
Aerosol and Air Quality Research, 17: 878–887, 2017
Copyright © Taiwan Association for Aerosol Research
ISSN: 1680-8584 print / 2071-1409 online
doi: 10.4209/aaqr.2016.09.0383
Mixed Chloride Aerosols and their Atmospheric Implications: A Review
Hao Wang
1
, Xinfeng Wang
1*
, Xue Yang
1
, Weijun Li
1
, Likun Xue
1
, Tao Wang
1,2
, Jianmin Chen
1
,
Wenxing Wang
1
1
Environment Research Institute, Shandong University, Ji’nan, China
2
Department of Civil and Environmental Engineering, the Hong Kong Polytechnic University, Hong Kong, China
ABSTRACT
Natural and anthropogenic chloride aerosols make up a significant fraction of atmospheric particulate matter and play
important roles in the boundary layer chemistry. Here we provide a review of the mixing characteristics of chloride
aerosols and the subsequent atmospheric implications, which are rarely considered in current field and modeling studies.
Single-particle analytical techniques have shown that a large fraction of chlorides mix internally with other components, in
particular inorganic salts and organic matters, instead of existing separately. In marine and coastal regions, high
proportions of chloride aerosols usually mix with inorganic substances (e.g., Mg, Ca, K, N, S), while small quantities of
them are coated by organic matter. In forest, grassland, and agricultural areas, most chlorides in biomass burning particles
mix with or are coated by organics. In industrialized urban areas, the chloride aerosols often co-exist with heavy/transition
metals (e.g., Zn, Pb) and are coated by organic materials in aged plumes. Moreover, secondary chlorides also mix with
mineral dusts, nitrates, and sulfates. The mixing of chloride aerosols with insoluble substances can inhibit their
hygroscopic properties, which in turn affects the cloud condensation nuclei activation and heterogeneous reactivity. The
encasing of chloride aerosols within light-absorbing substances changes their optical properties and subsequently causes
atmospheric warming. This paper emphasizes the complexity of the mixing of chloride aerosols, as well as the potential
atmospheric implications thereof, and proposes some research topics deserving future study.
Keywords: Chloride aerosol; Mixing state; Hygroscopicity; Reactivity; Optical properties.
INTRODUCTION
Chloride aerosols have been identified as a major
constituent of atmospheric particulate matter, and play
important roles in tropospheric chemistry (Erickson and
Duce 1988; Carslaw et al., 2010; Laskin et al., 2012).
They exist in various chemical forms and different sizes in
diverse locations. In marine and coastal regions, sea salts,
dominated by sodium chloride, contribute a large fraction
to the mass and number concentrations of aerosols, most of
which exist in super-micro particles (Erickson and Duce
1988; Gong et al., 2002; O’Dowd and de Leeuw, 2007). In
forest, grassland, and agricultural areas, aerosols
containing potassium chloride contribute almost half of the
number concentration of fine particles in biomass burning
plumes (Pratt et al., 2011). In industrialized urban areas,
aerosols containing metal chlorides contribute as much as
73% to the fine particles emitted in industrial smoke
*
Corresponding author.
Tel.: 86-531-88364675; Fax: 86-531-88361990
E-mail address: xinfengwa[email protected]u.cn
(Moffet et al., 2008a). Chloride aerosols can be activated
via reactions with strong acids or acid anhydrides, and in
turn further anticipate the tropospheric chemistry. Previous
field studies have confirmed that particulate-phase chlorides
(e.g., sodium chloride and potassium chloride) react with
sulfuric acid and/or nitric acid to release gas-phase HCl,
which further reacts with NH
3
to produce ammonium
chloride or with dust to produce chloride-dust (Sullivan et al.,
2007a). Recent studies have revealed that the heterogeneous
reactions of N
2
O
5
on chloride aerosols release ClNO
2
,
which serves as an important source of Cl atoms (Osthoff
et al., 2008; Thornton et al., 2010; Wang et al., 2016).
With the development and application of single-particle
analytical techniques, detailed information has been
obtained on the chemical constituents and mixing states of
individual aerosols, showing that a large fraction of chloride
aerosols mix with other substances, which alters the
behavior of chloride aerosols. Scanning electron microscopy
(SEM) and transmission electron microscopy (TEM) can
be used to identify the size and elemental distribution of
individual chloride aerosols in the atmosphere, together with
the mixing characteristics with other constituents (Barkay et
al., 2005; Laskin et al., 2006; Adachi and Buseck, 2008;
Chi et al., 2015). The recently developed aerosol time-of-
Wang et al., Aerosol and Air Quality Research, 17: 878–887, 2017
879
fight mass spectrometry (ATOFMS) can provide the size
and chemical composition of a large number of individual
aerosols in real time (Moffet et al., 2008b; Bi et al., 2011;
Prather et al., 2013). These single-particle techniques have
provided new insights and a deeper understanding of the
mixing characteristics of chlorine-rich aerosols including
sea salts, particles in biomass burning plumes and industrial
smoke, and secondary chloride aerosols. Andreae et al.
(1986) unexpectedly found that a large fraction of mineral
substances and sulfates in the marine boundary layer were
internally mixed with sodium chlorides. Subsequently, several
field studies have confirmed that a significant proportion of
sea salts are internally mixed with sulfates and nitrates (Mouri
et al., 1993; Laskin et al., 2002; Li et al., 2003a), while
other research has revealed that the chloride components in
sea salts acquire a coating of organic surfactants in polluted
coastal environments or even at remote islands (Tervahattu
et al., 2002a, b; Laskin et al., 2012; Chi et al., 2015).
Electron microscopy and single-particle mass spectrometry
have indicated that potassium chlorides in biomass burning
smoke mix with soot and organics, and some even acquire
an organic surface coating (Silva et al., 1999; Li et al.,
2003b). In urban areas, field studies have detected plentiful
submicron metal chloride containing particles, which are
typically mixed with carbonaceous materials in combustion
emissions (Moffet et al., 2008b; Li et al., 2009; Geng et al.,
2010; Hu et al., 2015). Additionally, in long-range transport
dust plumes over the Pacific, Sullivan et al. (2007b) observed
that a large amount of secondary particulate chlorides
(produced from gas-phase HCl) was internally mixed with
dust particles. The mixing of various chloride aerosols with
other substances, particularly insoluble and light-absorbing
materials, may significantly alter the hygroscopic, reactivity,
and optical properties, as well as their effect on the climate,
thus causing the apparent deviation between model
predictions and ambient observations. In spite of the above
studies, the current knowledge on the mixing state of chloride
aerosols is generally fragmentary, and a comprehensive
understanding is required.
This study reviews the currently available, scattered
studies on mixed chloride aerosols (the major field campaigns
are shown in Fig. 1) to obtain a clearer understanding of
their mixing properties and the subsequent atmospheric
implications. First, we provide a brief introduction to the
commonly used single-particle analytical methods. Then, we
summarize the previous results on the composition of mixed
chloride aerosols, their mixing characteristics, the co-existing
constituents and the necessary conditions for aerosol
mixing, according to a classification into sea salt, biomass
burning particle, industrial smoke dust, and secondary
chloride particle. Finally, we summarize and discuss the effect
of the mixing of chloride aerosols on the hygroscopicity,
reactivity, and optical properties of the particles.
