

This document comprises a prospectus for the purposes of Article 6 of Regulation (EU) 2017/1129 (as it forms part of retained
European Union (“EU”) law as defined in the European Union (Withdrawal) Act 2018) (the “Prospectus Regulation”), relating to
Haleon plc (the “Company” and together with its subsidiaries, the “Group”) and has been approved by the Financial Conduct
Authority of the United Kingdom (“FCA”), as competent authority under the Prospectus Regulation, in accordance with section
87A of the Financial Services and Markets Act 2000 of England and Wales, as amended (“FSMA”), and prepared and made
available to the public in accordance with the Prospectus Regulation Rules of the FCA made under section 73A of FSMA (the
“Prospectus Regulation Rules”). The FCA only approves this Prospectus as meeting the standards of completeness,
comprehensibility and consistency imposed by the Prospectus Regulation and such approval should not be considered as an
endorsement of the issuer that is, or the quality of the securities that are, the subject of this Prospectus. Investors should make
their own assessment as to the suitability of investing in the securities. This Prospectus is not an offer or invitation to the public to
subscribe for or purchase fully paid ordinary shares in the capital of the Company (“Haleon Shares”) but is issued solely in
connection with the admission of Haleon Shares to the premium listing segment of the Official List of the FCA and to the London
Stock Exchange’s main market for listed securities (“Admission”). It is proposed that Admission will take place shortly following
the Demerger and, unless the context requires otherwise, this Prospectus has been prepared on the assumption that the
Demerger Resolution will be passed at the GSK General Meeting and that the Demerger will become effective as proposed.
Application will be made to the FCA for all Haleon Shares to be admitted to the premium listing segment of the Official List of the
FCA and to the London Stock Exchange for Haleon Shares to be admitted to trading on the London Stock Exchange’s main
market for listed securities. It is expected that Admission will become effective, and that dealings in Haleon Shares will
commence on the London Stock Exchange, at 8.00 a.m. on 18 July 2022 (International Security Identification Number:
GB00BMX86B70).
This Prospectus is issued solely in connection with Admission. This Prospectus does not constitute or form part of an
offer or invitation to sell or issue, or any solicitation of an offer to purchase or subscribe for, any securities by any
person. No offer of Haleon Shares is being made in any jurisdiction.
This Prospectus should be read in its entirety. In particular, investors should take account of the section entitled “Risk Factors”
which contains a discussion of certain risks relating to the business of the Company. Investors should not solely rely on the
information summarised in the section entitled “Summary”.
(incorporated in England and Wales under the Companies Act 2006 with registered number 13691224)
Introduction to the premium listing segment of the Official List and
admission to trading on the main market of the London Stock Exchange
Joint Sponsors
Citigroup Global Markets Limited
Goldman Sachs International
Merrill Lynch International
Citigroup Global Markets Limited (“Citi”), which is authorised by the Prudential Regulation Authority and regulated in the United
Kingdom by the Prudential Regulation Authority and the Financial Conduct Authority, is acting exclusively for the Company and
no one else in connection with Admission and the Demerger and it will not regard any other person (whether or not a recipient of
this Prospectus) as a client in relation to Admission or the Demerger and will not be responsible to anyone other than the
Company for providing the protections afforded to its clients or for providing advice in relation to Admission or the Demerger or
any other transaction, matter, or arrangement referred to in this Prospectus.
Apart from the responsibilities and liabilities, if any, which may be imposed on Citi by FSMA or the regulatory regime established
thereunder or under the regulatory regime of any other applicable jurisdiction where exclusion of liability under the relevant
regulatory regime would be illegal, void or unenforceable, neither Citi nor any of its affiliates accepts any responsibility
whatsoever for the contents of this Prospectus including its accuracy, completeness and verification or for any other statement
made or purported to be made by it, or on its behalf, in connection with the Company or its subsidiaries, Haleon Shares or
Admission or the Demerger. Citi and its affiliates accordingly disclaim, to the fullest extent permitted by applicable law, all and
any liability whether arising in tort, contract or otherwise (save as referred to above) which they might otherwise be found to have
in respect of this Prospectus or any such statement. No representation or warranty, express or implied, is made by Citi or any of
its affiliates as to the accuracy, completeness, verification or sufficiency of the information set out in this Prospectus, and nothing
in this Prospectus will be relied upon as a promise or representation in this respect, whether or not to the past or future.
Goldman Sachs International (“Goldman Sachs”), which is authorised by the Prudential Regulation Authority and regulated in
the United Kingdom by the Prudential Regulation Authority and the Financial Conduct Authority, is acting exclusively for the
Company and no one else in connection with Admission and the Demerger and it will not regard any other person (whether or
not a recipient of this Prospectus) as a client in relation to Admission or the Demerger and will not be responsible to anyone
other than the Company for providing the protections afforded to its clients or for providing advice in relation to Admission or the
Demerger or any other transaction, matter, or arrangement referred to in this Prospectus.
Apart from the responsibilities and liabilities, if any, which may be imposed on Goldman Sachs by FSMA or the regulatory regime
established thereunder or under the regulatory regime of any other applicable jurisdiction where exclusion of liability under the
relevant regulatory regime would be illegal, void or unenforceable, neither Goldman Sachs nor any of its affiliates accepts any
responsibility whatsoever for the contents of this Prospectus including its accuracy, completeness and verification or for any
other statement made or purported to be made by it, or on its behalf, in connection with the Company or its subsidiaries, Haleon
Shares or Admission or the Demerger. Goldman Sachs and its affiliates accordingly disclaim, to the fullest extent permitted by
applicable law, all and any liability whether arising in tort, contract or otherwise (save as referred to above) which they might
otherwise be found to have in respect of this Prospectus or any such statement. No representation or warranty, express or
implied, is made by Goldman Sachs or any of its affiliates as to the accuracy, completeness, verification or sufficiency of the
information set out in this Prospectus, and nothing in this Prospectus will be relied upon as a promise or representation in this
respect, whether or not to the past or future.
Merrill Lynch International (“BofA Securities”), which is authorised by the Prudential Regulation Authority and regulated in the
United Kingdom by the Prudential Regulation Authority and the Financial Conduct Authority, is acting exclusively for the
Company and no one else in connection with Admission and the Demerger and it will not regard any other person (whether or
not a recipient of this Prospectus) as a client in relation to Admission or the Demerger and will not be responsible to anyone
other than the Company for providing the protections afforded to its clients or for providing advice in relation to Admission or the
Demerger or any other transaction, matter, or arrangement referred to in this Prospectus.
Apart from the responsibilities and liabilities, if any, which may be imposed on BofA Securities by FSMA or the regulatory regime
established thereunder or under the regulatory regime of any other applicable jurisdiction where exclusion of liability under the
relevant regulatory regime would be illegal, void or unenforceable, neither BofA Securities nor any of its affiliates accepts any
responsibility whatsoever for the contents of this Prospectus including its accuracy, completeness and verification or for any
other statement made or purported to be made by it, or on its behalf, in connection with the Company or its subsidiaries, Haleon
Shares or Admission or the Demerger. BofA Securities and its affiliates accordingly disclaim, to the fullest extent permitted by
applicable law, all and any liability whether arising in tort, contract or otherwise (save as referred to above) which they might
otherwise be found to have in respect of this Prospectus or any such statement. No representation or warranty, express or
implied, is made by BofA Securities or any of its affiliates as to the accuracy, completeness, verification or sufficiency of the
information set out in this Prospectus, and nothing in this Prospectus will be relied upon as a promise or representation in this
respect, whether or not to the past or future.
The distribution of this Prospectus in certain jurisdictions may be restricted by law and therefore persons into whose possession
this Prospectus comes should inform themselves about and observe any such restrictions in relation to Haleon Shares or this
Prospectus, including those in the paragraphs that follow. Any failure to comply with these restrictions may constitute a violation
of the securities laws of any such jurisdiction. Except in the United Kingdom, no action has been taken or will be taken in any
jurisdiction that would permit possession or distribution of this Prospectus in any country or jurisdiction where action for that
purpose is required. Accordingly, this Prospectus may not be distributed or published in any jurisdiction where to do so would
breach any securities laws or regulations of any such jurisdiction or give rise to an obligation to obtain any consent, approval or
permission, or to make any application, filing or registration. Failure to comply with these restrictions may constitute a violation of
the securities laws or regulations of such jurisdictions.
Australia
This document and the offer of Haleon Shares are only made available in Australia to persons to whom an offer of securities can
be made without disclosure in accordance with applicable exemptions under the Australian Corporations Act 2001 (Cth) as
modified by ASIC Instrument 22-0413 (the “Corporations Act”). This document is not a prospectus, product disclosure
statement or any other formal “disclosure document” for the purposes of Australian law and is not required to, and does not,
contain all the information which would be required in a “disclosure document” under Australian law. This document has not been
and will not be lodged or registered with the Australian Securities & Investments Commission or the Australian Securities
Exchange and the Company is not subject to the continuous disclosure requirements that apply in Australia.
Nothing in this document should be construed as legal, business or tax advice nor as financial product advice for the purposes of
Chapter 7 of the Corporations Act. Australia resident shareholders should be aware that the offer of Haleon Shares for resale in
Australia within 12 months of their issue may, under section 707(3) of the Corporations Act, require disclosure to investors under
Part 6D.2 of the Corporations Act if none of the exemptions in section 708 of the Corporations Act apply to the re-sale.
Canada
The Haleon Shares to be delivered to Shareholders resident in Canada have not been qualified for distribution to the public in
Canada and may not be resold in Canada except pursuant to a prospectus filed with the relevant Canadian securities regulatory
authorities, or under an exemption from the prospectus requirements of applicable Canadian securities laws. No securities
commission in Canada has reviewed this document or the merits of the Demerger. Haleon is not a reporting issuer in any
province or territory of Canada and the Haleon Shares are not listed on any stock exchange in Canada, and there is currently no
public market for the Haleon Shares in Canada. Shareholders resident in Canada should consult their own advisors prior to any
resale of the Haleon Shares they receive in connection with the Demerger.
China
This document does not constitute a public offer of the Haleon Shares, whether by sale or subscription, in the People’s Republic
of China (the “PRC”). The Haleon Shares are not being offered or sold directly or indirectly in the PRC to or for the benefits of
legal or natural persons of the PRC.
Further, no legal or natural persons of the PRC may directly or indirectly purchase any of the Haleon Shares or any beneficial
interest therein without obtaining all prior PRC’s governmental approvals that are required, whether statutorily or otherwise.
Persons who come into possession of this document are required by the Company and its representatives to observe these
restrictions.
2
Hong Kong
The contents of this document have not been reviewed or approved by any regulatory authority in Hong Kong. You are advised
to exercise caution in relation to the delivery of the Haleon Shares. If you are in any doubt about any of the contents of this
document, you should obtain independent professional advice.
The Haleon Shares may not be offered or sold by means of any document other than (i) in circumstances which do not constitute
an offer to the public within the meaning of the Companies (Winding Up and Miscellaneous Provisions) Ordinance (Cap.32, Laws
of Hong Kong), or (ii) to “professional investors” within the meaning of the Securities and Futures Ordinance (Cap.571, Laws of
Hong Kong) and any rules made thereunder, or (iii) in other circumstances which do not result in the document being a
“prospectus” within the meaning of the Companies (Winding Up and Miscellaneous Provisions) Ordinance (Cap.32, Laws of
Hong Kong), and no advertisement, invitation or document relating to the Haleon Shares may be issued or may be in the
possession of any person for the purpose of issue (in each case whether in Hong Kong or elsewhere), which is directed at, or the
contents of which are likely to be accessed or read by, the public in Hong Kong (except if permitted to do so under the laws of
Hong Kong) other than with respect to Haleon Shares which are or are intended to be disposed of only to persons outside Hong
Kong or only to “professional investors” within the meaning of the Securities and Futures Ordinance (Cap. 571, Laws of Hong
Kong) and any rules made thereunder.
This document is confidential to the person to whom it is addressed and no person to whom a copy of this document is issued
may issue, circulate, distribute, publish, reproduce or disclose (in whole or in part) this document to any other person in Hong
Kong without the consent of GSK.
New Zealand
The Haleon Shares are not being offered to the public within New Zealand. In New Zealand, the Haleon Shares are being issued
only to existing security holders of GSK with registered addresses in New Zealand in reliance on the Financial Markets Conduct
(Haleon plc) Exemption Notice 2022.
This document has been prepared in compliance with the laws of the United Kingdom. This document is not a product disclosure
statement under the Financial Markets Conduct Act 2013 (the “FMC Act”) or other similar offering or disclosure document under
New Zealand law and has not been registered, filed with, or approved by any New Zealand regulatory authority or under or in
accordance with the FMC Act or any other relevant law in New Zealand. It does not contain all the information that a product
disclosure document, under New Zealand law, is required to contain.
Singapore
The offer of the Haleon Shares is made only to and directed at, and the Haleon Shares are only available to, persons in
Singapore who are existing Shareholders of the Company.
This document has not been registered as a prospectus with the Monetary Authority of Singapore. Accordingly, this document
and any other document or material in connection with the offer or sale, or invitation for subscription or purchase, of the Haleon
Shares may not be circulated or distributed, nor may the Haleon Shares be offered or sold, or be made the subject of an
invitation for subscription or purchase, whether directly or indirectly, to persons in Singapore other than: (i) to existing
Shareholders of the Company under Section 273(1)(cd)(i) of the Securities and Futures Act, 2001 of Singapore (the “SFA”); or
(ii) otherwise pursuant to, and in accordance with the conditions of, any other applicable provision of the SFA.
Switzerland
This document does not constitute a prospectus pursuant to the Swiss Financial Services Act (“FinSA”). No application has or
will be made to admit the shares to trading on any trading venue (exchange or multilateral trading facility) in Switzerland. This
document is distributed in Switzerland only relying on the exemption of article 37(1)(e) FinSA provided for demergers and only
together with the GSK Shareholder Circular.
The Company intends to file with the U.S. Securities and Exchange Commission (the “SEC”) a registration statement on Form
20-F (the “Form 20-F”) under the U.S. Securities Exchange Act of 1934, as amended (the “US Exchange Act”), with respect to
the American depositary shares each representing 2 Haleon Shares (the “Haleon ADSs”). This Prospectus does not contain all
the information included in the Form 20-F. For further information with respect to the Haleon ADSs, please refer to the Form
20-F. The Company also intends to make an application to the New York Stock Exchange (the “NYSE”) for the Haleon ADSs to
be admitted to listing and trading on the NYSE.
This Prospectus is dated 1 June 2022.
3
TABLE OF CONTENTS
SUMMARY 5
EXPECTED TIMETABLE OF PRINCIPAL EVENTS 16
RISK FACTORS 17
PRESENTATION OF FINANCIAL AND OTHER INFORMATION 46
DIRECTORS, COMPANY SECRETARY, REGISTERED OFFICE AND ADVISERS 57
PART I KEY HIGHLIGHTS AND DEVELOPMENT OF THE GROUP 59
PART II MARKET OVERVIEW 65
PART III BUSINESS OVERVIEW 71
PART IV OVERVIEW OF THE DEMERGER AND SEPARATION 115
PART V DIRECTORS, SENIOR MANAGERS, CORPORATE GOVERNANCE AND
REMUNERATION
120
PART VI SELECTED FINANCIAL INFORMATION 131
PART VII OPERATING AND FINANCIAL REVIEW 147
PART VIII CAPITALISATION AND INDEBTEDNESS 208
PART IX UNAUDITED PRO FORMA FINANCIAL INFORMATION OF THE GROUP 210
PART X REGULATORY OVERVIEW 218
PART XI TAXATION 235
PART XII ADDITIONAL INFORMATION 242
SCHEDULE I DEFINITIONS AND GLOSSARY 320
SCHEDULE II HISTORICAL FINANCIAL INFORMATION OF GLAXOSMITHKLINE
CONSUMER HEALTHCARE HOLDINGS (NO.2) LIMITED
334
SCHEDULE III PFIZER HISTORICAL FINANCIAL INFORMATION 422
4
SUMMARY
1. INTRODUCTION AND WARNINGS
1.1 Details of the issuer
The issuer is Haleon plc (the “Company”), a public limited company incorporated in England
and Wales with registered number 13691224.
The Company’s registered and head office is at 980 Great West Road, Brentford, Middlesex TW8
9GS, United Kingdom. The telephone number of the Company’s registered office is
+44 (0)20 8047 5000 and the legal entity identifier of the Company is 549300PSB3WWEODCUP19.
1.2 Details of the securities
On Admission, the Haleon Shares will be registered with an ISIN of GB00BMX86B70 and
SEDOL of BMX86B7. It is expected that the Haleon Shares will be traded on the main market
for listed securities of the London Stock Exchange under the ticker symbol “HLN”.
1.3 Identity and contact details of the competent authority approving this Prospectus
This Prospectus has been approved by the FCA, as competent authority, with its head office at
12 Endeavour Square, London E20 1JN and telephone number: +44 (0)20 7066 1000, in
accordance with the Prospectus Regulation.
This Prospectus was approved by the FCA on 1 June 2022.
1.4 Warnings
This summary has been prepared in accordance with Article 7 of the Prospectus Regulation
and should be read as an introduction to this Prospectus. Any decision to invest in the Haleon
Shares should be based on consideration of the Prospectus as a whole by the investor. Any
investor could lose all or part of their invested capital and, where any investor’s liability is not
limited to the amount of the investment, it could lose more than the invested capital.
Civil liability attaches only to those persons who have tabled the summary, including any
translation thereof, but only if the summary is misleading, inaccurate or inconsistent when read
together with the other parts of the Prospectus or if it does not provide, when read together
with the other parts of the Prospectus, key information in order to aid investors when
considering whether to invest in the Haleon Shares.
2. KEY INFORMATION ON THE ISSUER
2.1 Who is the issuer of the securities?
The Company was incorporated in England and Wales on 20 October 2021 as DRVW 2022
Limited with registered number 13691224. DRVW 2022 Limited was re-registered as a public
limited company (DRVW 2022 plc) on 23 February 2022 and changed its name to Haleon plc
on 28 February 2022. The Company is domiciled in England and Wales and its legal entity
identifier is 549300PSB3WWEODCUP19.
(A) Principal activity
Following the Demerger, the principal activity of the Company will be to act as the ultimate
holding company of the Group. The principal legislation under which the Company operates is
the Companies Act and regulations made thereunder.
5

(B) Major shareholders
The Company was incorporated in anticipation of the Demerger, and is not a member of the
GSK Group. As at the date of this Prospectus, the entire issued share capital of the Company
is held and controlled by David Redfern, Adam Walker, Victoria Whyte and Subesh Williams,
who each hold four fully paid ordinary shares of £1.25 in the capital of the Company.
As at the Latest Practicable Date, and so far as is known to the Company by virtue of the
notifications made to GSK plc pursuant to the Companies Act, the Market Abuse Regulation
and/or the Disclosure Guidance and Transparency Rules, as a result of the Demerger and the
Share Exchanges, the following will, on Admission, be directly or indirectly interested in
3 per cent. or more of the Company’s issued share capital:
Name of shareholder Percentage of total voting rights
Pfizer 32
GSK Up to 6
SLP1* 4.74
BlackRock, Inc.** 3.60
* The other SLPs, being SLP2 and SLP3, will each have a less than 3 per cent. holding in the
Company on Admission so are not included in this table.
**BlackRock, Inc. is included in this table on the basis of its major shareholding in GSK of 6.40
per cent. (as at the date of notification to GSK).
(C) Key managing directors
The Executive Directors of the Company are:
Director Position
Brian McNamara Chief Executive Officer
Tobias Hestler Chief Financial Officer
(D) Statutory auditor
The auditor of GlaxoSmithKline Consumer Healthcare Holdings (No.2) Limited since the date
of its incorporation has been Deloitte, whose registered office is at 1 New Street Square,
London, EC4A 3HQ. Deloitte is registered to carry out audit work in the United Kingdom by the
Institute of Chartered Accountants in England and Wales. Deloitte has audited the statutory
consolidated annual accounts of GlaxoSmithKline Consumer Healthcare Holdings (No.2)
Limited as at and for the years ended 31 December 2021, 2020 and 2019.
The Company was incorporated on 20 October 2021. On 3 February 2022, the Company
appointed Deloitte as its UK statutory auditor for the year ending 31 December 2022.
In connection with the Company’s registration of securities under the U.S. Securities Exchange
Act of 1934, on 24 March 2022, the Company appointed KPMG LLP to audit the Company’s
financial statements for the year ending 31 December 2022 under the rules of the SEC and
U.S. Public Company Accounting Oversight Board (PCAOB).
The Company expects to conduct an audit tender process for the audit of the Company’s
financial statements for the year ending 31 December 2023, which would be subject to
shareholder approval at the Company’s 2023 annual general meeting.
6

2.2 What is the key financial information regarding the issuer?
Selected historical key financial information
The tables below set out selected key financial information for the Group for the financial years
ended 31 December 2021, 2020 and 2019 and for the three months ended 31 March 2022 and
31 March 2021:
Unaudited consolidated income statement
For the three months ended 31 March 2022 and 31 March 2021 2022 2021
£m £m
Revenue 2,627 2,306
Cost of sales (1,014) (904)
Gross Profit 1,613 1,402
Selling, general and administration (1,086) (1,009)
Research and development (64) (54)
Other operating income 3 9
Operating profit 466 348
Finance income 76
Finance expense (8) (4)
Net finance (costs)/income (1) 2
Profit before tax 465 350
Income tax (108) (101)
Profit after tax for the quarter 357 249
Profit attributable to shareholders 343 233
Profit attributable to non-controlling interests 14 16
Basic earnings per share (pence)
1
34,300 23,300
Diluted earnings per share (pence)
1
34,300 23,300
1
The number of shares in issue above is not representative of the number of shares in issue
in the future. For further detail, please see Note 15 of Schedule II (Historical Financial
Information of GlaxoSmithKline Consumer Healthcare Holdings (No.2) Limited).
7

Consolidated balance sheet
As at 31 March 2022 and 31 March 2021 31 March 2022 31 December 2021
£m
(unaudited)
£m
(audited)
Non-current assets
Property, plant and equipment 1,587 1,563
Right of use assets 100 99
Intangible assets 27,692 27,195
Deferred tax assets 314 312
Post-employment benefit assets 11 11
Derivative financial instruments 8 12
Other non-current assets 13 8
Total non-current assets 29,725 29,200
Current assets
Inventories 986 951
Trade and other receivables 2,415 2,207
Loan amounts owing from related parties 11,330 1,508
Cash and cash equivalents and liquid investments 383 414
Derivative financial instruments 18 5
Current tax recoverable 166 166
Total current assets 15,298 5,251
Total assets 45,023 34,451
Current liabilities
Short-term borrowings (80) (79)
Trade and other payables (3,142) (3,002)
Loan amounts owing to related parties (1,461) (825)
Derivative financial instruments (15) (18)
Current tax payable (242) (202)
Short-term provisions (86) (112)
Total current liabilities (5,026) (4,238)
Non-current liabilities
Long-term borrowings (9,363) (87)
Deferred tax liabilities (3,472) (3,357)
Pensions and other post-employment benefits (256) (253)
Derivative financial instruments (21) (1)
Other provisions (30) (27)
Other non-current liabilities (6) (8)
Total non-current liabilities (13,148) (3,733)
Total liabilities (18,174) (7,971)
Net assets 26,849 26,480
Equity
Share capital 11
Other reserves (11,502) (11,632)
Retained earnings 38,211 37,986
Shareholders’ equity 26,710 26,355
Non-controlling interests 139 125
Total equity 26,849 26,480
8

Consolidated income statement
For the years ended 31 December 2021, 31 December 2020 and 31 December 2019
£m 2021 2020 2019
Revenue 9,545 9,892 8,480
Cost of sales (3,595) (3,982) (3,678)
Gross Profit 5,950 5,910 4,802
Selling, general and administration (4,086) (4,220) (3,596)
Research and development (257) (304) (292)
Other operating income/(expense) 31 212 (17)
Operating profit 1,638 1,598 897
Finance income 17 20 24
Finance expense (19) (27) (35)
Net finance costs (2) (7) (11)
Profit before tax 1,636 1,591 886
Income tax (197) (410) (199)
Profit after tax 1,439 1,181 687
Profit attributable to shareholders 1,390 1,145 655
Profit attributable to non-controlling interests 49 36 32
Consolidated balance sheet
As at 31 December 2021, 31 December 2020 and 31 December 2019
£m 2021 2020 2019
Non-current assets 29,200 29,122 29,900
Current assets 5,251 5,008 5,811
Total Assets 34,451 34,130 35,711
Current liabilities (4,238) (4,014) (4,269)
Non-current liabilities (3,733) (3,893) (4,030)
Total liabilities (7,971) (7,907) (8,299)
Net assets 26,480 26,223 27,412
Consolidated cash flow statement
For the years ended 31 December 2021, 31 December 2020 and 31 December 2019
£m 2021 2020 2019
Cash flow from operating activities
Profit after tax 1,439 1,181 687
Adjustments reconciling profit after tax to cash generated from
operations 227 780 408
Cash generated from operations 1,666 1,961 1,095
Taxation paid (310) (554) (309)
Net cash inflow from operating activities 1,356 1,407 786
Net cash (outflow)/inflow from investing activities (33) 1,030 291
Net cash (outflow) from financing activities (1,236) (2,437) (925)
Increase in cash and bank overdrafts 87 - 152
Cash and bank overdrafts at the beginning of the year 323 329 191
Exchange adjustments (5) (6) (14)
Increase in cash and bank overdrafts 87 - 152
Cash and cash equivalents at end of year 405 323 329
(A) Selected pro forma key financial information
This Prospectus presents certain pro forma financial information for the Group to illustrate the
impact of the Notes Proceeds Loans, the receipt of related party loans, additional borrowings
and the payment of the Pre-Separation Dividends (together, the “Pro Forma Transactions”)
on the net assets of the Group as if the Pro Forma Transactions had taken place on 31 March
2022.
9

The unaudited pro forma statement of net assets of the Group has been prepared on the basis
of the unaudited interim financial information of the Group as at 31 March 2022, the date to
which the latest unaudited financial information in relation to the Group was prepared. The
unaudited pro forma statement of net assets of the Group has been prepared in accordance
with Annex 20 of the PR Regulation and pursuant to Listing Rule 13.3.3R in a manner
consistent with the accounting policies of the Company.
Because of its nature, the unaudited pro forma statement of net assets addresses a
hypothetical situation and, therefore, does not represent the Group’s actual financial position or
results. It may not, therefore, give a true picture of the Group’s financial position or results nor
is it indicative of the results that may, or may not, be expected to be achieved in the future. The
pro forma statement of net assets has been prepared for illustrative purposes only and in
accordance with Annex 20 of the PR Regulation.
Unaudited pro forma Statement of Net Assets
Pro forma adjustments related to the
Transactions
Group Net
Assets at 31
March 2022
Receipt of
Notes
Proceeds
Loans and
related party
loans
Additional
borrowings
Pre-Separation
Dividends
Transaction
costs
Unaudited
pro forma at
31 March
2022
£m
(Note 1)
£m
(Note 2)
£m
(Note 3)
£m
(Note 4)
£m
(Note 5)
£m
(Note 6)
Non-current Assets
Property, plant and equipment 1,587 - - - - 1,587
Right of use assets 100 - - - - 100
Intangible assets 27,692 - - - - 27,692
Deferred tax assets 314 - - - - 314
Post-employment benefit assets 11 - - - - 11
Derivative financial instruments 8 8
Other non-current assets 13 - - - - 13
Total non-current assets 29,725 - - - - 29,725
Current assets -
Inventories 986 - - - - 986
Trade and other receivables 2,415 - - - - 2,415
Loan amounts owing from related parties 11,330 (11,330) - - - -
Cash and cash equivalents and liquid
investments
383 9,869 1,435 (11,039) (84) 564
Derivative financial instruments 18 - - - - 18
Current tax recoverable 166 -
-
--166
Total current assets 15,298 (1,461) 1,435 (11,039) (84) 4,149
Total assets 45,023 (1,461) 1,435 (11,039) (84) 33,874
Current liabilities
Short-term borrowings (80) - - - - (80)
Trade and other payables (3,142) - - - - (3,142)
Loan amounts owing to related parties (1,461) 1,461 - - - -
Derivative financial instruments (15) - - - - (15)
Current tax payable (242) - - - - (242)
Short-term provisions (86) - - - - (86)
Total current liabilities (5,026) 1,461 - - - (3,565)
Non-current liabilities
Long-term borrowings (9,363) - (1,435) (25) - (10,823)
Deferred tax liabilities (3,472) - - - (3,472)
Pensions and other post-employment
benefits
(256) - - - (256)
Derivative financial instruments (21) (21)
Other provisions (30) - - - (30)
Other non-current liabilities (6) - - -
(6)
Total non-current liabilities (13,148) - (1,435) (25) - (14,608)
Total liabilities (18,174) 1,461 (1,435) (25) - (18,173)
Net assets 26,849 - -
(11,064) (84) 15,701
Notes
(1) The net assets of the Group as at 31 March 2022 have been extracted without material adjustment from the consolidation schedules
used to prepare the Interim Financial Information for the Group for the three months ended 31 March 2022 set out in Part VI (Selected
Financial Information).
10

(2) This adjustment reflects the receipt of the Notes Proceeds Loans and related party loans. Under the terms of the Notes Proceeds Loan
Agreements, the Notes Proceeds Loans will be repaid in full upon notice that the Demerger Resolution has been approved by GSK and
Pfizer.
Notes £m
Receipt of loan amounts owing from related parties a 11,330
Payment of loan amounts owing to related parties b
(1,461)
Total 9,869
a. Receipt of loan amounts owing from related parties includes Notes Proceeds Loans of £9,210 million and loan amounts
owing from GSK as part of the Group’s banking arrangements of £2,120 million.
b. Payment of loan amounts owing to related parties includes loan amounts owing to GSK as part of the Group’s banking
arrangements of £1,461 million.
(3) Additional borrowings to fund the payment of the Pre-Demerger Dividend, including, but not limited to, the Term Loan Facility. See also
paragraph 7.4 of Part VII (Operating and Financial Review).
(4) The Pre-Separation Dividends include the Balancing Dividend, Pre-Demerger Dividend, and the Sweep-up Dividend.
Notes £m
Balancing Dividend a 53
Pre-Demerger Dividend b 10,345
Sweep-up Dividend c 641
Pre-Separation Dividends 11,039
a. The Balancing Dividend reflects the cash dividend of £53 million to be paid by the Group to GSKCHH prior to Separation
in connection with the £25 million of Non-Voting Preference Shares issued to Pfizer recognised in long-term borrowings.
b. The Pre-Demerger Dividend reflects the cash dividend of £10,345 million to be paid by the Group to GSKCHH and
PFCHH ahead of Separation, in accordance with the terms of the Pfizer SHA, which, in summary, requires an amount
equal to the Pre-Separation Debt Proceeds of the Group less £300 million to be paid to GSKCHH and PFCHH prior to
Separation.
£m
Pre-Separation Debt Proceeds 10,645
Less £300m (300)
Pre-Demerger Dividend 10,345
c. The Sweep-up Dividend reflects the cash dividend of £641 million to be paid by the Group to GSKCHH and PFCHH, in
accordance with the terms of the Pfizer SHA which, in summary, requires all readily available cash in excess of
£300 million to be paid to GSKCHH and PFCHH prior to Separation. The actual amount paid is subject to, amongst other
things, additional cash flow generated by, or additional investments made by, or dividends paid in the ordinary course of
business by the Group up until the point of Separation. As such, the actual amount of the Sweep-up Dividend may
therefore differ from the amount referred to above.
£m
Cash and cash equivalents and liquid investments as of 31 March 2022 383
Receipt of Notes Proceeds Loans and related party loans 9,869
Additional borrowings 1,435
Payment of Pre-Demerger Dividend (10,345)
Transaction costs (84)
Balancing Dividend (53)
Less trapped cash* (264)
Less £300m (300)
Sweep-up Dividend 641
* Cash and cash equivalents that are in jurisdictions that have absolute cross-border restrictions on transfers of cash between
members of the Group.
(5) Transaction costs comprise charges for services relating to the Transactions. The Group expects to incur a cumulative total
£117 million of transaction costs in relation to the Transactions. The Group has incurred £33 million of transaction related costs as of
31 March 2022. Therefore, a transaction cost adjustment of £84 million has been made.
(6) The Pro Forma Financial Information does not reflect any changes in the trading results or financial position of the Group since
31 March 2022. None of the adjustments are expected to have a continuing effect on the Group.
11

(7) On a pro forma basis, net debt of the Group as at 31 March 2022 would have been £10,349 million.
Group net debt
at 31 March
2022
Pro forma
adjustments
Notes
Unaudited pro forma
at 31 March 2022
£m £m £m
Short-term borrowings 80 80
Long-term borrowings 9,363 1,460 a 10,823
Derivative financial liabilities 36 36
Cash and cash equivalents and liquid
investments
(383) (181) b (564)
Derivative financial assets
(26) (26)
Net debt 9,070 1,279 10,349
a. Additional borrowings to fund the payment of the Pre-Demerger Dividend, including, but not limited to, the Term Loan
Facility. See also paragraph 7.4 of Part VII (Operating and Financial Review).
b. Receipt of Notes Proceeds Loans and related party loans of £9,869 million, additional borrowings of £1,435 million,
payment of Pre-Separation Dividends of £11,039 million and the payment of Transaction costs of £84 million.
2.3 What are the key risks that are specific to the issuer?
The Group operates in a highly competitive market and failure to successfully compete with
competitors could have a material adverse effect on the Group’s business.
The Group’s success depends on its ability to anticipate and respond to changes in consumer
preferences and a failure to adapt its strategy appropriately may have a material adverse effect
on the Group’s business and/or financial condition.
The Group’s business results are impacted by the Group’s ability to manage disruptions in the
Group’s global supply chain and a failure to manage disruptions appropriately may have a
material adverse effect on the Group’s business and/or financial condition.
Increasing dependence on key retail customers, changes in the policies of the Group’s retail
customers, the emergence of alternative retail channels and the rapidly changing retail
landscape may materially and adversely affect the Group’s business.
The Group may not be able to develop and commercialise new products effectively, which may
materially and adversely affect the results of the Group’s operations and financial condition.
Failure to retain key personnel or attract new personnel may materially and adversely affect the
Group’s business.
Damage to the Group’s reputation could have a material adverse effect on the Group’s
business.
Failure to respond effectively to the challenges raised by climate change and other
sustainability matters may have a material adverse effect on the Group’s business and results
of operations.
The Group’s business is subject to legal and regulatory risks in all the markets in which it
operates, which may have a material adverse effect on the Group’s business operations and
financial condition.
The Group faces risks relating to the regulation and perception of the ingredients it uses in its
products, which could materially and adversely impact the Group’s business, prospects,
financial condition and results of operations.
The Group’s business is subject to market fluctuations and general economic conditions,
including inflationary pressures, each of which may materially and adversely affect the Group’s
business, financial condition, results of operations and prospects.
The Group may fail to realise any or all of the anticipated benefits of the Demerger and
Separation.
12
3. KEY INFORMATION ON THE SECURITIES
3.1 What are the main features of the securities?
(A) Type, class and ISIN of the securities
The Haleon Shares are fully paid ordinary shares in the capital of the Company with a nominal
value of £1.25 each (to be reduced to 1 pence following the Capital Reduction).
On Admission, the Haleon Shares will be registered with an ISIN of GB00BMX86B70 and
SEDOL of BMX86B7. It is expected that the Haleon Shares will be traded on the main market
for listed securities of the London Stock Exchange under the ticker symbol “HLN”.
(B) Currency of the securities
The Haleon Shares are and, on Admission will be, denominated in Pounds Sterling.
(C) Number of issued and fully paid securities
Immediately following completion of the Demerger, the number of Haleon Shares in issue will
be equal to the number of GSK Shares in issue at the Shareholder Record Time. As at the
Latest Practicable Date, there were 5,084,048,734 GSK Shares in issue (excluding ordinary
shares held in treasury).
Shortly after completion of the Demerger, the following share-for-share exchanges will occur:
(i) GSK will transfer its entire holding of GSKCHH B Ordinary Shares,
representing an 8.01 per cent. stake in GSKCHH’s ordinary share capital, to
the Company in exchange for 502,868,434 Haleon Shares, less a number of
Haleon Shares that is equal to the number of Excess GSK Shares. As at the
Latest Practicable Date, the number of Haleon Shares expected to be held by
GSK at Admission is expected to represent up to 6 per cent. of the total issued
share capital of the Company;
(ii) the SLPs (being certain Scottish limited partnerships set up to provide a
funding mechanism pursuant to which GSK will provide additional funding for
GSK’s UK Pension Schemes) will transfer their respective holdings of
GSKCHH C Ordinary Shares, representing 11.03 per cent. (in aggregate) of
GSKCHH’s ordinary share capital, to the Company in exchange for such
number of new Haleon Shares as is required so that, on Admission, the SLPs
will together hold Haleon Shares representing 7.5 per cent. (in aggregate and
to the nearest whole Haleon Share) of the total issued share capital of the
Company; and
(iii) Pfizer will transfer its entire holding in PFCHH to the Company in exchange for:
(a) 25 million Non-Voting Preference Shares; and (b) such number of Haleon
Shares as will result in Pfizer holding, on Admission, Haleon Shares
representing 32 per cent. of the total issued share capital of the Company (to
the nearest whole Haleon Share). Pfizer will sell its entire holding in the
Non-Voting Preference Shares to one or more third party investor(s)
immediately following the share-for-share exchange with the Company
described in this paragraph 3.1(C)(iii).
Therefore, it is expected that the Company will have 9,234,573,831 Haleon Shares in issue on
Admission. The Haleon Shares have a nominal value of £1.25 each (to be reduced to 1 pence
following the Capital Reduction) and will be fully paid.
The Non-Voting Preference Shares have a nominal value of £1 each and will be fully paid. The
Non-Voting Preference Shares will not be listed on the London Stock Exchange or any other
exchanges.
13
(D) Rights attaching to the securities
All Haleon Shares will rank pari passu with all other Haleon Shares in all respects, there being
no conversion or exchange rights attaching thereto, and all Haleon Shares will have equal
rights to participate in capital, dividend and profit distributions by the Company.
Subject to the provisions of the Companies Act, any equity securities issued by the Company
for cash must first be offered to the holders of Haleon Shares in proportion to their holdings of
Haleon Shares. The Companies Act and Listing Rules allow for the disapplication of
pre-emption rights which may be waived by a special resolution of the Haleon Shareholders,
whether generally or specifically, for a maximum period not exceeding five years.
On a show of hands every Haleon Shareholder who is present in person and every person
holding a valid proxy shall have one vote and on a poll every Haleon Shareholder present in
person or by proxy shall have one vote per Haleon Share.
(E) Rank of securities in the event of insolvency
The Haleon Shares do not carry any rights with respect to capital to participate in a distribution
(including on a winding-up) other than those that exist as a matter of law. The Haleon Shares
will rank pari passu with all other Haleon Shares in all respects.
The Haleon Shares will rank behind the Non-Voting Preference Shares in the event of the
insolvency of the Company as the Non-Voting Preference Shares carry preferential rights to
participate in a distribution of capital in the event of an insolvency (including on a winding-up)
up to an amount equal to their nominal value plus accrued dividend and any arrears or
deficiency in amount of the cumulative dividend.
(F) Description of restrictions on free transferability of the securities
Holders of Haleon Shares who the Company believes are or may be Designated Persons are
not permitted to dispose of their Haleon Shares or any legal or beneficial interest in any of
them without the prior written consent of the Company. The Haleon Shares are otherwise
freely transferable and there are no restrictions on transfer.
(G) Dividend policy
Following the Demerger, the Company will adopt a dividend policy, which will reflect the long-
term earnings and cash flow potential of the Group, consistent with maintaining sufficient
financial flexibility and meeting the Group’s capital allocation priorities. The initial dividend is
expected to be at the lower end of a 30 to 50 per cent. pay-out ratio, subject to Board approval.
The Company expects to pay a dividend to Haleon Shareholders in relation to the second half
of 2022 in H1 2023, subject to Board approval and following approval of the Company’s FY
2022 results.
3.2 Where will the securities be traded?
Application will be made for all the Haleon Shares to be admitted to the premium listing
segment of the Official List of the FCA and to trading on the LSE’s main market for listed
securities. Application will also be made to the NYSE for American depositary shares each
representing 2 Haleon Shares (the “Haleon ADSs”) to be admitted to listing and trading on the
NYSE. No application has been made or is currently intended to be made for Haleon Shares to
be admitted to listing or trading on any other exchange.
3.3 What are the key risks that are specific to the securities?
There is no existing market for the Haleon Shares and an active trading market for the Haleon
Shares may not develop or be sustained.
The Pfizer Group will retain a significant interest in the Company following Admission and its
interests may differ from those of the other Haleon Shareholders.
There can be no assurance that dividends will be paid on Haleon Shares.
14
4. KEY INFORMATION ON THE ADMISSION TO TRADING ON A REGULATED MARKET
4.1 Why is this Prospectus being produced?
This Prospectus does not constitute an offer or invitation to any person to subscribe for or
purchase any shares in the Company. It has been produced in connection with the application
to be made to the FCA for the Haleon Shares to be admitted to the premium listing segment of
the Official List and to the LSE for the Haleon Shares to be admitted to trading on its main
market for listed securities. It is expected that Admission will become effective and that
dealings in the Haleon Shares will commence on the LSE by no later than 8.00 a.m. (London
time) on 18 July 2022.
Application will also be made to the NYSE for the Haleon ADSs to be admitted to listing and
trading on the NYSE.
No application has been made for admission of Haleon Shares to trading on any other stock
exchange (nor is it the current intention of the Company to make any such application in
future).
15
EXPECTED TIMETABLE OF PRINCIPAL EVENTS
The times and dates set out in the timetables below and throughout this Prospectus that fall after the
date of publication of this Prospectus are indicative only and based on the Company’s current
expectations and may be subject to change without further notice.
Event Time and date
(1)
ADS Holder Voting Record Time for determining entitlement to
attend and vote at the GSK General Meeting
(2)
5 p.m. New York City time on
27 May 2022
Publication of the GSK Shareholder Circular and this Prospectus
1 June 2022
Latest time and date for receipt of Forms of Directions
(2)
2.30 p.m. on 30 June 2022
Latest time and date for receipt by Depositary of Voting
Instruction Cards from ADS Holders on the ADR Register
12 p.m. New York City time on
30 June 2022
Latest time and date for receipt of Proxy Forms, CREST Proxy
Instruction and electronic proxy appointments
(2)
2.30 p.m. on 4 July 2022
Shareholder Voting Record Time for determining entitlement
to attend and vote at the General Meeting
6.30 p.m. on 4 July 2022
(3)
GSK General Meeting 2.30 p.m. on 6 July 2022
Announcement of the results of the GSK General Meeting 6 July 2022
(after the GSK General Meeting)
Closing of the GSK ADS issuance and cancellation books
(4)
8 a.m. New York City time on
14 July 2022
Latest time and date for transfers of GSK Shares to be
registered on the Register at the Shareholder Record Time
6 p.m. on 15 July 2022
Shareholder Record Time for determining the entitlement to
the Demerger Dividend
6 p.m. on 15 July 2022
ADS Holder Record Time for determining the entitlement to
the Demerger Dividend
5 p.m. New York City time on
15 July 2022
Demerger Dividend to Qualifying GSK Shareholders After 6 p.m. on 15 July 2022
Completion of Share Exchanges 17 July 2022
Admission and commencement of dealings in Haleon Shares
on the LSE
8 a.m. on 18 July 2022
CREST accounts credited in respect of Haleon Shares in
uncertificated form
As soon as practicable after 8 a.m.
on 18 July 2022
Admission and commencement of dealings in Haleon ADSs
on the NYSE
9.30 a.m. New York City
time on 22 July 2022
Opening of the GSK ADS issuance and cancellation books
(4)
8 a.m. New York City time on
25 July 2022
Latest date for despatch of definitive share certificates (where
applicable) for Haleon Shares in certificated form to Qualifying
GSK Shareholders on the Register
By 1 August 2022
Latest date for despatch of opening statement for Haleon CSN
(5)
By 1 August 2022
Notes
(1) Unless otherwise indicated, all references to time in this timetable are to UK time.
(2) If you hold GSK Shares or GSK ADSs via a bank, broker or nominee you should contact your respective bank, broker or
nominee service provider for further information on the appropriate dates and times relevant for your particular holding.
(3) If the GSK General Meeting is adjourned for any reason, the Shareholder Voting Record Time for the adjourned meeting
will be 2.30 p.m. UK time on the date that is two business days before the date set for the adjourned meeting. The
Depositary will inform ADS Holders of any change to the ADS Holder Voting Record Time.
(4) The Depositary will suspend the issuance and cancellation of GSK ADSs from 14 July 2022 until 25 July 2022. This means
that during this time, you will not be able to convert your GSK ADSs into GSK Shares, surrender your GSK ADSs and
receive underlying GSK Shares, or deposit your GSK Shares and receive GSK ADSs. However, the closing of the issuance
and cancellation books does not impact trading, and you may continue to trade your GSK ADSs during this period.
(5) Subject to the timing of the Capital Reduction.
16
RISK FACTORS
The risks and uncertainties relating to the Haleon Shares, the Group’s business and the industry in
which it operates, described below, together with all other information contained in this Prospectus,
should be carefully considered in light of Admission.
The risks and uncertainties relating to the Haleon Shares, the Group’s business and the industry in
which it operates summarised in the part of this Prospectus headed “Summary” are the risks that the
Directors believe to be the most essential to an assessment of Haleon Shares. However, as the risks
which the Group faces relate to events and depend on circumstances that may or may not occur in the
future, you should consider not only the information on the key risks summarised in the part of this
Prospectus headed “Summary” but also, among other things, the risks and uncertainties described
below.
The risks and uncertainties described below represent those the Directors consider to be material as at
the date of this Prospectus. However, these risks and uncertainties are not the only ones facing the
Group. Additional risks and uncertainties not presently known to the Directors, or that the Directors
currently consider to be immaterial, may individually or cumulatively also materially and adversely
affect the business, results of operations, financial condition and/or prospects of the Group. If any or a
combination of these risks actually occurs, the business, results of operations, financial condition and/
or prospects of the Group could be materially and adversely affected. In such case, the market price of
Haleon Shares could decline. You should carefully consider the information in this Prospectus in light
of your personal circumstances.
1. RISKS RELATING TO THE GROUP’S BUSINESS AND INDUSTRY
1.1 The Group operates in a highly competitive market and failure to successfully compete
with competitors could have a material adverse effect on the Group’s business
The Group faces substantial and increasing competition in all of its product categories and
geographic markets. There are relatively low barriers to entry in certain product categories in
many of the markets in which the Group operates (particularly in the VMS category) and
accordingly the Group’s businesses compete with companies of all sizes on many different
fronts, including cost-effectiveness, product effectiveness and quality, brand recognition and
loyalty, technological innovations, consumer convenience, promotional activities, new product
introductions and expansion into new markets and channels.
The Group expects to continue to see heightened activity from its competitors worldwide,
including an increase in the introduction and aggressive marketing of new products in high
demand healthcare areas. In particular, the Group expects to experience: (i) increasing and
aggressive competition from smaller, high growth companies which often operate on a regional
basis, and may disrupt existing route-to-market models; (ii) increasing competition from
multinational corporations moving for the first time into, or expanding or focusing their presence
(whether through acquisitions, disposals, demergers or other means) in the global consumer
healthcare market in order to benefit from the higher profit margins on offer and greater
consumer interest in health products and services; and (iii) continuing competition from “private
label” products, which are brands sold exclusively by a particular retailer.
Some of the Group’s competitors may spend more aggressively on, or have more effective,
advertising and promotion activities than the Group does, introduce competing products more
quickly and/or respond more effectively to business and economic conditions and changing
consumer preferences, including by launching innovative new products. The Group’s ability to
compete also depends on the strength of its brands and on its ability to enforce and defend its
intellectual property against infringement and legal challenges by competitors.
The Group may be unable to anticipate the timing and scale of the threats posed by the many
competitors across its markets or to successfully respond to them, which could harm the
Group’s business. In addition, the cost of responding to the increasingly significant and
17
widespread competition worldwide, including management time, out-of-pocket expenses and
price reductions, may materially and adversely affect the Group’s performance. Ultimately, a
prolonged failure by the Group to compete effectively in its key markets could have a material
adverse effect on the Group’s business, prospects, results of operations and financial
condition.
1.2 The Group’s success depends on its ability to anticipate and respond to changes in
consumer preferences and a failure to adapt its strategy appropriately may have a
material adverse effect on the Group’s business and/or financial condition
As a consumer products business, the Group relies on its ability to leverage its existing brands
and products to drive increased sales and profits. This in turn depends on the Group’s ability to
identify and offer products at attractive prices that appeal to consumer tastes and preferences,
which are difficult to predict and evolve over time. The Group’s ability to implement this
strategy depends on, among other things, its ability to:
• continue to offer products that consumers want at competitive prices;
• develop and maintain consumer interest in its brands and increase its brand recognition
and loyalty;
• innovate successfully on its existing products; and
• effectively utilise a range of distribution channels in its key markets.
The Group may not be able to execute this strategy successfully, which could have a material
adverse effect on the Group’s business, prospects, results of operations and/or financial
condition.
In addition, any reduction in consumer demand for the types of products which the Group
offers as a result of changes in consumer lifestyle, environmental concerns, economic
downturns or other considerations could have a material adverse effect on the Group’s
business, prospects, financial condition and results of operations. For example, in recent years,
there is increasing awareness of the environmental impact and sustainability of practices and
products in the market (see paragraph 1.8 of Risk Factors below).
1.3 The Group’s business results are impacted by the Group’s ability to manage disruptions
in the Group’s global supply chain and a failure to manage disruptions appropriately
may have a material adverse effect on the Group’s business and/or financial condition
The Group is engaged in manufacturing and sourcing of products and materials on a global
scale. The Group’s operations and those of its suppliers, contract manufacturers and logistics
providers have been and may continue to be disrupted by a number of factors, including, but
not limited to:
• increased and/or changing regulation, as well as regulatory compliance issues;
• environmental events, including natural disasters (such as fires, floods and earthquakes)
and any potential effect of climate change;
• widespread health emergencies, such as COVID-19 or other pandemics or epidemics,
leading to delays in deliveries and constraints on shipping and logistics due to local
lockdowns, such as the recent lockdowns in China that may impact the delivery to and
from China of the Group’s products, as well as resources required for its products;
• strikes and other labour disputes;
• disruptions in logistics;
18
• cybersecurity failures or incidents;
• loss, impairment, closure or disruption of key manufacturing sites;
• loss of key suppliers or contract manufacturers;
• supplier capacity constraints;
• raw material and product quality or safety issues (on which see further risk factor 1.10
below);
• industrial accidents or other occupational health and safety issues;
• the impact on the Group’s suppliers of tighter credit or capital markets;
• the lack of availability of qualified personnel;
• global shipping, logistics, transport and warehousing constraints;
• governmental incentives and controls (including import and export restrictions, such as
new or increased tariffs, sanctions, quotas or trade barriers);
• acts of war (on which see further risk factor 2.7 below) or terrorism, political unrest or
uncertainty, fires or explosions, and other external factors over which the Group has no
control; and
• increases in ingredient, commodity and oil prices.
While the product ranges of the Group’s leading brands are manufactured by multiple sources,
some of the Group’s products are currently primarily manufactured at a single location. The
loss of the use of all or a portion of any of the Group’s manufacturing facilities or the loss of the
use of key suppliers could have a material adverse effect on the Group’s business, financial
condition and results of operations.
In addition, the Group purchases certain raw and packaging materials from single-source
suppliers or a limited number of suppliers and new suppliers may have to be qualified under
industry, governmental and its own standards, which can require additional investment and
take a significant period of time.
Although the Group has contingency plans in place, such as dual sourcing programmes and
alternative supply arrangements, those plans may not be sufficient to mitigate manufacturing or
supplier interruptions, and the Group may also be limited in its ability to pass on any price
increases in the prices it charges for its products. For example, the Group has entered into,
and may in the future enter into, fixed price contracts or hedging arrangements in order to
address increases in commodity prices and their effect on the Group’s ability to source
materials for its products. However, if prices decrease, the Group will be unable to realise the
benefit of the decrease due to fixed price contracts in place.
A significant disruption to the manufacturing or sourcing of products or materials for any
reason, including those mentioned above, could interrupt product supply and, if not remedied,
could lead to litigation or regulatory action, product delistings by retailers, financial penalties,
and reputational damage that could materially and adversely affect the Group’s business,
results of operations and financial condition.
19
1.4 Increasing dependence on key retail customers, changes in the policies of the Group’s
retail customers, the emergence of alternative retail channels and the rapidly changing
retail landscape may materially and adversely affect the Group’s business
The Group’s products are sold in a highly competitive global marketplace which has
experienced increased trade concentration and the growing presence of large-scale retailers,
including pharmacies, as well as discounters and e-commerce retailers. With the growing trend
towards retail trade consolidation, increased cross-border trade, the rapid growth of
e-commerce and the integration of traditional and digital operations at key retailers, the Group
is increasingly dependent on certain retailers, and some of these retailers have and may
continue to have greater bargaining strength than the Group does. For example, similar to its
competitors, while the Group maintains relationships with a variety of significant retailers
across its key markets, sales attributable to its top five largest retailers account for over half of
the Group’s revenue in the US market.
The Group’s large-scale retail customers, including pharmacies, may use their leverage to
demand higher trade discounts, allowances, display fees or increased investment, including
through display media, paid search, preparation fees and other programmes, which could lead
to reduced sales or profitability. The loss of a key retailer or a significant reduction in sales to a
key retailer could materially and adversely affect the Group’s business, prospects, results of
operations and financial condition. The Group’s business might also be negatively affected by
the growing presence and bargaining strength of customers who operate internationally and
retail buying alliances (horizontal alliances of retailers, retail chains or entire retailer groups that
cooperate in pooling their resources) and the enhanced leverage that such alliances possess.
The Group has also been and may continue to be negatively affected by changes in the
policies or practices of the Group’s retail trade and pharmacy customers, such as inventory
de-stocking, limitations on access to shelf space, delisting of the Group’s products, or
environmental, sustainability, supply chain or packaging initiatives and other conditions. For
example, a determination by a key retailer that any of the Group’s ingredients should not be
used in certain consumer products or that the Group’s packaging does not comply with certain
environmental, supply chain or packaging standards or initiatives could materially and
adversely impact the Group’s business, prospects, results of operations and financial condition.
“Private label” products sold by the Group’s retail customers, which are typically sold at lower
prices than branded products, are a source of competition for certain of the Group’s products.
In addition, the retail landscape in many of the Group’s markets continues to evolve as a result
of the rapid growth of e-commerce retailers (who are able to generate “private label” products
and capitalise on access to data) and price comparison sites, changing consumer preferences
(as consumers increasingly shop online), and, in certain categories (particularly VMS), the
increased presence of alternative retail channels, such as subscription services, sales through
social media platforms and direct-to-consumer businesses (especially those which specialise in
rapid distribution). The strong growth in e-commerce and the emergence of alternative retail
channels may create pricing and margin pressures and/or adversely affect the Group’s
relationships with key retailers. If the Group is not able to successfully manage and adapt to
these changes in the retail landscape, the Group’s business, prospects, results of operations
and financial condition could be materially and adversely affected.
1.5 The Group may not be able to develop and commercialise new products effectively,
which may materially and adversely affect the results of the Group’s operations and
financial condition
The future growth of the Group is to a significant extent dependent on its ability to develop new
products or new formulations of existing products. The Group’s ability to launch new products
and to expand into adjacent categories, channels of distribution or markets is affected by
whether the Group can successfully:
• identify, develop and fund technological innovations;
• obtain and maintain necessary intellectual property protection and avoid infringing
intellectual property rights of others;
20
• obtain and maintain approvals and registrations of regulated products, including from the
FDA, the EMA, the NMPA and other regulatory bodies in the countries in which the Group
has business operations, including in relation to Rx-to-OTC switches;
• anticipate, quickly respond to, and benefit from the needs and preferences of consumers
and customers by, among other things, effectively utilising digital technology and marketing
and data analytics to gain new commercial insights and develop relevant marketing and
advertising to identify new products that will align with consumer preferences; and
• successfully compete to in-license products.
The identification, development and introduction of innovative new products that drive
incremental sales involves considerable costs and effort, and any new product may not
generate sufficient customer and consumer interest and sales to become a profitable product
or to cover the costs of its development and promotion. The Group’s ability to achieve a
successful launch of a new product could also be adversely affected by pre-emptive actions
taken by competitors in response to the launch, such as increased promotional activities and
advertising. In addition, new products may not be accepted quickly or significantly in the
marketplace.
The product development process is both time-consuming and costly and involves a high
degree of business risk. In particular, the Group’s OTC products, including those in respect of
which it is undertaking an Rx-to-OTC switch, are subject to lengthy development programmes
and regulatory approval periods which can restrict the Group’s ability to innovate in this product
area. The Group must develop, test and manufacture products to meet its own internal
specifications and standards as well as all applicable regulatory and safety requirements, and it
is possible that a new product can fail to make it to market at any stage of this process. Whilst
the Group has a good track record of developing new products and executing Rx-to-OTC
switches, there can be no guarantee that the Group will continue to be able to develop and
commercialise new products at the rate required to retain or grow market share or that suitable
opportunities for further Rx-to-OTC switches will become available to the Group. Any failure to
develop and commercialise new products in a timely fashion may decrease revenue and/or
increase R&D costs and, consequently, may materially and adversely affect the results of the
Group’s operations and financial condition.
1.6 Failure to retain key personnel or attract new personnel may materially and adversely
affect the Group’s business
The Group relies upon a number of key executives and employees who have an in-depth
understanding of the consumer healthcare industry and the Group’s technologies, products,
programmes, collaborative relationships and strategic goals. While the Group follows a
disciplined, ongoing succession planning process and has succession plans in place for Senior
Management and other key executives, these do not guarantee that the services of qualified
senior executives will continue to be available to the Group at all times. Competition for such
personnel in the consumer healthcare industry is intense, and there can be no assurance that
the Group will be able to continue to attract and retain such personnel, particularly as
competitors may attempt to recruit them.
Further, the Group’s ability to implement its strategy depends on the ability and experience of
its Senior Management and other key employees. If the Group is unable to recruit, attract and
retain talented, highly qualified Senior Management and other key people, including through
competitive remuneration and benefits packages, appropriate career development, employee
resilience and engagement programmes, the Group’s business, prospects, results of
operations and financial condition could be materially and adversely affected. The Group is
also working to advance cultural change through the implementation of diversity, equality and
inclusion initiatives and through the implementation of a new purpose, strategy and culture
programme throughout the organisation. If the Group does not (or is perceived not to)
successfully implement these plans and initiatives, its ability to recruit, attract and retain talent
may be materially and adversely impacted, which may in turn materially and adversely affect
the Group’s business, results of operations and financial condition.
21
1.7 Damage to the Group’s reputation could have a material adverse effect on the Group’s
business
Maintaining the Group’s strong reputation and trust with consumers and the Group’s customers
globally is critical to selling the Group’s branded products. Negative publicity about the Group,
the Group’s industry, the Group’s brands and products, the Group’s advertising and promotion
practices, the Group’s use, storage and securing of technology and data, including personal
data, the Group’s supply chain, the Group’s ingredients, the Group’s packaging, the Group’s
research practices, threatened or pending litigation or regulatory proceedings, the Group’s
public policy engagement, the Group’s environmental, social and governance practices,
including as they relate to diversity, equality and inclusion, the health, safety and welfare of
employees or other stakeholders, or relations with the Group’s employees, or regulatory
infractions, violations of sanctions or anti-bribery rules, whether or not deserved, could
jeopardise the Group’s reputation and/or expose it to adverse press and social media attention.
The Group’s reputation may also be adversely affected if third parties with whom the Group
contracts, including its suppliers, manufacturers and customers, fail to maintain high ethical,
social and environmental standards, comply with local laws and regulations or become subject
to other negative events or adverse publicity. Such third parties may also enter into
relationships with or be acquired by other third parties whose values, business practices and/or
reputation expose the Group to the risk of adverse publicity and damage to its existing
relationships by association. While the Group has policies and procedures for managing third
party relationships, it may not be possible to fully ensure that third parties adhere to the same
standards and values as the Group or to replace third party relationships in a timely and/or
cost-effective manner.
In addition, widespread use of digital and social media by consumers has greatly increased the
accessibility of information and the speed of its dissemination. Negative publicity, posts or
comments on social media about the Group, the Group’s brands, the Group’s products,
including any ingredients used in its products, the Group’s packaging or the Group’s
employees, whether true or untrue, could damage the Group’s brands and its reputation and/or
lead to boycotts of its products. For example, during the COVID-19 pandemic, sales of Advil
(an ibuprofen-based product) were adversely impacted by negative media coverage regarding
the use of ibuprofen products in treating the symptoms of COVID-19. Moreover, the Group’s
reputation could be harmed as a result of inappropriate use of its branded products being
promoted on social media and any associated negative publicity. The success of the Group’s
brands could also suffer if the Group’s marketing initiatives do not have the desired impact on a
brand’s image or its ability to attract consumers.
Counterfeiting is a common issue for successful brands and has been amplified by
e-commerce. Although the Group has an anti-counterfeiting programme in place, third parties
continue to sell counterfeit versions of the Group’s products, such as Sensodyne, Panadol and
ENO, including on online platforms and on social media. These counterfeits are inferior in
quality to the genuine Group products and may pose safety risks to consumers. Consumers of
the Group’s brands could confuse the Group’s products with these counterfeit products,
purchasing the counterfeit products in error instead of the genuine Group products. The
consumption of inferior quality products, which consumers believe to be genuine (and, in some
instances, may cause consumer safety issues) could also damage the reputation of the Group
and its brands and lead to a reduction in market share with affected consumers choosing in the
future to buy competitors’ brands instead.
Damage to the Group’s reputation or loss of consumer confidence in the Group’s products for
these or any other reasons could materially and adversely affect the Group’s business, results
of operations, cash flows and financial condition, as well as require resources to rebuild the
Group’s reputation.
22
1.8 Failure to respond effectively to the challenges raised by climate change and other
sustainability matters may have a material adverse effect on the Group’s business and
results of operations
Concern over climate change and other sustainability matters has increased the focus on the
sustainability of practices and products in the market and may result in new or additional legal
and regulatory requirements to reduce or mitigate environmental or social impacts. Areas of
focus relevant to the Group’s business include, among others, responsible sourcing and
deforestation, human and labour rights, the use of plastic, energy and water, the recyclability or
recoverability of packaging, including single-use and other plastic packaging, and the use of
certain materials, such as palm oil where the sourcing or environmental impact of the material
has been linked to sustainability issues and therefore attracts scrutiny. If new or additional legal
and regulatory requirements relating to sustainability matters are more stringent than the
Group’s current legal and regulatory obligations and/or the Group’s existing practices and
procedures are inadequate to meet these requirements, this may require the Group to revise
its operations and supply chain management, including, for example, by collecting used
products, packaging or other materials from consumers and reintroducing them to the Group’s
manufacturing cycle. There may also be financial impacts as governments implement taxation,
such as extended producer responsibility taxes or carbon taxes to help to recover the cost of
managing plastic waste and the impacts of climate change. These developments may result in
increased costs and disruption to the Group’s operations, which could materially and adversely
affect the Group’s business, results of operations, cash flows and financial condition.
The Group’s reputation is also affected by its perceived sustainability credentials and its ability
to meet its sustainability goals. There is increased public attention, including by
non-governmental organisations, investors, customers, consumers, the Group’s employees
and other stakeholders, on climate change and other sustainability matters. Despite the
Group’s sustainability efforts, any failure or perceived failure to achieve its sustainability goals,
including, among others, to reduce net scope 1 and 2 emissions by 100 per cent. by 2030
(versus its 2020 baseline) and to make all product packaging recyclable or reusable by 2030
(versus its 2020 baseline and quality, safety and regulations permitting), or the perception
(whether or not valid) that the Group has failed to act responsibly with respect to such matters
or to effectively respond to new or additional legal or regulatory requirements regarding climate
change, could result in adverse publicity and/or litigation which could materially and adversely
affect the Group’s business and reputation. This could result in product delistings with
customers or loss of preference with consumers, investors, employees or other stakeholders,
which could materially and adversely affect the Group’s business, results of operations, cash
flows and financial condition.
The Group is dependent on shifts in the wider industry to meet some of its sustainability goals
and there is a risk that the Group will not meet its goals if those shifts do not take place. In
order to reduce its scope 3 carbon footprint, the Group depends on shifts in the energy grid
away from fossil fuels and towards renewable sources in the areas the Group sources from
and sells its products. The Group’s transition to more sustainable packaging formats and
circular business models is dependent on, among other things: the supply of recycled content
or alternative non-virgin petroleum-based plastic materials; regulatory approval for use of
alternative materials; the availability of new packaging technologies; and improvements in
recycling infrastructure. In order to meet its sustainable sourcing goals, the Group also
depends on the availability of sustainably sourced commodities at a reasonable cost. Adverse
developments in respect of such dependencies may result in the Group failing to meet its
sustainability goals and could lead to a material adverse effect on the Group’s reputation
which, in turn, could materially and adversely affect its business, results of operations, cash
flows and financial condition.
1.9 The Group may not be successful in obtaining, maintaining and enforcing sufficient
intellectual property rights to protect its business, or in avoiding claims that the Group
infringes on the intellectual property rights of others
The Group relies on various types of intellectual property rights such as trade marks, patents,
copyrights and designs, whether registered or unregistered, as well as unpatented proprietary
23
knowledge and trade secrets, to protect its business. However, these rights do not afford
complete protection against third parties’ claims and infringements. For example, trade marks,
patents, copyrights and designs are territorial; thus, the Group’s business can only claim
optimal IP protection in jurisdictions where the Group has obtained trade mark, patent, design
and copyright registrations, or has obtained licences to use third-party trade marks, patents,
copyrights or registered designs. While IP laws are fairly harmonised around the world, certain
countries’ laws may not protect the Group’s intellectual property rights to the same extent as
afforded in the UK and the USA. Additionally, there can be no assurance that third parties will
not independently develop knowledge and trade secrets that are similar to the Group’s, or
develop products or brands that compete effectively with the Group’s products and brands
without infringing, misusing or otherwise violating any of the Group’s intellectual property rights.
The Directors cannot be certain that any of the Group’s registered (granted or pending) or
unregistered trade marks, patents, copyrights, or designs will provide the Group with sufficient
protection from competitors, or that any intellectual property rights which the Group does hold
will not be invalidated, circumvented or challenged in the future. In the event of such a
challenge, the Group could incur significant costs to defend its intellectual property rights, even
if it is ultimately successful. Additionally, there is a risk that the Group will not be able to obtain
and perfect or, where appropriate, obtain licences for the intellectual property rights necessary
to support new product introductions and product innovations. Additionally, the Group has
licensed, and may license in the future, trade marks, patents, trade secrets and other
intellectual property rights to third parties. While the Group attempts to ensure that its
intellectual property rights are protected when entering into business relationships, third parties
may take actions that could materially and adversely affect the Group’s rights or the value of its
intellectual property rights.
The Group also uses intellectual property rights in-licensed from licensors. The Group’s
licences to such intellectual property rights may not provide exclusive or unrestricted rights in
all fields of use and in all territories in which the Group may wish to develop or commercialise
its products in the future and may restrict its rights to offer certain products in certain markets,
including through non-compete provisions, or impose other obligations on the Group in
exchange for its rights to the licensed intellectual property. In addition, the Group may not have
full control over the maintenance, protection, enforcement or use of the intellectual property
rights in-licensed from licensors, and therefore the Group may be reliant on the licensors to
conduct such activities.
Disputes may arise between the Group and its licensors regarding the scope of rights or
obligations under the relevant intellectual property licence agreements, including the scope of
the Group’s rights to use the licensed intellectual property, the Group’s rights with respect to
third parties, the Group’s and its licensors’ obligations with respect to the maintenance and
protection of the licensed intellectual property, financial obligations of the Group to the licensor,
and other interpretation-related issues. The agreements under which the Group licenses
intellectual property rights from others are complex, and the provisions of such agreements
may be susceptible to multiple interpretations. The resolution of any contract interpretation
disagreement that may arise could narrow what the Directors believe to be the scope of the
Group’s rights to the intellectual property being licensed, or increase what the Directors believe
to be its financial or other obligations under the relevant agreement. Termination of or disputes
over such licences could result in the loss of significant rights.
Third parties may copy or otherwise obtain and misuse the Group’s proprietary knowledge,
trade secrets, trade marks, patents, designs or copyrights, or infringe or otherwise violate the
Group’s intellectual property rights. For example, the Group’s brands are well-established in
the market and have attracted trade mark and patent infringers in the past. Additionally, the
Group may not be able to prevent current and former employees, contractors and other parties
from misappropriating the Group’s confidential and proprietary knowledge. Infringement,
misuse or other violation of any of the Group’s intellectual property rights may dilute or diminish
the value and goodwill of its brands and products in the marketplace, which could materially
and adversely affect the Group’s results of operations and make it more difficult for the Group
to maintain a strong market position. While the Group protects its intellectual property rights,
24
including through litigation, where necessary, it cannot economically prevent all infringements,
misuses or other violations, and any litigation could be protracted and costly and could have a
material adverse effect on the Group’s business and results of operations regardless of its
outcome.
1.10 The Group may incur liabilities or be forced to recall products as a result of real or
perceived product quality or other product-related issues
Failure to comply with good manufacturing or good distribution practices and regulations, as
well as other regulations in relation to product quality, throughout the Group’s in-house and
contract manufacturing supply and distribution chains could lead to product supply
interruptions, product recalls or withdrawals, litigation and/or regulatory enforcement action and
fines from regulators, such as the FDA, EMA and NMPA, despite employee training, promotion
of a health and safety culture, and control measures and systems being in place that are
designed to ensure that the safety and quality of the Group’s products is maintained. By way of
example, raw materials which the Group sources for production may become contaminated
through the supply chain and other product defects may occur due to human error or
equipment failure, among other things. Additionally, products may be contaminated or
tampered with during distribution or at stores. The Group is increasingly using new technology
to enhance the manufacture and testing of its products, such as the deployment of new
electronic documentation systems and advanced laboratory information management tools.
Such technology is inherently susceptible to the threat of cyberattacks which pose an ongoing
risk to the integrity of product quality data and its audit trail. The Group also continues to be
reliant on third parties and is continuing to undertake a global network rationalisation
programme to reduce the number of manufacturing sites it uses, both of which are factors that
may increase the risks to safe and timely supply of products.
Product recalls or withdrawals arising as a result of real or perceived product quality or other
product related issues, whether initiated on a voluntary basis or otherwise, can result in a
range of adverse consequences to the Group, including lost sales, the requirement to hold
increased inventories of substitute products, damaged relationships with regulators, loss of
market share to competitors, adverse publicity and reputational harm, in addition to the direct
costs of implementing any recall. Furthermore, such product quality or other product related
issues also expose the Group to a significant risk of litigation, particularly product liability
claims, and regulatory action (on which see further risk factor 2.4 below).
Failure by the Group to manufacture its products in accordance with good manufacturing
practices could have the potential to do significant damage to the Group’s reputation and
materially and adversely affect the results of its operations and financial condition. In addition,
if any of the Group’s competitors or customers supply faulty or contaminated products to the
market, the Group’s industry could be negatively impacted, which in turn could have material
adverse effects on the Group’s business.
1.11 A cyber-security incident, data breach or a failure of a key information technology
system could materially and adversely impact the Group’s business
The Group relies extensively on information technology systems (“IT Systems”), including
some which are managed, hosted, provided and/or used by third parties, including cloud-based
service providers, and their vendors, in order to conduct its business.
Although the Group has a broad array of information security measures in place, the Group’s
IT Systems, including those of third-party service providers with whom it has contracted, have
been, and will likely continue to be, subject to computer viruses or other malicious codes,
unauthorised access attempts, phishing and other cyber-attacks.
Cyber-attacks and other cyber incidents are occurring more frequently, are constantly evolving
in nature, are becoming more sophisticated and are being made by groups, individuals and
nation states with a wide range of expertise and motives. Such cyber-attacks and cyber
incidents can take many forms, including cyber extortion, social engineering, password theft or
introduction of viruses or malware, such as ransomware through phishing emails. For example,
25
the Group experienced an increase in cyber-attacks and other cyber incidents in the months
before Russia’s invasion of Ukraine, and there is a heightened risk of further cyber-attacks,
including from state actors (see also paragraph 2.7 of Risk Factors below). While the Group
has implemented systems, monitoring and training to prevent cyber-attacks and other cyber
incidents from being successful, the Group cannot guarantee that its security efforts will
prevent breaches or breakdowns of its, or its third-party service providers’, IT Systems since
the techniques used in these attacks change frequently and may be difficult to detect for
periods of time, and so such cyber-attacks may from time to time succeed. In addition, the
Group cannot guarantee that it or its third-party service providers’ response to any such
incidents will fully remedy the extent of the damage caused by these incidents. Although the
Group has policies and procedures in place to ensure that all personal information collected by
it or its third-party service providers is securely maintained, data breaches due to human error
or intentional or unintentional conduct may still occur in future.
Furthermore, the Group periodically upgrades its IT Systems or adopts new technologies. If
such an upgrade or new technology does not function as designed, does not go as planned or
increases the Group’s exposure to a cyber-attack or cyber incident, it may adversely impact the
Group’s business, including its ability to ship products to customers, issue invoices and
process payments or order raw and packaging materials. If the Group were to suffer a
significant loss or disclosure of confidential business or stakeholder information as a result of a
breach of its IT Systems, including those of third-party service providers with whom it has
contracted, or otherwise, the Group may suffer reputational, competitive and/or business harm,
incur significant costs and be subject to government investigations, litigation, fines and/or
damages, which may materially and adversely impact the Group’s business, prospects, results
of operations and financial condition.
While the Group has disaster recovery and business continuity plans in place, if its IT Systems
were damaged, breached or were to cease to function properly for any reason, including the
poor performance of, failure of or cyber-attack on, third-party service providers, catastrophic
events, power outages, cyber-security breaches, network outages, failed upgrades or other
similar events and if the disaster recovery and business continuity plans do not effectively
resolve such issues on a timely basis, the Group may suffer interruptions in its ability to
manage or conduct business as well as reputational harm, and may be subject to
governmental investigations and litigation, any of which may materially and adversely impact
the Group’s business, prospects, results of operations and financial condition.
1.12 The Group relies on third parties in many aspects of its business and ineffective
management of these relationships could increase the Group’s financial, legal,
reputational and operational risk
Due to the scale and scope of the Group’s business, the Group relies on relationships with
third parties, including its suppliers, contract manufacturers, distributors, contractors,
commercial banks, joint venture partners and external business partners, for route-to-market
and for certain functions (including the outsourcing of certain back office and consumer
relations services). If the Group is unable to effectively manage and maintain its third-party
relationships and the agreements under which the Group’s third-party partners operate, its
results of operations could be adversely impacted.
For example, in China, part of the Group’s business is conducted through Sino-American
Tianjin Smith Kline & French Laboratories Ltd., which is a joint venture between
GlaxoSmithKline Consumer Healthcare (Overseas) Limited, the Tianjin Pharmaceutical Group
and the Tianjin Zhongxin Pharmaceutical Group (the “TSK&F Joint Venture”), pursuant to a
joint venture agreement which is due to expire in September 2024. If the Group does not renew
these arrangements or implement alternative measures, in either case on acceptable terms,
then the continuity and development of part of its operations and route-to-market in China, as
well as its business, results of operations and cash flows in that market, may be adversely
affected.
Failure of third parties to meet their obligations to the Group or substantial disruptions in the
relationships between the Group and third parties could adversely impact the Group’s
26
operations and financial results. Additionally, while the Group has policies and procedures for
managing these relationships, they inherently involve a lesser degree of control over business
operations, and compliance with laws, regulations and Group policies and practices than is
available for the Group’s own operations and compliance, thereby potentially increasing the
Group’s financial, reputational, operational and legal risk, including in respect of health and
safety, environmental, social and governance issues, modern slavery, anti-bribery and
corruption.
1.13 The Group faces various risks related to pandemics, epidemics or similar widespread
public health concerns, the ultimate impact of which is outside the Group’s control and
which may materially and adversely affect the Group’s operations, cash flows and
financial condition
The Group faces various risks related to pandemics, epidemics or similar widespread public
health concerns, including the COVID-19 pandemic. A pandemic, epidemic or similar
widespread health concern could have, and COVID-19 has had and will continue to have, a
variety of impacts on the Group’s business, results of operations, cash flows and financial
condition, including:
• the Group’s ability to continue to maintain and support the health, safety and well-being of
the Group’s employees, including key employees;
• volatility in the demand for and availability of the Group’s products, which may be caused
by the temporary inability of the Group’s consumers to purchase the Group’s products due
to illness, financial hardship, quarantine, government actions mandating the closure of the
Group’s distributors or retailers or imposing travel or movement restrictions, shifts in
demand and consumption away from more discretionary or higher priced products to
lower-priced products, or pantry-loading activity;
• increases in demand for certain of the Group’s products requiring the Group to increase its
production capacity or acquire additional capacity at an additional cost and expense;
• decreases in demand and sales for certain of the Group’s key products such as Theraflu
and Robitussin due to a particularly weaker cold and flu season;
• changes in regulatory policy, including restrictions on sales of certain products. For
example, amid the COVID-19 pandemic, in certain countries specific restrictions were
introduced on the sale of cough and cold medicines in an attempt to prevent patients from
self-medicating against COVID-19 at home. In China, in early 2020, certain local
authorities introduced temporary restrictions on the sale of such medicines, which limited
sales of Contac (nasal decongestant tablets that also relieve pain and reduce fever) and
Fenbid (ibuprofen-based relief medicine) by the Group in 2020, adversely affecting the
Group’s revenue in APAC in FY 2020;
• changes in purchasing patterns of the Group’s consumers, including the frequency of
in-store visits by consumers to retailers and dental and skin health professionals and a shift
to purchasing the Group’s products online from e-commerce retailers;
• disruptions to the Group’s global supply chain (including the closure of manufacturing and
distribution facilities) due to, among other things, the availability of raw and packaging
materials or manufacturing components; a decrease in the Group’s workforce or in the
efficiency of such workforce, including as a result of illness, travel restrictions, absenteeism
or governmental regulations; and transportation and logistics challenges, including as a
result of port and border closures and other governmental restrictions or reduced shipping
capacity;
• failure of third parties on which the Group relies, including the Group’s retailers, suppliers,
contract manufacturers, logistics providers, customers, commercial banks, joint venture
27
partners and external business partners, to meet their obligations to the Group, or
significant disruptions in their ability to do so, which may be caused by their own financial
or operational difficulties;
• significant changes in the economic and political conditions of the markets in which the
Group operates, which could restrict and have restricted the Group’s employees’ ability to
work and travel, could mandate and have mandated or caused the closure of certain
distributors or retailers, the Group’s offices, shared business service centres and/or
operating and manufacturing facilities, or otherwise could prevent and have prevented the
Group as well as the Group’s third-party partners, suppliers or customers from sufficiently
staffing operations, including operations necessary for the manufacture, distribution, sale
and support of the Group’s products;
• disruptions and volatility in the global capital markets, which may increase the cost of
capital and/or adversely impact the Group’s access to capital; and/or
• volatility in foreign exchange rates and in raw and packaging materials and logistics costs.
Despite the Group’s efforts to manage these impacts, their ultimate impact also depends on
factors beyond the Group’s knowledge or control, including the duration, severity and
geographic scope of an outbreak, such as COVID-19, the availability, widespread distribution
and use of safe and effective vaccines and the actions taken to contain its spread and mitigate
its public health and economic effects.
1.14 The implementation of complex strategic, operational and/or change initiatives gives
rise to significant execution risks, which may affect the operational capacity of the
Group and may materially and adversely impact the Group if these initiatives fail to meet
their objectives
The Group has undertaken a number of, and may from time to time commence, strategic,
operational and/or change initiatives. For example, the Group has previously implemented
strategic initiatives to effectively integrate the Novartis and Pfizer consumer healthcare
businesses and execute a targeted programme of non-core asset divestments. There may be
financial, operational, regulatory, customer and reputational implications if such initiatives fail
(either wholly or in part) to meet their objectives, which could place strain on the operational
capacity of the Group. The scale and nature of the programmes and management challenges
may cause disruption to resourcing through heightened uncertainty, increased workloads and
short-term resource stretch, which, in turn, could result in the disruption of business as usual
activities. Implementing further strategic, operational and/or change initiatives may amplify
these risks. Any disruption caused by, or failure to successfully implement any such initiatives
could have a material adverse effect on the Group’s ordinary course business and,
consequently, its financial condition, results of operations and prospects, or otherwise harm the
Group’s reputation.
1.15 The Group’s business is affected by seasonality, which could have a negative impact on
the Group’s financial condition
Portions of the Group’s business are seasonal. This is driven by seasonal demand for certain
products, including its cough, cold and flu, allergy and decongestant products, such as Theraflu
and Robitussin. In respect of such products, if the seasonal effects which help to deliver
performance are negatively impacted, including due to unfavourable economic conditions, this
could have a material adverse effect on the Group’s financial condition and results of
operations for the entire year. Government measures imposed in response to COVID-19, such
as lockdowns and social distancing restrictions, have tempered the usual seasonal spikes in
the incidence of flu and cold, thus reducing demand for the Group’s cold and flu product lines
during FY 2021. Because of quarterly fluctuations caused by these and other factors,
comparisons of the Group’s operating results across different fiscal quarters may not be
accurate indicators of the Group’s future performance.
28
1.16 The Group may not successfully acquire and integrate other businesses, licence rights
to technologies or products, form and manage alliances, or divest businesses
The Group may decide in the future to pursue acquisitions, technology licensing arrangements,
strategic alliances or divestitures as part of its business strategy. The Group may not complete
these transactions in a timely manner, on a cost-effective basis or at all. In addition, the Group
may be subject to regulatory constraints or limitations or other unforeseen factors that prevent
it from realising the expected benefits of such transactions. Even if the Group is successful in
completing an acquisition, the products, intellectual property and technologies that are
acquired may not be successful or may require significantly greater resources and investments
than originally anticipated. The Group may be unable to integrate acquisitions successfully into
its existing business, and the Group may be unable to achieve expected operating margin
improvements, synergies or efficiencies. The Group could also incur or assume significant debt
and unknown or contingent liabilities in connection with acquisitions. The Group’s reported
operating results could be negatively affected by acquisition or disposition-related charges,
amortisation of expenses related to intangibles and charges for impairment of long-term
assets. The Group may be subject to litigation in connection with, or as a result of, acquisitions,
dispositions, licences or other alliances, including claims from terminated employees,
customers or third parties, and the Group may be liable for future or existing litigation and
claims related to the acquired business, disposition, licence or other alliance because either
the Group is not indemnified for such claims or the scope or availability of indemnification is
limited. These effects could cause the Group to incur significant expenses and could materially
and adversely affect the Group’s business, results of operations and financial condition.
1.17 The Group’s leverage and debt service obligations could materially and adversely affect
its business, financial condition or results of operations
Prior to the date of this Prospectus, the Group has incurred financial indebtedness in order to
fund the Pre-Demerger Dividend. As a result, the Group has higher leverage levels than are
reflected in the Group’s longer-term strategy and has significant debt service obligations. The
Group’s longer-term strategy to improve its financial risk profile, including by reducing levels of
indebtedness, may not be successful.
As at the Latest Practicable Date, the Group has the following financial indebtedness
outstanding:
• £300,000,000 2.875 per cent. notes due 29 October 2028 and £400,000,000 3.375 per
cent. notes due 29 March 2038, each issued by GSK Consumer Healthcare Capital UK plc
pursuant to the Programme;
• €850,000,000 1.250 per cent. notes due 29 March 2026, €750,000,000 1.750 per cent.
notes due 29 March 2030 and €750,000,000 2.125 per cent. notes due 29 March 2034,
each issued by GSK Consumer Healthcare Capital NL B.V. pursuant to the Programme;
• $700,000,000 3.024 per cent. callable fixed rate senior notes due 2024, $300,000,000
callable floating rate senior notes due 2024, $2,000,000,000 3.375 per cent. fixed rate
senior notes due 2027, $1,000,000,000 3.375 per cent. fixed rate senior notes due 2029,
$2,000,000,000 3.625 per cent. fixed rate senior notes due 2032 and $1,000,000,000
4.000 per cent. fixed rate senior notes due 2052, each issued by the US Issuer pursuant to
a private placement to institutional investors in the USA and outside the USA;
• $1,750,000,000 3.125 per cent. fixed rate senior notes due 2025 issued by GSK Consumer
Healthcare Capital UK plc pursuant to a private placement to institutional investors in the
USA and outside the USA;
• £nil under the Term Loan Facility (noting the Term Loan Facility is expected to be drawn on
or prior to the date of the Pre-Demerger Dividend); and
• £nil and $nil of RCF Loans.
29
Following payment of the Pre-Demerger Dividend, an amount equal to the Pre-Separation Debt
Proceeds less £300 million will have been distributed out of the Group, with no recourse, to
GSK and Pfizer and none of the Group’s debt will continue to benefit from guarantees provided
by GSK.
The degree to which the Group is leveraged could have important consequences to the
Group’s business, including, but not limited to:
• increasing the Group’s vulnerability to, and reducing its flexibility to respond to, a downturn
in the Group’s business or general adverse economic and industry conditions;
• limiting the Group’s ability to obtain additional financing in the longer term;
• requiring the dedication of a substantial portion of the Group’s cash flow from operations to
the payment of interest on the Group’s indebtedness and the repayment of principal,
thereby reducing the availability of such cash flow to fund capital expenditures, dividends,
joint ventures, acquisitions or other general corporate purposes;
• increasing the cost of future borrowings for the Group;
• a downgrade in the Group’s credit rating, which may, in turn, increase the cost of the
Group’s financing arrangements and make it difficult for the Group to access financing on
commercially acceptable terms or at all;
• limiting the Group’s flexibility in planning for, or reacting to, changes in the Group’s
business and the competitive environment and the industry in which it operates; and
• placing the Group at a competitive disadvantage as compared to some of its competitors,
to the extent that they are not as highly leveraged.
Any of these or other consequences or events could have a material adverse effect on the
Group’s business, financial condition and results of operations.
In addition, the Group may incur substantial additional indebtedness in the future. In
accordance with the terms and conditions of the Programme, the EMTN Issuers have capacity
to issue up to £10,000,000,000 in principal amount of notes (inclusive of the Pre-Separation
Programme Notes that have already been issued) which could further increase the Group’s
leverage and financial indebtedness. In addition, the Group’s Revolving Credit Facilities make
available £1,000,000,000 and $1,400,000,000 of commitments to provide RCF Loans which
remain undrawn as at the Latest Practicable Date. The covenants in existing financing
instruments do not fully prohibit the Company or its subsidiaries from incurring more
indebtedness. If new debt is added to the Group’s current debt levels, the risks that it now
faces could intensify. The incurrence of additional indebtedness would increase the leverage-
related risks described herein and would increase the risk of a downgrade in the Group’s credit
rating.
1.18 The Group’s business and results of operations are affected by fluctuations in interest
rates
The Group is subject to risk from financial instruments that bear interest at floating rates,
including one series of the Pre-Separation USD Notes and borrowings under the Group’s bank
financing facilities. These interest rates could rise significantly in the future, thereby increasing
the Group’s interest expenses associated with these obligations and reducing cash flow
available for other purposes.
The Group currently hedges a portion of the interest rates on its financial instruments with the
aim of achieving an appropriate balance of fixed-rate and floating-rate exposures. However, it
may not be able to replace or extend such hedges on terms that are acceptable to the Group,
or at all, and either the Group’s overall strategy or any individual hedge may not be fully
effective, which would expose the Group to interest rate risk.
30
1.19 Goodwill and indefinite-life intangible assets are a material component of the Group’s
balance sheet and impairments of these assets could have a significant impact on its
results
The Group has recorded a significant amount of goodwill and indefinite-life intangible assets,
representing £26.45 billion as of 31 December 2021, on its balance sheet. The Group tests the
carrying values of goodwill and indefinite-life intangible assets for impairment at least annually
and whenever events or circumstances indicate the carrying value may not be recoverable.
The estimates and assumptions about future results of operations and cash flows made in
connection with impairment testing could differ from future actual results of operations and
cash flows. While the Directors have concluded that the Group’s goodwill and indefinite-life
intangible assets are not impaired, future events could cause the Directors to conclude that the
goodwill associated with a given segment, or one of the Group’s indefinite-life intangible
assets, may have become impaired. Any resulting impairment charge, although non-cash,
could have a material adverse effect on the Group’s results of operations and financial
condition.
2. RISKS RELATING TO CHANGES IN LAW AND THE POLITICAL AND ECONOMIC
ENVIRONMENT, REGULATION AND LEGISLATION
2.1 The Group’s business is subject to legal and regulatory risks in all the markets in which
it operates, which may have a material adverse effect on the Group’s business
operations and financial condition
The Group’s business is subject to extensive legal and regulatory requirements in all the
markets in which it operates. Such legal and regulatory requirements apply to most aspects of
the Group’s products, including their development, ingredients, formulation, manufacture,
packaging content, labelling, storage, transportation, distribution, export, import, advertising,
promotion beyond therapeutic indications, sale and environmental impact. Many different
governmental and regulatory authorities in the Group’s markets regulate and have jurisdiction
over different aspects of the Group’s business activities. In addition, the Group’s selling
practices are regulated by competition law authorities in the UK, as well as in the EU, the USA
and other markets.
For example, in China, where the Group has significant sales and operations, governmental
authorities introduced changes in regulations relating to registrations of all generic medicines
(including OTC products) and recently introduced changes for oral health products. These
affect both new and existing products and impose increased data submission requirements for
products the Group markets in China. There is a risk that commercialisation of certain products
of the Group may be restricted in China if the Group is unable to comply with these regulatory
changes on the required timetable.
New or more stringent legal or regulatory requirements, or more restrictive interpretations of
existing requirements, could materially and adversely impact the Group’s business, results of
operations and financial condition. For example, regulators have decided, and might decide in
the future, that certain products of the Group should be prescription only or otherwise
reclassified, resulting in new regulations and laws, including in respect of claims, becoming
applicable to such products.
Because of the Group’s extensive international operations, the Group could be materially and
adversely affected by violations of worldwide anti-bribery laws, including those that prohibit
companies and their intermediaries from making improper payments to government officials or
other third parties for the purpose of obtaining or retaining business, such as the US Foreign
Corrupt Practices Act, the UK Bribery Act 2010, and other laws that prohibit commercial
bribery. Additionally, in certain jurisdictions, the Group’s engagement with healthcare
professionals and other external leaders is subject to applicable restrictions. While the Group’s
policies mandate compliance with such laws, the Group cannot provide assurance that the
Group’s internal control policies and procedures will always protect the Group from reckless or
criminal acts committed by its employees, joint venture partners or agents. Similarly, due to the
Group’s international operations, the Group could also be materially and adversely affected by
31
any violations of international sanctions laws, which continue to evolve in response to
geopolitical events (see also paragraph 2.7 of Risk Factors below). Violations of these laws, or
allegations of such violations, could disrupt the Group’s business and materially and adversely
affect its reputation and the Group’s business, prospects, results of operations and financial
condition.
While it is the Group’s policy to comply with all legal and regulatory requirements applicable to
the Group’s business, there can be no guarantee that the Group will always achieve full
compliance and a finding that the Group is in violation of, or out of compliance with, applicable
laws or regulations could subject the Group to civil remedies, including fines, damages,
injunctions or product recalls, or criminal sanctions, any of which could materially and
adversely affect the Group’s business, results of operations and financial condition. Even if a
claim is unsuccessful, is without merit or is not fully pursued, the cost of responding to such a
claim, including management time and out-of-pocket expenses, and the negative publicity
surrounding such assertions regarding the Group’s products, processes or business practices
could materially and adversely affect the Group’s reputation, brand image and the Group’s
business, prospects, results of operations and financial condition.
2.2 The Group faces risks relating to the regulation and perception of the ingredients it uses
in its products, which could materially and adversely impact the Group’s business,
prospects, financial condition and results of operations
Regulatory bodies and consumer groups may, from time to time, request or conduct reviews of
the use of certain ingredients that are used in manufacturing the Group’s products, the results
of which may have a material adverse effect on the Group’s business as the Group may need
to reformulate its products. For example, certain materials in consumer products are under
scrutiny in the EU, such as Titanium Dioxide, Synthetic Amorphous Silica and the potential, in
medicines, for Nitrosamine formation. If the result of such reviews is an inability to use or
restrictions on the use of certain ingredients and/or any requirement for remedial action, the
Group may incur significant additional costs and/or need to invest substantial resources to
make formulation adjustments to its products. Additionally, the Group may be adversely
affected by the findings and any remedial actions resulting from the EU’s ongoing
investigations into the impact of pharmaceuticals in the environment, such as the levels of
diclofenac measured in water in the EU.
While the Group monitors and seeks to respond to and address the impact of any emerging
regulatory and legislative developments, new or more stringent ingredient legislation could
have a negative impact on the Group’s business, undermine the Group’s reputation and
goodwill and affect consumer demand or trade customer demand for products containing such
ingredients. If the Group voluntarily removes, or is required to remove, certain ingredients from
its products, it may not be able to develop an alternative formulation, successfully modify its
existing products or obtain necessary regulatory approvals on a timely basis, or at all, which
could materially and adversely impact the Group’s business, prospects, financial condition and
results of operations.
2.3 The Group’s business is subject to market fluctuations and general economic
conditions, including inflationary pressures, each of which may materially and
adversely affect the Group’s business, financial condition, results of operations and
prospects
Uncertainty, fluctuations or negative trends in the international economic climate have had and
could continue to have a material adverse effect on the Group’s business and profitability.
There will be market fluctuations and economic factors that will be beyond the Group’s control,
but that will have the potential to materially and adversely affect its business, revenue, financial
condition and operating results.
Such factors include: (i) inflation or deflation; (ii) changes in government, fiscal and monetary
policies; (iii) changes in the financial standing of the Group’s customers, suppliers and
consumers, including levels of employment, real disposable income, salaries and wage rates;
(iv) consumer confidence and consumer perception of economic conditions; (v) retailers’
perception of consumer spending habits; (vi) technological change; (vii) exposure to possibly
adverse governmental or regulatory actions in countries where the Group operates or conducts
32
business; (viii) levels of volatility in global markets; (ix) exposure to the effects of economic
sanctions or other restrictive economic measures as a result of the Group’s global presence;
and (x) any change or development in global, national or regional economic and political
conditions.
For example, the Group is exposed to inflationary pressures and commodity prices, which
generally affect the Group through their impact on payroll and supply costs (including freight).
Inflationary pressures in FY 2021 increased the Group’s commodity, freight and payroll costs,
which had an adverse impact on the Group’s operating profit and operating profit margin.
Whilst the Group may increase product prices in order to mitigate the impact of inflation,
competitive pressures may constrain the Group’s ability to fully recover any increased costs in
this way, and so the Group may remain subject to market risk with respect to inflationary
pressures and increases in commodity prices. In addition, the Group’s initiatives to offset
headwinds from inflation in input prices and commodities, including forward buying, value
engineering and alternative supply arrangements, may not be sufficient to mitigate these risks.
Whilst the Group’s diversified geographic presence, product offering and consumer profile may
help to mitigate its exposure to risks that are localised or product- or consumer group-specific,
there can be no assurance that these risks would arise in such a way. The occurrence of any
of these risks could materially and adversely affect the business, revenue, financial condition
and operating results of the Group.
2.4 Litigation, disputes and regulatory investigations may materially and adversely affect
the Group’s business, financial condition, results of operations and prospects
The Group is, and may in the future be, subject to legal proceedings, disputes and regulatory
and governmental investigations in various contexts, including consumer fraud actions,
competitor and regulatory challenges to product and marketing claims, competition law
investigations, product liability and quality claims, human resources claims, contractual
disputes and other disputes or claims arising in the ordinary course of its business operations.
These legal actions, disputes and investigations may relate to aspects of the Group’s
businesses and operations that are specific to the Group, or that are common to companies
that operate in the Group’s markets, and this risk may be enhanced in circumstances where
the Group is operating in new markets. Legal actions and disputes may arise under contracts,
regulations or from a course of conduct taken by the Group, and may be class actions.
For example, in the USA, the Group is a defendant in ongoing proton pump inhibitor (“PPI”)
litigation, in which plaintiffs have alleged that their use of PPIs caused serious bodily injuries.
The Group has filed motions to dismiss several hundred cases, but the court has not yet ruled
on those motions. In addition, certain members of the GSK Group and the Pfizer Group are
party to proceedings relating to the detection of N-Nitroso-dimethylamine in Zantac (ranitidine)
products. Pursuant to the Pfizer SAPA, the Group has certain indemnification obligations to the
GSK Group and the Pfizer Group in respect of “Purchaser Liabilities” and “Assumed Liabilities”
(each as defined in the Pfizer SAPA), which may include liabilities related to OTC Zantac (see
paragraph 3.5 of Risk Factors below). Further, in 2013, GlaxoSmithKline Consumer Healthcare
GmbH & Co. KG and other members of a German trade mark association were fined by the
Federal Cartel Office of Germany, as a result of the exchange of certain information during
meetings from 2004 to 2006. Following the fine, the Group has become party to several civil
proceedings in Germany for follow-on damages. An adverse outcome in such proceedings (or
any other related proceedings) may have a material adverse effect on the Group’s business,
reputation, results of operations and financial condition.
Although the Group has developed and implemented a set of standards, controls, and policies
and procedures that are highly tailored to the specific requirements of the Group and the
regulatory regimes of the jurisdictions in which it operates, there is no guarantee that those
standards, controls, and policies and procedures will totally shield the Group from liability, and
the Group remains exposed to the risk of potential civil and/or criminal actions leading to
damages, fines and sanctions. For example, the risk of consumer fraud class actions,
competitor, regulatory and governmental challenges to product and marketing claims, and
product liability lawsuits remains significant. Governmental agencies such as the FTC are very
33
active in oversight of consumer products as they seek to prevent consumer fraud. The FTC
may have changing enforcement priorities in this area, for example, the use of expert
endorsements/testimonials, COVID-19-related marketing claims, all-natural marketing claims
and environmental marketing claims. Consumer fraud actions, and competitor, regulatory and
governmental challenges to product and marketing claims, and class-action lawsuits affecting
the Group have the potential to do significant damage to the Group’s reputation and materially
and adversely affect the results of its operations and financial condition.
Given the large or indeterminate amounts of damages sometimes sought by claimants, other
sanctions that might be imposed (including the Group no longer being able to use key claims)
and the inherent unpredictability of litigation and disputes, it is possible that an adverse
outcome to any litigation, dispute, government or regulatory investigation could have a material
adverse effect on the Group’s business, financial condition, results of operations and
prospects. At 31 December 2021, the Group had £14 million of provisions for legal disputes
and other matters, including amounts relating to legal and administrative proceedings.
2.5 The Group faces risks associated with significant international operations, which could
negatively impact the Group’s business
The Group operates on a global basis with 96.6 per cent. of the Group’s revenue in FY 2021
originating in markets outside the UK. While geographic diversity helps to reduce the Group’s
exposure to risks in any one country or part of the world, it also means that the Group faces
risks associated with significant international operations, including, but not limited to:
• changes in exchange rates for foreign currencies (as set out in more detail in paragraph
2.10 of Risk Factors below);
• exchange controls, export controls, economic sanctions and other limits on the Group’s
ability to import or export raw materials or finished products, including as a result of the
COVID-19 pandemic, or to repatriate earnings from overseas;
• political or economic instability (on which see also paragraph 2.7 of Risk Factors below),
geopolitical events (such as environmental events), widespread health emergencies (such
as the COVID-19 pandemic or other pandemics or epidemics), natural disasters or social
or labour unrest;
• rising geopolitical trade tensions in the Group’s key markets, such as between the USA,
Western Europe and China;
• changing macroeconomic conditions in the Group’s markets;
• lack of well-established, reliable and/or impartial legal systems in certain countries where
the Group operates and difficulties in enforcing contractual, intellectual property or other
legal rights;
• foreign ownership and investment restrictions and the potential for nationalisation or
expropriation of property or other resources;
• changes to trade policies and agreements and other foreign or domestic legal and
regulatory requirements, including those resulting in potentially adverse tax consequences
or the imposition of and/or the increase in onerous trade restrictions, tariffs and/or price
controls (including requirements to exclusively utilise local manufacturing); and
• changes to labour laws, travel or immigration restrictions, including as a result of the
COVID-19 pandemic or other pandemics or epidemics.
Any or all of the foregoing risks could have a significant impact on the Group’s ability to sell its
products on a competitive basis in international markets and may materially and adversely
34
affect its business, prospects, results of operations and financial condition. In addition, a
number of these risks may adversely impact consumer confidence and consumption, which
could reduce sales volumes of the Group’s products or result in a shift in its product mix from
higher margin to lower margin product offerings.
2.6 Volatility in material and other costs could materially and adversely impact the Group’s
profitability
Increases in the costs of and/or a reduction in the availability of materials, including active
pharmaceutical ingredients and excipients and raw and packaging material commodities, as
well as labour, energy, logistics and other necessary services, such as those seen during the
COVID-19 pandemic, may adversely affect the Group’s profit margins. If material and other
cost increases continue in the future and the Group is unable to pass along such higher costs
in the form of price increases, achieve cost efficiencies, such as in manufacturing and
distribution, or otherwise manage the exposure through sourcing strategies, ongoing
productivity initiatives and the potential use of commodity hedging contracts, the Group’s
business, results of operations and financial condition could be materially and adversely
impacted. In addition, even if the Group were able to increase the prices of its products in
response to material and other cost increases, the Group may not be able to sustain the price
increases. Also, sustained price increases may lead to declines in sales volumes as
competitors may not adjust their prices or consumers may decide not to pay higher prices,
which could lead to sales declines and loss of market share and could materially and adversely
affect the Group’s business, results of operations and financial condition.
2.7 The Group’s business may be impacted by the effects of Russia’s invasion of Ukraine
The Group is monitoring the effects of Russia’s invasion of Ukraine, with the GSK Board
overseeing and monitoring key risks. The Board will assume oversight and management of
these risks after Separation. The Group’s operations and presence in Russia and Ukraine is
limited and these markets accounted for less than 3 per cent. of each of the Group’s revenue
and Adjusted operating profit in FY 2021. However, the broader economic consequences of
the invasion are currently difficult to predict, and geopolitical instability, the imposition of
sanctions and other restrictive measures against Russia and any retaliatory actions taken by
Russia in response to such measures could adversely affect the global markets and the global
geopolitical and economic environment, which could in turn adversely impact the Group’s
business and/or the trading prices of its securities. Specifically, the Group faces the following
risks:
• The Group’s business includes employees based in Russia and Ukraine and revenue
deriving from sales in Russia and Ukraine. Although the Group does not consider that its
business in Russia and Ukraine constitutes a material part of the Group’s business, the
situation remains highly uncertain and the Group is actively monitoring the situation, the
risks to its employees and the significant risk of disruption to its operations, including in
relation to the importation and distribution of its products, in Russia and Ukraine and other
countries in the region.
• The Group generates revenue from sales of its products in Russia in the Russian Ruble,
while significant costs (notably, manufacturing and supply chain costs) associated with
those products are denominated in other currencies, such as Euro and US Dollar. The
international response to the invasion, including the imposition of international sanctions
against Russia, has had a significant adverse effect on the value of the Russian Ruble,
which has reduced the Group’s revenue from its operations in Russia without a
corresponding reduction in costs, and the Group may not be able to offset the devaluation
of the Russian Ruble through increased prices of its products. In addition, the imposition of
exchange controls may limit the Group’s ability to repatriate profits from its operations in
Russia.
• The Group’s customers in Russia and Ukraine have been significantly negatively affected
by the factors described above, which exposes the Group to increased counterparty risk in
relation to these customers and receivables from these customers.
35
• Given the Group’s international presence, it is subject to various global sanctions regimes,
and similar laws, regulations or orders imposed in response to the invasion, many of which
are evolving rapidly. The Group is monitoring changes to applicable global sanctions
regimes to ensure it remains in compliance with its obligations, as any failure to comply
with the evolving sanctions could present legal and reputational risks, which could, in turn,
have a material and adverse effect on the Group’s business. In addition, there is a risk that
Russia’s response to the global sanctions regime, as well as additional international
sanctions against Russia, creates regulatory uncertainty and presents further compliance
challenges for the Group’s operations, which will increase compliance costs and make it
difficult to continue operations in Russia.
• There may be certain reputational risks associated with the Group’s continued presence in
the Russian market. Negative publicity surrounding the Group’s continued presence and/or
supply of products to the general public in Russia could damage the Group’s brands and
its reputation, lead to boycotts of its products (outside of Russia) and/or have
consequences on the continuation of operations and/or sales in Russia, including a
determination by the Group to discontinue all sales in Russia. For further details on similar
risks facing the Group, please also see paragraph 1.7 of Risk Factors.
• As of the date of this Prospectus, the Russian government has indicated it has drawn up
plans to seize the assets of western companies leaving Russia. While the scope of such
measures is not presently clear, if the Group ceased its activities and/or suspended its
operations in Russia and did not resume its presence in Russia within a certain period of
time, there is a risk the Russian government could: (i) nationalise the Group’s assets
located in Russia; (ii) allow the Group’s patents and trade marks to be used within Russia
without the Group’s consent; and/or (iii) introduce restrictions on, or impose unfavourable
terms in respect of, payments made from Russia or relating to assets in Russia.
In addition to the specific implications for the Group’s operations in Russia and Ukraine, the
Group may be affected by broader impacts on the global geopolitical and economic
environment, including (but not limited to) changes in commodity, freight, logistics and input
costs.
The situation remains highly uncertain and there may be additional risks to the Group arising
out of or relating to the Russian invasion of Ukraine, and the escalating military conflict in the
region, which could also have a material and adverse impact on the Group’s business.
2.8 Failure to comply with regulation regarding the use of personal data could lead to
significant fines and regulatory action against the Group
The Group is subject to regulations in the jurisdictions in which it operates regarding the use of
personal data. The Group collects and processes personal data from its consumers,
customers, business contacts and employees as part of the operation of its business, and
therefore it must comply with data protection and privacy laws. Those laws generally impose
certain requirements on the Group in respect of the collection, retention, use and processing of
such personal information. Notwithstanding its efforts, the Group is exposed to the risk that this
data could be wrongfully appropriated, lost, disclosed, retained, stolen or processed in breach
of data protection laws. In addition, increased regulatory restrictions on the use of cookies may
materially and adversely affect the Group’s marketing practices as well as the cost efficiency of
such strategies. Failure to operate effective data collection controls could potentially lead to
regulatory censure, fines, reputational and financial costs.
The EU GDPR and the UK GDPR, as well as the increased data protection regulation in other
jurisdictions, such as the Personal Information Protection Law 2021, Cybersecurity Law 2016
and Data Security Law 2021 in China (each of which has a significant impact on the processing
of data in China), the Federal Law No. 152-FZ on Personal Data in Russia, and the California
Consumer Privacy Act of 2018 in California, USA, introduced the potential for significant new
levels of fines for non-compliance based on turnover. The Group will continue to review and
develop existing processes to ensure that customer personal data is processed in compliance
36
with applicable requirements, to the extent that they are applicable, and it may be required to
expend significant capital or other resources and/or modify its operations to meet such
requirements, any or a combination of which could have a material adverse effect on the
Group’s business, financial condition and financial results, or otherwise harm its reputation.
2.9 Failure to comply, or the costs of complying, with environmental and health and safety
regulations could materially and adversely affect the Group’s operations
The Group is subject to regulation relating to the protection of the environment and health and
safety, including regulations governing air emission, effluent discharge, and the use,
generation, manufacture, storage, handling and disposal of certain materials. The Directors
believe that it is in compliance in all material respects with all such laws, rules, regulations and
policies applicable to the Group. However, there can be no assurance that the Group will not
be required to incur significant costs to comply with such environmental and health and safety
laws and regulations in the future. Additionally, failure to manage environmental, health and
safety and sustainability risks could lead to significant harm to people, the environment and
communities in which the Group operates, fines, failure to meet stakeholder expectations and
regulatory requirements, litigation or regulatory action and damage to the Group’s reputation
and could materially and adversely affect the Group’s financial results. Additionally, working
conditions in global supply chains are subject to increased scrutiny and growing regulatory and
legislative requirements, including for companies to evidence their human rights due diligence
assessments. Failure to comply with such requirements could result in sanctions, including
injunctions, fines, civil liability and exclusion from public procurement being imposed on the
Group.
In addition, most product, component and raw material supply chains present a number of
potential reputational risks relating to: labour standards; health, safety and environmental
standards; raw material sourcing; and the social, ethical and environmental performance of
third party manufacturers and other suppliers. The Group mandates minimum requirements
regarding these issues, in line with international guidelines, for the Group’s own manufacturing
sites, third party manufacturers and suppliers. If it is perceived that the Group is not respecting
or advancing the economic and social progress and safety of the local communities it works in,
the Group’s reputation could be damaged, which could have a negative impact on the Group’s
‘social licence to operate’, the Group’s ability to secure new resources and labour and the
Group’s financial performance.
2.10 The Group is exposed to risks relating to fluctuations in currency exchange rates and
related hedging activities, which could negatively impact the Group’s financial condition
and prospects
As further described in paragraph 2.5 of Risk Factors above, the Group operates internationally
and holds assets, incurs liabilities, generates sales and pays expenses in a variety of
currencies other than Pounds Sterling (the currency in which it reports its financial results). The
Group’s operations outside the UK generated 96.6 per cent. of revenue in FY 2021.
Fluctuations in exchange rates for foreign currencies have reduced and could continue to
reduce the Pounds Sterling value of sales, earnings and cash flows the Group receives from
markets outside the UK, increase its supply costs (as measured in Pounds Sterling) in those
markets, negatively impact its competitiveness in those markets or otherwise materially and
adversely impact its business or financial condition. The Group’s foreign currency exposure will
be greater for so long as the leverage levels of the Group are higher than are reflected in the
Group’s longer-term strategy, the success of which cannot be guaranteed. The Group aims to
manage this risk through hedging where possible and practical; however, there are risks
associated with the use of hedging instruments (including derivative financial instruments).
While limiting to some degree the Group’s risk from fluctuations in currency exchange, such
hedging activities may be ineffective or may not offset more than a portion of the adverse
financial effect resulting from variations to such rates. The Group is also exposed to
counterparty credit (or repayment) risk in respect of counterparties to hedging contracts.
37
To the extent any hedging activities of the Group are wholly or partially ineffective, or to the
extent a hedging counterparty fails to meet its obligations under any hedging agreement, this
could result in losses which could have a material adverse effect on the Group’s business,
results of operations and financial condition.
2.11 Determinations made by the Group with respect to the application of tax law may result
in challenges from or disputes with tax authorities which result in the payment of
additional amounts for tax
The Group has a significant exposure to business operations which are subject to taxation
across multiple jurisdictions. The worldwide nature of the Group’s operations means that
intellectual property, R&D and manufacturing operations are centred in a number of locations.
A consequence of this is that the Group’s cross-border supply routes, which are necessary to
ensure supplies of healthcare products into numerous end markets, can be subject to complex
tax laws and can result in conflicting claims from tax authorities as to the profits to be taxed in
individual countries. Additionally, the Group is subject to many different forms of taxation within
any given jurisdiction in which it operates (including, but not limited to, corporate income taxes,
capital gains taxes on direct or indirect transfers of ownership, stamp duty and similar transfer
taxes, value added taxes, property taxes and social security and other payroll taxes) and many
tax regimes - domestically as well as cross-border - are increasingly complex (such that the
proper interpretation and application of tax laws is not always clear). This means that the
Group may be subject to domestic and cross-border tax authority disputes (potentially including
disputes between tax authorities), including with respect to the actions taken, or to be taken, in
connection with Separation, which could result in the payment of additional amounts of tax.
Such potential disputes and the resulting payment obligations could have a material and
adverse effect on the Group’s business, results of operations and financial condition. At
31 December 2021, the Group had recognised provisions of £150 million in respect of
uncertain tax positions.
2.12 The Company’s status as a non-US corporation for US federal income tax purposes
could be affected by a potential change in law
Corporations such as the Company that are organised outside the USA are generally treated
as non-US corporations for US federal income tax purposes. However, section 7874 of the
Code and the Treasury regulations thereunder can cause a corporation organised outside the
United States to be treated as a US corporation for US federal income tax purposes if: (i) the
corporation (the “Acquiring Non-US Corporation”) directly or indirectly acquires substantially all
of the properties of a US corporation (the “Acquired US Corporation”); (ii) the shareholders of
the Acquired US Corporation are treated as holding at least 80 per cent. of the shares of the
Acquiring Non-US Corporation after the acquisition by reason of holding shares in the Acquired
US Corporation (adjusting, for this purpose, for certain transactions such as certain
contributions and distributions and for certain fact patterns); and (iii) certain other requirements
are met.
A corporation that is treated as a US corporation as a result of the application of these rules
generally is subject to US federal income tax on its worldwide income, and dividends it pays to
shareholders that are not US Holders are subject to US withholding taxes, among other
adverse consequences. Because such a corporation would be a dual resident for tax purposes,
these taxes may apply in addition to (and not instead of) the taxes imposed by the jurisdiction
in which such corporation is otherwise resident, and there may be other adverse tax
consequences of being dual resident for tax purposes (such as restrictions on use of certain
reliefs). In addition, even if the Acquiring Non-US Corporation is not treated as a US
corporation under the test described above, in certain circumstances section 7874 can instead
cause the Acquiring Non-US Corporation to be subject to different adverse US federal income
tax consequences (including the unavailability of the preferential rate applicable to “qualified
dividends”).
The rules for determining whether a transaction is subject to section 7874 are complex and
subject to varying interpretations and potential legislative and regulatory changes. The
38
Company believes that under current law the Company should be treated as a non-US
corporation (and should not be subject to the other adverse consequences of section 7874 as
described above). However, several proposals to significantly expand the scope of section
7874 have been advanced over the years, including by the Biden administration and most
recently in December 2021 as part of the US Senate’s consideration of the Build Back Better
Act, which was not enacted into law. Accordingly, it is possible that such a proposal will be
enacted (possibly with retroactive effect), and there can be no assurance that section 7874 and
the Treasury regulations thereunder will not be amended in a way that could cause the
Company, as a result of Separation, either to be treated as a US corporation or to be subject to
the other adverse consequences of section 7874 as described above.
2.13 If the Company loses its foreign private issuer status in the USA in the future, it may
incur significant additional expenses which could have a material adverse effect on the
Group’s business, prospects, results of operations and financial condition
The Company is a “foreign private issuer” in the USA, as such term is defined under the US
Exchange Act, and, therefore, the Company is not required to comply with all the periodic
disclosure and current reporting requirements of the US Exchange Act and related rules and
regulations. Under the US Exchange Act, the determination of foreign private issuer status is
made annually on the last business day of an issuer’s most recently completed second fiscal
quarter and, accordingly, the next determination will be made with respect to the Company on
30 June 2022.
In the future, the Company would lose its foreign private issuer status if a majority of its shares
are owned by US residents and: (i) a majority of its directors or executive officers are US
citizens or residents; (ii) more than 50 per cent. of its assets are located in the USA; or (iii) its
business is administered principally in the USA. As of 31 December 2021, 37 per cent. of the
Group’s assets were located in the USA. The regulatory and compliance costs to the Company
under US securities laws as a US domestic issuer may be significantly more than costs the
Company incurs as a foreign private issuer. If the Company is not a foreign private issuer, it
would be required to file periodic reports and registration statements on US domestic issuer
forms with the Securities and Exchange Commission (the “SEC”), which are more detailed and
extensive in certain respects than the forms available to a foreign private issuer. The Company
would also have to mandatorily comply with US federal proxy requirements, and its executive
officers, directors and principal shareholders would become subject to the short-swing profit
disclosure and recovery provisions of Section 16 of the US Exchange Act. Further, the
Company would be required under current SEC rules to prepare its financial statements in
accordance with US generally accepted accounting principles and modify certain of its policies
to comply with corporate governance practices associated with US domestic issuers. In
addition, the Company may lose its ability to rely upon exemptions from certain corporate
governance requirements on US stock exchanges that are available to foreign private issuers.
Such transition and modifications would involve additional costs and may divert management’s
attention from other business concerns, which could have a material adverse effect on the
Company’s business, financial condition and results of operations.
3. RISKS RELATING TO THE DEMERGER AND SEPARATION
3.1 The Group may fail to realise any or all of the anticipated benefits of the Demerger and
Separation
The extent to which the anticipated benefits of the Demerger and Separation, including, among
others, the creation of a standalone public company with a leadership team with independent
control of its strategy and capital allocation decisions and the maximisation of shareholder
value, may be realised, is subject to a number of factors, including many which are outside of
the Group’s control. There can be no guarantee that the anticipated benefits of the Demerger
and Separation will be realised in full or in part, or as to the timing when any such benefits may
be realised. Failure to realise the anticipated benefits of the Demerger and Separation, in full or
in part, or in a timely manner, could result in a delay in the execution of the strategic objectives
of the Group and/or have a disruptive effect on the Group’s management and employees. This
39
could in turn have a material adverse effect on the Group’s business, results of operations,
financial condition and prospects.
3.2 The Company will incur new costs in its transition to a standalone public company and
its management team will be required to devote substantial time to new compliance
matters
As a standalone public company, the Company will incur additional legal, accounting, financing
and other expenses, including the costs of recruiting and retaining non-executive directors,
costs resulting from public company reporting obligations and the rules and regulations
regarding corporate governance practices, including the listing requirements of the LSE and
the NYSE. There can be no assurance that, under a changed Board structure and ownership,
and in an environment where it is subject to greater scrutiny and disclosure requirements, the
Group will be able to manage its operations in the same manner as it has done as part of the
GSK Group (see also paragraph 3.3 of Risk Factors below).
In particular, the Group will be subject to increased regulatory obligations as a result of being
listed, and its management team will need to devote a substantial amount of time to ensure
that the Group complies with all of these requirements. The implementation of new policies and
procedures across the Group could require significant time and energy that would otherwise be
devoted to the business’ operating activities and strategy. In addition, the reporting
requirements, rules and regulations will increase the Group’s legal and financial compliance
costs and make some activities more time-consuming and costly.
The Sarbanes-Oxley Act of 2002 (“Sarbanes-Oxley”), as well as regulations subsequently
adopted by the SEC and the NYSE, have imposed various requirements on public companies,
including rules regarding corporate governance practices. Sarbanes-Oxley requires, among
other things, that the Group maintain and periodically evaluate its internal controls over
financial reporting and disclosure controls and procedures. The Group and its management
team will have to perform system and process evaluation and testing of the Group’s internal
controls over financial reporting to allow management and the Group’s reporting accountants to
report on the effectiveness of the Group’s internal controls over financial reporting, as required
by section 404 of Sarbanes-Oxley.
The Group currently tests its internal controls over financial reporting on a regular basis, in
accordance with the financial reporting practices and policies of the GSK Group. However,
doing so as a standalone entity may require the Group’s management team and other
employees to devote a substantial amount of time to comply with these requirements and also
increase the Group’s legal and financial compliance costs. In particular, compliance with
section 404 of Sarbanes-Oxley after Separation will require additional expenses and
management efforts.
3.3 Following the Demerger and Separation, the Company will need to operate as an
independent publicly listed company and the Group could fail to meet the challenges
involved in operating successfully as a standalone business
Following the Demerger and Separation, the Company will need to operate as an independent
publicly listed company.
The Group’s operations have historically benefited from certain GSK central office resources,
including, among other things, access to its larger finance and treasury, corporate secretariat,
legal, procurement, information technology, investor relations and human resources teams.
The Group has also benefited from negotiated arrangements with third-party suppliers,
distributors, licensors, lessors, other business partners and/or counterparties as part of the
larger GSK Group. It cannot be assured that the Group will be able to maintain such
arrangements or replace them on similar terms.
Following the Demerger and Separation, the Group will take on additional responsibility for
these activities and, in preparation, it has enhanced its standalone arrangements in a wide
40
range of areas, including finance and treasury, corporate secretariat and investor relations.
Further, the Group will continue to have access to certain resources of the GSK Group under
the terms of the Transition Services Agreement (see paragraph 3.4 of Risk Factors below).
However, there remains a risk that the Group could suffer operational difficulties without
access to the support and services from GSK following the Demerger and Separation, which
could have a material adverse effect on the Group’s business. These challenges include:
(i) demonstrating to interested parties that the Demerger and Separation will not result in
adverse changes in standards of business and impairment of relationships with consumers,
customers, regulators or employees; (ii) retaining key personnel; (iii) distraction of
management; (iv) difficulty in marketing and communicating effectively the capabilities of the
Group as a standalone business; and (v) successfully negotiating the rebranding exercise such
that consumers accept the new branding under the Company name. Furthermore, there
remains a risk that operating as an independent group may reduce the Group’s flexibility to
deal with unexpected events and require additional resources.
In addition, there is a risk that the actual costs of the standalone arrangements could be higher
than expected, that there could be unanticipated dis-synergies and/or that the Group will need
to further invest in new services and functions. These risks, individually or together, could have
a material adverse effect on the Group’s business, financial condition, results of operations and
prospects.
3.4 For a period following the Demerger and Separation, the Company will be reliant on the
GSK Group for the provision of certain services and any disruption to such services
could be costly and materially and adversely affect the Group’s business, results of
operations, financial conditions and prospects
In connection with the Demerger and Separation, GSK and the Company entered into a
Transition Services Agreement. Services to be procured by the Group under the Transition
Services Agreement include certain information services, back office services and distribution
services for a transitional period as required by the Group. The majority of services will be
provided for a fixed period of not more than 12 months, and certain services may be extended
subject to certain conditions. As the Group does not currently have the capabilities to provide
these services internally, on a standalone basis, without third-party support, the Transition
Services Agreement provides contractual protections for the continued provision of these
services during the relevant transitional period, absent which the Group would need to procure
these services from other third-party providers. As a result, any significant disruption or other
issues in the services provided by the GSK Group under the Transition Services Agreement,
even if they give rise to a contractual claim, may cause operational difficulties that could
negatively impact the Group’s performance and results of operations.
Following the transitional periods set out in the Transition Services Agreement, the Group will
be required to provide these services internally or obtain these services from a third-party
provider. If the Group does not effectively develop and implement these capabilities, or it is
unable to source further arrangements from third-party providers, its business, results of
operations, financial condition and prospects could be materially and adversely affected.
3.5 The Group has indemnification obligations in favour of the GSK Group and the Pfizer
Group, which could be significant and have a material adverse effect on the financial
condition, results of operations and/or prospects of the Group
GSK, Pfizer and CH JVCo, entered into the Pfizer SAPA on 19 December 2018 pursuant to
which GSK, Pfizer and CH JVCo agreed to form a new global consumer healthcare joint
venture. The Pfizer SAPA, as amended from time to time, including by the Pfizer SAPA
Amendment Agreement, contains certain cross indemnities among the GSK Group, the Pfizer
Group and the Group. Among other provisions, CH JVCo is required to indemnify the GSK
Group and the Pfizer Group in respect of “Purchaser Liabilities” and “Assumed Liabilities”. The
Company is also required to guarantee such indemnity obligations of CH JVCo which may
include liabilities related to OTC Zantac. Certain members of the GSK Group and the Pfizer
41
Group are party to certain proceedings relating to the detection of N-Nitroso-dimethylamine in
Zantac (ranitidine) products. While Pfizer and GSK have each served the Group with notice of
potential claims under the relevant indemnification provisions in the Pfizer SAPA in relation to
possible liabilities connected with OTC Zantac, it is not possible, at this stage, to meaningfully
assess or to quantify or reliably estimate what liability (if any) that the Group may have to the
GSK Group and/or the Pfizer Group under the relevant indemnities.
Pursuant to certain other agreements entered into between the GSK Group and the Group in
connection with Separation, including the Asset Transfer Framework Agreement, the GSK
Group and the Group have provided certain cross indemnities in relation to certain businesses,
assets, liabilities and employees transferring from the GSK Group to the Group, as well as from
the Group to the GSK Group. For example, these include certain manufacturing sites in
Argentina and Brazil to be transferred from the GSK Group to the Group following Separation.
Among other requirements, CH JVCo is required to indemnify GSK in respect of losses
resulting from or arising out of past, present or future ownership, operation, use or conduct of
certain aspects of such assets and/or businesses transferring from the GSK Group to the
Group.
In addition, on or around the date of this Prospectus, GSK, Pfizer, the Company, CH JVCo and
GSKCHH entered into the Tax Covenant, which is to be effective from the time of the
Demerger. The Tax Covenant contains certain indemnities (subject to certain financial and
other limitations) in respect of taxation given from GSK and Pfizer to the Company (and vice
versa).
Such indemnities will survive completion of the Demerger and Separation. If any amounts
payable by the Group under the indemnities (or additional taxes imposed on the Group that are
not indemnified by GSK and/or Pfizer under the Tax Covenant) are substantial, this could have
a material adverse effect on the financial condition, results of operations and/or prospects of
the Group.
3.6 The Tax Covenant will restrict the Company’s ability to engage in certain transactions
As discussed above, the Company entered into the Tax Covenant on or around the date of this
Prospectus, which is to be effective from the time of the Demerger. The Tax Covenant imposes
certain restrictions on the Company, including certain restrictions with respect to actions
following completion of the Demerger that could cause Separation to fail to qualify for its
intended US federal income tax treatment. The restrictions primarily require the Company to
maintain the corporate structure of certain parts of the Group as it was immediately prior to the
Demerger. For example, there are restrictions on liquidating certain subsidiaries of the
Company, or issuing or redeeming shares in those subsidiaries. In addition, there are
restrictions on some intra-group disposals as well as certain non-ordinary course of business
transactions. As a result of these restrictions (some of which could be in place for at least two
years), the Company’s ability to engage in certain transactions, such as the disposition of
certain assets and certain repurchases of its stock, may be limited (although the Group will
nonetheless be entitled to take actions which would otherwise be restricted if the Company first
(i) obtains the consent of (or, in certain instances, if it consults with) GSK or Pfizer (as
applicable) or, in some cases, (ii) obtains an opinion from an appropriately qualified adviser or
a ruling from the IRS regarding the tax consequences of the proposed actions which, in either
case, is reasonably satisfactory to GSK or Pfizer (as applicable)). Although the Company does
not currently anticipate that these restrictions would have a material adverse impact on the
Company, these restrictions may reduce the Company’s ability to engage in certain business
transactions that otherwise might be advantageous.
3.7 GSK and Pfizer may compete with the Group
GSK and Pfizer will not be restricted from competing with the Group in the consumer
healthcare business, including as a result of acquiring a company that operates a consumer
healthcare business. Due to the significant resources of GSK and Pfizer, including brand
recognition, financial resources and know-how resulting from the previous management of the
42
Group’s business, GSK and Pfizer could have a significant competitive advantage over the
Group should they decide to engage in the type of business the Group conducts, which may
materially and adversely affect the Group’s business, results of operations and financial
condition.
4. RISKS RELATING TO THE HALEON SHARES
4.1 There is no existing market for the Haleon Shares and an active trading market for the
Haleon Shares may not develop or be sustained
Prior to Admission, there has been no public trading market for the Haleon Shares.
Although the Company intends to apply to the FCA for admission to the premium listing
segment of the Official List and intends to apply t o the LSE for admission to trading on its
main market for listed securities, the Company can give no assurance that an active
trading market for the Haleon Shares will develop or, if developed, could be sustained
following the closing of the Demerger and Separation. If an active trading market is not
developed or maintained, the liquidity and trading price of the Haleon Shares could be
materially and adversely affected.
4.2 The Pfizer Group will retain a significant interest in the Company following Admission
and its interests may differ from those of the other Haleon Shareholders
The Pfizer Group will retain a significant interest in the Company following Admission, including
32 per cent. of the Haleon Shares and thus of the voting rights of the Company. As a result, the
Pfizer Group will possess sufficient voting power to exercise significant influence over all
matters requiring shareholder approval, including the election or removal of directors and
advisers, the declaration of dividends, whether to accept the terms of a takeover offer and
other matters to be determined by the Haleon Shareholders.
In addition, the Pfizer Group has the right to nominate two persons to be appointed to the
Board as representative directors for so long as it continues to hold 20 per cent. or more of the
Haleon Shares in issue and a right to nominate one person to be appointed to the Board as a
representative director for so long as it continues to hold less than 20 per cent. but at least
10 per cent. of the Haleon Shares in issue. As at the date of this Prospectus, the Pfizer Group
has nominated Bryan Supran and John Young, who will become directors on Admission. In
exercising its voting rights, the Pfizer Group may be motivated by interests that differ from
those of the other Haleon Shareholders and the interests of the Pfizer Group could conflict with
or differ from the Company’s interests. The Company has entered into the Pfizer Relationship
Agreement to regulate its relationship with the Pfizer Group following Admission and, in
particular, to help ensure that the Company will be capable of operating and making decisions
for the benefit of Haleon Shareholders as a whole and independently of the Pfizer Group
following Admission. Notwithstanding the Pfizer Relationship Agreement, the concentration of
ownership in the Pfizer Group may have the effect of delaying, deferring or preventing a
change of control of the Company or impeding a merger, takeover or other business
combination which may otherwise be favourable for the Company or the Group. This in turn
could have a material adverse effect on the trading price of the Haleon Shares.
So long as the Pfizer Group continues to own, whether directly or indirectly, a significant
amount of the equity of the Company, the Pfizer Group will continue to be able to substantially
influence the Group’s ability to enter into any corporate transactions.
4.3 There can be no assurance that dividends will be paid on Haleon Shares
The Company may determine not to pay dividends. If it determines that it will pay dividends,
there can be no assurance that it will be able to pay dividends in the future. Under UK
company law, a company can only pay cash dividends to the extent that it has distributable
reserves and cash available for this purpose. As a holding company, the Company’s ability to
pay dividends in the future will be affected by a number of factors, including having sufficient
43
distributable reserves and its ability to receive sufficient dividends from subsidiaries. The ability
of companies within the Group to pay dividends and the Company’s ability to receive
distributions from its investments in other entities are subject to restrictions, including, but not
limited to, the existence of sufficient distributable reserves and cash. Any of the foregoing could
have a material adverse impact on the market price of the Haleon Shares.
4.4 The market price of the Haleon Shares may fluctuate
Haleon Shareholders should be aware that the value of an investment in the Group may
fluctuate and could be highly volatile. The price at which Haleon Shares may be quoted and
the price which investors may realise for their Haleon Shares will be influenced by a large
number of factors, some specific to the Group and its operations, and some which may affect
the Group’s industry as a whole, other comparable companies or publicly traded companies as
a whole.
The sentiments of the public market regarding the Demerger and Separation will be one such
factor. Following Admission of the Haleon Shares, there may be a period of relatively high-
volume trading in the Haleon Shares as the Company’s shareholder register finds its natural
composition. For example, the Haleon Shares may become less attractive to certain classes of
existing investors. The Company is unable to predict whether substantial amounts of the
Haleon Shares will be sold in the open market following Admission. Sales of a substantial
number of the Haleon Shares in the public market after Admission, or the perception that these
sales might occur, could depress the market price of the Haleon Shares. See also paragraph
4.5 of Risk Factors below.
This potential factor, together with other factors including actual or anticipated fluctuations in
the financial performance of the Group and its competitors, market fluctuations and/or factors
generally affecting consumers could lead to the market price of the Haleon Shares fluctuating.
4.5 Future sales of Haleon Shares, or the perception such sales might occur, could depress
the market price of the Haleon Shares
Following Admission, GSK will hold up to 6 per cent. of the Company’s issued share capital
and Pfizer will hold 32 per cent. of the Company’s share capital. Furthermore, as part of certain
arrangements pursuant to which GSK will provide additional support to GSK’s UK Pension
Schemes, the SLPs (being Scottish limited partnerships controlled by GSK and set up to
provide a funding mechanism pursuant to which GSK will provide additional funding for GSK’s
UK Pension Schemes) will in aggregate hold 7.5 per cent. of the total issued share capital of
the Company.
The Haleon Shares owned by GSK, Pfizer and the SLPs are subject to certain lock-up
restrictions. Following the expiration of the applicable lock-up period, or the waiver of such
lock-up restrictions, GSK, Pfizer and the SLPs will be able to sell their respective Haleon
Shares. During the period immediately prior to expiration of, and following the periods of sales
restrictions provided for by these lock-up arrangements, the market price for the Haleon
Shares may fall in anticipation of a sale of Haleon Shares. The perception that such sales
could occur may also materially and adversely affect the market price of the Haleon Shares.
This may make it more difficult for Haleon Shareholders to sell the Haleon Shares at a time
and price that they deem appropriate, and could also impede the Company’s ability to issue
equity securities in the future.
4.6 The Company may decide to offer additional Haleon Shares in the future, diluting the
interests of existing Haleon Shareholders and potentially materially and adversely
affecting the market price of Haleon Shares
Other than in connection with Admission or pursuant to employee share plans, the Company
has no current plans for an offer of shares. However, if the Company decides to offer additional
Haleon Shares or other securities convertible into Haleon Shares in the future, including as
44
consideration for any acquisitions, this could dilute the interests of existing Haleon
Shareholders and/or have an adverse impact on the market price of Haleon Shares as could
the public perception that an offering may occur.
4.7 Haleon Shareholders may not be able to exercise pre-emption rights or participate in
certain future issues of Haleon Shares and Overseas Shareholders may not be able to
participate in future issues of Haleon Shares
In the case of a future allotment of new Haleon Shares for cash, existing Haleon Shareholders
have certain statutory pre-emption rights, unless those rights are disapplied by a special
resolution of the Haleon Shareholders at a general meeting. An issue of new Haleon Shares
not for cash or when pre-emption rights have been disapplied could dilute the interests of the
then-existing Haleon Shareholders.
Securities laws of certain jurisdictions may restrict the Company’s ability to allow participation
by Haleon Shareholders in future offerings. In particular, shareholders in the USA may not be
entitled to exercise these rights, unless either the Haleon Shares and any other securities that
are offered and sold are registered under the US Securities Act, or the Haleon Shares and
such other securities are offered pursuant to an exemption from, or in a transaction not subject
to, the registration requirements of the US Securities Act. The Company cannot assure
prospective investors it will register any such offers or sales under the US Securities Act, that
any exemption from such overseas securities law requirements would be available to enable
US or other Haleon Shareholders to exercise their pre-emption rights or, if available, that the
Company will utilise any such exemption.
4.8 The ability of Overseas Shareholders to bring actions or enforce judgments against the
Company or the Directors may be limited
The ability of an Overseas Shareholder to bring an action against the Company may be limited
under law. The Company is a public limited company incorporated in England and Wales. The
rights of holders of the Haleon Shares are governed by English law and by the Articles of
Association. These rights differ from the rights of shareholders in typical US corporations and
some other non-UK companies. In particular, English law currently limits significantly the
circumstances under which the shareholders of English companies may bring derivative
actions. Under English law, in most cases, only the Company may be the proper plaintiff for the
purposes of maintaining proceedings in respect of wrongful acts committed against it and,
generally, neither an individual shareholder, nor any group of shareholders, has any right of
action in such circumstances. English law does not afford appraisal rights to dissenting
shareholders in the form typically available to shareholders in a US company. In addition, it
may not be possible for an Overseas Shareholder to enforce any judgments in civil or
commercial matters or any judgments in securities laws of countries other than the UK against
some or all of the Directors or executive officers of the Company who are resident in the UK or
countries other than those in which judgment is made.
4.9 Overseas Shareholders may be subject to exchange rate risk
The Haleon Shares are, and any dividends to be paid in respect of them will be, denominated
in Pounds Sterling. An investment in Haleon Shares by an investor whose principal currency is
not Pounds Sterling exposes the investor to foreign currency exchange rate risk. Any
depreciation of Pounds Sterling in relation to such foreign currency will reduce the value of the
investment in the Haleon Shares or any dividends in foreign currency terms.
45
PRESENTATION OF FINANCIAL AND OTHER INFORMATION
1. GENERAL
The contents of this Prospectus are not to be construed as legal, business or tax advice.
Recipients of this Prospectus should consult their own lawyer, financial adviser or tax adviser
for legal, financial or tax advice, as appropriate. Furthermore, the Company and the Directors
accept no responsibility for the accuracy or completeness of any information reported by the
press or other media, or the fairness or appropriateness of any forecasts, views or opinions
expressed by the press or other media regarding the Demerger and Separation, Admission,
GSK plc or the Group. The Company and the Directors make no representation as to the
appropriateness, accuracy, completeness or reliability of any such information or publication.
Unless otherwise stated, no representation or warranty, express or implied, is made and no
responsibility or liability is accepted by any person other than the Company and the Directors,
as to the accuracy, completeness, verification or sufficiency of, the information contained
herein, and nothing in this Prospectus is, or may be relied upon as, a promise or representation
by any of the Company’s advisers (including the Joint Sponsors) or any of their respective
affiliates in this respect, as to the past or future, and any of the Company’s advisers or their
respective affiliates, directors, officers, employees or advisers accordingly disclaim, to the
fullest extent permitted by applicable law, any and all liability whether arising in tort, contract or
otherwise which they might otherwise be found to have in respect of this document or any such
statement.
When considering what action you should take, you should seek your own independent
financial advice immediately from your stockbroker, bank manager, solicitor, accountant, fund
manager or other independent financial adviser authorised under FSMA if you are in the United
Kingdom or, if not, from another appropriately authorised independent financial adviser.
Without prejudice to any obligation of the Company to publish a supplementary prospectus
pursuant to section 87G of FSMA and PR 3.4.1 of the Prospectus Regulation Rules, neither
the publication of this Prospectus nor any distribution of Haleon Shares shall, under any
circumstances, create any implication that there has been no change in the business or affairs
of the Group taken as a whole since the date of this Prospectus or that the information
contained herein is correct as of any time subsequent to its date.
No person has been authorised to give any information or to make any representation in
connection with Admission other than the information and representations contained in this
Prospectus and, if any other information or representations is or are given or made, such
information or representations must not be relied upon as having been authorised by or on
behalf of the Company, the Directors or the Joint Sponsors.
Recipients of this Prospectus may not reproduce or distribute this Prospectus, in whole or in
part, and may not disclose any of the contents of this Prospectus or use any information herein
for any purpose other than considering Admission. Such recipients of this Prospectus agree to
the foregoing by accepting delivery of this Prospectus.
This Prospectus is not intended to provide the basis of any credit or other evaluation and
should not be considered as a recommendation by any of the Company, the Directors, any of
the Company’s advisers (including the Joint Sponsors) or any of their affiliates or
representatives regarding the securities of the Company.
This Prospectus has been approved by the FCA in accordance with section 87A of FSMA.
Admission to trading on the LSE’s main market for listed securities constitutes admission to
trading on a regulated market.
2. NO INCORPORATION OF WEBSITE INFORMATION
The contents of the Company’s website, any website mentioned in this Prospectus or any
website, directly or indirectly, linked to these websites have not been verified and do not form
part of this Prospectus, and information contained therein should not be relied upon.
46
3. FORWARD-LOOKING STATEMENTS
Certain statements in this Prospectus relate to the future, including forward-looking statements
relating to the Group’s financial position and strategy. Forward-looking statements give the
Group’s current expectations or forecasts of future events. In some cases, these forward-
looking statements can be identified by the use of forward-looking terminology, including
(without limitation) the terms “intend”, “aim”, “project”, “anticipate”, “estimate”, “plan”, “believe”,
“expect”, “may”, “should”, “will”, “continue” or other similar words. These statements discuss
future expectations concerning the Group’s results of operations or financial condition, or
provide other forward-looking statements. In particular, these include statements relating to
future actions, prospective products or product approvals, future performance or results of
current and anticipated products, sales efforts, expenses, the outcome of contingencies such
as legal proceedings, dividend payments and financial results. Any forward-looking statements
made by or on behalf of the Group speak only as of the date they are made and are based
upon the knowledge and information available to the Directors on the date of this Prospectus.
These forward-looking statements are not guarantees or predictions of future performance,
may be based on a number of assumptions (which may or may not themselves prove to be
correct) and, by their nature, involve known and unknown risks, uncertainties and other factors,
including the risk factors set out in the section entitled “Risk Factors”, many of which are
beyond the Group’s control, and which may cause the actual results to differ materially from
those expressed in the statements contained in this Prospectus. The Group’s actual results of
operations, financial condition and the development of the business sectors in which the Group
operates may differ materially from those expressed or implied in any forward-looking
statement contained in this Prospectus due to certain factors including, but not limited to,
domestic and global economic and business conditions, market-related risks pertaining to the
consumer healthcare industry as a whole, the policies and actions of regulatory authorities,
geopolitical developments, market developments, the impact of competition, technological
development, inflation, deflation, foreign currency exchange rates, the timing, impact and other
uncertainties of any future acquisitions, combinations or divestments within relevant industries,
as well as the impact of tax and other legislation and other regulations in the jurisdictions in
which the Group operates. In addition, even if the Group’s actual results of operations, financial
condition and the development of the business sectors in which it operates are consistent with
the forward-looking statements contained in this Prospectus, those results or developments
may not be indicative of results or developments in subsequent periods. Recipients of this
Prospectus are cautioned not to put undue reliance on forward-looking statements.
Other than as required by English law, none of the Company, its officers, advisers or any other
person gives any representation, assurance or guarantee that the occurrence of the events
expressed or implied in any forward-looking statements in this Prospectus will actually occur, in
part or in whole, and undertakes no obligation to update any forward-looking statements,
whether as a result of new information, future events or otherwise.
Additionally, statements of the intentions of the Board and/or Directors reflect the present
intentions of the Board and/or Directors, respectively, as at the date of this Prospectus and
may be subject to change as the composition of the Board alters, or as circumstances require.
Forward-looking statements contained in this Prospectus speak only as of the date of this
Prospectus. The Company, the Directors and the Company’s advisers expressly disclaim any
obligation or undertaking to update these forward-looking statements contained in the
document to reflect any change in their expectations or any change in events, conditions, or
circumstances on which such statements are based unless required to do so by applicable law,
the Prospectus Regulation Rules, the Listing Rules, the Disclosure Guidance and
Transparency Rules or the Market Abuse Regulation. The reader should, however, consult any
additional disclosures that the Group may make in any documents which it publishes and/or
files with the SEC. All readers, wherever located, should take note of these disclosures. The
statements above related to forward-looking statements should not be construed as a
qualification of the working capital statement contained in paragraph 17 of Part XII (Additional
Information).
47
4. MARKET AND INDUSTRY DATA
Other than in respect of statements of the type described in the paragraph below, unless the
source is otherwise stated, the market and industry data in this Prospectus constitute the
Directors’ estimates, using underlying data from independent third parties. Such data includes
market research, consultant surveys, publicly available information, reports of governmental
agencies and industry publications and surveys (including publications and data compiled by
Nicholas Hall and Euromonitor). Estimates extrapolated from this data involve risks and
uncertainties and are subject to change based on various factors.
Unless otherwise stated, statements of market position are on the basis of sales to consumers
in the relevant geographical market or product category in 2021, as reported by: (i) in the case
of statements relating to OTC/VMS, Nicholas Hall’s DB6 Consumer Healthcare Database at
manufacturer’s selling prices; and (ii) in the case of statements relating to Oral Health,
Euromonitor Passport ‘Oral Care’ at retail selling prices. The value of a market or product
category and market size are provided on the basis of sales to consumers in 2021 in the
relevant geographical market or product category, as reported by: (i) in the case of statements
relating to OTC/VMS, Nicholas Hall’s DB6 Consumer Healthcare Database at manufacturer’s
selling prices; and (ii) in the case of statements relating to Oral Health, Euromonitor Passport
‘Oral Care’ at manufacturer’s selling prices.
The Group confirms that all third-party data contained in this Prospectus has been accurately
reproduced and, so far as the Group is aware and able to ascertain from information published
by that third party, no facts have been omitted that would render the reproduced information
inaccurate or misleading.
Where third-party information has been used in this Prospectus, the source of such information
has been identified. While industry surveys, publications, consultant surveys and forecasts
generally state that the information contained therein has been obtained from sources believed
to be reliable, the accuracy and completeness of such information is not guaranteed. The
Group has not independently verified any of the data obtained from third-party sources
(whether identified in this Prospectus by source or used as a basis for the Directors’ beliefs and
estimates), or any of the assumptions underlying such data. Similarly, internal surveys, industry
forecasts and market research, which the Company believes to be reliable, have not been
independently verified.
5. TRADE MARKS, TRADE NAMES AND TRADE DRESS
This Prospectus includes trade marks, trade names and trade dress of other companies. Use
or display by the Group of other parties’ trade marks, trade names or trade dress or products is
not intended to and does not imply a relationship with, or endorsement or sponsorship by the
Group of, the trade mark, trade name or trade dress owners. Solely for the convenience of
investors, brands are referred to in this Prospectus without the
®
symbol, but the absence of
these references is not intended to indicate in any way that the Group will not assert its rights
to these brands to the fullest extent permitted by law.
6. PRESENTATION OF FINANCIAL INFORMATION
6.1 Overview
The Company has a complex financial history for the purposes of Article 18(3) of the
Prospectus Regulation. To comply with the requirements of the Prospectus Regulation, the
following historical financial information is presented in this Prospectus:
• consolidated financial information of the Group for the financial years ended 31 December
2021, 31 December 2020 and 31 December 2019; and
• combined historical financial information of the Pfizer Contributed CH Business for the
seven months ended 31 July 2019.
48
6.2 Historical Financial Information
The Group’s Historical Financial Information included in Schedule II (Historical Financial
Information of GlaxoSmithKline Consumer Healthcare Holdings (No.2) Limited) has been
prepared in accordance with the requirements of the Prospectus Regulation (including the
requirements regarding issuers with complex financial histories), the Listing Rules and the
basis of preparation included in Note 1 of Schedule II (Historical Financial Information of
GlaxoSmithKline Consumer Healthcare Holdings (No.2) Limited). The basis of preparation
describes the extent to which the Historical Financial Information has been prepared in
accordance with IFRS. The accounting policies applied and stated in the Historical Financial
Information are set out in Note 2 of Schedule II (Historical Financial Information of
GlaxoSmithKline Consumer Healthcare Holdings (No.2) Limited).
On 31 July 2019, the Group completed a transaction with Pfizer to combine substantially all of
GSK and Pfizer’s respective consumer healthcare businesses into a new world-leading
consumer healthcare joint venture (the “Pfizer Transaction”, as further described in paragraph
2.3 of Part I (Key Highlights and Development of the Group)). Since the completion of the
Pfizer Transaction, GSK has owned 68 per cent. of the ordinary shares in CH JVCo, being the
entity through which both GSK and Pfizer hold their equity interests in the joint venture and the
current holding company of the Group’s business, with Pfizer holding the remaining 32 per
cent. of the equity interest. The Pfizer Contributed CH Business was consolidated within the
Group’s financial statements from 1 August 2019.
Unless otherwise stated in this Prospectus, financial information relating to the Group has been
extracted without material adjustment from Schedule II (Historical Financial Information of
GlaxoSmithKline Consumer Healthcare Holdings (No.2) Limited).
6.3 Comparison of Historical Financial Information and Interim Financial Information to GSK
Segment Financials
Whilst a part of the GSK Group, a significant majority of the Group has historically been
reported as an operating segment under IFRS 8 in GSK’s annual report and interim financial
reporting (the “Consumer Healthcare Segment”). The Historical Financial Information and the
Interim Financial Information have been prepared to reflect the legal perimeter of the Group in
connection with the anticipated Demerger and Separation of the Company from the GSK
Group and therefore differ both in purpose and basis of preparation to the Consumer
Healthcare Segment as presented historically in GSK’s financial reporting. As a result, whilst
the two sets of financial information are similar, they are not the same because of certain
differences in accounting and disclosure under IFRS.
These differences primarily include:
(A) the inclusion in the Consumer Healthcare Segment of certain distribution and local
commercial activities performed by a limited number of other GSK Group entities in
relation to consumer healthcare products;
(B) the basis of allocation of certain cost-sharing and royalty agreements as attributed by a
limited number of other GSK Group entities for the purposes of the Consumer
Healthcare Segment;
(C) the inclusion of Horlicks and other consumer healthcare nutrition products in India and
certain other markets in the Consumer Healthcare Segment; and
(D) the sale of ThermaCare products until their disposal in 2020 which have been excluded
from the Consumer Healthcare Segment (but included in the Historical Financial
Information).
49

