
Biosafety in
Microbiological
and Biomedical
Laboratories
6th Edition
Centers for Disease Control and Prevention
National Institutes of Health
Inside front cover

Biosafety in
Microbiological
and Biomedical
Laboratories
6th Edition
U.S. Department of Health and Human Services
Public Health Service
Centers for Disease Control and Prevention
National Institutes of Health
Revised June 2020
iiiForeword
Foreword
Biosafety in Microbiological and Biomedical Laboratories (BMBL) has served
as the cornerstone of biosafety practice in the United States since its initial
release. We wish to emphasize that the sixth edition of BMBL remains an
advisory document recommending best practices for the safe conduct of work
in biomedical and clinical laboratories from a biosafety perspective. The BMBL
is not intended to be a regulatory document although we recognize that some
may use it in that way. The core principle of this document is protocol-driven risk
assessment; it is not possible for a single document to identify all of the possible
combinations of risks and mitigations feasible in biomedical and clinical labora-
tories. The BMBL should be used as a tool in the assessment and proposed
mitigation steps in biomedical and clinical laboratories.
This edition of BMBL includes revised sections, agent summary statements,
and appendices. We harmonized the recommendations included in this edition
with guidance issued and regulations promulgated by other organizations and
federal agencies. Wherever possible, we claried both the language and intent
of the information provided. In order to serve the needs of our community better,
this edition includes new appendices on the following topics: inactivation and
verication; laboratory sustainability; large-scale biosafety; and clinical laboratory
biosafety.
Over 200 of our scientic and professional colleagues contributed to the prepa-
ration of the sixth edition through participation in technical working groups and
serving as reviewers, guest editors, and subject matter experts. We wish to thank
them all for their dedication and hard work. Without them, the sixth edition of
BMBL would not be possible. We also recognize the hard work and contributions
made by all who participated in preparation of the previous editions of BMBL; we
have built on their solid work and commitment.
It would have been impossible to publish this revision without recognizing the
visionary leadership of the previous BMBL editors—Drs. John Richardson,
W. Emmett Barkley, Jonathan Richmond, Robert W. McKinney, Casey Chosewood,
and Deborah Wilson—without whom the BMBL would not be the respected
resource it is today. The Steering Committee members, Drs. Christy Myrick,
Richard G. Baumann, Margy Lambert, Patricia Delarosa, and Theresa Lawrence,
were instrumental in identifying authors, selecting additions to this edition, and
reviewing submissions. Their signicant contribution to this edition is sincerely
appreciated.
We are truly grateful to Ms. Shaina Mangino and Dr. Mallory Pomales of Eagle
Medical Services, LLC for their expertise and patience in assisting us with this
undertaking. Their superb organizational and editing skills were critical in the
creation of this document.
iv Biosafety in Microbiological and Biomedical Laboratories
We hope you nd the sixth edition of Biosafety in Microbiological and Biomedical
Laboratories complete, timely, and most of all, easy to use. Thank you for your
patience and understanding during the long and comprehensive revision process.
Paul J. Meechan, PhD, MPH, RBP, CBSP(ABSA)
Associate Director for Laboratory Safety
Oce of Laboratory Science and Safety
Centers for Disease Control and Prevention
Atlanta, GA
Jerey Potts, MPH, CBSP(ABSA)
Chief, Biorisk Management Branch
Division of Occupational Health and Safety
National Institutes of Health
Bethesda, MD
vForeword
Participants
Senior Co-Editors
Paul J. Meechan, PhD, MPH, RBP, CBSP(ABSA)
Associate Director for Laboratory Safety
Oce of Laboratory Science and Safety
Centers for Disease Control and Prevention
Jerey Potts, MPH, CBSP(ABSA)
Chief, Biorisk Management Branch
Division of Occupational Health and Safety
National Institutes of Health
Steering Committee
Richard G. Baumann, PhD, SM(NRCM)
Biological Safety Ocer
Division of Occupational Health and Safety
National Institutes of Health
Patricia Delarosa, PhD, CBSP(ABSA), CTM(ATAP)
Health Scientist, Biosafety
Oce of Strategy, Policy, Planning, and Requirements
Oce of the Assistant Secretary for Preparedness and Response
Department of Health and Human Services
Margy Lambert, PhD
Health Scientist
Oce of Science and Technology Assessment
Occupational Safety and Health Administration
CAPT Theresa Lawrence, PhD
Senior Science Ocer
Oce of the Associate Director for Preparedness and Response
U.S. Department of Health and Human Services
Paul J. Meechan, PhD, MPH, RBP, CBSP(ABSA)
Associate Director for Laboratory Safety
Oce of Laboratory Science and Safety
Centers for Disease Control and Prevention
vi Biosafety in Microbiological and Biomedical Laboratories
Christy Myrick, PhD, RBP(ABSA)
Lead Auditor
U.S. National Authority for Containment for Polioviruses
Center for Preparedness and Response
Centers for Disease Control and Prevention
Jerey Potts, MPH, CBSP(ABSA)
Chief, Biorisk Management Branch
Division of Occupational Health and Safety
National Institutes of Health
Deborah E. Wilson, DrPH, CBSP(ABSA)
RADM (ret.), U.S. Public Health Service,
Division of Occupational Health and Safety—Director
Oce of Research Services
National Institutes of Health
Technical Editors
Shaina Mangino
Senior Publications Editor
Eagle Medical Services, LLC
Mallory J. Pomales, DO
Senior Program Manager for Scientic Writers
Senior Scientic Editor
Eagle Medical Services, LLC
Primary Authors
Matthew J. Arduino, MS, DrPH, FSHEA, M(ASCP)CM
Sr. Adviser, Environmental Hygiene and Infection Prevention
Oce of the Director
Division of Healthcare Quality Promotion
Centers for Disease Control and Prevention
William D. Arndt
Health Scientist—Biorisk
Division of Laboratory Systems
Centers for Disease Control and Prevention
Heike Bailin, MD
Sta Physician, Occupational Medical Service
Division of Occupational Health and Safety
National Institutes of Health
viiForeword
Richard G. Baumann, PhD, SM(NRCM)
Biological Safety Ocer
Division of Occupational Health and Safety
National Institutes of Health
Richard S. Bradbury, PhD, FFSc RCPA
Division of Parasitic Diseases and Malaria
Center for Global Health
Centers for Disease Control and Prevention
Mary E. Brandt, PhD
Oce of Laboratory Science and Safety
Centers for Disease Control and Prevention
Cristina Bressler, MBA
Health Scientist
Occupational Health and Safety Oce
Oce of Safety, Security and Asset Management
Centers for Disease Control and Prevention
Byron Caughey, PhD
Senior Investigator
Laboratory of Persistent Viral Diseases, Rocky Mountain Laboratories
National Institute for Allergy and Infectious Diseases
National Institutes of Health
Rear Admiral Terri R. Clark, DVM, DACLAM
Director (ret.), Oce of Animal Care and Use
Assistant Surgeon General
USPHS Commission Corps
National Institutes of Health
Danielle Daniely, PhD, RBP(ABSA)
Director, Research Safety Programs and High Containment Laboratories
Oce of Research Integrity
Georgia State University
Patricia Delarosa, PhD, CBSP(ABSA), CTM(ATAP)
Health Scientist, Biosafety
Oce of Strategy, Policy, Planning, and Requirements
Oce of the Assistant Secretary for Preparedness and Response
Department of Health and Human Services
viii Biosafety in Microbiological and Biomedical Laboratories
Stephen Denny, DVM, MS, DACLAM, DACVPM
Acting Director, Oce of Animal Care and Use
Oce of Intramural Research
National Institutes of Health
Eileen Edmonson
Transportation Regulations Specialist
Standards and Rulemaking Division
Pipeline and Hazardous Materials Safety Administration
U.S. Department of Transportation
Samuel S. Edwin, PhD
Director
Division of Select Agents and Toxins
Center for Preparedness and Response
Centers for Disease Control and Prevention
Elizabeth (Zeba) Floyd, AIA, LEED AP BD+C ID+C
Project Director
Sustainable Design Consulting, LLC
Karen M. Frank, MD, PhD, D(ABMM)
Chief, Department of Laboratory Medicine
Clinical Center
National Institutes of Health
Mark D. Gibson, MS, CIH
Senior Industrial Hygienist
Division of Occupational Health and Safety
National Institutes of Health
Eduardo Gomez-Saladin, PhD, SM, RBP, CBSP(ABSA)
Deputy Director
Oce of Laboratory Safety
Centers for Disease Control and Prevention
Natasha K. Grith, MS
Chief, Quality and Safety Systems Branch
Division of Laboratory Systems
Centers for Disease Control and Prevention
ixForeword
Ted Hackstadt, PhD
Chief, Host-Parasite Interactions Section
Laboratory of Bacteriology
Rocky Mountain Laboratories
National Institute for Allergy and Infectious Diseases
National Institutes of Health
Susan B. Harper, DVM, MS, DACLAM, DACVPM, RBP(ABSA)
Oce of National Programs
USDA Agricultural Research Service
Kathryn L. Harris PhD, RBP(ABSA)
Senior Outreach and Education Specialist
Biosafety, Biosecurity and Emerging Biotechnology Policy Division
Oce of Science Policy
National Institutes of Health
Mark L. Hemphill, MS
Deputy Director, Division of Select Agents and Toxins
Center for Preparedness and Response
Centers for Disease Control and Prevention
Barbara L. Herwaldt, MD, MPH
Medical Epidemiologist, Division of Parasitic Diseases and Malaria
Centers for Disease Control and Prevention
Nancy P. Hoe, PhD, CBSP(ABSA)
Biosafety Ocer/Responsible Ocial for Rocky Mountain Laboratories
Division of Occupational Health and Safety
Oce of the Director, National Institutes of Health
Joseph P. Kozlovac, MS, RBP, CBSP(ABSA), SM(NRCM)
Agency Biosafety Ocer
USDA Agricultural Research Service
Oce of National Programs
Margy Lambert, PhD
Health Scientist
Oce of Science and Technology Assessment
Occupational Safety and Health Administration
George W. Lathrop
Veterinary Medical Ocer
National Center for Emerging and Zoonotic Infectious Diseases
Centers for Disease Control and Prevention
x Biosafety in Microbiological and Biomedical Laboratories
Susan Lawrence, PhD
Branch Chief
Microbiology Branch
Environmental Protection Agency
Susan A. Lippold, MD, MPH
Medical Director
Occupational Health Clinic
Centers for Disease Control and Prevention
R. Trevor Lubbert
Board Certied Senior Sta Entomologist
Community Health Branch
Division of Occupational Health and Safety
Oce of Research Safety
National Institutes of Health
Carolina Lúquez, PhD
National Botulism and Enteric Toxins Team
Enteric Diseases Laboratory Branch
Division of Foodborne, Waterborne, and Environmental Diseases
National Center for Emerging and Zoonotic Infectious Diseases
Centers for Disease Control and Prevention
Patrick M. McNutt, PhD
Principal Investigator
Department of Neuroscience
U.S. Army Medical Research Institute of Chemical Defense
Paul J. Meechan, PhD, MPH, RBP, CBSP(ABSA)
Associate Director for Laboratory Safety
Oce of Laboratory Science and Safety
Centers for Disease Control and Prevention
Barbara Owen, MPH, CBSP(ABSA), RBP, NRCM, CMM
Director, Global Safety and Environment
Corporate Biosafety Ocer
Merck & Co., Inc.
Steven Piguet, AIA, LEED Fellow, Fitwel Ambassador
Associate Principal
Sustainable Design Consulting, LLC
xiForeword
Segaran P. Pillai, PhD, SM(NRCM), SM(ASCP), FAAM
Director, Oce of Laboratory Science and Safety
Oce of the Commissioner
Food and Drug Administration
Jerey Potts, MPH, CBSP(ABSA)
Chief, Biorisk Management Branch
Division of Occupational Health and Safety
National Institutes of Health
Nathaniel Powell Jr., DVM, MS
Chief, Comparative Medicine Branch
Division of Scientic Resources
National Center for Emerging and Zoonotic Infectious Diseases
Centers for Disease Control and Prevention
Ann M. Powers, PhD
Lead, Virology Team
Arboviral Diseases Branch
Division of Vector-Borne Diseases
Centers for Disease Control and Prevention
Reynolds M. Salerno, PhD
Director, Division of Laboratory Systems
Centers for Disease Control and Prevention
Dr. Martin L. Sanders, PhD, CSP
Director, Safety, Emergency, and Environmental Compliance
Department of Health and Human Services
James M. Schmitt, MD, MS
Medical Director
Occupational Medical Service
Division of Occupational Health and Safety
National Institutes of Health
Stephen Tomasino, PhD
Senior Scientist
Environmental Protection Agency
Elizabeth G. Weirich, MS, SM(NRCM), CBSP(ABSA)
Division of Laboratory Systems
Centers for Disease Control and Prevention
xii Biosafety in Microbiological and Biomedical Laboratories
David M. White, DVM, PhD, RBP(ABSA), DACVM
Safety & Security Unit Lead
National Centers for Animal Health
USDA Animal and Plant Health Inspection Service
Deborah E. Wilson, DrPH, CBSP(ABSA)
RADM (ret.), U.S. Public Health Service,
Division of Occupational Health and Safety—Director
Oce of Research Services
National Institutes of Health
Liz E. York, FAIA
Chief Sustainability Ocer
Oce of the Chief Operating Ocer
Centers for Disease Control and Prevention
xiiiForeword xiiiForeword
Contributors
Michael Adler
Karen Anderson
Rebecca V. Anderson
Matthew J. Arduino
David Asher
John Balog
Shawn A. Bean
David E. Bentzel
Cary R. Binder
Brad Blitvich
Kathryn Board
William P. Bozza
Megan Morgan Brose
Robert Bull
Cara Burns
Sheldon Campbell
Cynthia Cary
Nick Chaplinski
Mark Chappell
Konstantin Chumakov
Jerey Cohen
Eugene Cole
Nancy Cornish
Whitni Davidson
C. Todd Davis
Sabrina Debose
Johnathan R. Deeds
Thomas Denagamage
Beverly Dickson
Gerhard Dobler
Mike Drebot
Edward Dubovi
Eilyn Fabregas
Chadi Filli
Betty A. Forbes
Marshall Gayton
Sarah Genzer
Christopher Good
Andrew Haddow
Vibeke (Vips)
Halkjaer-Knudsen
Alexander Hamberg
Glen Hansen
David Harbourt
Kathryn L. Harris
Courtney Harrison
Michael W. Hart
Robert Hawley
Henry Hays
John Henneman
Stephen Higgs
Julia Hilliard
Michael Holbrook
Bill Homovec
Romney Humphries
Debra Hunt
Freeda Isaac
Eddie L. Jackson
Peter Jahrling
Robert Jambou
Eric Jeppesen
Barbara Johnson
Crystal Johnson
Eric A. Johnson
Julie Johnson
Estella Z. Jones
Joanne Jones-Meehan
Gerardo Kaplan
Subhashinie
Kariyawasam
Jaqueline Katz
Arifa Khan
Lydia Kibiuk
Chris Kiley
Manley Kiser
Rajen Koshy
Laura Kramer
Philip Krause
Jens Kuhn
Anna Llewellyn
Maria Lorenzo
Luis Lugo-Roman
Nicole Lukovsky-
Akhsanov
Marian Major
Monear Makvandi
Alison Mawle
Erin McElvania
Thomas A. McKeon
David Scott McVey
Thomas P. Monath
Rashida Moore
David Morens
Eric C. Mossel
xiv Biosafety in Microbiological and Biomedical Laboratoriesxiv Biosafety in Microbiological and Biomedical Laboratories
Krista Murray
Christy Myrick
Brandy Nelson
Joseph Newsome
Stuart Nichol
Kenneth E. Nusbaum
Steve Oberste
Patricia Olinger
Lillian Orciari
Eugene O’Reilly
Mark Pagala
Subbian Satheshkumar
(Sathesh) Panayampalli
Eun-Chung Park
Amar Patil
Michael A. Pentella
William Peters
Brett Petersen
Brian R. Petuch
Susan C. Piguet
Ewan Plant
Kristin Prentice
Suzette Priola
Amy Pullman
Richard Rebar
Yvonne Reed
Ryan F. Relich
Pierre Rollin
Eugene Rosenthal
Scott Rusk
Janice Rusnak
Arick P. Sabin
Mo D. Salman
Lawrence B.
Schonberger
Lynne M. Sehulster
Brianna Skinner
Halley Smith
James Synder
Marisa Elkins St. Claire
James Stevens
Molly Stitt-Fischer
James R. Swearengen
William M. Switzer
Sandra Tallent
Cassandra Tansey
Robert Tesh
Anil J. Thachil
Eileen L. Thacker
Natalie J. Thornburg
William H. Tolleson
John Tonkiss
Althea C. Treacy
Anita Trichel
Jessica Tucker
Terrence Tumpey
Timothy Uyeki
Francisco A. Uzal
Nikos Vasilakis
Linfa (Lin-Fa) Wang
David Warnock
Scott Weaver
Zachary Weiner
Rebecca Weingarten
Robbin Weyant
Mark Whary
Diana Whipple
Temeri Wilder-Koe
Axel Wol
Zhiping Ye
Kyoung-Jin Yoon
Edward You
Jessica Young
Baolin Zhang
xvContents
Contents
Foreword ............................................................................................................ iii
Participants
......................................................................................................... v
Senior Co-Editors
........................................................................................... v
Steering Committee
....................................................................................... v
Technical Editors
............................................................................................vi
Primary Authors
.............................................................................................vi
Section I—Introduction
...................................................................................... 1
The Occurrence of Laboratory-associated Infections ..................................... 1
Evolution of National Biosafety Guidelines
..................................................... 3
Risk Criteria for Establishing Ascending Levels of Containment
.................... 4
Agent Summary Statements
.......................................................................... 5
Laboratory Biosecurity
................................................................................... 6
Using Biosafety in Microbiological and Biomedical Laboratories
.................... 6
Looking Ahead
............................................................................................... 7
References
.................................................................................................... 7
Section II—Biological Risk Assessment
........................................................... 9
The Risk Management Process
................................................................... 10
Risk Communication
.................................................................................... 19
Facilitating a Culture of Safety through Risk Assessment
........................... 19
Conclusion
................................................................................................... 20
References
.................................................................................................. 20
Section III—Principles of Biosafety
................................................................. 24
Safety Equipment (Primary Barriers)
............................................................ 24
Personal Protective Equipment
.................................................................... 25
Facility Design and Construction (Secondary Barriers)
................................ 25
Facility Practices and Procedures
................................................................ 26
Biosafety Levels
........................................................................................... 27
Animal Facilities
........................................................................................... 30
Clinical Laboratories
.................................................................................... 30
Laboratory Biosecurity
................................................................................. 31
References
.................................................................................................. 31
Section IV—Laboratory Biosafety Level Criteria
............................................ 32
Biosafety Level 1
.......................................................................................... 32
A. Standard Microbiological Practices ................................................ 32
B. Special Practices ........................................................................... 36
xvi Biosafety in Microbiological and Biomedical Laboratories
C. Safety Equipment (Primary Barriers and Personal
Protective Equipment)
............................................................ 36
D. Laboratory Facilities (Secondary Barriers) ..................................... 36
Biosafety Level 2
.......................................................................................... 37
A. Standard Microbiological Practices ................................................ 37
B. Special Practices ........................................................................... 40
C. Safety Equipment (Primary Barriers and Personal
Protective Equipment).
............................................................ 41
D. Laboratory Facilities (Secondary Barriers) ..................................... 42
Biosafety Level 3
.......................................................................................... 43
A. Standard Microbiological Practices ................................................ 43
B. Special Practices ........................................................................... 46
C. Safety Equipment (Primary Barriers and Personal
Protective Equipment)
............................................................. 48
D. Laboratory Facilities (Secondary Barriers) ..................................... 48
Biosafety Level 4
.......................................................................................... 51
A. Standard Microbiological Practices ................................................ 52
B. Special Practices ........................................................................... 55
C. Safety Equipment (Primary Barriers and Personal
Protective Equipment)
............................................................. 57
D. Laboratory Facilities (Secondary Barriers) ..................................... 59
Section V—Vertebrate Animal Biosafety Level Criteria for
Vivarium Research Facilities............................................................................ 70
Animal Biosafety Level 1
.............................................................................. 71
A. Standard Microbiological Practices ................................................ 72
B. Special Practices ........................................................................... 76
C. Safety Equipment (Primary Barriers and Personal
Protective Equipment)
............................................................ 76
D. Animal Facilities (Secondary Barriers) ........................................... 76
Animal Biosafety Level 2
.............................................................................. 78
A. Standard Microbiological Practices ................................................ 78
B. Special Practices ........................................................................... 82
C. Safety Equipment (Primary Barriers and Personal
Protective Equipment).
............................................................ 83
D. Animal Facilities (Secondary Barriers) ........................................... 84
Animal Biosafety Level 3
.............................................................................. 87
A. Standard Microbiological Practices ................................................ 87
B. Special Practices ........................................................................... 91
xviiContents
C. Safety Equipment (Primary Barriers and Personal
Protective Equipment)
............................................................. 93
D. Animal Facilities (Secondary Barriers) ........................................... 94
Animal Biosafety Level 4
.............................................................................. 98
A. Standard Microbiological Practices ................................................ 98
B. Special Practices ......................................................................... 102
C. Safety Equipment (Primary Barriers and Personal
Protective Equipment)
........................................................... 104
D. Animal Facilities (Secondary Barriers)
............................................ 108
References
................................................................................................ 117
Section VI—Principles of Laboratory Biosecurity
........................................ 119
Biosafety and Laboratory Biosecurity
......................................................... 120
Developing a Laboratory Biosecurity Program
........................................... 122
Example Guidance: A Laboratory Biosecurity Risk Assessment
and Management Process
...................................................................... 123
Elements of a Laboratory Biosecurity Program
.......................................... 124
References
................................................................................................ 128
Section VII—Occupational Health Support for Biomedical Research......... 130
Framework for Occupational Health Support of Biomedical
Research
............................................................................................... 130
Elements of an Occupational Health Program Supporting
Biomedical Research
.............................................................................. 133
Conclusion
................................................................................................. 141
References
................................................................................................ 141
Section VIII—Agent Summary Statements
.................................................... 147
References
................................................................................................ 147
Section VIII-A: Bacterial Agents
..................................................................... 148
Bacillus anthracis
....................................................................................... 148
Bordetella pertussis
................................................................................... 150
Brucella species
......................................................................................... 152
Burkholderia mallei
.................................................................................... 154
Burkholderia pseudomallei
......................................................................... 156
Campylobacter species
.............................................................................. 157
Chlamydia psittaci, C. trachomatis, C. pneumoniae
................................... 158
Clostridium botulinum and neurotoxin-producing species
of Clostridia
............................................................................................ 161
Clostridioides (formerly Clostridium) dicile
............................................... 162
xviii Biosafety in Microbiological and Biomedical Laboratories
Clostridium tetani and Tetanus toxin .......................................................... 163
Corynebacterium diphtheriae
..................................................................... 164
Francisella tularensis
................................................................................. 165
Helicobacter species
.................................................................................. 167
Legionella pneumophila and other Legionella spp.
.................................... 168
Leptospira
.................................................................................................. 169
Listeria monocytogenes
............................................................................. 171
Mycobacterium leprae
................................................................................ 172
Mycobacterium tuberculosis complex
........................................................ 173
Mycobacterium spp. other than M. tuberculosis complex
and M. leprae
......................................................................................... 175
Neisseria gonorrhoeae
............................................................................... 177
Neisseria meningitidis
................................................................................ 178
Salmonella serotypes, other than S. enterica serotype
Typhi (S. Typhi)
...................................................................................... 179
Salmonella enterica serotype Typhi (S. Typhi)
........................................... 181
Shiga toxin (Verocytotoxin)-producing Escherichia coli
............................ 182
Shigella
...................................................................................................... 184
Staphylococcus aureus (Methicillin-Resistant, Vancomycin-
Resistant, or Vancomycin-Intermediate)
................................................. 185
Treponema pallidum
................................................................................... 187
Vibrio species
............................................................................................ 188
Yersinia pestis
............................................................................................ 189
References
................................................................................................ 191
Section VIII-B: Fungal Agents
........................................................................ 212
Blastomyces dermatitidis and Blastomyces gilchristii
................................. 212
Coccidioides immitis and Coccidioides posadasii
...................................... 213
Histoplasma capsulatum
............................................................................ 215
Sporothrix schenckii species complex
........................................................ 216
Miscellaneous Yeast and mold organisms causing human infection
.......... 217
References
................................................................................................ 219
Section VIII-C: Parasitic Agents
..................................................................... 223
General Issues
........................................................................................... 223
Blood and Tissue Protozoal Parasites
........................................................ 223
Intestinal Protozoal Parasites
..................................................................... 228
xixContents
Cestode Parasites ...................................................................................... 230
Trematode Parasites
.................................................................................. 232
Nematode Parasites
.................................................................................. 233
References
................................................................................................ 237
Section VIII-D: Rickettsial Agents
.................................................................. 239
Coxiella burnetii
......................................................................................... 239
Rickettsia species and Orientia tsutsugamushi
.......................................... 241
References
................................................................................................ 244
Section VIII-E: Viral Agents
............................................................................ 247
Hantaviruses
.............................................................................................. 247
Hendra Virus (formerly known as Equine Morbillivirus) and
Nipah Virus
............................................................................................. 248
Hepatitis A Virus, Hepatitis E Virus
............................................................. 250
Hepatitis B Virus, Hepatitis C Virus, Hepatitis D Virus
................................ 251
Macacine alphaherpevirus 1 (Herpesvirus Simiae, Cerocopithecine
herpesvirus I, Herpes B Virus)
................................................................ 253
Human Herpes Virus
.................................................................................. 256
Inuenza Viruses
....................................................................................... 259
Lymphocytic Choriomeningitis Virus
........................................................... 264
Poliovirus
................................................................................................... 265
Poxviruses
................................................................................................. 268
Rabies Virus and related lyssaviruses
....................................................... 271
Retroviruses, including Human and Simian Immunodeciency
Viruses (HIV and SIV)
............................................................................ 273
Severe Acute Respiratory Syndrome (SARS) and Middle East
Respiratory Syndrome (MERS) Coronaviruses
...................................... 276
References
................................................................................................ 280
Section VIII-F: Arboviruses and Related Zoonotic Viruses
.......................... 292
Risk Group 2 Viruses with BSL-2 Containment Recommended
................. 294
Risk Group 3 Viruses with BSL-3 Containment Recommended
................. 295
West Nile Virus (WNV)
............................................................................... 300
Eastern Equine Encephalitis Virus (EEEV), Venezuelan Equine
Encephalitis Virus (VEEV), and Western Equine Encephalitis
Virus (WEEV)
......................................................................................... 302
Rift Valley Fever Virus (RVFV)
................................................................... 304
References
................................................................................................ 330
xx Biosafety in Microbiological and Biomedical Laboratories
Section VIII-G: Toxin Agents .......................................................................... 334
Botulinum Neurotoxin
................................................................................. 334
Staphylococcal Enterotoxins (SE)
.............................................................. 337
Ricin
........................................................................................................... 340
Selected Low Molecular Weight (LMW) Toxins
.......................................... 343
References
................................................................................................ 347
Section VIII-H: Prion Diseases
....................................................................... 355
Bovine Spongiform Encephalopathy
......................................................... 360
Handling and processing of tissues from patients with suspected
prion disease
......................................................................................... 361
Handling and processing of multiple human prion tissue samples
............ 361
References
................................................................................................ 363
Appendix A—Primary Containment for Biohazards: Selection,
Installation, and Use of Biological Safety Cabinets
..................................... 367
Part 1—Introduction
................................................................................... 367
Part 2—High-Eciency Particulate Air (HEPA) Filters and the
Development of Biological Containment Devices
................................... 368
Part 3—Biological Safety Cabinets
............................................................ 370
Part 4—Other Laboratory Hazards and Risk Assessment
.......................... 376
Part 5—BSC Use by the Investigator: Work Practices and Procedures
..... 378
Part 6—Facility and Engineering Requirements
......................................... 384
Part 7—Certication of BSCs
..................................................................... 386
Acknowledgments
...................................................................................... 396
References
................................................................................................ 397
Appendix B—Decontamination and Disinfection of Laboratory
Surfaces and Items
......................................................................................... 400
Purpose and Scope
................................................................................... 400
Antimicrobial Products—U.S. Regulations
................................................ 400
Environmentally-Mediated Transmission of Infection
................................ 401
Principles of Cleaning, Disinfection, and Sterilization................................. 401
Decontamination
....................................................................................... 403
References
................................................................................................ 410
Appendix C—Transportation of Infectious Substances
............................... 415
International Harmonization of Shipping and Transport Regulations
.......... 415
Transportation Regulations
........................................................................ 415
Select Agents
............................................................................................. 416
Regulations
............................................................................................... 416
xxiContents
Importation and Transfers .......................................................................... 417
Transfer of USDA Plant Pests
.................................................................... 418
DOT Packaging of Infectious Substances
.................................................. 418
Intrafacility Specimen and Sample Transfers
............................................. 421
References
................................................................................................ 421
Appendix D—Biosafety and Biocontainment for Pathogens
Aecting Agricultural Animals and Animals that are Loose-Housed
or in Open Penning
......................................................................................... 423
Introduction
................................................................................................ 423
Potential Enhancements for BSL-2 and ABSL-2 Facilities for
Conducting Work with Pathogens Aecting Agricultural Animals
............ 426
Potential Enhancements for BSL-3 and ABSL-3 Facilities for
Conducting Work with Pathogens Aecting Agricultural Animals
............ 427
BSL-4 and ABSL-4 Facilities that Work with Pathogens Aecting
Agricultural Animals
................................................................................ 429
Potential Enhancements for Animal Biosafety Level 2-Agriculture
(ABSL-2Ag) Facilities for Conducting Work with Animals that are
Loose-Housed or in Open Penning......................................................... 429
Animal Biosafety Level 3-Agriculture (ABSL-3Ag) Facilities required
for activities involving the use of hazardous biological agents
designated as High-Consequence Foreign Animal Diseases and
Pests by USDA APHIS in animals that are loose-housed or in
open penning
.......................................................................................... 433
Animal Biosafety Level 4-Agriculture (ABSL-4Ag) Facilities for
Conducting Work with Animals that are Loose-Housed or in
Open Penning
........................................................................................ 443
References
................................................................................................ 456
Appendix E—Arthropod Containment Guidelines (ACG)
............................ 458
References
................................................................................................ 459
Appendix F—Select Agents and Toxins
........................................................ 460
Appendix G—Integrated Pest Management (IPM)
........................................ 463
References
................................................................................................ 465
Appendix H—Working with Human, Non-Human Primate (NHP),
and Other Mammalian Cells and Tissues
...................................................... 466
Bloodborne pathogens and risk assessment related to material
source and type
...................................................................................... 466
Risk Mitigation
........................................................................................... 468
References
................................................................................................ 468
xxii Biosafety in Microbiological and Biomedical Laboratories
Appendix I—Guidelines for Work with Toxins of Biological Origin ............ 470
General Considerations for Toxin Use
........................................................ 470
Training and Laboratory Planning
.............................................................. 471
Safety Equipment and Containment
........................................................... 472
Inadvertent Toxin Aerosols
......................................................................... 473
Mechanical Injuries
.................................................................................... 474
Additional Precautions
............................................................................... 475
Decontamination and Spills
........................................................................ 476
Select Toxins
.............................................................................................. 478
References
................................................................................................ 481
Appendix J—NIH Oversight of Research Involving Recombinant
Biosafety Issues
.............................................................................................. 484
Appendix K—Inactivation and Verication
................................................... 486
Background
................................................................................................ 486
Filtration and Centrifugation
....................................................................... 487
Development of Inactivation Procedures
.................................................... 487
Validation of Inactivation Procedures
........................................................ 495
Alternative Strategies
................................................................................. 495
Attenuation Methods
.................................................................................. 496
Process Verication
................................................................................... 498
Institutional Verication
.............................................................................. 498
Tracking of and Communication about Inactivated Samples
...................... 498
Ongoing Review and Oversight of Inactivation and Verication
Procedures
............................................................................................. 499
Other Important Considerations
................................................................. 500
Conclusion
................................................................................................. 500
References
................................................................................................ 501
Appendix L—Sustainability
............................................................................ 504
Introduction and Issues
.............................................................................. 504
Strategies for Existing Laboratories and Operations
.................................. 504
Strategies for New and Renovated Laboratories
........................................ 507
References
................................................................................................ 512
Appendix M—Large-Scale Biosafety
............................................................. 515
Introduction
................................................................................................ 515
Risk Assessment
........................................................................................ 515
Conclusion
................................................................................................. 526
References
................................................................................................ 526
xxiiiContents
Appendix N—Clinical Laboratories ............................................................... 529
Clinical Laboratory Biosafety
...................................................................... 529
Conducting Risk Assessments in a Clinical Laboratory Environment
......... 529
Implementing Mitigation Measures in the Clinical Laboratory
Environment
........................................................................................... 531
Challenges in a Clinical Laboratory Environment
....................................... 537
Implementing Performance Management in a Clinical Laboratory
Environment
........................................................................................... 539
Risk Ethics in a Clinical Laboratory Environment
....................................... 540
Summary
................................................................................................... 541
References
................................................................................................ 541
Appendix O—Acronyms
................................................................................. 544
Glossary
.......................................................................................................... 552
Index
................................................................................................................ 559
Accessibility Descriptions of Figures
........................................................... 571
Appendix A—Primary Containment for Biohazards
.................................... 571
Appendix C—Transportation of Infectious Substances
.............................. 574

xxiv Biosafety in Microbiological and Biomedical Laboratories
Tables
Section IV—Laboratory Biosafety Level Criteria
Table 1. Summary of Laboratory Biosafety Levels (BSLs)
Section VIII-B: Fungal Agents
Table 1. Miscellaneous Yeast and Mold
Section VIII-E: Viral Agents
Table 1. Viruses currently included in the genus Lyssavirus
Section VIII-F: Arboviruses and Related Zoonotic Viruses
Table 1. Vaccine Strains of Specic Viruses that May Be Handled at BSL-2
Table 2. Explanation of Symbols Used in Tables 3 and 4 to Dene Basis for
Assignment of Viruses to Biosafety Levels
Table 3. Alphabetic Listing of Arboviruses and Hemorrhagic Fever Viruses*
Table 4. Alphabetic Listing of Arboviruses and Hemorrhagic Fever Viruses*
Table 5. Laboratories working with the viruses at BSL-3 listed below are
recommended to HEPA lter the exhaust air
Section VIII-H: Prion Diseases
Table 1. Human Prion Diseases
Table 2. Animal Prion Diseases
Table 3. Tissue Preparation for Human CJD and Related Diseases
Table 4. Prion Inactivation Methods for Reusable Instruments and Surfaces
Appendix A—Primary Containment for Biohazards: Selection,
Installation, and Use of Biological Safety Cabinets
Table 1. Selection of a Safety Cabinet through Risk Assessment
Table 2. Comparison of Biosafety Cabinet Characteristics
Appendix B—Decontamination and Disinfection of Laboratory
Surfaces and Items
Table 1. Activity Levels of Selected Liquid Chemical Disinfectants
Appendix D—Biosafety and Biocontainment for Pathogens
Aecting Agricultural Animals and Animals that are Loose-Housed
or in Open Penning
Table 1. Bacteria
Table 2. Fungi and Molds
Table 3. Nematodes, Trematodes, Cestodes, Protozoa, and Ectoparasites
Table 4. Viruses
Table 5. Toxins
Table 6. Prions

xxvContents
Table Key 1. Natural Host Range
Table Key 2. Natural Routes of Transmission
Table Key 3. Environmental Stability
Appendix I—Guidelines for Work with Toxins of Biological Origin
Table 1. Physical Inactivation of Toxins
Table 2. Chemical Inactivation of Toxins
Appendix K—Inactivation and Verication
Table 1. Advantages of Physical Inactivation
Table 2. Disadvantages of physical inactivation
Table 3. Advantages of chemical inactivation
Table 4. Disadvantages of chemical inactivation
Table 5. Advantages of chemical activated by physical treatment
Table 6. Disadvantages of chemical activated by physical treatment
Table 7. Advantages of natural and emerging antimicrobial strategies
Table 8. Disadvantages of natural and emerging antimicrobial strategies
Table 9. Advantages of combination methods
Table 10. Disadvantages of combination methods
Table 11. Advantages of novel methods to attenuate pathogens
Table 12. Disadvantages of novel methods to attenuate pathogens

xxvi Biosafety in Microbiological and Biomedical Laboratories
Figures
Appendix A—Primary Containment for Biohazards: Selection,
Installation, and Use of Biological Safety Cabinets
Figure 1. HEPA Filters
Figure 2. The Class I BSC
Figure 3. The Class II, Type A BSC
Figure 4. Canopy (thimble) unit for ducting a Class II, Type A BSC
Figure 5a. The Class II, Type B1 BSC (classic design)
Figure 5b. The Class II, Type B1 BSC (benchtop design)
Figure 6. The Class II, Type B2 BSC
Figure 7a. The Class II, Type C1 BSC (not connected to building
exhaust system)
Figure 7b. The Class II, Type C1 BSC (connected to building
exhaust system)
Figure 8. The Class III BSC
Figure 9a. The Horizontal Laminar ow Clean Bench
Figure 9b. The Vertical Laminar Flow Clean Bench
Figure 10. Clean to Dirty
Figure 11. Protection of a house vacuum
Figure 12. Bag-in/bag-out lter enclosure
Appendix B—Decontamination and Disinfection of Laboratory
Surfaces and Items
Figure 1. Descending Order of Relative Resistance to
Disinfectant Chemicals
Appendix C—Transportation of Infectious Substances
Figure 1. A Category A UN Standard Triple Packaging
Figure 2. A Category B Non-specication Triple Packaging
1Section 1—Introduction
Section I—Introduction
Biosafety in Microbiological and Biomedical Laboratories
(BMBL) has become
the overarching guidance document for the practice of biosafety in the U.S.—
the mechanism for addressing the safe handling and containment of infectious
microorganisms and hazardous biological materials. The principles of biosafety
introduced in 1984 in the rst edition of BMBL
and carried through this edition
remain steadfast. These principles are containment and risk assessment.
The fundamentals of containment include the microbiological practices,
safety equipment, and facility safeguards that protect laboratory workers, the
environment, and the public from exposure to infectious microorganisms that
are handled and stored in the laboratory. Risk assessment is the process that
enables the appropriate selection of microbiological practices, safety equipment,
and facility safeguards that can help prevent Laboratory-associated infections
(LAI). The purpose of periodic updates of BMBL is to rene guidance based
on new knowledge and experiences and to address contemporary issues that
present new risks that confront laboratory workers and the public health. In this
way, the guidance provided within the BMBL will continue to serve the microbio-
logical and biomedical community as a relevant and valuable reference.
1
The uncertainty and change regarding the identication of emerging agents
and the requirements for containment and safe storage of pathogens continues
to accelerate since the last edition of the BMBL was published. New infectious
agents and diseases have emerged. Work with infectious agents in public and
private research, public health, clinical and diagnostic laboratories, and in animal
care facilities has expanded. World events have demonstrated new threats
of bioterrorism. For these reasons, organizations and laboratory directors are
compelled to evaluate and ensure the eectiveness of their biosafety programs,
the prociency of their workers, as well as the capability of equipment, facilities,
and management practices to provide containment and security of microbiological
agents. Similarly, individual workers who handle pathogenic microorganisms
must understand the containment conditions under which infectious agents can
be safely manipulated and secured. Application of this knowledge and the use
of appropriate techniques and equipment will enable the microbiological and
biomedical community to help prevent personal, laboratory, and environmental
exposure to potentially infectious agents or biohazards.
The Occurrence of Laboratory-associated Infections
Published reports of LAIs rst appeared around the start of the 20th century. By
1978, four studies by Pike and Sulkin collectively identied 4,079 LAIs resulting
in 168 deaths occurring between 1930 and 1978.
These studies found that
the ten most common causative agents of overt infections among workers were
Brucella spp., Coxiella burnetii, hepatitis B virus (HBV), Salmonella enterica
serotype Typhi, Francisella tularensis, Mycobacterium tuberculosis, Blastomyces
2–5
2 Biosafety in Microbiological and Biomedical Laboratories
dermatitidis, Venezuelan equine encephalitis virus, Chlamydia psittaci, and
Coccidioides immitis. The authors acknowledged that the 4,079 cases did not
represent all LAIs that occurred during this period, since many laboratories chose
not to report overt cases or conduct surveillance programs to identify subclinical
or asymptomatic infections.
In addition, historical reports of LAIs seldom provided data sucient to determine
incidence rates, complicating quantitative assessments of risk. Similarly, there
were no distinguishable accidents or exposure events identied in more than
80% of the LAIs reported before 1978. Studies did show that, in many cases,
the infected person worked with a microbiological agent or was in the vicinity of
another person handling an agent.
2–6
During the 20 years following the Pike and Sulkin publications, a worldwide
literature search by Harding and Byers revealed 1,267 overt infections with 22
deaths.
7
Five deaths were of fetuses aborted as the consequence of a maternal
LAI. Mycobacterium tuberculosis, Coxiella burnetii, hantavirus, arboviruses,
HBV, Brucella spp., Salmonella spp., Shigella spp., hepatitis C virus, and Crypto-
sporidium spp. accounted for 1,074 of the 1,267 infections. The authors also
identied an additional 663 cases that presented as subclinical infections. Like
Pike and Sulkin, Harding and Byers reported that only a small number of the LAI
involved a documented specic incident. The non-specic associations reported
most often by these authors were working with a microbiological agent, being in
or around the laboratory, or being around infected animals.
The ndings of Harding and Byers indicated that clinical (diagnostic) and research
laboratories accounted for 45% and 51%, respectively, of the total LAIs reported.
This is a marked dierence from the LAIs reported by Pike and Sulkin prior to
1979, which indicated that clinical and research laboratories accounted for 17%
and 59%, respectively. The relative increase of LAIs in clinical laboratories may
be due in part to improved employee health surveillance programs that are able
to detect subclinical infections, or to the use of inadequate containment proce-
dures during the early stages of culture identication.
Comparison of the more recent LAIs reported by Harding and Byers with those
reported by Pike and Sulkin suggests that the number is decreasing. Harding and
Byers note that improvements in containment equipment, engineering controls,
and greater emphasis on safety training may be contributing factors to the
apparent reduction in LAIs over two decades. However, due to the lack of infor-
mation on the actual numbers of infections and the population at risk, it is dicult
to determine the true incidence of LAIs.
Publication of the occurrence of LAIs provides an invaluable resource for the
microbiological and biomedical community. For example, one report of occupa-
tional exposures associated with Brucella melitensis, an organism capable of
3Section 1—Introduction
transmission by the aerosol route, described how a sta member in a clinical
microbiology laboratory accidentally sub-cultured B. melitensis on the open
bench.
8
This error and a breach in containment practices resulted in eight LAIs
with B. melitensis among 26 laboratory members—an attack rate of 31%.
Reports of LAIs can serve as lessons in the importance of maintaining safe
conditions in biomedical and clinical laboratories.
Evolution of National Biosafety Guidelines
National biosafety guidelines evolved from the eorts of the microbiological and
biomedical community to promote the use of safe microbiological practices,
safety equipment, and facility safeguards that reduce LAIs and protect public
health and the environment. The historical accounts of LAIs raised awareness
about the hazards of infectious microorganisms and the health risks to laboratory
workers who handle them. Many published accounts suggested practices and
methods that might prevent LAIs.
9
Arnold G. Wedum was the Director of Industrial
Health and Safety at the United States Army Biological Research Laboratories,
Fort Detrick, from 1944 to 1969. His pioneering work in biosafety provided the
foundation for evaluating the risks of handling infectious microorganisms and
for recognizing biological hazards and developing practices, equipment, and
facility safeguards for their control. Fort Detrick also advanced the eld by aiding
the development of biosafety programs at the United States Department of
Agriculture (USDA), National Animal Research Center (NARC) and the United
States Department of Health and Human Services (DHHS), Centers for Disease
Control and Prevention (CDC), and National Institutes of Health (NIH). These
governmental organizations subsequently developed several national biosafety
guidelines that preceded the rst edition of BMBL.
In 1974, the CDC published Classication of Etiologic Agents on the Basis of
Hazard.
10
This report introduced the concept for establishing ascending levels of
containment that correspond to risks associated with handling infectious microor-
ganisms that present similar hazardous characteristics. Human pathogens were
grouped into four classes according to mode of transmission and the severity
of disease they caused. A fth class included non-indigenous animal pathogens
whose entry into the United States was restricted by USDA policy.
The NIH published National Cancer Institute Safety Standards for Research
Involving Oncogenic Viruses in 1974.
11
These guidelines established three
levels of containment based on an assessment of the hypothetical risk of cancer
in humans from exposure to animal oncogenic viruses or a suspected human
oncogenic virus isolate.
12,13
In 1976, NIH rst published the NIH Guidelines for
Research Involving Recombinant DNA Molecules (NIH Guidelines).
14
The current
NIH Guidelines described in detail the microbiological practices, equipment,
and facility safeguards that correspond to four ascending levels of physical
4 Biosafety in Microbiological and Biomedical Laboratories
containment and established criteria for assigning experiments to a containment
level based on an assessment of potential hazards of this continually evolving
technology.
15
The evolution of these guidelines set the foundation for developing
a code of practice for biosafety in microbiological and biomedical laboratories.
Led by the CDC and NIH, a broad collaborative initiative involving scientists,
laboratory directors, occupational physicians, epidemiologists, public health
ocials, and health and safety professionals developed the rst edition of BMBL
in 1984.
16
The BMBL provided the technical content not previously available in
biosafety guidelines by adding summary statements conveying guidance pertinent
to infectious microorganisms that had caused LAIs. The sixth edition of BMBL is
also the product of a broad collaborative initiative committed to perpetuate the
value of this national biosafety code of practice.
Risk Criteria for Establishing Ascending Levels of Containment
The primary risk criteria used to dene the four ascending levels of containment,
referred to as Biosafety Levels 1 through 4, are infectivity, severity of disease,
transmissibility, and the nature of the work being conducted. Another important
risk factor for agents that cause moderate to severe disease is the origin of the
agent, whether indigenous or exotic. Each level of containment describes the
microbiological practices, safety equipment, and facility safeguards for the corre-
sponding level of risk associated with handling an agent. The facility safeguards
associated with Biosafety Levels 1 through 4 help protect non-laboratory
occupants of the facility, the public health, and the environment.
Biosafety Level 1 (BSL-1) is the basic level of protection and is appropriate for
dened and characterized strains of viable biological agents that are not known
to cause disease in immunocompetent adult humans. Biosafety Level 2 (BSL-2)
is appropriate for handling moderate-risk agents that cause human disease of
varying severity by ingestion or through percutaneous or mucous membrane
exposure. Biosafety Level 3 (BSL-3) is appropriate for agents with a known
potential for aerosol transmission, for agents that may cause serious and poten-
tially lethal infections, and that are indigenous or exotic in origin. Exotic agents
that pose a high individual risk of life-threatening disease by infectious aerosols
and for which no treatment is available are restricted to high containment labora-
tories that meet Biosafety Level 4 (BSL-4) guidelines.
It is important to emphasize that the causative incident for most LAIs is
unknown.
7,8
Less obvious exposures such as the inhalation of infectious
aerosols or direct contact of broken skin or mucous membranes with droplets
containing an infectious microorganism or surfaces contaminated by droplets
may possibly explain the incident responsible for a number of LAIs. Manipulations
of liquid suspensions of microorganisms may produce aerosols and droplets.
Small-particle aerosols have respirable size particles that may contain one or
several microorganisms. These small particles stay airborne and easily disperse
5Section 1—Introduction
throughout the laboratory. When inhaled, the human lung will retain these
particles. Larger particle droplets rapidly fall out of the air, contaminating gloves,
the immediate work area, and the mucous membranes of unprotected workers.
A procedure’s potential to release microorganisms into the air as aerosols and
droplets is the most important operational risk factor that supports the need for
containment equipment and facility safeguards.
Agent Summary Statements
The sixth edition, as in all previous editions, includes agent summary statements
that describe the hazards, recommended precautions, and levels of containment
appropriate for handling specic human and zoonotic pathogens in the laboratory
and in facilities that house laboratory vertebrate animals. Agent summary
statements are included for agents that meet one or more of the following three
criteria:
1. The agent is a proven hazard to laboratory personnel working with
infectious materials;
2. The agent is suspected to have a high potential for causing LAIs
even though no documented cases exist; and
3. The agent causes grave disease or presents a signicant public
health hazard.
Scientists, clinicians, and biosafety professionals prepared the statements by
assessing the risks of handling the agents using standard protocols followed in
many laboratories. No one should conclude that the absence of an agent
summary statement for a human pathogen means that the agent is safe to
handle at BSL-1 or without a risk assessment to determine the appropriate
level of containment. Laboratory directors should also conduct independent
risk assessments before beginning work with an agent or procedure new to the
laboratory, even though an agent summary statement is available. There may
be situations where a laboratory director should consider modifying the precau-
tionary measures or recommended practices, equipment, and facility safeguards
described in an agent summary statement. In addition, laboratory directors
should seek guidance when conducting risk assessments. Knowledgeable
colleagues, institutional safety committees, institutional biosafety committees,
biosafety ocers, and public health, biosafety, and scientic associations are
excellent resources.
The agent summary statements in the fth edition of BMBL were reviewed in the
course of preparing the sixth edition. There are new and updated agent summary
statements including those for agents classied as Select Agents. For example,
there is an updated section on arboviruses and related zoonotic viruses including
new agent summary statements as well as statements for recently emerged
agents such as Middle East Respiratory Syndrome coronavirus (MERS-CoV).

6 Biosafety in Microbiological and Biomedical Laboratories
The sixth edition includes a substantially revised section on risk assessment that
emphasizes the critical importance of this process in selecting the appropriate
practices and level of containment. That section intentionally follows this intro-
duction because risk assessment is the core principle that supports a code of
practice for safe handling of infectious agents in microbiological and biomedical
laboratories.
Laboratory Biosecurity
The nation also continues to face a challenge in safeguarding the public health
from potential domestic or international bioterrorism. Existing standards and
practices may require adaptation to ensure protection from such hostile actions.
Federal regulations mandate increased security within the microbiological and
biomedical community in order to protect high consequence biological pathogens
and toxins from theft, loss, or misuse. The sixth edition of BMBL includes an
update on laboratory biosecurity—the discipline addressing the security of
microbiological agents and toxins and the threats posed to human and animal
health, the environment, and the economy by deliberate misuse or release. A
careful review of the laboratory biosecurity concepts and guidelines in Section VI
is essential for all laboratory workers.
Using Biosafety in Microbiological and Biomedical Laboratories
BMBL is a code of practice and an authoritative reference. Knowledge sucient to
work safely with hazardous microorganisms requires a careful review of multiple
sections of the BMBL. This will oer the reader an understanding of the biosafety
principles that serve as the basis for the concepts and recommendations included
in this reference. Reading only selected sections will not adequately prepare even
an experienced laboratory worker to handle potentially infectious agents safely.
The recommended practices, safety equipment, and facility safeguards described
in the BMBL are advisory. The intent was and is to establish a voluntary code of
practice, one that all members of a laboratory community will together embrace to
safeguard themselves and their colleagues, and to protect the public health and
environment.
Additional appendices have been added to the sixth edition of the BMBL,
including: Appendix K—Inactivation and Verication; Appendix L—Sustainability;
Appendix M—Large Scale Biosafety; and Appendix N—Clinical Laboratories. In
Appendix K, content has been added on inactivation verication, as recent events
have demonstrated that it may be insucient to follow a published inactivation
procedure and assume that it is capable of providing complete inactivation
of all pathogenic organisms present in a sample. In Appendix L, content has
been added to assist laboratories with nding methods to reduce the signicant
operating costs associated with laboratories. In Appendix M, biosafety consider-
ations for large-scale production of agents has been added, in recognition of the

7Section 1—Introduction
interest in the use of biological agents in the generation of biopharmaceuticals.
Finally, in Appendix N, content on the safe handling of biological materials
in clinical laboratories has been added, as the risk assessment of handling
specimens with unconrmed but suspected high-risk agents can be signicantly
dierent from the assessment traditionally generated in microbiology laboratories.
The BMBL should not be used as a single source of biosafety information; it
provides the basis for a rational risk assessment to be developed and reviewed
by the competent stakeholders at an institution. Inclusion of all relevant stake-
holders, including the biosafety oce or ocer, animal care sta, facilities sta,
management, and the Institutional Biosafety Committee, or equivalent resource,
is needed to ensure all relevant parties provide input and reach consensus on
the risk assessment.
Looking Ahead
Although Laboratory-associated infections are infrequent, it is critical that the
microbiological and biomedical communities continue their resolve to remain
vigilant and avoid complacency. The widely reported incidents of accidental
shipments of or potential exposures to high-consequence pathogens over the
last several years demonstrate that accidents and unrecognized exposures
continue to occur. The absence of clear evidence of the means of transmission
in most documented LAIs should motivate persons at risk to be alert to all
potential routes of exposure. The accidental release of microbial aerosols is a
probable cause of many LAIs,
17
which demonstrates the importance of worker
training and the ability to recognize potential hazards and correct unsafe
habits. Attention to and procient use of work practices, safety equipment, and
engineering controls are also essential.
Understanding the principles of biosafety, the use of well-executed risk assess-
ments, and the adherence to the microbiological practices, containment, and
facility safeguards described in BMBL will continue to contribute to a safer and
healthier working environment for laboratory sta, adjacent personnel, and the
community.
References
1. Richardson JH, Barkley WE, editors. Biosafety in Microbiological and
Biomedical Laboratories. 1st ed. Washington (DC); 1984.
2. Sulkin SE, Pike RM. Survey of laboratory-acquired infections. Am J Pub
Hlth Nations Hlth. 1951;41(7):769–81.
3. Pike RM, Sulkin SE, Schulze ML. Continuing importance of laboratory-
acquired infections. Am J Pub Hlth Nations Hlth. 1965;55:190–9.
4. Pike RM. Laboratory-associated infections: summary and analysis of 3921
cases. Health Lab Sci. 1976;13(2):105–14.
8 Biosafety in Microbiological and Biomedical Laboratories
5. Pike RM. Past and present hazards of working with infectious agents.
Arch Pathol Lab Med. 1978;102(7):333–6.
6. Pike RM. Laboratory-associated infections: incidence, fatalities, causes,
and prevention. Annu Rev Microbiol. 1979;33:41–66.
7. Harding AL, Byers KB. Epidemiology of Laboratory-associated infections.
In: Fleming DO, Hunt DL, editors. Biological Safety: Principles and
Practices. 3rd ed. Washington (DC): ASM Press; 2000. p. 35–54.
8. Staskiewicz J, Lewis CM, Colville J, Zervos M, Band J. Outbreak of Brucella
melitensis among microbiology laboratory workers in a community hospital.
J Clin Microbiol. 1991;29(2):287–90.
9. Wedum AG. Laboratory safety in research with infectious diseases. Public
Health Rep. 1964;79(7):619–33.
10. Ad Hoc Committee on the Safe Shipment and Handling of Etiologic Agents;
Center for Disease Control. Classication of etiologic agents on the basis
of hazard. 4th ed. Atlanta (GA): U.S. Department of Health, Education,
and Welfare, Public Health Service, Center for Disease Control, Oce of
Biosafety; 1974.
11. National Cancer Institute, Oce of Research Safety. National Cancer
Institute safety standards for research involving oncogenic viruses.
Bethesda: The National Institutes of Health (US); 1974.
12. Wedum AG. History and epidemiology of laboratory-acquired infections
(in relation to the cancer research program). JABSA. 1997;2(1):12–29.
13. West DL, Twardzik DR, McKinney RW, Barkley WE, Hellman A.
Identication, analysis, and control of biohazards in viral cancer research.
In: Fuscaldo AA, Erlick BJ, Hindman B, editors. Laboratory safety theory
and practice. New York: Academic Press; 1980. p. 167–223.
14. National Institutes of Health. NIH Guidelines for Research Involving
Recombinant DNA Molecules (NIH Guidelines). Bethesda (MD): National
Institutes of Health; 1976.
15. National Institutes of Health. NIH Guidelines for Research Involving
Recombinant or Synthetic Nucleic Acid Molecules (NIH Guidelines).
Bethesda (MD): National Institutes of Health, Oce of Science Policy; 2019.
16. Centers for Disease Control and Prevention; National Institutes of
Health. Biosafety in Microbiological and Biomedical Laboratories. 1st ed.
Richardson JH, Barkley WE, eds. Atlanta (GA): CDC; Bethesda (MD): NIH;
Washington (DC): U.S. G.P.O.; 1984.
17. Rusnak JM, Kortepeter MG, Hawley RJ, Anderson AO, Boudreau E, Eitzen E.
Risk of occupationally acquired illnesses from biological threat agents in
unvaccinated laboratory workers. Biosecur Bioterror. 2004;2(4):281–93.
9Section II—Biological Risk Assessment
Section II—Biological Risk Assessment
The ongoing practice of biological risk assessment is the foundation of safe
laboratory operations. Risk assessment requires careful judgment and is an
important responsibility for directors and principal investigators (PI) of micro-
biological and biomedical laboratories. Institutional leadership and oversight
resources, such as Institutional Biosafety Committees (IBCs) or equivalent
resources, animal care and use committees, biological safety professionals,
occupational health sta, and laboratory animal veterinarians also share in this
responsibility. When assessing risk, it is essential to broadly engage stakeholders,
including laboratory and facility sta and subject matter experts, in committee
reviews of work and discussions of past studies of Laboratory-associated infec-
tions (LAIs) and other published research. The biological risk assessment process
is used to identify the hazardous characteristics of an infectious or potentially
infectious agent or material, if known; the activities that can result in a person’s
exposure to an agent; the likelihood that such exposure will cause an LAI; and
the probable consequences of such an infection. The information identied by
risk assessment will provide a guide for the selection of appropriate mitigations,
including the application of Biosafety Levels and good microbiological practices,
safety equipment, and facility safeguards that can help prevent LAIs.
Promoting a positive culture of safety by integrating a risk management process
into daily laboratory operations results in the ongoing identication of hazards and
prioritization of risks and the establishment of risk mitigation protocols tailored
to specic situations. To be successful, this process must be collaborative and
inclusive of all stakeholders. Further, it must recognize a hierarchy of controls,
beginning with the elimination or reduction of hazards, then progress to imple-
menting the appropriate engineering and/or administrative controls to address
residual risks, and, if necessary, identifying personal protective equipment (PPE)
to protect the worker.
1
For the purposes of this section, hazards are dened as substances or situations
capable of causing adverse eects to health or safety.
2
Risks occur when people
interact with hazards and are a function of both the probability of adverse events
and expected consequences of a potential incident.
2
The product of probability
and consequence estimates provide a relative value that can be used to prioritize
risks. Since it is impossible to eliminate all risk, unless the associated hazard is
eliminated, the risk assessment evaluates recognized risks associated with a
particular hazard and reduces risk to an institutionally acceptable level through a
documented process. For the biological laboratory, this process is usually quali-
tative with classications from high- to low-risk. This section provides guidance on
conducting a risk assessment, implementing a risk mitigation program, commu-
nicating during and after the assessment, and developing practices to support
ongoing application of the risk assessment process.

10 Biosafety in Microbiological and Biomedical Laboratories
Risks are best mitigated by combining and overlapping risk management
practices and risk mitigation controls to oer redundant protections for the worker,
community, and the environment. Working through the risk assessment process
identies best practices for manipulating biological agents, how to integrate
multiple containment or protection strategies, and how to respond if something
does not go as planned. When performed comprehensively, it accounts for
changing methodologies, procedures, and regulations as the work evolves.
Adverse consequences, like LAIs, are more likely to occur if the risks are uniden-
tied or underestimated. By contrast, imposition of safeguards more rigorous
than needed may result in additional expense and burden for the laboratory
with little enhancement of laboratory safety. However, where there is insucient
information to make a clear determination of risk, consider the need for additional
safeguards until more data are available.
The Risk Management Process
The sixth edition of Biosafety in Microbiological and Biomedical Laboratories
(BMBL) provides guidance on risk mitigation measures to address common
agent and protocol risks. As all possible adverse incidents can’t be predicted,
judgments and decisions about control measures sometimes need to be based
on incomplete information. Special risks, associated with a particular type of
laboratory, may require more caution in the risk assessment; for example, clinical
laboratories rarely have the benet of agent information, as they are typically
looking to identify the causative agent for a medical diagnosis. Please refer to
Appendix N for additional information on clinical laboratories.
This section describes a six-step approach that gives structure to the risk
management process and reinforces an ongoing positive culture of safety. Other
methodologies may be useful, including the process described in the WHO
Laboratory Biosafety Manual.
The initial factors to consider in risk assessment fall into two broad categories:
agent hazards and laboratory procedure hazards. Following the assessment
of the inherent risk, the Biosafety Level and any additional indicated mitigation
strategies are determined. Before implementation of the controls, the risk
assessment and selected safeguards should be reviewed with a biosafety
professional, subject matter expert, and the IBC or equivalent resource. Then,
as part of an ongoing assessment of risk management, the prociency of sta
regarding safe practices and the integrity of safety equipment is evaluated and
training or competency gaps are addressed. Finally, the management strategies
are revisited regularly to reassess risks and mitigations and are updated when
appropriate.

11Section II—Biological Risk Assessment
First, identify hazardous characteristics of the agent and perform an
assessment of the inherent risk, which is the risk in the absence of
mitigating factors. Consider the principal hazardous characteristics of the agent,
which include its capability to infect and cause disease in a susceptible host,
severity of disease, and the availability of preventive measures and eective
treatments. Also consider possible routes of transmission of infection in the
laboratory, infectious dose (ID), stability in the environment, host range, whether
the agent is indigenous or exotic to the local environment, and the genetic
characteristics of the agent.
3–6
Several excellent resources provide information and guidance for making an
initial risk assessment. Section VIII of BMBL provides agent summary statements
for many agents that are associated with LAIs or are of increased public concern.
Agent summary statements also identify known and suspected routes of trans-
mission of Laboratory-associated infections and, when available, information on
infective dose, host range, agent stability in the environment, protective immuni-
zations, and attenuated strains of the agent. Safety documents from reputable
sources are also valuable, such as the Pathogen Data Safety Sheets generated
by the Public Health Agency of Canada (PHAC); the Pathogen Data Safety
Sheets are available at https://www.canada.ca/en/public-health/services/labora-
tory-biosafety-biosecurity/pathogen-safety-data-sheets-risk-assessment.html. A
thorough examination of the agent hazards is necessary when the intended use
of an agent does not correspond with the general conditions described in the
agent summary statement or when an agent summary statement is not available.
In addition, it is always helpful to seek guidance from colleagues with experience
in handling the agent and from biological safety professionals.
The NIH Guidelines for Research Involving Recombinant or Synthetic Nucleic
Acid Molecules (NIH Guidelines) has incorporated an agent Risk Group (RG)
classication for laboratory use that describes four general Risk Groups based
on these principle characteristics and the route of transmission of the natural
disease; this list is found in Appendix B of the NIH Guidelines. ABSA International
also has a compendium of organisms and Risk Group assignments from several
countries and organizations available at https://my.absa.org/Riskgroups. Agent
Risk Group assignments assist with an initial estimate of the pathogen’s risk;
the assessment must be modied appropriately based on the unique risks faced
by each laboratory for the specic work being done. The four groups address
the risk to both the laboratory worker and the community and correlate
with, but do not equate to, Biosafety Levels. See Section III for additional
information about Risk Groups and Biosafety Levels.

12 Biosafety in Microbiological and Biomedical Laboratories
Genetically modied agent hazardous characteristics The identication and
assessment of hazardous characteristics of genetically modied agents involve
consideration of the same factors used in risk assessment of the wild-type
organism. It is particularly important to address the possibility that the genetic
modication could increase or decrease an agent’s pathogenicity or aect its
susceptibility to antibiotics or other eective treatments. The risk assessment
can be dicult or incomplete because important information may not be available
for a newly engineered agent. Several investigators have reported that they
observed unanticipated enhanced virulence in recent studies with engineered
agents;
7–10
these observations give reasons to remain alert to the possibility that
experimental alteration of virulence genes may lead to altered risk and reinforce
the nature of risk assessment as a continuing process that requires updating as
research progresses.
The NIH Guidelines are the key reference in assessing risk and establishing
an appropriate Biosafety Level for work involving recombinant DNA molecules.
Please refer to Appendix J for more information about the NIH Guidelines and
the NIH Oce of Science Policy (OSP). The NIH Guidelines are available at
https://osp.od.nih.gov/wp-content/uploads/NIH_Guidelines.pdf.
11
Cell Cultures Workers who handle or manipulate human or animal cells and
tissues are at risk for possible exposure to potentially infectious latent and
adventitious agents that may be present in those cells and tissues. This risk is
illustrated by the reactivation of herpes viruses from latency,
12,13
the inadvertent
transmission of disease to organ recipients,
14,15
and the persistence of human
immunodeciency virus (HIV), hepatitis B virus (HBV), and hepatitis C virus
(HCV) within infected individuals in the U.S. population.
16
In addition, human and
animal cell lines that are not well characterized or are obtained from secondary
sources may introduce an infectious hazard to the laboratory. For example, the
handling of nude mice inoculated with a tumor cell line unknowingly infected with
lymphocytic choriomeningitis virus resulted in multiple LAIs.
17
See Appendix H for
additional information.
Other hazardous characteristics of an agent include probable routes of trans-
mission in the laboratory, infective dose, stability in the environment, host range,
and its endemic nature. In addition, reports of LAIs are a clear indicator of hazard
and often are sources of information helpful for identifying agent and procedural
hazards, and the precautions for their control. The absence of a report does not
indicate minimal risk. The number of infections reported for a single agent may
be an indication of the frequency of use as well as risk. Reporting of LAIs by
laboratory directors in scientic and medical literature is encouraged. The agent
summary statements in BMBL include specic references to reports on LAIs.
13Section II—Biological Risk Assessment
Once the inherent risk associated with the agent is considered, the next step in
the process involves addressing the possibility of transmission of the agent. The
most likely routes of transmission in the laboratory are:
1. Direct skin, eye or mucosal membrane exposure to an agent;
2. Parenteral inoculation by a syringe needle or other contaminated sharp,
or by bites from infected animals and arthropod vectors;
3. Ingestion of liquid suspension of an infectious agent, or by contaminated
hand to mouth exposure; and
4. Inhalation of infectious aerosols.
An awareness of the routes of transmission for the natural human disease is
helpful in identifying probable routes of transmission in the laboratory and the
potential for any risk to public health. For example, transmission of infectious
agents can occur by direct contact with discharges from respiratory mucous
membranes of infected persons, which would be a clear indication that a
laboratory worker is at risk of infection from mucosal membrane exposure to
droplets generated while handling that agent. Additional information used to
identify both natural and often noted laboratory modes of transmission can
be found in the Control of Communicable Diseases Manual.
3
It is important to
remember that the nature and severity of disease caused by a Laboratory-asso-
ciated infection and the probable route of transmission of the infectious agent in
the laboratory may dier from the route of transmission and severity associated
with the naturally-acquired disease.
18
An agent capable of transmitting disease through respiratory exposure to infec-
tious aerosols is a serious laboratory hazard, both for the person handling the
agent and for other laboratory occupants. Infective dose and agent stability are
particularly important in establishing the risk of airborne transmission of disease.
For example, the reports of multiple infections in laboratories associated with
the use of Coxiella burnetii are explained by its low inhalation infective dose,
which is estimated to be 10 inhaled infectious particles, and its resistance to
environmental stresses that enables the agent to survive outside of a living host
or culture media long enough to become an aerosol hazard.
19
When work involves the use of laboratory animals, the hazardous charac-
teristics of zoonotic agents require careful consideration when completing
a risk assessment. Evidence that experimental animals can shed zoonotic
agents and other infectious agents under study in saliva, urine, or feces is an
important indicator of hazard. The death of a primate center laboratory worker
from Macacine herpesvirus 1 (MHV-1, also known as Monkey B virus) infection
following an ocular splash exposure to biologic material from a rhesus macaque
emphasizes the seriousness of this hazard.
20
Experiments that demonstrate

14 Biosafety in Microbiological and Biomedical Laboratories
transmission of disease from an infected animal to a normal animal housed in the
same cage are reliable indicators of hazard. Experiments that do not demonstrate
transmission, however, do not rule out the hazard. For example, experimental
animals infected with Francisella tularensis, Coxiella burnetii, Coccidioides
immitis, or Chlamydia psittaci—agents that have caused many LAIs—rarely infect
cagemates.
21
The origin of the agent is also important when conducting a risk assessment.
Non-indigenous agents are of special concern because of their potential to
transmit or spread infectious diseases from foreign countries into the United
States. Importation of agents of human disease requires a permit from the CDC.
Importation of many agents of livestock, poultry, and other animal diseases
requires a permit from the USDA’s Animal and Plant Health Inspection Service
(APHIS). For additional details, see Appendix C.
Often, there is not sucient information to make an appropriate assessment
of risk. For example, the hazard of an unknown agent that may be present in a
specimen may not be known until the completion of agent identication and typing
procedures. It would be prudent to assume the specimen contains an unknown
agent presenting the hazardous classication that correlates with a minimum
of BSL-2 containment, unless additional information suggests the presence of
an agent of higher risk. Identication of agent hazards associated with newly
emergent pathogens also requires judgments based on incomplete information.
Often, epidemiologic ndings are the best sources for information in these cases.
When assessing the hazards of a newly attenuated pathogen, experimental data
should support a judgment that the attenuated pathogen is less hazardous than
the wild-type parent pathogen before making any reduction in the containment
recommended for that pathogen.
Second, identify laboratory procedure hazards. The principal laboratory
procedure hazards are agent concentration, suspension volume, equipment and
procedures that generate small particle aerosols and larger airborne particles
(droplets), and use of sharps. Procedures involving animals can present a
number of hazards such as bites and scratches, exposure to zoonotic agents,
and the handling of experimentally generated infectious aerosols.
Investigations of LAIs have identied the following routes of transmission: paren-
teral inoculations with syringe needles or other contaminated sharps, spills and
splashes onto skin and mucous membranes, ingestion through mouth pipetting,
animal bites and scratches, and inhalation exposures to infectious aerosols. The
rst four routes of laboratory transmission were easy to detect but accounted
for less than 20% of the LAIs reported in the 1979 retrospective review by
Pike.
22
Subsequent research on LAIs has conrmed that the probable sources of
infection are frequently not known.
23
15Section II—Biological Risk Assessment
Aerosols and droplets Aerosols are a serious hazard because they are
ubiquitous in laboratory procedures, are usually undetected, and are extremely
pervasive, placing the laboratory worker carrying out the procedure and other
persons in the laboratory at risk of exposure. There is general agreement among
biosafety professionals, laboratory directors, and principal investigators who have
investigated LAIs that an aerosol generated by procedures and operations is the
probable source of many LAIs, particularly in cases involving workers whose only
known risk factor was that they worked with an agent or were in an area where
that work was done.
Procedures that impart energy to a microbial suspension will produce aerosols.
Equipment used for handling and analyzing infectious agents in laboratories,
such as pipettes, blenders, centrifuges, sonicators, vortex mixers, cell sorters,
and matrix-assisted laser desorption/ionization-time of ight (MALDI-TOF) mass
spectrometers are potential sources of aerosols.
24,25
These procedures and
equipment generate respirable-size particles that remain airborne for protracted
periods. These particles can remain in the lungs if inhaled or create an exposure
hazard for coworkers in the laboratory or persons occupying adjacent spaces
open to airow from the laboratory. A number of investigators have determined
the aerosol output of common laboratory procedures. In addition, investigators
have proposed a model for estimating inhalation dosage from a laboratory
aerosol source. Parameters that characterize aerosol hazards include an agent’s
inhalation infective dose, its viability in an aerosol, aerosol concentration, and
particle size.
26–28
A careful and procient worker will minimize the generation of aerosols. For
example, the hurried worker may operate a sonic homogenizer with maximum
aeration, but the careful worker will consistently operate the device to ensure
minimal aeration. Experiments show that the aerosol burden with maximal
aeration is approximately 200 times greater than aerosol burden with minimal
aeration.
26
Similar results were shown for improper pipetting which generated
bubbles versus pipetting with minimal bubble generation.
Procedures and equipment that generate respirable size particles also generate
larger size droplets that settle out of the air rapidly, contaminating hands, work
surfaces, and possibly the mucous membranes of the persons performing the
procedure. An evaluation of the release of both respirable particles and droplets
from laboratory operations determined that the respirable component is relatively
small; in contrast, hand and surface contamination can be substantial.
29
The
potential risk from exposure to droplet contamination requires as much attention
in a risk assessment as the respirable component of aerosols.
Personal Protective Equipment (PPE) and Safety Equipment Hazards There
may be hazards that require specialized PPE in addition to safety glasses,

16 Biosafety in Microbiological and Biomedical Laboratories
laboratory gowns, and gloves. For example, a procedure that presents a splash
hazard may require the use of a mask and a face shield to provide adequate
protection. Inadequate training in the proper use of PPE may reduce its eec-
tiveness, provide a false sense of security, and could increase the risk to the
laboratory worker. For example, a respirator worn incorrectly may impart a risk to
the wearer independent of the agents being manipulated.
Safety equipment such as biological safety cabinets (BSCs), centrifuge safety
cups, and sealed rotors are used to provide a high degree of protection for the
laboratory worker from exposure to microbial aerosols and droplets. Safety
equipment that is not working properly is hazardous, especially when the user is
unaware of the malfunction. Poor location, room air currents, decreased airow,
leaking lters, raised sashes, crowded work surfaces, and poor user technique
compromise the containment capability of a BSC. The safety characteristics of
modern centrifuges are only eective if the equipment is operated properly.
Facility Control Hazards Facility safeguards help prevent the accidental release
of an agent from the laboratory. For example, one facility safeguard is directional
airow, which helps to prevent aerosol transmission from a laboratory into other
areas of the building. Directional airow is dependent on the operational integrity
of the laboratory’s heating, ventilation, and air conditioning (HVAC) system.
HVAC systems require careful monitoring and periodic maintenance to sustain
operational integrity. Loss of directional airow may compromise safe laboratory
operation. BSL-4 containment facilities provide more complex safeguards that
require signicant expertise to design and operate.
Consideration of facility safeguards is an integral part of the risk assessment.
A biological safety professional, building and facilities sta, and the IBC, or
equivalent safety committee, should help assess the facility’s capability to
provide appropriate protection for the planned work and recommend changes as
necessary. Risk assessment may support the need to include additional facility
safeguards in the construction of new or renovation of old facilities.
Third, make a determination of the appropriate Biosafety Level and select
additional precautions indicated by the risk assessment. The selection of the
appropriate Biosafety Level and the selection of any additional laboratory precau-
tions require a comprehensive understanding of the practices, safety equipment,
and facility safeguards described in Sections III, IV, and V of this publication.
There will be situations where the intended use of an agent requires greater
precautions than those described in the agent’s summary statement. These
situations will require the careful selection of additional precautions. An obvious
example would be a procedure for exposing animals to experimentally generated
infectious aerosols.
17Section II—Biological Risk Assessment
It is unusual that a risk assessment would indicate a need to alter the recom-
mended facility safeguards specied for the selected Biosafety Level. If this does
occur, it is important that a biological safety professional validate this judgment
before augmenting any facility secondary barrier.
While an entity’s biosafety plan is based on a risk assessment, the biosafety
plan may be inuenced by federal regulations and guidelines. For example,
the 2017 notice published by the National Science Foundation (NSF) denes
standard terms and conditions for federal research grants.
30
A listing of statutory,
regulatory, and executive requirements is provided in Appendix C of the updated
National Policy Requirements Matrix.
31
The biosafety plan required by the Federal
Select Agents and Toxins regulations (9 CFR Part 121, 42 CFR Part 73) must
be based on an assessment that addresses the risk of the Select Agent or Toxin
given its intended use and consider, where appropriate, the NIH Guidelines for
Research Involving Recombinant or Synthetic Nucleic Acid Molecules. It is also
important to recognize that individuals in the laboratory may dier in their suscep-
tibility to disease. Pre-existing conditions, medications, compromised immunity,
and pregnancy or breast-feeding that may increase exposure of infants to certain
agents are some of the conditions that may increase the risk of an individual for
acquiring an LAI. Consultation with an occupational health care provider knowl-
edgeable in infectious diseases is advisable in these circumstances.
Laboratory directors and principal investigators, or their designees, are respon-
sible for ensuring that the identied controls (equipment, administrative, and
PPE) have been made available and are adhered to or operating properly. For
example, a BSC that is not certied represents a potentially serious hazard to
the laboratory worker using it and to others in the laboratory. The director should
have all equipment deciencies corrected before starting work with an agent.
Vaccination(s) may be recommended for laboratory personnel based on safety
and availability; however, the protection aorded by a vaccine to an individual
depends on the eectiveness of the vaccine and duration of immunity. Vaccination
does not substitute for engineering and administrative risk mitigation controls.
Institutions must address risk perception by setting risk tolerance limits or perfor-
mance expectations on program elements and equipment identied as critical to
operations.
32,33
Risk mitigation requires nding a balanced approach that includes
ongoing hazard identication and review of control measures with a commitment
at all levels to reduce identied risk to a level tolerable to the institution. Risk
acceptance is not equal acceptance of all risks; a level of biological risk may be
essential to performing research, while acceptance of an equal risk of scientic
misconduct is not.
18 Biosafety in Microbiological and Biomedical Laboratories
Fourth, before implementation of the controls, review the risk assessment
and selected safeguards with a biosafety professional, subject matter
expert, and the IBC or equivalent resource. This review is strongly recom-
mended and may be required by regulatory or funding agencies. Review of
potentially high-risk protocols by the IBC should become standard practice.
Adopting this step voluntarily will promote the use of safe practices in work with
hazardous agents in microbiological and biomedical laboratories.
Fifth, as part of an ongoing process, evaluate the prociencies of sta
regarding safe practices and the integrity of safety equipment. The
protection of laboratory workers, other persons associated with the laboratory,
and the public will depend ultimately on the laboratory workers themselves. The
laboratory director or principal investigator should ensure that laboratory workers
have acquired the technical prociency in the use of microbiological practices and
safety equipment required for the safe handling of the agent and have developed
good habits that sustain excellence in the performance of those practices. Sta
at all skill levels need to know how to identify hazards in the laboratory and
how to obtain assistance in protecting themselves and others in the laboratory.
An evaluation of a worker’s training, experience in handling infectious agents,
prociency in following good microbiological practices, correct use of safety
equipment, consistent use of standard operating procedures (SOPs) for specic
laboratory activities, ability to respond to emergencies, and willingness to accept
responsibility for protecting one’s self and others is an important indication that a
laboratory worker is capable of working safely.
An assessment should identify any potential deciencies in the knowledge,
competency, and practices of the laboratory workers. Carelessness is a serious
concern because it can compromise any safeguards of the laboratory and
increase the risk for coworkers. Fatigue and its adverse eects on safety have
been well documented.
34
Training, experience, knowledge of the agent and
procedure hazards, good habits, caution, attentiveness, and concern for the
health of coworkers are prerequisites for laboratory sta in order to reduce the
risks associated with work with hazardous agents. Not all workers who join a
laboratory sta will have these prerequisite traits even though they may possess
excellent scientic credentials. Laboratory directors or principal investigators
should consider the use of competency assessment(s) to train and retrain new
sta to the point where aseptic techniques and safety precautions become
second nature.
35–37
Sixth, revisit regularly and verify risk management strategies and determine
if changes are necessary. Continue the risk management cycle, and adjust and
adapt as the need arises. This includes a regular update of biosafety manuals
and SOPs when changes in procedures or equipment occur. A cyclical, adaptable
19Section II—Biological Risk Assessment
risk management process forms the basis for a robust culture of safety in the
biological laboratory.
Risk Communication
An eective culture of safety depends on the eective communication and
reporting of risk indicators, including incidents and near misses, in a non-
punitive manner.
38
Documents communicating the fundamental elements of a
safety program are an important part of this culture and form the basis of the
risk assessment; this includes hazard communication to all stakeholders.
39
Institutional leadership can engage workers at all levels by collaborating with
institutional safety programs and committing to and supporting a safe working
environment.
Institutions that work with infectious agents and toxins need an appropriate
organizational and governance structure to ensure compliance with biosafety,
biocontainment, and laboratory biosecurity regulations and guidelines, and
to communicate risks.
40
In particular, the principal investigator or the facility
equivalent has the primary responsibility for communicating hazards and risks
in the laboratory. Sta must have the ability to report issues, including incidents
and near misses without fear of reprisal. Laboratory sta, IBCs or equivalent
resource, biosafety professionals, Institutional Animal Care and Use Committees
(IACUCs), and laboratory animal veterinarians also have responsibility for
identifying biological risks associated with laboratory work and communicating
institute-wide risk management practices. A biosafety ocer (BSO) and/or other
safety personnel can coordinate the institution’s safety program and may assist
in the development of risk communication documents including incident trends
and mitigations, SOPs, biosafety manuals, hazard control plans, and emergency
response plans. Risk management can identify deciencies in laboratory
worker performance or institutional policies and assists institutional leadership
responsible to make the necessary changes to safety programs to address those
deciencies. Biosafety program changes that promote the building of a culture
of safety are most eectively communicated across the institution using multiple
communication routes to ensure that all sta are informed. Good communication
practices include messages from leadership, risk management documents, IBCs
or equivalent resource, and other committee reviews, as necessary.
Facilitating a Culture of Safety through Risk Assessment
The goal of your risk assessment is to address all realistic, perceivable risks to
protect personnel, the community, and the environment. Research progress,
changes in personnel, and changes in regulation over time drive programmatic
change and demand reconsideration of all factors, as periodically necessary. Risk
assessment is an ongoing process, and all personnel have a role in its success.

20 Biosafety in Microbiological and Biomedical Laboratories
The challenge is to develop good habits and procedures through training and
competency checks with the support of leadership. Once established, these
practices will persist to further instill a culture of safety. A sound risk commu-
nication strategy is also critical for both hazard identication and successful
implementation. While policies and plans are tangible assets derived from the
risk assessment process, the ultimate success will be measured by whether you
establish, strengthen, and sustain a culture of safety while encouraging commu-
nication about risks between management and sta to prevent accidents before
they happen.
The regular review of all hazards, prioritization of risk, multidisciplinary review
of priority risks, and establishment of risk mitigation measures demonstrate the
institution’s commitment to a safe and secure working environment and form the
cornerstone of a biosafety program. The approach to risk assessment outlined
in the preceding section is not static and benets from active participation by all
relevant stakeholders. Aim for ongoing evaluation and periodic readjustments to
stay aligned with the changing needs of the institution and to protect all persons
from potential exposure to biological materials in laboratories and associated
facilities.
Conclusion
The BMBL is designed to assist organizations with the protection of workers
in biological laboratories and associated facilities from Laboratory-associated
infections. Risk assessment is the basis for the safeguards developed by the
CDC, the NIH, and the microbiological and biomedical community to protect
the health of laboratory workers and the public from the risks associated with
the use of hazardous biological agents in laboratories. Experience shows that
these established safe practices, equipment, and facility safeguards work; new
knowledge and experience may justify altering these safeguards.
References
1. United States Department of Labor [Internet]. Washington (DC):
Occupational Safety and Health Administration; c2016 [cited 2019 Jan 7].
Recommended Practices for Safety and Health Programs. Available from:
https://www.osha.gov/shpguidelines/docs/OSHA_SHP_Recommended_
Practices.pdf
2. Caskey S, Sevilla-Reyes EE. Risk Assessment. In: Laboratory Biorisk
Management: Biosafety and Biosecurity. Salerno RM, Gaudioso, J, editors.
Boca Raton: CRC Press; 2015. p. 45–63.
3. Heymann DL, editor. Control of Communicable Diseases Manual. 20th ed.
Washington (DC): American Public Health Association Press; 2014.

21Section II—Biological Risk Assessment
4. Bennett JE, Dolin R, Blaser MJ, editors. Mandell, Douglas, and Bennett’s
Principles and Practice of Infectious Diseases. 8th ed. Philadelphia (PA);
Elsevier Saunders; 2015.
5. Government of Canada [Internet]. Canada: Public Health Agency of
Canada; c2018 [cited 2019 Jan 7]. Pathogen Safety Data Sheets. Available
from: https://www.canada.ca/en/public-health/services/laboratory-biosafety-
biosecurity/pathogen-safety-data-sheets-risk-assessment.html
6. absa.org [Internet]. Mundelein (IL): American Biological Safety Association
International; [cited 2019 Jan 7]. Available from: https://absa.org.
7. Jackson RJ, Ramsay AJ, Christensen CD, Beaton S, Hall DF, Ramshaw
IA. Expression of Mouse Interleukin-4 by a Recombinant Ectromelia Virus
Suppresses Cytolytic Lymphocyte Responses and Overcomes Genetic
Resistance to Mousepox. J Virol. 2001;75(3):1205–10.
8. Shimono N, Morici L, Casali N, Cantrell S, Sidders B, Ehrt S, et
al. Hypervirulent mutant of Mycobacterium tuberculosis resulting
from disruption of the mce1 operon. Proc Natl Acad Sci USA.
2003;100(26):15918–23.
9. Cunningham ML, Titus RG, Turco SJ, Beverley SM. Regulation of
Dierentiation to the Infective Stage of the Protozoan Parasite Leishmania
major by Tetrahydrobiopterin. Science. 2001;292(5515):285–7.
10. Kobasa A, Takada A, Shinya K, Hatta M, Halfmann P, Theriault S, et al.
Enhanced Virulence of Inuenza A Viruses with the Haemagglutinin of the
1918 Pandemic Virus. Nature. 2004;431(7009):703–7.
11. National Institutes of Health. NIH Guidelines for Research Involving
Recombinant or Synthetic Nucleic Acid Molecules (NIH Guidelines).
Bethesda (MD): National Institutes of Health, Oce of Science Policy; 2019.
12. Efstathiou S, Preston CM. Towards an Understanding of the Molecular Basis
of Herpes Simplex Virus Latency. Virus Res. 2005;111(2):108–19.
13. Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Gelb LD, et
al. A Vaccine to Prevent Herpes Zoster and Postherpetic Neuralgia in Older
Adults. N Engl J Med. 2005;352(22):2271–84.
14. Centers for Disease Control and Prevention. Investigation of Rabies
Infections in Organ Donor and Transplant Recipients–Alabama,
Arkansas, Oklahoma, and Texas, 2004. MMWR Morb Mortal Wkly Rep.
2004;53(26):586–9.
15. Centers for Disease Control and Prevention. Lymphocytic Choriomeningitis
Virus Infection in Organ Transplant Recipients–Massachusetts, Rhode
Island, 2005. MMWR Morb Mortal Wkly Rep. 2005;54(21):537–9.
16. Bloodborne pathogens, 29 C.F.R. Part 1910.1030 (1992).
22 Biosafety in Microbiological and Biomedical Laboratories
17. Dykewicz CA, Dato VM, Fisher-Hoch SP, Howarth MV, Perez-Oronoz GI,
Ostro SM, et al. Lymphocytic Choriomeningitis Outbreak Associated with
Nude Mice in a Research Institute. JAMA. 1992;267(10):1349–53.
18. Lennette EH, Koprowski H. Human Infection With Venezuelan Equine
Encephalomyelitis Virus: A Report on Eight Cases of Infection Acquired in
the Laboratory. JAMA. 1943;123(17):1088–95.
19. Tigertt WD, Benenson AS, Gochenour WS. Airborne Q Fever. Bacteriol Rev.
1961;25:285–93.
20. Centers for Disease Control and Prevention. Fatal Cercopithecine
herpesvirus 1 (B virus) Infection Following a Mucocutaneous Exposure and
Interim Recommendations for Worker Protection. MMWR Morb Mortal Wkly
Rep. 1998;47(49):1073–6, 1083.
21. Wedum AG, Barkley WE, Hellman A. Handling of Infectious Agents. J Am
Vet Med Assoc. 1972;161(11):1557–67.
22. Pike RM. Laboratory-associated infections: Incidence, Fatalities, Causes
and Prevention. Annu Rev Microbiol. 1979;33:41–66.
23. Byers KB, Harding, AL. Laboratory-associated infections. In: Wooley DP,
Byers KB, editors. Biological Safety: Principles and Practices. 5th ed.
Washington (DC): ASM Press; 2017. p. 59–92.
24. Pomerleau-Normandin D, Heisz M, Su M. Misidentication of Risk Group
3/Security Sensitive Biological Agents by MALDI-TOF MS in Canada:
November 2015–October 2017. Can Commun Dis Rep. 2018;44(5):100–15.
25. Holmes KL. Characterization of aerosols produced by cell sorters and
evaluation of containment. Cytometry A. 2011;79(12):1000–8.
26. Dimmick RL, Fogl WF, Chatigny MA. Potential for Accidental Microbial
Aerosol Transmission in the Biology Laboratory. In: Hellman A, Oxman MN,
Pollack R, editors. Biohazards in Biological Research: Proceedings of a
conference held at the Asilomar conference center; 1973 Jan 22–24; Pacic
Grove (CA). New York: Cold Spring Harbor Laboratory; 1973. p. 246–66.
27. Kenny MT, Sabel FL. Particle Size Distribution of Serratia marcescens
Aerosols Created During Common Laboratory Procedures and Simulated
Laboratory Accidents. Appl Microbiol. 1968;16(8):1146–50.
28. Chatigny MA, Barkley WE, Vogl WF. Aerosol biohazard in microbiological
laboratories and how it is aected by air conditioning systems. ASHRAE
Transactions. 1974;80(Pt 1):463–9.
29. Chatigny MA, Hatch MT, Wolochow H, et al. Studies on release and survival
of biological substances used in recombinant DNA laboratory procedures.
National Institutes of Health Recombinant DNA Technical Bulletin.
1979;2:62–7.

23Section II—Biological Risk Assessment
30. National Science Foundation. Final Notice of Research Terms and
Conditions (RTC) To Address and Implement the Uniform Administrative
Requirements, Cost Principles, and Audit Requirements for Federal Awards
Issued by the U.S. Oce of Management and Budget (OMB). Fed Regist.
2017;82(48):13660–1.
31. National Science Foundation [Internet]. Alexandria (VA): Research Terms
and Conditions; c2017 [cited 2019 Jan 8]. Appendix C National Policy
Requirements. Available from: https://www.nsf.gov/bfa/dias/policy/fedrtc/
appc_march17.pdf
32. Vaughan D. The Challenger Launch Decision: Risky Technology, Culture,
and Deviance at NASA. Chicago: The University of Chicago Press; 1996.
33. History as Cause: Columbia and Challenger. Washington (DC): Columbia
Accident Investigation Board; 2003 Aug. Report Vol.: 1. p. 195–204.
34. Caldwell JA, Caldwell JL, Tompson LA, Liberman, HR. Fatigue and its
management in the workplace. Neurosci Biobehav Rev. 2019;96:272–89.
35. Lennette EH. Panel V common sense in the laboratory: recommendations
and priorities. In: Hellman A, Oxman MN, Pollack R, editors. Biohazards
in Biological Research: Proceedings of a conference held at the Asilomar
conference center; 1973 Jan 22–24; Pacic Grove (CA). New York: Cold
Spring Harbor Laboratory; 1973. p. 353.
36. Delany JR, Pentella MA, Rodriguez JA, Shah KV, Baxley KP, Holmes
DE; Centers for Disease Control and Prevention. Guidelines for biosafety
laboratory competency: CDC and the Association of Public Health
Laboratories. MMWR Suppl. 2011;60(2):1–23.
37. Ned-Sykes R, Johnson C, Ridderhof JC, Perlman E, Pollock A, DeBoy JM;
Centers for Disease Control and Prevention (CDC). Competency Guidelines
for Public Health Laboratory Professionals: CDC and the Association of
Public Health Laboratories. MMWR Suppl. 2015;64(1):1–81.
38. Behaviors that undermine a culture of safety. Sentinel Event Alert.
2008;(40):1–3.
39. United States Department of Labor [Internet]. Washington (DC):
Occupational Safety and Health Administration; c2016 [cited 2019 Jan 7].
Recommended Practices for Safety and Health Programs. Available from:
https://www.osha.gov/shpguidelines/docs/OSHA_SHP_Recommended_
Practices.pdf
40. U.S. Department of Health & Human Services [Internet]. Washington (DC):
Federal Experts Security Advisory Panel; c2017 [cited 2019 Jan 7]. Guiding
Principles for Biosafety Governance: Ensuring Institutional Compliance with
Biosafety, Biocontainment, and Laboratory Biosecurity Regulations and
Guidelines. Available from: https://www.phe.gov/s3/Documents/FESAP-
guiding-principles.pdf

24 Biosafety in Microbiological and Biomedical Laboratories
Section III—Principles of Biosafety
A fundamental objective of any biosafety program is the containment of potentially
hazardous biological agents and toxins. The term containment describes a
combination of primary and secondary barriers, facility practices and procedures,
and other safety equipment, including personal protective equipment (PPE), for
managing the risks associated with handling and storing hazardous biological
agents and toxins in a laboratory environment. The purpose of containment is to
reduce the risk of exposure to sta and the unintentional release of hazardous
biological agents or toxins into the surrounding community and environment. Final
determination on the combination of containment measures required to address
the relevant biosafety risk present at a facility should be based on a compre-
hensive biosafety risk assessment. A comprehensive biosafety risk assessment
is a key component of a successful biosafety program and should be part of
an all-hazards risk assessment; it should be conducted on a continual basis to
address evolving risks within the laboratory environment. Detailed information on
the biological risk assessment process is found in Section II of BMBL.
Management and leadership, with support from the facility’s biosafety profes-
sionals and other health and safety personnel, must perform and review the risk
assessment using the best available information. Management and leadership
are responsible for assessing the risks and selecting the appropriate combination
of risk mitigation measures. All persons in the institution are responsible for
performing their work in a manner that ensures the successful implementation
and performance of the safety measures identied in the risk assessment and
review.
Safety Equipment (Primary Barriers)
Primary barrier or primary containment is dened as physical containment
measure(s) placed directly at the level of the hazard. Safety equipment such
as biological safety cabinets (BSCs), enclosed containers, and other biosafety
controls are designed to protect personnel, the surrounding community, and the
environment from possible exposure to hazardous biological agents and toxins.
Primary barriers can function to either provide containment (e.g., BSCs) or direct
personal protection from the hazardous biological agents and toxins used. The
BSC is the standard device used to provide containment of hazardous biological
agents and toxins when conducting microbiological activities. Three primary
types of BSCs (Class I, II, III) are used in laboratory facilities and selection of
the appropriate BSC should be based on the risks identied for each respective
laboratory. The three classes of BSCs are described and illustrated in Appendix A
of BMBL.
Additional primary containment devices may include sealed containers
(e.g., sealed rotors and centrifuge safety cups). These enclosed containers
25Section III—Principles of Biosafety
are designed to contain aerosols, droplets, and leakage of hazardous biological
agents and toxins that may result during certain activities (e.g., centrifugation).
Sealed containers provide containment for transfers between laboratories
within a facility, between facilities, and depending upon risk assessment, within
a laboratory. Selection of the appropriate primary containment device should
be based on the risks identied for those activities likely to produce aerosols,
droplets, or result in potential leakage of hazardous biological agents and toxins.
Note that in some cases, such as when working with large animals, secondary
barriers may become primary barriers. This lack of traditional primary barriers
(e.g., BSC) can lead to additional risks to personnel, the surrounding community,
and the environment. In these cases, the facility becomes the primary barrier
and personnel must rely on administrative and personal protective equipment
to reduce the risk of exposure. This type of facility may require additional
enginerring controls and precautions (e.g., HEPA ltration on the exhaust air)
to mitigate the risks posed to personnel, the surrounding community, and the
environment.
Personal Protective Equipment
Personal protective equipment (PPE) helps protect the user’s body from injury
from a variety of sources (e.g., physical, electrical, heat, noise, chemical) or
potential exposure to biological hazards and airborne particulate matter. PPE
includes gloves, coats, gowns, shoe covers, closed-toe laboratory footwear,
respirators, face shields, safety glasses, goggles, or ear plugs. PPE is usually
used in combination with other biosafety controls (e.g., BSCs, centrifuge safety
cups, and small animal caging systems) that contain the hazardous biological
agents and toxins, animals, or materials being handled. In situations where a
BSC cannot be used, PPE may become the primary barrier between personnel
and the hazardous biological agents and toxins. Examples include eldwork,
resource-limited settings, certain animal studies, animal necropsy, and activities
relating to operations, maintenance, service, or support of the laboratory facility.
Selection of the appropriate PPE should be based on the risks identied for each
respective laboratory.
Facility Design and Construction (Secondary Barriers)
The design and construction of the laboratory facility provide a means of
secondary containment of hazardous biological agents and toxins. The secondary
barriers, together with other biosafety controls, help provide protection of
personnel, the surrounding community, and the environment from possible
exposure to hazardous biological agents and toxins.
When the risk of infection by aerosol or droplet exposure is present, higher levels
of secondary containment and multiple primary barriers may be used in combi-
nation with other controls to minimize the risk of exposure to personnel and the
26 Biosafety in Microbiological and Biomedical Laboratories
unintentional release into the surrounding community or the environment.
Such design features may include, but are not limited to the following:
■
Ventilation strategies to ensure containment of the hazards;
■
Euent decontamination systems; and
■
Specialized building/suite/laboratory congurations, including:
□
Controlled access zones to support the separation of the
laboratory from oce and public spaces;
□
Anterooms; and
□
Airlocks.
Design engineers may refer to specic ventilation recommendations as found in
the American Society of Heating, Refrigerating and Air-Conditioning Engineers
(ASHRAE) Laboratory Design Guide.
1
Please note that depending on the
laboratory facility, design professionals may need to follow or consult with the
current versions of additional design recommendations and requirements such as:
■
The National Institutes of Health (NIH) Design Requirements Manual
(DRM);
■
World Health Organization (WHO) Laboratory Biosafety Manual;
■
World Organization for Animal Health (OIE) Manual of Diagnostic Tests
and Vaccines for Terrestrial Animals; and/or
■
Other similar national or international design reference documents.
Facility Practices and Procedures
Established facility-specic best practices and procedures are essential to
support the implementation and sustainability of a successful biosafety program.
Persons working in facilities that handle and store hazardous biological agents
and toxins must be able to properly identify all potential hazards and be trained
and procient in necessary safe practices and procedures. Management and
leadership are responsible for providing and arranging the appropriate training of
all personnel based on their functional roles and responsibilities in support of the
biosafety program. Strict adherence to documented laboratory best practices and
procedures is an essential element of a robust biosafety program since failure
to follow the established procedures could result in an accidental exposure to
personnel or unintentional release of hazardous biological agents and toxins into
the surrounding community or the environment.
All facilities should develop and implement a biosafety program that identies
the hazards and species risk mitigation strategies to eliminate or reduce the
likelihood of exposures and unintentional releases of hazardous materials.
Management and leadership are ultimately responsible for the work conducted
within laboratory facilities. When existing safety practices and procedures are not
sucient to minimize the risk(s) associated with a particular hazardous biological
agent and/or toxin to an acceptable level, additional risk mitigation measures may

27Section III—Principles of Biosafety
be needed. Safety best practices and procedures must be developed and imple-
mented in coordination with other components of the overall biosafety program.
Biosafety Levels
The four primary Biosafety Levels (BSLs) for laboratories described in Section IV
of BMBL consist of combinations of facility design features and safety equipment
(primary and secondary barriers), facility practices and procedures, and personal
protective equipment. Selection of the appropriate combinations to safely conduct
the work should be based upon a comprehensive facility-specic biosafety risk
assessment that documents the properties of the biological agents and toxins
to be used, potential host characteristics, potential routes of infection, and the
laboratory work practices and procedures conducted or anticipated to be used in
the future. Recommended Biosafety Level(s) for the biological agents and toxins
in Section VIII of BMBL represent suggested practices for work with an agent or
toxin using standard protocols. Not all biological agents and toxins capable of
causing disease in humans are included in Section VIII.
When working with well-dened organisms, identication of the appropriate
biosafety controls should be based on the comprehensive biosafety risk
assessment. However, when information is available to suggest that virulence,
pathogenicity, antibiotic resistance patterns, vaccine and treatment availability,
or other factors are signicantly altered, an adjustment to the stringency of
biosafety controls may be needed. For example, handling large volumes or high
concentrations of a biological agent or toxin may require additional practices
outlined in Sections IV and V of BMBL. Similarly, procedures that produce large
amounts of aerosols may also require additional biosafety controls to reduce the
likelihood of exposures to personnel and the unintentional release of a biological
agent or toxin in the surrounding community or the environment. Furthermore,
vaccines should not be considered non-pathogenic simply because they are
vaccine strains.
It is important to note that the four Biosafety Levels described below are not to
be confused and equated with Agent Risk Groups as described in the National
Institutes of Health Guidelines for Research Involving Recombinant or Synthetic
Nucleic Acid Molecules (NIH Guidelines). The Risk Group (RG) of an agent is an
important factor to be considered during the biosafety risk assessment process.
Biological agents and toxins are assigned to their relevant Risk Groups based
on their ability to cause disease in healthy human adults and spread within the
community. However, just because a biological agent is listed as a Risk Group 3
agent, it does not mean the activities conducted with that biological agent must
occur in a BSL-3 laboratory.

28 Biosafety in Microbiological and Biomedical Laboratories
Biosafety Level 1
Biosafety Level 1 (BSL-1) standard practices, safety equipment, and facility speci-
cations are generally appropriate for undergraduate and secondary educational
training and teaching laboratories and for other laboratories that work with dened
and characterized strains of viable biological agents not known to consistently
cause disease in healthy adult humans. Bacillus subtilis, Naegleria gruberi,
infectious canine hepatitis virus, and exempt organisms under the NIH Guidelines
are examples of the biological agents meeting these criteria. BSL-1 represents a
basic level of containment that relies on standard, microbiological best practices
and procedures with no special primary or secondary barriers, other than a door,
a sink for handwashing, and non-porous work surfaces that are cleanable and
easy to decontaminate.
Biosafety Level 2
Biosafety Level 2 (BSL-2) standard practices, safety equipment, and facility
specications are applicable to laboratories in which work is performed using a
broad-spectrum of biological agents and toxins that are associated with causing
disease in humans of varying severity. With good practices and procedures, these
agents and toxins can generally be handled safely on an open bench, provided
the potential for producing splashes and aerosols is low. Hepatitis B virus, human
immunodeciency virus (HIV), Salmonella, and Toxoplasma are examples of the
biological agents that meet these criteria. Work done with any human, animal, or
plant-derived specimens (e.g., blood, body uids, tissues, or primary cell lines),
where the presence of a biological agent or toxin may be unknown, can often be
safely conducted under conditions typically associated with BSL-2.
3–5
Personnel
working with human-derived materials should refer to the OSHA Bloodborne
Pathogens Standard for specic required precautions.
2
The primary routes of exposure to personnel working with these types of
biological agents and toxins relate to accidents including exposure via the
percutaneous or mucosal routes and ingestion of potentially infectious materials.
Extreme caution should be taken with contaminated needles and other sharp
materials. Even though the biological agents and toxins routinely manipulated
at BSL-2 are not known to be transmissible by the aerosol route, procedures
with aerosol or high splash potential are conducted within primary containment
equipment, such as a BSC or safety centrifuge cups. Furthermore, the use of
primary containment equipment is also recommended when high-risk infectious
agents are suspected to be present in any human, animal, or plant-derived
specimens. Selection of the appropriate personal protective equipment should be
based on the risks identied for each respective laboratory. Special practices for
BSL-2 and ABSL-2 are recommended in Sections IV and V.
29Section III—Principles of Biosafety
Secondary barriers should include those previously mentioned for BSL-1. Waste
decontamination capabilities to reduce the potential of environmental contami-
nation and the separation of laboratory spaces from oce and public spaces to
reduce the risk of exposure to other personnel should be considered.
Biosafety Level 3
Biosafety Level 3 (BSL-3) standard practices, safety equipment, and facility
specications are applicable to laboratories in which work is performed using
indigenous or exotic biological agents with a potential for respiratory transmission
and those that may cause serious and potentially lethal infection. Mycobacterium
tuberculosis, St. Louis encephalitis virus, and Coxiella burnetii are examples of
the biological agents that meet these criteria.
The primary routes of exposure to personnel working with these types of
biological agents and toxins relate to accidental exposure via the percutaneous or
mucosal routes and inhalation of potentially infectious aerosols. At BSL-3, more
emphasis is placed on primary and secondary barriers to protect personnel, the
surrounding community, and the environment from exposure to potentially infec-
tious aerosols. All procedures involving the manipulation of infectious materials
are conducted within a BSC or other primary containment device. No work with
open vessels is conducted on the bench. When a procedure cannot be performed
within a BSC, a combination of personal protective equipment and other primary
containment strategies (e.g., centrifuge safety cups, sealed rotors or softwall
containment enclosures) are implemented based on a risk assessment. Loading
and unloading of the rotors and centrifuge safety cups take place in the BSC or
another containment device.
Secondary barriers for BSL-3 laboratories include those previously mentioned for
BSL-1 and BSL-2 laboratories. They also include enhanced ventilation strategies
to ensure inward directional airow, controlled access zones to limit access to
only laboratory approved personnel, and may contain anterooms, airlocks, exit
showers, and/or exhaust HEPA ltration.
Biosafety Level 4
Biosafety Level 4 (BSL-4) standard practices, safety equipment, and facility speci-
cations are applicable primarily for laboratories working with dangerous and
exotic biological agents that pose a high individual risk of life-threatening disease
that may be transmitted via the aerosol route and for which there is no available
vaccine or therapy. Marburg virus and Congo-Crimean hemorrhagic fever virus
are examples of the biological agents that meet these criteria. Agents with a close
or identical antigenic relationship to agents requiring BSL-4 containment must be
handled at this level until sucient data are obtained either to conrm continued
work at this level or to re-designate the level.

30 Biosafety in Microbiological and Biomedical Laboratories
The primary routes of exposure to personnel working with these types of
biological agents relate to accidental exposure via the percutaneous and mucous
membrane routes and inhalation of potentially infectious aerosols. The laboratory
worker’s complete isolation from aerosolized infectious materials is accomplished
primarily by working in a Class III BSC or in a Class II BSC with a full-body,
air-supplied positive-pressure personnel suit.
Secondary barriers for BSL-4 laboratories should include those previously
mentioned for previous Biosafety Levels. Additionally, the BSL-4 facility itself is
often a separate building or completely isolated zone with complex, specialized
ventilation requirements and waste management systems, for both solid and
liquid waste, to prevent the release of hazardous biological agents into the
surrounding community and the environment.
Animal Facilities
Four primary Biosafety Levels are also described for activities involving
hazardous biological agent and toxin work conducted with animals. These four
combinations of facility design and construction, safety equipment, and practices
and procedures are designated Animal Biosafety Levels (ABSL) 1, 2, 3, and 4,
and provide increasing levels of protection to personnel, the surrounding
community, and the environment.
One additional Biosafety Level, designated Animal Biosafety Level 3-Agriculture
(ABSL-3Ag) addresses activities involving the use of hazardous biological
agents and toxins designated as High-Consequence Foreign Animal Diseases
and Pests by the U.S. Department of Agriculture’s (USDA) Animal and Plant
Health Inspection Service (APHIS) in large or loose-housed animals. ABSL-3Ag
laboratories are designed so that the laboratory building itself serves as a primary
barrier to prevent the unintentional release of these high consequence agents
into the environment. More information on the design and operation of ABSL-3Ag
facilities and USDA/APHIS High-Consequence Foreign Animal Diseases and
Pests is provided in Appendix D of BMBL. Appendix D also provides guidance
for containment of loose-housed or open penned animals at other containment
levels, designated ABSL-2Ag and ABSL-4Ag.
Clinical Laboratories
Clinical laboratories routinely work with unknown specimens and specimens that
have the potential to be infected with multiple pathogens; as such, the occupa-
tional risks in a clinical laboratory environment dier from those of a research
or teaching laboratory. Most public and animal health clinical laboratories use
Biosafety Level 2 (BSL-2) facility, engineering, and biosafety practices.
5
Clinical
diagnostic laboratory personnel may not know what infectious agent or other
hazard(s) exist in the specimen they handle and process. More information on
clinical laboratory biosafety is provided in Appendix N.

31Section III—Principles of Biosafety
Laboratory Biosecurity
In recent years, with the passing of federal legislation regulating the possession,
use, and transfer of biological Select Agents and Toxins with high adverse public
health and/or agricultural consequences (DHHS, USDA APHIS Select Agents),
a much greater emphasis has been placed in the emerging eld of biosecurity.
Biosecurity and Select Agent issues are covered in detail in Section VI and
Appendix F of BMBL. While biosafety focuses on the protection of personnel,
the surrounding community, and the environment from the unintentional release
of hazardous biological agents and toxins, the eld of laboratory biosecurity is
focused on the prevention of the theft, loss, and misuse of hazardous biological
agents and toxins, equipment, and/or valuable information by an individual(s) for
malicious use. Nonetheless, a successful containment strategy must incorporate
aspects of both biosafety and laboratory biosecurity to adequately address the
risks present at the facility.
References
1. American Society of Heating, Refrigerating and Air-Conditioning Engineers.
ASHRAE Laboratory Design Guide: Planning and Operation of Laboratory
HVAC Systems. 2nd ed. Atlanta (GA): ASHRAE; 2015.
2. Bloodborne pathogens, 29 C.F.R. Part 1910.1030 (1992).
3. Centers for Disease Control and Prevention. Update: universal precautions
for prevention of transmission of human immunodeciency virus, hepatitis B
virus, and other bloodborne pathogens in health-care settings. MMWR Morb
Mortal Wkly Rep. 1988;37(24):377–82, 387–8.
4. Garner JS. Guideline for isolation precautions in hospitals. The Hospital
Infection Control Practices Advisory Committee. Infect Control Hosp
Epidemiol. 1996;17(1):53–80. Erratum in: Infect Control Hosp Epidemiol.
1996;17(4):214.
5. CLSI. Protection of Laboratory Workers from Occupationally Acquired
Infections: Approved Guideline—Fourth Edition. CLSI document M29-A4.
Wayne (PA): Clinical and Laboratory Standards Institute; 2014.

32 Biosafety in Microbiological and Biomedical Laboratories
Section IV—Laboratory Biosafety Level Criteria
The essential elements of the Biosafety Levels 1–4 are standard microbiological
practices, special practices, safety equipment, and laboratory facilities as
discussed in Section III; these elements apply to activities involving infectious
microorganisms, toxins, and laboratory animals. The four levels are organized
in ascending order by the degree of protection provided to personnel, the
environment, and the community. Special practices address any unique risks
associated with the handling of agents requiring increasing levels of containment.
Appropriate safety equipment and laboratory facilities enhance worker and
environmental protection.
The features of each Biosafety Level (BSL) are summarized in Table 1 of this
section. Adjustments to the containment levels described are based on an
assessment of all risks, as detailed in Section II. Each facility ensures that worker
safety and health concerns are coordinated with the Institutional Biosafety
Committee (IBC), or equivalent resource, and/or other applicable institutional safety
committee(s) and that all hazards are addressed as part of the protocol review
process. Additional occupational health information is provided in Section VII.
Biosafety Level 1
Biosafety Level 1 (BSL-1) is suitable for work involving well-characterized agents
not known to consistently cause disease in immunocompetent adult humans
and that present minimal potential hazard to laboratory personnel and the
environment. BSL-1 laboratories are not necessarily separated from the general
trac patterns in the building. Work is typically conducted on open benchtops
using standard microbiological practices. Special containment equipment or
facility design is not generally required but may be used as determined by
appropriate risk assessment. Laboratory personnel receive specic training in the
procedures conducted in the laboratory and are supervised by a scientist with
training in microbiology or a related science.
The following standard practices, safety equipment, and facility specications are
recommended for BSL-1.
A. Standard Microbiological Practices
1. The laboratory supervisor enforces the institutional policies that control
safety in and access to the laboratory.
2. The laboratory supervisor ensures that laboratory personnel receive
appropriate training regarding their duties, potential hazards, manipula-
tions of infectious agents, necessary precautions to minimize exposures,
and hazard/exposure evaluation procedures (e.g., physical hazards,
splashes, aerosolization) and that appropriate records are maintained.

33Section IV—Laboratory Biosafety Level Criteria
Personnel receive annual updates and additional training when
equipment, procedures, or policies change. All persons entering the
facility are advised of the potential hazards, are instructed on the appro-
priate safeguards, and read and follow instructions on practices and
procedures. An institutional policy regarding visitor training, occupational
health requirements, and safety communication is considered.
3. Personal health status may aect an individual’s susceptibility to
infection and ability to receive available immunizations or prophylactic
interventions. Therefore, all personnel, and particularly those of
reproductive age and/or those having conditions that may predispose
them to increased risk for infection (e.g., organ transplant, medical
immunosuppressive agents), are provided information regarding immune
competence and susceptibility to infectious agents. Individuals having
such conditions are encouraged to self-identify to the institution’s
healthcare provider for appropriate counseling and guidance. See
Section VII.
4. A safety manual specic to the facility is prepared or adopted in consul-
tation with the facility director and appropriate safety professionals. The
safety manual is available, accessible, and periodically reviewed and
updated, as necessary.
a. The safety manual contains sucient information to describe the
biosafety and containment procedures for the organisms and
biological materials in use, appropriate agent-specic decontami-
nation methods, and the work performed.
b. The safety manual contains or references protocols for emergency
situations, including exposures, medical emergencies, facility
malfunctions, and other potential emergencies. Training in
emergency response procedures is provided to emergency
response personnel and other responsible sta according to institu-
tional policies.
5. A sign is posted at the entrance to the laboratory when infectious
materials are present. Posted information includes: the laboratory’s
Biosafety Level, the supervisor’s or other responsible personnel’s name
and telephone number, PPE requirements, general occupational health
requirements (e.g., immunizations, respiratory protection), and required
procedures for entering and exiting the laboratory. Agent information is
posted in accordance with the institutional policy.
6. Long hair is restrained so that it cannot contact hands, specimens,
containers, or equipment.
34 Biosafety in Microbiological and Biomedical Laboratories
7. Gloves are worn to protect hands from exposure to hazardous materials.
a. Glove selection is based on an appropriate risk assessment.
b. Gloves are not worn outside the laboratory.
c. Change gloves when contaminated, glove integrity is compromised,
or when otherwise necessary.
d. Do not wash or reuse disposable gloves, and dispose of used
gloves with other contaminated laboratory waste.
8. Gloves and other PPE are removed in a manner that minimizes personal
contamination and transfer of infectious materials outside of the areas
where infectious materials and/or animals are housed or manipulated.
9. Persons wash their hands after working with potentially hazardous
materials and before leaving the laboratory.
10. Eating, drinking, smoking, handling contact lenses, applying cosmetics,
and storing food for human consumption are not permitted in laboratory
areas. Food is stored outside the laboratory area.
11. Mouth pipetting is prohibited. Mechanical pipetting devices are used.
12. Policies for the safe handling of sharps, such as needles, scalpels,
pipettes, and broken glassware are developed, implemented, and
followed; policies are consistent with applicable state, federal, and
local requirements. Whenever practical, laboratory supervisors adopt
improved engineering and work practice controls that reduce risk of
sharps injuries. Precautions are always taken with sharp items. These
include:
a. Plasticware is substituted for glassware whenever possible.
b. Use of needles and syringes or other sharp instruments is limited
in the laboratory and is restricted to situations where there is no
alternative (e.g., parenteral injection, blood collection, or aspiration
of uids from laboratory animals or diaphragm bottles). Active or
passive needle-based safety devices are to be used whenever
possible.
i. Uncapping of needles is performed in such a manner to reduce
the potential for recoil causing an accidental needlestick.
ii. Needles are not bent, sheared, broken, recapped, removed
from disposable syringes, or otherwise manipulated by hand
before disposal.
iii. If absolutely necessary to remove a needle from a syringe
(e.g., to prevent lysing blood cells) or recap a needle

35Section IV—Laboratory Biosafety Level Criteria
(e.g., loading syringes in one room and injecting animals in
another), a hands-free device or comparable safety procedure
must be used (e.g., a needle remover on a sharps container,
the use of forceps to hold the cap when recapping a needle).
iv. Used, disposable needles and syringes are carefully placed
in puncture-resistant containers used for sharps disposal
immediately after use. The sharps disposal container is located
as close to the point of use as possible.
c. Non-disposable sharps are placed in a hard-walled container for
transport to a processing area for decontamination, preferably by
autoclaving.
d. Broken glassware is not handled directly. Instead, it is removed
using a brush and dustpan, tongs, or forceps.
13. Perform all procedures to minimize the creation of splashes and/or
aerosols.
14. Decontaminate work surfaces after completion of work and after any spill
or splash of potentially infectious material with appropriate disinfectant.
Spills involving infectious materials are contained, decontaminated, and
cleaned up by sta who are properly trained and equipped to work with
infectious material. A spill procedure is developed and posted within the
laboratory.
15. Decontaminate all cultures, stocks, and other potentially infectious
materials before disposal using an eective method, consistent with
applicable institutional, local, and state requirements. Depending on
where the decontamination will be performed, the following methods are
used prior to transport:
a. Materials to be decontaminated outside of the immediate laboratory
are placed in a durable, leak-proof container and secured for
transport. For infectious materials, the outer surface of the container
is disinfected prior to moving materials and the transport container
has a universal biohazard label.
b. Materials to be removed from the facility for decontamination are
packed in accordance with applicable local, state, and federal
regulations.
16. An eective integrated pest management program is implemented. See
Appendix G.
17. Animals and plants not associated with the work being performed are not
permitted in the laboratory.
36 Biosafety in Microbiological and Biomedical Laboratories
B. Special Practices
None required.
C. Safety Equipment (Primary Barriers and Personal Protective Equipment)
1. Special containment devices or equipment, such as biosafety cabinets
(BSCs), are not generally required.
2. Protective laboratory coats, gowns, or uniforms are worn to prevent
contamination of personal clothing.
3. Protective eyewear is worn by personnel when conducting procedures
that have the potential to create splashes and sprays of microorganisms
or other hazardous materials. Eye protection and face protection are
disposed of with other contaminated laboratory waste or decontaminated
after use.
4. In circumstances where research animals are present in the laboratory,
the risk assessment considers appropriate eye, face, and respiratory
protection, as well as potential animal allergens.
D. Laboratory Facilities (Secondary Barriers)
1. Laboratories have doors for access control.
2. Laboratories have a sink for handwashing.
3. An eyewash station is readily available in the laboratory.
4. The laboratory is designed so that it can be easily cleaned.
a. Carpets and rugs in laboratories are not appropriate.
b. Spaces between benches, cabinets, and equipment are accessible
for cleaning.
5. Laboratory furniture can support anticipated loads and uses.
a. Benchtops are impervious to water and resistant to heat, organic
solvents, acids, alkalis, and other chemicals.
b. Chairs used in laboratory work are covered with a non-porous
material that can be easily cleaned and decontaminated with appro-
priate disinfectant.
6. Laboratory windows that open to the exterior are tted with screens.
7. Illumination is adequate for all activities and avoids reections and glare
that could impede vision.

37Section IV—Laboratory Biosafety Level Criteria
Biosafety Level 2
Biosafety Level 2 (BSL-2) builds upon BSL-1. BSL-2 is suitable for work with
agents associated with human disease and pose moderate hazards to personnel
and the environment. BSL-2 diers from BSL-1 primarily because: 1) laboratory
personnel receive specic training in handling pathogenic agents and are
supervised by scientists competent in handling infectious agents and associated
procedures; 2) access to the laboratory is restricted when work is being
conducted; and 3) all procedures in which infectious aerosols or splashes may be
created are conducted in BSCs or other physical containment equipment.
The following standard and special practices, safety equipment, and facility
specications are recommended for BSL-2.
A. Standard Microbiological Practices
1. The laboratory supervisor enforces the institutional policies that control
safety in and access to the laboratory.
2. The laboratory supervisor ensures that laboratory personnel receive
appropriate training regarding their duties, potential hazards, manipula-
tions of infectious agents, necessary precautions to minimize exposures,
and hazard/exposure evaluation procedures (e.g., physical hazards,
splashes, aerosolization) and that appropriate records are maintained.
Personnel receive annual updates and additional training when
equipment, procedures, or policies change. All persons entering the
facility are advised of the potential hazards, are instructed on the appro-
priate safeguards, and read and follow instructions on practices and
procedures. An institutional policy regarding visitor training, occupational
health requirements, and safety communication is considered.
3. Personal health status may aect an individual’s susceptibility to
infection and ability to receive available immunizations or prophylactic
interventions. Therefore, all personnel, and particularly those of
reproductive age and/or those having conditions that may predispose
them to increased risk for infection (e.g., organ transplant, medical
immunosuppressive agents), are provided information regarding immune
competence and susceptibility to infectious agents. Individuals having
such conditions are encouraged to self-identify to the institution’s
healthcare provider for appropriate counseling and guidance. See
Section VII.
4. A safety manual specic to the facility is prepared or adopted in consul-
tation with the facility director and appropriate safety professionals. The
safety manual is available, accessible, and periodically reviewed and
updated as necessary.
38 Biosafety in Microbiological and Biomedical Laboratories
a. The safety manual contains sucient information to describe the
biosafety and containment procedures for the organisms and
biological materials in use, appropriate agent-specic decontami-
nation methods, and the work performed.
b. The safety manual contains or references protocols for emergency
situations, including exposures, medical emergencies, facility
malfunctions, and other potential emergencies. Training in
emergency response procedures is provided to emergency
response personnel and other responsible sta according to institu-
tional policies.
5. A sign incorporating the universal biohazard symbol is posted at the
entrance to the laboratory when infectious materials are present. Posted
information includes: the laboratory’s Biosafety Level, the supervisor’s
or other responsible personnel’s name and telephone number, PPE
requirements, general occupational health requirements (e.g., immuniza-
tions, respiratory protection), and required procedures for entering and
exiting the laboratory. Agent information is posted in accordance with the
institutional policy.
6. Long hair is restrained so that it cannot contact hands, specimens,
containers, or equipment.
7. Gloves are worn to protect hands from exposure to hazardous materials.
a. Glove selection is based on an appropriate risk assessment.
b. Gloves are not worn outside the laboratory.
c. Change gloves when contaminated, glove integrity is compromised,
or when otherwise necessary.
d. Do not wash or reuse disposable gloves, and dispose of used
gloves with other contaminated laboratory waste.
8. Gloves and other PPE are removed in a manner that minimizes personal
contamination and transfer of infectious materials outside of the areas
where infectious materials and/or animals are housed or manipulated.
9. Persons wash their hands after working with potentially hazardous
materials and before leaving the laboratory.
10. Eating, drinking, smoking, handling contact lenses, applying cosmetics,
and storing food for human consumption are not permitted in laboratory
areas. Food is stored outside the laboratory area.
11. Mouth pipetting is prohibited. Mechanical pipetting devices are used.
39Section IV—Laboratory Biosafety Level Criteria
12. Policies for the safe handling of sharps, such as needles, scalpels,
pipettes, and broken glassware are developed, implemented, and
followed; policies are consistent with applicable state, federal, and
local requirements. Whenever practical, laboratory supervisors adopt
improved engineering and work practice controls that reduce risk of
sharps injuries. Precautions are always taken with sharp items. These
include:
a. Plasticware is substituted for glassware whenever possible.
b. Use of needles and syringes or other sharp instruments is limited
in the laboratory and is restricted to situations where there is no
alternative (e.g., parenteral injection, blood collection, or aspiration
of uids from laboratory animals or diaphragm bottles). Active or
passive needle-based safety devices are to be used whenever
possible.
i. Uncapping of needles is performed in such a manner to reduce
the potential for recoil causing an accidental needlestick.
ii. Needles are not bent, sheared, broken, recapped, removed
from disposable syringes, or otherwise manipulated by hand
before disposal.
iii. If absolutely necessary to remove a needle from a syringe
(e.g., to prevent lysing blood cells) or recap a needle (e.g.,
loading syringes in one room and injecting animals in another),
a hands-free device or comparable safety procedure must be
used (e.g., a needle remover on a sharps container, the use of
forceps to hold the cap when recapping a needle).
iv. Used, disposable needles and syringes are carefully placed
in puncture-resistant containers used for sharps disposal
immediately after use. The sharps disposal container is located
as close to the point of use as possible.
c. Non-disposable sharps are placed in a hard-walled container for
transport to a processing area for decontamination, preferably by
autoclaving.
d. Broken glassware is not handled directly. Instead, it is removed
using a brush and dustpan, tongs, or forceps.
13. Perform all procedures to minimize the creation of splashes and/or
aerosols.

40 Biosafety in Microbiological and Biomedical Laboratories
14. Decontaminate work surfaces after completion of work and after any spill
or splash of potentially infectious material with appropriate disinfectant.
Spills involving infectious materials are contained, decontaminated, and
cleaned up by sta who are properly trained and equipped to work with
infectious material. A spill procedure is developed and posted within the
laboratory.
15. Decontaminate all cultures, stocks, and other potentially infectious
materials before disposal using an eective method, consistent with
applicable institutional, local, and state requirements. Depending on
where the decontamination will be performed, the following methods are
used prior to transport:
a. Materials to be decontaminated outside of the immediate laboratory
are placed in a durable, leak-proof container and secured for
transport. For infectious materials, the outer surface of the container
is disinfected prior to moving materials and the transport container
has a universal biohazard label.
b. Materials to be removed from the facility for decontamination are
packed in accordance with applicable local, state, and federal
regulations.
16. An eective integrated pest management program is implemented. See
Appendix G.
17. Animals and plants not associated with the work being performed are not
permitted in the laboratory.
B. Special Practices
1. Access to the laboratory is controlled when work is being conducted.
2. The laboratory supervisor is responsible for ensuring that laboratory
personnel demonstrate prociency in standard microbiological practices
and techniques for working with agents requiring BSL-2 containment.
3. Laboratory personnel are provided medical surveillance, as appropriate,
and oered available immunizations for agents handled or potentially
present in the laboratory.
4. Properly maintained BSCs or other physical containment devices are
used, when possible, whenever:
a. Procedures with a potential for creating infectious aerosols or
splashes are conducted. These include pipetting, centrifuging,
grinding, blending, shaking, mixing, sonicating, opening containers
of infectious materials, inoculating animals intranasally, and
harvesting infected tissues from animals or eggs.
41Section IV—Laboratory Biosafety Level Criteria
b. High concentrations or large volumes of infectious agents are used.
Such materials may be centrifuged in the open laboratory using
sealed rotors or centrifuge safety cups with loading and unloading
of the rotors and centrifuge safety cups in the BSC or another
containment device.
c. If it is not possible to perform a procedure within a BSC or other
physical containment device, a combination of appropriate personal
protective equipment and administrative controls are used, based on
a risk assessment.
5. Laboratory equipment is decontaminated routinely; after spills, splashes,
or other potential contamination; and before repair, maintenance, or
removal from the laboratory.
6. A method for decontaminating all laboratory waste is available (e.g.,
autoclave, chemical disinfection, incineration, or other validated decon-
tamination method).
7. Incidents that may result in exposure to infectious materials are immedi-
ately evaluated per institutional policies. All such incidents are reported
to the laboratory supervisor and any other personnel designated by the
institution. Appropriate records are maintained.
C. Safety Equipment (Primary Barriers and Personal Protective Equipment)
1. Protective laboratory coats, gowns, or uniforms designated for laboratory
use are worn while working with hazardous materials and removed
before leaving for non-laboratory areas (e.g., cafeteria, library, and
administrative offices). Protective clothing is disposed of appropriately or
deposited for laundering by the institution. Laboratory clothing is not
taken home.
2.
Eye protection and face pr
otection (e.g., safety glass
es, goggles, mask,
face shield or other splatter
guard) are used for manipulations or activities
that may result in splashes or sprays of infectious
or other hazardous
materials. Eye protection and face protection are disposed of with other
contaminated laboratory waste or decontaminated after use.
3. The risk assessment considers whether respiratory protection is needed
for the work with hazardous materials. If needed, relevant staff are
enrolled in a properly constituted respiratory protection program.
4. In circumstances where research animals are present in the laboratory,
the risk assessment considers appropriate eye, face, and respiratory
protection, as well as potential animal allergens.

42 Biosafety in Microbiological and Biomedical Laboratories
D. Laboratory Facilities (Secondary Barriers)
1. Laboratory doors are self-closing and have locks in accordance with the
institutional policies.
2. Laboratories have a sink for handwashing. It should be located near the
exit door.
3. An eyewash station is readily available in the laboratory.
4. The laboratory is designed so that it can be easily cleaned.
a. Carpets and rugs in laboratories are not appropriate.
b. Spaces between benches, cabinets, and equipment are accessible
for cleaning.
5. Laboratory furniture can support anticipated loads and uses.
a. Benchtops are impervious to water and resistant to heat, organic
solvents, acids, alkalis, and other chemicals.
b. Chairs used in laboratory work are covered with a non-porous
material that can be easily cleaned and decontaminated with appro-
priate disinfectant.
6. Laboratory windows that open to the exterior are not recommended.
However, if a laboratory does have windows that open to the exterior,
they are tted with screens.
7. Illumination is adequate for all activities and avoids reections and glare
that could impede vision.
8. Vacuum lines in use are protected with liquid disinfectant traps and
in-line HEPA lters or their equivalent. See Appendix A, Figure 11. Filters
are replaced, as needed, or are on a replacement schedule determined
by a risk assessment.
9. There are no specic requirements for ventilation systems. However, the
planning of new facilities considers mechanical ventilation systems that
provide an inward ow of air without recirculation to spaces outside of
the laboratory.
10. BSCs and other primary containment barrier systems are installed and
operated in a manner to ensure their eectiveness. See Appendix A.
a. BSCs are installed so that uctuations of the room air supply and
exhaust do not interfere with proper operations. BSCs are located
away from doors, windows that can be opened, heavily traveled
laboratory areas, and other possible airow disruptions.

43Section IV—Laboratory Biosafety Level Criteria
b. BSCs can be connected to the laboratory exhaust system by either
a canopy connection (Class IIA only) or directly exhausted to the
outside through a hard connection (Class IIB, IIC, or III). Class IIA or
IIC BSC exhaust can be safely recirculated back into the laboratory
environment if no volatile toxic chemicals are used in the cabinet.
c. BSCs are certied at least annually to ensure correct performance,
or as specied in Appendix A, Part 7.
Biosafety Level 3
Biosafety Level 3 (BSL-3) is suitable for work with indigenous or exotic agents
that may cause serious or potentially lethal disease through the inhalation route
of exposure. Laboratory personnel receive specic training in handling pathogenic
and potentially lethal agents, and they are supervised by scientists competent in
handling infectious agents and associated procedures.
A BSL-3 laboratory has special engineering and design features.
The following standard and special practices, safety equipment, and facility
specications are recommended for BSL-3.
A. Standard Microbiological Practices
1. The laboratory supervisor enforces the institutional policies that control
safety in and access to the laboratory.
2. The laboratory supervisor ensures that laboratory personnel receive
appropriate training regarding their duties, potential hazards, manipula-
tions of infectious agents, necessary precautions to minimize exposures,
and hazard/exposure evaluation procedures (e.g., physical hazards,
splashes, aerosolization) and that appropriate records are maintained.
Personnel receive annual updates and additional training when
equipment, procedures, or policies change. All persons entering the
facility are advised of the potential hazards, are instructed on the appro-
priate safeguards, and read and follow instructions on practices and
procedures. An institutional policy regarding visitor training, occupational
health requirements, and safety communication is considered.
3. Personal health status may aect an individual’s susceptibility to
infection and ability to receive available immunizations or prophylactic
interventions. Therefore, all personnel, and particularly those of
reproductive age and/or those having conditions that may predispose
them to increased risk for infection (e.g., organ transplant, medical
immunosuppressive agents), are provided information regarding immune
competence and susceptibility to infectious agents. Individuals having

44 Biosafety in Microbiological and Biomedical Laboratories
such conditions are encouraged to self-identify to the institution’s
healthcare provider for appropriate counseling and guidance. See
Section VII.
4. A safety manual specic to the facility is prepared or adopted in consul-
tation with the facility director and appropriate safety professionals. The
safety manual is available, accessible, and periodically reviewed and
updated as necessary.
a. The safety manual contains sucient information to describe the
biosafety and containment procedures for the organisms and
biological materials in use, appropriate agent-specic decontami-
nation methods, and the work performed.
b. The safety manual contains or references protocols for emergency
situations, including exposures, medical emergencies, facility
malfunctions, and other potential emergencies. Training in
emergency response procedures is provided to emergency
response personnel and other responsible sta according to institu-
tional policies.
5. A sign incorporating the universal biohazard symbol is posted at the
entrance to the laboratory when infectious materials are present. Posted
information includes: the laboratory’s Biosafety Level, the supervisor’s
or other responsible personnel’s name and telephone number, PPE
requirements, general occupational health requirements (e.g., immuniza-
tions, respiratory protection), and required procedures for entering and
exiting the laboratory. Agent information is posted in accordance with the
institutional policy.
6. Long hair is restrained so that it cannot contact hands, specimens,
containers, or equipment.
7. Gloves are worn to protect hands from exposure to hazardous materials.
a. Glove selection is based on an appropriate risk assessment.
b. Gloves are not worn outside the laboratory.
c. Change gloves when contaminated, glove integrity is compromised,
or when otherwise necessary.
d. Do not wash or reuse disposable gloves and dispose of used gloves
with other contaminated laboratory waste.
8. Gloves and other PPE are removed in a manner that minimizes personal
contamination and transfer of infectious materials outside of the areas
where infectious materials and/or animals are housed or manipulated.
45Section IV—Laboratory Biosafety Level Criteria
9. Persons wash their hands after working with potentially hazardous
materials and before leaving the laboratory.
10. Eating, drinking, smoking, handling contact lenses, applying cosmetics,
and storing food for human consumption are not permitted in laboratory
areas. Food is stored outside the laboratory area.
11. Mouth pipetting is prohibited. Mechanical pipetting devices are used.
12. Policies for the safe handling of sharps, such as needles, scalpels,
pipettes, and broken glassware are developed, implemented, and
followed; policies are consistent with applicable state, federal, and
local requirements. Whenever practical, laboratory supervisors adopt
improved engineering and work practice controls that reduce risk of
sharps injuries. Precautions are always taken with sharp items. These
include:
a. Plasticware is substituted for glassware whenever possible.
b. Use of needles and syringes or other sharp instruments is limited
in the laboratory and is restricted to situations where there is no
alternative (e.g., parenteral injection, blood collection, or aspiration
of uids from laboratory animals or diaphragm bottles). Active or
passive needle-based safety devices are to be used whenever
possible.
i. Uncapping of needles is performed in such a manner to reduce
the potential for recoil causing an accidental needlestick.
ii. Needles are not bent, sheared, broken, recapped, removed
from disposable syringes, or otherwise manipulated by hand
before disposal.
iii. If absolutely necessary to remove a needle from a syringe
(e.g., to prevent lysing blood cells) or recap a needle (e.g.,
loading syringes in one room and injecting animals in another),
a hands-free device or comparable safety procedure must be
used (e.g., a needle remover on a sharps container, the use of
forceps to hold the cap when recapping a needle).
iv. Used, disposable needles and syringes are carefully placed
in puncture-resistant containers used for sharps disposal
immediately after use. The sharps disposal container is located
as close to the point of use as possible.
c. Non-disposable sharps are placed in a hard-walled container for
transport to a processing area for decontamination, preferably by
autoclaving.

46 Biosafety in Microbiological and Biomedical Laboratories
d. Broken glassware is not handled directly. Instead, it is removed
using a brush and dustpan, tongs, or forceps.
13. Perform all procedures to minimize the creation of splashes and/or
aerosols.
14. Decontaminate work surfaces after completion of work and after any spill
or splash of potentially infectious material with appropriate disinfectant.
Spills involving infectious materials are contained, decontaminated, and
cleaned up by sta who are properly trained and equipped to work with
infectious material. A spill procedure is developed and posted within the
laboratory.
15. Decontaminate all cultures, stocks, and other potentially infectious
materials before disposal using an eective method, consistent with
applicable institutional, local, and state requirements. Depending on
where the decontamination will be performed, the following methods are
used prior to transport:
a. Materials to be decontaminated outside of the immediate laboratory
are placed in a durable, leak-proof container and secured for
transport. For infectious materials, the outer surface of the container
is disinfected prior to moving materials and the transport container
has a universal biohazard label.
b. Materials to be removed from the facility for decontamination are
packed in accordance with applicable local, state, and federal
regulations.
16. An eective integrated pest management program is implemented. See
Appendix G.
17. Animals and plants not associated with the work being performed are not
permitted in the laboratory.
B. Special Practices
1. All persons entering the laboratory are advised of the potential hazards
and meet specic entry/exit requirements in accordance with institutional
policies. Only persons whose presence in the facility or laboratory areas
is required for scientic or support purposes are authorized to enter.
2. All persons who enter operational laboratory areas are provided
information on signs and symptoms of disease and receive occupational
medical services including medical evaluation, surveillance, and
treatment, as appropriate, and oered available immunizations for
agents handled or potentially present in the laboratory.

47Section IV—Laboratory Biosafety Level Criteria
3. The laboratory supervisor is responsible for ensuring that laboratory
personnel demonstrate prociency in standard microbiological practices
and techniques for working with agents requiring BSL-3 containment.
4. A system is established for reporting and documenting near misses,
laboratory accidents, exposures, unanticipated absences due to potential
Laboratory-associated infection, and for the medical surveillance of
potential laboratory-associated illnesses.
5. Incidents that result in exposure to infectious materials are immediately
evaluated per institutional policy. All such incidents are reported to the
laboratory supervisor, institutional management, and appropriate safety,
compliance, and security personnel according to institutional policy.
Appropriate records are maintained.
6. Biological materials that require BSL-3 containment are placed in a
durable leak-proof sealed primary container and then enclosed in a
non-breakable, sealed secondary container prior to removal from the
laboratory. Once removed, the primary container is opened within a BSC
in BSL-3 containment unless a validated inactivation method is used.
See Appendix K. The inactivation method is documented in-house with
viability testing data to support the method.
7. All procedures involving the manipulation of infectious materials are
conducted within a BSC or other physical containment device, when
possible. No work with open vessels is conducted on the bench. If it
is not possible to perform a procedure within a BSC or other physical
containment device, a combination of personal protective equipment
and other administrative and/or engineering controls, such as centrifuge
safety cups or sealed rotors, are used, based on a risk assessment.
Loading and unloading of the rotors and centrifuge safety cups take
place in the BSC or another containment device.
8. Laboratory equipment is routinely decontaminated after spills, splashes,
or other potential contamination, and before repair, maintenance, or
removal from the laboratory.
a. Equipment or material that might be damaged by high temperatures
or steam is decontaminated using an eective and veried method,
such as a gaseous or vapor method.
9. A method for decontaminating all laboratory waste is available in the
facility, preferably within the laboratory (e.g., autoclave, chemical disin-
fection, or other validated decontamination method).
48 Biosafety in Microbiological and Biomedical Laboratories
10. Decontamination of the entire laboratory is considered when there has
been gross contamination of the space, signicant changes in laboratory
usage, major renovations, or maintenance shutdowns. Selection of the
appropriate materials and methods used to decontaminate the laboratory
is based on a risk assessment.
11. Decontamination processes are veried on a routine basis.
C. Safety Equipment (Primary Barriers and Personal Protective Equipment)
1. Laboratory workers wear protective clothing with a solid-front, such as
tie-back or wrap-around gowns, scrub suits, or coveralls. Protective
clothing is not worn outside of the laboratory. Reusable clothing is
decontaminated before being laundered. Clothing is changed when
contaminated.
2. Based on work being performed, additional PPE may be required.
a. Eye protection and face protection (e.g., safety glasses, goggles,
mask, face shield or other splash guard) are used for manipulations
or activities that may result in splashes or sprays of infectious or
other hazardous materials. Eye protection and face protection are
disposed of with other contaminated laboratory waste or decontami-
nated after use.
b. Two pairs of gloves are worn when appropriate.
c. Respiratory protection is considered. Sta wearing respiratory
protection are enrolled in a properly constituted respiratory
protection program.
d. Shoe covers are considered.
3. In circumstances where research animals are present in the laboratory,
the risk assessment considers appropriate eye, face, and respiratory
protection, as well as potential animal allergens.
D. Laboratory Facilities (Secondary Barriers)
1. The laboratory is separated from areas that are open to unrestricted
trac ow within the building.
a. Laboratory access is restricted. Laboratory doors are lockable in
accordance with institutional policies. Access to the laboratory is
through two consecutive self-closing doors. A clothing change room
and/or an anteroom may be included in the passageway between
the two self-closing doors.
2. Laboratories have a sink for handwashing. The sink is hands-free
or automatically operated and should be located near the exit door.

49Section IV—Laboratory Biosafety Level Criteria
If a laboratory suite is segregated into dierent zones, a sink is also
available for handwashing in each zone.
3. An eyewash station is readily available in the laboratory.
4. The laboratory is designed, constructed, and maintained to facilitate
cleaning, decontamination, and housekeeping.
a. Carpets and rugs are not permitted.
b. Spaces between benches, cabinets, and equipment are accessible
for cleaning.
c. Seams, oors, walls, and ceiling surfaces are sealed. Spaces
around doors and ventilation openings are capable of being sealed
to facilitate space decontamination.
d. Floors are slip-resistant, impervious to liquids, and resistant to
chemicals. Flooring is seamless, sealed, or poured with integral
cove bases.
e. Walls and ceilings are constructed to produce a sealed smooth
nish that can be easily cleaned and decontaminated.
5. Laboratory furniture can support anticipated loads and uses.
a. Benchtops are impervious to water and resistant to heat, organic
solvents, acids, alkalis, and other chemicals.
b. Chairs used in laboratory work are covered with a non-porous
material that can be easily cleaned and decontaminated with an
appropriate disinfectant.
6. All windows in the laboratory are sealed.
7. Illumination is adequate for all activities and avoids reections and glare
that could impede vision.
8. Vacuum lines in use are protected with liquid disinfectant traps and
in-line HEPA lters or their equivalent. See Appendix A, Figure 11. Filters
are replaced, as needed, or are on a replacement schedule determined
by a risk assessment. Vacuum lines not protected as described are
capped. The placement of an additional HEPA lter immediately prior to
a central vacuum pump is considered.
9. A ducted mechanical air ventilation system is required. This system
provides sustained directional airow by drawing air into the laboratory
from “clean” areas toward “potentially contaminated” areas. The
laboratory is designed such that under failure conditions the airow will
not be reversed at the containment barrier.

50 Biosafety in Microbiological and Biomedical Laboratories
a. A visual monitoring device that conrms directional airow is
provided at the laboratory entry. Audible alarms to notify personnel
of airow disruption are considered.
b. The laboratory exhaust air is not re-circulated to any other area in
the building.
c. The laboratory exhaust air is dispersed away from occupied areas
and from building air intake locations or the exhaust air is HEPA
ltered.
10. BSCs and other primary containment barrier systems are installed and
operated in a manner to ensure their eectiveness. See Appendix A.
a. BSCs are installed so that uctuations of the room air supply and
exhaust do not interfere with proper operations. BSCs are located
away from doors, heavily traveled laboratory areas, and other
possible airow disruptions.
b. BSCs can be connected to the laboratory exhaust system by either
a canopy connection (Class IIA only) or directly exhausted to the
outside through a hard connection (Class IIB, IIC, or III). Class IIA or
IIC BSC exhaust can be safely recirculated back into the laboratory
environment if no volatile toxic chemicals are used in the cabinet.
c. BSCs are certied at least annually to ensure correct performance,
or as specied in Appendix A, Part 7.
d. Class III BSCs are provided supply air in such a manner that
prevents positive pressurization of the cabinet or the room.
11. Equipment that may produce infectious aerosols is used within primary
barrier devices that exhaust air through HEPA ltration or other equiv-
alent technology before being discharged into the laboratory. These
HEPA lters are tested annually and replaced as needed.
12. Facility is constructed to allow decontamination of the entire laboratory
when there has been gross contamination of the space, signicant
changes in usage, major renovations, or maintenance shutdowns.
Selection of the appropriate materials and methods used to decontam-
inate the laboratory is based on the risk assessment.
a. Facility design consideration is given to means of decontaminating
large pieces of equipment before removal from the laboratory.
13. Enhanced environmental and personal protection may be necessary
based on risk assessment and applicable local, state, or federal
regulations. These laboratory enhancements may include one or more of
the following: an anteroom for clean storage of equipment and supplies
51Section IV—Laboratory Biosafety Level Criteria
with dress-in, shower-out capabilities; gas-tight dampers to facilitate
laboratory isolation; nal HEPA ltration of the laboratory exhaust air;
laboratory euent decontamination; containment of other piped services;
or advanced access control devices, such as biometrics.
14. When present, HEPA lter housings have gas-tight isolation dampers,
decontamination ports, and/or bag-in/bag-out (with appropriate decon-
tamination procedures) capability. All HEPA lters are located as near
as practicable to the laboratory to minimize the length of potentially
contaminated ductwork. The HEPA lter housings allow for leak testing
of each lter and assembly. The lters and housings are certied at least
annually.
15. The BSL-3 facility design, operational parameters, and procedures are
veried and documented prior to operation. Facilities are tested annually
or after signicant modication to ensure operational parameters are
met. Verication criteria are modied as necessary by operational
experience.
16. Appropriate communication systems are provided between the
laboratory and the outside (e.g., voice, fax, and computer). Provisions
for emergency communication and emergency access or egress are
developed and implemented.
Biosafety Level 4
Biosafety Level 4 (BSL-4) is required for work with dangerous and exotic agents
that pose a high individual risk of aerosol-transmitted laboratory infections and
life-threatening diseases that are frequently fatal, agents for which there are no
vaccines or treatments, or work with a related agent with unknown risk of trans-
mission. Agents with a close or identical antigenic relationship to agents requiring
BSL-4 containment are handled at this level until sucient data are obtained to
re-designate the level. Laboratory sta receive specic and thorough training in
handling extremely hazardous infectious agents. Laboratory sta understand the
primary and secondary containment functions of standard and special practices,
containment equipment, and laboratory design characteristics. All laboratory
sta and supervisors are competent in handling agents and procedures requiring
BSL-4 containment. The laboratory supervisor controls access to the laboratory
in accordance with institutional policies.
There are two models for BSL-4 laboratories:
1. Cabinet Laboratory: manipulation of agents is performed in a Class III
BSC; and
2. Suit Laboratory: personnel wear a positive-pressure supplied-air
protective suit.

52 Biosafety in Microbiological and Biomedical Laboratories
BSL-4 cabinet and suit laboratories have special engineering and design features
to prevent microorganisms from dissemination into the environment.
The following standard and special practices, safety equipment, and facility
specications are necessary for BSL-4.
A. Standard Microbiological Practices
1. The laboratory supervisor enforces the institutional policies that control
safety in and access to the laboratory.
2. The laboratory supervisor ensures that laboratory personnel receive
appropriate training regarding their duties, potential hazards, manipula-
tions of infectious agents, necessary precautions to minimize exposures,
and hazard/exposure evaluation procedures (e.g., physical hazards,
splashes, aerosolization) and that appropriate records are maintained.
Personnel receive annual updates and additional training when
equipment, procedures, or policies change. All persons entering the
facility are advised of the potential hazards, are instructed on the appro-
priate safeguards, and read and follow instructions on practices and
procedures. An institutional policy regarding visitor training, occupational
health requirements, and safety communication is considered.
3. Personal health status may aect an individual’s susceptibility to
infection and ability to receive available immunizations or prophylactic
interventions. Therefore, all personnel, and particularly those of
reproductive age and/or those having conditions that may predispose
them to increased risk for infection (e.g., organ transplant, medical
immunosuppressive agents), are provided information regarding immune
competence and susceptibility to infectious agents. Individuals having
such conditions are encouraged to self-identify to the institution’s
healthcare provider for appropriate counseling and guidance. See
Section VII.
4. A safety manual specic to the facility is prepared or adopted in consul-
tation with the facility director and appropriate safety professionals. The
safety manual is available, accessible, and periodically reviewed and
updated as necessary.
a. The safety manual contains sucient information to describe the
biosafety and containment procedures for the organisms and
biological materials in use, appropriate agent-specic decontami-
nation methods, and the work performed.
b. The safety manual contains or references protocols for emergency
situations, including exposures, medical emergencies, facility
53Section IV—Laboratory Biosafety Level Criteria
malfunctions, and other potential emergencies. Training in
emergency response procedures is provided to emergency
response personnel and other responsible sta according to institu-
tional policies.
5. A sign incorporating the universal biohazard symbol is posted at the
entrance to the laboratory when infectious materials are present. Posted
information includes: the laboratory’s Biosafety Level, the supervisor’s
or other responsible personnel’s name and telephone number, PPE
requirements, general occupational health requirements (e.g., immuniza-
tions, respiratory protection), and required procedures for entering and
exiting the laboratory. Agent information is posted in accordance with the
institutional policy.
6. Long hair is restrained so that it cannot contact hands, specimen,
containers, or equipment
7. Gloves are worn to protect hands from exposure to hazardous materials.
a. Glove selection is based on an appropriate risk assessment.
b. Inner gloves are not worn outside the laboratory.
c. Change inner gloves when contaminated, glove integrity is compro-
mised, or when otherwise necessary.
d. Do not wash or reuse disposable gloves,and dispose of used gloves
with other contaminated laboratory waste.
8. Gloves and other PPE are removed in a manner that minimizes personal
contamination and transfer of infectious materials outside of the areas
where infectious materials and/or animals are housed or manipulated.
9. Eating, drinking, smoking, handling contact lenses, applying cosmetics,
and storing food for human consumption are not permitted in laboratory
areas. Food is stored outside the laboratory area.
10. Mouth pipetting is prohibited. Mechanical pipetting devices are used.
11. Policies for the safe handling of sharps, such as needles, scalpels,
pipettes, and broken glassware are developed, implemented, and
followed; policies are consistent with applicable state, federal, and
local requirements. Whenever practical, laboratory supervisors adopt
improved engineering and work practice controls that reduce risk of
sharps injuries. Precautions are always taken with sharp items. These
include:
a. Plasticware is substituted for glassware whenever possible.
54 Biosafety in Microbiological and Biomedical Laboratories
b. Use of needles and syringes or other sharp instruments is limited
in the laboratory and is restricted to situations where there is no
alternative (e.g., parenteral injection, blood collection, or aspiration
of uids from laboratory animals or diaphragm bottles). Active or
passive needle-based safety devices are to be used whenever
possible.
i. Uncapping of needles is performed in such a manner to reduce
the potential for recoil causing an accidental needlestick.
ii. Needles are not bent, sheared, broken, recapped, removed
from disposable syringes, or otherwise manipulated by hand
before disposal.
iii. If absolutely necessary to remove a needle from a syringe
(e.g., to prevent lysing blood cells) or recap a needle (e.g.,
loading syringes in one room and injecting animals in another),
a hands-free device or comparable safety procedure must be
used (e.g., a needle remover on a sharps container, the use of
forceps to hold the cap when recapping a needle).
iv. Used, disposable needles and syringes are carefully placed
in puncture-resistant containers used for sharps disposal
immediately after use. The sharps disposal container is located
as close to the point of use as possible.
c. Non-disposable sharps are placed in a hard-walled container for
transport to a processing area for decontamination, preferably by
autoclaving.
d. Broken glassware is not handled directly. Instead, it is removed
using a brush and dustpan, tongs, or forceps.
12. Perform all procedures to minimize the creation of splashes and/or
aerosols.
13. Decontaminate work surfaces after completion of work and after any spill
or splash of potentially infectious material with appropriate disinfectant.
Spills involving infectious materials are contained, decontaminated, and
cleaned up by sta who are properly trained and equipped to work with
infectious material. A spill procedure is developed and posted within the
laboratory.
14. Decontaminate all cultures, stocks, and other potentially infectious
materials before disposal using an eective method, consistent with
applicable institutional, local, and state requirements. A method for
decontaminating all laboratory wastes is available in the laboratory

55Section IV—Laboratory Biosafety Level Criteria
(e.g., autoclave, chemical disinfection, incineration, or other validated
decontamination method). See B. Special Practices, #7 in the following
sub-section for additional details.
15. An eective integrated pest management program is implemented. See
Appendix G.
16. Animals and plants not associated with the work being performed are not
permitted in the laboratory.
B. Special Practices
1. All persons entering the laboratory are advised of the potential hazards
and meet specic entry/exit requirements in accordance with institutional
policies. Only persons whose presence in the facility or individual
laboratory rooms is required for scientic or support purposes are
authorized to enter. Additional training/security requirements may be
required prior to gaining independent access to BSL-4 laboratories.
2. All persons who enter operational laboratory areas are provided
information on signs and symptoms of disease and receive occupational
medical services including medical evaluation, surveillance, and
treatment, as appropriate, and oered available immunizations for
agents handled or potentially present in the laboratory.
a. An essential adjunct to such an occupational medical services
system is the availability of a facility for the isolation and medical
care of personnel with potential or known Laboratory-associated
infections.
3. Laboratory personnel and support sta are trained and approved to work
in the facility. The laboratory supervisor is responsible for ensuring that,
prior to working independently with agents requiring BSL-4 containment,
laboratory personnel demonstrate high prociency in standard and
special microbiological practices and techniques for working with agents
requiring BSL-4 containment. Personnel are required to read and follow
instructions on practices, and procedural changes are addressed as part
of the protocol review.
4. A system is established for reporting and documenting near misses,
laboratory accidents, exposures, unanticipated absence due to potential
Laboratory-associated infection, and for the medical surveillance of
potential laboratory-associated illnesses.
5. Incidents that result in exposure to infectious materials are immediately
evaluated per institutional policy. All such incidents are reported to the
laboratory supervisor, institutional management, and appropriate safety,

56 Biosafety in Microbiological and Biomedical Laboratories
compliance, and security personnel according to institutional policy.
Appropriate records are maintained.
6. Biological materials that require BSL-4 containment are placed in a
durable, leak-proof sealed primary container and then enclosed in a
non-breakable, sealed secondary container prior to removal from the
BSL-4 facility by authorized personnel. These materials are transferred
through a disinfectant dunk tank, fumigation chamber, or decontam-
ination shower for receipt by authorized personnel. Once removed,
the primary container is not to be opened outside BSL-4 containment
unless a validated inactivation method is used (e.g., gamma irradiation).
See Appendix K. The inactivation method is documented in-house with
viability testing data to support the method.
7. All waste is decontaminated by a veried method prior to removal from
the laboratory.
8. Equipment is routinely decontaminated and is decontaminated after
spills, splashes, or other potential contamination and before repair,
maintenance, or removal from the laboratory.
a. Equipment or material that might be damaged by high temperatures
or steam is decontaminated using an eective and veried method,
such as a gaseous or vapor method, in an airlock or chamber
designed for this purpose.
9. A logbook, or other means of documenting the date and time of all
persons entering and leaving the laboratory, is maintained.
10. An inventory system for agents stored within the laboratory is in place.
11. While the laboratory is operational, personnel enter and exit the
laboratory through the clothing change and shower rooms except during
emergencies. All personal clothing and jewelry (except eyeglasses)
are removed in the outer clothing change room. All persons entering
the laboratory use laboratory clothing, including undergarments, pants,
shirts, socks, jumpsuits, shoes, and gloves, as appropriate. All persons
leaving the laboratory take a personal body shower. Used laboratory
clothing and other waste, including gloves, are not removed from the
inner change room through the personal shower. These items are treated
as contaminated materials and decontaminated before laundering or
disposal.
12. After the laboratory has been completely decontaminated by verication
of a validated method and all infectious agents are secured, necessary
sta may enter and exit without following the clothing change and
shower requirements described above.
57Section IV—Laboratory Biosafety Level Criteria
13. Daily inspections of essential containment and life support systems are
completed and documented before laboratory work is initiated to ensure
that the laboratory is operating according to established parameters.
14. Only necessary equipment and supplies are stored inside the laboratory.
All equipment and supplies taken inside the laboratory are decontami-
nated before removal from the laboratory.
a. Supplies and materials that are not brought into the laboratory
through the change room are brought in through a dunk tank,
previously decontaminated double-door autoclave, fumigation
chamber, or airlock. After securing the outer doors, personnel within
the laboratory retrieve the materials by opening the interior doors
of the autoclave, fumigation chamber, or airlock. The inner door
is secured after materials are brought into the facility. The outer
door of the autoclave or fumigation chamber is not opened until
the autoclave, fumigation chamber, or airlock has been operated
through a successful decontamination cycle.
C. Safety Equipment (Primary Barriers and Personal Protective Equipment)
Cabinet Laboratory
1. All procedures involving the manipulation of infectious materials are
conducted within a Class III BSC.
2. A Class III BSC contains:
a. Double-door, pass-through autoclave for decontaminating materials
passing out of the Class III BSC(s). The autoclave doors are
interlocked so that only one door can be opened at any time and are
automatically controlled so that the outside door to the autoclave
can only be opened after a successful decontamination cycle has
been completed.
b. A pass-through dunk tank, fumigation chamber, or equivalent decon-
tamination method so that materials and equipment that cannot be
decontaminated in the autoclave can be safely removed from the
cabinet. Containment between the cabinet and the surrounding
laboratory is maintained at all times.
c. A HEPA lter on the supply air intake and two HEPA lters in series
on the exhaust outlet of the unit. Supply air is provided in such a
manner that prevents positive pressurization of the cabinet. There
are gas-tight dampers on the supply and exhaust ducts of the
cabinet to permit gas or vapor decontamination of the unit. Ports for
injection of test medium are present on all HEPA lter housings.
58 Biosafety in Microbiological and Biomedical Laboratories
d. An interior constructed with smooth nishes that can be easily
cleaned and decontaminated. All sharp edges on cabinet nishes
are eliminated to reduce the potential for cuts and tears of gloves.
Equipment to be placed in the Class III BSC is also free of sharp
edges or other surfaces that may damage or puncture the cabinet
gloves.
e. Gloves that are inspected for damage prior to use and changed
if necessary. Gloves are replaced annually during cabinet
recertication.
3. The cabinet is designed to permit maintenance and repairs of cabinet
mechanical systems (e.g., refrigeration, incubators, centrifuges) to be
performed from the exterior of the cabinet whenever possible.
4. Manipulation of high concentrations or large volumes of infectious agents
within the Class III BSC is performed using physical containment devices
inside the cabinet whenever practical. Such materials are centrifuged
inside the cabinet using sealed rotors or centrifuge safety cups.
5. The interior of the Class III BSC and all contaminated plenums, fans, and
lters are decontaminated using a validated gaseous or vapor method
when there have been signicant changes in cabinet usage, before
major renovations or maintenance shutdowns, and in other situations,
as determined by risk assessment. Success of the decontamination is
veried before accessing the interior spaces of the cabinet.
6. The Class III BSC is certied at least annually.
7. For Class III BSCs directly connected via a double-door, pass-through to
a BSL-4 suit laboratory, materials may be placed into and removed from
the Class III BSC via the suit laboratory.
8. Workers in the laboratory wear protective laboratory clothing with a solid
front, such as tie-back or wrap-around gowns, scrubs, or coveralls. Shoe
coverings are considered based on a risk assessment.
a. Upon exit, all protective clothing is removed in the inner change
room before showering.
b. Prescription eyeglasses are decontaminated before removal through
the personal body shower.
9. Disposable gloves are worn underneath cabinet gloves to protect the
worker from exposure should a break or tear occur in a cabinet glove.
59Section IV—Laboratory Biosafety Level Criteria
Suit Laboratory
1. All procedures involving the manipulation of infectious materials are
conducted within a BSC or other physical containment devices. No work
with open vessels is conducted on the bench.
2. Equipment that may produce aerosols is used within primary barrier
devices that exhaust air through HEPA ltration before being discharged
into the laboratory or facility exhaust system. These HEPA lters are
tested annually and replaced as needed.
3. Materials centrifuged in the laboratory use sealed rotors or centrifuge
safety cups. Loading and unloading of the rotors and centrifuge safety
cups take place in the BSC or another containment device.
4. All procedures are conducted by personnel wearing a one-piece,
positive-pressure supplied-air suit.
a. All persons don laboratory clothing, such as scrubs, before entering
the room used for donning positive-pressure suits.
b. Procedures are in place to control and verify the operation of the
one-piece positive-pressure supplied-air suit, including gloves,
before each use.
c. Decontamination of outer suit gloves is performed during the course
of normal laboratory operations to remove gross contamination and
minimize further contamination of the laboratory.
d. Inner disposable gloves are worn to protect the laboratorian should
a break or tear in the outer suit gloves occur. Disposable inner
gloves are not worn outside the inner change area.
e. Upon exit from the chemical shower, inner gloves and all laboratory
clothing are removed and discarded or collected for autoclaving
before laundering prior to entering the personal shower.
f. Prescription eyeglasses are decontaminated before removal through
the personal body shower.
D. Laboratory Facilities (Secondary Barriers)
Cabinet Laboratory
1. The BSL-4 cabinet facility may be located in a separate building or a
clearly demarcated and isolated zone within a building.
a. Facility access is restricted. Laboratory doors are lockable.
60 Biosafety in Microbiological and Biomedical Laboratories
b. Exit from the laboratory is by sequential passage through an inner
(i.e., dirty) changing area, a personal shower, and an outer (i.e.,
clean) change room upon exiting the cabinet laboratory.
2. An automatically activated emergency power source is provided, at a
minimum, for the laboratory exhaust system, alarms, lighting, entry and
exit controls, BSCs, and door gaskets.
a. Monitoring and control systems for air supply, exhaust, life support,
alarms, entry and exit controls, and security systems are on an
uninterrupted power supply (UPS).
3. A double-door autoclave, dunk tank, fumigation chamber, or ventilated
airlock is provided at the containment barrier for the passage of
materials, supplies, or equipment.
4. A hands-free sink is provided near the door of the cabinet laboratory(ies)
and the inner change room. A sink is provided in the outer change room.
5. An eyewash station is readily available in the laboratory.
6. Walls, oors, and ceilings of the cabinet laboratory are constructed to
form a sealed internal shell to facilitate fumigation and prohibit animal
and insect intrusion. The internal surfaces of this shell are resistant to
liquids and chemicals used for cleaning and decontamination of the area.
Floors are monolithic, sealed, and coved.
a. All penetrations in the internal shell of the cabinet laboratory and
inner change room are sealed.
b. Openings around doors into the cabinet laboratory and inner
change room are minimized and capable of being sealed to facilitate
decontamination.
7. Services and plumbing that penetrate the cabinet laboratory walls, oors,
or ceiling are installed to ensure that no backow from the laboratory
occurs. These penetrations are tted with two (in series) backow
prevention devices. Consideration is given to locating these devices
outside of containment. Atmospheric venting systems are provided with
two HEPA lters in series and are sealed up to the second lter.
8. Furniture is minimized, of simple construction, and capable of supporting
anticipated loads and uses.
a. Spaces between benches, cabinets, and equipment are accessible
for cleaning and decontamination.
b. Benchtops are impervious to water and resistant to heat, organic
solvents, acids, alkalis, and other chemicals.

61Section IV—Laboratory Biosafety Level Criteria
c. Chairs used in laboratory work are covered with a non-porous
material that can be easily cleaned and decontaminated.
9. Windows are break-resistant and sealed.
10. Illumination is adequate for all activities and avoids reections and glare
that could impede vision.
11. If Class II BSCs or other primary containment barrier systems are
needed in the cabinet laboratory, they are installed and operated in a
manner to ensure their eectiveness. See Appendix A.
a. BSCs are installed so that uctuations of the room air supply and
exhaust do not interfere with proper operations. BSCs are located
away from doors, heavily traveled laboratory areas, and other
possible airow disruptions.
b. BSCs can be connected to the laboratory exhaust system by either
a canopy connection (Class IIA only) or directly exhausted to the
outside through a hard connection (Class IIB, IIC, or III). Cabinet
exhaust air passes through two HEPA lters, including the HEPA
in the BSC, prior to release outside. Class IIA or IIC BSC exhaust
can be safely recirculated back into the laboratory environment if no
volatile toxic chemicals are used in the cabinet.
c. BSCs are certied at least annually to ensure correct performance,
or as specied in Appendix A, Part 7.
12. Central vacuum systems are discouraged. If there is a central vacuum
system, it does not serve areas outside the cabinet. Two in-line HEPA
lters are placed near each use point and overow collection is provided
while in use. Filters are installed to permit in-place decontamination and
replacement.
13. A dedicated, non-recirculating ventilation system is provided. Only
cabinet laboratories with the same HVAC requirements (i.e., other BSL-4
cabinet laboratories, ABSL-4 cabinet facilities) may share ventilation
systems if gas-tight dampers and HEPA lters isolate each individual
laboratory system.
a. The supply and exhaust components of the ventilation system
are designed to maintain the laboratory at negative pressure to
surrounding areas and provide dierential pressure or directional
airow, as appropriate, between adjacent areas within the laboratory.
b. Redundant supply fans are recommended. Redundant exhaust fans
are required. Supply and exhaust fans are interlocked to prevent
positive pressurization of the cabinet laboratory.
62 Biosafety in Microbiological and Biomedical Laboratories
c. The ventilation system is monitored and alarmed to indicate
malfunction or deviation from design parameters. A visual monitoring
device is installed outside of containment so proper dierential
pressures within the laboratory may be veried prior to entry and
during regular checklist procedures. Visual monitoring is also in
place within containment.
d. Supply air to and exhaust air from the cabinet laboratory, inner
change room, and fumigation/decontamination chambers pass
through a HEPA lter. The air exhaust discharge is located away
from occupied spaces and building air intakes.
e. All HEPA lters are located as near as practicable to the cabinet
and laboratory to minimize the length of potentially contaminated
ductwork. All HEPA lters are tested and certied annually.
f. The HEPA lter housings are designed to allow for in situ decon-
tamination and verication of the validated decontamination process
prior to removal. The design of the HEPA lter housing has gas-tight
isolation dampers, decontamination ports, and the ability to individ-
ually scan each lter in the assembly for leaks.
14. Pass-through dunk tanks, fumigation chambers, or equivalent decon-
tamination methods are provided so that materials and equipment that
cannot be decontaminated in the autoclave can be safely removed from
the cabinet laboratory(ies). Access to the exit side of the pass-through is
limited to those with authorized access to the BSL-4 laboratory and with
specic clearance, if required.
15. Liquid euents from cabinet laboratory sinks, oor drains, autoclave
chambers, and other sources within the cabinet laboratory are decon-
taminated by a proven method, preferably heat treatment, before being
discharged to the sanitary sewer.
a. Decontamination of all liquid euents is documented. The decon-
tamination process for liquid euents is validated physically and
biologically. Biological validation is performed at least annually or
more often, if required by institutional policy.
b. Euents from personal body showers and toilets may be discharged
to the sanitary sewer without treatment.
16. A double-door, pass-through autoclave is provided for decontaminating
materials passing out of the cabinet laboratory. Autoclaves that open
outside of the laboratory are sealed to the wall through which the
autoclave passes. This bioseal is durable, airtight, and capable of
expansion and contraction. Positioning the bioseal so that the equipment
63Section IV—Laboratory Biosafety Level Criteria
can be accessed and maintained from outside the laboratory is strongly
recommended. The autoclave doors are interlocked so that only one
can be opened at any time and are automatically controlled so that the
outside door to the autoclave can only be opened after the decontami-
nation cycle has been completed.
a. Gas discharge from the autoclave chamber is HEPA-ltered
or decontaminated. Autoclave decontamination processes are
designed so that unltered air or steam exposed to infectious
material cannot be released to the environment.
17. The facility design parameters and operational procedures are
documented. The facility is tested to verify that the design and opera-
tional parameters have been met prior to operation. Facilities are also
re-tested annually or after signicant modication to ensure operational
parameters are met. Verication criteria are modied, as necessary, by
operational experience.
18. Appropriate communication systems are provided between the
laboratory and the outside (e.g., voice, fax, video, and computer).
Provisions for emergency communication and emergency access or
egress are developed and implemented.
Suit Laboratory
1. The BSL-4 suit facility may be located in a separate building or a clearly
demarcated and isolated zone within a building.
a. Facility access is restricted. Laboratory doors are lockable.
b. Entry into the laboratory is through an airlock tted with airtight
doors.
c. Exit from the laboratory is by sequential passage through the
chemical shower, inner (i.e., dirty) change room, personal shower,
and outer (i.e., clean) changing area.
2. Personnel who enter this area wear a positive-pressure suit supplied
with HEPA-ltered breathing air. The breathing air systems have
redundant compressors, failure alarms, and emergency back-up capable
of supporting all workers within the laboratory to allow the personnel to
safely exit the laboratory.
3. A chemical shower is provided to decontaminate the surface of the
positive-pressure suit before the worker leaves the laboratory. In the
event of an emergency exit or failure of the chemical shower system, a
method for decontaminating positive-pressure suits, such as a gravity-fed
supply of chemical disinfectant, is provided.
64 Biosafety in Microbiological and Biomedical Laboratories
4. An automatically activated emergency power source is provided at a
minimum for the laboratory exhaust system, alarms, lighting, entry and
exit controls, BSCs, and door gaskets.
a. Monitoring and control systems for air supply, exhaust, life support,
alarms, entry and exit controls, and security systems are on an
uninterrupted power supply (UPS).
5. A double-door autoclave, dunk tank, or fumigation chamber is provided
at the containment barrier for the passage of materials, supplies, or
equipment in or out of the laboratory.
6. Hands-free sinks inside the suit laboratory are placed near procedure
areas.
7. An eyewash station for use during maintenance is readily available in the
laboratory area.
8. Walls, oors, and ceilings of the laboratory are constructed to form a
sealed internal shell to facilitate fumigation and prohibit animal and
insect intrusion. The internal surfaces of this shell are resistant to liquids
and chemicals used for cleaning and decontamination of the area. Floors
are monolithic, sealed, and coved.
a. All penetrations in the internal shell of the laboratory, suit storage
room, and the inner change room are sealed.
9. Services and plumbing that penetrate the laboratory walls, oors, or
ceiling are installed to ensure that no backow from the laboratory
occurs. Breathing air systems are exempt from this provision. These
penetrations are tted with two (in series) backow prevention devices.
Consideration is given to locating these devices outside of containment.
Atmospheric venting systems are provided with two HEPA lters in series
and are sealed up to the second lter.
10. Decontamination of the entire laboratory is performed using a validated
gaseous or vapor method when there have been signicant changes
in usage, before major renovations or maintenance shutdowns, and in
other situations, as determined by risk assessment. Decontamination is
veried prior to any change in the status of the laboratory.
11. Furniture is minimized, of simple construction, and capable of supporting
anticipated loads and uses.
a. Spaces between benches, cabinets, and equipment are accessible
for cleaning, decontamination, and unencumbered movement of
personnel.

