1
FSIS Cooking Guideline for
Meat and Poultry Products
(Revised Appendix A)
December, 2021
Document ID: FSIS-GD-2021-14
This guideline provides information on the
Agency regulatory requirements
associated with safe production of ready-
to-eat (RTE) products with respect to the
destruction of Salmonella and other
pathogens. It applies to small and very
small meat and poultry official
establishments although all meat and
poultry establishments may apply the
recommendations in this guideline. It
relates to 9 CFR 318.17(a)(1), 9 CFR
318.23, 381.150(a)(1), and 9 CFR 417.

2
Table of Contents
Preface ............................................................................................................................ 4
Purpose of this Guideline .......................................................................................... 4
History of this Guideline and Reason for Reissuance ............................................... 5
Changes from the Previous Versions ....................................................................... 6
How to Effectively Use this Guideline ....................................................................... 8
Questions Regarding Topics in this Guideline .......................................................... 9
Background ................................................................................................................... 10
What is Lethality? ................................................................................................... 10
Products and Processes Covered by this Guideline ............................................... 10
Products and Processes Not Covered by this Guideline ........................................ 11
Biological Hazards of Concern During Cooking ...................................................... 13
General Considerations for Designing HACCP Systems to Achieve Lethality by Cooking
. ..................................................................................................................................... 18
Addressing Lethality in the HACCP System ........................................................... 18
Alternative Lethality ................................................................................................ 20
Monitoring, Calibration, and Recordkeeping ........................................................... 20
Corrective Actions under HACCP Cooking Deviations ........................................... 22
FSIS Critical Operating Parameters for Cooking ........................................................... 23
Come-Up-Time (CUT) ............................................................................................ 23
Relative Humidity .................................................................................................... 25
Table 1. Critical Operating Parameters for FSIS Humidity Options........................ 26
Relative Humidity Resources .................................................................................. 28
Situations when Humidity is Not Needed ................................................................ 31
Endpoint Time-Temperature ................................................................................... 34
Table 2. Time-Temperature Combinations for Meat Products to Achieve Lethality
. .............................................................................................................................. 35
Additional Critical Operating Parameters for Poultry Products................................ 36
Table 3. Time-Temperature Combinations for Chicken Products to Achieve
Lethality .................................................................................................................. 37
Table 4. Time-Temperature Combinations for Turkey Products to Achieve Lethality
. .............................................................................................................................. 38
Resources for Customized and Alternative Support ...................................................... 40
Scientific Gaps Identified by FSIS .................................................................................. 41
Table 5. Scientific Gaps where Critical Operating Parameters From Older
Guidance May be Used .......................................................................................... 43
References .................................................................................................................... 49
Attachment A1. Customized Processes and Alternative Lethality Support ................... 55

3
Supporting an Alternative Lethality Target (e.g., 5-Log) .......................................... 57
Table 6. Time-Temperature Combinations for Meat Products to Achieve a 5-Log
Reduction ............................................................................................................... 59
Predictive Microbial Modeling to Support CUT ....................................................... 62
Designing Challenge Studies for Cooking .............................................................. 63
Attachment A2. Cooking Deviations .............................................................................. 66
Corrective Actions to Perform When a Cooking Deviation Occurs ......................... 66
Type 1. Missed Endpoint Time-Temperature ......................................................... 67
Type 2. Insufficient Humidity During Cooking ........................................................ 69
Type 3. Long Heating CUT .................................................................................... 70
Predictive Microbial Modeling ................................................................................. 72
Product Testing ...................................................................................................... 77
Table 7. FSIS Recommendations for Product Sampling and Testing After Each
Type of Cooking Deviation to Determine Product Disposition ................................. 77
Disposition after Testing Results ............................................................................ 79
Attachment A3. When can Products be Labeled as Pasteurized? ................................ 81
Attachment A4. Sources of Salmonella Contamination in RTE Products and Best
Practices to Address It ................................................................................................... 82
Under-Processing ................................................................................................... 82
Cross-Contamination .............................................................................................. 82
Ingredients Added After the Lethality Treatment ..................................................... 84
Food Handlers ........................................................................................................ 86
Animals .................................................................................................................. 86
Attachment A5. RTE Salmonella Self-Assessment Tool ............................................... 87
Attachment A6. Cooking Country-Cured Hams ............................................................ 90
4
Preface
This is a revised version of the FSIS Cooking Guideline for Meat and Poultry Products
(Revised Appendix A). It has been updated in response to comments received on the
previous version and renamed. In addition, the guideline has been revised to include
recommendations from previous versions and new updates based on up-to-date
science. The guideline also includes changes to improve its readability.
This guideline represents FSIS’s current thinking on these topics. Establishments that
utilized previous versions of Appendix A as support should either:
• Update to this 2021 FSIS Cooking Guideline (Revised Appendix A) or
• Identify alternative support by December 14, 2022.
The information in this guideline is provided to assist meat and poultry establishments in
meeting the regulatory requirements. The contents of this document do not have the
force and effect of law and are not meant to bind the public in any way. This document
is intended only to provide clarity to industry regarding existing requirements under the
regulations. Under the regulations, meat and poultry establishments may choose to
implement different procedures than those outlined in this guideline, but they would
need to validate and support how those procedures are effective.
This guideline is focused on small and very small plants in support of the Small
Business Administration’s initiative to provide small businesses with compliance
assistance under the Small Business Regulatory Enforcement Fairness Act (SBREFA).
