Pregnancy Test with
Weeks Indicator
The only test that tells her
how many weeks
Pregnancy
Professional Series

About Clearblue®
Clearblue is the world’s number one selling brand in home pregnancy and fertility tests.
a
Consumers trust the Clearblue brand because it delivers the accurate information they
want. The Clearblue product range is built on a strong foundation of peer-reviewed
science and consumer understanding. Clearblue is supported by over 30 years of
expertise, quality and innovation in consumer diagnostics.
If you are a healthcare professional and wish to contact a member of the Clearblue support
team about any product in the Clearblue range, please send an email to
spdproductsupport@spdspark.com

Revolutionising home pregnancy testing
The Clearblue Pregnancy Test with Weeks Indicator is the latest innovation in pregnancy
testing. It is an over-the-counter semi-quantitative urine test for human chorionic
gonadotrophin (hCG), which is intended for the detection of pregnancy. It is a simple-to-use
product that is unique because it not only informs the user whether she is pregnant or not,
but also indicates the time since conception occurred, shown on screen as 1–2, 2–3 and 3+,
indicating weeks since conception.
• The Clearblue Pregnancy Test with Weeks Indicator is over 99% accurate at detecting
pregnancy from the day of the expected period
1,b
• The Weeks Indicator feature uses known levels of hCG in urine relative to the day of the
luteinising hormone (LH) surge to estimate time since conception
• Agreement between Clearblue Pregnancy Test with Weeks Indicator results and time
since conception by LH surge detection (± 1 day) is 93%
2
• It is also sensitive enough to be used up to 5 days before the missed period (which
corresponds to 4 days before the expected period)
1,c
• As with other home pregnancy tests, the Clearblue Pregnancy Test with Weeks Indicator
detects hCG, the hormone produced early in pregnancy and excreted in urine
3
• The hormone hCG is the marker of choice for detecting pregnancy and has a long and
proven history in pregnancy testing
4
• Uniquely, in addition to detecting the presence of hCG, the Clearblue Pregnancy
Test with Weeks Indicator also measures the level of hCG, which indicates the time
since conception occurred, shown on screen as 1–2, 2–3 and 3+, indicating weeks
since conception
• The Weeks Indicator feature assumes that conception occurs on the day after the
urinary LH surge (which stimulates ovulation)
5,6
and works by measuring threshold
levels of urinary hCG (Table One).
2
These thresholds are based on extensive
research of the urinary hCG rise in early pregnancy
7,8
Pregnancy
Clearblue
Pregnancy Test
with Weeks Indicator
The only test that tells her how
many weeks
Innovation using established technology
Pregnant
2-3
3
4
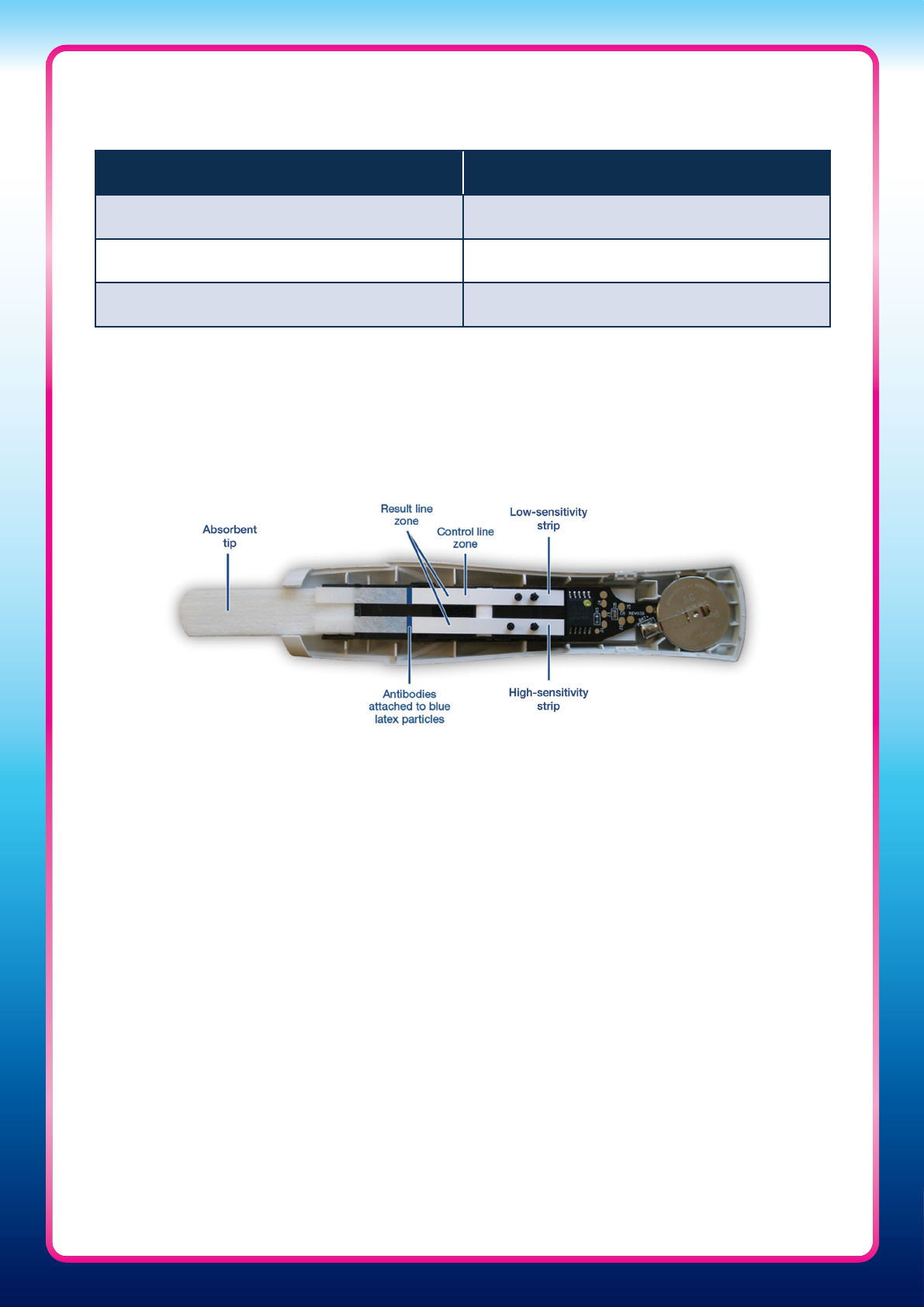
The Clearblue Pregnancy Test with Weeks Indicator
• The Clearblue Pregnancy Test with Weeks Indicator uses a pioneering new approach and
novel algorithm for measuring urinary hCG
• It contains two hCG measurement strips, a low-sensitivity strip and a high-sensitivity
strip, to enable the device to detect and analyse the wide dynamic range of hCG
concentrations found in urine during pregnancy. The high-sensitivity strip detects low
levels of hCG, expected in early pregnancy, and the low-sensitivity strip detects higher
levels of hCG, typical in pregnancies over 3 weeks since conception
• The test monitors the ‘control’ line, which is present on the low-sensitivity strip, and the
‘result’ lines, which are present on both strips. Only when a valid control line is detected
will the result be determined
