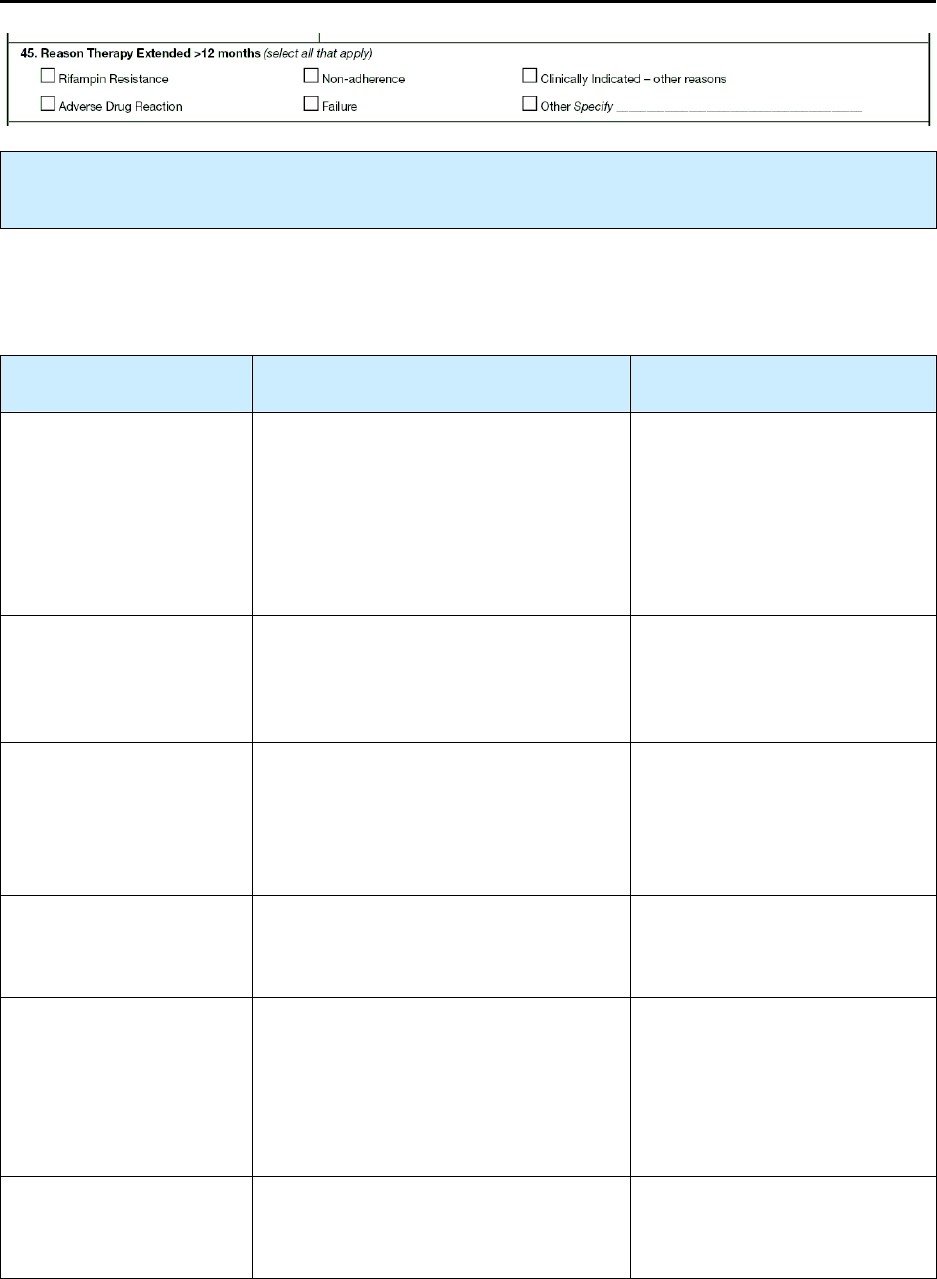
186
45.
Reason Therapy Extended >12 Months
Primary Purpose: Program function. Data are used to document reason for extended treatment and to
calculate program indicators.
Use the information entered for
Date Therapy Started
(item 36) and
Date Therapy Stopped
(item 43)
to calculate the length of anti-TB therapy. Sources for the reason(s) therapy was extended include patient
medical records, patient interview, and health care provider interview.
Option
(select all that apply)
Description Comment
Rifampin resistance
Patient had drug-resistant TB that
would require a treatment protocol
lasting more than 12 months (e.g.,
resistance to at least rifampin)
according to the ATS/CDC/IDSA
Official Joint Statement on the
Treatment of TB.
Adverse drug reaction
Patient had a significant adverse drug
reaction or experienced an adverse
treatment event due to anti-TB
medications that prolonged therapy.
Non-adherence
There were barriers to the patient’s
adherence to anti-TB therapy (e.g.,
treatment interruption), or the patient’s
lack of adherence resulted in extension
of therapy beyond 12 months.
Failure
A sputum specimen tested positive 4 or
more months after treatment began.
Criteria for determining failure
should be specified by the
clinician.
Clinically indicated―
other reasons
Clinical indications (other than
adverse drug reactions) include
central nervous system TB (e.g.,
meningitis), severe liver disease, or
other criteria as specified by the
clinician.
Other
Reason does not include any of the
choices listed above.
Use additional space at the
bottom of the page to write
comments regarding Other
reasons.
187
Exercise
45.
Reason Therapy Extended >12 months
What is the Reason Therapy was Extended for > 12 Months for the following patients?
(Choose the one best answer by matching the Reason Therapy was Extended for > 12 Months
with the patient. Write the letter for the Reason Therapy was Extended next to the question
number.)
Patient Reason Therapy was
Extended > 12 months
___
45.1
Ralph, a homeless man, begins DOT and
has a sputum culture conversion from
positive to negative by 2 months. But at 4
months his sputum results are positive.
___
45.2
Amy, a 3-year-old, has TB meningitis.
___
45.3
Jose and his brother Raul go on an
extended camping trip to Yosemite
National Park. He loses his medicine and
does not get more medicine until he returns
to the clinic 2 months later.
___
45.4
Bob’s initial drug susceptibility results
indicate that the isolate is resistant to
isoniazid and rifampin.
___
45.5
Nancy experiences severe breathing
difficulties and hives a week into therapy.
Her doctor changes her anti-TB
medications.
A.
B.
C.
D.
E.
Rifampin resistance
Adverse drug reaction
Non-adherence
Failure
Clinical indications –
other reasons
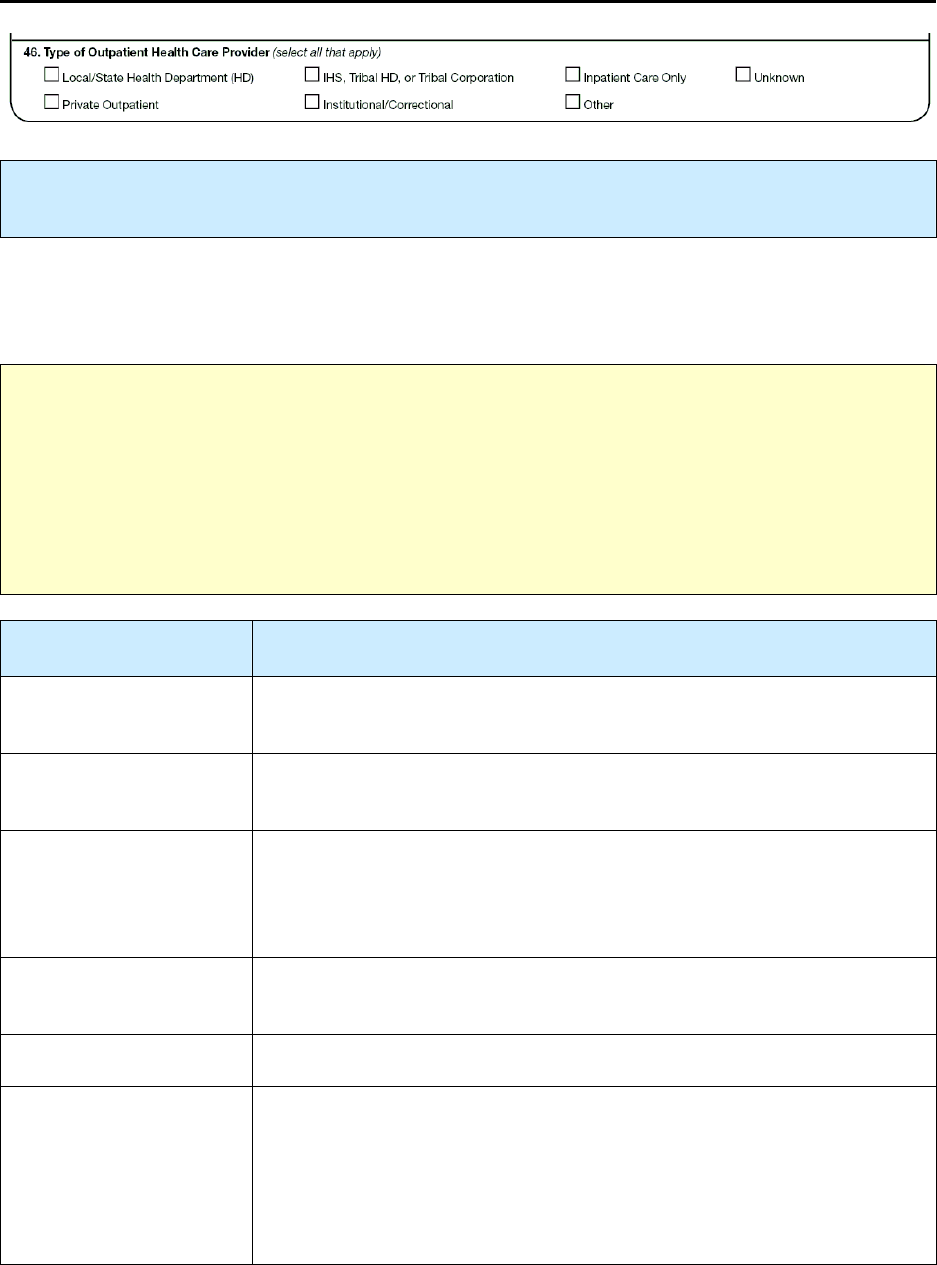
188
46.
Type of Outpatient Health Care Provider
Primary Purpose: Programmatic function. Data are used to guide TB programs in allocating
resources.
Definition for
Type of Outpatient Health Care Provider:
setting or affiliation of the provider who has
primary responsibility for clinical outpatient decision making (excluding diagnostic workup, contact
investigations, anti-TB medications, and directly observed therapy [DOT]).
Note:
•
Outpatient refers to a setting that is not a hospital and that does not provide acute care, such as a
clinic or a physician’s office.
•
Inpatient refers to a hospital or acute-care setting.
Here, these terms refer to the physician, not to the patient. These terms also denote the type of services
that are provided. Some institutions, such as a hospital, correctional facility, or long-term care facility,
may have both outpatient and inpatient settings.
Option
(select all that apply)
Description
Local/state health
department (HD)
Includes a TB program or a health clinic of a health department.
Private outpatient
Includes private physician or health care provider, health maintenance
organization (HMO), and private managed health care provider.
IHS, tribal HD, or tribal
corporation
Primary responsibility for clinical outpatient decision making rests with
the Indian Health Service (IHS); a tribal health department, such as the
American Indian or Alaska Native Tribal Health Department; or a tribal
corporation, such as the Tribal Healthcare Corporation.
Institutional/
correctional
Includes nursing homes and assisted living facilities, and all types of
correctional facilities.
Inpatient care only
Patient did not receive outpatient TB care. Care provided in a hospital.
Other
The provider is not included in the other categories and is not Unknown
(e.g., state TB chest hospital providing outpatient care, city/county/state-
owned hospitals that are not part of the health department providing
outpatient care, private hospital providing outpatient care, Veterans
Administration hospital, federal program, military facility, or community-
based organization [CBO]).

189
Unknown
Type of health care provider is not known. If you select Unknown, do not
select any other option for type of health care provider.
Comment:
Private outpatient
This category includes the private provider who has the primary responsibility for clinical outpatient
decision making for a TB patient, even though the TB control program or local/state health department
may be periodically contacting the private provider for the purpose of completing the RVCT and
ensuring proper TB case management.
Comment:
Inpatient care only
Examples of inpatient care only include
•
TB diagnosed at autopsy
•
Patients who were in the hospital but died before receiving outpatient TB care
•
Patients who received all of their TB care as an inpatient in a hospital
Comment: Multiple options
If a patient first received care from a private health care provider, but after a time (e.g., 3 months) lost his
or her medical insurance and began receiving care from the local or state health department, select both
Private
and
Local/State Health Department.

190
Exercise
46.
Type of Outpatient Health Care Provider
What is the Type of Outpatient Health Care Provider for the following patients?
(Choose the one best answer by matching the Type of outpatient health care provider with the
Patient. Write the letter for the type of provider next to the question number.)
Patient Type of Outpatient Health
Care Provider
___
46.1
Clyde is arrested for armed robbery. He is
diagnosed and treated for TB disease
during the 6 months that he is in the Bonnie
Springs Jail.