ANALYTICAL TECHNIQUES FOR PARTICLE
MIXING STATE
The development of single-particle techniques has allowed
substantial progress in research into particle mixing states,
and offers considerable advantages compared with the
Fig. 1. Map showing the previous field studies on mixing characteristics of chloride aerosols with various techniques.
Wang et al., Aerosol and Air Quality Research, 17: 878–887, 2017
880
traditional analytical methods for bulk aerosols. Bulk
aerosol analytical methods (e.g., online and offline ion
chromatography and aerosol mass spectrometry) provide only
information on the concentrations of different components
for large amounts of particles. To obtain the mixing
characteristics of different constituents in individual particles,
several advanced single-particle techniques have been applied
or developed in the past decades, including SEM, TEM,
ATOFMS, scanning transmission X-ray microscopy with
near-edge X-ray absorption fine-structure spectroscopy
(STXM-NEXAFS), nanoscale secondary-ion mass
spectrometry (NanoSIMS), and time-of-flight secondary-
ion mass spectrometry (TOF-SIMS).
SEM is a widely used technique in which the surface of
a single particle is imaged by scanning it with a high-energy
beam of electrons in a raster scan pattern. SEM equipped
with energy-dispersive X-ray spectrometry (EDX) is an
effective approach for identifying the surface composition
(elemental mapping) of individual particles with diameters
greater than 100 nm. The mixing characteristics of different
constituents in the surface layer of the particle can be
obtained from information on the shape and chemical
composition by SEM-EDX (Barkay et al., 2005; Laskin et
al., 2006; Pachauri et al., 2013).
TEM is an electron microscopic technique in which a
beam of electrons is transmitted through the particle.
TEM-EDX can be used to readily observe the mixing state,
structure, and elemental composition of the entire particle
(Li et al., 2003a; Adachi and Buseck, 2008; Li et al., 2016a).
Note that the ordinary EDX can only detect elements heavier
than carbon and the TEM requires labor-intensive operation.
The application of ultra-thin window detectors in SEM and
TEM permits the detection of light elements and thus
achieves nearly total element analysis. In addition, the TEM
and SEM suffer from a defect of possible changes of particle
samples in the microstructure, composition, and mass (e.g.,
semi-volatile compounds) during examination.
STXM-NEXAFS can also image the particle shape,
albeit with poor resolution. This combined method provides
information on the spatially resolved bonding and oxidation
state of the investigated particles with enhanced chemical
sensitivity. STXM-NEXAFS can be used to obtain the
mixing properties of certain specific constituents, particularly
organics and light-absorbing components (Moffet et al.,
2010; Laskin et al., 2012; Ault et al., 2013a).
ATOFMS is an online, field-deployable form of aerosol
mass spectrometry that couples aerodynamic particle sizing
with time-of-flight mass spectrometry in a single instrument.
ATOFMS provides real-time, high-time-resolution data on
the sizes, compositions, and mixing properties of individual
particles (Dall’Osto et al., 2004; Dall’Osto and Harrison,
2006; DeCarlo et al., 2006; Moffet et al., 2008a, b; Bi et
al., 2011). It provides good statistic information on single
particles; however, the specific components of the particles
are difficult to identify from the mass spectra.
Recently, TOF-SIMS and NanoSIMS have been used to
study single particles. In these methods, a primary-ion
beam is fired at the particle surface, and the secondary ions
produced by the impact are detected by advanced mass
spectrometers. TOF-SIMS and NanoSIMS provide the
distributions of all elements and isotopes, as well as certain
molecules, at the particle surface with very high sensitivity,
accuracy, and lateral resolution. Compared with TOF-SIMS,
NanoSIMS generally has higher sensitivity and a higher
lateral and mass resolution and obtains more detailed
information on the mixing of particles (Hagenhoff, 2000;
Tervahattu et al., 2002b, 2005; Marino et al., 2006; Ghosal
et al., 2014; Chi et al., 2015; Li et al., 2016b).
MIXING CHARACTERISTICS OF CHLORIDE
AEROSOLS IN ATMOSPHERE
Mixing of Chlorides in Sea Salts
Generally, a large fraction of the chlorides in sea salts are
internally mixed with inorganic materials in the atmosphere
and co-exist with many different substances (see the
schematic diagrams in Fig. 2). In the relatively clean
atmosphere over the Pacific Ocean, Atlantic Ocean, and
coastal Antarctica, field observations have indicated that
much of the sodium chloride in fresh sea salts is capped by
a small quantity of other inorganic elements (as shown in
Fig. 2(a)) (Andreae et al., 1986; Murphy et al., 1998; Li et
al., 2003a; Hara et al., 2005; Li et al., 2016a; Young et al.,
2016). For example, Andreae et al. (1986) observed by
SEM that regular sodium chloride crystals contacted with
various silicates and sulfates in the equatorial Pacific
Ocean between Ecuador and Hawaii, and Li et al. (2016a)
observed by SEM and TEM that square sodium chloride
particles were associated with certain amounts of magnesium
chlorides and calcium sulfates in the Yellow Sea of China.
In coastal regions with anthropogenic influences, the
compositions and mixing characteristics of sea salts
significantly change along with the atmospheric transport
and aging processes. A fraction of the particulate chlorides
in sea salts is converted into gas-phase compounds (e.g.,
HCl and ClNO
2
), with most of the remaining chlorides
completely encased within a large quantity of inorganic
elements (as shown in Fig. 2(b)) (e.g., Mg and S at Cape
Grim (Murphy et al., 1998), K, Ca, and S in Incheon (Geng
et al., 2010), Mg, K, Ca, and S in Antarctica (Hara et al.,
2005), and Mg, N, and S in Houston, Macao, and Hong Kong
(Laskin et al., 2002; Li et al., 2010, 2016a)). Amorphous
sodium chloride particles were found to be completely bound
with sodium nitrates and sodium sulfates in coastal Hong
Kong (Li et al., 2016a). An investigation of sea spray
aerosols generated in the laboratory by Ault et al. (2013b)
showed that elemental redistribution occurred within the
sea salt particles, and their internal structures were altered
by reaction with nitric acid. In addition, field studies have
found that the chlorides in sea salts were closely bound
with aluminum compounds in the coastal town of M’Bour,
Senegal (Deboudt et al., 2010), and iron compounds in the
industrial region of Dunkerque (Choël et al., 2010).