• The control and result lines cannot be read by eye. Instead, the test uses an optical
system to measure the density of the lines – a red light shines onto the control and result
line zones, a photodiode detects reflected light and a micro-processor converts the light
signal into clear results, visible on a liquid crystal display
A semi-quantitative assay
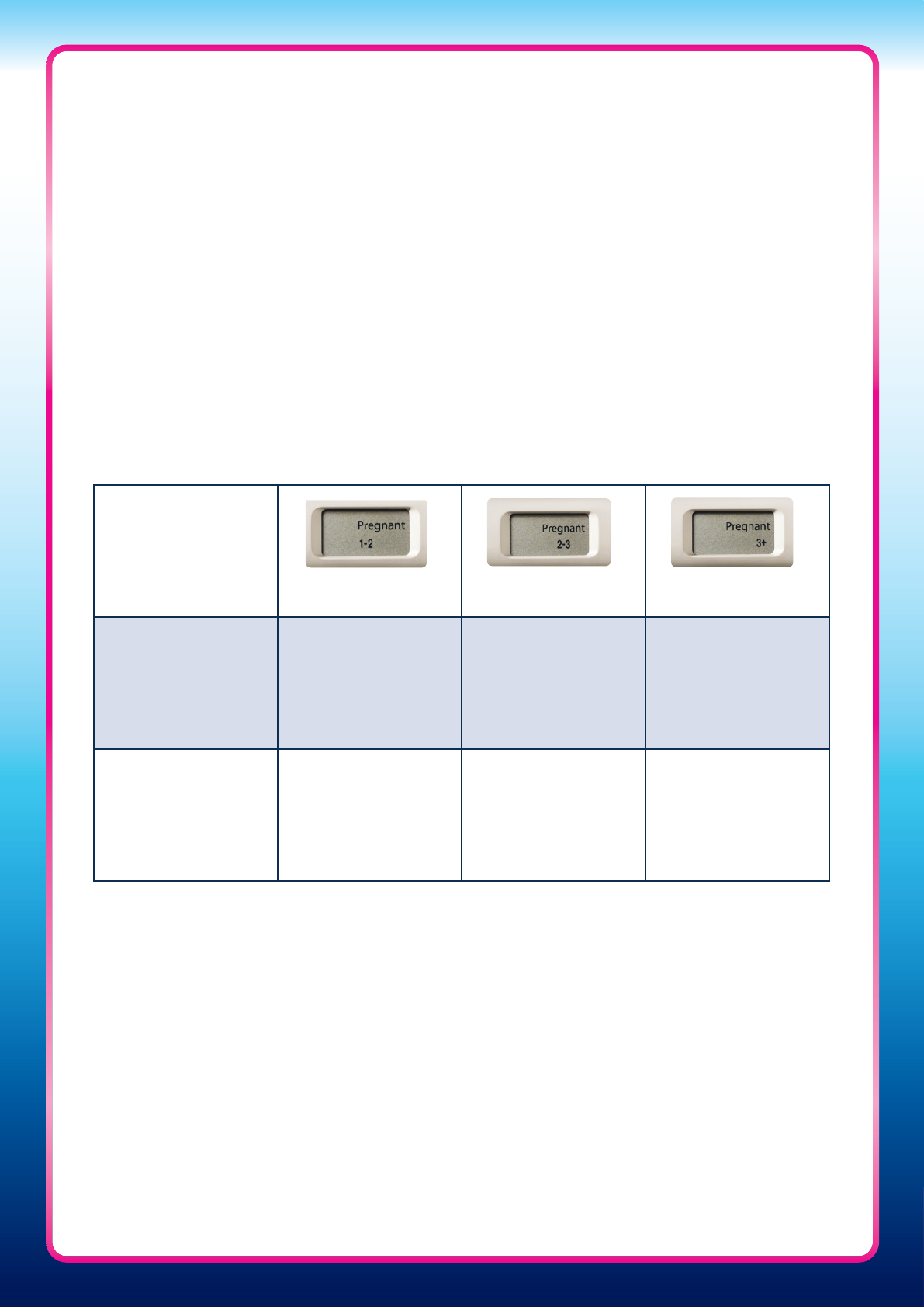
Table One: Urinary hCG threshold levels used in the Clearblue Pregnancy Test with Weeks
Indicator to determine time since conception
Weeks since conception Urinary hCG threshold (mIU/mL)
1–2 10
2–3 153
3+ 2,753

5
Over 99% accurate from the day of the expected period
b
The Clearblue Pregnancy Test with Weeks Indicator demonstrated over 99% accuracy in
detecting pregnancy when compared with a laboratory method (quantitative AutoDELFIA).
1
The study, using urine samples from 300 women aged 18–45 years across three batches,
found 99.4% overall agreement in detecting pregnancy between the Clearblue Pregnancy
Test with Weeks Indicator and quantitative hCG measurements.
1
Can be used up to 5 days before the missed period
c
The Clearblue Pregnancy Test with Weeks Indicator is highly sensitive and can be used up
to 5 days before the missed period. In a study, 135 women provided their first morning urine
samples every day during the cycle in which they became pregnant.
The Clearblue Pregnancy Test with Weeks Indicator detected 65% of pregnancies 5 days
before the missed period (which corresponds to 4 days before the expected period) and
90% of pregnancies 4 days before the missed period (which corresponds to 3 days before
the expected period) (Table Two).
1
Has excellent specificity
Concentrations of hCG in non-pregnant women increase with age, which can potentially
result in some peri- or post-menopausal women obtaining inaccurate false-positive results
with some types of pregnancy tests. The Clearblue Pregnancy Test with Weeks Indicator is
over 99% accurate from the day of the expected period, regardless of whether women are
pre-, peri- or post-menopausal.
1
A total of 301 individual female urine samples were collected from pre-menopausal (n=100),
peri-menopausal (n=101) and post-menopausal (n=100) age groups. Qualitative hCG analysis
was performed on all samples prior to conducting the study. In this non-pregnant
population, all 301 urine samples were tested with three batches of the Clearblue Pregnancy
Test with Weeks Indicator. All tests conducted on these samples were negative, with no
false-positive results reported.
1
Table Two: Percentage of pregnancies detected 0–4 days before the expected period with
the Clearblue Pregnancy Test with Weeks Indicator
1
Days before the expected period 4 3 2 1 0
Percentage of pregnancies detected 65% 90% 97% 98% 99%

6
Background on how the Clearblue Pregnancy Test
with Weeks Indicator provides an estimate of time
since conception
LH surge – An accurate method for referencing time since conception
Identifying the day of fertilisation is the most accurate method for dating pregnancy. It can
be estimated from the day of ovulation, as studies have shown that the egg only survives for
up to 24 hours
9
and a woman’s fertile period ends on the day of ovulation.
10–12
LH is a hormone produced by the anterior pituitary gland. A surge in LH triggers ovulation
and initiates the conversion of the residual follicle into a corpus luteum that, in turn,
produces progesterone to prepare the endometrium for a possible implantation. The
World Health Organization has defined the rise in circulating LH as the best indicator for
impending ovulation, and the day of ovulation is considered a surrogate marker for
conception, as conception can only occur within 24 hours of egg release.