However, all meat and poultry establishments may apply the recommendations in this
guideline. It is important that small and very small establishments have access to a full
range of scientific and technical support, and the assistance needed to establish safe
and effective Hazards Analysis and Critical Control Point (HACCP) systems. Although
large plants can benefit from the information, focusing the guideline on the needs of
small and very small establishments provides them with assistance that may be
otherwise unavailable to them.
Purpose of this Guideline
This guideline contains information to assist meat and poultry establishments producing
products that undergo cooking in complying with the HACCP regulatory requirements in
9 CFR 417. This guideline includes information on:
• Biological hazards during cooking.
• Regulatory requirements associated with the safe production of cooked ready-to-
eat (RTE) products.
• Options establishments can use to achieve lethality of Salmonella and other
pathogens.
5
• Processes that do not have validated research available (referred to as “scientific
gaps”) and options establishments can use until research is available.
• Resources for alternative support.
• Recommendations for evaluating cooking deviations.
Establishments can always seek guidance from State university extension service
specialists and HACCP Coordinators on developing programs and plans not provided in
this guideline to comply with HACCP regulatory requirements.
History of this Guideline and Reason for Reissuance
In the 1970s and 1980s, FSIS included prescriptive time, temperature, and humidity
operating parameters in the regulations for cooked beef, roast beef, and cooked corned
beef (42 FR 44217; 47 FR 31854; 48 FR 24314) in response to several outbreaks
associated with these products and research performed to determine how to prepare
them safely. When the Pathogen Reduction/Hazard Analysis and Critical Control Points
(PR/HACCP) final rule published in 1996, FSIS eliminated the prescriptive cooking
regulations and replaced them with performance standards requiring a 6.5-Log
reduction in Salmonella or alternative lethality for roast beef, cooked beef, and corned
beef, minimum internal temperature and holding times for fully cooked patties that
achieve a 5-Log reduction in Salmonella, and a 7-Log reduction in Salmonella or
alternative lethality for poultry products (9 CFR 318.17(a)(1), 9 CFR 318.23, 9 CFR
381.150(a)(1); see General Considerations for Designing HACCP Systems to Achieve
Lethality by Cooking, page 18. FSIS converted these former regulations to “Safe
Harbors” in an appendix to the final rule called Appendix A (64 FR 732).
Establishments have been using FSIS’s Appendix A, as published in 1999, as support
for cooking processes for many years. The original requirements and subsequent
guidance have been important to prevent human illness outbreaks and ensure the
production of safe food. See General Considerations for Designing HACCP Systems to
Achieve Lethality by Cooking, page 18 for more information on the current regulatory
requirements.
Over time, FSIS determined that some of its recommendations in the 1999 version of
Appendix A were vague, putting establishments at risk of producing unsafe products.
Additionally, some elements of the 1999 version of Appendix A were misunderstood or
overlooked, resulting in FSIS guidance being applied in ways that increased food safety
risks to consumers and potential risks to industry, including the risk of foodborne illness
outbreaks. FSIS also determined establishments were broadly applying the
recommendations for operating parameters in Appendix A beyond those meat and
poultry products it was originally designed to support.
To provide the needed updates and clarifications, FSIS issued revisions of both its
Cooking (Appendix A) and Stabilization (Appendix B) guidelines in 2017. The 2017
version of the guidelines took into account new and emerging technologies, processes,
and science. FSIS has updated this guideline in response to comments received on the
2017 version and has included additional options for cooking support based on updated

6
science and technology. The Agency is releasing this current 2021 version of the
FSIS Cooking Guideline for Meat and Poultry Products (Revised Appendix A) to
replace all previous versions.
Changes from the Previous Versions
This guideline dated December 14, 2021 is final. FSIS will update this guideline, as
necessary, should new information become available.
FSIS made the following changes to this guideline to reflect the comments received on
the previous version during the comment period and to include additional scientific
information.
For Appendix A, FSIS made changes to specify:
• The following products are not covered by the guideline (page 11): Fish of the
Order Siluriformes, pork rind pellets, rendered lard and tallow, dried products
processed under dry conditions, partially heat-treated NRTE products, and RTE
multi-hurdle products.
• The food safety significance of FSIS’s recommendations for relative humidity
(page 17).
• That relative humidity should be addressed for all cooked products (including
poultry) unless the establishment can support that humidity does not need to be
addressed. FSIS has not changed the relative humidity options (page 26) other
than re-emphasizing that they apply to all products.
• Additional resources for selecting a relative humidity option when following
FSIS’s cooking guidance (page 28).
• The situations when relative humidity does not need to be addressed including
by providing more information about situations considered to be direct heating
(page 31) (e.g., by clarifying that relative humidity does not need to be addressed
for meat patties cooked using FSIS’s time-temperature table for meat, if the
patties are cooked using direct heat (on page 31)). Previous guidance indicated
it did not need to be addressed for meat patties with the assumption all meat
patties are cooked using direct heat which is no longer the case.
• That natural casings become semipermeable during cooking, maintaining
moisture in the product, so that additional documentation to address relative
humidity is not needed (page 33).
• More detailed information for evaluating product safety following a heating
deviation (page 66). The revision also removes the recommendation for using
the ComBase model for Staphylococcus aureus growth (which was not validated)
7
because of the development and validation of the Danish Meat Research
Institute (DMRI) Staphtox model in 2018.