6.4 Reporting Framework
The financial information presented in this Prospectus reflects the operating and financial
performance of the Group, its cash flows and financial position and resources. The Group’s
results as reported in accordance with IFRS represent the Group’s overall performance. The
Group also uses a number of adjusted, non-IFRS, measures to report the performance of its
business, as described below.
Description of Key Line Items in the Group’s Financial Statements
The following descriptions of key line items in the financial statements are relevant to the
discussion of the Group’s results of operations in Part VII (Operating and Financial Review).
Item Represents
Revenue Revenue from sales of goods to external customers
against received orders. Revenue represents net
invoice value including fixed and variable consideration.
Variable consideration arises on the sale of goods as a
result of discounts and allowances given and accruals
for estimated future returns and rebates. Revenue is not
recognised in full until it is highly probable that a
significant reversal in the amount of cumulative revenue
recognised will not occur. The methodology and
assumptions used to estimate rebates and returns are
monitored and adjusted regularly in light of contractual
and legal obligations, historical trends, past experience
and projected market conditions. Once the uncertainty
associated with the returns and rebates is resolved,
revenue is adjusted accordingly. Value added tax and
other sales taxes are excluded from revenue.
Cost of sales Cost of sales includes all costs directly related to
bringing products to their final selling destination. This
includes purchasing and receiving costs and direct and
indirect costs to manufacture products, including
materials, labour and overhead expenses necessary to
acquire and convert purchased materials and supplies
into finished goods. Cost of sales also includes royalties
on certain licensed products, inspection costs, freight
charges, costs to operate equipment and depreciation
and amortisation.
Selling, general and administration
(“SG&A”)
SG&A expenses comprise advertising and promotion
costs, selling costs, warehouse and distribution costs,
corporate overheads, other administrative expenses
and depreciation and amortisation.
Research and development (“R&D”) R&D expenditure comprises expenditure that is directly
attributable to the research and development of new
products, including the costs attributable to the
generation of intellectual property and product
registrations, and depreciation and amortisation of
equipment, real estate and IT assets used by the R&D
function.
50

Other operating
(expense)/income
Other operating (expense)/income includes income and
expense from all other operating activities which are not
related to the ordinary course business of the Group,
such as gains/losses from disposals and transaction
costs.
Net finance costs Net finance costs comprise finance costs and finance
income, including net finance costs in relation to
pensions and similar obligations. Finance income
includes income on cash and cash equivalents and
income on other financial assets. Finance costs include
interest costs in relation to financial liabilities. This
includes interest on lease liabilities, which represents
the unwind of the discount rate applied to lease
liabilities.
Income tax Income tax is the expense resulting from the corporate
income tax payable in the different countries in which
the Group operates.
Adjusted Results and other non-IFRS financial measures
This Prospectus contains a number of non-IFRS measures to report the performance of the
Group’s business. Non-IFRS measures exclude amounts that are included in, or include
amounts that are excluded from, the most directly comparable measure calculated and
presented in accordance with IFRS, or are calculated using financial measures that are not
calculated in accordance with IFRS. Adjusted Results and other non-IFRS measures may be
considered in addition to, but not as a substitute for or superior to, information presented in
accordance with IFRS.
The Directors consider these metrics to be the non-IFRS financial measures used by the
Group to help evaluate growth trends, establish budgets and assess operational performance
and efficiencies. The Directors believe that these non-IFRS financial measures, in addition to
IFRS measures, provide an enhanced understanding of the Group’s results and related trends,
therefore increasing transparency and clarity of the Group’s results and business.
There are no generally accepted accounting principles governing the calculation of these
measures and the criteria upon which these measures are based can vary from company to
company. The non-IFRS financial measures presented in this Prospectus may not be
comparable to other similarly titled measures used by other companies, have limitations as
analytical tools and should not be considered in isolation or as a substitute for analysis of the
Group’s operating results as reported under IFRS. The Directors encourage investors and
analysts not to rely on any single financial measure but to review the Group’s financial and
non-financial information in its entirety.
The following non-IFRS measures are presented in this Prospectus:
Measure
Adjusted EBITDA Adjusted EBITDA is one of the measures used by
management to assess the financial performance of the
Group’s business. It is defined as profit after tax
excluding income tax, finance income, finance expense,
Adjusting Items (as defined in paragraph 5.2 of Part VI
(Selected Financial Information)), depreciation of
property plant and equipment, impairment of property
plant and equipment, right-of-use assets and computer
software net of reversals, depreciation of right-of-use
assets, and amortisation of software intangibles.
51
Adjusted EBITDA eliminates differences in performance
caused by variations in capital structures (affecting net
finance costs), tax positions (such as the availability of
net operating losses against which to relieve taxable
profits), the cost and age of tangible assets (affecting
relative depreciation expense) and the extent to which
intangible assets are identifiable (affecting relative
amortisation expense). Accordingly, the Directors
believe that Adjusted EBITDA provides useful
information to investors and others in understanding
and evaluating the Group’s operating results in the
same manner as the Group’s management.
Adjusted EBITDA has limitations as a financial measure
and investors should not consider it in isolation or as a
substitute for analysis of the Group’s results of
operations as reported under IFRS. In addition to the
limitations inherent to all Adjusted Results (as defined
below), some other limitations are:
• Although depreciation and amortisation are
non-cash charges, the assets being depreciated
and amortised may have to be replaced in the future
and Adjusted EBITDA does not reflect capital
expenditure requirements for such replacements or
for new capital expenditure or lease extensions; and
• Adjusted EBITDA does not reflect net finance
expense/income, cash requirements for the Group’s
working capital, transaction related costs,
separation and admission costs and disposal costs.
Adjusted Results Adjusted Results comprise Adjusted gross profit,
Adjusted gross profit margin, Adjusted operating profit,
Adjusted operating profit margin, Adjusted profit before
taxation, Adjusted profit after taxation, Adjusted profit
attributable to shareholders, Adjusted basic earnings
per share, Adjusted diluted earnings per share,
Adjusted cost of sales, Adjusted SG&A, Adjusted R&D,
Adjusted other operating income, Adjusted net finance
costs, Adjusted taxation charge, and Adjusted profit
attributable to non-controlling interests. Adjusted
Results exclude Net amortisation and impairment of
intangible assets, Restructuring costs, Transaction-
related costs, Separation and Admission costs, and
Disposals and others, in each case net of the impact of
taxes (where applicable) (collectively, the “Adjusting
Items”, which are defined at paragraph 5.2 of Part VI
(Selected Financial Information)).
The Directors believe that Adjusted Results, when
considered together with the Group’s operating results
as reported under IFRS, provide investors, analysts and
other stakeholders with helpful complementary
information to understand the financial performance and
position of the Group from period to period and allow
the Group’s performance to be more easily compared
against the majority of its peer competitors.
52

As Adjusted Results include the benefits of restructuring
programmes but exclude significant costs (such as
Restructuring costs, Transaction-related costs and
Separation and Admission costs) they should not be
regarded as a complete picture of the Group’s financial
performance as presented in accordance with IFRS. In
particular, when significant impairments, Restructuring
costs and Separation and Admission costs are
excluded, Adjusted Results will be higher than IFRS
results.
For information on the Adjusting Items and further
commentary on Adjusted Results, see paragraph 4.2 of
Part VI (Selected Financial Information).
Constant currency The Group’s reporting currency is Pounds Sterling, but
the Group’s significant international operations give rise
to fluctuations in foreign exchange rates. To neutralise
foreign exchange impact and to better illustrate the
change from one year to the next, the Group discusses
its results both on an “as reported basis” or using
“actual exchange rates” (“AER”) (local currency results
translated into Pounds Sterling at the prevailing foreign
exchange rate) and using constant currency exchange
rates (“CER”). To calculate results on a constant
currency basis, prior year exchange rates are used to
restate current year comparatives. The currencies
which most influence the constant currency results of
the Group and their exchange rates are shown in the
below table.
2021 2020 2019
Average rates:
USD/£ 1.38 1.29 1.28
Euro/£ 1.16 1.13 1.14
Swiss Franc/£ 1.25 1.21 1.27
CNY/£ 8.86 8.91 8.82
Free cash flow Free cash flow is calculated as net cash inflow from
operating activities plus cash inflows from the sale of
intangible assets, the sale of property, plant and
equipment and interest received, less cash outflows for
the purchase of intangible assets, the purchase of
property, plant and equipment, distributions to
non-controlling interests and interest paid.
The Directors believe free cash flow is meaningful to
investors because it is the measure of the funds
generated by the Group available for distribution of
dividends, repayment of debt or to fund the Group’s
strategic initiatives, including acquisitions. The purpose
of presenting free cash flow is to indicate the ongoing
cash generation within the control of the Group after
taking account of the necessary cash expenditures for
maintaining the capital and operating structure of the
53
Group (in the form of payments of interest, corporate
taxation and capital expenditure).
Free cash flow conversion Free cash flow conversion is calculated as free cash
flow, as defined above, divided by profit after tax.
Free cash flow conversion is used by the Directors to
evaluate the cash generation of the business relative to
its profit, by measuring the proportion of profit after tax
that is converted into free cash flow as defined above.
Net debt Net debt at a period end is calculated as short-term
borrowings (including bank overdrafts and short-term
lease liabilities), long-term borrowings (including long-
term lease liabilities), and derivative financial liabilities
less cash and cash equivalents and derivative financial
assets.
The Directors analyse the key cash flow items driving
the movement in net debt to understand and assess
cash performance and utilisation in order to maximise
the efficiency with which resources are allocated. The
analysis of cash movements in net debt allows the
Directors to more clearly identify the level of cash
generated from operations that remains available for
distribution after servicing the Group’s debt.
Organic revenue growth Organic revenue growth represents the change in
organic revenue at CER from one accounting period to
the next.
Organic revenue represents revenue, as determined
under IFRS and excluding the impact of acquisitions,
divestments and closures of brands or businesses,
revenue attributable to manufacturing service
agreements (“MSAs”) relating to divestments and the
closure of sites or brands, and the impact of currency
exchange movements.
Revenue attributable to MSAs relating to divestments
and production site or brand closures has been
removed from organic revenue because these
agreements are transitional and, with respect to
production site closures, include a ramp-down period in
which revenue attributable to MSAs gradually reduces
several months before the production site closes. This
revenue reduces the comparability of prior and current
year revenue and is therefore adjusted for in the
calculation of organic revenue growth.
Organic revenue is calculated period-to-period as
follows, using prior year exchange rates to restate
current year comparatives:
• current year organic revenue excludes revenue
from brands or businesses acquired in the current
accounting period;
54
• current year organic revenue excludes revenue
attributable to brands or businesses acquired in the
prior year from 1 January to the date of completion
of the acquisition;
• prior year organic revenue excludes revenue in
respect of brands or businesses divested or closed
in the current accounting period from 12 months
prior to the completion of the disposal or closure
until the end of the prior accounting period;
• prior year organic revenue excludes revenue in
respect of brands or businesses divested or closed
in the previous accounting period in full; and
• prior year and current year organic revenue
excludes revenue attributable to MSAs relating to
divestments and production site closures taking
place in either the current or prior year,
each an “Organic Adjustment”.
To calculate organic revenue growth for the period,
organic revenue for the prior year is subtracted from
organic revenue in the current year and divided by
organic revenue in the prior year.
By way of example:
• The Pfizer Transaction completed on 31 July 2019.
Organic revenue growth for the period FY 2019 to
FY 2020 excludes revenue attributable to brands
acquired as part of the Pfizer Transaction in
respect of the period 1 January 2020 to 31 July
2020.
• The Group completed the disposal of Breathe Right
on 1 October 2020. Organic revenue growth for the
period FY 2019 to FY 2020 excludes revenue
attributable to Breathe Right from the period
1 October 2019 to 31 December 2019. Organic
revenue growth for the period FY 2020 to FY 2021
excludes revenue attributable to Breathe Right in
FY 2020.
The Directors believe that discussing organic revenue
growth contributes to the understanding of the Group’s
performance and trends because it allows for a
year-on-year comparison of revenue in a meaningful
and consistent manner.
For a reconciliation of the closest measures prepared in accordance with IFRS to the
applicable non-IFRS measures, see paragraph 5 of Part VI (Selected Financial Information).
6.5 Currency presentation
The Group’s financial information is presented in Pounds Sterling. The abbreviations ‘£m’ or
‘£million’ represent millions of Pounds Sterling, and references to ‘pence’ and ‘p’ represent
pence in Pounds Sterling.
55
6.6 Rounding of figures
Certain financial information presented in tables in this Prospectus has been rounded to the
nearest whole number or the nearest decimal place. Therefore, the sum of the numbers in a
column may not conform exactly to the total figure given for that column. In addition, certain
percentages presented in the tables in this Prospectus reflect calculations based upon the
underlying information prior to rounding, and, accordingly, may not conform exactly to the
percentages that would be derived if the relevant calculations were based upon the rounded
numbers. Certain percentage shareholdings have also been rounded and therefore totals of
such percentage shareholdings may vary slightly from their actual arithmetic totals.
7. Pfizer’s interest in the Group
As at the date of this Prospectus, Pfizer’s 32 per cent. interest in the Group is held by PFCHH,
which holds all of the JVCo B Ordinary Shares, representing 32 per cent. of the voting rights
and 24.62 per cent. of the nominal value of CH JVCo. PFCHH is a direct wholly owned
subsidiary of Anacor and both are wholly owned subsidiaries of Pfizer. Prior to the Demerger,
Pfizer intends to undertake an intragroup reorganisation resulting in Pfizer becoming the direct
sole owner of PFCHH.
Accordingly, except where otherwise stated, references in this Prospectus, including in the
structure charts in Part IV (Overview of the Demerger and Separation), to the ownership of, or
transfer to the Company (pursuant to the terms of the Pfizer Exchange Agreement), of PFCHH
by Pfizer and to the issuance of Haleon Shares and Non-Voting Preference Shares to Pfizer
assume that this reorganisation takes place prior to the Demerger as expected. However, in
the event that the PFCHH Transfer is not completed by the time of completion of the
Demerger, then Anacor shall be the entity holding the ownership interests in PFCHH that are to
be transferred to the Company pursuant to the Pfizer Exchange Agreement and, in
consideration of such transfer, the Company shall issue Haleon Shares and Non-Voting
Preference Shares to Anacor.
In addition, references in this Prospectus to Pfizer’s or Anacor’s interest in 32 per cent. of the
Haleon Shares include both Haleon Shares and Haleon ADSs in respect of such Haleon
Shares.
Pfizer will continue to own its 32 per cent. ownership interest in the Company following
Separation. Pfizer has informed the Company that it intends to exit its position in the Company
in a disciplined fashion, with an objective of maximising value for Pfizer shareholders.
8. DEFINITIONS
Certain terms used in this Prospectus, including all capitalised terms and certain technical and
other terms, are defined and explained in Schedule I (Definitions and Glossary).
56
DIRECTORS, COMPANY SECRETARY, REGISTERED OFFICE AND ADVISERS
Directors Sir Dave Lewis
Brian McNamara
Tobias Hestler
Manvinder Singh (Vindi) Banga*
Marie-Anne Aymerich*
Tracy Clarke*
Dame Vivienne Cox*
Asmita Dubey*
Deirdre Mahlan*
Bryan Supran*
John Young*
(*those persons who will become directors of the Company on
Admission)
Company Secretary Amanda Mellor
Registered office 980 Great West Road
Brentford
Middlesex TW8 9GS
United Kingdom
Joint Sponsors Citigroup Global Markets Limited
Citigroup Centre
Canada Square
Canary Wharf
London E14 5LB
United Kingdom
Goldman Sachs International
Plumtree Court
25 Shoe Lane
London EC4A 4AU
United Kingdom
Merrill Lynch International
2 King Edward Street
London EC1A 1HQ
United Kingdom
Reporting accountants
and auditor
Deloitte LLP
1 New Street Square
London EC4A 3HQ
United Kingdom
Legal advisers to the
Company (as to English
law)
Slaughter and May
One Bunhill Row
London EC1Y 8YY
United Kingdom
Legal advisers to the
Company (as to US
law)
Cleary Gottlieb Steen & Hamilton LLP
2 London Wall Place
London EC2Y 5AU
United Kingdom
57
Legal advisers to the
Joint Sponsors
Ashurst LLP
London Fruit & Wool Exchange
1 Duval Square
London E1 6PW
United Kingdom
Registrar Equiniti Limited
Aspect House
Spencer Road
Lancing, BN99 6DA
United Kingdom
58

PART I
KEY HIGHLIGHTS AND DEVELOPMENT OF THE GROUP
1. Key highlights
1
The Directors believe that the Group is an exceptional business: a business with significant
global scale and reach with leading market share positions, differentiated by its 100 per cent.
focus on consumer healthcare and driven by its purpose of delivering better everyday health
with humanity. Its leading brands are built on science, innovation and human understanding
and are trusted by millions of consumers globally.
The Group is a world leader in consumer healthcare and is the leading business by sales in
OTC, in VMS and Therapeutic Oral Health.
2
The Group’s portfolio of category-leading brands
includes the world’s number one Toothpaste for sensitivity,
3
the world’s leading Multivitamin,
the world’s leading Topical Pain Relief brand, the world’s leading Denture Care brand and a
broad range of other large-scale, well-known consumer healthcare brands with a leading global
or regional presence.
The Group operates in a market that was worth over £160 billion in 2021 and is more relevant
than ever following the COVID-19 pandemic. Consumers are increasingly conscious of their
health and, supported by greater digital resources, are willing to take greater ownership of
treatment and prevention. This trend is further accelerated in emerging markets by a growing
middle-class population with a greater willingness to pay for OTC and wellness products. In
addition, an ageing population across many countries drives greater demand for many of the
products in the Group’s categories; for example, the requirements for arthritis pain relief and for
Denture Care products are linked with age. Similarly, governments, facing pressure on
healthcare spending driven by an ageing population, have adopted policy measures designed
to increase the use of OTC drugs (which are not generally reimbursed by governments)
relative to prescription drugs (typically reimbursed). The Directors expect these trends to
continue. Underpinned by these and other favourable market factors, the Directors anticipate
typical annual market growth of between 3 and 4 per cent. over the medium-term.
The Group has a strong footprint in the world’s consumer healthcare markets, including a
commercial presence in over 170 markets and a number one or two OTC/VMS market position
in countries which represented over 70 per cent. of the world’s OTC/VMS markets by value in
2021. This includes OTC/VMS market leadership in the USA and the leading OTC/VMS
multinational position in the higher growth markets of China and India.
The Group’s scale and brand portfolio is complemented by its well-developed capabilities in
trusted science and human understanding. The Group has a longstanding in-house scientific
capability deriving from its pharmaceutical heritage, which allows it to both innovate and build
trust through constructive engagement with the scientific community. The Group’s scientific
capabilities are combined with a deep understanding of the health needs of its consumers,
supported by consumer insights and broad engagement with healthcare professionals.
Significantly, the Group engages directly with approximately one third of the approximately
10 million healthcare professionals relevant to its categories. The power of this combination is
illustrated by the double-digit growth of Sensodyne over the past decade, which has been
driven by increasing public awareness of tooth sensitivity as a treatable condition, consumer-
centric scientific innovation and the generation of evidence-based claims to support expert
recommendations.
1
See paragraph 1 (Market and Industry Data)ofPresentation of Financial and Other Information for further information on the
use of market and industry data in this Prospectus.
2
Source: Therapeutic Oral Health ranking is based on Group analysis of third party data from Nielsen, IRI, Intage, IQVIA
Consumption Sales Data (2021-2022). Therapeutic Oral Health is defined as Therapeutic Toothpaste and Total Dental
Appliance Care.
3
Group analysis of 2020 third party market data.
59

The Group has been transformed through the synergistic combination of three leading
consumer healthcare businesses since 2015, alongside a targeted programme to optimise its
operating model, cost base and capabilities for the future. An extensive programme of
divestments has sharpened the focus of the business through the divestment of growth-dilutive
brands and those outside of the Group’s core categories. In addition, the extensive scientific
and consumer products experience of its legacy businesses has been significantly enhanced
by targeted investment in commercial and scientific capabilities, technologies and facilities,
most notably in the digital sphere. The separation of the Group from GSK now offers a number
of further intangible benefits, including increased management focus, an infrastructure and
organisational design more closely aligned to consumer healthcare requirements, capital
allocation priorities tailored to the needs of the business and management incentives which
can be focused on the specific priorities of the Group.
Despite the net negative impact of the COVID-19 pandemic, the business delivered above-
market organic revenue growth in both FY 2020 and FY 2021 whilst successfully integrating
the Pfizer consumer healthcare business which became part of the Group on 31 July 2019.
Over the period FY 2019 to FY 2021, the Group also achieved a meaningful improvement in
Adjusted operating profit margin driven by the delivery of integration synergies and operational
efficiencies, while still increasing investment in its brands and capabilities. This was achieved in
spite of adverse currency movements and the dilutive impact of divestments. Building on this
base, the Group has a clear and focused strategy which the Directors believe will drive
sustainable above-market growth and attractive shareholder returns based on four key pillars:
• Driving portfolio growth by increasing household penetration. While the Group’s
category-leading brands touch millions of consumers around the world, there remains
significant headroom for further penetration
4
across the portfolio. The Group has a clear
strategy for driving penetration-led growth with the consumer as its focus and which it plans
to accelerate and apply across its broader portfolio.
• Capitalising on new and emerging growth opportunities. The Group plans to build on
its significant recent growth in e-commerce, leveraging its rapidly developing capabilities in
this area. Additionally, the Group’s brand portfolio, extensive scale and powerful
route-to-market provide the opportunity to expand brands into new markets where it has the
reach and scale to succeed. Similarly, the greater size of the combined legacy GSK and
Pfizer consumer healthcare businesses in certain markets (relative to the legacy GSK and
Pfizer businesses alone) continues to offer the opportunity to scale up key brands which
previously lacked local distribution scale. Further opportunities exist in Rx-to-OTC switches
in the USA, an area where the Group has led the market over the last decade, and in
accelerating consumer trends such as the growth of the Naturals segment (as defined
below), where multiple launches are already underway with more planned.
• Performance underpinned by strong execution and financial discipline. The Group is
focused on first class commercial execution, increasingly supported by digital tools. In
addition, it has a strong culture of financial discipline and continuous improvement. This
combination has allowed it to deliver meaningful margin improvements since FY 2019,
whilst increasing investment in its brands. The Group’s strategy is to build on this track
record, maintaining its focus on commercial execution, business optimisation and cost
control, thereby enabling it to deliver sustainable moderate margin expansion while
continuing to invest for future growth.
• Running a responsible business. Running a responsible business is integral to the
Group’s purpose of delivering better everyday health with humanity and it believes it is well
placed to have a positive impact. Its environmental, social and governance (“ESG”) goals
focus on tackling the environmental and social barriers to everyday health and driving
health inclusivity through the promotion and delivery of sustainable solutions.
4
Penetration is the proportion of a population (in a defined geographic market or product category) that has purchased the
relevant category, brand or product at least once in the stated period.
60
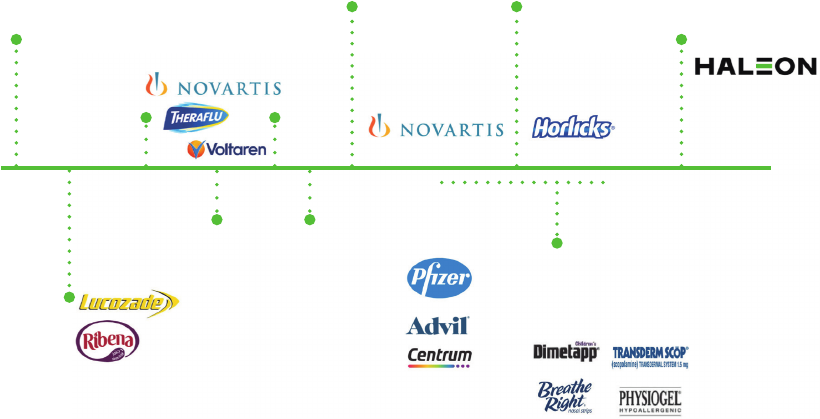
The Directors expect the Group’s strategy to deliver medium-term annual organic revenue
growth of 4 to 6 per cent. combined with sustainable moderate margin expansion on a constant
currency basis, whilst supporting continued investment for growth in the business. In turn, the
Directors expect this anticipated growth to deliver attractive shareholder returns underpinned
by strong cash generation and an initial dividend which is expected to be at the lower end of a
30 to 50 per cent. pay-out ratio (subject to Board approval). The Group proposes to maintain a
strong investment grade balance sheet with a target net debt to Adjusted EBITDA ratio of less
than 3x by the end of 2024.
The listing of the Company is the culmination of a seven year journey since the merger of the
legacy GSK and Novartis consumer healthcare businesses to create a global leader in
consumer healthcare. The Group’s world-class portfolio of brands, attractive geographic
footprint and strong capabilities leave it well positioned to benefit from favourable underlying
sector fundamentals.
2. Evolution of the Group
The Group has been transformed since 2012 through progressive strategic M&A and
divestments to create a world leader in consumer healthcare.
The Group’s scale has greatly expanded through the successful combination of the legacy
GSK consumer healthcare business with the Novartis consumer healthcare business in 2015,
and the subsequent combination of this business with the Pfizer consumer healthcare business
in 2019, reaching revenue of £9.5 billion in FY 2021. In addition, the Group’s focus has been
sharpened since 2012 through the progressive divestment of the GSK Group’s Nutritionals
businesses (including Lucozade, Ribena and Horlicks) and the divestment by the Group of
non-strategic OTC brands including its recent programme of divestments of non-strategic and
growth-dilutive brands (with aggregate net proceeds from divested brands of £1.1 billion)
during the period from FY 2019 to FY 2021. This deliberate strategy has resulted in a portfolio
more focused on higher-growth categories, markets and channels. These transactions also
provided a catalyst for a broader transformation of the Group (see paragraph 2.5
Transformation of the Group).
The key M&A milestones since 2012 in the Group’s business are summarised below:
Divest
Non-strategic
OTC
Exit of
beverages:
Lucozade and
Ribena to
Suntory
with Pfizer
Non-strategic and growth dilutive
assets disposals, £1.1bn proceeds
2012
2013
2015 2017
2019 2021
2014
2016
2018 2020
with Novartis
Full buy out of
Novartis from JV
for $13bn
Exit non strategic
categories, Horlicks
to Unilever
Divest
Divest
2022JV formation
JV formation
Significant divestment programme
Buy out
2.1 Legacy GSK consumer healthcare business
Prior to its combination with the Novartis consumer healthcare business in 2015, GSK’s
consumer healthcare business was already one of the world’s leading OTC and Oral Health
companies with a long heritage in consumer healthcare products dating back to the 18th
century, when its founding companies in Britain, the USA and Germany sold herbal products,
laxatives, vitamins and soaps.
61

The Group sold a range of leading OTC brands (including Panadol, Fenbid, Tums and ENO)
across Respiratory Health, Pain Relief, Digestive Health, Skin Health and Smokers’ Health,
together with a strong portfolio of Oral Health brands (including Sensodyne, Polident and
parodontax). Geographically, the GSK consumer healthcare business had a strong presence in
higher-growth emerging markets in the Middle East, Africa and Asia, which complemented its
businesses in Europe and North America.
2.2 Joint venture with Novartis
On 2 March 2015, GSK and Novartis formed a consumer healthcare joint venture to combine
the majority of GSK’s consumer healthcare business and all of Novartis’ OTC business (as
further described in paragraph 15.1 of Part XII (Additional Information)). Novartis’ business
provided GSK with a meaningful incremental presence in OTC, including several major brands,
notably Voltaren,
5
Theraflu, Excedrin and Otrivin. The combination added a leading portfolio of
globally recognised consumer-preferred and expert-recommended brands in the Pain Relief,
Respiratory Health, Smokers’ Health and Skin Health categories to the Group’s business.
Geographically, Novartis’ presence in Central and Eastern Europe combined with GSK’s
strength in these and other emerging markets presented multiple new growth opportunities
across the combined portfolio.
In June 2018, GSK acquired Novartis’ shareholding in the GSK/Novartis JV for $13 billion,
enabling GSK to take full operational and strategic control of the business (as further described
in paragraph 15.1 of Part XII (Additional Information)).
2.3 Joint venture with Pfizer
On 31 July 2019, GSK completed a transaction with Pfizer to combine substantially all of GSK
and Pfizer’s respective consumer healthcare businesses into a new world-leading consumer
healthcare joint venture (as further described in paragraph 15.2 of Part XII (Additional
Information)).
The transaction, which was transformational to the scale of the Group’s business, brought
together two businesses with highly complementary geographic footprints and brand portfolios.
While the Group retained its strong European footprint, completion of the transaction also
provided the Group with incremental geographical scale in the USA, where it became the
leader in OTC/VMS, and in China, where it became the leading OTC/VMS multinational. From
a portfolio perspective, the transaction provided the Group with global leadership in the higher-
growth VMS market (key brands: Centrum, Caltrate and Emergen-C), as well as a leading
presence in the US pain relief market through the acquisition of Advil, complementing the
Group’s existing Pain Relief portfolio under the Panadol, Voltaren, Fenbid and Excedrin
brands. Since completion, GSK has owned 68 per cent. of the ordinary shares in
GlaxoSmithKline Consumer Healthcare Holdings (No.2) Limited (the entity through which both
GSK and Pfizer hold their equity interests in the joint venture and the current holding company
of the Group’s business) with Pfizer holding the remaining 32 per cent. of the ordinary shares
in CH JVCo. The legacy Pfizer business has now been fully integrated into the Group.
2.4 Divestment of OTC and skin care non-core brands
Alongside integration of the Pfizer consumer healthcare business, the Group exited
approximately 50 non-strategic and growth-dilutive OTC and skincare assets from 2019 to
2021 to raise £1.1 billion of net proceeds. These disposals have further focused the business
on higher-growth categories, markets and channels and thereby enhanced the growth profile of
the Group.
5
Voltaren is a Novartis brand licensed to the Group exclusively for OTC products.
62

2.5 Transformation of the Group
The transactions summarised above have acted as a catalyst for a much broader
transformation of the Group, which is summarised below.
1. Portfolio reshaped, well positioned for growth
The portfolio changes since 2015 have resulted in a group that has been repositioned towards
higher, above-market growth. The share of sales driven from the Group’s nine large-scale
multinational power brands: Panadol, Voltaren, Advil, Otrivin, Theraflu, Sensodyne, Polident,
parodontax and Centrum (collectively, the “Power Brands”), which together have higher
revenue growth than the overall Group (and generally have higher gross margins), has
increased from 44 per cent. in 2015 to 58 per cent. in FY 2021 and the 2019-21 divestment
programme has eliminated a significant drag on overall growth.
6
The Pfizer Transaction
provided the Group with a significantly greater presence in higher-growth categories, notably
building a leadership position in VMS which has a higher-growth rate than other categories
7
and represented 16 per cent. of Group revenue in FY 2021 compared to 1 per cent. in 2015.
Similarly, investments made in digital commerce have meaningfully increased the Group’s
presence in the high growth e-commerce/digital channel, which grew from less than 1 per cent.
of revenue in 2015 to 8 per cent. of revenue in 2021. The Group is also well-positioned in key
geographies following the Novartis and Pfizer transactions. The Group has leading positions in
the world’s top two OTC/VMS markets with OTC/VMS market leadership in the USA (first in
2021 compared to fourth in 2015) and the leading OTC/VMS multinational position in China
(second overall in 2021 compared to fourteenth in 2015). These two markets accounted for
over 40 per cent. of Group revenue in FY 2021 and its leading presence in these two markets
provides the Group with a strong platform for future growth.
2. Optimised operating model, lean cost base and capabilities improved
Since 2015, the Group has made significant improvements to its footprint and operating model,
thereby delivering a sustainable increase in operating profit margin to support reinvestment in
brands, capabilities and tools to support growth.
The Group has significantly reduced its manufacturing site footprint. The 41 sites inherited from
the legacy Novartis, Pfizer and GSK consumer healthcare businesses since 2015 have been
reduced to 24 in 2022. Similarly, warehousing and distribution centres have been reduced from
over 200 inherited to approximately 90 in 2022 and R&D sites have been consolidated from
nine inherited to four in 2022.
In parallel, the Group has significantly improved the efficiency and effectiveness of its
advertising and promotion spend. In particular, the Group doubled its digital media spend
between FY 2019 and FY 2021, with enhanced targeting and a focus on return on investment.
Digital media spend represented approximately 50 per cent. of total media expenditure in FY
2021 and in the USA and China in particular, most of the Group’s advertising and promotion
spend is now digital with more to come in other markets. The Group has also rebalanced the
spend behind its Power Brands to drive future growth from its biggest opportunities, and
increased consumer-facing advertising and promotion.
Finally, the Group has evolved its operating model and enhanced its capabilities to support
stronger execution. Local markets have been increasingly empowered to innovate, improving
the Group’s agility to adapt to changing local needs. Significant investments have been made
in data and tools to drive improved data-led decision-making and stronger returns on the
Group’s investments. In addition, specialised tools have been built that enable better
6
Over 90 per cent. of the sales of OTC and skincare brands divested had negative growth based on compound revenue growth
on a CER basis over the two years prior to divestment for brands divested in 2019 and three years for brands divested in 2020
or 2021.
7
Source: Nicholas Hall Consumer Healthcare 2017-21 sales growth at MSP.
63

execution, including, for example, the Group’s shopper science labs, which enable commercial
teams to experiment with retail experiences and provide category management analysis in
partnership with retailers in each of the Group’s regions.
3. Delivering momentum while investing for growth
The Group’s strategy since 2019 has delivered strong financial results with good momentum
for the future, despite a net negative effect from the COVID-19 pandemic and the focus on
integration of the Pfizer assets and separation activities.
Since FY 2019, the Group’s revenue has increased by 12.6 per cent. to £9.5 billion in FY 2021.
This reflects the incorporation of the Pfizer business (only 5 months was included in FY 2019
as the transaction closed on 31 July 2019) and underlying business growth, partially offset by
divestments and adverse foreign exchange movements. The Group’s organic revenue growth
exceeded 2019-2021 market growth, with 2.8 per cent. organic revenue growth in FY 2020
8
and 3.8 per cent. organic revenue growth in FY 2021. The Group’s FY 2020 organic revenue
growth does not, however, fully reflect the FY 2019 to FY 2020 growth of the current brand
portfolio as it excludes the January to July revenue for the legacy Pfizer brands in FY 2019 and
FY 2020 and the figures also include growth-dilutive brands which no longer form part of the
Group’s portfolio.
In terms of profitability, the Group delivered a robust gross profit margin of 62 per cent. and
Adjusted gross profit margin of 63 per cent. in FY 2021, demonstrating the strength of its
brands, its optimised manufacturing footprint, and continued focus on price, cost of goods sold
and efficiencies to offset inflation. The Directors believe this margin is sustainable. In addition,
the Group has almost fully delivered on the £500 million synergies projected at the time of the
Pfizer Transaction in 2019 and expects to realise around a further £120 million of synergies in
2022, taking the total to around £600 million. Overall, the Group delivered an operating profit
margin of 17.2 per cent. and Adjusted operating profit margin of 22.8 per cent. in FY 2021, an
increase of 6.6 percentage points and 3.3 percentage points, respectively, since FY 2019
despite adverse currency impacts. Over the same period, the Group reinvested a share of
operating cost savings into advertising and promotion spend on brands to support future
growth. Finally, in terms of cash flow, the Group delivered £1.4 billion net cash inflow from
operating activities in both FY 2020 and FY 2021 driven by the underlying profitability of the
business, a disciplined approach to working capital (including a reduction in inventory and
debtor days) and stable capital expenditure.
8
The FY 2020 growth rate calculated on an organic basis was negatively impacted by uneven consumer buying patterns in FY
2020 during the COVID-19 pandemic which overlapped with the first twelve months following the Pfizer Transaction.
Specifically, the calculation of organic revenue in FY 2020 excludes revenue attributable to the brands acquired as part of the
Pfizer Transaction in the period 1 January 2020 to 31 July 2020 (see Presentation of Financial and Other Information above)
and includes revenue attributable to these brands for the period 1 August 2020 to 31 December 2020. Revenue during the
former period was high, driven by accelerated consumer purchases at the beginning of the COVID-19 pandemic. Revenue
during the latter period was negatively impacted by a reduction in consumer inventories (see also paragraphs 2.1, 2.14 and 5.1
of Operating and Financial Review). The calculation also includes revenue up to the point of sale for low growth divested
brands, which no longer form part of the Group’s portfolio.
64
PART II
MARKET OVERVIEW
1. Consumer Healthcare: a £160+ billion market
9
The global consumer healthcare market is one of the largest, most resilient and fastest-growing
across the FMCG sectors. However, unlike other standard FMCG markets, its definition varies
across competitors and common industry data sources. OTC/VMS is a major part of the
market, a primary focus of the Group’s key competitors and is currently valued at over
£135 billion. In addition to OTC/VMS, many peer companies compete in adjacent consumer
healthcare markets. For example, the Group and two of its largest consumer healthcare peers
compete in Oral Health, currently valued at £25 billion globally.
Therefore, the Group’s definition of the consumer healthcare market comprises: OTC/VMS and
Oral Health, which have an aggregate global market size of over £160 billion.
10
Further
information on the Group’s categories is set out in paragraph 3 of this Part II (Market Overview)
below.
Consumer Healthcare Market
180.0
160.0
140.0
120.0
100.0
80.0
60.0
40.0
20.0
2017
OTC Vitamins, Minerals & Supplements Oral Health
2018 2019 2020 2021
-
2017-2021 £bn
The Group’s largest single market is the USA, which is the number one consumer healthcare
market globally with £41 billion in consumer sales in 2021, representing approximately 27 per
cent. of the global market. The Group also has strong presence across Europe and China, as
well as in many other higher-growth markets, in particular China, which present an attractive
opportunity to increase household penetration of the consumer healthcare category.
2. Key market drivers
The market fundamentals shaping future growth in the consumer healthcare market, which
current expectations suggest could grow at a rate of 3-4 per cent. per annum over the medium-
term, include the five key drivers below.
Increased consumer focus on health and wellness
In the period prior to the COVID-19 pandemic, global consumers were increasingly taking a
more active role in self-management of their health and wellbeing. Since the outbreak of the
9
See paragraph 4 (Market and Industry Data)ofPresentation of Financial and Other Information for further information on the
use of market and industry data in this Prospectus.
10
Total 2021 market size of £161 billion based on Group analysis of third party market data.
65

pandemic, personal healthcare has become even more relevant and this trend has
accelerated. 2020 customer research found that 42 per cent. of consumers try to make
wellness a priority in their day-to-day life, and 79 per cent. think wellness is important. 71 per
cent. of those consumers place a higher priority on their health than they did two to three years
ago, and 70 per cent. anticipate health growing in their list of priorities looking forward.
11
This
represents an important driver in the growth of self-care and underpins favourable trends for
the sector as a whole.
Ageing populations
The proportion of people aged 65 years and over is expected to increase from 9.3 per cent. of
the global population in 2020 to 16.0 per cent., or approximately one in six people globally, in
2050.
12
This change in demographics brings with it increased need for self-care and
preventative care.
Emerging middle class
The emerging middle class in higher-growth economies has been a long term growth driver for
the consumer healthcare market as greater buying power has led to greater per capita usage.
Emerging and higher-growth economies continue to represent a sizeable growth opportunity
for the industry: per capita usage for combined OTC/VMS products in the USA was £110 per
capita in 2021; and Western European OTC/VMS usage per capita was £53 in the same
period.
13
By comparison, per capita usage in higher-growth markets, including China (2021
OTC/VMS of £22 per capita), Central and Eastern Europe (2021 OTC/VMS of £29 per capita),
India (2021 OTC/VMS of £2 per capita) and Latin America (2021 OTC/VMS of £13 per
capita),
14
is still relatively low, which presents an attractive opportunity to increase household
penetration of the consumer healthcare category.
Growing self-care in the face of increasing pressure on public health systems
Prior to the COVID-19 pandemic, pressure on public health had been rising over the long term.
In 2018, global spending on health reached $8.3 trillion, or 10 per cent. of global GDP, growing
slightly below GDP for the first time in five years. The COVID-19 pandemic has had, and is
continuing to have, a significant adverse impact on health systems globally, and the aftermath
of the pandemic may be accompanied by a potentially deep global economic crisis which could
have a long-lasting impact on future health financing.
15
As such, the consumer healthcare
market, and more specifically the ability to help consumers to self-care in general, represents a
major opportunity to reduce the current significant burden on public health.
Sizeable unmet consumer needs
Competition in the consumer healthcare market is partly driven by innovation designed to meet
unmet consumer needs. Through targeted innovation to address emerging trends – such as
the growing demand for natural ingredients, as well as premiumisation (where consumers
switch their purchases to premium alternatives), increased consumer interest in personalised
products, and emerging technologies that allow consumers to more directly manage their own
health – the Directors believe there is a sizeable opportunity for further growth.
11
Source: McKinsey & Company, The Future of Wellness H1 2021 Report. Based on consumer research in Brazil, China, Germany,
Japan, the US and the UK.
12
Source: UN Population Facts, October 2020.
13
Source: Nicholas Hall’s DB6 Consumer Healthcare Database at manufacturer’s selling prices.
14
Source: Nicholas Hall’s DB6 Consumer Healthcare Database at manufacturer’s selling prices.
15
Source: WHO, 2020.
66

3. The key market categories for the Group
OTC/VMS
Within the consumer healthcare market, OTC is distinct in that it is defined primarily by its
regulatory status (see also Part X (Regulatory Overview) for further information on relevant
regulations). OTC medicines are readily available to consumers in retail distribution channels
(including pharmacies) without the need for a doctor’s prescription. OTC comprises several
categories defined by specific consumer needs and competition is at the category level.
The Group’s OTC business is focused on three of the largest categories: Respiratory Health
(£23 billion market), Pain Relief (£16 billion market) and Digestive Health and Other (£44 billion
market). In Digestive Health and Other, the Group has a significant presence in Digestive
Health (£15 billion market), Skin Health (OTC Dermatologicals only, £17 billion market) and
Smokers’ Health (£1.3 billion market of the broader £13 billion Lifestyle OTC market). Current
expectations suggest that the OTC sector could grow by approximately 2 to 3 per cent. per
annum over the medium-term.
16
In contrast to the broader FMCG marketplace, OTC is highly regulated, with a regulatory
environment that differs by country and respective regulator. Most OTC innovations and
consumer benefit claims must pass a rigorous approval process including pharmaceutical-like
clinical testing. Distribution is also heavily regulated: in many countries, OTC medicines are
typically available only via the pharmacy channel, although the USA, Australia and UK, where
mass market distribution is permitted, are notable exceptions. While the associated regulatory
environment tends to lead to a slower innovation cycle versus typical FMCG, it provides a
significant competitive advantage to businesses such as the Group with strong scientific
capabilities and strong pharmacy and retail channel execution infrastructure and capabilities.
Competition in OTC is characterised by scientific innovation designed to fulfil unmet consumer
needs and is supported by FMCG consumer branding and marketing. Innovations can include
improved efficacy, new product formats, innovative packaging, and new consumer benefit
claims. Historically, the Rx-to-OTC switch, through which a medicine or class of medicines
previously only available via prescription is made readily available to retail consumers, has
been a significant growth driver. Switches take a relatively long time and require specific
capabilities and expertise, including scientific and regulatory resources, the ability to manage
clinical trials, and the ability to actively engage with key opinion leaders and regulators.
Respiratory Health comprises several sub-categories. The Group is the market leader in global
Respiratory Health with a global number two position (excluding traditional Chinese Medicine)
in the largest sub-category, Seasonal Cold and Flu, the number one position in Topical
Decongestants and the number four position in Allergy Care.
Pain Relief can be further segmented into Systemic Pain Relief (where the medicine is
ingested) and Topical Pain Relief (where the medicine is applied to the skin). The Group is the
global market leader in Pain Relief overall as well as in both of these sub-categories.
Digestive Health comprises a range of treatments to support healthy functioning of the
gastrointestinal tract including, amongst others: Antacids, Laxatives, and fibre products. The
Group is the market leader in Digestive Health globally, due to strong leadership in immediate
relief antacids in both developed and emerging markets.
Skin Health is highly fragmented, with multiple subcategories. The largest of these are Wound
Healers (the Group is number three globally), Antiseptics and Disinfectants, Anti-itch (number
four globally), Acne Remedies, General Antifungals (number three globally), Feminine Intimate
Care and Lip Care (number two globally). Additionally, the Group holds a global leadership
position in OTC Cold Sore Treatments.
16
Group’s projection for medium term (3 – 5 year) market growth rates based on analysis of third party data. Projection is based
on the Group’s current brand / market footprint.
67

Smokers’ Health, in which the Group holds the global number two position,
17
is one of several
sub-categories comprising the Lifestyle OTC category.
VMS is a £52 billion market and is broad-based, highly fragmented and aligned to multiple
specific consumer benefits. While it forms part of the broader OTC/VMS market, it is also
adjacent to the broader Nutrition market and, as a result, different competitors may take
different views of the market (Nutrition, Dietary Supplements, etc.). The current expectation is
that the VMS sector could grow by 4 - 5 per cent. per annum over the medium-term.
18
The
Group competes in VMS products usually intended to supplement a consumer’s diet,
containing one or more dietary ingredients (including vitamins, minerals, herbs or other
botanicals, amino acids, and other supplements). Formats can include pills, powders, food-like
forms (e.g. gummies), capsules, tablets, or liquids. The Group holds global number one
positions in three of the five largest VMS sub-categories: Multivitamins, Vitamin C Supplements
and Calcium Supplements. Unlike OTC medicines, VMS products are generally regulated in
the same way as foods by relevant government authorities (see also paragraph 4 of Part X
(Regulatory Overview)), and products within this category are distributed across a wide range
of consumer channels, including pharmacy, mass and specialty retail, and e-commerce. The
less complex VMS regulatory environment allows for a more rapid innovation cycle. However,
the comparatively limited constraints and barriers to entry enable smaller or local players to
enter and compete within this growing category.
Oral Health
The £25 billion Oral Health market is the most representative of a “true” FMCG category within
the Group’s consumer healthcare portfolio, albeit one that often requires differentiating
scientific capabilities to successfully compete for market share. The Group holds the global
number three market share position in Oral Health overall and the number one position in the
Therapeutic Oral Health sub-category.
19
The Group also has leading positions in Toothpaste
(number two in a £13 billion market) and Denture Care (number one in a £915 million market).
Other major sub-categories include Toothbrushes, Mouthwash, and Whitening. The current
expectation is that the Oral Health sector could grow by approximately 3 to 4 per cent. per
annum over the medium-term.
20
Regulation in relation to innovation, consumer benefit claims, and distribution is generally less
complex in the Oral Health market when compared to the OTC market, although some
products in the Group’s portfolio are classified as medicines and medical devices, particularly
in Therapeutic Oral Health and Denture Care. Therefore, innovation cycles are typically shorter
and outperforming the market requires differentiation and strong consumer marketing
capabilities combined with a high degree of agility. Distribution is relatively widespread, with
most Oral Health brands readily available to consumers across all major distribution channels
(including e-commerce).
4. Other key themes impacting the consumer healthcare market
Competitive environment
Competitive dynamics: The consumer healthcare market is highly competitive, with brands
differentiating themselves through scientific claims, consumer-driven innovation (including new
product development and claims), premiumisation and distinguished branding. Competition
also leverages traditional FMCG capabilities including consumer and channel marketing.
17
Note the Group’s US Nicorette trade mark, under which the CH Group’s US Smokers’ Health business is commercialised, is
licensed from Johnson & Johnson.
18
Group’s projection for medium term (3 – 5 year) market growth rates based on analysis of third party data. Projection is based
on the Group’s current brand / market footprint.
19
Therapeutic Oral Health ranking is based on Group analysis of third party data from (Nielsen, IRI, Intage, IQVIA Consumption
Sales Data (2021-2022). Therapeutic Oral Health is defined as Therapeutic Toothpaste and Total Dental Appliance Care.
20
Group’s projection for medium term (3 – 5 year) market growth rates based on analysis of third party data. Projection is based
on the Group’s current brand / market footprint.
68

Market consolidation: The OTC/VMS market is highly fragmented, with the top five players
holding a combined global share of 17 per cent. in 2021. Smaller competitors are also highly
regionalised, slowing the pace of consolidation. In contrast, Oral Health is highly consolidated
with the five largest competitors holding 61 per cent. of the market in 2021.
Major competitors: The Group’s competitors fall into four major groups: consumer healthcare
businesses within large pharmaceutical companies; FMCG companies with businesses in
overlapping or adjacent categories; local competitors in specific markets (particularly in China);
and retailer private label companies in the USA, UK, and Australia.
Regional dynamics: The Group’s two most significant markets are the USA and China. These
markets had aggregate market revenue of £41 billion and £35 billion
21
respectively in 2021. In
the USA, the Group holds the number one position in OTC/VMS and the number four position
in Oral Health, with a number three position in Toothpaste and the leading position in Denture
Care. In China, the Group holds the number two position in OTC/VMS (the number one
multinational) and is among the top ten in Oral Health.
Retail and distribution
OTC distribution is heavily weighted to the pharmacy channel globally (68 per cent. of global
revenue), with approximately 25 per cent. in other retail (primarily mass market in the USA and
UK and hospitals in China) and 7 per cent. of revenue in e-commerce. By contrast, VMS has a
greater weighting in e-commerce, with 30 per cent. in e-commerce, 28 per cent. in other retail
and 42 per cent. in pharmacy. Oral Health distribution closely mirrors the broader FMCG
space, with 55 per cent. of 2021 revenue in mass/grocery, 22 per cent. in pharmacy, and
12 per cent. in e-commerce. A further 11 per cent. of Oral Health distribution comes from other
much smaller channels, such as convenience.
22
Pharmacy channel: Pharmacy is the primary distribution channel for both OTC and VMS,
comprising 68 per cent. and 42 per cent. respectively of distribution globally. The Western
European pharmacy channel is both fragmented and highly regulated: Germany, France and
Spain do not permit corporate ownership of pharmacies and, as a result, market participants
must have the capabilities and infrastructure required to partner effectively with a large number
of individual store owners. While Italy is similarly regulated, corporate ownership of pharmacies
is permitted. The UK operates a parallel model, with mass market sales for some OTC/VMS
products permitted, while other OTC products are confined to the traditional pharmacy. Similar
to Western Europe, the bulk of Central and Eastern Europe operate on a pharmacy-regulated
model, with some corporate ownership permitted. This is also the predominant model in Latin
America and Asia. China also follows a primarily pharmacy model with a significant portion of
OTC medicines distributed through in-hospital pharmacies. In North America, the pharmacy
channel primarily consists of large drug store chains (for example, CVS, Walgreens). These
chains share many similarities with the mass/grocery channel (see below).
Mass/grocery: Mass sales of OTC medicines are widely permitted in the USA and permitted for
most OTC/VMS products in the UK and Australia. As a result, competition in these markets
requires strong FMCG-based customer marketing capabilities, including category management
and collaborative planning with major retailers; and the scale necessary to partner with the
world’s largest retailers. Notably, mass market retailers are both a distribution channel and
direct competition in the form of private label, making the ability to compete with private label
via differentiating innovation and strong brand loyalty critical to success in the mass market.
E-commerce: Online consumer healthcare sales have consistently grown at double digit rates
since 2018 and this trend was accelerated by the pandemic in 2020 and 2021 across all
21
Sales of traditional medicines are included where they are packaged and positioned alongside registered OTCs.
22
Source: Euromonitor Passport 2021 consumer sales at manufacturers’ selling prices. Mass/grocery as per Euromonitor’s Grocery
Retailers definition, pharmacy & drugstores as per Euromonitor’s Health and Beauty Specialist Retailers, e-commerce as per
Euromonitor’s e-commerce definitions.
69
regions. Online revenues are most significant to the Group in the USA and China, with
Germany and the UK leading online revenues in Europe. While this trend could be viewed as
disruptive to the traditional status quo and distribution, the resulting increased consumer
availability also represents an opportunity to drive a longer-term increase in both penetration
and category growth. Increasing market share in this evolving segment is dependent on having
the right capabilities to capitalise on this trend, as well as having invested sufficiently to equip
the business to adapt to fulfilling consumer needs in this channel.
70

PART III
BUSINESS OVERVIEW
1. Strengths
23
The Group is one of the world’s leading consumer healthcare businesses with an exceptional
portfolio of brands across its key categories and a strong footprint across the world’s largest
and fastest growing OTC/VMS and Oral Health markets.
The Group is further distinguished by leading consumer healthcare-focused scientific
capabilities, a well-developed organisational understanding of human health behaviours, strong
capabilities in brand building, innovation and digital commerce and a powerful route-to-market.
The Directors believe these represent important competitive strengths, which will support
sustainable above-market medium-term growth and attractive shareholder returns.
1.1 Exceptional portfolio of category-leading brands
The Group’s business is built on an exceptional and focused portfolio of trusted
consumer healthcare brands in attractive categories which provide meaningful
opportunities for growth.
The Group is a global leader in the consumer healthcare market with number one global
category positions in Therapeutic Oral Health,
24
VMS, Pain Relief, Respiratory Health and
Digestive Health. Across these key categories, the Group has an exceptional portfolio of
trusted brands with category-leading positions at a global or local level, including four out of the
world’s top ten OTC/VMS brands by revenue.
25
The Group’s leading brands
Oral Health
Market
Rank
Power brands
Local strategic
brands
VMS Pain Relief Respiratory Health
Digestive Health
& Other
£2.7bn sales 2021 £1.5bn sales 2021 £2.2bn sales 2021 £1.1bn sales 2021
£2.0bn sales 2021
The Group’s portfolio includes nine large-scale multinational Power Brands: Panadol, Voltaren,
Advil, Otrivin, Theraflu, Sensodyne, Polident, parodontax and Centrum, which represented
58 per cent. of revenue in FY 2021. Of these nine brands, Voltaren, Advil, Otrivin, Sensodyne,
Polident and Centrum are the number one or number two brand in their respective
23
See paragraph 1 (Market and Industry Data)ofPresentation of Financial and Other Information for further information on the
use of market and industry data in this Prospectus.
24
Group analysis of third party data from (Nielsen, IRI, Intage, IQVIA Consumption Sales Data (2021-2022). Therapeutic Oral
Health is defined as Therapeutic Toothpaste and Total Dental Appliance Care.
25
Excluding traditional Chinese medicine.
71

sub-categories globally.
26
In addition, Panadol is the leading Systemic Pain Relief brand
outside of the USA, Theraflu has a strong regional European and North American presence in
Systemic Cold and Flu and parodontax is amongst the world’s fastest growing global
Toothpaste brands.
27
The Power Brands are complemented by local strategic brands, which have scale and
leadership positions in key markets. These include, among others, Fenbid (the number two
Systemic Pain Relief brand in China), Emergen-C (the number one immunity VMS brand in the
USA), Grand-Pa (the number one Pain Relief brand in South Africa), Dr.BEST (the leading
Manual Toothbrush brand in Germany), ENO (the number one Digestive Health brand in Brazil
and India) and Tums (the leading Heartburn brand in the USA).
28
The combination of the Power Brands and local strategic brands provides the Group with a
focused, complementary and trusted portfolio which offers scale advantages, meaningful
opportunities for growth and positions the Group well to maximise the return on innovation,
advertising and promotion.
1.2 Attractive geographic footprint with strong presence in large and higher-growth markets
The Group has an extensive footprint across the global consumer healthcare market
and a leading position in the large US and Chinese markets. The Group’s revenue is
well-balanced between developed and emerging markets.
The Group has market-leading scale in the world’s consumer healthcare markets, including a
commercial presence in over 170 markets and a number one or two OTC/VMS position in
countries representing over 70 per cent. of the global OTC/VMS market by value in 2021.
The Group holds a leadership position in key scale and growth markets. This includes
leadership in the approximately £37 billion US OTC/VMS market (the largest market,
representing over 27 per cent. of the total OTC/VMS market) and regional leadership in the
approximately £31 billion European OTC/VMS market (approximately 23 per cent. of the total
OTC/VMS market). The Group also holds the leading multinational (“MNC”) position in the key
OTC/VMS growth markets of China and India (in both cases the Group is number two overall),
together with market leadership in the Asia Pacific region and in the Middle East and Africa
(“MEA”), (together, representing 44 per cent. of the total OTC/VMS market). In China, the
Group is currently the number one multinational consumer healthcare business.
26
Global rankings: Sensodyne #1 Sensitive Toothpaste (ZS 2021), Polident #1 Denture Care (Euromonitor 2021), Centrum #1
VMS, Voltaren #1 Topical Pain Relief, Otrivin #1 Topical Decongestant, Advil #2 Systemic Pain Relief (Nicholas Hall 2021).
27
parodontax is amongst the fastest growing global Toothpaste brands based on Group analysis of Euromonitor Passport data
(2021).
28
Source: Nicholas Hall’s DB6 Consumer Healthcare (OTC/VMS) Database (other than with respect to Dr. BEST); Euromonitor
Passport Database with respect to Dr. BEST.
72
The following chart shows the Group’s combined market ranking in OTC and VMS.
29
The Group’s scale in OTC/VMS is reinforced through its leadership position in Therapeutic Oral
Health
30
and overall number three position in Oral Health.
The Group benefits from a balance of revenue between developed and emerging markets with
approximately one third of the Group’s revenue delivered from emerging markets in FY 2021.
The Directors expect emerging markets in aggregate to grow ahead of developed markets over
the medium-term driven by rising incomes and health awareness.
1.3 Human understanding and trusted science, exclusively focused on consumer health
The Group has leading scientific capabilities focused exclusively on consumer
healthcare combined with well-developed human understanding generated through
dedicated in-house expertise and a range of proprietary consumer insight tools. The
Directors believe that the combination of human understanding and trusted science
drives better innovation and meaningful and positive engagement with both consumers
and experts.
Given the Group’s pharmaceutical heritage, trusted science is firmly embedded in the Group’s
culture and approach, and the Directors believe it possesses scientific capabilities that
differentiate it from other FMCG companies. The Group has a dedicated consumer healthcare
R&D organisation with a multidisciplinary talent pool of approximately 1,400 highly skilled
scientists and a strong network of external partnerships. This is further supported by high
quality R&D facilities which provide a range of capabilities, including fast prototyping, imaging,
product chemistry, microbiology, stability analysis and scale-up and technical transfer.
Since 2017, the Group has conducted nearly 70 clinical studies involving approximately 6,000
participants and has a strong track record of peer-reviewed journal publications and patent
applications. In addition, through its regulatory organisation which has a direct presence across
approximately 60 markets, it has completed approximately 19,000 regulatory applications and
approvals since 2019 in support of both new launches and the continuation of existing
products.
The Group’s focus on trusted science and the generation of evidence-based claims supported
by scientific research is also critical to the Group’s ability to engage with experts and
healthcare professionals with whom it is widely recognised as a partner of choice. Trust is
fundamental to these relationships and is embedded in the Group’s culture.
29
Source: Nicholas Hall’s DB6 Consumer Healthcare (OTC/VMS) Database, 2021 Store and E-commerce sales.
30
Group analysis of third party data from (Nielsen, IRI, Intage, IQVIA Consumption Sales Data (2021-2022). Therapeutic Oral
Health is defined as Therapeutic Toothpaste and Total Dental Appliance Care.
73

Alongside its scientific strengths, the Group has developed a range of capabilities focused on
understanding the health needs of its consumers and the barriers to treatment, and has
invested heavily in consumer insights, data analytics and a range of digital tools. These include
in-house shopper research facilities which enable sophisticated testing of consumer responses
to different retail scenarios, future trend spotting capabilities, and highly developed sensory
labs to source consumer feedback on the taste, texture and smell of the Group’s products. Its
social listening capability draws insights from over 70 million posts a year and its proprietary
‘Observatory’ library holds 53,000 findings on concepts, conditions or culture, as they relate to
health. The Group’s extensive expert engagement generates further insights, including early
visibility of unmet consumer needs.
The Directors believe the synthesis of trusted science and human understanding provides the
Group with competitive advantages in product development and commercialisation. The
Group’s consumer understanding supports the identification and development of products
addressing real consumer needs. Through its scientific capabilities, the Group is able to
develop innovative products which address these needs with claims backed by science and
supporting expert recommendation, which is a key driver of consumer healthcare performance.
In parallel, the Group’s consumer understanding supports product messaging which appeals to
consumers on an emotional level, as well as allowing the Group to target products and product
messaging to the relevant consumer audiences.
1.4 Strong brand building, innovation and digital capabilities combined with a leading
route-to-market
The Group has well-proven capabilities in building brands and campaigns that resonate
with consumers and in delivering innovation to meet consumer healthcare needs
together with the ability to reach across all key channels for consumer healthcare
products, including e-commerce.
The Group has a track record of building trusted and enduring brands across different
geographies and consumer populations and seeks to build real and personal connections in its
communications with consumers, underpinned by its trusted science. This means making a link
to the broader context of consumers’ needs, not merely focusing on functional benefits. One
example is the successful 2020 launch of Voltaren Arthritis Pain in the USA, following its
successful switch from prescription status. The promotional campaign focused on the brand’s
ability to restore the joy of movement to sufferers of arthritis pain, building upon the strong
functional claims of the product but focusing on the human impact of pain relief. The launch
was supported by an innovative website, tailored to the needs of arthritis sufferers with voice
search functionality, large tap targets, scalable font sizes, and the ability to view hands-free
content via videos and head-gesture-scrolling. In its first year of sales, the launch outperformed
any competitive OTC launch in the US Pain Relief market since 2011
31
and drove more than
80 per cent. of Topical Pain Relief category growth in the US market in the year of launch.
32
The Group’s commercial organisation is supported by innovative facilities and tools including
its shopper science labs, the Group’s own in-house content production studio, “CaST”, and
proprietary artificial intelligence tools which support dynamic content and media optimisation.
The foundations of the Group’s business lie in addressing real everyday health needs and
thereby delivering penetration and growth. The Group has demonstrable capabilities in the
delivery of innovation based on human insights to address consumer needs. For example, in
North America, the Group launched Tums Chewy Bites in 2017 having identified that
millennials, whilst having high rates of heartburn, were reluctant to treat the condition due to
negative perceptions of the product taste and perceived lack of relevance to them. The
colourful and fruit-flavoured chewy bites format addressed this perception whilst providing
31
Source: IRI Consumption Data from Market Advantage and Xlerate, FY2011-FY2021.
32
Source: Group analysis based on external data (IRI Market Advantage, Consumption Data).
74

convenience, a characteristic highly valued by this group. Building on the launch in 2017, the
Group has continued to innovate through products with new flavours and sensory properties
(for example, Chewy Bites Cooling Sensation), as well as products treating both heartburn and
bloating. Continued innovation brought 3.8 million new consumers into the category in the
USA
33
and drove US Tums Chewy 3 year compound consumer sales growth of 31 per cent. to
November 2021.
34
In addition to its marketing and innovation capabilities, the Group has a strong and established
presence in all key channels relevant for consumer healthcare and a scale which allows it to
effectively engage with retail partners of all sizes, buying groups, distributors, pharmacy chains
and individual pharmacies. In Europe, where the Group’s products are primarily sold through
small-scale pharmacies, this supports the Group’s number one ranking in the pharmacy
channel and, in key European markets, country average weighted distribution
35
levels in
pharmacy for the Group’s Power Brands of between 70 and 98 per cent. In mass market retail,
the Group is ranked second and has weighted distribution in key European markets for its
Power Brands of between 76 and 94 per cent.
36
In the mass channel in the USA and
elsewhere, the Group benefits from strategic partnerships with large retailers, supported by its
state-of-the-art shopper science labs which facilitate joint business planning.
The Group has also invested significantly in building local e-commerce capabilities and
strengthening strategic partnerships with global and local leaders. Alongside its digital
marketing capabilities, this has enabled the Group to more than double its e-commerce sales
between FY 2019 and FY 2021.
A key part of the Group’s global reach is also its ability to engage with experts and healthcare
professionals who play a significant part in product recommendation. For example, in OTC/
VMS, 85 per cent. of pharmacist recommendations lead to a purchase, and in Oral Health,
studies have shown that dentist recommendations have a significant influence over oral health
behaviours. As a result of its reach and focus on trusted science, many of the Group’s leading
brands across the portfolio are the number one recommended in their categories by experts in
the Group’s major markets.
37
2. Strategy
The Group has a clear and focused strategy to drive sustainable above-market growth and
attractive returns, guided by its purpose of delivering better everyday health with humanity.
This strategy is built on four pillars: growing the portfolio by driving household penetration,
capitalising on new and emerging growth opportunities, strong execution and financial
discipline and running a responsible business.
33
Source: IRI National Consumer Panel Data, FY2016-FY2021.
34
Source: IRI point of sale data, multi-outlet (MULO) + convenience + ecommerce Nov 2021.
35
Weighted distribution is the percentage of points of sale where a product is available, assigning to each point of sale, a weight
proportional to its sales.
36
Based on Group analysis of distribution data for the Group’s Power Brands (excluding Advil which is primarily a North American
brand) in a cross section of European markets, including Poland, Germany, Great Britain, Italy and Spain.
37
Based on surveys of healthcare professionals carried out in 2020 by Ipsos across 30 markets in the OTC and/or Oral Health
categories. In the majority of cases, the Group’s brands covered emerged as the brand recommended most often to patients by
the healthcare professionals surveyed.
75

2.1 Drive portfolio growth by increasing household penetration
The Directors believe there is significant opportunity for further penetration of its
brands across its categories. The Group has a clear and proven approach to driving
penetration growth.
Penetration in some of the Group’s key categories is still relatively low, and the Directors
expect to deliver significant continued growth from the Group’s current portfolio. In Oral Health,
nearly 1 in 3 adults have experienced sensitive teeth, but only 1 in 3 of those experiencing
sensitivity use a sensitivity toothpaste like Sensodyne.
38
In Pain Relief, 9 out of 10 people
suffer from pain, but only 1 in 3 of them immediately treat their pain.
39
In China, where calcium
intake is less than 50 per cent. of the daily recommended level
40
only approximately 17 per
cent. of people take a calcium supplement like Caltrate.
41
The Group has a clear and proven approach to driving penetration growth which utilises its key
capabilities in human understanding, trusted science, innovation and marketing, supported by
strong commercial execution. This can be illustrated by the successful growth of Sensodyne,
which delivered over 10 per cent. compound revenue growth between FY 2011 and FY 2021.
The Group’s strategy for Sensodyne incorporates the building of condition awareness and
relevance. Many sufferers of tooth sensitivity do not recognise it as a health condition and one
which can be treated. The Group’s communications address all sensitivity sufferers, using a
data-driven approach to tailor relevant messages for different target audiences across
channels.
Product innovation based on consumer insights has been a key driver of Sensodyne’s growth.
The Sensodyne product range has expanded well beyond the original formulation to offer a
range of benefits in response to different consumer needs. The range now includes, amongst
others, Sensodyne Rapid Relief offering pain relief within 60 seconds; Sensodyne Pronamel
which protects teeth against acid erosion; Sensodyne Repair and Protect which repairs,
strengthens and protects sensitive teeth; and, more recently, Sensodyne Sensitivity and Gum,
which has a clinically proven dual action formula for sensitive teeth and gum problems.
In addition, expert advocacy has been a core strength of Sensodyne which is the number one
dentist-recommended brand for sensitive teeth in 24 of 30 markets tracked.
42
Dental
recommendation has been built on the foundation of the Group’s trusted science with science -
backed claims developed by the Group and a large expert field force combined with
Healthpartner, the Group’s website for healthcare professionals ensuring awareness of the
benefits of Sensodyne products.
Finally, the Group’s commercial execution, both online and in-store, ensures that Sensodyne
has high levels of visibility and the right assortment of packs to support commercial
opportunities.
38
Source: Oral Health Population Data – IPSOS Incidence Study Calculations 2015: figures are averages.
39
Source: Edelman Intelligence, GP14, 2020, 19 markets, 19,000 respondents.
40
Source: Chinese Center for Disease Control and Prevention (2021).
41
Source: Penetration data from Kantar (2020).
42
Source: Group analysis based on Ipsos data from expert performance tracking study 2019-2020.
76

The Group is applying the same approach as on Sensodyne across other brands and markets.
For example, the Group’s parodontax messaging focuses on raising consumer awareness of
gum disease with many sufferers of bleeding gums unaware that this is a sign of gum disease
with the long-term risk of tooth loss. This has been supported by science-based product claims
(“parodontax is a toothpaste that is clinically proven to help reduce bleeding gums”) and
innovation of a range of different products focusing on different consumer needs including gum
health, whitening, gum repair and complete protection.
2.2 Capitalise on new and emerging growth opportunities
The Group plans to leverage its growing capabilities in e-commerce and expand its key
brands across leading markets. It will continue to pursue Rx-to-OTC switches in the
USA and capitalise on accelerating consumer trends.
Channel expansion: e-commerce
Over recent years, partly driven by the COVID-19 pandemic, there has been a significant
consumer shift to e-commerce with market compound e-commerce growth of approximately
24 per cent. per annum between 2018 and 2021.
43
The Directors expect this momentum to
continue.
As a result of the investments made in digital capabilities and strategic partnerships with
leading e-commerce companies, the Directors consider the Group to be well positioned to
capitalise on e-commerce growth. This is grounded in the Group’s strong recent performance.
Since 2019, the Group has delivered above-market growth in e-commerce sales, which have
grown from 4 per cent. of revenue in FY 2019 to 8 per cent. in FY 2021 (constituting growth of
over £0.4 billion in digital revenue). Significantly, the Group holds strong positions in more
developed e-commerce markets, such as China and the USA. In China, e-commerce revenue
represented 20 per cent. of FY 2021 revenue with FY 2020 to FY 2021 growth of over 40 per
cent. In the USA, e-commerce revenue represented 12 per cent. of FY 2021 revenue and with
FY 2020 to FY 2021 growth of over 35 per cent. E-commerce growth is a clear priority and an
area where the Group aims to build on the investments it has already made.
Geographic expansion
The Directors believe that the Group’s brand portfolio and powerful route-to-market capabilities
offer multiple opportunities to expand its brands beyond their existing geographies.
For example, in India and in many MEA markets, the business has a consistent performance
track record and strong route-to-market with 4 million distribution points in India and 80 per
cent. weighted distribution across MEA. The Group has experienced double digit growth in
MEA over the last two years and strong double digit growth in India over the last five years. In
each case, however, the majority of revenue currently comes from a small number of brands.
In FY 2021, over 75 per cent. of revenue in India was accounted for by ENO and Sensodyne
and over 48 per cent. of revenue in MEA was derived from Panadol and Sensodyne. The
Directors believe there is significant opportunity to leverage local capabilities to expand other
brands in the portfolio into these and other markets.
The Directors believe that its leading portfolio includes a number of brands which are well-
positioned for geographic expansion. For example, poor gum health, a common condition
worldwide, offers opportunity for the expansion of parodontax into additional markets through
application of the same growth model as Sensodyne. The brand was launched in India in the
second quarter of 2021 and has delivered strong initial performance.
Additionally, Centrum, although present in over 70 markets, is highly concentrated
geographically with approximately two thirds of revenue in FY 2021 coming from five markets.
The Directors see an opportunity to grow the brand within its existing footprint by leveraging
43
Source: Group analysis based on market data sourced from Nicholas Hall and Euromonitor.
77

the Group’s scale and route-to-market. This is supported by recent experience in the EMEA
and LatAm region with approximately 14 per cent. Centrum revenue growth in this region in FY
2021 compared to FY 2020.
Portfolio expansion: Rx-to-OTC switches
The Group continues to pursue Rx-to-OTC switches which have historically been a key source
of innovation and growth in OTC, especially in the USA. The Group has a strong track record
of switching both GSK and non-GSK products driven by a long-standing dedicated in-house
team and has implemented four Rx-to-OTC switches in the USA since 2014. This includes the
most recent switch of Voltaren Arthritis Pain in 2020, which drove 80 per cent. of market
revenue growth in the Topical Pain Relief category in the USA in 2020. The Group currently
has two active switch projects in its pipeline with expected launches (if successful and
approved) in 2025 and 2026 and is exploring further opportunities both within and outside of its
key categories.
Portfolio expansion: emerging consumer trends including Naturals
The Group intends to continue to capitalise on accelerating consumer trends, for example, the
growing consumer trend for natural products. Consumer healthcare products that are
non-medicated, ‘free from’ particular ingredients, or that include plant-based, herbal or other
naturally occurring ingredients (“Naturals”) are increasingly popular, especially amongst
younger consumers, with growth exceeding the market average. Consumers are increasingly
looking for natural products across disease prevention, treatment and recovery and the
Directors believe this trend will continue.
The Group sees an opportunity to expand its Naturals offering across relevant portfolios and
has launched 10 Naturals innovations since the beginning of FY 2021, including launches such
as Voltanatura, Centrum Whole Food, Sensodyne Nourish and Tums Naturals. 25 further
Naturals products are in the pipeline.
2.3 Underpin performance with strong execution and financial discipline
The Group will continue to focus on driving efficiency, effectiveness and agility to make
every investment count.
The Group has made significant progress in recent years in driving efficiency effectiveness
across its operations. Over the last seven years, the Group has optimised the manufacturing
footprint inherited from the legacy GSK, Novartis and Pfizer consumer healthcare businesses
from 41 sites to 24, whilst restructuring its supply chain such that manufacturing is increasingly
co-located in the same region as the end consumer (80 per cent. of product supply sourced
within the same region). This allows the Group to manufacture at scale, whilst retaining the
cost and responsiveness benefits of local sourcing. In the same period, the Group has more
than halved its number of distribution centres and has reduced its contract manufacturers from
approximately 250 inherited from the legacy GSK, Novartis and Pfizer businesses to
approximately 180 in 2022, thereby gaining scale benefits and reducing management costs.
Similarly, between 2019 and 2021, the Group’s marketing organisation reduced its creative
media and production agencies from 200 to 56 and the organisation has continued to optimise
how advertising and promotion spend is deployed with an increased focus on digital (doubling
between FY 2019 and FY 2021 to almost half of advertising and promotion spend in FY 2021).
Investments in AI tools such as People-Cloud and consumer data have significantly increased
the efficiency of this expenditure. In 2021, the Group was able to deliver a 185 per cent. return
on data-driven media spend.
44
44
Data-driven media spend is digital media spend targeting new consumers identified by data-driven consumer segmentation. These
consumers are served with media and messages relevant to their specific profiles. The 185 per cent. return refers to incremental
revenue generated relative to digital media expenditure.
78
In terms of in-market commercial execution, the Group has empowered its local markets to
innovate, increasing the Group’s agility in adapting to changing consumer healthcare needs.
800 R&D and category roles have been moved or re-aligned to local markets for 2022 and in
the USA, for example, approximately 68 per cent. of 2022 innovation projects are expected to
be locally managed. The Group has also built specialised tools that enable better local
commercial execution. For example, the Group’s sales teams across EMEA and LatAm are
supported by a customer relationship management system which ensures sales representative
calls are efficient and effective. Through this system, representatives are able to complete their
commercial activities, capture instore excellence key performance indicators, and deliver
category and product training and education. The Group is also now utilising image recognition
and machine learning across many retail stores in order to ascertain distribution and visibility
metrics that can then drive improvements with the objective of optimising sales. Similarly, the
Group’s shopper science labs inform its commercial practices to improve the experience of
retailers and consumers.
A sharp focus on net revenue management has been a further lever through which the Group
has sought to optimise its margins, including strategic initiatives such as increased penetration
of Power Brands in key markets such as India (typically Power Brands have higher margins
than the Group as a whole) and increased focus on improving returns on trade investment
spend. These initiatives have positively impacted margins and supported approximately 2.2 per
cent. price growth in 2021 (excluding divested brands and at CER), complementing an
approximately 1.8 per cent. growth in volumes (excluding divested brands).
The Group’s strategy of efficient commercial execution and cost discipline allows it to deliver
moderate operating profit margin expansion whilst reinvesting a share of cost savings delivered
in future growth through targeted investment in advertising and promotion, and innovation. This
in turn supports delivery of increased growth and growth in free cash flow creating further
operating leverage and efficiencies.
Between FY 2019 and FY 2021, the Group successfully increased operating profit margin and
Adjusted operating profit margin by 6.6 percentage points and 3.3 percentage points,
respectively, despite an adverse foreign exchange movement and an adverse impact from
divestment of growth-dilutive brands which received limited advertising and promotion support.
Over the same period, the Group reinvested a share of operating cost savings into advertising
and promotion spend on brands to support future growth.
The Group has additionally delivered net cash inflow from operating activities of £3.5 billion
and free cash flow of £3.8 billion, in each case across the period FY 2019-2021. Healthy cash
flows from operations have been strongly supported by a sharp focus on working capital
discipline and stable capital investment.
The Group is a business with strong operating profit margin (FY 2021: operating profit margin
of 17.2 per cent. and Adjusted operating profit margin of 22.8 per cent.) with above-market
growth supported by robust investment in its brands (FY 2021: advertising and promotion
expenditure as a percentage of revenue of 20.3 per cent., R&D costs as a percentage of
revenue of 2.7 per cent. and Adjusted R&D costs as a percentage of revenue of 2.6 per cent.).
The Group’s strategy is to maintain its sharp focus on business optimisation and cost control
whilst reinvesting a share of savings for future growth. This includes the delivery of the
remaining synergies from the integration of the Pfizer consumer healthcare business, other
ongoing projects in net revenue management and manufacturing and further operational costs
savings.
79

2.4 Run a responsible business
The Group’s responsible business agenda is intrinsically linked to its sector focus and
its purpose of delivering better everyday health with humanity. The Group has a
structurally advantaged environmental footprint in its sector and is strongly positioned
to advance health inclusivity. The Group is committed to building strong corporate
governance across the business.
Running a responsible business is intrinsically linked to the Group’s purpose and integral to
how the organisation operates. The Group recognises that the health of the world’s
environment affects the health of people and is committed to tackling the environmental and
social barriers to everyday health. The Group’s brands have clear and positive roles to play in
protecting and improving everyday health and doing so in inclusive and responsible ways.
The Group has a relatively small environmental footprint in terms of carbon intensity (2020
Carbon intensity scope 1-3 0.2kg CO2e/£ of revenue) and plastic packaging (2020 plastic
packaging footprint of approximately 50,000 tonnes). This is driven by the nature of its product
portfolio, a significant part of which is made up of precisely-dosed, small-sized premium
products which are bought and typically used over an extended period of time and which
therefore utilise less energy for manufacturing and plastic for packaging per £ of revenue. A
further structural advantage is that the Group’s products are less exposed to agricultural
ingredients which require high resource intensity in manufacturing. Such structural advantages
provide the Group with a strong foundation in terms of reduced risk exposure and the required
capital expenditure to further advance its environmental and social agenda. In addition, the
Group has a lower financial exposure to carbon taxation, plastic regulations or taxation, and
rising energy costs.
The Group’s environmental focus is to tackle the barriers to everyday health, focusing on
carbon footprint and climate change, sustainable healthcare packaging, and using trusted
ingredients that are sustainably sourced. The Group’s targets include: 100 per cent. reduction
in scope 1 & 2 (internal operational) carbon emissions by 2030 (versus its 2020 baseline);
42 per cent. reduction in scope 3 (from source to sale) by 2030; and 100 per cent. recyclable or
reusable packaging by 2030 versus its 2020 baseline (where quality, safety and regulations
permit, given the strict regulation of packaging requirements for certain healthcare products).
As a standalone business, the Group will set a longer-term carbon net zero goal informed by
the latest Science Based Targets initiative (“SBTi”) guidance.
45
The Group has a track record of delivering against these targets: 100 per cent. of electricity
used by the Group comes from renewable sources; renewable electricity generation has been
implemented at 12 out of its 24 manufacturing sites; and new solutions are being developed to
support delivery of a significant reduction in the use of virgin petroleum-based plastic. These
include sustainably-sourced Pulpex pulp-based packaging alternatives; new bamboo and
biobased plastic toothbrushes; and recycle-ready toothpaste tubes on track to reach one billion
tubes using recyclable plastic by 2025 (in line with Group’s target for all packaging to be
recyclable or reusable by 2030).
The Group sees health inclusivity or a lack of it as a critical factor for everyday health and
believes its leading global position in the consumer healthcare market offers it the opportunity
to make a meaningful positive impact in this area. The Group has set a target to help 50 million
people per annum by 2025 to gain access to opportunities for better everyday health
irrespective of their age, physical and mental capabilities, gender, ethnicity or sexual
orientation. The Group has identified a range of different programmes to achieve this. Across
its brand portfolio the Group will seek to provide inclusive products, services and resources
that help more people to access the care and support they need. The Group will continue to
focus on educating consumers and empowering self-care, supporting health literacy and
educational programmes for individuals and healthcare professionals. Finally, the Group will
45
SBTi is a collaboration between the United Nations, the World Resources Institute, the World Wide Fund for Nature and CDP, an
environmental reporting charity. SBTi provides companies with accreditation of science-based climate targets. SBTi however,
does not accept separate targets for business divisions within a wider company and therefore the Group will seek SBTi
accreditation post demerger from GSK.
80

utilise its reach, resources and expertise to cooperate with other experienced partners in the
field of healthcare and inclusivity. One such example is its partnership with the Economist
Intelligence Unit and leading academics to create the Health Inclusivity Index which is
expected to launch in July 2022 to facilitate dialogue with and among key stakeholder groups
and to identify opportunities and actions.
Within its own organisation, the Group is committed to inclusion, equality and diversity. This will
include, but not be limited to, the setting of ambitious targets on female and ethnic minority
representation in leadership roles.
The Group has a robust operational governance structure and is committed to building strong
corporate governance practices across its business.
3. Financial outlook and dividend policy
The Directors believe that the Group offers a compelling growth outlook supporting attractive
shareholder returns. Over the medium-term, the Directors expect the Group to deliver above-
market annual organic revenue growth of 4 to 6 per cent., alongside sustainable moderate
margin expansion and strong cash generation and conversion. The Group’s strong cash flow
supports its capital allocation priorities which are geared to investment in growth, a strong
investment grade balance sheet and an initial dividend which is expected to be at the lower
end of a 30 to 50 per cent. pay-out ratio, subject to Board approval.
Medium-term outlook
The Directors expect to deliver 4 to 6 per cent. annual organic revenue growth over the
medium-term, ahead of expected medium-term annual growth in the Group’s end markets of
approximately 3 to 4 per cent.
46
The Group grew ahead of the market in both 2020 and 2021, delivering organic revenue
growth of 2.8 per cent. in FY 2020
47
and of 3.8 per cent. in FY 2021. The growth of both the
Group’s business and the overall consumer healthcare market was adversely impacted by the
COVID-19 pandemic over this period.
The Directors expect the COVID-19 headwind to diminish from 2022 onwards and project
annual market growth of 3 to 4 per cent. in the medium-term. The Directors expect the Group
to deliver an acceleration of its organic revenue growth to 4 to 6 per cent. annually over the
medium-term with this above-market revenue growth driven by the Group’s strategy and
underpinned by its scale, exceptional brand portfolio and well-developed capabilities.
Sustainable moderate expansion in operating profit margin
The period FY 2019 to FY 2021 was characterised by a strong uplift in Adjusted operating
profit margin from 19.5 per cent. in FY 2019 to 22.8 per cent. in FY 2021, driven by the
integration benefits from the combination with the Pfizer consumer healthcare business,
coupled with operating leverage from revenue growth and efficient cost management in spite of
headwinds from currency rate movements and divestments. Over the same period, the Group
reinvested over £200 million of cost savings in A&P to support accelerated revenue growth.
Although the Directors expect the Group to incur incremental costs in order to operate as a
standalone business (see Separation and standalone costs below), the Directors expect the
46
Group’s projection for medium term (3 – 5 year) market growth rates based on analysis of third party data. Projection is based
on the Group’s current brand / market footprint.
47
Organic revenue in FY 2020 excludes revenue attributable to the brands acquired as part of the Pfizer Transaction in the period
1 January 2020 to 31 July 2020 (see paragraph 6.3 of Presentation of Financial and Other Information), which made a significant
contribution to the Group’s revenue in the VMS and Pain Relief categories during the period, with the effect that the overall
growth of the Group from 2019 to 2020 was reduced on an organic basis.
81
Group to deliver sustainable, moderate expansion in Adjusted operating profit margin over the
medium-term, assuming constant exchange rates. This reflects the benefits of net price and
product mix optimisation, efficiencies in the supply chain and continued cost discipline, offset in
part by reinvestment in innovation and A&P to drive growth.
Separation and Admission costs, Restructuring costs and standalone operating costs
The Directors expect the Group to incur Separation and Admission costs of approximately
£0.4 billion between FY 2022 and FY 2024, most of which are expected to be incurred in FY
2022. These include Admission costs of up to £0.1 billion. In addition, the Directors expect the
Group to incur Restructuring costs of approximately £0.2 billion between FY 2022 and FY 2024
in connection with projects to support further efficiencies in its operations. The Directors do not
currently anticipate any further significant restructuring programmes beyond this. Both
Restructuring costs and Separation and Admission costs are treated as an Adjusting Item for
the purpose of calculating Adjusted operating profit.
The Directors also expect the Group to incur recurring standalone operating costs of
approximately £175-200 million per annum from 2022 onwards to provide the capabilities to
operate successfully as a standalone UK public listed company, including in technology and
infrastructure, and in corporate support functions.
Strong cash conversion
The Group delivered £3.8 billion free cash flow over the period 2019 to 2021 driven by a sharp
focus on working capital discipline and stable capital investment of approximately 3 per cent. of
revenue per annum.
Over the medium-term, the Directors expect the Group to deliver strong free cash flow
conversion, enabled by the Group’s robust capital base with stable capex, a sharp focus on
working capital, and cost and cash discipline.
Disciplined capital allocation
The Group will apply a disciplined approach to capital allocation. The Group’s first capital
allocation priority will be focused re-investment to drive sustainable revenue growth and
attractive returns. Second, the Group will pursue a dividend policy which will reflect the long-
term earnings and cash flow potential of the Group, consistent with maintaining sufficient
financial flexibility and meeting the Group’s capital allocation priorities (see Dividend Policy
below). Third, the Group will pursue selective “bolt-on” acquisitions where the opportunities are
commercially compelling and consistent with the Group’s strategy. The Group’s capital
allocation priorities will be delivered in the context of maintaining an investment grade balance
sheet with a target net debt to Adjusted EBITDA ratio of less than 3x by the end of 2024.
Considerations for 2022
The Directors expect the Group to achieve organic revenue growth in the range of 4 to 6 per
cent. for FY 2022.
The Adjusted operating profit margin in FY 2022 is expected to benefit from the following:
• favourable volume mix benefits, reflecting continued strong growth and outperformance
from its Power Brands;
• the annualisation of pricing action taken in 2021 and further planned pricing;
• further supply chain efficiencies; and
• additional cost synergies resulting from the completed integration of the Pfizer
consumer healthcare portfolio of approximately £600 million (£100 million higher than
previously announced) with approximately £120 million expected to be delivered in FY
2022.
82
In addition, there are several factors which are expected to have a negative impact on the
Adjusted operating profit margin in FY 2022:
• the Group will continue to invest in its brands, maintaining its approach of investing in
A&P ahead of revenue growth on a targeted basis;
• inflationary cost pressures affecting the cost of commodity-related inputs, salaries and
wages and other activities performed by the Group, although the Directors believe a
range of possible mitigating actions are available to the Group; and
• the 2022 margin will reflect a full year of the new annual costs associated with running
the Company as a standalone public company of £175-200 million.
These revenue and margin expectations for FY 2022 assume no major unforeseen
macroeconomic or geopolitical developments arise after the publication of this Prospectus.
Dividend policy
Following the Demerger, the Company will adopt a dividend policy, which will reflect the long-
term earnings and cash flow potential of the Group, consistent with maintaining sufficient
financial flexibility and meeting the Group’s capital allocation priorities. The initial dividend is
expected to be at the lower end of a 30 to 50 per cent. pay-out ratio, subject to Board approval.
The Company expects to pay a dividend to Shareholders in relation to the second half of 2022
in H1 2023, subject to Board approval and following approval of the Company’s FY 2022
results.
4. Categories and brands
The Group operates across five categories. These are (i) Oral Health; (ii) VMS; (iii) Pain Relief;
(iv) Respiratory Health; and (v) Digestive Health and Other.
FY 2021 Revenue Split by Product Category
Respiratory Health
VMS
Digesve Health and Other
Pain Relief
Oral Health
29%
23%
20%
16%
12%
0% 5% 10% 15% 20% 25% 30%
The Group is the global market leader in OTC/VMS (which includes Pain Relief, Respiratory
Health, Digestive Health and VMS), as well as the third ranked player in Oral Health.
83

The Group has a portfolio of established consumer brands with strong brand equities at a
national, regional and global level. Following a progressive rationalisation and focusing of the
Group’s portfolio, 58 per cent. of the Group’s 2021 revenue was accounted for by the nine
Power Brands: Panadol, Voltaren, Advil, Otrivin, Theraflu, Sensodyne, Polident, parodontax
and Centrum. These are large-scale brands that drive the greatest growth for the Group with
market-leading positions, an attractive geographic footprint and long-term growth potential.
The world’s leading Sensitivity Toothpaste (and no.2
overall Toothpaste).
The world’s leading Denture Care brand.
Among the world’s fastest growing global Toothpaste
brands.
48
The world’s leading Topical Pain Relief brand.
The world’s no. 2 Systemic Pain Relief brand.
The leading Systemic Pain Relief brand outside the US.
Europe’s no. 2 Systemic Cold and Flu brand.
North America’s no. 3 Systemic Cold and Flu brand.
The world’s leading Nasal Decongestant brand.
The world’s leading Multivitamin.
Additionally, the Group has a range of local strategic brands which have scale and leadership
positions in key markets and contribute significantly to the Group’s business, particularly in the
USA and China.
The Group’s leading brands are detailed by category below.
4.1 Oral Health
The Group has one of the world’s leading Oral Health businesses, with operations in over 120
markets and a nearly 100-year track record in successful innovation and manufacture of quality
Oral Health products. The Group specialises in Therapeutic Oral Health, where it is the global
market leader,
49
providing therapeutic solutions to consumers for the prevention and treatment
of specific oral health conditions including sensitivity, acid erosion, gum disease, denture care
48
parodontax is among the world’s fastest growing global Toothpaste brands based on Group analysis Euromonitor Passport data
(2021).
49
Source: Group analysis of third party data from (Nielsen, IRI, Intage, IQVIA Consumption Sales Data (2021-2022). Therapeutic
Oral Health is defined as Therapeutic Toothpaste and Total Dental Appliance Care.
84

and dry mouth. This focus both allows the Group to leverage its leading capabilities in scientific
research, expert engagement and human understanding and allows it to focus on some of the
fastest growing premium oral health segments. This approach has enabled the Group to
command a price premium over the market and grow ahead of the global Toothpaste market
every year since 2015.
50
The Group operates in a highly competitive Oral Health market, estimated to be worth
£25 billion worldwide. The Group focuses primarily on Toothpaste and Denture Care, although
it also has a significant presence in the Mouthwash and Manual Toothbrush markets (including
its leading Manual Toothbrush brand in Germany, Dr.BEST). In Toothpaste, the Group is one
of a small number of global players and it is ranked second in the market worldwide. In Denture
Care it is the market leader.
In FY 2021, the Group generated revenue of £2.724 billion across its Oral Health portfolio.
Sensodyne
Sensodyne is the number two Toothpaste brand globally and the number one dentist-
recommended Toothpaste worldwide for sensitivity. Its purpose is to “help humanity reclaim
life’s small pleasures without the restrictions of sensitive teeth” and, since its launch in 1961, it
has become the brand most associated by consumers with the care of sensitive teeth across
virtually all of its key markets.
Tooth sensitivity is a common condition with nearly one third of the global population
experiencing symptoms, nevertheless only one third of sufferers purchase a sensitivity
toothpaste, thereby offering the opportunity for significant penetration-led growth.
51
The
combination of growing consumer awareness, recommendations of sensitivity toothpastes by
dental experts, favourable demographics and rising incomes in higher-growth economies,
provides an opportunity for the Group to leverage Sensodyne’s brand recognition and
innovation capability.
Building on its long history of innovation and pioneering science, Sensodyne has moved
beyond a primary focus on sensitivity-related conditions to encompass other oral health
conditions. These include innovative products developed in response to a range of identified
consumer needs, such as speed of relief and whitening. For the former, the Group first
launched Sensodyne Rapid Relief in 2010 offering fast pain relief from sensitivity within 60
seconds. An improved, clinically proven formulation providing rapid relief combined with the
additional benefit of long-lasting protection was launched in 2017. For the latter, the Group has
launched tailored whitening variants of Sensodyne Repair and Protect, a product that can
repair sensitive areas of teeth to relieve dentine hypersensitivity. These have increased
penetration with sensitivity sufferers who also want the benefit of whitening.
The Group continues to enhance Sensodyne’s product mix, (which also includes mouthwashes
and toothbrushes) and increase value through premiumisation. Most recently, Sensodyne
Nourish was launched, which is formulated with a bio-active mineral to nourish and strengthen
teeth, and is blended with natural mint and essential oils. The range is vegan-friendly with a
recycle-ready tube, cap and carton.
The brand has continued to outperform the global toothpaste market, reflecting underlying
brand strength, successful innovation and strong consumer uptake in traditional retail and
e-commerce channels.
50
Growth rates for both Toothpaste market size and the Group performance calculated in Retail Value RSP, GBP Million, fixed
2021 exchange rates.
51
Source: Oral Health Population Data – IPSOS Incidence Study Calculations 2015.
85

Polident
The Group is the global market leader in Denture Care (fixatives and cleansers) with
leadership positions in eight of the top ten Denture Care markets. Its denture care products are
sold under three major brands – Polident, Corega and Poligrip (“Poli/Corega”) – across
approximately 60 countries. Poli/Corega is the leading Denture Care brand family, operating in
both cleansers and fixatives at a global scale, and is highly recommended by dentists.
The Group has a deep human understanding of the profound impact and burden that wearing a
denture can have on people’s lives. Poli/Corega’s purpose is to lighten the load for all dental
appliance wearers, providing solutions that give people the confidence to live life without
worrying about their dentures or appliance, granting them the security to eat, speak, kiss and
smile freely. Polident denture cleansers have a low-abrasive formula and help keep dentures
clean and fresh, killing 99.99 per cent. of odour-causing bacteria in lab tests. Polident denture
fixatives provide strong hold, food seal and comfort. The Poli/Corega range includes products
for denture wearers who wear either full or partial dentures, as well as for people with
appliances including mouthguards and retainers.
The Group continues to innovate and develop new product offerings under the Poli/Corega
brand family. In the USA, Poligrip Max Seal was developed as a fixative with a precision nozzle
to help block out food particles. Poligrip Cushion and Comfort utilises Adaptagrip technology
and forms a unique gel layer which acts as a cushion to help provide gum comfort. Polident
ProGuard and Retainer has been launched in the USA as a fast and easy-to-use cleanser in
both tablet and foam form, and is compatible with materials often used for removable dental
appliances.
parodontax family
parodontax is among the world’s fastest growing global Toothpaste brands
52
and the largest
gum health brand (outside of China). parodontax is dedicated to winning the fight against the
devastating progression of gum disease and, alongside its sister brands, Corsodyl and
Chlorhexamed (together, the “parodontax family”), it offers a range of specialist gum care
products, which are designed for people looking to keep their gums healthy. While the Group
has successfully built a sizeable presence in certain markets, including the USA, the
parodontax family is yet to be introduced in many others which offer the potential for
geographic expansion.
There are 2.5 billion people globally who suffer from gum disease, with an incidence of one in
three people spitting blood when they brush their teeth (a sign of gum disease).
53
parodontax
has a distinctive brand equity which seeks to de-normalise bleeding gums and thereby help
address the world’s sixth largest disease. Developed in 1937, parodontax has a long heritage
in Europe in the prevention of gum disease. parodontax Original, in most markets outside of
the USA, is formulated with sodium bicarbonate to help break down plaque, making it easier to
remove, and it is clinically proven to be four times more effective at targeting the cause of
bleeding gums compared to regular toothpaste. In the US, stannous fluoride is used as the
active ingredient, making parodontax toothpaste three times more effective than a sodium
monofluorophosphate toothpaste at removing plaque bacteria.
The parodontax family product range also includes mouthwash, toothbrushes, gel and spray.
Corsodyl Intensive Treatment is used to treat the one third of gum sufferers who have
persistent bleeding gums. Corsodyl Treatment mouthwash contains 0.2 per cent.
chlorhexidine, which starts to kill the bacteria that cause plaque within 30 seconds; it also
forms a protective antibacterial layer over the teeth and gums to prevent plaque build-up for up
to 12 hours. The Group continues to expand its product range under parodontax. In response
to growing consumer demand for natural ingredient based products, parodontax Herbal
52
Source: Group analysis of Euromonitor Passport data (2021).
53
Source: Oral Health Population Data – IPSOS Incidence Study Calculations 2015.
86
toothpaste was launched in 2019 and rolled out across key EMEA markets in 2020. In Q1
2021, in the USA, the Group launched parodontax Active Gum Repair Toothpaste, a
formulation designed to help reverse early signs of gum damage by killing plaque bacteria at
the gum line.
Other Oral Health brands
The Group also has a number of locally important brands, which complement the Power
Brands above. For example, Dr.BEST is the leading Manual Toothbrush brand in Germany.
Reflective of its ambition to become the most sustainable toothbrush manufacturer globally, in
2021 the Group launched its first externally certified climate neutral toothbrush, Dr.BEST
GreenClean, which features a handle made from renewable cellulose and wood-based
bioplastic bristles made of 100 per cent. renewable castor oil and 100 per cent. plastic-free
packaging.
4.2 VMS
The Group is the global market leader in the VMS category, owning three of the top 20 global
brands: Centrum, Caltrate and Emergen-C, which together delivered revenue of £1.3 billion in
FY 2021. Importantly, approximately 54 per cent. of the Group’s VMS portfolio revenue in FY
2021 was in the key markets of the USA and China, which are expected to account for
approximately 72 per cent. of total VMS market growth between 2021 and 2026.
Consumers around the world are taking an increasingly proactive approach to managing their
own health. The Group’s vision is to empower consumers to do this by providing solutions to
help them achieve their holistic wellness goals. With its market leadership, recognised brands,
innovation capabilities and scientifically supported claims, the Group is well positioned to
support consumers on this journey.
In addition to the favourable market dynamics and geographic reach of its business, the Group
has identified a number of key opportunities to grow its VMS portfolio, including increasing the
penetration of the Group’s VMS brands across digital channels. Since the formation of the
GSK/Pfizer JV, which incorporated the Group’s leading VMS brands, the Group has made
meaningful investments in its digital capabilities, which are now beginning to favourably impact
performance. In FY 2021, the Group generated revenue of £1.501 billion across its VMS
portfolio.
Centrum
Centrum is the world’s number one selling multivitamin brand, offering a wide variety of
formulations that support energy, immunity and metabolism, as well as eye, heart, bone and
brain health. Launched in the USA in 1978, Centrum has built on decades of research and
innovation and its purpose is to “build every body from the inside out”. Centrum is now
available in over 50 markets and is the best-selling multivitamin brand in over 25 markets. It is
the most clinically studied multivitamin in the world.
Centrum multivitamin products are designed to help adults and children meet their diverse
nutritional needs and contain a variety of essential nutrients. They are devised with formulas
which take into account the latest dietary guidelines, scientific research, and recommended
dietary allowances from the US National Academy of Sciences and other regional/country
specific nutrition bodies. Exemplary of its long history of innovation, Centrum was the first
major brand to add many key nutrients to its products, such as beta-carotene in 1988, lutein in
1999 and lycopene in 2003.
Centrum has continued to innovate by introducing products which target the distinct needs of
consumers. For example, the Centrum Benefit Blends range was launched in eight variants in
Australia in 2021. Available in the form of tablets or capsules to meet differing consumer
preferences. Centrum Benefit Blends supplements are tailored to deliver specific health
87

benefits, for example, to support immune function or to reduce tiredness and fatigue. A further
example is Centrum Minis which were launched in the USA in 2020 in order to address the
consumer need for smaller pills which are easier to swallow.
As part of its growth strategy, the Group aims to maximise the reach of the Centrum brand by
targeting wellness-focused consumers. In the USA, such consumers constitute 25 per cent. of
consumers in the VMS market. The Group continues to develop its innovation and
communication eco-system to support these consumers on their healthcare journey, including
through engagement with social and healthcare influencers. The sustainable eco-system
combines new product and content innovation with proactive data insights to deliver an
increasingly frictionless, personalised experience for consumers.
Other VMS brands
In addition to Centrum, a number of other locally important brands also provide the Group with
leading positions in key markets. Two of these brands, Emergen-C and Caltrate, are
highlighted below.
In the USA, Emergen-C leads the Vitamin C Supplement market. Its purpose is to “fortify
immune health to help consumers emerge their best”. Starting as a niche vitamin dietary
supplement drink (sold as an effervescent powder), Emergen-C has since demonstrated
strong, consistent growth by expanding its penetration with more diverse consumer segments,
increasing year-round usage and delivering innovation such as the gummy format and the
botanicals line made from natural, plant-based ingredients.
Caltrate is a leading brand for bone and joint supplements and the Group’s largest brand in
China. In China, Caltrate, with its efficient high-volume calcium formula, is the second ranked
Calcium Supplement brand and the fifth largest VMS brand overall. Caltrate is focused on
providing support for strong bones and healthy and active movement. It has a wide range of
products containing bone-essential nutrients such as calcium and vitamin D3 in the form of
tablets, gummies and chewable tablets, with dedicated offerings for pregnant women, children
and the elderly. The brand has a longstanding equity in bone health and a core consumer base
of females over 45. However, since 2018, Caltrate has successfully expanded into joint health
with its Caltrate Gluco range offering products that contain nutrients such as glucosamine and
undenatured type II collagen (UC II). Caltrate has also successfully expanded its consumer
base beyond females and into younger age groups.
4.3 Pain Relief
The Group is the global market leader in OTC pain relief, leading in both topical (creams and
gels) and systemic (ingested products) pain relief with a portfolio of well-known and trusted
products to relieve pain and reduce inflammation. Its global Power Brands, Panadol, Voltaren
and Advil, as well as its other market-leading brands, including, among others, Fenbid,
Excedrin and Grand-Pa, bring comfort and ease to millions through clinically proven
therapeutic benefits, helping people manage their symptoms so that they can enjoy life to the
full. Pain Relief is a focused category, with the Group’s top five brands accounting for 95 per
cent. of the Group’s total Pain Relief category revenue in FY 2021. In FY 2021, the Group
generated revenue of £2.237 billion across its Pain Relief portfolio.
Voltaren
54
Voltaren is dedicated to restoring the “joy of movement” for body pain sufferers worldwide and
is the number one OTC Topical Pain Relief brand and the third largest OTC brand globally.
The brand has a global footprint with sales in over 87 countries, including the USA where there
was a successful Rx-to-OTC switch of Voltaren products in 2020. Voltaren and its active
ingredient enjoy high levels of recommendation by health care professionals and medical
associations worldwide, such as the American College of Rheumatology and the European
League against Rheumatism.
54
Voltaren operates under multiple different brand names around the world, including Iodex (India), Voltadol (Italy, Spain) Voltarol
(United Kingdom, Ireland, Norway) and Cataflam (Brazil).
88
Voltaren is primarily sold as a topical gel and it offers a range of other products across different
markets, including patches, pills and liquid capsules. Most products contain diclofenac, a
powerful nonsteroidal anti-inflammatory drug (“NSAID”) recommended for the treatment of
osteoarthritis, musculoskeletal disorders, soft-tissue injuries and acute or chronic pain. The
Voltaren range has been expanded through continual innovation and includes a wide range of
formulations for different consumer needs; for example, Voltaren 12 Hour Emulgel provides
consumers with an extended release formulation. Voltaren 12 Hour Emulgel has been
formulated for optimal absorption from skin to the site of pain and is also the only clinically
proven formulation to achieve deep penetration. It provides up to 12 hours of pain relief and the
active ingredient diclofenac reduces inflammation directly at the source.
The Group continues to respond to consumer needs under the Voltaren brand based on
insights generated from both consumers themselves and from the feedback of healthcare
professionals. The US launch of Voltaren Arthritis Pain was the first switch of a prescription-
strength OTC NSAID topical gel for arthritis pain, helping the nearly 30 million people in the
USA with osteoarthritis. This product followed the global launch of a new easy-open cap for
Voltaren products to cater for the ageing consumer – an inclusive innovation that won the
prestigious Drum Award for Packaging. In 2021, the Group introduced Voltanatura, an organic
plant-based gel for soothing tense, contracted muscles.
Advil
Advil is the number two Pain Relief brand in North America and the fourth largest OTC brand
globally. The brand is dedicated to helping people “reclaim life’s possibilities” and for over 35
years, consumers and doctors have trusted Advil to deliver powerful relief from various kinds of
acute pain, including headache, muscle ache, backache, minor arthritis, other joint pain and
menstrual cramps, as well as the aches and pains of the common cold. Advil, which is
ibuprofen-based and effective at relieving pain and fever, is the number one doctor-
recommended NSAID among OTC adult Pain Relief brands in the USA.
The Advil product range includes tablets, caplets, gel caplets, liquid-filled capsules,
suspensions and children’s drops to address a broad range of pain relief needs. Advil PM
combines the number one selling ibuprofen brand with the number one selling sleep medicine
(diphenhydramine) to help relieve night time pain and sleeplessness. In 2017, the Group
introduced Advil Liqui-Gels Minis for consumers who find capsules difficult to swallow,
providing the concentrated power of Advil in a capsule that is 33 per cent. smaller than the
standard Liqui-Gels. The Group also sells Advil Migraine, which is clinically proven to relieve
migraine pain and related symptoms, and Children’s Advil, for effective fever reduction,
providing up to 8 hours of relief in one dose.
In 2020, the Group launched the first ingredient innovation in the US OTC Systemic Pain Relief
category in 25 years. Advil Dual Action is the first and only FDA-approved pain relief
medication to combine the top two doctor-recommended and most widely used OTC pain
relievers, acetaminophen (paracetamol) and ibuprofen, into a single pill. The Group’s research
shows that consumers want to take as few medicines as possible, yet many use both ibuprofen
and acetaminophen – which work in different ways – when treating their pain. Advil Dual Action
allows consumers to take a lower daily dose of each medication in a single product that is
scientifically proven to provide greater efficacy than the individual components, providing
powerful, 8-hour relief.
As part of the Group’s work to achieve greater sustainability, in 2021 Advil announced it was
using a first-of-its-kind technology for OTC medicines which decreases the amount of plastic
resin required to mould and craft 80 million Advil bottles by 20 per cent. This innovation is
expected to reduce the amount of plastic in the environment by nearly 227 tonnes by 2022
alone.
89
Panadol
Panadol has a global footprint covering over 90 countries and it is the number one systemic
pain reliever outside of the USA and the sixth largest OTC brand globally. Panadol offers
leading paracetamol-based products that provide fast and effective pain relief from headache,
joint pain, fever and cold symptoms.
Panadol’s purpose is to “bring freedom from pain so the human spirit can shine” and, with a
track record of over 65 years in delivering innovative, high-quality and efficacious products, and
a reputation for being effective but gentle, it has established itself as the most trusted pain
relief brand in many of the Group’s markets.
Panadol’s expanding product range is designed to satisfy diverse and evolving consumer
needs, with products also dedicated to night pain and period pain. During the COVID-19
pandemic, the Group built additional capacity to respond to the growing demand for Panadol to
help treat the symptoms of pain and fever associated with COVID-19, including a highly
successful post-vaccine programme, which won gold in Nicholas Hall’s APAC Marketing
Awards. Unlike standard paracetamol tablets, Panadol Advance ranges contain innovative
Optizorb technology. This allows the tablets to disperse up to five times faster compared to
ordinary paracetamol tablets, enabling rapid relief and helping consumers enjoy life to the full
again.
Other Pain Relief brands
The Group’s global Power Brands are augmented by a number of locally important brands
which provide market-leading positions in key markets. Three of these are highlighted below.
Excedrin is a leading Systemic Pain Relief brand in the USA (ranked fifth overall) focused on
the relief of headaches and migraines, and has been providing trusted, fast headache and
migraine relief to US consumers for over 60 years. Excedrin’s purpose is to deliver fast relief
for different types of headaches, with consumers having the choice between Excedrin Extra
Strength, Excedrin Migraine, Excedrin Tension Headache and Excedrin PM Headache. The
research-backed effectiveness of Excedrin’s products makes the brand a trusted leader in
head pain relief, with Excedrin Migraine being the number one neurologist-recommended OTC
migraine treatment approved by the FDA.
Fenbid is the number two Systemic Pain Relief brand in China and the market-leading Pain
Relief brand in China outside of traditional Chinese medicine. Its purpose is to “enable
consumers to move forward and leave their pain behind”. Fenbid provides solutions and
formulations that are backed by science, including its popular 12-hour sustained release
formula.
Grand-Pa is the number one Pain Relief brand and largest OTC brand in South Africa. Used by
families for over 100 years, Grand-Pa’s purpose is to “liberate people to keep moving their
communities forward”, by providing fast and effective relief for different types of pain, driven by
formats that deliver fast absorption. Grand-Pa’s headache powder provides symptomatic relief
from mild to moderate pain and fever. In 2021, supported by consumer-tested concepts, the
Group modernised the Grand-Pa brand with the introduction of stick packs, which use sleek
design packaging for ease of consumption on the go.
4.4 Respiratory Health
The Group is the global market leader in Respiratory Health. Respiratory Health is a more
fragmented category, with local needs and consumer preferences in treating respiratory
ailments far more diversified compared to other consumer healthcare categories. The Group’s
portfolio is accordingly positioned, with a larger number of brands catering to local needs. Key
areas of the category where the Group competes include the £5.6 billion seasonal cold and flu
market, the £1.9 billion Topical Nasal Decongestants market and the £3.9 billion Allergy Care
market.
90

The Group’s focused approach to the Respiratory Health category is highlighted below through
its Power Brands, Otrivin and Theraflu, as well as through examples of its other locally
important brands, such as Flonase, Robitussin and Contac. In FY 2021, the Group generated
revenue of £1.132 billion across its Respiratory Health portfolio.
Unlike the Group’s other categories, seasonality has a significant impact on Respiratory Health
revenue. See paragraph 2.8 of Part VII (Operating and Financial Review) for a discussion of
seasonality in relation to the Group’s Respiratory Health portfolio.
Otrivin
55
Otrivin is the number one Topical Nasal Decongestant brand worldwide, with a presence in
over 40 markets and exists to “release the wonders of breathing well”. Otrivin provides
consumers with a complete suite of nasal care products, including both medicated and
non-medicated nasal sprays for adults and children. The medicated sprays, such as the
Medicated Complete Nasal Care Triple Action Nasal Spray, are designed to rapidly relieve the
symptoms of nasal congestion and rhinorrhoea and provide long-lasting benefits. This is
achieved using the active ingredients xylometazoline and ipratropium, which unblock the nose
within minutes, and lasts 6-8 hours, for better breathing. For consumers who prefer a
non-medicated solution, Otrivin Naturals uses seawater and sea salt solutions to cleanse away
excess mucus, gently restoring nasal function.
In 2021, the Group launched the Otrivin BreatheClean range in response to growing consumer
concerns about the impact of environmental pollution on breathing. Otrivin BreatheClean
contains isotonic seawater that helps to remove the trapped particulate pollution by washing it
away. The range was successfully launched in India and Poland in December 2020 and is
contributing to strong growth for the brand across both markets.
Theraflu
56
Theraflu is one of the world’s leading brands in the seasonal cold and flu market, operating in
over 50 markets with over 50 years of history and innovation. Its purpose is rooted in “fighting
for a flu-safe world”, with products that deliver effective relief from cold and flu symptoms. The
Theraflu range, consisting of syrups, hot liquid powders, caplets and capsules, provides
products in multiple forms to meet consumer as well as market preferences.
The leading format in the Theraflu product range is its Hot Liquid Powders. Available in a range
of flavours and drawing on extensive flu expertise, Theraflu provides symptomatic relief from
cold and flu.
Other Respiratory Health brands
The Group’s global Respiratory Health Power Brands are augmented by a number of locally
important brands, which provide the Group with leading positions in key markets. Three of
these, Flonase, Robitussin and Contac, are highlighted below.
Flonase is a leading allergy remedy in the USA with a presence across multiple other markets.
Its purpose is to deliver allergy relief that lasts. Flonase nasal sprays provide 24-hour all-in-one
non-drowsy allergy relief, targeting sneezing, runny nose, itchy and watery eyes plus nasal
congestion, which most allergy pills are unable to treat. The Flonase range consists of the
Flonase Allergy Relief Nasal Spray, an OTC medicine which incorporates fluticasone
propionate, the number one prescribed allergy medicine, and the Flonase Sensimist Allergy
Relief, made with MistPro Technology that creates a fine, gentle mist that is scent free.
55
Otrivin operates under multiple different brand names around the world, including Rinazina (Italy), Rhinomer (Spain),
ProRhinel (France) and Vibrocil (Portugal).
56
Theraflu operates under multiple different brand names around the world, including NeoCitran (Canada and Switzerland).
91

Robitussin is a leading US cough remedy (ranked second in the US) with a history of over 70
years and a purpose to “deliver cough and other cold symptom relief solutions consumers can
count on”. It has a portfolio of products that provide effective relief for multiple needs. The
product range includes both medicated and 100 per cent. natural, drug-free remedies for both
adults and children, available in a variety of formats. Robitussin is also available across
multiple markets outside the USA.
Contac is a well-known cold and flu brand in China. It has a range of Respiratory Health
products known for their strong efficacy, including multi-symptom cold and flu medicines, nasal
decongestion sprays and topical decongestion products.
4.5 Digestive Health and Other
Digestive Health
The Group is the market leader in the global Digestive Health market with a portfolio of trusted,
leading brands focused on key markets, in particular the USA (ranked first), India (ranked first)
and Brazil (ranked second), each of which are in the top ten markets for Digestive Health
products globally. The Group’s key brands are described below.
In FY 2021, the Group generated revenue of £1.951 billion across its Digestive Health and
Other portfolio.
Tums
Tums is the leading OTC Heartburn Treatment in the USA with a range of products for the fast
and effective treatment of heartburn and acid indigestion. Its purpose is to enable consumers
to “fight back against heartburn fast”. To maintain its position as the market leader, the
90-year-old brand continues to reinvent itself through innovation and creative communications.
Tums offers a varied portfolio of products in order to attract different consumer groups and
broaden its utility. For example, Tums Chewy Bites, an antacid with a tasty outer shell and soft
centre, aims to provide an enjoyable taste experience, attracting young category entrants,
whereas Tums Naturals is an antacid containing no artificial flavours or dyes, appealing to
health-conscious consumers seeking a more natural solution to their medicinal needs.
ENO
ENO is the number one OTC Heartburn Treatment in India and Brazil, with a range of antacid
products (powders, liquids and tablets) that provide temporary relief from the symptoms of
heartburn and gastric discomfort. ENO’s purpose is to “free appetite for life” for people
suffering from acid reflux or heartburn, through delivery of smart solutions to aid healthier
digestion. ENO powder is notable for its speed of relief as it begins to work in six seconds post-
consumption. In India, it is the Group’s single most distributed consumer healthcare brand, with
presence in over four million outlets. In Brazil, despite the challenges faced during the
COVID-19 pandemic, the brand continued to increase its market share in 2020. This growth
was partly driven by the launch of new products with innovative flavours and formats, such as
ENO in liquid format.
The Group also sells a broad range of other Digestive Health products, particularly in the USA,
where in addition to Tums its portfolio includes Nexium, Gas-X and Benefiber, among other
brands.
Nexium is the leading PPI heartburn treatment in the USA (ranked third in Heartburn overall)
and the number one choice for doctors for their own heartburn.
57
Nexium24HR is an effective
57
Among primary care physicians who use a branded OTC proton pump inhibitor (Report by FRC, A Lieberman Company,
September 2020).
92
treatment for frequent heartburn. It works by blocking acid directly at the source to provide
consumers with 24-hour protection, and potentially preventing heartburn before it even starts.
As a long-acting treatment the brand complements the Group’s Tums portfolio, which is an
effective treatment for occasional heartburn, and its antacid formulation provides consumers
with fast-acting relief. The Group holds worldwide OTC rights to Nexium (excluding Brazil)
under an agreement with AstraZeneca, which involved an Rx-to-OTC switch for the brand in
2014.
Gas-X and Benefiber broaden the Group’s Digestive Health portfolio beyond heartburn relief.
Gas-X is the number one Antiflatulent brand in the USA and Benefiber is a leading laxative in
the USA (ranked fourth).
Other
The Group focuses on certain sub-categories in Skin Health, including Lip Care, Haemorrhoid
Treatments and Wound Healers. In each of these sub-categories, the Group has leading
positions in key markets as illustrated by some of its locally important brands including:
ChapStick, the number two Lip Care brand in the USA; Bactroban, the leading Wound Healers
brand in China; Preparation H, the market-leading haemorrhoid treatment in the USA; and
Zovirax and Abreva, the world’s two leading Cold Sore Treatments. Further important Skin
Health brands include the Lamisil antifungal brand and Fenistil, a treatment for skin irritations.
The Group also has leading positions in Smokers’ Health through brands such as Nicorette,
the leading brand in the USA, and Nicotinell, the number two brand globally.
5. Global reach
Overview
As one of the world’s leading consumer healthcare businesses, the Group has a broad global
reach with a number 1 or number 2 OTC/VMS presence in countries which represented over
70 per cent. of the world’s OTC/VMS markets by value in 2021.
The Group’s commercial organisation leverages the benefits of its global scale whilst
maintaining accountability and agility at a local level. A global commercial organisation
provides global brand management and marketing, insights and analytics, and digital
commerce capabilities, where centralised expertise, scale and consistency provide value.
Commercial execution is driven by business units at a local level structured into three regions:
(i) North America; (ii) EMEA and LatAm; and (iii) APAC. See Part VII (Operating and Financial
Review) for a discussion of revenue for each region in FY 2021, FY 2020 and FY 2019.
The Group is the leading OTC/VMS business across all three regions, as well as one of the
leading businesses in Oral Health, on the basis of sales to consumers in FY 2021.
93
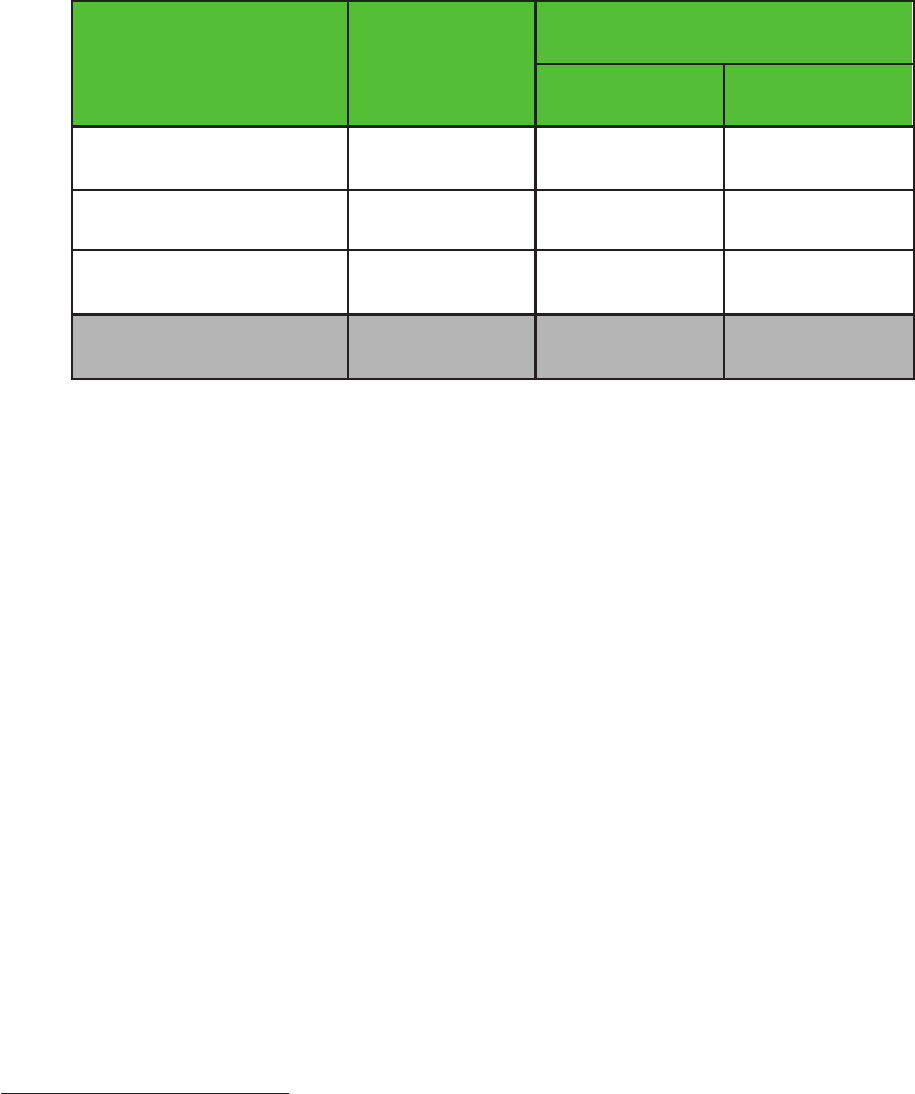
Group ranking by region
Region
North America
EMEA and LatAm
1
st
3
rd
59
60
3
rd
3
rd
4
th
1
st
1
st
1
st
1
st
1
st
1
st
1
st
APAC
Overall
OTC/VMS
Oral Health
Overall Therapeutic
58
North America
The North America region includes the USA, Canada and Puerto Rico, and is home to 5 per cent.
of the world’s population and 27 per cent. of global GDP.
61
The region is distinguished by a well-
developed consumer healthcare market with a significant presence of mass retail and large drug
store chains. Key market trends include a growing consumer interest in wellness products and
alleviating healthcare issues, increased product personalisation to meet specific needs, and a
growing e-commerce market, partially driven by the impact of the COVID-19 pandemic.
The North America region delivered £3.5 billion in revenue in FY 2021, representing 37 per
cent. of the Group’s total revenue. Approximately 4,600 people
62
work in the North America
region and the business is supported by five manufacturing sites, which work closely with the
commercial organisation to support consumer needs.
The Group is the market leader in North America in OTC/VMS benefitting from a 7.6 per cent.
market share, with leadership in Digestive Health and leading positions across Pain Relief
(ranked second), Respiratory Health (ranked fifth), Skin Health (ranked second) and VMS
(ranked third). It is ranked within the top four companies in Oral Health (ranked third equal
63
)
with a top three ranking in Toothpaste and leadership in Denture Care.
58
Group analysis based on third party data from Nielsen, IRI, Intage, IQVIA, Consumption Sales Data (2021-2022). Therapeutic
Oral Health is defined as Therapeutic Toothpaste and Total Dental Appliance Care.
59
The Group is ranked 3rd in Oral Health in North America by Euromonitor in 2021 but the difference with the 4th ranked player
is within the margin of error.
60
The Group is tanked 4th Oral Health in Asia Pacific+Australasia combined by Euromonitor in 2021 but the difference with the
5th ranked player is within the margin of error.
61
Source: World Bank (2020).
62
Full-time equivalent employees and agency staff as at 31 March 2022 (rounded to the nearest 100).
63
The Group is ranked 3rd in Oral Health in North America by Euromonitor in 2021 but the difference with the 4th ranked player
is within the margin of error.
94

The Group has an extensive portfolio of brands in the region, including four of the top 20 OTC/
VMS brands in the USA and a number of category-leading positions in the region, some of
which are highlighted below.
No. 1 Therapeutic Toothpaste
64
No. 3 Toothpaste overall
No. 1 Vitamin C Supplement
No. 2 OTC Systemic Analgesic
No. 1 OTC Heartburn
Treatment
No. 1 OTC Topical Analgesic
No. 1 PPI
65
Heartburn
Treatment
No. 1 Multivitamin
No. 1 Smokers’ Health
treatment
Consistent with its scale, the Group has broad distribution capability with over 245,000 points
of distribution in the USA across all channels, including mass retailers, pharmacies, clubs, food
and convenience stores and digital commerce. While the Group maintains relationships with a
variety of significant retailers across its key markets, sales attributable to its top five largest
retailers accounted for 60 per cent. of the Group’s revenue in the North American market in
2021 reflecting the concentrated retailer landscape in the USA. Nevertheless, the Group’s
revenue is relatively balanced across key customers, with no customer accounting for more
than 25 per cent. of the Group’s revenue in the region in 2021.
The Group is a partner of choice among its top ten customers in North America. The Group
has dedicated multifunctional top customer and channels teams in the region that cover sales,
category development, consumer engagement, supply and finance.
Close partnerships are supported by the Group’s two shopper science labs in New Jersey and
Arkansas and through its consumer insights platforms, which enable the Group to conduct
joint-business planning with key retailers. The Group’s strategic partnership with Walgreens in
the Pain Relief category supported the training of 75,000 in-store retail team members in the
delivery of empathetic fit-for-purpose treatment for pain sufferers based on insights derived
from the Group’s shopper science lab. A similar partnership seeks to upskill the store sales
teams in the VMS category based on the Group’s science and insights generated in the
shopper science lab. The Group has been recognised by several leading retailers since 2019,
including Walmart, Walgreens and CVS with awards including “Supplier of the Year”.
In the USA, the Group has invested heavily in digital commerce capabilities and marketing
support and has significantly grown e-commerce sales since FY 2019, for example, having
achieved market-leading positions on Amazon in Toothpaste and in Topical Pain with Voltaren.
Increased first-party data in the USA is facilitating the generation of insights that are leveraged
back into the business. The Group has also made progress towards improving its customer
experiences, including launching its first direct-to-consumer online store for ChapStick in the
USA in 2020.
EMEA and LatAm
The EMEA and LatAm region is managed as a segment within the Group’s structure. This is a
large and diverse region, which is home to 44 per cent. of the world’s population and 37 per
cent. of global GDP.
66
Covering approximately 150 markets, the region is managed under
seven business units: Northern Europe, Southern Europe, Central and Eastern Europe
(including the Commonwealth of Independent States), Russia, DACH (Germany, Austria and
Switzerland), Middle East and Africa and LatAm (Brazil, Colombia, Wider LatAm).
64
Source: Group analysis based on Euromonitor Passport (2021).
65
Proton pump inhibitor, a class of drug which reduces acid production by the stomach and has a longer duration of action than
traditional antacids.
66
Source: World Bank (2020).
95
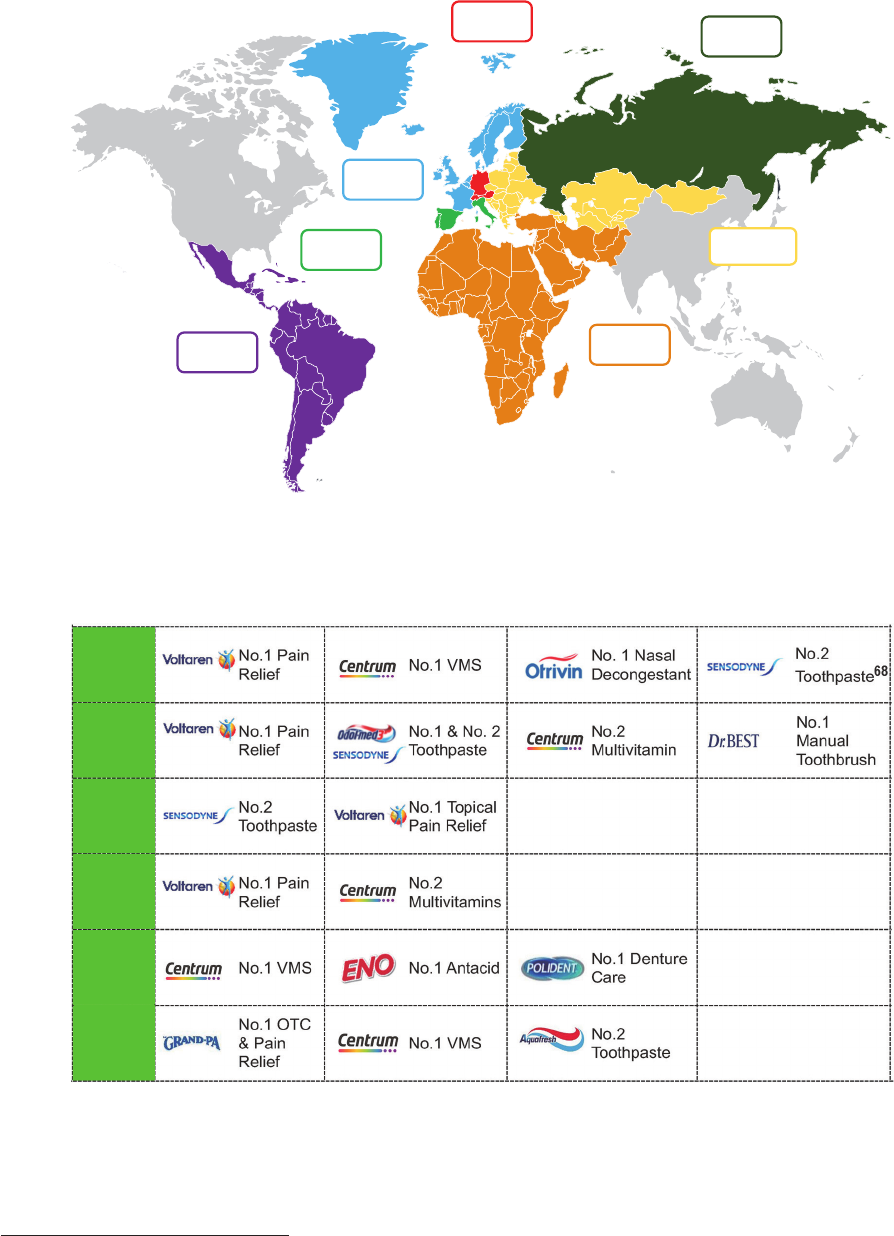
The EMEA and LatAm regions delivered £3.9 billion of revenue in FY 2021, representing
41 per cent. of the Group’s revenue. Approximately 12,300 people
67
work in the EMEA and
LatAm regions and the business is supported by 13 regional manufacturing sites located
across the region, enabling local innovation and closer response to consumer demands.
LatAm
(Brazil, Colombia,
Wider LatAm)
Middle East and
Africa (MEA)
Russia
Southern Europe
(Italy, Spain,
Portugal)
Northern Europe
(GB&I, France,
Benelux, Nordics
)
DACH
(Germany, Austria,
Switzerland)
Central & Eastern
Europe (CEE)
The Group is the largest consumer healthcare business across EMEA and LatAm with leading
positions across multiple categories, including Pain Relief (ranked first), Respiratory Health
(ranked first), VMS (ranked third) and Oral Health (ranked third).
Overall
UK
Brazil
Germany
Italy
South
Africa
In many EMEA and LatAm markets, OTC products are exclusively sold through the pharmacy
channel. Nevertheless, mass market retail is significant for Oral Health and VMS products and,
in certain markets for OTC products, notably in the UK, Netherlands and Mexico. Overall the
pharmacy channel represented approximately 60 per cent. of revenue in the region in FY 2021,
67
Full-time equivalent employees and agency staff as at 31 March 2022 (rounded to the nearest 100).
68
Sensodyne is ranked second across EMEA and LatAm. Within this, Sensodyne is ranked second in toothpaste in EMEA and
third in LatAm.
96
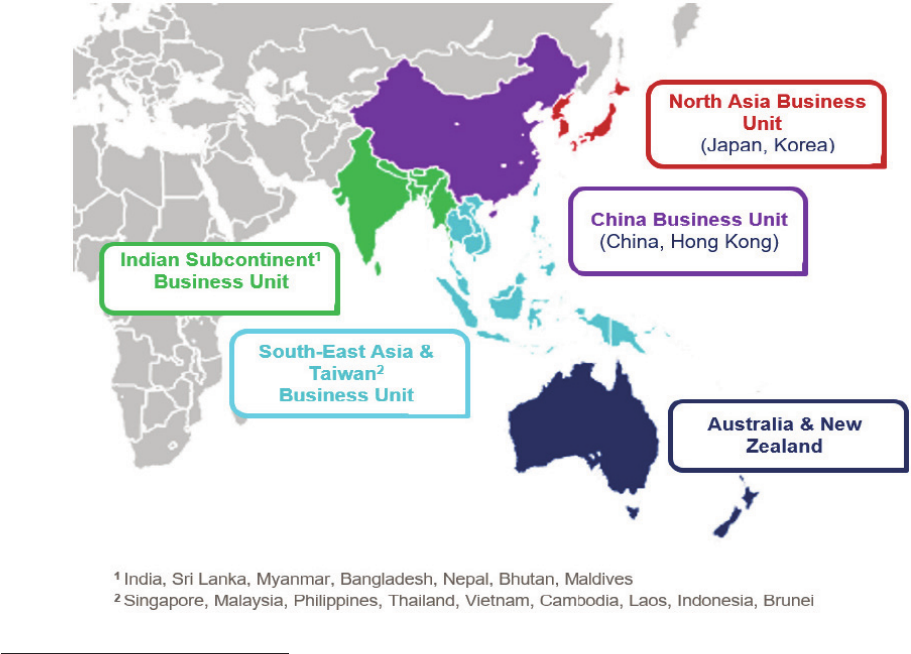
mass market retail represented approximately 35 per cent. in FY 2021 and e-commerce made
up the remaining approximately 5 per cent.
69
Given the importance of independent pharmacies and pharmacy chains in EMEA and LatAm,
the Group maintains a large, dedicated sales force. The sales force provides account
management, and drives excellence in store execution and expert advocacy on the Group’s
brands. In mass market retail, the Group has a weighted distribution level of over 80 per cent.
and is ranked second by share of sales. E-commerce is a relatively smaller proportion of the
region’s sales, but growing at around 23 per cent. per year and its contribution to the overall
sales mix in the region varies from 1 per cent. to 15 per cent. given the variety of regulatory
environments and digital maturity across the countries.
As in North America, the Group supports its customers to improve their category performance,
leveraging its shopper science and advanced technologies to support ranging, merchandising
and space planning. For example, it uses Dragonfly AI to replicate the human eye and
understand what grabs shoppers’ attention, as well as augmented and virtual reality to present
new concepts for point of sale both instore and on line. The Group is also using image-
recognition technology to track distribution and the visibility of key products, across the points of
sale. This enables the Group to collect insights to deliver efficient in-store execution of its brands.
APAC
APAC is a large, diverse and higher-growth region, home to 51 per cent. of the world’s population
and 36 per cent. of global GDP.
70
The region is split across five business units serving 22 markets
incorporating both well-established markets such as Japan, South Korea and Australia, as well as
rapidly growing markets including China, India and South East Asia. The region is distinguished by
a rapidly emerging middle class fuelling a demand for increased self-care and product
premiumisation, together with high levels of e-commerce in key markets, most notably China.
Asia Pacific region
69
Source: Group analysis based on external data (Nielsen).
70
Source: World Bank (2020).
97
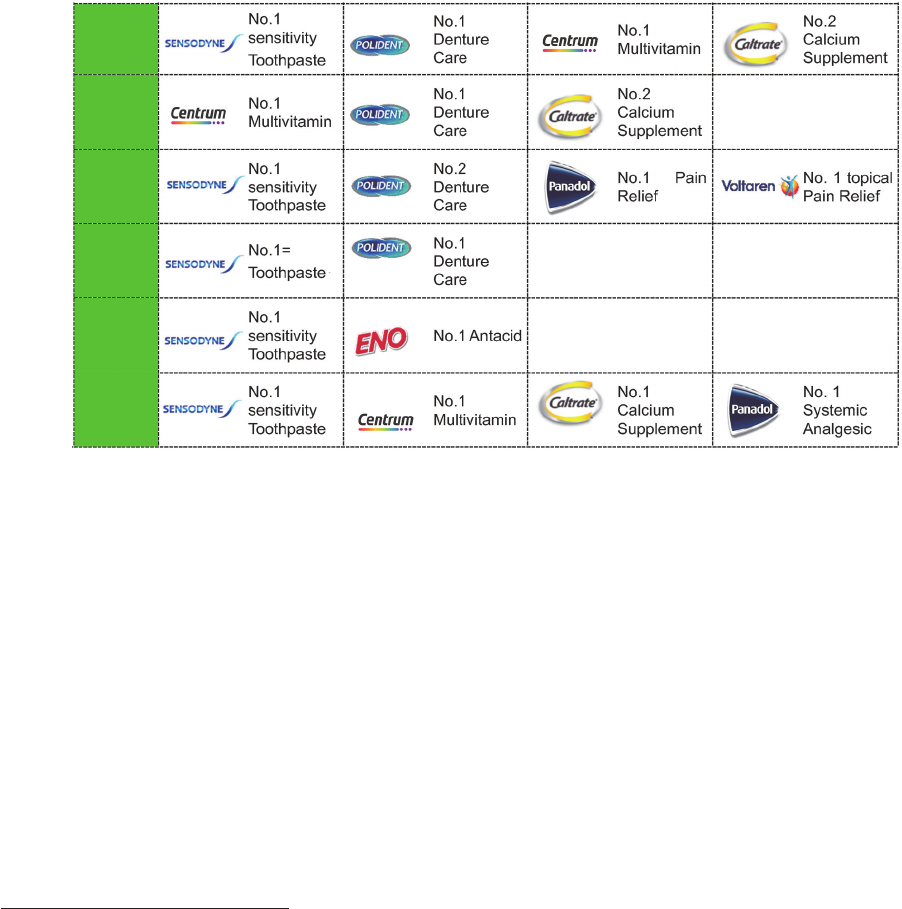
APAC markets delivered £2.1 billion of revenue in FY 2021, representing 22 per cent. of the
Group’s revenue. Approximately 5,900 people
71
work in the APAC region and the Group’s R&D
centre in Suzhou, China develops new products for the region based on local needs and
insights and collaborates with the six regional manufacturing sites to facilitate their introduction.
The Group is the market leader in the APAC region in OTC/VMS, with leading positions in Pain
Relief (ranked first) and VMS (ranked second). In Oral Health, the Group holds the leadership
position in Denture Care and Sensitivity Toothpaste (among the top five in Toothpaste overall).
This has been achieved through a highly focused portfolio in which nine brands
72
with market
leadership positions accounted for 84 per cent. of total APAC revenue in FY 2021.
Overall
Australia
India
China
Japan
Taiwan
74
73
The Group’s business in APAC is supported by broad local capabilities and expertise which
provide it with the agility to respond to evolving consumer needs across the dynamic markets
of the region. In addition to local commercial execution, the region’s employees also support
R&D, marketing strategy and manufacturing. The Group’s R&D centre in Suzhou, China
develops new products for the region based on local needs and insights and collaborates with
the six regional manufacturing sites to facilitate their introduction. Through a strong regional
supply network, approximately 80 per cent. of the Group’s business in APAC is supplied within
the APAC region.
In recognition of the diverse retailer and regulatory market landscape within APAC, the Group
takes a varied approach to its distribution strategy across the region, which variously consists
of direct sales to retailers, indirect sales made through distributors, or a combination of both
methods depending on the channel dynamic of the given market and the scale of the Group’s
operations. In India, the Group’s products are distributed by Hindustan Unilever Ltd.
The sales force within APAC is also deployed according to the structure of the relevant market.
Centralised buying functions are managed by centralised account management in more
71
Full-time equivalent employees and agency staff as at 31 March 2022 (rounded to the nearest 100).
72
Sensodyne (#1 sensitive Toothpaste brand in APAC), Caltrate (#2 Calcium Supplement in APAC), Centrum (#1 Multivitamin in
APAC), Panadol (# 1 systemic analgesic in APAC), Polident (#1 Denture care brand in APAC), Voltaren (#3 Topical Analgesic
in APAC and leading pain brand outside traditional Chinese medicine (“TCM”)), ENO (#1 number one Digestive Health brand in
India), Fenbid (#2 Systemic Pain Relief brand in China and leading Pain Relief brand outside of TCM), Bactroban (#1 wound
healer in APAC).
73
Source: Group analysis based on third party data from Nielsen, IRI, Intage, IQVIA Consumption Sales Data (2021-2022).
74
Sensodyne is ranked 1st in toothpaste in Japan by Euromonitor in 2021 but the difference with the 2nd ranked player is within
the margin of error.
98

developed markets, whereas smaller, independent customers are managed by territory
managers and sales representatives. The Group’s sales force in APAC consists of
approximately 2,500 employees who are further supported by distributor sales representatives
in certain markets. These teams are supported by the Group’s shopper science lab in
Singapore, which enables category management partnerships with key retail partners.
The APAC region leads the world in digital commerce with 61 per cent. of global retail
e-commerce sales in 2020 (according to eMarketer, May 2021). The Group has invested
heavily in this area. Through the prioritisation of digital programmes in APAC, e-commerce
revenue grew by 36.3 per cent. in FY 2021.
One such programme in China is based on the latest developments in the online-to-offline
(“O2O”) services market. O2O services enable a seamless digital purchase experience for the
consumer by combining physical retail pharmacy locations for sourcing and platform courier
teams for collection and delivery. These services enable consumers to find product information
and order medicines through O2O platforms and receive at home or to office delivery, typically
within 30 minutes. The Group identified the potential for O2O services and established a
dedicated O2O team to establish strategic collaborations with leading O2O platforms such as
Meituan and Eleme (part of the Alibaba group).
The Group has established flagship e-commerce brand-specific stores run on Alibaba’s T-mall
platforms and collaborates with online health and consultation platforms such as We-Doctor
and JD Health to enable consumer access to online advice, educational content, brand content
and on some platforms also direct product purchase.
The Group has also developed strategic collaborations with the Alibaba group where its Digital
Captaincy status in the VMS category enables access to a greater degree of data granularity
better informing its planning and commercial execution capability.
Besides its strong position on established e-commerce platforms such as T-mall, the Group is
also actively expanding into social commerce
75
on popular social engagement platforms such
as Douyin. The Group has recently established stores for several of its brands on Douyin’s
platform so that consumers can immediately purchase products on the platform whilst
engaging with the brand through content and livestreaming.
The Group has also established an in-house audience management platform that collates data
to provide a single source of information on consumers and healthcare professionals in order
to deliver personalised experiences. This enables richer and better targeted consumer
engagement to meet consumers’ healthcare needs more effectively through CRM and
marketing programmes.
6. Engagement with Consumers
As a leading consumer healthcare business, the Group has broad capabilities in marketing,
expert marketing, design, and consumer and business insights and analytics. These
capabilities are complemented by a strong and growing capability in digital commerce.
75
Selling products via e-commerce through social media.
99