13–15
Levels of LH
in urine correlate 100% with ultrasound detection of ovulation.
16
A prospective study using the Clearblue Fertility Monitor found that the LH surge preceded
the day of ovulation in 76% of cycles where there was a surge, and was within ±1 day of
agreement for 97% of these cycles. Therefore, ovulation can be presumed to occur on the
day of the LH surge +1 (with a ±1 day range).
17
hCG – An accurate scientific marker of time since conception
The peptide hormone hCG is produced by the embryo soon after conception, and later by
the syncytiotrophoblast (part of the placenta). An important role of hCG is to prevent the
disintegration of the corpus luteum and thereby maintain progesterone production, which is
critical for pregnancy in humans.
7
The Weeks Indicator uses urinary hCG levels to estimate the time since conception,
as hCG levels change predictably with pregnancy duration.
• Levels of hCG in serum and urine rise rapidly during the first days of pregnancy
18,19
and
are first detectable by sensitive laboratory assays 7 days before the missed period (which
corresponds to 6 days before the expected period), when the day of the missed period
is estimated using the day of ovulation
10
• In studies, absolute levels of hCG have been used to estimate gestational age
20,21
• Rigorous trials on approximately 3,000 individual samples show there is a consistent
pattern to urinary hCG levels during early pregnancy
9
• Three separate studies conducted in the UK and US over several years have each
provided identical evidence that hCG rises consistently in early pregnancy (Figure One)
7
• No dierences in the daily rise in urinary hCG concentration in early pregnancy are
observed between dierent ethnicities or races
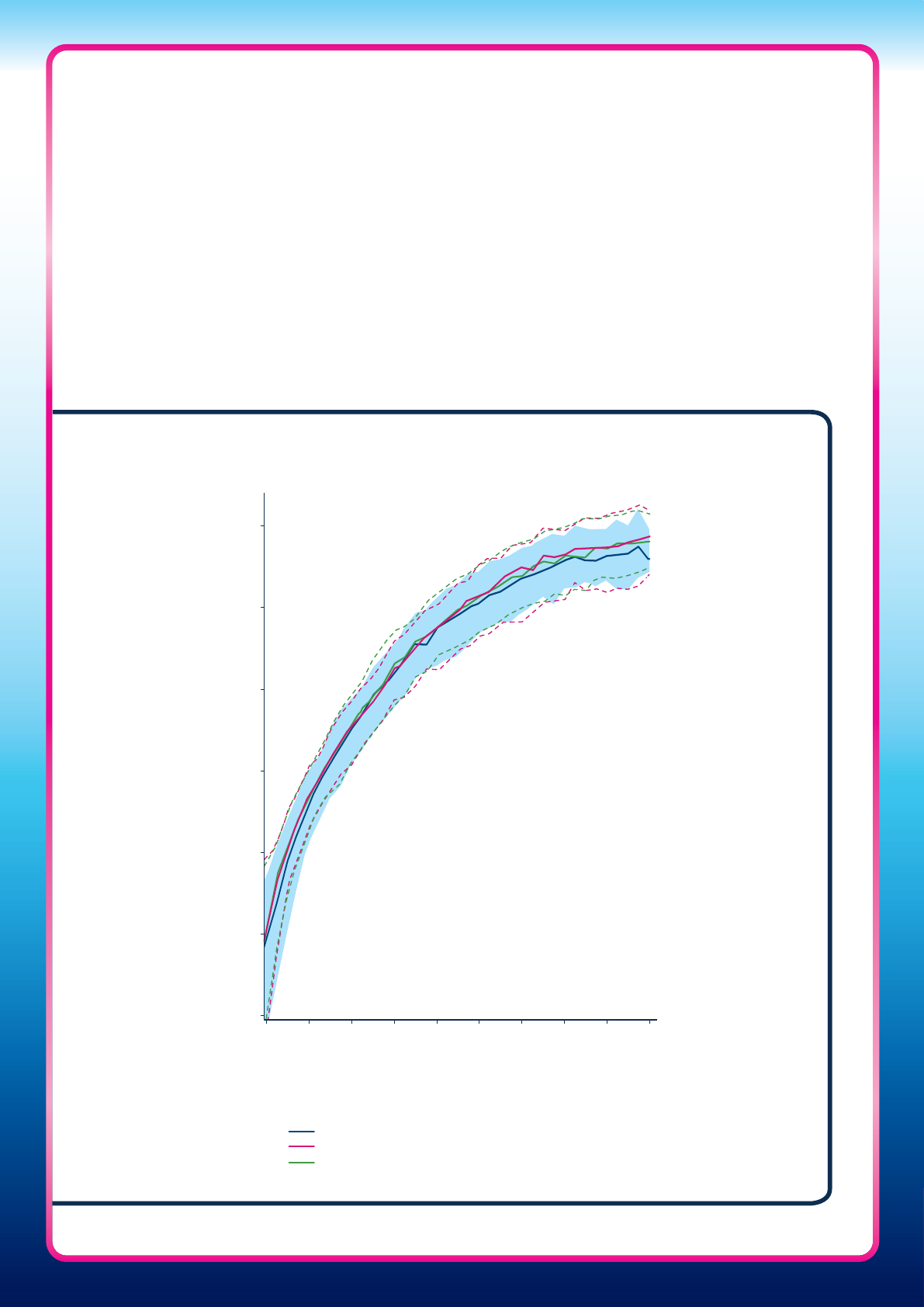
7,8
Figure One: The daily rise in urinary hCG detected in early pregnancy in three
dierent studies
7
0.1
1
10
100
1000
10,000
100,000
8
12 16 20 24 28 32 36 40 44
Days from calculated day of ovulation (LH surge +1)
Median, 10
th
and 90
th
centiles presented
Median hCG concentration (mIU/mL)
US Gestational Ages Study (2012, n=146)
UK Standard-of-Care Ultrasound Study (2008, n=91)
UK Early Pregnancy Study (2005, n=155)
100,000
10,000
1,000
100
10
1
0.1
8 12 16 20 24 28 32 36 40 44
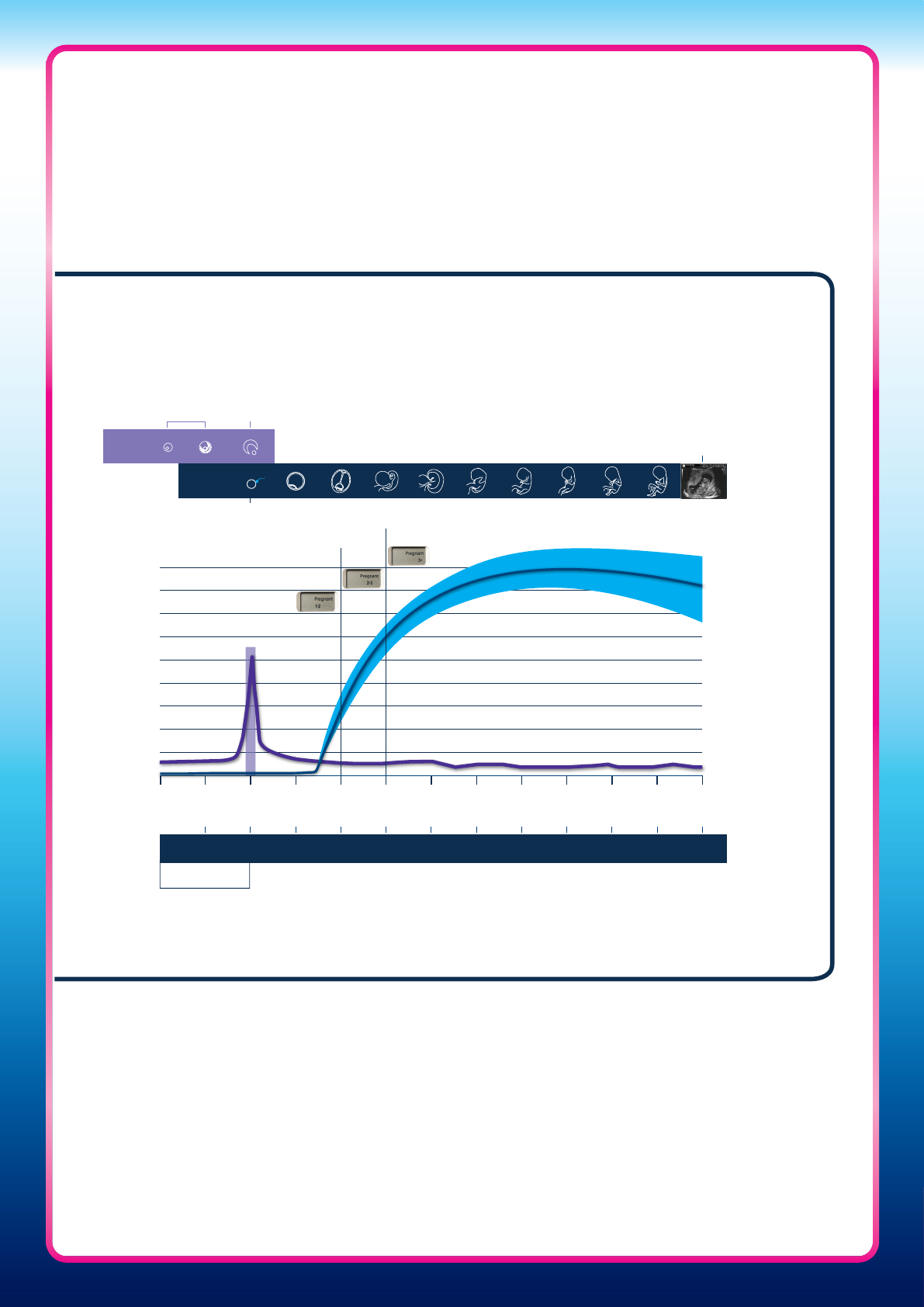
8
Figure Two: Derivation of the weeks since conception indications based
on hCG levels relative to the day of ovulation (day following LH surge), in
comparison to dating of pregnancy by LMP
LMP 1 2 3 4
Weeks since conception
Weeks since LMP
Hormone concentration
5 6 7 8 9 10
Threshold A
Threshold B
Follicle
matures
Egg release
(Ovulation)
Fertilisation
12 weeks
scan
Average
level
of hCG
1 2 3 4 5 6 7 8 9 10 11 12
Gestational
age
Idealised 14 days
follicular phase
LH
Day of
ovulation
Ovum
Foetus
• Pregnancy duration has been shown to be related to rising hCG levels in urine, and
urinary hCG can be used to estimate time since ovulation in weeks, relative to the LH
surge (Figure Two).
7,8,18,20
Studies have shown that gestational age estimated by hCG
concentration (measured by automated immunoassay) agrees with gestational age
