
Report to Congressional Requesters
United States General Accountin
g
Office
GA
O
December 2003
PRESCRIPTION
DRUGS
OxyContin Abuse and
Diversion and Efforts
to Address the
Problem
GAO-04-110

www.gao.gov/cgi-bin/getrpt?GAO-04-110.
To view the full product, including the scope
and methodology, click on the link above.
For more information, contact Marcia Crosse
at (202) 512-7119.
Highlights of GAO-04-110, a report to
congressional requesters
December 2003
PRESCRIPTION DRUGS
OxyContin Abuse and Diversion and
Efforts to Address the Problem
Purdue conducted an extensive campaign to market and promote OxyContin
using an expanded sales force to encourage physicians, including primary
care specialists, to prescribe OxyContin not only for cancer pain but also as
an initial opioid treatment for moderate-to-severe noncancer pain.
OxyContin prescriptions, particularly those for noncancer pain, grew
rapidly, and by 2003 nearly half of all OxyContin prescribers were primary
care physicians. The Drug Enforcement Administration (DEA) has
expressed concern that Purdue’s aggressive marketing of OxyContin focused
on promoting the drug to treat a wide range of conditions to physicians who
may not have been adequately trained in pain management. FDA has taken
two actions against Purdue for OxyContin advertising violations. Further,
Purdue did not submit an OxyContin promotional video for FDA review
upon its initial use in 1998, as required by FDA regulations.
Several factors may have contributed to the abuse and diversion of
OxyContin. The active ingredient in OxyContin is twice as potent as
morphine, which may have made it an attractive target for misuse. Further,
the original label’s safety warning advising patients not to crush the tablets
because of the possible rapid release of a potentially toxic amount of
oxycodone may have inadvertently alerted abusers to methods for abuse.
Moreover, the significant increase in OxyContin’s availability in the
marketplace may have increased opportunities to obtain the drug illicitly in
some states. Finally, the history of abuse and diversion of prescription
drugs, including opioids, in some states may have predisposed certain areas
to problems with OxyContin. However, GAO could not assess the
relationship between the increased availability of OxyContin and locations
of abuse and diversion because the data on abuse and diversion are not
reliable, comprehensive, or timely.
Federal and state agencies and Purdue have taken actions to address the
abuse and diversion of OxyContin. FDA approved a stronger safety warning
on OxyContin’s label. In addition, FDA and Purdue collaborated on a risk
management plan to help detect and prevent OxyContin abuse and diversion,
an approach that was not used at the time OxyContin was approved. FDA
plans to provide guidance to the pharmaceutical industry by September 2004
on risk management plans, which are an optional feature of new drug
applications. DEA has established a national action plan to prevent abuse
and diversion of OxyContin. State agencies have investigated reports of
abuse and diversion. In addition to developing a risk management plan,
Purdue has initiated several OxyContin-related educational programs, taken
disciplinary action against sales representatives who improperly promoted
OxyContin, and referred physicians suspected of improper prescribing
practices to the authorities.
Amid heightened awareness that
many patients with cancer and
other chronic diseases suffer from
undertreated pain, the Food and
Drug Administration (FDA)
approved Purdue Pharma’s
controlled-release pain reliever
OxyContin in 1995. Sales grew
rapidly, and by 2001 OxyContin had
become the most prescribed brand-
name narcotic medication for
treating moderate-to-severe pain.
In early 2000, reports began to
surface about abuse and diversion
for illicit use of OxyContin, which
contains the opioid oxycodone.
GAO was asked to examine
concerns about these issues.
Specifically, GAO reviewed (1) how
OxyContin was marketed and
promoted, (2) what factors
contributed to the abuse and
diversion of OxyContin, and
(3) what actions have been taken to
address OxyContin abuse and
diversion.
To improve efforts to prevent or
identify abuse and diversion of
controlled substances such as
OxyContin, FDA’s risk
management plan guidance should
encourage pharmaceutical
manufacturers with new drug
applications to submit plans that
contain a strategy for identifying
potential problems with abuse and
diversion. FDA concurred with
GAO’s recommendation. DEA
agreed that such risk management
plans are important, and Purdue
stated that the report appeared to
be fair and balanced.

Page i GAO-04-110 OxyContin Abuse and Diversion
Letter 1
Results in Brief 4
Background 7
Purdue Conducted an Extensive Campaign to Market and Promote
OxyContin 16
Several Factors May Have Contributed to OxyContin Abuse and
Diversion, but Relationship to Availability Cannot Be Assessed 29
Federal and State Agencies and Purdue Have Taken Actions to
Prevent Abuse and Diversion of OxyContin 34
Conclusions 41
Recommendation for Executive Action 42
Agency and Purdue Comments and Our Evaluation 43
Appendix I Scope and Methodology 46
Appendix II Summary of FDA Changes to the Original Approved
OxyContin Label 48
Appendix III Databases Used to Monitor Abuse and Diversion of
OxyContin and Its Active Ingredient Oxycodone 51
DAWN Data 51
NFLIS Data 51
STRIDE Data 52
National Survey on Drug Use and Health Data 52
Monitoring the Future Survey Data 52
Appendix IV Comments from the Food and Drug Administration 53
Appendix V Comments from the Drug Enforcement
Administration 56
Contents
Page ii GAO-04-110 OxyContin Abuse and Diversion
Tables
Table 1: Sales Representative Positions Available for OxyContin
Promotion, 1996 through 2002 19
Table 2: Total OxyContin Sales and Prescriptions for 1996 through
2002 with Percentage Increases from Year to Year 31
Table 3: Selected Language Approved by FDA in Warning Sections
of OxyContin Labels, 1995 and 2001 35
Table 4: Selected Language Approved by FDA in the Indication
Sections of OxyContin Labels, 1995 and 2001 35
Table 5: FDA Changes to the Original OxyContin Label Made from
June 1996 through July 2001 48
Figure
Figure 1: Promotional Spending for Three Opioid Analgesics in
First 6 Years of Sales 22

Page iii GAO-04-110 OxyContin Abuse and Diversion
Abbreviations
DAWN Drug Abuse Warning Network
DEA Drug Enforcement Administration
FDA Food and Drug Administration
FD&C Act Federal Food, Drug and Cosmetic Act
HHS Department of Health and Human Services
HIDTA High Intensity Drug Trafficking Area
JCAHO Joint Commission on Accreditation of Healthcare
Organizations
NFLIS National Forensic Laboratory Information System
ONDCP Office of National Drug Control Policy
PDUFA Prescription Drug User Fee Act of 1992
PhRMA Pharmaceutical Research and Manufacturers of America
RADARS Researched Abuse, Diversion, and Addiction-Related
Surveillance
SAMHSA Substance Abuse and Mental Health Services
Administration
STRIDE System to Retrieve Information from Drug Evidence
WHO World Health Organization
This is a work of the U.S. government and is not subject to copyright protection in the
United States. It may be reproduced and distributed in its entirety without further
permission from GAO. However, because this work may contain copyrighted images or
other material, permission from the copyright holder may be necessary if you wish to
reproduce this material separately.

Page 1 GAO-04-110 OxyContin Abuse and Diversion
December 23, 2003
The Honorable Frank R. Wolf
Chairman
Subcommittee on Commerce, Justice, State, and the Judiciary,
and Related Agencies
Committee on Appropriations
House of Representatives
The Honorable James C. Greenwood
Chairman
Subcommittee on Oversight and Investigations
Committee on Energy and Commerce
House of Representatives
The Honorable Harold Rogers
House of Representatives
Patients with cancer may suffer from fairly constant pain for months or
years. Patients with other diseases or conditions, such as rheumatoid
arthritis, osteoarthritis, chronic back pain, or sickle cell anemia, may also
suffer from pain that lasts for extended periods of time. Since 1986, the
World Health Organization (WHO) and others have reported that the
inadequate treatment of cancer and noncancer pain is a serious public
health concern. To address this concern, efforts have been made to better
educate health care professionals on the need to improve the treatment of
both cancer and noncancer pain, including the appropriate role of
prescription drugs.
Amid the heightened awareness that many people were suffering from
undertreated pain, in 1995 the Food and Drug Administration (FDA)
approved the new drug OxyContin, a controlled-release semisynthetic
opioid analgesic manufactured by Purdue Pharma L.P.,
1
for the treatment
of moderate-to-severe pain lasting more than a few days.
2
According to
1
OxyContin is an opioid analgesic—a narcotic substance that relieves a person’s pain
without causing the loss of consciousness. Hereafter, we refer to the company as Purdue.
2
As discussed later in this report, FDA approved the revised OxyContin label in July 2001 to
describe the time frame as “when a continuous around-the-clock analgesic is needed for an
extended period of time.”
United States General Accounting Office
Washington, DC 20548

Page 2 GAO-04-110 OxyContin Abuse and Diversion
Purdue, OxyContin provides patients with continuous relief from pain
over a 12-hour period, reduces pain fluctuations, requires fewer daily
doses to help patients adhere to their prescribed regimen more easily,
allows them to sleep through the night, and allows a physician to increase
the OxyContin dose for a patient as needed to relieve pain.
3
Sales of the
drug increased rapidly following its introduction to the marketplace in
1996. By 2001, sales had exceeded $1 billion annually, and OxyContin had
become the most frequently prescribed brand-name narcotic medication
for treating moderate-to-severe pain in the United States.
In early 2000, media reports began to surface in several states that
OxyContin was being abused—that is, used for nontherapeutic purposes
or for purposes other than those for which it was prescribed—and illegally
diverted.
4
According to FDA and the Drug Enforcement Administration
(DEA), the abuse of OxyContin is associated with serious consequences,
including addiction, overdose, and death.
5
When OxyContin was approved,
the federal government classified it as a schedule II controlled substance
under the Controlled Substances Act because it has a high potential for
abuse and may lead to severe psychological or physical dependence.
6
DEA
has characterized the pharmacological effects of OxyContin, and its active
ingredient oxycodone, as similar to those of heroin. Media reports
indicated that abusers were crushing OxyContin tablets and snorting the
powder or dissolving it in water and injecting it to defeat the intended
controlled-release effect of the drug and attain a “rush” or “high” through
3
According to FDA, there is no known limit to the amount of oxycodone, the active
ingredient in OxyContin, that can be used to treat pain.
4
Prescription drug diversion can involve such activities as “doctor shopping” by individuals
who visit numerous physicians to obtain multiple prescriptions, prescription forgery, and
pharmacy theft. Diversion can also involve illegal sales of prescription drugs by physicians,
patients, or pharmacists, as well as obtaining controlled substances from Internet
pharmacies without a valid prescription.
5
According to the National Institute on Drug Abuse, addiction is a chronic, relapsing
disease, characterized by compulsive drug seeking and use and by neurochemical and
molecular changes in the brain, whereas physical dependence is an adaptive physiological
state that can occur with regular drug use and results in withdrawal symptoms when drug
use is discontinued.
6
Under the Controlled Substances Act, which was enacted in 1970, drugs are classified as
controlled substances and placed into one of five schedules based on their medicinal value,
potential for abuse, and safety or dependence liability. Schedule I drugs have no medicinal
value; have not been approved by FDA; and along with schedule II drugs, have the highest
potential for abuse. Schedule II drugs have the highest potential for abuse of any approved
drugs.

Page 3 GAO-04-110 OxyContin Abuse and Diversion
the body’s rapid absorption of oxycodone. During a December 2001
congressional hearing, witnesses from DEA and other law enforcement
officials from Kentucky, Virginia, and West Virginia described the growing
problem of abuse and diversion of OxyContin.
7
Questions were raised
about what factors may have caused the abuse and diversion, including
whether Purdue’s efforts to market the drug may have contributed to the
problem. In February 2002, another congressional hearing was conducted
on federal, state, and local efforts to decrease the abuse and diversion of
OxyContin.
8
Because of your concerns about these issues, you asked us to examine the
marketing and promotion of OxyContin and its abuse and diversion.
Specifically, we addressed the following questions:
1. How has Purdue marketed and promoted OxyContin?
2. What factors contributed to the abuse and diversion of OxyContin?
3. What actions have been taken to address OxyContin abuse and
diversion?
To identify how Purdue marketed and promoted OxyContin, we
interviewed Purdue officials and analyzed company documents and data.
We also interviewed selected Purdue sales representatives who were high
and midrange sales performers during 2001 and physicians who were
among the highest prescribers of OxyContin. To determine how Purdue’s
marketing and promotion of OxyContin compared to that of other drugs,
we examined the promotional materials and information related to FDA
actions and interviewed officials from companies that manufacture and
market three other opioid drugs, Avinza, Kadian, and Oramorph SR, that
like OxyContin are classified as schedule II controlled substances.
9
Because of their concern about the proprietary nature of the information,
7
OxyContin, Hearings of the Subcommittee on the Departments of Commerce, Justice, and
State, the Judiciary, and Related Agencies, House Committee on Appropriations, 107th
Cong. Part 10 (Dec. 11, 2001).
8
OxyContin: Balancing Risks and Benefits, Hearing of the Senate Committee on Health,
Education, Labor, and Pensions, 107th Cong. 287 (Feb. 12, 2002).
9
Avinza was approved by FDA in 2002 and is marketed by Ligand Pharmaceuticals; Kadian
was approved in 1996 and is marketed by Alpharma-US Human Pharmaceuticals; and
Oramorph SR was approved in 1991 and is now owned by Élan Corporation, which told us
it is not currently marketing the drug.

