AN ISO 9001:2000 CERTIFIED ACADEMY
cliniminds
Academy for Clinical Research Training & Management
An ISO 9001:2000 Certified Academy
TM
Vision
TO ESTABLISH WORLDCLASS INSTITUION TO
OFFER WIDEST RANGE OF HGH QUALITY
CLINCIAL RESEARCH, HEALTHCARE AND
PHARMACEUTICAL PROGRAMS AND BUSINESS
SOLUTIONS
AN ISO 9001:2000 CERTIFIED ACADEMY
cliniminds
Academy for Clinical Research Training & Management
An ISO 9001:2000 Certified Academy
TM
• Tenet Health Edutech Pvt. Ltd. was established in 2004 by a team of
professionals from Clinical Research, Healthcare & Pharma industry.
• Cliniminds offers wide range of educational programs and training
solutions for the clinical research, pharma and healthcare industry..
• At present we offer over 23 educational and professional training
programs in pharma and clinical research using classroom; online and
corporate workshops mediums. Programs are backed up by the high
quality copyright content and faculty with global perspective.
• Cliniminds is Headquartered at Delhi, and have branches in Bangalore,
Hyderabad, Mumbai, Ahmedabad, Cochin, Bhopal and Vijaywada. New
branches are being set up in other cities as well.
• Also offer E-Learning solutions for the healthcare & pharma companies
and CROs.
Cliniminds Background
AN ISO 9001:2000 CERTIFIED ACADEMY
cliniminds
Academy for Clinical Research Training & Management
An ISO 9001:2000 Certified Academy
TM
• Classroom Professional Educational Programs in the
field of Clinical Research & Phrama in India –
Diploma & Certificate Programs.
• Online/Distance Learning – Global market
• Corporate Training Solutions
• E-Learning Solutions for Corporates
• Consulting Services
• Medical & Scientific Writing
Key Activities
AN ISO 9001:2000 CERTIFIED ACADEMY
cliniminds
Academy for Clinical Research Training & Management
An ISO 9001:2000 Certified Academy
TM
• Objective of this presentation is to seek International
Certification / Accreditation or Co-Certification of
Cliniminds’ Clinical Research etraining programs.
• Offer some of your clinical research and pharma industry
training and educational programs in India.
• Explore other options of some of Cliniminds programs at
your institution.
OBJECTIVE OF PRESENTATION
AN ISO 9001:2000 CERTIFIED ACADEMY
cliniminds
Academy for Clinical Research Training & Management
An ISO 9001:2000 Certified Academy
TM
BACKGROUND
Clinical research jobs are amongst most sought after
jobs segment in the healthcare industry with over
40% new top job offerings
Major gap in Demand & Supply of trained manpower
– An unmet need
With the shift of work to India; expected market
size of $2 billion and entry of new players, over
50,000 professionals will be required in 2012.
Current demand is over 12,000 professionals per
year.
Example : Qunitiles Corp, USA have alone hired over
2,500 new employees in the last 3 years.
AN ISO 9001:2000 CERTIFIED ACADEMY
cliniminds
Academy for Clinical Research Training & Management
An ISO 9001:2000 Certified Academy
TM
Background
The current facilities are able to train only
4,000 professionals.
Employers required well trained
professionals.
Attractive Salaries Offered & High Annual
Salary Growth.
AN ISO 9001:2000 CERTIFIED ACADEMY
cliniminds
Academy for Clinical Research Training & Management
An ISO 9001:2000 Certified Academy
TM
OPPORTUNITY
Global Clinical Research Training Size $2 billion
Market Size in 2005-06 $200 million
Estimated Market Size in 2009 $600 million
2012 Projected Industry Spending on
CRO Services and Investigator Grants $2 billion
Number of CROs - current 80
Number of MNC & Indian Pharmacos in
Clinical Research 70
Clinical Research Market Growth (Annual) 40%
Full time & Site Staff Required in 2012 50,000
New Protocols received by Indian Regulator
everyday 20
AN ISO 9001:2000 CERTIFIED ACADEMY
cliniminds
Academy for Clinical Research Training & Management
An ISO 9001:2000 Certified Academy
TM
Patients / Subjects Required in 2012 350,000
Over 3,000 new Certified Investigators will
be required in 2008 and over 12,000 by
2012
AN ISO 9001:2000 CERTIFIED ACADEMY
cliniminds
Academy for Clinical Research Training & Management
An ISO 9001:2000 Certified Academy
TM
INDIA HUB OF GLOBAL CLINCIAL
RESEARCH
India is one of the top 3 countries where companies plan to spend
the most R&D dollars over the next 3 years.
Favoured destination ahead of countries like Israel, Philippines,
Canada, China, Ireland & Russia in terms of Overall Climate
(Gartner Report, January 2003)
Powerhouse in R&D (e.g. GVK Wyeth Gloobal R&D Deal)
Over 60 CROs offer Phase I to IV trials complying with ICH-GCP
guidelines.
Over US$500 million foreign investment expected in the next 18
months
Over 200 hospitals serving as sites for clinical trials, India is
emerging as one of the fastest recruiter of subjects across the world.