estimated using the day of ovulation by 96% for 1–2 weeks gestation, 93% for 2–3 weeks
and 95% for more than 3 weeks
8
LMP, last menstrual period
9
Day of LMP – A frequently inaccurate method
Traditionally, the date of the first day of a woman’s LMP is used to date pregnancy, as this
is often the only information available in early pregnancy upon which to base an estimate.
However, LMP is frequently inaccurate due to a variety of reasons:
• Many women do not know or are uncertain of their LMP:
°
Examination of US birth records found that 16–20% of women have no recorded
LMP,
22,23
and another study found that 16% of women had an unknown LMP
24
°
Only 32% of women are truly certain of their LMP
25
°
The high incidence of ‘round number’ preference observed when women are asked
to recall their LMP supports the uncertainty of many women; 2.5 times more women
than would be expected chose the 15
th
day of the month as their LMP
26
• Bleeding in early pregnancy can be mistaken for the LMP
• Recent contraceptive use or underlying endocrine problems can lead to an inaccurate
estimate of the LMP
• For those women whose LMP is certain, there is an assumption that the follicular phase
is 14 days long; however, the follicular phase can range from 5–24 days
27
resulting in
as many as 10% of women who are certain of their LMP date being more than 7 days
inaccurate in their estimation of gestational age
28
• LMP provides a value to the same week in only 46% of women (within +1 week in 78%,
within +2 weeks in 87%)
29
Ultrasound – Standard of care for dating pregnancy in many countries
First-trimester ultrasound is a more accurate method for dating pregnancy than LMP.
Ultrasound at approximately 11
+0
to 13
+6
weeks after LMP is the standard of care for dating
pregnancy in many countries. This method estimates gestational age based on crown
rump length (CRL) converted using validated formulae,
30–32
which include an idealised
14-day follicular phase, to align the result to LMP dating. Dating using ultrasound CRL is
typically quoted as providing an accurate estimation of gestational age ±5 days.
33–36
Comparison of the Clearblue Pregnancy Test
with Weeks Indicator to standard methods
of dating pregnancy
10
How the Clearblue Pregnancy Test with Weeks
Indicator results relate to clinical care
The test is not intended to be a replacement for clinical care, but rather provides information
that a woman is immediately interested in once she has received a pregnant result, i.e. “How
long is it since I conceived?” Knowing the answer to this question can help women when
speaking with healthcare professionals (HCPs). The instruction leaflet makes it clear that the
woman should seek guidance from an HCP on receipt of a pregnant result and also helps
her to put the results into context with other methods of pregnancy dating: i.e. this test
estimates time since conception, which can be related to the way a doctor dates pregnancy
if an idealised follicular phase (14 days) is added to the Weeks Indicator result.
The table below is included in the instruction leaflet to help women understand how the
result from the Weeks Indicator relates to pregnancy dating by HCPs (Table Three).