Page 4 GAO-04-110 OxyContin Abuse and Diversion
the three companies that market these drugs did not provide us with the
same level of detail about the marketing and promotion of their drugs as
did Purdue. We also examined data from DEA on promotional
expenditures for OxyContin and two other schedule II controlled
substances. To examine what factors may have contributed to the abuse
and diversion of OxyContin, we interviewed officials from DEA, FDA, and
Purdue and physicians who prescribe OxyContin. We also analyzed IMS
Health data on sales of OxyContin nationwide and Purdue’s distribution of
sales representatives, as part of an effort to compare the areas with large
sales growth and more sales representatives per capita with the areas
where abuse and diversion problems were identified. However, limitations
on the abuse and diversion data prevented an assessment of the
relationship between the availability of OxyContin and areas where the
drug was abused or diverted. To determine what actions have been taken
to address OxyContin abuse and diversion, we interviewed FDA officials
and examined FDA information regarding the drug’s approval and
marketing and promotion. We also interviewed DEA officials and
examined how DEA determined the prevalence of OxyContin abuse and
diversion nationally. In addition, we examined state efforts to identify
those involved in the abuse and diversion of OxyContin. We also reviewed
actions taken by Purdue to address this problem. (See app. I for a detailed
discussion of our methodology.)
We performed our work from August 2002 through October 2003, in
accordance with generally accepted government auditing standards.
Purdue conducted an extensive campaign to market and promote
OxyContin using an expanded sales force and multiple promotional
approaches to encourage physicians, including primary care specialists, to
prescribe OxyContin as an initial opioid treatment for noncancer pain.
OxyContin sales and prescriptions grew rapidly following its market
introduction in 1996, with the growth in prescriptions for noncancer pain
outpacing the growth in prescriptions for cancer pain from 1997 through
2002. By 2003, nearly half of all OxyContin prescribers were primary care
physicians. DEA has expressed concern that Purdue’s aggressive
marketing of OxyContin focused on promoting the drug to treat a wide
range of conditions to physicians who may not have been adequately
trained in pain management. Purdue has been cited twice by FDA for using
potentially false or misleading medical journal advertisements for
OxyContin that violated the Federal Food, Drug and Cosmetic Act (FD&C
Act), including one advertisement that failed to include warnings about the
potentially fatal risks associated with OxyContin use. Further, Purdue did
Results in Brief
Page 5 GAO-04-110 OxyContin Abuse and Diversion
not submit an OxyContin promotional video for FDA review at the time of
its initial distribution in 1998, as required by FDA regulations. Therefore,
FDA did not have the opportunity to review the video at the time of its
distribution to ensure that the information it contained was truthful,
balanced, and accurately communicated. FDA reviewed a similar video in
2002 and told us that the video appeared to have made unsubstantiated
claims about OxyContin and minimized its risks.
Several factors may have contributed to OxyContin’s abuse and diversion.
OxyContin’s controlled-release formulation, which made the drug
beneficial for the relief of moderate-to-severe pain over an extended
period of time, enabled the drug to contain more of the active ingredient
oxycodone than other, non-controlled-release oxycodone-containing
drugs. This feature may have made OxyContin an attractive target for
abuse and diversion, according to DEA. OxyContin’s controlled-release
formulation, which delayed the drug’s absorption, also led FDA to include
language in the original label stating that OxyContin had a lower potential
for abuse than other oxycodone products. FDA officials thought that the
controlled-release feature would make the drug less attractive to abusers.
However, FDA did not recognize that the drug could be dissolved in water
and injected, which disrupted the controlled-release characteristics and
created an immediate rush or high, thereby increasing the potential for
abuse. In addition, the safety warning on the label that advised patients
not to crush the tablets because a rapid release of a potentially toxic
amount of the drug could result—a customary precaution for controlled-
release medications—may have inadvertently alerted abusers to a possible
method for misusing the drug. The rapid growth in OxyContin sales, which
increased the drug’s availability in the marketplace, may have made it
easier for abusers to obtain the drug for illicit purposes. Further, some
geographic areas have been shown to have a history of prescription drug
abuse and diversion that may have predisposed some states to the abuse
and diversion of OxyContin. However, we could not assess the
relationship between the increased availability of OxyContin and locations
where it is being abused and diverted because the data on abuse and
diversion are not reliable, comprehensive, or timely.
Since 2000, federal and state agencies and Purdue have taken several
actions to try to address abuse and diversion of OxyContin. In July 2001,
FDA approved a revised OxyContin label adding the highest level of safety
warning that FDA can place on an approved drug product. The agency also
collaborated with Purdue to develop and implement a risk management
plan to help detect and prevent abuse and diversion of OxyContin. Risk
management plans were not used at the time OxyContin was approved.
Page 6 GAO-04-110 OxyContin Abuse and Diversion
The plans are an optional feature of new drug applications that are
intended to decrease product risks by using one or more interventions or
tools beyond the approved product labeling. FDA plans to provide
guidance on risk management plans to the pharmaceutical industry by
September 2004. Also at the federal level, DEA initiated 257 OxyContin-
related abuse and diversion cases in fiscal years 2001 and 2002, which
resulted in 302 arrests and about $1 million in fines. At the state level,
Medicaid fraud control units have investigated OxyContin abuse and
diversion; however, they do not maintain precise data on the number of
investigations and enforcement actions completed. Similarly, state medical
licensure boards have investigated complaints about physicians who were
suspected of abuse and diversion of controlled substances, but they could
not provide data on the number of investigations involving OxyContin.
Purdue has initiated education programs and other activities for
physicians, pharmacists, and the public to address OxyContin abuse and
diversion. Purdue has also taken disciplinary action against its sales
representatives who improperly promoted OxyContin and has referred
physicians who were suspected of misprescribing OxyContin to the
appropriate authorities. Although Purdue has used very specific
information on physician prescribing practices to market and promote
OxyContin since its approval, it was not until October 2002 that Purdue
began to use this information and other indicators to identify patterns of
prescribing that could point to possible improper sales representative
promotion or physician abuse and diversion of OxyContin.
To improve efforts to prevent or identify the abuse and diversion of
schedule II controlled substances such as oxycodone, we recommend that
FDA’s risk management plan guidance encourage the pharmaceutical
manufacturers that submit new drug applications for these substances to
include plans that contain a strategy for monitoring the use of these drugs
and identifying potential abuse and diversion problems.
We received comments on a draft of this report from FDA, DEA, and
Purdue. FDA agreed with our recommendation that risk management
plans for schedule II controlled substances contain a strategy for
monitoring and identifying potential abuse and diversion problems. DEA
reiterated its statement that Purdue’s aggressive marketing of OxyContin
exacerbated the abuse and diversion problems and noted that it is
essential that risk management plans be put in place prior to the
introduction of controlled substances into the marketplace. Purdue said
the report appeared to be fair and balanced, but that we should add the
media as one of the factors contributing to abuse and diversion problems
Page 7 GAO-04-110 OxyContin Abuse and Diversion
with OxyContin. We incorporated their technical comments where
appropriate.
Ensuring that pharmaceuticals are available for those with legitimate
medical need while combating the abuse and diversion of prescription
drugs involves the efforts of both federal and state government agencies.
Under the FD&C Act, FDA is responsible for ensuring that drugs are safe
and effective before they are available in the marketplace. The Controlled
Substances Act,
10
which is administered by DEA, provides the legal
framework for the federal government’s oversight of the manufacture and
wholesale distribution of controlled substances, that is, drugs and other
chemicals that have a potential for abuse. The states address certain issues
involving controlled substances through their own controlled substances
acts and their regulation of the practice of medicine and pharmacy. In
response to concerns about the influence of pharmaceutical marketing
and promotional activities on physician prescribing practices, both the
pharmaceutical industry and the Department of Health and Human
Services’s (HHS) Office of Inspector General have issued voluntary
guidelines on appropriate marketing and promotion of prescription drugs.
As the incidence and prevalence of painful diseases have grown along with
the aging of the population, there has been a growing acknowledgment of
the importance of providing effective pain relief. Pain can be characterized
in terms of intensity—mild to severe—and duration—acute (sudden onset)
or chronic (long term). The appropriate medical treatment varies
according to these two dimensions.
In 1986, WHO determined that cancer pain could be relieved in most if not
all patients, and it encouraged physicians to prescribe opioid analgesics.
WHO developed a three-step analgesic ladder as a practice guideline to
provide a sequential use of different drugs for cancer pain management.
For the first pain step, treatment with nonopioid analgesics, such as
aspirin or ibuprofen, is recommended. If pain is not relieved, then an
opioid such as codeine should be used for mild-to-moderate pain as the
second step. For the third step—moderate-to-severe pain—opioids such as
morphine should be used.
10
Title II of the Comprehensive Drug Abuse Prevention and Control Act of 1970 (Pub. L. No.
91-513, §§100 et seq., 84 Stat. 1236, 1242 et seq.).
Background
Medical Treatment of Pain

Page 8 GAO-04-110 OxyContin Abuse and Diversion
Beginning in the mid-1990s, various national pain-related organizations
issued pain treatment and management guidelines, which included the use
of opioid analgesics in treating both cancer and noncancer pain. In 1995,
the American Pain Society recommended that pain should be treated as
the fifth vital sign
11
to ensure that it would become common practice for
health care providers to ask about pain when conducting patient
evaluations. The practice guidelines issued by the Agency for Health Care
Policy and Research provided physicians and other health care
professionals with information on the management of acute pain in 1992
and cancer pain in 1994, respectively.
12
Health care providers and hospitals
were further required to ensure that their patients received appropriate
pain treatment when the Joint Commission on Accreditation of Healthcare
Organizations (JCAHO), a national health care facility standards-setting
and accrediting body, implemented its pain standards for hospital
accreditation in 2001.
OxyContin, a schedule II drug manufactured by Purdue Pharma L.P., was
approved by FDA in 1995 for the treatment of moderate-to-severe pain
lasting more than a few days, as indicated in the original label.
13
OxyContin
followed Purdue’s older product, MS Contin, a morphine-based product
that was approved in 1984 for a similar intensity and duration of pain and
during its early years of marketing was promoted for the treatment of
cancer pain. The active ingredient in OxyContin tablets is oxycodone, a
compound that is similar to morphine and is also found in oxycodone-
combination pain relief drugs such as Percocet, Percodan, and Tylox.
Because of its controlled-release property, OxyContin contains more
active ingredient and needs to be taken less often (twice a day) than these
11
The other four vital signs physicians use to assess patients are pulse, blood pressure, core
temperature, and respiration.
12
In 1999, the name of the Agency for Health Care Policy and Research was changed to the
Agency for Healthcare Research and Quality. The agency, which is part of HHS, is
responsible for supporting research designed to improve the quality of health care, reduce
its costs, and broaden access to essential services.
13
When we refer to OxyContin’s label we are also referring to the drug’s package insert that
contains the same information about the product.
OxyContin

Page 9 GAO-04-110 OxyContin Abuse and Diversion
other oxycodone-containing drugs.
14
The OxyContin label originally
approved by FDA indicated that the controlled-release characteristics of
OxyContin were believed to reduce its potential for abuse. The label also
contained a warning that OxyContin tablets were to be swallowed whole,
and were not to be broken, chewed, or crushed because this could lead to
the rapid release and absorption of a potentially toxic dose of oxycodone.
Such a safety warning is customary for schedule II controlled-release
medications. FDA first approved the marketing and use of OxyContin in
10-, 20-, and 40-milligram controlled-release tablets. FDA later approved
80- and 160-milligram controlled-release tablets for use by patients who
were already taking opioids.
15
In July 2001, FDA approved the revised label
to state that the drug is approved for the treatment of moderate-to-severe
pain in patients who require “a continuous around-the-clock analgesic for
an extended period of time.” (See app. II for a summary of the changes
that were made by FDA to the original OxyContin label.)
OxyContin sales and prescriptions grew rapidly following its market
introduction in 1996. Fortuitous timing may have contributed to this
growth, as the launching of the drug occurred during the national focus on
the inadequacy of patient pain treatment and management. In 1997,
OxyContin’s sales and prescriptions began increasing significantly, and
they continued to increase through 2002. In both 2001 and 2002,
OxyContin’s sales exceeded $1 billion, and prescriptions were over 7
million. The drug became Purdue’s main product, accounting for 90
percent of the company’s total prescription sales by 2001.
Media reports of OxyContin abuse and diversion began to surface in 2000.
These reports first appeared in rural areas of some states, generally in the
Appalachian region, and continued to spread to other rural areas and
larger cities in several states. Rural communities in Maine, Kentucky, Ohio,
Pennsylvania, Virginia, and West Virginia were reportedly being devastated
by the abuse and diversion of OxyContin. For example, media reports told
of persons and communities that had been adversely affected by the rise of
addiction and deaths related to OxyContin. One report noted that drug
14
For example, according to Purdue’s comparable dose guide a patient taking one Percodan
4.5-milligram tablet or one Tylox 5-milligram tablet every 6 hours can be converted to
either a 10- or a 20-milligram OxyContin tablet to be taken every 12 hours. For a 12-hour
dosing period, one OxyContin tablet replaces two Percodan or Tylox tablets, and one
OxyContin tablet contains twice as much oxycodone as one of the other tablets.
15
In April 2001, Purdue discontinued distribution of the 160-milligram tablets because of
OxyContin abuse and diversion concerns.

Page 10 GAO-04-110 OxyContin Abuse and Diversion
treatment centers and emergency rooms in a particular area were
receiving new patients who were addicted to OxyContin as early as 1999.
Pain patients, teens, and recreational drug users who had abused
OxyContin reportedly entered drug treatment centers sweating and
vomiting from withdrawal. In West Virginia, as many as one-half of the
approximately 300 patients admitted to a drug treatment clinic in 2000
were treated for OxyContin addiction. The media also reported on deaths
due to OxyContin. For example, a newspaper’s investigation of autopsy
reports involving oxycodone-related deaths found that OxyContin had
been involved in over 200 overdose deaths in Florida since 2000.
16
In
another case, a forensic toxicologist commented that he had reviewed a
number of fatal overdose cases in which individuals took a large dose of
OxyContin, in combination with alcohol or other drugs.
After learning about the initial reports of abuse and diversion of
OxyContin in Maine in 2000, Purdue formed a response team made up of
its top executives and physicians to initiate meetings with federal and
state officials in Maine to gain an understanding of the scope of the
problem and to devise strategies for preventing abuse and diversion. After
these meetings, Purdue distributed brochures to health care professionals
that described several steps that could be taken to prevent prescription
drug abuse and diversion. In response to the abuse and diversion reports,
DEA analyzed data collected from medical examiner autopsy reports and
crime scene investigation reports. The most recent data available from
DEA show that as of February 2002, the agency had verified 146 deaths
nationally involving OxyContin in 2000 and 2001.
According to Purdue, as of early October 2003, over 300 lawsuits
concerning OxyContin were pending against Purdue, and 50 additional
lawsuits had been dismissed. The cases involve many allegations,
including, for example, that Purdue used improper sales tactics and
overpromoted OxyContin causing the drug to be inappropriately
prescribed by physicians, and that Purdue took inadequate actions to
prevent addiction, abuse, and diversion of the drug. The lawsuits have
been brought in 25 states and the District of Columbia in both federal and
state courts.
16
Doris Bloodsworth, “Pain Pill Leaves Death Trail: A Nine-Month Investigation Raises
Many Questions about Purdue Pharma’s Powerful Drug OxyContin,” Orlando Sentinel,
Oct. 19, 2003.

Page 11 GAO-04-110 OxyContin Abuse and Diversion
The Controlled Substances Act established a classification structure for
drugs and chemicals used in the manufacture of drugs that are designated
as controlled substances.
17
Controlled substances are classified by DEA
into five schedules on the basis of their medicinal value, potential for
abuse, and safety or dependence liability. Schedule I drugs—including
heroin, marijuana, and LSD—have a high potential for abuse and no
currently accepted medical use. Schedule II drugs—which include opioids
such as morphine and oxycodone, the primary ingredient in OxyContin—
have a high potential for abuse among drugs with an accepted medical use
and may lead to severe psychological or physical dependence. Drugs on
schedules III through V have medical uses and successively lower
potentials for abuse and dependence. Schedule III drugs include anabolic
steroids, codeine, hydrocodone in combination with aspirin or
acetaminophen, and some barbiturates. Schedule IV contains such drugs
as the antianxiety drugs diazepam (Valium) and alprazolam (Xanax).
Schedule V includes preparations such as cough syrups with codeine. All
scheduled drugs except those in schedule I are legally available to the
public with a prescription.
18
Under the FD&C Act and implementing regulations, FDA is responsible for
ensuring that all new drugs are safe and effective. FDA reviews scientific
and clinical data to decide whether to approve drugs based on their
intended use, effectiveness, and the risks and benefits for the intended
population, and also monitors drugs for continued safety after they are in
use.
FDA also regulates the advertising and promotion of prescription drugs
under the FD&C Act. FDA carries out this responsibility by ensuring that
prescription drug advertising and promotion is truthful, balanced, and
accurately communicated.
19
The FD&C Act makes no distinction between
17
Section 201, classified to 21 U.S.C. § 811.
18
Some schedule V drugs that contain limited quantities of certain narcotic and stimulant
drugs are available over the counter, without a prescription.
19
FDA regulations require that promotional labeling and advertisements be submitted to
FDA at the time of initial dissemination (for labeling) and initial publication (for
advertisements). The FD&C Act defines labeling to include all labels and other written,
printed, or graphic matter accompanying an article. For example, promotional materials
commonly shown or given to physicians, such as sales aids and branded promotional items,
are regulated as promotional labeling. FDA may also regulate promotion by sales
representatives on computer programs, through fax machines, or on electronic bulletin
boards.
Controlled Substances Act
FDA’s Regulation of
Prescription Drugs

Page 12 GAO-04-110 OxyContin Abuse and Diversion
controlled substances and other prescription drugs in the oversight of
promotional activities. FDA told us that the agency takes a risk-based
approach to enforcement, whereby drugs with more serious risks, such as
opioids, are given closer scrutiny in monitoring promotional messages and
activities, but the agency has no specific guidance or policy on this
approach. The FD&C Act and its implementing regulations require that all
promotional materials for prescription drugs be submitted to FDA at the
time the materials are first disseminated or used, but it generally is not
required that these materials be approved by FDA before their use. As a
result, FDA’s actions to address violations occur after the materials have
already appeared in public. In fiscal year 2002, FDA had 39 staff positions
dedicated to oversight of drug advertising and promotion of all
pharmaceuticals distributed in the United States. According to FDA, most
of the staff focuses on the oversight of promotional communications to
physicians. FDA officials told us that in 2001 it received approximately
34,000 pieces of promotional material, including consumer advertisements
and promotions to physicians, and received and reviewed 230 complaints
about allegedly misleading advertisements, including materials directed at
health professionals.
20
FDA issues two types of letters to address violations of the FD&C Act:
untitled letters and warning letters. Untitled letters are issued for
violations such as overstating the effectiveness of the drug, suggesting a
broader range of indicated uses than the drug has been approved for, and
making misleading claims because of inadequate context or lack of
balanced information. Warning letters are issued for more serious
violations, such as those involving safety or health risks, or for continued
violations of the act. Warning letters generally advise a pharmaceutical
manufacturer that FDA may take further enforcement actions, such as
seeking judicial remediation, without notifying the company and may ask
the manufacturer to conduct a new advertising campaign to correct
inaccurate impressions left by the advertisements.
Under the Controlled Substances Act, FDA notifies DEA if FDA is
reviewing a new drug application for a drug that has a stimulant,
depressant, or hallucinogenic effect on the central nervous system and has
abuse potential. FDA performs a medical and scientific assessment as
20
For details on FDA’s oversight of drug advertising see U.S. General Accounting Office,
Prescription Drugs: FDA Oversight of Direct-to-Consumer Advertising Has Limitations,
GAO-03-177 (Washington, D.C.: Oct. 28, 2002).