Some of the top medical/technical universities in Asia
AN ISO 9001:2000 CERTIFIED ACADEMY
cliniminds
Academy for Clinical Research Training & Management
An ISO 9001:2000 Certified Academy
TM
INDIA HUB OF CLINICAL OF CLINCIAL RESEARCH
The clinical community is populated with English speaking,
western-trained graduates
Sophisticated technological infrastructure
100 million plus English speaking / trained professionals
(Largest outside US)
Over 2 million science post graduates and growing…
Large pool of treatment naïve patients from multiethnic and
multiracial backgrounds
Better patient recruitment, retention and compliance
Participants generally benefit, as the trials conducted in India,
mostly in phase II - IV, provide improved care and
cost savings
as procedures and drugs are provided at no charge
AN ISO 9001:2000 CERTIFIED ACADEMY
cliniminds
Academy for Clinical Research Training & Management
An ISO 9001:2000 Certified Academy
TM
Cost effective operations
Higher GMP/GLP/GCP Compliance
Maximum number of approved GMP plants outside USA
Excellent quality management, Technology and
infrastructure
Increasing presence of all Pharma majors, CROs & also
in-house CROs set up by leading pharma companies
Strong IT industry availability of IT skilled manpower

AN ISO 9001:2000 CERTIFIED ACADEMY
cliniminds
Academy for Clinical Research Training & Management
An ISO 9001:2000 Certified Academy
TM
INDIA’S VITAL CLINICAL STATISTICS
Cancer: 3 million
Diabetes: 34 million
HIV: 8-10 million
Epilepsy: 8 million
Hypertension: 150 million
Schizophrenia: 1 million
Asthma: 40 million
Alzheimer's: 1.5 million
Cardiac-Related Deaths: 2 million
Recruits for genetic studies
600,000 practicing physicians
14,000 hospitals
700,000 beds
17,000 medical graduates per year
AN ISO 9001:2000 CERTIFIED ACADEMY
cliniminds
Academy for Clinical Research Training & Management
An ISO 9001:2000 Certified Academy
TM
REGULATORY FRAMEWORK
Positive Regulatory Environment – Protocols Approved by
DCGI / Schedule Y
CDSCO (Central Drugs Standard Control Organisation) to
regulate Clinical Research
Further strengthening of environment by setting up
National Drug Authority
Intellectual property protection
• As of January 2005, recognize product patents
from1995 to present
Clinical Trial Protocol Approval Time is reducing
Duty Free Import of Clinical Trial Supplies
Easier Drug Importation Procedure
ICMR Guidelines on the Safety of Human Subjects
USFDA OFFICE IN INDIA