Besides co-existing with other inorganic matter, a
significant fraction of the chlorides in sea salts also mix
with organic compounds in polluted coastal regions (see the
schematic diagrams in Fig. 3). The identified organic species
co-existing with sea salts varies by location (e.g., fatty acids
Wang et al., Aerosol and Air Quality Research, 17: 878–887, 2017
881
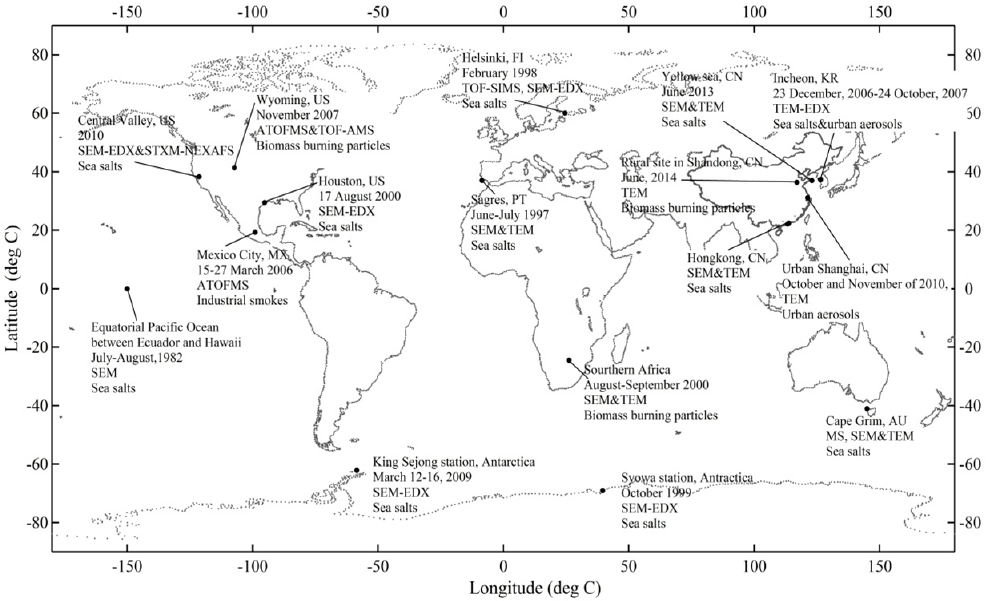
Fig. 2. Schematic diagrams showing the mixing of chlorides in sea salts with inorganic substances, referred from Li et al.
(2016a). (a) Typical square NaCl attaching with the mixture of Mg, K, Ca and S in the fresh sea salt; (b) Shapeless NaCl
enclosed by Na
2
SO
4
and NaNO
3
in the aged sea salt.
Fig. 3. Schematic diagrams showing the chlorides in sea salts coated by organic matters, referred from Ault et al. (2013a)
and Chi et al. (2015). (a) A typical fresh sea salt (square NaCl) and (b) an aged sea salt (NaCl core) coated by organic
materials.
in Helsinki (Tervahattu et al., 2002a, b), methanesulfonates
in King George Island, Antarctica (Maskey et al., 2011),
and multiple organic compounds at Cape Grim (Middlebrook
et al., 1998; Murphy et al., 1998) and in coastal central
California (De Bock et al., 2000)). In the sea salts mixed
with organics, most of the sodium chlorides were surface-
coated by organic materials. Laskin et al. (2012) found by
SEM-EDX and STXM-NEXAFS that the chloride core in
aged sea salts was coated by a substantial C-containing
constituent layer. Subsequent ocean-in-lab experiments with
sea spray aerosols generated from sea water after the addition
of biological and organic materials also discovered an
apparent organic coating layer at the surface of the sodium
chloride core (Ault et al., 2013a; Prather et al., 2013).
Mixing of Chlorides in Biomass Burning Particles
Chloride aerosols from biomass burning normally mix with
carbonaceous materials in young smoke and are completely
coated by organics after partial replacement in aged smoke
(see the schematic diagrams in Fig. 4). Li et al. (2003b)
investigated chloride aerosol particles from biomass
burning in the southern African boundary layer and free
troposphere using TEM. In fresh biomass burning plumes,
most of the chlorides existed as potassium chloride, and
the potassium chloride crystals contacted with carbonaceous
materials. Further biomass burning experiments in the open
field by Li et al. (2016a) confirmed that the co-existing
carbonaceous materials could be either organic matter or
soot, and their mixing characteristics varied depending on
the type of biomass fuel and the burning conditions. Geng
et al. (2010) also observed chlorides in tar ball particles
generated from household wood combustion in Incheon in
winter. In aged biomass burning smoke, potassium chlorides
were partly replaced by potassium sulfates and potassium
nitrates through heterogeneous reactions, and the remaining
chlorides and potassium were completely coated by organic
compounds (Li et al., 2003b).
Mixing of Chlorides in Industrial Smoke
Chloride aerosol particles directly emitted in industrial
smoke mostly co-exist with transition/heavy metals. Geng
et al. (2010), using TEM, observed abundant Zn/Pb/Cl-
Wang et al., Aerosol and Air Quality Research, 17: 878–887, 2017
882
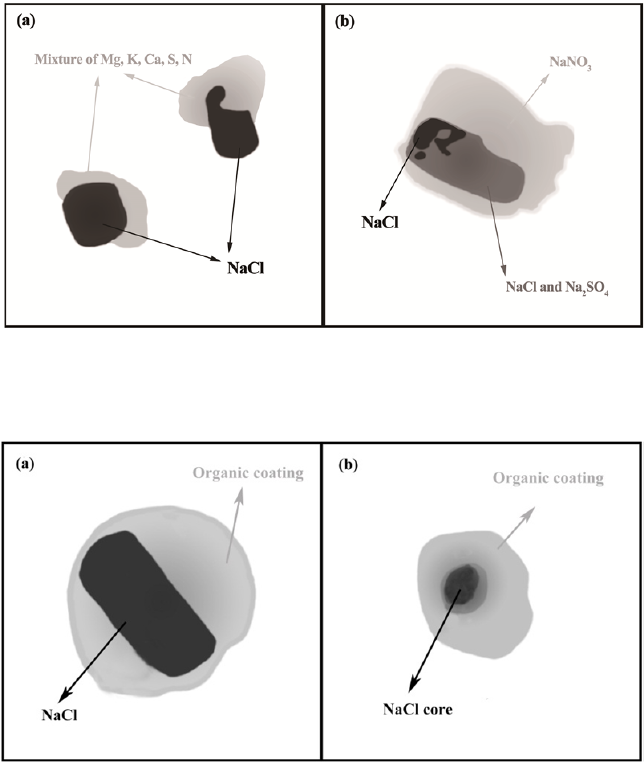
Fig. 4. Schematic diagrams showing the mixing characteristics of chlorides in biomass burning particles, referred from Li
et al. (2003b) and Li et al. (2016a). (a) KCl crystals touching with organics and soot in young biomass burning smokes. (b)
Shapeless KCl, and secondary K
2
SO
4
and KNO
3
coated by organic compounds after aging processes in aged smokes.
containing particles
in polluted urban Incheon, which were
attributed to industrial processes and coal-fired power
generation. The gas-phase zinc and lead chlorides emitted
from high-temperature combustion sources condensed into
solid-phase submicron Cl-containing particles upon cooling
(Hu et al., 2003). Online measurements of single particles
by ATOFMS in an industrial/residential section of Mexico
City by Moffet et al. (2008a, b) demonstrated that the
metal/Cl-containing particles accounted for a large fraction
of the fine-mode particles, and the fraction reached 73% in
the early morning hours. Other studies have also observed
numerous micron-sized Zn/Pb/Cl-containing particles in
lead smelter plumes (Ettler et al., 2005), in incinerator fly
ash (Tan et al., 2002; Zhang et al., 2009), in mixed urban
plumes (Lu et al., 2012), and in haze episodes (Li et al., 2009;
Hu et al., 2015). The chloride particles from industrial smoke
were often coated by organic matter after aging (see the
schematic diagram in Fig. 5). TEM images of aerosol
particles collected in urban Shanghai by Fu et al. (2012)
clearly showed that zinc chloride particles from industrial
plumes were completely enclosed within organic compounds.