• Where gaps exist, recommendations from its older cooking guidance can be
used until research is completed (see, Table 5. Scientific Gaps where Critical
Operating Parameters From Older Guidance May be Used, page 43) for:
1. Products cooked for short times at high temperatures.
2. Products cooked using microwave cooking methods that are not
designed to control relative humidity.
3. Products cooked using cooking methods that are not designed to
control relative humidity.
4. Other processes that may inherently maintain relative humidity
around the meat and poultry filling but cannot follow one of the relative
humidity options.
5. Processes where the drying step comes before cooking under moist
conditions.
6. Products with long heating come-up-times (CUTs).
• That information is included about a listeriosis outbreak associated with a cooked
country-cured ham product and recommendations for establishments that cook a
similar product once (page 90).
For Appendix A, FSIS removed:
• Information about how establishments could remove poultry rolls from the
cooking medium before product has achieved the target endpoint temperature
and immediately apply another heating or processing method (64 FR 732).
Since FSIS has clarified that limiting heating CUT is a critical operating
parameter for applying any of FSIS cooking guidance (including these older
options), the parameter to “immediately fully cook” poultry rolls subject to multiple
heating mediums and processes has been removed.
• Specific recommendations for conducting a Salmonella baseline study on raw
source materials as support for using cooking critical operating parameters that
achieve a 5-Log reduction in Salmonella for meat products instead of a 6.5 or 7-
Log reduction. This information was removed since it was interpreted to apply to
all establishments when it was only intended for establishments that wanted to
support a lower level of pathogen reduction from cooking. In addition, FSIS is
not aware of any establishments that have pursued such baseline sampling.
In addition to these changes, the guidelines format was restructured to make it easier to
use as described in the next section. This list of changes is not comprehensive, so

8
establishments should read the section titled FSIS Critical Operating Parameters for
Cooking and other relevant sections as needed.
How to Effectively Use this Guideline
As explained above in the Changes from the Previous Versions, the guidelines format
was restructured to make it easier to use. Specifically, the guideline is organized to
include the following topics in the body of the guideline:
• Biological hazards during cooking.
• Regulatory requirements associated with the safe production of cooked ready-to-
eat (RTE) products.
• Options establishments can use to achieve lethality of Salmonella and other
pathogens.
• Processes that do not have validated research available (referred to as “scientific
gaps”) and options establishments can use until research is available.
Information included in the body of the guideline is intended as scientific support that
can be used alone by establishments to meet Element 1 of validation (9 CFR
417.4(a)(1)) and to support decisions in the hazard analysis (9 CFR 417.5(a)(1)).
The following topics are included in attachments to the guideline:
• Resources for alternative support and
• Recommendations for evaluating cooking deviations.
Information provided in the attachments is not sufficient to use as sole support and
additional documentation is needed. For example, Attachment A1. Customized
Processes and Alternative Lethality Support (page 55), contains descriptions or brief
summaries of available scientific articles. However, the summaries are not considered
adequate support on their own because they do not contain the details of each study.
For this reason, establishments must have the full copy of the article on-file as scientific
support for their HACCP System. The summaries are provided to help establishments
identify journal articles related to their process. Each establishment needs to determine
if the operating parameters of a particular study match the establishment’s process.
Establishments are not limited to using the scientific articles listed and summarized as
support. In addition, Attachment A2. Cooking Deviations (page 66), contains
recommendations for evaluating product safety in the event of a deviation but this
information is not considered adequate support on its own because establishments
should perform predictive microbial modeling and may conduct sampling and testing in
order to support product disposition. Other information included in attachments is
intended to be supplementary.

9
Questions Regarding Topics in this Guideline
If after reading this guideline you still have questions, FSIS recommends searching the
publicly posted Knowledge Articles (“Public Q&As”) in the askFSIS database. If after
searching the database, you still have questions, refer them to the Office of Policy and
Program Development through askFSIS and select HACCP Deviation & HACCP
Validation as the Inquiry Type or by telephone at 1-800-233-3935.
Documenting these questions helps FSIS improve and refine present and future
versions of the guideline and associated issuances.

10
FSIS Cooking Guideline for Meat and Poultry Products
(Revised Appendix A)
Background
What is Lethality?
Lethality treatments are processes used by
establishments to eliminate Salmonella and other
pathogens in RTE products. Lethality treatments achieve
a specific reduction in the number of Salmonella and other
pathogens in the product (i.e., an “X-Log10 colony forming
units per gram
1
(CFU/g)” reduction). The combination of
one or more lethality treatments must be sufficient to
eliminate or adequately reduce Salmonella and other
pathogens to undetectable levels and prevent the
production of toxins or toxic metabolites in the RTE
product (e.g., from Staphylococcus aureus).
Establishments may use a variety of different lethality
processes, such as:
• Cooking the product (covered in this guideline).
• Fermentation.
• Drying.
• Salt-curing.
• Other processes that make the product safe for
consumption.
Products and Processes Covered by this
Guideline
This guideline addresses lethality of pathogens (e.g.,
Salmonella) in meat and poultry products
2
by heat
treatment (cooking) including for products that are cooked
to lethality but classified under a not-ready-to-eat HACCP
plan.
NOTE: FSIS has provided additional information about
the safe production of meat and poultry jerky products in
1
In the rest of this document, Log
10
colony forming units per gram (Log
10
CFU/g) will be annotated simply
as “Log.” All notations of “Log” should be read as in the unit Log
10
CFU/g unless other information is
provided.