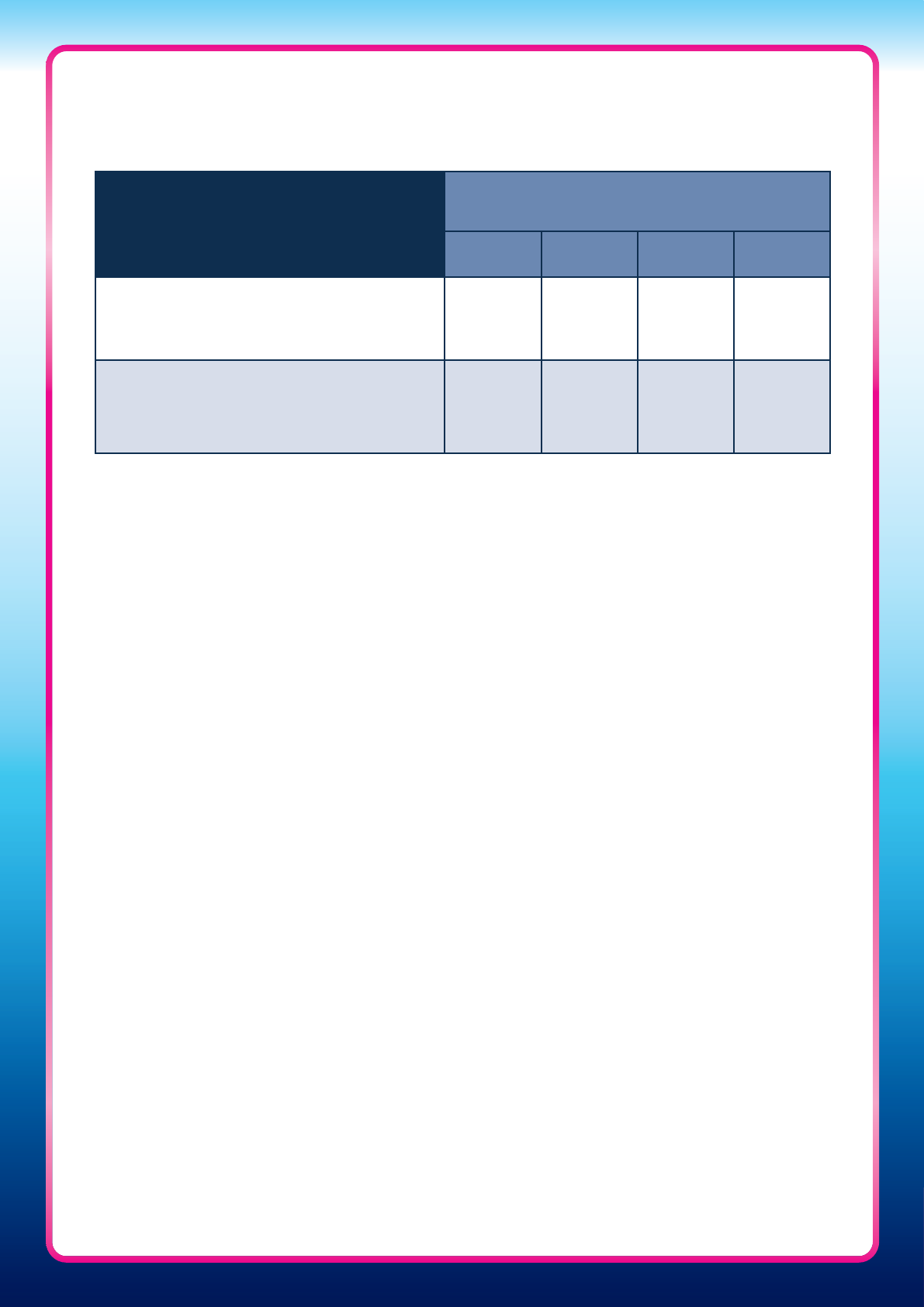
Table Three: How the Clearblue Pregnancy Test with Weeks Indicator result relates to
pregnancy dating by HCPs using LMP
Result
Pregnant 1–2 Pregnant 2–3 Pregnant 3+
What does this
mean?
Pregnant and
conceived
approximately 1–2
weeks ago
Pregnant and
conceived
approximately 2–3
weeks ago
Pregnant and
conceived more than
3 weeks ago
Pregnancy
dating by HCP
(weeks pregnant)*
3–4 weeks 4–5 weeks 5+ weeks
* Based on date of LMP

11
Clearblue Pregnancy Test with Weeks Indicator
compared with reference methods for determining
gestational age
A multi-centre, prospective study was conducted to compare the Weeks Indicator
results with reference methods for determining gestational age. Women were recruited
pre-conception, providing 153 pregnancies for analysis. Urine samples were collected by
participants throughout their cycle and for 4 weeks from the date their period was due if
pregnancy occurred. Quantitative measurement of urinary LH was used to determine the
LH surge (with LH surge +1 day defined as the day of ovulation), and ultrasound dating
scans were conducted at 11
+0
–13
+6
weeks’ gestation following Fetal Medical Foundation
guidelines.
37
The Clearblue Pregnancy Test with Weeks Indicator was tested on a random
set of urine samples, from 4 days prior to the day the period was due until 4 weeks later,
ensuring equal representation per volunteer and per week, and ensuring analysis accounted
for inter-cycle variation.
In this study, agreement between Clearblue Pregnancy Test with Weeks Indicator results and
time since conception (ovulation) by LH surge (±1 day) was 93%. If the ±1 day variation in
timing of ovulation from LH surge is not accounted for, agreement was 87%. The breakdown
by weeks category is shown in Table Four.
2
When comparing Clearblue Pregnancy Test with Weeks Indicator results with the CRL
measurement taken later in the same pregnancy, consideration has to be given to the
formula used to convert this CRL measurement into gestational age. Formula choice
can have a profound influence on agreement analysis due to the systematic bias evident
with some formulae. In addition, CRL measurement has a measurement error associated
within it; therefore, in cases of disagreement between methods, it can be unclear
whether this is due to the CRL measurement or the Clearblue Pregnancy Test with Weeks
Indicator. Typically, for scans conducted in early pregnancy, a ±5-day range is applied
to any measurement. Therefore, analysis of the multi-centre prospective study results
was conducted applying this clinical practice. Table Five summarises the agreements
found between the Clearblue Pregnancy Test with Weeks Indicator and ultrasound using
dierent formulae and allowing or not allowing for ultrasound measurement error.
Table Four: Accuracy of the Clearblue Pregnancy Test with Weeks Indicator compared with
ovulation day
2
Time since ovulation
Accuracy allowing for measurement error
(without allowance for measurement error)
1–2 weeks 96.0% (92.0%)
2–3 weeks 84.0% (72.0%)
3+ weeks 97.0% (94.0%)
Overall 93.0% (87.0%)
12
When gestational age estimated by LMP was compared in this study with gestational age
estimates based on ultrasound and day of ovulation, it was found to agree in 78% and
82% of cases, respectively (when CRL was converted using the Hadlock formula, with
bias adjustment). This is significantly lower than the agreements observed between the
Clearblue Pregnancy Test with Weeks Indicator and these reference methods (P<0.05).
2
Comparison with standard-of-care ultrasound
In a study conducted in the UK, the Clearblue Pregnancy Test with Weeks Indicator was
compared with standard-of-care ultrasound for assessing pregnancy duration in a real-
life observational setting. Data were available from 52 pregnant women and this study
reported an 82% agreement between the two methods. However, when a ±5-day range
was applied to the ultrasound reading (as per routine UK clinical practice), the level of
agreement increased to 98%.