AN ISO 9001:2000 CERTIFIED ACADEMY
cliniminds
Academy for Clinical Research Training & Management
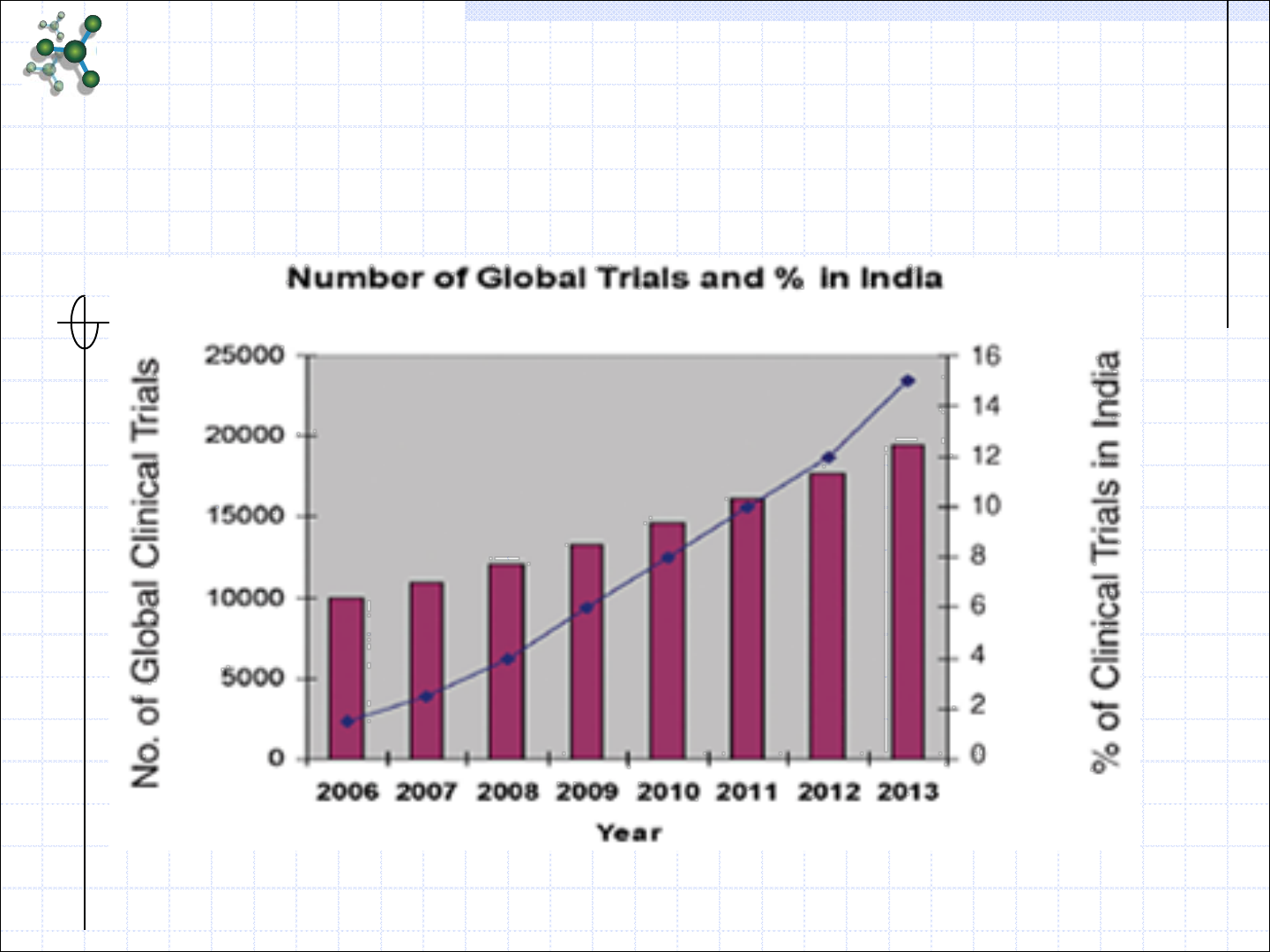
Percent of Global Trials in India
AN ISO 9001:2000 CERTIFIED ACADEMY
cliniminds
Academy for Clinical Research Training & Management
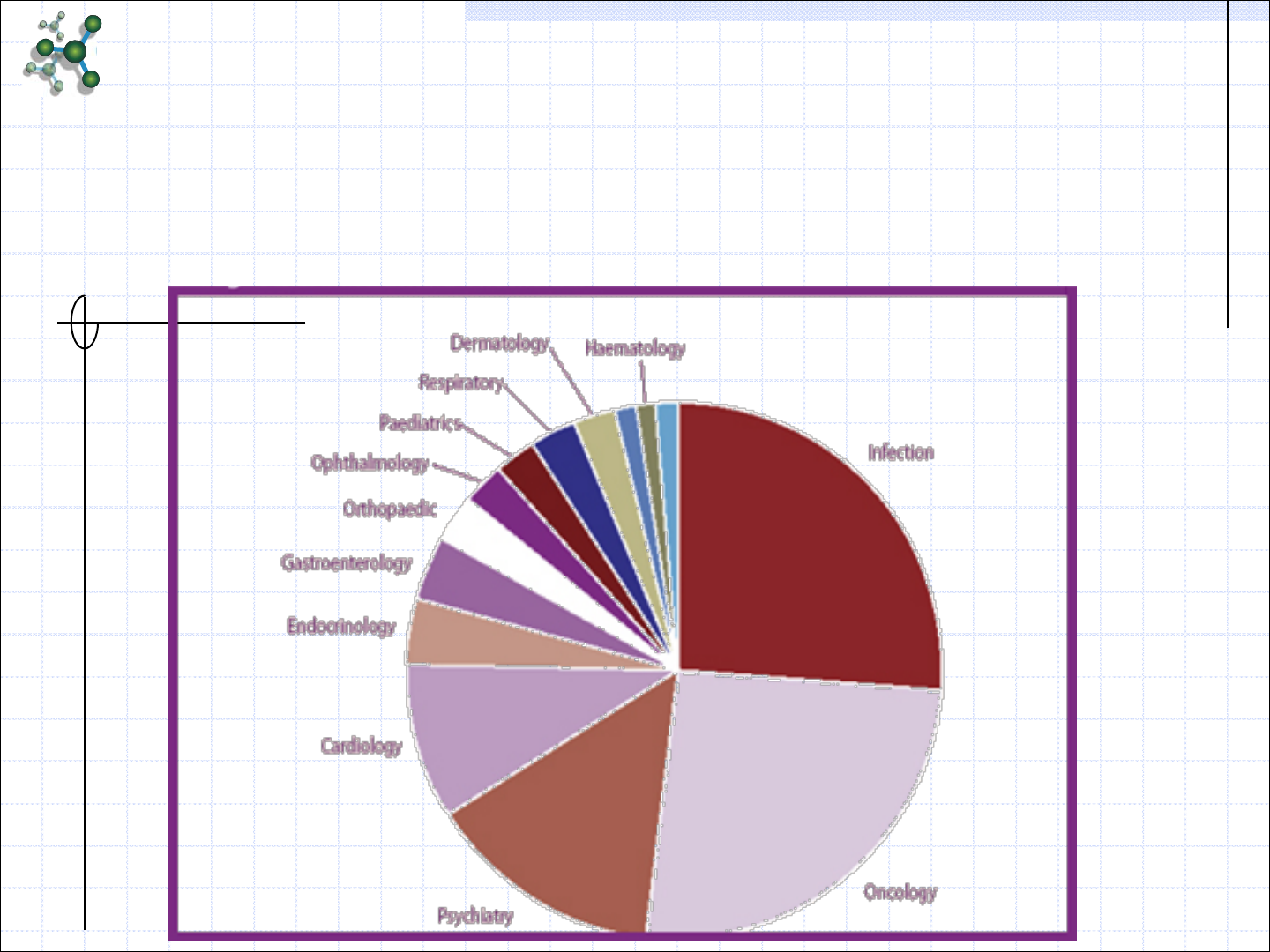
Clinical Trials Done in India
AN ISO 9001:2000 CERTIFIED ACADEMY
cliniminds
Academy for Clinical Research Training & Management
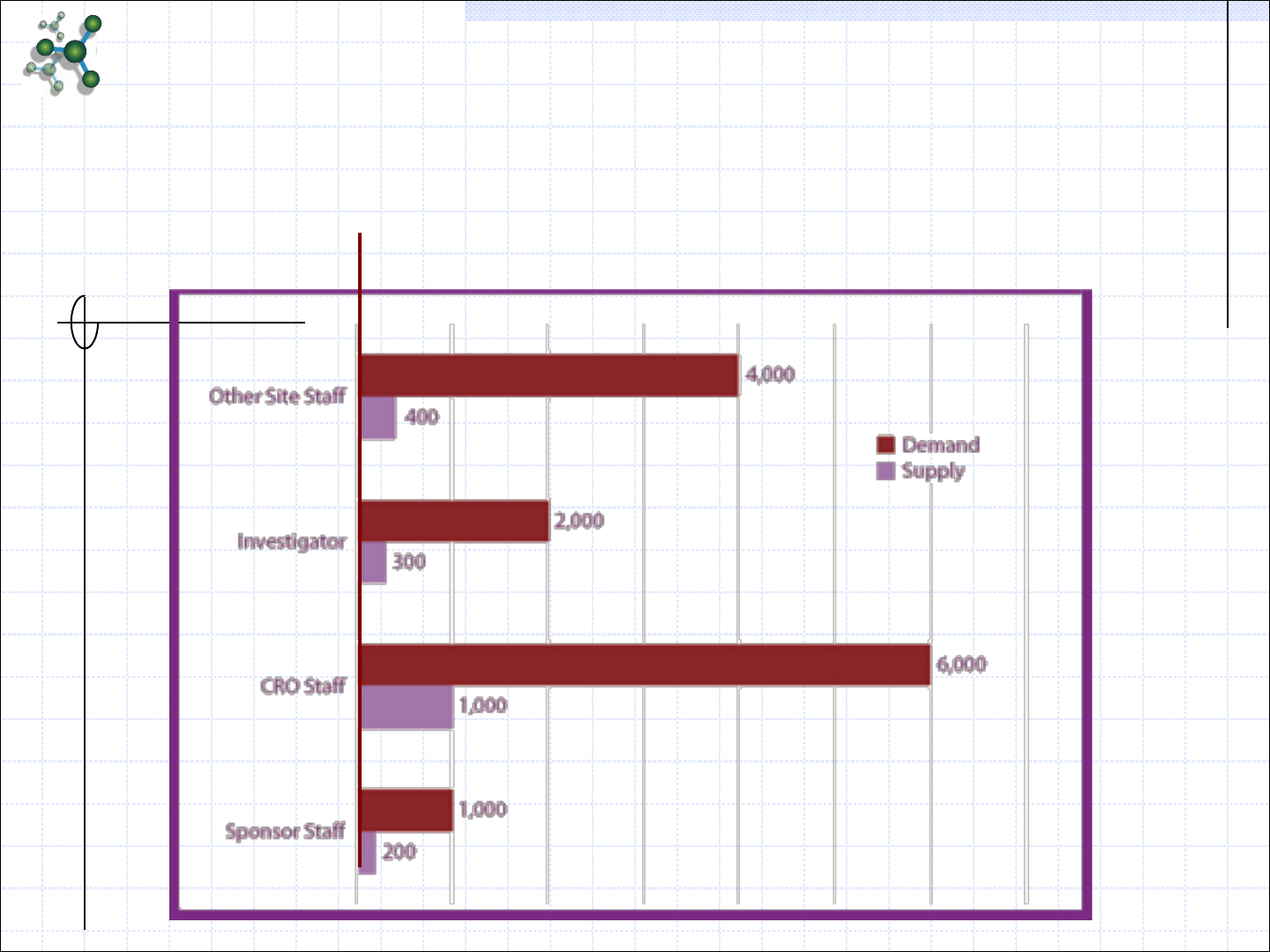
Demand Supply Gap in India
AN ISO 9001:2000 CERTIFIED ACADEMY
cliniminds
Academy for Clinical Research Training & Management
An ISO 9001:2000 Certified Academy
TM
CR Career Pathway
Pharmaceutical Companies
Clinical CROs (Contract Research Organizations)
BA/BE Centers
SMOs (Site Management Organizations)
Data Management CROs
IT Companies in Healthcare / Clinical Domain
• EDC Service Providers
• Central Laboratories
• Packaging & Labeling & Contract Manufacturers
• Investigator & Site Staff
• Training Centers.
AN ISO 9001:2000 CERTIFIED ACADEMY
cliniminds
Academy for Clinical Research Training & Management
An ISO 9001:2000 Certified Academy
TM
Functions in Career Pathways
CLINICAL
RESEARCH
CRO
SMO
Spons
or
Site
Data Management
Pharmacovigilance
Quality Assurance
DCGI
AN ISO 9001:2000 CERTIFIED ACADEMY
cliniminds
Academy for Clinical Research Training & Management
An ISO 9001:2000 Certified Academy
TM
Career in CROs / Pharma / Biotech Companies
Clinical Trial Assistant (CTA)
Clinical Research Associate (CRA)
Senior CRA
Clinical Team Leader
Project Manager
Senior Project Manager
Manager Medical & Regulatory
Manager –Safety / Patents
Manager Quality Assurance
Medical Director
Associate Director –Clinical
Associate Director –Projects
Director –Business Development
Director / Head (Clinical Operations)
General Manger / CEO / President
Phase I / II / III / IV Trial
Project Management
Drug Development Planning
Monitoring
Source Data Verification
Safety Reporting
Regulatory Approval
QA Audits
Business Development
AN ISO 9001:2000 CERTIFIED ACADEMY
cliniminds
Academy for Clinical Research Training & Management
An ISO 9001:2000 Certified Academy
TM
Career in SMO
Clinical Research Coordinators (CRC) / Study Coordinators
Principal Investigators / Co-Investigators
Medical Monitors
Project Manager / Senior Project Manager
Manager Medical & Regulatory
Manager Quality Assurance
Manager –Business Development
Medical Director
Associate Director –Clinical