Marketing
The Group has a clear and differentiated purpose to deliver better everyday health with
humanity and this drives the way the Group develops and commercialises its products. The
Directors believe the Group has a competitive advantage in everyday health with its human
understanding, combined with its trusted science. The Group’s brands are purposeful, founded
in science and focused, not only on care, but on quality of life, empathy and inclusion.
Restoring the joy of movementHelping humanity reclaim
life’s small pleasures
Lightening the load for all
appliance wearers
Releasing the wonders of
breathing well
Championing confidence from
the gums up
Freedom from pain so the
human spirit can shine
Fighting for a flu-safe world
Building every body
from the inside out
Reclaim life’s possibilities
By putting brand purpose at the centre of highly integrated campaigns, which are aligned
across commercial, expert, marketing and R&D, the Group’s human-centric brands help to
deliver more emotional connections and relatable consumer-centric experiences to support
better health outcomes.
For example, Voltaren’s purpose is to restore the joy of movement for body pain sufferers
worldwide:
A number of the Group’s campaigns are enduringly famous, reflected in and recognised by the
wide range of global and national marketing awards the Group has received. Sensodyne’s
famous dentist testimonial advertising was launched in 2005 and continues to offer authentic
dentist recommendation to consumers across the world. Over 5,000 dentists have offered to
recommend Sensodyne in the media, reflecting the significant numbers who do so every day.
100

Newer campaigns have also received considerable acclaim. For example, Theraflu’s “roll up
your sleeves” campaign and #FightingFluTogether to better meet the needs of underserved
communities drove reappraisal of the brand and improved social sentiment scores for Theraflu.
The Group prioritises marketing with a positive social or environmental impact and incorporates
social and sustainability goals aligned with brand purpose within its marketing strategies. For
example, Otrivin’s brand purpose is to “release the wonders of breathing well”. Accordingly,
Otrivin aims to raise awareness of the impact of air pollution on health reflecting growing
consumer concerns. In 2021, Otrivin partnered with a European biotech company to build a
playground that actively purifies the air as more children play in it. This is particularly pertinent
given that 93 per cent. of the world’s children play in spaces with unacceptable levels of air
quality. This installation featured at COP26 and drove significant levels of earned media.
Historically, the Group has achieved multiple successes for its marketing campaigns, including
design, creative impact, effectiveness and digital. This includes high-profile Gold and Grand
Prix awards at Cannes Lions, the Institute of Practitioners in Advertising, the Effies, Red Dot
and D&AD.
The Group continues to evolve its marketing operations and in 2021 it opened its pioneering
in-house content studio, CaST. CaST provides end-to-end content production, allowing the
Group to be agile and cost-effective in its content development, delivered via in-house subject
matter experts and underpinned by technology. This production model also helps the Group to
advance its creative effectiveness through dynamic creative optimisation (“DCO”). Since 2021,
CaST has delivered nearly 30 DCO campaigns across 13 markets, with 90 per cent. of these
delivering performance improvements against benchmarks (e.g. cost per click, cost per
completed view and ‘viewability’). The marketing function also continues to embrace
technology, such as artificial intelligence (“ AI”) and in 2020, Sensodyne launched Trio, the
world’s first machine learning and AI-enabled mobile experience which assesses people’s risk
of having tooth sensitivity. Trio is designed to give users personalised treatment advice and a
sample product or coupon, based on a picture of a user’s mouth and a short questionnaire
filled out by the user. Trio has been launched as a pilot across multiple markets, including
some of Sensodyne’s fastest growth markets, such as China and India. Additionally, the Group
utilises an industry-leading AI tool designed in partnership with Google and Picasso Labs –
Creative X – which scans over 20,000 video assets in 56 markets and provides
recommendations for creative improvements following YouTube best practices.
Commercial insights and analytics
Achieving a deep understanding of consumers, shoppers, experts and retailers is pivotal to the
mission of the Group.
The Group benefits from investments in in-house research facilities to solicit live shopper
feedback. This is used to improve pack designs, point of sale materials and shelf layouts,
including research tools to reach shoppers in their own homes, which the Group utilised
extensively during the COVID-19 pandemic.
101
In addition, the Group’s shopper science labs provide real-life and digital store environments
across all retail channels to recreate shopping scenarios to understand shopping behaviour, so
that it can tailor and personalise category and brand execution. This is supported by its centre
of excellence for shopper psychology, shopping insights and category management.
The Group uses its observatory tool to provide marketers, R&D and other teams across the
organisation with direct access to the full breadth and depth of its knowledge base and to
support their collaboration. This tool encompasses over 8,000 insight and analytics projects,
nearly 1,000 tested concepts, over 20 specialist subject libraries, and links to over 30 other
dashboards and research sources. It is updated in real time and new insights are added nearly
every day.
Consumer insight and understanding are further built through a number of complementary
marketing initiatives. For example, by partnering externally with InSites Consulting, the Group
has developed an extensive toolset to provide enhanced consumer insight. This has been
supported by more traditional large-scale key audience survey data and qualitative interview
information.
By enabling on-demand engagement with almost “any audience anywhere” via established
external partnerships to provide insight globally, the marketing organisation has flexibility to
identify and utilise the optimal market research approaches to identify commercial
opportunities, while taking into consideration commercial objectives, target audiences and
timing requirements.
The Group uses an extensive toolset to spot emerging consumer trends with disruptive
potential in order to help it shape the future of everyday health. Its toolset is designed to
discover and frame trends impacting health and wellness, to monitor their expression over time
and to prioritise those that will rise and endure. The Group’s proprietary and comprehensive
global trends framework monitors forces of change, trend territories and over 20 trends which
have the highest adoption and disruption potential. Combined with tools to monitor fresh trend
signals from search, social and in-market competitor activity, this enables the Group to identify
and react to global and local innovation opportunities, thereby driving incremental sales.
Digital capability
Deep human understanding attained through the Group’s data partnerships and insights
process is enhanced by digital capability. The Group was an early adopter of the Tech Stack
(Google), creating direct ownership of and access to audience data. Additionally, the Group
uses Publicis’ PeopleCloud (cloud-based marketing platform) to leverage relevant data sets to
better identify and connect with its growth audiences. The Group has systematically applied a
data-driven approach to marketing via PeopleCloud across its markets, which enables it to
target similar customers and continuously learn and grow its customer base. By responsibly
balancing privacy and personalisation, the Group is able to build long-term relevant
relationships with consumers across all of its brands.
Marketing campaigns are planned via digital-first connection planning, generating and placing
content made relevant for events, seasons, formats, cultural occasions and even weather
patterns. One of the Group’s advancements in predictive marketing is a proprietary tool called
TRGR, or Trigger. This pulls in data signals that help pinpoint where and when the Group’s
audiences are more or less receptive to its messages. It employs a set of bespoke and
customised business rules to provide dynamically personalised content designed to drive the
Group’s visibility in key moments, while delivering improved cost efficiency and stronger
performance. Digital media spend has reached approximately 50 per cent. of media spend,
fuelled by robust return on investment metrics from marketing effectiveness tools.
More broadly, the Group has increased its investment in digital capability across the business
to improve overall speed and efficiency. Data, a key enabler for growth, is a particular area of
focus as it allows the Group to better understand its consumers and customers. In 2020, a
dedicated data team of data scientists, innovation specialists, user experience designers and
102
data apprentices was set up to build the data strategy and governance processes in readiness
for a future standalone business. The team is also focused on building data literacy across the
business to enable it to extract the most value from its data, which will accelerate the Group’s
digital transformation and support more effective decision making.
As part of the recent transformation of the business, the Group places significant focus on
enhancing the digital capability and literacy of all of its people. In 2020, it launched the Digital
Commerce Academy, an online learning platform with training modules, playbooks, planning
frameworks and other resources to help embed core digital commerce learnings and
behaviours. Since launch in August 2020, more than 5,600 employees across over 84
countries have completed training through the platform as of March 2022. The academy
complements the digital accelerator programme, which rolled out in 2020 in the EMEA region,
following a successful launch in APAC in 2019. The programme is designed to drive sales
through digital commerce and promote a digital-first culture by integrating external digital
experts within teams. Building on this, in 2021, the Group announced a partnership with
University College London to create an industry first, exclusive digital commerce mini-MBA,
which is available for all of the Group’s employees. As a university-level educational
certification, the programme represents a first for the consumer industry.
The acceleration of investments into digital infrastructure and media channels has created a
more efficient, transparent and connected path to consumers. The Group has re-balanced its
digital investment to reflect consumer changes, while the increased use of digital channels has
also enabled it to analyse data to a greater degree, delivering key consumer insights and
enabling the targeting of specific audiences and consumer needs that previously may not have
been addressed.
The Group has begun to see significant success where it has made investments in digital. In
the 2020 launch of Voltaren in the USA, e-commerce formed a key pillar of the successful
brand launch. Two billion media impressions were earned through traditional TV and online
video advertisements. Similarly, during the Advil Dual Action launch, 588 million YouTube
impressions were made.
Notably, the Group’s first US website for Voltaren, VoltarenGel.com, was recognised by the
Arthritis Foundation as the world’s first arthritis-friendly website and obtained Gold Distinction
in the 13
th
Annual Shorty Awards. Among other features, the website implemented accessibility
features such as voice search and scalable font sizes to account for the possibility that users
might have arthritis in their hands that would make it difficult to navigate a website.
Overall, the Group’s e-commerce revenue grew from 4 per cent. of overall revenue in 2019 to
8 per cent. in 2021 and the Group expects e-commerce growth to continue.
7. Engagement with Experts
Overview
The ability to engage appropriately with experts within the healthcare community is a key driver
of the Group’s performance. The Group’s capabilities in expert engagement are one of its key
strengths and it is widely recognised as a partner of choice by healthcare professionals.
There are approximately 10 million healthcare professionals globally addressing the conditions
the Group serves and collectively they have the capacity to make an astonishing 52 billion
recommendations every year. Importantly, consumers take these expert recommendations
seriously and often act upon them. For example, 85 per cent. of pharmacist recommendations
lead to a purchase.
The Group has a dedicated approach to building relationships with experts, healthcare
professionals and external leaders based on trusted advice, recommendations and trial of its
products. This is supported by its purpose to “deliver better everyday health with humanity” and
103
is sustained by the knowledge that expert engagement has a direct impact on everyday health
behaviours. The Group is differentiated by its commitment to trusted science and strict policies
on scientific engagement, both of which provide a strong foundation for its engagement with
the healthcare community.
Nurturing genuine relationships with the scientific and healthcare community also generates
significant benefits for the Group which go beyond the direct generation of sales through expert
recommendations. Expert engagement generates insights that inform product design and
support clear communication of product benefits to consumer populations. It also helps to
ensure that the Group has early visibility on unmet category needs.
Expert field force
The Group maintains a large, dedicated consumer healthcare expert field force that engages
with doctors, dentists, pharmacists and other healthcare professionals across all of its key
markets and has a reputation that scores positively in terms of service. The Group’s research
indicates that the field force’s “called-on” experts make more recommendations per week than
experts that are not called on and this has a direct impact on product performance.
Digital
In addition to its expert field force, the Group engages with a broad group of healthcare
professionals through its digital tools. The Group has dedicated digital channels for experts in
38 markets and has a specialist digital team that engages with experts and healthcare
professionals via a dedicated portal, webinars, personalised learning, email marketing, social
media, searches and paid media channels.
Conferences and events
The Directors believe that one of the most effective ways of ensuring that consumers and
patients feel heard and reassured is by respecting, valuing and supporting the experts that
care for them. As a result, it runs a number of above brand and audience-led initiatives that
support the wellbeing, professional development and, where appropriate, business acumen of
expert audiences.
In addition, the Group has a presence at all major healthcare professional conferences, which
are growing in reach with the addition of virtual capabilities. The Group also presents
symposiums on topics relevant to its products and categories and publishes in peer-reviewed
publications globally.
Partnerships and initiatives
The Group also engages with experts across a range of global and regional initiatives which
are relevant to its brands and categories. These allow the Group to build trust with the
healthcare community and they also provide useful insights to support future innovation and
increase engagement for the Group and its products.
The Group recently partnered with the International Federation of Pharmacists (“FIP”) to
commission research amongst their four million professional members on the impact of air
pollution on respiratory health. FIP is the global federation of national associations of
pharmacists and pharmaceutical scientists and it has 146 member organisations worldwide.
The partnership has resulted in pioneering research on the impact of air pollution on respiratory
health and a thorough understanding of the barriers that exist to optimal self-care, including
health literacy.
In a similar way, the Group’s partnership with world-leading scientific experts and Smile Train,
a non-profit organisation providing corrective surgery for children with cleft lips and palates, led
to the first comprehensive cleft care guidelines that helped provide the evidence for the
inclusion of orofacial clefts as a priority issue in the WHO resolution and resulting Global Oral
Health Strategy.
104
8. R&D
The Group’s dedicated consumer healthcare R&D organisation has a track record of
successful innovation and the generation of product claims supported by scientifically robust
clinical evidence. It also has a critical role in supporting the compliance of the Group’s existing
and new products with varied, complex and moving regulatory requirements in over 170
markets in which the Group operates. It is differentiated by its global reach, broad capabilities,
its leading position in Rx-to-OTC switches and its ability to combine cutting edge science with
deep consumer understanding.
Capabilities
The Group’s R&D organisation’s multidisciplinary talent pool of approximately 1,400 highly
skilled scientists combines OTC and FMCG experience and represents a wide range of
scientific disciplines including scientists, medics, dentists, nutritionists, formulators, engineers,
regulatory professionals and flavour scientists. The Group’s category-level marketing and R&D
teams lead the strategic agenda of the Power Brands and drive and execute at scale the
Group’s innovation pipeline. Local marketing and R&D teams drive the growth and innovation
agenda of the local strategic brands and execute local market-relevant innovations for the
Power Brands. This organisational setup maximises speed of implementation and tailors for
consumer specificities. See also paragraphs 5 and 9 of this Part III ( Business Overview).
The Group has three state-of-the-art R&D centres in Richmond, Virginia, USA (OTC/VMS),
Weybridge, UK (Oral Health), and Suzhou, China (all categories in the Chinese market) providing
it with a broad range of in-house scientific capabilities. Among other capabilities, these sites
possess: (i) fast prototyping and pilot scale equipment for early stage development; (ii) imaging
capability with high specification instrumentation; and (iii) analytical chemistry, product chemistry,
sensory, packaging, process engineering, microbiology and stability capabilities. The R&D
centres also support scale-up and technical transfers to manufacturing and provide end-to-end
support for small-scale manufacturing. These capabilities are augmented by further consumer-
centric capabilities, including consumer behavioural facilities and sensory and flavour science
laboratories. In addition, the Group has embedded innovation resources which support local
business units and enable the Group to recruit R&D talent globally to develop products closer to
its consumers and tailor innovation for local consumer needs.
The Directors believe that the ability to support its products and claims with trusted science is
critical to its relationship with consumers and healthcare professionals. In support of this, the
Group has dedicated capabilities to support both clinical trials and real world studies of its
products and innovations. The Group has conducted over 65 clinical studies involving over
6,000 participants over the last five years, whilst the successful Rx-to-OTC switch of Voltaren
in the USA in 2020 was supported using data in markets where Voltaren is already an OTC
medicine. The R&D function’s expertise is also reflected in its track record of publications,
peer-reviewed journal contributions and patents, including 296 publications and over 70 priority
patent applications filed within the five years to end of 2021.
The Group’s R&D organisation places the understanding of the consumer at the heart of its
innovation processes and draws on its dedicated in-house sensory and flavour science labs
and consumer and shopper facilities to design products with the consumer in mind. The
Group’s R&D scientists regularly connect with consumers via digital channels to obtain early
feedback on innovation and the Group also monitors e-commerce reviews and utilises data-
driven tools to better understand trends, unmet needs and areas of opportunity. Advanced
visualisation techniques enable the Group to translate scientific benefits to consumers in an
accessible manner.
The Group also maintains world class regulatory and medical teams who are embedded
across multiple markets in each region. This enables the Group to rapidly launch innovations,
maintain compliance on existing products, and engage in and enhance the self-care regulatory
landscape – a capability which is a barrier for other smaller players. To illustrate this point, the
Group has completed over 19,000 regulatory applications and approvals around the world in
the last three years.
105
The Group augments its internal capabilities through dedicated business development and
external innovation teams that scout and in-license leading technologies. Innovation is further
supported by a large external ecosystem including established suppliers and contract
manufacturing organisations. Over 30 per cent. of the Group’s pipeline originates from external
partnerships.
Rx-to-OTC switches
The Group has an enduring and world-leading capability in Rx-to-OTC switches. The switch of
prescription products to OTC status is a key source of innovation and growth in OTC and
requires expertise in medical, regulatory and commercial matters. The Group has a
differentiated switch and direct-to-OTC consumer capability with a proven track record, having
successfully completed four switches (Nexium, Flonase, Sensimist and Voltaren) in the USA in
the last eight years, more than twice as many as any other business. The Group’s capabilities
in switches are long-standing, with the Group and its predecessors having switched 19
products since 1990. Additionally, the Group successfully completed one new drug application
in 2020 (Advil Dual Action). These capabilities are a key differentiator and the Group has a
long-standing dedicated in-house team composed of R&D and commercial experts, with a
track record of switching both GSK and non-GSK products.
Going forward, the Directors expect new switch opportunities to be increasingly supported by
digital technology to increase product awareness and availability, to enable better self-care and
to deepen direct relationships with consumers.
Selected innovations
The Contac product range includes a tablet that gives effective relief of seven cold and flu
symptoms. In China, to address the Ministry of Health’s policy restricting pseudoephedrine-
containing OTC medicines, the Group was able to in-license appropriate technology and use
its scientific and market expertise to overcome significant regulatory and technical challenges
to launch the Contac Revive innovation in August 2021.
In 2021, the Group upgraded its core Sensodyne Repair & Protect franchise with the launch of
Sensodyne Repair & Protect Deep Repair. The Group leveraged a previously acquired novel
technology, NovaMin, which was based on findings from bone implant technology. This
enables a deep and targeted occlusion of dentine tubules, in turn helping to reduce dentine
hypersensitivity.
A further recent innovation in Oral Health is the launch of Sensodyne Complete Protection in
January 2022, where the Group has created a formulation which offers all-round oral care
benefits, such as cavity protection and enamel strengthening, while still providing Sensodyne’s
clinically proven sensitivity protection.
In February 2021, the Group launched its Centrum Probiotics range in China. The R&D
function developed the products using certified probiotic strains imported from Denmark. The
launch was the Group’s first major move to expand Centrum beyond being a purely multi-
vitamin and minerals brand, and was achieved within seven months from project initiation,
reflecting the Group’s agile R&D capabilities.
In 2020, the FDA approved Advil Dual Action with acetaminophen as an OTC product for pain
relief. The exclusive formula, launched in September 2020, is the first FDA-approved OTC
combination of ibuprofen and acetaminophen in the USA.
Across 2019 and 2020, the Group upgraded its Voltaren franchise across Europe with the
introduction of two award-winning packaging solutions (with the one launched in 2019 already
patented, and a patent application pending for the one launched in 2020). Detailed consumer
work highlighted that convenience and ease of opening were key trial barriers. A “no mess”
applicator removed the need for direct product contact with hands. Additionally, observing the
target arthritis group’s use of the original product led to the introduction of the arthritis friendly
“easy open” cap.
106
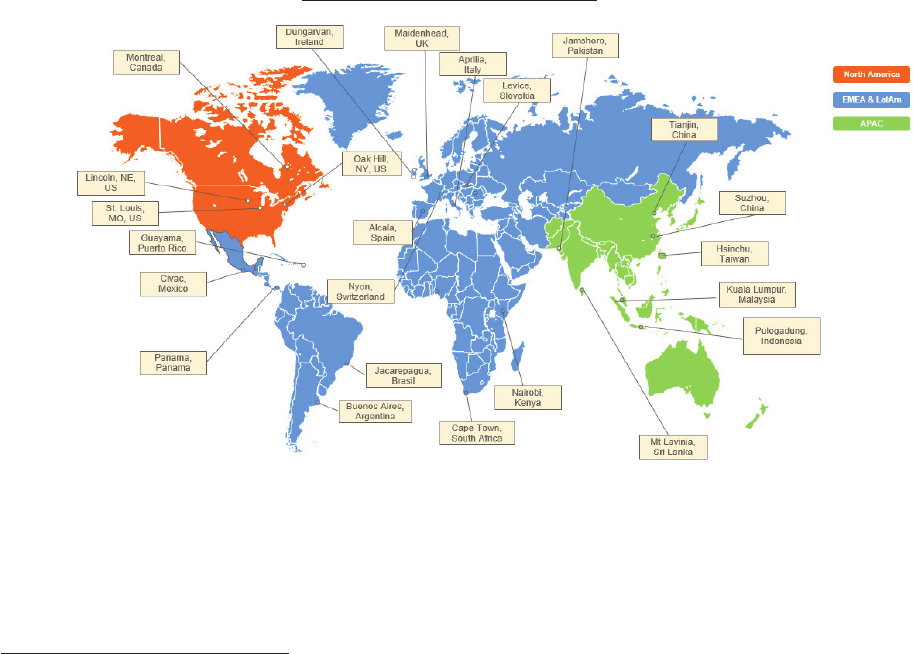
As part of its wider sustainability strategy, the Group has committed to developing solutions for
all of its packaging to be fully recyclable or reusable by 2030 (where quality, safety and
regulations permit). Within Oral Health, extensive stability and quality testing is underway to
ensure that all of the one-billion-plus toothpaste tubes produced by the Group each year are
made recyclable (first wave launched in July 2021 for Sensodyne Pronamel in Europe). The
Group is also redesigning its toothpaste caps with ergonomic upgrades to reduce plastic use
by more than 10 per cent. The Dr.BEST bamboo toothbrush (launched in 2020) and the
Dr.BEST first climate-neutral toothbrush made from renewable resources (launched in 2021)
are further recent innovations highlighting the Group’s sustainability strategy.
For further information on the Group’s R&D-driven innovations, see paragraph 4 of this Part III
(Business Overview) above.
9. Quality and supply chain
Overview
The Group operates a supply chain which combines a network of 24 in-house dedicated
consumer healthcare manufacturing sites with a number of third-party contract manufacturing
organisations (“CMOs”). The Group derives important commercial and competitive benefits from
the large historical investments it has made in its footprint, infrastructure, quality control systems
and people. It benefits both from the economies of scale from its large multi-region manufacturing
sites and from its ability to manufacture with agility and scale on a regional level, close to its
consumers. In addition, the quality and supply chain organisation’s track record in consistently
meeting the rigorous compliance requirements of national regulatory bodies, demonstrated via
successful quality inspection outcomes, is the result of investment, expertise and a cultural
commitment to quality which are difficult to replicate. See paragraph 2.7 of Part VII (Operating
and Financial Review) for a discussion of the key factors impacting the Group’s supply chain.
Group Internal Supply Network
76
Scale manufacturing at a local level
As one of the largest consumer healthcare companies in the world, the Group is able to deliver
the cost benefits of scale manufacturing both at multi-region supply sites such as Dungarvan
and Nyon, and through regionally focused sites close to its customers such as Suzhou and
Oak Hill.
76
The manufacturing sites in Argentina and Brazil will be transferred to the Group following Separation. See paragraph 15.4 of
Part XII (Additional Information) for further information.
107
Every year, the Group supplies more than 3.5 billion consumer packs globally, including
approximately 1.7 billion tubes of toothpaste, approximately 55 billion individual tablets and
high volumes of liquid doses, gels and creams. Approximately 70 per cent. of consumer packs
are sourced internally within the Group, with the remainder through a network of CMOs.
The Group’s ability to manufacture locally at scale is illustrated by some of the Group’s key
sites:
Levice, Slovakia Supply of the Group’s full portfolio of toothpaste at competitive cost
across EMEA at a volume in excess of 600 million tubes annually.
Dungarvan, Ireland Supply of up to six billion tablets of Panadol annually in addition to the
full range of denture cleansers and fixatives.
Nyon, Switzerland Supply of more than 200 million units of the Power Brands Voltaren
and Otrivin.
Guayama, Puerto
Rico
Supply of all Advil, Centrum and Emergen-C products for North America.
The production of Emergen-C was consolidated into Guayama as part of
the Group’s ongoing supply chain efficiency programme following
completion of the Pfizer Transaction to add further scale leverage.
Suzhou, China Dedicated China supply site combining agility for new product
introduction and manufacture at scale for key local products. The
recent addition of a second facility was designed to support growth
and expansion of the VMS product range.
The Group’s manufacturing footprint is well aligned geographically with its key markets around
the world. This allows products to be regionally sourced and more easily tailored to local needs
whilst also reducing the costs and risks associated with single-sourced global manufacturing.
For example, the six in-region manufacturing sites in APAC supply 82 per cent. of the region’s
products and work closely together with the Suzhou R&D organisation to facilitate the rapid
introduction of innovative region-specific products. The geographically aligned sourcing of
products also provides a natural currency hedge, helping to mitigate the impact of foreign
exchange movements.
Complementary mix of internal and external supply
The Group’s supply chain includes approximately 180 external CMOs which supply
approximately 30 per cent. of its consumer packs. While the Group has consolidated its CMO
network in recent years as part of its ongoing supply chain efficiency programme, it has many
ongoing long-established relationships with high quality and trusted CMOs. These relationships
allow the Group to access specialist dose forms, for example, in sprays and patches, while
supporting the Group’s agility in meeting changing consumer demands and providing
innovation and responsive new product introduction in all geographies.
Fully invested systems infrastructure
In support of the internal and external network of manufacturing sites, the Group maintains a
robust and up-to-date systems infrastructure including a single SAP enterprise resource
planning system at 22 of its 24 internal sites, separate from that of the GSK Group and
covering demand forecasting, supply planning, new product introduction and artwork
management. These investments enable seamless interaction across the supply chain whether
production is sourced internally or externally from CMOs.
108
Ongoing synergy delivery from the integration of the Novartis and Pfizer consumer healthcare
businesses
The Group continues to benefit from the scale advantages arising from the combination of both
the Novartis and Pfizer consumer healthcare businesses with GSK’s consumer healthcare
business. Over the past six years, the network rationalisation programme has reduced the
Group’s internal network from 41 sites inherited from the legacy GSK Group, Novartis and
Pfizer businesses to 24 and the Group’s supply network continues to deliver significant
synergies from the integration of the six legacy Pfizer sites and CMO network. In FY 2021 over
£90m cost of sales synergies were delivered with additional synergies projected in 2022.
Robust quality and compliance
The Group’s supply chain infrastructure is distinguished by high quality standards and rigorous
compliance procedures which are applied both to internal sites and the Group’s CMO partners.
These allow consumers, healthcare professionals and regulators to be confident in the Group’s
products.
The effectiveness of the Group’s quality management systems is validated by ongoing strong
performance in external regulator audits. The Group’s supply chain is subject to multiple
regulatory inspections every year by national medical regulatory bodies including the FDA and
MHRA. Since 2019, there have been more than 200 inspections by national regulatory bodies
with a 99.5 per cent. success rate across the internal supply network.
The Group has continued to invest to sustain this strong quality and compliance record in order
to keep ahead of evolving regulatory requirements. Recent investments include an updated
quality management system, enabling enhanced end-to-end compliance capability and
efficiency and electronic batch records deployment into key internal manufacturing sites.
Efficient and customer-oriented warehousing and logistics network
Following the integration of both the Pfizer and Novartis consumer healthcare businesses, the
Group’s warehousing and logistics network has been reviewed and optimised to meet
efficiency and customer service requirements for the combined portfolio and channel mix.
In markets with well-developed infrastructure and established third-party OTC distribution
capabilities, for example in North America and Western Europe, the Group operates through
large-scale distribution centres. In Europe, to further leverage scale and to enable efficient
inventory and service management, the Group operates warehouses covering multiple
markets, often in conjunction with multi-language packs. Typically, distribution centres are
operated with expert third parties in order to leverage scale, expertise and technology
platforms. In all cases, distribution centres, whether in house or third-party, must comply with
the Group’s rigorous quality compliance standards and are subject to the Group’s audit
process.
Ensuring supply continuity
The Group benefits from a comprehensive risk management programme which applies across
all of its sites to minimise potential disruption of supply to customers and patients. This is
supported by independent risk assessment of each manufacturing site. The Group continues to
drive down risk in its sites with a robust risk management approach and a strong
environmental, health and safety risk reduction programme. Supply continuity is also supported
by the Group’s history of strong relations with its site-based employees and union
representatives – presently, fewer than 50 per cent. of the Group’s sites are unionised.
The Group sources from approximately 2,500 direct material suppliers in approximately 65
countries. While, in broad terms, packaging supply and raw material supply takes place at a
local or regional level, the sourcing and supply of active pharmaceutical ingredients and
109
excipients is typically at a global level. To assist the mitigation of packaging and raw material
sourcing risks, the Group operates a dual-sourcing programme, which prioritises critical items
where risk is highest and revenue dependency is significant. As of 31 December 2021,
75-80 per cent. of the Group’s materials supply by spend was sourced from more than one
supplier and it expects this to increase to 85-90 per cent. by the end of 2023. Where dual
sourcing is not expedient or feasible, for example with unique specification materials (such as
supplier IP-owned flavours, or bespoke/IP-owned packaging applications), the Group mitigates
risk through holding higher inventories and sourcing from multiple production sites owned by
the same supplier.
Enabling the Group’s sustainability agenda
The supply network plays a key part in the delivery of the Group’s ambitious sustainability
goals with a number of key initiatives.
To support the global efforts to mitigate the impacts of climate change, the Group has
implemented renewable electricity generation at 12 of its 24 internal sites and is aiming to
reduce its net Scope 1 and 2 carbon emissions by 100 per cent. by 2030 (versus its 2020
baseline).
The Group’s supply network is also implementing a broad range of other environmental and
sustainability initiatives. For example, the Group achieved Zero Waste to Landfill certification
across its network in 2021 and expects to achieve sustainable sourcing of palm oil in 2025. From
a product perspective, the introduction of 100 per cent. recyclable toothpaste tubes and carbon-
neutral plastic-free toothbrushes has commenced in Europe and the Group is working towards
the implementation of pioneering recycling solutions for tubes and blister packs by 2030.
10. Intellectual Property
10.1 Trade marks
The Group’s meaningful and distinctive brands are of central importance to its business.
Accordingly, the Group employs a global trade mark strategy to ensure that it has extensive
and geographically wide-reaching trade mark coverage to protect their reputation and goodwill.
As of 1 March 2022, the Group owns 30,368 trade mark registrations and applications in
multiple jurisdictions worldwide. These are managed centrally at Group level in order to ensure
robust portfolio management and consistency.
The Group’s Power Brands have the most extensive level of trade mark coverage, with rights
filed to cover relevant word marks and logos in all major markets. Local strategic brands in the
Group’s key markets have coverage in their relevant markets. There is also substantial
coverage for the Group’s other leading brands worldwide. Along with the comprehensive
worldwide coverage, the Group has particularly extensive trade mark protection for its products
in the USA and China, both being key markets for the Group.
The Group has adopted the trade mark HALEON as its corporate name. Prior to adoption,
extensive brand clearance work was carried out in all key markets across the full range of
goods and services that the Group operates in, and anticipates operating in, and clearance in
such key markets was obtained. Trade mark applications have been filed in all countries in
which the Group operates.
The Group has worldwide exclusive, royalty-free and sub-licensable licences for certain OTC
products from both Novartis (including Voltaren and Lamisil) and GSK (including Flixonase,
Bactroban and Zovirax). These licences to the shared brands are perpetual, subject to material
breach by or insolvency of the relevant member of the Group, and exclusive in relation to
consumer healthcare products, subject to customary exclusions.
110
Additionally, the Group licenses certain brands to and from third parties, including Nexium
(OTC only), which is a worldwide in-licence from AstraZeneca (excluding Brazil); Nicorette,
which is a US in-licence from Johnson & Johnson; and Nicoderm, which is a US in-licence from
Sanofi.
The TSK&F Joint Venture in China markets several of the Group’s OTC brands locally
(including Contac, Fenbid and Bactroban). The main trade marks for the marketed products
are generally owned by, or licensed to (from GSK or Novartis as shared brands), the Group
and licensed to the TSK&F Joint Venture.
The Group routinely monitors the trade mark activities of competitors and takes timely legal
action to appropriately enforce its trade mark rights against infringing third parties and ensure
that the Group’s brand reputation, goodwill and value are protected. This action is employed
through infringement litigation in courts, enforcement actions at national intellectual property
authorities or by direct negotiations.
10.2 Patents
The Group employs a global patent strategy that endeavours to protect the Group’s R&D
innovations and commercial products and strengthen the Group’s competitive position in the
global consumer healthcare market. The Group currently has, as of 1 March 2022,
approximately 1,500 granted patents and approximately 300 patent applications globally,
including patents and patent applications relating to many of the Group’s Power Brands.
Patents in consumer healthcare play a vital, but different, role than patents in other health-
oriented fields such as pharmaceuticals and vaccines. Consumer health-based patents rarely,
if ever, solely cover a new active pharmaceutical compound by itself, because nearly all
consumer health-based products use well-known, established active pharmaceutical
compounds whose original patents have long since expired. Instead, consumer health-based
patents focus on all aspects of a product itself, such as the formulation, method of
manufacturing, delivery device, packaging, method of treatment, and design. Through careful
evaluation of the different unique features of each consumer healthcare product, the Group’s
products are often protected by multiple patents covering a variety of distinct features of the
product. This results in less reliance on individual patents for a product’s commercial success,
and the inability to obtain patent protection for one feature of the product can often be offset by
patent protection of a different feature. Consequently, the Group does not consider any single
patent to be critical to its overall financial health and success.
The Group’s global patent portfolio provides a number of competitive advantages in the
consumer healthcare industry. The Group’s diversified approach of patenting multiple features
of a product make the market entry of competitors with copy products difficult. Moreover, many
of the Group’s patents cover technology closely related to the Group’s products, creating a
further buffer of patent protection. Particularly during the early stages of new product launches,
patent protection delays competitor entry into the marketplace and provides a competitive
advantage of time for exclusive development of brand goodwill and market share prior to later
entry of competing products. The existence of granted patent rights also enables the Group to
advertise, promote, and mark its products as being patented. This supports the Group’s
marketing campaigns, builds endorsements from healthcare professionals and increases
consumer awareness of innovative technology being used in the Group’s products. Further,
some of the Group’s US patents, including certain patents covering some Advil products, cover
products which qualify for listing in the US FDA Orange Book, which provides statutory
exclusivity periods.
In addition to mitigating patent infringement risks, including through the active use of Freedom
to Operate clearances early in the R&D process to assess potential liabilities presented by
competitor patents, the Group’s global patent strategy invests resources in offensively
enforcing patent rights. The Group routinely monitors the activities of competitors and takes
timely legal action to assert the Group’s patent rights when appropriate. This allows the Group
to retain the competitive advantages provided by the patent portfolio and to protect market
share by seeking legal remedies, such as injunctions or monetary damages, to deter, prevent,
or delay competitor entry.
111
The Group’s patent portfolio further generates value to the Group through the licensing of
patents to outside parties covering technology that is not of commercial value to the Group.
The patent portfolio can also be utilised as leverage during business negotiations, facilitating
the creation of cross-licensing arrangements with competitors in lieu of litigation.
The Group in-licenses certain third-party patents that support the Group’s business goals,
including patents from companies with technical expertise in particular areas that the Group
seeks to commercialise. The Group also partners with third parties to accelerate, develop, and/
or commercialise new products in a cost-effective manner by utilising the knowledge of
external experts in a particular field.
10.3 Designs and other IP
In addition to the Group’s large patent and trade mark portfolios, the Group further strengthens
brand value through protection of distinct brand designs, such as packaging designs. As of
1 March 2022, the Group has 1,057 granted and pending designs which are managed centrally
at Group level. There is also copyright and unregistered intellectual property protection for pack
designs and unregistered brands to the extent available in a given country.
10.4 Domains
As of 1 March 2022, the Group owns 5,815 domain names, which are managed centrally at
Group level. Domain names for the Group’s corporate names have also been reserved,
including “Haleon.com” and “Haleon.cn”.
11. Environmental, social and governance
The Group’s purpose is to deliver everyday health with humanity and this informs the Group’s
relationships with all of its stakeholders as well its approach to stewardship of natural
resources. The Group has ambitious goals in relation to health inclusivity, its environmental
impact, and supporting the communities where it operates together with a commitment to
robust corporate governance practices across its business so as to enable it to maximise its
positive impact on society.
The Group’s ESG strategy is led centrally by a core team of experts with representation on the
management team and with a track record of ESG programme delivery in the consumer goods
and healthcare industries. Central strategy and coordination is complemented at a business
unit level by the incorporation of ESG objectives into the Group’s operational and performance
targets, ensuring that ESG is integral to how it manages its business and drives value creation
for all of its stakeholders.
Tackling environmental issues impacting everyday health
The Group’s commitments to a healthy environment can be seen in four areas in particular:
(i) carbon and climate change; (ii) sustainable healthcare packaging; (iii) trusted ingredients,
sustainably sourced; and (iv) operational waste and water. The commitments are brought to life
through product innovations.
With respect to carbon and climate change, the Group understands the impacts of fossil fuel
emissions and a warming planet on human health, whether it be through the impacts of
extreme weather events, the exposure of new regions to climate-driven infectious diseases or
the direct risks posed by air pollution to respiratory and cardiovascular health. Building on the
steps already taken while part of the GSK Group, the Group is taking a robust approach to
addressing its carbon footprint.
To support the global efforts to mitigate the impacts of climate change, the Group has
implemented renewable electricity generation at 12 of its 24 internal sites and is aiming to
112

reduce its carbon net Scope 1 and 2 carbon emissions by 100 per cent. by 2030 (versus its
2020 baseline). The Group intends to self-generate renewable electricity or purchase
renewable energy certificates to cover the Group’s total electricity usage by the end of 2022. In
addition, the Group aims to reduce its Scope 3 emissions by 42 per cent. from source to sale
by 2030 (versus its 2020 baseline). This level of carbon emissions reduction is aligned to the
Intergovernmental Panel on Climate Change 1.5
o
C pathway. Following Separation, the Group
intends to seek formal accreditation of its carbon commitments by the SBTi. The Group will
also set a long-term carbon net zero goal informed by the latest SBTi guidance.
In packaging, the Group aims to develop solutions for all of its product packaging to be recycle-
ready by 2025 and to be fully recyclable or reusable by 2030 where quality, safety and
regulations permit. The Group aims to reduce its use of virgin petroleum-based plastic by
10 per cent. by 2025 and one-third by 2030 versus its 2020 baseline. The Group will work with
partners to drive global and local initiatives to collect, sort and recycle consumer healthcare
packaging at scale by 2030.
The Group looks to leverage external partnerships to achieve its environmental goals. It is part
of the Pulpex partner consortium with Diageo, Unilever and PepsiCo, which looks at
introducing first-in-class pulp packaging made from sustainably sourced pulp. The Group is
further exploring the design and piloting of Pulpex bottles across several key brands: in its Oral
Health and OTC/VMS portfolios, including its global Power Brand Centrum.
The Group aims for all of its agricultural, forest and marine derived materials to be sustainably
sourced and deforestation free by 2030. In 2020 the Group’s sites that were its largest users of
glycerine, its most material palm oil derivative, achieved Roundtable on Sustainable Palm Oil
77
mass balance certification. As a next step in continuously improving the Group’s sourcing of
sustainable palm oil, it is progressing towards buying physically certified palm oil derivatives.
From 2022, the Group is providing funding towards several projects with ASD (Action for
Sustainable Derivatives) to drive positive impact on the ground by improving the livelihoods of
smallholders and providing support to increase the availability of sustainable sourced palm oil.
With respect to operational waste and water, the Group aims for all of its sites to achieve the
Alliance for Water Stewardship (“AWS”) Standard
78
by 2025, with all sites in water-stressed
basins to be water-neutral by 2030. One achievement in this area is at the Cape Town site,
which is located in a high-stress water basin. Here, the Group has reduced its water
consumption by over 50 per cent. since 2010 and the site is on track to become the first water-
neutral site across the manufacturing network by the end of 2022.
The Group aims for all of its manufacturing sites to achieve TRUE certification by 2030.
79
Additionally, the Group has been implementing its sustainability agenda through its product
innovations. The carbon-neutral Dr.BEST Green Clean toothbrush is an example of
sustainable innovation. It begins with renewable raw material sourcing from sustainable forests
and bio-composite material from the woodwork industry for the handle, has bristles made from
100 per cent. castor oil, and has plastic-free packaging.
To better understand the brush’s improved carbon footprint, the Group engaged with specialist
consultancy firm, Climate Partner, which found the footprint was reduced by more than
50 per cent. when compared with the standard Dr.BEST toothbrush. The remaining footprint is
77
Roundtable on Sustainable Palm Oil (“RSPO”) is a not for profit organisation with over 4000 members, dedicated to developing
and implementing global standards for sustainable palm oil. The RSPO has developed a set of environmental and social criteria
which companies must comply with in order to produce Certified Sustainable Palm Oil. When they are properly applied, these
criteria can help to minimise the negative impact of palm oil cultivation on the environment and communities in palm oil
producing regions.
78
AWS is a global alliance between businesses, NGOs and the public sector promoting good water stewardship. The AWS
Standard is a globally applicable framework for major water users to understand their water use and impacts, and to work
collaboratively and transparently for sustainable water management.
79
TRUE is the first zero waste certification programme dedicated to measuring, improving and recognising zero waste
performance by encouraging the adoption of sustainable materials management and reduction practices which contribute to
positive environmental, health and economic outcomes.
113
offset through a community-based Climate Partner project in Madagascar. The Group is
working on further innovation to reduce the carbon footprint even further with the ultimate goal
of reducing it to zero.
Inclusivity
Health inclusivity is fundamental to the Group’s purpose to deliver everyday health with
humanity, as everyday health is impacted by social exclusion and as bias and stigma prevent
people from accessing better everyday health. The Group is taking a leading position in
educating and empowering people to achieve better self-care and to provide accessible,
affordable health care solutions through its products.
The Group aims to empower millions of people a year to be more included in opportunities for
better everyday health, empowering 50 million people a year by 2025. It aims to achieve this in
three key ways: (i) driving change through its brands; (ii) empowering self-care; and
(iii) investing in thought leadership and research. The Group’s social impact from such
activities will be measured according to the nature of the activity. For example, the Group
developed an inclusive easy-to-open cap for Voltarol pain relief gel and will count the number
of potential arthritis sufferers rather than the total number of purchases in its reporting.
The Group is collaborating with The Economist Group to develop an index to promote
awareness of health inclusivity and ongoing and productive dialogue with policymakers and
healthcare professionals. The programme assesses the state of health inclusivity in 40
countries across four to five indicators (increasing to 80 countries in later phases), with the
data being made available to consumers and stakeholders though an interactive index hub.
The index will be hosted on The Economist website in an interactive hub to enable open
access so that stakeholders can use the data to perform their own analyses. In the first year,
this output will be used to raise awareness and build further understanding of the drivers of
health inclusivity. It is intended to facilitate meaningful dialogue with and among key
stakeholder groups who share an interest in improving health inclusivity, particularly investors,
policymakers, healthcare professionals and external experts, as well as the media, consumers
and customers. The outputs will equip the Group with insights to inform its own actions and
help identify opportunities for partnerships and wider coalitions of action in the medium to
longer term to drive health inclusivity.
The Group has also seen the synergistic benefits of its inclusivity focus in driving preference
and growth among retailers in multiple markets. For example, the US Tums “Bring Diversity to
the Table” campaign run at Walmart in 2020, which donated culinary and nutrition scholarships
with the Thurgood Marshall College fund, was awarded incremental displays in over 3,300
Walmart stores during the competitive holiday period and helped to deliver an increase in
revenue for the Tums brand.
Corporate governance
The Group strives for best-in-class corporate governance, which can be illustrated in three
areas. First, at a Board level, the Chair and Directors have been selected based upon the
capabilities, experience, diversity and regulatory requirements for a consumer healthcare
company. The Directors have a commitment to transparent reporting and disclosure and are
subject to a robust code of conduct.
Second, Board-level governance and committees have been established to ensure alignment
with all requirements of the UK Corporate Governance Code (see paragraph 3 of Part V
(Directors, Senior Managers, Corporate Governance and Remuneration)).
Third, the Group’s internal and external operational governance links in directly to Board-level
governance, enabling rapid escalation and visibility. This includes a focus on key performance
indicators, principal risks, supplier code of conduct and quality requirements, internal employee
training and the use of responsibility scorecards to promote the right behaviours.
114
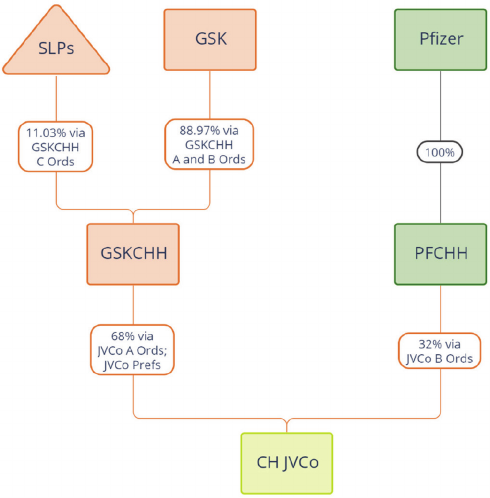
PART IV
OVERVIEW OF THE DEMERGER AND SEPARATION
1. The Demerger and further preparatory steps
On 23 June 2021, GSK announced its intention to effect the separation of the Consumer
Healthcare Business by way of a demerger of at least 80 per cent. of GSK’s 68 per cent.
holding in the Group. The Demerger is conditional on, amongst other things, the approval of
GSK Shareholders at the GSK General Meeting, the receipt of certain mandatory
governmental/regulatory approvals in India, Japan and South Korea, and the approval of the
Demerger Dividend by the GSK Board.
Pursuant to the proposed Demerger, GSK Shareholders who are registered on the Register at
the Shareholder Record Time will be entitled to receive one Haleon Share for each GSK Share
held by them at the Shareholder Record Time. GSK Shareholders will continue to own their
GSK Shares unless they sell or transfer them in the usual course.
1.1 Overview of the Demerger and Share Exchanges
Pursuant to the proposed Demerger and subsequent Share Exchanges described below, the
Company will come to own the entire issued share capital of each of GSKCHH and PFCHH
which, together, own the entire issued share capital of CH JVCo, the current parent company
of the Group.
Current structure of the Group
At present, the structure of the Group is as follows:
The share capital of CH JVCo consists of: (i) 680,000 JVCo A Ordinary Shares of £1 each; (ii)
300,000 non-voting JVCo Preference Shares of £1 each; and (iii) 320,000 JVCo B Ordinary
Shares of £1 each. The JVCo A Ordinary Shares and JVCo B Ordinary Shares each carry one
vote per share. Holders of the JVCo Preference Shares are entitled to 0.01 per cent. of the
aggregate amount of any dividends declared by CH JVCo, and are not entitled to any
proportion of the assets of CH JVCo available for distribution to shareholders on a return of
capital on a winding-up of CH JVCo (excluding any intra-group re-organisation on a solvent
basis). All JVCo A Ordinary Shares and JVCo Preference Shares are held by GSKCHH. All
JVCo B Ordinary Shares are held by PFCHH, which is a wholly owned subsidiary of Pfizer.
115

Accordingly, the share capital of CH JVCo is held as follows:
Shareholder Class Voting rights Nominal
Value
GSKCHH JVCo A Ordinary Shares 68 per cent. 75.38 per cent.
JVCo Preference Shares N/A
PFCHH JVCo B Ordinary Shares 32 per cent. 24.62 per cent.
The share capital of GSKCHH is comprised of three classes of shares: (i) GSKCHH A Ordinary
Shares; (ii) GSKCHH B Ordinary Shares; and (iii) GSKCHH C Ordinary Shares. At present, all
of the GSKCHH A Ordinary Shares and GSKCHH B Ordinary Shares are held by GSK. As part
of certain arrangements to fund GSK’s UK pension benefit obligations, on 25 March 2022, GSK
transferred its entire holding of GSKCHH C Ordinary Shares to the SLPs (being Scottish
Limited Partnerships controlled by GSK).
Demerger
The Demerger will be implemented by GSK declaring an interim dividend in specie to be
satisfied by: (i) the transfer by GSK of the GSKCHH A Ordinary Shares to the Company, in
return for (ii) the issuance of Haleon Shares by the Company to GSK Shareholders who are
registered on the Register at the Shareholder Record Time on the basis of one Haleon Share
for each GSK Share held by such GSK Shareholders at the Shareholder Record Time, save
that the number of Haleon Shares to be allotted and issued to each of the four initial
shareholders of the Company (each of whom is a GSK Shareholder) will be reduced by the
number of Haleon Shares already held by them at the Shareholder Record Time. GSK
Shareholders will continue to own their GSK Shares unless they sell or transfer them in the
usual course.
Share Exchanges
Shortly following completion of the Demerger, a series of share-for-share exchanges will occur,
under which the Company will come to own the entire issued share capital of GSKCHH and
PFCHH, which together own the entire issued share capital of CH JVCo. The purpose of the
share-for-share exchanges is to rationalise the Company’s shareholder structure such that all
persons with an interest in the Group do so through holding shares in the Company, as listed
parent company, and not further down the Group structure. Accordingly:
(A) GSK will transfer its entire shareholding of GSKCHH B Ordinary Shares, representing an
8.01 per cent. stake in the ordinary share capital of GSKCHH, to the Company for
502,868,434 Haleon Shares, less a number of Haleon Shares that is equal to the number
of Excess GSK Shares.
80
As at the Latest Practicable Date, the number of Haleon
Shares expected to be held by GSK at Admission is expected to represent up to 6 per
cent. of the total issued share capital of the Company (the “GSK Share Exchange”);
(B) each of the SLPs will transfer their respective holdings of GSKCHH C Ordinary Shares,
representing 11.03 per cent. in aggregate of the ordinary share capital of GSKCHH, to
the Company in consideration for such number of new Haleon Shares as is required so
that, on Admission, the SLPs will together hold Haleon Shares representing 7.5 per cent.
(in aggregate and to the nearest whole Haleon Share) of the total issued share capital of
the Company; and
80
To the extent any shares are issued by GSK (e.g. in respect of GSK employee share options) between the Latest Practicable
Date and the Shareholder Record Time, this would affect the post-Separation shareholdings in the Company. In summary, the
effect of any such issuance would be that: (i) the total number of Haleon Shares issued to Shareholders under the Demerger
would increase by the number of GSK Excess Shares; and (ii) there would be a corresponding reduction in the total number of
Haleon Shares issued to GSK under the GSK Share Exchange.
116
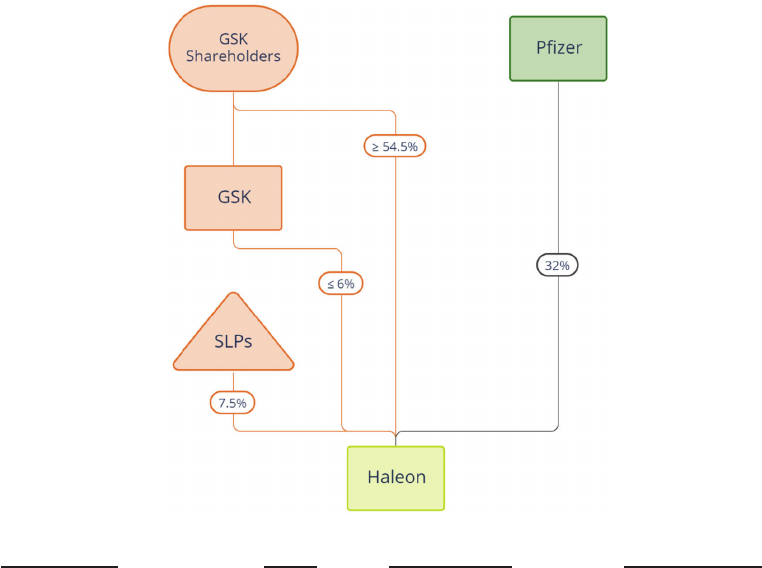
(C) Pfizer will transfer its entire holding in PFCHH to the Company in consideration for:
(i) such number of new Haleon Shares as is required so that, on Admission, Pfizer will
hold Haleon Shares representing 32 per cent. of the total issued share capital of the
Company (to the nearest whole Haleon Share); and (ii) 25 million Non-Voting Preference
Shares (the “Pfizer Share Exchange”),
together, the “ Share Exchanges”.
Immediately following the Pfizer Share Exchange, Pfizer will sell its entire holding in the
Non-Voting Preference Shares to one or more third party investor(s) (the “NVPS Sale”).
Through the Demerger, the Share Exchanges and the NVPS Sale, the ordinary share capital of
the Company will be held as follows:
Shareholder Class Voting rights Nominal Value
GSK Shareholders Ordinary At least 54.5 per cent. At least 54.5 per cent.
Pfizer Ordinary 32 per cent. 32 per cent.
SLPs (Scottish partnerships
controlled by GSK)
Ordinary 7.5 per cent. 7.5 per cent.
GSK Ordinary Up to 6 per cent. Up to 6 per cent.
1.2 Pre-Separation Dividends
Prior to Separation, the Group will pay certain dividends to GSKCHH and/or PFCHH. These
dividends will include:
(A) a cash dividend to be paid by the Group to GSKCHH in connection with the issuance of
the Non-Voting Preference Shares to Pfizer (the “Balancing Dividend”);
(B) a cash dividend to be paid by the Group to GSKCHH and PFCHH, in accordance with the
terms of the Pfizer SHA, which, in summary, requires an amount equal to the
Pre-Separation Debt Proceeds of the Group less £300 million to be paid to GSKCHH and
PFCHH prior to Separation (the “Pre-Demerger Dividend”); and
(C) following the payment of the Balancing Dividend and the Pre-Demerger Dividend, a cash
dividend to be paid by the Group to GSKCHH and PFCHH, in accordance with the terms
117
of the Pfizer SHA which, in summary, requires all readily available cash in excess of
£300 million to be paid to GSKCHH and PFCHH prior to Separation (the “Sweep-up
Dividend”),
(together, the “Pre-Separation Dividends”).
The minimum cash amount of £300 million in the calculation of the Sweep-Up Dividend reflects
the base cash amount required under the Pfizer SHA. The recipients of the Pre-Separation
Dividends reflect the current structure of the Group, as set out above.
Prior to the Pre-Separation Dividends, the Group will continue to pay its ordinary course,
quarterly dividends to GSKCHH and PFCHH in accordance with the terms of the Pfizer SHA
(including, a dividend to be paid in respect of the Group’s financial performance for Q1 2022).
1.3 Pre-Separation bond issuances
As part of the preparation for the Demerger, on 16 March 2022, GSK Consumer Healthcare
Capital UK plc and GSK Consumer Healthcare Capital NL B.V. acting as issuers (the “EMTN
Issuers”) established a £10,000,000,000 Euro Medium Term Note Programme (the
“Programme”) pursuant to which the EMTN Issuers may issue notes from time to time. As at
the date of this Prospectus, the EMTN Issuers have issued under the Programme:
£300,000,000 2.875 per cent. notes due 29 October 2028, £400,000,000 3.375 per cent. notes
due 29 March 2038, €850,000,000 1.250 per cent. notes due 29 March 2026, €750,000,000
1.750 per cent. notes due 29 March 2030 and €750,000,000 2.125 per cent. notes due
29 March 2034 (together, the “Pre-Separation Programme Notes”).
In addition, on 24 March 2022, GSK Consumer Healthcare Capital US LLC (the “US Issuer”)
issued $700,000,000 3.024 per cent. callable fixed rate senior notes due 2024, $300,000,000
callable floating rate senior notes due 2024, $2,000,000,000 3.375 per cent. fixed rate senior
notes due 2027, $1,000,000,000 3.375 per cent. fixed rate senior notes due 2029,
$2,000,000,000 3.625 per cent. fixed rate senior notes due 2032 and $1,000,000,000 4.000
per cent. fixed rate senior notes due 2052 and GSK Consumer Healthcare Capital UK plc
issued $1,750,000,000 3.125 per cent. fixed rate senior notes due 2025 (the “Pre-Separation
USD Notes”) in each case, pursuant to a private placement to institutional investors in the USA
and outside the USA.
The payment of all amounts owing in respect of: (i) notes issued under the Programme
(including the Pre-Separation Programme Notes); and (ii) the Pre-Separation USD Notes is, as
at the date of this Prospectus, guaranteed by GSK (see paragraph 15.13 of Part XII (Additional
Information) for further information). Following completion of the GSK Share Exchange, the
guarantee provided by GSK will cease to be effective and a guarantee provided by the
Company will come into full force and effect. Further details of the terms and conditions
governing the notes issued under the Programme and the Pre-Separation USD Notes can be
found at paragraph 15.13 of Part XII (Additional Information).
The net proceeds of the Pre-Separation Programme Notes and the Pre-Separation USD Notes
have been made available to GlaxoSmithKline Consumer Healthcare Finance Limited in order
to fund the making of certain upstream loans to wholly-owned subsidiaries of GSK and Pfizer.
As such:
(A) on 24 March 2022, GlaxoSmithKline Consumer Healthcare Finance Limited made a loan
of £4,465,197,183.55 to GlaxoSmithKline Finance plc and a loan of £2,101,269,262.85 to
Pfizer Service Company Ireland Unlimited Company; and
(B) on 29 March 2022, GlaxoSmithKline Consumer Healthcare Finance Limited made a loan
of £1,798,139,950.68 to GlaxoSmithKline Finance plc and a loan of £846,183,506.20 to
Pfizer Service Company Ireland Unlimited Company,
(together, the “Notes Proceeds Loans”) pursuant to certain upstream loan agreements as
amended from time to time (the “Notes Proceeds Loan Agreements”).
118
The terms of the Notes Proceeds Loan Agreements require, among other things, that the
Notes Proceeds Loans will be repaid in full to GlaxoSmithKline Consumer Healthcare Finance
Limited on 13 July 2022 or such other date as agreed between the parties in writing. Following
repayment of the Notes Proceeds Loans, the amounts received by GlaxoSmithKline Consumer
Healthcare Finance Limited will be made available to CH JVCo in order to fund a portion of the
Pre-Demerger Dividend.
1.4 The Group’s asset perimeter
On or around the date of this Prospectus, GSK, GSKCHH and CH JVCo entered into the Asset
Transfer Framework Agreement, which sets out the framework for transferring certain
businesses, assets, liabilities and employees that were excluded from the original perimeter of
the GSK/Pfizer JV as contemplated in the Pfizer SAPA and others that were included in the
original perimeter of the GSK/Pfizer JV but had not yet legally transferred or to record the
transfer of “wrong pocket” assets under the Pfizer SAPA, in each case from the GSK Group to
the Group (where a “wrong pocket” asset or liability is one that parties have identified as
incorrectly being transferred, or not transferred, to the other party in line with the principles of
the Pfizer SAPA) (see also paragraph 15.2 of Part XII (Additional Information)).
The Asset Transfer Framework Agreement also sets out the framework for transferring certain
businesses, assets, liabilities and employees from the Group to the GSK Group to record the
transfer of “wrong pocket” assets under the Pfizer SAPA, and to remove assets from the Group
that do not relate to the Consumer Healthcare Business, in each case from the Group to the
GSK Group. For further information on the Asset Transfer Framework Agreement, please see
paragraph 15.4 of Part XII (Additional Information).
1.5 Further information
The GSK Shareholder Circular also contains detailed information about how the Demerger and
other related steps will be effected. GSK Shareholders should only rely on the information in
the GSK Shareholder Circular.
Further information regarding the continuing relationships between the Company, GSK and
Pfizer following the Separation is set out at paragraph 15.10 of Part XII (Additional
Information).
119

PART V
DIRECTORS, SENIOR MANAGERS, CORPORATE GOVERNANCE AND REMUNERATION
1. DIRECTORS
The Directors and their principal functions within the Company, together with a brief description
of their management experience and expertise and principal business activities outside the
Company, are set out below. The business address of each of the Directors (in such capacity)
is 980 Great West Road, Brentford, Middlesex, TW8 9GS, United Kingdom.
Name Position Age
Sir Dave Lewis Non-Executive Chair 57
Brian McNamara Chief Executive Officer 56
Tobias Hestler Chief Financial Officer 50
Manvinder Singh (Vindi)
Banga*
Senior Independent
Non-Executive Director
67
Marie-Anne Aymerich* Non-Executive Director 56
Tracy Clarke* Non-Executive Director 55
Dame Vivienne Cox* Non-Executive Director 63
Asmita Dubey* Non-Executive Director 48
Deirdre Mahlan* Non-Executive Director 59
Bryan Supran* Non-Executive Director
(Pfizer nominee)
51
John Young* Non-Executive Director
(Pfizer nominee)
58
*indicates those persons who will become directors on Admission.
The management experience and expertise of each of the Directors is set out below.
Dave Lewis
Dave Lewis served as Group Chief Executive Officer of Tesco plc, a multinational grocery and
general merchandise retailer, from 2014 until September 2020.
Prior to joining Tesco, he served in a variety of management positions with Unilever plc, a
global consumer products company, from 1987 to 2014, including a variety of leadership roles
in Europe, Asia and the Americas, including as President, Personal Care from 2011 to 2014;
President, Americas from 2010 to 2011; and Chair, United Kingdom and Ireland from 2007 to
2010.
Dave has served on the Pepsico Inc. board since November 2020 and as Chair of Xlinks since
September 2021. He was appointed to serve as Co-Chair of the UK government’s Supply
Chain Advisory Group in October 2021. He previously served on the Sky plc board from 2012
to 2016.
Dave also serves on the boards of several non-profit and charitable organisations, including as
Chair of World Wildlife Fund - UK and as a trustee of Leverhulme Trust, a UK charitable
foundation. He was also Chair of Champions 12.3, a UN programme seeking to add
momentum to the achievement of the UN Sustainable Development Target 12.3 by 2030, and
Co-Chair of the Consumer, Retail and Life Sciences Business Council, which was established
to advise the Prime Minister of the United Kingdom.
120
In recognition of his contribution to business and the food industry in the United Kingdom, Dave
was knighted by Her Majesty Queen Elizabeth II in the 2021 New Year’s Honours List.
Brian McNamara
Brian was appointed Chief Executive Officer designate of the Company in July 2021, having
been Chief Executive Officer of the consumer healthcare division since 2016, where he
focused on shaping a strategy for growth that puts purpose at its heart. He leads approximately
23,000 people across more than 100 countries, who are working every day to deliver better
everyday health with humanity.
Prior to joining GSK, initially as Head of Europe and the Americas in 2015, Brian spent over a
decade at Novartis in senior leadership roles in the OTC division. He began his career at
Procter & Gamble, where over a 16-year tenure, he gained extensive experience in product
supply, brand marketing, and customer leadership.
Brian is a board member of the Consumer Goods Forum. He has previously served as a board
member and Chairman of the Global Self Care Federation and was an active member of the
board of trustees for Treloar’s – a trust providing support and independence education for
young people with physical disabilities.
Tobias Hestler
Tobias was appointed Chief Financial Officer designate of the Company in December 2021,
having been Chief Financial Officer of the consumer healthcare division since 2017.
Prior to joining GSK, Tobias was the Chief Financial Officer at Sandoz – Novartis’ generics
division. In 2010, he was named Chief Financial Officer for the former Novartis consumer
health division, a position that evolved into Tobias’ role as Chief Financial Officer for the
Novartis OTC division. Earlier in his career he held both local and global finance leadership
roles at Novartis in the USA, Germany and Switzerland, for example as Global Head of
Finance for the animal health business, Global Controller for Sandoz and Chief Financial
Officer for Hexal.
Manvinder Singh (Vindi) Banga
Vindi is currently Senior Independent Director at GSK. He has been Chairman of UK
Government Investments Limited (UKGI) since September 2021.
Prior to joining GSK, Vindi spent 33 years at Unilever plc in various roles, his last role was
President of the Global Foods, Home and Personal Care businesses, and a member of the
Unilever Executive Board. Vindi sat on the Prime Minister of India’s Council of Trade & Industry
from 2004 to 2014 and was on the Board of Governors of the Indian Institute of Management
(IIM), Ahmedabad.
Prior non-executive roles include Non-Executive Director of the Confederation of British
Industry (CBI) and Thomson Reuters Corp, Chairman of the Supervisory Board of Mauser
Group, Chairman of Kalle GmbH, Senior Independent Director of Marks & Spencer Group plc,
and Chair, Exec Committee, Diversey Inc. Vindi also holds a number of external appointments,
for example as Partner at Clayton Dubilier & Rice, Non-Executive Director at The Economist
Newspaper Limited, Member of Hakluyt International Advisory Board, Board Member of the
International Chamber of Commerce UK, Member of the Governing Board of the Indian School
of Business, Hyderabad, and Chair of the Board of Trustees at Marie Curie.
Marie-Anne Aymerich
Marie-Anne Aymerich has led a successful 30 plus year career in the consumer and luxury
sectors, growing businesses and shaping brands through innovation and creativity. Most
recently, Marie-Anne was Executive Vice President of the worldwide Oral Care category at
Unilever, where she developed new brands including Zendium and Regenerate. Prior to that,
121
Marie-Anne was General Manager of the Dior Perfume and Beauty business at LVMH where
she was responsible for strategy, equity, innovation, advertising, and digital. Before joining
LVMH, Marie-Anne was Marketing Director of the Home and Personal Care business at
Unilever. In addition to her executive career, Marie-Anne has been a Non-Executive Director of
Pierre Fabre since 2019.
Tracy Clarke
Tracy is currently Non-Executive Director and Chair of Remuneration at TP ICAP plc, Starling
Bank and the All England Netball Association. She is also Chair of a start-up called
SchoolOnline and Advisor and Non-Executive Director at Acin Ltd.
Tracy has extensive experience in international banking and financial services and in commercial
and leadership roles having held a range of senior executive positions over 30 years at Standard
Chartered Bank, where her last role was Private Bank CEO and Regional CEO, Europe &
Americas. During her time at Standard Chartered she served as a director of Standard Chartered
Bank UK, a Non-Executive Director of Standard Chartered First Bank in Korea, director of Zodia
Holdings Limited and Zodia Custody Ltd. She also chaired the Supervisory Board of Standard
Chartered Bank AG and Standard Chartered Yatirim Bankasi Turk A.S.
Prior non-executive roles include Chair of the Remuneration Committees of Sky plc and Eaga
plc and Non-Executive Director and Remuneration Committee member of Inmarsat plc. Tracy
has also served on the boards of the China Britain Business Council and TheCityUK.
Dame Vivienne Cox
Vivienne is currently an independent Non-Executive Director, and Workforce Engagement
Director at GSK.
Prior to joining GSK Vivienne worked at BP plc for 28 years, in Britain and Continental Europe
in various posts, including Executive Vice President and Chief Executive of BP’s gas, power
and renewable business and its alternative energy unit. Vivienne was previously a
Non-Executive Director of BG Group plc and Rio Tinto plc, the Senior Independent Director of
Pearson plc, Chairman of the Supervisory Board of Vallourec and the Lead Independent
Director at the UK Government’s Department for International Development.
Vivienne holds a number of external appointments, including Chair of Victrex plc,
Non-Executive Director of Stena AB, Vice President of the Energy Institute, Advisory Board
Member of Montrose Associates and Chair of the Rosalind Franklin Institute.
Asmita Dubey
Asmita Dubey has 25 years of experience working in consumer businesses. She has worked at
L’Ore´al since 2013 and is currently Chief Digital and Marketing Officer and a member of the
L’Ore´al Executive Committee. Asmita brings extensive expertise of marketing in the digital age
and is leading L’Ore´ al on a digital transformation journey to evolve the marketing models,
adopt new data-driven solutions, and accelerate emerging business models for the largest
beauty company in the world. Asmita also brings strong experience working in China where
she strengthened L’Ore´al’s digital footprint in the region, built L’Ore´ al’s first joint-business
partnerships with Alibaba and Tencent and established a broader start-up ecosystem. In
addition to her executive career, Asmita served on GSK’s Digital Advisory Board for two years.
Deirdre Mahlan
Deirdre is Chair of the Audit Committee at Experian plc, Non-Executive Director at Kimberly-
Clark Corporation and Chair of the Audit Committee of The Duckhorn Portfolio, Inc.
Deirdre has deep finance and consumer product experience and is a qualified accountant. She
has held a number of senior finance and general management roles over her 27 year career at
Diageo, including President of Diageo North America, Chief Financial Officer, Deputy Chief
122

Financial Officer, Head of Tax and Treasury at Diageo plc, Senior Vice President, Chief Financial
Officer at Diageo North America, and Vice President of Finance at Diageo Guinness USA. She
has also held various senior finance roles in Joseph Seagram and Sons, Inc. and PwC.
Bryan Supran
Bryan’s career as a leading corporate and transactional attorney spans more than 25 years,
including 17 years at Pfizer and Wyeth. Since 2015, Bryan has served as Senior Vice
President & Deputy General Counsel of Pfizer, a role that includes counselling management
and the Board of Directors on strategic initiatives and business development transactions, such
as Pfizer’s COVID-19 vaccine collaboration with BioNTech, successful split-off of its animal
health business as Zoetis, and joint venture with GSK to create a premier global consumer
healthcare business that has become Haleon. Bryan’s role also has included leadership of
Pfizer’s intellectual property and international legal teams, as well as legal support for Pfizer’s
R&D and manufacturing organizations. Previously, Bryan practiced law at Ropes & Gray LLP
in Boston and New York, where he helped establish the firm’s life sciences practice.
John Young
John has had an extensive career at Pfizer over 34 years and is currently Senior Adviser to the
CEO of Pfizer. In his previous role as Chief Business Officer he played an important role in
cultivating the collaborations that led to the successful development and delivery of the
Pfizer-BioNTech COVID-19 vaccine. John has led many large-scale transformations, including
the closing of the joint venture with GSK to create a premier global consumer healthcare
business in 2019. John has extensive healthcare, commercial and international experience.
2. SENIOR MANAGERS
In addition to the Directors, the current members of the senior executive team with
responsibility for day-to-day management of the Group’s business are set out below. The
business address of each of the Senior Managers (in such capacity) is 980 Great West Road,
Brentford, Middlesex TW8 9GS, United Kingdom.
Name Position Age
Dana Bolden Head of Corporate Affairs 55
Keith Choy Head of Asia Pacific 54
Bart Derde Head of Quality and Supply Chain 53
Amy Landucci Head of Digital and Technology 48
Filippo Lanzi Head of EMEA and LatAm 49
Jooyong Lee Head of Strategy and Office of the CEO 45
Teri Lyng Head of Transformation and Sustainability 60
Mairéad Nayager Chief Human Resources Officer 47
Lisa Paley Head of US and North America 56
Franck Riot Head of R&D 55
Tamara Rogers Chief Marketing Officer 53
Bjarne P Tellmann General Counsel 55
123
The management experience and expertise of each of the Senior Managers is set out below.
Dana Bolden
Dana joined GSK consumer healthcare as Head of Corporate Affairs in January 2021. He is
responsible for corporate communications, government affairs and responsible business
engagement.
Previously, Dana was Chief Communications Officer at Corteva Agriscience and prior to this,
he worked for The Coca-Cola Company, having started his career at global PR consultancy,
Cohn and Wolfe.
Dana is accredited by the Public Relations Society of America and is an active member of the
Arthur W. Page Society, the world’s leading professional association for senior public relations
and corporate communications executives and educators.
Keith Choy
Keith was appointed Region Head of Asia Pacific for GSK consumer healthcare in 2019.
Before joining GSK, Keith was the President for international markets of Pfizer’s consumer
healthcare business. He previously served as the Regional President of APAC for Pfizer
consumer healthcare and before that as China General Manager, where he oversaw significant
sales growth in the China OTC category. Across his 28 years of commercial experience in the
consumer-packaged goods and healthcare industries, Keith has also held various roles of
sales, brand marketing, and general management in Wyeth, Gillette and Joyco (acquired by
Wrigley Company).
He was awarded the Internationalist of the Year for 2017.
Bart Derde
Bart was appointed Head of Quality and Supply Chain for GSK consumer healthcare in 2018.
Prior to joining GSK, Bart spent 14 years at Reckitt where he was Head of Quality, Safety
Sustainability and Compliance as well as Head of Global Operations for Reckitt Health and
held various other roles in the global health supply chain.
Prior to joining Reckitt, Bart worked at Unilever for over a decade in a number of roles across
manufacturing, procurement, planning and strategic projects in the UK and the Netherlands.
Amy Landucci
Amy was appointed Chief Digital and Technology Officer for GSK consumer healthcare in
2021.
Prior to joining GSK in 2017, as Chief Information Officer for consumer healthcare, Amy spent
over a decade at Novartis, where she was most recently the Global Head of Digital Medicines.
She was responsible for defining and delivering the “beyond the pill” strategy and solutions to
accelerate Novartis’ entrance into digital medicines. Prior to this role, Amy was Chief
Information Officer for the Novartis OTC division.
Amy previously served on the board of directors for Healthy Women, the leading independent,
non-profit health information source for women in the USA with a mission to educate and
empower women to make informed health choices for themselves and their families.
Filippo Lanzi
Filippo was appointed Head of EMEA and LatAm in 2021, having previously served as EMEA
Lead from 2019 to 2021.
124
Having joined GSK consumer healthcare in 2012, he previously served in various roles across
Central, Eastern and Southern Europe before leading the APAC business.
Prior to that role, he was the Operating Unit Head in Italy and Greece for Novartis’ OTC
business. Previously, Filippo spent five years at Johnson & Johnson, managing the diabetes
division in Italy and then as Head of Mediterranean Cluster for the Ethicon/Endo franchise.
Before Johnson & Johnson, he spent a decade with Nestlé, holding various roles across
marketing, sales and finance.
Jooyong Lee
Jooyong Lee joined GSK consumer healthcare as Head of Strategy and Office of the Chief
Executive Officer in March 2019.
Previously at Diageo, Jooyong oversaw market strategy across all global markets with
particular focus on emerging markets across Asia, Africa and Latin America, and drove key
corporate strategic initiatives to unlock new sources of growth. Prior to this role, Jooyong was
Vice President of Strategy for InterContinental Hotels Group overseeing the Asia, Middle East
and Africa growth strategy, based in Singapore.
Jooyong is a former management consultant with McKinsey & Company, having started her
career at Procter & Gamble.
Teri Lyng
Teri was appointed as Head of Transformation and Sustainability at GSK consumer healthcare
in 2019 and has been responsible for the development and execution of the ESG strategy, as
well as the integration of the joint venture with Pfizer’s consumer healthcare business and the
preparation and operational readiness of the formal separation from GSK.
Previously, Teri led the quality function for GSK consumer healthcare and before that held
similar roles in the OTC division at Novartis and the consumer health divisions of both Wyeth
and Merck.
Mairéad Nayager
Mairéad was appointed as Chief Human Resources Officer in March 2022 and will be
responsible for developing global talent capabilities pre- and post-formal separation from GSK.
Previously, Mairéad was Chief Human Resources Officer at Diageo for over seven years, having
already held a number of HR executive roles across a number of Diageo’s regional businesses
over the previous decade. At Diageo she played a pivotal role in successfully leading the
business through multiple business transformations, developing and delivering a dynamic
performance culture and spearheading Diageo’s plans to help create a more inclusive world.
Prior to joining Diageo, Mairéad spent three years at the Irish Business and Employers’
Confederation where she represented companies across various sectors in industrial relations.
Lisa Paley
Lisa has led the North America region for GSK consumer healthcare since 2021, having
previously been General Manager, USA and Puerto Rico.
She joined GSK in 2019 from Pfizer’s consumer healthcare business, where she had been
President of North America, responsible for the US, Puerto Rico and Canadian markets. In her
decade at Pfizer, Lisa had senior roles in sales strategy; was General Manager of Puerto
Rico & Caribbean; and was the US Chief Customer Officer and leader of Global E-Commerce.
125
Previously, Lisa worked at Johnson & Johnson as a Vice President in US Sales and also spent
18 years with the Pfizer consumer healthcare business where she held positions of increasing
responsibility in sales, customer development, sales strategy, operations, category
management & insights and general management.
She is a former Certified Public Accountant and worked for Deloitte.
Lisa is a board member of several US industry association boards including CHPA Executive
Committee, NACDS Retail Advisory Board, and the WE Board.
Franck Riot
Franck was appointed Head of Global R&D for GSK consumer healthcare in 2019. Previously,
Franck was Vice President of Research and Innovation at Danone for the essential dairy and
plant-based world-wide business unit from 2017 to 2019.
Franck began his career as an R&D engineer in the beauty and personal care sector, then
spent 14 years at Danone, with progressively larger R&D roles in Europe. He left Danone for
six years to run the global R&D organisation for IGLO (owned by private equity firm Permira),
which was sold to Nomad Foods. Franck was part of the Nomad Foods team that then took the
company public in 2015, after which he returned to Danone to run research and innovation for
the newly created essential dairy and plant-based world-wide business unit after the acquisition
of the world leading plant-based food company WhiteWave in 2017.
Tamara Rogers
Tamara was appointed Chief Marketing Officer for GSK consumer healthcare in 2019, having
previously served as Region Head for EMEA.
Prior to joining GSK, Tamara spent 25 years at Unilever, having joined as a Management
Trainee in the UK. She held significant leadership positions such as EVP Region Head
Personal Care for Unilever North America and prior to that, EVP Global Deodorants Category.
Tamara has nearly 30 years of experience in FMCG with numerous commercial roles across
marketing, advertising, customer development and general management, in local, regional and
global capacities. Her experience includes the development of business growth strategies,
strategic portfolio management, innovation development, brand building, design, customer
development and trade marketing. Her responsibilities have also included media, consumer
business insights & analytics, marketing and digital commerce capability.
Tamara is a board member of the Global Self-Care Federation, which exists to create a
healthier world through better self-care.
Bjarne P Tellmann
Bjarne was appointed General Counsel of GSK consumer healthcare in 2020.
Prior to his role at GSK, Bjarne was Chief Legal Officer, General Counsel and member of the
executive team at Pearson, where he led the company’s legal, compliance, and company
secretary functions. He previously worked in numerous locations across Europe, Asia and the
USA in senior executive capacities with Coca-Cola, most recently as Associate General
Counsel of The Coca-Cola Company. He has also held positions at Kimberly-Clark, and the
law firms of Sullivan & Cromwell LLP and White & Case LLP.
Bjarne serves as a Non-Executive Director on the board and audit committee of Mowi ASA, the
world’s leading seafood company and the largest producer of Atlantic salmon. He previously
sat on the boards of Coca-Cola West Co.,Ltd., Coca-Cola Erfrischungsgetränke AG and Hire
an Esquire, Inc.
126
3. THE BOARD AND CORPORATE GOVERNANCE
3.1 The Board
The Board is responsible for leading and controlling the Group and has overall authority for the
management and conduct of the Group’s business and its strategy and development.
3.2 Compliance with corporate governance requirements
(A) Compliance with UK Corporate Governance Code
From Admission, the UK Corporate Governance Code will apply to the Group and the
Group will comply, and intends to continue to comply, with the UK Corporate
Governance Code.
(B) Board and Committee independence
The UK Corporate Governance Code recommends that at least half the board of
directors of a UK listed company (excluding the chair) should comprise ‘independent’
non-executive directors, being individuals determined by the Board to be independent
in character and judgement and free from relationships or circumstances which may
affect, or could appear to affect, the directors’ judgement. It also recommends that a
UK listed company should establish remuneration and audit committees of
independent non-executive directors, each comprising at least three members, as well
as a nomination committee, the majority of members of which should be independent
non-executive directors.
From Admission, the Board will comprise eleven members: two executive Directors
and nine non-executive Directors. The Board considers Dave Lewis, Manvinder Singh
(Vindi) Banga, Tracy Clarke, Dame Vivienne Cox, Deirdre Mahlan, Asmita Dubey and
Marie-Anne Aymerich to be independent for the purposes of the UK Corporate
Governance Code. The Board does not consider Bryan Supran and John Young to be
independent as they are representatives of Pfizer, a significant shareholder.
The Board therefore considers that the Company complies with the relevant
requirements of the UK Corporate Governance Code in relation to the balance of
executive and independent non-executive Directors on the Board and with the
requirements for the composition of the Company’s Audit & Risk Committee,
Remuneration Committee and Nomination Committee.
(C) Senior Independent Non-Executive Director
The UK Corporate Governance Code also recommends that the board of directors of a
UK listed company should appoint one of its independent non-executive directors to be
the senior independent non-executive director. The senior independent non-executive
director should provide a sounding board for the chair and serve as an intermediary for
the other directors and shareholders. He or she should be available to shareholders if
they have concerns that the normal channels of chair, chief executive officer or other
executive directors have failed to resolve or for which such channel of communication
is inappropriate. Manvinder Singh (Vindi) Banga has been appointed as the Company’s
Senior Independent Non-Executive Director subject to Admission.
(D) Workforce Engagement Director
The UK Corporate Governance Code requires that the Board understands the views of the
Company’s other key stakeholders, including its workforce. To facilitate effective
engagement with the Company’s workforce, the UK Corporate Governance Code
recommends that a listed company adopt one or a combination of: (a) a director appointed
127
from the workforce; (b) a formal workforce advisory panel; or (c) a designated
non-executive director. Dame Vivienne Cox has been appointed as the Company’s
dedicated non-executive workforce engagement director subject to Admission.
(E) Re-election
The UK Corporate Governance Code recommends that all directors of UK listed
companies should be subject to annual re-election. The Directors therefore intend to
put themselves up for election at the Company’s next annual general meeting
(expected to be held in the second quarter of 2023). It is also intended that the
Directors will continue to put themselves up for annual re-election voluntarily at each
further annual general meeting of the Company. In addition, prior to recommending
their re-election to Haleon Shareholders, the Board intends to carry out an annual
re-assessment of the ongoing independence of each of the non-executive Directors
and to make an appropriate statement disclosing their status in the Company’s annual
report.
3.3 Board committees
The Board has established a number of committees, whose terms of reference are
documented formally and updated as necessary. If the need should arise, the Board may set
up additional committees as appropriate.
(A) Audit & Risk Committee
The Audit & Risk Committee will be chaired by Deirdre Mahlan and its other members
will be Manvinder Singh (Vindi) Banga, Tracy Clarke and Dame Vivienne Cox. The
Audit & Risk Committee will meet at least four times a year, and otherwise as the
Audit & Risk Committee’s role and responsibilities require. The external auditors or a
member of the Audit & Risk Committee may also request a meeting if they consider
that one is necessary.
The Audit & Risk Committee’s terms of reference state that the Audit & Risk Committee
must comprise a minimum of three members all of which must be independent
non-executive directors of the Company. Appointments to the Audit & Risk Committee
will be made by the Board, on recommendation by the Nominations & Governance
Committee. The Chair, from time to time, is not eligible to be a member. At least one
member of the Audit & Risk Committee shall have recent and relevant financial
experience and the committee as a whole will be financially literate and have
competence relevant to the sector in which the Company operates. At least annually
the Board shall consider whether to designate one or more of the Audit & Risk
Committee as “Audit Committee financial experts” in accordance with US federal
securities laws and regulations.
The responsibilities of the Audit & Risk Committee include but are not limited to:
(i) receiving and reviewing reports from the Company’s external auditors, monitoring
their effectiveness and independence and making recommendations to the Board in
respect of their remuneration, appointment and dismissal; (ii) monitoring and reviewing
internal audit activities, reports and findings; (iii) reviewing the financial statements of
the Company; and (iv) reviewing, on behalf of the Board, the effectiveness of the
Group’s system of internal financial controls and internal control systems.
The Chief Financial Officer, General Counsel, Group Financial Controller, Head of
Audit & Assurance, Chief Compliance Officer, and a representative of the external
auditors shall be invited to attend meetings on a regular basis, although the Audit &
Risk Committee may meet without any executives of the Company being present. The
Chair, Chief Executive Officer and others may be invited to attend for all or part of any
meeting, as and when appropriate. The Audit & Risk Committee will also meet
128
separately at least once a year with the Group’s external auditors, the Head of Audit &
Assurance and the Chief Compliance Officer without the executive directors and other
management present.
The Audit & Risk Committee will prepare a report describing the work of the Audit & Risk
Committee to be included in the Company’s annual report. Among other matters, the report
will include an explanation of how auditor objectivity and independence is safeguarded
where the external auditor provides non-audit services. The chair of the Audit & Risk
Committee will be available at annual general meetings of the Company to make a
statement on the Audit & Risk Committee’s activities and to respond to questions from
Haleon Shareholders on matters within the Audit & Risk Committee’s area of responsibility.
(B) Remuneration Committee
The Remuneration Committee will be chaired by Tracy Clarke, and its other members
will be Manvinder Singh (Vindi) Banga, Dame Vivienne Cox and Deidre Mahlan. The
Remuneration Committee will meet at least four times a year, and otherwise as the
Remuneration Committee’s role and responsibilities require. A member of the
Remuneration Committee may also request a meeting if they consider that one is
necessary.
The Remuneration Committee’s terms of reference state that the Remuneration
Committee must comprise at least three independent non-executive directors.
Appointments to the Remuneration Committee will be made by the Board, on
recommendation by the Nominations & Governance Committee in consultation with the
chair of the Remuneration Committee.
The chair of the Remuneration Committee is appointed by the Board. In accordance
with the UK Corporate Governance Code, the chair should have served on a
remuneration committee for at least 12 months. On Admission, Tracy Clarke will satisfy
this provision of the UK Corporate Governance Code, having served as Chair of the
Remuneration Committees of Sky plc and Eaga plc and as a member of the
Remuneration Committee of Inmarsat plc.
The responsibilities of the Remuneration Committee include but are not limited to
determining the policy for Directors’ remuneration and within this setting remuneration
packages for the Chair, executive directors, senior management and the company
secretary and such other executives as required, in accordance with the UK Corporate
Governance Code.
Only members of the Remuneration Committee have the right to attend Remuneration
Committee meetings. The Chair, the Chief Executive Officer, the Chief HR Officer,
Head of Reward, external advisors and others, as appropriate, may attend meetings at
the invitation of the Committee, except when issues regarding their own remuneration
are discussed.
The Remuneration Committee will also prepare a report describing the activities of the
Remuneration Committee to be included in the Company’s annual report. The chair of
the Remuneration Committee will be available at annual general meetings of the
Company to respond to questions from Haleon Shareholders on the Remuneration
Committee’s activities.
(C) Nominations & Governance Committee
The Nominations & Governance Committee will be chaired by Dave Lewis, and its other
members will be Manvinder Singh (Vindi) Banga, Tracy Clarke and Deidre Mahlan. The
Nominations & Governance Committee will meet at least two times a year, and otherwise
as the Nominations & Governance Committee’s role and responsibilities require.
129
The Nominations & Governance Committee’s terms of reference state that the
Nominations & Governance Committee must comprise at least three members, the
majority of whom must be independent non-executive directors. The Chair is appointed
by the Board and should be either the Chair of the Board or a member of the
Nominations & Governance Committee. The Chair of the Board may not chair the
Nominations & Governance Committee when it is dealing with the appointment of his
or her successor or performance. Appointments to the Nominations & Governance
Committee will be made by the Board.
The responsibilities of the Nominations & Governance Committee include but are not
limited to: (i) reviewing the structure, size and composition, including the skills,
knowledge, experience and diversity (including of gender, social and ethnic
backgrounds and cognitive and personal strengths) of the Board and its Committees
and making recommendations to the Board with regard to any changes; (ii) identifying
and nominating for approval candidates to fill any vacancies on the Board and its
Committees; and (iii) ensuring plans are in place for orderly succession of the Board
and its Committees.
The Nominations & Governance Committee will also prepare a report describing the work
of the Nominations & Governance Committee to be included in the Company’s annual
report. The chair of the Nominations & Governance Committee will be available at annual
general meetings of the Company to respond to questions from Haleon Shareholders on
matters within the Nominations & Governance Committee’s area of responsibility.
4. CONTROLLING SHAREHOLDER
Pfizer is expected to be the beneficial owner of 32 per cent. of the issued Haleon Shares
immediately following Admission (to the nearest whole Haleon Share).
The Company has entered into a relationship agreement with Pfizer (the “ Pfizer Relationship
Agreement”).
The principal purpose of the Pfizer Relationship Agreement is to regulate the continuing
relationship between the Company and the Pfizer Group after Admission, including ensuring
that the Company is capable at all times of carrying on its business independently from Pfizer
as a controlling shareholder (as defined in Appendix I to the Listing Rules) and any of Pfizer’s
associates (as defined in Appendix I to the Listing Rules). A description of the terms of the
Pfizer Relationship Agreement is given in paragraph 15.10 of Part XII (Additional Information).
5. LOCK-UP ARRANGEMENTS
Pursuant to a lock-up deed entered into on or around the date of this Prospectus between
GSK, Pfizer, the SLPs (together, the “Shareholder Parties”), Citi and Morgan Stanley (the
“Lock-up Deed”), the Haleon Shares are subject to certain lock-up arrangements on
customary terms. In particular, subject to certain exceptions, the Lock-Up Deed prohibits the
offer, sale, lending, pledging or other disposal of Haleon Shares and ADSs in respect of such
Haleon Shares by the Shareholder Parties (and requires each of the Shareholder Parties to
further procure that each member of its corporate group likewise abides by the same
restrictions) for a period commencing on completion of the Share Exchanges and ending on
the day after the earlier of: (i) 10 November 2022; and (ii) the date of the first announcement by
Haleon of a quarterly trading update for a quarterly period ending after 30 June 2022. The
Lock-up Deed provides that the lock-up may be released during such period (which shall apply
pro rata to the Pfizer Group, on the one hand, and the GSK Group (including the SLPs), on the
other hand, in accordance with their relative ownership of Haleon Shares as of the date of the
release) upon the mutual written agreement of Citi and Morgan Stanley.
6. REMUNERATION AND PENSION BENEFITS
Details regarding remuneration of Directors are set out in paragraph 9 of Part XII (Additional
Information).
130

PART VI
SELECTED FINANCIAL INFORMATION
The selected consolidated historical financial information relating to the Group set out below has been
extracted, without material adjustment, from Schedule II (Historical Financial Information of
GlaxoSmithKline Consumer Healthcare Holdings (No.2) Limited). The selected non-IFRS financial
information and operating information relating to the Group set out below has been calculated on the
basis set out in “Presentation of Financial and Other Information”. The selected financial and operating
information presented below should be read in conjunction with Part VII (Operating and Financial
Review).
1. Consolidated income statement
For the years ended 31 December 2021, 31 December 2020 and 31 December 2019.
£m 2021 2020 2019
Revenue 9,545 9,892 8,480
Cost of sales (3,595) (3,982) (3,678)
Gross Profit 5,950 5,910 4,802
Selling, general and administration (4,086) (4,220) (3,596)
Research and development (257) (304) (292)
Other operating income/(expense) 31 212 (17)
Operating profit 1,638 1,598 897
Finance income 17 20 24
Finance expense (19) (27) (35)
Net finance costs (2) (7) (11)
Profit before tax 1,636 1,591 886
Income tax (197) (410) (199)
Profit after tax 1,439 1,181 687
Profit attributable to shareholders 1,390 1,145 655
Profit attributable to non-controlling interests 49 36 32
2. Consolidated balance sheet
As at 31 December 2021, 31 December 2020 and 31 December 2019.
£m 2021 2020 2019
Non-current assets 29,200 29,122 29,900
Current assets 5,251 5,008 5,811
Total Assets 34,451 34,130 35,711
Current liabilities (4,238) (4,014) (4,269)
Non-current liabilities (3,733) (3,893) (4,030)
Total liabilities (7,971) (7,907) (8,299)
Net assets 26,480 26,223 27,412
131

3. Consolidated cash flow statement
For the years ended 31 December 2021, 31 December 2020 and 31 December 2019.
£m 2021 2020 2019
Cash flow from operating activities
Profit after tax 1,439 1,181 687
Adjustments reconciling profit after tax to cash generated from
operations 227 780 408
Cash generated from operations 1,666 1,961 1,095
Taxation paid (310) (554) (309)
Net cash inflow from operating activities 1,356 1,407 786
Net cash (outflow)/inflow from investing activities (33) 1,030 291
Net cash (outflow) from financing activities (1,236) (2,437) (925)
Increase in cash and bank overdrafts 87 - 152
Cash and bank overdrafts at the beginning of the year 323 329 191
Exchange adjustments (5) (6) (14)
Increase in cash and bank overdrafts 87 - 152
Cash and cash equivalents at end of year 405 323 329
4. Q1 2022 Interim Financial Information
The unaudited consolidated income statement for the three months ended 31 March 2022 and
31 March 2021 and the unaudited consolidated balance sheet as at 31 March 2022 have been
extracted without material adjustment from the Company’s consolidation schedules (the
“Interim Financial Information”). The Interim Financial Information has been prepared on a
basis consistent with Historical Financial Information set out in Schedule II (Historical Financial
Information of GlaxoSmithKline Consumer Healthcare Holdings (No.2) Limited). The Interim
Financial Information as of and for the three months ended 31 March 2022 and 31 March 2021
are neither audited nor reviewed by an accountant. The audited consolidated balance sheet as
at 31 December 2021 has been extracted without material adjustment from the Historical
Financial Information for the period ended 31 December 2021 set out in Schedule II (Historical
Financial Information of GlaxoSmithKline Consumer Healthcare Holdings (No.2) Limited). This
Interim Financial Information should be read in conjunction with the Historical Financial
Information included elsewhere in this document.
132

4.1 Unaudited consolidated income statement
For the three months ended 31 March 2022 and 31 March 2021.
2022 2021
£m £m
Revenue 2,627 2,306
Cost of sales (1,014) (904)
Gross Profit 1,613 1,402
Selling, general and administration (1,086) (1,009)
Research and development (64) (54)
Other operating income 3 9
Operating profit 466 348
Finance income 7 6
Finance expense (8) (4)
Net finance (costs)/income (1) 2
Profit before tax 465 350
Income tax (108) (101)
Profit after tax for the quarter 357 249
Profit attributable to shareholders 343 233
Profit attributable to non-controlling interests 14 16
Basic earnings per share (pence)
1
34,300 23,300
Diluted earnings per share (pence)
1
34,300 23,300
Note
1
The number of shares in issue above is not representative of the number of shares in issue in the
future. For further detail, please see Note 15 of Schedule II (Historical Financial Information of
GlaxoSmithKline Consumer Healthcare Holdings (No.2) Limited).
133

4.2 Consolidated balance sheet
As at 31 March 2022 and 31 December 2021.
31 March
2022
31 December
2021
£m
(unaudited)
£m
(audited)
Non-current assets
Property, plant and equipment 1,587 1,563
Right of use assets 100 99
Intangible assets 27,692 27,195
Deferred tax assets 314 312
Post-employment benefit assets 11 11
Derivative financial instruments 8 12
Other non-current assets 13 8
Total non-current assets 29,725 29,200
Current assets
Inventories 986 951
Trade and other receivables 2,415 2,207
Loan amounts owing from related parties 11,330 1,508
Cash and cash equivalents and liquid investments 383 414
Derivative financial instruments 18 5
Current tax recoverable 166 166
Total current assets 15,298 5,251
Total assets 45,023 34,451
Current liabilities
Short-term borrowings (80) (79)
Trade and other payables (3,142) (3,002)
Loan amounts owing to related parties (1,461) (825)
Derivative financial instruments (15) (18)
Current tax payable (242) (202)
Short-term provisions (86) (112)
Total current liabilities (5,026) (4,238)
Non-current liabilities
Long-term borrowings (9,363) (87)
Deferred tax liabilities (3,472) (3,357)
Pensions and other post-employment benefits (256) (253)
Derivative financial instruments (21) (1)
Other provisions (30) (27)
Other non-current liabilities (6) (8)
Total non-current liabilities (13,148) (3,733)
Total liabilities (18,174) (7,971)
Net assets 26,849 26,480
Equity
Share capital 11
Other reserves (11,502) (11,632)
Retained earnings 38,211 37,986
Shareholders’ equity 26,710 26,355
Non-controlling interests 139 125
Total equity 26,849 26,480
134

5. Non-IFRS financial measures
5.1 Adjusted Results
The following tables set out a reconciliation between IFRS and Adjusted Results.
Q1 2022 Adjusted Results
£m
IFRS
Result
Net Amortisation
and Impairment
of Intangible
Assets
Restructuring
Costs
Transaction
Related Costs
Separation and
Admission
Costs
Disposal
and others
Adjusted
Results
Revenue 2,627 - - - - - 2,627
Cost of Sales (1,014) 28 1 - - - (985)
Selling, general
and admin (1,086) - 14 - 127 - (945)
Research and
development (64) - (2) - - - (66)
Other operating
income 3 - - - - (3) -
Operating Profit 466 28 13 - 127 (3) 631
Operating Profit
Margin % 17.7% 24.0%
Finance income 7 - - - - - 7
Finance expense (8) - - - - - (8)
Profit before tax 465 28 13 - 127 (3) 630
Income tax (108) 1 (3) - (24) (5) (139)
Tax rate % 23.2% 22.1%
Profit after tax for
the quarter 357 29 10 - 103 (8) 491
135

Q1 2021 Adjusted Results
£m
IFRS
Result
Net Amortisation
and Impairment
of Intangible
Assets
Restructuring
Costs
Transaction
Related Costs
Separation and
Admission
Costs
Disposal
and others
Adjusted
Results
Revenue 2,306 - - - - - 2,306
Cost of Sales (904) 11 14 - - - (879)
Selling, general
and admin (1,009) - 32 - 34 50 (893)
Research and
development (54) - 2 - - - (52)
Other operating
income 9 - - - - (9) -
Operating Profit 348 11 48 - 34 41 482
Operating Profit
Margin % 15.1% 20.9%
Finance income 6 - - - - - 6
Finance expense (4) - - - - - (4)
Profit before tax 350 11 48 - 34 41 484
Income tax (101) (2) (10) (7) - (120)
Tax rate % 29% 25%
Profit after tax for
the quarter 249 9 38 - 27 41 364
136
2021 Adjusted Results
£m
IFRS
Result
Net Amortisation
and Impairment
of Intangible
Asset
Restructuring
Costs
Transaction
Related Costs
Separation and
Admission
Costs
Disposals
and others
Adjusted
Results
Revenue 9,545 - - - - - 9,545
Cost of Sales (3,595) 8 44 - - - (3,543)
Gross Profit 5,950 8 44 - - - 6,002
Gross Profit
Margin % 62.3% 62.9%
Selling, general
and admin (4,086) - 150 - 278 76 (3,582)
Research and
development (257) 8 1 - - - (248)
Other operating
income 31 - - - - (31) -
Operating
Profit 1,638 16 195 - 278 45 2,172
Operating Profit
Margin % 17.2% 22.8%
Finance income 17 - - - - - 17
Finance
expense (19) - - - - - (19)
Profit before
tax 1,636 16 195 - 278 45 2,170
Income tax (197) 8 (36) - (47) (197) (469)
Tax rate % 12.0% 21.6%
Profit after tax 1,439 24 159 - 231 (152) 1,701
Profit for the
year attributable
to:
Shareholders of
the Group 1,390 24 159 - 231 (152) 1,652
Non-controlling
interests 49 - - - - - 49
Basic earnings
per Share 139,000p 2,400p 15,900p 0p 23,100p (15,200)p 165,200p
Weighted
average number
of shares
(Basic) 1,000,000 1,000,000
Diluted
earnings per
Share 139,000p 2,400p 15,900p 0p 23,100p (15,200)p 165,200p
Weighted
average number
of shares
(Diluted) 1,000,000 1,000,000
137

2020 Adjusted Results
£m
IFRS
Result
Net Amortisation
and Impairment
of Intangible
Asset
Restructuring
Costs
Transaction
Related Costs
Separation and
Admission
Costs
Disposals
and others
Adjusted
Results
Revenue 9,892 - - - - - 9,892
Cost of Sales (3,982) 81 89 91 - 2 (3,719)
Gross Profit 5,910 81 89 91 - 2 6,173
Gross Profit
Margin % 59.7% 62.4%
Selling, general
and admin (4,220) - 314 - 66 21 (3,819)
Research and
development (304) 16 8 - - - (280)
Other operating
income 212 - - - - (212) -
Operating
Profit 1,598 97 411 91 66 (189) 2,074
Operating Profit
Margin % 16.2% 21.0%
Finance income 20 - - - - - 20
Finance
expense (27) - - - - - (27)
Profit before
tax 1,591 97 411 91 66 (189) 2,067
Income tax (410) (19) (90) (20) (13) 69 (483)
Tax rate % 25.8% 23.4%
Profit after tax 1,181 78 321 71 53 (120) 1,584
Profit for the
year attributable
to:
Shareholders of
the Group 1,145 78 319 71 53 (120) 1,546
Non-controlling
interests 36 - 2 - - - 38
Basic earnings
per Share 114,500p 7,800p 31,900p 7,100p 5,300p (12,000)p 154,600p
Weighted
average number
of shares (Basic) 1,000,000 1,000,000
Diluted
earnings per
Share 114,500p 7,800p 31,900p 7,100p 5,300p (12,000)p 154,600p
Weighted
average number
of shares
(Diluted) 1,000,000 1,000,000
138
2019 Adjusted Results
£m
IFRS
Result
Net Amortisation
and Impairment
of Intangible
Assets
Restructuring
Costs
Transaction
Related Costs
Separation and
Admission
Costs
Disposals
and others
Adjusted
Results
Revenue 8,480 - - - - - 8,480
Cost of Sales (3,678) 36 69 366 - - (3,207)
Gross Profit 4,802 36 69 366 - - 5,273
Gross Profit
Margin % 56.6% 62.2%
Selling, general
and admin (3,596) - 236 - - 8 (3,352)
Research and
development (292) - 25 - - - (267)
Other operating
expense (17) - - - - 17 -
Operating
Profit 897 36 330 366 - 25 1,654
Operating Profit
Margin % 10.6% 19.5%
Finance income 24 - - - - - 24
Finance
expense (35) - - - - - (35)
Profit before
tax 886 36 330 366 - 25 1,643
Income tax (199) (5) (59) (81) - (21) (365)
Tax rate % 22.5% 22.2%
Profit after tax 687 31 271 285 - 4 1,278
Profit for the
year attributable
to:
Shareholders of
the Group 655 31 271 285 - 4 1,246
Non-controlling
interests 32 - - - - - 32
Basic earnings
per Share 65,500p 3,100p 27,100p 28,500p 0p 400p 124,600p
Weighted
average number
of shares (Basic) 1,000,000 1,000,000
Diluted
earnings per
Share 65,500p 3,100p 27,100p 28,500p 0p 400p 124,600p
Weighted
average number
of shares
(Diluted) 1,000,000 1,000,000
139

5.2 Adjusting Items
Adjusted Results exclude the following items (net of the impact of taxes, where applicable):
Net amortisation and impairment of intangible assets
Impairment of intangibles and goodwill and amortisation of intangibles excluding computer
software. Intangible amortisation and impairments arising from intangibles acquired in business
combinations are adjusted to reflect the performance of the business excluding the effect of
acquisition accounting.
It is the Group’s view that acquired intangible assets by their nature are fundamentally different
from other depreciating assets that are replaced on a predictable cycle. The Group excludes the
impact of non-cash amortisation associated with acquired intangible assets as this is not directly
attributable to the sale of the Group’s products and varies from period to period, which affects
comparability of the Group’s financial results. The costs to operate, maintain and extend the life of
acquired intangible assets and purchased intellectual property are reflected in the Group’s
operating costs as labour, overheads, etc.
Restructuring costs
Include personnel costs associated with restructuring programmes, impairments of tangible assets
and computer software relating to specific programmes approved by the Board from time to time
that are structural and of a significant scale, where the costs of individual or related projects
exceed £15 million. Restructuring costs also include integration costs following an acquisition,
including in relation to personnel, manufacturing sites, real estate and IT infrastructure. These
programmes can take several years to complete and are not directly attributable to the sale of the
Group’s products. Further, costs associated with these programmes vary from period to period,
which affects comparability of the Group’s financial results.
Restructuring costs do not include Separation and Admission costs (see “Separation and
Admission costs” below).
Transaction-related costs
Transaction-related accounting or other adjustments related to significant acquisitions. These
costs are adjusted as they arise as a result of business combinations. In FY 2019 and FY 2020,
these costs were related to the unwind of inventory fair value adjustments in connection with the
Pfizer Transaction, which was completed by the end of FY 2020. These costs are not directly
attributable to the sale of the Group’s products and vary from period to period, which affects
comparability of the Group’s financial results.
Separation and Admission costs
Costs incurred in relation to and in connection with the Demerger, Separation, Admission, and
registration of Haleon Shares represented by Haleon ADSs and of Haleon ADSs under the US
Exchange Act and listing of Haleon ADSs on the NYSE. These costs are not directly attributable to
the sale of the Group’s products and specifically relate to the foregoing activities, affecting
comparability of the Group’s financial results in historic and future reporting periods.
Disposals and others
Gains and losses on disposals of assets, businesses and tax indemnities related to business
combinations, and other items. These gains and losses are not directly attributable to the sale of
the Group’s products and vary from period to period, which affects comparability of the Group’s
financial results.
140

5.3 Organic revenue growth
The following tables reconcile reported revenue growth for the three months ended 31 March 2022
and 31 March 2021 to organic revenue growth for the same period by geographical segment and
by product category.
Geographic Segments
£m APAC EMEA/LatAm N America Total
Q1 2022 vs Q1 2021 (%)
Revenue Growth 15.8% 8.0% 20.1% 13.9%
Organic Adjustments
1
0.2% 2.0% 0.8% 1.2%
of which:
Effect of Acquisitions - - - -
Effect of Divestments - 1.1% 0.5% 0.6%
Effect of MSAs 0.2% 0.9% 0.3% 0.6%
Effect of Exchange Rates (0.8)% 4.5% (3.6)% 0.5%
Organic Revenue Growth 15.2% 14.5% 17.3% 15.6%
Product Categories
£m
Oral
Health VMS
Pain
Relief
Respiratory
Health
Digestive
Health and
Other Total
Q1 2022 vs Q1 2021 (%)
Revenue Growth 5.7% 16.4% 18.0% 51.0% 0.6% 13.9%
Organic Adjustments
1
- 0.1% 0.3% - 4.8% 1.2%
of which:
Effect of Acquisitions - - - - - -
Effect of Divestments - - 0.3% - 2.5% 0.6%
Effect of MSAs - 0.1% - - 2.3% 0.6%
Effect of Exchange Rates 2.2% (1.6)% 0.6% 1.9% (1.1)% 0.5%
Organic Revenue Growth 7.9% 14.9% 18.9% 52.9% 4.3% 15.6%
Note
1. As defined in paragraph 6.4 of Presentation of Financial and Other Information.
141

The following tables reconcile reported revenue growth for the years ended 31 December 2021,
31 December 2020 and 31 December 2019 to organic revenue growth for the same period by
geographical segment and by product category.
Geographic Segments
£m APAC EMEA/LatAm N America Total
2021 vs 2020 (%)
Revenue Growth 4.3% (4.5%) (6.7%) (3.5%)
Organic Adjustments
1
2.0% 3.4% 2.4% 2.7%
of which:
Effect of Acquisitions - - - -
Effect of Divestments 2.2% 3.1% 2.5% 2.7%
Effect of MSAs (0.2%) 0.3% (0.1%) -
Effect of Exchange Rates 2.8% 4.6% 5.6% 4.6%
Organic Revenue Growth 9.1% 3.5% 1.3% 3.8%
2020 vs 2019 (%)
Revenue Growth 20.7% 4.1% 31.2% 16.7%
Organic Adjustments
1
(15.9%) (5.0%) (32.1%) (16.6%)
of which:
Effect of Acquisitions (19.9%) (8.8%) (33.9%) (19.7%)
Effect of Divestments 4.0% 4.5% 1.2% 3.2%
Effect of MSAs - (0.7%) 0.6% (0.1%)
Effect of Exchange Rates 0.9% 4.0% 1.6% 2.7%
Organic Revenue Growth 5.7% 3.1% 0.7% 2.8%
Note
1. As defined in paragraph 6.4 of Presentation of Financial and Other Information.
142

Product Categories
£m
Oral
Health VMS
Pain
Relief
Respiratory
Health
Digestive
Health
and
Other Total
2021 vs 2020 (%)
Revenue Growth (0.8%) 0.5% 2.1% (12.8%) (9.8%) (3.5%)
Organic Adjustments
1
- 0.3% 0.3% 6.4% 7.6% 2.7%
of which:
Effect of Acquisitions - - - - - -
Effect of Divestments - 0.3% 0.3% 6.4% 7.5% 2.7%
Effect of MSAs - - - - 0.1% -
Effect of Exchange Rates 5.2% 3.4% 4.1% 4.6% 5.3% 4.6%
Organic Revenue Growth 4.4% 4.2% 6.5% (1.8%) 3.1% 3.8%
2020 vs 2019 (%)
Revenue Growth 3.3% 150.3% 25.8% (1.5%) (0.1%) 16.7%
Organic Adjustments
1
- (133.5%) (23.5%) (6.7%) (5.4%) (16.6%)
of which:
Effect of Acquisitions - (133.9%) (23.7%) (10.5%) (14.2%) (19.7%)
Effect of Divestments - 0.4% 0.2% 3.8% 9.4% 3.2%
Effect of MSAs - - - - (0.6%) (0.1%)
Effect of Exchange Rates 2.6% 2.5% 2.6% 1.9% 3.0% 2.7%
Organic Revenue Growth 5.9% 19.3% 4.9% (6.3%) (2.5%) 2.8%
Notes
1. As defined in paragraph 6.4 of Presentation of Financial and Other Information.
2. Organic revenue growth for the period FY 2019 to FY 2020 excludes revenue attributable to
brands acquired as part of the Pfizer Transaction for the period 1 January 2020 to 31 July 2020
and includes revenue attributable to these brands for the period 1 August 2020 to 31 December
2020. Sales patterns during these two periods were materially impacted by the COVID-19
pandemic with increased sales during the former period driven by accelerated purchases by
consumers combined with increased consumption and sales during the latter period negatively
impacted by a reduction in consumer inventories and weak cold and flu incidence (see
paragraphs 2.1, 2.14 and 6.1 of Part VII (Operating and Financial Review)).
143
5.4 Adjusted EBITDA
The reconciliation between profit after tax and Adjusted EBITDA for the years ended
31 December 2021, 31 December 2020 and 31 December 2019 is provided below.
£m 2021 2020 2019
Profit after tax 1,439 1,181 687
Add Back: Income Tax 197 410 199
Less: Finance Income (17) (20) (24)
Add Back: Finance Expense 19 27 35
Operating Profit 1,638 1,598 897
Net Amortisation and Impairment of Intangible Assets 16 97 36
Restructuring Costs 195 411 330
Transaction Related Costs - 91 366
Separation and Admission Costs 278 66 -
Disposals and Others 45 (189) 25
Adjusted Operating Profit 2,172 2,074 1,654
Add Back: Depreciation of property, plant and equipment 139 167 167
Add Back: Depreciation of right-of-use assets 35 48 31
Add Back: Amortisation – of software intangible assets 54 40 35
Add Back: Impairment of property, plant and equipment rights of use
assets and computer software net of impairment reversals 13 22 (3)
Adjusted EBITDA 2,413 2,351 1,884
5.5 Free cash flow
The reconciliation of net cash inflow from operating activities to free cash flow for the years
ended 31 December 2021, 31 December 2020 and 31 December 2019 is provided below.
£m 2021 2020 2019
Net cash inflow from operating activities 1,356 1,407 786
Purchase of property, plant and equipment (228) (222) (190)
Proceeds from sale of property, plant, and equipment 12 6 51
Purchase of intangible assets (70) (96) (53)
Proceeds from sale of intangible assets 137 924 120
Distributions to non-controlling interests (35) (31) (28)
Interest paid (15) (19) (29)
Interest received 16 19 24
Free cash flow 1,173 1,988 681
5.6 Free cash flow conversion
The reconciliation of free cash flow conversion for the years ended 31 December 2021,
31 December 2020 and 31 December 2019 is provided below.
£m 2021 2020 2019
Free cash flow 1,173 1,988 681
Profit after tax 1,439 1,181 687
Free cash flow conversion 82% 168% 99%
144

5.7 Net debt
The reconciliation of net debt to the different balance sheet items for the years ended
31 December 2021, 31 December 2020 and 31 December 2019 is provided below.
£m 2021 2020 2019
Short-term borrowings (79) (82) (64)
Long-term borrowings (87) (105) (121)
Derivative financial liabilities (19) (25) (2)
Cash and cash equivalents and liquid investments 414 334 340
Derivative financial assets 17 6 12
Net debt
1
246 128 165
Note
1. The sum of the Group’s cash and cash equivalents and liquid investments and derivative
financial assets were greater than the sum of its short-term borrowings, long-term borrowings
and derivative financial liabilities in the period 2019-2021 (a net cash position). ‘Net debt’ is
defined differently to ‘indebtedness’ referenced in Part VII – Operating and Financial Review.
6. Other selected financial and operating information
6.1 Regional performance
The tables below set out the Group’s regional revenue and operating profit for the years ended
31 December 2021, 31 December 2020 and 31 December 2019.
1
Revenue (£m) Revenue change FY20-FY21 % Revenue change FY19-FY20 %
2021 2020 2019
Reported
rates
Constant
currency Organic
Reported
rates
Constant
currency Organic
2
North America 3,525 3,779 2,880 (6.7) (1.3) 1.3 31.2 32.6 0.7
EMEA and LatAm 3,877 4,059 3,898 (4.5) - 3.5 4.1 8.4 3.1
APAC 2,143 2,054 1,702 4.3 7.1 9.1 20.7 21.8 5.7
Total 9,545 9,892 8,480 (3.5%) 1.0% 3.8% 16.7% 19.3% 2.8%
Adjusted operating profit (£m)
1
Adjusted operating profit margin %
2021 2020 2019 2021 2020 2019
North America 828 897 660 23.5% 23.7% 22.9%
EMEA and LatAm 960 857 746 24.8% 21.1% 19.1%
APAC 461 377 311 21.5% 18.4% 18.3%
Central and unallocated (77) (57) (63) n/a n/a n/a
Total 2,172 2,074 1,654 22.8% 21.0% 19.5%
Reconciling items
3
(534) (476) (757) n/a n/a n/a
Group operating profit 1,638 1,598 897 17.2% 16.2% 10.6%
Notes
1. On a segment basis, Adjusted operating profit is the measure of segment profit or loss
reviewed by the Company’s chief operating decision maker. Adjusting Items are not allocated
by segment, as these items are managed and funded centrally by the Group, and therefore are
not part of the measure of segment profit or loss reviewed by the Company’s chief operating
decision maker. See Note 6 of Schedule II (Historical Financial Information of GlaxoSmithKline
Consumer Healthcare Holdings (No.2) Limited).
2. Organic revenue growth for the period FY 2019 to FY 2020 excludes revenue attributable to
brands acquired as part of the Pfizer Transaction for the period 1 January 2020 to 31 July 2020
and includes revenue attributable to these brands for the period 1 August 2020 to
31 December 2020. Sales patterns during these two periods were materially impacted by the
COVID-19 pandemic with increased sales during the former period driven by accelerated
145

purchases by consumers combined with increased consumption and sales during the latter
period negatively impacted by a reduction in consumer inventories and weak cold and flu
incidence (see paragraphs 2.1, 2.14 and 6.1 of Part VII (Operating and Financial Review)).
3. Reconciling items for these purposes are the Adjusting Items, which are defined at paragraph
5.2 of this Part VI (Selected Financial Information) above. A reconciliation between IFRS and
Adjusted Results is included at paragraph 5.1 of this Part VI (Selected Financial Information)
above.
6.2 Revenue by product category
The table below sets out the Group’s revenue by product category for the years ended
31 December 2021, 31 December 2020 and 31 December 2019.
Revenue (£m) Revenue change FY20-FY21 % Revenue change FY19-FY20 %
2021 2020 2019
Reported
rates
Constant
currency Organic
Reported
rates
Constant
currency Organic
Oral Health 2,724 2,745 2,657 (0.8) 4.4 4.4 3.3 5.9 5.9
VMS 1,501 1,494 597 0.5 3.9 4.2 150.3 154.6 19.3
Pain Relief 2,237 2,192 1,742 2.1 6.2 6.5 25.8 28.6 4.9
Respiratory Health 1,132 1,298 1,318 (12.8) (8.6) (1.8) (1.5) 0.5 (6.3)
Digestive Health and Other 1,951 2,163 2,166 (9.8) (5.0) 3.1 (0.1) 2.5 (2.5)
Total 9,545 9,892 8,480 (3.5%) 1.0% 3.8% 16.7% 19.3% 2.8%
146

PART VII
OPERATING AND FINANCIAL REVIEW
This Part VII (Operating and Financial Review) should be read in conjunction with the section entitled
“Presentation of Financial and Other Information”, as well as Part II (Market Overview), Part III
(Business Overview) and Schedule II (Historical Financial Information of GlaxoSmithKline Consumer
Healthcare Holdings (No.2) Limited), including accompanying notes. Unless otherwise indicated, the
financial information contained in this Part VII (Operating and Financial Review) is extracted from the
financial information set out in the Historical Financial Information.
The following discussion of the Group’s results of operations and financial condition contains certain
forward-looking statements. The Group’s actual results could differ materially from those discussed in
these forward-looking statements. Factors that could cause or contribute to such differences include
those discussed elsewhere in this Prospectus, particularly in the section entitled “Risk Factors”. The
Group does not undertake any obligation to revise or publicly release the results of any revision to
these forward-looking statements.
1. Overview
The Group is a world-leading consumer healthcare business, with a portfolio of category
leading brands and approximately 23,000 people worldwide engaged in the research and
development, manufacture and sale of a broad range of consumer healthcare products.
The Group conducts business internationally across five consumer healthcare categories: Oral
Health, Pain Relief, VMS, Respiratory Health and Digestive Health and Other.
The Group’s international presence is organised into three geographic regions, which are the
Group’s reporting segments: North America, EMEA and LatAm and APAC. Each geographic
region consists of a number of countries, clusters of countries and markets.
North America
The North America region consists of the USA, Canada and Puerto Rico. As at 31 March 2022,
approximately 4,600 personnel
81
were engaged in the Group’s operations in North America.
North America represented 36.9 per cent. of the Group’s revenue for FY 2021. Revenue
attributable to North America fell by 6.7 per cent. at AER and 1.3 per cent. at CER from FY
2020 to FY 2021. Organic revenue growth in North America was 1.3 per cent. for this period,
as compared to 0.7 per cent. for the period FY 2019 to FY 2020.
EMEA and LatAm
The diverse EMEA and LatAm region is divided into seven business units: Northern Europe,
Southern Europe, Central and Eastern Europe (including the Commonwealth of Independent
States), Russia, DACH (Germany, Austria and Switzerland), Middle East and Africa and LatAm
(Brazil, Colombia and Wider LatAm). As at 31 March 2022, approximately 12,300 personnel
82
were engaged in the Group’s operations in EMEA and LatAm.
EMEA and LatAm accounted for 40.6 per cent. of the Group’s revenue for FY 2021. Revenue
attributable to EMEA and LatAm fell by 4.5 per cent. at AER and remained flat at CER from FY
2020 to FY 2021. Organic revenue growth in EMEA and LatAm was 3.5 per cent. for this
period, as compared to 3.1 per cent. for the period FY 2019 to FY 2020.
81
Full-time equivalent employees and agency staff (rounded to the nearest 100).
82
Full-time equivalent employees and agency staff (rounded to the nearest 100).
147

APAC
The APAC region covers the Asia Pacific markets and is divided into five business units:
Greater China, Australia and New Zealand, Indian Sub-Continent, North Asia (Japan and
South Korea) and South East Asia and Taiwan. As at 31 March 2022, approximately 5,900
personnel
83
were engaged in the Group’s operations in APAC.
APAC contributed 22.5 per cent. of the Group’s revenue for FY 2021. Revenue attributable to
APAC grew by 4.3 per cent. at AER and 7.1 per cent. at CER from FY 2020 to FY 2021.
Organic revenue growth in APAC was 9.1 per cent. for this period, as compared to 5.7 per
cent. for the period FY 2019 to FY 2020.
2. Key factors affecting the Group’s results of operations and financial position
The Group’s results of operations and financial condition are affected by a variety of factors, a
number of which are outside the control of the Group. Set out below is a discussion of the most
significant factors that have affected the Group’s financial results during the periods under
review and which the Directors currently expect to affect its financial results in the future.
Factors other than those presented below could also have a significant impact on the Group’s
results of operations and financial condition in the future.
2.1 Impact of macroeconomic factors and market trends on discretionary consumer
spending
The Group’s business is impacted by fluctuations in demand for the Group’s products as a
result of changes in discretionary consumer spending.
Demand for the Group’s products is generally impacted by macroeconomic conditions which
affect disposable income of consumers and discretionary consumer spending. The prevailing
global economic climate, inflation, levels of employment, disposable income, salaries, wage
rates, interest rates, geopolitical and political uncertainty, fiscal policy (particularly on public
healthcare), taxation, consumer confidence, consumer perception of economic conditions and
global pandemics, are all factors that affect the Group’s business. For example, in FY 2020,
the COVID-19 pandemic had a significant impact on the Group’s operations (see paragraph
2.14 of this Part VII (Operating and Financial Review) below). In addition, Russia’s invasion of
Ukraine has had an adverse effect on the global geopolitical and economic environment,
although the broader economic consequences of the invasion are currently difficult to predict
(see paragraph 3 of this Part VII (Operating and Financial Review) below).
Demand is also influenced by evolving consumer tastes and trends in discretionary consumer
spending on consumer healthcare products. Trends evidenced during the periods under review
include an increasing focus on self-management of health and wellbeing, an ageing population,
an increasing demand for natural products (consumer healthcare products that are
non-medicated, ‘free from’ particular ingredients, or that include plant-based, herbal or other
naturally occurring ingredients), premiumisation (where consumers switch their purchases to
premium alternatives) and the countervailing trend toward “private-label” products (brands sold
exclusively by a particular retailer, which are typically sold at lower prices than branded
products), sustainability, and the convergence of digital and healthcare.
The Group benefits from the increasing consumer focus on health and wellbeing, with the most
pronounced impact in the Pain Relief and VMS categories. Further, increased demand for
83
Full-time equivalent employees and agency staff (rounded to the nearest 100).
148
preventative and therapeutic products associated with an ageing population has a positive
impact on the Group’s Pain Relief and Oral Health categories. In the periods under review, the
Group’s results were impacted by premiumisation trends. For example, in FY 2020, this trend
adversely impacted sales of Aquafresh, the Group’s everyday toothpaste brand, whereas
Sensodyne, as a premium brand, benefited from the trend. Revenue in FY 2020 and FY 2021
was also affected by a number of “private-label” product launches; for example, such launches
resulted in reduced demand in the USA for Abreva in FY 2020 and Voltaren in FY 2021.
The success of the Group depends on its ability to navigate and respond to changes in
discretionary consumer spending. In turn, this ability is contingent on a number of factors,
including competitive and market dynamics, brand strength, product offering, innovation and
pricing (see paragraphs 2.2, 2.3, and 2.4 of this Part VII (Operating and Financial Review)
below and paragraph 1.2 of Risk Factors).
2.2 Competition in the consumer healthcare market
The Group’s operating and financial performance is affected by competition in the geographic
regions and product categories in which it operates. The Group faces competition from
companies that produce and sell competing products in an industry that is experiencing
consolidation. Consumer preference for branded, generic or private label products sold by
competitors adversely impacts the Group’s financial performance.
The Group’s competitors, which differ within individual geographic markets, include large-scale
retailers, smaller high-growth companies (which often operate on a regional basis and offer
aggressive competition), multinational corporations moving into or expanding their presence in
the consumer healthcare market, and “private-label” products sold by retailers (principally in
the US market) (see paragraph 1.4 of Risk Factors). While the convergence of digital
commerce and health aids the prospects for consumer healthcare market size growth, the
Group faces considerable competition from e-commerce retailers and large-scale retailers with
expansive digital operations.
Competitive factors impacting the Group’s business include market dynamics and evolving
consumer preferences, brand image, a diversified product portfolio, new product innovations
and product development, pricing that is attractive to consumers, and cost inputs (see
paragraphs 2.1, 2.3, 2.4 and 2.10 of this Part VII (Operating and Financial Review)). Other
competitive factors include supply chain (procurement, manufacturing and distribution) (see
paragraph 2.7 of this Part VII (Operating and Financial Review) below), the management of
sales and marketing activities, and access to cash for investment in the Group’s business (see
paragraph 2.11 of this Part VII (Operating and Financial Review) below).
2.3 Brand and product portfolio
The Group’s success is driven, to a large extent, by the strength of its brands and its ability to
leverage its diversified portfolio to drive increased sales, profits and cash generation. The
Group invests in R&D and marketing activities in order to maintain the long-term health of its
brands and products and grow value, with investment allocation varying year-to-year across
the portfolio.
The Group’s product portfolio is split among five categories: Oral Health, Pain Relief, VMS,
Respiratory Health and Digestive Health and Other. The Group’s largest category by revenue
is Oral Health, which accounted for 28.5 per cent. of the Group’s revenue in FY 2021. The Pain
149
Relief and Digestive Health and Other categories also significantly contribute to revenue,
respectively contributing 23.4 per cent. and 20.4 per cent. of revenue in FY 2021. VMS and
Respiratory Health respectively accounted for 15.7 per cent. and 11.9 per cent. of revenue in
FY 2021. Operating profit margin also varies across the Group’s portfolio.
The Group’s sales volumes and profits depends in part on the Group’s ability to identify and
offer products at prices that appeal to changing consumer needs and preferences. Through net
revenue management, the Group analyses pricing, discounting and promotional initiatives
across the regions in which it operates in order to optimise sales volumes and revenue and
support revenue growth and margin expansion. Pricing and discounting of the Group’s
products is influenced by a number of factors, including competitive and market dynamics
(including competitive pricing behaviours), brand recognition and brand loyalty, innovation and
marketing activities, supply chain (procurement, manufacturing and distribution), regulation and
other cost inputs (see paragraphs 2.1, 2.2, 2.3, 2.4, 2.7, 2.10 and 2.16 of this Part VII
(Operating and Financial Review)). Price increases contribute to revenue growth and cash
generation and support operating profit margin. Conversely, discounting dilutes operating profit
margin and impacts revenue growth and cash generation. During the periods under review, a
number of price increases and discounting initiatives affected the Group’s financial
performance.
The Group’s Power Brands benefit from strong brand recognition and loyalty among
consumers. While maintaining this recognition and loyalty requires greater investment in
advertising and promotion relative to the rest of the portfolio, the Power Brands have higher
gross margins. The Power Brands also typically account for the majority of the Group’s
revenue and have the highest growth. Accordingly, the Power Brands contribute significantly to
revenue growth. The Group’s local strategic brands also benefit from strong brand recognition
and loyalty among consumers. As a result, the profitability of local strategic brands is typically
comparable to the profitability of the Power Brands, and a number of the local strategic brands
in particular contribute significantly to revenue across the regions (for example, Tums,
Emergen-C, Flonase and Excedrin in the US, Caltrate and Fenbid in China and Dr. Best in
Germany). Investment in and growth rates of local strategic brands vary. Other brands in the
Group’s portfolio typically have lower levels of investment and have lower gross margins, but
these brands support overall profitability and consolidate the Group’s competitive position in
the geographic regions and categories in which it operates. The Group’s results may fluctuate
from year to year depending on the proportion of sales volume represented by higher-margin
brands.
The Group relies on its ability to leverage brand recognition and consumer loyalty in
responding to changing consumer trends and unmet consumer needs. Further, the success of
new product launches depends in part on the strength of the Group’s existing brands. In the
periods under review, a number of products were launched under the Group’s Power Brands
and local strategic brands (see paragraph 4 of Part III (Business Overview)). These launches
contributed significantly to growth in FY 2020 and FY 2021.
Competitive pressures from companies that produce and sell competing branded, generic and
“private-label” products affect consumer preference for the Group’s brands and products, which
in turn impacts the Group’s operating and financial performance (see paragraphs 2.1 and 2.2
of this Part VII (Operating and Financial Review) above).
The Group’s performance is also influenced by consumer perception of its brands and
products. In FY 2020, negative media coverage impacted consumer perception of the efficacy
150
of ibuprofen products in treating the symptoms of COVID-19, which adversely impacted sales
of Advil (an ibuprofen-based product) in FY 2020. The impact of this on the Group’s overall
performance during this period was more than mitigated by growth in Panadol (a paracetamol-
based product), illustrating that the Group’s diversified and balanced portfolio of products can
provide a certain level of protection against negative consumer trends and adverse
macroeconomic factors. However, the Group’s reliance on the strength of its brands to drive
revenue, operating profit margin and cash generation makes it vulnerable to reputational
damage and changes in consumer perception.
The Group actively manages its portfolio. In the periods under review, the brands acquired as
part of the Pfizer Transaction made an important contribution to the Group’s geographic
presence, revenue and operating profit margin (see paragraph 2.12 of this Part VII (Operating
and Financial Review) below). Alongside the acquisition of the Pfizer brands, the Group
continued to optimise and rationalise its portfolio by divesting a number of lower-growth brands
with limited synergies with the rest of the portfolio (see paragraph 2.13 of this Part VII
(Operating and Financial Review) below). The Group will continue to assess further brand
acquisitions and disposals in the context of its strategy and capital allocation priorities (see
paragraph 2 of Part III (Business Overview)).
2.4 Innovation
The development and introduction of new products to the Group’s portfolio contributes to
revenue growth and cash generation and supports operating profit margins. Product
development also supports portfolio diversification, which helps to minimise the effect of
negative consumer trends and adverse market cycles (see paragraph 2.3 of this Part VII
(Operating and Financial Review) above). The Group typically incurs incremental R&D costs in
the period prior to the launch of a new product, with advertising and promotion costs generally
incurred in connection with the launch and in subsequent periods. Investment in advertising
and promotion impacts visibility of the product in the market, which in turn influences the
success of a new product. Revenue growth attributable to a newly launched product is typically
higher in the period immediately following launch, reflecting peak consumer interest, following
which sales of the product begin to stabilise and the impact of market factors (such as
competition from competitors) on revenue becomes more pronounced.
Throughout the periods under review, the Group continued to develop and diversify its product
portfolio. As a consequence, the Group benefited from a number of product launches,
particularly in relation to the Power Brands and local strategic brands. For example, in APAC,
Caltrate revenue growth in the periods under review was partly driven by new product
development and launches, such as the launch of a range of Caltrate calcium supplements
with gender specific positioning.
The switch of prescription products to OTC status (see paragraph 8 of Part III (Business
Overview)) forms part of the Group’s innovation strategy. The process for such switches is
highly regulated and requires significant organisational resource and expenditure over a
number of years. This includes expenditure to support clinical studies, label comprehension (in
order to ensure that consumers understand the product and any side effects) and consumer
studies.
Following approval by the regulator, the Group typically incurs advertising and promotion costs
in connection with the launch of an Rx-to-OTC switch product and in subsequent periods. Any
failure to develop and commercialise new products in a timely fashion may decrease revenue
and/or increase R&D costs (see paragraph 1.5 of Risk Factors). The Group may face
151
competition from similar “private-label” products following launch of an Rx-to-OTC switch
product, which may negatively impact revenue growth attributable to the product. Typically, the
launch of similar “private-label” products following an Rx-to-OTC switch results in a steep
short-term reduction in the revenue growth attributable to the product followed by a return to
growth albeit not at the same level as prior to the private label launch.
In FY 2020 the Group successfully completed the Rx-to-OTC switch of Voltaren in the USA,
which was a key driver for growth in North America that year. In FY 2021, Voltaren revenue
was adversely impacted by reduced demand in the USA resulting from increased competition
from “private-label” products and the stabilisation of revenue streams following launch. The
FDA approval in 2020 (and the subsequent launch in H2 2020) of Advil Dual Action as an OTC
product contributed to revenue growth in the second half of FY 2020. The Group increased
marketing investments in order to support these launches.
2.5 Expansion of e-commerce and digital capabilities
Growing the Group’s e-commerce sales has been and will continue to be a key focus for the
Group. Revenue from e-commerce has grown from approximately 4 per cent. of total revenue
in FY 2019 to around 8 per cent. of total revenue in FY 2021. The majority of the Group’s
e-commerce sales are generated on third party platforms (e.g. Amazon, Alibaba), as opposed
to platforms hosted by the Group.
During the periods under review, the Group also increased its investment in digital media and
digital capabilities across the business, including in relation to advertising and promotion, R&D
and supply chain. For example, in the context of advertising and promotion, digital media
advertising accounted for approximately half of the Group’s total media spend in FY 2021. The
Group also invested in a range of digital tools and training programmes in order to enhance its
digital capability and support growth of the business in an increasingly digital world.
2.6 Geographic market presence
The Group has a strong global footprint and is focused on growth across the markets in which
it already operates. The Group serves approximately 170 markets, with 36.9 per cent. of the
Group’s revenue in FY 2021 generated in North America, 40.6 per cent. in EMEA and LatAm,
and 22.5 per cent. in APAC. Adjusted operating profit margin across the geographic regions
varies. In FY 2021, Adjusted operating profit margin was 23.5 per cent., 24.8 per cent., and
21.5 per cent. in North America, EMEA and LatAm and APAC, respectively. This variation is
ultimately driven by a number of factors, including sales volume of higher margin brands,
supply chain (procurement, manufacturing and distribution) and competitive dynamics, together
with required investment in R&D and marketing activities and other cost inputs.
North America
The North America region accounted for 36.9 per cent. (£3,525 million) of Group revenue in FY
2021. The region includes the Group’s largest single market, the USA, which accounted for
89.0 per cent. (£3,138 million) of revenue in North America and 32.9 per cent. of Group
revenue in FY 2021.
During the periods under review, the majority of the Group’s revenue in North America was
attributable to the Oral Health, Pain Relief and Digestive Health and Other categories. The
strength of brands such as Sensodyne, Tums and Flonase was a key driver of growth during
152

these periods. The Pfizer Transaction provided the Group with meaningful incremental scale in
North America, and brands acquired as part of the Pfizer Transaction (including Centrum and
Emergen-C) have also contributed significantly to Group revenue since it completed.
EMEA and LatAm
The EMEA and LatAm region was the largest contributor to Group revenue in FY 2021,
accounting for 40.6 per cent. (£3,877 million) of Group revenue. The region is equally split
between emerging and developed markets
84
with emerging markets making up four of the
seven business units (Central and Eastern Europe, Russia, Middle East and Africa and
LatAm).
During the periods under review, the majority of the Group’s revenue in EMEA and LatAm was
attributable to the Oral Health and Pain Relief categories. The strength of brands such as
Sensodyne, parodontax, Panadol and Voltaren was a key driver of growth during these
periods.
APAC
The APAC region accounted for 22.5 per cent. (£2,143 million) of the Group’s revenue in FY
2021. China is the largest market in APAC, accounting for 37.4 per cent. (£801 million) of
revenue in APAC and 8.4 per cent. of Group revenue in FY 2021.
During the periods under review, the majority of the Group’s revenue in APAC was attributable
to the Oral Health, VMS and Pain Relief categories. The strength of brands such as
Sensodyne, Panadol and Voltaren, as well as brands acquired as part of the Pfizer
Transaction, such as Centrum and Caltrate, was a key driver of revenue during these periods.
2.7 Supply chain
The Group relies on global supply chains and manufacturing and distribution operations which
are complex and subject to increasing regulatory requirements. The Group is exposed to a
number of factors that affect the sourcing, manufacturing, supply and pricing of the Group’s
products on a short-term basis, including macroeconomic events. In particular, the Group has
experienced and may continue to experience delays in deliveries due to supply chain
interruptions caused by the COVID-19 pandemic. For example, recent lockdowns in China
have had an impact on delivery of the Group’s products and resources required for the
manufacture of the Group’s products to and from China. Further, the Group has been exposed
to increases in commodity and oil prices following Russia’s invasion of Ukraine (see also
paragraph 3 below). The Group has entered and may in the future enter into fixed price
contracts or hedging arrangements in order to address increases in commodity prices and their
effect on the Group’s ability to source materials for its products. However, if prices decrease,
the Group will be unable to realise the benefit of the decrease due to fixed price contracts in
place. The Group may also be limited in its ability to pass on these price increases in the prices
it charges for its products. Competitive factors relating to the distribution of the Group’s
products also influence the Group’s performance. See also paragraph 1.3 of Risk Factors.
The Group prioritises reliability of supply to ensure its customers and consumers receive the
Group’s products. The Group is exposed to factors that may affect its ability to provide this
reliable supply from time to time. These factors include interruptions in supply from the Group’s
84
Classification of developed and emerging markets sourced from The International Monetary Fund DataMapper 2022.
153
own facilities or from third party facilities. Examples include issues at certain of the Group’s
own facilities affecting Advil supply in FY 2019 and FY 2021, Excedrin supply in FY 2019 and
FY 2020 and Preparation H supply in FY 2021.
The Group also manages its supply chain with a focus on supply continuity. For example,
where feasible, the Group aims to manage packaging and raw material sourcing risks through
dual sourcing (where key inputs are sourced from more than one location in order to limit the
risk of supply chain disruption). As of 31 December 2021, 75-80 per cent. of the Group’s
materials supply by spend was sourced from more than one supplier and it expects this to
increase to 85-90 per cent. by the end of 2023. The Group also runs capacity planning
processes in order to proactively identify pressures on supply chain capacity and conducts
assessments in order to assist management in determining the appropriate levels of
investment in facilities and equipment.
The Group has manufacturing sites located around the world. However, where possible, the
Group seeks to bring the manufacturing of its products within the region of sale. As a result,
more than 80 per cent. of the Group’s products are sourced in the region in which they are
sold. Not only does this enable the Group to respond more efficiently to the needs of
consumers, but it also forms part of the Group’s natural hedging strategy (see paragraph 2.15
of this Part VII (Operating and Financial Review)).
2.8 Inflationary pressures and commodity prices
The Group is exposed to inflationary pressures and commodity prices, which generally affect
the Group through their impact on payroll and supply costs (including freight). For example,
inflationary pressures in FY 2021 increased the Group’s commodity, freight and payroll costs,
which had an adverse impact on the Group’s operating profit and operating profit margin. While
the Group may increase product prices in order to mitigate the impact of inflation, competitive
pressures may constrain the Group’s ability to fully recover any increased costs in this way,
and so the Group may remain subject to market risk with respect to inflationary pressures and
increases in commodity prices. Where possible, the Group aims to manage its exposure to this
risk primarily through forward buying of commodities.
2.9 Seasonality
Revenue and cash flow in Respiratory Health is typically driven by seasonal demand for certain
of the Group’s products, including its cough, cold and flu, allergy and decongestant products.
The impact of seasonality on revenue in the Respiratory Health category is largely the same
across the Group’s geographic regions. However, as Respiratory Health revenue accounts for
a larger percentage of total revenue in North America and EMEA and LatAm, the impact of
seasonality is greater in these regions than in APAC. For example, FY 2021 Respiratory Health
revenue accounted for 11.9 per cent. and 14.2 per cent. of total revenue in North America and
EMEA and LatAm, respectively, and 7.6 per cent. of total revenue in APAC.
Sales in countries in the Northern Hemisphere typically peak from September through to
March, driven mainly by consumers purchasing products to self-medicate against cold and flu
symptoms. Most of the seasonality impact is experienced in the Northern Hemisphere, where
the Group’s operations are concentrated. Because of the timing of these seasonal peaks, the
Group’s first and fourth quarter results tend to show significantly more revenue in the
Respiratory Health category compared to its second and third quarter results, although this
trend was less pronounced in FY 2021, when 53.2 per cent. of the Group’s sales in Respiratory
154
Health occurred in the first and fourth quarters. It follows that the Group’s results for the first
and fourth quarters of each year may not necessarily be indicative of the results that may be
expected for a full financial year.
The Group incurs significant additional expenses in advance of and during September through
to Q1 of the following financial year in anticipation of higher sales during that period, including
costs of additional inventory and marketing. Further, the Group’s cash flow is affected by this
seasonality, with the Group experiencing lower cash flow during the second and third quarters
of the financial year due to increases in seasonal inventory and lower sales during this period,
in preparation for increased sales during the fourth quarter of the financial year and the first
quarter of the following financial year.
Although the Group’s SG&A and R&D costs, including personnel costs and administrative
costs, are more evenly distributed during the financial year, these costs are exposed to some
variations. Cash flows in these areas also experience some variation. For example, employee
bonus payments are accrued throughout the year but settled in March.
The seasonality effect of cold and flu season was impacted by the COVID-19 pandemic. This
had a material adverse effect on revenue in the Respiratory Health category in the period
1 August 2020 to and including the first half of FY 2021, reflecting the historically weak cold
and flu season driven by government restrictions in response to the COVID-19 pandemic (see
paragraph 2.14 of this Part VII (Operating and Financial Review) below). However, revenue in
Respiratory Health recovered during the second half of FY 2021, with Respiratory Health
revenue returning to levels broadly consistent with the corresponding period in FY 2019
towards the end of the year.
During the period FY 2019 to FY 2020, the seasonality impact was more pronounced on an
organic revenue growth basis. This is because organic revenue for the period FY 2019 to FY
2020 excludes revenue attributable to brands acquired as part of the Pfizer Transaction (such
as Robitussin) in respect of the period 1 January 2020 to 31 July 2020, which significantly
contributed to Group revenue in the period. Organic revenue for the period FY 2019 to FY
2020 includes revenue attributable to brands acquired as part of the Pfizer Transaction in
respect of the period 1 August 2020 to 31 December 2020; however, this period was
significantly affected by the impact of the COVID-19 pandemic, including in relation to the
seasonality effect of cold and flu season (as described above).
The Group is also impacted by the allergy season, the severity of which varies from year to
year across the geographic regions in which the Group operates depending on a number of
factors, including the weather and the intensity, timing and length of pollen season. A stronger
allergy season tends to result in higher revenue attributable to the Group’s Topical
Decongestant and Allergy Care products. For example, a stronger allergy season in Q2 2021
relative to the prior year comparator was a key driver for revenue growth in Flonase.
2.10 Commercial execution and financial discipline
The Group’s cost inputs are influenced by a number of factors, including competitive and
market dynamics, commercial capabilities, innovation and marketing activities, supply chain
(including procurement, manufacturing and distribution), acquisitions and divestments, and
seasonality. As an innovation-led business, R&D activities account for a significant proportion
of the Group’s costs. Further, the continued strength of the Group’s brands is contingent on a
competitive level of expenditure on advertising and promotion.
155
The Group seeks to drive the disciplined and efficient use of resources in order to provide
funding for brand growth and innovation, while delivering sustainable margin expansion and
cash generation (see paragraph 2.11 of this Part VII (Operating and Financial Review) below).
Following the transactions with Novartis and Pfizer (see paragraphs 2.2 and 2.3 of Part I (Key
Highlights and Development of the Group )), the Group has continued to implement a number
of initiatives to drive sustainable manufacturing and supply chain efficiency improvements
including:
• Streamlining of the Group’s manufacturing and supply footprint from 41 manufacturing
sites (inherited by the Group through arrangements as part of the GSK Group and the
transactions with Novartis and Pfizer) to 24 in 2021.
• Reducing the number of contract manufacturing partners from more than 250 (inherited by
the Group through arrangements as part of the GSK Group and the transactions with
Novartis and Pfizer) to approximately 180 in 2022.
• Streamlining and refreshing of the Group’s distribution centre footprint from more than 200
in 2015 (inherited by the Group through arrangements as part of the GSK Group and the
transactions with Novartis and Pfizer) to approximately 90 in 2021.
• Implementing ongoing initiatives to drive value from third-party expenditure and offset
headwinds from inflation in input prices and commodities, including forward buying, value
engineering and new supplier introduction, as well as initiatives to ensure continuity of
supply from the Group’s third party partners through programmes such as the approval of
alternate suppliers for critical materials.
• Implementing ongoing initiatives to drive site productivity and/or security of supply,
including dual sourcing and localisation of Power Brands such as Voltaren, Sensodyne and
Centrum.
The Group has also sought to drive effective and efficient use of resources in the research,
development, advertising and promotion of its brands, as well as the administration of the
Group’s operations, through initiatives including:
• Cost synergies generated by the Pfizer Transaction (see paragraph 2.12 of this Part VII
(Operating and Financial Review) below).
• De-duplication of R&D functions, localisation of R&D roles and projects and rationalisation
of the Group’s R&D footprint from 9 sites in 2015 (inherited by the Group through
arrangements as part of the GSK Group and the transactions with Novartis and Pfizer) to 4
sites in 2021.
• Rationalisation of creative production and media agencies from greater than 200 in 2019 to
an expected total of 56 by the end of 2022.
• Optimisation of e-commerce strength through increased investment in digital media (see
paragraph 2.5 of this Part VII (Operating and Financial Review)), as well as strengthening
execution in other channels, such as retail and pharmacy. For example, in the pharmacy
channel, where engagement with pharmacy and healthcare professionals is a core tenet of
the Group’s strategy, the Group has expanded its digital capabilities in order to support
these relationships and expand reach (e.g. the proprietary Healthpartner portal and
webinars).
156
• Ongoing non-manufacturing procurement initiatives leveraging the Group’s global scale
and strategic supplier relationships, as well as targeted efforts to maximise the
effectiveness of its investment in media and the efficiency of its support functions.
During the periods under review, costs incurred in connection with the delivery of synergies
arising out of the transaction with Novartis and the Pfizer Transaction were reflected as
Restructuring costs and are treated as an Adjusting Item for the purposes of Adjusted
operating profit. Other costs incurred in connection with the initiatives referred to in this
paragraph were not excluded from Adjusted operating profit.
2.11 Cash generation
The Group took a number of steps in the periods under review to drive cash generation from its
operations, including:
• tight management of receivables, inventory and payables;
• disciplined capital expenditure, with strategic investment focused on supply chain and
digital capabilities. This was reflected in a small increase in capital expenditure as a
percentage of revenue to 3.1 per cent. in FY 2021 from 2.9 per cent. in FY 2019; and
• the receipt of proceeds from the divestment of a number of the Group’s non-core brands
(see paragraph 2.13 of this Part VII (Operating and Financial Review) below).
2.12 Pfizer Transaction
The Pfizer Transaction completed on 31 July 2019 (see paragraph 2.3 of Part I (Key Highlights
and Development of the Group)). The Group’s results of operations and financial position have
been affected in the periods under review, and will continue to be affected, as a consequence
of the Pfizer Transaction, including steps taken in connection with and following the
transaction. For example, in FY 2022 the Directors expect the Group to continue to benefit
from synergies arising in connection with the Pfizer Transaction.
The Group’s revenue in FY 2020 was £9,892 million. The Group’s revenue in FY 2019
(including the revenue of the Pfizer Contributed CH Business from 1 August 2019, when it was
consolidated) was £8,480 million. Revenue of the Pfizer Contributed CH Business for the
seven months to 31 July 2019 was £1,523 million. From FY 2019 to FY 2020, the Group’s
revenue increased by 16.7 per cent. (£1,412 million) at AER. The increase was primarily driven
by the inclusion of the full year of revenue of the Pfizer Contributed CH Business in FY 2020,
compared to five months of revenue in FY 2019, together with other factors (see paragraph
6.1(A) of this Part VII (Operating and Financial Review) below). The impact of the Pfizer
Transaction therefore limits the comparability of the financial information of the Group for FY
2019 and FY 2020.
In the periods under review, the Group has achieved cost synergies as a consequence of the
Pfizer Transaction. In particular, the Group has adopted a leaner structure to drive cost savings
across a number of areas of the Group’s business, including the supply chain network, logistics
and infrastructure, advertising and marketing, sales and distribution and functional support (see
paragraph 2.10 of this Part VII (Operating and Financial Review) above). On 19 December
2018, GSK announced that the Pfizer Transaction was expected to realise annual cost savings
157
of £0.5 billion by 2022 for expected total cash costs of £0.9 billion and non-cash charges of
£0.3 billion. The Group has realised substantial cost synergies and expects to exceed this
target to realise a total of £0.6 billion of annual cost savings. The total cash costs are expected
to be £0.7 billion and non-cash charges are expected to be £0.1 billion, plus additional capital
expenditure of £0.2 billion. Up to 25 per cent. of the cost savings are intended to be reinvested
in the business to support innovation and other growth opportunities.
Restructuring costs and Transaction-related costs incurred by the Group during the period FY
2019 to FY 2021 were largely as a consequence of the Pfizer Transaction. Restructuring costs
were primarily attributable to steps taken in connection with the integration of the Pfizer
Contributed CH Business. Restructuring costs and Transaction-related costs are treated as
Adjusting Items for the purposes of Adjusted operating profit. Integration and restructuring
related to the Pfizer Transaction was substantially completed by the end of FY 2021, and the
Directors expect the final charges to occur in FY 2022.
2.13 Divestments
As part of the Group’s strategy in the periods under review, the Group made a number of
divestments in order to bring greater focus to its portfolio of brands. Divestments have a
negative impact on revenue growth year-on-year. Operating profit margin is also affected as a
result of gains and/or losses arising from divestments, together with costs incurred in
connection with the divestments. These costs are treated as an Adjusting Item for the purposes
of Adjusted operating profit.
The Group’s programme of disposals realised £1.1 billion in proceeds net of costs and taxes
from FY 2019 to FY 2021. The disposal programme had a negative impact on the Group’s
operating profit margin as the Group no longer received the associated revenue following the
divestments, but due to the relatively low investment in these brands and the lack of dedicated
resources supporting them, the majority of the costs previously attributed to these brands
remained following their disposal.
The sale of ThermaCare and related manufacturing sites, which completed in Q1 2020, was a
key divestment in the context of the Group’s operations and was made in connection with
certain regulatory conditions imposed on the Group as a result of the Pfizer Transaction.
Following the sale, restrictions on the integration of the Pfizer Contributed CH Business were
lifted. Other disposals included brands in the Group’s Skin Health portfolio.
The Group continues to actively manage its portfolio (see paragraph 2.3 of this Part VII
(Operating and Financial Review) above).
2.14 Impact of COVID-19
The COVID-19 pandemic and the implementation of associated responsive measures by
governments in the jurisdictions in which the Group operates affected the Group’s performance
in FY 2020 and FY 2021.
Widespread consumer stockpiling in Q1 2020 resulted in an increase in revenue across all of
the Group’s categories in North America and EMEA. Stockpiling was followed by falls in
demand, driven by consumers using up stockpiled supplies, together with fewer consumer
visits to pharmacies and retail outlets during government-imposed lockdowns. This negatively
impacted revenue in Q2 to Q4 of FY 2020, with the largest impact in Q2. Lockdowns also
impacted the market for Denture Care products at certain points during FY 2020 and FY 2021,
given the reduced incidence of social occasions.
158
The performance of the Group’s Respiratory Health category in FY 2020 and the first half of
2021 was materially adversely affected by the COVID-19 pandemic. Revenue in Respiratory
Health is typically driven by seasonal demand for certain of the Group’s products (see
paragraph 2.9 of this Part VII (Operating and Financial Review) above). However, Government
measures imposed in response to COVID-19, such as the widespread use of face masks and
implementation of lockdowns, social distancing measures and improved hygiene practices,
lead to a significant reduction in the number of respiratory illnesses, such as the common cold
and flu, in the Group’s key geographic markets. The introduction of specific restrictions on the
sale of cough and cold medicines in China further depressed the Group’s revenue (see
paragraph 2.16 of this Part VII (Operating and Financial Review) below). Respiratory Health
revenue consequently decreased by 1.5 per cent. at AER and increased by 0.5 per cent. at
CER from FY 2019 to FY 2020. Whilst revenue grew at CER due to the inclusion of full year
revenue of the brands acquired as part of the Pfizer Transaction (including Robitussin), the
category experienced negative organic revenue growth of 6.3 per cent. in FY 2020. In FY
2021, while Respiratory Health revenue continued to be adversely impacted in the first half of
the year, revenue recovered in the second half, as restrictions began to ease and seasonal
virus levels began to return to more normalised levels of respiratory illnesses.
The COVID-19 pandemic caused a surge in demand for certain OTC and VMS products, as
consumers became concerned with bolstering their immune systems and treating the
symptoms of COVID-19 and side effects of COVID-19 vaccinations. In particular, in FY 2020
and FY 2021, an increase in demand for Panadol (a paracetamol-based product) led to growth
in the Group’s Pain Relief category. This growth in Panadol revenue may also have been
attributable to negative media coverage regarding the use of ibuprofen products in treating the
symptoms of COVID-19, which adversely impacted sales of Advil (an ibuprofen-based product)
during FY 2020. In the VMS category, the increasing trend towards self-management of health
and wellbeing, which was accelerated by the COVID-19 pandemic, contributed to increased
demand for Centrum and Emergen-C during FY 2020 and FY 2021.
Among its far-reaching impacts, COVID-19 has also accelerated the convergence of the digital
environment and health, including the rapid expansion of e-commerce for consumer healthcare
products. Sales of the Group’s products through the online channel grew from around 4 per
cent. of revenue in FY 2019 to around 8 per cent. of revenue in FY 2021. In the periods under
review, the Group continued investment in the Group’s e-commerce platforms, including in
relation to digital content for online retailer platforms, the direct-to-consumer channel in the
USA (e.g., personalised Chapstick), and tools to measure the performance of the Group’s
“digital shelf” (e.g., presence of imagery, quality of content and shelf availability).
Costs associated with the Group’s supply chain were also impacted by the COVID-19
pandemic, with increased costs in relation to freight and staffing. Increased staffing costs were
driven by increased demand for the Group’s products, the purchase of additional personal
protective equipment, and changes to staff shift patterns in order to accommodate social
distancing requirements. Further, the Group’s shipping costs increased as a result of the global
macroeconomic impact of the COVID-19 pandemic.
2.15 Foreign exchange
The Group operates internationally and holds assets, incurs liabilities, generates sales and
pays expenses in a variety of currencies other than Pounds Sterling, which is the currency in
which it reports its consolidated financial results. As a result, the Group’s results of operations
are affected by exchange rate fluctuations between Pounds Sterling and other currencies in
159
which it conducts and will continue to conduct transactions, including US Dollar, Euro, Swiss
Franc and Chinese Renminbi. For example, the impact of movements in foreign currencies
against Pounds Sterling had a negative effect on the Group’s results of operations in FY 2021,
with a £443 million unfavourable revenue impact driven primarily by the depreciation of a
number of currencies, including the US Dollar, certain currencies in Latin America, the
Japanese Yen, the Turkish Lira, the Euro and the Russian Ruble, in each case against Pounds
Sterling during that period. As discussed in paragraph 3 of this Part VII (Operating and
Financial Review) below, Russia’s invasion of Ukraine has had an adverse effect on the value
of the Russian Ruble, which may negatively impact the Group’s operations in Russia, as
revenue from products sold in Russia are incurred in Russian Rubles, while costs associated
with those products are denominated in other currencies, such as Euro or US Dollars.
In order to reduce foreign currency translation exposure, the Group seeks to denominate
borrowings in the currencies of its principal assets and cash flows. The Group manages foreign
currency transactional exposure by selectively hedging exposure arising on external and
internal trade flows. The Group also adopts a natural hedging strategy and, to the extent
reasonably possible, seeks to align the currency of inflows and outflows to minimise foreign
exchange exposure. In certain cases (such as the purchase of some manufacturing inputs or
some export sales made to distributors in markets where the Group does not have an entity
presence) transactional foreign exchange exposure exists (see paragraph 2.9 of Risk Factors).
2.16 Regulation
The consumer healthcare market is heavily regulated by governments and other regulatory
bodies in the countries in which the Group operates. Within the consumer healthcare market,
the OTC segment tends to be subject to greater regulation, including in relation to pricing.
The Group expends significant resources within SG&A to support compliance with a broad and
varied range of regulatory requirements, including pharmacovigilance obligations, maintenance
of product registrations and compliance with rigorous quality standards. The Group is also
subject to periodic requirements to assess and address new potential risks to consumers, such
as the recent requirement imposed by a number of regulators to assess nitrosamine levels in
OTC medicines. Failure to comply with regulations could lead to supply interruptions, product
recalls and/or regulatory enforcement action and fines from regulators. See paragraph 2.1 of
Risk Factors.
Changes to the laws and regulations to which the Group and its operations are subject,
whether as a result of new or more stringent requirements, or more stringent interpretations of
existing requirements, can also impose significant compliance costs and impact the way in
which the Group conducts its business. For example, in China, in early 2020, certain local
authorities introduced temporary restrictions on the sale of certain cough and cold medicines in
an attempt to prevent patients from self-medicating against COVID-19 at home, which limited
sales of Contac (nasal decongestant tablets that also relieve pain and reduce a fever) and
Fenbid (ibuprofen-based pain relief medicine) by the Group in 2020. These restrictions had an
adverse impact on the Group’s revenue in FY 2020 and FY 2021. Similar restrictions may be
imposed in the future, depending on the development of the COVID-19 pandemic.
The Group has also been impacted, and expects to continue to be impacted, by recent reforms
regarding government price management of drugs in China (see paragraph 9 of Part X
(Regulatory Overview)). The nationwide volume-based procurement program, which was
introduced in 2018, has negatively impacted revenue in respect of Fenbid sold in the country
160
through the state-owned hospital channel, and other brands may be brought within the scope
of this initiative in future. In order to mitigate the impact, the Group is seeking to increase sales
through retail and e-commerce channels.
2.17 Separation and future restructuring
The Group has taken a number of actions in preparation for Separation, including to ensure
that the Group has the necessary infrastructure to operate as an independent publicly listed
company. These actions include the establishment of independent IT infrastructure and
independent corporate functions and governance of the Group, including building key
technology infrastructure and capability within corporate functions to support a publicly-listed
group.
During FY 2020 and FY 2021, the Group incurred costs in connection with preparation for
Separation of £66 million and £278 million, respectively. The Directors expect the Group to
incur Separation and Admission costs of approximately £0.4 billion between 2022 and 2024
(including Admission costs of up to £0.1 billion), most of which are expected to be incurred in
2022.
Following Separation, the Group expects to incur Restructuring costs of approximately
£0.2 billion between 2022 and 2024 in connection with projects to support further efficiencies in
its operations. The Directors do not currently anticipate further significant restructuring
programmes. Restructuring costs are treated as an Adjusting Item for the purpose of
calculating Adjusted operating profit.
The Directors also expect the Group to incur recurring operating costs of approximately
£175 million to £200 million per annum from 2022 onwards to provide the capabilities to
operate successfully as a standalone UK public listed company following Separation, including
in technology and infrastructure, and in corporate functions.
The Group has historically benefited from negotiated arrangements with third-party suppliers,
distributors, licensors, lessors, other business partners and/or counterparties as part of the
larger GSK Group. While certain of these arrangements will change as a result of Separation,
the cost impact is not expected to be material.
The Group has entered into a Transition Services Agreement with the GSK Group in
connection with Separation. The Transition Services Agreement is short-term and limited in
scope (see paragraph 15.14 of Part XII (Additional Information)). Additionally, the Group has
entered into Manufacturing and Supply Agreements with the GSK Group, which are also
limited in scope (see paragraph 15.15 of Part XII (Additional Information)).
3. Recent developments
The Group is monitoring the effects of Russia’s invasion of Ukraine, with the GSK Board
overseeing and monitoring key risks. The Board will assume oversight and management of
these risks after Separation. The Group’s operations and presence in these markets is limited
and Russia and Ukraine accounted for less than 3 per cent. of each of the Group’s revenue
and Adjusted operating profit in FY 2021. The Group has no manufacturing operations in these
markets and imports products sold there. Since the start of the conflict, the Group has been
constantly evaluating its activities in Russia and has stopped advertising and promotional
activity in Russia. The Group is prioritising supply of OTC medicines and reducing its oral
health products, ensuring the Group continues to meet basic consumer health needs. The
Group is no longer importing food supplements and vitamins into Russia.
161
There is a significant risk to disruption of the Group’s operations in Russia and Ukraine.
• Despite the limited scope of the Group’s activities in Russia and the Group’s focus on
meeting consumers’ everyday health needs, there may be certain reputational risks
associated with the Group’s presence in the Russian market.
• The Group generates revenue from sales of its products in Russia in the Russian Ruble,
while significant costs (notably, manufacturing and supply chain costs) associated with
those products are denominated in other currencies, such as Euro and US Dollar. The
international response to the invasion, including the imposition of international sanctions
against Russia, has had a significant adverse effect on the value of the Russian Ruble,
which has reduced the Group’s revenue from its operations in Russia without a
corresponding reduction in costs, and the Group may not be able to offset the devaluation
of the Russian Ruble through increased prices of its products. In addition, the imposition of
exchange controls may limit the Group’s ability to repatriate profits from its operations in
Russia.
• The Group’s ability to supply customers may be negatively affected by disruption to supply
and distribution channels.
• The Group’s customers in Russia and Ukraine may have been significantly negatively
affected by the factors described above, which exposes the Group to increased
counterparty risk in relation to these customers and receivables from these customers.
• The imposition of sanctions and other restrictive measures against Russia, Russia’s
response to the global sanctions regime, as well as additional international sanctions
against Russia, may create regulatory uncertainty and present further compliance
challenges for the Group’s operations, which may increase compliance costs and make it
difficult to continue operations in Russia.
• The Group tested all assets that presented impairment indicators as a result of the conflict
between Russia and Ukraine in Q1 2022 and recognised write-downs and impairments of
assets representing less than 0.1 per cent. of the Group’s total assets as at 31 March 2022
in relation to certain brands, finished goods inventory and receivables. Depending on the
long term outlook of the Group’s business in Russia and Ukraine, the carrying value of
certain of the Group’s intangible assets may be impacted. However, any such impact is not
anticipated to be material to the Group.
• As at the date of the Prospectus, the Russian government has indicated it has drawn up
plans to seize the assets of western companies leaving Russia. While the scope of such
measures is not presently clear, if the Group ceased its activities and/or suspended its
operations in Russia and did not resume its presence in Russia within a certain period of
time, the Russian government could: (i) nationalise the Group’s assets located in Russia;
(ii) allow the Group’s patents and trade marks to be used within Russia without the Group’s
consent; and/or (iii) introduce restrictions on, or impose unfavourable terms in respect of,
payments made from Russia or relating to assets in Russia.
In addition to the specific implications for the Group’s operations in Russia and Ukraine, the
Group may be affected by broader impacts on the global geopolitical and economic environment,
including (but not limited to) changes in commodity, freight, logistics and input costs.
162