___
46.2
Victor, a Vietnam War veteran, comes to a
Virginia Veterans Administration Hospital
to receive DOT.
___
46.3
Howard, an HIV patient, is admitted to
National Jewish Health hospital for
treatment of XDR TB, where he receives
all of his TB treatment.
___
46.4
Nita is treated for TB disease at the
Cherokee Indian Hospital.
___
46.5
Curtis is diagnosed with active TB at the
Dallas County Health Department and
starts DOT.
___
46.6
Joel is diagnosed and treated for TB disease
by his physician at the Kaiser Permanente
Health Center.
A.
B.
C.
D.
E.
F.
G.
Local/
State Health
Department
Private Outpatient
IHS, Tribal HD, or
Tribal Corporation
Institutional/
Correctional
Inpatient Care Only
Other
Unknown
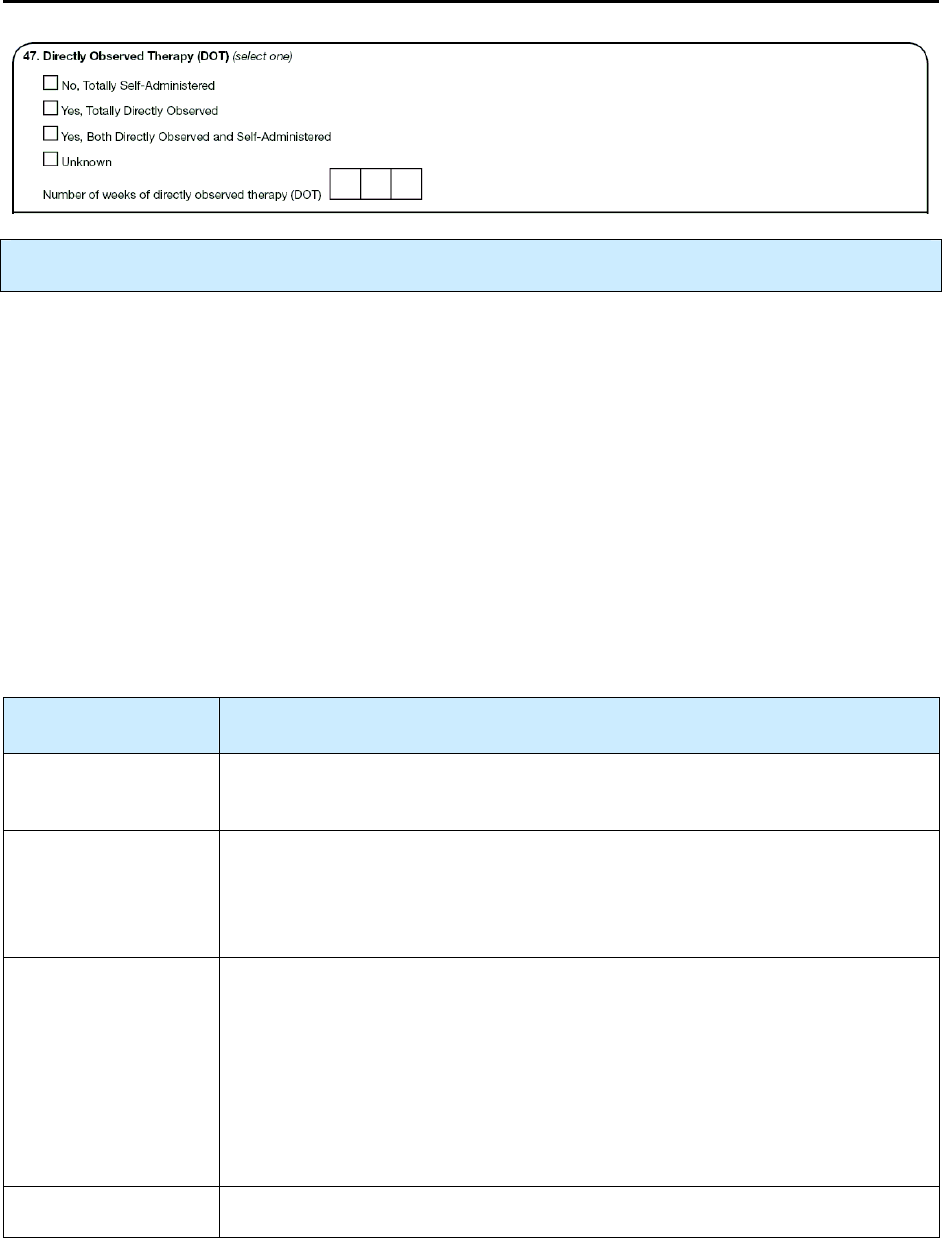
191
47.
Directly Observed Therapy (DOT)
Primary Purpose: Case management. Data are used to document administration of TB medications.
Directly observed therapy (DOT), or supervised therapy, involves the direct visual observation by a health
care provider (e.g., public health nurse, outreach worker, nurse, nurse’s aide) or other reliable trained
person (e.g., worker in a homeless shelter) of a patient’s ingestion of medication. Delivering medication
to a patient without visual confirmation of ingestion does not constitute DOT. However, a live video
camera confirmation of ingestion of medicine of
carefully selected patients
(e.g., stable and compliant)
constitutes DOT.
Anti-TB medication may be
1)
Self-administered (e.g., patient ingests medication dose[s]
without
direct visual observation
by a health care provider or other reliable person)
or
2)
Given by using DOT
or
3)
A combination of self-administered and given by using DOT
Option
(select one)
Description
No, totally
self-administered
No doses of medication were given under direct supervision.
Yes, totally directly
observed
Response applies if DOT was used for all doses for a patient who was taking
medication 1–5 times a week. Response also applies if the patient was taking
medication 7 times a week and DOT was used for at least 5 of those doses (i.e.,
patient self-administered the dose[s] during weekends and holidays).
Yes, both directly
observed and
self-administered
Response applies if the patient self-administered any dose while taking
medication 1–5 times a week. Response does not apply if the patient was
taking medication 7 times a week and DOT was used for at least 5 of those
doses (i.e., patient self-administered the dose[s] during weekends and
holidays).
Response also applies if patient took several months of self-administered
therapy and several months of DOT.
Unknown
It is not known whether any doses were given under direct supervision.

192
If you selected any
Yes
option, enter the
Number of weeks of directly observed therapy (DOT).
Option
(select one)
Description Comment
Number of weeks of
directly observed
therapy (DOT)
Based on the total number of
regimen-appropriate weeks and
doses ingested under directly
observed supervision (e.g., 026)
The total number of DOT weeks must
be less than or equal to the time
between Date Therapy Started (item
36) and Date Therapy Stopped (item
43).
To calculate
Number of weeks of directly observed therapy
(DOT weeks), use the following methods:
•
Review the patient’s medication records to determine the number of doses
given by DOT
each week
Review the patient’s medication records to determine the number of doses given by DOT each
week, or 7-day period. The number of days in a week is 7, but the calculation of DOT (or
medication) weeks should be independent of, or not restricted to, calendar weeks (i.e., Sunday
through Saturday).
•
Example: Medication week
A medication week can be, for example, Monday through Sunday or Wednesday through
Tuesday, as long as the week consists of 7 consecutive days.
•
Missed DOT dose
If a patient misses a DOT dose or there is a holiday during a medication week (i.e., DOT cannot
be given that week), as long as DOT is used when the missed dose(s) is made up at the end of
therapy, the dose(s) given at the end of therapy can be combined with the last “partial DOT
week” and counted as a “full DOT week.”
•
Count as a DOT week
Count as a DOT week any week during which DOT was used for every dose for a patient who
was taking medication 1–5 times a week. If the patient was taking medication 7 times a week,
DOT must have been used for at least 5 doses.
Often, the health department or the person completing the RVCT form does not have direct access to the
entire patient medical record or medication log because the TB patient is or was cared for by a provider
other than the health department (e.g., private health care provider). A private health care provider usually
does not provide DOT; rather, a public health care provider (e.g., public health nurse) provides DOT and
maintains the medication log and medication dosage calendar. The health department periodically follows
up with the provider, and when therapy is completed or the case is closed, the health department usually
completes a “close-out” form. In such instances, the health department should request a copy of the
medication log or review the log with the person who provided DOT (e.g., public health nurse) to
determine the amount of medication that was given by DOT.
193
Exercise
47.
Directly Observed Therapy (DOT)
Case study – Maximo
Maximo is diagnosed with TB in June 2009 after being evaluated through a free clinic in
Laredo, Texas. He is provided housing by the health department and starts on a 4-drug
DOT regimen administered by the health department on June 9, 2009. On August 4
(after 8 weeks of daily 4-drug therapy), his DOT regimen changes to twice-weekly INH
and RIF. He misses doses on August 25 and 27, October 8, and November 26
(Thanksgiving day). He successfully completes treatment and adheres to DOT up to
December 17, 2009.
47.1 What would you select for Directly Observed Therapy?
(circle the one best answer)
A.
No, totally self-administered
B.
Yes, totally directly observed
C.
Yes, both directly observed and self-administered
D.
Unknown
Use this calendar to determine weeks of DOT.
May June July August
1 2 1 2 3 4 5 6 1 2 3 4 1
3 4 5 6 7 8 9 7 8 9 10 11 12 13
5 6 7 8 9 10 11
2 3 4 5 6 7 8
10 11 12 13 14 15 16
14
15 16 17 18 19 20
12
13 14 15 16 17 18
9 10 11 12 13 14 15
17 18 19 20 21 22 23
21
22 23 24 25 26 27
19
20 21 22 23 24 25
16
17 18 19 20 21 22
24 25 26 27 28 29 30
28
29 30 26
27 28 29 30 31 23
24 25 26 27 28 29
31 30
31
September October November December
1 2 3 4 5 1 2 3 1 2 3 4 5 6 7 1 2 3 4 5
6 7 8 9 10 11 12
4 5 6 7 8 9 10
8 9 10 11 12 13 14
6 7 8 9 10 11 12
13 14 15 16 17 18 19
11
12 13 14 15 16 17
15
16 17 18 19 20 21
13
14 15 16 17 18 19
20 21 22 23 24 25 26
18
19 20 21 22 23 24
22
23 24 25 26 27 28
20
21 22 23 24 25 26
27 28 29 30 25
26 27 28 29 30 31
29
30 27
28 29 30 31
47.2. What would you enter for the number of weeks of DOT?
(circle the one best answer)
A.
0 2 0
B.
0 2 6
C.
0 3 2
194
Case study for Items 43–47 – Maximo (continued)
Now imagine that Maximo self-administers doses on August 25, August 27, and
September 1 because he was out of town visiting his sister that week. He still missed the
October 8 dose and the November 26 dose.
How would you complete items 43–47 on the RVCT?
47.3
Item 43: Date Therapy Stopped
(circle the one best answer)
A.
December 12, 2009
B.
December 17, 2009
47.4
Item 44: Reason Therapy Stopped or Never Started
(circle the one best answer)
A.
Completed Therapy
B.
Unknown
47.5
Item 45: Reason Therapy Extended > 12 Months
(circle the one best answer)
A.
Nonadherence
B.
Not applicable, leave blank
195
47.6
Item 46: Type of Outpatient Health Care Provider
(circle the one best answer)
A.
Local/State Health Department
B.
Private
47.7
Item 47: Directly Observed Therapy (DOT)
(circle the one best answer)
A.
Yes, both directly observed and self-administered
B.
Unknown
47.8 Item 47: Directly Observed Therapy (DOT). How many weeks of DOT were
provided?
(circle the one best answer)
A.
020
B.