Associate Director –Projects
Director / Head (Clinical Operations)
General Manager / CEO / President
AN ISO 9001:2000 CERTIFIED ACADEMY
cliniminds
Academy for Clinical Research Training & Management
An ISO 9001:2000 Certified Academy
TM
Career opportunities in DM
Data Entry Operator
Data Validation Executive
QA Executive
Data Manager
QA Manager
Statistical Programmer
Statistician
Data Reviewer
Data Base Designer
Medical Writer
Head –Data Management
AN ISO 9001:2000 CERTIFIED ACADEMY
cliniminds
Academy for Clinical Research Training & Management
An ISO 9001:2000 Certified Academy
TM
Key Cities in India for Clinical Research
Delhi & NCR Region
Mumbai
Pune
Ahmedabad
Vadodara
Hyderabad
Bangalore
Chennai
Cochin
Trivandrum
Chandigarh
Bhopal
Indore
Coimbatore
Visakhapatnam
AN ISO 9001:2000 CERTIFIED ACADEMY
cliniminds
Academy for Clinical Research Training & Management
An ISO 9001:2000 Certified Academy
TM
KEY PHARMA COMPANIES IN CLINCIAL
RESEARCH
Abbott, Mumbai
Chiron, Mumbai
Astra Zeneca Pharma India Ltd, Bangalore
Astra Zeneca Foundation, Bangalore
AventisPasteur, Delhi
Pfizer Ltd, Mumbai
Pfizer Biometrics, Mumbai
Altana(Zydus), Mumbai
Eli Lilly, Delhi
Boston Scientific, Delhi
Hospira, Delhi
Merck ,Delhi
Sanofi Aventis Syntho Lab, Mumbai
GSK, Glaxo SmithKline Pharmaceuticals Ltd,Mumbai
Novartis International Clinical Development Center, Mumbai
Novartis Pharma, Mumbai
Roche,Mumbai
Sandoz, Mumbai
Wyeth, Mumbai
BMS, Mumbai
Novo Nordisk, Bangalore
Lundbeck, Bangalore
Eisai Pharmaceuticals, Mumbai
AN ISO 9001:2000 CERTIFIED ACADEMY
cliniminds
Academy for Clinical Research Training & Management
An ISO 9001:2000 Certified Academy
TM
LG Life Sciences, Delhi
Bayer, Mumbai
GE, Delhi
Johnson & Johnson, Jansenn Cilag, Mumbai,
Cordi Baxter, Delhi
BD Biosciences, Delhi
Bharat Biotech, Hyderabad
Bharat Serum, Mumbai
Cadila Pharmaceuticals
Cipla, Mumbai
Emcure, Pune
Fulford India Mumbai
Indus Biotherapeutics, Ahmedabad
IPCA, Mumbai
Shreya Biotech,Pune
Shantha BiotechnicsPvt. Ltd. Hyderabad
Sun Pharma, Mumbai
Torrent Pharmaceutical Ltd, Gandhi nagar,
USV Ltd. Mumbai
Wockhardt, Mumbai
Zydus Cadilla, Ahmedabad
Biocon,Bangalore
Cadila Pharmaceuticals, Ahmedabad
Intas Pharmaceuticals Ltd., Ahmedabad
AN ISO 9001:2000 CERTIFIED ACADEMY
cliniminds
Academy for Clinical Research Training & Management
An ISO 9001:2000 Certified Academy
TM
Glenmark Pharmaceuticals Ltd., Mumbai
Himalaya Drugs ,Bangalore
Lupin Ltd., Pune