Page 13 GAO-04-110 OxyContin Abuse and Diversion
required by the Controlled Substances Act, and recommends to DEA an
initial schedule level to be assigned to a new controlled substance.
FDA plans to provide guidance to the pharmaceutical industry on the
development, implementation, and evaluation of risk management plans as
a result of the reauthorization of the Prescription Drug User Fee Act of
1992 (PDUFA).
21
FDA expects to issue this guidance by September 30,
2004. FDA defines a risk management program as a strategic safety
program that is designed to decrease product risks by using one or more
interventions or tools beyond the approved product labeling. Interventions
used in risk management plans may include postmarketing surveillance,
education and outreach programs to health professionals or consumers,
informed consent agreements for patients, limitations on the supply or
refills of products, and restrictions on individuals who may prescribe and
dispense drug products. All drug manufacturers have the option to develop
and submit risk management plans to FDA as part of their new drug
applications.
DEA is the primary federal agency responsible for enforcing the
Controlled Substances Act. DEA has the authority to regulate transactions
involving the sale and distribution of controlled substances at the
manufacturer and wholesale distributor levels. DEA registers legitimate
handlers of controlled substances—including manufacturers, distributors,
hospitals, pharmacies, practitioners, and researchers—who must comply
with regulations relating to drug security and accountability through the
maintenance of inventories and records. All registrants, including
pharmacies, are required to maintain records of controlled substances that
have been manufactured, purchased, and sold. Manufacturers and
distributors are also required to report their annual inventories of
controlled substances to DEA. The data provided to DEA are available for
use in monitoring the distribution of controlled substances throughout the
United States and identifying retail-level registrants that received unusual
quantities of controlled substances. DEA regulations for schedule II
prescription drugs, unlike those for other prescription drugs, require that
each prescription must be written and signed by the physician and may
not be telephoned in to the pharmacy except in an emergency. Also, a
21
The Prescription Drug User Fee Act of 1992, Pub. L. No. 102-571, title I, 106 Stat. 4491, was
reauthorized by the Food and Drug Modernization Act of 1997, Pub. L. No. 105-115, 111
Stat. 2296, and, most recently, by the Prescription Drug User Fee Amendments of 2002,
Pub. L. No. 107-188, title V, subtitle A, 116 Stat. 594, 687.
DEA’s Regulation of
Controlled Substances

Page 14 GAO-04-110 OxyContin Abuse and Diversion
prescription for a schedule II drug may not be refilled. A physician is
required to provide a new prescription each time a patient obtains more of
the drug. DEA also sets limits on the quantity of schedule II controlled
substances that may be produced in the United States in any given year.
Specifically, DEA sets aggregate production quotas that limit the
production of bulk raw materials used in the manufacture of controlled
substances. DEA determines these quotas based on a variety of data
including sales, production, inventories, and exports. Individual
companies must apply to DEA for manufacturing or procurement quotas
for specific pharmaceutical products. For example, Purdue has a
procurement quota for oxycodone, the principle ingredient in OxyContin,
that allows the company to purchase specified quantities of oxycodone
from bulk manufacturers.
State laws govern the prescribing and dispensing of prescription drugs by
licensed health care professionals. Each state requires that physicians
practicing in the state be licensed, and state medical practice laws
generally outline standards for the practice of medicine and delegate the
responsibility of regulating physicians to state medical boards. States also
require pharmacists and pharmacies to be licensed. The regulation of the
practice of pharmacy is based on state pharmacy practice acts and
regulations enforced by the state boards of pharmacy. According to the
National Association of Boards of Pharmacy, all state pharmacy laws
require that records of prescription drugs dispensed to patients be
maintained and that state pharmacy boards have access to the prescription
records. State regulatory boards face new challenges with the advent of
Internet pharmacies, because they enable pharmacies and physicians to
anonymously reach across state borders to prescribe, sell, and dispense
prescription drugs without complying with state requirements.
22
In some
cases, consumers can purchase prescription drugs, including controlled
substances, such as OxyContin, from Internet pharmacies without a valid
prescription.
22
For more details on Internet pharmacies, see U.S. General Accounting Office, Internet
Pharmacies: Adding Disclosure Requirements Would Aid State and Federal Oversight,
GAO-01-69 (Washington, D.C.: Oct. 19, 2000).
States’ Regulation of the
Practice of Medicine and
Pharmacy and Role in
Monitoring Illegal Use and
Diversion of Prescription
Drugs

Page 15 GAO-04-110 OxyContin Abuse and Diversion
In addition to these regulatory boards, 15 states operate prescription drug
monitoring programs as a means to control the illegal diversion of
prescription drugs that are controlled substances. Prescription drug
monitoring programs are designed to facilitate the collection, analysis, and
reporting of information on the prescribing, dispensing, and use of
controlled substances within a state. They provide data and analysis to
state law enforcement and regulatory agencies to assist in identifying and
investigating activities potentially related to the illegal prescribing,
dispensing, and procuring of controlled substances. For example,
physicians in Kentucky can use the program to check a patient’s
prescription drug history to determine if the individual may be “doctor
shopping” to seek multiple controlled substance prescriptions. An
overriding goal of prescription drug monitoring programs is to support
both the state laws ensuring access to appropriate pharmaceutical care by
citizens and the state laws deterring diversion. As we have reported, state
prescription drug monitoring programs offer state regulators an efficient
means of detecting and deterring illegal diversion. However, few states
proactively analyze prescription data to identify individuals, physicians, or
pharmacies that have unusual use, prescribing, or dispensing patterns that
may suggest potential drug diversion or abuse. Although three states can
respond to requests for information within 3 to 4 hours, providing
information on suspected illegal prescribing, dispensing, or doctor
shopping at the time a prescription is written or sold would require states
to improve computer capabilities. In addition, state prescription drug
monitoring programs may require additional legal authority to analyze data
proactively.
23
At the time that OxyContin was first marketed, there were no industry or
federal guidelines for the promotion of prescription drugs. Voluntary
guidelines regarding how drug companies should market and promote
their drugs to health care professionals were issued in July 2002 by the
Pharmaceutical Research and Manufacturers of America (PhRMA). In
April 2003, HHS’s Office of Inspector General issued voluntary guidelines
for how drug companies should market and promote their products to
federal health care programs. Neither set of guidelines distinguishes
between controlled and noncontrolled substances.
23
For more details on these programs, see U.S. General Accounting Office, Prescription
Drugs: State Monitoring Programs Provide Useful Tool to Reduce Diversion, GAO-02-634
(Washington, D.C.: May 17, 2002).
Guidelines for Marketing
Drugs to Health Care
Professionals
Page 16 GAO-04-110 OxyContin Abuse and Diversion
PhRMA’s voluntary code of conduct for sales representatives states that
interactions with health care professionals should be to inform these
professionals about products, to provide scientific and educational
information, and to support medical research and education.
24
The
question-and-answer section of the code addresses companies’ use of
branded promotional items, stating, for example, that golf balls and sports
bags should not be distributed because they are not primarily for the
benefit of patients, but that speaker training programs held at golf resorts
may be acceptable if participants are receiving extensive training. Purdue
adopted the code.
In April 2003, HHS’s Office of Inspector General issued final voluntary
guidance for drug companies’ interactions with health care professionals
in connection with federal health care programs, including Medicare and
Medicaid. Among the guidelines were cautions for companies against
offering inappropriate travel, meals, and gifts to influence the prescribing
of drugs; making excessive payments to physicians for consulting and
research services; and paying physicians to switch their patients from
competitors’ drugs.
Purdue conducted an extensive campaign to market and promote
OxyContin that focused on encouraging physicians, including those in
primary care specialties, to prescribe the drug for noncancer as well as
cancer pain. To implement its OxyContin campaign, Purdue significantly
increased its sales force and used multiple promotional approaches.
OxyContin sales and prescriptions grew rapidly following its market
introduction, with the growth in prescriptions for noncancer pain
outpacing the growth in prescriptions for cancer pain. DEA has expressed
concern that Purdue marketed OxyContin for a wide variety of conditions
to physicians who may not have been adequately trained in pain
management. Purdue has been cited twice by FDA for OxyContin
advertisements in medical journals that violated the FD&C Act. FDA has
also taken similar actions against manufacturers of two of the three
comparable schedule II controlled substances we examined, to ensure that
24
In addition, the American Medical Association, a professional association for physicians,
issued guidelines in 1990 regarding gifts given to physicians by drug industry
representatives. For example, physicians may accept individual gifts of nominal value that
are related to their work, such as notepads and pens, and may attend conferences
sponsored by drug companies that are educational and for which appropriate disclosure of
financial support or conflicts of interest is made.
Purdue Conducted an
Extensive Campaign
to Market and
Promote OxyContin

Page 17 GAO-04-110 OxyContin Abuse and Diversion
their marketing and promotion were truthful, balanced, and accurately
communicated. In addition, Purdue provided two promotional videos to
physicians that, according to FDA appear to have made unsubstantiated
claims and minimized the risks of OxyContin. The first video was available
for about 3 years without being submitted to FDA for review.
From the outset of the OxyContin marketing campaign, Purdue promoted
the drug to physicians for noncancer pain conditions that can be caused
by arthritis, injuries, and chronic diseases, in addition to cancer pain.
Purdue directed its sales representatives to focus on the physicians in
their sales territories who were high opioid prescribers. This group
included cancer and pain specialists, primary care physicians, and
physicians who were high prescribers of Purdue’s older product, MS
Contin. One of Purdue’s goals was to identify primary care physicians who
would expand the company’s OxyContin prescribing base. Sales
representatives were also directed to call on oncology nurses, consultant
pharmacists, hospices, hospitals, and nursing homes.
From OxyContin’s launch until its July 2001 label change, Purdue used two
key promotional messages for primary care physicians and other high
prescribers. The first was that physicians should prescribe OxyContin for
their pain patients both as the drug “to start with and to stay with.” The
second contrasted dosing with other opioid pain relievers with OxyContin
dosing as “the hard way versus the easy way” to dose because OxyContin’s
twice-a-day dosing was more convenient for patients.
25
Purdue’s sales
representatives promoted OxyContin to physicians as an initial opioid
treatment for moderate-to-severe pain lasting more than a few days, to be
prescribed instead of other single-entity opioid analgesics or short-acting
combination opioid pain relievers. Purdue has stated that by 2003 primary
care physicians had grown to constitute nearly half of all OxyContin
prescribers, based on data from IMS Health, an information service
providing pharmaceutical market research. DEA’s analysis of physicians
prescribing OxyContin found that the scope of medical specialties was
wider for OxyContin than five other controlled-release, schedule II
narcotic analgesics. DEA expressed concern that this resulted in
25
Following OxyContin’s July 2001 label change, Purdue modified its promotional messages
but continued to focus on encouraging physicians to prescribe OxyContin for patients
taking pain relievers every 4 to 6 hours. In 2003, Purdue began using the promotional claim
“there can be life with relief” in OxyContin promotion.
Purdue Focused on
Promoting OxyContin for
Treatment of Noncancer
Pain