025
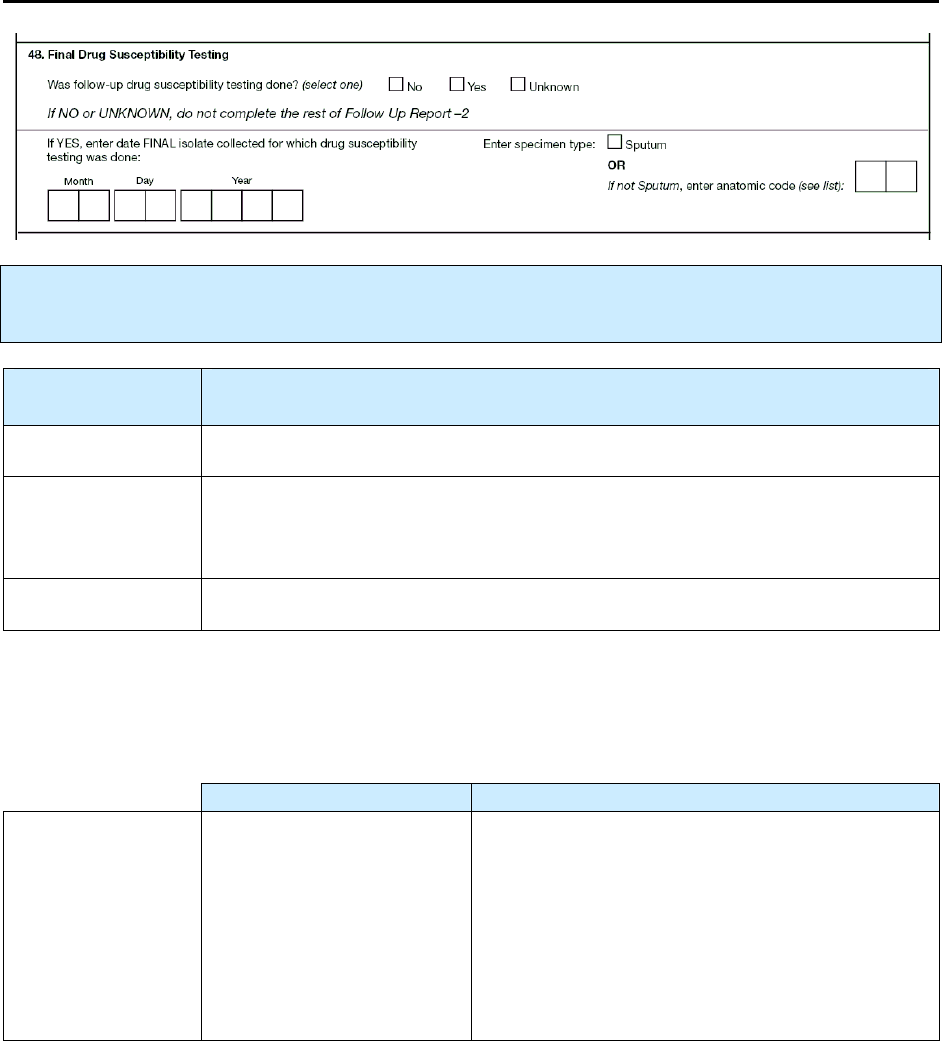
196
48.
Final Drug Susceptibility Testing
Primary Purpose: Surveillance. Data are used to observe trends in drug-resistant TB and to learn about
its epidemiology.
Option
(select one)
Description
No
Follow-up drug susceptibility testing was not performed.
Yes
Drug susceptibility testing was performed on a specimen that was collected
30 or more days after the specimen on which initial drug susceptibility testing
was performed.
Unknown
It is not known whether follow-up drug susceptibility testing was performed.
Comment:
This variable will help assess the frequency of acquired drug resistance.
If you selected
Yes
, enter the following information.
Description Comment
Date FINAL
specimen
collected on which
drug
susceptibility
testing was done
Month, day, and year
(e.g., 01/17/2009)
This date should be 30 or more days after the
collection date of the initial specimen on which
drug susceptibility testing was done (item 39).
This information is usually available from medical
records or laboratory reports.
If the month or day is unknown, enter 99 as the
default value (e.g., 01/99/2009).
197
Select the
Specimen Type
on which the final drug susceptibility testing was done.
Option
(select one)
Description
Sputum
Not sputum
Enter appropriate anatomic code (e.g., 30 for pericarditis) from Appendix C –
Anatomic Codes

198
Exercise
48.
Final Drug Susceptibility Testing
48.1 When must the final specimen be collected on which Final Drug Susceptibility
Testing is done?
(circle the one best answer)
A.
The date can be any time after the collection date for the initial specimen on
which drug susceptibility was done.
B.
The date should be less than 30 days after the collection date for the initial
specimen on which drug susceptibility testing was done.
C.
The date should be 30 or more days after the collection date for the initial
specimen on which drug susceptibility testing was done.
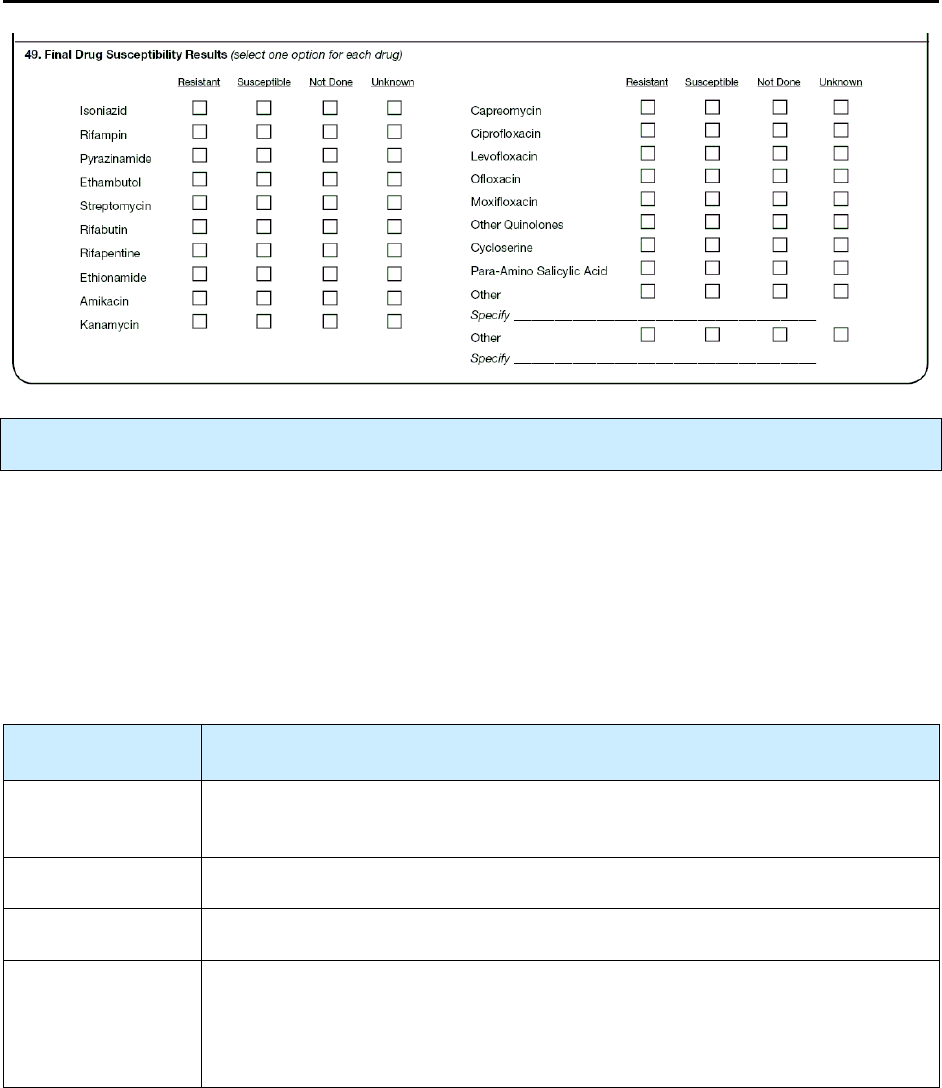
199
49.
Final Drug Susceptibility Results
Primary Purpose: Programmatic function. Data are used to monitor trends in drug resistance.
Record results for the
final
specimen on which drug susceptibility testing was performed. Drug
susceptibility testing procedures should comply with approved and accepted guidelines. If drug
susceptibility testing was performed on multiple specimens, select the most appropriate specimen: the one
associated with the primary, or major, site of disease; the final specimen from the major site of disease that
yields the best or most information concerning drug susceptibility results; or the final specimen that tested
positive.
For
each
drug listed, select one option from the following.
Option
(select one)
Description
Resistant
Drug has any degree of resistance (even partial resistance or resistance at a low
concentration of the drug, or other than completely susceptible result).
Susceptible
Select only if completely susceptible.
Not done
Susceptibility testing was not done for this drug.
Unknown
It is not known whether the test was performed.
or
Results were not available or result is not known for a reason other than pending
results.

200
Note: Other Quinolones
excludes ciprofloxacin, levofloxacin, moxifloxacin, and ofloxacin
because they are listed on the form.
Use the space at the bottom of the form to write comments (e.g., name of the laboratory that
performed drug susceptibility testing) regarding the case of TB reported on this form (Case
Completion Report).
If radiometric and conventional results on the same specimen differ (e.g., one is resistant, the other is
susceptible), discuss the results with your state TB laboratory director and complete the item
accordingly.
201
Exercise
49.
Final Drug Susceptibility Results
Case study for Items 48 and 49 – Lana
Lana is diagnosed with sputum culture-positive TB in October 2009. Initial drug
susceptibility tests showed her TB is sensitive to all of the first-line drugs that are
prescribed. She has a number of treatment interruptions and develops rifampin resistance,
which is discovered upon repeat drug susceptibility testing of sputum collected on
December 13, 2009. The isolate remains susceptible to isoniazid, pyrazinamide, and
ethambutol. The isolate is also found to be susceptible to rifabutin, ciprofloxacin,
moxifloxacin, streptomycin, amikacin, and kanamycin. No other drugs are tested.
What are the Final Drug Susceptibility Results for Lana?
(Choose the one best answer by checking the box indicating Final Drug Susceptibility Results
from the laboratory for each drug.)
A.
Resistant
B.
Susceptible
C.
Not Done
D.
Unknown
A.
Resistant
B.
Susceptible
C.
Not Done
D.
Unknown
49.1
Isoniazid
□ □
□
□
49.11 Capreomycin
□ □
□
□
49.2
Rifampin
□
□
□
□
49.12 Ciprofloxacin
□
□
□
□
49.3
Pyrazinamide
□
□
□
□
49.13 Levofloxacin
□
□
□
□
49.4
Ethambutol
□ □
□
□
49.14 Ofloxacin
□ □
□
□
49.5
Streptomycin
□
□
□
□
49.15 Moxifloxacin
□
□
□
□
49.6
Rifabutin
□
□
□
□
49.16
Other
Quinolones
□
□
□
□
49.7
Rifapentine
□ □
□
□
49.17 Cycloserine
□ □
□
□
49.8
Ethionamide
□
□
□
□
□
□
□
□
49.9
Amikacin
□
□
□
□
49.18
Para-Amino
Salicylic
Acid
49.10
Kanamycin
□
□
□
□
49.19 Other
□
□
□
□
Specify ____________________________________________
49.20
Other
□
□
□
□
Specify ____________________________________________
202

203
Appendix A
Appendix AAppendix A
Appendix A
Tuberculosis Case Definition for Public Health Surveillance
Tuberculosis Case Definition for Public Health SurveillanceTuberculosis Case Definition for Public Health Surveillance
Tuberculosis Case Definition for Public Health Surveillance
(Revised May 13, 2009)
Clinical description
A chronic bacterial infection caused by Mycobacterium tuberculosis, usually characterized
pathologically by the formation of granulomas. The most common site of infection is the lung,
but other organs may be involved.
Clinical case definition
A case that meets all of the following criteria:
• A positive tuberculin skin test result or positive interferon gamma release assay for M.
tuberculosis
• Other signs and symptoms compatible with tuberculosis (TB) (e.g., abnormal chest
radiograph, abnormal chest computerized tomography scan or other chest imaging study,
or clinical evidence of current disease)
• Treatment with two or more anti-TB medications
• A completed diagnostic evaluation
Laboratory criteria for diagnosis
• Isolation of M. tuberculosis complex from a clinical specimen,
*
or
• Demonstration of M. tuberculosis complex from a clinical specimen by nucleic acid
amplification test,
†
or
• Demonstration of acid-fast bacilli in a clinical specimen when a culture has not been or
cannot be obtained or is falsely negative or contaminated.
Case classification
Confirmed: a case that meets the clinical case definition or is laboratory confirmed
Comment
A case should not be counted twice within any consecutive 12-month period. However, a case
occurring in a patient who had previously had verified TB disease should be reported and
counted again if more than 12 months have elapsed since the patient completed therapy. A case
should also be reported and counted again if the patient was lost to supervision for greater than
12 months and TB disease can be verified again. Mycobacterial diseases other than those caused
by M. tuberculosis complex should not be counted in tuberculosis morbidity statistics unless
there is concurrent tuberculosis.
______________________________
*
Use of rapid identification techniques for M. tuberculosis (e.g., DNA probes and mycolic acid high-pressure liquid
chromatography performed on a culture from a clinical specimen) are acceptable under this criterion.
†
Nucleic acid amplification (NAA) tests must be accompanied by culture for mycobacteria species for clinical
purposes. A culture isolate of M. tuberculosis complex is required for complete drug susceptibility testing and also
genotyping. However, for surveillance purposes, CDC will accept results obtained from NAA tests approved by the
Food and Drug Administration (FDA) and used according to the approved product labeling on the package insert,
or a test produced and validated in accordance with applicable FDA and Clinical Laboratory Improvement
Amendments (CLIA) regulations.
204

205
Appendix B
Appendix BAppendix B
Appendix B
Recommendations for Reporting and Counting Tuberculosis Cases
Recommendations for Reporting and Counting Tuberculosis CasesRecommendations for Reporting and Counting Tuberculosis Cases
Recommendations for Reporting and Counting Tuberculosis Cases
(Revised May 13, 2009)
Since publication of the “Recommendations for Counting Reported Tuberculosis Cases”
1
in
July 1997, numerous changes have occurred, and many issues have been raised within the
field of tuberculosis (TB) surveillance. This current version updates and supersedes the
previous version.
A distinction should be made between reporting TB cases to a health department and
counting TB cases for determining incidence of disease. Throughout each year, TB cases
and suspected cases are reported to public health authorities by sources such as clinics,
hospitals, laboratories, and health care providers. From these reports, the state or local TB
control officer must determine which cases meet the current surveillance definition for TB
disease and whether the case is countable. These countable TB cases are then reported to the
Centers for Disease Control and Prevention (CDC).