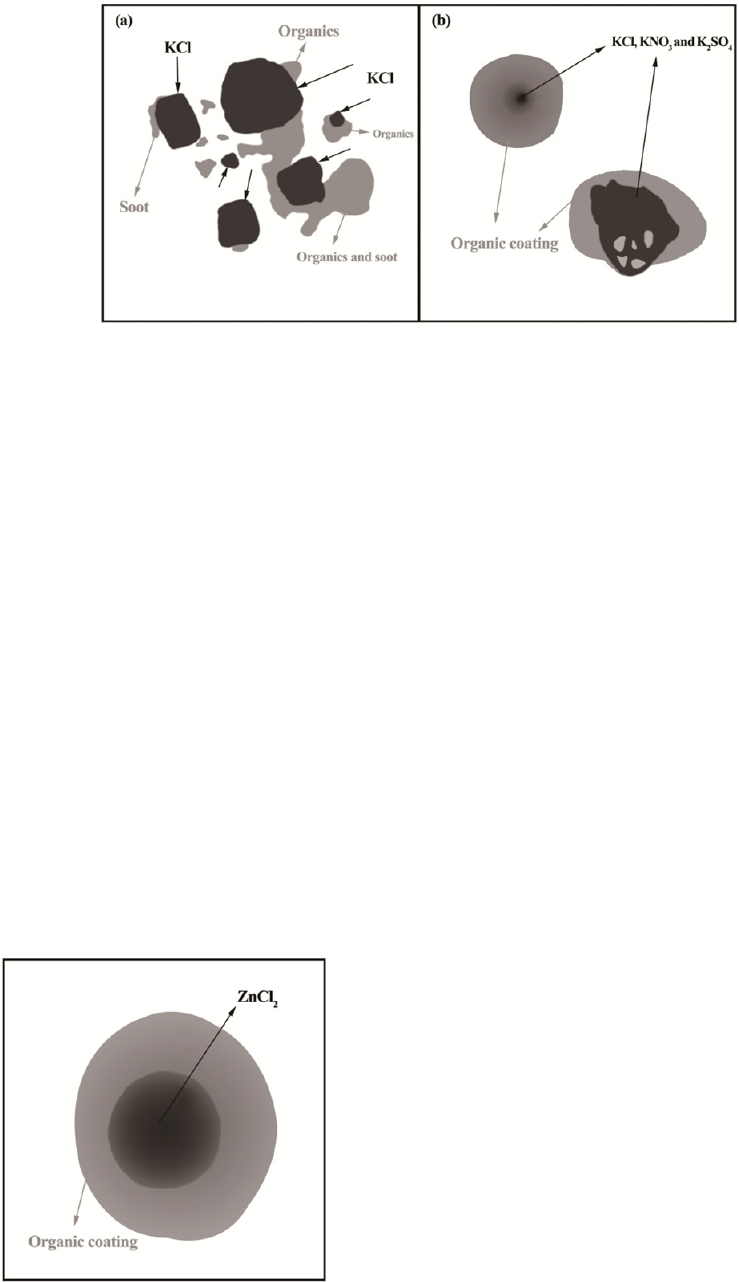
Fig. 5. Schematic diagram showing the typical industrial
ZnCl
2
particle coated by organic matters, referred from Fu
et al. (2012).
Mixing of Secondary Chloride Particles
The secondary chloride aerosols produced by the reaction
of HCl with alkaline substances (e.g., NH
3
and mineral
dusts) often mix with dust, nitrate, and sometimes sulfate.
Sullivan et al. (2007b) observed a large fraction of chlorides
mixing with mineral dusts (i.e., Al, Ca, and Fe compounds)
in long-range transport dust plumes by using ATOFMS
during shipboard measurements over the Pacific Ocean
between Hawaii and Japan. Further study by Sullivan et al.
(2007a) showed that the abundant chlorides in dust particles
originated from the heterogeneous reactions of HCl released
from chemically aged sea salt particles. The newly added
secondary chlorides in the mixed dust particles contributed
4–9% of the individual dust particle mass, and secondary
chloride-containing dust particles constituted as much as
65% of the total dust particles during a major dust storm.
Tobo et al. (2010) also noticed the chloride depletion in
sea salt particles and a coatings containing CaCl
2
on the
surface of dust core at Kanazawa site during an Asian dust
storm period. Moreover, Sullivan et al. (2007b) observed a
significant fraction of secondary chloride particles mixing
with nitrates (14.8% of the chloride-dust and nitrate-dust
particles) and a small proportion mixing with sulfates
(0.8% of the chloride-dust and sulfate-dust particles).
ATMOSPHERIC IMPLICATIONS OF MIXED
CHLORIDE AEROSOLS
Alteration to Hygroscopicity and Cloud Condensation
Nuclei Activation
The mixing of chloride aerosols with other inorganic
and organic constituents (as summarized in Table 1) can
significantly alter their hygroscopicity and further influence
the activation of cloud condensation nuclei (CCN) when
compared with the pure chloride salts. Salt particles primarily
consisting of pure hydrophilic chlorides are hygroscopic,
with deliquescence relative humidity (DRH) values of around
80% (e.g., 75.3% for NaCl, 84.2% for KCl, and 80.0% for
NH
4
Cl at 298 K) (Tang et al., 1978; Tang and Munkelwitz,
1993). The mixing of chloride salts with other inorganic salts,

Wang et al., Aerosol and Air Quality Research, 17: 878–887, 2017
883
Table 1. Summary of mixed chloride aerosols in atmosphere.
Type Location Chloride form Mixed element or
substance
Mixing pattern
Sea salts Marine and coastal
region
NaCl, minor MgCl
2
Mg, Ca, K, N, S,
organics
Enclosed with inorganics, coated
by organics
Biomass burning
particles
Vegetation covered
area
KCl N, S, K, soot,
organics
Touching with or coated by
carbons, replaced by N and S
Industrial smokes Industrialized city Zn-Pb-Cl Metals, organics Co-existing with metals, coated
by organics
Secondary chloride
particles
Marine and other
locations
NH
4
Cl, CaCl
2
Al, Ca, Fe, N, S Mixed with dust, nitrates, and
minor sulfates
particularly low-DRH salts like nitrates and hydrosulfates,
heavily decreases the DRH of the mixed aerosol particles
and thus enhances their CCN activation. Wexler and
Seinfeld (1991) compared the DRHs of mixtures of nitrates,
chlorides, and sulfates and those of the individual salts,
and found apparent decreases in DRH after mixing. Wise
et al. (2009) observed a significant reduction in deliquescence
humidity when sodium chloride was mixed with a small
amount of various inorganic substances, with the DRH
dropping to 66% for laboratory-synthesized sea salts and
to 57% for natural sea salts. For sulfate-coated and Mg-
rich chloride-coated sea salt particles collected from the
ambient atmosphere, Semeniuk et al. (2007a) also observed
that the initial water uptake occurred at lower relative
humidity (RH) than for common marine aerosol particles,
and the coated sea salts underwent a complex multi-step
deliquescence. Nevertheless, the mixing of chlorides with
less hygroscopic (i.e., organic compounds) and insoluble
substances (e.g., soot) can significantly suppress their
hygroscopicity and CCN efficiency. Semeniuk et al. (2007b)
investigated the hygroscopic behavior of single particles of
potassium chlorides mixing with organic matter at RH
values of 0–100%, and found that the mixed chloride particles
did not appear to deliquesce. For ambient particles comprising
organic substances and chloride and/or other inorganic
components, online field measurements indicated that the
CCN efficiency decreased from 12.8 ± 6.1% to 4.5 ± 2.6%
when the organic fraction increased from 10–20% to 30–
40% (Zhang et al., 2012).
