
Catalyst Clinical Services Pvt. Ltd.
UG-11, Upper Ground Floor, Aggarwal Prestige Mall, Plot No. 02, Road No. 44, Pitampura, New Delhi - 110034 (INDIA)
Ph: +91-9818356273, 8826806862
Email: info@catalystclinicalservices.com; Web: www.catalystclinicalservices.com
®
Professional Diploma In Clinical Research (PDCR )
Information Brochure
Program Objective
®
Professional Diploma in Clinical Research (PDCR ) is a skill development program (purely through correspondence) of 6 months duration with a primary
focus on Drug discovery and clinical trial processes, Good Clinical Practices (GCP) guidelines, Drug regulatory affairs, Roles and responsibilities of various
clinical trial stakeholders. The prime objective is to provide a high-end training thereby enhancing the employment prospects of the participants.
Program Highlights
· Optimal Duration: 6 months (requires approximately 80 hours of total reading)
· Ease of Training and Evaluation: Assignment based evaluation, no written examination
· Optimal Course Fee
· Wide Recognition and Acceptance of the program throughout the clinical research industry
With a track record of running 100 successful batches (over last 17 years), 10000+ candidates from 200+ cities across 27+ countries have enrolled for
®
PDCR program till date establishing itself as the largest clinical research training program of the country. Majority of successful PDCRians are working
for leading Pharmaceutical Companies, Contract Research Organizations (CROs) and Research Institutions across 300+ organizations.
Clinical Research and It's Potential
India became a member of WTO/GATT/TRIPS in 1995 and implemented the product patent regime in 2005. As part of WTO/GATT/TRIPS,
Pharmaceutical Industry has the rights to patent products as well as processes throughout the world including India. This has led to a significant growth
in pharmaceutical industry and increased stakes of multi-national companies in Indian operations.
In light of these changes, Clinical Research has emerged as a leading knowledge based industry of the new millennium. Clinical research is carried out on
healthy volunteers and patients with diseases to ensure that the drug, which is to be marketed, is safe and effective. It takes approximately 12 years and
US $1.3 billion to introduce a new drug to the market.
Clinical Research industry in India is growing rapidly and the country is projected to conduct nearly 5% of all global clinical trials in next 5 years. Being a
sunrise industry it is offering exciting career avenues as well as an accelerated growth path. Being a signatory of GATT/TRIPS, India is being looked upon
as a favorable destination for conducting global clinical trials. India offers unique advantages for global clinical research that include:
· Lower drug development cost
· Abundance of patients with genetic diversity
· Wide spectrum of disease
· Trained medical professionals
· Skilled manpower and IT enabled infrastructure at a lower cost
· Proficiency in English language
Clinical Research offers employment opportunities to the medicine/pharmacy/life sciences graduate and post-graduate students. However, clinical
research is a highly specialized and regulated profession therefore it requires specific skill sets to carry out various operations, as per the global norms.
We at Catalyst Clinical Services Pvt. Ltd. are committed towards developing India as a hub for global clinical research by catering to the ever-growing
training and compliance needs of the profession, through specialized training courses and workshops.
Job Prospects
Following positions are available to pursue a career in clinical research:
Clinical Research Coordinator/
Clinical Research Associate/
Pharmacovigilance Associate
Senior Clinical Research Associate
Project Manager/Team Lead/QA Associate
Manager Quality Assurance
Manager Clinical Operations
Director/VP
7-10 Yrs.
5-7 Yrs.
4-6 Yrs.
3-5 Yrs.
2-3 Yrs.
0-2 Yrs.
QA : Quality Assurance; VP: Vice President
Company Profile: Catalyst Clinical Services Pvt. Ltd.
Catalyst Clinical Services Pvt. Ltd. is a contract research organization with a prime focus on clinical research training and development activities.
With regards to clinical research training Catalyst has made pioneering initiatives such as:
®
· Professional Diploma in Clinical Research (PDCR );
· Advance Certificate Program(s) in Clinical Research;
· Professional Certificate in Pharmacovigilance (PCPV);
· Professional Diploma in Clinical Research (ASU&H);
TM
· Oncology Clinical Trials Training (OCTT );
®
· Advance Certificate in Clinical Research (ACCR );
· GCP Training Workshop(s)
· 21 CFR Part 11 Training
Program Director
Sanjay Gupta (M.B.A., M.Pharm) is a well known clinical research expert having over 22 years of extensive clinical research experience. He has personally
conducted and supervised over 100 clinical trials (Global registration trials, Exploratory Phase-II trials, Phase-I trials, Investigator initiated trials etc.)
across a wide range of therapeutic areas including Oncology, Endocrinology, Psychiatry, Critical Care, Infectious Diseases, Andrology, Ophthalmology
etc.
He has presented his research work in various International Journals and Conferences including American Society of Clinical Oncology (ASCO), Seminars
in Oncology, British Journal of Cancer (BJC), Gastric and Breast Cancer (GBC) and British Journal of Radiology (BJR). He has authored 9 books and written
thought provoking articles on clinical research field for periodicals Chronicle Pharmabiz and Express Pharma Pulse. His recent books “The Big Book of
Clinical Research” and “All You Need to Know about Clinical Research” has been widely acclaimed by key stakeholders across the clinical research
industry.