40
Comparison with ultrasound assessment in combined data from the US
Gestational Ages
41
and UK Standard-of-Care Ultrasound
40
studies
Two separate studies have examined the agreement between the Clearblue Pregnancy
Test with Weeks Indicator and ultrasound; both studies found a high level of agreement
(98%) despite being conducted in dierent geographies and using slightly dierent analysis
methods.
40,41
To provide a consistent, consolidated agreement figure between the Clearblue
Pregnancy Test with Weeks Indicator and ultrasound, a new analysis on all the available
ultrasound data was conducted using the same methodology. Data from 143 women from
the US Gestational Ages study
41
and 44 women from the UK Standard-of-Care Ultrasound
40
study were combined. Analysis of this combined dataset confirmed a high level of
agreement (97%) between the Clearblue Pregnancy Test with Weeks Indicator result and
gestational age based on estimates using an ultrasound dating scan, thus indicating that
the Clearblue Pregnancy Test with Weeks Indicator results are comparable to an ultrasound
dating scan.
42,d
Table Five: Agreement between Clearblue Pregnancy Test with Weeks Indicator
result and ultrasound estimate using dierent methods with or without allowing for
ultrasound error
35–39
Reference method and method of
comparison
Clearblue Pregnancy Test
with Weeks Indicator result
1–2 2–3 3+ Overall
Robinson
30
with adjustment for
ultrasound error (without adjustment), %
95.5
(49.5)
38
94.6
(63.7)
38
100.0
(98.8)
38
98.0
(82.5)
38
Hadlock
31
with Pexsters
39
adjustment for
bias with adjustment for ultrasound error
(without adjustment), %
99.1 (76.5) 97.4 (71.1)
99.9
(96.2)
98.9
(86.0)
Notes on formulae: The Hadlock formula is frequently used in clinical care and is a preset formula for most
ultrasound equipment. Evidence shows that this formula has a systematic bias of +2 days,
39
which has no
consequence in clinical practice, but when used as a reference method can provide a low agreement.
The Pexsters adjustment removes this bias, indicating that the low level of agreement at 1–2 weeks was
algorithm dependent.
13
Comparison with delivery date
A prospective study was conducted to compare the Clearblue Pregnancy Test with Weeks
Indicator results with the ultrasound CRL measurement for the prediction of final delivery
date. Urine samples were collected pre-conception until approximately 8 weeks’ gestational
age from 46 women with a natural delivery from the US Gestational Ages
41
and UK
Standard-of-Care Ultrasound
40
studies. The mean time from the Weeks Indicator result
(time since ovulation) to delivery was 37.47 weeks, while the mean time from ovulation
to delivery date based on ultrasound CRL measurement was 37.40 weeks. The Clearblue
Pregnancy Test with Weeks Indicator provides a prediction of delivery date comparable to
ultrasound CRL measurement, and the mean duration of time from ovulation to delivery is
also consistent with the typically reported 38 weeks.
43
Comparison with serum levels of hCG
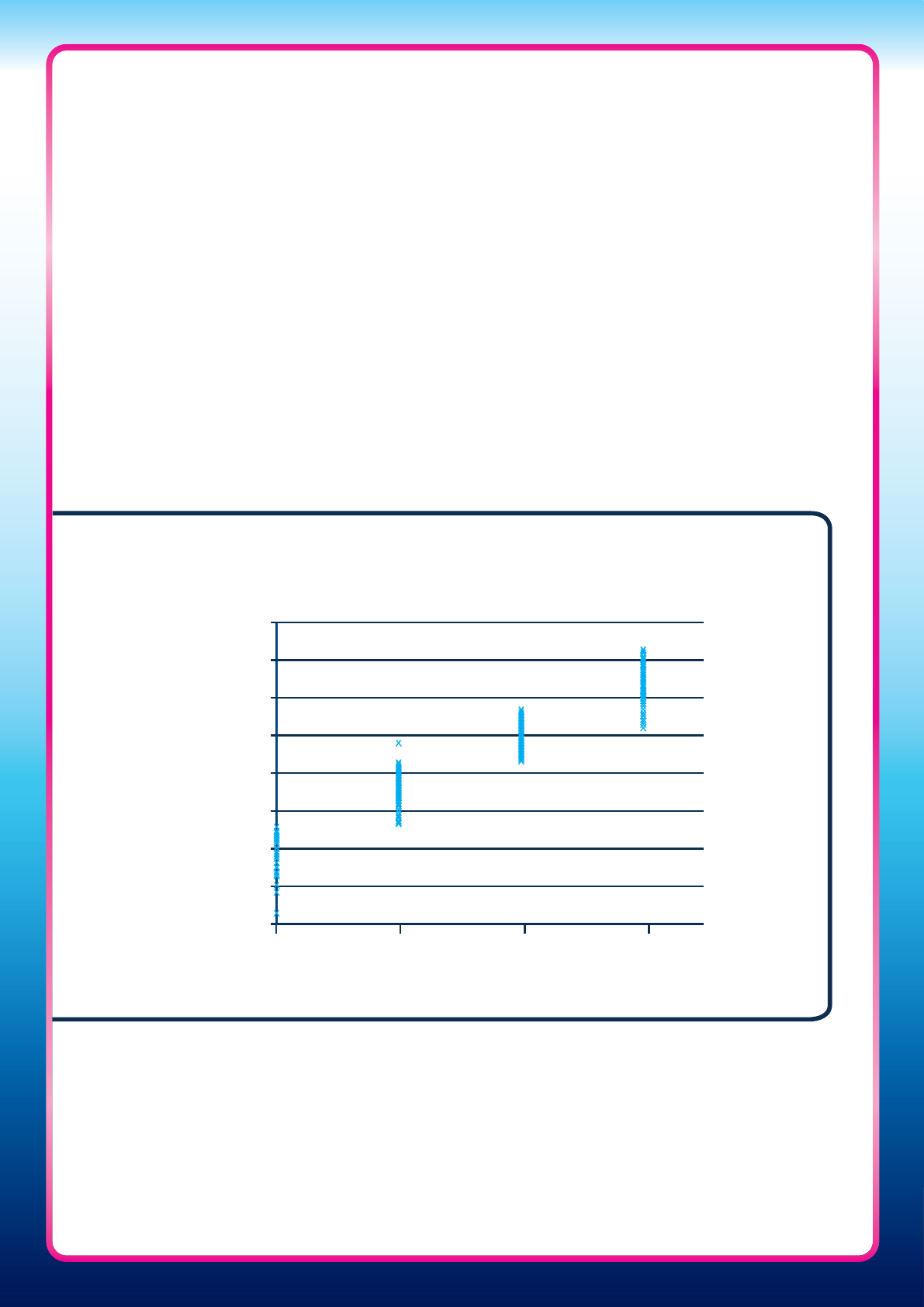
Results of the Clearblue Pregnancy Test with Weeks Indicator have also been compared with
serum levels of hCG (Figure Three). It can be seen that there is little overlap between the
Clearblue Pregnancy Test with Weeks Indicator result and the serum hCG concentrations.