65Section IV—Laboratory Biosafety Level Criteria
b. Benchtops are impervious to water and resistant to heat, organic
solvents, acids, alkalis, and other chemicals.
c. Chairs used in laboratory work are covered with a non-porous
material that can be easily cleaned and decontaminated.
d. Sharp edges and corners are avoided.
12. Windows are break-resistant and sealed.
13. Illumination is adequate for all activities and avoids reections and glare
that could impede vision.
14. BSCs and other primary containment barrier systems are installed and
operated in a manner to ensure their eectiveness. See Appendix A.
a. BSCs are installed so that uctuations of the room air supply and
exhaust do not interfere with proper operations. BSCs are located
away from doors, windows that can be opened, heavily traveled
laboratory areas, and other possible airow disruptions.
b. BSCs can be connected to the laboratory exhaust system by either
a canopy connection (Class IIA only) or directly exhausted to the
outside through a hard connection (Class IIB, IIC, or III), which
contains a HEPA lter.
c. Class IIA or IIC BSC exhaust can be safely recirculated back into
the laboratory environment if no volatile toxic chemicals are used in
the cabinet.
d. BSCs are certied at least annually to ensure correct performance,
or as specied in Appendix A, Part 7.
e. Class III BSCs are provided supply air in such a manner that
prevents positive pressurization of the cabinet or the room.
15. Central vacuum systems are discouraged. If there is a central vacuum
system, it does not serve areas outside the laboratory. Two in-line HEPA
lters are placed near each use point and overow collection is provided
while in use. Filters are installed to permit in-place decontamination and
replacement. Consideration is made to the provision of two HEPA lters
in series as close to the vacuum pump as possible.
16. A dedicated, non-recirculating ventilation system is provided. Only
laboratories or facilities with the same HVAC requirements (i.e., other
BSL-4 laboratories, ABSL-4, ABSL-3Ag, ABSL-4Ag facilities) may share
ventilation systems if gas-tight dampers and HEPA lters isolate each
individual laboratory system.
66 Biosafety in Microbiological and Biomedical Laboratories
a. The ventilation system is designed to maintain the laboratory at
negative pressure to surrounding areas and provide dierential
pressure or directional airow as appropriate between adjacent
areas within the laboratory.
b. Redundant supply fans are recommended. Redundant exhaust fans
are required. Supply and exhaust fans are interlocked to prevent
positive pressurization of the laboratory.
c. The ventilation system is monitored and alarmed to indicate
malfunction or deviation from design parameters. A visual monitoring
device is installed outside of containment so proper dierential
pressures within the laboratory may be veried prior to entry and
during regular checklist procedures. Visual monitoring is also in
place within containment.
d. Supply air to the laboratory, including the decontamination shower,
passes through a HEPA lter. All exhaust air from the suit laboratory,
decontamination shower, and fumigation or decontamination
chambers passes through two HEPA lters, in series, before
discharge to the outside. The exhaust air discharge is located away
from occupied spaces and air intakes.
e. All HEPA lters are located as near as practicable to the laboratory
to minimize the length of potentially contaminated ductwork. All
HEPA lters are tested and certied annually.
f. The HEPA lter housings are designed to allow for in situ decon-
tamination of the lter and verication of the validated process prior
to removal. The design of the HEPA lter housing has gas-tight
isolation dampers, decontamination ports, and the ability to individ-
ually scan each lter in the assembly for leaks.
17. Pass-through dunk tanks, fumigation chambers, or equivalent decon-
tamination methods are provided so that materials and equipment that
cannot be decontaminated in the autoclave can be safely removed from
the laboratory. Access to the exit side of the pass-through is limited to
those individuals authorized to be in the facility and provided appropriate
clearance if required.
18. Liquid euents from chemical showers, sinks, oor drains, autoclave
chambers, and other sources within the laboratory are decontaminated
by a proven method, preferably heat treatment, before being discharged
to the sanitary sewer.
a. Decontamination of all liquid euents is documented. The decon-
tamination process for liquid euents is validated physically and
67Section IV—Laboratory Biosafety Level Criteria
biologically. Biological validation is performed at least annually or
more often if required by institutional policy.
b. Euents from personal body showers and toilets may be discharged
to the sanitary sewer without treatment.
19. A double-door, pass-through autoclave(s) is provided for decontam-
inating materials passing out of the laboratory. Autoclaves that open
outside of the laboratory are sealed to the wall through which the
autoclave passes. This bioseal is durable, airtight, and capable of
expansion and contraction. Positioning the bioseal so that the equipment
can be accessed and maintained from outside the laboratory is strongly
recommended. The autoclave doors are interlocked so that only one
can be opened at any time and be automatically controlled so that the
outside door to the autoclave can only be opened after a successful
decontamination cycle has been completed.
a. Gas discharge from the autoclave chamber is HEPA-ltered or
is decontaminated. Autoclave decontamination processes are
designed so that unltered air or steam exposed to infectious
material cannot be released to the environment.
20. The facility design parameters and operational procedures are
documented. The facility is tested to verify that the design and opera-
tional parameters have been met prior to operation. Facilities are also
re-tested annually or after signicant modication to ensure operational
parameters are maintained. Verication criteria are modied, as
necessary, by operational experience.
21. Appropriate communication systems are provided between the
laboratory and the outside (e.g., voice, fax, video, and computer).
Provisions for emergency communication and emergency access or
egress are developed and implemented.

68 Biosafety in Microbiological and Biomedical Laboratories
Table 1. Summary of Laboratory Biosafety Levels (BSLs)
BSL Agents
Special
Practices
a
Primary Barrier
and Personal
Protective
Equipment
a
Facilities
(Secondary
Barriers)
a
1 Well-characterized
agents not known
to consistently
cause disease in
immunocompetent
adult humans and
present minimal
potential hazard to
laboratory personnel
and the environment.
Standard
microbiological
practices
No primary barriers
required; protective
laboratory clothing;
protective face,
eyewear, as needed
Laboratory
doors; sink for
handwashing;
laboratory bench;
windows tted with
screens; lighting
adequate for all
activities
2 Agents associated
with human disease
and pose moderate
hazards to personnel
and the environment
Limited access;
occupational medical
services including
medical evaluation,
surveillance, and
treatment, as
appropriate; all
procedures that
may generate an
aerosol or splash
conducted in a BSC;
decontamination
process needed for
laboratory equipment
BSCs or other
primary containment
device used for
manipulations of
agents that may
cause splashes or
aerosols; protective
laboratory clothing;
other PPE,
including respiratory
protection, as
needed
Self-closing doors;
sink located near
exit; windows
sealed or tted with
screens; autoclave
available
3 Indigenous or
exotic agents; may
cause serious or
potentially lethal
disease through the
inhalation route of
exposure
Access limited to
those with need to
enter; viable material
removed from
laboratory in primary
and secondary
containers; opened
only in BSL-3 or
ABSL-3 laboratories;
all procedures with
infectious materials
performed in a BSC
BSCs for all
procedures with
viable agents; solid
front gowns, scrubs,
or coveralls; two
pairs of gloves,
when appropriate;
protective eyewear,
respiratory
protection, as
needed
Physical
separation from
access corridors;
access through
two consecutive
self-closing doors;
hands-free sink near
exit; windows are
sealed; ducted air
ventilation system
with negative airow
into laboratory;
autoclave available,
preferably in
laboratory
Continued on next page ►

69Section IV—Laboratory Biosafety Level Criteria
BSL Agents
Special
Practices
a
Primary Barrier
and Personal
Protective
Equipment
a
Facilities
(Secondary
Barriers)
a
4 Dangerous and
exotic agents
that pose high
individual risk of
aerosol-transmitted
laboratory infections
and life-threatening
disease that are
frequently fatal,
for which there
are no vaccines
or treatments; and
related agents with
unknown risk of
transmission
Clothing change
before entry;
daily inspections
of essential
containment
and life support
systems; all wastes
decontaminated
prior to removal from
laboratory; shower
on exit
BSCs for all
procedures with
viable agents;
solid front gowns,
scrubs, or coveralls;
b
gloves;
b
full-body,
air-supplied, positive-
pressure suit
c
Entry sequence;
entry through airlock
with airtight doors;
c
walls, oors, ceilings
form sealed internal
shell; dedicated,
non-recirculating
ventilation
system required;
double-door,
pass-through
autoclave required
a. Each successive BSL contains the recommendations of the preceding level(s) and the criteria in the cell.
b. Applies to Cabinet Laboratory
c. Applies to Suit Laboratory
70 Biosafety in Microbiological and Biomedical Laboratories
Section V—Vertebrate Animal Biosafety Level Criteria for
Vivarium Research Facilities
This guidance is provided for the use of experimentally infected animals housed
in indoor research facilities (e.g., vivarium research facilities) and applies to the
maintenance of laboratory animals that may naturally harbor zoonotic infectious
agents. In both instances, institutional management provides facilities, sta, and
established practices that reasonably ensure appropriate levels of environmental
quality, safety, security, and care for the laboratory animal.
1
Laboratory animal
facilities are to be considered a special type of laboratory. As a general principle,
the Biosafety Level (e.g., facilities, practices, and operational requirements)
recommended for working with infectious agents in vivo and in vitro are
comparable.
The animal room can present unique concerns. Animals may generate aerosols,
may bite and scratch, and/or may be infected with a zoonotic agent. The appli-
cation of the Animal Biosafety Levels (ABSL) is determined by a protocol-driven
risk assessment.
These recommendations presuppose that laboratory animal facilities, operational
practices, and quality of animal care are approved by an Institutional Animal Care
and Use Committee (IACUC)
2
and meet applicable standards and regulations
(e.g., Guide for the Care and Use of Laboratory Animals,
3
Animal Welfare
Regulations).
4,5
In addition, the organization has an occupational health and
safety program that addresses potential hazards associated with the conduct of
laboratory animal research. Occupational Health and Safety in the Care and Use
of Research Animals,
6
published by the Institute for Laboratory Animal Research
(ILAR), is most helpful in this regard. Additional safety guidance on working with
non-human primates (NHPs) is available in the ILAR publication, Occupational
Health and Safety in the Care and Use of Nonhuman Primates.
7
Personnel receive specic training in humane animal care and handling
in accordance with the appropriate regulatory requirements and guidance
documents (e.g., Animal Welfare Regulations,
4
Guide for the Care and Use of
Laboratory Animals,
3
and taxon-specic publications for wild/exotic animals)
as well as animal facility procedures, and are supervised by an individual with
adequate knowledge of potential hazards and experimental animal procedures.
This includes training on proper use of engineering controls, including biosafety
cabinets (BSCs) or downdraft tables, as well as personal protective equipment
(PPE) appropriate to the ABSL as determined by a risk assessment. The
biosafety ocer (BSO), the IBC, or equivalent resource, and/or other applicable
committees are responsible for the review of protocols and policies to protect
personnel who manipulate and care for animals from hazardous exposures.
71Section V—Vertebrate Animal Biosafety Level Criteria for Vivarium Research Facilities
Facilities for laboratory animals used in studies of infectious or non-infectious
disease should be physically separate from other activities, such as animal
production, quarantine, clinical laboratories, and from facilities providing patient
care. Trac ow that will minimize the risk of cross-contamination should be
incorporated into the facility.
The recommendations detailed below describe four combinations of practices,
safety equipment, and facilities for experiments with animals involved in infectious
disease research and other studies that may require containment. These four
combinations, designated ABSL-1–4, provide increasing levels of protection to
personnel and to the environment, and are recommended as minimum standards
for activities involving infected laboratory animals. The four ABSLs describe
animal facilities and practices applicable to work with animals infected with agents
requiring BSL-1–4 containment, respectively. Investigators who are inexperienced
should seek help in designing their experiments from individuals experienced in
this specialized work.
In addition to the ABSLs described in this section, the USDA has developed
facility parameters and work practices for handling agents of agricultural signi-
cance. Appendix D includes a discussion on Animal Biosafety Levels 2, 3, and 4
Agriculture (ABSL-2Ag, ABSL-3Ag, ABSL-4Ag). The “Ag” designation is used for
animals that are loose-housed or in open penning and may be exposed to agents
of concern from an agricultural perspective. USDA requirements are unique to
agriculture because of the necessity to protect the environment from pathogens
of economic or environmental impact. Appendix D also describes some of the
enhancements beyond standard recommendation at ABSL-2–4 that may be
required by USDA APHIS when working in the laboratory or vivarium with certain
veterinary agents of concern.
Facility standards and practices for invertebrate vectors and hosts are not
specically addressed in this section. Please refer to Appendix E for additional
information on the Arthropod Containment Guidelines (ACG).
Animal Biosafety Level 1
Animal Biosafety Level 1 (ABSL-1) is suitable for animal work involving
well-characterized agents that are not known to consistently cause disease
in immunocompetent adult humans and present minimal potential hazard to
personnel and the environment.
Special containment equipment or facility design may be required as determined
by risk assessment. See Section II for additional information on the Biological
Risk Assessment.
72 Biosafety in Microbiological and Biomedical Laboratories
Personnel receive specic training in animal facility procedures and are
supervised by an individual with adequate knowledge of potential hazards and
experimental animal procedures.
The following standard practices, safety equipment, and facility specications are
recommended for ABSL-1.
A. Standard Microbiological Practices
1. The animal facility director establishes and enforces policies, proce-
dures, and protocols for biosafety, biosecurity, and emergencies within
the animal facility.
2. Access to the animal room is limited. Only those persons required for
experimental, husbandry, or support purposes are authorized to enter
the facility.
3. Each institution ensures that worker safety and health concerns are
addressed as part of the animal protocol review process. Consideration
is given to specic biohazards unique to the animal species and protocol
in use. Prior to beginning a study, animal protocols are reviewed and
approved by the Institutional Animal Care and Use Committee (IACUC)
as well as the Institutional Biosafety Committee (IBC), as appropriate.
4. The supervisor ensures that animal care, facility, and support personnel
receive appropriate training regarding their duties, animal husbandry
procedures, potential hazards, manipulations of infectious agents,
necessary precautions to minimize exposures, and hazard/exposure
evaluation procedures (e.g., physical hazards, splashes, aerosolization).
Personnel receive annual updates and additional training when
equipment, procedures, or policies change. Records are maintained
for all hazard evaluations, training sessions, and sta attendance. All
persons, including facility equipment personnel, service workers, and
visitors, are advised of the potential hazards (e.g., naturally acquired
or research pathogens, allergens); are instructed on the appropriate
safeguards; and read and follow instructions on practices and proce-
dures. An institutional policy regarding visitor training, occupational
health requirements, and safety communication is considered.
5. Personal health status may aect an individual’s susceptibility to
infection or ability to receive available immunizations or prophylactic
interventions. Therefore, all personnel, and particularly those of
reproductive age and/or those having conditions that may predispose
them to increased risk for infection (e.g., organ transplant, medical
immunosuppressive agents), are provided information regarding immune

73Section V—Vertebrate Animal Biosafety Level Criteria for Vivarium Research Facilities
competence and susceptibility to infectious agents. Individuals having
such conditions are encouraged to self-identify to the institution’s
healthcare provider for appropriate counseling and guidance. See
Section VII. Facility supervisors ensure that medical sta are informed of
potential occupational hazards within the animal facility, to include those
associated with research, animal husbandry duties, animal care, and
manipulations.
6. Appropriate occupational medical services are in place, as determined
by risk assessment.
a. An animal allergy prevention program is part of the medical
surveillance.
b. Personnel using respirators for animal allergy prevention are
enrolled in an appropriately constituted respiratory protection
program.
7. A safety manual specic to the facility is prepared or adopted in consul-
tation with the facility director and appropriate safety professionals. The
safety manual is available, accessible, and periodically reviewed and
updated, as necessary.
a. The safety manual contains sucient information to describe the
biosafety and containment procedures for the experimental animals,
organisms, and biological materials in use, appropriate agent-spe-
cic decontamination methods, and the work performed.
b. The safety manual contains or references protocols for emergency
situations, including exposures, medical emergencies, facility
malfunctions, escape of animals within the animal facility, and other
potential emergencies. A plan for the disposition of animals during
emergency situations is included. Training in emergency response
procedures is provided to emergency response personnel and other
responsible sta according to institutional policies.
8. A sign is posted at the entrance to the animal room when infectious
agents are present. Posted information includes: the room’s Animal
Biosafety Level, the supervisor’s or other responsible personnel’s name
and telephone number, PPE requirements, general occupational health
requirements (e.g., immunizations, respiratory protection), and required
procedures for entering and exiting the animal room. Agent information is
posted in accordance with the institutional policy.
9. Long hair is restrained so that it cannot contact hands, animals,
specimens, containers, or equipment.
74 Biosafety in Microbiological and Biomedical Laboratories
10. Gloves are worn to protect hands from exposure to hazardous materials
and when handling animals.
a. Glove selection is based on an appropriate risk assessment.
8–12
b. Consider the need for bite and/or scratch-resistant gloves.
c. Gloves worn inside the animal facility are not worn outside the
animal facility.
d. Change gloves when contaminated, glove integrity is compromised,
or when otherwise necessary.
e. Do not wash or reuse disposable gloves, and dispose of used
gloves with other contaminated animal facility waste.
11. Gloves and other PPE are removed in a manner that minimizes personal
contamination and transfer of infectious materials outside of the areas
where infectious materials and/or animals are housed or manipulated.
12. Persons wash their hands after handling animals and before leaving
the areas where infectious materials and/or animals are housed or
manipulated.
13. Eating, drinking, smoking, handling contact lenses, applying cosmetics,
and storing food for human consumption are not permitted in animal
areas.
14. Mouth pipetting is prohibited. Mechanical pipetting devices are used.
15. Policies for the safe handling of sharps, such as needles, scalpels,
pipettes, and broken glassware are developed, implemented, and
followed; policies are consistent with applicable state, federal, and
local requirements.
13
Whenever practical, supervisors adopt improved
engineering and work practice controls that reduce the risk of sharps
injuries. Precautions are always taken with sharp items. These include:
a. Plasticware is substituted for glassware whenever possible.
b. Use of needles and syringes or other sharp instruments is limited
in the animal facility and is restricted to situations where there is no
alternative (e.g., parenteral injection, blood collection, or aspiration
of uids from laboratory animals or diaphragm bottles). Active or
passive needle-based safety devices are used whenever possible.
i. Uncapping of needles is performed in such a manner to reduce
the potential for recoil causing an accidental needlestick.
ii. Needles are not bent, sheared, broken, recapped, removed
from disposable syringes, or otherwise manipulated by hand
before disposal.

75Section V—Vertebrate Animal Biosafety Level Criteria for Vivarium Research Facilities
iii. If absolutely necessary to remove a needle from a syringe
(e.g., to prevent lysing blood cells) or recap a needle (e.g.,
loading syringes in one room and injecting animals in another),
a hands-free device or comparable safety procedure must be
used (e.g., a needle remover on a sharps container, or the use
of forceps to hold the cap when recapping a needle).
iv. Used, disposable needles and syringes are carefully placed
in puncture-resistant containers used for sharps disposal
immediately after use. The sharps disposal container is located
as close to the point of use as possible.
c. Non-disposable sharps (e.g., necropsy instruments such as forceps,
pins, reusable scalpels) are placed in a hard-walled container for
transport to a processing area for decontamination.
d. Broken glassware is not handled directly. Instead, it is removed
using a brush and dustpan, tongs, or forceps.
16. All procedures are carefully performed to minimize the creation of
aerosols or splatters of infectious materials and waste.
17. Decontaminate work surfaces after completion of work and after any spill
or splash of potentially infectious material with appropriate disinfectant.
Spills involving infectious materials are contained, decontaminated, and
cleaned up by sta who are properly trained and equipped to work with
infectious material. A spill procedure is developed and posted within the
animal facility.
18. Decontaminate all cultures, stocks, and other potentially infectious
materials before disposal using an eective method, consistent with
applicable institutional, local and state requirements. Depending on
where the decontamination will be performed, the following methods are
used prior to transport:
a. Materials to be decontaminated outside of the immediate animal
room are placed in a durable, leak-proof container and secured for
transport. For infectious materials, the outer surface of the container
is disinfected prior to moving materials and the transport container
has a universal biohazard label.
b. Materials to be removed from the facility for decontamination are
packed in accordance with applicable local, state, and federal
regulations.
19. An eective integrated pest management program is required. See
Appendix G.
76 Biosafety in Microbiological and Biomedical Laboratories
20. Animals and plants not associated with the work being performed are
not permitted in the areas where infectious materials and/or animals are
housed or manipulated.
B. Special Practices
None required.
C. Safety Equipment (Primary Barriers and Personal Protective Equipment)
1. Specialized devices or equipment for restraint or containment may be
required as determined by appropriate risk assessment.
2. Laboratory coats, gowns, or uniforms are the minimum recommended
to prevent contamination of personal clothing. Protective outer clothing
is not worn outside areas where infectious materials and/or animals are
housed or manipulated. Gowns and uniforms are not worn outside the
animal facility.
3. Eye protection and face protection (e.g., safety glasses, goggles,
mask, face shield, or other splatter guard) are used for manipulations
or activities that may result in splashes or sprays of infectious or other
hazardous materials. Eye protection and face protection are disposed of
with other contaminated facility waste or decontaminated after use.
4. Persons having contact with NHPs assess the risk of mucous membrane
exposure and wear protective equipment (e.g., face shield, surgical
mask, goggles), as appropriate.
5. Additional PPE is considered for persons working with large animals.
D. Animal Facilities (Secondary Barriers)
1. ABSL-1 facilities should be separated from the general trac patterns of
the building and restricted as appropriate. Consider placing animal areas
away from exterior walls of buildings to minimize the impact from the
outside environment temperatures.
a. External facility doors are self-closing and self-locking.
b. Access to the animal facility is restricted.
c. Doors to areas where infectious materials and/or animals are
housed open inward, are self-closing, are kept closed when
experimental animals are present, and never propped open. Doors
to cubicles inside an animal room may open outward or slide
horizontally or vertically.
77Section V—Vertebrate Animal Biosafety Level Criteria for Vivarium Research Facilities
2. The animal facility has a sink for handwashing.
a. Emergency eyewash and shower are readily available, easily
accessible, and appropriately maintained.
b. Sink traps are lled with water and/or appropriate disinfectant to
prevent the migration of vermin and gases.
c. If open oor drains are provided, the traps are lled with water and/
or appropriate disinfectant or sealed to prevent the migration of
vermin and gases.
3. The animal facility is designed, constructed, and maintained to facilitate
cleaning and housekeeping. The interior surfaces (e.g., walls, oors,
ceilings) are water-resistant.
a. Floors are slip-resistant, impervious to liquids, and resistant to
chemicals. Floors with drains are sloped toward drains to facilitate
cleaning.
b. It is recommended that penetrations in oors, walls, and ceilings be
sealed, including openings around ducts, doors, doorframes, outlets,
and switch plates to facilitate pest control and proper cleaning.
c. Internal facility xtures, such as light features, air ducts, and utility
pipes, are designed and installed to minimize horizontal surface
areas to facilitate cleaning and minimize the accumulation of debris
or fomites.
d. External windows are not recommended; if present, they are
resistant to breakage. Where possible, windows are sealed. If
the animal facility has windows that open, they are tted with y
screens.
e. Illumination is adequate for all activities and avoids reections and
glare that could impede vision.
4. Furniture can support anticipated loads and uses.
a. Benchtops are impervious to water and resistant to heat, organic
solvents, acids, alkalis, and other chemicals.
b. Chairs used in animal areas are covered with a non-porous material
that can be easily cleaned and decontaminated with an appropriate
disinfectant and sealed to prevent harboring of insects/vermin.
c. Equipment and furnishings are carefully evaluated to minimize
exposure of personnel to pinch points and sharp edges and corners.
78 Biosafety in Microbiological and Biomedical Laboratories
5. Ventilation is provided in accordance with the Guide for the Care and
Use of Laboratory Animals.
3
a. Ventilation system design considers the heat and high moisture load
produced during the cleaning of animal rooms and the cage wash
process.
6. Cages are washed manually or preferably in a mechanical cage washer.
The mechanical cage washers have a nal rinse temperature of at
least 180°F. If manual cage washing is utilized, ensure that appropriate
disinfectants are selected.
Animal Biosafety Level 2
Animal Biosafety Level 2 (ABSL-2) builds upon the practices, procedures,
containment equipment, and facility requirements of ABSL-1. ABSL-2 is suitable
for work involving laboratory animals infected with agents associated with human
disease and posing a moderate hazard to personnel and the environment. It
also addresses hazards from ingestion and from percutaneous and mucous
membrane exposure.
ABSL-2 requires that, in addition to the requirements for ABSL-1, a BSC or other
physical containment equipment is used when procedures involve the manipu-
lation of infectious materials or where aerosols or splashes may be created.
Appropriate PPE is worn to reduce exposure to infectious agents, animals, and
contaminated equipment. An appropriate occupational health program is in place,
as determined by risk assessment.
The following standard and special practices, safety equipment, and facility
specications are recommended for ABSL-2.
A. Standard Microbiological Practices
1. The animal facility director establishes and enforces policies, proce-
dures, and protocols for biosafety, biosecurity, and emergencies within
the animal facility.
2. Access to the animal room is limited. Only those persons required for
experimental, husbandry, or support purposes are authorized to enter
the facility.
3. Each institution ensures that worker safety and health concerns are
addressed as part of the animal protocol review process. Consideration
is given to specic biohazards unique to the animal species and protocol
in use. Prior to beginning a study, animal protocols are also reviewed
and approved by the Institutional Animal Care and Use Committee

79Section V—Vertebrate Animal Biosafety Level Criteria for Vivarium Research Facilities
(IACUC) and the Institutional Biosafety Committee (IBC), or equivalent
resource, as appropriate.
4. The supervisor ensures that animal care, facility, and support personnel
receive appropriate training regarding their duties, animal husbandry
procedures, potential hazards, manipulations of infectious agents,
necessary precautions to minimize exposures, and hazard/exposure
evaluation procedures (e.g., physical hazards, splashes, aerosolization).
Personnel receive annual updates and additional training when
equipment, procedures, or policies change. Records are maintained
for all hazard evaluations, training sessions, and sta attendance. All
persons, including facility equipment personnel, service workers, and
visitors, are advised of the potential hazards (e.g., naturally acquired
or research pathogens, allergens); are instructed on the appropriate
safeguards; and read and follow instructions on practices and proce-
dures. An institutional policy regarding visitor training, occupational
health requirements, and safety communication is considered.
5. Personal health status may aect an individual’s susceptibility to
infection and ability to receive available immunizations or prophylactic
interventions. Therefore, all personnel, and particularly those of
reproductive age and/or those having conditions that may predispose
them to increased risk for infection (e.g., organ transplant, medical
immunosuppressive agents), are provided information regarding immune
competence and susceptibility to infectious agents. Individuals having
such conditions are encouraged to self-identify to the institution’s
healthcare provider for appropriate counseling and guidance. See
Section VII. Facility supervisors ensure that medical sta are informed of
potential occupational hazards within the animal facility, to include those
associated with research, animal husbandry duties, animal care, and
manipulations.
6. Appropriate occupational medical services are in place, as determined
by risk assessment.
a. An animal allergy prevention program is part of the medical
surveillance.
b. Personnel using respirators for animal allergy prevention are
enrolled in an appropriately constituted respiratory protection
program.
7. A safety manual specic to the facility is prepared or adopted in consul-
tation with the facility director and appropriate safety professionals. The
safety manual is available, accessible, and periodically reviewed and
updated as necessary.
80 Biosafety in Microbiological and Biomedical Laboratories
a. The safety manual contains sucient information to describe the
biosafety and containment procedures for the experimental animals,
organisms, biological materials in use, and the work performed.
b. The safety manual contains or references protocols for emergency
situations, including exposures, medical emergencies, facility
malfunctions, escape of animals within the animal facility, and other
potential emergencies. A plan for the disposition of animals during
emergency situations is included. Training in emergency response
procedures is provided to emergency response personnel and other
responsible sta according to institutional policies.
8. A sign is posted at the entrance to the animal room when infectious
agents are present. Posted information includes: the universal biohazard
symbol, the room’s Animal Biosafety Level, the supervisor’s or other
responsible personnel’s name and telephone number, PPE require-
ments, general occupational health requirements (e.g., immunization,
respiratory protection), and required procedures for entering and exiting
the animal room. Agent information is posted in accordance with the
institutional policy.
9. Long hair is restrained so that it cannot contact hands, animals,
specimens, containers, or equipment.
10. Gloves are worn to protect hands from exposure to hazardous materials
and when handling animals.
a. Glove selection is based on an appropriate risk assessment.
b. Consider the need for bite and/or scratch-resistant gloves.
c. Gloves worn inside the animal facility are not worn outside the
animal facility.
d. Change gloves when contaminated, glove integrity is compromised,
or when otherwise necessary.
e. Do not wash or reuse disposable gloves, and dispose of used
gloves with other contaminated animal facility waste.
11. Gloves and other PPE are removed in a manner that minimizes personal
contamination and transfer of infectious materials outside of the areas
where infectious materials and/or animals are housed or manipulated.
12. Persons wash their hands after handling animals and before leaving
the areas where infectious materials and/or animals are housed or
manipulated.
81Section V—Vertebrate Animal Biosafety Level Criteria for Vivarium Research Facilities
13. Eating, drinking, smoking, handling contact lenses, applying cosmetics,
and storing food for human consumption are not permitted in animal areas.
14. Mouth pipetting is prohibited. Mechanical pipetting devices are used.
15. Policies for the safe handling of sharps, such as needles, scalpels,
pipettes, and broken glassware are developed, implemented, and
followed; policies are consistent with applicable state, federal, and
local requirements. Whenever practical, supervisors adopt improved
engineering and work practice controls that reduce the risk of sharps
injuries. Precautions are always taken with sharp items. These include:
a. Plasticware is substituted for glassware whenever possible.
b. Use of needles and syringes or other sharp instruments is limited
in the animal facility and is restricted to situations where there is no
alternative (e.g., parenteral injection, blood collection, or aspiration
of uids from laboratory animals or diaphragm bottles). Active or
passive needle-based safety devices are to be used whenever
possible.
i. Uncapping of needles is performed in such a manner to reduce
the potential for recoil causing an accidental needlestick.
ii. Needles are not bent, sheared, broken, recapped, removed
from disposable syringes, or otherwise manipulated by hand
before disposal.
iii. If absolutely necessary to remove a needle from a syringe
(e.g., to prevent lysing blood cells) or recap a needle (e.g.,
loading syringes in one room and injecting animals in another),
a hands-free device or comparable safety procedure must be
used (e.g., a needle remover on a sharps container, or the use
of forceps to hold the cap when recapping a needle).
iv. Used, disposable needles and syringes are carefully placed
in puncture-resistant containers used for sharps disposal
immediately after use. The sharps disposal container is located
as close to the point of use as possible.
c. Non-disposable sharps (e.g., necropsy instruments such as forceps,
pins, reusable scalpels) are placed in a hard-walled container for
transport to a processing area for decontamination.
d. Broken glassware is not handled directly. Instead, it is removed
using a brush and dustpan, tongs, or forceps.

82 Biosafety in Microbiological and Biomedical Laboratories
16. All procedures are carefully performed to minimize the creation of
aerosols or splatters of infectious materials and waste.
17. Decontaminate work surfaces after completion of work and after any spill
or splash of potentially infectious material with appropriate disinfectant.
Decontaminate all potentially infectious materials before transport or
disposal using an eective method. Spills involving infectious materials
are contained, decontaminated, and cleaned up by sta who are properly
trained and equipped to work with infectious material. A spill procedure is
developed and posted within the animal facility.
18. Decontaminate all cultures, stocks, and other potentially infectious
materials before disposal using an eective method, consistent with
applicable institutional, local and state requirements. Depending on
where the decontamination will be performed, the following methods are
used prior to transport:
a. Materials to be decontaminated outside of the immediate animal
room are placed in a durable, leak-proof container and secured for
transport. For infectious materials, the outer surface of the container
is disinfected prior to moving materials and the transport container
has a universal biohazard label.
b. Materials to be removed from the facility for decontamination are
packed in accordance with applicable local, state, and federal
regulations.
19. An eective integrated pest management program is required. See
Appendix G.
20. Animals and plants not associated with the work being performed are
not permitted in the areas where infectious materials and/or animals are
housed or manipulated.
B. Special Practices
1. Animal care sta are provided information on signs and symptoms
of disease, receive occupational medical services including medical
evaluation, surveillance, and treatment, as appropriate, and are oered
available immunizations for agents handled or potentially present in the
facility.
2. All procedures involving the manipulation of infectious materials that
may generate an aerosol are conducted within a BSC or other physical
containment device, when possible. If it is not possible to perform
a procedure within a BSC or other physical containment device, a
83Section V—Vertebrate Animal Biosafety Level Criteria for Vivarium Research Facilities
combination of appropriate personal protective equipment, administrative
and/or engineering controls (e.g., downdraft table) are used, based on a
risk assessment.
a. Restraint devices and practices that reduce the risk of exposure
during animal manipulations (e.g., physical restraint, chemical
restraint) are used whenever possible.
b. Equipment, cages, and racks are handled in a manner that
minimizes contamination of other areas. Cages are decontaminated
prior to washing.
3. Develop and implement an appropriate decontamination program in
compliance with applicable institutional, local, and state requirements.
a. Equipment is decontaminated before repair, maintenance, or
removal from the animal facility. A method for decontaminating
routine husbandry equipment and sensitive electronic or medical
equipment is identied and implemented.
b. Decontamination of an entire animal room is considered when there
has been gross contamination of the space, signicant changes
in usage, and for major renovations or maintenance shutdowns.
Selection of the appropriate materials and methods used to decon-
taminate the animal room is based on the risk assessment.
c. Decontamination processes are veried on a routine basis.
4. Incidents that may result in exposure to infectious materials are immedi-
ately evaluated per institutional policies. All such incidents are reported
to the animal facility supervisor and any other personnel designated by
the institution. Appropriate records are maintained.
C. Safety Equipment (Primary Barriers and Personal Protective Equipment).
1. Properly maintained BSCs and other physical containment devices or
equipment are used whenever conducting procedures with a potential for
creating aerosols, splashes, or other potential exposures to hazardous
materials. These include the necropsy of infected animals, harvesting
of tissues or uids from infected animals or eggs, and intranasal inocu-
lation of animals. A risk assessment dictates the type of other physical
containment devices used when BSCs may not be suitable.
a. When indicated by risk assessment, animals are housed in primary
biosafety containment equipment appropriate for the animal species,
such as solid wall and bottom cages covered with micro-isolator lids
or other equivalent primary containment systems for larger animals.
84 Biosafety in Microbiological and Biomedical Laboratories
b. If used, actively ventilated caging systems are designed to contain
microorganisms. Exhaust plenums for these systems are sealed.
Safety mechanisms are in place to prevent the cage and exhaust
plenums from becoming positively pressurized if the exhaust fan
fails. The system is also alarmed to indicate operational malfunc-
tions. Exhaust HEPA lters and lter housings are certied annually.
2. Protective clothing, such as gowns, uniforms, scrubs, or laboratory
coats, and other PPE are worn while in the areas where infectious
materials and/or animals are housed or manipulated.
a. Scrubs and uniforms are removed before leaving the animal facility.
b. Reusable clothing is appropriately contained and decontaminated
before being laundered. Animal facility and protective clothing is
never taken home.
c. Disposable PPE and other contaminated waste are appropriately
contained and decontaminated prior to disposal.
3. Eye protection and face protection (e.g., safety glasses, goggles, mask,
face shield, or other splatter guard) are used for manipulations or
activities that may result in splashes or sprays from infectious or other
hazardous materials when the animal or microorganisms is handled
outside the BSC or another containment device. Eye protection and
face protection are disposed of with other contaminated facility waste or
decontaminated after use.
4. Persons having contact with NHPs assess the risk of mucous membrane
exposure and wear protective equipment (e.g., face shield, surgical
mask, goggles), as appropriate.
5. Additional PPE is considered for persons working with large animals.
6. Based on the pathogen and work performed, respiratory protection may
be considered for sta enrolled in a properly constituted respiratory
protection program.
D. Animal Facilities (Secondary Barriers)
1. ABSL-2 facilities should be separated from the general trac patterns
of the building and restricted, as appropriate. Consider placing animal
areas away from exterior walls of buildings to minimize the impact from
the outside environment temperatures.
a. External facility doors are self-closing and self-locking.
b. Access to the animal facility is restricted.
c. Doors to areas where infectious materials and/or animals are
85Section V—Vertebrate Animal Biosafety Level Criteria for Vivarium Research Facilities
housed open inward, are self-closing, are kept closed when exper-
imental animals are present, and are never to be propped open.
Doors to cubicles inside an animal room may open outward or slide
horizontally or vertically.
2. A handwashing sink is located at the exit of the areas where infectious
materials and/or animals are housed or manipulated. Additional sinks for
handwashing are located in other appropriate locations within the facility.
If the animal facility has segregated areas where infectious materials
and/or animals are housed or manipulated, a sink is also available for
handwashing at the exit from each segregated area.
a. Emergency eyewash and shower are readily available, easily
accessible, and appropriately maintained.
b. Sink traps are lled with water and/or appropriate disinfectant to
prevent the migration of vermin and gases.
c. If open oor drains are provided, the traps are lled with water and/
or appropriate disinfectant or sealed to prevent the migration of
vermin and gases.
3. The animal facility is designed, constructed, and maintained to facilitate
cleaning and housekeeping. The interior surfaces (e.g., walls, oors, and
ceilings) are water-resistant.
a. Floors are slip-resistant, impervious to liquids, and resistant to
chemicals. Floors with drains are sloped toward drains to facilitate
cleaning.
b. Penetrations in oors, walls, and ceiling surfaces are sealed,
including openings around ducts, doors, doorframes, outlets, and
switch plates to facilitate pest control and proper cleaning.
c. Internal facility xtures, such as light features, air ducts, and utility
pipes, are designed and installed to minimize horizontal surface
areas to facilitate cleaning and minimize the accumulation of debris
or fomites.
d. External windows are not recommended; if present, they are sealed
and resistant to breakage.
e. Illumination is adequate for all activities and avoids reections and
glare that could impede vision.
4. Furniture is minimized and can support anticipated loads and uses.
a. Benchtops are impervious to water and resistant to heat, organic
solvents, acids, alkalis, and other chemicals.
86 Biosafety in Microbiological and Biomedical Laboratories
b. Chairs used in animal areas are covered with a non-porous material
that can be easily cleaned and decontaminated with an appropriate
disinfectant and sealed to prevent harboring of insects/vermin.
c. Equipment and furnishings are carefully evaluated to minimize
exposure of personnel to pinch points and sharp edges and corners.
5. Ventilation is provided in accordance with the Guide for the Care and
Use of Laboratory Animals.
3
a. Ventilation system design considers the heat and high moisture
load produced during the cleaning of animal rooms and the cage
wash process.
b. The direction of airow into the animal facility is inward; animal
rooms maintain inward directional airow compared to adjoining
hallways.
c. A ducted exhaust air ventilation system is provided.
d. Exhaust air is discharged to the outside without being recirculated
to other rooms.
6. Mechanical cage washers have a nal rinse temperature of at least
180°F. The cage wash area is designed to accommodate the use of
high-pressure spray systems, humidity, strong chemical disinfectants,
and 180°F water temperatures during the cage/equipment cleaning
process.
7. BSCs and other primary containment barrier systems are installed and
operated in a manner to ensure their eectiveness. See Appendix A.
a. BSCs are installed so that uctuations of the room air supply and
exhaust do not interfere with proper operations. BSCs are located
away from doors, windows that can be opened, heavily traveled
areas, and other possible airow disruptions.
b. BSCs can be connected to the animal facility exhaust system by
either a canopy connection (Class IIA only) or directly exhausted to
the outside through a hard connection (Class IIB, IIC, or III). Class
IIA or IIC BSC exhaust can be safely recirculated back into the
animal facility environment if no volatile toxic chemicals are used in
the cabinet.
c. BSCs are certied at least annually to ensure correct performance,
or as specied in Appendix A, Part 7.
8. Vacuum lines in use are protected with liquid disinfectant traps and
in-line HEPA lters or their equivalent. See Appendix A, Figure 11. Filters
87Section V—Vertebrate Animal Biosafety Level Criteria for Vivarium Research Facilities
are replaced, as needed, or on a replacement schedule determined by a
risk assessment.
9. An autoclave is present in the animal facility to facilitate decontamination
of infectious materials and waste. A validated alternative process (e.g.,
alkaline digestion, incineration) may be used for decontamination and
disposal of carcasses.
Animal Biosafety Level 3
Animal Biosafety Level 3 (ABSL-3) involves practices suitable for work with
laboratory animals infected with indigenous or exotic agents, agents that present
a potential for aerosol transmission, and agents causing serious or potentially
lethal disease. ABSL-3 builds upon the standard practices, procedures,
containment equipment, and facility requirements of ABSL-2.
The ABSL-3 facility has special engineering and design features.
ABSL-3 requires that in addition to the requirements for ABSL-2, all procedures
are conducted in BSCs or by use of other physical containment equipment.
Inward airow at the containment boundary is maintained. Handwashing sinks are
capable of hands-free operation.
Appropriate PPE is worn to reduce exposure to infectious agents, animals, and
contaminated equipment.
The following standard and special safety practices, safety equipment, and facility
specications are necessary for ABSL-3.
A. Standard Microbiological Practices
1. The animal facility director establishes and enforces policies, proce-
dures, and protocols for biosafety, biosecurity, and emergencies within
the animal facility.
2. Access to the animal room is limited. Only those persons required for
experimental, husbandry, or support purposes are authorized to enter
the facility.
3. Each institution ensures that worker safety and health concerns are
addressed as part of the animal protocol review process. Consideration
is given to specic biohazards unique to the animal species and protocol
in use. Prior to beginning a study, animal protocols are also reviewed
and approved by the Institutional Animal Care and Use Committee
(IACUC) and the Institutional Biosafety Committee (IBC), or equivalent
resource, as appropriate.

88 Biosafety in Microbiological and Biomedical Laboratories
4. The supervisor ensures that animal care, facility, and support personnel
receive appropriate training regarding their duties, animal husbandry
procedures, potential hazards, manipulations of infectious agents,
necessary precautions to minimize exposures, and hazard/exposure
evaluation procedures (e.g., physical hazards, splashes, aerosolization).
Personnel receive annual updates and additional training when
equipment, procedures, or policies change. Records are maintained
for all hazard evaluations, training sessions, and sta attendance. All
persons, including facility equipment personnel, service workers, and
visitors, are advised of the potential hazards (e.g., naturally acquired
or research pathogens, allergens); are instructed on the appropriate
safeguards; and read and follow instructions on practices and proce-
dures. An institutional policy regarding visitor training, occupational
health requirements, and safety communication is considered.
5. Personal health status may aect an individual’s susceptibility to
infection, ability to receive available immunizations or prophylactic
interventions. Therefore, all personnel, and particularly those of
reproductive age and/or those having conditions that may predispose
them to increased risk for infection (e.g., organ transplant, medical
immunosuppressive agents), are provided information regarding immune
competence and susceptibility to infectious agents. Individuals having
such conditions are encouraged to self-identify to the institution’s
healthcare provider for appropriate counseling and guidance. See
Section VII. Facility supervisors ensure that medical sta are informed of
potential occupational hazards within the animal facility, to include those
associated with research, animal husbandry duties, animal care, and
manipulations.
6. Appropriate occupational medical services are in place, as determined
by risk assessment.
a. An animal allergy prevention program is part of the medical
surveillance.
b. Personnel using respirators for animal allergy prevention are
enrolled in an appropriately constituted respiratory protection
program.
7. A safety manual specic to the facility is prepared or adopted in consul-
tation with the facility director and appropriate safety professionals. The
safety manual is available, accessible, and periodically reviewed and
updated as necessary.
89Section V—Vertebrate Animal Biosafety Level Criteria for Vivarium Research Facilities
a. The safety manual contains sucient information to describe the
biosafety and containment procedures for the experimental animals,
organisms, biological materials in use, and the work performed.
b. The safety manual contains or references protocols for emergency
situations, including exposures, medical emergencies, facility
malfunctions, escape of animals within the animal facility, and other
potential emergencies. A plan for the disposition of animals during
emergency situations is included. Training in emergency response
procedures is provided to emergency response personnel and other
responsible sta according to institutional policies.
8. A sign is posted at the entrance to the animal room when infectious
agents are present. Posted information includes: the universal biohazard
symbol, the room’s Animal Biosafety Level, the supervisor’s or other
responsible personnel’s name and telephone number, PPE require-
ments, general occupational health requirements (e.g., immunization,
respiratory protection), and required procedures for entering and exiting
the animal room. Agent information is posted in accordance with the
institutional policy.
9. Long hair is restrained so that it cannot contact hands, animals,
specimens, containers, or equipment.
10. Gloves are worn to protect hands from exposure to hazardous materials
and when handling animals.
a. Glove selection is based on an appropriate risk assessment.
b. Consider the need for bite and/or scratch-resistant gloves.
c. Gloves worn inside the animal facility are not worn outside the
animal facility.
d. Change gloves when contaminated, glove integrity is compromised,
or when otherwise necessary.
e. Do not wash or reuse disposable gloves, and dispose of used
gloves with other contaminated facility waste.
11. Gloves and other PPE are removed in a manner that minimizes personal
contamination and transfer of infectious materials outside of the areas
where infectious materials and/or animals are housed or manipulated.
12. Persons wash their hands after handling animals and before leaving
the areas where infectious materials and/or animals are housed or
manipulated.
90 Biosafety in Microbiological and Biomedical Laboratories
13. Eating, drinking, smoking, handling contact lenses, applying cosmetics,
and storing food for human consumption are not permitted in animal
areas.
14. Mouth pipetting is prohibited. Mechanical pipetting devices are used.
15. Policies for the safe handling of sharps, such as needles, scalpels,
pipettes, and broken glassware are developed, implemented, and
followed; policies are consistent with applicable state, federal, and
local requirements. Whenever practical, supervisors adopt improved
engineering and work practice controls that reduce the risk of sharps
injuries. Precautions are always taken with sharp items. These include:
a. Plasticware is substituted for glassware whenever possible.
b. Use of needles and syringes or other sharp instruments is limited
in the animal facility and is restricted to situations where there is no
alternative (e.g., parenteral injection, blood collection, or aspiration
of uids from laboratory animals or diaphragm bottles). Active or
passive needle-based safety devices are to be used whenever
possible.
i. Uncapping of needles is performed in such a manner to reduce
the potential for recoil causing an accidental needlestick.
ii. Needles are not bent, sheared, broken, recapped, removed
from disposable syringes, or otherwise manipulated by hand
before disposal.
iii. If absolutely necessary to remove a needle from a syringe
(e.g., to prevent lysing blood cells) or recap a needle (e.g.,
loading syringes in one room and injecting animals in another),
a hands-free device or comparable safety procedure must be
used (e.g., a needle remover on a sharps container, or the use
of forceps to hold the cap when recapping a needle).
iv. Used, disposable needles and syringes are carefully placed
in puncture-resistant containers used for sharps disposal
immediately after use. The sharps disposal container is located
as close to the point of use as possible.
c. Non-disposable sharps (e.g., necropsy instruments such as forceps,
pins, reusable scalpels) are placed in a hard-walled container for
transport to a processing area for decontamination.
d. Broken glassware is not handled directly. Instead, it is removed
using a brush and dustpan, tongs, or forceps.

91Section V—Vertebrate Animal Biosafety Level Criteria for Vivarium Research Facilities
16. All procedures are carefully performed to minimize the creation of
aerosols or splatters of infectious materials and waste.
17. Decontaminate work surfaces after completion of work and after any spill
or splash of potentially infectious material with appropriate disinfectant.
Spills involving infectious materials are contained, decontaminated, and
cleaned up by sta who are properly trained and equipped to work with
infectious material. A spill procedure is developed and posted within the
animal facility.
18. Decontaminate all cultures, stocks, and other potentially infectious
materials before disposal using an eective method, consistent with
applicable institutional, local and state requirements. Depending on
where the decontamination will be performed, the following methods are
used prior to transport:
a. Materials to be decontaminated outside of the immediate animal
room are placed in a durable, leak-proof container and secured for
transport. For infectious materials, the outer surface of the container
is disinfected prior to moving materials and the transport container
has a universal biohazard label.
b. Materials to be removed from the facility for decontamination are
packed in accordance with applicable local, state, and federal
regulations.
19. An eective integrated pest management program is required. See
Appendix G.
20. Animals and plants not associated with the work being performed are
not permitted in the areas where infectious materials and/or animals are
housed or manipulated.
B. Special Practices
1. Animal care sta are provided information on signs and symptoms of
disease, receive occupational medical services including medical evalu-
ation, surveillance, and treatment as appropriate, and are oered available
immunizations for agents handled or potentially present in the facility.
2. A system is established for reporting and documenting near misses,
animal facility accidents, exposures, unanticipated absences due to
potential Laboratory-associated infection, and for the medical surveil-
lance of potential laboratory-associated illnesses.
3. Incidents that result in exposure to infectious materials are immediately
evaluated per institutional policy. All such incidents are reported to the
animal facility director, facility supervisor, institutional management, and

92 Biosafety in Microbiological and Biomedical Laboratories
appropriate facility safety, compliance, and security personnel according
to institutional policy. Appropriate records are maintained.
4. Only necessary equipment and supplies are recommended to be taken
inside the animal facility.
5. All procedures involving the manipulation of infectious materials are
conducted within a BSC or other physical containment device, when
possible. If it is not possible to perform a procedure within a BSC
or other physical containment device, a combination of appropriate
personal protective equipment, administrative and/or engineering
controls (e.g., downdraft table) are used, based on a risk assessment.
a. Restraint devices and practices that reduce the risk of exposure
during animal manipulations (e.g., physical restraint, chemical
restraint) are used whenever possible.
b. Equipment, cages, and racks are handled in a manner that
minimizes contamination of other areas.
6. Biological materials that are to remain in a viable state during removal
from the animal facility are placed in a durable leak-proof sealed primary
container and then enclosed in a non-breakable, sealed secondary
container prior to removal from the facility by authorized personnel.
Once removed, the primary container is opened within a BSC in BSL-3
or ABSL-3 containment unless a validated inactivated method is used.
See Appendix K. The inactivation method is documented in-house with
viability testing data to support the method.
7. Develop and implement an appropriate decontamination program
in compliance with applicable institutional, local, state, and federal
requirements.
a. Equipment is decontaminated before repair, maintenance, or
removal from the areas where infectious materials and/or animals
are housed or manipulated. A method for decontaminating routine
husbandry equipment and sensitive electronic or medical equipment
is identied and implemented.
b. Decontamination of an entire animal room is considered when there
has been gross contamination of the space, signicant changes in
usage, major renovations, or maintenance shutdowns. Selection of
the appropriate materials and methods used to decontaminate the
animal room is based on the risk assessment.
c. Decontamination processes are veried on a routine basis.

93Section V—Vertebrate Animal Biosafety Level Criteria for Vivarium Research Facilities
C. Safety Equipment (Primary Barriers and Personal Protective Equipment)
1. Properly maintained BSCs and other physical containment devices
or equipment are used for manipulations of infectious materials and
animals as determined by risk assessment.
a. The risk of infectious aerosols from infected animals or their
bedding can be reduced if animals are housed in containment
caging systems, such as solid wall and bottom cages covered with
micro-isolator lids, open cages placed in inward ow ventilated
enclosures, HEPA lter isolators and caging systems, or other
equivalent primary containment systems.
i. Actively ventilated caging systems are designed to prevent the
escape of microorganisms from the cage. Exhaust plenums
for these systems are sealed to prevent the escape of micro-
organisms if the ventilation system becomes static, and the
exhaust is HEPA-ltered. Safety mechanisms are in place to
prevent the cage and exhaust plenums from becoming positive
to the surrounding area should the exhaust fan fail. The system
is alarmed to indicate operational malfunctions.
b. When animals cannot be housed in ventilated containment cages/
units, certain features of the animal room act as the primary barriers.
The procedures in place include how workers are protected from
agents shed by the animals (e.g., PPE enhancements) as well as
how the environment is protected from such agents through the use
of biocontainment enhancements such as some combination of boot
or PPE change or surface decontamination at the door, a personal
shower at the room level, and/or other procedures.
2. Special consideration is given to the potential for cross-contamination
when open caging is used. See Appendix D for additional information.
3. Personnel within the animal facility wear protective clothing, such as
uniforms or scrubs.
a. Disposable PPE such as non-woven, olen cover-all suits, or
wrap-around or solid-front gowns are worn over this clothing before
entering areas where infectious materials and/or animals are housed
or manipulated. Front-button, laboratory coats are unsuitable.
b. Reusable clothing is appropriately contained and decontaminated
before being laundered. Animal facility and protective clothing is
never taken home.
94 Biosafety in Microbiological and Biomedical Laboratories
c. Disposable PPE is removed when leaving the areas where infec-
tious materials and/or animals are housed or manipulated. Scrubs
and uniforms are removed before leaving the animal facility.
d. Disposable PPE and other contaminated waste are appropriately
contained and decontaminated prior to disposal.
4. All personnel entering areas where infectious materials and/or animals
are housed or manipulated wear appropriate head covering, eye, face,
and respiratory protection. To prevent cross-contamination, boots, shoe
covers, or other protective footwear are used where indicated and
disposed of or decontaminated after use.
5. Head covering, eye protection, and face protection are disposed of with
other contaminated animal facility waste or decontaminated after use.
6. Procedures may require wearing two pairs of gloves (i.e., double-glove).
Change outer gloves when contaminated, glove integrity is compro-
mised, or when otherwise necessary.
7. Additional PPE is considered for persons working with large animals.
D. Animal Facilities (Secondary Barriers)
1. ABSL-3 facilities should be separated from the general trac patterns of
the building and restricted as appropriate. Consider placing animal areas
away from exterior walls of buildings to minimize the impact from the
outside environment temperatures.
a. External facility doors are self-closing and self-locking.
b. Access to the animal facility is restricted.
c. Doors to areas where infectious materials and/or animals are
housed open inward, are self-closing, are kept closed when experi-
mental animals are present, and are never propped open.
d. Entry into the containment area is via a double-door entry, which
constitutes an anteroom/airlock and a change room. Exit showers
may be considered based on risk assessment. An additional
double-door anteroom or double-doored autoclave may be provided
for movement of supplies and wastes into and out of the facility.
2. A handwashing sink is located at the exit of the areas where infectious
materials and/or animals are housed or manipulated. Additional sinks for
handwashing are located in other appropriate locations within the facility.
If the animal facility has segregated areas where infectious materials
and/or animals are housed or manipulated, a handwashing sink is also
available near the exit from each segregated area.
95Section V—Vertebrate Animal Biosafety Level Criteria for Vivarium Research Facilities
a. The sink is hands-free or automatically operated.
b. Emergency eyewash and shower are readily available, easily
accessible, and appropriately maintained.
c. Sink traps are lled with water and/or appropriate disinfectant or
sealed to prevent the migration of vermin and gases.
d. Floor drains are maintained and lled with water and/or appropriate
disinfectant or sealed to prevent the migration of vermin and gases.
3. The animal facility is designed, constructed, and maintained to facilitate
cleaning, decontamination, and housekeeping. The interior surfaces
(e.g., walls, oors, and ceilings) are water-resistant.
a. Floors are slip-resistant, impervious to liquids, and resistant to
chemicals. Flooring is seamless, sealed, or poured with integral
cove bases. Floors slope to drain, if present.
b. Penetrations in oors, walls, and ceiling surfaces are sealed,
including openings around ducts, outlets, switch plates, and
doorframes, to facilitate pest control, proper cleaning, and decon-
tamination. Walls, oors, and ceilings form a sanitizable and sealed
surface.
c. Internal facility xtures, such as light features, air ducts, and utility
pipes, are designed and installed to minimize horizontal surface
areas to facilitate cleaning and minimize the accumulation of debris
or fomites.
d. External windows are not recommended; if present, they are sealed
and resistant to breakage.
e. Illumination is adequate for all activities and avoids reections and
glare that could impede vision.
4. Furniture is minimized and can support anticipated loads and uses.
a. Benchtops are impervious to water and resistant to heat, organic
solvents, acids, alkalis, and other chemicals.
b. Chairs used in animal areas are covered with a non-porous material
that can be easily cleaned and decontaminated with an appropriate
disinfectant and sealed to prevent harboring of insects/vermin.
c. Equipment and furnishings are carefully evaluated to minimize
exposure of personnel to pinch points and sharp edges and corners.

96 Biosafety in Microbiological and Biomedical Laboratories
5. Ventilation is provided in accordance with the Guide for the Care and
Use of Laboratory Animals.
3
a. Ventilation system design considers the heat and high moisture
load produced during the cleaning of animal rooms and the cage
wash process.
b. The direction of airow into the animal facility is inward; animal
rooms maintain inward directional airow compared to adjoining
hallways. A visual monitoring device, which conrms directional
airow, is provided at the animal room entrance.
c. A ducted exhaust air ventilation system is provided. Exhaust air is
discharged to the outside without being recirculated to other rooms.
This system creates directional airow, which draws air into the
animal room from “clean” areas and toward “contaminated” areas.
d. The exhaust air is dispersed away from occupied areas and from
building air intake locations or the exhaust air is HEPA-ltered.
e. The ABSL-3 animal facility is designed such that under failure
conditions the airow will not be reversed at the containment barrier.
Alarms are considered to notify personnel of ventilation and HVAC
system failure.
6. Cages are decontaminated prior to removal from the containment barrier
and prior to washing in a mechanical cage washer. The cage wash area
is designed to accommodate the use of high-pressure spray systems,
humidity, strong chemical disinfectants, and 180°F water temperatures
during the cage/equipment cleaning process.
7. BSCs and other primary containment barrier systems are installed and
operated in a manner to ensure their eectiveness. See Appendix A.
a. BSCs are installed so that uctuations of the room air supply and
exhaust do not interfere with proper operations. BSCs are located
away from doors, windows that can be opened, heavily traveled
areas, and other possible airow disruptions.
b. BSCs can be connected to the animal facility exhaust system by
either a canopy connection (Class IIA only) or directly exhausted to
the outside through a hard connection (Class IIB, IIC, or III). Class
IIA or IIC BSC exhaust can be safely recirculated back into the
animal facility environment if no volatile toxic chemicals are used in
the cabinet.

97Section V—Vertebrate Animal Biosafety Level Criteria for Vivarium Research Facilities
c. BSCs are certied at least annually to ensure correct performance,
or as specied in Appendix A, Part 7.
d. Class III BSCs are provided supply air in such a manner that
prevents positive pressurization of the cabinet or the animal room.
8. Equipment that may produce infectious aerosols is contained in primary
barrier devices that exhaust air through HEPA ltration, or other equiv-
alent technology, before being discharged into the animal facility. These
HEPA lters are tested annually and replaced as needed.
9. All vacuum lines are protected with HEPA lters, or their equivalent, or
are capped. Vacuum lines in use are protected with liquid disinfectant
traps and in-line HEPA lters or their equivalent. See Appendix A, Figure
11. Filters are replaced, as needed, or are on a replacement schedule
determined by a risk assessment. The placement of an additional HEPA
lter immediately prior to a central vacuum pump is considered.
10. An autoclave is available within the containment barrier. The autoclave
is utilized to decontaminate infectious materials and waste before
moving these materials to the other areas of the facility. If not within
the containment barrier, special practices are developed for the
transport of infectious materials to designated alternate locations
for decontamination. A validated alternative process (e.g., alkaline
digestion, incineration) may be used for decontamination and disposal
of carcasses.
11. The ABSL-3 facility design, operational parameters, and procedures are
veried and documented prior to operation. Facilities are tested annually
or after signicant modication to ensure operational parameters are
met. Verication criteria are modied as necessary by operational
experience.
12. Enhanced environmental and personal protection may be necessary
based on risk assessment and applicable local, state, or federal regula-
tions. These enhancements may include one or more of the following:
an anteroom for clean storage of equipment and supplies with dress-in,
shower-out capabilities; gas-tight dampers to facilitate animal room
isolation; nal HEPA ltration of the animal room exhaust air; animal
room euent decontamination; containment of other piped services; or
advanced access control devices, such as biometrics.

98 Biosafety in Microbiological and Biomedical Laboratories
Animal Biosafety Level 4
Animal Biosafety Level 4 (ABSL-4) is required for work with animals infected with
dangerous and exotic agents that pose a high individual risk of aerosol-trans-
mitted laboratory infections and life-threatening diseases that are frequently fatal,
agents for which there are no vaccines or treatments, or work with a related agent
with unknown risk of transmission. Agents with a close or identical antigenic
relationship to agents requiring ABSL-4 containment are handled at this level until
sucient data are obtained to re-designate the level. Animal care sta receive
specic and thorough training in handling extremely hazardous, infectious agents
and infected animals. Animal care sta understand the primary and secondary
containment functions of standard and special practices, containment equipment,
and facility design characteristics. All animal care sta and supervisors are
competent in handling animals, agents, and procedures requiring ABSL-4
containment. The animal facility director and/or supervisor control(s) access to
the ABSL-4 animal facility in accordance with institutional policies.
There are two models for ABSL-4 facilities:
1. Cabinet Facility: All handling of agents, infected animals, and housing of
infected animals is performed in Class III BSCs. See Appendix A; and
2. Suit Facility: Personnel wear a positive-pressure suit. The animal room
maintains negative pressure relative to the surrounding areas and
have HEPA-ltered supply and exhaust systems. A site-specic risk
assessment that considers the agent, the potential for agent shedding,
and aerosol generation from infected animals is conducted to determine
appropriate animal housing. Most infected animals are housed in a
primary containment system and handled under a primary barrier system
such as a Class II BSC or another containment system.
ABSL-4 builds upon the standard practices, procedures, containment equipment,
and facility requirements of ABSL-3. However, ABSL-4 cabinet and suit facilities
have special engineering and design features to prevent microorganisms from
dissemination into the environment and to protect personnel.
The ABSL-4 cabinet facility is distinctly dierent from an ABSL-3 facility containing
a Class III BSC.
The following standard and special practices, safety equipment, and facility
specications are necessary for ABSL-4.
A. Standard Microbiological Practices
1. The animal facility director establishes and enforces policies, proce-
dures, and protocols for biosafety, biosecurity, and emergencies within
the animal facility.

99Section V—Vertebrate Animal Biosafety Level Criteria for Vivarium Research Facilities
2. Access to the animal room is limited. Only those persons required for
experimental, husbandry, or support purposes are authorized to enter
the facility.
3. Each institution ensures that worker safety and health concerns are
addressed as part of the animal protocol review process. Consideration
is given to specic biohazards unique to the animal species and protocol
in use. Prior to beginning a study, animal protocols are also reviewed
and approved by the Institutional Animal Care and Use Committee
(IACUC) and the Institutional Biosafety Committee (IBC), or equivalent
resource, as appropriate.
4. The supervisor ensures that animal care, facility, and support personnel
receive appropriate training regarding their duties, animal husbandry
procedures, potential hazards, manipulations of infectious agents,
necessary precautions to minimize exposures, and hazard/exposure
evaluation procedures (e.g., physical hazards, splashes, aerosolization).
Personnel receive annual updates and additional training when
equipment, procedures or policies change. Records are maintained
for all hazard evaluations, training sessions, and sta attendance. All
persons, including facility equipment personnel, service workers, and
visitors, are advised of the potential hazards (e.g., naturally acquired
or research pathogens, allergens); are instructed on the appropriate
safeguards; and read and follow instructions on practices and proce-
dures. An institutional policy regarding visitor training, occupational
health requirements, and safety communication is considered.
5. Personal health status may aect an individual’s susceptibility to
infection and ability to receive available immunizations or prophylactic
interventions. Therefore, all personnel, and particularly those of
reproductive age and/or those having conditions that may predispose
them to increased risk for infection (e.g., organ transplant, medical
immunosuppressive agents), are provided information regarding
immune competence and susceptibility to infectious agents. Individuals
having such conditions are encouraged to self-identify to the institu-
tion’s healthcare provider for appropriate counseling and guidance. See
Section VII. Facility supervisors ensure that medical sta are informed
of potential occupational hazards within the animal facility, to include
those associated with research, animal husbandry duties, animal care,
and manipulations.
6. Appropriate occupational medical services are in place, as determined
by risk assessment.
100 Biosafety in Microbiological and Biomedical Laboratories
a. An animal allergy prevention program is part of the medical
surveillance.
b. Personnel using respirators for animal allergy prevention are
enrolled in an appropriately constituted respiratory protection
program.
7. A safety manual specic to the facility is prepared or adopted in consul-
tation with the facility director and appropriate safety professionals. The
safety manual is available, accessible, and periodically reviewed and
updated as necessary.
a. The safety manual contains sucient information to describe the
biosafety and containment procedures for the experimental animals,
organisms and biological materials in use, appropriate agent-specic
decontamination methods, and the work performed.
b. The safety manual contains or references protocols for emergency
situations, including exposures, medical emergencies, facility
malfunctions, escape of animals within the animal facility, and other
potential emergencies. A plan for the disposition of animals during
emergency situations is included. Training in emergency response
procedures is provided to emergency response personnel and other
responsible sta according to institutional policies.
8. A sign is posted at the entrance to the animal room when infectious
agents are present. Posted information includes: the universal biohazard
symbol, the room’s Animal Biosafety Level, the supervisor’s or other
responsible personnel’s name and telephone number, general occupa-
tional health requirements (e.g., immunization, respiratory protection),
PPE requirements and required procedures for entering and exiting
the animal room. Agent information is posted in accordance with the
institutional policy.
9. Gloves are worn to protect hands from exposure to hazardous materials
and when handling animals.
a. Glove selection is based on an appropriate risk assessment.
b. Inner gloves worn inside the animal facility are not worn outside the
animal facility.
c. Change inner gloves when contaminated, glove integrity is compro-
mised, or when otherwise necessary.
d. Do not wash or reuse disposable gloves, and dispose of used
gloves with other contaminated animal facility waste.
101Section V—Vertebrate Animal Biosafety Level Criteria for Vivarium Research Facilities
10. Gloves and other PPE are removed in a manner that minimizes personal
contamination and transfer of infectious materials outside of the areas
where infectious materials and/or animals are housed or manipulated.
11. Eating, drinking, smoking, handling contact lenses, applying cosmetics,
and storing food for human consumption are not permitted in animal
areas.
12. Mouth pipetting is prohibited. Mechanical pipetting devices are used.
13. Policies for the safe handling of sharps, such as needles, scalpels,
pipettes, and broken glassware are developed, implemented, and
followed; policies are consistent with applicable state, federal, and
local requirements. Whenever practical, supervisors adopt improved
engineering and work practice controls that reduce the risk of sharps
injuries. Precautions are always taken with sharp items. These include:
a. Plasticware is substituted for glassware whenever possible.
b. Use of needles and syringes or other sharp instruments is limited
in the animal facility and is restricted to situations where there is no
alternative (e.g., parenteral injection, blood collection, or aspiration
of uids from laboratory animals or diaphragm bottles). Active or
passive needle-based safety devices are to be used whenever
possible.
i. Uncapping of needles is performed in such a manner to reduce
the potential for recoil causing an accidental needlestick.
ii. Needles are not bent, sheared, broken, recapped, removed
from disposable syringes, or otherwise manipulated by hand
before disposal.
iii. If absolutely necessary to remove a needle from a syringe
(e.g., to prevent lysing blood cells) or recap a needle (e.g.,
loading syringes in one room and injecting animals in another),
a hands-free device or comparable safety procedure must be
used (e.g., a needle remover on a sharps container, or the use
of forceps to hold the cap when recapping a needle).
iv. Used, disposable needles and syringes are carefully placed
in puncture-resistant containers used for sharps disposal
immediately after use. The sharps disposal container is located
as close to the point of use as possible.
c. Non-disposable sharps (e.g., necropsy instruments such as forceps,
pins, reusable scalpels) are placed in a hard-walled container for
transport to a processing area for decontamination.

102 Biosafety in Microbiological and Biomedical Laboratories
d. Broken glassware is not handled directly. Instead, it is removed
using a brush and dustpan, tongs, or forceps.
14. All procedures are carefully performed to minimize the creation of
aerosols or splatters of infectious materials and waste.
15. Decontaminate work surfaces after completion of work and after any spill
or splash of potentially infectious material with appropriate disinfectant.
Spills involving infectious materials are contained, decontaminated, and
cleaned up by sta who are properly trained and equipped to work with
infectious material. A spill procedure is developed and posted within the
animal facility.
16. All wastes from the animal room, including animal tissues, carcasses,
and bedding are transported from the animal room in leak-proof, covered
containers for appropriate disposal consistent with applicable institu-
tional, local, and state requirements. See B. Special Practices, #7 in the
following sub-section for additional details.
17. An eective integrated pest management program is required. See
Appendix G.
18. Animals and plants not associated with the work being performed are
not permitted in the areas where infectious materials and/or animals are
housed or manipulated.
B. Special Practices
1. All persons entering the animal facility are advised of the potential
hazards and meet specic entry/exit requirements in accordance with
institutional policies. Only persons whose presence in the facility or
individual animal rooms is required for scientic or support purposes are
authorized to enter. Additional training/security requirements may be
required prior to gaining independent access to the animal facility.
2. All persons who enter operational animal areas are provided information
on signs and symptoms of disease and receive occupational medical
services including medical evaluation, surveillance, and treatment, as
appropriate, and oered available immunizations for agents handled or
potentially present in the facility.
a. An essential adjunct to such an occupational medical services
system is the availability of a facility for the isolation and medical
care of personnel with potential or known Laboratory-associated
infections.
3. The facility supervisor is responsible for ensuring that, prior to working
independently in ABSL-4 containment, personnel demonstrate high

103Section V—Vertebrate Animal Biosafety Level Criteria for Vivarium Research Facilities
prociency in standard and special microbiological practices, and
techniques for working with agents requiring ABSL-4 containment.
4. A system is established for reporting and documenting near misses,
accidents, exposures, unanticipated absences due to potential Labora-
tory-associated infection, and for the medical surveillance of potential
laboratory-associated illnesses.
5. Incidents that may result in exposure to infectious materials are immedi-
ately evaluated per institutional policy. All incidents are reported to the
animal facility director, facility supervisor, institutional management, and
appropriate facility safety, compliance, and security personnel according
to institutional policy. Appropriate records are maintained.
6. Biological materials that are to remain in a viable state during removal
from the animal facility are placed in a durable leak-proof sealed primary
container and then enclosed in a non-breakable, sealed secondary
container prior to removal from the facility by authorized personnel.
These materials are transferred through a disinfectant dunk tank,
fumigation chamber, or decontamination shower. Once removed, the
primary container is not opened outside BSL-4 or ABSL-4 containment
unless a validated inactivation method is used (e.g., gamma irradiation).
See Appendix K. The inactivation method is documented in-house with
viability testing data to support the method.
7. All wastes (including animal tissues, carcasses, and contaminated
bedding) and other materials are decontaminated by a veried method
before removal from the ABSL-4 facility.
8. Equipment is routinely decontaminated and is decontaminated before
repair, maintenance, or removal from the animal facility. Equipment,
cages, and racks are handled in a manner that minimizes contamination
of other areas. Cages are autoclaved or thoroughly decontaminated
before they are cleaned and washed.
a. Equipment (e.g., sensitive electronic, medical, or routine husbandry
equipment) or material that might be damaged by high tempera-
tures or steam is decontaminated using an eective and veried
procedure such as a gaseous or vapor method in a sealable airlock
or chamber designed for this purpose.
9. Procedures to reduce possible worker exposure are instituted, such as
use of squeeze cages, working only with anesthetized animals, or other
appropriate practices. Personnel assigned to work with infected animals
may be required to work in pairs as directed by institutional policies.
104 Biosafety in Microbiological and Biomedical Laboratories
10. A logbook, or other means of documenting the date and time of all
persons entering and leaving the animal facility, is maintained.
11. While the facility is operational, personnel enter and exit the animal
facility through the clothing change and shower rooms except during
emergencies. All personal clothing and jewelry (except eyeglasses)
are removed in the outer clothing change room. All persons entering
the facility use animal facility clothing, including undergarments, pants,
shirts, jumpsuits, shoes, and gloves, as appropriate. All persons leaving
the animal facility are required to take a personal body shower. Used
animal facility clothing and other waste, including gloves, are treated
as contaminated materials and decontaminated before laundering or
disposal.
12. After the facility has been completely decontaminated by verication
of a validated method, necessary sta may enter and exit the animal
facility without following the clothing change and shower requirements
described above.
13. Daily inspections of essential containment and life support systems are
completed and documented before laboratory work is initiated to ensure
that the animal rooms and animal facilities are operating according to
established parameters.
14. Only necessary equipment and supplies are stored inside the animal
facility. All equipment and supplies taken inside the facility are decontam-
inated before removal from the laboratory.
a. Supplies and materials that are not brought into the animal facility
through the change room are brought in through a dunk tank,
previously decontaminated double-door autoclave, fumigation
chamber, or airlock. After securing the outer doors, personnel within
the laboratory retrieve the materials by opening the interior doors
of the autoclave, fumigation chamber, or airlock. The inner door
is secured after materials are brought into the facility. The outer
door of the autoclave or fumigation chamber is not opened until
the autoclave, fumigation chamber, or airlock has been operated
through a successful decontamination cycle.
C. Safety Equipment (Primary Barriers and Personal Protective Equipment)
Cabinet Facility
1. All procedures involving the manipulation of infectious animals and
materials are conducted within a Class III BSC.
105Section V—Vertebrate Animal Biosafety Level Criteria for Vivarium Research Facilities
2. A Class III BSC contains:
a. Double-door, pass-through autoclave for decontaminating materials
passing out of the Class III BSC(s). The autoclave doors are
interlocked so that only one door can be opened at any time and are
automatically controlled so that the outside door to the autoclave
can only be opened after a successful decontamination cycle has
been completed.
b. A pass-through dunk tank, fumigation chamber, or equivalent decon-
tamination method so that materials and equipment that cannot be
decontaminated in the autoclave can be safely removed from the
cabinet. Containment between the cabinet and the surrounding
animal room is maintained at all times.
c. A HEPA lter on the supply air intake and two HEPA lters in series
on the exhaust outlet of the unit. Supply air is provided in such a
manner that prevents positive pressurization of the cabinet. There
are gas-tight dampers on the supply and exhaust ducts of the
cabinet to permit gas or vapor decontamination of the unit. Ports for
injection of test medium are present on all HEPA lter housings for
annual lter recertication.
d. An interior constructed with smooth nishes that can be easily
cleaned and decontaminated. All sharp edges on cabinet nishes
are eliminated to reduce the potential for cuts and tears of the
cabinet gloves. Equipment to be placed in the Class III BSC is also
free of sharp edges or other surfaces that may damage or puncture
the cabinet gloves.
e. Class III cabinet gloves are inspected for leaks periodically and
changed if necessary. Gloves are replaced annually during cabinet
recertication.
3. The cabinet is designed to permit maintenance and repairs of cabinet
mechanical systems (e.g., refrigeration, incubators, centrifuges) to be
performed from the exterior of the cabinet whenever possible.
4. Manipulation of high concentrations or large volumes of infectious agents
within the Class III BSC is performed using physical containment devices
inside the cabinet whenever practical. Such materials are centrifuged
inside the cabinet using sealed rotors or centrifuge safety cups.
5. The interior of the Class III BSC and all contaminated plenums, fans, and
lters are decontaminated using a validated gaseous or vapor method
when there have been signicant changes in cabinet usage, before
major renovations or maintenance shutdowns, and in other situations,
106 Biosafety in Microbiological and Biomedical Laboratories
as determined by risk assessment. Success of the decontamination is
veried before accessing the interior spaces of the cabinet.
6. The Class III BSC is certied at least annually.
7. For Class III BSCs directly connected via a double door pass through to
an ABSL-4 suit facility, materials may be placed into and removed from
the Class III BSC via the suit facility.
8. Restraint devices and practices that reduce the risk of exposure during
animal manipulations are used where practicable (e.g., physical restraint
devices, chemical restraint medications, mesh, or Kevlar gloves).
9. Workers in the animal facility wear protective animal facility clothing
with a solid front, such as tie-back or wrap-around gowns, scrubs, or
coveralls. Additional PPE may be required based on risk assessment.
a. Upon exit, all protective clothing is removed in the inner change
room before showering.
b. Prescription eyeglasses are decontaminated before removal through
the personal body shower.
10. Disposable gloves are worn underneath cabinet gloves to protect the
worker from exposure should a break or tear occur in a cabinet glove.
Suit Facility
1. All procedures involving the manipulation of infectious materials
or infected animals are conducted within a BSC or other physical
containment devices.
2. Infected animals are housed in a primary containment system. Primary
containment systems include: actively ventilated caging systems; open
cages placed in ventilated enclosures; solid wall and bottom cages
covered with micro-isolator lids and opened in laminar oor hoods or
HEPA-ltered downdraft tables; or other equivalent primary containment
systems.
a. Actively ventilated caging systems are designed to prevent the
escape of microorganisms from the cage. Exhaust plenums for
these systems are sealed to prevent the escape of microorganisms
if the ventilation system becomes static, and the exhaust is
HEPA-ltered. These HEPA lters are tested annually and replaced
as needed. Safety mechanisms are in place to prevent the cage
and exhaust plenums from becoming positive to the surrounding
area should the exhaust fan fail. The system is alarmed to indicate
operational malfunctions.

107Section V—Vertebrate Animal Biosafety Level Criteria for Vivarium Research Facilities
3. Infected animals may be housed in open cages within a dedicated
animal-holding room that serves as the primary barrier. A room serving as
a primary barrier is air-tight and capable of being decontaminated using
fumigation. If animals are to be contained in a dedicated animal-holding
room serving as the primary barrier, the following conditions are met:
a. Prior to fumigation of the animal-holding room, cages may be
removed for autoclaving or chemical decontamination.
b. Caging is chosen to reduce the amount of animal detritus that can be
thrown out of the cage and onto the oor of the animal holding room.
c. The ow of personnel, material, and equipment is directed in order
to minimize the spread of contamination from the animal-holding
room into adjacent areas of the animal facility.
4. When large animals cannot be housed in a primary containment system
or ventilated containment cages/units, certain features of the animal
room (e.g., HEPA exhaust lters and the sealed and pressure-tested
room surfaces) act as the primary barriers.
a. Loose-housed or open penned animals may require ABSL-3Ag or
ABSL-4Ag containment. See Appendix D for additional information.
5. Equipment that may produce aerosols is used within primary
containment devices that exhaust air through HEPA ltration before
being discharged into the animal room or facility exhaust system. These
HEPA lters are tested annually and replaced as needed.
6. All procedures are conducted by personnel wearing a one-piece,
positive-pressure supplied-air suit.
a. All persons don animal facility clothing, such as scrubs, before
entering the room used for donning positive-pressure suits.
b. Procedures are in place to control and verify the operation of the
one-piece positive-pressure supplied-air suit, including gloves,
before each use.
c. Decontamination of outer suit gloves is performed during the course
of normal operations to remove gross contamination and minimize
further contamination of the animal room.
d. Inner disposable gloves are worn to protect the laboratorian should
a break or tear in the outer suit gloves occur. Disposable inner
gloves are not worn outside the inner change area.
108 Biosafety in Microbiological and Biomedical Laboratories
e. Upon exit from the chemical shower, inner gloves and all animal
facility clothing are removed and discarded or collected for
autoclaving before laundering prior to entering the personal shower.
f. Prescription eyeglasses are decontaminated before removal through
the personal body shower.
D. Animal Facilities (Secondary Barriers)
Cabinet Facility
1. The ABSL-4 cabinet facility consists of either a separate building or a
clearly demarcated and isolated zone within a building. Consider placing
animal areas away from exterior walls of buildings to minimize the impact
from the outside environment temperatures.
a. Facility access is restricted. Facility doors are lockable.
b. Exit from the animal facility is by sequential passage through an
inner (i.e., dirty) changing area, a personal shower, and an outer
(i.e., clean) change room upon exiting the cabinet facility.
2. An automatically activated emergency power source is provided at a
minimum for the animal facility exhaust system, alarms, lighting, entry
and exit controls, BSCs, and door gaskets.
a. Monitoring and control systems for air supply, exhaust, life support,
alarms, entry and exit controls, and security systems are on an
uninterrupted power supply (UPS).
b. The emergency power system(s) is tested at least annually.
3. A double-door autoclave, dunk tank, fumigation chamber, or ventilated
airlock is provided at the containment barrier for the passage of
materials, supplies, or equipment.
4. A hands-free sink is provided near the door from the cabinet room to the
inner change rooms. A sink is provided in the outer change room.
5. An eyewash station is readily available in the animal area.
6. Walls, oors, and ceilings of the cabinet facility are constructed to form
a sealed internal shell to facilitate fumigation and prohibit animal and
insect intrusion. The internal surfaces of this shell are resistant to liquids
and chemicals used for cleaning and decontamination of the area. Floors
are monolithic, sealed, and coved.
a. All penetrations in the internal shell of the facility are sealed.
b. Openings around doors into the facility are minimized and capable
of being sealed to facilitate decontamination.

109Section V—Vertebrate Animal Biosafety Level Criteria for Vivarium Research Facilities
7. Services and plumbing that penetrate the facility walls, oors, or ceiling
are installed to ensure that no backow from the facility occurs. These
penetrations are tted with two (in series) backow prevention devices.
Consideration is given to locating these devices outside of containment.
Atmospheric venting systems are provided with two HEPA lters in series
and are sealed up to the second lter.
8. Furniture is minimized, of simple construction, and capable of supporting
anticipated loads and uses.
a. Spaces between benches, cabinets, and equipment are accessible
for cleaning and decontamination.
b. Benchtops are impervious to water and resistant to heat, organic
solvents, acids, alkalis, and other chemicals.
c. Chairs used in animal areas are covered with a non-porous material
that can be easily cleaned and decontaminated as appropriate and
sealed to prevent harboring of insects/vermin.
d. Equipment and furnishings are carefully evaluated to minimize
exposure of personnel to pinch points and sharp edges and corners.
9. Illumination is adequate for all activities and avoids reections and glare
that could impede vision.
10. Windows are break-resistant and sealed.
11. If Class II BSCs or other primary containment barrier systems are
needed in the cabinet laboratory, they are installed and operated in a
manner to ensure their eectiveness. See Appendix A.
a. BSCs are installed so that uctuations of the room air supply and
exhaust do not interfere with proper operations. BSCs are located
away from doors, windows that can be opened, heavily traveled
areas, and other possible airow disruptions.
b. BSCs can be connected to the animal facility exhaust system by
either a canopy connection (Class IIA only) or directly exhausted to
the outside through a hard connection (Class IIB, IIC, or III). Cabinet
exhaust air passes through two HEPA lters, including the HEPA in
the BSC, prior to release outside. Class IIA or IIC BSC exhaust can
be safely recirculated back into the animal facility environment if no
volatile toxic chemicals are used in the cabinet.
c. BSCs are certied at least annually to ensure correct performance,
or as specied in Appendix A, Part 7.
110 Biosafety in Microbiological and Biomedical Laboratories
12. Central vacuum systems are discouraged. If there is a central vacuum
system, it does not serve areas outside the cabinet. Two in-line HEPA
lters are placed near each use point and overow collection is provided
while in use. Filters are installed to permit in-place decontamination and
replacement.
13. A dedicated, non-recirculating ventilation system is provided. Only
facilities with the same HVAC requirements (i.e., other BSL-4 labora-
tories, ABSL-4, ABSL-3Ag, ABSL-4Ag facilities) may share ventilation
systems if gas-tight dampers and HEPA lters isolate each individual
room system.
a. The supply and exhaust components of the ventilation system are
designed to maintain the cabinet facility at negative pressure to
surrounding areas and provide dierential pressure or directional
airow, as appropriate, between adjacent areas within the facility.
b. Redundant supply fans are recommended. Redundant exhaust fans
are required. Supply and exhaust fans are interlocked to prevent
positive pressurization of the facility.
c. The ventilation system is monitored and alarmed to indicate
malfunction or deviation from design parameters. A visual monitoring
device is installed outside of containment so proper dierential
pressures within the facility may be veried prior to entry and during
regular checklist procedures. Visual monitoring is also in place
within the cabinet room.
d. Supply air to and exhaust air from the cabinet room, inner change
room, and fumigation/decontamination chambers pass through
a HEPA lter. The air exhaust discharge is located away from
occupied spaces and building air intakes.
e. All HEPA lters are located as near as practicable to the cabinet
room to minimize the length of potentially contaminated ductwork.
All HEPA lters are tested and certied annually.
f. The HEPA lter housings are designed to allow for in situ decontam-
ination of the lter and verication of the validated decontamination
process prior to removal. The design of the HEPA lter housing has
gas-tight isolation dampers, decontamination ports, and the ability to
individually scan each lter in the assembly for leaks.
14. Pass-through dunk tanks, fumigation chambers, or equivalent decon-
tamination methods are provided so that materials and equipment that
cannot be decontaminated in the autoclave can be safely removed from
the cabinet room(s). Access to the exit side of the pass-through is limited
111Section V—Vertebrate Animal Biosafety Level Criteria for Vivarium Research Facilities
to those with authorized access to the animal facility and with specic
clearance, if required.
15. Liquid euents from cabinet room sinks, oor drains, autoclave
chambers, and other sources within the cabinet facility are decontam-
inated by a proven method, preferably heat treatment, before being
discharged to the sanitary sewer.
a. Decontamination of all liquid euents is documented. The decon-
tamination process for liquid euents is validated physically and
biologically. Biological validation is performed annually or more often
as required by institutional policy.
b. Euents from personal body showers and toilets may be discharged
to the sanitary sewer without treatment.
16. A double-door, pass-through autoclave is provided for decontaminating
materials passing out of the cabinet facility. Autoclaves that open
outside of the facility are sealed to the wall through which the autoclave
passes. This bioseal is durable, airtight, and capable of expansion
and contraction. Positioning the bioseal so that the equipment can be
accessed and maintained from outside the facility is strongly recom-
mended. The autoclave doors are interlocked so that only one can be
opened at any time and are automatically controlled so that the outside
door to the autoclave can only be opened after the decontamination
cycle has been completed.
a. Gas discharge from the autoclave chamber is HEPA-ltered
or decontaminated. Autoclave decontamination processes are
designed so that unltered air or steam exposed to infectious
material cannot be released to the environment.
b. The size of the autoclave is sucient to accommodate the expected
volume of waste, size of equipment and cages, and any future
programmatic needs.
17. Cages are decontaminated prior to removal from the cabinet. The cage
wash area is designed to accommodate the use of high-pressure spray
systems, humidity, strong chemical disinfectants, and 180°F water
temperatures during the cage/equipment cleaning process.
18. The animal facility design parameters and operational procedures
are documented. The facility is tested to verify that the design and
operational parameters have been met prior to operation. Facilities
are also re-tested annually or after signicant modication to ensure
operational parameters are maintained. Verication criteria are modied,
as necessary, by operational experience.
112 Biosafety in Microbiological and Biomedical Laboratories
19. Appropriate communication systems are provided between the animal
facility and the outside (e.g., voice, fax, video, and computer). Provisions
for emergency communication and emergency access or egress are
developed and implemented.
Suit Facility
1. The ABSL-4 suit facility may be located in a separate building or a
clearly demarcated and isolated zone within a building. Consider placing
animal areas away from exterior walls of buildings to minimize the impact
from the outside environment temperatures.
a. Facility access is restricted. Facility doors are lockable.
b. Entry into the animal facility is through an airlock tted with airtight
doors.
c. Exit from the facility is by sequential passage through the chemical
shower, inner (i.e., dirty) change room, personal shower, and outer
(i.e., clean) changing area.
2. Personnel who enter this area wear a positive-pressure suit supplied
with HEPA-ltered breathing air. The breathing air systems have
redundant compressors, failure alarms, and emergency back-up capable
of supporting all workers within the facility to allow the personnel to
safely exit the facility.
3. A chemical shower is provided to decontaminate the surface of the
positive-pressure suit before the worker leaves the facility. In the event
of an emergency exit or failure of the chemical shower system, a method
for decontaminating positive-pressure suits, such as a gravity-fed supply
of chemical disinfectant, is provided.
4. An automatically activated emergency power source is provided at a
minimum for the animal facility exhaust system, alarms, lighting, entry
and exit controls, BSCs, and door gaskets.
a. Monitoring and control systems for air supply, exhaust, life support,
alarms, entry and exit controls, and security systems are on an
uninterrupted power supply (UPS).
5. A double-door autoclave, dunk tank, or fumigation chamber is provided
at the containment barrier for the passage of materials, supplies, or
equipment in or out of the facility.
6. Hands-free sinks inside the animal facility are placed near procedure
areas.
113Section V—Vertebrate Animal Biosafety Level Criteria for Vivarium Research Facilities
7. An eyewash station for use during maintenance is readily available in
the animal area.
8. Walls, oors, and ceilings of the animal facility are constructed to form
a sealed internal shell to facilitate fumigation and prohibit animal and
insect intrusion. The internal surfaces of this shell are resistant to
liquids and chemicals used for cleaning and decontamination of the
area. Floors are monolithic, sealed, and coved.
a. All penetrations in the internal shell of the animal room(s), suit
storage room, and the inner change room are sealed.
9. Services and plumbing that penetrate the facility walls, oors, or
ceiling are installed to ensure that no backow from the facility
occurs. Breathing air systems are exempt from this provision. These
penetrations are tted with two (in series) backow prevention devices.
Consideration is given to locating these devices outside of containment.
Atmospheric venting systems are provided with two HEPA lters in
series, are sealed up to the second lter, and have protection against
insect and animal intrusion.
10. Decontamination of the entire facility is performed using a validated
gaseous or vapor method when there has been a signicant change in
facility usage, before major renovations or maintenance shutdowns, and
in other situations, as determined by risk assessment. Decontamination
is veried prior to any change in the status of the facility.
11. Furniture is minimized, of simple construction, and capable of supporting
anticipated loads and uses.
a. Spaces between benches, cabinets, and equipment are accessible
for cleaning, decontamination and unencumbered movement of
personnel.
b. Benchtops are impervious to water and resistant to heat, organic
solvents, acids, alkalis, and other chemicals.
c. Chairs used in animal areas are covered with a non-porous material
that can be easily cleaned and decontaminated as appropriate and
sealed to prevent harboring of insects/vermin.
d. Equipment and furnishings are carefully evaluated to minimize
exposure of personnel to pinch points and sharp edges and corners.
12. Windows are break-resistant and sealed.
13. Illumination is adequate for all activities and avoids reections and glare
that could impede vision.

114 Biosafety in Microbiological and Biomedical Laboratories
14. BSCs and other primary containment barrier systems are installed in a
manner to ensure their eectiveness. See Appendix A.
a. BSCs are installed so that uctuations of the room air supply and
exhaust do not interfere with proper operations. BSCs are located
away from doors, heavily traveled areas, and other possible airow
disruptions.
b. BSCs can be connected to the animal facility exhaust system by
either a canopy connection (Class IIA only) or directly exhausted to
the outside through a hard connection (Class IIB, IIC, or III), which
contains a HEPA lter. Class IIA or IIC BSC exhaust can be safely
recirculated back into the facility environment if no volatile toxic
chemicals are used in the cabinet.
c. BSCs are certied at least annually to ensure correct performance,
or as specied in Appendix A, Part 7.
d. Class III BSCs are provided supply air in such a manner that
prevents positive pressurization of the cabinet or the animal room.
15. Central vacuum systems are discouraged. If there is a central vacuum
system, it does not serve areas outside the ABSL-4 facility. Two in-line
HEPA lters are placed near each use point and overow collection is
provided while in use. Filters are installed to permit in-place decontam-
ination and replacement. Consideration is made to the provision of two
HEPA lters in series as close to the vacuum pump as possible.
16. A dedicated, non-recirculating ventilation system is provided. Only
laboratories or facilities with the same HVAC requirements (i.e., other
BSL-4 laboratories, ABSL-4, ABSL-3Ag, ABSL-4Ag facilities) may share
ventilation systems if gas-tight dampers and HEPA lters isolate each
individual animal room.
a. The supply and exhaust components of the ventilation system are
designed to maintain the ABSL-4 facility at negative pressure to
surrounding areas and provide dierential pressure or directional
airow as appropriate between adjacent areas within the facility.
b. Redundant supply fans are recommended. Redundant exhaust fans
are required. Supply and exhaust fans are interlocked to prevent
positive pressurization of the facility.
c. The ventilation system is monitored and alarmed to indicate
malfunction or deviation from design parameters. A visual monitoring
device is installed outside of containment so proper dierential
pressures within the facility may be veried prior to entry and during
115Section V—Vertebrate Animal Biosafety Level Criteria for Vivarium Research Facilities
regular checklist procedures. Visual monitoring is also in place
within containment.
d. Supply air to the animal facility, including the decontamination
shower, passes through a HEPA lter. All exhaust air from the suit
facility, decontamination shower, and fumigation or decontamination
chambers passes through two HEPA lters, in series, before
discharge to the outside. The exhaust air discharge is located away
from occupied spaces and air intakes.
e. All HEPA lters are located as near as practicable to the areas
where infectious materials and/or animals are housed or manipu-
lated to minimize the length of potentially contaminated ductwork.
All HEPA lters are tested and certied annually.
f. The HEPA lter housings are designed to allow for in situ decontam-
ination of the lter and verication of the validated decontamination
process prior to removal. The design of the HEPA lter housing has
gas-tight isolation dampers, decontamination ports, and the ability to
individually scan each lter in the assembly for leaks.
17. Pass-through dunk tanks, fumigation chambers, or equivalent decon-
tamination methods are provided so that materials and equipment that
cannot be decontaminated in the autoclave can be safely removed from
the animal facility. Access to the exit side of the pass-through is limited to
those individuals authorized to be in the animal facility and provided with
appropriate clearance if required.
18. Liquid euents from chemical showers, sinks, oor drains, autoclave
chambers, and other sources within the facility are decontaminated by
a proven method, preferably heat treatment, before being discharged to
the sanitary sewer.
a. Decontamination of all liquid euents is documented. The decon-
tamination process for liquid euents is validated physically and
biologically. Biological validation is performed at least annually or
more often as required by institutional policy.
b. Euents from personal body showers and toilets may be discharged
to the sanitary sewer without treatment.
19. A double-door, pass-through autoclave(s) is provided for decontam-
inating materials passing out of the facility. Autoclaves that open
outside of the facility are sealed to the wall through which the autoclave
passes. This bioseal is durable, airtight, and capable of expansion
and contraction. Positioning the bioseal so that the equipment can
be accessed and maintained from outside the facility is strongly
116 Biosafety in Microbiological and Biomedical Laboratories
recommended. The autoclave doors are interlocked so that only one
can be opened at any time and be automatically controlled so that the
outside door to the autoclave can only be opened after the decontami-
nation cycle has been completed.
a. Gas discharge from the autoclave chamber is HEPA-ltered or
is decontaminated. Autoclave decontamination processes are
designed so that unltered air or steam exposed to infectious
material cannot be released to the environment.
b. The size of the autoclave is sucient to accommodate the expected
volume of waste, size of equipment and cages, and any future
programmatic needs.
20. Cages are decontaminated prior to removal from the animal facility. The
cage wash area is designed to accommodate the use of high-pressure
spray systems, humidity, strong chemical disinfectants, and 180°F water
temperatures during the cage/equipment cleaning process.
21. The ABSL-4 facility design parameters and operational procedures
are documented. The facility is tested to verify that the design and
operational parameters have been met prior to operation. Facilities
are also re-tested annually or after signicant modication to ensure
operational parameters are maintained. Verication criteria are modied,
as necessary, by operational experience.
22. Appropriate communication systems are provided between the facility
and the outside (e.g., voice, fax, video, and computer). Provisions
for emergency communication and emergency access or egress are
developed and implemented.
23. Facilities housing animals in open caging have the following design
elements:
a. Access to the animal holding room from service corridors outside of
the containment space requires passage through two sets of doors,
and the innermost door is an air pressure resistant (APR) door.
b. For any animal holding room considered to be a primary barrier,
the APR door(s) providing direct ingress from the exterior service
corridor is tted with appropriate and redundant lockout mechanisms
to prevent access when the animal-holding room is contaminated
and in use. There is more than one mechanism to ensure that this
primary barrier door cannot be opened when the animal room is
contaminated and the APR door does not serve as an emergency
exit from the animal facility. The APR door is appropriately tested to

117Section V—Vertebrate Animal Biosafety Level Criteria for Vivarium Research Facilities
demonstrate that in the closed, locked-out mode, the door provides
an air-tight barrier proven by pressure decay testing or other equiv-
alent method(s).
c. Any door(s) allowing access into an internal corridor from which
there is direct ingress to an animal holding room is tted with either:
1) an APR door; or 2) a non-APR door, providing directional airow
is maintained from the corridor space into the animal room. For the
purpose of fumigation, animal rooms equipped with non-APR doors
opening into the adjacent interior corridors are considered one
space (i.e., areas between air-tight doors are fumigated together).
d. Any door(s) used for access to the out-of-containment service
corridor (the secondary barrier) are self-closing and of solid
construction, designed not to corrode, split, or warp.
e. Access to the service corridor inside the secondary barrier is
restricted and strictly controlled when animal rooms are in use.
Whenever possible, the secondary barrier door(s) is tted with
safety interlock switches designed to prevent it from opening
when an animal-holding room door (the primary barrier) is opened
following room decontamination; if interlock devices cannot be
used, specic administrative procedures are implemented to control
access to the service corridor.
f. The out-of-containment service corridor maintains a negative
pressure (inward directional airow) relative to adjoining trac
corridors.
24. Loose-housed or open penned animals may be subject to the require-
ments of ABSL-3Ag or ABSL-4Ag. See Appendix D for additional
information.
References
1. USDA [Internet]. Washington (DC): Oce of the Chief Information Ocer;
c2002 [cited 2019 April 30]. USDA Security Policies and Procedures for
Biosafety Level 3 Facilities. Available from: https://www.ocio.usda.gov/
document/departmental-manual-9610-001
2. National Institutes of Health, Oce of Laboratory Animal Welfare. Public
Health Service policy on humane care and use of laboratory animals.
Bethesda (MD): U.S. Department of Health and Human Services; 2015.
3. Institute for Laboratory Animal Research. Guide for the Care and Use of
Laboratory Animals. 8th ed. Washington (DC): The National Academy
Press; 2011.

118 Biosafety in Microbiological and Biomedical Laboratories
4. Animal Welfare Act and Amendment, 9 C.F.R. Subchapter A, Parts 1, 2, 3
(1976).
5. Tabak LA. Appendix G-II-D-2-1. Containment for Animal Research. Fed
Regist. 2016;81(73):22287.
6. National Research Council. Occupational Health and Safety in the Care
and Use of Research Animals. Washington (DC): National Academy
Press; 1997.
7. National Research Council; Institute for Laboratory Animal Research.
Occupational health and safety in the care and use of nonhuman primates.
Washington (DC): The National Academy Press; 2003.
8. Grammer LC, Greenberger PA. Patterson’s Allergic Diseases. Baltimore
(MD). Lippincott Williams & Wilkins; 2009.
9. Hunt LW, Fransway AF, Reed CE, Miller LK, Jones RT, Swanson MC, et al.
An epidemic of occupational allergy to latex involving health care workers.
J Occup Environ Med. 1995;37(10):1204–9.
10. Centers for Disease Control and Prevention [Internet]. Atlanta (GA):
The National Institute for Occupational Safety and Health; c1997 [cited
2019 April 30]. Preventing Allergic Reactions to Natural Rubber Latex in
the Workplace. Available from: https://www.cdc.gov/niosh/docs/97-135/
default.html
11. United States Department of Labor [Internet]. Washington (DC):
Occupational Safety and Health Administration; c2008 [cited 2019 April
30]. Potential for Sensitization and Possible Allergic Reaction to Natural
Rubber Latex Gloves and other Natural Rubber Products. DHHS (NIOSH)
Publication Number 97-135. Available from: https://www.osha.gov/dts/shib/
shib012808.html
12. Allmers H, Brehler R, Chen Z, Raulf-Heimsoth M, Fels H, Baur X. Reduction
of latex aeroallergens and latex-specic IgE antibodies in sensitized workers
after removal of powdered natural rubber latex gloves in a hospital. J Allergy
Clin Immunol. 1998;102(5):841–6.
13. Bloodborne pathogens, 29 C.F.R. Part 1910.1030 (1992).

119Section VI—Principles of Laboratory Biosecurity
Section VI—Principles of Laboratory Biosecurity
The anthrax attacks on U.S. citizens in October 2001 and the subsequent
expansion of the United States Select Agent regulations in December 2003 have
led scientists, laboratory managers, security specialists, biosafety professionals,
and other scientic and institutional leaders to consider the need for developing,
implementing, and/or improving the security of biological agents and toxins
within their facilities.
1
Since the publication of the fth edition of BMBL, laboratory
biosecurity was better dened by biorisk management documents including
the International Standard Organization (ISO) 35001, Biorisk Management for
Laboratories and Other Related Organizations. Other eorts include pre-access
suitability, personnel reliability, and threat management approaches that identify
and manage behavioral problems that could result in laboratory biosecurity risks.
This section describes laboratory biosecurity planning for microbiological and
biomedical laboratories. As indicated below, laboratories with good biosafety
programs already fulll many of the basic requirements needed to secure
biological materials. For laboratories not handling Select Agents, the access
controls and training requirements specied for BSL-2 and BSL-3 in Section IV
of BMBL may provide sucient security for the materials being studied. Security
assessments and additional security measures should be considered when
Select Agents, other agents of high public health, environmental, and agriculture
concerns, or agents of high economic/commercial value such as patented vaccine
candidates are introduced into the laboratory.
The recommendations presented in this section are advisory. Excluding the
Select Agent regulations, Executive Order (EO) 13546, and the Global Health
Security Agenda EO 13747 (GHSA), there is no current federal requirement for
the development of a laboratory biosecurity program. However, the application
of these principles and the assessment process may enhance overall laboratory
management, safety, and security. Laboratories that fall under the Select Agent
regulations should consult Appendix F.
2–4
The term biosecurity has multiple denitions. In the plant and animal industry,
agricultural biosecurity relates to policies, measures, and regulatory frameworks,
based in science, applied to protect, manage, and respond to risks associated
with food, agriculture, health, and the environment. In some countries, biosecurity
is used in place of the term biosafety. For the purposes of this section, the term
laboratory biosecurity
5
will refer to measures designed to prevent loss, theft, or
deliberate misuse of biological material, technology, or research-related infor-
mation from laboratories or laboratory-associated facilities. See Appendix D for
additional information about agricultural biosecurity.
Security is not a new concept in laboratories handling biological agents and
materials. Several of the security measures discussed in this section are
120 Biosafety in Microbiological and Biomedical Laboratories
embedded in the Biosafety Levels that serve as the foundation for good
laboratory practices throughout the biological laboratory community. Most
biomedical and microbiological laboratories do not have Select Agents or Toxins;
however, they maintain control over and account for research materials, protect
relevant sensitive information, and work in facilities with access controls commen-
surate with the potential public health, agricultural, environmental, and economic
impact of the biological agents in their collections. These measures are in place
in most laboratories that apply good laboratory management practices and have
appropriate biosafety programs.
Biosafety and Laboratory Biosecurity
Biosafety and laboratory biosecurity are related concepts, but they are not
identical. Biosafety programs reduce exposure of individuals and the environment
to potentially hazardous biological agents. Biosafety is achieved by implementing
various degrees of performance-based control and containment measures
for biological materials, through infrastructure design and access restrictions,
personnel expertise and training, use of containment equipment, and safe
methods of managing infectious materials.
Laboratory biosecurity, the prevention of the theft, loss, or misuse of biological
material, technology, or research-related information, is accomplished through
personnel vetting, personnel reliability, violence prevention programs, laboratory
biosecurity training, dual-use research oversight process, cybersecurity
standards, material and facility control, and accountability standards; however,
laboratory biosecurity is not limited to this list.
While the objectives are dierent, biosafety and laboratory biosecurity measures
are usually complementary and share common components. Both are based
upon risk assessment and management methodology; personnel expertise
and responsibility; control and accountability for research materials including
microorganisms and culture stocks; access control elements; material transfer
documentation; training; emergency planning; and program management.
Both programs assess personnel qualications. The biosafety program ensures
that personnel are qualied to perform their jobs safely through training and
documentation of technical expertise. Sta must exhibit the appropriate level
of professional responsibility for the management of research materials by
adherence to appropriate materials management procedures. Biosafety practices
require laboratory access to be limited when work is in progress. Laboratory
biosecurity practices ensure that access to the laboratory facility and biological
materials are limited and controlled as necessary. Facilities should have pre-
established reporting mechanisms regarding any concerning behavior/incidents
in order to alleviate laboratory biosecurity insider threat concerns. An inventory
or material management process for control and tracking of biological stocks or
121Section VI—Principles of Laboratory Biosecurity
other sensitive materials is also a component of both programs. For biosafety,
the shipment of infectious biological materials must adhere to safe packaging,
containment, and appropriate transport procedures; laboratory biosecurity
ensures that transfers are controlled, tracked, and documented commensurate
with the potential risks. Both programs must engage laboratory personnel in the
development of practices and procedures that fulll the biosafety and laboratory
biosecurity program objectives but that do not hinder research or clinical/
diagnostic activities. The success of both programs hinges on a laboratory culture
that understands and accepts the rationale for biosafety and laboratory biose-
curity programs and the corresponding management oversight.
In some cases, laboratory biosecurity practices may conict with biosafety
practices, requiring personnel and management to devise policies that accom-
modate both sets of objectives (e.g. signage). Standard biosafety practice
requires that signage be posted on laboratory doors to alert people to the hazards
that may be present within the laboratory. The biohazard sign normally includes
the name of the agent, specic hazards, and precautions (e.g., PPE) associated
with the use or handling of the agent and contact information for the investigator.
These hazard communication practices may conict with security objectives.
Therefore, biosafety and laboratory biosecurity considerations must be balanced
and proportional to the identied risks when developing institutional policies.
Alternative solutions may be developed and implemented to meet both sets of
objectives.
Designing a laboratory biosecurity program that does not jeopardize laboratory
operations or interfere with the conduct of research requires a familiarity with
microbiology and the materials that require protection. Protecting pathogens
and other sensitive biological materials while preserving the free exchange
of research materials and information may present signicant institutional
challenges. Therefore, a combination or tiered approach to protecting biological
materials, commensurate with the identied risks, often provides the best
resolution to conicts that may arise. However, in the absence of legal require-
ments for a laboratory biosecurity program, the health and safety of laboratory
personnel, and the surrounding environment should take precedence over
laboratory biosecurity concerns.
A risk management methodology can be used to identify the need for a laboratory
biosecurity program. A risk management approach to laboratory biosecurity:
1. Establishes which, if any, agents, technology, and/or research-related
information require laboratory biosecurity measures to prevent loss,
theft, diversion, or intentional misuse; and
2. Ensures that the protective measures provided, and the costs associated
with that protection, are proportional to the risk.
122 Biosafety in Microbiological and Biomedical Laboratories
The need for a laboratory biosecurity program should be based on the possible
impact of the theft, loss, diversion, or intentional misuse of the materials, recog-
nizing that dierent agents and toxins will pose dierent levels of risk. Resources
are not innite. Laboratory biosecurity policies and procedures should not seek
to protect against every conceivable risk. The risks need to be identied and
prioritized, and resources need to be allocated based on that prioritization. Not
all institutions will rank the same agent at the same risk level. Risk management
methodology takes into consideration available institutional resources and the risk
tolerance of the institution.
Developing a Laboratory Biosecurity Program
Management, researchers and laboratory supervisors must be committed to
being responsible stewards of infectious agents and toxins. Development and
implementation of a laboratory biosecurity program should be a collaborative
process involving all stakeholders. The stakeholders include, but are not
limited to: senior management; scientic sta; human resource ocials; infor-
mation technology sta; and safety, security, and engineering personnel. The
involvement of organizations and/or personnel responsible for a facility’s overall
security is critical because many potential laboratory biosecurity measures may
already be in place as part of an existing safety or security program. This coordi-
nated approach is essential in ensuring that the laboratory biosecurity program
provides reasonable, timely, and cost-eective solutions addressing the identied
security risks without unduly aecting the scientic or business enterprise or the
provision of clinical and/or diagnostic services.
There is a need to include law enforcement and security communities in the
development of preventive measures and enforcement principles going beyond
response and consequence management, especially for laboratories working at
BSL-3 or BSL-4. The FBI has a Weapons of Mass Destruction (WMD) Coordi-
nator assigned to each of its eld oces across the U.S. WMD Coordinators
are responsible for conducting laboratory biosecurity outreach in their area of
responsibility and being a point of contact for any concerns/threats involving
WMD, including biological agents and materials.
The need for a laboratory biosecurity program should reect sound risk
management practices based on a site-specic risk assessment. A laboratory
biosecurity risk assessment should analyze the probability and consequences
of loss, theft, and potential misuse of biological material, technology, or
research-related information.
6
Most importantly, the laboratory biosecurity risk
assessment should be used as the basis for making risk management decisions
that are balanced with the needs of the biosafety risk assessment.
123Section VI—Principles of Laboratory Biosecurity
Example Guidance: A Laboratory Biosecurity Risk Assessment and
Management Process
Dierent models exist regarding laboratory biosecurity risk assessment.
Most models share common components such as asset identication, threat,
vulnerability, and mitigation. What follows is one example of how a laboratory
biosecurity risk assessment may be conducted. In this example, the entire risk
assessment and risk management process may be divided into ve main steps,
each of which can be further subdivided. Example guidance for these ve steps
is provided below.
Step 1: Identify and Prioritize Biological Materials, Research-Related Information,
and Technology
■
Identify the biological materials, research-related information, and
technology that exist at the institution.
■
Identify the form of the material, location, and quantities, including
non-replicating materials (e.g., toxins).
■
Evaluate the potential for misuse of these assets.
■
Evaluate the consequences of misuse of these assets.
Prioritize the assets based on the consequences of misuse (i.e., risk of malicious
use). At this point, an institution may nd that none of its biologic materials,
research-related information, or technology merit the development and
implementation of a separate laboratory biosecurity program or that the existing
security at the facility is adequate. In this event, no additional steps would need
to be completed.
Step 2: Identify and Prioritize the Threat to Biological Materials, Research-Re-
lated Information, and Technology
■
Identify the types of “Insiders” who may pose a threat to the biologic
materials, research-related information, and technology at the institution.
■
Identify the types of “Outsiders” (if any) who may pose a threat to the
biologic materials, research-related information, and technology at the
institution.
■
Evaluate and prioritize the motive, means, and opportunity of these
various potential adversaries.
Step 3: Analyze the Risk of Specic Security Scenarios
■
Develop a list of possible laboratory biosecurity scenarios or undesired
events that could occur at the institution. Each scenario is a combination
of an item, an adversary, and an action. Consider:
□
Access to the item within the laboratory;
□
How the undesired event could occur;
□
Protective measures in place to prevent occurrence; and
124 Biosafety in Microbiological and Biomedical Laboratories
□
How the existing protection measures could be breached
(i.e., vulnerabilities).
■
Evaluate the probability of each scenario materializing (i.e., the likelihood)
and its associated consequences. Assumptions include:
□
Although a wide range of threats are possible, certain threats are
more probable than others; and
□
All agents/assets are not equally attractive to an adversary; valid
and credible threats, existing precautions, and the potential need
for select enhanced precautions are considered.
■
Prioritize or rank the scenarios by risk for review by management.
Step 4: Develop an Overall Risk Management Program
■
Management commits to oversight, implementation, training, and mainte-
nance of the laboratory biosecurity program.
■
Management develops a laboratory biosecurity risk statement,
documenting which laboratory biosecurity scenarios represent an
unacceptable risk and must be mitigated vs. those risks appropriately
handled through existing protection control.
■
Management develops a laboratory biosecurity plan to describe how the
institution will mitigate those unacceptable risks including:
□
A written security plan, standard operating procedures, and
incident response plans; and
□
Written protocols for employee training on potential hazards, the
laboratory biosecurity program, and incident response plans.
■
Management ensures necessary resources to achieve the protection
measures documented in the laboratory biosecurity plan.
Step 5: Re-evaluate the Institution’s Risk Posture and Protection Objectives
■
Management regularly reevaluates and makes necessary modications
to the:
□
Laboratory biosecurity risk statement;
□
Laboratory biosecurity risk assessment process;
□
Institution’s laboratory biosecurity program/plan; and
□
Institution’s laboratory biosecurity systems.
■
Management assures the daily implementation, training, annual re-evalu-
ation and practice drills of the security program.
Elements of a Laboratory Biosecurity Program
Many facilities may determine that existing safety and security programs provide
adequate mitigation for the security concerns identied through the laboratory
biosecurity risk assessment. This section oers examples and suggestions for
components of a laboratory biosecurity program should the risk assessment
reveal that further protections may be warranted. Program components should be
site-specic and based upon organizational threat/vulnerability assessment and
125Section VI—Principles of Laboratory Biosecurity
as determined appropriate by facility management. Elements discussed below
should be implemented, as needed, based upon the risk assessment process.
They should not be construed as minimum requirements or minimum standards
for a laboratory biosecurity program.
Program Management
If a laboratory biosecurity plan is implemented, institutional management must
support the laboratory biosecurity program. Appropriate authority must be
delegated for implementation and the necessary resources provided to assure
program goals are being met. An organizational structure for the laboratory
biosecurity program that clearly denes the chain of command, roles, and
responsibilities should be distributed to the sta. Program management should
ensure that laboratory biosecurity plans are created, implemented, exercised,
and revised as needed. The laboratory biosecurity program should be integrated
into relevant institutional policies and plans.
Physical Security—Access Control and Monitoring
The physical security elements of a laboratory biosecurity program are intended
to prevent the introduction and removal of assets for non-ocial purposes. An
evaluation of the physical security measures should include a thorough review of
the building(s) and premises, the laboratories, and the biological material storage
areas. Many requirements for a laboratory biosecurity plan may already exist in a
facility’s overall security plan.
Access should be limited to authorized and designated employees based on the
need to enter sensitive areas. Methods for limiting access could be as simple as
locking doors or having a card key system in place. Evaluations of the levels of
access should consider all facets of the laboratory’s operations and programs
(e.g., laboratory entrance requirements, freezer access). The need for entry by
visitors, laboratory workers, management ocials, students, cleaning and mainte-
nance sta, and emergency response personnel should be considered.
Personnel Management
Personnel management includes identifying the roles and responsibilities
for employees who handle, use, store, and transport pathogens and/or other
important assets. The eectiveness of a laboratory biosecurity program against
identied threats depends, rst and foremost, on the integrity and awareness of
those individuals who have access to pathogens, toxins, sensitive information
and/or other assets. Employee vetting/screening policies and procedures are
used to help evaluate these individuals. To maintain a personnel reliability and
violence prevention plan, management should conduct periodic reviews of sta,
establish an anonymous peer and threat reporting system, institute an Employee
Health and Wellness Program, and foster leadership accountability to address
submitted reports. Policies should also be developed for personnel and visitor

126 Biosafety in Microbiological and Biomedical Laboratories
identication, visitor management, access procedures, and reporting of security
incidents.
Inventory and Accountability
Material accountability procedures should be established to track the inventory
of biological materials and toxins; storage including physical and digital; the use,
transfer, and destruction of dangerous biological materials and assets when
no longer needed; and the inactivation of biological materials, particularly prior
to transport outside the facility. See Appendix K. The objective is to know what
assets exist at a facility, where they are located, and who is responsible for them.
To achieve this, management should dene:
1. The materials (or forms of materials) subject to accountability measures;
2. Records to be maintained and timelines for record retention;
3. Operating procedures associated with inventory maintenance (e.g., how
material is identied, where it can be used and stored); and
4. Documentation and reporting requirements.
It is important to emphasize that microbiological agents are capable of replication
and are often propagated. Therefore, knowing the exact quantity of organisms
at any given time may be impractical. Depending on the risks associated with a
pathogen or toxin, management can designate an individual who is accountable,
knowledgeable about the materials in use, and responsible for the security of the
materials under his or her control.
Information Security
Policies should be established for handling sensitive information associated with
the laboratory biosecurity program. For the purpose of these policies, “sensitive
information” is information that is related to the security of pathogens and toxins
or other critical infrastructure information. Examples of sensitive information may
include facility security plans, access control codes, newly developed technol-
ogies or methodologies, agent inventories, and storage locations.
Discussion of information security in this section does not pertain to information
that has been designated “classied” by the United States pursuant to Executive
Order 12958, as amended, and is governed by United States law or to
research-related information that is typically unregulated or unrestricted through
the peer-review and approval processes.
The objectives of an information security program are to ensure data integrity,
protect information from unauthorized release, and ensure that the appropriate
level of condentiality is preserved. Facilities should develop policies that govern
the proper identication, marking, handling, securing, and storage of sensitive
information including electronic les and removable electronic media (e.g., CDs,
127Section VI—Principles of Laboratory Biosecurity
external hard drives, USB ash drives). The information security program should
be tailored to meet the needs of the business environment, support the mission
of the organization, and mitigate the identied threats. It is critical that access to
sensitive information be controlled.
Transport of Biological Agents
Material transport policies should include accountability measures for the
movement of materials within an institution (e.g., between laboratories, during
shipping and receiving activities) and outside of the facility (e.g., between insti-
tutions or locations). Transport policies should address the need for appropriate
documentation and material accountability and control procedures for biological
materials and toxins in transit between locations. Transport security measures
should be instituted to ensure that appropriate authorizations have been received
and that adequate communication between facilities has occurred before,
during, and after transport of pathogens or other potentially hazardous biological
materials. Personnel should be adequately trained and familiar with regulatory
and institutional procedures for proper containment, packaging, labeling,
documentation, and transport of biological materials.
Accident, Injury, and Incident Response Plans
Laboratory security policies should consider situations that may require
emergency responders or public safety personnel to enter the facility in response
to an accident, injury, or other safety issue or security threat. The preservation of
human life and the safety and health of laboratory employees and the surrounding
community must take precedence over laboratory biosecurity and biosafety
concerns in an emergency.
Facilities are encouraged to coordinate with medical, re, police, and other
emergency ocials when preparing emergency and security breach response
plans. Standard Operating Procedures (SOPs) should be developed that
minimize the potential exposure of responding personnel to potentially hazardous
biological materials. Laboratory emergency response plans should be integrated
with relevant facility-wide or site-specic security plans. These plans should also
consider such adverse events as bomb threats, natural disasters and severe
weather, power outages, and other facility emergencies that may introduce
security threats.
Reporting and Communication
Communication is an important aspect of a laboratory biosecurity program. A
“chain-of-notication” should be established in advance of an actual event. This
communication chain should include laboratory and program ocials, institution
management, and any relevant regulatory or public authorities. The roles and
responsibilities of all involved ocials and programs should be clearly dened.

128 Biosafety in Microbiological and Biomedical Laboratories
Policies should address the reporting and investigation of potential security
breaches (e.g., missing biological agents, unusual or threatening phone calls,
unauthorized personnel in restricted areas, unauthorized transfer of assets to and
from the facility).
Training and Practice Drills
Laboratory biosecurity training is essential for the successful implementation of a
laboratory biosecurity program. Program management should establish training
programs that inform and educate individuals regarding their responsibilities
within the laboratory and the institution. For example, it might be dicult to
identify suspicious activity that warrants attention without appropriate training on
security awareness, laboratory biosecurity best practices, and the facility’s estab-
lished reporting mechanisms. Practice drills should address a variety of scenarios
such as loss or theft of materials, emergency response to accidents and injuries,
incident reporting, and identication of and response to security breaches. These
scenarios may be incorporated into existing emergency response drills such as
re drills or building evacuation drills associated with bomb threats. Incorporating
laboratory biosecurity measures into existing procedures and response plans
often provide ecient use of resources, saves time, and can minimize confusion
during emergencies.
Security Updates and Re-evaluations
The laboratory biosecurity risk assessment and program should be reviewed
and updated routinely and following any laboratory biosecurity-related incident.
Re-evaluation is a necessary and on-going process in the dynamic environments
of today’s biomedical and research laboratories. Laboratory biosecurity program
managers should develop and conduct laboratory biosecurity program audits
and implement corrective actions as needed. Audit results and corrective actions
should be documented. The appropriate program ocials should maintain
records.
Select Agents
If a laboratory possesses, uses, or transfers Select Agents, it must comply with all
requirements of the National Select Agent Program. See Appendix F for additional
information
References
1. Richmond, JY, Nesby-O’Dell, SL. Laboratory security and emergency
response guidance for laboratories working with select agents. MMWR
Recomm Rep. 2002;51(RR-19):1–8.
2. Possession, use, and transfer of select agents and toxins; Final Rule,
42 C.F.R. Part 73 (2005).
129Section VI—Principles of Laboratory Biosecurity
3. Possession, use, and transfer of biological agents and toxins, 7 C.F.R.
Part 331 (2005).
4. Possession, use, and transfer of biological agents and toxins, 9 C.F.R.
Part 121 (2005).
5. World Health Organization. Biorisk Management. Laboratory Biosecurity
Guidance. Geneva: World Health Organization; 2006.
6. Casadevall A, Pirofski L. The weapon potential of a microbe. Trends in
Microbiology. 2004;12(6):259–63.

130 Biosafety in Microbiological and Biomedical Laboratories
Section VII—Occupational Health Support for Biomedical
Research
The occupational health provider is integral in the promotion of a workplace
culture of safety in biomedical and microbiological research. An occupational
health program that supports sta with access to biological hazards, such as
infectious agents or toxins, should aim to alleviate the risk of adverse health
consequences due to potential exposures to biohazards in the workplace. Health
services should be risk-based and tailored to meet the needs of individual sta
and the research institution based on risk assessment. Ideally, the program
focuses on work-related healthcare to avoid potential conicts of interest. An
institution must carefully consider available options for implementing robust
occupational health support as an essential component of its risk management
strategy.
1,2
Framework for Occupational Health Support of Biomedical Research
Basic Concepts for Providing Work-Related Healthcare in a Research Setting
Occupational health services that support a biomedical research community
should be based on detailed risk assessments of hazards in the workplace.
3
See Section II for additional information. Services should complement the
hierarchy of exposure controls and provide relief in case of potential exposure to
a hazard.
4
Medical countermeasures such as vaccines, wound decontamination,
or pharmaceutical agents may reduce the risk of harm, but they do not eliminate
it (e.g., vaccine failure or antibiotic resistance).
5,6
Dierent elements of occupational health support may be indicated at various
stages of employment, ranging from anticipatory risk mitigation (e.g., preplacement
evaluation or vaccination) to incident-driven medical measures such as post-
exposure immuno- or chemoprophylaxis. A change in a sta member’s health
status suggestive of a Laboratory-associated infection (LAI) requires clinical care
and an interdisciplinary investigation into a possible antecedent occupational
exposure. At each juncture, the healthcare provider must take care to tailor
services to mitigate the individual sta member’s risk for harm.
1,7
Before research involving biological hazards begins, stakeholders should have
plans in place for providing occupational health support for sta commensurate
with the potential health risks of the proposed work (i.e., pathogens, activities,
and work environment or facility).
8
An institution may require, as a condition of
employment, its sta to participate in relevant occupational health programs
designed to reduce risks associated with research on biological agents that may
pose grave threats to human health and society (high-consequence pathogens).
9
The provider may consider establishing contact with subject matter experts
(SMEs) for consultation on procedural and clinical elements of the program,
especially agent-specic occupational exposure and illness response plans

131Section VII—Occupational Health Support for Biomedical Research
concerning high-consequence pathogens or bioengineered infectious particles
whose pathogenic potential is not established.
10,11
Continual collaboration among stakeholders is key to optimal protection of
biomedical research sta. The designated occupational healthcare provider
should work with institutional safety sta, principal investigators (PIs), and
clinically-oriented SMEs (i.e., infectious diseases specialists) to ensure optimal
work-related health care of laboratorians and their support sta.
Practical and Regulatory Requirements for Occupational Health Programs
Occupational health services may be administered through a variety of arrange-
ments and may be employer- or community-based, provided they are readily
available, allow timely evaluation, and appropriate treatment. Regardless of
employment status, all workers should have access to a comparable level
of care and occupational health services based on their risk of occupational
hazard exposure. Contractors, students, volunteers, and visitors should receive
work-related occupational health services through their employer or sponsor
equivalent to those provided by the host institution for its employees.
The designated occupational health provider should be familiar with the
nature of hazards in the work environment and the controls used to prevent
exposures. The program should have the means to implement promptly any
indicated pre- and post-exposure medical measures and related counseling. The
provider should ensure that services rendered remain consistent and conform to
current practices such as recommended immunization schedules and infection
control.
12–14
Expanded discussions of principles of standard occupational health
practices are available in authoritative texts.
15,16
The provider should be aware
of and abide by guidance or regulations including but not limited to the NIH
Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid
Molecules (NIH Guidelines); Title 42 of the Code of Federal Regulations, Part
73; relevant Occupational Safety and Health Administration (OSHA) standards;
the Americans with Disabilities Act (ADA) of 1990 and related regulations; the
Pregnancy Discrimination Act of 1978; and patient condentiality laws including
Health Insurance Portability and Accountability Act of 1996 (HIPAA).
17–24
Risk-based Design of Occupational Health Services
The scope of an occupational health program should match the clinical and
research portfolio of the institution it supports. Institutional biosafety and security
policies may require additional occupational health support. This publication
advises stakeholders in sta members’ safety and health in microbiological and
biomedical research laboratories on recommendations for working safely with
biological agents ranging from Risk Groups (RG) 1 to 4. Please refer to Sections
II, III, and IV for additional information on RG classication and Biosafety Level
(BSL) requirements. Work with microbes that are not associated with disease in
132 Biosafety in Microbiological and Biomedical Laboratories
healthy adults (RG1) likely requires minimal occupational health support, although
the provider should be aware of other non-biological hazards that may be present
in the laboratory. Sta with access to RG2, RG3, or RG4 biological agents
should be provided with occupational health services that stand to decrease
the risk of potential harm. The program will need to commit resources that are
likely proportionate to the severity of potential health risks of these agents and
the residual risk of exposure after implementation of applicable controls. This
consideration becomes especially pronounced for programs that support RG3
and RG4 pathogen research where the elevated cost of emergency preparedness
reects the need to mitigate a wide range of risks, including those associated with
high-impact, low-probability events.
25–27
With increasingly widespread applications of advances in bioengineering, occupa-
tional medical sta must be prepared to adapt established practices to evolving
workplace hazards.
28–30
The principles of expert risk-based occupational health
support for work with naturally occurring biological agents apply to work with
genetically modied organisms, designer biologics, or novel genetic constructs.
For example, viral vectors deployed in gene therapy or vaccinology may be
engineered to incorporate safety features at the genomic level to decrease infec-
tivity or virulence. However, even highly genetically altered particles should not be
presumed to be risk-free to sta who are exposed to them, as illustrated by the
replacement of rst-generation lentiviral platforms with third- or fourth-generation
HIV-derived vectors.
31
Until immediate and long-term health risks of genetically
modied organisms or synthetic constructs are better characterized (e.g., inser-
tional mutagenesis), the provider must appreciate that an agent’s genome-level
safety features may not fully protect exposed sta from potential health risks.
The NIH Oce of Science Policy provides guidance on assessing and mitigating
potential harm from recombinant nucleic acids, genetically modied organisms, or
entirely new constructs with varying capacity to infect human cells.
17,32
Sta may require additional occupational health services besides those targeting
biological agents under scientic investigation. For example, researchers
engaged in human subjects research activities or animal care and veterinary sta
who support the use of laboratory animals should receive all applicable medical
care and counseling.
33
Laboratory animals may become zoonotic disease vectors
when a sta member is exposed to an infected animal’s body uids or tissues
(e.g., Macacine alphaherpesvirus 1 [B virus] or Simian immunodeciency virus
[SIV]).
34,35
In turn, susceptible research animals must be protected from reverse
zoonotic transmission of human pathogens. For example, Measles morbillivirus
or Mycobacterium tuberculosis (Mtb) may devastate non-human primates
(NHPs) and cause substantial losses.
36
Other potential hazards may add to the
complexity of pertinent occupational health support; some with established risk
factors such as human-derived materials; chemical, physical, or environmental
hazards; and others with less well-circumscribed risk to sta (e.g., hazards
133Section VII—Occupational Health Support for Biomedical Research
associated with eld research or outbreak response). OSHA provides general
guidance on safety and health in a laboratory environment such as respiratory
protection and hearing conservation.
19,37,38
The occupational health program
should collaborate with institutional biosafety, management, and subject matter
experts to customize services that complement risk mitigation in biomedical
research.
Pre- and Post-exposure Communications
All biomedical research laboratories should maintain a laboratory-specic
biosafety manual that species the steps all sta should take immediately after an
incident. An eective incident response, including medical care of aected sta,
relies on the coordinated execution of the plan and concise, prompt communi-
cations.
39
Laying the foundation for proper post-exposure risk mitigation begins
before an occupational exposure occurs (e.g., with risk awareness training in the
workplace and targeted preplacement occupational health evaluations). Incident
response protocols should describe requisite notications at the time of a potential
exposure, including how to access medical care.
40
All sta should identify and
work to remove barriers to prompt, qualied post-exposure medical care. Commu-
nity-based medical care of a sta member after a potential occupational exposure
may require additional steps to ensure optimal assessment and treatment of the
sta member, including connecting the healthcare provider with SMEs.
Occupational Health and Risk Management
The designated occupational health program should design a quality assurance
program to monitor internal operations and interdisciplinary processes with a
healthcare component.
41
Each occupational health support oering and procedure
should be reviewed regularly with respect to the most current practice guidelines
and relevance to the research supported. The occupational health program is
uniquely positioned to contribute to the institution’s ongoing risk management
activities. For example, prevention of future exposures should be informed by the
collection and analysis of work-related injury and illness statistics.
42,43
Elements of an Occupational Health Program Supporting Biomedical
Research
Preplacement Medical Evaluations
Supervisors should inform all workers about workplace hazards and exposure
controls and refer newly hired sta with proposed access to biological hazards
(e.g., biological agents, human subjects, laboratory animals, or their respective
body uids or tissues) to the occupational health program for a risk-based
preplacement medical evaluation.
1,19
The healthcare provider must review sta
members’ personal and occupational health history in light of the supervisors’
input on potential hazards and minimum functional requirements of the position.
This standard review includes past and current medical conditions and treatment;
134 Biosafety in Microbiological and Biomedical Laboratories
present use of medications (prescription and non-prescription); allergies and
adverse reactions to medicines, vaccines, animals, and other environmental
allergens; and a complete immunization history, including serology results,
when appropriate, or relevant prior infections. The provider should discuss
agent-specic risk factors and incidental hazards (e.g., zoonotic infections, toxic
chemicals, or laboratory animal allergens), and the provider should dispense
information on health conditions that might increase susceptibility to infection and
complications after an occupational exposure. The provider should ensure sta
members’ familiarity with the need for standard rst aid after an exposure, and
the need to promptly report work-related injuries and illnesses. The importance
of exposure prevention should be emphasized while cautioning against overre-
liance on medical countermeasures for curbing work-related health risks. For
example, minimizing exposure to likely allergens (e.g., animal proteins or latex)
is paramount to the control of occupational allergies. Sensitization to specic
allergens may not be reversible even with treatment. Sta should be directed to
supervisors and safety professionals for training and proper use of applicable
exposure control strategies, including personal protective equipment (PPE).
8
The provider should also advise sta on steps to take in cases of potentially
work-related illness(es), such as signs or symptoms suggestive of an LAI or an
occupationally-acquired allergy.
The occupational health program should oer only those services that constitute
eective medical support related to workplace hazards and duties. For example,
testing for immunity to a specic pathogen is rarely indicated as a condition for
employment. Pre-immunization serology should be performed in accordance
with established risk-based guidelines.
13,44
Serum banking, the practice of
collecting and storing frozen serum samples, is of questionable value to the care
of research or clinical laboratory sta; it should not be oered routinely without
a clear indication. An exception may be made if a risk assessment suggests
that work conditions are likely to lead to unrecognized exposures, especially to
pathogens with long latency periods or with the potential for subclinical infection.
If serum banking is utilized, the provider must implement it with the requisite
precautions to ensure accurate retrieval, proper storage and disposal, patient
privacy, and observance of applicable ethics standards.
1,45
Serum sampling and
short-term storage should be considered on a case-by-case basis with properly
designed testing strategies for post-incident screening of potentially exposed sta
or investigation of possible LAIs.
1
Vaccines
The Advisory Committee on Immunization Practices (ACIP) provides expert
advice on the most eective immunization strategies against vaccine-preventable
diseases. The occupational health program should utilize ACIP guidelines for
routine administration of vaccines and oer any licensed vaccine indicated to

135Section VII—Occupational Health Support for Biomedical Research
provide risk-based agent-specic immune protection.
1,13,44
Please refer to the
agent summary statements in Section VIII for additional information on available
vaccines for various biological agents.
With few exceptions, acceptance of vaccinations that are medically indicated
should not be a precondition of employment in biomedical research laboratories.
However, under specic legal situations, an institution may be able to exclude a
worker who declines to receive a potentially protective licensed vaccine against
a virulent pathogen strain from working directly with that agent. Each institution
must determine the best risk management strategy for its laboratory-based
workforce. The healthcare provider should counsel sta who refuse recom-
mended immunization against a vaccine-preventable disease and document the
sta members’ lack of protection in the medical record.
Periodic Medical Evaluations
In most cases, there is no medical basis for requiring periodic medical evaluations
for the vast majority of sta solely because they work with biological hazards.
Institutions may require specic work groups to participate in periodic medical
evaluations provided it is justied by a substantial risk of exposure to biohazards.
The possibility of increased health risks due to potential changes in sta health
status should not serve as a basis for requiring workers in biomedical research
to be subjected to periodic medical evaluations; rather, sta should be oered
the chance to seek medical advice when such changes occur. Sta with specic
concerns, such as working with biohazards while immunocompromised or the
eects of hazards on their reproductive capacity, should be directed to seek
condential medical counseling with a qualied clinician.
Screening programs for work-related infections of sta, such as post-exposure
medical surveillance, contact investigations, or research settings associated with
evidently elevated exposure risk to specic pathogens, should also be risk-based.
Periodic testing, ostensibly to detect unrecognized workplace exposures,
should be avoided unless there is an unusual constellation of risk factors that
could preclude the timely recognition of LAIs. For example, a workplace risk
assessment may conclude that there is sucient residual exposure risk to
Mtb, an easily transmissible agent with a low infectious dose and long latent
period, to warrant surveillance of sta to avoid dire health consequences for
unknowingly infected sta and their contacts. Before an occupational health
program endeavors to screen asymptomatic sta without a recognized exposure
to a specic pathogen, the provider should justify the benet of such testing,
clearly dene criteria for interpretation of results, and develop plans for further
investigation of indeterminate and positive test results. Any medical surveillance
must meet requisite criteria.
46–49
136 Biosafety in Microbiological and Biomedical Laboratories
Occupational Health Support for Occupational Injuries and Potential Exposures
In case of a potential hazard exposure, the sta member must immediately
perform proper rst aid and follow all established agent-specic protocols. All
occupational injuries, including potential exposures to a biohazard, should be
reported to the occupational healthcare provider immediately. The provider should
notify the supervisor and safety sta if the sta member has not already done so.
The provider must take a suciently detailed account of the incident to quickly
determine its clinical signicance. The primary source of information is typically
the aected sta member. Collateral sources include safety professionals
investigating the incident, the supervisor or PI, and others with knowledge of the
circumstances of the incident or source materials involved. The following key
factors in this step include:
■
Exposure controls used at the time of the incident and work activities
performed leading up to it;
■
The mechanism of the potential exposure (e.g., percutaneous injury,
splash to mucous membranes or skin, inhalation of an infectious aerosol);
■
The nature of the potential biohazard (e.g., animal body uid, culture
medium, contaminated fomite) and inoculum size (concentration, volume);
■
Characteristics of agent(s) known or suspected to be involved (e.g.,
species, strain); transmission in natural infection or LAI; minimum
infectious or lethal dose to humans; incubation period; drug susceptibility
or resistance;
■
Agent viability (i.e., inactivation by chemical or physical means prior to
incident) and genetic modications (to enhance viral vector safety); and
■
First aid performed at the workplace (e.g., duration and cleansing agent
used, time elapsed from exposure to initiation).
The two most critical determinants that diminish the risk of infection are the
immediate and adequate cleansing of the aected body area and avoidance
of delays in starting appropriate post-exposure prophylaxis (PEP). When in
doubt, the provider should repeat rst aid. The provider should take a pertinent
health and social history focused on mitigating the risk of adverse health
consequences for the aected sta member and the community due to the
potential exposure. This should include factors that may aect the individual’s
susceptibility to infection with the pathogen of concern, barriers to adherence
to proposed medical management, and the potential for exposure of others
during the incident or close contacts. Prior agent-specic immunization does not
obviate the need for a post-exposure medical evaluation because vaccination
may not fully protect against disease. PEP should be oered whenever such
treatment may prevent or ameliorate illness. The provider may consult clinical
specialists who have experience with the biological agents of concern. If need
be, the sta member should be transferred to a medical facility that can provide
137Section VII—Occupational Health Support for Biomedical Research
the necessary level of care.
10
The occupational health program should ensure
adequate medical support is available for incidents where multiple sta may
have been exposed.
Clinically-Oriented, Post-Exposure Risk Assessment
In case of an occupational hazard exposure, the clinician’s rst priority is
mitigating against the risk of further harm to the aected sta member. The
occupational health program may contribute further by documenting lessons
learned from each incident, thereby decreasing the chances for future exposures.
To achieve both goals, it may help to distinguish between a potential biohazard
and specic pathogens of concern and to stratify the risk of exposure (RoE) and
risk of adverse health consequences or disease (RoD) separately.
1,50
It may be
unknown at the time of an incident whether the source material (hazard) involved
harbors any potentially harmful biological agents. Some biological materials
(i.e., animal or human body uids and tissues) may present a mixed hazard with
more than one specic pathogen of concern, each warranting separate RoE and
RoD estimates. The RoE to a pathogen informs agent-specic subsequent clinical
decision-making (e.g., initiating treatment to lower the initial RoD).
For a biohazard exposure to occur two conditions must be met: (1) a biohazard
must be present (i.e., released from containment by aerosolization, splash, spill,
or mishandling of a contaminated object), and (2) the sta member must come
into direct contact with the biohazard. The provider must determine whether a
pathogen may have been transmitted to the sta member and the mechanism of
exposure is compatible with transmission of an agent of concern. Whenever the
possibility of transmission of a specic biological agent cannot be excluded, the
provider must estimate the level of RoD. Risk factors for infection, illness, and
potential for complications include circumstances of the incident, characteristics
of the biological agents involved, host factors such as immune function or
pre-exposure vaccination, and the utilization of post-exposure medical counter-
measures. Generally, initial estimates of RoE and RoD levels will correlate.
Post-exposure medical measures such as immediate wound decontamination
and PEP may lower the initial RoD estimate but they cannot eliminate the
possibility of an LAI.
Post-Exposure Follow-Up Care and Testing
The provider should counsel each sta member who reports a potential occupa-
tional exposure on the signicance of the incident and clearly communicate the
post-exposure care plan, including treatment options, alternatives to treatment,
testing procedures, and interpretation and implications of laboratory results.
When PEP is recommended, the sta member should be followed closely for
signs of an LAI, compliance with treatment and possible adverse medication
eects. Sta exposed to infectious agents for which there is no eective PEP
138 Biosafety in Microbiological and Biomedical Laboratories
must receive appropriate post-incident care tailored to the agent involved and
the worker’s personal health. Sta may be asked to adhere to an agent-specic
monitoring protocol to facilitate early detection of a symptomatic LAI. The provider
may recommend isolation of a sta member to avoid secondary transmission
during the prodromal phase associated with pathogens that may render a person
infectious prior to the onset of symptoms (e.g., inuenza).
The optimal post-exposure testing strategy for evidence of infection depends on
the pathogen of concern, potential spectrum of illness, performance of available
commercial assays, and the aected worker’s host risk factors. Awaiting test
results, including pregnancy testing, should not delay initiation of clinically
indicated and appropriately selected PEP. Certain PEP protocols, such as
antiretroviral regimens, may justify targeted baseline laboratory testing.
51
A serum
specimen collected at the time of the incident may be useful for exposure-related
surveillance; however, screening for pre-existing infection with an agent of
concern should not be conducted routinely. When there are no signs or symptoms
of an LAI, subsequent laboratory or imaging studies to assess if transmission
occurred should be avoided in most cases. However, when there is clinical value
in detecting acute infections that may remain asymptomatic for prolonged periods,
post-exposure testing strategies should aim for early detection. For example,
nucleic acid testing for Hepacivirus C (HCV) even before antibodies may be
present or screening for latent Mtb infection could lead to timely recognition of
the need for treatment of an LAI. For serologic assays, comparison of results
from paired serum samples, collected at appropriate time points, constitutes
more reliable laboratory evidence of recent infection than results of screening of
a single serum specimen. Ideally, the provider performs serial serological assays,
simultaneously testing aliquots of baseline serum and samples collected when
specic immune markers are assumed to become detectable. The clinician may
consider blinding the testing facility to the times the samples were obtained.
Documented seroconversion, or a signicant increase in antibody titer (at least
four-fold) associated with a compatible clinical syndrome, is usually highly
suggestive of acute infection. The typical timing of serial serum collections
in each case may be modied by circumstances of the exposure, the agent’s
characteristics, host factors, and medical countermeasures taken. For example,
screening too soon may fail to detect low levels of early immune markers. Repeat
screening at appropriate intervals may be indicated when seroconversion may
be delayed; for example, repeat screening may be indicated due to the nature of
the agent (e.g., human retroviruses), the immediate use of PEP (e.g., B virus), or
the aected sta members’ immune system function. If a sta member is to be
screened with a non-commercial assay based on expert consensus, the provider
should submit samples from uninfected source(s) as negative controls, positive
control samples, whenever possible, and blind the testing facility to sources
and timing of sample collection. The provider should caution the exposed sta
139Section VII—Occupational Health Support for Biomedical Research
member that the clinical utility of such assays is not the same as licensed tests
and must be interpreted with extreme caution.
Post-exposure occupational health care of an aected sta member may be
informed by establishing whether the biological material involved harbored
specic pathogens of concern. The provider should work with the principal inves-
tigator, veterinarian, or clinician responsible for the source material to determine if
testing appropriate samples could help establish if a specic infectious agent was
present. Negative results may not indicate the absence of a specic infectious
agent and should be interpreted with caution.
Occupational Health Support for Occupational Illnesses
Sta in biomedical research and clinical laboratories should be encouraged to
seek timely care for illnesses attributable to their work. Full implementation of
laboratory exposure controls at recommended Biosafety Levels clearly reduces
the chance of LAIs.
26,52
However, there is little evidence to corroborate the
eectiveness of biocontainment practices in preventing occupational exposures
due to underreporting and a lack of centralized data-sharing on biological hazard
exposures and LAIs.
53
The true incidence of LAIs remains unknown and, although
increased adherence to safer work practices in biomedical and microbiological
laboratories has eliminated many opportunities for occupational exposures, sta
remain at risk for LAIs.
52,54
Historically, sta with proven LAIs often did not recall
an antecedent exposure. Unexpectedly, serious illnesses have resulted from
exposures that were deemed trivial at the time of the incident or were not recog-
nized as an LAI at initial presentation.
55–57
Research and clinical laboratorians who
work with human pathogens, or access spaces where such agents are handled,
should maintain an awareness of the timing of a febrile illness in light of their work
activities. They should be encouraged (e.g., at preplacement or post-exposure
medical evaluations) to have a low threshold for contacting the designated
occupational health provider with the earliest signs and symptoms that could be
compatible with an LAI.
The provider must conduct a risk assessment for any acutely ill sta member
who handled a potential pathogen during a time span prior to the onset of
symptoms equal to the pathogen’s range of incubation period. In addition to a
focused clinical history, the interview should include an inquiry into recent work
with biological materials, potential breaches of exposure controls, adherence
to biosafety practices, sick contacts at work and outside, and other plausible
exposure opportunities to infectious agents (e.g., hobbies or travel). Clinicians
should be aware that in cases of occupational exposures, a pathogen’s typical
incubation period or initial clinical presentation may dier markedly from naturally
acquired infections (e.g., due to disparate exposure mechanisms or an agent’s
genetic modications). Prior vaccination or infection with certain pathogens

140 Biosafety in Microbiological and Biomedical Laboratories
may also aect the clinical course of an LAI with a related infectious agent
(e.g., tick-borne encephalitis or dengue). Close-working relationships among all
stakeholders and ready access to expert medical care are absolutely essential to
an adequate LAI response. Risk stratication of a possible LAI follows the same
considerations as a post-incident evaluation except in a retrospective fashion and
with increased emphasis on risk for the ill sta member’s close contacts who may
be subject to contemporaneous workplace exposure or secondary transmission.
The occupational health program should be prepared to work with supervisors
and biosafety professionals to conduct workplace contact investigations or case
nding, taking care to balance the needs for privacy protection and infection
control. An LAI that meets criteria for a reportable disease requires notication of
public health authorities.
Additional workplace hazards and ergonomic conditions in the laboratory
environment may give rise to work-related health conditions that may diminish
sta’s ability to work safely with human pathogens such as work-related muscu-
loskeletal disorders or occupationally acquired allergies. In most cases, allergies
to laboratory animals develop within the rst year of occupational exposure
to the allergens. Of the 20 to 30% of workers who become allergic to animal
proteins, 5% may progress to asthma that may, rarely, threaten workers’ lives
and livelihood due to anaphylaxis.
1,7
The occupational health program should be
prepared to evaluate and treat these conditions to ensure a safe return of sta to
full duty.
Occupational Health Support of Sta in High and Maximum Biocontainment
Adequate occupational health support of research in BSL-3 and BSL-4 labora-
tories may pose special challenges for occupational health providers.
58
BSL-3,
BSL-4, and associated animal facilities (i.e., ABSL-3, ABSL-4, and the high
containment facilities described for open penned or loose-housed animals in
Appendix D) are designed to minimize the risk of exposure to high-consequence
biological agents for workers, the community, and the environment.
59,60
See
Sections III, IV, V, and Appendix D for additional information. BSL-3 or BSL-4
researchers who participate in eld research or outbreak response involving
RG3 or RG4 pathogens may need additional occupational health services due to
increased exposure risks.
61
The same principles of incident and illness response outlined above apply to
potential hazard exposures and LAIs in a BSL-3 or BSL-4 laboratory environment,
but with an increased concern for public health and potential harm to society if
RG3 or RG4 agents were to be released, diverted, or intentionally misused. See
Section VI for additional information about laboratory biosecurity. A sta member
with access to RG3 or RG4 pathogens who develops an unexplained acute
febrile illness should seek medical consultation at the earliest onset of symptoms.

141Section VII—Occupational Health Support for Biomedical Research
Supervisory sta may encourage RG3 and RG4 agent researchers to contact the
designated medical provider in case of a possible LAI, rather than seeking care
from a community-based medical provider who may be less familiar with hazards
involved. Depending on risk, a fever watch for the duration of the incubation
period, with calls to the occupational health program in the event of a fever,
may be a useful component of institutional emergency preparedness. Advance
planning for appropriate care in case of an occupational exposure or possible
LAI is a fundamental component of an occupational health program supporting
research of RG3 or RG4 pathogens.
9
The designated medical provider may forge
liaisons with clinical programs capable of the requisite advanced level of care
for patients infected with high-consequence pathogens.
10,50,62
Incident and illness
response plans should also include timely and appropriate notication of local
health authorities as warranted by the circumstances in each case.
Conclusion
Occupational health support for a biomedical research community should
consist of select, expert services tailored to address the risks identied for the
individual sta member and the institution and commensurate with the scope
of work involving potential biological hazards. The strength of an occupational
health program supporting sta in laboratories or animal care facilities where
such biological materials are present depends on sound coordination with each
component of the institution’s occupational safety and health operations. The
occupational healthcare provider has a vital role in the health, safety, and security
of sta in the biomedical research environment and the establishment of a robust
culture of safety.
References
1. Schmitt JM. Occupational medicine in a biomedical research setting.
In: Wooley DP, Byers KB, editors. Biological Safety: Principles and
Practices. 5th ed. Washington (DC): ASM Press; 2017. p. 511–17.
2. Burnett LC. Developing a biorisk management program to support biorisk
management culture. In: Wooley DP, Byers KB, editors. Biological Safety:
Principles and Practices. 5th ed. Washington (DC): ASM Press; 2017.
p. 495–510.
3. Wooley DP, Fleming DO. Risk assessment of biological hazards. In: Wooley
DP, Byers KB, editors. Biological Safety: Principles and Practices. 5th ed.
Washington (DC): ASM Press; 2017. p. 95–104.
4. Centers for Disease Control and Prevention [Internet]. Atlanta (GA): The
National Institute for Occupational Safety and Health (NIOSH); c2015 [cited
2019 Mar 6]. Hierarchy of Controls. Available from: https://www.cdc.gov/
niosh/topics/hierarchy/default.html

142 Biosafety in Microbiological and Biomedical Laboratories
5. Wiedermann U, Garner-Spitzer E, Wagner A. Primary vaccine failure
to routine vaccines: Why and what to do?. Hum Vaccin Immunother.
2016;12(1):239–43.
6. Wilde H. Failures of post-exposure rabies prophylaxis. Vaccine.
2007;25(44):7605–9.
7. Schmitt JM, Wilson DE, Raber JM. Occupational Safety and Health.
In: Weichbrod RH, Thompson GA, Norton JN, editors. Management of
animal care and use programs in research, education, and testing. 2nd ed.
Boca Raton (FL): CRC Press; 2018. p. 279–318.
8. Delany JR, Pentella MA, Rodriguez JA, Shah KV, Baxley KP, Holmes
DE; Centers for Disease Control and Prevention. Guidelines for biosafety
and laboratory competency: CDC and the Association of Public Health
Laboratories. MMWR Suppl. 2011;60(2):1–23.
9. Federal Select Agent Program [Internet]. Atlanta (GA); Riverdale (MD):
Centers for Disease Control and Prevention; Animal and Plant Inspection
Service; c2018 [cited 2019 Mar 6]. Occupational Health Program. Available
from: https://www.selectagents.gov/ohp-intro.html
10. Jahrling P, Rodak C, Bray M, Davey RT. Triage and management of
accidental laboratory exposures to biosafety level-3 and -4 agents. Biosecur
Bioterror 2009;7(2):135–43.
11. Fischman ML, Goldstein DA, Cullen MR. Emerging Technologies.
In: Rosenstock L, Cullen MR, Brodkin CA, Redlich CA, editors. Textbook
of Clinical Occupational and Environmental Medicine. 2nd ed. Philadelphia:
Elsevier Saunders; 2005. p. 263–71.
12. Kim DK, Riley LE, Hunger P. Advisory Committee on Immunization Practices
recommended immunization schedule for adults aged 19 years or older—
United States, 2018. MMWR Morb Mortal Wkly Rep. 2018;67(5):158–60.
13. Centers for Disease Control and Prevention [Internet]. Atlanta (GA):
National Center for Immunization and Respiratory Disease; c2019 [cited
2019 Mar 6]. ACIP Vaccine Recommendations and Guidelines. Available
from: https://www.cdc.gov/vaccines/hcp/acip-recs/index.html
14. Centers for Disease Control and Prevention [Internet]. Atlanta (GA):
National Center for Emerging and Zoonotic Infectious Diseases, Division of
Healthcare Quality Promotion; c2016 [cited 2019 Mar 6]. Infection Control.
Available from: https://www.cdc.gov/infectioncontrol/index.html
15. Menckel EWA, Westerholm P, editors. Evaluation in occupational health
practice. 1st ed. Oxford: Butterworth-Heinemann; 1999.
16. Levy BS, Wegman DH, editors. Occupational Health: Recognizing and
Preventing Work-related Disease and Injury. 4th ed. Philadelphia: Lippincott
Williams & Wilkins; 2000.

143Section VII—Occupational Health Support for Biomedical Research
17. National Institutes of Health. NIH Guidelines for Research Involving
Recombinant or Synthetic Nucleic Acid Molecules (NIH Guidelines).
Bethesda (MD): National Institutes of Health, Oce of Science Policy; 2019.
18. Federal Select Agent Program [Internet]. Atlanta (GA); Riverdale (MD):
Centers for Disease Control and Prevention; Animal and Plant Health
Inspection Service; c2017 [cited 2019 Mar 6]. Select Agents Regulations.
Available from: https://www.selectagents.gov/regulations.html
19. Occupational Safety and Health Administration. Laboratory Safety Guidance.
OSHA 3404-11R. Washington (DC): U.S. Department of Labor; 2011.
20. Bloodborne pathogens, 29 C.F.R. Part 1910.1030 (1992).
21. Americans with Disabilities Act [Internet]. Washington (DC): Department
of Justice Civil Rights Division; [cited 2019 Mar 6]. Fighting Discrimination
in Employment Under the ADA. Available from: https://www.ada.gov/
employment.htm
22. U.S. Equal Employment Opportunity Commission [Internet]. Washington (DC):
Oce of Legal Counsel; c2015 [cited 2019 Mar 6]. Enforcement Guidance:
Pregnancy Discrimination and Related Issues. No. 915.003. Available from:
https://www.eeoc.gov/laws/guidance/pregnancy_guidance.cfm
23. U.S. Department of Health and Human Services [Internet]. Washington
(DC): Oce for Civil Rights Headquarters; c2017 [cited 2019 Mar 6].
Your Rights Under HIPAA. Available from: https://www.hhs.gov/hipaa/
for-individuals/guidance-materials-for-consumers/index.html
24. American Medical Association [Internet]. Chicago (IL): Ethics; c1995–2019
[2019 Mar 6]. Code of Medical Ethics: Privacy, condentiality & medical
records. Available from: https://www.ama-assn.org/delivering-care/ethics/
code-medical-ethics-privacy-condentiality-medical-records
25. National Institutes of Health [Internet]. Bethesda (MD): National Institute
of Allergy and Infectious Diseases; c2018 [cited 2019 Mar 6]. The Need
for Biosafety Labs. Available from: https://www.niaid.nih.gov/research/
biosafety-labs-needed
26. Wurtz N, Papa A, Hukic M, Di Caro A, Leparc-Goart I, Leroy E, et al.
Survey of laboratory-acquired infections around the world in biosafety level
3 and 4 laboratories. Eur J Clin Microbiol Infect Dis. 2016;35(8):1247–58.
27. Richards SL, Pompei VC, Anderson A. BSL-3 laboratory practices in the
United States: comparison of select agent and non-select agent facilities.
Biosecur Bioterror. 2014;12(1):1–7.
28. Condreay JP, Kost TA, Mickelson CA. Emerging considerations in virus-
based gene transfer systems. In: Wooley DP, Byers KB, editors. Biological
Safety: Principles and Practices. 5th ed. Washington (DC): ASM Press;
2017. p. 221–46.

144 Biosafety in Microbiological and Biomedical Laboratories
29. Howard J, Murashov V, Schulte P. Synthetic biology and occupational risk.
J Occup Environ Hyg. 2017;14(3):224–36.
30. Wooley DP. Molecular agents. In: Wooley DP, Byers KB, editors. Biological
Safety: Principles and Practices. 5th ed. Washington (DC): ASM Press;
2017. p. 269–83.
31. Schlimgen R, Howard J, Wooley D, Thompson M, Baden LR, Yang OO,
et al. Risks associated with lentiviral vectors exposures and prevention
strategies. J Occup Environ Med. 2016;58(12):1159–66.
32. National Institutes of Health [Internet]. Bethesda (MD): Oce of
Science Policy; [cited 2019 Mar 6]. Biosafety, Biosecurity, and
Emerging Biotechnology. Available from: https://osp.od.nih.gov/
biosafety-biosecurity-and-emerging-biotechnology/
33. National Research Council. Occupational Health and Safety in the Care
and Use of Research Animals. Washington (DC): National Academy Press;
1997.
34. Hankenson FC, Johnston NA, Weigler BJ, Di Giacomo RF. Zoonoses of
occupational health importance in contemporary laboratory animal research.
Comp Med. 2003;53(6):579–601.
35. Bailey C, Manseld K. Emerging and Reemerging Infectious Diseases
of Nonhuman Primates in the Laboratory Setting. Vet Pathol.
2010;47(3):462–81.
36. Willy ME, Woodward RA, Thornton VB, Wol AV, Flynn BM, Heath JL, et al.
Management of a measles outbreak among Old World nonhuman primates.
Lab Anim Sci. 1999;49(1):42–8.
37. Occupational Safety and Health Standards. Respiratory protection, 29
C.F.R. Sect. 1910.134 (2006).
38. OSHA Instruction. Hearing Conservation Program, PER 04-00-004 (2008).
39. Miller JM, Astles R, Baszler T, Chapin K, Carey R, Garcia L, et al.
Guidelines for safe work practices in human and animal medical diagnostic
laboratories. Recommendations of a CDC-convened, Biosafety Blue Ribbon
Panel. MMWR Suppl. 2012;61(1):1–102. Erratum in: MMWR Surveill Summ.
2012;61(12):214.
40. Centers for Disease Control and Prevention; Animal and Plant Health
Inspection Service. Incident Response Plan Guidance [Internet]. Federal
Select Agent Program; 2018 [cited 2019 Aug 9]. Available from:
https://www.selectagents.gov/resources/Incident_Response_Plan.pdf
41. Belk HD. Implementing continuous quality improvement in occupational
health programs. J Occup Med. 1990;32(12):1184–8.

145Section VII—Occupational Health Support for Biomedical Research
42. U.S. Department of Labor [Internet]. Washington (DC): Bureau of Labor
Statistics; c2013 [cited 2019 Mar 6]. Using workplace safety and health data
for injury prevention. Available from: https://www.bls.gov/opub/mlr/2013/
article/using-workplace-safety-data-for-prevention.htm
43. Peterson JS, Morland MA. Measuring biosafety program eectiveness.
In: Wooley DP, Byers KB, editors. Biological Safety: Principles and
Practices. 5th ed. Washington (DC): ASM Press; 2017. p. 519–36.
44. Advisory Committee on Immunization Practices; Centers for Disease
Control and Prevention (CDC). Immunization of Health-Care Personnel:
Recommendations of the Advisory Committee on Immunization Practices
(ACIP). MMWR Recomm Rep. 2011;60(RR-7):1–45.
45. Tuck MK, Chan DW, Chia D, Godwin AK, Grizzle WE, Krueger KE, et al.
Standard operating procedures for serum and plasma collection: early
detection research network consensus statement standard operating
procedure integration working group. J Proteome Res. 2009;8(1):113–7.
46. Occupational Safety and Health Administration Medical Screening and
Surveillance Requirements in OSHA Standards: A Guide. OSHA 3162-01R.
Washington (DC): U.S. Department of Labor; 2014.
47. Baker EL, Matte TP. Occupational Health Surveillance. In: Rosenstock
L, Cullen MR, Brodkin CA, Redlich CA, editors. Textbook of Clinical
Occupational and Environmental Medicine. 2nd ed. Philadelphia: Elsevier
Saunders; 2005. p. 76–82.
48. Koh D, Aw T-C. Surveillance in occupational health. Occup Environ Med.
2003;60:705–10.
49. Manno M, Sito F, Licciardi L. Ethics of biomonitoring for occupational health.
Toxicol Lett. 2014;231(2):111–21.
50. Rusnak JM, Kortepeter MG, Aldis J, Boudreau E. Experience in the medical
management of potential laboratory exposures to agents of bioterrorism on
the basis of risk assessment at the Unites States Army Medical Research
Institute of Infectious Diseases (USAMRIID). J Occup Environ Med.
2004;46(8):801–11.
51. Kuhar DT, Henderson DK, Struble KA, Heneine W, Thomas V, Cheever LW,
et al. Updated US Public Health Service Guidelines for the Management
of Occupational Exposures to Human Immunodeciency Virus and
Recommendations for Postexposure Prophylaxis. Infect Control Hosp
Epidemiol. 2013:34(9);875–92.
52. Byers KB, Harding AL. Laboratory-associated infections. In: Wooley DP,
Byers KB, editors. Biological Safety: Principles and Practices. 5th ed.
Washington (DC): ASM Press; 2017. p. 59–92.
146 Biosafety in Microbiological and Biomedical Laboratories
53. Kimman TG, Smit E, Klein MR. Evidence-Based Biosafety: a Review of the
Principles and Eectiveness of Microbiological Containment Measures. Clin
Microbiol Rev. 2008;21(3):403–25.
54. Siengsanan-Lamont J, Blacksell SD. A Review of Laboratory-Acquired
Infections in the Asia-Pacic: Understanding Risk and the Need for
Improved Biosafety for Veterinary and Zoonotic Diseases. Trop Med Infect
Dis. 2018;3(2). pii: E36.
55. Cohen JI, Davenport DS, Stewart JA, Deitchman S, Hilliard JK, Chapman
LE, et al. Recommendations for prevention of and therapy for exposure to B
virus (Cercopithecine herpesvirus 1). Clin Infect Dis 2002;35(10):1191–203.
56. Centers for Disease Control and Prevention. Fatal Laboratory-Acquired
Infection with an Attenuated Yersinia pestis Strain–Chicago, Illinois, 2009.
MMWR Morb Mortal Wkly Rep. 2011;60(7):201–5.
57. Sheets CD, Harriman K, Zipprich J, Louie JK, Probert WS, Horowitz M, et al.
Fatal Meningococcal Disease in a Laboratory Worker–California, 2012.
MMWR Morb Mortal Wkly Rep. 2014;63(35):770–2.
58. Crane JT, Richmond JY. Design of biomedical laboratory and specialized
biocontainment facilities. In: Wooley DP, Byers KB, editors. Biological
Safety: Principles and Practices. 5th ed. Washington (DC): ASM Press;
2017. p. 343–66.
59. Rusnak JM, Kortepeter MG, Hawley RJ, Anderson AO, Boudreau E, Eitzen
E. Risk of occupationally acquired illnesses from biological threat agents in
unvaccinated laboratory researchers. Biosecur Bioterror. 2004;2(4):281–93.
60. Bressler DS, Hawley RJ. Safety considerations in the biosafety level
4 maximum-containment laboratory. In: Wooley DP, Byers KB, editors.
Biological Safety: Principles and Practices. 5th ed. Washington (DC): ASM
Press; 2017. p. 695–717.
61. Kortepeter MG, Cieslak TJ, Kwon EH, Smith PW, Kratochvil CJ, Hewlett
AL. Comment on “Ebola virus infection among Western healthcare
workers unable to recall the transmission route.” Biomed Res Int.
2017;2017:7458242.
62. Risi GF, Bloom ME, Hoe NP, Arminio T, Carlson P, Powers T, et al.
Preparing a community hospital to manage work-related exposures to
infectious agents in biosafety level 3 and 4 laboratories, Emerg Infect Dis.
2010;16(3):373–8.