Page 18 GAO-04-110 OxyContin Abuse and Diversion
OxyContin’s being promoted to physicians who were not adequately
trained in pain management.
Purdue’s promotion of OxyContin for the treatment of noncancer pain
contributed to a greater increase in prescriptions for noncancer pain than
for cancer pain from 1997 through 2002.
26
According to IMS Health data,
the annual number of OxyContin prescriptions for noncancer pain
increased nearly tenfold, from about 670,000 in 1997 to about 6.2 million in
2002.
27
In contrast, during the same 6 years, the annual number of
OxyContin prescriptions for cancer pain increased about fourfold, from
about 250,000 in 1997 to just over 1 million in 2002. The noncancer
prescriptions therefore increased from about 73 percent of total
OxyContin prescriptions to about 85 percent during that period, while the
cancer prescriptions decreased from about 27 percent of the total to about
15 percent. IMS Health data indicated that prescriptions for other schedule
II opioid drugs, such as Duragesic
28
and morphine products, for noncancer
pain also increased during this period. Duragesic prescriptions for
noncancer pain were about 46 percent of its total prescriptions in 1997,
and increased to about 72 percent of its total in 2002. Morphine products,
including, for example, Purdue’s MS Contin, also experienced an increase
in their noncancer prescriptions during the same period. Their noncancer
prescriptions were about 42 percent of total prescriptions in 1997, and
increased to about 65 percent in 2002. DEA has cited Purdue’s focus on
promoting OxyContin for treating a wide range of conditions as one of the
reasons the agency considered Purdue’s marketing of OxyContin to be
overly aggressive.
26
IMS Health reported noncancer prescriptions written for the following types of pain
conditions: surgical aftercare; musculoskeletal disorders including back and neck
disorders, arthritis conditions, and injuries and trauma including bone fractures; central
nervous system disorders including headache conditions such as migraines; genitourinary
disorders including kidney stones; and other types of general pain.
27
The IMS Health data included information from the National Disease and Therapeutics
Index and the National Prescription Audit. The National Disease and Therapeutics Index
does not capture data from anesthesiologists and dental specialties. The National
Prescription Audit data include retail pharmacy, long-term-care, and mail-order
prescriptions.
28
Duragesic is a skin patch used to deliver the opioid pain reliever fentanyl over a 72-hour
period.
Page 19 GAO-04-110 OxyContin Abuse and Diversion
Purdue significantly increased its sales force to market and promote
OxyContin to physicians and other health care practitioners. In 1996,
Purdue began promoting OxyContin with a sales force of approximately
300 representatives in its Prescription Sales Division.
29
Through a 1996
copromotion agreement, Abbott Laboratories provided at least another
300 representatives, doubling the total OxyContin sales force.
30
By 2000,
Purdue had more than doubled its own internal sales force to 671. The
expanded sales force included sales representatives from the Hospital
Specialty Division, which was created in 2000 to increase promotional
visits on physicians located in hospitals. (See table 1.)
Table 1: Sales Representative Positions Available for OxyContin Promotion, 1996
through 2002
Positions available
a
1996 1997 1998 1999 2000 2001 2002
Purdue Prescription Sales Division 318 319 377 471 562 641 641
Purdue Hospital Specialty Division 0 0 0 0 109 125 126
Subtotal—All Purdue sales
representatives
318 319 377 471 671 766 767
Abbott Laboratories sales
representatives
b
300 300 300 300 300 300 300
Total 618 619 677 771 971 1,066 1,067
Source: GAO analysis of Purdue data.
a
All positions were not necessarily filled in a given year.
b
Under the OxyContin copromotion agreement, Abbott Laboratories provided at least 300 sales
representatives each year.
The manufacturers of two of the three comparable schedule II drugs have
smaller sales forces than Purdue. Currently, the manufacturer of Kadian
has about 100 sales representatives and is considering entering into a
copromotion agreement. Elan, the current owner of Oramorph SR, has
approximately 300 representatives, but told us that it is not currently
marketing Oramorph SR. The manufacturer of Avinza had approximately
50 representatives at its product launch. In early 2003, Avinza’s
manufacturer announced that more than 700 additional sales
29
These sales representatives were also responsible for promoting other Purdue products.
30
Abbott Laboratories sales representatives’ promotion of OxyContin is limited to hospital-
based anesthesiologists and surgeons and major hospitals, medical centers, and
freestanding pain clinics.
Purdue Significantly
Increased Its Sales Force
to Market and Promote
OxyContin
Page 20 GAO-04-110 OxyContin Abuse and Diversion
representatives would be promoting the drug under its copromotion
agreement with the pharmaceutical manufacturer Organon—for a total of
more than 800 representatives.
By more than doubling its total sales representatives, Purdue significantly
increased the number of physicians to whom it was promoting OxyContin.
Each Purdue sales representative has a specific sales territory and is
responsible for developing a list of about 105 to 140 physicians to call on
who already prescribe opioids or who are candidates for prescribing
opioids. In 1996, the 300-plus Purdue sales representatives had a total
physician call list of approximately 33,400 to 44,500. By 2000, the nearly
700 representatives had a total call list of approximately 70,500 to 94,000
physicians. Each Purdue sales representative is expected to make about 35
physician calls per week and typically calls on each physician every 3 to 4
weeks. Each hospital sales representative is expected to make about 50
calls per week and typically calls on each facility every 4 weeks.
Purdue stated it offered a “better than industry average” salary and sales
bonuses to attract top sales representatives and provide incentives to
boost OxyContin sales as it had done for MS Contin. Although the sales
representatives were primarily focused on OxyContin promotion, the
amount of the bonus depended on whether a representative met the sales
quotas in his or her sales territory for all company products. As
OxyContin’s sales increased, Purdue’s growth-based portion of the bonus
formula increased the OxyContin sales quotas necessary to earn the same
base sales bonus amounts. The amount of total bonuses that Purdue
estimated were tied to OxyContin sales increased significantly from about
$1 million in 1996, when OxyContin was first marketed, to about $40
million in 2001. Beginning in 2000, when the newly created hospital
specialty representatives began promoting OxyContin, their estimated
total bonuses were approximately $6 million annually. In 2001, the average
annual salary for a Purdue sales representative was $55,000, and the
average annual bonus was $71,500. During the same year, the highest
annual sales bonus was nearly $240,000, and the lowest was nearly
$15,000. In 2001, Purdue decided to limit the sales bonus a representative
could earn based on the growth in prescribing of a single physician after a
meeting with the U.S. Attorney for the Western District of Virginia at
which the company was informed of the possibility that a bonus could be
based on the prescribing of one physician.

Page 21 GAO-04-110 OxyContin Abuse and Diversion
In addition to expanding its sales force, Purdue used multiple approaches
to market and promote OxyContin. These approaches included expanding
its physician speaker bureau and conducting speaker training conferences,
sponsoring pain-related educational programs, issuing OxyContin starter
coupons for patients’ initial prescriptions, sponsoring pain-related Web
sites, advertising OxyContin in medical journals, and distributing
OxyContin marketing items to health care professionals.
In our report on direct-to-consumer advertising, we found that most
promotional spending is targeted to physicians.
31
For example, in 2001, 29
percent of spending on pharmaceutical promotional activities was related
to activities of pharmaceutical sales representatives directed to
physicians, and 2 percent was for journal advertising—both activities
Purdue uses for its OxyContin promotion. The remaining 69 percent of
pharmaceutical promotional spending involved sampling (55 percent),
which is the practice of providing drug samples during sales visits to
physician offices, and direct-to-consumer advertising (14 percent)—both
activities that Purdue has stated it does not use for OxyContin.
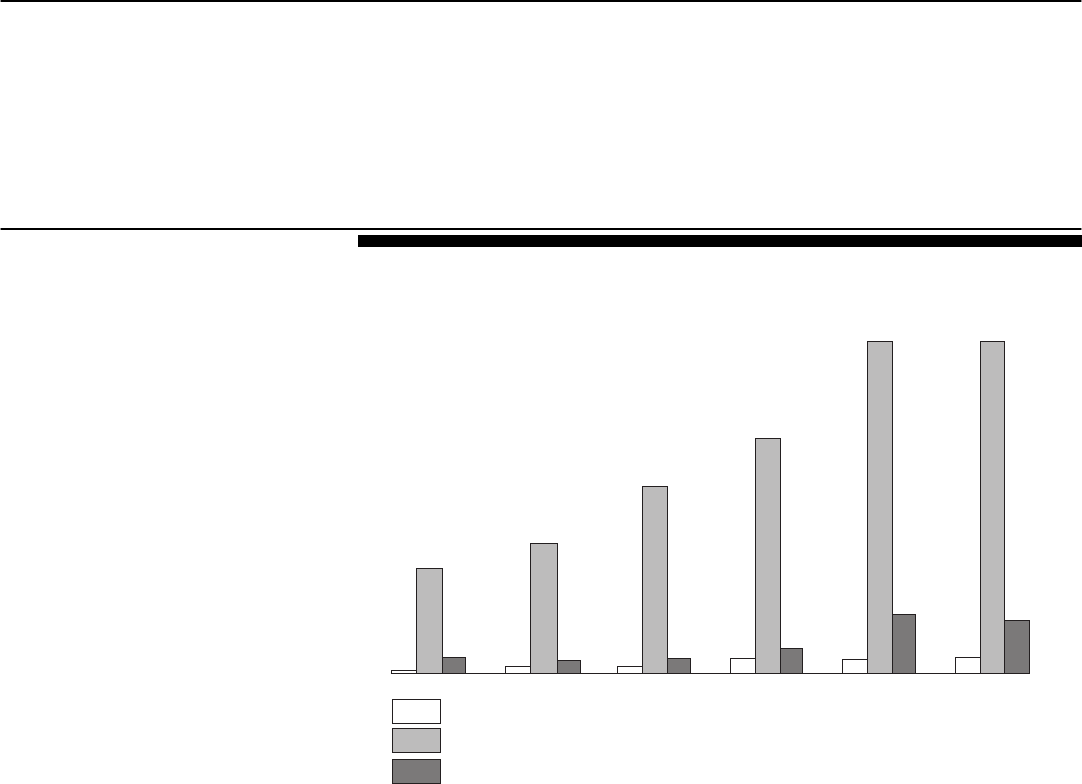
According to DEA’s analysis of IMS Health data, Purdue spent
approximately 6 to 12 times more on promotional efforts during
OxyContin’s first 6 years on the market than it had spent on its older
product, MS Contin, during its first 6 years, or than had been spent by
Janssen Pharmaceutical Products, L.P., for one of OxyContin’s drug
competitors, Duragesic. (See fig. 1.)
31
U.S. General Accounting Office, Prescription Drugs: FDA Oversight of Direct-to-
Consumer Advertising Has Limitations, GAO-03-177 (Washington, D.C.: Oct. 28, 2002).
Purdue Employed Multiple
Approaches to Market and
Promote OxyContin
Page 22 GAO-04-110 OxyContin Abuse and Diversion
Figure 1: Promotional Spending for Three Opioid Analgesics in First 6 Years of
Sales
Note: Dollars are 2002 adjusted.
During the first 5 years that OxyContin was marketed, Purdue conducted
over 40 national pain management and speaker training conferences,
usually in resort locations such as Boca Raton, Florida, and Scottsdale,
Arizona, to recruit and train health care practitioners for its national
speaker bureau. The trained speakers were then made available to speak
about the appropriate use of opioids, including oxycodone, the active
ingredient in OxyContin, to their colleagues in various settings, such as
local medical conferences and grand round presentations in hospitals
involving physicians, residents, and interns. Over the 5 years, these
conferences were attended by more than 5,000 physicians, pharmacists,
and nurses, whose travel, lodging, and meal costs were paid by the
company. Purdue told us that less than 1 percent annually of the
physicians called on by Purdue sales representatives attended these
conferences. Purdue told us it discontinued conducting these conferences
in fall 2000. Purdue’s speaker bureau list from 1996 through mid-2002
included nearly 2,500 physicians, of whom over 1,000 were active
participants. Purdue has paid participants a fee for speaking based on the
physician’s qualifications; the type of program and time commitment
30
25
20
15
10
5
0
MS Contin: 1984-1989
OxyContin: 1996-2001
Duragesic: 1991-1996
Year 1 Year 2 Year 3 Year 4 Year 5 Year 6
Source: DEA and IMS Health, Integrated Promotional Service Audit.
Absolute dollars in millions

Page 23 GAO-04-110 OxyContin Abuse and Diversion
involved; and expenses such as airfare, hotel, and food. The company
currently marketing the comparable drug Avinza has a physician speaker
bureau, but does not sponsor speaker training and conferences at resort
locations. Kadian’s current company does not have a physician speaker
bureau and has not held any conferences.
From 1996, when OxyContin was introduced to the market, to July 2002,
Purdue has funded over 20,000 pain-related educational programs through
direct sponsorship or financial grants. These grants included support for
programs to provide physicians with opportunities to earn required
continuing medical education credits, such as grand round presentations
at hospitals and medical education seminars at state and local medical
conferences. During 2001 and 2002, Purdue funded a series of nine
programs throughout the country to educate hospital physicians and staff
on how to comply with JCAHO’s pain standards for hospitals and to
discuss postoperative pain treatment. Purdue was one of only two drug
companies that provided funding for JCAHO’s pain management
educational programs.
32
Under an agreement with JCAHO, Purdue was the
only drug company allowed to distribute certain educational videos and a
book about pain management; these materials were also available for
purchase from JCAHO’s Web site. Purdue’s participation in these activities
with JCAHO may have facilitated its access to hospitals to promote
OxyContin.
For the first time in marketing any of its products, Purdue used a patient
starter coupon program for OxyContin to provide patients with a free
limited-time prescription. Unlike patient assistance programs, which
provide free prescriptions to patients in financial need, a coupon program
is intended to enable a patient to try a new drug through a one-time free
prescription. A sales representative distributes coupons to a physician,
who decides whether to offer one to a patient, and then the patient
redeems it for a free prescription through a participating pharmacy. The
program began in 1998 and ran intermittently for 4 years. In 1998 and 1999,
each sales representative had 25 coupons that were redeemable for a free
30-day supply. In 2000 each representative had 90 coupons for a 7-day
supply, and in 2001 each had 10 coupons for a 7-day supply.
Approximately 34,000 coupons had been redeemed nationally when the
32
During 2000 through 2002, JCAHO sponsored a series of educational programs on pain
management standards with various cosponsors, including pain-related groups such as the
American Pain Society and the American Academy of Pain Medicine.

Page 24 GAO-04-110 OxyContin Abuse and Diversion
program was terminated following the July 2001 OxyContin label change.
The manufacturers of two of the comparable drugs we examined—Avinza
and Kadian—used coupon programs to introduce patients to their
products. Avinza’s coupon program requires patients to make a copayment
to cover part of the drug’s cost.
Purdue has also used Web sites to provide pain-related information to
consumers and others. In addition to its corporate Web site, which
provides product information, Purdue established the “Partners Against
Pain” Web site in 1997 to provide consumers with information about pain
management and pain treatment options. According to FDA, the Web site
also contained information about OxyContin. Separate sections provide
information for patients and caregivers, medical professionals, and
institutions. The Web site includes a “Find a Doctor” feature to enable
consumers to find physicians who treat pain in their geographic area.
33
As
of July 2002, over 33,000 physicians were included. Ligand, which markets
Avinza, one of the comparable drugs, has also used a corporate Web site to
provide product information. Purdue has also funded Web sites, such as
FamilyPractice.com, that provide physicians with free continuing medical
educational programs on pain management.
34
Purdue has also provided
funding for Web site development and support for health care groups such
as the American Chronic Pain Association and the American Academy of
Pain Medicine. In addition, Purdue is one of 28 corporate donors—which
include all three comparable drug companies—listed on the Web site of
the American Pain Society, the mission of which is to improve pain-related
education, treatment, and professional practice. Purdue also sponsors
painfullyobvious.com, which it describes as a youth-focused “message
campaign designed to provide information—and stimulate open
discussions—on the dangers of abusing prescription drugs.”
Purdue also provided its sales representatives with 14,000 copies of a
promotional video in 1999 to distribute to physicians. Entitled From One
Pain Patient to Another: Advice from Patients Who Have Found Relief,
the video was to encourage patients to report their pain and to alleviate
patients’ concerns about taking opioids. Purdue stated that the video was
to be used “in physician waiting rooms, as a ‘check out’ item for an office’s
33
The “Find a Doctor” feature is a physician listing service provided by the National
Physicians DataSource, LLC.
34
Purdue has also helped to fund the Dannemiller Memorial Education Foundation and the
American Academy of Physician Assistants Web sites.

Page 25 GAO-04-110 OxyContin Abuse and Diversion
patient education library, or as an educational tool for office or hospital
staff to utilize with patients and their families.” Copies of the video were
also available for ordering on the “Partners Against Pain” Web site from
June 2000 through July 2001. The video did not need to be submitted to
FDA for its review because it did not contain any information about
OxyContin. However, the video included a statement that opioid
analgesics have been shown to cause addiction in less than 1 percent of
patients. According to FDA, this statement has not been substantiated.
As part of its marketing campaign, Purdue distributed several types of
branded promotional items to health care practitioners. Among these
items were OxyContin fishing hats, stuffed plush toys, coffee mugs with
heat-activated messages, music compact discs, luggage tags, and pens
containing a pullout conversion chart showing physicians how to calculate
the dosage to convert a patient to OxyContin from other opioid pain
relievers.
35
In May 2002, in anticipation of PhRMA’s voluntary guidance for
sales representatives’ interactions with health care professionals, Purdue
instructed its sales force to destroy any remaining inventory of non-health-
related promotional items, such as stuffed toys or golf balls. In early 2003,
Purdue began distributing an OxyContin branded goniometer—a range
and motion measurement guide. According to DEA, Purdue’s use of
branded promotional items to market OxyContin was unprecedented
among schedule II opioids, and was an indicator of Purdue’s aggressive
and inappropriate marketing of OxyContin.
Another approach Purdue used to promote OxyContin was to place
advertisements in medical journals. Purdue’s annual spending for
OxyContin advertisements increased from about $700,000 in 1996 to about
$4.6 million in 2001. All three companies that marketed the comparable
drugs have also used medical journal advertisements to promote their
products.
Purdue has been cited twice by FDA for using advertisements in
professional medical journals that violated the FD&C Act. In May 2000,
FDA issued an untitled letter to Purdue regarding a professional medical
35
It is common drug industry practice for companies to provide conversion tables for sales
representatives to distribute to health care practitioners. Purdue used a similar pen for its
older product, MS Contin.
OxyContin Advertisements
Violated the FD&C Act

Page 26 GAO-04-110 OxyContin Abuse and Diversion
journal advertisement for OxyContin.
36
FDA noted that among other
problems, the advertisement implied that OxyContin had been studied for
all types of arthritis pain when it had been studied only in patients with
moderate-to-severe osteoarthritis pain, the advertisement suggested
OxyContin could be used as an initial therapy for the treatment of
osteoarthritis pain without substantial evidence to support this claim, and
the advertisement promoted OxyContin in a selected class of patients—
the elderly—without presenting risk information applicable to that class of
patients.
37
Purdue agreed to stop dissemination of the advertisement. The
second action taken by FDA was more serious. In January 2003, FDA
issued a warning letter to Purdue regarding two professional medical
journal advertisements for OxyContin that minimized its risks and
overstated its efficacy, by failing to prominently present information from
the boxed warning on the potentially fatal risks associated with OxyContin
and its abuse liability, along with omitting important information about the
limitations on the indicated use of OxyContin.
38
The FDA requested that
Purdue cease disseminating these advertisements and any similar violative
materials and provide a plan of corrective action. In response, Purdue
issued a corrected advertisement, which called attention to the warning
letter and the cited violations and directed the reader to the prominently
featured boxed warning and indication information for OxyContin.
39
The
FDA letter was one of only four warning letters issued to drug
manufacturers during the first 8 months of 2003.
40
In addition, in follow-up discussions with Purdue officials on the January
2003 warning letter, FDA expressed concerns about some of the
information on Purdue’s “Partners Against Pain” Web site. The Web site
appeared to suggest unapproved uses of OxyContin for postoperative pain
that may have been inconsistent with OxyContin’s labeling and lacked risk
36
FDA indicated that in 2000, it issued 75 untitled letters to 46 drug manufacturers, as well
as 4 warning letters to 4 drug manufacturers, for using promotional activities that violated
the FD&C Act.
37
The advertisement appeared in the New England Journal of Medicine in May 2000.
38
The advertisements appeared in the Journal of the American Medical Association in
October and November 2002.
39
According to FDA, the corrective advertisement ran for 3 months and appeared in
approximately 30 medical journals.
40
FDA indicated that from January through August 2003, it issued 4 warning letters to four
manufacturers and 12 untitled letters to seven drug manufacturers for using promotional
activities that violated the FD&C Act.