2
Throughout this document references to “meat and poultry products” may be considered inclusive of
meat by-products, meat food products, and poultry food products as defined in 9 CFR 301.2 and 9 CFR
381.1, unless otherwise stated (e.g., Products and Processes Not Covered by This Guidance).
KEY DEFINITIONS
A ready-to-eat (RTE)
product is defined as a
meat or poultry product
that is in a form that is
edible by the end
consumer without
additional preparation to
achieve food safety and
that may receive
additional preparation for
palatability, aesthetic, or
culinary purposes (9
CFR 430.1).
Lethality is the process
(or combination of
processes) that ensure a
specific, reduction in the
number of Salmonella and
other pathogens in the
product (i.e., an “x-Log”
reduction). Lethality
processes eliminate or
adequately reduce
Salmonella and other
pathogens and prevent the
formation of their toxins or
toxic metabolites,
facilitating the production
of a safe RTE food
product.
11
the FSIS Compliance Guideline for Meat and Poultry Jerky Produced by Small and Very
Small Establishments. The information for jerky production remains in a separate
guideline because of the complexities of the process, including drying procedures, and
to help address questions from small and very small processing establishments.
Products and Processes Not Covered by this Guideline
The recommendations in this guideline do not apply to the following specific products:
Fish of the Order Siluriformes (e.g., catfish)
FSIS cooking guidance was not validated for fish of the order Siluriformes. Therefore,
this guidance should not be used for fish.
Fish establishments may use the cooking guidance in Table A-3 of The Food and Drug
Administration’s (FDA’s) Fish and Fishery Products Hazards and Control Guidance as
support for the cooking step of fish products. The time-temperature recommendations
are designed to achieve a 6-Log reduction in Listeria monocytogenes (Lm).
Pork Rind Pellets
Establishments may cook pork skins in pork fat or oil for several hours rendering the fat
and reducing the skin into pellets. This intermediate product is then further processed
by frying to produce a finished product such as pork rinds, cracklins (cracklings), or
chicharrones. FSIS cooking guidance does not apply to the cooking or rendering of
pork skins into a pellet. Establishments may use the cooking requirements in 9 CFR
94.8(b)(4) as support for cooking pork skins into a pellet. Although these are Animal
Plant and Health Inspection Service (APHIS) requirements for imported pork skins from
countries where foot-and-mouth disease, African swine fever, classical swine fever, or
swine vesicular disease exist, these cooking requirements ensure at least a 6.5-Log
reduction of Salmonella (Juneja, et al., 2001a; Murphy et al., 2003; Murphy et al., 2004).
NOTE: FSIS cooking guidance may be used for cooking of pork skins for products other
than pork rind pellets (e.g., for use in pickled products) and for frying of pork rind pellets
into popped pork skins. Guidance for monitoring the cooking critical limit for these
products can be found in the Key Question on page 21.
Rendered Lard and Tallow
FSIS cooking guidance does not apply to the rendering of animal fats, such as lard and
tallow, which, due to the high fat content, generally need to reach higher temperatures
and longer dwell
3
times to achieve the same reductions in Salmonella (Ramirez-
Hernandez et al., 2018). However, based on the D values (time at a constant
temperature necessary to destroy 90% or 1-Log of the target organism) reported by
Ramirez-Hernandez et al. (2018), the cooking requirements for rendering in 9 CFR
315.1(a) are adequate to ensure an animal fat rendering process achieves at least 6.5-
3
“Dwell time” ref ers to the time a product is held at a specific temperature. Other commonly used terms
such as “hold time” or “rest time” may be considered synonymous for the purpose of this guideline.

12
Log reductions of Salmonella. Therefore establishments may
use 9 CFR 315.1 as support for a lard or rendering process,
provided the critical operational parameters (≥ 170°F for ≥ 30
minutes) are met throughout the product.
Dried Products Processed Under Dry Conditions
FSIS cooking guidance does not support lethality for a
process that relies on drying alone (e.g., biltong), nor does
this guidance support a process where the drying step comes
before a cooking step that does not apply humidity or does
not apply humidity during cooking at sufficient levels to
rehydrate the product surface (e.g., biltong or country-cured
ham that is cooked in an unsealed oven after drying). This
guidance also does not support lethality for a dried product
cooked under moist conditions several times after drying
(e.g., country-cured ham that is cooked in a sealed oven
several times after the hams have been salt-cured and dried).
Such dried products are typically considered intermediate
moisture foods (i.e., those foods that do not require
refrigeration to control pathogens). The water activity range
of foods considered intermediate moisture varies in the
literature. For example, FDA classifies intermediate moisture
foods as those with a water activity between 0.60 and 0.85
(FDA, 2018). However, some meat and poultry products may
have a water activity > 0.85 and still be considered
KEY DEFINITIONS
Stabilization is the
process of preventing or
limiting the growth of
spore-forming bacteria
capable of producing
toxins either in the
product or in the human
intestine after
consumption.
Stabilization processes
may include cooling, hot-
holding, or meeting and
maintaining a certain pH
or water activity level and
other processes, such as
drying and fermentation/
acidification that render
the product shelf-stable or
safe at room
temperatures.
“intermediate moisture” because of other factors such as pH and salt concentration
(Leistner, 1987). For example, country-cured ham has an average water activity of 0.88
but is considered shelf-stable due to the combination of water activity, high salt, and
nitrite (Mikel and Newman, 2003; Reynolds et al., 2001).