He is the founder member of Society for the Promotion of Ethical Clinical Trials (SPECT) in India and also the Network Coordinator for a cancer trials
network set-up by University of Oxford, London (India and UK).
®
PDCR Program Curriculum
® ®
All the students enrolling for PDCR program are committed to uphold the highest standards of personnel and professional ethics. The PDCR program
consists of four modules in all. The components of four modules are as follows:
Module No.
Module Title and Components
Introduction to Pharmaceutical Medicine
· The Drug Development Process
· New Drug Discovery
· Clinical Development of Drug
· Essential Clinical Trial Documents
· Clinical Trials Terminology
1
2
3
Good Clinical Practice (GCP) Foundations
· History of GCP - milestones in the evolution of GCP
· Principles of GCP
· Applicable GCP Guidelines
· Declaration of Helsinki
· Clinical Study Process
· The Management of Clinical Studies (Sponsor)
· Ethics in Clinical Research
· Informed Consent
· Serious Adverse Event (SAE)
· Challenges in the Implementation of GCP Guidelines
· Biostatistics
Drug Regulatory Affairs (Clinical Trials)
· Overview of Regulatory Environment in USA, Australia, Europe and India
· Clinical Trial Application Requirements in India
· Import- Export of Clinical Trial Drugs in India
· Health Insurance Portability and Accountability Act (HIPAA) Privacy Rule.
· IND/ANDA/New Drug Application
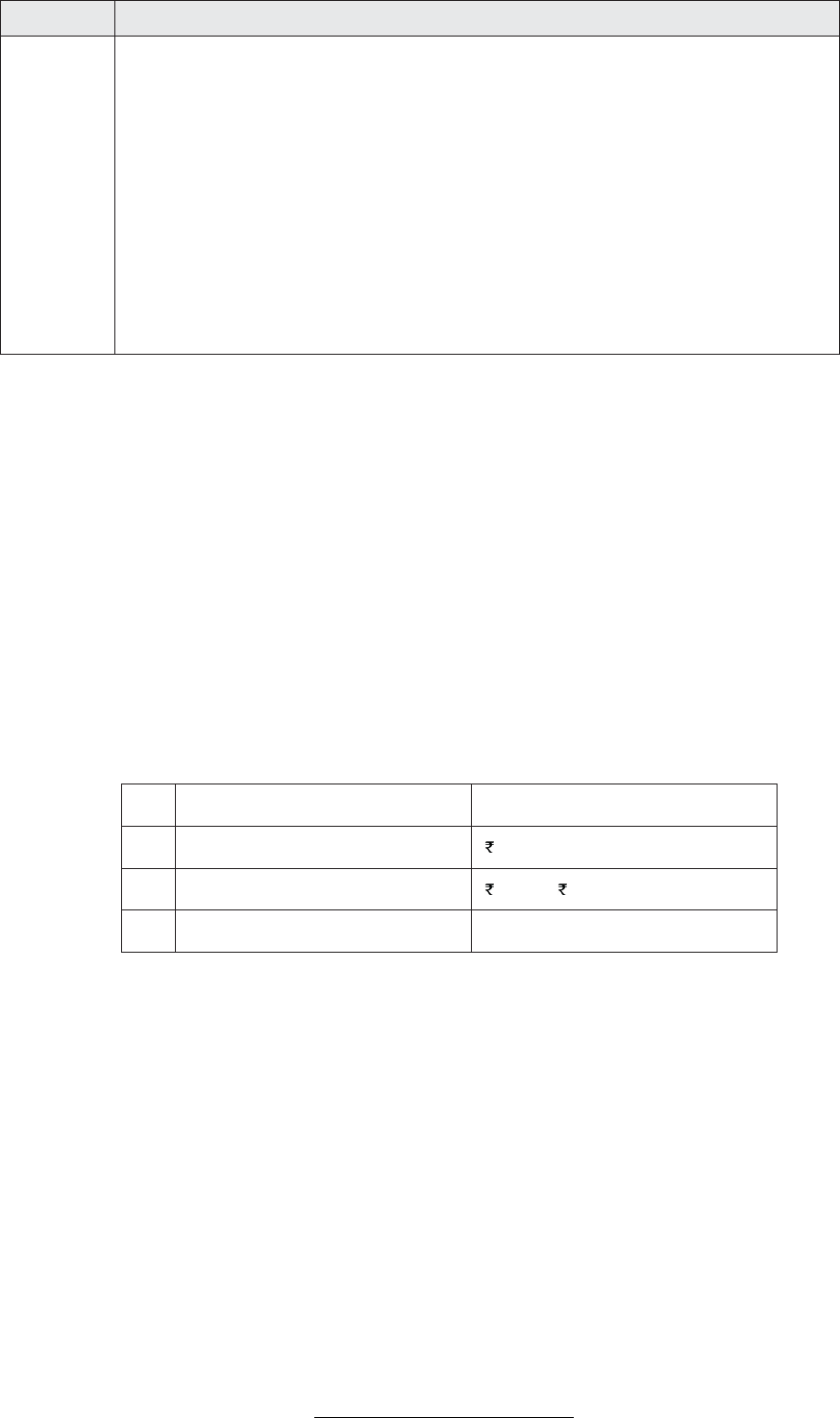
Module No.