Nicholas Piramal,Mumbai
Panacea Biotech, Delhi
Ranbaxy Research Laboratories, Delhi
Ranbaxy Research Laboratories, Gurgaon
Serum Institute of India, Pune
Torrent, Ahmedabad
AN ISO 9001:2000 CERTIFIED ACADEMY
cliniminds
Academy for Clinical Research Training & Management
An ISO 9001:2000 Certified Academy
TM
KEY CROs IN INDIA
Quintiles Ahmadabad / Bangalore
Synchron Ahmedabad /Bangalore
Lambda Ahmedabad
Genpact Delhi/Bangalore
Siro Clinpharm Mumbai
I-Gate Mumbai
Reliance Clinical Services Mumbai
PPD Mumbai
Onmnicare Bangalore
ICON Bangalore
Clin Trac Bangalore
PharmaNet Bangalore
Pharm-Olam Bangalore
Aizant Hyderabad
Lotus Labs Bangalore
Vimta Hyderabad
GVK Hyderabad
BioServe Clinical Research Hyderabad
Apothecaries Delhi
Clinsys Delhi/Noida
Fortis Delhi/Noida
Kendle Delhi/Gurgaon
Bioassay Baroda
Clinworld Bangalore
Perinclinical Mumbai
Quest Life Sciences
AN ISO 9001:2000 CERTIFIED ACADEMY
cliniminds
Academy for Clinical Research Training & Management
An ISO 9001:2000 Certified Academy
TM
WHY CAREER IN CLINCIAL RESEARCH
Fastest growing segment in the healthcare /
pharmaceutical industry
Excellent Opportunity to Develop Combination of
Technical and Management Skills
Part of the Global Growth Opportunity
Wider Job Horizon
Rapidly Growing Opportunities…and growing
(internal and external)
Attractive Compensation and future growth
Higher Job Satisfaction
Continuous Training Opportunities
International Opportunities
AN ISO 9001:2000 CERTIFIED ACADEMY
cliniminds
Academy for Clinical Research Training & Management
An ISO 9001:2000 Certified Academy
TM
CHALLENGES FOR HUMAN RESORUCES
Very rapid growth in number and size of
companies
Spectrum of skills required is in scarcity
Lack of specialization
Limited pool of experienced people
Employee Retention
Continuous Training
AN ISO 9001:2000 CERTIFIED ACADEMY
cliniminds
Academy for Clinical Research Training & Management
An ISO 9001:2000 Certified Academy
TM
Established 23 programs with extensive, high quality course content
and faculty: Online; E-Learning; Distance Learning & Classroom. More
industry oriented programs currently in pipeline.
Presence in 8 key cities of India – Delhi; Mumbai; Hyderabad;
Bangalore; Ahmedabad, Cochin, Bhopal and Vijaywada. 4 more cities
to start soon.
Program content is targeted towards all major global markets and
address the global regulatory environment. Overseas students are
growing from Americas; Europe; Middle East; Africa and other
countries.