Establishments that apply these types of processes must identify other support for their
HACCP System (9 CFR 417.5(a)(1) and 9 CFR 417.4(a)(1)).
NOTE: This guidance includes critical operating parameters for cooking products which
are dried, then cooked under moist conditions. Scientific Gaps Identified by FSIS
describes critical operating parameters (page 47) and Attachment A6. Cooking
Country-Cured Hams includes additional tips, specific to country-cured hams (page
90).
Partially Heat-Treated NRTE Products
This guideline does not cover partially heat-treated products that are not ready-to-eat
(NRTE) and did not reach a validated lethality time-temperature combination (for
example: partially heat-treated bacon and hams). These products are addressed in the
FSIS Stabilization Guideline for Meat and Poultry Products because cumulative growth
of Clostridium perfringens and Clostridium botulinum are hazards of concern over the
course of partial cooking and cooling processes.

13
NOTE: As noted under the Products and Processes Covered by this Guideline, this
guideline may be used for products that are cooked to lethality but classified under a
Not RTE (NRTE) HACCP plan. For such products, please refer to the product
reclassification guidance in the Listeria Guideline, Attachment 1.2 on pages 22-23 and
Appendix 1.2 on pages 28-29 for guidance related to labeling, HACCP categorization,
and intended use.
RTE Multi-hurdle Products
This guidance does not address the safe production of products that rely on multiple
hurdles to achieve lethality and shelf-stability (e.g., fermented and dried sausage).
However, some regulatory information associated with such products is included in
General Considerations for Designing HACCP Systems to Achieve Lethality by
Cooking, page 18.
NOTE: Stabilization requirements and recommendations for cooling meat and poultry
products after heat treatment are described in the FSIS Stabilization Guideline for Meat
and Poultry Products.
Biological Hazards of Concern During Cooking
The following section is designed to complement FSIS’s Meat and Poultry Hazards and
Control Guide and to further assist establishments in conducting a hazard analysis for
cooked meat and poultry products as required by 9 CFR 417.2(a)(1) and for supporting
decisions in their hazard analysis as required by 9 CFR 417.5(a)(1).
The following hazard is present in raw products whose outgrowth during the
heating come-up time should be controlled:
• Staphylococcus aureus (S. aureus)
The following are hazards present in raw products that the lethality treatment
should be designed to destroy:
• Salmonella
• Shiga toxin-producing Escherichia E. coli (STEC) (in beef)
• Campylobacter (in poultry)
• Lm
• Trichinae spiralis and Toxoplasma gondii (in pork, especially feral or non-
confinement raised swine)
NOTE: Although all of these hazards are a concern, Salmonella is considered an
indicator of lethality because the thermal destruction of Salmonella in cooked products
would indicate the destruction of most other pathogens (64 FR 732).
More details about S. aureus and Salmonella (an indicator of lethality) can be found on
the following page.
14
S. aureus
S. aureus is a bacterial pathogen that causes nausea, vomiting, and abdominal
cramping with or without diarrhea. The Centers for Disease Control and Prevention
(CDC) estimates over 240,000 illnesses annually in the U.S. are attributed to S. aureus
(Scallan et al., 2011). S. aureus causes illness when the bacteria grows to high levels
in food and one or more heat-stable enterotoxins are produced (Kadariya et al., 2014).
Various types of foods serve as the optimum vehicle for S. aureus. The pathogen has
been identified in meat products, such as fermented salami and brine-injected hams. In
the 1980s, S. aureus enterotoxin outbreaks were frequently attributed to hams.
Continued outbreaks at hotels, restaurants and institutions as documented in the
National Outbreak Reporting System (NORS)
4
highlight that S. aureus is still a concern
in hams particularly when prepared in these settings. For example, between 2013 to
2018, at least six S. aureus enterotoxin outbreaks at hotels, restaurants and institutions
were reported in NORS in which ham was the suspected food vehicle. S. aureus can
contaminate raw meat and poultry from the animal hide, skin, or tissue during slaughter.
After slaughter and cooking, RTE meat or poultry products can be contaminated with S.
aureus from handling by individuals carrying the organism. This pathogen is the main
food safety concern during long heating come-up-times (CUT) (that is the amount of
time product temperature is between 50 to 130°F while heating). S. aureus can be
present on the raw meat or poultry and grow to high enough levels to produce a toxin in
the food. Growth occurs from 45 to 118°F, but effectively begins at 60˚F, especially in
raw meats where the growth of other bacteria is inhibited by nitrite or salt. The critical
level for human illness is 5-Log or higher which allows enterotoxin production (Kadariya
et al., 2014). The toxin is not destroyed by the critical operating parameters described
in this cooking guideline.
FSIS recommends limiting the growth of S. aureus during processing to 2-Log or less.
Normal levels of S. aureus in raw meat are usually 2-Log (Doyle and Buchanan, 2013;
IFT, 2003; Waldroup, 1996). Limiting growth to 2-Log or less allows for a margin of
safety before S. aureus would produce toxins. Conditions that allow 3-Log growth are
considered a public health concern because they would result in a total of 5-Log S.
aureus in the product which is considered the minimum critical level for human illness
(Kadariya et al., 2014).