Beginning in 2009, state and local TB control officers may also report to CDC those TB
cases that are verified but not countable for morbidity statistics, as a measure of
programmatic and case management burden. The noncountable report can include persons
with TB disease recurring within a consecutive 12-month period after the patient completed
TB therapy.
I. Reporting TB Cases. CDC recommends that health care providers and laboratories
be required to report all TB cases or suspected cases to state and local health
departments based on the current “Tuberculosis Case Definition for Public Health
Surveillance” (Appendix A). This notification is essential in order for TB programs to
• Ensure case supervision
• Ensure completion of appropriate therapy
• Ensure completion of contact investigations
• Evaluate program effectiveness
• Assess trends and characteristics of TB morbidity
II. TB Surveillance. For purposes of surveillance, a case of TB is defined on the basis of
laboratory or clinical evidence of active disease due to M. tuberculosis complex.*
________________________________
* Because most laboratories use tests that do not routinely distinguish Mycobacterium tuberculosis from very
closely related species, these laboratories report culture results as being positive or negative for “Mycobacterium
tuberculosis complex.” Although in almost all cases of human disease, isolates in the M. tuberculosis complex are,
in fact, M. tuberculosis, other species are possible. For example, one study in San Diego found that 6% of human
tuberculosis was caused by Mycobacterium bovis; cultures from these cases would be reported by most laboratories
as being positive for M. tuberculosis complex. Other species in the Mycobacterium tuberculosis complex include M.
africanum, M. microti, M. canetii, M. caprae, and M. pinnipedii. Although M. microti, M. canetii, M. caprae, and
M. pinnipedii are newly described species, their inclusion in M. tuberculosis complex should not impact public
health laboratories or programs, because only a few laboratories identify to the species level. These seven species
are almost identical in DNA homology studies. In terms of their ability to cause clinical disease or be transmissible
from person to person, M. bovis, M. africanum, M. microti, M. canetii, M. caprae, and M. pinnipedii behave like M.
tuberculosis; therefore, disease caused by any of the organisms should be reported as TB, using the Report of
206
Verified Case of Tuberculosis (RVCT). The only exception is the BCG strain of M. bovis, which may be isolated
from persons who have received the vaccine for protection against TB or as cancer immunotherapy; disease caused
by the BCG strain of M. bovis should not be reported as TB.
a. Laboratory Case Definition
• Isolation of M. tuberculosis complex from a clinical specimen. The use of rapid
identification techniques for M. tuberculosis performed on a culture from a clinical
specimen, such as DNA probes and high-pressure liquid chromatography (HPLC), is
acceptable under this criterion.
OR
• Demonstration of M. tuberculosis from a clinical specimen by nucleic acid
amplification (NAA) test. NAA tests must be accompanied by cultures of
mycobacterial species. However, for surveillance purposes, CDC will accept results
obtained from NAA tests approved by the Food and Drug Administration (FDA) and
used according to the approved product labeling on the package insert, or a test
produced and validated in accordance with applicable FDA and Clinical Laboratory
Improvement Amendments (CLIA) regulations.
OR
• Demonstration of acid-fast bacilli (AFB) in a clinical specimen when a culture has
not been or cannot be obtained or is falsely negative or contaminated; historically this
criterion has been most commonly used to diagnose TB in the postmortem setting.
b. Clinical Case Definition. In the absence of laboratory confirmation of M. tuberculosis
complex after a diagnostic process has been completed, persons must have all of the
following criteria for clinical TB:
• Evidence of TB infection based on a positive tuberculin skin test result or positive
interferon gamma release assay for M. tuberculosis
AND
• One of the following:
(1) Signs and symptoms compatible with current TB disease, such as an
abnormal chest radiograph or abnormal chest computerized tomography scan or
other chest imaging study,
OR
(2) Clinical evidence of current disease (e.g., fever, night sweats, cough, weight
loss, hemoptysis)
AND
• Current treatment with two or more anti-TB medications

207
NOTE: The software for TB surveillance developed by CDC includes a calculated variable
called “Vercrit,” for which one of the values is “Provider Diagnosis.” “Provider Diagnosis” is
selected when the user chooses to override a “Suspect” default value in the case verification
screen as “Verified by Provider Diagnosis.” Thus, “Provider Diagnosis” is not a component of
the case definition for TB in the current “Tuberculosis Case Definition for Public Health
Surveillance” (Appendix A). CDC’s national morbidity reports have traditionally included all
TB cases that are considered verified by the reporting areas, without a requirement that cases
meet the published case definition.
III. Counting TB Cases. Cases that meet the current CDC surveillance case
definition for verified TB are counted by 52 reporting areas with count authority
(50 states, District of Columbia, and New York City) to determine annual
incidence for the United States. The remaining 8 reporting areas (American
Samoa, Federated States of Micronesia, Guam, Marshall Islands, Northern
Mariana Islands, Puerto Rico, Republic of Palau, and U.S. Virgin Islands) report
cases to CDC but are not included in the annual incidence for the United States.
The laboratory and clinical case definitions are the two diagnostic categories used
in the CDC “Tuberculosis Case Definition for Public Health Surveillance.”
Most verified TB cases are accepted for counting based on laboratory
confirmation of M. tuberculosis complex from a clinical specimen.
A person may have more than one discrete (separate and distinct) episode of
TB. If disease recurs in a person within any 12-consecutive-month period after
the patient completed therapy, count only one episode as a case. However, if TB
disease recurs in a person, and if more than 12 months have elapsed since the
person completed TB therapy or was lost to supervision, the TB case is
considered a separate episode and should be counted as a new case.
Mycobacterial diseases other than those caused by M. tuberculosis complex
should not be counted in TB morbidity statistics unless there is concurrent TB.
a. Verified TB Cases
COUNT
Count only verified TB cases that meet the laboratory or clinical case
definitions (see Section II). The diagnosis of TB must be verified by the TB
control officer or designee. The current CDC surveillance case definition for
TB describes and defines the criteria to be used in the case definition for TB
disease.
DO NOT COUNT
If diagnostic procedures have not been completed, do not count; wait for
confirmation of disease. Do not count as a case the patient for which two or
more anti-TB medications have been prescribed for preventive therapy for
exposure to multidrug- resistant (MDR) TB, or while the diagnosis is still
pending.
208
b. Nontuberculous Mycobacterial Diseases (NTM)
COUNT
An episode of TB disease diagnosed concurrently with another nontuberculous
mycobacterial disease should be counted as a TB case.
DO NOT COUNT
Disease attributed to or caused by nontuberculous mycobacteria alone should
not be counted as a TB case.
c. TB Cases Reported at Death
COUNT
TB cases first reported to the health department at the time of a person’s death
are counted as incident cases, provided the person had current disease at the
time of death. The TB control officer should verify the diagnosis of TB.
DO NOT COUNT
Do not count as a case of TB if there is no evidence of current disease at the
time of death or at autopsy.
d. Immigrants, Refugees, Permanent Resident Aliens, Border Crossers,* and
Foreign Visitors
2
COUNT
Immigrants and refugees who are examined after arriving in the United States
and diagnosed with clinically active TB requiring anti-TB medications should
be reported and counted by the locality of their current residence at the time of
diagnosis regardless of citizenship status.
Border crossers* who are diagnosed with TB and plan to receive anti-TB
therapy from a locality in the United States for 90 days or more should be
reported and counted by the locality where they receive anti-TB therapy.
Foreign visitors (e.g., students, commercial representatives, and diplomatic
personnel) who are diagnosed with TB, are receiving anti-TB therapy, and have
been, or plan to remain in, the United States for 90 days or more should be
reported and counted by the locality of current residence.
*Border crosser — defined, by the U.S. Citizenship and Immigration Services
(USCIS)
2
as “an alien resident of the United States reentering the country after
an absence of less than six months in Canada or Mexico, or a nonresident alien
entering the United States across the Canadian border for stays of no more than
six months, or across the Mexican border for stays of no more than 72 hours.”
Border crossers may go back and forth across the border many times in a short
period.
209
DO NOT COUNT
Any person who was diagnosed and started on anti-TB drugs in another country
should not be counted as a new case but should be reported as a verified
noncountable TB case.
Border crossers* and foreign visitors who are diagnosed with TB and receive
anti-TB therapy from a locality in the United States for less than 90 days but
plan to return to their native country to continue therapy should not be reported
or counted by the locality where they receive anti-TB therapy.
e. Out-of-State or Out-of-Area Residents
COUNT
A person’s TB case should be counted by the locality in which he or she resides
at the time of diagnosis. TB in a person who has no address should be counted
by the locality that diagnosed and is treating the TB. The TB control officer
should notify the appropriate out-of-state or out-of-area TB control officer of
the person’s home locality to (1) determine whether the case has already been
counted to avoid “double counting,” and (2) agree on which TB control office
should count the case if it has not yet been counted.
DO NOT COUNT
Do not count a case in a newly diagnosed TB patient who is an out-of-area
resident and whose TB has already been counted by the out-of-area TB control
office.
f. Migrants and Other Transients
COUNT
Persons without any fixed U.S. residence are considered to be the public health
responsibility of their present locality and their TB case should be reported and
counted where diagnosed.
DO NOT COUNT
Cases in transient TB patients should not be counted when there is evidence that
they have already been counted by another locality.
g. Federal Facilities (e.g., Military and Veterans Administration Facilities)
COUNT
Cases in military personnel, dependents, or veterans should be reported and
counted by the locality where the persons are residing in the United States at the
time of diagnosis and initiation of treatment.
However, if military personnel or dependents are discovered to have TB at a
military base outside the United States but are referred elsewhere for treatment
(e.g., a military base located within the United States), the TB case should be
reported and counted where treated and not where the diagnosis was made.
210
DO NOT COUNT
Do not count if the case was already counted by another locality in the United
States.
h. Indian Health Service
COUNT
TB should be reported to the local health authority (e.g., state or county) and
counted where diagnosed and treatment initiated. However, for a specific group
such as the Navajo Nation, which is geographically located in multiple states,
health departments should discuss each case and determine which locality
should count the case.
DO NOT COUNT
Do not count if the case was already counted by another locality.
i. Correctional Facilities (e.g., Local, State, Federal, and Military)
COUNT
Persons who reside in local, state, federal, or military correctional facilities may
frequently be transferred or relocated within and/or between various
correctional facilities. TB in these persons should be reported to the local health
authority and counted by the locality where the diagnosis was made and
treatment plans were initiated.
DO NOT COUNT
Do not count correctional facility residents’ TB cases that were counted
elsewhere by another locality or correctional facility, even if treatment
continues at another locale or correctional facility.
j. Peace Corps, Missionaries, and Other Citizens Residing Outside the United
States
DO NOT COUNT
TB in persons diagnosed outside the United States should not be counted. TB in
these persons should be counted by the country in which they are residing,
regardless of their plans to return to the United States for further work-up or
treatment.
IV. Suggested Administrative Practices
To promote uniformity in TB case counting, the following administrative
procedures are recommended:
(a) All TB cases verified by the 52 reporting areas with count authority (50 states,
District of Columbia, and New York City) during the calendar year (by
December 31) will be included in the annual U.S. incidence count for that
year. All tuberculosis cases verified during the calendar year by a reporting
area with count authority from one of the remaining 8 reporting areas
(American Samoa, Federated States of Micronesia, Guam, Marshall Islands,
211
Northern Mariana Islands, Puerto Rico, Republic of Palau, and U.S. Virgin
Islands) are also counted but are not included in the annual incidence for the
United States. Cases for which bacteriologic results are pending or for which
confirmation of disease is questionable for any other reason should not be
counted until their status is clearly determined; they should be counted at the
time they meet the criteria for counting. This means that a case reported in one
calendar year could be included in the morbidity count for the following year.
The reporting area with count authority should ensure that there is agreement
between final local and state TB figures reported to CDC. Currently, some
reporting areas may not use this suggested protocol. Some of these areas may
wait until the beginning of the following year when they have received and
processed all of the TB cases for inclusion in the annual case count for the
previous year. If reporting areas decide to revise their protocols, they should
be aware that their TB trends may change.
(b) TB is occasionally reported to health departments over the telephone, by letter
or fax, or on forms other than the Report of Verified Case of Tuberculosis
(RVCT). Such information should be accepted as an official morbidity report
if sufficient details are provided; otherwise, the notification should be used as
an indicator of a possible TB case (suspect) which should be investigated
promptly for confirmation.
V. TB Surveillance Definitions
Case - an episode of TB disease in a person meeting the laboratory or clinical
criteria for TB as defined in the document “Tuberculosis Case Definition for
Public Health Surveillance” (see Section II for criteria).
Suspect - a person for whom there is a high index of suspicion for active TB
(e.g., a known contact to an active TB case or a person with signs or symptoms
consistent with TB) who is currently under evaluation for TB disease.
Verification of a TB case - the process whereby a TB case, after the diagnostic
evaluation is complete, is reviewed at the local level (e.g., state or county) by a
TB control official who is familiar with TB surveillance definitions; if all the
criteria for a TB case are met, the TB case is then verified and eligible for
counting.