44
Figure Three: Comparison of Clearblue Pregnancy Test with Weeks Indicator
result with serum beta hCG concentration in 500 urine samples from 200
pregnant women
44
100,000
1,000,000
10,000
1,000
100
10
1
0.1
0.01
Not
Pregnant
1–2 2–3 3+
Serum beta hCG concentration (mIU/mL)
Time since ovulation (weeks)
14
What do consumers think of the Clearblue
Pregnancy Test with Weeks Indicator?
• Easy to use – Women prefer its midstream test stick format over other formats such as
cassette or strip-based pregnancy tests. In a study, more than 95% of women stated that
they preferred the midstream test stick format, stating hygiene and ease of use as some
of the reasons for this preference
45
• Trusted by women – Women feel great confidence in the digital results which are easy
to read, giving women reassurance, confidence and trust in the accuracy of the result.
46
In studies, 98% of women were confident of the results of a pregnancy test when the
results were displayed in words, compared with less than 50% for some traditional
line-based tests, and less than 30% for strip- or cassette-based tests.
45
Furthermore, it has
been shown that one in four women can misread traditional line pregnancy test results
47
• Women favour a Weeks Indicator feature – A pregnancy test with a Weeks Indicator
feature is preferred over a standard pregnancy test
48
Limitations
• The manufacturer’s instructions regarding any medication being taken should be read
before conducting the test
• When testing before the day of the expected period, the first morning urine sample
should always be used. This is not necessary when testing on or after the day of the
expected period
• Testing within 14 days after administration of a fertility drug containing hCG can give
a false pregnant result. Other fertility therapies (such as clomiphene citrate), painkillers
and hormonal contraceptives (e.g. the contraceptive pill) should not aect the result.
Recent cessation of hormonal contraceptives or use of fertility therapies like clomiphene
citrate may cause irregular periods, in which case the user may test too soon
• Excessive fluid intake should be avoided before testing, as a urine sample that is too
dilute may give a false-negative (non-pregnant) result
• Ectopic pregnancy and ovarian cysts can give misleading results.
49
Multiple pregnancies
can also give misleading results
• Elevated levels of hCG that are caused by an increase of pituitary hCG production in
peri-menopause, chemotherapy-induced suppression of gonadal function or gestational
trophoblastic disease can give misleading results
49
• A recent pregnancy, miscarriage or termination can give misleading results, as hCG can
be found in the body for several weeks after giving birth
50
and after a miscarriage
or termination
51
• If a positive (pregnant) result is obtained and the woman later obtains a non-pregnant
result, or her period starts, it may be due to natural loss during the early stage of
pregnancy. This is not uncommon, as around one in four pregnancies end in early
pregnancy loss
52,53
• Women should discuss any unexpected results with an HCP
15
1. Johnson SR, et al. Analytical performance of home pregnancy test that estimates time since ovulation based on hCG threshold concentration at week
boundaries. Clin Chem. (2013) S209: B45.
2. Johnson S, et al. Accuracy of a home-based device for giving an early estimate of pregnancy duration compared with reference methods. Fertil Steril.
(2013) 100: 1635–1641.
3. Vaitukaitis JL. Development of the home pregnancy test. Ann N Y Acad Sci. (2004) 1038: 220–222.
4. Stephenson JN. Pregnancy testing and counseling. Pediatr Clin North Am. (1989) 36: 681–696.
5. Burger HG. Estradiol: the physiological basis of the fertile period. Int J Gynaecol Obstet. (1989) Suppl 1: 5–9.
6. Collins WP. Hormonal indices of ovulation and the fertile period. Adv Contracept. (1985) 1: 279–294.
7. Gnoth C, Johnson S. Strips of hope: Accuracy of home pregnancy tests and new developments. Geburtsh Frauenheilk. (2014) 74: 661–669.
8. Larsen J, et al. Human chorionic gonadotropin as a measure of pregnancy duration. Int J Gynaecol Obstet. (2013) 123: 189–195.
9. Ferreira-Poblete A. The probability of conception on different days of the cycle with respect to ovulation: an overview. Adv Contracept. (1997) 13: 83–95.
10. Wilcox AJ, et al. Timing of sexual intercourse in relation to ovulation. Effects on the probability of conception, survival of the pregnancy, and sex of the
baby. N Engl J Med. (1995) 333: 1517–1521.
11. Dunson DB, et al. Day-specific probabilities of clinical pregnancy based on two studies with imperfect measures of ovulation. Hum Reprod.
(1999) 14: 1835–1839.
12. Keulers MJ, et al. The length of the fertile window is associated with the chance of spontaneously conceiving an ongoing pregnancy in subfertile couples.
Hum Reprod. (2007) 22: 1652–1656.
13. Roos J, et al. Monitoring the menstrual cycle: Comparison of urinary and serum reproductive hormones referenced to true ovulation. Eur J Contracept
Reprod Health Care. (2015) 20: 438–450.
14. Johnson S, et al. Development of the first urinary reproductive hormone ranges referenced to independently determined ovulation day. Clin Chem Lab
Med (2015) 53: 1099–1108.
15. WHO Task Force. Temporal relationships between ovulation and defined changes in the concentration of plasma estradiol-17 beta, luteinising hormone,
follicle stimulating hormone, and progesterone. I. Probit analysis. Am J Obstet Gynecol. (1980) 138: 383–390.
16. Guida M, et al. Efficacy of methods for determining ovulation in a natural family planning program. Fertil Steril. (1999) 72: 900–904.
17. Behre HM, et al. Prediction of ovulation by urinary hormone measurements with the home use ClearPlan Fertility Monitor: comparison with transvaginal
ultrasound scans and serum hormone measurements. Hum Reprod. (2000) 15: 2478–2482.
18. Nepomnaschy PA, et al. Urinary hCG patterns during the week following implantation. Hum Reprod. (2008) 23: 271–277.
19. Zegers-Hochschild F, et al. Predictive value of human chorionic gonadotrophin in the outcome of early pregnancy after in-vitro fertilization and
spontaneous conception. Hum Reprod. (1994) 9: 1550–1555.
20. Rule AH, et al. Use of beta-human chorionic gonadotrophin in gestational aging. Ann Clin Lab Sci. (1985) 15: 428–434.
21. Fritz MA, Guo SM. Doubling time of human chorionic gonadotrophin (hCG) in early normal pregnancy: relationship to hCG concentration and gestational
age. Fertil Steril. (1987) 47: 584–589.
22. Dietz PM, et al. A comparison of LMP-based and ultrasound-based estimates of gestational age using linked California live birth and prenatal screening
records. Paediatr Perinat Epidemiol. (2007) 21: 62–71.
23. Taffel S, et al. A method of imputing length of gestation on birth certificates. Vital Health Stat. (1982) 93: 1–11.
24. Buekens P, et al. Epidemiology of pregnancies with unknown last menstrual period. J Epidemiol Community Health. (1984) 38: 79–80.