To limit S. aureus growth, some establishments formulate products with antimicrobials
such as phosphate or lactate. But the most common practice is to limit the amount of
time products spend in the temperature range where S. aureus grows the fastest (i.e.,
50 to 130°F). This guideline identifies CUT as a critical operating parameter to ensure
lethality by cooking when applying the time-temperature tables (see FSIS Critical
Operating Parameters for Cooking on page 23). FSIS is aware that establishments
preparing some products (e.g., ham or beef brisket) may not be able to follow FSIS’s
Come-Up-Time Option because of the thermodynamics of the heating process.
Therefore, FSIS identified long CUT as a Scientific Gap since support does not exist for
many common processes (page 48). This gap supports the use of any of FSIS’s
applicable time-temperature combinations (pages 35, 37, 38) and relative humidity,
4
https://www.cdc.gov/nors/index.html
15
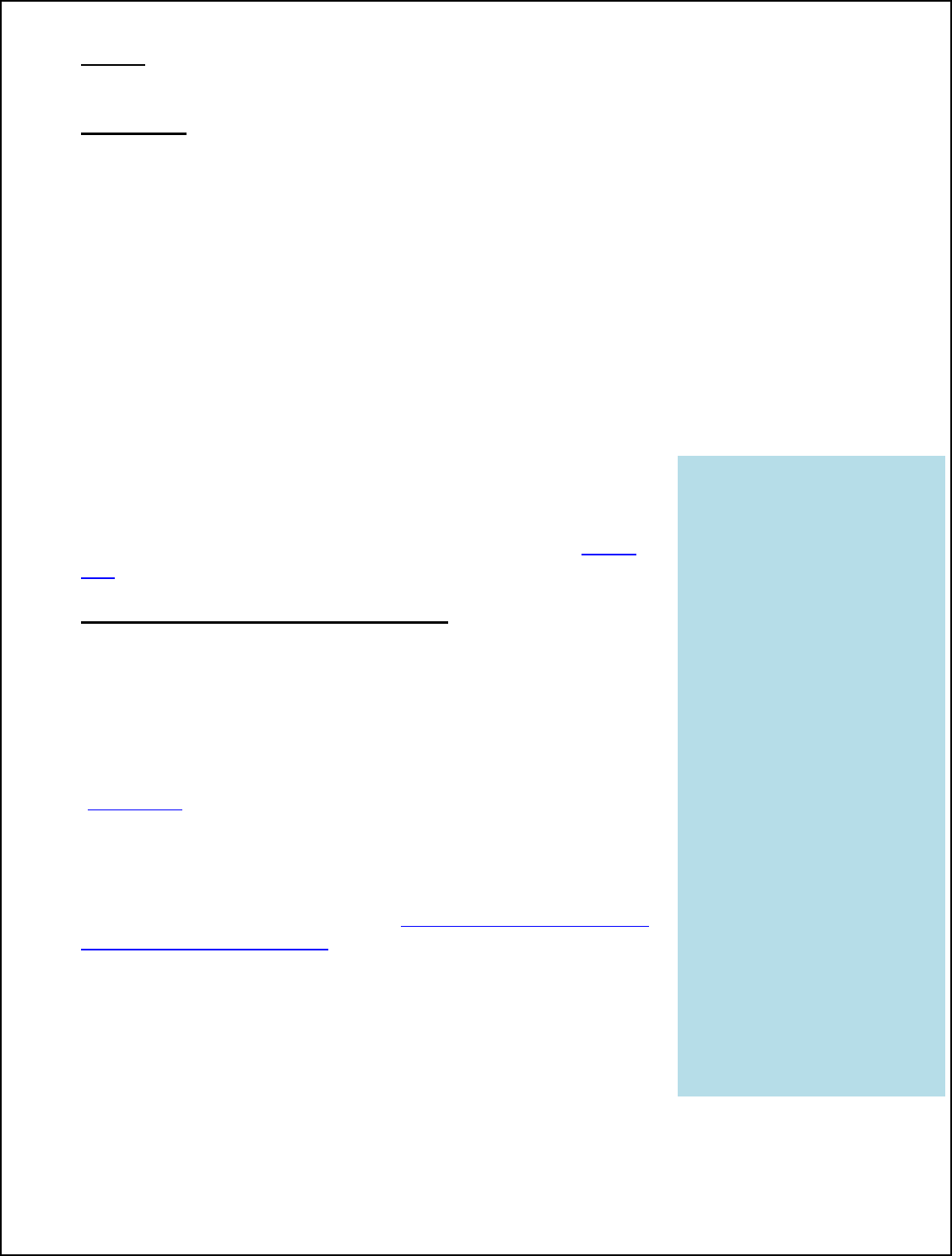
KEY DEFINITIONS
Critical operating
parameters are those
parameters of an
intervention that must be
met for the intervention to
operate effectively and as
intended. Such
parameters include but
are not limited to time,
temperature, water
activity, concentration,
relative humidity, and type
of equipment (to the
extent that the use of
different equipment would
result in an inability to
achieve the critical
parameters of the study).
without considering CUT as a critical operating parameter until research can be
complete.
Salmonella
Salmonella is a bacterial pathogen that causes diarrhea and fever. Infection with
Salmonella may result in arthritis (Ajene et al., 2013). The CDC reports that
nontyphoidal Salmonella species (spp.) is one of the leading causes of foodborne
illness, with an estimated 1 million cases of foodborne Salmonella infection annually in
the U.S (Scallan et al., 2011). Salmonella spp. infections are the second leading cause
of foodborne illness in the United States. Meat and poultry outbreaks are frequently
associated with Salmonella spp.