4. Current financial update
GSK intends to publish its quarterly and half-yearly results for the three and six months ended 30
June 2022 on 27 July 2022, which will include financial information in relation to the Consumer
Healthcare Segment prepared under IFRS 5 “Non-current assets held for sale and discontinued
operations”. The Group also intends to publish an update on trading for the six months ending 30
June 2022 on 27 July 2022 and will acknowledge certain financial information published by GSK in
relation to the Consumer Healthcare Segment. The Group intends to publish its half-yearly results
for the six months ended 30 June 2022 on 19 September 2022.
4.1 Revenue
Revenue for the three months ended 31 March 2022 compared to the three months ended
31 March 2021
The Group’s revenue in Q1 2022 and Q1 2021 was £2,627 million and £2,306 million,
respectively. The Group’s revenue increased by 13.9 per cent. at AER and increased by
14.4 per cent. at CER during Q1 2022. The Group’s organic revenue growth was 15.6 per cent.
Revenue growth at AER and CER reflected growth across all regions and categories, and
benefitted from favourable prior year comparators especially in Respiratory Health which saw a
strong rebound following the historically low cold and flu season in Q1 2021, with cold and flu
sales contributing approximately five percentage points to total growth. In addition, advance
retailer and wholesaler stock-in, and initial distributor sell-in due to the systems cutover and
distribution business model change ahead of Separation contributed approximately two
percentage points to total growth. Strong sales growth in Pain Relief benefited from increased
demand during the COVID-19 Omicron wave and an improved capacity in VMS.
Organic revenue growth was primarily driven by the same factors but excluded a 0.5 per cent.
decrease in revenue growth as a result of the net adverse exchange rate movements (included
in AER) and a 1.2 per cent. decrease in revenue growth as a result of the impact of
divestments and MSAs (included both in AER and CER).
4.2 Revenue by product category
The table below sets out the Group’s revenue by product category for the three months ended
31 March 2022 and 31 March 2021.
Revenue (£m) Revenue change Q1 21 - Q1 22 %
Q1 2022 Q1 2021
Reported
rates
Constant
currency Organic
Oral Health 741 701 5.7% 7.9% 7.9%
VMS 405 348 16.4% 14.8% 14.9%
Pain Relief 635 538 18.0% 18.6% 18.9%
Respiratory Health 367 243 51.0% 52.9% 52.9%
Digestive Health and Other 479 476 0.6% -0.5% 4.3%
Total 2,627 2,306 13.9% 14.4% 15.6%
(A) Oral Health
Revenue in the Oral Health category grew by 5.7 per cent. at AER and 7.9 per cent. at
CER. Sensodyne delivered high-single digit per cent. revenue growth reflecting
underlying brand strength, continued innovation and strong growth across key markets
including the US, India, Japan, Middle East and Africa. Gum Health delivered high-
single digits per cent. revenue growth. Denture Care grew low teens per cent. following
the decrease of sales during the COVID-19 pandemic.
163

(B) VMS
Revenue in the VMS category grew by 16.4 per cent. at AER and 14.8 per cent. at CER.
Centrum grew high -teens per cent., with particularly strong growth in China due to
consumer focus on immunity as a result of the COVID-19 pandemic. Emergen-C grew
high -thirties per cent. versus a high -twenties per cent. decrease in Q1 2021. Caltrate
revenue growth declined low-single digits per cent., with mid-single digit per cent. growth
in China being insufficient to offset a decline in the US and South East Asia.
(C) Pain Relief
Revenue in the Pain Relief category grew by 18.0 per cent. at AER and 18.6 per cent.
at CER. Panadol grew low -forties per cent. due to a successful campaign aimed at
post vaccination use and increased demand during the COVID-19 Omicron wave. Advil
grew high -twenties per cent. benefitting from retail stocking patterns in the US versus
a decrease in Q1 2021. Excedrin grew high-single digit per cent. and Voltaren was
stable with growth in China offset by a decrease in Germany.
(D) Respiratory Health
Revenue in the Respiratory Health category grew by 51.0 per cent. at AER and
52.9 per cent. at CER. The category rebounded strongly from the historically low
demand for cold and flu products in Q1 2021. Cold and flu product sales more than
doubled in the US and were strong in Europe, Middle East and Africa and Latin
America, with sales ahead of pre-pandemic levels in 2019.
(E) Digestive Health and Other
In Digestive Health and Other, revenue grew by 0.6 per cent. at AER and declined by
0.5 per cent. at CER. This was primarily driven by strong revenue growth in Tums and
Eno partially offset by a low-single digit per cent. decline in Nexium.
4.3 Revenue by region
The table below sets out the Group’s revenue by region for the quarter ended 31 March 2022
and 31 March 2021.
Revenue (£m) Revenue change Q1 21 - Q1 22 %
Q1 2022 Q1 2021
Reported
rates
Constant
currency Organic
North America 940 783 20.1% 16.5% 17.3%
EMEA and LatAm 1,057 979 8.0% 12.5% 14.5%
APAC 630 544 15.8% 15.0% 15.2%
Total 2,627 2,306 13.9% 14.4% 15.6%
(A) North America
The Group’s revenue attributable to North America was £940 million and £783 million
in Q1 2022 and Q1 2021, respectively. The Group’s revenue grew 20.1 per cent. at
AER and 16.5 per cent. at CER. Organic revenue growth was 17.3 per cent.
Revenue growth at AER and CER was largely driven by mid-fifties per cent. growth in
Respiratory Health as a result of strong execution and a rebound following the
164
historically low cold and flu season in Q1 2021, and low-twenties per cent. growth in
Pain Relief driven by sales in Advil. VMS also experienced low-twenties per cent.
revenue growth, reflecting sales growth of Centrum and Emergen-C.
Organic revenue growth was primarily driven by the same principal factors but
excluded a 3.6 per cent. increase in revenue growth as a result of favourable exchange
rate movements (included in AER) and a 0.8 per cent. decrease in revenue growth as
a result of the impact of divestments and MSAs (included both in AER and CER).
(B) EMEA and LatAm
The Group’s revenue attributable to EMEA and LatAm was £1,057 million and
£979 million in Q1 2022 and Q1 2021, respectively. The Group’s revenue grew 8.0 per
cent. at AER and 12.5 per cent. at CER. Organic revenue growth was 14.5 per cent.
Revenue growth at AER and CER was largely driven by high-fifties per cent. growth in
Respiratory Health, which saw a strong rebound following the historically low cold and
flu season in Q1 2021, which led to an increase in consumption of Otrivin, Panadol and
Theraflu. In addition, Panadol sales were also supported by a campaign aimed at post
vaccination use and increased demand during the COVID-19 Omicron wave. This
drove high-single digit per cent. growth in the Pain Relief category and offset a decline
in sales of Voltaren. Low-teens per cent. revenue growth in VMS and high-single digit
per cent. revenue growth in Oral Health were driven by higher consumption. A sell-in
ahead of a systems cutover and distribution model change contributed approximately 4
percentage points to revenue growth in the region.
Organic revenue growth was primarily driven by the same principal factors but
excluded a 4.5 per cent. decrease in revenue growth as a result of adverse exchange
rate movements (included in AER) and a 2.0 per cent. decrease in revenue growth as
a result of the impact of divestments and MSAs (included both in AER and CER).
(C) APAC
The Group’s revenue attributable to APAC was £630 million and £544 million in Q1
2022 and Q1 2021, respectively. The Group’s revenue grew 15.8 per cent. at AER and
15.0 per cent. at CER. Organic revenue growth was 15.2 per cent.
Revenue growth at AER and CER was largely driven by high-thirties per cent. growth
in Pain Relief principally in Australia. Low double-digit per cent. revenue growth in VMS
was driven by continued consumption increases of Centrum in China and South East
Asia. High single-digit per cent. revenue growth in Oral Health was driven by increased
Sensodyne consumption, with growth principally coming from China, India, Japan and
South East Asia.
Organic revenue growth was primarily driven by the same principal factors but
excluded a 0.8 per cent. increase in revenue growth as a result of favourable exchange
rate movements (included in AER) and a 0.2 per cent. decrease in revenue growth as
a result of the impact of MSAs (included both in AER and CER).
165
4.4 Operating profit and operating profit margin
Operating profit and operating profit margin for the three months ended 31 March 2022
compared to the three months ended 31 March 2021
The Group’s operating profit was £466 million in Q1 2022 and £348 million in Q1 2021, with an
operating profit margin of 17.7 per cent. and 15.1 per cent. in each of those periods
respectively. Adjusted operating profit was £631 million in Q1 2022 and £482 million in Q1
2021, with an Adjusted operating profit margin of 24.0 per cent. and 20.9 per cent. in each of
those periods respectively.
The increase in operating profit and operating profit margin primarily reflected organic revenue
growth, strong leverage from volume growth and product price increases, supply chain
efficiencies and increases in incremental synergy benefits from the Pfizer Transaction of
approximately £30 million. This was partially offset by increased commodity and freight costs,
increased advertising and promotion investment and approximately £20 million of costs
associated with providing the Group with the capabilities to operate as a standalone UK public
listed company following Separation.
Adjusting Items within operating profit totalled £165 million and £134 million in Q1 2022 and Q1
2021 respectively. The £31m increase is driven by an increase in Separation and Admission
costs and Net amortisation and impairment of intangible assets, partially offset by decreases in
restructuring costs associated with the Pfizer Transaction and other operating income from
disposal and others.
The increases in Adjusted operating profit and Adjusted operating profit margin were driven by
the same principal factors affecting operating profit and operating profit margin but excluded
the impact of the increase in Adjusting Items.
4.5 Profit after tax
Profit after tax for the three months ended 31 March 2022 compared to the three months
ended 31 March 2021
Taking into account net finance costs, which included the impact of the issuance of the
Pre-Separation Programme Notes and the Pre-Separation USD Notes, profit after tax was
£357 million in Q1 2022, increasing by £108 million from £249 million in Q1 2021. This
reflected higher operating profit during the period, as described above, and a lower effective
tax rate in Q1 2022.
Adjusted profit after tax was £491 million in Q1 2022, increasing by £127 million from
£364 million in Q1 2021. This reflected increased Adjusted operating profit during the period,
as described above, and a lower Adjusted effective tax rate in Q1 2022, which decreased from
25 per cent. in Q1 2021 to 22 per cent. in Q1 2022.
4.6 Non-current assets
The Group’s non-current assets as at 31 March 2022 and 31 December 2021 were
£29,725 million and £29,200 million, respectively. The Group’s non-current assets increased by
£525 million, primarily driven by an increase in intangible assets, due to exchange rate
fluctuations and the transfer of two local brands from GSK into the JV as part of the
Separation, offset by amortisation.
166

4.7 Current assets
The Group’s current assets as at 31 March 2022 and 31 December 2021 were £15,298 million
and £5,251 million, respectively. The Group’s current assets increased by £10,047 million,
primarily driven by an increase in loan amounts owing from related parties in connection with
the Notes Proceeds Loans. In addition, trade receivables increased due to higher sales and
exchange rate fluctuations.
4.8 Non-current liabilities
The Group’s non-current liabilities as at 31 March 2022 and 31 December 2021 were
£13,148 million and £3,733 million, respectively. The Group’s non-current liabilities increased
by £9,415 million, primarily driven by an increase in long-term borrowings, attributable to the
issuance of the Pre-Separation Programme Notes and the Pre-Separation USD Notes during
the period and exchange rate fluctuations.
4.9 Current liabilities
The Group’s current liabilities as at 31 March 2022 and 31 December 2021 were £5,026 million
and £4,238 million, respectively. The Group’s current liabilities increased by £788 million,
largely due to higher loan amounts owing to related parties as part of the Group’s existing
banking arrangements with GSK finance entities and exchange rate fluctuations.
5. Key performance indicators and non-IFRS financial measures
In evaluating the Group’s results of operations, the Directors consider the following key
performance indicators and non-IFRS financial measures. Further information on the definition
and purpose of these metrics is included at paragraph 6.4 of Presentation of Financial and
Other Information. For a reconciliation of non-IFRS financial measures, see paragraph 5 of
Part VI (Selected Financial Information).
2021 2020 2019
Revenue (£m) 9,545 9,892 8,480
Revenue growth (%) (3.5) 16.7 -
Organic revenue growth (%) 3.8 2.8 -
Gross profit (£m)
1
5,950 5,910 4,802
Adjusted gross profit (£m) 6,002 6,173 5,273
Gross profit margin (%)
1
62.3 59.7 56.6
Adjusted gross profit margin (%) 62.9 62.4 62.2
Operating profit (£m)
1
1,638 1,598 897
Adjusted operating profit (£m) 2,172 2,074 1,654
Operating profit margin (%)
1
17.2 16.2 10.6
Adjusted operating profit margin (%) 22.8 21.0 19.5
Profit after tax (£m)
1
1,439 1,181 687
Adjusted EBITDA (£m) 2,413 2,351 1,884
Net cash inflows from operating activities (£m)
1
1,356 1,407 786
Free cash flow (£m) 1,173 1,988 681
Free cash flow conversion (%) 82 168 99
Note
1. Not considered to be key performance indicator, but included as the nearest IFRS
measure to the relevant non-IFRS measure presented in the table above.
167
6. Results of Operations
6.1 Description of the Group’s results of operations
(A) Revenue
Revenue for the financial year ended 31 December 2021 compared to the financial
year ended 31 December 2020
The Group’s revenue in FY 2021 and FY 2020 was £9,545 million and £9,892 million,
respectively.
The Group’s revenue decreased by 3.5 per cent. at AER, and increased by 1.0 per
cent. at CER reflecting dilution from divestments given the completion of the
divestment programme during FY 2021. The Group’s organic revenue growth was
3.8 per cent.
The decline in revenue at AER reflected adverse exchange rate movements of
£443 million as Pounds Sterling, the Group’s reporting currency, strengthened against
other currencies such as the US Dollar, Euro, certain currencies in Latin America, the
Japanese Yen, the Turkish Lira and the Russian Ruble.
Revenue growth at AER and CER was impacted by a decline attributable to the full
year revenue effect of divestments made during the course of FY 2020, including
Physiogel, Breathe Right, Venoruton and Coldrex, as well as a number of divestments
made during FY 2021 including Transderm Scop, Acne Aid, Baldriparan and
Spectraban.
Organic revenue growth was primarily driven by growth in revenue attributable to
brands in the Oral Health, Pain Relief and VMS categories (including Sensodyne,
parodontax, Voltaren, Panadol, Excedrin, Centrum and Caltrate), as well as growth in
Otrivin, Flonase, Tums, ENO and brands in the Smokers’ Health sub-category of
Digestive Health and Other. This reflects the underlying strength of brands across the
Group’s portfolio and categories and continuing growth in e-commerce.
The Group delivered revenue growth at CER in the Oral Health, Pain Relief, and VMS
categories. Respiratory Health experienced a decline in revenue at CER due to the
revenue impact of divestments of brands in that category, as well as the continued
impact of the COVID-19 pandemic and associated responsive measures, while the
Digestive Health and Other category experienced revenue decline at CER due to the
revenue impact of divestments of brands in that category.
• In Oral Health, revenue declined by 0.8 per cent. at AER and grew by 4.4 per cent.
at CER. Revenue growth at CER was driven by continued strong demand in
Sensodyne. Sensodyne delivered mid-single digit per cent. revenue growth at CER,
which reflected underlying brand strength, continued innovation (for example, the
Repair and Protect and Pronamel products), and increased consumption, particularly
in India and China. Additionally there was growth in respect of parodontax. This
growth was partially offset by a decline in Aquafresh revenue at CER, which was
attributable to a shift in promotional focus to other brands in the Oral Health
category. Denture Care revenue was flat year-on-year, driven by measures
implemented in connection with the COVID-19 pandemic and increased competition
in certain markets, although growth returned in the last quarter of FY 2021.
168
• Revenue in the VMS category grew by 0.5 per cent. at AER and 3.9 per cent. at
CER. Growth at CER built on the significant revenue growth experienced by the
category in FY 2020. Revenue growth in FY 2021 was primarily attributable to the
sustained, double-digit year-on-year per cent. growth in Centrum and mid-single
digit growth in Caltrate, partially offset by a high-single digit per cent. decline in
Emergen-C. The strong performance of Centrum reflected the increasing
consumer trend towards self-management of health and wellbeing, particularly in
the APAC region, as well as successful innovation and improved supply capacity in
the US. The decline in Emergen-C revenue was due to a particularly strong
comparator in FY 2020, when Emergen-C revenue increased by half compared to
FY 2019 as a result of a surge in demand during the early stages of the COVID-19
pandemic. However, Emergen-C revenue in FY 2021 was significantly above
revenue attributable to the brand in FY 2019.
• Revenue in the Pain Relief category grew by 2.1 per cent. at AER and 6.2 per cent.
at CER. Revenue growth at CER was largely due to growth in revenue attributable
to Panadol, Voltaren, and Excedrin. Panadol revenue growth at CER in the low
mid-teens was driven by the strength of the brand and an increase in demand
driven by self-medication of symptoms associated with COVID-19 and the
COVID-19 vaccination. Voltaren experienced double-digit per cent. revenue growth
at CER, with growth in EMEA and LatAm and APAC partially offset by the impact
of the introduction of “private-label” products in the US in FY 2021. Excedrin
revenue recovered during FY 2021 following temporary supply chain interruption in
FY 2020. Advil revenue was impacted by temporary disruption in third party supply.
• Revenue in the Respiratory Health category declined by 12.8 per cent. at AER and
8.6 per cent. at CER. The decline at CER was primarily attributable to the full year
revenue impact of the divestment of Breathe Right. While revenue in Respiratory
Health was adversely impacted by an exceptionally low cold and flu incidence in
the first half of FY 2021, revenue for the category recovered in the second half of
the year (see paragraph 2.14 of this Part VII (Operating and Financial Review)
above). Flonase experienced single -digit per cent. revenue growth year-on-year,
driven by a stronger allergy season relative to the prior year comparator.
• In Digestive Health and Other, revenue declined by 9.8 per cent. at AER and
5.0 per cent. at CER. Revenue decline at CER was largely attributable to the full
year revenue impact of the divestments of Physiogel, Venoruton and Coldrex
during FY 2020. This was partially offset by an increase in revenue attributable to
Tums, ENO and Chapstick. Tums revenue growth was largely due to growth in the
market for Antacids, improved supply chain capacity, and innovation launches in
the Naturals segment. ENO revenue growth was due to product price increases
and increased consumption. An increase in Chapstick revenue was attributable to
increased in-store purchases, coinciding with the easing of COVID-19 lockdowns.
Revenue for the financial year ended 31 December 2020 compared to the financial
year ended 31 December 2019
The Group’s revenue in FY 2020 was £9,892 million. The Group’s revenue in FY 2019
(including the revenue of the Pfizer Contributed CH Business from 1 August 2019,
when it was consolidated) was £8,480 million. Revenue of the Pfizer Contributed CH
Business for the seven months to 31 July 2019 was £1,523 million.
169
The Group’s revenue increased by 16.7 per cent. at AER and 19.3 per cent. at CER,
and the Group’s organic revenue growth was 2.8 per cent.
Revenue growth at AER and CER was primarily driven by the inclusion of the full year
of revenue of the Pfizer Contributed CH Business in FY 2020, compared to five months
of revenue in FY 2019, together with growth in revenue attributable to Sensodyne,
Voltaren and Panadol. Revenue growth at AER and CER was partially offset by a
decline in revenue attributable to divestments made during the course of FY 2020,
including Physiogel, Breathe Right, Venoruton, Oilatum and Coldrex. Growth in
revenue at AER was further offset by adverse exchange rate movements as Pounds
Sterling, the Group’s presentation currency, strengthened against other currencies
such as the US Dollar, certain currencies in Latin America, the South African Rand and
the Russian Ruble.
Organic revenue growth was primarily attributable to growth in organic revenue across
the Group’s VMS, Pain Relief and Oral Health categories (including in respect of
Sensodyne, Centrum, Voltaren and Panadol). Revenue growth in the VMS and Pain
Relief categories was stronger in the period 1 January 2020 to 31 July 2020 as a result
of increased demand for products during the COVID-19 pandemic designed to address
fever symptoms, together with the impact of consumer stockpiling (described further
below). Organic revenue for the period excludes revenue attributable to brands
acquired as part of the Pfizer Transaction in the period 1 January 2020 to 31 July 2020
(see paragraph 6.3 of Presentation of Financial and Other Information), which made a
significant contribution to the Group’s revenue in the VMS and Pain Relief categories
during the period, with the effect that the overall revenue growth of the Group was
reduced on an organic basis. Furthermore, brands divested in FY 2021 (notably
Transderm Scop in the Digestive Health and Other category, in respect of which
revenue declined due to competition from generic products) are included in organic
revenue growth for the period FY 2019 to FY 2020, and this also had a significant
impact on the organic growth measure in this period.
The Group delivered strong revenue growth at CER in the VMS and Pain Relief
categories, single-digit growth in the Oral Health and Digestive Health and Other
categories, and flat revenue growth in Respiratory Health.
• In Oral Health, revenue grew by 3.3 per cent. at AER and 5.9 per cent. at CER.
Growth at CER was driven by strong demand in Sensodyne, as well as growth in
respect of parodontax, largely offset by a decline in Aquafresh revenue and revenue
attributable to brands in the Denture Care sub-category of Oral Health, the latter of
which was driven by fewer opportunities for social occasions during the COVID-19
pandemic (see paragraph 2.14 of this Part VII (Operating and Financial Review)
above).
• In the VMS category, revenue grew by 150.3 per cent. at AER and 154.6 per cent. at
CER. Growth at CER was primarily attributable to the inclusion of the full year
revenue of brands acquired as part of the Pfizer Transaction (including Centrum,
Caltrate and Emergen-C), together with an increasing consumer trend towards self-
management of health and wellbeing, which was accelerated by the COVID-19
pandemic. The Group also increased advertising and promotion investment in the
VMS category.
170
• Revenue grew by 25.8 per cent. at AER and 28.6 per cent. at CER in the Pain
Relief category. Growth at CER was largely attributable to the inclusion of the full
year of revenue of brands acquired as part of the Pfizer Transaction (including
Advil), together with the Rx-to-OTC switch of Voltaren and a strong performance
from Panadol. Advil revenue was adversely affected by negative media coverage
regarding the use of ibuprofen products in treating the symptoms of COVID-19
(see paragraph 2.3 of this Part VII (Operating and Financial Review) above).
Excedrin revenue in North America was affected by temporary supply chain
interruption.
• In Respiratory Health, revenue decreased by 1.5 per cent. at AER and was flat at
CER. Flat revenue at CER was primarily attributable to the inclusion of a full year
of revenue attributable to brands acquired as part of the Pfizer Transaction
(including Robitussin). This was largely offset by a decline in revenue attributable
to Otrivin and Theraflu, which were negatively impacted by the COVID-19
pandemic, as well as the impact of the divestment of certain brands in this
category. In particular, there was an exceptionally weak cold, cough and flu season
in Q3 and Q4 of 2020 as a result of measures taken in response to COVID-19,
together with fewer consumer visits to stores following the implementation of
lockdowns.
• In Digestive Health and Other, revenue decreased by 0.1 per cent. at AER and
increased by 2.5 per cent. at CER. Growth at CER was largely attributable to the
inclusion of a full year of revenue attributable to brands acquired as part of the
Pfizer Transaction (including Preparation H and Chapstick). This was partially
offset by a decrease in revenue attributable to Fenistil and Abreva, driven by fewer
consumer visits to stores following the implementation of lockdowns during the
COVID-19 pandemic. These factors also negatively impacted Preparation H and
Chapstick revenue. Further, revenue was negatively impacted as a result of the
divestment of certain brands.
(B) Cost of sales
Cost of sales for the financial year ended 31 December 2021 compared to the financial
year ended 31 December 2020
The Group’s cost of sales was £3,595 million and £3,982 million in FY 2021 and FY
2020, respectively. The Group’s cost of sales decreased by 9.7 per cent. (£387
million). This decrease reflects a reduction in Adjusting Items (as outlined below), the
full year impact of divestments in FY 2020 and further integration savings driven by the
Pfizer Transaction, which more than offset investment in supply chain to improve
continuity and capacity, as well as inflationary pressures, principally in relation to
commodity and freight costs.
Adjusting Items within costs of sales totalled £52 million in FY 2021, which
predominantly related to costs of restructuring programmes, together with Net
impairment and amortisation of intangible assets across a number of the Group’s
brands. Adjusting Items within costs of sales totalled £263 million in FY 2020. The
decrease primarily reflected reduced costs relating to the Pfizer Transaction (mainly
Transaction-related costs and Restructuring costs), as well as reduced Net
amortisation and impairment of intangible assets across a number of the Group’s
brands.
171
Adjusted cost of sales was £3,543 million and £3,719 million in FY 2021 and FY 2020,
respectively. Adjusted cost of sales decreased by 4.7 per cent. (£176 million). This was
primarily driven by the full year impact on cost of sales of divestments made in FY
2020 and further synergies from the Pfizer Transaction, which more than offset
increased investment in supply chain, inflationary pressures and the increase in sales
and freight costs in each region due to disruption caused by the COVID-19 pandemic.
Cost of sales as a percentage of revenue reduced to 37.7 per cent. from 40.3 per cent.
in FY 2020, largely driven by the reduction in Adjusting Items, further synergies from
the Pfizer Transaction, product price increases and supply chain efficiencies. This was
partially offset by increased investment in supply chain and increases in freight,
commodities and other costs (for example, employee costs), which reflected
inflationary pressures in the supply chain. Adjusted cost of sales as a percentage of
revenue reduced to 37.1 per cent. in FY 2021 from 37.6 per cent. in FY 2020.
Cost of sales for the financial year ended 31 December 2020 compared to the financial
year ended 31 December 2019
The Group’s cost of sales in FY 2020 was £3,982 million. The Group’s cost of sales in
FY 2019 (including the cost of sales of the Pfizer Contributed CH Business from
1 August 2019, when it was consolidated) was £3,678 million. Cost of sales of the
Pfizer Contributed CH Business for the seven months to 31 July 2019 was
£570 million.
The Group’s cost of sales increased by 8.3 per cent. (£304 million). This increase was
primarily driven by the inclusion of the full year of cost of sales of the Pfizer Contributed
CH Business in FY 2020, compared to five months in FY 2019, partially offset by a
reduction in Adjusting Items (as outlined below), as well as the impact of divestments
and ongoing supply chain productivity efforts.
Adjusting Items within costs of sales totalled £263 million in FY 2020, which
predominantly related to further Adjusting Items associated with the Pfizer Transaction
(mainly Transaction-related costs and Restructuring costs), together with Net
amortisation and impairment of intangible assets across a number of Group’s brands.
Adjusting Items within cost of sales totalled £471 million in FY 2019. These Adjusting
Items principally related to the Pfizer Transaction (mainly Transaction-related costs and
Restructuring costs).
Adjusted cost of sales was £3,719 million and £3,207 million in FY 2020 and FY 2019,
respectively. Adjusted cost of sales increased by 16.0 per cent. (£512 million), primarily
driven by the inclusion of the full year of Adjusted cost of sales of the Pfizer
Contributed CH Business in FY 2020, compared to five months in FY 2019.
Cost of sales as a percentage of revenue reduced to 40.3 per cent. in FY 2020 from
43.4 per cent. in FY 2019, largely driven by a reduction in Transaction-related costs,
product price increases, improvements in brand mix, synergies and ongoing supply
chain productivity efforts. These factors were partially offset by increased freight and
staffing costs driven by the COVID-19 pandemic. Adjusted cost of sales as a
percentage of revenue reduced to 37.6 per cent. in FY 2020 from 37.8 per cent. in FY
2019.
172
(C) Gross profit
Gross profit for the financial year ended 31 December 2021 compared to the financial
year ended 31 December 2020
The Group’s gross profit in FY 2021 was £5,950 million, producing a gross profit
margin of 62.3 per cent. The Group’s gross profit in FY 2020 was £5,910 million,
producing a gross profit margin of 59.7 per cent.
The Group’s gross profit increased by 0.7 per cent. (£40 million) in FY 2021, which
primarily reflected a reduction in Adjusting Items within cost of sales associated with
the Pfizer Transaction (mainly Transaction-related costs and Restructuring costs). This
more than offset the year-on-year decline in the Group’s revenue at AER. Gross profit
margin increased by 2.6 percentage points, largely reflecting the reduction in these
Adjusting Items associated with the Pfizer Transaction, as well as product price
increases, improvements in brand mix (including the divestment of several brands) and
synergies from the Pfizer Transaction, as well as other ongoing supply chain and
manufacturing efficiency efforts. These factors were partially offset by investment in the
supply chain, as well as increased freight costs, commodity prices and staffing costs
arising in connection with the COVID-19 pandemic.
Adjusted gross profit was £6,002 million and £6,173 million in FY 2021 and FY 2020,
respectively. Adjusted gross profit decreased by 2.8 per cent. (£171 million), broadly in
line with the year-on-year decline in the Group’s revenue at AER.
Adjusted gross profit margin for the Group was 62.9 per cent. and 62.4 per cent. for FY
2021 and FY 2020, respectively. The factors driving the year-on-year increase of 0.5
percentage points were the same as for gross profit margin, except in respect of
changes in Adjusting Items, which are excluded from Adjusted gross profit margin.
Gross profit for the financial year ended 31 December 2020 compared to the financial
year ended 31 December 2019
The Group’s gross profit was £5,910 million in FY 2020, producing a gross profit
margin of 59.7 per cent. The Group’s gross profit for FY 2019 (including the gross profit
of the Pfizer Contributed CH Business from 1 August 2019, when it was consolidated)
was £4,802 million, producing a gross profit margin of 56.6 per cent. Gross profit of the
Pfizer Contributed CH Business for the seven months to 31 July 2019 was
£953 million, with a gross profit margin of 62.6 per cent.
The Group’s gross profit increased by 23.1 per cent. (£1,108 million), primarily driven
by the inclusion of the full year of gross profit of brands acquired as part of the Pfizer
Transaction in FY 2020, compared to five months of gross profit in FY 2019. Gross
profit margin increased by 3.1 percentage points from FY 2019 to FY 2020. This
increase was largely driven by a reduction in Adjusting Items in relation to the Pfizer
Transaction (mainly Transaction-related costs and Restructuring costs), product price
increases, improvements in brand mix, synergies resulting from the Pfizer Transaction,
and ongoing supply chain productivity efforts. These factors were partially offset by
increased freight and staffing costs arising as a result of the COVID-19 pandemic.
Adjusted gross profit was £6,173 million and £5,273 million in FY 2020 and FY 2019,
respectively. Adjusted gross profit increased by 17.1 per cent. (£900 million), primarily
173
driven by the inclusion of the full year of gross profit of the Pfizer Contributed CH
Business in FY 2020, compared to five months in FY 2019.
Adjusted gross profit margin for the Group was 62.4 per cent. and 62.2 per cent. for FY
2020 and FY 2019, respectively. The factors driving the year-on-year increase of 0.2
percentage points were the same as for gross profit margin, save in respect of changes
in Adjusting Items, which are excluded from Adjusted gross profit margin.
(D) Selling, general and administration (“ SG&A”)
SG&A costs for the financial year ended 31 December 2021 compared to the financial
year ended 31 December 2020
The Group’s SG&A costs were £4,086 million and £4,220 million in FY 2021 and FY
2020, respectively. The Group’s SG&A costs decreased by 3.2 per cent. (£134 million)
in FY 2021, primarily driven by the continued benefit of synergies from the Pfizer
Transaction and the tight control of ongoing costs, partially offset by an increase in
Adjusting Items, primarily in respect of Separation and Admission costs.
Adjusting Items within SG&A totalled £504 million in FY 2021. These principally related
to Separation and Admission costs as well as further Restructuring costs associated
with the Pfizer Transaction. Adjusting Items within SG&A totalled £401 million in FY
2020. These principally related to Restructuring costs associated with the Pfizer
Transaction, as well as the initial costs in connection with Separation and Admission.
Adjusted SG&A costs were £3,582 million and £3,819 million in FY 2021 and FY 2020,
respectively. Adjusted SG&A costs decreased by 6.2 per cent. (£237 million). This
primarily reflected the continuing benefit of synergies from the Pfizer Transaction and
tight control of ongoing costs.
SG&A costs as a percentage of revenue increased to 42.8 per cent. in FY 2021 from
42.7 per cent. in FY 2020. This primarily reflected the impact of an increase in
Adjusting Items, in particular costs related to the Pfizer Transaction and in connection
with Separation and Admission. This was partially mitigated by synergies from the
Pfizer Transaction and tight cost control. While the Group decreased advertising and
promotion investment in the Respiratory Health category in response to reduced
demand resulting from the impact of the COVID-19 pandemic and associated
responsive measures (see paragraph 2.14 of this Part VII (Operating and Financial
Review) above), investment was redirected to the VMS and Pain Relief categories.
Adjusted SG&A costs as a percentage of revenue decreased to 37.5 per cent. in FY
2021 from 38.6 per cent. in FY 2020.
SG&A costs for the financial year ended 31 December 2020 compared to the financial
year ended 31 December 2019
The Group’s SG&A costs in FY 2020 were £4,220 million. The Group’s SG&A costs in
FY 2019 (including the SG&A costs of the Pfizer Contributed CH Business from
1 August 2019, when it was consolidated) were £3,596 million. SG&A costs of the
Pfizer Contributed CH Business for the seven months to 31 July 2019 were
£630 million.
174
The Group’s SG&A costs increased by 17.4 per cent. (£624 million), primarily driven by
the inclusion of the full year of SG&A costs of the Pfizer Contributed CH Business in
FY 2020, compared to five months in FY 2019, as well as an increase in Adjusting
Items (as outlined below), partially offset by synergy savings following the Pfizer
Transaction and cost control.
Adjusting Items within SG&A totalled £401 million in FY 2020. These principally related
to further Restructuring costs associated with the Pfizer Transaction, as well as the
initial costs in connection with the Separation and Admission. Adjusting Items within
SG&A costs totalled £244 million in FY 2019. These principally related to Restructuring
costs arising from the Pfizer Transaction.
Adjusted SG&A costs were £3,819 million and £3,352 million in FY 2020 and FY 2019,
respectively. Adjusted SG&A costs increased by 13.9 per cent. (£467 million), primarily
driven by the inclusion of the full year of Adjusted SG&A costs of the Pfizer Contributed
CH Business in FY 2020, compared to five months in FY 2019.
SG&A costs as a percentage of revenue increased to 42.7 per cent. in FY 2020 from
42.4 per cent. in FY 2019. This reflected an increase in Adjusting Items, partially offset
by synergies resulting from the Pfizer Transaction and cost control, in each case as
referred to above. At a category level, the Group increased advertising and promotion
investment in the VMS and Pain Relief Categories. In respect of the latter, this was in
part to support the OTC launch of Voltaren and Advil Dual Action in North America (see
paragraph 2.4 of this Part VII (Operating and Financial Review) above). This increase
in advertising and promotion spend in the VMS and Pain Relief categories was in turn
partially offset by decreased investment in certain brands in the Group’s portfolio where
the COVID-19 pandemic had a temporary negative impact on demand for certain of the
Group’s products. Adjusted SG&A costs as a percentage of revenue decreased to
38.6 per cent. in FY 2020 from 39.5 per cent. in FY 2019.
(E) Research and development (“R&D”)
R&D costs for the financial year ended 31 December 2021 compared to the financial
year ended 31 December 2020
The Group’s R&D costs were £257 million and £304 million in FY 2021 and FY 2020,
respectively. The Group’s R&D costs decreased by 15.5 per cent. (£47 million) in FY
2021, which primarily reflected continuing Pfizer synergies (including in relation to
rationalisation of the site footprint and de-duplication of certain functions) and a
decrease in Adjusting Items, partially offset by costs associated with an increased
focus on innovation at a regional level.
Adjusting Items within R&D costs totalled £9 million in FY 2021. These primarily related
to Net impairment and amortisation of intangible assets. Adjusting Items within R&D
costs totalled £24 million in FY 2020. These principally related to Net amortisation and
impairment of intangible assets, in addition to Restructuring costs associated with the
Pfizer Transaction.
Adjusted R&D costs were £248 million and £280 million in FY 2021 and FY 2020,
respectively. Adjusted R&D costs decreased by 11.4 per cent. (£32 million), primarily
driven by Pfizer synergies, and partially offset by costs associated with an increased
focus on local innovation at a regional level.
175
R&D costs as a percentage of revenue decreased to 2.7 per cent. in FY 2021 from
3.1 per cent. in FY 2020. This reflected the full year benefit of the Pfizer synergies and
reduced Restructuring costs. Adjusted R&D costs as a percentage of revenue
decreased to 2.6 per cent. in FY 2021 from 2.8 per cent. in FY 2020.
R&D costs for the financial year ended 31 December 2020 compared to the financial
year ended 31 December 2019
The Group’s R&D costs in FY 2020 were £304 million. The Group’s R&D costs in FY
2019 (including the R&D costs of the Pfizer Contributed CH Business from 1 August
2019, when it was consolidated) were £292 million. R&D costs of the Pfizer
Contributed CH Business for the seven months to 31 July 2019 were £67 million.
The Group’s R&D costs increased by 4.1 per cent. (£12 million), primarily driven by the
inclusion of the full year of R&D costs of the Pfizer Contributed CH Business in FY
2020, compared to five months in FY 2019, partially offset by headcount reduction and
optimisation of the Group’s R&D operations, which included laboratory closures in
Warren (New Jersey, USA) and Barnard Castle (UK).
Adjusting Items within R&D costs totalled £24 million in FY 2020. These primarily
related to Net amortisation and impairment of intangible assets, in addition to further
Restructuring costs associated with the Pfizer Transaction. Adjusting Items within R&D
costs totalled £25 million in FY 2019. These principally related to Restructuring costs in
connection with the Pfizer Transaction.
Adjusted R&D costs were £280 million and £267 million in FY 2020 and FY 2019,
respectively. Adjusted R&D costs increased by 4.9 per cent. (£13 million), primarily
driven by the inclusion of a full year of Adjusted R&D costs of the Pfizer Contributed
CH Business, partly offset by headcount reduction and optimisation of the Group’s
R&D operations, which included laboratory closures in Warren (New Jersey, USA) and
Barnard Castle (UK).
R&D costs as a percentage of revenue decreased to 3.1 per cent. in FY 2020 from
3.4 per cent. in FY 2019. This reflected headcount reduction and optimisation of the
Group’s R&D operations, as referred to above. Adjusted R&D costs as a percentage of
revenue decreased to 2.8 per cent. in FY 2020 from 3.1 per cent. in FY 2019.
(F) Other operating (expense)/income
Other operating income of £31 million in FY 2021 primarily reflected the net gain on
disposal of Transderm Scop, Acne-Aid, Spectraban and Baldriparan.
Other operating income of £212 million in FY 2020 primarily reflected the net gain on
the sale of a number of brands of the Group, including ThermaCare, Breathe Right,
Physiogel, Coldrex, Venoruton and Oilatum.
Other operating expenses of £17 million in FY 2019 predominantly related to
transaction costs incurred in connection with the disposal of ThermaCare. Transaction
costs in relation to the disposal of certain other businesses and assets of the Group
also contributed to operating expenses in FY 2019.
176
(G) Operating profit and operating profit margin
Operating profit and operating profit margin for the financial year ended 31 December
2021 compared to the financial year ended 31 December 2020
The Group’s operating profit in FY 2021 was £1,638 million, producing an operating
profit margin of 17.2 per cent. The Group’s operating profit in FY 2020 was
£1,598 million, producing an operating profit margin of 16.2 per cent.
The Group’s operating profit increased by 2.5 per cent. (£40 million) in FY 2021,
primarily reflecting organic revenue growth, a reduction in Adjusting Items and tight
cost control, largely offset by the impact of divestments during FY 2021 and FY 2020,
increased advertising and promotion investment, and increased commodity and freight
costs. Operating profit margin increased by 1.0 percentage point, primarily driven by
the benefit of a full year of Pfizer synergies, product price increases, product and
pricing mix and tight cost control, which more than offset the increased advertising and
promotion investment, increased commodity and freight costs, and investment in
manufacturing sites.
Adjusting Items within operating profit totalled £534 million in FY 2021, which
represented costs totalling £565 million in relation to Restructuring costs associated
with the Pfizer Transaction, Separation and Admission costs and Net impairment and
amortisation of intangible assets, offset by income from divestments of £31 million.
Adjusting Items within operating profit totalled £476 million in FY 2020, which
represented £688 million in relation to Transaction-related costs and Restructuring
costs associated with the Pfizer Transaction and Separation and Admission costs, as
well as Net impairment and amortisation of intangible assets, partially offset by income
from divestments of £212 million.
Adjusted operating profit was £2,172 million and £2,074 million in FY 2021 and FY
2020, respectively. Adjusted operating profit increased by 4.7 per cent. (£98 million),
primarily reflecting organic revenue growth, reductions in SG&A costs and R&D costs
resulting from synergies resulting from the Pfizer Transaction, increased gross profit
margins and tight cost control, partially offset by increased advertising and promotion
investment and increased commodity and freight costs.
The Group’s Adjusted operating profit margin was 22.8 per cent. and 21.0 per cent. in
FY 2021 and FY 2020, respectively. Adjusted operating profit margin increased by 1.8
percentage points. The principal factors affecting Adjusted operating profit margin were
the same as for operating profit margin.
Operating profit and operating profit margin for the financial year ended 31 December
2020 compared to the financial year ended 31 December 2019
The Group’s operating profit in FY 2020 was £1,598 million, producing an operating
profit margin of 16.2 per cent. The Group’s operating profit for FY 2019 (including the
operating profit of the Pfizer Contributed CH Business from 1 August 2019, when it was
consolidated) was £897 million, producing an operating profit margin of 10.6 per cent.
Operating profit of the Pfizer Contributed CH Business for the seven months to 31 July
2019 was £257 million, with an operating profit margin of 16.8 per cent.
177
The Group’s operating profit increased by 78.1 per cent. (£701 million), primarily driven
by the inclusion of the full year of operating profit of brands acquired as part of the
Pfizer Transaction in FY 2020, compared to five months of operating profit in FY 2019,
together with related synergies, a net reduction in Adjusting Items in relation to cost of
sales, R&D and SG&A as described above (mainly related to the Pfizer Transaction)
and other operating income from the sale of brands. Operating profit margin increased
by 5.6 percentage points from FY 2019 to FY 2020. This increase was primarily driven
by synergies resulting from the Pfizer Transaction, as well as business growth and
product pricing and mix, partially offset by the full year impact of the brands acquired
as part of the Pfizer Transaction (albeit reduced as a result of cost controls
implemented by the Group), the impact of divestitures and additional supply chain
costs. Operating profit margin was also affected by changes in Adjusting Items.
Adjusting Items within operating profit totalled £476 million in FY 2020, which
represented Transaction costs, Restructuring costs associated with the Pfizer
Transaction and Separation and Admission costs, as well as Net impairment and
amortisation of intangible assets, partially offset by income from divestments of
£212 million. Adjusting Items within operating profit totalled £757 million in FY 2019,
which mainly reflected costs totalling £696 million in relation to Restructuring costs and
Transaction-related costs associated with the Pfizer Transaction.
Adjusted operating profit was £2,074 million and £1,654 million in FY 2020 and FY
2019, respectively. Adjusted operating profit increased by 25.4 per cent. (£420 million).
As above, this principally reflected the inclusion of the full year of operating profit of
brands acquired as part of the Pfizer Transaction in FY 2020, compared to five months
of operating profit in FY 2019, together with related synergies, combined with business
growth and tight cost control, partially offset by the impact of divestments. The
Adjusting Items in respect of operating profit are described in paragraphs 6.1(B) to
6.1(E) of this Part VII (Operating and Financial Review) above.
The Group’s Adjusted operating profit margin was 21.0 per cent. and 19.5 per cent. in
FY 2020 and FY 2019, respectively. Adjusted operating profit margin increased by 1.5
percentage points. The factors affecting Adjusted operating profit margin were the
same as for operating profit margin, except in respect of changes in Adjusting Items,
which are excluded from Adjusted operating profit margin.
(H) Net finance costs
Net finance costs for the financial year ended 31 December 2021 compared to the
financial year ended 31 December 2020
Net finance costs on both an IFRS and Adjusted basis reduced to £2 million in FY 2021
from £7 million in FY 2020, primarily due to a decrease in payable balances with
finance entities in the GSK Group and other loans.
There were no Adjusting Items that affected Net finance costs in FY 2021 and FY
2020.
Net finance costs for the financial year ended 31 December 2020 compared to the
financial year ended 31 December 2019
Net finance costs on both an IFRS and Adjusted basis reduced to £7 million in FY 2020
from £11 million in FY 2019, primarily attributable to the revaluation of derivatives and
financial instruments.
178
There were no Adjusting Items that affected Net finance costs in FY 2020 and FY
2019.
(I) Profit before tax
Profit before tax for the financial year ended 31 December 2021 compared to the
financial year ended 31 December 2020
Taking into account net finance costs, profit before tax was £1,636 million in FY 2021,
increasing by £45 million from £1,591 million in FY 2020. This reflected increased
operating profit during the period, as described above.
Adjusted profit before tax was £2,170 million in FY 2021, increasing by £103 million
from £2,067 million in FY 2020. This reflected increased Adjusted operating profit
during the period, as described above.
Profit before tax for the financial year ended 31 December 2020 compared to the
financial year ended 31 December 2019
Taking into account net finance costs, profit before tax was £1,591 million in FY 2020,
increasing by £705 million from £886 million in FY 2019. This reflected increased
operating profit during the period, as described above.
Adjusted profit before tax was £2,067 million in FY 2020, increasing by £424 million
from £1,643 million in FY 2019. This reflected increased Adjusted operating profit
during the period, as described above.
(J) Income tax and effective tax rate
In FY 2021, the corporate tax charge was £197 million on profit before tax of
£1,636 million. The IFRS effective tax rate was 12.0 per cent, which reflected the
impacts of the applicable tax treatment on Adjusting Items. Permanent differences on
disposals, acquisitions and transfers including tax credits relating to an uplift in the tax
basis of certain brands transferred intragroup resulted in a reduction of the IFRS
effective rate. The Adjusted effective tax rate of 21.6 per cent. was higher than the UK
statutory rate of 19.0 per cent. due to profit generated in jurisdictions with higher tax
rates (such as the USA and China), tax losses not recognised, and changes in tax
rates in certain jurisdictions, partially offset by the benefits of tax rulings in territories
such as Switzerland and Puerto Rico, the availability of R&D credit, re-assessments of
prior year estimates and other permanent differences.
In FY 2020, the corporate tax charge was £410 million on profit before tax of
£1,591 million. The IFRS effective tax rate was 25.8 per cent, which reflected the
impacts of the applicable tax treatment on Adjusting Items, and was also adversely
impacted by revaluing the rates applicable to various deferred tax balances. The
Adjusted effective tax rate of 23.4 per cent. was higher than the UK statutory rate of
19.0 per cent. due to profit generated in jurisdictions with higher tax rates (such as the
USA and China), partially offset by the benefits of tax rulings in territories such as
Switzerland and Puerto Rico, the availability of R&D credit, re-assessment of prior year
estimates and other permanent differences.
179
In FY 2019, the corporate tax charge was £199 million on profit before tax of
£886 million. The IFRS effective tax rate was 22.5 per cent., which reflected the
impacts of the applicable tax treatment on Adjusting Items. The Adjusted effective tax
rate of 22.2 per cent. was higher than the UK statutory rate of 19.0 per cent. due to
profit generated in jurisdictions with higher tax rates (such as the USA, Italy and
China), partially offset by the benefits of tax rulings in territories such as Switzerland
and Puerto Rico, the availability of R&D credits and other non-taxable items.
(K) Profit after tax
Profit after tax for the financial year ended 31 December 2021 compared to the
financial year ended 31 December 2020
Profit after tax was £1,439 million in FY 2021, increasing by £258 million from
£1,181 million in FY 2020. This reflected the reduced effective tax rate and tax charge
in FY 2021, as well as increased operating profit during the period, as described
above.
Adjusted profit after tax was £1,701 million in FY 2021, increasing by £117 million from
£1,584 million in FY 2020. This reflected the reduced effective tax rate and tax charge
in FY 2021, as well as increased Adjusted operating profit during the period, as
described above.
Profit after tax for the financial year ended 31 December 2020 compared to the
financial year ended 31 December 2019
Profit after tax was £1,181 million in FY 2020, increasing by £494 million from
£687 million in FY 2019. This reflected increased operating profit during the period, as
described above, partially offset by an increased effective tax rate and tax charge in FY
2020.
Adjusted profit after tax was £1,584 million in FY 2020, increasing by £306 million from
£1,278 million in FY 2019. This reflected increased Adjusted operating profit during the
period, as described above, partially offset by the increased Adjusted effective tax rate
and tax charge in FY 2020.
(L) Profit attributable to non-controlling interests
Profit attributable to non-controlling interests for the financial year ended 31 December
2021 compared to the financial year ended 31 December 2020
Profit attributable to non-controlling interests in FY 2021 was £49 million, increasing by
£13 million from £36 million in FY 2020. Adjusted profit attributable to non-controlling
interests in FY 2021 was £49 million, increasing by £11 million from £38 million in FY
2020. These increases reflect reduced Adjusted effective tax rate and tax charge in FY
2021, in addition to higher Adjusted operating profit during the period, as described
above.
There were no Adjusting Items that affected non-controlling interests in FY 2021, as
compared to £2 million in FY 2020 (as described below).
180
Profit attributable to non-controlling interests for the financial year ended 31 December
2020 compared to the financial year ended 31 December 2019
Profit attributable to non-controlling interests in FY 2020 was £36 million, increasing by
£4 million from £32 million in FY 2019. Adjusted profit attributable to non-controlling
interests in FY 2020 was £38 million, increasing by £6 million from £32 million in FY
2019. These increases reflect higher Adjusted operating profit during the period, as
described above, partially offset by the increased Adjusted effective tax rate and tax
charge in FY 2020.
Adjusting Items attributable to non-controlling interests totalled £2 million in FY 2020.
These principally related to costs incurred in relation to restructuring programmes in
respect of certain Group subsidiaries with non-controlling interests.
(M) Earnings per share (“EPS”)
Earnings per share for the financial year ended 31 December 2021 compared to the
financial year ended 31 December 2020
In FY 2021, Basic EPS and Diluted EPS were both 139,000p. On an Adjusted basis,
these were 165,200p. In FY 2020, Basic EPS and Diluted EPS were both 114,500p.
On an Adjusted basis, these were 154,600p. The year-on-year increase reflected
increased profit after tax during the period, as described above.
The number of shares in issue used to calculate these amounts may not be
representative of the number of shares in issue in the future.
Earnings per share for the financial year ended 31 December 2020 compared to the
financial year ended 31 December 2019
In FY 2020, Basic EPS and Diluted EPS were both 114,500p. On an Adjusted basis,
these were 154,600p. In FY 2019, Basic EPS and Diluted EPS were both 65,500p. On
an Adjusted basis, these were 124,600p. The year-on-year increase reflected
increased profit after tax during the period, as described above.
The number of shares in issue used to calculate these amounts may not be
representative of the number of shares in issue in the future.
(N) Adjusted EBITDA
Adjusted EBITDA for the financial year ended 31 December 2021 compared to the
financial year ended 31 December 2020
The Group’s Adjusted EBITDA was £2,413 million and £2,351 million in FY 2021 and
FY 2020 respectively.
The Group’s Adjusted EBITDA increased by 2.6 per cent. (£62 million), primarily driven
by the increase in the Adjusted operating profit during the period, as described above,
partially offset by a net decrease in depreciation and amortisation.
181
Adjusted EBITDA for the financial year ended 31 December 2020 compared to the
financial year ended 31 December 2019
The Group’s Adjusted EBITDA was £2,351 million in FY 2020. The Group’s Adjusted
EBITDA in FY 2019 (including the Adjusted EBITDA of the Pfizer Contributed CH
Business from 1 August 2019, when it was consolidated) was £1,884 million.
The Group’s Adjusted EBITDA increased by 24.8 per cent. (£467 million), primarily
driven by the inclusion of the full year of profit of the Pfizer Contributed CH Business in
FY 2020, compared to five months in FY 2019. The remaining growth largely resulted
from synergies arising from the Pfizer Transaction and an increase in depreciation and
amortisation, partially offset by increased supply chain costs arising as a result of the
COVID-19 pandemic.
6.2 Regional performance
(A) Regional performance for the financial year ended 31 December 2021 compared to
31 December 2020
(i) North America
(a) Revenue
The Group’s revenue attributable to North America was £3,525 million and
£3,779 million in FY 2021 and FY 2020, respectively.
The Group’s revenue declined 6.7 per cent. at AER, and 1.3 per cent. at CER.
Organic revenue growth in North America was 1.3 per cent. for the period FY
2020 to FY 2021. This principally reflected growth across the Oral Health, Pain
Relief and Digestive Health categories, partially offset by a decline in revenue
in the VMS and Respiratory Health categories.
The decline in revenue at AER and CER was primarily driven by the full year
revenue impact of divestments made in FY 2020, principally the divestment of
Breathe Right, ThermaCare, Dimetapp and Anbesol. This was compounded by
a further decline in Respiratory Health revenue as compared to FY 2020,
driven by an exceptionally low incidence of cold and flu in the first half of FY
2021 (see paragraph 2.14 of this Part VII (Operating and Financial Review)
above). Revenue at AER was further impacted by adverse currency exchange
movements of £204 million as Pounds Sterling strengthened against the
US Dollar. These negative trends were partially offset by continued growth in
Sensodyne, Tums, Centrum and Flonase revenue and recovery in Excedrin
revenue following supply chain disruption in FY 2020.
The decline in revenue at CER was attributable to several factors across the
categories in which the Group operates:
• Mid-single digit per cent. revenue growth at CER in the Oral Health
category was primarily driven by Sensodyne, which reflected the strength
of the brand in the USA, continued innovation and product price increases.
182
Increased demand for parodontax also contributed to growth in Oral
Health. Revenue in respect of brands in the Denture Care sub-category
declined due to increased competition.
• There was a low-single digit per cent. revenue decline at CER in the VMS
category driven by reduced demand for Emergen-C, partially offset by
growth in Centrum. Emergen-C revenue decreased, relative to a
particularly strong comparator in FY 2020, when Emergen-C experienced a
surge in demand associated with the COVID-19 pandemic. The continued
growth in Centrum revenue reflected the continued consumer trend
towards self-management of health and wellbeing, as well as successful
innovation and improved supply capacity in the US.
• Mid-single digit per cent. revenue growth at CER in the Pain Relief
category was mainly driven by a recovery in Excedrin following supply
chain interruption in FY 2020. This was partially offset by revenue decline
in Advil due to the impact of temporary disruption in third party supply.
Voltaren revenue declined due to increased competition from “private-
label” Diclofenac products, which was in line with expectations (see
paragraph 2.4 of this Part VII (Operating and Financial Review)).
• In the Respiratory Health category, a mid-teens per cent. revenue decline
at CER was mainly attributable to a decline in Robitussin and Theraflu
revenue, driven by exceptionally low cold and flu incidence, together with
the full year revenue impact of the divestment of Breathe Right and other
cold and flu brands in FY 2020. This was partially offset by increased
demand for Flonase, driven by a stronger allergy season relative to the
prior year comparator.
• In Digestive Health and Other, the low-single digit per cent. revenue
decline at CER was primarily attributable to the full year impact of the
divestments of ThermaCare, Dimetapp and Anbesol in FY 2020, as well as
a decline in Preparation H revenue, in part due to a temporary supply chain
disruption. Chapstick revenue started to recover after being negatively
impacted by fewer consumer visits to stores in FY 2020 during the earlier
stages of the COVID-19 pandemic. Revenue attributable to Tums
continued to grow, driven by an increase in the size of the Antacids market.
(b) Adjusted operating profit
Adjusted operating profit for the North America region in FY 2021 was
£828 million, producing an Adjusted operating profit margin of 23.5 per cent.
Adjusted operating profit for the North America region in FY 2020 was
£897 million, producing an Adjusted operating profit margin of 23.7 per cent.
The year-on-year decrease in Adjusted operating profit margin of 0.2
percentage points reflected a number of factors, including investment in brands
in the VMS and Pain Relief categories and inflationary pressures (such
increased commodity prices, supply chain costs and payroll), partially offset by:
• synergies resulting from the Pfizer Transaction;
• continued lower levels of travel, meeting and other expenses due to
the COVID-19 pandemic; and
183
• net savings in advertising and promotion spend as a percentage of
revenue, resulting from the continuation of cost containment measures
in respect of the Digestive Health and Other and Respiratory Health
categories and divested brands.
(ii) EMEA and LatAm
(a) Revenue
The Group’s revenue attributable to EMEA and LatAm was £3,877 million and
£4,059 million in FY 2021 and FY 2020, respectively.
In FY 2021, the Group’s revenue attributable to EMEA and LatAm decreased
by 4.5 per cent. at AER, whilst revenue growth at CER was flat.
Organic revenue growth in EMEA and LatAm was 3.5 per cent. This was
principally driven by growth across the Pain Relief and Digestive Health
categories, combined with lower revenue growth in the Oral Health, VMS and
Respiratory Health categories.
The decline in revenue at AER and flat growth in revenue at CER were largely
a result of the full year revenue impact of divestments made during FY 2020,
including Physiogel, Breathe Right, ThermaCare, Venoruton and Coldrex,
partially offset by growth in a number of brands, including Sensodyne,
parodontax, Voltaren, Panadol and Centrum. Revenue at AER was negatively
impacted by adverse currency exchange movements of £183 million as
Pounds Sterling strengthened, primarily against the Brazilian Real, Turkish
Lira, Russian Ruble, Argentine Peso and South African Rand.
The EMEA and LatAm region delivered flat revenue at CER, which was
attributable to several factors across the categories in which the Group
operates:
• Single digit per cent. revenue growth at CER in the Oral Health category
was driven by an increase in revenue attributable to Sensodyne,
parodontax and, to a lesser extent, the Denture Care sub-category.
Sensodyne revenue growth reflected price increases, as well as new
product launches. Growth was partially offset by a decline in other brands
in the Oral Health category.
• Revenue growth at CER in the VMS category was flat. There were
increases in Centrum and Calsource revenue, which were offset by
declines in revenue of smaller brands and the impact of divestments.
• Mid-single digit per cent. revenue growth at CER in the Pain Relief
category was primarily driven by growth in Panadol revenue, which
reflected the strength of the brand and increased demand for paracetamol
during the COVID-19 pandemic. Voltaren also benefited from strong
revenue growth, which reflected price rises in key markets and a
combination of innovation and promotional activity.
184
• A mid-single digit per cent. revenue decline at CER in the Respiratory
Health category was largely attributable to the full year revenue impact of
divestments made in FY 2020, including Breathe Right and Coldrex. In
addition, there was an exceptionally weak cold and flu season. However,
revenue for the category recovered in the second half of the year (see
paragraph 2.14 of this Part VII (Operating and Financial Review) above).
• A high-single digit per cent. revenue decline at CER in Digestive Health
and Other revenue was primarily a result of the full year revenue impact of
a number of divestments made during FY 2020, (including ThermaCare,
Physiogel and Venoruton) and the impact of the divestment of brands in FY
2021 (including Transderm Scop and Baldriparan), in addition to a
decrease in third party contract manufacturing sales related to previous
divestments. This decline was partially offset by an increase in revenue
attributable to smaller brands.
(b) Adjusted operating profit
Adjusted operating profit for EMEA and LatAm in FY 2021 was £960 million,
producing an Adjusted operating profit margin of 24.8 per cent. Adjusted
operating profit for EMEA and LatAm in FY 2020 was £857 million, with an
Adjusted operating profit margin of 21.1 per cent. The increase in Adjusted
operating profit margin of 3.7 per cent. reflected the following factors:
• the further benefit of synergies resulting from the Pfizer Transaction
and tight cost control; and
• a favourable product mix and net revenue management initiatives,
together with manufacturing efficiencies.
The increase in Adjusted operating profit margin was partially offset by
increased advertising and promotion spend to drive greater demand in a
number of markets in the region.
(iii) APAC
(a) Revenue
The Group’s revenue attributable to APAC in FY 2021 and FY 2020 was
£2,143 million and £2,054 million, respectively.
In FY 2021, the Group’s revenue attributable to APAC increased by 4.3 per
cent. at AER, and 7.1 per cent. at CER.
Organic revenue growth in APAC was 9.1 per cent. in the period FY 2020 to
FY 2021. This principally reflected growth across the Pain Relief and VMS
categories, combined with lower revenue growth in the Oral Health, Digestive
Health and Other and Respiratory Health categories.
Growth in revenue at AER and CER was primarily driven by growth in revenue
attributable to the VMS, Oral Health and Pain Relief categories. Revenue
185
growth at AER was affected by adverse foreign exchange movements of
£56 million as Pound Sterling strengthened against the Japanese Yen, Indian
Rupee, Philippine Peso and certain other currencies in the region.
Revenue growth at CER was attributable to several factors across the
categories in which the Group operates:
• A single digit per cent. revenue growth at CER in the Oral Health category
was primarily due to growth in Sensodyne driven by India, China and
Japan, combined with revenue growth of parodontax, as well as growth in
Denture Care, where reduced social occasions impacted demand.
• In the VMS category, low mid-teens per cent. revenue growth at CER was
primarily attributable to growth in Caltrate and Centrum, supported by
campaigns focused on educating consumers about their immune systems.
• In the Pain Relief category, low-teens per cent. revenue growth at CER
was principally a result of revenue growth at CER in Panadol, which
benefited from increased demand associated with COVID-19 vaccination
campaigns in South East Asia and Taiwan and Australia, and revenue
growth at CER in Voltaren, driven by distribution expansion in China and
Australia and new product launches in India. Fenbid sales in China were
flat, due to the continuation of a temporary ban on the sale of fever
medicine in parts of the country during the COVID-19 pandemic.
• A low-single digit per cent. revenue decline at CER in the Respiratory
Health category was primarily due to the full year impact of the disposal of
Breathe Right and a decline in Theraflu revenue, driven by low cold and flu
incidence. Contac revenue was adversely impacted by the continuation of
specific bans on the over-the-counter sale of cough and cold medicines in
China.
• A low-single digit per cent. revenue decline at CER in the Digestive Health
and Other category was largely driven by the full year revenue impact of
the divestment of Physiogel in FY 2020 and the divestment of Acne Aid in
FY 2021. This decline was partially offset by growth in ENO revenue,
mainly as a result of growth in India.
(b) Adjusted operating profit
Adjusted operating profit for the APAC region in FY 2021 was £461 million,
producing an Adjusted operating profit margin of 21.5 per cent. Adjusted
operating profit for the APAC region in FY 2020 was £377 million, producing an
Adjusted operating profit margin of 18.4 per cent.
The increase in the Adjusted operating profit margin of 3.1 percentage points
principally reflected synergies arising from the Pfizer Transaction, disciplined
overhead cost control and other operational efficiencies within manufacturing.
This was partially offset by higher advertising and promotion investment as a
percentage of revenue, reflecting the Group’s launch of targeted public
186
campaigns (for example, to educate consumers about their immune systems),
increased digital advertising to drive growth in Sensodyne and Voltaren, and
increased freight costs resulting from the COVID-19 pandemic.
(B) Regional performance for the financial year ended 31 December 2020 compared to
31 December 2019
(i) North America
(a) Revenue
The Group’s revenue attributable to North America in FY 2020 was
£3,779 million. The Group’s revenue attributable to North America in FY 2019
(including the revenue of the Pfizer Contributed CH Business attributable to
North America from 1 August 2019, when it was consolidated) was
£2,880 million. Revenue attributable to North America of the Pfizer Contributed
CH Business for the seven months to 31 July 2019 was £845 million.
The Group’s revenue grew 31.2 per cent. at AER and 32.6 per cent. at CER.
Organic revenue growth in the North America region was 0.7 per cent. for the
period FY 2019 to FY 2020. This principally reflected growth in organic revenue
across the VMS, Pain Relief and Oral Health categories. This was partly offset
by a decline in revenue in the Respiratory Health category, due to the impact of
the exceptionally low cold and flu incidence, and in the Digestive Health and
Other category, including in respect of the brand Transderm Scop, which
experienced revenue decline as a result of generic competition. Transderm
Scop was subsequently disposed of during FY 2021. Furthermore, organic
revenue growth for the period FY 2019 to FY 2020 excludes revenue
attributable to brands acquired as part of the Pfizer Transaction for the seven
months to 31 July 2020, during which these brands experienced a strong
positive revenue impact from consumer stockpiling and increased consumption
as a result of the COVID-19 pandemic. Accordingly, the overall growth of the
North America region in the period FY 2019 to FY 2020 was reduced when
measured on an organic basis.
Growth in revenue at AER and CER was primarily driven by the inclusion of the
full year revenue of brands acquired as part of the Pfizer Transaction (including
Advil, Centrum and Emergen-C) in FY 2020, compared to five months in FY
2019, together with growth in revenue attributable to Sensodyne and Voltaren,
partially offset by a decline in Excedrin revenue and a number of divestments,
including the divestment of Breathe Right. Revenue growth at AER was further
offset by adverse currency exchange movements of £42 million as Pounds
Sterling strengthened against the US Dollar.
The North America region delivered revenue growth at CER, which was
attributable to a number of factors across the categories in which the Group
operates:
• High-single digit per cent. revenue growth at CER in the Oral Health
category was primarily driven by Sensodyne and parodontax, while
187
revenue in respect of brands in the Denture Care sub-category of Oral
Health and Biotène remained broadly stable. Growth in Sensodyne was
reflective of the strength of the brand in the USA, as well as a number of
launches, including Sensodyne Sensitivity and Gum and Sensodyne
Pronamel Intensive Enamel Repair.
• Significant triple digit per cent. revenue growth at CER in the VMS category
was primarily attributable to the inclusion of the full year revenue of brands
acquired as part of the Pfizer Transaction (including Emergen-C and
Centrum), together with a growing consumer trend towards self-
management of health and wellbeing.
• High double digit per cent. revenue growth at CER in the Pain Relief
category was mainly driven by the Rx-to-OTC switch of Voltaren, together
with the inclusion of the full year of revenue of Advil, which was acquired
as part of the Pfizer Transaction. This was partially offset by temporary
disruption to Excedrin supply.
• In the Respiratory Health category, mid-teens per cent. revenue growth at
CER was mainly attributable to the inclusion of the full year revenue of
brands acquired as part of the Pfizer Transaction (including Robitussin).
Revenue in this category was however adversely impacted by the
exceptionally low cold and flu incidence (see paragraph 2.14 of this Part VII
(Operating and Financial Review) above).
• Similarly, in Digestive Health and Other, mid-teens per cent. revenue
growth at CER was primarily attributable to the inclusion of the full year
revenue of brands acquired as part of the Pfizer Transaction (including
Chapstick and Preparation H). Abreva revenue declined due to the launch
of a number of private-label brands in the USA, as well as fewer consumer
visits to stores following the implementation of lockdowns during the
COVID-19 pandemic. Chapstick revenue was also negatively impacted by
fewer consumer visits to stores during the COVID-19 pandemic.
(b) Adjusted operating profit
Adjusted operating profit for the North America region in FY 2020 was
£897 million, producing an Adjusted operating profit margin of 23.7 per cent.
Adjusted operating profit for the North America region in FY 2019 (including the
operating profit of the Pfizer Contributed CH Business attributable to North
America from 1 August 2019, when it was consolidated) was £660 million,
producing an Adjusted operating profit margin of 22.9 per cent.
The year-on-year change in Adjusted operating profit margin of 0.8 percentage
points reflected a number of factors, including:
• synergies resulting from the Pfizer Transaction, principally in relation to
reductions in SG&A costs as a result of headcount reductions; and
• net savings in advertising and promotion spend as a percentage of
revenue, resulting from cost containment measures taken in respect of the
188
Digestive Health and Other and Respiratory Health categories and
divested brands, partially offset by investment in the VMS category and
Advil and Voltaren in the Pain Relief category.
(ii) EMEA and LatAm
(a) Revenue
The Group’s revenue attributable to EMEA and LatAm was £4,059 million in FY
2020. The Group’s revenue attributable to EMEA and LatAm in FY 2019
(including the revenue of the Pfizer Contributed CH Business attributable to
EMEA and LatAm from 1 August 2019, when it was consolidated) was
£3,898 million. Revenue attributable to EMEA and LatAm from the Pfizer
Contributed CH Business for the seven months to 31 July 2019 was
£362 million.
In FY 2020, the Group’s revenue attributable to EMEA and LatAm increased by
4.1 per cent. at AER, and 8.4 per cent. at CER. Organic revenue growth in
EMEA and LatAm was 3.1 per cent. for the period FY 2019 to FY 2020.
Growth at AER and CER was primarily driven by the inclusion of the full year of
revenue in FY 2020 of brands acquired as part of the Pfizer Transaction,
compared to five months in FY 2019, together with growth in Sensodyne,
parodontax and Panadol revenue, partially offset by declines in Fenistil and
Otrivin revenue. Revenue at AER was negatively impacted by adverse
currency exchange movements of £166 million primarily due to Pounds Sterling
strengthening against the Brazilian Real, Russian Ruble, Argentine Peso and
South African Rand.
Organic revenue growth principally reflected double-digit per cent. growth in
the VMS category, together with growth across the Pain Relief and Oral Health
categories, partially offset by decline in organic revenue in the Respiratory
Health and Digestive Health and Other categories.
The EMEA and LatAm region delivered revenue growth at CER, which
reflected a number of factors across the categories in which the Group
operates:
• Mid-single digit per cent. revenue growth at CER in the Oral Health
category was driven by an increase in revenue attributable to Sensodyne
and parodontax.
• Low triple digit per cent. revenue growth at CER in the VMS category was
primarily attributable to the inclusion of the full year of revenue of brands
acquired as part of the Pfizer Transaction (including Centrum), together
with an increasing consumer trend towards self-management of health and
wellbeing.
• Low-teens per cent. revenue growth at CER in the Pain Relief category
was primarily driven by Panadol, reflecting the strength of the brand and
increased demand for paracetamol during the COVID-19 pandemic.
Voltaren revenue remained broadly flat.
189
• A mid-single digit per cent. revenue decline at CER in the Respiratory
Health category was attributable to a reduction in respiratory illnesses such
as cold and flu as a result of measures implemented in response to the
COVID-19 pandemic. This negatively impacted Otrivin revenue. Theraflu
remained broadly stable.
• There was a mid-single digit per cent. revenue decline at CER in the
Digestive Health and Other category, with a reduction in Fenistil and
brands in the Smokers’ Health sub-category of Digestive Health and Other,
largely stemming from the COVID-19 pandemic. This was partially offset by
growth in ENO, driven by the Group’s growth strategy in Brazil and
increased consumption in the Middle East and Africa.
(b) Adjusted operating profit
Adjusted operating profit for EMEA and LatAm in FY 2020 was £857 million,
producing an Adjusted operating profit margin of 21.1 per cent. Adjusted
operating profit for EMEA and LatAm in FY 2019 (including the Adjusted
operating profit of the Pfizer Contributed CH Business attributable to EMEA
and LatAm from 1 August 2019, when it was consolidated) was £746 million,
with an Adjusted operating profit margin of 19.1 per cent.
The increase in Adjusted operating profit margin of 2.0 percentage points
reflected the following factors:
• synergies resulting from the Pfizer Transaction, principally in relation to
reductions in SG&A costs as a result of headcount reductions; and
• disciplined resource allocation in advertising and promotion spend as a
percentage of revenue and net revenue management initiatives.
(iii) APAC
(a) Revenue
The Group’s revenue attributable to APAC in FY 2020 was £2,054 million. The
Group’s revenue attributable to APAC in FY 2019 (including the revenue of the
Pfizer Contributed CH Business attributable to APAC from 1 August 2019,
when it was consolidated) was £1,702 million. The revenue attributable to
APAC from the Pfizer Contributed CH Business for the seven months to
31 July 2019 was £316 million.
The Group’s revenue attributable to APAC increased by 20.7 per cent. at AER
and 21.8 per cent. at CER. Organic revenue growth in APAC was 5.7 per cent.
in the period FY 2019 to FY 2020.
Growth in revenue at AER and CER was primarily driven by the inclusion of the
full year of revenue of the Pfizer Contributed CH Business in FY 2020,
compared to five months in FY 2019, particularly in the VMS category, together
with growth in revenue attributable to Sensodyne and Voltaren. Revenue
growth at AER was further affected by adverse foreign exchange movements
of £19 million as Pounds Sterling strengthened against Japanese Yen, Taiwan
Dollar and Philippine Peso and certain other currencies in the region.
190
Growth in organic revenue principally reflected strong, mid-thirties per cent.
growth in the VMS category, together with low-single digit per cent. revenue
growth in the Pain Relief category and high-single digit per cent. revenue
growth in the Oral Health category, partially offset by a single digit per cent.
revenue decline in the Digestive Health and Other categories and a double
digit per cent. revenue decline in the Respiratory Health category. The latter
reflected the historically weak cold and flu season and government restrictions
in response to the COVID-19 pandemic.
Revenue growth at CER was attributable to a number of factors across the
categories in which the Group operates:
• High-single digit per cent. revenue growth at CER in the Oral Health
category was primarily driven by Sensodyne, while revenue in respect of
brands in the Denture Care sub-category of Oral Health remained broadly
stable. Sensodyne growth was principally driven in China, Japan, Australia
and India.
• Low triple digit per cent. revenue growth at CER in the VMS category was
primarily attributable to the inclusion of the full year of revenue of brands
acquired as part of the Pfizer Transaction (including Centrum), together
with an increasing consumer trend towards self-management of health and
wellbeing. Growth in Centrum was driven by China, the Philippines, Taiwan
and Korea, where in FY 2020 the Group launched a public awareness
campaign with the purpose of educating consumers about their immune
systems. Caltrate revenue was driven by increased penetration in the
online and retail channels.
• Mid-single digit per cent. revenue growth at CER in the Pain Relief
category was driven by strong growth in Voltaren, supported by price
increases, together with product launches in India, partially offset by a
reduction in Fenbid sales in China as certain local authorities introduced
temporary restrictions on the sale of cough and cold medicines during the
COVID-19 pandemic.
• A low double digit per cent. revenue decline at CER in the Digestive Health
and Other category reflected a decline in Zentel and Physiogel, which was
divested part way through FY20, partially offset by growth in Bactroban and
ENO.
• A low double digit per cent. revenue decline at CER in the Respiratory
Health category was due to lower instances of respiratory illnesses as a
result of the implementation of measures in response to the COVID-19
pandemic. Contac and Robitussin were also impacted by COVID-19
related temporary restrictions on the sale of cough and cold medicines in
certain parts of China.
(b) Adjusted operating profit
Adjusted operating profit for the APAC region in FY 2020 was £377 million,
producing an Adjusted operating profit margin of 18.4 per cent. Adjusted
191
operating profit for the APAC region in FY 2019 (including the Adjusted
operating profit of the Pfizer Contributed CH Business attributable to APAC
from 1 August 2019, when it was consolidated) was £311 million, producing an
Adjusted operating profit margin of 18.3 per cent.
The increase in the Adjusted operating profit margin of 0.1 percentage points
reflected synergies resulting from the Pfizer Transaction, principally in relation
to reductions in SG&A costs as a result of headcount reductions and higher
gross margin due to product mix, supply chain efficiencies and tight cost
control. The increase was partially offset by greater advertising and promotion
investment as a percent of revenue, reflecting targeted public campaigns
launched by the Group to educate consumers about their immune systems,
increased digital advertising to drive growth in Sensodyne and Voltaren, and
increased advertising in relation to the launch of new products.
6.3 Adjusting Items
Adjusting Items for the financial year ended 31 December 2021 compared to the financial year
ended 31 December 2020
Net intangible amortisation and impairment charges (pre-tax) decreased to £16 million
(£24 million net of tax) in FY 2021 from £97 million (£78 million net of tax) in FY 2020. This
reflected decreased impairment charges on indefinite and definite life brands, which reduced to
£12 million in FY 2021 from £45 million in FY 2020, in addition to a smaller decrease in
amortisation of definite life brands to £40 million in FY 2021 from £50 million in FY 2020,
partially offset by an increase in the reversal of impairments of definite life brands to £36 million
in FY 2021 from £18 million in FY 2020. In FY 2020, the impairment charge mainly included
impairments of Zyrtec, capitalised costs for a discontinued oral care project and a discontinued
pain relief device and the reversal of impairments related to Transderm Scop.
Restructuring costs (pre-tax) decreased to £195 million (£159 million net of tax) in FY 2021
from £411 million (£321 million net of tax in FY 2020). This reflected the reduction in integration
costs related to the Pfizer Transaction.
There were no Transaction-related costs (pre-tax) FY 2021, compared to £91 million
(£71 million net of tax) in FY 2020. This was due to completion of the fair value unwind on
inventory acquired as part of the Pfizer Transaction that took place during FY 2019 and FY
2020.
Separation and Admission costs (pre-tax) increased to £278 million (£231 million net of tax) in
FY 2021 from £66 million (£53 million net of tax) in FY 2020. These costs in FY 2021 mainly
consisted of £257 million of costs in connection with Separation and £19 million of costs in
connection with Admission, which reflected an increase in operational separation activity,
compared with £66 million of costs in connection with Separation in FY 2020. The £191 million
year-on-year increase in Separation costs reflected an increase in operational separation
activity ahead of Separation and Admission.
Disposals and others (pre-tax) resulted in net expense of £45 million (£152 million net of tax) in
FY 2021, compared to net income of £189 million (£120 million net of tax) in FY 2020. In FY
2021, permanent differences on disposals, acquisitions and transfers including tax credits
relating to an uplift in the tax basis of certain brands transferred intragroup resulted in a
192
reduction in the corporate tax charge of £164 million in the year. Additionally, this included
£60 million of historical adjustments, mainly relating to the write-off of expired tax indemnities,
£14 million of loss on the disposal of Transderm Scop and Scopoderm and £16 million relating
to a tax indemnity payment to the Pfizer Group. These were partially offset by £42 million of
profit on the disposal of a number of brands and other credits of £4 million.
Adjusting Items for the financial year ended 31 December 2020 compared to the financial year
ended 31 December 2019
Net intangible amortisation and impairment charges (pre-tax) increased to £97 million
(£78 million net of tax) in FY 2020 from £36 million (£31 million net of tax) in FY 2019. This
primarily reflected increased impairment charges on indefinite and definite life brands, which
grew to £45 million in FY 2020 from £19 million in FY 2019, in addition to an increase in
amortisation of definite life brands to £50 million in FY 2020 from £27 million in FY 2019,
partially offset by an increase in the reversal of impairments of definite life brands to £18 million
in FY 2020 from £10 million in FY 2019. In FY 2020, the impairment charge mainly included
impairments of Zyrtec, capitalised costs for a discontinued oral care project and a discontinued
pain relief device and the reversal of impairments related to Transderm Scop. In FY 2019, the
impairment charge included impairments of Savlon, Eurax and Abreva and the reversal of
impairments related to Prevacid.
Restructuring costs (pre-tax) increased to £411 million (£321 million net of tax) in FY 2020 from
£330 million (£271 million net of tax) in FY 2019, reflecting increased integration costs following
the Pfizer Transaction, in addition to other restructuring and programme costs.
Transaction-related costs (pre-tax) decreased to £91 million (£71 million net of tax) in FY 2020
from £366 million (£285 million net of tax) in FY 2019. This was due to the fact that the majority
of the fair value unwind on inventory acquired as part of the Pfizer Transaction took place
during FY 2019.
Separation and Admission costs (pre-tax) of £66 million (£53 million net of tax) in FY 2020
relate to preparation for Separation and Admission, which was commenced in FY 2020.
Disposals and others (pre-tax) resulted in net income of £189 million (£120 million net of tax) in
FY 2020, compared to a net expense of £25 million (£4 million net of tax) in FY 2019, arising
from the net profit from the disposal of a number of consumer healthcare brands.
7. Liquidity and capital resources
7.1 Overview
The principal source of the Group’s liquidity is cash generated from operations. The Group also
has access to the debt capital markets through the Programme (as described below), as well
as the Revolving Credit Facilities (as described below) and a number of local borrowing
facilities in a variety of currencies and at floating rates in order to meet specific funding needs
of certain subsidiaries in the Group. As at the date of this Prospectus, certain notes have been
issued under the Programme, an overview of the terms of which are set out at paragraph
7.4(A) of this Part VII (Operating and Financial Review) below. The Group also expects to
establish ‘Euro’ and US Dollar commercial paper programmes, pursuant to which subsidiaries
of the Group may issue commercial paper from time to time. It is expected that the Company
will guarantee payment of amounts owing in respect of any commercial paper issued under
such programmes.
193
The Group’s liquidity requirements primarily relate to servicing its ongoing debt obligations
(including under the Programme, Pre-Separation USD Notes and the Revolving Credit
Facilities), its working capital requirements, funding its operating expenses and capital
expenditures (including its investments in R&D and advertising and promotion activities),
funding dividend payments, and implementing the Group’s growth strategies. In addition, it is
expected that the Term Loan Facility will be drawn in order to fund the Pre-Demerger Dividend.
From completion of the Pfizer Transaction, liquidity management has been governed by certain
provisions of the Pfizer SHA (see paragraph 15.5 of Part XII (Additional Information)), including
in relation to borrowings, cash management and shareholder funding and dividend payments.
In order to manage any shortfall between cash in hand and an agreed amount of readily
available cash of £300 million, the Group entered into an uncommitted facility with a
relationship bank, which has not been utilised. The Group manages liquidity risk through cash
management and forecasting processes under which the Group reviews its cash balances and
measures its actual performance against forecasts in order to manage liquidity risk. The Group
also monitors its exposure to foreign exchange rates and adopts hedging when it deems
appropriate.
The Group intends to continue to apply a disciplined approach to capital allocation, investing
for growth whilst maintaining an investment grade credit rating (see paragraph 3 of Part III
(Business Overview)).
As part of the Group’s existing banking arrangements, a significant proportion of the Group’s
cash is on-lent to the GSK Group. As of 31 December 2021, cash and cash equivalents
(including those amounts on-lent to the GSK Group) were primarily comprised US Dollar,
Chinese Yuan, Euro and Pounds Sterling. Cash and cash equivalents included £67 million not
available for general use due to restrictions applying in the subsidiaries where it is held,
including exchange controls and taxes on repatriation.
7.2 Cash flow
The table below summarises the principal components of the Group’s consolidated cash flows
for the periods under review, which has been extracted from the Historical Financial
Information.
£m 2021 2020 2019
Cash flow from operating activities
Profit after tax 1,439 1,181 687
Adjustments reconciling profit after tax to cash generated
from operations 227 780 408
Cash generated from operations 1,666 1,961 1,095
Taxation paid (310) (554) (309)
Net cash inflow from operating activities 1,356 1,407 786
Net cash inflow from investing activities (33) 1,030 291
Net cash (outflow) from financing activities (1,236) (2,437) (925)
Increase in cash and bank overdrafts 87 - 152
Cash and bank overdrafts at the beginning of the year 323 329 191
Exchange adjustments (5) (6) (14)
Increase in cash and bank overdrafts 87 - 152
Cash and cash equivalents at end of year 405 323 329
194

Net cash (outflow)/inflow from operating activities
Net cash inflow from operating activities was £1,356 million and £1,407 million in FY 2021 and
FY 2020, respectively. Net cash inflow from operating activities was £786 million in FY 2019.
Net cash inflow from operating activities for the Pfizer Contributed CH Business for the seven
months to 31 July 2019 was £58 million.
The year-on-year decrease of £51 million from FY 2020 to FY 2021 was largely due to a
decrease in cash generated from operations, which decreased by £295 million to
£1,666 million in FY 2021 from £1,961 million in FY 2020, partially offset by a £244 million
reduction in tax paid. The decrease in cash generated from operations was primarily
attributable to a larger net outflow from working capital, as outlined below, partially offset by
higher operating profits.
The year-on-year increase of £621 million from FY 2019 to FY 2020 was largely due to cash
generated from operations, which increased by £866 million to £1,961 million in FY 2020 from
£1,095 million in FY 2019, primarily attributable to the full year impact in FY 2020 of brands
acquired as part of the Pfizer Transaction, as compared to five months in FY 2019. The
increase was also due to strong underlying growth in each of EMEA and LatAm, North America
and APAC. In addition, reductions in working capital had a positive impact on cash flow, as
outlined below.
Working capital
The Group’s working capital movements comprise movements in trade and other receivables,
inventory and trade and other payables.
The following table sets out changes in the Group’s working capital for the periods indicated:
Financial Year
£m 2021 2020 2019
Decrease/(increase) in inventories (17) 130 232
Decrease/(increase) in trade receivables 14 18 (57)
(Decrease)/increase in trade payables 41 140 (256)
Net change in other receivables and payables (190) (273) (380)
Changes in working capital (152) 15 (461)
Inventory
Inventory increased by £2 million to £951 million at 31 December 2021 from £949 million at
31 December 2020. This resulted in a negative cash flow of £17 million in FY 2021. This
impact on cash flow was principally driven by inventory available in North America following
increases in manufacturing output. Inventory was also affected by non-cash movements,
including inventory transferred to assets held for sale and foreign exchange movements.
Inventory reduced by £262 million to £949 million at 31 December 2020 from £1,211 million at
31 December 2019. This resulted in a positive cash flow of £130 million in FY 2020. This
impact on cash flow was driven by activities designed to optimise inventory levels across the
supply chain, as well as the divestment of a number of brands and a reduction in inventories
for brands that experienced higher demand driven by the COVID-19 pandemic. Inventory was
also affected by non-cash movements, including fair value adjustments related to the Pfizer
Transaction and exchange rate movements.
195
Trade receivables
Trade receivables declined by £30 million to £1,318 million at 31 December 2021 from
£1,348 million at 31 December 2020, driven by improvements to cash collection and exchange
rate changes. This resulted in a positive cash flow of £14 million in FY 2021. Non-cash
movements were related to foreign exchange fluctuations.
Trade receivables declined by £49 million to £1,348 million at 31 December 2020 from
£1,397 million at 31 December 2019, driven by exchange rate changes, customers of the
Pfizer Contributed CH Business agreeing to adopt the shorter payment settlement periods of
the GSK Group in the USA and accelerated settlement in APAC. This resulted in a positive
cash flow of £18 million in FY 2020. Cash movements included the positive impact of
customers of the Pfizer Contributed CH Business adopting the lower payment settlement
periods of the GSK Group in the USA, partially offset by higher receivables associated with
increased sales in APAC. Trade receivables were also impacted by non-cash movements
related to foreign exchange fluctuations.
Trade payables
Trade payables increased by £29 million to £1,369 million at 31 December 2021 from
£1,340 million at 31 December 2020. This was driven by higher marketing spend in the fourth
quarter of 2021, partially offset by changes in exchange rates. This resulted in a positive cash
flow impact of £41 million in FY 2021.
Trade payables increased by £139 million to £1,340 million at 31 December 2020 from
£1,201 million at 31 December 2019. This was driven by higher marketing spend and capital
expenditure in the second half of the year, together with payables balances acquired by the
Group as part of the Pfizer Transaction after 31 July 2019. This resulted in a positive cash flow
impact of £140 million in FY 2020.
Other receivables and payables
Other receivables declined by £121 million to £889 million at 31 December 2021 from
£1,010 million at 31 December 2020. Other receivables primarily consist of prepayments and
receivables with the Pfizer Group, the GSK Group and other third parties. Other payables
decreased by £295 million to £1,633 million at 31 December 2021 from £1,928 million at
31 December 2020. Other payables primarily consist of customer return and rebate accruals,
wage, salary and social security accruals, VAT and deferred income. The net change in other
receivables and payables resulted in a negative cash outflow of £190 million. This was
primarily driven by an increase in balances receivable from GSK in relation to the Group’s right
to receive profits of certain brands and businesses still legally owned by GSK and a decrease
in operating balances payable to GSK in relation to certain payments made by GSK on behalf
of the Group. Non-cash movements related to foreign exchange fluctuations.
Other receivables declined by £72 million to £1,010 million at 31 December 2020 from
£1,082 million at 31 December 2019. Other receivables were the same as set out above. Other
payables decreased by £291 million to £1,928 million at 31 December 2020 from £2,219 million
at 31 December 2019. Other payables were the same as set out above. The net change in
other receivables and payables resulted in a negative cash outflow of £273 million, primarily
driven by a decline in third party receivables related to the Pfizer Transaction. Non-cash
movements related to foreign exchange fluctuations.
196

Net cash (outflow)/inflow from investing activities
Net cash (used in)/generated from investing activities was a £33 million outflow and a
£1,030 million inflow in FY 2021 and FY 2020, respectively. Net cash generated from investing
activities was £291 million in FY 2019. Net cash used in investing activities for the Pfizer
Contributed CH Business for the seven months to 31 July 2019 was £25 million.
The year-on-year decrease of £1,063 million from FY 2020 to FY 2021 principally reflected a
decrease in the proceeds from the sale of intangible assets and proceeds from the divestment
programme. The net cash of £33 million used in investing activities in FY 2021 was primarily
related to investment in property, plant and equipment and software.
The year-on-year increase of £739 million from FY 2019 to FY 2020 was principally driven by
proceeds from the sale of intangible assets, which increased by £804 million to £924 million in
FY 2020 from £120 million in FY 2019, reflecting the divestment of a number of smaller brands
in the Group’s portfolio, including Breathe Right and Physiogel. Disposal of businesses
increased to £221 million in FY 2020 due to the disposal of ThermaCare (see paragraph 2.13
of this Part VII (Operating and Financial Review) above).
Net cash (outflow)/inflow from financing activities
Net cash used in from financing activities decreased by £1,201 million to £1,236 million in FY
2021 from £2,437 million in FY 2020, reflecting decreased dividend payments. Dividends paid
to shareholders decreased by £1,223 million to £1,148 million in FY 2021 from £2,371 million in
FY 2020, which, to a large degree, reflects the decrease in proceeds from the sale of intangible
assets and proceeds from the divestment programme. The quantum of dividend payments
made during the period also reflected arrangements entered into as part of the Pfizer
Transaction, which will terminate with effect from Admission.
Net cash used in from financing activities increased by £1,512 million to £2,437 million in FY
2020 from £925 million in FY 2019 due to increased dividend payments. Dividends paid to
shareholders increased by £1,219 million to £2,371 million in FY 2020 from £1,152 million in
FY 2019, which reflected the increased cash generation of the business following completion of
the Pfizer Transaction. The quantum of dividend payments made during the period also
reflected arrangements entered into as part of the Pfizer Transaction, which will terminate with
effect from Admission. Whilst no capital contributions were made in FY 2020, in FY 2019 a
capital contribution of £335 million was made into the Group relating to the completion of the
Pfizer Transaction.
Free cash flow and free cash flow conversion
During the periods under review the Group delivered a total of £3.8 billion free cash flow,
driven by proceeds from divestments, a sharp focus on working capital discipline and stable
capital investment of approximately 3 per cent. of revenue per annum, partially offset by spend
in relation to Restructuring costs and Separation and Admission costs.
Financial Year
2021 2020 2019
Net cash inflows from operating activities (£m)
1
1,356 1,407 786
Free cash flow (£m) 1,173 1,988 681
Free cash flow conversion (%) 82 168 99
197

Note
1. Included as the nearest IFRS measure to the non-IFRS measures presented in the
table above.
Free cash flow in FY 2021 was £1,173 million, with a free cash flow conversion rate of 82 per
cent. Free cash flow in FY 2020 was £1,988 million, with a free cash flow conversion of 168 per
cent. Free cash flow in FY 2019 was £681 million, with a free cash flow conversion of 99 per
cent.
Free cash flow decreased by 41.0 per cent. (£815 million) from FY 2020 to FY 2021. The
decrease in free cash flow was primarily attributable to a decline in the proceeds from sale of
intangible assets, proceeds from the divestment programme and the decrease in net cash
inflow from operating activities. These factors were partially offset by a decrease in the
purchase of intangible assets.
Free cash flow increased by 191.9 per cent. (£1,307 million) from FY 2019 to FY 2020. The
increase in free cash flow was primarily attributable to the impact of proceeds received from
the disposal of a number of brands (see “Net cash flows (used in)/generated from investing
activities” above) of £924 million (FY 2019: £120 million). The increase in free cash flow was
also attributable to the inclusion of the full year of operating cash flows in FY 2020 of brands
acquired as part of the Pfizer Transaction and strong performance in the Group’s VMS, Pain
Relief and Oral Health categories, together with synergy savings and cost control. These
factors were partially offset by increased capital expenditure (see paragraph 7.5 of this Part VII
(Operating and Financial Review) below).
7.3 Net debt
During the periods under review, the Group’s principal source of liquidity was cash generated
from operations. The Group did not have any long-term debt, excluding lease liabilities, in its
capital structure. In the period following completion of the Pfizer Transaction, excess cash was
distributed to GSKCHH and PFCHH by way of dividends in accordance with the terms of the
Pfizer SHA, which will terminate with effect from Admission. Cash and cash equivalents
retained on the balance sheet following the payment of these dividends was primarily used by
the Group for working capital purposes, funding operating expenses and capital expenditures,
and implementing the Group’s growth strategies. As at 31 December 2021, the Group’s net
debt consisted of lease liabilities, short-term bank borrowings and derivative financial liabilities,
more than offset by cash and cash equivalents, liquid investments and derivative financial
assets.
In preparation for Separation, GSK Consumer Healthcare Capital UK plc, GSK Consumer
Healthcare Capital NL B.V. and GSK Consumer Healthcare Capital US LLC each issued notes,
the net proceeds of which have been made available to GlaxoSmithKline Consumer
Healthcare Finance Limited in order to fund the making of certain upstream loans to wholly-
owned subsidiaries of GSK and Pfizer (see paragraph 7.4 of this Part VII (Operating and
Financial Review) below).
As at 31 December 2021, the Group had £991 million of outstanding gross indebtedness
85
,
comprising £79 million of short-term borrowings, £87 million of long-term borrowings and
85
Indebtedness excludes loan amounts receivable from related parties of £1,508 million as at 31 December 2021 where there is
no right to offset.
198

£825 million of loan amounts owing to related parties. As at 31 March 2022, the Group had
£10,904 million of outstanding gross indebtedness
86
, comprising £80 million of short-term
borrowings, £9,363 million of long-term borrowings and £1,461 million of loan amounts owing
to related parties.
7.4 Capital Resources and Indebtedness
See Part VIII (Capitalisation and Indebtedness) for details relating the Group’s capitalisation
and indebtedness as at the dates indicated therein. Further details of the capital resources of
the Group are set out in the summaries below.
The unaudited pro forma statement of net assets of the Group set out in Section A of Part IX
(Unaudited Pro Forma Financial Information of the Group) has been prepared to illustrate the
impact of the Notes Proceeds Loans, the receipt of related party loans, additional borrowings
and the payment of the Pre-Demerger Dividend on the net assets of the Group as if such
transactions had taken place on 31 March 2022. In particular, Note (7) illustrates the impact of
these transactions on the net debt of the Group as if such transactions had taken place on
31 March 2022.
(A) Bond issuances
As part of the preparation for Separation, on 16 March 2022, GSK Consumer
Healthcare Capital UK plc and GSK Consumer Healthcare Capital NL B.V. (the “EMTN
Issuers”) established a £10,000,000,000 Euro Medium Term Note Programme (the
“Programme”) pursuant to which the EMTN Issuers may issue notes from time to time.
As at the date of this Prospectus, the EMTN Issuers have issued the notes described
under “Pre-Separation Programme Notes” below under the Programme.
In addition, on 24 March 2022 the US Issuer and GSK Consumer Healthcare Capital
UK plc issued a number of standalone bonds pursuant to a private placement to
institutional investors in the USA and outside the USA in reliance on exemptions from
the registration requirements of the US Securities Act (the “ Pre-Separation USD
Notes”) (as described under “Pre-Separation USD Notes” below).
The payment of all amounts owing in respect of: (i) notes issued under the Programme
(including the notes in issuance as at the date of this Prospectus, as described under
“Pre-Separation Programme Notes” below); and (ii) the Pre-Separation USD Notes is,
as at the date of this Prospectus, guaranteed by GSK. Following completion of the
GSK Share Exchange, the guarantee provided by GSK will cease to be effective and a
guarantee provided by the Company will come into full force and effect.
Pre-Separation Programme Notes
A list of the notes issued under the Programme as at the date of this Prospectus (the
“Pre-Separation Programme Notes”) and an overview of the terms applicable to such
notes are set out below:
• £300,000,000 2.875 per cent. notes due 29 October 2028 (the “2.875 per
cent. Notes”) - The 2.875 per cent. Notes were issued by GSK Consumer
86
Indebtedness excludes loan amounts receivable from related parties of £11,330 million as at 31 March 2022 where there is no
right to offset.
199
Healthcare Capital UK plc and bear interest at a rate of 2.875 per cent. per
annum, payable annually in arrear. Unless previously redeemed or
purchased and cancelled the 2.875 per cent. Notes will be redeemed by
GSK Consumer Healthcare Capital UK plc on 29 October 2028.
• £400,000,000 3.375 per cent. notes due 29 March 2038 (the “3.375 per
cent. Notes”) - The 3.375 per cent. Notes were issued by GSK Consumer
Healthcare Capital UK plc and bear interest at a rate of 3.375 per cent. per
annum, payable annually in arrear. Unless previously redeemed or
purchased and cancelled the 3.375 per cent. Notes will be redeemed by
GSK Consumer Healthcare Capital UK plc on 29 March 2038.
• €850,000,000 1.250 per cent. notes due 29 March 2026 (the “1.250 per
cent. Notes”) - The 1.250 per cent. Notes were issued by GSK Consumer
Healthcare Capital NL B.V. and bear interest at a rate of 1.250 per cent.
per annum, payable annually in arrear. Unless previously redeemed or
purchased and cancelled the 1.250 per cent. Notes will be redeemed by
GSK Consumer Healthcare Capital NL B.V. on 29 March 2026.
• €750,000,000 1.750 per cent. notes due 29 March 2030 (the “1.750 per
cent. Notes”) - The 1.750 per cent. Notes were issued by GSK Consumer
Healthcare Capital NL B.V. and bear interest at a rate of 1.750 per cent.
per annum, payable annually in arrear. Unless previously redeemed or
purchased and cancelled the 1.750 per cent. Notes will be redeemed by
GSK Consumer Healthcare Capital NL B.V. on 29 March 2030.
• €750,000,000 2.125 per cent. notes due 29 March 2034 (the “2.125 per
cent. Notes”) - The 2.125 per cent. Notes were issued by GSK Consumer
Healthcare Capital NL B.V. and bear interest at a rate of 2.125 per cent.
per annum, payable annually in arrear. Unless previously redeemed or
purchased and cancelled the 2.125 per cent. Notes will be redeemed by
GSK Consumer Healthcare Capital NL B.V. on 29 March 2034.
Each series of Pre-Separation Programme Notes additionally contains a limited
negative pledge and customary events of default (including cross-acceleration
provisions). The occurrence of any event of default under the Pre-Separation
Programme Notes would permit, amongst other things, the acceleration of the relevant
series of Pre-Separation Programme Notes in accordance with the terms and
conditions of the Pre-Separation Programme Notes.
Each series of Pre-Separation Programme Notes can be redeemed prior to maturity at
the option of the relevant issuer in accordance with the terms and conditions of the
Programme. In addition to other customary call features, each series of Pre-Separation
Programme Notes includes a make-whole call option, which permits the relevant issuer
to redeem the relevant notes on not less than 15 nor more than 60 days’ notice at any
time, subject to payment of the sum of the present values of the remaining scheduled
payments of principal and interest through to maturity (subject to a customary discount
calculated using an applicable bond reference rate plus an agreed margin).
200

Pre-Separation USD Notes
The $700,000,000 3.024 per cent. callable fixed rate senior notes due 2024 (the
“3.024 per cent. Notes”) were issued by the US Issuer and bear interest at a rate of
3.024 per cent. per annum, payable semi-annually in arrear. Unless previously
redeemed or purchased and cancelled the 3.024 per cent. Notes will be redeemed by
the US Issuer on 24 March 2024.
The $300,000,000 callable floating rate senior notes due 2024 (the “Floating Rate
Notes”) were issued by the US Issuer and bear interest at a floating rate, payable
quarterly in arrear. Unless previously redeemed or purchased and cancelled the
Floating Rate Notes will be redeemed by the US Issuer on 24 March 2024.
The $2,000,000,000 3.375 per cent. fixed rate senior notes due 2027 (the “2027
3.375 per cent. Notes”) were issued by the US Issuer and bear interest at a rate of
3.375 per cent. per annum, payable semi-annually in arrear. Unless previously
redeemed or purchased and cancelled the 2027 3.375 per cent. Notes will be
redeemed by the US Issuer on 24 March 2027.
The $1,000,000,000 3.375 per cent. fixed rate senior notes due 2029 (the “2029
3.375 per cent. Notes”) were issued by the US Issuer and bear interest at a rate of
3.375 per cent. per annum, payable semi-annually in arrear. Unless previously
redeemed or purchased and cancelled the 2029 3.375 per cent. Notes will be
redeemed by the US Issuer on 24 March 2029.
The $2,000,000,000 3.625 per cent. fixed rate senior notes due 2032 (the “3.625 per
cent. Notes”) were issued by the US Issuer and bear interest at a rate of 3.625 per
cent. per annum, payable semi-annually in arrear. Unless previously redeemed or
purchased and cancelled the 3.625 per cent. Notes will be redeemed by the US Issuer
on 24 March 2032.
The $1,000,000,000 4.000 per cent. fixed rate senior notes due 2052 (the “4.000 per
cent. Notes”) were issued by the US Issuer and bear interest at a rate of 4.000 per
cent. per annum, payable semi-annually in arrear. Unless previously redeemed or
purchased and cancelled the 4.000 per cent. Notes will be redeemed by the US Issuer
on 24 March 2052.
The $1,750,000,000 3.125 per cent. fixed rate senior notes due 2025 (the “3.125 per
cent. Notes”) were issued by GSK Consumer Healthcare Capital UK plc and bear
interest at a rate of 3.125 per cent. per annum, payable semi-annually in arrear. Unless
previously redeemed or purchased and cancelled the 3.125 per cent. Notes will be
redeemed by GSK Consumer Healthcare Capital UK plc on 24 March 2025.
The Pre-Separation USD Notes additionally contain a limited negative pledge and
customary events of default (including cross-acceleration provisions). The occurrence
of any event of default under the Pre-Separation USD Notes would permit, amongst
other things, the acceleration of the relevant series of the Pre-Separation USD Notes in
accordance with the terms and conditions of the Pre-Separation USD Notes.
Each series of the Pre-Separation USD Notes can be redeemed prior to maturity at the
option of the relevant issuer in accordance with the terms and conditions of the
Pre-Separation USD Notes.
201
The 3.024 per cent. Notes, the 2027 3.375 per cent. Notes, the 2029 3.375 per cent.
Notes, the 3.625 per cent. Notes and the 4.000 per cent. Notes include a make-whole
call option, which permits the US Issuer to redeem the relevant series of notes on not
less than 15 nor more than 60 days’ notice at any time prior to the applicable par call
date set out in the terms and conditions of the Pre-Separation USD Notes (the “Par
Call Date”), subject to payment of the greater of (i) 100 per cent. of the principal
amount of the relevant notes to be redeemed on that redemption date and (ii) the sum
of the present values of the remaining scheduled payments of principal and interest
that would be due if the relevant series of the notes matured on the applicable Par Call
Date (subject to a customary discount calculated using an applicable bond reference
rate plus an agreed margin), plus accrued and unpaid interest, if any, thereon to, but
excluding, the redemption date. On or after the applicable Par Call Date, the US Issuer
may redeem the relevant series of notes at a redemption price equal to 100 per cent. of
the principal amount of the applicable series of notes to be redeemed, plus accrued
and unpaid interest, if any, thereon to, but excluding, the redemption date.
The 3.125 per cent. Notes include a make-whole call option, which permits GSK
Consumer Healthcare Capital UK plc to redeem the notes on not less than 15 nor more
than 60 days’ notice at any time, subject to payment of the greater of (i) 100 per cent.
of the principal amount of the notes to be redeemed on that redemption date and
(ii) the sum of the present values of the remaining scheduled payments of principal and
interest (subject to a customary discount calculated using an applicable bond reference
rate plus an agreed margin), plus accrued and unpaid interest, if any, thereon to, but
excluding, the redemption date.
The Floating Rate Notes include a par call option, which permits the US Issuer to
redeem the Floating Rate Notes, in whole or in part, at its option at any time and from
time to time on or after 24 March 2023 at a redemption price equal to 100 per cent. of
the principal amount of the Floating Rate Notes to be redeemed, plus accrued and
unpaid interest, if any, thereon to, but excluding, the redemption date. Notwithstanding
the foregoing, instalments of interest on the Floating Rate Notes to be redeemed that
are due and payable on a Floating Rate Notes interest payment date falling on or prior
to a redemption date will be payable on the Floating Rate Notes interest payment date
to the registered holders as of the close of business on the relevant regular record date
according to the Floating Rate Notes and the Indenture, as applicable.
(B) Note Proceeds Loans
The net proceeds of the Pre-Separation Programme Notes and the Pre-Separation
USD Notes have been made available to GlaxoSmithKline Consumer Healthcare
Finance Limited in order to fund the making of certain upstream loans to wholly-owned
subsidiaries of GSK and Pfizer. As such:
(i) on 24 March 2022, GlaxoSmithKline Consumer Healthcare Finance Limited
made a loan of £4,465,197,183.55 to GlaxoSmithKline Finance plc and a loan
of £2,101,269,262.85 to Pfizer Service Company Ireland Unlimited Company;
and
(ii) on 29 March 2022, GlaxoSmithKline Consumer Healthcare Finance Limited
made a loan of £1,798,139,950.68 to GlaxoSmithKline Finance plc and a loan
of £846,183,506.20 to Pfizer Service Company Ireland Unlimited Company,
202
(together, the “Notes Proceeds Loans”) pursuant to certain upstream loan
agreements as amended from time to time (the “Notes Proceeds Loan
Agreements”).
The Note Proceeds Loan Agreements provide for interest on the Note Proceeds Loans
at a rate of 1.365 per cent. per annum, payable semi-annually in arrear. The Note
Proceeds Loan Agreements require the relevant borrower to make limited
representations and covenants and contain limited events of default (including cross-
acceleration provisions) and (subject as provided below) prepayment events. The
occurrence of any event of default under either Note Proceeds Loan Agreement would
permit GlaxoSmithKline Consumer Healthcare Finance Limited to, amongst other
things, accelerate the relevant Note Proceeds Loan.
The purpose of the Notes Proceeds Loans was to make the net proceeds of the
Pre-Separation Programme Notes and the Pre-Separation USD Notes available to the
GSK Group and the Pfizer Group in advance of the date on which they would receive
those proceeds as part of the Pre-Demerger Dividend. Accordingly, the terms of the
Notes Proceeds Loan Agreements require, among other things, that the Notes
Proceeds Loans will be repaid in full to GlaxoSmithKline Consumer Healthcare Finance
Limited on 13 July 2022 or such other date as agreed between the parties in writing.
Following repayment of the Notes Proceeds Loans, the amounts received by
GlaxoSmithKline Consumer Healthcare Finance Limited will be made available to CH
JVCo in order to fund a portion of the Pre-Demerger Dividend.
(C) Revolving Credit Facilities
On 18 February 2022, CH JVCo entered into syndicated revolving credit facilities (the
“Revolving Credit Facilities” and loans extended thereunder the “RCF Loans”). The
commitments under the Revolving Credit Facilities are provided by (i) Banco Bilbao
Vizcaya Argentaria, S.A., London Branch; (ii) Banco Santander, S.A., London Branch;
(iii) Bank of America, N.A.; (iv) Bank of America, N.A., London Branch; (v) Barclays
Bank PLC; (vi) BNP Paribas, London Branch; (vii) Citibank, N.A.; (viii) Citibank, N.A.,
London Branch; (ix) Deutsche Bank AG, London Branch; (x) Deutsche Bank AG New
York Branch; (xi) Goldman Sachs Bank USA; (xii) HSBC Bank plc; (xiii) ING Bank N.V.,
London Branch; (xiv) JPMorgan Chase Bank, N.A.; (xv) JPMorgan Chase Bank, N.A.,
London Branch; (xvi) Lloyds Bank plc; (xvii) Mizuho Bank, Ltd.; (xviii) Morgan Stanley
Bank N.A.; (xix) Royal Bank of Canada; and (xx) Standard Chartered Bank (Hong
Kong) Limited.
The initial borrower under each of the Revolving Credit Facilities is CH JVCo but,
following completion of the GSK Share Exchange and in accordance with the terms of
the Revolving Credit Facilities, the Company will accede to the Revolving Credit
Facilities and replace CH JVCo as borrower under the Revolving Credit Facilities (the
borrower under the Revolving Credit Facilities from time-to-time, the “RCF Borrower”).
Following its accession as borrower under the Revolving Credit Facilities, the Company
will guarantee the obligations of any other member of the Group that accedes to the
Revolving Credit Facilities as an additional borrower.
The Revolving Credit Facilities provide the RCF Borrower with access to:
• a multicurrency facility denominated in Pounds Sterling, with a commitment of
£1,000,000,000 and an initial maturity date of 24 September 2025 (the “GBP
Facility”); and
203
• a US Dollar facility, incorporating a swingline facility (the “Swingline Facility”), with
an aggregate commitment of $1,400,000,000 and an initial maturity date of
24 September 2023 (the “USD Facility”).
As at the Latest Practicable Date, each of the GBP Facility and the USD Facility is
undrawn.
With certain exceptions, RCF Loans bear interest at a rate equal to the aggregate of:
(i) the applicable risk free rate (which for loans drawn in Pounds Sterling is the Bank of
England’s Sterling Overnight Interbank Average Rate (‘SONIA’) and for loans drawn in
US dollars is the New York Federal Reserves Secured Overnight Financing Rate
(‘SOFR’)) and (ii) a margin determined in accordance with the terms of the Revolving
Credit Facilities, which is dependent on the corporate rating assigned to the Company.
The proceeds of each RCF Loan are available for the general corporate purposes of
the Group and such specific purposes as may be determined by the RCF Borrower.
The Swingline Facility is available for financing or refinancing the payment of (or in
respect of) any indebtedness or other obligations of the Group (including commercial
paper, but excluding any other drawing from the Swingline Facility).
The Revolving Credit Facilities require the RCF Borrower to make certain customary
representations and warranties at various times throughout the term of the Revolving
Credit Facilities. In addition, the terms of the Revolving Credit Facilities contain
customary restrictions on the operations of the RCF Borrower and the Group. These
include customary positive and negative covenants, including a negative pledge and,
with effect from the GSK Share Exchange, restrictions on disposals. The Revolving
Credit Facilities do not contain any financial covenants, but the RCF Borrower is
required to comply with certain information covenants, including the delivery of financial
information.
The Revolving Credit Facilities contain customary events of default, including cross-
acceleration provisions. The occurrence of any event of default under the Revolving
Credit Facilities at a time when any RCF Loans are outstanding would permit, amongst
other things, the acceleration of all RCF Loans.
(D) Term Loan Facility
On 18 February 2022, CH JVCo entered into a term loan facility with a total
commitment of £1,500,000,000 (the “Term Loan Facility”) provided by (i) Bank of
America, N.A., London Branch; (ii) Banco Santander, S.A., London Branch;
(iii) Barclays Bank PLC; (iv) BNP Paribas Fortis SA/NV; (v) BNP Paribas; (vi) Citibank,
N.A., London Branch; (vii) Deutsche Bank AG, London Branch; (viii) Goldman Sachs
Bank USA; (ix) HSBC Bank plc; (x) JPMorgan Chase Bank, N.A., London Branch;
(xi) Mizuho Bank, Ltd.; (xii) Morgan Stanley Bank N.A.; and (xiii) Standard Chartered
Bank (Hong Kong) Limited.
The payment of amounts owing in respect of the Term Loan Facility are, as at the date
of this Prospectus, not guaranteed. Following completion of the GSK Share Exchange,
the Company will accede to the Term Loan Facility as a guarantor of the Term Loan
Facility in accordance with the terms of the Term Loan Facility.
204
The Term Loan Facility is denominated in Pounds Sterling and permits a single term
loan to be borrowed. As at the Latest Practicable Date no amount has been borrowed
under the Term Loan Facility, although it is expected to be drawn on or prior to the date
of the Pre-Demerger Dividend.
Any loan drawn under the Term Loan Facility will bear interest at a rate equal to the
aggregate of: (i) the applicable risk free rate (being the Bank of England’s Sterling
Overnight Interbank Average Rate (‘SONIA’)); and (ii) a margin determined in
accordance with the terms of the Term Loan Facility, which is dependent on the
corporate rating assigned to the Company.
The Term Loan Facility is made available on customary ‘certain funds’ terms and the
proceeds of any utilisation under the Term Loan Facility are available for use, directly
or indirectly, towards the payment of the Pre-Demerger Dividend. The Term Loan
Facility has a maturity date falling 36 months after the date on which it was entered
into.
The Term Loan Facility requires CH JVCo and, from the point at which it accedes to
the Term Loan Facility, the Company to make certain customary representations and
warranties at various times throughout the term of the Term Loan Facility. In addition,
the Term Loan Facility contains customary restrictions on the operations of CH JVCo,
the Group and, from the point at which it accedes to the Term Loan Facility, the
Company. These include customary positive and negative covenants, including a
negative pledge and, with effect from the GSK Share Exchange, restrictions on
disposals. The Term Loan Facility does not contain any financial covenants, but CH
JVCo and, from the point at which it accedes to the Term Loan Facility, the Company
are required to comply with certain information covenants, including the delivery of
financial information.
The Term Loan Facility contains customary events of default, including cross-
acceleration provisions. The occurrence of any event of default under the Term Loan
Facility at a time when any amount is outstanding under the Term Loan Facility would
permit, amongst other things, the acceleration of such amounts.
(E) Commercial Paper Programmes
The Group expects to establish ‘Euro’ and US Dollar commercial paper programmes
pursuant to which members of the Group may issue commercial paper from time to
time. It is expected that the Company will guarantee payment of amounts owing in
respect of any commercial paper issued under such programmes.
7.5 Capital expenditure
During the periods under review, the Group’s capital expenditure primarily related to property,
plant and equipment, including a number of projects as part of restructuring the Group’s
business, and the purchase of intangible assets, largely related to computer software.
205

The table below summarises the Group’s capital expenditure for the periods under review.
Financial Year
£m 2021 2020 2019
Purchase of property, plant and equipment 228 222 190
Purchase of intangible assets 70 96 53
Total capital expenditure 298 318 243
Total capital expenditure
The Group’s capital expenditure was £298 million and £318 million in FY 2021 and FY 2020,
respectively. The Group’s capital expenditure was £243 million in FY 2019. Capital expenditure
for the Pfizer Contributed CH Business for the seven months to 31 July 2019 was £25 million.
The year-on-year decrease in capital expenditure from FY 2020 to FY 2021 reflected a
reduction in the purchase of intangible assets, partially offset by a small increase in the
purchase of property, plant and equipment.
The year-on-year increase in capital expenditure from FY 2019 to FY 2020 was largely driven
by investments in supply chain and technology as part of restructuring the business, as well as
the full year impact of the Pfizer Contributed CH Business in FY 2020.
Property, plant and equipment
Purchase of property, plant and equipment was £228 million, £222 million and £190 million in
FY 2021, FY 2020 and FY 2019, respectively. Spend in FY 2019 and FY 2020 was
predominantly driven by large scale integration projects following the completion of the Pfizer
Transaction. In FY 2021 the investment profile switched to focus on business as usual projects
and investment (including continuous improvement to property, plant and equipment and the
renewal of site infrastructure).
The year-on-year increase of £6 million from FY 2020 to FY 2021 reflected expenditure on a
large number of small projects across various sites, including in relation to site closures,
technology systems integation and optimisation of supply chain.
The year-on-year increase of £32 million from FY 2019 to FY 2020 was primarily driven by the
increase in large projects across various sites as part of the restructuring of the Group’s
business in FY 2020, including in relation to site closures, technology systems integration and
optimisation of supply chain. In FY 2019, the purchase of property, plant and equipment was
primarily attributable to a number of large projects, including in relation to site closures and
rationalisation and optimisation of supply chain.
Intangible assets
The Group’s purchase of intangible assets (which largely related to computer software) was
£70 million, £96 million and £53 million in FY 2021, FY 2020 and FY 2019, respectively.
The year-on-year decrease of £26 million from FY 2020 to FY 2021 was driven by decreased
expenditure on the integration of the Pfizer Contributed CH Business into the Group. The
increase of £43 million from FY 2019 to FY 2020 was driven by increased expenses following
the integration of the Pfizer Contributed CH Business into the Group. Across all three years,
spend included integrating production sites and commercial entities, upgrading the system
infrastructure in production sites and general software for the Group.
206
8. Risk disclosures
For a description of the Group’s management of liquidity, market, foreign exchange, wholesale
and retail credit, credit and treasury-related risk, see Note 33 of Schedule II (Historical
Financial Information of GlaxoSmithKline Consumer Healthcare Holdings (No.2) Limited).
9. Accounting policies
The accounting policies of the Group are set out in Notes 1 and 2 of Schedule II (Historical
Financial Information of GlaxoSmithKline Consumer Healthcare Holdings (No.2) Limited). The
judgements made in applying accounting policies are set out in Note 3 of Schedule II
(Historical Financial Information of GlaxoSmithKline Consumer Healthcare Holdings (No.2)
Limited).
207

PART VIII
CAPITALISATION AND INDEBTEDNESS
Both the capitalisation and indebtedness information has been extracted without material adjustment
from the unaudited accounting records of the Group as at 31 March 2022.
The table below sets out the Group’s shareholder indebtedness as at 31 March 2022.
31 March
2022
£m
Note (unaudited)
Short-term borrowings
Guaranteed ............................................................................... -
Secured .................................................................................. 1 30
Unguaranteed / unsecured .................................................................. 2 1,511
Total short-term borrowings ............................................................... 1,541
Long-term borrowings
Guaranteed ............................................................................... 3 9,275
Secured .................................................................................. 4 88
Unguaranteed / unsecured ................................................................... -
Total long-term borrowings ................................................................. 9,363
The table below sets out the Group’s capitalisation as at 31 March 2022.
31 March
2022
£m
Note (unaudited)
Share capital .............................................................................. 1
Share premium ............................................................................ -
Retained earnings .......................................................................... 5 37,986
Other reserves ............................................................................. (11,502)
Shareholders’ equity ....................................................................... 26,485
Non-controlling interests ..................................................................... 139
Total equity ............................................................................... 26,624
(1) Secured short-term borrowings represent the current portion of lease liabilities as at 31 March
2022.
(2) Unguaranteed / unsecured short-term borrowings represent the sum of bank loan and
overdrafts and loan amounts owing to related parties in the Group’s net indebtedness table
under Total short-term borrowings and loan amounts owing to related parties in the Group’s net
indebtedness table.
(3) Guaranteed long-term borrowings include the net proceeds from the issuance of the
Pre-Separation Programme Notes and the Pre-Separation USD Notes (the “ Pre-Separation
Notes”). Long-term borrowings reflect the proceeds received from the Pre-Separation Notes,
less transaction costs of £34 million incurred, which are capitalised and will be amortised over
the term of each note. The payment of all amounts owing in respect of: (i) notes issued under
the Programme (including the Pre-Separation Programme Notes); and (ii) the Pre-Separation
USD Notes is, as at the date of this Prospectus, guaranteed by GSK. Following completion of
the GSK Share Exchange, the guarantee provided by GSK will cease to be effective and a
guarantee provided by the Company will come into full force and effect. Further details of the
terms and conditions governing the notes issued under the Programme and the Pre-Separation
USD Notes can be found at paragraph 15.12 of Part XII (Additional Information).
208

(4) Secured long-term borrowings represent the non-current portion of lease liabilities as at
31 March 2022.
(5) Retained earnings as at 31 December 2021.
The Group’s capitalisation in the table above does not take into account the Transactions described in
Part IX (Unaudited Pro Forma Financial Information of the Group). Except as set out in the preceding
sentence, there has been no material change in the Group’s total capitalisation since 31 March 2022.
The following table sets out the Group’s net indebtedness as at 31 March 2022.
31 March
2022
£m Note (unaudited)
Cash ..................................................................................... 344
Cash equivalents ........................................................................... 38
Other current financial assets ................................................................. 1
Liquidity ................................................................................. 383
Current financial receivable ................................................................ 6 11,330
Bank loan and overdrafts .................................................................... 50
Lease liabilities ............................................................................ 30
Loan amounts owing to related parties ......................................................... 1,461
Total short-term borrowings and loan amounts owing to related parties ......................... 1,541
Net current financial indebtedness .......................................................... 10,172
Lease liabilities ............................................................................ 88
£300,000,000 2.875 per cent. notes due 2028 ................................................... 299
£400,000,000 3.375 per cent. notes due 2038 ................................................... 398
€850,000,000 1.250 per cent. notes due 2026 ................................................... 711
€750,000,000 1.750 per cent. notes due 2030 ................................................... 632
€750,000,000 2.125 per cent. notes due 2034 ................................................... 628
$700,000,000 3.024 per cent. callable notes due 2024 ............................................ 533
$300,000,000 floating rate callable notes due 2024 ............................................... 229
$2,000,000,000 3.375 per cent. notes due 2027 ................................................. 1,516
$1,000,000,000 3.375 per cent. notes due 2029 ................................................. 753
$2,000,000,000 3.625 per cent. notes due 2032 ................................................. 1,515
$1,000,000,000 4.000 per cent. notes due 2052 ................................................. 741
$1,750,000,000 3.125 per cent. notes due 2025 ................................................. 1,320
Total long-term borrowings ................................................................. 7 9,363
Net financial indebtedness ................................................................. 809
(6) Current financial receivable incudes £2,120 million of loan amounts owing from GSK financing
companies as part of the Group’s banking arrangements and £9,210 million owing in respect of
the Notes Proceeds Loans as at 31 March 2022.
(7) In addition to the above, the Group had a committed term loan facility of £1,500 million and
RCF loans of £1,000 million and $1,400 million that were undrawn as at 31 March 2022.
The Group had no indirect or contingent indebtedness as at 31 March 2022.
The Group’s net financial indebtedness in the table above does not take into account the Transactions
described in Part IX (Unaudited Pro Forma Financial Information of the Group). Except as set out in the
preceding sentence, there has been no material change in the Group’s net indebtedness since
31 March 2022.
209
PART IX
UNAUDITED PRO FORMA FINANCIAL INFORMATION OF THE GROUP
Section A: Unaudited pro forma Statement of Net Assets
The unaudited pro forma statement of net assets of the Group set out below (the “Unaudited Pro
Forma Financial Information”) has been prepared in accordance with Annex 20 of the Prospectus
Regulation (as supplemented by Commission Delegated Regulation (EU) 2019/980) and on the basis
of the notes set out below to illustrate the impact of the Notes Proceeds Loans, the receipt of related
party loans, additional borrowings and the payment of the Pre-Separation Dividends (together the
“Transactions”) on the net assets of the Group as if the Transactions had taken place on 31 March
2022.
The Unaudited Pro Forma Financial Information has been prepared on the basis of the unaudited
interim financial information of the Group as at 31 March 2022, the date to which the latest unaudited
financial information in relation to the Group was prepared. The Unaudited Pro Forma Financial
Information has been prepared in accordance with Annex 20 of the PR Regulation and pursuant to
Listing Rule 13.3.3R in a manner consistent with the accounting policies of the Company.
The Unaudited Pro Forma Financial Information is shown for illustrative purposes only and because of
its nature addresses a hypothetical situation. It does not represent the Group’s actual financial position
or results. It may not, therefore, give a true picture of the Group’s financial position or results nor is it
indicative of the results that may, or may not, be expected to be achieved in the future.
The unaudited pro forma information does not constitute financial statements within the meaning of
section 434(3) of the Companies Act. Investors should read the whole of this Prospectus and not rely
solely on the pro forma financial information contained in this Part IX (Unaudited Pro Forma Financial
Information of the Group).
Deloitte LLP’s report on the Unaudited Pro Forma Financial Information is set out in Section B of this
Part IX (Unaudited Pro Forma Financial Information of the Group).
210

Pro forma adjustments related to the
Transactions
Group Net
Assets at 31
March 2022
Receipt of
Notes
Proceeds
Loans and
related party
loans
Additional
borrowings
Pre-
Separation
Dividends
Transaction
costs
Unaudited
pro forma at
31 March
2022
£m
(Note 1)
£m
(Note 2)
£m
(Note 3)
£m
(Note 4)
£m
(Note 5)
£m
(Note 6)
Non-current Assets
Property, plant and equipment 1,587 - - - - 1,587
Right of use assets 100 - - - - 100
Intangible assets 27,692 - - - - 27,692
Deferred tax assets 314 - - - - 314
Post-employment benefit assets 11 - - - - 11
Derivative financial instruments 8 8
Other non-current assets 13 - - - - 13
Total non-current assets 29,725 - - - - 29,725
Current assets -
Inventories 986 - - - - 986
Trade and other receivables 2,415 - - - - 2,415
Loan amounts owing from related parties 11,330 (11,330) - - - -
Cash and cash equivalents and liquid
investments
383 9,869 1,435 (11,039) (84) 564
Derivative financial instruments 18 - - --18
Current tax recoverable 166 - - - - 166
Total current assets 15,298 (1,461) 1,435 (11,039) (84) 4,149
Total assets 45,023 (1,461) 1,435 (11,039) (84) 33,874
Current liabilities
Short-term borrowings (80) - - - - (80)
Trade and other payables (3,142) - - - - (3,142)
Loan amounts owing to related parties (1,461) 1,461 - - - -
Derivative financial instruments (15) - - - - (15)
Current tax payable (242) - - - - (242)
Short-term provisions (86) - - - - (86)
Total current liabilities (5,026) 1,461 - - - (3,565)
Non-current liabilities
Long-term borrowings (9,363) - (1,435) (25) - (10,823)
Deferred tax liabilities (3,472) - - - (3,472)
Pensions and other post-employment benefits (256) - - - (256)
Derivative financial instruments (21) (21)
Other provisions (30) - - - (30)
Other non-current liabilities (6) - - - (6)
Total non-current liabilities (13,148) - (1,435) (25) - (14,608)
Total liabilities (18,174) 1,461 (1,435) (25) - (18,173)
Net assets 26,849 - - (11,064) (84) 15,701
211