147Section VIII—Agent Summary Statements
Section VIII—Agent Summary Statements
The agent summary statements contained in Section VIII of the sixth edition of
Biosafety in Microbiological and Biomedical Laboratories (BMBL) are designed to
assist the reader with the risk assessment for their work, as directed in Section II.
The statements are assembled by subject matter experts and represent a
summary of key information regarding pathogens with signicance to the
biomedical community. Although the statements provide recommendations
regarding containment for specic activities, they should serve only as the starting
point for a laboratory’s risk assessment and should not serve as a substitute
for an assessment. The statements cannot fully factor in the change in risk due
to the size of a sample, concentration of agent present, change in virulence or
pathogenicity, nor any change in ability to provide medical countermeasures due
to antibiotic or antiviral resistance.
The following list of agents is also not comprehensive, and the reader is directed
to other information to assist in the risk assessment, including the Public Health
Agency of Canada’s Pathogen Safety Data Sheets (PSDS),
1
the American Public
Health Association’s Control of Communicable Diseases Manual,
2
American
Society for Microbiology Manual of Clinical Microbiology,
3
and the ABSA Interna-
tional Risk Group Database.
4
References
1. Government of Canada [Internet]. Canada: Public Health Agency of
Canada; c2018 [cited 2018 Dec 20]. Pathogen Safety Data Sheets.
Available from: https://www.canada.ca/en/public-health/services/laboratory-
biosafety-biosecurity/pathogen-safety-data-sheets-risk-assessment.html
2. Heymann DL, editor. Control of Communicable Diseases Manual. 20th ed.
Washington (DC): American Public Health Association; 2014.
3. Jorgensen JH, Pfaller MA, Carroll KC, Funke G, Landry ML, Richter SS,
et al, editors. Manual of Clinical Microbiology. 11th ed. Washington (DC):
American Society for Microbiology; 2015.
4. American Biological Safety Association [Internet]. ABSA International; c2018
[cited 2018 Dec 20]. Risk Group Database. Available from: https://my.absa.
org/tiki-index.php?page=Riskgroups
148 Biosafety in Microbiological and Biomedical Laboratories
Section VIII-A: Bacterial Agents
Bacillus anthracis
Bacillus anthracis, a Gram-positive, non-hemolytic, and non-motile bacillus,
is the etiologic agent of anthrax, an acute bacterial disease among wild and
domestic mammals, including humans. Like all members of the genus Bacillus,
under adverse conditions, B. anthracis has the ability to produce spores that
allow the organism to persist for long periods (i.e., years), withstanding heat and
drying, until the return of more favorable conditions for vegetative growth.
1
It is
because of this ability to produce spores coupled with signicant pathogenic
potential in humans that this organism is considered one of the most serious and
threatening biowarfare or bioterrorism agents.
2
Most mammals are susceptible
to anthrax; it mostly aects herbivores that ingest spores from contaminated soil
and, to a lesser extent, carnivores that scavenge on the carcasses of diseased
animals. In the United States, it occurs sporadically in animals in parts of the
West, Midwest, and Southwest. Human case rates for anthrax are highest in
Africa and central and southern Asia.
3
The infectious dose varies greatly from
species to species and is route-dependent. The inhalation anthrax infectious
dose (ID) for humans has been primarily extrapolated from inhalation challenges
of non-human primates (NHPs) or studies done in contaminated wool mills.
Estimates vary greatly but the median lethal dose (LD50) is likely within the range
of 2,500–55,000 spores.
4
It is believed that very few spores (ten or fewer) are
required for cutaneous anthrax infection.
5
Anthrax cases have been rare in the
United States since the rst half of the 20th century. The mortality rates have
been reported to be approximately 20% for cutaneous anthrax without antibiotics,
25–75% for gastrointestinal anthrax, and 80% or more for inhalation anthrax. With
treatment, <1% of cutaneous anthrax cases are fatal. The fatality rate of a series
of inhalation anthrax cases in 2001 was 36% with antibiotics.
6,7
Bacillus cereus
biovar anthracis, if inhaled, can produce symptoms similar to inhalation anthrax.
Rapid rule-out tests to dierentiate B. cereus biovar anthracis from other Bacillus
spp. are currently not available.
6
Occupational Infections
Occupational infections are possible when in contact with contaminated animals,
animal products, or pure cultures of B. anthracis, and may include ranchers,
veterinarians, and laboratory workers. Although numerous cases of laboratory-
associated anthrax (primarily cutaneous) were reported in earlier literature, in
recent years, cases of anthrax due to laboratory accidents have been rare in the
United States.
8,9
Natural Modes of Infection
The clinical forms of anthrax in humans that result from dierent routes of
infection include:
149Section VIII-A: Bacterial Agents
1. Cutaneous (via broken skin);
2. Gastrointestinal (via ingestion);
3. Inhalation anthrax;
10
and
4. Injection (to date, identied in heroin-injecting drug users in northern
Europe).
11,12
Cutaneous anthrax is the most common (> 95% of human cases worldwide) and
is a readily treatable form of the disease. While naturally occurring disease is no
longer a signicant public health problem in the United States, B. anthracis has
become a bioterrorism concern. In 2001, 22 people were diagnosed with anthrax
acquired from spores sent through the mail, including 11 cases of inhalation
anthrax with ve deaths and 11 cutaneous cases.
13
A report of accidental
shipment of live organisms highlights the importance of adherence to handling
guidelines.
14
The approach to prevention and treatment of anthrax diers from
that for other bacterial infections. When selecting post-exposure prophylaxis or
a combination of antimicrobial drugs for treatment of anthrax, it is recommended
to consider the production of toxin, the potential for antimicrobial drug resistance,
the frequent occurrence of meningitis, and the presence of latent spores.
15
Laboratory Safety and Containment Recommendations
B. anthracis may be present in blood, skin lesion exudates, cerebrospinal uid
(CSF), pleural uid, sputum, and rarely, in urine and feces.
12
Primary hazards to
laboratory personnel are: direct and indirect contact of broken skin with cultures
and contaminated laboratory surfaces, accidental parenteral inoculation and,
rarely, exposure to infectious aerosols. Spores are resistant to many disinfectants
and may remain viable on some surfaces for years.
BSL-3 practices, containment equipment, and facilities are recommended for
work involving production quantities or high concentrations of cultures, screening
environmental or unknown samples (especially powders) from anthrax-contami-
nated locations, diagnostics or suspected anthrax samples, and for activities with
a high potential for aerosol production. As soon as B. anthracis is suspected in
the sample, BSL-3 practices are recommended for further culture and analysis.
BSL-2 practices, containment equipment, and facilities are recommended for
primary inoculation of cultures from potentially infectious clinical materials.
ABSL-2 practices, containment equipment, and facilities are recommended for
studies utilizing experimentally infected laboratory rodents. It is recommended
that all centrifugation be performed using autoclavable, aerosol-tight rotors
or safety cups that are opened within the BSC after each run. In addition, it is
recommended to collect routine surveillance swabs for culture inside the rotor
and rotor lid and, if contaminated, it is recommended to autoclave rotors before
re-use.

150 Biosafety in Microbiological and Biomedical Laboratories
Special Issues
Be advised of possible misidentication using automated systems. For identi-
cation using MALDI-TOF MS, it is recommended to use alternative tube extraction
that kills viable organisms in the BSC, followed by ltration through a 0.1–0.2 um
lter to remove any remaining viable cells or spores, and not direct spotting of
plates in the open laboratory.
15,16
Vaccines Control of anthrax begins with control of the disease in livestock,
and vaccination of livestock has long been central to control programs. Human
anthrax is best controlled through prevention, including (a) pre-exposure
vaccination for persons at high-risk for encountering aerosolized B. anthracis
spores, (b) reduction of animal illness by vaccination of livestock at risk for
anthrax, and (c) environmental controls to decrease exposure to contaminated
animal products, such as imported hair and skins. After a person is exposed to
aerosolized B. anthracis spores, a combination of antimicrobials and vaccine
provides the best available protection.
17
A licensed vaccine for anthrax in humans
is available, the anthrax vaccine adsorbed (AVA). AVA is produced from the
protective antigen of an attenuated non-encapsulated strain of B. anthracis. The
vaccine is approved by the Food and Drug Administration (FDA) for at-risk adults
before exposure to anthrax. Guidelines for its use in occupational settings are
available from the ACIP.
18
CDC has reviewed and updated guidelines for anthrax
post-exposure prophylaxis and treatment.
17
Vaccination is not recommended for
workers involved in routine processing of clinical specimens or environmental
swabs in general clinical diagnostic laboratories. Of interest, Obiltoxaximab, a
novel monoclonal antibody directed against the protective antigen of B. anthracis,
which plays a key role in the pathogenesis of anthrax, has received approval
for treatment and prevention of inhalational anthrax.
19
Because of the limited
potential of antibiotic treatment once toxemia has already set in, numerous
strategies are being explored for therapy directed against the action of anthrax
toxins.
20
Select Agent B. anthracis and Bacillus cereus biovar anthracis are Select Agents
requiring registration with CDC and/or USDA for possession, use, storage and/or
transfer. See Appendix F for additional information.
Transfer of Agent Importation of this agent requires CDC and/or USDA impor-
tation permits. Domestic transport of this agent may require a permit from USDA
APHIS VS. A Department of Commerce (DoC) permit may be required for the
export of this agent to another country. See Appendix C for additional information.
Bordetella pertussis
Bordetella pertussis, an exclusively human respiratory pathogen of worldwide
distribution, is the etiologic agent of whooping cough or pertussis. The organism
151Section VIII-A: Bacterial Agents
is a fastidious, small, Gram-negative coccobacillus that requires specialized
culture and transport media for cultivation in the laboratory.
21
Alternatively,
infection may be diagnosed by molecular methodologies on a direct specimen.
Its natural habitat is the human respiratory tract.
Occupational Infections
Occupational transmission of pertussis has been reported, primarily among
healthcare workers.
22
Outbreaks, including secondary transmission, among
workers have been documented in hospitals, long-term care institutions, and
laboratories. Nosocomial transmission has been reported in healthcare settings
and laboratory-associated pertussis has also been documented.
23,24
Natural Modes of Infection
Pertussis is highly communicable, with person-to-person transmission occurring
via aerosolized respiratory secretions (droplets) containing the organism. The
attack rate among susceptible hosts is aected by the frequency, proximity, and
time of exposure to infected individuals; however, transmission rates to suscep-
tible contacts may be close to 90% with the infectious dose only around 100
CFU.
21
Although the number of reported pertussis cases declined by over 99%
following the introduction of vaccination programs in the 1940s, the incidence
of pertussis remains cyclical, with epidemic peaks occurring every three to ve
years within a given region.
25
In 2015, the World Health Organization reported
142,512 pertussis cases globally and estimated that there were 89,000 deaths
attributed to pertussis.
26
However, a recent publication modeling pertussis case
and death estimates proposed that there were 24.1 million pertussis cases and
160,700 deaths in children younger than ve years in 2014 worldwide.
27
Of
signicance, B. pertussis continues to circulate in populations despite high vacci-
nation of infants and children because protection wanes after several years.
28
Nevertheless, in vaccinating countries, although pertussis is primarily observed
in neonates, infections are found in under-vaccinated or unvaccinated individuals
of all ages, including young infants, older school children, adolescents, and
adults.
27–29
Adults and adolescents with atypical or undiagnosed B. pertussis
infections are a primary reservoir. Pertactin is an outer membrane protein and
virulence factor for B. pertussis, and it should be noted that pertactin-negative
strains may evade vaccine-mediated immunity.
30
Laboratory Safety and Containment Recommendations
The agent may be present in high levels in respiratory secretions and may be
found in other clinical material, such as blood and lung tissue.
31,32
Aerosol gener-
ation during the manipulation of cultures and contaminated clinical specimens
generate the greatest potential hazard. Direct contact is also a hazard with the
agent being able to survive a number of days on surfaces such as clothing.

152 Biosafety in Microbiological and Biomedical Laboratories
BSL-3 practices, containment equipment, and facilities are appropriate for
production operations. BSL-2 practices, containment equipment, and facilities
are recommended for all activities involving the use or manipulation of known
or potentially infectious clinical material and cultures. ABSL-2 practices and
containment equipment are recommended for housing experimentally infected
animals. Primary containment devices and equipment, including biological safety
cabinets, safety centrifuge cups, or sealed rotors are recommended for activities
likely to generate potentially infectious aerosols.
Special Issues
Vaccines A number of pertussis vaccines are available for infants, children,
preteens, teens, and adults. DTaP (Diphtheria/Tetanus/Pertussis) is the childhood
vaccine, and Tdap (Tetanus/Diphtheria/Pertussis) is the pertussis booster vaccine
for preteens, teens, and adults.
33
Transfer of Agent Importation of this agent requires CDC and/or USDA impor-
tation permits. Domestic transport of this agent may require a permit from USDA
APHIS VS. A DoC permit may be required for the export of this agent to another
country. See Appendix C for additional information.
Brucella species
The genus Brucella consists of slow-growing, very small, Gram-negative
coccobacilli whose natural hosts are mammals. The taxonomy of Brucella species
remains in ux; however, this genus currently includes 10 recognized species:
■
Six terrestrial
□
B. melitensis (preferred hosts: sheep, goats, and camels)
□
B. suis (preferred hosts: swine and other wild animals)
□
B. abortus (natural hosts: cattle and bualo)
□
B. canis (natural host: dogs)
□
B. ovis (natural host: rams)
□
B. neotomae (natural host: desert and wood rats)
■
Three marine
□
B. delphini
□
B. pinnipedialis
□
B. ceti
■
One proposed species of unknown origin.
34
High-risk species for human infections include Brucella abortus, B. melitensis,
and B. suis. There is a wide spectrum of clinical manifestations, and patients may
have an extended recovery period. Mortality is estimated to be less than 1%.
34,35
153Section VIII-A: Bacterial Agents
Occupational Infections
Brucellosis is a frequently reported Laboratory-associated infection.
34–38
Airborne
and mucocutaneous exposures can produce Laboratory-associated infections.
Many cases of laboratory-associated disease appear to be due to mishandling
and misidentication of the organism.
39
The need to improve compliance with
recommended guidelines was highlighted when 916 laboratory workers were
exposed to the RB51 vaccine strain, which is known to cause human illness,
due to mishandling of a prociency test sample.
41
Brucellosis is an occupational
disease for workers who handle infected animals or their tissues. Accidental
self-inoculation with vaccine strains is an occupational hazard for veterinarians
and other animal handlers.
Natural Modes of Infection
Brucellosis (Undulant fever, Malta fever, Mediterranean fever) is a zoonotic
disease of worldwide occurrence. Mammals, particularly cattle, goats, swine, and
sheep, act as reservoirs for Brucella spp. as animals are generally asymptomatic.
Multiple routes of transmission have been identied, including direct contact with
infected animal tissues or products, ingestion of contaminated milk, and airborne
exposure in animal pens and stables.
Laboratory Safety and Containment Recommendations
Brucella may be found in a wide variety of body tissues, including blood, CSF,
semen, pulmonary excretions, placenta, and occasionally urine. Most laboratory-
associated cases occur in research facilities and involve exposures to zoonotic
Brucella organisms grown in large quantities or exposure to placental tissues
containing zoonotic Brucella spp. Cases have also occurred in clinical laboratory
settings from sning bacteriological cultures or working on open benchtops.
42,43
Human infections are commonly attributed to exposure to aerosols or direct skin
contact with cultures or infectious animal specimens.
43,44
The infectious dose of
Brucella is 10–100 organisms by aerosol or subcutaneous routes in laboratory
animals.
45,46
Brucella spp. are environmentally stable, surviving days to months in
carcasses and organs, in soil and on surfaces.
45,46
BSL-3 practices, containment equipment, and facilities are recommended for all
manipulations of cultures of pathogenic Brucella spp. BSL-3 practices are recom-
mended when handling products of conception or clinical specimens suspected to
contain Brucella.
12
ABSL-3 practices are recommended for experimental animal
studies. BSL-2 practices, containment equipment, and facilities are recommended
for routine handling of clinical specimens of human or animal origin.

154 Biosafety in Microbiological and Biomedical Laboratories
Special Issues
Be advised of possible misidentication using automated systems. For identi-
cation using MALDI-TOF MS, it is recommended to use alternative tube extraction
that kills viable organisms and not direct spotting of plates in the open laboratory.
Vaccines Human Brucella vaccines have been developed and tested in other
countries with limited success.
49
Although a number of successful vaccines are
available for immunization of animals, no licensed human vaccines are currently
available. Some recently described ribosomal proteins and fusion proteins
demonstrate a protective eect against Brucella based on antibody and cell-me-
diated responses, which may prove useful in potential vaccines.
34
Select Agent Brucella abortus, B. melitensis, and B. suis are Select Agents
requiring registration with CDC and/or USDA for possession, use, storage and/or
transfer. See Appendix F for additional information.
Transfer of Agent Importation of this agent requires CDC and/or USDA impor-
tation permits. Domestic transport of this agent may require a permit from USDA
APHIS VS. A DoC permit may be required for the export of this agent to another
country. See Appendix C for additional information.
Burkholderia mallei
Burkholderia mallei is a non-motile, Gram-negative rod associated with glanders,
a disease primarily of equine species, but which can be seen in humans. While
endemic foci of infection exist in some areas of the world, glanders due to natural
infection is extremely rare in the United States with the last naturally occurring
case reported in 1934.
50
Reported mortality rates are over 90% if left untreated,
and up to 50% with treatment.
50
Occupational Infections
Glanders occurs almost exclusively among individuals who work with equine
species and/or handle B. mallei cultures in the laboratory. B. mallei can be very
infectious in the laboratory setting. The only reported case of human glanders in
the United States over the past 50 years resulted from a laboratory exposure.
51
Modes of transmission may include inhalation and/or mucocutaneous exposure.
Natural Modes of Infection
Glanders is a highly communicable disease of solipeds (such as horses, goats,
and donkeys). Zoonotic transmission occurs to humans, but person-to-person
transmission is rare. Glanders in solipeds and humans has been eradicated from
North America and Western Europe. However, sporadic infections of animals
are still reported in Far East Asia, South America, Eastern Europe, North Africa,
and the Middle East.
50
Clinical manifestations in humans include localized

155Section VIII-A: Bacterial Agents
infection, pulmonary infection, bacteremia, or chronic infection, characterized by
suppurative tissue abscesses. The organism is transmitted by direct invasion of
abraded or lacerated skin; inhalation with deep lung deposition; and by bacterial
invasion of the nasal, oral, and conjunctival mucous membranes. Occupational
exposures most often occur through exposed skin.
50
Laboratory Safety and Containment Recommendations
B. mallei can be hazardous in a laboratory setting. Laboratory-associated
infections have resulted from aerosol and cutaneous exposure. A laboratory-
associated infection in 2001 was the rst case of glanders reported in the United
States in over 50 years.
51,52
The ability of B. mallei to survive for up to 30 days
in water at room temperature should be a consideration in development and
implementation of safety, disinfection, and containment procedures for labora-
tories and animal facilities handling this agent.
BSL-3 and ABSL-3 practices, containment equipment, and facilities are recom-
mended for all manipulations of suspect cultures, animal necropsies, and for
experimental animal studies. BSL-3 practices are recommended for preparatory
work on cultures or contaminated materials for automated identication systems.
BSL-3 practices, containment equipment, and facilities are appropriate for
production operations. BSL-2 practices, containment equipment, and facilities
are recommended for primary inoculation of cultures from potentially infectious
clinical materials. Primary containment devices and equipment, including
biological safety cabinets, safety centrifuge cups, or sealed rotors are recom-
mended for activities likely to generate potentially infectious aerosols.
Special Issues
Be advised of possible misidentication using automated systems. For identi-
cation using MALDI-TOF MS, it is recommended to use alternative tube extraction
that kills viable organisms and not direct spotting of plates in the open laboratory.
Vaccines Vaccine research and development has been conducted, but there is
no available vaccine.
53
Select Agent B. mallei is a Select Agent requiring registration with CDC and/or
USDA for possession, use, storage and/or transfer. See Appendix F for additional
information.
Transfer of Agent Importation of this agent requires CDC and/or USDA impor-
tation permits. Domestic transport of this agent may require a permit from USDA
APHIS VS. A DoC permit may be required for the export of this agent to another
country. See Appendix C for additional information.
156 Biosafety in Microbiological and Biomedical Laboratories
Burkholderia pseudomallei
Burkholderia pseudomallei is a motile, Gram-negative, oxidase-positive rod that
is found in soil and water environments of equatorial regions, including Southeast
Asia, Northern Australia, Madagascar, Africa, India, China, Taiwan, Central
America, and South America.
54
This organism, the causative agent of melioidosis,
is capable of infecting both humans and animals. A recent study estimates the
global incidence of melioidosis is 165,000 cases with 89,000 deaths.
55
Occupational Infections
Melioidosis is a disease associated with activities that expose people to soil
and water such as rice farming or gardening; however, B. pseudomallei can be
hazardous for laboratory workers, with two possible cases of aerosol transmission
of melioidosis in laboratory sta.
56–58
Natural Modes of Infection
Natural modes of transmission usually occur through direct contact with an
environmental source (usually water or soil) by ingestion, percutaneous inocu-
lation, or inhalation of the organism. In endemic areas, a signicant number
of agricultural workers have positive antibody titers to B. pseudomallei in the
absence of overt disease.
59
Manifestations include localized disease, pulmonary
disease, bacteremia, and disseminated disease. Abscesses can be seen in a
variety of tissues and organs. However, the majority of persons exposed to this
organism do not develop clinical infection.
54
Latent infection with subsequent
reactivation is well recognized. Risk factors for contracting melioidosis include
diabetes, liver or renal disease, chronic lung disease, thalassemia, malignancy,
and immunosuppression.
54,60,61
Laboratory Safety and Containment Recommendations
B. pseudomallei can cause systemic disease in human patients. Infected tissues
and purulent drainage from cutaneous or tissue abscesses can be sources of
infection as can blood and sputum. The ability of B. pseudomallei to survive for
years in water (as well as soil) should be a consideration in development and
implementation of safety, disinfection, and containment procedures for labora-
tories and animal facilities handling this agent.
62,63
BSL-3 and ABSL-3 practices, containment equipment, and facilities are recom-
mended for all manipulations of suspect cultures, animal necropsies, and for
experimental animal studies. BSL-3 practices are recommended for preparatory
work on cultures or contaminated materials for automated identication systems.
BSL-3 practices, containment equipment, and facilities are appropriate for
production operations. BSL-2 practices, containment equipment, and facilities
are recommended for primary inoculation of cultures from potentially infectious
clinical materials.

157Section VIII-A: Bacterial Agents
Special Issues
Be advised of possible misidentication using automated systems. For identi-
cation using MALDI-TOF MS, it is recommended to use alternative tube extraction
that kills viable organisms and not direct spotting of plates in the open laboratory.
Select Agent B. pseudomallei is a Select Agent requiring registration with CDC
and/or USDA for possession, use, storage and/or transfer.
64
See Appendix F for
additional information.
Transfer of Agent Importation of this agent requires CDC and/or USDA impor-
tation permits. Domestic transport of this agent may require a permit from USDA
APHIS VS. A DoC permit may be required for the export of this agent to another
country. See Appendix C for additional information.
Campylobacter species
Campylobacters are curved, S-shaped, or spiral Gram-negative rods associated
with gastrointestinal infections, bacteremia, and sepsis. Organisms are isolated
from stool specimens using selective media, reduced oxygen tension, and
elevated incubation temperature (43°C) for some species, or they may be
detected by molecular testing of primary clinical specimens.
Occupational Infections
These organisms rarely cause Laboratory-associated infections (LAI), although
laboratory-associated cases have been documented.
65–67
Infected animals are
also a potential source of infection.
68
Natural Modes of Infection
Numerous domestic and wild animals, including poultry, pets, farm animals,
laboratory animals, and wild birds, are known reservoirs and are a potential
source of infection for laboratory and animal care personnel. While the infective
dose is not rmly established, ingestion of as few as 350–800 organisms has
caused symptomatic infection.
69–71
Natural transmission usually occurs from
ingestion of organisms in contaminated food such as poultry and milk products,
contaminated water, or from direct contact with infected pets and farm animals—
particularly exposure to cow manure.
72
Person-to-person transmission has been
documented.
73
Although the illness is usually self-limiting, relapses can occur in
untreated cases and in association with some immunocompromised conditions.
74
Although infection can be mild, signicant complications can occur in pregnant
women, including septic abortion.
75,76
Laboratory Safety and Containment Recommendations
Pathogenic Campylobacter spp. may occur in fecal specimens in large numbers.
C. fetus subsp. fetus may also be present in blood, exudates from abscesses,

158 Biosafety in Microbiological and Biomedical Laboratories
tissues, and sputa. Campylobacter spp. can survive for many weeks in water at
4°C. The primary laboratory hazards are ingestion and parenteral inoculation of
the organism. The signicance of aerosol exposure is not known.
BSL-2 practices, containment equipment, and facilities are recommended
for activities with cultures or potentially infectious clinical materials. ABSL-2
practices, containment equipment, and facilities are recommended for activities
with naturally or experimentally infected animals.
Special Issues
Transfer of Agent Importation of this agent requires CDC and/or USDA impor-
tation permits. Domestic transport of this agent may require a permit from USDA
APHIS VS. A DoC permit may be required for the export of this agent to another
country. See Appendix C for additional information.
Chlamydia psittaci, C. trachomatis, C. pneumoniae
Chlamydia psittaci, C. pneumoniae, and C. trachomatis are the three species of
Chlamydia known to infect humans. Alternative nomenclature may include the
names Chlamydophila pneumoniae and Chlamydophila psittaci. Chlamydiae are
non-motile, bacterial pathogens with obligate intracellular life cycles. These three
species of Chlamydia vary in host spectrum, pathogenicity, and in the clinical
spectrum of disease. C. psittaci is a zoonotic agent that commonly infects
psittacine (i.e., parrot family) birds and is highly pathogenic for humans. With
appropriate treatment, the mortality rate for C. psittaci is about 1%.
77–79
C. trachomatis is historically considered an exclusively human pathogen.
C. pneumoniae is considered the least pathogenic species, often resulting in
subclinical or asymptomatic infections in both animals and humans. Chlamydiae
have a biphasic life cycle: elementary bodies form the extracellular stage and
are infective, while the reticulate bodies are intracellular and replicate by binary
ssion in vacuoles.
78–80
Occupational Infections
Chlamydial infections caused by C. psittaci and C. trachomatis lymphogranuloma
venereum (LGV) strains were at one time among the commonly reported
laboratory-associated bacterial infections.
36,83
In cases reported before 1955, the
majority of infections were psittacosis, and these had the highest case fatality rate
of laboratory-associated infectious agents.
84
The major sources of laboratory-
associated psittacosis are contact with and exposure to infectious aerosols in
the handling, care, or the necropsy of naturally or experimentally infected birds.
Infected mice and eggs also are important sources of C. psittaci. Most reports
of Laboratory-associated infections with C. trachomatis attribute the infection
to inhalation of large quantities of aerosolized organisms during purication or
sonication procedures. Early reports commonly attributed infections to exposure
159Section VIII-A: Bacterial Agents
to aerosols formed during nasal inoculation of mice or inoculation of egg yolk
sacs and harvest of chlamydial elementary bodies. Infections are associated
with fever, chills, malaise, and headache; a dry cough is also associated with
C. psittaci infection. Some workers exposed to C. trachomatis have developed
conditions including mediastinal and supraclavicular lymphadenitis, pneumonitis,
conjunctivitis, and keratitis.
81,85
Seroconversion to chlamydial antigens is common
and often striking; however, early antibiotic treatment may prevent an antibody
response. Antibiotics are eective against chlamydial infections. A case of
Laboratory-associated infection attributed to inhalation of droplet aerosols with
C. pneumoniae has been reported.
86
There has been a report of an outbreak
attributed to exposure to equine fetal membranes.
87,88
With all species of
Chlamydia, occupational exposures that can lead to infection most often occur
through exposure to mucosal tissues in the eyes, nose, and respiratory tract.
Natural Modes of Infection
C. psittaci is the cause of psittacosis, a respiratory infection that can lead to
severe pneumonia requiring intensive care support and possible death. Sequelae
include endocarditis, hepatitis, abortion, and neurological complications.
78
Natural infections are acquired by inhaling dried secretions from infected birds.
Psittacine birds commonly kept as pets (e.g., parrots, parakeets, cockatiels) and
poultry are most frequently involved in transmission. C. trachomatis can cause
a spectrum of clinical manifestations including genital tract infections, inclusion
conjunctivitis, trachoma, pneumonia in infants, and LGV. The LGV strains cause
more severe and systemic disease than do genital strains. C. trachomatis genital
tract infections are sexually transmitted and ocular infections (trachoma) are
transmitted by exposure to secretions from infected persons through contact or
fomite transmission. C. pneumoniae is a common cause of respiratory infection;
up to 50% of adults have serologic evidence of previous exposure. Infections with
C. pneumoniae are transmitted by droplet aerosolization and are most often mild
or asymptomatic, although there is research on the possible association of this
agent with chronic diseases such as atherosclerosis, asthma, and others.
82,89
Laboratory Safety and Containment Recommendations
C. psittaci may be present in the tissues, feces, nasal secretions, and blood
of infected birds, and in the blood, sputum, and tissues of infected humans.
C. psittaci can remain infectious in the environment for months and on dry,
inanimate surfaces for 15 days.
90
C. trachomatis may be present in genital,
bubo, and conjunctival uids of infected humans. Exposure to infectious aerosols
and droplets, created during the handling of infected birds and tissues, are
the primary hazards to laboratory personnel working with C. psittaci.
91,92
The
primary laboratory hazards of C. trachomatis and C. pneumoniae are accidental
parenteral inoculation and direct and indirect exposure of mucous membranes
of the eyes, nose, and mouth to genital, bubo, or conjunctival uids, cell culture

160 Biosafety in Microbiological and Biomedical Laboratories
materials, and uids from infected cell cultures or eggs. Infectious aerosols,
including those that may be created as a result of centrifugation, also pose a risk
for infection.
BSL-3 practices and containment equipment are recommended for activities
involving work with cultures, specimens, or clinical isolates known to contain or
be potentially infected with the LGV serovars (L1 through L3) of C. trachomatis.
BSL-3 practices, containment equipment, and facilities are indicated for activities
with high potential for droplet or aerosol production and for activities involving
large quantities or concentrations of infectious materials.
BSL-3 practices, containment equipment, and facilities are also recommended
for activities involving the necropsy of infected birds and the diagnostic
examination of tissues or cultures known to contain or be potentially infected
with C. psittaci strains of avian origin. Wetting the feathers of infected birds
with a detergent-disinfectant prior to necropsy can appreciably reduce the risk
of aerosols of infected feces and nasal secretions on the feathers and external
surfaces of the bird. ABSL-3 practices, containment equipment, and facilities and
respiratory protection are recommended for personnel working with naturally or
experimentally infected caged birds.
Activities involving non-avian strains of C. psittaci may be performed in a BSL-2
facility as long as BSL-3 practices are followed. Laboratory work with the LGV
serovars of C. trachomatis can be conducted in a BSL-2 facility as long as BSL-3
practices are followed when handling potentially infectious materials.
BSL-2 practices, containment equipment, and facilities are recommended
for personnel working with clinical specimens and cultures or other materials
known or suspected to contain the ocular or genital serovars of C. trachomatis
or C. pneumoniae. ABSL-2 practices, containment equipment, and facilities are
recommended for activities with animals that have been experimentally infected
with genital serovars of C. trachomatis or C. pneumoniae.
Special Issues
C. trachomatis genital infections are reportable infectious diseases.
Vaccines There are no human vaccines against Chlamydia spp.
Transfer of Agent Importation of this agent requires CDC and/or USDA impor-
tation permits. Domestic transport of this agent may require a permit from USDA
APHIS VS. A DoC permit may be required for the export of this agent to another
country. See Appendix C for additional information.

161Section VIII-A: Bacterial Agents
Clostridium botulinum and neurotoxin-producing species of Clostridia
Clostridium botulinum, and rare strains of C. baratii and C. butyricum, are
anaerobic, spore-forming, Gram-positive bacilli that cause botulism, a life-threat-
ening foodborne illness. The pathogenicity of these organisms results from the
production of botulinum toxin under anaerobic conditions in which C. botulinum
spores germinate. Please refer to Botulinum neurotoxins in Section VIII-G for
biosafety guidance in handling toxin preparations.
Laboratory Safety and Containment Recommendations
Neurotoxin producing Clostridia species or its toxin may be present in a variety
of food products, clinical materials (serum, feces), and environmental samples
(soil, surface water) handled in the laboratory.
93
In addition, bacterial cultures
may produce very high levels of toxin.
94
In healthy adults, it is typically the
toxin and not the organism that causes disease. Risk of laboratory exposure
is primarily due to the presence of the toxin, as opposed to infection from the
organism that produces the toxin. Toxin exposure may occur through ingestion,
contact with non-intact skin or mucosal membranes, or inhalation. Although
spore-forming, there is no known risk from spore exposure except for the
potential presence of residual toxin associated with pure spore preparations. It is
recommended to use laboratory safety protocols that focus on the prevention of
accidental exposure to the toxin produced by these Clostridia species.
BSL-3 practices and containment are recommended for activities with a high
potential for aerosol or droplet production or for those requiring routine handling
of larger quantities of the organism or toxin. ABSL-2 and BSL-2 practices,
containment equipment, and facilities are recommended for diagnostic studies
and titration of toxin. Before the collection of specimens, it is recommended to
call the designated public health laboratory regarding any case of suspected
botulism for guidance on diagnosis, treatment, specimen collection, and
investigation.
95
BSL-2 practices, containment equipment, and facilities are
recommended for activities that involve the organism or the toxin including the
handling of potentially contaminated food.
96
Special Issues
Select Agent Neurotoxin-producing Clostridia species are Select Agents requiring
registration with CDC and/or USDA for possession, use, storage and/or transfer.
See Appendix F for additional information. See the C. botulinum Toxin Agent
Summary Statement in Section VIII-G and Appendix I for additional information.
Transfer of Agent Importation of this agent requires CDC and/or USDA impor-
tation permits. Domestic transport of this agent may require a permit from USDA
APHIS VS. A DoC permit may be required for the export of this agent or its toxin
to another country. See Appendix C for additional information.
162 Biosafety in Microbiological and Biomedical Laboratories
Clostridioides (formerly Clostridium) dicile
Clostridioides (formerly Clostridium) dicile is a Gram-positive, spore-forming,
obligate anaerobic bacillus, and it is the most common cause of infectious
diarrhea in hospitalized patients.
97
The incidence of infection in the United States
has increased dramatically since 2000. There were a half a million cases and
29,000 deaths reported in the United States in 2011.
98
Increases in incidence
have also been observed worldwide.
99
Clinical presentations range from
asymptomatic colonization to mild self-limiting diarrhea to fulminant pseudomem-
branous colitis, toxic megacolon, and multi-organ failure, requiring emergency
colectomy.
100
Because individuals may be asymptomatically colonized with
toxigenic or non-toxigenic strains of C. dicile, testing in the clinical diagnostic
laboratory may involve one of several one, two, or three-step algorithms in an
attempt to optimize sensitivity and specicity. Tests include enzyme immuno-
assays for free toxin or glutamate dehydrogenase, toxigenic culture, and nucleic
acid amplication tests for toxin.
101
Occupational Infections
There is a report of laboratory-associated C. dicile infection based on a clinical
laboratory survey,
102
but cases are rare.
Natural Modes of Infection
Transmission is primarily via the fecal-oral route through hand-to-hand contact.
Airborne environmental dispersal is also a route of transmission.
103,104
Most
infections present during or shortly after a course of antimicrobial therapy, which
disrupts the intestinal microbial composition, permitting C. dicile colonization
and toxin production. Clindamycin, other macrolides, third-generation cephalo-
sporins, penicillins, and uoroquinolones are frequently associated with
C. dicile infection.
105
Between 20–35% of patients fail initial therapy, and
60% of patients with multiple prior recurrences will fail subsequent therapy.
Fecal transplantation has become a successful therapeutic option for many
patients.
106,107
Asymptomatic colonization in neonates and infants (<2 years)
is quite common. There is concern for an increasing incidence in children
beyond this age.
108
C. dicile virulence factors include the exotoxins TcdA and
TcdB, which bind to receptors on epithelial cells. NAP1, PCR ribotype 027 is a
hypervirulent strain of Clostridioides dicile, which also contains a binary toxin
(CDT) and a deletion in the tcdC gene that aects the production of toxins.
100
It
is characterized by high-level uoroquinolone resistance, ecient sporulation,
enhanced cytotoxicity, and high toxin production. There is an associated higher
mortality rate, as patients are more likely to develop life-threatening complica-
tions.
109,110
Infection or asymptomatic carriage can also occur in domestic, farm,
and wild animals. C. dicile can be recovered from retail meats.
104

163Section VIII-A: Bacterial Agents
Laboratory Safety and Containment Recommendations
Infectious fecal specimens are the most common C. dicile-containing specimens
received in the laboratory. Endospores of C. dicile are impervious to desiccation,
temperature uctuations, freezing, irradiation, and many antiseptic solutions,
including alcohol-based gels and quaternary ammonium-based agents.
106
Spores
can survive in the environment for months to years.
104
Guidelines are available
for management of healthcare-associated infections due to C. dicile and for
cleaning to reduce the spread of the organism.
111
BSL-2 practices, containment equipment, and facilities are recommended for
all activities utilizing known or potentially infected clinical materials or cultures.
ABSL-2 facilities are recommended for studies utilizing infected laboratory
animals.
Special Issues
Transfer of Agent Importation of this agent requires CDC and/or USDA impor-
tation permits. Domestic transport of this agent may require a permit from USDA
APHIS VS. A DoC permit may be required for the export of this agent to another
country. See Appendix C for additional information.
Clostridium tetani and Tetanus toxin
Clostridium tetani is an anaerobic, endospore-forming, Gram-positive rod
found in the soil and is an intestinal tract commensal. It produces a potent
neurotoxin, tetanospasmin, which causes tetanus, an acute neurologic condition
characterized by painful muscular contractions. Tetanospasmin is an exceedingly
potent protein toxin that consists of a heavy chain subunit that binds the toxin to
receptors on neuronal cells and a light chain subunit that blocks the release of
inhibitory neural transmitter molecules within the central nervous system. The
incidence of tetanus in the United States has declined steadily since the intro-
duction of tetanus toxoid vaccines in the 1940s.
112,113
Occupational Infections
Although the risk of infection to laboratory personnel is low, there have been
some incidents of laboratory personnel exposure recorded.
84,114
Natural Modes of Infection
Contamination of wounds by soil is the usual mechanism of transmission for
tetanus. Of the 233 cases of tetanus reported to CDC from 1998 through 2000,
acute injury (puncture, laceration, abrasion) was the most frequent predisposing
condition. Elevated incidence rates also were observed for persons aged over
60 years, diabetics, and intravenous drug users.
112,113
When introduced into a
suitable anaerobic or microaerophilic environment, C. tetani spores germinate

164 Biosafety in Microbiological and Biomedical Laboratories
and produce tetanospasmin. The incubation period ranges from three to 21 days.
The observed symptoms are primarily associated with the presence of the toxin.
Wound cultures are not generally useful for diagnosing tetanus.
95,115
Tetanus is
a medical emergency and immediate treatment with human tetanus immune
globulin is indicated.
113
Laboratory Safety and Containment Recommendations
The organism may be found in soil, intestinal, or fecal samples. Accidental
parenteral inoculation of the toxin is the primary hazard to laboratory personnel.
Because it is uncertain if tetanus toxin can be absorbed through mucous
membranes, the hazards associated with aerosols and droplets remain unclear.
BSL-2 practices, containment equipment, and facilities are recommended for
activities involving the manipulation of cultures or toxins. ABSL-2 practices,
containment equipment, and facilities are recommended for animal studies.
Special Issues
Vaccines It is recommended that vaccination status be considered in a risk
assessment for work with this organism and/or toxin. While the risk of laboratory-
associated tetanus is low, vaccination is recommended for some following risk
assessment, and review of the current recommendations of the ACIP.
116
Transfer of Agent Importation of this agent or its toxin may require CDC and/or
USDA importation permits. Domestic transport of this agent may require a permit
from USDA APHIS VS. A DoC permit may be required for the export of this agent
to another country. See Appendix C for additional information.
Corynebacterium diphtheriae
Corynebacterium diphtheriae is a pleomorphic, Gram-positive rod that is isolated
from the nasopharynx and skin of humans. The organism will grow on media
containing 5% sheep blood, but it is recommended that primary plating include
one selective agar such as cysteine-tellurite blood agar or fresh Tinsdale
media incubated in 5% CO2-enriched atmosphere to separate from normal oral
ora.
117
C. diphtheriae produces a potent exotoxin and is the causative agent of
diphtheria, one of the most widespread bacterial diseases of the pre-vaccine era.
The exotoxin gene is found on the beta-corynebacteriophage, which can infect
non-toxigenic strains of C. ulcerans or C. pseudotuberculosis, leading to the
production of toxin by these species.
118
Occupational Infections
Laboratory-associated infections with C. diphtheriae have been documented.
84,119
Zoonotic infections with C. diphtheriae have not been recorded. C. ulcerans is
a zoonotic pathogen that has been cultured from untreated milk and companion
animals and infrequently associated with toxic infections in humans.
120,121

165Section VIII-A: Bacterial Agents
Inhalation, accidental parenteral inoculation, and ingestion are the primary
laboratory hazards.
Natural Modes of Infection
The agent may be present in exudates or secretions of the nose, throat (tonsil),
pharynx and larynx, in wounds, blood, and on the skin. C. diphtheriae can be
present for weeks to months in the nasopharynx and skin lesions of infected
individuals and for a lifetime in asymptomatic individuals. C. diphtheriae can
survive for up to six months on dry inanimate surfaces. Travel to endemic areas
or close contact with persons who have returned recently from such areas
increases risk.
122
Transmission usually occurs via direct contact with patients or
carriers, and more rarely, with articles such as clothing contaminated with secre-
tions from infected people. Naturally occurring diphtheria is characterized by the
development of grayish-white, membranous lesions involving the tonsils, pharynx,
larynx, or nasal mucosa. Systemic sequelae are associated with the production of
diphtheria toxin, and the toxic dose of diphtheria toxin in humans is <100 ng per
kg body weight.
123
An eective vaccine is available for diphtheria, and this disease
has become a rarity in countries with vaccination programs.
Laboratory Safety and Containment Recommendations
BSL-2 practices, containment equipment, and facilities are recommended for
all activities utilizing known or potentially infected clinical materials or cultures.
ABSL-2 facilities are recommended for studies utilizing infected laboratory
animals.
Special Issues
Vaccines A licensed vaccine is available. The reader is advised to consult the
current recommendations of the ACIP.
124
While the risk of laboratory-associated
diphtheria is low, the administration of an adult diphtheria-tetanus toxoid at
ten-year intervals may further reduce the risk of illness to laboratory and animal
care personnel.
124
Transfer of Agent Importation of this agent requires CDC and/or USDA
importation permits. A DoC permit may be required for the export of this agent to
another country. See Appendix C for additional information.
Francisella tularensis
Francisella tularensis is a small, Gram-negative coccobacillus that infects
numerous animal species, especially lagomorphs (including rabbits); it is the
causal agent of tularemia (Rabbit fever, Deer y fever, Ohara disease, or
Francis disease) in humans. F. tularensis can be divided into three subspecies:
F. tularensis (Type A), F. holarctica (Type B), and F. mediasiatica. F. tularensis
subsp. novicida is now considered to be a separate species and referred to as

166 Biosafety in Microbiological and Biomedical Laboratories
F. novicida. Type A and Type B strains are highly infectious, requiring only 10–50
organisms to cause disease, and are the main cause of tularemia worldwide.
125
The overall fatality rate of infections is <2%, but can be up to 24% for particular
strains.
126
Person-to-person transmission of tularemia has not been documented.
The incubation period varies with the virulence of the strain, dose, and route of
introduction, but ranges from 1–14 days with most cases exhibiting symptoms in
three to ve days.
127
Symptoms include sudden fever, chills, headaches, diarrhea,
muscle aches, joint pain, dry cough, and progressive weakness, with possible
development of pneumonia. Other symptoms may include skin or mouth ulcers,
swollen and painful lymph nodes, sore throat, and swollen, painful eyes.
Occupational Infections
Tularemia has been a commonly reported laboratory-associated bacterial
infection.
84,128
Most cases have occurred at facilities involved in tularemia
research; however, cases have been reported in diagnostic laboratories as well.
Occasional cases are linked to work with naturally or experimentally infected
animals or their ectoparasites.
Natural Modes of Infection
Arthropod bites (e.g., tick, deer y, horse y, mosquito), handling or ingesting
infectious animal tissues or uids, ingestion of contaminated water or food, and
inhalation of infective aerosols are the primary transmission modes in nature.
Occasionally, infections have occurred from bites or scratches by carnivores with
contaminated mouthparts or claws.
Laboratory Safety and Containment Recommendations
The agent may be present in lesion exudates, respiratory secretions, CSF, blood
or lymph node aspirates from patients, tissues from infected animals, uids from
infected animals, and uids from infected arthropods. Direct contact of skin or
mucous membranes with infectious materials, accidental parenteral inoculation,
ingestion, and exposure to aerosols and infectious droplets have resulted in
infection. Infection has been more commonly associated with cultures than with
clinical materials and infected animals.
128
According to the Public Health Agency
of Canada’s (PHAC) Pathogen Safety Data Sheet for F. tularensis, the agent
can survive for months to years in carcasses, organs, and straw. Additional
information is available at https://www.canada.ca/en/public-health/services/
laboratory-biosafety-biosecurity/pathogen-safety-data-sheets-risk-assessment/
francisella-tularensis-material-safety-data-sheets-msds.html.
BSL-3 and ABSL-3 practices, containment equipment, and facilities are recom-
mended for all manipulations of suspect cultures, animal necropsies, and for
experimental animal studies. BSL-3 practices are recommended for preparatory
work prior to the use of automatic instruments that involves manipulation of

167Section VIII-A: Bacterial Agents
cultures. Characterized strains of reduced virulence such as LVS and SCHU
S4ΔclpB can be handled with BSL-2 practices. F. novicida strains can also be
handled with BSL-2 practices. BSL-2 practices, containment equipment, and
facilities are recommended for initial activities involving clinical materials of
human or animal origin suspected to contain F. tularensis.
Special Issues
Be advised of possible misidentication using automated systems. For identi-
cation of samples suspected of containing F. tularensis using MALDI-TOF MS, it
is recommended to use alternative tube extraction that kills viable organisms and
not direct spotting of plates in the open laboratory.
Vaccines A vaccine for tularemia is under review by the Food and Drug Adminis-
tration and is not currently available in the United States.
130
Select Agent F. tularensis is a Select Agent requiring registration with CDC
and/or USDA for possession, use, storage and/or transfer. See Appendix F for
additional information.
Transfer of Agent Importation of this agent requires CDC and/or USDA impor-
tation permits. Domestic transport of this agent may require a permit from USDA
APHIS VS. A DoC permit may be required for the export of this agent to another
country. See Appendix C for additional information.
Helicobacter species
Helicobacter species are spiral or curved, Gram-negative rods isolated from
gastrointestinal and hepatobiliary tracts of mammals and birds. There are
currently 37 recognized species, including at least 14 isolated from humans.
Helicobacter pylori is the main cause of peptic ulcer disease and a major risk
factor for gastric cancer. The main habitat of H. pylori is the human gastric
mucosa. Other Helicobacter spp. (H. cinaedi, H. canadensis, H. canis,
H. pullorum, and H. fennelliae) may cause asymptomatic infection as well as
proctitis, proctocolitis, enteritis and extraintestinal infections in humans.
131
Prevalence of H. pylori infection is decreasing worldwide, but infection is higher
in certain ethnic groups and in migrants.
132
Occupational Infections
Both experimental and accidental LAIs with H. pylori have been reported.
133,134
Ingestion is the primary known laboratory hazard. The importance of aerosol
exposures is unknown.
Natural Modes of Infection
Chronic gastritis and duodenal ulcers are associated with H. pylori infection.
Epidemiologic associations have also been made with gastric adenocarcinoma.
135

168 Biosafety in Microbiological and Biomedical Laboratories
Human infection with H. pylori may be long in duration with few or no symptoms
or may present as an acute gastric illness. Transmission, while incompletely
understood, is thought to be by the fecal-oral or oral-oral route.
Laboratory Safety and Containment Recommendations
H. pylori may be present in gastric and oral secretions and stool. The enterohe-
patic Helicobacter spp. (e.g., H. canadensis, H. canis, H. cinaedi, H. fennelliae,
H. pullorum, and H. winghamensis) may be isolated from stool specimens,
rectal swabs, and blood cultures.
131
It is recommended to incorporate processes
for containment of potential aerosols or droplets into procedures involving
homogenization or vortexing of gastric specimens.
136
BSL-2 practices, containment equipment, and facilities are recommended for
activities with clinical materials and cultures known to contain or potentially
contain the Helicobacter spp. ABSL-2 practices, containment equipment,
and facilities are recommended for activities with experimentally or naturally
infected animals.
Special Issues
Transfer of Agent Importation of this agent requires CDC and/or USDA impor-
tation permits. Domestic transport of this agent may require a permit from USDA
APHIS VS. A DoC permit may be required for the export of this agent to another
country. See Appendix C for additional information.
Legionella pneumophila and other Legionella spp.
Legionella spp. are small, faintly staining, Gram-negative bacteria. They are
obligately aerobic, slow-growing, nonfermentative organisms that have a unique
requirement for L-cysteine and iron salts for in vitro growth. Legionellae are
readily found in natural aquatic bodies and some species (L. longbeachae) have
been recovered from soil.
137,138
They are able to colonize hot-water tanks at a
temperature range from 40 to 50°C. There are currently 59 known Legionella
species, three subspecies, and over 70 distinct serogroups of Legionella. While
30 species are known to cause human infection, the most frequent cause of
human infection is L. pneumophila serogroup 1.
137
Occupational Infections
Although laboratory-associated cases of legionellosis have not been reported
in the literature, at least one case due to presumed aerosol or droplet exposure
during animal challenge studies with L. pneumophila has been recorded.
139
There has been one reported case of probable human-to-human transmission of
Legionella spp.
140

169Section VIII-A: Bacterial Agents
Natural Modes of Infection
Legionella is commonly found in environmental sources, typically in man-made,
warm water systems. The mode of transmission from these reservoirs is aerosol-
ization, aspiration, or direct inoculation into the airway.
137
Legionella spp. may be
present in amoebae from contaminated water. Legionella spp. have the ability to
persist outside of hosts in biolms, surviving for months in distilled water and for
over a year in tap water.
141
The spectrum of illness caused by Legionella species
ranges from a mild, self-limited, u-like illness (Pontiac fever) to a disseminated
and often fatal disease characterized by pneumonia and respiratory failure
(Legionnaires’ disease). Although rare, Legionella has been implicated in cases
of sinusitis, cellulitis, pericarditis, and endocarditis.
138
Legionellosis may be either
community-acquired or nosocomial. Risk factors include smoking, chronic lung
disease, and immunosuppression. Surgery, especially involving transplantation,
has been implicated as a risk factor for nosocomial transmission.
Laboratory Safety and Containment Recommendations
The agent may be present in respiratory tract specimens (i.e., sputum, pleural
uid, bronchoscopy specimens, lung tissue) and in extrapulmonary sites.
A potential hazard may exist for the generation of aerosols containing high
concentrations of the agent.
For activities likely to produce extensive aerosols or when large quantities of
Legionella spp. are manipulated, BSL-2 with BSL-3 practices are recommended.
BSL-2 practices, containment equipment, and facilities are recommended
for all activities involving materials or cultures suspected or known to contain
Legionella spp.
ABSL-2 practices, containment equipment, and facilities are recommended for
activities with experimentally-infected animals. Routine processing of environ-
mental water samples for Legionella may be performed with standard BSL-2
practices.
Special Issues
Transfer of Agent Importation of this agent requires CDC and/or USDA impor-
tation permits. Domestic transport of this agent may require a permit from USDA
APHIS VS. A DoC permit may be required for the export of this agent to another
country. See Appendix C for additional information.
Leptospira
The genus Leptospira is composed of spiral-shaped bacteria with hooked ends.
Leptospires are ubiquitous in nature; they are either free-living in freshwater or
associated with renal infection in animals. Historically, these organisms have
been classied into pathogenic (L. interrogans) and saprophytic (L. biexa)
170 Biosafety in Microbiological and Biomedical Laboratories
groups, but recent studies have identied more than 21 species based on genetic
analysis, nine of which are denitive pathogens.
142
These organisms also have
been characterized serologically, with more than 200 pathogenic and 60 sapro-
phytic serovars identied.
142
These organisms are the cause of leptospirosis, a
zoonotic disease of worldwide distribution. Growth of leptospires in the laboratory
requires specialized media and culture techniques, and cases of leptospirosis are
usually diagnosed by serology.
Occupational Infections
Leptospirosis is a well-documented, laboratory hazard. In older literature, 70 LAIs
and ten deaths have been reported.
36,84
Direct and indirect contact with uids and
tissues of experimentally or naturally infected mammals during handling, care,
or necropsy are potential sources of infection.
143,144
A laboratory-associated case
caused by percutaneous exposure to broth cultures of Leptospira was reported
in 2004.
145
It is important to remember that rodents are natural carriers of lepto-
spires. Animals with chronic renal infection shed large numbers of leptospires in
the urine continuously or intermittently for long periods of time. Leptospira spp.
may persist for weeks in soil contaminated with infected urine. Rarely, infection
may be transmitted by bites of infected animals.
143
Natural Modes of Infection
Human leptospirosis typically results from direct contact with infected animals,
contaminated animal products, or contaminated water sources. Common routes
of infection are abrasions, cuts in the skin or via the conjunctiva. Higher rates of
infection are observed in agricultural workers and workers in other occupations
associated with animal contact. Human-to-human transmission is rare. Leptospi-
rosis can cause the following symptoms: fever, headache, chills, muscle aches,
vomiting, jaundice, red eyes, abdominal pain, diarrhea, and rash. After an initial
phase of illness, the patient may recover, then become ill again with another
more severe phase that can involve kidney failure, liver failure, or meningitis
(Weil’s Disease).
146
Laboratory Safety and Containment Recommendations
The organism may be present in urine, blood, and tissues of infected animals
and humans. Asymptomatic infection may occur in carrier animals and humans.
Ingestion, parenteral inoculation, and direct and indirect contact of skin or
mucous membranes, particularly the conjunctiva, with cultures or infected
tissues or body uids are the primary laboratory hazards. The importance of
aerosol exposure is unclear, but occasional cases of inhalation of droplets of
urine or water have been suspected.
147
BSL-2 practices, containment equipment, and facilities are recommended for
all activities involving the use or manipulation of known or potentially infective

171Section VIII-A: Bacterial Agents
tissues, body uids, and cultures. ABSL-2 practices are recommended for the
housing and manipulation of infected animals.
Special Issues
Transfer of Agent Importation of this agent requires CDC and/or USDA impor-
tation permits. Domestic transport of this agent may require a permit from USDA
APHIS VS. A DoC permit may be required for the export of this agent to another
country. See Appendix C for additional information.
Listeria monocytogenes
Listeria monocytogenes is a Gram-positive, catalase-positive, non-spore
forming, aerobic bacillus that is weakly beta-hemolytic on sheep blood agar.
148
The organism has been isolated from soil, animal feed (silage), and a wide
range of human foods and food processing environments. It may also be
isolated from symptomatic/asymptomatic animals (particularly ruminants) and
humans.
149
This organism is the causative agent of listeriosis, a foodborne
disease of humans and animals.
Occupational Infections
Cutaneous listeriosis, characterized by pustular or papular lesions on the arms
and hands, has been described in veterinarians and farmers.
150
Asymptomatic
carriage has been reported in laboratorians.
151
Natural Modes of Infection
Most human cases of listeriosis result from eating contaminated foods, notably
soft cheeses, ready-to-eat meat products (e.g., hot dogs, luncheon meats),
pâté, and smoked sh/seafood.
149
Listeriosis can present in healthy adults with
symptoms of fever and gastroenteritis; pregnant women and their fetuses;
newborns; and persons with impaired immune function are at greatest risk of
developing severe infections including sepsis, meningitis, and fetal demise.
In pregnant women, L. monocytogents infections occur most often in the third
trimester and may precipitate labor. Transplacental transmission of L. monocyto-
genes poses a grave risk to the fetus.
152
Laboratory Safety and Containment Recommendations
Listeria monocytogenes may be found in feces, CSF, and blood, as well as
numerous food and environmental samples.
149
L. monocytogenes is somewhat
heat-resistant, can tolerate (and replicate in) cold temperatures, can survive at
low pH conditions, and can be resistant to some disinfectants such as quaternary
ammonium compounds.
153,154
Naturally or experimentally infected animals are
a source of exposure to laboratory workers, animal care personnel, and other
animals. While ingestion is the most common route of exposure, Listeria can also
cause eye and skin infections following direct contact with the organism.

172 Biosafety in Microbiological and Biomedical Laboratories
BSL-2 practices, containment equipment, and facilities are recommended when
working with clinical specimens and cultures known or suspected to contain
Listeria. ABSL-2 practices, containment equipment, and facilities are recom-
mended for activities involving experimentally or naturally infected animals. Due
to potential risks to the fetus, it is recommended that pregnant women be advised
of the risk of exposure to L. monocytogenes.
Special Issues
Transfer of Agent Importation of this agent requires CDC and/or USDA impor-
tation permits. Domestic transport of this agent may require a permit from USDA
APHIS VS. A DoC permit may be required for the export of this agent to another
country. See Appendix C for additional information.
Mycobacterium leprae
Mycobacterium leprae is a Gram-positive bacterium and is the causative agent
of leprosy, also called Hansen’s disease. M. leprae are intracellular bacteria
that cannot be cultured using laboratory medium. Bacteria can be recovered
from infected tissues and propagated in laboratory animals, specically the
nine-banded armadillo. M. lepromatosis are related bacteria that have now been
identied to cause similar disease.
155
Occupational Infections
There are no cases of occupational acquisition of M. leprae reported as a result
of working in a laboratory or being in contact with clinical materials of human or
animal origin.
Natural Modes of Infection
Leprosy is transmitted from person-to-person following prolonged exposure,
presumably via contact with respiratory secretions from infected individuals or
animals. Naturally-occurring leprosy has been reported in armadillos, with both
humans and armadillos recognized as reservoirs for infection.
156,157
Although
transmission from armadillos to humans has not been denitively proven, it is
likely since contact with armadillos is a signicant risk factor for acquisition of
human disease.
158,159
Cases in the United States have recently been seen in
Texas, Florida, and Louisiana.
160,161
Endemic animal forms of the disease have
been described due to related organisms.
162
Laboratory Safety and Containment Recommendations
M. leprae may be present in tissues and exudates from lesions of infected
humans and experimentally or naturally infected animals. Direct contact of the
skin and mucous membranes with infectious materials and parenteral inoculation
are the primary potential laboratory hazards associated with handling infectious
clinical materials.

173Section VIII-A: Bacterial Agents
Selection of an appropriate disinfectant is an important consideration for labora-
tories working with mycobacteria. See Appendix B for additional information.
BSL-2 practices, containment equipment, and facilities are recommended for all
activities with known or potentially infectious materials from humans and animals.
It is recommended to use extraordinary care to avoid accidental parenteral
inoculation with contaminated sharp instruments. ABSL-2 practices, containment
equipment, and facilities are recommended for animal studies utilizing rodents,
armadillos, and NHPs.
Special Issues
Transfer of Agent Importation of this agent requires CDC and/or USDA impor-
tation permits. Domestic transport of this agent may require a permit from USDA
APHIS VS. A DoC permit may be required for the export of this agent to another
country. See Appendix C for additional information.
Mycobacterium tuberculosis complex
The Mycobacterium tuberculosis complex includes the species M. tuberculosis,
M. bovis, M. africanum, M. caprae, M. microti, M. canettii, M. pinnipedii, and the
recently described species M. mungi and M. orygis.
163,164
M. tuberculosis grows
slowly, typically requiring several weeks for formation of colonies on solid media.
Incubation in broth culture can at times reduce the incubation time to less than
one week if the inoculum is sucient.
163
The organism has a thick, lipid-rich cell
wall that renders bacilli resistant to harsh treatments including alkali and deter-
gents. Mycolic acid in the cell wall results in a positive acid-fast stain.
Occupational Infections
M. tuberculosis and M. bovis infections are a proven hazard to laboratory
personnel and others who may be exposed to infectious aerosols in the
laboratory, autopsy rooms, and other healthcare facilities.
36,84,165–169
The incidence
of tuberculosis in health care personnel working with M. tuberculosis-infected
patients has been reported to be signicantly higher than that of those not
working with the agent.
170
Multidrug-resistant (MDR) and extensively drug-
resistant (XDR) strains are of particular concern.
109,171
Naturally or experimentally
infected NHPs are a proven source of human infection.
172
Experimentally-infected
guinea pigs and mice do not pose the same hazard because droplet nuclei are
not produced by coughing in these species; however, litter from infected animal
cages may become contaminated and serve as a source of infectious aerosols.
Natural Modes of Infection
M. tuberculosis is the etiologic agent of tuberculosis, a leading cause of morbidity
and mortality worldwide. Infectious aerosols produced by coughing spread
disease from person to person. Some individuals will develop active disease

174 Biosafety in Microbiological and Biomedical Laboratories
within months of infection, and some of those will clear the infection completely.
Others will achieve immunological control with latent (but viable) organisms, with
potential for reactivation later upon immunosuppression. Approximately 5–10%
of latent infections progress to active infections. The primary focus of infection
is the lungs, but extra-pulmonary disease does occur, primarily in immunocom-
promised individuals. Miliary (disseminated) tuberculosis has the most serious
consequences with meningitis developing in 50% of cases, along with a high
fatality rate if not treated eectively. HIV infection is a serious risk factor for the
development of active disease. M. bovis is primarily found in animals but can
also infect humans. It is spread to humans, primarily children, by consumption of
non-pasteurized milk and dairy products, by handling of infected carcasses, or by
inhalation. Human-to-human transmission of M. bovis via aerosols is possible.
Laboratory Safety and Containment Recommendations
Tubercle bacilli may be present in sputum, gastric lavage uids, CSF, urine,
and in a variety of tissues. Exposure to laboratory-generated aerosols is the
most important laboratory hazard encountered. Tubercle bacilli may survive in
heat-xed smears and, if present, may be aerosolized in the preparation of frozen
tissue sections.
171
Because of the low infective dose of M. tuberculosis (<10
bacilli), it is recommended that sputa and other clinical specimens from suspected
or known cases of tuberculosis be considered potentially infectious and handled
with appropriate precautions. Mycobacteria can be resistant to disinfection
and may survive on inanimate surfaces for long periods. Needlesticks are also
a recognized hazard. Selection of an appropriate disinfectant is an important
consideration for laboratories working with mycobacteria. See Appendix B for
additional information.
BSL-3 practices, containment equipment, and facilities are recommended for
laboratory activities in the propagation and manipulation of cultures of any of
the subspecies of the M. tuberculosis complex. Use of a slide-warming tray,
rather than a ame, is recommended for xation of slides. ABSL-3 practices
are recommended for animal studies using experimentally or naturally infected
NHPs or immunocompromised mice, as high titers may be found in organs from
immunocompromised animals. Animal studies using rodents (e.g., guinea pigs,
rats, rabbits, mice) can be conducted at ABSL-2 with ABSL-3 practices.
174
All
airborne infections of rodents using M. tuberculosis must be performed in an
appropriate ABSL-3 laboratory.
BSL-2 practices and procedures, containment equipment, and facilities are
recommended for non-aerosol-producing manipulations of clinical specimens.
Manipulation of small quantities of the attenuated vaccine strain M. bovis Bacillus
Calmette-Guérin (BCG) can be performed at BSL-2 in laboratories that do not

175Section VIII-A: Bacterial Agents
culture M. tuberculosis and do not have BSL-3 facilities. However, considerable
care is suggested to verify the identity of the strain and to ensure that cultures are
not contaminated with virulent M. tuberculosis or other M. bovis strains.
Special Issues
Be advised of possible misidentication using automated systems. For
identication using MALDI-TOF MS, it is recommended to use alternative tube
extraction that kills viable organisms in the BSC, and not direct spotting of plates
in the open laboratory.
Surveillance Annual or semi-annual skin testing with puried protein derivative
(PPD) or FDA-approved Interferon-Gamma Release Assay (IGRA) of previously
skin-test-negative personnel can be used as a surveillance procedure.
175
Vaccines The attenuated live BCG is available and used in other countries but is
not generally recommended for use in the United States.
Transfer of Agent Importation of this agent requires CDC and/or USDA impor-
tation permits. Domestic transport of this agent may require a permit from USDA
APHIS VS. A DoC permit may be required for the export of this agent to another
country. See Appendix C for additional information.
Mycobacterium spp. other than M. tuberculosis complex and M. leprae
There are over 150 Mycobacterium species including both slowly and rapidly
growing species.
163
In the past, mycobacterial isolates that were not identied
as M. tuberculosis complex were often called atypical mycobacteria, but these
are now more commonly referred to as nontuberculous mycobacteria (NTM) or
mycobacteria other than tuberculosis (MOTT). The majority of mycobacterial
species are common environmental organisms. There has been a perceived
increase in NTM isolated from hospitalized patients over the past 20 years.
176,177
Approximately 25 species are associated with human infections, with a number
of additional species associated with infections in immunocompromised
persons.
178
All of these species are considered opportunistic pathogens in
humans, and they are not considered generally communicable; however, there
is evidence of transmission between some individuals with chronic diseases.
179
The most common types of infections and causes are:
1. Pulmonary disease with a clinical presentation resembling tuberculosis
caused by M. kansasii, M. avium, and M. intracellulare;
2. Lymphadenitis associated with M. avium, M. scrofulaceum, and other
rapidly growing mycobacteria;
180
3. Disseminated infections in immunocompromised individuals caused by
M. avium and M. intracellulare;

176 Biosafety in Microbiological and Biomedical Laboratories
4. Pulmonary infection or colonization of patients with cystic brosis
caused by M. avium complex, M. kansasii, M. abscessus, and other
rapidly growing mycobacteria;
181,182
and
5. Skin ulcers and soft tissue wound infections including Buruli ulcer
caused by M. ulcerans, granulomas caused by M. marinum associated
with exposure to organisms in freshwater and saltwater and sh tanks,
and tissue infections resulting from trauma or surgical procedures
caused by M. fortuitum, M. chelonae, and M. abscessus.
Occupational Infections
A Laboratory-associated infection with Mycobacterium spp. other than M. tuber-
culosis complex was reported when a laboratory worker injected bacteria into his
thumb while performing experiments on mice.
183
Natural Modes of Infection
Person-to-person transmission is not considered common, but there is
evidence for transmission in some populations.
179
Presumably, pulmonary
infections are most often the result of inhalation of aerosolized bacilli,
most likely from the surface of contaminated water. Mycobacteria are
widely distributed in the environment and in animals, and zoonoses have
occurred.
184,185
They are also common in potable water supplies, perhaps as
the result of the formation of biolms.
Laboratory Safety and Containment Recommendations
Various species of mycobacteria may be present in sputa, exudates from
lesions, tissues, and in environmental samples. Mycobacteria can be resistant to
disinfection and survive on inanimate surfaces and for long periods in natural and
tap water sources. Direct contact of skin or mucous membranes with infectious
materials, ingestion, and parenteral inoculation are the primary laboratory
hazards associated with clinical materials and cultures. Aerosols created during
the manipulation of broth cultures or tissue homogenates of these organisms also
pose a potential infection hazard.
BSL-2 practices, containment equipment, and facilities are recommended for
activities with clinical materials and cultures of Mycobacterium other than
M. tuberculosis complex. Clinical specimens may also contain M. tuberculosis
and laboratory workers are advised to exercise caution to ensure the correct
identication of mycobacterial isolates. Special caution is recommended in
handling M. ulcerans and M. marinum to avoid skin exposure. ABSL-2 practices,
containment equipment, and facilities are recommended for animal studies.
Selection of an appropriate tuberculocidal disinfectant is an important consider-
ation for laboratories working with mycobacteria. See Appendix B for additional
information.

177Section VIII-A: Bacterial Agents
Special Issues
Transfer of Agent Importation of this agent requires CDC and/or USDA impor-
tation permits. Domestic transport of this agent may require a permit from USDA
APHIS VS. A DoC permit may be required for the export of this agent to another
country. See Appendix C for additional information.
Neisseria gonorrhoeae
Neisseria gonorrhoeae is a Gram-negative, oxidase-positive diplococcus
associated with gonorrhea, a sexually transmitted disease of humans. The
organism may be isolated from clinical specimens and cultivated in the laboratory
using specialized growth media.
186
Infection is often diagnosed using molecular
methods on direct clinical specimens.
Occupational Infections
Laboratory-associated gonococcal infections have been reported in the United
States and elsewhere.
187–189
These infections have presented as conjunctivitis,
with either direct nger-to-eye contact or exposure to splashes of either liquid
cultures or contaminated solutions proposed as the most likely means of
transmission.
Natural Modes of Infection
Gonorrhea is a sexually transmitted disease of worldwide importance. The 2016
rate of reported infection for this disease in the United States was 145.8 per
100,000 population, a steady increase from a low of 98.1 infections per 100,000
population recorded in 2009.
191
The natural mode of infection is through direct
contact with exudates from mucous membranes of infected individuals. This
usually occurs by sexual activity, although newborns may also become infected
during birth.
186
Laboratory Safety and Containment Recommendations
The agent may be present in conjunctival, urethral and cervical exudates,
synovial uid, urine, feces, blood, and CSF. Parenteral inoculation and direct
or indirect contact of mucous membranes with infectious clinical materials are
known primary laboratory hazards. Laboratory-associated illness due to aerosol
transmission has not been documented.
Additional primary containment and personnel precautions such as those
described for BSL-3 may be indicated when there is high risk of aerosol or droplet
production and for activities involving production quantities or high concentrations
of infectious materials. BSL-2 practices, containment equipment, and facilities
are recommended for all activities involving the use or manipulation of clinical
materials or cultures. Animal studies may be performed at ABSL-2.

178 Biosafety in Microbiological and Biomedical Laboratories
Special Issues
Neisseria gonorrhoeae has gained resistance to several classes of antimicrobials
over the last few decades, making the organism increasingly dicult to treat.
Fluoroquinolones, oral cephalosporins such as cexime, and doxycycline are no
longer recommended for treatment of uncomplicated gonorrhea. An extensively
drug-resistant (XDR) strain has been reported and is being monitored, and
currently, there are no other eective treatments for XDR gonorrhea.
192
Transfer of Agent Importation of this agent requires CDC and/or USDA impor-
tation permits. Domestic transport of this agent may require a permit from USDA
APHIS VS. A DoC permit may be required for the export of this agent to another
country. See Appendix C for additional information.
Neisseria meningitidis
Neisseria meningitidis is a Gram-negative diplococcus which can cause serious
invasive bacterial infections, with clinical manifestations including serious
acute meningitis and septicemia in humans. Virulence is associated with the
expression of a polysaccharide capsule. Among the thirteen dened N. menin-
gitidis capsular serogroups, six are the main causes of invasive meningococcal
disease (serogroups A, B, C, W, X and Y). The handling of N. meningitidis
isolates, particularly from sterile body sites, and/or clinical specimens containing
live N. meningitidis may increase the risk of transmission for microbiologists.
193
Occupational Infections
Manipulating suspensions of N. meningitidis outside a BSC is associated with
a high risk for contracting meningococcal disease.
193,194
Microbiologists have
been shown to have a much higher infection rate compared to that of the United
States’ general population aged 30–59 years, and a case fatality rate of 50%—
substantially higher than the 12–15% associated with disease among the general
population. Almost all the microbiologists identied as having an LAI had manip-
ulated invasive N. meningitidis isolates on an open laboratory bench.
195
Rigorous
protection from droplets or aerosols (including the use of a BSC) is recommended
when microbiological procedures are performed on all N. meningitidis isolates.
Although there are some molecular assays that can detect N. meningitidis directly
in clinical specimens, cultures are still routinely performed.
Natural Modes of Infection
The human upper respiratory tract is the natural reservoir for N. meningitidis.
Invasion of organisms from the respiratory mucosa into the circulatory system
causes infection that can range in severity from subclinical to fulminant fatal
disease. Transmission occurs from person-to-person and is usually mediated by
direct contact with respiratory droplets from infected individuals.

179Section VIII-A: Bacterial Agents
Laboratory Safety and Containment Recommendations
N. meningitidis may be present in pharyngeal exudates, CSF, blood, saliva,
sterile body sites (most commonly CSF and blood), and in rare cases, urine or
urethral (genital) discharge. Parenteral inoculation, droplet exposure of mucous
membranes, infectious aerosol generation and ingestion are the primary hazards
to laboratory personnel. Based on the mechanism of natural infection and the risk
associated with the handling of isolates on an open laboratory bench, exposure
to droplets or aerosols of N. meningitidis is the most likely risk for infection in
the laboratory. Although N. meningitidis does not survive well outside of a host,
the organism is able to survive on plastic and glass from hours to days at room
temperature.
BSL-3 practices and procedures are indicated for activities with a high potential
for droplet or aerosol production and for activities involving production quantities
or high concentrations of infectious materials. BSL-2 practices, containment
equipment, and facilities are recommended for handling bacterial cultures and
inoculation of clinical materials. It is recommended to handle all N. meningitidis
cultures within a BSC. ABSL-2 conditions are recommended for animal studies.
Special Issues
Vaccines For protection against N. meningitidis serogroups A, C, Y, and W-135,
there are commercially available polysaccharide and conjugate vaccines. These
are recommended to be administered to otherwise healthy children in adoles-
cence with a booster in late adolescence.
193
Recently, a meningococcal serogroup
B vaccine has become available. Both vaccines are necessary for full protection
as one does not confer immunity for the other.
196
Vaccination with both vaccines is
recommended for laboratorians who handle live bacteria and may be exposed to
N. meningitidis.
193,197,198
Transfer of Agent Importation of this agent requires CDC and/or USDA impor-
tation permits. Domestic transport of this agent may require a permit from USDA
APHIS VS. A DoC permit may be required for the export of this agent to another
country. See Appendix C for additional information.
Salmonella serotypes, other than S. enterica serotype Typhi (S. Typhi)
Salmonellae are Gram-negative, enteric bacteria associated with diarrheal illness
in humans. They are motile oxidase-negative organisms that are easily cultivated
on standard bacteriologic media, although enrichment and selective media may
be required for isolation from clinical specimens. Salmonellae can easily be
isolated using selective and dierential media or may be detected by molecular
testing of primary clinical specimens. Taxonomic studies have organized this
genus into two species, S. enterica and S. bongori, containing more than 2,500
antigenically distinct serotypes.
199,200
S. enterica contains the vast majority of
180 Biosafety in Microbiological and Biomedical Laboratories
serotypes associated with human disease. S. enterica serotypes Typhimurium
and Enteritidis are the serotypes most frequently encountered in the United
States. This summary statement covers all serotypes except S. Typhi.
Occupational Infections
Salmonellosis is a documented hazard to laboratory personnel.
114,201–204
Primary
reservoir hosts include a broad-spectrum of domestic and wild animals, including
birds, mammals, and reptiles, all of which may serve as a source of infection to
laboratory personnel. Case reports of LAIs indicate a presentation of symptoms
similar to those of naturally-acquired infections.
205
Natural Modes of Infection
Salmonellosis is a foodborne disease of worldwide distribution. An estimated one
million foodborne cases of salmonellosis occur annually in the United States,
and the global burden of non-typhoidal disease is estimated to be 94 million
cases and 155,000 deaths annually.
206–208
A wide range of domestic and feral
animals (e.g., poultry, swine, rodents, cattle, iguanas, turtles, chicks, dogs, cats,
and others) may serve as reservoirs for this disease, as well as humans.
209,210
Some human carriers shed the bacteria for years and some patients recovering
from S. enterica infections may shed the bacteria for months. Animals can
also have a latent or carrier state with long-term shedding of the bacteria. The
most common mode of transmission is by ingestion of food from contaminated
animals or contamination during processing. The disease usually presents
as acute enterocolitis (fever, severe diarrhea, abdominal cramping), with an
incubation period ranging from six to 72 hours, most often lasting four to seven
days and patients tend to recover without treatment. Antimicrobial therapy is not
recommended for uncomplicated Salmonella-related gastroenteritis.
206
Bacte-
remia occurs in 3–10% of individuals infected with S. enterica. Antimicrobial
resistance of Salmonella spp. is becoming a problem worldwide, and this is a
concern for invasive disease.
211
Laboratory Safety and Containment Recommendations
The agent may be present in feces, blood, urine, food, feed, and environmental
materials. Some Salmonella spp. may survive for long periods in food, feces,
water, and on surfaces. Ingestion and parenteral inoculations are the primary
laboratory hazards. Naturally or experimentally infected animals are a potential
source of infection for laboratory and animal care personnel and for other animals.
BSL-2 practices, containment equipment, and facilities are recommended for
activities using clinical materials and diagnostic quantities of infectious cultures.
It is recommended that special emphasis be placed on personal protective
equipment, handwashing, manipulation of faucet handles, and decontamination of
work surfaces to decrease the risk of LAI. For work involving production quantities
or high concentrations of cultures, and for activities with a high potential for

181Section VIII-A: Bacterial Agents
aerosol production, it is recommended that a BSC be used and that centrifugation
be performed using autoclavable, aerosol-tight rotors and safety cups. ABSL-2
facilities and practices are recommended for activities with experimentally
infected animals.
199
Special Issues
Vaccines Human vaccines against non-typhoidal strains are not available.
212
Transfer of Agent Importation of this agent requires CDC and/or USDA impor-
tation permits. Domestic transport of this agent may require a permit from USDA
APHIS VS. A DoC permit may be required for the export of this agent to another
country. See Appendix C for additional information.
Salmonella enterica serotype Typhi (S. Typhi)
The genus Salmonella is divided into two species, S. enterica and S. bongori,
containing more than 2,500 antigenically distinct subtypes or serotypes.
200
S. enterica contains the vast majority of serotypes associated with human
disease. S. enterica serotype Typhi, commonly designated S. Typhi, is the
causative agent of typhoid fever. Untreated case mortality for typhoid fever is
>10%.
213
S. Typhi is a motile, Gram-negative, enteric bacterium that is easily
cultivated on standard bacteriologic media, although enrichment and selective
media may be required for isolation of this organism from clinical materials.
S. Typhi can easily be isolated using selective and dierential media, or it may
be detected by molecular testing of primary clinical specimens. S. enterica
serotype Paratyphi (S. Paratyphi) is also considered a typhoidal serovar causing
a similar illness.
Occupational Infections
Typhoid fever is a demonstrated hazard to laboratory personnel and students
working with S. Typhi in teaching laboratories with many Laboratory-associated
infections and several resulting fatalities being reported.
84,114,203
Ingestion and, less
frequently, parenteral inoculation are the most signicant modes of transmission
in the laboratory. Secondary transmission to other individuals outside of the
laboratory is also a concern. Laboratory-associated S. Typhi infections usually
present with headache, abdominal pain, high fever, and possible septicemia.
203
Natural Modes of Infection
Typhoid fever is a serious, potentially lethal, bloodstream infection associated
with sustained high fever and headaches. It is common in the developing world
with 25 million infections and >200,000 deaths annually but rare in the United
States with only 400 cases annually.
214–216
Less than 1% of cases in the U.S. are
lethal, and these cases are often associated with foreign travel. Humans are the
sole reservoir, and asymptomatic carriers may occur. The infectious dose is low

182 Biosafety in Microbiological and Biomedical Laboratories
(<1000 organisms), and the incubation period may vary from one to six weeks
depending upon the dose of the organism. The natural mode of transmission
is by ingestion of food or water contaminated by feces or urine of patients or
asymptomatic carriers.
199,206
Antimicrobial resistance of S. Typhi is a signicant
global concern.
217
Laboratory Safety and Containment Recommendations
The agent may be present in feces, blood, bile, and urine. Humans are the only
known natural reservoir of infection. Ingestion and parenteral inoculation of the
organism represent the primary laboratory hazards. The importance of aerosol
exposure in previous cases is not known. To avoid possible secondary trans-
mission related to contaminated surfaces and clothing in teaching laboratories,
the use of nonpathogenic strains is recommended.
BSL-3 practices and equipment are recommended for activities likely to produce
signicant aerosols or for activities involving production quantities of organisms.
BSL-2 practices, containment equipment, and facilities are recommended for
activities using clinical materials and diagnostic quantities of infectious cultures.
It is recommended that special emphasis be placed on personal protective
equipment, handwashing, manipulation of faucet handles, and decontamination of
work surfaces to decrease the risk of LAI.
It is recommended that centrifugation be performed using autoclavable aerosol-
tight rotors or safety cups. ABSL-2 facilities, practices, and equipment are
recommended for activities with experimentally infected animals.
Special Issues
Vaccines Vaccines for S. Typhi are available and it is recommended that
personnel regularly working with potentially infectious materials consider
vaccination. The reader is advised to consult the current recommendations of the
Advisory Committee on Immunization Practices (ACIP).
218
Transfer of Agent Importation of this agent requires CDC and/or USDA impor-
tation permits. Domestic transport of this agent may require a permit from USDA
APHIS VS. A DoC permit may be required for the export of this agent to another
country. See Appendix C for additional information.
Shiga toxin (Verocytotoxin)-producing Escherichia coli
Escherichia coli (E. coli) is one of six species in the Gram-negative genus Esche-
richia. This organism is a common inhabitant of the bowel ora of healthy humans
and other mammals and is one of the most extensively studied prokaryotes.
An extensive serotyping system has been developed for E. coli based on the
O (somatic) and H (agellar) antigens expressed by these organisms. Certain
183Section VIII-A: Bacterial Agents
pathogenic clones of E. coli may cause urinary tract infections, bacteremia,
meningitis, and diarrheal disease in humans, and these clones are associated
with specic serotypes.
199
The diarrheagenic E. coli strains have been characterized into at least ve basic
pathogenicity groups: Shiga toxin (Verocytotoxin)-producing E. coli (a subset are
referred to as enterohemorrhagic E. coli), enterotoxigenic E. coli, enteropatho-
genic E. coli, enteroinvasive E. coli, and enteroaggregative E. coli.
199
In addition
to clinical signicance, E. coli strains are routinely used as hosts for cloning
experiments and other genetic manipulations in the laboratory. This summary
statement only provides recommendations for safe manipulation of Shiga
toxin-producing E. coli strains.
Occupational Infections
Shiga toxin-producing E. coli strains, including strains of serotype O157:H7,
are a demonstrated hazard to laboratory personnel with the majority of reported
Laboratory-associated infections being caused by enterohemorrhagic E. coli.
219–223
Sources of infection include ingestion from contaminated hands and contact
with infected animals. The infectious dose is estimated to be low, similar to that
reported for Shigella spp., at 10–100 organisms.
223
Natural Modes of Infection
Cattle represent the most common natural reservoir of Shiga toxin-producing
E. coli, but it has also been detected in wild birds and rodents in close proximity
to farms.
224
Transmission usually occurs by ingestion of contaminated food,
including raw milk, fruits, vegetables, and particularly ground beef. Human-
to-human transmission has been observed in families, daycare centers, and
custodial institutions. Waterborne transmission has been reported from outbreaks
associated with swimming in a crowded lake and drinking unchlorinated municipal
water.
225–227
E. coli has the ability to survive from hours to months on inanimate
surfaces. In a small number of patients (usually children) infected with these
organisms, the disease progresses to hemolytic uremic syndrome or death.
Laboratory Safety and Containment Recommendations
Shiga toxin-producing E. coli are usually isolated from feces. However, a variety
of food specimens contaminated with the organisms including uncooked ground
beef, unpasteurized dairy products, and contaminated produce may present
laboratory hazards. This agent may also be found in blood or urine specimens
from infected humans or animals. Ingestion is the primary laboratory hazard. The
importance of aerosol exposure is not known.
BSL-2 practices, containment equipment, and facilities are recommended for
activities using clinical materials and diagnostic quantities of infectious cultures.

184 Biosafety in Microbiological and Biomedical Laboratories
It is recommended that special emphasis be placed on personal protective
equipment, handwashing, manipulation of faucet handles, and decontamination of
work surfaces to decrease the risk of LAI. For work involving production quantities
or high concentrations of cultures, and for activities with a high potential for
aerosol production, it is recommended that a BSC be used and that centrifugation
be performed using autoclavable aerosol-tight rotors and safety cups. ABSL-2
facilities and practices are recommended for activities with experimentally
infected animals.
Special Issues
Transfer of Agent Importation of this agent requires CDC and/or USDA impor-
tation permits. Domestic transport of this agent may require a permit from USDA
APHIS VS. A DoC permit may be required for the export of this agent to another
country. See Appendix C for additional information.
Shigella
The genus Shigella is composed of non-motile, Gram-negative bacteria in the
family Enterobacteriaceae. There are four subgroups that have been historically
treated as separate species including: subgroup A (Shigella dysenteriae),
subgroup B (S. exneri), subgroup C (S. boydii), and subgroup D (S. sonnei).
Members of the genus Shigella have been recognized since the late 19th century
as causative agents of bacillary dysentery, or shigellosis.
199
Shigella can easily be
isolated using selective and dierential media, or it may be detected by molecular
testing of primary clinical specimens.
Occupational Infections
Shigellosis is one of the most frequently reported Laboratory-associated
infections in the United States.
102,114
A survey of 397 laboratories in the United
Kingdom revealed that in 1994–1995, four of nine reported Laboratory-associated
infections were caused by Shigella.
228
The direct handling of isolates and animal
work, such as experimentally infecting guinea pigs, other rodents, and NHPs are
proven sources of Laboratory-associated infection.
114,229
Natural Modes of Infection
Humans and other large primates are the only natural reservoirs of Shigella
bacteria. Most transmission is by the fecal-oral route; infection also is caused by
ingestion of contaminated food or water.
199
Infection with Shigella dysenteriae
type 1 causes more severe, prolonged, and frequently fatal illness than does
infection with other Shigella spp., with a fatality rate up to 20%. Complications of
shigellosis can include hemolytic uremic syndrome and reactive arthritis (Reiter’s
syndrome).
230

185Section VIII-A: Bacterial Agents
Laboratory Safety and Containment Recommendations
The agent may be present in feces and, rarely, in the blood of infected humans
or animals. The organism can be shed for weeks after infection and it is commu-
nicable as long as the organism is present in the feces. Shigella spp. can survive
for days in feces and water. Ingestion is the primary laboratory hazard and to a
lesser extent, parenteral inoculation of the agent and person-to-person trans-
mission are potential laboratory hazards. Although rare, experimentally-infected
guinea pigs and other rodents can transmit infection to laboratory sta. The
50% infectious dose (oral) of Shigella for humans is only 180 organisms.
114
The
importance of aerosol exposure is not known.
BSL-2 practices, containment equipment, and facilities are recommended for
activities using clinical materials and diagnostic quantities of infectious cultures.
It is recommended that special emphasis be placed on personal protective
equipment, handwashing, manipulation of faucet handles, and decontamination
of work surfaces to decrease the risk of LAI. For work involving production
quantities or high concentrations of cultures, and for activities with a high
potential for aerosol production, it is recommended that a BSC be used and
that centrifugation be performed using autoclavable, aerosol-tight rotors and
safety cups. ABSL-2 facilities and practices are recommended for activities with
experimentally-infected animals.
Special Issues
Vaccines Vaccines are currently not available for use in humans.
Transfer of Agent Importation of this agent requires CDC and/or USDA impor-
tation permits. Domestic transport of this agent may require a permit from USDA
APHIS VS. A DoC permit may be required for the export of this agent to another
country. See Appendix C for additional information.
Staphylococcus aureus (Methicillin-Resistant, Vancomycin-Resistant, or
Vancomycin-Intermediate)
Staphylococcus aureus is a Gram-positive bacterium associated with a wide
spectrum of diseases in humans, ranging from minor to severe. S. aureus is
a catalase-positive coccus that is a non-motile, non-spore forming facultative
anaerobe. S. aureus isolates express a coagulase factor, which dierentiates
them from other staphylococci that colonize humans. S. aureus is easily
cultivated on standard and selective media, such as high mannitol salt agar.
Several molecular tests are also available for testing from clinical specimens.
Methicillin-resistant S. aureus (MRSA) is common in most areas of the world,
with a resistance rate of 30% in most of North America. Vancomycin is currently
the treatment of choice for MRSA.
231
Vancomycin-resistant S. aureus (VRSA)
(vancomycin MIC ≥ 16 μg/mL) is rare, with only 14 cases documented in the
186 Biosafety in Microbiological and Biomedical Laboratories
United States, in addition to unconrmed cases in India and Iran.
232
Vancomycin-
intermediate S. aureus (VISA) (i.e., isolates with reduced susceptibility to vanco-
mycin, dened as a MIC of 4–8 μg/mL) have been documented at a higher rate,
but remain uncommon in most hospitals.
233
To date, all isolates of VRSA and
VISA have remained susceptible to other FDA-approved drugs.
Occupational Infections
Several cases of laboratory-associated MRSA infections have been
documented.
234–236
To date, no laboratory or occupational infections due to VISA
or VRSA have been reported. Case reports of Laboratory-associated infections
include nasal colonization and minor skin infections. Guidelines have been
provided for investigation and control of VRSA in healthcare settings.
235
Natural Modes of Infection
S. aureus (including MRSA and VISA) is part of the normal human ora,
found primarily in the nares and on the skin of primarily the groin and axillae.
Approximately 20% of the population is persistently colonized by S. aureus, and
60% are colonized intermittently.
238
Animals may act as reservoirs, including
livestock and companion animals.
239
S. aureus is an opportunistic pathogen that
causes a wide variety of diseases in humans. The organism is a leading cause
of foodborne gastroenteritis, as a result of consumption of food contaminated
with enterotoxins expressed by some strains. Skin conditions caused by
S. aureus include cellulitis, scalded skin syndrome, furuncles, carbuncles,
impetigo, and abscesses. Certain strains of S. aureus express toxic shock
syndrome toxin-1 (TSST-1), which is responsible for toxic shock syndrome.
S. aureus is also a common cause of surgical site infections, endocarditis,
peritonitis, pneumonia, bacteremia, meningitis, osteomyelitis, and septic arthritis.
Infection modes include ingestion of food containing enterotoxins and person-to-
person transmission via contact with colonized health care workers to patients.
Nasal colonization can lead to auto-infection.
Laboratory Safety and Containment Recommendations
The agent may be present in many human specimens and in food. Primary
hazards to laboratory personnel are direct and indirect contact of broken skin or
mucous membranes with cultures and contaminated laboratory surfaces, paren-
teral inoculation, and ingestion of contaminated materials.
BSL-2 practices, containment equipment, and facilities are recommended for all
activities utilizing known or potentially infected clinical materials or cultures.
ABSL-2 facilities are recommended for studies utilizing infected laboratory animals.

187Section VIII-A: Bacterial Agents
Special Issues
Vaccines Vaccines are currently not available for use in humans.
Transfer of Agent Importation of this agent requires CDC and/or USDA impor-
tation permits. Domestic transport of this agent may require a permit from USDA
APHIS VS. A DoC permit may be required for the export of this agent to another
country. See Appendix C for additional information.
Treponema pallidum
Treponema pallidum is a species of extremely fastidious spirochetes that die
readily upon desiccation or exposure to atmospheric levels of oxygen and have
not been cultured continuously in vitro.
240
T. pallidum cells have lipid-rich outer
membranes and are highly susceptible to disinfection with common alcohols
(i.e., 70% isopropanol). This species contains three subspecies including
T. pallidum subsp. pallidum (associated with venereal syphilis), T. pallidum subsp.
endemicum (associated with endemic syphilis), and T. pallidum subsp. pertenue
(associated with yaws). These organisms are obligate human pathogens.
Occupational Infections
T. pallidum is a documented hazard to laboratory personnel, but there have been
no reported cases since the 1970s.
84,241
Experimentally-infected animals are a
potential source of infection. Syphilis has been transmitted to personnel working
with a concentrated suspension of T. pallidum obtained from an experimental
rabbit orchitis.
242
Rabbit-adapted T. pallidum (Nichols strain and possibly others)
retains virulence for humans, and rabbits are used in both clinical and research
laboratories to isolate clinical strains and model venereal syphilis, respectively.
243
A murine model was recently developed to study venereal syphilis.
244
Natural Modes of Infection
Humans are the only known natural reservoir of T. pallidum; though, non-human
primates may be a potential reservoir.
245
Transmission occurs via direct
sexual contact (venereal syphilis), direct skin contact (yaws), or direct mucous
membrane contact (endemic syphilis). Venereal syphilis is a sexually transmitted
disease that occurs worldwide, whereas yaws occurs in tropical areas of Africa,
South America, the Caribbean, and Indonesia. Endemic syphilis is limited to arid
areas of Africa and the Middle East.
246
Laboratory Safety and Containment Recommendations
The agent may be present in materials collected from cutaneous and mucosal
lesions and in blood. T. pallidum has a low infectious dose (57 organisms) by
injection. Parenteral inoculation and contact of mucous membranes or broken
skin with infectious clinical materials are the primary hazards to laboratory
personnel.

188 Biosafety in Microbiological and Biomedical Laboratories
BSL-2 practices, containment equipment, and facilities are recommended for all
activities involving the use or manipulation of blood or other clinical specimens
from humans or infected animals. ABSL-2 practices, containment equipment, and
facilities are recommended for work with infected animals.
Special Issues
Vaccines Vaccines are currently not available for use in humans.
Transfer of Agent Importation of this agent requires CDC and/or USDA impor-
tation permits. Domestic transport of this agent may require a permit from USDA
APHIS VS. A DoC permit may be required for the export of this agent to another
country. See Appendix C for additional information.
Vibrio species
Vibrio species are straight or curved motile Gram-negative rods. Growth of Vibrio
spp. is stimulated by sodium, and the natural habitats of these organisms are
primarily aquatic environments. Though rare in the U.S., cholera is an acute
intestinal infection caused by V. cholerae with 3–5 million cases and 100,000
deaths each year, globally.
247
There are at least 12 dierent Vibrio spp. isolated
from clinical specimens. V. cholerae and V. parahaemolyticus are common
causes of human enteritis, and V. alginolyticus and V. vulnicus are common
causes of extraintestinal infections including wound infections and septicemia.
248
Vibrio spp. can easily be isolated using selective and dierential media, or can
be detected by molecular testing of primary clinical specimens.
Occupational Infections
Rare cases of bacterial enteritis due to Laboratory-associated infections with
either V. cholerae or V. parahaemolyticus have been reported.
84,249–251
Naturally-
and experimentally-infected animals and shellsh are potential sources for such
illnesses. No other Vibrio spp. have been implicated in Laboratory-associated
infections.
Natural Modes of Infection
The most common natural mode of infection is the ingestion of contaminated
food or water. The human oral infecting dose of V. cholerae in healthy, non-achlo-
rhydric individuals is approximately 106–1011 colony-forming units, while that of
V. parahaemolyticus ranges from 105–107 cells.
252,253
The importance of aerosol
exposure is unknown; although, it has been implicated in at least one instance.
251
The risk of infection following oral exposure is increased in persons with abnormal
gastrointestinal physiology, including individuals on antacids, with achlorhydria,
or with partial or complete gastrectomies. Fatal cases of septicemia may occur in
individuals who are immunocompromised or have pre-existing medical conditions
such as liver disease, cancer, or diabetes.

189Section VIII-A: Bacterial Agents
Laboratory Safety and Containment Recommendations
Pathogenic Vibrio spp. can be present in human fecal samples or in the meats
and the exterior surfaces of marine invertebrates such as shellsh. Survival
and growth of Vibrio spp. in water is dependent on high salinity. Other clinical
specimens from which Vibrio spp. may be isolated include blood, arm or leg
wounds, eye, ear, and gallbladder.
250
LAIs of V. cholerae or V. parahaemolyticus
have been observed in laboratory researchers after the use of syringes, decon-
tamination of a laboratory spill, or the handling of infected animals.
249–251
Exposure
of open wounds to Vibrio spp. in contaminated seawater or shellsh can result in
infections and septicemia.
BSL-2 practices, containment equipment, and facilities are recommended
for activities with cultures or potentially infectious clinical materials. ABSL-2
practices, containment equipment, and facilities are recommended for activities
with naturally or experimentally infected animals.
Special Issues
Vaccines A cholera vaccine is licensed and available in the United States. It is
currently only recommended for adult travelers to areas of active cholera trans-
mission.
254
There are currently no human vaccines against V. parahaemolyticus.
Transfer of Agent Importation of this agent requires CDC and/or USDA impor-
tation permits. Domestic transport of this agent may require a permit from USDA
APHIS VS. A DoC permit may be required for the export of this agent to another
country. See Appendix C for additional information.
Yersinia pestis
Yersinia pestis, the causative agent of plague, is a Gram-negative bacillus
frequently characterized by a “safety pin” appearance on stained preparations
from specimens. The incubation period for bubonic plague ranges from two to six
days while the incubation period for pneumonic plague is one to six days.
Occupational Infections
Y. pestis is a documented laboratory hazard. A number of LAIs have been
reported in the United States, some of which were fatal.
84,255
One lethal case
in a laboratory researcher was due to the attenuated strain KIM D27.
256
The
condition of hereditary hemochromatosis coupled with diabetes in the researcher
is believed to have contributed to the fatal course of disease. Veterinary sta and
pet owners have become infected when handling domestic cats with oropha-
ryngeal or pneumonic plague.
190 Biosafety in Microbiological and Biomedical Laboratories
Natural Modes of Infection
There is a natural zoonotic cycle of Y. pestis between wild rodents and their eas.
Infective eabites are the most common mode of transmission, but direct human
contact with infected tissues or body uids of animals and humans may also
serve as sources of infection.
Plague has a high mortality rate if untreated (50%) and caused three major
pandemics, including the Black Death of the 14th century. There are three
manifestations of disease: bubonic, septicemic, and pneumonic. Bubonic plague
results in tender and painful lymph nodes (buboes). Septicemic plague, which
may develop directly or from untreated bubonic plague, can lead to shock and
bleeding into the skin and tissues, potentially causing necrosis. Pneumonic
plague results in a rapidly developing pneumonia and can be spread from person
to person via respiratory droplets. Plague occurs in multiple countries of the
world, with the highest incidence in Africa. Most cases in the United States occur
in rural, western states. Sporadic cases in the United States average about seven
cases per year. Contact with infected sylvatic rodents, such as prairie dogs and
ground squirrels, has resulted in human infections.
257
Laboratory Safety and Containment Recommendations
Y. pestis has been isolated from bubo aspirates, blood, sputum, CSF and autopsy
tissues (spleen, liver, lung), depending on the clinical form and stage of the
disease; feces, urine or bone marrow samples may be positive for Y. pestis DNA
or antigen but not the organism itself. Primary hazards to laboratory personnel
include direct contact with cultures and infectious materials from humans or
animal hosts and inhalation of infectious aerosols or droplets generated during
their manipulation. Laboratory animal studies have shown the lethal and infec-
tious doses of Y. pestis to be quite low, less than 100 colony-forming units.
258
Y. pestis can survive for months in human blood and tissues. Fleas may remain
infective for months. It is recommended that laboratory and eld personnel be
counseled on methods to avoid ea bites and autoinoculation when handling
potentially infected live or dead animals.
BSL-3 and ABSL-3 practices, containment equipment, and facilities are
recommended for all manipulations of suspect cultures, animal necropsies, and
for experimental animal studies. BSL-3 practices, containment equipment, and
facilities are appropriate for production operations. Characterized strains of
reduced virulence such as Y. pestis strain A1122 can be manipulated at BSL-2.
BSL-2 practices, containment equipment, and facilities are recommended for
primary inoculation of cultures from potentially infectious clinical materials.
When performing eldwork involving animals that may have eas, gloves and
appropriate clothing should be worn to prevent contact with skin, and insect

191Section VIII-A: Bacterial Agents
repellent can be used to reduce the risk of ea bites. Arthropod Containment
Level 3 (ACL-3) facilities and practices are recommended for all laboratory work
involving infected arthropods.
255
See Appendix G for additional information on
Arthropod Containment Guidelines.
Special Issues
Be advised of possible misidentication using automated systems. For identi-
cation of samples suspected of containing Y. pestis using MALDI-TOF MS, it is
recommended to use alternative tube extraction that kills viable organisms and
not direct spotting of plates in the open laboratory.
Vaccines There are no licensed vaccines currently available in the United
States.
259
New plague vaccines are in development but are not expected to be
commercially available in the immediate future.
206
Select Agent Y. pestis is a Select Agent requiring registration with CDC and/or
USDA for possession, use, storage and/or transfer. See Appendix F for additional
information.
Transfer of Agent Importation of this agent requires CDC and/or USDA impor-
tation permits. Domestic transport of this agent may require a permit from USDA
APHIS VS. A DoC permit may be required for the export of this agent to another
country. See Appendix C for additional information.
References
1. Dragon DC, Rennie RP. The ecology of anthrax spores: tough but not
invincible. Can Vet J. 1995;36(5):295–301.
2. Wright JG, Quinn CP, Shadomy S, Messonnier N. Use of anthrax vaccine
in the United States: recommendations of the Advisory Committee
on Immunization Practices (ACIP), 2009. MMWR Recomm Rep.
2010;59(RR-6):1–30.
3. World Health Organization. Anthrax in humans and animals. 4th ed.
Geneva: WHO Press; 2008.
4. Inglesby TV, O’Toole T, Henderson DA, Bartlett JG, Ascher MS, Eitzen E,
et al. Anthrax as a biological weapon, 2002: updated recommendations for
management. JAMA. 2002;287(17):2236–52. Erratum in: JAMA 2002 Oct
16;288(15):1849.
5. Watson A, Keir D. Information on which to base assessments of risk
from environments contaminated with anthrax spores. Epidemiol Infect.
1994;113(3):479–90.
6. Bottone EJ. Bacillus cereus, a volatile human pathogen. Clin Microbiol Rev.
2010;23(2):382–98.

192 Biosafety in Microbiological and Biomedical Laboratories
7. U.S. Department of Health and Human Services [Internet]. Silver
Spring (MD): U.S. Food & Drug Administration; c2018 Feb 05
[cited 2018 Oct 26]. Anthrax. Available from: https://www.fda.gov/
BiologicsBloodVaccines/Vaccines/ucm061751.htm
8. Centers for Disease Control and Prevention. Human anthrax associated
with an epizootic among livestock–North Dakota, 2000. MMWR Morb Mortal
Wkly Rep. 2001;50(32):677–80.
9. Centers for Disease Control and Prevention. Suspected cutaneous anthrax
in a laboratory worker–Texas, 2002. MMWR Morb Mortal Wkly Rep.
2002;51(13):279–81.
10. Grith J, Blaney D, Shadomy S, Lehman M, Pesik N, Tostenson S, et al.
Investigation of inhalation anthrax case, United States. Emerg Infect Dis.
2014;20(2):280–3.
11. Palmateer NE, Hope VD, Roy K, Marongiu A, White JM, Grant KA, et
al. Infections with spore-forming bacteria in persons who inject drugs,
2000–2009. Emerg Infect Dis. 2013;19(1):29–34.
12. U.S. Department of Health and Human Services [Internet]. Washington
(DC): Association of Public Health Laboratories and American
Society for Microbiology; c2016 [cited 2018 Oct 26]. Clinical and
Laboratory Preparedness and Response Guide; [332 p.]. Available
from: https://asprtracie.hhs.gov/technical-resources/resource/6102/
clinical-laboratory-preparedness-and-response-guide
13. Jernigan DB, Raghunathan PL, Bell BP, Brechner R, Bresnitz EA, Butler
JC, et al. Investigation of bioterrorism-related anthrax, United States, 2001:
epidemiologic ndings. Emerg Infect Dis. 2002;8(10):1019–28.
14. Weiss S, Yitzhaki S, Shapira SC. Lessons to be Learned from
Recent Biosafety Incidents in the United States. Isr Med Assoc J.
2015;17(5):269–73.
15. Centers for disease control and prevention. Use of Anthrax Vaccine in
the United States: Recommendations of the Advisory Committee on
Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019; 68(4) 1–14.
16. Weller SA, Stokes MG, Lukaszewski RA: 2015. Observations on
the Inactivation Ecacy of a MALDI-TOF MS Chemical Extraction
Method on Bacillus anthracis Vegetative Cells and Spores. PLoS One,
10(12):e0143870.
17. Tracz DM, Antonation KS, Corbett CR: 2016. Verication of a Matrix-
Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry
Method for Diagnostic Identication of High-Consequence Bacterial
Pathogens. J Clin Microbiol, 54(3):764–767.

193Section VIII-A: Bacterial Agents
18. Centers for Disease Control and Prevention. Use of anthrax vaccine in
response to terrorism: supplemental recommendations of the Advisory
Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep.
2002;51(45):1024–6.
19. Greig SL. Obiltoxaximab: First Global Approval. Drugs. 2016;76(7):823–30.
20. Kaur M, Singh S, Bhatnagar R. Anthrax vaccines: present status and future
prospects. Expert Rev Vaccines. 2013;12(8):955–70.
21. Kilgore PE, Salim AM, Zervos MJ, Schmitt HJ. Pertussis: Microbiology,
Disease, Treatment, and Prevention. Clin Microbiol Rev. 2016;29(3):449–86.
22. Mattoo S, Cherry JD. Molecular pathogenesis, epidemiology, and clinical
manifestations of respiratory infections due to Bordetella pertussis and other
Bordetella subspecies. Clin Microbiol Rev. 2005;18(2):326–82.
23. Beall B, Cassiday PK, Sanden GN. Analysis of Bordetella pertussis isolates
from an epidemic by pulsed-eld gel electrophoresis. J Clin Microbiol.
1995;33(12):3083–6.
24. Burstyn DG, Bara LJ, Peppler MS, Leake RD, St Geme J, Jr., Manclark
CR. Serological response to lamentous hemagglutinin and lymphocytosis-
promoting toxin of Bordetella pertussis. Infect Immun. 1983;41(3):1150–6.
25. Pinto MV, Merkel TJ. Pertussis disease and transmission and host
responses: insights from the baboon model of pertussis. J Infect. 2017;74
Suppl 1:S114–S9.
26. Centers for Disease Control and Prevention [Internet]. Atlanta (GA):
National Center for Immunization and Respiratory Diseases, Division
of Bacterial Diseases; c2017 [cited 2018 Oct 26]. Pertussis (Whooping
Cough). Available from: https://www.cdc.gov/pertussis/countries/index.html
27. Yeung KHT, Duclos P, Nelson EAS, Hutubessy RCW. An update of the
global burden of pertussis in children younger than 5 years: a modelling
study. Lancet Infect Dis. 2017;17(9):974–80.
28. Guiso N. Bordetella pertussis and pertussis vaccines. Clin Infect Dis.
2009;49(10):1565–9.
29. Ward JI, Cherry JD, Chang SJ, Partridge S, Keitel W, Edwards K, et al.
Bordetella Pertussis infections in vaccinated and unvaccinated adolescents
and adults, as assessed in a national prospective randomized Acellular
Pertussis Vaccine Trial (APERT). Clin Infect Dis. 2006;43(2):151–7.
30. Queenan AM, Cassiday PK, Evangelista A. Pertactin-negative variants of
Bordetella pertussis in the United States. N Engl J Med. 2013;368(6):583–4.
31. Centers for Disease Control and Prevention. Fatal case of unsuspected
pertussis diagnosed from a blood culture–Minnesota, 2003. MMWR Morb
Mortal Wkly Rep. 2004;53(6):131–2.

194 Biosafety in Microbiological and Biomedical Laboratories
32. Janda WM, Santos E, Stevens J, Celig D, Terrile L, Schreckenberger PC.
Unexpected isolation of Bordetella pertussis from a blood culture. J Clin
Microbiol. 1994;32(11):2851–3.
33. Centers for Disease Control and Prevention [Internet]. Atlanta (GA):
National Center for Immunization and Respiratory Diseases; c2017
[cited 2018 Oct 26]. Vaccines and Preventable Diseases. Available from:
https://www.cdc.gov/vaccines/vpd/pertussis/recs-summary.html
34. Araj GF. Brucella. In: Jorgensen JH, Pfaller MA, Carroll KC, Funke
G, Landry ML, Richter SS, Warnock DW, editors. Manual of Clinical
Microbiology, Volume 1. 11th ed. Washington (DC): ASM Press; 2015.
p. 863–72.
35. Pappas G, Akritidis N, Bosilkovski M, Tsianos E. Brucellosis. N Engl J Med.
2005;352(22):2325–36.
36. Miller CD, Songer JR, Sullivan JF. A twenty-ve year review of laboratory-
acquired human infections at the National Animal Disease Center. Am Ind
Hyg Assoc J. 1987;48(3):271–5.
37. Olle-Goig JE, Canela-Soler J. An outbreak of Brucella melitensis infection
by airborne transmission among laboratory workers. Am J Public Health.
1987;77(3):335–8.
38. Singh K. Laboratory-acquired infections. Clin Infect Dis. 2009;49(1):142–7.
39. Traxler RM, Lehman MW, Bosserman EA, Guerra MA, Smith TL. A
literature review of laboratory-acquired brucellosis. J Clin Microbiol.
2013;51(9):3055–62.
40. biosafety.be [Internet]. Belgium: Belgian Biosafety Server; c2018
[cited 2018 Oct 26]. Available from: https://www.biosafety.be/.
41. Centers for Disease Control and Prevention. Update: potential exposures
to attenuated vaccine strain Brucella abortus RB51 during a laboratory
prociency test–United States and Canada, 2007. MMWR Morb Mortal
Wkly Rep. 2008;57(2):36–9.
42. Grammont-Cupillard M, Berthet-Badetti L, Dellamonica P. Brucellosis from
sning bacteriological cultures. Lancet. 1996;348(9043):1733–4.
43. Huddleson IF, Munger M. A Study of an Epidemic of Brucellosis Due to
Brucella melitensis. Am J Public Health Nations Health. 1940;30(8):944–54.
44. Staszkiewicz J, Lewis CM, Colville J, Zervos M, Band J. Outbreak of
Brucella melitensis among microbiology laboratory workers in a community
hospital. J Clin Microbiol. 1991;29(2):287–90.
195Section VIII-A: Bacterial Agents
45. Mense MG, Borschel RH, Wilhelmsen CL, Pitt ML, Hoover DL. Pathologic
changes associated with brucellosis experimentally induced by aerosol
exposure in rhesus macaques (Macaca mulatta). Am J Vet Res.
2004;65(5):644–52.
46. Pardon P, Marly J. Resistance of normal or immunized guinea pigs
against a subcutaneous challenge of Brucella abortus. Ann Rech Vet.
1978;9(3):419–25.
47. Almiron MA, Roset MS, Sanjuan N. The Aggregation of Brucella abortus
Occurs Under Microaerobic Conditions and Promotes Desiccation
Tolerance and Biolm Formation. Open Microbiol J. 2013;7:87–91.
48. Corbel MJ. Brucellosis: an overview. Emerg Infect Dis. 1997;3(2):213–21.
49. Seleem MN, Boyle SM, Sriranganathan N. Brucellosis: a re-emerging
zoonosis. Vet Microbiol. 2010;140(3–4):392–8.
50. Van Zandt KE, Greer MT, Gelhaus HC. Glanders: an overview of infection
in humans. Orphanet J Rare Dis. 2013;8:131.
51. Centers for Disease Control and Prevention. Laboratory-acquired
human glanders–Maryland, May 2000. MMWR Morb Mortal Wkly Rep.
2000;49(24):532–5.
52. Srinivasan A, Kraus CN, DeShazer D, Becker PM, Dick JD, Spacek L,
et al. Glanders in a military research microbiologist. N Engl J Med.
2001;345(4):256–8.
53. Titball RW, Burtnick MN, Bancroft GJ, Brett P. Burkholderia pseudomallei
and Burkholderia mallei vaccines: Are we close to clinical trials? Vaccine.
2017;35(44):5981–9.
54. Lipuma JJ, Currie BJ, Peacock SJ, Vandamme PAR. Burkholderia,
Stenotrophomonas, Ralstonia, Cupriavidus, Pandoraea, Brevundimonas,
Comamonas, Delftia, and Acidovorax. In: Jorgensen JH, Pfaller MA, Carroll
KC, Funke G, Landry ML, Richter SS, Warnock DW, editors. Manual of
Clinical Microbiology, Volume 1. 11th ed. Washington (DC): ASM Press;
2015. p. 791–812.
55. Limmathurotsakul D, Golding N, Dance DA, Messina JP, Pigott DM, Moyes
CL, et al. Predicted global distribution of Burkholderia pseudomallei and
burden of melioidosis. Nat Microbiol. 2016;1(1).
56. Green RN, Tunell PG. Laboratory acquired melioidosis. Am J Med.
1968;44(4):599–605.
57. Peacock SJ, Schweizer HP, Dance DA, Smith TL, Gee JE, Wuthiekanun V,
et al. Management of accidental laboratory exposure to Burkholderia
pseudomallei and B. mallei. Emerg Infect Dis. 2008;14(7):e2.

196 Biosafety in Microbiological and Biomedical Laboratories
58. Schlech WF 3rd, Turchik JB, Westlake RE Jr, Klein GC, Band JD, Weaver
RE. Laboratory-acquired infection with Pseudomonas pseudomallei
(melioidosis). N Engl J Med. 1981;305(19):1133–5.
59. Kohler C, Dunachie SJ, Muller E, Kohler A, Jenjaroen K, Teparrukkul P, et al.
Rapid and Sensitive Multiplex Detection of Burkholderia pseudomallei-
Specic Antibodies in Melioidosis Patients Based on a Protein Microarray
Approach. PLoS Negl Trop Dis. 2016;10(7):e0004847.
60. Dance DA. Ecology of Burkholderia pseudomallei and the interactions
between environmental Burkholderia spp. and human-animal hosts.
Acta Trop. 2000;74(2–3):159–68.
61. Centers for Disease Control and Prevention [Internet]. Atlanta (GA):
National Center for Emerging and Zoonotic Infectious Diseases, Division of
High Consequence Pathogens and Pathology; c2012 [cited 2018 Oct 29].
Melioidosis. Available from: https://www.cdc.gov/melioidosis/
62. Robertson J, Levy A, Sagripanti JL, Inglis TJ. The survival of Burkholderia
pseudomallei in liquid media. Am J Trop Med Hyg. 2010;82(1):88–94.
63. Shams AM, Rose LJ, Hodges L, Arduino MJ. Survival of Burkholderia
pseudomallei on Environmental Surfaces. Appl Environ Microbiol.
2007;73(24):8001–4.
64. Benoit TJ, Blaney DD, Gee JE, Elrod MG, Homaster AR, Doker TJ, et al.
Melioidosis Cases and Selected Reports of Occupational Exposures to
Burkholderia pseudomallei–United States, 2008–2013. MMWR Surveill
Summ. 2015;64(5):1–9.
65. Masuda T, Isokawa T. [Biohazard in clinical laboratories in Japan].
Kansenshogaku Zasshi. 1991;65(2):209–15.
66. Oates JD, Hodgin UG Jr. Laboratory-acquired Campylobacter enteritis.
South Med J. 1981;74(1):83.
67. Penner JL, Hennessy JN, Mills SD, Bradbury WC. Application of serotyping
and chromosomal restriction endonuclease digest analysis in investigating
a laboratory-acquired case of Campylobacter jejuni enteritis. J Clin
Microbiol. 1983;18(6):1427–8.
68. Saunders S, Smith K, Schott R, Dobbins G, Scheftel J. Outbreak of
Campylobacteriosis Associated with Raccoon Contact at a Wildlife
Rehabilitation Centre, Minnesota, 2013. Zoonoses Public Health.
2017;64(3):222–7.
69. Hara-Kudo Y, Takatori K. Contamination level and ingestion dose of
foodborne pathogens associated with infections. Epidemiol Infect.
2011;139(10):1505–10.
197Section VIII-A: Bacterial Agents
70. Black RE, Levine MM, Clements ML, Hughes TP, Blaser MJ. Experimental
Campylobacter jejuni infection in humans. J Infect Dis. 1988;157(3):472–9.
71. Robinson DA. Infective dose of Campylobacter jejuni in milk. Br Med J
(Clin Res Ed). 1981;282(6276):1584.
72. Ravel A, Hurst M, Petrica N, David J, Mutschall SK, Pintar K, et
al. Source attribution of human campylobacteriosis at the point of
exposure by combining comparative exposure assessment and subtype
comparison based on comparative genomic ngerprinting. PLoS One.
2017;12(8):e0183790.
73. Marchand-Senecal X, Bekal S, Pilon PA, Sylvestre JL, Gaudreau
C. Campylobacter fetus Cluster Among Men Who Have Sex With
Men, Montreal, Quebec, Canada, 2014–2016. Clin Infect Dis.
2017;65(10):1751–3.
74. Kaakoush NO, Castano-Rodriguez N, Mitchell HM, Man SM. Global
Epidemiology of Campylobacter Infection. Clin Microbiol Rev.
2015;28(3):687–720.
75. Skuhala T, Skerk V, Markotic A, Bukovski S, Desnica B. Septic
abortion caused by Campylobacter jejuni bacteraemia. J Chemother.
2016;28(4):335–6.
76. Smith JL. Campylobacter jejuni infection during pregnancy: long-term
consequences of associated bacteremia, Guillain-Barre syndrome, and
reactive arthritis. J Food Prot. 2002;65(4):696–708.
77. Hogerwerf L, DE Gier B, Baan B, Van Der Hoek W. Chlamydia psittaci
(psittacosis) as a cause of community-acquired pneumonia: a systematic
review and meta-analysis. Epidemiol Infect. 2017;145(15):3096–105.
78. Knittler MR, Sachse K. Chlamydia psittaci: update on an underestimated
zoonotic agent. Pathog Dis. 2015;73(1):1–15.
79. Petrovay F, Balla E. Two fatal cases of psittacosis caused by Chlamydophila
psittaci. J Med Microbiol. 2008;57(Pt 10):1296–8.
80. Beeckman DS, Vanrompay DC. Zoonotic Chlamydophila psittaci infections
from a clinical perspective. Clin Microbiol Infect. 2009;15(1):11–7.
81. Corsaro D, Greub G. Pathogenic potential of novel Chlamydiae and
diagnostic approaches to infections due to these obligate intracellular
bacteria. Clin Microbiol Rev. 2006;19(2):283–97.
82. Gaydos C, Essig A. Chlamydiaceae. In: Jorgensen JH, Pfaller MA, Carroll
KC, Funke G, Landry ML, Richter SS, Warnock DW, editors. Manual of
Clinical Microbiology, Volume 1. 11th ed. Washington (DC): ASM Press;
2015. p. 1106–21.
198 Biosafety in Microbiological and Biomedical Laboratories
83. Van Droogenbroeck C, Beeckman DS, Verminnen K, Marien M, Nauwynck
H, Boesinghe Lde T, et al. Simultaneous zoonotic transmission of
Chlamydophila psittaci genotypes D, F and E/B to a veterinary scientist. Vet
Microbiol. 2009;135(1–2):78–81.
84. Pike RM. Laboratory-associated infections: summary and analysis of 3921
cases. Health Lab Sci. 1976;13(2):105–14.
85. Bernstein DI, Hubbard T, Wenman WM, Johnson BL Jr, Holmes KK,
Liebhaber H, et al. Mediastinal and supraclavicular lymphadenitis and
pneumonitis due to Chlamydia trachomatis serovars L1 and L2. N Engl J
Med. 1984;311(24):1543–6.
86. Hyman CL, Augenbraun MH, Roblin PM, Schachter J, Hammerschlag MR.
Asymptomatic respiratory tract infection with Chlamydia pneumoniae TWAR.
J Clin Microbiol. 1991;29(9):2082–3.
87. Chan J, Doyle B, Branley J, Sheppeard V, Gabor M, Viney K, et al. An
outbreak of psittacosis at a veterinary school demonstrating a novel source
of infection. One Health. 2017;3:29–33.
88. Taylor KA, Durrheim D, Heller J, O’Rourke B, Hope K, Merritt T, et al.
Equine chlamydiosis-An emerging infectious disease requiring a one health
surveillance approach. Zoonoses Public Health. 2018;65(1):218–21.
89. Foster LH, Portell CA. The role of infectious agents, antibiotics, and antiviral
therapy in the treatment of extranodal marginal zone lymphoma and other
low-grade lymphomas. Curr Treat Options Oncol. 2015;16(6):28.
90. Kramer A, Schwebke I, Kampf G. How long do nosocomial pathogens
persist on inanimate surfaces? A systematic review. BMC Infect Dis.
2006;6:130.
91. Kaleta EF, Taday EM. Avian host range of Chlamydophila spp. based on
isolation, antigen detection and serology. Avian Pathol. 2003;32(5):435–61.
92. Krauss H, Weber A, Appel M, Enders B, Isenberg HD, Schiefer HG,
et al. Bacterial Zoonoses. In: Krauss H, Weber A, Appel M, Enders B,
Isenberg HD, Schiefer HG, et al, authors. Zoonoses: Infectious Diseases
Transmissible from Animals to Humans. 3rd ed. Washington (DC): ASM
Press; 2003. p. 173–252.
93. Smith LDS, Sugiyama H. Botulism: the organism, its toxins, the disease.
2nd ed. Barlows A, editor. Springeld (IL): Charles C. Thomas; 1988.
94. Siegel LS, Metzger JF. Toxin production by Clostridium botulinum
type A under various fermentation conditions. Appl Environ Microbiol.
1979;38(4):606–11.
199Section VIII-A: Bacterial Agents
95. Stevens DL, Bryant AE, Carroll K. Clostridium. In: Jorgensen JH, Pfaller MA,
Carroll KC, Funke G, Landry ML, Richter SS, Warnock DW, editors. Manual
of Clinical Microbiology, Volume 1. 11th ed. Washington (DC): ASM Press;
2015. p. 940–66.
96. Maksymowych AB, Simpson LL. A brief guide to the safe handling of
biological toxin. In: Aktories K, editor. Bacterial toxins: Tools in Cell Biology
and Pharmacology. London: Chapman and Hall; 1997. p. 295–300.
97. Lawson PA, Citron DM, Tyrrell KL Finegold SM. Reclassication of
Clostridium dicile as Clostridioides dicile (Hall and O’Toole 1935) Prevot
1938. Anaerobe. 2016;40:95–9.
98. Lessa FC, Mu Y, Bamberg WM, Beldavs ZG, Dumyati GK, Dunn JR, et al.
Burden of Clostridium dicile infection in the United States. N Engl J Med.
2015;372(9):825–34.
99. Lo Vecchio A, Zacur GM. Clostridium dicile infection: an update on
epidemiology, risk factors, and therapeutic options. Curr Opin Gastroenterol.
2012;28(1):1–9.
100. Schaer H, Breitruck A. Clostridium dicile—From Colonization to Infection.
Front Microbiol. 2018;9:646.
101. Gateau C, Couturier J, Coia J, Barbut F. How to: diagnose infection caused
by Clostridium dicile. Clin Microbiol Infect. 2018;24(5):463–8.
102. Baron EJ, Miller JM. Bacterial and fungal infections among diagnostic
laboratory workers: evaluating the risks. Diagn Microbiol Infect Dis.
2008;60(3):241–6.
103. Best EL, Fawley WN, Parnell P, Wilcox MH. The potential for airborne
dispersal of Clostridium dicile from symptomatic patients. Clin Infect Dis.
2010;50(11):1450–7.
104. Crobach MJT, Vernon JJ, Loo VG, Kong LY, Pechine S, Wilcox MH, et
al. Understanding Clostridium dicile Colonization. Clin Microbiol Rev.
2018;31(2).
105. Leer DA, Lamont JT. Clostridium dicile infection. N Engl J Med.
2015;372(16):1539–48.
106. Hopkins RJ, Wilson RB. Treatment of recurrent Clostridium dicile colitis:
a narrative review. Gastroenterol Rep (Oxf). 2018;6(1):21–8.
107. Kelly CR, Kahn S, Kashyap P, Laine L, Rubin D, Atreja A, et al. Update
on Fecal Microbiota Transplantation 2015: Indications, Methodologies,
Mechanisms, and Outlook. Gastroenterology. 2015;149(1):223–37.

200 Biosafety in Microbiological and Biomedical Laboratories
108. Lo Vecchio A, Lancella L, Tagliabue C, De Giacomo C, Garazzino S,
Mainetti M, et al. Clostridium dicile infection in children: epidemiology and
risk of recurrence in a low-prevalence country. Eur J Clin Microbiol Infect
Dis. 2017;36(1):177–85.
109. U.S. Department of Health and Human Services. Antibiotic Resistance
Threats in the United States, 2013. Atlanta (GA): Centers for Disease
Control and Prevention; 2013. 114 p.
110. McDonald LC, Killgore GE, Thompson A, Owens RC Jr, Kazakova SV,
Sambol SP, et al. An epidemic, toxin gene-variant strain of Clostridium
dicile. N Engl J Med. 2005;353(23):2433–41.
111. Centers for Disease Control and Prevention [Internet]. Atlanta (GA): National
Center for Emerging Zoonotic Infectious Diseases, Division of Healthcare
Quality Promotion; c2015 [cited 2018 Oct 29]. Clostridium dicile Infection.
Available from: https://www.cdc.gov/hai/organisms/cdi/cdi_infect.html
112. Centers for Disease Control and Prevention. Tetanus surveillance—United
States, 2001–2008. MMWR Morb Mortal Wkly Rep. 2011;60(12):365–9.
113. Centers for Disease Control and Prevention [Internet]. Atlanta (GA):
National Center for Immunization and Respiratory Diseases, Division of
Bacterial Diseases; c2017 [cited 2018 Oct 30]. Tetanus. Available from:
https://www.cdc.gov/tetanus/index.html
114. Sewell DL. Laboratory-associated infections and biosafety. Clin Microbiol
Rev. 1995;8(3):389–405.
115. Campbell JI, Lam TM, Huynh TL, To SD, Tran TT, Nguyen VM, et al.
Microbiologic characterization and antimicrobial susceptibility of
Clostridium tetani isolated from wounds of patients with clinically
diagnosed tetanus. Am J Trop Med Hyg. 2009;80(5):827–31.
116. Centers for Disease Control and Prevention. Updated recommendations for
use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis (Tdap)
vaccine from the Advisory Committee on Immunization Practices, 2010.
117. Funke G, Bernard KA. Coryneform Gram-Positive Rods. In: Jorgensen JH,
Pfaller MA, Carroll KC, Funke G, Landry ML, Richter SS, Warnock DW,
editors. Manual of Clinical Microbiology, Volume 1. 11th ed. Washington
(DC): ASM Press; 2015. p. 474–503.
118. Tiwari T, Warton M. Diphtheria Toxoid. In: Plotkin SA, Orenstein WA, Ot PA,
Edwards KM, authors. Plotkin’s Vaccines. 7th ed. Philadelphia (PA):
Elsevier; 2018. p. 261–75.
119. Thilo W, Kiehl W, Geiss HK. A case report of laboratory-acquired diphtheria.
Euro Surveill. 1997;2(8):67–8.

201Section VIII-A: Bacterial Agents
120. Galbraith NS, Forbes P, Cliord C. Communicable disease associated with
milk and dairy products in England and Wales 1951–80. Br Med J (Clin Res
Ed). 1982;284(6331):1761–5.
121. Hogg RA, Wessels J, Hart J, Efstratiou A, De Zoysa A, Mann G, et al.
Possible zoonotic transmission of toxigenic Corynebacterium ulcerans
from companion animals in a human case of fatal diphtheria. Vet Rec.
2009;165(23):691–2.
122. May ML, McDougall RJ, Robson JM. Corynebacterium diphtheriae and the
returned tropical traveler. J Travel Med. 2014;21(1):39–44.
123. Gill DM. Bacterial toxins: a table of lethal amounts. Microbiol Rev.
1982;46(1):86–94.
124. Advisory Committee on Immunization Practices; Centers for Disease
Control and Prevention (CDC). Immunization of health-care personnel:
recommendations of the Advisory Committee on Immunization Practices
(ACIP). MMWR Recomm Rep. 2011;60(RR-7):1–45.
125. Maurin M, Gyuranecz M. Tularaemia: clinical aspects in Europe. Lancet
Infect Dis. 2016;16(1):113–24.
126. Centers for Disease Control and Prevention. Tularemia—United States,
2001–2010. MMWR Morb Mortal Wkly Rep. 2013;62(47):963–6.
127. Eliasson H, Broman T, Forsman M, Back E. Tularemia: current epidemiology
and disease management. Infect Dis Clin North Am. 2006;20(2):289–311, ix.
128. Wurtz N, Papa A, Hukic M, Di Caro A, Leparc-Goart I, Leroy E, et al.
Survey of laboratory-acquired infections around the world in biosafety level
3 and 4 laboratories. Eur J Clin Microbiol Infect Dis. 2016;35(8):1247–58.
129. Jones CL, Napier BA, Sampson TR, Llewellyn AC, Schroeder MR, Weiss
DS. Subversion of host recognition and defense systems by Francisella spp.
Microbiol Mol Biol Rev. 2012;76(2):383–404.
130. Centers for Disease Control and Prevention [Internet]. Atlanta (GA):
National Center for Emerging and Zoonotic Infectious Diseases, Division
of Vector-Borne Diseases; c2016 [cited 2018 Oct 30]. Tularemia. Available
from: https://www.cdc.gov/tularemia
131. De Witte C, Schulz C, Smet A, Malfertheiner P, Haesebrouck F. Other
Helicobacters and gastric microbiota. Helicobacter. 2016;21 Suppl 1:62–8.
132. Burucoa C, Axon A. Epidemiology of Helicobacter pylori infection.
Helicobacter. 2017;22 Suppl 1.
133. Matysiak-Budnik T, Briet F, Heyman M, Megraud F. Laboratory-acquired
Helicobacter pylori infection. Lancet. 1995;346(8988):1489–90.

202 Biosafety in Microbiological and Biomedical Laboratories
134. Marshall BJ, Armstrong JA, McGechie DB, Glancy RJ. Attempt
to full Koch’s postulates for pyloric Campylobacter. Med J Aust.
1985;142(8):436–9.
135. Venerito M, Vasapolli R, Rokkas T, Delchier JC, Malfertheiner P.
Helicobacter pylori, gastric cancer and other gastrointestinal malignancies.
Helicobacter. 2017;22 Suppl 1.
136. Pillai DR. Helicobacter pylori Cultures. In: Leber AL, editor. Clinical
Microbiology Procedures Handbook, Volume 1. 4th ed. Washington (DC):
ASM Press; 2016. p. 3.8.4.1–3.8.4.5.
137. Burillo A, Pedro-Botet ML, Bouza E. Microbiology and Epidemiology of
Legionnaire’s Disease. Infect Dis Clin North Am. 2017;31(1):7–27.
138. Edelstein PH, Luck C. Legionella. In: Jorgensen JH, Pfaller MA, Carroll KC,
Funke G, Landry ML, Richter SS, Warnock DW, editors. Manual of Clinical
Microbiology, Volume 1. 11th ed. Washington (DC): ASM Press; 2015.
p. 887–904.
139. Centers for Disease Control and Prevention. Unpublished data. Center for
Infectious Diseases. HEW, Public Health Service. 1976.
140. Correia AM, Ferreira JS, Borges V, Nunes A, Gomes B, Capucho R, et al.
Probable Person-to-Person Transmission of Legionnaires’ Disease. N Engl
J Med. 2016;374(5):497–8.
141. Schwake DO, Alum A, Abbaszadegan M. Impact of environmental factors
on Legionella populations in drinking water. Pathogens. 2015;4(2):269–82.
142. Levett PN. Leptospira. In: Jorgensen JH, Pfaller MA, Carroll KC, Funke
G, Landry ML, Richter SS, Warnock DW, editors. Manual of Clinical
Microbiology, Volume 1. 11th ed. Washington (DC): ASM Press; 2016.
p. 1028–36.
143. Barkin RM, Guckian JC, Glosser JW. Infection by Leptospira ballum:
a laboratory-associated case. South Med J. 1974;67(2):155 passim.
144. Bolin CA, Koellner P. Human-to-human transmission of Leptospira
interrogans by milk. J Infect Dis. 1988;158(1):246–7.
145. Sugunan AP, Natarajaseenivasan K, Vijayachari P, Sehgal SC.
Percutaneous exposure resulting in laboratory-acquired leptospirosis—
a case report. J Med Microbiol. 2004;53(Pt 12):1259–62.
146. Centers for Disease Control and Prevention [Internet]. Atlanta (GA):
National Center for Emerging and Zoonotic Infectious Diseases, Division of
High-Consequence Pathogens and Pathology; c2017 [cited 2018 Oct 30].
Leptospirosis. Available from: https://www.cdc.gov/leptospirosis/
147. World Health Organization [Internet]. Geneva; c2018 [cited 2018 Oct 30].
Leptospirosis. Available from: https://www.who.int/topics/leptospirosis/en/
203Section VIII-A: Bacterial Agents
148. Schuchat A, Swaminathan B, Broome CV. Epidemiology of human
listeriosis. Clin Microbiol Rev. 1991;4(2):169–83.
149. Wellinghausen N. Listeria and Erysipelothrix. In: Jorgensen JH, Pfaller MA,
Carroll KC, Funke G, Landry ML, Richter SS, Warnock DW, editors. Manual
of Clinical Microbiology, Volume 1. 11th ed. Washington (DC): ASM Press;
2016. p. 462–73.
150. Godshall CE, Suh G, Lorber B. Cutaneous listeriosis. J Clin Microbiol.
2013;51(11):3591–6.
151. Ortel S. [Listeriosis during pregnancy and excretion of Listeria by laboratory
workers (author’s transl)]. Zentralbl Bakteriol Orig A. 1975;231(4):491–502.
152. Vazquez-Boland JA, Krypotou E, Scortti M. Listeria Placental Infection.
MBio. 2017;8(3). pii: e00949–17.
153. Gandhi M, Chikindas ML. Listeria: A foodborne pathogen that knows how to
survive. Int J Food Microbiol. 2007;113(1):1–15.
154. Kovacevic J, Ziegler J, Walecka-Zacharska E, Reimer A, Kitts DD,
Gilmour MW. Tolerance of Listeria monocytogenes to Quaternary
Ammonium Sanitizers Is Mediated by a Novel Eux Pump Encoded by
emrE. Appl Environ Microbiol. 2015;82(3):939–53.
155. Vera-Cabrera L, Escalante-Fuentes WG, Gomez-Flores M, Ocampo-
Candiani J, Busso P, Singh P, et al. Case of diuse lepromatous
leprosy associated with “Mycobacterium lepromatosis”. J Clin Microbiol.
2011;49(12):4366–8.
156. Truman RW, Kumaresan JA, McDonough CM, Job CK, Hastings RC.
Seasonal and spatial trends in the detectability of leprosy in wild armadillos.
Epidemiol Infect. 1991;106(3):549f–60.
157. Walsh GP, Meyers WM, Binford CH. Naturally acquired leprosy in the
nine-banded armadillo: a decade of experience 1975–1985. J Leukoc Biol.
1986;40(5):645–56.
158. Bruce S, Schroeder TL, Ellner K, Rubin H, Williams T, Wolf JE Jr. Armadillo
exposure and Hansen’s disease: an epidemiologic survey in southern
Texas. J Am Acad Dermatol. 2000;43(2 Pt 1):223–8.
159. Clark BM, Murray CK, Horvath LL, Deye GA, Rasnake MS, Longeld RN.
Case-control study of armadillo contact and Hansen’s disease. Am J Trop
Med Hyg. 2008;78(6):962–7.
160. Domozych R, Kim E, Hart S, Greenwald J. Increasing incidence of leprosy
and transmission from armadillos in Central Florida: A case series. JAAD
Case Rep. 2016;2(3):189–92.
204 Biosafety in Microbiological and Biomedical Laboratories
161. Sharma R, Singh P, Loughry WJ, Lockhart JM, Inman WB, Duthie MS, et al.
Zoonotic Leprosy in the Southeastern United States. Emerg Infect Dis.
2015;21(12):2127–34.
162. O’Brien CR, Malik R, Globan M, Reppas G, McCowan C, Fyfe JA. Feline
leprosy due to Candidatus ‘Mycobacterium lepraefelis’: Further clinical
and molecular characterisation of eight previously reported cases and an
additional 30 cases. J Feline Med Surg. 2017;19(9):919–32.
163. Pfyer GE. Mycobacterium: General Characteristics, Laboratory Detection,
and Staining Procedures. In: Richter SS, editor. Manual of Clinical
Microbiology 11th ed. 1. Washington, DC: ASM Press; 2015. p. 536–69.
164. Esteban J, Munoz-Egea MC. Mycobacterium bovis and Other Uncommon
Members of the Mycobacterium tuberculosis Complex. Microbiol Spectr.
2016;4(6).
165. Grist NR, Emslie J. Infections in British clinical laboratories, 1982–3. J Clin
Pathol. 1985;38(7):721–5.
166. Muller HE. Laboratory-acquired mycobacterial infection. Lancet.
1988;2(8606):331.
167. Pike RM, Sulkin SE, Schulze ML. Continuing Importance of Laboratory-
Acquired Infections. Am J Public Health Nations Health. 1965;55:190–9.
168. Belchior I, Seabra B, Duarte R. Primary inoculation skin tuberculosis by
accidental needlestick. BMJ Case Rep. 2011;2011. pii: bcr1120103496.
169. Menzies D, Fanning A, Yuan L, FitzGerald JM, Canadian Collaborative
Group in Nosocomial Transmission of Tuberculosis. Factors associated
with tuberculin conversion in Canadian microbiology and pathology
workers. Am J Respir Crit Care Med. 2003;167(4):599–602.
170. Reid DD. Incidence of tuberculosis among workers in medical laboratories.
Br Med J. 1957;2(5035):10–4.
171. Centers for Disease Control and Prevention. Acquired multidrug-resistant
tuberculosis–Buenaventura, Colombia, 1998. MMWR Morb Mortal Wkly
Rep. 1998;47(36):759–61.
172. Kaufmann AF, Anderson DC. Tuberculosis control in nonhuman primates.
In: Montali RJ, editor. The Symposia of the National Zoological Park of
Zoo Animals: Proceedings of a Symposium held at the Conservation
and Research Center, National Zoological Park, Smithsonian Institution,
October 6–8 1976. Washington (DC): Smithsonian Institution Press; 1978.
p. 227–34.
173. Allen BW. Survival of tubercle bacilli in heat-xed sputum smears. J Clin
Pathol. 1981;34(7):719–22.

205Section VIII-A: Bacterial Agents
174. Herman P, Fauville-Dufaux M, Breyer D, Van Vaerenbergh B, Pauwels K,
Dai Do Thi C, et al. Biosafety Recommendations for the Contained Use of
Mycobacterium tuberculosis Complex Isolates in Industrialized Countries.
Scientic Institute of Public Health [Internet]. 2006 [cited 2019 May
8]:[about 17 p]. Available from: https://www.biosafety.be/sites/default/les/
mtub_nal_dl.pdf
175. Centers for Disease Control and Prevention [Internet]. Atlanta (GA): Division
of Tuberculosis Elimination; c2015 [cited 2018 Oct 30]. Tuberculosis (TB).
Available from: https://www.cdc.gov/tb/publications/factsheets/testing/igra.htm
176. Al Houqani M, Jamieson F, Chedore P, Mehta M, May K, Marras TK.
Isolation prevalence of pulmonary nontuberculous mycobacteria in Ontario
in 2007. Can Respir J. 2011;18(1):19–24.
177. Billinger ME, Olivier KN, Viboud C, de Oca RM, Steiner C, Holland SM, et al.
Nontuberculous mycobacteria-associated lung disease in hospitalized
persons, United States, 1998–2005. Emerg Infect Dis. 2009;15(10):1562–9.
178. van Ingen J. Diagnosis of nontuberculous mycobacterial infections. Semin
Respir Crit Care Med. 2013;34(1):103–9.
179. Sabin AP, Ferrieri P, Kline S. Mycobacterium abscessus Complex Infections
in Children: A Review. Curr Infect Dis Rep. 2017;19(11):46.
180. Lindeboom JA, Kuijper EJ, Bruijnesteijn van Coppenraet ES, Lindeboom R,
Prins JM. Surgical excision versus antibiotic treatment for nontuberculous
mycobacterial cervicofacial lymphadenitis in children: a multicenter,
randomized, controlled trial. Clin Infect Dis. 2007;44(8):1057–64.
181. Bar-On O, Mussa H, Mei-Zahav M, Prais D, Steuer G, Staer P, et al.
Increasing nontuberculous mycobacteria infection in cystic brosis. J Cyst
Fibros. 2015;14(1):53–62.
182. Candido PH, Nunes Lde S, Marques EA, Folescu TW, Coelho FS, de Moura
VC, et al. Multidrug-resistant nontuberculous mycobacteria isolated from
cystic brosis patients. J Clin Microbiol. 2014;52(8):2990–7.
183. Chappler RR, Hoke AW, Borchardt KA. Primary inoculation with
Mycobacterium marinum. Arch Dermatol. 1977;113(3):380.
184. Biet F, Boschiroli ML, Thorel MF, Guilloteau LA. Zoonotic aspects of
Mycobacterium bovis and Mycobacterium avium-intracellulare complex
(MAC). Vet Res. 2005;36(3):411–36.
185. Kim SY, Shin SJ, Lee NY, Koh WJ. First case of pulmonary disease caused
by a Mycobacterium avium complex strain of presumed veterinary origin in
an adult human patient. J Clin Microbiol. 2013;51(6):1993–5.
206 Biosafety in Microbiological and Biomedical Laboratories
186. Elias J, Frosch M, Vogel U. Neisseria. In: Jorgensen JH, Pfaller MA, Carroll
KC, Funke G, Landry ML, Richter SS, Warnock DW, editors. Manual of
Clinical Microbiology, Volume 1. 11th ed. Washington (DC): ASM Press;
2015. p. 635–51.
187. Bruins SC, Tight RR. Laboratory-acquired gonococcal conjunctivitis. JAMA.
1979;241(3):274.
188. Diena BB, Wallace R, Ashton FE, Johnson W, Platenaude B. Gonococcal
conjunctivitis: accidental infection. Can Med Assoc J. 1976;115(7):609, 12.
189. Malhotra R, Karim QN, Acheson JF. Hospital-acquired adult gonococcal
conjunctivitis. J Infect. 1998;37(3):305.
190. Zajdowicz TR, Kerbs SB, Berg SW, Harrison WO. Laboratory-acquired
gonococcal conjunctivitis: successful treatment with single-dose ceftriaxone.
Sex Transm Dis. 1984;11(1):28–9.
191. U.S. Department of Health and Human Services. Sexually Transmitted
Disease Surveillance 2016. Atlanta (GA): Centers for Disease Control and
Prevention; 2017. 164 p.
192. Centers for Disease Control and Prevention. Sexually Transmitted
Diseases Treatment Guicelines, 2015. Morbidity and Mortality Weekly
Report 64 (3). 2015.
193. Cohn AC, MacNeil JR, Clark TA, Ortega-Sanchez IR, Briere EZ,
Meissner HC, et al. Prevention and control of meningococcal disease:
recommendations of the Advisory Committee on Immunization Practices
(ACIP). MMWR Recomm Rep. 2013;62(RR-2):1–28.
194. Sheets CD, Harriman K, Zipprich J, Louie JK, Probert WS, Horowitz M, et al.
Fatal meningococcal disease in a laboratory worker–California, 2012.
MMWR Morb Mortal Wkly Rep. 2014;63(35):770–2.
195. Centers for Disease Control and Prevention. Laboratory-acquired
meningococcal disease–United States, 2000. MMWR Morb Mortal Wkly
Rep. 2002;51(7):141–4.
196. Patton ME, Stephens D, Moore K, MacNeil JR. Updated Recommendations
for Use of MenB-FHbp Serogroup B Meningococcal Vaccine—Advisory
Committee on Immunization Practices, 2016. MMWR Morb Mortal Wkly
Rep. 2017;66(19):509–13.
197. Immunization of health-care workers: recommendations of the Advisory
Committee on Immunization Practices (ACIP) and the Hospital Infection
Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep.
1997;46(RR-18):1–42.
198. Grogan J, Roos K. Serogroup B Meningococcus Outbreaks, Prevalence,
and the Case for Standard Vaccination. Curr Infect Dis Rep. 2017;19(9):30.

207Section VIII-A: Bacterial Agents
199. Strockbine NA, Bopp CA, Fields PI, Kaper JB, Nataro JP. Escherichia,
Shigella, and Salmonella. In: Jorgensen JH, Pfaller MA, Carroll KC,
Funke G, Landry ML, Richter SS, Warnock DW, editors. Manual of Clinical
Microbiology, Volume 1. 11th ed. Washington (DC): ASM Press; 2015.
p. 685–713.
200. Dekker JP, Frank KM. Salmonella, Shigella, and Yersinia. Clin Lab Med.
2015;35(2):225–46.
201. Alexander DC, Fitzgerald SF, DePaulo R, Kitzul R, Daku D, Levett PN,
et al. Laboratory-Acquired Infection with Salmonella enterica serovar
Typhimurium Exposed by Whole-Genome Sequencing. J Clin Microbiol.
2016;54(1):190–3.
202. Barker A, Duster M, Van Hoof S, Safdar N. Nontyphoidal Salmonella:
An Occupational Hazard for Clinical Laboratory Workers. Appl Biosaf.
2015;20(2):72–4.
203. Grist NR, Emslie JA. Infections in British clinical laboratories, 1984–5.
J Clin Pathol. 1987;40(8):826–9.
204. Nicklas W. Introduction of Salmonellae into a centralized laboratory animal
facility by infected day old chicks. Lab Anim. 1987;21(2):161–3.
205. Steckelberg JM, Terrell CL, Edson RS. Laboratory-acquired Salmonella
Typhimurium enteritis: association with erythema nodosum and reactive
arthritis. Am J Med. 1988;85(5):705–7.
206. Centers for Disease Control and Prevention [Internet]. Atlanta (GA):
National Center for Emerging and Zoonotic Infectious Diseases, Division
of Vector-Borne Diseases; c2018 [cited Oct 30]. Plague. Available from:
https://www.cdc.gov/plague/index.html
207. Majowicz SE, Musto J, Scallan E, Angulo FJ, Kirk M, O’Brien SJ, et al. The
global burden of nontyphoidal Salmonella gastroenteritis. Clin Infect Dis.
2010;50(6):882–9.
208. Ao TT, Feasey NA, Gordon MA, Keddy KH, Angulo FJ, Crump JA. Global
burden of invasive nontyphoidal Salmonella disease, 2010. Emerg Infect
Dis. 2015;21(6).
209. Gambino-Shirley K, Stevenson L, Wargo K, Burnworth L, Roberts J,
Garrett N, et al. Notes from the Field: Four Multistate Outbreaks of Human
Salmonella Infections Linked to Small Turtle Exposure—United States,
2015. MMWR Morb Mortal Wkly Rep. 2016;65(25):655–6.
210. Whiley H, Gardner MG, Ross K. A Review of Salmonella and Squamates
(Lizards, Snakes and Amphibians): Implications for Public Health.
Pathogens. 2017;6(3). pii: E38.

208 Biosafety in Microbiological and Biomedical Laboratories
211. Kariuki S, Gordon MA, Feasey N, Parry CM. Antimicrobial resistance and
management of invasive Salmonella disease. Vaccine. 2015;33 Suppl
3:C21–9.
212. Fuche FJ, Sow O, Simon R, Tennant SM. Salmonella Serogroup C: Current
Status of Vaccines and Why They Are Needed. Clin Vaccine Immunol.
2016;23(9):737–45.
213. Stuart BM, Pullen RL. Typhoid; clinical analysis of 360 cases. Arch Intern
Med (Chic). 1946;78(6):629–61.
214. Buckle GC, Walker CL, Black RE. Typhoid fever and paratyphoid fever:
Systematic review to estimate global morbidity and mortality for 2010.
J Glob Health. 2012;2(1):010401.
215. Crump JA, Luby SP, Mintz ED. The global burden of typhoid fever. Bull
World Health Organ. 2004;82(5):346–53.
216. Centers for Disease Control and Prevention [Internet]. Atlanta (GA): National
Center for Emerging and Zoonotic Infectious Diseases, Division of Foodborne,
Waterborne, and Environmental Diseases; c2018 [cited 2018 Oct 31].
Salmonella. Available from: https://www.cdc.gov/salmonella/index.html
217. Crump JA, Sjolund-Karlsson M, Gordon MA, Parry CM. Epidemiology,
Clinical Presentation, Laboratory Diagnosis, Antimicrobial Resistance, and
Antimicrobial Management of Invasive Salmonella Infections. Clin Microbiol
Rev. 2015;28(4):901–37.
218. Jackson BR, Iqbal S, Mahon B; Centers for Disease Control and Prevention.
Updated recommendations for the use of typhoid vaccine–Advisory
Committee on Immunization Practices, United States, 2015. MMWR Morb
Mortal Wkly Rep. 2015;64(11):305–8.
219. Laboratory acquired infection with Escherichia coli O157. Commun Dis Rep
CDR Wkly. 1994;4(7):29.
220. Escherichia coli O157 infection acquired in the laboratory. Commun Dis
Rep CDR Wkly. 1996;6(28):239.
221. Booth L, Rowe B. Possible occupational acquisition of Escherichia coli
O157 infection. Lancet. 1993;342(8882):1298–9.
222. Burnens AP, Zbinden R, Kaempf L, Heinzer I, Nicolet J. A case of laboratory
acquired infection with Escherichia coli O157:H7. Zentralbl Bakteriol.
1993;279(4):512–7.
223. Rao GG, Saunders BP, Masterton RG. Laboratory acquired
verotoxin producing Escherichia coli (VTEC) infection. J Hosp Infect.
1996;33(3):228–30.
209Section VIII-A: Bacterial Agents
224. Nielsen EM, Skov MN, Madsen JJ, Lodal J, Jespersen JB, Baggesen DL.
Verocytotoxin-producing Escherichia coli in wild birds and rodents in close
proximity to farms. Appl Environ Microbiol. 2004;70(11):6944–7.
225. Bopp DJ, Sauders BD, Waring AL, Ackelsberg J, Dumas N, Braun-Howland E,
et al. Detection, isolation, and molecular subtyping of Escherichia coli
O157:H7 and Campylobacter jejuni associated with a large waterborne
outbreak. J Clin Microbiol. 2003;41(1):174–80.
226. Friedman MS, Roels T, Koehler JE, Feldman L, Bibb WF, Blake P.
Escherichia coli O157:H7 outbreak associated with an improperly
chlorinated swimming pool. Clin Infect Dis. 1999;29(2):298–303.
227. Swerdlow DL, Woodru BA, Brady RC, Grin PM, Tippen S, Donnell
HD Jr, et al. A waterborne outbreak in Missouri of Escherichia coli
O157:H7 associated with bloody diarrhea and death. Ann Intern Med.
1992;117(10):812–9.
228. Walker D, Campbell D. A survey of infections in United Kingdom
laboratories, 1994–1995. J Clin Pathol. 1999;52(6):415–8.
229. National Research Council. Zoonoses. In: National Research Council.
Occupational Health and Safety in the Care and Use of Research Animals.
Washington (DC): National Academy Press; 1997. p. 65–105.
230. Batz MB, Henke E, Kowalcyk B. Long-term consequences of foodborne
infections. Infect Dis Clin North Am. 2013;27(3):599–616.
231. Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, et al.
Clinical practice guidelines by the infectious diseases society of america for
the treatment of methicillin-resistant Staphylococcus aureus infections in
adults and children: executive summary. Clin Infect Dis. 2011;52(3):285–92.
232. Banerjee T, Anupurba S. Colonization with vancomycin-intermediate
Staphylococcus aureus strains containing the vanA resistance gene in a
tertiary-care center in north India. J Clin Microbiol. 2012;50(5):1730–2.
233. Howden BP, Davies JK, Johnson PD, Stinear TP, Grayson ML. Reduced
vancomycin susceptibility in Staphylococcus aureus, including vancomycin-
intermediate and heterogeneous vancomycin-intermediate strains:
resistance mechanisms, laboratory detection, and clinical implications.
Clin Microbiol Rev. 2010;23(1):99–139.
234. Duman Y, Yakupogullari Y, Otlu B, Tekerekoglu MS. Laboratory-acquired
skin infections in a clinical microbiologist: Is wearing only gloves really safe?
Am J Infect Control. 2016;44(8):935–7.
235. Gosbell IB, Mercer JL, Neville SA. Laboratory-acquired EMRSA-15
infection. J Hosp Infect. 2003;54(4):323–5.

210 Biosafety in Microbiological and Biomedical Laboratories
236. Wagenvoort JH, De Brauwer EI, Gronenschild JM, Toenbreker HM,
Bonnemayers GP, Bilkert-Mooiman MA. Laboratory-acquired meticillin-
resistant Staphylococcus aureus (MRSA) in two microbiology laboratory
technicians. Eur J Clin Microbiol Infect Dis. 2006;25(7):470–2.
237. Centers for Disease Control and Prevention [Internet]. Atlanta (GA):
National Center for Emerging and Zoonotic Infectious Diseases, Division
of Healthcare Quality Promotion; c2015 [cited 2018 Oct 31]. Healthcare-
associated Infections. Available from: https://www.cdc.gov/hai/organisms/
visa_vrsa/visa_vrsa.html
238. Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus
aureus: epidemiology, underlying mechanisms, and associated risks. Clin
Microbiol Rev. 1997;10(3):505–20.
239. Pantosti A. Methicillin-Resistant Staphylococcus aureus Associated with
Animals and Its Relevance to Human Health. Front Microbiol. 2012;3:127.
240. Peeling RW, Mabey DC. Syphilis. Nat Rev Microbiol. 2004;2(6):448–9.
241. Fitzgerald JJ, Johnson RC, Smith M. Accidental laboratory infection with
Treponema pallidum, Nichols strain. J Am Vener Dis Assoc. 1976;3(2 Pt 1):
76–8.
242. Chacko CW. Accidental human infection in the laboratory with Nichols
rabbit-adapted virulent strain of Treponema pallidum. Bull World Health
Organ. 1966;35(5):809–10.
243. Turner TB, Hardy PH, Newman B. Infectivity tests in syphilis. Br J Vener Dis.
1969;45(3):183–95.
244. Silver AC, Dunne DW, Zeiss CJ, Bockenstedt LK, Radolf JD, Salazar JC,
et al. MyD88 deciency markedly worsens tissue inammation and
bacterial clearance in mice infected with Treponema pallidum, the agent
of syphilis. PLoS One. 2013;8(8):e71388.
245. Knauf S, Liu H, Harper KN. Treponemal infection in nonhuman primates as
possible reservoir for human yaws. Emerg Infect Dis. 2013;19(12):2058–60.
246. Sena AC, Pillay A, Cox DL, Radolf JD. Treponema and Brachyspira, Human
Host-Associated Spirochetes. In: Jorgensen JH, Pfaller MA, Carroll KC,
Funke G, Landry ML, Richter SS, Warnock DW, editors. Manual of Clinical
Microbiology, Volume 1. 11th ed. Washington (DC): ASM Press; 2015.
p. 1055–81.
247. Centers for Disease Control and Prevention [Internet]. Atlanta (GA):
National Center for Emerging and Zoonotic Infectious Diseases, Division of
Foodborne, Waterborne, and Environmental Diseases; c2018 [cited 2018
Oct 31]. Cholera—Vibrio cholerae infection. Available from: https://www.cdc.
gov/cholera/index.html

211Section VIII-A: Bacterial Agents
248. Centers for Disease Control and Prevention [Internet]. Atlanta (GA):
National Center for Emerging and Zoonotic Infectious Diseases, Division of
Foodborne, Waterborne, and Environmental Diseases; c2018 [cited 2018
Oct 31]. Vibrio Species Causing Vibriosis. Available from: https://www.cdc.
gov/vibrio/surveillance.html
249. Huhulescu S, Leitner E, Feierl G, Allerberger F. Laboratory-acquired
Vibrio cholerae O1 infection in Austria, 2008. Clin Microbiol Infect.
2010;16(8):1303–4.
250. Sheehy TW, Sprinz H, Augerson WS, Formal SB. Laboratory Vibrio cholerae
infection in the United States. JAMA. 1966;197(5):321–6.
251. Lee KK, Liu PC, Huang CY. Vibrio parahaemolyticus infectious for both
humans and edible mollusk abalone. Microbes Infect. 2003;5(6):481–5.
252. Daniels NA, Ray B, Easton A, Marano N, Kahn E, McShan AL 2nd, et al.
Emergence of a new Vibrio parahaemolyticus serotype in raw oysters:
A prevention quandary. JAMA. 2000;284(12):1541–5. Erratum in: JAMA.
2001;285(2):169.
253. American Public Health Association. Cholera and other vibrioses. In:
Heymann DL, editor. Control of Communicable Diseases Manual. 20th ed.
Washington (DC): APHA Press; 2015. p. 102–14.
254. Wong KK, Burdette E, Mahon BE, Mintz ED, Ryan ET, Reingold
AL. Recommendations of the Advisory Committee on Immunization
Practices for Use of Cholera Vaccine. MMWR Morb Mortal Wkly Rep.
2017;66(18):482–5.
255. American Committee of Medical Entomology; American Society of Tropical
Medicine and Hygiene. Arthropod Containment Guidelines, Version 3.2.
A project of the American Committee of Medical Entomology and American
Society of Tropical Medicine and Hygiene. Vector Borne Zoonotic Dis.
2019;19(3):152–73.
256. Centers for Disease Control and Prevention. Fatal laboratory-acquired
infection with an attenuated Yersinia pestis Strain–Chicago, Illinois, 2009.
MMWR Morb Mortal Wkly Rep. 2011;60(7):201–5.
257. Eads DA, Hoogland JL. Precipitation, Climate Change, and
Parasitism of Prairie Dogs by Fleas that Transmit Plague. J Parasitol.
2017;103(4):309–19.
258. Burmeister RW, Tigertt WD, Overholt EL. Laboratory-acquired pneumonic
plague. Report of a case and review of previous cases. Ann Intern Med.
1962;56:789–800.
259. Titball RW, Williamson ED. Yersinia pestis (plague) vaccines. Expert Opin
Biol Ther. 2004;4(6):965–73.
212 Biosafety in Microbiological and Biomedical Laboratories
Section VIII-B: Fungal Agents
Blastomyces dermatitidis and Blastomyces gilchristii
Blastomyces dermatitidis is a dimorphic fungal pathogen existing in nature and
in laboratory cultures at room temperature as a lamentous mold with asexual
spores (conidia) that are the infectious particles; conidia convert to large budding
yeasts under the appropriate culture conditions in vitro at 37°C and in the
parasitic phase in vivo in warm-blooded animals. Infections with B. dermatitidis
occur when conidia are inhaled or when yeast forms are injected. The sexual
stage is an Ascomycete with infectious ascospores. Blastomyces gilchristii was
recently recognized as a novel species found predominantly in northwestern
Ontario, Wisconsin, and Minnesota.
1
Occupational Infections
Three groups are at greatest risk of Laboratory-associated infection (LAI):
microbiologists, veterinarians, and pathologists.
2
Laboratory-associated local
infections have been reported following accidental parenteral inoculation with
infected tissues or cultures containing yeast forms of B. dermatitidis.
3–9
Laboratory
infections have also occurred following the presumed inhalation of conidia
from mold-form cultures.
10,11
Infection with B. dermatitidis can be pulmonary,
cutaneous, or disseminated. Disseminated blastomycosis usually begins with
pulmonary infection. Transmission occurs rarely via animal bites, sexual means,
or vertical transmission. Forestry workers and other workers with outdoor occupa-
tions have developed blastomycosis after exposure to contaminated soil or plant
material, particularly moist soil with decaying vegetation.
12
At least 11 reported
LAIs with two fatalities have occurred.
13,14
Natural Modes of Infection
The fungus has been reported in multiple geographically separated countries, but
it is best known as a fungus endemic to North America and in association with
plant material in the environment. Infections are not communicable but require
common exposure from a point source. Although presumed to dwell within the
soil of endemic areas, B. dermatitidis is extremely dicult to isolate from soil.
Outbreaks associated with the exposure of people to decaying wood have been
reported. However, outdoor activities were not a risk factor in the largest outbreak
reported through 2017; instead, the large Hmong population in the area of
Wisconsin that was involved in the outbreak may have had an underlying genetic
predisposition.
15
B. dermatitidis infections are most common in humans and dogs
though other animals, such as cats and horses, may also develop blastomycosis.
Human-to-human transmission occurs rarely via perinatal or sexual transmission.

213Section VIII-B: Fungal Agents
Laboratory Safety and Containment Recommendations
Yeast forms may be present in the tissues of infected animals and in clinical
specimens. Parenteral (subcutaneous) inoculation of these materials may
cause local skin infection and granulomas. Mold-form cultures of B. dermatitidis
containing infectious conidia and processing of soil or other environmental
samples may pose a hazard of aerosol exposure.
BSL-3 practices, containment equipment, and facilities are recommended for
handling sporulating mold-form cultures already identied as B. dermatitidis and
soil or other environmental samples known or likely to contain infectious conidia.
BSL-2 and ABSL-2 practices, containment equipment, and facilities are recom-
mended for activities with clinical materials, animal tissues, yeast-form cultures,
and infected animals.
Special Issues
Transfer of Agent Importation of this agent requires CDC and/or USDA impor-
tation permits. Domestic transport of this agent may require a permit from USDA
APHIS VS. A DoC permit may be required for the export of this agent to another
country. See Appendix C for additional information.
Coccidioides immitis and Coccidioides posadasii
Coccidioides spp. are endemic to the Sonoran Desert of the western hemisphere
including northern Mexico, southern Arizona, central and southern California, and
western Texas. In recent decades, C. immitis has been divided into two species:
C. immitis and C. posadasii.
16
These species are dimorphic fungal pathogens
existing in nature and in laboratory cultures at room temperature as lamentous
molds with asexual spores (single-cell arthroconidia three to ve microns in size)
that are the infectious particles. The arthroconidia convert to spherules under
the appropriate culture conditions in vitro at 37°C and in vivo in warm-blooded
animals.
Occupational Infections
Laboratory-associated coccidioidomycosis is a documented hazard of working with
sporulating cultures of Coccidioides spp.
17–19
Occupational exposure in archeolo-
gists and prison employees in endemic regions has been associated with high dust
exposure.
20,21
Attack rates for laboratory and occupational exposures where a larger
number of spores are inhaled are higher than for non-occupational environmental
exposures. Smith reported that 28 of 31 (90%) Laboratory-associated infections in
his institution resulted in clinical disease, but more than half of infections acquired in
nature were asymptomatic.
22
Risk of respiratory infection from exposure to infected
tissue or aerosols of infected secretions is very low. Accidental percutaneous
inoculation has typically resulted in localized granuloma formation.
23
214 Biosafety in Microbiological and Biomedical Laboratories
Natural Modes of Infection
Single spores in environmental exposures can produce infections by the respi-
ratory route. Peak exposures occur during arid seasons, and exposure can also
occur during natural disasters such as earthquakes.
24
Coccidioides spp. grow in
infected tissue as larger multicellular spherules up to 70 microns in diameter and
pose little or no risk of infection from direct exposure.
Most infections from environmental exposure are subclinical and result in
life-long protection from subsequent exposures. The incubation period is one
to three weeks, and the disease manifests as community-acquired pneumonia
with immunologically mediated fatigue, skin rashes, and joint pain. One of the
synonyms for coccidioidomycosis is desert rheumatism. A small proportion of
infections are complicated by hematogenous dissemination from the lungs to
other organs, most frequently skin, the skeleton, and the meninges. Disseminated
infection is much more likely in persons with cellular immunodeciencies (e.g.,
AIDS, organ transplant recipient, lymphoma, receipt of tumor necrosis factor
[TNF] inhibitors) and in pregnant women in the third trimester.
Laboratory Safety and Containment Recommendations
Because of their size, arthroconidia are conducive to ready dispersal in air and
retention in the deep pulmonary spaces. The much larger size of the spherule
considerably reduces the eectiveness of this form of the fungus as an airborne
pathogen.
Spherules of the fungus may be present in clinical specimens and animal tissues,
and infectious arthroconidia may be present in mold cultures and soil or other
samples from natural sites. Inhalation of arthroconidia from either environmental
samples or mold isolates is a serious laboratory hazard.
19
Most exposures occur
due to personnel handling cultures of unknown infectious status on the bench,
rather than in a BSC. Personnel should be aware that infected animal or human
clinical specimens or tissues stored or shipped under temperature and nutrient
conditions that could promote germination of arthroconidia pose a theoretical
laboratory hazard. Slide cultures should never be prepared from unknown hyaline
(colorless) isolates, as they could contain Coccidioides spp.
BSL-3 practices, containment equipment, and facilities are recommended for
propagating and manipulating sporulating cultures already identied as Coccid-
ioides spp. and for processing soil or other environmental materials known or
suspected to contain infectious arthroconidia. Experimental animal studies should
be done at BSL-3 when challenge is via the intranasal or pulmonary route.
BSL-2 practices, containment equipment, and facilities are recommended for
handling and processing clinical specimens, identifying isolates, and processing
animal tissues that may contain Coccidioides spp. ABSL-2 practices, containment

215Section VIII-B: Fungal Agents
equipment, and facilities are appropriate for experimental animal studies when
the route of challenge is parenteral.
Special Issues
Transfer of Agent Importation of this agent requires CDC and/or USDA impor-
tation permits. Domestic transport of this agent may require a permit from USDA
APHIS VS. A DoC permit may be required for the export of this agent to another
country. See Appendix C for additional information.
Histoplasma capsulatum
Histoplasma capsulatum is a dimorphic fungal pathogen existing in nature and
in laboratory cultures at room temperature as a lamentous mold with asexual
spores (macro-and/or microconidia); microconidia are the infectious particles that
convert to small budding yeasts under the appropriate culture conditions in vitro
at 37°C and in the parasitic phase in vivo. The sexual stage is an Ascomycete
with infectious ascospores.
Specic hazards/risks associated with Histoplasma include:
1. Immunocompromised individuals are at increased risk of infection and
experience more severe infections and higher mortality;
2. Dissemination throughout body has resulted in death but usually results
in chronic infection;
3. Previously controlled infections can be re-activated when cellular
immunity is impaired;
4. The adrenal gland can be destroyed by visceral infection; and
5. 5–20% of cases involve the central nervous system and appear as
chronic meningitis or focal brain lesions.
Occupational Infections
Laboratory-associated histoplasmosis is a documented hazard in facilities
conducting diagnostic or investigative work.
9,25–27
Pulmonary infections have
resulted from handling mold form cultures.
28,29
Local infection has resulted from
skin puncture during autopsy of an infected human,
30
from accidental needle
inoculation of a viable culture,
31
from accidental inoculation with a lymph node
biopsy sample from an infected patient,
32
and from spray into the eye.
33
Collecting
and processing soil samples from endemic areas has caused pulmonary infec-
tions in laboratory workers,
34
and one death was reported in 1962.
35
Conidia are
resistant to drying and may remain viable for long periods of time. The small size
of the infective conidia (less than ve microns) is conducive to airborne dispersal
and intrapulmonary retention. Work with experimental animals suggests that
hyphal fragments are also capable of serving as viable inocula.
25

216 Biosafety in Microbiological and Biomedical Laboratories
Natural Modes of Infection
The fungus is distributed worldwide in the environment and is associated with
bird and bat feces. It has been isolated from soil, often in river valleys, between
latitudes 45°N and 45°S. Histoplasmosis is naturally acquired by the inhalation of
infectious microconidia, which can survive in excess of ten years in soil.
25
Infec-
tions are not transmissible from person-to-person but require common exposure
to a point source. Large outbreaks have been reported from exposure to soil or
plant material contaminated with bird or bat feces
36,37
and from exposure to soil
during construction projects.
38
Laboratory Safety and Containment Recommendations
The infective stage of this dimorphic fungus (microconidia) is present in
sporulating mold form cultures and in soil from endemic areas. The yeast form is
present in tissues or uids from infected animals and may produce local infection
following parenteral inoculation or splash onto mucous membranes.
BSL-3 practices, containment equipment, and facilities are recommended for
propagating sporulating cultures of H. capsulatum in the mold form, as well as
for processing soil or other environmental materials known or likely to contain
infectious conidia.
BSL-2 and ABSL-2 practices, containment equipment, and facilities are
recommended for handling and processing clinical specimens; identifying
isolates, animal tissues, and mold cultures; identifying cultures that may contain
Histoplasma in routine diagnostic laboratories; and for inoculating experimental
animals, regardless of route. Any culture identifying dimorphic fungi should be
handled in a Class II BSC. Protective eyewear should be worn when splash(es)
to mucous membranes may occur.
Special Issues
Transfer of Agent Importation of this agent requires CDC and/or USDA impor-
tation permits. Domestic transport of this agent may require a permit from USDA
APHIS VS. A DoC permit may be required for the export of this agent to another
country. See Appendix C for additional information.
Sporothrix schenckii species complex
The Sporothrix schenckii species complex is composed of at least six species
(Sporothrix brasiliensis, Sporothrix mexicana, Sporothrix globosa, S. schenckii
sensu stricto, Sporothrix luriei, and Sporothrix albicans) of dimorphic fungal
pathogens existing in nature and in laboratory cultures at room temperature as
lamentous mold with asexual spores (conidia); the conidia are the infectious
particles that convert to small budding yeasts in the parasitic phase in vivo.
39
The sexual stage is unknown.