Counting of a TB case - the process whereby a reporting area with count
authority evaluates verified TB cases against count criteria (e.g., assesses for
case duplication). These cases are then counted for morbidity in that locality
(e.g., state or county) and reported to CDC for national morbidity counting.
Noncountable, verified cases may also be sent to CDC.
Mycobacterium tuberculosis complex (M. tuberculosis complex) - Because
most laboratories use tests that do not routinely distinguish Mycobacterium
tuberculosis from very closely related species, these laboratories report culture
results as being positive or negative for “Mycobacterium tuberculosis complex.”
Although in almost all cases of human disease, isolates in the M. tuberculosis
complex are, in fact, M. tuberculosis, other species are possible. For example,
212
one study in San Diego found that 6% of human tuberculosis was caused by
Mycobacterium bovis; cultures from these cases would be reported by most
laboratories as being positive for M. tuberculosis complex. Other species in the
Mycobacterium tuberculosis complex include M. africanum, M. microti, M.
canetii, M. caprae, and M. pinnipedii. Although M. microti, M. canetii, M.
caprae, and M. pinnipedii are newly described species, their inclusion in M.
tuberculosis complex should not impact public health laboratories or programs
because only a few laboratories identify to the species level. These seven
species are almost identical in DNA homology studies. In terms of their ability
to cause clinical disease or be transmissible from person to person, M. bovis, M.
africanum, M. microti, M. canetti, M. caprae, and M. pinnipedii behave like M.
tuberculosis; therefore, disease caused by any of the organisms should be
reported as TB, using the Report of Verified Case of Tuberculosis (RVCT). The
only exception is the BCG strain of M. bovis, which may be isolated from
persons who have received the vaccine for protection against TB or as cancer
immunotherapy; disease caused by the BCG strain of M. bovis should not be
reported as TB.
Nontuberculous mycobacteria (NTM) - mycobacteria other than
Mycobacterium tuberculosis complex that can cause human infection or disease.
Common nontuberculous mycobacteria include M. avium complex or MAC (M.
avium, M. intracellulare), M. kansasii, M. marinum, M. scrofulaceum, M.
chelonae, M. fortuitum, and M. simiae. Other terms have been used to represent
NTM, including MOTT (mycobacteria other than TB) and “atypical”
mycobacteria.
Reporting area - areas responsible for counting and reporting verified TB cases
to CDC. Currently there are 60 reporting areas: the 50 states, District of
Columbia, New York City, American Samoa, Federated States of Micronesia,
Guam, Marshall Islands, Northern Mariana Islands, Puerto Rico, Republic of
Palau, and U.S. Virgin Islands. The annual incidence of tuberculosis for the
United States is based on 52 reporting areas (the 50 states, District of Columbia,
and New York City).
Alien - defined by the U.S. Citizenship and Immigration Services (USCIS)
2
as
“any person not a citizen or national of the United States.”
Border crosser - defined, by the U.S. Citizenship and Immigration Services
(USCIS)
2
as “an alien resident of the United States reentering the country after
an absence of less than six months in Canada or Mexico, or a nonresident alien
entering the United States across the Canadian border for stays of no more than
six months, or across the Mexican border for stays of no more than 72 hours.”
Border crossers may go back and forth across the border many times in a short
period.
Class A TB with waiver
3
All applicants who have tuberculosis disease and have been granted a waiver.
213
Class B1 TB, Pulmonary
3
No treatment
• Applicants who have medical history, physical exam, HIV, or CXR
findings suggestive of pulmonary TB but have negative AFB sputum
smears and cultures and are not diagnosed with TB or can wait to have
TB treatment started after immigration.
Completed treatment
• Applicants who were diagnosed with pulmonary TB and successfully
completed directly observed therapy prior to immigration. The cover
sheet should indicate if the initial sputum smears and cultures were
positive and if drug susceptibility testing results are available.
Class B1 TB, Extrapulmonary
3
Applicants with evidence of extrapulmonary TB. Document the anatomic site of
infection.
Class B2 TB, Latent TB Infection (LTBI) Evaluation
3
Applicants who have a tuberculin skin test ≥10 mm but otherwise have a
negative evaluation for TB. The size of the TST reaction, the applicant’s status
with respect to LTBI treatment, and the medication(s) used should be
documented. For applicants who had more than one TST, whether the applicant
converted the TST should be documented (i.e., initial TST <10 mm but
subsequent TST ≥10 mm).
Class B3 TB, Contact Evaluation
3
Applicants who are a recent contact of a known tuberculosis case. The size of
the applicant’s TST reaction should be documented. Information about the
source case, name, alien number, relationship to contact, and type of
tuberculosis should also be documented.
Immigrant - defined by the USCIS
2
as “an alien admitted to the United States
as a lawful permanent resident. Immigrants are those persons lawfully accorded
the privilege of residing permanently in the United States. They may be issued
immigrant visas by the Department of State overseas or adjusted to permanent
resident status by the USCIS of the United States.”
Permanent Resident Alien - see Immigrant.

214
Waivers
3
- A provision allows applicants undergoing pulmonary or laryngeal
tuberculosis treatment to petition for a Class A TB with waiver. Waivers should
be pursued for any immigrant or refugee who has a complicated clinical course
and would benefit from receiving treatment of their tuberculosis in the United
States. Applicants diagnosed with tuberculosis disease who are both smear- and
culture-negative and will be traveling to the United States prior to start of
treatment do not need to complete the waiver process.
References
1. Recommendations for Counting Reported TB Cases. Atlanta: CDC, July
1997.
2. U.S. Department of Homeland Security, U.S. Citizenship and Immigration
Services; http://uscis.gov. Accessed March 2009.
3. 2007 Technical Instructions for Tuberculosis Screening and Treatment for
Panel Physicians. Atlanta: CDC, Division of Global Migration and
Quarantine. http://www.cdc.gov/ncidod/dq/panel_2007.htm. Accessed
March 2009.
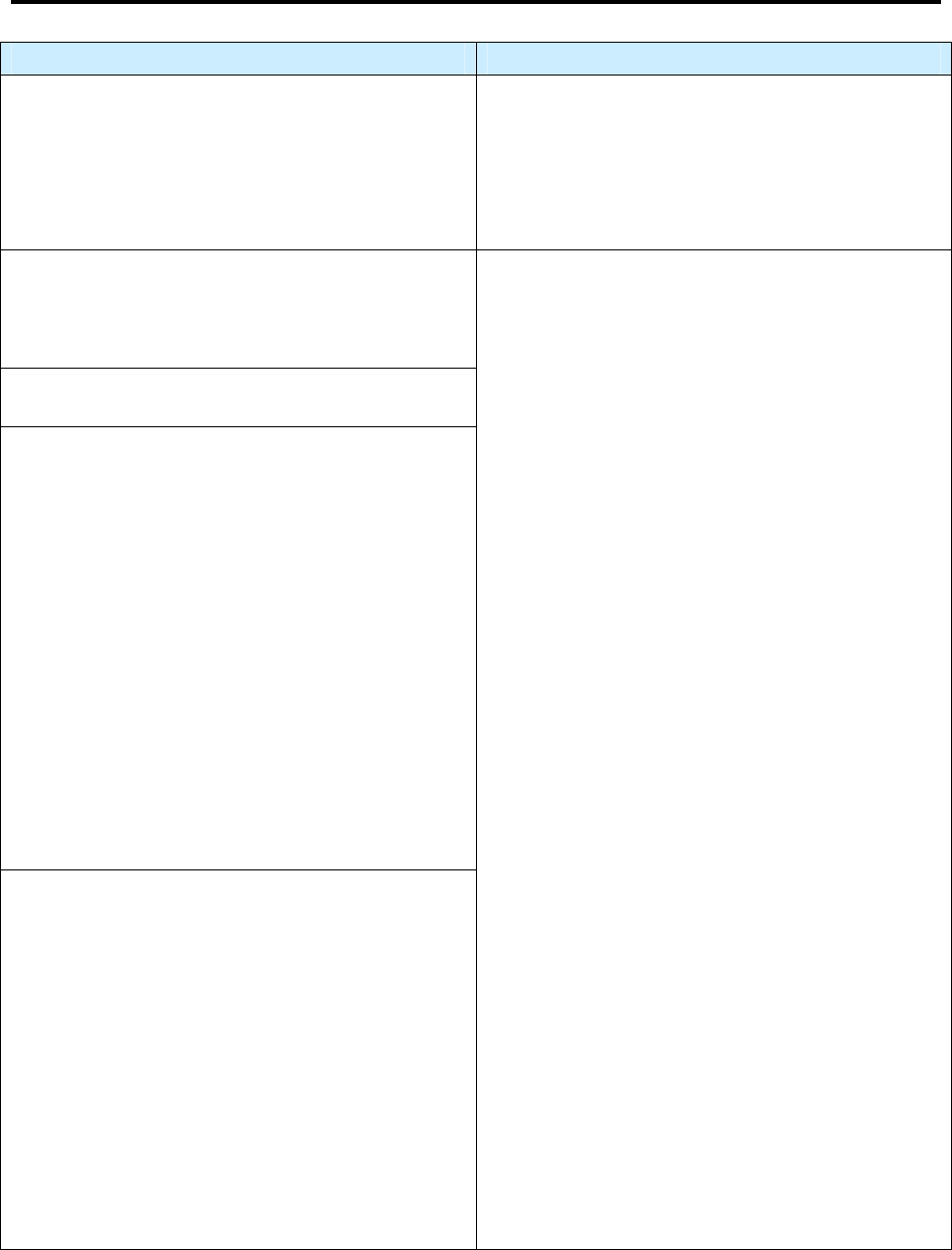
215
Appendix C
Appendix CAppendix C
Appendix C
Anatomic Codes
Anatomic CodesAnatomic Codes
Anatomic Codes
Anatomic Code Anatomic Code
Dermal System
00 * Skin and skin appendages
01 * Subcutaneous Tissue
02 * Breast
03 Milk
Cardiovascular System
30 * Pericardium
31 * Heart
32 * Cardiac valve
33 Pericardial fluid
34 * Blood vessel
Hematopoietic System
04 * Bone marrow
05 * Spleen
06 * Blood
Lymphatic System
07 Lymph node
Musculoskeletal System
08 Bone, NOS (Not Otherwise Specified)
09 Skeletal system (Bones of head, ribcage,
and vertebral column)
10 Skeletal system (Bones of shoulder,
girdle, pelvis, and extremities)
11 Soft tissue, NOS (Not Otherwise
Specified)
12 Soft tissue (Muscles of head, neck,
mouth, and upper extremity)
13 Soft tissue (Muscles of trunk, perineum,
and lower extremity)
14 Tendon and tendon sheath
15 Ligament and fascia
16 Joints (Synovial tissue)
17 Synovial fluid
Respiratory System
18 * Nose
19 * Accessory Sinus
20 * Nasopharynx
21 * Epiglottis
22 * Trachea
23 Bronchus
24 Bronchiole
25 Lung
26 Pleura
27 Upper respiratory fluids or tracheal fluids
28 Bronchial fluid
29 Pleural fluid
Gastrointestinal System
35 * Mouth
36 * Lip
37 * Tongue
38 * Tooth, gum, and supporting structures
of the tooth
39 * Salivary gland
40 * Liver
41 * Gallbladder
42 * Extrahepatic bile duct
43 * Pancreas
44 Saliva
45 Bile and pancreatic fluid
46 * Pharynx, oropharynx, and hypopharynx
47 * Tonsils and adenoids
48 * Esophagus
49 * Stomach
50 * Small intestine - duodenum
51 * Small intestine - jejunum & ileum
52 * Appendix
53 * Colon
54 * Rectum
55 * Anus
56 Gastric aspirate
57 Gastrointestinal contents (feces)
58 Omentum and peritoneum
59 Peritoneal fluid
* Only codes marked with an asterisk (*) should be used when a Site of Disease (item 16) is Other.