Notes
(1) The net assets of the Group as at 31 March 2022 have been extracted without material
adjustment from the consolidation schedules used to prepare the Interim Financial Information
for the Group for the three months ended 31 March 2022 set out in Part VI (Selected Financial
Information).
(2) This adjustment reflects the receipt of the Notes Proceeds Loans and related party loans.
Under the terms of the Notes Proceeds Loan Agreements, the Notes Proceeds Loans will be
repaid in full upon notice that the Demerger Resolution has been approved by GSK and Pfizer.
Notes £m
Receipt of loan amounts owing from related
parties
a 11,330
Payment of loan amounts owing to related
parties b (1,461)
Total 9,869
a. Receipt of loan amounts owing from related parties includes Notes Proceeds Loans of
£9,210 million and loan amounts owing from GSK as part of the Group’s banking
arrangements of £2,120 million.
b. Payment of loan amounts owing to related parties includes loan amounts owing to GSK
as part of the Group’s banking arrangements of £1,461 million.
(3) Additional borrowings of £1,435 million to fund the payment of the Pre-Demerger Dividend,
including, but not limited to, the Term Loan Facility. See also paragraph 7.4 of Part VII
(Operating and Financial Review).
(4) The Pre-Separation Dividends include the Balancing Dividend, Pre-Demerger Dividend, and
the Sweep-up Dividend.
Notes £m
Balancing Dividend a 53
Pre-Demerger Dividend b 10,345
Sweep-up Dividend c 641
Pre-Separation Dividends 11,039
a. The Balancing Dividend reflects the cash dividend of £53 million to be paid by the
Group to GSKCHH prior to Separation in connection with the £25 million of Non-Voting
Preference Shares issued to Pfizer recognised in long-term borrowings.
b. The Pre-Demerger Dividend reflects the cash dividend of £10,345 million to be paid by
the Group to GSKCHH and PFCHH ahead of Separation, in accordance with the terms
of the Pfizer SHA, which, in summary, requires an amount equal to the Pre-Separation
212

Debt Proceeds of the Group less £300 million to be paid to GSKCHH and PFCHH prior
to Separation.
£m
Pre-Separation Debt Proceeds 10,645
Less £300m (300)
Pre-Demerger Dividend 10,345
c. The Sweep-up Dividend reflects the cash dividend of £641 million to be paid by the
Group to GSKCHH and PFCHH, in accordance with the terms of the Pfizer SHA
which, in summary, requires all readily available cash in excess of £300 million to be
paid to GSKCHH and PFCHH prior to Separation. The actual amount paid is subject
to, amongst other things, additional cash flow generated by, or additional investments
made by, or dividends paid in the ordinary course of business by the Group up until
the point of Separation. As such, the actual amount of the Sweep-up Dividend may
therefore differ from the amount referred to above.
£m
Cash and cash equivalents and liquid investments
as of 31 March 2022 383
Receipt of Notes Proceeds Loans and related party
loans
9,869
Additional borrowings 1,435
Payment of Pre-Demerger Dividend (10,345)
Transaction costs (84)
Balancing Dividend (53)
Less trapped cash * (264)
Less £300m (300)
Sweep-up Dividend 641
* Cash and cash equivalents that are in jurisdictions that have absolute cross-
border restrictions on transfers of cash between members of the Group.
(5) Transaction costs comprise charges for services relating to the Transactions. The Group
expects to incur a cumulative total £117 million of transaction costs in relation to the
Transactions. The Group has incurred £33 million of transaction related costs as of 31 March
2022. Therefore, a transaction cost adjustment of £84 million has been made.
(6) The Pro Forma Financial Information does not reflect any changes in the trading results or
financial position of the Group since 31 March 2022. None of the adjustments are expected to
have a continuing effect on the Group.
213

(7) On a pro forma basis, net debt of the Group as at 31 March 2022 would have been
£10,349 million.
Group net
debt at 31
March
2022
Pro forma
adjustments
Notes
Unaudited pro
forma at 31
March 2022
£m £m £m
Short-term borrowings 80 80
Long-term borrowings 9,363 1,460 a 10,823
Derivative financial liabilities 36 36
Cash and cash equivalents and liquid
investments
(383) (181) b (564)
Derivative financial assets (26) (26)
Net debt 9,070 1,279 10,349
a. Additional borrowings to fund the payment of the Pre-Demerger Dividend, including, but not
limited to, the Term Loan Facility. See also paragraph 7.4 of Part VII (Operating and Financial
Review).
b. Receipt of Notes Proceeds Loans and related party loans of £9,869 million, additional
borrowings of £1,435 million, payment of Pre-Separation Dividends of £11,039 million and the
payment of Transaction costs of £84 million.
214

Section B: Accountants Report on Pro Forma Financial Information
Deloitte LLP
Hill House
1 Little New Street
London
EC4A 3TR
The Board of Directors
on behalf of Haleon plc
980 Great West Road,
Brentford, Middlesex,
TW8 9GS,
United Kingdom
Goldman Sachs International
Plumtree Court,
25 Shoe Lane,
London,
EC4A 4AU
Citigroup Global Markets Limited
Citigroup Centre,
Canada Square,
Canary Wharf,
London,
E14 5LB
Merrill Lynch International
2 King Edward Street,
London
EC1A 1HQ
1 June 2022
Dear Sirs/Mesdames,
Haleon plc (the “Company”)
We report on the pro forma financial information (the “Pro forma Financial Information”) set out in
Part IX of the prospectus dated 1 June 2022 (the “Prospectus”). This report is required by the PR
Regulation and is given for the purpose of complying with that regulation and for no other purpose.
Opinion
In our opinion:
(A) the Pro forma financial information has been properly compiled on the basis stated;
and
(B) such basis is consistent with the accounting policies of the Company.
215
Responsibilities
It is the responsibility of the directors of the Company (the “Directors”) to prepare the Pro forma
financial information in accordance with Annex 20 sections 1 and 2 of the PR Regulation.
It is our responsibility to form an opinion, as to the proper compilation of the Pro forma financial
information and to report that opinion to you in accordance with Annex 20 section 3 of the PR
Regulation.
Save for any responsibility arising under Prospectus Regulation Rule 5.3.2R(2)(f) to any person as and
to the extent there provided, to the fullest extent permitted by law we do not assume any responsibility
and will not accept any liability to any other person for any loss suffered by any such other person as a
result of, arising out of, or in connection with this report or our statement, required by and given solely
for the purposes of complying with Annex 1 item 1.3 of the PR Regulation, consenting to its inclusion in
the Prospectus.
In providing this opinion we are not updating or refreshing any reports or opinions previously made by
us on any financial information used in the compilation of the pro forma financial information, nor do we
accept responsibility for such reports or opinions beyond that owed to those to whom those reports or
opinions were addressed at the date of their issue.
Basis of preparation
The Pro Forma Financial Information has been prepared on the basis described in the notes therein,
for illustrative purposes only, to provide information about how the transaction might have affected the
financial information presented on the basis of the accounting policies adopted by the Company in
preparing the Historical Financial Information for the period ended 31 December 2021.
Basis of Opinion
We conducted our work in accordance with the Standards for Investment Reporting issued by the
Financial Reporting Council in the United Kingdom. We are independent of the Company in
accordance with the Financial Reporting Council’s Ethical Standard as applied to Investment Circular
Reporting Engagements, and we have fulfilled our other ethical responsibilities in accordance with
these requirements.
The work that we performed for the purpose of making this report, which involved no independent
examination of any of the underlying financial information, consisted primarily of comparing the
unadjusted financial information with the source documents, considering the evidence supporting the
adjustments and discussing the Pro Forma Financial Information with the Directors.
We planned and performed our work so as to obtain the information and explanations we considered
necessary in order to provide us with reasonable assurance that the Pro Forma Financial Information
has been properly compiled on the basis stated and that such basis is consistent with the accounting
policies of the Company.
Our work has not been carried out in accordance with auditing or other standards and practices
generally accepted in jurisdictions outside the United Kingdom, including the United States of America,
and accordingly should not be relied upon as if it had been carried out in accordance with those
standards or practices.
216
Declaration
For the purposes of Prospectus Regulation Rule 5.3.2R(2)(f) we are responsible for this report as part
of the Prospectus and declare that to the best of our knowledge, the information contained in this
report is, in accordance with the facts and that the report makes no omission likely to affect its import.
This declaration is included in the Prospectus in compliance with Annex 1 item 1.2 of the PR
Regulation.
Yours faithfully
Deloitte LLP
Deloitte LLP is a limited liability partnership registered in England and Wales with registered number
OC303675 and its registered office at 1 New Street Square, London EC4A 3HQ, United Kingdom.
Deloitte LLP is the United Kingdom affiliate of Deloitte NSE LLP, a member firm of Deloitte Touche
Tohmatsu Limited, a UK private company limited by guarantee (“DTTL”). DTTL and each of its member
firms are legally separate and independent entities. DTTL and Deloitte NSE LLP do not provide
services to clients.
217
PART X
REGULATORY OVERVIEW
1. Overview
The Group’s activities are subject to a rigorous regulatory framework on a local and
international level that conditions and affects the Group’s activities. The process of obtaining
regulatory approvals and ongoing compliance with applicable laws, regulations and other
requirements require the expenditure of substantial time and financial resources.
The following is a summary of the regulatory landscape applicable to the Group’s business in
the key markets in which the Group operates. Where there are material differences, the
applicable local regulatory framework is also summarised in respect of the USA, EU and/or
China, being key markets for the Group’s business from a regulatory perspective. Paragraphs
2 to 5 of this Part X (Regulatory Overview) below summarise the applicable laws, regulations
and other requirements that are materially relevant to the Group’s consumer healthcare
products.
The Group has products in a number of different regulatory classifications. From a regulatory
perspective, the majority of the Group’s products can be categorised according to four principal
regulatory classifications: (i) OTC medicines; (ii) medical devices; (iii) foods; and (iv) cosmetics.
These classifications and their application to a given product in a given market may vary
according to jurisdiction, the nature of the product and changes in law, among other variables.
For example, while supplements are typically regulated as foods, in certain jurisdictions they
may be regulated as medicines where they mitigate disease states or their ingredient levels
exceed locally defined maximum thresholds for supplements. Accordingly, certain products will
be subject to varying levels of regulation in different markets.
Additional laws, regulations and other requirements materially relevant to the Group’s business
are summarised in paragraphs 6 to 12 of this Part X (Regulatory Overview) below.
2. OTC medicines
Medicines are broadly defined as any product (or any ingredient(s) of such product) with an
intended use to treat, prevent or cure a disease or medical condition. There are two main
classifications of medicines: (i) those requiring a prescription; and (ii) those that can be bought
over-the-counter without a prescription. Examples of OTC medicines include analgesics such
as ibuprofen and paracetamol (known as acetaminophen in the USA); indigestion remedies
such as antacids; and decongestants such as xylometazoline and oxymetazoline.
2.1 Regulation of OTC medicines
In general, regulations applicable to prescription medicines also apply to OTC medicines.
Regulations relating to manufacturing, testing, facility registration and inspection, clinical trials,
importation, safety monitoring and risk management apply equally to both classifications. The
principles followed are the guidelines and standards published by the International Council for
Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (“ICH”), of
which the USA, the EU and China are all members. The ICH brings together regulatory
authorities and the pharmaceutical industry to discuss scientific and technical aspects of
pharmaceuticals and to develop ICH guidelines. The ICH guidelines cover quality, efficacy and
safety aspects, among other topics. There are additionally often specific regulations that apply
to OTC products to address unique issues common to these types of medicines.
218
USA
In the USA, the Group must comply with laws, regulations and other requirements promulgated
by numerous federal and state authorities, including the United States Food and Drug
Administration (“FDA”) and other agencies and divisions of the Department of Health and
Human Services, the Drug Enforcement Administration and other agencies of the Department
of Justice, the Consumer Product Safety Commission, the Environmental Protection Agency,
Customs and Border Protection (for imports and exports), the Federal Trade Commission
(“FTC”) and state agencies. Applicable legal requirements govern, to varying degrees, the
research, development, manufacturing, commercialisation and sale of the Group’s products,
including pre-clinical and clinical testing, approval, production, labelling, sale, distribution,
import, export, post-market surveillance, advertising, dissemination of information and
promotion. Failure to comply with applicable legal requirements can result in product recalls,
seizures, injunctions, refusal to approve or withdrawal of approval of product applications,
monetary fines or criminal prosecution.
The FDA is the principal regulator of OTC medicines. Its authority comes primarily from the
Federal Food, Drug and Cosmetic Act of 1938, as amended. In addition to reviewing New Drug
Applications (“NDAs”) for branded drugs and Abbreviated New Drug Applications (“ANDAs”)
for generic drugs and overseeing the OTC drug monograph framework, the FDA has the
authority to ensure that drugs introduced into interstate commerce are not “adulterated”. For
these purposes, adulterated means that the product or its manufacture does not comply with
FDA quality and related standards. A drug is adulterated if, among other things: (i) it is
prepared under unsanitary conditions such that it may have been contaminated or may cause
injury to patients; (ii) its manufacture does not comply with Good Manufacturing Practice
(“GMP”); (iii) it does not comply with an official compendium; (iv) its strength, purity or quality
differs from that which it purports to possess; or (v) it is manufactured, processed or held in a
facility which refuses FDA inspection.
EU
In the EU, medicinal products are subject to extensive pre- and post-marketing regulation by
regulatory authorities at both the EU and Member State (national) levels. The EU system is
based on a closely coordinated regulatory network of national competent authorities in the EEA
working together with the European Medicines Agency (“EMA”) and the European
Commission, whose principal role is to take binding decisions based on the scientific
recommendations delivered by the EMA. The network was built to help ensure that safe,
effective and high-quality medicines are authorised throughout the EU, and that patients,
healthcare professionals and citizens are provided with adequate and consistent information
about medicines.
China
In China, the National Medical Products Administration (“NMPA”) is the primary regulatory
authority. Its objectives are: (i) to supervise the safety of drugs (including traditional Chinese
medicines and ethno-medicines), medical devices and cosmetics; (ii) to regulate the
registration of drugs, medical devices and cosmetics; and (iii) to undertake associated
standards management. There are a number of institutions affiliated with the NMPA, including:
the National Institutes for Food and Drug Control; the Chinese Pharmacopoeia Commission;
the Center for Drug Evaluation (“CDE”); the Center for Food and Drug Inspection; the Center
for Drug Reevaluation; and the Center for Medical Device Evaluation. China has significantly
219
updated its regulatory framework under the Drug Administration Law of 2019, issuing new
regulations to modernise the healthcare system.
2.2 Marketing authorisation process
A licence is generally required to market a medicine. Regulatory agencies issue a product
licence based on a marketing authorisation application (“MAA”) dossier. A dossier is compiled
and submitted to regulatory authorities in accordance with local regulations. Once a licence is
granted, the marketed product must be compliant with its registered details and any change to
the technical details requires an update to the registration. However, in some instances, local
regulations may allow marketing without a specific prior approval, provided defined criteria are
met.
The licence indicates the legal status of the product: prescription or OTC, as well as any other
restrictions on the marketing or use of the product. Regulatory agencies may have different
views on whether a particular product is appropriate to be marketed as OTC in their countries.
In some instances, a regulatory agency may issue certain conditions for approval, referred to
as post-marketing commitments or obligations. This may mean that the company must conduct
a Phase IV (or post-marketing) study in order to provide the agency with additional information
about the use of the medicine in the general population under marketing conditions. Failure to
comply can result in licence revocation.
Site inspections
Site inspections are a routine aspect of a regulatory authority’s review of the MAA to ensure
medicines are manufactured in accordance with GMP. Inspections by the FDA or EU agencies
may be recognised for registration in international markets outside of the USA and EU.
However, some local regulatory authorities require conduct of their own site inspections.
Scheduling and waiting for the results of these site inspections is time consuming, often adding
one to two years to the registration process.
USA
In order to market and sell a new drug product in the USA, a drug manufacturer must either:
(i) file an NDA that shows the quality, safety and effectiveness of the new drug; (ii) file an
ANDA that demonstrates equivalence of a generic to another company’s branded drug
product; or (iii) comply with the OTC drug monograph requirements, which are the rules for
each therapeutic category establishing conditions, such as active ingredients, uses, doses and
testing, under which an OTC drug is generally recognised as safe and effective and can be
marketed without an NDA and FDA pre-market approval.
EU
In the EU, application dossier content requirements for medicinal products are set by the
European Commission and, like the USA and many other markets, are aligned with ICH
guidelines. There are several administrative mechanisms to request regulatory approval of a
medicine (both prescription and non-prescription): (i) the centralised procedure, which is an EU
authorisation route resulting in a single marketing authorisation valid in all EU Member States
and EEA countries; (ii) the mutual recognition procedure, resulting in a mutually recognised
product (used where a product is already authorised in at least one Member State and
220
approval is sought in at least one other Member State); (iii) the decentralised procedure,
resulting in a mutually recognised product (used where a product is not already authorised in
any Member State and the centralised procedure is not available or selected); and (iv) the
standalone national procedure for authorisation in a single Member State.
China
In China, applications to market medicinal products are covered under the Drug Registration
Regulation 2020, which covers, among other requirements, GMP and requirements of good
clinical practice (“GCP”). “Technical guidance”, issued by the CDE, indicates Chemistry,
Manufacturing and Control data and both clinical and non-clinical requirements. The key
elements of any regulatory application are quality, safety and efficacy, and, until recently, there
has been one process for the registration of all medicines in China, irrespective of prescription
or OTC status. However, the new Drug Registration Regulation 2020 provides an alternate
process for OTC medicines, which maintains the principles of quality, safety and efficacy.
2.3 Post-marketing authorisation
Compliance with registered details and post-marketing changes
Once the marketing authorisation licence is granted, the company is required to comply with
the conditions of approval and must always ensure that the product label used in the market is
compliant with the registration and that the product is manufactured and supplied in
compliance with registered details.
Non-compliance can lead to product recalls or other action by the regulatory authority, often
posted publicly. This may involve fines, licence revocation and/or increased inspection of the
manufacturing sites and/or other products marketed by the company.
Any changes to registered details relating to the marketing authorisation require registration
updates, which may require regulatory authority approval (and potentially a review fee) prior to
implementation. This mostly applies to changes that could impact product quality
(manufacturing implications), safety or efficacy (e.g. a new indication). When such approval is
required, the review times vary depending on the type and extent of the change. Following
approval, the changes form part of the licence requirement and must be implemented within
the timeframe required by the local regulator.
Licence maintenance and expiration
Many regulatory authorities grant licences that are effective for a specified period of time, after
which a renewal application must be submitted to continue to market the product. This renewal
period is typically every five years. Once a licence is granted, it is the company’s obligation to
keep the licence effective. If a product is never marketed or if a renewal application is not
submitted on time, the licence is lost.
2.4 Other OTC medicines regulations
Rx-to-OTC switches
An Rx-to-OTC switch refers to the process by which the legal classification of a drug changes
from Rx (prescription) to OTC (non-prescription) status. This involves the generation of
221
extensive supportive data to establish that the drug can be used safely and effectively by
consumers based only on their understanding of the product labelling and without the
intervention of a healthcare professional.
Rx-to-OTC switches require in-depth consideration of the inherent safety profile and efficacy of
a drug, balanced with mitigation of the potential increased risks associated with OTC
availability. They also require consideration of the capability of consumers to use the product
appropriately based on labelling and associated instructions.
For a drug to be suitable for OTC status: (i) the indication must be for a condition that a
consumer can recognise themselves; (ii) the benefit of the product must exceed the risks
(including any potential adverse events being of low incidence and easily identifiable by
consumers); (iii) the potential to misuse or abuse the drug must be low; (iv) the labelling of the
product must be compliant (see paragraph 7 of this Part X (Regulatory Overview) below); and
(v) there must be data demonstrating the efficacy and safety of the product. This supporting
data includes detailed analysis of the drug’s safety from clinical studies and in-market use, as
well as label comprehension studies and sometimes “actual use” studies, which demonstrate
appropriate consumer selection/deselection and product use that complies with label
instructions.
Other market-specific requirements
National authorities sometimes have requirements and internal procedures for assessing
product quality, efficacy and safety for marketing authorisations, for example, requiring local
market study data to demonstrate relevance to the target market population. However, many
requirements can be managed by providing additional certificates or notarising documentation
to provide assurance of data authenticity from markets where permits or approvals have
already been obtained.
Certificate of Pharmaceutical Product
The WHO, in an effort to assist smaller regulatory authorities with marketing authorisation
applications, particularly those that may not have the ability to assess product quality
independently, established a recommended format for a Certificate of Pharmaceutical Product
(“CoPP”). This certificate is required by the importing country as part of the local registration
procedure in certain markets. CoPPs are issued for each drug product. Many countries require,
or strongly prefer, a CoPP issued by the regulatory authority in the country of manufacture (or
“source” CoPP). Some countries accept a “non-source” CoPP, but this is typically by exception.
Before requesting a CoPP, the medicine must first be registered and, in many cases,
marketed.
Import and export
USA
Importers of medicines to the USA must comply with United States Customs and Border
Protection documentation requirements and examination. Perceived violations will be refused
admission and a notice of detention and hearing may be issued. If the FDA ultimately refuses
admission, a redelivery notice may be received with a notice for damages for up to three times
the product value.
222
Products for export from the USA are subject to the import requirements of the importing
foreign country and, if the product is not approved in the USA, the company must apply to the
FDA for appropriate export documentation.
EU
Manufacturers and importers of medicinal products located in the EEA must hold a
manufacturing authorisation issued by the national competent authority of the Member State
where such activities are being carried out. The manufacturer or importer must have a qualified
person who is responsible for certifying that each batch of product has been manufactured in
accordance with EU GMP before releasing the product for commercial distribution in the EU or
for use in a clinical trial. Manufacturing facilities are subject to periodic inspections by the
competent regulatory authorities for compliance with GMP.
China
Imports to and exports from China follow the same principles as the USA and EU. Chinese
importers must provide necessary documents (including, for example, import drug licence,
GSP licence, business licence, imported batch product’s certificate of analysis / country of
origin / order / commercial invoice / packing list / bill of lading, local drug test report and
historical import evidence, in each case as applicable) to the provincial healthcare authorities
or NMPA, who in turn provide a customs form for the importer to present at China customs.
Failure to meet the requirements results in the rejection of goods at the border.
Where medicines are being imported, an import drug licence is required, which grants the
manufacturer the right to register, import, sell and use the imported drug in China. In the
application process for the import drug licence, the NMPA: reviews a dossier documenting the
quality, safety and efficacy of the drug; verifies the quality specification of the drug; and
performs a sample test on three batches of the drug. Once granted, the licence remains active
for a five-year renewable period.
3. Medical devices
Medical devices are broadly defined as products which a manufacturer intends to be used to
diagnose, prevent, monitor, predict, treat or alleviate disease. Devices generally achieve their
purpose by physical modes of action; the principal intended action may not be
pharmacological, immunological or metabolic.
3.1 Classification of medical devices
Although different regulatory authorities have different systems of review before a medical
device can be marketed, they all apply a risk management approach to classify devices. All
medical devices must satisfy safety and performance, quality system (some low-risk devices
may be exempt) and labelling requirements. The degree of regulatory scrutiny increases with
the potential risks of the medical device.
The purpose of risk classification is to ensure that the regulatory controls applied to a medical
device are proportionate to the risk. Most markets have an overall I-III classification system,
with class I being the lowest risk and class III being the highest. Class III devices usually
support life, present high risk of illness or injury, or are implanted. Examples include
pacemakers and catheters. Class II devices present more moderate risk to the user and
223
include, for example (under the MDR (as defined below) in the EU), denture cleansers, denture
adhesives, pain-relieving heat patches and therapeutic toothpastes. Class I devices have the
lowest perceived risk and include devices such as, liquid medicine measuring cups, spectacles
and bandages. The Group’s products, throughout its global portfolio, are largely classified
nationally as Class II or Class I medical devices.
The regulatory requirements increase as the device risk class increases. These regulatory
controls may include, for example, those in relation to: (i) the operation of a quality system for
all devices; (ii) the need for and frequency of independent external audit of the manufacturer’s
quality system; (iii) a reference technical file with support data defining performance and
controls; (iv) independent external review of the technical data; (v) product testing using
in-house or independent resources; and (vi) documentation of relevant clinical evidence to
support the manufacturer’s claims.
3.2 Market authorisation
USA
In the USA, most Class III devices and new devices that are not substantially equivalent to an
already legally marketed product require clearance through a Pre-Market Approval (“PMA”).
There must be documented safety and effectiveness data for the device and clinical data is
required. Where a PMA is not needed, most Class II and some Class I devices require a 510k
submission, which must demonstrate how the proposed medical device is substantially
equivalent to a medical device that is already on the US market and an FDA clearance
decision is generally received within 150 days. Most Class I and some Class II devices are
exempt from a 510k submission before sale, but are still subject to general control
requirements. For low-risk products for which there is no legally marketed substantially
equivalent device in the USA (and therefore the 510k submission is inappropriate or has
resulted in a not substantially equivalent determination), there is the De Novo classification
request. This provides a pathway to classify novel medical devices for which general controls
alone, or general and special controls, provide reasonable assurance of safety and
effectiveness for the intended use. The De Novo review is a stringent process. Devices that are
classified into Class I or Class II through a De Novo request may be marketed and used as
predicates for future 510k submissions.
EU
In the EU, manufacturers may self-certify compliance of Class I devices with simple
notifications to the competent authority, with files open to inspection should a competent
authority wish to do so. Class II devices, as well as some Class I devices (those with a
measuring function or sterility requirements), require the involvement of an approved notified
body which audits files / manufacturers on behalf of the competent regulatory authority.
Class III devices generally require the involvement of a notified body and often the competent
authority as well. Following product clearance, the manufacturer signs a declaration of
conformity and places the Conformité Européenne CE mark on or with the device.
In May 2021, the Medical Device Regulation (Regulation (EU) 2017/745) (“MDR”) came into
effect in the EU, with new in-vitro diagnostic regulations anticipated in 2022. The MDR is more
comprehensive than the Medical Device Directive (93/42/EEC). The MDR greatly increases the
rigor and robustness of the regulations governing medical device products in EU markets.
There is no “grandfathering” of products: all products are expected to meet the MDR
224
requirements. Additionally, all products and their manufacturers are subject to re-review by the
notified body on a yearly cycle (for Class IIb and Class III devices) or every two years (for
Class IIa devices) or a “periodic” review up to every four years (for Class I devices).
China
In China, the NMPA is the institution responsible for both medical devices and medicines. For
locally manufactured devices in China, Class I and Class II devices go respectively to the
municipal and provincial authorities to obtain market authorisation approval. All Class III
devices and devices not manufactured in China go to the NMPA. In the latter case,
manufacturers must send the appropriate documentation showing that the device has been
approved in its country of origin.
To register a device, type testing is required. In most cases, device samples are provided to an
NMPA-accredited institute for testing. It may also be required to provide supportive clinical data
along with the application, especially for higher risk devices.
Foreign manufacturers must also have China-based agents that will represent their interests in
China. The responsibilities of the designated agents include providing technical service and
maintenance support for the device, assisting with device recall (if recall is required),
overseeing the registration process, and providing support for the manufacturer in case
adverse events occur due to device malfunction.
Medical device registrations in China are valid for five years. Market authorisation holders
must: (i) ensure the quality of their products; (ii) show that their products meet all applicable
requirements; (iii) submit self-inspection reports to relevant authorities every year; and
(iv) maintain their products’ information in the NMPA’s unique device identification database.
4. Food (dietary supplements)
4.1 General
Products in the food classification include vitamins, minerals and supplements to be ingested
as part of a daily diet. Most food products do not require pre-market authorisation, although
specific categories of foods (such as food supplements, foods for special medical purposes or
dietary supplements in China) may require notification of sale to applicable regulatory bodies.
In some countries, such as China, products classified as functional health foods also require a
formal pre-market review and registration process.
The food industry typically avoids registration as a form of food control in favour of systems
based on Hazard Analysis Critical Control Point (“HACCP”), an internationally recognised
method of assessing, preventing and managing food safety risks, with low-risk foods subject to
few controls and high-risk foods subject to more controls.
The majority of products marketed by the Group in this classification are regulated as dietary
supplements. However, some supplements may instead be regulated as medicines, for
example, where they mitigate disease states or their ingredient levels exceed locally defined,
e.g. recommended dietary allowances, to be categorised as a supplement.
225
4.2 Market authorisation
Dietary supplements
A dietary supplement is a product taken by mouth that contains a dietary ingredient intended to
supplement the diet. A “dietary ingredient” is one, or any combination, of a vitamin, a mineral, a
herb or other botanical, an amino acid, a dietary substance for use by the consumer to
supplement the diet by increasing the total dietary intake (e.g. enzymes or tissues from organs
or glands), or a concentrate, metabolite, constituent or extract.
In most markets, dietary supplements do not require a submission or approval prior to launch,
although novel ingredients may require supporting submissions, as may new claims.
Notification procedures prior to or immediately after sale commences are typically required.
4.3 Food safety
Safety and packaging
For the most part, food laws adopt a principled, risk-management approach to ensure safety of
the food chain. They lay down basic good hygiene and safety prerequisites, and require food
businesses to assess ingredient risk and to remove or mitigate those risks following the
HAACP framework.
One area of food safety that poses a specific risk to consumers different from most cosmetics
or medical devices and some medicinal products is the degree of exposure to substances that
migrate from food contact packaging materials into foodstuffs. Therefore, primary food
packaging in contact with food is subject to a suite of complex, food-specific legislation driven
by these safety concerns.
Composition
Composition is intimately linked to safety. Any food ingredient without a history of safe
consumption is termed a novel food or new dietary ingredient and cannot be sold as a food or
added to a food without pre-authorisation by relevant regulatory agencies. This authorisation
requires the applicant to rigorously demonstrate the safety of the substance, and this complex
process can sometimes take years to accomplish.
Food products often require and use additives (e.g. colours, preservatives, stabilisers,
emulsifiers). There are lists of permitted additives that are regularly evaluated and many have
maximum permitted levels. The use of a new additive, or the use of an existing additive for a
purpose not explicitly permitted by law, requires pre-authorisation, and food manufacturers are
required to demonstrate both safety and technical need before a new additive use is
authorised.
5. Cosmetics
5.1 General
Cosmetics are products that are applied to external parts of the human body (generally also
including the teeth and mucous membranes of the oral cavity) for cleansing, beautifying,
promoting attractiveness or altering the appearance without affecting the body’s structure or
226
functions. They typically include examples such as shampoo, deodorant, perfume and some
toothpastes. However, products can be classified differently by country or region, and a
cosmetic in one country may be classified as a medicine, or even a medical device, in another
country. For example, fluoride toothpaste is a cosmetic in the EU and a drug in the USA. Other
products regulated as drugs in the USA include mouthwashes marketed with therapeutic
claims, skin protectants (such as lip balms) and treatments for dandruff or acne.
5.2 Market authorisation
Regulations on market authorisation for cosmetics differ by country or region. Some countries
require pre-market approvals, while others require no registration. Where approval is required,
the standard of documentation required to market cosmetics differs by country. For example,
some countries require a robust dossier (which will include safety assessments, detailed
manufacturing information, raw material functionality and other pertinent information) where
manufacturers present documentation to the health ministries to be approved or denied. In
some other countries, documentation is not required to be presented and can remain on file
with the manufacturer.
6. Clinical trials for OTC medicines
The beginning of the development phase for a new drug involves pre-clinical in vitro and in vivo
laboratory studies to assess the potential effects of substances and examine chemical-physical
properties, toxicological data and other information. Following positive pre-trial results and
approval, the drug in question is tested in humans in clinical trials which consist of four phases,
each phase requiring increasingly large, complex, costly and time-consuming clinical studies.
The first three phases must take place before market authorisation is obtained (see paragraph
2.2 of this Part X (Regulatory Overview) above).
Clinical trials are subject to the GCP requirements set out by the ICH in the USA, the EU,
China and other ICH markets. These include the requirement that all research patients provide
their informed consent in writing for their participation in any clinical trial.
USA
The pre-clinical and clinical development paths in the USA are broadly similar to those in the
EU (described below) and are governed by the same GCP requirements. Before commencing
the clinical trial, an Investigational New Drug Application is submitted to the FDA and the
sponsor must also obtain a favourable opinion from an independent ethics committee. A
protocol and any subsequent protocol amendments must be submitted to the FDA. In addition,
an institutional review board at each institution participating in the trial must review and
approve the plan for any clinical trial before it commences at that institution. Information about
certain clinical trials must be submitted within specific timeframes to the National Institutes of
Health for public dissemination on their website. Regulatory authorities, institutional review
boards or the sponsors may suspend or terminate a clinical trial at any time on various
grounds, including a finding that the research patients are being exposed to an unacceptable
health risk.
EU
In the EU, prior to commencing a clinical trial, the sponsor must obtain a Clinical Trial
Authorisation (“CTA”) from the competent authority of the Member State in which the trial will
be conducted, and a positive opinion from an independent ethics committee. In the EU, the
227
European Medicines Agency manages this process centrally through the Clinical Trials
Information System (“CTIS”) which allows assessment, authorisation and maintenance of
clinical trials through a single entry point for both health authority and ethics committee
considerations across all Member States. This harmonises the process for all Member States
and ensures the same GCP standards are applied across all Member States. Every clinical trial
must have a sponsor and any sponsor that is not established within the EEA must appoint a
legal representative in the jurisdiction. The CTA application includes, among other things, a
trial protocol detailing the objectives, design, methodology, statistical considerations and
organisation of the trial, and an investigational medicinal product dossier with information about
the manufacture and quality of the drug being investigated. During the trial, any substantial
changes to the protocol or any adverse side effects reported must be notified to the competent
authority and ethics committee via the CTIS portal. Following the trial, the sponsors must post
clinical trial results in the European Union Drug Regulating Authorities Clinical Trials database.
China
As in the EU and USA, clinical trials in China require compliance with ICH and GCP principles
and pre-trial approval from both the regulator, the NMPA, and an ethics committee. For certain
studies, approval from both central and local ethics committees may be required. Approval may
also be required before commencing studies of certain cosmetics, such as toothpaste, or when
new claims or ingredients are being proposed. For clinical studies collecting human biological
samples, additional approval is needed from the Office of Human Genetic Resources
Administration. China has stringent requirements relating to site governance, managed through
GCP offices located within hospitals. Any amendments to trial protocol or safety concerns must
be reported to the relevant authorities, who have the power to suspend or terminate a trial
based on various grounds. Following a clinical trial, the sponsor must publish the trial results in
a publicly accessible registry.
7. Claims and labelling
The labelling for all product classifications which the Group markets, including OTC medicines,
medical devices, foods and cosmetics, is subject to applicable laws in all of the markets in
which the Group operates. Labelling regulations differ by market and product classification.
They may specify text format and the order of information, as well as require specific
information and statements. For example, they may require inclusion of, among other things,
product identity, product ingredients, the name and place of business of the manufacturer,
packer or distributor, net quantity of contents, expiry date, batch number, registration number
and instructions for appropriate use.
Claims in advertisements and on labels must be truthful, not misleading, not unfair, and
substantiated, and regulatory authorities may take enforcement action against businesses
which fail to comply with relevant rules. The extent of substantiation required for a claim, as
well as the level of regulatory scrutiny applied by authorities, is dependent on the product
classification and product’s risk profile, with OTC medicines typically requiring greater
substantiation, and varies from country to country.
USA
The FTC, FDA and other government agencies enforce compliance with applicable laws on
product claims, which broadly require claims to be truthful, not misleading and sufficiently
substantiated with scientific evidence on the benefits and safety of the product. The FTC will
228
also consider how consumers will interpret claims, including in circumstances where the claim
may be technically true, but the advertisement and what is implied may nonetheless be
misleading.
There are specific requirements for different product classifications. For example, while
cosmetic labelling does not require FDA approval prior to going on market, the FDA regulates
cosmetic labelling claims and monitors, and takes action against, claims which are not truthful,
are misleading or make medicinal claims. Under the Federal Food, Drug, and Cosmetic Act,
the FDA may take action against “misbranding” violations, which include where the cosmetic’s
label does not include all required information or such information is not adequately prominent
and conspicuous. Similarly, for foods, businesses are responsible for evaluating the safety and
labelling of their products before marketing to ensure that they meet all the requirements of
applicable FDA regulations and the Dietary Supplement Health and Education Act of 1994. The
FDA is responsible for taking action against any adulterated or misbranded dietary supplement
product after it reaches the market.
EU
Advertising of products is subject both to general consumer advertising requirements pursuant
to the Unfair Commercial Practices Directive (Directive 2005/29/EC), which states a general
prohibition on misleading and aggressive advertising, as well as more specifically in respect of
each product classification. For example, advertisements of medicinal products must: (i) be set
out in such a way that it is clear that the message is an advertisement and that the product is
clearly identified as a medicinal product; (ii) not refer, in improper, alarming or misleading
terms, to claims of recovery; and (iii) not give the impression that a medical consultation or
surgical operation is unnecessary (pursuant to Directive 2001/83/EC).
The advertising and promotion of a medical device must be undertaken in accordance with its
intended purpose. The MDR prohibits use of text, names, trade marks, pictures and figurative
or other signs that may mislead the user or the patient with regard to the device’s intended
purpose, safety and performance.
New health claims made on foods need to be reviewed with a positive opinion by the European
Food Safety Agency and approved by the European Commission. The objective is to ensure
that any claim made on a food’s labelling, presentation or advertising in the EU is clear,
accurate and based on scientific evidence.
The EU has also established a legal framework for cosmetic labelling claims based on the
Cosmetics Products Regulation (Regulation (EC) No 1223/2009). Responsible persons must
ensure that a cosmetic product made available on the market is safe for human health when
used under normal or reasonably foreseeable conditions of use, taking into account, in
particular: (i) presentation; (ii) labelling; (iii) instructions for use and disposal; and (iv) any other
indication or information provided by the responsible person.
China
In China, there is similarly an extensive regulatory framework on advertising and product
claims. Among other requirements, OTC medicine advertisements must not contain difficult or
confusing medical or pharmaceutical terms which may mislead the public about the effect and
safety of the proposed products. An advertisement must make clear that the product is OTC by
including the OTC logo. All medical device advertisements must contain the name of the
229
approved medical device, name of the manufacturing enterprise, registration certificate number
and advertisement licence number. All information must conform to the product certificate
issued by the NMPA. The Cosmetic Supervision Administration and Regulation, which came
into force on 1 January 2021, enhances regulatory requirements in respect of cosmetic claims,
including that applicants must submit sufficient scientific evidence on the NMPA’s website for
claim substantiation. The NMPA also enforces categories of permissible and prohibited claims
in respect of foods.
8. Consumer safety and quality
Consumer safety
Manufacturers of OTC medicines, cosmetics, medical devices and foods must ensure that their
products are safe for consumers to use. Vigilance regulations across the world play an
important role in ensuring the safety of all products whether in the development pipeline,
already approved for marketing, or post-launch. These regulations are different for each type of
product but in all cases require the collection, detection, assessment, monitoring and
prevention of adverse events/undesirable effects. Once approved for marketing, the holder of a
medicinal marketing authorisation must also establish and maintain a pharmacovigilance
system as described in ICH guidelines. The obligations include expedited reporting of adverse
reactions, submission of periodic safety update reports and proactive detection of signals/
trends. Pharmacovigilance systems can be subject to inspection by health authorities and
corrective actions may be required to address any deficiencies identified. For medical devices,
manufacturers must also expedite reporting of serious safety events, prepare periodic safety
update reports and proactively analyse trends, with documentation held at manufacturing
facilities for inspection. For cosmetics and foods, “serious undesirable events”/adverse events
are tracked and analysed to ensure that products are fit for use.
Quality
Quality regulations across the world play an important role in ensuring the safety and efficacy
of consumer healthcare products. The regulations are required both for new innovations and
already existing products. Every country has its own regulations which apply to innovation,
manufacturing / good manufacturing practices, testing, marketing, post-marketing studies and
reporting by product classification (e.g. medicines, medical devices, cosmetics and dietary
supplements).
Regulators conduct pre-approval and post-approval inspections of facilities involved in the
development, manufacturing, packaging and testing of drugs to ensure GMP compliance. If an
inspection results in a finding, corrective actions to address the deficiencies must be
performed. Adverse inspections can lead to inspectional observations, warning letters, seizure,
recalls, injunctions and shutdown of facilities.
Medical devices are subject to quality system regulations. A quality system is the
organisational structure, responsibilities, procedures, processes and resources needed to
implement quality management for medical devices. Quality system regulations cover the
methods, facilities and controls used by the manufacturer in the design, manufacture,
packaging, labelling, storage, installation, servicing and post market handling of medical
devices. Quality system requirements can impact all phases in the medical device life span,
including approval of the device. Applicable requirements depend on the risk class of the
device and on the regulatory system of the country.
230
9. Pricing
The Group’s activities are subject to price control laws and regulations in some of the markets
in which it operates. The range and extent of these requirements vary by market.
In China, prices are mainly determined by a mix of regulations and market competition. In
respect of medicines (both Rx and OTC) in the hospital channel, the government regulates
prices through a centralised procurement mechanism, medical insurance reimbursement
standards and strengthened regulation of medical and pricing practices.
In November 2018, China introduced a national volume-based procurement pilot programme
for medicines that are sold in the hospital channel in which companies submit bids for several
generic drugs, with the winners gaining a guaranteed sale volume of the total market for those
drugs for 1-3 years. Since 2019, this pilot programme has been expanded nationwide and also
includes a provincial volume-based procurement programme that allows provincial
governments to include drugs that are outside the national volume-based procurement scope.
Outside the hospital channel, some medicine prices are indirectly managed by certain policies.
In certain cities, such as Shanghai and Nanjing, retail prices are indirectly affected by volume-
based procurement and price control policies, such that the prices are to varying degrees
linked to the hospital channel bidding prices. In many other provinces retail pharmacies can set
prices freely but with an upper limit to reimbursement.
10. Environment and health and safety
The Group’s operations, like those of other healthcare companies, involve the use of
substances regulated under environmental laws, primarily in manufacturing processes and, as
such, the Group is subject to numerous local, national and international environmental
protection and health and safety laws and regulations. Environmental laws are complex,
frequently amended and have generally become more stringent over time.
Certain environmental laws impose strict (i.e. may be imposed regardless of fault) and joint and
several liability on current or previous owners of real property and current or previous owners
or operators of facilities for the costs of investigation, removal or remediation of hazardous
substances or materials at such properties or at properties at which parties have disposed of
hazardous substances. These laws may require that the Group reimburses the government for
costs incurred at these sites or otherwise pays for the cost of investigation and clean-up of
these sites, including compensation for damage to natural resources.
In addition, the Group is subject to increasingly extensive reporting obligations in respect of its
relationship with the environment and climate change. These include, among others, reporting
requirements under the framework of the Task Force on Climate-related Financial Disclosures,
as well as legislation in relation to Streamlined Energy and Carbon Reporting. Disclosure and
reporting requirements in relation to wider ESG matters are also increasingly extensive and
subject to greater regulatory scrutiny. For example, the Group must comply with the Modern
Slavery Act 2015 and make specific disclosures on its engagement with stakeholders,
including employees.
The Group must also comply with applicable safety laws to protect employees against
occupational injuries. Under such laws, employers typically must establish and maintain
working conditions and workplaces that effectively prevent danger to employees. In particular,
231
employers must comply with certain medical and hygiene standards and meet certain health
and safety requirements at work, such as carrying out risk assessments and implementing
measures for the safety of employees.
11. ABAC, AML and sanctions
The Group is required to comply with applicable anti-bribery and corruption (“ABAC”), anti-
money laundering (“AML”) and sanctions regulations in the jurisdictions in which it operates.
These include, among others, the US Foreign Corrupt Practices Act 1977, the UK Bribery Act
2010, the UK Money Laundering, Terrorist Financing and Transfer of Funds (Information on the
Payer) Regulations 2017, and the China Criminal Law of the PRC 2020. Additionally, in certain
jurisdictions, the Group’s engagement with healthcare professionals and other external leaders
is subject to applicable restrictions. For example, in the USA, there are federal and state anti-
kickback laws that prohibit the payment or receipt of kickbacks, bribes or other remuneration
intended to induce the purchase or recommendation of healthcare products and services
covered by government healthcare programmes or reward past purchases or
recommendations.
12. Data privacy
USA
In the USA, the Group is subject to a range of consumer privacy laws, whose specific
requirements vary from state to state. For example, in California, the Group is subject to the
California Consumer Privacy Act of 2018 (“CCPA”). The CCPA requires businesses to comply
with various requirements relating to the collection, use and disclosure of personal information
of California consumers. Notably, the CCPA grants consumers the right to opt-out of the sale of
their personal information by businesses. “Selling” is defined broadly to include almost any
transfer of personal information to a third party for valuable consideration, including through
intra-group transfers. Businesses are required to have a “do not sell my personal information”
button available to consumers on their website homepage, and consumers must be given
explicit notice when their personal information is sold. Companies subject to the CCPA must
also create and publish a privacy policy that discloses: (i) the categories of personal
information the business collects; (ii) the sources(s) from which the personal information is
collected; and (iii) the purpose for which the personal information is collected and/or sold. The
CCPA has been amended by the California Privacy Rights Act 2020 (“CPRA”), which will come
into effect in 2023. The CPRA expands consumer privacy rights and includes an opt-out for
any sharing of personal data for cross-contextual behavioural advertising, whereby businesses
track consumer behaviour across unaffiliated websites and use the data collected for
advertising purposes. Virginia, Colorado and Utah have also passed similar comprehensive
privacy laws, and several more states may join them in the coming months. Like CPRA, the
Virginia, Colorado and Utah laws each include a provision allowing consumers to, among other
things, opt out of the use of their personal information for purposes of online tracking and
targeted advertising. These comprehensive privacy laws are in addition to an existing set of
multi-state laws requiring notification in cases of personal data breach.
EU and UK
Both the EU GDPR and the UK GDPR regulate the processing of the personal data of living
individuals (“data subjects”) by, among others: (i) companies that collect or receive personal
data and control the use of that data (“data controllers”); and (ii) companies that process
232
personal data on behalf of data controllers (“data processors”). The Group is a data controller
and is required to comply with both the EU GDPR (as the Group carries out certain activities in
the EU) and the UK GDPR.
According to the EU GDPR and the UK GDPR, personal data includes any information relating
to data subjects who can be identified from that information. It can therefore include: personal
details such as name, address, email address, telephone number and date of birth; information
relating to the individual, whether in their personal, family or professional life; and any
expression of opinion about an individual or indications of a company’s (or any other person’s)
intentions in respect of that individual; and it includes persistent online identifiers such as IP
address, machine ID and other technical data that can be tied to an individual. The processing
of personal data covers any activity done to or in relation to the personal data. In addition, to
the extent a company processes, controls or otherwise uses “special category” personal data
(including individuals’ health or medical information, genetic information and biometric
information), more stringent rules apply, further limiting the circumstances and the manner in
which a company is legally permitted to process that data.
EU GDPR and UK GDPR entail strict requirements, including with respect to (i) international
data transfers, (ii) data mapping and accountability obligations, (iii) the involvement of a data
processor, (iv) the appointment of a data protection officer, (v) data subjects’ rights (e.g.,
notices, right to data portability and right to be forgotten), (vi) the need to carry out a data
privacy impact assessment regarding data processing activities using new technologies likely
to result in a high risk to the rights and freedom of natural persons, and (vii) notification
obligations in case of a data breach.
There are costs and administrative burdens associated with compliance with the EU GDPR
and the UK GDPR. Any failure or perceived failure to comply carries with it the risk of
significant penalties and sanctions, of up to £17.5 million or 4 per cent. of global turnover for
failure to comply with UK GDPR and up to €20 million or 4 per cent. of global turnover for
failure to comply with EU GDPR.
In addition, the EU is in the process of agreeing a new e-Privacy Regulation (“ePR”), which will
replace the e-Privacy Directive. This will be directly implemented in the laws of each Member
State without the need for further enactment and it is conceivable that the UK will also consider
alignment. The draft ePR imposes new rules around, among other areas, confidentiality of
online communications, the use of cookies and direct marketing. EU regulators recently have
focused attention on advertising technologies that track user behaviour and serve ads based
on online activities and profiles. These initiatives will increase the regulatory burden in respect
of certain business activities including, in particular, the way a business conducts online
research, and online marketing and advertising activities, including efforts to understand users’
internet usage and online purchasing habits.
China
In China, the Group is also subject to a range of data privacy and security regulations,
including the Personal Information Protection Law 2021, the Cybersecurity Law 2016 and the
Data Security Law 2021. Such data regulations have a significant impact on the processing of
data in China, as well as the cost of dedicated systems, teams and infrastructure that may be
required for businesses to comply.
The Personal Information Protection Law 2021 is the first comprehensive legislation on
personal information protection in China. It specifies the scope of personal information, clarifies
233
the legal bases for processing personal information, lays down the obligations and
responsibilities imposed on data processors and imposes stringent requirements on data
localisation. Consequences of non-compliance may include monetary fines of up to 5 per cent.
of the previous year’s turnover, termination of data transfers and personal liability imposed on
those directly responsible.
The Group must also comply with the Cybersecurity Law 2016, which requires the
establishment of internal security management systems that meet the requirements of a
classified protection system for cyber security. This includes: appointing dedicated cyber
security personnel; taking technical measures to prevent computer viruses, network attacks
and intrusions; taking technical measures to monitor and record network operation status and
cyber security incidents; and adopting data security measures, such as data classification,
back-ups and encryption. Where facilities are deemed to be part of China’s “critical information
infrastructure”, the Cybersecurity Law sets high requirements for operational security, including
data localisation and national security review requirements for any products or services that
may impact national security.
The Data Security Law 2021 regulates core state data and other important data. It sets out
requirements for data security management and requires that security assessment reports are
submitted to regulators. In addition, it prohibits an entity from providing any such data stored in
China to a foreign judicial or law enforcement agency without the approval of the relevant
Chinese regulator. Penalties for non-compliance may include monetary fines, cessation of
business and revocation of business licences.
234
PART XI
TAXATION
Part A: United Kingdom Taxation
The following paragraphs are intended only as a general guide to current UK tax law and HMRC’s
current published practice (which may not be binding on HMRC) as at the date of this Prospectus,
which are both subject to change at any time, possibly with retrospective effect. All rates and
allowances referenced below are those currently in force. Furthermore, they are not exhaustive and
relate only to certain limited aspects of the UK tax consequences for Haleon Shareholders of holding or
disposing of Haleon Shares.
Except where expressly stated otherwise, the paragraphs below are intended to apply only to Haleon
Shareholders: (a) who are for UK tax purposes resident and, if individuals, domiciled or deemed
domiciled in (and only in) the United Kingdom for UK tax purposes; (b) to whom split-year treatment
does not apply; (c) who are the absolute beneficial owners of their Haleon Shares and any dividends
paid in respect of them; and (d) who hold their Haleon Shares as investments (otherwise than through
an individual savings account or an exempt pension arrangement or as carried interest) and not as
securities to be realised in the course of a trade.
The paragraphs below may not apply to certain shareholders, such as charities, dealers in securities,
trustees, broker dealers, market makers, insurance companies and collective investment schemes,
pension schemes, persons subject to UK tax on the remittance basis, persons who are otherwise
exempt from UK taxation and persons who have (or are deemed to have) acquired their Haleon Shares
by virtue of an office or employment or persons who are treated as holding their Haleon Shares as
carried interest. Such shareholders may be subject to special rules.
The material set out in the paragraphs below does not constitute tax advice. Any person who is
in any doubt as to their tax position or who is or may be subject to tax in a jurisdiction other
than the United Kingdom should consult an appropriate professional adviser. Investors should
be aware that the tax legislation of the investor’s jurisdiction and/or the tax legislation of the
United Kingdom may have an impact on the income received from the Haleon Shares.
1. Direct taxation of dividends on the Haleon Shares
(A) UK withholding tax
The Company will not be required to withhold tax at source from dividend payments it
makes.
(B) Individual Haleon Shareholders within the charge to UK income tax
The general tax treatment of dividends paid by the Company to individual Haleon
Shareholders is as follows:
• dividends received by an individual Haleon Shareholder from the Company (or
from other sources) will form part of the Haleon Shareholder’s total income for
income tax purposes;
• a nil rate of income tax applies to the first part of taxable dividend income received
by an individual Haleon Shareholder in a tax year (the “Nil Rate Amount”),
regardless of the tax rate that would otherwise apply. For the tax year from 6 April
2022 to 5 April 2023, the Nil Rate Amount is £2,000; and
235
• any taxable dividend income received by an individual Haleon Shareholder in a tax
year in excess of the Nil Rate Amount will be taxed at the rates set out below.
Where a Haleon Shareholder’s taxable dividend income for a tax year exceeds the Nil
Rate Amount, the excess amount (the “Relevant Dividend Income”) will, subject to
the availability of any income tax personal allowance, be subject to income tax, at the
following rates for the 2022/2023 tax year:
• 8.75 per cent., to the extent that the Relevant Dividend Income falls below the
threshold for the higher rate of income tax;
• 33.75 per cent., to the extent that the Relevant Dividend Income falls above the
threshold for the higher rate of income tax but below the threshold for the
additional rate of income tax; and
• 39.35 per cent., to the extent that the Relevant Dividend Income falls above the
threshold for the additional rate of income tax.
In determining whether and, if so, to what extent the Relevant Dividend Income falls
above or below the threshold for the higher rate of income tax or, as the case may be,
the additional rate of income tax, the Haleon Shareholder’s total taxable dividend
income for the tax year in question (including the part within the Nil Rate Amount) will
be treated as the highest part of the Haleon Shareholder’s total income for income tax
purposes.
(C) Corporate Haleon Shareholders within the charge to UK corporation tax
Haleon Shareholders within the charge to UK corporation tax which are “small
companies” (for the purposes of Chapter 2 of Part 9A of the Corporation Tax Act 2009)
will not generally be subject to UK corporation tax on any dividend received from the
Company on the Haleon Shares, provided certain conditions are met.
Other Haleon Shareholders within the charge to UK corporation tax will be subject to
UK corporation tax on dividends received from the Company on the Haleon Shares,
unless the dividends fall within an exempt class and certain conditions are met. In
general: (a) dividends paid on non-redeemable shares that do not carry any present or
future preferential rights to dividends or to the company’s assets on its winding up, and
(b) dividends paid to a person holding less than 10 per cent. of the issued share capital
of the payer (or, if there is more than one class of share, the same class of that share
capital in respect of which the dividends are paid) and who is entitled to less than
10 per cent. of the profits available for distribution to holders of the same class of
shares and would be entitled to less than 10 per cent. of the assets available for
distribution to holders of the same class of shares on a winding-up, are examples of
dividends that fall within an exempt class, subject to certain targeted and general anti-
avoidance rules.
2. Chargeable gains
(A) Individuals
A disposal or deemed disposal of Haleon Shares may give rise to a chargeable gain
(or allowable loss) for the purposes of UK capital gains tax, depending upon the
Haleon Shareholder’s circumstances and subject to any available exemption or relief.
236
A capital gains tax annual exemption (which is £12,300 for individuals in the 2022/2023
tax year) may, however, be available to the extent it has not already been utilised by
the individual Haleon Shareholder.
The rate of capital gains tax on share disposals (after taking advantage of the annual
exemption and deducting any allowable capital losses) is currently 10 per cent. to the
extent that individuals are subject to income tax at the basic rate and any chargeable
gain does not exceed the unused part of their basic rate income tax band. Where an
individual is subject to income tax at the basic rate but any chargeable gain exceeds
the unused part of their basic rate income tax band, the rate of capital gains tax on the
excess is 20 per cent. The rate of capital gains tax is also 20 per cent. for individuals
who are subject to income tax at the higher or additional rates.
(B) Companies
A disposal or deemed disposal of Haleon Shares may give rise to a chargeable gain
(or allowable loss) for the purposes of UK corporation tax, depending on the
circumstances and subject to any available exemption or relief. Corporation tax is
charged on chargeable gains at the rate of corporation tax applicable to that company.
(C) Capital Reduction
The Capital Reduction should be treated as a reorganisation of the Company’s share
capital. The effect of this should be that a Haleon Shareholder’s resultant holding of
Haleon Shares following the Capital Reduction should be treated as the same asset,
acquired at the same time and for the same consideration, as the holding of Haleon
Shares held by that Haleon Shareholder immediately prior to the Capital Reduction.
Haleon Shareholders therefore should not be treated as having made a disposal for the
purposes of UK tax on chargeable gains.
3. UK Stamp Duty and Stamp Duty Reserve Tax (“SDRT”)
The following statements are intended as a general and non-exhaustive guide to the current
UK stamp duty and SDRT position and apply regardless of whether or not a Haleon
Shareholder is resident in the United Kingdom. It should be noted that certain categories of
person, including market makers, brokers, dealers, persons connected with clearance services
and depositary receipt systems and other specified market intermediaries, may not be liable to
stamp duty or SDRT or may be liable at a higher rate or may, although not primarily liable for
tax, be required to notify and account for it under the Stamp Duty Reserve Tax Regulations
1986.
(A) General
A sale of Haleon Shares will generally be subject to UK stamp duty (if the Haleon
Shares are held in certificated form) or SDRT (if the sale is settled electronically
through the UK’s CREST system of paperless transfers), in either case at the rate of
0.5 per cent. of the amount or value of the consideration paid for the Haleon Shares.
Transfers of Haleon Shares to a connected company of a Haleon Shareholder (or its
nominee) may be subject to stamp duty and/or SDRT based on the market value of the
Haleon Shares at the time of the transfer, if that is higher than the amount or value of
the consideration actually paid for the Haleon Shares, subject to any relief which may
be available for intragroup transfers.
237
Any stamp duty payable (as opposed to SDRT) is rounded up to the nearest £5. No
stamp duty (as opposed to SDRT) will be payable if the amount or value of the
consideration is (and is certified on the instrument of transfer to be) £1,000 or under
and the transfer does not form part of a larger transaction, or series of transactions,
where the aggregate consideration exceeds £1,000. In practice, only one of either
stamp duty or SDRT would be paid and is usually paid or borne by the purchaser.
(B) Depositary receipt issuers and clearance services
Stamp duty and SDRT may arise upon the deposit of an underlying Haleon Share with
a depositary receipt issuer, generally at the higher rate of 1.5 per cent. of the issue
price or, as the case may be, of the amount or value of the consideration for or, in
certain circumstances, the value of the transfer. Following litigation, however, HMRC
have confirmed that they will no longer seek to apply the 1.5 per cent. SDRT charge on
an issue of shares to a depositary receipt issuer or to a person providing clearance
services (or their nominee or agent) on the basis that this is not compatible with EU
law. HMRC’s published view is that this remains the position under the terms of the
European Union (Withdrawal) Act 2018 following the end of the transition period unless
the stamp taxes on shares legislation is amended. HMRC’s view is that the 1.5 per
cent. SDRT or stamp duty charge will continue to apply to a transfer of shares or
securities to a depositary receipt system or clearance service where the transfer is not
an integral part of an issue of share capital.
Based on HMRC’s published practice, no stamp duty will be payable on the acquisition
or transfer of depositary receipts. Furthermore, an agreement to transfer depositary
receipts will not give rise to a liability to SDRT.
Special rules, which are similar in many respects to those described above in relation
to depositary receipt systems, apply to the issue or transfer of shares to a clearance
service (and to transfers of shares within a clearance service).
Part B: US Taxation
United States Federal Income Tax Considerations
The following is a summary of material US federal income tax considerations generally applicable to
US Holders (as defined below) of owning and disposing of the Haleon Shares.
This summary is based on provisions of the Internal Revenue Code of 1986, as amended (the “Code”),
and regulations, rulings and judicial interpretations thereof, in force as of the date hereof, and the
Convention Between the Government of the United States of America and the Government of the
United Kingdom of Great Britain and Northern Ireland for the Avoidance of Double Taxation and the
Prevention of Fiscal Evasion with Respect to Taxes on Income and on Capital Gains, in force as of
31 March 2003 (as amended by any subsequent protocols, including the protocol in force as of
31 March 2003) (the “Treaty”). Those authorities may be changed at any time, perhaps retroactively,
so as to result in US federal income tax consequences different from those summarised below.
This summary is directed only to US Holders that hold their Haleon Shares as capital assets and does
not address particular tax consequences that may be applicable to US Holders who may be subject to
special tax rules, such as US Holders of Haleon ADSs, banks, brokers or dealers in securities or
currencies, traders in securities electing to mark to market, financial institutions, life insurance
companies, tax-exempt entities, regulated investment companies, entities or arrangements that are
treated as partnerships for US federal income tax purposes (or partners therein), holders that own or
238
are treated as owning 10 per cent. or more of the Company’s stock by vote or value, persons holding
ordinary shares as part of a hedging or conversion transaction or a straddle, or persons whose
functional currency is not the USD. Moreover, this summary does not address state, local or foreign
taxes, the US federal estate and gift taxes, the Medicare contribution tax applicable to net investment
income of certain non-corporate US Holders, or alternative minimum tax consequences of acquiring,
holding or disposing of ordinary shares.
For purposes of this summary, a “US Holder” is a beneficial owner of Haleon Shares that is a citizen or
resident of the United States or a US domestic corporation or that otherwise is subject to US federal
income taxation on a net income basis in respect of such Haleon Shares.
You should consult your own tax advisors about the consequences to you of the acquisition,
ownership, and disposition of Haleon Shares, including the relevance to your particular
situation of the considerations discussed below and any consequences arising under foreign,
state, local or other tax laws.
US Federal Income Tax Consequences of Owning and Disposing of Haleon Shares Received in
the Demerger
Taxation of Dividends
Subject to the discussion below under “Passive Foreign Investment Company Status,” the gross
amount of any distribution of cash or property with respect to Haleon Shares that is paid out of the
Company’s current or accumulated earnings and profits (as determined for US federal income tax
purposes) will generally be includible in a US Holder’s taxable income as ordinary dividend income on
the day on which the US Holder receives the dividend and will not be eligible for the dividends-received
deduction allowed to corporations under the Code.
The Company does not expect to maintain calculations of its earnings and profits in accordance with
US federal income tax principles. US Holders therefore should expect that distributions generally will
be reported as dividends for US federal income tax purposes.
Dividends paid in Pounds Sterling generally will be includible in the US Holder’s income in a USD
amount calculated by reference to the exchange rate in effect on the day the dividends are distributed.
Any gain or loss on a subsequent sale, conversion or other disposition of such non-US currency by
such US Holder generally will be treated as ordinary income or loss and generally will be income or
loss from sources within the United States.
Subject to certain exceptions for short-term positions, the USD amount of dividends received by an
individual with respect to Haleon Shares will be subject to taxation at a preferential rate if the dividends
are “qualified dividends.” Dividends paid on Haleon Shares will be treated as qualified dividends if:
• the Company is eligible for the benefits of a comprehensive tax treaty with the United
States that the US Treasury determines is satisfactory for purposes of this provision and
that includes an exchange of information program; and
• the Company was not, in the year prior to the year in which the dividend was paid, and is
not, in the year in which the dividend is paid, a passive foreign investment company (a
“PFIC”).
The US Treasury has determined that the Treaty meets the requirements for reduced rates of taxation,
and the Company believes it is eligible for the benefits of the Treaty. The Company was not treated as
239
a corporation for US federal income tax purposes for its 2021 taxable year, and thus the PFIC rules
were not applicable to the Company for such year. In addition, based on the Company’s audited
financial statements and its current expectations regarding the value and nature of its assets, and the
sources and nature of its income, it does not anticipate becoming a PFIC for its 2022 taxable year or in
the foreseeable future. Holders should consult their own tax advisors regarding the availability of the
reduced dividend tax rate in light of their own particular circumstances.
Dividend distributions with respect to Haleon Shares generally will be treated as “passive category”
income from sources outside the United States for purposes of determining a US Holder’s US foreign
tax credit limitation.
Taxation of Dispositions of Haleon Shares
Subject to the discussion below under “Passive Foreign Investment Company Status,” upon a sale,
exchange or other taxable disposition of the Haleon Shares, a US Holder will realise gain or loss for
US federal income tax purposes in an amount equal to the difference between the amount realised on
the disposition and the US Holder’s adjusted tax basis in the shares, as determined in USD as
discussed below. Such gain or loss will be capital gain or loss, and will generally be long-term capital
gain or loss if the shares have been held for more than one year. Long-term capital gain realised by a
US Holder that is an individual generally is subject to taxation at a preferential rate. The deductibility of
capital losses is subject to limitations.
Gain, if any, realised by a US Holder on the sale or other disposition of the shares generally will be
treated as US source income for US foreign tax credit purposes.
If a US Holder sells or otherwise disposes of Haleon Shares in exchange for currency other than USD,
the amount realised generally will be the USD value of the currency received at the spot rate in effect
on the date of sale or other disposition (or, if the shares are traded on an established securities market
at such time, in the case of cash basis and electing accrual basis US Holders, the settlement date). An
accrual basis US Holder that does not elect to determine the amount realised using the spot exchange
rate on the settlement date will recognise foreign currency gain or loss equal to the difference between
the USD value of the amount received based on the spot exchange rates in effect on the date of the
sale or other disposition and the settlement date. A US Holder generally will have a tax basis in the
currency received equal to the USD value of the currency received at the spot rate in effect on the
settlement date. Any currency gain or loss realised on the settlement date or the subsequent sale,
conversion, or other disposition of the non-US currency received for a different USD amount generally
will be US-source ordinary income or loss, and will not be eligible for the reduced tax rate applicable to
long-term capital gains. If an accrual basis US Holder makes the election described in the first
sentence of this paragraph, it must be applied consistently from year to year and cannot be revoked
without the consent of the IRS. A US Holder should consult its own tax advisors regarding the
treatment of any foreign currency gain or loss realised with respect to any currency received in a sale
or other disposition of the shares.
Capital Reduction
It is not clear whether the Capital Reduction would be treated as a realisation event for US federal
income tax purposes, although even if the Capital Reduction were to be treated as such a realisation
event, the Company intends for the Capital Reduction to qualify as tax-free under sections 368(a)(1)(E)
and/or 1036 of the Code. Assuming that the Capital Reduction qualifies for tax-free treatment, (i) a US
Holder will not recognise any gain or loss upon the Capital Reduction, and (ii) the US Holder’s
aggregate adjusted basis and holding period in the US Holder’s Haleon Shares should be the same as
the US Holder’s aggregate basis and holding period in the Haleon Shares exchanged therefor. US
240
Holders that acquired their Haleon Shares on different dates and at different prices should consult their
tax advisors regarding the allocation of the tax basis of such shares.
Passive Foreign Investment Company Status
Special US tax rules apply to companies that are considered to be PFICs. The Company will be
classified as a PFIC in a particular taxable year if, taking into account its proportionate share of the
income and assets of its subsidiaries under applicable “look-through” rules, either:
• 75 per cent. or more of its gross income for the taxable year is passive income; or
• the average percentage of the value of its assets that produce or are held for the
production of passive income is at least 50 per cent.
For this purpose, passive income generally includes dividends, interest, gains from certain
commodities transactions, rents, royalties and the excess of gains over losses from the disposition of
assets that produce passive income.
The Company does not expect to become a PFIC in 2022 or the foreseeable future. However, the
PFIC tests must be applied each year, and it is possible that the Company may become a PFIC in a
future year. In the event that, contrary to the Company’s expectation, the Company is classified as a
PFIC in any year, a US Holder generally would be subject to additional taxes on certain distributions
and any gain realised from the sale or other taxable disposition of Haleon Shares regardless of
whether the Company continued to be a PFIC in any subsequent year, unless such US Holder elects
to mark their Haleon Shares to market for tax purposes on an annual basis. US Holders are
encouraged to consult their own tax advisor as to the Company’s status as a PFIC and the tax
consequences to the US Holder of such status.
Foreign Financial Asset Reporting
Individual US Holders that own “specified foreign financial assets” with an aggregate value in excess of
$50,000 on the last day of the taxable year or $75,000 at any time during the taxable year are
generally required to file an information statement along with their tax returns, currently on Form 8938,
with respect to such assets. “Specified foreign financial assets” include any financial accounts held at a
non-US financial institution, as well as securities issued by a non-US issuer that are not held in
accounts maintained by financial institutions. Higher reporting thresholds apply to certain individuals
living abroad and to certain married individuals. Regulations extend this reporting requirement to
certain entities that are treated as formed or availed of to hold direct or indirect interests in specified
foreign financial assets based on objective criteria. US Holders who fail to report the required
information could be subject to substantial penalties. In addition, the statute of limitations for
assessment of tax would be suspended, in whole or part. Prospective investors are encouraged to
consult with their own tax advisors regarding the possible application of these rules, including the
application of the rules to their particular circumstances.
Backup Withholding and Information Reporting
Dividends paid on, and proceeds from the sale or other disposition of, the shares to a US taxpayer
generally may be subject to the information reporting requirements of the Code and may be subject to
backup withholding unless the US taxpayer provides an accurate taxpayer identification number and
makes any other required certification or otherwise establishes an exemption. Backup withholding is
not an additional tax. The amount of any backup withholding from a payment to a US taxpayer will be
allowed as a refund or credit against the US taxpayer’s US federal income tax liability, provided the
required information is furnished to the US Internal Revenue Service in a timely manner.
A holder that is not a US taxpayer may be required to comply with certification and identification
procedures in order to establish its exemption from information reporting and backup withholding.
241

PART XII
ADDITIONAL INFORMATION
1. RESPONSIBILITY STATEMENT
The Directors, whose names appear at paragraph 1 of Part V (Directors, Senior Managers,
Corporate Governance and Remuneration), and the Company accept responsibility for the
information contained in this Prospectus. To the best of the knowledge of the Directors and the
Company, the information contained in this Prospectus is in accordance with the facts and this
Prospectus makes no omission likely to affect its import.
2. INCORPORATION AND ACTIVITY OF THE COMPANY
The Company was incorporated and registered in England and Wales under the Companies
Act as a private company limited by shares on 20 October 2021 under the name DRVW 2022
Limited with registered number 13691224. The principal legislation under which the Company
operates is the Companies Act and regulations made thereunder. DRVW 2022 Limited was
re-registered as a public limited company (DRVW 2022 plc) on 23 February 2022 and changed
its name to Haleon plc on 28 February 2022.
Following the Demerger, the principal activity of the Company will be to act as the ultimate
holding company of the Group.
The Company is domiciled in England and Wales with its registered and head office at 980
Great West Road, Brentford, Middlesex TW8 9GS, United Kingdom. The telephone number of
the Company’s registered office is +44 (0)20 8047 5000 and its website is www.haleon.com,
which will go live following Separation. The information on the Company’s website does not
form part of this Prospectus.
The legal entity identifier of the Company is 549300PSB3WWEODCUP19.
3. SHARE CAPITAL OF THE COMPANY
3.1 Issued share capital of the Company on Admission
Following the Demerger (but before performance and completion of the Exchange
Agreements), the number of Haleon Shares in issue will be equal to the number of GSK
Shares in issue at the Shareholder Record Time. As at the Latest Practicable Date, there were
5,084,048,734 GSK Shares in issue (excluding ordinary shares held in treasury). Further detail
on the Demerger is set out at paragraph 1 of Part IV (Overview of the Demerger and
Separation).
Shortly following the Demerger and prior to Admission, the Company will issue: (i) new Haleon
Shares to each of GSK, Pfizer and the SLPs and (ii) Non-Voting Preference Shares to Pfizer,
in each case pursuant to the Exchange Agreements, such that, on Admission, the ordinary
share capital of the Company will be held as follows:
Shareholder Class Voting rights Nominal Value
GSK Shareholders Ordinary Shares At least
54.5 per cent.
At least
54.5 per cent.
Pfizer Ordinary Shares 32 per cent. 32 per cent.
SLPs Ordinary Shares 7.5 per cent. 7.5 per cent.
GSK Ordinary Shares Up to 6 per cent. Up to 6 per cent.
The Haleon Shares have a nominal value of £1.25 each (to be reduced to 1 pence following
the Capital Reduction) and will be fully paid. The Non-Voting Preference Shares have a
nominal value of £1 each and will be fully paid.
242
Holders of Haleon Shares who the Company believes are or may be Designated Persons are
not permitted to dispose of their Haleon Shares or any legal or beneficial interest in any of
them without the prior written consent of the Company. The Haleon Shares are otherwise
freely transferable and there are no restrictions on transfer.
The Haleon Shares will be registered with ISIN number GB00BMX86B70 and SEDOL number
BMX86B7.
3.2 History of the share capital and pending reduction of capital
On incorporation, two ordinary shares of £1 each in the capital of the Company were issued
and have been fully paid up in cash. Subsequently, two further ordinary shares of £1 each in
the capital of the Company were issued and have been fully paid up in cash. In addition,
redeemable preference shares of £1 each (the “Redeemable Shares”) were issued and were
fully paid up in cash. The Redeemable Shares were redeemed by the Company on 11 April
2022.
On 23 May 2022, the Company issued 16 ordinary shares of £1 each which were fully paid up
in cash. Immediately following that issuance, the Company consolidated its 20 ordinary shares
of £1 each into four ordinary shares of £5 each and then sub-divided such shares into sixteen
ordinary shares of £1.25 each .
Prior to Admission, it is expected that David Redfern, Adam Walker, Victoria Whyte and
Subesh Williams, in their capacity as shareholders of the Company, will pass a special
resolution of the Company approving the Capital Reduction pursuant to section 641(1)(b) of the
Companies Act. Implementation of the Capital Reduction is conditional on:
(A) Admission having occurred;
(B) the Court having granted the Court Order confirming the Capital Reduction; and
(C) Companies House having issued a certificate of registration registering the Capital
Reduction.
The purpose of the Capital Reduction is to create additional distributable reserves in the
Company, which the Company can then use to support future distributions to shareholders in
accordance with its stated dividend policy, as set out in paragraph 3 of Part III (Business
Overview). It is expected that aggregate distributable reserves of up to approximately £29.4
billion will be created by the Capital Reduction.
The Capital Reduction is expected to be confirmed by the Court as soon as practicable after
Admission, subject to court availability for scheduling a hearing date, and an application to
register the Capital Reduction, including a copy of the Court Order and the required statement
of capital approved by the Court, will be delivered to Companies House as soon as practicable
thereafter. Companies House is required to register the Capital Reduction on delivery of the
Court Order and statement of capital, and must then issue a certificate of registration
registering the Capital Reduction pursuant to section 649(5) and (6) of the Companies Act. The
Capital Reduction will take effect upon Companies House registering the Court Order and
accompanying statement of capital, at which point the nominal value of each Haleon Share will
be reduced from £1.25 to 1 pence.
Following the date of this Prospectus, and prior to Admission, GSK and the Company intend to
implement the Demerger as described in paragraph 1 of Part IV (Overview of the Demerger
and Separation) of this Prospectus, which will result in, among other things, the Company
becoming the ultimate holding company of the Group and GSK Shareholders receiving one
Haleon Share for every one GSK Share held by such GSK Shareholders at the Shareholder
Record Time.
243
Shortly following the Demerger, GSK, Pfizer, the SLPs and the Company intend to implement
the Share Exchanges as described in paragraph 15.9 of this Part XII (Additional Information)
below, which will result in, among other things, the following alterations to the share capital of
the Company:
(A) the Company will allot and issue to GSK 502,868,434 Haleon Shares, less a number of
Haleon Shares that is equal to the number of Excess GSK Shares. As at the Latest
Practicable Date, the number of Haleon Shares expected to be held by GSK at
Admission is expected to represent up to 6 per cent. of the total issued share capital of
the Company;
(B) the Company will allot and issue to the SLPs such number of new Haleon Shares as is
required so that, on Admission, the SLPs will together hold Haleon Shares
representing 7.5 per cent. of the total issued share capital of the Company (to the
nearest whole Haleon Share); and
(C) the Company will allot and issue to Pfizer: (i) 25 million Non-Voting Preference Shares;
and (ii) such number of Haleon Shares as will result in Pfizer holding, on Admission,
Haleon Shares representing 32 per cent. of the total issued share capital of the
Company (to the nearest whole Haleon Share).
Immediately following the issue of shares described in paragraph (C) above, Pfizer will carry
out the NVPS Sale.
Further details of the Demerger and the Share Exchanges are set out in paragraph 1 of Part IV
(Overview of the Demerger and Separation).
4. INFORMATION ABOUT THE HALEON SHARES AND NON-VOTING PREFERENCE
SHARES
4.1 Description and type of securities
The Haleon Shares will, when issued, be fully paid ordinary shares with a nominal value of
£1.25 each (to be reduced to 1 pence following the Capital Reduction). The Company has and,
following Separation and Admission, will have one class of ordinary shares.
The Non-Voting Preference Shares will, when issued, be fully paid non-voting preference
shares with a nominal value of £1 each carrying preferential rights in respect of both dividends
and distributions of capital. The Company has and, following Separation and Admission, will
have one class of preference shares in issue.
The Haleon Shares and the Non-Voting Preference Shares will, when issued, be credited as
fully paid and free from all liens, equities, charges, encumbrances and other interests.
The Non-Voting Preference Shares will rank pari passu with all other Non-Voting Preference
Shares and carry preferential dividend rights ahead of the Haleon Shares, entitling the holder to
quarterly cumulative dividends at a fixed rate of 9.5 per cent. per annum for a period of five years
from the date of the issue of the Non-Voting Preference Shares, following which the rate shall be
reset for each subsequent period of five consecutive years at the rate which is equal to the Bank
of England base rate prevailing at the time of reset plus 7.5 per cent. Dividends on the Non-Voting
Preference Shares which have become due and payable in accordance with the Articles are
required to be approved and paid in full before any repurchases or distributions can be made with
respect to the Haleon Shares. The Non-Voting Preference Shares will also carry preferential rights
to participate in any distribution of capital in the event of the insolvency of the Company (including
on a winding-up of the Company) up to an amount equal to their nominal value plus accrued
dividend and any arrears or deficiency in amount of the cumulative dividend.
The Haleon Shares will rank behind the Non-Voting Preference Shares, as described in the
preceding paragraph, and pari passu with all other Haleon Shares for dividends and
distributions on shares of the Company declared, made or paid after their issue.
244
Further detail on the rights attaching to the Haleon Shares and the Non-Voting Preference
Shares is set out in paragraph 4.5 of this Part XII (Additional Information) below.
4.2 Legislation under which the Haleon Shares were created
The Haleon Shares and the Non-Voting Preference Shares have been created under the
Companies Act.
4.3 Listing
An application will be made to the FCA for the Haleon Shares to be admitted to the premium
listing segment of the Official List. An application will also be made to the LSE for the Haleon
Shares to be admitted to trading on its main market for listed securities. It is expected that
Admission will become effective and that dealings in the Haleon Shares will commence on the
LSE by no later than 8.00 a.m. (London time) on 18 July 2022. The Company is expected to be
eligible for inclusion in the FTSE UK Index Series from Admission.
The Company also plans to make an application to the NYSE for the Haleon ADSs to be
admitted to listing and trading on the NYSE.
No application has been made for admission of Haleon Shares to trading on any other stock
exchange (nor is it the current intention of the Company to make any such application in
future).
There is no prior trading record for the Haleon Shares.
No application has been made for admission of the Non-Voting Preference Shares to trading
on any stock exchange, nor is it the current intention of the Company to make any such
application in future. There is no prior trading record for the Non-Voting Preference Shares.
4.4 Form and currency of the Haleon Shares and the Non-Voting Preference Shares
The Haleon Shares and the Non-Voting Preference Shares will be in registered form and will
be capable of being held in certificated and uncertificated form. The registrar of the Company is
Equiniti.
Title to the certificated Haleon Shares and Non-Voting Preference Shares will be evidenced by
entry in the register of members of the Company and title to uncertificated Haleon Shares and
Non-Voting Preference Shares will be evidenced by entry in the operator register maintained
by Equiniti (which will form part of the register of members of the Company).
No share certificates will be issued in respect of Haleon Shares or Non-Voting Preference
Shares in uncertificated form. No temporary documents of title have been or will be issued in
respect of the Haleon Shares or the Non-Voting Preference Shares.
It is currently anticipated that the Haleon Shares and the Non-Voting Preference Shares will be
eligible to join CREST, the computerised, paperless system for settlement of sales and
purchases of shares in the London securities market, with effect immediately upon Admission
and the commencement of dealings on the LSE.
The Haleon Shares and the Non-Voting Preference Shares will be denominated in Pounds
Sterling and the Haleon Shares will be quoted in Pounds Sterling on the LSE.
245
4.5 Rights attached to the Haleon Shares and the Non-Voting Preference Shares
Haleon Shares
All the Haleon Shares will rank pari passu in all respects. There are no conversion or exchange
rights attaching to the Haleon Shares, and all the Haleon Shares will have equal rights to
participate in capital, dividend and profit distributions by the Company.
Subject to the provisions of the Companies Act, any equity securities issued by the Company
for cash must first be offered to Haleon Shareholders in proportion to their holdings of Haleon
Shares. The Companies Act and the Listing Rules allow for the disapplication of pre-emption
rights which may be approved by a special resolution of the Haleon Shareholders, either
generally or specifically, for a maximum period not exceeding five years. A resolution to this
effect was passed on 23 May 2022 and is summarised in sub-paragraph (B) of paragraph 4.6
of this Part XII (Additional Information) below.
Except in relation to dividends which have been declared and rights on a liquidation of the
Company, the Haleon Shareholders have no rights to share in the profits of the Company.
The Haleon Shares are not redeemable. However, the Company may purchase or contract to
purchase any of the Haleon Shares on- or off-market, subject to the Companies Act and the
requirements of the Listing Rules. The Company may purchase Haleon Shares only out of
distributable reserves or the proceeds of a new issue of shares made to fund the repurchase.
Further details of the rights attached to the Haleon Shares in relation to attendance and voting
at general meetings, entitlements on a winding-up of the Company, transferability of Haleon
Shares and dividends are set out in paragraph 5 of this Part XII (Additional Information) below.
Non-Voting Preference Shares
The Non-Voting Preference Shares are fully paid non-voting preference shares with a nominal
value of £1 each. Each Non-Voting Preference Share is redeemable in whole at the option of
the Company or redeemable at the option of each relevant Non-Voting Preference Shareholder
in respect of its entire holding of Non-Voting Preference Shares on any date falling not less
than five years after the date on which that Non-Voting Preference Share was issued or, if
earlier, on the Company undergoing a change of control. Such redemption shall be at the
nominal value of the relevant Non-Voting Preference Shares plus the amount, if any, of all
accrued but unpaid dividends on the Non-Voting Preference Shares. The Company has and,
following Separation and Admission, will have, one class of non-voting preference shares.
The Non-Voting Preference Shares will not confer any voting rights, other than in respect of
matters that entail a variation of the class rights attaching to the Non-Voting Preference
Shares, in which case each Non-Voting Preference Share will confer one vote at a separate
class meeting of the Non-Voting Preference Shareholders convened in order to consider a
proposed variation of class rights.
The Non-Voting Preference Shares will rank pari passu with all other Non-Voting Preference
Shares and have preferential dividend rights ahead of the Haleon Shares, entitling Non-Voting
Preference Shareholders to quarterly cumulative dividends at a fixed rate of 9.5 per cent. per
annum for a period of five years from the date of the issue of the Non-Voting Preference
Shares, following which the rate shall be reset for each subsequent period of five consecutive
years at the rate which is equal to the Bank of England base rate prevailing at the time of reset
plus 7.5 per cent. Dividends on the Non-Voting Preference Shares which have become due
and payable in accordance with the Articles are required to be approved and paid in full before
any repurchases or distributions can be made with respect to the Haleon Shares. The
Non-Voting Preference Shares will also carry preferential rights to participate in a distribution of
capital in the event of insolvency (including on a winding-up) up to an amount equal to their
nominal value plus accrued dividend and any arrears or deficiency in amount of the cumulative
dividend.
246
The Haleon Shares will rank behind the Non-Voting Preference Shares, as described in the
preceding paragraph, and pari passu with all other Haleon Shares for dividends and
distributions on ordinary shares of the Company declared, made or paid after their issue.
4.6 Resolutions passed by initial shareholders of the Company
Authorisations relating to the share capital of the Company
On 23 May 2022, David Redfern, Adam Walker, Victoria Whyte and Subesh Williams, in their
capacity as the only shareholders of the Company passed the following resolutions relating to
the share capital of the Company, each of which is subject to and conditional upon Admission
occurring:
(A) an ordinary resolution that the Directors be generally and unconditionally authorised, in
accordance with section 551 of the Companies Act, in substitution for all subsisting
authorities, to exercise all powers of the Company to allot shares in the Company and
to grant rights to subscribe for or convert any security into shares in the Company up
to an aggregate nominal amount of £3,847,723,920 which authority shall expire at the
end of the first annual general meeting of the Company following Admission or, if
earlier, at the close of business on 30 June 2023 (unless previously revoked or varied
by the Company in general meeting) save that under such authority the Company may,
before such expiry, make an offer or agreement which would or might require shares to
be allotted or rights to subscribe for or convert any security into shares to be granted
after such expiry and the Directors may allot shares or grant rights to subscribe for or
convert any security into shares in pursuance of such an offer or agreement as if the
relevant authority conferred hereby had not expired;
(B) a special resolution that, subject to the passing of the resolution described in
paragraph (A) above, and in substitution for all subsisting authorities, the Directors be
empowered to allot equity securities (as defined in the Companies Act) for cash under
the authority given by that resolution and/or to sell ordinary shares held by the
Company as treasury shares for cash as if section 561 of the Companies Act did not
apply to any such allotment or sale, such power to be limited:
(i) to the allotment of equity securities and sale of treasury shares in connection
with an offer of, or invitation to apply for, equity securities:
(a) to ordinary shareholders in proportion (as nearly as may be
practicable) to their existing holdings; and
(b) to holders of other equity securities, as required by the rights of those
securities, or as the Directors otherwise consider necessary,
but so that the Directors may impose any limits or restrictions and make any
arrangements which they consider necessary or appropriate to deal with
treasury shares, fractional entitlements, record dates, legal, regulatory or
practical problems in, or under the laws of, any territory or any other matter
whatsoever; and
(ii) to the allotment of equity securities or sale of treasury shares (otherwise than
under paragraph (i) above) up to a nominal amount of £577,158,587
(calculated, in the case of equity securities which are rights to subscribe for, or
convert, securities into, ordinary shares by reference to the aggregate nominal
amount of relevant shares which may be allotted pursuant to such rights),
such power to expire at the end of the first annual general meeting of the Company
following Admission (or, if earlier, at the close of business on 30 June 2023) but, in
each case, prior to its expiry the Company may make offers, and enter into
247
agreements, which would, or might, require equity securities to be allotted (and
treasury shares to be sold) after the power expires and the Directors may allot equity
securities (and sell treasury shares) under any such offer or agreement as if the power
had not expired;
(C) a special resolution that, subject to the passing of the authority described in paragraph
(A) above, the Directors be empowered in addition to any authority described in
paragraph (B) above to allot equity securities (as defined in the Companies Act) for
cash under the authority described in paragraph (A) and/or to sell ordinary shares held
by the Company as treasury shares for cash as if section 561 of the Companies Act
did not apply to any such allotment or sale, such power to be:
(i) limited to the allotment of equity securities or sale of treasury shares up to a
nominal amount of £577,158,587 (calculated, in the case of equity securities
which are rights to subscribe for, or convert, securities into, ordinary shares by
reference to the aggregate nominal amount of relevant shares which may be
allotted pursuant to such rights); and
(ii) used only for the purposes of financing (or refinancing, if the authority is to be
used within six months after the original transaction) a transaction which the
Directors determine to be an acquisition or other capital investment of a kind
contemplated by the Statement of Principles on Disapplying Pre-Emption
Rights most recently published by the Pre-Emption Group prior to the date of
this Prospectus,
such power to expire at the end of the first annual general meeting of the Company
following Admission (or, if earlier, at the close of business on 30 June 2023) but, in
each case, prior to its expiry the Company may make offers, and enter into
agreements, which would, or might, require equity securities to be allotted (and
treasury shares to be sold) after the power expires and the Directors may allot equity
securities (and sell treasury shares) under any such offer or agreement as if the power
had not expired;
(D) a special resolution that the Company be generally and unconditionally authorised for
the purposes of section 701 of the Companies Act to make market purchases (within
the meaning of section 693(4) of the Companies Act) of its own ordinary shares
provided that the:
(i) maximum number of ordinary shares hereby authorised to be purchased is
923,453,741;
(ii) minimum price, exclusive of expenses, which may be paid for each ordinary
share is the nominal value of such share;
(iii) maximum price, exclusive of expenses, which may be paid for each ordinary
share shall be the higher of (i) an amount equal to five per cent. above the
average market value for the Company’s ordinary shares for the five business
days immediately preceding the day on which the ordinary share is contracted
to be purchased; and (ii) the higher of the price of the last independent trade
and the highest current independent purchase bid at the time on the trading
venue on which the purchase is carried out; and
(iv) authority conferred as described under this paragraph (D) shall, unless
renewed prior to such time, expire at the end of the next annual general
meeting of the Company (or, if earlier, at the close of business on 30 June
2023), save that the Company may, before such expiry, enter into a contract
for the purchase of ordinary shares which would or might be completed wholly
or partly after such expiry and the Company may purchase ordinary shares
pursuant to any such contract as if this authority had not expired.
248
Board undertaking in relation to share capital authorities
Following the Capital Reduction, the aggregate nominal value of the Company’s issued
ordinary share capital will reduce and, as a result, the headroom contained within certain of the
share capital authorities above will, until the Company’s next annual general meeting (or, if
earlier, at the close of business on 30 June 2023), be in excess of the level of standing annual
share capital authorities generally considered to be appropriate for a listed company.
Accordingly, in order to demonstrate that the Group does not intend to breach, inter alia, the
guidance of investment protection committees (such as the IA, PLSA and PIRC) or the
Pre-Emption Group’s “Statement of Principles” regarding routine disapplication of pre-emption
rights, the Board has resolved that:
(A) to the extent that the authority conferred by the resolution described at sub-paragraph
(A) above is in respect of an aggregate nominal amount which exceeds one-third of the
aggregate nominal amount of the Company’s issued ordinary share capital on
Admission (the “Admission Capital”), it will not exercise that authority in respect of
such excess without first seeking shareholder approval;
(B) it will limit the exercise of the power conferred by the resolution described at
sub-paragraph (B) above, as limited by limb (ii) of that sub-paragraph, to the
disapplication of pre-emption rights in respect of allotments of the Company’s shares
up to an aggregate nominal amount which is not more than five per cent of the
Admission Capital; and
(C) it will limit the exercise of the power conferred by the resolution described at
sub-paragraph (C) above, as limited by limb (i) of that sub-paragraph, to the
disapplication of pre-emption rights in respect of allotments of the Company’s shares
up to an aggregate nominal amount which is not more than five per cent of the
Admission Capital.
Authority to make donations to political organisations and political expenditure
On 23 May 2022, David Redfern, Adam Walker, Victoria Whyte and Subesh Williams, in their
capacity as the only shareholders of the Company passed the following ordinary resolution:
for the purposes of sections 366 and 367 of the Companies Act, the Company and all
companies that are or become, at any time during the period for which this
authorisation has effect, subsidiaries of the Company, are authorised in aggregate to:
(A) make political donations, as defined in section 364 of the Companies Act, to
political parties and/or independent electoral candidates, as defined in section
363 of the Companies Act, not exceeding £50,000 in total;
(B) make political donations to political organisations other than political parties, as
defined in section 363 of the Companies Act, not exceeding £50,000 in total;
and
(C) incur political expenditure, as defined in section 365 of the Companies Act, not
exceeding £50,000 in total,
in each case during the period beginning with the date of passing this resolution and
ending at the end of the next annual general meeting of the Company (or, if earlier, at
the close of business on 30 June 2023). In any event, the aggregate amount of political
donations and political expenditure made or incurred under this authority shall not
exceed £100,000.
249
Authority to call general meetings on 14 days’ notice
On 23 May 2022, David Redfern, Adam Walker, Victoria Whyte and Subesh Williams, in their
capacity as the only shareholders of the Company passed the following special resolution:
that a general meeting of the Company other than an annual general meeting may be
called on no less than 14 clear days’ notice.
Authority for the Audit & Risk Committee to determine the remuneration of the auditors
On 23 May 2022, David Redfern, Adam Walker, Victoria Whyte and Subesh Williams, in their
capacity as the only shareholders of the Company passed the following ordinary resolution:
that the Audit & Risk Committee of the Company be authorised to determine the
remuneration of the auditors.
4.7 Taxation
Certain information on taxation in the UK and the USA is set out in Part XI (Taxation). The
information contained in Part XI (Taxation) is intended only as a general guide to the current
tax position in the UK and the USA for the Haleon Shareholders described therein.
4.8 Haleon plc ADR Programme
In the following description, a “Holder” is the person registered with JPMorgan Chase Bank
N.A., as depositary for the Haleon ADSs (the “Depositary”), references to American depositary
receipts or ADRs mean ADRs evidencing Haleon ADSs, “ADSs” refer to the Haleon ADSs,
“shares” refer to Haleon Shares and “Custodian” refers to JPMorgan Chase Bank N.A.,
custodian of the Haleon Shares underlying the Haleon ADSs.
General
ADRs evidencing ADSs are issuable pursuant the Deposit Agreement between the Company
and JPMorgan Chase Bank, N.A., as depositary, and the Holders of the ADRs (the “Deposit
Agreement”). The principal executive office of the Depositary is 383 Madison Avenue, Floor
11, New York, New York 10179. Each ADS represents the right to receive two Haleon Shares.
An ADR may evidence any number of ADSs.
The Company intends to apply to list the ADSs on the NYSE under the ticker symbol “HLN”.
Voting
The Depositary or, if the deposited securities are registered in the name of or held by its
nominee, its nominee, subject to and in accordance with the constituent documents of the
Company, irrevocably appointed each Holder for the time being on the record date (the
“Voting Record Date”) fixed by the Depositary in respect of any meeting (at which holders of
deposited securities are entitled to vote) as its proxy to attend, vote and speak at the relevant
meeting (or any adjournment thereof) in respect of the deposited securities represented by the
ADSs registered on the books of the Depositary in the name of such Holder on the Voting
Record Date. In respect of any such meeting each such Holder can appoint any person as its
substitute proxy to attend, vote and speak on behalf of the Holder subject to and in accordance
with the provisions of the Deposit Agreement and the constituent documents of the Company.
As soon as practicable after receipt of notice of any meeting at which the holders of deposited
securities are entitled to vote, or of solicitation of consents or proxies from holders of deposited
securities, the Depositary shall fix the Voting Record Date in respect of such meeting or
solicitation. The Depositary or, if the Company so determines, the Company shall, distribute to
250
Holders of record on such Voting Record Date: (a) such information as is contained in such
notice of meeting or in the solicitation materials, (b) an ADR proxy card in a form prepared by
the Depositary, (c) a statement that each Holder at the close of business on the Voting Record
Date will be entitled, subject to any applicable law, the Company’s constituent documents and
the provisions of or governing the deposited securities, either (i) to use such ADR proxy card in
order to attend, vote and speak at such meeting as the proxy of the Depositary or its nominee
solely with respect to the ordinary shares or other deposited securities represented by ADSs
evidenced by such Holder’s ADRs, or (ii) to appoint any other person as the substitute proxy of
such Holder, solely with respect to the ordinary shares or other deposited securities
represented by ADSs evidenced by such Holder’s ADRs, or (iii) to renounce the proxy initially
provided by the Depositary or its nominee to such Holder or such Holder’s substitute proxy and
to provide voting instructions to the Depositary as to the exercise of the voting rights, pertaining
to the ordinary shares or other deposited securities represented by ADSs evidenced by their
respective ADRs (“Voting Instructions”), and (d) if the Depositary is to be given Voting
Instructions by such Holders, a brief statements as to the manner in which Voting Instructions
may be given to the Depositary. Each Holder shall be solely responsible for the forwarding of
voting information to the persons or entities having a beneficial ownership interest in ADSs (the
“Beneficial Owners”) registered in such Holder’s name. There is no guarantee that Holders
and Beneficial Owners generally or any Holder or Beneficial Owner in particular will receive the
notice described above with sufficient time to enable such Holder or Beneficial Owner to return
any Voting Instructions to the Depositary in a timely manner or for the Holder to arrange to
attend, vote and/or speak at the relevant meeting. The Company shall provide notice to the
Depositary of such vote or meeting in a timely manner and at least 30 days prior to the date of
such vote or meeting (unless less than 30 days’ notice of the meeting has been given in
accordance with the Company’s Articles of Association and English law, in which case the
Company will provide to the Depositary such advance notice of the meeting as may be
possible under the circumstances); provided that if the Depositary receives less than 30 days’
notice of such vote or meeting, the Depositary shall only make such distribution to the extent it
deems it to be practicable.
Upon actual receipt by the ADR department responsible for proxies and Voting Instructions
(including, without limitation, instructions of any entity or entities acting on behalf of the
nominee for DTC) of a Holder on the Voting Record Date in the manner and on or before the
time established by the Depositary for such purpose, the Depositary shall endeavour, insofar
as practicable and permitted under applicable law, the provisions of the Company’s constituent
documents and the provisions of the deposited securities, to vote or cause to be voted the
deposited securities represented by the ADSs evidenced by such Holder’s ADRs in
accordance with such Voting Instructions insofar as practicable and permitted under the
provisions of or governing deposited securities. The Depositary will not itself exercise any
voting discretion in respect of any deposited securities. Ordinary shares or other deposited
securities represented by ADSs for which no specific Voting Instructions are received by the
Depositary from the Holder shall not be voted by the Depositary but may be directly voted by
such Holder in attendance at meetings of shareholders as proxy for the Depositary or its
nominee, subject to, and in accordance with, the Deposit Agreement and the Company’s
constituent documents.
Holders and their substitute proxy (other than the Depositary) shall only be permitted to attend,
vote and speak at meetings at which holders of deposited securities are entitled to vote as the
proxy of the Depositary or its nominee with respect to the whole number of ordinary shares
represented by the ADSs evidenced by ADRs held by such Holders on the record date set by
the Depositary. For the avoidance of doubt, when the Depositary receives Voting Instructions
from a substitute proxy of a Holder (including, without limitation, instructions from Broadridge
Financial Solutions or any other entity acting on behalf of participants and/or customers of
participants within DTC) or their agents, and such registered Holder has notified the Depositary
that it holds ADRs on behalf of such substitute proxies, the Depositary shall treat such Voting
Instructions as coming from an entity that holds ADRs on behalf of such substitute proxies and
the Depositary shall vote or cause to be voted the deposited securities in accordance with such
instructions.
Holders are strongly encouraged to forward their Voting Instructions as soon as possible. Voting
Instructions will not be deemed received until such time as the ADR department responsible for
251
proxies and voting has received such Voting Instructions notwithstanding that such Voting
Instructions may have been physically received by the Depositary prior to such time.
Notwithstanding anything contained in the Deposit Agreement or any ADR, the Depositary
may, to the extent not prohibited by any law, rule or regulation or the rules and/or requirements
of the stock exchange on which the ADSs are listed, in lieu of distribution of the materials
provided to the Depositary in connection with any meeting of or solicitation of consents or
proxies from holders of deposited securities, distribute to the Holders a notice, after consulting
the Company as to the form of such notice to the extent practicable, that provides Holders with,
or otherwise publicises to Holders, instructions on how to retrieve such materials or receive
such materials upon request (i.e., by reference to a website containing the materials for
retrieval or a contact for requesting copies of the materials).
Procedures for transmitting notices, reports and proxy soliciting material
In addition to the procedures for transmitting notices discussed above under “Voting,” the
Depositary or its agent will keep, at a designated transfer office (the “Transfer Office”), (i) a
register (the “ADR Register”) for the registration, registration of transfer, combination and
split-up of ADRs and (ii) facilities for the delivery and receipt of ADRs. Title to an ADR (and to
deposited securities represented by the ADSs), upon delivery to the Depositary of proper
instruments of transfer, is transferable by delivery with the same effect as in the case of
negotiable instruments under the laws of the State of New York; provided that the Depositary,
notwithstanding any notice to the contrary, may treat the person in whose name such ADR is
registered on the ADR Register as the absolute owner hereof for all purposes and neither the
Depositary nor the Company will have any obligation or be subject to any liability under the
Deposit Agreement or any ADR to any Beneficial Owner, unless such Beneficial Owner is the
Holder hereof. Such ADR is transferable on the ADR Register and may be split into other
ADRs or combined with other ADRs into one ADR, evidencing the aggregate number of ADSs
surrendered for split-up or combination, by the Holder hereof or by duly authorised attorney
upon surrender of ADRs at the Transfer Office or upon delivery to the Depositary of proper
instruments of transfer, duly stamped as may be required by applicable law; provided that the
Depositary may close the ADR Register at any time or from time to time when deemed
expedient by it and it shall also close the issuance book portion of the ADR Register when
reasonably requested by the Company in order to enable the Company to comply with
applicable law.
The Deposit Agreement, the provisions of or governing deposited securities and any written
communications from the Company, which are both received by the Custodian or its nominee
as a holder of deposited securities and made generally available to the holders of deposited
securities, are available for inspection by Holders at the offices of the Depositary and its agent
or agents, at the Transfer Office, on the SEC website, or upon request from the Depositary
(which request may be refused by the Depositary at its discretion). The Depositary will
distribute copies of such communications (or English translations or summaries thereof) to
Holders when furnished by the Company. The Company is subject to the periodic reporting
requirements of the US Exchange Act and accordingly files certain reports with the SEC.
“Direct Registration ADR” means an ADR, the ownership of which is recorded on the Direct
Registration System.
“Direct Registration System” means the system for the uncertificated registration of
ownership of securities established by DTC and utilised by the Depositary pursuant to which
the Depositary may record the ownership of ADRs without the issuance of a certificate, which
ownership shall be evidenced by periodic statements issued by the Depositary to the Holders
entitled thereto.
Sale or exercising of Rights
The Depositary will distribute to each Holder entitled thereto on the record date set by the
Depositary therefor at such Holder’s address shown on the ADR Register, in proportion to the
number of deposited securities (on which the following distributions on deposited securities are
252
received by the Custodian) represented by ADSs evidenced by such Holder’s ADRs:
(i) warrants or other instruments in the discretion of the Depositary representing rights to
acquire additional ADRs in respect of any rights to subscribe for additional shares or rights of
any nature available to the Depositary as a result of a distribution on deposited securities
(“Rights”), to the extent that the Company timely furnishes to the Depositary evidence
satisfactory to the Depositary that the Depositary may lawfully distribute the same (the
Company has no obligation to so furnish such evidence), or (ii) to the extent the Company
does not so furnish such evidence and sales of Rights are practicable, any USD available to
the Depositary from the net proceeds of sales of Rights as in the case of cash, or (iii) to the
extent the Company does not so furnish such evidence and such sales cannot practicably be
accomplished by reason of the non-transferability of the Rights, limited markets therefor, their
short duration or otherwise, nothing (and any Rights may lapse).
Deposit or sale of securities resulting from dividends, splits or plans of reorganisation
If the Company makes a dividend payable at the election of the holders of ordinary shares in
either cash or additional ordinary shares that it wishes to be made available to the Holders, the
Company shall give notice thereof to the Depositary at least 30 days prior to the proposed
distribution stating whether or not it wishes such elective distribution to be made available to
the Holders. The Depositary shall make such elective distribution available to the Holders only
if, among other things, the Company has timely requested that the elective distribution is
available to the Holders and the Depositary shall have determined that such distribution is
reasonably practicable. If the conditions for making the elective distribution available to the
Holders are satisfied, the Depositary shall establish a record date and procedures to enable
the Holders to elect the receipt of either cash or additional ADSs. If the conditions for making
the elective distribution available to the Holders are not satisfied, the Depositary shall, to the
extent permitted by law, distribute either cash or additional ADSs to the Holders on the basis of
the same determination as is made in the local market in respect of the ordinary shares for
which no election is made. There can be no assurance that Holders or Beneficial Owners
generally, or any Holder and/or Beneficial Owner in particular, will be given the opportunity to
receive elective distributions on the same terms and conditions as the holders of ordinary
shares.
To the extent the Depositary deems distribution of securities or property available to the
Depositary resulting from any distribution on deposited securities (other than cash, ordinary
shares or Rights) not to be equitable and practicable, the Depositary may distribute any US
Dollars available to the Depositary from net proceeds of sale of such securities or property.
The Depositary may, in its discretion, and shall if reasonably requested by the Company,
distribute additional or amended ADRs or cash, securities or property to reflect any change in
par value, split-up, consolidation, cancellation or other reclassification of deposited securities,
any ordinary share distributions or other distributions not distributed to Holders or any cash,
securities or property available to the Depositary in respect of deposited securities from (and
the Depositary is hereby authorised to surrender any deposited securities to any person and,
irrespective of whether such deposited securities are surrendered or otherwise cancelled by
operation of law, rule, regulation or otherwise, to sell by public or private sale any property
received in connection with) any recapitalisation, reorganisation, merger, consolidation,
liquidation, receivership, bankruptcy or sale of all or substantially all the assets of the
Company. To the extent the Depositary does not amend ADRs or make a distribution to
Holders to reflect any of the foregoing, or the net proceeds thereof, whatever cash, securities
or property results from any of the foregoing shall constitute deposited securities and each
ADS evidenced by an ADR shall automatically represent its pro rata interest in the deposited
securities as then constituted. Promptly upon the occurrence of any of the aforementioned
changes affecting deposited securities, the Company shall notify the Depositary in writing of
such occurrence and as soon as practicable after receipt of such notice from the Company,
may instruct the Depositary to give notice thereof, at the Company’s expense, to Holders in
accordance with the provisions hereof. Upon receipt of such instruction, the Depositary shall
give notice to the Holders in accordance with the terms thereof, as soon as reasonably
practicable.
253
For all cash dividends and other cash distributions that are made available to the Depositary
after the date that will be published on www.adr.com (as updated by the Depositary from time
to time, “ADR.com”) and communicated to then current Holders by mail, the Depositary will
distribute any cash to Holders solely via electronic funds transfer, except as otherwise provided
in this paragraph. In order to receive such amounts, Holders must provide their bank deposit
details to the Depositary in accordance with the instructions provided by the Depositary for this
purpose. Subject to the last sentence of this paragraph, all such amounts owing to Holders
who do not provide such bank deposit details shall be held by the Depositary on behalf of such
Holders until such bank deposit details have been provided. All amounts so held by the
Depositary will be reported for tax purposes as if paid to all Holders as of the date that funds
are first made available to Holders and will neither accrue interest nor be invested for Holders
while they are being held. A Holder will be unable to receive cash dividends or other cash
distributions to which it is entitled until such time as such Holder either (i) provides its bank
deposit details to the Depositary in accordance with the instructions provided by the Depositary
for this purpose, (ii) transfers such Holder’s ADS position into DTC or (iii) cancels its ADSs
(whereupon, in the case of a transfer to DTC or a cancellation, such Holder will receive a check
for the aggregate amount of cash dividends and/or cash distributions being held on its behalf).
Notwithstanding the foregoing, the Depositary shall, if instructed by the Company, distribute
cash dividends and other cash distributions by check or by such other means as the Company
and the Depositary may agree.
Foreign exchange related matters
To facilitate the administration of various depositary receipt transactions, including
disbursement of dividends or other cash distributions and other corporate actions, the
Depositary may engage the foreign exchange desk within JPMorgan Chase Bank, N.A. (the
“Bank”) and/or its affiliates in order to enter into spot foreign exchange transactions to convert
foreign currency into US Dollars (“FX Transactions”). For certain currencies, FX Transactions
are entered into with the Bank or an affiliate, as the case may be, acting in a principal capacity.
For other currencies, FX Transactions are routed directly to and managed by an unaffiliated
local custodian (or other third party local liquidity provider), and neither the Bank nor any of its
affiliates is a party to such FX Transactions.
The foreign exchange rate applied to an FX Transaction will be either (a) a published
benchmark rate, or (b) a rate determined by a third party local liquidity provider, in each case
plus or minus a spread, as applicable. The Depositary will disclose which foreign exchange
rate and spread, if any, apply to such currency on ADR.com. Such applicable foreign exchange
rate and spread may (and neither the Depositary, the Bank nor any of their affiliates is under
any obligation to ensure that such rate does not) differ from rates and spreads at which
comparable transactions are entered into with other customers or the range of foreign
exchange rates and spreads at which the Bank or any of its affiliates enters into foreign
exchange transactions in the relevant currency pair on the date of the FX Transaction.
Additionally, the timing of execution of an FX Transaction varies according to local market
dynamics, which may include regulatory requirements, market hours and liquidity in the foreign
exchange market or other factors. Furthermore, the Bank and its affiliates may manage the
associated risks of their position in the market in a manner they deem appropriate without
regard to the impact of such activities on the Company, the Depositary, Holders or Beneficial
Owners. The spread applied does not reflect any gains or losses that may be earned or
incurred by the Bank and its affiliates as a result of risk management or other hedging related
activity. To the extent the Company provides US Dollars to the Depositary, neither the Bank
nor any of its affiliates will execute an FX Transaction. In such case, the Depositary will
distribute the US Dollars received from the Company.
Further details relating to the applicable foreign exchange rate, the applicable spread and the
execution of FX Transactions will be provided by the Depositary on ADR.com. The Company,
Holders and Beneficial Owners each acknowledge and agree that the terms applicable to FX
Transactions disclosed from time to time on ADR.com will apply to any FX Transaction
executed pursuant to the Deposit Agreement.
254
Amendment and termination of the Deposit Agreement
The form of ADRs evidencing ADSs and any provisions of the Deposit Agreement relating to
those ADRs may be amended by the Company and the Depositary. Any amendment that
imposes or increases any fees or charges, other than taxes and other governmental charges,
transfer or registration fees, transmission costs, delivery costs or other such expenses, or that
otherwise prejudices any substantial existing right of the Holders or Beneficial Owners, will not
take effect as to any ADRs until 30 days after notice of the amendment has been given to the
Holders. Every Holder and Beneficial Owner of any ADR, at the time an amendment becomes
effective, will be deemed to continue to hold such ADR and to consent and agree to the
amendment and to be bound by the Deposit Agreement or the ADR as amended. No
amendment may impair the right of any Holder to surrender ADRs and receive in return the
deposited securities represented by the ADSs. If any governmental body or regulatory body
should adopt new laws, rules or regulations which would require amendment or supplement of
the Deposit Agreement or the form of ADR to ensure compliance therewith, the Company and
the Depositary may amend or supplement the Deposit Agreement and the ADR at any time in
accordance with such changed laws, rules or regulations. Such amendment or supplement to
the Deposit Agreement in such circumstances may become effective before a notice of such
amendment or supplement is given to Holders or within any other period of time as required for
compliance.
Whenever the Company directs, the Depositary has agreed to terminate the Deposit
Agreement as to ADRs evidencing ADSs by mailing a termination notice to the Holders then
outstanding at least 30 days before the date fixed in the notice of termination. The Depositary
may likewise terminate the Deposit Agreement as to ADRs evidencing ADSs by mailing a
termination notice to the Company and the Holders then outstanding at least 30 days before
the date of termination, under the following circumstances: (i) in the event of the Company’s
bankruptcy or insolvency, (ii) if the ordinary shares cease to be listed on an internationally
recognised stock exchange, (iii) if the Company effects (or will effect) a redemption of all or
substantially all of the deposited securities, or a cash or share distribution representing a return
of all or substantially all of the value of the deposited securities, or (iv) there occurs a merger,
consolidation, sale of assets or other transaction as a result of which securities or other
property are delivered in exchange for or in lieu of deposited securities, except where such
transaction was commenced, announced by the Company or notified to the Depositary prior to
the effective date of the Deposit Agreement.
After the date so fixed for termination, the Depositary and its agents will perform no further acts
under the Deposit Agreement and the ADRs, except to receive and hold (or sell) distributions
on deposited securities and deliver deposited securities being withdrawn. As soon as
practicable after the date so fixed for termination, the Depositary shall use its reasonable
efforts to sell the deposited securities and shall thereafter (as long as it may lawfully do so)
hold in an account (which may be a segregated or unsegregated account) the net proceeds of
such sales, together with any other cash then held by it under the Deposit Agreement, without
liability for interest, in trust for the pro rata benefit of the Holders of ADRs not theretofore
surrendered. After making such sale, the Depositary shall be discharged from all obligations in
respect of the Deposit Agreement and the ADRs, except to account for such net proceeds and
other cash. After the date so fixed for termination, the Company shall be discharged from all
obligations under the Deposit Agreement except for its obligations to the Depositary and its
agents.
Rights of Holders to inspect the transfer books of the Depositary and the list of Holders
The Depositary will keep books for the registration and transfer of ADRs as well as facilities for
the delivery and receipt of ADRs at the Transfer Office. These books will be open for inspection
by Holders at all reasonable times. However, this inspection may not be for the purpose of
communicating with Holders in the interest of a business or object other than the Company
business or a matter related to the Deposit Agreement or the ADRs.
255
Restrictions on the right to transfer or withdraw the underlying securities
As a condition precedent to the issue, registration, registration of transfer, split-up or
combination of any ADR, the delivery of any distribution in respect thereof, or the withdrawal of
any deposited securities, the Company, the Depositary, or Custodian may require payment of a
sum sufficient to reimburse it for any tax or other governmental charge and any stock transfer
or registration fee with respect thereto (including any such tax or charge and fee with respect to
ordinary shares or other deposited securities being registered) and payment of any applicable
fees as therein provided, may require the production of proof satisfactory to it as to the identity
and genuineness of any signature, as well as such other information, including without
limitation, information as to citizenship, residence, exchange control approval, beneficial or
other ownership of, or interest in any securities, compliance with applicable law, regulations,
provisions of or governing deposited securities and terms of the Deposit Agreement and ADR,
as it may deem necessary or proper, and may also require compliance as the Depositary may
deem reasonably necessary or appropriate to comply with any applicable laws, rules,
regulations or industry standards or to avoid, prevent or mitigate any potential liability to the
Depositary.
The issuance of ADRs, the acceptance of deposits of ordinary shares, the registration,
registration of transfer, split-up or combination of ADRs or the withdrawal of deposited
securities may be suspended, generally or in particular instances, when the ADR Register or
any register for deposited securities is closed or when any such action is deemed advisable by
the Depositary or the Company at any time or from time to time.
Limitations on the Depositary’s liability
The Depositary shall not incur any liability to any Holder or Beneficial Owners of ADRs, if by
reason of any provision of any present or future law, rule, regulation, fiat, order or decree of the
United Kingdom, US, or any other country or jurisdiction, or of any governmental or regulatory
authority or any securities exchange or market or automated quotation system, or the
provisions of or governing any deposited securities, or by reason of any provision, present or
future, of the Company’s charter, or by reason of any act of God, war, terrorism, epidemic,
pandemic, cyber ransomware or malware attack or other circumstances beyond its control, the
Depositary shall be prevented or forbidden from or be subject to any civil or criminal penalty on
account of doing or performing any act or thing which by the terms of the Deposit Agreement it
is provided shall be done or performed; nor shall the Depositary incur any liability to any Holder
or Beneficial Owner of any ADR by reason of any non-performance or delay, caused as
aforesaid, in the performance of any act or thing which by the terms of the Deposit Agreement
it is provided shall or may be done or performed, or by reason of any exercise of, or failure to
exercise, any discretion provided for in the Deposit Agreement.
The Depositary assumes no obligation nor shall it be subject to any liability under the Deposit
Agreement to any Holders or Beneficial Owners of any ADR (including, without limitation,
liability with respect to the validity or worth of any deposited securities), except that it agrees to
perform its obligations specifically set forth in the Deposit Agreement without gross negligence
or wilful misconduct. The Depositary shall not be a fiduciary or have any fiduciary duty to
Holders or Beneficial Owners.
The Depositary and its agents shall not be under any obligation to appear in, prosecute or
defend any action, suit or other proceeding in respect of any deposited securities, the ADSs or
in respect of the ADRs. The Depositary shall not be liable to Holders or Beneficial Owners for
any action or non-action by it in reliance upon the advice of or information from the Company,
legal counsel, accountants, any person presenting ordinary shares for deposit, any Holder or
any other person believed by it to be competent to give such advice or information. The
Depositary shall not be liable for the acts or omissions made by, or the insolvency of, any
securities depository, clearing agency or settlement system.
The Depositary shall not be responsible for, and shall incur no liability in connection with or
arising from, the insolvency of any custodian that is not a branch or affiliate of JPMorgan
256
Chase Bank, N.A. The Depositary shall not have any liability for the price received in
connection with any sale of securities, the timing thereof or any delay in action or omission to
act nor shall it be responsible for any error or delay in action, omission to act, default or
negligence on the part of the party so retained in connection with any such sale or proposed
sale. The Depositary shall not be liable for any acts or omissions to act on the part of the
Custodian, except to the extent that any Holder has incurred liability directly as a result of the
Custodian having (i) committed fraud or wilful misconduct in the provision of custodial services
to the Depositary or (ii) failed to use reasonable care in the provision of custodial services to
the Depositary as determined in accordance with the standards prevailing in the jurisdiction in
which the Custodian is located.
The Depositary and its respective agents may rely and shall be protected in acting upon any
written notice, request, direction, instruction or document believed by it to be genuine and to
have been signed, presented or given by the proper party or parties.
The Depositary shall be under no obligation to inform Holders or Beneficial Owners about the
requirements of the laws, rules or regulations or any changes therein or thereto of any country
or jurisdiction or of any governmental or regulatory authority or any securities exchange or
market or automated quotation system.
The Depositary and its agents will not be responsible for any failure to carry out any Voting
Instructions to vote any of the deposited securities, for the manner in which Voting Instructions
are given, including instructions to give a discretionary proxy to a person designed by the
Company, for the manner in which any such vote is cast, including without limitation any vote
cast by a person to whom the Depositary is required to grant a discretionary proxy pursuant to
the Deposit Agreement, for any act or omission to act on the part of Holders, Beneficial
Owners, the Company or its agents in connection with voting at a meeting, or for the effect of
any such vote.
The Depositary may rely upon instructions from the Company or its counsel in respect of any
approval or licence required for any currency conversion, transfer or distribution.
The Depositary and its agents may own and deal in any class of securities of the Company and
its affiliates and in ADRs.
Notwithstanding anything to the contrary set forth in the Deposit Agreement or any ADR, the
Depositary and its agents may fully respond to any and all demands or requests for information
maintained by or on its behalf in connection with the Deposit Agreement, any Holder or
Holders, any ADR or ADRs or otherwise related hereto or thereto to the extent such
information is requested or required by or pursuant to any lawful authority, including without
limitation laws, rules, regulations, administrative or judicial process, banking, securities or other
regulators.
The Depositary shall not be liable for the failure by any Holder or Beneficial Owner to obtain
the benefits of credits or refunds of non-US tax paid against such Holder’s or Beneficial
Owner’s income tax liability.
The Depositary is under no obligation to provide the Holders and Beneficial Owners, or any of
them, with any information about the tax status of the Company. The Depositary shall not incur
any liability for any tax or tax consequences that may be incurred by Holders and Beneficial
Owners on account of their ownership or disposition of the ADRs or ADSs.
The Depositary shall not incur any liability for the content of any information submitted to it by
or on behalf of the Company for distribution to the Holders or for any inaccuracy of any
translation thereof, for any investment risk associated with acquiring an interest in the
deposited securities, for the validity or worth of the deposited securities, for the credit-
worthiness of any third party, for allowing any rights to lapse upon the terms of the Deposit
Agreement or for the failure or timeliness of any notice from the Company.
257
Notwithstanding anything to the contrary set forth in the Deposit Agreement or any ADR, the
Depositary may use third party delivery services and providers of information regarding matters
such as, but not limited to pricing, proxy voting, corporate actions, class action litigation and
other services in connection herewith and the Deposit Agreement, and use local agents to
provide services, such as, but not limited to, attendance at meetings of holders of securities of
issuers. Although the Depositary will use reasonable care (and cause their agents to use
reasonable care) in the selection and retention of such third party providers and local agents,
they will not be responsible for any errors or omissions made by them in providing the relevant
information or services.
The Depositary shall not be liable for any acts or omissions made by a successor depositary
whether in connection with a previous act or omission of the Depositary or in connection with
any matter arising wholly after the removal or resignation of the Depositary.
By holding or owning an ADR or ADS or an interest therein, Holders and Beneficial Owners
each irrevocably agree that any legal suit, action or proceeding against or involving the Holders
or Beneficial Owners brought by the Depositary, arising out of or based upon the Deposit
Agreement, the ADSs, the ADRs or the transactions contemplated herein, therein or hereby,
may be instituted in a state or federal court in New York, New York, and by holding or owning
an ADR or an ADS or an interest therein each irrevocably waives any objection that it may now
or hereafter have to the laying of venue of any such proceeding, and irrevocably submits to the
non-exclusive jurisdiction of such courts in any such suit, action or proceeding. By holding or
owning an ADR or ADS or an interest therein, Holders and Beneficial Owners each also
irrevocably agree that any legal suit, action or proceeding against or involving the Depositary
and/or the Company brought by Holders or Beneficial Owners, arising out of or based upon the
Deposit Agreement, the ADSs, the ADRs or the transactions contemplated therein, herein,
thereby or hereby, including, without limitation, claims under the US Securities Act, may be
only instituted in the United States District Court for the Southern District of New York (or in the
state courts of New York County in New York if either (i) the United States District Court for the
Southern District of New York lacks subject matter jurisdiction over a particular dispute or
(ii) the designation of the United States District Court for the Southern District of New York as
the exclusive forum for any particular dispute is, or becomes, invalid, illegal or unenforceable).
The Company has agreed to indemnify the Depositary under certain circumstances and the
Depositary has agreed to indemnify the Company under certain circumstances.
Notwithstanding any other provision of the Deposit Agreement or the ADRs to the contrary,
neither the Company nor the Depositary, nor any of their respective agents shall be liable to
the other for any indirect, special, punitive or consequential damages or lost profits, in each
case of any form incurred by any of them or any other person or entity (including, without
limitation, Holders and Beneficial Owners), whether or not foreseeable and regardless of the
type of action in which such a claim may be brought (collectively “Special Damages”) except
(i) to the extent such Special Damages arise from the gross negligence or wilful misconduct of
the party from whom indemnification is sought or (ii) to the extent Special Damages arise from
or out of a claim brought by a third party (including, without limitation, Holders and Beneficial
Owners) against the Depositary or its agents acting under the Deposit Agreement, except to
the extent such Special Damages arise out of the gross negligence or wilful misconduct of the
party seeking indemnification hereunder.
Notwithstanding the limitations on the Depositary’s liability set forth in the Deposit Agreement,
no provision of the Deposit Agreement is intended to constitute a waiver or limitation of any
rights which any Holders or Beneficial Owners of ADRs may have under the US Securities Act
or the US Exchange Act, to the extent applicable.
Fees and charges payable by Holders
Pursuant to the Deposit Agreement, Holders may be required to pay various fees to the
Depositary, and the Depositary may refuse to provide any service for which a fee is assessed
until the applicable fee has been paid. In particular, the Depositary, under the terms of the
258
Deposit Agreement, shall charge (i) a fee of $5.00 per 100 ADSs (or portion thereof) for the
issuance, delivery, reduction, cancellation or surrender (as the case may be) of ADSs, (ii) a fee
of $0.05 or less per ADS held (A) upon which any cash distribution is made pursuant to the
Deposit Agreement or (B) in the case of an elective cash/stock dividend, upon which a cash
distribution or an issuance of additional ADSs is made as a result of such elective dividend,
(iii) a fee for the distribution or sale of securities, such fee being in an amount equal to the fee
for the execution and delivery of ADSs referred to above which would have been charged as a
result of the deposit of such securities but which securities or the net cash proceeds from the
sale thereof are instead distributed by the Depositary to Holders entitled thereto, (iv) an
aggregate fee of $0.05 or less per ADS per calendar year (or portion thereof) for services
performed by the Depositary in administering the ADRs (which fee may be charged on a
periodic basis during each calendar year and shall be assessed against Holders as of the
record date or record dates set by the Depositary during each calendar year and shall be
payable at the sole discretion of the Depositary by billing such Holders or by deducting such
charge from one or more cash dividends or other cash distributions), and (v) a fee for the
reimbursement of such fees, charges and expenses as are incurred by the Depositary and/or
any of its agents (including, without limitation, the Custodian) and expenses incurred on behalf
of Holders in connection with compliance with foreign exchange control regulations or any law
or regulation relating to foreign investment) in connection with the servicing of the ordinary
shares or other deposited securities, the sale of securities (including, without limitation,
deposited securities), the delivery of deposited securities or otherwise in connection with the
Depositary’s or its Custodian’s compliance with applicable law, rule or regulation (which fees
and charges shall be assessed on a proportionate basis against Holders as of the record date
or dates set by the Depositary and shall be payable at the sole discretion of the Depositary by
billing such Holders or by deducting such charge from one or more cash dividends or other
cash distributions).
The Company will pay all other fees, charges and expenses of the Depositary and any agent of
the Depositary (except the Custodian) pursuant to agreements from time to time between the
Company and the Depositary, except (i) stock transfer or other taxes and other governmental
charges (which are payable by Holders or persons depositing shares), (ii) cancellation
transaction (including SWIFT, telex and facsimile transmission) fees and delivery expenses
incurred at the request of persons depositing, or Holders delivering shares, as disclosed on the
“Disclosures” age (or successor page) of www.ADR.com, ADRs or deposited securities (which
are payable by such persons or Holders), (iii) transfer or registration expenses for the
registration or transfer of deposited securities on any applicable register in connection with the
deposit or withdrawal of deposited securities (which are payable by persons depositing
ordinary shares or Holders withdrawing deposited securities).
Direct and indirect payments by the Depositary
The Depositary anticipates reimbursing the Company for certain expenses incurred by the
Company that are related to the establishment and maintenance of the ADR programme upon
such terms and conditions as the Company and the Depositary may agree from time to time.
The Depositary may make available to the Company a set amount or a portion of the
Depositary fees charged in respect of the ADR programme or otherwise upon such terms and
conditions as the Company and the Depositary may agree from time to time.
5. ARTICLES OF ASSOCIATION
The Articles of Association of the Company, which were adopted on 31 May 2022, contain
(amongst others) provisions to the following effect.
5.1 Unrestricted objects
The objects of the Company are unrestricted.
259
5.2 Limited liability
The liability of the Haleon Shareholders is limited to the amount, if any, unpaid on the Haleon
Shares held by them.
5.3 Share rights
Subject to any rights attached to existing Haleon Shares and Non-Voting Preference Shares,
the Company may issue shares with such rights and restrictions as the Company may by
ordinary resolution decide, or (if there is no such resolution or so far as it does not make
specific provision) as the Board may decide. Such rights and restrictions apply as if they were
set out in the Articles of Association. The Company may issue redeemable shares, subject to
any rights attached to existing Haleon Shares and the Non-Voting Preference Shares. The
Board may determine the terms and conditions and the manner of redemption of any
redeemable shares so issued. Such terms and conditions apply to the relevant shares as if
they were set out in the Articles of Association.
5.4 Voting rights
Haleon Shareholders are entitled to vote at a general meeting or class meeting on a poll.
Under the Articles of Association, any resolution put to a vote at a general meeting of the
Company shall be decided on a poll. The Companies Act and the Articles of Association of the
Company provide that on a poll every Haleon Shareholder has one vote per Haleon Share held
by them and a Haleon Shareholder may vote in person or by one or more proxies. Where a
Haleon Shareholder appoints more than one proxy, the proxies appointed by them taken
together have the same voting rights as the Haleon Shareholder could exercise in person.
In the case of joint holders of a Haleon Share the vote of the senior who tenders a vote,
whether in person or by proxy, is accepted to the exclusion of the votes of the other joint
holders and, for this purpose, seniority is determined by the order in which the names stand in
the register in respect of the joint holding.
Non-Voting Preference Shares do not confer any right to vote at a general meeting. Non-Voting
Preference Shareholders are, however, entitled to vote in respect of their Non-Voting
Preference Shares at any class meeting of Non-Voting Preference Shareholders.
5.5 Restrictions on voting
A Haleon Shareholder is not entitled to vote at any general meeting or class meeting in respect
of any Haleon Share held by them if any call or other sum then payable by them in respect of
that Haleon Share remains unpaid or if that Haleon Shareholder has been served with a
restriction notice (as defined in the Articles of Association) after failure to provide the Company
with information concerning interests in those Haleon Shares required to be provided under the
Companies Act.
5.6 Dividends and other distributions
The Company may by ordinary resolution from time to time declare dividends not exceeding
the amount recommended by the Board. Subject to the Companies Act, the Board may pay
interim dividends, and also any fixed rate dividend, whenever the financial position of the
Company, in the opinion of the Board, justifies its payment. If the Board acts in good faith, it is
not liable to holders of Haleon Shares or Non-Voting Preference Shares with preferred or pari
passu rights for losses arising from the payment of interim or fixed dividends on other Haleon
Shares or Non-Voting Preference Shares.
The Board may withhold payment of all or any part of any dividends or other moneys payable
in respect of Haleon Shares from a person with a 0.25 per cent. or greater holding, in number
or nominal value, of such shares (in each case, calculated exclusive of any such shares held
as treasury shares) (in this paragraph, a “0.25 per cent. interest”) if such a person has been
served with a restriction notice (as defined in the Articles of Association) after failure to provide
the Company with information concerning interests in those Haleon Shares required to be
provided under the Companies Act.
260
The Non-Voting Preference Shares rank pari passu with all other Non-Voting Preference
Shares and have preferential dividend rights ahead of the Haleon Shares, entitling Non-Voting
Preference Shareholders to quarterly cumulative dividends at a fixed rate of 9.5 per cent. per
annum for a period of five years from the date of the issue of the Non-Voting Preference
Shares, following which the rate shall be reset for each subsequent period of five consecutive
years at the rate which is equal to the Bank of England base rate prevailing at the time of reset
plus 7.5 per cent. Dividends on the Non-Voting Preference Shares which have become due
and payable in accordance with the Articles are required to be approved and paid in full before
any repurchases or distributions can be made with respect to the Haleon Shares.
Except insofar as the rights attaching to, or the terms of issue of, any Haleon Share otherwise
provide, all dividends are apportioned and paid pro rata as between the Haleon Shares
according to the amounts paid up on the Haleon Share during any portion of the period in
respect of which the dividend is paid. Dividends may be declared or paid in any currency.
The Board may, if authorised by an ordinary resolution of the Company, offer Haleon
Shareholders (excluding any Haleon Shareholder holding Haleon Shares as treasury shares) in
respect of any dividend the right to elect to receive Haleon Shares by way of scrip dividend
instead of cash.
Any dividend unclaimed after a period of six years from the date when it was declared or
became due for payment is forfeited and reverts to the Company unless the Board decides
otherwise.
The Board may decide on the way dividends or other money payable in cash relating to a
Haleon Share are paid, including deciding on different methods of payment for different Haleon
Shareholders or groups of Haleon Shareholders. If the Board has decided on different methods
of payment, it may also give Haleon Shareholders the option of choosing in which of these
ways they would like to receive payment or it may specify that a particular method of payment
will be used unless Haleon Shareholders choose otherwise. If Haleon Shareholders fail to
provide the necessary details to enable payment of the dividend or other amount payable to
them or if payment cannot be made using the details provided by the Haleon Shareholder, the
dividend or other amount payable will be treated as unclaimed.
The Company may cease to employ any means of payment, including intra-bank transfers or
other electronic means, for dividends if (i) for any one dividend the payment by any method has
failed (including where the payment has been rejected or refunded) and reasonable enquiries
have failed to establish any new account of the registered holder; or (ii) in respect of any
payments to be made via cheque, any hard copy notice, document or other information served
on or sent or supplied to a member of the Company has been returned to the Company
undelivered and the relevant member has not supplied to the Company (or its agent) a new
registered address, or a postal address within the United Kingdom or the United States for the
service of notices and the despatch or supply of documents and other information. The
Company must recommence sending dividend payments if the holder or person entitled by
transmission requests such recommencement in writing (and provides any information
reasonably required by the Company to enable it to do so).
5.7 Rights on a winding up
The Non-Voting Preference Shares carry preferential rights to participate in a distribution of
capital in the event of insolvency (including on a winding-up) up to an amount equal to their
nominal value plus accrued dividend and any arrears or deficiency in amount of the cumulative
dividend.
The Haleon Shares do not carry any rights to participate in a capital distribution (including on a
liquidation) other than those that exist as a matter of law. Under the Companies Act, upon a
liquidation, after the claims of creditors have been satisfied and subject to any special rights
attaching to any other class of shares in the Company (including the Non-Voting Preference
Shares), surplus assets (if any) are distributed among the Haleon Shareholders in proportion to
the number and nominal amounts of their Haleon Shares.
261
5.8 Pre-emption rights
The rights of Haleon Shareholders to participate pre-emptively in any allotment of equity
securities are prescribed by the Companies Act. Under the Companies Act, subject to certain
statutory exceptions, a company proposing to allot equity securities (which includes the grant
of rights to subscribe for shares) must first offer them on the same or more favourable terms to
each holder of shares pro rata to their existing shareholding. The statutory pre-emption right
also applies to a sale of shares that, immediately before the sale, were held by the Company
as treasury shares. The Companies Act allows this statutory pre-emption right to be disapplied
by special resolution so that the Directors may allot shares as if the pre-emption provisions did
not apply, either in relation to a general authority to allot shares or in relation to a specified
allotment of equity securities.
The statutory pre-emption regime does not apply to: the allotment or transfer of Haleon Shares
under an employees’ share scheme; the allotment of bonus shares; or an allotment of equity
securities that are paid up wholly or partly otherwise than in cash.
5.9 Transfer of shares
The Haleon Shares and Non-Voting Preference Shares are in registered form. Any Haleon
Share or Non-Voting Preference Share may be held in uncertificated form and, subject to the
Articles of Association, title to uncertificated Haleon Shares or Non-Voting Preference Shares
may be transferred by means of a relevant system. Provisions of the Articles of Association do
not apply to any uncertificated Haleon Shares or Non-Voting Preference Shares to the extent
that such provisions are inconsistent with the holding of Haleon Shares or Non-Voting
Preference Shares (as applicable) in uncertificated form, with the transfer of Haleon Shares or
Non-Voting Preference Shares (as applicable) by means of a relevant system, with any
provision of the legislation relating to the holding, evidencing of title to, or transfer of
uncertificated shares.
Subject to the Articles of Association, any Haleon Shareholder or Non-Voting Preference
Shareholder may transfer all or any of their certificated Haleon Shares or Non-Voting
Preference Shares by an instrument of transfer in any usual form or in any other form which
the Board may approve. The instrument of transfer must be signed by or on behalf of the
transferor and (in the case of a partly-paid Haleon Share) the transferee.
The transferor of a Haleon Share or Non-Voting Preference Share is deemed to remain the
holder until the transferee’s name is entered in the register.
The Board can decline to register any transfer of any Haleon Share or Non-Voting Preference
Share which is not a fully paid Haleon Share or Non-Voting Preference Share. The Board may
also decline to register a transfer of a certificated Haleon Share or Non-Voting Preference
Share unless the instrument of transfer:
(A) is duly stamped or certified or otherwise shown to the satisfaction of the Board to be
exempt from stamp duty and is accompanied by the relevant share certificate and such
other evidence of the right to transfer as the Board may reasonably require;
(B) is in respect of only one class of Haleon Share or Non-Voting Preference Share; and
(C) if to joint transferees, is in favour of not more than four such transferees.
Registration of a transfer of an uncertificated Haleon Share or a Non-Voting Preference Share
may be refused in the circumstances set out in the uncertificated securities rules (as defined in
the Articles of Association) and where, in the case of a transfer to joint holders, the number of
joint holders to whom the uncertificated Haleon Share or Non-Voting Preference Share (as
applicable) is to be transferred exceeds four.
262
The Board may decline to register a transfer of any of the Company’s certificated Haleon
Shares by a person with a 0.25 per cent. interest if such a person has been served with a
restriction notice (as defined in the Articles of Association) after failure to provide the Company
with information concerning interests in those shares required to be provided under the
Companies Act, unless the transfer is shown to the Board to be pursuant to an arm’s length
sale (as defined in the Articles of Association).
5.10 Redemption of Non-Voting Preference Shares
Each Non-Voting Preference Share is redeemable in whole at the option of the Company or
redeemable at the option of each relevant Non-Voting Preference Shareholder in respect of its
entire holding of Non-Voting Preference Shares on any date falling not less than five years
after the date on which that Non-Voting Preference Share was issued or, if earlier, on the
Company undergoing a change of control. Such redemption shall be at the nominal value of
the relevant Non-Voting Preference Shares plus the amount, if any, of all accrued but unpaid
dividends on the Non-Voting Preference Shares.
5.11 Sub-division of share capital
Any resolution authorising the Company to sub-divide any of its Haleon Shares may determine
that, as between the Haleon Shares resulting from the sub-division, any of them may have a
preference, advantage or deferred or other right or be subject to any restriction as compared
with the others.
5.12 General meetings
The Articles of Association rely on the Companies Act provisions dealing with the calling of
general meetings. Under the Companies Act an annual general meeting must be called by
notice of at least 21 days. Upon listing, the Company will be a “traded company” for the
purposes of the Companies Act and as such will be required to give at least 21 days’ notice of
any other general meeting unless a special resolution reducing the period to not less than 14
days has been passed at the immediately preceding annual general meeting or at a general
meeting held since that annual general meeting or, pending the Company’s first annual general
meeting, at any general meeting. Notice of a general meeting must be given in hard copy form,
in electronic form, or by means of a website and must be sent to every Haleon Shareholder
and every Director. It must state the time and date and the place of the meeting and the
general nature of the business to be dealt with at the meeting. As the Company will be a traded
company, the notice must also state the website address where information about the meeting
can be found in advance of the meeting, the voting record time, the procedures for attending
and voting at the meeting, details of any forms for appointing a proxy, procedures for voting in
advance (if any are offered), and the right of Haleon Shareholders to ask questions at the
meeting. In addition, a notice calling an annual general meeting must state that the meeting is
an annual general meeting.
The Board may decide to allow persons entitled to attend and participate in a general meeting
to do so by simultaneous attendance and participation by means of an electronic facility with no
member necessarily in physical attendance at the electronic meeting, and to permit directors or
others to attend and speak, and the chair of the meeting to preside, by electronic means.
Shareholders present in person or by proxy by means of such electronic facility will be counted
in the quorum for, and entitled to participate in, the relevant general meeting.
5.13 Restrictions in respect of Designated Persons
The Articles of Association contain provisions empowering the Company to apply certain
restrictions and to take certain actions in relation to Haleon Shares and Non-Voting Preference
Shares (“Restricted Shares”) where the Company believes the holder of such shares is or
may be a Designated Person (a “Restricted Person”).
263

In respect of any Restricted Shares:
(A) all of the rights attaching to the Restricted Shares, including (but not limited to) any rights
to attend and vote at general meetings of the Company, rights to receive dividends and
other distributions from the Company and to otherwise participate in the assets of the
Company (including on a winding up) are suspended and cease to have effect;
(B) no interest accrues on any dividend (or capital return) paid to the Company’s
shareholders generally but withheld from the Restricted Person in accordance with
sub-paragraph (A);
(C) the directors of the Company are entitled to take steps to ensure that any Restricted
Shares held in uncertificated form are immediately converted into certificated form, and
that any Restricted Shares held in certificated form are not converted into
uncertificated form;
(D) the Restricted Person is prohibited from disposing of the Restricted Shares or any
legal or beneficial interest in any of them without the prior written consent of the
Company; and
(E) the Company may, on giving written notice to the relevant Restricted Person, authorise
any director of the Company or the company secretary (who are deemed to be
appointed as the Restricted Person’s attorney) to transfer the Restricted Shares to a
subsidiary undertaking of the Company (a “Restricted Share Trustee”) to hold on
trust for the Restricted Person on the terms set out in the Articles of Association.
The restrictions described above will apply to any Restricted Shares held by a Restricted
Person unless and until the directors are satisfied that the Restricted Person has ceased to be
a Designated Person (a “Released Person”). Any person whose shares in the Company are
Restricted Shares and who believes that they have ceased to be a Designated Person may
give written notice to the Company confirming that they believe that they have ceased to be a
Designated Person and the date(s) on which such change became effective (a “Release
Notice”). However, the decision as to whether and when a person’s shares cease to be
Restricted Shares is ultimately a decision for the directors (at their sole discretion); the
directors do not have to receive a Release Notice, for example, before determining that a
Restricted Person has become a Released Person.
87
If at any time the directors determine that a Restricted Person has become a Released Person,
the restrictions described above will cease to apply in respect of that person’s shares with
immediate effect from the time of such determination and the Company is required, as soon as
reasonably practicable and if applicable, to: (i) procure that any Restricted Shares converted
into certificated form are converted back into uncertificated form; (ii) pay, without interest, to
the Released Person or their nominee (provided that such nominee is not itself a Designated
Person) any moneys relating to the Released Person’s shares which were withheld from the
Released Person while their shares in the Company were Restricted Shares; and (iii) procure
that the legal title to any Restricted Shares transferred to a Restricted Share Trustee is
returned to the Released Person or their nominee (provided that such nominee is not itself a
Designated Person).
87
In order to assist the directors with this determination, the registrar and/or another independent adviser to the Company will
routinely review the register of members and section 808 register of beneficial ownership against the sanctions lists using a
market-standard screening software. In addition, the register of members and section 808 register of beneficial ownership will
be screened against the sanctions lists as at the record date of any dividend. Additional screening will be carried out as and
when the Company and its advisers think it is necessary. If the Company receives a Release Notice from a Designated
Person, the Company would verify that such person is no longer a Designated Person by checking against the sanctions lists.
264
6. ORGANISATIONAL STRUCTURE
Following the Demerger, the Company will be the ultimate holding company of the Group.
The following table shows details of CH JVCo’s significant subsidiaries.
Name Country of
incorporation or
registration
Proportion of
ownership
interest (%)
Principal activity
GlaxoSmithKline
Consumer
Healthcare
(Overseas) Limited
England and Wales 100 Holding and
service company
GlaxoSmithKline
Consumer
Healthcare Holdings
(US) LLC
USA 100 Holding company,
IP owner, service,
manufacture and
distribution
GlaxoSmithKline
Consumer
Healthcare L.L.C.
USA 100 Holding company
GSK Consumer
Healthcare Holdings
(No. 1) Limited
England and Wales 100 Holding company
GSK Consumer
Healthcare Holdings
(No. 7) Limited
England and Wales 100 Holding company
GSK Consumer
Healthcare Holdings
(US) Inc.
USA 100 Holding company
and US treasury
functions
GSK Consumer
Healthcare Holdings
No. 2 LLC
USA 100 Holding company
GSK Consumer
Healthcare SARL
Switzerland 100 Holding company,
trading and
distribution, IP and
manufacturing site
owner
7. INTERESTS OF MAJOR SHAREHOLDERS
The Company was incorporated in anticipation of the Separation, and is not a member of the
GSK Group. As at the date of this Prospectus, the entire issued share capital of the Company
is held and controlled by David Redfern, Adam Walker, Victoria Whyte and Subesh Williams.
As at the Latest Practicable Date, and so far as is known to the Company by virtue of the
notifications made to GSK pursuant to the Companies Act, the Market Abuse Regulation and/
or the Disclosure Guidance and Transparency Rules, as a result of the Demerger and the
265

Share Exchanges, the following will, on Admission, be directly or indirectly interested in 3 per
cent. or more of the Company’s issued ordinary share capital:
Name of shareholder Percentage of total voting rights
Pfizer 32
GSK Up to 6
SLP1* 4.74
BlackRock, Inc.** 3.60
* The other SLPs, being SLP2 and SLP3, will each have a less than 3 per cent. holding in the
Company on Admission so are not included in this table.
** BlackRock, Inc. is included in this table on the basis of its major shareholding in GSK of 6.40
per cent. (as at the date of notification to GSK).
Following Admission, no Haleon Shareholder has or will have different voting rights from any
other holder of Haleon Shares in respect of any Haleon Shares held by them and the Haleon
Shares held by them will rank pari passu in all respects with all other Haleon Shares.
Any holder of Non-Voting Preference Shares will have no voting rights, other than in respect of
matters that entail a variation of the class rights attaching to the Non-Voting Preference
Shares, in which case each Non-Voting Preference Share will confer one vote at a separate
class meeting of the Non-Voting Preference Shareholders convened to consider a proposed
variation of class rights.
8. DIRECTORS AND SENIOR MANAGEMENT
8.1 Directorships and partnerships outside the Group
The details of those companies and partnerships outside the Group of which the Directors and
Senior Managers are currently directors or partners, or have been directors or partners at any
time during the five years prior to the publication of this Prospectus, are as follows:
Name Current directorships
and partnerships
Previous directorships
and partnerships
Directors
Dave Lewis Xlinks Tesco plc
Pepsico Inc.
World Wildlife Fund – UK
A Bird’s Eye View
Brian McNamara Consumer Goods Forum GlaxoSmithKline
Consumer Healthcare
Holdings Limited
Global Self-Care
Federation
Treloar Trust
Tobias Hestler - GlaxoSmithKline
Consumer Healthcare
Holdings Limited
Hexal AG
266