Change in Heterogeneous Reactivity
Changes in the liquid water and soluble chloride contents
in mixed chloride aerosols subsequently affect the reactivity
of the chlorides in related heterogeneous reactions. Generally,
the mixing of chlorides with inorganic salts tends to promote
reactivity, while mixing with or coating by less hygroscopic
and insoluble substances is prone to suppress reactivity.
Saul et al. (2006) determined the reactive uptake coefficient
of nitric acid on both pure sodium chlorides and sodium
chlorides mixed with magnesium chlorides at RH values of
10–85%, and found that the uptake coefficient on the mixed
chloride aerosols remained high (> 0.1) even at 10% RH, and
was much higher than that on pure sodium chloride aerosols.
This enhancement suggests that the displacement of chloride
by nitrate is facilitated by the mixing of chlorides. Stewart
et al. (2004) also observed a higher N
2
O
5
uptake coefficient
on natural sea salts (e.g., 0.048 at 50% RH) than that on
pure sodium chlorides (e.g., 0.033 at 50% RH), but they
attributed this phenomenon to the variation in the salt size,
which led to a limitation in the uptake rate on small particles.
An investigation of the heterogeneous loss of N
2
O
5
on
submicron sea salt particles with a hexanoic acid coating
by Thornton and Abbatt (2005) showed that the presence of
millimolar levels of hexanoic acid greatly decreased the N
2
O
5
uptake coefficient by a factor of 3–4 (i.e., approximately from
0.025 to 0.008, at 70% RH).
Effects on Optical Properties and Radiative Forcing
The mixing of chloride aerosols with organic matter alters
not only the hygroscopicity but also the optical properties
of the aerosols, which has a further effect on the radiative
forcing. Generally, mixing with non-absorbing organic matter
suppresses the uptake of water, particularly at high humidity,
and thus reduces the aerosol scattering efficiency and the
subsequent cooling effect on the atmosphere. Abo Riziq et
al. (2007) measured the optical properties of mixed chloride
particles and found that the extinction efficiency of mixed
particles of sodium chloride with glutaric acid decreased
significantly in comparison with pure sodium chloride
particles. A modeling study by Randles et al. (2004) predicted
that an internal mixture of 90% sodium chloride with 10%
non-absorbing organics would cause 3% less cooling than
100% sodium chloride particles in the visible spectrum
over clear-sky oceans. The mixing of chloride with light-
absorbing substances (e.g., black carbon (soot) and brown
carbon (nitroaromatic compounds, humic-like substances,
and imidazoles)) generally enhances the absorption of light
and/or decreases the scattering of light by aerosols when
compared with the individual components, and thus causes
a warming effect. Mallet et al. (2004) compared the single-
scattering albedos of internally mixed sea salt with black
carbon particles, the corresponding externally mixed particles,
and unmixed sea salts at a coastal industrialized region in
France, and found that the single-scattering albedo of the
internally mixed particles (0.75) was significantly lower than
those of the externally mixed particles (0.85) and unmixed
sea salts (0.99). A modeling study by Randles et al. (2004)
predicted that the internal mixing of 90% NaCl with 10%
mildly absorbing organic compounds would substantially
reduce the radiative cooling compared with 100% NaCl
aerosols. In addition, Hoffer et al. (2006) found that humic-
like substances in biomass burning particles at a tropical
Wang et al., Aerosol and Air Quality Research, 17: 878–887, 2017
884
pasture site in Brazil contributed 50% to the absorption of
light by aerosols at 300 nm and 7% for the entire spectrum.
Chakrabarty et al. (2010) also reported that the presence of
brown carbon in biomass burning particles collected from
a laboratory chamber led to an increase in light absorption and
the radiative forcing efficiency. The global mean radiative
forcing of biomass burning aerosols is approximately 0.04
W m
–2
, with a median of 0.02 W m
–2
(Forster et al., 2007).
FUTURE PERSPECTIVES
A number of field studies on single particles have
confirmed that a large fraction of the chloride aerosols from
various sources are internally mixed with other inorganic
and organic substances in diverse locations. Laboratory
experiments and a few field measurements and modeling
simulations have provided some evidence that the mixing
of chloride aerosols with other constituents has significant
effects on their hygroscopicity, CCN activity, heterogeneous
reactivity, optical properties, and radiative forcing when
compared with the pure chloride salts. However, additional
studies are required to obtain a comprehensive understanding
of the mixing characteristics of chloride particles and their
subsequent atmospheric effects, including (a) more field
studies on the mixing properties of chloride aerosols in
megacity centers, suburban areas, and rural locations to
fully understand the influences of anthropogenic activities
on the mixing state as well as the mixing processes; (b)
further laboratory studies to clarify the complex effects of
different mixing patterns on the size, density, morphology,
porosity, hygroscopicity, reactivity, and optical properties
of chloride aerosols; (c) the development and improvement
of modeling techniques by taking into account the mixing
state of the chloride aerosols and the subsequent changes
in their physical and chemical properties; and (d) modeling
simulations to evaluate the potential environmental effects
(e.g., radiative forcing, CCN activation, and precipitation)
of mixed chloride aerosols.
CONCLUSION
In this review, we have summarized the existing studies
and findings on the mixing characteristics of chloride
aerosols, and the atmospheric consequences of this mixing.
Besides the traditionally used SEM and TEM, several new
single-particle techniques have been developed and applied
in research on the mixing state and chemical composition
of aerosol particles, including STXM-NEXAFS, ATOFMS,
TOF-SIMS, and NanoSIMS. Field studies in various locations
have shown that a large proportion of chlorides internally
mix with other inorganic and organic constituents rather
than existing alone. Specifically, sodium chlorides in fresh
sea salts are usually attached to a small quantity of other
inorganic substances, while in aged sea salts they are
completely encased within a large quantity of inorganic
materials. Sodium chlorides in polluted coastal regions also
co-exist with multiple organic compounds, with an organic
layer coated on the surface. Potassium chlorides in biomass
burning particles are generally attached to carbonaceous
materials in young smoke, and they are completely coated by
organic matter in aged smoke. Most chloride aerosols emitted
in industrial smoke are mixed with transition/heavy metals,
and a coating of organics and/or sulfates appears on aged
particles. In addition, some secondary chlorides are produced
by the reactions of HCl with alkaline substances, and can
mix with mineral dusts, nitrates, and sulfates. The mixing
of chloride with other components can significantly alter
the physical and chemical properties of the aerosol
particles. Generally, mixing with inorganic salts enhances
the hygroscopicity, heterogeneous reactivity, and CCN
activation of the aerosols, while mixing with organic matter
suppresses these traits. The mixing of chloride aerosols
with organic compounds can decrease the scattering of light
or increase its absorption by aerosols, thus leading to a
warming effect to the atmosphere. With consideration of
the apparent effects of mixing on the properties of chloride
aerosols and subsequently on the climate and the limited
current studies in this community, we also provide
suggestions for necessary future research.