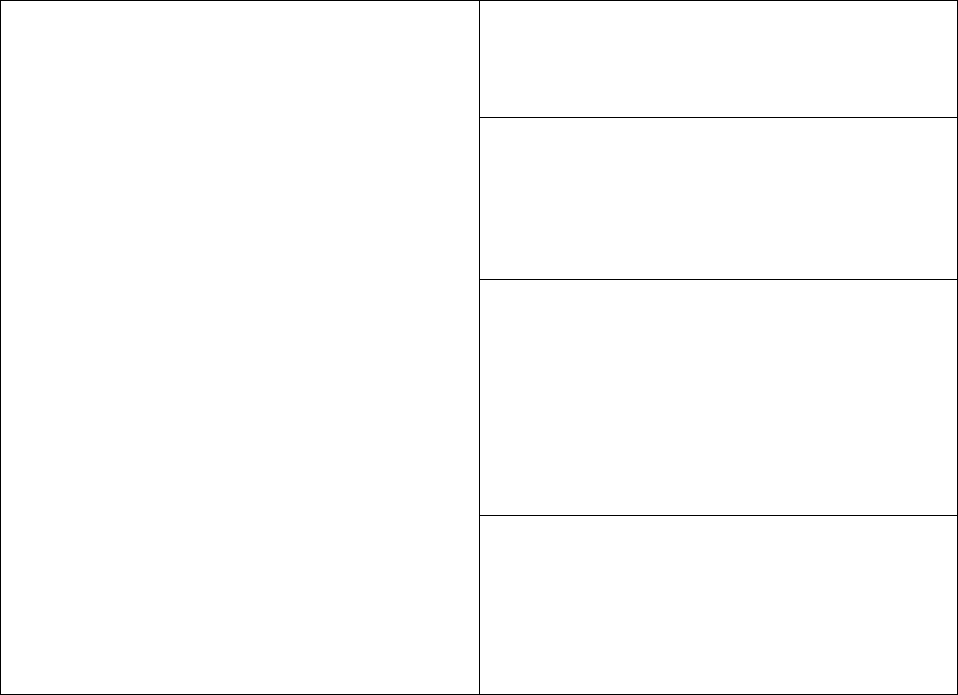
216
Fetal Structures
80 * Placenta, umbilical cord, and
implantation site
81 * Fetus and embryo
Endocrine System
82 * Pituitary gland
83 * Adrenal gland
84 * Thyroid or parathyroid gland(s)
85 * Thymus
Neurological System
86 CSF (Cerebral spinal fluid)
87 Meninges, dural sinus, choroid plexus
88 * Brain
89 * Spinal cord
90 * Cranial, spinal, and peripheral nerve
91 * Eye and ear appendages
92 * Ear and mastoid cells
Urogenital System
60 Kidney
61 Renal pelvis
62 Ureter
63 Urinary bladder
64 Urethra
65 Penis
66 Prostate and seminal vesicle
67 Testis
68 Epididymis, vas deferens, spermatic
cord, and scrotum
69 Urine
70 Male genital fluids
71 Vulva, labia, clitoris, and Bartholin's
gland
72 Vagina
73 Uterus
74 Cervix
75 Endometrium
76 Myometrium
77 Fallopian tube, broad ligament,
parametrium, and parovarian region
78 Ovary
79 Female genital fluids
Other
93 Pus
94 * Other
95 Multiple Sites
99 Unknown
* Only codes marked with an asterisk (*) should be used when a Site of Disease (item 16) is Other.
217
Appendix D
Appendix DAppendix D
Appendix D
Reporting Area Codes
Reporting Area CodesReporting Area Codes
Reporting Area Codes
Reporting Area Codes
Name Alpha Code Name Alpha Code
Alabama AL 01 Nebraska NE 31
Alaska AK 02 Nevada NV 32
Arizona AZ 04 New Hampshire NH 33
Arkansas AR 05 New Jersey NJ 34
California CA 06 New Mexico NM 35
Colorado CO 08 New York NY 36
Connecticut CT 09 New York City NO 975772
Delaware DE 10 North Carolina NC 37
Florida FL 12 North Dakota ND 38
Georgia GA 13 Ohio OH 39
Hawaii HI 15 Oklahoma OK 40
Idaho ID 16 Oregon OR 41
Illinois IL 17 Pennsylvania PA 42
Indiana IN 18 Rhode Island RI 44
Iowa IA 19 South Carolina SC 45
Kansas KS 20 South Dakota SD 46
Kentucky KY 21 Tennessee TN 47
Louisiana LA 22 Texas TX 48
Maine ME 23 Utah UT 49
Maryland MD 24 Vermont VT 50
Massachusetts MA 25 Virginia VA 51
Michigan MI 26 Washington WA 53
Minnesota MN 27 Washington D.C. DC 11
Mississippi MS 28 West Virginia WV 54
Missouri MO 29 Wisconsin WI 55
Montana MT 30 Wyoming WY 56
U.S. Island Reporting Area Codes
For information on citizenship and “U.S.-born” for the U.S. Island Areas see Country of Birth (item 12)
Name Alpha
Code Name Alpha
Code
American Samoa AQ 60 Palau PS 70
Federated States of
Micronesia
FM 64 Puerto Rico PR 72
Guam GU 66 Republic of Marshall
Islands
RM 68
Northern Mariana
Islands
CQ 69 Virgin Islands VQ 78
218
219
Appendix E
Appendix EAppendix E
Appendix E
Country Codes
Country CodesCountry Codes
Country Codes
Country Alpha Code
Afghanistan AFG
Albania ALB
Algeria DZA
American Samoa ASM
Andorra AND
Angola AGO
Anguilla AIA
Antarctica ATA
Antigua and Barbuda ATG
Argentina ARG
Armenia ARM
Aruba ABW
Ashmore and Cartier Islands AT
Australia AUS
Austria AUT
Azerbaijan AZE
Bahamas, The BHS
Bahrain BHR
Baker Island FQ
Bangladesh BGD
Barbados BRB
Bassas Da India BS
Belarus BLR
Belgium BEL
Belize BLZ
Benin BEN
Bermuda BMU
Bhutan BTN
Bolivia BOL
Bosnia and Herzegovina BIH
Botswana BWA
Bouvet Island BVT
British Indian Ocean Territory IOT
Brazil BRA
British Virgin Islands VGB
Brunei BRN
Bulgaria BGR
Burkina (Upper Volta) BFA
Burma BUMM
Burundi BDI
Cambodia KHM
Cameroon CMR
Canada CAN
Cape Verde CPV
Cayman Islands CYM
220
Country Alpha Code
Central African Republic CAF
Chad TCD
Chile CHL
China CHN
Christmas Island CXR
Clipperton Island IP
Cocos (Keeling) Islands CCK
Colombia COL
Comoros COM
Congo COG
Cook Islands COK
Coral Sea Islands CR
Costa Rica CRI
Croatia HRV
Cuba CUB
Cyprus CYP
Czech Republic CZE
Czechoslovakia CSHH
Denmark DNK
Djibouti DJI
Dominica DMA
Dominican Republic DOM
Ecuador ECU
Egypt EGY
El Salvador SLV
Equatorial Guinea GNQ
Eritrea ERI
Estonia EST
Ethiopia ETH
Europa Island EU
Falkland Islands (Malvinas) FLK
Faroe Islands FRO
Federated States of Micronesia FSM
Fiji FJI
Finland FIN
French Southern and Antarctic Lands ATF
France FRA
French Guiana GUF
French Polynesia PYF
Gabon GAB
Gambia, The GMB
Gaza Strip GZ
Georgia GEO
Germany DEU
Ghana GHA
Gibraltar GIB
Glorioso Islands GO
Greece GRC
Greenland GRL
Grenada GRD
221
Country Alpha Code
Guadeloupe GLP
Guam GUM
Guatemala GTM
Guernsey GGY
Guinea GIN
Guinea-Bissau GNB
Guyana GUY
Haiti HTI
Heard Island and McDonald Islands HMD
Honduras HND
Hong Kong HKG
Howland Island HQ
Hungary HUN
Iceland ISL
India IND
Indonesia IDN
Iran IRN
Iraq IRQ
Iraq-Saudi Arabia Neutral Zone NTHH
Ireland IRL
Israel ISR
Italy ITA
Ivory Coast CIV
Jamaica JAM
Jan Mayen JN
Japan JPN
Jarvis Island DQ
Jersey JEY
Johnston Atoll JQ
Jordan JOR
Juan De Nova Island JU
Kazakhstan KAZ
Kenya KEN
Kingman Reef KQ
Kiribati KIR
Korea, Republic of KOR
Korea, Democratic People’s Republic PRK
Kuwait KWT
Kyrgyzstan KGZ
Laos LAO
Latvia LVA
Lebanon LBN
Lesotho LSO
Liberia LBR
Libya LBY
Liechtenstein LIE
Lithuania LTU
Luxembourg LUX
Macau MAC
Macedonia MKD
222
Country Alpha Code
Madagascar MDG
Malawi MWI
Malaysia MYS
Maldives MDV
Mali MLI
Malta MLT
Man, Isle of IMN
Marshall Islands MHL
Martinique MTQ
Mauritania MRT
Mauritius MUS
Mayotte MYT
Mexico MEX
Midway Island MIUM
Moldova MDA
Monaco MCO
Mongolia MNG
Montenegro MNE
Montserrat MSR
Morocco MAR
Mozambique MOZ
Myanmar MMR
Namibia NAM
Nauru NRU
Navassa Island BQ
Nepal NPL
Netherlands NLD
Netherlands Antilles ANT
New Caledonia NCL
New Zealand NZL
Nicaragua NIC
Niger NER
Nigeria NGA
Niue NIU
Norfolk Island NFK
Northern Mariana Islands MNP
Norway NOR
Not Specified NI
Oman OMN
Pakistan PAK
Palau PLW
Palmyra Atoll LQ
Panama PAN
Papua New Guinea PNG
Paracel Islands PF
Paraguay PRY
Peru PER
Philippines PHL
Pitcairn Islands PCN
Poland POL
223
Country Alpha Code
Portugal PRT
Portuguese Timor TPTL
Puerto Rico PRI
Qatar QAT
Reunion REU
Romania ROU
Russia RUS
Rwanda RWA
South Georgia/South Sandwich Islands SGS
San Marino SMR
Sao Tome and Principe STP
Saudi Arabia SAU
Senegal SEN
Serbia SRB
Seychelles SYC
Sierra Leone SLE
Singapore SGP
Slovak Republic SVK
Slovenia SVN
Solomon Islands SLB
Somalia SOM
South Africa ZAF
Soviet Union SUHH
Spain ESP
Spratly Islands PG
Sri Lanka LKA
St Lucia LCA
St. Helena SHN
St. Kitts and Nevis KNA
St. Pierre and Miquelon SPM
St. Vincent/Grenadines VCT
Sudan SDN
Suriname SUR
Svalbard SJM
Swaziland SWZ
Sweden SWE
Switzerland CHE
Syria SYR
Taiwan TWN
Tajikistan TJK
Tanzania, United Republic of TZA
Thailand THA
Timor-Leste TLS
Togo TGO
Tokelau TKL
Tonga, Kingdom of TON
Trinidad and Tobago TTO
Tromelin Island TE
Tunisia TUN
Turkey TUR
224
Country Alpha Code
Turkmenistan TKM
Turks and Caicos Islands TCA
Tuvalu TUV
U.S. Minor Outlying Islands UMI
Uganda UGA
Ukraine UKR
United Arab Emirates ARE
United Kingdom GBR
United States USA
Uruguay URY
U.S. Miscellaneous Pacific Islands PUUM
Uzbekistan UZB
Vanuatu (New Hebrides) VUT
Vatican City VAT
Venezuela VEN
Vietnam VNM
Virgin Islands VIR
Wake Island WKUM
Wallis and Futuna WLF
West Bank WE
Western Sahara ESH
Western Samoa WSM
Yemen YEM
Yugoslavia YUCS
Zaire ZRCD
Zambia ZMB
Zimbabwe ZWE
225
Appendix F
Appendix FAppendix F
Appendix F
Glossary
GlossaryGlossary
Glossary
Term Definition
Acid-fast bacilli (AFB)
Microorganisms that when stained, retain color even after they have been
washed in an acid solution; may be detected under a microscope in a stained
smear.
Active case finding
Looking for undiagnosed cases by screening a population.
Active TB disease
An illness, caused by bacteria called Mycobacterium tuberculosis, in which
tuberculosis (TB) bacteria are multiplying and attacking parts of the body,
most commonly the lungs. A person with active TB disease is capable of
spreading the disease to others if the TB bacteria are active in the lungs or
throat. The symptoms of active TB disease include weakness, weight loss,
fever, no appetite, chills, and sweating at night. Other symptoms may
include a bad cough, pain in the chest, and coughing up blood.
Adherence to treatment
Following the recommended course of treatment by taking all the
prescribed medications for the entire length of time necessary.
Adverse effect
Negative side effect resulting from the use of a drug (for example, hepatitis,
nausea, headache).
Bronchoscopy
A procedure used to obtain pulmonary secretions or lung tissue with an
instrument called a bronchoscope.
Case management
A system in which a specific health department employee is assigned
primary responsibility for the patient, systematic regular review of patient
progress is conducted, and plans are made to address any barriers to
adherence.
Case rate
The number of cases that occur during a certain time period, divided by the
size of the population during that time period; the case rate is often
expressed in terms of a population size of 100,000 persons.
Case reporting
Informing the state or local health department when a new case (an
occurrence) of TB disease has been diagnosed or is suspected.
Cavity
A hollow space within the lung, visible on a chest x-ray or CT scan.
Clinical evaluation
An evaluation done to find out whether a patient has symptoms of TB
disease or is responding to treatment; also done to check for adverse
reaction to TB medications.
Clinician
A physician, physician’s assistant, or nurse.
Congregate setting
A setting in which a group of usually unrelated persons reside in close
physical proximity. These settings may include hospitals, long-term care
facilities, assisted living facilities, correctional facilities, or homeless
shelters (see residential facilities).
226
Contact investigation
A procedure for interviewing a person who has TB disease to determine
who may have been exposed to TB. People who have been exposed to TB
are tested for latent TB infection (LTBI) and TB disease.
Contacts
People exposed to someone with infectious TB disease, generally including
family members, roommates or housemates, close friends, coworkers,
classmates, and others.