Developed feature rich state-of-the-art user friendly ONLINE
LEARNING SYSTEM. System now used by major pharma companies
for in-house training.
Have been regularly conducting clinical research training
programs for National Institute of Health, USA in the Indian
market.
Cliniminds - Key Achievements
AN ISO 9001:2000 CERTIFIED ACADEMY
cliniminds
Academy for Clinical Research Training & Management
An ISO 9001:2000 Certified Academy
TM
Awaiting the accreditation of our programs by Government of India.
Top of the Line Faculty & Industry Driven academic Council.
Co-certified Training Programs with NIH USA, MSD, Bioserve, Max
Healthcare and other companies.
Key Clients for Training Workshops and Online Training Programs
: NIH USA; MSD; Fresenius Kabi, Germany; Dabur; Novartis; Quintiles;
Ranbaxy; GSK; Bioserve; Max Healthcare; Johnson & Johnson;
Panacea Biotec
Strong & Multiple industry tie ups / collaborations to provide practical
orientation
Trained over 1,000 professionals, with increasing number of international
students for online programs from North America, South America, Europe and
Middle East.
Accreditation / Certification from Pharmaceutical Society of India & ISO
9001:2000 by JAS ANZ.
Key Achievements
AN ISO 9001:2000 CERTIFIED ACADEMY
cliniminds
Academy for Clinical Research Training & Management
An ISO 9001:2000 Certified Academy
TM
Conducted several industry workshops for leading corporates, viz. NIH,
MSD (Merck), Novartis, Biocon, Quintiles, Bioserve, Ranbaxy,
Panacea, Max Healthcare, Max Neeman, Apollo Hospitals, Fresenius
Kabi, Merck & Co., Panacea Biotec, Asian Clinical Trials, Quintiles.
Very High student satisfaction levels and excellent placements.
Experience in conducting multi location training programs using
Videoconferencing technology.
Key Achievements
AN ISO 9001:2000 CERTIFIED ACADEMY
cliniminds
Academy for Clinical Research Training & Management
An ISO 9001:2000 Certified Academy
TM
Training Workshops Offered
• Over 35 Workshops for major Indian and MNCs have been conducted in the
following areas :
Conduct & Management of Clinical Trials
Monitoring of Clinical Trials
ICH GCP Workshop on Clinical Research
Quality Assurance in Clinical Research
Auditing & Inspections of Clinical Trials
Pharmacovgilance
Regulatory Affairs
Medical & Scientific Writing
Ethics in Clinical Research
Roles & Responsibilities of Investigators
Conduct of Cancer Clinical Trials
Conduct & Management of BA/BE Studies
• Duration of these workshops have been from 1 – 3 days.
• Workshops are also blended with Online Learning System for follow up
training.
• Programs can be customised to suit your needs.
AN ISO 9001:2000 CERTIFIED ACADEMY
cliniminds
Academy for Clinical Research Training & Management
An ISO 9001:2000 Certified Academy
TM
Certifications offered by Cliniminds
Advanced Post Graduate Diploma in Clinical Research & Pharmacovigilance
Advanced Post Graduate Diploma in Clinical Research & Regulatory Affairs in Pharma
& C R
Advanced Post Graduate Diploma in Clinical Research & Regulatory Affairs in Pharma
& C R-Class Room
Advanced Post Graduate Diploma in Clinical Research & Clinical Data Management &
Biostats +SAS
Advanced Post Graduate Diploma in Clinical Research & Clinical Data Management &
Biostats +SAS-Class Room
Advanced Post Graduate Diploma in Clinical Research
Diploma in Clinical Research
Post Graduate Diploma in Pharmacovigilance
Certificate Program in Conducting & Managing Bioequivalence & Bioavailability
Studies
Certificate Program in Conducting & Managing Clinical Trials for Cancer Patients
AN ISO 9001:2000 CERTIFIED ACADEMY
cliniminds
Academy for Clinical Research Training & Management
An ISO 9001:2000 Certified Academy
TM
Certifications offered by Cliniminds
Post Graduate Diploma in Clinical Data Management & SAS
Certificate Program For Clinical Trial Investigators & Site Personnel
Certificate Program in Monitoring of Clinical Trials
Post Graduate Diploma in Clinical Trials Management
Post Graduate Diploma in Clinical Research for Nurses
Certificate Program in Quality Assurance in Clinical Research
Post Graduate Diploma in Regulatory Affairs
Post Graduate Diploma in Biostatistics
ICH GCP Certificate Program In Clinical Research For Medical Practitioners
Integrated Post Graduate Diploma In Clinical Research & Pharmacovigilance
Post Graduate Diploma in Pharmacovigilance - Class Room
Integrated Post Graduate Diploma in Clinical Research & Pharmacovigilance - Class Room
Post Graduate Diploma in Medical and Scientific Content Writing
Post Graduate Diploma in cGMP – Good Manufacturing Practices
Certificate Program in Clinical Trial Regulations & GCP in Europe & UK
Certificate Program in Clinical Trials Auditing & Inspection
Post Graduate Diploma in Clinical Trials Management - Class Room
Post Graduate Diploma in Clinical Data Management & SAS - Class Room
AN ISO 9001:2000 CERTIFIED ACADEMY
cliniminds
Academy for Clinical Research Training & Management
An ISO 9001:2000 Certified Academy
TM
Cliniminds Students
1. Cliniminds students are from the medical, dental, homeopathic,
pharmacy, biotechnology, biochemistry, microbiology and other life
sciences background with Advanced Post Graduate Diploma in
Clinical Research; Post Graduate Diploma in Clinical Trials
Management; Pharmacovigilance; Regulatory Affairs; Data
Management & SAS; Monitoring.