ACKNOWLDEGEMENTS
This work was supported by the National Natural Science
Foundation of China (Nos. 91544213, 41275123, 21407094)
and the Natural Science Foundation of Shandong Province
(No. ZR2014BQ031).
REFERENCES
Abo Riziq, A., Erlick, C., Dinar, E. and Rudich, Y. (2007).
Optical properties of absorbing and non-absorbing aerosols
retrieved by cavity ring down (CRD) spectroscopy.
Atmos. Chem. Phys. 7: 1523–1536.
Adachi, K. and Buseck, P. (2008). Internally mixed soot,
sulfates, and organic matter in aerosol particles from
Mexico City. Atmos. Chem. Phys. 8: 6469–6481.
Andreae, M.O., Charlson, R.J., Bruynseels, F., Storms, H.,
Van Grieken, R. and Maenhaut, W. (1986). Internal
mixture of sea salt, silicates, and excess sulfate in
marine aerosols. Science 232: 1620–1623.
Ault, A.P., Moffet, R.C., Baltrusaitis, J., Collins, D.B.,
Ruppel, M.J., Cuadra-Rodriguez, L.A., Zhao, D., Guasco,
T.L., Ebben, C.J. and Geiger, F.M. (2013a).
Size-
dependent changes in sea spray aerosol composition and
properties with different seawater conditions. Environ.
Sci. Technol. 47: 5603–5612.
Ault, A.P., Guasco, T.L., Ryder, O.S., Baltrusaitis, J.,
Cuadra-Rodriguez, L.A., Collins, D.B., Ruppel, M.J.,
Bertram, T.H., Prather, K.A. and Grassian, V.H. (2013b).
Inside versus outside: Ion redistribution in nitric acid
reacted sea spray aerosol particles as determined by single
particle analysis J. Am. Chem. Soc. 135: 14528–14531.
Barkay, Z., Teller, A., Ganor, E., Levin, Z. and Shapira, Y.
(2005). Atomic force and scanning electron microscopy
of atmospheric particles. Microsc. Res. Tech. 68: 107–
114.
Bi, X., Zhang, G., Li, L., Wang, X., Li, M., Sheng, G., Fu,
J. and Zhou, Z. (2011). Mixing state of biomass burning
Wang et al., Aerosol and Air Quality Research, 17: 878–887, 2017
885
particles by single particle aerosol mass spectrometer in
the urban area of PRD, China. Atmos. Environ. 45:
3447–3453.
Carslaw, K., Boucher, O., Spracklen, D., Mann, G., Rae, J.,
Woodward, S. and Kulmala, M. (2010). A review of
natural aerosol interactions and feedbacks within the
earth system. Atmos. Chem. Phys. 10: 1701–1737.
Chakrabarty, R., Moosmüller, H., Chen, L.W., Lewis, K.,
Arnott, W., Mazzoleni, C., Dubey, M., Wold, C., Hao,
W. and Kreidenweis, S. (2010). Brown carbon in tar
balls from smoldering biomass combustion. Atmos.
Chem. Phys. 10: 6363–6370.
Chi, J., Li, W., Zhang, D., Zhang, J., Lin, Y., Shen, X., Sun,
J., Chen, J., Zhang, X., Zhang, Y. (2015). Sea salt
aerosols as a reactive surface for inorganic and organic
acidic gases in the Arctic troposphere. Atmos. Chem.
Phys. 15: 11341–11353.
Choël, M., Deboudt, K. and Flament, P. (2010). Development
of time-resolved description of aerosol properties at the
particle scale during an episode of industrial pollution
plume. Water Air Soil Poll. 209: 93–107.
Dall'Osto, M., Beddows, D.C.S., Kinnersley, R.P., Harrison,
R.M., Donovan, R.J. and Heal, M.R. (2004).
Characterization of individual airborne particles by
using aerosol time-of-flight mass spectrometry at Mace
Head, Ireland. J. Geophys. Res. 109: D21302.
Dall'Osto, M. and Harrison, R. (2006). Chemical
characterisation of single airborne particles in Athens
(Greece) by ATOFMS. Atmos. Environ. 40: 7614–7631.
De Bock, L.A., Joos, P.E., Noone, K.J., Pockalny, R.A. and
Van Grieken, R.E. (2000). Single particle analysis of
aerosols, observed in the marine boundary layer during
the Monterey Area Ship Tracks Experiment (MAST),
with respect to cloud droplet formation. J. Atmos. Chem.
37: 299–329.
Deboudt, K., Flament, P., Choël, M., Gloter, A., Sobanska,
S. and Colliex, C. (2010). Mixing state of aerosols and
direct observation of carbonaceous and marine coatings
on African dust by individual particle analysis. J.
Geophys. Res. 115: D24207.
DeCarlo, P.F., Kimmel, J.R., Trimborn, A., Northway,
M.J., Jayne, J.T., Aiken, A.C., Gonin, M., Fuhrer, K.,
Horvath, T., Docherty, K.S., Worsnop, D.R. and Jimenez,
J.L. (2006). Field-deployable, high-resolution, time-of-
flight aerosol mass spectrometer. Anal. Chem. 78: 8281–
8289.
Erickson, D.J. and Duce, R.A. (1988). On the global flux
of atmospheric sea salt. J. Geophys. Res. 93: 14079–
14088.
Ettler, V., Zdenek, J., Baronnet, A., Jankovsky, F., Gilles,
C., Mihaljevic, M., Sebek, O., Strnad, L. and Bezdicka,
P. (2005).
Mineralogy of air-pollution-control residues
from a secondary lead smelter: environmental implications.
Environ. Sci. Technol. 39: 9309–9316.
Forster, P., Ramaswamy, V., Artaxo, P., Berntsen, T., Betts,
R., Fahey, D.W., Haywood, J., Lean, J., Lowe, D.C. and
Myhre, G. (2007). Changes in atmospheric constituents
and in radiative forcing. Chapter 2, In Climate Change
2007. The Physical Science Basis. Solomon, S., Qin, D.,
Manning, M., Chen, Z., Marquis, M., Averyt, K.B.,
Tignor, M. and Miller, H.L. (Eds.), Cambridge University
Press, Cambridge, United Kingdom and New York, NY,
USA, pp. 129–234.
Fu, H., Zhang, M., Li, W., Chen, J., Wang, L., Quan, X.
and Wang, W. (2012). Morphology, composition and
mixing state of individual carbonaceous aerosol in urban
Shanghai. Atmos. Chem. Phys. 12: 693–707.
Geng, H., Kang, S., Jung, H.J., Choël, M., Kim, H. and Ro,
C.U. (2010). Characterization of individual submicrometer
aerosol particles collected in Incheon, Korea, by
quantitative transmission electron microscopy energy-
dispersive X-ray spectrometry. J. Geophys. Res. 115:
D15306.
Ghosal, S., Weber, P.K. and Laskin, A. (2014). Spatially
resolved chemical imaging of individual atmospheric
particles using nanoscale imaging mass spectrometry:
Insight into particle origin and chemistry. Anal. Methods
6: 2444–2451.