217Section VIII-B: Fungal Agents
Occupational Infections
Most cases of sporotrichosis are reported sporadically following accidental
inoculation with contaminated material. Large outbreaks have been documented
in persons occupationally or recreationally exposed to soil or plant material
containing the fungus. However, members of the S. schenckii species complex
have caused a substantial number of local skin or eye infections in laboratory
personnel.
40
Most occupational cases have been associated with accidents and
have involved splashing culture material into the eye,
41,42
scratching,
43
injecting
infected material into the skin,
44
or being bitten by an experimentally infected
animal.
45,46
Skin infections without any apparent trauma to the skin have also
resulted from handling cultures
47–49
and from the necropsy of animals.
50
Laboratory Safety and Containment Recommendations
Although localized skin and eye infections have occurred in an occupational
setting, no pulmonary infections have been reported as a result of laboratory
exposure. It should be noted that serious disseminated infections have been
reported in immunocompromised persons.
51
BSL-2 and ABSL-2 practices, containment equipment, and facilities are recom-
mended for laboratory handling of clinical specimens suspected of containing
infectious particles, soil and vegetation suspected to contain S. schenckii, and
experimental animal activities with S. schenckii. Any culture identifying dimorphic
fungi should be handled in a Class II BSC. Protective eyewear should be worn
when splash(es) to mucous membranes may occur.
Special Issues
Transfer of Agent Importation of this agent requires CDC and/or USDA impor-
tation permits. Domestic transport of this agent may require a permit from USDA
APHIS VS. A DoC permit may be required for the export of this agent to another
country. See Appendix C for additional information.
Miscellaneous Yeast and mold organisms causing human infection
The majority of mold organisms in Table 1 cause infection in compromised hosts.
Risk factors may include neutropenia, previous exposure to antibiotics, treatment
for cancer, especially leukemia and lymphoma, organ or stem cell transplant,
severe burns, HIV infection with low CD4 cell counts, and placement of central
lines or other monitoring devices.
The majority of these organisms are found in the environment and are transmitted
through exposure to air, water, or dust. Mold conidia can be inhaled or injected
subcutaneously through trauma or other accidental inoculation. Dermatophytes
can be transmitted through the person-to-person route, the animal-to-person
route, and the environment-to-person route.
218 Biosafety in Microbiological and Biomedical Laboratories
Candida yeasts are found as part of the normal human respiratory or gastroin-
testinal ora and may cause infection after exposure to antibiotics, abdominal
surgery, or other causes. Yeast outbreaks in hospitals can occur through
exposure to contaminated hospital equipment, foods, or medications. Some
yeast species, most notably Candida auris,
52
cause concern because they display
resistance to multiple antifungal drugs. Cryptococcus basidiospores are found
in the environment largely associated with bird droppings or certain trees. They
cause infection in compromised hosts after inhaling fungal spores.
BSL-2 and ABSL-2 practices, containment equipment, and facilities are
recommended for propagating and manipulating cultures known to contain these
agents. All unknown mold cultures should be handled in a Class II BSC.
Table 1. Miscellaneous Yeast and Mold
Agent
Occupational
Infection
Natural Mode of
Infection Biosafety Level
Candida species Not common From point source in
environment; from
gastrointestinal tract
into bloodstream
BSL-2
Cryptococcus
neoformans and C. gattii
Occasional
inoculation into skin
when working with
laboratory animals
Inhalation from point
source in environment.
No person-to-person
transmission reported.
BSL-2 (handle in BSC
to prevent laboratory
contamination)
Dermatophyte
molds: Trichophyton,
Microsporum,
Epidermophyton species
Occasional direct
inoculation from
handling isolates
or contaminated
materials
Person-to-person;
common exposure to a
point source; handling
infected animals
BSL-2
Hyaline Molds:
Aspergillus spp.,
Fusarium spp.
Not common Presumed inhalation;
subcutaneous
inoculation from
environmental source
BSL-2 (handle in BSC
to prevent laboratory
contamination)
Talaromyces (Penicillium)
marneei
Occasional direct
inoculation when
working with
laboratory animals;
rare inhalation in
immunocompromised
individual
Mostly inhalation (in
immunocompromised
hosts)
BSL-2 (handle in BSC
to prevent laboratory
contamination)
Continued on next page ►

219Section VIII-B: Fungal Agents
Agent
Occupational
Infection
Natural Mode of
Infection Biosafety Level
Dematiaceous
Molds: Bipolaris spp.;
Cladophialophora
bantiana; Exophiala
spp; Exserohilum
rostratum; Fonsecaea
spp.; Pseudallescheria
spp.; Rhinocladiella spp.;
Scedosporium spp.;
Verruconis (Ochroconis)
gallopava
Not reported,
but inhalation or
subcutaneous
inoculation are
possible routes of
exposure
Presumed inhalation;
subcutaneous
inoculation from
environmental source.
C. bantiana,
E. dermatitidis,
V. gallopava, and
R. mackenziei are
neurotropic.
C. bantiana can cause
disseminated infection
in otherwise healthy
hosts.
BSL-2 (handle in BSC
to prevent laboratory
contamination)
Mucormycete molds:
Mucor spp.; Rhizopus
spp.; Rhizomucor spp.;
Lichtheimia (Absidia) spp.
Not reported Presumed inhalation;
subcutaneous
inoculation from
environmental source;
ingestion
BSL-2 (handle in BSC
to prevent laboratory
contamination)
References
1. Brown EM, McTaggart LR, Zhang SX, Low DE, Stevens, DA, Richardson SE.
Phylogenetic analysis reveals a cryptic species Blastomyces gilchristii, sp.
nov. within the human pathogenic fungus Blastomyces dermatitidis. PLoS
One. 2013;8(3):e59237. Erratum in: PLoS One. 2016.
2. DiSalvo AF. The epidemiology of blastomycosis. In: Al-Doory Y, DiSalvo AF,
editors. Blastomycosis. New York: Plenum Medical Book Company; 1992.
p. 75–104.
3. Evans N. A clinical report of a case of blastomycosis of the skin from
accidental inoculation. JAMA. 1903;40(26):1172–5.
4. Harrell ER. The known and the unknown of the occupational mycoses.
In: University of Michigan School of Public Health, author. Occupational
diseases acquired from animals. Continued education series, No. 124. Ann
Arbor (MI): University of Michigan, School of Public Health; 1964. p. 176–8.
5. Larsh HW, Schwarz J. Accidental inoculation blastomycosis. Cutis.
1977;19(3):334–5, 337.
6. Larson DM, Eckman MR, Alber RL, Goldschmidt VG. Primary cutaneous
(inoculation) blastomycosis: an occupational hazard to pathologists. Amer J
Clin Pathol. 1983;79(2):253–5.
7. Wilson JW, Cawley EP, Weidman FD, Gilmer WS. Primary cutaneous North
American blastomycosis. AMA Arch Dermatol. 1955;71(1):39–45.
8. Graham WR Jr, Callaway JL. Primary inoculation blastomycosis in a
veterinarian. J Am Acad Dermatol.1982;7(6):785–6.
220 Biosafety in Microbiological and Biomedical Laboratories
9. Schwarz J, Kauman CA. Occupational hazards from deep mycoses. Arch
Dermatol. 1977;113(9):1270–5.
10. Baum GL, Lerner PI. Primary pulmonary blastomycosis: a laboratory
acquired infection. Ann Intern Med. 1970;73(2):263–5.
11. Denton JF, Di Salvo AF, Hirsch ML. Laboratory-acquired North American
blastomycosis. JAMA. 1967;199(12):935–6.
12. Centers for Disease Control and Prevention. Blastomycosis acquired
occupationally during prairie dog relocation—Colorado, 1998. MMWR Morb
Mortal Wkly Rept. 1999;48(5):98–100.
13. Pike RM. Laboratory-associated infections. Summary and analysis of 3921
cases. Health Lab Sci. 1976;13(2):105–14.
14. Schell WA. Mycotic Agents. In: Wooley DP, Byers KB, editors. Biological
Safety: Principles and Practices. 5th ed. Washington (DC): ASM Press;
2017. p. 147–62.
15. Roy M, Benedict K, Deak E, Kirby MA, McNeil JT, Stickler CJ, et al. A large
community outbreak of blastomycosis in Wisconsin with geographic and
ethnic clustering. Clin Infect Dis. 2013;57(5):655–62.
16. Fisher MC, Koenig GL, White TJ, Taylor JW. Molecular and phenotypic
description of Coccidioides posadasii sp nov, previously recognized
as the non-California population of Coccidioides immitis. Mycologia.
2002;94(1):73–84.
17. Pappagianis D. Coccidioidomycosis (San Joaquin or Valley Fever).
In: DiSalvo AF, editor. Occupational mycoses. Philadelphia (PA): Lea and
Febiger; 1983. p. 13–28.
18. Nabarro JD. Primary pulmonary coccidioidomycosis: case of laboratory
infection in England. Lancet. 1948;1(6513):982–4.
19. Stevens DA, Clemons KV, Levine HB, Pappagianis D, Baron EJ, Hamilton
JR, et al. Expert opinion: what to do when there is Coccidioides exposure in
a laboratory. Clin Infect Dis. 2009;49(6):919–23.
20. Petersen LR, Marshall SL, Barton-Dickson C, Hajjeh RA, Lindsley MD,
Warnock DW, et al. Coccidioidomycosis among workers at an archeological
site, northeastern Utah. Emerg Infect Dis. 2004;10(4):637–42.
21. de Perio MA, Niemeier RT, Burr GA. Coccidioides exposure and
coccidioidomycosis among prison employees, California, United States.
Emerg Infect Dis. 2015;21(6):1031–3.
22. Wilson JW, Smith CE, Plunkett OA. Primary cutaneous coccidioidomycosis:
the criteria for diagnosis and a report of a case. Calif Med.
1953;79(3):233–9.
221Section VIII-B: Fungal Agents
23. Tomlinson CC, Bancroft P. Granuloma Coccidioides: report of a case
responding favorably to antimony and potassium tartrate. JAMA.
1928;91(13):947–51.
24. Schneider E, Hajjeh RA, Spiegel RA, Jibson RW, Harp EL, Marshall
GA, et al. A coccidioidomycosis outbreak following the Northridge, Calif,
earthquake. JAMA. 1997;277(11):904–8.
25. Furcolow ML. Airborne histoplasmosis. Bacteriol Rev. 1961;25:301–9.
26. Pike RM. Past and present hazards of working with infectious agents. Arch
Pathol Lab Med. 1978;102(7):333–6.
27. Pike RM. Laboratory-associated infections: Summary and analysis of 3921
cases. Health Lab Sci. 1976;13(2):105–14.
28. Murray JF, Howard D. Laboratory-acquired histoplasmosis. Am Rev Respir
Dis. 1964;89:631–40.
29. Sewell DL. Laboratory-associated infections and biosafety. Clin Microbiol
Rev. 1995;8(3):389–405.
30. Tosh FE, Balhuizen J, Yates JL, Brasher CA. Primary cutaneous
histoplasmosis: report of a case. Arch Intern Med. 1964;114:118–9.
31. Tesh RB, Schneidau JD. Primary cutaneous histoplasmosis. N Engl J Med.
1966;275(11):597–9.
32. Buitrago MJ, Gonzalo-Jimenez N, Navarro M, Rodriguez-Tudela JL,
Cuenca-Estrella M. A case of primary cutaneous histoplasmosis acquired
in the laboratory. Mycoses 2011;54(6):e859–61.
33. Spicknall CG, Ryan RW, Cain A. Laboratory-acquired histoplasmosis.
N Engl J Med. 1956;254(5):210–4.
34. Vanselow NA, Davey WN, Bocobo FC. Acute pulmonary histoplasmosis in
laboratory workers: report of 2 cases. J Lab Clin Med. 1962;59:236–43.
35. Pike RM. Laboratory-associated infections: incidence, fatalities, causes,
and prevention. Annu Rev Microbiol. 1979;33:41–66.
36. Chamany S, Mirza SA, Fleming JW, Howell JF, Lenhart SW, Mortimer VD,
et al. A large histoplasmosis outbreak among high school students in
Indiana, 2001. Pediatr Infect Dis J. 2004;23(10):909–14.
37. Ho GL, Bigler WJ. The role of bats in the propagation and spread of
histoplasmosis: a review. J Wildl Dis. 1981;17:191–6.
38. Morgan J, Cano MV, Feikin DR, Phelan M, Monroy OV, Morales PK, et al.
A large outbreak of histoplasmosis among American travelers associated
with a hotel in Acapulco, Mexico, Spring 2001. Am J Trop Med Hyg.
2003;69(6):663–9.
222 Biosafety in Microbiological and Biomedical Laboratories
39. Lopez-Romero E, Reyes-Montes Mdel R, Perez-Torres A, Ruiz-Baca
E, Villagomez-Castro JC, Mora-Montes HM, et al. Sporothrix schenckii
complex and sporotrichosis, an emerging public health problem. Future
Microbiol. 2011;6(1):85–102.
40. Ishizaki H, Ikeda M, Kurata Y. Lymphocutaneous sporotrichosis caused by
accidental inoculation. J Dermatol. 1979;6(5):321–3.
41. Fava A. Un cas de sporotrichose conjonctivale et palpébrale primitives.
Ann d’ocul. 1909:338–43. French.
42. Wilder WH, McCullough CP. Sporotrichosis of the eye. JAMA.
1914;62(15):1156–60.
43. Carougeau M. Premier cas Africain de sporotrichose de deBeurmann:
transmission de la sporotrichose du mulet a l’homme. Bull Mém Soc Méd
Hôp de Paris. 1909;28:507–10. French.
44. Thompson DW, Kaplan W. Laboratory-acquired sporotrichosis.
Sabouraudia. 1977;15(2):167–70.
45. Jeanselme E, Chevallier P. Chancres sporotrichosiques des doigts produits
par la morsure d’un rat inoculé de sporotrichose. Bull Mém Soc Méd Hôp
de Paris. 1910:176–8. French.
46. Jeanselme E, Chevallier P. Transmission de la sporotrichose a l’homme
par les morsures d’un rat blanc inoculé avec une nouvelle variété de
Sporotrichum: Lymphangite gommeuse ascendante. Bull Mém Soc Méd
Hôp de Paris. 1911;31(3):287–301. French.
47. Meyer KF. The relation of animal to human sporotrichosis: studies on
American sporotrichosis III. JAMA. 1915;65(7):579–85.
48. Norden A. Sporotrichosis; clinical and laboratory features and a serologic
study in experimental animals and humans. Acta Pathol Microbiol Scand
Suppl. 1951;89:1–119.
49. Cooper CR, Dixon DM, Salkin IF. Laboratory-acquired sporotrichosis.
J Med Vet Mycol. 1992;30(2):169–71.
50. Fielitz H. Ueber eine Laboratoriumsinfektion mit dem Sporotrichum de
Beurmanni. Centralbl. f. Bakt. etc. I. Abt. Originale. 1910;55(5):361–70.
German.
51. Sugar AM, Lyman CA. Sporothrix schenckii. In: Sugar AM, Lyman CA. A
practical guide to medically important fungi and the diseases they cause.
Philadelphia (PA): Lippincott-Raven; 1997. p. 86–8.
52. McCarthy MW, Walsh TJ. Containment strategies to address the expanding
threat of multidrug-resistant Candida auris. Expert Rev Anti Infect Ther.
2017;15(12):1095–99.

223Section VIII-C: Parasitic Agents
Section VIII-C: Parasitic Agents
General Issues
This section focuses on potential hazards of working in settings in which
exposures to viable parasites could occur, and approaches to decrease the
likelihood of accidental exposures. Available data are limited; the perspective
provided is based on review of the literature regarding reported cases of occupa-
tionally-acquired parasitic infections, available information for selected parasites
regarding potential intervention measures (e.g., disinfection approaches), and
knowledge about parasite biology and about the epidemiology and clinical
aspects of parasitic infections. Additional details regarding occupationally-
acquired cases of parasitic infections and recommendations for post-exposure
management are available elsewhere,
1–3
as is further perspective about
zoonoses of occupational health importance in laboratory animal research.
4
Information about diagnosing and treating parasitic infections and perspective
regarding special considerations for persons who are immunocompromised
or pregnant can be obtained from various reference materials, including the
website of CDC’s Division of Parasitic Diseases and Malaria, and are available
at https://www.cdc.gov/parasites. Diagnostic resources and information about
parasitic life cycles, including routes of transmission, are available through
CDC’s DPDx website at https://www.cdc.gov/dpdx.
Note: Microsporidia historically were considered parasites but are now recognized
by most experts as fungi. However, because of their traditional association with
parasitology, microsporidia are discussed in Section VIII-C: Parasitic Agents.
Blood and Tissue Protozoal Parasites
In descending order of total number of reported cases of infection reported in the
literature, the blood and tissue protozoal parasites that have been associated with
documented cases of occupationally acquired infection are: Trypanosoma cruzi,
Plasmodium spp., Toxoplasma gondii, Leishmania spp., and Trypanosoma brucei
subspp.
1
Other blood/tissue protozoa of potential concern include Babesia spp.,
the free-living amebae, including Acanthamoeba spp., Balamuthia mandrillaris,
Naegleria fowleri, and Sappinia pedata; and the Sarcocystis spp. that can cause
intramuscular sarcocystosis. In addition, various genera/species of microsporidia
(now classied as fungi) may pose an occupational risk for extraintestinal infection;
see below regarding an occupationally-acquired case of microsporidiosis.
In alphabetical order: Leishmania spp. cause various syndromes, including
visceral, cutaneous, and mucosal leishmaniasis (clinical presentation is in part
species dependent); Plasmodium spp. cause malaria; T. gondii causes toxoplas-
mosis; T. cruzi causes American trypanosomiasis (Chagas disease); and T. brucei
subsp. gambiense and subsp. rhodesiense cause human African trypanosomiasis
224 Biosafety in Microbiological and Biomedical Laboratories
(sleeping sickness). Depending in part on parasite and host factors, infective
stages of these parasites may be found in the bloodstream, either briey
(e.g., during a particular phase of the infection), intermittently, or during all or
most of the course of the infection. Among these parasites, tissue tropisms
vary by genus and species, including which, if any, tissues/organs may become
infected and whether the tissue and blood stages of the parasite dier. Some
of these pathogens have been reported to be transmitted via blood transfusion,
organ/tissue transplantation, and congenitally.
5–7
Occupational Infections
Occupationally-acquired cases of infection with Leishmania spp., Plasmodium
spp., T. gondii, and Trypanosoma spp. have been reported. The most commonly
reported modes of transmission have included sharps (e.g., needlestick) injuries
and other percutaneous exposures (e.g., through preexisting cuts, breaks,
or microabrasions).
1,2
Vector-borne transmission to laboratorians has been
reported, particularly for Plasmodium spp. (P. falciparum, P. vivax, and the
simian parasite P. cynomolgi) but also for T. cruzi and Leishmania major.
1
Other
reported laboratory routes of transmission have included mucous membrane
exposures (T. gondii, Leishmania spp., and T. cruzi) and ingestion (T. gondii).
1,2
Laboratory-associated cases of infection with Leishmania spp., T. gondii, and
T. cruzi have also been reported in persons who were working with these
organisms but did not recall a discrete accident or exposure.
1,2
Laboratory-associated cases of infection with blood/tissue protozoa may range
from asymptomatic to severe. One individual with a reported case of laboratory-
associated Leishmania infection developed clinical manifestations consistent
with visceral involvement (e.g., fever, splenomegaly, leukopenia);
1,2
this case
was caused either by L. donovani or by L. infantum, which is in the L. donovani
species complex. The other laboratorians with reported cases of occupational-
ly-acquired Leishmania infection (including, but not limited to, the other persons
infected with parasites in the L. donovani species complex) developed skin
lesions (cutaneous leishmaniasis), with or without associated lymphadenopathy.
1,2
One of the individuals who developed cutaneous leishmaniasis ultimately
developed mucosal leishmaniasis as a sequela. In this instance, the etiologic
agent was L. amazonensis, a species found in parts of South America. Overall,
the exposure routes for the reported laboratory-associated cases of Leishmania
infection have included accidental needlestick injuries, preexisting non-intact skin,
mucosal contact, and the bite of an infected sand y in an insectary.
1
Occupationally-acquired Plasmodium infection may be associated with clinical
manifestations such as fever, chills, fatigue, and hemolytic anemia. Malaria
may be severe and life-threatening, particularly if caused by P. falciparum.
Mosquito-transmitted (sporozoite-induced) Plasmodium infections have been
225Section VIII-C: Parasitic Agents
documented repeatedly in laboratory settings.
1
The other reported cases
of occupationally-acquired Plasmodium infection have occurred in persons
(including healthcare workers) who had accidental sharps injuries or exposures
of non-intact skin.
1,2
Laboratory-associated T. gondii infection may range from asymptomatic to
relatively mild (e.g., u-like symptoms, rash, lymphadenopathy) to life-threatening
(e.g., myocarditis and encephalitis). Laboratorians have become infected with
T. gondii via ingestion of sporulated oocysts from feline fecal specimens, as
well as via percutaneous (e.g., through needlestick injuries or non-intact skin) or
mucosal contact with tachyzoites or bradyzoites from human or animal specimens
(e.g., peritoneal uid from experimentally infected rodents) or cultures.
1,2
The clinical manifestations of the acute phase of T. cruzi infection may include
swelling and redness at the site of exposure, fever, rash, and lymphadenopathy.
Life-threatening myocarditis and meningoencephalitis may develop. Approxi-
mately 20% to 30% of chronically infected persons ultimately develop clinical
manifestations, typically cardiac and less often gastrointestinal (megaesophagus
or megacolon). Laboratorians have become infected with T. cruzi via percuta-
neous or mucosal exposures, such as to blood from experimentally infected
animals or to feces from infected triatomine bugs.
Infection with T. b. rhodesiense (East African) and T. b. gambiense (West
African), which are vector-borne in nature (see below), may cause swelling
and redness at the site of exposure, as well as various clinical manifestations
during the hemolymphatic stage of the infection. East African trypanosomiasis
typically is associated with a more acute course than the West African form,
with early invasion of the central nervous system (CNS). After the parasite (of
either subspecies) invades the CNS, the infection typically is fatal unless treated.
Laboratorians have become infected with T. brucei subspp. through sharps
injuries or non-intact skin.
1,2
Various genera/species of microsporidia found naturally in non-human animals
can cause extraintestinal infection in humans. Tissue tropisms vary by genus/
species and also may be aected by host factors. Spores (i.e., the infective form)
of microsporidia are hardy and can survive for long periods in the environment;
ingestion is the primary route of transmission in nature, whereas other exposure
routes could cause infection in laboratory settings. The one reported laboratory-
associated case of microsporidiosis—a case of keratoconjunctivitis without
systemic symptoms—occurred in an immunocompetent laboratorian who was
accidentally exposed to Encephalitozoon cuniculi “when several drops of culture
supernatant containing several million spores were spilled into both eyes.”
8
No laboratory-associated cases of intramuscular sarcocystosis have been
reported. However, humans who ingest fecally shed oocysts or sporocysts of
226 Biosafety in Microbiological and Biomedical Laboratories
Sarcocystis nesbitti or of various unidentied Sarcocystis spp. with unknown
carnivorous denitive hosts may develop intramuscular cysts.
9
Babesia microti and other Babesia spp., which can cause human babesiosis
(piroplasmosis), are transmitted in nature by the bite of an infected tick. Although no
laboratory-associated cases of Babesia infection have been reported, such cases
could be acquired through percutaneous contact with contaminated blood from
infected persons or animals or, for culturable Babesia spp., with cultured parasites.
Bites from naturally or experimentally infected ticks may also pose a risk.
Among the free-living amebae (FLA), Naegleria fowleri causes primary amebic
meningitis, which typically progresses rapidly and causes death, whereas
Acanthamoeba spp., B. mandrillaris, and S. pedata may cause granulomatous
amebic encephalitis, which typically is more subacute or chronic. FLA may also
cause disguring skin lesions (Acanthamoeba spp. and B. mandrillaris) and
potentially blinding keratoconjunctivitis, particularly in association with the use of
contact lenses or the presence of corneal abrasions (Acanthamoeba spp.). No
laboratory-associated cases of infection with FLA have been reported. However,
potentially infective stages of FLA may be found in tissue, cerebrospinal uid,
and other types of specimens from infected persons and in laboratory cultures of
the organisms.
Natural Modes of Infection
Leishmania spp., Plasmodium spp., and American and African trypanosomes
are transmitted in nature by blood-sucking insects. Sandies in the genera
Phlebotomus and Lutzomyia transmit Leishmania spp.; mosquitoes in the genus
Anopheles transmit Plasmodium spp.; triatomine bugs, including Triatoma,
Rhodnius, and Panstrongylus spp., transmit T. cruzi, which is found in the feces
rather than the saliva of the bugs; tsetse ies in the genus Glossina transmit
African trypanosomes; and ixodid (hard) ticks transmit Babesia spp.
Malaria is widely distributed in the tropics, although the prevalence and incidence
rates of Plasmodium infection vary in and among areas of endemicity. In
aggregate, seven Plasmodium spp. have been documented to infect humans in
nature, primarily P. falciparum, P. vivax, P. ovale, and P. malariae but also the
simian species P. knowlesi, P. cynomolgi, and P. simium.
Leishmaniasis is endemic in parts of the tropics, subtropics, and southern
Europe. Many Leishmania spp. are zoonotic (e.g., have rodent or canine reservoir
hosts); however, infected humans serve as epidemiologically important reservoir
hosts in some settings for some species, including L. donovani and L. tropica.
Only cats and other felines can serve as denitive hosts for T. gondii, which is
found worldwide. Birds and mammals, including sheep, pigs, rodents, cattle,
deer, and humans, can become infected via ingestion of tissue cysts or mature

227Section VIII-C: Parasitic Agents
(sporulated) fecal oocysts and subsequently develop tissue cysts (e.g., in skeletal
muscle, myocardium, brain, eyes). Chagas disease is endemic in Mexico, Central
America, and South America; sporadic vector-borne cases also occur in focal
areas of the southern United States. Various domestic and wild mammals are
found naturally infected with T. cruzi. African trypanosomiasis is endemic in
sub-Saharan Africa but is highly focal in its distribution. T. b. gambiense occurs in
parts of western and central Africa, whereas T. b. rhodesiense occurs in parts of
eastern and southern Africa. T. b. rhodesiense is a zoonotic infection with cattle
or, in a more limited role, game animals serving as reservoir hosts, whereas
humans are the only epidemiologically important hosts for T. b. gambiense.
Babesia infections are found worldwide in animals, and multiple Babesia spp.
have been documented to infect humans; examples of animal reservoir hosts
include white-footed mice (Peromyscus leucopus) and other small mammals for
B. microti and cattle for B. divergens.
Laboratory Safety and Containment Recommendations
BSL-2 and ABSL-2 practices, including containment equipment/facilities and
laboratory personal protective equipment (PPE), are recommended for activities
involving infective stages of the parasites discussed in this section.
Depending in part on the parasite and the phase of the infection, infective stages
of blood and tissue protozoa may be present in blood and various body uids and
tissue specimens, including in cultures and homogenates, from infected humans
and from experimentally or naturally infected animals, including arthropod vectors
if pertinent. See above regarding the primary laboratory hazards. The risks for
accidental exposures and occupationally-acquired infections in persons working
with cultures, tissue homogenates, blood, or other specimens that contain any
of the organisms discussed here, including during procedures that might create
aerosols or droplets, should be reduced by use of PPE (e.g., long-sleeved
laboratory coat/gown, gloves, face shield, sturdy closed footwear, clothing that
covers exposed legs), in conjunction with containment in a biosafety cabinet
(BSC). For work with infected arthropod vectors, the prevention measures include
using the relevant PPE, as well as maintaining and transporting vectors in facil-
ities or transport containers that reasonably preclude the exposure of personnel
or the escape of the arthropods. See Appendix E for additional information.
Special Issues
Transfer of Agent Importation of any of these agents requires CDC and/or
USDA importation permits. Domestic transport of these agents may require a
permit from USDA APHIS VS. A Department of Commerce (DoC) permit may be
required for the export of these agents to another country. See Appendix C for
additional information.
228 Biosafety in Microbiological and Biomedical Laboratories
Intestinal Protozoal Parasites
Intestinal protozoal parasites that pose an occupational risk include Cryptospo-
ridium spp., which cause cryptosporidiosis; Cyclospora cayetanensis, which
causes cyclosporiasis; Cystoisospora belli, which causes cystoisosporiasis;
Entamoeba histolytica, which causes intestinal and extraintestinal (e.g., liver
abscess) amebiasis; Giardia duodenalis, which causes giardiasis; and Sarco-
cystis hominis (from beef) and S. suihominis (from pork), which cause intestinal
sarcocystosis
9
(see above regarding Sarcocystis spp. that can cause intramus-
cular sarcocystosis). Dientamoeba fragilis (for which a cyst stage recently was
identied)
10
and Blastocystis spp.
11
are additional intestinal protozoal parasites
that may pose risk to laboratory workers, although their pathogenic potentials
in humans continue to be debated.
10,12
Multiple genera/species of microsporidia
(now classied as fungi) can cause intestinal microsporidiosis in humans.
Occupational Infections
Laboratory-associated infections with Cryptosporidium spp., E. histolytica,
G. duodenalis, and C. belli have been reported.
1–3
The reported cases typically
have been associated with ingestion of the parasite and, if symptomatic, with
gastrointestinal symptoms. Laboratory work that does or may entail exposure
to Cryptosporidium oocysts warrants special care. Occupationally-acquired
infections have occurred quite commonly in personnel working with this agent,
especially if infected calves were the source of the oocysts.
1,2
Other infected
animals pose potential risks as well. Circumstantial evidence suggests that
airborne transmission of oocysts via droplets of this small organism (i.e., 4–6
µm in diameter) might occur.
1,2
Rigid adherence to protocol (see below) should
reduce the risks for accidental exposures and occupationally-acquired infections
in laboratory and animal care personnel.
Natural Modes of Infection
All of these intestinal protozoa have cosmopolitan distributions. In nature, the
primary route of transmission is ingestion of an environmentally hardy oocyst
(for the coccidia), cyst (for E. histolytica and G. duodenalis), or spore for the
microsporidia. The ID50 has been best established for the zoonotic species
Cryptosporidium parvum: the reported ID50 has ranged from 12 to 2,066 ingested
oocysts, depending on the strain tested;
13
and the ID50 for one strain of C. hominis
ranged from 10 to 83 oocysts.
14
Because intestinal protozoa multiply in the host,
ingestion of even small inocula could cause infection and illness. The role, if any,
for non-human reservoir hosts diers among the intestinal protozoa. Cattle, other
mammals, and birds can be infected with various Cryptosporidium spp.
Humans are the primary hosts for E. histolytica and C. belli and are the only
established hosts for C. cayetanensis. Most human cases of G. duodenalis
infection likely are acquired via direct or indirect human-to-human transmission,
229Section VIII-C: Parasitic Agents
although zoonotic transmission may rarely occur, particularly from companion
cats and dogs. The parasites discussed in this paragraph do not require more
than one host to complete their life cycle.
Laboratory Safety and Containment Recommendations
BSL-2 and ABSL-2 practices, including containment equipment/facilities and
laboratory personal protective equipment (PPE), are recommended for activities
involving infective stages of the parasites discussed in this section.
Depending on the organism, infective stages of these parasites and of
microsporidia may be present in the feces and/or in other body uids (e.g., bile)
and tissues. Appropriate standard precautions are recommended, with special
attention to personal hygiene (e.g., handwashing), the use of PPE, and laboratory
practices that reduce the risk for accidental ingestion of these organisms. Use
of a BSC and/or face shield should also reduce the possibility of airborne trans-
mission via contaminated droplets (e.g., when working with liquid suspensions of
Cryptosporidium oocysts). Cryptosporidium oocysts are infectious when shed in
stool because they have already fully sporulated and do not require further devel-
opment outside the host; the oocysts are often present in high numbers in stool
and are environmentally hardy. In contrast, the oocysts of Cystoisospora belli
and Cyclospora cayetanensis require an extrinsic maturation period to become
infective, which, under favorable environmental conditions, may be relatively short
(potentially, <24 hours) for C. belli but is quite long (typically, at least 1–2 weeks)
for C. cayetanensis.
For disinfection of contaminated surfaces (e.g., benchtops and equipment),
commercially available iodine-containing disinfectants are eective against
E. histolytica and G. duodenalis, when used as directed, as are high concen-
trations of chlorine (one cup of full-strength commercial bleach [~5% chlorine]
per gallon of water [1:16, vol/vol]).
1,2
Because undiluted 3% (10 volumes)
commercial hydrogen peroxide is known to kill Cryptosporidium oocysts after a
suciently long contact time (data for Cystoisospora and Cyclospora oocysts are
not available), the following approach can be used to decontaminate a surface
aected by a laboratory spill containing Cryptosporidium oocysts.
1
After removing
organic material from the contaminated surface (e.g., by using a conventional
laboratory detergent/cleaner) and absorbing the bulk of the spill with disposable
paper towels, ood and completely cover the surface with undiluted hydrogen
peroxide. Dispense hydrogen peroxide repeatedly, as needed, to keep aected
surfaces covered and wet/moist for approximately 30 minutes. Absorb residual
hydrogen peroxide with disposable paper towels, and allow surfaces to dry
thoroughly (10 to 30 minutes) before use. Care should be taken to autoclave
or similarly disinfect all paper towel litter and other disposable materials before
disposal. Reusable laboratory items can be disinfected and washed in a
laboratory dishwasher by using the sanitize cycle and a detergent containing

230 Biosafety in Microbiological and Biomedical Laboratories
chlorine. Alternatively, contaminated items may be immersed for approximately
one hour in a water bath preheated to 50ºC and washed thereafter in a detergent/
disinfectant solution.
Special Issues
Transfer of Agent Importation of any of these agents requires CDC and/or USDA
importation permits. Domestic transport of these agents may require a permit
from USDA APHIS VS. A Department of Commerce (DoC) permit may be required
for the export of these agents to another country. See Appendix C for additional
information.
Cestode Parasites
Cestode parasites that pose an occupational risk include Echinococcus spp.,
Hymenolepis (Rodentolepis) nana, and Taenia solium. Echinococcosis is caused
by cestodes in the genus Echinococcus: E. granulosus causes cystic echino-
coccosis, E. multilocularis causes alveolar echinococcosis, and E. vogeli and
E. oligarthrus cause polycystic echinococcosis. Humans serve as intermediate
hosts and harbor the metacestode or larval stage, which produces a hydatid
cyst. Hymenolepis nana, the dwarf tapeworm, is cosmopolitan in distribution and
causes hymenolepiasis, which is intestinal infection with the adult tapeworm.
Taenia solium, the pork tapeworm, causes taeniasis, which is the infection of the
intestinal tract with the adult worm, and cysticercosis, which is the development of
larval/tissue cysts (i.e., cysticerci) in various parts of the body, such as brain and
subcutaneous tissue.
Occupational Infections
No Laboratory-associated infections with any cestode parasite have been reported.
Natural Modes of Infection
H. nana may act as a one-host parasite and does not require maturation in an
intermediate host. H. nana is directly transmissible by ingestion of eggs shed in
the feces of denitive hosts (i.e., infected humans or rodents). The life cycles of
Echinococcus and Taenia spp. require two hosts. Canids, including dogs, wolves,
foxes, coyotes, and jackals, serve as denitive hosts for E. granulosus; and
various herbivores, such as sheep, cattle, deer, and horses, serve as intermediate
hosts. Foxes and coyotes are the principal denitive hosts for E. multilocularis,
although various canids and felids also can become infected. Rodents serve
as intermediate hosts. Bush dogs and pacas serve as the denitive and
intermediate hosts, respectively, for E. vogeli. Dogs also may be infected. Wild
felines, including cougars, jaguarondi, jaguars, ocelots, and pampas cats, are the
denitive hosts for E. oligarthrus. Various rodents, such as agoutis, pacas, spiny
rats, and rabbits, serve as intermediate hosts. Humans become infected with

231Section VIII-C: Parasitic Agents
Echinococcus spp. when eggs shed by denitive hosts are accidentally ingested.
For T. solium, humans serve as denitive hosts (i.e., harbor the adult tapeworm)
but also may serve as accidental intermediate hosts (i.e., harbor cysticerci, larval/
tissue cysts). Pigs, which are the usual intermediate hosts, become infected as
they scavenge human stool that contains T. solium eggs.
Laboratory Safety and Containment Recommendations
Infective eggs of Echinococcus spp. may be present in the feces of carnivore
denitive hosts.
4
E. granulosus poses the greatest risk because it is the most
common and widely distributed Echinococcus sp. and because dogs are the
primary denitive hosts. For T. solium, infective eggs in the feces of humans
serve as the source of infection; accidental ingestion of infective eggs is the
primary laboratory hazard. Ingestion of cysticerci of T. solium or Taenia asiatica
in pork and T. saginata in beef could cause human intestinal infection with the
adult tapeworm. Ingestion of the eggs of H. nana shed in the feces of denitive
hosts (humans or rodents) could result in intestinal infection.
Although no Laboratory-associated infections with Echinococcus spp. or T. solium
have been reported, the consequences of such infections could be serious. For
echinococcal infections, the severity and nature of the signs and symptoms, if
any, depend in part on the location of the cysts, their size, and condition (alive
vs. dead). Clinical manifestations associated with a liver cyst could include
hepatosplenomegaly, abdominal pain, and nausea, whereas a lung cyst may
cause chest pain, dyspnea, and hemoptysis. For T. solium, ingestion of eggs
from human feces can result in cysticercosis. Subcutaneous or intramuscular
T. solium cysts may be asymptomatic; although cysts in the CNS also may be
asymptomatic, they may cause seizures and other neurologic manifestations.
For laboratory work with infective stages of the cestode parasites discussed
here, BSL-2 and ABSL-2 practices, including containment equipment/facilities
and laboratory personal protective equipment (PPE), are recommended, with
special attention to personal hygiene (e.g., handwashing), the use of PPE, and
laboratory practices that reduce the risk for accidental ingestion of infective eggs.
For example, gloves should be worn when there may be direct contact with feces
or with surfaces contaminated with fresh feces either from carnivores potentially
infected with Echinococcus spp., humans potentially infected with T. solium, or
humans or rodents potentially infected with H. nana.
Special Issues
Transfer of Agent Importation of any of these agents requires CDC and/or
USDA importation permits. Domestic transport of these agents may require a
permit from USDA APHIS VS. A Department of Commerce (DoC) permit may be
required for the export of these agents to another country. See Appendix C for
additional information.
232 Biosafety in Microbiological and Biomedical Laboratories
Trematode Parasites
The trematode parasites that pose the greatest occupational risk are the
Schistosoma spp., although others, including Fasciola spp., are of concern.
Schistosoma mansoni causes intestinal schistosomiasis. The adult ukes
typically reside in the venules of the bowel and rectum. Fasciola hepatica, the
sheep liver uke, causes fascioliasis, in which the adult ukes live in the bile
ducts of the human or animal host.
Occupational Infections
Laboratory-associated infections with S. mansoni and F. hepatica (one possible
such case) have been reported, but accidental infections with other Schistosoma
spp. could also occur.
1,2
Laboratory-associated infections with F. hepatica may
be asymptomatic or associated with various clinical manifestations, such as
right upper quadrant pain, depending in part on the phase of the infection. Most
laboratory exposures to schistosomes would result in low worm and egg burdens,
with low-risk for long-term morbidity, although acute infection may be associated
with clinical manifestations (e.g., dermatitis, fever, cough, hepatosplenomegaly,
lymphadenopathy).
Natural Modes of Infection
F. hepatica has a cosmopolitan distribution and is most common in sheep-raising
areas; other natural hosts include goats, cattle, hogs, deer, and rodents. Snails in
the family Lymnaeidae, primarily species of Lymnaea, serve as intermediate hosts
for F. hepatica and release cercariae that encyst on vegetation. Humans become
infected with F. hepatica by eating raw or inadequately cooked vegetation,
especially green leafy plants, such as watercress, on which metacercariae have
encysted. The same route of transmission is applicable to Fasciola gigantica
(giant liver uke) and Fasciolopsis buski (an intestinal uke). Infection with other
trematodes requires consumption of the infected intermediate host (mainly sh or
crustaceans); therefore, the laboratory risk posed by these pathogens is minimal
if appropriate standard precautions are followed, including the use of PPE.
S. mansoni is endemic in parts of Africa, South America, and the Caribbean.
Free-swimming cercariae in contaminated bodies of water infect humans via
skin penetration. The natural snail hosts capable of supporting development of
S. mansoni are various species of Biomphalaria.
Laboratory Safety and Containment Recommendations
Infective stages of F. hepatica (metacercariae) and S. mansoni (cercariae) may
be found, respectively, encysted on aquatic plants or free-living in the water in
laboratory aquaria used to maintain snail intermediate hosts. Ingestion of uke
metacercariae and skin penetration by schistosome cercariae are the primary
laboratory hazards. Dissection or crushing of schistosome-infected snails may

233Section VIII-C: Parasitic Agents
also result in exposure of skin or mucous membranes to cercariae-containing
droplets. Additionally, metacercariae may be inadvertently transferred from
hand to mouth by ngers or gloves, following contact with contaminated aquatic
vegetation or aquaria.
All of the reported cases of laboratory-associated schistosomiasis have been
caused by S. mansoni, which probably in part reects the fact that a laboratory
life cycle for S. mansoni can be maintained using mice, which is not possible for
the other Schistosoma spp. However, accidental infection with S. haematobium,
S. japonicum, S. mekongi, S. intercalatum, or S. guineensis could easily
occur via transdermal penetration if infected snail intermediate hosts are kept
in aquaria or if laboratorians work with water samples that contain infective
cercariae. In addition, exposure to cercariae of non-human (e.g., avian) species
of schistosomes may cause mild-to-severe dermatitis (i.e., swimmer’s itch).
BSL-2 and ABSL-2 practices, including appropriate PPE and containment
equipment/facilities, are recommended for laboratory work with infective stages
of the trematode parasites discussed here (i.e., when there may be direct contact
with water containing cercariae or vegetation with encysted metacercariae from
naturally or experimentally infected snail intermediate hosts). For example,
in addition to gloves, long-sleeved laboratory coats and face shields or other
protective garb should be worn when working in the immediate area of aquaria or
other water sources that may contain schistosome cercariae. Cercariae can be
killed on contact with 70% ethanol.
15
Therefore, precautionary measures include
having squirt bottles that contain 70% ethanol as well as bottles that contain hand
sanitizers for which alcohol is the active ingredient strategically placed around
the laboratory to facilitate immediate access after accidental spills/exposures.
15
Various approaches (e.g., ethanol, bleach, heat) can be used to kill snails and
cercariae in the water of laboratory aquaria before discharge to sanitary sewers.
For example, heating the water to ≥50°C will kill the cercariae within seconds.
15
Special Issues
Transfer of Agent Importation of any of these agents requires CDC and/or
USDA importation permits. Domestic transport of these agents may require a
permit from USDA APHIS VS. A Department of Commerce (DoC) permit may be
required for the export of these agents to another country. See Appendix C for
additional information.
Nematode Parasites
Nematode parasites that pose an occupational risk include the ascarids; Strongy-
loides stercoralis; hookworms (both human and animal); Enterobius vermicularis
(human pinworm); and the human lariae, primarily Wuchereria bancrofti and
Brugia spp. Three hookworm species cause patent disease in humans: Necator
americanus, Ancylostoma duodenale, and Ancylostoma ceylanicum (which also
234 Biosafety in Microbiological and Biomedical Laboratories
causes patent disease in cats and dogs). Ancylostoma braziliense, A. caninum,
and Uncinaria stenocephala cause hookworm infection in cats and dogs and can
also cause cutaneous larva migrans in humans. Ascaris lumbricoides causes
ascariasis in humans and pigs. Baylisascaris procyonis (a parasite of raccoons),
Toxocara canis (dog reservoir), and Toxocara cati (cat reservoir) cause visceral,
ocular, and neural larva migrans in humans. Larval anisakid nematodes (in sh
and squid) cause anisakiasis. Trichuris trichiura (whipworm) causes trichuriasis
in humans. E. vermicularis (pinworm; humans only) causes enterobiasis
(oxyuriasis). S. stercoralis (humans and dogs) causes strongyloidiasis; animal
Strongyloides spp. may cause cutaneous larva migrans. Angiostrongylus canton-
ensis causes eosinophilic meningitis, and Trichinella spp. cause trichinellosis.
Occupational Infections
Laboratory-associated infections with human hookworms, A. lumbricoides,
E. vermicularis, and Strongyloides stercoralis have been reported.
1–3
Laboratory
infections with hookworm and Strongyloides spp. presumptively acquired
from infected animals have also been reported.
1–3
Allergic reactions to various
antigenic components of human and animal ascarids and anisakids from sh
(e.g., aerosolized antigens) may pose risk to sensitized persons.
Laboratory-associated infections with these nematodes may be asymptomatic
or associated with a range of clinical manifestations, depending in part on the
parasite species and the location(s) of the parasite in the host. The clinical
manifestations of infection with A. lumbricoides may include cough, fever, and
pneumonitis as larvae migrate through the lungs; the larvae develop into adult
worms in the small intestine. Infection with E. vermicularis usually causes
perianal pruritus, with intense itching.
Natural Modes of Infection
Human hookworm and S. stercoralis infections are acquired via transdermal
penetration of the skin by infective lariform larvae. These nematodes are
commonly found in tropical and subtropical regions of the world and cause
infection in the small intestine. In contrast to hookworms, S. stercoralis is
autoinfective and infection may be lifelong if untreated. Intradermal migration
of S. stercoralis larvae can be associated with a rapidly moving, serpiginous,
pruritic eruption referred to as larva currens (“racing” or “running” larva). The time
required for Strongyloides larvae passed in stool to develop into infective lar-
iform larvae may be as short as approximately two days (i.e., 48 hours); the time
required for hookworm larvae to become infective may be as short as three days.
Human cutaneous larva migrans (creeping eruption) occurs when infective larvae
of animal hookworms (typically dog and cat hookworms) or of animal Strongy-
loides spp. penetrate the skin and begin wandering. Hookworm infections in dogs
and cats and Strongyloides spp. infections in animals are endemic worldwide.
235Section VIII-C: Parasitic Agents
A. caninum larvae can also cause infection if ingested. On rare occasions,
ingested A. caninum larvae have developed into non-gravid adult worms in the
human gut, leading to eosinophilic enteritis.
A. lumbricoides and T. trichiura infections are endemic in tropical and subtropical
regions of the world. T. canis and T. cati are found worldwide in dogs and cats,
respectively. B. procyonis is found primarily in raccoons but may also infect dogs.
All of these parasites are transmitted via ingestion of embryonated (larvated)
eggs. Unembryonated eggs passed in the stool require 2–3 weeks to larvate and
become infectious. The eggs are very hardy in the environment and are resistant
to most disinfectants (see below).
E. vermicularis is found worldwide, but pinworm infection tends to be more
common in school-age children than adults and in temperate than tropical
regions. Pinworm infection is acquired by ingestion of eggs (e.g., eggs on
contaminated ngers after scratching the perianal skin). Eggs passed by female
worms are not immediately infective but require only several hours to become
fully infectious. Pinworm infection is of relatively short duration (approximately
60 days on average) unless reinfection occurs.
Some anisakid larvae (Anisakis spp., Pseudoterranova decipiens, and
Contracecum spp.) are infective to humans via ingestion. The larvae may be
coughed up, be vomited, or form eosinophilic granulomas in the gastrointestinal
tract. These nematodes also are antigenic and may cause immediate hypersen-
sitivity reactions (e.g., urticaria, anaphylaxis) when infected sh are ingested.
Laboratory Safety and Containment Recommendations
Eggs and larvae of most nematodes are not infective in freshly passed feces;
development to the infective stages may require from less than one day to several
weeks, depending in part on the genus/species and the environmental conditions.
Ingestion of infective eggs or transdermal penetration by infective larvae are the
primary hazards to laboratory sta and animal care personnel.
To minimize the risk for transdermal penetration when working with cultures
or fecal specimens that may contain infective hookworm or Strongyloides spp.
larvae, PPE should be used to cover exposed skin. In an investigation in which
S. stercoralis–positive stool specimens were reexamined after they had been
stored at 4°C for 24, 48, and 72 hours, 23% of the 74 specimens examined still
had viable larvae after refrigeration for 72 hours.
16
The following iodine concen-
trations have been shown to kill infective larvae immersed in an aqueous iodine
solution for one to ve minutes: 50 ppm iodine for S. stercoralis larvae, 60 ppm
for N. americanus (hookworm) larvae, and 70 ppm for A. caninum (hookworm)
larvae.
17
In vitro exposure to 70% ethanol has been shown to kill infective
S. stercoralis larvae within 4.3 ± 1 minutes (mean ± standard deviation).
18
In
vitro exposure to 70% ethanol has been shown to kill 95.6% of 45 infective

236 Biosafety in Microbiological and Biomedical Laboratories
N. americanus larvae within ve minutes and to kill all such larvae within 10
minutes.
19
Taking into consideration the data summarized in this paragraph,
Lugol’s iodine (1% povidine iodine; 10,000 ppm) may be used to kill N. americanus
and S. stercoralis infective larvae on exposed skin and 70% ethanol (which leaves
far less residue on surfaces) may be used to disinfect contaminated laboratory
surfaces and equipment.
Ascarid (A. lumbricoides, Toxocara spp., B. procyonis) and E. vermicularis eggs
are sticky; special care is warranted to ensure that contaminated surfaces and
equipment are thoroughly cleaned. Precautions are warranted even when working
with formalin-xed stool specimens. Ascarid eggs, which are exceptionally
environmentally resistant, may continue to develop to the infective stage in
formalin;
20
they also may continue to develop despite exposure to high concentra-
tions of disinfectants for long periods. However, ascarid eggs can be deactivated
by the use of heat at or above 60ºC for more than 15 minutes.
Accidental ingestion of larvated (infectious) eggs of Toxocara and B. procyonis
could lead to visceral migration of larvae, including invasion of the eyes and CNS.
The larvae of Trichinella in fresh or digested animal tissue, or of A. cantonensis
in fresh or digested mollusk tissue, could cause infection if accidentally ingested.
Vector arthropods infected with larial parasites pose a potential hazard to
laboratory personnel. The prevention measures include using the relevant
PPE (e.g., gowns, gloves, closed shoes); maintaining and transporting vectors
in facilities or transport containers that reasonably preclude the exposure of
personnel or the escape of infected arthropods are also essential. See Appendix E
for additional information.
The use of primary containment (e.g., BSC) during work that may be associated
with aerosolization should reduce the potential for exposure to aerosolized
antigens of ascarids and anisakids, which can cause allergic reactions in sensi-
tized persons. Special attention to use of PPE and to personal hygiene (e.g.,
handwashing) is warranted when working with any of the nematode pathogens
discussed here.
Special Issues
Transfer of Agent Importation of any of these agents requires CDC and/or
USDA importation permits. Domestic transport of these agents may require a
permit from USDA APHIS VS. A Department of Commerce (DoC) permit may be
required for the export of these agents to another country. See Appendix C for
additional information.
237Section VIII-C: Parasitic Agents
References
1. Herwaldt BL. Protozoa and helminths. In: Wooley DP, Byers KB, editors.
Biological Safety: Principles and Practices. 5th ed. Washington (DC): ASM
Press; 2017. p. 105–45.
2. Herwaldt BL. Laboratory-acquired parasitic infections from accidental
exposures. Clin Microbiol Rev. 2001;14(4):659–88.
3. Pike RM. Laboratory-associated infections: summary and analysis of 3921
cases. Health Lab Sci. 1976;13(2):105–14.
4. Hankenson FC, Johnston NA, Weigler BJ, Di Giacomo RF. Zoonoses of
occupational health importance in contemporary laboratory animal research.
Comp Med. 2003;53(6):579–601.
5. Wendel S, Leiby DA. Parasitic infections in the blood supply: assessing and
countering the threat. Dev Biol (Basel). 2007;127:17–41.
6. Schwartz BS, Mawhorter SD; AST Infectious Diseases Community of
Practice. Parasitic infections in solid organ transplantation. Am J Transplant.
2013;13 Suppl 4:280–303.
7. Carlier Y, Truyens C, Deloron P, Peyron F. Congenital parasitic infections:
a review. Acta Trop. 2012;121(2):55–70.
8. van Gool T, Biderre C, Delbac F, Wentink-Bonnema E, Peek R, Vivares
CP. Serodiagnostic studies in an immunocompetent individual infected with
Encephalitozoon cuniculi. J Infect Dis. 2004;189(12):2243–9.
9. Fayer R, Esposito DH, Dubey JP. Human infections with Sarcocystis
species. Clin Microbiol Rev. 2015;28(2):295–311.
10. Stark D, Barratt J, Chan D, Ellis JT. Dientamoeba fragilis, the neglected
trichomonad of the human bowel. Clin Microbiol Rev. 2016;29(3):553–80.
11. Rajah Salim H, Suresh Kumar G, Vellayan S, Mak JW, Khairul Anuar A, Init I,
et al. Blastocystis in animal handlers. Parasitol Res. 1999;85(12):1032–3.
12. Roberts T, Stark D, Harkness J, Ellis J. Update on the pathogenic potential
and treatment options for Blastocystis sp. Gut Pathog. 2014;6:17.
13. Messner MJ, Chappell CL, Okhuysen PC. Risk assessment for
Cryptosporidium: a hierarchical Bayesian analysis of human dose response
data. Water Res. 2001;35(16):3934–40.
14. Chappell CL, Okhuysen PC, Langer-Curry R, Widmer G, Akiyoshi DE,
Tanriverdi S, et al. Cryptosporidium hominis: experimental challenge of
healthy adults. Am J Trop Med Hyg. 2006;75(5):851–7.
15. Tucker MS, Karunaratne LB, Lewis FA, Freitas TC, Liang YS.
Schistosomiasis. Curr Protoc Immunol. 2013;103:Unit 19.1.1–19.1.58.
238 Biosafety in Microbiological and Biomedical Laboratories
16. Inês Ede J, Souza JN, Santos RC, Souze ES, Santos FL, Silva ML, et al.
Ecacy of parasitological methods for the diagnosis of Strongyloides
stercoralis and hookworm in faecal specimens. Acta Trop. 2011;120(3):
206–10.
17. Thitasut P. Action of aqueous solutions of iodine on fresh vegetables and on
the infective stages of some common intestinal nematodes. Am J Trop Med
Hyg. 1961;10:39–43.
18. Hirata T, Kishimoto K, Uchima N, Kinjo N, Hokama A, Kinjo F, et al. Ecacy
of high-level disinfectants for gastrointestinal endoscope disinfection against
Strongyloides stercoralis. Digestive Endoscopy. 2006;18:269–71.
19. Speare R, Melrose W, Cooke S, Croese J. Techniques to kill infective
larvae of human hookworm Necator americanus in the laboratory and a
new Material Safety Data Sheet. Aust J Med Sci. 2008;29(3):91–6.
20. Ash LR, Orihel TC. Parasites: A Guide to Laboratory Procedures and
Identication. Chicago: ASCP Press; 1991.
239Section VIII-D: Rickettsial Agents
Section VIII-D: Rickettsial Agents
Coxiella burnetii
Coxiella burnetii is a bacterial obligate intracellular pathogen that is the etiologic
agent of Q (query) fever. It undergoes its developmental cycle within an acidic
vacuolar compartment, exhibiting many characteristics of a phagolysosome. The
biphasic developmental cycle consists of a small cell variant (SCV) and a large
cell variant (LCV). The SCV is the more structurally-stable cell variant, persisting
for extended periods of time outside of host cells and exhibiting resistance to
extracellular stresses (drying, extreme temperatures, environmental conditions).
The LCV is the larger, metabolically-active variant, which facilitates replication of
the agent.
1–4
The organism undergoes a virulent (phase I) to avirulent (phase II)
transition upon serial laboratory passage in eggs or tissue culture.
The ID of phase I organisms in laboratory animals has been calculated to be as
small as a single organism.
5
The estimated human ID for development of Q fever
by inhalation is approximately 10 organisms.
6
Typically, the disease manifests
with u-like symptoms including fever, headache, and myalgia, but can also
present with pneumonia and hepatomegaly. Infections range from subclinical
to severe, and primary/acute infections respond readily to antibiotic treatment.
Although rare, C. burnetii can cause chronic infections such as endocarditis,
granulomatous hepatitis, or vascular infections.
7
Occupational Infections
Q fever is the second most commonly reported Laboratory-associated infection
(LAI) in Pike’s compilation with outbreaks involving 15 or more persons recorded
in several institutions.
8,9
Infectious aerosols are the most likely route of LAI.
Experimentally infected animals may also serve as potential sources of infection
for laboratory and animal care personnel. Exposure to naturally infected, often
asymptomatic, sheep and their birth products is a documented hazard to
personnel.
10,11
Natural Modes of Infection
Q fever occurs worldwide. A broad range of domestic and wild mammals are
natural hosts for Q fever and may serve as potential sources of infection.
Parturient animals and their birth products are common sources of infection.
The placenta of infected sheep may contain as many as 10
9
organisms per
gram of tissue
12
and milk may contain 10
5
organisms per gram. The resistance
of the organism to drying and its low infectious dose can lead to dispersal from
contaminated sites. The agent may also be present in infected arthropods, and it
may be present in the blood, urine, feces, milk, and tissues of infected animals or
human hosts.

240 Biosafety in Microbiological and Biomedical Laboratories
Laboratory Safety and Containment Recommendations
Recent advances leading to cell-free media supporting the growth of C. burnetii
13
have greatly reduced the necessity of using embryonated eggs or cell culture
techniques for propagation and accompanying extensive purication procedures.
Exposure to infectious aerosols and parenteral inoculation remain the most likely
sources of infection to laboratory and animal care personnel.
8,9
BSL-3 practices and facilities are recommended for activities involving the
inoculation, incubation, and harvesting of C. burnetii, the necropsy of infected
animals, and the manipulation of infected tissues. Because infected rodents may
shed the organisms in urine or feces,
8
experimentally infected animals should be
maintained under ABSL-3. A specic plaque-puried clonal isolate of an avirulent
(phase II, Nine Mile Strain, plaque puried clone 4) strain is exempt from the
Select Agent Regulations and may be safely handled under BSL-2 conditions.
14
BSL-2 practices and facilities are recommended for nonpropagative laboratory
procedures, including serological examinations and staining of impression
smears.
Special Issues
C. burnetii is among the most environmentally stable of non-spore forming
bacteria with a known capacity for extended survival in soil or other contami-
nated materials, such as animal products, for years.
4
The ID approaches a single
organism,
5
thus the capacity for airborne or aerosol transmission is high. Infec-
tions are frequently asymptomatic, or cause relatively mild, u-like symptoms,
but can be severe. Chronic infections (i.e., endocarditis) are possible, particularly
in those with pre-existing valvular damage or immunocompromised individuals.
Q fever is a known hazard during pregnancy.
15
Exposure to naturally infected, often asymptomatic, sheep and their birth products
is a documented hazard to personnel.
10,11
Recommended precautions for facilities
using sheep as experimental animals are described by Spinelli and Bernard.
10,16
Vaccines Q fever vaccines are not commercially available in the United States.
Individuals with valvular heart disease should not work with C. burnetii. Work
with C. burnetii should be avoided during pregnancy. See Section VII for
additional information.
Select Agent C. burnetii is considered a Select Agent under the Code of Federal
Regulations (42 CFR Part 73). All rules concerning the possession, storage, use,
and transfer of Select Agents apply. Appendix F contains additional information
on Select Agents, including contact information for registration and obtaining
appropriate permits for importing, exporting, or transporting this agent.

241Section VIII-D: Rickettsial Agents
Transfer of Agent Importation of this agent requires CDC and/or USDA impor-
tation permits. Domestic transport of this agent may require a permit from USDA
APHIS VS. A Department of Commerce (DoC) permit may be required for the
export of this agent to another country. See Appendix C for additional information.
Rickettsia species and Orientia tsutsugamushi
Rickettsia prowazekii, Rickettsia typhi, the Spotted Fever Group agents of human
disease (Rickettsia rickettsii, Rickettsia conorii, Rickettsia akari, Rickettsia
australis, Rickettsia sibirica, and Rickettsia japonica), Orientia tsutsugamushi,
Rickettsia philipii (Rickettsia 364D), Rickettsia parkeri, and various other
Rickettsia spp. either known as or suspected to be human pathogens of varying
pathogenicity are the respective etiologic agents of epidemic typhus, endemic
(murine) typhus, Rocky Mountain spotted fever, Mediterranean spotted fever,
rickettsialpox, Queensland tick typhus, North Asian spotted fever, Japanese
spotted fever, scrub typhus, Pacic Coast tick fever (PCTF), and Rickettsia
parkeri rickettsiosis.
Rickettsia spp. are bacterial obligate intracellular pathogens that are transmitted
by arthropod vectors and replicate within the cytoplasm of eukaryotic host cells.
Rickettsia spp. are broken into four groups within the genus: the typhus group,
the Spotted Fever Group, a transitional group, and an ancestral group.
17
The
more distantly related scrub typhus group is now considered a distinct genus,
Orientia. Rickettsiae are primarily associated with arthropod vectors in which they
may exist as endosymbionts that infect mammals, including humans, through the
bite of infected ticks, lice, eas, or mites.
Occupational Infections
Although not a natural route of infection, some Rickettsia spp. can be infectious
by an aerosol route, thus adherence to BSL-3 practices is essential. Parenteral
inoculation/needlestick injuries are also among the more common routes of
laboratory infection. Infections can also be acquired by conjunctival inoculation.
Pike reported 56 cases of epidemic typhus with three deaths, 68 cases of murine
typhus, and 57 cases of laboratory-associated typhus (type not specied).
8
Three
cases of murine typhus were reported from a research facility.
18
Two of these
three cases were associated with the handling of infectious materials on the open
bench; the third case resulted from an accidental parenteral inoculation.
Rocky Mountain spotted fever (RMSF) is a documented hazard to laboratory
personnel. Pike reported 63 laboratory-associated cases, 11 of which were fatal
and occurred prior to 1940.
8
Since that time, two fatalities occurred, in the same
facility and presumably from the same exposure, among a laboratory worker and
a custodian in 1977. These illnesses were presumed to be employment-related.
19
242 Biosafety in Microbiological and Biomedical Laboratories
Oster reported nine cases occurring from 1971 to 1976 in one laboratory, which
were believed to have been acquired as a result of exposure to infectious
aerosols.
20
Natural Modes of Infection
The epidemiology of rickettsial infections is a reection of the prevalence of the
rickettsiae in the vector population and the interactions of the arthropod vector
with humans. Epidemic typhus is unusual among rickettsiae in that humans are
considered the primary host. Transmission is by the human body louse, and
outbreaks are now associated with breakdowns of social conditions.
21
Under
these conditions, even with appropriate treatment, mortality averaged about 4%.
22
Endemic typhus is maintained in rodents and transmitted to humans by eas. The
various spotted fever group rickettsiae are limited geographically, probably by the
distribution of the arthropod vector (usually ticks), although specic spotted fever
group rickettsiae are found on all continents.
23
Laboratory Safety and Containment Requirements
Accidental parenteral inoculation and exposure to infectious aerosols are the
most likely sources of Laboratory-associated infection.
24
Aerosol transmission of
R. rickettsii has been experimentally documented in non-human primates.
25
Five
cases of rickettsialpox recorded by Pike were associated with exposure to bites of
infected mites.
8
The tissues of naturally and experimentally infected mammals and their ectopar-
asites are potential sources of human infection. The organisms are relatively
unstable under ambient environmental conditions.
BSL-3 practices and containment equipment are recommended for activities
involving culture propagation or specimen preparation and propagation of clinical
isolates known to contain or potentially containing Rickettsia spp. pathogenic to
humans.
Arthropod Containment Level 3 (ACL-3) practices and facilities are recommended
for animal studies with arthropods naturally or experimentally infected with
rickettsial agents of human disease.
26
Laboratory work with Rickettsia spp. may be conducted in a BSL-2 facility with
enhanced special practices including strict access control, competency, and
adherence to BSL-3 practices. Laboratories should be locked and access to
non-essential personnel should be prohibited. BSL-3 practices include, but are
not limited to, appropriate personal protective equipment (e.g., rear-closing
gowns, gloves, eye protection, and respiratory protection such as N95 respirators
or PAPRs), use of BSCs when handling any open container with potentially
infectious material, and primary containment, such as sealed centrifuge rotors
243Section VIII-D: Rickettsial Agents
and other means of containment outside the BSC. Disruption of infected cells or
yolk sacs should be accomplished within the BSC using an enclosed chamber
to minimize the potential for aerosols. If eggs are used for propagation, the site
of inoculation should be sealed with an appropriate sealant prior to transfer to
an incubator. BSL-2 facilities with BSL-3 practices are recommended for all
manipulations of known or potentially infectious materials, including the necropsy
of experimentally infected animals and trituration of their tissues, and inoculation,
incubation, and harvesting of embryonated eggs or cell cultures. Use of sharps
should be minimized. When use of sharps is necessary, they should be disposed
of and decontaminated appropriately. All contaminated materials should be
eectively decontaminated before removal from the laboratory. If transport to an
autoclave is necessary, materials should be double-bagged.
BSL-2 practices and facilities are recommended for nonpropagative laboratory
procedures with inactivated samples, including serological and uorescent
antibody procedures, nucleic acid amplication, and for the staining of impression
smears after xation.
ABSL-2 practices and facilities are recommended for the holding of experimentally
infected mammals other than arthropods. Several species including R. montanensis,
R. rhipicephali, R. bellii, R. amblyommatis, and R. canadensis are not known to
cause human disease and may be handled under BSL-2 conditions. New species
are frequently described and should be evaluated for appropriate containment on
a case-by-case basis.
Because of the proven value of antibiotic therapy in the early stages of infection,
it is essential that laboratories working with rickettsiae have an eective
system for reporting febrile illnesses in the laboratory, animal facility, and
support personnel; medical evaluation of potential cases; and the institution of
appropriate antibiotic therapy when indicated. Prophylactic antibiotic treatment
following a potential exposure is discouraged in the absence of clinically
compatible signs and symptoms and could delay onset of disease. Vaccines are
not currently available for use in humans.
Laboratory Surveillance
Since 1940, only two laboratory fatalities have occurred due to R. rickettsii.
19,27,28
This incident emphasizes the necessity of controlling access to the laboratory
and expeditious reporting of any exposure or unexplained illness.
Special Issues
Occupational Health Recommendations Under natural circumstances, the
severity of disease caused by rickettsial agents varies considerably.
23,29
In the
laboratory, very large inocula are possible, which might produce unusual and
very serious responses. Surveillance of personnel for Laboratory-associated

244 Biosafety in Microbiological and Biomedical Laboratories
infections with rickettsial agents can dramatically reduce the risk of serious
consequences of disease. See Section VII for additional information.
Infections adequately treated with specic anti-rickettsial chemotherapy on the
rst day of disease do not generally present serious problems. However, delay
in instituting appropriate chemotherapy may result in debilitating or severe acute
disease ranging from increased periods of convalescence in typhus and scrub
typhus to death in R. rickettsii infections. The key to reducing the severity of
disease from LAIs is a reliable surveillance system, which includes:
1. Round-the-clock availability of an experienced medical ocer knowl-
edgeable about infectious disease;
2. Education of all personnel on signs and symptoms of disease and the
advantages of early therapy;
3. A non-punitive, anonymous reporting system for all recognized
accidents; and
4. The reporting of all febrile illnesses, especially those associated with
headache, malaise, and prostration when no other certain cause exists.
Select Agent R. prowazekii is considered a Select Agent under the Code of
Federal Regulations (42 CFR Part 73). All rules concerning the possession,
storage, use, and transfer of Select Agents apply. Appendix F contains additional
information on Select Agents, including contact information for registration and
obtaining appropriate permits for importing, exporting or transporting this agent.
Transfer of Agent Importation of this agent requires CDC and/or USDA impor-
tation permits. Domestic transport of this agent may require a permit from USDA
APHIS VS. A Department of Commerce (DoC) permit may be required for the
export of this agent to another country. See Appendix C for additional information.
References
1. Babudieri B. Q fever: a zoonosis. Adv Vet Sci. 1959;5:81–182.
2. Ignatovich VF. The course of inactivation of Rickettsia burnetii in uid media.
J Microbiol Epidemiol Immunol. 1959;30(9):134–41.
3. Sawyer LA, Fishbein DB, McDade JE. Q fever: current concepts. Rev Infect
Dis. 1987;9(5):935–46.
4. Heinzen RA, Hackstadt T, Samuel JE. Developmental biology of Coxiella
burnetii. Trends Microbiol. 1999;7(4):149–54.
5. Ormsbee R, Peacock M, Gerlo R, Tallent G, Wike D. Limits of rickettsial
infectivity. Infect Immun. 1978;19(1):239–45.
6. Wedum AG, Barkley WE, Hellman A. Handling of infectious agents. J Am
Vet Med Assoc. 1972;161(11):1557–67.
245Section VIII-D: Rickettsial Agents
7. Maurin M, Raoult D. Q fever. Clin Microbiol Rev. 1999;12(4):518–53.
8. Pike RM. Laboratory-associated infections: Summary and analysis of 3921
cases. Hlth Lab Sci. 1976;13(2):105–14.
9. Johnson JE, Kadull PJ. Laboratory-acquired Q fever. A report of fty cases.
Am J Med. 1966;41(3):391–403.
10. Spinelli JS, Ascher MS, Brooks DL, Dritz SK, Lewis HA, Morrish RH, et al.
Q fever crisis in San Francisco: Controlling a sheep zoonosis in a lab
animal facility. Lab Anim. 1981:24–7.
11. Meiklejohn G, Reimer LG, Graves PS, Helmick C. Cryptic epidemic of Q
fever in a medical school. J Infect Dis. 1981;144(2):107–13.
12. Welsh HH, Lennette EH, Abinanti FR, and Winn JF. Q fever in California. IV.
Occurrence of Coxiella burnetii in the placenta of naturally infected sheep.
Public Health Rep. 1951;66(45):1473–7.
13. Omsland A, Cockrell DC, Howe D, Fischer ER, Virtaneva K, Sturdevant DE,
et al. Host cell-free growth of the Q fever bacterium Coxiella burnetii. Proc
Natl Acad Sci U S A. 2009;106(11):4430–4.
14. Hackstadt T. Biosafety concerns and Coxiella burnetii [letter]. Trends
Microbiol. 1996;4(9):341–2.
15. Eldin C, Melenotte C, Mediannikov O, Ghigo E, Million M, Edouard S, et al.
From Q Fever to Coxiella burnetii infection: a Paradigm Change. Clin
Microbiol Rev. 2017;30(1):115–90.
16. Bernard KW, Parham GL, Winkler WG, Helmick CG. Q fever control
measures: Recommendations for research of facilities using sheep. Infect
Control. 1982;3(6):461–5.
17. Gillespie JJ, Williams K, Shukla M, Snyder EE, Nordberg EK, Ceraul
SM, et al. Rickettsia phylogenomics: unwinding the intricacies of obligate
intracellular life. PLoS One. 2008;3(4):e2018.
18. Bellanca J, Iannin P, Hamory B, Miner WF, Salaki J, Stek M. Laboratory-
acquired endemic typhus—Maryland. MMWR. 1978;27(26):215–6.
19. Hazard PB, McCroan JE. Fatal Rocky Mountain Spotted Fever—Georgia.
MMWR. 1977;26:84.
20. Oster CN, Burke DS, Kenyon RH, Ascher MS, Harber P, Pedersen CE Jr.
Laboratory-acquired Rocky Mountain Spotted Fever. The hazard of aerosol
transmission. N Engl J Med. 1977;297(16):859–63.
21. A large outbreak of epidemic louse-borne typhus in Burundi. Wkly Epidemiol
Rec. 1997;72(21):152–3.
22. Bechah Y, Capo C, Mege JL, Raoult D. Epidemic typhus. Lancet Infect Dis.
2008;8(7):417–26.
246 Biosafety in Microbiological and Biomedical Laboratories
23. Richards AL. Worldwide detection and identication of new and old
Rickettsiae and rickettsial diseases. FEMS Immunol Med Microbiol.
2012;64(1):107–10.
24. Hattwick MA, O’Brien RJ, Hanson BF. Rocky Mountain Spotted Fever:
epidemiology of an increasing problem. Ann Intern Med. 1976;84(6):732–9.
25. Saslaw S, Carlisle HN. Aerosol infection of monkeys with Rickettsia
rickettsii. Bacteriol Rev. 1966;30(3):636–45.
26. Vanlandingham DL, Higgs S, Huang YJS. Arthropod Vector Biocontainment.
In: Wooley DP, Byers KB, editors. Biological Safety Principles and Practices.
5th ed. Washington (DC): ASM Press; 2017. p. 399–410.
27. Wurtz N, Papa A, Hukic M, Di Caro A, Leparc-Goart I, Leroy E, et al.
Survey of laboratory-acquired infections around the world in Biosafety Level
3 and 4 laboratories. Eur J Clin Microbiol Infect Dis. 2016;35(8):1247–58.
28. Harding LH, Byers KB. Laboratory-associated infections. In: Wooley DP,
Byers KB, editors. Biological Safety: Principles and Practices. 5th ed.
Washington (DC): ASM Press; 2017. p. 59–92.
29. Hackstadt T. The biology of Rickettsiae. Infect Agents Dis.
1996;5(3):127–43.
247Section VIII-E: Viral Agents
Section VIII-E: Viral Agents
Hantaviruses
Hantaviruses are negative-sense RNA viruses belonging to the genus Hanta-
virus within the family Bunyaviridae. The natural hosts of hantaviruses are
rodent species and they occur worldwide. Hantavirus pulmonary syndrome
(HPS) is a severe disease caused by hantaviruses such as Sin Nombre virus
or Andes virus whose hosts are rodents in the subfamily Sigmodontinae. This
subfamily only occurs in the New World, so HPS is not seen outside North
and South America. Hantaviruses in Europe and Asia frequently cause kidney
disease, called nephropathica epidemica in Europe, and hemorrhagic fever with
renal syndrome (HFRS) in Asia. HFRS caused by Seoul or Seoul-like viruses
originating from Rattus sp. has been described worldwide. Hantaviruses have
been recently described worldwide in shrews, but no human disease has been
described yet from these viruses.
Occupational Infections
Documented Laboratory-associated infections have occurred in individuals
working with hantaviruses.
1–4
Extreme caution must be used in performing any
laboratory operation that may create aerosols (e.g., centrifugation, vortex-
mixing). Operations involving rats, voles, and other laboratory rodents should
be conducted with special caution because of the extreme hazard of aerosol
infection, especially from infected rodent urine.
Natural Modes of Infection
HPS is a severe, often fatal disease that is caused by Sin Nombre and Andes or
related viruses.
5,6
Most cases of human illness have resulted from exposures to
naturally infected wild rodents or to their excreta. Human infections and illness
(caused by Seoul-like virus) have been reported in Europe and the U.S. in people
raising and trading pet rats.
7,8
Person-to-person transmission does not occur, with
the exception of a few rare instances documented, for Andes virus.
9,10
Arthropod
vectors are not known to transmit hantaviruses.
Laboratory Safety and Containment Recommendations
Laboratory transmission of hantaviruses from rodents to humans via the
aerosol route is well documented.
4–6,10
Exposures to rodent excreta, especially
aerosolized infectious urine, fresh necropsy material, and animal bedding
are presumed to be associated with risk. Other potential routes of laboratory
infection include ingestion, contact of infectious materials with mucous
membranes or broken skin and, in particular, animal bites. Viral RNA has been
detected in necropsy specimens and in patient blood and plasma obtained early
in the course of HPS;
11,12
however, the infectivity of blood or tissues is unknown.

248 Biosafety in Microbiological and Biomedical Laboratories
All work involving inoculation of virus-containing material into rodent species
permissive for chronic infection should be conducted at ABSL-4. Cell-culture virus
propagation and purication should be carried out in a BSL-3 facility using BSL-3
practices, containment equipment, and procedures. Serum or tissue samples from
potentially infected rodents should be handled at BSL-2 using BSL-3 practices,
containment equipment, and procedures. Potentially infected tissue samples
should be handled in BSL-2 facilities following BSL-3 practices and procedures.
BSL-2 practices, containment equipment, and facilities are recommended for
laboratory handling of sera from persons potentially infected with hantaviruses.
The use of a BSC is recommended for all handling of human body uids when
potential exists for splatter or aerosol. Experimentally infected rodent species
known not to excrete the virus can be housed in ABSL-2 facilities using ABSL-2
practices and procedures.
Special Issues
Transfer of Agent Importation of this agent requires CDC and/or USDA impor-
tation permits. Domestic transport of this agent may require a permit from USDA
APHIS VS. A DoC permit may be required for the export of this agent to another
country. See Appendix C for additional information.
Hendra Virus (formerly known as Equine Morbillivirus) and Nipah Virus
Hendra virus (HeV) and Nipah virus (NiV) are members of the genus called
Henipavirus, within the family Paramyxoviridae.
13
Outbreaks of a previously
unrecognized paramyxovirus, at rst called equine morbillivirus, later named
Hendra virus, occurred in horses in Australia in 1994 and 1995. From 1994 to
2017, there have been more than 90 conrmed cases of Hendra virus infection
in horses in Queensland and in northeast New South Wales. Following contacts
with infected horses, four out of the seven human cases described were fatal
and associated with encephalitis or respiratory disease. During 1998–1999, an
outbreak of illness caused by a similar but distinct virus, now known as Nipah
virus, occurred in Malaysia and Singapore. Human illness, characterized by fever,
severe headache, myalgia, and signs of encephalitis occurred, in individuals
in close contact with infected pigs (i.e., pig farmers and abattoir workers).
14–16
A few patients developed a respiratory disease. Approximately 40% of cases
resulted in fatalities. Following the 1998–1999 outbreak in Malaysia, the WHO
Regional Oce for South-East Asia reported 16 outbreaks in Bangladesh and
India between 2001 and 2012, totaling 263 cases. Person-to-person transmission
of Nipah virus in Bangladesh and India are reported regularly. Transmission also
occurs from direct exposure to infected bats and through consumption of raw
date palm sap contaminated with infectious bat excretions. In 2014, an outbreak
of Nipah virus occurred in the Philippines that resulted in deaths of horses and
humans. Outbreaks of Nipah in South-East Asia have a strong seasonal pattern,
249Section VIII-E: Viral Agents
occurring between December and May, possibly due to bat breeding season
or the date palm sap harvesting season.
17–19
A new henipavirus, Cedar virus,
has been isolated from pteropid bats and has signicantly reduced virulence in
several animal models. The reduced virulence is likely related to alterations found
in the P gene, which ablates the production of innate immune antagonist proteins.
Occupational Infections
No Laboratory-associated infections are known to have occurred because of
Hendra or Nipah virus exposure. However, people in close contact with Hendra
virus-infected horses, especially veterinary professionals (i.e., four cases with two
fatalities), are at high risk of contracting the disease.
20–24
Natural Modes of Infection
The natural reservoir hosts for the Hendra and Nipah viruses appear to be fruit
bats of the genus Pteropus.
25–27
Studies suggest that a locally occurring member
of the genus, Pteropus giganteus, is the reservoir for the virus in Bangladesh.
28
Individuals who had regular contact with bats had no evidence of infection
(i.e., antibody) in one study in Australia.
29
Human-to-human transmission has
been described in familial clusters and associated with close care of severely ill
patients.
30
Laboratory Safety and Containment Recommendations
The exact mode of transmission of these viruses has not been established. Most
clinical cases to date have been associated with close contact with horses, equine
blood or body uids (Australia), or pigs (Malaysia/Singapore), but presumed
transmission from Pteropus bats to humans via palm date juice has been recorded
in Bangladesh. Live virus has been detected in bat urine, implying the important
role of urine in transmitting henipaviruses to spillover hosts. Hendra and Nipah
viruses have been isolated from tissues of infected animals. In the outbreaks
in Malaysia and Singapore, viral antigen was found in central nervous system,
kidney, and lung tissues of fatal human cases, and virus was present in secretions
of patients, albeit at low levels.
31,32
Active surveillance for infection of healthcare
workers in Malaysia has not detected evidence of Laboratory-associated infections
in this setting.
33
Because of the unknown risks to laboratory workers and the potential impact on
indigenous livestock, should the virus escape a diagnostic or research laboratory,
health ocials and laboratory managers should evaluate the need to work with
the virus and the containment capability of the facility before undertaking any
work with Hendra, Nipah, or suspected related viruses. BSL-4 is required for all
work with these viruses. Once a diagnosis of Nipah or Hendra virus is suspected,
all diagnostic specimens also must be handled at BSL-4. ABSL-4 is required for
any work with infected animals.

250 Biosafety in Microbiological and Biomedical Laboratories
Work with Cedar virus in a new animal model should be performed at ABSL-3
until it is demonstrated that the virus does not result in observable illness. Work
with Cedar virus in susceptible animal hosts can be performed at ABSL-2 if it has
been demonstrated that the virus is avirulent/non-pathogenic and following a risk
assessment of the proposed work.
Special Issues
Vaccines Vaccines are not available for use in humans, but Hendra vaccine is
available in Australia for horses.
Select Agent Hendra and Nipah virus are Select Agents requiring registration
with CDC or USDA for possession, use, storage, and/or transfer. See Appendix F
for additional information.
Transfer of Agent Importation of this agent requires CDC and/or USDA impor-
tation permits. Domestic transport of this agent may require a permit from USDA
APHIS VS. A DoC permit may be required for the export of this agent to another
country. See Appendix C for additional information.
Hepatitis A Virus, Hepatitis E Virus
Hepatitis A virus (HAV) is a positive-sense single-stranded RNA virus, the type
species of the Hepatovirus genus in the family Picornaviridae. Hepatitis E virus
(HEV) is a positive-sense single-stranded RNA virus of the genus Orthohepevirus
in the family Hepeviridae. There are four major hepatitis E genotypes that infect
humans: genotypes 1, 2, 3, and 4.
Occupational Infections
Laboratory-associated infections with hepatitis A or E viruses do not appear to be
an important occupational risk among laboratory personnel. However, hepatitis
A is a documented hazard in animal handlers and others working with naturally
or experimentally infected chimpanzees and other non-human primates.
34
Workers handling other susceptible primates (e.g., owl monkeys, marmosets)
also may be at risk for hepatitis A infection. Hepatitis E virus appears to be less
of a risk to laboratory personnel than hepatitis A virus, except during pregnancy,
when infection with HEV genotype 1 can result in increased maternal and
fetal morbidity or mortality. Exposure to HEV-infected pigs, the primary animal
reservoir for hepatitis E virus, rabbits, or macaques may pose an occupational
hazard to animal handlers, but the extent of this risk is unknown.
Natural Modes of Infection
Most infections with hepatitis A are foodborne and occasionally waterborne. The
virus has, on rare occasions, been transmitted through blood, blood-derived
products, and other potentially infectious materials. Usually, infectious virus is

251Section VIII-E: Viral Agents
present in feces and blood during the incubation period, prodromal phase of
the disease, and one week after jaundice onset, but it is not transmitted later in
infection and the convalescence period. Hepatitis E virus genotypes 1 and 2 are
transmitted via the fecal-oral route primarily by contaminated water in developing
countries resulting in sporadic cases and occasionally large outbreaks. Hepatitis
E virus genotypes 3 and 4 are associated with zoonotic hepatitis E infections
transmitted to humans mainly through consumption of raw or undercooked pork
and game meat or by contact with infected animals. This occurs in developed
countries and results in sporadic cases. Transmission through blood and
blood-derived products has been reported. Infection generally causes an acute
self-limiting disease after an incubation period of two to six weeks but chronic
infection with genotype 3 has been reported in immunocompromised individuals.
Laboratory Safety and Containment Recommendations
These agents may be present in feces and blood of infected humans and
non-human primates. Feces, stool suspensions, and other contaminated
materials are the primary hazards to laboratory personnel. Care should be taken
to avoid puncture wounds when handling contaminated blood from humans
or non-human primates. There is no evidence that aerosol exposure results in
infection. Although hepatitis A virus is known to be one of the most stable viruses
in the environment, hepatitis E virus is also very stable.
BSL-2 practices, containment equipment, and facilities are recommended for the
manipulation of hepatitis A and E viruses, infected feces, blood, or other tissues.
ABSL-2 practices and facilities are recommended for activities using naturally or
experimentally-infected non-human primates or other animal models that may
shed the virus.
Special Issues
Vaccines FDA-licensed inactivated vaccines against hepatitis A are available.
There are no FDA-licensed vaccines against hepatitis E in the U.S., but a vaccine
is currently available in China.
Transfer of Agent Importation of this agent requires CDC and/or USDA impor-
tation permits. Domestic transport of this agent may require a permit from USDA
APHIS VS. A DoC permit may be required for the export of this agent to another
country. See Appendix C for additional information.
Hepatitis B Virus, Hepatitis C Virus, Hepatitis D Virus
Hepatitis B virus (HBV) is the type species of the Orthohepadnavirus genus in
the family Hepadnaviridae. Hepatitis C virus (HCV), with six genotypes, is the
type species of the Hepacivirus genus in the family Flaviviridae. Hepatitis D virus
(HDV) is the only member of the genus Deltavirus.
252 Biosafety in Microbiological and Biomedical Laboratories
Occupational Infections
Hepatitis B has been one of the most frequently occurring Laboratory-associated
infections, and laboratory workers are recognized as a high-risk group for
acquiring such infections.
35,36,38
Hepatitis C virus infection can occur in the laboratory as well.
37
The prevalence
of the antibody to hepatitis C (anti-HCV) is slightly higher in medical care workers
than in the general population. Epidemiologic evidence indicates that HCV is
spread predominantly by the parenteral route.
39
Natural Modes of Infection
These viruses are naturally acquired from a carrier during blood transfusion,
injection, tattooing, or body piercing with inadequately sterilized instruments.
Non-parenteral routes, such as domestic contact and unprotected (heterosexual
and homosexual) intercourse, are potential modes of transmission. Vertical
transmission (i.e., mother to child) is also possible.
Individuals who are infected with the HBV are at risk of infection with HDV, a
defective RNA virus that requires the presence of HBV for replication. Infection
with HDV usually exacerbates the symptoms caused by HBV infection.
Laboratory Safety and Containment Recommendations
HBV may be present in blood and blood products of human origin, in urine,
semen, CSF, and saliva. Parenteral inoculation, droplet exposure of mucous
membranes, and contact exposure of broken skin are the primary laboratory
hazards.
40
The virus may be stable in dried blood or blood components for
several days. Attenuated or avirulent strains have not been identied.
HCV has been detected primarily in blood and serum, less frequently in saliva,
and rarely or not at all in urine or semen. It appears to be somewhat stable
at room temperature on surfaces or equipment.
41,42
Infectivity of the virus is
sensitive to repeated freezing and thawing.
BSL-2 facilities with additional primary containment and personnel precautions,
such as those described for BSL-3, may be indicated for activities with potential
for droplet or aerosol production and for activities involving production quantities
or concentrations of infectious materials. BSL-2 practices, containment
equipment, and facilities are recommended for all activities utilizing known or
potentially infectious body uids and tissues. ABSL-2 practices, containment
equipment, and facilities are recommended for activities utilizing naturally or
experimentally infected chimpanzees or other non-human primates (NHPs).
Gloves should be worn when working with infected animals and when there
is the likelihood of skin contact with infectious materials. In addition to these

253Section VIII-E: Viral Agents
recommended precautions, persons working with HBV, HCV, or other bloodborne
pathogens should consult the OSHA Bloodborne Pathogen Standard.
43
Special Issues
Vaccines Licensed recombinant vaccines against hepatitis B are available and
are highly recommended for laboratory personnel.
35,36,38
Vaccines against hepatitis
C and D are not yet available for use in humans, but vaccination against HBV will
also prevent HDV infection.
Transfer of Agent Importation of this agent requires CDC and/or USDA impor-
tation permits. Domestic transport of this agent may require a permit from USDA
APHIS VS. A DoC permit may be required for the export of this agent to another
country. See Appendix C for additional information.
Macacine alphaherpevirus 1 (Herpesvirus Simiae, Cerocopithecine
herpesvirus I, Herpes B Virus)
B virus is a member of the Alphaherpesvirus genus (simplexvirus) in the family
Herpesviridae. It occurs naturally in macaque monkeys, of which there are nine
distinct species. Macaques may have primary, recurrent, and latent infections,
often with no apparent symptoms or lesions. B virus is the only member of the
family of simplex herpesviruses that can cause zoonotic infections. Human
infections have been identied in at least 50 instances, with approximately
80% mortality when untreated.
44
There have been no reported fatal cases
where prompt rst aid with wound or exposure site cleansing was performed
within minutes after the exposure and post-exposure prophylaxis was given.
Reactivated ocular disease has occurred in one individual,
45
and three infections
resulting in seroconversion to B virus have occurred in the last decade. Cases
prior to 1970 were not treated with antiviral agents because none were available.
Morbidity and mortality associated with zoonotic infection result from invasion of
the central nervous system, resulting in ascending paralysis ultimately with loss
of ability to sustain respiration in the absence of mechanical ventilation. From
1987–2016, ve additional fatal infections brought the number of lethal infections
to 21 since the discovery of B virus in 1932.
46
Occupational Infections
B virus is a hazard in facilities where macaque monkeys are present. Mucosal
secretions (i.e., saliva, genital secretions, and conjunctival secretions) are the
primary body uids associated with the risk of B virus transmission. However, it
is possible for other materials to become contaminated. For instance, in 1997
a research assistant at the Yerkes Primate Center suered a mucosal splash
without injury while transporting a caged macaque; the individual subsequently
died.
47
Based on the work being performed, the activity was considered
254 Biosafety in Microbiological and Biomedical Laboratories
low-risk at that time. However, feces, urine, or other uids and surfaces may be
contaminated with virus shed from mucosal uids. Zoonoses have been reported
following virus transmission through a bite, scratch, or splash accident, but
in at least two cases, no recognized exposure could be recalled. In one such
case, fatality occurred. Multiple cases of B virus have also been reported after
exposure to monkey cell cultures and to central nervous system tissue. There is
often no apparent evidence of B virus infection in the animals or their cells and
tissues, making it imperative that all suspect exposures be treated according
to recommended standards.
44
However, the risks associated with this hazard
are readily reduced by practicing barrier precautions and by rapid and thorough
cleansing immediately following possible site contamination. Precautions
should be taken when work requires the use of any macaque species, even
antibody-negative animals. Animals that are seronegative may be acutely infected
and shedding virus but not yet antibody positive. In most documented cases of
B virus zoonosis, the virus was not recovered from potential sources except in
four cases, making speculations that some macaque species may be safer than
others unfounded. The loss of ve lives in the past three decades underscores
that B virus infections have a low probability of occurrence, but when they do
occur there are high consequences.
Specic, regular training for B virus hazards, including understanding the modes
of exposure and transmission, should be provided to individuals encountering B
virus hazards. Training should also include proper use of engineering controls
and personal protective equipment, which is essential to prevention. Immediate
and thorough cleansing following bites, scratches, splashes, or contact with
potential fomites in high-risk areas appears to be helpful in prevention of B virus
infections.
47
First aid and emergency medical assistance procedures are most
eective when institutions set the standard to be practiced by all individuals
encountering B virus hazards.
Natural Modes of Infection
B virus occurs as a natural infection of Asiatic macaque monkeys and approxi-
mately 10% of newly caught rhesus monkeys have antibodies against the virus,
which is frequently present in kidney cell cultures of this animal. In macaque
species, the virus can cause vesicular lesions on the tongue and lips and
sometimes of the skin. B virus is not present in blood or serum in healthy infected
macaques. Transmission of B virus appears to increase when macaques reach
sexual maturity.
Laboratory Safety and Containment Recommendations
The National Academies Press published the Institute for Laboratory Animal
Research’s (ILAR) guidelines for working with non-human primates.
48
The guide-
lines provide additional information regarding risks and mitigation strategies when
handling non-human primates.
255Section VIII-E: Viral Agents
Asymptomatic B virus shedding accounts for most transmission among monkeys
and human workers, but those working in the laboratory with potentially infected
cells or tissues from macaques are also at risk. Exposure via mucous membranes
or skin breaks provides this agent access to a new host, whether the virus is
being shed from a macaque or human, or is present in or on contaminated cells,
tissues, or surfaces.
44
B virus is not generally found in serum or blood, but these
products obtained through venipuncture should be handled carefully because
contamination of needles via skin can occur. When working with macaques
directly, the virus can be transmitted through bites, scratches, or splashes only
when the animal is shedding virus from mucosal sites. Fomites or contaminated
surfaces (e.g., cages, surgical equipment, tables) should always be considered
sources of B virus unless veried as decontaminated or sterilized. Zoonotically
infected humans should be cautioned about autoinoculation of other susceptible
sites when shedding virus during acute infection.
BSL-4 facilities are recommended for the propagation of viruses obtained
from diagnostic samples or stocks. Experimental infections of macaques as
well as small animal models with B virus are recommended to be restricted to
ABSL-4 containment. BSL-3 practices are recommended for handling diagnostic
materials with possible B virus. BSL-2 practices and facilities are suitable for all
activities involving the use or manipulation of tissues, cells, blood, or serum from
macaques with appropriate personal protective equipment.
All macaques regardless of their origin should be considered potentially infected.
Animals with no detectable antibody are not necessarily B virus-free. Macaques
should be handled with strict barrier precaution protocols and injuries should be
tended immediately according to the recommendations of the B Virus Working
Group led by NIH and CDC.
44
Barrier precautions and appropriate rst aid are the keys to prevention of severe
morbidity and mortality often associated with B virus zoonoses. These prevention
tools were not implemented in each of the ve B virus fatalities during the past
three decades. Guidelines are available for safely working with macaques and
should be consulted.
44,49
The correct use of gloves, masks, and protective coats,
gowns, aprons, or overalls is recommended for all personnel while working with
non-human primates, especially macaques and other Old World species; this
is inclusive for all persons entering animal rooms where non-human primates
are housed. To minimize the potential for mucous membrane exposure, some
form of barrier is required to prevent droplet splashes to eyes, mouth, and nasal
passages. Types and use of personal protective equipment (e.g., goggles or
glasses with solid side shields and masks, or wrap-around face shields) should
be determined with reference to the institutional risk assessment. Specications
of protective equipment must be balanced with the work to be performed so that

256 Biosafety in Microbiological and Biomedical Laboratories
the barriers selected do not increase workplace risk by obscuring vision and
contributing to increased risk of bites, needlesticks, scratches, or splashes.
Special Issues
Post-exposure prophylaxis with oral acyclovir or valacyclovir should be
considered when exposures are thought to have occurred. Even a slight scratch
can result in transmission. Therapy with intravenous acyclovir and/or ganciclovir
in documented B virus infections is also important in the reduction of morbidity
following B virus zoonotic infection.
44
Ganciclovir is generally reserved for
symptomatic cases conrmed by CSF evaluation. Because of the seriousness
of B virus infection, experienced medical and laboratory personnel should be
consulted to develop individual case management. Barrier precautions should be
observed with conrmed cases. B virus infection, as with all alphaherpesviruses,
is lifelong in macaques.
50
There are no eective vaccines available and no
curative therapeutics for humans.
Transfer of Agent Importation of this agent requires CDC and/or USDA impor-
tation permits. Domestic transport of this agent may require a permit from USDA
APHIS VS. A DoC permit may be required for the export of this agent to another
country. See Appendix C for additional information.
Human Herpes Virus
The herpesviruses are ubiquitous human pathogens and are commonly present
in a variety of clinical materials submitted for virus isolation. Thus far, nine
herpesviruses have been isolated from humans: herpes simplex virus-1 (HSV-1),
herpes simplex virus-2 (HSV-2), human cytomegalovirus (HCMV), varicella-
zoster virus (VZV), Epstein-Barr virus (EBV), and human herpesviruses (HHV)
6A, 6B, 7, and 8.51
Because these viruses establish lifelong latency in human tissues, they may
manifest either as primary or recurrent infections. HSV primary and recurrent
infections are usually characterized by localized vesicular lesions at or near the
site of the initial infection. Primary infection with HSV-1 often occurs in early
childhood and may be mild and unapparent. Symptoms such as fever or malaise
can sometimes occur. HSV-1 is a frequent cause of viral meningoencephalitis.
Genital infections, usually caused by HSV-2, generally occur in adults and are
sexually transmissible.
Disseminated disease and encephalitis that may occur in neonatal infections can
be fatal. EBV is the most frequent cause of infectious mononucleosis and is also
associated with the pathogenesis of several lymphomas and nasopharyngeal
cancer.
52,53
EBV-associated cancers normally have viral genomes integrated into
the transformed cells. HCMV is often undiagnosed, presenting as a nonspecic
257Section VIII-E: Viral Agents
febrile illness with features of infectious mononucleosis. HCMV can cause severe
congenital syndrome, which may manifest as mental retardation, microcephaly,
motor disabilities, and chronic liver disease in infants who were exposed to the
virus in utero.
51
Congenital HCMV is also a frequent cause of deafness in children
who were exposed to the virus in utero.
Primary infection with VZV causes chickenpox, while recurrences of this
viral infection cause herpes zoster (shingles). Primary infection with HHV-6B
or HHV-7 can cause exanthem subitum (roseola), a common childhood
rash-associated illness and can also be a cause of infectious mononucleosis
syndrome.
53,54
Other clinical manifestations of roseola include nonspecic febrile
illness and febrile seizures. Reactivation of HHV-6 is usually identied only in
the severely immunocompromised, when it may be associated with encephalitis
or other manifestations. Disease caused by HHV-6A, which is a less common
infection that usually occurs after early childhood, is less well-understood.
HHV-8 is the causative agent of Kaposi’s sarcoma and of primary eusion
lymphoma.
55
High-risk groups for HHV-8 include HIV-infected men who have
sex with men and individuals from areas of high endemicity, such as Africa or
the Mediterranean.
56
The prevalence of HHV-8 is also higher among intravenous
drug users than in the general population.
56
At least one report has provided
evidence that, in African children, HHV-8 infection may be transmitted from
mother to child.
57
While few of the human herpesviruses have been demonstrated to cause Labora-
tory-associated infections, they are both primary and opportunistic pathogens,
especially in immunocompromised hosts, in whom recurrent infections can be
particularly severe and even life-threatening. Macacine alphaherpesvirus 1
(B-virus, Monkey B virus) is not a human herpesvirus and is discussed separately
in the preceding agent summary statement.
Occupational Infections
Few of the human herpesviruses have been documented as sources of
Laboratory-associated infections. Although this diverse group of viral agents has
not demonstrated a high potential hazard for Laboratory-associated infection,
frequent presence in clinical materials and common use in research warrant the
application of appropriate laboratory containment and safe practices.
Natural Modes of Infection
Given the wide array of viruses included in this family, the natural modes of
infection vary greatly, as does the pathogenesis of the various viruses. These
viruses both infect and establish latency in dierent types of cells leading to some
of the major clinical dierences in the disease that they cause. Transmission
of human herpesviruses in nature is generally associated with close, intimate
258 Biosafety in Microbiological and Biomedical Laboratories
contact with a person excreting the virus in their saliva, urine, or other bodily
uids.
57
For example, VZV is transmitted person-to-person through direct contact,
aerosolized vesicular uids, and respiratory secretions. HHV-8 and CMV can be
transmitted through organ transplantation
58,59
and blood transfusion.
60
The ability
of HHV-6 to integrate into the human genome allows vertical transmission in a
small percentage of cases.
Laboratory Safety and Containment Recommendations
Clinical materials, including blood, urine, and saliva, and isolates of human
herpesviruses may pose a risk of infection following ingestion, parenteral
inoculation, and droplet exposure of the mucous membranes of the eyes, nose,
or mouth, exposure to non-intact skin, or inhalation of concentrated aerosolized
materials. Clinical specimens containing the more virulent Macacine alphaher-
pesvirus 1 (B-virus) may be inadvertently submitted for diagnosis of suspected
herpes simplex infection, though the combination of a suspected herpes simplex
infection with exposure to a rhesus macaque should trigger serious concern in
the treating physician, and ideally would involve special labelling and consultation
with the microbiology laboratory. HCMV may pose a special risk to pregnant
women because of potential infection of the fetus. All human herpesviruses pose
an increased risk to persons who are immunocompromised and are not previ-
ously immune to these viruses.
BSL-2 facilities with additional containment and procedures, such as those
described for BSL-3, should be considered when producing, purifying, and
concentrating human herpesviruses, based on risk assessment. BSL-2 practices,
containment equipment, and facilities are recommended for activities utilizing
known or potentially infectious clinical materials or cultures of indigenous viral
agents that are associated or identied as a primary pathogen of human disease.
Although there is little evidence that infectious aerosols are a signicant source
of LAIs, it is prudent to avoid the generation of aerosols during the handling of
clinical materials or isolates or during the necropsy of animals.
Autologous transformation of B cells using EBV should not be performed.
Containment recommendations for Macacine alphaherpesvirus 1 (B-virus,
Monkey B virus) are described in the preceding agent summary statement.
Special Issues
Vaccines Vaccines for varicella-zoster are licensed and available in the United
States. In the event of a laboratory exposure to a non-immune individual, varicella
vaccine is likely to prevent or at least reduce the severity of disease.
61
Treatment Antiviral medications are available for treatment or prevention of
infections with several of the human herpesviruses.

259Section VIII-E: Viral Agents
Transfer of Agent Importation of this agent requires CDC and/or USDA impor-
tation permits. Domestic transport of this agent may require a permit from USDA
APHIS VS. A DoC permit may be required for the export of this agent to another
country. See Appendix C for additional information.
Inuenza Viruses
Inuenza is an acute viral disease of the respiratory tract. The most common
clinical manifestations are fever, headache, malaise, sore throat, cough, and
muscle aches. GI tract manifestations (e.g., nausea, vomiting, diarrhea) are rare
but may accompany the respiratory phase in children. The two most important
features of inuenza are the epidemic nature of illness and the mortality that
arises from pulmonary complications of the disease.
62
Inuenza virus infection may be associated with extrapulmonary complications,
including viral myocarditis and viral encephalitis. Cardiovascular deaths during
inuenza epidemics have increased indicating that cardiovascular complications,
including exacerbation of chronic underlying conditions, are important contributors
to inuenza-related morbidity and mortality.
63,64
Inuenza viruses are enveloped RNA viruses belonging to the family Orthomyxo-
viridae. There are four serotypes of inuenza viruses—A, B, C, and D, of which
human infections have been virologically conrmed for all except inuenza D
viruses. Inuenza A viruses are further classied into subtypes by the surface
glycoproteins hemagglutinin (H) and neuraminidase (N). Emergence of new
subtypes (antigenic shift) in humans occurs at irregular intervals with Type A
viruses. New subtypes can result from reassortment of human, swine, and avian
inuenza A virus genes. If there is little or no population immunity and the viruses
are able to spread in a sustained manner from human-to-human, they can be
responsible for rare pandemics. Minor antigenic changes within a circulating
seasonal inuenza A virus subtype or inuenza B virus lineage (antigenic drift)
are ongoing processes that are responsible for annual epidemics that make the
annual reformulation of inuenza vaccines necessary.
Inuenza A viruses of dierent antigenic subtypes occur naturally in many
domestic and wild avian species and have formed sustained lineages in swine,
equine, and canine species. Avian origin inuenza A viruses also sporadically
infect multiple other mammalian species. Two inuenza A virus subtypes have
only been detected in bats. Novel inuenza A virus infections of humans (zoonotic
transmission of avian or variant [swine-origin] inuenza A viruses) occur sporad-
ically.
65
Limited, non-sustained human-to-human transmission of some novel
inuenza A viruses has been reported following prolonged unprotected exposures
to an ill index case.
66–68
Interspecies transmission and reassortment of inuenza A
viruses have been reported to occur among humans, pigs, and wild and domestic
fowl. The inuenza A viruses responsible for the 1918, 1957, 1968, and 2009
260 Biosafety in Microbiological and Biomedical Laboratories
pandemics contained gene segments closely related to those of avian or swine
inuenza A viruses.
69–71
Control of inuenza is a continuing human and veterinary
public health concern.
Occupational Infections
LAIs, in the absence of animals, have not been well documented in the literature.
However, it is believed that there is a risk of possible exposure to infectious
inuenza virus in the laboratory, especially through work with high concentrations
of virus and/or experimental operations that generate aerosols (e.g., centrifu-
gation, vortex-mixing). Animal-associated infections in the laboratory or the eld
have been reported.
72–74
LAIs may result from inoculation of mucous membranes
including the upper respiratory tract through fomite transmission (e.g., touching
virus-contaminated gloves to one’s face following handling of tissues, feces,
or secretions from infected animals; touching contaminated door handles or
computer keyboards and then touching mucous membranes).
Natural Modes of Infection
Near-range inhalation through droplet/airborne spread is the predominant mode
of inuenza virus transmission among humans. Transmission may also theoret-
ically occur through direct contact of contaminated surfaces and subsequent
inoculation of mucous membranes including the upper respiratory tract since
inuenza viruses may persist for hours on surfaces particularly in the cold and
under conditions of low humidity.
69
The incubation period is from one to four days.
Recommendations for antiviral treatment and chemoprophylaxis of inuenza are
available.
75
Laboratory Safety and Containment Recommendations
The agent may be present in respiratory tissues or secretions of humans and
infected animals and birds. In addition, the agent may be present in the intestines
and cloacae of many infected avian species. Inuenza viruses may be dissemi-
nated in multiple organs in some infected animal species. The primary laboratory
hazard is inhalation of the virus from aerosols generated by infecting animals
or by aspirating, dispensing, mixing, centrifuging, or otherwise manipulating
virus-infected materials. Genetic manipulation has the potential for altering the
host range, pathogenicity, and antigenic composition of inuenza viruses. The
potential for introducing inuenza viruses with novel genetic composition into
humans is unknown.
Seasonal Human Inuenza Viruses BSL-2 facilities, practices, and procedures
are recommended for diagnostic research and production activities utilizing
contemporary inuenza A, B, and C viruses circulating among humans (e.g., H1/
H3/B). ABSL-2 is appropriate for work with these viruses in animal models.
261Section VIII-E: Viral Agents
Zoonotic and Animal Inuenza A Viruses BSL-3 or ABSL-3 containment, with
enhancements directed by regulatory authorities, should be used for laboratory
work with low pathogenicity avian inuenza (LPAI) A viruses that have caused
zoonotic infections, particularly those with fatal outcomes (e.g., H7N4, H10N8).
Work with Asian lineage A(H7N9) and non-U.S.LPAI A viruses should also be
conducted in BSL-3 or ABSL-3 laboratories with practices, procedures, and
facilities enhancements, as directed by regulatory authorities.
BSL-2 with enhanced facilities, practices, and procedures, as directed by regulatory
authorities, should be used for working with domestic LPAI A viruses (e.g., H1–4,
H6, H8–16) and equine, canine, and swine inuenza A viruses. ABSL-2 with
enhancements directed by regulatory authorities is appropriate for work with
these viruses in animal models. Asian lineage A(H7N9) LPAI viruses have caused
sporadic zoonotic infections with high mortality in humans since 2013.
76
Non-Contemporary Human Inuenza Viruses Non-contemporary, wild-type
human inuenza A(H2N2) viruses or reassortants containing the H2 or N2 RNA
segments should be handled with increased caution. Important considerations in
working with these viruses are the number of years since an antigenically related
virus last circulated and the potential presence of a susceptible population.
BSL-3 and ABSL-3 practices, procedures, and facilities are recommended with
rigorous adherence to respiratory protection and clothing change protocols.
Negative pressure, HEPA-ltered respirators and eye protection, or positive
air-purifying respirators (PAPRs) are recommended for use. Cold-adapted, live
attenuated A(H2N2) vaccine viruses may be worked with at BSL-2, but it is
recommended that a risk assessment be performed before working with such
viruses, and attention should be paid to prevent generation of reassortants that
have H2 and/or N2 RNA segments and lack attenuating features of the parental
attenuated viruses.
Historical, wild-type human inuenza A(H1N1) and A(H3N2) viruses that have
not circulated among humans in many years should be handled with increased
precaution since younger adult workers and children have little or no immunity
against such viruses. It is recommended that a risk assessment be performed
before working with such viruses; this would include consideration of the number
of years since a closely related virus last circulated among humans. For example,
pre-2009 A(H1N1) viruses have not circulated in humans since the 2009–2010
season and there is little antigenic similarity between these viruses and the
A(H1N1)pdm09 viruses that were responsible for the 2009 inuenza pandemic.
Other examples may arise in the future. In such cases, a more cautious approach
to containment utilizing elevated Biosafety Levels and practices is warranted
(e.g., BSL-2 with enhanced practices, procedures, and facilities).

262 Biosafety in Microbiological and Biomedical Laboratories
1918 Inuenza A(H1N1) Pandemic Virus Any research involving reverse
genetics of the 1918 inuenza A(H1N1) pandemic virus should proceed with
extreme caution. Research ndings suggest that exposure to A(H1N1)pdm09
virus through immunization or infection would provide protection against the
reconstructed 1918 A(H1N1) virus.
77
Moreover, several serological studies of the
A(H1N1)pdm09 virus have provided evidence for the presence of preexisting,
cross-reactive antibodies to a 1918-like H1N1 virus from previous vaccinations
or infections.
78,79
However, the 1918 A(H1N1) virus is still considered to pose
both biosafety and biosecurity threats. The following practices and conditions are
recommended for manipulation of reconstructed 1918 inuenza A(H1N1) viruses
and laboratory animals infected with the viruses. These following practices and
procedures are considered minimum standards for work with the fully recon-
structed virus.
■
BSL-3 and ABSL-3 practices, procedures, and facilities;
■
Animals, including non-human primates (NHPs), should be housed in
primary barrier systems in ABSL-3 facilities;
■
Use of negative pressure, HEPA-ltered respirators, or PAPRs;
■
Rigorous adherence to respiratory protection and clothing change
protocols;
■
HEPA ltration for treatment of exhaust air; and
■
Personal showers prior to exiting the laboratory.
Highly Pathogenic Avian Inluenza (HPAI) A Viruses Manipulating HPAI A
viruses (e.g., H5, H7) in biomedical research laboratories also requires additional
precautions because some viruses may pose increased risk to laboratory workers
and have signicant agricultural and economic implications. BSL-3 and ABSL-3
with enhanced practices, procedures, and facilities, as directed by regulatory
authorities, are required, including clothing change and personal showering
protocols. Loose-housed animals infected with HPAI A viruses must be contained
within ABSL-3Ag facilities. See Appendix D for additional information. Negative
pressure, HEPA-ltered respirators and eye protection, or positive air-purifying
respirators are recommended for HPAI A viruses with potential to infect humans.
Other Inuenza Recombinant or Reassortant Viruses When considering the
biocontainment level and attendant practices and procedures for work with other
inuenza recombinant or reassortant viruses, the IBC, or equivalent resource,
should consider but not limit consideration to the following in the conduct of
protocol-driven risk assessment.
■
The gene constellation used;
■
Any mutations that are introduced and may result in enhancement of a
pathogen’s transmissibility and/or virulence;
80
■
Clear evidence of reduced virus replication in the respiratory tract of
appropriate animal models, compared with the level of replication of the
wild-type parent virus from which it was derived;

263Section VIII-E: Viral Agents
■
Evidence of clonal purity and phenotypic stability; and
■
The number of years since a virus that was antigenically related to the
donor of the hemagglutinin and neuraminidase genes last circulated.
If adequate risk assessment data are not available, a more cautious approach to
containment, utilizing elevated Biosafety Levels and practices, is warranted.
Special Issues
Occupational Health Considerations Institutions performing work with HPAI and
LPAI A viruses that have infected humans; non-contemporary wild-type human
inuenza A viruses, including recombinants and reassortants; and viruses created
by reverse genetics of extinct virus strains (e.g., 1918 strain) should develop
and implement a specic medical surveillance and response plan. At a minimum,
these plans should: 1) strongly recommend annual vaccination with a currently
licensed inuenza vaccine for such individuals; 2) provide employee counseling
regarding disease signs and symptoms including fever, conjunctivitis, and
respiratory symptoms; 3) establish a protocol for monitoring personnel for these
symptoms; 4) include collection of acute and convalescent serum samples in the
event of a possible LAI; and 5) establish a clear medical protocol for responding
to suspected Laboratory-associated infections. Antiviral drugs (e.g., oseltamivir,
zanamivir) should be available for treatment of illness or post-exposure treatment/
chemoprophylaxis, as necessary.
75
It is recommended that the virus under study
be tested for susceptibility to antiviral drugs. All personnel should be enrolled in
an appropriately constituted respiratory protection program.
Select Agent The reconstructed 1918 inuenza A(H1N1) virus and HPAI viruses
are Select Agents requiring registration with CDC or USDA for possession, use,
storage, and/or transfer. See Appendix F for additional information.
Transfer of Agent Importation and transfer of animal-origin viruses and
diagnostic specimens obtained from animals require APHIS importation permits.
CDC/PHS import permits are required for importation of seasonal inuenza A, B,
and C viruses and specimens obtained from humans. CDC/PHS permits may also
be required for importation of animal-origin inuenza viruses of known zoonotic
potential. Importation and transfer of Select Agent viruses require APHIS/CDC
importation permits. APHIS permit-driven containment, facility requirements, and
personnel practices and/or restrictions may be applied for the possession and
handling of animal-origin and zoonotic viruses. This may also include laboratory
data/results to exclude the possibility of contamination with HPAI Select Agent
viruses in specimens. A DoC export license or license exemption may be required
for the export of Select Agent viruses to another country. See Appendix C for
additional information.
264 Biosafety in Microbiological and Biomedical Laboratories
Lymphocytic Choriomeningitis Virus
Lymphocytic choriomeningitis (LCM) is a rodent-borne viral infectious disease
that presents as aseptic meningitis, encephalitis, or meningoencephalitis. The
causative agent is the LCM virus (LCMV) that was initially isolated in 1933.
The virus is the prototypical member of the family Arenaviridae.
Occupational Infections
LAIs with LCM virus are well documented. Most infections occur when chronic
viral infection exists in laboratory or pet rodents, especially mice, hamsters, and
guinea pigs.
81–83
Nude and severe combined immune decient (SCID) mice may
pose a special risk of harboring silent chronic infections. Mice shedding the virus
may be asymptomatic. Inadvertently infected cell cultures also present a potential
source of infection and dissemination of the agent.
Natural Modes of Infection
LCMV infections have been reported in Europe, the Americas, Australia, and
Japan, and may occur wherever infected rodent hosts are found. Several
serologic studies conducted in urban areas have shown that the prevalence
of LCMV infection among humans ranges from 2% to 10%. Seroprevalence of
37.5% has been reported in humans in the Slovak Republic.
84
The common house mouse, Mus musculus, naturally spreads LCMV. Once
infected, these mice can become chronically infected as demonstrated by the
presence of virus in blood and/or by persistently shedding virus in urine. Infec-
tions by Callitrichid hepatitis virus, a strain of LCMV, have also occurred in NHPs
in zoos, including macaques and marmosets.
Humans become infected by inhaling infectious aerosolized particles of rodent
urine, feces, or saliva; by ingesting food contaminated with the virus; by
contamination of mucous membranes with infected body uids; or by directly
exposing cuts or other open wounds to virus-infected blood. Several clusters of
organ recipients from donors with unrecognized acute LCMV infection have been
described with poor survival rates in the immunosuppressed recipients.
85–89
The
source of donors’ infection is usually untraceable except in one case where a pet
hamster that was not overtly ill was incriminated.
89
Pregnant women infected with
LCMV have transmitted the virus to their fetuses that resulted in death or serious
central nervous system malformation as a consequence.
90
Laboratory Safety and Containment Recommendations
The agent may be present in blood, CSF, urine, secretions of the nasopharynx,
feces, and tissues of infected animal hosts and humans. Parenteral inoculation,
inhalation, contamination of mucous membranes or broken skin with infectious
tissues or uids from infected animals are common hazards. Aerosol transmission
is well documented.
81

265Section VIII-E: Viral Agents
Of special note, tumors may acquire LCMV as an adventitious virus without
obvious eects on the tumor. The virus may survive freezing and storage in liquid
nitrogen for long periods. When infected tumor cells are transplanted, subsequent
infection of the host and virus excretion may occur.
Women of childbearing age should be made aware of risks posed by LCMV or
rodents potentially infected by LCMV. Women who are pregnant or planning to
become pregnant should be provided medical counseling that informs them of
these risks with LCMV or animals potentially infected with LCMV.
Strains of LCMV that are shown to be lethal in non-human primates should be
handled at BSL-3. BSL-3 is also required for activities with high potential for
aerosol production, work with production quantities or high concentrations of
infectious materials, and for manipulation of infected transplantable tumors, eld
isolates, and clinical materials from human cases. Work with infected hamsters
should be done at ABSL-3.
BSL-2 practices, containment equipment, and facilities are suitable for activities
utilizing known or potentially infectious body uids and for cell culture passage
of laboratory-adapted strains. ABSL-2 practices, containment equipment, and
facilities are suitable for studies in adult mice with mouse brain-passaged strains
requiring BSL-2 containment.
Special Issues
Vaccines Vaccines are not available for use in humans.
Transfer of Agent Importation of this agent requires CDC and/or USDA impor-
tation permits. Domestic transport of this agent may require a permit from USDA
APHIS VS. A DoC permit may be required for the export of this agent to another
country. See Appendix C for additional information.
Poliovirus
Poliovirus is the type species of the Enterovirus genus in the family Picorna-
viridae. Picornaviruses are small viruses with an RNA genome. Enteroviruses
are likely transient inhabitants of the gastrointestinal tract and are stable at acid
pH. There are three poliovirus serotypes: PV1, PV2, and PV3. Immunity to one
serotype does not produce signicant immunity to the other two.
Occupational Infections
Laboratory-associated poliomyelitis is uncommon. Twelve cases, including
two deaths, were reported between 1941 and 1976.
91,92
Several instances of
asymptomatic laboratory infections with poliovirus have been reported, but
no laboratory-associated poliomyelitis has been reported for over 40 years.
Both inactivated poliovirus vaccine (IPV) and oral poliovirus vaccine (OPV) are
266 Biosafety in Microbiological and Biomedical Laboratories
highly eective in preventing disease. OPV alone induces mucosal immunity,
which gradually fades over subsequent years. Poliovirus infections among
immunized laboratory workers remain undetermined in the absence of laboratory
conrmation. An immunized laboratory worker may unknowingly be a source of
poliovirus transmission to susceptible persons in the community.
93
In April 2017,
a spill of WPV2 in a production facility in the Netherlands infected one operator
whose stool tested positive for poliovirus. This incident highlights the risk of
containment breach and emphasizes the need for appropriate incident response
planning and government oversight.
94
Natural Modes of Infection
Humans are the only known reservoir of poliovirus, which is transmitted most
frequently by persons with inapparent infections. Person-to-person spread of
poliovirus via the fecal-oral route is the most common route of transmission,
although the oral-oral route may account for some cases. Only one in several
hundred infections of unimmunized persons with wild poliovirus leads to paralytic
disease, with the vast majority of infections being asymptomatic or accompanied
by minor, u-like symptoms.
At one time, poliovirus infection occurred throughout the world. Transmission of
wild poliovirus ceased in the United States by 1979. A polio eradication program
conducted by the Pan American Health Organization led to elimination of polio
from the Western Hemisphere in 1991. The Global Polio Eradication Program,
led by the World Health Organization, has dramatically reduced the number of
paralytic cases.
The last case of wild PV2 (WPV2) was detected in 1999, and certication of
WPV2 eradication occurred in 2015. Since WPV2 was eradicated, all polio cases
associated with PV2 have been caused by oral polio vaccine (OPV) directly
(vaccine-associated paralytic polio [VAPP]) or by vaccine-derived polio type 2
virus (VDPV2). Due to continued occurrence of VAPP and outbreaks and chronic
infections associated with VDPV2, WHO discontinued all routine OPV2 use as of
May 1, 2016 by coordinating a global switch from trivalent OPV to bivalent OPV,
containing only OPV1 and 3, along with the introduction of a single dose of inacti-
vated polio vaccine (IPV). The last case of WPV3 occurred in Nigeria in 2012
and certication of WPV3 eradication occurred in 2019. As of 2019, only three
countries (Pakistan, Afghanistan, and Nigeria) are considered to be endemic for
WPV1. Complete polio eradication is expected in the near future.
Laboratory Safety and Containment Recommendations
Poliovirus is present in stool and in throat secretions of infected persons and
in lymph nodes, brain tissue, and spinal cord tissue in fatal cases. In addition,
poliovirus may be present in environmental samples (e.g., sewage).
267Section VIII-E: Viral Agents
Ingestion and parenteral inoculation are the primary routes of infection for
laboratory workers. For immunized persons parenteral inoculation likely
presents a lower risk. The potential for aerosol exposure is unknown. Laboratory
animal-associated infections have not been reported, but infected non-human
primates should be considered to present a risk.
Laboratory personnel working with and visitors with access to known poliovirus or
infectious materials potentially containing poliovirus must have documented polio
vaccination. Persons who have had a primary series of OPV or IPV and who are
at an increased occupational risk should receive another dose of IPV. Available
data do not indicate the need for more than a single lifetime IPV booster dose for
adults.
95
Type 2 and WPV3 Declaration of WPV2 eradication and the termination of routine
OPV2 use initiated the containment of PV2 under the WHO Global Action Plan
III (GAPIII).
96
GAPIII seeks to decrease the risk of reintroduction of eradicated
polioviruses from laboratories and other facilities by calling for the destruction of
non-essential poliovirus materials and containment of retained poliovirus material
in certied poliovirus-essential facilities that adhere to the containment measures
specied in GAP III. These measures include a biorisk management system,
biosafety, security, and physical laboratory features and, at the time of this writing,
apply to WPV2 and VDPV types 2 and 3, VDPV2, and OPV2 infectious materials
as well as WPV and VDPV potentially infectious materials (e.g., fecal, respiratory
secretion, and environmental samples collected at a time and in a place where
WPV or VDPV was present). The U.S. National Authority for Containment (NAC)
of Poliovirus at the CDC is responsible for working with poliovirus facilities
to achieve certication. At the time of nal eradication of all poliovirus types,
additional GAPIII physical laboratory containment measures will be required for
WPV and VDPV materials.
OPV2 potentially infectious materials are subject to the Guidance for non-
poliovirus facilities to minimize risk of sample collections potentially infectious
for polioviruses.
97,98
This document assigns risk categories based on the material
and work performed and outlines specic risk mitigation measures that are much
less stringent than GAPIII.
Type 1 and OPV3 When nal eradication is declared, GAPIII containment will
also apply to types 1 and OPV3. Laboratories and other facilities are encouraged
to destroy all PV1 and OPV3 materials not essential for research or other work.
BSL-2 and ABSL-2 practices, containment equipment, and facilities are recom-
mended for all activities using poliovirus infectious and potentially infectious
materials, including environmental and clinical samples. Contact the U.S. NAC
for enhanced measures for work with eradicated poliovirus types and strains.

268 Biosafety in Microbiological and Biomedical Laboratories
Laboratories should work with attenuated Sabin OPV strains unless there are
strong scientic reasons for working with wild polioviruses. Contact the NAC for
additional measures for work with WPV and VDPV types 2 and 3, and OPV2
infectious materials.
Special Issues
Transfer of Agent Importation of this agent requires CDC and/or USDA impor-
tation permits. Domestic transport of this agent may require a permit from USDA
APHIS VS. A DoC permit may be required for the export of this agent to another
country. See Appendix C for additional information. Contact the NAC prior to
transfers of polioviruses.
Poxviruses
Four genera within the Chordopoxvirinae subfamily (family Poxviridae) contain
species that can cause human disease: Orthopoxvirus, Parapoxvirus, Yatapox-
virus, and Molluscipoxvirus.
99
Most species in these genera are zoonotic with
the exception of variola virus (Orthopoxvirus) and molluscum contagiosum virus
(Molluscipoxvirus), which are solely human pathogens.
100,101
As most Laboratory-
associated infections involve accidents associated with orthopoxviruses, only
species of this genus will be discussed further.
Occupational Infections
Vaccinia virus is the prototypical orthopoxvirus, and its well-studied character-
istics make it commonly used in both general and biomedical research.
102
Thus,
vaccinia virus is the leading agent of laboratory-associated poxvirus infections.
LAIs with replication-competent species, including wild-type and modied strains
of vaccinia virus, have occurred even in previously vaccinated laboratorians.
Other persons at risk for occupational exposure include animal care personnel
having direct contact with vaccinated or infected animals or their secretions, or
healthcare personnel who care for vaccinated or infected patients or administer
a live vaccinia virus.
102,103
The manifestation of infection is dependent upon factors such as virus species,
route of entry, and host immune status. Infection results in the development of
one to several lesions (localized) or a generalized rash (systemic) on the skin
and/or mucous membranes. Infection with variola or monkeypox virus causes
a febrile prodrome that is preceded by a distinct systemic rash illness. Vaccinia
virus and cowpox virus typically cause a single lesion at the site of infection;
however, multiple lesions and a generalized rash may also take place. Uncom-
plicated disease typically resolves within several weeks.
99,100
269Section VIII-E: Viral Agents
Natural Modes of Infection
The most well-known orthopoxvirus is variola virus, which causes smallpox. After
an extensive vaccination campaign, smallpox was declared eradicated in 1980.
Monkeypox occurs sporadically in several West and Central African countries
but remains endemic in the Democratic Republic of Congo. The importation
of wild-caught animals from Ghana into the United States resulted in a 2003
monkeypox outbreak that aected multiple states. Vaccinia virus is used to make
the current smallpox vaccine. Naturally-acquired infections with vaccinia virus
exist outside of the United States.
104
Cases of human cowpox occur in Europe
and Asia. Rodents are known or suspected to play a part in the transmission of
monkeypox, cowpox, and vaccinia viruses.
99–101
Laboratory Safety and Containment Recommendations
Vaccination with vaccinia virus can aord protection against infection from other
species of orthopoxviruses. Smallpox vaccination occurs via scarication using
a multi-puncture method with a bifurcated needle. The current U.S.-licensed
smallpox vaccine, ACAM2000, uses a replication-competent vaccinia virus strain.
Symptoms such as fever, headache, and swollen lymph nodes are prevalent
following vaccination. Adverse events include localized reactions (e.g., robust
take), unintentional transfer of virus (e.g., self-inoculation, ocular vaccinia), diuse
dermatologic complications (e.g., eczema vaccinatum, non-specic post-vac-
cination rash), progressive vaccinia, cardiac complications, fetal vaccinia, and
postvaccinial central nervous system disease. Due to the severity of complica-
tions that can arise from vaccination, the vaccine is not recommended for persons
with certain contraindications.
99,103,105,106
Orthopoxviruses are stable in a wide range of environmental temperatures and
humidity. Virus may enter the body through the mucous membranes (e.g., eye
splashes, inhalation of droplets or ne-particle aerosols), broken skin (e.g.,
needlesticks, scalpel cut), ingestion, or by parenteral inoculation. Sources of
exposure include fomites, infected human or animal tissue, excretions or respi-
ratory secretions, or infectious cultures.
106
Routine vaccination with ACAM2000 is recommended for laboratory personnel
who directly handle cultures or animals contaminated or infected with repli-
cation-competent vaccinia virus, recombinant vaccinia viruses derived from
replication-competent vaccinia strains (i.e., those that are capable of causing
clinical infection and producing infectious virus in humans), or other orthopoxvi-
ruses that infect humans (e.g., monkeypox, cowpox, and variola).
106
Vaccination
is advised every three years for work with monkeypox and variola viruses, and
every 10 years for cowpox and vaccinia viruses. Vaccination is not required
for individuals working in laboratories that only manipulate replication-decient
strains of vaccinia virus (modied virus Ankara [MVA], NYVAC, TROVAC,
270 Biosafety in Microbiological and Biomedical Laboratories
and ALVAC). Vaccination may be oered to healthcare workers, animal care
personnel, and vaccinators who have contact with contaminated materials.
Vaccination does not protect against non-Orthopoxvirus species.
103,106
Research with variola virus is restricted to two WHO-approved BSL-4 and ABSL-4
facilities; one is the CDC in Atlanta, GA, and the other is the State Research
Center of Virology and Biotechnology (VECTOR) in Koltsovo, Russia. ABSL-3
practices, containment equipment, and facilities are recommended for monkeypox
work in experimentally or naturally infected animals. BSL-2 facilities with BSL-3
practices are advised if vaccinated personnel perform laboratory work with
monkeypox virus. BSL-2 and ABSL-2 containment plus vaccination are recom-
mended for work with vaccinia and other human pathogenic poxviruses. The
lowering of containment to BSL-1 for the manipulation of attenuated poxviruses
and vectors (e.g., modied virus Ankara [MVA], NYVAC, TROVAC, and ALVAC) in
areas where no other human orthopoxviruses are being used may be considered.
However, higher levels of containment are recommended if these strains are
used in work areas where other orthopoxviruses are manipulated. Vaccination
is not required for individuals working only in laboratories where no other ortho-
poxviruses or recombinants are handled. BSL-2 and ABSL-2 plus vaccination
are recommended for work with most other poxviruses. Note that for research
subject to the NIH Guidelines, approval to lower containment from BSL-2 must be
requested from NIH Oce of Science Policy.
107
Special Issues
The CDC provides information on a variety of topics relating to variola,
monkeypox, and vaccinia viruses online at https://www.cdc.gov. For non-emer-
gency information on potential human infections, smallpox vaccination, or
treatment options, the CDC Poxvirus Inquiry Line can be contacted at 404-639-
4129 or CDC-Info can be reached at 800-232-4636. To obtain smallpox vaccine,
CDC Drug Services can be reached by phone at 404-639-3670 or by email at
[email protected]. Clinicians or health departments may contact the CDC
Emergency Operations Center in critical circumstances.
Select Agent Congo Basin monkeypox, Variola major, and Variola minor are
Select Agents requiring registration with CDC for possession, use, storage, and/or
transfer. See Appendix F for additional information.
Transfer of Agent The importation of poxviruses into the United States and/or
their interstate transport may be subject to the rules and regulations of the CDC
Import Permit Program, CDC Division of Select Agents and Toxins, and/or the
USDA Animal and Plant Health Inspection Service. The exportation of poxviruses
may require a DoC permit.
271Section VIII-E: Viral Agents
Rabies Virus and related lyssaviruses
Rabies is an acute, progressive, fatal encephalitis caused by negative-stranded
RNA viruses in the genus Lyssavirus, family Rhabdoviridae.
108,109
Rabies lyssa-
virus (formerly Rabies virus) is the representative member (type species) of the
genus and is responsible for the majority of human and animal cases of rabies
worldwide. Currently, there are 14 recognized viral species within the genus
Lyssavirus, which can be found in Table 1.
Occupational Infections
Rabies LAIs are extremely rare; two cases have been documented. Both cases
resulted from presumed exposure to high concentrations of infectious aerosols—
one generated in a vaccine production facility
110
and the other in a research
facility.
111
Naturally or experimentally-infected animals, their tissues, and their
excretions are also a potential source of exposure for laboratory and animal care
personnel.
Natural Modes of Infection
The natural hosts of rabies virus are many bat species and terrestrial carnivores,
but any mammal can be infected. The saliva of infected animals is highly infec-
tious, and bites are the usual means of transmission, although infection through
supercial skin lesions or mucosa is possible.
Laboratory Safety and Containment Recommendations
When working with infected animals, the highest viral concentrations are present
in central nervous system (CNS) tissue, salivary glands, saliva, and lacrimal
secretions, but rabies viral antigens may be detected in all innervated tissues.
The most likely sources for exposure of laboratory and animal care personnel
are accidental parenteral inoculation, cuts, or needlesticks with contaminated
laboratory equipment, bites by infected animals, and exposure of mucous
membranes or broken skin to infectious tissue or uids. Infectious aerosols
have not been a demonstrated hazard to personnel working with routine clinical
materials or conducting diagnostic examinations. Fixed and attenuated strains of
virus are presumed to be less hazardous, but the two recorded cases of labora-
tory-associated rabies resulted from presumed exposure to the xed Challenge
Virus Standard and Street Alabama Duerin strains, respectively.
110, 111
Additional precautions (such as BSL-2 with BSL-3 practices) should be
considered when working with lyssaviruses other than rabies virus; refer to
Table 1. BSL-2 and/or ABSL-2 practices, containment equipment, and facilities
are recommended for all activities utilizing known or potentially infectious
materials or animals. Pre-exposure rabies vaccination is recommended for all
individuals prior to working with lyssaviruses or infected animals or engaging
in diagnostic, production, or research activities with these viruses.
112
Rabies
272 Biosafety in Microbiological and Biomedical Laboratories
vaccination is also recommended for all individuals entering or working in the
same room where lyssaviruses or infected animals are used. The presence of
virus-neutralizing antibodies in vaccinated individuals should be ascertained.
112,113
Prompt administration of post-exposure booster vaccinations is recommended
following recognized exposures in previously vaccinated individuals per current
guidelines.
112,113
In cases where it is not possible to open the skull or remove the brain within a
BSC, such as an autopsy or routine diagnostics, use appropriate methods and
personal protective equipment (PPE), including dedicated laboratory clothing,
heavy or chainmail gloves to avoid cuts or sticks from cutting instruments or
bone fragments, and an N95 respirator combined with a face shield or a PAPR
to protect the skin and mucous membranes of the eyes, nose, and mouth from
exposure to tissue fragments or infectious droplets. Ample coverage of a 10%
bleach solution should be used during and after the procedure for decontami-
nation of exposed or contaminated surfaces and equipment.
114
To prevent the generation of aerosols, a handsaw is recommended instead of
an oscillating saw and contact of the saw with brain tissue is avoided. Additional
primary containment and personnel precautions, such as those described for
BSL-3, are indicated for activities with a high potential for droplet or aerosol
production, and for activities involving large production quantities or high concen-
trations of infectious materials.
Table 1. Viruses currently included in the genus Lyssavirus
Species Acronym Recommended Biosafety Level
Aravan lyssavirus* ARAV 2
Australian bat lyssavirus ABLV 2
Bokeloh bat lyssavirus* BBLV 2
Duvenhage lyssavirus DUVV 2
European bat 1 lyssavirus EBLV-1 2
European bat 2 lyssavirus EBLV-2 2
Ikoma lyssavirus* IKOV 3
Irkut lyssavirus IRKV 2
Khujand lyssavirus* KHUV 2
Lagos bat lyssavirus* LBV 3
Mokola lyssavirus MOKV 3
Rabies lyssavirus RABV 2
Shimoni bat lyssavirus* SHIBV 3
West Caucasian bat lyssavirus* WCBV 3
*No human cases have been documented
Notes: This table is nal as of publication, but it will be updated in future editions of BMBL
to reect the discovery of new, divergent lyssaviruses. When handled in a BSL-2 laboratory,
BSL-3 pracitices and procedures should be used.

273Section VIII-E: Viral Agents
Special Issues
The CDC provides information on a variety of topics relating to Rabies virus,
lyssaviruses, and pre/post-exposure prophylaxis online at https://www.cdc.gov.
For non-emergency information on potential human infections, or treatment
options, the CDC Rabies Duty Ocer can be contacted at 404-639-1050 or
CDC-Info can be reached at 800-232-4636.
Transfer of Agent Importation of this agent requires CDC and/or USDA impor-
tation permits. Domestic transport of this agent may require a permit from USDA
APHIS VS. A DoC permit may be required for the export of this agent to another
country. See Appendix C for additional information.
Retroviruses, including Human and Simian Immunodeciency Viruses
(HIV and SIV)
The family Retroviridae is divided into two subfamilies: 1) the Orthoretrovirinae
with six genera including the genus Lentivirus, which includes HIV-1, HIV-2,
and SIVs; the genus Deltaretrovirus, which includes human and simian
T-lymphotropic viruses (HTLV-1, HTLV-2, HTLV-3, HTLV-4, and STLVs); and
the genus Betaretrovirus, which includes simian type D retrovirus (SRV); and
2) the Spumaretrovirinae, which has recently been updated to contain ve
genera,
115
including the genus Simiispumavirus, which includes simian foamy
viruses (SFVs) that can occasionally infect humans in close contact with infected
non-human primates (NHPs). Of these, only HIV and HTLV are pathogenic in
humans and are now classied as known human carcinogens in the National
Toxicology Program’s Report on Carcinogens.
53
SIV/HIV genetic recombinants,
known as SHIVs, are used in NHPs as models of HIV infection. The composition
of SHIVs can vary but generally consist of an SIV genetic backbone containing
specic HIV genes or gene regions.
Occupational Infections
Since 1991, data on occupational HIV transmission in health care workers
(HCW) have been collected through a CDC-supported National HIV Surveillance
system following a standardized case investigation protocol by state health
department HIV sta with help from CDC.
116,117
For surveillance purposes,
laboratory workers are dened as those persons, including students and
trainees, who have worked in a clinical or HIV laboratory setting anytime since
1978. Cases reported in this system are classied as either documented
or possible occupational transmission. Those classied as documented
occupational transmission had evidence of HIV seroconversion (i.e., a negative
HIV-antibody test at the time of the exposure that converted to positive) following
a discrete percutaneous or mucocutaneous occupational exposure to blood,
body uids, or other clinical or laboratory specimens. As of 2013, conrmed HIV
infections among 58 HCWs were reported, including 20 laboratory workers, of
274 Biosafety in Microbiological and Biomedical Laboratories
which only one involved a laboratory worker who sustained a needle exposure
while working with an HIV-infected culture. There were another 49 HCWs
exposed to HIV-infected blood, including four persons exposed to concentrated
virus in a laboratory.
116,117
Workers have been reported to develop antibodies to simian immunodeciency
virus (SIV) following exposures.
118–120
One case was associated with a needlestick
that occurred while the worker was manipulating a contaminated needle after
bleeding an SIV-infected macaque monkey.
121
Another case involved a laboratory
worker who handled macaque SIV-infected blood specimens without gloves.
Though no specic incident was recalled, this worker had dermatitis on the
forearms and hands while working with the infected blood specimens.
118
A third
worker was exposed to SIV-infected primate blood through a needlestick and
subsequently developed antibodies to SIV.
118
Of these three persons, only the
worker exposed via dermatitis showed evidence of a persistent infection. To date,
there is no evidence of illness or immunological incompetence in any of these
workers. However, workers who have been occupationally exposed to HIV/SIV
are recommended to immediately start an antiretroviral regimen. SFV infections
in humans have occurred due to cross-species transmission following a variety
of NHP exposures (e.g., working with NHPs, hunting and butchering NHPs)
resulting in life-long, persistent infection but without any evidence for disease.
Higher prevalences have been reported in individuals exposed to NHPs by bites,
especially those reporting severe bite wounds. There has been a report of a
laboratory infection while handling SFV.
119
Laboratory infection with SRV has been
reported in two workers but without molecular evidence of persistent infection or
disease.
122
SRV infection was also reported in one AIDS patient with lymphoma
but without a history of NHP contact. Dual infection of a laboratory worker with
SFV and SRV has also been reported but without evidence of secondary trans-
mission of disease.
122
STLV infection of laboratory workers has not been reported
but is known to occur in persons who hunt NHPs.
123,124
Natural Modes of Infection
Retroviruses are widely distributed as infectious agents of vertebrates, including
NHPs. Within the human population, the spread of HIV and HTLV is by close
sexual contact, parenteral exposure through blood, blood-derived products, or
other potentially infectious materials and from mother to child. Transmission of
SFV and SRV from infected persons has not been reported.
122,124,125
SIV infection of NHPs rarely causes disease but can lead to immunodeciency
and AIDS-like illness similar to that seen in HIV-infected humans.
123
STLV
infection of NHPs has been reported to cause T-cell lymphomas and leukemia,
generalized skin lesions, and splenomegaly.
123
SRV-infected macaques can
show symptoms similar to AIDS in humans, and this presentation is called simian
AIDS (SAIDS).
123
SRV-infected macaques have also displayed retroperitoneal
275Section VIII-E: Viral Agents
bromatosis, necrotizing stomatitis with osteomyelitis, acute death, splenomegaly,
lymphadenopathy, and broproliferative disorders. Disease has not been
associated with NHPs naturally infected with SFV.
123
Laboratory Safety and Containment Recommendations
HIV and HTLV have been isolated from blood, semen, saliva, urine, CSF, amniotic
uid, breast milk, cervical secretions, and tissues of infected persons and experi-
mentally infected NHPs. Additionally, HIV has been isolated from tears of infected
persons.
SIV, SHIV, and STLV have been isolated from blood, CSF, and a variety of tissues
of infected NHPs.
123
Limited data exist on the concentration of virus in semen,
saliva, cervical secretions, urine, breast milk, and amniotic uid. Virus should be
presumed to be present in all primate-derived tissue cultures, in animals exper-
imentally infected or inoculated with SIV, SHIV, or STLV, in all materials derived
from SIV, SHIV, and STLV cultures, and in/on all equipment and devices coming
into direct contact with any of these materials.
126
SFV and SRV have been isolated from NHP blood and a variety of other tissues
and can be cultured in vitro. Virus should be presumed to be present in all
NHP-derived tissue cultures, in animals experimentally infected or inoculated
with SFV or SRV, in all materials derived from SFV or SRV cultures, and in/on
all equipment and devices coming into direct contact with any of these materials,
similar to the handling of human clinical materials.
123
Although the risk of occupationally-acquired infection with retroviruses is primarily
through exposure to infected blood, it is also prudent to wear gloves when manip-
ulating other body uids such as feces, saliva, urine, tears, sweat, vomitus, and
human breast milk.
In the laboratory, retroviruses should be presumed to be present in all blood
or clinical specimens contaminated with blood, in any unxed tissue or organ
(other than intact skin) from a human (living or dead), in retrovirus cultures, in all
materials derived from retrovirus cultures, and in/on all equipment and devices
coming into direct contact with any of these materials.
The skin (especially when scratches, cuts, abrasions, dermatitis, or other lesions
are present) and mucous membranes of the eye, nose, and mouth should be
considered as potential pathways for entry of these retroviruses during laboratory
activities. It is unknown whether infection can occur via the respiratory tract. The
need for using sharps in the laboratory should be evaluated. Needles, sharp
instruments, broken glass, and other sharp objects must be carefully handled and
properly discarded. Care must be taken to avoid spilling and splashing infected
cell-culture liquid and other potentially infected materials.
276 Biosafety in Microbiological and Biomedical Laboratories
Activities involving large-scale volumes or preparation of concentrated retro-
viruses, including HIV, SIV, or SHIV, should be conducted at BSL-3. Activities,
such as producing research-laboratory-scale quantities of retroviruses, including
HIV, SIV or SHIV, manipulating concentrated virus preparations, and conducting
procedures that may produce droplets or aerosols, can be performed in a BSL-2
facility using BSL-3 practices.
Standard Precautions and personal protective equipment should be used when
working with all body uids even if the infection status of the individual or animal
is unknown.
126
BSL-2 practices, containment equipment, and facilities are recom-
mended for activities involving blood-contaminated clinical specimens, body
uids, and tissues from NHPs and humans infected with retroviruses. ABSL-2 is
appropriate for NHPs and other animals infected with retroviruses, including HIV,
SIV, or SHIV. Human serum from any source that is used as a control or reagent
in a test procedure should be handled at BSL-2. Since 1996, post-exposure
prophylaxis with antiretrovirals has been recommended to prevent infection
following occupational exposures.
127
In addition to the aforementioned recommendations, persons working with any
retrovirus, including HIV, SIV, or SHIV, or other bloodborne pathogens, should
consult the OSHA Bloodborne Pathogen Standard.
43
Special Issues
It is recommended that all institutions establish written policies (e.g., treatment,
prophylaxis protocols) regarding the management of laboratory exposure to
retroviruses (HIV, SIV). See Section VII for additional information.
The risk associated with retroviral vector systems can vary signicantly, especially
lentiviral vectors. Because the risk associated with each gene transfer system
can vary, it is recommended that all gene transfer protocols be reviewed by the
institution’s biosafety review committee or IBC.
Transfer of Agent Importation of this agent or materials containing this agent
may require CDC and/or USDA importation permits. Domestic transport of this
agent may require a permit from USDA APHIS VS. A DoC permit may be required
for the export of this agent to another country. See Appendix C for additional
information.
Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory
Syndrome (MERS) Coronaviruses
Note: the 6th edition of the BMBL had already undergone nal clearance at the
time of the 2019 coronavirus pandemic. For the latest biosafety recommendations
regarding work with SARS Coronavirus 2 (SARS-CoV-2) please consult the CDC
COVID-19 website at (https://www.cdc.gov/coronavirus/2019-nCoV/index.html).
277Section VIII-E: Viral Agents
Several human coronaviruses have been identied that can be broadly classied
into low and high pathogenicity. Low pathogenic human coronaviruses include
229E, HKU1, OC43, and NL63. High pathogenic coronaviruses include SARS
and MERS-CoV. SARS is a viral respiratory illness caused by SARS-associated
coronavirus (SARS-CoV) within the family Coronaviridae. SARS was retro-
spectively recognized in China in November 2002. Over the next few months,
the illness spread to other Southeast Asian countries, North America, South
America, and Europe following major airline routes.
128
The majority of disease-
spread occurred in hospitals, among family members, and contacts of hospital
workers. From November 2002 through July 2003, when the global outbreak
was contained, a total of 8,098 probable cases of SARS were reported to the
WHO from 29 countries.
In general, SARS patients present with fever (temperature greater than 100.4°F
[>38.0°C]), malaise, and myalgia quickly followed by respiratory symptoms
including shortness of breath and cough. Ten to 20% of patients may have
diarrhea. Review of probable cases indicates that the shortness of breath
sometimes rapidly progresses to respiratory failure requiring ventilation. The
case fatality rate is about 11%.
A second human coronavirus that causes severe disease, Middle East Respi-
ratory Syndrome coronavirus (MERS-CoV), was rst identied in Saudi Arabia in
September 2012.
128–130
Between 2012 and mid-2017, the WHO conrmed 1,952
cases with 693 deaths.
131
Cases have been conrmed in 27 countries, though
all cases have been linked to residents of the Arabian Peninsula.
131
A wide
clinical spectrum of MERS-CoV infections has been reported with asymptomatic
infection identied during outbreaks, acute respiratory illness in most symptomatic
patients, or severe presentation including rapidly progressive pneumonitis,
respiratory failure, septic shock, or multi-organ failure resulting in death.
132
Globally, 35–40% of cases reported to WHO have resulted in fatality. Common
signs and symptoms at hospital admission include fever, chills/rigors, headache,
non-productive cough, dyspnea, and myalgia.
Occupational Infections
Three dierent episodes of SARS-CoV transmission to laboratory workers
occurred in 2003 and 2004 in research laboratories in Singapore, Taiwan, and
Beijing.
133–135
The events in 2004 involved two dierent laboratory personnel,
with one case resulting in secondary and tertiary transmission of the virus to
close contacts and healthcare providers.
133
Each occurrence was linked to a
deviation from protocol or established laboratory practices.
134,135
Additionally, no
laboratory-associated cases have been associated with the routine processing
of SARS or MERS diagnostic specimens for detection of virus; however, both
coronaviruses represent an emerging infectious disease for which risk to the
medical and laboratory community is not fully understood; therefore, caution
278 Biosafety in Microbiological and Biomedical Laboratories
should be exercised when handling specimens that could potentially contain
SARS or MERS-CoV.
Natural Modes of Infection
The mode of transmission in nature is not well understood. It appears that SARS
is transmitted from person-to-person through close contact such as caring for,
living with, or having direct contact with respiratory secretions or body uids of a
suspected or probable case.
136
SARS is thought to be spread primarily through
droplets, aerosols, and possibly fomites. The natural reservoir for SARS-CoV is
unknown.
MERS-CoV transmission can occur in hospital settings through close contact.
In the community, transmission can occur between ill people and others through
close contact. Transmission may also occur in the community through close
contact with infected dromedary camels who may be a reservoir for the virus. The
incubation period of MERS-CoV is usually two to ve days; however, it can range
from two to 14 days.
131
Healthcare workers are at increased risk of acquiring SARS or MERS from an
infected patient, especially if involved in pulmonary/respiratory procedures such
as endotracheal intubation, nebulization of medications, diagnostic specimen
collection, sputum induction, airway suctioning, positive-pressure ventilation, and
high-frequency oscillatory ventilation.
Laboratory Safety and Containment Recommendations
SARS and MERS coronaviruses may be detected in respiratory, blood, urine, or
stool specimens. The exact mode of transmission of coronavirus Laboratory-
associated infections have not been established, but in clinical settings, the
primary mode of transmission appears to be through direct or indirect contact of
mucous membranes with infectious respiratory droplets.
136,137
SARS and MERS coronavirus propagation in cell culture and the initial charac-
terization of viral agents recovered in cultures of clinical specimens must be
performed at BSL-3. Respiratory protection should be used by all personnel.
Inoculation of animals for potential recovery of SARS- or MERS-CoV for
characterization of putative SARS or MERS agents must be performed in ABSL-3
facilities using ABSL-3 work practices. Respiratory protection should be used.
Activities involving manipulation of untreated specimens should be performed
in BSL-2 facilities using BSL-3 practices. In the rare event that a procedure or
process involving untreated specimens cannot be conducted in a BSC, gloves,
gown, eye protection, and respiratory protection should be used.

279Section VIII-E: Viral Agents
In clinical laboratories, respiratory specimens, whole blood, serum, plasma, and
urine specimens should be handled using Standard Precautions at BSL-2.
138
Work using intact, full-length genomic RNA should be conducted at BSL-2.
In the event of any break in laboratory procedure or accident (e.g., accidental
spillage of material suspected of containing SARS- or MERS-CoV), procedures
for emergency exposure management and environmental decontamination should
be immediately implemented and the supervisor should be notied. The worker
and the supervisor, in consultation with occupational health or infection control
personnel, should evaluate the break in procedure to determine if an exposure
occurred. See Special Issues below.
Special Issues
Occupational Health Considerations Personnel working with the virus or
samples containing or potentially containing the virus should be trained regarding
the symptoms of SARS- and MERS-CoV infection and counseled to report any
fever or respiratory symptoms to their supervisor immediately. Post-exposure
baseline serum samples should be taken following any potential exposures.
Personnel should be evaluated for possible exposure and the clinical features
and course of their illness should be closely monitored for any signs or symptoms
of disease. Institutions performing work with SARS- or MERS-CoV or handling
specimens likely to contain the agent should develop and implement a specic
occupational medical plan with respect to this agent. The plan, at a minimum,
should contain procedures for managing:
■
Deviation from protocol or established laboratory procedures;
■
Exposed workers without symptoms;
■
Exposed workers who develop symptoms within ten days of an
exposure; and
■
Symptomatic laboratory workers with no recognized exposure.
Further information and guidance regarding the development of a personnel
exposure response plan are available from the CDC.
139
Laboratory workers who
are believed to have had a laboratory exposure to SARS- or MERS-CoV should
be evaluated, counseled about the risk of SARS- and MERS-CoV transmission
to others, and monitored for fever or lower respiratory symptoms as well as for
any of the following: sore throat, rhinorrhea, chills, rigors, myalgia, headache, and
diarrhea.
Local and/or state public health departments should be promptly notied of
laboratory exposures and illness in exposed laboratory workers.
Select Agent SARS-CoV is a Select Agent requiring registration with CDC or
USDA for possession, use, storage, and/or transfer. See Appendix F for additional
information.
280 Biosafety in Microbiological and Biomedical Laboratories
Transfer of Agent The importation of SARS- and MERS-CoV into the United
States and/or its interstate transport may be subject to the rules and regulations
of the CDC Import Permit Program, CDC Division of Select Agents and Toxins,
and/or the USDA Animal and Plant Health Inspection Service. The exportation of
SARS-CoV may require a DoC permit.
References
1. Desmyter J, LeDuc JW, Johnson KM, Brasseur F, Deckers C, van Ypersele
de Strihou C. Laboratory rat associated outbreak of haemorrhagic fever
with renal syndrome due to Hantaan-like virus in Belgium. Lancet.
1983;2(8365–66):1445–8.
2. Lloyd G, Bowen ET, Jones N, Pendry A. HFRS outbreak associated with
laboratory rats in UK. Lancet. 1984;1(8387):1175–6.
3. Tsai TF. Hemorrhagic fever with renal syndrome: mode of transmission to
humans. Lab Animal Sci. 1987;37(4):428–30.
4. Umenai T, Lee HW, Lee PW, Saito T, Hongo M, Yoshinaga K, et al.
Korean haemorrhagic fever in sta in an animal laboratory. Lancet.
1979;1(8130):1314–6.
5. Centers for Disease Control and Prevention. Laboratory management of
agents associated with hantavirus pulmonary syndrome: interim biosafety
guidelines. MMWR Recomm Rep. 1994;43(RR-7):1–7.
6. Lopez N, Padula P, Rossi C, Lazaro ME, Franze-Fernandez MT. Genetic
identication of a new hantavirus causing severe pulmonary syndrome in
Argentina. Virology. 1996;220(1):223–6.
7. Jameson LJ, Taori SK, Atkinson B, Levick P, Featherstone CA, van der
Burgt G, et al. Pet rats as a source of hantavirus in England and Wales,
2013. Euro Surveill. 2013;18(9).pii:20415.
8. Kerins JL, Koske SE, Kazmierczak J, Austin C, Gowdy K, Dibernardo A, et
al. Outbreak of Seoul Virus Among Rats and Rat Owners—United States
and Canada, 2017. MMWR Morb Mortal Wkly Rep. 2018;67(4):131–4.
9. Martinez-Valdebenito C, Calvo M, Vial C, Mansilla R, Marco C, Palma RE,
et al. Person-to-person household and nosocomial transmission of andes
hantavirus, Southern Chile, 2011. Emerg Infect Dis. 2014;20(10):1629–36.
10. Padula PJ, Edelstein A, Miguel SD, Lopez NM, Rossi CM, Rabinovich RD.
Hantavirus pulmonary syndrome in Argentina: molecular evidence of person
to person transmission of Andes virus. Virology. 1998;241(2):323–30.
11. Hjelle B, Spiropoulou CF, Torrez-Martinez N, Morzunov S, Peters CJ, Nichol
ST. Detection of Muerto Canyon virus RNA in peripheral blood mononuclear
cells from patients with hantavirus pulmonary syndrome. J Infect Dis.
1994;170(4):1013–7.

281Section VIII-E: Viral Agents
12. Nichol ST, Spiropoulou CF, Morzunov S, Rollin PE, Ksiazek TG, Feldmann
H, et al. Genetic identication of a hantavirus associated with an outbreak of
acute respiratory illness. Science. 1993;262(5135):914–7.
13. Eaton BT, Broder CC, Middleton D, Wang LF. Hendra and Nipah viruses:
dierent and dangerous. Nat Rev Microbiol. 2006;4(1):23–35.
14. Chua KB, Bellini WJ, Rota PA, Harcourt BH, Tamin A, Lam SK, et al.
Nipah virus: a recently emergent deadly paramyxovirus. Science.
2000;288(5470):1432–5.
15. Chua KB, Goh KJ, Wong KT, Kamarulzaman A, Tan PS, Ksiazek TG, et al.
Fatal encephalitis due to Nipah virus among pig-farmers in Malaysia.
Lancet. 1999;354(9186):1257–9.
16. Paton NI, Leo YS, Zaki SR, Auchus AP, Lee KE, Ling AE, et al. Outbreak
of Nipah-virus infection among abattoir workers in Singapore. Lancet.
1999;354(9186):1253–6.
17. Luby SP, Gurley ES. Epidemiology of Henipavirus disease in humans. Curr
Top Microbiol Immunol. 2012;359:25–40.
18. Rahman MA, Hossain MJ, Sultana S, Homaira N, Khan SU, Rahman M,
et al. Date palm sap linked to Nipah virus outbreak in Bangladesh, 2008.
Vector Borne Zoonotic Dis. 2012;12(1):65–72.
19. World Health Organization [Internet]. Regional Oce for South-East
Asia; c2018 [cited 2018 Nov 27]. Nipah virus outbreaks in the WHO
South-East Asia Region. Available from: http://www.searo.who.int/entity/
emerging_diseases/links/nipah_virus_outbreaks_sear/en/
20. Hooper PT, Gould AR, Russell GM, Kattenbelt JA, Mitchell G. The
retrospective diagnosis of a second outbreak of equine morbillivirus
infection. Aust Veterinary J. 1996;74(3):244–5.
21. Murray K, Selleck P, Hooper P, Hyatt A, Gould A, Gleeson L, et al. A
morbillivirus that caused fatal disease in horses and humans. Science.
1995;268(5207):94–7.
22. Rogers RJ, Douglas IC, Baldock FC, Glanville RJ, Seppanen KT, Gleeson
LJ, et al. Investigation of a second focus of equine morbillivirus infection in
coastal Queensland. Aust Vet J. 1996;74(3):243–4.
23. Selvey LA, Wells RM, McCormack JG, Ansford AJ, Murray K, Rogers RJ, et al.
Infection of humans and horses by a newly described morbillivirus. Med J Aust.
1995;162(12):642–5.
24. Yu M, Hansson E, Shiell B, Michalski W, Eaton BT, Wang LF. Sequence
analysis of the Hendra virus nucleoprotein gene: comparison with other
members of the subfamily Paramyxovirinae. J Gen Virol. 1998;79(Pt 7):
1775–80.

282 Biosafety in Microbiological and Biomedical Laboratories
25. Field H, Crameri G, Kung NY, Wang LF. Ecological aspects of hendra virus.
Curr Top Microbiol Immunol. 2012;359:11–23.
26. Halpin K, Hyatt AD, Fogarty R, et al. Pteropid bats are conrmed as the
reservoir hosts of henipaviruses: a comprehensive experimental study of
virus transmission. Am J Trop Med Hyg. 2011;85(5):946–51.
27. Yob JM, Field H, Rashdi AM, Morrissy C, van der Heide B, Rota P, et al.
Nipah virus infection in bats (order Chiroptera) in peninsular Malaysia. Emer
Infect Dis. 2001;7(3):439–41.
28. Iccdr B. Outbreaks of encephalitis due to Nipah/Hendra-like viruses,
Western Bangladesh. Hlth Sci Bull. 2003;1:1–6.
29. Selvey L, Taylor R, Arklay A, Gerrard J. Screening of bat carers for
antibodies to equine morbillivirus. Comm Dis Intell. 1996;20(22):477–8.
30. Luby SP. The pandemic potential of Nipah virus. Antiviral Res.
2013;100(1):38–43.
31. Chua KB, Lam SK, Goh KJ, Hooi PS, Ksiazek TG, Kamarulzaman A,
et al. The presence of Nipah virus in respiratory secretions and urine of
patients during an outbreak of Nipah virus encephalitis in Malaysia. J Infect.
2001;42(1):40–3.
32. Wong KT, Shieh WJ, Zaki SR, Tan CT. Nipah virus infection, an emerging
paramyxoviral zoonosis. Springer Semin Immunopathol. 2002;24(2):215–28.
33. Mounts AW, Kaur H, Parashar UD, Ksiazek TG, Cannon D,
Arokiasamy JT, et al. A cohort study of health care workers to assess
nosocomial transmissibility of Nipah virus, Malaysia, 1999. J Infect Dis.
2001;183(5):810–3.
34. Pike RM. Laboratory-associated infections: incidence, fatalities, causes, and
prevention. Annu Rev Microbiol. 1979;33:41–66.
35. Schillie S, Murphy TV, Sawyer M, Ly K, Hughes E, Jiles R, et al. CDC
guidance for evaluating health-care personnel for Hepatitis B virus
protection and for administering postexposure management. MMWR
Recomm Rep. 2013;62(RR-10):1–19.
36. Centers for Disease Control and Prevention [Internet]. Atlanta (GA): Division
of Viral Hepatitis and National Center for HIV/AIDS, Viral Hepatitis, STD,
and TB Prevention; c2018 [cited 2018 Nov 30]. Hepatitis B Questions and
Answers for Health Professionals. Available from: https://www.cdc.gov/
hepatitis/hbv/hbvfaq.htm#overview
37. Centers for Disease Control and Prevention. Recommendations for
follow-up of health-care workers after occupational exposure to Hepatitis C
virus. MMWR Morb Mortal Wkly Rep. 1997;46(26):603–6.

283Section VIII-E: Viral Agents
38. Centers for Disease Control and Prevention. Recommendation of the
Immunization Practices Advisory Committee (ACIP). Inactivated Hepatitis B
virus vaccine. MMWR Morb Mortal Wkly Rep. 1982;31(24):317–22, 327–8.
39. Chung H, Kudo M, Kumada T, Katsushima S, Okano A, Nakamura T, et al.
Risk of HCV transmission after needlestick injury, and the ecacy of short-
duration interferon administration to prevent HCV transmission to medical
personnel. J Gastroenterol. 2003;38(9):877–9.
40. Buster E, van der Eijk AA, Schalm SW. Doctor to patient transmission
of Hepatitis B virus: implications of HBV DNA levels and potential new
solutions. Antiviral Res. 2003;60(2):79–85.
41. Binka M, Paintsil E, Patel A, Lindenbach BD, Heimer R. Survival of Hepatitis
C Virus in Syringes Is Dependent on the Design of the Syringe-Needle and
Dead Space Volume. PloS One. 2015;10(11):e0139737. Erratum in: PLoS
One. 2015;10(12):e0146088.
42. Paintsil E, Binka M, Patel A, Lindenbach BD, Heimer R. Hepatitis C
virus maintains infectivity for weeks after drying on inanimate surfaces
at room temperature: implications for risks of transmission. J Infect Dis.
2014;209(8):1205–11.
43. Occupational exposure to bloodborne pathogens; correction–OSHA. Final
rule, correction. Fed Regist. 1992;57(127):29206.
44. Cohen JI, Davenport DS, Stewart JA, Deitchman S, Hilliard JK, Chapman LE,
et al. Recommendations for prevention of and therapy for exposure to B virus
(Cercopithecine herpesvirus 1). Clin Infect Dis. 2002;35(10):1191–203.
45. Calvo C, Friedlander S, Hilliard J, Swarts R, Nielsen J, Dhindsa H, et al.
Case Report: Reactivation Of Latent B Virus (Macacine Herpesvirus 1)
Presenting As Bilateral Uveitis, Retinal Vasculitis And Necrotizing Herpetic
Retinitis. Investigative Ophthalmology & Visual Science. 2011;52(14):2975.
46. Centers for Disease Control and Prevention [Internet]. Atlanta (GA):
National Center for Immunization and Respiratory Diseases, Division of Viral
Diseases; c2016 [cited 2018 Nov 30]. B Virus (herpes B, monkey B virus,
herpesvirus simiae, and herpesvirus B). Available from: https://www.cdc.
gov/herpesbvirus/cause-incidence.html
47. Centers for Disease Control and Prevention. Fatal Cercopithecine
herpesvirus 1 (B virus) infection following a mucocutaneous exposure and
interim recommendations for worker protection. MMWR Morb Mortal Wkly
Rep. 1998;47(49):1073–6, 1083.
48. Committee on Occupational Health and Safety in the Care and Use of
Non-Human Primates. Occupational Health and Safety in the Care and Use of
Nonhuman Primates. Washington (DC): The National Academies Press; 2003.