2. All students are provided hands on practical training on regulatory
affairs; clinical trials conduct & management; ethics; subject
recruitment & retention; roles & responsibilities; essential documents,
viz. protocol, CRF, ICF, Trial Master File and other documents,
monitoring; project planning & management, medical & scientific
writing; drug safety, data management, basics of bio stats and other
areas.
3. Large number of CROs, pharma companies and hospitals have
recruited number of clinical research students from Cliniminds.
4. There are large number of overseas students from North America,
Europe, Middle East, South America
AN ISO 9001:2000 CERTIFIED ACADEMY
cliniminds
Academy for Clinical Research Training & Management
An ISO 9001:2000 Certified Academy
TM
ONLINE PROGRAMS
Cliniminds Online Learning Systems has been designed to meet the needs
of both individual students and corporates. Large number of companies
have used Cliniminds system for internal training, viz. Ranbaxy; Quintilesl
GSK; Bioserve; Asian Clinical Trials, etc.
Following are the key features :
Ready to use and user friendly
Bulk user license with control panel
Customisation / mix and match from Cliniminds Programs.
System allows you to upload some of your internal training documents
for users. Only accessible for your own teams
Users account could be created or blocked in no time
Realtime Records of Training for HR / Training manager to monitor the
progress of the users
Online Evaluation
Online data can not be saved, download or copy/pasted. Printing could
be allowed.
Effective for site staff / investigators
Fully protected.
99.999% uptime
Cost effective
Confidentiality.
AN ISO 9001:2000 CERTIFIED ACADEMY
cliniminds
Academy for Clinical Research Training & Management
An ISO 9001:2000 Certified Academy
TM
Our Capabilities / Strengths
Top of the line industry experts and faculty with extensive industry
experience
Expertise in content development
Strong academic team and faculty
Strong practical training and placement assistance to students
Over 20 years of experience in advising, setting up and managing
education, healthcare and clinical research businesses.
Experience in setting up turnkey projects in the clinical research &
healthcare sector
Promoters come with strong Industry experience and clear vision for the
clinical research and healthcare industry
Strong regulatory experience
Strong understanding of marketing and distribution
AN ISO 9001:2000 CERTIFIED ACADEMY
cliniminds
Academy for Clinical Research Training & Management
An ISO 9001:2000 Certified Academy
TM
TESTIMONIALS
AN ISO 9001:2000 CERTIFIED ACADEMY
cliniminds
Academy for Clinical Research Training & Management
QA Specialist
NIH
US Government
The sessions were particularly informative for me, who
was not versed in the Indian regulations.
I think the Schedule Y information was of particular
interest to me.
It was of particular benefit to the group that Cliniminds
focused on GCP, Tox studies, Pre-Clin studies, and
Phase I-II-III. The assessment at the completion of the
presented materials was great. It is always a must to
assess competency as part of a training. All speakers
were great presenters and had very good talks.
AN ISO 9001:2000 CERTIFIED ACADEMY
cliniminds
Academy for Clinical Research Training & Management
It’s a great privilege for me to give a testimonial about my training
institute. I can say that it was a perfect time for me to enhance my
skills for widening my opportunities in Clinical Research. I appreciate
the study materials because it was a blend of both simplicity and
authenticity covering every nook and corner pertaining to my
specialization. The coaching classes cum workshops I attended, was
completely Industry oriented and professionally executed. I can surely
say to the new aspiring candidates, that the courses provided by
Cliniminds will be the first of its kind where you get the real touch of the
Clinical Research Industry and new energetic start.
Dr. Praveen. S
Clinical Research Coordinator
Max Neeman International
New Delhi
AN ISO 9001:2000 CERTIFIED ACADEMY
cliniminds
Academy for Clinical Research Training & Management
Its really my pleasure and proud to say that I am a student of Cliniminds. This
institution helped me a lot to shape up my career beyond my expectation. I am
very thankful to the institution to made me to procure a good job in good
company. The classes were really in practical sense, which helped me, a lot in
this industry to cope up with the work environment. I recommend this
institution for those who aspires their careers in Clinical Research.
Dr.Monika Tyagi
AN ISO 9001:2000 CERTIFIED ACADEMY
cliniminds
Academy for Clinical Research Training & Management
I joined Cliniminds in March,08 batch of Advanced P.G.Programme for
Clinical Research.It was a very interesting, informative course. In addition,I
also attended 3 workshops organized by Cliniminds on ICH-GCP, Quality
Assurance and Pharmacovigilance.The workshops were of very good quality
in terms of content and faculty.Overall I have found the staff very helpful and
the material provided of good quality.I joined Max Neeman International in
April,2008 as a CRC.Cliniminds actively helped me to get the job.I have now
been promoted to Drug Safety Manager-Medical Monitoring.I sincerely
thank Cliniminds and wish best of luck to the institute for the future.
Dr. Sutirtha Mukhopadhyay
Manager – Drug Safety - Icon
AN ISO 9001:2000 CERTIFIED ACADEMY
cliniminds
Academy for Clinical Research Training & Management
I was a part of the Cliniminds 2008 batch for APCR at the NEW
DELHI The programme helped me in knowing the Clinical Research field
which was relatively new to me ,the interactive session and the periodic
projects and test helped us to grow our knowledge the teachers were help full
The institute helped me in getting a Placement at MAX NEEMAN
INTERNATIONAL while i was doing the course and by the time i
completed the course i was promoted The regular counseling helped me
in planning my career I WISH THE BEST OF LUCK TO ALL THE
CURRENT AND FUTURE STUDENTS OF CLINIMINDS.