Page 27 GAO-04-110 OxyContin Abuse and Diversion
information about the drug. For example, one section of the Web site did
not disclose that OxyContin is not indicated for pain in the immediate
postoperative period—the first 12 to 24 hours following surgery—for
patients not previously taking the drug, because its safety in this setting
has not been established. The Web site also did not disclose that
OxyContin is indicated for postoperative pain in patients already taking
the drug or for use after the first 24 hours following surgery only if the
pain is moderate to severe and expected to persist for an extended period
of time. Purdue voluntarily removed all sections of the Web site that were
of concern to FDA.
FDA has also sent enforcement letters to other manufacturers of
controlled substances for marketing and promotion violations of the
FD&C Act. For example, in 1996, FDA issued an untitled letter to Zeneca
Pharmaceuticals, at the time the promoter of Kadian,
41
for providing
information about the drug to a health professional prior to its approval in
the United States. Roxane Laboratories, the manufacturer of Oramorph
SR, was issued four untitled letters between 1993 and 1995 for making
misleading and possibly false statements. Roxane used children in an
advertisement even though Oramorph SR had not been evaluated in
children, and a Roxane sales representative issued a promotional letter to
a pharmacist that claimed, among other things, that Oramorph SR was
superior to MS Contin in providing pain relief. FDA has sent no
enforcement letters to Ligand Pharmaceuticals concerning Avinza.
Beginning in 1998, Purdue, as part of its marketing and promotion of
OxyContin, distributed 15,000 copies of an OxyContin video to physicians
without submitting it to FDA for review. This video, entitled I Got My Life
Back: Patients in Pain Tell Their Story, presented the pain relief
experiences of various patients and the pain medications, including
OxyContin, they had been prescribed. FDA regulations require
pharmaceutical manufacturers to submit all promotional materials for
approved prescription drug products to the agency at the time of their
initial use. Because Purdue did not comply with this regulation, FDA did
not have an opportunity to review the video to ensure that the information
it contained was truthful, balanced, and accurately communicated. Purdue
has acknowledged the oversight of not submitting the video to FDA for
41
Zeneca Pharmaceuticals promoted Kadian for Faulding Laboratories, the drug’s
manufacturer at that time.
Purdue Distributed an
OxyContin Video without
FDA’s Review That
Appears to Have Made
Unsubstantiated Claims
and Minimized Risks
Page 28 GAO-04-110 OxyContin Abuse and Diversion
review. In February 2001, Purdue submitted a second version of the video
to FDA, which included information about the 160-milligram OxyContin
tablet. FDA did not review this second version until October 2002, after we
inquired about its content. FDA told us it found that the second version of
the video appeared to make unsubstantiated claims regarding OxyContin’s
effect on patients’ quality of life and ability to perform daily activities and
minimized the risks associated with the drug.
The 1998 video used a physician spokesperson to describe patients with
different pain syndromes and the limitations that each patient faced in his
or her daily activities. Each patient’s pain treatment was discussed, along
with the dose amounts and brand names of the prescription drugs,
including OxyContin, that either had been prescribed in the past or were
being prescribed at that time. The physician in the videos also stated that
opioid analgesics have been shown to cause addiction in less than 1
percent of patients—a fact that FDA has stated has not been substantiated.
At the end of the video, the OxyContin label was scrolled for the viewer.
In 2000, Purdue submitted another promotional video to FDA entitled I
Got My Life Back: A Two Year Follow up of Patients in Pain, and it
submitted a second version of this video in 2001, which also included
information on the 160-milligram OxyContin tablet. Purdue distributed
12,000 copies of these videos to physicians. Both versions scrolled the
OxyContin label at the end of the videos. FDA stated that it did not review
either of these videos for enforcement purposes because of limited
resources. Distribution of all four Purdue videos was discontinued by July
2001, in response to OxyContin’s labeling changes, which required the
company to modify all of its promotional materials, but copies of the
videos that had already been distributed were not retrieved and destroyed.
FDA said that it receives numerous marketing and promotional materials
for promoted prescription drugs and that while every effort is made to
review the materials, it cannot guarantee that all materials are reviewed
because of limited resources and competing priorities. FDA officials also
stated that pharmaceutical companies do not always submit promotional
materials as required by regulations and that in such instances FDA would
not have a record of the promotional pieces.
Page 29 GAO-04-110 OxyContin Abuse and Diversion
There are several factors that may have contributed to the abuse and
diversion of OxyContin. OxyContin’s formulation as a controlled-release
opioid that is twice as potent as morphine may have made it an attractive
target for abuse and diversion. In addition, the original label’s safety
warning advising patients not to crush the tablets because of the possible
rapid release of a potentially toxic amount of oxycodone may have
inadvertently alerted abusers to possible methods for misuse. Further, the
rapid growth in OxyContin sales increased the drug’s availability in the
marketplace and may have contributed to opportunities to obtain the drug
illicitly. The history of abuse and diversion of prescription drugs in some
geographic areas, such as those within the Appalachian region, may have
predisposed some states to problems with OxyContin. However, we could
not assess the relationship between the growth in OxyContin prescriptions
or increased availability with the drug’s abuse and diversion because the
data on abuse and diversion are not reliable, comprehensive, or timely.
While OxyContin’s potency and controlled-release feature may have made
the drug beneficial for the relief of moderate-to-severe pain over an
extended period of time, DEA has stated that those attributes of its
formulation have also made it an attractive target for abuse and diversion.
According to recent studies, oxycodone, the active ingredient in
OxyContin, is twice as potent as morphine.
42
In addition, OxyContin’s
controlled-release feature allows a tablet to contain more active ingredient
than other, non-controlled-release oxycodone-containing drugs.
One factor that may have contributed to the abuse and diversion of
OxyContin was FDA’s original decision to label the drug as having less
abuse potential than other oxycodone products because of its controlled-
release formulation. FDA officials said when OxyContin was approved the
agency believed that the controlled-release formulation would result in
less abuse potential because, when taken properly, the drug would be
absorbed slowly, without an immediate rush or high. FDA officials
acknowledged that the initial wording of OxyContin’s label was
“unfortunate” but was based on what was known about the product at that
time.
42
See, for example, G.B. Curtis, et al. “Relative Potency of Controlled-Release Oxycodone
and Morphine in a Postoperative Pain Model,” European Journal of Clinical
Pharmacology, vol. 55, no. 6 (1999): 55:425-429.
Several Factors May
Have Contributed to
OxyContin Abuse and
Diversion, but
Relationship to
Availability Cannot Be
Assessed
OxyContin’s Formulation
May Have Made It an
Inviting Drug for Abuse
and Diversion

Page 30 GAO-04-110 OxyContin Abuse and Diversion
FDA officials told us that abusers typically seek a drug that is intense and
fast-acting. When OxyContin was approved, FDA did not recognize that if
the drug is dissolved in water and injected its controlled-release
characteristics could be disrupted, creating an immediate rush or high and
thereby increasing the potential for misuse and abuse. DEA officials told
us that OxyContin became a target for abusers and diverters because the
tablet contained larger amounts of active ingredient and the controlled-
release formulation was easy for abusers to compromise.
The safety warning on the OxyContin label may also have contributed to
the drug’s potential for abuse and diversion, by inadvertently providing
abusers with information on how the drug could be misused. The label
included the warning that the tablets should not be broken, chewed, or
crushed because such action could result in the rapid release and
absorption of a potentially toxic dose of oxycodone. FDA places similar
safety warnings on other drugs to ensure that they are used properly. FDA
officials stated that neither they nor other experts anticipated that
crushing the controlled-release tablet and intravenously injecting or
snorting the drug would become widespread and lead to a high level of
abuse.
The large amount of OxyContin available in the marketplace may have
increased opportunities for abuse and diversion. Both DEA and Purdue
have stated that an increase in a drug’s availability in the marketplace may
be a factor that attracts interest by those who abuse and divert drugs.
Following its market introduction in 1996, OxyContin sales and
prescriptions grew rapidly through 2002. In 2001 and 2002 combined, sales
of OxyContin approached $3 billion, and over 14 million prescriptions for
the drug were dispensed. (See table 2.) OxyContin also became the top-
selling brand-name narcotic pain reliever in 2001 and was ranked 15th on a
list of the nation’s top 50 prescription drugs by retail sales.
43
43
This information is from the National Institute for Health Care Management’s Prescription
Drug Expenditures reports for 2000 and 2001, prepared using American Institutes for
Research analysis of Scott-Levin Prescription Audit Data. OxyContin was ranked 18th in
2000.
OxyContin’s Wide
Availability May Have
Increased Opportunities
for Illicit Use
Page 31 GAO-04-110 OxyContin Abuse and Diversion
Table 2: Total OxyContin Sales and Prescriptions for 1996 through 2002 with
Percentage Increases from Year to Year
Year Sales
Percentage
increase
Number of
prescriptions
Percentage
increase
1996 $44,790,000 N/A 316,786 N/A
1997 125,464,000 180 924,375 192
1998 286,486,000 128 1,910,944 107
1999 555,239,000 94 3,504,827 83
2000 981,643,000 77 5,932,981 69
2001 1,354,717,000 38 7,183,327 21
2002 1,536,816,000 13 7,234,204 7
Sources: Purdue and IMS Health.
Legend: N/A = not applicable.
Note: GAO analysis of OxyContin sales and prescription data from Purdue and IMS Health, which
includes data from all 50 states and the District of Columbia. Sales include combined retail and
nonretail sales in drugstores, hospitals, and long-term-care facilities from the IMS Health U.S.
National Sales database. Prescriptions include retail pharmacy, long-term-care, and mail-order
prescriptions from IMS Health’s National Prescriptions Audit.
According to DEA, the abuse and diversion of OxyContin in some states
may have reflected the geographic area’s history of prescription drug
abuse. The White House Office of National Drug Control Policy (ONDCP)
designates geographic areas with illegal drug trade activities for allocation
of federal resources to link local, state, and federal drug investigation and
enforcement efforts. These areas, known as High-Intensity Drug
Trafficking Areas (HIDTA), are designated by ONDCP in consultation with
the Attorney General, the Secretary of the Treasury, heads of drug control
agencies, and governors in the states involved.
44
According to a 2001 HIDTA report,
45
the Appalachian region, which
encompasses parts of Kentucky, Tennessee, Virginia, and West Virginia,
44
In making a designation, ONDCP considers whether the geographic area is a center of
drug production, manufacturing, importation, or distribution; whether state and local law
enforcement agencies have committed resources to respond aggressively to the drug
trafficking problem; whether drug activities in the area are having a harmful impact on
other areas of the country; and whether a significant increase in federal resources is
necessary to respond to the area’s drug-related activities.
45
Appalachia High Intensity Drug Trafficking Area Task Force, The OxyContin Threat in
Appalachia (London, Ky.: August 2001).
History of Prescription
Drug Abuse in Some States
May Have Predisposed
Them to Problems with
OxyContin
Page 32 GAO-04-110 OxyContin Abuse and Diversion
has been severely affected by prescription drug abuse, particularly pain
relievers, including oxycodone, for many years. Three of the four states—
Kentucky, Virginia, and West Virginia—were among the initial states to
report OxyContin abuse and diversion. Historically, oxycodone,
manufactured under brand names such as Percocet, Percodan, and Tylox,
was among the most diverted prescription drugs in Appalachia. According
to the report, OxyContin has become the drug of choice of abusers in
several areas within the region. The report indicates that many areas of the
Appalachian region are rural and poverty-stricken, and the profit potential
resulting from the illicit sale of OxyContin may have contributed to its
diversion and abuse. In some parts of Kentucky, a 20-milligram OxyContin
tablet, which can be purchased by legitimate patients for about $2, can be
sold illicitly for as much as $25. The potential to supplement their incomes
can lure legitimate patients into selling some of their OxyContin to street
dealers, according to the HIDTA report.
The databases DEA uses to track the abuse and diversion of controlled
substances all have limitations that prevent an assessment of the
relationship between the availability of OxyContin and areas where the
drug is being abused or diverted. Specifically, these databases, which
generally do not provide information on specific brand-name drugs such
as OxyContin, are based on data gathered from limited sources in specific
geographic areas and have a significant time lag. As a result, they do not
provide reliable, complete, or timely information that could be used to
identify abuse and diversion of a specific drug.
DEA officials told us that it is difficult to obtain reliable data on what
controlled substances are being abused by individuals and diverted from
pharmacies because available drug abuse and diversion tracking systems
do not capture data on a specific brand-name product or indicate where a
drug product is being abused and diverted on a state and local level.
Because of the time lags in reporting information, the data reflect a
delayed response to any emerging drug abuse and diversion problem. For
example, the Drug Abuse Warning Network (DAWN) estimates national
drug-related emergency department visits or deaths involving abused
drugs using data collected by the Substance Abuse and Mental Health
Services Administration (SAMHSA). The data are collected from hospital
emergency departments in 21 metropolitan areas that have agreed to
voluntarily report drug-abuse-related information from a sample of patient
Limitations on Abuse and
Diversion Data Prevent
Assessment of the
Relationship with
OxyContin’s Availability