Gong, S., Barrie, L. and Lazare, M. (2002). Canadian
Aerosol Module (CAM): A size-segregated simulation
of atmospheric aerosol processes for climate and air
quality models 2. Global sea-salt aerosol and its budgets.
J. Geophys. Res. 107: D244779.
Hagenhoff, B. (2000). High resolution surface analysis by
TOF-SIMS. Microchim. Acta 132: 259–271.
Hara, K., Osada, K., Kido, M., Matsunaga, K., Iwasaka, Y.,
Hashida, G. and Yamanouchi, T. (2005). Variations of
constituents of individual sea-salt particles at Syowa
station, Antarctica. Tellus Ser. B 57: 230–246.
Hoffer, A., Gelencsér, A., Guyon, P., Kiss, G., Schmid, O.,
Frank, G., Artaxo, P. and Andreae, M. (2006). Optical
properties of humic-like substances (HULIS) in biomass-
burning aerosols. Atmos. Chem. Phys. 6: 3563–3570.
Hu, C.W., Chao, M.R., Wu, K.Y., Chang-Chien, G.P., Lee,
W.J., Chang, L.W. and Lee, W.S. (2003). Characterization
of multiple airborne particulate metals in the surroundings
of a municipal waste incinerator in Taiwan. Atmos.
Environ. 37: 2845–2852.
Hu, Y., Lin, J., Zhang, S., Kong, L., Fu, H. and Chen J.
(2015). Identification of the typical metal particles among
haze, fog, and clear episodes in the Beijing atmosphere.
Sci. Total Environ. 511: 369–390.
Laskin, A., Iedema, M.J. and Cowin, J.P. (2002).
Quantitative time-resolved monitoring of nitrate formation
in sea salt particles using a CCSEM/EDX single particle
analysis. Environ. Sci. Technol. 36: 4948–4955.
Laskin, A., Cowin, J.P. and Iedema, M.J. (2006).
Analysis
of individual environmental particles using modern
methods of electron microscopy and X-ray microanalysis.
J. Electron Spectrosc. 150: 260–274.
Laskin, A., Moffet, R.C., Gilles, M.K., Fast, J.D., Zaveri,
R.A., Wang, B., Nigge, P. and Shutthanandan, J. (2012).
Tropospheric chemistry of internally mixed sea salt and
organic particles: Surprising reactivity of NaCl with
weak organic acids. J. Geophys. Res. 117: D15302.
Li, J., Anderson, J.R. and Buseck, P.R. (2003a).
TEM study
of aerosol particles from clean and polluted marine
boundary layers over the North Atlantic. J. Geophys.
Wang et al., Aerosol and Air Quality Research, 17: 878–887, 2017
886
Res. 108: D64189.
Li, J., Pósfai, M., Hobbs, P.V. and Buseck, P.R. (2003b).
Individual aerosol particles from biomass burning in
southern Africa: 2. Compositions and aging of inorganic
particles. J. Geophys. Res. 108: D138484.
Li, K., Sinha, B. and Hoppe, P. (2016b). Speciation of
nitrogen bearing species using negative and positive
secondary ion spectra with Nano Secondary Ion Mass
Spectrometry. Anal. Chem. 88: 3281–3288.
Li, W. and Shao, L. (2009). Transmission electron microscopy
study of aerosol particles from the brown hazes in
northern China. J. Geophys. Res. 114: D09302.
Li, W., Shao, L., Wang, Z., Shen, R., Yang, S. and Tang, U.
(2010). Size, composition, and mixing state of individual
aerosol particles in a South China coastal city. J.
Environ. Sci. 22: 561–569.
Li, W., Shao, L., Zhang, D., Ro, C.U., Hu, M., Bi, X.,
Geng, H., Matsuki, A., Niu, H. and Chen, J. (2016a). A
review of single aerosol particle studies in the
atmosphere of East Asia: Morphology, mixing state,
source, and heterogeneous reactions. J. Cleaner Prod.
112: 1330–1349.
Lu, S., Zhang, R., Yao, Z., Yi, F., Ren, J., Wu, M., Feng,
M. and Wang, Q. (2012). Size distribution of chemical
elements and their source apportionment in ambient
coarse, fine, and ultrafine particles in Shanghai urban
summer atmosphere. J. Environ. Sci. 24: 882–890.
Mallet, M., Roger, J., Despiau, S., Putaud, J. and Dubovik,
O. (2004). A study of the mixing state of black carbon in
urban zone. J. Geophys. Res. 109: D04202.
Marino, B., Boon, J.J., Hendriks, E., Horreard, F. and
Hilion, F. (2006). Imaging TOF-SIMS and nano-SIMS
studies of barite-celestite particles in grounds from
paintings by Van Gogh. e-Preserv. Sci. 3: 41–50.
Maskey, S., Geng, H., Song, Y.C., Hwang, H., Yoon, Y.J.,
Ahn, K.H. and Ro, C.U. (2011). Single-particle
characterization of summertime Antarctic aerosols
collected at King George Island using quantitative energy-
dispersive electron probe X-ray microanalysis and
attenuated total reflection Fourier transform-infrared
imaging techniques. Environ. Sci. Technol. 45: 6275–6282.
Middlebrook, A.M., Murphy, D.M. and Thomson, D.S.
(1998). Observations of organic material in individual
marine particles at Cape Grim during the First Aerosol
Characterization Experiment (ACE 1). J. Geophys. Res.
103: 16475–16483.
Moffet, R.C.,
de Foy, B., Molina, L.T., Molina, M.J. and
Prather, K.A. (2008a). Measurement of ambient aerosols
in northern Mexico City by single particle mass
spectrometry. Atmos. Chem. Phys. 8: 4499–4516.
Moffet, R.C., Desyaterik, Y., Hopkins, R.J., Tivanski, A.V.,
Gilles, M.K., Wang, Y., Shutthanandan, V., Molina, L.T.,
Abraham, R.G. and Johnson, K.S. (2008b).
Characterization of aerosols containing Zn, Pb, and Cl
from an industrial region of Mexico City. Environ. Sci.
Technol. 42: 7091–7097.
Moffet, R.C., Henn, T., Laskin, A. and Gilles, M.K. (2010).
Automated chemical analysis of internally mixed aerosol
particles using X-ray spectromicroscopy at the carbon
K-edge. Anal. Chem. 82: 7906–7914.
Mouri, H., Okada, K. and Shigehara, K. (1993). Variation
of Mg, S, K and Ca contents in individual sea-salt
particles. Tellus Ser. B 45: 80–85.
Murphy, D., Anderson, J., Quinn, P., McInnes, L., Brechtel,
F., Kreidenweis, S., Middlebrook, A., Pósfai, M., Thomson,
D. and Buseck, P. (1998). Influence of sea-salt on aerosol
radiative properties in the Southern Ocean marine
boundary layer. Nature 392: 62–65.
O'Dowd, C.D. and De Leeuw, G. (2007). Marine Aerosol
production: A review of the current knowledge. Philos.
Trans. R. Soc. London, Ser. A 365: 1753–1774.
Osthoff, H.D., Roberts, J.M., Ravishankara, A., Williams,
E.J., Lerner, B.M., Sommariva, R., Bates, T.S., Coffman,
D., Quinn, P.K. and Dibb, J.E. (2008).