Salmonella occurs naturally in raw animal products; however, Salmonella should not be
found in RTE meat and poultry products because these products have undergone a
lethality treatment. Also, RTE products are intended to be consumed without further
preparation for safety (i.e., cooking), and if pathogens are present, their consumption
may cause illness. FSIS considers all RTE meat and poultry products that are
contaminated with Salmonella, as well as Listeria
monocytogenes and STEC, to be adulterated under the Federal
Meat Inspection Act and Poultry Products Inspection Act (21
U.S.C. 601(m)(1)) and 453(g)(1)). Any detectable Salmonella or
other pathogens of concern adulterates RTE products (64 FR
732).
Salmonella as an Indicator of Lethality
Meat and poultry products may be contaminated with Salmonella
during the slaughter and dressing process and by cross-
contamination in the processing environment when insanitary
conditions are present. For cooked products, FSIS recommends
that establishments use Salmonella as an indicator of lethality
because the thermal destruction of Salmonella in cooked
products would indicate the destruction of most other pathogens
(64 FR 732). If the establishment’s scientific support
demonstrates that the lethality treatment achieves sufficient
reduction in Salmonella, it does not need to provide additional
support that adequate reduction of other pathogens such as
STEC, Campylobacter, Lm, Trichinae spiralis or Toxoplasma
gondii is achieved. As stated in the FSIS Compliance Guideline
HACCP Systems Validation, establishments should not use
pathogens other than Salmonella as indicators of lethality for
cooked products unless the alternate pathogen displays similar
or higher resistance to the lethality processes.
NOTE: While Salmonella is considered an indicator of lethality
for validation purposes, in the event of a deviation where the
establishment missed its time-temperature parameters or
applied insufficient relative humidity, FSIS recommends testing for other pathogens of
concern (e.g., E. coli O157:H7 and Lm) because the absence of Salmonella does not

16
assure the absence of other pathogens since the establishment was unable to follow
the critical operational parameters in its scientific support. In addition, depending on the
type of deviation, other pathogens may also be of concern (e.g., C. perfringens and C.
botulinum). For more information see Attachment A2. Cooking Deviations, page 66.
How to Control Salmonella
Establishments must ensure the target Log reduction of Salmonella and other
vegetative pathogens is achieved throughout the product. To ensure vegetative
pathogens, including Salmonella, are killed on the interior of the product, the endpoint
time-temperature combination the product achieves is a critical operating parameter.
Most often, the target temperatures used during cooking reported in scientific support
documents and this guideline are the internal temperatures that the product should
reach. FSIS has found that some establishments use the recommendations established
for internal product temperature to set critical limits for the oven temperature. However,
setting the oven temperature to the temperature identified in the FSIS time-temperature
tables is not appropriate because doing so does not ensure that the product will reach
the same target internal temperature.
In addition to the product temperature, the amount of time the product is held at this
temperature (also known as the dwell time) is also critical to ensuring that adequate
lethality is achieved. If the product is held at the target temperature for less time than
specified in the time-temperature tables in this guideline, then adequate lethality may
not be achieved.
To ensure a process achieves the target Log reductions of Salmonella on the surface of
the product, moisture during cooking is a critical factor. Moisture (e.g., relative humidity)
around a product during cooking promotes lethality on the product surface in two ways:
• Moist cooking reduces surface evaporation from the product during heating
(evaporative cooling). Producing products under conditions of high moisture
early in the cooking process reduces evaporative cooling allowing product
surfaces to reach higher temperatures resulting in a greater reduction in
microorganisms; and
• Moist cooking keeps the product surface (and any pathogens) wet which
prevents product drying. Product drying reduces the water activity and
concentrates solutes (e.g., sugar and salt). Research has demonstrated that
bacteria can become more heat tolerant as their moisture levels decrease, and
increased concentrations of solutes, especially salt, increase the heat resistance
of bacteria (Buege et al., (2006), Boles et al., (2004), and Sindelar et al., (2016)).
Therefore, drying of the product surface before pathogens are destroyed will
increase pathogen heat resistance and allow the pathogens to survive the
heating process.
By incorporating moisture (e.g., relative humidity) to minimize evaporation and the loss
of surface moisture from the product, the D values (time at a constant temperature
necessary to destroy 90% or 1-Log of the target organism) that are the basis for the

17
time-temperature combinations, will remain valid (Goepfert, 1970; Goodfellow and
Brown, 1978). If evaporation, drying, or an increase in solute concentration is likely to
occur, the times and temperatures in scientific studies and supporting documentation
are not likely to be sufficient to provide the required lethality.