Page 33 GAO-04-110 OxyContin Abuse and Diversion
medical records, and from medical examiners in 42 metropolitan areas.
46
However, DAWN cannot make estimates for rural areas, where initial
OxyContin abuse and diversion problems were reported to be most
prevalent, nor does it usually provide drug-product-specific information,
and its data have a lag time of about 1 year. DEA stated that development
of enhanced data collection systems is needed to provide “credible, legally
defensible evidence concerning drug abuse trends in America.”
47
DEA relies primarily on reports from its field offices to determine where
abuse and diversion are occurring. DEA officials stated that the initial
areas that experienced OxyContin abuse and diversion problems included
rural areas within 8 states—Alaska, Kentucky, Maine, Maryland, Ohio,
Pennsylvania, Virginia, and West Virginia. In July 2002, DEA told us that it
learned that OxyContin abuse and diversion problems had spread into
larger areas of the initial 8 states, as well as parts of 15 other states, to
involve almost half of the 50 states.
48
According to DEA officials, while
DEA field offices continue to report OxyContin as a drug of choice among
abusers, OxyContin has not been and is not now considered the most
highly abused and diverted prescription drug nationally.
49
OxyContin is the
most abused single-entity prescription product according to those DEA
state and divisional offices that report OxyContin abuse.
46
The reliability of the data collected depends on whether the emergency room patient visit
was reported as drug related, whether the patient reported taking a particular drug, and
whether the emergency room physician indicated a drug’s brand name in the patient’s
medical record.
47
See app. III for more details on the abuse and diversion databases DEA uses.
48
The 15 states are Alabama, Arizona, Colorado, Connecticut, Florida, Louisiana,
Massachusetts, Mississippi, Missouri, New Jersey, North Carolina, South Carolina, Texas,
Washington, and Wisconsin.
49
Hydrocodone products, such as Anexsia, Hycodan, Lorcet, Lortab, and Vicodin, remain
among the most abused and diverted scheduled prescription drugs nationally.
Page 34 GAO-04-110 OxyContin Abuse and Diversion
Since becoming aware of reports of abuse and diversion of OxyContin,
federal and state agencies and Purdue have taken actions intended to
address these problems. To protect the public health, FDA has
strengthened OxyContin label warnings and requested that Purdue
develop and implement an OxyContin risk management plan. In addition,
DEA has stepped up law enforcement actions to prevent abuse and
diversion of OxyContin. State Medicaid fraud control units have also
attempted to identify those involved in the abuse and diversion of
OxyContin. Purdue has initiated drug abuse and diversion education
programs, taken disciplinary actions against sales representatives who
improperly promote OxyContin, and referred physicians who were
suspected of improperly prescribing OxyContin to the appropriate
authorities. However, until fall 2002 Purdue did not analyze its
comprehensive physician prescribing reports, which it routinely uses in
marketing and promoting OxyContin, and other indicators to identify
possible physician abuse and diversion.
Reports of abuse and diversion of OxyContin that were associated with an
increasing incidence of addiction, overdose, and death prompted FDA to
revise the drug’s label and take other actions to protect the public health.
In July 2001, FDA reevaluated OxyContin’s label and made several changes
in an effort to strengthen the “Warnings” section of the label. FDA added a
subsection—“Misuse, Abuse, and Diversion of Opioids”—to stress that
physicians and pharmacists should be alert to the risk of misuse, abuse,
and diversion when prescribing or dispensing OxyContin. FDA also added
a black box warning—the highest level of warning FDA can place on an
approved drug product. FDA highlighted the language from the original
1995 label—stating that OxyContin is a schedule II controlled substance
with an abuse liability similar to morphine—by moving it into the black
box. Also, while the original label suggested that taking broken, chewed,
or crushed OxyContin tablets “could lead to the rapid release and
absorption of a potentially toxic dose of oxycodone,” a more strongly
worded warning in the black box stated that taking the drug in this manner
“leads to rapid release and absorption of a potentially fatal dose of
oxycodone” (emphasis added). (See table 3.) In addition to the black box
warning, FDA also changed the language in the original label that
described the incidence of addiction inadvertently induced by physician
prescribing as rare if opioids are legitimately used in the management of
pain. The revised label stated that data are not available to “establish the
true incidence of addiction in chronic patients.”
Federal and State
Agencies and Purdue
Have Taken Actions
to Prevent Abuse and
Diversion of
OxyContin
Reports of Abuse and
Diversion Led to Label
Changes and Other Actions
by FDA
Page 35 GAO-04-110 OxyContin Abuse and Diversion
Table 3: Selected Language Approved by FDA in Warning Sections of OxyContin
Labels, 1995 and 2001
Warning label in 1995 Black box warning in 2001
“Warning:
OxyContin Tablets are to be swallowed
whole, and are not to be broken, chewed, or
crushed. Taking broken, chewed, or crushed
OxyContin Tablets could lead to the rapid
release and absorption of a potentially toxic
dose of oxycodone.”
“Warning: OxyContin is an opioid agonist
and a Schedule II controlled substance
with an abuse liability similar to
morphine.”
“OxyContin Tablets are to be swallowed
whole and are not to be broken, chewed,
or crushed. Taking broken, chewed, or
crushed OxyContin Tablets leads to rapid
release and absorption of a potentially
fatal dose of oxycodone.” (emphasis
added)
Source: FDA-approved label for Purdue’s OxyContin.
As mentioned earlier, the indication described in the original label was
also revised to clarify the appropriate time period for which OxyContin
should be prescribed for patients experiencing moderate-to-severe pain.
The language in the 1995 label was changed from “where use of an opioid
analgesic is appropriate for more than a few days” to “when a continuous,
around-the-clock analgesic is needed for an extended period of time.” (See
table 4.) A summary of changes made by FDA to the original OxyContin
label is given in appendix II.
Table 4: Selected Language Approved by FDA in the Indication Sections of
OxyContin Labels, 1995 and 2001
Indication in 1995 Black box indication change in 2001
“OxyContin Tablets are a controlled-release
oral formulation of oxycodone hydrochloride
indicated for the management of moderate-
to-severe pain where use of an opioid
analgesic is appropriate for more than a few
days.”
“OxyContin Tablets are a controlled-release
oral formulation of oxycodone hydrochloride
indicated for the management of moderate-
to-severe pain when a continuous,
around-the-clock analgesic is needed
for an extended period of time.”
(emphasis added)
Source: FDA-approved label for Purdue’s OxyContin.
Beginning in early 2001, FDA collaborated with Purdue to develop and
implement a risk management plan to help identify and prevent abuse and
diversion of OxyContin. As a part of the risk management plan in
connection with the labeling changes, Purdue was asked by FDA to revise
all of its promotional materials for OxyContin to reflect the labeling

Page 36 GAO-04-110 OxyContin Abuse and Diversion
changes. In August 2001, FDA sent a letter to Purdue stating that all future
promotional materials for OxyContin should prominently disclose the
information contained in the boxed warning; the new warnings that
address misuse, abuse, diversion, and addiction; and the new precautions
and revised indication for OxyContin. Purdue agreed to comply with this
request.
FDA officials told us that it is standard procedure to contact a drug
manufacturer when the agency becomes aware of reports of abuse and
diversion of a drug product so that FDA and the drug manufacturer can
tailor a specific response to the problem. While FDA’s experience with
risk management plans is relatively new, agency officials told us that
OxyContin provided the opportunity to explore the use of the plans to help
identify abuse and diversion problems. FDA is currently making decisions
about whether risk management plans will be requested for selected
opioid products. Also, in September 2003, FDA’s Anesthetic and Life
Support Drugs Advisory Committee held a public hearing to discuss its
current review of proposed risk management plans for opioid analgesic
drug products to develop strategies for providing patients with access to
pain treatment while limiting the abuse and diversion of these products.
FDA has also taken other actions to address the abuse and diversion of
OxyContin. It put information on its Web site for patients regarding the
appropriate use of OxyContin.
50
FDA worked with Purdue to develop
“Dear Health Care Professional” letters, which the company distributed
widely to health care professionals to alert them that the package insert
had been revised to clarify the indication and strengthen the warnings
related to misuse, abuse, and diversion. FDA also has worked with DEA,
SAMHSA, the National Institute on Drug Abuse, ONDCP, and the Centers
for Disease Control and Prevention to share information and insights on
the problem of abuse and diversion of OxyContin.
In April 2001, DEA developed a national action plan to deter abuse and
diversion of OxyContin. According to DEA officials, this marked the first
time the agency had targeted a specific brand-name product for
monitoring because of the level and frequency of abuse and diversion
associated with the drug. Key components of the action plan include
coordinating enforcement and intelligence operations with other law
50
See www.fda.gov/cder/drug/infopage/oxycontin/default.htm.
DEA Developed an Action
Plan to Deter OxyContin
Abuse and Diversion
Page 37 GAO-04-110 OxyContin Abuse and Diversion
enforcement agencies to target people and organizations involved in abuse
and diversion of OxyContin, pursuing regulatory and administrative action
to limit abusers’ access to OxyContin, and building national outreach
efforts to educate the public on the dangers related to the abuse and
diversion of OxyContin. DEA has also set Purdue’s procurement quota for
oxycodone at levels lower than the levels requested by Purdue.
DEA has increased enforcement efforts to prevent abuse and diversion of
OxyContin. From fiscal year 1996 through fiscal year 2002, DEA initiated
313 investigations involving OxyContin, resulting in 401 arrests. Most of
the investigations and arrests occurred after the initiation of the action
plan. Since the plan was enacted, DEA initiated 257 investigations and
made 302 arrests in fiscal years 2001 and 2002. Among those arrested were
several physicians and pharmacists. Fifteen health care professionals
either voluntarily surrendered their controlled substance registrations or
were immediately suspended from registration by DEA. In addition, DEA
reported that $1,077,500 in fines was assessed and $742,678 in cash was
seized by law enforcement agencies in OxyContin-related cases in 2001
and 2002.
Among several regulatory and administrative actions taken to limit
abusers’ access to OxyContin and controlled substances, DEA’s Office of
Diversion Control, in collaboration with the Department of Justice’s Office
of Justice Programs, Bureau of Justice Assistance, provides grants to
states for the establishment of prescription drug monitoring programs. The
conference committee report for the fiscal year 2002 appropriation to the
Department of Justice directed the Office of Justice Programs to make a
$2 million grant in support of the Harold Rogers Prescription Drug
Monitoring Program, which enhances the capacity of regulatory and law
enforcement agencies to collect and analyze controlled substance
prescription data. The program provided grants to establish new
monitoring programs in Ohio, Pennsylvania, Virginia, and West Virginia.
California, Kentucky, Massachusetts, Nevada, and Utah also received
grants to enhance existing monitoring programs.
DEA has also attempted to raise national awareness of the dangers
associated with abuse and diversion of OxyContin. In October 2001 DEA
joined 21 national pain and health organizations in issuing a consensus
statement calling for a balanced policy on prescription medication use.
According to the statement, such a policy would acknowledge that health
care professionals and DEA share responsibility for ensuring that
prescription medications, such as OxyContin, are available to patients who
need them and for preventing these drugs from becoming a source of

Page 38 GAO-04-110 OxyContin Abuse and Diversion
abuse and diversion. DEA and the health organizations also called for a
renewed focus on educating health professionals, law enforcement, and
the public about the appropriate use of opioid pain medications in order to
promote responsible prescribing and limit instances of abuse and
diversion. DEA is also working with FDA to encourage state medical
boards to require, as a condition of their state licensing, that physicians
obtain continuing medical education on pain management.
When OxyContin was first introduced to the market in 1996, DEA granted
Purdue’s initial procurement quota request for oxycodone. According to
DEA, increases in the quota were granted for the first several years.
Subsequently, concern over the dramatic increases in sales caused DEA to
request additional information to support Purdue’s requests to increase
the quota. In the last several years, DEA has taken the additional step of
lowering the procurement quota requested by Purdue for the manufacture
of OxyContin as a means for addressing abuse and diversion. However,
DEA has cited the difficulty of determining an appropriate level while
ensuring that adequate quantities were available for legitimate medical
use, as there are no direct measures available to establish legitimate
medical need.
State Medicaid fraud control units and medical licensure boards have
taken action in response to reports of abuse and diversion of OxyContin.
State Medicaid fraud control units have conducted investigations of abuse
and diversion of OxyContin, but generally do not maintain precise data on
the number of investigations and enforcement actions completed.
Although complete information was not available from directors of state
Medicaid fraud control units in Kentucky, Maryland, Pennsylvania,
Virginia, and West Virginia with whom we spoke, each of those directors
told us that abuse and diversion of OxyContin is a problem in his or her
state. The directors told us that they had investigated cases that involved
physicians or individuals who had either been indicted or prosecuted for
writing medically unnecessary OxyContin prescriptions in exchange for
cash or sexual relationships.
State medical licensure boards have also responded to complaints about
physicians who were suspected of abuse and diversion of controlled
substances, but like the Medicaid fraud control units, the boards generally
do not maintain data on the number of investigations that involved
OxyContin. Representatives of state boards of medicine in Kentucky,
Pennsylvania, Virginia, and West Virginia told us that they have received
complaints from various sources, such as government agencies, health
State Agencies Have
Responded to Reports of
OxyContin Abuse and
Diversion

Page 39 GAO-04-110 OxyContin Abuse and Diversion
care professionals, and anonymous tipsters, about physicians suspected of
abuse and diversion of controlled substances. However, each of the four
representatives stated that his or her board does not track the complaints
by specific drug type and consequently cannot determine whether the
complaints received allege physicians’ misuse of OxyContin. Each of the
four representatives also told us that his or her medical licensure board
has adopted or strengthened guidelines or regulations for physicians on
prescribing, administering, and dispensing controlled substances in the
treatment of chronic pain. For example, in March 2001, the Kentucky
Board of Medical Licensure adopted guidelines to clarify the board’s
position on the use of controlled substances for nonterminal/nonmalignant
chronic pain.
51
The boards of medicine in Pennsylvania, Virginia, and West
Virginia each have guidelines for the appropriate use of controlled
substances that are similar to those adopted by Kentucky.
In response to concerns about abuse and diversion of OxyContin, in April
2001 FDA and Purdue began to discuss the development of a risk
management plan to help detect and prevent abuse and diversion of
OxyContin. Purdue submitted its risk management plan to FDA for review
in August 2001.
52
The plan includes some actions that Purdue proposed to
take, as well as others that it has already taken. Purdue’s risk management
plan includes actions such as strengthening the safety warnings on
OxyContin’s label for professionals and patients, training Purdue’s sales
force on the revised label, conducting comprehensive education programs
for health care professionals, and developing a database for identifying
and monitoring abuse and diversion of OxyContin.
Under the risk management plan, OxyContin’s label was strengthened,
effective in July 2001, by revising the physician prescribing information
and adding a black box warning to call attention to OxyContin’s potential
51
The Kentucky guidelines for the use of controlled substances in pain treatment provide
that (1) a complete medical history and examination be conducted and documented in
patient medical records, (2) a written treatment plan state objectives for determining
treatment success, (3) the risks and benefits of the use of controlled substances be
discussed by physician and patient, (4) periodic review of the course of treatment be
conducted, (5) consultation or referral to an expert in pain management be considered for
patients who are at risk for substance abuse, (6) patient’s medical record be kept accurate
and complete, and (7) physicians be in compliance with applicable federal and state
controlled substance laws and regulations.
52
Amended versions of Purdue’s risk management plan for OxyContin were submitted to
FDA for review in April 2002 and in March 2003.
Purdue Is Implementing a
Risk Management Plan for
OxyContin

Page 40 GAO-04-110 OxyContin Abuse and Diversion
for misuse, abuse, and diversion. (See app. II.) Purdue trained its sales
force on the specifics of the revised label and provided sales
representatives with updated information on the appropriate use of opioid
analgesics, legal guidelines associated with promotion of its products, and
their responsibility and role in reporting adverse events. Purdue also
reiterated to its sales representatives that failure to promote products
according to the approved label, promotional materials, and applicable
FDA standards would result in disciplinary action by the company.
According to Purdue, from April 2001 through May 2003 at least 10 Purdue
employees were disciplined for using unapproved materials in promoting
OxyContin. Disciplinary actions included warning letters, suspension
without pay, and termination.
Purdue also has provided education programs for health care
professionals and the public under its risk management plan. For example,
in 2001 Purdue supported seminars that examined ways health care
professionals can help prevent abuse and diversion of opioids. Purdue
worked with DEA and other law enforcement agencies to develop and
implement antidiversion educational programs. In 2002, Purdue also
launched the Web site painfullyobvious.com to educate teenagers, parents,
law enforcement officers, and discussion leaders about the dangers of
prescription drug abuse.
Because reliable data on the abuse and diversion of controlled substance
drugs are not available, Purdue developed the Researched Abuse,
Diversion, and Addiction-Related Surveillance (RADARS) System, as part
of its risk management plan, to study the nature and extent of abuse of
OxyContin and other schedule II and III prescription medications and to
implement interventions to reduce abuse and diversion.
53
According to
Purdue, RADARS collects and computes abuse, diversion, and addiction
rates for certain drugs based on population and determines national and
local trends.
Since the launch of OxyContin, Purdue has provided its sales force with
considerable information to help target physicians and prioritize sales
contacts within a sales territory. Sales representatives routinely receive
daily, weekly, monthly, and quarterly physician prescribing reports based
53
RADARS will collect information on brand-name and generic versions of buprenorphine,
fentanyl, hydrocodone, hydromorphone, oxycodone, morphine, and methadone.
Benzodiazepine is scheduled to be added to RADARS in late 2003.