Module Title and Components
4
Roles and Responsibilities of Clinical Trial Personnel
· Roles and Responsibilities of Sponsor
· Roles and Responsibilities of Investigator
· Roles and Responsibilities of ERB/IRB/IEC
· Roles and Responsibilities of CRA /Monitor
· Roles and Responsibilities of Auditor
· Roles and Responsibilities of Clinical Research Coordinator or Site Manager
· Roles and Responsibilities of CRO's
· Roles and Responsibilities of Regulatory Authorities
· Roles and Responsibilities of Clinical Data Manager (CDM )
· Roles and Responsibilities of Clinical Biostatistician
Duration
The program duration is 6 months. However, it can be stretched up to a maximum of 10 months (due to the inability to qualify in the first attempt),
failure to which would lead to cancellation of the candidature thereby requiring a fresh enrolment.
Eligibility
· B.Pharm, M.Pharm, Ph.D
· M.B.B.S, M.D, M.S, D.N.B, D.M
· B.D.S, M.D.S, B.P.T, B.Tech
· B.A.M.S, B.H.M.S, B.U.M.S
· B.Sc, M.Sc, Ph.D
· Working Professionals
Third year students of above curriculums are also eligible for enrolment in the program.
Application and Fee
The program fee of Professional Diploma in Clinical Research (PDCR®) is as follows:
Candidates are required to send their Application Form along with a copy of highest qualification proof and program fee (through Demand Draft drawn
in the favor of “Catalyst Clinical Services Pvt. Ltd.” payable at Delhi). The candidates are advised to write their name and address on the back of demand
draft.
®
The enrolment in PDCR program is subject to the realization of Program fees.
Application Form completed in all respect should be sent to:
®
Course Coordinator- PDCR
Catalyst Clinical Services Pvt. Ltd.
UG-11, Upper Ground Floor, Aggarwal Prestige Mall, Plot No. 02, Road No. 44, Pitampura, New Delhi - 110034 (INDIA)
Ph: +91-9818356273, 8826806862
E-mail: info@catalystclinicalservices.com
Participants
Indian Participants
Indian Participants Residing Overseas
1
2
15500
15500 + 3000 towards postal charges
Foreign Nationals
3
US$ 325
S. No. Fees

Evaluation
The evaluation is based on the grading of assignments. The assignments are required to be submitted to the Course Coordinator on or before the
scheduled date. Assignments reaching after the scheduled dates will not be considered for the evaluation.
Letter grade system is used for grading the assignments. These letter grades are:
A
B
C
D
E
Excellent
Very Good
Good
Satisfactory
Unsatisfactory
80% and above
≥ 60% and < 80%
≥ 50% and < 60%
≥ 40% and < 50%
< 40%
®
Candidates securing D grade and above in all four modules would be eligible to receive Professional Diploma in Clinical Research (PDCR ). If a
candidate fails to secure minimum “D“ grade in the assignment of any particular module, he/she would be given another chance to resubmit the fresh
set of assignments. The fresh set of assignments would be issued to the candidates on request. If a candidate fails to pass his/her second attempt,
he/she would be ineligible for the award of certificate. In that case the candidate has to apply for a fresh registration. There is no provision for the re-
evaluation of assignments. The evaluated Assignments will be discarded after 6 months from the date of declaration of result for the respective batch.
The decision of Catalyst Clinical Services Pvt. Ltd. would be final and binding to all the students. All legal disputes would be subject to New Delhi
jurisdiction only. Catalyst Clinical Services Pvt. Ltd. reserves the right to change the rules and regulations from time to time in its sole and absolute
discretion. If any such change is made, the latest amended rule/ regulation would be applicable.
Issue of Fresh Set of Assignments
Candidates who are unable to qualify the program in the first attempt can make a written request for the issue of fresh set of assignments for the
module(s) in which they are unable to qualify. For all such requests a processing fee of 5000/- will be payable in the form of bank draft drawn in the
favor of “Catalyst Clinical Services Pvt. Ltd.” payable at Delhi. The same fee is applicable to those candidates also who are not able to submit the
assignments within the stipulated time frame*.
Issue of Duplicate Copy of Grade Card and Certificate
Request for issue of duplicate copy of grade card and certificate can be made to the Course Coordinator stating the reason thereof. For the issue of
duplicate copy of grade card or certificate, a processing fee of 500/- will be payable in the form of bank draft drawn in the favor of “Catalyst Clinical
Services Pvt. Ltd.” payable at Delhi.
Class Room Training (Optional)
Classroom training of 16 hrs. (2 days) is available to Indian students for an extra fee of 5000/- at selected cities. The classroom training will only be
arranged if at least 25 students opt for it in a particular city. The most probable locations are Delhi, Mumbai, Bangalore, and Chennai**.
* The total program duration can not exceed 10 months from the start date of a batch.
** Venues are subject to change depending upon minimum registration of 25 participants per city.