284 Biosafety in Microbiological and Biomedical Laboratories
49. Guidelines for prevention of Herpesvirus simiae (B virus) infection
in monkey handlers. The B Virus Working Group. J Med Primatol.
1988;17(2):77–83.
50. Hu JL, Eberle R, Capitanio J, Zhou SS, Barry PA. Dierential detection
of B virus and rhesus cytomegalovirus in rhesus macaques. J Gen Virol.
2003;84(Pt 1):83–92.
51. Roizman B, Pellett PE. Herpesviridae. In: Knipe DM, Howley PM, editors.
Fields Virology. Vol 2. 6th ed. Philadelphia: Lippincott Williams & Wilkins;
2013. p. 1802–22.
52. Heymann DL, editor. Control of Communicable Diseases Manual. 20th ed.
Washington (DC): American Public Health Association; 2015.
53. U.S. Department of Health and Human Services [Internet]. Washington
(DC): National Toxicology Program; c2018 [cited 2018 Dec 3]. 14th Report
on Carcinogens. Available from: https://ntp.niehs.nih.gov/pubhealth/roc/
index-1.html#toc1
54. Cohen, JI. Human herpesvirus types 6 and 7. In: Bennett JE, Dolin R,
Blaser MJ, editors. Mandell, Douglas, and Bennett’s principles and
practice of infectious diseases. Vol 2. 8th ed. Philadelphia: Elsevier; 2015.
p. 1772–6.
55. Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, Knowles DM, et
al. Identication of herpesvirus-like DNA sequences in AIDS-associated
Kaposi’s sarcoma. Science. 1994;266(5192):1865–9.
56. Dukers NH, Rezza G. Human herpesvirus 8 epidemiology: what we do and
do not know. AIDS. 2003;17(12):1717–30.
57. Plancoulaine S, Abel L, van Beveren M, Treqouet DA, Joubert M, Tortevoye
P, et al. Human herpesvirus 8 transmission from mother to child and
between siblings in an endemic population. Lancet. 2000;356(9235):1062–5.
58. Regamey N, Tamm M, Wernli M, Witschi A, Thiel G, Cathomas G, et al.
Transmission of human herpesvirus 8 infection from renal-transplant donors
to recipients. N Engl J Med. 1998;339(19):1358–63.
59. Luppi M, Barozzi P, Guaraldi G, Ravazzini L, Rasini V, Spano C, et al.
Human herpesvirus 8-associated diseases in solid-organ transplantation:
importance of viral transmission from the donor. Clin Infect Dis.
2003;37(4):606–7.
60. Mbulaiteye SM, Biggar RJ, Bakaki PM, Pfeier RM, Whitby D, Owor AM,
et al. Human herpesvirus 8 infection and transfusion history in children with
sickle-cell disease in Uganda. J Natl Cancer Inst. 2003;95(17):1330–5.
285Section VIII-E: Viral Agents
61. Marin M, Guris D, Chaves SS, Schmid S, Seward JF; Advisory Committee
on Immunization Practices, Centers for Disease Control and Prevention.
Prevention of Varicella: recommendations of the Advisory Committee on
Immunization Practices (ACIP). MMWR Recomm Rep. 2007;56(RR-4):1–40.
62. Treanor JJ. Inuenza virus. In: Bennett JE, Dolin R, Blaser MJ, editors.
Mandell, Douglas, and Bennett’s principles and practice of infectious
diseases. Vol 2. 8th ed. Philadelphia: Elsevier; 2015. p. 2000–4.
63. Kwong JC, Schwartz KL, Campitelli MA. Acute Myocardial Infarction
after Laboratory-Conrmed Inuenza Infection. N Engl J Medicine.
2018;378(26):2540–1.
64. Sellers SA, Hagan RS, Hayden FG, Fischer WA 2nd. The hidden burden
of inuenza: A review of the extra-pulmonary complications of inuenza
infection. Inuenza Other Respir Viruses. 2017;11(5):372–93.
65. Uyeki TM, Katz JM, Jernigan DB. Novel inuenza A viruses and pandemic
threats. Lancet. 2017;389(10085):2172–74.
66. Jhung MA, Epperson S, Biggersta M, Allen D, Balish A, Barnes N, et al.
Outbreak of variant inuenza A(H3N2) virus in the United States. Clini Infect
Dis. 2013;57(12):1703–12.
67. Wang H, Feng Z, Shu Y, Yu H, Zhou L, Zu R, et al. Probable limited person-
to-person transmission of highly pathogenic avian inuenza A(H5N1) virus
in China. Lancet. 2008;371(9622):1427–34.
68. Zhou L, Chen E, Bao C, Xiang N, Wu J, Wu S, et al. Clusters of Human
Infection and Human-to-Human Transmission of Avian Inuenza A(H7N9)
Virus, 2013–2017. Emerg Infect Dis. 2018;24(2).
69. Inuenza. In: Heymann DL, editor. Control of communicable diseases
manual. 20th ed. Washington (DC): American Public Health Association;
2015. p. 306–22.
70. Dowdle WR, Hattwick MA. Swine inuenza virus infections in humans.
J. Infect Dis. 1977;136 Suppl:S386–5399.
71. Tang JW, Shetty N, Lam TT, Hon KL. Emerging, novel, and known inuenza
virus infections in humans. Infect Dis Clin North Am. 2010;24(3):603–17.
72. Bouvier NM. Animal models for inuenza virus transmission studies: a
historical perspective. Curr Opin Virol. 2015;13:101–8.
73. Lee CT, Slavinski S, Schi C, Merlino M, Daskalakis D, Liu D, et al.
Outbreak of Inuenza A(H7N2) Among Cats in an Animal Shelter With
Cat-to-Human Transmission—New York City, 2016. Clin Infect Dis.
2017;65(11):1927–29.
74. Webster RG, Geraci J, Petursson G, Skirnisson K. Conjunctivitis in
human beings caused by inuenza A virus of seals. N Engl J Med.
1981;304(15):911.

286 Biosafety in Microbiological and Biomedical Laboratories
75. Fiore AE, Shay DK, Broder K, Iskander JK, Uyeki TM, Mootrey G, et al.
Prevention and control of inuenza: recommendations of the Advisory
Committee on Immunization Practices (ACIP), 2008. MMWR Recomm Rep.
2008;57(RR-7):1–60.
76. Su S, Gu M, Liu D, Cui J, Gao GF, Zhou J, et al. Epidemiology, Evolution,
and Pathogenesis of H7N9 Inuenza Viruses in Five Epidemic Waves since
2013 in China. Trends Microbiol. 2017;25(9):713–28.
77. Pearce MB, Belser JA, Gustin KM, Pappas C, Houser KV, Sun X, et al.
Seasonal trivalent inactivated inuenza vaccine protects against 1918
Spanish inuenza virus infection in ferrets. J Virol. 2012;86(13):7118–25.
78. Hancock K, Veguilla V, Lu X, Zhong W, Butler EN, Sun H, et al. Cross-
reactive antibody responses to the 2009 pandemic H1N1 inuenza virus.
N Engl J Med. 2009;361(20):1945–52.
79. Medina RA, Manicassamy B, Stertz S, Seibert CW, Hai R, Belshe RB, et al.
Pandemic 2009 H1N1 vaccine protects against 1918 Spanish inuenza
virus. Nat Commun. 2010;1:28.
80. Science Safety Security [Internet]. Washington (DC): U.S. Department of
Health & Human Services; c2017 [cited 2018 Dec 3]. Dual Use Research of
Concern. Available from: https://www.phe.gov/s3/dualuse/Pages/default.aspx
81. Bowen GS, Calisher CH, Winkler WG, Kraus AL, Fowler EH, Garman RH,
et al. Laboratory studies of a lymphocytic choriomeningitis virus outbreak in
man and laboratory animals. Am J Epidemiol. 1975;102(3):233–40.
82. Jahrling PB, Peters CJ. Lymphocytic choriomeningitis virus. A neglected
pathogen of man. Arch Pathol Lab Med. 1992;116(5):486–8.
83. Knust B, Ströher U, Edison L, Albarino CG, Lovejoy J, Armeanu E,
et al. Lymphocytic Choriomeningitis Virus in Employees and Mice at
Multipremises Feeder-Rodent Operation, United States, 2012. Emerg Infect
Dis. 2014;20(2):240–7.
84. Reiserová L, Kaluzová M, Kaluz S, Willis AC, Zavada J, Zavadska E,
et al. Identication of MaTu-MX Agent as a New Strain of Lymphocytic
Choriomeningitis Virus (LCMV) and Serological Indication of Horizontal
Spread of LCMV in Human Population. Virology. 1999;257(1):73–83.
85. Fischer SA, Graham MB, Kuehnert MJ, Kotton CN, Srinivasan A, Marty
FM, Comer JA, et al. Transmission of lymphocytic choriomeningitis virus by
organ transplantation. N Engl J Med. 2006;354(21):2235–49.
86. Macneil A, Stroher U, Farnon E, Campbell S, Cannon D, Paddock CD, et
al. Solid organ transplant-associated lymphocytic choriomeningitis, United
States, 2011. Emerg Infect Dis. 2012;18(8):1256–62.
287Section VIII-E: Viral Agents
87. Mathur G, Yadav K, Ford B, Schafer IJ, Basavaraju SV, Knust B, et al. High
clinical suspicion of donor-derived disease leads to timely recognition and
early intervention to treat solid organ transplant-transmitted lymphocytic
choriomeningitis virus. Transpl Infect Dis. 2017;19(4).
88. Palacios G, Druce J, Du L, Tran T, Birch C, Briese T, et al. A new arenavirus
in a cluster of fatal transplant-associated diseases. N Engl J Med.
2008;358(10):991–8.
89. Centers for Disease Control and Prevention. Lymphocytic choriomeningitis
virus infection in organ transplant recipients–Massachusetts, Rhode Island,
2005. MMWR Morb Mortal Wkly Rep. 2005;54(21):537–9.
90. Wright R, Johnson D, Neumann M, Ksiazek TG, Rollin P, Keech RV, et al.
Congenital lymphocytic choriomeningitis virus syndrome: a disease that
mimics congenital toxoplasmosis or Cytomegalovirus infection. Pediatrics.
1997;100(1):E9.
91. Dowdle WR, Gary HE, Sanders R, van Loon AM. Can post-eradication
laboratory containment of wild polioviruses be achieved?. Bull World Health
Organ. 2002;80(4):311–6.
92. Pike RM. Laboratory-associated infections: summary and analysis of 3921
cases. Hlth Lab Sci. 1976;13(2):105–14.
93. Mulders MN, Reimerink JH, Koopmans MP, van Loon AM, van der
Avoort HG. Genetic analysis of wild-type poliovirus importation into The
Netherlands (1979–1995). J Infect Dis. 1997;176(3):617–24.
94. Previsani N, Singh H, St Pierre J, Boualam L, Fournier-Caruana J, Sutter
RW, et al. Progress Toward Containment of Poliovirus Type 2—Worldwide,
2017. MMWR Morb Mortal Wkly Rep. 2017;66(24):649–52.
95. Prevots DR, Burr RK, Sutter RW, Murphy TV; Advisory Committee on
Immunization Practices. Poliomyelitis prevention in the United States.
Updated recommendations of the Advisory Committee on Immunization
Practices (ACIP). MMWR Recomm Rep. 2000;49(RR-5):1–22; quiz CE1–7.
96. World Health Organization. WHO Global Action Plan to minimize poliovirus
facility-associated risk after type-specic eradication of wild polioviruses and
sequential cessation of oral polio vaccine use. Geneva: WHO Press; 2015.
97. World Health Organization. Guidance for non-poliovirus facilities to minimize
risk of sample collections potentially infectious for polioviruses. Geneva:
World Health Organization; 2018.
98. Annex 2. In: World Health Organization. Guidance for non-poliovirus
facilities to minimize risk of sample collections potentially infectious for
polioviruses. Geneva: World Health Organization; 2018. p. 18–9.

288 Biosafety in Microbiological and Biomedical Laboratories
99. Damon IK. Poxviruses. In: Knipe DM, Howley PM, editors. Fields Virology.
Vol 2. 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2013. p. 2160–84.
100. Lewis-Jones S. Zoonotic poxvirus infections in humans. Curr Opin Infect
Dis. 2004;17(2):81–9.
101. Reed KD, Melski JW, Graham MB, Regnery RL, Sotir MJ, Wegner MV, et al.
The detection of monkeypox in humans in the Western Hemisphere. N Engl
J Med. 2004;350(4):342–50.
102. MacNeil A, Reynolds MG, Damon IK. Risks associated with vaccinia virus in
the laboratory. Virology. 2009;385(1):1–4.
103. Wharton M, Strikas RA, Harpaz R, Rotz LD, Schwartz B, Casey CG,
et al. Recommendations for using smallpox vaccine in a pre-event
vaccination program. Supplemental recommendations of the Advisory
Committee on Immunization Practices (ACIP) and the Healthcare Infection
Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep.
2003;52(RR-7):1–16.
104. Peres MG, Bacchiega TS, Appolinario CM, Vicente AF, Mioni MSR, Ribeiro
BLD, et al. Vaccinia Virus in Blood Samples of Humans, Domestic and Wild
Mammals in Brazil. Viruses. 2018;10(1). pii: E42.
105. Casey C, Vellozzi C, Mootrey GT, Chapman LE, McCauley M, Roper MH,
et al. Surveillance guidelines for smallpox vaccine (vaccinia) adverse
reactions. MMWR Recomm Rep. 2006;55(RR-1):1–16.
106. Petersen BW, Harms TJ, Reynolds MG, Harrison LH. Use of Vaccinia
Virus Smallpox Vaccine in Laboratory and Health Care Personnel at Risk
for Occupational Exposure to Orthopoxviruses—Recommendations of the
Advisory Committee on Immunization Practices (ACIP), 2015. MMWR Morb
Mortal Wkly Rep. 2016;65(10):257–62.
107. National Institutes of Health. NIH Guidelines for Research Involving
Recombinant or Synthetic Nucleic Acid Molecules (NIH Guidelines).
Bethesda (MD): National Institutes of Health, Oce of Science Policy; 2019.
108. Rupprecht CE, Hanlon CA, Hemachudha T. Rabies re-examined. Lancet
Infect Dis. 2002;2(6):327–43.
109. International Committee on Taxonomy of Viruses [Internet]. Taxonomy;
c2019 [cited 2019 Mar 12]. Virus Taxonomy: 2018b Release. Available from:
https://talk.ictvonline.org/taxonomy/
110. Winkler WG, Fashinell TR, Lengwell L, Howard P, Conomy P. Airborne
rabies transmission in a laboratory worker. JAMA. 1973;226(10):1219–21.
111. Centers for Disease Control and Prevention. Rabies in a laboratory
worker—New York. MMWR Morb Mortal Wkly Rep. 1977;26(22):183–4.
289Section VIII-E: Viral Agents
112. Manning SE, Rupprecht CE, Fishbein D, Hanlon CA, Lumlertdacha B,
Guerra M, et al. Human rabies prevention–United States, 2008:
Recommendations of the Advisory Committee on Immunization Practices.
MMWR Recomm Rep. 2008;57(RR-3):1–28.
113. Rupprecht CE, Gibbons RV. Clinical practice. Prophylaxis against rabies.
N Engl Journal Med. 2004;351(25):2626–35.
114. Centers for Disease Control and Prevention. Human rabies—Kentucky/
Indiana, 2009. MMWR Morb Mortal Wkly Rep. 2010;59(13):393–6.
115. Khan AS, Bodem J, Buseyne F, Gessain A, Johnson W, Kuhn JH, et al.
Spumaretroviruses: Updated taxonomy and nomenclature. Virology.
2018;516:158–64.
116. Centers for Disease Control and Prevention. HIV/AIDS surveillance report.
U.S. HIV and AIDS cases reported through June 1998. Midyear Edition.
1998;10(1).
117. Joyce MP, Kuhar D, Brooks JT. Notes from the eld: Occupationally
acquired HIV infection among health care workers—United States,
1985–2013. MMWR Morb Mortal Wkly Rep. 2015;63(53):1245–6.
118. Centers for Disease Control and Prevention. Seroconversion to simian
immunodeciency virus in two laboratory workers. MMWR Morb Mortal Wkly
Rep. 1992;41(36):678–81.
119. Schweizer M, Turek R, Hahn H, Schliephake A, Netzker KO, Eder G, et al.
Markers of foamy virus infections in monkeys, apes, and accidentally
infected humans: appropriate testing fails to conrm suspected foamy virus
prevalence in humans. AIDS Res Hum Retroviruses. 1995;11(1):161–70.
120. Sotir M, Switzer W, Schable C, Schmitt J, Vitek C, Khabbaz RF. Risk of
occupational exposure to potentially infectious nonhuman primate materials
and to simian immunodeciency virus. J Med Primatol. 1997;26(5):233–40.
121. Khabbaz RF, Rowe T, Murphey-Corb M, Heneine WM, Schable CA,
George JR, et al. Simian immunodeciency virus needlestick accident in a
laboratory worker. Lancet. 1992;340(8814):271–3.
122. Lerche NW, Switzer WM, Yee JL, Shanmugam V, Rosenthal AN, Chapman LE,
et al. Evidence of infection with simian type D retrovirus in persons
occupationally exposed to nonhuman primates. J Virol. 2001;75(4):1783–9.
123. Murphy HW, Miller M, Ramer J, Travis D, Barbiers R, Wolfe ND, et al.
Implications of simian retroviruses for captive primate population
management and the occupational safety of primate handlers. J Zoo Wildl
Med. 2006;37(3):219–33.
124. Switzer WM, Bhullar V, Shanmugam V, Conge ME, Parekh B, Lerche NW, et
al. Frequent simian foamy virus infection in persons occupationally exposed
to nonhuman primates. J Virol. 2004;78(6):2780–9.

290 Biosafety in Microbiological and Biomedical Laboratories
125. Switzer WM, Heneine W. Foamy Virus. In: Liu D, editor. Molecular Detection
of Human Viral Pathogens: Boca Raton: CRC Press; 2011. p. 131–46.
126. Centers for Disease Control and Prevention [Internet]. Atlanta (GA):
National Center for Zoonotic and Emerging Infectious Diseases, Division of
Healthcare Quality Promotion; c2016. Standard Precautions for All Patient
Care. Available from: https://www.cdc.gov/infectioncontrol/basics/standard-
precautions.html.
127. Kuhar DT, Henderson DK, Struble KA, Heneine W, Thomas V, Cheever LW,
et al. Updated US Public Health Service guidelines for the management
of occupational exposures to human immunodeciency virus and
recommendations for postexposure prophylaxis. Infect Control Hosp
Epidemiol. 2013;34(9):875–93. Erratum in: Infect Control Hosp Epidemiol.
2013;34(11):1238.
128. van Boheemen S, de Graaf M, Lauber C, Bestebroer TM, Raj VS, Zaki AM,
et al. Genomic characterization of a newly discovered coronavirus
associated with acute respiratory distress syndrome in humans. MBio.
2012;3(6). pii: e00473–12.
129. Assiri A, Al-Tawq JA, Al-Rabeeah AA, Al-Rabiah FA, Al-Hajjar S, Al-Barrack A,
et al. Epidemiological, demographic, and clinical characteristics of 47 cases
of Middle East respiratory syndrome coronavirus disease from Saudi Arabia:
a descriptive study. Lancet Infect Dis. 2013;13(9):752–61.
130. Centers for Disease Control and Prevention. Severe respiratory illness
associated with a novel coronavirus–Saudi Arabia and Qatar, 2012. MMWR
Morb Mortal Wkly Rep. 2012;61(40):820.
131. World Health Organization [Internet]. Geneva. c2018 [cited 2018 Dec 3].
Middle East respiratory syndrome coronavirus (MERS-CoV). Available from:
https://www.who.int/emergencies/mers-cov/en/
132. Rasmussen SA, Gerber SI, Swerdlow DL. Middle East respiratory syndrome
coronavirus: update for clinicians. Clin Infect Dis. 2015;60(11):1686–9.
133. Centers for Disease Control and Prevention [Internet]. Atlanta (GA):
National Center for Immunization and Respiratory Diseases, Division of Viral
Diseases; c2017 [cited 2018 Dec 3]. Severe Acute Respiratory Syndrome
(SARS). Available from: https://www.cdc.gov/sars/
134. American Biological Safety Association [Internet]. c2014 [cited 2018 Dec 3].
Laboratory-Acquired Infection (LAI) Database. Available from: https://my.
absa.org/LAI
135. Lim PL, Kurup A, Gopalakrishna G, Chan KP, Wong CW, Ng LC, et al.
Laboratory-acquired severe acute respiratory syndrome. N Engl J Med.
2004;350(17):1740–5.

291Section VIII-E: Viral Agents
136. SARS, MERS, and other coronavirus infections. In: Heymann DL, editor.
Control of Communicable Diseases Manual. 20th ed. Washington (DC):
American Public Health Association; 2015. p. 539–49.
137. Chow PK, Ooi EE, Tan HK, Ong KW, Sil BK, Teo M, et al. Healthcare worker
seroconversion in SARS outbreak. Emerg Infect Dis. 2004;10(2):249–50.
138. Centers for Disease Control and Prevention [Internet]. Atlanta (GA):
National Center for Zoonotic and Emerging Infectious Diseases, Division of
Healthcare Quality Promotion; c2016. Standard Precautions for All Patient
Care. Available from: https://www.cdc.gov/infectioncontrol/basics/standard-
precautions.html.
139. Centers for Disease Control and Prevention. Severe Acute Respiratory
Syndrome. Public Health Guidance for Community-Level Preparedness and
Response to Severe Acute Respiratory Syndrome (SARS) Version 2.
Supplement F: Laboratory Guidance. Department of Health & Human
Services; 2004.

292 Biosafety in Microbiological and Biomedical Laboratories
Section VIII-F: Arboviruses and Related Zoonotic Viruses
In 1979, and again in 1985, the American Committee on Arthropod-Borne
Viruses (ACAV) Subcommittee on Arbovirus Laboratory Safety (SALS) provided
biosafety recommendations for each of the approximately 500 viruses registered
in the International Catalogue of Arboviruses, including Certain Other Viruses
of Vertebrates.
1
Since the last print publication of the Catalog, SALS, the CDC,
and the NIH have periodically reviewed these viruses as well as newly identied
arboviruses and provided recommended biosafety practices and containment
for arboviruses identied or registered since that time. These recommendations
are based, in part, on risk assessments derived from information provided by
a worldwide survey of laboratories working with arboviruses, newly published
reports on the viruses, reports of laboratory infections, and discussions with
scientists working with each virus.
A series of signicant tables are provided throughout Section VIII-F. Table 1
contains a list of vaccine strains of viruses that may be handled at BSL-2. Table 3
provides an alphabetical listing of the recognized arboviruses at the time of
publication and includes the common name, acronym, virus family or genus,
Biosafety Level (BSL) recommendation, basis for the rating, and antigenic group
(if known).
2
Many of the organisms are classied as Select Agents and require
special security measures to possess, use, or transfer; see Appendix F for
additional information. Table 2 provides a key for the SALS basis for assignment
of viruses listed in Tables 3 and 4. Table 4 provides an alphabetical listing of
the arthropod-only arboviruses and includes the common name, acronym, virus
family or genus, BSL recommendation, basis for the rating, and whether the virus
has been isolated. Table 5 provides a list of agents that may be handled at BSL-3
with HEPA-ltered exhaust air. The agents in Tables 1, 3, 4 and 5 require permits
from APHIS, DOC, and/or CDC.
It is important to assess the risks of each member of the arbovirus family individ-
ually. While arboviral families may share many similarities, each can present their
own unique biosafety risks. Viruses that have positive-sense single-stranded
RNA carry unique infection risks that are not a consideration for other pathogens.
Positive-sense viral RNA can directly cause infection since its RNA can serve as
mRNA to direct viral protein synthesis by the host cell.
3
Additionally, disinfection
methods aimed at inactivating an enveloped virus may not be eective at
rendering a positive-sense single-stranded RNA non-infectious.
4
In addition to the true arboviruses (i.e., viruses that replicate in both vertebrates
and invertebrates), a signicant number of arthropod-only viruses (i.e., viruses
not known to replicate in vertebrate cells) that are closely related to arboviral
counterparts have been identied.
5
While there is no evidence that these viruses

293Section VIII-F: Arboviruses and Related Zoonotic Viruses
replicate or cause disease in vertebrate cells, most have not been characterized
fully enough to conrm this and have been designated as “arthropod-only” based
on genetic relationships. The infectivity of these viruses by routes of infection
common to the laboratory may be unknown. For this reason, all of these viruses
have been assigned Risk Group 2 (RG2) classication based on relationships
to the small number that have been characterized. Table 4 lists these viruses as
known to date. Table 3 also contains viruses from the family Arenaviridae that
are rodent-borne with members known to cause hemorrhagic fever, including
Lymphocytic choriomeningitis virus (see Section VIII-E), Guanarito, Junin, Lassa,
Machupo, and Sabia virus. Also included are Orthohantaviruses, including Andes,
Sin Nombre, and Hantaan, that can be transmitted to humans by rodent urine,
saliva, or feces.
Agent summary statements have been included for certain arboviruses. They
were submitted by a panel of experts for more detailed consideration due to one
or more of the following factors:
■
At the time of writing this edition, the organism represented an
emerging public health threat in the United States;
■
The organism presented unique biocontainment challenge(s) that
required further detail; and/or
■
The organism presented a signicant risk of Laboratory-associated
infection.
These recommendations were made in the winter of 2017; requirements for
biosafety, shipping, and Select Agent registration can change. Please be sure to
conrm the requirements with the appropriate Federal agency. If the pathogen
of interest is one listed in Appendix D, contact APHIS for additional biosafety
requirements. APHIS guidance may supersede the information found in this
section.
Recommendations for the containment of infected arthropod vectors were drafted
by a subcommittee of the American Committee on Medical Entomology (ACME)
and updated in 2019 as the Arthropod Containment Guidelines version 3.2; see
Appendix E for additional information.
6
Some commonly used vaccine strains for which attenuation has been rmly
established are recognized by SALS; these vaccine strains may be handled safely
at BSL-2 and are listed in Table 1.

294 Biosafety in Microbiological and Biomedical Laboratories
Table 1. Vaccine Strains of Specic Viruses that May Be Handled at BSL-2
Virus Vaccine Strain
Chikungunya 181/25
Junin Candid
Rift Valley fever #1 MP-12
Venezuelan equine encephalomyelitis TC83 & V3526
Yellow fever 17-D
Japanese encephalitis 14-14-2
Based on the recommendations listed with the tables, the following guidelines
should be adhered to where applicable.
Risk Group 2 Viruses with BSL-2 Containment Recommended
The recommendations for conducting work with the viruses listed in Table 3 at
BSL-2 are based on the existence of historical laboratory experience adequate
to assess the risks when working with this group of viruses. This indicates 1)
no overt Laboratory-associated infections are reported; 2) infections resulted
from exposures other than by infectious aerosols; or 3) if disease from aerosol
exposure is documented, it is uncommon.
Laboratory Safety and Containment Recommendations
Agents listed in this group may be present in blood, CSF, various tissues, and/
or infected arthropods depending on the agent and the stage of infection. The
primary laboratory hazards are accidental parenteral inoculation, contact of the
virus with broken skin or mucous membranes, and bites of infected laboratory
rodents or arthropods. Properly maintained BSCs, preferably Class II, or other
appropriate personal protective equipment (PPE) or physical containment devices
are used whenever procedures with a potential for creating infectious aerosols or
splashes are conducted.
BSL-2 practices, containment equipment, and facilities are recommended for
activities with potentially infectious clinical materials and arthropods and for
manipulations of infected tissue cultures, embryonated hen’s eggs, and small
vertebrate animals.
Large quantities and/or high concentrations of any virus have the potential to
overwhelm both innate immune mechanisms and vaccine-induced immunity.
When a virus normally handled at BSL-2 is being produced in large quantities or
in high concentrations, additional risk assessment is required. This might indicate
BSL-3 practices, including respiratory protection, based on a risk assessment.
295Section VIII-F: Arboviruses and Related Zoonotic Viruses
West Nile virus (WNV) and St. Louis Encephalitis virus (SLE) risk assessments
have been revised to indicate BSL-2 containment may be acceptable for routine
work. Prior to moving existing work with either virus from BSL-3 laboratories to
BSL-2, a thorough assessment should be made to assess the possible risk from
contamination of samples with other agents needing BSL-3 containment.
Risk Group 3 Viruses with BSL-3 Containment Recommended
The recommendations for viruses listed in Table 3 that require BSL-3 containment
are based on multiple criteria. SALS considered the laboratory experience
for some viruses to be inadequate to assess risk, regardless of the available
information regarding disease severity. In some cases, SALS recorded overt
Laboratory-associated infections (LAI) transmitted by the aerosol route in the
absence or non-use of protective vaccines and considered that the natural
disease in humans is potentially severe, life-threatening, or causes residual
damage.
1
Arboviruses also were classied as requiring BSL-3 containment if they
caused diseases in domestic animals in countries outside of the United States.
Laboratory Safety and Containment Recommendations
The agents listed in this group may be present in blood, CSF, urine, semen, and
exudates, depending on the specic agent and stage of disease. The primary
laboratory hazards are exposure to aerosols of infectious solutions and animal
bedding, accidental parenteral inoculation, and contact with broken skin. Some of
these agents (e.g., VEE virus) may be relatively stable in dried blood or exudates.
BSL-3 practices, containment equipment, and facilities are recommended for
activities using potentially infectious clinical materials and infected tissue cultures,
animals, or arthropods.
A licensed attenuated live virus is available for immunization against yellow fever.
It is recommended for all personnel who work with this agent or with infected
animals and for those entering rooms where the agents or infected animals are
present.
BSL-3 containment is still recommended for Junin virus provided that all at-risk
personnel are immunized and the laboratory is equipped with HEPA-ltered
exhaust.
SALS also has reclassied Central European tick-borne encephalitis viruses
(TBEV-CE subtype) as needing BSL-3 containment, provided all at-risk personnel
are immunized. TBEV-CE subtype refers to the following group of very closely
related, if not essentially identical, tick-borne aviviruses isolated from Czecho-
slovakia, Finland, and Russia: Absettarov, Hanzalova, Hypr, and Kumlinge
viruses. While there is a vaccine available that confers immunity to the TBEV-CE
subtype group of genetically (>98%) homogeneous viruses, the ecacy of this

296 Biosafety in Microbiological and Biomedical Laboratories
vaccine against Russian spring-summer encephalitis virus (RSSEV) (TBEV-FE;
Far Eastern subtype) infections has not been established. Thus, the TBEV-CE
subtype group of viruses has been reclassied as needing BSL-3 containment
when personnel are immunized with TBEV-CE subtype vaccine, while RSSEV
(TBEV-FE subtype) remains classied as needing BSL-4 containment.
Select Agent TBEV-CE viruses are Select Agents requiring registration with CDC
and/or USDA for possession, use, storage, and/or transfer. See Appendix F for
additional information.
Transfer of Agent Importation of these agents may require CDC and/or USDA
importation permits. Domestic transport of these agents may require a permit
from USDA APHIS VS. A Department of Commerce (DoC) permit may be required
for the export of these agents to another country. See Appendix C for additional
information.
Vaccines Investigational vaccines for persons working with eastern equine
encephalomyelitis virus (EEEV), Venezuelan equine encephalitis virus (VEEV),
western equine encephalomyelitis virus (WEEV), and Rift Valley fever viruses
(RVFV) may be available in limited quantities and administered on-site at the
Special Immunization Program of USAMRIID, located at Ft. Detrick, Frederick,
MD. These, and other vaccines that are investigational new drugs (IND), are
administered under a cooperative agreement between the Special Immunization
Program and the individual’s requesting organization.
The use of these investigational vaccines for laboratory personnel should be
considered if the vaccine is available. Initial studies have shown these vaccines
to be eective in producing an appropriate immunologic response, and the
adverse eects of vaccination are within acceptable parameters.
7,8,9
The decision
to recommend vaccines for laboratory personnel must be carefully considered
and based on a risk assessment that includes a review of the characteristics
of the agent and the disease, benets vs. the risk of vaccination, experience of
the laboratory personnel, laboratory procedures to be used with the agent, and
contraindications for vaccination including the health status of the employee.
If the investigational vaccine is contraindicated or laboratory personnel refuse
vaccination, the use of enhanced engineering controls, practices, or personal
protective equipment may provide an alternative. Respiratory protection, such as
use of a PAPR, is a best practice when using organisms with a well-established
risk of aerosol infections in the laboratory, such as VEE viruses.
Any respiratory protection equipment must be provided in conjunction with an
appropriately constituted respiratory protection program. Other methods of respi-
ratory protection may be warranted based on an assessment of risk as dened
in Section II of this manual. All personnel in a laboratory with the infectious agent

297Section VIII-F: Arboviruses and Related Zoonotic Viruses
must use comparable personal protective equipment that meets or exceeds the
requirements, even if they are not working with the organism. Sharps precautions
as described in Section IV must be continually and strictly reinforced, regardless
of whether investigational vaccines are used.
Enhanced BSL-3 Containment
HEPA ltration of the exhaust air is recommended for viruses handled at BSL-3
and listed in Table 5.
Situations may arise for which enhancements to BSL-3 practices and equipment
are required; for example, when a BSL-3 laboratory performs diagnostic testing
on specimens from patients with hemorrhagic fevers thought to be due to dengue
or yellow fever viruses. When the origin of these specimens is Africa, the Middle
East, or South America, such specimens might contain etiologic agents, such
as arenaviruses, loviruses, or other viruses that are usually manipulated in a
BSL-4 laboratory. Examples of enhancements to BSL-3 laboratories include:
1) enhanced respiratory protection of personnel against aerosols; 2) HEPA
ltration of exhaust air from the laboratory; and 3) personal body shower upon
exit. Additional appropriate training is recommended for all sta, including animal
care personnel.
Risk Group 4 Viruses with BSL-4 Containment Recommended
The recommendations for viruses assigned to BSL-4 containment are based
on documented cases of severe and frequently fatal, naturally occurring human
infections and aerosol-transmitted laboratory infections. SALS recommends that
certain agents with a close antigenic or genetic relationship to agents assigned
to BSL-4 also be provisionally handled at this level until sucient laboratory data
indicates that work with the agent may be assigned to a lower Biosafety Level.
Laboratory Safety and Containment Recommendations
The infectious agents may be present in blood, urine, respiratory and throat
secretions, semen, and other uids and tissues from human or animal hosts as
well as in arthropods, rodents, and non-human primates (NHPs). Respiratory
exposure to infectious aerosols, mucous membrane exposure to infectious
droplets, and accidental parenteral inoculation are the primary hazards to
laboratory or animal care personnel.
10,11
BSL-4 practices, containment equipment, and facilities are recommended for
all activities using materials of human, animal, or arthropod origin that may be
infected with one of the agents listed in this summary. Clinical specimens from
persons suspected of being infected with one of the agents listed in this summary
should be submitted to a laboratory with a BSL-4 facility.
12
298 Biosafety in Microbiological and Biomedical Laboratories
Dealing with Unknown Arboviruses The ACAV has published reports
documenting laboratory workers who acquired arbovirus infections during the
course of their duties.
2,13
In the rst such report, it was recognized that these
laboratory infections typically occurred by unnatural routes such as percutaneous
or aerosol exposure, that “lab-adapted” strains were still pathogenic for humans,
and that as more laboratories worked with newly identied agents, the frequency
of LAIs was increasing. Therefore, to assess the risk of these viruses and provide
safety guidelines to those working with them, ACAV appointed SALS to evaluate
the hazards of working with arboviruses in the laboratory setting.
2,14,15
The SALS committee made a series of recommendations, published in 1980,
describing four levels of laboratory practices and containment guidelines
that were progressively more restrictive. These levels were determined after
widely-distributed surveys evaluated numerous criteria for each particular virus
including: 1) past occurrence of LAIs correlated with facilities and practices used;
2) volume of work performed as a measure of potential exposure risk; 3) immune
status of laboratory personnel; 4) incidence and severity of naturally-acquired
infections in adults; and 5) incidence of disease in animals outside the United
States (to assess import risk).
While these criteria are still important factors to consider in any risk assessment
for manipulating arboviruses in the laboratory, it is important to note that there
have been many modications to personal laboratory practices (e.g., working in
a BSC while wearing personal protective equipment in contrast to working with
viruses on an open benchtop) and signicant changes in laboratory equipment,
facilities, and PPE (e.g., BSC, PAPR) available since the initial SALS evaluation.
When dealing with a newly recognized or poorly characterized arbovirus, where
there is insucient previous experience to characterize the risk, investigators
should consider using additional safety measures. Additionally, when working
with eld-collected mosquitoes that may contain arboviruses, additional protective
measures should be considered, particularly with procedures that can generate
aerosols. New methods allow the relationships between newly discovered viruses
and other disease-causing arboviruses to be established with less work and less
potential for exposure. One criterion for a newly identied arbovirus is a thorough
description of how the virus will be handled and investigated. For example,
experiments involving pure genetic analysis could be handled dierently than
those where the virus will be put into animals or arthropods.
16,17
Therefore, in
addition to those established by SALS, additional assessment criteria should be
considered in the risk assessment.
Most of the identied arboviruses have recommended Biosafety Levels for
routine handling; however, a number of those that are infrequently studied, newly
identied, or have only single isolation events may not have been fully evaluated
by SALS, ACAV, CDC, or the NIH. Thorough risk assessment is important for all
299Section VIII-F: Arboviruses and Related Zoonotic Viruses
arboviral research and it is of particular importance for work involving unclassied
viruses. Additionally, an individual risk assessment should consider the fact that
not all strains of a particular virus exhibit the same degree of pathogenicity or
transmissibility. A careful assessment by the laboratory director, institutional
biosafety ocer and safety committee, and outside experts, as necessary,
functions to minimize the risk of human, animal, and environmental exposure
while allowing research to progress.
Chimeric Viruses The ability to construct cDNA clones encoding a complete
RNA viral genome has led to the generation of recombinant viruses containing a
mixture of genes from two or more dierent viruses. Chimeric, full-length viruses
and truncated replicons have been constructed from numerous alphaviruses and
aviviruses. For example, alphavirus replicons encoding foreign genes have been
used widely as immunogens against bunyavirus, lovirus, arenavirus, and other
antigens. These replicons have been safe and usually immunogenic in rodent
hosts leading to their development as candidate human vaccines against several
virus groups including retroviruses.
18–21
Because chimeric viruses contain portions of multiple viruses, the IBC or equiv-
alent resource, in conjunction with the biosafety ocer and the researchers, must
conduct a risk assessment that, in addition to standard criteria, includes specic
elements that need to be considered before assigning appropriate Biosafety
Levels and containment practices. These elements include: 1) the ability of the
chimeric virus to replicate in cell culture and animal model systems in comparison
with its parental strains;
22
2) altered virulence characteristics or attenuation
compared with the parental viruses in animal models;
23
3) virulence or attenuation
patterns by intracranial routes using large doses for agents aecting the CNS;
24,25
and 4) demonstration of lack of reversion to virulence or parental phenotype.
Additionally, while variable pathogenicity occurs frequently with naturally identied
strains, it is of particular note for strains that are modied in the laboratory. It may
be tempting to assign Biosafety Levels to hybrid or chimeric strains based on the
parental types but due to possible altered biohazard potential, a separate risk
assessment needs to be completed, and an assignment to a dierent Biosafety
Level may be justied.
26
A clear description of the strains involved should
accompany any risk assessment.
Many patterns of attenuation have been observed with chimeric aviviruses and
alphaviruses using the criteria described above, and some of these chimeras
have undergone testing as human vaccines.
27
Chimeric viruses may have some safety features not associated with parental
viruses. For example, they are generated from genetically stable cDNA clones
without the need for animal or cell culture passage. This minimizes the possibility
of mutations that could alter virulence properties. Because some chimeric strains
300 Biosafety in Microbiological and Biomedical Laboratories
incorporate genomic segments lacking gene regions or genetic elements critical
for virulence, there may be a limited possibility of genetic changes that could
generate strains exhibiting wild-type virulence.
Ongoing surveillance and laboratory studies suggest that many arboviruses
continue to be a risk to human and animal populations. The attenuation of
all chimeric strains should be veried using the most rigorous containment
requirements of the parental strains. The local IBC, or equivalent resource,
should evaluate containment recommendations for each chimeric virus on a
case-by-case basis, using virulence data from an appropriate animal model.
Additional guidance from the NIH Oce of Science Policy may be necessary.
West Nile Virus (WNV)
This virus belongs to the family Flaviviridae and the genus Flavivirus, Japanese
encephalitis virus antigenic complex. The complex currently includes Alfuy,
Cacipacore, Japanese encephalitis, Koutango, Kunjin, Murray Valley encephalitis,
St. Louis encephalitis, Rocio, Stratford, Usutu, West Nile, and Yaounde viruses.
Flaviviruses share a common size (40–60nm), symmetry (enveloped, icosahedral
nucleocapsid), nucleic acid (positive-sense, single-stranded RNA approximately
10,000–11,000 bases), and virus morphology. The virus was rst isolated from a
febrile, adult woman in the West Nile District of Uganda in 1937.
28
The ecology
was characterized in Egypt in the 1950s; equine disease was rst noted in Egypt
and France in the early 1960s.
29,30
It rst appeared in North America in 1999
causing encephalitis in humans and horses.
31
The virus has now been detected in
Africa, Europe, the Middle East, west and central Asia, Oceania (subtype Kunjin
virus), and North and South America.
WNV spread over the past 20 years throughout temperate regions of Europe and
North America. As the ecological and epidemiological patterns of this virus in the
new geographic regions evolved, WNV is now endemic throughout the U.S. and
is one of the most extensively studied arboviruses in this country.
While WNV can cause serious neurologic disease, most people infected
with WNV do not have symptoms. About one in ve people who are infected
develop a fever with other symptoms such as headache, body aches, joint
pains, vomiting, diarrhea, or rash. About one out of 150 infected people develop
a serious, sometimes fatal, illness aecting the central nervous system such
as encephalitis (inammation of the brain) or meningitis (inammation of the
membranes that surround the brain and spinal cord). Symptoms of severe
illness include high fever, headache, neck stiness, stupor, disorientation, coma,
tremors, convulsions, muscle weakness, vision loss, numbness, and paralysis.
There are no vaccines to prevent WNV in people; treatment is supportive.

301Section VIII-F: Arboviruses and Related Zoonotic Viruses
Occupational Infections
LAIs with WNV have been reported in the literature. SALS reported 15 human
infections from laboratory accidents in 1980.
2
One of these infections was
attributed to aerosol exposure. However, with the development of improved
laboratory and PPE equipment, only three LAIs (due to parenteral inoculations
during work with sharps) have been published in the past two decades.
32,33
Natural Modes of Infection
In the U.S., infected mosquitoes, primarily members of the Culex genus, transmit
WNV. Virus amplication occurs during periods of adult mosquito blood-feeding
by continuous transmission between mosquito vectors and bird reservoir hosts.
Humans, horses, and most other mammals are not known to develop infectious
viremias very often, and thus, are probably “dead-end” or incidental hosts.
Laboratory Safety and Containment Recommendations
WNV may be present in blood, serum, tissues, and CSF of infected humans,
birds, mammals, and reptiles. The virus has been found in oral uids and feces
of birds. Parenteral inoculation with contaminated materials poses the greatest
hazard; contact exposure of broken skin is a possible risk. Sharps precautions
should be strictly adhered to when handling potentially infectious materials.
Workers performing necropsies on infected animals or exposed to feces of
infected birds may be at higher risk of infection.
Given the signicant number of laboratories working with WNV (with only three
parenteral LAIs) and the nearly complete endemicity across the U.S., BSL-2
practices, containment equipment, and facilities are now recommended for all
manipulations of WNV. BSL-2 practices and facilities are similarly recommended
for the closely related and also endemic St. Louis encephalitis virus. As
always, each laboratory should perform a risk assessment to determine if the
procedures being conducted might warrant additional containment measures.
For example, if working with extremely high titers of virus or aerosol-generating
procedures, BSL-3 containment might be considered. For laboratories seeking
to move existing work with WNV from BSL-3 laboratories to BSL-2, a thorough
assessment should be made to assess the possible risk from contamination of
samples with other agents needing BSL-3 containment.
Special Issues
Transfer of Agent Importation of this agent may require CDC and/or APHIS
importation permits. Domestic transport of this agent may require a permit from
USDA APHIS VS. A DoC permit may be required for the export of this agent to
another country. See Appendix C for additional information.
302 Biosafety in Microbiological and Biomedical Laboratories
Eastern Equine Encephalitis Virus (EEEV), Venezuelan Equine Encephalitis
Virus (VEEV), and Western Equine Encephalitis Virus (WEEV)
VEEV, EEEV, and WEEV are members of the genus Alphavirus in the family
Togaviridae. They are small, enveloped viruses with a genome consisting of a
single strand of positive-sense RNA. All three viruses can cause encephalitis
often accompanied by long-term neurological sequelae. The incubation period
ranges from one to 10 days, and the duration of acute illness is typically days
to weeks depending upon severity of the illness. Although not the natural route
of transmission, the viruses are highly infectious by the aerosol route, and LAIs
have been documented.
34
Of note, strains of EEEV from South America are now
designated as Madariaga virus (MADV) and are no longer considered EEEV
viruses.
35
Madariaga virus strains, while still within the EEE antigenic complex,
are genetically and ecologically distinct from North American strains of EEEV.
They typically do not cause large epizootics, and their capacity to cause human
illness is not well-characterized.
The encephalitic alphaviruses are all capable of causing lethal encephalitis in
humans and horses; however, the patterns of disease, disease severity, and
incidence vary greatly. Most reported cases represent severe forms of disease
as the majority of infections are either mild, u-like illness, or asymptomatic.
WEEV is currently the rarest, with no human infections detected since 1988, and
fewer than 700 total cases reported in the United States since the 1960s. Young
children (<12 months) are the most susceptible to severe disease with an overall
mortality rate estimated at about 4%. EEEV is also rare in the United States with
an average of seven neurological cases each year. However, encephalitic cases
of EEEV infection can have a mortality rate estimated at 30–70% and survivors
often experience severe permanent neurological sequelae. VEEV mortality
rates are typically around 1% and severe cases are typically in children. One
of the largest VEEV outbreaks occurred in Columbia in 1995 and aected
approximately 75,000 individuals. Of these, 3,000 developed neurological
manifestations with a total of approximately 300 deaths. There are no licensed
vaccines or therapeutics available.
Occupational Infections
These alphaviruses, especially VEEV, are infectious by aerosol in laboratory
studies and more than 160 EEEV, VEEV, or WEEV LAIs have been documented.
Many infections were due to procedures involving high virus concentrations and
aerosol-generating activities such as centrifugation and mouth pipetting. Proce-
dures involving animals (e.g., infection of newly hatched chicks with EEEV and
WEEV) and mosquitoes are also particularly hazardous.
303Section VIII-F: Arboviruses and Related Zoonotic Viruses
Natural Modes of Infection
Alphaviruses are zoonoses maintained and amplied in natural transmission
cycles involving a variety of mosquito species and either small rodents or birds.
Humans and equines are accidental hosts with naturally acquired alphavirus
infections resulting from the bites of infected mosquitoes.
EEEV occurs in focal locations along the eastern seaboard, the Gulf Coast, and
some inland Midwestern locations of the United States, in Canada, and some
Caribbean Islands; the related MADV occurs in Central and South America.
35,36
Small outbreaks of human disease have occurred in the United States, the
Dominican Republic, Cuba, and Jamaica. In the United States, equine epizootics
are common occurrences during the summer in coastal regions bordering the
Atlantic and Gulf of Mexico, in other eastern and Midwestern states, and as far
north as Quebec, Ontario, and Alberta in Canada.
In Central and South America, focal outbreaks due to VEE virus occur periodically
with rare large regional epizootics involving thousands of equine cases and
deaths in predominantly rural settings. These epizootic/epidemic viruses are
theorized to emerge periodically from mutations occurring in the continuously
circulating enzootic VEE viruses in northern South America. The classical
epizootic varieties of the virus are not present in the United States. An enzootic
subtype, Everglades virus (VEE antigenic complex subtype II virus), exists
naturally in southern Florida; endemic foci of Bijou Bridge virus (VEE antigenic
complex subtype III-B virus), have been described in the western United States.
37
WEEV is found mainly in western parts of the United States and Canada.
Sporadic infections also occur in Central and South America.
Laboratory Safety and Containment Recommendations
Alphaviruses may be present in blood, CSF, other tissues (e.g., brain), or throat
washings. The primary laboratory hazards are parenteral inoculation, contact of
the virus with broken skin or mucous membranes, bites of infected animals or
arthropods, or aerosol inhalation.
Diagnostic and research activities involving clinical material, infectious cultures,
and infected animals or arthropods should be performed with BSL-3 practices,
containment equipment, and facilities. Due to the high risk of aerosol infection,
respiratory protection is a best practice for non-immune personnel. Animal work
with VEEV, EEEV, and WEEV should be performed under ABSL-3 conditions.
HEPA ltration is required on the exhaust system of laboratory and animal facil-
ities using VEEV.

304 Biosafety in Microbiological and Biomedical Laboratories
Special Issues
Vaccines Two strains of VEEV (TC-83 and V3526) are highly attenuated in
vertebrate studies and are excluded from Select Agent regulations. Because of
the low level of pathogenicity, these strains may be safely handled under BSL-2
conditions without vaccination or additional personal protective equipment
(e.g., respiratory protection).
Investigational vaccine protocols have been developed to immunize at-risk
laboratory or eld personnel against these alphaviruses; however, the vaccines
are available only on a limited basis and may be contraindicated for some
personnel. Therefore, additional personal protective equipment may be warranted
if vaccination can’t be administered. For personnel who have no neutralizing
antibody titer (from previous vaccination or natural infection), respiratory
protection should be considered for all procedures.
Select Agent Epizootic (equine amplication-competent) subtype strains of VEEV
(subtypes IAB and IC) and EEEV (but not MADV) are Select Agents requiring
registration with CDC and/or APHIS for possession, use, storage, and/or transfer.
See Appendix F for additional information.
Transfer of Agent Importation of this agent may require CDC and/or APHIS
importation permits. Domestic transport of this agent may require a permit from
USDA APHIS VS. A Department of Commerce (DoC) permit may be required
for the export of this agent to another country. See Appendix C for additional
information.
Rift Valley Fever Virus (RVFV)
RVFV was rst isolated in Kenya in 1936 and subsequently shown to be
endemically present in almost all areas of sub-Saharan Africa.
38
In periods of
heavy rainfall, large epizootics occur involving primarily sheep, cattle, and human
disease, although many other species are infected. The primordial vertebrate
reservoir is unknown, but the introduction of large herds of highly susceptible
domestic breeds in the last few decades has provided a substrate for massive
virus amplication. The virus has been introduced into Egypt, Saudi Arabia, and
Yemen and caused epizootics and epidemics in those countries. The largest of
these was from 1977 to 1979 in Egypt with many thousands of human cases and
610 reported deaths.
39
Most human infections are symptomatic and the most common syndrome
consists of fever, myalgia, malaise, anorexia, and other non-specic symptoms.
Recovery within one to two weeks is usual, but hemorrhagic fever, encephalitis,
or retinitis also occur. Hemorrhagic fever develops as the primary illness
progresses and is characterized by disseminated intravascular coagulation and
hepatitis. Perhaps 2% of cases will develop this complication and the mortality
305Section VIII-F: Arboviruses and Related Zoonotic Viruses
is high. Encephalitis follows apparent recovery in <1% of cases and results in a
substantial mortality and sequelae. Retinal vasculitis occurs in convalescence of
a substantial, but not precisely known, proportion of cases. The retinal lesions are
often macular and permanent, leading to substantial loss of visual acuity.
Infected sheep and cattle suer a mortality rate of 10–35%, and spontaneous
abortion occurs virtually in all pregnant females. Other animals studied have
lower viremia and lesser mortality but may abort. This virus is a World Organi-
zation for Animal Health (OIE) List A disease and triggers export sanctions.
Occupational Infections
The potential for infection of humans by routes other than arthropod transmission
was rst recognized in veterinarians performing necropsies. Subsequently, it
became apparent that contact with infected animal tissues and infectious aerosols
were dangerous; many infections were documented in herders, slaughterhouse
workers, and veterinarians. Most of these infections resulted from exposure to
blood and other tissues including aborted fetal tissues of sick animals.
There have been 47 reported laboratory infections; before modern containment
and vaccination became available, virtually every laboratory that began work with
the virus suered infections suggestive of aerosol transmission.
40,41
Natural Modes of Infection
Field studies show RVFV to be transmitted predominantly by mosquitoes;
although, other arthropods may be infected and transmit. Mechanical trans-
mission also has been documented in the laboratory. Floodwater Aedes species
are the primary vector and transovarial transmission is an important part of the
maintenance cycle.
42
However, many dierent mosquito species are implicated in
horizontal transmission in eld studies, and laboratory studies have shown a large
number of mosquito species worldwide to be competent vectors, including North
American mosquitoes.
It is currently believed that the virus passes dry seasons in the ova of ood-water
Aedes mosquitoes. Rain allows infectious mosquitoes to emerge and feed on
vertebrates. Several mosquito species can be responsible for horizontal spread,
particularly in epizootic/epidemic situations. The vertebrate ampliers are usually
sheep and cattle, with two caveats: 1) a native African vertebrate amplier is
thought to exist but is yet to be dened, and 2) very high viremias in humans are
thought to play some role in viral amplications.
43
Transmission of disease occurs between infected animals but is of low eciency;
virus titers in throat swabs are low. Nosocomial infection rarely, if ever, occurs.
There are no examples of latency with RVFV, although virus may be isolated from
lymphoid organs of mice and sheep for four to six weeks post-infection.
306 Biosafety in Microbiological and Biomedical Laboratories
Laboratory Safety and Containment Recommendations
Concentrations of RVFV in blood and tissues of sick animals are often very high.
Placenta, amniotic uid, and fetuses from aborted domestic animals are highly
infectious. Large numbers of infectious virus particles also are generated in cell
cultures and laboratory animals.
BSL-3 practices, containment equipment, and facilities are recommended for
processing human or animal material in endemic zones or in non-endemic areas
in emergency circumstances. Particular care should be given to stringent aerosol
containment practices, autoclaving waste, decontamination of work areas, and
control of egress of material from the laboratory. Other cultures, cells, or similar
biological material that could potentially harbor RVFV should not be used in an
RVFV laboratory and subsequently removed.
Diagnostic or research studies outside endemic areas should be performed
in a BSL-3 laboratory. Personnel also must have respiratory protection (e.g.,
PAPR) or be vaccinated for RVFV. In addition, APHIS may require full ABSL-3Ag
containment for research conducted in non-endemic areas using loose-housed
animals. See Appendix D for additional information.
Special Issues
Vaccines Two apparently eective vaccines have been developed by the
Department of Defense (DOD) and have been used in volunteers, laboratory sta,
and eldworkers under investigational protocols, but neither vaccine is available
at this time.
Select Agent RVFV is a Select Agent requiring registration with CDC and/or
APHIS for possession, use, storage and/or transfer. See Appendix F for additional
information.
The live-attenuated MP-12 vaccine strain and the ΔNSs-ΔNSm-ZH501 strain are
excluded from the Select Agent regulations. In general, BSL-2 containment is
recommended for working with these strains.
APHIS may require ABSL-3 enhanced, ABSL-3, or ABSL-3Ag facilities and
practices for working with RVFV in the United States; see Appendix D for
additional information. Investigators should contact APHIS for further guidance
before initiating research.
Transfer of Agent Importation of this agent may require CDC and/or APHIS
importation permits. Domestic transport of this agent may require a permit from
USDA APHIS VS. A Department of Commerce (DoC) permit may be required
for the export of this agent to another country. See Appendix C for additional
information.

307Section VIII-F: Arboviruses and Related Zoonotic Viruses
Table 2. Explanation of Symbols Used in Tables 3 and 4 to Dene Basis for
Assignment of Viruses to Biosafety Levels
Symbol Denition
S
Results of SALS survey and information from the Catalog.
1
IE
Insucient experience with virus in laboratory facilities with low biocontainment.
A
Additional Criteria (A1–A8)
A1
Disease in sheep, cattle, or horses.
A2
Fatal human laboratory infection—probably aerosol.
A3
Extensive laboratory experience and mild nature of aerosol laboratory infections justify
BSL-2.
A4
Placed in BSL-4 based on the close antigenic relationship with a known agent handled at
BSL-4 plus insucient experience.
A5
Arenaviruses handled at BSL-2 are not known to cause serious acute disease in humans
and are not acutely pathogenic for laboratory animals including primates. It is strongly
recommended that work with high concentrations of these arenaviruses be done at
BSL-3.
A6
Level assigned to prototype or wild-type virus. A lower level may be recommended for
variants with well-dened reduced virulence characteristics.
A7
Placed at this Biosafety Level based on close antigenic or genetic relationship to other
viruses in a group of three or more viruses, all of which are classied at this level.
A8
Hantaviruses handled at BSL-2 are not known to cause laboratory infections, overt
disease in humans, or severe disease in experimental primates. Because of antigenic
and biologic relationships to highly pathogenic hantaviruses and the likelihood that
experimentally infected rodents may shed large amounts of virus, it is recommended that
work with high concentrations of virus or experimentally infected rodents be conducted at
BSL-3.
308 Biosafety in Microbiological and Biomedical Laboratories
Table 3. Alphabetic Listing of Arboviruses and Hemorrhagic Fever Viruses*
Virus Name Acronym Family Genus
Recommended
BSL
Basis of
Rating
Antigenic Group
Abadina ABAV Reoviridae Orbivirus 2 A7 N/A
Above Maiden ABMV Reoviridae Orbivirus 2 A7 N/A
Abras ABRV Peribunyaviridae Orthobunyavirus 2 A7 Patois
Absettarov ABSV Flaviviridae Flavivirus 4 A4
Tick-borne Encephalitis—
CE subtype
Abu Hammad AHV Nairoviridae Orthonairovirus 2 S Dera Ghazi Khan
Abu Mina ABMV Nairoviridae Orthonairovirus 2 A7 N/A
Acado ACDV Reoviridae Orbivirus 2 S Corriparta
Acara ACAV Peribunyaviridae Orthobunyavirus 2 S Capim
Achiote ACHOV Peribunyaviridae Orthobunyavirus 2 A7 California
Adana ADAV Phenuiviridae Phlebovirus 2 A7 Salehabad
Adelaide River ARV Rhabdoviridae Ephemerovirus 2 IE Bovine Ephemeral Fever
Adria ADRV Phenuiviridae Phlebovirus 2 A7 N/A
African horse
sickness
AHSV Reoviridae Orbivirus 3
b
A1 African Horse Sickness
African swine fever ASFV Asfarviridae Asvirus 3
b
IE Asvirus
Aguacate AGUV Phenuiviridae Phlebovirus 2 S Phlebotomus Fever
Aino AINOV Peribunyaviridae Orthobunyavirus 2 S Simbu
Akabane AKAV Peribunyaviridae Orthobunyavirus 3
b
S Simbu
Alajuela ALJV Peribunyaviridae Orthobunyavirus 2 A7 N/A
Alcube
N/A Phenuiviridae Phlebovirus 2 A7 N/A
Alenquer ALEV Phenuiviridae Phlebovirus 2 IE Phlebotomus Fever
Alfuy ALFV Flaviviridae Flavivirus 2 S N/A
Alkhurma AHFV Flaviviridae Flavivirus 4 A4
Tick-borne Encephalitis—
CE subtype
Allpahuayo ALLPV Arenaviridae Mammarenavirus 3 IE Tacaribe
Almeirim ALMV Reoviridae Orbivirus 2 IE Changuinola
Almpiwar ALMV Rhabdoviridae Sripuvirus 2 S N/A
Altamira ALTV Reoviridae Orbivirus 2 IE Changuinola
Amaparí AMAV Arenaviridae Mammarenavirus 2 A5 Tacaribe
Ambe AMBEV Phenuiviridae Phlebovirus 2 IE N/A
Amga MGAV Hantaviridae Orthohantavirus 3
a
A7 N/A
Amur/Soochong ASV Hantaviridae Orthohantavirus 3
a
A7 N/A
Anadyr ANADV Peribunyaviridae Orthobunyavirus 2 A7 N/A
Anajatuba ANJV Hantaviridae Orthohantavirus 3
a
A7 N/A
Ananindeua ANUV Peribunyaviridae Orthobunyavirus 2 A7 Guama
Andasibe ANDV Reoviridae Orbivirus 2 A7 N/A
Andes ANDV Hantavirudae Orthohantavirus 3
a
IE Hantaan
Anhanga ANHV Phenuiviridae Phlebovirus 2 S Phlebotomus Fever
Anhembi AMBV Peribunyaviridae Orthobunyavirus 2 S Bunyamwera
Anopheles A ANAV
Peribunyaviridae Orthobunyavirus 2 S Anopheles A
Anopheles B ANBV Peribunyaviridae Orthobunyavirus 2 S Anopheles B
Antequera ANTV
Unclassied
Bunyavirales
not applicable
2 IE Antequera
Apeú APEUV Peribunyaviridae Orthobunyavirus 2 S N/A
Apoi APOIV Flaviviridae Flavivirus 2 S N/A
Araguari ARAV Orthomyxoviridae Unassigned 3 IE N/A
Continued on next page ►
309Section VIII-F: Arboviruses and Related Zoonotic Viruses
Virus Name Acronym Family Genus
Recommended
BSL
Basis of
Rating
Antigenic Group
Aransas Bay ABV Orthomyxoviridae Thogotovirus 2 IE Upolu
Araraquara ARQV Hantaviridae Orthohantavirus 3
a
A7 N/A
Araucaria ARAUV Hantaviridae Orthohantavirus 3
a
A7 N/A
Arbia ARBV Phenuiviridae Phlebovirus 2 IE Phlebotomus Fever
Arboledas ADSV Phenuiviridae Phlebovirus 2 A7 Phlebotomus Fever
Arbroath ABRV Reoviridae Orbivirus 2 A7 N/A
Aride ARIV Unclassied virus 2 S N/A
Ariquemes ARQV Phenuiviridae Phlebovirus 2 A7 Phlebotomus Fever
Arkonam ARKV Reoviridae Orbivirus 2 S N/A
Armero ARMV Phenuiviridae Phlebovirus 2 A7 Phlebotomus Fever
Aroa AROAV Flaviviridae Flavivirus 2 S N/A
Arrabida ARRV Phenuiviridae Phlebovirus 2 A7 N/A
Artashat ARTSV Nairoviridae Orthonairovirus 3 IE N/A
Aruac ARUV Rhabdoviridae Unassigned 2 S N/A
Arumateua ARMTV Peribunyaviridae Orthobunyavirus 2 A7 N/A
Arumowot AMTV Phenuiviridae Phlebovirus 2 S Phlebotomus Fever
Asama ASAV Hantaviridae Orthohantavirus 3
a
A7 N/A
Asikkala ASIV Hantaviridae Orthohantavirus 3
a
A7 N/A
Aura AURAV Togaviridae Alphavirus 2 S
Western Equine
Encephalitis
Avalon AVAV Nairoviridae Orthonairovirus 2 S Sakhalin
Babahoyo BABV Peribunyaviridae Orthobunyavirus 2 A7 Patois
Babanki BBKV Togaviridae Alphavirus 2 A7
Western Equine
Encephalitis
Bagaza BAGV Flaviviridae Flavivirus 2 S N/A
Bahig BAHV Peribunyaviridae Orthobunyavirus 2 S Tete
Bakau BAKV Peribunyaviridae Orthobunyavirus 2 S Bakau
Bakel BAKV Nairoviridae Orthonairovirus 2 A7 N/A
Baku BAKUV Reoviridae Orbivirus 2 S Kemerovo
Balkan BALKV Phenuiviridae Phlebovirus 2 A7 N/A
Bandia BDAV Nairoviridae Orthonairovirus 2 S Qalyub
Bangoran BGNV Rhabdoviridae Unassigned 2 S N/A
Bangui BGIV
Unclassied
Bunyavirales
N/A 2 S N/A
Banna BAV Reoviridae Seadornavirus 3 IE N/A
Banzi BANV Flaviviridae Flavivirus 2 S N/A
Barmah Forest BFV Togaviridae Alphavirus 2 A7 Barmah Forest
Barranqueras BQSV
Unclassied
Bunyavirales
N/A 2 IE Antequera
Barur BARV Rhabdoviridae Ledantevirus 2 S Kern Canyon
Batai BATV Peribunyaviridae Orthobunyavirus 2 S Bunyamwera
Batama BMAV Peribunyaviridae
Orthobunyavirus 2 A7 Tete
Batken BKNV Orthomyxoviridae Thogotovirus 2 IE N/A
Batu Cave BCV Flaviviridae Flavivirus 2 A7 N/A
Bauline BAUV Reoviridae Orbivirus 2 S Kemerovo
Bayou BAYV Hantaviridae Orthohantavirus 3
a
A7 N/A
BeAr 328208 B AV Peribunyaviridae Orthobunyavirus 2 A7 N/A
Bear Canyon BCNV Arenaviridae Mammarenavirus 3 A7 N/A
Continued on next page ►
310 Biosafety in Microbiological and Biomedical Laboratories
Virus Name Acronym Family Genus
Recommended
BSL
Basis of
Rating
Antigenic Group
Beatrice Hill BHV Rhabdoviridae Tibrovirus 2 IE N/A
Beaumont BEAUV Rhabdoviridae Unassigned 2 A7 N/A
Bebaru BEBV Togaviridae Alphavirus 2 S Semliki Forest
Belem BLMV
Unclassied
Bunyavirales
N/A 2 IE N/A
Belmont BELV
Unclassied
Bunyavirales
N/A 2 S N/A
Belterra BELTV Phenuiviridae Phlebovirus 2 A7 Phlebotomus Fever
Benevides BENV Peribunyaviridae Orthobunyavirus 2 A7 Capim
Benca BNFV Peribunyaviridae Orthobunyavirus 2 A7 Capim
Bermejo BMJV Hantaviridae Orthohantavirus 3
a
IE Hantaan
Berrimah BRMV Rhabdoviridae Ephemerovirus 2 IE Bovine Ephemeral Fever
Bertioga BERV Peribunyaviridae Orthobunyavirus 2 S Guama
Bhanja BHAV Phenuiviridae Phlebovirus 3 S Bhanja
Big Brushy Tank BBTV Arenaviridae Mammarenavirus 3 IE N/A
Big Cypress BCPOV Reoviridae Orbivirus 2 A7 N/A
Bimbo BBOV Rhabdoviridae Unassigned 2 IE N/A
Bimiti BIMV Peribunyaviridae Orthobunyavirus 2 S Guama
Birao BIRV Peribunyaviridae Orthobunyavirus 2 S Bunyamwera
Bivens Arm BAV Rhabdoviridae Tibrovirus 2 IE N/A
Black Creek Canal
BCCV Hantaviridae Orthohantavirus 3
a
A7 N/A
Bloodland Lake BLLV Hantaviridae Orthohantavirus 2a A8 N/A
Blue River BRV Hantaviridae Orthohantavirus 3
a
A7 N/A
Bluetongue
(exotic serotypes)
BTV Reoviridae Orbivirus 3
b
S Bluetoungue
Bluetongue
(non-exotic)
BTV Reoviridae Orbivirus 2
b
S Bluetoungue
Bobaya BOBV
Unclassied
Bunyavirales
N/A 2 IE N/A
Bobia BIAV Peribunyaviridae Orthobunyavirus 2 IE Olifantsvlei
Boracéia BORV Peribunyaviridae Orthobunyavirus 2 S Anopheles B
Botambi BOTV Peribunyaviridae Orthobunyavirus 2 S Olifantsvlei
Boteke BTKV Rhabdoviridae Vesiculovirus 2 S Vesicular Stomatitis
Bouboui BOUV Flaviviridae Flavivirus 2 S Bouboui
Bourbon BRBV Orthomyxoviridae Thogotovirus 2 A7 N/A
Bovine ephemeral
fever
BEFV Rhabdoviridae Ephemerovirus 3 A1 Bovine Ephemeral Fever
Bowe BOWV Hantaviridae Orthohantavirus 3
a
A7 N/A
Bozo BOZOV Peribunyaviridae Orthobunyavirus 2 A7 Bunyamwera
Brazoran Peribunyaviridae Unassigned 2 A7 N/A
Breu Branco BRBV Reoviridae Orbivirus 2 A7 N/A
Broadhaven BRDV Reoviridae Orbivirus 2 A7 N/A
Bruconha BRUV Peribunyaviridae Orthobunyavirus 2 A7 N/A
Bruges BRGV Hantaviridae
Orthohantavirus 3
a
A7 N/A
Buenaventura BUEV Phenuiviridae Phlebovirus 2 IE Phlebotomous Fever
Buggy Creek Togaviridae Alphavirus 2 A7
Western Equine
Encephalitis
Bujaru BUJV Phenuiviridae Phlebovirus 2 S N/A
Bukalasa bat BBV Flaviviridae Flavivirus 2 A7 N/A
Bundibugyo BDBV Filoviridae Ebolavirus 4 A4 Ebola
Continued on next page ►
311Section VIII-F: Arboviruses and Related Zoonotic Viruses
Virus Name Acronym Family Genus
Recommended
BSL
Basis of
Rating
Antigenic Group
Bunyamwera BUNV Peribunyaviridae Orthobunyavirus 2 S Bunyamwera
Bunyip Creek BCV Reoviridae Orbivirus 2 S N/A
Burana BURV Nairoviridae Orthonairovirus 2 A7 N/A
Burg El Arab BEAV
Unclassied
Bunyavirales
N/A 2 S N/A
Bushbush BSBV Peribunyaviridae Orthobunyavirus 2 S N/A
Bussuquara BSQV Flaviviridae Flavivirus 2 S N/A
Buttonwillow BUTV Peribunyaviridae Orthobunyavirus 2 S N/A
Bwamba BWAV Peribunyaviridae Orthobunyavirus 2 S N/A
Cabassou CABV Togaviridae Alphavirus 3 IE
Venezuelan Equine
Encephalitis
Cacao CACV Phenuiviridae Phlebovirus 2 S N/A
Cache Valley CVV Peribunyaviridae Orthobunyavirus 2 S N/A
Cachoeira Portiera CPOV Peribunyaviridae Orthobunyavirus 2 A7 N/A
Cacipacoré CPCV Flaviviridae Flavivirus 2 IE N/A
Caimito CAIV Phenuiviridae Phlebovirus 2 S N/A
Calchaqui CQIV Peribunyaviridae Unassigned 2 A7 Gamboa
California
encephalitis
CEV Peribunyaviridae Orthobunyavirus 2 S California
Calovo CVOV Peribunyaviridae Orthobunyavirus 2 S N/A
Campana CMAV Phenuiviridae Phlebovirus 2 A7 Punta Toro
Cananeia
CNAV Peribunyaviridae Orthobunyavirus 2 IE N/A
Candiru CDUV Phenuiviridae Phlebovirus 2 S Candiru
Caninde CANV Reoviridae Orbivirus 2 IE Changuinola
Cano Delgadito CADV Hantaviridae Orthohantavirus 3
a
IE Hantaan
Cao Bang CBNV Hantaviridae Orthohantavirus 3
a
A7 N/A
Cape Wrath CWV Reoviridae Orbivirus 2 S Kemerovo
Capim CAPV Peribunyaviridae Orthobunyavirus 2 S Capim
Capira CAPV Phenuiviridae Phlebovirus 2 A7 Punta Toro
Caraipé CRPV Peribunyaviridae Orthobunyavirus 2 A7 N/A
Carajás CRJV Rhabdoviridae Vesiculovirus 2 A7 Vesicular Stomatitis
Caraparú CARV Peribunyaviridae Orthobunyavirus 2 S N/A
Carey Island CIV Flaviviridae Flavivirus 2 S N/A
Caspiy CASV Nairoviridae Orthonairovirus 2 A7 N/A
Castelo dos Sonhos CASV Hantaviridae Orthohantavirus 3
a
IE N/A
Cat Que CQV Peribunyaviridae Orthobunyavirus 2 A7 N/A
Catarina CTNV Arenaviridae Mammarenavirus 3 IE N/A
Catú CATUV Peribunyaviridae Orthobunyavirus 2 S Guama
Chaco CHOV Rhabdoviridae Sripuvirus 2 S Timbo
Chagres CHGV Phenuiviridae Phlebovirus 2 S Phlebotomus Fever
Chandipura CHPV
Rhabdoviridae Vesiculovirus 2 S Vesicular Stomatitis
Changuinola CGLV Reoviridae Orbivirus 2 S Changuinola
Chapare CHAPV Arenaviridae Mammarenavirus 4 A4 N/A
Charleville CHVV Rhabdoviridae Unassigned 2 S Rab
Chenuda CNUV Reoviridae Orbivirus 2 S Kemerovo
Chikungunya CHIKV Togaviridae Alphavirus 3 S Semliki Forest
Chilibre CHIV Phenuiviridae Phlebovirus 2 S Phlebotomus Fever
Chim CHIMV Nairoviridae Orthonairovirus 2 IE N/A
Continued on next page ►
312 Biosafety in Microbiological and Biomedical Laboratories
Virus Name Acronym Family Genus
Recommended
BSL
Basis of
Rating
Antigenic Group
Chizé CHZV Phenuiviridae Phlebovirus 2 A7 N/A
Chobar Gorge CGV Reoviridae Orbivirus 2 S Chobar Gorge
Choclo CHOV Hantavirus Orthohantavirus 3
a
A7 N/A
Clo Mor CMV Nairoviridae Orthonairovirus 2 S Sakhalin
CoAr 1071 CA1071V Peribunyaviridae Orthobunyavirus 2 A7 N/A
CoAr 3627 CA3627V Peribunyaviridae Orthobunyavirus 2 A7 N/A
Coastal Plains CPV Rhabdoviridae Tibrovirus 2 IE Tibrogargan
Cocal COCV Rhabdoviridae Vesiculovirus 2 A3 Vesicular Stomatitis
Cocle CCLV Phenuiviridae Phlebovirus 2 A7 Punta Toro
Codajas CDJV Reoviridae Orbivirus 2 A7 N/A
Colony COYV Reoviridae Orbivirus 2 A7 N/A
Colony B North CBNV Reoviridae Orbivirus 2 A7 N/A
Colorado tick fever CTFV Reoviridae Coltivirus 2 S Colorado Tick Fever
Crimean-Congo
hemorrhagic fever
CCHFV Nairoviridae Orthonairovirus 4 A7
Crimean-Congo
hemorrhagic fever
Connecticut CNTV Rhabdoviridae Unassigned 2 IE Sawgrass
Corfou CFUV Phenuiviridae Phlebovirus 2 A7 Phlebotomus Fever
Corriparta CORV Reoviridae Orbivirus 2 S Corriparta
Cotia COTV Poxviridae Unassigned 2 S N/A
Cowbone Ridge
CRV Flaviviridae Flavivirus 2 S N/A
Csiro Village CVGV Reoviridae Orbivirus 2 S Palyam
Cuiaba CUIV Rhabdoviridae Unassigned 2 S N/A
Cupixi CPXV Arenaviridae Mammarenavirus 3 IE N/A
Curionopolis CRNPV Rhabdoviridae Curiovirus 2 A7 N/A
Dabakala DABV Peribunyaviridae Orthobunyavirus 2 A7 Olifantsvlei
Dabieshan DBSV Hantaviridae Orthohantavirus 3
a
A7 N/A
D’Aguilar DAGV Reoviridae Orbivirus 2 S Palyam
Dakar bat DBV Flaviviridae Flavivirus 2 S N/A
Dandenong DANV Arenaviridae Mammarenavirus 2 A5 N/A
Dashli DASHV Phenuiviridae Phlebovirus 2 A7 N/A
Deer tick DRTV Flaviviridae Flavivirus 3 A7 N/A
Dengue virus 1 DENV-1 Flaviviridae Flavivirus 2 S N/A
Dengue virus 2 DENV-2 Flaviviridae Flavivirus 2 S N/A
Dengue virus 3 DENV-3 Flaviviridae Flavivirus 2 S N/A
Dengue virus 4 DENV-4 Flaviviridae Flavivirus 2 S N/A
Dera Ghazi Khan DGKV Nairoviridae Orthonairovirus 2 S Dera Ghazi Khan
Dobrava-Belgrade DOBV Hantaviridae Orthohantavirus 3a IE N/A
Dhori DHOV Orthomyxoviridae Thogotovirus 2 S N/A
Douglas DOUV
Peribunyaviridae Orthobunyavirus 3 IE Simbu
Durania DURV Phenuiviridae Phlebovirus 2 A7 Phlebotomus Fever
Durham DURV Rhabdoviridae Tupavirus 2 IE N/A
Dugbe DUGV Nairoviridae Orthonairovirus 3 S Nairobi Sheep Disease
Eastern equine
encephalitis
EEEV Togaviridae Alphavirus 3
b
S
Eastern Equine
Encephalitis
Ebola EBOV Filoviridae Ebolavirus 4 S Ebola
Edge Hill EHV Flaviviridae Flavivirus 2 S N/A
EgAN 1825-61 EGAV Phenuiviridae Phlebovirus 2 A7 N/A
El Huayo EHUV Peribunyaviridae Orthobunyavirus 2 A7 N/A
Continued on next page ►
313Section VIII-F: Arboviruses and Related Zoonotic Viruses
Virus Name Acronym Family Genus
Recommended
BSL
Basis of
Rating
Antigenic Group
El Moro Canyon ELMCV Hantaviridae Orthohantavirus 3a A7 N/A
Ellidaey ELLV Reoviridae Orbivirus 2 A7 N/A
Enseada ENSV
Unclassied
Bunyavirales
N/A 3 IE N/A
Entebbe bat ENTV Flaviviridae Flavivirus 2 S N/A
Epizootic
hemorrhagic disease
EHDV Reoviridae Orbivirus 2 S
Epizootic Hemorrhagic
Disease
Equine encephalosis EEV Reoviridae Orbivirus 3 A1 N/A
Eret ERETV Peribunyaviridae Orthobunyavirus 2 A7 N/A
Erve ERVEV Nairoviridae Orthonairovirus 2 S Thiafora
Escharte ESCV Phenuiviridae Phlebovirus 3 IE N/A
Essaouira ESSV Reoviridae Orbivirus 2 A7 N/A
Estero Real ERV Peribunyaviridae Orthobunyavirus 2 IE Patois
Eubenangee EUBV Reoviridae Orbivirus 2 S Eubenangee
Everglades EVEV Togaviridae Alphavirus 3 S
Venezuelan Equine
Encephalitis
Eyach EYAV Reoviridae Coltivirus 2 S Colorado Tick Fever
Facey’s Paddock FPV Peribunyaviridae Orthobunyavirus 2 A7 N/A
Farallon FARV Nairoviridae Orthonairovirus 2 A7 N/A
Farmington FRMV Rhabdoviridae Unassigned 2 A7 N/A
Fermo FERV Phenuiviridae Phlebovirus 2 A7 Sandy Fever Naples
Fikirini
FKRV Rhabdoviridae Ledantevirus 2 A7 N/A
Fin V 707 FINV Phenuiviridae Phlebovirus 2 A7 N/A
Finch Creek FINCV Nairoviridae Orthonairovirus 2 A7 N/A
Fitzroy River FRV Flaviviridae Flavivirus 3 A7 Yellow Fever
Flanders FLAV Rhabdoviridae Hapavirus 2 S Hart Park
Flexal FLEV Arenaviridae Mammarenavirus 3 S Tacaribe
Fomede FV Reoviridae Orbivirus 2 A7 Chobar Gorge
Forécariah FORV Phenuiviridae Phlebovirus 2 A7 Bhanja
Fort Morgan FMV Togaviridae Alphavirus 2 S
Western Equine
Encephalitis
Fort Sherman FSV Peribunyaviridae Orthobunyavirus 2 A7 Bunyamwera
Foula FOUV Reoviridae Orbivirus 2 A7 N/A
Fraser Point FPV Nairoviridae Orthonairovirus 2 A7 N/A
Frijoles FRIV Phenuiviridae Phlebovirus 2 S Phlebotomus Fever
Fugong FUGV Hantaviridae Orthohantavirus 3
a
IE N/A
Fukuoka FUKV Rhabdoviridae Ledantevirus 2 A7 N/A
Fusong FUSV Hantaviridae Orthohantavirus 3 A7 N/A
Gabek Forest GFV Phenuiviridae Phlebovirus 2 A7 Phlebotomus Fever
Gadgets Gully GGYV Flaviviridae Flavivirus 2 IE N/A
Gairo GAIV Arenaviridae Mammarenavirus 3 A7 N/A
Gamboa GAMV
Peribunyaviridae Orthobunyavirus 2 S Gamboa
Gan Gan GGV Peribunyaviridae Orthobunyavirus 2 A7 Mapputta
Garatuba GTBV Peribunyaviridae Orthobunyavirus 2 A7 N/A
Garba GARV Rhabdoviridae Unassigned 2 IE Matariva
Garissa GRSV Peribunyaviridae Orthobunyavirus 3 A7 Bunyamwera
Geran GERV Nairoviridae Orthonairovirus 2 A7 N/A
Germiston GERV Peribunyaviridae Orthobunyavirus 3 Bunyamwera
Getah GETV Togaviridae Alphavirus 2 A1 Semliki Forest
Continued on next page ►
314 Biosafety in Microbiological and Biomedical Laboratories
Virus Name Acronym Family Genus
Recommended
BSL
Basis of
Rating
Antigenic Group
Gomoka GOMV Reoviridae Orbivirus 2 S Ieri
Gordil GORV Phenuiviridae Phlebovirus 2 IE Phlebotomus Fever
Gossas GOSV Nairoviridae Orthonairovirus 2 S N/A
Gou GOUV Hantaviridae Orthohantavirus 2
a
IE N/A
Gouleako GOLV Phenuiviridae Goukovirus 3 IE N/A
Granada GRAV Phenuiviridae Phlebovirus 2 A7 N/A
Grand Arbaud GAV Phenuiviridae Phlebovirus 2 S Uukuniemi
Gray Lodge GLOV Rhabdoviridae Hapavirus 2 IE Vesicular Stomatitis
Great Island GIV Reoviridae Orbivirus 2 S Kemerovo
Great Saltee GRSV Nairoviridae Orthonairovirus 2 A7 N/A
Great Saltee Island GSIV Reoviridae Orbivirus 2 A7 N/A
Grimsey GSYV Reoviridae Orbivirus 2 A7 N/A
Guajará GJAV Peribunyaviridae Orthobunyavirus 2 S Capim
Guamá GMAV Peribunyaviridae Orthobunyavirus 2 S Guama
Guanarito GTOV Arenaviridae Mammarenavirus 4 A4 Tacaribe
Guaratuba GTBV Peribunyaviridae Orthobunyavirus 2 A7 Guama
Guaroa GROV Peribunyaviridae Orthobunyavirus 2 S California
Gumbo Limbo GLV Peribunyaviridae Orthobunyavirus 2 S N/A
Gurupi
GURV Reoviridae Orbivirus 2 IE Changuinola
Gweru GWV Reoviridae Orbivirus 2 A7 N/A
Hantaan HTNV Hantaviridae Orthohantavirus 3
a
S Hantaan
Hanzalova HANV Flaviviridae Flavivirus 4 A4
Tick-borne Encephalitis—
CE subtype
Hart Park HPV Rhabdoviridae Hapavirus 2 S Hart Park
Hazara HAZV Nairoviridae Orthonairovirus 2 S CCHF
Heartland HRTV Phenuiviridae Phlebovirus 3 IE N/A
Highlands J HJV Togaviridae Alphavirus 2 S
Western Equine
Encephalitis
Huacho HUAV Reoviridae Orbivirus 2 S Kemerovo
Hughes HUGV Nairoviridae Orthonairovirus 2 S Hughes
Hunter Island HUIV Phenuiviridae Phlebovirus 3 IE N/A
Hypr HYPRV Flaviviridae Flavivirus 4 S
Tick-borne Encephalitis—
CE subtype
Iaco IACOV Peribunyaviridae Orthobunyavirus 2 IE Bunyamwera
Ibaraki IBAV Reoviridae Orbivirus 2 IE
Epizootic Hemorrhagic
Disease
Icoaraci ICOV Phenuiviridae Phlebovirus 2 S Phlebotomus Fever
Ieri IERIV Reoviridae Orbivirus 2 S Ieri
Ife IFEV Reoviridae Orbivirus 2 IE N/A
Iguape IGUV Flaviviridae Flavivirus 2 A7 N/A
Ilesha ILEV Peribunyaviridae Orthobunyavirus 2 S Bunyamwera
Ilhéus ILHV
Flaviviridae Flavivirus 2 S N/A
Imjin MJNV Hantaviridae Orthohantavirus 3
a
IE N/A
Inrmatus INFV Peribunyaviridae Orthobunyavirus 2 A7 California
Ingwavuma INGV Peribunyaviridae Orthobunyavirus 2 S Simbu
Inhangapi INHV Rhabdoviridae Unassigned 2 IE N/A
Inini INIV Peribunyaviridae Orthobunyavirus 2 IE Simbu
Inkoo INKV Peribunyaviridae Orthobunyavirus 2 S California
Inner Farne INFV Reoviridae Orbivirus 2 A7 N/A
Continued on next page ►
315Section VIII-F: Arboviruses and Related Zoonotic Viruses
Virus Name Acronym Family Genus
Recommended
BSL
Basis of
Rating
Antigenic Group
Ippy IPPYV Arenaviridae Mammarenavirus 2 S Tacaribe
Iquitos IQTV Peribunyaviridae Orthobunyavirus 2 A7 N/A
Iriri IRRV Rhabdoviridae Curiovirus 2 A7 N/A
Irituia IRIV Reoviridae Orbivirus 2 S Changuinola
Isfahan ISFV Rhabdoviridae Vesiculovirus 2 S Vesicular Stomatitis
Israel turkey
meningoencephalitis
ITV Flaviviridae Flavivirus 2 with 3 practices S N/A
Issyk-Kul ISKV Nairoviridae Orthonairovirus 3 IE N/A
Itacaiunas ITCNV Rhabdoviridae Curiovirus 2 A7 N/A
Itaituba ITAV Phenuiviridae Phlebovirus 2 IE Phlebotomus Fever
Itaporanga ITPV Phenuiviridae Phlebovirus 2 S Phlebotomus Fever
Itaquí ITQV Peribunyaviridae Orthobunyavirus 2 S N/A
Itaya Peribunyaviridae Orthobunyavirus 2 A7 N/A
Itimirim ITIV Peribunyaviridae Orthobunyavirus 2 IE Guama
Itupiranga ITUV Reoviridae Orbivirus 2 II N/A
Ixcanal IXCV Phenuiviridae Phlebovirus 2 A7 Phlebotomus Fever
Jacareacanga JACV Reoviridae Orbivirus 2 IE Corriparta
Jacunda JCNV Phenuiviridae Phlebovirus 2 A7 Phlebotomus Fever
Jamanxi JAMV Reoviridae Orbivirus 2 IE Changuinola
Jamestown Canyon JCV
Peribunyaviridae Orthobunyavirus 2 S California
Japanaut JAPV Reoviridae Orbivirus 2 S N/A
Japanese
encephalitis
JEV Flaviviridae Flavivirus 3
b
S N/A
Jari JARIV Reoviridae Orbivirus 2 IE Changuinola
Jatobal JTBV Preibunyaviridae Orthobunyavirus 2 A7 N/A
Jeju JJUV Hantaviridae Orthohantavirus 3
a
A7 N/A
Jerry Slough JSV Peribunyaviridae Orthobunyavirus 2 S California
Joa JOAV Phenuiviridae Phlebovirus 2 A7 N/A
Johnston Atoll J AV Orthomyxoviridae Quaranjavirus 2 S Quaranl
Joinjakaka JOIV Rhabdoviridae Hapavirus 2 S N/A
Juan Diaz JDV Peribunyaviridae Orthobunyavirus 2 S Capim
Jugra JUGV Flaviviridae Flavivirus 2 S N/A
Junín JUNV Arenaviridae Mammarenavirus 4 A6 Tacaribe
Juquitiba JUQV Hantaviridae Orthohantavirus 3
a
A7 N/A
Jurona JURV Rhabdoviridae Vesiculovirus 2 S Vesicular Stomatitis
Juruaca JRCV Picornaviridae Unassigned 2 A7 N/A
Jutiapa JUTV Flaviviridae Flavivirus 2 S N/A
Kabuto Mountain KAMV Phenuiviridae Phlebovirus 2 A7 N/A
Kachemak Bay KBV Nairoviridae Orthonairovirus 2 A7 N/A
Kadam KADV Flaviviridae
Flavivirus 2 S N/A
Kaeng Khoi KKV Peribunyaviridae Orthobunyavirus 2 S N/A
Kaikalur KAIV Peribunyaviridae Orthobunyavirus 2 S Simbu
Kairi KRIV Peribunyaviridae Orthobunyavirus 2 A1 Bunyamwera
Kaisodi KSOV
Unclassied
Bunyavirales
N/A 2 S Kaisodi
Kala Iris KIRV Reoviridae Orbivirus 2 A7 N/A
Kamese KAMV Rhabdoviridae Hapavirus 2 S Hart Park
Kammavanpettai KMPV Reoviridae Orbivirus 2 S N/A
Continued on next page ►
316 Biosafety in Microbiological and Biomedical Laboratories
Virus Name Acronym Family Genus
Recommended
BSL
Basis of
Rating
Antigenic Group
Kannamangalam KANV Rhabdoviridae Unassigned 2 S N/A
Kanyawara KYAV Rhabdoviridae Ledantevirus 2 A7 N/A
Kao Shuan KSV Nairoviridae Orthonairovirus 2 S N/A
Karimabad KARV Phenuiviridae Phlebovirus 2 S N/A
Karshi KSIV Flaviviridae Flavivirus 2 S N/A
Kasba KASV Reoviridae Orbivirus 2 S N/A
Kasokero KASV Nairoviridae Orthonairovirus 2 A7 N/A
Kédougou KEDV Flaviviridae Flavivirus 2 A7 N/A
Kemerovo KEMV Reoviridae Orbivirus 2 S N/A
Kenai KENV Reoviridae Orbivirus 2 A7 N/A
Kenkeme KKMV Hantaviridae Orthohantavirus 3
a
A7 N/A
Kern Canyon KCV Rhabdoviridae Ledantevirus 2 S N/A
Ketapang KETV Peribunyaviridae Orthobunyavirus 2 S N/A
Keterah KTRV Nairoviridae Orthonairovirus 2 S N/A
Keuraliba KEUV Rhabdoviridae Ledantevirus 2 S N/A
Keystone KEYV Peribunyaviridae Orthobunyavirus 2 S California
Khabarovsk KHAV Hantaviridae Orthohantavirus 3
a
IE Hantaan
Kharagysh KHAV Reoviridae Orbivirus 2 A7 N/A
Khasan
KHAV Phenuiviridae Phlebovirus 2 IE CCHF
Khatanga KHATV Peribunyaviridae Orthobunyavirus 2 A7 N/A
Kimberley KIMV Rhabdoviridae Ephemerovirus 2 A7 Bovine Ephemeral Fever
Kindia KINV Reoviridae Orbivirus 2 A7 Palyam
Kismayo KISV Phenuiviridae Phlebovirus 2 S Bhanja
Klamath KLAV Rhabdoviridae Tupavirus 2 S Vesicular Stomatitis
Kokobera KOKV Flaviviridae Flavivirus 2 S N/A
Kolente KOLEV Rhabdoviridae Ledantevirus 2 A7 N/A
Kolongo KOLV Rhabdoviridae Unassigned 2 S Rab
Komandory KOMV Phenuiviridae Phlebovirus 2 IE N/A
Koongol KOOV Peribunyaviridae Orthobunyavirus 2 S Koongol
Kotonkan KOTV Rhabdoviridae Ephemerovirus 2 S Rab
Koutango KOUV Flaviviridae Flavivirus 3 S N/A
Kowanyama KOWV Peribunyaviridae Orthobunyavirus 2 S N/A
Kumlinge KUMV Flaviviridae Flavivirus 4 A4
Tick-borne Encephalitis—
CE subtype
Kunjin KUNV Flaviviridae Flavivirus 2 S N/A
Kununurra KNAV Rhabdoviridae Unassigned 2 S N/A
Kupe KUPV Nairoviridae Orthonairovirus 3 IE N/A
Kwatta KWAV
Rhabdoviridae Unassigned 2 S Vesicular Stomatitis
Kyasanur Forest
disease
KFDV
Flaviviridae Flavivirus 4 S N/A
Kyzylagach KYZV Togaviridae Alphavirus 2 IE
Western Equine
Encephalitis
La Crosse LACV Peribunyaviridae Orthobunyavirus 2 S California
Lagos bat LBV Rhabdoviridae Lyssavirus 2 S Rab
Laguna Negra LANV Hantaviridae Orthohantavirus 3
a
IE N/A
Laibin LAIV Hantaviridae Orthohantavirus 3
a
IE N/A
La Joya LJV Rhabdoviridae Hapavirus 2 S Vesicular Stomatitis
Lake Chad LKCV Orthomyxoviridae Quaranjavirus 2 A7 N/A
Continued on next page ►
317Section VIII-F: Arboviruses and Related Zoonotic Viruses
Virus Name Acronym Family Genus
Recommended
BSL
Basis of
Rating
Antigenic Group
Lake Clarendon LCV Reoviridae Orbivirus 2 IE N/A
Landjia LJAV Rhabdoviridae Hapavirus 2 S N/A
Langat LGTV Flaviviridae Flavivirus 2 S N/A
Lanjan LJNV
Unclassied
Bunyavirales
N/A 2 S Kaisodi
Las Maloyas LMV Peribunyaviridae Orthobunyavirus 2 A7 Anopheles A
Lassa LASV Arenaviridae Mammarenavirus 4 S N/A
Latino LATV Arenaviridae Mammarenavirus 2 A5 Tacaribe
Leanyer LEAV Peribunyaviridae Orthobunyavirus 2 A7 N/A
Lebombo LEBV Reoviridae Orbivirus 2 S N/A
Lechiguanas LECHV Hantaviridae Orthohantavirus 3
a
IE Hantaan
Le Dantec LDV Rhabdoviridae Ledantevirus 2 S Le Dantec
Lednice LEDV Peribunyaviridae Orthobunyavirus 2 A7 Turlock
Leopards Hill LPHV Nairoviridae Orthonairovirus 2 A7 N/A
Leticia LTCV Phenuiviridae Phlebovirus 2 A7 Punta Toro
Lipovnik LIPV Reoviridae Orbivirus 2 S Kemerovo
Llano Seco LLSV Reoviridae Orbivirus 2 IE Umatilla
Loei River LORV Arenaviridae Mammarenavirus 3 IE N/A
Lokern LOKV Peribunyaviridae Orthobunyavirus 2 S Bunyamwera
Lone Star
LSV Phenuiviridae Phlebovirus 2 S N/A
Longquan LQUV Hantaviridae Orthohantavirus 3
a
IE N/A
Louping Ill LIV Flaviviridae Flavivirus 3
b
S N/A
Lujo LUJV Arenaviridae Mammarenavirus 4 A4 N/A
Lukuni LUKV Peribunyaviridae Orthobunyavirus 2 S Anopheles A
Lumbo LUMV Peribunyaviridae Orthobunyavirus 2 A7 N/A
Luna LUNV Arenaviridae Mammarenavirus 3 A7 N/A
Lundy LUNV Reoviridae Orbivirus 2 A7 N/A
Lunk LNKV Arenaviridae Mammarenavirus 3 IE N/A
Luxi LUXV Hantaviridae Orthohantavirus 3
a
IE N/A
Lymphocytic
choriomeningitis
LCMV Arenaviridae Mammarenavirus 2 A5 N/A
Macaua MCAV Peribunyaviridae Orthobunyavirus 2 IE Bunyamwera
Machupo MACV Arenaviridae Mammarenavirus 4 S Tacaribe
Maciel MCLV Hantaviridae Orthohantavirus 3
a
IE N/A
Madariaga MADV Togaviridae Alphavirus 3 A7
Eastern Equine
Encephalitis
Madre de Dios MDDV Peribunyaviridae Orthobunyavirus 2 A7 N/A
Madrid MADV Peribunyaviridae Orthobunyavirus 2 S N/A
Maguari MAGV Peribunyaviridae Orthobunyavirus 2 S Bunyamwera
Mahogany Hammock MHV Peribunyaviridae Orthobunyavirus 2 S Guama
Maiden MDNV
Reoviridae Orbivirus 2 A7 N/A
Main Drain MDV Peribunyaviridae Orthobunyavirus 2 S Bunyamwera
Malakal MALV Rhabdoviridae Ephemerovirus 2 S Bovine Ephemeral
Maldonado MLOV Phenuiviridae Phlebovirus 2 A7 Candiru
Malsoor MALV Phenuiviridae Phlebovirus 3 IE N/A
Manawa M WAV Phenuiviridae Phlebovirus 2 S Uukuniemi
Manitoba MNTBV Rhabdoviridae Hapavirus 2 A7 N/A
Manzanilla MANV Peribunyaviridae Orthobunyavirus 2 S Simbu
Continued on next page ►
318 Biosafety in Microbiological and Biomedical Laboratories
Virus Name Acronym Family Genus
Recommended
BSL
Basis of
Rating
Antigenic Group
Mapputta MAPV Peribunyaviridae Orthobunyavirus 2 S Mapputta
Maporal MAPV Hantaviridae Orthohantavirus 3
a
IE Hantaan
Maprik MPKV Peribunyaviridae Orthobunyavirus 2 S Mapputta
Maraba MARAV Rhabdoviridae Vesiculovirus 2 A7 N/A
Marajo MRJV Unclassied virus N/A 2 IE N/A
Marburg MARV Filoviridae Marburgvirus 4 S Marburg
Marco MCOV Rhabdoviridae Hapavirus 2 S N/A
Mariental MRLV Arenaviridae Mammarenavirus 3 IE N/A
Maripa MARV Hantaviridae Orthohantavirus 3
a
IE N/A
Mariquita MRQV Phenuiviridae Phlebovirus 2 A7 N/A
Marituba MTBV Peribunyaviridae Orthobunyavirus 2 S N/A
Marondera MRDV Reoviridae Orbivirus 2 A7 N/A
Marrakai MARV Reoviridae Orbivirus 2 S N/A
Massila MASV Phenuiviridae Phlebovirus 2 A7 N/A
Matariya MTYV Rhabdoviridae Unassigned 2 S N/A
Matruh MTRV Peribunyaviridae Orthobunyavirus 2 S N/A
Matucare MATV Reoviridae Orbivirus 2 S N/A
Mayaro MAYV Togaviridae Alphavirus 2 S Semliki Forest
Mboke
MBOV Peribunyaviridae Orthobunyavirus 2 A7 N/A
Mburo MBUV Peribunyaviridae Orthobunyavirus 2 A7 N/A
Meaban MEAV Flaviviridae Flavivirus 2 IE N/A
Medjerda Valley MVV Phenuiviridae Phlebovirus 2 A7 N/A
Melao MELV Peribunyaviridae Orthobunyavirus 2 S California
Merino Walk MWV Arenaviridae Mammarenavirus 3 IE N/A
Mermet MERV Peribunyaviridae Orthobunyavirus 2 S Simbu
Middelburg MIDV Togaviridae Alphavirus 2 A1 Middelburg
Mill Door MDR Reoviridae Orbivirus 2 A7 N/A
Minacu N/A Reoviridae Orbivirus 2 IE N/A
Minatitlan MNTV Peribunyaviridae Orthobunyavirus 2 S Minatitlan
Minnal MINV Reoviridae Orbivirus 2 S Umatilla
Mirim MIRV Peribunyaviridae Orthobunyavirus 2 S Guama
Mitchell River MRV Reoviridae Orbivirus 2 S N/A
Mobala MOBV Arenaviridae Mammarenavirus 3 A7 Tacaribe
Modoc MODV Flaviviridae Flavivirus 2 S N/A
Moju MOJUV Peribunyaviridae Orthobunyavirus 2 S Guama
Mojui Dos Campos MDCV Peribunyaviridae Orthobunyavirus 2 IE N/A
Mono Lake MLV Reoviridae Orbivirus 2
S Kemerovo
Monongahela MGLV
Hantaviridae Orthohantavirus 3
a
A7 N/A
Montana myotis
leukoencephalitis
MMLV Flaviviridae Flavivirus 2 S N/A
Montano MTNV Hantaviridae Orthohantavirus 3
a
A7 N/A
Monte Dourado MDOV Reoviridae Orbivirus 2 IE Changuinola
Mopeia MOPV Arenaviridae Mammarenavirus 3 A7 N/A
Moriche MORV Peribunyaviridae Orthobunyavirus 2 S Capim
Morolillo MOLV Phenuiviridae Phlebovirus 3 IE N/A
Morreton MORV Rhabdoviridae Vesiculovirus 2 A7 Vesicular Stomatitis
Morro Bay MBV Peribunyaviridae Orthobunyavirus 2 IE California
Continued on next page ►
319Section VIII-F: Arboviruses and Related Zoonotic Viruses
Virus Name Acronym Family Genus
Recommended
BSL
Basis of
Rating
Antigenic Group
Morogoro MORV Arenaviridae Mammarenavirus 3 A7 N/A
Morumbi MRMBV Phenuiviridae Phlebovirus 2 A7 Phlebotomus Fever
Mosqueiro MQOV Rhabdoviridae Hapavirus 2 A7 Hart Park
Mosso das Pedras MDPV Togaviridae Alphavirus 3 A7
Venezuelan Equine
Encephalitis
Mossuril MOSV Rhabdoviridae Hapavirus 2 S Hart Park
Mount Elgon bat MEBV Rhabdoviridae Ledantevirus 2 S Vesicular Stomatitis
Mudjinbarry MUDV Reoviridae Orbivirus 2 A7 N/A
Muju MUJV Hantaviridae Orthohantavirus 2
a
A8 N/A
Muleshoe MULV Hantaviridae Orthohantavirus 2
a
A8 N/A
M’Poko MPOV Peribunyaviridae Orthobunyavirus 2 S Turlock
Mucambo MUCV Togaviridae Alphavirus 3 S
Venezuelan Equine
Encephalitis
Mucura MCRV Phenuiviridae Phlebovirus 2 A7 Phlebotomus Fever
Munguba MUNV Phenuiviridae Phlebovirus 2 IE Phlebotomus Fever
Murray Valley
encephalitis
MVEV Flaviviridae Flavivirus 3 S N/A
Murre MURV Phenuiviridae Phlebovirus 2 A7 N/A
Murutucú MURV Peribunyaviridae Orthobunyavirus 2 S N/A
Mykines MYKV Reoviridae Orbivirus 2 A7 Kemerovo
Nairobi sheep
disease
NSDV Nairoviridae Orthonairovirus 3
b
A1 Nairobi Sheep Disease
Nanjianyin N/A Flaviviridae Flavivirus 4 A4
Tick-borne Encephalitis—
CE subtype
Naranjal NJLV Flaviviridae Flavivirus 2 IE N/A
Nasoule NASV Rhabdoviridae Unassigned 2 A7 Rab
Navarro NAVV Rhabdoviridae Unassigned 2 S N/A
Ndumu NDUV Togaviridae Alphavirus 2 A1 Ndumu
Necocli NECV Hantaviridae Orthohantavirus 3
a
A7 N/A
Negishi NEGV Flaviviridae Flavivirus 3 S
Tick-borne Encephalitis—
CE subtype
Nepuyo NEPV Peribunyaviridae Orthobunyavirus 2 S N/A
Netivot NETV Reoviridae Orbivirus 2 A7 N/A
New Minto NMV Rhabdoviridae Unassigned 2 IE Sawgrass
New York NYOV Hantaviridae Orthohantavirus 3
a
A7 N/A
Ngaingan NGAV Rhabdoviridae Hapavirus 2 S Tibrogargan
Ngaric NRIV Peribunyaviridae Orthobunyavirus 3 A7 Bunyamwera
Ngoupe NGOV Reoviridae Orbivirus 2 A7 Eubenangee
Ninarumi NRUV Reoviridae Orbivirus 3 A7 N/A
Nique NIQV Phenuiviridae Phlebovirus 2 S Phlebotomus Fever
Nkolbisson NKOV Rhabdoviridae Ledantevirus 2 S Kern Canyon
Nodamura NOV Nodaviridae Alphanodavirus 2 IE N/A
Nola NOLAV Peribunyaviridae Orthobunyavirus 2 S Bakau
North Clett NCLV
Reoviridae Orbivirus 2 A7 N/A
North Creek NORCV Rhabdoviridae Unassigned 2 A7 N/A
North End NEDV Reoviridae Orbivirus 2 A7 N/A
Northway NORV Peribunyaviridae Orthobunyavirus 2 IE Bunyamwera
Nova NVAV Hantaviridae Orthohantavirus 3
a
IE N/A
Ntaya NTAV Flaviviridae Flavivirus 2 S N/A
Nugget NUGV Reoviridae Orbivirus 2 S Kemerovo
Continued on next page ►
320 Biosafety in Microbiological and Biomedical Laboratories
Virus Name Acronym Family Genus
Recommended
BSL
Basis of
Rating
Antigenic Group
Nyabira NYAV Reoviridae Orbivirus 2 A7 N/A
Nyamanini NYMV Nyamaninidae Nyavirus 2 S Nyamanini
Nyando NDV Peribunyaviridae Orthobunyavirus 2 S Nyando
Oceanside OCV Phenuiviridae Phlebovirus 2 A7 N/A
Oak Vale OVV Rhabdoviridae Unassigned 2 A7 N/A
Ockelbo N/A Togaviridae Alphavirus 2 A7
Western Equine
Encephalitis
Odrenisrou ODRV Phenuiviridae Phlebovirus 2 A7 Phlebotomus Fever
Oita OITAV Rhabdoviridae Ledantevirus 2 A7 N/A
Okahandja OKAV Arenaviridae Mammarenavirus 3 IE N/A
Okhotskiy OKHV Reoviridae Orbivirus 2 S Kemerovo
Okola OKOV
Unclassied
Bunyavirales
2 S Tanga
Olbia OLBV Phenuiviridae Phlebovirus 2 A7 N/A
Olifantsvlei OLIV Peribunyaviridae Orthobunyavirus 2 S Olifantsvlei
Oliveros OLVV Arenaviridae Mammarenavirus 3 A7 N/A
Omo OMOV Nairoviridae Orthonairovirus 2 A7 Qalyub
Omsk hemorrhagic
fever
OHFV Flaviviridae Flavivirus 4 S N/A
O’nyong-nyong ONNV Togaviridae Alphavirus 2 S Semliki Forest
Orán ORANV Hantaviridae Orthohantavirus 3
a
IE Hantaan
Oriboca ORIV Peribunyaviridae Orthobunyavirus 2 S N/A
Oriximiná ORXV Phenuiviridae Phlebovirus 2 IE Phlebotomus Fever
Oropouche OROV Peribunyaviridae Orthobunyavirus 2 S Simbu
Orungo ORUV Reoviridae Orbivirus 2 S Orungo
Ossa OSSAV Peribunyaviridae Orthobunyavirus 2 S N/A
Ouango OUAV Rhabdoviridae Unassigned 2 IE N/A
Oubangui OUBV Poxviridae Unassigned 2 IE N/A
Oubi OUBIV Peribunyaviridae Orthobunyavirus 2 A7 Olifantsvlei
Ourem OURV Reoviridae Orbivirus 2 IE Changuinola
Oxbow OXBV Hantaviridae Orthohantavirus 3
a
A7 N/A
Pacora PCAV
Unclassied
Bunyavirales
2 S N/A
Pacui PACV Peribunyaviridae Unassigned 2 S N/A
Pahayokee PAHV Peribunyaviridae Orthobunyavirus 2 S Patois
Palma PMAV Phenuiviridae Phlebovirus 2 IE Bhanja
Palestina PLSV Peribunyaviridae Orthobunyavirus 2 IE Minatitlan
Palyam PALV Reoviridae Orbivirus 2 S Palyam
Para PARAV Peribunyaviridae Unassigned 2 IE Simbu
Paramushir PMRV Nairoviridae Orthonairovirus 2 IE Sakhalin
Paraná PARV Arenaviridae Mammarenavirus 2 A5 Tacaribe
Paranoá PARV Hantaviridae Orthohantavirus
3
a
IE N/A
Paroo River PRV Reoviridae Orbivirus 2 IE N/A
Parry’s Lagoon PLV Reoviridae Orbivirus 2 IE N/A
Pata PATAV Reoviridae Orbivirus 2 S N/A
Pathum Thani PTHV Nairoviridae Orthonairovirus 2 S Dera Ghazi Khan
Patois PATV Peribunyaviridae Orthobunyavirus 2 S Patois
Peaton PEAV Peribunyaviridae Orthobunyavirus 2 A1 Simbu
Perdões N/A Peribunyaviridae Orthobunyavirus 2 A7 N/A
Continued on next page ►
321Section VIII-F: Arboviruses and Related Zoonotic Viruses
Virus Name Acronym Family Genus
Recommended
BSL
Basis of
Rating
Antigenic Group
Pergamino PRGV Hantaviridae Orthohantavirus 3
a
IE N/A
Perinet PERV Rhabdoviridae Vesiculovirus 2 A7 Vesicular Stomatitis
Peruvian horse
sickness
PHSV Reoviridae Orbivirus 3 A1 N/A
Petevo PETV Reoviridae Orbivirus 2 A7 Palyam
Phnom Penh bat PPBV Flaviviridae Flavivirus 2 S N/A
Pichindé PICHV Arenaviridae Mammarenavirus 2 A5 Tacaribe
Picola PIAV Reoviridae Orbivirus 2 IE Wongorr
Pintupo N/A Peribunyaviridae Orthobunyavirus 2 A7 N/A
Pirital PIRV Arenaviridae Mammarenavirus 3 IE N/A
Piry PIRYV Rhabdoviridae Vesiculovirus 3 S Vesicular Stomatitis
Pixuna PIXV Togaviridae Alphavirus 2 S
Venezuelan equine
encephalitis
Playas PLAV Peribunyaviridae Orthobunyavirus 2 IE Bunyamwera
Pongola PGAV Peribunyaviridae Orthobunyavirus 2 S Bwamba
Ponteves PTVV Phenuiviridae Phlebovirus 2 A7 Uukuniemi
Poovoot POOV Reoviridae Orbivirus 2 A7 N/A
Potiskum POTV Flaviviridae Flavivirus 2 A7 N/A
Potosi POTV Peribunyaviridae Orthobunyavirus 2 IE Bunyamwera
Powassan POWV Flaviviridae Flavivirus 3 S N/A
Precarious Point
PPV Phenuiviridae Phlebovirus 2 A7 Uukuniemi
Pretoria PREV Nairoviridae Orthonairovirus 2 S Dera Ghazi Khan
Prospect Hill PHV Hantaviridae Orthohantavirus 2 A8 Hantaan
Puchong PUCV Rhabdoviridae Ephemerovirus 2 S Bovine Ephemeral Fever
Pueblo Viejo PVV Peribunyaviridae Orthobunyavirus 2 IE Gamboa
Pun Island PIV Nairoviridae Orthonairovirus 2 A7 N/A
Punique PUNV Phenuiviridae Phlebovirus 2 A7 Sandy Fever Naples
Punta Salinas PSV Nairoviridae Orthonairovirus 2 S Hughes
Punta Toro PTV Phenuiviridae Phlebovirus 2 S Phlebotomus Fever
Purus PURV Reoviridae Orbivirus 2 IE Changuinola
Puumala PUUV Hantaviridae Orthohantavirus 3
a
IE Hantaan
Qalyub QYBV Nairoviridae Orthonairovirus 2 S Qalyub
Quaranl QRFV Orthomyxoviridae Quaranjavirus 2 S Quaranl
Quezon QZNV Hantaviridae Orthohantavirus 3
a
IE N/A
Radi RADIV Rhabdoviridae Vesiculovirus 2 A7 Vesicular Stomatitis
Ravn RAVV Filoviridae Marburgvirus 4 S Marburg
Raza RAZAV Nairoviridae Orthonairovirus 2 A7 N/A
Razdan RAZV Phenuiviridae Unassigned 2 IE N/A
Resistencia RTAV
Unclassied
Bunyavirales
2 IE Antequera
Restan RESV Peribunyaviridae
Orthobunyavirus 2 S N/A
Reston REST Filoviridae Ebolavirus 4 S Ebola
Rift Valley fever RVFV Phenuiviridae Phlebovirus 3
b
S Phlebotomus Fever
Rio Bravo RBV Flaviviridae Flavivirus 2 S N/A
Rio Grande RGV Phenuiviridae Phlebovirus 2 S Phlebotomus Fever
Rio Mamoré RIOMV Hantaviridae Orthohantavirus 3
a
A7 N/A
Rio Negro RNV Togaviridae Alphavirus 3 A7
Venezuelan Equine
Encephalitis
Rio Pracupi N/A Peribunyaviridae Orthobunyavirus 2 A7 N/A
Continued on next page ►
322 Biosafety in Microbiological and Biomedical Laboratories
Virus Name Acronym Family Genus
Recommended
BSL
Basis of
Rating
Antigenic Group
Rio Preto da Eva RIOPV Phenuiviridae Unassigned 2 IE N/A
Riverside RISV Rhabdoviridae Unassigned 2 IE N/A
RML 105355 RMLV Phenuiviridae Phlebovirus 2 A7 N/A
Rochambeau RBUV Rhabdoviridae Curiovirus 2 IE Rab
Rocio ROCV Flaviviridae Flavivirus 3 S N/A
Rockport RKPV Hantaviridae Orthohantavirus 3
a
IE N/A
Ross River RRV Togaviridae Alphavirus 2 S Semliki Forest
Rost Island RSTV Reoviridae Orbivirus 2 A7 Kemerovo
Royal Farm RFV Flaviviridae Flavivirus 2 S N/A
Rukutama RUKV Phenuiviridae Phlebovirus 2 A7 N/A
Russian spring-
summer encephalitis
RSSEV Flaviviridae Flavivirus 4 S
Tick-borne Encephalitis—
FE subtype
Ryukyu RYKV Arenaviridae Mammarenavirus 2 A5 N/A
Saaremaa SAAV Hantaviridae Orthohantavirus 3
a
IE Hantaan
Sabiá SABV Arenaviridae Mammarenavirus 4 A4 N/A
Sabo SABOV Peribunyaviridae Orthobunyavirus 2 S Simbu
Saboya SABV Flaviviridae Flavivirus 2 S N/A
Saddaguia SADV Phenuiviridae Phlebovirus 2 A7 N/A
Sagiyama SAGV Togaviridae Alphavirus 2 A1 Semliki Forest
Saint-Floris
SAFV Phenuiviridae Phlebovirus 2 S Phlebotomus Fever
Sakhalin SAKV Nairoviridae Orthonairovirus 2 S Sakhalin
Salanga SGAV Poxviridae Unassigned 2 IE SGA
Salehabad SALV Phenuiviridae Phlebovirus 2 S Phlebotomus Fever
Salmon River SAVV Reoviridae Coltivirus 2 IE Colorado Tick Fever
Salobo SBOV Phenuiviridae Phlebovirus 3 IE N/A
Sal Vieja SVV Flaviviridae Flavivirus 2 A7 N/A
San Angelo SAV Peribunyaviridae Orthobunyavirus 2 S California
Sandy fever Cyprus N/A Phenuiviridae Phlebovirus 2 IE N/A
Sandy fever
Ethiopia
N/A Phenuiviridae Phlebovirus 2 IE N/A
Sandy fever Naples SFNV Phenuiviridae Phlebovirus 2 S Phlebotomus Fever
Sandy fever Sicilian SFSV Phenuiviridae Phlebovirus 2 S Phlebotomus Fever
Sandy fever Turkey SFTV Phenuiviridae Phlebovirus 2 IE N/A
Sandjimba SJAV Rhabdoviridae Unassigned 2 S Rab
Sangassou SANGV Hantaviridae Orthohantavirus 3 A7 N/A
Sango SANV Peribunyaviridae Orthobunyavirus 2 S Simbu
San Juan SJV Peribunyaviridae Orthobunyavirus 2 IE Gamboa
San Perlita SPV Flaviviridae Flavivirus 2 A7 N/A
Santarem STMV
Unclassied
Bunyavirales
N/A 2 IE N/A
Santa Rosa SARV
Peribunyaviridae Orthobunyavirus 2 IE Bunyamwera
Sapphire II SAPV Nairoviridae Orthonairovirus 2 A7 N/A
Saraca SRAV Reoviridae Orbivirus 2 IE Changuinola
Sathuperi SATV Peribunyaviridae Orthobunyavirus 2 S Simbu
Sathuvachari SVIV Reoviridae Orbivirus 2 A7 N/A
Saumarez Reef SREV Flaviviridae Flavivirus 2 IE N/A
Sawgrass SAWV Rhabdoviridae Unassigned 2 S Sawgrass
Schmallenberg SBV Peribunyaviridae Orthobunyavirus 2 A7 N/A
Continued on next page ►
323Section VIII-F: Arboviruses and Related Zoonotic Viruses
Virus Name Acronym Family Genus
Recommended
BSL
Basis of
Rating
Antigenic Group
Sebokele SEBV Picornaviridae Parechovirus 2 S N/A
Sedlec SEDV Peribunyaviridae Orthobunyavirus 2 A7 N/A
Seletar SELV Reoviridae Orbivirus 2 S Kemerovo
Sembalam SEMV Unclassied virus N/A 2 S N/A
Semliki Forest SFV Togaviridae Alphavirus 3 A2 Semliki Forest
Sena Madureira SMV Rhabdoviridae Sripuvirus 2 IE Timbo
Seoul SEOV Hantaviridae Orthohantavirus 3
a
IE Hantaan
Sepik SEPV Flaviviridae Flavivirus 2 IE N/A
Serra Do Navio SDNV Peribunyaviridae Orthobunyavirus 2 A7 California
Serra Norte SRNV Phenuiviridae Phlebovirus 2 A7 N/A
Severe fever with
thrombocytopenia
syndrome
SFTSV Phenuiviridae Phlebovirus 3 IE N/A
Shamonda SHAV Peribunyaviridae Orthobunyavirus 2 S Simbu
Shark River SRV Peribunyaviridae Orthobunyavirus 2 S Patois
Shiant Island SHIV Reoviridae Orbivirus 2 A7 N/A
Shokwe SHOV Peribunyaviridae Orthobunyavirus 2 IE Bunyamwera
Shuni SHUV Peribunyaviridae Orthobunyavirus 2 S Simbu
Silverwater SILV Phenuiviridae Phlebovirus 2 S Kaisodi
Simbu SIMV Peribunyaviridae Orthobunyavirus 2 S Simbu
Sindbis
SINV Togaviridae Alphavirus 2 S
Western Equine
Encephalitis
Sin Nombre SNV Hantaviridae Orthohantavirus 3
a
IE Hantaan
Sixgun City SCV Reoviridae Orbivirus 2 S Kemerovo
Skinner Tank SKTV Arenaviridae Mammarenavirus 2 A5 N/A
Snowshoe hare SSHV Peribunyaviridae Orthobunyavirus 2 S California
Sokoluk SOKV Flaviviridae Flavivirus 2 S N/A
Soldado SOLV Nairoviridae Orthonairovirus 2 S Hughes
Solwezi SOLV Arenaviridae Mammarenavirus 3 IE N/A
Somone SOMV Unclassied virus 3 IE Somone
Sororoca SORV Peribunyaviridae Orthobunyavirus 2 S Bunyamwera
Souris SOUV Arenaviridae Mammarenavirus 2 A5 N/A
South Bay SBV
Unclassied
Bunyavirales
N/A 3 IE N/A
South River SORV Peribunyaviridae Orthobunyavirus 2 A7 N/A
Spondweni SPOV Flaviviridae Flavivirus 2 S N/A
Sripur SRIV Rhabdoviridae Sripuvirus 3 IE N/A
St. Abbs Head SAHV Phenuiviridae Phlebovirus 2 A7 N/A
St. Louis encephalitis SLEV Flaviviridae Flavivirus 2 S N/A
Staneld N/A Peribunyaviridae Orthobunyavirus 2 A7 N/A
Stratford STRV Flaviviridae Flavivirus 2 S N/A
Sudan SUDV Filoviridae
Ebolavirus 4 S Ebola
Sunday Canyon SCAV Phenuiviridae Phlebovirus 2 S N/A
Sweetwater Branch SWBV Rhabdoviridae Tibrovirus 2 IE N/A
Tacaiuma TCMV Peribunyaviridae Orthobunyavirus 2 S Anopheles A
Tacaribe TCRV Arenaviridae Mammarenavirus 2 A5 Tacaribe
Tǎchéng tick 1 TTV-1 Nairoviridae Orthonairovirus 2 IE N/A
Taggert TAGV Nairoviridae Orthonairovirus 2 S Sakhalin
Tahyña TAHV Peribunyaviridae Orthobunyavirus 2 S California
Continued on next page ►
324 Biosafety in Microbiological and Biomedical Laboratories
Virus Name Acronym Family Genus
Recommended
BSL
Basis of
Rating
Antigenic Group
Taiassui TAIAV Peribunyaviridae Orthobunyavirus 2 A7 N/A
Taï Forest TAFV Filoviridae Ebolavirus 4 S Ebola
Tamdy TDYV Nairoviridae Orthonairovirus 2 IE N/A
Tamiami TMMV Arenaviridae Mammarenavirus 2 A5 Tacaribe
Tanga TANV
Unclassied
Bunyavirales
N/A 2 S Tanga
Tanjong Rabok TRV Peribunyaviridae Orthobunyavirus 2 S Bakau
Tapara TAPV Phenuiviridae Phlebovirus 2 A7 N/A
Tataguine TATV Peribunyaviridae Orthobunyavirus 2 S N/A
Tehran TEHV Phenuiviridae Phlebovirus 2 A7 Phlebotomus Fever
Telok Forest TFV Peribunyaviridae Orthobunyavirus 2 IE Bakau
Tembe TMEV Reoviridae Orbivirus 2 S N/A
Tembusu TMUV Flaviviridae Flavivirus 2 S N/A
Tensaw TENV Peribunyaviridae Orthobunyavirus 2 S Bunyamwera
Termeil TERV Peribunyaviridae Orthobunyavirus 2 IE N/A
Tete TETEV Peribunyaviridae Orthobunyavirus 2 S Tete
Thailand THAIV Hantaviridae Orthohantavirus 3 A7 N/A
Thiafora TFAV Nairoviridae Orthonairovirus 2 A7 Thiafora
Thimiri THIV Peribunyaviridae Orthobunyavirus 2 S Simbu
Thogoto
THOV Orthomyxoviridae Thogotovirus 2 S Thogoto
Thormodseyjarlettur THRV Reoviridae Orbivirus 2 A7 N/A
Thottapalayam TPMV Hantaviridae Orthohantavirus 2 S Hantaan
Tibrogargan TIBV Rhabdoviridae Tibrovirus 2 S Tibrogargan
Tillamook TILLV Nairoviridae Orthonairovirus 2 A7 N/A
Tilligerry TILV Reoviridae Orbivirus 2 IE Eubenangee
Timbo TIMV Rhabdoviridae Unassigned 2 S Timbo
Timboteua TBTV Peribunyaviridae Orthobunyavirus 2 A7 Guama
Tinaroo TINV Peribunyaviridae Orthobunyavirus 2 IE Simbu
Tindholmur TDMV Reoviridae Orbivirus 2 A7 Kemerovo
Tlacotalpan TLAV Peribunyaviridae Orthobunyavirus 2 IE Bunyamwera
Toa TFLV Nairoviridae Orthonairovirus 2 IE N/A
Tonate TONV Togaviridae Alphavirus 3 IE
Venezuelan Equine
Encephalitis
Tonto Creek TTCV Arenaviridae Mammarenavirus 2 A5 N/A
Topografov TOPV Hantaviridae Orthohantavirus 3
a
IE Hantaan
Toscana TOSV Phenuiviridae Phlebovirus 2 S Phlebotomus Fever
Toure TOUV Arenavirudae Unassigned 2 S Tacaribe
Tracambe TRCV Reoviridae Orbivirus 2 A7 N/A
Tribeč TRBV Reoviridae Orbivirus 2 S Kemerovo
Triniti TNTV
Togaviridae Unassigned 2 S N/A
Trivittatus TVTV Peribunyaviridae Orthobunyavirus 2 S California
Trocara TROV Togaviridae Alphavirus 2 IE Trocara
Trombetas TRMV Peribunyaviridae Orthobunyavirus 2 A7 N/A
Trubanaman TRUV Peribunyaviridae Orthobunyavirus 2 S Mapputta
Tsuruse TSUV Peribunyaviridae Orthobunyavirus 2 S Tete
Tucunduba TUCV Peribunyaviridae Orthobunyavirus 2 A7 N/A
Tucurui TUCRV Peribunyaviridae Orthobunyavirus 2 A7 N/A
Tula TULV Hantaviridae Orthohantavirus 2
a
A8 N/A
Continued on next page ►
325Section VIII-F: Arboviruses and Related Zoonotic Viruses
Virus Name Acronym Family Genus
Recommended
BSL
Basis of
Rating
Antigenic Group
Tunari TUNV Hantaviridae Orthohantavirus 3a A7 N/A
Tunis TUNV Phenuiviridae Phlebovirus 2 A7 Phlebotomus Fever
Turlock TURV Peribunyaviridae Orthobunyavirus 2 S Turlock
Turuna TUAV Phenuiviridae Phlebovirus 2 IE Phlebotomus Fever
Tyulek TLKV Orthomyxoviridae Quaranjavirus 2 A7 N/A
Tyuleniy TYUV Flaviviridae Flavivirus 2 S N/A
Uganda S UGSV Flaviviridae Flavivirus 2 S N/A
Umatilla UMAV Reoviridae Orbivirus 2 S Umatilla
Umbre UMBV Peribunyaviridae Orthobunyavirus 2 S Turlock
Una UNAV Togaviridae Alphavirus 2 S Semliki Forest
Upolu UPOV Orthomyxoviridae Thogotovirus 2 S Upolu
Uriurana UURV Phenuiviridae Phlebovirus 2 A7 Phlebotomus Fever
Urucuri URUV Phenuiviridae Phlebovirus 2 S Phlebotomus Fever
Usutu USUV Flaviviridae Flavivirus 2 S N/A
Utinga UTIV Peribunyaviridae Orthobunyavirus 2 IE Simbu
Utive UVV Peribunyaviridae Orthobunyavirus 2 A7 N/A
Uukuniemi UUKV Phenuiviridae Phlebovirus 2 S Uukuniemi
Uzun-Agach UZAV Nairoviridae Orthonairovirus 2 A7 N/A
Vaeroy
VAEV Reoviridae Orbivirus 2 A7 N/A
Vellore VELV Reoviridae Orbivirus 2 S Palyam
Venezuelan equine
encephalitis
VEEV Togaviridae Alphavirus 3
b
S
Venezuelan Equine
Encephalitis
Venkatapuram VKTV Unclassied virus N/A 2 S N/A
Vesicular stomatitis—
Alagoas
VSAV Rhabdoviridae Vesiculovirus 2
b
S Vesicular Stomatitis
Vesicular stomatitis—
Indiana
VSIV Rhabdoviridae Vesiculovirus 2
b
A3 Vesicular Stomatitis
Vesicular stomatitis—
New Jersey
VSNJV Rhabdoviridae Vesiculovirus 2
b
A3 Vesicular Stomatitis
Vinces VINV Peribunyaviridae Orthobunyavirus 2 A7 N/A
Vinegar Hill VHV Nairoviridae Orthonairovirus 2 A7 N/A
Virgin River VRV Peribunyaviridae Orthobunyavirus 2 A7 N/A
Wad Medani WMV Reoviridae Orbivirus 2 S Kemerovo
Wallal WALV Reoviridae Orbivirus 2 S Wallal
Wanowrie WANV
Unclassied
Bunyavirales
N/A 2 S N/A
Warrego WARV Reoviridae Orbivirus 2 S Warrego
Warrego K WARKV Reoviridae Orbivirus 2 A7 N/A
Weldona WELV Peribunyaviridae Orthobunyavirus 2 A7 N/A
Wēnzhōu WENV Arenaviridae Mammarenavirus 3 IE N/A
Wēnzhōu tick WTV Nairoviridae Orthonairovirus 2 A7 N/A
Wesselsbron WESSV Flaviviridae Flavivirus 3
b
S N/A
Western equine
encephalitis
WEEV Togaviridae Alphavirus 3 S
Western Equine
Encephalitis
West Nile WNV Flaviviridae Flavivirus 2 S N/A
Wexford WEXV Reoviridae Orbivirus 2 A7 N/A
Whataroa WHAV Togaviridae Alphavirus 2 S
Western Equine
Encephalitis
Whitewater Arroyo WWAV Arenaviridae Mammarenavirus 3 IE Tacaribe
Witwatersrand WITV Peribunyaviridae Orthobunyavirus 2 S N/A
Continued on next page ►

326 Biosafety in Microbiological and Biomedical Laboratories
Virus Name Acronym Family Genus
Recommended
BSL
Basis of
Rating
Antigenic Group
Wolkberg WBV Peribunyaviridae Orthobunyavirus 2 IE N/A
Wongal WONV Peribunyaviridae Orthobunyavirus 2 S Koongol
Wongorr WGRV Reoviridae Orbivirus 2 S Wongorr
Wyeomyia WYOV Peribunyaviridae Orthobunyavirus 2 S Bunyamwera
Xiburema XIBV Rhabdoviridae Unassigned 2 IE N/A
Xingu XINV Peribunyaviridae Orthobunyavirus 3 N/A Bunyamwera
Yaba-1 Y1V Peribunyaviridae Orthobunyavirus 2 A7 N/A
Yaba-7 Y7V Peribunyaviridae Orthobunyavirus 3 IE N/A
Yacaaba YACV Peribunyaviridae Orthobunyavirus 2 IE N/A
Yakeshi YKSV Hantaviridae Orthohantavirus 3
a
IE N/A
Yaoundé YAOV Flaviviridae Flavivirus 2 A7 N/A
Yaquina Head YHV Reoviridae Orbivirus 2 S Kemerovo
Yata YATAV Rhabdoviridae Ephemerovirus 2 S N/A
Yellow fever YFV Flaviviridae Flavivirus 3 S N/A
Yogue YOGV Nairoviridae Orthonairovirus 2 S Yogue
Yoka YOKAV Poxviridae Unassigned 2 IE N/A
Yokose YOKV Flaviviridae Flavivirus 2 A7 N/A
Yug Bogdanovac YBV Rhabdoviridae Vesiculovirus 2 IE Vesicular Stomatitis
Yunnan orbivirus
YOUV Reoviridae Orbivirus 3 IE N/A
Zaliv Terpeniya ZTV Phenuiviridae Phlebovirus 2 S Uukuniemi
Zegla ZEGV Peribunyaviridae Orthobunyavirus 2 S Patois
Zerdali ZERV Phenuiviridae Phlebovirus 2 A7 Phlebotomus Fever
Zika ZIKV Flaviviridae Flavivirus 2 S N/A
Zirqa ZIRV Nairoviridae Orthonairovirus 2 S Hughes
Zungarococha ZUNV Peribunyaviridae Orthobunyavirus 2 A7 N/A
*Federal regulations, import/export requirements, and taxonomic status are subject to changes. Check with the
appropriate federal agency to conrm regulations and ICTV for most current taxonomic status.
a. Containment requirements will vary based on virus concentration, animal species, or virus type. See the Hantavirus
agent summary statement in Section VIII-E.
b. These organisms are considered pathogens of signicant agricultural importance by APHIS (see Appendix D) and
may require additional containment up to and including ABSL-3Ag containment. Not all strains of each organism
are necessarily of concern to APHIS. Contact APHIS for more information regarding exact containment/permit
requirements before initiating work.
c. Garissa virus is considered an isolate of this virus, so same containment requirements apply.
327Section VIII-F: Arboviruses and Related Zoonotic Viruses
Table 4. Alphabetic Listing of Arboviruses and Hemorrhagic Fever Viruses*
Virus Name Acronym Family Genus
Recommended
Biosafety Level
Basis of
Rating
Isolate
Aedes aegypti densovirus AaeDNV Parvoviridae Brevidensovirus 2 IE Yes
Aedes albopictus densovirus AalDNV Parvoviridae Brevidensovirus 2 IE Yes
Aedes cinereus avivirus AeciFV Flaviviridae Unassigned 2 IE ?
Aedes galloisi avivirus AGFV Flaviviridae Unassigned 2 IE ?
Aedes avivirus AEFV Flaviviridae Unassigned 2 IE Yes
Aedes pseudoscutellaris
densovirus
N/A Parvoviridae Brevidensovirus 2 IE ?
Aedes pseudoscutellaris reovirus N/A Reoviridae Dinovernavirus 2 IE Yes
Aedes vexans avivirus AeveFV Flaviviridae Unassigned 2 IE ?
Anopheles avivirus N/A Flaviviridae Unassigned 2 IE ?
Anopheles gambiae densovirus AgDNV Parvoviridae Unassigned 2 IE Yes
Arboretum ABTV Rhabdoviridae Almendravirus 2 IE Yes
Aripo N/A Flaviviridae Unassigned 2 IE Yes
Assam N/A Flaviviridae Unassigned 2 IE ?
Badu BADUV Phenuiviridae Phasivirus 2 IE Yes
Balsa BALV Rhabdoviridae Almendravirus 2 IE Yes
Barkedji BJV Flaviviridae Unassigned 2 IE ?
Bontang Baru BBaV Mesoniviridae Unassigned 2 IE Yes
Brejeira BRJV Unassigned Negevirus 2 IE Yes
Calbertado CLBOV Flaviviridae Unassigned 2 IE ?
Casuarina CASV Mesoniviridae Unassigned 2 IE Yes
Cavally CavV Mesoniviridae Alphamesonivirus 2 IE Yes
Cell Fusing Agent CFAV Flaviviridae Unassigned 2 IE Ye s
Chaoyang CHAOV Flaviviridae Unassigned 2 IE Ye s
Coot Bay CBV Rhabdoviridae Almendravirus 2 IE Yes
Culex avivirus CxFV Flaviviridae Unassigned 2 IE Ye s
Culex Y N/A Birnaviridae Entomobirnavirus 2 IE Yes
Culex theileri avivirus
CxthFV/
CTFV
Flaviviridae Unassigned 2 IE Yes
Culiseta avivirus CsFV Flaviviridae Unassigned 2 IE Yes
Cumuto CUMV Bunyavirales Goukovirus 2 IE Yes
Czech Aedes vexans avivirus
Czech
AeveFV
Flaviviridae Unassigned 2 IE ?
Dak Nong DKNG Mesoniviridae Unassigned 2 IE Yes
Dezidougou DEZV Unassigned Negevirus 2 IE Ye s
Donggang DONV Flaviviridae Unassigned 2 IE ?
Eilat EILV Togaviridae Alphavirus 2 IE Yes
Ecuador Paraiso Escondido EPEV Flaviviridae Unassigned 2 IE Ye s
Espirito Santo ESV Birnaviridae Unassigned 2 IE Yes
Gouleako GOUV Bunyaviridae Goukovirus 2 IE Ye s
Goutanap GANV Unassigned Negevirus 2 IE Ye s
Guaico Culex GCXV Jingmenvirus Unassigned 2 IE Ye s
Hana HanaV Mesoniviridae Unassigned 2 IE Ye s
Hanko HANKV Flaviviridae Unassigned 2 IE Yes
Herbert HEBV Peribunyaviridae Herbevirus 2 IE Ye s
High Island HISLV Reoviridae Idnovirus 2 IE Ye s
Huángpi tick 1 HTV-1 Nairoviridae Orthonairovirus 2 IE ?
Continued on next page ►
328 Biosafety in Microbiological and Biomedical Laboratories
Virus Name Acronym Family Genus
Recommended
Biosafety Level
Basis of
Rating
Isolate
Ilomantsi ILOV Flaviviridae Unassigned 2 IE Yes
Kamiti River KRV Flaviviridae Unassigned 2 A7 Yes
Kamphaeng Phet KPhV Mesoniviridae Unassigned 2 IE Ye s
Kampung Karu KPKV Flaviviridae Unassigned 2 IE Ye s
Karang Sari KSaV Mesoniviridae Unassigned 2 IE Yes
Kibale KIBV Peribunyaviridae Herbevirus 2 IE Yes
Lammi LAMV Flaviviridae Unassigned 2 IE Yes
La Tina LTNV Flaviviridae Unassigned 2 IE Yes
Long Island tick rhabdovirus LITRV Rhabdoviridae Unassigned 2 IE ?
Long Pine Key LPKV Flaviiviridae Unassigned 2 IE Ye s
Loreto PeAR2612/77 LORV Unassigned Negevirus 2 IE Yes
Marisma mosquito MMV Flaviviridae Unassigned 2 IE Yes
Méno MénoV Mesoniviridae Unassigned 2 IE Yes
Mercadeo MECDV Flaviviridae Unassigned 2 IE Yes
Mosquito X MXV Birnaviridae Entomobirnavirus 2 IE Yes
Moumo MoumoV Mesoniviridae N/A 2 IE ?
Moussa MOUV Rhabdoviridae Unassigned 2 IE Ye s
Nakiwogo NAKV Flaviviridae Unassigned 2 IE Ye s
Nam Dinh NDiV Mesoniviridae Alphamesonivirus 2 IE Yes
Nanay NANV Flaviviridae Unassigned 2 IE Ye s
Negev NEGV Unassigned Negevirus 2 IE Yes
Ngewotan NWTV Unassigned Negevirus 2 IE Ye s
Ngoye NGOV Flaviviridae Unassigned 2 IE ?
Nhumirim NHUV Flaviviridae Unassigned 2 IE Ye s
Nienokoue NIEV Flaviviridae Unassigned 2 IE Yes
Nounané NOUV Flaviviridae Unassigned 2 IE Yes
Nsé NseV Mesoniviridae Unassigned 2 IE Yes
Ochlerotatus caspius avivirus OCFV Flaviviridae Unassigned 2 IE Yes
Okushiri OKV Unassigned Negevirus 2 IE Ye s
Palm Creek PCV Flaviviridae Unassigned 2 IE Yes
Parramatta River PaRV Flaviviridae Unassigned 2 IE Ye s
Phelbotomine-associated avivirus N/A Flaviviridae Unassigned 2 IE ?
Piura PIUV Unassigned Negevirus 2 IE Ye s
Puerto Almendras PTAMV Rhabdoviridae Almendravirus 2 IE Yes
Quảng Binh QBV Flaviviridae Unassigned 2 IE Yes
Santana SANV Unassigned Negevirus 2 IE Ye s
Sarawak SWKV Alphatetraviridae Betatetravirus 2 IE Ye s
Spanish Culex avivirus SCxFV Flaviviridae Unassigned 2 IE Ye s
Spanish Ochlerotatus avivirus SOcFV Flaviviridae Unassigned 2 IE Yes
St. Croix River SCRV Reoviridae Orbivirus 2 IE Yes
Tai TAIV Peribunyaviridae Herbevirus 2 IE Ye s
Tanay TANAV Unassigned Negevirus 2 IE Yes
Wallereld WALV Unassigned Negevirus 2 IE Yes
Wang Thong WTV Flaviviridae Unassigned 2 IE Ye s
Xishuangbanna avivirus XFV Flaviviridae Unassigned 2 IE Yes
Yamada avivirus YDFV Flaviviridae Unassigned 2 IE Yes
Yunnan Culex avivirus YNCxFV Flaviviridae Unassigned 2 IE Yes
329Section VIII-F: Arboviruses and Related Zoonotic Viruses
Table 5. Laboratories working with the viruses at BSL-3 listed below are
recommended to HEPA lter the exhaust air
Virus Name
African Horse Sickness**
African Swine Fever**
Akabane**
Cabassou
Chikungunya
Everglades
Germiston
Louping III
Mucambo
Oropouche
Rift Valley Fever**
Rocio
Tonate
Venezuelan Equine Encephalitis
Wesselsbron**
Yellow Fever
** These organisms are considered pathogens of signicant agricultural importance by the USDA (see Appendix D) and
may require additional containment (up to and including ABSL-3Ag containment). Not all strains of each organism
are necessarily of concern to the USDA. Contact USDA for more information regarding exact containment/permit
requirements before initiating work.

330 Biosafety in Microbiological and Biomedical Laboratories
References
1. American Committee on Arthropod-Borne Viruses. Information Exchange
Subcommittee; American Society of Tropical Medicine and Hygiene.
International catalogue of arboviruses: including certain other viruses
of vertebrates. Vol 2. 3rd ed. Karabatsos N, editor. San Antonio (TX):
American Society of Tropical Medicine and Hygiene; 1985.
2. Laboratory safety for arboviruses and certain other viruses of
vertebrates. The Subcommittee on Arbovirus Laboratory Safety of the
American Committee on Arthropod-Borne Viruses. Am J Trop Med Hyg.
1980;29(6):1359–81.
3. Stobart CC, Moore ML. RNA virus reverse genetics and vaccine design.
Viruses. 2014;6(7):2531–50.
4. Lemire, KA, Rodriguez YY, McIntosh MT. Alkaline hydrolysis to remove
potentially infectious viral RNA contaminants from DNA. Virol J. 2016;13:88.
5. Calisher CH, Higgs S. The Discovery of Arthropod-Specic Viruses
in Hematophagous Arthropods: An Open Door to Understanding the
Mechanisms of Arbovirus and Arthropod Evolution?. Annu Rev Entomol.
2018;63:87–103.
6. Mary Ann Liebert, Inc. [Internet]. New Rochelle (NY): A project of The
American Committee of Medical Entomology of the American Society of
Tropical Medicine and Hygiene; c2019 [cited 2019 Mar 13]. Arthropod
Containment Guidelines, Version 3.2. Available from: https://www.liebertpub.
com/doi/10.1089/vbz.2018.2431
7. Bartelloni PJ, McKinney RW, Duy TP, Cole FE Jr. An inactivated eastern
equine encephalomyelitis vaccine propagated in chick-embryo cell
culture. II. Clinical and serologic responses in man. Am J Trop Med Hyg.
1970;19(1):123–6.
8. Pittman PR, Makuch RS, Mangiaco JA, Cannon TL, Gibbs PH, Peters CJ.
Long-term duration of detectable neutralizing antibodies after administration
of live-attenuated VEE vaccine and following booster vaccination with
inactivated VEE vaccine. Vaccine. 1996;14(4):337–43.
9. Bartelloni PJ, McKinney RW, Calia FM, Ramsburg HH, Cole FE Jr.
Inactivated western equine encephalomyelitis vaccine propagated in chick
embryo cell culture. Clinical and serological evaluation in man. Am J Trop
Med Hyg. 1971;20(1):146–9.
10. Leifer E, Gocke DJ, Bourne H. Lassa fever, a new virus disease of man
from West Africa. II. Report of a laboratory-acquired infection treated with
plasma from a person recently recovered from the disease. Am J Trop Med
Hyg. 1970;19(4):667–9.
331Section VIII-F: Arboviruses and Related Zoonotic Viruses
11. Weissenbacher MC, Grela ME, Sabattini MS, Maiztequi JI, Coto CE,
Frigerio MJ, et al. Inapparent infections with Junin virus among laboratory
workers. J Infect Dis. 1978;137(3):309–13.
12. Ad Hoc Committee on the Safe Shipment and Handling of Etiologic Agents;
Center for Disease Control. Classication of etiologic agents on the basis of
hazard. 4th ed. U.S. Department of Health, Education, and Welfare, Public
Health Service, Center for Disease Control, Oce of Biosafety; 1974.
13. Hanson RP, Sulkin SE, Beuscher EL, Hammon WM, McKinney RW,
Work TH. Arbovirus infections of laboratory workers. Extent of problem
emphasizes the need for more eective measures to reduce hazards.
Science. 1967;158(3806):1283–6.
14. Karabatsos N. Supplement to international catalogue of arboviruses
including certain other viruses of vertebrates. Am J Trop Med Hyg.
1978;27(2 Pt 2 Suppl):372–440.
15. American Committee on Arthropod Borne Viruses Subcommittee on
Information Exchange; Center for Disease Control. International catalogue of
arboviruses: including certain other viruses of vertebrates. Vol 75. Issue 8301.
2nd ed. Berge TO, editor. Washington (DC): Public Health Service; 1975.
16. Tabachnick WJ. Laboratory containment practices for arthropod vectors of
human and animal pathogens. Lab Anim (NY). 2006;35(3):28–33.
17. Hunt GJ, Tabachnick WJ. Handling small arbovirus vectors safely during
Biosafety Level 3 containment: Culicoides variipennis sonorensis (Diptera:
Ceratopogonidae) and exotic bluetongue viruses. J Med Entomol.
1996;33(3):271–7.
18. Berglund P, Quesada-Rolander M, Putkonen P, Biberfeld G, Thorstensson R,
Liljestrom P. Outcome of immunization of cynomolgus monkeys with
recombinant Semliki Forest virus encoding human immunodeciency virus
type 1 envelope protein and challenge with a high dose of SHIV-4 virus.
AIDS Res Hum Retroviruses. 1997;13(17):1487–95.
19. Davis NL, Caley IJ, Brown KW, Betts MR, Irlbeck DM, McGrath KM, et al.
Vaccination of macaques against pathogenic simian immunodeciency
virus with Venezuelan equine encephalitis virus replicon particles. J Virol.
2000;74(1):371–8.
20. Fernandez IM, Golding H, Benaissa-Trouw BJ, de Vos NM, Harmsen M,
Nottet HS, et al. Induction of HIV-1 IIIb neutralizing antibodies in BALB/c
mice by a chimaeric peptide consisting of a T-helper cell epitope of Semliki
Forest virus and a B-cell epitope of HIV. Vaccine. 1998;16(20):1936–40.
332 Biosafety in Microbiological and Biomedical Laboratories
21. Notka F, Stahl-Hennig C, Dittmer U, Wolf H, Wagner R. Construction
and characterization of recombinant VLPs and Semliki-Forest virus live
vectors for comparative evaluation in the SHIV monkey model. Biol
Chem.1999;380(3):341–52.
22. Kuhn RJ, Grin DE, Owen KE, Niesters HG, Strauss JH. Chimeric
Sindbis-Ross River viruses to study interactions between alphavirus
nonstructural and structural regions. J Virol. 1996;70(11):7900–9.
23. Schoepp RJ, Smith JF, Parker MD. Recombinant chimeric western and
eastern equine encephalitis viruses as potential vaccine candidates.
Virology. 2002;302(2):299–309.
24. Paessler S, Fayzulin RZ, Anishchenko M, Greene IP, Weaver SC, Frolov I.
Recombinant Sindbis/Venezuelan equine encephalitis virus is highly
attenuated and immunogenic. J Virol. 2003;77(17):9278–86.
25. Arroyo J, Miller CA, Catalan J, Monath TP. Yellow fever vector live-virus
vaccines: West Nile virus vaccine development. Trends Mol Med.
2001;7(8):350–4.
26. Warne SR. The safety of work with genetically modied viruses. In: Ring
CJA, Blair ED, editors. Genetically Engineered Viruses: Development and
Applications. Oxford: BIOS Scientic Publishers; 2001. p. 255–73.
27. Monath TP, McCarthy K, Bedford P, Johnson CT, Nichols R, Yoksan S, et al.
Clinical proof of principle for ChimeriVax: recombinant live, attenuated
vaccines against avivirus infections. Vaccine. 2002;20(7–8):1004–18.
28. Smithburn KC, Hughes TP, Burke AW, Paul JH. A neurotropic virus isolated
from the blood of a native of Uganda. Am J Trop Med Hyg. 1940;20:471–92.
29. Melnick JL, Paul JR, Riordan JT, Barnett VH, Goldblum N, Zabin E.
Isolation from human sera in Egypt of a virus apparently identical to West
Nile virus. Proc Soc Exp Biol Med. 1951;77(4):661–5.
30. Taylor RM, Work TH, Hurlbut HS, Rizk F. A study of the ecology of West Nile
virus in Egypt. Am J Trop Med Hyg. 1956;5(4):579–620.
31. Gerhardt R. West Nile virus in the United States (1999–2005). J Am Anim
Hosp Assoc. 2006;42(3):170–7.
32. Centers for Disease Control and Prevention. Laboratory-acquired West
Nile virus infections—United States, 2002. MMWR Morb Mortal Wkly Rep.
2002;51(50):1133–5.
33. Venter M, Burt FJ, Blumberg L, Fickl H, Paweska J, Swanepoel R. Cytokine
induction after laboratory-acquired West Nile virus infection. N Engl J Med.
2009;360(12):1260–2.
333Section VIII-F: Arboviruses and Related Zoonotic Viruses
34. Rusnak JM, Kortepeter MG, Hawley RJ, Anderson AO, Boudreau E, Eitzen E.
Risk of occupationally acquired illnesses from biological threat agents in
unvaccinated laboratory workers. Biosecur Bioterror. 2004;2(4):281–93.
35. Arrigo NC, Adams AP, Weaver SC. Evolutionary patterns of eastern equine
encephalitis virus in North versus South America suggest ecological
dierences and taxonomic revision. J Virol. 2010;84(2):1014–25.
36. Morris CD. Eastern Equine Encephalitis. In: Monath TP, editor. The Arboviruses:
Epidemiology and Ecology. Vol 3. Boca Raton: CRC Press; 1988. p. 2–20.
37. Kinney RM, Trent DW, France JK. Comparative immunological and
biochemical analyses of viruses in the Venezuelan equine encephalitis
complex. J Gen Virol. 1983;64(Pt 1):135–47.
38. Flick R, Bouloy M. Rift Valley fever virus. Curr Mol Med. 2005;5(8):827–34.
39. Imam IZ, Darwish MA. A preliminary report on an epidemic of Rift Valley
fever (RVF) in Egypt. J Egypt Public Health Assoc. 1977;52(6):417–8.
40. Francis T, Magill TP. Rift valley fever: a report of three cases of laboratory
infection and the experimental transmission of the disease to ferrets. J Exp
Med. 1935;62(3):433–48.
41. Smithburn KC, Haddow AJ, Mahay AF, Kitchen SF. Rift valley
fever; accidental infections among laboratory workers. J Immunol.
1949;62(2):213–27.
42. Linthicum KJ, Anyamba A, Tucker CJ, Kelley PW, Myers MF, Peters CJ.
Climate and satellite indicators to forecast Rift Valley fever epidemics in
Kenya. Science. 1999;285(5426):397–400.
43. Weaver SC. Host range, amplication and arboviral disease emergence.
Arch Virol Suppl. 2005;(19):33–44.
334 Biosafety in Microbiological and Biomedical Laboratories
Section VIII-G: Toxin Agents
Botulinum Neurotoxin
Seven immunologically distinct serotypes of botulinum neurotoxin (BoNT) have
been isolated (A, B, C1, D, E, F, and G), which are dened by neutralization
of toxicity using specic homologous polyclonal antibodies. Recently, two
novel BoNT have been proposed as new serotypes, but additional validation
is needed to conrm these toxins as distinct types. Each BoNT holotoxin is a
disulde-bonded heterodimer, composed of a zinc metalloprotease light chain
(approximately 50 kDa) and a heavy chain (approximately 100 kDa), which
binds with high anity to peripheral cholinergic nerve terminals and facilitates
the translocation of the catalytic light chain into the nerve terminal cytosol.
1,2
BoNT-mediated toxicity (i.e., muscle weakness and autonomic dysfunction)
results from the activity of the light chain, which cleaves soluble N-ethylmaleim-
ide-sensitive factor attachment protein receptor (SNARE) proteins, required for
neurotransmitter release. BoNTs are produced by Clostridium botulinum and rare
strains of Clostridium baratii, Clostridium butyricum, and Clostridium argentinense
as protein complexes, with one to six accessory neurotoxin-associated proteins
that stabilize the toxin in biological systems and facilitate its absorption from the
gastrointestinal tract, making BoNT highly toxic by the oral route.
1
Serotypes A, B, E and, less commonly, F are responsible for most human
poisoning through contaminated food, wound infection, or colonization of the
gastrointestinal tract. Wild animals and livestock may be at greater risk for
poisoning with serotypes B, C1, and D.
3,4
To date, no conrmed cases of human
or animal intoxication have been reported with serotype G. It is important to
recognize that all BoNT serotypes are potentially lethal by injection, aerosol
delivery, and oral ingestion. BoNT is one of the most toxic proteins known;
absorption of extremely small amounts of toxin can cause severe incapacitation
and death, depending upon the serotype and the route of exposure.
5,6
Diagnosis of Laboratory Exposures
Botulism is initially diagnosed by the presence of characteristic clinical signs and
symptoms, which are similar for all serotypes and routes of intoxication.
7
The
onset of botulism is generally preceded by a latency of several hours to days,
even with aerosol exposure. The duration of the latent period varies inversely with
the amount of toxin absorbed.
Botulism generally begins with bilateral, symmetric cranial nerve palsies that
may progress to descending accid paralysis, including respiratory failure. Signs
and symptoms generally include dysphagia, facial paralysis, ptosis, dysarthria,
diplopia, and impaired gag reex. Asymmetric cranial nerve palsies are rarely
reported.
8

335Section VIII-G: Toxin Agents
Sophisticated tests, such as nerve conduction studies and single-ber electro-
myography, can support the diagnosis of botulism and distinguish it from other
neuromuscular conditions presenting with similar symptoms, such as Guillain-
Barré Syndrome or myasthenia gravis.
7
Detection of BoNT in clinical or food
specimens conrms clinically diagnosed cases. Laboratory tests such as mouse
bioassay and mass spectrometry should be used mainly for conrmation of the
clinical diagnosis, not as a basis for initiating treatment with antitoxin. Since
individual variations in the presentation of signs have been documented, botulism
should be suspected after a potential exposure even if some of the characteristic
signs are absent.
Laboratory Safety and Containment Recommendations
Solutions of sodium hypochlorite (NaOCl, 0.1%) or sodium hydroxide (NaOH,
0.1N) readily inactivate BoNT and are recommended for decontamination of
work surfaces and for spills. Sodium hypochlorite (0.6%) also inactivates cells
and spores of BoNT-producing species of Clostridium. Sterilization in a steam
autoclave at 121°C for 30 minutes eectively inactivates BoNT and BoNT-pro-
ducing species of Clostridium, including spores. Additional considerations for the
safe use and inactivation of toxins of biological origin are found in Appendix I.
Because BoNT-producing species of Clostridium require an anaerobic
environment for growth and are essentially not transmissible among individuals,
exposure to pre-formed BoNT is the primary concern for laboratory workers.
Two of the most signicant hazards in working with BoNT and cultures of BoNT-
producing species of Clostridium are unintentional aerosol generation, especially
during centrifugation, and accidental needlestick. Although BoNT does not
penetrate intact skin, the toxin can be absorbed through broken or lacerated skin
as well as by contact with eyes and mucous membranes.
BSL-2 practices, containment equipment, and facilities including the use of
appropriate PPE (i.e., disposable gloves, laboratory coat, and eye protection)
are recommended for routine dilutions, titrations, or diagnostic studies with
materials known to contain or have the potential to contain BoNT. Activities that
may generate aerosols should be performed within a BSC (Class II). Needlesticks
can be minimized by careful arrangement of the workspace and maintaining
operational awareness at all times. Additional primary containment and personnel
precautions, such as those recommended for BSL-3, should be considered on a
case-by-case basis for activities that require handling of large quantities of toxin.
Workers in diagnostic laboratories should be aware that BoNT-producing species
of Clostridium could be stable for weeks or longer in a variety of food products,
clinical samples (e.g., feces), and environmental samples (e.g., soil). Stability of
the toxin itself will depend upon the sterility, temperature, pH, and ionic strength
of the sample matrix.
4,9,10
BoNT retains its activity for long periods (at least 6–12

336 Biosafety in Microbiological and Biomedical Laboratories
months) in a variety of frozen foods, especially under acidic conditions (pH
4.5–5.0) and/or high ionic strength, but the toxin is readily inactivated by heating
at 100°C for ten minutes.
10
A documented incident of laboratory intoxication with BoNT occurred in workers
who were performing necropsies on animals that had been exposed 24 hours
earlier to aerosolized BoNT serotype A. The laboratory workers presumably
inhaled aerosols generated from the animal fur; the report does not describe
protective precautions. The intoxications were relatively mild, and all aected
individuals recovered after a week of hospitalization.
11
Despite the low incidence
of laboratory-associated botulism, the high toxicity of BoNT necessitates that
laboratory workers exercise caution during all experimental procedures.
Personnel not directly involved in laboratory studies involving BoNT, such as
maintenance personnel, should be discouraged from entering the laboratory when
a toxin is in use, until after the work has ceased and all work surfaces have been
decontaminated (see Appendix I for additional information). Puried preparations
of toxin sub-units (e.g., isolated BoNT light chains or heavy chains) should be
handled as if contaminated with holotoxin unless proven otherwise by toxicity
bioassays. Recombinant BoNT produced in heterologous expression hosts
should be considered toxic and handled with equal precautionary measures as
endogenously produced BoNT.
Special Issues
Vaccines There are currently no approved vaccines for BoNT. A pentavalent
(serotypes A, B, C, D, and E) botulinum toxoid vaccine was available through
the CDC as an investigational new drug (IND) until 2011, but it was discontinued
due to a decline in immunogenicity of some of the serotypes and an increase
in occurrence of moderate local reactions. Vaccine candidates are currently in
clinical trials.
12
Treatment Hospitalization is usually required, and respiratory support may be
necessary for severe botulism. In 2013, FDA approved an antitoxin designated
as Botulism Antitoxin Heptavalent (A, B, C, D, E, F, G)—(Equine), BAT
®
for the
treatment of botulism in adult and pediatric patients. BAT
®
is currently the only
approved specic treatment for botulism and can eectively neutralize each of the
seven known serotypes of BoNT. BAT
®
, manufactured by Emergent BioSolutions
(formally Cangene), can decrease the severity of intoxication by neutralizing
BoNT that remains in the bloodstream.
13
BAT
®
is available from the U.S. Strategic
National Stockpile (SNS) and is supplied by the Oce of the Assistant Secretary
for Preparedness and Response (ASPR). BabyBIG
®
(Botulism Immune Globulin)
is available for infant botulism through the California Infant Botulism Treatment
and Prevention Program.

337Section VIII-G: Toxin Agents
Select Agents and Toxins BoNT and BoNT-producing species of Clostridium
have the potential to pose a severe threat to human health and are therefore
included on the HHS list of Tier 1 Select Agents and Toxins. Entities that possess,
use, store, or transfer BoNT-producing species of Clostridium are required to be
registered with the Federal Select Agent Program (FSAP). Entities that intend
to possess, use, store, or transfer quantities of BoNT above the permissible
amount are also required to be registered with FSAP. See Appendix F for more
information.
Transfer of Agent Domestic transfer or importation of BoNT-producing species
of Clostridium or BoNT above the permissible amount require prior approval from
FSAP. A DoC permit may be required for the export of these agents and toxin to
another country. See Appendix C for additional information.
Staphylococcal Enterotoxins (SE)
Staphylococcal Enterotoxins (SE) are a group of closely related extracellular
protein toxins of 22 to 29 kD molecular weight that are produced by distinct gene
clusters found in a wide variety of S. aureus strains.
14–16
SE belong to a large
family of homologous pyrogenic exotoxins from staphylococci, streptococci,
and mycoplasma, which are capable of causing a range of illnesses in humans
through pathological amplication of the normal T-cell receptor response,
cytokine/lymphokine release, immunosuppression, and endotoxic shock.
15,17
Classic SE include ve serotypes A–E (SEA, SEB, SEC, SED, and SEE, respec-
tively), but genomic analysis has further identied and characterized previously
unrecognized SE, such as serotype H (SEH), that has been linked to foodborne
incidents.
18,19
Symptoms from SE may vary with the exposure route and dose. SEA is a
common cause of severe gastroenteritis in humans.
20–22
In cases from accidental
food poisoning, it is estimated that gastric exposure to as little as 0.05–1 µg of
SEA causes incapacitating illness.
23–27
Comparative human toxicity for dierent
serotypes of SE is largely unknown, but human volunteers exposed to 20–25 µg
of SE serotype B (SEB) experienced enteritis similar to that caused by SEA.
28
SE are highly toxic by intravenous and inhalation routes of exposure, with lethal
doses causing death in NHPs mainly due to shock and/or pulmonary edema.
29–33
By inference from accidental exposure of laboratory workers and controlled
experiments with NHPs, it is estimated that inhalation of less than 1 ng/kg can
incapacitate more than 50% of exposed humans and that the inhalation LD
50
in
humans may be as low as 20 ng/kg for SEB.
34
Exposure of mucous membranes to SEB in a laboratory setting or in clinical
studies has been reported to cause conjunctivitis and localized cutaneous
swelling, with some laboratory workers also experiencing incapacitating
338 Biosafety in Microbiological and Biomedical Laboratories
gastrointestinal symptoms.
35–37
Intradermal or dermal exposure to concentrated
SE solutions or patch tests (≥ 1μg/cm
2
) has resulted in erythema, induration, or
dermatitis.
36–39
Diagnosis of Laboratory Exposures
Diagnosis of SE intoxication is based on clinical and epidemiologic features.
Gastric intoxication with SE begins rapidly after exposure (generally 1 to 6
hours) and is characterized by nausea, vomiting, and abdominal cramps; it is
often accompanied by diarrhea, but generally occurs without a high fever.
23,31
At higher exposure levels, intoxication progresses to hypovolemia, dehydration,
vasodilatation in the kidneys, and lethal shock.
21
While fever is uncommon after
SE ingestion, inhalation of SE commonly results in an acute febrile illness. After a
latent period of 3 to 12 hours (range 1.5 to 18 hours), inhalation of SEB results in
rapid onset of illness, generally characterized by high fever (range often 103⁰ to
105⁰F), chills, headache, malaise, myalgia, and a non-productive cough.
35
Some
individuals may develop retrosternal chest pain and dyspnea. Severe cases
may develop pulmonary edema or acute respiratory distress syndrome (ARDS).
Inhalational SEB intoxication may also be associated with upper respiratory
tract signs and symptoms (e.g., sore throat, rhinorrhea, sinus congestion, and/
or profuse postnasal drip), conjunctival injection, and/or pharyngeal erythema.
35,37
GI symptoms may also occur after SEB inhalation. Symptoms from SE ingestion
usually resolve in 24 to 48 hours, and it is rarely fatal. Symptoms from SEB
inhalation due to laboratory exposures generally persist for a duration of 2 to 5
days, but the cough may persist for up to four weeks.
40
Nonspecic laboratory
ndings in inhalational SEB include a neutrophilic leukocytosis. WBC counts
are often >10,000 cells/mm
3
and have ranged from 8,000 to 28,000 cells/mm
3
.
The chest X-ray is often normal but may show abnormalities consistent with
pulmonary edema in severe cases.
40
Dierential diagnosis of SE inhalation may be unclear initially because the
symptoms are similar to disease caused by several respiratory pathogens
(e.g., inuenza, adenovirus, and mycoplasma). However, naturally occurring
pneumonia or inuenza typically involve symptoms presenting over a more
prolonged interval of time, whereas SE intoxication tends to involve symptoms
that rapidly plateau. Unrecognized SEB exposure has often been initially misdi-
agnosed as community-acquired pneumonia, with SEB exposure suspected only
after onset of illness in other at-risk laboratory workers within a 12-hour period.
34
Laboratory conrmation of intoxication includes SE detection by immunoassay
of environmental and clinical samples and gene amplication to detect staphylo-
coccal genes in environmental samples.
24,41,42,43
SE may be undetectable in the
serum at the time symptoms occur; nevertheless, a serum specimen should be
drawn as early as possible after exposure. Data from animal studies suggest the
presence of SE in the serum or urine is transient.
44
Respiratory secretions and

339Section VIII-G: Toxin Agents
nasal swabs may demonstrate the toxin within 24 hours of inhalation exposure.
Evaluation of neutralizing antibody titers in acute and convalescent sera of
exposed individuals can be undertaken, but it may yield false positives resulting
from pre-existing antibodies produced in response to natural SE exposure.
40
Laboratory Safety and Containment Recommendations
General considerations for the safe use and inactivation of toxins of biological
origin are found in Appendix I. Inhalational exposure, mucous membrane
exposure (via aerosol or droplet exposure or direct contact with contaminated
gloves), accidental ingestion, and parenteral inoculation are believed to be the
primary hazards of SE for laboratory and animal-care personnel.
24,27,35
SE are
relatively stable, monomeric proteins, readily soluble in water, and resistant to
proteolytic degradation, temperature uctuations, and low pH conditions. The
physical/chemical stability of SE suggests that additional care must be taken by
laboratory workers to avoid exposure to residual toxin that may persist in the
environment.
Active SE toxins may be present in clinical samples, lesion uids, respiratory
secretions, fur, or tissues of exposed animals. Additional care should be taken
during cage cleaning and the necropsy of exposed animals and in the handling
of clinical stool samples because SE toxins retain toxic activity throughout the
digestive tract.
Accidental laboratory exposures to SEB have been reviewed.
35
Documented
accidents included inhalation of SE aerosols generated from pressurized
equipment failure and re-aerosolization of residual toxin from the fur of exposed
animals. The most common cause of laboratory intoxication with SE is currently
expected to result from accidental self-exposure via the mucous membranes by
touching contaminated hands or gloves to the face or eyes.
BSL-2 practices, containment equipment, and facilities should be used when
handling SE or potentially contaminated material. Because SE is highly active
by the oral or ocular exposure route, the use of a laboratory coat, gloves, and
safety glasses is mandatory when handling toxin or toxin-contaminated solutions.
Frequent, careful handwashing and laboratory decontamination should be
strictly enforced when working with SE. Depending upon a risk assessment of
the laboratory operation, the use of a face mask and goggles may be required
to avoid ocular and oropharyngeal exposure due to inadvertent touching of the
face and mucous membranes with contaminated gloves. Additional primary
containment and personnel precautions, such as those recommended for BSL-3
(e.g., respirator), should be considered on a case-by-case basis for activities with
a high potential for aerosol or droplet production and those involving the use of
large quantities of SE.