Name Current directorships
and partnerships
Previous directorships
and partnerships
Marie-Anne Aymerich Pierre Fabre Group -
Respire Ventures
Academy of St Martin in the
Fields
Manvinder Singh
(Vindi) Banga
UK Government
Investments (“UKGI”)
Kalle GmbH
International Chamber of
Commerce UK
Mauser Group
The Economist Newspaper
Limited
Parksons Packaging Limited
GSK plc High Ridge Brands
Kedaara Capital I Limited Thomson Reuters
Foundation
Kedaara Capital Investment
Managers Ltd
Marks and Spencer Group
plc
Kedaara Holdings Ltd
Clayton Dubilier & Rice LLP
Tracy Clarke Acin Ltd Standard Chartered AG
Starling Bank Limited Standard Chartered
Yatirim Bankasi Turk A.S
School Reviewer Limited Zodia Holdings Limited
TP ICAP plc Zodia Custody Ltd
All England Netball
Association
TheCityUK
Inmarsat plc
Sky plc
Dame Vivienne Cox GSK plc Vallourec SA
Victrex plc Pearson plc
Venterra Group plc UK Government’s
Department for International
Development (DFID)
Said Business School
Stena AB
The Rosalind Franklin
Institute
African Leadership Institute
Montrose Associates
Asmita Dubey - -
Deirdre Mahlan Experian plc The Distilled Spirits
Council
Kimberly-Clark Corporation
The Duckhorn Portfolio, Inc.
267

Name Current directorships
and partnerships
Previous directorships
and partnerships
Bryan Supran* Anacor Upjohn Inc.
Arena Pharmaceuticals, Inc. Medivation LLC
Array BioPharma Inc. JMI-Daniels
Pharmaceuticals, Inc.
GenTrac, Inc.
Purepac Pharmaceutical
Holdings LLC
GI Europe, Inc.
GI Japan, Inc. Antioch Merger Sub, Inc.
Hospira, Inc. Arlington Acquisition Sub
Inc.
InnoPharma, Inc.
Excaliard Pharmaceuticals,
Inc.
Parkedale Pharmaceuticals,
Inc.
* Each entity outside of the Group of which Bryan Supran is currently a director or partner, or
has been a director or partner at any time during the five years prior to the publication of this
Prospectus, is a subsidiary or former subsidiary of Pfizer.
John Young Johnson Controls
International (JCI)
Biotechnology Innovation
Organization
Imbria Pharmaceuticals European Federation of
Pharmaceutical Industries
and Associations
Senior Managers
Dana Bolden Washington & Lee University -
Keith Choy - -
Bart Derde - -
Amy Landucci - -
Filippo Lanzi - -
Jooyong Lee - -
Teri Lyng - -
Maire´ad Nayager - -
Lisa Paley - -
Franck Riot - -
Tamara Rogers - -
Bjarne Philip Tellmann Mowi ASA Hire an Esquire, Inc.
268
Save as set out above, none of the Directors or the Senior Managers has any business
interests, or performs any activities, outside the Group which are significant with respect to the
Group.
8.2 Conflicts of interest
Save as set out below, there are no actual or potential conflicts of interest between the duties
owed by the Directors or the Senior Managers to the Company and the private interests and/or
other duties that they may also have.
The Pfizer Directors represent the Pfizer Group. Amongst other things, the Pfizer Group may
from time to time acquire and hold interests in businesses that compete directly or indirectly
with the Group, or with which the Group conducts business. Each of the Directors has a
statutory duty under the Companies Act to avoid conflicts of interests with the Company and to
disclose the nature and extent of any such interest to the Board. Under the Articles of
Association and, as permitted by the Companies Act, the Board may authorise any matter
which would otherwise involve a Director breaching this duty to avoid conflicts of interest and
may attach to any such authorisation such conditions and/or restrictions as the Board deems
appropriate (including in respect of the receipt of information or restrictions on participation at
certain Board meetings), in accordance with the Articles of Association (as summarised in
paragraph 5 of this Part XII (Additional Information) above).
8.3 Directors and Senior Managers’ confirmations
(A) Other than the Pfizer Directors, no Director or Senior Manager was selected to act in
such capacity pursuant to any arrangement or understanding with any shareholder,
consumer, supplier or any other person having a business connection with the Group.
(B) Other than as set out at paragraph (E) below, as at the date of this Prospectus, no
Director or Senior Manager has during the last five years:
(i) been convicted in relation to fraudulent offences;
(ii) been associated with any bankruptcy, receivership, liquidation or companies
put into administration while acting in the capacity of a member of the
administrative, management or supervisory body or of senior manager of any
company;
(iii) been subject to any official public incrimination and/or sanctions by any
statutory or regulatory authorities (including designated professional bodies);
or
(iv) been disqualified by a court from acting as a member of the administrative,
management or supervisory body of a company or from acting in the
management or conduct of the affairs of any company.
(C) There are no family relationships between any of the Directors and/or the Senior
Managers.
(D) There are no outstanding loans or guarantees granted or provided by any member of
the Group for the benefit of any of the Directors or Senior Managers.
(E) Along with other Clayton, Dubilier & Rice (“CD&R”, a private equity firm) personnel,
Vindi Banga was a director of High Ridge Brands Co., a portfolio company of CD&R
affiliated funds, in the USA. The company filed for bankruptcy under Chapter 11 in
December 2019, a matter of public record, upon which Vindi Banga and the other
CD&R personnel ceased to be directors of the company upon confirmation of the plan.
269
8.4 Interests of Directors and Senior Managers in the share capital of GSK and of the
Company
As at the date of this Prospectus, the Directors and Senior Managers have no interest in the
share capital of the Company. Following Admission, the interests of the Directors and Senior
Managers in the share capital of the Company will be based on the number of GSK Shares
owned and the number of GSK Shares subject to awards that will be unvested at that time,
which, as at the Latest Practicable Date, is expected to be as follows.
(A) Issued GSK plc share capital
Set out below are the interests as at the Latest Practicable Date of the Directors and the Senior
Managers in the share capital of GSK, including over awards over GSK Shares. On completion
of the Demerger, each Director and Senior Manager will receive one Haleon Share for every
one GSK Share held at the Shareholder Record Time.
Executive Directors’ interests in shares (as at Latest Practicable Date)
Unvested interests in GSK Employee
Share Schemes
Total
Directors’
interests
(1)
Beneficial
interests
(2)
Not subject to
performance
Subject to
performance
GSK
Shares/
GSK
ADSs
GSK
Shares/
GSK
ADSs
(3, 4)
GSK
Options
(5)
GSK Shares/
GSK ADSs
(6)
GSK ADSs
Brian
McNamara 139,884 121,233 18,651 - 232,810
GSK Shares
Tobias
Hestler 31,480 11,380 20,100 870 25,971
(1) Total directors’ interests include beneficial interests and unvested share plan interests not
subject to performance.
(2) Beneficial interests for Tobias Hestler include 997 shares purchased through the
GlaxoSmithKline plc ShareReward Plan.
(3) Unvested ADSs not subject to performance for Brian McNamara represent bonus deferrals
(as described in note 8 below).
(4) Unvested shares not subject to performance for Tobias Hestler represent GlaxoSmithKline
Share Value Plan shares.
(5) Unvested options not subject to performance for Tobias Hestler represent options granted
under the GlaxoSmithKline plc ShareSave Plan 2012.
(6) Unvested ADSs/shares subject to performance represent unvested Performance Share
Plan awards.
(7) Vested but unexercised options: none of the Directors hold vested but unexercised options.
(8) DABP: The table below shows bonus deferrals and subsequent reinvestment of dividends
under the DABP. The amounts represent the gross ADS balances prior to the sale of any ADS
to satisfy tax liabilities on vesting.
270

Deferred Annual Bonus Plan
(Bonus deferrals) (as at Latest Practicable Date)
ADS
Brian McNamara 18,651
Non-Executive Directors’ interests in shares (as at Latest Practicable Date)
Total directors
interests
(1)
Beneficial interests
GSK Shares
Dave Lewis - -
Vindi Banga 106,583 71,800
Marie-Anne Aymerich - -
Tracy Clarke - -
Dame Vivienne Cox 11,527 -
Asmita Dubey - -
Deirdre Mahlan - -
Bryan Supran - -
John Young - -
(1) For Vindi Banga and Dame Vivienne Cox, total directors’ interests include beneficial
interests and any shares received as all or part of their fees under the GSK Non-Executive
Directors’ share allocation plan.
271

Senior Managers’ interests in shares (as at Latest Practicable Date)
Unvested interests in GSK
Employee Share Schemes
Total
interests
(1)
Beneficial
interests
(2)
Not subject to
performance
Subject to
performance
GSK
Shares/
GSK
ADSs
GSK
Shares/
GSK
ADSs
(3)
GSK
Options
(4)
GSK Shares/
GSK ADSs
(5)
GSK
ADSs
Dana
Bolden 7,660 - 7,660 - 9,330
Amy
Landucci 17,118 5,638 11,480 - 14,801
Teri Lyng 32,113 18,903 13,210 - 14,801
Lisa Paley 20,282 7,072 13,210 - 14,801
Bjarne
Philip
Tellmann 13,806 - 13,806 - 64,080
GSK
Shares
Keith Choy 47,703 17,725 29,977 - 25,971
Bart Derde 49,481 21,921 27,560 745 35,611
Amy
Landucci 18,231 18,231 - - -
Filippo
Lanzi 66,886 43,766 23,120 - 25,971
Jooyong
Lee 12,239 355 11,884 870 6,690
Máiréad
Nayager - - - - -
Franck
Riot 24,815 1,365 23,450 - 25,971
Tamara
Rogers 41,853 14,293 27,560 - 35,611
(1) Total interests include beneficial interests and unvested share plan interests not subject to
performance.
(2) Beneficial interests include shares purchased through the GlaxoSmithKline plc
ShareReward Plan: 803 shares for Bart Derde; 376 shares for Amy Landucci; 355 shares for
Jooyong Lee; and 902 shares for Tamara Rogers.
(3) Unvested shares/ADSs not subject to performance represent GlaxoSmithKline Share Value
Plan shares. For Keith Choy, unvested shares not subject to performance also include
GlaxoSmithKline 2017 Deferred Annual Bonus Plan shares.
(4) Unvested options not subject to performance represent options granted under the GSK UK
Sharesave scheme for Bart Derde and Jooyong Lee.
272
(5) Unvested ADSs/shares subject to performance represent unvested GlaxoSmithKline 2017
Performance Share Plan awards.
(6) Vested but unexercised options: none of the Senior Managers hold vested but unexercised
options.
(B) Awards over GSK Shares
On completion of the Demerger, Directors and Senior Managers with options and awards over
GSK Shares will be deemed to have left employment with GSK and be treated as so-called
‘good leavers’ under the rules of the respective GSK share plans (and subject, where relevant,
to GSK’s Recoupment Policy).
In accordance with those ‘good leaver’ rules, in relation to awards held by Directors and Senior
Managers under GSK’s ‘Share Value Plan’, the 2020 award and two thirds of the 2021 award
will vest earlier than initially scheduled. Applying the equivalent rules for awards held by
Directors and Senior Managers under GSK’s ‘Performance Share Plan’, the 2020 award will be
rounded up (as if employment with GSK had continued until the end of 2022) and will vest,
subject to performance, in the first quarter of 2023, and two thirds of the 2021 award will vest,
subject to performance, in the first quarter of 2023.
For senior employees covered by GSK’s Recoupment Policy (namely the CEO and certain US
senior executives), vesting of the relevant GSK awards will be delayed for 12 months post-
Demerger.
In addition to any ordinary course annual awards made under the Haleon Group’s discretionary
share plans following the Demerger, Haleon Group employees who hold 2021 awards under
the GSK plans referred to above will receive an award (referred to as a ‘refill award’) under the
Haleon Group’s equivalent plans, over Haleon Shares on substantially equivalent terms and
with a value equivalent to the value of GSK Shares subject to the relevant GSK award that did
not vest because of the early vesting of the GSK award (and that award having been time pro-
rated).
All long-term incentive grants over Haleon Shares awarded or vesting following the Demerger,
including refill awards, will be under the governance of, and subject to the approval of, the
Remuneration Committee.
Participants in GSK’s all-employee Share Save and Share Reward Plans will be treated in
accordance with the normal rules for ‘good leavers’ under those plans.
Save as set out above, no Director or Senior Manager has any interests in the share capital or
any other securities of the Company.
9. DIRECTORS’ SERVICE CONTRACTS AND LETTERS OF APPOINTMENT
9.1 Executive Directors
The Executive Directors have service agreements, entered into on: (i) 9 May 2022 by Brian
McNamara; and (ii) 10 May 2022 by Tobias Hestler, each in anticipation of Admission, with
GlaxoSmithKline Consumer Healthcare Overseas Limited. The terms outlined in the Executive
Directors’ service agreements and their related offer letters will take effect upon the date of the
Demerger (though if, for any reason, the Demerger has not occurred by 31 December 2022,
the terms set out in the offer letter will expire and the Executive Directors will remain employed
on their existing terms and conditions).
273

The key terms of their appointments are as follows:
(A) General terms
Name Position
Commencement of
employment Notice period
Brian McNamara Chief Executive Officer 1 September 2004 12 months
Tobias Hestler Chief Financial Officer 1 October 2017 12 months
The Executive Directors are each entitled to a remuneration package comprising annual basic
salary (which is to be reviewed, though not necessarily increased, annually), participation in
discretionary performance-related annual bonus and long-term incentives under such bonus
and incentive scheme(s) as the Haleon Group operates from time to time (including any
all-employee share plans established by the Haleon Group), the option of pension contributions
or a fixed cash allowance in lieu of pension contributions, and participation in the Haleon
Group’s benefit plans, including (but not limited to) membership of any private healthcare
scheme operated by the Haleon Group (including eligibility for the Executive Director’s spouse
or partner and eligible dependent children), life assurance/death in service benefit and
membership of a Group Income Protection plan. The Executive Directors also benefit from the
indemnity provided by the Company in the form provided to all Directors. Further details are set
out in paragraph 9.4 of this Part XII (Additional Information).
In addition to the eight normal public holidays observed in England, the Executive Directors are
entitled to 28 working days of paid holiday in each complete holiday year.
(B) Termination provisions
Under the service agreements, either party may terminate the relevant agreement on 12
months’ written notice. GlaxoSmithKline Consumer Healthcare Overseas Limited in its
discretion is entitled to terminate each Executive Director’s service agreement at any time by
making a payment (or phased payments) in lieu of the basic salary only that would otherwise
have been payable during all (or the remaining part of) the notice period. The Executive
Directors can be placed on garden leave for all or part of their notice period.
In addition, GlaxoSmithKline Consumer Healthcare Overseas Limited may terminate each
service agreement without notice or payment in lieu of notice in certain circumstances,
including where an Executive Director is guilty of (i) wilfully neglecting his duties, or
(ii) committing any serious or persistent breach of his service agreement or gross misconduct.
If GlaxoSmithKline Consumer Healthcare Overseas Limited terminates the CFO’s employment
at a time when the CFO is in receipt of payments under a Group Income Protection plan,
GlaxoSmithKline Consumer Healthcare Overseas Limited will use reasonable endeavours to
request consent from the applicable insurer to continue those payments to the CFO following
such termination.
Each Executive Director is subject to a confidentiality undertaking which is unlimited in time
and remains in effect both during (including any period of garden leave), and following the
termination of, his employment under his service agreement.
In addition, each Executive Director is subject to (i) a non-compete post-termination restrictive
covenant for a period of 6 months, relating to working for or setting up a business which
competes with GlaxoSmithKline Consumer Healthcare Overseas Limited or any company
associated with it; (ii) a post-termination restrictive covenant for a period of 6 months,
restricting his shareholding in a business which competes with GlaxoSmithKline Consumer
Healthcare Overseas Limited or any company associated with it; (iii) a non-dealing post-
termination restrictive covenant for a period of 12 months, relating to dealing with customers
and prospective customers of GlaxoSmithKline Consumer Healthcare Overseas Limited or any
274

company associated with it; (iv) a non-solicitation post-termination restrictive covenant for a
period of 12 months, relating to soliciting customers, prospective customers and certain key
people in relation to GlaxoSmithKline Consumer Healthcare Overseas Limited or any company
associated with it; (v) a post-termination restrictive covenant for a period of 12 months, in
relation to interfering with suppliers to GlaxoSmithKline Consumer Healthcare Overseas
Limited or any company associated with it; and (vi) a post-termination restrictive covenant
which runs for an unlimited amount of time, in relation to holding himself out to be connected
with, or making derogatory comments about, GlaxoSmithKline Consumer Healthcare Overseas
Limited or any company associated with it. The relevant time period in the case of each post-
termination restrictive covenant runs from the termination of his employment or, if earlier, the
commencement of any period of garden leave.
9.2 Non-Executive Directors
On Admission, the Company will have nine Non-Executive Directors: the Chair, six
independent Non-Executive Directors and two Non-Executive Directors nominated by Pfizer.
The Non-Executive Directors (excluding the Chair) were each appointed by a letter of
appointment conditional on and effective from Admission. The Chair was appointed as Chair
Designate on 17 December 2021 and as a Non-Executive Director on 23 May 2022; his
appointment as Chair will take effect on Admission. The key terms of these appointments are
as follows:
(A) General terms
Name Position Notice period
Dave Lewis Chair Three months
Manvinder Singh
(Vindi) Banga
Senior Independent
Non-Executive Director
Three months
Marie-Anne Aymerich Non-Executive Director Three months
Tracy Clarke Non-Executive Director Three months
Dame Vivienne Cox Non-Executive Director Three months
Asmita Dubey Non-Executive Director Three months
Deidre Mahlan Non-Executive Director Three months
Bryan Supran Non-Executive Director Three months
John Young Non-Executive Director Three months
The Chair is entitled to receive a fee of £700,000 per annum. The base fee for each other
Non-Executive Director is £95,000 per annum. Additional fees will be payable as follows:
£50,000 per annum for the Senior Independent Director, £30,000 per annum for the Workforce
Engagement Director; £40,000 per annum for chairing the Audit & Risk Committee; and
£40,000 per annum for chairing the Remuneration Committee. The fees for each Non-
Executive Director (including the Chair) are to be reviewed annually (but with no obligation to
increase them). In addition, each Non-Executive Director (including the Chair) is entitled to be
reimbursed for reasonable and properly documented expenses necessarily incurred in the
proper performance of their duties. They are not eligible to participate in any pension or share
scheme operated by the Haleon Group or to receive any bonus. Each Non-Executive Director
275
(including the Chair) has the benefit of: (i) a personal accident insurance policy maintained by
the Company; (ii) directors’ and officers’ liability insurance maintained by the Company; and (iii)
the indemnity provided by the Company in the form provided to all Directors.
Each Non-Executive Director (including the Chair) is subject to confidentiality undertakings
without limitation in time (subject, in the case of the Pfizer-nominated directors, to the
provisions of the Pfizer Relationship Agreement (see paragraph 15.10 of this Part XII
(Additional Information)) and a non-compete restrictive covenant for the duration of their
appointment.
(B) Termination provisions
In the case of each Non-Executive Director (including the Chair), either party may terminate the
appointment on three months’ written notice. Each Non-Executive Director’s (including the
Chair’s) appointment terminates automatically in certain circumstances, including where
(i) their office as a Director is vacated or (ii) they fail to be elected or re-elected at any annual
general meeting. Their appointment may also be terminated by the Company with immediate
effect in certain circumstances, including where they: (i) are convicted of an arrestable criminal
offence (other than a road traffic offence for which a non-custodial penalty is imposed) or
otherwise engage in conduct which brings or is likely to bring themselves or the Company into
disrepute; or (ii) commit any serious or repeated breach of their duties to the Company. Their
appointment may also be terminated at any time by the Company in accordance with the
Articles of Association or the Companies Act or, in the case of the Pfizer-nominated directors,
in accordance with the Pfizer Relationship Agreement (see paragraph 15.10 of this Part XII
(Additional Information)).
9.3 Directors’ and Senior Managers’ remuneration
The aggregate amount of remuneration paid (including any contingent or deferred
compensation) and all benefits in kind granted to the Directors and the Senior Managers (in
Financial Year 2021 the Senior Managers consisted of 11 individuals), in all capacities for
services to the Group for the year ended 31 December 2021 rounded to the nearest £1,000
was £17,676,000, constituting £5,889,000 in salary and fees, £1,758,000 in benefits,
£1,348,000 in retirement benefits, £2,948,000 in annual variable remuneration and £5,733,000
in share-based payments. Of this amount, £6,147,000 was paid to the Directors as set out
below, and £11,529,000 was paid to the Senior Managers.
Details of remuneration paid to the Directors for the year ended 31 December 2021 are set out
below:
Name Salary
and
fees
(£000s)
Retirement
benefits or
cash in lieu
of pension
(£000s)
Annual
variable
remuneration
(£000s)
Taxable
benefits
(£000s)
Share-
based
payments
(£000s)
(3)
Total
(£000s)
Brian
McNamara
£823 £323
(1)
£537 £618
(2)
£2,114 £4,415
Tobias
Hestler
£466 £79 £221 £23 £661 £1,450
Manvinder
Singh
(Vindi)
Banga
£109 - - £1 £36 £146
276

Name Salary
and
fees
(£000s)
Retirement
benefits or
cash in lieu
of pension
(£000s)
Annual
variable
remuneration
(£000s)
Taxable
benefits
(£000s)
Share-
based
payments
(£000s)
(3)
Total
(£000s)
Dame
Vivienne
Cox
£101 - - £1 £34 £136
Total £1,499 £402 £758 £643 £2,845 £6,147
(1) For Brian McNamara’s future pension arrangements, see summary of the directors’
remuneration policy in paragraph 9.4 of this Part XII (Additional Information).
(2) Brian McNamara’s benefits included international assignment related costs, healthcare
cover, life assurance cover, personal financial advice, a car allowance and other expenses; for
Brian McNamara’s future taxable benefits arrangements, see summary of the directors’
remuneration policy in paragraph 9.4 of this Part XII (Additional Information).
(3) Share-based payments for Brian McNamara and Tobias Hestler in respect of their vested
long-term incentive awards, and for Vindi Banga and Dame Vivienne Cox include shares
received as all or part of their fees.
9.4 Remuneration policy
The Company’s strategy is to establish a directors’ remuneration policy that:
• drives the success of Haleon and the delivery of its business strategy for the benefit
of consumers and other key stakeholders;
• creates shareholder value;
• provides an appropriately competitive package to attract, retain and motivate
executive talent for a standalone consumer goods business, which will source talent
globally; and
• is aligned with the Company’s business priorities, culture, wider workforce pay
policies, and best practice.
Consistent with this strategy, overall remuneration packages for the Executive Directors have
been set at levels that are considered by the Board (having taken independent advice) to be
appropriate for the size and nature of the business following Admission.
The information below and in paragraphs 9.1 and 9.2 above, together with the details of the
share-based incentive plans set out in paragraphs 10.1 to 10.7 of this Part XII (Additional
Information), summarises the key components of the Executive Director and Non-Executive
Director remuneration arrangements which will apply from Admission.
The Company’s remuneration policies and processes are fully compliant with all regulatory
requirements and may be amended from time to time to ensure continued compliance with
these requirements.
The Company will formally propose a directors’ remuneration policy for approval by Haleon
Shareholders at the first annual general meeting of the Company following Admission, in
accordance with section 439A of the Companies Act 2006 and regulations set out in the Large
and Medium-sized Companies and Groups (Accounts and Reports) Regulations 2008 (as
amended). It is currently intended that, if approved, that policy will apply for three years from
the date of that annual general meeting.
277
That policy will allow implementation of the remuneration strategy through a combination of
base salary, benefits, pensions, annual bonus, long-term incentives, and all-employee share
plans.
A summary of the key terms of the directors’ remuneration policy that will operate from
Admission until the shareholder-approved directors’ remuneration policy is put in place is
provided below.
(A) Base Salary
Base salaries for Executive Directors are set at a level appropriate to secure and retain high
calibre individuals needed to deliver Haleon’s strategic priorities. Upon Admission, the base
salaries will be £1,250,000 for the CEO and £700,000 for the CFO respectively. The CEO’s
salary level takes into account the fact that he will be localised onto a UK contract and that his
prior remuneration package included international assignment benefits and a US pension, both
of which end under the new arrangement.
The individual’s role, experience and performance, and independently sourced data for
relevant comparator groups, will be considered when determining salary levels.
There is no formal maximum limit and, ordinarily, salary increases will be broadly in line with
the average increases for the wider Haleon workforce. However, increases may be higher to
reflect a change in the scope of the individual’s role, responsibilities or experience. Salary
adjustments may also reflect wider market conditions in the geography in which the individual
operates.
In line with market practice, the Remuneration Committee will review directors’ base salaries
annually with the next review scheduled for 2023 and with any increase normally taking effect
from 1 April.
(B) Benefits
Executive Directors are eligible to receive benefits in line with the policy for other employees
which may vary by location. These include, but are not limited to, private healthcare (including
eligibility for the Executive Director’s spouse or partner and eligible dependent children), life
assurance/death in service benefit, membership of a Group Income Protection plan, personal
tax and financial planning, and any contractual post-retirement benefits. The Executive
Directors are also entitled to car travel or a car allowance and the CEO is eligible to receive
home security services. Other benefits include expenses properly incurred in the ordinary
course of business, which are deemed to be taxable benefits on the individual. The Executive
Directors also benefit from the indemnity provided by the Company in the form provided to all
Directors.
In line with the policy for other employees, Executive Directors may be eligible to receive
overseas relocation allowances and international transfer-related benefits when appropriate.
To facilitate Brian McNamara’s employment arrangements being moved from an international
assignee package to a standard, local market, basis, a one-off payment of £300,000 (subject to
deductions for tax and National Insurance contributions) will be made to him in 2022.
Executive Directors in the UK are also eligible to participate in any all-employee share
schemes established by the Haleon Group, on the same terms as other employees.
Benefit provision is tailored to reflect market practice in the geography in which the Executive
Director is based and different policies may apply if current or future Executive Directors are
based in a different country.
278
(C) Pension arrangements
The approach to pensions arrangements for Executive Directors is in line with the broader
workforce. In the United Kingdom, Executive Directors will receive their pensions entitlements
from the date of their appointment.
Executive Directors are eligible to participate in the Haleon Group’s defined contribution
pension plan in the United Kingdom, with the employee contributing a core amount equal to
2 per cent. of their base salary and GlaxoSmithKline Consumer Healthcare Overseas Limited
contributing a core amount equal to 7 per cent. of their base salary and matching additional
employee contributions up to 3 per cent. of their base salary (subject to any relevant cap on
tax-advantaged contributions and in line with implementation principles for other members of
the pension plan). To the extent that any such cap applies, or where the Executive Director
does not participate in the Haleon Group’s defined contribution pension plan, GlaxoSmithKline
Consumer Healthcare Overseas Limited’s contribution of 7 per cent. of base salary not paid
into that pension plan will be paid to that Executive Director as a cash allowance in lieu of the
relevant part of GlaxoSmithKline Consumer Healthcare Overseas Limited’s contributions to
that pension plan.
(D) Annual bonus
Executive Directors are eligible to participate in the Haleon plc Annual Incentive Plan which is
intended to incentivise and recognise execution of the business strategy on an annual basis.
Executive Directors are required to defer 50 per cent. of any bonus earned into an award over
Haleon Shares or Haleon ADSs under the Haleon plc Deferred Annual Bonus Plan 2022 (the
“DABP”), which will normally vest on the third anniversary of grant.
The bonus opportunities for on-target performance are 100 per cent. of base salary for each of
the CEO and the CFO. The maximum bonus opportunities for outstanding performance are two
times target.
Performance measures are based on a combination of financial targets and individual and
business objectives, with the weighting of measures determined by the Remuneration
Committee each year according to business priorities.
The Remuneration Committee may apply judgement in making appropriate adjustments to
bonus outcomes (either up or down) to ensure they reflect underlying business performance.
The proportion of any bonus satisfied in cash will be subject to the malus and clawback
provisions summarised in paragraph 9.4(I) of this Part XII (Additional Information). The period
during which any cash award may be recovered will be two years from the date the relevant
bonus is paid. The proportion of any bonus deferred into a DABP Award will be subject to the
leaver and malus and clawback provisions summarised in paragraph 9.4(I) of this Part XII
(Additional Information).
(E) Long-term incentives
The Board has adopted the Haleon plc Performance Share Plan 2022 (the “PSP”), conditional
on Admission. Executive Directors are eligible to participate in the PSP, which is intended to
incentivise and recognise delivery of longer-term business priorities, financial growth and
increases in shareholder value.
Under the PSP, awards will be in the form of conditional share awards or nil-cost options. It is
the Remuneration Committee’s current intention that the normal maximum awards that may be
granted under the PSP in respect of any financial year are 450 per cent. of salary for the CEO
and 350 per cent. of salary for the CFO.
279
These awards to Executive Directors will be subject to performance conditions set by the
Remuneration Committee. Further details of performance conditions and weightings will be set
out in the first Directors’ Remuneration Report.
It is envisaged that awards will be granted annually to Executive Directors under the PSP and
will have a three-year performance period and a further post-vesting two-year holding period.
The Remuneration Committee may adjust the formulaic vesting outcome (either up or down) to
ensure that the overall outcome reflects underlying business performance over the vesting
period.
Awards are eligible for dividend equivalent payments in respect of dividends that would have
been paid on the Haleon Shares or Haleon ADSs that vest under the PSP Awards up to the
date the awards vest (or to the end of any relevant post-vesting holding period).
A PSP Award will be subject to the leaver and malus and clawback provisions summarised in
paragraph 9.4(I) of this Part XII (Additional Information).
A summary of the principal terms of the PSP is set out in paragraph 10.1 of this Part XII
(Additional information).
(F) Share ownership requirements
To align their interests with those of shareholders, Executive Directors are required to build and
maintain significant holdings of shares in the Company over time. The requirements for the
CEO and CFO are 450 per cent. and 350 per cent. of salary respectively. Until the relevant
share ownership requirements have been met, Executive Directors are required to hold all
Haleon Shares acquired under the PSP and/or DABP (net of income tax and National
Insurance contributions). Non-Executive Directors (including the Chair) are also encouraged to
build up a personal holding in the shares of the Company equal to the value of one year of their
annual base fee.
Executive Directors are required to comply with shareholding requirements for two years after
departure, at a level equal to the lower of their shareholding requirement immediately prior to
departure or their actual shareholding on departure.
(G) Recruitment policy
The remuneration package of new Executive Directors will be determined on a case-by-case
basis, in line with the provisions of the directors’ remuneration policy in force at the time.
The Remuneration Committee is mindful of the sensitivity relating to recruitment packages and,
in particular, the ‘buying out’ of rights relating to previous employment. The intent is to seek to
minimise such arrangements. However, in certain circumstances, the Remuneration
Committee may determine that such arrangements are in the best interests of the Company
and its shareholders, and such arrangements will, where possible, be on a like-for-like basis
with the forfeited remuneration terms.
(H) Termination policy
In the event of termination, the Executive Directors’ service agreements provide for payments of
base salary, pensions and benefits over the notice period or for GlaxoSmithKline Consumer
Healthcare Overseas Limited to terminate immediately on making a payment (or phased payments)
in lieu of notice equivalent to base salary only for the notice period (or the remainder of such
period). Notice (or payment in lieu) will not be payable in certain circumstances, including where an
Executive Director is guilty of (i) wilfully neglecting his duties, or (ii) committing any serious or
persistent breach of his service agreement or gross misconduct. There is no contractual right to any
bonus or long term incentive payment in the event of a notice of termination being given or received
on or before the date on which the bonus or long term incentive payment would otherwise have
been paid, although the Remuneration Committee may exercise its discretion to pay such a bonus
or long term incentive payment.
280
(I) Malus and clawback
In certain circumstances, the Remuneration Committee may at any time prior to the second
anniversary of the date the cash element of an annual bonus is paid or a PSP, DABP or SVP
Award (an “Executive Award”) vests: (i) reduce (to zero if appropriate) the cash bonus or
Executive Award; (ii) impose additional conditions on the cash bonus or Executive Award;
(iii) increase the length of the performance period applicable to an Executive Award (or delay
the payment of a cash bonus or the vesting of an Executive Award); or (iv) require that a
participant either return some or all of the Haleon Shares or Haleon ADSs acquired under an
Executive Award or make a cash payment to the Company in respect of the Haleon Shares or
Haleon ADSs delivered under an Executive Award or cash bonus paid.
The Remuneration Committee may only invoke these malus and clawback provisions in
accordance with the Haleon malus and clawback policy from time to time, in circumstances
such as a material misstatement of results; a failure of risk management resulting in material
financial loss; an error or material misstatement which results in an overpayment (such as in
the assessment of performance); a corporate failure of the Haleon Group; employee
misconduct; or material reputational damage to the Haleon Group.
10. SHARE-BASED INCENTIVE PLANS
Following Admission, the Company intends to operate three discretionary share-based
incentive plans: the PSP, the DABP and a share value plan (the “SVP”) (together, the
“Executive Plans”). The Company also intends to operate two tax-advantaged all-employee
share-based incentive plans: a share acquisition and free share plan, known as a UK Share
Reward Plan (“Share Reward Plan”) and a savings-related share option plan, known as a UK
Sharesave plan (the “Sharesave Plan”) (the Share Reward Plan and the Sharesave Plan,
together with the Executive Plans, the “Plans”). The main features of each of the Plans are set
out below, with the common terms of the Executive Plans set out in paragraph 10.6 of this
Part XII (Additional Information) and the common terms of the Plans as a whole set out in
paragraph 10.7 of this Part XII (Additional Information).
10.1 PSP
The PSP was adopted by the Board on 23 May 2022, conditional on Admission. The PSP is a
discretionary share plan, under which the Remuneration Committee may grant awards over
Haleon Shares or Haleon ADSs (“PSP Awards”) to incentivise and retain eligible employees.
The PSP will be administered by the Remuneration Committee or by any sub-committee or
person duly authorised by it.
(A) Individual limit
Awards will not be granted to an Executive Director under the PSP over Haleon Shares or
Haleon ADSs with a market value (as determined by the Remuneration Committee) in excess
of the relevant limit set out in the prevailing directors’ remuneration policy, in respect of any
financial year of the Company.
It is the Remuneration Committee’s current intention that PSP Awards to be granted to the
Executive Directors in respect of the financial year ended 31 December 2022 will be calculated
by reference to 450 per cent. of salary for the CEO and 350 per cent. of salary for the CFO
(these amounts are exclusive of any ‘refill awards’).
In applying this limit, no account will be taken of Haleon Shares or Haleon ADSs representing
notional dividends on PSP Awards or Haleon Shares or Haleon ADSs which have been
awarded to ensure that a participant is not financially disadvantaged if they agree to satisfy the
employer’s national insurance or social security liability in relation to their PSP Award.
281
(B) Performance conditions
The vesting of PSP Awards will be subject to the satisfaction of performance conditions. The
performance conditions will normally be measured over a period of at least three financial
years for PSP Awards granted to Executive Directors and any member of the Executive Team.
Any performance condition may be amended in accordance with its terms or if there is a
situation which causes the Remuneration Committee to consider that any amended
performance condition would be a fairer measure of performance.
(C) Vesting of PSP Awards
Performance conditions will be assessed as soon as reasonably practicable after the end of
the relevant performance period. The Remuneration Committee will determine the extent to
which the PSP Awards will then vest, taking into account the extent that the performance
conditions have been satisfied. To the extent that they vest, PSP Awards will normally vest on
the vesting date set by the Remuneration Committee at grant, which will normally be the third
anniversary of the date of grant.
(D) Holding period
The Remuneration Committee may also determine at grant that a PSP Award is subject to an
additional holding period following vesting. Where a holding period applies, PSP Awards will
either vest at the end of the holding period or they may vest before the start of the holding
period but some or all of the vested Haleon Shares or Haleon ADSs will be subject to
restrictions during the holding period.
10.2 DABP
The DABP was adopted by the Board on 23 May 2022, conditional on Admission. The DABP is
a discretionary share plan implemented so that a portion of a participant’s annual bonus can be
deferred into an award of Haleon Shares or Haleon ADSs (a “DABP Award”). The DABP will
be administered by the Remuneration Committee or by any sub-committee or person duly
authorised by it.
DABP Awards will normally vest on such date or dates as the Remuneration Committee may
determine when the DABP Award is granted.
10.3 SVP
The SVP was adopted by the Board on 23 May 2022, conditional on Admission. The SVP is a
discretionary share plan, under which the Remuneration Committee may grant awards over
Haleon Shares or Haleon ADSs (“SVP Awards”) to incentivise and retain eligible employees of
the Haleon Group. Save for any ‘refill awards’ referred to in paragraph 8.4 of this Part XII
(Additional Information), SVP Awards cannot be granted to Executive Directors or to any
Senior Manager who, by analogy with the corresponding GSK ‘Share Value Plan’, would not
have been eligible to participate in that GSK plan. As part of its programme to incentivise and
retain its employees, the Company will explore a broadly-based one-time award, subject to
vesting conditions, that may be settled in newly issued Haleon Shares or Haleon ADSs (not
exceeding 2,600,000 Haleon Shares (or the equivalent value of Haleon ADSs) in total). The
SVP will be administered by the Remuneration Committee or by any sub-committee or person
duly authorised by it.
(A) Individual limit
Except in exceptional circumstances, SVP Awards will not normally be granted to an eligible
employee under the SVP over Haleon Shares or Haleon ADSs with a market value (as
determined by the Remuneration Committee) in excess of 300 per cent. of salary, in respect of
any financial year of the Company.
282
In applying this limit, no account will be taken of Haleon Shares or Haleon ADSs representing
notional dividends on SVP Awards or Haleon Shares or Haleon ADSs which have been
awarded to ensure that a participant is not financially disadvantaged if they agree to satisfy the
employer’s national insurance or social security liability in relation to their SVP Award.
(B) Additional conditions
The vesting of SVP Awards may be subject to the satisfaction of additional conditions set by
the Remuneration Committee at the time of grant. Any such condition may be amended or
waived by the Remuneration Committee. It is not currently intended that vesting of SVP
Awards will be subject to performance or other conditions.
(C) Vesting of SVP Awards
The Remuneration Committee will determine the extent to which the SVP Awards will vest,
taking into account the extent that any additional conditions have been satisfied. To the extent
that they vest, SVP Awards will normally vest on the vesting date set by the Remuneration
Committee at grant.
10.4 Share Reward Plan
The Share Reward Plan was adopted by the Board on 23 May 2022, conditional on Admission.
The Share Reward Plan is an all-employee share ownership plan established by the Company
which has been designed to meet HMRC requirements so that Shares can be acquired by UK
employees in a tax-efficient manner.
(A) Grant of Share Reward Plan awards
Under the Share Reward Plan, eligible employees may be: (i) offered the opportunity to buy
Haleon Shares up to a maximum value of the lesser of £1,800 and 10 per cent. of their pre-tax
salary each year (“Partnership Shares”); (ii) given up to two free Haleon Shares (“Matching
Shares”) for each Partnership Share bought; (iii) awarded free Haleon Shares up to a value of
£3,600 (“Free Shares”) each year; and/or (iv) allowed or required to purchase Haleon Shares
using dividends received on Shares held in the Share Reward Plan (“Dividend Shares”). The
Board may change these limits in the future should the relevant legislation change the
maximum levels of participation referred to above.
(B) Share Reward Plan Trust
The Share Reward Plan operates through a UK-resident trust (the “Trust”). The trustee of the
Trust acquires the Haleon Shares that are awarded to or purchased on behalf of participants. A
participant will be the beneficial owner of any Haleon Shares held on their behalf by the trustee
of the Trust.
(C) Eligibility
Each time the Board decides to make an award under the Share Reward Plan, all employees
(including Executive Directors) of the Company and its subsidiaries participating in the Share
Reward Plan, where those employees are UK resident taxpayers, must be offered the
opportunity to participate. Other employees of the Haleon Group may be permitted to
participate at the Board’s discretion. Employees who are invited to participate must have
completed any specified minimum qualifying period of employment (as determined by the
Board in line with the relevant legislation, which currently may not exceed 18 months) before
they can participate.
283
(D) Partnership Shares
The Board may allow a participant to use pre-tax salary to buy Partnership Shares at their then
market value or (where pre-tax salary is accumulated) by reference to the market value either
at the start or end of the relevant accumulation period. Once acquired, Partnership Shares may
be withdrawn from the Trust by the participant at any time.
Participants can stop contributing at any time. The participant’s contributions may be used to
buy Partnership Shares immediately or may be accumulated for up to 12 months before they
are used to buy Haleon Shares.
(E) Matching Shares
The Board may, in its discretion, offer free Matching Shares to a participant who has
purchased Partnership Shares. There is a holding period of between three and five years (or
such other period as may be permitted by the relevant legislation from time to time) during
which the participant cannot withdraw the Matching Shares from the Trust, unless the
participant ceases to be employed by the Haleon Group. The precise duration of this holding
period will be determined by the Board each time Matching Shares are awarded. The Board, in
its discretion, may provide that the Matching Shares will be forfeited if the participant ceases to
be employed by the Haleon Group other than because of death, retirement, injury, disability,
redundancy, or the sale of the individual’s employing company or business out of the Haleon
Group (each a “Good Leaver Reason”) or if the related Partnership Shares are withdrawn
from the Trust.
(F) Free Shares
There will be a holding period of between three and five years (or such other period as may be
permitted by the relevant legislation from time to time) during which the participant cannot
withdraw the Free Shares from the Trust unless the participant ceases to be employed by a
member of the Haleon Group. The precise duration of this holding period will be determined by
the Board each time Free Shares are awarded. The Board, in its discretion, may provide that
the Free Shares will be forfeited if the participant ceases to be employed by the Haleon Group
other than for a Good Leaver Reason. Free Shares must generally be offered to all eligible
employees on similar terms, and the award may be subject to performance measures. “Similar
terms” means the terms may only be varied by reference to remuneration, length of service or
hours worked.
(G) Reinvestment of dividends
The Board may allow or require a participant to reinvest (and acquire Haleon Shares with) the
whole or part of any dividends paid on Haleon Shares held in the Trust on their behalf.
Dividend Shares must be held in the Trust for no less than three years, unless the participant
ceases to be employed by the Haleon Group.
(H) Voting Rights
Participants may be offered the opportunity to direct the trustee of the Trust how to exercise
the voting rights attached to the Haleon Shares held on their behalf. The trustee will not
exercise the voting rights unless they receive the participants’ instructions.
(I) Corporate events
In the event of a general offer being made to the Company’s shareholders (or a similar
takeover event taking place), participants will be able to direct the trustee of the Trust as to
how to act in relation to their Haleon Shares held in the Share Reward Plan. In the event of an
internal reorganisation of the Haleon Group, any Haleon Shares held by participants may be
replaced by equivalent shares in a new holding company.
284
(J) Adjustments
Haleon Shares acquired on a variation of the share capital of the Company will usually be
treated in the same way as the Haleon Shares originally acquired or awarded under the Share
Reward Plan in respect of which the rights were conferred and as if they were acquired or
awarded at the same time.
(K) Rights attaching to Haleon Shares
Any Haleon Shares issued to the trustee of the Trust will rank equally with other Haleon Shares
then in issue (except for rights arising by reference to a record time or date prior to the time or
date of issue). In the event of a rights issue, participants will be able to direct the trustee of the
Trust as to how to act in respect of the Haleon Shares held in the Share Reward Plan on their
behalf.
10.5 Sharesave Plan
The Sharesave Plan was adopted by the Board on 23 May 2022, conditional on Admission.
The Sharesave Plan is an all-employee share option plan established by the Company which
has been designed to meet HMRC requirements so that UK employees can acquire fully paid
Haleon Shares in a tax-efficient manner.
(A) Eligibility
Each time the Board decides to issue an invitation to employees to participate in the
Sharesave Plan, all employees (including Executive Directors) of the Company and its
subsidiaries participating in the Sharesave Plan, where those employees are UK resident
taxpayers, must be offered the opportunity to participate. Other employees of the Haleon
Group may be permitted to participate at the Board’s discretion. Employees who are invited to
participate must have completed any specified minimum qualifying period of employment (as
determined by the Board in line with the relevant legislation, which currently may not exceed
five years) before they can participate.
(B) Savings contract
Under the Sharesave Plan, eligible employees must enter into a linked savings contract to
make savings over a period of three or five years. The Board has the discretion to set the
length of the savings contract at three and/or five years. Monthly savings by a participant under
all savings contracts linked to options granted under any tax-advantaged savings-related share
option plan may not exceed the statutory maximum, which is currently set at £500 per month.
The Board may set a lower limit in relation to any particular grant. At the end of the savings
contract, participants may either withdraw their savings on a tax-free basis or use their savings
(plus any interest or bonus) to acquire Haleon Shares.
(C) Option price
The proceeds of the savings contract can be used to exercise an option to acquire Haleon
Shares at an option price per Haleon Share. The Board sets the option price which must not be
manifestly less than 80 per cent. of the market value of a Haleon Share on the business day
before the date of the invitation, or on the date specified in the invitation, or the average market
value over the three preceding business days.
(D) Exercise of options
Options can normally only be exercised within six months of the date that the bonus becomes
payable (or would have become payable if a bonus was due) under the terms of the savings
contract. Options not exercised by the end of this period will lapse.
285
(E) Leaving employment
Generally, an option will lapse on the date the eligible employee ceases to hold office or
employment.
However, where a participant ceases to hold office or employment because of injury, disability,
redundancy, retirement or the sale of the individual’s employing company or business out of
the Haleon Group, their option may be exercised within six months after the participant’s
cessation of office or employment. If a participant ceases to hold office or employment more
than three years after the option was granted, their option may be exercised within six months
after their cessation of office or employment provided the reason for the cessation is not
misconduct. If a participant dies, their option may be exercised within one year by their
personal representatives.
(F) Corporate events
On a takeover, scheme of arrangement, compulsory acquisition or certain other corporate
reorganisations, options can generally be exercised early to the extent of the savings made up
to the date of exercise. Alternatively, participants may be permitted to exchange their options
for options over shares in the acquiring company.
(G) Adjustments
In the event of a variation in the Company’s share capital, the Board may adjust the number of
Haleon Shares subject to options and/or the option price applicable to options in such manner
as it considers appropriate and as is permitted by the Sharesave legislation.
(H) Rights attached to Haleon Shares
Options granted under the Sharesave Plan will not confer shareholder rights on a participant
until that participant has exercised their option and received the underlying Haleon Shares. Any
Haleon Shares issued will rank equally with other Haleon Shares then in issue (except for
rights arising by reference to a record date prior to their issue).
10.6 Terms common to the Executive Plans
(A) Eligibility
All employees (including the Executive Directors) of the Haleon Group are eligible for selection
to participate in the Executive Plans at the discretion of the Remuneration Committee, save for
the SVP under which no awards may be granted to Executive Directors or (save for any ‘refill
awards’ referred to in paragraph 8.4 of this Part XII (Additional Information)) to any Senior
Manager who, by analogy with the corresponding GSK ‘Share Value Plan’, would not have
been eligible to participate in that GSK plan.
(B) Timing of awards
Executive Awards will normally be granted (i) during the 42 days beginning on: (a) Admission;
(b) the first day after the announcement of the Company’s results for any period; (c) in the case
of the PSP and DABP only, the day on which the directors’ remuneration policy (or amendment
to it) is approved by the Company’s shareholders; (d) the day on which changes to the
legislation or regulations affecting share plans are announced, effected or made; or (e) to the
extent that share dealing restrictions apply in any of the preceding four periods, the first dealing
day on which such dealing restrictions are lifted, or (ii) on any other day on which the
Remuneration Committee determines that exceptional circumstances exist which justify the
making of an Executive Award at that time.
286
(C) Form of awards
The Remuneration Committee may grant Executive Awards as conditional awards of Haleon
Shares, or nil-cost options over Haleon Shares. No payment is required for the grant of an
Executive Award. Executive Awards structured as nil-cost options will normally be exercisable
from the point of vesting (or, where a PSP Award is subject to a holding period, the end of that
holding period) until the tenth anniversary of the grant date, except for SVP Awards which will
normally be exercisable for six months from vesting.
(D) Settlement
The Remuneration Committee may, in its discretion, decide to satisfy an Executive Award with
a cash payment equal to the market value of the Haleon Shares that the participant would have
received had the relevant Executive Award been satisfied with Haleon Shares.
(E) Dividend equivalents
If the Remuneration Committee so determines, participants will receive an amount (in
additional Haleon Shares, unless the Remuneration Committee decides it will be paid (in full or
in part) in cash) equal to the value of any dividends declared during the period beginning on
the grant date and ending on the date on which the Executive Award vests or, if there is a
holding period applicable to a PSP Award, when that holding period ends, and which would
have been paid on the Haleon Shares subject to an Executive Award which vest. This amount
may assume the reinvestment of dividends and exclude or include special dividends.
(F) Malus and clawback
The malus and clawback policy summarised in paragraph 9.4(I) above will apply to Executive
Awards.
(G) Cessation of employment
PSP Awards
An unvested PSP Award will usually lapse when a participant ceases to be an employee or
director of the Haleon Group.
If, however, a participant ceases to be an employee or director of the Haleon Group because of
their death, ill-health, injury, disability, redundancy, retirement with the agreement of their
employing company, the sale of the participant’s employing company or business out of the
Haleon Group or in other circumstances at the discretion of the Remuneration Committee (i.e.
they leave as a “good leaver”), their PSP Award will normally continue to vest (and be
released) on the date when it would have vested (and been released) if they had not ceased to
be an employee or director of the Haleon Group.
The extent to which PSP Awards vest in these circumstances will be determined by the
Remuneration Committee, taking into account the satisfaction of any performance conditions
applicable to PSP Awards measured over the original performance period. The Remuneration
Committee retains discretion, however, to allow the PSP Award to vest (and be released) on
the individual’s cessation of office or employment or such other date as it decides, taking into
account any applicable performance conditions measured up to such point as it decides.
Unless the Remuneration Committee decides otherwise, the extent to which a PSP Award
vests will also take into account the proportion of the performance period (or, in the case of a
PSP Award not subject to performance conditions, the vesting period) which has elapsed on
the cessation of the participant’s office or employment with the Haleon Group.
If a participant ceases to be an employee or director of the Haleon Group during a holding
period in respect of a PSP Award for any reason other than gross misconduct or summary
dismissal, their PSP Award will normally be released at the end of the holding period. If a
participant leaves for gross misconduct or is summarily dismissed, any outstanding PSP
Awards they hold will immediately lapse.
287
DABP Awards
A DABP Award will vest in full as if the participant had not ceased to be an employee or
director of the Haleon Group unless the Remuneration Committee determines that the DABP
Award will vest in its entirety on a different date. If a participant leaves for gross misconduct or
is summarily dismissed, any DABP Awards they hold will immediately lapse.
SVP Awards
An unvested SVP Award will usually lapse when a participant ceases to be an employee or
director of the Haleon Group.
If, however, a participant ceases to be an employee or director of the Haleon Group because of
their ill-health, injury, disability, redundancy, retirement with the agreement of their employing
company, the sale of the participant’s employing company or business out of the Haleon Group
or in other circumstances at the discretion of the Remuneration Committee (i.e. they leave as a
“good leaver”), their SVP Award will vest on the individual’s cessation of office or employment
or such other date as the Remuneration Committee may decide (not being more than 30 days
after cessation of the participant’s office or employment with the Haleon Group).
If a participant dies, their SVP Award will vest on the date of their death on the basis set out for
other “good leavers” below.
Unless the Remuneration Committee decides otherwise, the extent to which an SVP Award
vests will take into account the proportion of the vesting period which has elapsed on the
cessation of the participant’s office or employment with the Haleon Group (rounded up to the
nearest whole year), and vesting will occur regardless of the satisfaction of any conditions
applicable to that SVP Award.
Executive Awards structured as nil-cost options
Executive Awards structured as nil-cost options which do not lapse may normally be exercised
to the extent vested for a period of 12 months (six months in the case of the SVP) after vesting
(or, where PSP Awards are subject to a holding period, release).
Where nil-cost options have already vested (and, where relevant, been released) on the date
of cessation of office or employment, those options may normally be exercised for a period of
12 months from the date of cessation (or six months for a nil-cost option granted under the
SVP), unless the participant leaves for gross misconduct or is summarily dismissed, in which
case their options will lapse.
(H) Corporate events
If there is a takeover of the Company, Executive Awards may vest (and be released) early. The
proportion of any unvested PSP Awards which vest will be determined by the Remuneration
Committee, taking into account performance up to that time and, unless the Remuneration
Committee determines otherwise, the proportion of the performance period which has elapsed.
DABP Awards will vest in full. SVP Awards will vest in full, unless the Remuneration Committee
determines otherwise.
Awards structured as nil-cost options may then normally be exercised for a period of six weeks,
and will be automatically exercised at the end of that period. Alternatively, the Remuneration
Committee may require that Executive Awards are exchanged for equivalent awards over
shares in another company (subject to the acquiring company’s consent). In the case of PSP
Awards, the replacement awards will be subject to appropriate performance conditions.
In the event of a demerger, special dividend or other transaction which in the Remuneration
Committee’s opinion may affect the value of Haleon Shares, the Remuneration Committee may
allow the Executive Award to vest (in full or in part) or provide for the Executive Award to be
automatically exchanged for an equivalent award over shares in another company.
288
(I) Variation of capital
If there is a variation of the share capital of the Company or in the event of a demerger, special
dividend or other transaction which in the Remuneration Committee’s opinion may affect the
value of Haleon Shares, the Remuneration Committee may make such adjustments to the
number or class of shares subject to Executive Awards and/or change the identity of the
company whose shares are subject to the Executive Award, in each case as it considers
appropriate.
(J) Rights attaching to Haleon Shares
Haleon Shares issued and/or transferred under the Executive Plans will not confer rights on
any participant until that participant has received the underlying Haleon Shares. Any Haleon
Shares issued will rank equally with Haleon Shares then in issue (except for rights arising by
reference to a record date prior to their issue).
10.7 Terms common to the Plans
(A) Overall limits
The Plans may operate over new issue Haleon Shares, treasury Haleon Shares or Haleon
Shares purchased in the market. The rules of the Plans provide that, in any ten year rolling
period, the number of Haleon Shares which may be issued under the Plans and any other
employee share plan adopted by the Company may not exceed 10 per cent. of the issued
ordinary share capital of the Company from time to time. In addition, the number of Haleon
Shares which may be issued under the Executive Plans and any other discretionary employee
share plan adopted by the Company may not exceed 5 per cent. of the issued ordinary share
capital of the Company from time to time in the same period.
Haleon Shares used to settle ‘refill awards’ granted under the Executive Plans will count
towards these limits.
Haleon Shares transferred out of treasury will count towards these limits for so long as this is
required under institutional shareholder guidelines. However, awards which are relinquished or
lapse will be disregarded for the purposes of these limits.
(B) Amendments
The Board (or, in the case of the Executive Plans, the Remuneration Committee) may, at any
time, amend the provisions of the Plans in any respect. The prior approval of the Company’s
shareholders must be obtained in the case of any amendment which is made to the advantage
of eligible employees and/or participants and relates to the provisions relating to eligibility,
individual or overall limits, the basis for determining the entitlement to, and the terms of,
awards, the adjustments that may be made in the event of any variation to the share capital of
the Company and/or the rule relating to such prior approval. There are, however, exceptions to
this requirement to obtain shareholder approval for any minor amendments to benefit the
administration of the Plans, to take account of the provisions of any legislation, or to obtain or
maintain favourable tax, exchange control or regulatory treatment for any participant or
member of the Haleon Group.
(C) Non-transferability
Awards (other than where indicated otherwise in connection with the Share Reward Plan under
paragraph 10.4 of this Part XII (Additional Information)) are not transferable other than to the
participant’s personal representatives in the event of the participant’s death.
289
(D) Benefits not pensionable
Benefits received under the Plans are not pensionable.
(E) Overseas plans
The Board may, at any time, establish further plans based on the Plans for overseas territories.
Any such plan will be similar to the Plans but may be modified to take account of local tax,
exchange control or securities laws. Any Haleon Shares made available under such further
overseas plans must be treated as counting against the limits on individual and overall
participation under the Plans.
The PSP and SVP incorporate separate schedules for employees who are resident in the
United States of America, France and Switzerland in compliance with the relevant securities
and tax laws.
(F) Termination
No awards may be granted under the Plans more than ten years after Admission.
10.8 The Company’s employee benefit trust
The Company intends to establish following Admission an employee benefit trust or trusts (the
“Trusts”) to operate in connection with the Plans. The Company will have the power to appoint
and remove the trustee(s) of the Trusts. The Trusts will benefit current and former employees
and directors (other than Non-Executive Directors) of the Haleon Group and certain members
of their families (excluding any person resident in the jurisdiction where a Trust is constituted
for tax purposes, as appropriate).
The trustee(s) of the Trusts will have the power to acquire Haleon Shares or Haleon ADSs and,
with effect from Admission, any Haleon Shares or Haleon ADSs acquired may be used for the
purposes of the Plans, other employee share plans established by the Haleon Group from time
to time or otherwise for the benefit of the beneficiaries of the Trusts.
With effect from Admission, the Haleon Group may fund the Trusts by loan or gift to acquire
Haleon Shares or Haleon ADSs either by market purchase or by subscription. Any awards to
subscribe for Haleon Shares or Haleon ADSs granted to the Trusts or Haleon Shares or
Haleon ADSs issued to the Trusts will be treated as counting against the overall limits that
apply to the Plans. Following Admission, the trustee(s) of the Trusts will not, without Haleon
Shareholder approval, hold or acquire more than 5 per cent. of the Company’s issued ordinary
share capital from time to time (disregarding any Haleon Shares or Haleon ADSs held by it as
a nominee).
11. PENSIONS
Defined benefit pension schemes
One of the former sponsoring employers in GSK’s UK Pension Schemes and the other material
funded defined benefit pension scheme in the UK, the SmithKline Beecham Senior Executive
Pension Plan (the “SBSEPP”), is a company within the Haleon Group (the “Haleon
Employer”). With effect from the end of 31 March 2022, the Haleon Employer ceased to
participate in each of the UK Pension Schemes and the SBSEPP. All of the Haleon Employer’s
liabilities in respect of the UK Pension Schemes and the SBSEPP were apportioned to
GlaxoSmithKline Services Unlimited (another of the sponsoring employers and a company in
the GSK Group following the Demerger) by way of a statutory apportionment mechanism.
The US defined benefit plan has been closed to future accrual since 1 January 2021. The US
defined benefit plan liabilities and assets related to Group employees are being retained by the
GSK Group following the Demerger. The majority of the US post-retirement healthcare plan
liabilities will be retained by the GSK Group following the Demerger, with the exception of the
liability for active Haleon Group employees which will transfer to the Group.
290

The majority of the legacy defined benefit pension liabilities transferring to the Group relate to
arrangements outside of the UK and USA. Over 90 per cent. of these liabilities relate to the
pension arrangements in Ireland, Switzerland and Germany. The Group will assume liabilities
for current and (where applicable) former employees under such arrangements and, where
such plans are funded, a share of the plan assets will be transferred to the plan set up by the
Group.
Ongoing pension provision for Haleon Group employees
In the UK, since 1 April 2022, Group employees have been provided with ongoing accrual via
LifeSight, a defined contribution master trust arrangement provided by Willis Towers Watson.
Certain risk benefits, such as life assurance, are provided to Group employees through a
separate arrangement with Legal & General. The Demerger does not change these
arrangements with LifeSight and Legal & General.
In the USA, ongoing accrual is provided via a GSK Group defined contribution arrangement.
The Group’s US employees are currently members of this plan, but a defined contribution plan
is being established for them and they will join this plan at or before the date of Demerger. The
US defined contribution plan assets related to Group employees will be transferred to this plan.
Outside of the UK and the USA, the Group has committed to replicating GSK Group benefits
where possible. Some pension arrangements operate on a stand-alone basis for the Group,
and these will continue beyond the Demerger, but other arrangements cover both GSK Group
and Group employees. In the latter case, arrangements have been made to separate these
plans. In most cases, the GSK Group will retain the existing plan and the Group will establish a
new plan, with a transfer of the part of the assets of the GSK plan attributable to Group
employees being made to the plan established by the Group.
12. EMPLOYEES
The average monthly number of personnel
88
(including directors) employed by the Group for
the three years ended 31 December 2019, 2020 and 2021 was 19,000, 21,900 and 22,800
respectively. The monthly average number of Group personnel by region is included below:
Region 2021 2020 2019
North America 5,800 5,300 4,000
EMEA and LatAm 11,700 10,900 10,000
APAC 5,300 5,700 5,000
Total 22,800 21,900 19,000
The number of personnel from 2019 to 2021 increased mainly as a result of the integration of
employees from the Pfizer Group upon the completion of the Pfizer Transaction. The
completion date of the Pfizer Transaction was 31 July 2019 however, the transfer of the
employees took place in phases. As markets have been integrated, this has subsequently
resulted in employees being transferred to the Group during the latter part of 2019 through to
2021. The increase was partially offset by a reduction in personnel due to existing restructuring
programmes and synergies achieved from the Pfizer Transaction.
Total personnel in March 2022 was broadly in line with the 2021 average with North American
reductions (driven by restructuring programmes and synergies) during the course of 2021
largely offset by the recruitment of additional personnel in 2022 in both EMEA and LatAm and
APAC to support Group administrative operations previously carried out by GSK. Total APAC
personnel also increased due to the transfer of the Pulogadung site from GSK into the Group’s
manufacturing network in late 2021.
88
Full-time equivalent employees and agency staff (rounded to the nearest 100). The average number of agency staff in 2021
was 2,400.
291
13. RELATED PARTY TRANSACTIONS
Details of related party transactions entered into by members of the Group during the period
covered by the Historical Financial Information are as set out in Note 30 of the Historical
Financial Information. The nature of the related party transactions of the Group has not
changed between the period covered by the Historical Financial Information and the Latest
Practicable Date from those described in Note 30 to the Historical Financial Information. Save
as set out above and as described in paragraph 15 of this Part XII (Additional Information),
there have been no other related party transactions entered into by members of the Group
which require disclosure under IFRS between the period covered by the Historical Financial
Information and the Latest Practicable Date.
14. LITIGATION
Save as described below, there are no governmental, legal or arbitration proceedings
(including any such proceedings which are pending or threatened of which the Company is
aware) during the period covering the 12 months preceding the date of this document which
may have, or have had in the recent past, significant effects on the Company’s and/or the
Group’s financial position or profitability.
The Group is currently, and may from time to time be, involved in significant legal and
administrative proceedings, principally product liability, intellectual property, tax, antitrust,
securities law, employment and governmental investigations, as well as related private
litigation, further details of which are set out below. The Group makes provision for these
proceedings on a regular basis, as noted below. The Group may become involved in significant
legal proceedings in respect of which it is not possible to make a reliable estimate of the
expected financial effect, if any, that could result from ultimate resolution of the proceedings.
With respect to each of the legal proceedings described below, other than those for which a
provision has been made, the Group is unable to make a reliable estimate of the expected
financial effect at this stage. In particular, the Group does not believe that information about the
amount sought by plaintiffs, if that is known, would be meaningful with respect to those legal
proceedings. This is due to a number of factors, including, but not limited to, the stage of
proceedings, the entitlement of parties to appeal a decision and clarity as to theories of liability,
damages and governing law.
At 31 December 2021, the Group had £14 million of provisions for legal disputes and other
matters, including amounts relating to legal and administrative proceedings, which are included
within “Other Provisions” as set out in Note 26 to the Historical Financial Information of
GlaxoSmithKline Consumer Healthcare Holdings (No.2) Limited Schedule II.
The ultimate liability for legal claims may vary from the amounts provided and is dependent
upon the outcome of litigation proceedings, investigations and possible settlement
negotiations. The Group’s position could change over time, and, therefore, there can be no
assurance that any losses that result from the outcome of any legal proceedings will not
exceed by a material amount the amount of the provisions reported in the Group’s financial
accounts. If this were to happen, it could have a material adverse impact on the results of
operation of the Group in the reporting period in which the judgments are incurred or the
settlements entered into.
14.1 Zantac litigation
In 2019, the GSK Group was contacted by several regulatory authorities regarding the
detection of N-Nitroso-dimethylamine (“NDMA”) in Zantac (ranitidine) products. Based on
information available at the time and correspondence with regulators, the GSK Group made
the decision to suspend the release, distribution and supply of all dose forms of Zantac to all
markets pending the outcome of the ongoing tests and investigations. Also, as a precautionary
action, the GSK Group made the decision to initiate a voluntary pharmacy/retail level recall of
Zantac products globally.
292
On 30 April 2020, the EMA recommended the suspension of ranitidine medicines. Following
the publication of the EMA’s recommendation, the GSK Group communicated a decision not to
re-enter the market. In the USA, the FDA requested that all manufacturers withdraw ranitidine
products from the market.
GSK, GlaxoSmithKline LLC, GlaxoSmithKline (America) Inc. and/or Pfizer have been named
as defendants (alongside other manufacturers of ranitidine, as well as retailers and distributors)
in over 2,200 US personal injury lawsuits involving Zantac. There are also numerous unfiled
claims added to a registry implemented by the court presiding over the Zantac MDL
proceeding. Class actions alleging economic injury and medical monitoring also have been
filed in federal court. Outside the USA, there are seven class actions (two active) and forty
individual actions pending in Canada, along with a class action in Israel.
On 6 February 2020, the US product liability litigation was assigned MDL status in the
Southern District of Florida. On 24 August 2020, the GSK Group and the Pfizer Group filed
motions to dismiss the MDL claims based on innovator liability, preemption and deficiencies in
the pleadings. On 31 December 2020 and 8 January 2021, the court granted the GSK Group’s
and the Pfizer Group’s motion on innovator liability, the generic defendants’ motion on
preemption and the motion of all defendants on deficiencies in the pleadings with leave to
replead. The plaintiffs have filed notices of appeal related to the decisions on innovator liability
and generic preemption. Plaintiffs filed amended master complaints, which the defendants
moved to dismiss on 24 March 2021. On 30 June 2021, the court issued its rulings on the
additional motions. The court granted the GSK Group’s and the Pfizer Group’s motions to
dismiss on innovator liability and “failure to warn through the FDA” claims. The court also
dismissed the claim under the Racketeer Influenced and Corrupt Organizations Act with
prejudice.
On 20 March 2020, the Department of Justice (the “DOJ”) sent the GSK Group notice of a civil
investigation it had opened into allegations of False Claims Act violations by the GSK Group
related to Zantac. On 18 June 2020, the DOJ served a civil investigative demand on the GSK
Group, formalising its request for documents. On the same day, the New Mexico Attorney
General filed a lawsuit against multiple defendants, including the GSK Group and the Pfizer
Group, alleging violations of state consumer protection and false advertising statutes, among
other claims. The City of Baltimore filed a similar action on 12 November 2020.
In addition to the product liability cases filed in the MDL, cases have been filed against the
GSK Group and the Pfizer Group in several State Courts, including a consolidated action in
California State Court. The first trial in relation to Zantac is set to commence on 22 August
2022 in the Circuit Court of the Third Judicial District, Madison County Illinois, followed by the
first trial in the Superior Court of California, Alameda, scheduled to commence in February
2023, with three further bellwether trials to be scheduled in 2023.
With respect to the USA, the OTC rights to Zantac were originally owned by a joint venture
established between the GSK Group and Warner Lambert in 1993. Following the grant of FDA
approval for the OTC formulation in 1995, OTC Zantac was marketed by the GSK-Warner
Lambert joint venture until 1998 when the joint venture was terminated and, following which,
Warner Lambert retained the exclusive rights to the OTC product. In 2000, Warner Lambert
was acquired by Pfizer. In 2006, Johnson & Johnson acquired Pfizer’s OTC business, including
the rights to OTC Zantac, which were on-sold to Boehringer Ingelheim as a condition to merger
control approval. In 2017, Boehringer Ingelheim sold its consumer healthcare business
(including OTC Zantac) to Sanofi.
Under the Pfizer SAPA, CH JVCo is required to indemnify the GSK Group and the Pfizer
Group in respect of “Purchaser Liabilities” and “Assumed Liabilities”, further detail on which is
set out in paragraph 15.2 of this Part XII (Additional Information) below.
Whilst Pfizer and GSK have each served CH JVCo with notice of potential claims under the
relevant indemnification provisions in the Pfizer SAPA in relation to possible liabilities
connected with OTC Zantac, it is not possible, at this stage, to meaningfully assess whether
293
the outcome will result in a probable outflow, or to quantify or reliably estimate what liability (if
any) that CH JVCo may have to the GSK Group and/or the Pfizer Group under the relevant
indemnities. This is due to a number of factors and uncertainties, including:
(A) the complex factual matrix relating to the third-party tort claims and the implications of
the history of ownership of the US OTC Zantac rights, including: (i) the inability to
establish whether the patients took the OTC and/or the prescription Zantac product
and over what period(s); (ii) the application of (and interaction between) the various
liability allocation and indemnification regimes entered into in connection with the
successive transfers of ownership of US OTC Zantac, as well as under the Pfizer
SAPA; and (iii) how that complex factual matrix and/or ownership history interacts with
the terms of the Pfizer SAPA to determine the application and scope of CH JVCo’s
indemnification obligations to the GSK Group and/or the Pfizer Group; and
(B) the current status of the respective proceedings, which remain at an early stage.
14.2 PPI litigation
Certain members of the Group are defendants in the ongoing proton pump inhibitor (“PPI”)
litigation, in which plaintiffs allege that their use of PPIs caused serious bodily injuries, including
acute kidney injury, chronic kidney disease or end-stage renal failure. As of January 2022,
there are approximately 1,500 Prevacid24HR (OTC) personal injury lawsuits and
approximately 2,300 Nexium 24HR (OTC) lawsuits filed and pending against the Group, nearly
all of which are pending in an MDL in the District of New Jersey. In addition to the MDL cases,
there is a small subset of cases pending in several state courts.
Manufacturers of other PPIs, including both prescription and OTC products, are named as
co-defendants in the MDL. The Group has filed motions to dismiss several hundred cases, but
the MDL court has not yet ruled on those motions.
The first bellwether trial in the MDL is set for October 2022 and will focus on prescription
products manufactured by other co-defendants. The Group and its Prevacid24HR (OTC) or
Nexium 24HR (OTC) products will not be involved in the first trial. Additional trials involving
other defendants, including the Group, may be scheduled for 2023 or 2024.
The Group divested the rights to Prevacid in the USA in 2019, but retained certain historical
litigation liabilities. Prevacid was originally acquired by the Group as part of the GSK/Novartis
JV, and therefore, to the extent that the litigation, in whole or in part, gives rise to any liabilities
that result from, or otherwise relate to, acts or omissions of the Novartis group, or any
circumstances or events in existence or arising, in the period prior to completion of the GSK/
Novartis JV, the Group may be entitled to indemnification by Novartis (subject to the applicable
limitations and financial thresholds set out in the Novartis Contribution Agreement) (see
paragraph 15.1 of this Part XII (Additional Information) below).
14.3 German competition litigation
In 2013, GlaxoSmithKline Consumer Healthcare GmbH & Co. KG and other members of a
working group, Körperpflege, Wasch- und Reinigungsmittel (“KWR”), of a German trade mark
association, Markenverband e.V., were fined by the Federal Cartel Office of Germany, as a
result of the exchange of certain information during meetings from 2004 to 2006. The
information exchanged related primarily to annual terms negotiations with retailers and to the
timing and the order of magnitude of list price increases. A total fine of approximately
€63 million was imposed in 2013 on 15 companies, including €5.1 million against the Group.
Following the fine imposed by the Federal Cartel Office in 2013, the Group is party to eight
active civil proceedings in Germany for damages against the Group and other manufacturers of
branded drugstore products. The claimants allege that the exchange of information within KWR
led to higher purchase prices being paid by the retailers, and therefore the Group and other
KWR members are jointly and severally liable for potential damages. The proceedings are
taking place in different courts across Germany and are at different stages.
294
Separate proceedings have been brought against the Group and certain other members of
KWR by the insolvency administrator of Schlecker (formerly a large drugstore retailer in
Germany) and other retailers, including Müller, Rossmann, Kaufland and Budnikowsky. Two of
these actions have been dismissed in lower courts but are subject to appeal. For one of these
actions, the Federal Court of Justice has set a date for the oral hearing on the appeal for 5 July
2022. Two related proceedings brought by Norma have concluded as the claimants withdrew
their action.
Additionally, the Group has intervened as a third party on the defendants’ side in three
separate proceedings brought by Bartels-Langness and Kaufland (in two separate
proceedings).
15. MATERIAL CONTRACTS
The contracts listed below have been entered into by the Company or a member of the Group:
(i) within the two years immediately preceding publication of this Prospectus and are material
to the Company or any member of the Group, or (ii) at any time and contain any provision
under which the Company or any member of the Group has any obligation or entitlement which
is material to the Company or any member of the Group as at the date of this Prospectus, in
each case not including contracts entered into in the ordinary course of business.
15.1 Novartis Contribution Agreement
Pursuant to a contribution agreement dated 22 April 2014 between GSK, Novartis and
GSKCHH (as amended and restated on 29 May 2014 and 2 March 2015) (the “Novartis
Contribution Agreement”), GSK and Novartis formed a joint venture combining the majority of
GSK’s consumer healthcare business with all of Novartis’ OTC business (see paragraph 2.2 of
Part I (Key Highlights and Development of the Group) for further details) (the “GSK/Novartis
JV”). GSKCHH was the entity through which both GSK and Novartis held their equity interests
in the GSK/Novartis JV.
On 27 March 2018, GSK, Novartis, GSKCHH and the respective GSK and Novartis
shareholders in the GSK/Novartis JV entered into a put option implementation agreement,
pursuant to which the parties agreed that Novartis would be bought out of the GSK/Novartis JV
for consideration of $13 billion (the “Novartis Buyout”). The Novartis Buyout completed on
1 June 2018.
Under the Novartis Contribution Agreement, Novartis provided indemnities to GSKCHH in
respect of: (i) pre-completion liabilities and liabilities resulting from pre-completion actions in
respect of the OTC business contributed by Novartis to the GSK/Novartis JV; and (ii) any
pre-completion tax liabilities of the Novartis companies contributed by Novartis to the GSK/
Novartis JV, in each case, subject to certain limited exceptions. These indemnities survived the
Novartis Buyout and the creation of the subsequent GSK/Pfizer JV (see paragraph 15.2 of this
Part XII ( Additional Information) below).
Following completion of the Novartis Buyout, GSKCHH released and discharged GSK from any
and all existing and future obligations under (and waived any and all rights to make any claim
under) the reciprocal indemnities that were provided by GSK to GSKCHH pursuant to the
Novartis Contribution Agreement in respect of the business and companies contributed by
GSK to the GSK/Novartis JV.
15.2 Pfizer Stock and Asset Purchase Agreement
Pursuant to a stock and asset purchase agreement dated 19 December 2018 and amended
and restated on 31 July 2019 (the “Pfizer SAPA”), GSK, Pfizer and CH JVCo agreed to form a
new global consumer healthcare joint venture (the “GSK/Pfizer JV”), through: (i) the
acquisition by CH JVCo of the Pfizer Contributed CH Business (as defined below) from Pfizer
and (ii) the transfer by GSK to CH JVCo of those parts of the GSK Contributed CH Business
295
(as defined below) not already owned by GSKCHH (the former holding company of the Group)
following the creation of the GSK/Novartis JV. Completion of the transaction (“Pfizer
Completion”) took place on 31 July 2019 (the “Pfizer Completion Date”).
Asset Perimeter: GSK Contributed CH Business
The “GSK Contributed CH Business” has the meaning given to “Purchaser Business” in the
Pfizer SAPA, which was defined as follows:
(A) the worldwide business of researching, developing, manufacturing, marketing,
commercialising, distributing and selling the products sold under the brand names
listed for GSK in an annex to the Pfizer SAPA as conducted by GSK (directly and
indirectly) as of the date of the Pfizer SAPA and as of immediately prior to Pfizer
Completion;
(B) the business reflected in certain specified financial statements of the GSK Contributed
CH Business, including the assets, rights, properties, activities, operations and
liabilities that comprised such business;
(C) the business of marketing, commercialising, distributing and selling any
over-the-counter healthcare or medicine products, wellness products and other
personal care, oral care, nutrition, skin health, cosmetic and related products (the
“Consumer Healthcare Products”) as conducted by GlaxoSmithKline Asia Private
Limited (including pursuant to the Consignment Selling Agreement) as of the date of
the Pfizer SAPA and as of immediately prior to Pfizer Completion; and
(D) to the extent not otherwise reflected in the financial statements referred to in paragraph
(B) above, the research and development of any Consumer Healthcare Products, as
conducted by GSK (directly and indirectly) through its consumer healthcare business
(directly or indirectly pursuant to a contractual arrangement with any other GSK
business, to the extent of the GSK consumer healthcare business’ right pursuant to
such contractual arrangement), as of the date of the Pfizer SAPA and as of
immediately prior to Pfizer Completion,
but excluded:
(i) the worldwide business of researching, developing, manufacturing, marketing,
commercialising, distributing and selling pharmaceutical products to the extent
such business and the economic benefit attached to such business was not
reflected in the financial statements referred to in paragraph (B) above; and
(ii) the excluded assets listed for GSK in an annex to the Pfizer SAPA, namely:
(a) the assets within the scope of (and proceeds of) GSK’s divestment of
the Horlicks brand and other consumer healthcare nutrition products in
India to Unilever N.V. (which completed on 1 April 2020);
(b) GlaxoSmithKline Consumer Healthcare Limited (GSK’s listed
subsidiary in India);
(c) GlaxoSmithKline Bangladesh Limited;
(d) GlaxoSmithKline Consumer Nigeria plc;
(e) Imitrex and Ventolin; and
(f) certain manufacturing sites in Argentina, Brazil, Indonesia, India and
Nigeria.
296
The parties subsequently agreed to transfer manufacturing sites in Indonesia, Argentina and
Brazil into the Group - see section entitled “Asset Transfer Framework Agreement—Asset
Perimeter - GSK Group to Group” in paragraph 15.4 of this Part XII (Additional Information)
below.
Asset Perimeter: Pfizer Contributed CH Business
The “Pfizer Contributed CH Business” has the meaning given to “Business” in the Pfizer
SAPA, which was defined as the worldwide business of researching, developing,
manufacturing, marketing, commercialising, distributing and selling:
(A) the products sold under the brand names listed for Pfizer in an annex to the Pfizer
SAPA, as conducted by Pfizer (directly and indirectly) as of the date of the Pfizer
SAPA and as of immediately prior to Pfizer Completion; and
(B) any over-the-counter consumer healthcare or medicine products, wellness products
and other personal care, oral care, nutrition, skin health, cosmetic and related
products, as conducted by Pfizer (directly and indirectly) through its Pfizer consumer
healthcare business unit (directly or indirectly pursuant to a contractual arrangement
with any other Pfizer business unit, to the extent of the Pfizer consumer healthcare
business unit’s rights pursuant to such contractual arrangement) as of the date of the
Pfizer SAPA and as of immediately prior to Pfizer Completion,
but excluded:
(i) any product marketed, commercialised, distributed or sold under the brands
Diflucan One, Feldene Gel or Ponstan (or any other products containing the
same or similar compounds as such products) in any jurisdiction;
(ii) any pharmaceutical products or pharmaceutical products that have become or
may in the future become, in whole or in part, over-the-counter products (other
than the products included in the definition of “Business”); and
(iii) any product containing any of the following compounds (or marketed,
commercialised, distributed or sold under any of the following brands) in any
jurisdiction: (a) Sildenafil citrate (Viagra); (b) Celecoxib (Celebrex); (c)
Varenicline (Chantix/Champix); (d) Atorvastatin (Lipitor); (e) Gabapentin
(Neutontin); and (f) Fesoterodine (Toviaz).
Representations and warranties
Pursuant to the Pfizer SAPA, GSK and Pfizer each gave customary and broadly reciprocal
representations and warranties to each other and to CH JVCo. The majority of these
representations and warranties have now since expired, other than certain fundamental
warranties including in respect of title to the shares and assets contributed by GSK and Pfizer,
respectively, to the Group, which are due to expire on 31 July 2022 (being the third anniversary
of the Pfizer Completion Date).
Indemnities
Under the Pfizer SAPA, GSK and Pfizer each agreed to indemnify each other and the Group in
respect of losses (other than losses relating to tax, which were subject to a separate regime –
see below) relating to certain liabilities that the parties agreed would be retained by the GSK
Group or the Pfizer Group, respectively, relating to, among other things: (i) the assets that
were excluded from the GSK Contributed CH Business or the Pfizer Contributed CH Business
respectively (as described above); (ii) liabilities under any pension or other employee benefit
plans not sponsored by GSKCHH or another member of the Group, subject to certain
exceptions; and (iii) any liabilities arising from any third party claim in respect of products
containing talc or asbestos distributed or sold by the GSK Group or the Pfizer Group at any
time before Pfizer Completion.
297
CH JVCo is required to indemnify the GSK Group and the Pfizer Group in respect of
“Purchaser Liabilities” and “Assumed Liabilities”, which were defined as follows:
“Purchaser Liabilities” means any and all liabilities (other than certain specified exceptions –
including those liabilities GSK agreed to indemnify the Group in respect of, as summarised
above) of GSK or any of its affiliates, whether arising prior to, on or after Pfizer Completion, to
the extent resulting from or arising out of the past, present or future ownership, operation, use
or conduct of the Purchaser Business, where “Purchaser Business” has the meaning described
above under the section entitled “Pfizer Stock and Asset Purchase Agreement—Asset
Perimeter: GSK Contributed CH Business”; and
“Assumed Liabilities” means any and all liabilities (other than certain specified exceptions –
including those liabilities Pfizer agreed to indemnify the Group in respect of, as summarised
above) of Pfizer or any of its affiliates, whether arising prior to, on or after Pfizer Completion, to
the extent resulting from or arising out of the past, present or future ownership, operation, use
or conduct of the Business, where “Business” has the meaning described above under “Pfizer
Stock and Asset Purchase Agreement—Asset Perimeter: Pfizer Contributed CH Business”.
The Pfizer SAPA Amendment Agreement will also extend CH JVCo’s indemnification
obligations in favour of GSK and Pfizer to include, among other things, all losses (other than
losses relating to tax, which were subject to a separate regime (see below)) relating to liabilities
to the extent resulting from or arising out of the past, present or future ownership, operation,
use or conduct of the Consumer Healthcare Business since Pfizer Completion, subject to
certain exceptions (see paragraph 15.3 of this Part XII (Additional Information) below).
In respect of tax, each of GSK and Pfizer provided an indemnity, subject to customary exclusions
and limitations, to the Group in respect of, amongst other things, tax liabilities of the companies
contributed to the GSK/Pfizer JV arising in respect of the period prior to Pfizer Completion.
CH JVCo retained its rights to indemnification against Novartis under the Novartis Contribution
Agreement (see paragraph 15.1 of this Part XII (Additional Information) above).
The indemnities provided by each of GSK, Pfizer and CH JVCo under the Pfizer SAPA will
survive completion of the Demerger and Separation.
15.3 Pfizer SAPA Amendment Agreement
On or around the date of this Prospectus, GSK, Pfizer, CH JVCo and the Company entered
into the second amendment agreement to the Pfizer SAPA (the “Pfizer SAPA Amendment
Agreement”) to implement certain amendments, including: (i) amendments to the Pfizer SAPA
that were deemed appropriate as a result of the Group being an independent, separate
business from the GSK Group and the Pfizer Group from Separation; (ii) amendments that
were deemed appropriate as a result of an overlap with certain other ancillary agreements that
are currently being entered into as part of the Separation; and (iii) to include the Company in
the Pfizer SAPA indemnity framework by way of a guarantee given by the Company of CH
JVCo’s indemnification obligations under the Pfizer SAPA.
Pursuant to the Pfizer SAPA Amendment Agreement:
(A) CH JVCo’s indemnification obligations under the Pfizer SAPA (as described above
under “Pfizer Stock and Asset Purchase Agreement— Indemnities”), shall be extended
to include, among other things, all losses (other than losses relating to tax, which were
subject to a separate regime) relating to liabilities to the extent resulting from or arising
out of the past, present or future ownership, operation, use or conduct of the
Consumer Healthcare Business since Pfizer Completion, subject to certain exceptions
primarily related to liabilities retained by each of Pfizer and GSK, respectively, under
the Pfizer SAPA; and
298
(B) the Company, which is deemed a ‘Purchaser Indemnified Party’ under the Pfizer SAPA
and has the benefit of the indemnities given to CH JVCo under the Pfizer SAPA, has
provided a guarantee of CH JVCo’s indemnity obligations under the Pfizer SAPA (as
described above under “Pfizer Stock and Asset Purchase Agreement— Indemnities”),
as amended by the Pfizer SAPA Amendment Agreement.
The Pfizer SAPA Amendment Agreement is conditional upon (among other things) the passing
of the Related Party Transactions Resolution at the GSK General Meeting and, if such
approval is not obtained by 31 December 2022 or if GSK abandons the Separation prior to
completion of the Demerger, the Pfizer SAPA Amendment Agreement shall automatically
terminate.
The Pfizer SAPA Amendment Agreement also includes provisions related to the release of
guarantees given by the Pfizer Group for the benefit of companies in the Group (or vice versa).
15.4 Asset Transfer Framework Agreement
On or around the date of this Prospectus, GSK, GSKCHH and CH JVCo entered into an asset
transfer framework agreement (the “Asset Transfer Framework Agreement”), setting out the
framework for transferring certain businesses, assets, liabilities and employees that were
excluded from the original perimeter of the GSK/Pfizer JV as contemplated in the Pfizer SAPA
and others that were included in the original perimeter of the GSK/Pfizer JV but had not yet
legally transferred or to record the transfer of “wrong pocket” assets under the Pfizer SAPA
(where a “wrong pocket” asset or liability is one that parties have identified as incorrectly being
transferred, or not transferred, to the other party in line with the principles of the Pfizer SAPA),
in each case from the GSK Group to the Group (see paragraph 15.2 of this Part XII (Additional
Information) above). The Asset Transfer Framework Agreement also sets out the framework
for transferring certain businesses, assets, liabilities and employees from the Group to the GSK
Group to record the transfer of “wrong pocket” assets under the Pfizer SAPA, and to remove
assets from the Group that do not relate to the Consumer Healthcare Business, in each case
from the Group to the GSK Group.
Asset Perimeter - GSK Group to Group
The businesses, assets, liabilities and employees within the perimeter of the Asset Transfer
Framework Agreement has the meaning given to “Transferring Businesses” and “Transferring
Assets” in the Asset Transfer Framework Agreement, which include the following:
(A) a certain manufacturing site in Indonesia;
(B) certain distribution businesses relating to the Consumer Healthcare Business in Chile,
Egypt, Peru, Morocco, Nigeria, Singapore, Vietnam, Laos and Cambodia, Uruguay,
Dominican Republic, Ecuador, El Salvador, Guatemala, Honduras, Jamaica and
Trinidad & Tobago (referred to as “alliance markets”);
(C) certain employees and assets providing administrative and support services to the
Consumer Healthcare Business by support services entities in the UK and US, and
support service centre hubs in Costa Rica, India, Malaysia and Poland; and
(D) certain assets and liabilities relating to PPE (plant, property and equipment) and
people-related fixed assets to be transferred from the GSK Group to the Group, in
Singapore, Philippines, Turkey, France, South Africa, Japan, Panama, Netherlands,
Pakistan, Mexico and Brazil.
There are also certain “wrong pocket” assets and liabilities that relate to the Consumer
Healthcare Business and are within the Pfizer SAPA perimeter which transfer from the GSK
Group to the Group, including:
(i) GlaxoSmithKline Bangladesh Private Limited;
299
(ii) certain intellectual property rights relating to the Consumer Healthcare
Business’ brands; and
(iii) certain marketing authorisations relating to the Consumer Healthcare
Business’ products.
Certain manufacturing sites in Argentina and Brazil were agreed to transfer from the GSK
Group into the Group following Demerger pursuant to the terms of a net economic benefit
arrangement letter agreement dated on or around the date of this Prospectus and a Brazil
asset transfer framework agreement dated on or around the date of this Prospectus
(respectively). The terms on which these manufacturing sites in Argentina and Brazil will
transfer into the Group are broadly in line with the terms of the Asset Transfer Framework
Agreement.
Asset Perimeter - Group to GSK Group
The Asset Transfer Framework Agreement also transfers certain businesses, assets, liabilities
and employees from the Group to the GSK Group, including:
(A) certain “wrong pocket” assets and liabilities that do not relate to the Consumer
Healthcare Business but currently sit within the Group (so these will be transferred
from the Group to the GSK Group as part of the Separation), including certain
intellectual property rights and marketing authorisations that do not relate to the
Consumer Healthcare Business’ brands or products; and
(B) certain assets and liabilities relating to GSK’s business in Sri Lanka.
Warranties
Pursuant to the Asset Transfer Framework Agreement, GSK gave CH JVCo customary
business warranties relating to the Transferring Assets and Transferring Business (where
“Transferring Assets” and/or “Transferring Businesses” has the meaning described above
under the section entitled “Asset Transfer Framework Agreement—Asset Perimeter - GSK
Group to Group”), and CH JVCo gave GSK customary capacity warranties. Certain
fundamental warranties given by GSK are due to expire in July 2025 (3 years following the
Demerger), and the remainder of the warranties are due to expire in October 2023 (15 months
following the Demerger).
Indemnities
The Asset Transfer Framework Agreement contains a substantially equivalent indemnification
regime to the Pfizer SAPA indemnification regime described in paragraph 15.2 of this Part XII
(Additional Information) above.
In respect of taxes relating to the “Transferring Assets” and/or “Transferring Businesses”
(where “Transferring Assets” and/or “Transferring Businesses” has the meaning described
above under the section entitled “Asset Transfer Framework Agreement—Asset Perimeter -
GSK Group to Group”),
(A) GSK and CH JVCo shall each be responsible for half of any transfer taxes imposed on
the businesses, assets and liabilities transferred;
(B) the transferee shall bear the cost of any VAT imposed on the transfers (except in the
case of Egypt where the parties have agreed that the amount of any irrecoverable VAT
shall be split equally between them); and
(C) CH JVCo will assume all liabilities for taxes imposed with respect to the Transferring
Businesses or the Transferring Assets (other than GlaxoSmithKline Bangladesh
Private Limited), other than tax liabilities relating to the period pre-closing, which are
retained by GSK.
300
Employees and pensions
The Asset Transfer Framework Agreement provides for the transfer of employees and
associated employment-related liabilities from the GSK Group to the Group in relation to any
employees transferring from the GSK Group to the Group, and reciprocal provisions in relation
to employees transferring from the Group to the GSK Group, including provisions in respect of
the apportionment of such inherited liabilities and associated reimbursements. It also
addresses the treatment of equity awards under the GSK equity plan for the employees of the
Group and the allocation of certain liabilities with respect to such awards.
The Asset Transfer Framework Agreement also provides for a transfer of unfunded pension
liabilities from the GSK Group to the Group in relation to any employees transferring from the
GSK Group to the Group, and a transfer of pension liabilities from the Group to the GSK Group
in relation to employees transferring from the Group to the GSK Group. Where an employee
transferring from the GSK Group to the Haleon Group is a member of a funded pension plan,
the Asset Transfer Framework Agreement requires the parties to use their reasonable
endeavours to procure the transfer of pension liabilities and assets attributable to such
employees from the GSK Group pension plan to the Group pension plan (and vice versa in
respect of an employee transferring from the Group to the GSK Group).
15.5 Pfizer Shareholders’ Agreement
The shareholders’ agreement, as amended or supplemented from time to time, in relation to
the GSK/Pfizer JV was entered into on 31 July 2019 among GSKCHH, Pfizer, PFCHH, GSK
and CH JVCo (the “Pfizer SHA”). The Pfizer SHA governs the relationship between the
shareholders of CH JVCo and its ongoing management and operation. Pursuant to the SCIA
(see paragraph 15.8 of this Part XII (Additional Information) below), the parties have agreed
that the Pfizer SHA will be terminated in its entirety with effect from Admission.
15.6 Demerger Agreement
The Company and GSK entered into a demerger agreement on or around the date of this
Prospectus (the “Demerger Agreement”) to effect the Demerger and to govern aspects of the
relationship between the Company and GSK following completion of the Demerger, including in
respect of, among other things, confidentiality and certain indemnity obligations in connection
with the issuance of shares by the Company in connection with the Demerger. Certain aspects
of the Demerger Agreement are conditional upon (among other things):
(A) the passing of the Demerger Resolution and the Related Party Transactions
Resolution by GSK Shareholders at the GSK General Meeting;
(B) the payment of certain interim dividends required to be paid by CH JVCo to GSKCHH
and PFCHH ahead of Separation (including the Pre-Demerger Dividend);
(C) approval of the Demerger Dividend by the GSK Board;
(D) approval of the Competition Commission of India (the “India Condition”);
(E) approval of the Korea Fair Trade Commission pursuant to certain South Korean
merger control laws (the “South Korea Condition”);
(F) approval of the Fair Trade Commission pursuant to certain Japanese merger control
laws (the “Japan Condition” and, together with the India Condition and the South Korea
Condition, the “Regulatory Conditions” and each a “Regulatory Condition”);
(G) no order, injunction or decree issued by a governmental entity of competent jurisdiction
or other legal restraint or prohibition preventing the consummation of the Demerger
being in effect;
301
(H) the Sponsors’ Agreement and GSK Sponsors’ Agreement not having terminated in
accordance with their terms;
(I) the FCA having acknowledged to the Company or its agent (and such
acknowledgement not having been withdrawn) that the application for Admission:
(i) has been approved; and (ii) will become effective as soon as a dealing notice has
been issued by the FCA and any Listing Conditions have been satisfied;
(J) the LSE having acknowledged to the Company or its agent (and such
acknowledgement not having been withdrawn) that the Haleon Shares will be admitted
to trading on its main market for listed securities;
(K) (i) the Form 20-F having been declared effective by the SEC, and (ii) no stop order
suspending its effectiveness being in effect, and no proceedings for such purpose
being pending before or threatened by the SEC;
(L) the ADSs having been approved for listing on the New York Stock Exchange, subject
only to official notice of issuance;
(M) the Exchange Agreements having been duly executed, continuing to bind all parties
thereto and having become unconditional in all respects (save for any condition
relating to completion of the Demerger or the Demerger Agreement being
unconditional) such that the Share Exchanges shall be capable of occurring, subject
only to the due performance of the relevant agreement(s), and all parties thereto shall
stand ready to perform such agreements and complete the Share Exchanges, no later
than the Sunday after completion of the Demerger; and
(N) the PFCHH Transfer having completed (provided that such condition will be deemed
satisfied if the PFCHH Transfer has not completed by 8.00 p.m. London time on the
date that is three (3) business days after satisfaction of the Regulatory Conditions).
In the event that any of the Regulatory Conditions remain unsatisfied at 8.00 p.m. on 12 July
2022, then:
(i) completion of the Demerger shall be delayed past the currently expected time;
(ii) completion of the Demerger shall instead take place at 8.00 p.m. (and in any
event after the close of business in London) on the first Friday that is at least
three (3) business days after satisfaction of all of the Regulatory Conditions,
provided that all other conditions (except for any conditions which will only be
satisfied on completion of the Demerger) are satisfied or deemed satisfied by
such time, and further provided that none of the events specified in paragraphs
(G), (H) and (K)(ii) above have occurred at such time (such Friday being the
“Delayed Demerger Completion Date”); and
(iii) the Share Exchanges shall be scheduled to occur on the first Sunday after the
Delayed Demerger Completion Date and Admission shall be scheduled to
occur on the first Monday after the Delayed Demerger Completion Date.
Subject to the Pfizer SHA, GSK has the right in its absolute discretion by notice to the
Company at any time prior to completion of the Demerger to terminate the Demerger
Agreement in connection with an abandonment of the Demerger.
The Demerger Agreement contains certain customary indemnities under which GSK
indemnifies the Company in respect of liabilities, losses demands, claims, costs, taxes and
damages arising, directly or indirectly, from or in consequence of certain claims.
The Demerger Agreement also sets out how guarantees given by the GSK Group for the
benefit of companies in the Group (or vice versa) will be dealt with following the Demerger.
302
15.7 Tax Covenant
In accordance with the SCIA, the Company, CH JVCo, GSK, GSKCHH and Pfizer entered into
a tax covenant on or around the date of this Prospectus, which is to be effective from the time
of the Demerger (the “Tax Covenant”).
Subject to certain financial and other customary limitations, the Tax Covenant contains certain
indemnities in respect of taxation given from GSK and Pfizer to the Company (and vice versa)
where it has been agreed that such taxes are properly allocable to the indemnifying party.
Amongst other things, GSK and Pfizer provide the Company with indemnities for tax arising (if
any) pursuant to certain pre-Demerger reorganisation steps within the Group and the steps
which comprise the Separation. As is customary for demerger transactions, the Company
provides a more limited set of tax indemnities to GSK and Pfizer.
The indemnities in the Tax Covenant cover only liabilities which have been notified by the
indemnified party to the indemnifying party by the end of the period ending 30 days after the
expiry of the period specified by statute during which an assessment of the relevant underlying
tax liability may be issued by the relevant tax authority or, if there is no such period, by the end
of the period ending six years and 30 days after the end of the accounting period in which the
Demerger occurs.
The Tax Covenant also imposes certain restrictions on the Company, including certain
restrictions with respect to actions following completion of the Demerger that could cause
Separation to fail to qualify for its intended US federal income tax treatment. The restrictions
primarily require the Company to maintain the corporate structure of certain parts of the Group
as it was immediately prior to the Demerger. For example, there are restrictions on liquidating
certain subsidiaries of the Company, or issuing or redeeming shares in those subsidiaries. In
addition, there are restrictions on some intra-group disposals as well as non-ordinary course of
business transactions. As a result of these restrictions (some of which could be in place for at
least two years), the Company’s ability to engage in certain transactions, such as the
disposition of certain assets and certain repurchases of its stock, may be limited (although the
Group will nonetheless be entitled to take actions which would otherwise be restricted if the
Company first (i) obtains the consent of (or, in certain instances, if it consults with) GSK or
Pfizer (as applicable) or, in some cases, (ii) obtains an opinion from an appropriately qualified
adviser or a ruling from the IRS regarding the tax consequences of the proposed actions
which, in either case, is reasonably satisfactory to GSK or Pfizer (as applicable)). Although the
Company does not currently anticipate that these restrictions would have a material adverse
impact on the Company, these restrictions may reduce the Company’s ability to engage in
certain business transactions that otherwise might be advantageous.
The Tax Covenant also contains provisions on administrative matters and the conduct of tax
authority audits or disputes (including, in each case, how the parties should bear the costs and
expenses of the same). It also contains certain mechanical provisions to ensure that the tax
indemnities in the Pfizer SAPA operate properly post-Demerger (i.e. once the Company is the
head of a standalone group).
15.8 Separation Co-operation and Implementation Agreement
The Separation Co-operation and Implementation Agreement (the “SCIA”) was entered into on
or around the date of this Prospectus among GSK, Pfizer, Anacor, GSKCHH, PFCHH, CH
JVCo and the Company, and details certain actions to be taken and arrangements to be
implemented to effect completion of, or which otherwise relate to, the Separation. The SCIA
records the obligations of the parties relating to such matters and contains certain terms on
which relations between the parties will be governed following completion of the Separation.
The parties to the SCIA have agreed to co-operate to achieve completion of the Separation
and have undertaken to take all steps required, and to enter into (or procure the entry into of)
all documents required, to effect the Separation.
303
The parties to the SCIA have agreed and acknowledged that GSK has, subject to the terms of
the Pfizer SHA, the right in its absolute discretion by notice in writing to the other parties to the
SCIA at any time prior to completion of the Demerger to abandon the Separation and, if GSK
provides such notice, the SCIA shall automatically terminate.
The parties to the SCIA have agreed and acknowledged that they shall each take all actions
that they are able to take (and shall procure so far as they are able to do so, that the members
of each of their respective groups do the same), so that certain dividends to be declared and
paid in connection with the Separation are so declared and paid prior to the commencement of
the Demerger Completion Steps and, in respect of certain dividends, following receipt of
certain regulatory approvals. In the event that any such dividends are in any respect defective
or are susceptible to legal challenge, the Company has agreed and has undertaken to take all
possible lawful steps, (including, without limitation, distributable reserves planning and
management; rectification and ratification steps; and procuring that none of CH JVCo,
GSKCHH or PFCHH or any other member of the Group take steps to seek recovery of prior
dividend payments), such that any amounts received by any member of the GSK Group or any
member of the Pfizer Group or to which they are entitled by way of dividend can be retained by
the relevant member(s) of the GSK Group or the Pfizer Group (as applicable). The Company
has further agreed, subject to certain exceptions, to indemnify GSK, each member of the GSK
Group, Pfizer and each member of the Pfizer Group from and against any and all liabilities and
certain costs arising before, on, or after completion of the Separation in respect of: (i) any
defect in, or any actual or potential claim, proceeding, suit or action brought by any member of
the Group that arises out of or in connection with any of the relevant dividends; and (ii) any
failure by the Company to take all possible steps required by the SCIA to ensure that any
amounts received by any member of the GSK Group or any member of the Pfizer Group by
way of any relevant dividend can be retained by the relevant member(s) of the GSK Group
and/or the Pfizer Group (as applicable).
The SCIA also sets out certain other rights and obligations of the parties relating to, among
other things, information rights and confidentiality. Pursuant to the terms of the SCIA, Pfizer
has certain rights to certain information regarding the Company and the Haleon Group. Subject
to certain exceptions, those rights will not apply if and when Pfizer and members of Pfizer’s
group cease to hold, in aggregate, Haleon Shares or Haleon ADSs in respect of such Haleon
Shares representing at least ten per cent. of the Haleon Shares in issue (or the ordinary shares
of any ultimate holding company thereof from time to time).
The parties to the SCIA have agreed that the Pfizer SHA shall terminate with effect from
Admission (without prejudice to any rights or liabilities arising under the Pfizer SHA prior to
such termination) and that, notwithstanding any provision of the Pfizer SHA, the provisions of
the Pfizer SHA that are expressly stated to continue after termination of the Pfizer SHA shall
not continue and, instead, certain of such provisions shall be set out or otherwise implemented
in the SCIA or other documents to be entered into in connection with the Separation.
15.9 Exchange Agreements
Subject to and shortly after completion of the Demerger, a series of share-for-share exchanges
(together, the “Share Exchanges”) will occur pursuant to the share exchange agreements
summarised below in order to rationalise the Company’s shareholding structure such that GSK,
the SLPs and Pfizer will hold their remaining interests in the Consumer Healthcare Business by
holding shares in the Company, as the listed parent company.
(A) GSK Exchange Agreement
On or around the date of this Prospectus, GSK and the Company entered into an
exchange agreement (the “GSK Exchange Agreement”) pursuant to which GSK will,
conditional on completion of the Demerger, transfer all of its GSKCHH B Ordinary
Shares to the Company in exchange for the issuance by the Company of 502,868,434
Haleon Shares, less a number of Haleon Shares that is equal to the number of Excess
GSK Shares. As at the Latest Practicable Date, the number of Haleon Shares
expected to be held by GSK at Admission is expected to represent up to 6 per cent. of
the total issued share capital of the Company.
304
(B) SLP Exchange Agreement
On or around the date of this Prospectus, the SLPs and the Company entered into an
exchange agreement (the “SLP Exchange Agreement”) pursuant to which the SLPs
will, conditional on completion of the Demerger, transfer all of their GSKCHH C
Ordinary Shares to the Company in exchange for the issuance by the Company of
Haleon Shares, representing 7.5 per cent. (in aggregate and to the nearest whole
Haleon Share) of the issued and outstanding Haleon Shares immediately following
Separation.
Following completion of the Demerger Agreement, the GSK Exchange Agreement and
the SLP Exchange Agreement, the Company will own the entire issued share capital of
GSKCHH, which in turn owns 68 per cent. of the ordinary shares in CH JVCo.
(C) Pfizer Exchange Agreement
On or around the date of this Prospectus, Pfizer, Anacor and the Company entered into
an exchange agreement (the “Pfizer Exchange Agreement”) pursuant to which Pfizer
will, conditional on completion of the Demerger, transfer all of its interests in PFCHH
(the company that holds 32 per cent. of the ordinary shares in CH JVCo) to the
Company in exchange for the issuance by the Company of Haleon Shares to Pfizer
and the Depositary (on behalf of Pfizer), representing in aggregate 32 per cent. of the
issued and outstanding Haleon Shares immediately following Separation (to the
nearest whole Haleon Share), and 25 million Non-Voting Preference Shares.
Following completion of the Demerger Agreement and the Exchange Agreements
summarised above, the Company will, indirectly, own 100 per cent. of CH JVCo.
15.10 Pfizer Relationship Agreement
The relationship agreement between the Company and Pfizer was entered into as a deed on or
around the date of this Prospectus (the “Pfizer Relationship Agreement”). The principal
purpose of the Pfizer Relationship Agreement is to regulate the continuing relationship
between the Company and Pfizer after Admission. References to aggregate interests in Haleon
Shares in the Pfizer Relationship Agreement include both direct holdings of Haleon Shares and
interests in Haleon Shares held indirectly through holdings of Haleon ADSs.
Pursuant to the Pfizer Relationship Agreement, Pfizer has undertaken as required by LR
6.5.4R, that, for so long as Pfizer is a controlling shareholder (as defined in Appendix I to the
Listing Rules), it shall (and shall procure that its associates (as defined in Appendix I of the
Listing Rules) shall): (i) conduct all transactions and arrangements with the Company and the
Group at arm’s length and on normal commercial terms; (ii) not take any action that would have
the effect of preventing the Company from complying with its obligations under the Listing
Rules; and (iii) not propose or procure the proposal of a shareholder resolution of the Company
which is intended or appears to be intended to circumvent the proper application of the Listing
Rules (together the “Independence Provisions”). For so long as Pfizer is a controlling
shareholder, it shall (and shall, so far as it is legally able to do so, procure that its associates
shall) not take any action which precludes the Company or any other member of the Group
from carrying on an independent business as its main activity.
Under the Pfizer Relationship Agreement, Pfizer is granted the right to nominate two persons
to be appointed to the Board as representative directors for so long as it and its affiliates
together continue to hold 20 per cent. or more of the Haleon Shares in issue and a right to
nominate one person to be appointed to the Board as a representative director for so long as it
and its affiliates together continue to hold less than 20 per cent. but at least 10 per cent. of the
Haleon Shares in issue. Pfizer is subject to customary standstill provisions, subject to certain
exceptions, and the Pfizer Relationship Agreement imposes certain obligations on the
Company in connection with seeking shareholder authority to carry out share repurchases to
ensure that no such repurchases result in a requirement for Pfizer to make a general offer for
Haleon Shares in accordance with Rule 9 of the City Code (provided that Pfizer has not itself
entered into any disqualifying transactions).
305
Under the Pfizer Relationship Agreement, Pfizer agrees to procure that any member of its
group holding an interest in Haleon Shares on Admission (including for the avoidance of doubt,
Anacor) shall, for such time as that member of Pfizer’s group holds an interest in Haleon
Shares, comply with the provisions of the Pfizer Relationship Agreement as if that member of
Pfizer’s group were a party to the Pfizer Relationship Agreement with the same obligations as
Pfizer.
The Pfizer Relationship Agreement will terminate on the date that Pfizer and its affiliates cease
to hold at least 10 per cent. of the Haleon Shares in issue.
15.11 Registration Rights Agreement
The Registration Rights Agreement (the “Registration Rights Agreement”) was entered into
on or around the date of this Prospectus among the Company, Pfizer, GSK and the SLPs.
GSK, Pfizer and the SLPs, together with their respective affiliates, successors or permitted
assigns, to the extent they are holders or beneficial owners of the Company’s registrable
securities, are referred to in the Registration Rights Agreement as “Holders”. The Company’s
registrable securities include all shares and ADSs held by the Holders in the Company after
Separation and equity securities issued in exchange or replacement thereof.
The Registration Rights Agreement provides for certain demand and piggyback registration
rights to the Holders. Pursuant to the demand registration rights:
(A) the Company shall, no later than 60 calendar days after Separation, file with the SEC a
shelf registration statement covering the resale under the US Exchange Act of all
registrable securities and shall use its reasonable best efforts to have such shelf
registration statement declared effective no later than the earlier of: (i) 90 calendar
days following Separation if the SEC elects to “review” the shelf registration statement;
and (ii) 10 business days after Company is notified by the SEC that such shelf
registration statement will not be “reviewed” or will not be subject to further review;
(B) following the expiration of the lock-up restrictions in the Lock-up Deed, each Holder
shall have the right to sell any part of its registrable securities in an underwritten
offering pursuant to the shelf registration statement (the “Shelf Underwriting ”) by
delivering a written request to the Company. The Company shall give notice of such
request to the Holders of other registrable securities registered on the shelf registration
statement, and, subject to certain limitations, include in the Shelf Underwriting the
registrable securities of the other requesting Holders;
(C) if, following the expiration of the lock-up restrictions in the Lock-up Deed, the shelf
registration statement is not available for use by the Holders, each Holder may require
the Company to file one or more registration statements covering all or any part of its
registrable securities, subject to certain limitations. The Company shall use its
reasonable best efforts to file or confidentially submit with the SEC the registration
statement no later than 60 days from receipt of request from the Holder if the
registration is on Form F-1 or Form S-1 (or 30 days if the registration is on Form F-3 or
Form S-3); and
(D) the Registration Rights Agreement includes customary provisions that permit the
Company to postpone filing or confidentially submitting a registration statement, or if a
registration statement has been filed or confidentially submitted, suspend use of, or
withdraw, such registration statement for a limited duration to avoid disclosing material
non-public information in certain circumstances.
Pursuant to the piggyback registration right, if the Company registers any of its equity
securities for its own account or for the account of any other shareholder under the US
Securities Act, the Company shall give written notice of its intention to do so to each of the
Holders of record of registrable securities. Upon the written request from a Holder, the
Company shall, subject to certain limitations, use its reasonable best efforts to cause such
Holder’s registrable securities to be registered under the US Securities Act.
306
The Registration Rights Agreement requires the Company to provide a standard indemnity to
the Holders against any claims relating to any untrue statement of a material fact (or omission
of a material fact) in any registration statement, a prospectus or the information conveyed by
the Company to any purchaser at the time of the sale to such purchaser, or any violation by the
Company of applicable law. The Registration Rights Agreement also requires each Holder to
indemnify the Company, subject to certain limitations and with an opportunity to cure, with
respect to any untrue statement of a material fact (or omission of a material fact) in any
registration statement or prospectus, if such statement or omission was made in reliance upon
and in strict conformity with written information furnished to the Company by such Holder
specifically for the use therein.
The Registration Rights Agreement requires the Company to pay all expenses associated with
the registration of the registrable securities under the Registration Rights Agreement, but
excluding transfer taxes and commissions payable in an underwritten offering, which will be
payable by the Holders.
15.12 Sponsors’ Agreement
In connection with the Separation and Admission, the Company, CH JVCo and the Joint
Sponsors entered into a Sponsors’ Agreement on or around the date of this document (the
“Sponsors’ Agreement”), pursuant to which:
(A) the Company appointed the Joint Sponsors as sponsors in connection with the
production and publication of this Prospectus and the Application for Admission, and
the Joint Sponsors accepted such appointment;
(B) the Joint Sponsors have been granted all powers, authorities and discretions which are
necessary for or incidental to the performance of their responsibilities under the Listing
Rules;
(C) the Company has agreed to deliver certain documents to the Joint Sponsors relating to
this Prospectus and Admission and the Joint Sponsors’ responsibilities under the
Listing Rules;
(D) the Company has given customary representations, warranties, and indemnities to the
Joint Sponsors; and
(E) the Joint Sponsors have the right to terminate the Sponsors’ Agreement in certain
circumstances prior to Admission. These circumstances include (amongst others): (i) if
any statement in this Prospectus (and/or certain associated announcements) is or has
become untrue, inaccurate or misleading in a manner which, in the opinion of the Joint
Sponsors (acting in good faith), is material in the context of the Group taken as a
whole, the Separation, Admission, or this Prospectus (and/or certain associated
announcements); and (ii) the breach by the Company of any of the warranties or
undertakings contained in the Sponsors’ Agreement where the effect of such breach,
in the opinion of the Joint Sponsors (acting in good faith), is material in the context of
the Group taken as a whole, the Separation, Admission or the Joint Sponsors’ roles.
15.13 The CH Debt Documents
(A) EMTN Programme
As part of the preparation for the Demerger, on 16 March 2022, GSK Consumer
Healthcare Capital UK plc and GSK Consumer Healthcare Capital NL B.V. acting as
issuers (the “EMTN Issuers”) established a £10,000,000,000 Euro Medium Term Note
Programme (the “Programme”) pursuant to which the EMTN Issuers may issue notes
from time to time. As at the date of this Prospectus, the EMTN Issuers have issued
under the Programme: £300,000,000 2.875 per cent. notes due 2028, £400,000,000
3.375 per cent. notes due 2038, €850,000,000 1.250 per cent. notes due 2026,
€750,000,000 1.750 per cent. notes due 2030 and €750,000,000 2.125 per cent. notes
due 2034 (together, the “Pre-Separation Programme Notes”).
307
A list of the Pre-Separation Programme Notes and an overview of the terms applicable
to such notes is set out below:
• £300,000,000 2.875 per cent. notes due 29 October 2028 (the “2.875 per cent.
Notes”) - The 2.875 per cent. Notes were issued by GSK Consumer Healthcare
Capital UK plc and bear interest at a rate of 2.875 per cent. per annum, payable
annually in arrear. Unless previously redeemed or purchased and cancelled the
2.875 per cent. Notes will be redeemed by GSK Consumer Healthcare Capital UK
plc on 29 October 2028.
• £400,000,000 3.375 per cent. notes due 29 March 2038 (the “3.375 per cent.
Notes”) - The 3.375 per cent. Notes were issued by GSK Consumer Healthcare
Capital UK plc and bear interest at a rate of 3.375 per cent. per annum, payable
annually in arrear. Unless previously redeemed or purchased and cancelled the
3.375 per cent. Notes will be redeemed by GSK Consumer Healthcare Capital UK
plc on 29 March 2038.
• €850,000,000 1.250 per cent. notes due 29 March 2026 (the “1.250 per cent.
Notes”) - The 1.250 per cent. Notes were issued by GSK Consumer Healthcare
Capital NL B.V. and bear interest at a rate of 1.250 per cent. per annum, payable
annually in arrear. Unless previously redeemed or purchased and cancelled the
1.250 per cent. Notes will be redeemed by GSK Consumer Healthcare Capital NL
B.V. on 29 March 2026.
• €750,000,000 1.750 per cent. notes due 29 March 2030 (the “1.750 per cent.
Notes”) - The 1.750 per cent. Notes were issued by GSK Consumer Healthcare
Capital NL B.V. and bear interest at a rate of 1.750 per cent. per annum, payable
annually in arrear. Unless previously redeemed or purchased and cancelled the
1.750 per cent. Notes will be redeemed by GSK Consumer Healthcare Capital NL
B.V. on 29 March 2030.
• €750,000,000 2.125 per cent. notes due 29 March 2034 (the “2.125 per cent.
Notes”) - The 2.125 per cent. Notes were issued by GSK Consumer Healthcare
Capital NL B.V. and bear interest at a rate of 2.125 per cent. per annum, payable
annually in arrear. Unless previously redeemed or purchased and cancelled the
2.125 per cent. Notes will be redeemed by GSK Consumer Healthcare Capital NL
B.V. on 29 March 2034.
Each series of Pre-Separation Programme Notes additionally contains a limited
negative pledge and customary events of default (including cross-acceleration
provisions). The occurrence of any event of default under the Pre-Separation
Programme Notes would permit, amongst other things, the acceleration of the relevant
series of Pre-Separation Programme Notes.
Each series of Pre-Separation Programme Notes can be redeemed prior to maturity at
the option of the relevant issuer in accordance with the terms and conditions of the
relevant series of Pre-Separation Programme Notes. In addition to other customary
redemption features, each series of Pre-Separation Programme Notes includes a
make-whole redemption option, which permits the relevant issuer to redeem all or
some only of the notes on not less than 15 nor more than 60 days’ notice at any time,
subject to payment of the present value of the remaining scheduled payments of
principal and interest through to maturity (subject to a customary discount calculated
using an applicable bond reference rate plus an agreed margin).
The payment of all amounts owing in respect of notes issued under the Programme
(including the Pre-Separation Programme Notes) is, as at the date of this Prospectus,
unconditionally and irrevocably guaranteed by GSK. From and including the date of
completion of the Demerger, the guarantee provided by GSK will cease to be effective
and the notes will be unconditionally and irrevocably guaranteed by the Company.
308
(B) Pre-Separation USD Notes
In addition, on 24 March 2022:
(i) GSK Consumer Healthcare Capital US LLC (the “US Issuer”) issued
$700,000,000 3.024 per cent. fixed rate senior notes due 2024, $300,000,000
floating rate senior notes due 2024, $2,000,000,000 3.375 per cent. fixed rate
senior notes due 2027, $1,000,000,000 3.375 per cent. fixed rate senior notes
due 2029, $2,000,000,000 3.625 per cent. fixed rate senior notes due 2032
and $1,000,000,000 4.000 per cent. fixed rate senior notes due 2052; and
(ii) GSK Consumer Healthcare Capital UK plc issued $1,750,000,000 3.125 per
cent. fixed rate senior notes due 2025,
each pursuant to a private placement to institutional investors in the USA and
outside the USA in reliance on exemptions from the registration requirements
of the US Securities Act (the “Pre-Separation USD Notes”).
A list of the Pre-Separation USD Notes issued as at the date of this Prospectus and an
overview of the terms applicable to such notes is set out below:
• The $700,000,000 3.024 per cent. notes due 2024 (the “3.024 per cent. Notes”)
were issued by the US Issuer and bear interest at a rate of 3.024 per cent. per
annum, payable semi-annually in arrear. Unless previously redeemed or
purchased and cancelled the 3.024 per cent. Notes will be redeemed by the US
Issuer on 24 March 2024.
• The $300,000,000 floating rate notes due 2024 (the “Floating Rate Notes”) were
issued by the US Issuer and bear interest at a floating rate, payable quarterly in
arrear. Unless previously redeemed or purchased and cancelled the Floating Rate
Notes will be redeemed by the US Issuer on 24 March 2024.
• The $2,000,000,000 3.375 per cent. notes due 2027 (the “2027 3.375 per cent.
Notes”) were issued by the US Issuer and bear interest at a rate of 3.375 per cent.
per annum, payable semi-annually in arrear. Unless previously redeemed or
purchased and cancelled the 2027 3.375 per cent. Notes will be redeemed by the
US Issuer on 24 March 2027.
• The $1,000,000,000 3.375 per cent. notes due 2029 (the “2029 3.375 per cent.
Notes”) were issued by the US Issuer and bear interest at a rate of 3.375 per cent.
per annum, payable semi-annually in arrear. Unless previously redeemed or
purchased and cancelled the 2029 3.375 per cent. Notes will be redeemed by the
US Issuer on 24 March 2029.
• The $2,000,000,000 3.625 per cent. notes due 2032 (the “3.625 per cent. Notes”)
were issued by the US Issuer and bear interest at a rate of 3.625 per cent. per
annum, payable semi-annually in arrear. Unless previously redeemed or
purchased and cancelled the 3.625 per cent. Notes will be redeemed by the US
Issuer on 24 March 2032.
• The $1,000,000,000 4.000 per cent. notes due 2052 (the “4.000 per cent. Notes”)
were issued by the US Issuer and bear interest at a rate of 4.000 per cent. per
annum, payable semi-annually in arrear. Unless previously redeemed or
purchased and cancelled the 4.000 per cent. Notes will be redeemed by the US
Issuer on 24 March 2052.
• The $1,750,000,000 3.125 per cent. notes due 2025 (the “3.125 per cent. Notes”)
were issued by GSK Consumer Healthcare Capital UK plc and bear interest at a
309
rate of 3.125 per cent. per annum, payable semi-annually in arrear. Unless
previously redeemed or purchased and cancelled the 3.125 per cent. Notes will be
redeemed by GSK Consumer Healthcare Capital UK plc on 24 March 2025.
The Pre-Separation USD Notes additionally contain a limited negative pledge and
customary events of default (including cross-acceleration provisions). The occurrence
of any event of default under the Pre-Separation USD Notes would permit, amongst
other things, the acceleration of the Pre-Separation USD Notes.
Each series of the Pre-Separation USD Notes can be redeemed prior to maturity at the
option of the relevant issuer in accordance with the terms and conditions of the
Pre-Separation USD Notes.
The 3.024 per cent. Notes, the 2027 3.375 per cent. Notes, the 2029 3.375 per cent.
Notes, the 3.625 per cent. Notes and the 4.000 per cent. Notes include a make-whole
call option, which permits the US Issuer to redeem the relevant series of notes on not
less than 15 nor more than 60 days’ notice at any time prior to the applicable par call
date set out in the terms and conditions of the Pre-Separation USD Notes (the “Par
Call Date”), subject to payment of the greater of (i) 100 per cent. of the principal
amount of the relevant notes to be redeemed on that redemption date and (ii) the
present value of the remaining scheduled payments of principal and interest that would
be due if the relevant series of the notes matured on the applicable Par Call Date
(subject to a customary discount calculated using an applicable bond reference rate
plus an agreed margin), plus accrued and unpaid interest, if any, thereon to, but
excluding, the redemption date. On or after the applicable Par Call Date, the US Issuer
may redeem the relevant series of notes at a redemption price equal to 100 per cent. of
the principal amount of the applicable series of notes to be redeemed, plus accrued
and unpaid interest, if any, thereon to, but excluding, the redemption date.
The 3.125 per cent. Notes include a make-whole call option, which permits GSK
Consumer Healthcare Capital UK plc to redeem the notes on not less than 15 nor more
than 60 days’ notice at any time, subject to payment of the greater of (i) 100 per cent.
of the principal amount of the notes to be redeemed on that redemption date and
(ii) the present value of the remaining scheduled payments of principal and interest
(subject to a customary discount calculated using an applicable bond reference rate
plus an agreed margin), plus accrued and unpaid interest, if any, thereon to, but
excluding, the redemption date.
The Floating Rate Notes include a par call option, which permits the US Issuer to
redeem the Floating Rate Notes, in whole or in part, at its option at any time and from
time to time on or after 24 March 2023 at a redemption price equal to 100 per cent. of
the principal amount of the Floating Rate Notes to be redeemed, plus accrued and
unpaid interest, if any, thereon to, but excluding, the redemption date. Notwithstanding
the foregoing, instalments of interest on the Floating Rate Notes to be redeemed that
are due and payable on a Floating Rate Notes interest payment date falling on or prior
to a redemption date will be payable on the Floating Rate Notes interest payment date
to the registered holders as of the close of business on the relevant regular record date
according to the Floating Rate Notes and the Indenture, as applicable.
The payment of all amounts owing in respect of the Pre-Separation USD Notes is, as
at the date of this Prospectus, guaranteed by GSK. Following completion of the GSK
Share Exchange, the guarantee provided by GSK will cease to be effective and a
guarantee provided by the Company will come into full force and effect.
(C) Revolving Credit Facilities
On 18 February 2022, CH JVCo entered into syndicated revolving credit facilities (the
“Revolving Credit Facilities” and loans extended thereunder the “RCF Loans”). The
commitments under the Revolving Credit Facilities are provided by (i) Banco Bilbao
310
Vizcaya Argentaria, S.A., London Branch; (ii) Banco Santander, S.A., London Branch;
(iii) Bank of America, N.A.; (iv) Bank of America, N.A., London Branch; (v) Barclays
Bank PLC; (vi) BNP Paribas, London Branch; (vii) Citibank, N.A.; (viii) Citibank, N.A.,
London Branch; (ix) Deutsche Bank AG, London Branch; (x) Deutsche Bank AG New
York Branch; (xi) Goldman Sachs Bank USA; (xii) HSBC Bank plc; (xiii) ING Bank N.V.,
London Branch; (xiv) JPMorgan Chase Bank, N.A.; (xv) JPMorgan Chase Bank, N.A.,
London Branch; (xvi) Lloyds Bank plc; (xvii) Mizuho Bank, Ltd.; (xviii) Morgan Stanley
Bank N.A.; (xix) Royal Bank of Canada; and (xx) Standard Chartered Bank (Hong
Kong) Limited.
The initial borrower under each of the Revolving Credit Facilities is CH JVCo but,
following completion of the GSK Share Exchange and in accordance with the terms of
the Revolving Credit Facilities, the Company will accede to the Revolving Credit
Facilities and replace CH JVCo as borrower under the Revolving Credit Facilities (the
borrower under the Revolving Credit Facilities from time-to-time, the “RCF Borrower”).
Following its accession as borrower under the Revolving Credit Facilities, the Company
will guarantee the obligations of any other member of the Group that accedes to the
Revolving Credit Facilities as an additional borrower.
The Revolving Credit Facilities provide the RCF Borrower with access to:
• a multicurrency facility denominated in Pounds Sterling, with a commitment of
£1,000,000,000 and an initial maturity date of 24 September 2025 (the “GBP
Facility”); and
• a US Dollar facility, incorporating a swingline facility (the “ Swingline Facility”),
with an aggregate commitment of $1,400,000,000 and an initial maturity date of
24 September 2023 (the “USD Facility”).
As at the Latest Practicable Date, each of the GBP Facility and the USD Facility is
undrawn.
With certain exceptions, RCF Loans bear interest at a rate equal to the aggregate of:
(i) the applicable risk free rate (which for loans drawn in Pounds Sterling is the Bank of
England’s Sterling Overnight Interbank Average Rate (‘SONIA’)) and for loans drawn in
US dollars is the New York Federal Reserves Secured Overnight Financing Rate
(‘SOFR’)) and (ii) a margin determined in accordance with the terms of the Revolving
Credit Facilities, which is dependent on the corporate rating assigned to the Company.
The proceeds of each RCF Loan are available for the general corporate purposes of
the Group and such specific purposes as may be determined by the RCF Borrower.
The Swingline Facility is available for financing or refinancing the payment of (or in
respect of) any indebtedness or other obligations of the Group (including commercial
paper, but excluding any other drawing from the Swingline Facility).
The Revolving Credit Facilities require the RCF Borrower to make certain customary
representations and warranties at various times throughout the term of the Revolving
Credit Facilities. In addition, the terms of the Revolving Credit Facilities contain
customary restrictions on the operations of the RCF Borrower and the Group. These
include customary positive and negative covenants, including a negative pledge and,
with effect from the GSK Share Exchange, restrictions on disposals. The Revolving
Credit Facilities do not contain any financial covenants, but the RCF Borrower is
required to comply with certain information covenants, including the delivery of financial
information.
The Revolving Credit Facilities contain customary events of default, including cross-
acceleration provisions. The occurrence of any event of default under the Revolving
Credit Facilities at a time when any RCF Loans are outstanding would permit, amongst
other things, the acceleration of all RCF Loans.
311
(D) Term Loan Facility
On 18 February 2022, CH JVCo entered into a term loan facility with a total
commitment of £1,500,000,000 (the “Term Loan Facility”) provided by (i) Bank of
America, N.A., London Branch; (ii) Banco Santander, S.A., London Branch;
(iii) Barclays Bank PLC; (iv) BNP Paribas Fortis SA/NV; (v) BNP Paribas; (vi) Citibank,
N.A., London Branch; (vii) Deutsche Bank AG, London Branch; (viii) Goldman Sachs
Bank USA; (ix) HSBC Bank plc; (x) JPMorgan Chase Bank, N.A., London Branch;
(xi) Mizuho Bank, Ltd.; (xii) Morgan Stanley Bank N.A.; and (xiii) Standard Chartered
Bank (Hong Kong) Limited.
The payment of amounts owing in respect of the Term Loan Facility are, as at the date
of this Prospectus, not guaranteed. Following completion of the GSK Share Exchange,
the Company will accede to the Term Loan Facility as a guarantor of the Term Loan
Facility in accordance with the terms of the Term Loan Facility.
The Term Loan Facility is denominated in Pounds Sterling and permits a single term
loan to be borrowed. As at the Latest Practicable Date no amount has been borrowed
under the Term Loan Facility.
Any loan drawn under the Term Loan Facility will bear interest at a rate equal to the
aggregate of: (i) the applicable risk free rate (being the Bank of England’s Sterling
Overnight Interbank Average Rate (‘SONIA’)); and (ii) a margin determined in
accordance with the terms of the Term Loan Facility, which is dependent on the
corporate rating assigned to the Company.
The Term Loan Facility is made available on customary ‘certain funds’ terms and the
proceeds of any utilisation under the Term Loan Facility are available for use, directly
or indirectly, towards the payment of the Pre-Demerger Dividend. The Term Loan
Facility has a maturity date falling 36 months after the date on which it was entered
into.
The Term Loan Facility requires CH JVCo and, from the point at which it accedes to
the Term Loan Facility, the Company to make certain customary representations and
warranties at various times throughout the term of the Term Loan Facility. In addition,
the Term Loan Facility contains customary restrictions on the operations of CH JVCo,
the Group and, from the point at which it accedes to the Term Loan Facility, the
Company. These include customary positive and negative covenants, including a
negative pledge and, with effect from the GSK Share Exchange, restrictions on
disposals. The Term Loan Facility does not contain any financial covenants, but CH
JVCo and, from the point at which it accedes to the Term Loan Facility, the Company
are required to comply with certain information covenants, including the delivery of
financial information.
The Term Loan Facility contains customary events of default, including cross-
acceleration provisions. The occurrence of any event of default under the Term Loan
Facility at a time when any amount is outstanding under the Term Loan Facility would
permit, amongst other things, the acceleration of such amounts.
15.14 Transition Services Agreement
In connection with the Separation, GlaxoSmithKline Services Unlimited, GlaxoSmithKline LLC,
GlaxoSmithKline Consumer Healthcare (Overseas) Limited and GlaxoSmithKline Consumer
Healthcare Holdings (US) LLC entered into a Transition Services Agreement on or around the
date of this Prospectus (the “Transition Services Agreement”), pursuant to which each group
will provide limited services to the other on commercial terms and on an arms’ length basis for
a transitional period, effective from completion of the Demerger.
312
Under the Transition Services Agreement, the GSK Group will provide services to the Group
including: (i) the continued provision of and access to technology applications and platforms;
(ii) the continued provision of various back office services and support across finance, facilities
and office space; (iii) supply chain (including quality, warehouse, distribution and logistics
support); and (iv) certain other services, including regulatory compliance and
pharmacovigilance. The Group will also provide certain limited reverse services to the GSK
Group.
Term and Termination
The Transition Services Agreement provides that the majority of services will be provided for a
fixed period of not more than 12 months, and certain services may be extended subject to
certain conditions. No fixed period service will continue beyond 24 months unless an extension
is required as a result of force majeure, the service provider’s material breach, applicable law
or an act or omission of a regulator. For event-based services, which are linked to an external
trigger/event, the default position is that the service will be provided until such trigger / event
occurs, plus an additional tail period of up to 6 months. These services can be extended for a
period equalling 6 months (less the duration of any tail period).
The service recipient under the Transition Services Agreement may, with respect to any
service, terminate (in whole or in part) such service: (a) for convenience upon giving at least 90
days’ notice to the service provider (such early termination may be subject to early termination
costs); and (b) for services provided in connection with a delayed asset (i.e. an asset used by
the service recipient to conduct the Consumer Healthcare Business as at the Demerger but
which has not yet been transferred to the service recipient on the effective date of the
Transition Services Agreement) provided 60 days’ notice is given to the service provider. The
service provider may terminate a service (in whole or in part) in the event its contract with a
subcontractor who provides the service is terminated. In addition, if a force majeure event
arises, then where the service recipient has obtained the affected service(s) from a substitute
source, the service recipient may terminate the agreement in respect of such affected
service(s) if the service recipient wishes to continue with such substitute source, with the
relevant exit costs under the applicable work package being paid to the service provider on
termination.
Either the service provider or the service recipient may terminate the Transition Services
Agreement upon prior written notice (in the case of limb (ii), of at least 90 days wherever
possible) to the other party if: (i) the other party has materially breached or materially failed to
perform any of its obligations under the agreement, and such breach has not been remedied
within a cure period of at least 45 days after receipt of written notice of such failure by the
non-breaching party; or (ii) the other party suffers an insolvency event. GlaxoSmithKline
Services Unlimited is not entitled to terminate the agreement due to any non-compliance of the
Consumer Healthcare Business with certain policies, procedures and practices of the service
provider or its affiliates or applicable laws before the effective date of the Transition Services
Agreement or any circumstances or conditions with respect to the Consumer Healthcare
Business that existed prior to the effective date of the Transition Services Agreement, provided
that GlaxoSmithKline Consumer Healthcare (Overseas) Limited must use all reasonable
endeavours to remediate such noncompliance or conditions or circumstances.
Indemnities
Pursuant to the Transition Services Agreement, the service provider has agreed to indemnify
the service recipient and its affiliates in respect of losses resulting from the fraud, gross
negligence or wilful misconduct of the service provider or the service provider’s affiliates and
subcontractors and certain liabilities relating to service provider employees.
Pursuant to the Transition Services Agreement, the service recipient has agreed to indemnify
the service provider in respect of losses resulting from the provision of services under the
Transition Services Agreement, except where such losses result from: (i) the fraud, gross
negligence or wilful misconduct of the service provider or the service provider’s affiliates or
313
subcontractors; (ii) a breach of the Transition Services Agreement or local country agreements
by the service provider or the service provider’s affiliates or subcontractors; (iii) certain
liabilities relating to service provider employees; or (iv) any third party IP violation resulting
from the service provider not seeking a required consent.
15.15 Manufacturing and Supply Agreements
In connection with the Separation, each of GlaxoSmithKline Trading Services Limited and
GlaxoSmithKline Consumer Trading Services Limited entered into two separate (but mirrored
in all material respects) Manufacturing and Supply Agreements with the other on or around the
date of this Prospectus (each a “Manufacturing and Supply Agreement” and together, the
“Manufacturing and Supply Agreements”). Pursuant to each Manufacturing and Supply
Agreement, one party will, to the extent required, supply the other with pharmaceutical or
Consumer Healthcare Products (as the case may be) from the relevant manufacturing sites
owned by each Group after the Demerger on commercial terms and on an arms’ length basis.
Each Manufacturing and Supply Agreement provides for the supply of pharmaceutical or
Consumer Healthcare Products (as applicable) from the relevant sites at which the same
products were manufactured prior to the Demerger and which have not transferred to the
relevant party that requires receipt of the supply. The products will either be supplied on a full
service or toll/consignment basis for consistency, with pricing subject to: (i) a margin applied to
the standard cost (in the case of full service) or conversion cost (in the case of toll/
consignment); and (ii) an annual price review and an annual payment, if net costs for materials
and freight have increased.
Term and Termination
Each Manufacturing and Supply Agreement will run for an initial period of five years, with a
potential extension for 12 months and otherwise, by agreement, terminable for convenience
from year three, by either party, upon (i) with respect to the GlaxoSmithKline Trading Services
Limited to GlaxoSmithKline Consumer Trading Services Limited Manufacturing and Supply
Agreement, giving 18 months’ written notice; and (ii) with respect to the GlaxoSmithKline
Consumer Trading Services Limited to GlaxoSmithKline Trading Services Limited
Manufacturing and Supply Agreement, giving 24 months’ written notice. Each party may
otherwise terminate the agreement immediately: (i) in whole or in part (including on a
product-by-product basis) at any time by mutual written agreement; (ii) for the other party’s
material breach of the agreement or applicable law, which if capable of remedy, is not
remedied within a cure period of 30 days; (iii) where the other party has failed to comply with
anti-bribery and corruption requirements; (iv) if the other party suffers an insolvency event; (v) if
a force majeure circumstance arises; or (vi) in respect of a specific product and relevant
territory if: (x) the marketing authorisation for such product is revoked by a governmental entity
due to a health, safety or efficacy concern; or (y) any governmental entity intervenes to prevent
manufacture of that product for a significant technical or regulatory reason.
Warranties and Indemnities
Each Manufacturing and Supply Agreement contains customary warranty and indemnity
provisions.
16. SIGNIFICANT CHANGE
There has been no significant change in the financial position or financial performance of the
Group since 31 December 2021, being the date to which the latest historical financial
information of the Group was published.
17. WORKING CAPITAL STATEMENT
The Company is of the opinion that, after taking into account the bank and other facilities
available, the working capital available for the Group is sufficient for its present requirements,
that is, for at least the next 12 months from the date of publication of this Prospectus.
314
18. CONSENTS
The Company has received the following written consents, which are available for inspection at
the times and locations set out in paragraph 22 of this Part XII (Additional Information) below,
in connection with the publication of this Prospectus:
(A) Deloitte has given and not withdrawn its written consent to the inclusion in this
Prospectus of:
(i) the report set out in paragraph B of Part IX (Unaudited Pro Forma Financial
Information of the Group);
(ii) the report set out in Section A of Schedule II (Historical Financial Information of
GlaxoSmithKline Consumer Healthcare Holdings (No.2) Limited); and
(iii) the report set out in Section A of Schedule III (Pfizer Historical Financial
Information),
and has authorised the contents of its reports for the purposes of item 5.3.2R(2)(f) of
the Prospectus Regulation Rules.
19. AUDITOR
The auditor of GlaxoSmithKline Consumer Healthcare Holdings (No.2) Limited since the date
of its incorporation has been Deloitte, whose registered office is at 1 New Street Square,
London, EC4A 3HQ. Deloitte is registered to carry out audit work in the United Kingdom by the
Institute of Chartered Accountants in England and Wales. Deloitte has audited the statutory
consolidated annual accounts of GlaxoSmithKline Consumer Healthcare Holdings (No.2)
Limited as at and for the years ended 31 December 2021, 2020 and 2019.
The Company was incorporated on 20 October 2021. On 3 February 2022, the Company
appointed Deloitte as its UK statutory auditor for the year ending 31 December 2022.
In connection with the Company’s registration of securities under the U.S. Securities Exchange
Act of 1934, on 24 March 2022, the Company appointed KPMG LLP to audit the Company’s
financial statements for the year ending 31 December 2022 under the rules of the SEC and
U.S. Public Company Accounting Oversight Board (PCAOB).
The Company expects to conduct an audit tender process for the audit of the Company’s
financial statements for the year ending 31 December 2023, which would be subject to
shareholder approval at the Company’s 2023 annual general meeting.
20. FRUSTRATING ACTIONS, MANDATORY BIDS AND COMPULSORY ACQUISITION RULES
RELATING TO ORDINARY SHARES
Other than as provided by the City Code and Chapter 28 of the Companies Act, there are no
rules or provisions relating to frustrating actions, mandatory bids and/or squeeze-out and
sell-out rules relating to the Company.
20.1 Frustrating actions
The City Code applies to the Company. During the course of an offer period, or when the board
has reason to believe that a bona fide offer may be imminent, Rule 21.1 of the City Code
prohibits the board from taking any action which may result in an offer or bona fide possible
offer being frustrated, or in shareholders being denied the opportunity to decide on its merits,
or from taking certain frustrating actions, without the approval of shareholders in a general
meeting.
315
20.2 Mandatory bids
Rule 9.1 of the City Code states that, except with the consent of the Takeover Panel, when:
(A) any person acquires, whether by a series of transactions over a period of time or not,
an interest in shares which (taken together with shares in which persons acting in
concert with that person are interested) carry 30 per cent. or more of the voting rights
of a company; or
(B) any person, together with persons acting in concert with that person, is interested in
shares which in the aggregate carry not less than 30 per cent. of the voting rights of a
company, but does not hold shares carrying more than 50 per cent. of such voting
rights, and such person, or any persons acting in concert with that person, acquires an
interest in any other shares which increases the percentage of the shares carrying
voting rights in which that person is interested,
such person shall extend offers, on the basis set out in Rules 9.3, 9.4 and 9.5 of the City Code,
to the holders of any class of equity share capital whether voting or non-voting and also to the
holders of any other class of transferable securities carrying voting rights. Offers for different
classes of equity share capital must be comparable and the Takeover Panel should be
consulted in advance in such cases.
Persons acting in concert (and concert parties) comprise persons who, pursuant to an
agreement or understanding (whether formal or informal), co-operate to obtain or consolidate
control of a company or to frustrate the successful outcome of an offer for a company. Certain
categories of people are deemed under the City Code to be acting in concert with each other
unless the contrary is established.
“Interests in shares” is defined broadly in the City Code. A person who has long economic
exposure, whether absolute or conditional, to changes in the price of shares will be treated as
interested in those shares. A person who only has a short position in shares will not be treated
as interested in those shares.
“Voting rights” for these purposes means all the voting rights attributable to the share capital of
a company which are then exercisable at a general meeting.
20.3 Authority of the Company to redeem or purchase its own shares
When a company redeems or purchases its own voting shares, under Rule 37 of the City Code
any resulting increase in the percentage of shares carrying voting rights in which a person or
group of persons acting in concert is interested will be treated as an acquisition for the purpose
of Rule 9 of the City Code. Rule 37 of the City Code provides that, subject to prior consultation,
the Takeover Panel will normally waive any resulting obligation to make a general offer if there
is a vote of independent shareholders and a procedure along the lines of that set out in
Appendix 1 to the City Code is followed. Appendix 1 to the City Code sets out the procedure
which should be followed in obtaining that consent of independent shareholders. Under Note 1
on Rule 37.1 of the City Code, a person who comes to exceed the limits in Rule 9.1 in
consequence of a company’s redemption or purchase of its own shares will not normally incur
an obligation to make a mandatory offer unless that person is a director, or the relationship of
the person with any one or more of the directors is such that the person is, or is presumed to
be, acting in concert with any of the directors. A person who has appointed a representative to
the board of the company will be treated for these purposes as a director. However, there is no
presumption that all the directors (or any two or more directors) are acting in concert solely by
reason of a proposed redemption or purchase by a company of its own shares, or the decision
to seek shareholders’ authority for any such purchase. For so long as the Pfizer Directors (or
any other directors nominated by Pfizer or its concert parties from time to time) are director(s)
of the Company, Note 1 on Rule 37.1 of the City Code will not exempt Pfizer (or its concert
parties) from the effects of Rule 37.1 of the City Code.
316
Under Note 2 on Rule 37.1 of the City Code, the exception in Note 1 on Rule 37.1 described
above will not apply, and an obligation to make a mandatory offer may therefore be imposed, if
a person (or any relevant member of a group of persons acting in concert) has acquired an
interest in shares at a time when they had reason to believe that such a redemption or
purchase by the company of its own shares would take place. Note 2 will not normally be
relevant unless the relevant person knows that a purchase for which requisite shareholder
authority exists is being, or is likely to be, implemented (whether in whole or in part).
The Takeover Panel must be consulted in advance in any case where Rule 9 of the City Code
might be relevant. This will include any case where a person or group of persons acting in
concert is interested in shares carrying 30 per cent. or more but does not hold shares carrying
more than 50 per cent. of the voting rights of a company, or may become interested in 30 per
cent. or more on full implementation of the proposed purchase or redemption by the company
of its own shares. In addition, the Takeover Panel should always be consulted if the aggregate
interests in shares of the directors and any other persons acting in concert, or presumed to be
acting in concert, with any of the directors amount to 30 per cent. or more, or may be increased
to 30 per cent. or more on full implementation of the proposed purchase or redemption by the
company of its own shares.
Subject to certain limits, the Company has authority to purchase Haleon Shares under the
terms of the shareholder resolution summarised in paragraph 4.6 of this Part XII (Additional
Information) above (the “Buyback Authority”). The maximum aggregate number of Haleon
Shares authorised to be purchased under the Buyback Authority is 10 per cent. of the
Company’s issued share capital immediately following Admission (subject to the conditions
summarised in paragraph 4.6 of this Part XII (Additional Information) above).
The Buyback Authority is due to expire at the conclusion of the first annual general meeting of
the Company (or, if earlier, at the close of business on 30 June 2023) but, in each case, so that
the Company may, before the expiry of the Buyback Authority, enter into a contract to
purchase Haleon Shares which will or may be executed wholly or partly after the expiry of such
Buyback Authority.
If, prior to such expiry: (a) the Company were to exercise the Buyback Authority in full; (b) the
aggregate percentage beneficial holding of Pfizer in the Company immediately following
Admission is approximately 32 per cent. of the issued share capital of the Company; and
(c) none of the Haleon Shares which Pfizer holds are purchased by the Company under the
Buyback Authority and no Haleon Shares have been newly issued by the Company between
the date of Admission and the date that the Buyback Authority is fully exercised, then the
shareholding of Pfizer in the Company would increase to approximately 35.56 per cent. This
increase would be less to the extent that any of the Haleon Shares held by Pfizer are
purchased by the Company.
In the present case, if the Company were to exercise its Buyback Authority then the effect of
Rule 37.1 of the City Code is that, unless independent Haleon Shareholders approve a waiver
of Rule 9 or the Takeover Panel grants a dispensation, a mandatory offer by Pfizer under Rule
9 would be required.
In respect of the period from Admission up to the conclusion of the first annual general meeting
of the Company (or, if earlier, at the close of business on 30 June 2023), the Takeover Panel
has confirmed that, notwithstanding Rule 37.1 of the City Code, any potential increase in the
shareholding of Pfizer in the Company as a result of the exercise of the Buyback Authority will
not require Pfizer to make a mandatory offer pursuant to Rule 9 of the City Code, and therefore
a resolution of the independent shareholders of the Company will not be necessary. This
confirmation has been given on the basis that: (a) the Buyback Authority was passed on
23 May 2022; and (b) the consequences of any buyback pursuant to the Buyback Authority
have been fully disclosed in this Prospectus.
For so long as Pfizer holds in aggregate an interest in 30 per cent. or more of the voting rights
in the Company and subject (where necessary) to the prior consent of the Takeover Panel, the
Company has undertaken to procure that at the first annual general meeting of the Company
317
and thereafter once in every calendar year, the Company will propose to its independent
shareholders a resolution to waive, in accordance with Appendix 1 to the City Code, all
obligations of Pfizer to make a mandatory offer under Rule 9 that may otherwise arise as a
result of the Company purchasing or effecting any other transaction in relation to the Haleon
Shares.
20.4 Squeeze-out rules
Under the Companies Act, if a “takeover offer” (as defined in section 974 of the Companies
Act) is made by an offeror to acquire all of the shares in the Company not already owned by it
and the offeror were to acquire, or unconditionally contract to acquire, not less than 90 per
cent. in value of the shares to which such offer relates, and not less than 90 per cent. of the
voting rights attached to such shares, within three months of the last day on which its offer can
be accepted, the offeror could then compulsorily acquire the remaining shares not assented to
the offer. The offeror would do so by sending a notice to the outstanding members informing
them that it will compulsorily acquire their shares and, six weeks later, it would deliver a
transfer of the outstanding shares in its favour to the Company which would execute the
transfers on behalf of the relevant members, and pay the consideration for the outstanding
shares to the Company which would hold the consideration on trust for the relevant members.
The consideration offered to the members whose shares are compulsorily acquired under this
procedure must, in general, be the same as the consideration that was available under the
original offer unless a member can show that the offer value is unfair.
20.5 Sell-out rules
The Companies Act also gives minority members a right to be bought out in certain
circumstances by an offeror who has made a takeover offer. If a takeover offer related to all the
shares in the Company and, at any time before the end of the period within which the offer
could be accepted, the offeror held or had agreed to acquire not less than 90 per cent. in value
of the shares and not less than 90 per cent. of the voting rights carried by the shares in the
Company, any holder of shares to which the offer related who had not accepted the offer could
by a written communication to the offeror require it to acquire those shares. The offeror would
be required to give any member notice of his or her right to be bought out within one month of
that right arising. The offeror may impose a time limit on the rights of minority members to be
bought out, but that period cannot end less than three months after the end of the acceptance
period or, if later, three months from the date on which notice is served on members notifying
them of their sell-out rights. If a member exercises his or her rights, the offeror is entitled and
bound to acquire those shares on the terms of the offer or on such other terms as may be
agreed.
21. LIMITATIONS ON ENFORCEMENT OF US LAWS
The Company is a global consumer healthcare products company domiciled in the UK. Many
of the Directors and executive officers (as well as certain directors, managers and executive
officers of the Company’s finance subsidiaries) reside outside the USA, and a substantial
portion of the Group’s assets and the assets of such persons are located outside the USA. As
a result, it may be difficult for holders of Haleon Shares to serve legal process on the Company
or the Directors and executive officers or have any of them appear in a US court. There is
some doubt as to the enforceability in the UK, in original actions or in actions for enforcement
of judgments of US courts, of civil liabilities based solely on the federal securities laws of the
USA. In addition, awards for punitive damages in actions brought in the USA or elsewhere may
be unenforceable in the UK.
22. DOCUMENTS AVAILABLE FOR INSPECTION
Copies of the following documents may be inspected at www.haleon.com for a period of
12 months from the date of publication of this Prospectus:
• the Articles of Association;
• the written consent letters referred to in paragraph 18 of this Part XII (Additional
Information) above;
318
• the report by Deloitte set out in Section B of Part IX (Unaudited Pro Forma Financial
Information of the Group);
• the report by Deloitte set out in Section A of Schedule II (Historical Financial Information of
GlaxoSmithKline Consumer Healthcare Holdings (No.2) Limited);
• the report by Deloitte set out in Section A of Schedule III (Pfizer Historical Financial
Information); and
• this Prospectus.
For the purposes of Rule 3.2 of the Prospectus Regulation Rules, this Prospectus will be
published in printed form and available free of charge, during normal business hours on any
weekday (Saturdays, Sundays and public holidays excepted) for a period of 28 days from the
date of publication of this Prospectus at 980 Great West Road, Brentford, Middlesex TW8 9GS,
United Kingdom. In addition, the Prospectus will be published in electronic form and be
available at www.haleon.com.
319
Schedule I
DEFINITIONS AND GLOSSARY
“A&P” advertising and promotion;
“Admission” admission of the Haleon Shares to the premium listing segment
of the Official List and to trading on the LSE’s main market for
listed securities;
“ADR” American depositary receipt evidencing ADSs;
“ADS” American depositary share;
“ADS Holder Record Time” 5 p.m. New York City time on 15 July 2022;
“ADS Holder Voting Record
Time”
5 p.m. New York City time on 27 May 2022;
“ADS Holders” the holders of GSK ADSs;
“ADR Programme” the American depositary receipt programme to be established for
the Company on or around Admission;
“Anacor” Anacor Pharmaceuticals, Inc., a corporation incorporated under
the laws of Delaware whose registered office is at 235 East 42nd
Street, New York, New York 10017;
“APAC” Asia Pacific;
“Articles of Association” the articles of association of the Company from time to time;
“Asset Transfer Framework
Agreement”
has the meaning given in paragraph 15.4 of Part XII (Additional
Information);
“Assumed Liabilities ” has the meaning given in paragraph 15.2 of Part XII (Additional
Information);
“Audit & Risk Committee” the audit and risk committee of the Company;
“Balancing Dividend” has the meaning given in paragraph 1.2 of Part IV (Overview of
the Demerger and Separation);
“Board” the board of directors of the Company from time to time;
“BofA Securities” Merrill Lynch International;
“Buyback Authority” the Company’s authority to purchase Haleon Shares under the
terms of the shareholder resolution summarised in paragraph 4.6
of Part XII (Additional Information);
“CAGR” compound annual growth rate;
320
“Capital Reduction” the reduction of capital to be implemented by the Company in
accordance with Section 641(1)(b) of the Companies Act
pursuant to which the Company shall:
(A) cancel and extinguish £1.24 of the nominal value of each
Haleon Share; and
(B) cancel and extinguish all amounts standing to the credit
of the Company’s share premium account,
with all amounts so reduced being credited to the Company’s
profit and loss reserve;
“certificated” or “in certificated
form”
in relation to a share or other security, a share or other security
title to which is recorded in the relevant register of the share or
other security concerned as being held in certificated form (that
is, not in CREST);
“cGMP” current Good Manufacturing Practices;
“Chair” the chair of the Board;
“CH JV Group” CH JVCo together with its subsidiaries and subsidiary
undertakings from time to time;
“CH JVCo” GlaxoSmithKline Consumer Healthcare Holdings (No.2) Limited;
“Citi” Citigroup Global Markets Limited;
“City Code” the City Code on Takeovers and Mergers;
“Consignment Selling
Agreement”
the consignment selling agreement entered into between
Hindustan Unilever Limited and GlaxoSmithKline Asia Private
Limited dated 1 April 2020;
“Companies Act” the Companies Act 2006 of the UK, as amended;
“Company” Haleon plc, a public limited company incorporated in England and
Wales with registered number 13691224 whose registered office
is 980 Great West Road, Brentford, Middlesex, United Kingdom,
TW8 9GS;
“Consumer Healthcare
Business”
(A) prior to Separation, the business of researching and
developing, manufacturing, distributing, marketing,
selling, promoting and/or otherwise commercialising
Consumer Healthcare Products, in each case as
conducted by the CH JV Group as at the date of this
Prospectus; and
321
(B) following Separation, the business of researching and
developing, manufacturing, distributing, marketing,
selling, promoting and/or otherwise commercialising
Consumer Healthcare Products, in each case as
conducted by the Haleon Group, together with any assets
and/or entities that will form part of the Group pursuant to
the Asset Transfer Framework Agreement and other
ancillary and implementing agreements;
“Consumer Healthcare
Products”
has the meaning given in paragraph 15.2 of Part XII (Additional
Information);
“Court” the High Court of Justice in England and Wales;
“Court Order” an order of the Court confirming the Capital Reduction, granted
pursuant to section 648 of the Companies Act;
“CREST” the system for the paperless settlement of trades in securities and
the holding of uncertificated securities in accordance with the
CREST Regulations operated by Euroclear;
“CREST Manual” the rules governing the operation of CREST as published by
Euroclear;
“CREST Proxy Instruction” a proxy appointment or instruction made via CREST,
authenticated in accordance with Euroclear’s specifications and
containing the information set out in the CREST Manual;
“CREST Regulations” the Uncertificated Securities Regulations 2001 (SI 2001
No. 3755), as amended;
“Delayed Demerger Completion
Date”
has the meaning given in paragraph 15.6 of Part XII (Additional
Information);
“Deloitte” Deloitte LLP;
“Demerger” the proposed demerger of the predominant part of GSK plc’s
interest in the CH JV Group, to be effected by way of the
Demerger Dividend on the terms and subject to the conditions set
out in the Demerger Agreement;
“Demerger Agreement” has the meaning given in paragraph 15.6 of Part XII (Additional
Information);
“Demerger Completion Steps” (A) the delivery by GSK plc to the Company of a duly
executed transfer of the GSKCHH A Ordinary Shares in
favour of the Company, together with the relevant share
certificate(s);
(B) the procurement by the Company that the names of the
Qualifying GSK Shareholders to whom Haleon Shares
are to be allotted and issued pursuant to the Demerger
322
Agreement are entered into the Company register of
members; and
(C) the delivery, or procurement thereof, by each of GSK plc
and the Company of duly executed copies of certain
agreements to effect the structural and operational
separation of the Consumer Healthcare Business from
GSK plc and related ancillary agreements;
“Demerger Dividend” the interim dividend, in specie, proposed to be declared by the
GSK Board to be satisfied by: (i) the transfer by GSK of the
GSKCHH A Ordinary Shares to the Company in consideration for:
(ii) the issuance by the Company of Haleon Shares to Qualifying
GSK Shareholders in accordance with the Demerger Agreement;
“Demerger Resolution” the ordinary resolution numbered 1, set out in the Notice of GSK
General Meeting;
“Designated Person” (A) any person listed on a Sanctions List (as defined in the
Articles of Association); or (B) any other person, in each case
where it would be unlawful, by virtue of any Sanctions Law (as
defined in the Articles of Association) applicable to the Company,
for the Company or any of its directors, officers, or employees to
make available to such person, or to otherwise facilitate dealings
by such person in, any shares in the company or the benefit of
any rights attaching to such shares;
“Directors” the directors of the Company as at the date of this Prospectus
and those persons who will become directors of the Company on
Admission, whose names are all set out in paragraph 1 of Part V
(Directors, Senior Managers, Corporate Governance and
Remuneration);
“Disclosure Guidance and
Transparency Rules”
the disclosure guidance and transparency rules made by the FCA
under Part VI of FSMA (as set out in the FCA’s Handbook of
Rules and Guidance), as amended;
“EEA” the European Economic Area;
“EMA” the European Medicines Agency;
“EMEA and LatAm” Europe, Middle East and Africa and Latin America;
“EMTN Issuers” GSK Consumer Healthcare Capital UK plc and GSK Consumer
Healthcare Capital NL B.V.;
“Equiniti FS” Equiniti Financial Services Limited, a private company registered
in England and Wales with registered number 06208699 whose
registered office is Aspect House, Spencer Road, Lancing, West
Sussex BN99 6DA, being the FCA authorised and regulated
entity that provides and manages the GSK CSN and the Haleon
CSN;
“ERP” enterprise resource planning;
323
“ESG” environmental, social and governance;
“EU” the European Union;
“EU GDPR” or “GDPR” Regulation (EU) No 2016/679 of the European Parliament and of
the Council of 27 April 2016 on the protection of natural persons
with regard to the processing of personal data and on the free
movement of such data, and repealing Directive 95/46/EC
(General Data Protection Regulation), as amended;
“EU Member State” or “Member
State”
a member state of the EU;
“Euro” o r “€” the lawful currency of the EU;
“Euroclear” Euroclear UK & International Limited, the operator of CREST;
“Excess GSK Shares” any GSK Shares in issue at the Shareholder Record Time in
excess of (X) ((X) being the number of GSK Shares in issue at
the Latest Practicable Date);
“Exchange Agreements” the GSK Exchange Agreement, the SLP Exchange Agreement
and the Pfizer Exchange Agreement;
“FCA” the Financial Conduct Authority of the UK;
“FDA” the US Food and Drug Administration;
“FMCG” fast-moving consumer goods;
“Form of Direction” the voting form for GSK CSN holders to vote at the GSK General
Meeting;
“Form 20-F” a registration statement on Form 20-F under the US Exchange
Act;
“FSMA” the Financial Services and Markets Act 2000 of the UK, as
amended;
“FTC” the US Federal Trade Commission;
“GBP Facility” has the meaning given in paragraph 7.4 of Part VII (Operating
and Financial Review);
“GMP” Good Manufacturing Practice;
“Goldman Sachs” Goldman Sachs International;
“Group” (A) prior to Separation, the CH JV Group; and
(B) following Separation, the Haleon Group;
“GSK” GSK plc, a public limited company incorporated in England and
Wales with registered number 038887792 whose registered office
is at 980 Great West Road, Brentford, Middlesex, TW8 9GS;
324
“GSK ADS” an ADS representing two GSK Shares, evidenced by an ADR;
“GSK Board” the board of directors of GSK plc from time to time;
“GSK Contributed CH
Business”
has the meaning given in paragraph 15.2 of Part XII (Additional
Information);
“GSK CSN” the GSK plc Nominee Service provided by Equiniti FS;
“GSK Exchange Agreement” has the meaning given in paragraph 15.9 of Part XII (Additional
Information);
“GSK General Meeting” the general meeting of GSK plc proposed to be held at 2.30 p.m.
London time on 6 July to approve the Demerger Resolution and
certain other resolutions;
“GSK Group” in respect of any time prior to the Demerger, GSK plc and its
subsidiaries and subsidiary undertakings from time to time; and in
respect of any period following the Demerger, the Post-Demerger
GSK Group;
“GSK/Novartis JV” has the meaning given in paragraph 15.1 of Part XII (Additional
Information);
“GSK/Pfizer JV” has the meaning given in paragraph 15.2 of Part XII (Additional
Information);
“GSK Share Exchange” has the meaning given in paragraph 1.1 of Part IV (Overview of
the Demerger and Separation);
“GSK Shareholder” a holder of GSK Shares from time to time;
“GSK Shareholder Circular” the circular published by GSK plc in relation to the Demerger;
“GSK Shares” the fully paid ordinary shares in the capital of GSK plc;
“GSK Sponsors’ Agreement” the Sponsors’ Agreement entered into between GSK and the
Joint Sponsors on or around the date of this document in
connection with Separation;
“GSKCHH” GlaxoSmithKline Consumer Healthcare Holdings Limited, the
GSK subsidiary which holds GSK’s interests in CH JVCo;
“GSKCHH A Ordinary Shares” the fully paid A Ordinary Shares in the capital of GSKCHH;
“GSKCHH B Ordinary Shares” the fully paid B Ordinary Shares in the capital of GSKCHH;
“GSKCHH C Ordinary Shares” the fully paid C Ordinary Shares in the capital of GSKCHH;
“Haleon ADSs” the American depositary shares, each representing 2 Haleon
Shares;
“Haleon CSN” the Haleon nominee service provided by Equiniti FS;
“Haleon Group” the Company together with its subsidiaries and subsidiary
undertakings from time to time;
325
“Haleon Shareholder” a holder of Haleon Shares from time to time;
“Haleon Shares” the fully paid ordinary shares of £1.25 each in the capital of the
Company;
“Historical Financial
Information”
the historical financial information of the Group set out in
Schedule II (Historical Financial Information of GlaxoSmithKline
Consumer Healthcare Holdings (No.2) Limited);
“HMRC” HM Revenue & Customs in the UK;
“IFRS” the International Financial Reporting Standards;
“Indenture” an indenture dated as of 24 March 2022 among the US Issuer,
GSK Consumer Healthcare Capital UK plc, GSK and the
Company as guarantors and Deutsche Bank Trust Company
Americas, as trustee, registrar, paying agent, transfer agent and
calculation agent;
“India Condition” has the meaning given in paragraph 15.6(D) of Part XII
(Additional Information);
“Interim Financial Information” has the meaning given in paragraph 4 of Part VI (Selected
Financial Information);
“IP” intellectual property;
“Japan Condition” has the meaning given in paragraph 15.6(F) of Part XII
(Additional Information);
“Joint Sponsors” BofA Securities, Citi and Goldman Sachs;
“JVCo A Ordinary Shares” the fully paid A Ordinary Shares in the capital of CH JVCo;
“JVCo B Ordinary Shares” the fully paid B Ordinary Shares in the capital of CH JVCo;
“JVCo Preference Shares” the fully paid Preference Shares in the capital of CH JVCo;
“Latest Practicable Date” 30 May 2022, being the last practicable date prior to publication
of this Prospectus;
“Listing Conditions” the conditions to which an approval by the FCA of the admission
of Haleon Shares to the Official List with a premium listing is
express to be subject;
“Listing Rules” the rules made by the FCA in its capacity as the UK Listing
Authority under Part VI of FSMA (and contained in the UK Listing
Authority’s publication of the same name), as amended from time
to time;
“Lock-up Deed” has the meaning given in paragraph 5 of Part V (Directors, Senior
Managers, Corporate Governance and Remuneration);
“LSE” London Stock Exchange plc or the market conducted by it, as the
context requires;
326
“Manufacturing and Supply
Agreements”
has the meaning given in paragraph 15.15 of Part XII ( Additional
Information);
“Market Abuse Regulation” or
“MAR”
Regulation (EU) No 596/2014 of the European Parliament and of
the Council of 16 April 2014 on market abuse (Market Abuse
Regulation) and repealing Directive 2003/6/EC of the European
Parliament and of the Council and Commission Directives
2003/124/EC, 2003/125/EC and 2004/72/EC and the delegated
acts, implementing acts and technical standards thereunder, as
such legislation forms part of retained EU law as defined in the
European Union (Withdrawal) Act 2018;
“MDL” Multidistrict Litigation;
“MHRA” the UK Medicines and Healthcare products Regulatory Agency;
“Morgan Stanley” Morgan Stanley & Co. International plc;
“NMPA” the China National Medical Products Administration;
“Nominations & Governance
Committee”
the nominations and governance committee of the Company;
“Non-Voting Preference
Shareholder”
a holder of Non-Voting Preference Shares from time to time;
“Non-Voting Preference
Shares”
the fully paid non-voting preference shares of £1 each in the
capital of the Company;
“Notes Proceeds Loan
Agreements”
the loan agreements governing the Notes Proceeds Loans as
amended from time to time;
“Notes Proceeds Loans” has the meaning given in paragraph 1.3 of Part IV (Overview of
the Demerger and Separation);
“Notice of GSK General
Meeting”
the notice of the GSK General Meeting which is set out in the
GSK Shareholder Circular;
“Novartis” Novartis International A.G.;
“Novartis Buyout” has the meaning given in paragraph 15.1 of Part XII (Additional
Information);
“Novartis Completion” 2 March 2015;
“Novartis Contribution
Agreement”
has the meaning given in paragraph 15.1 of Part XII (Additional
Information);
“NSAID” nonsteroidal anti-inflammatory drug;
“NVPS Sale” has the meaning given in paragraph 1.1 of Part IV (Overview of
the Demerger and Separation);
“NYSE” New York Stock Exchange;
“Official List” the Official List of the FCA;
“OTC” over-the-counter;
327
“Overseas Shareholders” holders of GSK Shares with registered addresses outside the UK;
“Par Call Date” has the meaning given in paragraph 7.4 of Part VII ( Operating
and Financial Review);
“parodontax family” parodontax, Corsodyl and Chlorhexamed;
“PFCHH” PF Consumer Healthcare Holdings LLC, a wholly-owned
subsidiary of Pfizer which holds Pfizer’s interest in CH JVCo;
“PFCHH Interests” all of the common interests in the capital of PFCHH in issue
immediately prior to completion of the Pfizer Share Exchange,
which comprise all ownership interests of whatever nature in
PFCHH and all of which are held by Anacor as of the date of this
Prospectus and all of which, from completion of the PFCHH
Transfer until the completion of the Pfizer Share Exchange, shall
be held by Pfizer;
“PFCHH Transfer” the series of transactions pursuant to which the PFCHH Interests
will be transferred, distributed or otherwise assigned from Anacor
to Pfizer;
“Pfizer” Pfizer Inc.;
“Pfizer Contributed CH
Business”
has the meaning given in paragraph 15.2 of Part XII (Additional
Information);
“Pfizer Completion” has the meaning given in paragraph 15.2 of Part XII (Additional
Information);
“Pfizer Completion Date” 31 July 2019;
“Pfizer Directors” the Directors nominated by the Pfizer Group;
“Pfizer Exchange Agreement” has the meaning given in paragraph 15.9 of Part XII (Additional
Information);
“Pfizer Group” Pfizer together with its subsidiaries and subsidiary undertakings
from time to time;
“Pfizer Relationship
Agreement”
has the meaning given in paragraph 15.10 of Part XII ( Additional
Information);
“Pfizer SAPA” has the meaning given to it in paragraph 15.2 of Part XII
(Additional Information);
“Pfizer SAPA Amendment
Agreement”
has the meaning given in paragraph 15.3 of Part XII (Additional
Information);
“Pfizer SHA” has the meaning given in paragraph 15.5 of Part XII (Additional
Information);
“Pfizer Share Exchange” has the meaning given in paragraph 1.1 of Part IV (Overview of
the Demerger and Separation);
328
“Pfizer Transaction” has the meaning given in paragraph 2.12 of Part VII (Operating
and Financial Review);
“Polident Family” Polident, Corega and Poligrip;
“Post-Demerger GSK Group” GSK plc and its subsidiaries and subsidiary undertakings from
time to time, excluding those companies which form part of the
Group;
“Pounds Sterling”, “£” or
“pence”
the lawful currency of the UK;
“Power Brands” has the meaning given to it in paragraph 2.5 of Part I (Key
Highlights and Development of the Group);
“PR Regulation” Commission Delegated Regulation (EU) 2019/980 of 14 March
2019 supplementing Regulation (EU) 2017/1129 of the European
Parliament and of the Council as regards the format, content,
scrutiny and approval of the prospectus to be published when
securities are offered to the public or admitted to trading on a
regulated market, and repealing Commission Regulation (EC) No
809/2004, as it forms part of retained EU law as defined in the
European Union (Withdrawal) Act 2018;
“Pre-Demerger Dividend” has the meaning given in paragraph 1.2 of Part IV (Overview of
the Demerger and Separation);
“Pre-Separation Dividends” has the meaning given in paragraph 1.2 of Part IV (Overview of
the Demerger and Separation);
“Pre-Separation Notes” the Pre-Separation Programme Notes and the Pre-Separation
USD Notes;
“Pre-Separation Debt
Proceeds”
the amounts received by members of the Haleon Group on
repayment of the Notes Proceeds Loans together with any
amounts drawn by members of the Haleon Group under any
additional borrowings (including, but not limited to, the Term Loan
Facility) as at the date of the Pre-Demerger Dividend;
“Pre-Separation Programme
Notes”
has the meaning given in paragraph 1.3 of Part IV (Overview of
the Demerger and Separation);
“Pre-Separation USD Notes” has the meaning given in paragraph 1.3 of Part IV (Overview of
the Demerger and Separation);
“Programme” has the meaning given in paragraph 1.3 of Part IV (Overview of
the Demerger and Separation);
“Prospectus” this document dated 1 June 2022, comprising a prospectus
relating to the Company for the purpose of the Admission;
329
“Prospectus Regulation” Regulation (EU) 2017/1129 of the European Parliament and of
the Council of 14 June 2017 on the prospectus to be published
when securities are offered to the public or admitted to trading on
a regulated market, and repealing Directive 2003/71/EC, including
the delegated acts, implementing acts and technical standards
thereunder, as such legislation forms part of retained EU law as
defined in the European Union (Withdrawal) Act 2018;
“Prospectus Regulation Rules” Prospectus Regulation Rules of the FCA made under section 73A
of FSMA;
“Proxy Form” the form of proxy enclosed with the GSK Shareholder Circular for
the use by GSK Shareholders in connection with the GSK
General Meeting;
“Purchaser Liabilities” has the meaning given in paragraph 15.2 of Part XII (Additional
Information);
“QSC” quality and supply chain;
“Qualifying GSK Shareholder” a holder of GSK Shares who is registered on the Register at the
Shareholder Record Time, including GSK Shareholders in the
GSK CSN;
“R&D” research and development;
“RCF Borrower” has the meaning given in paragraph 7.4 of Part VII (Operating
and Financial Review);
“RCF Loans” has the meaning given in paragraph 7.4 of Part VII (Operating
and Financial Review);
“Record Time” the Shareholder Record Time for the GSK Shareholders and the
ADS Holder Record Time for the ADS Holders;
“Redeemable Shares” the redeemable preference shares of £1 each in the capital of the
Company;
“Register” the register of members of GSK;
“Registrar” or “Equiniti” Equiniti Limited, Aspect House, Spencer Road, Lancing
BN99 6DA;
“Registration Rights
Agreement”
has the meaning given in paragraph 15.11 of Part XII ( Additional
Information);
“Regulatory Conditions” has the meaning given in paragraph 15.6(F) of Part XII
(Additional Information);
“Related Party Transactions
Resolution”
the ordinary resolution numbered 2, set out in the Notice of GSK
General Meeting;
“Remuneration Committee” the remuneration committee of the Company;
330
“Revolving Credit Facilities” has the meaning given to it in paragraph 7.4 of Part VII
(Operating and Financial Review);
“Rx” requiring a prescription;
“Rx-to-OTC switch” switch of Rx products to OTC status;
“Sarbanes-Oxley” has the meaning given in paragraph 3.2 of Risk Factors;
“SCIA” has the meaning given in paragraph 15.8 of Part XII (Additional
Information);
“SDRT” UK Stamp Duty and Stamp Duty Reserve Tax;
“SEC” the US Securities and Exchange Commission;
“Senior Management” or
“Senior Managers”
those individuals identified as Senior Managers in paragraph 2 of
Part V (Directors, Senior Managers, Corporate Governance and
Remuneration);
“Separation” the Demerger, Share Exchanges, Admission and other steps
pursuant to which, among other things, the Company will become
a listed company holding the Consumer Healthcare Business;
“Separation Agreements” the Transition Services Agreement and Manufacturing and Supply
Agreements;
“SG&A” selling, general and administration;
“Share Exchanges” has the meaning given in paragraph 1.1 of Part IV (Overview of
the Demerger and Separation);
“Shareholder Record Time” 6 p.m. (UK time) on 15 July 2022;
“Shareholder Voting Record
Time”
6.30 p.m. (UK time) on 4 July 2022;
“SLP Exchange Agreement” has the meaning given in paragraph 15.9 of Part XII (Additional
Information);
“SLPs” (A) GSK (No. 1) Scottish Limited Partnership, a private fund
limited partnership registered in Scotland with registration
number SL035527 and whose principal place of business
is at 50 Lothian Road, Festival Square, Edinburgh,
EH3 9WJ;
(B) GSK (No. 2) Scottish Limited Partnership, a private fund
limited partnership registered in Scotland with registration
number SL035526 and whose principal place of business
is at 50 Lothian Road, Festival Square, Edinburgh,
EH3 9WJ; and
331
(C) GSK (No. 3) Scottish Limited Partnership, a private fund
limited partnership registered in Scotland with registration
number SL035525 and whose principal place of business
is at 50 Lothian Road, Festival Square, Edinburgh,
EH3 9WJ,
being the Scottish limited partnerships that will each
receive shares in the Company pursuant to the SLP
Exchange Agreement, and “SLP” shall be construed
accordingly;
“SLP1” GSK (No. 1) Scottish Limited Partnership, a private fund limited
partnership registered in Scotland with registration number
SL035527 and whose principal place of business is at 50 Lothian
Road, Festival Square, Edinburgh, EH3 9WJ;
“South Korea Condition” has the meaning given in paragraph 15.6(E) of Part XII
(Additional Information);
“Sponsors’ Agreement” has the meaning given in paragraph 15.12 of Part XII (Additional
Information);
“subsidiary” a subsidiary as that term is defined in section 1159 of the
Companies Act;
“subsidiary undertaking” a subsidiary undertaking as that term is defined in section 1162 of
the Companies Act;
“Sweep-Up Dividend” has the meaning given in paragraph 1.2 of Part IV (Overview of
the Demerger and Separation);
“Swingline Facility” has the meaning given in paragraph 7.4 of Part VII (Operating
and Financial Review);
“Takeover Panel” the Panel on Takeovers and Mergers;
“Tax Covenant” has the meaning given in paragraph 15.7 of Part XII (Additional
Information);
“Term Loan Facility” has the meaning given to it in paragraph 7.4 of Part VII
(Operating and Financial Review);
“Transition Services
Agreement”
has the meaning given to it in paragraph 15.14 of Part XII
(Additional Information);
“TSK&F Joint Venture” Sino-American Tianjin Smith Kline & French Laboratories Ltd;
“UK GDPR” GDPR (as it forms part of retained EU law as defined in the
European Union (Withdrawal) Act 2018);
“UK Listing Authority” or
“UKLA”
the FCA acting in its capacity as the competent authority for the
purposes of Part VI of FSMA;
332
“UK Pension Schemes” the GSK Pension Scheme, the GSK Pension Fund and the
SmithKline Beecham Pension Plan, and “UK Pension Scheme”
means any one of them;
“uncertificated” or “in
uncertificated form”
refers to a share or other security recorded on the relevant
register of the share or security concerned as being held in
uncertificated form in CREST and title to which may be
transferred by using CREST;
“United Kingdom” or “UK” the United Kingdom of Great Britain and Northern Ireland;
“United States”, “USA” or “US” the United States of America, its territories and possessions, any
state of the United States of America, the District of Columbia and
all other areas subject to its jurisdiction;
“US Exchange Act” the US Securities Exchange Act of 1934, as amended;
“US Dollar”, “US Dollars” or “$” the lawful currency of the United States;
“US Holder” a beneficial owner of Haleon Shares that is a citizen or resident of
the United States or a US domestic corporation or that otherwise
is subject to US federal income taxation on a net income basis in
respect of such Haleon Shares;
“US Issuer” GSK Consumer Healthcare Capital US LLC;
“US Securities Act” the US Securities Act of 1933, as amended;
“USD Facility” has the meaning given in paragraph 7.4 of Part VII ( Operating
and Financial Review);
“VMS” vitamins, minerals and supplements;
“Voting Record Time” the Shareholder Voting Record Time for the GSK Shareholders
and the ADS Holder Voting Record Time for the ADS Holders;
and
“WHO” the World Health Organisation.
333
Schedule II
HISTORICAL FINANCIAL INFORMATION OF GLAXOSMITHKLINE CONSUMER HEALTHCARE
HOLDINGS (NO.2) LIMITED
SECTION A: ACCOUNTANT’S REPORT IN RESPECT OF THE HISTORICAL FINANCIAL
INFORMATION OF THE CH GROUP FOR THE YEARS ENDED 31 DECEMBER 2021,
31 DECEMBER 2020 AND 31 DECEMBER 2019.
334