25. Geirsson RT, Busby-Earle RM. Certain dates may not provide a reliable estimate of gestational age. Br J Obstet Gynaecol. (1991) 98: 108–109.
26. Waller DK, et al. Assessing number-specific error in the recall of onset of last menstrual period. Paediatr Perinat Epidemiol. (2000) 14: 262–267.
27. Lenton EA, et al. Normal variation in the length of the follicular phase of the menstrual cycle. Br J Obstet Gynaecol. (1984) 91: 681–684.
28. Geirsson RT. Ultrasound instead of last menstrual period as the basis of gestational age assignment. Ultrasound Obstet Gynecol. (1991) 1: 212–219.
29. Mustafa G, David RJ. Comparative accuracy of clinical estimate versus menstrual gestational age in computerized birth certificates. Public Health Rep.
(2001) 116: 15–21.
30. Robinson HP, Fleming JEE. A critical evaluation of sonar ‘crown-rump length’ measurements. Br J Obstet Gynaecol. (1975) 82: 702–710.
31. Hadlock FP, et al. Fetal crown-rump length: Re-evaluation of relation to menstrual age (5–18 weeks) with high resolution real-time US. Radiology.
(1992) 182: 501–505.
32. Verburg BO, et al. New charts for ultrasound dating of pregnancy and assessment of fetal growth: Longitudinal data from a population-based cohort.
Ultrasound Obstet Gynecol. (2008) 31: 388–396.
33. Kalish RB, et al. First- and second- trimester ultrasound assessment of gestational age. Am J Obstet Gynecol. (2004) 191: 975–978.
34. Volleberg JHA, et al. The accuracy of ultrasonic measurement of fetal crown-rump length. Eur J Obstet Gynecol Reprod Biol. (1989) 60: 253–256.
35. Piantelli G, et al. Ultrasound dating-curve analysis in the assessment of gestational age. Clin Exp Obstet Gynecol. (1994) 2: 108–118.
36. American College of Obstetrics and Gynecologists. Ultrasonography in pregnancy. ACOG Technical Bulletin no187: December 1993.
37. Nicolaides KH. Screening for chromosomal defects. Ultrasound Obstet Gynecol. (2003) 21: 313–321.
38. SPD data on file: Agreement between the gestational age results obtained using the Clearblue Pregnancy Test with Weeks Indicator compared to
ultrasound CRL–based measurements converted using the Robinson formula was 95.5%, 94.6%, 100.0% and 98.0% for 1-2 weeks, 2-3 weeks, 3+ weeks
and overall, respectively.
39. Pexsters A, et al. New crown–rump length curve based on over 3500 pregnancies. Ultrasound Obstet Gynecol. (2010) 35: 650–655.
40. Johnson S, et al. Agreement between the Clearblue Digital Pregnancy Test Conception Indicator and standard-of-care ultrasound dating in the
assessment of pregnancy duration. Curr Med Res Opin. (2011) 27: 393–401.
41. Johnson SR, et al. Levels of urinary human chorionic gonadotrophin (hCG) following conception and variability of menstrual cycle length in a cohort of
women attempting to conceive. Curr Med Res Opin. (2009) 25: 741–748.
42. SPD data on file: Analysis of a combined dataset from 143 women from the US Gestational Ages study
22
and 44 women from the UK Standard-of-Care
23
study reported high-level agreement (97%) between Clearblue Pregnancy Test with Weeks Indicator and ultrasound dating scan results.
43. Johnson S, Godbert S. Comparison of home pregnancy test with weeks estimator and ultrasound crown rump measurement to predict delivery date.
Fertil Steril. (2013) 100: S330.
44. Johnson S. Home pregnancy test and urinary hCG compared to ultrasound assessment of pregnancy duration. 1
st
International Conference of Obstetrics
Gynecology, Guangzhou, China. (2012) Abstract and Oral Presentation.
45. Pike J, et al. Comparison of volunteers’ experience of using, and accuracy of reading, different types of home pregnancy formats. Expert Opin Med Diagn.
(2013) 7: 435–441.
46. SPD data on file: In a study of 114 consumers, 93.8% rated their confidence in the result of the Clearblue Pregnancy Test with Weeks Indicator as 1–3 on a 7
point scale (1=very confident; 7=not very confident).
47. Tomlinson C, et al. Comparison of accuracy and certainty of results of six home pregnancy tests available over-the-counter. Curr Med Res Opin.
(2008) 24: 1645–1649.
48. SPD data on file: In a study of 114 consumers, 92.1% stated they were more likely to use a pregnancy test with a Weeks Indicator feature than a standard
pregnancy test.
49. Stenman UH, et al. The classification, functions and clinical use of different isoforms of HCG. Hum Reprod Update. (2006) 12: 769–784.
50. Korhonen J, et al. Disappearance of human chorionic gonadotropin and its alpha- and beta- subunits after term pregnancy. Clin Chem.
(1997) 43: 2155–2163.
51. Steier JA, et al. Human chorionic gonadotropin in maternal plasma after induced abortion, spontaneous abortion, and removed ectopic pregnancy.
Obstet Gynecol. (1984) 64: 391–394.
52. Chard T. Frequency of implantation and early pregnancy loss in natural cycles. Baillieres Clin Obstet Gynaecol. (1991) 5: 179–189.
53. Macklon NS, et al. Conception to ongoing pregnancy: the ‘black box’ of early pregnancy loss. Hum Reprod Update. (2002) 8: 333–343.
References

a
Based on international sales compiled using independent market research data (data on file).
b
The Clearblue Pregnancy Test with Weeks Indicator has been shown to be over 99% accurate from the day of the expected period when compared
with a reference method in laboratory studies using urine samples supplied for pregnancy testing.
c
Five days before the missed period corresponds to 4 days before the expected period. In laboratory testing, 98% of pregnant results were detected on
the day before the expected period, 97% were detected 2 days before, 90% were detected 3 days before and 65% were detected
4 days before the expected period (5 days before the missed period).
d
The Clearblue Pregnancy Test with Weeks Indicator result does not replace the need for a pregnant woman to attend routine ultrasound examinations.
e
Based on studies of 143 women comparing ultrasound dating to weeks result (up to 3+ weeks).
2
Clearblue Pregnancy Test
with Weeks Indicator is:
Accurate – over 99% accurate from the day of the expected period
1,b
Clinically proven – correlates with estimation of gestational aging by routine ultrasound
e
Sensitive – can be used up to 5 days before the missed period
2,c
Unmistakably clear – results displayed in words and numbers on a digital screen
For more information about the Clearblue Pregnancy Test with Weeks Indicator, please visit our websites:
This material is intended for healthcare professionals only. It is for general information only with no warranties,
representations or undertakings, express or implied, and does not constitute medical advice. Product images are for
illustration only. Clearblue
® is a registered trade mark of SPD Swiss Precision Diagnostics GmbH (“SPD”). © 2017 SPD
(except for any third-party content identified as such). All rights reserved.
www.clearblue.com
www.swissprecisiondiagnostics.com
SPD Swiss Precision Diagnostics GmbH, 1213 Petit Lancy, Geneva, Switzerland
Clearblue Professional Series: HCP-0018.5