340 Biosafety in Microbiological and Biomedical Laboratories
Special Issues
Vaccines No approved vaccine or specic antidote is currently available for
human use, but experimental, recombinant vaccines are under development.
Select Agents and Toxins SEA, SEB, SEC, SED, and SEE are included in the
HHS Select Agents and Toxins List. Entities that intend to possess, use, store
or transfer quantities of SE above the permissible amount are required to be
registered with FSAP. See Appendix F for more information.
Transfer of Agent Domestic transfer or importation of SE above the permissible
amount requires prior approval from FSAP. A DoC permit may be required for the
export of this agent to another country. See Appendix C for additional information.
Ricin
Ricin is produced in maturing seeds of the castor plant Ricinus communis L.,
which has been recognized for centuries as a highly poisonous plant for humans
and livestock.
45
The castor seed contains castor oil, an important chemical
feedstock for lubricants, polyamides, polyurethanes, plasticizers, and cosmetics,
but also contains as much as 6% ricin and Ricinus communis agglutinin (w/w).
46
Thus, processing castor seed for castor oil results in a seed meal that is a
crude form of ricin. Ricin belongs to a family of type 2 ribosome-inactivating
proteins (RIPs) from plants, including abrin, modeccin, and viscumin, that share
a similar overall structure and mechanism of action.
47
The ricin holotoxin is a
disulde-bonded heterodimer composed of an A-chain (approximately 34 kD
polypeptide) and a B-chain (approximately 32 kD). The A-chain is an N-glyco-
sidase enzyme that removes a specic adenine base from the 28S ribosomal
RNA, resulting in loss of protein synthesis by inactivation of the ribosome.
The B-chain is a relatively non-toxic lectin that facilitates toxin binding and
internalization through interaction with glycolipids and glycoproteins that line
the surface of the target cell.
45
The Ricinus communis agglutinin (RCA
120
) is a
tetramer composed of 2 A-chains and 2 B-chains that are homologous to ricin
A-chain (93%) and B-chain (84%) at the protein sequence level.
48
There are
monoclonal antibodies that distinguish ricin from RCA
120
and comparisons among
dierent castor cultivars indicate ricin content exceeds that of RCA
120
by a factor
of 2.5–3.
49
As isolated from the seed, ricin is composed of various glycosylated
forms and isoforms.
50
Ricin is much less toxic by weight than BoNT or SE, and published case reports
suggest that gastric ingestion of ricin is rarely fatal in adults, with ingestion of
castor beans the common route for gastric exposure.
51
Animal studies and human
poisonings suggest that the eects of ricin depend upon the route of exposure,
with inhalation and intravenous exposure being the most toxic. In laboratory mice,
the LD
50
has been estimated as 3 to 5 μg/kg by inhalation, 5 μg/kg by intravenous
injection, 22 μg/kg by intraperitoneal injection, 24 μg/kg by subcutaneous
341Section VIII-G: Toxin Agents
injection, and 20 mg/kg by intragastric administration.
52
Before more stringent
safety precautions were introduced, workers in castor oil processing plants and
nearby residents were exposed to dust from the seed meal. While there were very
few reported deaths from ricin exposure, severe allergic responses including skin
reactions and asthma were common.
53
The human lethal dose has not been established rigorously but is estimated
at 5–10 µg/kg by injection, intramuscular or intravenous, and 5–10 µg/kg by
inhalation.
54
The RCA
120
is considerably less toxic than ricin, with 300 times as
much RCA
120
needed to kill 50% of Vero cells in a cell toxicity study.
50
Diagnosis of Laboratory Exposures
The primary diagnosis is through clinical signs and symptoms that vary greatly
depending upon the route of exposure. Following inhalation exposure, symptoms
may appear within eight hours and include cough, labored respiration, and fever,
which may progress to respiratory distress and death.
55
Most of the pathology
occurs in the upper and lower respiratory tract, including inammation, bloody
sputum, and pulmonary edema. Toxicity from ricin inhalation will progress despite
treatment with antibiotics, as opposed to a treatable bacterial infection. There is
no mediastinitis as seen with inhalation anthrax. Ricin patients will not plateau
clinically as occurs after inhalation of SEB.
Gastric ingestion of ricin causes nausea, vomiting, diarrhea, abdominal cramps,
and dehydration. Initial symptoms may appear more rapidly following gastric
ingestion (1–5 hours) but generally require exposure to much higher levels of
toxin compared with the inhalation route. Following injection of ricin, symptoms
may appear within six hours and include nausea, vomiting, anorexia, and high
fever. The site of ricin injection typically shows signs of inammation with marked
swelling and induration. One case of poisoning by ricin injection resulted in fever,
vomiting, irregular blood pressure, and death by vascular collapse after a period
of several days; it is unclear in this case if the toxin was deposited intramuscularly
or in the bloodstream.
56
After aerosol exposure to ricin, additional supportive clinical or diagnostic features
may include the following: bilateral inltrates on chest radiographs, arterial
hypoxemia, neutrophilic leukocytosis, and a bronchial aspirate rich in protein.
52
Numerous methods for detecting and quantifying ricin have been developed.
Specic immunoassay of serum and respiratory secretions, immunohistochemical
stains of tissue, or detection of the castor seed alkaloid ricinine in urine may be
used to conrm a diagnosis.
57
An immuno-PCR method is able to detect pg/ml of
ricin in sera and feces of intoxicated mice.
58
PCR can detect residual castor bean
DNA in most ricin preparations. Likewise, ELISA, mass spectrometry techniques,
and cell viability assays are amongst the most common assays used to detect

342 Biosafety in Microbiological and Biomedical Laboratories
ricin from contaminated samples.
59
Ricin is an extremely immunogenic toxin,
and paired acute and convalescent sera should be obtained from survivors for
measurement of antibody response.
Laboratory Safety and Containment Recommendations
General considerations for the safe use and inactivation of toxins of biological
origin are found in Appendix I. Precautions should be extended to handling
potentially contaminated clinical, diagnostic, and post-mortem samples because
ricin may retain toxicity in the lesion uids, respiratory secretions, or unxed
tissues of exposed animals.
When the ricin A-chain is separated from the B-chain and administered paren-
terally to animals, its toxicity is diminished by >1,000-fold compared with ricin
holotoxin.
60
However, puried preparations of natural ricin A-chain or B-chain and
crude extracts from castor beans should be handled as if contaminated by ricin
until proven otherwise by bioassay.
Ricin is a relatively non-specic cytotoxin and irritant that should be handled in
the laboratory as a non-volatile toxic chemical. Based upon animal studies, the
inhalation of air-borne dust particles or small liquid droplets carrying ricin into the
lungs is still considered the most dangerous route of exposure. BSL-2 practices,
containment equipment, and facilities are recommended, including laboratory
coat, gloves, and eye protection, when handling ricin toxin or potentially contam-
inated materials. A full-face respirator should be worn if there is a potential for
creating a toxin aerosol. A BSC is used if there is any chance that ricin aerosols
will be generated. Solutions of ricin can be inactivated by treatment with sodium
hypochlorite bleach, and crude ricin powder is inactivated by autoclaving with
calcium oxide (lime).
Special Issues
Vaccines No approved vaccine or specic antidote is currently available for
human use, but experimental, recombinant vaccines are under development.
There is at least one commercial ricin vaccine in Phase 1 clinical trials.
61
Select Agents and Toxins Ricin is included in the HHS list of Select Agents
and Toxins. Entities that intend to possess, use, store or transfer quantities of
ricin above the permissible amount are required to be registered with FSAP. See
Appendix F for more information.
Transfer of Agent Domestic transfer or importation of ricin above the permissible
amount requires prior approval from FSAP. A DoC permit may be required for the
export of this agent to another country. See Appendix C for additional information.
343Section VIII-G: Toxin Agents
Selected Low Molecular Weight (LMW) Toxins
Low Molecular Weight (LMW) Toxins comprise a structurally and functionally
diverse class of natural poisons, ranging in size from several hundred to a few
thousand daltons. LMW toxins include complex organic structures and disulde
cross-linked and cyclic polypeptides. Tremendous structural diversity may occur
within a particular type of LMW toxin, often resulting in incomplete toxicological
or pharmacological characterization of minor isoforms. Grouping LMW toxins
together has primarily been a means of distinguishing them from protein toxins
with respect to key biophysical characteristics. Compared with proteins, the
LMW toxins are of smaller size, which alters properties such as ltration and
distribution; are generally more stable and persistent in the environment; and
some compounds may exhibit poor water-solubility necessitating the use of
organic solvents. These characteristics pose special challenges for safe handling,
containment, and decontamination of LMW toxins within the laboratory.
The set of LMW toxins selected for discussion herein are employed routinely
as laboratory reagents and/or have been designated as potential public health
threats by the CDC, including: T-2 mycotoxin, produced by Fusarium fungi;
62,63
saxitoxin and related paralytic shellsh poisons, produced by select marine
dinoagellates within the genus Alexandrium, Gymnodinium, and Pyrodinium, as
well as certain freshwater cyanobacteria;
64
tetrodotoxin from a number of marine
animals;
65
brevetoxins from the dinoagellate Karenia brevis;
66
palytoxins from
select marine coelenterates belonging to the genus Palythoa and from marine
dinoagellates belonging to the genus Ostreopsis;
67,68
polypeptide conotoxins
α-GI (includes GIA) and α-MI from the Conus genus of gastropod mollusks;
69
the amino acid analog domoic acid from select marine diatoms from the genus
Pseudo-nitzschia;
70
and the monocyclic polypeptide microcystins from select
freshwater cyanobacteria such as Microcystis aeruginosa.
71
Trichothecene mycotoxins comprise a broad class of structurally complex,
non-volatile sesquiterpene compounds that are potent inhibitors of protein
synthesis.
62,63
Mycotoxin exposure occurs by consumption of moldy grains, and
at least one of these toxins, designated T-2, has been implicated as a potential
biological warfare agent.
63
T-2 is a lipid-soluble molecule that can be absorbed
into the body rapidly through exposed mucosal surfaces.
72
Toxic eects are most
pronounced in metabolically active target organs and include emesis, diarrhea,
weight loss, nervous disorder, cardiovascular alterations, immunodepression,
hemostatic derangement, bone marrow damage, skin toxicity, decreased
reproductive capacity, and death.
63
The LD
50
for T-2 in laboratory animals ranges
from 0.2 to 10 mg/kg, depending on the route of exposure, with aerosol toxicity
estimated to be 20 to 50 times greater than parenteral exposure.
63
Of special
note, T-2 is a potent vesicant capable of directly damaging skin or corneas. Skin
lesions, including frank blisters, have been observed in animals with local, topical
application of 50 to 100 ng of toxin.
63,72
344 Biosafety in Microbiological and Biomedical Laboratories
Saxitoxin and tetrodotoxin are paralytic marine alkaloid toxins that interfere
with normal function of voltage-activated sodium channels in excitable cells of
heart, muscle, and neuronal tissue by blocking ion ow, causing potentially lethal
paralytic shellsh poisoning and puersh poisoning, respectively.
73
Animals
exposed to 1–10 µg/kg of either of these toxins by parenteral routes typically
develop a rapid onset of excitability, muscle spasm, and respiratory distress;
death may occur within 10–15 minutes in extreme cases from respiratory
paralysis.
64,74
Humans ingesting seafood contaminated with saxitoxin or tetrodo-
toxin show similar signs of toxicity, typically preceded by paresthesias of the lips,
face, and extremities.
73,75
Brevetoxins are ladder-frame-polyether, shellsh neurotoxins produced by marine
dinoagellates that accumulate in lter-feeding mollusks and cause non-lethal
human intoxications from ingestion of contaminated seafood, known as neuro-
toxic shellsh poisoning, or by respiratory irritation from sea spray containing
the toxins.
73
This toxin group lowers the activation potential in voltage-activated
sodium channels resulting in channel opening at normal resting membrane
potentials, eectively making the sodium channel of aected nerve or muscle
cells hyper-excitable. Symptoms of human ingestion include paresthesias of
the face, throat, and ngers or toes, followed by dizziness, chills, muscle pains,
nausea, gastroenteritis, and clinical signs including reduced heart rate. Brevetoxin
has a parenteral LD
50
of 200 µg/kg in mice and guinea pigs. Guinea pigs exposed
to a slow infusion of brevetoxin develop fatal respiratory failure within 30 minutes
of exposure to 20 µg/kg toxin.
74
Palytoxin, and related toxins such as ovatoxins, are structurally complex,
articulated fatty alcohols associated with certain colonial anemones such as
Palythoa toxica and select marine dinoagellates of the genus Ostreopsis.
67
This
toxin group is capable of binding and converting the essential cellular Na+/K+
pump into a non-selective cation channel.
68,76
Palytoxin is among the most potent
coronary vasoconstrictors known, killing animals within minutes by cutting o
oxygen to the myocardium.
77
Symptoms in aected individuals can vary based
on the route of exposure and may include rhabdomyolysis due to consumption
of contaminated seafood, respiratory distress, and fever from inhalation of
aerosolized toxins, and skin and ocular irritation from topical exposure.
67,78
The
LD
50
for intravenous administration ranges from 0.025 to 0.45 µg/kg in dierent
species of laboratory animals.
77
Palytoxin is lethal by several parenteral routes but
is about 200-fold less toxic if administered to the alimentary tract (oral or rectal)
compared with intravenous administration.
77
Palytoxin causes corneal damage
and can cause irreversible blindness at topically applied levels of approximately
400 ng/kg, despite extensive rinsing after ocular instillation.
77
Like brevetoxins,
palytoxins cause respiratory irritation from exposure to marine aerosols when the
345Section VIII-G: Toxin Agents
causative dinoagellates are present in high numbers, but unlike brevetoxins,
palytoxins are also associated with u-like symptoms with high fever.
78
Conotoxins are polypeptides, typically 10–30 amino acids long and stabilized by
distinct patterns of disulde bonds that have been isolated from the toxic venom
of marine snails and shown to be neurologically active or toxic in mammals.
69
Of the estimated >105 dierent polypeptides (conopeptides) present in venom
of over 500 known species of Conus, only a few have been rigorously tested for
animal toxicity. Of the isolated conotoxin subtypes that have been analyzed, at
least two post-synaptic paralytic toxins, designated α-GI (includes GIA) and α-MI,
have been reported to be toxic in laboratory mice with LD
50
values in the range
of 10–100 µg/kg depending upon the species and route of exposure. Workers
should be aware that human toxicity of whole or partially fractionated Conus
venom, as well as synthetic combinations of isolated conotoxins, may exceed that
of individual components. For example, untreated cases of human poisoning with
venom of C. geographus result in an approximately 70% fatality rate, probably as
a result of the presence of mixtures of various α- and µ-conotoxins with common
or synergistic biological targets.
69,79
The α-conotoxins act as potent nicotinic
antagonists, and the µ-conotoxins block the sodium channel.
69
Symptoms of
envenomation depend upon the Conus species involved, generally occur rapidly
after exposure (minutes), and range from severe pain to spreading numbness.
80
Severe intoxication results in muscle paralysis, blurred or double vision, diculty
breathing and swallowing, and respiratory or cardiovascular collapse.
80
Domoic acid is a kainic acid analog neurotoxin that causes amnesic shellsh
poisoning after the consumption of contaminated seafood. Domoic acid has a
high anity for glutamate receptors in the hippocampus resulting in excitotoxicity
and neuronal degeneration.
81
Symptoms of exposure include vomiting, nausea,
diarrhea and abdominal cramps, headache, dizziness, confusion, disorientation,
short-term memory loss, motor weakness, seizures, cardiac arrhythmias, and
coma with possible death in extreme cases.
Microcystins (also called cyanoginosins) are monocyclic heptapeptides composed
of specic combinations of L- and D-amino acids, some with uncommon side
chain structures, that are produced by various freshwater cyanobacteria.
82
The
toxins are potent inhibitors of liver protein phosphatase type 1 and are capable
of causing massive hepatic hemorrhage and death.
82
One of the more potent
toxins in this family, microcystin-LR, has a parenteral LD
50
of 30 to 200 µg/kg in
rodents.
71
Exposure to microcystin-LR causes animals to become listless and
prone in the cage; death occurs in 16 to 24 hours. The toxic eects of microcystin
vary depending upon the route of exposure and may include hypotension and
cardiogenic shock, in addition to hepatotoxicity.
71,83

346 Biosafety in Microbiological and Biomedical Laboratories
Diagnosis of Laboratory Exposures
LMW toxins are a diverse set of molecules with a correspondingly wide range
of signs and symptoms of laboratory exposure, as discussed above for each
toxin. Common symptoms can be expected for LMW toxins with common
mechanisms of action. For example, several paralytic marine toxins that interfere
with normal sodium channel function cause rapid paresthesias of the lips, face,
and digits after ingestion. The rapid onset of illness or injury (minutes to hours)
generally supports a diagnosis of chemical or LMW toxin exposure. Painful skin
lesions may occur almost immediately after contact with T-2 mycotoxin, and
ocular irritation or lesions will occur in minutes to hours after contact with T-2 or
palytoxin.
Specic diagnosis of LMW toxins in the form of a rapid diagnostic test is not
presently available in the eld. Serum and urine should be collected for testing
at specialized reference laboratories by methods including antigen detection,
receptor-binding assays, or liquid chromatographic analyses of metabolites.
Parent compounds and metabolites of several marine and freshwater toxins,
including saxitoxin, tetrodotoxin, domoic acid, brevetoxins, and microcystins are
well-studied as part of routine regulation of food and water supplies.
73
Likewise,
T-2 mycotoxin absorption and distribution in the body has been studied, and its
metabolites can be detected as late as 28 days after exposure.
63
Marine toxins
are highly stable in food and are typically not aected by cooking or freezing.
Once consumed, most marine toxins are metabolized and rapidly excreted
through the urine, in some cases, such as saxitoxin, tetrodotoxin, and domoic
acid, within 24–72 hours.
81,84
In contrast, freshwater microcystins bind covalently
to target protein phosphatases in the liver, making analysis of clinical samples
dicult even in postmortem analysis of livestock that died from suspected micro-
cystin contamination of drinking water.
85
Clinical specimens can include blood,
urine, lung, liver, and stomach contents. Few clinical tests have been validated
for these toxins. Far more methods are available for the testing of environmental
or food samples including a variety of screening and conrmatory techniques,
depending on the toxin.
Laboratory Safety and Containment Recommendations
General considerations for the safe use and inactivation of toxins of biological
origin are found in Appendix I. Ingestion, parenteral inoculation, skin and eye
contamination, and droplet or aerosol exposure of mucous membranes are the
primary hazards to laboratory and animal care personnel. LMW toxins also can
contaminate food sources or small-volume water supplies. Additionally, the T-2
mycotoxin is a potent vesicant and requires additional safety precautions to
prevent contact with exposed skin or eyes. Palytoxin also is highly toxic by the
ocular route of exposure.

347Section VIII-G: Toxin Agents
In addition to their high toxicity, the physical and chemical stability of the LMW
toxins contributes to the risks involved in handling them in the laboratory
environment. Unlike many protein toxins, the LMW toxins can contaminate
surfaces as a stable, dry lm that may pose an essentially indenite contact threat
to laboratory workers. Special emphasis, therefore, must be placed upon proper
decontamination of work surfaces and equipment.
86
When handling LMW toxins or potentially contaminated material, BSL-2 practices,
containment equipment, and facilities are recommended, especially the wearing
of a laboratory coat, safety glasses, and disposable gloves; the gloves must be
impervious to organic solvents or other diluents employed with the toxin.
The use of respiratory protection is considered if there is potential for aerosol-
ization of the toxin. A BSC (Class II, Type B1 or B2) or a chemical fume hood
equipped with exhaust HEPA lters are also indicated for activities with a potential
for aerosol, such as powder samples, and/or the use of large quantities of toxin.
For LMW toxins that are not easily decontaminated with bleach solutions, it
is recommended to use pre-positioned, disposable liners for laboratory work
surfaces to facilitate clean-up and decontamination.
Special Issues
Vaccines No approved vaccines are currently available for human use. Experi-
mental therapeutics for LMW toxins have been reviewed.
87
Select Agents and Toxins Some LMW toxins are listed as Select Agents
and Toxins. Entities that intend to possess, use, store or transfer quantities of
regulated LMW toxins above their permissible amount are required to be regis-
tered with FSAP. See Appendix F for more information.
Transfer of Agent Domestic transfer or importation of regulated LMW toxins
above their permissible amount requires prior approval from FSAP. A DoC permit
may be required for the export of this agent to another country. See Appendix C
for additional information.
References
1. Pirazzini M, Rossetto O, Eleopra R, Montecucco C. Botulinum neurotoxins:
biology, pharmacology, and toxicology. Pharmacol Rev. 2017;69(2):200–35.
2. Simpson L. The life history of a botulinum toxin molecule. Toxicon
2013;68:40–59
3. Gangarosa EJ, Donadio JA, Armstrong RW, Meyer KF, Brachman PS,
Dowell VR. Botulism in the United States, 1899–1969. Am J Epidemiol.
1971;93(2):93–101.
348 Biosafety in Microbiological and Biomedical Laboratories
4. Hatheway CL. Botulism. In: Balows A, Hausler WJ, Ohashi M, Turano
A, editors. Laboratory Diagnosis of Infectious Diseases: Principles and
Practice. Vol. 1. New York: Springer-Verlag; 1988. p. 111–33.
5. Adler M, Franz DR. Toxicity of Botulinum Neurotoxin by Inhalation:
Implications in Bioterrorism. In: Salem H, Katz SA, editors. Aerobiology:
The Toxicology of Airborne Pathogens and Toxins. Cambridge: The Royal
Society of Chemistry Press; 2016. p. 167–82.
6. Johnson EA, Montecucco C. Botulism. In: Engel AG, editor. Handbook of
Clinical Neurology. Vol. 91. Elsevier; 2008. p. 333–68.
7. Shapiro RL, Hatheway C, Swerdlow DL. Botulism in the United States:
a clinical and epidemiologic review. Ann Intern Med. 1998;129(3):221–8.
8. Filozov A, Kattan JA, Jitendranath L, Smith CG, Lúquez C, Phan QN, et al.
Asymmetric Type F botulism with cranial nerve demyelination. Emerg Infect
Dis. 2012;18(1):102–4.
9. Woolford AL, Schantz EJ, Woodburn M. Heat inactivation of botulinum
toxin type A in some convenience foods after frozen storage. J Food Sci.
1978;43(2):622–4.
10. Siegel LS. Destruction of botulinum toxins in food and water. In: Hauschild
AHW, Dodds KL. Clostridium botulinum: Ecology and Control in Foods. New
York: Marcel Dekker; 1993. p. 323–42.
11. Holzer E. Botulismus durch inhalation. Med Klin. 1962;41:1735–40. German.
12. Webb RP, Smith LA. What next for vaccine development? Expert Rev
Vaccines. 2013;12(5):481–92.
13. Yu PA, Lin NH, Mahon BE, Sobel J, Yu Y, Mody RK, et al. Safety and
Improved Clinical Outcomes in Patients Treated With New Equine-Derived
Heptavalent Botulinum Antitoxin. Clin Infect Dis. 2017;66(suppl_1):S57–S64.
14. Argudin MA, Mendoza MC, Rodicio MR. Food poisoning and
Staphylococcus aureus Enterotoxins. Toxins (Basel). 2010;2(7):1751–73.
15. Jarraud S, Peyrat MA, Lim A, Tristan A, Bes M, Mougel C, et al. egc, a
highly prevalent operon of enterotoxin gene, forms a putative nursery of
superantigens in Staphylococcus aureus. J Immunol. 2001;166(1):669–77.
Erratum in: J Immunol. 2001;166(6):following 4259.
16. Llewelyn M, Cohen J. Superantigens: microbial agents that corrupt
immunity. Lancet Infect Dis. 2002;2(3):156–62.
17. Marrack P, Kappler J. The Staphylococcal enterotoxins and their
relatives. Science. 1990;248(4956):705–11. Erratum in: Science.
1990;248(4959):1066.
349Section VIII-G: Toxin Agents
18. Jørgensen HJ, Mathisen T, Løvseth A, Omoe K, Qvale KS, Loncarevic
S. An outbreak of staphylococcal food poisoning caused by enterotoxin
H in mashed potato made with raw milk. FEMS Microbiol Lett.
2005;252(2):267–72.
19. Ikeda T, Tamate N, Yamaguchi K, Makino S. Mass Outbreak of Food
Poisoning Disease Caused by Small Amounts of Staphylococcal
Enterotoxins A and H. Appl Environ Microbiol. 2005;71(5):2793–5.
20. Balaban N, Rasooly A. Staphylococcal enterotoxins. Int J Food Microbiol.
2000;61(1):1–10.
21. Jett M, Ionin B, Das R, Neill R. The Staphylococcal Enterotoxins. In:
Sussman M, editor. Molecular Medical Microbiology. Vol. 2. San Diego:
Academic Press; 2002. p. 1089–116.
22. Pinchuk IV, Beswick EJ, Reyes VE. Staphylococcal enterotoxins. Toxins
(Basel). 2010;2(8):2177–97.
23. Asao T, Kumeda Y, Kawai T, Shibata T, Oda H, Haruki K, et al. An extensive
outbreak of staphylococcal food poisoning due to low-fat milk in Japan:
estimation of enterotoxin A in the incriminated milk and powdered skim milk.
Epidemiol Infect. 2003;130(1):33–40.
24. Bergdoll MS. Enterotoxins. In: Montie TC, Kadis S, Ajl SJ, editors. Microbial
toxins: bacterial protein toxins. Vol. 3. New York: Academic Press; 1970.
p. 265–326.
25. Do Carmo LS, Cummings C, Linardi VR, Dias RS, De Souza JM, De Sena
MJ, et al. A case study of a massive staphylococcal food poisoning incident.
Foodborne Pathog Dis. 2004;1(4):241–6.
26. Evenson ML, Hinds MW, Bernstein RS, Bergdoll MS. Estimation of
human dose of staphylococcal enterotoxin A from a large outbreak of
staphylococcal food poisoning involving chocolate milk. Int J Food Microbiol.
1988;7(4):311–6.
27. Hennekinne JA, De Buyser ML, Dragacci S. Staphylococcus aureus and its
food poisoning toxins: characterization and outbreak investigation. FEMS
Microbiol Rev. 2012;36(4):815–36.
28. Raj HD, Bergdoll MS. Eect of enterotoxin B on human volunteers.
J Bacteriol. 1969;98(2):833–4.
29. Finegold MJ. Interstitial pulmonary edema. An electron microscopic
study of the pathology of entertoxemia in Rhesus monkeys. Lab Invest.
1967;16(6):912–24.
30. Hodoval LF, Morris EL, Crawley GJ, Beisel WR. Pathogenesis of lethal
shock after intravenous staphylococcal enterotoxin B in monkeys. Appl
Microbiol. 1968;16(2):187–92.
350 Biosafety in Microbiological and Biomedical Laboratories
31. Krakauer T, Stiles BG. The staphylococcal enterotoxin (SE) family: SEB and
siblings. Virulence. 2013;4(8):759–73
32. Mattix ME, Hunt RE, Wilhelmsen CL, Johnson AJ, Baze WB. Aerosolized
staphylococcal enterotoxin B-induced pulmonary lesions in Rhesus
monkeys (Macaca mulatta). Toxiciol Pathol. 1995;23(3):262–8.
33. Weng CF, Komisar JL, Hunt RE, Johnson AJ, Pitt ML, Ruble DL, et al.
Immediate responses of leukocytes, cytokines and glucocorticoid hormones
in the blood circulation of monkeys following challenge with aerosolized
staphylococcal enterotoxin B. Int Immunol. 1997;9(12):1825–36.
34. LeClaire RD, Pitt MLM. Biological Weapons Defense: Eect Levels. In:
Lindler LE, Lebeda FJ, Korch GW, editors. Biological Weapons Defense:
Infectious Diseases and Counterbioterrorism. Totowa (NJ): Humana Press,
Inc.; 2005. p. 41–61.
35. Rusnak JM, Kortepeter M, Ulrich R, Poli M, Boudreau E. Laboratory
exposures to Staphylococcal enterotoxin B. Emerg Infect Dis.
2004;10(9):1544–9.
36. Strange P, Skov L, Lisby S, Nielsen PL, Baasgaard O. Staphylococcal
enterotoxin B applied on intact normal and intact atopic skin induces
dermatitis. Arch Dermatol. 1996;132(1):27–33.
37. Wedum AG. The Detrick experience as a guide to the probable ecacy of
P4 microbiological containment facilities for studies in microbial recombinant
DNA molecules. ABSA. 1996;1(1):7–25.
38. Rusnak JM, Kortepeter MG. Ocular and percutaneous exposures to
staphylococcal enterotoxins A and B manifested as conjunctivitis with
periocular swelling and gastrointestinal symptoms or localized skin lesions.
International Conference on Emerging and Infectious Diseases 2004:
Program and Abstracts Book; 2004 Feb 29–Mar 3; Atlanta, GA. 2004.
39. Scheuber PH, Golecki JR, Kickhofen B, Scheel D, Beck G, Hammer DK.
Skin reactivity of unsensitized monkeys upon challenge with staphylococcal
enterotoxin B: a new approach for investigating the site of toxin action.
Infect Immun. 1985;50(3):869–76.
40. Saikh KU, Ulrich RG, Krakauer T. Staphylococcal Enterotoxin B and Related
Toxins Produced by Staphylococcus aureus and Streptococcus pyogenes.
In: Bozue J, Cote CK, Glass PJ, editor. Textbooks of Military Medicine:
Medical Aspects of Biological Warfare. Fort Sam Houston (TX): Oce of
The Surgeon General, Borden Institute; 2018. p. 403–14.
41. Biological toxins: Staphylococcal Enterotoxin B (SEB). In: Withers MR,
lead editor. USAMRIID’s Medical Management of Biological Casualties
Handbook. 8th ed. Fort Detrick (MD): U.S. Army Medical Research Institute
of Infectious Diseases; 2014. p. 129–33.
351Section VIII-G: Toxin Agents
42. Kadariya J, Smith TC, Thapaliya D. Staphylococcal aureus and
staphylococcal food-borne disease: an ongoing challenge in public health.
Biomed Res Int. 2014;2014:827965.
43. Aitichou M, Henkens R, Sultana AM, Ulrich RG, Ibrahim MS. Detection of
Staphylococcus aureus enterotoxin A and B genes with PCR-EIA and a
hand-held electrochemical sensor. Mol Cell Probes. 2004;18(6):373–7.
44. Cook E, Wang X, Robiou N, Fries BC. Measurement of staphylococcal
enterotoxin B in serum and other supernatant with a capture enzyme-linked
immunosorbent assay. Clin Vaccine Immunol. 2007;14(9):1094–101.
45. Olsnes S. The history of ricin, abrin and related toxins. Toxicon.
2004;44(4):361–70.
46. McKeon TA. Castor (Ricinus communis L.). In: McKeon TA, Hayes DG,
Hildebrand DF, Weselake RJ, editors. Industrial Oil Crops. AOCS Press;
2016. p. 75–112.
47. Hartley MR, Lord JM. Cytotoxic ribosome-inactivating lectins from plants.
Biochim Biophys Acta. 2004;1701(1–2):1–14.
48. Roberts LM, Lamb FI, Pappin DJ, Lord JM. The primary sequence
of Ricinus communis agglutinin: Comparison with ricin. J Biol Chem.
1985;260(29):15682–6.
49. Brandon DL, McKeon TA, Pateld SA, Kong Q, He X. Analysis of castor by
ELISAs that distinguish ricin and Ricinus communis agglutinin (RCA). J Am
Oil Chem Soc. 2016;93(3):359–63.
50. Worbs S, Skiba M, Soderstrom M, Rapinoja ML, Zeleny R, Russman H, et al.
Characterization of ricin and R. communis agglutinin reference materials.
Toxins (Basel). 2015;7(12):4906–34.
51. Doan LG. Ricin: mechanism of toxicity, clinical manifestations, and vaccine
development. A review. J Toxicol Clin Toxicol. 2004;42(2):201–8.
52. Franz DR, Jaax NK. Ricin Toxin. In: Sidell FR, Takafuji ET, Franz DR,
editors. Textbooks of Military Medicine: Medical Aspects of Chemical and
Biological Warfare. The TMM Series. Part 1: Warfare, Weaponry, and
the Casualty. Washington (DC): Oce of The Surgeon General at TMM
Publications; 1997. p. 631–42.
53. Apen EM, Cooper WC, Horton RJM, Scheel LD. Health Aspects of Castor
Bean Dust: Review and Bibliography. Cincinnati (OH): U.S. Department of
Health, Education, and Welfare; 1967. 132 p.
54. Bradberry SM, Dickens KJ, Rice P, Griths GD, Vale JA. Ricin poisoning.
Toxicol Rev. 2003;22(1):65–70.
55. Audi J, Belson M, Patel M, Schier J, Osterloh J. Ricin poisoning, a
comprehensive review. JAMA. 2005;294(18):2342–51.
352 Biosafety in Microbiological and Biomedical Laboratories
56. Knight B. Ricin–a potent homicidal poison. Br Med J. 1979;1(6159):350–1
57. Johnson RC, Lemire SW, Wooltt AR, Ospina M, Preston KP, Olson CT, et al.
Quantication of ricinine in rat and human urine: a biomarker for ricin
exposure. J Anal Toxicol. 2005;29(3):149–55.
58. He X, McMahon S, Henderson TD 2nd, Griey SM, Cheng LW. Ricin
toxicokinetics and its sensitive detection in mouse sera or feces using
immune-PCR. PLoS One. 2010;5(9):e12858.
59. Bozza WP, Tolleson WH, Rivera Rosado LA, Zhang B. Ricin detection:
tracking active toxin. Biotechnol Adv. 2015;33(1):117–23.
60. Soler-Rodriguez AM, Uhr JW, Richardson J, Vitetta ES. The toxicity of
chemically deglycosylated ricin A-chain in mice. Int J Immunopharmacol.
1992;14(2):281–91.
61. Pittman PR, Reisler RB, Lindsey CY, Guerena F, Rivard R, Clizbe DP, et al.
Safety and immunogenicity of ricin vaccine, RVEcTM, in a Phase 1 clinical
trial. Vaccine. 2015;33(51):7299–306.
62. Bamburg JR. Chemical and Biochemical Studies of the Trichothecene
Mycotoxins. In: Rodricks, JV, editor. Mycotoxins and Other Fungal Related
Food Problems. Vol 149. Washington (DC): American Chemical Society;
1976. p. 144–62.
63. Wannemacher RW, Wiener SL. Trichothecene Mycotoxins. In: Sidell FR,
Takafuji ET, Franz DR, editors. Textbooks of Military Medicine: Medical
Aspects of Chemical and Biological Warfare. The TMM Series. Part 1:
Warfare, Weaponry, and the Casualty. Washington (DC): Oce of The
Surgeon General at TMM Publications; 1997. p. 655–76.
64. Schantz EJ. Chemistry and biology of saxitoxin and related toxins. Ann N Y
Acad Sci. 1986;479:15–23.
65. Yasumoto T, Nagai H, Yasumura D, Michishita T, Endo A, Yotsu M, et al.
Interspecies distribution and possible origin of tetrodotoxin. Ann N Y Acad
Sci. 1986;479:44–51.
66. Baden DG, Mende TJ, Lichter W, Welham H. Crystallization and toxicology
of T34: a major toxin from Florida’s red tide organism (Ptychodiscus brevis).
Toxicon. 1981;19(4):455–62.
67. Deeds JR, Schwartz MD. Human risk associated with palytoxin exposure.
Toxicon. 2010;56(2):150–62.
68. Moore RE, Scheuer PJ. Palytoxin: a new marine toxin from a coelenterate.
Science. 1971;172(3982):495–8.
69. Olivera BM, Cruz LJ. Conotoxins, in retrospect. Toxicon. 2001;39(1):7–14.
353Section VIII-G: Toxin Agents
70. Lefebvre KA, Robertson A. Domoic acid and human exposure risks:
A review. Toxicon. 2010;56(2):218–30
71. Carmichael WW. Algal toxins. In: Callow JA, editor. Advances in botanical
research. Vol. 12. London: Academic Press; 1986. p. 47–101.
72. Bunner BL, Wannemacher RW Jr, Dinterman RE, Broski FH. Cutaneous
absorption and decontamination of [3H]T-2 toxin in the rat model. J Toxicol
Environ Health. 1989;26(4):413–23.
73. Poli MA. Foodborne Marine biotoxins. In: Miliotis MD, Bier JW, editors.
International handbook of foodborne pathogens. New York: Marcel Dekker;
2003. p. 445–58.
74. Franz DR, LeClaire RD. Respiratory eects of brevetoxin and saxitoxin in
awake guinea pigs. Toxicon. 1989;27(6):647–54.
75. Kao CY. Tetrodotoxin, saxitoxin and their signicance in the study of
excitation phenomena. Pharmacol Rev. 1966;18(2):997–1049.
76. Artigas P, Gadsby DC. Na+/K+-pump ligands modulate gating of palytoxin-
induced ion channels. Proc Natl Acad Sci USA. 2003;100(2):501–5.
77. Wiles JS, Vick JA, Christensen MK. Toxicological evaluation of palytoxin in
several animal species. Toxicon. 1974;12(4):427–33.
78. Tubaro A, Durando P, Del Favero G, Ansaldi F, Icardi G, Deeds JR, et al.
Case denitions for human poisonings postulated to palytoxins exposure.
Toxicon. 2011;57(3):478–95.
79. Cruz LJ, White J. Clinical Toxicology of Conus Snail Stings. In: Meier J,
White J, editors. Handbook of Clinical Toxicology of Animal Venoms and
Poisons. Boca Raton: CRC Press; 1995. p. 117–27.
80. McIntosh JM, Jones RM. Cone venom–from accidental stings to deliberate
injection. Toxicon. 2001;39(10):1447–51.
81. Pulido OM. Domoic acid toxicologic pathology: A review. Mar Drugs.
2008;6(2):180–219.
82. Dawson RM. The toxicology of microcystins. Toxicon. 1998;36(7):953–62.
83. LeClaire RD, Parker GW, Franz DR. Hemodynamic and calorimetric
changes induced by microcystin-LR in the rat. J Appl Toxicol.
1995;15(4):303–11.
84. DeGrasse S, Rivera V, Roach J, White K, Callahan J, Couture D, et al.
Paralytic shellsh toxins in clinical matrices: Extension of AOAC ocial
method 2005.06 to human urine and serum and application to a 2007 case
study in Maine. Deep-Sea Res II. 2014;103:368–75.
354 Biosafety in Microbiological and Biomedical Laboratories
85. MacKintosh RW, Dalby KN, Campbell DG, Cohen PT, Cohen P, MacKintosh
C. The cyanobacterial toxin microcystin binds covalently to cysteine-273 on
protein phosphatase 1. FEBS Lett. 1995;371(3):236–40.
86. Wannemacher RW. Procedures for inactivation and safety containment of
toxins. In: Proceedings for the symposium on agents of biological origin.
1989; Aberdeen Proving Ground, MD. Aberdeen, Maryland: U.S. Army
Chemical Research, Development and Engineering Center; 1989. p. 115–22.
87. Padle BM. Therapy and prophylaxis of inhaled biological toxins. J Appl
Toxicol. 2003;23(3):139–70.
355Section VIII-H: Prion Diseases
Section VIII-H: Prion Diseases
Transmissible spongiform encephalopathies (TSE) or prion diseases are
neurodegenerative diseases, which aect humans and a variety of domestic
and wild animal species.
1–4
A central biochemical feature of prion diseases is the
conversion of normal prion protein (PrP) to an abnormal, misfolded, pathogenic
isoform designated PrPS
c
after the prototypic prion disease—scrapie. The
infectious agents that transmit prion diseases are known as prions and contain
no known prion-specic nucleic acids or virus-like particles. Prions are composed
mainly, if not entirely, of PrPS
c
. They are highly resistant to inactivation by
heat and chemicals and thus require special biosafety precautions. Prions are
transmissible by inoculation, ingestion, or transplantation of infected tissues
or homogenates. Prion infectivity is high in the brain and other central nervous
system tissues and lower in lymphoid tissues including the spleen, lymph node,
gut, bone marrow, and blood. A 2017 study indicates the presence of low levels
of prion infectivity in the skin of sporadic Creutzfeldt-Jakob disease (sCJD)
decedents.
5
A chromosomal gene (PRNP) encodes PrP
C
, the cellular isoform of PrP. PrPS
c
is derived from PrP
C
by a post-translational process whereby PrPS
c
acquires a
high beta-sheet content and a resistance to inactivation by normal disinfection
processes. PrPS
c
is less soluble in aqueous buers and is partially protease-
resistant. As a result, when prion-containing samples are incubated with
proteases such as proteinase K, PrPS
c
can often be distinguished from PrP
C
,
which is completely protease-sensitive.
Occupational Infections
Although sCJD infections have occurred in medical specialists and health
professionals, including pathologists who encounter cases of CJD post-mortem,
no overall increased occupational risk for health professionals has been found.
6
However, despite the lack of a clearly identied source, the atypical pathology of
CJD in at least one neurosurgeon suggests that this case was more likely to have
been an acquired, rather than sporadic, form of CJD.
7
Modes of Infection and Spread
Recognized diseases caused by prions are listed in Table 1 (human diseases)
and Table 2 (animal diseases). Besides certain medical procedures using prion
contaminated materials (e.g., dura matter), the only clear risk factor for natural
disease transmission is the consumption of infected tissues, such as human
brain in the case of Kuru, and meat, including nervous tissue, in the case of
bovine spongiform encephalopathy (BSE) and related diseases such as feline
spongiform encephalopathy (FSE). Familial forms of CJD are acquired by inheri-
tance of a mutant PRNP gene through the germline.
356 Biosafety in Microbiological and Biomedical Laboratories
Although the exact mechanism of infection and spread among sheep and
goats developing natural scrapie is unknown, there is considerable evidence
that one of the primary sources is oral ingestion of placental membranes from
infected ewes. There is no evidence of transmission of scrapie to humans even
though the disease has been recognized in sheep for over 200 years. The TSE
diseases, transmissible mink encephalopathy (TME), BSE, FSE, and exotic
ungulate encephalopathy (EUE), are all thought to occur after the consumption
of prion-infected foods.
8
The exact mechanism of chronic wasting disease (CWD)
spread among mule deer, white-tailed deer, and Rocky Mountain elk is unknown.
3
There is strong evidence that CWD is laterally transmitted and environmental
contamination may play an important role in local maintenance of the disease.
Under experimental conditions, CWD and other prion diseases have been
transmitted via aerosols, but there is no evidence that this is a natural route of
transmission.
9–11
Prions are usually most ecient at infecting the homologous species, but
cross-species infection with a reduced eciency is also possible. After cross-
species infection, there is often a gradual adaptation of specicity for the new
host, especially if there is spread from individual to individual. This process of
cross-species adaptation can vary among individuals within the same species.
Therefore, the rate of adaptation and nal species specicity of the resultant prion
is dicult to predict. Such considerations help to form the basis for the biosafety
classication of dierent prions.
Table 1. Human Prion Diseases
Disease Abbreviation Mechanism of Pathogenesis
Kuru N/A Infection through ritualistic cannibalism
Sporadic CJD sCJD Unknown mechanism; possibly somatic mutation or
spontaneous conversion of PrP
C
to PrP
Sc
Variant CJD vCJD Infection presumably from consumption of
BSE-contaminated cattle products or secondary
bloodborne transmission
Familial or genetic
CJD
fCJD or gCJD Germline mutations in PRNP gene
Iatrogenic CJD iCJD Infection from contaminated corneal or dura mater grafts,
pituitary hormone, or neurosurgical equipment
Gerstmann–
Sträussler–Scheinker
syndrome
GSS Germline mutations in PRNP gene
Continued on next page ►

357Section VIII-H: Prion Diseases
Disease Abbreviation Mechanism of Pathogenesis
Fatal Familial
Insomnia
FFI Germline mutations in PRNP gene
Sporadic Fatal
Insomnia
sFI Presumably same as sCJD (see above)
Variably Protease-
Sensitive Prionopathy
VPSPr Presumably same as sCJD (see above)
Table 2. Animal Prion Diseases
Disease Abbreviation Natural Host Mechanism of Pathogenesis
Scrapie N/A Sheep, goats,
mouon
Infection in genetically susceptible
animals
Bovine Spongiform
Encephalopathy
BSE Cattle Infection with prion-contaminated
feedstus (classical BSE); unknown/
possible spontaneous misfolding of
PrP
C
to PrP
Sc
(atypical BSE)
Chronic Wasting
Disease
CWD Mule deer, white-
tailed deer, Rocky
Mountain elk,
reindeer, moose
Unknown mechanism; probably from
direct animal contact with infected
feces, urine, drool, or indirectly from
contaminated environment (e.g., feed,
water, dirt)
Exotic Ungulate
Encephalopathy
EUE Nyala, greater
kudu, and onyx
Infection with BSE-contaminated
feedstus
Feline Spongiform
Encephalopathy
FSE Domestic cats,
wild cats in
captivity
Infection with BSE-contaminated
feedstus
Transmissible Mink
Encephalopathy
TME Mink
(farm-raised)
Infection with prion-contaminated
feedstus
Laboratory Safety and Containment Recommendations
In the laboratory setting, prions from human tissue and human prions propagated
in animals can be manipulated at BSL-2 or higher. Due to concerns about
BSE prions infecting humans and cattle, certain circumstances may call for
the use of BSL-3 facilities and/or practices, with a sealed secondary container
used for transport of samples inside the laboratory. Use of containment and
prion-dedicated equipment is recommended whenever possible in order to limit
contamination as well as the area and materials that would need to undergo
inactivation procedures.
All other animal prions may be manipulated at BSL-2 with standard BSL-2
practices. However, when a prion from one species is inoculated into another
the resultant infected animal should be treated according to the biosafety
358 Biosafety in Microbiological and Biomedical Laboratories
guidelines applying to either the source or recipient of the inoculum, whichever
is more stringent.
In the care of patients diagnosed with human prion disease, Standard Precautions
are considered adequate. Human prion diseases in the clinical setting have not
been found to be communicable or contagious other than through invasive
procedures resulting in iatrogenic exposures.
12
One study reports nding
detectable infectivity and prion seeding activity in the skin of sCJD cadavers
though at much lower levels than what is found in brain tissues of sCJD patients.
If such infectivity were also to be found in asymptomatic prion infected persons
or early in the course of the sCJD illness, this could heighten concern for the
potential of iatrogenic sCJD transmission through invasive skin procedures.
5
There is no evidence of contact or aerosol transmission of prions from one human
to another. However, human prions have been transmitted via some routes. Kuru
has been transmitted through ritualistic cannibalism in New Guinea. Iatrogenic
CJD has been caused by the contamination of medical devices, administration of
prion-contaminated growth hormone, or the transplantation of prion-contaminated
dura mater and corneal grafts. It is highly suspected that variant CJD can also be
transmitted by blood transfusion.
13
However, there is no evidence for bloodborne
transmission of non-variant forms of CJD.
14
Familial CJD, Gerstmann–Sträussler–
Scheinker syndrome (GSS), and fatal familial insomnia (FFI) are all dominantly-
inherited prion diseases; many dierent mutations of the PRNP gene have been
shown to be genetically linked to the development of inherited prion disease.
Studies of prions from many cases of inherited prion disease have demonstrated
transmission to apes, monkeys, and mice, especially those carrying human PRNP
transgenes.
Special Issues
Inactivation of Prions Prions are characterized by relative resistance to conven-
tional inactivation procedures including irradiation, boiling, dry heat, and harsh
chemicals such as formalin, betapropiolactone, and alcohols. While prion infec-
tivity in puried samples is diminished by prolonged digestion with proteases, the
results from boiling in sodium dodecyl sulfate (SDS) and urea alone are variable.
More eective treatments include enzymatic treatments with SDS,
15
vaporized
hydrogen peroxide,
16
4% SDS in 1% acetic acid at 65–134°C,
17,18
or mildly acidic
hypochlorous acid.
19
Denaturing organic solvents such as phenol or chaotropic
reagents (e.g., guanidine isothiocyanate) have resulted in greatly reduced, but
not always complete, inactivation. Similarly, the use of conventional autoclaves as
the sole inactivating treatment has not always resulted in complete inactivation of
prions.
20,21
Formalin-xed and paran-embedded tissues, especially of the brain,
remain infectious.
22
Some investigators recommend that formalin-xed tissues
from suspected cases of prion disease be immersed for 30 minutes in 96%
359Section VIII-H: Prion Diseases
formic acid or phenol before histopathologic processing (see Table 3), but such
treatments may severely distort the microscopic neuropathology and may not
completely inactivate infectivity.
The safest and most unambiguous method for ensuring that there is no risk of
residual infectivity on contaminated instruments and other materials is to discard
and destroy them by incineration.
23
Current recommendations for inactivation
of prions on instruments and other materials are based on the use of sodium
hypochlorite, NaOH, Environ LpH (no longer commercially available),
24
and the
moist heat of autoclaving. Combinations of heat and chemical inactivation are
likely to be most reliable (See Table 4).
20,23,25
A less caustic hypochlorous acid
solution can also decontaminate prions on stainless steel,
19
but further validation
of this treatment is warranted.
Surgical Procedures Precautions for surgical procedures on patients diagnosed
with prion disease are outlined in an infection control guideline for transmissible
spongiform encephalopathies developed by a consultation convened by the WHO
in 1999.
23,25
Sterilization of reusable surgical instruments and decontamination of
surfaces are performed in accordance with recommendations described by the
CDC and the WHO infection control guidelines.
23
Table 4 summarizes the key
recommendations for decontamination of reusable instruments and surfaces.
Contaminated disposable instruments or materials can be incinerated at 1000°C
(1832°F) or greater.
26,27
Autopsies Routine autopsies and the processing of small amounts of forma-
lin-xed tissues containing human prions can safely be done using Standard
Precautions.
28,29
The absence of any known eective treatment for prion
disease demands caution. The highest concentrations of prions are in the
central nervous system and its coverings. Based on animal studies, it is likely
that prions are also found in the spleen, thymus, lymph nodes, skin, blood,
and intestine. The main precaution to be taken by laboratorians working with
prion-infected or contaminated material is to avoid accidental puncture of the
skin.
12
If possible, cut resistant gloves are worn when handling contaminated
specimens. If accidental contamination of unbroken skin occurs, the area is
washed with detergent and abundant quantities of warm water (avoid scrubbing);
brief exposure (1 minute to 1 N NaOH or a 1:10 dilution of bleach) or more
prolonged soaking in a commercial hypochlorous acid preparation (BrioHOCl
®
)
can be considered for additional safety.
19,23
Additional guidance related to
occupational injury is provided in the WHO infection control guidelines.
23
Unxed
samples of brain, spinal cord, and other tissues containing human prions should
be processed with extreme care in a BSL-2 facility, optimally with restricted
access, additional PPE, and dedicated equipment.
360 Biosafety in Microbiological and Biomedical Laboratories
Bovine Spongiform Encephalopathy
Although the eventual total number of variant CJD cases resulting from BSE
transmission to humans is unknown, a review of the epidemiological data from
the United Kingdom indicates that BSE transmission to humans is not ecient.
30
The most prudent approach is to study BSE prions at a minimum in a BSL-2
facility utilizing appropriate BSL-3 practices.
When performing necropsies on large animals where there is an opportunity
that the worker may be accidentally splashed or have contact with high-risk
materials (e.g., spinal column, brain), personnel wear full-body coverage personal
protective equipment (e.g., gloves, rear closing gown, and face shield). Use of
disposable plasticware, which can be discarded as a dry regulated medical waste
or incinerated, is highly recommended.
Aerosol transmission of prions has been observed experimentally,
9–11
but there
is no evidence that this occurs under natural conditions or in clinical settings.
It is still prudent to avoid the generation of aerosols or droplets during the manip-
ulation of tissues or uids and during the necropsy of experimental animals. It is
further strongly recommended that impervious gloves be worn for activities that
provide the opportunity for skin contact with infectious tissues and uids.
Animal carcasses and other tissue waste can be disposed by incineration with a
minimum secondary temperature of 1000°C (1832°F).
23,26
Pathological inciner-
ators should maintain a primary chamber temperature in compliance with design
and applicable state regulations and employ good combustion practices. Medical
waste incinerators should comply with applicable state and federal regulations.
The alkaline hydrolysis process, using a vessel that exposes the carcass or
tissues to NaOH or KOH heated to 95°–150°C, can be used as an alternative to
incineration for the disposal of carcasses and tissue.
20,31
The process has been
shown to completely inactivate some strains of prions when used for the recom-
mended period.
Table 3. Tissue Preparation for Human CJD and Related Diseases
Step Instructions
1 Histology technicians wear gloves, apron, laboratory coat, and face protection.
2 Adequate xation of small tissue samples (e.g., biopsies) from a patient with suspected prion
disease can be followed by post-xation in 96% absolute formic acid for 30 minutes, followed
by 45 hours in fresh 10% formalin.
3 Liquid waste can be collected in a 4 L waste bottle initially containing 600 ml 6 N NaOH.
4 Gloves, embedding molds, and all handling materials are disposed as regulated medical waste.
Continued on next page ►

361Section VIII-H: Prion Diseases
Step Instructions
5 Tissue cassettes can be processed in a TSE-dedicated processor or manually to prevent
contamination of general use tissue processors.
6 Tissues are embedded in a disposable embedding mold. If used, forceps are decontaminated
as in Table 4.
7 In preparing sections, cut-resistant gloves can be worn; section waste is collected and
disposed of in a regulated medical waste receptacle. The knife stage is wiped with 2 N
NaOH, or sodium hypochlorite (20,000 ppm) followed by distilled water. The knife used is
discarded immediately in a “regulated medical waste sharps” receptacle. Slides are labeled
with “CJD Precautions.” The sectioned block is sealed with paran.
8 Routine staining:
a. slides are processed by hand using disposable specimen cups or in a TSE-dedicated
stainer;
b. after placing the coverslip on, slides are decontaminated by soaking them for 10–60
min in 2 N NaOH or sodium hypochlorite (20,000 ppm) followed by distilled water; and
c. slides are labeled as “Infectious-CJD.”
9 Other suggestions:
a. disposable specimen cups or slide mailers may be used for reagents;
b. slides for immunocytochemistry may be processed in disposable Petri dishes; and
c. equipment is decontaminated as described above or disposed as regulated medical
waste.
Handling and processing of tissues from patients with suspected prion
disease
The special characteristics of work with prions require attention to the facilities,
equipment, policies, and procedures involved.
10
The related considerations
outlined in Table 3 should be incorporated into the laboratory’s risk management
for this work.
Handling and processing of multiple human prion tissue samples
In research environments where multiple human prion positive tissues may be
processed and stained, a prion-dedicated tissue processor, self-contained stainer
(i.e., discharge is collected and not discarded into the drain), dedicated specimen
cups, and staining dishes can be used. The same personal protective equipment,
decontamination procedures, and waste disposal procedures listed in Table 3 are
also applicable. In addition, large volumes of aqueous liquid waste generated by
the tissue processor and stainer can be mixed with moisture-absorbing pellets,
sealed in a container, and incinerated at 1000°C (1832°F ) or greater.

362 Biosafety in Microbiological and Biomedical Laboratories
Table 4. Prion Inactivation Methods for Reusable Instruments
and Surfaces
19,21,24,25
Method Instructions
1 Immerse in 1 N NaOH or sodium hypochlorite (20,000 ppm available chlorine) for
1 hour. Transfer into water and autoclave (gravity displacement) at 121ºC for 1 hour.
Clean and sterilize by conventional means. [Note: Sodium hypochlorite may be
corrosive to some instruments, including autoclaves.]
2 Immerse in a pan containing 1 N NaOH, heat in a gravity displacement autoclave at
121ºC for 30 minutes. Clean-rinse in water and sterilize by conventional means.
3 Immerse in 1 N NaOH or sodium hypochlorite (20,000 ppm) for 1 hour. Remove
and rinse instruments with water, transfer to open pan and autoclave at 121ºC
(gravity displacement) or 134ºC (porous load) for 1 hour. Clean and sterilize by
conventional means.
4 Surfaces or heat-sensitive instruments can be treated with 2 N NaOH or sodium
hypochlorite (20,000 ppm) for 1 hour. Ensure surfaces remain wet for entire period, then
rinse well with water. Before chemical treatment, it is strongly recommended that gross
contamination of surfaces be reduced because the presence of excess organic material
will reduce the strength of either NaOH or sodium hypochlorite solutions.
5 2% Environ LpH
®
(EPA Reg. No. 1043-118; no longer commercially available) may be
used on washable, hard, non-porous surfaces (such as oors, tables, equipment, and
counters), items, such as non-disposable instruments, sharps, and sharp containers,
and/or laboratory waste solutions (such as formalin or other liquids). This product is
currently being used under FIFRA Section 18 exemptions in a number of states. Users
should consult with the state environmental protection oce prior to use. Items may be
immersed for 0.5–16 h, rinsed with water, and sterilized using conventional methods.
(Adapted from https://www.cdc.gov)
The FDA has not yet approved any product for decontaminating, disinfecting, or
sterilizing prions. The methods described are considered research use only.
Working Solutions: 1 N NaOH equals 40 grams of NaOH per liter of water.
Solution should be prepared daily. A stock solution of 10 N NaOH can be
prepared and 1:10 dilutions (1 part 10 N NaOH plus 9 parts water) should be
prepared frequently enough to maintain a fully eective alkalinity.
Note, 20,000 ppm sodium hypochlorite equals a 2% solution. Many commercial
household bleach sources in the United States contain 6.15% sodium
hypochlorite; for such sources, a 1:3 v/v dilution (1 part bleach plus 2 parts water)
would produce a solution with 20,500 ppm available chlorine. This relatively
easy method provides a slightly more concentrated solution (extra 500 ppm)
that should not pose a problem with decontamination procedures or signicantly
increase chemical risks in the laboratory. Bleach solutions can o-gas and
working solutions should be prepared frequently enough to maintain adequate
available chlorine levels.
363Section VIII-H: Prion Diseases
CAUTION: Above solutions are corrosive and require suitable personal protective
equipment and proper secondary containment. These strong corrosive solutions
require careful disposal in accordance with local regulations. Sodium hypochlorite
and sodium hydroxide solutions may corrode autoclaves.
Precautions for using NaOH or sodium hypochlorite solutions in
autoclaves NaOH spills or gas may damage the autoclave if proper containers
are not used. The use of containers with a rim and lid designed for condensation
to collect and drip back into the pan is recommended. Aluminum should not
be used. Persons who use this procedure should be cautious in handling hot
NaOH solution (post-autoclave) and in avoiding potential exposure to gaseous
NaOH; exercise caution during all sterilization steps; and allow the autoclave,
instruments, and solutions to cool down before removal.
25,32
Immersion in sodium
hypochlorite bleach can cause severe damage to some instruments. Neutral-
ization of hypochlorite with thiosulfate prior to autoclaving is recommended to
prevent the release of chlorine gas.
33
Biosafety cabinet (BSC) decontamination Because the paraformaldehyde
vaporization procedure does not diminish prion titers, BSCs must be decontami-
nated with 1 N NaOH or 50% v/v of 5.25% sodium hypochlorite household bleach
and rinsed with water. BSC technicians should chemically treat the HEPA lter
and chamber while removing it from its housing. HEPA lters can be wrapped in
a double layer of plastic and incinerated. The use of respirators may be advisable
to protect against chemical vapors during decontamination.
References
1. Will RG, Ironside JW. Sporadic and Infectious Human Prion Diseases. Cold
Spring Harb Perspect Med. 2017;7(1).
2. Brown P, Brandel JP, Sato T, Nakamura Y, MacKenzie J, Will RG, et al.
Iatrogenic Creutzfeldt-Jakob disease, nal assessment. Emerging Infect
Dis. 2012;18(6):901–7.
3. Haley NJ, Hoover EA. Chronic wasting disease of cervids: current
knowledge and future perspectives. Annu Rev Anim Biosci. 2015;3:305–25.
4. Greenlee JJ, Greenlee MH. The transmissible spongiform encephalopathies
of livestock. ILAR J. 2015;56(1):7–25.
5. Orru CD, Yuan J, Appleby BS, Li B, Li Y, Winner D, et al. Prion seeding
activity and infectivity in skin samples from patients with sporadic
Creutzfeldt-Jakob disease. Sci Transl Med. 2017;9(417).
6. Alcalde-Cabero E, Almazan-Isla J, Brandel JP, Breithaupt M, Catarino J,
Collins S, et al. Health professions and risk of sporadic Creutzfeldt-Jakob
disease, 1965 to 2010. Euro Surveill. 2012;17(15).
364 Biosafety in Microbiological and Biomedical Laboratories
7. Kobayashi A, Parchi P, Yamada M, Brown P, Saverioni D, Matsuura Y, et al.
Transmission properties of atypical Creutzfeldt-Jakob disease: a clue to
disease etiology?. J Virol. 2015;89(7):3939–46.
8. Marin-Moreno A, Fernandez-Borges N, Espinosa JC, Andreoletti O, Torres JM.
Transmission and Replication of Prions. Prog Mol Biol Transl Sci.
2017;150:181–201.
9. Denkers ND, Seelig DM, Telling GC, Hoover EA. Aerosol and nasal
transmission of chronic wasting disease in cervidized mice. J Gen Virol.
2010;91(Pt 6):1651–8.
10. Haybaeck J, Heikenwalder M, Klevenz B, Schwarz P, Margalith I, Bridel C,
et al. Aerosols transmit prions to immunocompetent and immunodecient
mice. PLoS Pathog. 2011;7(1):e1001257. Erratum in: Correction: Aerosols
transmit prions to immunocompetent and immunodecient mice. PLoS
Pathog. 2016.
11. Denkers ND, Hayes-Klug J, Anderson KR, Seelig DM, Haley NJ, Dahmes SJ,
et al. Aerosol Transmission of Chronic Wasting Disease in white-tailed deer.
J Virol. 2013;87(3):1890–2.
12. Ridley RM, Baker HF. Occupational risk of Creutzfeldt-Jakob disease.
Lancet. 199;341(8845):641–2.
13. Llewelyn CA, Hewitt PE, Knight RS, Amar K, Cousens S, Mackenzie J, et al.
Possible transmission of variant Creutzfeldt-Jakob disease by blood
transfusion. Lancet. 2004;363(9407):417–21.
14. Crowder LA, Schonberger LB, Dodd RY, Steele WR. Creutzfeldt-Jakob
disease lookback study: 21 years of surveillance for transfusion
transmission risk. Transfusion. 2017;57(8):1875–8.
15. Jackson GS, McKintosh E, Flechsig E, Prodromidou K, Hirsch P, Linehan J,
et al. An enzyme-detergent method for eective prion decontamination of
surgical steel. J Gen Virol. 2005;86(Pt 3):869–78.
16. Fichet G, Comoy E, Duval C, Antloga K, Dehen C, Charbonnier A, et al.
Novel methods for disinfection of prion-contaminated medical devices.
Lancet. 2004;364(9433):521–6.
17. Peretz D, Supattapone S, Giles K, Vergara J, Freyman Y, Lessard P,
et al. Inactivation of prions by acidic sodium dodecyl sulfate. J Virol.
2006;80(1):322–31.
18. Giles K, Glidden DV, Beckwith R, Seoanes R, Peretz D, DeArmond SJ,
et al. Resistance of bovine spongiform encephalopathy (BSE) prions to
inactivation. PLoS Pathog. 2008;4(11):e1000206.

365Section VIII-H: Prion Diseases
19. Hughson AG, Race B, Kraus A, Sangare LR, Robins L, Groveman BR, et al.
Inactivation of Prions and Amyloid Seeds with Hypochlorous Acid. PLoS
Pathog. 2016;12(9):e1005914.
20. Taylor DM, Woodgate SL. Rendering practices and inactivation of
transmissible spongiform encephalopathy agents. Rev Sci Tech.
2003;22(1):297–310.
21. Ernst DR, Race RE. Comparative analysis of scrapie agent inactivation
methods. J Virol Methods. 1993;41(2):193–201.
22. Priola SA, Ward AE, McCall SA, Trilo M, Choi YP, Solforosi L, et al. Lack
of prion infectivity in xed heart tissue from patients with Creutzfeldt-Jakob
disease or amyloid heart disease. J Virol. 2013;87(17):9501–10.
23. Communicable Disease and Surveillance Control. WHO Infection Control
Guidelines for Transmissible Spongiform Encephalopathies. Report of a
WHO Consultation; 1999 Mar 23–26; Geneva, Switzerland. Geneva: World
Health Organization; 1999. p. 1–38.
24. Race RE, Raymond GJ. Inactivation of transmissible spongiform
encephalopathy (prion) agents by environ LpH. J Virol. 2004;78(4):2164–5.
25. Centers for Disease Control and Prevention [Internet]. Atlanta (GA): Division
of High-Consequence Pathogens and Pathology (DHCPP); c2018 [cited
2019 Mar 5]. Creutzfeldt-Jakob Disease, Classic (CJD). Infectious Control.
Available from: https://www.cdc.gov/prions/CJD/infection-control.html
26. Brown P, Rau EH, Johnson BK, Bacote AE, Gibbs CJ Jr, Gajdusek DC. New
studies on the heat resistance of hamster-adapted scrapie agent: threshold
survival after ashing at 600 degrees C suggests an inorganic template of
replication. Proc Natl Acad Sci U S A. 2000;97(7):3418–21.
27. Brown P, Rau EH, Lemieux P, Johnson BK, Bacote AE, Gajdusek DC.
Infectivity studies of both ash and air emissions from simulated incineration
of scrapie-contaminated tissues. Environ Sci Technol. 2004;38(22):6155–60.
28. Ironside JW, Bell JE. The ‘high-risk’ neuropathological autopsy in AIDS
and Creutzfeldt-Jakob disease: principles and practice. Neuropathol Appl
Neurobiol. 1996;22(5):388–93.
29. Hilton DA. Pathogenesis and prevalence of variant Creutzfeldt-Jakob
disease. J Pathol. 2006;208(2):134–41.
30. Diack AB, Head MW, McCutcheon S, Boyle A, Knight R, Ironside JW,
et al. Variant CJD. 18 years of research and surveillance. Prion.
2014;8(4):286–95.
31. Richmond JY, Hill RH, Weyant RS, Nesby-O’Dell SL, Vinson PE. What’s hot
in animal biosafety?. ILAR J. 2003;44(1):20–7.
366 Biosafety in Microbiological and Biomedical Laboratories
32. Brown SA, Merritt K. Use of containment pans and lids for autoclaving
caustic solutions. Am J Infect Control. 2003;31(4):257–60.
33. Hadar J, Tirosh T, Grafstein O, Korabelnikov E. Autoclave emissions—
hazardous or not. J Am Biol Safety Assoc. 1997;2(3):44–51.

367Appendix A—Primary Containment for Biohazards
Appendix A—Primary Containment for Biohazards:
Selection, Installation, and Use of Biological Safety
Cabinets
Part 1—Introduction
This document presents information on the design, selection, function, and use of
Biological Safety Cabinets (BSCs), also referred to as biosafety cabinets, which
are the primary means of containment for working safely with infectious microor-
ganisms and prions. Brief descriptions of the facility and engineering concepts for
the conduct of microbiological research are also provided. BSCs are only one part
of an overall biosafety program, which requires consistent use of good microbio-
logical practices, use of primary containment equipment, and proper containment
facility design. Detailed descriptions of acceptable work practices, procedures,
and facilities, known as Biosafety Levels (BSL) 1 to 4, are presented in Section IV
of BMBL.
BSCs are designed to provide personnel and environmental protection when
appropriate practices and procedures are followed. Three kinds of BSCs,
designated as Class I, II and III, have been developed to meet varying research
and clinical needs. Class II and Class III cabinets provide operator, product, and
environmental protection. Most BSCs use High-Eciency Particulate Air (HEPA)
lters in the exhaust and supply systems. Ultra-Low Particulate Air (ULPA) lters
are used for some special applications. The exception is a Class I BSC, which
has HEPA-ltered exhaust air only.
This appendix is divided into seven Parts. HEPA and ULPA lters and their use
in BSCs are briey described in Part 2. Part 3 presents a general description of
the special features of BSCs that provide varying degrees of personnel, environ-
mental, and product protection. Laboratory hazards and risk assessment are
discussed in Part 4. Part 5 presents work practices, procedures, and practical tips
to maximize the protection aorded by the most commonly used BSCs. Facility
and engineering requirements needed for the operation of each type of BSC are
presented in Part 6. Part 7 reviews requirements for routine certication intervals
to ensure proper operation and integrity of a Class II BSC.
These Parts are not meant to be denitive or all-encompassing. Rather, an
overview is provided to clarify the expectations, functions, and performance of
these critical primary barriers. This document has been written for the biosafety
professionals, laboratorians, engineers, and managers who desire a better
understanding of each type of cabinet; the factors considered for the selection of
a BSC to meet specic operational needs; and the services required to maintain
the operational integrity of the cabinet.

368 Biosafety in Microbiological and Biomedical Laboratories
Proper maintenance of BSCs used for work at all Biosafety Levels cannot be
overemphasized. Biosafety professionals and laboratorians need to understand
that an active BSC is a primary containment device. A BSC must be routinely
inspected and tested by trained personnel, following strict protocols, to verify that
it is working properly. This process, referred to as certication of the BSC, should
be performed at least annually, or as specied in Part 7 of this section.
Part 2—High-Eciency Particulate Air (HEPA) Filters and the Development
of Biological Containment Devices
Since the earliest Laboratory-associated infections (LAIs) with S. Typhi to the
contemporary hazards posed by bioterrorism, antibiotic-resistant bacteria,
and rapidly mutating viruses, threats to worker safety have stimulated the
development and renement of workstations where infectious microorganisms
could be safely handled. These workstations have helped maintain sterility of cell
lines, minimize cross-contamination, and maintain product integrity. The use of
proper procedures and equipment, as described in Section IV of BMBL, cannot be
overemphasized in providing primary personnel and environmental protection. For
example, high-speed blenders designed to reduce aerosol generation, needle-
locking syringes, micro burners, and safety centrifuge cups or sealed rotors are
among the engineered devices that protect laboratory workers from biological
hazards. An important piece of safety equipment is the BSC, in which manipula-
tions of infectious microorganisms are performed.
Background
Early prototype clean air cubicles were designed to protect the materials being
manipulated from environmental or worker-generated contamination rather than
to protect the worker from the risks associated with the manipulation of potentially
hazardous materials. Filtered air was blown across the work surface directly at
the worker. Therefore, these cubicles could not be used for handling infectious
agents because the worker was in a contaminated air stream.
To protect the worker during manipulations of infectious agents, a small
workstation was needed that could be installed in existing laboratories with
minimum modication to the room. The earliest designs for primary containment
devices were essentially non-ventilated boxes built of wood, and later of
stainless steel, in which simple operations such as weighing materials could be
accomplished.
1
Early versions of ventilated cabinets did not have adequate or controlled direc-
tional air movement. They were characterized by mass airow into the cabinets
with widely varying air volumes across openings. Mass airow into a cabinet
drew contaminated air away from the laboratory worker. This was the forerunner
of the Class I BSC. However, since the inow air was unltered, the cabinet
369Appendix A—Primary Containment for Biohazards
was contaminated with environmental microorganisms and other undesirable
particulate matter.
Control of airborne particulate materials became possible with the development of
lters that eciently removed microscopic contaminants from the air. The HEPA
lter was developed to create dust-free work environments (e.g., cleanrooms and
clean benches) in the 1940s.
1
HEPA and ULPA Filters HEPA lters used in most BSCs remove the Most
Penetrating Particle Size (MPPS) of approximately 0.3 µm with a minimum
eciency of 99.99%, while ULPA lters remove particles of average size 0.1–0.2
µm or 0.2–0.3 µm with minimum eciency of 99.999%.
2
Particles both larger
and smaller than the MPPS (including bacterial spores and viruses) are removed
with greater eciency. HEPA and ULPA lter eciency and the mechanics
of particle collection by these lters are well-studied and well- documented;
therefore, only a brief description is included here.
3,4
The typical HEPA lter medium is a single sheet of borosilicate bers treated with
a wet-strength, water-repellant binder. Advances in ltration science have also
seen the introduction of HEPA and ULPA lters with dierent media types such as
polytetrauoroethylene (PTFE [i.e., Teon]) for use in BSCs and similar devices.
The lter medium is pleated to increase the overall surface area inside the lter
frames and the pleats are often divided by corrugated aluminum separators
(Figure 1). The separators prevent the pleats from collapsing in the air stream
and provide a path for airow. Alternate designs providing substitutions for the
aluminum separators may also be used and are known as separatorless lters.
The lter is glued into a wood, metal, or plastic frame. Careless handling of the
lter (e.g., improper storage or dropping) can damage the medium at the glue
joint and cause tears or shifting of the lter resulting in leaks in the medium. This
is the primary reason why lter integrity must be tested when a BSC is installed
initially and each time it is moved or relocated (Part 7).
Various types of containment and similar devices incorporate the use of HEPA
and ULPA lters in the exhaust and/or supply air system to remove airborne
particulate material. It should be noted that, although ULPA lters can be used in
BSCs, there is not at this time a specic situation that requires them. ULPA lters
are more expensive to purchase and can raise energy costs and be detrimental
to the lifespan of the device motors due to the increased resistance through
the lter. Depending on the conguration of these lters and the direction of the
airow, varying degrees of personnel, environmental, and product protection can
be achieved.
5
Part 5 describes the proper practices and procedures necessary to
maximize the protection aorded by the various devices.
370 Biosafety in Microbiological and Biomedical Laboratories
Part 3—Biological Safety Cabinets
The similarities and dierences in protection oered by the various classes of
BSCs are reected in Table 1. Please also refer to Table 2 and Part 4 for further
considerations pertinent to BSC selection and risk assessment.
The Class I BSC
The Class I BSC provides personnel and environmental protection but no product
protection. It is similar in terms of air movement to a chemical fume hood but
has a HEPA lter in the exhaust system to protect the environment (Figure 2). In
the Class I BSC, unltered room air is drawn in through the work opening and
across the work surface. Personnel and environmental protection is provided by a
minimum inward airow velocity of 75 linear feet per minute (lfm) through the front
opening.
6
Because product protection is provided by the Class II BSCs, general
usage of the Class I BSC has declined. Class I BSCs are used where aerosols
may be generated and product protection is not required, such as for cage
dumping, culture aeration, or tissue homogenization, or to enclose equipment
(e.g., centrifuges, harvesting equipment, or small fermenters).
The classical Class I BSC is direct-connected to the building exhaust system and
the building exhaust fan provides the negative pressure necessary to draw room
air into the cabinet. The airow pattern into a Class I is similar to a chemical fume
hood where unltered laboratory air ows inward over the product. Any aerosols
and particulates are pulled into an exhaust plenum that contains a HEPA lter,
which lters out the aerosols and particulates.
Some Class I BSCs are equipped with an integral exhaust fan. In this case, the
cabinet air may be recirculated into the laboratory if no noxious or toxic gases
or vapors are used. This Class I BSC may also be canopy connected with an
exhaust alarm when hazardous gases or vapors are used.
A panel with openings to allow access for the hands and arms to the work surface
can be added to the Class I cabinet. The restricted opening results in increased
inward air velocity, increasing worker protection. For added safety, arm-length
gloves can be attached to the panel. Makeup air is then drawn through an
auxiliary air supply opening (which may contain a lter) and/or around a loose-
tting front panel.
Some Class I models used for animal cage changing are designed to allow
recirculation of air into the room after HEPA ltration and may require more
frequent lter replacement due to lter loading and odor from organic material
captured on the lter.
All Class I BSCs should be certied annually for sucient airow and lter
integrity.
371Appendix A—Primary Containment for Biohazards
The Class II BSC
As biomedical researchers began to use sterile animal tissue and cell culture
systems, particularly for the propagation of viruses, cabinets were needed that
also provided product protection. In the early 1960s, the laminar ow principle
evolved. Unidirectional air moving at a xed velocity along parallel lines was
demonstrated to reduce turbulence resulting in predictable particle behavior.
Biocontainment technology also incorporated this laminar or uniform, directional
ow principle with the use of the HEPA lter to aid in the capture and removal
of airborne contaminants from the air stream.
7
This combination of technologies
that exists in the Class II BSC serves to help protect the laboratory worker from
potentially infectious aerosols
4
generated within the cabinet and also provides
necessary product protection. Class II BSCs are partial barrier systems that rely
on the directional movement of air to provide containment. As the air curtain is
disrupted (e.g., movement of materials in and out of a cabinet, rapid or sweeping
movement of the arms) the potential for contaminant release into the laboratory
work environment is increased, as is the risk of product contamination.
The Class II (Types A1, A2, B1, B2, and C1)
8
BSCs provide personnel, environ-
mental, and product protection. Airow is drawn into the front grille of the cabinet,
providing personnel protection. In addition, the downward ow of HEPA-ltered
air provides product protection by minimizing the chance of cross-contamination
across the work surface of the cabinet. Because cabinet exhaust air is passed
through a certied HEPA lter, it is particulate-free (environmental protection), and
may be recirculated to the laboratory (Type A1, A2, and C1 BSCs) or discharged
from the building through a canopy (formerly thimble) connected to the building
exhaust.
It is possible to exhaust the air from a Type A1, A2, or C1 cabinet outside of
the building. When using volatile toxic chemicals, removal of the exhaust from
the laboratory is required. However, it must be done in a manner that does not
alter the balance of the cabinet exhaust system, thereby disturbing the internal
cabinet airow. The proper method of connecting a Type A1, A2, or C1 cabinet
to the building exhaust system is through use of a canopy connection,
8,9
which
provides a small opening or air gap (usually one inch) around the cabinet exhaust
lter housing (Figure 4). The airow of the building exhaust must be sucient to
maintain the ow of room air into the gap between the canopy unit and the lter
housing. The canopy must be removable or be designed to allow for operational
testing of the cabinet and must have an alarm to indicate insucient airow
through the canopy (Part 6). Class II, Type A1 or A2 cabinets should never be
direct-connected to the building exhaust system.
8
Fluctuations in air volume and
pressure that are common to all building exhaust systems can make it dicult to
match the airow requirements of the cabinet.
372 Biosafety in Microbiological and Biomedical Laboratories
Type B cabinets must be direct-connected, preferably to a dedicated, independent
exhaust system. Fans for laboratory exhaust systems should be located at the
terminal end of the ductwork to avoid pressurizing the exhaust ducts. A failure
in the building exhaust system may not be apparent to the user, as the supply
blowers in the cabinet will continue to operate. A pressure-independent monitor
and alarm must be installed to provide a warning and shut o the BSC supply fan,
should a failure in exhaust airow occur. Since this feature is not supplied by all
cabinet manufacturers, it is prudent to install a sensor such as a ow monitor and
alarm in the exhaust system as necessary. To maintain critical operations, labora-
tories using Type B BSCs should connect the exhaust blower to the emergency
power supply.
HEPA lters are eective at trapping particulates, and thus infectious agents, but
do not capture volatile chemicals or gases. Only canopy-connected Type A1, A2,
and C1 or Types B1 and B2 BSCs should be used when working with volatile,
toxic chemicals, but amounts must be limited (Table 2).
The mechanical design and air balance testing of the laboratory exhaust system
for Class IIB BSCs must use Concurrent Balance Values (CBV) as published in
the NSF/ANSI 49 Standard—a standard that describes the requirements for the
construction and function of a Class II BSC.
8
When a BSC is certied to NSF/
ANSI 49-2018, the standard method is to set the inow velocities using a direct
inow measurement (DIM) hood. When the HVAC system air balance is set, it is
typically done based on duct traverse air measurements taken at some point in
the ductwork. The two groups are attempting to measure and set the BSC inows,
but each is using a dierent type of instrument and taking airow measurements
at dierent locations. There can be a dierence in air volume measurements
between the two. The CBV provides each discipline the information they require
to properly test or certify the BSC.
All Class II cabinets are designed for work involving microorganisms assigned to
Risk Groups (RG) 1–4. Class II BSCs provide the microbe-free work environment
necessary for cell culture propagation and also may be used for the formulation
of nonvolatile antineoplastic or chemotherapeutic drugs.
10,11
Class II BSCs may
be used with organisms requiring BSL-4 containment in a BSL-4 suit laboratory
by a worker wearing a positive-pressure protective suit. Maximum containment
potential is achieved only through strict adherence to proper practices and
procedures.
Class II, Type A1 BSC An internal fan (Figure 3) draws sucient room air
through the front grille to maintain a minimum calculated or measured average
inow velocity of at least 75 lfm at the face opening of the cabinet. The supply
air ows through a HEPA lter and provides particulate-free air to the work
surface. Airow provided in this manner reduces turbulence in the work zone and
minimizes the potential for cross-contamination.
373Appendix A—Primary Containment for Biohazards
The downward moving air splits as it approaches the work surface; the fan draws
part of the air to the front grille and the remainder to the rear grille. Although there
are variations among dierent cabinets, this split generally occurs about halfway
between the front and rear grilles and two to six inches above the work surface.
The air is drawn through the front and rear grilles by the internal fan and pushed
into the space between the supply and exhaust lters. Due to the relative size of
these two lters, approximately 30% of the air passes through the exhaust HEPA
lter and 70% recirculates through the supply HEPA lter back into the work
zone of the cabinet. Most Class II, Type A1, and A2 cabinets have dampers to
modulate this division of airow.
Since 2010, a Class II A1 cabinet may not have a potentially contaminated
positively pressurized plenum that is not surrounded by a negatively pressurized
plenum. This change has minimized the dierence between an A1 and A2 cabinet
to the inow velocity.
Class II, Type A2 BSC (Formerly called A/B3) Only when this BSC (Figure 3)
is ducted to the outdoors does it meet the requirements of the former Class II,
Type B3.
8
The designation Class II B3 is no longer used. The Type A2 cabinet
has a minimum calculated or measured inow velocity of 100 lfm. All positive-
pressure contaminated plenums within the cabinet are surrounded by a negative
air pressure plenum thus ensuring that any leakage from a contaminated plenum
will be drawn into the cabinet and not released to the environment. Small
quantities of volatile toxic chemicals or radionuclides can be used in a Type A2
cabinet only if it exhausts to the outside via a properly functioning canopy with
exhaust alarm.
8
Class II, Type B1 BSC Some biomedical research requires the use of small
quantities of toxic volatile chemicals, such as organic solvents or carcinogens.
Carcinogens used in cell culture or microbial systems require both biological and
chemical containment.
9
The Class II, Type B cabinet originated with the National Cancer Institute
(NCI)-designed Type 212 (later called Type B) BSC (Figure 5a) and was
designed for manipulations of small quantities of toxic volatile chemicals with in
vitro biological systems. The NSF/ANSI 49-2018 denition of Type B1 cabinets
8
includes this classic NCI design Type B; cabinets without a supply HEPA lter
located immediately below the work surface (Figure 5b); and those with exhaust/
recirculation downow ratios other than 70/30%.
The cabinet supply blower draws room air (plus a portion of the cabinet’s
recirculated air) through the front grille and through the supply HEPA lter located
immediately below the work surface. This particulate-free air ows upward through
a plenum at each side of the cabinet and then downward to the work area through
374 Biosafety in Microbiological and Biomedical Laboratories
a backpressure plate. In some cabinets, there is an additional supply HEPA lter to
remove particulates that may be generated by the blower-motor system.
Room air is drawn through the face opening of the cabinet at a minimum
measured inow velocity of 100 lfm. As with the Type A1 and A2 cabinets, there
is a split in the down-owing air stream just above the work surface. In the Type
B1 cabinet, approximately 70% of the downow air exits through the rear grille,
passes through the exhaust HEPA lter, and is discharged from the building. The
remaining 30% of the downow air is drawn through the front grille. Since the air
that ows to the rear grille is discharged into the exhaust system, activities that
may generate toxic volatile chemical vapors or gases should be conducted toward
the rear of the cabinet work area.
12
Class II, Type B2 BSC This BSC is a total-exhaust cabinet; no air is recirculated
within it (Figure 6). This cabinet provides simultaneous primary biological and
chemical (small quantity) containment. Consideration must be given to the
chemicals used in BSCs as some chemicals can destroy the lter medium,
housings, and/or gaskets causing loss of containment. The supply blower draws
either room or outside air in at the top of the cabinet, passes it through a HEPA
lter and down into the work area of the cabinet. The building exhaust system
draws air through both the rear and front grilles, capturing the supply air plus
the additional amount of room air needed to produce a minimum calculated or
measured inow face velocity of 100 lfm. All air entering this cabinet is exhausted
and passes through a HEPA lter (and perhaps some other air-cleaning device,
such as a carbon lter, if required, for the work being performed prior to discharge
to the outside). This cabinet exhausts as much as 1,200 cubic feet per minute
of conditioned room air making this cabinet expensive to operate. The higher
static air pressure required to operate this cabinet also results in additional
costs associated with heavier gauge ductwork and higher capacity exhaust
fan. Therefore, the need for a Class II, Type B2 should be justied by the risk
assessment of the research to be conducted.
Should the building exhaust system fail, the cabinet will be pressurized, resulting
in a ow of air from the work area back into the laboratory.
Cabinets built since the early 1980s have an interlock system, installed by the
manufacturer, to prevent the supply blower from operating whenever the exhaust
ow is insucient; systems can be retrotted. Exhaust air movement should be
monitored by a pressure-independent device, such as a ow monitor.
Class II, Type C1 BSC This BSC is similar to a Type B1 BSC in that it has a
special region of the work area intended for work with toxic volatile chemicals
that are exhausted from the building (Figure 7a). However, it also has an internal
exhaust blower that allows the BSC to be either room recirculated if no volatile
toxic chemicals or vapors are present or canopy-connected with an exhaust alarm
375Appendix A—Primary Containment for Biohazards
if volatile toxic chemicals are used. Room air is drawn through the face opening of
the cabinet at a minimum measured inow velocity of 100 lfm. The down-owing
air stream just above the work surface is split by a specic grille pattern with a
portion of 70% to be exhausted and the remaining 30% recirculated. If the air that
ows over the specic region is discharged into the exhaust system, activities that
may generate toxic, volatile chemicals or gases must only be conducted in that
area of the cabinet work zone if connected to a properly functioning canopy with
alarm (Figure 7b). If canopy connected during a building system failure, the BSC
must be either interlocked with the cabinet blower(s) alarm to shut o the cabinet
or, if using a sealed and tested duct system and if permitted by a chemical risk
assessment, may continue to operate for up to ve minutes pressurizing the duct
and indicating the time remaining before the BSC is shut o.
Special Applications Class II BSCs can be modied to accommodate special
tasks. For example, the front sash can be modied by the manufacturer to accom-
modate the eyepieces of a microscope. The work surface can be designed to
accept a carboy, a centrifuge, or other equipment that may require containment.
A rigid plate with openings for the arms can be added if needed. Good cabinet
design, microbiological aerosol tracer testing of the modication, and appropriate
certication (Part 7) are required to ensure that the basic systems operate
properly after modication (Part 5).
The Class III BSC
The Class III BSC (Figure 8) was designed for work with highly infectious microbi-
ological agents and the conduct of hazardous operations and provides maximum
protection for the environment and the worker. It is a gas-tight (no leak greater
than 1x10-
7
cc/sec with 1% test gas at three inches pressure water gauge
13
)
enclosure with a non-opening view window. Access for passage of materials into
the cabinet is through a dunk tank that is accessible through the cabinet oor or a
double-door pass-through box (e.g., antechamber, autoclave) that can be decon-
taminated between uses. Reversing that process allows materials to be removed
from the Class III BSC safely. Both supply and exhaust air are HEPA-ltered on
a Class III cabinet. Exhaust air must pass through two HEPA lters, or a HEPA
lter and an air incinerator, before discharge directly to the outdoors. Class III
cabinets are not exhausted through the general laboratory exhaust system. Using
a dedicated exhaust system reduces the risk of outside ventilation inuences
on Class III containment performance. Airow is maintained by an exhaust
system exterior to the cabinet, which keeps the cabinet under negative pressure
(minimum of 0.5 in water gauge). This level of negative pressure is required to
minimize risk and maintain containment if a breach occurs such as holes or tears
in the glove system.
Long, heavy-duty rubber gloves are attached in a gas-tight manner to ports in
the cabinet to allow direct manipulation of the materials isolated inside. Although

376 Biosafety in Microbiological and Biomedical Laboratories
these gloves restrict movement, they prevent the user’s direct contact with the
hazardous materials. The trade-o is clearly on the side of maximizing personal
safety. Depending on the design of the cabinet, the supply HEPA lter provides
particulate-free, albeit somewhat turbulent, airow within the work environment.
Laminar or uniform airow is optional but not a typical characteristic of a Class III
cabinet.
Several Class III BSCs can be joined together in series to provide a larger work
area. Such cabinet lines are custom-built; the equipment installed in the cabinet
series (e.g., refrigerators, small elevators, shelves to hold small animal cage
racks, microscopes, centrifuges, incubators) is generally custom-built as well.
Horizontal Laminar Flow Clean Bench Horizontal laminar ow clean benches
(also referred to as clean air devices [CADs]) are not BSCs (Figure 9a). These
pieces of equipment discharge HEPA-ltered air from the back of the cabinet
across the work surface and toward the user. These devices only provide product
protection. They can be used for certain clean activities, such as the dust-free
assembly of sterile equipment or electronic devices. Clean benches should
never be used when handling cell culture materials, drug formulations, potentially
infectious materials, or any other potentially hazardous materials. The worker will
be exposed to the materials being manipulated on the clean bench potentially
resulting in hypersensitivity, toxicity, or infection depending on the materials being
handled. Horizontal airow clean benches must never be used as a substitute for
a biological safety cabinet. Users must be aware of the dierences between these
two devices.
Vertical Flow Clean Bench Vertical ow clean benches or CADs (Figure 9b) also
are not BSCs. They may be useful, for example, in hospital pharmacies when a
clean area is needed for preparation of intravenous solutions or for the prepa-
ration of nucleic acids for PCR. While these units generally have a sash, the air is
usually discharged into the room under the sash, resulting in the same potential
worker exposure issues presented by the horizontal laminar ow clean benches.
These benches should never be used when handling cell culture materials, drug
formulations, potentially infectious materials, or any other potentially hazardous
materials.
Part 4—Other Laboratory Hazards and Risk Assessment
Primary containment is an important strategy in minimizing exposure to the many
chemical, radiological and biological hazards encountered in the laboratory. In
Table 2, an overview is provided of the various classes of BSCs, the level of
containment aorded by each, and the appropriate risk assessment consider-
ations. Microbiological risk assessment is addressed in depth in Section II of
BMBL.

377Appendix A—Primary Containment for Biohazards
Working with Chemicals in BSCs
Work with infectious microorganisms often requires the use of various chemical
agents, and many commonly used chemicals vaporize easily. Therefore, evalu-
ation of the inherent hazards of the chemicals must be part of the risk assessment
when selecting a BSC. Flammable chemicals should not be used in Class II,
Type A1, A2, and non-ducted Type C1 cabinets since vapor buildup inside the
cabinet presents a re hazard. In order to determine the greatest chemical
concentration that might be entrained in the air stream following an accident or
spill, it is necessary to evaluate the quantities to be used. Mathematical models
are available to assist in these determinations.
12
For more information regarding
the risks associated with exposure to chemicals, the reader should consult the
Permissible Exposure Levels determined under OSHA regulations available
at https://www.osha.gov/dsg/annotated-pels/tablez-1.html and Threshold Limit
Values (TLVs) for various chemical substances established by the American
Conference of Governmental Industrial Hygienists.
14
The electrical systems of Class II BSCs are not spark-proof. Therefore, a
chemical concentration approaching the lower explosive limits of the compound
must be prohibited. Furthermore, since non-exhausted Class II, Type A1, A2, and
C1 cabinets return chemical vapors to the cabinet workspace and the room, they
may expose the operator and other room occupants to toxic chemical vapors.
A chemical fume hood should be used for procedures using volatile chemicals
instead of a BSC when biological containment is not needed. Chemical fume
hoods are connected to an independent exhaust system and operate with
single-pass air discharged, directly or through a manifold, outside the building.
They may also be used when manipulating chemical carcinogens.
9
When
manipulating small quantities of volatile, toxic chemicals, required for use in
microbiological studies, Class I and Class II (Type B1 and B2) BSCs, exhausted
to the outdoors, can be used. The Class II, Type A1, A2, and C1 canopy-
exhausted cabinets may be used with small quantities of volatile, toxic chemicals.
8
Many liquid chemicals, including nonvolatile antineoplastic agents, chemothera-
peutic drugs and low-level radionuclides, can be safely handled inside properly
canopy connected Class II, Type A, and C1 cabinets.
10,11
Class II BSCs should
not be used for labeling of biohazardous materials with radioactive iodine or other
volatile radionuclides. Hard-ducted, ventilated containment devices incorporating
both HEPA and charcoal lters in the exhaust systems are necessary for the
conduct of this type of work.
Many virology and cell culture laboratories use diluted preparations of chemical
carcinogens
15,16
and other toxic substances. Prior to maintenance, a careful evalu-
ation must be made of potential problems associated with decontaminating the
cabinet and the exhaust system. Air treatment systems, such as a charcoal lter
16

378 Biosafety in Microbiological and Biomedical Laboratories
may be required so that discharged air meets applicable emission regulations.
A bag-in/bag-out housing may be needed to reduce the exposure risk to workers
replacing chemically contaminated lters.
Radiological Hazards in the BSC
As indicated above, volatile radionuclides such as I
125
should not be used within
Class II BSCs. When using nonvolatile radionuclides inside a BSC, the same
hazards exist as if working with radioactive materials on the benchtop. Work with
nonvolatile radionuclides that has the potential for splatter or creation of aerosols
can be done within the BSC.
Radiologic monitoring must be performed. A straight, vertical (i.e., not sloping)
beta shield may be used inside the BSC to provide worker protection. A sloping
shield can disrupt the air curtain and increase the possibility of contaminated
air being released from the cabinet. A radiation safety professional should be
contacted for specic guidance.
Risk Assessment
The potential for adverse events must be evaluated to eliminate, or reduce to the
greatest extent possible, worker exposure to infectious organisms and to prevent
release to the environment. Agent summary statements, detailed in Section VIII
of BMBL or from other reputable sources, such as the Public Health Agency of
Canada, provide data for microorganisms known to have caused Laboratory-
associated infections that may be used in protocol-driven risk assessments.
Through the process of risk assessment, the laboratory environment and the work
to be conducted are evaluated to identify hazards and develop interventions to
reduce risks to an acceptable level.
A properly certied and operational BSC is an eective engineering control
(Part 6) that must be used in concert with the appropriate practices, procedures,
and other administrative controls to further reduce the risk of exposure to poten-
tially infectious microorganisms. Suggested work practices and procedures for
minimizing risks when working in a BSC are detailed in Part 5.
Part 5—BSC Use by the Investigator: Work Practices and Procedures
Preparing for Work within a Class II BSC
Preparing a written checklist of materials necessary for a particular activity
and placing necessary materials in the BSC before beginning work serves to
minimize the number and extent of air curtain disruptions compromising the
fragile air barrier of the cabinet. The rapid movement of a worker’s arms in
a sweeping motion into and out of the cabinet will disrupt the air curtain and
compromise the partial containment barrier provided by the BSC. Moving arms
in and out slowly, perpendicular to the face opening of the cabinet will reduce
379Appendix A—Primary Containment for Biohazards
this risk. Other personnel activities in the room (e.g., rapid movements near the
face of the cabinet, walking trac, room fans, open/closing room doors) may
also disrupt the cabinet air barrier.
6
Laboratory coats, preferably with knit or elastic cus, should be worn buttoned
over street clothing; latex, vinyl, nitrile, or other suitable gloves are worn to
provide hand protection. Increasing levels of PPE may be warranted as deter-
mined by an individual risk assessment. For example, a solid-front, back-closing
laboratory gown provides better protection of personal clothing than a traditional
laboratory coat and is a recommended practice at BSL-3.
Before beginning work, the investigator should adjust the stool height in an
ergonomic position with proper back and feet support so that his/her face
is above the front opening. Manipulation of materials should be delayed for
approximately one minute after placing the hands/arms inside the cabinet.
This allows the cabinet to stabilize, to air sweep the hands and arms, and to
allow time for turbulence reduction. When the user’s arms rest atly across
the front grille, occluding the grille opening, room air laden with particles may
ow directly into the work area, rather than being drawn down through the front
grille. Raising the arms slightly will alleviate this problem. Ergonomic elbow
rests can also be used that elevate the elbows above the front grille so as to
not disrupt the airow and keep the user’s arms and shoulders in a comfortable
position. The front grille must not be blocked with such things as toweling,
research notes, discarded plastic wrappers, and/or pipetting devices. All opera-
tions should be performed on the work surface at least four inches in from the
front grille. If there is a drain valve under the work surface, it should be closed
prior to beginning work in the BSC.
Materials or equipment placed inside the cabinet may cause disruption of the
airow, resulting in turbulence, possible cross-contamination, and/or breach of
containment. Extra supplies (e.g., additional gloves, culture plates or asks,
culture media) should be stored outside the cabinet. Only the materials and
equipment required for the immediate work should be placed in the BSC.
For some laboratory applications, specially designed BSCs containing large pieces
of specialized equipment such as cell analyzers, ow cytometers, incubators, and
centrifuges may be installed by the manufacturer and will require eld certication.
In those instances, the manufacturer should supply to the user the certication
testing methodology information that assures the BSC will pass containment to
NSF/ANSI 49-2018. In situations where a user places a new or dierent piece
of equipment in the BSC, whether it is a special BSC or standard model, smoke
visualization with equipment operational is required to eld verify containment
performance. The certier should consult with the manufacturer during smoke
visualization testing to provide guidance for the certication evaluation.
380 Biosafety in Microbiological and Biomedical Laboratories
BSCs are performance veried by the manufacturer for use by a single individual
at any given time. If it is deemed necessary by a facility for more than one person
to be working in a BSC at the same time it should only be done after performing
a comprehensive risk assessment for both product and personnel that encom-
passes hazard identication, exposure assessment, dose-response assessment,
risk characterization, and a risk mitigation strategy.
BSCs are designed for 24-hour per day operation and some investigators
believe that continuous operation of non-canopied Class IIA BSCs helps control
the laboratory’s level of dust and other airborne particulates. Although energy
conservation may suggest BSC operation only when needed, especially if the
cabinet is not used routinely, room air balance is an overriding consideration. Air
discharged through ducted BSCs must be considered in the overall air balance
of the laboratory. If night setback modes are used for BSC’s, they must be
interlocked to the laboratory supply and exhaust system to maintain negative
laboratory air balance.
If the cabinet has been shut down, the blowers should be operated at least ve
minutes before beginning work to allow the cabinet to purge. This purge will
remove any suspended particulates in the cabinet. The work surface, the interior
walls (except the supply lter diuser), and the interior surface of the window
should be wiped with 70% ethanol (EtOH), a 1:100 dilution of household bleach
(i.e., 0.05% sodium hypochlorite), or other disinfectant as determined by the
investigator to meet the requirements of the particular activity. When bleach
is used, a second wiping with sterile water is needed to remove the residual
chlorine, which may eventually corrode stainless steel surfaces. Wiping with
non-sterile water may recontaminate cabinet surfaces, which is a critical issue
when sterility is essential (e.g., maintenance of cell cultures).
Similarly, the surfaces of all materials and containers placed into the cabinet
should be wiped with 70% EtOH or other disinfectant determined to meet the
laboratory’s need to reduce the introduction of contaminants to the cabinet
environment. This simple step will reduce introduction of mold spores and
thereby minimize contamination of cultures. Further reduction of microbial load on
materials to be placed or used in BSCs may be achieved by periodic decontami-
nation of incubators and refrigerators.
Material Placement inside the BSC
Plastic-backed, absorbent toweling can be placed on the work surface but not on
the front or rear grille openings. The use of toweling facilitates routine cleanup
and reduces splatter and aerosol generation
17
during an overt spill. It can be
folded and placed in a biohazard bag or other appropriate waste receptacle when
work is completed.
381Appendix A—Primary Containment for Biohazards
All materials should be placed as far back in the cabinet as practical, toward
the rear edge of the work surface and away from the front and back grille of the
cabinet. Similarly, aerosol-generating equipment (e.g., vortex mixers, tabletop
centrifuges) should be placed toward the rear of the cabinet to take advantage
of the air split described in Part 3. Bulky items such as biohazard bags, discard
pipette trays, and vacuum collection asks should be placed to one side of the
interior of the cabinet. If placing those items in the cabinet requires opening the
sash, make sure that the sash is returned to its original position before work is
initiated. The correct sash position should be indicated on the front of the cabinet.
An audible alarm will sound if the sash is in the wrong position while the fan is
operating. Biological material or other hazardous agents should be placed in the
BSC last.
Certain common practices interfere with the operation of the BSC. The biohazard
collection bag should not be taped to the outside of the cabinet. This practice
encourages the BSC user to frequently move in and out of the BSC to move
discarded materials into the outside bag. Movement in and out of the BSC should
be minimized to reduce the risk of biohazardous materials being brought out
of the BSC or room contamination being brought into the BSC. Upright pipette
collection containers should neither be used in BSCs nor placed on the oor
outside the cabinet. The frequent inward/outward movement needed to place
objects in these containers is disruptive to the integrity of the cabinet air barrier
and can compromise both personnel and product protection. Horizontal pipette
discard trays, which may contain an appropriate chemical disinfectant, should be
used within the cabinet. Large sharps containers will interfere with the downward
airow and should not be used. Furthermore, potentially contaminated materials
should not be brought out of the cabinet until they have been surface decon-
taminated or placed into a closable waste container for transfer to an incubator,
autoclave, or another part of the laboratory. The closable waste container should
also be surface decontaminated prior to removal.
Operations within a Class II BSC
Laboratory Hazards Many procedures conducted in BSCs may create splatter
or aerosols. Good microbiological techniques should always be used when
working in a BSC. For example, techniques used to reduce splatter and aerosol
generation will also minimize the potential for personnel exposure to infectious
materials manipulated within the cabinet. Class II cabinets are designed so that
horizontally nebulized spores introduced into the cabinet will be captured by the
downward owing cabinet air within 14 in
8
of travel. Therefore, keeping clean
materials at least one foot away from aerosol-generating activities will minimize
the potential for cross-contamination.
382 Biosafety in Microbiological and Biomedical Laboratories
The workow should be from clean to dirty (Figure 10). Materials and supplies
should be placed in the cabinet in such a way as to limit the movement of dirty
items over clean ones.
Several measures can be taken to reduce the chance for cross-contamination of
materials when working in a BSC. Opened tubes or bottles should not be held in a
vertical position. Investigators working with Petri dishes and tissue culture plates
should hold the lid above the open sterile surface to minimize direct impaction of
downward air. Bottle or tube caps should not be placed on the toweling if used.
Items should be recapped or covered as soon as possible.
Open ames are neither required nor recommended in the near microbe-free
environment of a biological safety cabinet. On an open bench, aming the neck
of a culture vessel will create an upward air current that prevents microorganisms
from falling into the tube or ask. An open ame in a BSC, however, creates
turbulence that disrupts the pattern of HEPA-ltered air being supplied to the work
surface. When deemed absolutely necessary and approved by the appropriate
facility authorities after a thorough risk assessment, touch-plate micro burners
equipped with a pilot light to provide a ame on demand may be used. Internal
cabinet air disturbance and heat buildup will be minimized. The burner must
be turned o when work is completed. Small electric furnaces are available for
decontaminating bacteriological loops and needles and are preferable to an open
ame inside the BSC. Disposable loops should be used whenever possible.
Aspirator bottles or suction asks should be connected to an overow collection
ask containing appropriate disinfectant and to an in-line HEPA or equivalent lter
(Figure 11). Commercial equivalents are acceptable once validated for specic
laboratory use. This combination will provide protection to the central building
vacuum system or vacuum pump, as well as to the personnel who service this
equipment. Inactivation of aspirated materials can be accomplished by placing
a volume of a chemical decontamination solution having a concentration of
chemical sucient to decontaminate microorganisms when the ask is lled to
its maximum capacity into the ask to inactivate the microorganisms as they are
collected. Once inactivation occurs, liquid materials can be disposed of as nonin-
fectious waste. The ask material should be resistant to the decontamination
solution used.
Investigators must determine the appropriate method of decontaminating wastes
that will be removed from the BSC at the conclusion of the work. When chemical
means alone are appropriate, a suitable liquid disinfectant should be placed into
a discard pan before work begins. Items should be introduced into the pan with
minimum splatter, covered completely, and allowed appropriate contact time as
per manufacturer’s instructions. Alternatively, liquids can be autoclaved prior to
disposal. The liquid container should be placed in a suitable, secondary container,
383Appendix A—Primary Containment for Biohazards
and the outside of these containers wiped with a suitable liquid disinfectant, prior
to removal from the BSC.
When a steam autoclave is used for solid wastes, contaminated materials should
be placed into a biohazard bag or discard pan. Adding water to ensure steam
generation during the autoclave cycle needs to be determined experimentally.
The bag should be loosely closed (to allow steam to enter the bag) or the discard
pan should be covered in the BSC prior to transfer to the autoclave. The bag
should be transported and autoclaved in a leak-proof tray or pan. It is a prudent
practice to decontaminate the exterior surface of bags and pans just prior to
removal from the cabinet.
Decontamination
Cabinet Surface Decontamination With the cabinet blower running, all
containers and equipment should be surface decontaminated and removed
from the cabinet when work is completed. All biological materials and hazardous
agents should be removed rst. At the end of the workday, the nal surface
decontamination of the cabinet should include a wipe-down of the work surface,
the cabinet’s sides and back, and the interior of the glass. If necessary, the
cabinet should also be monitored for radioactivity and decontaminated when
necessary. Investigators should remove their gloves and gowns in a manner to
prevent contamination of unprotected skin and aerosol generation and wash their
hands as the nal step in safe microbiological practices. The cabinet blower may
be left on or turned o after these operations are completed.
Small spills within the operating BSC can be handled immediately by removing
the contaminated absorbent paper toweling and placing it into the biohazard bag
or receptacle. Small spills inside the BSC can be covered with paper towels, and
starting from the outside of the spill, covered in an appropriate disinfectant. Once
appropriate contact time is reached, usually 20 to 30 minutes, towels should be
pushed from the edge of the spill to the center and disposed of into a biohazard
bag or receptacle. Cabinet interior and items inside the BSC should be wiped
down with a towel dampened with disinfectant. Gloves should be changed after
the work surface is decontaminated and before placing clean absorbent toweling,
if used in the cabinet.
Spills large enough to result in liquids owing through the front or rear grilles
require decontamination that is more extensive. All items within the cabinet
should be surface decontaminated and removed. After ensuring that the drain
valve is closed, decontaminating solution can be poured onto the work surface
and through the grille(s) into the drain pan. The drain pan should be emptied into
a collection vessel containing disinfectant. A hose barb and exible tube should
be attached to the drain valve and be of sucient length to allow the open end
to be submerged in the disinfectant within the collection vessel. This procedure
384 Biosafety in Microbiological and Biomedical Laboratories
serves to minimize aerosol generation. The drain pan should be ushed with
water, the drain tube removed, and the drain valve closed.
Should the spilled liquid contain a hazardous chemical or radioactive material,
a similar procedure can be followed. The appropriate safety personnel should
be contacted for specic instructions.
Periodic removal of the cabinet work surface and/or grilles after the completion
of drain pan decontamination is recommended because of dirty drain pan
surfaces and grilles, which ultimately could occlude the drain valve or block
airow. However, extreme caution should be observed while wiping these
surfaces to avoid injury from sharp metal edges and other items (e.g., broken
glass, pipette tips) that may be present. Always use disposable paper toweling
and avoid applying harsh force. Wipe dirty surfaces gently. Never leave toweling
on the drain pan because the paper could block the drain valve or the air
passages in the cabinet.
Gas Decontamination BSCs that have been used for work involving infectious
materials must be decontaminated before HEPA lters are changed or internal
repair work is done.
8,18–20
Before a BSC is relocated, a risk assessment considering
the agents manipulated within the BSC must be performed to determine the need
and method for decontamination. The most common decontamination methods
use formaldehyde gas, hydrogen peroxide vapor,
8
or chlorine dioxide gas.
Part 6—Facility and Engineering Requirements
Secondary Barriers
BSCs are considered the primary containment barrier for manipulation of
infectious materials, and the laboratory room itself is considered the secondary
containment barrier.
21
Inward directional airow is established by
22
exhausting
a greater volume of air than is supplied to a given laboratory and by drawing
makeup air from the adjacent space. This is optional at BSL-2 but must be
maintained at BSL-3 and BSL-4.
23
The air balance for the entire facility should be
established and maintained to ensure that airow is from areas of least to greater
potential contamination.
Building Exhaust BSL-4 laboratory air must be directly exhausted to the outside
since it is considered potentially contaminated. This concept is referred to as a
dedicated, single-pass exhaust system. The exhausted room air can be HEPA-l-
tered when a high level of aerosol containment is needed, which is always true at
BSL-4, but is an enhancement at BSL-3 and recommended for work with some
organisms.
3
When the building exhaust system is used to vent a Class IIB BSC,
the exhaust system must be designed using the CBV and have sucient capacity
to maintain the exhaust ow if changes in the static pressure within the system
should occur.
8
The connection to a BSC must be constant air volume (CAV).
385Appendix A—Primary Containment for Biohazards
The HVAC exhaust system must be sized to handle both the room exhaust
and the exhaust requirements of all containment devices that may be present.
Adequate supply air must be provided to ensure appropriate function of the
exhaust system. Right-angle bends, changing duct diameters, and transitional
connections within the systems will add to the demand on the exhaust fan.
The building exhaust air should be discharged away from supply air intakes,
to prevent re-entrainment of laboratory exhaust air into the building air supply
system. Refer to recognized design guides for locating the exhaust terminus
relative to nearby air intakes.
24
Utility Services Utility services needed within a BSC must be planned carefully.
Protection of vacuum systems must be addressed (Figure 11). Electrical outlets
inside the cabinet must be protected by ground fault circuit interrupters and
should be supplied by an independent circuit. The use of open ames in the BSC
is not recommended.
8
In very rare instances, when propane or natural gas needs
to be provided, a clearly marked emergency gas shut-o valve outside the cabinet
must be installed for re safety. All non-electrical utility services should have
exposed, accessible shut-o valves. The use of compressed air within a BSC
must be carefully considered and controlled to prevent aerosol production and
reduce the potential for vessel pressurization.
Ultraviolet Lamps Ultraviolet (UV) lamps should not be used as the sole
disinfection method in a BSC. If installed, UV lamps should be cleaned regularly
to remove any lm that may block the output of the lamp. The lamps should be
evaluated regularly and checked with a UV meter to ensure that the appropriate
intensity of UV light is being emitted. Replace the bulb when the uence rate is
below 40 uW/cm
2
. Unshielded UV lamps must be turned o when the room is
occupied to protect eyes and skin from UV exposure. If the cabinet has a sliding
sash, close the sash when operating the UV lamp. Most new BSCs use sliding
sashes that are interlocked when operating the UV lamp to prevent exposure.
BSC Placement BSCs were developed as workstations to provide personnel,
environmental, and product protection during the manipulation of infectious
microorganisms. Certain considerations must be met to ensure maximum
eectiveness of these primary barriers. Whenever possible, adequate clearance
should be provided behind and on each side of the cabinet to allow easy access
for maintenance and to ensure that the cabinet air re-circulated to the laboratory
is not hindered. A 12–14 inch clearance above the cabinet is required to provide
for accurate air velocity measurement across the exhaust lter surface
25,26
and
for exhaust lter changes. When the BSC is hard-ducted (direct-connected) or
canopy connected to the ventilation system, adequate space must be provided
so that the conguration of the ductwork will not interfere with airow. The canopy
unit must provide adequate access to the exhaust HEPA lter for testing.
386 Biosafety in Microbiological and Biomedical Laboratories
The ideal location for the biological safety cabinet is remote from the entry
(i.e., the rear of the laboratory away from trac) since people walking parallel to
the face of a BSC can disrupt the air curtain.
8,16,27
The air curtain created at the
front of the cabinet is quite fragile, amounting to a nominal inward and downward
velocity of one mph. Open windows, air supply registers, portable fans, or
laboratory equipment that creates air movement (e.g., centrifuges, vacuum
pumps) should not be located near the BSC. Similarly, chemical fume hoods
must not be located close to BSCs.
HEPA Filters HEPA lters, whether part of a building exhaust system or part of
a cabinet, will require replacement when they become loaded to the extent that
sucient airow can no longer be maintained. In most instances, lters must be
decontaminated before removal. To contain the decontamination gas or vapor
used for microbiological decontamination, exhaust systems containing HEPA
lters require airtight dampers to be installed on both the inlet and discharge side
of the lter housing. This ensures containment of the gas or vapor inside the lter
housing during decontamination. Access panel ports in the lter housing also
allow for performance testing of the HEPA lter (Part 7).
A bag-in/bag-out lter assembly
3,28
(Figure 12) can be used in situations where
HEPA ltration is necessary for operations involving biohazardous materials and
hazardous or toxic chemicals. The bag-in/bag-out system is used when it is not
possible to gas or vapor decontaminate the HEPA lters, or when hazardous
chemicals or radionuclides have been used in the BSC, and provides protection
against exposure for the maintenance personnel and the environment. A bag-in/
bag-out system will require a method to decontaminate or safely dispose of the
lter once removed (e.g., a waste service that will decontaminate the lter, or a
large enough autoclave). Note, however, that this requirement must be identied
at the time of purchase and installation; a bag-in/bag-out assembly cannot be
added to a cabinet after-the-fact without an extensive engineering evaluation.
Part 7—Certication of BSCs
Development of Containment Standards
The evolution of containment equipment for varied research and diagnostic
applications created the need for consistency in construction and performance.
Federal Standard 209
29
was developed to establish classes of air cleanliness and
methods for monitoring clean workstations and cleanrooms where HEPA lters
are used to control airborne particulates. It has since been replaced with ISO
14644-2015.
30
The rst “standard” to be developed specically for BSCs
12
served as a Federal
procurement specication for the NIH Class II, Type 1 (now called Type A1)
BSC, which had a xed or hinged front window or a vertical sliding sash, vertical
downward airow, and HEPA-ltered supply and exhaust air. This specication
387Appendix A—Primary Containment for Biohazards
described design criteria and dened prototype tests for microbiological aerosol
challenge, velocity proles, and leak testing of the HEPA lters. A similar
procurement specication was generated
31
when the Class II, Type 2 (now called
Type B1) BSC was developed.
NSF/ANSI 49 for Class II BSCs was rst published in 1976, providing the rst
independent standard for design, manufacture, and testing of BSCs. This standard
replaced the NIH specications, which were being used by other institutions
and organizations purchasing BSCs. NSF/ANSI 49-2018
8
incorporates current
specications regarding design, construction, performance, and eld certication.
This Standard for BSCs establishes performance criteria and provides the minimum
testing requirements that are accepted in the United States. Cabinets that meet the
Standard and are certied by NSF bear an “NSF” mark.
NSF/ANSI 49-2018 pertains to all models of Class II cabinets (Type A1, A2, B1,
B2, C1) and provides a series of specications regarding:
■
Design/construction;
■
Performance;
■
Installation recommendations; and
■
Recommended microbiological decontamination procedures.
References and specications pertinent to Class II Biosafety Cabinetry, Annex F
of NSF/ANSI 49-2018, which covers eld testing of BSCs, is a normative part of
the Standard. This Standard is reviewed periodically by a committee of experts to
ensure that it remains consistent with developing technologies
The operational integrity of a BSC must be validated before it is placed into
service and after it has been repaired or relocated. Relocation may break the
HEPA lter seals or otherwise damage the lters or the cabinet. Each BSC should
be tested and certied at least annually to ensure continued, proper operation.
On-site eld certication (NSF/ANSI 49-2018, Annex F) must be performed
by experienced, qualied personnel. Some basic information is included in
the Standard to assist in understanding the frequency and kinds of tests to be
performed. In 1993, NSF began a program for accreditation of certiers based on
written and practical examinations. Education and training programs for persons
seeking accreditation as qualied to perform all eld certication tests are oered
by a variety of organizations. Selecting competent individuals to perform testing
and certication is important. It is suggested that the institutional biosafety ocer
(BSO) or Health and Safety Oce be consulted when identifying companies
qualied to conduct the necessary eld performance tests.
It is strongly recommended that, whenever possible, accredited eld certiers are
used to test and certify BSCs. If in-house personnel are performing the certica-
tions, then these individuals should become accredited.
388 Biosafety in Microbiological and Biomedical Laboratories
Performance Testing BSCs in the Field
Class II BSCs are the primary containment devices that protect the worker,
product, and environment from exposure to microbiological agents. BSC opera-
tions, as specied by NSF/ANSI 49-2018, Annex F need to be veried at the
time of installation and, as a minimum, annually thereafter. A cabinet should be
recertied whenever a HEPA or ULPA lter is replaced, maintenance repairs are
made to internal parts, or a cabinet is relocated.
Finally, accurate test results can only be assured when the testing equipment
is properly maintained and calibrated. It is appropriate to request the calibration
information for the test equipment being used by the certier.
Table 1. Selection of a Safety Cabinet through Risk Assessment
Biosafety
Level Personnel
Protection
Provided
Product Environmental BSC Class
BSL-1 to 3 Yes No Yes I
BSL-1 to 3 Yes Yes Yes II (A1, A2, B1, B2)
BSL-4 Yes Yes Yes III; II—when used in suit
room with suit
Table 2. Comparison of Biosafety Cabinet Characteristics
BSC
Class
Face
Velocity Airow Pattern
Application:
Nonvolatile Toxic
Chemicals and
Radionuclides
Application:
Volatile Toxic
Chemicals and
Radionuclides
I 75
In at front through HEPA to the outside or into the room through
HEPA (Figure 2)
Yes
When exhausted
outdoorsa,b
II, A1 75
70% recirculated to the cabinet work area through HEPA; 30%
balance can be exhausted through HEPA back into the room or to
outside through a canopy unit (Figure 3)
c
Yes (small amounts)
b
Yes (small amounts)
a,b
II, B1 100
30% recirculated, 70% exhausted. Exhaust cabinet air must pass
through a dedicated, internal cabinet duct to the outside through a
HEPA lter (Figures 5a 5b)
Yes Yes (small amounts)
a,b
I, B2 100
No recirculation; total exhaust to the outside through a HEPA lter
(Figure 6)
Yes Yes (small amounts)
a,b
II, A2 100
Similar to II, A1, but has 100 lfm intake air velocity exhaust air can
be ducted to the outside through a canopy unit (Figure 7)
Yes
When exhausted
outdoors (formally B3),
(small amounts)
a,b
II, C1 100
30% recirculated, 70% exhausted. Exhaust cabinet air must pass
through a dedicated, internal cabinet duct to the outside through a
blower and HEPA lter
Yes Yes (small amounts)
a,b
III N/A
Supply air is HEPA-ltered. Exhaust air passes through two
HEPA lters in series and is exhausted to the outside via a hard
connection (Figure 8)
Yes Yes (small amounts)
a,b
a. Installation requires a special duct to the outside, and may require an in-line charcoal lter, and/or a spark-proof
(explosion-proof) motor and other electrical components in the cabinet. Discharge of a Class I or Class II, Type A2
cabinet into a room should not occur if volatile chemicals are used.
b. A risk assessment should be completed by laboratory and safety facility personnel to determine amounts to be
used. In all cases, only the smallest amounts of the chemical(s) required for the work to be performed should
be used in the BSC. In no instance should the chemical concentration approach the lower explosion limits of the
compounds.
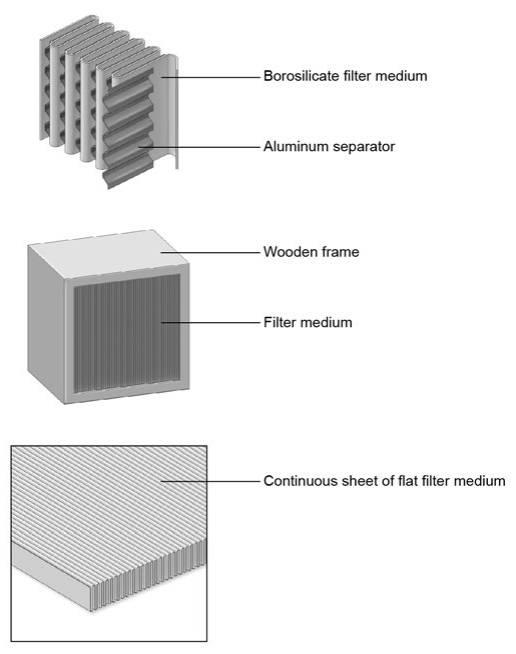
389Appendix A—Primary Containment for Biohazards
c. Class IIA1 cabinets built prior to 2010 were allowed to have potentially contaminated, positively pressurized
plenums. After 2010, All Class II cabinets must have potentially contaminated plenums under negative pressure
or surrounded by negatively pressurized plenums.
Figure 1. HEPA Filters
HEPA lters are typically constructed of paper-thin sheets of borosilicate medium,
pleated to increase surface area, and axed to a frame. Aluminum or plastic
separators are often added for stability.
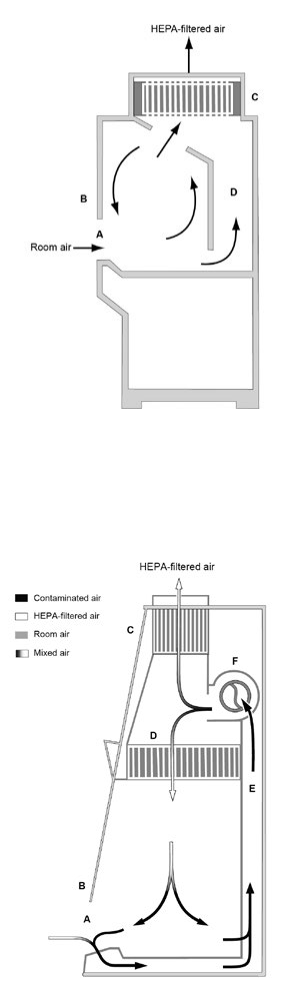
390 Biosafety in Microbiological and Biomedical Laboratories
Figure 2. The Class I BSC
(A) front opening; (B) sash; (C) exhaust HEPA lter; (D) exhaust plenum.
Note: this classical style cabinet needs to be direct-connected to the building
exhaust system.
Figure 3. The Class II, Type A BSC
(A) front opening; (B) sash; (C) exhaust HEPA lter; (D) supply HEPA lter;
(E) common plenum; (F) exhaust blower. Note: Since 2010 there is minimal
dierence between the Class II, Type A1 and Class II, Type A2 except for the
inow velocity.
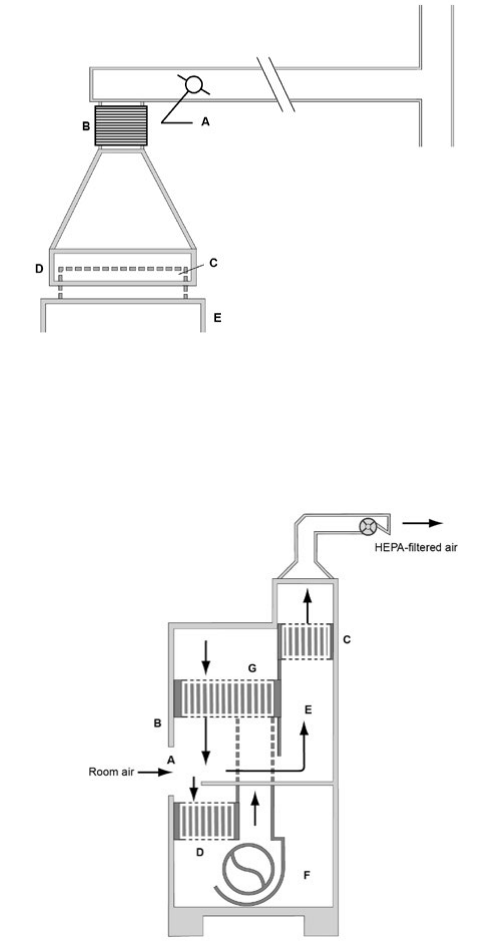
391Appendix A—Primary Containment for Biohazards
Figure 4. Canopy (thimble) unit for ducting a Class II, Type A BSC
(A) balancing damper; (B) exible connector to exhaust system; (C) cabinet
exhaust HEPA lter housing; (D) canopy unit; (E) BSC. Note: There is a gap
between the canopy unit (D) and the exhaust lter housing (C), through which
room air is exhausted.
Figure 5a. The Class II, Type B1 BSC (classic design)
(A) front opening; (B) sash; (C) exhaust HEPA lter; (D) supply HEPA lter;
(E) negative pressure dedicated exhaust plenum; (F) blower; (G) additional
HEPA lter for supply air. Note: The cabinet exhaust needs to be direct-
connected to the building exhaust system.
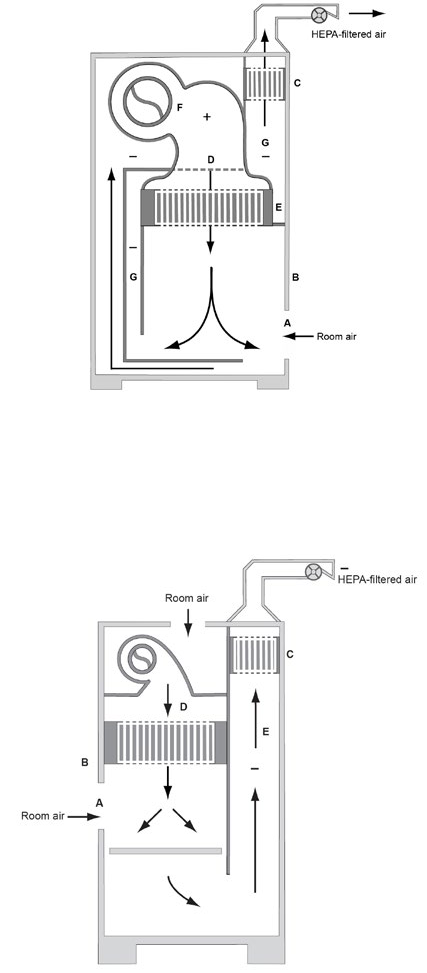
392 Biosafety in Microbiological and Biomedical Laboratories
Figure 5b. The Class II, Type B1 BSC (benchtop design)
(A) front opening; (B) sash; (C) exhaust HEPA lter; (D) supply plenum; (E) supply
HEPA lter; (F) blower; (G) negative pressure exhaust plenum. Note: The cabinet
exhaust needs to be direct-connected to the building exhaust system.
Figure 6. The Class II, Type B2 BSC
(A) front opening; (B) sash; (C) exhaust HEPA lter; (D) supply HEPA lter;
(E) negative pressure exhaust plenum. Note: The cabinet needs to be
direct-connected to the building exhaust system.
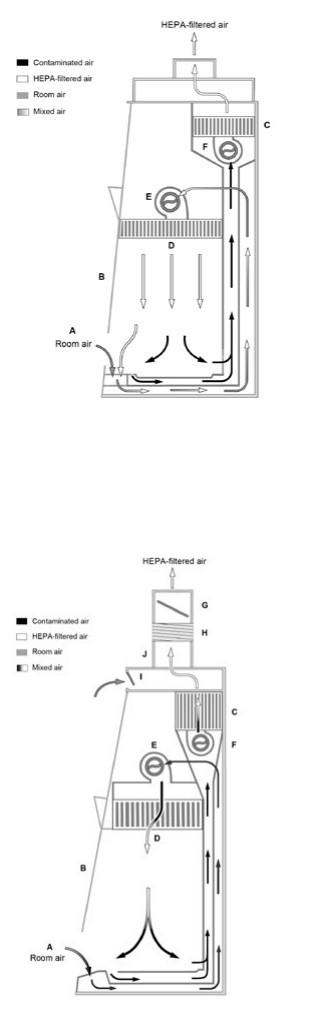
393Appendix A—Primary Containment for Biohazards
Figure 7a. The Class II, Type C1 BSC (not connected to building exhaust system)
(A) front opening; (B) sash; (C) exhaust HEPA lter; (D) supply lter; (E) supply
blower; (F) exhaust blower.
Figure 7b. The Class II, Type C1 BSC (connected to building exhaust system)
(A) front opening; (B) sash; (C) exhaust HEPA lter; (D) supply HEPA lter; (E)
supply blower; (F) exhaust blower; (G) balancing damper; (H) sealed exible duct
(optional); (I) canopy opening/gap; (J) exhaust duct.
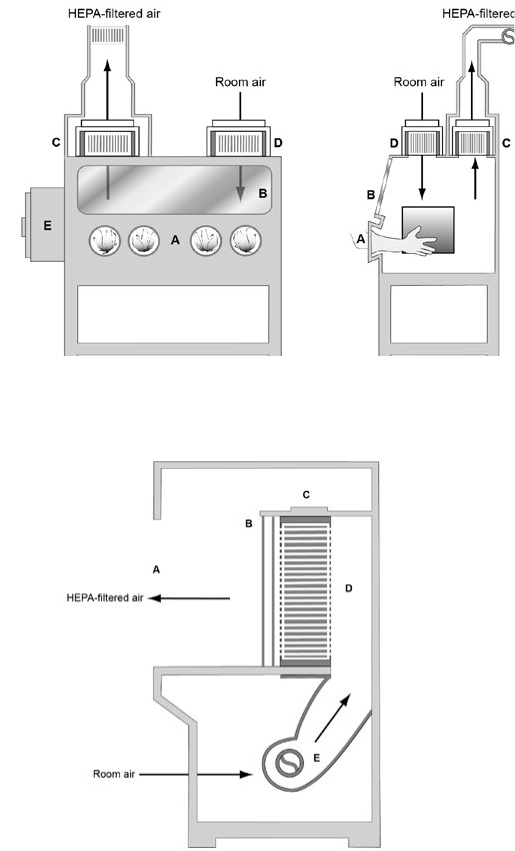
394 Biosafety in Microbiological and Biomedical Laboratories
Figure 8. The Class III BSC
(A) glove ports with O-ring for attaching arm-length gloves to cabinet;
(B) window; (C) exhaust HEPA lter; (D) supply HEPA lter; (E) double-ended
autoclave or pass-through box; (F) exhaust HEPA lter. Note: A chemical dunk
tank may be installed, which would be located beneath the work surface of the
BSC with access from above. The cabinet exhaust needs to be direct-connected
to an exhaust system where the fan is separate from the exhaust fans of the
facility ventilation system. The exhaust air must be double HEPA-ltered or
HEPA-ltered and incinerated.
Figure 9a. The Horizontal Laminar ow Clean Bench
(A) front opening; (B) supply grille; (C) supply HEPA lter; (D) supply plenum;
(E) blower.
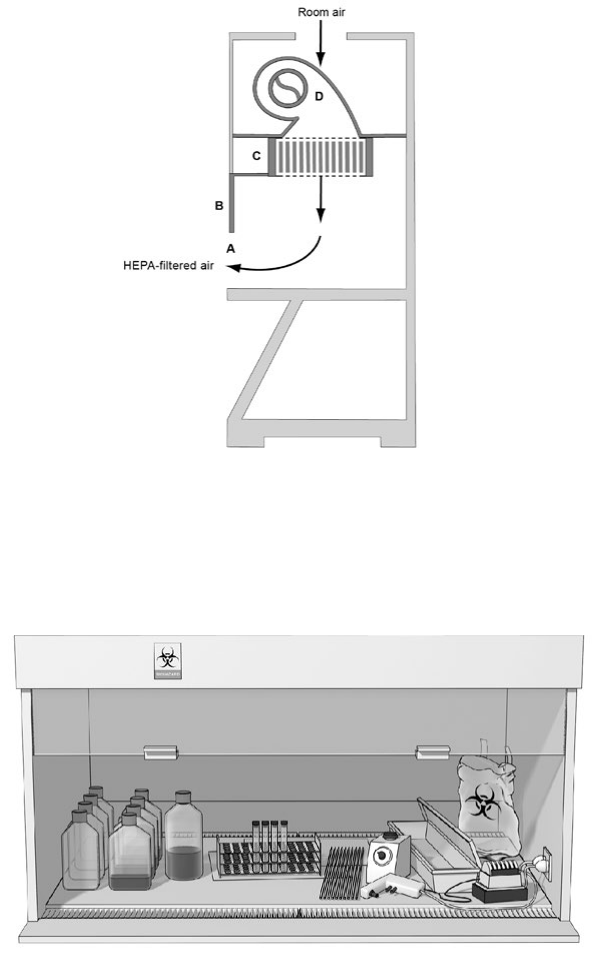
395Appendix A—Primary Containment for Biohazards
Figure 9b. The Vertical Laminar Flow Clean Bench
(A) front opening; (B) sash; (C) supply HEPA lter; (D) blower. Note: Some
vertical ow clean benches have recirculated air through front and/or rear grilles.
Figure 10. Clean to Dirty
A typical layout for working from the clean to the dirty side within a Class II BSC.
Clean cultures (left) can be inoculated (center); contaminated pipettes can be
discarded in the shallow pan and other contaminated materials can be placed in
the biohazard bag (right). This arrangement is reversed for left-handed persons.
396 Biosafety in Microbiological and Biomedical Laboratories
Figure 11. Protection of a house vacuum
Example method to protect a house vacuum system during aspiration of infec-
tious uids. The suction ask (A) is used to collect the contaminated uids into a
suitable decontamination solution; the right ask (B) serves as a uid overow
collection vessel. An in-line HEPA lter (C) is used to protect the vacuum system
(D) from aerosolized microorganisms.
Figure 12. Bag-in/bag-out lter enclosure
A bag-in/bag-out lter enclosure allows for the removal of the contaminated lter
without worker exposure. (A) lters; (B) bags; (C) safety straps; (D) cinching
straps; (E) shock cord located in the mouth of the PVC bag restricts the bag
around the second rib of the housing lip.
Acknowledgments
We gratefully acknowledge the Baker Company; Filtration Group, Inc.;
Flanders Filters; and Forma Scientic, Inc., for use of some drawings and
gures reproduced herein.
397Appendix A—Primary Containment for Biohazards
References
1. Kruse RH, Puckett WH, Richardson JH. Biological safety cabinetry. Clin
Microbiol Rev. 1991;4(2):207–41.
2. HEPA and ULPA Filters, IEST-RP-CC001 (2016).
3. First MW. Filters, high capacity lters and high-eciency lters: review and
production. In-Place Filter Testing Workshop; 1971; Boston, Massachusetts.
4. Dow Chemical U.S.A.; National Cancer Institute. A Workshop for
Certication of Biological Safety Cabinets. No. BH 74-01-11. Midland (MI):
Dow Chemical U.S.A.; 1974.
5. Richmond JY. Safe practices and procedures for working with human
specimens in biomedical research laboratories. J Clin Immunoassay.
1988;13:115–9.
6. Barbeito MS, Taylor LA. Containment of microbial aerosols in a
microbiological safety cabinet. Appl Microbiol. 1968;16(8):1255–9.
7. Whiteld WJ. A new approach to cleanroom design. Albuquerque (NM):
Sandia Corporation; 1962.
8. NSF International (NSF); American National Standards Institute
(ANSI). NSF/ANSI 49-2018. Biosafety Cabinetry: Design, Construction,
Performance, and Field Certication. Ann Arbor (MI): NSF/ANSI; 2018.
9. Jones RL Jr, Tepper B, Greenier TG, Stuart DG, Large S, Eagleson D.
Eects of Thimble Connections of Biological Safety Cabinets. Abstracts of
32nd Biological Safety Conference; 1989; New Orleans, LA.
10. Guidelines for Cytotoxic (Antineoplastic) Drugs. Standard 01-23-001,
Appendix A (1986).
11. Centers for Disease Control and Prevention; National Institute for
Occupational Safety and Health. NIOSH Alert: Preventing Occupational
Exposures to Antineoplastic and Other Hazardous Drugs in Health Care
Settings. Cincinnati (OH): NIOSH—Publications Dissemination; 2004.
12. Stuart DG, First MW, Jones RL Jr, Eagleson JM Jr. Comparison of
chemical vapor handling by three types of class II biological safety
cabinets. Particulate and Microbial Control. 1983.
13. Stuart D, Kiley M, Ghidoni D, Zarembo M. The Class III Biological Safety
Cabinet. In: Richmond JY, editor. Anthology of Biosafety VII: Biosafety
Level 3. Mundelein (IL): American Biological Safety Association; 2004.
p. 57–71.
14. American Conference of Governmental Industrial Hygienists (ACGIH).
Threshold limit values for chemical substances and physical agents and
biological exposure indices. Cincinnati (OH): ACGIH; 2006.

398 Biosafety in Microbiological and Biomedical Laboratories
15. National Institutes of Health. NIH guidelines for the laboratory use of
chemical carcinogens. Washington (DC): U.S. Department of Health &
Human Services; 1981.
16. National Cancer Institute; Oce of Research Safety. Laboratory safety
monograph: a supplement to the NIH guidelines for recombinant DNA
research. Bethesda (MD): National Institutes of Health; 1978.
17. Oce of Research Safety; National Cancer Institute. National Cancer
Institute Safety Standards for Research Involving Chemical Carcinogens.
Bethesda (MD): The National Institutes of Health; 1975.
18. Jones R, Drake J, Eagleson D. Using Hydrogen Peroxide Vapor to
Decontaminate Biological Safety Cabinets. Baker [Internet]. 1993 [cited
2019 Mar 11];1(1):[about 4 p.] Available from: https://bakerco.com/
communication/white-papers/
19. Jones R, Stuart D, Large S, Ghidoni D. Cycle Parameters for
Decontaminating a Biological Safety Cabinet Using H2O2 Vapor. Baker
[Internet]. 1993 [cited 2019 Mar 11];1(2):[about 4 p.] Available from:
https://bakerco.com/communication/white-papers/
20. Jones R, Stuart D, PhD, Large S, Ghidoni D. Decontamination of a HEPA
lter using hydrogen peroxide vapor. Acumen. 1993;1(3):1–4.
21. Fox D, editor. Proceedings of the National Cancer Institute symposium on
design of biomedical research facilities. Monograph Series. Vol 4; 1979 Oct
18–19; Frederick, MD. Litton Bionetics, Inc.; 1979.
22. Laboratories. In: American Society of Heating, Refrigerating and
Air-Conditioning Engineers. 2015 ASHRAE Handbook—HVAC Applications.
Atlanta (GA): ASHRAE; 2015.
23. Agricultural Research Service (ARS) [Internet]. Beltsville (MD): United
States Department of Agriculture; c2012 [cited 2019 Mar 12]. ARS Facilities
Design Standards. ARS—242.1. Available from: https://www.afm.ars.usda.
gov/ppweb/pdf/242-01m.pdf
24. American Conference of Governmental Industrial Hygienists (ACGIH).
Industrial Ventilation: A Manual of Recommended Practice for Design.
28th ed. Cincinnati (OH): ACGIH; 2015.
25. Jones RL Jr, Stuart DG, Eagleson D, Greenier TJ, Eagleson JM Jr. The
eects of changing intake and supply air ow on biological safety cabinet
performance. Appl Occup Environ Hyg. 1990;5(6):370–7.
26. Jones RL Jr, Stuart DG, Eagleson, D, et al. Eects of ceiling height on
determining calculated intake air velocities for biological safety cabinets.
Appl Occup Environ Hyg. 1991;6(8):683–8.
399Appendix A—Primary Containment for Biohazards
27. Rake BW. Inuence of crossdrafts on the performance of a biological safety
cabinet. Appl Environ Microbiol. 1978;36(2):278–83.
28. Barbeito MS, West DL, editors. Laboratory ventilation for hazard control.
Proceedings of a 1976 Cancer Research Safety Symposium; 1976 Oct
21–22; Frederick, MD. Frederick (MD): Frederick Cancer Research Center;
1976.
29. Airborne Particulate Cleanliness Classes in Clean rooms and Clean Zones,
Federal Standard No. 209 (1963).
30. Cleanrooms and associated controlled environments—Part 1: Classication
of air cleanliness by particle concentration, ISO 14644-1 (2015).
31. National Cancer Institute. Specications for general purpose clean air
biological safety cabinet. Bethesda (MD): National Institutes of Health; 1973.

400 Biosafety in Microbiological and Biomedical Laboratories
Appendix B—Decontamination and Disinfection of
Laboratory Surfaces and Items
Purpose and Scope
Appendix B provides basic guidance for the decontamination or disinfection
of environmental surfaces and items in the laboratory with antimicrobial
substances and other practices to mitigate the possibility of transmission of
pathogens to laboratory workers, the public, and the environment. The selection
of an appropriate antimicrobial product and adherence to the product label
instructions are critical to ensuring the product’s performance against the
target microorganism. Regulatory oversight, terminology, factors necessary for
environmentally-mediated transmission of infection (e.g., aerosol generation,
contact, indirect contact), methods for sterilization and disinfection, and the levels
of antimicrobial activity associated with liquid chemical disinfectants are reviewed
in this appendix. One must remember that aerosol-generating procedures should
be conducted in containment. Accidents involving infectious aerosols have been a
source of contamination within the laboratory setting and may impact the method
chosen for decontamination. General approaches are emphasized instead of
detailed protocols and methods. It is important to follow the manufacturer’s
instructions for use when performing decontamination practices in the laboratory.
Antimicrobial Products—U.S. Regulations
Antimicrobial pesticides (e.g., disinfectants) are classied as pesticides and are
regulated by both the United States Environmental Protection Agency under the
authority of the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA)
1,2
and the United States Food and Drug Administration, Center for Devices and
Radiologic Health by the Food Quality Protection Act (FQPA).
3
The laboratory
is responsible for selecting an appropriate EPA-registered product and using
it according to the manufacturer’s instructions on the product label. The more
commonly used public health antimicrobial products are described in the
Glossary (e.g., sporicides, disinfectants, and sanitizers). The lists of selected
EPA-registered disinfectants are available at https://www.epa.gov/oppad001/
chemregindex.htm.
The FDA has dened three types of liquid chemical germicides for processing
medical devices, and these germicides are regulated as auxiliary devices (FDA
1977 Policy Manual): (1) sterilant/high-level disinfectant; (2) intermediate-level
disinfectant; and (3) low-level disinfectant. See Glossary.
Disinfectants used in the laboratory include those recommended by equipment
manufacturers and a broad-spectrum product, typically an intermediate-level
disinfectant (i.e., a product with a mycobacteriology claim). Safe use of chemicals
within the laboratory falls under the OSHA Laboratory Standard.
4
401Appendix B—Decontamination and Disinfection of Laboratory Surfaces and Items
Environmentally-Mediated Transmission of Infection
Laboratory-associated infections (LAIs) can be transmitted directly or indirectly
from contaminated environmental sources within the laboratory (e.g., air, fomites
and laboratory instruments, aerosols, and splashes) to laboratory sta. Fortu-
nately, LAIs are relatively rare events because there are several requirements
necessary for environmental transmission to occur;
5,6
this is commonly referred
to as the chain of infection.
7,8
The requirements needed for environmental
transmission include the presence of a pathogen of sucient virulence, sucient
dose of a pathogen to cause infection (i.e., infectious dose), a mechanism of
transmission of the pathogen from the environment to the host, the correct portal
of entry to a susceptible host, and the immune status of the host.
To accomplish successful transmission from an environmental source, all the
requirements for the chain of infection must be present. The absence of any one
element will reduce and/or prevent the potential for transmission. Additionally,
the pathogen in question must overcome environmental stresses to retain
viability (e.g., ability to form biolms in low, nutrient-moist environments or
distribution systems, ability to survive dehydration), virulence, and the capability
to initiate infection in the host. In the laboratory setting, high concentrations of
pathogens are commonplace, and contamination of environmental surfaces
(e.g., benchtops, equipment, personal protective equipment) and hands of the
laboratorian may occur. Aerosol generation procedures and those that generate
splashes may also contaminate surfaces, personnel, and potentially expose
workers (e.g., inhalation, contact with mucous membranes) to pathogens.
Reduction of environmental microbial contamination by both containment
(e.g., performing aerosol-generating procedures in a biological safety cabinet
or glove box) and conventional cleaning procedures is often enough to reduce,
but not eliminate, the risk of environmentally-mediated transmission. It is the
general practice in laboratories to use both cleaning and surface disinfection or
sterilization procedures to mitigate the potential for transmission of infection. In
addition, proper hand hygiene and appropriate personal protective equipment
(e.g., gloves, lab coat/smock, safety glasses, goggles, respirators) use are also
important factors in preventing transmission to laboratory personnel.
Principles of Cleaning, Disinfection, and Sterilization
To implement a laboratory biosafety program, it is important to understand
the principles of cleaning and disinfection or sterilization. The terms are often
misused and misunderstood. The denitions and capabilities of each inactivation
procedure are discussed with an emphasis on achievement and, in some cases,
monitoring of each state.
Cleaning Cleaning is the removal of gross contamination from a surface to the
extent necessary for further processing for intended use. In these cases, cleaning
402 Biosafety in Microbiological and Biomedical Laboratories
can be used to remove microorganisms and other associated contaminants
(e.g., blood, tissues, culture media) from a surface by physical means but may
not provide any antimicrobial activity. Cleaning is often an essential pre-requisite
to disinfection or sterilization processes to ensure the optimal activity of the
antimicrobial eects of disinfectants or sterilization processes. Biolms may
be present in the laboratory (e.g., sinks, plumbing xtures, uid-lled lines of
laboratory equipment, water containing reservoirs, incubator humidication
systems) and are often dicult to treat/disinfect. Most biolms require physical
cleaning (e.g., scrubbing) and the use of compatible oxidative disinfectants
(e.g., chlorine dioxide, peroxyacetic acid, ozone). In some situations, replacing
tubing and distribution lines may be necessary.
Disinfection Disinfection is generally a less-lethal process than sterilization;
it eliminates nearly all recognized pathogenic microorganisms, but not neces-
sarily all microbial forms (e.g., bacterial spores) present on inanimate objects.
Disinfection does not ensure a kill level and lacks the margin of safety achieved
by sterilization procedures. The eectiveness of a disinfection procedure is
controlled by several factors, each one of which may have a pronounced eect
on the end results. Factors aecting disinfection include the following:
1. Nature and number of contaminating microorganisms (especially the
presence of bacterial spores);
2. Amount of organic matter present (e.g., soil, feces, blood);
3. Type and condition of surfaces, instruments, devices, and materials to
be disinfected;
4. Temperature; and
5. Contact (exposure) time.
By denition, chemical disinfection, especially high-level disinfection, diers from
chemical sterilization by the lack of sporicidal power. This is an over-simplication
of reality because a few chemical disinfectants do kill large numbers of spores
even though high concentrations and several hours of exposure may be required.
Non-sporicidal disinfectants may dier in their capacity to accomplish disinfection
or decontamination. Some disinfectants rapidly kill only the ordinary vegetative
forms of bacteria, such as staphylococci and streptococci, some forms of fungi,
and lipid-containing viruses; others are eective against such relatively resistant
organisms as Mycobacterium bovis or Mycobacterium terrae, non-enveloped
viruses, and most forms of fungi.
9
In general, most laboratories use a disinfectant that has a broad range of activity;
thus, most labs should select a product with a tuberculocidal/mycobactericidal
claim for routine purposes. Many of these products will also have claims that
meet the OSHA Bloodborne Pathogens Standard.
10,11
403Appendix B—Decontamination and Disinfection of Laboratory Surfaces and Items
Sterilization Any item, device, or solution is sterile when it is completely free of
all forms of living microorganisms, including spores and viruses. This denition
is categorical and absolute; an item is either sterile or it is not. Sterilization can
be accomplished by dry or moist heat, gases and vapors (e.g., chlorine dioxide,
ethylene oxide, formaldehyde, hydrogen peroxide, methyl bromide, nitrogen
dioxide, ozone, propylene oxide), plasma sterilization technology, and radiation
(e.g., gamma, e-beam in industry).
From an operational standpoint, a sterilization procedure cannot be categorically
dened because the likelihood that an individual microorganism survives is never
zero. Rather, the procedure is dened as a process, after which the probability
of a microorganism surviving on an item subjected to treatment is less than
one in one million. This is referred to as a sterility assurance level (SAL) of
10
-6
.
12–14
Laboratories use sterilization techniques for producing media, sterilizing
glassware, and other items, and for decontaminating waste.
Decontamination
Decontamination renders an area, device, item, or material safe to handle in the
context of being reasonably free from a risk of disease transmission. The primary
objective of decontamination is to reduce the level of microbial contamination
so that transmission of infection is prevented. The decontamination process
may involve the cleaning of an instrument, device, or area with ordinary soap
and water. In laboratory settings, decontamination of items, used laboratory
materials, and regulated laboratory wastes is often accomplished by a sterilization
procedure such as steam autoclaving, which may be the most cost-eective way
to decontaminate a device or an item.
The presence of any organic matter necessitates longer contact time with a
decontamination method if the item or area is not pre-cleaned. For example,
a steam cycle used to sterilize pre-cleaned items can be 20 minutes at 121°C.
When steam sterilization is used to decontaminate laboratory waste that contains
items that have a high bio-burden and there is no pre-cleaning (i.e., infectious
waste), the cycle times are generally longer and should be veried and validated
for the typical load. Validation involves the combined use of thermocouples and
biological indicators (BIs) placed throughout the load to ensure penetration of
steam into the waste. Verication can be accomplished by routine monitoring
of the steam sterilization cycles (i.e., cycle times, pressure, temperature) and
by placing BIs within the load.
15
In addition to time, temperature may also be
increased to ensure inactivation of pathogens.
16–18
Decontamination in laboratory
settings often requires longer exposure times because pathogenic microorganisms
may be protected from contact with steam.
Chemical disinfectants used for decontamination range in activity from high-level
disinfectants (e.g., high concentrations of sodium hypochlorite [chlorine bleach]),
404 Biosafety in Microbiological and Biomedical Laboratories
which might be used to decontaminate spills of cultured or concentrated infectious
agents in research or clinical laboratories, to low-level disinfectants or sanitizers
for general housekeeping purposes or spot decontamination of environmental
surfaces in healthcare settings. Resistance of selected organisms to decontam-
ination is presented in descending order in Figure 1. If dangerous and highly
infectious agents are present in a laboratory, the methods for decontamination of
spills, laboratory equipment, biological safety cabinet, or infectious waste are very
signicant and may include prolonged autoclave cycles, incineration, or gaseous
treatment of surfaces.
Figure 1. Descending Order of Relative Resistance to Disinfectant Chemicals
Prions
Bacterial Spores
Bacillus subtilis, Clostridium sporogenes, Clostridium dicile
Mycobacteria
Mycobacterium bovis, M. terrae, and other Nontuberculous mycobacteria
Non-enveloped or Small Viruses
Poliovirus, Coxsackievirus, Rhinovirus
Fungi
Trichophyton spp., Cryptococcus spp., Candida spp.
Vegetative Bacteria
Pseudomonas aeruginosa, Staphylococcus aureus,
Salmonella choleraesuis, Enterococci
Enveloped or Medium-size Viruses
Herpes simplex virus, CMV, Respiratory syncytial virus,
HBV, HCV, HIV, Hantavirus, Ebola virus
Note: There are exceptions to this list. Pseudomonas spp. are sensitive to
high-level disinfectants. However, in biolms, the protected cells and those
within free-living amoeba, or existing as persister cells (viable but not culturable)
within the biolm, can approach the resistance of bacterial spores to the same
disinfectant. The same is true for the resistance to glutaraldehyde by some
nontuberculous mycobacteria, some fungal ascospores of Microascus cinereus
and Chaetomium globosum, and the pink-pigmented Methylobacteria. Prions are
also resistant to most liquid chemical germicides and are discussed in the last
part of this appendix.
405Appendix B—Decontamination and Disinfection of Laboratory Surfaces and Items
Space Decontamination Space decontamination is a specialized activity and
should be performed by individuals with proper expertise, training, and personal
protective equipment.
19–24
Decontamination requirements for laboratory spaces
inuence the design of these facilities. The interior surfaces of laboratories must
be easy to clean and decontaminate. Penetrations in BSL-3 laboratory surfaces
should be sealed or capable of being sealed for decontamination purposes. Care
should be taken that penetrations in the walls, oors, and ceilings are kept to a
minimum and are sight sealed. Verication of the seals is highly recommended
but is usually not required for BSL-3 laboratories. The BSL-4 laboratory design
requires interior surfaces that are water-resistant and sealed to facilitate
fumigation. Periodic fumigation is required in the BSL-4 suit laboratory to allow
routine maintenance and certication of equipment.
Procedures for decontamination of large spaces such as incubators or rooms
are varied and inuenced signicantly by the type of etiologic agent involved, the
characteristics of the structure containing the space, and the materials present
in the space. The primary methods for space decontamination follow. Fumigants
that are currently used are either gases, vapors, mists, or fogs (dry mists).
Fumigants that are gases obey gas laws, can evenly distribute throughout the
room, and are easily scalable by increasing the volume of gases used. Fumigants
applied as mists or fogs do not behave like gases and are particles (<1–12 µ in
size) that settle onto surfaces being treated.
Paraformaldehyde and Formaldehyde Gas
Paraformaldehyde and solutions of formaldehyde have been used to generate
formaldehyde gas and mists; historically, they have been used in laboratory
settings for decontamination of large spaces and biological safety cabinets.
25,26
When using formaldehyde and paraformaldehyde, take safety precautions,
27,28
federal regulations, state regulations, and local regulations into consideration.
29
Formaldehyde is also recognized as a known human carcinogen.
30
There is at
least one EPA-registered paraformaldehyde product available for the decontam-
ination of laboratories. It is important that paraformaldehyde is used per labeling
instructions and that a fumigation management and safety plan that meets
federal, state, and local regulations is prepared in advance of application and is
implemented during application. For use as a space decontamination agent, the
standard concentration of formaldehyde is 0.3g/ft
3
(approximately 8,000 ppm) with
a relative humidity of between 60 and 85%.
31
Increasing the amount of parafor-
maldehyde is not advised, as the lower explosive limit for formaldehyde gas is 7%
(70,000 ppm).
32
It is recommended that formaldehyde gas decontamination be
performed only by highly experienced individuals.
406 Biosafety in Microbiological and Biomedical Laboratories
Hydrogen Peroxide Vapor
Hydrogen peroxide can be vaporized and used for the decontamination of glove
boxes and small room areas. Vapor phase hydrogen peroxide has been shown
to be an eective sporicide at concentrations ranging from 0.5 mg/L to <10 mg/L.
The optimal concentration of this agent is about 2.4 mg/L with a contact time of at
least one hour. This system can be used to decontaminate glove boxes, walk-in
incubators, and small rooms. An advantage of this system is that the end products
(i.e., water and oxygen) are not toxic. Low relative humidity can be used.
33–36
Chlorine Dioxide Gas
Chlorine dioxide gas sterilization can be used for decontamination of laboratory
rooms, equipment, glove boxes, and incubators. The concentration of gas at the
site of decontamination should be approximately 10 mg/L with a contact time of
one to two hours.
37–40
Chlorine dioxide possesses the bactericidal, virucidal, and sporicidal properties of
chlorine, but unlike chlorine, it does not lead to the formation of trihalomethanes
and does not combine with ammonia to form chlorinated organic products (chlora-
mines). The gas cannot be compressed and stored in high-pressure cylinders,
but it is generated upon demand using a column-based solid-phase generation
system. Gas is diluted to the use concentration, usually between 10 and 30 mg/L.
Within reasonable limits, a chlorine dioxide gas generation system is unaected
by the size or location of the ultimate destination for the gas. Relative humidity
does need to be controlled and high humidity is optimal. Although most often
used in closed sterilizers, the destination enclosure for the chlorine dioxide gas
does not need to be such a chamber. Because chlorine dioxide gas exits the
generator at a modest positive pressure and ow rate, the enclosure also need
not be evacuated and could be a sterility-testing isolator, a glove box or sealed
BSC, or even a small room that could be sealed to prevent gas egress.
40
Chlorine
dioxide gas is rapidly broken down by light; care must be taken to eliminate light
sources in spaces to be decontaminated.
Decontamination of Surfaces Liquid chemical disinfectants may be used for
decontamination of large surface areas. The usual procedure is to ood the area
with a disinfectant for periods up to several hours. This approach is messy, and
some of the disinfectants used represent a toxic hazard to laboratory sta. For
example, most of the high-level disinfectants on the United States market are
formulated for use on instruments and medical devices rather than on environ-
mental surfaces. Intermediate and low-level disinfectants are formulated for use
on fomites and environmental surfaces but lack the potency of high-level disin-
fectants. For the most part, intermediate and low-level disinfectants can be safely
used and, as with all EPA-registered disinfectants, the manufacturer’s instructions
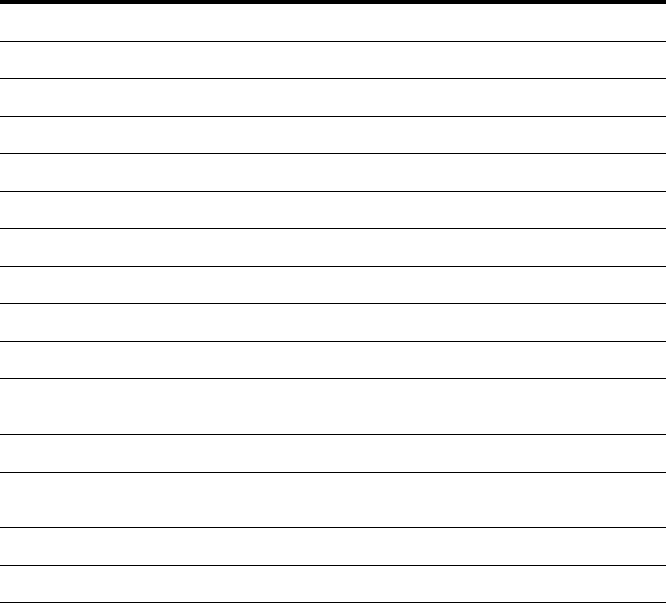
407Appendix B—Decontamination and Disinfection of Laboratory Surfaces and Items
should be followed.
41
Disinfectants that have been used for decontamination
include: sodium hypochlorite solutions at concentrations of 500 to 6000 parts per
million (ppm); oxidative disinfectants, such as hydrogen peroxide and peracetic
acid; phenols; and iodophors. Procedures for the use of chemical disinfectants
should include safety precautions, the use of appropriate personal protective
equipment, hazard communication, and training on spill response.
Concentrations and exposure times vary depending on the disinfectant
formulation and the manufacturer’s instructions for use. See Table 1 for a list
of chemical disinfectants and their activity levels. A spill control plan must be
available in the laboratory. This plan should include the rationale for selection
of the disinfectant, the approach to its application, contact time, and other
parameters. Biological agents requiring BSL-3 and BSL-4 containment pose a
high risk to workers and possibly to the environment, and these agents should
be managed by trained, professional sta who are equipped to work with
concentrated material.
Table 1. Activity Levels of Selected Liquid Chemical Disinfectants
Chemical
a
Concentration Activity level
Glutaraldehyde Variable Sterilization
Glutaraldehyde Variable Intermediate to high-level disinfection
Ortho-phthalaldehyde (OPA) 0.55% High-level disinfection
Hydrogen peroxide 6–30% Sterilization
Hydrogen peroxide 3–6% Intermediate to high-level disinfection
Formaldehyde
b
6–8% Sterilization
Formaldehyde 1–8% Low- to high-level disinfection
Chlorine dioxide Variable Sterilization
Chlorine dioxide Variable High-level disinfection
Peracetic Acid 0.08%–0.23% with peroxide
concentrations of 1–7.35%
Sterilization
Peracetic acid Variable High-level disinfection
Hypochlorites
c
500–6000 mg/L Free
available
Intermediate to high-level disinfection
Alcohols (ethyl, Isopropyl)
d
70% Intermediate-level disinfection
Phenolics 0.5–3% Low- to intermediate-level disinfection
Continued on next page ►
408 Biosafety in Microbiological and Biomedical Laboratories
Chemical
a
Concentration Activity level
Iodophors
e
30–50 mg/L Free Low- to intermediate-level disinfection
Quaternary Ammonium
Compounds
Variable Low-level disinfection
a. This list of chemical disinfectants centers on generic formulations. A large number of commercial products based
on these generic components can be considered for use. Users should ensure that commercial formulations
are registered with EPA or by the FDA. Users can search for EPA-registered products at https://www.epa.gov/
pesticide-labels.
b. Because formaldehyde is classied as a known human carcinogen and has a low permissible exposure limit
(PEL), the use of formaldehyde is limited to certain specic circumstances under carefully controlled conditions
(e.g., for the disinfection of certain hemodialysis equipment). There are no FDA-cleared liquid chemical sterilant/
disinfectants that contain formaldehyde.
c. Generic disinfectants containing chlorine are available in liquid or solid form (e.g., sodium or calcium hypochlorite).
The indicated concentrations are rapid-acting and broad-spectrum (i.e., tuberculocidal, bactericidal, fungicidal,
and virucidal). Note: Common household bleach is an excellent and inexpensive source of sodium hypochlorite.
Concentrations between 500 and 1000 ppm chlorine are appropriate for the vast majority of uses requiring an
intermediate-level of germicidal activity; higher concentrations are extremely corrosive as well as irritating to
personnel, and their use should be limited to situations where there may be spores or there is an excessive
amount of organic material or unusually high concentrations of microorganisms (e.g., spills of cultured material in
the laboratory). In situations where there is an excessive amount of organic material present, the surfaces should
be thoroughly cleaned to remove as much organic material as possible before applying sodium hypochlorite
solution to disinfect the surface (see product label instructions). The concentration of the sodium hypochlorite
should be determined in advance of use and the solution should be made fresh each day.
d. The eectiveness of alcohols as intermediate-level germicides is limited because they evaporate rapidly, resulting
in short contact times, and because they lack the ability to penetrate residual organic material. They are rapidly
tuberculocidal, bactericidal, and fungicidal, but may vary in spectrum of virucidal activity. Items to be disinfected
with alcohols should be carefully pre-cleaned then totally submerged for an appropriate exposure time.
e. Only those iodophors registered with EPA as hard-surface disinfectants should be used, closely following the
manufacturer’s instructions regarding proper dilution and product stability. Antiseptic iodophors are not suitable to
disinfect devices, environmental surfaces, or medical instruments.
Transmissible Spongiform Encephalopathy Agents (Prions) Prions are
exceptionally dicult to inactivate and decontaminate and are the causative agent
of Creutzfeldt-Jakob disease (CJD) and other transmissible spongiform enceph-
alopathies of the central nervous system in humans or animals. Studies show
that prions are resistant to conventional uses of heat and/or chemical germicides
for the sterilization of instruments and devices.
12,42,43
Treatment of tissues and
contaminated tissues is based on tissue infectivity.
44
See Section VIII-H: Prion
Diseases for additional information.
Inactivation of Select Agents Select agents can be inactivated using conven-
tional disinfection and sterilization procedures appropriate to the type of agent
(e.g., virus, spore-forming bacteria). Inactivation procedures typically leave cell
components intact that can then be used as reagents for assay development or
other studies while the purpose of disinfection is to kill and damage pathogens
with no attention to preserve cell components. Once inactivated, the agents are
no longer subject to the Select Agent Regulations. Problems have arisen when
spore-forming Select Agents such as Bacillus anthracis have not been completely
inactivated. This was highlighted in 2015 when irradiated spores were shipped to
non-select, agent-approved laboratories but were later found to be only partially
inactivated.
45
The Select Agent Regulations require that the inactivation process

409Appendix B—Decontamination and Disinfection of Laboratory Surfaces and Items
used for these agents be validated. Select Agent guidance is available at
https://www.selectagents.gov/resources/Inactivation_Guidance.pdf and at
https://www.selectagents.gov/resources/Biosafety_Guidance.pdf.
Chemical Safety When using chemical agents for decontamination, pay attention
to instructions for their use and Safety Data Sheets (SDS); ensure they are used
safely and that appropriate precautions and protections are used. Exposures to
disinfectants have resulted in occupational injuries such as cancer, hypersensitiv-
ities, dermatitis, and asthma.
46,47
Hand Hygiene Handwashing and hand decontamination are an underappreciated
part of risk mitigation for handling pathogens. Gloves should be worn when
handling biohazardous materials and hazardous chemicals, including those used
in disinfection and decontamination; this does not replace the need for regular
hand hygiene by laboratory personnel.
48
Hand hygiene should be performed
after removing gloves, after touching potentially contaminated surfaces with bare
hands, after completing work, and before exiting the laboratory. The main method
of hand hygiene in the laboratory is handwashing with soap and water.
When handwashing facilities are not available, an alcohol-based hand sanitizer
(ABHS) with an alcohol concentration between 60–95% may be used in
conjunction with or in lieu of immediate handwashing, based on agent type and
a risk assessment that accounts for potential reduced ecacy of hand sanitizers
for soiled hands and inactivating some microorganisms (i.e., bacterial spores,
parasites, and non-enveloped viruses). ABHS may be used for immediate hand
hygiene until a handwashing facility can be accessed only if hands are not
grossly contaminated. The limitations of AHBS should be communicated to sta.
Handwashing with soap and water remains the preferred method of performing
hand hygiene.
49
ABHS should be applied to cover the skin and nails (including
underneath the nail) of the hands for 20–30 seconds. Posters are available to
assist in demonstrating the proper method of hand sanitizing using ABHS at
https://www.cdc.gov/features/handhygiene.
If hands are grossly contaminated when exiting the laboratory, they should be
washed with soap or soap containing an antiseptic agent (i.e., antimicrobial
soap) and water.
49,50
When using soap and water, the entire procedure should
last 40–60 seconds from wetting hands to drying with a paper towel. Posters
are available to assist in demonstrating the proper method of handwashing at
https://www.cdc.gov/handwashing/posters.html. Posters are available to assist
in demonstrating the proper method of handwashing and use of an ABHS at
https://www.who.int/gpsc/tools/GPSC-HandRub-Wash.pdf.

410 Biosafety in Microbiological and Biomedical Laboratories
References
1. Environmental Protection Agency. Pesticide Labeling and Other Regulatory
Revisions, Final Rule (40 C.F.R. Parts 152 and 156). Fed Regist.
2001;66(241):64759–68.
2. Environmental Protection Agency. Data Requirements for Antimicrobial
Pesticides, Final Rule (40 C.F.R. Parts 158 and 161). Fed Regist.
2013;78(89):26936–93.
3. Food Quality Protection Act of 1996, Pub. L. No. 104-70, 104 Stat. 1627
(August 3, 1996).
4. Occupational Safety and Health Administration. Laboratory safety guidance
Washington (DC): U.S. Department of Labor; 2011.
5. Vesley D, Lauer J, Hawley R. Decontamination, sterilization, disinfection,
and antisepsis. In: Fleming DO, Hunt DL, editors. Biological Safety:
Principles and Practices. 3rd ed. Washington (DC): ASM Press; 2000.
p. 383–402.
6. Byers KB, Harding AL. Laboratory-associated infections. In: Wooley DP,
Byers KB, editors. Biological Safety: Principles and Practices. 5th ed.
Washington (DC): ASM Press; 2017. p. 59–92.
7. Greene VW. Microbiological contamination control in hospitals. 1.
Perspectives. Hospitals. 1969;43(20):78–88.
8. Boyce JM. The inanimate environment. In: Jarvis WR, editor. Bennett
& Brachman’s Hospital Infections. 4th ed. Philadelphia (PA): Lippincott
Williams & Wilkins; 2014.
9. Lin CS, Fuller J, Mayhall ES. Federal Regulation of Liquid Chemical
Germicides by the U.S. Food and Drug Administration. In: Block SS, editor.
Disinfection, Sterilization, and Preservation. 5th ed. Philadelphia (PA):
Lippincott Williams & Wilkins; 2001. p. 1293–1301.
10. Occupational Safety and Health Administration. Occupational Exposure to
Bloodborne Pathogens; Needlestick and Other Sharps Injuries; Final Rule
(29 C.F.R. 1910.1030). Fed Regist. 2001;66(12):5318–25.
11. Environmental Protection Agency [Internet]. Washington (DC): Pesticide
registration; c2017 [cited 2018 Oct 16]. Selected EPA-registered
Disinfectants. Available from: https://www.epa.gov/pesticide-registration/
selected-epa-registered-disinfectants
12. Centers for Disease Control and Prevention [Internet]. Atlanta (GA):
Disinfection and Sterilization; revised 2017 Feb 15 [cited 2018 Oct 16].
Guideline for disinfection and sterilization in healthcare facilities, 2008.
Available from: https://www.cdc.gov/infectioncontrol/pdf/guidelines/
disinfection-guidelines.pdf
411Appendix B—Decontamination and Disinfection of Laboratory Surfaces and Items
13. Favero MS. Sterility assurance: concepts for patient safety. In: Rutala WA,
editor. Disinfection, sterilization and antisepsis: principles and practices
in healthcare facilities. Washington (DC): Association for Professionals in
Infection Control and Epidemiology, Inc; 2001. p. 110–9.
14. Favero MS, Bond WW. The use of liquid chemical germicides. In: Morrissey
RF, Phillips GB, editors. Sterilization technology: A practical guide for
manufacturers and users of health care products. New York: Van Nostrand
Reinhold; 1993. p. 309–34.
15. Royalty-Hann W. Solutions for biological indicator problems from a quality
assurance viewpoint. Biocontrol Sci. 2007;12(2):77–81.
16. Lemieux P, Sieber R, Osborne A, Woodard A. Destruction of spores on
building decontamination residue in a commercial autoclave. Appl Environ
Microbiol. 2006;72(12):7687–93.
17. Lauer JL, Battles DR, Vesley D. Decontaminating infectious laboratory
waste by autoclaving. Appl Environ Microbiol. 1982;44(3):690–4.
18. Rutala WA, Stiegel MM, Sarubbi FA Jr. Decontamination of laboratory
microbiological waste by steam sterilization. Appl Environ Microbiol.
1982;43(6):1311–6.
19. Tearle P. Decontamination by fumigation. Commun Dis Public Health.
2003;6(2):166–8.
20. Girouard DJ, Czarneski MA. Room, suite scale, class III biological safety
cabinet, and sensitive equipment decontamination and validation using
gaseous chlorine dioxide. Applied Biosafety. 2016;21(1):34–44.
21. Kaspari O, Lemmer K, Becker S, Lochau P, Howaldt S, Nattermann H, et al.
Decontamination of a BSL3 laboratory by hydrogen peroxide fumigation
using three dierent surrogates for Bacillus anthracis spores. J Appl
Microbiol. 2014;117(4):1095–103.
22. Krishnan J, Fey G, Stanseld C, Landry L, Nguy H, Klassen S, et al.
Evaluation of a Dry Fogging System for Laboratory Decontamination.
Applied Biosafety. 2012;17(3):132–41.
23. Gordon D, Carruthers B-A, Theriault S. Gaseous decontamination methods
in high-containment laboratories. Applied Biosafety. 2012;17(1):31–9.
24. Czarneski MA, Lorcheim K. A discussion of biological safety cabinet
decontamination methods: formaldehyde, chlorine dioxide, and vapor phase
hydrogen peroxide. Applied Biosafety. 2011;16(1):26–33.
25. Ackland NR, Hinton MR, Denmeade KR. Controlled formaldehyde
fumigation system. Appl Environ Microbiol. 1980;39(3):480–7.