Deloitte LLP
Hill House
1 Little New Street
London
EC4A 3TR
The Board of Directors
on behalf of Haleon plc
980 Great West Road,
Brentford, Middlesex,
TW8 9GS,
United Kingdom
Goldman Sachs International
Plumtree Court,
25 Shoe Lane,
London,
EC4A 4AU
Citigroup Global Markets Limited
Citigroup Centre,
Canada Square,
Canary Wharf,
London,
E14 5LB
Merrill Lynch International
2 King Edward Street,
London
EC1A 1HQ
1 June 2022
Dear Sirs/Mesdames
GlaxoSmithKline Consumer Healthcare Holdings (No.2) Limited and, together with its
subsidiaries, the “CH JV Group”
We report on the financial information of CH JV Group and its subsidiary undertakings for the years
ended 31 December 2019, 31 December 2020 and 31 December 2021 set out in Schedule II Section B
of the prospectus dated 1 June 2022 of Haleon plc (the “Company”) (the “Prospectus”). This report is
required by Annex 1 item 18.3.1 of the PR Regulation and is given for the purpose of complying with
that requirement and for no other purpose.
Opinion on financial information
In our opinion, the financial information gives, for the purposes of the Prospectus, a true and fair view
of the state of affairs of the Group as at 31 December 2019, 31 December 2020 and 31 December
2021 and of its profits, cash flows and changes in equity and statement of comprehensive income for
the years then ended. in accordance with International Financial Reporting Standards as adopted by
the IASB (“IASB IFRS”) and International Financial Reporting Standards as adopted by the United
Kingdom (“UK IFRS”) (together “IFRS”).
335
Responsibilities
As described in Notes 1 & 2 the Directors of the Company are responsible for preparing the financial
information in accordance with IFRS.
It is our responsibility to form an opinion on the financial information and to report our opinion to you.
Save for any responsibility arising under Prospectus Regulation Rule 5.3.2R(2)(f) to any person as and
to the extent there provided, to the fullest extent permitted by law we do not assume any responsibility
and will not accept any liability to any other person for any loss suffered by any such other person as a
result of, arising out of, or in connection with this report or our statement, required by and given solely
for the purposes of complying with Annex 1 item 1.3 of the Prospectus Delegated Regulation,
consenting to its inclusion in the Prospectus.
Basis of preparation
This financial information has been prepared for inclusion in the Prospectus on the basis of the
accounting policies set out in note 1 to the financial information.
Basis of opinion
We conducted our work in accordance with Standards for Investment Reporting issued by the Financial
Reporting Council (“FRC”) in the United Kingdom. We are independent of the Company and the CH JV
Group in accordance with the FRC’s Ethical Standards applied to Investment Circular Reporting
Engagements, and we have fulfilled our other ethical responsibilities in accordance with these
requirements.
Our work included an assessment of evidence relevant to the amounts and disclosures in the financial
information. It also included an assessment of significant estimates and judgments made by those
responsible for the preparation of the financial information and whether the accounting policies are
appropriate to the entity’s circumstances, consistently applied and adequately disclosed.
We planned and performed our work so as to obtain all the information and explanations which we
considered necessary in order to provide us with sufficient evidence to give reasonable assurance that
the financial information is free from material misstatement whether caused by fraud or other
irregularity or error.
Our work has not been carried out in accordance with auditing or other standards and practices
generally accepted in jurisdictions outside the United Kingdom, including the United States of America,
and accordingly should not be relied upon as if it had been carried out in accordance with those
standards and practices.
Conclusions Relating to Going Concern
In performing this engagement on the financial information, we have concluded that the directors’ use
of the going concern basis of accounting in the preparation of the financial information is appropriate.
336
Declaration
For the purposes of Prospectus Regulation Rule 5.3.2R(2)(f), we are responsible for this report as part
of the Prospectus and declare that to the best of our knowledge the information contained in this report
is, in accordance with the facts and that this report makes no omission likely to affect its import. This
declaration is included in the Prospectus in compliance with Annex 1 item 1.2 of the PR Regulation and
for no other purpose.
Yours faithfully
Deloitte LLP
Deloitte LLP is a limited liability partnership registered in England and Wales with registered number
OC303675 and its registered office at 1 New Street Square, London EC4A 3HQ, United Kingdom.
Deloitte LLP is the United Kingdom affiliate of Deloitte NSE LLP, a member firm of Deloitte Touche
Tohmatsu Limited, a UK private company limited by guarantee (“DTTL”). DTTL and each of its member
firms are legally separate and independent entities. DTTL and Deloitte NSE LLP do not provide
services to clients.
337
SECTION B: HISTORICAL FINANCIAL INFORMATION OF GLAXOSMITHKLINE CONSUMER
HEALTHCARE HOLDINGS (NO.2) LIMITED FOR THE YEARS ENDED 31 DECEMBER 2021,
31 DECEMBER 2020 AND 31 DECEMBER 2019
338
GlaxoSmithKline Consumer Healthcare Holdings (No.2) Limited
Historical Financial Information
339
GlaxoSmithKline Consumer Healthcare Holdings (No.2) Limited
Consolidated income statement for the years ended 31 December
Note 2021 2020 2019
£m £m £m
Revenue 6 9,545 9,892 8,480
Cost of sales (3,595) (3,982) (3,678)
Gross profit 5,950 5,910 4,802
Selling, general and administration (4,086) (4,220) (3,596)
Research and development (257) (304) (292)
Other operating income/(expense) 7 31 212 (17)
Operating profit 8 1,638 1,598 897
Finance income 10 17 20 24
Finance expense 11 (19) (27) (35)
Net finance costs (2) (7) (11)
Profit before tax 1,636 1,591 886
Income tax 13 (197) (410) (199)
Profit after tax for the year 1,439 1,181 687
Profit attributable to shareholders 1,390 1,145 655
Profit attributable to non-controlling interests 49 36 32
Basic earnings per share (pence) 15 139,000 114,500 65,500
Diluted earnings per share (pence) 15 139,000 114,500 65,500
340
GlaxoSmithKline Consumer Healthcare Holdings (No.2) Limited
Consolidated statement of comprehensive income for the years ended 31 December
2021 2020 2019
£m £m £m
Profit after tax for the year 1,439 1,181 687
Other comprehensive expense for the year
Items that may be subsequently reclassified to income statement:
Exchange movements on overseas net assets and net investment hedges (34) (170) (708)
Fair value movements on cash flow hedges 11 - -
Deferred tax on fair value movements on cash flow hedges (2) - -
(25) (170) (708)
Items that will not be reclassified to income statement:
Exchange movements on overseas net assets - 1 -
Remeasurement gains/(losses) on defined benefit plan 27 (13) (13)
Deferred tax on actuarial movements in defined benefit plans (12) 13 3
15 1 (10)
Other comprehensive expense, net of tax for the year (10) (169) (718)
Total comprehensive income/(expense), net of tax for the year 1,429 1,012 (31)
Total comprehensive income/(expense) for the year attributable to:
Shareholders 1,380 975 (63)
Non-controlling interests 49 37 32
Total comprehensive income/(expense), net of tax for the year 1,429 1,012 (31)
341
GlaxoSmithKline Consumer Healthcare Holdings (No.2) Limited
Consolidated balance sheet as at 31 December
Note 2021 2020 2019
£m £m £m
Non-current assets
Property, plant and equipment 16 1,563 1,486 1,470
Right of use assets 17 99 116 151
Intangible assets 18 27,195 27,218 28,012
Deferred tax assets 13 312 251 254
Post-employment benefit assets 25 11 41 3
Derivative financial instruments 33 12 - -
Other non-current assets 8 10 10
Total non-current assets 29,200 29,122 29,900
Current assets
Inventories 19 951 949 1,211
Trade and other receivables 20 2,207 2,358 2,479
Loan amounts owing from related parties 30 1,508 1,119 1,461
Cash and cash equivalents and liquid investments 21 414 334 340
Assets held for sale 22 - 68 225
Derivative financial instruments 33 5 6 12
Current tax recoverable 166 174 83
Total current assets 5,251 5,008 5,811
Total assets 34,451 34,130 35,711
Current liabilities
Short-term borrowings 24 (79) (82) (64)
Trade and other payables 23 (3,002) (3,268) (3,420)
Loan amounts owing to related parties 30 (825) (300) (457)
Liabilities directly associated with assets held for sale 22 - - (29)
Derivative financial instruments 33 (18) (25) (2)
Current tax payable (202) (236) (196)
Short-term provisions 26 (112) (103) (101)
Total current liabilities (4,238) (4,014) (4,269)
Non-current liabilities
Long-term borrowings 24 (87) (105) (121)
Deferred tax liabilities 13 (3,357) (3,373) (3,514)
Pensions and other post-employment benefits 25 (253) (336) (298)
Derivative financial instruments 33 (1) - -
Other provisions 26 (27) (65) (76)
Other non-current liabilities (8) (14) (21)
Total non-current liabilities (3,733) (3,893) (4,030)
Total liabilities (7,971) (7,907) (8,299)
Net assets 26,480 26,223 27,412
Equity
Share capital 28 1 1 1
Share premium 28 - - 20,842
Other reserves 28 (11,632) (11,652) 1,372
Retained earnings 37,986 37,763 5,106
Shareholders’ equity 26,355 26,112 27,321
Non-controlling interests 125 111 91
Total equity 26,480 26,223 27,412
342
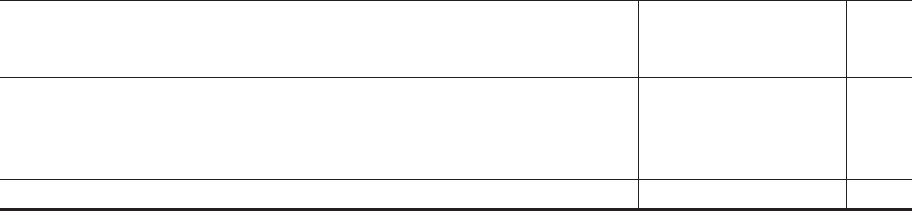
GlaxoSmithKline Consumer Healthcare Holdings (No.2) Limited
Consolidated statement of changes in equity for the years ended 31 December
Note
Share
capital
Share
premium
Other
reserves
Retained
earnings
Shareholders’
equity
Non-
controlling
interests
Total
Equity
£m £m £m £m £m £m £m
At 1 January 2021 1 - (11,652) 37,763 26,112 111 26,223
Profit after tax - - - 1,390 1,390 49 1,439
Other comprehensive expenses - - 9 (19) (10) - (10)
Total comprehensive income - - 9 1,371 1,380 49 1,429
Contribution from parent 28 - - 11 - 11 - 11
Distributions to non-controlling interests - - - - - (35) (35)
Dividends to shareholders 14 - - - (1,148) (1,148) - (1,148)
At 31 December 2021 1 - (11,632) 37,986 26,355 125 26,480
343
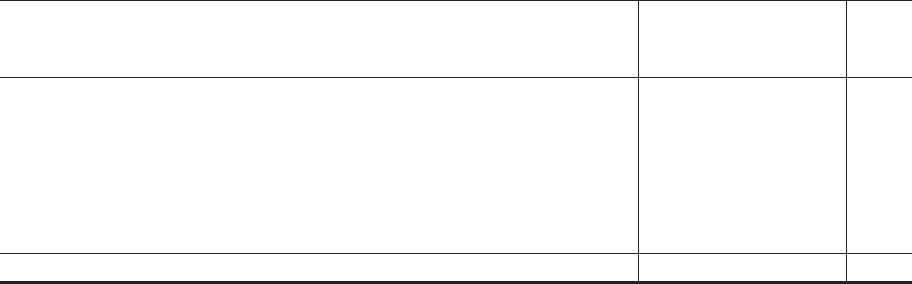
GlaxoSmithKline Consumer Healthcare Holdings (No.2) Limited
Consolidated statement of changes in equity for the years ended 31 December
Note
Share
capital
Share
premium
Other
reserves
Retained
earnings
Shareholders’
equity
Non-
controlling
interests
Total
Equity
£m £m £m £m £m £m £m
At 1 January 2020 1 20,842 1,372 5,106 27,321 91 27,412
Profit after tax - - - 1,145 1,145 36 1,181
Other comprehensive expenses - - - (170) (170) 1 (169)
Total comprehensive income - - - 975 975 37 1,012
Issue of share capital 28 13,166 - (13,166) - - - -
Capital reduction 28 (13,166) (20,842) (45) 34,053 - - -
Contribution (non-cash) from parent 28 - - 187 - 187 - 187
Acquisition of non-controlling interests 29 - - - - - 14 14
Distributions to non-controlling interests - - - - - (31) (31)
Dividends to shareholders 14 - - - (2,371) (2,371) - (2,371)
At 31 December 2020 1 - (11,652) 37,763 26,112 111 26,223
344
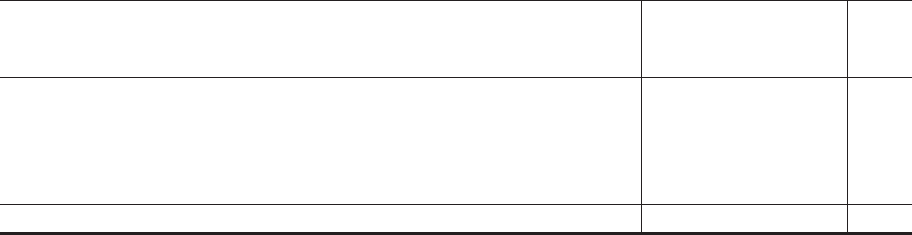
GlaxoSmithKline Consumer Healthcare Holdings (No.2) Limited
Consolidated statement of changes in equity for the years ended 31 December
Note
Share
capital
Share
premium
Other
reserves
Retained
earnings
Shareholders’
equity
Non-
controlling
interests
Total
Equity
£m £m £m £m £m £m £m
At 1 January 2019 - 20,321 (14,841) 6,321 11,801 67 11,868
Profit after tax - - - 655 655 32 687
Other comprehensive expenses - - - (718) (718) - (718)
Total comprehensive expenses - - - (63) (63) 32 (31)
Issue of share capital 28 1 521 16,213 - 16,735 - 16,735
Acquisition of non-controlling interests 29 - - - - - 20 20
Distributions to non-controlling interests - - - - - (28) (28)
Dividends to shareholders 14 - - - (1,152) (1,152) - (1,152)
At 31 December 2019 1 20,842 1,372 5,106 27,321 91 27,412
345

GlaxoSmithKline Consumer Healthcare Holdings (No.2) Limited
Consolidated cash flow statement for the years ended 31 December
Note 2021 2020 2019
£m £m £m
Cash flows from operating activities
Profit after tax 1,439 1,181 687
Adjustments reconciling profit after tax to cash generated from operations 31 227 780 408
Cash generated from operations 31 1,666 1,961 1,095
Taxation paid (310) (554) (309)
Net cash inflow from operating activities 1,356 1,407 786
Cash flows from investing activities
Purchase of property, plant and equipment (228) (222) (190)
Proceeds from sale of property, plant, and equipment 12 6 51
Purchase of intangible assets (70) (96) (53)
Proceeds from sale of intangible assets 137 924 120
Purchase of business, net of cash acquired 29 - 20 120
Proceeds from sale of businesses 29 - 221 -
Decrease in amounts invested with GSK finance companies 100 158 219
Interest received 16 19 24
Net cash (outflow)/inflow from investing activities (33) 1,030 291
Cash flows from financing activities
Repayment of lease liabilities (38) (44) (42)
Interest paid (15) (19) (29)
Dividends paid to shareholders (1,148) (2,371) (1,152)
Distributions to non-controlling interests (35) (31) (28)
Net contribution from parent 4 - 335
Repayment of borrowings - (10) -
Proceeds from borrowings 8 38 1
Other financing cash flows (12) - (10)
Net cash outflow from financing activities (1,236) (2,437) (925)
Increase in cash and bank overdrafts 87 - 152
Cash and bank overdrafts at the beginning of the year 323 329 191
Exchange adjustments (5) (6) (14)
Increase in cash and bank overdrafts 87 - 152
Cash and bank overdrafts at end of year 405 323 329
Cash and bank overdrafts at the end of year comprise:
Cash and cash equivalents 21 413 333 339
Overdrafts (8) (10) (10)
Cash and bank overdrafts at end of year 405 323 329
346
GlaxoSmithKline Consumer Healthcare Holdings (No.2) Limited
Notes to the consolidated Historical Financial Information for the years ended
31 December 2021, 2020 and 2019
1. Presentation of the Historical Financial Information
1.1 General information
GlaxoSmithKline Consumer Healthcare Holdings (No.2) Limited (“CHHL2”) and its subsidiary
undertakings (collectively, the “CH Group”) is a group of companies focused on developing
and marketing a range of Oral Health, Pain Relief, Vitamins, Minerals and Supplements,
Respiratory Health, Digestive Health and Other products for people in more than 100 countries.
CHHL2 is a private limited company incorporated in the United Kingdom on 24 April 2019 as a
subsidiary of GlaxoSmithKline plc. (“GSK Group”) and is a limited company registered in
England and Wales. The registered office is located at 980 Great West Road, Brentford,
Middlesex, TW8 9GS, United Kingdom.
The consolidated Historical Financial Information of the CH Group, as described further below,
for the three years ended 31 December 2021 (the “Historical Financial Information”) has
been prepared specifically for the purposes of this Prospectus and in accordance with
Regulation (EU) 2017/1129 as supplemented by Commission Delegated Regulation (EU)
2019/980 each as they form part of United Kingdom domestic law by virtue of the EU
Withdrawal Act 2018 and in accordance with the basis of preparation set out below.
1.2 Perimeter and basis of preparation
The consolidated Historical Financial Information comprises the CH Group for the three years
ended 31 December 2019, 2020 and 2021. The legal entities contained within the CH Group
are set out in Note 38.
On 19 December 2018, GSK Group reached an agreement with Pfizer Inc. (“Pfizer”) to
combine their respective consumer health businesses into a new world-leading Joint Venture
(the “GSK-Pfizer Joint Venture” or the “JV”) (the “Pfizer Transaction”). Subsequent to the
Pfizer Transaction, GSK Group held and still holds a majority controlling equity interest of 68%
in the CH Group whilst Pfizer held and still holds a non-controlling equity interest of 32% in the
CH Group.
The Pfizer Transaction was completed on 31 July 2019 and the Pfizer consumer health
business was consolidated and included in the CH Group’s consolidated Historical Financial
Information from that date.
Pfizer, GSK Group and CHHL2 entered into a stock and asset purchase agreement as
amended on 31 July 2019 (the “SAPA”). The SAPA reflected the fact that the majority of GSK’s
consumer healthcare business already sat within a relatively standalone corporate structure
within GSK Group, and under the terms of the SAPA, Pfizer agreed to transfer its consumer
healthcare business into GSK’s consumer healthcare business.
Basis of preparation
The consolidated Historical Financial Information has been prepared in accordance with the
requirements of International Financial Reporting Standards as issued by the International
Accounting Standards Board (“IASB IFRS”) and International Financial Reporting Standards as
adopted by the United Kingdom (“UK IFRS”) (together “IFRS”).
The CH Group is comprised predominantly of, but not entirely, a group of legal entities during
the periods presented and the consolidated Historical Financial Information reflects the assets,
liabilities and results of operations that represented Consumer Healthcare within GSK Group
(“GSK Consumer Healthcare”).
347
The following summarises the basis of accounting applied in preparing the consolidated
Historical Financial Information:
• The CH Group’s Historical Financial Information has been prepared following the
principles of IFRS 10 Consolidated financial statements, and comprise the assets and
liabilities, income and expenses, equity and reserves and cash flows for a certain group
of legal entities within GSK Consumer Healthcare business, as contemplated by the
SAPA, from 1 January 2019 to 31 July 2019 and the newly formed GSK-Pfizer Joint
Venture from 1 August 2019 onwards.
• On 20 June 2019, the existing GSK Consumer Healthcare entities identified within the
SAPA were transferred from GSK Group to CHHL2. The transfer of these investments to
CHHL2 was accounted for as a reorganisation of entities under common control.
Accordingly, all assets, liabilities and results of operations are recorded at their carrying
values within the GSK Group from 1 January 2019 to 20 June 2019 representing the
continuation of GSK’s Consumer Healthcare business and hence the Historical Financial
Information for this period has been prepared on a consolidated basis.
• On 31 July 2019, the Pfizer Transaction closed and CHHL2 acquired Pfizer’s consumer
healthcare business. The acquired assets and liabilities were accounted for under IFRS 3
at fair value.
• All intercompany transactions have been eliminated between subsidiaries of the CH
Group. Transactions and balances between the CH Group and the rest of the GSK Group
represent third-party transactions and balances from the perspective of the CH Group.
They have been presented alongside third-party transactions and balances in the
appropriate financial statement line items of the consolidated Historical Financial
Information to which such transactions and balances relate as well as being disclosed as
related party transactions.
The consolidated Historical Financial Information presented herein does not necessarily reflect
what the operating results and cash flows would have been had the CH Group been a
standalone group for all periods presented.
The accounting policies applied and disclosed below are consistent with those to be used by
the CH Group in its next annual financial statements for the year ending 31 December 2022
and these policies have been applied consistently to all periods presented unless stated
otherwise.
The consolidated Historical Financial Information is presented in Sterling (“ GBP”, “£”), the
functional currency of CHHL2 and presentation currency of the CH Group, and all values
stated in millions of GBP (“£m”), except where otherwise indicated, and have been prepared
on the historical cost basis, unless otherwise indicated in the accounting policies.
The consolidated Historical Financial Information does not constitute statutory accounts within
the meaning of section 434(3) of the Companies Act 2006.
Going concern
The consolidated Historical Financial Information has been prepared on a going concern basis
and under the historical cost convention, unless otherwise indicated in the accounting policies
below. The directors of the CH Group have reviewed cash flow forecasts and trading budgets
and after making appropriate enquiries, have formed the view that the CH Group will generate
sufficient cash to meet its ongoing requirements for at least the next 12 months; accordingly,
the going concern basis of preparation has been adopted.
348
2. Accounting principles and policies
(a) Consolidation
Entities over which the CH Group has the power to direct the relevant activities so as to affect
the returns to the CH Group, generally through control over the financial and operating policies
from either voting or contractual rights, are accounted for as subsidiaries. Interests acquired in
entities are consolidated from the date the CH Group acquires control and interests sold are
de-consolidated from the date control ceases.
Where, as part of a business combination, the CH Group is not able to exercise control over a
particular operation due to the existence of legal or other restrictions, the associated assets
and liabilities are not consolidated, and a financial asset or liability is recognised for the
economic benefit or obligation to be received under the contribution agreement. The assets
and liabilities are consolidated, and the associated financial asset or liability derecognised, on
the date at which the CH Group is able to exercise control over these operations.
Transactions and balances between subsidiaries are eliminated and no profit before tax is
recognised on sales between subsidiaries until the products are sold to customers outside the
CH Group. Transactions with non-controlling interests are recorded directly in equity. Deferred
tax relief on unrealised intra-group profit is accounted for only to the extent that it is considered
recoverable.
(b) Business combinations
Business combinations where common control exists at the time of the transaction relate to
businesses that were controlled by a part of the GSK Group, other than the CH Group and then
as a result of the combination were transferred into the CH Group at some point during the
periods covered by the Historical Financial Information. Such business combinations are
accounted for by recognising all assets and liabilities acquired at their previous carrying values
within the GSK Group with effect from the beginning of the earliest period reported in the
Historical Financial Information. No new goodwill arises from such transactions.
Business combinations where common control does not exist before the transaction are
accounted for using the acquisition accounting method. Identifiable assets, liabilities and
contingent liabilities acquired are measured at fair value at acquisition date. The consideration
transferred is measured at fair value and includes the fair value of any contingent
consideration. Where the consideration transferred, together with the non-controlling interest,
exceeds the fair value of the net assets, liabilities and contingent liabilities acquired, the excess
is recorded as goodwill, denominated in the currency of the operation acquired.
The costs related to business combinations are charged to the income statement in the period
in which they are incurred. Where not all the equity of a subsidiary is acquired the
non-controlling interest is recognised either at fair value or at the non-controlling interest’s
share of the net assets of the subsidiary, on a case-by-case basis.
Changes in the CH Group’s ownership percentage of subsidiaries are accounted for within
equity.
(c) Foreign currency translation
Foreign currency transactions are booked in the functional currency of the relevant company at
the exchange rate ruling on the date of transaction. Foreign currency monetary assets and
liabilities are retranslated into the functional currency at rates of exchange ruling at the balance
sheet date. Exchange differences are included in the income statement.
On consolidation, assets and liabilities, including related goodwill, of overseas subsidiaries are
translated into Sterling at rates of exchange ruling at the balance sheet date. The results and
cash flows of overseas subsidiaries are translated into Sterling using average rates of
exchange.
349
Exchange adjustments arising when the opening net assets and the profits for the period
retained by overseas subsidiaries are translated into Sterling are recognised in Other
Comprehensive Income.
(d) Revenue
The CH Group receives revenue for supply of goods to external customers against orders
received. The majority of contracts that the CH Group enters into relate to sales orders
containing single performance obligations for the delivery of consumer healthcare products.
The average duration of a sales order is less than 12 months.
Product revenue is recognised when control of the goods is passed to the customer. The point
at which control passes is determined by each customer arrangement, but generally occurs on
delivery to the customer.
Product revenue represents net invoice value including fixed and variable consideration.
Variable consideration arises on the sale of goods as a result of discounts and allowances
given and accruals for estimated future returns and rebates. Revenue is not recognised in full
until it is highly probable that a significant reversal in the amount of cumulative revenue
recognised will not occur. The methodology and assumptions used to estimate rebates and
returns are monitored and adjusted regularly in the light of contractual and legal obligations,
historical trends, past experience and projected market conditions. Once the uncertainty
associated with the returns and rebates is resolved, revenue is adjusted accordingly. Value
added tax and other sales taxes are excluded from revenue.
(e) Expenditure
Expenditure is recognised in respect of goods and services received when supplied in
accordance with contractual terms. Provision is made when an obligation exists for a future
liability in respect of a past event and where the amount of the obligation can be reliably
estimated. Manufacturing start-up costs between validation and the achievement of normal
production are expensed as incurred. Advertising and promotion expenditure are charged to
the income statement as incurred. Shipment costs on intercompany transfers are charged to
cost of sales, distribution costs on sales to customers are included in selling, general and
administrative (“SG&A”) expenditure.
Restructuring costs are recognised and provided for, where appropriate, in respect of the direct
expenditure of a business reorganisation where the plans are sufficiently detailed and well
advanced, and where a valid expectation to those affected has been created by either starting
to implement the restructuring plans or announcing its main features.
(f) Research and development
Research and development (“R&D”) expenditure is charged to the income statement in the
period in which it is incurred. R&D expenditure comprises expenditure that is directly
attributable to the research and development of new products, including the costs attributable
to the generation of intellectual property and product registrations, depreciation and
amortisation of equipment, real estate and IT assets used by the R&D function. Development
expenditure is capitalised from when the required regulatory approvals to launch a new product
are obtained and the criteria for recognising an asset are met. Property, plant and equipment
used for research and development is capitalised and depreciated in accordance with the CH
Group’s policy described below.
(g) Legal and other disputes
Provision is made for the anticipated settlement costs of legal or other disputes against the CH
Group where an outflow of resources is considered probable, and a reliable estimate can be
made of the likely outcome.
350

The CH Group may become involved in legal proceedings, in respect of which it is not possible
to make a reliable estimate of the expected financial effect, if any, that could result from
ultimate resolution of the proceedings. In these cases, appropriate disclosure about such cases
would be included but no provision would be made. Costs associated with claims made by the
CH Group against third parties are charged to the income statement as they are incurred.
(h) Pensions and other post-employment benefits
The costs of providing pensions under defined benefit schemes are calculated using the
projected unit credit method and spread over the period during which benefit is expected to be
derived from the employees’ services, consistent with the advice of qualified actuaries. Pension
obligations are measured as the present value of estimated future cash flows discounted at
rates reflecting the yields of high-quality corporate bonds. Pension scheme assets are
measured at fair value at the balance sheet date.
The costs of other post-employment liabilities are calculated in a similar way to defined benefit
pension schemes and spread over the period during which benefit is expected to be derived
from the employees’ services, in accordance with the advice of qualified actuaries. Future cash
flows discounted at rates reflecting the yields of high-quality corporate bonds. Pension scheme
assets are measured at fair value at the balance sheet date.
Actuarial gains and losses and the effect of changes in actuarial assumptions, are recognised
in the statement of comprehensive income in the period in which they arise.
The CH Group’s contributions to defined contribution plans are charged to the income
statement as incurred.
(i) Employee share plans
Incentives, in the form of shares in the CH Group’s ultimate parent company, GlaxoSmithKline
plc, are provided to employees under share option and share award schemes. These schemes
are operated by GSK affiliates.
The fair values of these options and awards are calculated at their grant dates using a Black-
Scholes option pricing model and charged to the income statement over the relevant vesting
periods. At the end of each reporting period, the GSK Group reviews its charge and revises it
accordingly based on the number of options expected to vest.
(j) Property, plant and equipment
Property, plant and equipment (“PP&E”) is stated at the cost of purchase or construction less
provisions for depreciation and impairment. Financing costs are capitalised within the cost of
qualifying assets under construction.
Depreciation is calculated to write off the cost less residual value of PP&E, excluding freehold
land, using the straight-line basis over the expected useful life. Residual values and lives are
reviewed, and where appropriate adjusted, annually. The normal expected useful lives of the
major categories of PP&E are:
Freehold buildings 20 to 50 years
Leasehold land and buildings Lease term or 20 to 50 years
Plant and machinery 10 to 20 years
Equipment and vehicles 3 to 10 years
On disposal of PP&E, the cost and related accumulated depreciation and impairments are
removed from the Historical Financial Information and the net amount, less any proceeds, is
taken to the income statement.
351
(k) Intangible assets
Goodwill
Goodwill arising on consolidation represents the excess of the cost of acquisition over the fair
value of the CH Group’s share of the identifiable assets and liabilities of the acquired
subsidiaries at the date of acquisition. Goodwill is tested annually for impairment, or more
frequently where indicators of impairment exist and is carried at cost less any accumulated
impairment losses.
Goodwill is allocated to cash generating units (“CGU”) for the purpose of impairment testing. A
CGU is identified at the lowest aggregation of assets that generate largely independent cash
inflows, and that which is looked at by management for monitoring and managing the business.
If the recoverable amount of the CGU is less than the carrying amount, an impairment loss is
allocated first to reduce the carrying amount of any goodwill allocated to the unit and then to
the other assets of the unit pro rata on the basis of the carrying amount of each asset in the
unit. Any impairment is immediately recognised in the consolidated income statement and an
impairment loss recognised for goodwill is not subsequently reversed.
On disposal, the attributable amount of goodwill is included in the determination of the gain or
loss on disposal.
Other intangible assets
Intangible assets are stated at cost less provisions for amortisation and impairments.
Licences, patents, know-how and marketing rights separately acquired or acquired as part of a
business combination are amortised over their estimated useful lives, generally not exceeding
20 years, using the straight-line basis from the time they are available for use. The estimated
useful lives for determining the amortisation charge consider patent lives, where applicable, as
well as the value obtained from periods of non-exclusivity. Asset lives are reviewed and, where
appropriate, adjusted annually.
Any development costs incurred by the CH Group and associated with acquired licences,
patents, know-how or marketing rights are written off to the income statement when incurred,
unless the criteria for recognition of an internally generated intangible asset are met, usually
when a regulatory filing has been made in a major market and approval is considered highly
probable.
Acquired brands are valued independently as part of the fair value of businesses acquired from
third parties where the brand has a value which is substantial and long term and where the
brands either are contractual or legal in nature or can be sold separately from the rest of the
businesses acquired. Brands are amortised over their estimated useful lives of up to 20 years,
except where it is considered that the useful economic life is indefinite.
The costs of acquiring and developing computer software for internal use and internet sites for
external use are capitalised as intangible fixed assets where the software or site supports a
significant business system and the expenditure leads to the creation of an asset. ERP
systems software is amortised over seven to ten years and other computer software over three
to five years.
(l) Leases
The CH Group recognises right of use assets under lease arrangements in which it is the
lessee, except for short term leases (defined as leases with a lease term of 12 months or less)
and leases of low value assets. Rights to use assets owned by third parties under lease
arrangements are capitalised at the inception of the lease and recognised on the balance
sheet. The corresponding liability to the lessor is recognised as a lease obligation within short
and long-term borrowings. The carrying amount is subsequently increased to reflect interest on
the lease liability and reduced by lease payments made.
352
For calculating the discounted lease liability on leases with annual payments of £2 million or
more, the implicit rate in the lease is used. If this is not available, the incremental borrowing
rate with a lease specific adjustment is used. If neither of these is available, and for leases with
annual payments of less than £2 million, the incremental borrowing rate is calculated at the
rate of interest at which the CH Group would have been able to borrow for a similar term and
with a similar security the funds necessary to obtain a similar asset in a similar market.
Finance costs are charged to the income statement to produce a constant periodic rate of
charge on the remaining balance of the obligations for each accounting period.
Variable rents are not part of the lease liability and the right of use asset. These payments are
charged to the income statement as incurred. Short-term and low value leases are not
capitalised, and lease rentals are also charged to the income statement as incurred.
Non-lease components are accounted for separately from the lease components in plant and
equipment leases but are not separately accounted for in land and buildings or vehicle leases.
If modifications or reassessments occur, the lease liability and right of use asset are
re-measured.
Right of use assets where title is expected to pass to the CH Group at a point in the future are
depreciated on a basis constant with similar owned assets. In other cases, right of use assets
are depreciated over the shorter of the useful life of the asset or the lease term.
(m) Impairment of non-current assets
The carrying values of all non-current assets are reviewed for impairment, either on a stand-
alone basis or as part of a larger CGU, when there is an indication that the assets might be
impaired. Additionally, goodwill, intangible assets with indefinite useful lives and intangible
assets which are not yet available for use are tested for impairment annually. Any provision for
impairment is charged to the income statement.
Impairments of goodwill are not reversed. Impairment losses on other non-current assets are
only reversed if there has been a change in estimates used to determine recoverable amounts
and only to the extent that the revised recoverable amounts do not exceed the carrying values
that would have existed, net of depreciation or amortisation, had no impairments been
recognised.
(n) Inventories
Inventories are included in the Historical Financial Information at the lower of cost (including
raw materials, direct labour, other direct costs and related production overheads) and net
realisable value. Cost is determined on a first in, first out basis.
(o) Trade payables
Trade payables are initially recognised at fair value and then held at amortised cost. Long-term
payables are discounted where the effect is material.
(p) Taxation
Current tax is provided at the amounts expected to be paid applying tax rates that have been
enacted or substantively enacted by the balance sheet date. A separate return approach is
used for calculating income tax provisions and related deferred tax assets and liabilities.
Deferred tax is provided in full on temporary differences arising between the tax bases of
assets and liabilities and their carrying amounts in the Historical Financial Information.
353
Deferred tax assets are recognised to the extent that it is probable that future taxable profits
will be available against which the temporary differences can be utilised. Deferred tax is
provided on temporary differences arising on investments in subsidiaries except where the
timing of the reversal of the temporary difference can be controlled and it is probable that the
temporary difference will not reverse in the foreseeable future. Deferred tax is provided using
rates of tax that have been enacted or substantively enacted by the balance sheet date.
(q) Financial instruments
Financial assets
Financial assets are measured at amortised cost, fair value through other comprehensive
income (FVTOCI) or fair value through profit or loss (FVTPL). The measurement basis is
determined by reference to both the business model for managing the financial asset and the
contractual cash flow characteristics of the financial asset. For financial assets other than trade
receivables a 12-month expected credit loss (ECL) allowance is recorded on initial recognition.
If there is subsequent evidence of a significant increase in the credit risk of an asset, the
allowance is increased to reflect the full lifetime ECL. If there is no realistic prospect of
recovery, the asset is written off.
Trade receivables
Trade receivables are measured in accordance with the business model under which each
portfolio of trade receivables is held. The CH Group has portfolios in two of the three business
models under IFRS 9 to collect the contractual cash flows (measured at amortised cost) and to
sell the contractual cash flows (measured at FVTPL).
Trade receivables measured at amortised cost are carried at the original invoice amount less
allowances for ECL. ECLs are calculated in accordance with the simplified approach permitted
by IFRS 9, using a provision matrix applying lifetime historical credit loss experience to the
trade receivables. The ECL rate varies depending on whether and the extent to which
settlement of the trade receivables is overdue and it is also adjusted as appropriate to reflect
current economic conditions and estimates of future conditions. For the purpose of determining
credit loss rates, customers are classified into groupings that have similar loss patterns. The
key drivers of the loss rate are the location and type of customer.
When a trade receivable is determined to have no reasonable expectation of recovery it is
written off, firstly against any expected credit loss allowance available and then to the income
statement.
Subsequent recoveries of amounts previously provided for or written off are credited to the
income statement. Long-term receivables are discounted where the effect is material.
Cash and cash equivalents
Cash held in deposit accounts is measured at amortised cost. Cash and short-term deposits in
the statement of financial position comprise cash at banks and on hand and short-term highly
liquid deposits with a maturity of three months or less, that are readily convertible to a known
amount of cash and subject to an insignificant risk of changes in value.
Borrowings
All borrowings are initially recorded at the amount of proceeds received, net of transaction
costs. Borrowings are subsequently carried at amortised cost, with the difference between the
proceeds, net of transaction costs, and the amount due on redemption being recognised as a
charge to the income statement over the period of the relevant borrowing.
354
Derivative financial instruments and hedging
Derivative financial instruments are used to manage exposure to market risks. The principal
derivative instruments used by the CH Group are forward foreign exchange contracts and
swaps. The CH Group does not hold or issue derivative financial instruments for trading or
speculative purposes.
Derivative financial instruments are classified as held-for-trading and are measured at fair
value. Derivatives designated as hedging instruments are classified on inception as cash flow
hedges, net investment hedges or fair value hedges.
Changes in the fair value of derivatives designated as cash flow hedges are recognised in
other comprehensive income to the extent that the hedges are effective. Ineffective portions
are recognised in profit or loss immediately. Amounts deferred in other comprehensive income
are reclassified to the income statement when the hedged item affects profit or loss.
Net investment hedges are accounted for in a similar way to cash flow hedges.
Changes in fair values of derivatives designated as fair value hedges are recorded in the
income statement together with the changes in the fair value of the hedged asset or liability.
Changes in the fair value of any derivative instruments that do not qualify for hedge accounting
are recognised immediately in the income statement.
3. Key accounting judgements and estimates
In preparing the consolidated Historical Financial Information, management is required to make
judgements about when or how items should be recognised in the consolidated Historical
Financial Information and estimates and assumptions that affect the amounts of assets,
liabilities, income and expenses reported in the consolidated Historical Financial Information.
Actual amounts and results could differ from those estimates. The following are the critical
accounting judgements and key sources of estimation uncertainty.
Taxation
Estimates
Management makes the judgement of whether there is sufficient information to be able to
make a reliable estimate of the outcome of the dispute. If insufficient information is available,
no provision is made.
If sufficient information is available, in estimating a potential tax liability, the CH Group applies
a risk-based approach to determine the transactions most likely to be subject to challenge,
assuming that the relevant tax authority will review and have full knowledge of all the relevant
information, and the probability that the CH Group would be able to obtain compensatory
adjustments under international tax treaties. These estimates consider the specific
circumstances of each dispute and relevant external advice, are inherently judgemental and
could change substantially over time as each dispute progresses and new facts emerge.
Further details and the factors affecting the tax charge in future years are set out in Note 13,
‘Taxation’. Where open tax matters exist, the ultimate liability for such matters may vary from
the amounts provided and is dependent upon the outcome of negotiations with the relevant tax
authorities or, if necessary, litigation proceedings. At 31 December 2021, the CH Group had
recognised provisions of £150 million in respect of such uncertain tax positions (2020:
£124 million and 2019: £123 million). Due to the number of uncertain tax positions held and the
number of jurisdictions to which these relate, it is not practicable to give meaningful sensitivity
estimates.
355
Indefinite life brands
Estimates
Estimation of recoverable amount of indefinite life brands. The CH Group tests at least
annually whether indefinite life brands have suffered any impairment, in accordance with the
accounting policy. The recoverable amounts of indefinite life brands have been determined
based on fair value less costs of disposal model. These calculations require the use of
estimates and assumptions consistent with the most up to date budgets and plans that have
been formally approved by management and are based on discounted cash flow forecasts
using estimated long-term growth rates. The key assumptions used and sensitivity analysis are
disclosed in Note 18, ‘Intangible assets’.
Legal and other disputes
Judgement and estimates
Management makes a judgement of whether it is remote, possible or probable that an outflow
of resources embodying economic benefits will be required to settle legal obligations. To the
extent that the potential outflow is assessed as possible but not probable or insufficient
information is available to make a judgement on whether a potential outflow is probable, no
provision is made and disclosure related to the claim is provided.
For legal obligations that are assessed as leading to a probable outflow and sufficient
information is available, the estimated provisions take into account the specific circumstances
of each dispute and relevant external advice, are inherently judgemental and could change
substantially over time as each dispute progresses and new facts emerge. Details of the status
and various uncertainties involved in the significant unresolved disputes are set out in Note 27,
‘Contingent Liabilities’. The CH Group’s Directors, having taken legal advice, have established
provisions after taking into account the relevant facts and circumstances of each matter and in
accordance with accounting requirements.
The CH Group may become involved in legal proceedings, in respect of which it is not possible
to make a reliable estimate of the expected financial effect, or practicable to give a meaningful
range of outcomes that could result from ultimate resolution of the proceedings. In these cases,
appropriate disclosure about such cases would be provided, but no provision would be made
and no contingent liability can be quantified. The ultimate liability for legal claims may vary from
the amounts provided and is dependent upon the outcome of litigation proceedings,
investigations, and possible settlement negotiations. The position could change over time and,
therefore, there can be no assurance that any losses that result from the outcome of any legal
proceedings will not exceed the amount of the provisions reported in the Group’s financial
statements by a material amount.
4. Adoption of new and revised standards
In 2020, the CH Group implemented an amendment to IFRS 3 ‘Business combinations’ which
was issued in October 2018. The amendment clarifies the definition of a business and permits
a simplified initial assessment of whether an acquired set of activities and assets is a group of
assets rather than a business. The amendment did not have a material impact on the results or
financial position of the CH Group in 2020.
‘Covid-19-Related Rent Concessions (Amendment to IFRS 16)’ was issued in May 2020. It
introduces a practical expedient to IFRS 16 ‘Leases’ which permits a lessee to elect not to
assess whether a COVID-19-related concession in respect of rent due for periods to 30 June
2021 is a lease modification. The amendment is applicable for annual reporting periods
beginning on or after 1 June 2020 and earlier application is permitted. The amendment did not
have a material impact on the results or financial position of the CH Group in 2020.
356
The CH Group previously accounted for SaaS (software as a service) configuration and
customisation costs as intangible assets. Following the IFRS IC (Interpretation Committee)
agenda decision on SaaS in April 2021, the CH Group has adopted the treatment set out in the
IFRS IC agenda decision and expensed configuration and customisation costs where the entity
does not control the software being configured. The impact of the change has not had a
material impact on the results or financial position of the CH Group.
Where the retirement benefit to which an employee is entitled is capped at a specified number
of consecutive years, the CH Group previously accounted for these employee benefits from the
employment commencement date. Following the IFRS IC agenda decision on Attributing
Benefit to Periods of Service in May 2021, the CH Group has adopted the treatment set out in
the IFRS IC agenda decision to account for the employee benefits during the last specified
number of years where the employee earn the benefit. The impact of the change has not had a
material impact on the results or financial position of the CH Group.
During the year, the CH Group implemented ‘Interest Rate Benchmark Reform Phase 2 -
Amendments to IFRS 9, IAS 39, IFRS 7, IFRS 4 and IFRS 16’ which was issued in August
2020. The amendments address issues that arise from implementation of the reforms,
including the replacement of one benchmark with an alternative one. A practical expedient is
provided such that the change to contractual cash flows for financial assets and liabilities
(including lease liabilities) is accounted for prospectively by revising the effective interest rate.
In addition, hedge accounting will not be discontinued solely because of the IBOR reform.
Further information is provided in note 33.
Certain new accounting standards, amendments to accounting standards and interpretations
have been published that are not mandatory for 31 December 2021 reporting periods and have
not been early adopted by the CH Group. These standards, amendments or interpretations are
not expected to have a material impact on the entity in the current or future reporting periods.
5. Exchange rates
The CH Group operates in many countries and earns revenues and incurs costs in many
currencies. The results of the CH Group, as reported in Sterling, are affected by movements in
exchange rates between Sterling and other currencies. Average exchange rates, as modified
by specific transaction rates for large transactions, prevailing during the year, are used to
translate the results and cash flows of overseas subsidiaries into Sterling. Year-end rates are
used to translate the net assets of those entities. The currencies which most influenced these
translations and the relevant exchange rates were:
Average rates Period end rates
2021 2020 2019 2021 2020 2019
Average rates:
USD/£ 1.38 1.29 1.28 1.35 1.36 1.32
Euro/£ 1.16 1.13 1.14 1.19 1.11 1.18
Swiss Franc/£ 1.25 1.21 1.27 1.23 1.20 1.28
CNY/£ 8.86 8.91 8.82 8.56 8.93 9.19
6. Revenue and segment information
Analysis of revenue by geography is included below, the composition of these geographical
segments is reviewed on an annual basis.
For management reporting purposes, the CH Group is organised into business units based on
geographical areas and has three reportable segments, as follows:
• North America
• Europe, Middle East, Africa and Latin America (EMEA and LatAm)
• Asia Pacific (APAC)
357

No operating segments have been aggregated to form the above reportable operating
segments.
The primary products sold by each of the reportable segments consist of Oral Health, Pain
Relief, Vitamins, Minerals and Supplements, Respiratory Health, Digestive Health and Other
products and the product portfolio is consistent across the reportable segments.
The Commercial Operations Board is the Chief Operating Decision Maker (“CODM”) who
monitors the operating results of the Group’s business units separately for the purpose of
making decisions about resource allocation and performance assessment. The CODM uses a
measure of adjusted operating profit to assess the performance of the reportable segments.
Adjusted Operating Profit is defined as operating profit less net intangible amortisation and
impairment of brands, licenses, and patents, restructuring costs, transaction related costs,
separation and admission costs, and disposals and other disposal related adjusting costs. The
CODM does not review IFRS operating profit or total assets on a segment basis.
2021 2020 2019
Revenue by segment £m £m £m
North America 3,525 3,779 2,880
EMEA and LatAm 3,877 4,059 3,898
APAC 2,143 2,054 1,702
Total revenue 9,545 9,892 8,480
2021 2020 2019
Adjusted operating profit by segment £m £m £m
North America 828 897 660
EMEA and LatAm 960 857 746
APAC 461 377 311
Corporate and other unallocated (77) (57) (63)
2,172 2,074 1,654
Reconciling items between adjusted operating
profit and operating profit:
Net amortisation and impairment of intangible assets 18,8 (16) (97) (36)
Restructuring costs 12 (195) (411) (330)
Transaction related costs 19 - (91) (366)
Separation and admission costs 8 (278) (66) -
Disposals and others 29 (45) 189 (25)
Group operating profit 1,638 1,598 897
Net finance costs (2) (7) (11)
Profit before taxation 1,636 1,591 886
Net amortisation and impairment of intangible assets includes amortisation and impairment of
intangible assets, excluding computer software, and impairment of goodwill, net of reversals of
impairment. Transaction related costs relate to transaction related accounting including the
unwind of uplift of fair value in inventory.
2021
2020 2019
Revenue by product category £m
£m £m
Oral health 2,724 2,745 2,657
Pain relief 2,237 2,192 1,742
Vitamins, minerals and supplements 1,501 1,494 597
Respiratory health 1,132 1,298 1,318
Digestive health and other 1,951 2,163 2,166
Total revenue 9,545 9,892 8,480
358
Revenue attributable to the country of domicile and all foreign countries of operation greater
than 10% are included below:
2021
2020 2019
Revenue by location of customer £m
£m £m
UK 327 374 380
US 3,138 3,360 2,559
China 801 700 474
Rest of the World 5,279 5,458 5,067
Total revenue 9,545 9,892 8,480
North
America
Europe,
Middle
East and
Africa,
Latin
America
Asia
Pacific
Other
reconciling
items
Total
CH
Group
Other segmental information £m £m £m £m £m
Year ended 31 December 2021
Impairment charges 5 5 2 25 37
Impairment reversal - - - (48) (48)
Year ended 31 December 2020
Impairment charges 6 10 6 68 90
Impairment reversal - - - (21) (21)
Year ended 31 December 2019
Impairment charges 5 1 - 19 25
Impairment reversal (9) - - (10) (19)
Non-current assets attributable to the country of domicile and all foreign countries of operation
greater than 10% are included below:
2021 2020 2019
Non-current assets by location of subsidiary £m £m £m
UK 430 410 471
US & Puerto Rico 7,884 7,827 8,262
Rest of the World 20,551 20,593 20,910
Non-current assets 28,865 28,830 29,643
Non-current assets by location excludes derivatives, deferred tax assets and post-retirement
benefit assets.
7. Other operating (expense)/income
In 2021, as part of the continued strategic review of the business, the CH Group sold several
assets including Transderm Scop, Acne-Aid and Baldriparan. The CH Group has recognised a
total net gain on disposals of £31 million in the year.
In 2020, as a result of the strategic review of the business, the CH Group sold several
businesses and assets including Breathe Right, Physiogel, Coldrex, Venoruton, certain
intellectual property rights of Horlicks and other assets and smaller businesses. Thermacare
has been disposed in 2020 to meet EMEA anti-trust requirements. The CH Group has
recognised a total net gain on disposals of £212 million in the year.
In 2019 Other operating expense includes net losses of £17 million relating to the deal costs
incurred for the disposal of intellectual property and businesses, mainly related to deal costs
preparing Thermacare for the divestment.
Certain Horlicks intellectual property rights amounting to £16 million legally owned by an entity
within the CH Group were disposed of in 2020. These intellectual property rights were legally
359

owned by an entity within the CH Group however the GSK Group has the beneficial title to
these. The intellectual property rights were sold for £74 million resulting in a gain on disposal of
£58 million. The proceeds of the sale were subsequently declared and paid as a dividend to
the GSK Group in 2020, refer to Note 14 ‘Dividends’.
8. Operating profit
2021 2020 2019
The following items have been included in operating profit: £m £m £m
Advertising and promotion 1,941 2,013 1,772
Distribution costs 209 226 194
Impairment of assets held for sale
Intangible assets - 20 -
Property, plant and equipment - 3 -
Net foreign exchange losses 11 12 7
Short term lease charge 1 1 1
Separation and admission costs 278 66 -
Separation and admission costs represent costs incurred in relation to and in connection with
the separation and potential listing of the CH Group as a standalone business. These costs are
not directly attributable to the sale of the CH Group’s products and specifically relate to the
activities mentioned above, affecting comparability of the CH Group’s financial results in
historic and future reporting periods.
9. Employee and Key Management Personnel costs
2021 2020 2019
£m £m £m
Wages and salaries 1,287 1,362 1,285
Social security costs 147 151 109
Pension and other post-employment costs (Note 25) 30 30 29
Cost of share-based incentive plans (Note 34) 59 63 58
Severance costs from integration and restructuring activities 95 77 89
1,618 1,683 1,570
The CH Group provides benefits to employees, commensurate with local practice in individual
countries, including, in some markets, healthcare insurance, subsidised car schemes and
personal life insurance.
All individuals performing services for the CH Group are employed and remunerated by CH
Group companies or other members of the GSK Group. Where a management charge for
wages and salaries has been made from entities outside the CH Group, such amounts are not
included in wages and salaries above as it is not practical to separate such amounts from other
management recharges.
360

Details of Key Management Personnel
Key Management Personnel comprises the Executive board members and the Consumer
Healthcare leadership team (“CHLT”). The compensation of Key Management Personnel in
respect of their services to the CH Group in aggregate was as follows:
2021 2020 2019
£m £m £m
Wages and salaries 11.7 13.9 13.8
Social security costs 1.1 1.1 1.1
Pension and other post-employment costs 1.7 1.6 1.4
Cost of share-based incentive plans 6.9 7.8 7.8
21.4 24.4 24.1
Retirement benefits accrued under defined benefit schemes sponsored by sister companies
within the GSK Group for one director in 2021, two directors in 2020 and two directors in 2019.
Two (2020: three, 2019: three) directors received share awards under long term incentive
plans in respect of qualifying services to the CH Group in 2021.
10. Finance income
2021 2020 2019
£m £m £m
Interest income arising from:
Cash and cash equivalents 3 2 2
Other receivables with GSK Group companies 10 12 18
Derivatives at fair value through profit or loss 4 4 4
Net gains arising from:
Financial instruments measured at fair value through profit or loss (35) (27) -
Retranslation of loans 35 29 -
17 20 24
2021 2020 2019
£m £m £m
Finance income arising from:
Financial assets measured at amortised cost 13 14 20
Financial assets measured at fair value through profit or loss 4 4 4
Net gains arising from:
Financial instruments measured at fair value through profit or loss (35) (27) -
Retranslation of loans 35 29 -
17 20 24
11. Finance expense
2021 2020 2019
£m £m £m
Interest expense arising on:
Financial liabilities at amortised cost (3) (2) (5)
Derivatives at fair value through profit or loss (5) (7) (12)
Loans with GSK Group companies (4) (6) (9)
Net losses arising from:
Financial instruments measured at fair value through profit or loss - - (4)
Retranslation of loans - - 3
Finance expense arising on lease liabilities (4) (7) (4)
Other finance expense (3) (5) (4)
(19) (27) (35)
361
12. Restructuring costs
Restructuring costs mainly relate to initiatives announced since formation of the CH Group and
include Personnel costs, impairments of tangible assets and computer software relating to
restructuring programmes.
Restructuring costs are those mainly related to specific Board-approved restructuring
programmes, including integration costs following material acquisitions, which are structural
and are of a significant scale in terms of the costs of individual or related projects. In 2021, CH
Group defined such programmes to comprise of any projects exceeding £15 million whilst in
prior periods, this was determined to be any projects exceeding £25 million. This change did
not result in any differences in the nature of restructuring programmes included as part of
restructuring costs for 2020 and 2019.
In 2021, Restructuring costs of £195 million (2020: £411 million, 2019: £330 million) have been
charged to the income statement and recognised in the expense categories outlined below.
Restructuring costs are mainly activities to generate synergies from the integration of Pfizer’s
Consumer Healthcare business into the CH Group’s business, following the Pfizer Transaction
completed on 31 July 2019.
The unutilised balances of the restructuring costs as at 31 December 2021, 2020 and 2019 are
included in Note 26 ‘Other provisions’.
2021 2020 2019
£m £m £m
Cost of sales 44 89 69
Selling, general and administration, and other operating expenses 150 314 236
Research and development 1 8 25
195 411 330
13. Taxation
The major components of income tax expense are:
2021 2020 2019
Taxation charge/(credit) based on profits for the period £m £m £m
Current year charge
361 540 196
Charge in respect of prior periods
(50) 11 21
Total current taxation
311 551 217
Total deferred taxation
(114) (141) (18)
Total taxation charge 197 410 199
The tax charge on total profits amounted to £197 million (2020: £410 million and 2019: £199
million) and represented an effective tax rate of 12% (2020: 26% and 2019: 23%).
2021
2020 2019
Reconciliation of the taxation rate on the CH Group profits £m £m £m
Profit before tax 1,636 1,591 886
UK statutory rate of taxation of 19% 311 302 167
Differences in overseas taxation rates 105 124 97
Benefit of substance-based tax rulings (18) (70) (29)
R&D tax credits (2) (2) (2)
Tax losses not recognised 3 8 -
Permanent differences on disposals, acquisitions and transfers (164) (20) -
Items non-deductible/taxable for tax purposes 3 25 (1)
Re-assessment of prior year estimates (70) 19 (29)
Changes in tax rates
29
24 (4)
Total tax charge 197 410 199
362
Permanent differences on disposals, acquisitions and transfers in 2021 reflects tax credits
arising on the transfer of intellectual property within the CH Group.
Future tax charges, and therefore the effective tax rate, may be affected by factors such as
acquisitions, disposals, restructurings, the location of research and development activity, tax
regime reforms, agreements with tax authorities and resolution of open matters as the CH
Group continue to bring the tax affairs up to date around the world. The CH Group operates in
countries where the tax rate differs from the UK tax rate and the taxable profits earned and tax
rates in those countries vary from year to year. The impact of these overseas taxes on the
overall rate of tax is shown above.
Taxation matters
The integrated nature of the CH Group’s worldwide operations involves significant investment
in research and manufacture at a limited number of locations, with consequential cross-border
supply routes into numerous end-markets. This gives rise to complexity and delay in
negotiations with revenue authorities as to the profits on which individual CH Group companies
are liable to tax.
In line with current OECD guidelines, the CH Group base its transfer pricing policy on the
‘arm’s length’ principle. However, different tax authorities may seek to attribute further profit to
activities being undertaken in their jurisdiction potentially resulting in double taxation. The CH
Group also has open items in several jurisdictions concerning such matters as the deductibility
of particular expenses and the tax treatment of certain business transactions. The CH Group
applies a risk based approach to determine the transactions most likely to be subject to
challenge and the probability that the CH Group would be able to obtain compensatory
adjustments under international tax treaties.
The calculation of the CH Group’s total tax charge therefore necessarily involves a degree of
estimation and judgement in respect of certain items whose tax treatment cannot be finally
determined until resolution has been reached with the relevant tax authority or, as appropriate,
through a formal legal process.
Whilst a newly standalone CH Group may be subject to additional and/or different scrutiny from
tax authorities than as part of a wider-GSK Group, the CH Group continues to believe that it
has made adequate provision for the liabilities it may bear in respect of periods which are open
and not yet agreed by tax authorities. The ultimate liability for such matters may vary from the
amounts provided and is dependent upon the outcome of agreements with relevant tax
authorities or litigation where appropriate. At 31 December 2021, the CH Group had
recognised provisions of £150 million in respect of such uncertain tax positions (2020:
£124 million and 2019: £123 million).
In 2021, the aggregate amount of unremitted profits at the balance sheet date was
approximately £1.7 billion (2020: £1.6 billion and 2019: £1.4 billion). UK legislation relating to
company distributions provides for exemption from tax for most repatriated profits, subject to
certain exceptions. Provision for deferred tax liabilities of £38 million (2020: £24 million and
2019: £14 million) has been made in respect of withholding taxation that would arise on the
distribution of profits by certain overseas subsidiaries. Deferred tax is not provided on
temporary differences of £147 million (2020: £135 million and 2019: nil) arising on unremitted
profits as management can control any future reversal and does not consider such a reversal
to be probable.
363

Movement in deferred tax assets and liabilities
Accelerated
capital
allowances
Intangibles
Pensions &
other post-
employment
benefits
Tax
losses
Other net
temporary
differences
Total
£m
£m £m £m £m £m
As at 1 January 2021 (45) (3,451) 82 26 266 (3,122)
Exchange adjustments
(6) (18) (8) - 9 (23)
(Charge)/credit to income
statement
(15) 31 (12) (17) 127 114
Credit to statement of
comprehensive income
- - (12) - (2) (14)
At 31 December 2021 (66) (3,438) 50 9 400 (3,045)
Accelerated
capital
allowances
Intangibles
Pensions &
other post-
employment
benefits
Tax
losses
Other net
temporary
differences
Total
£m
£m £m £m £m £m
As at 1 January 2020 (35) (3,563) 64 17 257 (3,260)
Exchange adjustments 1 11 1 1 - 14
(Charge)/credit to income
statement
(11) 131 4 8 9 141
Credit to statement of
comprehensive income
- - 13 - - 13
Transfers from liabilities
directly related to assets
held for sale
- (30) - - - (30)
At 31 December 2020 (45) (3,451) 82 26 266 (3,122)
Accelerated
capital
allowances
Intangibles
Pensions &
other post-
employment
benefits
Tax
losses
Other net
temporary
differences
Total
£m £m £m £m £m £m
As at 1 January 2019 (39) (1,103) 61 14 165 (902)
Exchange adjustments - 212 (3) (1) - 208
(Charge)/credit to income
statement
(1) (51) (10) (7) 91 22
Credit to statement of
comprehensive income
-- 1-23
Additions through business
combination
5 (2,621) 15 11 (1) (2,591)
At 31 December 2019 (35) (3,563) 64 17 257 (3,260)
364
Recognised tax losses comprise £9 million (2020: £26 million and 2019: £17 million) in respect
of net trading losses. Other net temporary differences include accrued expenses for which a
tax deduction is only available on a paid basis and deferred tax on intra-group profits arising on
intercompany inventories which are eliminated within the consolidated Historical Financial
Information. As intra-group profits are not eliminated from the individual entities’ tax returns a
temporary difference arises that will reverse at the point in time inventory is sold externally.
After offsetting deferred tax assets and liabilities where appropriate within territories, the net
deferred tax liability comprises:
2021
2020 2019
£m
£m £m
Deferred tax assets
312
251 254
Deferred tax liabilities
(3,357)
(3,373) (3,514)
(3,045) (3,122) (3,260)
For the periods presented, US entities within the CH Group remain party to the GSK Group
unitary state filing. US temporary differences therefore continue to be valued at the unitary
state tax blended rate applicable to the GSK Group. As a result of the demerger, the US
entities will no longer be part of the GSK Group unitary state filing and these entities will need
to prepare standalone state tax filings. This may result in a higher rate of state taxes applying
to the CH Group for both current and deferred tax liabilities.
2021
2021 2020 2020 2019 2019
Unrecognised tax
losses
Tax
losses
Unrecognised
asset
Tax
losses
Unrecognised
asset
Tax
losses
Unrecognised
asset
£m
£m £m £m £m £m
Trading losses expiring:
Within 10 years 15 3 26 3 6 1
More than 10 years 326 15 335 17 349 24
Available indefinitely 67 10 86 15 - -
As at 31 December 408 28 447 35 355 25
Deferred tax assets are recognised where it is probable that future taxable profit will be
available to utilise losses, as supported by management forecasts.
14. Dividends
During the years ended 31 December 2021, 2020 and 2019 the CH Group declared and paid
dividends as set forth below. No further dividends were declared or paid.
Date paid £ per share £m
Dividends paid in 2021:
30 March 2021 621 621
28 September 2021 49 49
21 December 2021 478 478
1,148
Dividends paid in 2020:
17 June 2020 54 54
19 June 2020 1,292 1,292
22 September 2020 20 20
23 September 2020 750 750
9 November 2020 255 255
2,371
365

Date paid £ per share £m
Dividends paid in 2019:
28 March 2019 72 43
24 May 2019 1,127 338
28 May 2019 75,000 15
7 June 2019 25,470,588 432
28 June 2019 1,829,268 300
28 June 2019 3,199 24
1,152
15. Earnings per share
2021 2020 2019
pence pence pence
Basic earnings per share 139,000 114,500 65,500
Diluted earnings per share 139,000 114,500 65,500
Basic earnings per share has been calculated by dividing the profit attributable to shareholders
by the weighted average number of shares in issue during the period, with 1,000,000 shares
outstanding on 1 January 2021, 2020 and 2019.
Diluted earnings per share has been calculated after adjusting the weighted average number of
shares used in the basic calculation to assume the conversion of all potentially dilutive shares.
However, since employees’ share options are satisfied in shares of GSK Group, there are no
dilutive equity instruments. The number of shares in issue above may not be representative of
the number of shares in issue in the future.
The numbers of shares used in calculating basic and diluted earnings per share are reconciled
below:
2021 2020 2019
Weighted average number of shares in issue ‘000 ‘000 ‘000
Basic 1,000 1,000 1,000
Dilution for share options and awards - - -
Diluted 1,000 1,000 1,000
366

16. Property, plant and equipment
Land and
buildings
Plant,
equipment
and vehicles
Assets
under
construction
Total
£m £m £m £m
Cost at 1 January 2019 762 1,321 204 2,287
Exchange adjustments 14 (14) (13) (13)
Additions 22 62 118 202
Additions through business combinations 145 175 34 354
Disposals and write-offs (10) (174) (22) (206)
Reclassifications (4) 102 (102) (4)
Transfer to assets held for sale - - (9) (9)
Cost at 31 December 2019 929 1,472 210 2,611
Exchange adjustments (8) 5 (4) (7)
Additions 4 9 217 230
Additions through business combinations - 6 - 6
Disposals and write-offs (27) (81) (11) (119)
Reclassifications 26 96 (130) (8)
Transfer to assets held for sale (14) (17) (4) (35)
Cost at 31 December 2020 910 1,490 278 2,678
Exchange adjustments 15 (27) (3) (15)
Additions 1 13 215 229
Disposals and write-offs (40) (132) (7) (179)
Reclassifications 34 150 (184) -
Transfer to assets held for sale - (8) - (8)
Cost at 31 December 2021 920 1,486 299 2,705
Depreciation at 1 January 2019 (195) (803) - (998)
Exchange adjustments (43) (16) - (59)
Charge for the year (33) (134) - (167)
Disposals and write-offs 6 122 - 128
Depreciation at 31 December 2019 (265) (831) - (1,096)
Exchange adjustments 1 (3) - (2)
Charge for the year (39) (128) - (167)
Disposals and write-offs 20 80 - 100
Transfer to assets held for sale 10 19 - 29
Depreciation at 31 December 2020 (273) (863) - (1,136)
Exchange adjustments - 17 - 17
Charge for the year (32) (107) - (139)
Disposals and write-offs 28 114 - 142
Transfer to assets held for sale - 6 - 6
Depreciation at 31 December 2021 (277) (833) - (1,110)
367

Land and
buildings
Plant,
equipment
and vehicles
Assets under
construction
Total
£m £m £m £m
Impairment at 1 January 2019 - (54) (8) (62)
Exchange adjustments - 2 5 7
Impairment losses (4) (1) (1) (6)
Disposals and write-offs - 6 1 7
Reversal of impairments - 8 1 9
Impairment at 31 December 2019
(4)
(39) (2) (45)
Exchange adjustments - 1 (1) -
Impairment losses (8) (10) (1) (19)
Disposals and write-offs 3 2 - 5
Reversal of impairments
3
--3
Impairment at 31 December 2020
(6)
(46) (4) (56)
Exchange adjustments (2) - - (2)
Impairment losses (6) (8) (3) (17)
Disposals and write-offs 8 20 3 31
Reversal of impairments - 12 - 12
Impairment at 31 December 2021 (6) (22) (4) (32)
Depreciation and impairment at 31
December 2019
(269) (870) (2) (1,141)
Depreciation and impairment at 31
December 2020
(279) (909) (4) (1,192)
Depreciation and impairment at 31
December 2021
(283) (855) (4) (1,142)
Net book value at 31 December 2019 660 602 208 1,470
Net book value at 31 December 2020 631 581 274 1,486
Net book value at 31 December 2021 637 631 295 1,563
For the three years ended 31 December 2021, the impairment losses principally arise from
decisions to rationalise facilities and are calculated based on higher of fair value less costs of
disposal and value in use. The fair value less costs of disposal valuation methodology uses
significant inputs which are not based on observable market data, and therefore this valuation
technique is classified as level 3 of the fair value hierarchy. These calculations determine the
net present value of the projected risk-adjusted, post-tax cash flows of the relevant asset or
cash generating unit, applying a discount rate of the CH Group’s post-tax weighted average
cost of capital (“WACC”) of 6%, adjusted where appropriate for relevant specific risks. For
value in use calculations, where an impairment is indicated and a pre-tax cash flow calculation
is expected to give a materially different result, the test would be re-performed using pre-tax
cash flows and a pre-tax discount rate.
Impairment losses of £2 million for 2021 (2020: £11 million and 2019: £3 million) have been
charged to cost of sales and £15 million for 2021 (2020: £8 million and 2019: £3 million) have
been charged to selling, general and administration expenses respectively.
Reversals of impairment arise from subsequent reviews of the impaired assets where the
conditions which gave rise to the original impairments are deemed no longer to apply. All of the
reversals have been credited to cost of sales.
Reclassifications of £8 million for 2020 (2019: £4 million) relating to assets under construction
that have been reclassified to computer software in intangible assets during the year. No
reclassifications were made in 2021.
Certain assets were transferred from property, plant and equipment to assets held for sale and
subsequently disposed of during the year. There were no assets and liabilities held for sale
remaining as at 31 December 2021.
368
17. Right of use assets
Land and
buildings
Plant and
equipment
Vehicles Total
£m £m
£m £m
Net book value at 1 January 2019 104 2 13 119
Exchange adjustments (4) (1) (1) (6)
Additions through business combinations 27 10 2 39
Additions 20 1 10 31
Depreciation (22) (2) (7) (31)
Disposals
- (1)
- (1)
Net book value at 31 December 2019 125 9 17 151
Exchange adjustments (3) - - (3)
Additions 28 1 10 39
Depreciation (39) (1) (8) (48)
Disposals and write-offs
(14) (4)
(5) (23)
Net book value at 31 December 2020 97 5 14 116
Exchange adjustments 1 - (1) -
Additions 27 4 6 37
Depreciation (27) - (8) (35)
Disposals and write-offs
(10) (8)
(1) (19)
Net book value at 31 December 2021
88 1
10 99
An analysis of lease liabilities is set out in Note 24, ‘Borrowings’. The total cash outflow for
leases amounted to £38 million for 2021 (2020: £44 million and 2019: £42 million). There were
no significant lease commitments for leases not commenced at year-ends.
18. Intangible assets
Goodwill
Indefinite
life
brands
Amortised
brands,
licences
and
patents
Computer
Software
Total
£m £m £m £m £m
Cost at 1 January 2019 2,613 8,524 401 279 11,817
Exchange adjustments (100) (1,035) (10) (8) (1,153)
Additions through business
combinations
5,658 12,357 - 31 18,046
Other additions - - 11 42 53
Disposals and asset write-offs - - (2) (2) (4)
Reclassification - (18) 18 4 4
Transfer to assets held for sale -
(227) (14)
- (241)
Cost at 31 December 2019 8,171 19,601 404 346 28,522
Exchange adjustments (29) (82) (9) (3) (123)
Additions through business
combinations
124 - - 2 126
Other additions - - 7 89 96
Disposals and asset write-offs (1) - (9) (13) (23)
Reclassification - (572) 572 8 8
Transfer to assets held for sale - (635) (253) - (888)
Cost at 31 December 2020 8,265 18,312 712 429 27,718
Exchange adjustments (19) 65 (2) (3) 41
Other additions - - 7 66 73
Disposals and asset write-offs - - (23) (20) (43)
Reclassification - (9) 9 - -
Transfer to assets held for sale - (43) (6) - (49)
Cost at 31 December 2021 8,246 18,325 697 472 27,740
369

Goodwill
Indefinite
life
brands
Amortised
brands,
licences
and
patents
Computer
Software
Total
£m £m £m £m £m
Amortisation at 1 January 2019
-
- (143) (105) (248)
Exchange adjustments - - 5 (1) 4
Charge for the period - - (27) (35) (62)
Disposals and asset write-offs - - 1 1 2
Transfer to assets held for sale
-
-3-3
Amortisation at 31 December 2019 - - (161) (140) (301)
Exchange adjustments - - 2 - 2
Charge for the period - - (50) (40) (90)
Disposals and asset write-offs - - 5 12 17
Transfer to assets held for sale
-
-44-44
Amortisation at 31 December 2020 - - (160) (168) (328)
Exchange adjustments - - 1 - 1
Charge for the period - - (40) (54) (94)
Disposals and asset write-offs - - - 3 3
Transfer to assets held for sale
-
-2-2
Amortisation at 31 December 2021
-
- (197) (219) (416)
Goodwill
Indefinite
life
brands
Amortised
brands,
licences
and
patents
Computer
Software
Total
£m £m £m £m £m
Impairment at 1 January 2019 - (240) (17) (2) (259)
Exchange adjustments - 1 1 (1) 1
Impairment losses - (2) (17) - (19)
Reversal of impairment losses - - 10 - 10
Transfer to assets held for sale
-53
5-58
Impairment at 31 December 2019 - (188) (18) (3) (209)
Exchange adjustments - 1 4 - 5
Impairment losses - (10) (35) - (45)
Reversal of impairment losses - - 18 - 18
Reclassification - 39 (39) - -
Transfer to assets held for sale - - 59 - 59
Impairment at 31 December 2020 - (158) (11) (3) (172)
Exchange adjustments - - - - -
Impairment losses - - (12) (8) (20)
Reversal of impairment losses - 36 - - 36
Disposals and asset write-offs
-
-23 427
Impairment at 31 December 2021
-
(122) - (7) (129)
Amortisation and impairment at
31 December 2019
- (188) (179) (143) (510)
Amortisation and impairment at
31 December 2020
- (158) (171) (171) (500)
Amortisation and impairment at
31 December 2021
- (122) (197) (226) (545)
Net book value at 31 December 2019 8,171 19,413 225 203 28,012
Net book value at 31 December 2020 8,265 18,154 541 258 27,218
Net book value at 31 December 2021 8,246 18,203 500 246 27,195
The net book value of computer software included £130 million (2020: £124 million and 2019:
£8 million) of internally generated costs.
370

Goodwill
Goodwill mainly arose from the Novartis transaction in 2015 (£2.6 billion) and the Pfizer
transaction in 2019 (£5.6 billion).
Goodwill is allocated to the CH Group’s CGUs as follows:
2021 2020 2019
£m £m £m
Asia Pacific 2,127 2,132 2,015
Europe, Middle East and Africa, and Latin America 2,902 2,908 2,919
North America 3,217 3,225 3,237
Net book value at 31 December 8,246 8,265 8,171
The CH Group tests goodwill annually for impairment, or more frequently if there are
indications that goodwill might be impaired. The recoverable amounts of the CGUs are
assessed using a fair value less costs of disposal model. Fair value less costs of disposal is
calculated using a discounted cash flow approach, with a post-tax discount rate applied to the
projected risk-adjusted post-tax cash flows and terminal value.
The discount rate used is based on the CH Group’s post-tax WACC of 6%, as most cash
generating units have integrated operations across large parts of the CH Group. The discount
rate is adjusted where appropriate for specific segment, country or currency risks. The
valuation methodology uses significant inputs which are not based on observable market data,
therefore this valuation technique is classified as level 3 in the fair value hierarchy.
Details relating to the discounted cash flow model used in the impairment tests of the Asia
Pacific (“APAC”), Europe, Middle East and Africa and Latin America (“EMEA and LatAm”),
and North America (“N America”) cash generating units are as follows:
Valuation basis Fair value less costs of disposal
Key assumptions Sales growth rates
Profit margins
Terminal growth rate
Discount rate
Taxation rate
Determination of
assumptions
Growth rates are internal forecasts based on both internal and
external market information.
Margins reflect past experience, adjusted for expected
changes.
Terminal growth rates based on management’s estimate of
future long-term average growth rates.
Discount rates based on the CH Group WACC, adjusted
where appropriate.
Taxation rates based on appropriate rates for each region.
Period of specific
projected cash flows
Five years
Terminal growth rate 2021 2020 2019
APAC 4.5% p.a. 4.5% p.a. 4.5% p.a.
EMEA and
LatAm
3.5% p.a. 3.5% p.a. 3.5% p.a.
N America 2.5% p.a. 2.5% p.a. 2.5% p.a.
Discount rate (post tax) 2021 2020 2019
APAC 6.7% 7.1% 6.8%
EMEA and
LatAm
7.6% 7.9% 7.5%
N America 6.0% 6.0% 6.0%
The terminal growth rate does not exceed the long-term projected growth rate for the CH
Group, reflects the impact of future competition and takes account of new product launches.
371

Goodwill is monitored for impairment at the segmental level. In each case the valuation
indicated sufficient headroom such that a reasonably possible change to key assumptions is
unlikely to result in an impairment of goodwill.
Indefinite life brands and other amortised brands
Indefinite life brands comprise a portfolio of Consumer Healthcare products primarily acquired
from GlaxoSmithKline legacy entities, Novartis businesses acquired in 2015 and Pfizer
businesses acquired in 2019. The indefinite life brands are valued at historical acquisition date.
The net book value of the major brands are as follows:
2021
£m
2020
£m
2019
£m
Advil 3,362 3,349 3,408
Voltaren 2,725 2,725 2,725
Centrum 1,828 1,824 1,808
Caltrate 1,731 1,678 1,648
Otrivin 1,385 1,385 1,385
Preparation H 1,152 1,139 1,171
Robitussin 1,126 1,111 1,138
Nexium 670 668 682
Fenistil 598 598 598
ChapStick 521 512 523
Emergen-C 439 433 447
Theraflu 436 433 438
Panadol 395 396 397
Lamisil - - 291
Sensodyne 270 270 270
Breathe Right - - 251
Nicotinell 246 246 246
Excedrin 177 174 180
Vitasprint 117 122 135
Biotene 121 120 123
Physiogel - - 114
Polident 114 114 114
Corega 102 102 102
Be-total 85 89 99
Other brands
603 666
1,120
18,203 18,154 19,413
Robitussin and Preparation H were affected by lower cold & flu incidence resulting from the
COVID-19 social distancing measures and by supply chain issues respectively which has
resulted in a reduced level of headroom. The CH Group has performed a sensitivity analysis
based on changes in key assumptions considered to be reasonably possible by management
leaving all other assumptions unchanged. Sensitivity analysis for the year ended 31 December
2021 has identified these two brands as being sensitive to reasonably possible changes in key
assumptions. In order for the recoverable amount to be equal to the carrying values of
Robitussin and Preparation H, either the discount rate would have to be increased by 0.5% and
0.1%, or the operating margin decreased by 4.1% and 1.5%, or the long term growth rate
decreased by 0.7% and 0.2% respectively. Sensitivity analysis for the year ended
31 December 2020 only identified Robitussin as being sensitive to reasonably possible
changes in key assumptions. In order for the recoverable amount to be equal to the carrying
value, the discount rate would have to be increased by 0.3% or operating margin decreased by
2.7% or the long term growth rate decreased by 0.4%. The CH Group considers that changes
in key assumptions of this magnitude are reasonably possible in the current environment.
During the year ended 31 December 2020, Breathe Right and Physiogel were transferred to
Assets Held for Sale and subsequently disposed of. In addition, certain brands including
Lamisil, were reclassified from indefinite life brands to amortised brands following a review by
the CH Group on the useful life of these brands. As at 31 December 2021, Lamisil had a
carrying value of £259 million (2020: £275 million and 2019: £291 million) with a remaining
amortisation period of 18 years.
372
Except as set out above, each of these brands is considered to have an indefinite life, given
the strength and durability of the brand and the level of marketing support. The brands are in
relatively similar, stable and profitable market sectors, with similar risk profiles, and their size,
diversification and market shares mean that the risk of market-related factors causing a
reduction in the lives of the brands is considered to be relatively low. The CH Group is not
aware of any material legal, regulatory, contractual, competitive, economic or other factor
which could limit their useful lives. Accordingly, they are not amortised.
Each brand is tested annually for impairment and other amortised intangible assets are tested
when indicators of impairment arise. This testing applies a fair value less costs of disposal
methodology, generally using post-tax cash flow forecasts with a terminal value calculation and
a discount rate equal to the CH Group post-tax WACC of 6% for 2020 and 2019 adjusted
where appropriate for country and currency specific risks. This valuation methodology uses
significant inputs which are not based on observable market data, and therefore this valuation
technique is classified as level 3 of the fair value hierarchy. The main assumptions include
future sales price and volume growth, product contribution and the future expenditure required
to maintain the product’s marketability and registration in the relevant jurisdictions. These
assumptions are based on past experience and are reviewed as part of management’s
budgeting and strategic planning cycle for changes in market conditions and sales erosion
through competition. The terminal growth rates applied of between -3% and 3% are
management’s estimates of future long-term average growth rates of the relevant markets.
Other than as disclosed above, the directors do not consider that any reasonably possible
changes in the key assumptions would cause the fair value less cost of sale of the individually
significant brands disclosed above to fall below their carrying values.
For 2021, the income statement charge for net impairment losses includes impairments of
Zyrtec, Treely and capitalised costs for a discontinued research and development project,
netted off by reversal of impairments in relation to Alvedon, Abreva and Solpadeine.
Certain assets were transferred from intangible assets to assets held for sale and subsequently
disposed of during the year. There were no assets and liabilities held of sale remaining as at
31 December 2021.
For 2020, the income statement charge for net impairment losses mainly includes impairments
of Zyrtec, capitalised costs for a discontinued oral care project and a discontinued pain relief
device, netted off by reversal of impairments in relation to Transderm Scop.
For 2019, the income statement charge for net impairment losses includes impairments of
Savlon, Eurax and Abreva, netted off by reversal of impairments in Prevacid.
Amortisation
Net impairment
(reversals)/losses
2021 2020 2019 2021 2020 2019
£m £m £m £m £m £m
Cost of sales
57
62 38 (32) 11 9
Selling, general and administration
37
25 24 8 - -
Research and development
-
3-816-
94 90 62 (16) 27 9
19. Inventories
2021 2020
2019
£m £m £m
Raw materials and consumables
233 231
240
Work in progress
47 70
147
Finished goods
671 648
824
951 949 1,211
373

The total cost of inventories recognised as an expense and included in cost of sales amounted
to £3,462 million in 2021 (2020: £3,666 million and 2019: £3,143 million). This includes
inventory write-down of £174 million (2020: £141 million and 2019: £132 million).
The reversals of prior year write-downs of inventories in 2021 is £63 million (2020: £43 million
and 2019: £24 million) and principally arise from the reassessment of usage or demand
expectations prior to inventory expiration.
Included in the balance as at 31 December 2019 is an uplift of the fair value of the inventory
acquired from Pfizer as part of the Pfizer transaction of £91 million. The uplift of the fair value
was fully unwound as at 31 December 2020. For the year ended 31 December 2019, the
amount of uplift of the fair value unwound was £366 million.
20. Trade and other receivables
2021 2020 2019
£m £m £m
Trade receivables, net of expected credit loss allowance 1,318 1,348 1,397
Other prepayments and accrued income 56 61 29
Interest receivable 1 1 2
Employee loans and advances 4 2 4
Other third-party receivables 286 452 592
Other receivables with Pfizer Group companies - 2 14
Other receivables with GSK Group companies 542 492 441
2,207 2,358 2,479
Expected credit loss allowance 2021 2020 2019
£m £m £m
At 1 January 51 35 19
Exchange adjustments (1) (1) (2)
Charge for the year 33 24 19
Subsequent recoveries of amounts provided for (30) (5) -
Utilised - (2) (1)
At 31 December 53 51 35
Details of other receivables with Pfizer and GSK Group companies can be found in Note 30,
‘Related party transactions’.
Set out below is the information about the credit risk exposure on the CH Group’s trade
receivables using a provision matrix:
Trade receivable
Days past due
Year ended
31 December
2021
Current
0-30
days
31-90
days
91-180
days
181 days-
1 year
Greater
than 1
year
Total
£m £m £m £m £m £m £m
Expected credit loss rate 1% 2% 17% 100% 100% 100%
Estimated total gross
carrying amount at default
1,255 46 30 16 7 17 1,371
Expected credit loss 7 1 5 16 7 17 53
Trade receivable
Days past due
Year ended
31 December
2020
Current
0-30
days
31-90
days
91-180
days
181 days-
1 year
Greater
than 1
year
Total
£m £m £m £m £m £m £m
Expected credit loss rate 1% 3% 18% 55% 100% 100%
Estimated total gross
carrying amount at default
1,298 30 28 20 12 11 1,399
Expected credit loss 11 1 5 11 12 11 51
374

21. Cash and cash equivalents and liquid investments
2021 2020 2019
£m £m £m
Cash at bank and in hand 413 333 339
Liquid investments
11
1
414 334 340
Cash and cash equivalents include £67 million in 2021 (2020: £53 million and 2019: £17
million) not available for general use due to restrictions applying in the subsidiaries where it is
held. Restrictions include exchange controls and taxes on repatriation.
22. Assets and liabilities held for sale
2021 2020 2019
£m £m
£m
Plant, equipment and vehicles - - 23
Other intangibles - 62 189
Inventory -613
Other liabilities
- - (29)
- 68 196
Non-current assets and non-current liabilities are transferred to assets held for sale and
liabilities held for sale when it is expected that their carrying amounts will be recovered
principally through disposal and a sale is considered highly probable. They are held at the
lower of carrying amount and fair value less costs to sell.
Assets of £47 million were transferred from Intangible assets and subsequently disposed of
during the year. There were no assets and liabilities held of sale remaining as at 31 December
2021.
Assets held for sale as at 31 December 2020, which were after impairment reversals and
exchange impact, were subsequently disposed of in 2021.
Assets held for sale and liabilities held for sale in 2019 primarily reflect the Thermacare
disposal group, which was acquired from Pfizer as part of its Consumer Healthcare business
but had to be sold by the CH Group in 2020 to meet anti-trust requirements.
Included within assets held for sale as at 31 December 2020 were inventory assets which were
written down to fair value less costs to sell of £6 million (2019: £13 million). The valuation
methodology uses significant inputs which are not based on observable market data; therefore,
this valuation is classified as level 3 in the fair value hierarchy.
23. Trade and other payables
2021 2020 2019
£m £m £m
Trade payables 1,369 1,340 1,201
Customer return and rebate accruals 661 594 506
Other accruals 434 459 564
Wages and salaries 237 259 254
Other payables - - 79
Social security 45 76 82
VAT payables 35 42 30
Deferred income 11 11 7
Other payables with Pfizer Group companies 7 26 40
Other payables with GSK Group companies
203 461
657
3,002 3,268 3,420
375

Customer return and rebate accruals are provided for by the CH Group at the point of sale in
respect of the estimated rebates, discounts or allowances payable to customers. Accruals are
made at the time of sale but the actual amounts paid are based on claims made some time
after the initial recognition of the sale. As the amounts are estimated they may not fully reflect
the final outcome and are subject to change dependent upon, amongst other things, the types
of buying group and product sales mix. The level of accrual is reviewed and adjusted quarterly
in the light of historical experience of actual rebates, discounts or allowances given and returns
made and any changes in arrangements. Future events could cause the assumptions on which
the accruals are based to change, which could affect the future results of the CH Group.
Details of payables with Pfizer and GSK Group companies can be found in Note 30, ‘Related
party transactions’.
24. Borrowings
2021 2020 2019
£m £m £m
Short-term borrowings
Loan and overdrafts (49) (48) (24)
Lease liabilities (30) (34) (40)
(79) (82) (64)
Long-term borrowings
Lease liabilities (87) (105) (121)
(87) (105) (121)
Total borrowings (166) (187) (185)
As at 31 December 2021, the CH Group had a short-term bank loan of £42 million (2020:
£37 million and 2019: £13 million). The weighted average interest rate on the short-term bank
loan as at 31 December 2020 and 2021 was 3.7% (2019: 3%).
Lease liabilities
The maturity analysis of lease liabilities recognised on the CH Group balance sheet is as
follows:
2021 2020 2019
£m £m £m
Rental payments due within one year
(30)
(34) (40)
Rental payments due between one and two years
(22) (33)
(45)
Rental payments due between two and three years
(15) (14)
(16)
Rental payments due between three and four years
(13) (12)
(15)
Rental payments due between four and five years
(10) (12)
(10)
Rental payments due after five years
(27) (34)
(35)
(117) (139) (161)
376

25. Pensions and other post-employment benefits
Defined benefit pension and other post-
employment costs
2021 2020 2019
£m £m £m
German pension schemes 4 3 4
Swiss pension schemes 5 7 12
Irish pension schemes 6 4 7
Other overseas pensions schemes 5 7 4
Unfunded post-retirement healthcare schemes 10 9 2
30 30 29
Analysed as:
Defined benefit schemes 22 26 26
Defined contribution pensions schemes 8 4 3
The costs of the defined benefit pension and post-retirement healthcare schemes are charged
in the income statement as follows:
Net
pensions
total
Other post
retirement
obligations
total
Total post
retirement
obligations
£m £m £m
2021
Cost of sales 10 10 20
Research and development - - -
Selling, general and administration 2 - 2
31 December 2021 12 10 22
2020
Cost of sales 14 9 23
Research and development - - -
Selling, general and administration 3 - 3
31 December 2020 17 9 26
2019
Cost of sales 13 2 15
Research and development 2 - 2
Selling, general and administration 9 - 9
31 December 2019 24 2 26
GSK Consumer Healthcare Holdings (No.2) Limited entities operate pension arrangements
which cover the CH Group’s material obligations to provide pensions to retired employees.
These arrangements have been developed in accordance with local practices in the countries
concerned. Pension benefits can be provided by state schemes, by defined contribution
schemes, whereby retirement benefits are determined by the value of funds arising from
contributions paid in respect of each employee, or by defined benefit schemes, whereby
retirement benefits are based on employee pensionable remuneration and length of service.
Pension costs of defined benefit schemes for accounting purposes have been calculated using
the projected unit method. In certain countries pension benefits are provided on an unfunded
basis, some administered by trustee companies. Formal, independent, actuarial valuations of
the CH Group’s main plans are undertaken regularly, normally at least every three years.
Actuarial movements in the period are recognised through the statement of comprehensive
income. Discount rates are derived from AA rated corporate bond yields except in countries
where there is no deep market in corporate bonds where government bond yields are used.
377
Discount rates are selected to reflect the term of the expected benefit payments. Projected
inflation rate and pension increases are long-term predictions based on the yield gap between
long-term index-linked and fixed interest Gilts.
In addition, GlaxoSmithKline plc affiliates operate certain pension schemes in which the CH
Group’s UK and US employees participate. These schemes include defined benefit
arrangements where the assets are held independently of the CH Group’s finances and which
are funded partly by contributions from members and partly by contributions from the
GlaxoSmithKline plc affiliates at rates advised by independent professionally qualified
actuaries.
For the UK plans, there is an interest rate and inflation hedging strategy in place. The targets
are based on an economic measure of the plan liabilities. Furthermore, the plans also currently
hedge a portion of their equity exposure with a staggered maturity profile. The interest rate risk
and credit rate risk in the US plans are partially hedged. The targets are based on an
accounting measure of the plan liabilities.
In the UK, the defined benefit pension schemes operated for the benefit of former Glaxo
Wellcome employees and former SmithKline Beecham employees remain separate. These
schemes were closed to new entrants in 2001 and subsequent UK employees, including
employees of the CH Group, are entitled to join a defined contribution scheme.
Following a period of consultation with impacted employees, it was announced on
17 December 2020 that the UK defined benefit plans would be closed to future accrual
effective from 31 March 2022. As a result, post closure the accrued benefits of active
participants will be revalued in line with inflation (RPI for the legacy Glaxo Wellcome plans and
CPI for the legacy SmithKline Beecham plans subject to the relevant caps for each
arrangement) rather than capped pay increases. In addition, all defined benefit plan
participants who are still active at 1 April 2022, including participants of the CH Group, will
receive a defined pension contribution of £10,000 each.
With respect to the US cash balance pension plans, it was announced on 9 September 2020
that they would be closed to future accrual from 1 January 2021.
In addition, there are a number of post-retirement healthcare schemes, the principal one of
which is in the US.
The management fee from GlaxoSmithKline plc group companies includes an element relating
to the pension arrangements for the CH Group’s UK and US employees calculated as if the
arrangements were on a defined contribution basis. The underlying assets and liabilities of the
schemes cover a number of UK and US undertakings and cannot readily be split between each
Group undertaking on a consistent and reliable basis. The cost of such defined contribution
arrangements is not included in the £12 million (2020: £17 million and 2019: £24 million)
charge analysed above.
378
The average life expectancy assumed now for an individual at the age of 60 and projected to
apply in the years stated below for an individual then at the age of 60 is as follows:
As at
31 December 2021
Germany Switzerland Ireland Rest of World
Male
Years
Female
Years
Male
Years
Female
Years
Male
Years
Female
Years
Male
Years
Female
Years
Current 25.4 29.2 26.6 28.5 26.7 29.3 27.2 28.5
Projected for 2041 28.4 31.5 28.4 30.2 29.2 31.5 28.7 30.0
As at
31 December 2020
Germany Switzerland Ireland Rest of World
Male
Years
Female
Years
Male
Years
Female
Years
Male
Years
Female
Years
Male
Years
Female
Years
Current 25.4 29.2 26.6 28.8 26.6 29.1 26.8 28.2
Projected for 2040 28.4 31.5 28.4 30.4 29.0 31.3 28.4 29.7
As at
31 December 2019
Germany Switzerland Ireland Rest of World
Male
Years
Female
Years
Male
Years
Female
Years
Male
Years
Female
Years
Male
Years
Female
Years
Current 25.3 29.1 26.5 28.7 26.5 29.1 27.1 28.8
Projected for 2039 28.3 31.4 28.4 30.4 29.0 31.3 28.8 30.4
The assets of funded schemes are generally held in separately administered trusts, either as
specific assets or as a proportion of a general fund or are insurance contracts. Assets are
invested in different classes in order to maintain a balance between risk and return.
Investments are diversified to limit the financial effect of the failure of any individual investment.
The Pension Plans are exposed to risk that arises because the estimated market value of the
Plans’ assets might decline, the investment returns might reduce, or the estimated value of the
Plans’ liabilities might increase.
In line with the agreed mix of return seeking assets to generate future returns and liability
matching assets to better match future pension obligations, the CH Group has defined an
overall long-term investment strategy for the Plans, with investments across a broad range of
assets. The main market risks within the asset and hedging portfolio are against credit risk,
interest rates, long-term inflation, equities, property, and bank counterparty risk.
The Plan liabilities are a series of future cash flows with relatively long duration. On an IAS 19
basis, these cash flows are sensitive to changes in the expected long-term inflation rate and
the discount rate (AA corporate bond yield curve) where an increase in long-term inflation
corresponds with an increase in the liabilities, and an increase in the discount rate corresponds
with a decrease in the liabilities.
379

The CH Group has applied the following financial assumptions in assessing the defined benefit
liabilities:
2021 2020 2019
%pa %pa %pa
Germany
Rate of increase of future earnings 3.00 3.00 3.00
Discount rate 1.10 0.50 1.10
Expected pension increases 2.10 1.40 1.50
Inflation rate
2.10 1.40
1.50
Switzerland
Rate of increase of future earnings 1.80 1.80 2.00
Discount rate 0.20 0.10 0.10
Expected pension increases - - -
Inflation rate
1.00 1.00 1.00
Ireland
Rate of increase of future earnings 2.00 2.00 2.00
Discount rate 1.30 0.80 1.30
Expected pension increases 3.00 - -
Inflation rate
2.10 1.50 1.60
Rest of World
Rate of increase of future earnings N/A N/A N/A
Discount rate 2.70 1.45 1.85
Expected pension increases N/A N/A N/A
Inflation rate
2.25 1.50
1.63
The amounts recorded in the income statement and statement of comprehensive income in
relation to the defined benefit pension and post-retirement healthcare schemes were as
follows:
Pensions
Other post-
employment
benefits
Total
£m £m £m
31 December 2021
Amounts charged to operating profit:
Current service cost 18 8 26
Past service cost/(credit) (4) - (4)
Gain from settlement (3) - (3)
Net interest cost 1 2 3
12 10 22
Re-measurements recorded in the statement of
comprehensive income
(8) (19) (27)
31 December 2020
Amounts charged to operating profit:
Current service cost 24 6 30
Past service cost/(credit) (7) - (7)
Net interest cost - 3 3
17 9 26
Re-measurements recorded in the statement of
comprehensive income
5813
31 December 2019
Amounts charged to operating profit:
Current service cost 21 2 23
Net interest cost 3 - 3
24 2 26
Re-measurements recorded in the statement of
comprehensive income
5813
380

The fair values of the assets and liabilities of the German, Swiss and Irish defined benefit
pension schemes, together with aggregated data for other defined benefit pension schemes in
the CH Group are as follows:
Germany Switzerland Ireland
Rest
of
World
Total
31 December 2021 £m £m £m £m £m
Equities: -Listed 54 98 102 6 260
Property: -Unlisted - 52 - - 52
Bonds: -Listed 55 89 130 19 293
Insurance contracts 27 55 - - 82
Other assets - 6 1 12 19
136 300 233 37 706
Asset ceiling
restriction
- (26) - - (26)
Fair value of assets
136 274 233 37 680
Present value of
scheme obligations
(246) (274) (254) (48) (822)
Recognised on the balance
sheet
(110) - (21) (11) (142)
Included in post-employment
benefits assets
- - 11 - 11
Included in post-employment
benefits obligations
(110) - (32) (11) (153)
(110) - (21) (11) (142)
Actual return (loss) on plan
assets
15 (14) (4) 1 (2)
Germany Switzerland Ireland
Rest
of
World
Total
31 December 2020 £m £m £m £m £m
Equities: -Listed 49 91 80 9 229
Property: -Unlisted - 45 - - 45
Bonds: -Listed 52 80 167 25 324
Insurance contracts 29 12 - 1 42
Other assets - 17 - 6 23
Fair value of assets 130 245 247 41 663
Present value of scheme obligations (262) (212) (306) (65) (845)
Recognised on the balance sheet (132) 33 (59) (24) (182)
Included in post-employment
benefits assets
-338-41
Included in post-employment
benefits obligations
(132) - (67) (24) (223)
(132) 33 (59) (24) (182)
Actual return on plan assets - 15 20 1 36
381

Germany Switzerland Ireland
Rest
of
World
Total
31 December 2019 £m £m £m £m £m
Equities: -Listed 50 83 70 8 211
Property: -Unlisted - 45 - - 45
Bonds: -Listed 49 70 141 25 285
Insurance contracts 23 8 - 1 32
Other assets - 16 - 7 23
Fair value of assets 122 222 211 41 596
Present value of scheme
obligations
(239) (232) (245) (58) (774)
Recognised on the balance sheet (117) (10) (34) (17) (178)
Included in post-employment
benefits assets
---33
Included in post-employment
benefits obligations
(117) (10) (34) (20) (181)
(117)
(10) (34) (17) (178)
Actual return on plan assets 16 44 31 - 91
The defined benefit pension obligation is analysed as follows:
2021 2020 2019
£m £m £m
Funded (812) (834) (709)
Unfunded (10) (11) (65)
(822) (845) (774)
382

The movement in the net defined benefit liability is as follows:
Fair value
of assets
Present
value of
obligation
Net
pensions
total
Net post
retirement
obligations
£m £m £m £m
At 1 January 2019 503 (658) (155) (56)
Exchange adjustments (17) 34 17 (4)
Additions through business combinations 5 (45) (40) (50)
Service cost - (21) (21) (2)
Interest income/(cost) 5 (8) (3) -
Re-measurements:
Return on plan assets, excluding amounts
included in interest 86 - 86 -
Gain from change in demographic assumptions - 7 7 -
Gain from change in financial assumptions - (88) (88) (8)
Experience losses - (10) (10) -
Employers contributions 30 - 30 3
Scheme participants’ contributions 7 (7) - -
Benefits paid (23) 22 (1) -
At 31 December 2019 596 (774) (178) (117)
Exchange adjustments 33 (44) (11) 20
Service cost - (23) (23) (6)
Past service cost - 7 7 -
Interest income/(cost) 5 (6) (1) (3)
Settlements and curtailments (19) 19 - -
Re-measurements:
Return on plan assets, excluding amounts
include in interest
31 - 31 -
Gain from change in demographic assumptions - 26 26 -
Gain from change in financial assumptions - (59) (59) (8)
Experience losses - (3) (3) -
Employers contributions 28 - 28 1
Scheme participants’ contributions 8 (7) 1 -
Benefits paid (19) 19 - -
At 31 December 2020 663 (845) (182) (113)
Exchange adjustments (34) 48 14 (2)
Service cost - (18) (18) (8)
Past service cost - 4 4 -
Interest income/(cost) 4 (5) (1) (2)
Settlements and curtailments (5) 8 3 -
Assets acquired/(liability assumed) from GSK
Group
1
39 (39) - -
Re-measurements:
Return on plan assets, excluding amounts
include in interest
(6) - (6) -
Gain from change in demographic assumptions - 7 7 -
Gain from change in financial assumptions - 33 33 19
Experience losses - (26) (26) -
Employers contributions 30 - 30 -
Scheme participants’ contributions 7 (7) - -
Benefits paid (18) 18 - 6
At 31 December 2021 680 (822) (142) (100)
1
There were £39 million of assets acquired and £39 million of liabilities assumed from the GSK
Group during the year ended 31 December 2021, as a result of the separation of the existing
GSK Group Pension Fund in Switzerland into two independent schemes for the Biopharma and
Consumer Healthcare businesses in preparation of the proposed separation of the CH Group
from the GSK Group. Under local plan rules the new GSK Group Scheme covering the
Biopharma businesses could not accept any retired members and therefore these members
were included in the CH Group scheme.
383

A reconciliation of the net post-employment benefit to the balances recognised on the
consolidated balance sheet is as follows:
2021 2020 2019
£m
£m £m
Net Pensions total (142) (182) (178)
Net post retirement obligations (100) (113) (117)
Net post-employment benefit (242) (295) (295)
Post-employment benefit assets recognised on the consolidated balance
sheet
11 41 3
Post-employment benefit obligations recognised on the consolidated balance
sheet
(253) (336) (298)
Net post-employment benefit
(242) (295) (295)
Employer contributions for 2022 are estimated to be approximately £28 million in respect of
defined benefit pension schemes and £6 million in respect of post-retirement medical benefits.
The defined benefit pension and post-retirement obligations analysed by membership category
is as follows:
Pension obligations Post-retirement obligations
2021 2020 2019 2021 2020 2019
£m
£m £m £m £m £m
Active (418) (462) (388) (97) (107) (117)
Retired (237) (202) (195) (3) (3) -
Deferred (167) (181) (191) - (3) -
(822) (845) (774) (100) (113) (117)
384

Sensitivity analysis
The approximate effect of changes in assumptions used on the benefit obligations and on the
annual defined benefit and post-retirement costs are detailed below. This information has been
determined by taking into account the duration of the liabilities and the overall profile of the
plan membership.
2021 2020 2019
£m £m £m
A 0.25% decrease in discount rate:
Increase in annual pension cost 0.8 0.8 1.2
Decrease in annual post-retirement benefits cost 0.1 0.1 0.1
Increase in pension obligation 34.8 39.4 37.7
Increase in post-retirement benefits obligation 2.9 3.4 3.1
A 0.25% increase in discount rate:
Decrease in annual pension cost (0.9) (1.0) (1.2)
Increase in annual post-retirement benefits cost (0.1) (0.1) (0.1)
Decrease in pension obligation (32.6) (37.0) (34.6)
Decrease in post-retirement benefits obligation (2.8) (3.2) (3.0)
A 0.25% increase in inflation:
Increase in annual pension cost 0.2 0.2 0.2
Increase in pension obligation 11.0 12.9 12.6
A 0.25% decrease in inflation:
Decrease in annual pension cost (0.2) (0.2) (0.2)
Decrease in pension obligation (10.7) (12.7) (12.3)
A one year increase in life expectancy:
Increase in annual pension cost 0.9 1.0 1.1
Increase in annual post-retirement benefits cost 0.2 0.2 0.2
Increase in pension obligation 27.8 31.9 28.6
Increase in post-retirement benefits obligation 2.0 2.4 2.2
The weighted average duration of the defined benefit obligation is as follows:
2021 2020 2019
years
years years
Pension benefits 18 19 20
Post-retirement benefits
16
17 16
385

26. Other provisions
Restructuring
programmes
Other
provisions
Total
£m £m
£m
Cost at 1 January 2019 (85) (38) (123)
Exchange adjustments 3 3 6
Charge for the period (92) (10) (102)
Reversed unused 22 4 26
Utilised 21 6 27
Additions through business combination - (13) (13)
Other movements
(2) 4
2
As at 31 December 2019 (133) (44) (177)
Exchange adjustments (1) - (1)
Charge for the period (139) (10) (149)
Reversed unused 45 4 49
Utilised 100 13 113
Other movements
(4) 1
(3)
As at 31 December 2020 (132) (36) (168)
Exchange adjustments 3 1 4
Charge for the period (52) (9) (61)
Reversed unused 9 4 13
Utilised 68 9 77
Other movements
(8) 4
(4)
As at 31 December 2021
(112) (27)
(139)
2021 2020 2019
£m £m
£m
To be settled within one year (112) (103) (101)
To be settled after one year (27) (65) (76)
Total provision (139) (168) (177)
Other provisions include employee-related, legal, environmental, and other provisions. Details
of restructuring provisions can be found in Note 12, ‘Restructuring costs’.
27. Contingent Liabilities
2021 2020 2019
£m £m
£m
Contingent Liabilities
33 28 47
At 31 December 2021, contingent liabilities, comprising guarantees and other items arising in
the normal course of business, amounted to £33 million (2020: £28 million and 2019: £47
million). Contingent liabilities arise when the CH Group has a present obligation as a result of a
past event and comprise guarantees and other items arising in the normal course of business.
Provision is made for the outcome of tax, legal and other disputes where it is both probable
that the CH Group will suffer an outflow of funds and it is possible to make a reliable estimate
of that outflow.
The CH Group is involved in significant legal and administrative proceedings, principally
product liability. The most significant of these matters, other than tax matters, are described
below.
Legal proceedings
The CH Group makes provision for these proceedings on a regular basis as summarised in
Note 2 ‘Accounting principles and policies’ and Note 26 ‘Other provisions’.
The CH Group may become involved in significant legal proceedings in respect of which it is
not possible to determine whether a potential outflow is probable. In these cases, appropriate
disclosures about such cases would be included in this note, but no provision would be made
for the cases.
386
With respect to each of the legal proceedings described below, other than those for which a
provision has been made, the CH Group is unable to make a reliable estimate of the expected
financial effect at this stage. The CH Group does not believe that information about the amount
sought by the plaintiffs, if that is known, would be meaningful with respect to those legal
proceedings. This is due to a number of factors, including, but not limited to, the stage of
proceedings, the entitlement of parties to appeal a decision and clarity as to theories of liability,
damages and governing law.
The CH Group’s position could change over time, and, therefore, there can be no assurance
that any losses that result from the outcome of any legal proceedings will not exceed by a
material amount the amount of the provisions reported in the CH Group’s financial statements.
If this were to happen, it could have a material adverse impact on the results of operations of
the CH Group in the reporting period in which the judgements are incurred or the settlements
entered into.
Zantac Litigation
GSK Group and/or the Pfizer Group have been named as a defendant (alongside other
manufacturers of ranitidine, as well as retailers and distributors) in approximately 2,150 US
personal injury lawsuits involving Zantac, the bulk of which are pending in a Multidistrict
Litigation (“MDL”) in the Southern District of Florida. There are also numerous unfiled claims
registered in a census required by the court presiding over the MDL. Class actions alleging
economic injury and medical monitoring have also been filed in federal court. In addition to the
product liability cases filed in the MDL, cases have been filed in several State Courts, including
a consolidated action in California State Court. Outside the USA, there are four class actions
pending against the GSK Group and the Pfizer Group in Canada, along with a class action
pending against the GSK Group in Israel. The GSK Group has also received notice of a civil
investigation opened by the Department of Justice (the “DOJ”) into allegation of False Claims
Act violations by the GSK Group related to Zantac. The New Mexico Attorney General filed a
lawsuit against multiple defendants, including the GSK Group and the Pfizer Group, alleging
violations of state consumer protection and false advertising statutes, among other claims.
Under the Pfizer SAPA, the CH Group is required to indemnify the GSK Group and the Pfizer
Group in respect of “Purchaser Liabilities” and “Assumed Liabilities”, which may include
liabilities related to OTC Zantac. Whilst Pfizer and GSK have each served the CH Group with
notice of potential claims under the relevant indemnification provisions in the Pfizer SAPA in
relation to possible liabilities connected with OTC Zantac, it is not possible, at this stage, to
meaningfully assess whether the outcome will result in a probable outflow, or to quantify or
reliably estimate the liability (if any) that the CH Group may have to the GSK Group and/or the
Pfizer Group under the relevant indemnities.
Proton Pump Inhibitor litigation
The CH Group is a defendant in the ongoing proton pump inhibitor (“PPI”) litigation, in which
plaintiffs allege that their use of PPIs caused serious bodily injuries, including acute kidney
injury, chronic kidney disease and end-stage renal failure. As of June 2021, there are
approximately 1,500 Prevacid24HR personal injury lawsuits and approximately 2,300
Nexium24HR cases pending against the CH Group, nearly all of which are pending in a
Multidistrict Litigation (“MDL”) proceeding in the District of New Jersey. Manufacturers of other
PPIs, including both prescription and OTC products, also are named as co-defendants in the
MDL. The CH Group has filed motions to dismiss several hundred cases, but the MDL court
has not yet ruled on those motions. In addition to the MDL cases, a small number of cases are
pending in state courts. The CH Group is unable to determine whether the outcome will result
in a probable outflow.
German Competition Litigation
In 2013, the CH Group and other members of a working group, Körperpflege, Wasch- und
Reinigungsmittel (“KWR”), of a German trademark association were fined by the Federal Cartel
387
Office of Germany, as a result of the exchange of certain information during meetings from
2004 to 2006. The information exchanged related primarily to annual terms negotiations with
retailers and to the timing and the order of magnitude of list price increases. Following the fine
imposed by the Federal Cartel Office in 2013, the CH Group is party to eight active civil
proceedings in Germany for damages against the CH Group and other manufacturers of
branded drugstore products. The claimants allege that the exchange of information within KWR
led to higher purchase prices being paid by the retailers, and therefore the Group and other
KWR members are jointly and severally liable for potential damages. The proceedings are
taking place in different courts across Germany and are at different stages.
Separate proceedings have been brought against the CH Group and certain other members of
KWR by the insolvency administrator of Schlecker (formerly a large drugstore retailer in
Germany) and other retailers. Two of these actions have been dismissed in lower courts but
are subject to appeal. Additionally, the CH Group has intervened as a third party on the
defendants’ side in three other separate proceedings. The CH Group is unable to determine
whether the outcome will result in a probable outflow.
28. Share capital, share premium and other reserves
At 1
January
2019
Issue of
share
capital
At 31
December
2019
Ordinary A shares at Number - 680,000 680,000
£1.00 each £’000 - 680 680
Ordinary B shares at Number - 320,000 320,000
£1.00 each £’000 - 320 320
Non redeemable Number - 300,000 300,000
Preference shares at £’000 - 300 300
£1.00 each
C Deferred share at Number - - -
£13,166,038,547.00 £’000 - - -
Share capital £’000 - 1,300 1,300
Share premium £’000 20,321 521 20,842
At 31
December
2019
Issue of
share
capital
Capital
Reduction
At 31
December
2020
Ordinary A shares at Number 680,000 --680,000
£1.00 each £’000 680 --680
Ordinary B shares at Number 320,000 --320,000
£1.00 each £’000 320 --320
Non redeemable Number 300,000 --300,000
Preference shares at £’000 300 --300
£1.00 each
C Deferred share at Number - 1 (1) -
£13,166,038,547.00 £’000 - 13,166,039 (13,166,039) -
Share capital £’000 1,300 13,166,039 (13,166,039) 1,300
Share premium £’000 20,842 - (20,842) -
388