Page 41 GAO-04-110 OxyContin Abuse and Diversion
on IMS Health data that specify the physicians who have written
prescriptions for OxyContin and other opioid analgesics, and the number
of prescriptions written. Although this information has always been
available for use by Purdue and its sales representatives, it was not until
fall 2002 that Purdue directed its sales representatives to begin using 11
indicators to identify possible abuse and diversion and to report the
incidents to Purdue’s General Counsel’s Office for investigation. Among
the possible indicators are a sudden unexplained change in a physician’s
prescribing patterns that is not accounted for by changes in patient
numbers, information from credible sources such as a pharmacist that a
physician or his or her patients are diverting medications, or a physician
who writes a large number of prescriptions for patients who pay with
cash. As of September 2003, Purdue—through its own investigations—had
identified 39 physicians and other health care professionals who were
referred to legal, medical, or regulatory authorities for further action. Most
of the 39 referrals stemmed from reports by Purdue’s sales force.
Other actions included in the plan that were taken by Purdue prior to
submission of its risk management plan include discontinuance of the 160-
milligram tablet of OxyContin to reduce the risk of overdose from this
dosage strength, the development of unique markings for OxyContin
tablets intended for distribution in Mexico and Canada to assist law
enforcement in identifying OxyContin illegally smuggled into the United
States, and the distribution of free tamper-resistant prescription pads
designed to prevent altering or copying of the prescription. Purdue also
implemented a program in 2001 to attempt to predict “hot spots” where
OxyContin abuse and diversion were likely to occur, but discontinued the
program in 2002 when Purdue concluded that nearly two-thirds of the
counties identified had no abuse and diversion.
At present, both federal agencies and the states have responsibilities
involving prescription drugs and their abuse and diversion. FDA is
responsible for approving new drugs and ensuring that the materials drug
companies use to market and promote these drugs are truthful, balanced,
and accurate. However, FDA examines these promotional materials only
after they have been used in the marketplace because the FD&C Act
generally does not give FDA authority to review these materials before the
drug companies use them. Moreover, the FD&C Act provisions governing
drug approval and promotional materials make no distinction between
controlled substances, such as OxyContin, and other prescription drugs.
DEA is responsible for registering handlers of controlled substances,
approving production quotas and monitoring distribution of controlled
Conclusions

Page 42 GAO-04-110 OxyContin Abuse and Diversion
substances to the retail level. It is the states, however, that are responsible
for overseeing the practice of medicine and pharmacy where drugs are
prescribed and dispensed. Some states have established prescription drug
monitoring programs to help them detect and deter abuse and diversion.
However, these programs exist in only 15 states and most do not
proactively analyze prescription data to identify individuals, physicians, or
pharmacies that have unusual use, prescribing, or dispensing patterns that
may suggest potential drug diversion or abuse.
The significant growth in the use of OxyContin to treat patients suffering
from chronic pain has been accompanied by widespread reports of abuse
and diversion that have in some cases led to deaths. The problem of abuse
and diversion has highlighted shortcomings at the time of approval in the
labeling of schedule II controlled substances, such as OxyContin, and in
the plans in place to detect misuse, as well as in the infrastructure for
detecting and preventing the abuse and diversion of schedule II controlled
substances already on the market.
Addressing abuse and diversion problems requires the collaborative
efforts of pharmaceutical manufacturers; the federal and state agencies
that oversee the approval and use of prescription drugs, particularly
controlled substances; the health care providers who prescribe and
dispense them; and law enforcement. After the problems with OxyContin
began to surface, FDA and Purdue collaborated on a risk management
plan to help detect and prevent abuse and diversion. Although risk
management plans were not in use when OxyContin was approved, they
are now an optional feature of new drug applications. FDA plans to
complete its guidance to the pharmaceutical industry on risk management
plans by September 30, 2004. The development of this guidance, coupled
with FDA’s current review of proposed risk management plans for
modified-release opioid analgesics, provides an opportunity to help ensure
that manufacturers include a strategy to monitor the use of these drugs
and to identify potential problems with abuse and diversion.
To improve efforts to prevent or identify the abuse and diversion of
schedule II controlled substances, we recommend that the Commissioner
of Food and Drugs ensure that FDA’s risk management plan guidance
encourages pharmaceutical manufacturers that submit new drug
applications for these substances to include plans that contain a strategy
for monitoring the use of these drugs and identifying potential abuse and
diversion problems.
Recommendation for
Executive Action

Page 43 GAO-04-110 OxyContin Abuse and Diversion
We provided a draft of this report to FDA, DEA, and Purdue, the
manufacturer of OxyContin, for their review. FDA and DEA provided
written comments. (See apps. IV and V.) Purdue’s representatives provided
oral comments.
FDA said that it agreed with our recommendation that its risk
management plan guidance should encourage all pharmaceutical
manufacturers submitting new drug applications for schedule II controlled
substances to include strategies to address abuse and diversion concerns.
FDA stated that the agency is working on the risk management plan
guidance. FDA also noted that the FD&C Act makes no distinction
between controlled substances and other prescription drugs in its
provisions regulating promotion, but that as a matter of general policy, the
agency more closely scrutinizes promotion of drugs with more serious risk
profiles. However, FDA does not have written guidance that specifies that
promotional materials for controlled substances receive priority or special
attention over similar materials for other prescription drugs. Furthermore,
our finding that FDA did not review any of the OxyContin promotional
videos provided by Purdue until we brought them to the agency’s attention
raises questions about whether FDA provides extra attention to
promotional materials for controlled substances that by definition have a
high potential for abuse and may lead to severe psychological or physical
dependence. FDA recommended that we clarify our description of the
content of the warning letter issued to Purdue and provide additional
information describing the extent of the corrective action taken by
Purdue. FDA also recommended noting in the report that part of the risk
management plan in connection with the 2001 labeling changes was a
requirement that all OxyContin promotional materials be revised to reflect
the labeling changes and all future materials prominently disclose this
information. Finally, FDA noted that the promotional videos discussed in
the report were submitted by Purdue prior to the labeling change and
discontinued as a result of the labeling change. As we note in the report,
Purdue acknowledged that all the promotional videos were not submitted
to FDA at the time they were distributed. Moreover, although Purdue told
us that these videos were no longer distributed after the label change,
those videos that had been distributed were not collected and destroyed.
We revised the report to reflect FDA’s general comments. FDA also
provided technical comments that we incorporated where appropriate.
In its written comments, DEA agreed that the data on abuse and diversion
are not reliable, comprehensive, or timely, as we reported. DEA reiterated
its previous statement that Purdue’s aggressive marketing of OxyContin
fueled demand for the drug and exacerbated the drug’s abuse and
Agency and Purdue
Comments and Our
Evaluation
Page 44 GAO-04-110 OxyContin Abuse and Diversion
diversion. DEA also stated that Purdue minimized the abuse risk
associated with OxyContin. We agree with DEA that Purdue conducted an
extensive campaign to market and promote OxyContin using an expanded
sales force and multiple promotional approaches to encourage physicians,
including primary care specialists, to prescribe OxyContin as an initial
opioid treatment for noncancer pain, and that these efforts may have
contributed to the problems with abuse and diversion by increasing the
availability of the drug in the marketplace. However, we also noted that
other factors may have contributed to these problems. We also agree that
Purdue marketed OxyContin as having a low abuse liability, but we noted
that this was based on information in the original label approved by FDA.
DEA also acknowledged that the lack of a real measure of legitimate
medical need for a specific product (OxyContin), substance (oxycodone),
or even a class of substances (controlled release opioid analgesics) makes
it difficult to limit manufacturing as a means of deterring abuse and
diversion. DEA also noted that it is essential that risk management plans
be put in place prior to the introduction of controlled substances into the
marketplace, consistent with our recommendation. We revised the report
to provide some additional detail on problems associated with OxyContin
and Purdue’s marketing efforts. DEA provided some technical comments
on the draft report that we incorporated where appropriate.
Purdue representatives provided oral comments on a draft of this report.
In general, they thought the report was fair and balanced; however, they
offered both general and technical comments. Specifically, Purdue stated
that the report should add the media as a factor contributing to the abuse
and diversion of OxyContin because media stories provided the public
with information on how to “get high” from using OxyContin incorrectly.
Our report notes that the safety warning on the original label may have
inadvertently alerted abusers to a possible method for misusing the drug.
However, we note that the original label was publicly available from FDA
once OxyContin was approved for marketing. Purdue also suggested that
we include Duragesic, also a schedule II opioid analgesic, as a fourth
comparable drug to OxyContin. The three comparable drugs we used in
the report were chosen in consultation with FDA as comparable opioid
analgesics to OxyContin, because they were time-released, morphine-
based schedule II drugs formulated as tablets like OxyContin. In contrast,
Duragesic, which contains the opioid analgesic fentanyl and provides pain
relief over a 72-hour period, is formulated as a skin patch to be worn
rather than as a tablet. Purdue representatives also provided technical
comments that were incorporated where appropriate.

Page 45 GAO-04-110 OxyContin Abuse and Diversion
We also provided sections of this draft report to the manufacturers of
three comparative drugs we examined. Two of the three companies with a
drug product used as a comparable drug to OxyContin reviewed the
portions of the draft report concerning their own product, and provided
technical comments, which were incorporated where appropriate. The
third company did not respond to our request for comments.
As agreed with your offices, unless you publicly announce this report’s
contents earlier, we plan no further distribution until 30 days after its issue
date. At that time, we will send copies of this report to the Commissioner
of Food and Drugs, the Administrator of the Drug Enforcement
Administration, Purdue, and the other pharmaceutical companies whose
drugs we examined. We will also make copies available to others upon
request. In addition, the report will be available at no charge on the GAO
Web site at http://www.gao.gov.
If you or your staffs have any questions about this report, please call me at
(202) 512-7119 or John Hansen at (202) 512-7105. Major contributors to
this report were George Bogart, Darryl Joyce, Roseanne Price, and Opal
Winebrenner.
Marcia Crosse
Director, Health Care—Public Health
and Military Health Care Issues
Appendix I: Scope and Methodology
Page 46 GAO-04-110 OxyContin Abuse and Diversion
To identify the strategies and approaches used by Purdue Pharma L.P.
(Purdue) to market and promote OxyContin, we interviewed Purdue
officials and analyzed company documents and data. Specifically, we
interviewed Purdue officials concerning its marketing and promotional
strategies for OxyContin, including its targeting of physicians with specific
specialties and its sales compensation plan to provide sales
representatives with incentives for the drug’s sales. We also interviewed
selected Purdue sales representatives who had high and midrange sales
during 2001 from Kentucky, Pennsylvania, Virginia, and West Virginia—
four states that were initially identified by the Drug Enforcement Agency
(DEA) as having a high incidence of OxyContin drug abuse and
diversion—and from California, Massachusetts, and New Jersey—three
states that DEA did not initially identify as having problems with
OxyContin. We asked the sales representatives about their training,
promotional strategies and activities, and targeting of physicians. We also
interviewed physicians who were among the highest prescribers of
OxyContin regarding their experiences with Purdue sales representatives,
including the strategies used to promote OxyContin, as well as their
experiences with sales representatives of manufacturers of other opioid
analgesics. We reviewed Purdue’s quarterly action plans for marketing and
promoting OxyContin for 1996 through 2003, Purdue’s sales representative
training materials, and materials from ongoing OxyContin-related
litigation. To obtain information on how Purdue’s marketing and
promotion of OxyContin compared to that of other companies, we
identified, in consultation with the Food and Drug Administration (FDA),
three opioid analgesics that were similar to OxyContin. The three drugs—
Avinza, Kadian, and Oramorph SR—are all time-released, morphine-based
analgesics that are classified as schedule II controlled substances. We
examined the promotional materials each drug’s manufacturer submitted
to FDA and any actions FDA had taken against the manufacturers related
to how the drugs were marketed or promoted. We also interviewed
company officials about how they marketed and promoted their respective
drugs. Because of their concerns about proprietary information, the three
companies did not provide us with the same level of detail about their
drugs’ marketing and promotion as did Purdue.
To examine factors that contributed to the abuse and diversion of
OxyContin, we reviewed DEA abuse and diversion data as part of an effort
to compare them with DEA’s OxyContin state distribution data and with
IMS Health data on the rates of OxyContin sales and prescription
dispensing to determine if they occurred in similar geographic areas. We
also analyzed the distribution of Purdue sales representatives by state and
compared them with the availability of OxyContin and abuse and diversion
Appendix I: Scope and Methodology
Appendix I: Scope and Methodology
Page 47 GAO-04-110 OxyContin Abuse and Diversion
data to determine whether states with high rates of OxyContin sales and
prescription dispensing and abuse and diversion problems had more sales
representatives per capita than other states. However, limitations in the
abuse and diversion data prevent an assessment of the relationship
between the availability of OxyContin and areas where the drug was
abused and diverted. We also reviewed the High Intensity Drug Trafficking
Area (HIDTA) reports on states with histories of illegal drug activities. We
interviewed DEA and FDA officials, physicians who prescribed
OxyContin, officials from physician licensing boards in selected states,
officials from national health practitioner groups, and company officials
and sales representatives about why OxyContin abuse and diversion have
occurred.
To determine the efforts federal and state agencies and Purdue have made
to identify and prevent abuse and diversion of controlled substances such
as OxyContin, we interviewed FDA officials and analyzed information
from FDA regarding the marketing and promotion of controlled
substances, specifically OxyContin; FDA’s decision to approve the original
label for OxyContin; and FDA’s subsequent decision to revise OxyContin’s
labeling, as well as FDA’s role in monitoring OxyContin’s marketing and
advertising activities. We also interviewed DEA officials about the
agency’s efforts to identify and prevent abuse and diversion, including its
national action plan for OxyContin, and how it determines the prevalence
of OxyContin abuse and diversion nationally. We also interviewed officials
from national practitioner associations, Medicaid fraud control units, and
physician licensing boards in states with initial reports of abuse and
diversion—Kentucky, Maryland, Pennsylvania, Virginia, and West
Virginia—regarding concerns they had about the abuse and diversion of
OxyContin. We reviewed Purdue’s OxyContin risk management plan
submissions to FDA from 2001 through 2003 to identify actions taken by
Purdue to address abuse and diversion of OxyContin.
Appendix II: Summary of FDA Changes to the
Original Approved OxyContin Label
Page 48 GAO-04-110 OxyContin Abuse and Diversion
Table 5 provides a description of the changes made by FDA to sections of
the original OxyContin approved label from June 1996 through July 2001.
These changes included a black box warning, the strongest warning an
FDA-approved drug can carry, and specifically addressed areas of concern
related to the opioid characteristics of oxycodone and its risk of abuse and
diversion.
Table 5: FDA Changes to the Original OxyContin Label Made from June 1996 through July 2001
Summary of FDA changes to original
OxyContin label in 2001 Language in OxyContin label approved in 2001
Black box warning was added to stress the opioid
nature of oxycodone and risks for abuse and
diversion of the drug.
“WARNING:
OxyContin is an opioid agonist and a Schedule II controlled substance
with an abuse liability similar to morphine.
Oxycodone can be abused in a manner similar to other opioid agonists, legal or
illicit. This should be considered when prescribing or dispensing OxyContin in
situations where the physician or pharmacist is concerned about an increased
risk of misuse, abuse, or diversion.
OxyContin Tablets are a controlled-release oral formulation of oxycodone
hydrochloride indicated for the management of moderate to severe pain
when a continuous, around-the-clock analgesic is needed for an extended
period of time.
OxyContin Tablets are NOT intended for use as a prn analgesic. OxyContin 80
mg and 160 mg Tablets ARE FOR USE IN OPIOID-TOLERANT PATIENTS
ONLY. These tablet strengths may cause fatal respiratory depression
when administered to patients not previously exposed to opioids.
OxyContin TABLETS ARE TO BE SWALLOWED WHOLE AND ARE NOT
TO BE BROKEN, CHEWED, OR CRUSHED. TAKING BROKEN, CHEWED,
OR CRUSHED OxyContin TABLETS LEADS TO RAPID RELEASE AND
ABSORPTION OF A POTENTIALLY FATAL DOSE OF OXYCODONE.”
Clinical pharmacology
—Provides a pharmacological description of
oxycodone as a pure opioid agonist whose principal
action is analgesia.
—Identifies other members of the opioid agonist
class, such as morphine, hydromorphone, fentanyl,
and hydrocodone.
—Describes the pharmacological properties of
opioids in general (anxiolysis, euphoria, feelings of
relaxation, respiratory depression, constipation,
miosis, cough suppression, and analgesia).
—Describes respiratory depression as one of the
most serious side effects of opioids that could lead
to overdose or death.
“CLINICAL PHARMACOLOGY
Oxycodone is a pure agonist opioid whose principal therapeutic action is
analgesia. Other members of the class known as opioid agonists include
substances such as morphine, hydromorphone, fentanyl, codeine, and
hydrocodone. Pharmacological effects of opioid agonists include anxiolysis,
euphoria, feelings of relaxation, respiratory depression, constipation, miosis,
and cough suppression, as well as analgesia. Like all pure opioid agonist
analgesics, with increasing doses there is increasing analgesia, unlike with
mixed agonist/antagonists or non-opioid analgesics, where there is a limit to the
analgesic effect with increasing doses. With pure opioid agonist analgesics,
there is no defined maximum dose; the ceiling to analgesic effectiveness is
imposed only by side effects, the more serious of which may include
somnolence and respiratory depression.”
Appendix II: Summary of FDA Changes to the
Original Approved OxyContin Label
Appendix II: Summary of FDA Changes to the
Original Approved OxyContin Label
Page 49 GAO-04-110 OxyContin Abuse and Diversion
Summary of FDA changes to original
OxyContin label in 2001 Language in OxyContin label approved in 2001
Misuse, abuse, and diversion of opioids
A subsection on misuse, abuse and diversion was
added to the WARNINGS section of the label.
—Characterizes oxycodone as an opioid agonist of
the morphine-type and stresses that opioid agonists
are sought by drug abusers and people with
addiction disorders and are subject to diversion.
—Makes clear that oxycodone can be abused in a
manner similar to other opioid agonists, legal or
illicit, and that physicians and pharmacists should
be aware of and alert to risk of misuse, abuse, and
diversion when prescribing or dispensing
oxycodone.
—Modifies original label statement that iatrogenic
addiction (addiction induced inadvertently by a
physician or a physician’s treatment) is rare if
opioids were legitimately used in the management
of pain to state that data are not available to
establish the true incidence of addiction in chronic
patients.
“Misuse, Abuse and Diversion of Opioids
Oxycodone is an opioid agonist of the morphine-type. Such drugs are sought by
drug abusers and people with addiction disorders and are subject to criminal
diversion.
Oxycodone can be abused in a manner similar to other opioid agonists, legal or
illicit. This should be considered when prescribing or dispensing OxyContin in
situations where the physician or pharmacist is concerned about an increased
risk of misuse, abuse, or diversion.
OxyContin has been reported as being abused by crushing, chewing, snorting,
or injecting the dissolved product. These practices will result in the uncontrolled
delivery of the opioid and pose a significant risk to the abuser that could result
in overdose and death (see WARNINGS and DRUG ABUSE AND
ADDICTION).
Concerns about abuse, addiction, and diversion should not prevent the proper
management of pain. The development of addiction to opioid analgesics in
properly managed patients with pain has been reported to be rare. However,
data are not available to establish the true incidence of addiction in chronic pain
patients.
Healthcare professionals should contact their State Professional Licensing
Board, or State Controlled Substances Authority for information on how to
prevent and detect abuse of this product.”