412 Biosafety in Microbiological and Biomedical Laboratories
26. Newsom SW, Walsingham BM. Sterilization of the biological safety cabinet.
J Clin Pathol. 1974;27(11):921–4.
27. Cheney JE, Collins CH. Formaldehyde disinfection in laboratories:
limitations and hazards. Br J Biomed Sci. 1995;52(3):195–201. Erratum in:
Br J Biomed Sci. 1995 Dec;52(4):332.
28. Fox JM, Shuttleworth G, Martin F. Methodology to reduce formaldehyde
exposure during laboratory fumigation. Int J Environ Health Res.
2013;23(5):400–6.
29. Formaldehyde, 29 C.F.R. Sect. 1910.1048 (2013).
30. National Toxicology Program [Internet]. Research Triangle Park (NC):
Department of Health and Human Services; c2016 [cited 2018 Oct 17].
Report on Carcinogens, Fourteenth Edition. Formaldehyde. CAS No.
50-00-0. Available from: https://ntp.niehs.nih.gov/ntp/roc/content/proles/
formaldehyde.pdf
31. Luftman HS. Neutralization of formaldehyde gas by ammonium bicarbonate
and ammonium carbonate. Applied Biosafety. 2005;10(2):101–6.
32. Centers for Disease Control and Prevention [Internet]. Atlanta (GA): The
National Institute for Occupational Safety and Health (NIOSH); c2014 [cited
2018 Oct 16]. Formaldehyde. Available from: https://www.cdc.gov/niosh/
idlh/50000.html
33. Klapes NA, Vesley D. Vapor-phase hydrogen peroxide as a surface
decontaminant and sterilant. Appl Environ Microbiol. 1990;56(2):503–6.
34. Johnson JW, Arnold JF, Nail SL, Renzi E. Vaporized hydrogen peroxide
sterilization of freeze dryers. J Parenter Sci Technol. 1992;46(6):215–25.
35. Krause J, McDonnell G, Riedesel H. Biodecontamination of animal rooms
and heat-sensitive equipment with vaporized hydrogen peroxide. Contemp
Top Lab Anim Sci. 2001;40(6):18–21.
36. Graham GS, Ricklo JR. Development of VHP sterilization technology.
J Healthc Mater Manage. 1992;10(8)54, 56–8.
37. Czarneski MA. Microbial decontamination of a 65-room new pharmaceutical
research facility. Applied Biosafety. 2009;14(2):81–8.
38. Lorcheim K, Lorcheim P. Mold remediation of a research facility in a
hospital. Applied Biosafety. 2013;18(4):191–6.
39. Luftman HS, Regits MA, Lorcheim P, Czarneski MA, Boyle T, Aceto H, et al.
Chlorine dioxide gas decontamination of large animal hospital intensive and
neonatal care units. Applied Biosafety. 2006;11(3):144–54.
40. Knapp JE, Battisti DL. Chloride dioxide. In: Block SS, editor. Disinfection,
sterilization, and preservation. 5th ed. Philadelphia (PA): Lippincott Williams
& Wilkins; 2001. p. 215–27.
413Appendix B—Decontamination and Disinfection of Laboratory Surfaces and Items
41. Favero MS, Bond WW. Chemical disinfection of medical and surgical
materials. In: Block SS, editor. Disinfection, sterilization, and preservation.
5th ed. Philadelphia (PA): Lippincott Williams & Wilkins; 2001. p. 881–917.
42. World Health Organization. WHO Infection Control Guidelines for
Transmissible Spongiform Encephalopathies: Report of a WHO
consultation Geneva, Switzerland, 23–26 March 1999. Report presented
at: WHO Consultation; 1999 Mar 23–26; Geneva, Switzerland.
43. World Health Organization [Internet]. Geneva: Blood Safety and Clinical
Technology Department Health Technology and Pharmaceutical Cluster;
c2003 [cited 2018 Oct 16]. WHO Guidelines on Transmissible Spongiform
Encephalopathies in relation to Biological and Pharmaceutical Products
Available from: https://www.who.int/bloodproducts/publications/en/WHO_
TSE_2003.pdf?ua=1
44. World Health Organization [Internet]. Geneva: WHO Tables on Tissue
Infectivity Distribution in Transmissible Spongiform Encephalopathies;
c2010 [cited 2018 Oct 16]. Available from: https://www.who.int/
bloodproducts/tablestissueinfectivity.pdf?ua=1
45. Department of Defense [Internet]. Washington (DC): Committee for
Comprehensive Review of DoD Laboratory Procedures, Processes, and
Protocols Associated with Inactivating Bacillus anthracis Spores; c2015
[cited 2018 Oct 16]. Review committee report: inadvertent shipment of live
Bacillus anthracis spores by DoD. Available from: https://dod.defense.gov/
Portals/1/features/2015/0615_lab-stats/Review-Committee-Report-Final.pdf
46. Casey ML, Hawley B, Edwards N, Cox-Ganser JM, Cummings KJ. Health
problems and disinfectant product exposure among sta at a large
multispecialty hospital. Am J Infect Control. 2017;45(10):1133–8. Erratum
in: 2018;46(5):599.
47. Weber DJ, Rutala WA. Occupational risks associated with the use of
selected disinfectants and sterilants. In: Rutala WA, editor. Disinfection,
Sterilization and Antisepsis in Healthcare. Washington (DC): The
Association for Professionals in Infection Control and Epidemiology, Inc.;
1998. p. 211–26.
48. World Health Organization. Laboratory Biosafety Manual. 3rd ed. Geneva
(Switzerland): World Health Organization; 2004.
49. World Health Organization. WHO Guidelines on Hand Hygiene in Health
Care. Geneva (Switzerland): WHO Press; 2009.
414 Biosafety in Microbiological and Biomedical Laboratories
50. Boyce JM, Pittet D; Healthcare Infection Control Practices Advisory
Committee; HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force.
Guideline for hand hygiene in health-care settings. Recommendations
of the Healthcare Infection Control Practices Advisory Committee and
the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Society for
Healthcare Epidemiology of America/Association for Professionals in
Infection Control/Infectious Diseases Society of America. MMWR Recomm
Rep. 2002;51(RR-16):1–45, quiz CE1–4.
415Appendix C—Transportation of Infectious Substances
Appendix C—Transportation of Infectious Substances
An infectious substance is a material known to contain or reasonably expected
to contain a pathogen. A pathogen is a microorganism (i.e., bacteria, viruses,
rickettsiae, parasites, fungi) or other agent (e.g., proteinaceous infectious particle
[prion]) that can cause disease in humans or animals. Infectious substances may
exist as puried and concentrated cultures but may also be present in a variety
of materials or physical states, such as body uids or tissues or lyophilized
materials. Infectious substances and materials that are known or suspected
to contain them are regulated as hazardous materials by the United States
Department of Transportation (DOT), when transported in commerce in, to, or
through the United States and by the International Civil Aviation Organization
(ICAO) when transported internationally.
International Harmonization of Shipping and Transport Regulations
The United States works to assure the compatibility of its hazardous materials
regulations with those of other bodies such as the United Nations, which issues
Recommendations on the Transport of Dangerous Goods. Specialized organiza-
tions within the United Nations, such as ICAO, issue detailed instructions based
on these recommendations that national governments, including the United
States, agree to comply with in full or in part. ICAO references, including the
International Air Transport Association (IATA) Dangerous Goods Regulations,
establish international standards for the air transport of infectious or toxic
materials.
1,2
The United States prescribes how to comply with these international
instructions in 49 CFR Part 171, Subpart C.
Transportation Regulations
International and domestic transport regulations for infectious substances are
designed to prevent the release of these materials in transit and to protect the
public, workers, property, and the environment from the harmful eects that
may occur from exposure to these materials. Protection is achieved through
packaging requirements and multiple types of hazard communication. Packages
must be designed to withstand rough handling and other forces experienced in
transportation, such as vibration, stacking, moisture, and changes in air pressure
and temperature. Hazard communication includes shipping papers, labels,
markings on the outside of packages, and other information necessary to enable
transport workers and emergency response personnel to correctly identify the
material and respond eciently in an emergency situation. Packaging and hazard
communication exceptions exist to avoid duplication with other governmental
regulations or to appropriately transport infectious substances with fewer risks.
In addition, shippers and carriers must be trained on these regulations so that
they can properly prepare shipments and recognize and respond to the risks
posed by these materials.

416 Biosafety in Microbiological and Biomedical Laboratories
Select Agents
Select Agents and Toxins are a subset of biological agents and toxins that the
Departments of Health and Human Services (HHS) and Agriculture (USDA)
have determined to have the potential to pose a severe threat to public health
and safety, to animal or plant health, or to animal or plant products. Persons or
organizations who either oer for transportation or transport Select Agents and
Toxins in commerce into or throughout the United States must comply with the
Select Agent regulations (42 CFR Part 73, 9 CFR Part 121, and 7 CFR Part
331), including requesting prior authorization to transfer or import the agents and
toxins. The APHIS/CDC Form 2, Request to Transfer Select Agents and Toxins,
is used by persons or organizations to request prior authorization of a transfer of
Select Agent(s) or Toxin(s) from the Federal Select Agent Program as required by
regulations (7 CFR Part 331, 9 CFR Part 121, and 42 CFR Part 73). Importation
and domestic movement permits are no longer required for public health or animal
health Select Agent pathogens. More information regarding Select Agents and
Toxins is available at https://www.selectagents.gov.
Persons who oer for transportation or transport Select Agents in commerce in,
to, or through the United States must develop and implement security plans for
such transportation. A security plan must include an assessment of the possible
transportation security risks for materials covered by the security plan and specic
measures to reduce or eliminate the assessed risks. At a minimum, a security
plan must include measures to address those risks associated with personnel
security, en route security, and unauthorized access.
Regulations
United States Department of Transportation. 49 CFR Parts 171–180, Hazardous
Materials Regulations. Applies to the shipment of infectious substances in
commercial transportation in, to, or through the United States. Information on
these regulations is available at https://www.phmsa.dot.gov/hazmat.
United States Postal Service (USPS). 39 CFR Part 20, International Postal
Service (International Mail Manual), and Part 111, General Information on
Postal Service (Domestic Mail Manual). Regulations on transporting infectious
substances through the USPS are codied in Section 601.10.17 of the Domestic
Mail Manual and Section 135 of the International Mail Manual. A copy of the
Domestic and International Mail Manuals may be obtained from the USPS Postal
Explorer website at https://pe.usps.com/DMM300/Index.
Occupational Health and Safety Administration (OSHA). 29 CFR Section
1910.1030, Occupational Exposure to Bloodborne Pathogens. These regulations
provide minimal packaging and labeling for blood and body uid when transported
within a laboratory or outside of it. Information may be obtained from your local
OSHA oce or at https://www.osha.gov.

417Appendix C—Transportation of Infectious Substances
Technical Instructions for the Safe Transport of Dangerous Goods by Air
(Technical Instructions). International Civil Aviation Organization (ICAO). These
regulations apply to the shipment of infectious substances by air and are
recognized in the United States and by most countries worldwide. A copy of these
regulations may be purchased from the ICAO Document Sales Unit on the ICAO
Dangerous Goods Regulations. International Air Transport Association (IATA).
Global standards are detailed in this widely recognized publication on require-
ments for the transport of biological and chemical hazards. They are issued
by IATA, an airline association, based on the ICAO Technical Instructions, and
followed by most airline carriers. A copy of these regulations may be purchased
from IATA at https://www.iata.org/publications/dgr/Pages/index.aspx or by email
Importation and Transfers
Regulations governing the transfer of biological agents are designed to ensure
that possession of these agents is in the best interest of the public and the nation.
These regulations require documentation of personnel and facilities, justication
of need, and pre-approval of the transfer by a federal authority. The following
regulations apply to this category:
Biological Agent or Vectors of Human Disease Import Permit. 42 CFR Section
71.54. Unless the material meets one of the regulatory exclusions, this regulation
requires a permit from the CDC Import Permit Program to import infectious
biological agents, infectious substances, and vectors of human disease into the
United States. More information is available at the CDC Import Permit Program
website at https://www.cdc.gov/cpr/ipp/index.htm.
Transfer of any Select Agents or Toxins requires the intended recipient to be
registered with the Select Agent Program and submit an APHIS/CDC Form 2
as required to obtain approval to import the Select Agent or Toxin prior to each
importation event (see 42 CFR Part 73, 9 CFR Part 12, and/or 7 CFR Part 330).
Importation of Pathogenic Agents of Livestock, Poultry and Other Animal
Diseases and Other Materials Derived from Livestock, Poultry or Other Animals.
9 CFR Part 122. Organisms and Vectors. The USDA, APHIS, Veterinary Services
(VS) requires that a permit be issued prior to the importation or domestic transfer
(interstate movement) of pathogenic disease agents of livestock, poultry, or other
animals. Information may be obtained at 301-851-3300 or from the USDA website
at https://www.aphis.usda.gov/aphis/ourfocus/animalhealth. Completed permit
applications may be submitted electronically at https://www.aphis.usda.gov/
permits/learn_epermits.shtml.

418 Biosafety in Microbiological and Biomedical Laboratories
Importation of Plant Pests. 7 CFR Part 330. Federal Plant Pest Regulations;
General; Plant Pests; Soil, Stone, and Quarry Products; Garbage. This regulation
requires a permit to move into or through the United States or by interstate any
plant pest or a regulated product, article, or means of conveyance in accordance
with this part. Information can be obtained by calling 301-851-2357 or at the
USDA APHIS website at https://www.aphis.usda.gov/aphis/ourfocus/planthealth/
import-information.
Transfer of USDA Plant Pests
The movement of Plant Pests is regulated under two distinct and separate
regulations: (1) 7 CFR Part 331—Possession, Use, and Transfer of Select Agents
and Toxins; and (2) 7 CFR Part 330—Federal Plant Pest Regulations; General;
Plant Pests; Soil; Stone and Quarry Products; Garbage. The regulation found at
7 CFR Part 331 requires an approved Transfer Form (APHIS/CDC Form 2) prior
to importation, interstate, or intrastate movement of a Select Agent Plant Pest.
In addition, under 7 CFR Part 330, the movement of a Plant Pest also requires
a PPQ Form 526 permit for movement in, to, or through the United States, or
interstate any plant pest or a regulated product, article, or means of conveyance
in accordance with this part. Information can be obtained by calling 301-851-2357
or at the Select Agent Program website at https://www.selectagents.gov.
Export of Human, Animal, and Plant Pathogens and Related Materials;
Department of Commerce (DoC); 15 CFR Parts 730–799. This regulation requires
that exporters of a wide variety of etiologic agents of human, plant, and animal
diseases, including genetic material, and products that might be used for culture
of large amounts of agents, will require an export license. Information may be
obtained by calling the DoC Bureau of Industry and Security (BIS) at 202-482-
4811 or at the DoC BIS website at https://www.bis.doc.gov. Additional web
resources include:
1. https://www.bis.doc.gov/index.php/regulations/
export-administration-regulations-ear
2. https://classic.ntis.gov/products/export-regs/
DOT Packaging of Infectious Substances
General DOT Packaging Requirements for Transport of Infectious Substances
by Aircraft
DOT-compliant packaging is required by domestic and international air carriers
for transport of infectious substances. DOT packaging regulations are also the
basis for infectious substance packaging designed for motor vehicle, railcar, and
vessel transport. The following is a summary of each packaging type and related
transportation requirements.
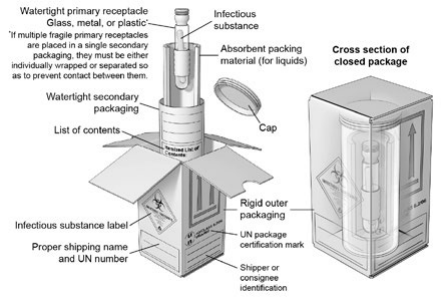
419Appendix C—Transportation of Infectious Substances
Category A Infectious Substance (UN 2814 and UN 2900): Figure 1. A Category
A material is an infectious substance that is transported in a form that is capable
of causing permanent disability or life-threatening or fatal disease to otherwise
healthy humans or animals when exposure to it occurs. An exposure occurs when
an infectious substance is released outside of its protective packaging, resulting
in physical contact with humans or animals. Category A infectious substances are
assigned to identication number “UN 2814” for substances that cause disease in
humans or in both humans and animals, or “UN 2900” for substances that cause
disease in animals only.
Figure 1 shows an example of the UN standard triple packaging system for
materials known or suspected of being a Category A infectious substance as
outlined in the Packaging Instruction of the IATA Dangerous Goods Regulations.
3
The package consists of a watertight primary receptacle or receptacles; a water-
tight secondary packaging; and a rigid outer packaging of adequate strength for
its capacity, mass, and intended use. Note that for liquid materials, the secondary
packaging must contain absorbent material in sucient quantities to absorb the
entire contents of all primary receptacles. A list of contents must be located on
or near the secondary packaging. Each surface of the external dimension of the
packaging must be 100 mm (3.9 inches) or more. The completed package must
pass specic performance tests, including a drop test and a water-spray test, and
must be capable of withstanding, without leakage, an internal pressure producing
a pressure dierential of not less than 95 kPa (0.95 bar, 14 psi). The completed
package must also be capable of withstanding, without leakage, temperatures in
the range of -40ºC to +55ºC (-40ºF to 131ºF). The completed package must be
marked “UN 2814, Infectious substance, aecting humans,” or “UN 2900, Infec-
tious substance, aecting animals,” and labeled with a Division 6.2 (infectious
substance) label. In addition, the package must be accompanied by appropriate
shipping documentation, including a shipping paper and emergency response
information.
Figure 1. A Category A UN Standard Triple Packaging
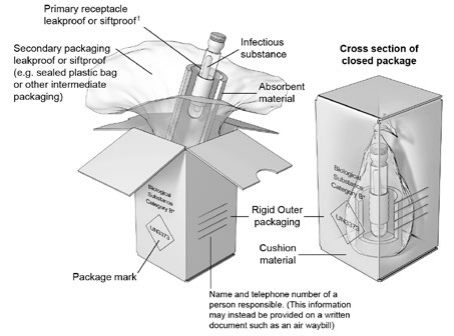
420 Biosafety in Microbiological and Biomedical Laboratories
Category B Biological specimen (UN 3373): Figure 2. A Category B infectious
substance is one that does not meet the criteria for inclusion in Category A.
A Category B infectious substance does not cause permanent disability or
life-threatening or fatal disease in otherwise healthy humans or animals when
exposure to it occurs. The proper shipping name for a Category B infectious
substance is “UN3373, Biological substance, Category B.”
Figure 2 shows an example of the triple packaging system for materials known
or suspected of containing a Category B infectious substance. A Category B
infectious substance must be placed in a packaging consisting of a leak-proof
primary receptacle, leak-proof secondary packaging, and rigid outer packaging.
At least one surface of the outer packaging must have a minimum dimension
of 100 mm by 100 mm (3.9 inches). The packaging must be of good quality
and strong enough to withstand the shocks and loadings normally encountered
during transportation. For liquid materials, the secondary packaging must
contain absorbent material in sucient quantities to absorb the entire contents
of all primary receptacles. For aircraft, the primary or secondary packaging must
be capable of withstanding, without leakage, an internal pressure producing
a pressure dierential of 95 kPa (0.95 bar, 14 psi). The package must be
constructed and closed to prevent any loss of contents that might be caused
under normal transportation conditions by vibration or changes in temperature,
humidity, or pressure. The completed package must be capable of passing a 1.2
meter (3.9 feet) drop test. The package must be marked with a diamond-shaped
marking containing the identication number “UN 3373” and labeled with the
proper shipping name “Biological substance, Category B.” In addition, the name,
address, and telephone number of a person knowledgeable about the material
must be provided on a written document, such as an air waybill, or on the
package itself.
Figure 2. A Category B Non-specication Triple Packaging

421Appendix C—Transportation of Infectious Substances
Intrafacility Specimen and Sample Transfers
Any movement of a pathogen between parts of an institution, which would
require transport in a motor vehicle, on public roads, would require compliance
with the requirements given previously in this Appendix. However, movement
of a pathogen on private roads within the connes of a contiguous facility
boundary (e.g., a campus) where public access is restricted is not commercial
transportation and, therefore, is not subject to these requirements. If movement
of a pathogen is on or crosses a public road, it also is not subject to these
requirements if access to the public road is restricted by signals, lights, gates, or
similar controls.
4–8
It is also common to need to move samples or cultures between laboratories,
between oors in a building, or by walking samples between buildings. When
a sample needs to be moved, care should be taken to minimize the transport
through public and oce areas. Avoid passenger elevators when possible, using
stairs and freight elevators instead. It is recommended that the sample(s) be
placed in a sealable bag or container to provide primary leak-proof containment.
Place absorbent in the bag or container to absorb any spilled material in the
event of a loss. Place the sealed bag or container in a durable, rigid outer
container for transport. Disinfect the exterior of the outer container as appro-
priate depending on the risk posed by the material to be transported. PPE to be
worn during transit is based on the institution’s risk assessment.
Transfer of specic, high-risk pathogens, even within an organization, may need
approval from USDA, CDC, or the Federal Select Agent Program.
References
1. International Civil Aviation Organization [Internet]. Montreal (Quebec):
Safety; c2017–2018 [cited 2018 Dec 4]. Technical Instructions for the Safe
Transport of Dangerous Goods by Air (Doc 9284). Available from: https://
www.icao.int/safety/dangerousgoods/pages/technical-instructions.aspx
2. 3.6.2 Division 6.2—Infectious Substances. In: International Air Transport
Association. IATA Dangerous Goods Regulations. 60th ed. Montreal: IATA;
2019. p. 177–81.
3. Packaging Instruction 650. In: International Air Transport Association. IATA
Dangerous Goods Regulations. 60th ed. Montreal: IATA; 2019. p. 557–9.
4. United States Department of Transportation [Internet]. Washington (DC):
Pipeline and Hazardous Materials Safety Administration; c2017 [cited 2018
Dec 4]. Interpretation Response #16-0134. Available from: https://www.
phmsa.dot.gov/regulations/title49/interp/16-0134

422 Biosafety in Microbiological and Biomedical Laboratories
5. United States Department of Transportation [Internet]. Washington (DC):
Pipeline and Hazardous Materials Safety Administration; c2009 [cited 2018
Dec 4]. Interpretation Response #08-0244. Available from: https://www.
phmsa.dot.gov/regulations/title49/interp/08-0244
6. United States Department of Transportation [Internet]. Washington (DC):
Pipeline and Hazardous Materials Safety Administration; c2006 [cited 2018
Dec 4]. Interpretation Response #06-0113. Available from: https://www.
phmsa.dot.gov/regulations/title49/interp/06-0113
7. United States Department of Transportation [Internet]. Washington (DC):
Pipeline and Hazardous Materials Safety Administration; c2006 [cited 2018
Dec 4]. Interpretation Response #06-0088. Available from: https://www.
phmsa.dot.gov/regulations/title49/interp/06-0088
8. United States Department of Transportation [Internet]. Washington (DC):
Pipeline and Hazardous Materials Safety Administration; c2004 [cited 2018
Dec 4]. Interpretation Response #04-0116. Available from: https://www.
phmsa.dot.gov/regulations/title49/interp/04-0116

423Appendix D—Biosafety and Biocontainment for Pathogens Aecting Agricultural Animals
Appendix D—Biosafety and Biocontainment for
Pathogens Aecting Agricultural Animals and Animals
that are Loose-Housed or in Open Penning
Questions regarding this information should be directed to USDA/Agricultural
Research Service (ARS) Oce of National Programs, Animal Production &
Protection, which is responsible for the content of this appendix.
Introduction
Appendix D focuses primarily on in vitro and in vivo research and diagnostic
activities involving pathogens that primarily aect agricultural animals and other
animal species that cannot be housed in primary containment isolators or an
equivalent means of primary containment following challenge. Basic biocon-
tainment principles used for human pathogen studies provide the foundation for
biosafety and biocontainment practices that reduce the risk of inadvertent release
of agriculture-specic agents into the environment or native animal populations;
see Sections IV and V for additional information. However, enhancements may
be necessary to address specic conditions and requirements essential for
research involving agriculture-specic pathogens. This is particularly important
when agricultural animals and wildlife used in research must be loose-housed
or maintained in open penning where the room or facility serves as the primary
biocontainment barrier.
The host range of these veterinary pathogens may be limited to animals,
although some may also have zoonotic potential and could pose a risk to both
animals and humans. The wide spectrum of animal species routinely used for
agricultural research includes those found in commercial agricultural production
facilities; commercial aquaculture; wildlife; and traditional laboratory animals.
The potential for accidental biohazard release due to research activities could
lead to signicant regional and national economic consequences from animal
morbidity, animal mortality, and international trade restrictions that may be
imposed. These additional economic and environmental risks must be considered
when developing biosafety and biocontainment risk assessments for working with
pathogens that infect agricultural animals. The implementation of biocontainment
guidelines and risk mitigation strategies for working with agriculturally important
pathogens should also be driven by protocols that clearly distinguish between
these pathogens and pathogens that are solely a real or potential threat to
human health. Additional emphasis is placed upon biocontainment measures in
assessing the risk of work with agricultural pathogens to reduce or eliminate the
risk of agent release into the environment.
424 Biosafety in Microbiological and Biomedical Laboratories
Due to their size and disposition, agricultural animals can cause injuries and/
or physical trauma to workers outside those expected with smaller laboratory
animals, and these incidents fall outside the scope generally considered in
biosafety oversight. All sta who work with large animals should at a minimum
receive species-specic training in animal behavior, eective handling practices,
and other physical safety precautions. Whenever possible, experienced sta
should be assigned to train and supervise new employees until skill and compe-
tency have been veried.
This appendix includes a section titled Potential Enhancements for Work with
Pathogens Aecting Agricultural Animals. This section describes enhancements
that exceed standard practices, procedures, containment equipment, and
facility design features common to traditional Biosafety Level 2 (BSL-2), Animal
Biosafety Level 2 (ABSL-2), BSL-3, ABSL-3, BSL-4, and ABSL-4 laboratories
and facilities. These enhancements should be considered for work with
pathogens that aect agricultural animals. The USDA’s Animal and Plant Health
Inspection Service-Veterinary Services (USDA APHIS VS), other regulatory
entities, or local policies and procedures may have additional requirements
for working with agricultural pathogens in an in vitro laboratory or with animals
maintained inside primary containment isolators.
This appendix also includes sections on ABSL-2-Agriculture (ABSL-2Ag), ABSL-
3Ag, and ABSL-4Ag for work with animals infected with high consequence or
otherwise regulated livestock pathogens (as dened in 9 CFR Section 122.2)
that are loose-housed or in open penning. These sections describe special
practices, procedures, containment equipment, and/or facility design features
needed for pathogen/agent studies requiring agricultural or wildlife species that
cannot be housed in a primary containment isolator. When animals are loose-
housed or in open penning, the room or facility serves as the primary barrier
for pathogen containment, so construction and operational design features are
critical biosafety components. For instance, biological safety cabinets (BSCs)
cannot be used for whole animal manipulations, and the volume of waste
generated often exceeds the capacity of typical laboratory-scale programs for
the disposal of animal waste, bedding, and carcasses.
Developing safe work practices, facility design requirements, and engineering
features needed to maintain optimal containment levels must also include risk
assessments for specic procedures that will be performed. These assessments
must include risks associated with agents to be studied, the selection of animal
models, and proposed work activities. Some agent characteristics that should be
considered include host range, infectious dose, mode of transmission, treatment
and immunization availability, environmental stability, and whether the agent is
indigenous or exotic to the location where it will be used. Factors that should be
considered during animal model selection include proposed species; breed or
425Appendix D—Biosafety and Biocontainment for Pathogens Aecting Agricultural Animals
strain (if applicable); age, size, and gender; animal source or vendor; inherent
susceptibility to infection; the eect stress may have on shedding of the agent;
ease of handling vs. animal behavior and responses to stress; and health and
immunization status of the animals. Elements of study design and proposed work
activities that should be evaluated include agent quantity, concentration, and
culture requirements (i.e., agar vs. broth); aerosol-generating procedures (e.g.,
high-pressure pen wash-downs during normal animal care); ability to use primary
containment equipment for manipulations and housing; exposure or challenge
methods; and decontamination methods. Identifying necessary personnel training
and experience in animal handling and general biosafety and biocontainment
practices are also critical to the health and safety of both workers and animals.
Although this appendix focuses on planned research involving known animal
pathogens, the information and concepts can also be applied to animal diagnostic
laboratories where work involves “unknown” or suspect diagnostic specimens.
These diagnostic laboratories receive a wide range of samples that often contain
pathogens, both specic to animals and zoonotic. The virulence of isolated
agents can vary greatly and can cause diseases in animals and humans that
range in signicance from low to high consequence. Carefully analyzing clinical
histories and other background information that accompanies diagnostic samples
is critical for determining an appropriate mitigation strategy to help to control
disease spread and minimize its impact on human and agricultural health.
Information in this appendix should be augmented with the recommendations
of the Biosafety Blue Ribbon Panel convened by the U.S. Centers for Disease
Control and Prevention (CDC)
1
for developing and implementing safety practices
and procedures for animal diagnostic laboratories, as well as taking into account
standard veterinary medical and husbandry best practices that protect veterinary
medical workers in the eld, such as on-farm management practices that prevent
disease introduction and cross-contamination of farms.
This appendix provides guidance for performing robust risk assessments and
determining optimal biosafety practices and containment features that should
be implemented to address those risks. It is not intended as a regulatory
document. USDA APHIS regulates all cultures or collections of organisms and
their derivatives (e.g., DNA, RNA) that may introduce or disseminate contagious
or infectious diseases of livestock and poultry, as well as plants. Institutions
that receive and work with these controlled materials must be approved for the
work and issued a permit before work begins, as well as adhering to the specic
conditions and requirements in the permit, other relevant regulatory requirements,
and/or applicable local rules, policies, and guidelines. The results of site-specic
risk assessments may inform the specic implementation of biocontainment
procedures necessary to meet regulatory requirements, but they do not
supersede them. In addition to the conduct of a robust local risk assessment,
institutions should consult with appropriate regulatory agencies before planning
426 Biosafety in Microbiological and Biomedical Laboratories
new construction or renovating existing facilities to ensure the completed project
is fully functional for all planned uses.
Tables 1–6 and Table Keys 1–3 provide information and guidance regarding the
potential hosts, routes of infection, environmental stability, and recommended
containment levels for in vitro research (BSL), in vivo research with small animals
(ABSL), and in vivo research with large animals (Ag requirements) for a number
of dierent agents and toxins. These tables can assist in the development of a
risk assessment and must be modied by a specic analysis of the work to be
performed and the specic agent used. Note that the agents listed are repre-
sentative of the genus and containment information provided and should not be
considered the denitive list of agents.
Potential Enhancements for BSL-2 and ABSL-2 Facilities for Conducting
Work with Pathogens Aecting Agricultural Animals
USDA APHIS VS, other regulatory authorities, or local policies may require
additional enhancements that exceed standard BSL-2 and ABSL-2 requirements
before approving the possession or study of certain controlled organisms and
their derivatives (e.g., DNA, RNA) that aect agricultural animals. These agents
may be zoonotic or primary animal pathogens; pose low to moderate economic
risk to the agricultural sector; and are generally classied as Risk Group 1 (RG-1)
or RG-2 human pathogens.
Specic enhancements that may be prescribed by regulatory authorities during
the permitting process are mandatory. Institutions may choose to supplement
the required features with additional enhancements based on a site-specic risk
assessment described in the introduction of this appendix and appropriate for
the proposed laboratory and vivarium procedures. The following is a partial list of
actions that may be appropriate:
1. Personnel may be required to use additional personal protective
equipment (PPE).
2. Agent and/or infected animal manipulations are performed exclusively
inside a BSC (if possible due to size limitations) or other primary
containment device.
3. Contaminated euent is collected for disinfection and validated for
inactivation before discharge into facility drainage system or drains to a
dedicated Drain Waste Vent system feeding an euent decontamination
system prior to sanitary sewer.
4. Administrative controls and policies are developed to limit contact
between containment sta and susceptible animals outside the BSL-2
or ABSL-2 enhanced containment space (i.e., o-premises personally
recognizant quarantine policy that is based on agent and species factors).
427Appendix D—Biosafety and Biocontainment for Pathogens Aecting Agricultural Animals
Potential Enhancements for BSL-3 and ABSL-3 Facilities for Conducting
Work with Pathogens Aecting Agricultural Animals
Baseline laboratory techniques, safety procedures, containment equipment, and
facility design features are commensurate with those found in standard BSL-3
and/or ABSL-3 facilities. In addition, supplemental administrative and engineering
controls that mitigate potential risks these agents pose to surrounding animal
populations and the environment may be necessary. The agents considered
here include pathogens of agricultural and wildlife species that pose moderate-
to high-risk to agricultural production and may also be zoonotic pathogens.
Specic control measures are strategically implemented based on a rigorous risk
assessment process.
USDA APHIS VS, other regulatory authorities, or local policies may specify
additional enhancements that exceed standard BSL-3 and ABSL-3 requirements
before approving the possession or study of certain controlled organisms and
their derivatives (e.g., DNA, RNA) that aect agricultural animals.
Additional restrictions apply to certain agents that are not indigenous to the
United States. Approval for possession and experimental use of these pathogens
is contingent on minimum physical containment and security requirements. In
some cases, a permit is required to import or otherwise acquire live organisms.
Potential enhancements, based on a risk assessment, to increase the safety
and biocontainment of BSL-3 and ABSL-3 containment facilities designed for in
vitro procedures and/or in vivo work with animals housed and manipulated within
primary containment are listed below:
1. Potential Facility Enhancements
a. Personnel enter through a series of barriers that provide complete
separation of potentially contaminated animals, materials, and
equipment in BSL-3 or ABSL-3 containment space from other areas
of the building. This can be accomplished through a combination of
procedures and basic facility design.
b. Mechanically interlocked entry/exit vestibule doors or an equivalent
mechanism or process (e.g., work practices) are used to prevent
opening both doors simultaneously.
c. Emergency exit doors are congured to allow safe egress but
cannot be used to gain unauthorized access to the facility. It is
recommended that emergency exits have vestibules to store
emergency decontamination materials.
428 Biosafety in Microbiological and Biomedical Laboratories
d. HEPA ltration of exhaust air. If necessary:
i. Exhaust HEPA lters outside of the BSL-3 or ABSL-3
containment barrier should be located as close to the
containment space as possible to minimize the length of
potentially contaminated air ducts.
ii. Construction of exhaust HEPA lter housings must allow
independent certication testing of each lter in place after
installation and either in-place decontamination or features
that permit the lter to be bagged-out for removal and
decontamination or disposal.
2
iii. The installation of redundant parallel exhaust HEPA lter units,
which accommodate lter changes without disrupting laboratory
activities, should be considered.
iv. Pre-lters should be installed at the room level before the
exhaust HEPA lter and changed frequently to improve
eectiveness and extend HEPA lter life.
e. Engineering features that protect supply air against airow reversals,
if necessary. These could include:
i. A dedicated fresh air supply, which has not been previously
circulated, is preferred; appropriate enhancements should
especially be considered if the air supply for the containment
space is drawn from contiguous non-containment space rather
than a dedicated outside supply.
ii. HEPA ltration of air supply and/or installation of fast-acting
bioseal dampers.
f. Installation of an euent decontamination system (EDS). If needed:
i. Construction should allow for cycle validation with biological
indicators or another equivalent ecacy verication method.
ii. The EDS should be installed in a containable space that is
designed to prevent contamination of adjacent space in the
event of a leak and be able to be adequately sealed to facilitate
space decontamination, if necessary. A site-specic risk
assessment should be performed to identify design features
needed in the containable space, including airlocks, exit
showers, special PPE, containment basins or diking of EDS
tanks, or exhaust air ltration.

429Appendix D—Biosafety and Biocontainment for Pathogens Aecting Agricultural Animals
iii. Plumbing should pass through piping tunnels that allow visual
line inspections; alternatively, double-walled piping with annular
leak detection should be installed in areas that do not allow
visual inspection and cannot be readily accessed for mainte-
nance and repair.
iv. Floor drains may be capped and sealed if an EDS is not present.
2. Potential Practice Enhancements
a. Personnel may be required to use additional PPE based upon
site-specic risk assessment.
b. Administrative controls and policies that limit contact between
containment sta and susceptible animals outside the BSL-3 or
ABSL-3 enhanced containment space (i.e., personally recognizant
quarantine policy).
BSL-4 and ABSL-4 Facilities that Work with Pathogens Aecting
Agricultural Animals
Standard BSL-4 and ABSL-4 practices, procedures, containment equipment,
and facility design features (Sections IV and V of BMBL) are generally
adequate for in vitro procedures involving Risk Group 4 agents in a BSL-4
laboratory or in vivo work involving animals housed inside primary containment
isolators in an ABSL-4 facility.
Potential Enhancements for Animal Biosafety Level 2-Agriculture (ABSL-
2Ag) Facilities for Conducting Work with Animals that are Loose-Housed
or in Open Penning
ABSL-2Ag is recommended for in vivo work involving agents requiring ABSL-2
containment/practices and that includes large livestock and wildlife species that
cannot be housed in primary containment isolators. The animals are maintained
in open penning or loosely housed within a pen/enclosure that may be a single
room, an area within a larger building (e.g., a suite of rooms), or an entire
building. Agents may be primary animal pathogens or zoonotic, are classied
as RG-1 or RG-2, and pose low to moderate economic risks to the agricultural
sector. An example could be a potentially serious agricultural pathogen that is
endemic in the location where the laboratory is situated.
ABSL-2Ag includes the standard practices, procedures, containment equipment,
and facility design features required for ABSL-2. The perimeter of the primary
containment zone is dened by the physical room and an outer containment
zone by the physical facility, making its construction and design features critically
important for risk mitigation and pathogen containment. Appropriate supplemental
enhancements should be selected after a robust risk assessment and should
430 Biosafety in Microbiological and Biomedical Laboratories
address specic conditions or requirements stipulated by USDA APHIS VS
(9 CFR Section 122.2), other relevant regulatory entities, or local policies and
procedures.
Potential enhancements to increase the safety of ABSL-2Ag containment facilities
designed for in vivo work with large livestock and wildlife species are listed below:
1. Potential Facility Enhancements
a. Entrance into the facility should be through a series of barriers
and/or procedures that provide a distinct separation between
containment and non-containment areas. Provisions should be
included for removing, disinfecting, and/or disposing of contami-
nated PPE, footwear, uniforms, and/or equipment before exiting the
ABSL-2Ag containment area.
b. A boot wash should be installed at the entry/exit of the animal room
or ABSL-2Ag containment barrier. The disinfectant solution should
be changed as needed to maintain ecacy.
c. A site-specic risk assessment should be performed to determine if
personal showers are needed for personnel exiting the ABSL-2Ag
containment space—at either the room level, the facility level, or both.
d. Penning, gating, and/or animal restraint systems must be appro-
priate for the species being housed and must be selected/designed
as part of a comprehensive risk assessment process performed
in consultation with the veterinary sta. Critical factors that should
be considered include animal size, proposed procedures, and safe
handling strategies. The equipment should be free of pinch points
and sharp edges that could injure animals or individuals working
in the ABSL-2Ag space and should be sealed or coated with a
nish that is resistant to disinfectants and water pressures used
for routine cleaning. Rooms equipped with modular or changeable
units may be advantageous since they can be used to house a
wider range of species.
e. A site-specic risk assessment should be performed to determine
the need for a ventilation system that is capable of maintaining
directional airow from low hazard areas to higher hazard areas,
which exhausts directly to the outside. Ventilation system options
can include natural ventilation or forced air systems that may
include a dedicated, ducted supply and exhaust system that
discharges to the outside.
431Appendix D—Biosafety and Biocontainment for Pathogens Aecting Agricultural Animals
i. Air handling systems serving areas where large animals are
housed or manipulated should be designed to maintain environ-
mental conditions that are consistent with relevant animal
welfare requirements in addition to the needs of minimizing
emissions (to include particulates).
ii. If an exhaust system is provided, the exhaust air cannot be
recirculated to non-animal room/barn areas and may only be
recirculated to other equivalent containment animal rooms
within the animal facility/barn (i.e., not to non-animal areas or
non-containment animal rooms). If exhaust air is recirculated,
the possibility of cross-contamination in the event of system
failure must be addressed in locally developed, site-specic
incident response plans.
iii. The site-specic risk assessment should determine the need
to provide particulate ltration for supply and/or exhaust air
systems that service ABSL-2Ag areas to prevent cross-contam-
ination between animals on study and other animals housed in
or near the facility, including wildlife.
f. Equipment and supplies must be available for cold storage and
decontamination of large animal carcasses, and adequate decon-
tamination of solid and/or liquid waste.
i. Examples of typical decontamination systems used in
ABSL-2Ag facilities include autoclaves, tissue digesters,
incinerators, and renderers.
ii. Alternate or redundant decontamination systems and proce-
dures should be available for when the primary system requires
maintenance or repairs.
iii. Composting or other nonconventional disposal methods may
be considered if their use is supported by a risk assessment
that specically considers the location, long-term stability,
and proximity of the disposal site relative to other susceptible
animals maintained outside the ABSL-2Ag facility.
iv. The eectiveness of all decontamination methods in use must
be validated.
v. A local risk assessment should be conducted to determine
if euent in animal room drainage systems can be safely
discharged to the sanitary sewer or if it must be collected for
disinfection before disposal.
432 Biosafety in Microbiological and Biomedical Laboratories
g. Cleaning supplies and equipment must be available to decontam-
inate penning, gates, transport crates, and other large devices in
direct contact with animals. Surfaces and design features of these
items should permit thorough cleaning and sanitation. Some articles
may need to be disassembled for complete decontamination.
h. Floors, ceilings, and walls in animal rooms must be constructed
of monolithic materials that are durable and resistant to damage
from animal impact and pressurized sprays, chemical disinfectants,
hot water, or steam that is used for sanitation. Electrical wiring
(e.g., outlets) and equipment (e.g., light xtures) installed in wet
or otherwise hazardous locations must be properly sealed and
grounded. Animal welfare issues (e.g., footing) must be considered
in material selection, application, and use.
2. Potential Practice Enhancements
a. A site-specic risk assessment should be conducted to identify local
practices, equipment, and facility design features that are needed to
protect workers, animals, and the environment.
i. The use of supplemental PPE (e.g., face shields, shin guards,
respirators) and/or facility equipment with advanced safety
features (e.g., quick release latches, self-closing gates) should
be considered to protect workers and animals from hazards
encountered while working with agricultural animals in close
quarters and to protect animals from accidental entrapment or
escape.
ii. Special exit procedures and/or facility features (e.g., anterooms
for PPE or clothing changes, boot-washing stations, shower
facilities) may be needed for workers to exit the containment
area safely.
b. Administrative controls and policies should be established that limit
contact between containment sta and susceptible animals outside
the ABSL-2Ag containment space (i.e., personally recognizant
quarantine policy).
c. It is recommended that administrative controls and policies be
established for a minimum of two workers to be present in the
containment area at all times (i.e., a “buddy system”) or other means
of monitoring worker safety in containment. Operational protocols
should also require all sta to be trained on appropriate response
procedures for time-sensitive emergencies involving workers pinned
or otherwise entangled by equipment or animals.

433Appendix D—Biosafety and Biocontainment for Pathogens Aecting Agricultural Animals
Animal Biosafety Level 3-Agriculture (ABSL-3Ag) Facilities required for
activities involving the use of hazardous biological agents designated as
High-Consequence Foreign Animal Diseases and Pests by USDA APHIS in
animals that are loose-housed or in open penning
ABSL-3Ag containment incorporates standard practices, procedures, containment
equipment, and facility design features common to ABSL-3 and ABSL-2Ag facil-
ities (see preceding sections) but also incorporates many of the facility features
usually reserved for ABSL-4 facilities as enhancements. This level of containment
is required for animals that must be housed in open cages or pens and that have
been infected with specic transboundary livestock or wildlife pathogens dened
by USDA APHIS VS. The agents involved may either be animal pathogens that
pose signicant economic risk to the agricultural sector or agents with zoonotic
potential that are classied as RG-1, RG-2, or RG-3 pathogens. Many of the
agents listed in Tables 1–6 that require ABSL-3Ag are veterinary pathogens and
typically do not pose a severe or high-likelihood risk to human health. Specic
enhancements for research involving these agents can be found in the USDA
Agricultural Research Services Facilities Design Standards 242.1M-ARS
3–8
available at https://www.afm.ars.usda.gov/; however, USDA APHIS VS Select
Agent Regulations (9 CFR Part 121) will specify required facility enhancements
that exceed standard ABSL-3 requirements for research involving agricultural
pathogens that pose signicant economic risk to local, regional, or national
agricultural sectors.
Because large animals and wildlife species involved in research and diagnostic
activities cannot be housed in primary containment isolators, the room perimeter
serves as the primary containment barrier. The containment zone may consist
of a single room, a suite of rooms within a larger facility, or may occupy an
entire building. The area of containment functions as a “box within a box” and
is completely isolated from non-containment areas. Access is strictly controlled
and is limited to personnel who have been properly trained and cleared. Special
physical security features often associated with ABSL-4 facilities may be incorpo-
rated to safeguard against unauthorized entries.
A site-specic risk assessment should be completed that documents the various
ABSL-3 and ABSL-2Ag enhancements (see preceding sections) considered for
implementation. Supplemental enhancements should be based on the results of
this risk assessment and on any specic conditions or requirements stipulated
by USDA APHIS VS, other relevant regulatory entities, or local policies and
procedures.
At minimum, ABSL-3Ag containment facilities must meet requirements associated
with ABSL-3 and ABSL-2Ag containment; and incorporate some enhancements
usually found in ABSL-4 facilities. Potential enhancements to increase the safety
of ABSL-3Ag containment facilities designed for in vivo work with large animals
434 Biosafety in Microbiological and Biomedical Laboratories
are listed below. USDA APHIS VS, other relevant regulatory entities, or local
policies and procedures will determine which enhancements are required based
on the specic details of the proposed work.
1. Potential Facility Enhancements
a. Access to containment areas should be restricted to authorized
personnel. All entry and exit points should be secured with locks
or equivalent electronic access systems and protected by alarms
that will alert authorities of unauthorized movement into or out of
the facility.
b. The entrance to the ABSL-3Ag containment facility should have
a double door vestibule that separates containment areas from
non-containment areas; the doors should be mechanically inter-
locked to prevent simultaneous opening.
i. When two doors are interlocked, at least one of the doors must
meet air pressure resistant (APR) specications, preferably the
door that opens into non-containment space (i.e., the door from
the facility shower to non-containment space).
ii. Personnel must be trained to close the APR doors completely
without damaging them when entering or exiting.
iii. A site-specic risk assessment should be performed to
assess the benets of installing a second APR door between
the animal room exit and the door used to exit the biocon-
tainment facility to maintain correct air pressure dierentials.
Unless specically identied as a permitting or regulatory
requirement, a room-level APR door may not be needed if
the containment area is maintained under negative pressure
such that when the facility-level APR door is opened, air is
inward-directional and contains any pathogens that may have
escaped the animal room.
iv. APR doors may be equipped with pneumatic or mechanical
compression seals. A risk assessment must be conducted
to determine if pneumatic doors should be equipped with
redundant seals (i.e., two-layer or separate seals that are not
linked but rather are lled independently to ensure a defect in
one does not cause the second to fail) to ensure the system’s
integrity. Mechanical compression seals should be checked
and adjusted at regular intervals to ensure full contact when
the seal is engaged.
435Appendix D—Biosafety and Biocontainment for Pathogens Aecting Agricultural Animals
v. Pneumatic lines that inate the gaskets on APR doors should
be equipped with HEPA lters and check valves, as air from
the containment space can enter the lines if a hole occurs in
the gasket.
vi. Integral features of all APR doors (e.g., hinges, latches, knobs,
locking mechanisms, viewing panels) must be sealed and
veried airtight through pressure decay testing. Airtight veri-
cation is dened in Appendix 9B of the USDA ARS Facilities
Design Standards 242.1M-ARS.8 Pressure decay testing must
be completed (1) before occupying the facility, (2) following any
structural modications to the facility, and (3) at regular intervals
determined through a site-specic risk assessment while the
facility is in use.
vii. APR doors may require reinforcements or structural enhance-
ments to ensure the integrity of the door seal if they are at
risk of being physically damaged by large animals. A factory
acceptance test that simulates anticipated impact load is
recommended to ensure the door unit will meet minimum load
requirements.
viii. The facility may include separate dedicated receiving bays
or vestibules equipped with interlocked APR doors that are
separate from the main entrance and/or equivalent transport
systems (e.g., pass-through dunk tanks, gaseous fumigation
chambers, autoclaves). These separate facilities can be used
as dedicated storage areas (e.g., feed and bedding) or to
move equipment and supplies into and out of the ABSL-3Ag
containment space. A local risk assessment may identify
acceptable operational alternatives that provide an equivalent
level of containment, such as combining inward-directional
airow with a single APR door between the contained corridor
and the vestibule, rather than two interlocked doors.
c. Decontamination of personnel exiting the containment zone should
involve two separate transitions to ensure maximum environmental
protection: the rst transition involves exiting the animal room
and entering the change room, and the second transition involves
exiting the change room and then the containment zone or facility.
While various design and procedural options are available for
incorporating these transition zones, selection should be based
on the results of a local risk assessment and relevant regulatory
436 Biosafety in Microbiological and Biomedical Laboratories
and permit requirements. From a design perspective, ABSL-3Ag
facilities must have a personal shower at the containment-non-con-
tainment boundary even if alternate exit strategies are implemented
that do not always require a second shower by personnel. Some
strategies include:
i. A comprehensive solution where two separate personal
shower facilities are utilized. Personnel shower two times:
(1) before entering the change room from the animal room
(i.e., a room-level shower), and (2) after exiting the animal
room shower and before leaving the facility (i.e., a facility-level
shower).
ii. Personnel are required to have a complete clothing change
before entering each animal room or suite (i.e., experimental
unit) to ensure clothing worn inside the animal room is separate
and distinct from that worn in spaces outside the animal
room (e.g., hallways, laboratories). This facilitates a transition
between containment zones (i.e., ABSL-3Ag to a containment
corridor that accesses other containment spaces [BSL-3 or
other ABSL-3Ag animal rooms]) and should be paired with a
facility-level shower to ensure a contained to non-containment
space personal decontamination step.
iii. Decontamination of outer PPE within the animal containment
room or suite, followed by PPE removal as the worker steps
into a transition area located between containment and lower
containment or non-containment zones. The process must
be carefully documented to ensure the procedures can be
performed easily and consistently by personnel working in these
areas and must be paired with an animal room level exit shower
to ensure adequate personal decontamination and environ-
mental protection step.
iv. Provisions for disinfecting and changing contaminated boots
before moving between or exiting containment units (i.e., animal
rooms) should be employed.
d. Penning, gating, and/or animal restraint systems must be appropriate
for the species housed, and these systems should be selected/
designed as part of a comprehensive risk assessment performed
in consultation with the veterinary sta. Critical factors to consider
include animal size, proposed procedures, and safe handling strat-
egies. The equipment should be free of pinch points and sharp edges
437Appendix D—Biosafety and Biocontainment for Pathogens Aecting Agricultural Animals
that could injure animals or individuals working in the ABSL-3Ag
space and sealed or coated with a nish that is resistant to disin-
fectants and water pressures used for routine cleaning. Rooms
equipped with modular or changeable units may be advantageous
since they can be used to house a wider range of species.
e. Appropriate equipment and supplies should be available inside the
ABSL-3Ag facility to decontaminate large animal waste, carcasses,
and other contaminated refuse and articles that need to be removed
from the containment area. This equipment should include design
features that ensure the same level of containment as the primary
barrier.
i. Examples of typical decontamination systems used in
ABSL-3Ag facilities include autoclaves, tissue digesters, incin-
erators, renderers, gaseous decontamination chambers, liquid
disinfectant dunk tanks, and similar equipment. Autoclaves,
tissue digesters, renderers, and incinerators should be designed
or programmed to prevent opening of the outer door until the
decontamination cycle is completed and veried to have met
program parameters.
ii. The installation of equipment designed with pass-through
features that permit contaminated articles to be loaded into
an autoclave or sterilizer inside the containment zone and
decontaminated before removal on the non-containment side.
This equipment should be installed with mechanical elements
located or accessible outside the ABSL-3Ag facility to facilitate
routine maintenance and repairs.
iii. A site-specic risk assessment should be performed to
determine the need for ltration or decontamination of the
condensate and/or exhaust from decontamination equipment
(e.g., autoclaves).
f. Liquid euents from ABSL-3Ag containment areas must be
collected and decontaminated before disposal into a sanitary sewer.
Collection and decontamination methods should be selected after a
site-specic risk assessment.
i. Installation of a central liquid euent waste collection and
decontamination system is the preferred method.
ii. Heat decontamination systems must be designed so that the
contaminated euent can be held at specied temperatures,
pressures, and times to ensure complete inactivation of all
438 Biosafety in Microbiological and Biomedical Laboratories
hazardous materials. Systems should operate at a range of
temperatures and holding times to economically and eciently
process a wide range of euents.
iii. At minimum, euents from laboratory sinks, BSCs, and oor
drains should be directed into the waste collection system
for decontamination before discharge. A site-specic risk
assessment should be performed to (1) determine if euent
from autoclave chambers, shower rooms, and toilets should
be collected and decontaminated, and (2) identify the optimal
decontamination method that is required (i.e., validated
chemical treatment system or heated liquid waste decontami-
nation system).
iv. Facilities should be designed with basement access or piping
tunnels that allow the facility waste plumbing systems to be
inspected. Double containment piping systems with annular leak
detection capability should be used for plumbing that is buried,
concealed, or located outside the containment facility.
g. Waste handling procedures must adhere to the results of a site-
specic risk assessment and applicable regulations and local
policies and procedures.
i. Decontamination systems and procedures must be validated
using biological indicators, culture of treated waste, or another
equivalent process to ensure the selected cycle and operating
parameters are appropriate for the agents as well as the types
and volumes of waste generated.
ii. Operating parameters should be validated for each load type
that is treated, and periodically veried using an appropriate
method.
iii. In some cases, a two-step waste treatment process may be
indicated. For example, waste can rst be autoclaved for
removal from the containment facility and then destroyed
through incineration (i.e., locally at the facility or through a
commercial service). Regulations pertaining to the transport of
potentially infectious waste must be considered in this process.
iv. Disposal methods such as composting or spreading manure on
elds are not allowed.
h. ABSL-3Ag facilities are required to have dedicated, single-pass
ventilation systems that create and maintain an appropriate
inward-directional pressure gradient.
439Appendix D—Biosafety and Biocontainment for Pathogens Aecting Agricultural Animals
i. The air supply and exhaust system must be independent or
isolatable, and it must provide graded pressure dierentials
such that inward directional airow is maintained in containment
spaces relative to adjacent non-containment areas in the event
of a breach (e.g., opening doors). Pressure dierentials must
be designed such that air moves continuously from low hazard
areas to higher hazard areas in the event of a breach.
ii. A visual indicator that displays real-time pressure dierentials
should be available outside the containment facility to conrm
that personnel can enter safely.
iii. Audible or visual alarms are needed that can be heard and/or
seen both inside and outside of the containment space to alert
sta when pressure dierentials are outside the pre-set range.
The alarm system should be compatible with worker safety and
animal welfare (i.e., audible without being so loud that animals
are startled or stressed, or just visual). Intercom systems should
limit the type and number of overhead announcements that can
be disruptive and contribute to excessive noise levels.
iv. Ventilation system performance must be validated (1) before
the facility becomes operational, (2) at least annually while the
facility is operational, and (3) following any signicant modica-
tions to the ventilation system. Guidelines for standards to be
used in risk assessments and the development of site-specic
validation protocols can be found in the following:
1. USDA ARS Facilities Design Standards 242.1M-ARS3–8
2. ANSI/ASSE Z9.14 Testing and Performance Verication
Methodologies for Ventilation Systems for Biological Safety
Level 3 (BSL-3) and Animal Biological Safety Level 3
(ABSL-3) Facilities
2
i. HVAC system pressure dierentials should be designed after a
site-specic risk assessment to incorporate engineering features
that protect against sustained reversal of directional airow in the
event of a breach of containment (e.g., opening doors).
i. Air supply and exhaust systems should be interlocked to
prevent sustained reversal of directional airow during HVAC
failures or emergencies that can lead to positive pressurization
of containment spaces.
440 Biosafety in Microbiological and Biomedical Laboratories
ii. Supply air must pass through ductwork with either a HEPA lter
and/or a fast-acting bioseal (i.e., bubble-tight) damper that fails
in the closed position to prevent the reverse ow of contami-
nated air through supply ducts into other containment zones or
non-containment areas outside the facility.
iii. In the absence of a supply HEPA lter(s), a robust preventative
maintenance program that includes an annual validation
process must be implemented to ensure the fast-acting bioseal
damper operates as designed to prevent airow reversal.
iv. Bioseal dampers must consistently fail in the closed position
and continue to function properly during power failures (e.g.,
electrically held open, mechanically and automatically closed
in a power failure). Gaskets should be constructed of materials
that will seal properly, regardless of scheduled applications
of lubricants and/or sealants. The seal must be capable of
withstanding the air pressures applied as fans spin down in a
power failure.
j. The exhaust air from ABSL-3Ag facilities should pass through
ductwork with two HEPA lters installed in series prior to being
exhausted to the outside.
i. HEPA lters should be located outside of the containment zone
to facilitate routine maintenance and validation procedures.
They should also be located as close to the ABSL-3Ag facility
as possible to minimize the overall length of potentially contami-
nated ductwork outside the containment zone.
ii. Pressure decay testing must be used to conrm that HEPA
lter frames, housing, and the ductwork between the ABSL-3Ag
facility and the HEPA lter are airtight. This testing is described
in the USDA ARS Facilities Design Standards 242.1M-ARS.3–8
iii. Methods for eectively decontaminating sections of potentially
contaminated ductwork that extends outside the ABSL-3Ag
facility should be identied and validated.
iv. HEPA lter housings must be fabricated to allow the lters
to be scan-tested after installation and decontaminated in
place before removal. Parallel HEPA lter units that allow lter
changes and scan testing without disrupting facility operation
should be considered for maximum exibility and eciency.
Conguration and operation of parallel units should be carefully
evaluated to ensure continuous operation.
441Appendix D—Biosafety and Biocontainment for Pathogens Aecting Agricultural Animals
v. Redundant exhaust fans must be installed to ensure
containment parameters are maintained continuously during
equipment maintenance, and redundant supply fans are highly
recommended. Precautions should be taken if fast-acting
dampers are used in closed rooms instead of redundant supply
fans because extreme negative pressures can develop that can
injure personnel and animals or cause structural damage.
vi. Pre-lters (at least 80–90% eciency) should be installed in
air supply and exhaust ductwork to extend the functional life of
HEPA lters. Pre-lters should be installed inside the biocon-
tainment room or facility to facilitate frequent changes that can
be completed without decontaminating the exhaust system.
Used pre-lters should be regarded as contaminated and
disinfected or decontaminated by a validated method before
they are removed from the ABSL-3Ag facility for disposal.
vii. Air handling systems must be able to regulate the temperature
and humidity in areas where animals are housed or manip-
ulated, and the exhaust air cannot be recirculated to supply
non-animal areas.
k. Plumbing traps must be kept lled with liquid disinfectant or capped,
and the atmospheric vents associated with these traps must have
HEPA lters or their equivalent installed. Whenever possible,
deep-seal plumbing traps should be used to prevent potential
cross-contamination due to loss of seal, back pressure, or trap
siphonage.
l. HEPA lters must be installed on return lines of pneumatic systems
(e.g., plumbing vents, pneumatic lines for inatable door seals,
vacuum systems).
i. In general, central vacuum systems are discouraged. When a
vacuum source is needed, a HEPA lter should be installed near
the service cock or point of use.
ii. Installation should allow in-place lter decontamination and/or
replacement without exposing the local environment to potential
contamination.
m. Construction materials used in an ABSL-3Ag facility should be
appropriate for the intended end use. Walls must be constructed
slab-to-slab and must be contiguous with the oor and ceiling.

442 Biosafety in Microbiological and Biomedical Laboratories
i. All penetrations in the oors, walls, and ceilings must be
sealed and veried to be airtight to prevent cross-contam-
ination and to allow gaseous or vapor phase fumigation
within the containment facility without aecting adjacent
non-containment space (see specications in the USDA ARS
Facilities Design Standards 242.1M-ARS).
3–8
This includes
openings around ductwork; plumbing xtures; doorframes;
door hardware and gaskets; electrical boxes; and vents.
ii. When required, exterior windows and vision panels must
be sealed and constructed of breakage-resistant materials
sucient to withstand animal kicks or bites.
iii. The room envelope must meet the minimum criteria for a
primary containment barrier that is equivalent to performance
standards established for secondary barriers in ABSL-3 spaces.
Each ABSL-3Ag primary containment unit (i.e., room, suite)
must be veried as airtight.
n. Necropsy rooms must be equipped and large enough to safely
accommodate work on research animals housed in the containment
unit. Equipment (i.e., ceiling hoists, wall-mounted drag systems,
mobile tilt tables) and strategies to assist with the humane transport
of moribund animals and the carcasses of dead animals that are too
large for facility sta to move manually should be incorporated into
facility design and operations.
o. If BSCs are installed, they must be selected, located, installed,
operated, and maintained according to the manufacturer’s instruc-
tions and standards found in NSF/ANSI 49-2018 and Appendix A.
Due to the high rate of air exchange and room pressure uctuations
that occur with APR door operation in ABSL-3Ag facilities, all
ventilated equipment should undergo extensive functional testing
during installation and at an increased frequency while in operation
to ensure proper placement and operation.
2. Potential Practice Enhancements
a. Access to containment areas should be controlled, monitored,
and limited to personnel who are adequately trained, cleared, and
authorized to work in this area. A trained escort must be provided
for other individuals who enter the facility, such as inexperienced
workers, visitors, and service providers.
b. Personnel should follow a redundant, two-step decontamination
443Appendix D—Biosafety and Biocontainment for Pathogens Aecting Agricultural Animals
process when exiting the ABSL-3Ag facility to prevent accidental
contamination of non-containment space. Workers may be required
to shower or wear extra PPE that can be surface-decontaminated
upon exiting a primary containment room, followed by an additional
shower before exiting the containment facility. However, a wide
range of options are available to meet this requirement, and a
site-specic risk assessment that incorporates relevant regulatory
and permit requirements should be performed to determine appro-
priate decontamination protocols.
c. Administrative controls and policies should limit contact between
containment sta and susceptible animals outside the ABSL-3Ag
containment space (i.e., personally recognizant quarantine
policy based on site-specic risk assessment and regulatory
requirements).
d. Administrative controls and policies should recommend a minimum
of two workers to be present in the containment area at all times
(i.e., a “buddy system”) or other means of monitoring worker safety
in containment. All sta working in biocontainment areas should
be trained on appropriate response procedures for time-sensitive
emergencies involving workers pinned or entrapped by equipment
or animals.
Animal Biosafety Level 4-Agriculture (ABSL-4Ag) Facilities for Conducting
Work with Animals that are Loose-Housed or in Open Penning
ABSL-4Ag containment incorporates standard practices, procedures, containment
equipment, and facility design features common to ABSL-4 and ABSL-3Ag
facilities (see previous sections). This level of containment is required for animals
infected with zoonotic pathogens that would ordinarily require (1) facilities and
procedures commensurate with ABSL-4 containment as determined by relevant
regulatory authorities, or (2) a comprehensive local risk assessment, which also
assesses the cross-contamination risk, for animals that cannot be housed in
primary containment isolators (e.g., open caging units inside exible lm isolators
with inward-directional airow that is separate from the facility’s HVAC system).
Personnel working in the ABSL-4Ag containment zone must wear positive-
pressure suits.
Agents studied in ABSL-4Ag containment can pose a signicant economic risk to
the agricultural sector and are also zoonotic pathogens consistent with RG-3 or
RG-4 classication, for which eective treatments and/or preventative measures
are not available for humans. Animals used in this research are housed loosely
444 Biosafety in Microbiological and Biomedical Laboratories
or in open penning, so the room perimeter serves as the primary containment
barrier. The containment zone may consist of a single room, a suite of rooms
within a larger facility, or an entire building. The area of containment functions
as a “box within a box” and is completely isolated from non-containment areas.
Access is strictly controlled and limited to personnel who have been properly
trained and cleared. Special physical security features that are required for
standard ABSL-4 facilities must be incorporated to safeguard against unautho-
rized entries.
A site-specic risk assessment should be completed that documents the various
ABSL-4 and ABSL-3Ag enhancements that were considered. Supplemental
enhancements should be based on the results of this risk assessment and should
be implemented with specic conditions or requirements stipulated by USDA
APHIS VS, other relevant regulatory entities, or local policies and procedures.
At minimum, ABSL-4Ag containment facilities must meet requirements associated
with ABSL-4 and ABSL-3Ag containment. Potential enhancements to increase the
safety of ABSL-4Ag containment facilities designed for in vitro procedures and/or
in vivo work with animals are listed below:
1. Potential Facility Enhancements
a. APR doors must be equipped with pneumatic or mechanical
compression seals. A risk assessment should be conducted to
determine if pneumatic doors need redundant seals (i.e., two-layer
or separate seals that are not linked and are lled independently
to ensure a defect in one does not cause the second to fail) to
ensure the system’s integrity. Mechanical compression seals must
be checked and adjusted at regular intervals to ensure full contact
when the seal is engaged.
i. Pneumatic lines that inate the gaskets on APR doors must be
equipped with HEPA lters and check valves when there is any
possibility that air from the containment space is entering the
lines.
ii. Doors may be self-closing and may require reinforcements or
structural enhancements to ensure the integrity of door seals
if they are at risk from physical damage by large animals. A
factory acceptance test that simulates anticipated impact load
is recommended to ensure door units will meet minimum load
requirements.
iii. Pressure decay testing must be used to ensure integral features
of all doors (e.g., hinges, latches, knobs, locking mechanisms,
viewing panels) are veried to be sealed and airtight.
445Appendix D—Biosafety and Biocontainment for Pathogens Aecting Agricultural Animals
b. The ABSL-4Ag room includes a separate dedicated vestibule
equipped with interlocked APR doors that are separate from
the main entrance and/or equivalent transport systems
(e.g., pass-through dunk tanks, gaseous fumigation chambers,
autoclaves) that can be used as dedicated storage areas
(e.g., feed and bedding) or to move equipment and supplies into
and out of the ABSL-4Ag containment space. Construction of the
vestibule and APR doors must be compatible with chemicals used
to decontaminate or fumigate contaminated equipment, waste,
and supplies before removal from the containment facility.
c. A chemical decontamination shower will generally be required
before exiting the primary ABSL-4Ag containment zone (large
animal room). However, a simple physical decontamination step/
shower of the positive-pressure suit (e.g., water wash down shower)
may be sucient if moving between rooms of similarly treated
animals (i.e., infected with the same experimental agent).
i. To prevent cross-contamination of dierent experimental
groups, full PPE decontamination is generally required before
movement into a new containment zone (e.g., separate
BSL-4, ABSL-4, or other ABSL-4Ag areas) from an ABSL-4 Ag
room/zone. However, if you are employing a low- to high-risk
movement strategy (e.g., working with uninfected controls
before experimentally infected animals), a chemical decontam-
ination shower may not be required to move between specic
rooms. A project-specic risk assessment must be conducted
to address these issues, but a full chemical decontamination
shower is required before exiting the maximum containment
facility.
ii. Location and operational parameters of a decontamination
vestibule or chemical shower at the containment barrier should
be determined through a site-specic risk assessment that
includes factors such as containment requirements, research
needs, and experimental workow.
iii. Installation of boot washes and boot storage is recommended
adjacent to animal room exits and the decontamination shower.
iv. A risk assessment to determine selection of encapsulating suits
with or without integral boots will need to occur. In some cases,
it may be advantageous to utilize suits that do not have integral
boots to allow boot changes between rooms, where personnel
446 Biosafety in Microbiological and Biomedical Laboratories
will use a boot wash and maintain a set of boots in each
ABSL-4Ag animal room.
d. Ventilation system performance must be validated (1) before the
facility becomes operational, (2) at least annually while the facility
is operational, and (3) following any signicant modications to the
ventilation system. Local risk assessment processes and the devel-
opment of site-specic validation protocols should be conducted
using standards and guidelines found in the following:
i. USDA ARS Facilities Design Standards 242.1M-ARS3–8
ii. ANSI/ASSE Z9.14 Testing and Performance-Verication
Methodologies for Ventilation Systems for Biological Safety
Level 3 (BSL-3) and Animal Biological Safety Level 3
(ABSL-3) Facilities
2
e. Ceiling-mounted self-coiling air lines or tension tethers to suspend
air lines away from animals and equipment must be used to prevent
entanglement and damage to the lines. The system design should
accommodate the need for personnel to safely enter and exit pens
with animals, but work practices should minimize such contact as
much as possible with the use of chutes, isolation gates, and/or free
pens to facilitate movement of animals away from personnel unless
contact is absolutely required.
2. Potential Practice Enhancements
a. Personnel must wear a positive-pressure ventilated protective suit
with a safe breathing air source.
i. Pressurized suits should not have integral foot covers or boots;
separate work boots are recommended.
ii. The durability of pressurized suits should be evaluated to
conrm they are suitable for anticipated work conditions
involving agricultural animals that are housed loosely or in
open penning.
b. Personnel working in pressurized suits should be trained in the
strategic use of penning, gating, and animal restraint equipment to
minimize potential contact with animals, animal waste, and sharp
surfaces.
c. Administrative controls and policies should recommend a minimum
of two workers to be present in the containment area at all times
(i.e., a “buddy system”) or other means of monitoring worker safety

447Appendix D—Biosafety and Biocontainment for Pathogens Aecting Agricultural Animals
in containment. All sta should be trained on appropriate response
procedures for time-sensitive emergencies involving workers who
are pinned or otherwise trapped by equipment or animals.
Table 1. Bacteria
Genus Agent(s) Hosts
1
Routes
2
Stability
3
In vitro
Cont.
In vivo
Cont.
In vivo
Ag
Cont.
Other
Regs
Actinobacillus spp. A. pleuropneumoniae 3 3, 4, 5 2 2 2 2Ag–3Ag N/A
Aeromonas spp.
A. hydrophila,
A. salmonicida
5 3, 8 2 2 2 2Ag N/A
Anaplasma spp.
A. centrale,
A. marginale,
A. phagocytophilum
1a 2, 4 2 2 2 2Ag N/A
Arcobacter spp.
A. butzleri,
A. cryaerophilus,
A. skirrowii
1, 2, 3, 10b 1, 8 2 2 2 2Ag N/A
Bacillus spp.
B. anthracis,
B. cereus
1–10 2, 3, 8 1–3 2–3 2–3 2Ag–3Ag Y
Bartonella spp. B. henselae 7b, 9 2, 4 2 2 2 2Ag N/A
Bibersteinia spp. B. trehalosi 1 9 2 2 2 2Ag N/A
Borrelia spp. B. burgdorferi 2, 4, 7
, 10b 2 2 2 2 2Ag N/A
Brucella spp.
B. abortus,
B. canis,
B. melitensis,
B. ovis,
B. suis
1, 2, 3, 6,
7a, 10b
1, 3, 4,
5, 7, 8
2 2–3 2–3 2Ag–3Ag Y
Burkholderia spp.
B. mallei (Pseudomonas
mallei),
B. pseudomallei
1, 2, 3,
7, 10b
1, 3, 4, 5 2 3 3 2Ag–3Ag Y
Campylobacter spp.
C. coli,
C. fetus fetus,
C. fetus venerealis,
C. jejuni
1, 3, 4a 1, 8 1 2 2 2Ag N/A
Chlamydia spp.
C. caviae,
C. felis,
C. muridarum,
C. pecorum,
C. pneumoniae,
C. psittaci,
C. suis,
C. trachomatis
1, 2,
3, 4, 5,
6, 7, 8, 10
3, 4, 5 2 2–3 2–3 2Ag–3Ag N/A
Chlamydophilus spp. C. abortus 1c 3, 4, 5 2 2 2 2Ag–3Ag N/A
Clostridium spp.
C. botulinum,
C. dicile,
C. perfringens,
Types A, B, C, and D
1–10 1, 8 2–3 2–3 2–3 2Ag–3Ag Y
Coxiella spp. C. burnetii 1 3, 4, 5 3 3 3 2Ag–3Ag Y
Cronobacter spp.
C. sakazakii (Enterobacter
sakazakii)
10b 4 2 2 2 2Ag N/A
Ehrlichia spp.
E. canis,
E. chaeensis,
E. ewingii,
E. ondiri,
E. ruminantium
1, 6a, 7,
10b
2 1–2 2 2 2Ag N/A
Environmental
Mastitis
E. coli,
Streptococcus uberis,
Klebsiella,
Proteus,
Pseudomonas,
Serratia spp.
1a 9 2 2 2 2Ag N/A
Erysipelothrix spp. E. rhusiopathiae
1c
, 3, 4, 5,
6d, 7c, 10b
4, 8 3 2 2 3Ag N/A
Continued on next page ►

448 Biosafety in Microbiological and Biomedical Laboratories
Genus Agent(s) Hosts
1
Routes
2
Stability
3
In vitro
Cont.
In vivo
Cont.
In vivo
Ag
Cont.
Other
Regs
Escherichia spp. E. coli
1, 2, 3, 4, 6,
7, 8, 10
1, 4, 8 2 2 2 2Ag–3Ag N/A
Flavobacteria spp.
F. branchiophilum,
F. columnare,
F. psychrophilum
5 3, 7 1 2 2 2Ag N/A
Francisella spp. F. tularensis
1, 2, 3, 4, 5,
6, 7, 10
2, 3, 4 2 3 3–4 2Ag–3Ag Y
Histophilus spp.
H. somni (Haemophilus
somnus)
1a 9 2 2 2 2Ag N/A
Leptospira spp.
L. bratislava,
L. canicola,
L. grippotyphosa,
L. hardjo,
L. icterohaemorrhagiae,
L. interrogans,
L. pomona
1, 2, 3, 6,
7, 8, 10
9
2 2 2 2Ag N/A
Listeria spp. L. monocytogenes
1, 3, 4, 6,
7, 8, 10
1 2 2 2 2Ag N/A
Mannheimia spp. M. haemolytica 1a 9 2 2 2 2Ag N/A
Melissococcus spp. M. plutonius 9 2, 8 2 2 2 2Ag N/A
Moraxella spp. M. bovis 1a 2, 3, 4 2 2 2 2Ag N/A
Mycobacterium spp.
M. avium subsp
paratuberculosis,
M. bovis,
M. chelonae,
M. fortuitum,
M. marinum,
M. neoaurum,
M. scrofulaceum,
M. simiae
1, 5, 6a,
10b
1, 3, 4, 5 2 2–3 2–3 2Ag–3Ag Y
Mycoplasma spp.
M.agalactiae,
M. bovis,
M.capricolum
capripneumoniae (F 38),
M. gallisepticum,
M. hyopneumoniae,
M. mycoides capri (PG 3),
M. mycoides (large colony
type),
M. mycoides mycoides
(small colony type),
M. synoviae
1, 2
, 4
1, 3, 4, 5,
7, 9
2 2 2–3 2Ag–3Ag Y
Paenibacillus sp. P. larvae larvae 9 4, 7 2 2 2 2Ag N/A
Pasteurella spp. P. multocida 1a 9 2 2 2 2Ag N/A
Pleisomonas spp. P. shigelloides
1, 3, 4, 5,
6, 7, 10
1, 8 2 2 2 2Ag N/A
Pseudomonas spp. P. aeruginosa 10b 4 2 2 2–3 2Ag N/A
Renibacterium spp. R. salmoninarum 5a 4, 7 2 2 2 2Ag N/A
Rhodococcus spp. R. equi
1, 2, 3,
7b, 10b
2 2 2 2 2Ag N/A
Rickettsia spp.
R. felis,
R. prowazekii,
R. rickettsii,
R. typhi,
Orientia tsutsugamushi
6, 7a, 8,
9, 10b
2, 3
, 4 3 2 2–3 2Ag–3Ag Y
Salmonella spp.
S. enterica (including
serovars Abortusovis,
Choleraesuis, Dublin,
Enteritidis, Gallinarum,
and Typhimurium)
S. bongori
1a, 3, 4,
5, 6c
3, 4, 5, 7, 8 2 2 2 2Ag N/A
Shigella spp.
S. boydii,
S. dysenteriae,
S. exneri,
S. sonnei
1a, 4a, 10b 1, 8 2 2 2 2Ag N/A
Continued on next page ►
449Appendix D—Biosafety and Biocontainment for Pathogens Aecting Agricultural Animals
Genus Agent(s) Hosts
1
Routes
2
Stability
3
In vitro
Cont.
In vivo
Cont.
In vivo
Ag
Cont.
Other
Regs
Spirillum spp. S. minus 8, 10b 4, 8 2 2 2 2Ag N/A
Staphylococcus spp. S. aureus (mastitis)
1, 2, 3, 6,
7, 8, 10
8 2 2 2–3 2Ag N/A
Streptobacillus spp. S. moniliformis 8, 10b 4, 8 2 2 2 2Ag N/A
Streptococcus spp.
S. canis,
S. equi equi,
S. equi zooepidemicus,
S. iniae,
S. pyogenes,
S. suis
1–10 1, 3, 4, 5, 8 2 2 2 2Ag Y
Taylorella spp. T. equigenitalis 2 4, 5 2 2 2 2Ag Y
Vibrio spp.
V. cholerae,
V. parahaemolyticus,
V. vulnicus
5 8 2 2 2 2Ag N/A
Xenohaliotis spp. X. californiensis 5d 1
2 2 2 2Ag Y
Yersinia spp.
Y. enterocolitica,
Y. pestis,
Y. pseudotuberculosis,
Y. ruckeri
1, 2, 3, 5, 7,
8, 10b
3, 4, 8 1–2 2–3 3–4 2Ag–3Ag Y
Table 2. Fungi and Molds
Genus Agent(s) Hosts
1
Routes
2
Stability
3
In vitro
Cont.
In vivo
Cont.
In vivo
Ag
Cont.
Other
Regs
Aphanomyces spp.
A. astaci,
A. invadans
5 1, 3, 4 2 2 2 2Ag–3Ag Y
Batrachochytrium
spp.
B. dendrobatidis 6e 3, 4 2 2 2 2Ag N/A
Coccidioides spp.
C. immitis,
C. posadasii
1a, 2, 3,
7, 10b
3, 5 2 3 3 2Ag–3Ag N/A
Cryptococcus spp. C. neoformans
1, 2, 4, 6,
7, 10b
3 2 2 2 2Ag N/A
Epidermophyton
spp.
E. occosum
1, 2, 3,
7, 10b
4 2 2 2 2Ag N/A
Histoplasma spp.
H. capsulatum
farciminosum
2 2, 3, 4 2 3 3 2Ag–3Ag N/A
Microsporum spp.
M. canis,
M. gypseum,
M. nanum
1, 2, 3, 7 4 2 2 2 2Ag N/A
Nosema spp.
N. apis,
N. ceranae
9 3, 4 3 2 2 3Ag N/A
Pseudogymnoascus
spp.
P. destructans 6g 4 2 2 2 2Ag N/A
Saprolegnia spp. S. parasitica 5 3 2 2 2 2Ag N/A
Sporothrix spp. S. schenckii
1, 2, 3,
4, 5, 6,
7, 8, 10b
1, 4 2 2 2 2Ag N/A
Trichophyton spp.
T. equinum,
T. mentagrophytes,
T. verrucosum
1, 2, 3, 7 4 2 2 2 2Ag N/A

450 Biosafety in Microbiological and Biomedical Laboratories
Table 3. Nematodes, Trematodes, Cestodes, Protozoa, and Ectoparasites
Genus Agent(s) Hosts
1
Routes
2
Stability
3
In vitro
Cont.
In vivo
Cont.
In vivo
Ag
Cont.
Other
Regs
Acarapis spp. A. woodi 9 4 2 2 2 2Ag N/A
Aethina spp. A. tumida 9 4 2 2 2 2Ag N/A
Alaria spp. A. americana 6d, 7 6 1 2 2 2Ag N/A
Amblyomma spp.
A. americanum,
A. maculatum
1, 2, 3, 4,
6, 7, 8, 10
4 2 2 2 2Ag N/A
Amphimerus spp. A. pseudofelineus
5, 6b, 7,
10b
6, 8 2 2 2 2Ag N/A
Ancylostoma spp.
A. braziliense,
A. caninum,
A. duodenale
7, 10b 4 2 2 2 2Ag N/A
Anisakis spp.
A. pegrei,
A. simplex
5 8 2 2 2 2Ag N/A
Babesia spp.
B. bovis,
B. bigemina,
B. divergens,
B. major,
B. ovata,
B. occultans,
B. jakimovi
1, 2, 6a,
10b
2, 6 2 2 2 2Ag Y
Baylisascaris spp.
B. columnaris,
B. melis,
B. procyonis
1, 2, 3, 4,
6, 7, 10
1, 6 2 2 2 2Ag N/A
Besnoitia spp. B. besnoiti 1a 6 2 2 2 2Ag N/A
Bonamia spp.
B. ostreae,
B. exitiosa
5d 4 2 2 2 2Ag Y
Bunostomum spp. B. phlebotomum 1 1, 6 2 2 2 2Ag N/A
Ceratonova spp. C. shasta 5a 3 2 2 2 2Ag N/A
Chrysomya spp. C. bezziana
1, 2, 3, 4,
6, 7, 8, 10
4, 6 2 2 2 2Ag Y
Cochliomyia spp. C. hominivorax
1, 2, 3, 4,
6, 7, 8, 10
4, 6 2 2 2 2Ag Y
Cryptosporidium
spp.
C. parvum 1, 2, 3, 10b 1, 4 2 2 2 2Ag N/A
Dicrocoelium spp. D. dendriticum
1, 2, 6,
7, 10b
6 1 2 2 2Ag N/A
Diphyllobothrium
spp.
D. dendriticum,
D. latum
10b 6, 8 2 2 2 2Ag N/A
Echinococcus spp.
E. granulosa,
E.multilocularis,
E. oligarthrus,
E. shiquicus,
E. vogeli
1, 3, 7a,
10b
1 2 2 2 2Ag N/A
Echinostoma spp.
E. cinetorchis,
E. hortense,
E. liei,
E. revolutum
4, 5, 6,
7, 10b
1 2 2 2 2Ag N/A
Eimeria spp.
E. acervulina,
E. brunetti,
E. maxima,
E. meleagridis,
E. necatrix,
E. tenella
1, 2, 3,
4, 6d, 7
1 2 2 2 2Ag N/A
Entamoeba spp. E. histolytica 10 1, 5 3 2 2 2Ag N/A
Fasciola spp. F. hepatica 1, 6a 6 1 2 2 2Ag N/A
Fascioloides spp. F. magna 1, 6a 6 1 2 2 2Ag N/A
Giardia spp.
G. duodenalis,
G. intestinalis,
G. lambia
1, 3, 6,
8, 7, 10
1, 5 2 2 2 2Ag N/A
Gyrodactylus spp. G. salaris 5a 4 2 2 2 2Ag N/A
Continued on next page ►

451Appendix D—Biosafety and Biocontainment for Pathogens Aecting Agricultural Animals
Genus Agent(s) Hosts
1
Routes
2
Stability
3
In vitro
Cont.
In vivo
Cont.
In vivo
Ag
Cont.
Other
Regs
Histomonas spp. H. meleagridis 4 1, 4 2 2 2 2Ag N/A
Ichthyobodo spp. I. Necator 5 3 1 2 2 2Ag N/A
Ichthyophthirius spp. I. multiliis 5 3 2 2 2 2Ag N/A
Isospora spp.
I. burrowsi,
I. canis,
I. felis,
I. ohioensis,
I. neorivolta
3, 4, 6c,
7, 10b
1 2 2 2 2Ag N/A
Ixodes spp.
I. pacicus,
I. ricinus,
I. scapularis,
1, 2, 3, 4, 6,
7, 8, 10
4 2 2 2 2Ag N/A
Leishmania spp.
L. braziliensis,
L. chagasi,
L. infantum
2, 7, 10b 2 2 2 2 2Ag Y
Marteilia spp. M. refringens 5d 6 1 2 2 2Ag Y
Metagonimus spp. M. yokogawai 5, 6, 7, 10b 6, 8 2 2 2 2Ag N/A
Metorchis spp. M. conjunctus 5, 6, 7, 10b 6, 8 2 2 2 2Ag N/A
Mikrocytos
spp. M. mackini 5d 3, 4, 8 2 2 2 2Ag–3Ag N/A
Myxobolus spp. M. cerebralis 5a 6 2 2 2 2Ag N/A
Nanophyetus spp.
N. salmincola (Troglotrema
salmincola)
6b, 7a 6 1 2 2 2Ag N/A
Necator spp. N. americanus 10b 1 2 2 2 2Ag N/A
Oestrus spp. O. ovis 1, 6a 2 2 2 2 2Ag N/A
Opisthorchis spp.
O. felineus,
O. viverrini
5, 6, 7, 10b 6, 8 2 2 2 2Ag N/A
Paralaria spp. P. bovicola 1a 1, 6 2 2 2 2Ag N/A
Paragonimus spp.
P. kellicotti,
P. miyazakii,
P. westermani
5, 7, 10b 6, 8 2 2 2 2Ag N/A
Perkinsus spp.
P. marinus,
P. olensi
5d 1, 3, 9 2 2 2 2Ag Y
Psoroptes spp. P. ovis 1 4 2 2 2 2Ag Y
Rhipicephalus spp.
R. annulatus,
R. sanguineus
1, 2, 3, 4, 6,
7, 8, 10
4 2 2 2 2Ag N/A
Sarcocystis spp.
S. cruzi,
S. hirsuta,
S. hominis
1, 2, 3, 4, 6,
8, 10b
8 2 2 2 2Ag N/A
Sarcoptes spp. S. scabiei 7, 10b 4 2 2 2 2Ag Y
Taenia spp.
T. multiceps,
T. saginata,
T. solium
3, 10b 6, 8 2 2 2 2Ag N/A
Theileria spp.
T. annulata,
T. buei,
T. lestoquardi,
T. luwenshuni,
T. mutans,
T. orientalis,
T. parva,
T. sergenti,
T. uilenbergi
1, 6a 2 2 2 2 2Ag–3Ag Y
Toxocara spp.
T. canis,
T. cati
7, 10b 1, 7 2 2 2 2Ag N/A
Toxoplasma spp. T. gondii 7b 8 2 2 2 2Ag N/A
Trichinella spp. T. spiralis 3, 6, 10b 8 2 2 2 2Ag N/A
Trichodina spp. N/A 5 3 1 2 2 2Ag N/A
Trichomonas spp.
T. fetus,
T. gallinae,
T. stableri
4 1, 5 2 2 2 2Ag Y
Continued on next page ►
452 Biosafety in Microbiological and Biomedical Laboratories
Genus Agent(s) Hosts
1
Routes
2
Stability
3
In vitro
Cont.
In vivo
Cont.
In vivo
Ag
Cont.
Other
Regs
Trichuris spp.
T. suis,
T. trichiura,
T. vulpis
10b 1, 8 2 2 2 2Ag N/A
Tropilaelaps spp. T. clareae, T. mercedesae 9 6 2 2 2 2Ag N/A
Trypanosoma spp.
T. brucei,
T. congolense,
T. cruzi,
T. equiperdum,
T. evansi,
T. vivax
1, 2, 3, 6, 7,
8, 10
2, 4, 7 2 2 2 2Ag–3Ag Y
Uncinaria spp. U. stenocephala 7 1 2 2 2 2Ag N/A
Varroa spp. V. destructor 9 6 2 2 2 2Ag N/A
Table 4. Viruses
Genus Agent(s) Hosts
1
Routes
2
Stability
3
In vitro
Cont.
In vivo
Cont.
In vivo
Ag
Cont.
Other
Regs
Adenoviridae N/A 1–10 1,3 3 1–3 1–3 2Ag–3Ag N/A
Arenaviridae
Lymphocytic
Choriomeningitis Virus,
Viral Hemorrhagic Fever
7, 8, 10 1,3,4,5, 7,8 2 2–4 2–4 2Ag–4Ag Y
Asfarviridae African Swine Fever Virus 3 4,5,8 2 2 2–3 3Ag Y
Arteriviridae
Equine Viral Arteritis Virus,
Porcine Reproductive and
Respiratory Syndrome
Virus
2, 3 2,3,4,5,7 2 2 2–3 2Ag–3Ag Y
Astroviridae Astrovirus
1, 3, 4, 6a,
7, 8, 10b
1 2 2 2 2Ag N/A
Baculoviridae
Baculovirus penaei
(Crustaceans), Penaeus
monodon-type baculovirus
(Crustaceans)
5c 1,3,4,7,8 2 2 2 2Ag N/A
Birnaviridae
Infectious Bursal Disease
Virus, Infectious Pancreatic
Necrosis (Fish)
4a, 5a 1,3,4,7,9 2–3 2 2–3 2Ag N/A
Bornaviridae Borna Disease Virus
1, 2, 3, 4, 6,
7, 10
1,3,4,5,8 2 2 2 2Ag N/A
Bunyaviridae
Akabane Virus, Cache
Valley Virus, Crimean-
Congo Hemorrhagic Fever
Virus, Hantavirus, Nairobi
Sheep Disease Virus, Rift
Valley Fever Virus
1, 2, 3, 6, 7,
8, 9, 10
1,2,3,4, 5,8 1–2 2–4 2–4 2Ag–4Ag Y
Caliciviridae
European Brown Hare
Syndrome Virus, Hepatitis
E Virus, Noroviruses,
Rabbit Calicivirus Disease,
Sapovirus, Vesicular
Exanthema Virus of Swine
3, 4a, 5a, 6,
7, 8, 10b
1,2,4,5,8 2
2 2–3 2Ag–3Ag Y
Circoviridae Porcine Circovirus II 3 2,3,4 2 2 2 2Ag N/A
Coronaviridae
Avian Infectious Bronchitis,
Porcine Delta Coronavirus,
Porcine Epizootic Diarrhea,
SARS-Associated
Coronavirus, Transmissible
Gastroenteritis
3, 4a, 6,
10b
1,3,4,8 2 2 2–3 2Ag–3Ag Y
Filoviridae Viral Hemorrhagic Fever 10 1,3,4,5 2 4 4 2Ag–4Ag Y
Continued on next page ►

453Appendix D—Biosafety and Biocontainment for Pathogens Aecting Agricultural Animals
Genus Agent(s) Hosts
1
Routes
2
Stability
3
In vitro
Cont.
In vivo
Cont.
In vivo
Ag
Cont.
Other
Regs
Flaviviridae
Bovine Viral Diarrhea
Virus, Classical Swine
Fever Virus, Japanese
Encephalitis Virus, Louping
Ill Virus, Wesselsbron
Disease Virus, West Nile
Fever Virus
1, 2, 3, 4, 6,
7a, 9, 10b
1,2,3,4,
5,7,8
2–3 2–3 2–4 2Ag–3Ag Y
Herpesviridae
Bovine Herpes Virus 1,
Equine Herpes Virus,
Gallid Herpesvirus 1,
Gallid Alphaherpesvirus 2,
Koi Herpesvirus, Malignant
Catarrhal Fever Virus,
Pseudorabies Virus
1, 2, 3, 4,
5, 6a
1,3,4,5,7 1–2 2–3 2–3 2Ag–3Ag Y
Iridoviridae
Red Sea Bream Iridoviral
Disease
5 4 2 2 2 2Ag N/A
Nimaviridae
White Spot Syndrome
Virus (Crustaceans)
5c 4,7 2 2 2 2Ag N/A
Orthomyxoviridae
Avian Inuenza Virus
(highly pathogenic),
Infectious Salmon Anemia
Virus, Swine Inuenza
Virus, Syncytial Hepatitis
of Tilapia
3, 4, 5, 6c 1,3,4,5 1–2 2–3 2–3 2Ag–3Ag Y
Paramyxoviridae
Bovine Respiratory
Syncytial Virus, Hendra
Virus, Menangle Virus,
Newcastle Disease
Virus (Velogenic Strain),
Nipah Virus, Peste Des
Petits Ruminants Virus,
Rinderpest Virus, Turkey
Rhinotracheitis
1, 2, 3, 4, 6,
7, 10
1,3,4,5 1–3 2–4 2–4 2Ag–4Ag Y
Parvoviridae
Infectious Hypodermal and
Hematopoietic Necrosis
(Crustaceans), Aleutian
Mink Disease
5c 7,8 2 2 2 2Ag Y
Picornaviridae
Duck Hepatitis Virus,
Foot and Mouth Disease,
Hepatitis A Virus, Swine
Vesicular Disease Virus,
Taura Syndrome Virus
(Crustaceans), Teschen
Disease Virus
1, 3, 4b,
5c,
6, 10b
1,3,4,5,8 2 2 2–3 2Ag–3Ag Y
Poxviridae
Camelpox Virus,
Capripoxvirus, Contagious
Ecthyma, Monkeypox
Virus, Myxoma Virus
1, 6, 7c, 10 2,3,4,5,9 2–3 2–4 2–4 2Ag–3Ag Y
Reoviridae
African Horse Sickness
Virus, Bluetongue Virus,
Epizootic Hemorrhagic
Disease Virus, Equine
Encephalosis Virus,
Rotavirus
2, 4a, 5, 6,
7a, 8
2,4,8 2 2 2–3 2Ag–3Ag Y
Retroviridae
Bovine Leukemia Virus
(Enzootic), Caprine
Arthritis Encephalitis Virus,
Equine Infectious Anemia
Virus, Jembrana Virus,
Maedi-Visna
1, 2, 6a 2,3,4,5,7 1–3 2–3 2–3 2Ag–3Ag Y
Rhabdoviridae
Bovine Ephemeral
Fever Virus, Epizootic
Hematopoietic Necrosis
(Fish), Infectious
Hematopoietic Necrosis
Virus (Fish), Rabies,
Spring Viremia of Carp
Virus, Vesicular Stomatitis
Virus (exotic), Viral
Hemorrhagic Septicemia
Virus (Fish)
1, 2, 3, 5, 6,
7, 8, 10
1,2,3,4,
5,7,8
1–2 2–3 2–3 2Ag–3Ag Y
Continued on next page ►
454 Biosafety in Microbiological and Biomedical Laboratories
Genus Agent(s) Hosts
1
Routes
2
Stability
3
In vitro
Cont.
In vivo
Cont.
In vivo
Ag
Cont.
Other
Regs
Roniviridae
Yellowhead Virus
(Crustaceans)
5c 1,4 2 2 2 2Ag N/A
Togaviridae
Eastern Equine
Encephalitis Virus,
Getah Virus, Venezuelan
Equine Encephalitis
Virus, Western Equine
Encephalomyelitis Virus
2, 3, 8, 10 2,3 2 2–3 2–3 2Ag–3Ag Y
Table 5. Toxins
Toxin(s) Hosts
1
Routes
2
Stability
3
In vitro
Cont.
In vivo
Cont.
In vivo
Ag
Cont.
Other
Regs
Botulinum Neurotoxin
1a, 2, 4,
6c, 10b
8 2 2 2 2Ag Y
Clostridium perfringens epsilon toxin
1, 3, 4a,
10b
3,8 2–3 2 2 2Ag N/A
Shiga toxin 10b 8 3 2 2 3Ag
Staphylococcal enterotoxin (B, C) 10b 8 3 2 2 3Ag Y
T-2 Toxin
1, 3, 4,
5, 10b
8 2 2 2 2Ag Y
Table 6. Prions
Disease(s) Hosts
1
Routes
2
Stability
3
In vitro
Cont.
In vivo
Cont.
In vivo
Ag
Cont.
Other
Regs
Bovine Spongiform Encephalopathy 1,7b,10 8 3 2 2 2Ag–3Ag Y
Scrapie 1b,1c 7 3 2 2 2Ag Y
Chronic Wasting Disease 6a 1,5,7 2 2 2 2Ag Y
Table Key 1. Natural Host Range
Designation Meaning
1 Ruminant (multiple species)
1a Bovine
1b Caprine
1c Ovine
1d Camelids
2 Equine
3 Porcine (domestic and feral)
4 Domestic Fowl (multiple species)
Continued on next page ►
455Appendix D—Biosafety and Biocontainment for Pathogens Aecting Agricultural Animals
Designation Meaning
4a Chicken
4b Duck
4c Turkey
4d Geese
4e Ratites (e.g., ostriches, emus)
5 Aquatic (multiple species)
5a Salmonids
5b Catsh
5c Crustaceans
5d Mollusks
6 Wildlife (multiple species)
6a Wild Ruminant (e.g., wildebeests, bualo, cervids)
6b Wild Carnivores (e.g., wolf, coyote, raccoon)
6c Wild Fowl
6d Wild Lagomorphs
6e Wild and Captive Amphibians
6f Wild and Captive Reptiles
6g Bats
7 Domestic Companion Animals (multiple species, including hamsters, gerbils, guinea
pigs, non-laboratory mice/rats)
7a Canine
7b Feline
7c Domestic Lagomorphs
7d Ferrets
8 Rodent (multiple species)
9 Insects (honeybees)
10 Primates (humans and non-human)
Continued on next page ►
456 Biosafety in Microbiological and Biomedical Laboratories
Designation Meaning
10a Non-human Primates
10b Humans
Table Key 2. Natural Routes of Transmission
Designation Meaning
1 Fecal-Oral
2 Arthropod Vector (e.g., ticks, lice, eas, crustaceans, mosquitos)
3 Aerosol Transmission (e.g., sneezing, coughing, nasal discharges, dust,
particulates, water transmission in aquatic species)
4 Mechanical/Bloodborne (e.g., needles, palpation sleeves, injuries, direct contact,
poxviruses)
5 Secretions (e.g., milk, saliva, semen, vaginal secretions)
6 Intermediate Host (e.g., snails, tissue cysts [required for transmission])
7 Vertical Transmission (e.g., transplacental, mother-to-ospring)
8 Ingestion (e.g., toxins, grazing, contaminated feed)
9 Varies or Highly Variable (i.e., when route is dependent on environmental or
host factors)
Table Key 3. Environmental Stability
Designation Meaning
1 Readily inactivated by desiccation, direct sunlight, composting, exposure to
normal temperature uctuations, and/or eliminate access to arthropod vectors
and intermediate hosts.
2 Inactivation requires commercial disinfectants, detergents, temperature extremes
(pasteurization), or steam. For tick-borne diseases, stability reects tick persistence.
3
Inactivation requires specialized procedures (e.g., irradiation, incineration, bacterio-
phages, ultrasound, oxidation, mechanical stress, signicant alterations of pH).
References
1. Miller JM, Astles R, Baszler T, Carey R, Garcia L, Gray L, et al. Guidelines
for Safe Work Practices in Human and Animal Medical Diagnostic
Laboratories. Recommendation of a CDC-Convened, Biosafety Blue Ribbon
Panel. MMWR Suppl. 2012;61(1):1–102.
457Appendix D—Biosafety and Biocontainment for Pathogens Aecting Agricultural Animals
2. Testing and Performance-Verication Methodologies for Ventilation Systems
for Biological Safety Level 3 (BSL-3) and Animal Biological Safety Level 3
(ABSL-3) Facilities, ANSI/ASSE Z9.14 (2014).
3. 9.1 General. In: United States Department of Agriculture. ARS Facilities
Design Standards. Washington (DC): USDA ARS; 2012. p. 223–5.
4. 9.2 Hazard Classication and Choice of Containment. In: United States
Department of Agriculture. ARS Facilities Design Standards. Washington
(DC): USDA ARS; 2012. p. 226.
5. 9.3 Primary Barriers (Containment Equipment). In: United States
Department of Agriculture. ARS Facilities Design Standards. Washington
(DC): USDA ARS; 2012. p. 227.
6. 9.4 Secondary Barriers (Facility Design Features). In: United States
Department of Agriculture. ARS Facilities Design Standards. Washington
(DC): USDA ARS; 2012. p. 228–44.
7. 9.5 Special Design Issues. In: United States Department of Agriculture.
ARS Facilities Design Standards. Washington (DC): USDA ARS; 2012.
p. 245–53.
8. Appendix 9B: Testing and Certication Requirements for the Critical
Components of Biological Containment Systems. In: United States
Department of Agriculture. ARS Facilities Design Standards. Washington
(DC): USDA ARS; 2012. p. 268–74.

458 Biosafety in Microbiological and Biomedical Laboratories
Appendix E—Arthropod Containment Guidelines (ACG)
An ad hoc committee of concerned vector biologists including members of
the American Committee of Medical Entomology (ACME), a subcommittee of
the American Society of Tropical Medicine and Hygiene (ASTMH), and other
interested persons drafted the original Arthropod Containment Guidelines (ACG)
in 2003.
1
The guidelines provide principles and practices for risk assessment for
research on arthropods of public health importance. The risk assessment and
practices in the ACG are designed to be consistent with the NIH Guidelines for
recombinant DNA research and the BMBL.
The ACG were published in hard copy in the March 2019 issue of Vector-Borne
Zoonotic Diseases
2
and are freely downloadable from https://www.liebertpub.com/
doi/10.1089/vbz.2018.2431.
The ACG recommend biosafety measures specic for arthropods of public health
importance considering that:
■
Arthropods present unique containment challenges not encountered
with microbial pathogens; and
■
Arthropod containment has not been covered specically in BMBL or
the NIH Guidelines.
The ACG contain two sections of signicant interest to most researchers:
■
The Principles of Risk Assessment that discusses arthropods in the
usual context (e.g., those known to contain a pathogenic agent, those
with uncertain pathogens, and those with no agent). Arthropod risk
assessment is primarily a qualitative judgment that cannot be based
on a prescribed algorithm. Several factors must be considered in
combination: the agents transmitted, whether the arthropod is or may
be infected, the mobility and longevity of the arthropod, its reproductive
potential, biological containment, and epidemiological factors inu-
encing transmission in the proposed location or region at risk.
■
Factors considered in Arthropod Containment Level (ACL) classication
include:
□
Biological containment is a signicant factor that reduces the
hazards associated with accidental escape of arthropods;
□
Epidemiological context alters the risks of an escape and its
impact on the location or site in which the work is performed;
□
The phenotype of the vector, such as insecticide resistance; and
□
Genetically modied arthropods with an emphasis on phenotypic
change.
459Appendix E—Arthropod Containment Guidelines (ACG)
Four Arthropod Containment Levels (ACL 1–4) add increasingly stringent
measures and are similar to Biosafety Levels. The most exible level is ACL-2,
which covers most exotic and transgenic arthropods and those infected with
pathogens requiring BSL-2 containment. Like the BMBL, each level has four
components, with the following similar format:
■
Standard practices;
■
Special practices;
■
Equipment (primary barriers); and
■
Facilities (secondary barriers).
The ACG do not reect a formal endorsement by ACME or ASTMH. The guide-
lines are subject to change based on further consideration of the requirements
for containment of arthropods and vectors.
References
1. American Committee of Medical Entomology; American Society of Tropical
Medicine and Hygiene. Arthropod containment guidelines. A project of the
American Committee of Medical Entomology and American Society of
Tropical Medicine and Hygiene. Vector Borne Zoonotic Dis. 2003;3:61–98.
2. American Committee of Medical Entomology; American Society of Tropical
Medicine and Hygiene. Vector-Borne and Zoonotic Diseases. New Rochelle
(NY): Mary Ann Liebert, Inc.; 2019.
460 Biosafety in Microbiological and Biomedical Laboratories
Appendix F—Select Agents and Toxins
Following the anthrax attacks of 2001 that resulted in ve deaths, Congress
signicantly strengthened federal oversight of biological agents and toxins that
have the potential to pose a severe threat to public health; animal and plant
health; and animal and plant products (Select Agents and Toxins). The Public
Health Security and Bioterrorism Preparedness and Response Act of 2002
(Bioterrorism Response Act) required the Department of Health and Human
Services (HHS) to regulate the possession, use, and transfer of select biological
agents and toxins that have the potential to pose a severe threat to public health
and safety. Subtitle B of Title II of the Bioterrorism Response Act (cited as the
Agricultural Bioterrorism Protection Act of 2002) granted comparable regulatory
authorities to the U.S. Department of Agriculture (USDA) over select biological
agents and toxins that have the potential to pose a severe threat to animal and
plant health or products. The Bioterrorism Response Act also requires HHS and
USDA to coordinate activities regarding the zoonotic agents regulated by both
Departments.
These activities are implemented through the Federal Select Agent Program
(FSAP). FSAP is managed jointly by the Centers for Disease Control and
Prevention’s (CDC) Division of Select Agents and Toxins (DSAT) and the Animal
and Plant Health Inspection Service’s (APHIS) Agriculture Select Agent Services
(AgSAS). FSAP regulates the acquisition, use, storage and transfer of Select
Agents and Toxins through the development, implementation, and enforcement
of the federal Select Agent regulations—7 CFR Part 331 (APHIS-PPQ), 9 CFR
Part 121 (APHS-VS), and 42 CFR Part 73 (CDC).
FSAP provides national oversight of the safety and security of potentially
dangerous biological Select Agents and Toxins. Key elements of the Select
Agent regulations include:
■
All entities that possess, use, or transfer Select Agents and Toxins
must be registered with FSAP.
■
All individuals who have access to Select Agents and Toxins must rst
be approved by FSAP after a security risk assessment (SRA) performed
by the Federal Bureau of Investigation’s (FBI) Criminal Justice Infor-
mation Services Division (CJIS) to help guard against access to the
agents and toxins by those who may wish to misuse them.
■
Enforcement actions for regulatory violations may be taken to address
present risks and increase future compliance through administrative
actions and/or civil monetary penalties. An entity may be referred to
the HHS Oce of the Inspector General (OIG) or APHIS Investigative
and Enforcement Services (IES), or the FBI may be notied of the
incident for potential further investigation, as appropriate.
461Appendix F—Select Agents and Toxins
■
An entity’s registration may be denied, suspended, or revoked if it is
determined that such action is necessary to protect human, animal,
or plant health, or animal or plant products.
■
Each registered entity must designate a Responsible Ocial (RO),
an individual with the authority and responsibility to act on behalf of
the entity and charged with ensuring compliance with the Select Agent
regulations. The RO is able to respond to onsite incidents involving
Select Agents in a timely manner, ensures annual inspections are
conducted for each space where Select Agents are stored or used,
reviews the entity’s validated inactivation procedures and investigates
any failures, and reports the identication and nal disposal of any
Select Agent or Toxin in a diagnostic specimen or prociency test.
Alternate Responsible Ocial(s) (ARO) may be designated to serve
when the RO is not available; AROs have the same responsibilities
as ROs.
■
Each registered entity must develop and implement a written security
plan sucient to safeguard their Select Agents and/or Toxins against
unauthorized access, theft, loss, or release.
■
Each registered entity must develop and implement a written biosafety
plan commensurate with the risk of their Select Agents and/or Toxins,
given their intended use.
■
A registered entity must receive pre-approval for Restricted experiments
that pose heightened safety and security risks. See Section 13 of the
Select Agents and Toxins regulations for additional information.
■
Each registered entity must develop and implement a written incident
response plan specic to the hazards associated with their Select
Agents and/or Toxins.
■
Each registered entity must provide information and training on
biosafety, security, and incident response to individuals with access to
Select Agents and Toxins.
■
Any instances of the theft, loss, or release of a Select Agent or Toxin
must be promptly reported to FSAP in accordance with the Select
Agent and Toxin regulations.
■
An entity may only transfer a Select Agent or Toxin to another entity
registered to possess that agent or toxin, and the transfer must be
preauthorized by FSAP.
■
Each registered entity must maintain complete records and documen-
tation including, but not limited to: inventories, exposures, lists of
individuals with approved access, and entry into areas containing
Select Agents or Toxins.
■
FSAP may conduct inspections of an entity without prior notication
and prior to issuing a certicate of registration.

462 Biosafety in Microbiological and Biomedical Laboratories
■
There are specic exemptions or exclusions to the regulations
including specic attenuated strains or Select Toxins modied to be
less potent or toxic.
■
Entities must use validated inactivation procedures to inactivate Select
Agents. Please refer to the appendix on Inactivation and Verication.
As of January 2017, FSAP regulates 66 Select Agents and Toxins. The list of
Select Agents and Toxins is reviewed at least every two years to determine if
agents or toxins need to be added to or deleted from the list.
For more information on the regulations and guidance documents for implemen-
tation of a Select Agent program, please visit https://www.selectagents.gov.
463Appendix G—Integrated Pest Management (IPM)
Appendix G—Integrated Pest Management (IPM)
Integrated Pest Management (IPM) is an important part of managing a research
facility. Many pests, including ies and cockroaches, can mechanically transmit
disease pathogens and compromise the research environment. Even the
presence of innocuous insects can contribute to the perception of unsanitary
conditions.
The most common approach to pest control has been the application of pesti-
cides, either as a preventive or remedial measure. Pesticides can be eective
and may be necessary as a corrective measure, but they have limited long-term
eects when used alone. Pesticides also can contaminate the research
environment through pesticide drift and volatilization.
To manage pests and minimize the use of pesticides, it is necessary to employ a
comprehensive program approach that integrates housekeeping, maintenance,
and pest control services. This method of pest control is often referred to as IPM.
The primary goal of an IPM program is to prevent pest problems by managing
the facility environment to make it less conducive to pest infestation. Along with
limited applications of pesticides, pest control is achieved through proactive
operational and administrative intervention strategies to correct conditions that
promote pest problems.
Prior to developing any type of IPM program, it is important to dene an
operational framework process for IPM services that also helps promote collab-
oration between IPM specialists and facility personnel. This framework should
incorporate facility restrictions as well as operational and procedural issues into
the IPM program. An eective IPM program is an integral part of the facility’s
management. An IPM policy statement should be included in the facility’s
standard operating procedures to increase awareness of the program.
Training sources for the principles and practices of structural (indoor) IPM
programs are available through university entomology departments, county
extension oces, the Entomological Society of America, state departments
of agriculture, state pest control associations, the National Pest Management
Association (NPMA), suppliers of pest control equipment, and IPM consultants
and rms. Several universities oer correspondence courses, short courses, and
training conferences on structural pest management.
IPM is a strategy-based approach that considers not only the cost of the services
but also the eectiveness of the program’s components. Each IPM program is
site-specic and tailored to the environment where applied.

464 Biosafety in Microbiological and Biomedical Laboratories
Laboratory IPM services are dierent from those in an oce building or an
animal care facility. Interrelated components of environmental pest management
follow.
Facility Design IPM issues and requirements should be addressed in a
research facility’s planning, design, construction, and retrotting. This provides
an opportunity to incorporate features that help exclude pests, minimize pest
habitat, and promote proper sanitation in order to reduce future corrections that
can disrupt research operations. Examples can be obtained from the National
Institutes of Health Design Requirements Manual at https://www.orf.od.nih.gov/
TechnicalResources/Documents/DRM/DRM1.4042419.pdf.
Monitoring Monitoring is the central activity of an IPM program and is used to
minimize pesticide use. Traps, visual inspections, and sta interviews identify
areas and conditions that may foster pest activity.
Sanitation and Facility Maintenance Many pest problems can be prevented or
corrected by ensuring proper sanitation, reducing clutter and pest habitat, and
by performing repairs that exclude pests. Records of structural deciencies and
housekeeping conditions should be maintained to track problems and determine
if corrective actions were carried out and completed in a timely manner.
Communication A sta member should be designated to meet with IPM
personnel to assist in resolving facility issues that impact pest management.
Reports communicated verbally and in writing concerning pest activity and
improvement recommendations for personnel, practices, and facility conditions
should be provided to the designated personnel. Facility personnel should
receive training on pest identication, biology, and sanitation, which can promote
understanding and cooperation with the goals of the IPM program.
Recordkeeping A logbook should be used to record pest activity and conditions
pertinent to the IPM program. It may contain protocols and procedures for IPM
services in that facility, Safety Data Sheets on pesticides, pesticide labels,
treatment records, oor plans, and survey reports.
Non-pesticide Pest Control Pest management methods such as trapping,
exclusion, caulking, washing, heating, and freezing can be applied safely and
eectively when used in conjunction with proper sanitation and structural repair.
Pest Management with Pesticides Preventive applications of pesticides should
be discouraged, and treatments should be restricted to areas of known pest
activity. When pesticides are applied, the least toxic product(s) available should
be used and applied in the most eective and safe manner. Fogging should be
avoided.

465Appendix G—Integrated Pest Management (IPM)
Program Evaluation and Quality Assurance Quality assurance and program
review should be performed to provide an objective, ongoing evaluation of IPM
activities and eectiveness to ensure that the program does, in fact, manage
pests and meet the specic needs of the facility program(s) and its occupants.
Based on this review, current IPM protocols can be modied and new proce-
dures implemented.
Technical Expertise A qualied entomologist can provide helpful technical
guidance to develop and implement an IPM program. Pest management
personnel should be licensed and certied by the appropriate regulatory
agency(s).
Safety IPM minimizes the potential of pesticide exposure to the research
environment and the sta by limiting the scope of pesticide treatments.
References
1. Bennett GW, Owens JM, editors. Advances in urban pest management.
New York: Van Nostrand Reinhold Company; 1986.
2. Biocontrol Network [homepage on the Internet]. Murfreesboro (TN):
Biocontrol Network; c2018 [cited 2018 Sept 25]. Available from:
http://www.biconet.com.
3. National Institutes of Health, Oce of Management, Oce of Research
Facilities [Internet]. Bethesda (MD): Design Requirements Manual (DRM);
c2018 [cited 2018 Sept 25]. Available from: https://www.orf.od.nih.gov/
TechnicalResources/Documents/DRM/DRM1.4042419.pdf
4. National Pest Management Association [homepage on the Internet].
Fairfax (VA): NPMA Pestworld; c2018 [cited 2018 Sept 25]. Available from:
http://npmapestworld.org.
5. Olkowski W, Daar S, Olkowski H. Common-sense pest control: least-toxic
solutions for your home, garden, pests and community. Newton (CT):
The Taunton Press, Inc.; 1991.
6. Robinson WH. Urban entomology: insect and mite pests in the human
environment. New York: Chapman and Hall; 1996.
7. Robinson, WH. Urban Insects and Arachnids: a handbook of urban
entomology. New York: Cambridge University Press; 2011.
466 Biosafety in Microbiological and Biomedical Laboratories
Appendix H—Working with Human, Non-Human Primate
(NHP), and Other Mammalian Cells and Tissues
As with any other type of laboratory activity, a risk assessment should preface
work with eukaryotic cell cultures. Such work is generally considered low-risk,
but risk increases when working with human and other primate cell lines and
with primary cells from other mammalian species in the laboratory. This standard
recognizes that employees in both research and clinical work settings face
inherent risks working with human materials. Microbiological and biomedical
researchers can minimize or eliminate these risks using a combination of
engineering and work practice controls, personal protective clothing, safety
equipment, training, medical surveillance, vaccination, signs and labels, and
other provisions.
Bloodborne pathogens and risk assessment related to material source
and type
Bloodborne pathogens are pathogenic microorganisms present in human blood
and other potentially infectious materials (OPIM), which can infect and cause
disease in persons who are exposed to blood containing these pathogens.
Hepatitis B virus (HBV), hepatitis C virus (HCV), and human immunodeciency
virus (HIV) are the most common examples of such microorganisms. Work
with blood and OPIM involves risk of exposure not only to these agents, but
also other opportunistic pathogens transmitted primarily by other routes (e.g.,
contact, droplet, and airborne) that may be present in blood or the sample
material at the time it is being handled. For example, Mycobacterium tubercu-
losis may be transmitted via the airborne route and primarily present in human
lung tissues, while bacterial species such as Staphylococci may be contact
transmitted but present in localized tissues or blood during acute infections.
Prions, responsible for spongiform encephalopathies and other diseases,
may be more concentrated in neural tissues rather than blood, whereas viral
hemorrhagic fever-causing viruses can be considered bloodborne pathogens but
are often present in other body uids, such as urine.
1
Numerous pathogens can
be present in human materials and each agent may have a number of dierent
characteristics to consider pertaining to the process of infection. For this reason,
a risk assessment must be performed that takes into account material source,
type, characteristics, and the procedures being performed with the material.
Working with human, NHP, and other mammalian cell lines may present a risk
of exposure to bloodborne pathogens, as widely recognized and documented
in research and healthcare settings; guidance on how to respond to potential
exposures is available.
2–4
For institutions in the United States, the Occupational
Safety and Health Administration (OSHA) has developed a bloodborne
467Appendix H: Working with Human and NHP Cells/Tissues
pathogens standard that must be applied to all work with human blood and
OPIM, including body uids, tissues, and primary cell lines.
5
Tissue Source Each institution should conduct a risk assessment, which
can begin by appreciating the tissue source (species origin). The closer the
relationship of the material is to humans, the higher the risk since pathogens
usually have evolved species-specic requirements. Old World non-human
primate (NHP) specimens (i.e., macaques) may contain Macacine herpesvirus
(Herpes B) and Simian Immunodeciency Virus (SIV). This material should
always be considered potentially infected and should be handled with strict
barrier precautions and with swift occupational responses for potential
exposures. Herpes B virus infection in macaques is usually symptom-free, or
causes only mild oral lesions, but in humans, the infection can be fatal.
6
Also,
consider that some pathogens can cross between species (e.g., inuenzas,
SARS Co-V, West Nile virus). Working with other (non-human and non-NHP)
mammalian, avian, and invertebrate cell lines generally presents lower risks.
Cell or Tissue Type Another important consideration is cell or tissue type and
whether there is a hazard associated with the capability of the cell to form tumors
(e.g., oncogene expressing). Hematopoietic cells and lymphoid tissues can
have tumorigenic potential and therefore have an increased risk for handling.
Neural tissues and endothelial cells may be considered to have less risk, but an
assessment must determine the probability of whether such cells contain other
adventitious agents and take into account the tissue or cell source(s) and param-
eters related to the history of that source. Epithelial cells and broblasts present
the lowest risk in terms of cell type and tumorigenic potential.
7
Culture Type When working with cell lines, the culture type is another important
consideration. Primary cell lines are derived by sampling directly from in vivo
organ and tissue samples and have a higher risk of containing undetected
pathogens. Therefore, these culture types have shorter lifespans of unknown
characterization and present a higher potential risk while culturing. Continuous
cell lines (i.e., cells immortalized with viral agents such as EBV, SV-40, or other
viral agents) have been modied to grow for extended passages, perhaps even
indenitely. Continuous cultures can usually be more characterized with PCR
and cytometric analyses; however, cells carrying viral genomic material still
can pose increased risks in the event of inadvertent exposures, particularly
for immune-compromised individuals.
8
There has been a report of tumor
development from an accidental needlestick injury.
9
Permissive cell lines that
support viral replication may have a heightened risk of contamination with viral
pathogens. Well-established, and possibly even tested, cell lines are generally
considered safer, but the possibility of adventitious contamination by an

468 Biosafety in Microbiological and Biomedical Laboratories
unspecied pathogen during use must be considered during the risk assessment
process, and measures must be taken to lower the risk of contamination.
10
Additional Considerations When conducting a risk assessment, consider if
endogenous pathogens are present in the specimen or if the pathogens have
been added intentionally. Another key consideration is if agents may have been
added as a result of passaging of the line in animal model systems. Experimen-
tally infected cell lines should be handled following safety recommendations
for both potential endogenous pathogens and known pathogens added in the
course of research. Any cell line with known endogenous pathogens should
be handled following the safety recommendations for those pathogens. Risk
assessment should also consider if any recombinant materials are expressed
by the cell line and whether the cell line is a type that supports viral replication.
Consult with the Institutional Biosafety Committee, or equivalent resource, when
working with recombinant or synthetic nucleic acids in cell lines.
11
Helpful guide-
lines exist to increase awareness of the problems encountered when working
with cells in biomedical research and how to address them eectively.
12
Risk Mitigation
At a minimum, human and other primate cells should be treated as potentially
infectious and handled using BSL-2 practices, engineering controls, and
facilities.
13
The use of a biological safety cabinet (BSC) for culturing activities is
the universally accepted best practice. Higher containment must be considered
for cell lines harboring Risk Group 3 and 4 pathogens as indicated by the risk
assessment; higher containment must be considered if the agents present
become airborne when energy is imparted on the biological sample. Personal
protective equipment (PPE) such as laboratory coats, gloves, and eye protection
should be worn in tissue culture laboratories and additional PPE should be
added as indicated by risk assessment. All waste culture material must be
decontaminated before disposal. All laboratory sta working with human and
NHP cells and tissues should be enrolled in an occupational medical program
specic for bloodborne pathogens, and sta should work under the policies and
guidelines established by their institution’s Exposure Control Plan (ECP).
Please refer to Section II for additional information about the risk assessment
process and risk mitigation.
References
1. Kuhn JH, Clawson AN, Radoshitzky SR, Wahl-Jensen V, Bavari S, Jahrling
PB. Viral Hemorrhagic Fevers: History and Denitions. In: Singh SK, Ruzek
D, editors. Viral Hemorrhagic Fevers. Boca Raton (FL): CRC Press; 2013.
p. 3–13.

469Appendix H: Working with Human and NHP Cells/Tissues
2. Siegel JD, Rhinehart E, Jackson M, Chiarello L; Healthcare Infection Control
Practices Advisory Committee. 2007 Guideline for Isolation Precautions:
Preventing Transmission of Infectious Agents in Health Care Settings. Am J
Infect Control. 2007;35(10):S65–S164.
3. Kuhar DT, Henderson DK, Struble KA, Heneine W, Thomas V, Cheever LW,
et al. Updated U.S. Public Health Service Guidelines for the Management
of Occupational Exposures to Human Immunodeciency Virus and
Recommendations for Postexposure Prophylaxis. Infect Control Hosp
Epidemiol. 2013;34(9):875–92. Erratum in: Infect Control Hosp Epidemiol.
2013;34(11):1238.
4. US Public Health Service. Updated U.S. Public Health Service Guidelines
for the Management of Occupational Exposures to HBV, HCV, and HIV and
Recommendations for Postexposure Prophylaxis. MMWR Recomm Rep.
2001;50(RR-11):1–52.
5. Bloodborne pathogens, 29 C.F.R. Part 1910.1030 (1992).
6. NASPHV; Centers for Disease Control and Prevention; Council of State
and Territorial Epidemiologists; American Veterinary Medical Association.
Compendium of measures to prevent disease associated with animals
in public settings, 2009: National Association of State Public Health
Veterinarians, Inc. (NASPHV). MMWR Recomm Rep. 2009;58(RR-5):1–21.
7. Pauwels K, Herman P, Van Vaerenbergh B, Dai Do Thi C, Berghmans L,
Waeterloos G, et al. Animal Cell Cultures: Risk Assessment and Biosafety
Recommendations. Apple Biosaf. 2007;12(1):26–38.
8. Caputo JL. Safety Procedures. In: Freshney RI, Freshney MG, editors.
Culture of Immortalized Cells. New York: Wiley-Liss; 1996. p. 25–51.
9. Gugel EA, Sanders ME. Needle-stick transmission of human colonic
adenocarcinoma [letter]. N Engl J Med. 1986;315(23):1487.
10. McGarrity GJ. Spread and control of mycoplasmal infection of cell culture.
In Vitro. 1976;12(9):643–8.
11. National Institutes of Health. NIH Guidelines for Research Involving
Recombinant or Synthetic Nucleic Acid Molecules (NIH Guidelines).
Bethesda (MD): National Institutes of Health, Oce of Science Policy; 2019.
12. Geraghty RJ, Capes-Davis A, Davis JM, Downward J, Freshney RI,
Knezevic I, et al. Guidelines for the use of cell lines in biomedical research.
Br J Cancer. 2014;111(6):1021–46.
13. United States Department of Labor [Internet]. Washington (DC):
Occupational Safety and Health Administration; c1994 [cited 2019
April 10]. Applicability of 1910.1030 to establish human cell lines.
Available from: https://www.osha.gov/pls/oshaweb/owadisp.
show_document?p_table=INTERPRETATIONS&p_id=21519
470 Biosafety in Microbiological and Biomedical Laboratories
Appendix I—Guidelines for Work with Toxins of
Biological Origin
Biological toxins encompass a vast range of peptides, small molecules, and
macromolecular proteins that cause disease by interfering with biological
processes. As their name suggests, biological toxins reside between traditional
denitions of biological and chemical agents. They are produced by living
organisms, are unable to replicate, and do not result in communicable diseases.
The production of novel or existing toxins by synthetic means is becoming
increasingly accessible.
1,2
Many biological toxins have been evolutionarily
optimized to rapidly disrupt critical biological functions at low concentrations.
Their extraordinary, highly specic toxicity is mediated through a diverse set
of mechanisms, including enzymatic activity against critical cellular targets,
blockade of membrane ion channels and receptors, and perturbation of
essential cellular functions. The remarkable combination of specicity and
potency has resulted in the widespread use of diverse biological toxins for
clinical and research purposes, including botulinum neurotoxins, tetrodotoxin,
conotoxins, scorpion toxins, snake venom toxins, and immunotoxins. Because
laboratory workers in a wide range of medical and scientic disciplines are likely
to encounter biological toxins at some point during their career, it is critically
important that laboratory workers understand and are able to assess the risks
associated with their use.
Laboratory workers can be exposed to biological toxins through a variety
of routes, including inhalation of powders, aerosols, or volatile substances;
ingestion; injection; and absorption through dermal, mucosal or ocular tissues.
Many biological toxins are highly potent, and internalization of even relatively low
doses may result in death or severe incapacitation. Consequently, it is critically
important for those working with biological toxins to understand and implement
appropriate laboratory safety principles. A number of principles for the safe use
of many toxins commonly encountered in the clinical or research environment
are summarized below, including for those biological toxins regulated by the
Federal Select Agent Program as Select Toxins (see below).
General Considerations for Toxin Use
The primary risks during laboratory use of biological toxins result from accidental
injection, absorption through skin or mucous membranes, inhalation, and
ingestion. Laboratory work with most toxins in amounts routinely employed in the
biomedical sciences can be performed safely with minimal risk to the worker and
negligible risk to the surrounding community. Under most circumstances, toxins
can be handled using established general guidelines for toxic or highly-toxic
chemicals with the incorporation of additional safety and security measures
471Appendix I—Guidelines for Work with Toxins of Biological Origin
based upon a risk assessment for each specic toxin and laboratory operation.
3,4
Additionally, the mixed hazard nature of toxins and their associated organisms
should be considered in the risk assessment when determining appropriate
facilities, practices, and equipment use for situations where both biological
and chemical hazards are present. Standard use of engineering controls (e.g.,
Class II or Class III biosafety cabinets or open-front chemical fume hoods) and
personnel protective equipment (e.g., safety glasses or goggles, mask, gloves,
and lab coat) are generally sucient to avoid accidental inhalation or topical
exposure.
Training and Laboratory Planning
Each laboratory worker must be trained in the theory and practice of the toxins to
be used, with special emphasis on the nature of the practical hazards associated
with laboratory operations. These include risks associated with transfer of
solubilized toxins; manipulation of waste solutions, contamination of materials
and equipment; and decontamination after routine operations and spills. Workers
must be well-trained and suciently adept at all laboratory procedures and
safety practices before participating in toxin operations.
A risk assessment should be conducted to identify potential hazards and develop
safe operating procedures before undertaking toxin operations. For example, the
use of pre-operational checklists is highly recommended.
4
For complex opera-
tions, newly approved toxin workers should undergo supervised practice runs
in which the exact laboratory procedures to be undertaken are rehearsed using
nontoxic simulants. Technical rehearsals are particularly important to mitigate the
psychological stress of working with highly dangerous agents.
The inclusion of toxins can signicantly complicate otherwise routine laboratory
procedures. For example, equipment with potential to produce aerosols may
need to be placed in primary containment, such as a biosafety cabinet (BSC) or
fume hood, and decontaminated after each use. The use of personal protective
equipment (PPE) can reduce dexterity, and operations may be more dicult
when conducted in crowded hoods or BSCs. If toxins and infectious agents
are used together, then both must be considered in the risk assessment when
selecting containment equipment, developing safety procedures, and choosing
decontamination and disposal methods. Early endpoints need to be designed
to balance experimental objectives with safe and ethical application of toxins to
animals. The medical consequences of an accidental needlestick during animal
operations may be signicantly increased when toxin is involved. Team leaders
should be prepared to carefully review study procedures to identify how toxin
use may interfere with experimental execution and develop eective mitigation
strategies.
472 Biosafety in Microbiological and Biomedical Laboratories
Each laboratory that uses toxins must develop toxin-specic chemical hygiene
plans. The National Research Council has provided a review entitled “Prudent
Practices in the Laboratory: Handling and Management of Chemical Hazards”
with guidance on development of chemical hygiene plans and compliance
with regulations governing occupational safety and health, hazard communi-
cation, and environmental protection. The 2011 edition of this review can be
downloaded for free from https://www.nap.edu/catalog/12654/prudent-practic-
es-in-the-laboratory-handling-and-management-of-chemical. These procedures
are also summarized in the Occupational Safety and Health Administration’s
Laboratory Standard (29 CFR Section 1910.1450, Appendix A).
A number of engineering and human controls are available to decrease the risk
of accidental misuse of biological toxins. An inventory control system should
be established and audited on a regular basis (e.g., monthly or quarterly) to
account for toxin quantity, use, and disposition. While an inventory control
system is required for users of non-exempt quantities of Select Toxins (see
below for exempt quantity limits), it is also useful for ensuring that exempt
quantity users do not accidentally exceed permissible toxin limits. For additional
information select toxin exemption requirements, see the Federal Select Agent
Program website (www.selectagents.gov). Toxins should be stored in storage
containers with labels that clearly list the toxin contents, points of contact for
trained, responsible laboratory sta, and emergency contact information. The
use of locks on storage containers oers an additional level of oversight and
control over toxin access. Laboratory work with toxins should only be done in
designated rooms with controlled access and at pre-determined bench areas.
When toxins are in use, the room should have clearly posted signage stating,
for example, “Toxins in Use—Authorized Personnel Only.” Signage should
provide a knowledgeable point of contact and delineate minimum requirements
for PPE. Whenever possible, unrelated and nonessential work should be
avoided in laboratory or clinical areas where concentrated solutions of toxins
or of toxin-producing organisms are maintained. Laboratory visitors must be
briefed and monitored to prevent them from inadvertently handling contaminated
laboratory equipment or touching exposed surfaces without protection. Finally,
treatment plans for accidental exposures should be prepared and available
to emergency responders and, when possible, coordinated with primary care
facilities. While there is no way to completely eliminate the dangers of biological
toxin use, implementation of these controls can signicantly reduce the risks
associated with toxin storage and use.
Safety Equipment and Containment
Routine operations with dilute toxin solutions are conducted under BSL-2
conditions with the aid of PPE and a well-maintained BSC, chemical fume
473Appendix I—Guidelines for Work with Toxins of Biological Origin
hood, or comparable engineering controls.
5
Engineering controls should be
selected according to the risk assessment for each specic toxin operation. A
certied BSC or chemical fume hood will suce for routine operations with most
solubilized protein toxins. Work involving toxin powders, volatile chemicals, or
radionuclides combined with toxin solutions may require additional safeguards
or barriers based on the risks associated with each toxin preparation.
Handling of solubilized toxins should be conducted within the operationally
eective zone of a BSC or chemical fume hood. Before initiating work, each user
should verify the hood or BSC is properly working according to manufacturer
guidelines. When using a BSC or hood, workers should wear suitable laboratory
PPE to protect the hands, arms, and eyes, such as laboratory coats with knit
or elastic cus, smocks or coveralls, disposable gloves, and safety glasses.
When working with toxins that pose direct percutaneous hazards, special care
must be taken to select gloves that are impervious to the toxin and the diluents
or solvents employed. When conducting large volume liquid transfers and other
operations that pose a potential splash or droplet hazard in an open-front hood
or BSC, workers should wear a disposable facemask or face shield.
Toxin(s) should be removed from the hood or BSC only after the exterior of
the closed primary container has been decontaminated and placed in a clean
secondary container. Toxin solutions, especially concentrated stock solutions,
should be transported in leak/spill-proof secondary containers. The interior of
the hood or BSC should be decontaminated periodically; for example, at the end
of the day or after a spill. Until thoroughly decontaminated, the hood or BSC
should remain posted to indicate that toxins are present, and access should be
restricted to sta trained in toxin use and decontamination.
Selected operations with toxins may require modied BSL-3 practices and
procedures. The determination to use BSL-3 is made in consultation with
available biosafety sta and is based upon a risk assessment that considers
the variables of each specic laboratory operation, especially the toxin under
study, the physical state of the toxin (solution or dry form), the total quantity
of toxin used relative to the estimated human median lethal dose, the volume
of the material manipulated, the methodology, and any human or equipment
performance limitations.
Inadvertent Toxin Aerosols
Many biological toxins are highly potent, and emphasis must be placed on
evaluating and modifying experimental procedures to avoid inadvertent gener-
ation of toxin aerosols. Tubes containing solubilized toxin under pressure should
be only be opened in a BSC, chemical fume hood, or other ventilated enclosure.
474 Biosafety in Microbiological and Biomedical Laboratories
Operations that expose toxin solutions to vacuum or pressure should always be
handled in this manner, and the operator should also use appropriate respiratory
protection to minimize the accidental inhalation of aerosolized toxins or toxin
powder. If vacuum lines are used with toxin, they should be protected with a
HEPA lter to prevent entry of toxins into the line and include a vacuum ask
with decontamination solution between the vacuum source and vacuum line.
HEPA lters should be considered to be contaminated with toxin particles and
disposed of as described below.
Centrifugation of cultures or materials potentially containing toxins should only
be performed using sealed, thick-walled tubes in safety centrifuge cups or
sealed rotors. The outside surfaces of containers, safety cups (if applicable),
and rotors should be routinely cleaned before and after each use to prevent
contamination that may generate an aerosol. The sealed centrifuge safety cups
or sealed rotor should be taken from the centrifuge to a BSC prior to opening or
it should be taken to other suitable containment prior to breaking the seal and
removing centrifugation tubes.
Mechanical Injuries
Accidental needlesticks or mechanical injury from sharps (i.e., glass or
metal implements) pose a well-known risk to laboratory workers. When
these accidents occur during operations using biological toxins in amounts
that approach a human lethal dose, the consequences may be catastrophic.
Consequently, additional care must be taken prior to and during toxin
operations to reduce the risks of exposure through mechanical injury.
Only workers trained, competent, and experienced in handling animals and
toxin operations should be permitted to conduct operations involving animals,
especially injection of toxin solutions using hollow-bore needles. Discarded
needles/syringes and other sharps should never be recapped; instead, they
should be placed directly into properly labeled, puncture-resistant sharps
containers and decontaminated. Glassware should be replaced with plastic for
handling toxin solutions to minimize the risk of cuts or abrasions from contam-
inated surfaces. Thin-walled glass equipment should be completely avoided.
Glass Pasteur pipettes are particularly dangerous for transferring toxin solutions
and should be replaced with disposable plastic pipettes. Glass chromatography
columns under pressure must be enclosed within a plastic water jacket or other
secondary container.
475Appendix I—Guidelines for Work with Toxins of Biological Origin
Additional Precautions
Experiments should be planned to eliminate or minimize work with dry toxin
or toxin- containing formulations (e.g., lyophilized material or freeze-dried
preparations). Unavoidable operations with dry toxin should only be undertaken
with appropriate respiratory protection and engineering controls. Dry toxin can
be manipulated using a Class II BSC or with the use of secondary containment
such as a disposable glove bag or glove box within a hood. Static-free
disposable gloves should be worn when working with dry forms of toxins that
are subject to spread by electrostatic dispersal. If a Class II BSC is used, HEPA
lters should be considered to be contaminated with toxin particles and disposed
of as described below. Workers should wear respiratory protection suitable to
prevent accidental inhalation of toxin particles.
In specialized laboratories, the intentional, controlled generation of aerosols from
toxin solutions may be required to test antidotes or vaccines in experimental
animals. These are extremely hazardous operations that should only be
conducted after extensive validation of equipment and personnel using non-toxic
simulants. Aerosol exposure of animals should be done in a certied Class III
BSC or similar containment device. Workers should take additional precautions
to avoid accidental exposure to biological toxins when removing exposed
animals from the exposure area and for the subsequent 24 hours after exposure;
additional precautions include wearing protective clothing (e.g., disposable Tyvek
suit) and appropriate respiratory protection. To minimize the risk of dry toxin
generating a secondary aerosol, areas of animal skin or fur exposed to aerosols
should be gently wiped with a damp cloth containing water or buered cleaning
solution before the animals are returned to holding areas. Injections of toxin
solutions into animals can be conducted outside of a BSC, but attention must
be paid to avoiding needlesticks and ensuring that used syringes are stored and
disposed of properly to avoid accidental contamination or loss of toxin.
For high-risk operations involving dry forms of toxins, intentional aerosol
formation, or the use of hollow-bore needles in conjunction with amounts of toxin
estimated to be lethal for humans, consideration should be given to requiring the
presence of at least two knowledgeable individuals at all times in the laboratory.
6
This is particularly important when using toxins that have acute eects. While
the physicochemical properties of most toxins render interpersonal transmission
highly unlikely, emergency care providers should be aware of the possibility
of contamination in the environment or through direct transfer of bodily uids
(e.g., during mouth-to-mouth resuscitation). Laboratories using toxins that have
acute eects on cardiopulmonary function should have emergency resuscitation
training provided and equipment located in the near vicinity to sustain casualties
476 Biosafety in Microbiological and Biomedical Laboratories
until the toxic eect passes and emergency caregivers are on-scene. Resusci-
tation equipment should include mask-bag or oxygen delivery systems to reduce
the risk of exposure to emergency caregivers.
Vaccinations against some biological toxins are available and may be appro-
priate for laboratory workers, depending on the amount of toxin used, frequency
of use, and risk of toxin exposure.
Decontamination and Spills
Decontamination of a biological toxin(s) means the toxin is rendered inactive and
is no longer capable of exerting its toxic eect. Toxin stability varies considerably
outside of physiological conditions depending upon the temperature, pH, ionic
strength, presence of co-factors, and other characteristics of the surrounding
matrix. Literature values for dry heat inactivation of toxins can be misleading due
to variations in experimental conditions, matrix composition, and experimental
criteria for assessing toxin activity. Inactivation is not always a linear function
of heating time; some protein toxins possess a capacity to re-fold and partially
reverse inactivation caused by heating. In addition, the conditions for denaturing
toxins in aqueous solutions are not necessarily applicable for inactivating dry,
powdered toxin preparations.
General guidelines for laboratory decontamination of selected toxins are summa-
rized in Tables 1 and 2, but inactivation procedures should not be assumed to be
100% eective without validation using specic toxin bioassays. Most toxins are
susceptible to steam inactivation (121°C for one hour) or to chemical inactivation
with dilute sodium hydroxide (NaOH) at concentrations of 0.1–0.25N, and/or
sodium hypochlorite (NaOCl) solutions at concentrations of 0.1–2.5% (w/v).
Commercially available bleach solutions typically contain 3–6% (w/v) NaOCl.
Bleach decontamination solutions should always be prepared fresh (i.e., <24 h).
Contaminated materials and toxin waste solutions can be inactivated by
incineration, extensive autoclaving, or by soaking in a suitable decontamination
solution, depending on the toxin (Table 2). Once decontaminated, liquid
inactivated toxins can be absorbed onto a solid matrix (i.e., absorbent pad, lter
paper, or paper towel) for incineration as hazardous waste. Alternatively, liquid
inactivated toxins can be disposed of in the sink, depending on local regulations
and policies. All disposable contaminated solid material should be placed in
secondary containers and then autoclaved and/or disposed of as hazardous
waste for incineration. Contaminated or potentially contaminated protective
clothing and equipment (e.g., PPE) that is to be re-used should be decontami-
nated using suitable chemical methods or should be autoclaved after use, if the
toxin is heat-labile, and before it is re-used or removed from the laboratory for
cleaning or repair.
477Appendix I—Guidelines for Work with Toxins of Biological Origin
In the event of a liquid spill, avoid splashes or generating aerosols during
clean-up by covering the spill with dry paper towels or other disposable,
absorbent material. Ensure that appropriate PPE (at a minimum to include
mask, gloves, safety glasses or goggles, and laboratory coat) is worn during the
clean-up. Apply an appropriate decontamination solution to the spill, beginning
at the perimeter and working towards the center. Allow sucient contact time for
the decontamination solution to completely inactivate the toxin (Table 2). Restrict
access to the contaminated area until the decontamination is complete. Absorb
the decontaminated toxin onto a solid matrix and discard as hazardous waste for
incineration.
Spills involving toxin powder have an increased risk of inhalational exposure.
PPE should include respiratory protection, gloves, safety glasses or goggles,
and lab coat. If the spill occurs within the BSC, gently cover the powder spill
with damp absorbent paper towels to avoid raising dust. Apply the appropriate
chemical inactivating agent starting at the perimeter and working toward the
center, allowing for sucient contact time as specied in Table 2. Wipe the area
with a paper towel soaked in bleach solution or a decontamination solution
specic to the biological toxin; then, wash with soap and water. Dispose of
the decontaminated physical waste by autoclaving or as hazardous waste for
incineration. A powder spill outside the BSC should trigger the immediate evacu-
ation of the area. The spill should be managed and decontaminated as above;
however, access to the contaminated area should be carefully controlled in order
to minimize the possibility of disturbing the powder and causing an inhalational
exposure. Decontamination personnel should be equipped with respirators.
Depending on the size of the spill, the area may have to be quarantined and the
HVAC system turned o until the entire spill is contained and the area decontam-
inated. Filters in the HVAC system may need to be removed and discarded by
trained personnel.
Decontamination of large areas, buildings, or oces containing sensitive
equipment or documents poses special challenges. Large-scale decontami-
nation is not covered explicitly here, but careful extrapolation from the basic
principles may inform more extensive clean-up eorts.
Low molecular weight biological toxins tend to be highly stable and resistant to
decontamination. Chemical decontamination with NaOCl is currently the most
reliable method for inactivation.
7
Alternative methods have not proven very
eective. For example, 1 N sulfuric or hydrochloric acid does not inactivate T-2
mycotoxin and only partially inactivates microcystin-LR, saxitoxin, and brevetoxin
(PbTx-2). Tetrodotoxin and palytoxin are inactivated by hydrochloric acid, but
only at relatively high molar concentrations. T-2 is not inactivated by exposure
to 18% formaldehyde plus methanol (16 hours), 90% freon-113 + 10% acetic

478 Biosafety in Microbiological and Biomedical Laboratories
acid, calcium hypochlorite, sodium bisulfate, or mild oxidizing agents. Hydrogen
peroxide is ineective in inactivating T-2 mycotoxin. Hydrogen peroxide does
cause some inactivation of saxitoxin and tetrodotoxin but requires a 16-hour
contact time in the presence of ultraviolet light. The addition of 3% acetone after
bleach treatment has been suggested to prevent reformation of mycotoxins after
bleach treatment when decontaminating spills or glassware.
8
Select Toxins
HHS and the USDA have identied a group of toxins that pose a severe
threat to human, animal, and/or plant health as Select Toxins. The Federal
Select Agent Program oversees the possession, use, and transfer of these
toxins, to include botulinum neurotoxins (all serotypes and subtypes), abrin,
paralytic alpha conotoxins, diacetoxyscirpenol, ricin, saxitoxin, staphylococcal
enterotoxins (subtypes A–E), T-2 toxin, and tetrodotoxin. A current list of Select
Toxins and exempt quantities can be found at https://www.selectagents.gov/
SelectAgentsandToxins.html. Registration with the CDC or USDA is required
for possession, use, modication, production, storage, and/or transfer of
non-exempt quantities of Select Toxins, while exempt quantities should be
carefully managed by the responsible organization to prevent loss or misuse.
Most Select Toxins are highly potent, and corresponding antidotes are not
clinically available; thus, extreme care must be taken when using these agents
for clinical or research purposes. Risk assessments and emergency treatment
plans should be formulated that are specic to the dangers of each Select
Toxin, and responsible parties should undertake regular reviews of laboratory
procedures to ensure that laboratory procedures are understood and carefully
followed by technical personnel.
Table 1. Physical Inactivation of Toxins
Toxin
Steam
Autoclave
Dry Heat
(10 min)
Freeze-Thaw
Gamma
Irradiation
Botulinum
neurotoxin A–G
Yes
a
≥ 100° C
b
No
c
Incomplete
d
Staphylococcal
enterotoxin
Yes
e
≥ 100°
C
; refold
f
No
g
Incomplete
h
Ricin Yes
i
≥ 100° C
i
No
j
Incomplete
k
Microcystin No
l
≥ 260° C m No
n
ND
Saxitoxin No
l
≥ 260° C
m
No
n
ND
Palytoxin No
l
≥ 260° C
m
No
n
ND
Continued on next page ►

479Appendix I—Guidelines for Work with Toxins of Biological Origin
Toxin
Steam
Autoclave
Dry Heat
(10 min)
Freeze-Thaw
Gamma
Irradiation
Tetrodotoxin No
l
≥ 260° C
m
No
n
ND
T-2 mycotoxin No
l
≥ 815° C
m
No
n
ND
Brevetoxin
(PbTx-2)
No
l
≥ 815° C
m
No
n
ND
Abrin Yes
o
ND ND ND
Shiga toxin Yes
p
ND ND ND
ND indicates “not determined” from available literature.
a. Steam autoclaving should be at ≥121° C for 1 h For volumes larger than 1 liter, especially those containing
Clostridium botulinum spores, autoclave at ≥121° C for 2 h to ensure that sucient heat has penetrated to kill all
spores.
9,10
b. Exposure to 100° C for 10 min inactivates BoNT. Heat denaturation of BoNT as a function of time is biphasic with
most of the activity destroyed relatively rapidly, but with some residual toxin (e.g., 1–5%) inactivated much more
slowly.
11
c. Measured using BoNT serotype A at -20° C in food matrices at pH 4.1–6.2 over a period of 180 days.
12
d. Measured using BoNT serotypes A and B with gamma irradiation from a
60
Co source.
13,14
e. Protracted steam autoclaving, similar to that described for BoNT, followed by incineration is recommended for
disposal of SE-contaminated materials.
f. Inactivation may not be complete depending upon the extent of toxin re-folding after denaturation. Biological
activity of SE can be retained despite heat and pressure treatment routinely used in canned food product
processing.
15
g. SE toxins are resistant to degradation from freezing, chilling or storage at ambient temperature. Active SEB in the
freeze-dried state can be stored for years.
16
h. References
16,17
i. Dry heat of >100º C for 60 min in an ashing oven or steam autoclave treatment at >121º C for 1 h reduced the
activity of pure ricin by >99%.
7
Heat inactivation of impure toxin preparations (e.g., crude ricin plant extracts) may
vary. Heat-denatured ricin can undergo limited refolding (<1%) to yield active toxin.
j. Ricin holotoxin is not inactivated signicantly by freezing, chilling, or storage at ambient temperature. In the liquid
state with a preservative (sodium azide), ricin can be stored at 4º C for years with little loss in potency.
k. Irradiation causes a dose-dependent loss of activity for aqueous solutions of ricin, but complete inactivation
is dicult to achieve; 75 MRad reduced activity 90%, but complete inactivation was not achieved even at 100
MRad.
18
Gamma irradiation from a laboratory
60
Co source can be used to partially inactivate aqueous solutions of
ricin, but dried ricin powders are signicantly resistant to inactivation by this method.
l. Autoclaving with 17 lb pressure (123º C) for 30 min failed to inactivate LMW toxins.
7,19
All burnable waste from
LMW toxins should be incinerated at temperatures in excess of 815º C (1,500º F).
m. Toxin solutions were dried at 150º C in a crucible, placed in an ashing oven at various temperatures for either 10
or 30 min, reconstituted, and tested for concentration and/or activity; tabulated values are temperatures exceeding
those required to achieve 99% toxin inactivation.
7
n. LMW toxins are generally very resistant to temperature uctuations and can be stored in the freeze-dried state for
years and retain toxicity.
o. Reference
20
p. Reference
21,22
480 Biosafety in Microbiological and Biomedical Laboratories
Table 2. Chemical Inactivation of Toxins
Toxin
NaOCl (30
min)
NaOH Freeze-Thaw
Gamma
Irradiation
Botulinum
neurotoxin A–G
≥ 0.1%
a
≥ 0.25 N ND Yes
b
Staphylococcal
enterotoxin
≥ 0.5%
c
≥ 0.25 N ND ND
Ricin ≥ 1.0%
d
ND > 0.1% + 0.25 N
e
ND
Saxitoxin ≥ 0.1%
e
ND 0.25% + 0.25 N
e
ND
Palytoxin ≥ 0.1%
e
ND 0.25% + 0.25 N
e
ND
Microcystin ≥ 0.5%
e
ND 0.25% + 0.25 N
e
ND
Tetrodotoxin ≥ 0.5%
e
ND 0.25% + 0.25 N
e
ND
T-2 mycotoxin ≥ 2.5%
e,f
ND 0.25% + 0.25 N
e
ND
Brevetoxin (PbTx-2) ≥ 2.5%
e,f
ND 0.25% + 0.25 N
e
ND
Alpha conotoxins ≥ 0.5%
g
10 N
g
ND No
g
Abrin ≥ 0.7%
h
ND ND ND
Shiga toxin ≥ 0.5% ND 0.25% + 0.25 N
e
ND
ND indicates “not determined” from available literature.
a. Solutions of NaOCl (≥ 0.1% nal concentration; typically a 1:50 dilution of commercial bleach into distilled water)
or NaOH (> 0.25 N) for 30 min inactivate BoNT and are recommended for decontaminating work surfaces and
spills of C. botulinum or BoNT. Chlorine at a concentration of 0.3–0.5 mg/L as a solution of hypochlorite rapidly
inactivates BoNT (serotypes B or E tested) in water.
23
Chlorine dioxide inactivates BoNT, but chloramine is less
eective.
23,24
After decontamination, the solution is safe to discard in the sink as long as local ordinances are
obeyed. Alternatively, BoNT can be absorbed onto a disposable napkin, dried, and disposed of in hazardous
waste for incineration.
b. Ozone (> 2 mg/L) or powdered activated charcoal treatment also completely inactivate BoNT (serotypes A, B
tested) in water under dened conditions.
24,25
c. SEB is inactivated with 0.5% hypochlorite for 10–15 min.
26
d. Ricin is inactivated by a 30-min exposure to concentrations of NaOCl ranging from 0.1–2.5%, or by a mixture of
0.25% NaOCl plus 0.25 N NaOH.
7
In general, solutions of 1.0% NaOCl are eective for decontamination of ricin
from laboratory surfaces, equipment, animal cages, or small spills.
e. The minimal eective concentration of NaOCl was dependent on toxin and contact time; all LMW toxins tested
were inactivated at least 99% by treatment with 2.5% NaOCl, or with a combination of 0.25% NaOCl and 0.25 N
NaOH.
7
f. For T-2 mycotoxin and brevetoxin, liquid samples, accidental spills, and nonburnable waste should be soaked in
2.5% NaOCl with 0.25 N NaOH for 4 h. Cages and bedding from animals exposed to T-2 mycotoxin or brevetoxin
should be treated with 2.5% NaOCl and 0.25 N NaOH for 4 h. Exposure for 30 min to 1.0% NaOCl is an eective
procedure for the laboratory (working solutions, equipment, animal cages, working area and spills) for the
inactivation of saxitoxin or tetrodotoxin. Decontamination of equipment and waste contaminated with select
brevetoxins has been reviewed.
19
g. Conotoxins can also be inactivated using reducing agents such as dithiothreitol β- mercaptoethanol, or tris
(2-carboxyethyl) phosphine (100 mM) at 65–100° C for 15 min, followed by alkylation with 100 mM maleimide in
isopropanol at 65° C for 15 min. Alternatively, alpha conotoxins can be inactivated by hydrolysis in 10 N NaOH or
HCl at 100° C for 30 min.
27
h. Exposure of crude abrin solution and dried abrin to 0.67% NaOCl eliminated over 90% of cytotoxicity within 5 min.
28

481Appendix I—Guidelines for Work with Toxins of Biological Origin
References
1. Franz DR. Defense Against Toxin Weapons. In: Sidell FR, Takafuji ET,
Franz DR, editors. Medical Aspects of Chemical and Biological Warfare.
The TMM Series. Part 1: Warfare, Weaponry, and the Casualty.
Washington (DC): Oce of the Surgeon General at TMM Publications;
1997. p. 603–19.
2. Millard CB. Biological weapons defense: infectious diseases and
counterbioterrorism. Lindler LE, Lebeda FJ, Korch GW, editors. Totowa
(NJ): Humana Press. 2005. Medical defense against protein toxin
weapons: review and perspective; p. 255–84.
3. The biological defense safety program—technical safety requirements,
32 C.F.R. Part 627 (1993).
4. Johnson B, Mastnjak R, Resnick IG. Anthology of Biosafety II:
Facility Design Considerations. Richmond J, editor. Mundelein (IL):
American Biological Safety Association; 2000. Vol 2. Safety and Health
Considerations for Conducting Work with Biological Toxins; p. 88–111.
5. Kruse RH, Puckett WH, Richardson JH. Biological safety cabinetry. Clin
Microbiol Rev. 1991;4:207–41.
6. Kozlovac J, Hawley RJ. Biological toxins: safety and science. In:
Wooley DP, Byers KB, editors. Biological safety: principles and practice.
Washington (DC): ASM Press; 2017. p. 247–68.
7. Wannemacher RW, Bunner DL, Dinterman RE. Inactivation of low
molecular weight agents of biological origin. In: US Army Chemical
Research, Development & Engineering Center. Proceedings for the
Symposium on Agents of Biological Origins; 1989 Mar 21–23; Laurel (MD).
p. 115–22.
8. U.S. Food & Drug Administration. ORA Laboratory Manual. [Internet]. 2013
[cited 2018 Sept 28]; IV(7): [about 23 p.]. Available from: https://www.fda.
gov/ScienceResearch/FieldScience/LaboratoryManual/default.htm
9. Balows A, Hausler WJ Jr, Ohashi M, Turano A, editors. Laboratory
Diagnosis of Infectious Diseases: Principles and Practice. Vol 1. New York:
Springer-Verlag; 1988.
10. Hatheway CL. Botulism. In: Balows A, Hausler WJ Jr, Ohashi M, Turano A,
editors. Laboratory Diagnosis of Infectious Diseases: Principles and
Practice. Vol 1. New York: Springer-Verlag; 1988. p. 111–33.
11. Siegel LS. Destruction of botulinum toxins in food and water. In: Hauschild
AHW, Dodds KL, editors. Clostridium botulinum: Ecology and Control in
Foods. New York: Marcel Dekker, Inc.; 1993. p. 323–41.
12. Woolford A, Schantz EJ, Woodburn M. Heat inactivation of botulinum
toxin type A in some convenience foods after frozen storage. J Food Sci.
1978;43:622–4.
482 Biosafety in Microbiological and Biomedical Laboratories
13. Dack GM. Eect of irradiation on Clostridium botulinum toxin subjected
to ultracentrifugation. Natick (MA): Quartermaster Food and Container
Institute for the Armed Forces; 1956. Report No.: 7.
14. Wagenaar RO, Dack GM. Eect in surface ripened cheese of irradiation on
spores and toxin of Clostridium botulinum types A and B. Food Research
Institute. 1956;21:226–34.
15. Bennett RW, Berry MR. Serological reactivity and in vivo toxicity of
Staphylococcus aureus enterotoxin A and D in selected canned foods.
J Food Sci. 1987;52:416–8.
16. Concon JM. Bacterial Food Contaminants: Bacterial Toxins. In: Concon JM,
author. Food Toxicology (in two parts) Parts A and B. New York: Marcel
Dekker, Inc.; 1988. p. 771–841.
17. Modi NK, Rose SA, Tranter HS. The eects of irradiation and temperature
on the immunological activity of staphylococcal enterotoxin A. Int J Food
Microbiol. 1990;11:85–92.
18. Haigler HT, Woodbury DJ, Kempner ES. Radiation inactivation of ricin
occurs with transfer of destructive energy across a disulde bridge. Proc
Natl Acad Sci USA. 1985;82(16):5357–9.
19. Poli MA. Laboratory procedures for detoxication of equipment and waste
contaminated with brevetoxins PbTx-2 and PbTx-3. J Assoc O Anal
Chem. 1988;71(5):1000–2.
20. Tam CC, Henderson TD, Stanker LH, He X, Cheng LW. Abrin Toxicity
and Bioavailability after Temperature and pH Treatment. Toxins.
2017;9(10):320.
21. Rasooly R, Do PM. Shiga toxin Stx2 is heat-stable and not inactivated by
pasteurization. Int J Food Microbiol. 2010;136(3):290–4.
22. Lumor SE, Fredrickson NR, Ronningen I, Deen BD, Smith K,
Diez-Gonzalez F, et al. Comparison of the presence of Shiga toxin 1 in
food matrices as determined by an enzyme-linked immunosorbent assay
and a biological activity assay. J Food Prot. 2012;75(6):1036–42.
23. Notermans S, Havelaar AH. Removal and inactivation of botulinum toxins
during production of drinking water from surface water. Antonie Van
Leeuwenhoek. 1980;46:511–4.
24. Brazis AR, Bryant AR, Leslie JE, Woodward RL, Kabler PW. Eectiveness
of halogens or halogen compounds in detoxifying Clostridium botulinum
toxins. J Am Waterworks Assoc. 1959;51(7):902–12.
25. Graikoski JT, Blogoslawski WJ, Choromanski J. Ozone inactivation of
botulinum type E toxin. Ozone: Sci Eng. 1985;6:229–34.
26. Robinson, JP. Annex 2—Toxins. In: Public Health Response to Biological
and Chemical Weapons: WHO Guidance. 2nd ed. Geneva (Switzerland):
World Health Organization; 2004. p. 214–28.
483Appendix I—Guidelines for Work with Toxins of Biological Origin
27. Liu D, editor. Manual of Security Sensitive Microbes and Toxins. 1st ed.
Boca Raton (FL): CRC Press, Taylor & Francis Group; 2014.
28. Tolleson WH, Jackson LS, Triplett OA, Aluri B, Cappozzo J, Banaszewski K,
et al. Chemical inactivation of protein toxins on food contact surfaces.
J Agric Food Chem. 2012;60(26):6627–40.
484 Biosafety in Microbiological and Biomedical Laboratories
Appendix J—NIH Oversight of Research Involving
Recombinant Biosafety Issues
The locus for oversight of research subject to the NIH Guidelines for Research
Involving Recombinant or Synthetic Nucleic Acid Molecules (NIH Guidelines)
within NIH is the Oce of Science Policy (OSP), which is located within the
Oce of the Director of the NIH, and is responsible for the oversight of research
involving recombinant or synthetic nucleic acid molecules. The key elements
in the biosafety oversight framework for such research are the NIH Guidelines
and Institutional Biosafety Committees (IBCs) or equivalent resource. NIH
OSP promotes the science, safety, and ethics of research subject to the NIH
Guidelines with the primary goals of enabling the safe conduct of research and
of helping to advance all elds of science that employ recombinant or synthetic
nucleic acid molecules.
The NIH Guidelines specify appropriate biosafety practices and procedures for
research involving the construction and handling of recombinant or synthetic
nucleic acid molecules, as well as cells, organisms, and viruses that contain such
molecules. Recombinant or synthetic nucleic acid molecules are dened in the
NIH Guidelines as:
1. Molecules that a) are constructed by joining nucleic acid molecules and
b) that can replicate in a living cell (i.e., recombinant nucleic acids);
2. Nucleic acid molecules that are chemically, or by other means, synthe-
sized or amplied, including those that are chemically or otherwise
modied but can base pair with naturally occurring nucleic acid
molecules (i.e., synthetic nucleic acids); or
3. Molecules that result from the replication of those described in (1) or (2).
Compliance with the NIH Guidelines is a term and condition of NIH funding, and
the NIH Guidelines are applicable to all research conducted at or sponsored by
an institution that receives any funding from the NIH for recombinant or synthetic
nucleic acid molecule research, regardless of the funding source of an individual
project. The broad reach of the NIH Guidelines promotes the consistency of
biosafety practices across the institution to better protect the safety of laboratory
workers, the public, and the environment.
The NIH Guidelines were rst published in 1976 and are revised as technological,
scientic, and policy developments warrant. They outline the roles and respon-
sibilities of various entities involved in the conduct or oversight of recombinant
or synthetic nucleic acid molecule research, including institutions, investigators,
IBCs, biosafety ocers, and the NIH (Section IV of the NIH Guidelines). They
classify agents into one of four Risk Groups (Appendix B of the NIH Guidelines)
based on their potential to cause disease in a healthy adult human and describe
four levels of physical containment practices (Appendix G of the NIH Guidelines)

485Appendix J—NIH Oversight of Research Involving Recombinant Biosafety Issues
that should be employed for research with the agents based on the potential
risk. The NIH Guidelines establish dierent levels of review and approval for
recombinant or synthetic nucleic acid molecule research, based on the nature of
the activity. These levels are:
1. Approval from the NIH Director and the IBC before initiation of the
research.
2. Approval from NIH OSP and the IBC before initiation of the research.
3. Approval from the IBC before initiation of human gene transfer research.
4. Approval from the IBC prior to initiation of the research.
5. Notication of the IBC simultaneous with initiation of the research with
subsequent IBC review and approval.
See Section III of the NIH Guidelines for additional details. In all instances, it is
important to note that review and approval by an IBC is required.
The roles and responsibilities of IBCs, as well as membership, procedures, and
functions are outlined in Section IV-B-2 of the NIH Guidelines. Institutions that are
ultimately responsible for the eectiveness of IBCs may dene additional roles
and responsibilities for these committees in addition to those specied in the NIH
Guidelines. For example, some institutions may set a policy that their IBC will also
review certain research that is not subject to the NIH Guidelines (e.g., research
involving non-recombinant pathogens). The NIH Guidelines are available at
https://osp.od.nih.gov/biotechnology/nih-guidelines/.
Additional information regarding NIH OSP, the NIH Guidelines, and the roles and
responsibilities of IBCs can be found at http://osp.od.nih.gov.
486 Biosafety in Microbiological and Biomedical Laboratories
Appendix K—Inactivation and Verication
This appendix describes inactivation methods that enable retention of character-
istic(s) of interest in pathogens, viral nucleic acid sequences, or toxins in order
to accommodate the intended future use(s) of the material and verication of
inactivation procedures. Inactivation and verication of Select Agents and Toxins
must be in compliance with current regulations from the Federal Select Agent
Program.
1
Key Terminology discussed in this appendix is dened in the Glossary and
includes inactivation, validated inactivation procedure, viability testing protocol,
infectivity testing, toxicity testing, attenuation, process verication, institutional
verication, and validation.
1
Background
When choosing an inactivation method, consider key characteristics, including
the infectious agent (e.g., pathogen, viral nucleic acid sequences, or toxin),
resistance to treatment, and ability to recover from the treatment.
2,3
Environ-
mental stability is high for some agents including spores, pathogens residing
within biolms, and prions.
Dierent types of inactivation procedures target dierent components and/or
systems within the agent. Inactivation targets include: bacterial cell walls; lipid
envelopes or cell membranes; nucleic acids; and regulatory systems involved
in the agent’s virulence, replication, and/or transmissibility. Types of inactivation
methods may include:
■
Physical (e.g., heat,
4,5
ionizing irradiation,
6,7
254 nm ultraviolet [UV]
light
8–10
);
■
Chemical (e.g., chaotropic compounds such as guanidine hydro-
chloride,
11–14
oxidizers such as chlorine and hydrogen peroxide,
15–18
psoralen or titanium dioxide nanoparticles activated by UV-A
10,19–23
);
■
Natural antimicrobial strategies (e.g., enzymes such as lysozymes and
virolysins [bacteriophage-encoded lytic enzymes
24–26
], antimicrobial
peptides such as nisin,
27
and bacteriophages
28
); or
■
Combination (e.g., sublethal mild temperatures [<60 degrees Celsius]
with various nonthermal treatments,
2
antimicrobial compounds with
ionizing radiation,
29
and lysozyme with antimicrobial compounds
30
).
Some traditional disinfection methods can also serve as inactivation treatments.
For example, spores, vegetative bacteria, DNA viruses, and RNA viruses can be
eectively inactivated with peracetic acid with minimal eects on the ability to
do subsequent PCR and ELISA immunoassays.
18
Alternatives to antibiotics for
humans and animals, environmental decontamination methods, and food safety
processes could potentially lead to the development of inactivation procedures.
25
487Appendix K—Inactivation and Verication
Novel inactivation strategies include use of cell wall hydrolases, such as
lysozyme,
24
and antimicrobial peptides such as nisin.
27
When choosing an inactivation method, several factors need to be considered
including: specic controls; the balance between ecacy of inactivation vs.
the retention of desired characteristics; and the appropriate safety margin
(i.e., overkill amount). Additional advantages may include low cost and broad
applicability to dierent types of agents.
Filtration and Centrifugation
Filtration is a common pathogen removal method; ltration is also used to
supplement an inactivation method by removing or reducing the amount of active
pathogen, viral nucleic acid sequences, or toxin from biological uids, culture
supernatant, and other materials. Filtration may result in the loss of a signicant
fraction of the material to be used and will require viability testing to ensure no
agent passes through any defect in the lter. Centrifugation or centrifugation
combined with ltration can be used to supplement inactivation methods by
separating out and removing signicant amounts of the pathogen, viral nucleic
acid sequences, or toxin from the material that will be used for subsequent
purposes. Centrifugation may result in adverse eects on the structural integrity
of the residual material and requires additional time and processing steps to
recover the material for further use.
An extract (e.g., nucleic acids, antigens, lysate) is derived from a two-step process
with an initial step (e.g., lysis) where the agent is subjected to a treatment, followed
by a second step (e.g., ltration) to remove any residual active agent.
Development of Inactivation Procedures
The starting point for development of an inactivation procedure is deciding which
inactivation method(s) is appropriate, eective, and feasible to use for the specic
set of circumstances. Inactivation procedures considered can be based on:
1. A procedure developed in-house;
2. A procedure published in a peer-reviewed journal; or
3. A commonly accepted method (e.g., heat, dry or wet).
Many variables need to be considered when developing inactivation procedures;
these include the type and amount (i.e., volume and titer) of agent (e.g., pathogen,
nucleic acid or toxin) to be inactivated; matrix/solvent surrounding the agent;
concentration of starting matrix material; treatment time, temperature, pH, and dose
of treatment; process controls; type of container being used for inactivation; and
appropriate safety measures. The post-exposure environment may also play a role
in the ecacy of the inactivation; therefore, the subsequent environmental condi-
tions (e.g., temperature and nutrients in the matrix) should be controlled as well.
488 Biosafety in Microbiological and Biomedical Laboratories
In cases where limited samples are available, it may be appropriate to use
surrogate strains or agents to develop the inactivation procedures. If resistance
information is known, the most resistant strain or agent should be used as the
surrogate. Generally, suitable surrogates are bacteria from the same genus and
viruses from the same family. Another type of surrogate that may be appropriate
in some situations is a tissue surrogate. In this case, a sample of the tissue
adjacent to the tissue of interest that has also undergone the inactivation may be
used for conrmation of the inactivation procedure and verication that adequate
ecacy has been achieved in the process.
Use of dose-response (e.g., survival of the pathogen, viral nucleic acids, or toxin
vs. the inactivating treatment dose or time), spike-and-recovery experiments
(i.e., bioburden reduction studies), and building an adequate safety margin are
all important elements to incorporate into an inactivation procedure. Factors that
should be considered include:
1. Testing method(s) for the specic set of circumstances involved
(e.g., type, amount, and concentration of starting material);
2. Controls (process, negative, positive);
3. The limit of detection;
4. Interference of residual inactivation material and matrix materials
with viability, infectivity, or toxicity testing; and
5. Appropriate safety margins.
Tables 1–8 outline the key advantages and disadvantages of four broad inacti-
vation method categories—physical, chemical, chemical activated by physical,
and natural and emerging. Tables 9 and 10 outline advantages and disadvan-
tages of combination methods.
Physical inactivation includes heat (dry or wet),
4,5
ionizing radiation,
6,7
and
ultraviolet light (UV-C radiation).
8–10
Physical inactivation through heat involves
hot-air (dry) or steam under pressure (wet), which is used to irreversibly destroy
an agent’s protein structure (denaturation). Ionizing radiation induces single- and
double-strand breaks in nucleic acids. Ultraviolet light, especially at 254 nm, is an
eective treatment for reduction of bacteria; UV-C causes photochemical damage
to nucleic acids through formation of pyrimidine dimers, inhibiting DNA replication
and transcription.