At 31
December
2020
Issue of
share
capital
Capital
Reduction
At 31
December
2021
Ordinary A shares at Number 680,000 --680,000
£1.00 each £’000 680 --680
Ordinary B shares at Number 320,000 --320,000
£1.00 each £’000 320 --320
Non redeemable Number 300,000 --300,000
Preference shares at £’000 300 --300
£1.00 each
C Deferred share at Number - ---
£13,166,038,547.00 £’000 -
---
Share capital £’000 1,300
--1,300
Share premium £’000 -
---
Ordinary A shares and Ordinary B shares carry equal rights. Share premium was recognised
on shares issued by CHHL2 except where CHHL2 has applied to take merger relief under
Section 612 of the Companies Act 2006. In such cases the excess of the fair value of the
assets and liabilities recognised into the CH Group, over the nominal value of the share issued
has been added to the merger reserve as per table disclosed below.
During the year ended 31 December 2019, CHHL2 allotted 680,000 ordinary A shares of £1
each to GlaxoSmithKline Consumer Healthcare Holdings Limited and 320,000 ordinary B
shares of £1 each to PF Consumer Healthcare Holdings LLC. All these shares were allotted
with £1 million nominal value and £20,842 million share premium.
In addition, CHHL2 also issued 300,000 preference shares of £1 each to GlaxoSmithKline
Consumer Healthcare Holdings Limited during the year. The preference shares are
non-redeemable with a discretionary right to receive conditional dividends in which 0.01% of
the aggregate amount of dividend shall be payable to the holders of the preference shares,
therefore the preference shares are classified as equity.
During the year ended 31 December 2020, CHHL2 issued one C Deferred share of
£13,166,038,547 to GlaxoSmithKline Consumer Healthcare Holdings Limited. The C Deferred
share is non-redeemable and does not carry any voting rights, dividend rights or rights in the
event of a return of capital.
Subsequently during 2020, CHHL2 cancelled and extinguished in its entirety the share
premium balance of £20,842 million in accordance with section 642 of the Companies Act.
CHHL2 also cancelled and extinguished the fully paid up C Deferred share of £13,166 million in
the share capital of CHHL2 held by GlaxoSmithKline Consumer Healthcare Holdings Limited.
Details of other reserves are included below:
2021 2020
2019
Other reserves
£m £m
£m
As at 1 January
(11,652) 1,372 (14,841)
Other comprehensive income 9 - -
Issue of share capital - (13,166) 16,213
Capital reduction - (45) -
Contribution from parent 11 - -
Contribution (non-cash) from parent
- 187 -
As at 31 December
(11,632) (11,652) 1,372
Other Reserves include a merger reserve that arises as a result of acquisition of business.
389
29. Acquisitions and disposals
2020
Business acquisitions
On 28 September 2020, the CH Group completed the acquisition of legal ownership of
approximately 55% equity interests in the legal entity that holds the Hsinchu site in Taiwan
from the Pfizer Group in a non-cash transaction, whereby the CH Group has acquired the
business as part of the completion of the Pfizer Transaction on 31 July 2019. The CH Group
has measured the business at fair value.
Goodwill of £124m, which is not expected to be deductible for tax purpose, has been
recognised. The goodwill represents the potential for future synergies arising from combining
the acquired businesses with the CH Group’s existing business together with the value of the
workforce acquired.
The non-controlling interest for this acquisition recorded in the CH Group, calculated applying
the proportionate interests’ method, represents Pfizer Group’s share of the net assets of the
CH Group, excluding goodwill.
The majority of the Hsinchu site’s revenue was generated through manufacturing of Consumer
Healthcare products for companies within the CH Group and was eliminated on consolidation.
Therefore, the external revenue arising from the Hsinchu site since the acquisition on
28 September 2020 was immaterial and would remain immaterial if the business had been
acquired at the beginning of the year. The business has been integrated into the CH Group’s
existing activities and it is not practicable to identify the impact on the CH Group profit in the
period.
The fair value of the assets acquired in business combinations, including goodwill, are set out
in the table below.
Taiwan Hsinchu site business
£m
Net assets acquired:
Intangible assets 2
Property, plant and equipment 6
Inventory 5
Cash and cash equivalents 20
Other assets 6
Other liabilities (21)
Non-controlling interests (14)
Goodwill
124
Total
128
Consideration settled by shares in CHHL2 128
Cash consideration paid
-
Total consideration
128
2020
Business disposals
In 2020, the CH Group made several business disposals, resulting in the CH Group receiving
net cash consideration of £221 million. The business disposals mainly related to the divestment
of EMEA rights of Thermacare, which was acquired from Pfizer as part of its Consumer
Healthcare business following the completion of the Pfizer Transaction on 31 July 2019 and
was disposed by the CH Group on 30 March 2020 to meet anti-trust requirements.
390
The gain on the disposals of businesses in the year of £69 million was calculated as follows:
Total
£m
Cash consideration received 221
Net assets sold:
Goodwill (1)
Intangible assets (125)
Property, plant and equipment (12)
Inventory (5)
Other net assets
(1)
(144)
Transaction costs
(8)
Total gain on disposal
69
2019
Business acquisitions
The Pfizer Transaction was completed on 31 July 2019.
The GSK Group and Pfizer have contributed their respective Consumer Healthcare businesses
into the CH Group to form a new Consumer Healthcare Joint Venture in a non-cash
transaction, whereby the CH Group has acquired Pfizer’s Consumer Healthcare business in
return for shares in the CH Group. CHHL2 is the parent holding company of the new Joint
Venture and the CH Group is being held by the GSK Group with an equity interest of 68% and
Pfizer with an equity interest of 32%.
Goodwill of £5.6 billion, which is not expected to be deductible for tax purpose, has been
recognised. The goodwill represents the potential for future synergies arising from combining
the acquired businesses with the CH Group’s existing business together with the value of the
workforce acquired. Total transaction costs for the acquisition amounted to £77 million.
Since the acquisition on 31 July 2019, revenue of £1.2 billion arising from the Pfizer Consumer
Healthcare business has been included in CH Group revenue in 2019. If the business had
been acquired at the beginning of the year, CH Group revenue in 2019 would have been
£1.5 billion higher. The business has been integrated into the CH Group’s existing activities
and it is not practical to identify the impact on the CH Group profit in the period.
391
The fair value of the assets acquired in business combinations, including goodwill, are set out
in the table below.
Pfizer Consumer Healthcare
business
£m
Net assets acquired:
Intangible assets (indefinite life brands) 12,357
Property, plant and equipment 354
Rights of use assets 39
Inventory 986
Trade and other receivables 546
Other assets including cash and cash equivalents 302
Trade and other payables (779)
Net deferred tax liabilities (2,591)
Other liabilities (99)
Non-controlling interests (20)
Goodwill
5,658
Total
16,753
Consideration settled by shares in CHHL2 16,753
Cash consideration paid
-
Total consideration
16,753
2019
Business disposals
There were no business disposals during the year ended 31 December 2019.
30. Related party transactions
The CH Group undertook significant transactions with entities from within the GSK Group
during the years ended 31 December 2021, 31 December 2020 and 31 December 2019 and
with entities from within the Pfizer Group for the period from 1 August 2019 to 31 December
2019 and for the years ended 31 December 2020 and 2021.
Entities from within the GSK Group supplied goods to and purchased goods from the CH
Group during the period. The CH Group supplies goods to companies within the GSK Group
under Distribution Agreements in those countries where the CH Group does not have its own
local operating company. In addition, entities from within the GSK Group were engaged to
provide support function services to the CH Group under Support Services Agreements
(“SSA”) including: regulatory and safety services, financial management and reporting, human
resources, payroll services, IT support, property management, legal services, contract
manufacturing, management of the CH Group’s UK and US pension schemes, and
management of the CH Group’s employee share schemes. In addition, the CH Group operates
separate agreements with GSK affiliates for the provision of research and development and for
toll-manufacturing services. Cash amounts are also held with GSK financing companies.
Entities from within the Pfizer Group supplied services and goods to and purchased goods and
services from the CH Group via the Transitional Services Agreement during the period. All
related party transactions are undertaken at arm’s length in accordance with the CH Group
transfer pricing policy.
Where the legal completion of local transfer of assets and liabilities has been delayed, but the
CH Group is able to exercise control over the relevant activities, the relevant net assets and
profits have been recognised in the results.
Comparative disclosures included related party transactions with entities within the Pfizer
Group for the period from 1 August 2019 to 31 December 2019. Following the completion of
392

the Pfizer Transaction on 31 July 2019, transactions between the CH Group and Pfizer Group
are deemed related party transactions and are disclosed below for the period from 1 August
2019 onwards.
Pfizer Companies
2021 2020 2019
£m
£m £m
Sales of goods
-
17 2
Purchases of goods
-
(11) (1)
Services and royalty income
-
17 6
Services and royalty expense
(68)
(121) (62)
Dividend paid
367
735 -
Other amounts owing to related parties
(7)
(26) (40)
Other amounts owing from related parties
-
214
GlaxoSmithKline Companies
2021 2020 2019
£m
£m £m
Sales of goods
114
397 179
Purchases of goods
(81)
(81) (48)
Services and royalty income
20
49 80
Services and royalty expense
(354)
(384) (346)
Interest income
10
12 18
Interest expense
(4)
(6) (9)
Dividend paid
781 1,636
-
Other amounts owing to related parties
(203)
(461) (657)
Other amounts owing from related parties
542
483 429
Loan amounts owing to related parties
(825)
(300) (457)
Loan amounts owing from related parties
1,508 1,119 1,461
£825 million (2020: £300 million and 2019: £457 million) loan amounts owing to related parties
is held with GSK Financing companies as part of the CH Group’s banking arrangements.
These balances are unsecured with interest largely paid at the new risk free benchmark rates
+0.10% (2020: LIBOR + 0.25% and 2019: LIBOR + 0.25%) and are repayable on demand.
£1,508 million (2020: £1,119 million and 2019: £1,461 million) loan amounts owing from related
parties is held with GSK Financing companies as part of the CH Group’s banking
arrangements. These balances are unsecured with interest largely received at the new risk free
benchmark rate -0.05% (2020: LIBOR –0.125% and 2019: LIBOR –0.125%) and are repayable
on demand.
393

31. Adjustments reconciling profit after tax to operating cash flow
2021 2020 2019
£m £m £m
Profit after tax 1,439 1,181 687
Taxation charge 197 410 199
Net finance costs 2 7 11
Depreciation of property, plant and equipment and rights of use assets 174 215 198
Amortisation of intangible assets 94 90 62
Impairment and assets written off, net of reversals 1 88 12
(Gain)/loss on sale of intangible assets (27) (143) 5
Loss on sale of property, plant and equipment - 3 6
Gain on sale of business (4) (69) -
Fair value adjustment from Pfizer transaction - 91 366
Other non-cash movements (22) 100 (6)
Increase in other non-current financial liabilities - - (9)
(Decrease)/increase in pension and other provisions (36) (27) 25
Changes in working capital:
(Increase)/decrease in inventories (17) 130 232
Decrease/(increase) in trade receivables 14 18 (57)
Increase/(decrease) in trade payables 41 140 (256)
Net change in other receivables and payables (190) (273) (380)
227 780 408
Cash generated from operations 1,666 1,961 1,095
32. Commitments
2021 2020 2019
£m £m £m
Contracted for but not provided in the Historical Financial Information:
Intangible assets 68 36 48
Property, plant and equipment 80 90 62
Purchase commitments 410 745 1,035
Future finance charges on leases 12 16 20
Investments 49 53 78
619 940 1,243
Purchase commitments mainly include amounts committed for contract manufacturing
agreements.
33. Financial instruments and related disclosures
The CH Group reports in Sterling and paid dividends out of cash in Sterling. During the periods
presented, GSK Group’s Treasury function has been employed as a service provider to
manage and monitor the CH Group’s external and internal funding requirements and financial
risks in support of the CH Group’s strategic objectives. Treasury activities are governed by
policies approved by the CH Group Board of Directors.
The CH Group operates on a global basis, through a number of subsidiary companies and the
existing sales networks of the GSK Group.
A Treasury meeting, chaired by the GlaxoSmithKline Consumer Healthcare Chief Financial
Officer (CFO), takes place on a regular basis to review Treasury activities. Its members receive
management information relating to Treasury activities. The GSK Group’s internal auditors
review the Treasury internal control environment regularly as part of their review of the GSK
Group’s Treasury function.
394
The CH Group may use a variety of financial instruments to finance its operations and
derivative financial instruments to manage market risks from these operations. Derivatives
principally comprise of foreign exchange forward contracts and swaps which are used to swap
borrowings and liquid assets into currencies required for group purposes.
Derivatives are used exclusively for hedging purposes in relation to underlying business
activities and not as trading or speculative instruments.
Capital management
During the periods presented, the CH Group managed its capital to ensure that entities in the
CH Group were able to operate as going concerns whilst availing themselves of intercompany
funding where appropriate. The capital structure of the CH Group consists wholly of
shareholders’ equity as well as short-term bank loans (see “consolidated statement of changes
in equity” and Note 24 ‘Borrowings’). The Board reviews the CH Group’s dividend policy which
is established in accordance with parameters set in the Shareholders Agreement between the
GSK Group and the Pfizer Group.
Selling margins are sufficient to cover normal operating costs and operations are cash
generative.
Operating cash flow is used to fund investment in research and development of new products.
It is also used to make routine outflows of capital expenditure, tax and dividends.
Liquidity risk
The CH Group benefits from strong positive cash flow from its operating units and has
substantial cash and cash equivalents, which amounted to £413 million at 31 December 2021
(2020: £333 million and 2019: £339 million).
Market risk
Interest rate risk management
The CH Group has no significant external debt and therefore its interest expense is not
exposed to changes in interest rates. The CH Group earns interest income on its cash and
therefore benefits from an increase in interest rates. The impact of a decrease in interest rates
is limited (see interest rate sensitivity).
Forward starting interest rate swaps
The forward starting interest rate contracts, exchanging floating interest for fixed interest, have
been designated as cash flow hedges to hedge the interest variability of the interest cash flows
associated with the future fixed rate debt.
The critical terms of the forward starting interest rate swap contracts and their corresponding
hedged items are materially the same. A qualitative assessment of effectiveness is performed,
and it is expected that the value of the interest rate swap contracts and the value of the
corresponding hedged items will systematically change in opposite directions in response to
movements in the underlying interest rates. The main sources of ineffectiveness in these
hedge relationships are the effects of the Group’s own credit risk on the fair value of the
interest rate swap contracts, which are not reflected in the fair value of the hedged item
attributable to the change in interest rates. No other material sources of ineffectiveness
emerged from these hedging relationships.
395

The following tables provide information regarding forward starting interest rate swap contracts
outstanding and the related hedged items at 31 December 2021. Interest rate swap contract
assets and liabilities are presented in the line ‘Derivative financial instruments’ (either as assets
or liabilities) on the Consolidated balance sheet.
2021
Hedging
instruments
Average
contracted
fixed rate
Notional
principal
value
Change in fair
value for
recognising
hedge
ineffectiveness
Fair value
assets/
(liabilities)
%£m £m £m
5-10 years 1.1038% 668 4 4
10-30 years 1.3385% 935 3 3
>30 years 1.4515% 393 4 4
2021
Hedged items Change in value used for
calculating hedge
ineffectiveness
Balance in cash flow hedge
reserve for continuing hedges
£m £m
Pre-hedging of long-term
interest rate
(11) (9)
The following table details the effectiveness of the hedging relationships and the amounts
reclassified from the hedging reserve to profit or loss:
2021
Amount reclassified to profit or loss
Hedging
gains/(losses)
recognised in
other
comprehensive
income
Amount of
hedge
ineffectiveness
recognised in
profit or loss
Line item
in profit or
loss in
which
hedge
ineffectiveness
is included
Hedged
future cash
flows no
longer
expected to
occur
As
hedged
item
affects
profit or
loss
Line item in
which
reclassification
adjustment is
included
£m £m £m £m
Cash flow hedges
Pre-hedging of long-term interest rates
5-10 years 4 -
Finance
(income)/
expense
--
Finance
(income)/
expense
10-30 years 3 -
Finance
(income)/
expense
--
Finance
(income)/
expense
>30 years 4 -
Finance
(income)/
expense
--
Finance
(income)/
expense
Interest rate benchmark reform
‘Interest rate benchmark reform – Amendments to IFRS 9, IAS 39, IFRS 4, IFRS 7 and
IFRS 16’ Phase I and Phase II were issued by the IASB in September 2019 and August 2020.
These amendments modify specific hedge accounting requirements to allow hedge accounting
to continue for affected hedges during the period of uncertainty before the hedged items or
hedging instruments affected by the current interest rate benchmarks are amended as a result
of the ongoing interest rate benchmark reforms. Phase II also provides that, for financial
instruments measured using amortised cost measurement, changes to the basis for
determining the contractual cash flows required by interest rate benchmark reform should be
reflected by adjusting their effective interest rate and no immediate gain or loss should be
recognised.
396
The CH Group has closely monitored the market and the output from the various industry
working groups managing the transition to new benchmark interest rates. This includes
announcements made by LIBOR regulators, including the Financial Conduct Authority (FCA)
and the US Commodity Futures Trading Commission, regarding the transition away from
LIBOR (including GBP LIBOR, USD LIBOR and EURIBOR) to the Sterling Overnight Index
Average Rate (SONIA), the Secured Overnight Financing Rate (SOFR), and the Euro Short-
Term Rate (€STR) respectively.
At 31 December 2021, the CH Group was not directly exposed to interest rate benchmark
reform as it held no interest rate derivatives or floating rate debt that referenced to LIBOR. The
CH Group did not transition any material derivatives or floating rate debt into a new index as all
of the instruments referencing LIBOR matured before December 2021 and new derivative
instruments are referenced to SOFR.
Foreign exchange risk management
Foreign currency transaction exposures arising on internal and external trade flows are
selectively hedged. The CH Group’s objective is to minimise the exposure of overseas
operating subsidiaries to transaction risk by matching local currency income with local currency
costs where possible and by maintaining intercompany payment terms of 30 days or less.
Foreign currency cash flows may be hedged selectively as approved by the CFO. Cash
surpluses or borrowing requirements of subsidiary companies are usually managed centrally
using foreign exchange forward contracts and swaps to hedge future repayments back into the
originating currency.
397
Derivative financial instruments and hedging program
Derivative financial instruments are used to mitigate exposure to foreign exchange
transactional risks of the CH Group and are classified as a current asset or liability. The fair
value of a derivative financial instrument is classified as a non-current asset or liability if the
remaining maturity is more than 12 months and as a current asset or liability if the maturity is
less than 12 months. All foreign exchange contracts are for periods of 12 months or less.
2021 2020 2019
Fair value Fair value Fair value
Assets
£m
Liabilities
£m
Assets
£m
Liabilities
£m
Assets
£m
Liabilities
£m
Non-current
Cash flow hedges – Interest
rate swap contracts (principal
amount – £1,996 million
(2020 – £nil, 2019 – £nil)
12 (1) - - - -
Current
Cash flow hedges – Interest
rate swap contracts (principal
amount – £nil million (2020 –
£nil, 2019 – £nil)
- -----
Derivatives designated and
effective as hedging
instruments
12 (1) - - - -
Non-current
Embedded and other
derivatives
- -----
Current
Foreign exchange contracts
(principal amount –
£1,854 million (2020 –
£2,318 million, 2019 –
£1,668 million)
5 (18) 6 (25) 12 (2)
Embedded and other
derivatives
- -----
Derivatives classified as held
for trading
5 (18) 6 (25) 12 (2)
Total derivative instruments 17 (19) 6 (25) 12 (2)
Wholesale and retail credit risk
The CH Group employs the GSK Group as a service provider to monitor credit risk relating to
key wholesalers. These activities include a review of their quarterly financial information and
Standard & Poor’s credit ratings, development of internal risk ratings, and the establishment
and periodic review of credit limits. The results of these reviews are submitted to
GlaxoSmithKline Consumer Healthcare’s local management to support the risk management
process. No customer accounts for more than 5% of the CH Group’s trade receivables
balance.
All new customers are subject to a credit vetting process and existing customers will be subject
to a review at least annually. The vetting process and subsequent reviews involve obtaining
information including the customer’s status as a government or private sector entity, audited
financial statements, credit bureau reports, debt rating agency (e.g. Moody’s, Standard &
Poor’s) reports, payment performance history (from trade references, industry credit groups)
and bank references.
Historical and forward-looking information is considered to determine the appropriate expected
credit loss allowance.
398

Credit risk
Credit risk is the risk that a counterparty will default on its contractual obligations resulting in
financial loss to the Group and arises on cash and cash equivalents and favourable derivative
financial instruments held with banks and financial institutions as well as credit exposures to
wholesale and retail customers, including outstanding receivables.
The CH Group considers its maximum credit risk to be £3,894 million (2020: £3,588 million and
2019: £4,100 million) which is the total of the CH Group’s financial assets, excluding Other
investments which bear equity risk rather than credit risk. See next page for details on the
CH Group’s total financial assets.
The CH Group’s greatest concentration of credit risk at 31 December 2021 is £456 million
(2020: £412 million and 2019: £260 million) with GSK LLC (A/A2), and £229 million (2020:
£138 million and 2019: £254 million) with GSK IHC Ltd (A+/A2).
As at 31 December 2020, there was also credit risk of £313 million (2019: £nil) with
GlaxoSmithKline (China) R&D Company Limited (A/A2). As at 31 December 2019, the
CH Group was also exposed to concentration risk of £222 million with GSK Finance plc. There
has been no change in the estimation techniques or significant assumptions made during the
current reporting period in assessing the loss allowance for financial assets at amortised cost
since the adoption of IFRS 9.
Treasury-related credit risk
The CH Group has continued to maintain its conservative approach to counterparty risk
throughout 2021. The aggregate credit risk in respect of financial instruments that the
CH Group may have with one counterparty is limited by reference to the long-term credit
ratings assigned for that counterparty by Moody’s Investors Service (“Moody’s”) and Standard
and Poor’s. The table below sets out the credit ratings of counterparties for cash and cash
equivalents.
2021 AAA/Aaa AA/Aa A/A BBB/Baa
BB+/Ba1
and
below or
unrated Total
£m £m £m £m £m £m
Bank balances and deposits - - 393 14 3 410
Money Market Funds 3 - - - - 3
Government securities - 1 - - - 1
Third party financial derivatives - - 17 - - 17
3 1 410 14 3 431
2020 AAA/Aaa AA/Aa A/A BBB/Baa
BB+/Ba1
and
below or
unrated Total
£m £m £m £m £m £m
Bank balances and deposits
-
- 289 36 8 333
Third party financial derivatives
-
-6 - - 6
- - 295 36 8 339
2019 AAA/Aaa AA/Aa A/A BBB/Baa
BB+/Ba1
and
below or
unrated Total
£m £m £m £m £m £m
Bank balances and deposits
-
27 279 27 6 339
Third party financial derivatives
-
12 - - - 12
- 39 279 27 6 351
399

The credit ratings in the above tables are as assigned by Moody’s and Standard and Poor’s.
Where the opinion of the two rating agencies differs, the lower rating of the two is assigned to
the counterparty. Where local rating or Fitch data is the only source available, the ratings are
converted to global ratings equivalent to those of Standard and Poor’s or Moody’s using
published conversion tables.
The cash balances are used by subsidiary entities in funding their working capital
requirements. The £3 million (2020: £8 million and 2019: £6 million) of cash categorised as
held with unrated or sub-investment grade counterparties (lower than BBB-/Baa3) includes
£2 million (2020: £3 million and 2019: £3 million) held with BTV (unrated) in Austria and
£1 million (2020: £nil and 2019: £nil) held with Banco Popular (unrated) in Puerto Rico.
Global counterparty limits are assigned to each of GlaxoSmithKline Consumer Healthcare’s
banking and investment counterparties based on long-term credit ratings from Moody’s and
Standard and Poor’s. The CH Group’s usage of these limits is monitored daily by GSK Group’s
Corporate Compliance Officer (“CCO”) who operates independently from GSK Group’s
Treasury. Any breach of these limits would be reported to the CFO immediately. The CCO also
monitors the credit rating of these counterparties and, when changes in ratings occur, notifies
GSK Group’s Treasury so that changes can be made to investment levels or authority limits as
appropriate.
Financial assets and financial liabilities
2021 2020 2019
Carrying
value
Fair
value
Carrying
value
Fair
value
Carrying
value
Fair
value
£m £m £m £m £m £m
Financial assets measured at
amortised cost:
Cash and cash equivalents 410 410 333 333 339 339
Liquid investments 1 1 1 1 1 1
Financial assets at fair value
through profit or loss:
Trade and other receivables
and certain other non-current
assets
1,955 1,955 2,129 2,129 2,287 2,287
Loan amounts owing from
related parties
1,508 1,508 1,119 1,119 1,461 1,461
Held for trading derivatives that
are not in a designated and
effective hedging relationship
55 66 1212
Cash and cash equivalents
(Money Market Funds)
33 -- --
Financial assets at fair value
through other comprehensive
income:
Derivatives designated and
effective as hedging
instruments
12 12 - - - -
Total financial assets 3,894 3,894 3,588 3,588 4,100 4,100
400

Financial assets and financial liabilities (continued)
2021 2020 2019
Carrying
value
Fair
value
Carrying
value
Fair
value
Carrying
value
Fair
value
£m £m £m £m £m £m
Financial liabilities
measured at amortised
cost:
Short term loans and
overdrafts
(49) (49) (48) (48) (24) (24)
Total borrowings
excluding lease liabilities
(49) (49) (48) (48) (24) (24)
Trade and other payables,
Other provisions and certain
other non-current liabilities in
scope of IFRS 9
(2,673) (2,673) (2,888) (2,888) (3,056) (3,056)
Loan amounts owing to
related parties
(825) (825) (300) (300) (457) (457)
Financial liabilities at fair
value through profit or
loss:
Held for trading
derivatives that are not in
a designated and effective
hedging relationship
(18) (18) (25) (25) (2) (2)
Financial assets at fair
value through other
comprehensive income:
Derivatives designated
and effective as hedging
instruments
(1)(1)----
Total financial liabilities (3,566) (3,566) (3,261) (3,261) (3,539) (3,539)
Net financial assets and
financial liabilities
328 328 327 327 561 561
The table above presents the carrying amounts and the fair values of the CH Group’s financial
assets and liabilities. The fair values of the financial assets and liabilities are included at the
price that would be received to sell an asset or paid to transfer a liability in an orderly
transaction between market participants at the measurement date.
The following methods and assumptions were used to estimate the fair values:
• Cash and cash equivalents - approximates to the carrying amount
• Short-term loans and overdrafts - approximates to the carrying amount because of the
short maturity of these instruments
• Forward exchange contracts - based on present value of contractual cash flows using
market sourced data (exchange rates)
• Receivables and payables - approximates to the carrying amount
Trade and other receivables, Other non-current assets, Trade and other payables, Other
provisions and Other non-current liabilities are reconciled to the relevant balance sheet
amounts in tables below.
Financial instruments held at fair value
Financial assets and liabilities held at fair value are categorised by the valuation methodology
applied in determining their fair value. Where possible, quoted prices in active markets are
401

used (Level 1). Where such prices are not available, the asset is classified as Level 2, provided
all significant inputs to the valuation model used are based on observable market data. If one
or more of the significant inputs to the valuation model is not based on observable market data,
the instrument is classified as Level 3.
Level 1 Level 2 Level 3 Total
At 31 December 2021 £m £m £m £m
Financial assets at fair value through profit or
loss:
Held for trading derivatives that are not in a
designated and effective hedging relationship
-5 -5
Cash and cash equivalents (money market funds) 3 - - 3
Financial assets at fair value through other
comprehensive income
Derivatives designated and effective as hedging
instruments
-12 -12
317 -20
Financial liabilities at fair value through profit or
loss:
Held for trading derivatives that are not in a
designated and effective hedging relationship
- (18) - (18)
Financial assets at fair value through other
comprehensive income:
Derivatives designated and effective as hedging
instruments
- (1) - (1)
- (19) - (19)
Level 1 Level 2 Level 3 Total
At 31 December 2020 £m £m £m £m
Financial assets at fair value through profit or
loss:
Held for trading derivatives that are not in a
designated and effective hedging relationship
-6 6
-6 -6
Financial liabilities at fair value through profit or
loss:
Held for trading derivatives that are not in a
designated and effective hedging relationship
- (25) (25)
- (25) - (25)
Level 1 Level 2 Level 3 Total
At 31 December 2019 £m £m £m £m
Financial assets at fair value through profit or
loss:
Held for trading derivatives that are not in a
designated and effective hedging relationship
-12 12
-12 -12
Financial liabilities at fair value through profit or
loss:
Held for trading derivatives that are not in a
designated and effective hedging relationship
- (2) (2)
- (2) - (2)
Trade and other receivables and Other non-current assets in scope of IFRS 9
The following table reconciles financial instruments within Trade and other receivables and
Other non-current assets which fall within the scope of IFRS 9 to the relevant balance sheet
402
amounts. The financial assets are predominantly non-interest earning. Non-financial
instruments include tax receivables and prepayments, which are outside the scope of IFRS 9.
At 31 December 2021
Financial
instruments
Non-
financial
instruments
Total
£m £m £m
Trade and other receivables (Note 20) 1,947 260 2,207
Loans amount owing from related parties 1,508 - 1,508
Other non-current assets 8 - 8
3,463 260 3,723
At 31 December 2020
Financial
instruments
Non-
financial
instruments
Total
£m £m £m
Trade and other receivables (Note 20) 2,120 238 2,358
Loans amount owing from related parties 1,119 - 1,119
Other non-current assets 9 1 10
3,248 239 3,487
At 31 December 2019
Financial
instruments
Non-
financial
instruments
Total
£m £m £m
Trade and other receivables (Note 20) 2,278 201 2,479
Loans amount owing from related parties 1,461 - 1,461
Other non-current assets 9 1 10
3,748 202 3,950
403
Trade and other payables, Other provisions and Other non-current liabilities in scope of
IFRS9
The following table reconciles financial liabilities within Trade and other payables, Other
provisions and Other non-current liabilities which fall within the scope of IFRS 9 to the relevant
balance sheet amounts. Accrued wages and salaries are included within financial liabilities.
Non-financial instruments include payments on account, tax and social security payables and
provisions which do not arise from contractual obligations to deliver cash or another financial
asset, which are outside the scope of IFRS 9.
At 31 December 2021
Financial
instruments
Non-
financial
instruments
Total
£m £m £m
Trade and other payables (Note 23) (2,671) (331) (3,002)
Loan amounts owing to related parties (825) - (825)
Other provisions (Note 26) - (139) (139)
Other non-current liabilities (2) (6) (8)
(3,498) (476) (3,974)
At 31 December 2020
Financial
instruments
Non-
financial
instruments
Total
£m £m £m
Trade and other payables (Note 23) (2,881) (387) (3,268)
Loan amounts owing to related parties (300) - (300)
Other provisions (Note 26) - (168) (168)
Other non-current liabilities (7) (7) (14)
(3,188) (562) (3,750)
At 31 December 2019
Financial
instruments
Non-
financial
instruments
Total
£m £m £m
Trade and other payables (Note 23) (3,044) (376) (3,420)
Loan amounts owing to related parties (457) - (457)
Other provisions (Note 26) (3) (174) (177)
Other non-current liabilities (9) (12) (21)
(3,513) (562) (4,075)
Offsetting of financial assets and liabilities
Financial assets and liabilities are offset and the net amount reported in the balance sheet
where there is a legally enforceable right to offset the recognised amounts, and there is an
intention to settle on a net basis or realise the asset and settle the liability simultaneously.
There are also arrangements that do not meet the criteria for offsetting but still allow for the
related amounts to be offset in certain circumstances, such as bankruptcy or the termination of
a contract.
404

The following tables set out the financial assets and liabilities that are offset, or subject to
enforceable master netting arrangements and other similar agreements but not offset, as at
31 December 2021, 31 December 2020 and 31 December 2019. The column ‘Net amount’
shows the impact on the CH Group’s balance sheet if all offset rights were exercised.
Gross
financial
assets /
(liabilities)
Gross
financial
assets /
(liabilities)
set off
Net financial
assets/
(liabilities)
per
balance
sheet
Related
amounts not
offset
Net
amount
At 31 December 2021 £m £m £m £m £m
Financial assets
Derivative financial assets 17 - 17 (9) 8
Financial liabilities
Derivative financial liabilities (19) - (19) 9 (10)
Gross
financial
assets /
(liabilities)
Gross
financial
assets /
(liabilities)
set off
Net financial
assets/
(liabilities)
per
balance
sheet
Related
amounts not
offset
Net
amount
At 31 December 2020 £m £m £m £m £m
Financial assets
Derivative financial assets 6 - 6 (6) -
Financial liabilities
Derivative financial liabilities (25) - (25) 6 (19)
Gross
financial
assets /
(liabilities)
Gross
financial
assets /
(liabilities)
set off
Net financial
assets/
(liabilities)
per
balance
sheet
Related
amounts not
offset
Net
amount
At 31 December 2019 £m £m £m £m £m
Financial assets
Derivative financial assets 12 - 12 (1) 11
Financial liabilities
Derivative financial liabilities (2) - (2) 1 (1)
Amounts which do not meet the criteria for offsetting on the balance sheet but could be settled
net in certain circumstances principally relate to derivative transactions under ISDA
(International Swaps and Derivatives Association) agreements where each party has the option
to settle amounts on a net basis in the event of default of the other party. As there is presently
not a legally enforceable right of offset, these amounts have not been offset in the balance
sheet but have been presented separately in the tables above.
Sensitivity analysis
The sensitivity analysis has been prepared on the assumption that the amount of net cash
(cash and cash equivalents less overdrafts), the ratio of fixed to floating interest rates of the
debt and derivatives portfolio and the proportion of financial instruments in foreign currencies
are all constant. Financial instruments affected by market risk include borrowings, cash and
deposits and derivative financial instruments. The tables below illustrate the estimated impact
on the income statement and equity as a result of hypothetical market movements in foreign
exchange and interest rates in relation to the CH Group’s financial instruments. The range of
variables chosen for the sensitivity analysis reflects management’s view of changes which are
reasonably possible over a one-year period.
405

Foreign exchange sensitivity
The two major foreign currencies in which the CH Group’s financial instruments are
denominated are US Dollars and Euros. The CH Group has considered movements in these
currencies over the last three years and has concluded that a 10-cent movement in rates is a
reasonable benchmark. Financial instruments are only considered sensitive to foreign
exchange rates where they are not in the functional currency of the entity that holds them.
Intercompany loans which are fully hedged to maturity with a currency swap have been
excluded from this analysis.
2021 2020
2019
Increase/
(decrease)
in income
(Decrease)/
increase
in income
(Decrease)/
increase
in income
£m £m £m
10 cent appreciation of the US dollar 2 (14) (1)
10 cent depreciation of the US dollar (2) 12 1
10 cent appreciation of the Euro dollar (5) (7) (5)
10 cent depreciation of the Euro dollar 4 6 4
Interest rate sensitivity
The CH Group is exposed to interest rate risk on its outstanding borrowings and investments
where any changes in interest rates will affect future cash flows or the fair values of financial
instruments.
The table below shows the CH Group’s hypothetical sensitivity to changes in interest rates in
relation to Sterling, US dollar, Euro and Swiss franc variable rate financial assets and liabilities,
including derivatives. If the interest rates applicable to floating rate financial assets and
liabilities were to have increased by 1% (100 basis points), and assuming other variables had
remained constant, it is estimated that the CH Group’s finance income for 2021 would have
increased by approximately £1 million (2020: £3 million increase and 2019: £13 million
increase). A 1% (100 basis points) movement in US interest rates would cause an increase of
£197 million to equity (2020 – nil and 2019 – nil). A 1% (100 basis points) movement in interest
rates in relation to Sterling or Euro is not deemed to have a material effect on equity.
2021 2020 2019
(Decrease)/
increase
in income
(Decrease)/
increase
in income
Increase/
(decrease)
in income
£m £m £m
1% (100 basis points) increase in Sterling interest rates (13) (15) 16
1% (100 basis points) increase in US dollar interest rates 8 14 4
1% (100 basis points) increase in Euro interest rates 6 4 (6)
1% (100 basis points) increase in Swiss franc interest rates - - (1)
406

Contractual cash flows for non-derivative financial liabilities and derivative instruments
The following table provides an analysis of the anticipated contractual cash flows including
interest payable for the CH Group’s non-derivative financial liabilities on an undiscounted
basis. Cash flows in foreign currencies are translated using spot rates at 31 December. Cash
flows associated with onerous contracts have not been included in this disclosure as the
maturity of these cash flows is included in Note 32 ‘Commitments’.
Debt
Lease
liabilities
Trade
payables and
other
liabilities not
in net debt Total
At 31 December 2021 £m £m £m £m
Due in less than one year 49 30 3,496 3,575
Between one and two years - 22 2 24
Between two and three years - 15 - 15
Between three and four years - 13 - 13
Between four and five years - 10 - 10
After five years - 27 - 27
Gross contractual cash flows 49 117 3,498 3,664
Debt
Lease
liabilities
Trade
payables and
other
liabilities not
in net debt Total
At 31 December 2020 £m £m £m £m
Due in less than one year 48 34 3,181 3,263
Between one and two years - 33 7 40
Between two and three years - 14 - 14
Between three and four years - 12 - 12
Between four and five years - 12 - 12
After five years - 34 - 34
Gross contractual cash flows 48 139 3,188 3,375
Debt
Lease
liabilities
Trade
payables and
other
liabilities not
in net debt Total
At 31 December 2019 £m £m £m £m
Due in less than one year 24 40 3,501 3,565
Between one and two years - 45 9 54
Between two and three years - 16 - 16
Between three and four years - 15 - 15
Between four and five years - 10 - 10
After five years - 35 - 35
Gross contractual cash flows 24 161 3,510 3,695
407

The table below provides an analysis of the anticipated contractual cash flows for the
CH Group’s derivative instruments, using undiscounted cash flows. Cash flows in foreign
currencies are translated using spot rates at 31 December. The gross cash flows of foreign
exchange contracts are presented for the purposes of this table although, in practice, the
CH Group uses standard settlement arrangements to reduce its liquidity requirements on these
instruments.
Years ended 31 December
2021
2020 2019
Receivables Payables Receivables Payables Receivables Payables
£m £m £m £m £m £m
Foreign
exchange
contracts
Due in less
than one year
1,852 (1,865) 2,321 (2,340) 1,668 (1,660)
Interest rate
swap contracts
Due in less
than one year
- (13)
-- --
Between one
and two years
12 (26)
-- --
Between two
and three
years
24 (26)
-- --
Between three
and four years
28 (26)
-- --
Between four
and five years
28 (26)
-- --
After five
years
260 (221)
-- --
Gross
contractual
cash flows
2,204 (2,203) 2,321 (2,340) 1,668 (1,660)
34. Employee share schemes
Incentives in the form of shares in the CH Group’s ultimate parent company, GlaxoSmithKline
plc, are provided to employees under the following share option and share award schemes.
The share-based compensation charge for the above schemes has been recorded in the
income statement as administrative expenses of £59 million (2020: £63 million and 2019:
£58 million). This expense is incurred in the form of a charge from GlaxoSmithKline Services
Unlimited, as calculated under IFRS 2 “Share-Based Payments”.
Performance Share Plan Awards and Share value plan
The GSK Group operates a Performance Share Plan whereby share awards are granted to
senior executives at no cost. The percentage of each award that vests is based upon the
performance of the GSK Group and the CH Group over a three-year measurement period.
Grants of Performance Share Plan awards normally vest at the end of the three-year vesting
and performance period and are available for sale at that time. The GSK Group operates a
Share Value Plan whereby share awards are granted to employees at no cost. There are no
performance criteria attached. Grants of Share Value Plan Awards normally vest at the end of
the three-year vesting period and are available for sale at that time.
35. Principal CH group companies
The following represent the principal subsidiaries of the CH Group at 31 December 2021. The
equity share capital of these entities is wholly owned by the CH Group except where its
408

percentage interest is shown. All companies are incorporated in their principal country of
operation except where stated. A full list of CHHL2’s subsidiaries is available in Note 38 which
forms part of these Historical Financial Information.
England
GlaxoSmithKline Consumer Healthcare (Overseas) Limited
GlaxoSmithKline Consumer Healthcare (UK) Trading Limited
GlaxoSmithKline Consumer Healthcare (UK) IP Limited
GlaxoSmithKline Consumer Healthcare Finance Limited
GlaxoSmithKline Consumer Healthcare Finance No. 2 Limited
GlaxoSmithKline Consumer Trading Services Limited
Consumer Healthcare Holdings Limited
Consumer Healthcare Intermediate Holdings Limited
GlaxoSmithKline Consumer Healthcare Holdings (No.2) Limited
PRISM PCH Limited
Europe
Novartis Consumer Health S.A. (Switzerland)
GlaxoSmithKline Healthcare GmbH & Co. KG (Germany)
Stafford-Miller (Ireland) Limited
USA
Block Drug Company, Inc.
GlaxoSmithKline Consumer Healthcare Holdings (US) LLC
GlaxoSmithKline Consumer Healthcare, L.P. (88%)
PF Consumer Healthcare 1 LLC
Consumer Healthcare Intermediate Holdings LLC
PRISM PCH LLC
GSK Consumer Healthcare Holdings No. 1 LLC
GSK Consumer Healthcare Holdings No. 2 LLC
GlaxoSmithKline Consumer Healthcare Holdings (US) Inc.
Other
Pfizer Biotech Corporation (55%)
Sino-American Tianjin Smith Kline & French Laboratories Ltd (China) (55%)
Puerto Rico Consumer Branch
Wyeth Pharmaceutical Co. Ltd.
GlaxoSmithKline Consumer Healthcare Pte. Ltd. (Singapore)
36. Non-controlling interests
Non-controlling interests comprises interests in entities, of which the CH Group’s
non-controlling interests are individually not material.
37. Post balance sheet events
On 11 March 2022, the Company’s Directors approved the interim dividends totalling
£421 million to be paid to the Company’s equity shareholders on 30 March 2022. Out of the
total dividends of £421 million, £286 million is to be paid to GlaxoSmithKline Consumer
Healthcare Holdings Limited, and £135 million is to be paid to PF Consumer Healthcare
Holdings LLC.
On 16 March 2022, the CH Group issued £700 million and €2,350 million in notes under a £10
billion Euro Medium Term Note Programme. In addition, on 24 March 2022, the CH Group
issued $8,750 million in notes pursuant to a private placement to institutional investors.
In connection with these bond issuances, the CH Group recognised long-term borrowings of
£9,275 million and loan amounts owing from related parties of £9,210 million, representing the
bond proceeds on lent to the GSK Group and the Pfizer Group following the issuances.
409
In March 2022, the CH Group legally acquired two brands from the GSK Group for
approximately £170 million. The CH Group had already recognised the net profits generated by
these brands due to the existing contractual arrangements with the GSK Group which gave the
CH Group the right to the profits generated by these brands.
38. Subsidiaries
The full list of subsidiaries and other significant holdings of CHHL2 as at 31 December 2021
are as follows:
Company Direct
shares
held (%)
Indirect
shares
held (%)
Security Address of the
registered
office
Wholly owned subsidiaries
GlaxoSmithKline Consumer
Healthcare SRL
- 100 Ordinary 1-5 Costache Negri
Street,
Opera Center One, 6th
floor
(Zone 2), District 5,
Bucharest, Romania
GlaxoSmithKline Consumer
Healthcare, Produtos para
a Saude e Higiene, Lda
- 100 Ordinary Euro
Quota 1
Rua Dr Antonio
Loureiro Borges No 3,
Arquiparque,
Miraflores, Alges,
1495-131, Portugal
- 100 Ordinary Euro
Quota 2
- 100 Ordinary Euro
Quota 4
GlaxoSmithKline
Healthcare Ukraine O.O.O.
- 100 Ownership Pavla Tychyny avenue,
1-V,
Kiev 02152, Ukraineinterest
GlaxoSmithKline Panama
S.A.
100 - Ordinary Urbanizacion Industrial
Juan D, Calles A Y B,
Republic of
Panama, Panama
GSK CH Caricam Sociedad
de Responsabilidad
Limitada
- 100 Participation Urbanizacion Industrial
Juan D, Calles A Y B,
Republic of Panama,
Panama
GlaxoSmithKline Paraguay
S.A.
- 100 Ordinary Oficial Gilberto Aranda
333,
Planta Alta Casi
Salvador del
Mundo, Asuncion,
Paraguay
GSK Consumer Healthcare
Holdings (US) Inc.
- 100 Common Corporation Service
Company, 251 Little
Falls Drive, Wilmington,
Delaware, 19808,
United States
Alacer Corp. - 100 Common CSC – 2710 Gateway
Oaks Drive, Suite
150N, Sacramento,
California,
95833-3505, United
States
410
Company Direct
shares
held (%)
Indirect
shares
held (%)
Security Address of the
registered
office
GlaxoSmithKline Consumer
Healthcare Holdings (US)
LLC
- 100 LLC Interests Corporation Service
Company, 251 Little
Falls Drive, Wilmington,
Delaware, 19808,
United States
Block Drug Company, Inc. - 100 Common Corporation Service
Company, Princeton
South Corporate
Center, Suite 160, 100
Charles Ewing Blvd,
Ewing, New Jersey,
08628, United States
Block Drug Corporation - 100 Common Corporation Service
Company, Princeton
South Corporate
Center, Suite 160, 100
Charles Ewing Blvd,
Ewing, New Jersey,
08628, United States
Stafford-Miller Limited
(in liquidation)
- 100 Ordinary 55 Baker Street London
W1U 7EU England
GlaxoSmithKline Consumer
Healthcare (US) IP LLC
- 100 LLC Interests Corporation Service
Company, 251 Little
Falls Drive, Wilmington,
Delaware, 19808,
United States
GlaxoSmithKline Consumer
Healthcare L.L.C.
- 100 Limited Liability
Company -
Interests
Corporation Service
Company, 2595
Interstate Drive, Suite
103, Harrisburg,
Pennsylvania, 17110,
United States
GSK Consumer Health, Inc. - 100 Common -
no par value
Corporation Service
Company, 251 Little
Falls Drive, Wilmington,
Delaware, 19808,
United States
GSK Consumer Healthcare
Services, Inc.
- 100 Common Stock Corporation Service
Company, 251 Little
Falls Drive, Wilmington,
Delaware, 19808,
United States
PF Consumer Healthcare 1
LLC
- 100 Membership Interest Corporate Service
Company, 251 Little
Falls Drive Wilmington
DE 19808 USA
Wyeth Consumer
Healthcare LLC
- 100 Membership Interest CT Corporation
System, 600 N 2nd St,
Suite 401, Harrisburg,
Pennsylvania, 17101,
United States
Pfizer PFE Colombia S.A.S 100 - Common Carrera 7 No.113-43
Piso 4 Colombia
411
Company Direct
shares
held (%)
Indirect
shares
held (%)
Security Address of the
registered
office
PT GSK Consumer
Healthcare Indonesia
- 100 Ordinary Graha Paramita
Building, 5th F, Jalan
Denpasar Raya Blok
D-2, Kuningan,
JAKARTA SELATAN,
12940, Indonesia
Consumer Healthcare
Holdings Limited
100 - Ordinary 980 Great West Road
Brentford Middlesex
TW8 9GS England
Consumer Healthcare
Intermediate Holdings
Limited
- 100 Ordinary 980 Great West Road
Brentford Middlesex
TW8 9GS England
GSK Consumer Healthcare
Capital NL B.V.
- 100 Shares 980 Great West Road
Brentford Middlesex
TW8 9GS England
GSK Consumer Healthcare
Capital UK PLC
- 100 Ordinary 980 Great West Road
Brentford Middlesex
TW8 9GS England
GSK Consumer Healthcare
Holdings (No.1) Limited
- 100 Ordinary 980 Great West Road
Brentford Middlesex
TW8 9GS England
GSK Consumer Healthcare
Holdings (No.3) Limited
- 100 Ordinary 980 Great West Road
Brentford Middlesex
TW8 9GS England
GSK Consumer Healthcare
Holdings (No.4) Limited
- 100 Ordinary 980 Great West Road
Brentford Middlesex
TW8 9GS England
GSK Consumer Healthcare
Holdings (No.5) Limited
- 100 Ordinary 980 Great West Road
Brentford Middlesex
TW8 9GS England
GSK Consumer Healthcare
Holdings (No.6) Limited
- 100 Ordinary 980 Great West Road
Brentford Middlesex
TW8 9GS England
GSK Consumer Healthcare
Holdings (No.7) Limited
- 100 Ordinary 980 Great West Road
Brentford Middlesex
TW8 9GS England
GSK Consumer Healthcare
Holdings (No.8) Limited
- 100 Ordinary 980 Great West Road
Brentford Middlesex
TW8 9GS England
GSK Consumer Healthcare
Capital US LLC
- 100 LLC Interests Corporation Service
Company, 251 Little
Falls Drive, Wilmington
DE 19808, United
States
GSK Consumer Healthcare
Chile SpA
- 100 TBC Av. Andrés Bello
N°2687, 25th floor, Las
Condes, Chile
GSK Consumer Healthcare
Egypt Limited
- 100 Ordinary North 90th street,
Boomerang building,
5th District, Cairo,
Egypt
412
Company Direct
shares
held (%)
Indirect
shares
held (%)
Security Address of the
registered
office
GSK Consumer Healthcare
Egypt LLC
- 100 Quotas North 90th street,
Boomerang building,
5th District, Cairo,
Egypt
GSK Consumer Healthcare
Peru S.R.L
- 100 Ordinary Av Jorge Basadre 349,
piso 5,San Isidro, Lima,
05W-109, Peru
GSK Consumer Healthcare
S.A.
- 100 Ordinary Route de l’Etraz, 1197
Prangins, Switzerland
GSK Consumer Healthcare
Schweiz AG
- 100 Ordinary Suurstoffi 14, Rotkreuz,
6343, Switzerland
PRISM PCH Limited - 100 Voting Shares
Non Voting Shares
980 Great West Road
Brentford Middlesex
TW8 9GS
Ferrosan S.R.L. - 100 Registered Capital 178/C Calea Turzii,
Cluj-
Napoca, Cluj County,
Romania
Pfizer Consumer
Healthcare GmbH
- 100 Ordinary Linkstrasse 10, 10785
Berlin,
Germany
Pfizer Consumer
Manufacturing Italy S.r.l.
- 100 Quota 90, Via Nettunese,
04011,
Aprilia (Prov. di Latin),
Italy
Ferrosan ApS - 100 A-Share Capital: Nykaer 68, Brondby,
DK-26 05, Denmark
B-Share Capital
Ferrosan International ApS
(merged into Ferrosan ApS
with effect 1 Jan 2021)
- 100 Ordinary Nykaer 68, Brondby,
DK-26 05, Denmark
GlaxoSmithKline Consumer
Healthcare (Ireland)
Limited
- 100 Ordinary Euro
Redenominated
12 Riverwalk Citywest
Business Campus,
Dublin, 24, Ireland
GlaxoSmithKline Consumer
Healthcare (UK) (No.1)
Limited
- 100 Ordinary 980 Great West Road,
Brentford, Middlesex,
TW8 9GS, England
GlaxoSmithKline Consumer
Healthcare (UK) IP Limited
- 100 Ordinary 980 Great West Road,
Brentford, Middlesex,
TW8 9GS, England
GlaxoSmithKline Consumer
Healthcare Finance Limited
- 100 Ordinary 980 Great West Road,
Brentford, Middlesex,
TW8 9GS, England
GlaxoSmithKline Consumer
Healthcare Investments
(Ireland) (No.2) Unlimited
Company
- 100 Ordinary Knockbrack,
Dungarvan, Co
Waterford, X35 RY76,
Ireland
GlaxoSmithKline Consumer
Trading Services Limited
- 100 Ordinary 980 Great West Road,
Brentford, Middlesex,
TW8 9GS, England
GlaxoSmithKline
Dungarvan Limited
- 100 Ordinary (Euro) Knockbrack,
Dungarvan, Co
Waterford, X35 RY76,
Ireland
413
Company Direct
shares
held (%)
Indirect
shares
held (%)
Security Address of the
registered
office
GSK Consumer Healthcare
Holdings No. 2 LLC
- 100 Unit Corporation Service
Company, 251 Little
Falls Drive, Wilmington,
Delaware, DE, 19808,
United States
GSK Consumer Healthcare
Insurance Limited
- 100 Ordinary Dorey Court, Admiral
Park, St Peter Port,
GY1 4AT, Guernsey
GlaxoSmithKline Consumer
Healthcare Pte. Ltd
- 100 Ordinary 23 Rochester Park,
139234,
Singapore
P.T. Sterling Products
Indonesia
- 100 A Shares Graha Paramita
Building, 5th F, Jalan
Denpasar Raya Blok
D-2, Jakarta, 12940,
Indonesia
B Shares
GSK Consumer Healthcare
Singapore Pte. Ltd.
- 100 Ordinary 23 Rochester Park,
139234,
Singapore
GSK Consumer Healthcare
Trinidad and Tobago
Limited
- 100 Ordinary 5
th
Floor Algico Plaza,
91-93 St. Vincent
Street, Port of Spain,
Trinidad and Tobago
PF Consumer Healthcare
Brazil Importadora e
Distribuidora de
Medicamentos Ltda
- 100 Quota Barueri, State of Sao
Paulo, at Avenida Ceci,
No.1900, Block lll, Part
67, Tambore District,
Zip Code 06460-120,
Brazil
PF Consumer Healthcare
Singapore Pte. Ltd
- 100 Ordinary 23 Rochester Park
139234 Singapore
PF Consumer Healthcare
UK Limited
- 100 Ordinary 980 Great West Road
Brentford Middlesex
TW8 9GS
PF Consumer Ireland
Company Limited
- 100 Ordinary 9 Riverwalk, National
Digital
Park, Citywest
Business Park,
Dublin, 24, Ireland
Pfizer Laboratories PFE
(Pty) Ltd.
- 100 Common Flushing Meadows
Building,
The Campus, 57
Sloane,
Bryanston 2021, South
Africa
SmithKline Beecham
Research Limited
- 100 Ordinary 980 Great West Road,
Brentford, Middlesex,
TW8 9GS, England
Stafford-Miller (Ireland)
Limited
- 100 Ordinary Clocherane, Youghal
Road,
Dungarvan, Co.
Waterford,
Ireland
414
Company Direct
shares
held (%)
Indirect
shares
held (%)
Security Address of the
registered
office
Stiefel Consumer
Healthcare (UK) Limited
- 100 Ordinary 980 Great West Road,
Brentford, Middlesex,
TW8 9GS, England
Stiefel Laboratories
(Ireland) Limited (in
liquidation)
- 100 Ordinary Finisklin Business Park,
County Sligo, Ireland
GlaxoSmithKline Consumer
Healthcare Chile SpA
- 100 Joint Stock Av. Andrés Bello
N°2687
25th floor Las Condes
Chile
Treerly Health Co., Ltd - 100 Capital Contribution Unit 01A, Room 3901,
No 16. East Zhujiang
Road,Tianhe District,
Guangzhou City, the
PRC, China
GSK Consumer Healthcare
Export Limited
- 100 Ordinary 980 Great West Road
Brentford Middlesex
TW8 9GS England
Wyeth Pharmaceutical Co.
Ltd
- 100 Registered Capital 4 Baodai West Road,
Suzhou, Jiangsu
Province, 215128,
China
GlaxoSmithKline Consumer
Healthcare (UK) Trading
Limited
- 100 Ordinary 980 Great West Road,
Brentford, Middlesex,
TW8 9GS, England
GlaxoSmithKline Consumer
Healthcare S.A.
- 100 Ordinary Site Apollo, Avenue
Pascal 2-4-6, Wavre,
1300, Belgium
GlaxoSmithKline Consumer
Healthcare (Thailand)
Limited
- 100
1
Ordinary 13th Floor, Unit 13.05
and
13.06 Wave Place,
55 Wireless Road,
Lumpini, Pathumwan,
Bangkok, 10330,
Thailand
GlaxoSmithKline Consumer
Healthcare (Overseas)
Limited
- 100 Ordinary 980 Great West Road,
Brentford, Middlesex,
TW8 9GS, England
GSK Consumer Healthcare
Levice s.r.o.
- 100 Ordinary Priemyselny Park
Gena, Ul. E. Sachsa
4-6, 934 01, Levice,
Slovakia
Duncan Consumer
Healthcare Philippines Inc
- 100 Common 23rd Flr The Finance
Centre 26th Street
Corner 9th Avenue
Bonifacio Global City
Taguig City 1634
Philippines
Ex-Lax, Inc. - 100 Common The Prentice Hall
Corporation System,
Puerto Rico, Inc., c/o
Fast Solutions, LLC,
Citi Tower, 252 Ponce
de Leon Avenue, Floor
20, San Juan, 00918,
Puerto Rico
415
Company Direct
shares
held (%)
Indirect
shares
held (%)
Security Address of the
registered
office
GlaxoSmithKline Brasil
Produtos para Consumo e
Saude Ltda
- 100
1
Quotas Av des Americas 3500,
4th Floor, Rooms 407-
420, Riode Janeiro-RJ,
22621-000, Brazil
GlaxoSmithKline Consumer
Healthcare (China) Co. Ltd
- 100 Ordinary Floor 8, 168
Xizangzhong Road,
Huangpu District,
Shanghai, China
GlaxoSmithKline (Suzhou)
Trading Co., Ltd
- 100 Registered Capital No.699 Gangpu Road,
Wusongjiang Science
and Technology
Industrial Park,
Wuzhong Economic &
Technical Development
Zone, Suzhou, China
GlaxoSmithKline Consumer
Healthcare (Hong Kong)
Limited
- 100 Ordinary 23/F., Tower 6, The
Gateway, 9 Canton
Road, Tsimshatsui,
Kowloon , Hong Kong
GlaxoSmithKline Consumer
Healthcare A/S
- 100 Ordinary Nykaer 68, Brondby,
DK-2605, Denmark
GlaxoSmithKline Consumer
Healthcare AB
- 100 Ordinary Nykaer 68, DK-2605,
Brondby, Denmark
GlaxoSmithKline Consumer
Healthcare Aps
- 100 Ordinary Delta Park 37, 2665,
Vallensbæk Strand,
Denmark
GlaxoSmithKline Consumer
Healthcare Australia Pty ltd
- 100 Ordinary 82 Hughes Avenue,
Ermington, NSW, 2115,
Australia
GlaxoSmithKline Consumer
Healthcare B.V.
- 100 Ordinary Van Asch van
Wijckstraat 55G
Amersfoot 3811 LP,
Netherlands
GlaxoSmithKline Consumer
Healthcare Colombia SAS
- 100 Ordinary Carrera 7 No. 113 - 43
Piso 4, Colombia
GlaxoSmithKline Consumer
Healthcare Czech Republic
s.r.o.
- 100 Ordinary Hvezdova 1734/2c,
Prague, 4 140 00,
Czech Republic
GlaxoSmithKline Consumer
Healthcare Finance No.2
Limited
- 100 Ordinary 980 Great West Road,
Brentford, Middlesex,
TW8 9GS, England
GlaxoSmithKline Consumer
Healthcare Finland Oy
- 100 Ordinary Piispansilta 9A,
Fin-02230,
Espoo, Finland
GlaxoSmithKline Consumer
Healthcare GmbH
- 100 Ordinary Wagenseilgasse 3,
Euro Plaza, Gebaude I,
4. Stock, Vienna,
A-1120, Austria
GlaxoSmithKline Consumer
Healthcare Hellas Single
Member Societe Anonyme
- 100 Ordinary 274 Kifissias Avenue
Halandri, Athens, 152
32, Greece
416
Company Direct
shares
held (%)
Indirect
shares
held (%)
Security Address of the
registered
office
GlaxoSmithKline Consumer
Healthcare Investments
(Ireland) (No 3) Limited (in
liquidation)
- 100 Ordinary Knockbrack,
Dungarvan, Co
Waterford, X35 RY76,
Ireland
GlaxoSmithKline Consumer
Healthcare Japan K.K.
- 100 Ordinary 1-8-1 Asasaka
Minato-ku,
Tokyo, Japan
GlaxoSmithKline Consumer
Healthcare Korea Co., Ltd.
- 100 Ordinary 9F LS Yongsan Tower,
92,
Hangang-daero,
Yongsan-gu, Seoul,
140-702, Republic of
Korea
GlaxoSmithKline Consumer
Healthcare Mexico, S. De
R.L. de C.V.
- 100 Ordinary Calzada Mexico-
Xochimilco
4900, Colonia San
Lorenzo
Huipulco, Delegacion
Tlalpan, Mexico, D.F.
14370, Mexico
GlaxoSmithKline Consumer
Healthcare New Zealand
ULC
- 100 Ordinary Level 11, Zurich House,
21
Queen Street,
Auckland, 1010, New
Zealand
GlaxoSmithKline Consumer
Healthcare Norway AS
- 100 Ordinary Drammensveien 288,
Lysaker, 1326 Norway
GlaxoSmithKline Consumer
Healthcare Philippines Inc
- 100 Common 23rd Flr The Finance
Centre 26th Street
Corner 9th Avenue
Bonifacio Global City
Taguig City 1634
Philippines
GlaxoSmithKline Consumer
Healthcare S.A.
- 100 Ordinary Severo Ochoa, 2,
Parque
Tecnologico de Madrid,
Tres
Cantos, Madrid, 28760,
Spain
GlaxoSmithKline Consumer
Healthcare Sdn. Bhd.
- 100 Ordinary Lot 89, Jalan Enggang,
Ampang/Ulu Kelang
Industrial Estate,
Selangor, 54200,
Malaysia
GlaxoSmithKline Consumer
Healthcare Slovakia s. r. o.
- 100 Ownership interest Galvaniho 7/A,
Bratislava, 821 04,
Slovakia
GlaxoSmithKline Consumer
Healthcare South Africa
(Pty) Ltd
- 100 Ordinary Flushing Meadows
Building,
The Campus, 57
Sloane Street,
Bryanston 2021, South
Africa
GlaxoSmithKline Consumer
Healthcare Sp.z.o.o.
- 100 Ordinary Ul. Grunwaldzka 189,
Poznan, 60-322,
Poland
417
Company Direct
shares
held (%)
Indirect
shares
held (%)
Security Address of the
registered
office
GlaxoSmithKline Consumer
Healthcare Sri Lanka
Holdings Limited
- 100 Ordinary 980 Great West Road,
Brentford, Middlesex,
TW8 9GS, England
GlaxoSmithKline Consumer
Healthcare S.r.l
- 100 Ordinary Via Zambeletti snc
Baranzate 20021 Milan
Italy
GlaxoSmithKline Consumer
Healthcare Vietnam
Company Limited
- 100 Charter Capital Floor 16, Metropolitan,
235
Dong Khoi, Ben Nghe
Ward,
District 1, Ho Chi Minh
City,
Vietnam
GlaxoSmithKline Consumer
Private Limited
- 100 Equity Patiala Road, Nabha
147201, Dist Patiala,
Punjab, India
GlaxoSmithKline Costa
Rica S.A.
- 100 Ordinary San Jose 300 Este de
la
Rotonda Betania,
Carretera a Sabanilla,
Costa Rica
GlaxoSmithKline
Healthcare AO
- 100 Ordinary Premises III, Room 9,
floor 6, Presnenskaya
nab. 10,
Moscow, 123112,
Russian
Federation
GlaxoSmithKline
Healthcare GmbH
- 100 Ordinary Barthstr. 4, Munchen,
80339, Germany
GlaxoSmithKline Consumer
Healthcare GmbH & Co.
KG
- 100 Partnership
Capital
Barthstr. 4, Munchen,
80339, Germany
Panadol GmbH - 100 Ordinary Barthstr. 4, Munchen,
80339, Germany
Kuhs GmbH - 100 Ordinary Barthstr. 4, Munchen,
80339, Germany
GlaxoSmithKline Limited - 100 Ordinary Likoni Road, PO Box
78392,
Nairobi, Kenya
GlaxoSmithKline Sante
Grand Public SAS
- 100 Ordinary 23 rue Francois Jacob,
92500, Rueil-
Malmaison, France
GlaxoSmithKline
Technology (Taizhou) Co.,
Ltd
- 100 Ordinary Room 708 in Building
D, Phase II of New
Drug
Innovation Base,
Taizhou, 225300,
Jaingsu, Province,
China
418
Company Direct
shares
held (%)
Indirect
shares
held (%)
Security Address of the
registered
office
GlaxoSmithKline Tuketici
Sagligi Anonim Sirketi
- 100 Nominative Buyukdere Caddesi
No. 173, 1.Levent
Plaza B Blok
1.Levent, Istanbul,
34394,
Turkey
GlaxoSmithKline-Consumer
Hungary Limited Liability
Company
- 100 Membership H-1124, Csorsz utca
43,
Budapest, Hungary
GSK Canada Holding
Company Limited
- 100 Ordinary 980 Great West Road,
Brentford, Middlesex,
TW8 9GS, England
PF Consumer Healthcare
Canada ULC/ PF Soins De
Sante SRI
- 100 Common 595 Burrad Street,
Three
Bentall Centre, P.O
Box 49314, Suite 2600,
Vancouver, British
Columbia Canada V7X
1L3
GSK CH Kazakhstan LLP - 100 Charter Capital 32 A Manasa Str.,
Bostandyk
District, Almaty,
050008,
Kazakhstan
GSK Consumer Healthcare
Israel Ltd
- 100 Ordinary 25 Basel Street, Petech
Tikva 49510, Israel
GSK New Zealand Holding
Company Limited
- 100 Ordinary 980 Great West Road,
Brentford, Middlesex,
TW8 9GS, England
Iodosan S.p.A. - 100 Ordinary Via Zambeletti
snc,Baranzate, Milan,
20021, Italy
PF Consumer Healthcare
B.V.
- A-100 Class A: Van Asch Van
Wjckstaat 55G, 3811
LP Amersfoort,
Netherlands
B-100 Class B
PF Consumer Healthcare
Holding B.V.
- 100 Ordinary Van Asch Van
Wjckstaat 55G, 3811
LP Amersfoort,
Netherlands
Pfizer Consumer
Healthcare AB Wyeth
Pharmaceuticals
Company
- 100 Ordinary Vetenskapsvagen 10,
SE-191
90, Sollentuna, Sweden
State Road No. 3
Kilometer 142.1,
Guayama, 00784,
Puerto Rico
- 100 Partnership shares
SmithKline Beecham S.A. - 100 Ordinary Ctra de Ajalvir Km
2.500, Alcala de
Henares, Madrid,
28806, Spain
419
Company Direct
shares
held (%)
Indirect
shares
held (%)
Security Address of the
registered
office
Sterling Drug (Malaya) Sdn
Berhad
- 100 Ordinary Lot 89, Jalan Enggang,
Ampang/Ulu Kelang
Industrial Estate,
Selangor, 54200,
Malaysia
GlaxoSmithKline Consumer
Healthcare Saudi Limited
- 100 Ordinary 603 Salamah Tower,
6th Floor, Madinah
Road, Al-Salamah
District, Jeddah, 21425,
Saudi Arabia
Vog AU PTY Ltd - 100 Ordinary,
Redeemable
82 Hughes Avenue,
Ermington, NSW, 2115,
Australia
Preference
NCH—Nutrition Consumer
Health Ltd
- 100 Ordinary 14 Hamephalsim St,
Petach
Tikva, Israel
PT BINA Dentalindo (in
liquidation)
- 100 Ordinary Gedung Graha
Ganesha Lantai 3, JI
Raya Bekasi Km 17,
No5, Jakarta Timur,
13930, Indonesia
Sterling products
international, Incorporated
- 100 Common Corporation Service
Company, 251 Little
Falls Drive, Wilmington,
Delaware, 19808,
United States
GlaxoSmithKline Consumer
Healthcare ULC /
GlaxoSmithKline Soins De
Sante Aux Consommateurs
SRI
- 100 A Class 595 Burrard Street
Suite 2600 Three
Bentall Centre
P.O. Box 49314
Vancouver BC V7X 1L3
Canada
Preference
Common
PF Consumer Healthcare
Poland sp. z.o.o
- 100 Ordinary Rzymowskiego 53
street
02-697 Warsaw Poland
PF Consumer Taiwan LLC 100 - Common interests The Corporation Trust
Company Corporation
Trust
Center 1209 Orange
Street
Wilmington DE 19801
United States
Subsidiaries where the effective interest is less than 100%
Glaxo Wellcome Ceylon
Limited
- Ordinary:
99.9995
Ordinary
B: 100
Ordinary
Ordinary B
121 Galle Road,
Kaldemulla,
Moratuwa, Sri Lanka
SmithKline Beecham
(Private) Limited
- 99.6462 Ordinary World Trade Center,
Level 34, West Tower,
Echelon Square,
Colombo 1, Sri Lanka
420
Company Direct
shares
held (%)
Indirect
shares
held (%)
Security Address of the
registered
office
GlaxoSmithKline Consumer
Healthcare, L.P.
- 88 Partnership
Capital -General
Partner/ Limited
Partner
Corporation Service
Company, 251 Little
Falls Drive, Wilmington,
Delaware, 19808,
United States
Beecham Enterprises Inc. - 88 Common Corporation Service
Company, 251 Little
Falls Drive, Wilmington,
Delaware, 19808,
United States
GlaxoSmithKline Consumer
Healthcare Pakistan Limited
- 85.79 Ordinary The Sykes Building, 35
Dockyard Road, West
Wharf, Karachi, 74000,
Pakistan
GSK-Gebro Consumer
Healthcare GmbH
- 60 Ordinary Bahnhofbichl 13, 6391
Fieberbrunn, KitzbUhel,
Austria
Sino-American Tianjin
Smith Kline & French
Laboratories Ltd
- 55 Ordinary Cheng Lin Zhuang
Industrial
Zone, Dong Li District,
Tianjin, 300163, China
Pfizer Biotech Corporation 54.98 - Ordinary 24F, No.66, Sec 1,
Zhong Xiao W. Rd,
Taipei 100, Taiwan
Other significant shareholdings
Duncan Pharmaceuticals
Philippines Inc
- 23.27 Common 23rd Flr The Finance
Centre 26th Street
Corner 9th Avenue
Bonifacio Global City
Taguig City 1634
Philippines
GlaxoSmithKline
Philippines Inc
- 23.27 Ordinary 23rd Floor, The
Finance
Centre, 26th Street
Corner 9th Avenue,
Bonifacio Global City,
Taguig City, 1634,
Philippines
GlaxoSmithKline
Landholding Company Inc
- 9.29 Common 23rd Floor, The
Finance
Centre 26th Street
Corner 9th Avenue
Bonifacio Global City
Taguig City 1634
Philippines
1
The Company holds a direct shareholding in these subsidiaries of less than 0.01%
421
Schedule III
PFIZER HISTORICAL FINANCIAL INFORMATION
FINANCIAL INFORMATION OF PFIZER CONSUMER HEALTHCARE
SECTION A: ACCOUNTANT’S REPORT IN RESPECT OF THE FINANCIAL INFORMATION OF
PFIZER CONSUMER HEALTHCARE FOR THE SEVEN MONTHS ENDED 31 JULY 2019
422

Deloitte LLP
Hill House
1 Little New Street
London
EC4A 3TR
The Board of Directors
on behalf of Haleon plc
980 Great West Road,
Brentford, Middlesex,
TW8 9GS,
United Kingdom
Goldman Sachs International
Plumtree Court,
25 Shoe Lane,
London,
EC4A 4AU
Citigroup Global Markets Limited
Citigroup Centre,
Canada Square,
Canary Wharf,
London,
E14 5LB
Merrill Lynch International
2 King Edward Street,
London
EC1A 1HQ
1 June 2022
Dear Sirs/Mesdames
Pfizer Contributed CH Business
We report on the financial information of the Pfizer Contributed CH Business for the seven month
period ended 31 July 2019 set out in Schedule III Section B of the prospectus dated 1 June 2022 of
Haleon plc (the “Company”) (the “Prospectus”). This financial information has been prepared for
inclusion in the Prospectus on the basis of the accounting policies set out in notes 1 and 2 to the
financial information. This report is required by Annex 1 item 18.3.1 of the PR Regulation and is given
for the purpose of complying with that requirement and for no other purpose.
Opinion on financial information
In our opinion, the financial information gives, for the purposes of the Prospectus, a true and fair view
of the state of affairs of the Pfizer Contributed CH Business as at 31 July 2019 and of its profits, cash
flows and changes in equity and statement of comprehensive income for the seven months then ended
in accordance with the basis of preparation set out in Note 1 to the financial information.
423
Responsibilities
As described in Notes 1 & 2 the Directors of the Company are responsible for preparing the financial
information on the basis of preparation set out in Note 1 to the financial information.
It is our responsibility to form an opinion on the financial information and to report our opinion to you.
Save for any responsibility arising under Prospectus Regulation Rule 5.3.2R(2)(f) to any person as and
to the extent there provided, to the fullest extent permitted by law we do not assume any responsibility
and will not accept any liability to any other person for any loss suffered by any such other person as a
result of, arising out of, or in connection with this report or our statement, required by and given solely
for the purposes of complying with Annex 1 item 1.3 of the PR Regulation, consenting to its inclusion in
the Prospectus.
Basis of preparation
This financial information has been prepared for inclusion in the Prospectus on the basis of the
accounting policies set out in note 1 to the financial information.
Basis of opinion
We conducted our work in accordance with Standards for Investment Reporting issued by the Financial
Reporting Council (“FRC”) in the United Kingdom. We are independent of the Company and the Pfizer
Contributed CH Business in accordance with the FRC’s Ethical Standards applied to Investment
Circular Reporting Engagements, and we have fulfilled our other ethical responsibilities in accordance
with these requirements.
Our work included an assessment of evidence relevant to the amounts and disclosures in the financial
information. It also included an assessment of significant estimates and judgments made by those
responsible for the preparation of the financial information and whether the accounting policies are
appropriate to the entity’s circumstances, consistently applied and adequately disclosed.
We planned and performed our work so as to obtain all the information and explanations which we
considered necessary in order to provide us with sufficient evidence to give reasonable assurance that
the financial information is free from material misstatement whether caused by fraud or other
irregularity or error.
Our work has not been carried out in accordance with auditing or other standards and practices
generally accepted in jurisdictions outside the United Kingdom, including the United States of America,
and accordingly should not be relied upon as if it had been carried out in accordance with those
standards and practices.
Conclusions Relating to Going Concern
In performing this engagement on the financial information, we have concluded that the directors’ use
of the going concern basis of accounting in the preparation of the financial information is appropriate.
424
Declaration
For the purposes of Prospectus Regulation Rule 5.3.2R(2)(f), we are responsible for this report as part
of the Prospectus and declare that to the best of our knowledge the information contained in this report
is, in accordance with the facts and that this report makes no omission likely to affect its import. This
declaration is included in the Prospectus in compliance with Annex 1 item 1.2 of the PR Regulation and
for no other purpose.
Yours faithfully
Deloitte LLP
Deloitte LLP is a limited liability partnership registered in England and Wales with registered number
OC303675 and its registered office at 1 New Street Square, London EC4A 3HQ, United Kingdom.
Deloitte LLP is the United Kingdom affiliate of Deloitte NSE LLP, a member firm of Deloitte Touche
Tohmatsu Limited, a UK private company limited by guarantee (“DTTL”). DTTL and each of its member
firms are legally separate and independent entities. DTTL and Deloitte NSE LLP do not provide
services to clients.
425
SECTION B: HISTORICAL FINANCIAL INFORMATION FOR PFIZER CONSUMER
HEALTHCARE FOR THE SEVEN MONTHS ENDED 31 JULY 2019
426
Pfizer Consumer Health
Combined Historical Financial Information
427

Pfizer Consumer Healthcare
Combined income statement for the seven months ended 31 July
Note 2019
£m
Revenue 5 1,523
Cost of sales (570)
Selling, general and administration (630)
Research and development (67)
Other operating income 1
Operating profit 6 257
Finance income 8 25
Finance expense 9 (37)
Net Finance costs (12)
Profit before tax 245
Income tax 11 (52)
Profit after tax for the period
89
193
Profit attributable to shareholders 191
Profit attributable to non-controlling interests 2
89
As the Business has not historically constituted a separate legal entity or group the disclosures related to earnings per share
are not able to be presented.
428
Pfizer Consumer Healthcare
Combined statement of comprehensive income for the seven months ended 31 July
2019
£m
Profit after tax for the period 193
Other comprehensive income for the period
Items that may be subsequently reclassified to income statement:
Exchange movements on overseas net assets and net investment hedges
301
301
Total comprehensive income, net of tax for the period 494
Total comprehensive income for the period attributable to:
Shareholders 492
Non-controlling interests 2
Total comprehensive income, net of tax for the period 494
429
Pfizer Consumer Healthcare
Combined balance sheet as at 31 July
Note 2019
£m
Non-current assets
Property, plant and equipment 12 551
Right-of-use assets 13 39
Intangible assets 14 6,624
Deferred tax assets 11 107
Other non-current assets 76
Total non-current assets 7,397
Current assets
Inventories 15 505
Trade and other receivables 16 501
Cash and cash equivalents 17 38
Current tax recoverable 8
Total current assets 1,052
Total assets 8,449
Current liabilities
Short-term borrowings 19 (11)
Trade and other payables 18 (649)
Current tax payable (108)
Short-term provisions 21 (37)
Total current liabilities (805)
Non-current liabilities
Long-term borrowings 19 (30)
Deferred tax liabilities 11 (1,001)
Income taxes payable (47)
Pensions and other post-employment benefits 20 (25)
Other provisions 21 (20)
Other non-current liabilities (17)
Total non-current liabilities (1,140)
Total liabilities (1,945)
Net assets 6,504
Equity
Invested capital 23 6,504
Non-controlling interests -
Total equity 6,504
430
Pfizer Consumer Healthcare
Combined statement of changes in equity for the seven months ended 31 July
Note Invested
capital
Non-controlling
interests
Total
Equity
£m £m £m
At 1 January 2019 6,006 - 6,006
Profit after tax 191 2 193
Other comprehensive income 301 - 301
Total comprehensive income 492 2 494
Share-based payment transactions 28 20 - 20
Distributions to non-controlling interests - (2) (2)
Distributions to parent (14) - (14)
Balance at 31 July 2019 6,504 - 6,504
431

Pfizer Consumer Healthcare
Combined statement of cash flows for the seven months ended 31 July
Note 2019
£m
Cash flows from operating activities
Profit after tax 193
Adjustments reconciling profit after tax to cash generated from
operations
25 (51)
Cash generated from operations 25 142
Taxation paid (84)
Net cash inflow from operating activities 58
Cash flows from investing activities
Purchase of property, plant and equipment (25)
Net cash (outflow) from investing activities (25)
Cash flows from financing activities
Payment of principle portion of lease liabilities (6)
Interest paid (1)
Financing activities with Pfizer (22)
Other financing cash flows (3)
Net cash (outflow) from financing activities (32)
Increase in cash and cash equivalents 1
Cash at the beginning of the period 35
Exchange adjustments 2
Increase in cash and cash equivalents 1
Cash and cash equivalents at end of the period 17 38
432
Pfizer Consumer Healthcare
Notes to the combined Historical Financial Information for the seven months ended 31 July
2019
1. Presentation of the Historical Financial Information
1.1 General Information
Pfizer Consumer Healthcare (“PCH” or the “Business”) was a business unit of Pfizer Inc.
(“Pfizer”) during the seven-month period ended 31 July 2019. PCH develops, manufactures
and markets leading non-prescription medicines, vitamins, and personal care products. The
combined Historical Financial Information includes all the operations that comprise the Pfizer
Consumer Healthcare operations of Pfizer.
On 31 July 2019, Pfizer completed a transaction in which Pfizer and GlaxoSmithKline plc
(“GSK”) combined their respective consumer healthcare businesses into a new consumer
healthcare joint venture that operates globally under the GSK Consumer Healthcare name. In
exchange for contributing PCH to the joint venture, Pfizer received a 32% equity stake in the
new company and GSK owns the remaining 68%.
The combined Historical Financial Information of PCH, as described further below, for the
seven months ended 31 July 2019 (the “Historical Financial Information”) has been
prepared specifically for the purposes of this Prospectus and in accordance with Regulation
(EU) 2017/1129 as supplemented by Commission Delegated Regulation (EU) 2019/980 each
as they form part of United Kingdom domestic law by virtue of the EU Withdrawal Act
(2018) and in accordance with the basis of preparation set out below.
This Historical Financial Information does not constitute statutory accounts within the meaning
of section 434(3) of the Companies Act 2006.
1.2 Perimeter and basis of preparation
As the Business has not historically constituted a separate legal entity or group, the Historical
Financial Information, which has been presented for the purpose of this document has been
prepared on a basis that combines the results, assets and liabilities of the Business by
applying principles of consolidation as set out in IFRS 10 ‘Consolidated Financial Statements’.
The Historical Financial Information has been prepared in accordance with the requirements of
the Listing Rules and in accordance with this basis of preparation. This basis of preparation
describes how the Historical Financial Information has been prepared in accordance with
International Financial Reporting Standards as adopted pursuant to Regulation (EC) No
1606/2002 as it applies in the European Union (‘IFRS’) for the seven months ended 31 July
2019 except as noted below. The principal accounting policies that have been applied in
preparing the Historical Financial Information are set out below. The Historical Financial
Information has been prepared on a basis consistent with the accounting policies adopted in
the Consolidated Historical Financial Information of GSK Consumer Healthcare.
IFRS does not explicitly provide guidance for the preparation of combined Historical Financial
Information, therefore certain accounting conventions permitted for the preparation of Historical
Financial Information for inclusion in investment circulars, as described in the Standards for
Investment Reporting 2000 Annexure (the ‘Annexure‘), issued by the UK Audit Practices
Board, have been applied where IFRS does not provide specific accounting treatments.
The Historical Financial Information is presented in Sterling (£). For most of PCH’s international
operations, the local currencies have been determined to be the functional currencies. All
values are in millions of GBP (‘‘£m’’), except where otherwise indicated.
433
All significant intercompany balances and transactions between the legal entities that comprise
PCH have been eliminated. Balances due from or due to Pfizer that are expected to be cash-
settled, if any, are included, depending on the nature of the balance, in Trade and other
receivables, Other non-current assets, Trade and other payables and Other non-current
liabilities in the combined balance sheet. All balances and transactions among PCH and Pfizer
that are not cash-settled are shown as part of equity in the combined balance sheet and
represent the net of amounts settled without payment (to)/from Pfizer. For additional
information about balances and transactions among PCH and Pfizer, see Note 24.
The Historical Financial Information has been derived from the consolidated financial
statements and accounting records of Pfizer and include allocations for direct costs and
indirect costs attributable to the operations of the Consumer Healthcare business of Pfizer.
Income statement
• The combined income statement reflects all of the operating results of PCH that were
either specifically identifiable or directly attributable to PCH and its operations
associated with the product lines based on how the PCH business was managed as
part of Pfizer in the period presented, except for certain products (primarily a
prescription medicine that transferred to a non-prescription product) that were not part
of the transaction with GSK and remained with Pfizer.
• The combined income statement includes costs directly incurred by PCH for certain
support functions (Enabling Functions) and allocations to PCH for Enabling Functions
that were provided on a centralised basis within Pfizer, such as expenses for digital,
facilities, legal, finance, human resources, insurance, public affairs and procurement,
among others. Allocations are based on either a specific identification basis or, when
specific identification is not practical, proportional allocation methods (e.g., using third-
party sales, headcount, etc.), depending on the nature of the services.
• The combined income statement includes certain manufacturing and supply costs
directly incurred by PCH for manufacturing facilities, external supply, and logistics and
support as well as allocations of such costs incurred by manufacturing plants that were
shared with other Pfizer business units and centralised Pfizer Global Supply (PGS)
costs that Pfizer did not routinely allocate to its business units. These costs may
include manufacturing variances and changes in the standard costs of inventory,
among others. Where used, allocations are based on either a specific identification
basis or, when specific identification is not practical, proportional allocation methods,
such as consumer healthcare identified manufacturing costs, depending on the nature
of the costs.
• The combined income statement includes directly incurred costs for certain PCH
research and development (R&D) activities and allocations of certain R&D expenses
managed by Pfizer’s R&D organisation. Pfizer does not routinely allocate these costs
to any of its business units. These allocations are based on either a specific
identification basis or, when specific identification is not practical, estimates of the
costs incurred in connection with the R&D activities associated with PCH.
• The combined income statement also includes allocations from Enabling Functions
and PGS for restructuring charges, additional depreciation associated with asset
restructuring and implementation costs. Pfizer does not routinely allocate these costs
to any of its business units. For additional information about allocations of restructuring
charges and other costs associated with cost-reduction/productivity initiatives, see
Note 10.
• The combined income statement includes allocations of pension and post-retirement
service costs that have been deemed attributable to PCH operations. For information
about allocations of pension and post-retirement costs, see Note 20.
434
• The combined income statement includes allocations of other corporate and
commercial costs, which can include, but are not limited to, certain compensation
items, such as share-based compensation expenses and certain fringe benefit
expenses maintained on a centralised basis within Pfizer. Pfizer does not routinely
allocate these costs to any of its business units. The combined income statement also
includes a combination of allocations to PCH and directly incurred costs for other
corporate and commercial costs for certain strategy, business development, and other
related activities. Allocations are based on either a specific identification basis or, when
specific identification is not practical, proportional allocation methods (e.g., using third-
party sales, headcount, etc.), depending on the nature of the services.
• The combined income statement includes an allocation of income from insurance
recoveries related to the hurricanes in Puerto Rico toward the end of the third quarter
of 2017.
• The combined income statement includes the impact of certain specifically identified
assets as well as allocations of purchase accounting impacts resulting from business
combinations and trade and asset acquisitions. These impacts are primarily associated
with PCH related assets acquired as part of Pfizer’s acquisitions of Wyeth (2009),
Ferrosan’s consumer healthcare business (2011), Alacer Corporation (2012) and
Treerly Healthcare Co. Ltd (2016), and primarily include amortisation related to the
increase in fair value of the acquired definite life intangible assets, depreciation related
to the increase/decrease in fair value of the acquired fixed assets (primarily
manufacturing facilities), and the fair value changes associated with contingent
consideration.
• The combined income statement includes an allocation of interest-related expenses,
including the effect of hedging activities associated with the Pfizer corporate debt, and
an allocation for interest income associated with the Pfizer corporate investments,
primarily based on proportional earnings before interest, taxes, depreciation and
amortisation. PCH participated in Pfizer’s centralised hedging and offsetting programs.
As such, the combined income statement, includes an allocation for the impact of
Pfizer’s derivative financial instruments used for offsetting changes in foreign currency
rates net of the related exchange gains and losses for the portion that was deemed to
be associated with PCH operations primarily based on proportional earnings before
interest, taxes, depreciation and amortisation.
Balance sheet
• The combined balance sheet reflects all of the assets and liabilities of Pfizer that were
either specifically identifiable or were directly attributable to PCH and its operations.
Cash from PCH operations in subsidiaries that were not completely PCH dedicated is
not included in the combined balance sheet since this cash is swept into Pfizer’s
centralised cash management system. PCH participated in Pfizer’s centralised cash
management system and generally all excess cash was transferred to Pfizer on a daily
basis. Cash disbursements for operations and/or investing activities were funded as
needed by Pfizer. Accordingly, the PCH cash balance as of 31 July 2019 is not
representative of an independent company and could be significantly different at
another point in time.
• For benefit plans, the combined balance sheet only includes the assets and liabilities of
benefit plans dedicated to PCH employees–see Note 20.
• The combined Historical Financial Information do not include allocations of Pfizer
corporate debt as none is specifically related to the PCH operations.
Management believes that the allocations are a reasonable reflection of the services received
or the costs incurred on behalf of PCH and its operations and that the combined income
statement reflects all costs of the PCH Business.
435
The provision for taxes on income in the combined income statement has been calculated as if
PCH filed a tax return separate from Pfizer in the various jurisdictions where it does business.
The allocated expenses from Pfizer primarily include:
Cost of
sales
Selling, general
and
administrative
expenses
Research
and
development
expenses
Other
operating
income
Net
Finance
costs Total
£m £m £m £m £m £m
Enabling functions operating
expenses
1 100 5 - - 106
PGS manufacturing costs 4 - 1 - - 5
Research and development
expenses
--6--6
Restructuring charges 1 5 - - - 6
Additional depreciation associated
with asset restructuring
1----1
Implementation costs 2 1 1 - - 4
Fringe benefit expenses - 1 - - - 1
Share-based compensation
expense
3134--20
Net interest expense - - - - 18 18
Income from insurance recoveries - (11) - - - (11)
Net gains associated with Pfizer’s
investments and net currency
exchange gains
- - - (1) (3) (4)
Other corporate and commercial
costs
231--6
2. Accounting principles and policies
(a) Consolidation
Entities over which PCH has the power to direct the relevant activities so as to affect the
returns to PCH, generally through control over the financial and operating policies from either
voting or contractual rights, are accounted for as subsidiaries. Interests acquired in entities are
consolidated from the date PCH acquires control and interests sold are de-consolidated from
the date control ceases.
Where, as part of a business combination, PCH is not able to exercise control over a particular
operation due to the existence of legal or other restrictions, the associated assets and liabilities
are not consolidated, and a financial asset or liability is recognised for the economic benefit or
obligation to be received under the contribution agreement. The assets and liabilities are
consolidated, and the associated financial asset or liability derecognised, on the date at which
PCH is able to exercise control over these operations.
Transactions and balances between subsidiaries are eliminated and no profit before tax is
recognised on sales between subsidiaries until the products are sold to customers outside of
PCH. Transactions with non-controlling interests are recorded directly in equity.
The financial information included in the combined Historical Financial Information is as of
31 July 2019 and for the seven months from 1 January 2019 to 31 July 2019 for the US
subsidiaries and as of 30 June 2019 and for the seven months from 1 December 2018 to
30 June 2019 for subsidiaries operating outside the US. PCH is not able to combine its US and
International subsidiaries on the same reporting date as it is impracticable to do so. The
Historical Financial Information is adjusted for the effects of significant transactions or events
that occur between these dates, where applicable. No such adjustments were necessary in the
period presented in these combined financial statements.
436
(b) Business combinations
Business combinations where common control exists at the time of the transaction relate to
businesses that were controlled by a part of Pfizer, other than PCH and then as a result of the
combination were transferred into PCH at some point during the period covered by the
Historical Financial Information. Such business combinations are accounted for by recognising
all the assets and liabilities acquired at their previous carrying values within Pfizer with effect
from the beginning of the earliest period reported in the Historical Financial Information. No
new goodwill arises from such transactions.
Business combinations where common control does not exist before the transaction are
accounted for using the acquisition accounting method. Identifiable assets, liabilities and
contingent liabilities acquired are measured at fair value at acquisition date. The consideration
transferred is measured at fair value and includes the fair value of any contingent
consideration. Where the consideration transferred, together with the non-controlling interest,
exceeds the fair value of the net assets, liabilities and contingent liabilities acquired, the excess
is recorded as goodwill, denominated in the currency of the operation acquired.
The costs related to business combinations are charged to the income statement in the period
in which they are incurred. Where not all the equity of a subsidiary is acquired the
non-controlling interest is recognised either at fair value or at the non-controlling interest’s
share of the net assets of the subsidiary, on a case-by-case basis.
Changes in PCH’s ownership percentage of subsidiaries are accounted for within equity.
(c) Foreign currency translation
Foreign currency transactions are booked in the functional currency of the relevant company at
the exchange rate ruling on the date of transaction. Foreign currency monetary assets and
liabilities are retranslated into the functional currency at rates of exchange ruling at the balance
sheet date. Exchange differences are included in the income statement.
On consolidation, assets and liabilities, including related goodwill, of overseas subsidiaries are
translated into Sterling at rates of exchange ruling at the balance sheet date. The results and
cash flows of overseas subsidiaries are translated into Sterling using average rates of
exchange.
Exchange adjustments arising when the opening net assets and the profits for the period
retained by overseas subsidiaries are translated into Sterling are recognised in Other
Comprehensive Income.
(d) Revenue
PCH receives revenue for supply of goods to external customers against orders received. The
majority of contracts that PCH enters into relate to sales orders containing single performance
obligations for the delivery of consumer healthcare products. The average duration of a sales
order is less than 12 months.
Product revenue is recognised when control of the goods is passed to the customer. The point
at which control passes is determined by each customer arrangement, but generally occurs on
delivery to the customer.
Product revenue represents net invoice value including fixed and variable consideration.
Variable consideration arises on the sale of goods as a result of discounts and allowances
given and accruals for estimated future returns and rebates. Revenue is not recognised in full
until it is highly probable that a significant reversal in the amount of cumulative revenue
recognised will not occur. The methodology and assumptions used to estimate rebates and
returns are monitored and adjusted regularly in the light of contractual and legal obligations,
437
historical trends, past experience and projected market conditions. Once the uncertainty
associated with the returns and rebates is resolved, revenue is adjusted accordingly. Value
added tax and other sales taxes are excluded from revenue.
(e) Expenditure
Expenditure is recognised in respect of goods and services received when supplied in
accordance with contractual terms. Provision is made when an obligation exists for a future
liability in respect of a past event and where the amount of the obligation can be reliably
estimated. Manufacturing start-up costs between validation and the achievement of normal
production are expensed as incurred. Advertising and promotion expenditure are charged to
the income statement as incurred. Shipment costs on intercompany transfers are charged to
cost of sales, whereas distribution costs on sales to customers are included in selling, general
and administrative (‘SG&A’) expenditure.
Restructuring costs are recognised and provided for, where appropriate, in respect of the direct
expenditure of a business reorganisation where the plans are sufficiently detailed and well
advanced, and where a valid expectation to those affected has been created by either starting
to implement the restructuring plans or announcing its main features.
(f) Research and development
Research and development (‘R&D’) expenditure is charged to the income statement in the
period in which it is incurred. Development expenditure is capitalised from when the required
regulatory approvals to launch a new product are obtained and the criteria for recognising an
asset are met. Property, plant and equipment used for research and development is capitalised
and depreciated in accordance with the policy described below.
(g) Legal and other disputes
Provision is made for the anticipated settlement costs of legal or other disputes against PCH
where an outflow of resources is considered probable and a reliable estimate can be made of
the likely outcome.
PCH may become involved in legal proceedings, in respect of which it is not possible to make
a reliable estimate of the expected financial effect, if any, that could result from ultimate
resolution of the proceedings. In these cases, appropriate disclosure about such cases would
be included but no provision would be made. Costs associated with claims made by PCH
against third parties are charged to the income statement as they are incurred.
(h) Pensions and other post-employment benefits
The costs of providing pensions under defined benefit schemes are calculated using the
projected unit credit method and spread over the period during which benefit is expected to be
derived from the employees’ services, consistent with the advice of qualified actuaries. Pension
obligations are measured as the present value of estimated future cash flows discounted at
rates reflecting the yields of high-quality corporate bonds. Pension scheme assets are
measured at fair value at the balance sheet date.
The costs of other post-employment liabilities are calculated in a similar way to defined benefit
pension schemes and spread over the period during which benefit is expected to be derived
from the employees’ services, in accordance with the advice of qualified actuaries. Future cash
flows discounted at rates reflecting the yields of high-quality corporate bonds. Pension scheme
assets are measured at fair value at the balance sheet date.
Actuarial gains and losses and the effect of changes in actuarial assumptions, are recognised
in the statement of comprehensive income in the period in which they arise.
PCH’s contributions to defined contribution plans are charged to the income statement as
incurred.
438

(i) Employee share plans
Incentives in the form of shares in PCH’s ultimate parent company, Pfizer Inc., are provided to
employees under share option and share award schemes.
The fair values of these options and awards are calculated at their grant dates using a Black-
Scholes option pricing model or other appropriate methods, such as Monte Carlo simulation
model or intrinsic value method, and charged to the income statement over the relevant vesting
periods. At the end of each reporting period, the Pfizer Group revises its charge based on the
number of options expected to vest, where appropriate.
(j) Property, plant and equipment
Property, plant and equipment (PP&E) is stated at the cost of purchase or construction less
provisions for depreciation and impairment. Financing costs are capitalised within the cost of
qualifying assets under construction.
Depreciation is calculated to write off the cost less residual value of PP&E, excluding freehold
land, using the straight-line basis over the expected useful life. Residual values and lives are
reviewed, and where appropriate adjusted, annually. The normal expected useful lives of the
major categories of PP&E are:
Land -
Buildings 33 to 50 years
Plant and machinery 8 to 20 years
Equipment and vehicles 3 to 12 years
On disposal of PP&E, the cost and related accumulated depreciation and impairments are
removed from the Historical Financial Information and the net amount, less any proceeds, is
taken to the income statement.
(k) Intangible Assets
Goodwill
Goodwill arising on consolidation represents the excess of the cost of acquisition over the fair
value of PCH’s share of the identifiable assets and liabilities of the acquired subsidiaries at the
date of acquisition. Goodwill is tested annually for impairment, or more frequently where
indicators of impairment exist and is carried at cost less any accumulated impairment losses.
Goodwill is allocated to cash generating units (“CGU”) for the purpose of impairment testing. A
CGU is identified at the lowest aggregation of assets that generate largely independent cash
inflows, and that which is looked at by management for monitoring and managing the business.
If the recoverable amount of the CGU is less than the carrying amount, an impairment loss is
allocated first to reduce the carrying amount of any goodwill allocated to the unit and then to
the other assets of the unit pro rata on the basis of the carrying amount of each asset in the
unit. Any impairment is immediately recognised in the income statement and an impairment
loss recognised for goodwill is not subsequently reversed.
On disposal, the attributable amount of goodwill is included in the determination of the gain or
loss on disposal.
Other intangible assets
Intangible assets are stated at cost less provisions for amortisation and impairments.
439
Licences, patents, know-how and marketing rights separately acquired or acquired as part of a
business combination are amortised over their estimated useful lives, generally not exceeding
20 years, using the straight-line basis from the time they are available for use. The estimated
useful lives for determining the amortisation charge consider patent lives, where applicable, as
well as the value obtained from periods of non-exclusivity. Asset lives are reviewed, and where
appropriate, adjusted annually.
Any development costs incurred and associated with acquired licences, patents, know-how or
marketing rights are written off to the income statement when incurred, unless the criteria for
recognition of an internally generated intangible asset are met, usually when a regulatory filing
has been made in a major market and approval is considered highly probable.
Acquired brands are valued independently as part of the fair value of businesses acquired from
third parties where the brand has a value which is substantial and long term and where the
brands either are contractual or legal in nature or can be sold separately from the rest of the
businesses acquired. Brands are amortised over their estimated useful lives of up to 20 years,
except where it is considered that the useful economic life is indefinite.
The costs of acquiring and developing computer software for internal use and internet sites for
external use are capitalised as intangible fixed assets where the software or site supports a
significant business system and the expenditure leads to the creation of an asset. ERP
systems software is amortised over seven to ten years and other computer software over three
to five years.
(l) Leases
PCH recognises right of use assets under lease arrangements in which it is the lessee. Rights
to use assets owned by third parties under lease arrangements are capitalised at the inception
of the lease and recognised on the balance sheet. The corresponding liability to the lessor is
recognised as a lease obligation within short and long-term borrowings. The carrying amount is
subsequently increased to reflect interest on the lease liability and reduced by lease payments
made.
For calculating the discounted lease liability on leases the implicit rate in the lease is used. If
this is not available, the incremental borrowing rate with a lease specific adjustment is used. If
neither of these is available, the incremental borrowing rate is calculated at the rate of interest
at which PCH would have been able to borrow for a similar term and with a similar security the
funds necessary to obtain a similar asset in a similar market.
Finance costs are charged to the income statement so as to produce a constant periodic rate
of charge on the remaining balance of the obligations for each accounting period.
Variable rents are not part of the lease liability and the right of use asset. These payments are
charged to the income statement as incurred. Short-term and low value leases are not
capitalised, and lease rentals are charged to the income statement as incurred.
Non-lease components are accounted for separately from the lease components in plant and
equipment leases but are not separately accounted for in land and buildings or vehicle leases.
If modifications or reassessments occur, the lease liability and right of use asset are
re-measured.
Right of use assets where title is expected to pass to PCH at a point in the future are
depreciated on a basis constant with similar owned assets. In other cases, right of use assets
are depreciated over the shorter of the useful life of the asset or the lease term.
(m) Impairment of non-current assets
The carrying values of all non-current assets are reviewed for impairment, either on a stand-
alone basis or as part of a larger CGU, when there is an indication that the assets might be
440
impaired. Additionally, goodwill, intangible assets with indefinite useful lives and intangible
assets which are not yet available for use are tested for impairment annually. Any provision for
impairment is charged to the income statement.
Impairments of goodwill are not reversed. Impairment losses on other non-current assets are
only reversed if there has been a change in estimates used to determine recoverable amounts
and only to the extent that the revised recoverable amounts do not exceed the carrying values
that would have existed, net of depreciation or amortisation, had no impairments been
recognised.
(n) Inventories
Inventories are included in the Historical Financial Information at the lower of cost (including
raw materials, direct labour, other direct costs and related production overheads) and net
realisable value. Cost is generally determined on a first in, first out basis.
(o) Trade payables
Trade payables are initially recognised at fair value and then held at amortised cost. Long-term
payables are discounted where the effect is material.
(p) Taxation
Current tax is provided at the amounts expected to be paid applying tax rates that have been
enacted or substantively enacted by the balance sheet date.
Deferred tax is calculated on temporary differences arising between the tax bases of assets
and liabilities and their carrying amounts in the Historical Financial Information. Deferred tax
assets are recognised to the extent that it is probable that future taxable profits will be available
against which the temporary differences can be utilised. Deferred tax is provided on temporary
differences arising on investments in subsidiaries except where the timing of the reversal of the
temporary difference can be controlled and it is probable that the temporary difference will not
reverse in the foreseeable future. Deferred tax is provided using rates of tax that have been
enacted or substantively enacted by the balance sheet date. Deferred tax assets and liabilities
are offset if and only if there is a legally enforceable right to set off current tax assets and
current tax liabilities and the deferred tax assets and deferred tax liabilities relate to income
taxes levied by the same taxation authority on either the same taxable entity or different
taxable entities which intend either to settle current tax liabilities and assets on a net basis, or
to realise the assets and settle the liabilities simultaneously, in each future period in which
significant amounts of deferred tax liabilities or assets are expected to be settle or recovered.
Deferred tax relating to items recognised outside profit or loss is recognised outside profit or
loss. Deferred tax items are recognised in correlation to the underlying transaction either in
OCI or directly in equity.
(q) Financial instruments
Financial assets
Financial assets are measured at amortised cost, fair value through other comprehensive
income (FVTOCI) or fair value through profit or loss (FVTPL). The measurement basis is
determined by reference to both the business model for managing the financial asset and the
contractual cash flow characteristics of the financial asset. For financial assets other than trade
receivables a 12-month expected credit loss (ECL) allowance is recorded on initial recognition.
If there is subsequent evidence of a significant increase in the credit risk of an asset, the
allowance is increased to reflect the full lifetime ECL. If there is no realistic prospect of
recovery, the asset is written off.
441
Trade receivables
Trade receivables are measured in accordance with the business model under which each
portfolio of trade receivables is held. PCH has portfolios in two of the three business models
under IFRS 9 to collect the contractual cash flows (measured at amortised cost) and to sell the
contractual cash flows (measured at FVTPL).
Trade receivables measured at amortised cost are carried at the original invoice amount less
allowances for ECL. Expected credit losses are calculated in accordance with the simplified
approach permitted by IFRS 9, using a provision matrix applying lifetime historical credit loss
experience to the trade receivables. The expected credit loss rate varies depending on whether
and the extent to which settlement of the trade receivables is overdue and it is also adjusted as
appropriate to reflect current economic conditions and estimates of future conditions. For the
purpose of determining credit loss rates, customers are classified into groupings that have
similar loss patterns. The key drivers of the loss rate are the location and type of customer.
When a trade receivable is determined to have no reasonable expectation of recovery it is
written off, firstly against any expected credit loss allowance available and then to the income
statement.
Subsequent recoveries of amounts previously provided for or written off are credited to the
income statement. Long-term receivables are discounted where the effect is material.
Cash and cash equivalents
Cash held in deposit accounts is measured at amortised cost. Cash and short-term deposits in
the statement of financial position comprise cash at banks and on hand and short-term highly
liquid deposits with a maturity of three months or less, that are readily convertible to a known
amount of cash and subject to an insignificant risk of changes in value.
Borrowings
All borrowings are initially recorded at the amount of proceeds received, net of transaction
costs. Borrowings are subsequently carried at amortised cost, with the difference between the
proceeds, net of transaction costs, and the amount due on redemption being recognised as a
charge to the income statement over the period of the relevant borrowing.
Derivative financial instruments and hedging
Derivative financial instruments are used to manage exposure to market risks. During the
period presented, PCH did not directly use derivative financial instruments to manage its
exposure to market risks, instead the business participated in Pfizer’s centralised hedging
program. PCH does not hold or issue derivative financial instruments for trading or speculative
purposes.
Derivative financial instruments are classified as held-for-trading and are measured at fair
value. Derivatives designated as hedging instruments are classified on inception as cash flow
hedges, net investment hedges or fair value hedges.
Changes in the fair value of derivatives designated as cash flow hedges are recognised in
other comprehensive income to the extent that the hedges are effective. Ineffective portions
are recognised in profit or loss immediately. Amounts deferred in other comprehensive income
are reclassified to the income statement when the hedged item affects profit or loss.
Net investment hedges are accounted for in a similar way to cash flow hedges.
442
3. Key accounting judgements and estimates
In preparing the combined Historical Financial Information, management is required to make
judgements about when or how items should be recognised in the combined Historical
Financial Information and estimates and assumptions that affect the amounts of assets,
liabilities, income and expenses reported in the combined Historical Financial Information.
Actual amounts and results could differ from those estimates. The following are considered to
be the critical accounting judgements and key sources of estimation uncertainty.
Taxation
Uncertain Tax Positions
Management makes the judgement of whether there is sufficient information to be able to
make a reliable estimate of the outcome of the dispute. If insufficient information is available,
no provision is made.
If sufficient information is available, in estimating a potential tax liability, PCH applies a risk-
based approach to determine the transactions most likely to be subject to challenge, assuming
that the relevant tax authority will review and have full knowledge of all the relevant
information, and the probability that PCH would be able to obtain compensatory adjustments
under international tax treaties. These estimates consider the specific circumstances of each
dispute and relevant external advice, are inherently judgemental and could change
substantially over time as each dispute progresses and new facts emerge.
Further details and the factors affecting the tax charge in future years are set out in Note 11
‘Taxation’. Where open tax matters exist, the ultimate liability for such matters may vary from
the amounts provided and is dependent upon the outcome of negotiations with the relevant tax
authorities or, if necessary, litigation proceedings. Due to the number of uncertain tax positions
held and the number of jurisdictions to which these relate, it is not practical to give meaningful
sensitivity estimates.
Legal and other disputes
Judgement and estimates
Management makes a judgement of whether it is remote, possible or probable that an outflow
of resources embodying economic benefits will be required to settle legal obligations. To the
extent that the potential outflow is assessed as possible but not probable or insufficient
information is available to make a judgement on whether a potential outflow is probable, no
provision is made and disclosure related to the claim is provided.
For legal obligations that are assessed as leading to a probable outflow and sufficient
information is available, the estimated provisions take into account the specific circumstances
of each dispute and relevant external advice, are inherently judgemental and could change
substantially over time as each dispute progresses and new facts emerge. Details of the status
and various uncertainties involved in the significant unresolved disputes are set out in Note 22,
‘Contingent Liabilities’. Management, having taken legal advice, have established provisions
after taking into account the relevant facts and circumstances of each matter and in
accordance with accounting requirements.
PCH may become involved in legal proceedings, in respect of which it is not possible to make
a reliable estimate of the expected financial effect, or practicable to give a meaningful range of
outcomes that could result from ultimate resolution of the proceedings. In these cases,
appropriate disclosure about such cases would be provided, but no provision would be made
and no contingent liability can be quantified. The ultimate liability for legal claims may vary from
the amounts provided and is dependent upon the outcome of litigation proceedings,
investigations, and possible settlement negotiations. The position could change over time and,
therefore, there can be no assurance that any losses that result from the outcome of any legal
proceedings will not exceed the amount of the provisions reported in PCH’s combined financial
statements by a material amount.
443

4. Exchange rates
PCH operates in many countries and earns revenues and incurs costs in many currencies. The
results of PCH as presented in Sterling, are affected by movements in exchange rates between
Sterling and other currencies. Average exchange rates, as modified by specific transaction
rates for large transactions, prevailing during the year, are used to translate the results and
cash flows of overseas subsidiaries into Sterling. Year-end rates are used to translate the net
assets of those entities. The currencies which most influenced these translations and the
relevant exchange rates were:
Seven Months Ended 31 July 2019
Average rates Period end rates
CNY/£ 8.72 8.38
Euro/£ 1.14 1.09
US$/£ 1.28 1.22
5. Revenue
Analysis of revenue by geography is included below.
Seven Months Ended
31 July 2019
Revenue by geography £m
North America 845
APAC 316
EMEA 249
LATAM 113
Total revenue 1,523
North America consists of United States and Canada.
Asia Pacific (APAC) consists of China, India, Australia, Philippines, Taiwan, Korea and certain
other APAC countries.
Europe, Middle East, Africa (EMEA) consists of countries in Europe, Middle East, Africa,
including Germany, Italy, the U.K., France, South Africa and certain other EMEA countries.
Latin America (LATAM) consists of Brazil, Colombia, Mexico, Puerto Rico, Argentina and
certain other LATAM countries.
Analysis of revenue by major product category is included below.
Seven Months Ended
31 July 2019
Revenue by product £m
Dietary Supplements 698
Pain Management 427
Personal Care Group 72
Respiratory 142
Gastrointestinal 179
Other 5
Total revenue 1,523
444

6. Operating profit
Seven Months Ended
31 July 2019
The following items have been included in operating profit: £m
Advertising and promotion 253
Distribution costs 39
Net foreign exchange gains (7)
Short term lease charge 7
7. Employee and key management personnel costs
Seven Months Ended
31 July 2019
£m
Wages and salaries 169
Social security costs 60
Pension and other post-employment costs (Note 20) 18
Cost of share-based incentive plans (Note 28) 20
Severance costs from integration and restructuring activities 7
274
PCH provides benefits to employees, commensurate with local practice in individual countries,
including, in some markets, healthcare insurance, subsidised car schemes and personal life
insurance.
All individuals performing services for PCH are employed and remunerated by PCH companies
or other members of the Pfizer group. Where a management charge for wages and salaries
has been made from entities outside PCH, such amounts are not included in wages and
salaries above and it is not practical to separate such amounts from other management
recharges.
Details of Key Management Personnel
Key Management Personnel includes representatives from the PCH leadership team, prior to
the completion of the Pfizer Transaction on 31 July 2019. The compensation of Key
Management Personnel in respect of their services to PCH in aggregate was as follows:
Seven Months Ended
31 July 2019
£m
Wages and salaries 2
Social security costs 1
Pension and other post-employment costs -
Cost of share-based incentive plans 1
4
8. Finance income
Seven Months Ended
31 July 2019
£m
Interest income arising from:
Cash and cash equivalents 4
Net gains arising from:
Financial instruments measured at fair value through profit or loss 21
25
445

9. Finance expense
Seven Months Ended
31 July 2019
£m
Interest expense arising on:
Financial liabilities at amortised cost (24)
Derivatives at fair value through profit or loss (18)
Finance expense arising on lease liabilities (1)
Other finance expense (6)
(37)
10. Restructuring costs
Restructuring costs are primarily related to employee termination costs associated with cost-
reduction and productivity initiatives. Employee termination costs are generally recorded when
the actions are probable and estimable and include accrued severance benefits, pension and
post-retirement benefits, many of which may be paid out during periods after termination.
The balances of the restructuring costs as at 31 July 2019 are included in Note 21 Other
Provisions.
Seven Months Ended
31 July 2019
£m
Cost of sales 1
Selling, general and administration, and other operating expenses 8
9
11. Taxation
The major components of income tax expense are:
Seven Months Ended
31 July 2019
Taxation charge based on profits for the period
£m
Total current taxation 43
Total deferred taxation 9
Total taxation charge 52
The tax charge on total profits amounted to £52 million for the seven months ended 31 July
2019 and represented an effective tax rate of 21%.
Seven Months Ended
31 July 2019
Reconciliation of the taxation rate on Group profits
£m
Profit before tax 245
US statutory rate of taxation (21%) 51
Differences in overseas taxation rates 19
Tax settlements and resolution of uncertain tax positions (14)
State and local taxes, net of federal benefits
(4)
Total tax charge and effective tax rate (21%)
52
Future tax charges, and therefore the effective tax rate, may be affected by factors such as
acquisitions, disposals, restructurings, the location of research and development activity, tax
446
regime reforms, agreements with tax authorities and resolution of open matters as we continue
to bring the tax affairs up to date around the world. PCH operates in countries where the tax
rate differs from the US tax rate. The taxable profits and tax rates in those countries also vary
from year to year.
Taxation matters
The integrated nature of PCH’s worldwide operations involves significant investment in
research and manufacturing at a limited number of locations, with consequential cross-border
supply routes into numerous end-markets. This gives rise to complexity and delay in
negotiations with revenue authorities as to the profits on which individual Group companies are
liable to tax.
Management continues to believe that it has made adequate provision for the liabilities likely to
arise from periods which are open and not yet agreed by tax authorities. The ultimate liability
for such matters may vary from the amounts provided and is dependent upon the outcome of
agreements with relevant tax authorities or litigation where appropriate. At of 31 July 2019 PCH
had recognised provisions of £68 million in respect of such uncertain tax positions.
The aggregate amount of unremitted profits at the balance sheet date was approximately
£493 million. Provision for deferred tax liabilities of £2 million has been made in respect of
withholding taxation that would arise on the distribution of profits by certain overseas
subsidiaries. Deferred tax is not provided on the remainder of temporary differences arising on
unremitted profits as management can control any future reversal and does not consider such
a reversal to be probable.
Movement in deferred tax assets and liabilities
Accelerated
capital
allowances Intangibles
Pensions &
other post-
employment
benefits
Tax
losses
Other net
temporary
differences Total
£m £m £m £m £m £m
As at 1 January 2019 - (1,107) 51 100 123 (833)
Exchange adjustments (1) (50) 2 5 4 (40)
(Charge)/credit to income
statement
(13) (3) (1) 33 (25) (9)
Credit to statement of
comprehensive income
- - (2) (9) - (11)
Reclass - - - - (1) (1)
At 31 July 2019 (14) (1,160) 50 129 101 (894)
Recognised tax losses amount to £243 million as at 30 July of net trading losses. Other net
temporary differences include accrued expenses for which a tax deduction is only available on
a paid basis and deferred tax on intra-group profits arising on inter-company stock which are
eliminated within the combined and consolidated Historical Financial Information. As intra-
group profits are not eliminated from the individual entities’ tax returns a temporary difference
arises that will reverse at the point in time stock is sold externally. After offsetting deferred tax
assets and liabilities where appropriate within territories, the net deferred tax liability
comprises:
31 July 2019
£m
Deferred tax assets 107
Deferred tax liabilities
(1,001)
(894)
447
Seven Months Ended 31 July
2019
Unrecognised tax losses
Tax
losses
Unrecognised
asset
£m £m
Trading losses expiring:
Within 10 years 45 9
More than 10 years 36 7
Available indefinitely 305 52
As at July 31 386 68
Deferred tax assets are recognised where it is probable that future taxable profit will be
available to utilise losses, as supported by management forecasts.
12. Property, plant and equipment
Land and
buildings
Plant,
equipment
and
vehicles
Assets
under
construction Total
£m £m £m £m
Cost at 1 January 2019 448 502 37 987
Exchange adjustments 23 26 2 51
Additions - 4 17 21
Disposals and write-offs (1) (4) - (5)
Reclassifications 11 10 (21) -
Cost at 31 July 2019 481 538 35 1,054
Depreciation at 1 January 2019 (178) (279) - (457)
Exchange adjustments (10) (15) - (25)
Charge for the year (7) (16) - (23)
Disposals and write-offs - 2 - 2
Depreciation at 31 July 2019 (195) (308) - (503)
Net book value at 31 July 2019 286 230 35 551
13. Right-of-use assets
Land and
buildings
Plant and
equipment Vehicles Total
£m £m £m £m
Net book value at 1 January 2019 27 12 2 41
Exchange adjustments 1 - - 1
Additions 2 - - 2
Depreciation (3) (2) - (5)
Net book value at 31 July 2019 27 10 2 39
An analysis of lease liabilities is set out in Note 19, ‘Borrowings’. The total cash outflow for
leases amounted to £7 million for the seven-month period ended 31 July 2019. There were no
significant lease commitments for leases not commenced at the period-end.
448
14. Intangible assets
Goodwill
Indefinite
life
brands
Amortised
brands,
licences &
patents
Computer
software Total
£m £m £m £m £m
Cost at 1 January 2019 1,544 4,151 1,123 31 6,849
Exchange adjustments 73 189 55 2 319
Additions 11
Disposals and asset write-offs - - - (10) (10)
Cost at 31 July 2019 1,617 4,340 1,178 24 7,159
Amortisation at 1 January 2019 - - (452) (27) (479)
Exchange adjustments - - (24) (1) (25)
Charge for the period - - (39) (2) (41)
Disposals and asset write-offs - - - 10 10
Amortisation at 31 July 2019 - - (515) (20) (535)
Net book value at 31 July 2019 1,617 4,340 663 4 6,624
Goodwill
Goodwill was tested for impairment as at 31 December 2018, and no impairment was
recognised. On 31 July 2019 goodwill was assessed for any indicators of impairment. No
indicators were identified and therefore no further impairment assessment was performed.
Indefinite life brands and other amortised brands
The historical cost of the major brands are as follows:
As at
31 July 2019
£m
Advil 1,340
Centrum 884
Robitussin 562
Caltrate 427
Preparation H 389
Chapstick 392
Emergen-C 145
Other 201
4,340
Each of these brands is considered to have an indefinite life, given the strength and durability
of the brand and the level of marketing support. The brands are in relatively similar, stable and
profitable market sectors, with similar risk profiles, and their size, diversification and market
shares mean that the risk of market-related factors causing a reduction in the lives of the
brands is considered to be relatively low. PCH is not aware of any material legal, regulatory,
contractual, competitive, economic or other factor which could limit their useful lives.
Accordingly, they are not amortised.
Each brand is tested annually for impairment and other amortised intangible assets are tested
when indicators of impairment arise. Indefinite life brands were tested for impairment as at
31 December 2018, and no impairment was recognised. On 31 July 2019 indefinite life brands
were assessed for any indicators of impairment. No indicators were identified and therefore no
further impairment assessment was performed.
449

For the seven months ended 31 July 2019, amortisation and impairment losses, net of
reversals, have been charged in the income statement as follows.
Amortisation Net impairment losses
£m £m
Cost of sales 41 -
41 -
15. Inventories
As at
31 July 2019
£m
Raw materials and consumables
69
Work in progress
79
Finished goods
357
505
The total cost of inventories recognised as an expense and included in cost of sales amounted
to £521 million.
16. Trade and other receivables
As at
31 July 2019
£m
Trade receivables, net of expected credit loss allowance
438
Other prepayments and accrued income
23
Employee loans and advances
1
Other third-party receivables
39
501
As at
31 July 2019
Expected credit loss allowance £m
At 1 January 14
Exchange adjustments 1
Charge for the period 1
Utilised (3)
At 31 July 13
17. Cash and cash equivalents
As at
31 July 2019
£m
Cash at bank and in hand
38
38
450
18. Trade and other payables
As at
31 July 2019
£m
Trade payables (303)
Customer return and rebate accruals (74)
Other accruals (161)
Wages and salaries (45)
Other payables (11)
Social security (21)
VAT payables (34)
(649)
Customer return and rebate accruals are provided for by PCH at the point of sale in respect of
the estimated rebates, discounts or allowances payable to customers. Accruals are made at
the time of sale but the actual amounts paid are based on claims made some time after the
initial recognition of the sale. As the amounts are estimated they may not fully reflect the final
outcome and are subject to change. The level of accrual is reviewed and adjusted in the light of
historical experience of actual rebates, discounts or allowances given and returns made and
any changes in arrangements. Future events and uncertainties could cause the assumptions
on which the accruals are based to change, which could affect the future results of PCH.
19. Borrowings
As at
31 July 2019
£m
Short-term borrowings
Lease liabilities 11
11
Long-term borrowings
Lease liabilities 30
Total borrowings 41
Lease liabilities
The maturity analysis of lease liabilities recognised on the PCH balance sheet is as follows:
As at
31 July 2019
£m
Rental payments due within one year 11
Rental payments due between one and two years 7
Rental payments due between two and three years 6
Rental payments due between three and four years 6
Rental payments due between four and five years 5
Rental payments due after five years 6
41
451

20. Pensions and other post-employment benefits
Pension and other post-employment costs
Seven Months Ended
31 July 2019
£m
German pension schemes
0.5
French pension schemes
0.1
0.6
Analysed as:
Defined benefit schemes
0.6
There are four defined benefit PCH pension plans in Germany and one dedicated PCH
pension plan in France. Two of the plans in Germany are frozen to future benefit accruals.
Pension and other post-employment costs are primarily included in selling, general and
administration expenses.
Many of the PCH employees also participate in benefit plans sponsored by Pfizer. The
combined income statement includes the service cost associated with direct PCH employees
participating in plans that are not dedicated to PCH as well as an allocation of service cost that
has been deemed attributable to PCH operations. The combined balance sheet does not
include benefit plan assets and liabilities associated with PCH employees participating in plans
that are not dedicated to PCH.
The average life expectancy assumed now for an individual at the age of 65 and projected to
apply in 2044 for an individual then at the age of 65 is as follows:
As at 31 July 2019 Germany France
Male
Years
Female
Years
Male
Years
Female
Years
Current 20.0 23.6 19.0 23.0
Projected for 2044
23.5 26.4 19.0 23.0
The Pension Plans are exposed to risk that arises because the estimated market value of the
Plan assets might decline, the investment returns might reduce, or the estimated value of the
Plans’ liabilities might increase.
The Plan liabilities are a series of future cash flows with relatively long duration. On an IAS 19
basis, these cash flows are sensitive to changes in the expected rate of compensation increase
and the discount rate (investment grade corporate bond yield curve) where an increase in
expected compensation corresponds with an increase in the liabilities, and an increase in the
discount rate corresponds with a decrease in the liabilities.
PCH has applied the following financial assumptions in assessing the defined benefit liabilities:
Seven Months
Ended
31 July 2019
Germany France
%pa
%pa
Discount rate 2.05% 1.75%
Expected rate of compensation increase
2.13%
2.25%
452
The amounts recorded in the income statement and statement of comprehensive income in
relation to the defined benefit pension and post-retirement healthcare schemes were as
follows:
Pensions
£m
31 July 2019
Amounts charged to operating profit:
Current service cost 0.3
Net interest cost 0.3
0.6
Re-measurements recorded in the statement of comprehensive income -
The fair values of the assets and liabilities of the German and French defined benefit pension
schemes, together with aggregated data for other defined benefit pension schemes in the PCH
are as follows:
Germany France Total
31 July 2019 £m £m £m
Insurance contracts 5 - 5
Fair value of assets 5-5
Present value of scheme obligations (29) (1) (30)
Recognised on the balance sheet (24) (1) (25)
Included in post-employment benefits assets - - -
Included in post-employment benefits obligations (24) (1) (25)
Actual return on plan assets ---
All of PCH’s dedicated pension plan assets are associated with one PCH dedicated pension
plan in Germany. The assets associated with this plan are comprised of insurance contracts.
The estimated fair value of the insurance contracts was contract value. The remaining PCH
dedicated pension plans were unfunded at 31 July 2019.
The defined benefit pension obligation is analysed as follows:
Seven Months Ended
31 July 2019
£m
Funded (6)
Unfunded (24)
(30)
The movement in the net defined benefit liability is as follows:
Fair value
of assets
Present
value of
obligation
Net
pensions
total
£m £m £m
At 1 January 2019 5 (28) (23)
Exchange adjustments - (1.8) (1.8)
Service cost - (0.4) (0.4)
Interest income/(cost) - (0.3) (0.3)
Employers contributions 0.5 - 0.5
Benefits paid (0.5) 0.5 -
At 31 July 2019 5 (30) (25)
453

The defined benefit pension analysed by membership category is as follows:
Pension obligations
Seven Months Ended 31 July 2019
£m
Active (11)
Retired (6)
Deferred (13)
(30)
Sensitivity Analysis
The approximate effect of changes in assumptions used on the benefit obligations and on the
annual defined benefit and post-retirement costs are detailed below. This information has been
determined by considering the duration of the liabilities and the overall profile of the plan
membership.
Seven Months Ended 31 July 2019
£m
A 0.25% decrease in discount rate:
Increase in annual pension cost -
Increase in pension obligation 1
A 0.25% increase in discount rate:
Decrease in annual pension cost -
Decrease in pension obligation (1)
A 0.25% increase in inflation:
Increase in annual pension cost -
Increase in pension obligation 1
A 0.25% decrease in inflation:
Decrease in annual pension cost -
Decrease in pension obligation (1)
A one year increase in life expectancy:
Increase in annual pension cost -
Increase in pension obligation -
The weighted average duration of the defined benefit obligation is as follows:
2019
years
Pension benefits 16.5
Defined contribution plans
PCH employees are eligible to participate in Pfizer’s defined contribution plans, whereby
employees contribute a portion of their compensation, which is partially matched, in cash, by
Pfizer. Beginning on 1 January, 2011, for newly hired non-union employees, rehires and
transfers to the U.S. or Puerto Rico, Pfizer no longer offers a defined benefit pension plan and,
instead, offers a Retirement Savings Contribution (RSC) in the defined contribution plan. The
RSC is an annual non-contributory employer contribution (that is not dependent upon the
participant making a contribution) determined based on each employee’s eligible
compensation, age and years of service. Beginning on 1 January, 2018, all non-union
employees in Pfizer’s U.S. and Puerto Rico defined benefit plans transitioned to the RSC in the
defined contribution plans.
454
Contribution expense for direct PCH employees, associated with non-dedicated defined
contribution plans, totalled approximately £15 million in the seven months ended 31 July 2019.
Post-retirement plans
Many of the PCH employees are eligible to participate in post-retirement plans, consisting
primarily of medical insurance for retirees, sponsored by Pfizer. Pfizer does not fund post-
retirement plans, but contributes to the plans as benefits are paid. Service cost expense for
direct PCH employees in Canada, the U.S. and Puerto Rico, participating in post-retirement
benefit plans that are not dedicated to PCH totalled approximately £1.5 million in the seven
months ended 31 July 2019.
21. Other provisions
Restructuring
programmes
Other
provisions
Total
£m £m £m
As at 1 January 2019 (57) (19) (76)
Charge for the period (4) (1) (5)
Utilised 24 - 24
As at 31 July 2019 (37) (20) (57)
As at
31 July 2019
£m
To be settled within one year (37)
To be settled after one year (20)
Total provision (57)
Other provisions include legal related provision, asset retirement obligation and other
provisions. Details of restructuring provisions can be found in Note 10, ‘Restructuring costs’.
22. Contingent liabilities
Contingent liabilities arise when PCH has a present obligation as a result of a past event and
comprise guarantees and other items arising in the normal course of business.
Provision is made for the outcome of tax, legal and other disputes where it is both probable
that PCH will suffer an outflow of funds and it is possible to make a reliable estimate of that
outflow.
PCH is subject to numerous contingencies arising in the ordinary course of business. The most
significant of these matters, other than tax matters, are described below.
Legal proceedings
PCH makes provision for these proceedings on a regular basis as summarised in Note 2
‘Accounting principles and policies’ and Note 21 ‘Other provisions’.
PCH has assessed that claims and defences in these matters are substantial, but litigation is
inherently unpredictable and excessive verdicts do occur. PCH could incur judgments, enter
into settlements or revise expectations regarding the outcome of certain matters, and such
developments could have a material adverse effect on results of operations and/or cash flows
in the period in which the amounts are accrued and/or cash flows in the period in which the
amounts are paid.
455
Losses that are both probable and reasonably estimable have been accrued for. Substantially
all of PCH’s contingencies are subject to significant uncertainties and, therefore, determining
whether a loss is probable and/or the measurement of any loss (if probable) can be complex.
Consequently, it is not feasible to estimate the range of reasonably possible loss in excess of
amounts accrued. Assessments are based on estimates and assumptions that have been
deemed reasonable by management, but the assessment process relies heavily on estimates
and assumptions that may prove to be incomplete or inaccurate, and unanticipated events and
circumstances may occur that might cause us to change those estimates and assumptions.
Amounts recorded for legal and environmental contingencies can result from a complex series
of judgments about future events and uncertainties and can rely heavily on estimates and
assumptions.
The principal pending matters to which PCH is a party are discussed below. In determining
whether a pending matter is a principal matter, PCH considers both quantitative and qualitative
factors in order to assess materiality, such as, among other things, the amount of damages
and the nature of any other relief sought in the proceeding, if such damages and other relief
are specified; PCH’s view of the merits of the claims and of the strength of its defences;
whether the action purports to be, or is, a class action and, if not certified, views of the
likelihood that a class will be certified by the court; the jurisdiction in which the proceeding is
pending; whether related actions have been transferred to a multi-district litigation; any
experience that PCH or, to its knowledge, other companies have had in similar proceedings;
whether disclosure of the action would be important to a reader of the Historical Financial
Information, including whether disclosure might change a reader’s judgment about the
Historical Financial Information in light of all of the information that is available to the reader;
the potential impact of the proceeding on PCH’s reputation; and the extent of public interest in
the matter. In addition, with respect to patent matters in which PCH is the plaintiff,
consideration is given to, among other things, the financial significance of the product protected
by the patent. As a result of considering qualitative factors in the determination of principal
matters, there are some matters discussed below with respect to which management believes
that the likelihood of possible loss in excess of amounts accrued is remote.
Product litigation - Nexium24HR and Protonix
Several individual and multi-plaintiff lawsuits have been filed against Pfizer, certain of its
subsidiaries and/or other pharmaceutical manufacturers in various federal and state courts
alleging that the plaintiffs developed kidney-related injuries purportedly as a result of the
ingestion of certain proton pump inhibitors. The cases against Pfizer involve Nexium24HR and/
or Protonix (a Pfizer pharmaceutical product) and seek compensatory and punitive damages
and, in some cases, treble damages, restitution or disgorgement. In August 2017, the federal
actions were ordered transferred for coordinated pre-trial proceedings to a Multi-District
Litigation (In re: Proton-Pump Inhibitor Products Liability Litigation (No. II)) in the U.S. District
Court for the District of New Jersey. As part of the consumer healthcare joint venture
transaction with GSK, the joint venture has agreed to assume, and to indemnify Pfizer for,
liabilities arising out of such litigation to the extent related to Nexium24HR.
GSK Consumer Healthcare is now the defendant in the ongoing proton pump inhibitor (“PPI”)
litigation. As of June 2021, there are approximately 2,500 Nexium24HR personal injury
lawsuits pending against GSK Consumer Healthcare, nearly all of which are pending in a
Multidistrict Litigation (“MDL”) proceeding in the District of New Jersey. Manufacturers of other
PPIs also are named as co-defendants in the MDL. GSK Consumer Healthcare has filed
motions to dismiss several hundred cases, but the MDL court has not yet ruled on those
motions. In addition to the MDL cases, a small number of cases are pending in state courts.
Guarantees and indemnifications
In the ordinary course of business and in connection with the sale of assets and businesses,
PCH often indemnifies its counterparties against certain liabilities that may arise in connection
with the transaction or related to activities prior to or following the transaction. These
456

indemnifications typically pertain to environmental, tax, employee and/or product-related
matters and patent-infringement claims. If the indemnified party were to make a successful
claim pursuant to the terms of the indemnification, PCH may be required to reimburse the loss.
These indemnifications are generally subject to various restrictions and limitations. Historically,
the business has not paid significant amounts under these provisions and, as of 31 July 2019,
recorded amounts for the estimated fair value of these indemnifications are not significant.
23. Invested capital
Invested capital
£m
Invested capital
At 1 January 2019 6,006
Movements in the period 498
As at 31 July 2019 6,504
24. Related party transactions
The Historical Financial Information includes certain related party transactions.
There were no balances from transactions between PCH and Pfizer that are expected to be
cash-settled as of 31 July 2019. All balances and transactions between PCH and Pfizer that
are not cash-settled are shown as part of Invested Capital in the combined balance sheet and
represent the net of amounts settled without payment (to)/from Pfizer. Such amounts are
reflected in the combined statement of cash flows based on the cash flows made by Pfizer on
behalf of PCH, with the offset reflected in Net financing activities with Pfizer in the financing
section.
Pfizer uses a centralised approach to cash management and financing its operations. During
the period covered by these combined financial statements, excess cash was remitted to Pfizer
on a regular basis and is reflected within Invested Capital. Similarly, PCH cash disbursements
were predominantly funded through Pfizer’s cash accounts and are reflected within Invested
Capital.
There are no other amounts or loan amounts owing to or from related parties.
Historically, Pfizer has provided significant corporate, manufacturing and shared services
functions and resources to PCH. The combined income statement reflects allocations for these
costs, as described in Note 1.
25. Adjustments reconciling profit after tax to operating cash flow
Seven Months Ended
31 July 2019
£m
Profit after tax 193
Taxation charge 52
Net finance costs 11
Depreciation of property, plant and equipment and right of use assets 36
Amortisation of intangible assets 41
Impairment and assets written off 1
Other non-cash movements 11
Changes in working capital:
Increase in inventories (41)
Decrease in trade receivables 16
Decrease in trade payables (78)
Net change in other receivables and payables (100)
(51)
Cash generated from operations 142
457
26. Commitments
As of 31 July 2019, there are agreements totalling £66 million to purchase goods and services
that are enforceable and legally binding and include amounts primarily relating to advertising
commitments.
27. Financial instruments and related disclosures
PCH is a business unit of Pfizer. Pfizer’s Treasury function manages and monitors PCH’s
external and internal funding requirements and financial risks in support of PCH’s strategic
objectives.
Capital management
PCH participated in Pfizer’s centralized cash management system and generally all excess
cash is transferred to Pfizer on a daily basis. Accordingly, the PCH cash balance as of 31 July,
2019 is not representative of an independent company and could be significantly different at
another point in time. Cash disbursements for operations and/or investing activities are funded
as needed by Pfizer. All balances and transactions among PCH and Pfizer that are not cash
settled are shown as part of Invested Capital and represent the net of amounts settled without
payment (to)/from Pfizer. Historically, Pfizer provided significant corporate, manufacturing and
shared services functions and resources to PCH. The combined financial statements of PCH
reflect an allocation of these costs, which PCH believes is a reasonable reflection of the
services received. However, these allocations may not reflect the expenses that would have
been incurred if PCH had operated as an independent standalone company.
Liquidity risk
PCH participated in Pfizer’s centralized cash management system and generally all excess
cash is transferred to Pfizer on a daily basis. Accordingly, the PCH cash balance as of 31 July,
2019 of £38 million is not representative of an independent company and could be significantly
different at another point in time. Cash disbursements for operations and/or investing activities
are funded as needed by Pfizer.
Market risk
Interest rate risk management
PCH does not have external debt and therefore its interest expense is not significantly exposed
to changes in interest rates. Although PCH earns interest income on its cash and therefore
benefits from an increase in interest rates, PCH interest income is not significant because
generally all excess cash is transferred to Pfizer on a daily basis. Included in the PCH
combined financial statements is an allocation of Pfizer’s interest-related expenses associated
with the Pfizer corporate debt and an allocation of interest income associated with the Pfizer
corporate investments.
Interest rate benchmark reform
PCH is not directly exposed to interest rate benchmark reform since it has no interest rate
derivatives as of 31 July, 2019. As a business unit of Pfizer, PCH participated in Pfizer’s
centralized hedging activities.
Foreign exchange risk management
PCH is subject to foreign exchange risk in its commercial operations and assets and liabilities
that are denominated in foreign currencies. As a business unit of Pfizer, PCH participated in
Pfizer’s financial risk management program to minimize the impact of foreign exchange rate on
its earnings. Pfizer addresses this exposure through a combination of operational means and
458
financial instruments. Pfizer adapts its practices periodically as economic conditions change.
On the commercial side, a significant portion of PCH revenues and earnings is exposed to
changes in exchange rates. Where foreign exchange risk is not offset by other exposures
Pfizer may use foreign currency exchange contracts and/or foreign currency swaps to manage
that risk.
Wholesale and retail credit risk
PCH used Pfizer Inc. expertise to monitor wholesale credit risk. This risk was managed by
Pfizer which used their own internal metrics and systems for monitoring this type of credit risk.
Sensitivity analysis
The sensitivity analysis has been prepared on the assumption that the amount of cash and
cash equivalents and the proportion of financial instruments in foreign currencies are all
constant. Financial instruments affected by market risk are cash and deposits. The tables
below illustrate the estimated impact on the income statement and equity as a result of
hypothetical market movements in foreign exchange and interest rates in relation to PCH’s
financial instruments. The range of variables chosen for the sensitivity analysis reflects
management’s view of changes which are reasonably possible over a one-year period.
Credit risk
Credit risk is the risk that a counterparty will default on its contractual obligations resulting in
financial loss to PCH and arises on cash and cash equivalents held with banks and financial
institutions as well as credit exposures to wholesale and retail customers, including outstanding
receivables.
Treasury-related credit risk
The aggregate credit risk in respect of financial instruments that PCH may have with one
counterparty is limited by reference to the long-term credit ratings assigned for that
counterparty by Moody’s Investors Service (“Moody’s”) and Standard and Poor’s. The table
below sets out the credit ratings of counterparties for cash and cash equivalents.
2019 AAA/Aaa AA/Aa A/A BBB/Baa
BB+/Ba1
and
below or
unrated
Total
£m £m £m £m £m £m
Bank balances and deposits - 30 1 7 - 38
----38
The credit ratings in the above tables are as assigned by Moody’s. Where local rating data is
the only source available, the ratings are converted to global ratings equivalent to those of
Standard and Poor’s or Moody’s using published conversion tables.
Global counterparty limits are assigned to each of PCH’s banking and investment
counterparties based on long-term credit ratings from Moody’s and Standard and Poor’s.
PCH’s usage of these limits is monitored daily by Pfizer’s Corporate Treasury Group as well as
Pfizer’s Corporate Compliance Officer (CCO) who operates independently from Pfizer
Treasury. Any breach of these limits would be reported to the CFO immediately. The CCO also
monitors the credit rating of these counterparties and, when changes in ratings occur, notifies
Pfizer Treasury so that changes can be made to investment levels or authority limits as
appropriate.
459
Financial assets and financial liabilities
Seven Months Ended
31 July 2019
Carrying value Fair value
£m £m
Financial assets measured at amortised cost:
Cash and cash equivalents 38 38
Financial assets at fair value through profit or loss:
Trade and other receivables and certain other non-current assets 499 499
Total financial assets 537 537
Financial liabilities measured at amortised cost:
Short term loans and overdrafts - -
Trade and other payables, Other provisions and certain Other
non-current liabilities in scope of IFRS 9
(548) (548)
Total financial liabilities (548) (548)
Net financial assets and financial liabilities (11) (11)
The table above presents the carrying amounts and the fair values of PCH’s financial assets
and liabilities. The fair values of the financial assets and liabilities are included at the price that
would be received to sell an asset or paid to transfer a liability in an orderly transaction
between market participants at the measurement date.
The following methods and assumptions were used to estimate the fair values:
• Cash and cash equivalents - approximates to the carrying amount
• Receivables and payables - approximates to the carrying amount
Trade and other receivables, Other non-current assets, Trade and other payables, Other
provisions and Other non-current liabilities are reconciled to the relevant balance sheet
amounts in tables below.
Trade and other receivables and Other non-current assets in scope of IFRS 9
The following table reconciles financial instruments within Trade and other receivables and
Other non-current assets which fall within the scope of IFRS 9 to the relevant balance sheet
amounts. The financial assets are predominantly non-interest earning. Non-financial
instruments include tax receivables and prepayments, which are outside the scope of IFRS 9.
Seven Months Ended
31 July 2019
Financial
instruments
Non-
financial
instruments
Total
£m £m £m
Trade and other receivables (Note 16) 462 39 501
Other non-current assets 37 39 76
499 78 577
Trade and other payables, Other provisions and Other non-current liabilities in scope of
IFRS9
The following table reconciles financial liabilities within Trade and other payables, Other
provisions and Other non-current liabilities which fall within the scope of IFRS 9 to the relevant
balance sheet amounts. Accrued wages and salaries are included within financial liabilities.
Non-financial instruments include payments on account, tax and social security payables and
provisions which do not arise from contractual obligations to deliver cash or another financial
asset, which are outside the scope of IFRS 9.
460

Seven Months Ended
31 July 2019
Financial
instruments
Non-
financial
instruments
Total
£m £m £m
Trade and other payables (Note 18) (548) (101) (649)
Other provisions (Note 21) - (20) (20)
Other non-current liabilities - (17) (17)
(548) (138) (686)
Foreign exchange sensitivity
The two major foreign currencies in which PCH’s financial instruments are denominated are
Chinese Yuan and US Dollars. PCH has considered movements in these currencies and has
concluded that a 10% movement in rates is a reasonable benchmark. Financial instruments
are only considered sensitive to foreign exchange rates where they are not in the functional
currency of the entity that holds them.
Seven Months Ended
31 July 2019
Increase/(Decrease) in
income
£m
10% appreciation of the Chinese Yuan 0.3
10% appreciation of the US dollar 0.1
10% depreciation of the Chinese Yuan (0.3)
10% depreciation of the US dollar (0.1)
Interest rate sensitivity
PCH is exposed to interest rate risk on its outstanding borrowings and investments where any
changes in interest rates will affect future cash flows or the fair values of financial instruments.
The table below shows PCH’s hypothetical sensitivity to changes in interest rates in relation to
Canadian dollar, US dollar, Euro and South African Rand variable rate financial assets and
liabilities. If the interest rates applicable to floating rate financial assets and liabilities were to
have increased by 1% (100 basis points), and assuming other variables had remained
constant, it is estimated that PCH’s finance income for the period would have increased by
approximately £0.3 million. A 1% (100 basis points) movement in interest rates is not deemed
to have a material effect on equity.
Seven Months Ended
31 July 2019
Increase/(Decrease) in
income
£m
1% (100 basis points) increase in Canadian dollar interest rates 0.1
1% (100 basis points) increase in US dollar interest rates -
1% (100 basis points) increase in Euro interest rates 0.1
1% (100 basis points) increase in South African Rand interest rates 0.1
461
The following table provides an analysis of the anticipated contractual cash flows including
interest payable for PCH’s non-derivative financial liabilities on an undiscounted basis. Cash
flows in foreign currencies are translated using spot rates at 31 July 2019.
Lease
liabilities
Trade
payables and
other
liabilities not
in net debt
Total
At 31 July 2019 £m £m £m
Due in less than one year 11 594 605
Between one and two years 7 - 7
Between two and three years 6 - 6
Between three and four years 6 - 6
Between four and five years 5 - 5
After five years 6 - 6
Gross contractual cash flows 41 594 635
28. Employee share schemes
PCH compensation programs include grants under Pfizer’s share-based payment
programmes. The combined income statement includes all of the share-based payment
expenses directly attributable to PCH. The expenses include allocations of direct expenses as
well as expenses that have been deemed attributable to PCH operations. Compensation
programs at PCH can include share-based payments under various Pfizer employee stock and
incentive plans. The award value is determined by reference to the fair value of share-based
awards to similar employees in competitive survey data or industry peer groups used for
compensation purposes; and is allocated between different long term incentive vehicles, in the
form of Restricted Stock Units (RSUs), Stock Options, Total Shareholder Return Units
(TSRUs), Portfolio Performance Shares (PPSs) and Performance Share Awards (PSAs) and
Profit Units (PTUs), as determined by the Pfizer Compensation Committee.
The pre-tax share-based compensation charge for the above schemes (including allocated
amounts) has been recorded in the income statement of £19.6 million for the seven months
ended 31 July, 2019 (£2.8 million in Cost of sales; £13.5 million in Selling, general and
administrative expenses; and £3.3 million in Research and development expenses), which is
considered immaterial for further disclosure. This expense is incurred in the form of a charge
from Pfizer Inc. No further disclosures have been made since the share plans are with Pfizer
and not PCH and post-acquisition these plans do not transfer over with PCH to GSK Consumer
Healthcare.
462

29. Principal PCH companies
The following represent the principal subsidiaries of PCH at 31 July 2019.
England
PF Consumer Healthcare UK Ltd
PRISM PCH LP
Small Partner LP
New UK LP 2
Europe
Ferrosan A/S
Ferrosan International A/S
Vesterålens Naturprodukter A/S
Vesterålens Naturprodukter OY
Pfizer Santé Familiale
Pfizer Consumer Healthcare GmbH
PF Consumer Ireland Limited Company
Pfizer Consumer Healthcare Italy S.r.l
Pfizer Consumer Manufacturing Italy S.r.l.
PF Consumer Healthcare B.V.
PF Consumer Healthcare Holding B.V.
Vesterålens Naturprodukter AS
PF Consumer Healthcare Poland sp. z o.o.
Ferrosan S.R.L.
Pfizer Consumer Healthcare AB
Vesterålens Naturprodukter AB
USA
Wyeth Consumer Healthcare LLC (Puerto Rico Branch)
PF Consumer Healthcare B.V. (Puerto Rico Operations), LLC
PF Consumer Healthcare 1 LLC
Wyeth Consumer Healthcare LLC
New PCH LLC
Alacer Corp.
PF Consumer Taiwan LLC
PF Consumer Healthcare UK LLC
Consumer Healthcare Intermediate Holdings LLC
Consumer Healthcare Holdings LLC
PRISM PCH LLC
463

Other
Whitehall Laboratorios Peru
Wyeth Consumer Healthcare LLC (Peru Branch)
PF Healthcare Australia Pty Ltd
PF Consumer Healthcare New Zealand Limited
PF Consumer Healthcare Canada ULC / PF Soins De Sante SRI
Treerly Health Co., Ltd
Wyeth Pharmaceutical Co., Ltd.
PF Consumer Healthcare Singapore PTE. LTD.
Pfizer Laboratories PFE (Pty) Ltd
PF Consumer Taiwan Branch
PF Consumer UAE
30. Non-controlling interests
Non-controlling interests comprises interests in entities, of which PCH’s non-controlling
interests are individually not material.
31. Post balance sheet events
PCH has evaluated post balance sheet events from the balance sheet date through the date at
which the Historical Financial Information is available to be issued, and determined that there
are no other items to disclose.
464
Donnelley Financial Solutions 211680