Appendix II: Summary of FDA Changes to the
Original Approved OxyContin Label
Page 50 GAO-04-110 OxyContin Abuse and Diversion
Summary of FDA changes to original
OxyContin label in 2001 Language in OxyContin label approved in 2001
Drug abuse and addiction
—Emphasizes that the abuse potential of
oxycodone is equivalent to that of morphine.
—Describes the controlled status of OxyContin and
emphasizes that, like morphine and other opioids
used in analgesia, oxycodone can be abused and
is subject to criminal diversion.
—Stresses proper prescribing practices,
dispensing, and storage.
—Deletes statement that delayed absorption of
OxyContin was believed to reduce the abuse
liability of the drug.
—Stresses the risks associated with parenteral
injection of OxyContin and reiterates the original
label’s description of drug addiction and “drug-
seeking” behaviors commonly in addicts and
abusers.
“DRUG ABUSE AND ADDICTION
OxyContin is a mu-agonist with an abuse liability similar to morphine and
is a Schedule II controlled substance. Oxycodone, like morphine and
other opioids used in analgesia, can be abused and is subject to criminal
diversion.
Drug addiction is characterized by compulsive use, use for non-medical
purposes, and continued use despite harm or risk of harm. Drug addiction is a
treatable disease, utilizing a multi-disciplinary approach, but relapse is common.
“Drug-seeking” behavior is very common in addicts and drug abusers. Drug-
seeking tactics include emergency calls or visits near the end of office hours,
refusal to undergo appropriate examination, testing or referral, repeated “loss”
of prescriptions, tampering with prescriptions, and reluctance to provide prior
medical records or contact information for other treating physician(s). “Doctor
shopping” to obtain additional prescriptions is common among drug abusers
and people suffering from untreated addiction.
Abuse and addiction are separate and distinct from physical dependence and
tolerance. Physicians should be aware that addiction may not be accompanied
by concurrent tolerance and symptoms of physical dependence in all addicts. In
addition, abuse of opioids can occur in the absence of true addiction and is
characterized by misuse for non-medical purposes, often in combination with
other psychoactive substances. OxyContin, like other opioids, has been
diverted for non-medical use. Careful record keeping of prescribing information,
including quantity, frequency, and renewal requests is strongly advised.
Proper assessment of the patient, proper prescribing practices, periodic
reevaluation of therapy, and proper dispensing and storage are appropriate
measures that help to limit abuse of opioid drugs.
OxyContin consists of a dual-polymer matrix, intended for oral use only.
Abuse of the crushed tablet poses a hazard of overdose and death. This
risk is increased with concurrent abuse of alcohol and other substances.
With parenteral abuse, the tablet excipients, especially talc, can be
expected to result in local tissue necrosis, infection, pulmonary
granulomas, and increased risk of enocarditis and valvular heart injury.
Parenteral drug abuse is commonly associated with transmission of
infectious disease such as hepatitis and HIV.”
Safety and handling
—Emphasizes the controlled status of OxyContin.
—Alerts health care professionals that OxyContin
could be a target for theft and diversion and
instructs that they should contact their State
Professional Licensing Board or State Controlled
Substances Authority for information on how to
prevent and detect abuse or diversion of the
product.
“SAFETY AND HANDLING
OxyContin Tablets are solid dosage forms that contain oxycodone which is a
controlled substance. Like morphine, oxycodone is controlled under Schedule II
of the Controlled Substances Act.
OxyContin has been targeted for theft and diversion by criminals. Healthcare
professionals should contact their State Professional Licensing Board or State
Controlled Substances Authority for information on how to prevent and detect
abuse or diversion of this product.”
Source: FDA-approved label for Purdue’s OxyContin.
Appendix III: Databases Used to Monitor
Abuse and Diversion of OxyContin and Its
Active Ingredient Oxycodone
Page 51 GAO-04-110 OxyContin Abuse and Diversion
DEA uses several databases to monitor abuse and diversion of controlled
substances, including OxyContin and its active ingredient oxycodone.
Specifically, the agency monitors three major databases—the Drug Abuse
Warning Network (DAWN), the National Forensic Laboratory Information
System (NFLIS), and the System to Retrieve Information from Drug
Evidence (STRIDE).
1
DEA also monitors other data sources to identify
trends in OxyContin abuse and diversion, such as the Substance Abuse
and Mental Health Services Administration’s (SAMHSA) National Survey
on Drug Use and Health, formerly the National Household Survey on Drug
Abuse, and the Monitoring the Future Study funded by the National
Institute on Drug Abuse.
2
SAMHSA operates the DAWN system, which estimates national drug-
related emergency department visits and provides death counts involving
abused drugs. DAWN collects data semiannually on drug abuse from
hospital emergency department admission and medical examiner data
from 21 metropolitan areas and a limited number of metropolitan medical
examiners who agree to voluntarily report medical record samples. The
emergency department and medical examiner data generally do not
differentiate oxycodone from OxyContin, unless the individual provides
the information to the hospital or identifiable tablets are found with the
person. Although samples from hospitals outside the 21 metropolitan areas
are also available, DAWN is not able to make drug-related emergency
department visit or death estimates for rural or suburban areas.
NFLIS, a DEA-sponsored project initiated in 1997, collects the results of
state and local forensic laboratories’ analyses of drugs seized as evidence
by law enforcement agencies. NFLIS is used to track drug abuse and
trafficking involving both controlled and noncontrolled substances and
reports results by a drug’s substance, such as oxycodone, and not by its
brand name. DEA stated that because new laboratories are being added,
1
Other databases used by DEA to assess changes in drug abuse and diversion include the
Drug Early Warning System, the Drug and Alcohol Services Information System, the
Treatment Episode Data Set, the National Survey of Substance Abuse Treatment Services,
the Uniform Facility Data Set, the Poison Control Center Data or Toxic Exposure
Surveillance System, the Automation of Reports and Consolidated Ordering System, the
DEA Theft System, and the DEA Field Reports and Investigative Teletypes.
2
The National Institute on Drug Abuse is part of the National Institutes of Health within the
Department of Health and Human Services.
Appendix III: Databases Used to Monitor
Abuse and Diversion of OxyContin and Its
Active Ingredient Oxycodone
DAWN Data
NFLIS Data
Appendix III: Databases Used to Monitor
Abuse and Diversion of OxyContin and Its
Active Ingredient Oxycodone
Page 52 GAO-04-110 OxyContin Abuse and Diversion
its data should not yet be used for trending purposes. As of March 2003,
35 state laboratories and 52 local or municipal laboratories participated in
the project.
STRIDE, another DEA database, reports the results of chemical evidence
analysis done by DEA laboratories in drug diversion and trafficking cases.
Oxycodone data are reported by combining single and combination
oxycodone drugs and do not provide specific enough information to
distinguish OxyContin cases and exhibits. The database’s lag time, which
varies by laboratory, depends on how quickly the findings are entered
after the seizure of the drug substance and its analysis.
The National Survey on Drug Use and Health, another SAMHSA database,
is used to develop national and state estimates of trends in drug
consumption.
3
Prior to 2001, the self-reported survey asked participants if
they had illicitly used any drug containing oxycodone. In 2001, the survey
included a separate section for pain relievers, and asked participants if
they had used OxyContin, identifying it by its brand name, that had not
been prescribed for them. State samples from the survey are combined to
make national- and state-level estimates of drug use, and because the
estimated numbers derived for OxyContin are so small, it is not possible to
project illicit OxyContin use on a regional, state, or county basis.
The Monitoring the Future Survey, funded by the National Institute on
Drug Abuse and conducted by the University of Michigan, annually
monitors the illicit use of drugs by adolescent students in the 8th, 10th,
and 12th grades. The 2002 survey included new questions using the brand
names of four drugs, including OxyContin, in its survey on the annual and
30-day prevalence of drug use.
3
Self-reporting individuals are interviewed regarding their illicit drug use over three
periods—within the last 30 days, during the past year, and during their lifetime. The survey
data are limited, as it is not possible to determine specifically which year respondents may
have used a drug illicitly, because they are asked both whether they have ever used the
drug illicitly in their lifetime and whether they have used it during the past year.
STRIDE Data
National Survey on
Drug Use and Health
Data
Monitoring the Future
Survey Data

Appendix IV: Comments from the Food and
Drug Administration
Page 53 GAO-04-110 OxyContin Abuse and Diversion
Appendix IV: Comments from the Food and
Drug Administration

Appendix IV: Comments from the Food and
Drug Administration
Page 54 GAO-04-110 OxyContin Abuse and Diversion

Appendix IV: Comments from the Food and
Drug Administration
Page 55 GAO-04-110 OxyContin Abuse and Diversion

Appendix V: Comments from the Drug
Enforcement Administration
Page 56 GAO-04-110 OxyContin Abuse and Diversion
Appendix V: Comments from the Drug
Enforcement Administration

Appendix V: Comments from the Drug
Enforcement Administration
Page 57 GAO-04-110 OxyContin Abuse and Diversion
(290206)
The General Accounting Office, the audit, evaluation and investigative arm of
Congress, exists to support Congress in meeting its constitutional responsibilities
and to help improve the performance and accountability of the federal
government for the American people. GAO examines the use of public funds;
evaluates federal programs and policies; and provides analyses,
recommendations, and other assistance to help Congress make informed
oversight, policy, and funding decisions. GAO’s commitment to good government
is reflected in its core values of accountability, integrity, and reliability.
The fastest and easiest way to obtain copies of GAO documents at no cost is
through the Internet. GAO’s Web site (www.gao.gov) contains abstracts and full-
text files of current reports and testimony and an expanding archive of older
products. The Web site features a search engine to help you locate documents
using key words and phrases. You can print these documents in their entirety,
including charts and other graphics.
Each day, GAO issues a list of newly released reports, testimony, and
correspondence. GAO posts this list, known as “Today’s Reports,” on its Web site
daily. The list contains links to the full-text document files. To have GAO e-mail
this list to you every afternoon, go to www.gao.gov and select “Subscribe to e-mail
alerts” under the “Order GAO Products” heading.
The first copy of each printed report is free. Additional copies are $2 each. A
check or money order should be made out to the Superintendent of Documents.
GAO also accepts VISA and Mastercard. Orders for 100 or more copies mailed to a
single address are discounted 25 percent. Orders should be sent to:
U.S. General Accounting Office
441 G Street NW, Room LM
Washington, D.C. 20548
To order by Phone: Voice: (202) 512-6000
TDD: (202) 512-2537
Fax: (202) 512-6061
Contact:
Web site: www.gao.gov/fraudnet/fraudnet.htm
E-mail: fraudnet@gao.gov
Automated answering system: (800) 424-5454 or (202) 512-7470
Jeff Nelligan, Managing Director, Nelligan[email protected] (202) 512-4800
U.S. General Accounting Office, 441 G Street NW, Room 7149
Washington, D.C. 20548
GAO’s Mission
Obtaining Copies of
GAO Reports and
Testimony
Order by Mail or Phone
To Report Fraud,
Waste, and Abuse in
Federal Programs
Public Affairs