Country of birth
The country where a person was born.
Culture
To grow organisms on media (substances containing nutrients) so that they
or the product of this process can be identified.
Daily regimen
A treatment schedule in which the patient takes a dose of each prescribed
medication every day.
Diabetes mellitus
A disease in which the body's ability to use sugar is altered.
Diagnostic evaluation
An evaluation used to diagnose TB disease; includes a medical history, a
chest x-ray, the collection of specimens for bacteriologic examination, and
possibly a tuberculin skin test or an interferon-gamma release assay such as
the QuantiFERON
®
-TB Gold test.
Directly observed
therapy (DOT)
A designated person watches the TB patient swallow each dose of the
prescribed drugs.
Drug susceptibility test
A laboratory method for finding drug resistance in a microorganism.
Drug-resistant TB
TB caused by organisms that are able to grow in the presence of a particular
drug; TB that is resistant to at least one first-line antituberculosis drug.
End-stage renal disease
(ESRD)
A condition when chronic kidney failure has progressed to the point where
kidney function is less than 10% of normal; requires dialysis or
transplantation; also known as stage 5 chronic kidney disease. The most
common cause of ESRD in the United States is diabetes.
Ethambutol (EMB)
A drug used to treat TB disease; may cause vision problems. Ethambutol
should be used cautiously in children who are too young to be monitored for
changes in their vision.
Extrapulmonary TB
TB disease that occurs in places other than the lungs, such as the lymph
nodes, the pleura, the brain, the kidneys, or the bones; most types of
extrapulmonary TB are not infectious.
First-line TB drugs
The initial drugs used for treating TB disease. Include isoniazid (INH),
rifampin (RIF), pyrazinamide (PZA), and either ethambutol (EMB). or
streptomycin (SM).
Foreign-born persons
People born outside of the United States.
HIV
Human immunodeficiency virus, the virus that causes AIDS.
227
Immunosuppressive
therapy
Therapy that suppresses or weakens the immune system.
Interferon-gamma
(IFN-γ)
Protein that is normally produced by the body in response to infection.
Interferon-gamma
release assay (IGRA)
A type of blood test that measures a person’s immune reactivity to M.
tuberculosis by measuring release of IFN-γ. In the U.S., QuantiFERON
®
-
TB Gold, QuantiFERON
®
-TB Gold In-Tube, and T-SPOT
®
.TB are
currently available IGRAs.
Isolate
A sample from a specimen that was identified as a certain organism such as
M. tuberculosis complex.
Isoniazid (INH)
A drug that is used for treating LTBI and one of the drugs used to treat TB
disease; although relatively safe, it may cause hepatitis and other severe
adverse reaction in some patients.
Latent TB infection
(LTBI)
Refers to the condition when a person is infected with tubercle bacilli, but
TB disease has not developed. Persons with LTBI do not have TB disease
symptoms and they cannot spread TB germs to others. Persons with LTBI
usually have a positive result to the Mantoux tuberculin skin test or an
interferon-gamma release assay.
LTBI treatment
Medication that is given to people who have latent TB infection to prevent
them from developing TB disease.
Mantoux tuberculin skin
test (TST)
A method of testing for TB infection; a needle and syringe are used to inject
0.1 ml of 5 tuberculin units of liquid tuberculin between the layers of the
skin (intradermally), usually on the forearm; the reaction to this test, a
palpable swollen area (induration), is measured 48 to 72 hours after the
injection and is interpreted as positive or negative depending on the size of
the reaction and the patient’s risk factors for TB.
Miliary TB
Miliary TB is a serious type of tuberculosis infection. It is a histological or
radiologic finding, rather than a site of disease. It appears on radiograph as a
great number of small, well-defined nodules that look like millet seeds
scattered throughout the lungs, hence the name “miliary.”
Multidrug-resistant TB
(MDR TB)
Resistant to at least the drugs isoniazid and rifampin; MDR TB is more
difficult to treat than drug-susceptible TB.
Mycobacterium
tuberculosis
One of the organisms causing TB in humans, and sometimes called the
tubercle bacillus; belongs to a group of bacteria called mycobacteria.
Mycobacterium
tuberculosis complex
A group of closely related mycobacteria that can cause active TB (e.g., M.
tuberculosis, M. bovis, and M. africanum). Most TB in the United States is
caused by M. tuberculosis.
Nucleic acid
amplification (NAA)
A technique that amplifies (copies) DNA or RNA segments, in order to
directly identify microorganisms in sputum specimens.
228
Pulmonary TB
TB disease that occurs in the lungs, typically causing a cough and an
abnormal chest x-ray. Pulmonary TB is usually infectious if untreated. Most
TB cases reported in the United States are pulmonary TB.
Pyridoxine
Another name for vitamin B6; it is given to prevent peripheral neuropathy;
should always be given to pregnant and breastfeeding women on isoniazid.
QuantiFERON
®
-TB
Gold test (QFT-G)
A blood test used for diagnosing infection with M. tuberculosis. The QFT-G
measures a patient’s immune reactivity to M. tuberculosis by measuring the
response to TB proteins when they are mixed with a small amount of blood
(see IGRAs).
Recurrence
A patient who has either a
• Negative culture result while receiving anti-TB therapy, but at some
point after therapy is completed, either the culture result becomes
positive for M. tuberculosis or the patient has clinical or radiologic
deterioration that is consistent with TB disease.
or
• Negative smear and culture result (e.g., clinical case) at diagnosis
and while receiving anti-TB therapy, but at some point after therapy
is completed, either the patient has a culture result that is positive for
M. tuberculosis or has clinical or radiologic deterioration that is
consistent with TB disease.
Rifabutin
A drug used to treat TB disease; used as a substitute for rifampin (RIF) in
the treatment of all forms of TB.
Rifampin
A drug used to treat TB disease; also used for LTBI treatment. Rifampin
has several possible side effects (for example, hepatitis, turning body fluids
orange, and drug interactions).
Rifapentine
A drug used to treat TB disease; used once weekly with isoniazid during the
continuation phase with selected HIV-negative patients.
Second-line TB drugs
Drugs used to treat TB that is resistant to first-line TB drugs (for example,
capreomycin, kanamycin, ethionamide, cycloserine, ciprofloxacin,
amikacin).
Smear
A specimen that has been smeared onto a glass slide, stained, washed in an
acid solution, and then placed under the microscope for examination; used
to detect acid-fast bacilli in a specimen.
Specimen
A sample collected from a person for testing.
Sputum
Phlegm from deep in the lungs, collected in a sterile container for
processing and examination.
Susceptibility
An organism’s ability to be killed by a particular drug.
Suspect
A person for whom there is a high index of suspicion for active TB (e.g., a
known contact to an active TB case or to a person with signs or symptoms
consistent with TB) who is currently under evaluation for TB disease.

229
XDR TB
The occurrence of TB in persons whose M. tuberculosis isolates are
resistant to isoniazid and rifampin, plus resistant to any fluoroquinolone and
at least one of three injectable second-line drugs (i.e., amikacin, kanamycin,
or capreomycin).
230
231
Appendix
Appendix Appendix
Appendix G
GG
G
Answer Key for the Exercises
Answer Key for the ExercisesAnswer Key for the Exercises
Answer Key for the Exercises
Module A – RVCT (page 1) Items 1-16
# Correct
Answer
Notes for Answers
1.1 E
1.2 B
1.3 A
For this item, the month and year always need to be entered. But if the exact
day is not known, enter 99.
It is important to read the instructions for entering the dates for each item because
they can vary from item to item. For some items enter “99” for the unknown
month or day, and “9999” for the unknown year. This may vary from what will be
entered into a computer software program.
• 03 99 2009 – for March, unknown day, in 2009
• 99 99 2009 – for unknown month and day in 2009
• 01 02 9999 – for January 2, in a year that is unknown
1.4 A
The Date Reported is the date that a health department first suspects that the
patient might have TB or first receives a report (verbal or written notification)
from a health care provider. In this instance, the Date Reported is January 6.
January 10 is the date that the county health department received laboratory results
confirming TB diagnosis. January 27 is the Date Submitted.
2.1 B
2.2 B
Date Submitted is June 30 because this was the date that the RVCT form is
submitted to the reporting area. June 1 is the date reported and July 10 is the
date counted.
3.1 A
3.2 B
3.3 A
3.4 B
4.1 A
Laverne receives a new diagnosis in Milwaukee, but is an out-of-area resident and
returns home for treatment. The reporting address should be her address in
Chicago.
4.2 B
Eduardo, a migrant worker, receives a new diagnosis, but is an out-of-area
resident. Because of his migrant status, the reporting address for Eduardo should
be the area in which he lives at the time of diagnosis, which is Watsonville. In
addition, he should also be counted in the area in which he lives at the time of
diagnosis (Watsonville), even if he returned to his permanent home in Sacramento
for treatment. For more information on determining the reporting address for
patients, see the Guidelines to Determine Reporting Address in the instructions for
Reporting Address for Case Counting (item 4). For more information about
how cases should be counted, see Count Status (item 5).
5.1 D
232
5.2 C
Because his recurrence of TB disease was within 12 months of completion of
therapy this recurrence is not considered a separate episode of TB. Initial
diagnosis does not factor into this decision. A 2009 RVCT would be completed
and in Count Status (item 5), Verified Case: Recurrent TB within 12 months
would be selected.
5.3 A
It is Yes, because his recurrence of TB disease is more than 12 months after
completion of therapy and is considered a new case of TB. A 2010 RVCT would
be completed and for Count Status (item 5) count as a TB case would be selected.
5.4 B
5.5 A
5.6 A
5.7 A
Raul’s case is counted as a new case of TB in Arizona because his recurrence of
TB is more than 12 months after completion of therapy.
5.8 C
Communication between TB control programs to ensure continuity of care and
submission of reports regarding a patient who is moving from one area to another
should be conducted as securely and efficiently as possible (e.g., telephone, e-mail,
secure fax, express courier).
5.9 A
5.10 A
5.11 C
5.12 B
5.13 B
A 2009 RVCT would be completed by Georgia listing John’s count status in Item
5 as a verified case counted by another area. The 2009 form is completed by
Georgia and the 2009 reporting address should reflect his 2009 Georgia address to
assess level of burden of a noncountable case. However, Georgia cannot count him
in its 2009 case count because he has been counted as a case in Tennessee within
12 months. John never completed therapy in Tennessee, so Georgia should assist
John in completing therapy, and communicate with Tennessee to provide details
for case close-out.
6.1 C
6.2 A
6.3 B
6.4 C
The Date Counted is the date that the responsible count authority verifies the case
as a new case of TB and includes it in the official TB case count.
6.5 B
7.1 C
David is not considered to have a Previous Diagnosis of TB Disease because in
January 2009 he had LTBI and not TB disease.
8.1 C
9.1 A
Health care workers are dependent on the answers that the patients provide,
regardless of how the health care worker thinks the items should be answered.
However, asking the patient probing questions and reviewing the patient’s medical
records and other documentation can provide the most accurate answers for
biological sex at birth.
10.1 A
11.1 E
Joaquin says his mother is American Indian and his father is African American, so
the answer is E because both A and C are correct.
233
11.2 B
Trang is Filipino and Vietnamese, which are both considered Asian race. Even
though the Philippines are islands in the Pacific, Filipinos are considered an Asian
race.
12.1 A
When a patient was born abroad to a parent who is a U.S. citizen, the patient is
considered “U.S.-born.”
12.2 B
Because Wolfgang was actually born in Germany, the correct answer is Germany.
12.3 A
Bernard is considered “U.S.-born” because he was born in 1 of the 50 states or the
District of Columbia. Even though both of his parents were born in a foreign
country, he is still considered “U.S.-born.”
12.4 A
Bernard was born in the United States, so the answer would be United States.
12.5 B
Mayleen is not considered “U.S.-born” because she was not born in 1 of the 50
states or the District of Columbia and neither of her parents were U.S. citizens.
Mayleen is a U.S. citizen but not U.S.-born. Her parents are citizens of Palau.
However, if they had been born in Guam they would be considered U.S. citizens
and Mayleen would be considered “U.S.-born.”
12.6 B
Mayleen was born in Guam, so the answer would be Guam.
12.7 B
Jiguna was born abroad to parents who were not U.S. citizens. Therefore he is not
considered “U.S.-born.”
12.8 B
Because Jiguna was born in Kenya, the correct answer is Kenya.
13.1 A
When a patient is born abroad, the date arrived in the U.S. should be when the
patient entered the U.S. for the first time.
13.2 B
The answer is B. For patients born in another country, enter month and year first
arrived in the U.S. Ken was born in the Republic of the Marshall Islands and
moved to Arkansas. Even though his mother is a U.S. citizen and Ken is
“U.S.-born,” the item is asking when the patient first arrived in the U.S.
14.1 A
Although Pim’s parents are divorced, in this case it is probably best to enter her
mother’s and father’s countries of birth. The guardians are usually the parents.
There are only 2 lines, so it is not possible to indicate 3 people. In another
situation, it might be better to list the stepfather; it just depends on the situation. It
is important to identify not only the guardian who has the most contact with
the child, but also the guardian who may have a possible risk of TB exposure.