Dr SAURABH SAXENA B.D.S P.G.D
Clinical Research Associate MAX NEEMAN
INTERNATIONAL Max House,1st Floor,
1,Dr.Jha Marg, Okhla-III New Delhi-
110020

AN ISO 9001:2000 CERTIFIED ACADEMY
cliniminds
Academy for Clinical Research Training & Management
It would be injustice if I failed to appreciate my courses ( Certificate program
in Clinical Research ; Pharmacovigilance ; & Regulatory Affairs) from
Cliniminds. . The courses made me industry-ready, and also gave me the
edge that others do not have. The study manuals for all the courses were quite
comprehensive and easy to understand and in my opinion, it covered all
aspects, while also giving ample knowledge about the subject. I would really
like to thank Cliniminds for making me understand about the basics of
Clinical Research Industry and delivering the right knowledge in a structured
manner.All in all it was a great experience and one that I would be ready to
undergo any time again.
Dr. Sandeep Bhatia
Medical Advisor
Sanofi-Aventis
AN ISO 9001:2000 CERTIFIED ACADEMY
cliniminds
Academy for Clinical Research Training & Management
Thank you for organizing an ICH GCP Workshop at Max Healthcare at such
a short notice.
I am pleased to inform you that the workshop was very successful, and have
completely met out objectives.
We are pleased with the content quality and the faculty.
We would be glad to use you training services for our future Clinical
Research Training requirements.
Dr. Saroj Kumar Sabath
Manager-Clinical Research
Max Healthcare

AN ISO 9001:2000 CERTIFIED ACADEMY
cliniminds
Academy for Clinical Research Training & Management
I am really glad to inform you that I have joined MAX NEEMAN as CRC
from 16th March 09.
I am really thankful to you for recommending my name to Max Neeman.
It was great learning in Cliniminds from eminent and experienced teachers
from Clinical Research field.
Sincere thanks for all your support and encouragement.
Dr. Anuja Mathkari
AN ISO 9001:2000 CERTIFIED ACADEMY
cliniminds
Academy for Clinical Research Training & Management
I rate it is as best one, because here in our company we have online training
sessions just same as u have, but it is somewhat different more student
friendly and never encountered any problems interms of difficulty in
navigation and completion.
I salute the designer of site and content of study material, i also recommend
all my colleagues about the course.
I owe to your cliniminds for ignite the minds of students by such a awesome
course.
Waiting for your foster certification in course.
D.Raghavendra,
Quality Assurance Specialist,
Quality & Compliance
Management
GSK
Gurgaon, India.
AN ISO 9001:2000 CERTIFIED ACADEMY
cliniminds
Academy for Clinical Research Training & Management
I have taken the course "Integrated Post Graduate Diploma In Clinical
Research and Pharmacovigilance" during the period of May - July, 2009.Having found it myself
on-line, I found it very important to be sure the site was reliable. From the very first steps of my
on-line interaction with
the course providers I put whatever initial uncertainties I had aside. The authors were
committed, prompt, responsive and engaged. They would take all of my inquiries seriously and
usually get back to me within 24 hours, which was more than understandable due to the time
difference. This remained true
from the transfer of advance payment up to the moment I was issued the graduation certificate.
Above all though, it was the concept that caught my attention in the first place. The name of the
course matched my area of interest and once I got my access rights I learned that its content
lived up to it as well. Being put together in a comprehensive way, it also provided a user-
friendly learning
and knowledge testing interface and manageable timelines. I can recommend the course to
anyone who is looking for the value for their money. The absence of any of the international
English language evaluation
requirement also saves money and time, provided one is already familiar with the specifics of
the English used by the international clinical trials community, which no English course can be
of much help with anyway.
Tomas Novak,
PharmD July 9, 2009 Prague,
Czech Republic"
AN ISO 9001:2000 CERTIFIED ACADEMY
cliniminds
Academy for Clinical Research Training & Management
Very good faculty, very interactive and positive in accepting comments,
queries and answering to their best capability and knowledge.
The content of presentation was adequate and simple for understanding to
freshers as well.
I liked the institute and wish to enroll myself for future workshops and in the
ongoing courses.
Rashmi Kulshrestha
Associate Director Regulatory
Affairs
Ranbaxy Laboratories Ltd.
AN ISO 9001:2000 CERTIFIED ACADEMY
cliniminds
Academy for Clinical Research Training & Management
I found the workshop on ICH –GCP conducted at the Cliniminds highly
interesting and praiseworthy for the fact that those two days of brainstorming
sessions gave me a detailed insight into various aspects of Clinical Research
imperative for doctors and its growing demand in Pharmaceutical industry. The
faculty at the Cliniminds is highly skilled in their job and good enough to solve
my queries. I would like to wish the Cliniminds the best of wishes in all their
endeavors towards developing skilled and motivated Clinical Researchers I found
the workshop on ICH –GCP conducted at the Cliniminds highly interesting and
praiseworthy for the fact that those two days of brainstorming sessions gave me a
detailed insight into various aspects of Clinical Research imperative for doctors
and its growing demand in Pharmaceutical industry. The faculty at the Cliniminds
is highly skilled in their job and good enough to solve my queries. I would like to
wish the Cliniminds the best of wishes in all their endeavors towards developing
skilled and motivated Clinical Researchers
Dr. Manish Mahajan
M.D. Deptt. of Biophysics
A.I.I.M.S.
AN ISO 9001:2000 CERTIFIED ACADEMY
cliniminds
Academy for Clinical Research Training & Management
An ISO 9001:2000 Certified Academy
TM
Tenet Health Edutech Pvt. Ltd.
Cliniminds
C-55 Preet Vihar, 1
st
Floor
Main Vikas Marg
Delhi 110092
India
Tel : +91 11 30287800
Fax : +91 11 30287802
Email : info@cliniminds.com
Mobile : +91 9810068241
www.cliniminds.com