489Appendix K—Inactivation and Verication
Table 1. Advantages of Physical Inactivation
Consideration Heat Ionizing radiation Light (UV-C)
Ecacy Broad Broad
Inactivates viruses,
Gram-positive and
Gram-negative bacteria
Applicability Broad Broad Broad
Residual toxicity Low None None
Cost N/A N/A Low cost
Structural
maintenance
N/A
Proteins; 3-D structure
preserved
Most proteins
Penetration
Complete, depending on
length of treatment
Inactivation of denser
materials
Surface
Resistance N/A None observed None observed
Ease of use Simple and convenient N/A Short exposure time
Table 2. Disadvantages of physical inactivation
Consideration Heat Ionizing radiation Light (UV-C)
Acute Toxicity Thermal burns possible High toxicity
May damage exposed
skin
Structural
maintenance
Limited due to
denaturation of proteins;
may damage agent’s
ability to produce
immune response
N/A
DNA intrastrand
crosslinks limit use for
PCR and transcription
assays
Cost N/A High cost N/A
Penetration
Limited by access of all
material to steam or dry
heat; trapped air may
serve as insulation
N/A
Limited by capacity
of light; impacted by
opaqueness of liquid,
proportion of suspended
particles, soluble and
insoluble materials, and
distance from UV source
Ease of use N/A
Regulatory, security
constraints (irradiator);
long exposure times
N/A

490 Biosafety in Microbiological and Biomedical Laboratories
Chemical inactivation includes chaotropic agents
11–14
and oxidizers.
15–18
Chemical
inactivation through chaotropic agents utilizes guanidine-based denaturing
agents to disrupt cells and liberate nucleic acids; these agents have strong
protein denaturant properties when used at high concentrations. Oxidizing agents
oxidize cell membranes resulting in loss of structure leading to cell lysis and
death. Examples of oxidizing agents include: hypochlorous acid (HOCl), chlorine,
hydrogen peroxide, and peracetic acid.
Table 3. Advantages of chemical inactivation
Consideration Chaotropic agents Oxidizers
Ecacy
Inactivates viruses, Gram-positive and
Gram-negative bacteria
Broad; HOCl eective against prions
and spore-forming Bacillus spp., with
rapid inactivation
Applicability N/A Broad
Residual toxicity N/A
Low toxicity (weak acids safe
for contact with skin, mucous
membranes)
Cost N/A Low cost
Structural
maintenance
Nucleic acids preserved N/A
Ease of use
Non-volatile; eective at room
temperature; kits with prepared
reagents are available
N/A
Table 4. Disadvantages of chemical inactivation
Consideration Chaotropic agents Oxidizers
Ecacy Incomplete inactivation of spores N/A
Acute toxicity
Irritant, toxic, corrosive at high
concentrations
Irritant, toxic, corrosive at high
concentrations
Structural
maintenance
N/A
May damage agent’s ability to
produce an immune response
Ease of use
Need to be removed or neutralized to
assess inactivation
Limited storage stability; may need to
be neutralized to assess inactivation
Inactivation may also be achieved via a chemical inactivation activated by
physical treatment; examples include psoralen and UV-A radiation
19–21
and
titanium dioxide (TiO
2
) and UV-A radiation.
10,22,23
Psoralens, in the presence of
491Appendix K—Inactivation and Verication
UV-A (320–400 nm) radiation, inactivate viral agents. TiO
2
is a stable and inert
material that can continuously exhibit antimicrobial eects when illuminated.
Photocatalysis increases cell permeability with eux of intracellular contents
leading to cell death.
Table 5. Advantages of chemical activated by physical treatment
Consideration Psoralen + UV-A TiO
2
+ UV-A
Ecacy Aects a wide range of viruses
Wide range of agents, including
lethal toxin of Bacillus anthracis;
Nanoparticles (titania) exhibit
superior inactivation
Structural
maintenance
Viral surface epitopes and nucleic
acids preserved
N/A
Resistance None observed N/A
Ease of use N/A
Chemically stable; energy source
can be solar light
Table 6. Disadvantages of chemical activated by physical treatment
Consideration Psoralen + UV-A TiO
2
+ UV-A
Ecacy Limited to viruses
Eciency of technology needs
improvement
Structural
maintenance
N/A
Characteristics may be aected by
cell wall damage
Ease of use
Amotosalen (AMT) needs to be
removed or neutralized to assess
ecacy of inactivation
Requires close contact between agent
and TiO
2
Inactivation may also be achieved through natural and emerging antimicrobial
strategies including lysozyme,
24–26
antimicrobial peptides (AMP),
25,27
and bacterio-
phages.
25,28
Bacterial killing by lysozyme occurs through hydrolysis of cell walls.
It is eective against Gram-positive bacteria and is an important component in
the prevention of microbial growth in foods. Bacteriocins (i.e., bacterial proteins
or peptides) are AMPs widely used in food bio-preservation. Antimicrobial
peptides are the cornerstone of innate immunity. AMPs have various intracellular
and extracellular targets, but AMPs primarily bind to and form pores in cell
membranes. Bacteriophages (phages) are viruses capable of infecting and killing
bacteria. Phages are among the most abundant organisms in nature and are not
known to infect eukaryotes. Use of multiple closely related phages (i.e., cocktail)
has been shown to be more eective in killing microbial pathogens.

492 Biosafety in Microbiological and Biomedical Laboratories
Table 7. Advantages of natural and emerging antimicrobial strategies
Consideration Lysozyme AMPs Bacteriophages
Applicability Broad Broad N/A
Ecacy
Broad; eective against
Gram-positive bacteria
(acts to kill bacteria
immediately); food and
waterborne viruses
Broad; wide
spectrum of agents,
particularly bacteria;
non-immunogenic
Highly active with
targeted, specic host
range; particularly
eective against several
foodborne pathogens
Residual toxicity Low toxicity Low toxicity Low toxicity
Cost Low cost N/A Low cost
Recoverability N/A
Low; AMPs with
extracellular and
intracellular targets
provide a multi-pronged
attack (lessening
possibility of recovery)
Low; cocktail of related
phages increases
ecacy and limits
recoverability
Ease of use
Lysozyme is generally
heat stable and eective
at low concentrations
(~1%)
N/A N/A
Table 8. Disadvantages of natural and emerging antimicrobial strategies
Consideration Lysozyme AMPs Bacteriophages
Applicability
Not as eective on
Gram-negative bacteria
due to their complex cell
wall composition
N/A Narrow host range
Ecacy N/A N/A
Bacterial resistance
to phages may lead
to development
of bacteriophage
insensitive mutants;
ecacy may be
temperature-dependent
Structural
maintenance
Potential for destruction
of pathogen’s cell
wall may limit use of
inactivated materials
Potential for destruction
of pathogen’s cell
wall may limit use of
inactivated materials;
key intracellular
structural proteins of
pathogen important for
use may be aected
Lysis by phage may
limit recovery of cellular
materials
Continued on next page ►

493Appendix K—Inactivation and Verication
Consideration Lysozyme AMPs Bacteriophages
O-target eects N/A N/A
Phage-mediated
transfer of genetic
material to hosts; need
for careful monitoring to
ensure phage genome
is free from toxin and
virulence genes
Ease of use
Low stability
(short half-life)
Low stability
(AMPs inactivated
by proteases)
N/A
Ease of use
Lysozyme is generally
heat stable and eective
at low concentrations
(~1%)
N/A N/A
Finally, inactivation may be achieved through combination methods including
sub-lethal mild temperatures (<60°C) with non-thermal treatments,
2
antimi-
crobial compounds with ionizing radiation,
29
and antimicrobial compounds with
lysozyme.
30
Some common non-thermal treatments include High Pressure
Processing (HPP), Pulsed Electric Field (PEF), and ultrasound (US). The use of
anti-microbial compounds, such as AMPs, can facilitate reduction of the dose of
ionizing radiation treatment necessary for inactivation of pathogens. Synergistic
eects of antimicrobial compounds, such as AMPs with lysozyme, eectively
inactivate and/or kill Gram-positive bacteria. Antimicrobial compounds with
lysozyme are eective against a broader spectrum of pathogens. Resistance
mechanisms to antimicrobial compounds are well known and must be considered
as a potential risk.
31
Table 9. Advantages of combination methods
Consideration
Temperature +
non-thermal
Antimicrobial +
ionizing radiation
Antimicrobial +
lysozyme
Applicability Broad Broad
Combination treatment
results in higher ecacy
for a broader spectrum
of pathogens, including
germinating spores
Ecacy
Broad; eective on
a wide variety of
agents; ecacy greatly
enhanced by combined
use of sub-lethal mild
temperatures with
non-thermal treatments
Broad; eective
inactivation of agents
including a wide variety
of foodborne pathogenic
bacteria
Eective inactivation
of bacteria, particularly
Gram-positive bacteria,
and a wide variety of
foodborne pathogenic
bacteria
Continued on next page ►
494 Biosafety in Microbiological and Biomedical Laboratories
Consideration
Temperature +
non-thermal
Antimicrobial +
ionizing radiation
Antimicrobial +
lysozyme
Residual toxicity Low Toxicity
Lowered by combination
treatment since lower
dose of ionizing
radiation is eective
Low Toxicity
Structural
maintenance
N/A
Lower dose of ionizing
irradiation is key to
retention of desirable
qualities of animal and
plant products.
N/A
Ease of use
Combination allows
shorter processing times
N/A N/A
Table 10. Disadvantages of combination methods
Consideration
Temperature +
non-thermal
Antimicrobial +
ionizing radiation
Antimicrobial +
lysozyme
Applicability
Non-thermal techniques
are less eective
against spores
N/A
Generally ineective
against Gram-negative
bacteria
Cost N/A
High cost for some
natural antimicrobial
compounds
N/A
Recoverability
Not all pathogens
present are inactivated
at same time; potential
for sublethal injury and
possibility of recovery
N/A N/A
O-target eects N/A
Need to consider broad-
spectrum of eects by
antimicrobials, including
synthetic ones, on host
Need to consider broad-
spectrum of eects by
antimicrobials, including
synthetic ones, on host
Resistance N/A
Resistance to
antimicrobial peptides
await more in-depth
investigation
Resistance to
antimicrobial peptides
await more in-depth
investigation
Ease of use
Optimization
of combination
technologies to obtain
highest ecacy needed
Low stability (some
natural antimicrobial
compounds have nite
half-life)
Inactivation is not
immediate
495Appendix K—Inactivation and Verication
Validation of Inactivation Procedures
Conditions of an inactivation procedure must be optimized for ecacy and
tailored to the specic materials and circumstances present in that setting.
A validated inactivation procedure will designate a set of conditions that have
been determined to adequately render:
1. A pathogen non-viable, with ecacy established by viability testing data;
2. The isolated viral nucleic acid incapable of producing infectious forms of
virus, with ecacy established by infectivity testing data; or
3. A toxin no longer capable of exerting a toxic eect, with ecacy estab-
lished by toxicity testing data.
Viability testing procedures may include cell viability assays, growth analysis,
in vivo exposure, or a combination of these methods. A common viral infectivity
testing procedure consists of introducing the positive (+) strand RNA into
permissive cells to determine if that strand can produce an infectious virus.
Toxicity testing may include functional activity assays and in vivo exposure
assays.
The potential for incomplete inactivation, including errors that might result from
exceeding the capacity of the inactivating process to kill the pathogen, lack of
specicity, detection limits, and run-to-run variation should be considered when
setting specications for conrmed inactivation procedures. Sucient replicates
of the testing must be performed in order to determine the underlying variability
within the procedure in the hands of the laboratorians performing it. In addition to
the factors considered during development of an inactivation procedure, elements
1
that should be evaluated when conrming an inactivation procedure include:
. Any chemical inactivation treatments that need to be neutralized or
diluted prior to the conrmation testing; and
2. The statistical probability of inactivation (i.e., was the sample subject to
sucient inactivating material/process to provide a statistically signicant
probability of complete inactivation).
Alternative Strategies
Alternative strategies, such as sampling and use of surrogates, may be
considered when standard validation of an inactivation procedure is not a viable
option. Sampling of a subset of inactivated material may be the
strategy of choice
for situations where materials are limited or when other conditions make full
conrmation impractical. Depending on the type of inactivated material, sampling
could involve either testing a subset of the total number of samples that are
similar or testing a fraction of each of the samples.
The level of underlying variability is a key determinant of the level of conrmation
that should be done; factors to consider include the frequency of testing, the
496 Biosafety in Microbiological and Biomedical Laboratories
appropriate sampling strategy, the use of surrogates, and the percentage of the
sample(s) tested. The underlying variability depends on multiple factors including
the type of sample, the type of inactivation procedure, and the specic materials,
equipment, and conditions used in the inactivation procedure. Laboratories
should re-conrm inactivation procedures whenever changes (e.g., in reagents,
equipment, or environmental conditions) are introduced into the existing validated
inactivation methods. Inactivation procedures should also be re-assessed and
re-validated periodically due to the agent itself changing over time, either through
natural or deliberate means (e.g., mutation, recombination, reassortment of viral
genomes, horizontal gene transfer, synthetic derivation of agents, and modica-
tions resulting from gain-of-function studies).
The risk assessment is the basis for the institution setting a policy on a sampling
strategy that it considers sucient for future runs of the inactivation procedure.
It may be appropriate for inactivation procedures with lower risk materials or
ones that have minimal underlying variability to test only the process controls in
subsequent inactivation runs while it may be appropriate to do conrmation for all
subsequent inactivated samples for those inactivation procedures with higher risk
materials and/or those that have greater underlying variability.
More stringent viability testing is warranted for materials that have only
undergone agent removal (e.g., ltration) than for those materials that have
been treated with both an inactivation method and a removal step to lter out
any residual active agent. The risk for not doing infectivity testing for every viral
nucleic acid extract is mitigated by conrmation of the inactivation procedure,
inclusion of process controls, and an appropriate sampling strategy for subse-
quent inactivation by extraction.
Attenuation Methods
Attenuation is a method to minimize disease risk that involves using a weakened
form of a pathogen, viral nucleic acid sequences, or a toxin. Attenuated
pathogens generally have some combination of reduction in the agent’s virulence,
replication, and/or transmissibility (including host and tissue tropism). Attenuation
methods, while lowering risks and potentially enabling work at a lower Biosafety
Level, do not meet the criteria for classication as inactivation. A thorough risk
assessment is needed to determine whether attenuation of an agent merits
lowering of the Biosafety Level. Attenuation methods include anti-virulence
compounds that target bacterial secretion systems, disarming rather than killing
bacterial pathogens,
25,32–34
and engineering of micro-RNA (miRNA) regulation
systems to restrict viral tropism/host range.
35,36
Reduction of containment level
should never be considered for an attenuation system that results in only a
temporary reduction of virulence.
497Appendix K—Inactivation and Verication
Tables 11 and 12 outline the key advantages and disadvantages of two novel
attenuation methods. First, natural and emerging antimicrobial strategies utilize
anti-virulence compounds
25,32–34
targeting bacterial secretion systems to disarm,
rather than kill, bacterial pathogens. Bacterial secretion systems are capable of
directly translocating key macromolecules directly into a host to modulate defense
mechanisms, facilitating the survival of the agent. Anti-virulence compounds
deprive bacteria of their virulence functions while preserving characteristics useful
for research. Second, molecular biocontainment utilizes microRNA (miRNA)
regulation and tropism
35,36
to engineer miRNA (endogenous, small, non-protein
coding RNAs; important regulators of gene expression) systems to limit a
pathogen’s virulence, replication, and/or transmissibility, including tropism of viral
agents (host range).
Table 11. Advantages of novel methods to attenuate pathogens
Consideration Anti-virulence compounds miRNA regulation
Applicability N/A
Broad applicability through miRNA
engineering
Ecacy
Broad-spectrum activity (especially
Gram-negative)
Species-specic miRNA can attenuate
while retaining replication and
transmissibility in animal model(s)
Structural
maintenance
N/A
Desired characteristics are relatively
stable over long-term through
engineered miRNA regulation
Residual toxicity Low toxicity Low toxicity
Resistance Development of resistance delayed N/A
Table 12. Disadvantages of novel methods to attenuate pathogens
Consideration Anti-virulence compounds miRNA regulation
Applicability Limited to bacteria N/A
Recoverability N/A
Agent may regain infectivity;
monitoring is required
Ecacy Attenuation, not inactivation Attenuation, not inactivation
O-target eects Unknown
Regulation of multiple genes may
have unintended consequences
Continued on next page ►

498 Biosafety in Microbiological and Biomedical Laboratories
Consideration Anti-virulence compounds miRNA regulation
Ease of use
Attenuation occurs at dierent
times; diagnostic tests do not
distinguish between pathogenic
and non-pathogenic bacteria.
Compound(s) that only suppress
virulence while present should not
be considered suitable for reduction
in containment.
N/A
Process Verication
The validated inactivation procedure should be veried in the hands of the
laboratorian performing the procedure while using the reagent sources and
equipment intended for the routine process; verication occurs regardless of
procedure source (i.e., commonly accepted, published, or in-house procedure).
Run-to-run variability is due to the cumulative eect of variation, sometimes slight,
in a number of factors including materials, equipment, pathogen concentration,
environmental conditions, and the personnel performing that particular procedure.
Verication of a validated inactivation procedure is necessary because run-to-run
variations may result in somewhat dierent levels of ecacy.
Verication will need to be risk-based. For lower risk organisms, verication
may be the printout from an autoclave that demonstrated adequate time and
temperature for inactivation or results of a biological indicator. For higher risk
organisms, verication involves testing for the absence of viability, infectivity,
and toxicity; see Validation of Inactivation Procedures within this appendix.
The purpose of process verication is to demonstrate that adequate ecacy is
achieved despite these normal variations in run-to-run conditions.
Institutional Verication
While process verication applies to individual facilities at an institution, institu-
tional verication refers to armation by the institution that the set of conrmed
inactivation and separation/removal procedures used at that institution result in
end-products that achieve adequate inactivation ecacy. It is the institution’s
responsibility to ensure that pathogens, viral nucleic acid sequences, and toxins
handled at their institution are adequately inactivated (or decontaminated) in
order to protect their workers, the public, and the environment and to ensure
movement of the inactivated material to lower containment levels is appropriate.
Tracking of and Communication about Inactivated Samples
The institution should evaluate recordkeeping on the specics of the inactivation
protocol including its limitations; depending on the containment required for the
live organism, one may need data on the risk assessment performed; data from
499Appendix K—Inactivation and Verication
viability, infectivity, or toxicity testing; who performed the inactivation procedure;
the date it was done; and where it was performed. Clear sample labeling is
critical as it enables tracking of the identity of the material, inactivation status,
inactivation date, and other relevant information. Should an inactivation failure
occur, good recordkeeping will aid in informing any individuals who may have
been exposed and could also prevent the samples from being moved to a lower
containment level, if the failure is caught quickly, thus preventing potential
occupational exposures. Internal and external recipients of any material that is
not adequately inactivated must be notied promptly.
Good biosafety and laboratory biosecurity practices include communicating about
any hazards that may be present in inactivated samples, information on the risk
assessments performed for the inactivation and conrmation procedures, details
of the institution’s sampling strategy, appropriate labeling, robust training of the
laboratorians, and retention of experimental data associated with inactivation
verication. Thorough tracking of the inactivation and verication specics is
important for senders of shipments; internal recipients of the material and others
at the institution who may be potentially exposed; individuals who may potentially
be exposed during transport of the materials; and external recipients of shipments
of inactivated biological materials. Use of the original level of containment for the
intact pathogen may be merited if the inactivation status of incoming materials is
uncertain.
Ongoing Review and Oversight of Inactivation and Verication Procedures
Inactivation procedures and methods to verify ecacy of inactivation procedures
should be reviewed regularly (annually is recommended for high-risk agents,
based on risk assessment for lower risk agents); when conditions (e.g., higher
volumes or concentrations of material, temperature, matrix material) have diered
from the pre-determined inactivation procedure conditions set by the conrmation
study(ies); and when a previously veried inactivation procedure fails. The review
of inactivation and verication procedures on a regular, ongoing basis is also
essential in ensuring inactivation ecacy for evolving agents.
An investigation with a root cause analysis needs to be performed on failure of
any previously veried inactivation procedure to determine what went wrong and
how to prevent inactivation failures from happening in the future. Recurring issues
with an inactivation or verication procedure warrant modication of the inacti-
vation method or development of an alternative method(s) for future inactivation
and verication procedures. An institution’s sampling strategy should also be
re-assessed periodically.
500 Biosafety in Microbiological and Biomedical Laboratories
Other Important Considerations
Equipment and other components used in inactivation and verication procedures
need to be regularly maintained in order to ensure consistent inactivation ecacy
over time. Chemical and physical hazards of inactivation procedures should also
be regularly assessed as part of the routine review of the procedures. OSHA’s
Laboratory Safety Guidance provides information on regulations and guidance for
handling hazardous materials in the laboratory.
37
Training and evaluation of competency are key to achieving high levels of
biosafety; consistency minimizes the risks of an incident occurring and limits
negative consequences of an incident should one occur. Regular safety training
should include information on current inactivation and verication procedures
and information on any modied or new procedures; this information should be
provided to all aected sta. Re-training after inactivation failure is appropriate
to emphasize lessons learned from the root cause analysis of the inactivation
failure. The eectiveness of a safety program is highly dependent on the safety
culture at the institution—a strong safety culture with a proactive rather than a
reactive approach is a key safeguard in prevention of laboratory incidents.
Conclusion
Inactivation and verication procedures need to be tailored to the specic
procedural circumstances and based on a risk assessment. In-house testing is
recommended for all methodologies due to the wide variability in conditions at
dierent institutions; the inevitability of dierences in assay conditions, equipment
and/or reagent sources; and the varied conditions used for the dierent types
of inactivation procedures. Gaps in knowledge of inactivation and verication
methods mean there is often improvisation at the institutional level. One useful
way to ensure that information on eective inactivation and verication methods is
broadly shared with the scientic community is through inclusion of this important
data in the “Materials and Methods” sections of publications.
Novel inactivation methods that enable retention of desired agent characteristic(s)
are an area of active research in the eld of biosafety, but additional work is
needed. Advances in inactivation and verication procedures can improve safety
and security, enable reduction of the Biosafety Level used and the costs, and
allow forward movement in some valuable research projects that might otherwise
face obstacles.

501Appendix K—Inactivation and Verication
References
1. Federal Select Agent Program [Internet]. Atlanta and Riverdale: Centers
for Disease Control and Prevention, Division of Select Agents and Toxins
and Animal and Plant Health Inspection Services, Agriculture Select
Agent Services; c2017 [cited 2018 Dec 26]. Guidance on the Inactivation
or Removal of Select Agents and Toxins for Future Use. Available from:
https://www.selectagents.gov/resources/Inactivation_Guidance.pdf
2. Van Impe J, Smet C, Tiwari B, Greiner R, Ojha S, Stulić V, et al. State of
the art of nonthermal and thermal processing for inactivation of micro-
organisms. J Appl Microbiol. 2018;125(1):16–35.
3. Mbonimpa EG, Blatchley ER 3rd, Applegate B, Harper WF Jr. Ultraviolet
A and B wavelength-dependent inactivation of viruses and bacteria in the
water. J Water Health. 2018;16(5):796–806.
4. Farcet MR, Kreil TR. Zika virus is not thermostable: very eective virus
inactivation during heat treatment (pasteurization) of human serum albumin.
Transfusion. 2017;57(3pt2):797–801.
5. Spotts Whitney EA, Beatty ME, Taylor TH Jr, Weyant R, Sobel J, Arduino
MJ, et al. Inactivation of Bacillus anthracis spores. Emerg Infect Dis.
2003;9(6):623–7.
6. Cote CK, Buhr T, Bernhards CB, Bohmke MD, Calm AM, Esteban-Trexler JS,
et al. A Standard Method to Inactivate Bacillus anthracis Spores to Sterility
Using γ-Irradiation. Appl Environ Microbiol. 2018.
7. Elliott LH, McCormick JB, Johnson KM. Inactivation of Lassa, Marburg, and
Ebola viruses by gamma irradiation. J Clin Microbiol. 1982;16(4):704–8.
8. Vaidya V, Dhere R, Agnihotri S, Muley R, Patil S, Pawar A. Ultraviolet-C
irradiation for inactivation of viruses in foetal bovine serum. Vaccine.
2018;36(29):4215–21.
9. Blázquez E, Rodríguez C, Ródenas J, Pérez de Rozas A, Segalés J,
Pujols J, et al. Ultraviolet (UV-C) inactivation of Enterococcus faecium,
Salmonella choleraesuis and Salmonella Typhimurium in porcine plasma.
PLoS One. 2017;12(4):e0175289.
10. Vatansever F, Ferraresi C, de Sousa MV, Yin R, Rineh A, Sharma SK,
et al. Can biowarfare agents be defeated with light?. Virulence.
2013;4(8):796–825.
11. Blow JA, Dohm DJ, Negley DL, Mores CN. Virus inactivation by nucleic
acid extraction reagents. J Virol Methods. 2004;119(2):195–8.
12. Haddock E, Feldmann F, Feldmann H. Eective Chemical Inactivation of
Ebola Virus. Emerg Infect Dis. 2016;22(7):1292–4.
13. Rosenstierne MW, Karlberg H, Bragstad K, Lindegren G, Stoltz ML,
Salata C, et al. Rapid Bedside Inactivation of Ebola Virus for Safe Nucleic
Acid Tests. J Clin Microbiol. 2016;54(10):2521–9.
502 Biosafety in Microbiological and Biomedical Laboratories
14. Roberts PL, Lloyd D. Virus inactivation by protein denaturants used in
anity chromatography. Biologicals. 2007;35(4):343–7.
15. Hughson AG, Race B, Kraus A, Sangaré LR, Robins L, Groveman BR, et
al. Inactivation of Prions and Amyloid Seeds with Hypochlorous Acid. PLoS
Pathog: 2016;12(9):e1005914.
16. Rose LJ, Rice EW, Jensen B, Murga R, Peterson A, Donlan RM, et al.
Chlorine inactivation of bacterial bioterrorism agents. Appl Environ Microbiol.
2005;71(1):566–8.
17. Dembinski JL, Hungnes O, Hauge AG, Kristoersen AC, Haneberg B,
Mjaaland S. Hydrogen peroxide inactivation of inuenza virus preserves
antigenic structure and immunogenicity. J Virol Methods. 2014;207:232–7.
18. Sagripanti JL, Hülseweh B, Grote G, Voss L, Böhling K, Marschall HJ.
Microbial inactivation for safe and rapid diagnostics of infectious samples.
Appl Environ Microbiol. 2011;77(20):7289–95.
19. Schneider K, Wronka-Edwards L, Leggett-Embrey M, Walker E,
Sun P, Ondov B, et al. Psoralen Inactivation of Viruses: A Process
for the Safe Manipulation of Viral Antigen and Nucleic Acid. Viruses.
2015;7(11):5875–88.
20. Laughhunn A, Huang YS, Vanlandingham DL, Lanteri MC, Stassinopoulos A.
Inactivation of chikungunya virus in blood components treated with
amotosalen/ultraviolet A light or amustaline/glutathione. Transfusion.
2018;58(3):748–57.
21. Santa Maria F, Laughhunn A, Lanteri MC, Aubry M, Musso D,
Stassinopoulos A. Inactivation of Zika virus in platelet components using
amotosalen and ultraviolet A illumination. Transfusion. 2017;57(8):2016–25.
22. Nakano R, Ishiguro H, Yao Y, Kajioka J, Fujishima A, Sunada K, et al.
Photocatalytic inactivation of inuenza virus by titanium dioxide thin lm.
Photochem Photobiol Sci. 2012;11(8):1293–8.
23. Kashef N, Huang YY, Hamblin MR. Advances in antimicrobial photodynamic
inactivation at the nanoscale. Nanophotonics. 2017;6(5):853–79.
24. Takahashi H, Tsuchiya T, Takahashi M, Nakazawa M, Watanabe T,
Takeuchi A, et al. Viability of murine norovirus in salads and dressings
and its inactivation using heat-denatured lysozyme. Int J Food Microbiol.
2016;233:29–33.
25. Lambert MS. An update on alternatives to antibiotics–old and new
strategies. Appl Biosaf. 2011:16(3):184–7.
26. Takahashi M, Okakura Y, Takahashi H, Imamura M, Takeuchi A, Shidara H,
et al. Heat-denatured lysozyme could be a novel disinfectant for reducing
hepatitis A virus and murine norovirus on berry fruit. Int J Food Microbiol.
2018;266:104–8.

503Appendix K—Inactivation and Verication
27. Singh VP. Recent approaches in food bio-preservation—a review. Open
Vet J. 2018;8(1):104–11.
28. Tomat D, Casabonne C, Aquili V, Balagué C, Quiberoni A. Evaluation of
a novel cocktail of six lytic bacteriophages against Shiga toxin-producing
Escherichia coli in broth, milk and meat. Food Microbiol. 2018;76:434–42.
29. Gomes C, Moreira RG, Castell-Perez E. Microencapsulated antimicrobial
compounds as a means to enhance electron beam irradiation treatment
for inactivation of pathogens on fresh spinach leaves. J Food Sci.
2011;76(6):E479–88.
30. Chai C, Lee KS, Imm GS, Kim YS, Oh SW. Inactivation of Clostridium
dicile spore outgrowth by synergistic eects of nisin and lysozyme.
Can J Microbiol. 2017;63(7):638–43.
31. Joo HS, Fu CI, Otto M. Bacterial strategies of resistance to antimicrobial
peptides. Philos Trans R Soc Lond B Biol Sci. 2016;371(1695).
32. Baron C. Antivirulence drugs to target bacterial secretion systems.
Curr Opin Microbiol. 2010;13(1):100–5.
33. Paschos A, den Hartigh A, Smith MA, Atluri VL, Sivanesan D, Tsolis RM,
et al. An in vivo high-throughput screening approach targeting the type IV
secretion system component VirB8 identied inhibitors of Brucella abortus
2308 proliferation. Infect Immun. 2011;79(3):1033–43.
34. Sharifahmadian M, Arya T, Bessette B, Lecoq L, Ruediger E, Omichinski JG,
et al. Monomer-to-dimer transition of Brucella suis type IV secretion
system component VirB8 induces conformational changes. FEBS J.
2017;284(8):1218–32.
35. Langlois RA, Albrecht RA, Kimble B, Sutton T, Shapiro JS, Finch C, et al.
MicroRNA-based strategy to mitigate the risk of gain-of-function inuenza
studies. Nat Biotechnol. 2013;31(9):844–7.
36. Lambert MS. Safety overview of techniques involving miRNAs, siRNAs,
and other small regulatory RNAs. Appl Biosaf. 2009;14(3):150–2.
37. United States Department of Labor [Internet]. Washington (DC):
Occupational Safety and Health Administration; c2011 [cited 2018 Dec
27]. Laboratory Safety Guidance. Available from: https://www.osha.gov/
Publications/laboratory/OSHA3404laboratory-safety-guidance.pdf
504 Biosafety in Microbiological and Biomedical Laboratories
Appendix L—Sustainability
Introduction and Issues
Sustainability is the ability to satisfy current needs without depleting resources
needed for the future. The phrase “triple bottom line” (e.g., “people-planet-prot”)
is often associated with sustainability to explain the benets of balancing the
nancial bottom line with environmental and social goals in order to nd eective
solutions that can stand the test of time without compromising human health.
While safety remains of utmost importance in design and/or operation of a
laboratory, minimizing waste and safeguarding long-term human health through
protection of the environment is a high priority. Design, construction, and
operation of sustainable laboratories requires a holistic approach that considers
the interconnectedness of building systems. The project delivery process can
be optimized with an integrated design approach and by establishing multi-
disciplinary evaluation of issues regarding both the current uses and the
potential future uses of a building.
Laboratories consume more resources and energy per square foot than other
commercial buildings. Factors inuencing laboratory energy consumption include:
continuous operation, ventilation needs at exhaust devices, energy-intensive
and heat-generating equipment, and use of water for steam sterilization and
other processes. Furthermore, critical research and containment requirements in
laboratories often require electrical power system redundancy to remain fail-safe.
This appendix outlines potential opportunities to increase the eciency of the
laboratory portion of buildings to achieve energy and cost savings, decrease
pollution, and optimize material resource use. The appendix also highlights strat-
egies to improve indoor air quality and lighting in order to increase productivity,
improve worker comfort and well-being, and reduce maintenance issues related
to occupant comfort.
Strategies for Existing Laboratories and Operations
Sustainability approaches within laboratories usually focus on design and
construction of new facilities. However, improvements to operational and
management practices of existing laboratories can yield meaningful savings
and conserve material resources.
Commissioning
Commissioning, a process to verify systems are working as intended, has
demonstrated median savings of 15% in existing buildings; laboratories have
shown an average payback of retro-commissioning costs of one year or less.
1
Facility Managers might consider retro-commissioning, starting with an audit to
assess energy and water consumed in the laboratory. When auditing, include
505Appendix L—Sustainability
retro-commissioning of equipment when possible. Systematic evaluation of
equipment can identify problems that developed as equipment aged or as building
uses changed. For example, recalibrating a temperature sensor is inexpensive
but improves diagnostics and/or monitoring. Correcting a variable-frequency drive
motor controller that operates at an unnecessarily high-speed saves energy and
money over time without incurring signicant rst-cost.
Water and Energy Eciency
Evaluate measures to improve energy and water eciency in response to ndings
from the audit. Simple measures, such as upgrading to energy-ecient lighting
or implementing after-hours airow reduction (i.e., setbacks), can be taken.
Conserve water by adding shut-o sensors and clearly labeling xtures with
instructional signage for occupants.
Evaluating Energy Eciencies Using Audits
Develop a strategic approach prior to implementing the audit. Expand the audit
process to evaluate material waste and to determine the eectiveness of any
waste management strategies already in place. Follow the guidance in an
approved or appropriate document such as Document 203, Health Care Waste
Management Audit Procedures—Guidance, which was developed with the
support of the CDC.
2
1. Compare the percentage by weight of recyclable and non-recyclable
items to total waste to evaluate eectiveness of recycling strategies.
2. Identify and focus strategies to reduce major contributors to the waste
stream.
3. Donate unneeded, but functional, equipment instead of sending it to a
landll. Properly decommission and disinfect any potentially contami-
nated items prior to donating.
4. Evaluate recycling potential in terms of procurement goals. For example:
a. An audit in a non-containment laboratory showing an abundance
of PPE gloves could lead to a procurement preference for nitrile
gloves since nitrile gloves not used with infectious materials are
potentially recyclable.
b. Establish purchasing guidelines to dene minimum or recom-
mended amounts of recycled plastic in conical centrifuge tubes.
c. Purchase reusable autoclavable reagent reservoirs, where
feasible, to reduce plastic waste.
5. Include vivaria in waste inventories. Consider the following where
appropriate:
a. Compost non-infectious bedding and discarded feed instead of
landlling or incinerating it.
b. Change cage bedding based on use or ammonia level vs. on a
schedule.
506 Biosafety in Microbiological and Biomedical Laboratories
Energy Use in Laboratories and Potential Initiatives
Plug-in equipment such as autoclaves, centrifuges, and freezers account for up
to half of the energy used in a typical laboratory. In addition to generating heat
during operation, freezers consume a signicant portion of that energy demand.
Consider creating an internal competition or participating in the International
Laboratory Freezer Challenge, a competition designed to promote sample
integrity and reduce costs and energy.
3
Implement the best practices outlined in
the Challenge’s protocol: clean refrigerant coils to optimize performance; create
searchable inventories to shorten the time freezer doors are open and reduce
time spent locating samples; and reset Ultra-Low Temperature (ULT) Freezers
from -80ºC to -70ºC to reduce energy consumption without having a discernible
impact on temperature stability.
4
If equipment needs replacement, opt for more
ecient models. See 3. Strategies for New and Renovated Laboratories, below,
for recommendations.
Identify areas of potential ineciencies related to occupant behavior in laboratory
areas. For example:
1. Explore the impact of shutting chemical fume hoods using variable air
volume controls when not in use. Harvard University implemented a
“Shut the Sash” Program, which calculated utility savings of $200,000–
$250,000 per year in the Department of Chemistry and Chemical Biology
(houses 278 chemical fume hoods).
5
2. Turn o autoclaves (except for constant-bleed autoclaves or those that
are equipped with a sleep mode) at night and over weekends.
3. Forgo the drying stage in tunnel washers for Vivarium cages and allow
cleaned cages to air-dry.
Good practices emphasize laboratory-specic operations and control strategies
while better practices improve the ventilation design process with advanced
computer or physical modeling techniques.
6
Most energy use in laboratories is related to ventilation. Use tracer gas tests
following the ASHRAE Laboratory Design Guide to calculate the air-changes per
hour in an existing laboratory. Conduct airow simulations to evaluate scenarios
regarding spills or aerosols to reveal opportunities for improvement in ventilation
component eciency. Introduce neutrally buoyant helium-lled soap bubbles to
a space to provide a visual evaluation of laboratory airow. As the bubbles reach
room temperature, they follow tiny air currents.
Develop “Green Chemistry” initiatives and protocols to reduce chemical waste
at the source. Reduce or eliminate the use of hazardous chemical reagents,
solvents, and products to save space and water while reducing hazardous waste
and carbon dioxide releases. Understand the toxicology of chemicals in use
as well as the principles of Green Chemistry outlined by the EPA.
7
Conduct an
507Appendix L—Sustainability
inventory of hazardous chemicals in use and develop a systematic process to
reduce or eliminate those chemicals using alternate methods or replacing them.
Explore databases regarding alternative methods and alternative chemicals
such as the “Green” Alternatives Wizard, which is a searchable online database
developed by the Massachusetts Institute of Technology (MIT).
8
Try to use
chemicals that are less toxic, biodegradable after use, do not deplete ozone, and/
or do not form smog. Consider less hazardous chemical alternatives, such as the
use of uorous solvents instead of chlorinated ones.
Eliminate chemicals when feasible. Allow glass to dry instead of using acetone.
Avoid use of reaction solvents if crushing solids together will suce.
In addition to the strategies above, consider use of general operational and
maintenance guidance provided in well-established green building rating
systems.
9–13
Strategies for New and Renovated Laboratories
A sustainable design approach should result in a project with improved utility of
spaces, enhanced occupant comfort and well-being, right-sizing of equipment,
and protection of the environment.
Pre-Design
In terms of sustainability, the most critical activity in laboratory planning begins
before the design phase. The goal of pre-design activities is to provide infor-
mation necessary for a design team to develop a robust programming document,
which is the cornerstone of a sustainable, high-performance building.
Dene design intent by developing an Owner’s Project Requirements (OPR)
document. Identify performance requirements from the perspective of stake-
holders including the researchers, directors, technicians, operators, community,
and any other parties that will be aected by the outcome of the laboratory
design. Carefully outline the stakeholders’ specic requirements for the proposed
use of each space. Dierentiate between an actual requirement and a wish-list.
In addition to addressing aspects of safety requirements, dene the requirements
and base assumptions about the use of the laboratories and other spaces.
Include the hours and conditions when a space is likely to be occupied, partially
occupied, or unoccupied. Identify areas where worker schedules are most
predictable. This will allow coordination to evaluate lighting or other system
controls that may be shut o or adjusted automatically to save energy. Comment
on the acceptable time-period for system start-ups during unanticipated or
emergency use. Include considerations for potential changes in laboratory
uses or sizes over time. This enables a design team to explore the possible
impact on support utilities such as supply and exhaust of air as well as various
508 Biosafety in Microbiological and Biomedical Laboratories
congurations of laboratory benches/casework. Establish goals for energy and
water eciency. Include comments on how success in meeting those goals will be
measured. Identify laboratories that do not need a narrow range of humidity and/
or thermal control. Laboratories for the 21st Century
14
estimates that too narrow
a range of acceptable humidity can increase energy use by as much as 25%.
Identify spaces where daylight is appropriate and does not hinder the proposed
research. This enhances workers’ well-being and reduces the need for articial
illumination during the daytime.
Design
Engage a design team with proven experience in designing sustainable
laboratories. Require an “Integrative Process” meeting to be attended by key
laboratory personnel, facility managers, and as many members of the design
team as feasible. This meeting will support development of a formal program for
use by the design team as they develop design and construction documents.
At the meeting, collectively review the OPR described above. Have attendees
discuss their concerns and strategies for all primary objectives stated in the OPR.
Establish a protocol that requires consideration of multiple factors in addition to
safety. This includes life-cycle cost, exibility, site conditions, indoor environment,
environmental impact, renewable energy, and the ecient use of water, energy,
and materials. Determine how success of meeting the OPR will be measured at
each subsequent phase of the project.
Sustainable Design Strategies
Renovation or construction of new laboratories should avoid automatic repli-
cation of solutions from other laboratories. Solutions should be customized
but adaptable. Stakeholders may benet by becoming generally familiar
with laboratory construction recommendations that incorporate sustainability
topics.
15–18
Acoustics Specic equipment and activities in each laboratory may impact
communication and create noise that, if unaddressed, can increase occupant
fatigue. A laboratory space with noisy equipment (e.g., fume hood) should not be
designed with the same noise criterion (NC) as a dry, computational space or a
classroom.
19,20
Articial Lighting Eciency and Quality Moderate levels of acceptable,
ambient (i.e., general) lighting combined with task lighting (where specically
needed) are key components to ecient and eective lighting design. When
looking to save energy, use automatic shut-o or dim ambient lighting in spaces
or zones where schedules are predictable. The intensity and color of light as well
as the contrast level between lit surfaces will impact the workers’ visual comfort.
Lighting built into a fume hood or biosafety cabinet can be coordinated with the
color of ambient lighting to enhance that visual comfort.

509Appendix L—Sustainability
Flexible laboratory bay congurations requiring workbench mobility require
consideration regarding bench-mounted task lighting as well as the reduced
lighting level that may result when a bench has been moved away. Consideration
should be given to the chemicals in use near heat-generating, under-cabinet task
lights.
Evaluate the lighting aspects of laboratory bench conguration mock-ups.
Mock-ups should include the proposed color(s) for the work surface, a portion of
proposed ceiling, and any major ceiling elements (such as an air diuser) that
may impact the perception of light levels or visual contrast.
For additional information on New Buildings Institute Advanced Lighting
Guidelines (AGL Online), please visit https://newbuildings.org/resource/
advanced-lighting-guidelines. For additional information on the Illuminating
Engineering Society, please visit https://www.ies.org/, https://www.ihs.com/
products/iesna-standards.html or refer to the NIH Design Requirements Manual.
15
Automated Energy Monitoring and Control System (EMCS) Projects including
an EMCS can track the details of energy consumption and performance through
sub-meters that relay information to the EMCS. Loads for HVAC (heating, venti-
lation, and air-conditioning), lighting, and plug-in equipment should be monitored
separately, as should large loads like those for chillers.
Dynamic or demand control may be useful when a laboratory’s Biosafety Level
classication is low and chemical hazards are also low, based on risk assess-
ments. The control reduces air-change rates when sensors indicate good air
quality. Air quality is typically determined by establishing maximum thresholds of
total volatile organic compounds (TVOC) and small particulates.
Biophilia Biophilia suggests that humans have an instinctive aliation with
nature and other living systems. It can be used as a design strategy. Provide
visual connections to symbolic foliage, organic forms, and sunlight to foster
psychological well-being and cognitive function.
21
Chilled Beams Chilled beams are appropriate for laboratories without a high
density of fume hoods or for laboratories that do not require a high rate of airow
changes. They minimize energy used for tempering air by separating the heating
and cooling functions from the ventilation. The “beam” contains elements for
sensible cooling using cold water (with a temperature above the dew point) that
circulates through coils. Ventilation is provided by parallel elements tied to a
central air handling system. The air-temperature required to condition the space
with either the greatest heating or the greatest cooling load drives the design.
These systems require additional piping and are likely to incur more initial cost,
but they ultimately save money due to signicantly smaller central air-handling
510 Biosafety in Microbiological and Biomedical Laboratories
systems and ducts. There are currently limited data regarding the use of chilled
beam technology in high containment laboratories.
Commissioning See Strategies for Existing Laboratories and Operations, above,
for more information regarding Commissioning. Also see the ANSI Z9.
14
Standard,
Testing and Performance-Verication Methodologies for Ventilation Systems for
Biosafety Level 3 (BSL-3) and Animal Biosafety Level 3 (ABSL-3) Facilities.
22
Daylight and Glare Control Natural daylight is an ecient lighting source and
enhances occupant well-being. Design elements and devices to control and
prevent glare are critical to worker comfort. This should increase energy savings
through reduction of heat gain. Fortunately, numerous options are available for
new spaces. Options may include:
1. Interior sun shading devices, such as blinds or shades, outside of
laboratory space;
2. Exterior sun shading, which may be xed or can be automated to adjust
in response to time of day or sun angle; and
3. Glass that is fritted or coated with lm or that changes transparency
through electrochromic or thermodynamic properties. Note that this
glazing can also be specied with features that reduce bird collision.
Energy Recovery Transfer of heat energy generated in one space or system
to another space or system can save substantial amounts of energy and allow
for smaller, less costly heating and cooling systems. Enthalpy wheels, heat
pipes, and run-around loops, which transfer heat across air streams, should be
considered; concerns regarding odor, biological, and chemical contamination may
preclude their use. It should be noted that the heated air must be directed towards
the laboratories where the exhaust air came from to minimize the potential for any
cross-contamination in the event of a leak within the transfer system.
Evaluate energy recovery from common systems that serve laboratories with
varying (low and high) loads during operation. Heated air from laboratories with
heat-generating equipment and occupants can be used to pre-heat a space that
is too cool. Additional space may be required for some recovery systems, such
as heat pipe systems or rotary exchangers (e.g., enthalpy or desiccant wheels).
Exhaust Review energy eciency and exibility when evaluating fume hoods.
For BSL-1 and BSL-2 laboratories, consider allowing manifold exhaust.
Conducting a Computational Fluid-Dynamics (CFD) Model will evaluate airow
patterns. These performance-based simulations can be used to evaluate
safety and optimize airow in a given scenario (e.g., the time needed to clear
a chemical).
511Appendix L—Sustainability
Flexibility A building that is designed to be exible will accommodate future
needs without radical renovation; this could save material resources and funds.
The Whole Building Design Guide, a web-based portal with up-to-date information
on planning and designing research laboratories, provides recommendations for
incorporating exibility into laboratory design.
23
Passageway and doorway width
should be designed to accommodate larger equipment than originally scheduled,
such as autoclaves and cage racks. Provide wide pathways between loading
docks and locations for large equipment. Vertical expansion to accommodate
additional fume hoods should be considered.
Greywater Reuse Non-potable (e.g., greywater) is water that has not come
into contact with sewage, biological agents, radioisotopes, or toxic chemicals.
Greywater may be reused outside of the laboratory for functions, such as
toilet-ushing or landscape irrigation. Polished water (i.e., salts or microscopic
particulates are removed) resulting from laboratory processes is a potential
source of reusable water.
Ventilation The profound impact of ventilation on energy use makes evaluation
of the appropriate number of air-changes in each laboratory critical. Do not
automatically replicate design or air-changes from similar projects. Balance safety
and energy concerns by allowing designs for spaces with less stringent safety
classications to have fewer air-changes.
In addition to the design considerations noted above, review specications
for the proposed equipment in terms of energy and water eciency. Consider
giving preference to laboratory-grade refrigerators and/or freezers and
ultra-low temperature (ULT) freezers that do not exceed the maximum energy
consumption; the EPA’s Energy Star program provides such specications.
24
Freezer selection in new or renovated laboratories typically has the largest impact
on energy consumption of any single equipment group other than those related
to ventilation. Give preference to ULT freezers that use natural refrigerants and
vacuum-insulated panels. Note that an energy-ecient ULT operating at -80ºC
uses more energy than at -70ºC.
Additional items to consider:
1. Evaluate specifying autoclaves that use less water in the cooling
process, typically through regulation, sometimes via facility-chilled water
loop when chiller capacity allows.
2. Add a system to cool euent in retrot situations.
3. Specify water and energy-ecient vivarium cage washers.
a. Use nal rinse water for the initial cycle and incorporate heat
exchangers to recapture heat from overow rinse water in order
to reduce overall steam and cold water consumption.

512 Biosafety in Microbiological and Biomedical Laboratories
4. Incorporate a recirculation system that pumps water back to the vacuum
system of the autoclave.
a. Recirculation systems and some heat exchange systems with
improved autoclave functions can require more space.
References
1. Mills E, Bourassa N, Pipette MA, Friedman H, Haasl T, Powell T, et al.
The Cost-Eectiveness of Commissioning New and Existing Commercial
Buildings: Lessons from 224 Buildings. In: National Conference on Building
Commissioning; 2005 May 4–6. p. 1–22.
2. Health Care Without Harm [Internet]. Health Care Waste Management
Audit Procedures—Guidance; c2018 [cited 2018 Dec 14]. Document
203. Available from: https://noharm-global.org/documents/
health-care-waste-management-audit-procedures-guidance
3. freezerchallenge.org [Internet]. International Laboratory Freezer Challenge;
c2018 [cited 2018 Dec 14]. Available from: https://www.freezerchallenge.org/
4. Emerging Technologies Coordinating Council [Internet]. Ultra-Low
Temperature Freezers: Opening the Door to Energy Savings in Laboratories;
c2016 [cited 2018 Dec 14]. Available from: https://www.etcc-ca.com/reports/
ultra-low-temperature-freezers-opening-door-energy-savings-laboratories
5. Harvard University [Internet]. Cambridge (MA): Shut the Sash Program;
c2018 [cited 2018 Dec 14]. Validating Cost and Energy Savings from
Harvard’s Shut the Sash Program: Tackling Energy Use in Labs. Available
from: https://green.harvard.edu/programs/green-labs/shut-sash-program
6. Bell GC. Laboratories for the 21st Century: Best Practice Guide. Optimizing
Laboratory Ventilation Rates. Washington (DC): U.S. Environmental
Protection Agency; 2008.
7. U.S. Environmental Protection Agency [Internet]. Washington (DC): Green
Chemistry; c2017 [cited 2018 Dec 14]. Basics of Green Chemistry. Available
from: https://www.epa.gov/greenchemistry/basics-green-chemistry#twelve
8. ehs.mit.edu/greenchem [Internet]. “Green” Alternatives Wizard; c2018 [cited
2018 Dec 14]. Available from: http://ehs.mit.edu/greenchem/
9. BREEAM
®
[Internet]. San Francisco (CA): Refurbishment and Fit-Out
Technical Standard; c2018 [cited 2018 Dec 17]. Available from: https://www.
breeam.com/refurbishment-and-t-out
10. BREEAM
®
[Internet]. San Francisco (CA): New Construction Technical
Standards; c2018 [cited 2018 Dec 17]. Available from: https://www.breeam.
com/new-construction
11. LEED Reference Guide for Building Design and Construction. Washington
(DC): U.S. Green Building Council; 2013.

513Appendix L—Sustainability
12. LEED Reference Guide for Building Operations and Maintenance.
Washington (DC): U.S. Green Building Council; 2013.
13. International WELL Building Institute. The WELL Building Standard
®
.
Version 1.0. New York: Delos Living, LLC; 2014.
14. Langerich S, Lilly E, et al. Laboratories for the 21st Century: Best Practice
Guide. Commissioning Ventilated Containment Systems in the Laboratory.
Washington (DC): U.S. Environmental Protection Agency; 2008.
15. National Institutes of Health [Internet]. Bethesda (MD): Oce of Research
Facilities; c2018 [cited 2018 Dec 17]. Design Requirements Manual.
Available from: https://www.orf.od.nih.gov/PoliciesAndGuidelines/
BiomedicalandAnimalResearchFacilitiesDesignPoliciesandGuidelines/
Pages/DesignRequirementsManual2016.aspx
16. International Institute for Sustainable Laboratories [Internet]. Washington
(DC): U.S. Environmental Protection Agency, Department of Energy; c2018
[cited 2018 Dec 21]. Labs21 Tool Kit. Available from: http://www.i2sl.org/
resources/toolkit.html
17. wbdg.org [Internet]. Washington (DC): Whole Building Design Guide; c2018
[cited 2018 Dec 17]. Available from: http://www.wbdg.org/
18. Energy.gov [Internet]. Washington (DC): Oce of Energy Eciency
& Renewable Energy; c2018 [cited 2018 Dec 17]. Federal Energy
Management Program. Laboratories. Available from: https://www.energy.
gov/eere/femp/energy-eciency-laboratories
19. Acoustical Performance Criteria, Design Requirements, and Guidelines
for Schools, Part 1: Permanent Schools. ANSI/ASA S12.60-2010.
Acoustical Society of America. Accessed 2018 Dec 17: https://
successforkidswithhearingloss.com/wp-content/uploads/2012/01/
ANSI-ASA_S12.60-2010_PART_1_with_2011_sponsor_page.pdf
20. Lab Manager [Internet]. Canada: LabX; c2018 [cited 2018 Dec 17].
Laboratory Acoustics. Available from: https://www.labmanager.com/
lab-design-and-furnishings/2011/11/laboratory-acoustics#
21. Terrapin Bright Green [Internet]. New York: Terrapin Bright Green, LLC;
c2014 [cited 2018 Dec 17]. 14 Patterns of Biophilic Design: Improving
Health & Well-Being in the Built Environment. Available from: https://www.
terrapinbrightgreen.com/reports/14-patterns
22. Testing and Performance-Verication Methodologies for Ventilation Systems
for Biosafety Level 3 (BSL-3) and Animal Biosafety Level 3 (ABSL-3)
Facilities, ANSI/ASSE Z9.14 (2014).
23. Whole Building Design Guide [Internet]. Washington (DC): Design
Recommendations; c2017 [cited 2018 Dec 17]. Research Laboratory.
Available from: https://www.wbdg.org/building-types/research-facilities/
research-laboratory

514 Biosafety in Microbiological and Biomedical Laboratories
24. Energy Star [Internet]. Washington (DC): U.S. Environmental Protection
Agency, Department of Energy; c2016 [cited 2018 Dec 17]. ENERGY STAR
®
Program Requirements for Laboratory Grade Refrigerators and Freezers.
Available from: https://www.energystar.gov/sites/default/les/ENERGY%20
STAR%20V1.1%20Lab%20Grade%20Refrigerator%20and%20Freezer%20
Program%20Requirements.pdf

515Appendix M—Large-Scale Biosafety
Appendix M—Large-Scale Biosafety
Introduction
When working with biological agents in large-scale quantities, there are
unique considerations that must be addressed in order to ensure worker and
environmental protection. Large-scale biological production facilities should use
the laboratory scale principles of risk assessment set forth in BMBL Section II,
and by ISO 35001, Biorisk Management for Laboratories and Other Related
Organizations.
In addition to laboratory scale risk assessment requirements, the utilization
of larger equipment and volumes of chemicals or raw materials requires risk
management strategies beyond biological safety alone. The following sections
apply risk management steps to give readers the most pertinent information for
managing risk in large-scale production. The recommendations assume that
those performing risk assessments for large-scale work will involve industrial
hygienists and other process safety specialists when implementing risk
assessment and control measures for large-scale operations.
Appendix K of NIH Guidelines for Research Involving Recombinant or Synthetic
Nucleic Acid Molecules (NIH Guidelines) prescribes safety practices and
containment procedures for large-scale (i.e., >10 liters per container) facilities.
These guidelines can be applied to all large-scale work with biological materials
(e.g., genetically modied organisms [GMO] and non-GMO, human, and animal/
zoonotic pathogens). Please ensure familiarity with local regulations as these
may dier from recommendations in this text.
Risk Assessment
Integrate the steps and processes utilized in laboratory biological risk assessment
for any large-scale project. Risk assessment should be done during planning,
when elements of the process change, and during periodic reviews of existing
biological production processes, particularly after incidents or process failures.
Risk control measures must be installed to mitigate unacceptable risk. Systems
must be evaluated to determine their contribution to risk. The Good Practice
quality guidelines and regulations (GxP) include three commonly used GxPs:
Good Clinical Practices (GCP), Good Laboratory Practices (GLP),
1
and Good
Manufacturing Practices (GMP);
2
GxP product Impact Assessment (IA) analysis
can be extended to evaluate biosafety and laboratory biosecurity-related systems
that govern exposure control, process room and environmental protection,
decontamination, access control and accountability. Risk assessments should
focus on the biological, chemical, physical, product, and equipment biosafety and
laboratory biosecurity risk points. Production technologies and equipment with the
potential for misuse (laboratory biosecurity/dual-use/export control) may also be
516 Biosafety in Microbiological and Biomedical Laboratories
included in the risk assessment. Subject matter experts in engineering; Heating,
Ventilation, and Air Conditioning (HVAC); quality control; occupational health;
security; and health, safety, and environment (HSE) should always be consulted
when making risk-based determinations.
Hazard Identication
The rst step of risk assessment is hazard identication. Review additional factors
that are unique to large-scale biological processes. Additional factors include but
are not limited to:
1. Unique strains utilized primarily for research or manufacturing processes
(e.g., producing high titers of a toxin);
2. High volumes (>10 liters) and high concentrations of product;
3. Specialized equipment and processes with unique risk points require a
Hazard Analysis of Critical Control Points and/or Hazard and Operability
studies;
4. Pressurized vessels and lines for biological and chemical reactions
pose a risk for aerosol generation (e.g., bioreactors, fermenters, thermal
inactivation tanks); and
5. Atypical routes of transmission (e.g., inhalation of biological agents or
toxins not normally transmitted via the aerosol route).
Non-biological hazards to consider when performing a risk assessment may
include, but are not limited to:
1. Hazardous chemicals: formaldehyde or similar for inactivation, large
quantities of detergents, disinfectants and caustics, adjuvants, preser-
vatives, solvents for down-stream processing, allergens or toxins, and
asphyxiants;
2. Physical hazards: noise, steam, heat, cold, and radiation including UV
and lasers;
3. Life-safety hazards: conned space, working at heights, line breaking,
and pressurized systems;
4. Ergonomics;
5. Process safety-relevant controls (e.g., re/explosions; pressurized
systems);
6. Preventative maintenance (PM): solid and process euent waste
streams and control measures employed, including PM of relevant
equipment;
7. Processes to control release of material (i.e., human and environmental
risks), including corresponding emergency procedures; and
8. Risk points associated with equipment.
517Appendix M—Large-Scale Biosafety
Hazard Evaluation
As with laboratory risk assessment, the hazards associated with the biological
agent/material and process equipment must be evaluated. In addition, the
operational integrity of containment equipment and facility safeguards and the
capability of area sta to eectively control potential hazards must be considered.
Sta capability will depend on the training, technical prociency, and good habits
of all team members.
Large-scale research and production pose additional risks that require evaluation.
Increased growth, vessel size, and enhanced aeration magnify the aerosol
generation risk. By design, the biological agent concentration is greatly increased.
Therefore, protection from aerosol transmission must be considered for agents
normally transmitted by insect bite or injection.
Chemical risks are also increased due to handling of dry powders for media
preparation, pumping of acid or base for pH control, and preparation/addition of
inactivation chemicals for vaccine preparation. Closed system transfer technology
may be foreign to those with experience limited to the laboratory.
Risks due to hazardous energy (i.e., electrical, steam, pressurized gases) are
also magnied. Hazardous energy control procedures such as removing the
power cord or closing a supply valve become complex and may be poorly under-
stood by those with experience limited to the laboratory.
Risk Control
Risk mitigation strategies identied in large-scale research and production follow
the same principles (i.e., hierarchy of controls) established to control HSE risks.
3
Those performing risk assessments for large-scale work may be able to eliminate
a hazard or substitute to reduce risk. When this is not possible, engineering,
administrative and/or work practice controls, and PPE are utilized.
Engineering Controls
Selecting the proper engineering solution is an iterative process.
4,5
The design
provisions for a large-scale biological production facility will dier greatly
depending on whether the work is dealing with an exotic, indigenous, eradicated,
novel, or emerging disease-causing agent; a highly allergenic compound; a GMO,
carcinogenic or highly toxic product; or a well-characterized and attenuated
childhood vaccine.
Many controls must be considered in the process, including HSE-risk, biosafety,
and laboratory biosecurity. In addition, large-scale GxP facilities must evaluate
quality design controls for product as well as personnel and environmental
protection. Consider state and local regulations when implementing the design of
518 Biosafety in Microbiological and Biomedical Laboratories
a large-scale biological production facility. A large-scale facility balancing GxP and
biosafety requirements will need to evaluate the following basic facility principles:
Clean to Dirty The process design must include controls to prevent contam-
ination spread within the facility and to the environment. If applicable, an
assessment of conicts between GxP and biosafety requirements must also
occur to achieve two dierent denitions of clean. If there are two competing
requirements, implement controls that address the highest consequence events
and identify alternate methods to meet the intent of the competing requirement.
For example, if an operation requires positive-pressure environment to achieve
product protection, you can create an air pressure sink in an anteroom to ensure
containment of the biological agent.
Change Rooms and Barriers Establish donning and dong needs by creating
an operational ow diagram. This will help clarify how many actions an operator
must take for a given procedure or process step when passing through a
personnel barrier or door. The review should cover normal operations, planned
and unplanned maintenance, and emergencies. This process should identify the
potential demand in PPE for the facility, the number and locations of room(s), and
room size(s) necessary for storing PPE and changing. Facilities covered by GxP
requirements must consider PPE and workow requirements to achieve product
protection in addition to personnel and environmental protection.
Airlocks and high/low-risk rooms (i.e., biologicals vs. cleanrooms) The
design must address biosafety concerns as well as applicable GxP requirements
to achieve personnel, environment, and product protection, if required.
Surfaces Floor, wall and ceiling, door and window, and other exposed component
surfaces must be impervious and easy to clean. The materials must be resistant
to a host of chemicals including liquid and gaseous disinfectants, if needed, for
decontamination or prevention of cross-contamination. Construction attributes of
oor strength, ceiling height, segregation need, piping (i.e., materials, product,
and waste) and energy lines must support and promote large-scale processes.
HVAC system, room pressure, and airow The design of the airow must
provide personnel and environmental protection. In the event a process area
must be positive-pressure, consider designing the room airlock or changing
area as a pressure sink. Exhaust air ltering systems may be required, as in the
case of vaccine plants producing live attenuated vaccines, to prevent ductwork
contamination. GxP requirements may also require product protection design
considerations.
Gaseous Decontamination The HVAC system, walls, and wall penetrations must
be made such that the room can be decontaminated without a negative impact
to adjacent spaces. The decontaminant employed must be appropriate for the
519Appendix M—Large-Scale Biosafety
process and biological agents handled. Use the same principles for gaseous
decontamination of a laboratory, but the quantities used and the clearing times
will dier substantially.
Spill Containment When designing for spill containment, consider the biological,
chemical, and physical processes in an area. Always review spill scenarios while
designing a facility. Identify what and how much can be released, where spilled
materials will ow (e.g., are there drains leading to an euent decontamination
system (EDS) or will materials released be captured within a containment dike), if
manual inactivation will be required, and what emergency response activities will
encompass.
Kill Tanks/EDS Systems Ensure EDS systems can inactivate euent from
production waste and spills. It is particularly benecial to have a facility designed
with secondary failsafe systems when large amounts of material are processed.
The exact method used will depend on local regulations and the materials in
question. Numerous options exist, including chemical inactivation using acids or
caustics, and heat inactivation (batch or continuous). Ensure holding tanks have
stirrers when volumes are large. Most facilities employ hard piping, and a process
to clean and decontaminate these lines between production areas and the EDS
must be integrated into the plan.
Those performing risk assessments for large-scale work will also determine
the type of equipment to be used by considering production needs and risk
assessment results.
6
Historically, the standard has been xed equipment (i.e.,
stainless steel bioreactors) with a combination of hard and exible hose piping
for upstream (i.e., biological agent propagation) and downstream (i.e., biological
agent purication, concentration, and potentially inactivation) processes.
Increasingly, single-use (SU) equipment is replacing xed equipment for upstream
processes. The “ballroom” concept, where both upstream and downstream
processes are in one large production facility, is now accepted for select
biological processes.
7
The ballroom concept relies on maintaining closed systems
at all times.
1. Ballroom Layout Advantages
a. More exibility to accommodate dierent process trains;
b. Improved operational eciency and oversight (e.g., avoids
having to move equipment between rooms); and
c. Reduction of footprints and cost.
2. Ballroom Layout Disadvantages
a. Increased risk of contamination spread in upset conditions to
downstream processes;
b. Need for typically open operations (e.g., cell expansion, column
packing or powder addition) to be handled in closed systems;
520 Biosafety in Microbiological and Biomedical Laboratories
c. Need for enhanced environmental monitoring to be conducted
to detect a breach in any closed system and need to ensure
contamination or cross-contamination has not occurred; and
d. Challenging area and equipment decontamination when
production areas are shared.
A non-comprehensive list of containment requirements and associated risk
points is provided below to assist in the assessment of risks associated with SU
equipment.
Containment Requirements and Example Risk Points
7–10
1. Viable organisms should be handled in a closed system or other primary
containment.
a. Ensure the bioreactor bag is compatible with maximum output
temperature of heating control circuit;
b. Ensure the tubing is compatible with process media, including
pH control solutions and stability testing has been performed;
and
c. Implement procedures to ensure that probes are not removed
during operation.
2. Culture uids are not removed from a system until organisms are
inactivated.
a. Implement procedures for removing bioreactor bag(s) containing
infectious agent(s).
3. Inactivation of waste solutions and materials with respect to their
biohazard potential.
a. Implement procedures for processing used bioreactor bags
containing infectious agents;
b. Ensure presence of biosafety cabinet for removing reusable
components before destruction;
c. Ensure the waste disposal procedure compatible with
bioreactor bags;
d. Implement a procedure for safely autoclaving used bag;
e. Implement a procedure for safe packing and transport to
incinerator if the used bag will be directly incinerated; and
f. Ensure the incinerator facility can burn large quantities of
silicone tubing and bag lm.
4. Control of aerosols by engineering or procedural controls to prevent or
minimize release of organisms.
a. Implement controls to prevent bioreactor bag overlling during
additions;
b. Ensure proper procedure for tubing welding;
c. Ensure proper procedure for tube weld integrity test;
521Appendix M—Large-Scale Biosafety
d. Ensure regular PM of tubing welders to prevent misalignment; and
e. Ensure that plastic quick connectors (non-steamable) release
viable organism(s) when released.
5. Treatment of exhaust gases from a closed system to minimize or prevent
release of viable organisms.
a. Consider exhaust gas ltration;
b. Consider controls of exhaust lter clogging with foam and
humidity; and
c. Ensure there is an exhaust lter holder positioned to encourage
condensate drainage.
6. Closed system that has contained viable organisms not opened until
sterilized by a validated procedure.
a. Ensure the bioreactor bag is compatible with inactivation
chemical.
7. Closed system to be maintained at as low a pressure as possible to
maintain integrity of containment features.
a. Implement a process safety management study of gas overlay
and sparging system to determine susceptibility to overpressure,
including post-power failure;
b. Ensure bag installation procedures to prevent damage;
c. Ensure pressure control to limit aeration and overlay pressure;
d. Ensure the pressure alarms are interlocked to the gas supply;
e. Ensure pressure relief devices are installed on gas supplies and
properly sized;
f. Consider installing in-line pressure relief before the bioreactor to
protect against gas regulator failure; and
g. Ensure the gas supply valves fail closed upon power interruption.
8. Rotating seals and other penetrations into closed system designed to
prevent or minimize leakage.
a. Consider magnetic couplings to eliminate rotary seals;
b. Implement procedures to ensure stirrer operates during pre-use
integrity test;
c. Ensure rotary seals engineered to prevent infectious agent
release; and
d. Consider that over-speed may result in decoupling and in-bag
rupture.
9. Closed system shall incorporate monitoring or sensing devices to
monitor the integrity of containment.
a. Consider bioreactor bag pressure logging;
b. Ensure that loss of pressure (low-pressure alarm) results in
sparge/overlay shutdown; and
522 Biosafety in Microbiological and Biomedical Laboratories
c. Ensure that the sensors respond quickly enough to pressure
changes.
10. Validated integrity testing of the closed containment system.
a. Consider integrity test procedures pre-inoculation.
11. Emergency plans required for handling large losses of cultures.
a. Implement a leak detection system for bottom- or side-mounted
probes;
b. Consider bottom- or side-mounted sensors guarded to prevent
impact damage;
c. Consider respiratory PPE as part of operating PPE or ensure
respiratory PPE availability for emergency cleanup;
d. Ensure a contaminated worker emergency procedure available;
e. Ensure a large spill clean-up procedure available, including a
spill kit;
f. Ensure personnel trained in large-scale clean-up of infectious
organisms; and
g. Consider gas decontamination of production suite post-incident.
12. Requirements for controlled access area.
a. Ensure aerosol-containment within skid (i.e., process module);
b. Consider a spill containment pan to contain or divert entire
bioreactor contents for inactivation;
c. Ensure the pan will divert a worst-case leak scenario to biowaste
without spill to the oor;
d. Consider spill containment within the suite (dike, bund, raised
door threshold) to contain entire bioreactor contents for
inactivation;
e. Ensure the suite exhaust HEPA ltration for uid transfers
outside bioreactor containment; and
f. Ensure the suite is designed to prevent the release of infectious
aerosols using dierential pressure and sealing of room
penetrations.
Those performing risk assessments for large-scale work will also need to review
equipment types and assist in the evaluation of the choice that will best balance
the needs of GxP and biosafety. These equipment types include:
Pumps and Pipes The type of piping used will depend on how the process is
laid out. Hard piping will need clean-in-place (CIP) and sterilization-in-place
(SIP) for both GxP and biosafety reasons. Soft hoses allow for quick changes
and cleaning. The type of pump will have to meet the volume demands of
production. Peristaltic pumps are often used in combination with soft hoses.
The risk assessment must show what type of piping and pump to use to meet
523Appendix M—Large-Scale Biosafety
GxP (if applicable), biosafety, and general HSE demands. Make sure that points
where pipes penetrate walls are correctly sealed to promote safe gaseous
decontamination. Additionally, pump operation should be evaluated for hearing
protection implementation.
Compressed Air and Gases Compressed air is one means of transferring
uids between vessels. The safety review will identify elevated pressure points,
type of relief valve protection required, and rupture disc failure scenarios. Some
processes require asphyxiants, such as CO
2
or N
2
, and safety measures are to be
established to mitigate associated risk.
Electrical Power Power should be installed in a manner that prevents water
ingress in all production and failure modes. Planning and construction must follow
local electrical codes and the Occupational Safety and Health Administration
electrical standards. Large xed equipment fermenters and equipment often
require high voltage power, which creates the need for additional safety measures
including emergency stop buttons to shut down equipment and installation of
water and dustproof electrical enclosures.
11,12
Special care must be taken when
solvents are used in production; follow applicable national codes, such as
NFPA, UL, and OSHA. UPS needs must be evaluated based on the equipment
and facility needs. An emergency generator may be essential to maintain
biocontainment.
Production equipment including bioreactors, fermentors, ltration units and
centrifuges In all upstream and some downstream processes, equipment is used
while the product is still infectious. These units must be set up to eliminate the
risk of aerosol release. Prior to charging process equipment with live biological
material, the integrity of the closed system should be veried. Before opening
a closed system for maintenance or cleaning, in situ decontamination of the
vessel is required. To prevent an aerosol release occurring as a result of an upset
condition, small equipment can be placed inside a containment device such as a
biological safety cabinet. Larger equipment containing infectious agents should
reside in rooms under negative pressure. If negative pressure can’t be achieved,
room entry and exit airlocks may be used as negative air pressure “sinks” to
prevent the escape of aerosols into adjacent areas.
Work Practice and Administrative Controls
Good microbiological practices are vital and apply in the same way as they
do in biological research laboratories. Chemical hygiene, hearing protection
evaluations in equipment areas, ergonomic, and safety principles apply to large-
scale biological production areas as they do in other research laboratories and
production areas. Access should be restricted to trained personnel only. Other
administrative controls include:

524 Biosafety in Microbiological and Biomedical Laboratories
Occupational Health Employers should oer workers appropriate medical
surveillance programs to identify immune suppression and other underlying
medical conditions, which could be risk factors that necessitate adaptations or
accommodations. Occupational physicians should advise on, from a medical
point of view, protection measures and procedures (e.g., tness for duty to wear
respirators or perform specic tasks). Where appropriate, the physician will oer
vaccination, or provide vaccines, with follow up on titers. In addition to surveil-
lance, clinical treatment procedures for accidental exposure should be developed.
For biological agents susceptible to antibiotics, antimicrobial susceptibility testing
results should be obtained before large-scale operations begin.
Emergency Response Plans for dierent emergency situations should be estab-
lished, including spill protocols. Where appropriate, post-exposure prophylaxis
and policies for isolation of potentially infected people should be established. One
dierentiating factor between small and large spill clean-up is that, unless there
is an immediately dangerous for life and health (IDLH) situation, the operator in
a large-scale facility must remain in the room long enough to stop and contain
the release to minimize HSE consequences. Further information on emergency
preparedness and response can be found in Biological Safety: Principles and
Practices.
13
Laboratory Biosecurity The risk management strategy for a large-scale risk
assessment should dene both a biosafety containment strategy (refer to BMBL
Section II, NIH Guidelines’ Appendix K, and the area-specic risk assessment)
and a laboratory biosecurity strategy. The biosafety containment strategy denes
controls that mitigate risk from an unintentional release, and the laboratory
biosecurity strategy denes controls that prevent theft of biological agents that
are associated with human health and/or agricultural industry impact. Likewise,
materials, equipment, technology, and knowledge of dual-use potential needs to
be addressed and a strategy developed to address misuse.
14–18
Training Biosafety, laboratory biosecurity, and GxP training (if applicable)
are essential in large-scale biological production. For large-scale processes,
training should review the epidemiology, signs/symptoms of infection, mode of
transmission, risk-mitigating controls including donning and dong of PPE, and
emergency response procedures, area-specic SOPs, including spill response
protocols, required for the biological agent/material handled. Workers should
understand when PPE is required for product protection vs. personnel protection.
An understanding of the handling requirements for inactivated vs. unconrmed
inactivated materials is critical. Training should include a knowledge check.
Ergonomics The ergonomic issues associated with large-scale operations
dier from those encountered in the laboratory. Material handling in large-scale
operations will present a larger risk of ergonomic injury. To address the ergonomic
issues associated with material handling, include the nature of the load in the risk

525Appendix M—Large-Scale Biosafety
assessment (i.e., the weight distribution and shape of the load), the capabilities of
the individual performing the task, the duration and frequency of the task, and the
environment in which the material handling task is performed (e.g., space limited
or extreme temperature environments). Mitigate ergonomic risks by mechanical
means (e.g., lifts, hand trucks, pushcarts), redesign of the work area (e.g., ramps
to replace stairs, automated transfer of materials to replace manual transfer),
redesign of the work task (e.g., pushing rather than pulling), and training of
personnel (e.g., proper lifting technique).
Waste Handling The processes of waste handling are the same as for research
laboratories but larger amounts require dierent logistics. For guidance on
validation of decontamination agents and procedures, refer to Appendix B. Key
considerations include inactivation of organisms in situ vs. external to process
vessel or container. Consider inactivation methodologies for solid infectious waste
streams as well as wastewater from production euent (i.e., determine if there
will be an impact to the site wastewater treatment permit due to the presence of
organics including preservatives such as thimerosal or adjuvants).
Review and Checking of Risk Control Measures Risk control measures need
to be evaluated for ecacy in order to protect people and the environment. The
organization should maintain a risk control register, which should be periodically
reviewed. The strategy should address the major risk streams (e.g., chemical,
physical, biological, and ergonomic).
Preventative Maintenance Preventative maintenance is vital to avoiding process
contamination and to ensuring biocontainment. Safety and security-related
equipment and infrastructure should be incorporated into a preventive mainte-
nance program that incorporates a change control process. For example, rotary
seals in fermenters must be monitored for increased loss of seal water or steam
pressure and should be replaced before failure; high-pressure piston seals of
homogenizers must be replaced regularly to prevent aerosol release; autoclave
temperature and pressure sensors require regular calibration, and steam traps
must be maintained. Depending on design, autoclave bioseal or air dierential
seals should be tested (e.g., smoke, pressure hold, soap bubble, and helium
leak testing) to determine whether they have deteriorated. When required,
HEPA lters (i.e., HVAC and equipment) should be integrity tested annually and
critical barrier HEPAs should be monitored for pressure dierential. Thermal or
chemical inactivation systems should undergo regular inspection for corrosion
and preventative maintenance of gaskets, seals, and sensors, as well as addition
pumps, to ensure proper operation. Validation of inactivation parameters is also
required by using spore-based indicators or the actual production organisms.
Continuous ow thermal inactivation systems should undergo regular chemical
clean-in-place cycles to remove coagulated protein residues, which can reduce
system eciency.
526 Biosafety in Microbiological and Biomedical Laboratories
PPE/Gowning
PPE and gowning are used for both personnel and product protection. When
PPE is utilized for product protection, it is designed to prevent shedding of
foreign material into the production process and nal product and to contain skin
and respiratory shedding from the worker. Standard cotton or synthetic materials
are not acceptable because they are prone to shedding. When PPE is utilized
for worker protection, it should be assessed against physical, chemical, and
biological hazards. Cotton laboratory coats or jumpsuits are easily saturated
with chemical and biological liquids during a large release or spill and do not
provide adequate protection. Man-made, water-resistant polymers are a better
choice; they are less apt to become saturated. Refer to the material permeation
rate or breakthrough detection time. The most protective options for personnel
protection are gowns made of microporous laminated materials or jumpsuits with
covered zippers.
Depending on the chemicals and/or biological materials handled, large volumes
at high concentration plus the inherent increased risk of aerosol generation
may require respiratory protection. Common disposable, half-face respirators
(e.g., N95) may be sucient for biological material protection, but they are
not designed for chemical protection and may not be sucient to protect
against large volumes of a concentrated high-risk pathogen. Therefore, a risk
assessment should be performed to identify the appropriate respirator required
for the operation (i.e., ltering facepiece, tight-tting facepiece, PAPR or SCBA).
Conclusion
Large-scale growth of biological agents is necessary in a variety of settings
and requires an evaluation of both the GxP and biosafety requirements. With
careful planning and a robust risk assessment of the unique requirements of a
large-scale facility, it is possible to design and operate a facility that protects the
product, workers, and the environment.
References
1. Good Laboratory Practice for Nonclinical Laboratory Studies, 21 C.F.R.
Part 58 (2018).
2. U.S. Department of Health & Human Services. Guidance for Industry:
Quality Systems Approach to Pharmaceutical CGMP Regulations. Food
and Drug Administration; 2006. 32 p.
3. McGarrity GJ, Hoerner CL. Biological Safety in the Biotechnology Industry.
In: Fleming DO, Richardson JH, Tulis JH, Vesley D, editors. Laboratory
Safety: Principles and Practices. 2nd ed. Washington (DC): ASM Press;
1995. p. 119–31.
527Appendix M—Large-Scale Biosafety
4. Center for Chemical Process Safety of the American Institute of Chemical
Engineers. Guidelines for Process Safety in Bioprocess Manufacturing
Facilities. Hoboken (NJ): John Wiley & Sons, Inc.; 2011.
5. Hambleton P, Melling J, Salusbury TT, editors. Biosafety in Industrial
Biotechnology. Glasgow: Springer Science+Business Media Dordrecht; 1994.
6. Cipriano ML, Downing M, Petuch B. Biosafety Considerations for Large-
Scale Processes. In: Wooley DP, Byers KB, editors. Biological Safety:
Principles and Practices. 5th ed. Washington (DC): ASM Press; 2017.
p. 597–617.
7. Palberg T, Johnson J, Probst S, Gil P, Rogalewicz J, Kennedy M, et.al.
Challenging the Cleanroom Paradigm for Biopharmaceutical Manufacturing
of Bulk Drug Substance. BioPharm International. 2011;24(8):1–13.
8. Klutz S, Magnus J, Lobedann M, Schwan P, Maiser B, Niklas J, et al.
Developing the biofacility of the future based on continuous processing and
single-use technology. J Biotechnol. 2015;213:120–30.
9. Löelholz C, Kaiser SC, Kraume M, Eibl R, Eibl D. Dynamic Single-Use
Bioreactors Used in Modern Liter- and m(3)- Scale Biotechnological
Processes: Engineering Characteristics and Scaling Up. Adv Biochem Eng
Biotechnol. 2014;138:1–44.
10. Halkjaer-Knudsen V. Single-Use: The fully closed systems?. Am Pharma
Rev. 2011;14:68–74.
11. Occupational Safety and Health Administration. Controlling Electrical
Hazards. Washington (DC): U.S. Department of Labor; 2002.
12. National Fire Protection Association. Standard for Electrical Safety in the
Workplace. NFPA 70E. 2018.
13. Wooley DP, Byers KB. Biological Safety: Principles and Practices. 5th ed.
Washington (DC): ASM Press; 2017.
14. National Institutes of Health [Internet]. Bethesda: Oce of Science Policy;
c2018 [cited 2018 Nov 27]. Dual Use Research of Concern. Available from:
https://osp.od.nih.gov/biotechnology/dual-use-research-of-concern/
15. Science Safety Security [Internet]. Washington (DC): U.S. Department
of Health & Human Services; c2015 [cited 2018 Nov 27]. United
States Government Policy for Institutional Oversight of Life Sciences
DURC. Available from: https://www.phe.gov/s3/dualuse/Pages/
InstitutionalOversight.aspx
16. Science Safety Security [Internet]. Washington (DC): U.S. Department
of Health & Human Services; c2017 [cited 2017 Nov 27]. Dual Use
Research of Concern. Available from: https://www.phe.gov/s3/dualuse/
Pages/default.aspx
528 Biosafety in Microbiological and Biomedical Laboratories
17. Drew TW, Mueller-Doblies UU. Dual use issues in research—A subject of
increasing concern?. Vaccine. 2017;35(44):5990–4.
18. Sandia National Laboratories. Laboratory Biosafety and Biosecurity Risk
Assessment Technical Guidance Document: International Biological Threat
Reduction. Albuquerque and Livemore: Sandia Corporation; 2014.

529Appendix N—Clinical Laboratories
Appendix N—Clinical Laboratories
Clinical Laboratory Biosafety
Most contemporary medical decision-making utilizes the result(s) of at least one
diagnostic test conducted in a clinical laboratory as a part of evidence-based
care.
1,2
because they detect and report epidemiologically important organisms and
operation of clinical laboratories is critical for both the care of individual patients
and the health of laboratory professionals, the community, and the environment.
In 2016, following the U.S. Ebola crisis, the U.S. Clinical Laboratory Improvement
Advisory Committee (CLIAC) recognized “the matter of biosafety in clinical
laboratories as an urgent unmet national need.” In particular, CLIAC indicated the
need for concise, understandable guidance to help enable clinical laboratories
to assess and mitigate risks when the identity of the infectious agent is unknown
3
This appendix focuses on biorisk management (BRM) in a
and mitigate risks and evaluate the performance of the implemented controls in
reducing risks associated with the handling, storage, and disposal of hazardous
biological materials.
4
Conducting Risk Assessments in a Clinical Laboratory Environment
Risk assessment is the process of evaluating the risk(s) that arise from agent and
laboratory hazards, taking into account the adequacy of existing controls, priori-
tizing those risks, and deciding if the risks are acceptable.
5
The risk assessment
generates information that guides the selection of appropriate microbiological
practices, safety equipment, and facility safeguards that can reduce Laboratory-
associated infections (LAIs). In addition, the integration of the risk assessment
prioritization of risks and the establishment of risk mitigation protocols tailored
6
Please refer to
Section II for additional information.
Risk assessment is the foundation of every comprehensive BRM system.
The BRM approach is similar to the Quality Management System (QMS) or
Individualized Quality Control Plan (IQCP) that clinical laboratories commonly
use to establish quality standards for laboratory testing. QMS and IQCP include
processes for risk assessment, quality control planning, and quality assessment.
7
BRM includes processes for risk assessment, risk mitigation and performance
the Assessment Mitigation Performance (AMP) model.
4
Ideally, BRM and QMS
should be integrated and mutually supportive systems in a clinical laboratory.
530 Biosafety in Microbiological and Biomedical Laboratories
The clinical laboratory director is responsible for the overall operation and
administration of the laboratory. As stated in the Clinical Laboratory Improvement
Amendments (CLIA) regulations,
8
the laboratory director must:
1. Ensure that testing systems developed and used for each of the tests
performed in the laboratory provide quality laboratory services for all
aspects of test performance, and
2. Ensure that the physical plant and environmental conditions of the
laboratory are appropriate for the testing performed and provide a safe
environment in which employees are protected from physical, chemical,
and biological hazards.
However, the responsibility for ensuring the safe and secure handling of
hazardous materials in a clinical laboratory should be shared. Laboratory
leadership should not conduct risk assessments alone, but should depend on the
knowledge and expertise of the laboratory, infection prevention, and safety profes-
assessments. Risk assessments should be documented and routinely evaluated,
the laboratory environment. Additionally, risk assessments should be evaluated
when unanticipated or unusual events, near-misses, incidents, or accidents
occur. Implementation of a continual risk assessment process creates a proactive
approach to laboratory safety rather than a reactive one, potentially preventing
incidents and accidents before they happen.
The assessment team should determine what hazards may exist and the risks
associated with those hazards. When the agent hazards are unknown, it may be
helpful for clinical laboratories to monitor current disease outbreaks and compile
lists of commonly encountered pathogens for a population, region, or specimen
type. Knowledge of endemic diseases in an area and receipt of a specimen type
specimen from a patient with recurring fevers who has recently returned from
travel in central Africa may suggest the presence of the protozoan parasite,
Plasmodium falciparum, a causative agent of malaria. In addition, clinical labora-
tories can sometimes gain insight into a suspected diagnosis, or even a pathogen,
stain on a sputum specimen could suggest mycobacteria such as Mycobacterium
tuberculosis, the causative agent of tuberculosis. As a best practice, clinical
laboratories should encourage clinicians to notify the laboratory when they
suspect a patient(s) may have an infectious disease that could pose risks to the
laboratory professional.
To help structure biological risk assessments, clinical laboratories should
consider what procedures or activities will be performed, where the work will
be performed, who will perform the work, and what undesirable events could

531Appendix N—Clinical Laboratories
occur. It is also essential to evaluate the potential routes of transmission of the
suspected infectious agent (i.e., inhalation of aerosols, ingestion, percutaneous
inoculation from sharps or non-intact skin, and direct mucous membrane contact
inhalation risk, but there is a risk of percutaneous, mucous membrane contact,
ingestion, or non-intact skin exposure in clinical laboratories. Protecting portals
of entry (i.e., eyes, nose, mouth, and non-intact skin) can reduce initial exposure
to hazards, subsequent transmission of infectious agents, and potential LAIs.
The use of laboratory equipment, including instruments for analytical testing, may
also present safety risks. A recent study showed that during routine operation
of automated clinical laboratory equipment, potentially infectious aerosols
or microdroplets were recovered from laboratory equipment surfaces and
professionals.
9
Clinical laboratories should consider a wide range of potential hazards when
conducting a risk assessment. Examples of hazards unique to the clinical
laboratory that should also be considered are listed below:
Hazards associated with inadequate mitigation capabilities.
Implementing Mitigation Measures in the Clinical Laboratory Environment
Pathogens (BBP)
Standard
10
must be followed when clinical laboratories handle blood and body
employee exposure. Standard Precautions are an expansion of the major
(except sweat), non-intact skin, and mucous membranes may contain transmis-
an infectious agent.
11
Implementation of Standard Precautions constitutes the
primary strategy for the prevention of transmission of infectious agents among
healthcare personnel,
11
with additional controls implemented as indicated by the
risk assessment.
12
In general, clinical laboratories conduct the majority of their work at BSL-2,
including initial processing of clinical specimens for microbiology workup in a
Section IV for additional information. Traditionally,
the safety community has relied on a hierarchy of controls to select measures to
532 Biosafety in Microbiological and Biomedical Laboratories
eliminate or minimize exposure to hazards and their associated risks,
13
and the
Personal Protective Equipment (PPE).
investigations often indicate the need for new recommendations or the implemen-
tation of existing recommendations for high-risk pathogens. In a scenario in which
a clinical laboratory may be in possession of a specimen for which the facility
is unable to provide the appropriate mitigation, it may be advisable to consider
shipping the specimen to a facility with the equipment and experience to handle
the clinical specimens.
The following risk mitigation measures apply in the scenario where the clinical
Elimination and Substitution Elimination and substitution are concepts that
are more readily applied to a research environment than a clinical setting. In a
clinical laboratory, elimination might mean foregoing a diagnostic test in a case
because the risks are considered too high, or the existing mitigation measures
are considered inadequate. Substituting the agent hazard for something less
cases, substituting diagnostic equipment, instrumentation, or procedures may
not be desirable. In these situations, clinical laboratories rely on a combination
of additional engineering controls, administrative and work practice controls, and
PPE for their safety mitigation measures. Risk assessments should be used to
laboratory.
Engineering Controls Engineering controls can reduce hazardous conditions
or place a barrier between the laboratory professional and the hazard. Barriers
commonly used are Class II BSCs, sharps containers, centrifuge safety cups,
closed automation systems, automated decappers or cap-piercing test systems,
option may be to include alternative containment devices such as an enclosed
workstation in combination with additional work practices and/or enhanced PPE.
533Appendix N—Clinical Laboratories
Administrative and Work Practice Controls Administrative and work practice
controls target changes in work procedures that promote safe behaviors of
policies, such as establishing an active medical surveillance program and
occupational health program, and providing immunizations for infectious agents
that are commonly encountered by laboratory professionals (e.g., hepatitis B
and N. meningitidis). Other examples include written standard operating proce-
dures (SOPs), laboratory signage, and professional training programs. Work
practice controls involve the performance of tasks and adherence to standard
and special practices. Some examples are mandating frequent handwashing,
minimizing the generation of aerosols, limiting the use of sharps, using safer
sharps (e.g., self-sheathing needles and needleless systems), routinely decon-
taminating work areas and equipment, safely collecting and decontaminating
liquid waste from automated systems, disposing of biohazardous and other
hazardous waste properly, and working appropriately inside a BSC. Risks are
the engineering and administrative controls and work practices.
The importance of administrative and work practice controls is well-illustrated
through the response to the 2014–2016 Ebola outbreak. In 2014, when a
Ebola patient diagnosed in the
United States, the hospital lacked a dedicated and specialized biocontainment
unit to conduct diagnostic tests on specimens from the patient. Laboratory
management assessed the risks and implemented additional administrative
and work practice controls in their core laboratory to ensure the safety of their
laboratory professionals while conducting diagnostic tests for their patients.
As a result, the clinical laboratory professionals successfully handled patient
14
Some examples of
Ebola outbreak included:
Conducting diagnostic testing in a dedicated space within the core
laboratory with dedicated equipment.
Trigger Points Another practice becoming more common in clinical microbiology
laboratories is the recognition of trigger points during diagnostic testing that
prompt workers to conduct work in a BSC
12,15,16,17
or other containment device.
used to determine when to heighten the precautions or conditions for handling a
specimen or culture.
534 Biosafety in Microbiological and Biomedical Laboratories
The following list, which is not comprehensive, includes some examples of trigger
points for continuing further workup in a BSC:
Growth only on chocolate agar or better growth on chocolate agar
no growth on MacConkey agar, oxidase-positive, and Gram stain
showing small Gram-negative diplococci. Possible microorganism:
Neisseria meningitidis.
projections and ground-glass appearance, and Gram stain showing
boxcar-shaped, large Gram-positive rods with or without spores.
Possible microorganism: Bacillus anthracis.
Gram-negative rod (GNR) with bipolar staining (safety pin shape) and
“fried egg” appearance in older cultures. Possible microorganism:
Yersinia pestis.
®
Select Agents. There is no requirement that commercial test systems contain
Select Agents in their databases nor are they required to test Select Agents
9
Personal Protective Equipment (PPE) reduces exposure by blocking the clinical
route of transmission of an infectious agent can help the risk assessment process
determine what PPE is appropriate. Many pathogens are transmitted by multiple
Depending on the risk assessment, respiratory protection could be used when
working with an infectious agent that is known or suspected to be airborne trans-
missible. Working with an infectious agent that is known to be transmissible by
and mouth).
Risk assessments assist with determining what PPE should be worn for
closed laboratory coat or gown, eye protection, closed-toe shoes, and gloves.

535Appendix N—Clinical Laboratories
risks. Increasing the amount or use of PPE does not always indicate an increase
protection needed without compromising the health of the laboratory professional
or their ability to safely perform their duties. The National Institute for Occupa-
tional Safety and Health and OSHA each have additional information on PPE
selection and use.
18,19
See Section IV for additional information on PPE.
Worker Competencies and Training
Creation of a culture of safety and a safe work environment depends on
that make them risk-conscious and attentive to safety practices. Laboratory safety
competencies may include: understanding the hazards in the laboratory and the
in the laboratory. The quality of laboratory testing has been an expectation of
clinical laboratory accreditation and licensure agencies for many years, and
some agencies are now moving toward inclusion of laboratory safety as another
required competency for accreditation.
Training and practice on the use of PPE are critical for safe operations in the
clinical laboratory. If not used properly, the PPE will likely not achieve its intended
level of comfort and physical ability to perform those tasks.
Laboratory professionals should also be competent in decontamination of the
laboratory for routine cleaning, disposing of waste, and responding to spills. They
should understand the types and volumes of spills that they can safely handle,
and which require additional support. They should be trained on non-routine and
to predict how people will respond during non-routine or emergency situations,
but frequent training and drills will help identify gaps that were not recognized
previously and facilitate the revision of procedures.
A positive and proactive culture of safety can be reinforced by including safety
expectations in job descriptions, reviews of employee performance, and career
advancement.
20
Supervisors should ensure that all laboratory professionals:
536 Biosafety in Microbiological and Biomedical Laboratories
1. Understand the risks involved with their work and how to use the safety
2.
3. Demonstrate appropriate technical expertise to safely and accurately
4. Recognize the limitations of implemented controls and what to do if they
Emergency Response Procedures
Working in a clinical laboratory environment will always involve some level of risk,
and unintended events, including incidents and accidents, will occur. Therefore,
laboratories should have mitigation procedures outlined in an emergency
response plan to address those unintended events. This plan should cover both
events that could occur in the laboratory and events that could occur outside the
laboratory environment but directly impact laboratory operations. The clinical
-
response procedures according to the determined level of risk.
Examples of emergencies that could occur in the laboratory include spills inside
and outside of primary containment (e.g., BSC), exposure to hazardous materials
leaks. Examples of emergencies that could occur outside of the laboratory include
emergencies, and natural disasters.
may need to be involved in the risk assessment and the development of the
to exposure to a hazardous material may require coordination with infectious
disease specialists outside of the institution.
Laboratory management should ensure that an emergency response plan exists,
plan. In addition, hazard communication in the form of signage and posted SOPs
Considerations for emergency response may include, but are not limited to,
Discussion-based exercises (i.e., tabletop exercises) and operations-based
537Appendix N—Clinical Laboratories
the emergency response plan. These drills and exercises should include diverse
groups who would be involved in laboratory emergency response, including
institutional leadership, laboratory leadership, laboratory professionals, opera-
results of the drills and exercises should be documented, evaluated for successes
drills and exercises should be used to revise laboratory risk assessments, as well
as the laboratory emergency response plan.
Challenges in a Clinical Laboratory Environment
pre-analytical phase occurs prior to the specimen being tested in the laboratory or
at the point-of-care (POC). During this phase, specimens are collected, labeled,
packaged, and transported or shipped. The analytical phase encompasses
test results, the storage of specimens, and the disposal processes.
Handling Specimens with Unknown Pathogens
Diagnostic testing for a single patient may involve receipt of multiple types of
specimens (e.g. blood, sputum, urine) with little information regarding suspected
diagnoses. Clinicians assess the patient and often order a battery of diagnostic
for uncommon pathogens. When atypical pathogens are under consideration
Laboratory professionals, which include phlebotomists, are often not aware of the
hazards and subsequent risks posed by the specimens they draw or handle until
The risks associated with handling clinical specimens may not be fully recog-
nized. Some pathogens have low infectious doses and some clinical specimens
Additionally, multiple pathogens may be present in one clinical specimen. In
clinical microbiology laboratories, clinical microbiologists isolate, grow, and
expand populations of the pathogen(s) to obtain a pure culture for performing
manipulations and quantities of the pathogen(s), thereby increasing the risk for
microbiologists who handle those organisms.

538 Biosafety in Microbiological and Biomedical Laboratories
Diagnostic Testing Environments
Diagnostic testing incorporates many disciplines and is generally performed in
cytology, histology, microbiology). Each of these laboratories conducts a
variety of tests and utilizes various equipment/procedures. Routine laboratory
procedures may generate aerosols (e.g., pipetting, mixing, centrifuging,
vortexing, aliquoting, grinding, plating, and opening or removing caps).
12
Clinical
laboratories conduct a high volume of tests in a fast-paced, highly technical, and
repetitive testing environment. High-throughput instrumentation, such as large
chemical analyzers and other automated equipment, are often operated outside
of secondary containment and can potentially generate splashes, splatters, and
aerosols during operation.
Most clinical and public health laboratories incorporate BSL-2 standard and
special practices, safety equipment, and facility recommendations. However,
potentially contain pathogens may occur in an open environment, such as on a
laboratory benchtop. Consequently, mitigation strategies and controls must be
implemented to reduce the risk of exposure to laboratory professionals. Please
also see Section IV for additional information on BSL-2.
Point-of-care or bedside testing is performed with increasing frequency and
and medical assistants who do not routinely collect specimens or perform
laboratory analyses may be tasked with conducting these tests at the bedside
to provide immediate data for patient care. This practice occurs in critical care
Since these environments frequently lack the engineering controls of a properly
designed laboratory facility, additional procedural controls and PPE are used.
Clinical Laboratory Workforce
In contrast to research or academic settings, most clinical laboratories operate 24
often work rotating shifts or evening/night shifts to maintain critical operations.
judgment can be impaired
and existing safety measures may be overlooked.
The loss of skilled professionals because of high turnover, an aging workforce, a
reduction in educational and training programs, and a lack of time and resources
laboratory workforce.
539Appendix N—Clinical Laboratories
Laboratory-associated infections
21
Despite
the evolution of biosafety practices and equipment, laboratory exposures to
infectious agents and LAIs continue to occur. One recent American Society for
Microbiology (ASM) publication summarizes LAI data collected from 1930 to
most commonly reported agents has decreased, the total number of LAIs in
clinical laboratories has increased while LAIs from research laboratories has
decreased during the same timeframe.
22
The majority of the LAIs occurred in
clinical microbiology laboratories and were bacterial infections.
Understanding the origin of these LAIs is still often elusive. It is widely accepted
that the numbers of LAIs reported or documented represent a substantial
underestimation of the actual number of LAIs. Undocumented cases and lack of
denominator data continue to complicate the assessment of risk and determi-
nation of true
reported LAIs.
23,24
Many of these LAIs with unrecognized exposure events are
believed to be due to aerosol exposures. Sources of exposure that could
be explained included spills and splashes to mucous membranes, ingestion
(i.e., from contaminated surfaces or fomites to hands to mouth), and percuta-
neous inoculation from sharps, cuts, needlesticks, and non-intact skin.
Implementing Performance Management in a Clinical Laboratory Environment
Recent safety systems literature shows that every organization creates a culture
25
When incidents occur, they are almost never isolated errors committed by single
individuals. Instead, incidents generally result from multiple, small errors in
recognizes that procedures and human behavior will always change and adapt
over time and that human error is inevitable, especially in complex, high-stress
environments.
25
regularly work in the clinical laboratory. The risk assessment should not only
identify and prioritize risks and select the most appropriate control measures
but also establish how those control measures will be evaluated on a routine
basis.
26
The laboratory professionals should be primarily responsible for actively
540 Biosafety in Microbiological and Biomedical Laboratories
of the accident and then implements corrective actions. However, this reactive
at a moment in time.
Routine, proactive monitoring and evaluation will highlight daily accounts of
successful safety performance. Checklists (e.g., PPE and BSC) and process
decontaminating non-disposable, reusable PPE, and steps for discarding used
check, and performing surface disinfection.
Every laboratory should develop its own monitoring and evaluation methods. It
-
tiveness of the controls. In order to be successful, the laboratory professionals
will need to understand the risks that the controls are designed to mitigate and to
determine whether the controls are working as intended. One way to encourage
the engagement of laboratory professionals is to incorporate a non-punitive
approach for reporting operational problems and proposing solutions that improve
biosafety. Discussions about recent, unanticipated events in the laboratory could
result in changes in the way that risks are controlled before a safety incident
occurs.
Risk Ethics in a Clinical Laboratory Environment
There will always be risks associated with working in a clinical laboratory, and risk
ethics must be included in the clinical laboratory risk assessment process. Risk
ethics are principles that guide rational choices on risk-taking and risk exposure.
When a clinical laboratory conducts a risk assessment, numerous risk perception
Some of these include individual factors (e.g., knowledge, demographics, person-
ality, health, stress), context factors (e.g., culture, social relationships, political
analysis or negative media coverage).
Risk tolerance and risk aversion (or risk acceptability) will vary between and even
within institutions. Each clinical laboratory will assess its own risks and may reach
unique conclusions about the acceptability of those risks that are distinct from
conclusions reached in another laboratory for similar risks. Risk acceptability will

541Appendix N—Clinical Laboratories
also vary for normal operations compared to emergency operations. Determining
what is necessary for optimal patient care and what risks are acceptable for the
laboratory professional will ultimately depend on frequent communication and
transparent decision-making among everyone involved in the management and
operation of the clinical laboratory.
Summary
exposure to human blood or other potentially infectious materials and directs
the creation and implementation of a written Exposure Control Plan to eliminate
or minimize employee exposure. Existing guidance (e.g., CDC, MMWR, BMBL)
states that most clinical laboratories function as BSL-2 facilities with workers
following Standard Precautions and BSL-2 practices.
Recent events, including the 2014–2016 Ebola outbreak, demonstrate that
clinical laboratories need to adopt and support a risk management approach to
biosafety and quality that emphasizes the importance of conducting activity- and
integrating a rigorous performance evaluation process that embraces continual
improvement. The preceding discussion outlined a range of topics unique to the
clinical laboratory environment, and the content of this appendix should be used
as a starting point for the development of a robust culture of safety in the clinical
environment.
References
1.
6):487–8.
2. Badrick T. Evidence-based laboratory medicine. Clin Biochem Rev.
6.
3.
Summary Report: April 13–14, 2016. Available from: https://ftp.cdc.gov/pub/
CLIAC_meeting_presentations/pdf/CLIAC_Summary/cliac0416_summary.pdf
4. Gribble LA, Tria ES, Wallis L. The AMP Model. In: Salerno RM, Gaudioso J,
editors. Laboratory Biorisk Management: Biosafety and Biosecurity. Boca
5. CEN Workshop Agreement. Laboratory Biorisk Management—Guidelines
for the implementation of CWA 15793:2008. Brussels: European Committee

542 Biosafety in Microbiological and Biomedical Laboratories
6. Pentella MA. Components of a biosafety program for a clinical laboratory.
In: Wooley DP, Byers KB, editors. Biological Safety: Principles and
7.
https://www.cms.gov/Regulations-and-Guidance/Legislation/CLIA/
Individualized_Quality_Control_Plan_IQCP.html
8.
9.
10.
11. Siegel JD, Rhinehart E, Jackson M, Chiarello
Control Practices Advisory Committee. Guideline for Isolation Precautions:
Preventing Transmission of Infectious Agents in Healthcare Settings. Atlanta
12. Miller MJ, Astles R, Baszler T, Chapin K, Carey R, Garcia L, et al. Guidelines
for Safe Work Practices in Human and Animal Medical Diagnostic
Laboratories. Recommendations of a CDC-convened, Biosafety Blue Ribbon
13.
https://www.cdc.
gov/niosh/topics/hierarchy/default.html
14.
15.
(poster): https://msdh.ms.gov/msdhsite/index.cfm/14,0,188,547,html
16.
Pathogens. Available from:
17.
https://www.aphl.org/programs/preparedness/Pages/partnerships-outreach.aspx

543Appendix N—Clinical Laboratories
18.
Equipment. Available from: https://www.cdc.gov/niosh/topics/emres/ppe.html
19.
Personal Protective Equipment. Overview. Available from: https://www.osha.
gov/SLTC/personalprotectiveequipment/
20.
Centers for Disease Control and Prevention (CDC). Competency Guidelines
for Public Health Laboratory Professionals: CDC and the Association of
21. Kruse RH, Puckett WH, Richardson JH. Biological Safety Cabinetry.
22. Wooley DP, Byers KB. Biological Safety: Principles and Practices. 5th ed.
23. CLSI. Protection of Laboratory Workers from Occupationally Acquired
24. Baron EJ, Miller JM. Bacterial and fungal infections among diagnostic
laboratory workers: evaluating the risks. Diagn Microbiol Infect Dis.
6.
25. Salerno R, Gaudioso J, editors. Laboratory Biorisk Management—Biosafety
and
26. Burnett L, Olinger P. Evaluating Biorisk Management Performance.
In: Salerno RM, Gaudioso J, editors. Laboratory Biorisk Management:
Biosafety and
544 Biosafety in Microbiological and Biomedical Laboratories
Appendix O—Acronyms
A1HV-1 Alcelaphine Herpesvirus-1
ABSA American Biological Safety Association
ABHS Alcohol-Based Hand Sanitizer
ABSL Animal Biosafety Level
ABSL-2Ag Animal Biosafety Level 2-Agriculture
ABSL-3Ag Animal Biosafety Level 3-Agriculture
ABSL-4Ag Animal Biosafety Level 4-Agriculture
ACAV American Committee on Arthropod-Borne Viruses
ACIP Advisory Committee on Immunization Practices
ACG Arthropod Containment Guidelines
ACL Arthropod Containment Levels
ACME American Committee of Medical Entomology
ADA Americans with Disabilities Act
AHS African Horse Sickness
AHSV African Horse Sickness Virus
AIDS AcquiredImmuneDeciencySyndrome
AKAV Akabane Virus
AMP Antimicrobial Peptides
AMP Assessment Mitigation Performance
AMT Amotosalen
APHIS Animal and Plant Health Inspection Service
APMV-1 Avian Paramyxovirus Type 1
APR Air Pressure Resistant
ARS Agricultural Research Service
ARDS Acute Respiratory Distress Syndrome
ASF African Swine Fever
ASFV African Swine Fever Virus
ASHRAE American Society of Heating, Refrigerating, and Air-Conditioning
Engineers
ASTMH American Society of Tropical Medicine and Hygiene
AVA Anthrax Vaccine Adsorbed
BAT Botulism Antitoxin
BCG Bacillus Calmette-Guérin
BDV Border Disease Virus
BI Biological Indicator
BIS Bureau of Science and Industry
BMBL Biosafety in Microbiological and Biomedical Laboratories
BoNT Botulinum neurotoxin
545Appendix O—Acronyms
BREEAM Building Research Establishment Environmental Assessment Method
BRM Biorisk Management
BSAT Biological Select Agents and Toxins
BSC Biological Safety Cabinet
BSE Bovine Spongiform Encephalopathy
BSL Biosafety Level
BSO BiosafetyOcer
BTV Bluetongue Virus
BVDL Bovine Viral Diarrhea Virus
CAD Clean Air Device
CAV Constant Air Volume
CBPP Contagious Bovine Pleuropneumonia
CCPP Contagious Caprine Pleuropneumonia
CETBE Central European Tick-Borne Encephalitis
CDC Centers for Disease Control and Prevention
CFD Computational Fluid Dynamics
CFR Code of Federal Regulations
CFU Colony Forming Units
CIP Clean in Place
CJD Creutzfeldt-Jakob Disease
CJIS Criminal Justice Information Services Division
CLIA Clinical Laboratory Improvement Amendments
CLIAC Clinical Laboratory Improvement Advisory Committee
CNS Central Nervous System
CSF Cerebrospinal Fluid
CSFV Classical Swine Fever Virus
CWD Chronic Wasting Disease
DHHS Department of Health and Human Services
DNA Deoxyribonucleic Acid
DOC Department of Commerce
DOD Department of Defense
DOL Department of Labor
DOT Department of Transportation
DRM NIH Design Requirements Manual
DTaP Diphtheria Tetanus acellular Pertussis
EBV Epstein-Barr Virus
ECP Exposure Control Plan
EDS EuentDecontamination System
EEE Eastern Equine Encephalomyelitis
ELISA Enzyme-Linked Immunosorbent Assay
546 Biosafety in Microbiological and Biomedical Laboratories
EMCS Energy Monitoring and Control System
EO Executive Order
EPA Environmental Protection Agency
EtOH Ethanol
EUE Exotic Ungulate Encephalopathy
FBI Federal Bureau of Investigation
FDA Food and Drug Administration
FFI Fatal Familial Insomnia
FIFRA Federal Insecticide, Fungicide, and Rodenticide Act
FLA Free Living Amebae
FMD Foot and Mouth Disease
FMDV Foot and Mouth Disease Virus
FQPA Food Quality Protection Act
FSAP Federal Select Agent Program
FSE Feline Spongiform Encephalopathy
GAP III Global Action Plan III
GCP Good Clinical Practices
GHSA Global Health Security Agenda
GI Gastrointestinal Tract
GLP Good Laboratory Practices
GMO GeneticallyModiedOrganism
GMP Good Manufacturing Practices
GNR Gram-Negative Rod
GSS Gerstmann-Sträussler-Scheinker Syndrome
H Hemagglutinin
HAV Hepatitis A Virus
HEPA High-EciencyParticulateAir
HBV Hepatitis B Virus
HCMV Human Cytomegalovirus
HCV Hepatitis C Virus
HCW Healthcare Workers
HD Heartwater Disease
HDV Hepatitis D Virus
HEV Hepatitis E Virus
HeV Hendra Virus
HFRS Hemorrhagic Fever with Renal Syndrome
HHV Human Herpes Virus
HHV-6A Human Herpes Virus 6A
HHV-6B Human Herpes Virus 6B
HHV-7 Human Herpes Virus 7
547Appendix O—Acronyms
HHV-8 Human Herpes Virus 8
HIPPA Health Insurance Portability and Accountability Act
HIV HumanImmunodeciencyVirus
HPAI Highly Pathogenic Avian Inuenza
HPAIV Highly Pathogenic Avian InuenzaVirus
HPS Hantavirus Pulmonary Syndrome
HSE Health, Safety, and Environment
HSV-1 Herpes Simplex Virus 1
HSV-2 Herpes Simplex Virus 2
HTLV Human T-Lymphotropic Viruses
HVAC Heating, Ventilation, and Air Conditioning
IA Impact Assessment
IACUC Institutional Animal Care and Use Committee
IATA International Air Transport Association
IBC Institutional Biosafety Committee
ICAO International Civil Aviation Organization
ICTV International Committee on Taxonomy of Viruses
ID Infectious Dose
ID50 Number of organisms necessary to infect 50% of a group of animals
IDLH Immediately Dangerous for Life and Health
IES APHIS Investigative and Enforcement Services
IFU Instructions for Use
IgG Immunoglobulin
IGRA Interferon-Gamma Release Assay
ILAR Institute for Laboratory Animal Research
IND Investigational New Drug
IPM Integrated Pest Management
IPV Inactivated Poliovirus Vaccine
IQCP Individualized Quality Control Plan
ISA Infectious Salmon Anemia
ISAV Infectious Salmon Anemia Virus
LAI Laboratory-associated infections
LCM Lymphocytic Choriomeningitis
LCMV Lymphocytic Choriomeningitis Virus
LCV Large Cell Variant
LD Lethal Dose
LED Light Emitting Diode
LEED Leadership in Energy and Environmental Design
lfm Linear Feet Per Minute
LGV Lymphogranuloma Venereum
548 Biosafety in Microbiological and Biomedical Laboratories
LMW Low Molecular Weight
LSD Lumpy Skin Disease
LSDV Lumpy Skin Disease Virus
MALDI-TOF Matrix-Assisted Laser Desorption/Ionization-Time of
Flight Mass Spectrometry
MCF Malignant Catarrhal Fever
MDR Multidrug-Resistant
MenV Menangle Virus
MERS Middle East Respiratory Syndrome
MERS-CoV Middle East Respiratory Syndrome Coronavirus
MIT Massachusetts Institute of Technology
MMWR Morbidity and Mortality Weekly Report
MOTT Mycobacteria Other Than Tuberculosis
MPPS Most Penetrating Particle Size
MVA ModiedVaccinia Ankara
NaOCl Sodium Hypochlorite
NaOH Sodium Hydroxide
N Neuraminidase
NBL National Biocontainment Laboratory
NC Noise Criterion
NCI National Cancer Institute
ND Newcastle Disease
NDV Newcastle Disease Virus
NHP Non-human Primate
NIH National Institutes of Health
NiV Nipah Virus
NIOSH National Institute for Occupational Safety and Health
NSF National Science Foundation
NTM Non-tuberculous Mycobacterium
OIG OceoftheInspectorGeneral
OIE World Organization for Animal Health
OPIM Other Potential Infectious Material
OPM Owner’s Project Requirements
OPV Oral Poliovirus Vaccine
OSHA Occupational Safety and Health Administration
OSP OceofSciencePolicy
PAPR Positive Air-Purifying Respirator
PBT Pentavalent Botulinum Toxoid Vaccine
PCR Polymerase Chain Reaction
PEL Permissible Exposure Level

549Appendix O—Acronyms
PEP Post-exposure Prophylaxis
PI Principal Investigator
PM Preventative Maintenance
PPD PuriedProteinDerivative
PPE Personal Protective Equipment
PPM Parts Per Million
PPQ Plant Protection and Quarantine
PPRV Pest des Petits Ruminants Virus
PrP Prion Protein
PTFE Polytetrauoroethylene
PV1 Poliovirus serotype 1
PV2 Poliovirus serotype 2
PV3 Poliovirus serotype 3
QMS Quality Management System
RAC Recombinant DNA Advisory Committee
RBL Regional Biocontainment Laboratory
RG Risk Group
RIP Ribosome-Inactivating Protein
RNA Ribonucleic Acid
RO ResponsibleOcial
RoD Risk of Disease
RoE Risk of Exposure
RP Rinderpest
RPV Rinderpest Virus
RVFV Rift Valley Fever Virus
SAIDS Simian AIDS
SAL Sterility Assurance Level
SALS Subcommittee on Arbovirus Laboratory Safety
SARS Severe Acute Respiratory Syndrome
SARS-CoV SARS-Associated Coronavirus
SBA Sheep Blood Agar
SCBA Self-Contained Breathing Apparatus
SCID SevereCombinedImmunodeciency
sCJD Sporadic Creutzfeldt-Jakob-Disease
SC type Small-Colony type
SCV Small Cell Variant
SDS Safety Data Sheet (Appendix B)
SDS Sodium Dodecyl Sulfate (Section VIII-H)
SE Staphylococcal Enterotoxins
SEA SE Serotype A
550 Biosafety in Microbiological and Biomedical Laboratories
SEB SE Serotype B
SEC SE Serotype C
SED SE Serotype D
SEE SE Serotype E
SHE SE Serotype H
SFV Simian Foamy Virus
SHIV Simian/HumanImmunodeciencyVirus
SIP Sterilization in Place
SIV SimianImmunodeciencyVirus
SGP Sheep and Goat Pox
SGPV Sheep and Goat Pox Virus
SLE St. Louis Encephalitis virus
SME Subject Matter Expert
SNS US Strategic National Stockpile
SOP Standard Operating Procedure
SRA Security Risk Analysis
SRV Simian type D Retrovirus
STLV Simian T-Lymphotropic Virus
SU Single-Use
SVCV Spring Viremia of Carp Virus
SVD Swine Vesicular Disease
SVDV Swine Vesicular Disease Virus
TBEV-CE Tick-Borne Encephalitis Virus- Central European subtype
TBEV-FE Tick-Borne Encephalitis Virus- Far Eastern subtype
TLV Threshold Limit Values
TME Transmissible Mink Encephalopathy
TNF Tumor Necrosis Factor
TSE Transmissible Spongiform Encephalopathy
TVOC Total Volatile Organic Compounds
ULPA Ultra-Low Particulate Air
ULT Ultra-Low Temperature
UP Universal Precautions
UPS Uninterruptable Power Supply
UV Ultraviolet
USAMRIID U.S. Army Medical Research Institute of Infectious Diseases
USC U.S. Code
USDA U.S. Department of Agriculture
USPS United States Postal Service
VAPP Vaccine-Associated Paralytic Polio
VAV Variable Air Volume
551Appendix O—Acronyms
VDPV2 Vaccine-Derived Polio Type 2 Virus
VEEV Venezuelan Equine Encephalitis Virus
VS Veterinary Services
VZV Varicella-Zoster Virus
WBC White Blood Cell
WEEV Western Equine Encephalomyelitis Virus
WHO World Health Organization
WMD Weapons of Mass Destruction
WNV West Nile Virus
XDR Extensively Drug-Resistant
552 Biosafety in Microbiological and Biomedical Laboratories
Glossary
Agent: In a biological context, a microorganism, biological toxin, or human
endoparasite, either naturally occurring or genetically modied, with the potential
to cause infection, allergy, toxicity, or otherwise, create a hazard to human health.
Agricultural biosecurity: The scientically-based policies, measures, and
regulatory frameworks that are applied to protect, manage, and respond to risks
associated with food, agriculture, health, and the environment.
Air sweep: Within a BSC, use of the downow air after slowly placing arms and
hands inside the BSC to remove particulates prior to starting work.
Attenuation: A method to minimize disease risk that involves using a weakened
form of a pathogen, viral nucleic acid sequences, or a toxin.
Bioburden reduction studies: See spike-and-recovery experiments.
Biorisk: The eect of uncertainty expressed by the combination of the
consequences of an event and the associated likelihood of occurrence, where
biological material is the source of harm.
Biorisk management: Coordinated activities to direct and control an organization
with regard to biorisk.
Bloodborne pathogens: Pathogenic microorganisms present in human blood
and other potentially infectious materials (OPIM), which can infect and/or
cause disease in persons who are exposed to blood or OPIM containing these
pathogens.
Cell type: A classication that distinguishes between morphologically or
phenotypically dierent forms within an organism.
Clean bench: A device that directs HEPA-ltered air horizontally or vertically over
a surface, towards the user.
Cleanroom: A room that utilizes HEPA-ltered supply air to reduce the amount
particulate contamination to a designated level (e.g., ISO Class 4 allows no more
than 1.0 x 10
4
particles/m
3
with a size ≥ 0.1 µm).
Clean to dirty: In the context of workow, a process of working within a BSC that
segregates unused (e.g., clean) or sterile materials on one side of the cabinet
from used (e.g., dirty) materials on the other, with a central working area. For a
right-handed person, the clean material will generally be on the right, and the dirty
material will be on the left; the opposite orientation is appropriate for a left-handed
person. In the context of airow, it is the preferred direction of air movement, from
areas of lower potential contamination to those of higher potential contamination.
553Glossary
Cleaning: A process to reduce or remove adherent organic and inorganic soil
(e.g., blood proteins, debris and biological matter, and other material) from
surfaces usually with detergent and water.
Conrmed Inactivation Procedure: A method that has been tested and
determined under specied conditions to have adequate ecacy in rendering a
pathogen non-viable (i.e., viability testing); viral nucleic acid sequences that can
produce infectious forms of a virus non-infectious (i.e., infectivity testing); or a
toxin no longer capable of exerting a toxic eect (i.e., toxicity testing).
Contact time: The time required for a process or chemical treatment to
inactivate a microorganism on the surface or item, which may depend on the
number of organisms present and other variables (e.g., temperature, organic
load, water hardness).
Containment: A combination of primary and secondary containment barriers,
facility practices and procedures, and other safety equipment, such as personal
protective equipment (PPE), for managing the risks associated with handling and
storing hazardous biological agents and toxins in a laboratory environment.
Culture type: Animal cell cultures can be characterized into three types—
explants, primary cell lines, and immortal cell lines. The risk of explants
and primary cell lines directly derived from explants are frequently poorly
characterized and may pose unknown risks to the researcher.
Decontamination: The use of physical and/or chemical means to remove,
inactivate, or destroy microbial pathogens (e.g., bloodborne or aerosolized) on
a surface or item to the point where they are no longer capable of transmitting
infectious particles and the item or surface is rendered safe to handle; however,
this denition has been broadened by infection control specialists to include all
pathogens and physical spaces (e.g., patient rooms, laboratories, buildings).
Directional airow: Movement of air in one direction to minimize potential
cross-contamination from aerosols.
Disinfectant: A substance, or mixture of substances, that destroys or irreversibly
inactivates bacteria, fungi, and viruses, but not necessarily bacterial spores or
prions, in the inanimate environment.
Disinfection: A process that destroys pathogens and other microorganisms,
except prions, by physical or chemical means.
High-Level Disinfection: A lethal process utilizing a sterilant under less than
sterilizing conditions (e.g., 10–30 min contact time instead of 6–10 h needed for
sterilization). The process kills all forms of microbial life except for large numbers
of bacterial spores.
554 Biosafety in Microbiological and Biomedical Laboratories
■
Intermediate-Level Disinfection: A lethal process utilizing an agent
that kills viruses, mycobacteria, fungi, and vegetative bacteria, but no
bacterial spores.
■
Low-Level Disinfection: A lethal process utilizing an agent that kills
vegetative forms of bacteria, some fungi, and enveloped viruses.
Endogenous pathogens: Pathogens normally associated with a host and not
provided as part of an experimental protocol.
Etiologic agent: An agent capable of causing disease—usually a pathogen such
as a bacterium, virus, parasite, fungus, or toxin. Now replaced with the term
infectious materials or infectious substances in 49 CFR Parts 171–180.
Exempt Organisms: Organisms listed under Appendix C of the NIH Guidelines,
including K-12 derived strains of E. coli. These organisms are generally
considered not to pose a signicant risk to health or the environment and are
exempt from the requirements of the NIH Guidelines.
Facility: A building, or portion of a building, which houses laboratories or animal
facilities and all of their associated functions (e.g., autoclave rooms, equipment
rooms, feed rooms, cage wash areas). For higher containment areas, it may
include only the rooms within the containment boundary.
Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA): FIFRA provides
for federal regulation of pesticide distribution, sale, and use. All pesticides,
including antimicrobial pesticides, distributed or sold in the United States must be
registered (i.e., licensed) by the EPA. Manufacturers submit ecacy data to the
EPA to support product claims.
Inactivation: A procedure to render a pathogen non-viable, viral nucleic acid
sequences non-infectious, or a toxin non-toxic while retaining characteristic(s) of
interest for future use. Methods targeting tropism may be host-specic.
Infectious materials: Any material, solid or liquid, which contains biological
agents capable of causing infection in either humans, animals or both.
Infectious substances: Substances that are known or are reasonably expected
to contain pathogens. Infectious substances can include patient specimens,
biological cultures, medical or clinical wastes and/or biological products such as
vaccines.
Infectivity testing: A process to conrm ecacy of the inactivation procedure
by demonstrating that viral nucleic acid sequences are incapable of producing
infectious forms of viruses. Ecacy assessments on methods that target tropism
may be host-specic.
555Glossary
Institutional Biosafety Committee (IBC): The committee required under the NIH
Guidelines to review and approve research with recombinant or synthetic nucleic
acids. The committee may also take on additional tasks, such as review of all
work with biological agents. Sites not subject to the NIH Guidelines may choose
to establish an IBC or use a committee with a similar name (e.g., site biosafety
committee, institutional safety committee) to oversee research with recombinant
or synthetic nucleic acids and/or biological agents. IBC is the generic term used
in the BMBL.
Institutional verication: Armation by an entity that the set of conrmed
inactivation and separation/removal procedures used at that entity result in end
products that achieve adequate inactivation ecacy.
Instructions for use (IFU): Section of the product label that includes
manufacturer’s instructions for using a product safely (i.e., dilution, contact time,
how to apply). The manufacturer may also have an extended label that provides
additional instructions.
Laboratory: A room, or series of rooms, which may or may not be contiguous,
used for research under the control of a single supervisor or principal investigator.
Laboratory biosecurity: The measures designed to prevent loss, theft,
or deliberate misuse of biological material, technology, or research-related
information from laboratories or laboratory-associated facilities.
Laminar ow: Laminar ow occurs when the uid (i.e., air) ows in innitesimal
parallel layers with no disruption between them. In laminar ows, uid layers slide
in parallel, with no eddies, swirls or currents normal to the ow itself.
Mask: A covering over the the mouth and nose, not certied to provide respiratory
protection. May be used to provide mucous membrane protection from droplets.
Not equivalent to a respirator.
Material: In a biological context, any material comprised of, containing, or that
may contain biological agents and/or their harmful products, such as toxins or
allergens. Biological materials may be blood, secretions, or tissues of human or
animal origin, debris, organic material from nature, culture or preservation media,
human, animal, and plant cultures.
Microorganism: A biological agent that is often unicellular or acellular, capable
of replication or of transferring genetic material, including bacteria, viruses, fungi,
and parasites.
Pathogen: Microorganisms (e.g., bacteria, viruses, rickettsiae, parasites, fungi)
and other agents such as prions, which can cause disease in humans, animals,
or plants.
556 Biosafety in Microbiological and Biomedical Laboratories
Penetration: a deliberate hole or opening in a surface (e.g., wall, oor, ceiling)
that must be sealed to prevent air leakage from a facility or laboratory.
Persons: All individuals at a facility, whether employees, contractors, or visitors.
Pest: A pest is an organism living and growing where their presence is undesired
or unintentional. A pest can cause damage to plants, humans, structures, and
other creatures.
Pesticide label: Pesticide product labels provide critical information about
how to safely and legally handle and use pesticide products (e.g., antimicrobial
pesticides). Unlike most other types of product labels, pesticide labels are legally
enforceable, and all of them carry the statement: “It is a violation of Federal law to
use this product in a manner inconsistent with its labeling.”
Pre-cleaning: The removal of bulk contaminating material not part of the material
or surface being cleaned.
Process verication: Demonstration that use of an inactivation procedure that
employs the set of specied conditions established in the conrmation study(s)
has achieved adequate ecacy.
Product Labeling: This is any legend, artwork, or mark attached to disinfectant.
It will include IFUs, EPA registry number, and label claims (e.g., microorganisms
tested for EPA registry).
Purge (BSC): Process of providing time to allow BSC airow to lter cabinet
air and remove contaminants from the air prior to starting work or concluding
experiments in a BSC.
Respirator: A device to provide protection from aerosols or vapors, depending
on the ltration medium. It is approved by regulatory entities and requires
documented training, t testing, and medical surveillance.
Restricted experiment: Experiments that potentially provide drug resistance to
U.S. Select Agents, if the acquisition could compromise the control of the disease
agent, or experiments that deliberately create synthetic or recombinant genes for
the synthesis of Select Toxins lethal at a LD
50
of less than 100 ng/kg.
Risk ethics: Principles that morally guide rational choices on risk-taking and risk
exposure and are important considerations of risk management.
Room: The smallest physical subdivision of a laboratory or facility.
Root cause analysis: A collective term that describes a wide range of
approaches, tools, and techniques used to uncover causes of problems. The root
cause is the core issue that sets in motion the entire cause-and-eect reaction
that ultimately leads to the problem(s).
557Glossary
Sanitizers: A chemical preparation and an antimicrobial agent for killing at least
99.9% of microorganisms. Sanitizers are typically used on food contact surfaces,
carpet, in-tank toilet bowl additives, laundry additives, and air fresheners.
Sight sealed: Visual inspection of sealed areas in BSL-3 laboratories, including
walls, ceilings, and oors.
Spike-and-recovery experiments: Studies based on deliberately adding
a specic agent (i.e., spiking) and subsequently measuring the removal or
inactivation during inactivation steps. Also known as bioburden reduction studies.
Sporicide: A substance, or mixture of substances, that irreversibly inactivates
bacterial spores in the inanimate environment.
Sta: All full-time equivalent employees and part-time employees, as well as
other categories (e.g., students, fellows, and guest researchers) at a facility, who
are provided occupational health and other services by the institution.
Sterility assurance level (SAL): The probability of survival of microorganisms
after terminal sterilization, and a predictor of the ecacy of the process.
Sterilant: A substance or mixture of substances that destroys or eliminates
all forms of microbial life in the inanimate environment including all forms of
vegetative bacteria, bacterial spores, fungi, fungal spores, and viruses.
Sterilization: A physical or chemical process that kills or inactivates all microbial
life forms including highly resistant bacterial spores.
Tested cell lines: Human cell lines that have been tested for bloodborne
pathogens. Also refers to cell lines that have been tested to demonstrate the
absence of specic pathogens.
Tissue source: The organism, or the organ, from which a specic tissue was
removed for scientic use.
Tissue type: Animal tissue is categorized into one of four types—connective,
muscle, nervous, and epithelial.
Toxicity testing: A process to conrm ecacy of the inactivation procedure by
demonstrating the toxin is no longer capable of exerting a toxic eect.
Trigger point: A recognized combination of diagnostic ndings that can be
used to determine when to heighten the precautions or conditions for handling a
sample or culture.
Validated inactivation procedure: A procedure that renders a microorganism
non-viable but allows the microorganism to retain characteristics of interest for
future use; the ecacy is conrmed by data generated from a viability testing
protocol.
558 Biosafety in Microbiological and Biomedical Laboratories
Validation: Establishment of the performance characteristics of a method and
provision of objective evidence that the performance requirements for a specied
intended use are fullled.
Verication: Demonstration that a validated method functions in the user’s hands
according to the method’s specications determined in the validation study and is
t for purpose.
Viability testing protocol: A process to conrm ecacy of the inactivation
procedure by demonstrating the material is free of all viable pathogens.
559Index
Index
A
African horse sickness 308
Allergic reactions 234
Animal Biosafety Level-Agriculture
Animal Biosafety Level 2 Agriculture
ABSL-2Ag 30
,
71
,
424
,
429
,
430
,
431
,
432
,
433
,
544
Animal Biosafety Level 3 Agriculture
ABSL-3Ag 30
,
65
,
71
,
107
,
110
,
114
,
117
,
262
,
306
,
326
,
329
,
424
,
433
,
434
,
435
,
436
,
437
,
438
,
440
,
441
,
442
,
443
,
444
,
544
Animal Biosafety Level 4 Agriculture
ABSL-4Ag 30
,
65
,
71
,
107
,
110
,
114
,
117
,
424
,
443
,
444
,
445
,
446
,
544
Animal Biosafety Levels 30
,
70
,
71
ABSL 28
,
30
,
61
,
65
,
68
,
70
,
71
,
72
,
76
,
78
,
84
,
87
,
92
,
94
,
96
,
97
,
98
,
102
,
103
,
106
,
107
,
108
,
110
,
112
,
114
,
116
,
117
,
140
,
149
,
152
,
153
,
155
,
156
,
158
,
160
,
161
,
163
,
164
,
165
,
166
,
168
,
169
,
171
,
172
,
173
,
174
,
176
,
177
,
179
,
181
,
182
,
184
,
185
,
186
,
188
,
189
,
190
,
213
,
214
,
216
,
217
,
218
,
227
,
229
,
231
,
233
,
240
,
243
,
248
,
249
,
250
,
251
,
252
,
255
,
260
,
261
,
262
,
265
,
267
,
270
,
271
,
276
,
278
,
303
,
306
,
326
,
329
,
424
,
426
,
427
,
428
,
429
,
430
,
431
,
432
,
433
,
434
,
435
,
436
,
437
,
438
,
439
,
440
,
441
,
442
,
443
,
444
,
445
,
446
,
457
,
510
,
513
,
544
Animal Biosafety Level 1 71
ABSL-1 71
,
72
,
76
,
78
Animal Biosafety Level 2 78
,
424
,
429
,
544
ABSL-2 28
,
71
,
78
,
84
,
87
,
149
,
152
,
158
,
160
,
161
,
163
,
164
,
165
,
168
,
169
,
171
,
172
,
173
,
174
,
176
,
177
,
179
,
181
,
182
,
184
,
185
,
186
,
188
,
189
,
213
,
214
,
216
,
217
,
218
,
227
,
229
,
231
,
233
,
243
,
248
,
250
,
251
,
252
,
260
,
261
,
265
,
267
,
270
,
271
,
276
,
424
,
426
,
429
Animal Biosafety Level 3 30
,
87
,
433
,
510
,
513
,
544
ABSL-3 68
,
87
,
92
,
94
,
96
,
97
,
98
,
140
,
153
,
155
,
156
,
160
,
166
,
174
,
190
,
240
,
250
,
261
,
262
,
265
,
270
,
278
,
303
,
306
,
424
,
427
,
428
,
429
,
433
,
439
,
442
,
446
,
457
,
510
,
513
Animal Biosafety Level 4 98
,
443
,
544
ABSL-4 61
,
65
,
98
,
102
,
103
,
106
,
108
,
110
,
112
,
114
,
116
,
140
,
248
,
249
,
255
,
270
,
424
,
429
,
433
,
443
,
444
,
445
Anthrax 148
,
191
,
192
,
193
,
544
Arboviruses 292
,
295
,
298
,
308
,
327
,
333
560 Biosafety in Microbiological and Biomedical Laboratories
Arenaviruses 307
Arthropod containment 458
,
459
Ascaris 234
B
Bacillus anthracis 148
,
192
,
408
,
411
,
413
,
491
,
501
,
534
Bacillus subtilis 28
,
404
Biological agents 27
,
407
Biological safety cabinets
BSCs 16
,
24
,
25
,
36
,
37
,
40
,
42
,
43
,
50
,
58
,
60
,
61
,
64
,
65
,
68
,
69
,
70
,
83
,
86
,
87
,
93
,
96
,
97
,
98
,
106
,
108
,
109
,
112
,
114
,
242
,
294
,
363
,
367
,
368
,
369
,
370
,
371
,
372
,
374
,
375
,
376
,
377
,
378
,
379
,
380
,
381
,
384
,
385
,
386
,
387
,
388
,
424
,
438
,
442
,
471
,
532
,
535
Class I 24
,
367
,
368
,
370
,
377
,
388
,
390
Class II 30
,
61
,
98
,
109
,
216
,
217
,
218
,
294
,
335
,
347
,
367
,
370
,
371
,
372
,
373
,
374
,
375
,
377
,
378
,
381
,
386
,
387
,
388
,
389
,
390
,
391
,
392
,
393
,
395
,
471
,
475
,
532
Class III 30
,
50
,
51
,
57
,
58
,
65
,
97
,
98
,
104
,
105
,
106
,
114
,
367
,
375
,
376
,
394
,
397
,
471
,
475
Biorisk 20
,
119
,
129
,
515
,
541
,
543
,
545
,
552
Biosafety Levels 4
,
9
,
11
,
27
,
30
,
32
,
68
,
70
,
71
,
120
,
139
,
261
,
263
,
298
,
299
,
307
,
367
,
368
,
459
Biosafety Level 1 4
,
28
,
32
,
71
BSL-1 4
,
5
,
28
,
29
,
32
,
37
,
71
,
270
,
388
,
510
Biosafety Level 2 4
,
28
,
30
,
37
,
78
,
424
,
429
,
544
BSL-2 4
,
14
,
28
,
29
,
30
,
37
,
40
,
119
,
149
,
152
,
153
,
155
,
156
,
158
,
160
,
161
,
163
,
164
,
165
,
167
,
168
,
169
,
170
,
172
,
173
,
174
,
176
,
177
,
179
,
180
,
182
,
183
,
185
,
186
,
188
,
189
,
190
,
213
,
214
,
216
,
217
,
218
,
219
,
227
,
229
,
231
,
233
,
240
,
242
,
243
,
248
,
251
,
252
,
255
,
258
,
260
,
261
,
265
,
267
,
270
,
271
,
272
,
276
,
278
,
279
,
292
,
293
,
294
,
295
,
301
,
304
,
306
,
307
,
335
,
339
,
342
,
347
,
357
,
359
,
360
,
384
,
424
,
426
,
459
,
468
,
472
,
510
,
531
,
538
,
541
Biosafety Level 3 4
,
29
,
30
,
43
,
87
,
117
,
246
,
331
,
397
,
433
,
510
,
513
,
544
BSL-3 4
,
27
,
29
,
43
,
47
,
51
,
68
,
92
,
119
,
122
,
140
,
143
,
149
,
152
,
153
,
155
,
156
,
160
,
161
,
166
,
169
,
174
,
175
,
177
,
179
,
182
,
190
,
213
,
214
,
216
,
240
,
241
,
242
,
243
,
248
,
252
,
255
,
258
,
261
,
262
,
265
,
270
,
271
,
272
,
276
,
278
,
292
,
294
,
295
,
296
,
297
,
301
,
303
,
306
,
307
,
329
,
335
,
339
,
357
,
360
,
379
,
384
,
405
,
407
,
424
,
427
,
428
,
429
,
436
,
439
,
446
,
457
,
473
,
510
,
513
,
557
561Index
Biosafety Level 4 4
,
29
,
51
,
98
,
443
,
544
BSL-4 4
,
16
,
29
,
30
,
51
,
52
,
55
,
56
,
58
,
59
,
61
,
62
,
63
,
65
,
103
,
110
,
114
,
122
,
140
,
249
,
255
,
270
,
296
,
297
,
307
,
372
,
384
,
388
,
405
,
407
,
424
,
429
,
445
BSL 4
,
5
,
14
,
16
,
27
,
28
,
29
,
30
,
32
,
37
,
40
,
43
,
47
,
51
,
52
,
55
,
56
,
58
,
59
,
61
,
62
,
63
,
65
,
68
,
69
,
71
,
92
,
103
,
110
,
114
,
119
,
122
,
131
,
140
,
143
,
149
,
152
,
153
,
155
,
156
,
158
,
160
,
161
,
163
,
164
,
165
,
166
,
167
,
168
,
169
,
170
,
172
,
173
,
174
,
175
,
176
,
177
,
179
,
180
,
182
,
183
,
185
,
186
,
188
,
189
,
190
,
213
,
214
,
216
,
217
,
218
,
219
,
227
,
229
,
231
,
233
,
240
,
241
,
242
,
243
,
248
,
249
,
251
,
252
,
255
,
258
,
260
,
261
,
262
,
265
,
267
,
270
,
271
,
272
,
276
,
278
,
279
,
292
,
293
,
294
,
295
,
296
,
297
,
301
,
303
,
304
,
306
,
307
,
308
,
329
,
335
,
339
,
342
,
347
,
357
,
359
,
360
,
367
,
372
,
379
,
384
,
388
,
405
,
407
,
424
,
426
,
427
,
428
,
429
,
436
,
439
,
445
,
446
,
457
,
459
,
468
,
472
,
473
,
510
,
513
,
531
,
538
,
541
,
545
,
557
Biosafety Ocer 545
BSO 19
,
70
,
387
,
545
Biosecurity 6
,
20
,
23
,
31
,
119
,
120
,
122
,
123
,
124
,
129
,
144
,
524
,
528
,
541
,
543
Bioterrorism 348
,
460
Blastomyces dermatitidis 1
,
212
,
219
Bloodborne pathogens 21
,
31
,
118
,
143
,
466
,
469
,
542
,
552
Bordetella pertussis 150
,
193
,
194
Botulinum neurotoxin 478
,
480
,
544
Botulism 198
,
334
,
336
,
347
,
348
,
481
,
544
Brucella 1
,
2
,
8
,
152
,
153
,
154
,
194
,
195
,
447
,
503
abortus 152
,
154
,
194
,
195
,
447
,
503
canis 152
,
167
,
168
,
234
,
235
,
447
,
449
,
451
melitensis 2
,
8
,
152
,
154
,
194
,
447
suis 152
,
154
,
447
,
449
,
452
,
503
Brucellosis 153
,
194
,
195
Burkholderia mallei 154
,
195
Burkholderia pseudomallei 156
,
195
,
196
B virus 1
,
12
,
13
,
22
,
28
,
31
,
132
,
138
,
146
,
251
,
253
,
254
,
255
,
256
,
257
,
258
,
259
,
282
,
283
,
284
,
303
,
466
,
467
.
See also Macacine herpesvirus
C
Campylobacter 157
,
158
,
196
,
197
,
202
,
209
,
447
coli 182
,
183
,
208
,
209
,
447
,
448
,
503
,
554
562 Biosafety in Microbiological and Biomedical Laboratories
fetus 157
,
171
,
172
,
197
,
258
,
447
,
451
jejuni 196
,
197
,
209
,
447
Chimeric viruses 299
Chlamydia 2
,
14
,
158
,
159
,
160
,
197
,
198
,
447
pneumoniae 158
,
159
,
160
,
198
,
447
psittaci 2
,
14
,
158
,
159
,
160
,
197
,
198
,
447
trachomatis 158
,
159
,
160
,
198
,
447
Cholera 210
,
211
Clean 362
,
376
,
394
,
395
,
399
,
518
,
545
,
552
Clostridium 161
,
162
,
163
,
198
,
199
,
200
,
334
,
335
,
337
,
348
,
404
,
447
,
454
,
479
,
481
,
482
,
503
botulinum 161
,
198
,
334
,
336
,
347
,
348
,
447
,
470
,
478
,
479
,
480
,
481
,
482
tetani 163
,
200
Coccidioides immitis 2
,
14
,
213
,
220
Coccidioides posadasii 213
,
220
Congo-Crimean hemorrhagic fever 29
Conidia 215
Containment 4
,
57
,
71
,
105
,
118
,
146
,
149
,
151
,
153
,
155
,
156
,
157
,
159
,
161
,
163
,
164
,
165
,
166
,
168
,
169
,
170
,
171
,
172
,
174
,
176
,
177
,
179
,
180
,
182
,
183
,
185
,
186
,
187
,
189
,
190
,
191
,
211
,
213
,
214
,
216
,
217
,
222
,
227
,
229
,
231
,
232
,
235
,
240
,
242
,
247
,
249
,
251
,
252
,
254
,
258
,
260
,
264
,
266
,
267
,
269
,
271
,
275
,
278
,
287
,
293
,
294
,
295
,
297
,
301
,
303
,
306
,
326
,
330
,
335
,
339
,
342
,
346
,
357
,
367
,
368
,
386
,
397
,
457
,
458
,
459
,
472
,
513
,
519
,
520
,
544
,
553
,
571
Primary containment 106
,
152
,
155
,
376
Corynebacterium diphtheriae 164
,
201
Coxiella burnetii 1
,
2
,
13
,
14
,
29
,
239
,
244
,
245
Creutzfeldt-Jakob 355
,
363
,
364
,
365
,
408
,
545
,
549
Cytomegalovirus 287
,
546
D
Decontamination 48
,
59
,
62
,
64
,
66
,
83
,
92
,
107
,
111
,
113
,
115
,
383
,
384
,
398
,
400
,
403
,
405
,
406
,
410
,
411
,
435
,
436
,
438
,
476
,
477
,
480
,
518
,
545
,
553
Dengue 312
Dermatophytes 217
Epidermophyton 218
,
449
Microsporum 218
,
449
Trichophyton 218
,
404
,
449
563Index
Diphtheria 152
,
200
,
545
Disinfection 400
,
401
,
402
,
410
,
411
,
412
,
413
,
553
,
554
DOT Packaging Requirements 418
E
Ebola 146
,
310
,
312
,
321
,
323
,
324
,
404
,
501
,
529
,
533
,
541
Encephalitis 295
,
302
,
305
,
308
,
309
,
310
,
311
,
312
,
313
,
314
,
316
,
317
,
319
,
320
,
321
,
322
,
323
,
324
,
325
,
329
,
453
,
454
,
545
,
550
,
551
Epidermophyton 218
,
449
Escherichia coli 182
,
208
,
209
,
503
F
Facility 16
,
25
,
26
,
50
,
59
,
63
,
71
,
73
,
79
,
88
,
98
,
99
,
104
,
106
,
108
,
112
,
367
,
384
,
427
,
430
,
434
,
444
,
457
,
464
,
481
,
504
,
554
Facility design 50
Fasciola 232
,
450
Francisella tularensis 1
,
14
,
165
Fungal Agents 212
G
Giardia 228
,
450
Gloves 34
,
38
,
44
,
53
,
58
,
74
,
80
,
89
,
100
,
101
,
105
,
118
,
252
,
360
,
383
,
409
Gonorrhea 177
Guidelines 3
,
8
,
11
,
12
,
17
,
21
,
23
,
27
,
28
,
71
,
131
,
142
,
143
,
144
,
145
,
150
,
163
,
186
,
191
,
211
,
255
,
270
,
284
,
288
,
293
,
330
,
365
,
397
,
413
,
439
,
456
,
458
,
469
,
470
,
484
,
485
,
509
,
513
,
515
,
524
,
527
,
541
,
542
,
543
,
544
,
554
,
555
H
Hantaviruses 247
,
307
Helicobacter pylori 167
,
201
,
202
Hemorrhagic fever 280
,
304
Hendra virus 248
,
249
,
281
HEPA lter 49
,
51
,
57
,
62
,
65
,
66
,
93
,
97
,
105
,
110
,
114
,
115
,
329
,
363
,
369
,
370
,
371
,
372
,
373
,
374
,
375
,
376
,
385
,
386
,
387
,
388
,
390
,
391
,
392
,
393
,
394
,
395
,
396
,
398
,
428
,
440
,
441
,
474
Hepatitis 28
,
250
,
251
,
252
,
282
,
283
,
452
,
453
,
466
,
546
A virus 250
,
251
,
259
,
285
,
502
564 Biosafety in Microbiological and Biomedical Laboratories
B virus
HBV 1
,
2
,
12
,
251
,
252
,
253
,
283
,
404
,
466
,
469
,
546
C virus 2
,
12
,
251
,
252
,
282
,
283
,
466
HCV 12
,
138
,
251
,
252
,
253
,
283
,
404
,
466
,
469
,
546
D virus 251
HDV 251
,
252
,
253
,
546
E virus 250
,
251
Histoplasma 215
,
216
,
449
capsulatum 215
,
216
,
449
farciminosum 449
Human immunodeciency virus
HIV 12
,
28
,
132
,
174
,
217
,
257
,
273
,
274
,
275
,
276
,
282
,
289
,
331
,
404
,
466
,
469
,
547
Hypr 295
,
314
I
Inuenza 21
,
259
,
260
,
261
,
262
,
285
,
286
,
453
,
547
Institutional Biosafety Committee 7
,
32
,
72
,
79
,
87
,
99
,
468
,
547
,
555
IBC 10
,
16
,
18
,
32
,
70
,
72
,
79
,
87
,
99
,
262
,
276
,
299
,
300
,
485
,
547
,
555
Investigational New Drug 547
IND 296
,
336
,
547
J
Junin virus 295
,
331
K
Kuru 355
,
356
,
358
Kyasanur Forest disease 316
L
Laboratory-associated infection 2
,
6
,
8
,
11
,
21
,
22
,
70
,
118
,
122
,
129
,
130
,
141
,
142
,
143
,
144
,
145
,
148
,
168
,
169
,
171
,
172
,
191
,
193
,
194
,
195
,
196
,
197
,
198
,
199
,
200
,
201
,
203
,
204
,
205
,
207
,
208
,
209
,
210
,
211
,
219
,
220
,
221
,
222
,
224
,
226
,
228
,
235
,
237
,
238
,
239
,
245
,
246
,
247
,
257
,
258
,
280
,
281
,
282
,
283
,
284
,
285
,
286
,
287
,
288
,
289
,
290
,
293
,
330
,
331
,
332
,
333
,
334
,
335
,
338
,
340
,
345
,
347
,
348
,
349
,
350
,
351
,
353
,
355
,
360
,
361
,
362
,
363
,
364
,
365
,
367
,
370
,
371
,
374
,
378
,
379
,
384
,
388
,
392
,
398
,
401
,
403
,
406
,
407
,
408
,
410
,
411
,
412
,
419
,
421
,
426
,
433
,
447
,
448
,
449
,
450
,
451
,
452
,
453
,
454
,
455
,
456
,
467
,
469
,
475
,
565Index
476
,
479
,
480
,
482
,
486
,
488
,
491
,
501
,
502
,
503
,
506
,
512
,
519
,
529
,
541
,
542
,
543
,
553
,
561
,
564
LAI 1
,
2
,
6
,
9
,
17
,
21
,
130
,
134
,
136
,
137
,
138
,
139
,
140
,
141
,
142
,
144
,
145
,
157
,
168
,
169
,
171
,
172
,
178
,
180
,
182
,
184
,
185
,
194
,
195
,
196
,
197
,
199
,
200
,
203
,
204
,
207
,
208
,
209
,
212
,
224
,
226
,
239
,
263
,
281
,
282
,
284
,
285
,
286
,
287
,
288
,
290
,
295
,
332
,
340
,
345
,
347
,
348
,
350
,
351
,
360
,
365
,
406
,
407
,
408
,
410
,
411
,
448
,
451
,
456
,
469
,
480
,
502
,
503
,
539
,
541
,
542
,
543
,
547
Laboratory biosecurity 120
,
122
,
124
,
128
,
555
Laboratory security and emergency response 128
Legionella pneumophila 168
Leishmania spp. 223
,
224
,
226
,
451
Leprosy 172
,
204
Leptospira interrogans 202
Leptospirosis 170
,
202
Listeria monocytogenes 171
,
203
Loose-housed animals 262
Low molecular weight toxin
LMW 343
,
346
,
347
,
479
,
480
,
548
Lumpy skin disease virus
LSDV 548
Lymphocytic choriomeningitis 264
,
286
,
287
,
293
,
317
Lymphogranuloma venereum
LGV 158
,
159
,
160
,
547
M
Macacine herpesvirus 13
,
467
MHV-1 13
Malaria 223
,
224
,
226
Mammalian Cells and Tissues 466
Marburg 29
,
318
,
321
,
501
Mask 555
Menangle virus
MenV 548
Mice 22
,
264
,
286
566 Biosafety in Microbiological and Biomedical Laboratories
Microsporum 218
,
449
Molds 218
,
219
,
449
Monkeypox 269
,
453
Mycobacterium 1
,
2
,
21
,
29
,
132
,
172
,
173
,
175
,
176
,
203
,
204
,
205
,
402
,
404
,
448
,
466
,
530
,
548
avium 175
,
176
,
205
,
448
bovis 173
,
174
,
175
,
204
,
205
,
402
,
404
,
448
,
450
fortuitum 176
,
448
kansasii 175
,
176
leprae 172
,
175
marinum 176
,
205
,
448
scrofulaceum 175
,
448
ulcerans 164
,
176
,
201
N
Naegleria 28
,
223
,
226
fowleri 223
,
226
Nairobi sheep disease 319
Neisseria 177
,
178
,
206
,
534
gonorrhoeae 177
,
178
meningitidis 178
,
179
,
533
,
534
Nematode parasites 233
Newcastle disease virus
NDV 320
,
548
NIH Guidelines 3
,
8
,
11
,
12
,
17
,
21
,
27
,
28
,
131
,
143
,
270
,
288
,
458
,
469
,
484
,
485
,
515
,
524
,
554
,
555
O
Occupational health 118
,
130
,
131
,
141
Orientia 241
,
448
tsutsugamushi 241
,
448
P
Paramyxovirus 544
Parasitic agents
Blood and Tissue Protozoal Parasites 223
Cestode 230
567Index
Intestinal 228
Nematode 233
Trematode 232
Pathogens 28
,
192
,
196
,
202
,
207
,
290
,
348
,
365
,
402
,
410
,
416
,
418
,
423
,
424
,
426
,
427
,
429
,
531
,
537
,
542
,
554
Performance Testing BSCs 388
Personal protective equipment 25
,
468
Pest management 464
,
465
Pike and Sulkin 1
,
2
Plague 190
,
207
,
211
Plasmodium spp. 223
,
224
,
226
Poliovirus 265
,
266
,
267
,
287
,
404
,
547
,
548
,
549
Pontiac fever 169
Poxviruses 268
,
288
Primary barriers 24
Primates 70
,
144
,
283
,
455
,
456
Pseudomonas 196
,
404
,
447
,
448
pseudomallei 156
,
157
,
195
,
196
,
447
Q
Q fever 239
,
240
,
244
,
245
R
Rabies virus 271
,
273
Radiological Hazards 378
Regulations 23
,
70
,
131
,
143
,
240
,
244
,
400
,
408
,
415
,
416
,
417
,
418
,
419
,
421
,
433
,
438
,
526
,
542
,
545
Respirator 548
,
556
Retroviruses 273
,
274
,
289
,
331
Rickettsia 241
,
242
,
244
,
245
,
246
,
448
akari 241
australis 241
bellii 243
canadensis 167
,
168
,
243
conorii 241
montanensis 243
568 Biosafety in Microbiological and Biomedical Laboratories
prowazekii 241
,
244
,
448
rhipicephali 243
rickettsii 241
,
242
,
243
,
244
,
246
,
448
sibirica 241
spotted fever group 242
typhi 241
,
448
Rickettsial Agents 239
Rift Valley fever virus 333
Risk assessment 1
,
9
,
16
,
19
,
20
,
141
,
237
,
468
,
515
,
529
Rocky Mountain spotted fever 241
S
Safety equipment 16
,
24
Salmonella 1
,
2
,
28
,
179
,
180
,
181
,
207
,
208
,
404
,
448
,
501
S. enterica serotype Typhi 179
,
181
SALS 292
,
293
,
295
,
297
,
298
,
301
,
307
,
549
SARS-associated coronavirus 277
SARS-CoV 276
,
277
,
278
,
279
,
280
,
549
Schistosoma spp. 232
,
233
Secondary barriers 29
,
30
Select agents 408
Serum 134
,
248
,
346
Severe Acute Respiratory Syndrome 276
,
290
,
291
,
549
SARS 276
,
277
,
278
,
279
,
280
,
290
,
291
,
452
,
467
,
549
Sharps 297
,
301
,
410
Sheep and Goat Pox Virus 550
SGPV 550
Shiga toxin 182
,
183
,
454
,
479
,
480
,
482
,
503
Shigella spp. 2
,
183
,
184
,
185
,
448
Shigellosis 184
Shipment 8
,
331
Simian immunodeciency virus 132
,
289
SIV 132
,
273
,
274
,
275
,
276
,
467
,
550
Smallpox 269
,
288
Sporothrix schenckii 216
,
222
569Index
Spring viremia of carp virus
SVCV 550
Standard Precautions 276
,
279
,
290
,
291
,
358
,
359
,
531
,
541
Staphylococcal Enterotoxins 337
,
349
,
549
SE 204
,
207
,
337
,
338
,
339
,
340
,
350
,
479
,
549
,
550
Sterilization 335
,
359
,
401
,
403
,
407
,
410
,
411
,
412
,
413
,
550
Strongyloides spp 234
,
235
Surveillance 145
,
175
,
206
,
243
,
273
,
288
,
365
Swine vesicular disease virus
SVDV 550
Syphilis 187
,
210
T
Taenia solium 230
Tetanus 152
,
163
,
164
,
200
,
545
Theileria 451
annulata 451
bovis 173
,
174
,
175
,
204
,
205
,
402
,
404
,
448
,
450
Toxin Agents 334
Toxoplasma 28
,
223
,
451
Training 18
,
33
,
38
,
44
,
53
,
73
,
80
,
89
,
100
,
128
,
254
,
463
,
471
,
500
,
524
,
535
Transmissible spongiform encephalopathies 355
TSE 355
,
356
,
361
,
413
,
550
Trematode parasites
Fasciola 232
,
450
Schistosoma 232
,
233
Treponema pallidum 187
,
210
Trichophyton 218
,
404
,
449
Trypanosoma
cruzi 223
,
224
,
225
,
226
,
227
,
451
,
452
evansi 452
vivax 224
,
226
,
452
Tuberculosis 204
,
205
,
548
Tularemia 166
,
201
Typhoid fever 181
,
208
570 Biosafety in Microbiological and Biomedical Laboratories
U
Ultraviolet lamps
UV lamps 385
V
Vaccines 26
,
134
,
150
,
152
,
154
,
155
,
160
,
164
,
165
,
167
,
175
,
179
,
181
,
182
,
185
,
187
,
188
,
189
,
191
,
192
,
193
,
194
,
200
,
208
,
240
,
243
,
250
,
251
,
253
,
258
,
265
,
296
,
304
,
306
,
336
,
340
,
342
,
347
,
348
Vaccinia 268
,
269
,
288
,
548
Variola 270
Venezuelan equine encephalomyelitis 294
Vibrio 188
,
189
,
210
,
211
,
449
cholerae 188
,
189
,
210
,
211
,
449
parahaemolyticus 188
,
189
,
211
,
449

571Accessibility Descriptions of Figures
Accessibility Descriptions of Figures
Appendix A—Primary Containment for Biohazards
Figure 1. HEPA Filters
HEPA lter consisting of a square wooden frame that contains borosilicate lter
media that is wrapped around supporting aluminum columns.
RETURN TO FIGURE
Figure 2. The Class I BSC
Cut away side view of Class I BSC. Arrows show air owing into the unit from
the bottom front sash then through the plenum at the back of the unit, and then
exiting the unit through a HEPA lter at the top of the unit.
RETURN TO FIGURE
Figure 3. The Class II, Type A BSC
Cut away side view of Class II Type A BSC. Arrows show air owing into the unit
from the bottom front sash and then being pulled by a fan up through a plenum at
the back of the unit. Upon exiting the fan, 30% of the air is exhausted through a
HEPA lter at the top of the unit and 70% is driven through a separate HEPA lter
and down onto the work surface of the cabinet.
RETURN TO FIGURE
Figure 4. Canopy (thimble) unit for ducting a Class II, Type A BSC
Cut away side view of a thimble unit positioned over the exhaust port of a BSC.
The thimble overlaps the exhaust port by 1 inch on each side. The thimble is in
the shape of a pyramid, with the wide bottom positioned above the exhaust port
of the BSC and the narrow top connecting to a pipe representing the building
exhaust system.
RETURN TO FIGURE
Figure 5a. The Class II, Type B1 BSC (classic design)
Cut away side view of a Class II Type B1 BSC. The unit has three HEPA lters
positioned above the work surface, below the work surface, and at the exhaust
port at the top of the unit. A fan is positioned at the bottom of the unit, below the
HEPA lter under the work surface. Arrows show air ow into the unit through
the front sash and then in two directions within the unit. One direction is down
through the HEPA lter under the work surface and then up through a plenum to
the top of the unit, where it is driven down through a second HEPA lter to the
work surface. The second airow direction is through the back of the work surface
to a separate plenum, and then out through a HEPA lter at the exhaust port
at the top of the unit. The unit is directly connected (no thimble) to the building
exhaust system.
RETURN TO FIGURE
Figure 5b. The Class II, Type B1 BSC (benchtop design)
Cut away side view of a Class II type B1 BSC designed to sit on a bench top.
The unit has two HEPA lters, one positioned above the work surface and the
second at the exhaust port at the top of the unit. A fan is positioned in the top

572 Biosafety in Microbiological and Biomedical Laboratories
of the unit, above the HEPA lter that sits above the work surface. Arrows show
air ow into the unit through the front sash, down under the work surface, and
through a plenum which directs it to the fan, which drives us through a HEPA lter
down to the work surface. The HEPA-ltered air then splits just above the work
surface, with one portion returning to the fan through the original plenum and a
second portion owing through a separate plenum to the exhaust port, where it is
exhausted through a HEPA lter. The unit is directly connected (no thimble) to the
building exhaust system.
RETURN TO FIGURE
Figure 6. The Class II, Type B2 BSC
Cut away side view of a Class II Type B2 BSC. Two HEPA lters are shown. One
is positioned above the work surface and the second is positioned at the exhaust
port at the top of the unit. A fan is located in the top of the unit, above the HEPA
lter that is positioned above the work surface. Arrows show air being drawn into
the unit through the top of the unit and front sash, and then directed under the
work surface. Air is then pulled up through an exhaust plenum and HEPA lter
at the exhaust port. The unit is directly connected (no thimble) to the building
exhaust system.
RETURN TO FIGURE
Figure 7a. The Class II, Type C1 BSC (not connected to building exhaust system)
Cut away side view of a Class II, Type C1 BSC. Two HEPA lters are shown, one
positioned directly above the work surface and one located a the exhaust port
on the top of the unit. Two fans are shown, one located directly above the HEPA
lter that is located above the work surface, and one located directly below the
HEPA lter that is located at the exhaust port at the top of the unit. Arrows show air
owing into the unit through the front sash, down under the work surface, and then
up through a plenum to a space above the work surface, where it is driven by a fan
down through a HEPA lter to the work surface. The HEPA-ltered air then splits
slightly above the work surface and is either recirculated back through the original
plenum, or is pulled into a separate exhaust plenum and is exhausted through
the second HEPA lter. The unit is not connected to the building exhaust system.
RETURN TO FIGURE
Figure 7b. The Class II, Type C1 BSC (connected to building exhaust system)
Cut away side view of a Class II, Type C1 BSC that is connected to the building
exhaust system. Two HEPA lters are shown, one positioned directly above the
work surface and one located a the exhaust port on the top of the unit. Two fans
are shown, one located directly above the HEPA lter that is located above the
work surface, and one located directly below the HEPA lter that is located at the
exhaust port at the top of the unit. Arrows show air owing into the unit through
the front sash, down under the work surface, and then up through a plenum to a
space above the work surface, where it is driven by a fan down through a HEPA
lter to the work surface. The HEPA-ltered air then splits slightly above the work
573Accessibility Descriptions of Figures
surface and is either recirculated back through the original plenum, or is pulled
into a separate exhaust plenum and is exhausted through the second HEPA
lter. The BSC is connected to the building exhaust system by a thimble unit that
overlaps the exhaust port and provides a 1-inch gap to that allows for room air to
be drawn in to balance the building exhaust system.
RETURN TO FIGURE
Figure 8. The Class III BSC
Front view and cut away side view of Class III BSC. The front view shows two
sets of glove ports (four ports total) arranged in a line below a viewing window
that spans the width of the cabinet. A double ended pass through box is attached
to the left side of the cabinet to allow for the moving of materials into and out
of the cabinet. Two HEPA lters are located on the top of the cabinet; one is
located at the air intake port and the second is located at the exhaust port. The
cabinet is direct connected to and exhaust duct that contains an additional HEPA
lter, thus providing for double HEPA ltration of exhaust air. The cut away side
view shows a human hand inside of the glove port holding an item inside of the
cabinet. Two HEPA lters are located on the top of the cabinet; one is located at
the air intake port and the second is located at the exhaust port. The cabinet is
direct connected to and exhaust duct that contains an additional HEPA lter, thus
providing for double HEPA ltration of exhaust air.
RETURN TO FIGURE
Figure 9a. The Horizontal Laminar ow Clean Bench
Cut away side view of a horizontal laminar ow clean bench. There is a broad
opening in the front of the unit and a HEPA lter located in the rear of the work
area. There is a plenum between the HEPA lter and the back wall of the unit,
and a fan located in the bottom of the unit. Airow arrows show air entering the
unit from a port on the front and beneath the work surface. The fan drives the
incoming air up the plenum, through the HEPA lter, and across the work surface.
The HEPA ltered air then exits the unit out the front opening and toward the
worker.
RETURN TO FIGURE
Figure 9b. The Vertical Laminar Flow Clean Bench
Cut away side view of a vertical laminar ow clean bench. The unit contains a
HEPA lter located above the work surface and a fan in the space above the
HEPA lter. Airow arrows show air entering the unit through a port in the top of
the unit, and then being driven by the fan down through the HEPA lter and on
to the work surface. The HEPA ltered air then exits the unit through the front
opening toward the worker.
RETURN TO FIGURE
Figure 10. Clean to Dirty
Front view of a BSC that contains equipment and materials typically used in
biological manipulations. The equipment in the BSC is oriented for use by a
right-handed worker. “Clean” materials, such as sterile culture media or buer

574 Biosafety in Microbiological and Biomedical Laboratories
containers are located on the left side of the work surface. “Dirty” materials such
as waste containers are located on the right side of the work surface. This order
would be reversed if the worker was left-handed.
RETURN TO FIGURE
Figure 11. Protection of a house vacuum
Two vacuum asks and an in-line HEPA lter are connected in series by vacuum
lines to a port for house vacuum. Material is drawn into the rst ask, which
contains a decontamination solution. The rst ask is connected by a vacuum line
to a second empty ask, which provides overow protection for the rst ask. An
in-line HEPA lter is located between the overow ask and the house vacuum port.
RETURN TO FIGURE
Figure 12. Bag-in/bag-out lter enclosure
Exploded view of a large square HEPA housing unit that contains two HEPA lter
assemblies one stacked on the other. The Upper lter assembly is exploded out
to show the lter unit, removal bag, support straps, and housing lid are arranged
to allow for removal of a contaminated lter using the pre-packed bag already
present in the housing. The lower lter assembly is shown only with the housing lid
removed to illustrate how the lter and removal bag are packed into the housing.
RETURN TO FIGURE
Appendix C—Transportation of Infectious Substances
Figure 1. A Category A UN Standard Triple Packaging
Complete Category A packaging system, including the outer cardboard container
with required labels and a hard walled cylindrical secondary container with a
screw cap. The secondary container contains the sealable primary container
for the biological material, which may be glass, metal, or plastic, along with
absorbent material to capture any leakage within the secondary container.
RETURN TO FIGURE
Figure 2. A Category B Non-specication Triple Packaging
Complete Category B packaging system, including the outer cardboard container
with required labels, and a leak-proof secondary container, such as a sealable
plastic bag. The secondary container contains the sealable primary container
for the biological material, which may be glass, metal, or plastic, along with
absorbent material to capture any leakage within the secondary container.
RETURN TO FIGURE
Inside back cover

U.S. Department of Health and Human Services
Public Health Service
Centers for Disease Control and Prevention
National Institutes of Health
HHS Publication No. (CDC) 300859
Revised June 2020
CS308133-A