In this case, Pim’s father would be a higher priority than the stepfather because he
still has regular and frequent contact with her, and he also has a risk factor because
he immigrated from Thailand. If the biological father was not at risk of TB and
everything else was equal with the stepfather, then the stepfather would probably
be listed as the guardian. It just depends on how the state TB program views
guardianship and factors that put the patient at greatest risk for TB.
14.2 B
14.3 A
Antonio is “U.S.-born” because his father was born in the United States and is a
U.S. citizen.
14.4 A
The answer is El Salvador because Antonio was actually born in El Salvador.
234
14.5 B
The answer might depend on how the state TB program views guardianship.
Antonio’s legal guardians are his uncle and aunt, the Trujillos, both born in El
Salvador. His birth parents’ birth countries are El Salvador (mother) and the
United States (father). Following are 3 options for how this item could be
answered (depending on the state rules regarding parental care):
• If the TB program decided that Antonio still has contact with his father,
the relevant primary guardians might be from El Salvador (Trujillos) and
the United States (father).
• If the TB program decided that Antonio is primarily cared for by the
Trujillos, the relevant primary guardians might be from El Salvador
(Trujillos).
• If the TB program routinely captures data on the country of birth of the
pediatric patients’ mother and father, the relevant answer might be El
Salvador (mother) and United States (father).
14.6 A
Since Antonio was out of the United States for an uninterrupted period of more
than 2 months, select Yes.
14.7 B
Answer No because Regina was out of the United States for an uninterrupted
period less than 2 months. .
14.8 A
Answer Yes because Lisa was out of the United States for more than 2 months
uninterrupted.
15.1 A
15.2 B
Ruth’s status is dead at diagnosis. Even though she clearly had TB when she came
to the emergency room, TB was not suspected until after her death when the
autopsy was performed.
15.3 A
Yes, TB was a cause of death. Regardless of whether pneumonia was
misdiagnosed or was the immediate cause of death, TB was an underlying cause
of death based on the autopsy. Ruth has a verified case of TB based on positive
pathology results on lung tissue, which would be recorded in
Smear/Pathology/Cytology of Tissue and Other Body Fluids (item 19).
16.1 E
TB disease is found in the pulmonary, pleural, and lymphatic intrathoracic sites, so
all three sites should be selected. This item is usually completed by an MD
consultant.
16.2 E
Bone and/or joint is not correct. TB disease is found in both the blood and bone
marrow. Choose “Other: enter anatomic code(s).” The anatomic codes are listed in
Appendix C
– Anatomic Codes.
235
Module B – RVCT (page 2) Items 15-25
# Correct
Answer
Notes for Answers
17.1 A
Select positive if any of the results are positive.
17.2 A
January 13 is the date collected because that was the date when the first positive
sputum was collected.
18.1 A
18.2 B
18.3 B
January 16 was date that the first positive culture specimen was collected.
February 16 is the date that the first positive culture was reported.
18.4 B
Forbes Diagnostics Incorporated is a commercial laboratory because fees are
charged for each specimen processed or test performed.
18.5 C
National Jewish Health hospital laboratory is not considered a public health
laboratory or a commercial laboratory. This laboratory sometimes charges for
services, but for the purposes of the RVCT it is categorized as “Other.”
19.1 B
19.2 B
19.3 A
19.4 A
19.5 B
Any positive result supersedes a negative result in reporting TB diagnostic
criteria. Since the results are discrepant (smear negative, pathology positive),
Type of Exam should correspond to the result captured as positive. If both smear
and pathology are positive, both smear and pathology/cytology should be checked
under Type of Exam.
20.1 A
20.2 C
The date collected is the date for the specimen that came back positive. The initial
specimen collected on October 13 is contaminated. The second specimen
collected on October 25 has a positive result, so that is the date used for date
collected.
20.3 C
20.4 A
Public health laboratory was selected because the specimen was sent to the state
health laboratory.
21.1 E
The result was reported as indeterminate. Although the test was done, it was
inconclusive rather than positive or negative.
22.A 1 C
Emily’s result is C – Abnormal because her radiograph shows tiny, well-defined
nodules, indicating evidence of miliary TB.
22.A 2 A
Alice’s result is A – Normal because her chest radiograph shows no evidence of
TB
22.A 3 E
Lawrence’s result is E – Unknown because he remembers getting a chest
radiograph but the result is not known.
22.A 4 B
Roy’s result is B – Abnormal with evidence of cavitary lesion because he has
an abnormal chest radiograph with a cavitary lesion.
22.A 5 D
Frank’s result is D – Not Done because the radiograph was not done.

236
22.B 1 E
Site of Disease (item 16) does not include miliary TB. On the old RVCT, miliary
TB could be selected as a Site of Disease. However, in this version of the RVCT,
items 22A and 22B are the only items that indicate military TB. Since miliary TB
appeared in both the initial chest radiograph and the CT scan, the answer is “E.”
23.1 A
23.2 A
The documented TST was May 1995.
23.3 B
24.1 A
24.2 D
25.1 E
Health care worker is the answer because that reason supersedes
Employment/Administrative testing.
25.2 H
25.3 C
25.4 B
25.5 A
25.6 G
25.7 D
25.8 F
25.9 B
25.10 C
237
Module C – RVCT (page 3) Items 26-37
# Correct
Answer
Notes for Answers
26.1 A
27.1 B
Yes, because he was homeless at some time during the past year.
28.1 C
Local jail is the answer because he was at Lanner County Jail when the diagnostic
evaluation was performed, even though the result did not come back until he was in
the ICE Detention Center.
29.1 A
At the time of diagnosis, Gladys is living at home, not at the nursing facility. She
may have acquired TB while she lived at the nursing home, but that is not where she
was diagnosed. In addition, Gladys was not evaluated for TB when she was at the
nursing facility.
30.1 E
30.2 G
30.3 H
30.4 C
30.5 A
30.6 D
30.7 B
30.8 F
30.9 A
31.1 B
Even though Chaz denies that he injects drugs, his record shows that he was in an
injecting drug detoxification program within the past year.
32.1 A
According to Spider, he used non-injecting drugs in the past, but not within the past
12 months. You have to take his word for this because there is no proof that he did
use drugs within the past 12 months, and you do not suspect or see any evidence of
drug use.
33.1 B
Based on your observations of Jack Daniel, you have seen evidence that he has used
alcohol excessively during the past 12 months. In addition, he was in an alcohol
treatment program within the past year.
34.1 E
34.2 A
34.3 F
34.4 I
34.5 D
34.6 B
34.7 G
34.8 C
35.1 E
35.2 H
35.3 C
35.4 D
35.5 B
35.6 G

238
35.7 A
35.8 F
35.9 J
35.10
I
36.1 B
The documented date that the patient first ingests the drugs is the date that is
preferred. But, in this case study, Thelma was not sure about the date she started
taking the drugs, so the next documented date would be the date that the pharmacy
dispensed the drug, which in this case was February 15.
37.1 B
37.2 B
37.3 B
37.4 B
37.5 A
37.6 A
239
Module D – Initial Drug Susceptibility Report (Follow Up Report – 1) Items 38–40
# Correct
Answer
Notes for Answers
38.1 A
There are 12 spaces for genotyping accession number. Instructions are to complete
the number beginning at the left-most box. Do not add zeros to finish filling the
boxes. This genotyping accession number is from the California laboratory because
it has the 2-digit year (09) followed by L, then 4 digits. If the genotyping accession
number is from the CDC laboratory, it will have a hyphen after the 2-digit year; this
hyphen should also be entered into a box as part of the genotyping accession
number. If the genotyping accession number is from the Michigan laboratory, it will
have RF after the 2-digit year.
The last three columns above are the spoligotype, MIRU, and PCR type,
respectively. The second column is not a genotyping accession number because it
does not fit the numbering format of any of the three reference laboratories. RVCT
users should be aware that there may be additional local, state, submitter, cluster, or
other identification numbers listed on a genotyping report. RVCT users should be
able to identify the genotyping accession number by becoming familiar with the
format used by the NTGS laboratory serving their reporting area.
39.1 A
39.2 B
Item 39 refers to the first culture positive specimen on which drug susceptibility
testing was performed , regardless of which site it was taken from (e.g., it does not
have to be the major site of disease). Because the urine culture grew before the sputum
cultures, drug susceptibility testing was performed on the urine specimen. Therefore
the urine is the first specimen on which drug susceptibility testing was performed.
That is why July 3 is the answer for 39.1, and the specimen type is urine for 39.2. If
the July 5 sputum culture had grown first, drug susceptibility testing would have been
performed on that specimen; July 5 would have been the answer for 39.1, and sputum
would have been the answer for 39.2. Even though the major site of disease may be
pulmonary, this question is asking for the first specimen on which drug susceptibility
testing was performed.
40.1 A
Isoniazid: Any resistance is resistance, even if it is low.
40.2 B
40.3 B
40.4 A
40.5 C
40.6 A
40.7 C
40.8 D
Ethionamide: It is not known if the test was done, so Unknown should be selected.
Some laboratories report no resistance, which means susceptible.
40.9 B
40.10 C
240
Module E – Case Completion Report (Follow Up Report – 2) Items 41–49
# Correct
Answer
Notes for Answers
41.1 D
41.2 B
41.3 C
41.4 F
41.5 A
41.6 E
42.1 B
Moved is defined as a relocation of a patient’s residence, resulting in a change in
local health department jurisdiction. Therefore Johann moved in state, but out of
jurisdiction.
43.1 B
44.1 C
This question is asking what is the reason that therapy was stopped, not what is
the cause of death. The autopsy indicated that his death was likely due to an
adverse treatment event to INH. However, the reason that therapy was stopped was
that Hannibal died, and then it was determined the cause of death was related to TB
therapy.
If his therapy had been stopped before he died, the answer would still be the same
because he died. You would answer adverse treatment event for therapy stopped
only for a patient who had not died and his/her treatment was permanently stopped.
45.1 D
45.2 E
45.3 C
45.4 A
45.5 B
46.1 D
46.2 F
46.3 E
46.4 C
46.5 A
46.6 B
47.1 B
241
47.2 B
There always need to be 3 numbers entered into the boxes provided. In this case
there needs to be a leading 0 in the first box as opposed to entering “2” in the left-
most box. If the number of weeks of DOT is a single digit such as 8 weeks, this
would be entered as 008. If the patient has 2 full years of DOT, this would be
entered as 104 weeks.
Count the following weeks of DOT for Maximo:
• 8 weeks of daily 4-drug regimen
• 18 weeks of 2-drug regimen
This equals a total of 26 weeks of DOT.
Do not count the following doses of DOT for Maximo:
• The missed doses on August 25 and 27 when no drugs were given. (The
missed doses in August were “made up” on December 8 and 10 by adding
an extra week of therapy.)
• The week when only 1 dose of a twice-weekly regimen was given on
October 6 and missed on October 8. (The missed dose on October 8
was
“made up” on December 15. October 6 and December 15 contribute 0.5 of a
twice-weekly regimen each, adding up to 1 week.)
• The week when only 1 dose of a twice-weekly regimen was given on
November 24 and missed on November 26. (The missed dose on November
26 was “made up” on December 17. November 24 and December 17
contribute 0.5 of a twice-weekly regimen each, adding up to 1 week.)
47.3 B
Item 43: Date Therapy Stopped is December 17, 2009 (12/17/2009).
47.4 A
Item 44: Reason Therapy Stopped or Never Started is completed therapy.
47.5 B
Item 45: Reason Therapy Extended > 12 Months is not applicable, leave
blank.
47.6 A
Item 46: Type of Outpatient Health Care Provider is Local/state health
department.
47.7 A
Item 47: Directly Observed Therapy (DOT) is Yes, both directly observed and
self-administered.
47.8 B
Item 47: Directly Observed Therapy (DOT) Use the above calendar to count the
number of weeks of DOT. The total number of DOT weeks from initiation of
therapy to completion is entered as 025. There were 8 weeks of 4-drug regimen and
17 weeks of 2-drug regimen. Do not count the last week of August, when no DOT
doses were given. Then begin counting 7-day periods on September 7. The dose
on October 10 (whether missed or self-administered) is skipped, and October 5 and
12 administration forms a DOT week (2 doses appropriately given). Begin
counting whole weeks again the week of October 16. The December 12 dose
counts as 0.5 dose, but there is no dose to match it with, so it does not form a DOT
week.
48.1 C
49.1 B
49.2 A
49.3 B
49.4 B
49.5 B
49.6 B
49.7 C
242
49.8 C
49.9 B
49.10
B
49.11
C
49.12
B
49.13
C
49.14
C
49.15
B
49.16
C
49.17
C
49.18
C
49.19
C
All items have to be checked as either resistant, susceptible, not done, or unknown.
You can’t leave any of them blank unless the item is pending.
49.20
C
All items have to be checked as either resistant, susceptible, not done, or unknown.
You can’t leave any of them blank unless the item is pending.
243

244
For more information, contact
U.S. Department of Health and Human Services
Centers for Disease Control and Prevention
National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention
Division of Tuberculosis Elimination
1600 Clifton Road N.E.
MS E-10
Atlanta, GA 30333
Phone (404) 639-8120
Fax: (404) 639-8959