High levels of
nitryl chloride in the polluted subtropical marine boundary
layer. Nat. Geosci. 1: 324–328.
Pachauri, T., Singla, V., Satsangi, A., Lakhani, A., Kumari,
K.M. (2013). SEM-EDX characterization of individual
coarse particles in Agra, India. Aerosol Air Qual. Res.
13: 523–536.
Prather, K.A., Bertram, T.H., Grassian, V.H., Deane, G.B.,
Stokes, M.D., DeMott, P.J., Aluwihare, L.I., Palenik, B.P.,
Azam, F. and Seinfeld, J.H. (2013).
Bringing the ocean
into the laboratory to probe the chemical complexity of
sea spray aerosol. Proc. Natl. Acad. Sci. U.S.A. 110:
7550–7555.
Pratt, K., Murphy, S., Subramanian, R., DeMott, P., Kok,
G., Campos, T., Rogers, D., Prenni, A., Heymsfield, A. and
Seinfeld, J. (2011). Flight-based chemical characterization
of biomass burning aerosols within two prescribed burn
smoke plumes. Atmos. Chem. Phys. 11: 12549–12565.
Randles, C., Russell, L. and Ramaswamy, V. (2004).
Hygroscopic and optical properties of organic sea salt
aerosol and consequences for climate forcing. Geophys.
Res. Lett. 31: L16108.
Saul, T.D., Tolocka, M.P. and Johnston, M.V. (2006).
Reactive uptake of nitric acid onto sodium chloride
aerosols across a wide range of relative humidities. J.
Phys. Chem. A 110: 7614–7620.
Semeniuk, T.A., Wise, M.E., Martin, S.T., Russell, L.M.
and Buseck, P.R. (2007a). Water uptake characteristics
of individual atmospheric particles having coatings.
Atmos. Environ. 41: 6225–6235.
Semeniuk, T.A., Wise, M.E., Martin, S.T., Russell, L.M.
and Buseck, P.R. (2007b). Hygroscopic behavior of
aerosol particles from biomass fires using environmental
transmission electron microscopy. J. Atmos. Chem. 56:
259–273.
Silva, P.J., Liu, D.Y., Noble, C.A. and Prather, K.A.
(1999). Size and chemical characterization of individual
particles resulting from biomass burning of local southern
California species. Environ. Sci. Technol. 33: 3068–3076.
Stewart, D.J., Griffiths, P. and Cox, R. (2004). Reactive
uptake coefficients for heterogeneous reaction of N
2
O
5
with submicron aerosols of NaCl and natural sea salt.
Atmos. Chem. Phys. 4: 1381–1388.
Sullivan, R.C., Guazzotti, S.A., Sodeman, D.A., Tang, Y.,
Carmichael, G.R. and Prather, K.A. (2007a). Mineral
Wang et al., Aerosol and Air Quality Research, 17: 878–887, 2017
887
dust is a sink for chlorine in the marine boundary layer.
Atmos. Environ. 41: 7166–7179.
Sullivan, R.C., Guazzotti, S.A., Sodeman, D.A. and Prather,
K.A. (2007b). Direct observations of the atmospheric
processing of Asian mineral dust. Atmos. Chem. Phys. 7:
1213–1236.
Tan, P.V., Fila, M.S., Evans, G.J. and Jervis, R.E. (2002).
Aerosol laser ablation mass spectrometry of suspended
powders from PM sources and its implications to
receptor modeling. J. Air Waste Manage. Assoc. 52: 27–
40.
Tang, I.N., Munkelwitz, H. and Davis, J. (1978). Aerosol
growth studies — IV. Phase transformation of mixed salt
aerosols in a moist atmosphere. J. Aerosol Sci. 9: 505–
511.
Tang, I.N. and Munkelwitz, H.R. (1993).
Composition and
temperature dependence of the deliquescence properties
of hygroscopic aerosols. Atmos. Environ. 27: 467–473.
Tervahattu, H., Hartonen, K., Kerminen, V.M., Kupiainen,
K., Aarnio, P., Koskentalo, T., Tuck, A.F. and Vaida, V.
(2002a). New evidence of an organic layer on marine
aerosols. J. Geophys. Res. 107: D7.
Tervahattu, H., Juhanoja, J. and Kupiainen, K. (2002b).
Identification of an organic coating on marine aerosol
particles by TOF-SIMS. J. Geophys. Res. 107: D164319.
Tervahattu, H., Juhanoja, J., Vaida, V., Tuck, A.F., Niemi,
J.V., Kupiainen, K., Kulmala, M. and Vehkamäki, H.
(2005). Fatty acids on continental sulfate aerosol
particles. J. Geophys. Res. 110: D06207.
Thornton, J.A. and Abbatt, J.P. (2005). N
2
O
5
reaction on
submicron sea salt aerosol: Kinetics, products, and the
effect of surface active organics. J. Phys. Chem. A 109:
10004–10012.
Thornton, J.A., Kercher, J.P., Riedel, T.P., Wagner, N.L.,
Cozic, J., Holloway, J.S., Dubé, W.P., Wolfe, G.M.,
Quinn, P.K. and Middlebrook, A.M. (2010). A large
atomic chlorine source inferred from mid-continental
reactive nitrogen chemistry. Nature 464: 271–274.
Tobo, Y., Zhang, D., Matsuki, A. and Iwasaka, Y. (2010).
Asian dust particles converted into aqueous droplets
under remote marine atmospheric conditions. Proc.
Natl. Acad. Sci. U.S.A. 107: 17905–17910.
Wang, T., Tham, Y.J., Xue, L., Li, Q., Zha, Q., Wang, Z.,
Poon, S.C., Dubé, W.P., Blake, D.R. and Louie, P.K.
(2016).
Observations of nitryl chloride and modeling its
source and effect on ozone in the planetary boundary
layer of southern China. J. Geophys. Res. 121: 2476–2489.
Wexler, A.S. and Seinfeld, J.H. (1991). Second-generation
inorganic aerosol model. Atmos. Environ. 25: 2731–2748.
Wise, M.E., Freney, E.J., Tyree, C.A., Allen, J.O., Martin,
S.T., Russell, L.M. and Buseck, P.R. (2009).
Hygroscopic behavior and liquid-layer composition of
aerosol particles generated from natural and artificial
seawater. J. Geophys. Res. 114: D03201.
Young, G., Jones, H., Darbyshire, E., Baustian, K.,
McQuaid, J., Bower, K., Connolly, P., Gallagher, M. and
Choularton, T. (2016). Size-segregated compositional
analysis of aerosol particles collected in the European
Arctic during the ACCACIA campaign. Atmos. Chem.
Phys. 16: 4063–4079.
Zhang, Q., Meng, J., Quan, J., Gao, Y., Zhao, D., Chen, P.
and He, H. (2012). Impact of aerosol composition on
cloud condensation nuclei activity. Atmos. Chem. Phys.
12: 3783–3790.
Zhang, Y., Wang, X., Chen, H., Yang, X., Chen, J. and
Allen, J. (2009). Source apportionment of lead-containing
aerosol particles in Shanghai using single particle mass
spectrometry. Chemosphere 74: 501–507.
Received for review, September 2, 2016
Revised, December 19, 2016
Accepted, December 19, 2016