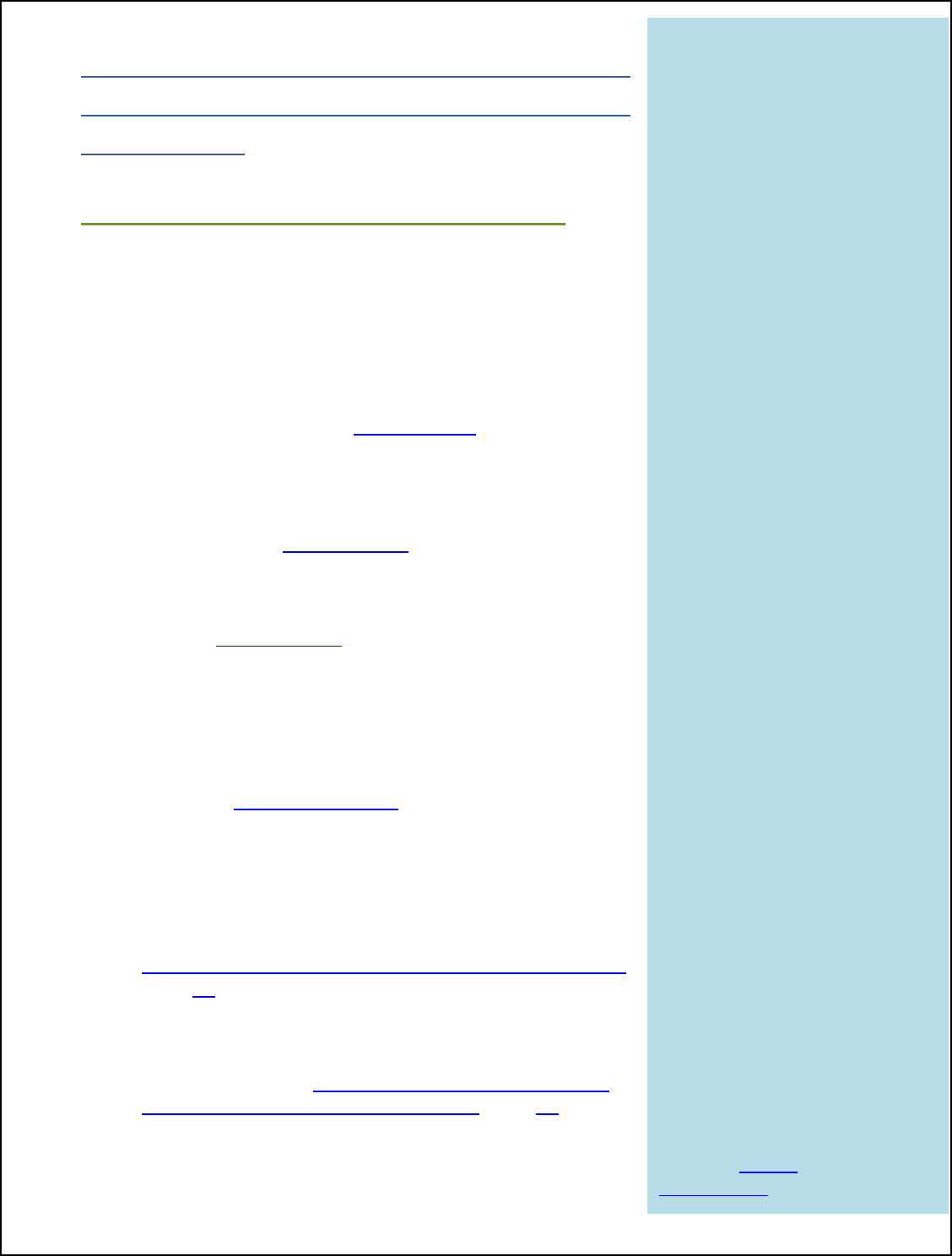
How does Moisture Ensure Bacteria on the Surface are Killed During Cooking?
During cooking, achieving a high oven temperature and internal product temperature
alone are not enough to ensure the final product is free of harmful bacteria.
Establishments need to make sure that cooking is done in a moist environment to
ensure lethality. When relative humidity is low, oven air is dry, and a process called
evaporative cooling increases, which is something we do not want. Evaporative
cooling is the same thing that allows humans to keep cool by sweating. When you get
too hot, you produce sweat, and when that sweat evaporates, it cools you down.
Evaporation equals cooling.
When you get too
hot…
…you produce
sweat.
When that sweat
evaporates…
…it cools you down.
Just like on a person’s skin, evaporative cooling cools down the surface of meat and
poultry during cooking. Although the oven is hot, because the surface of the product is
cooling down, that moisture evaporation can actually prevent the surface of the product
from becoming hot enough to kill off harmful bacteria. We can reduce evaporative
cooling by keeping the humidity in the oven high. That way the moisture in the product
does not evaporate as quickly, keeping the meat’s surface moist and hot and resulting
in an adequate bacterial kill. Why
does this work?
Imagine that you are in New
Mexico or Nevada where it is really
hot, but dry. If you’re outside,
you’re more likely to sweat and
that sweat will cool you down, so
you don’t feel as hot. Now imagine
you’re in Florida where it is not
only really hot, but also humid. If
you’re outside where it is humid,
your skin’s surface will stay sweaty
and hot, your sweat will not
Desert
Dry Heat
= Cooling Down
VS.
Tropical
More Humidity
= Less Cooling
evaporate, and you will not cool down. Since the air is already saturated, or full of
moisture (humid), there is less evaporation from your body and, therefore, less cooling.
The way humidity keeps you hot in Florida is the same way moisture keeps meat and
Evaporation
=
Cooling

18
poultry products hot, too.
19
General Considerations for Designing
HACCP Systems to Achieve Lethality
by Cooking
Addressing Lethality in the HACCP System
FSIS has established performance standards in the
regulations for specific ready-to-eat (RTE) products. The
performance standards for specific products set required
levels of Salmonella lethality during cooking as follows:
• Cooked poultry products must be processed to
achieve at least a 7-Log reduction of Salmonella or an
alternative lethality per 381.150(a)(1).
• Roast, cooked, and corned beef must be processed
to achieve at least a 6.5-Log reduction of Salmonella
or an alternative lethality (e.g., at least a 5-Log
reduction)) per 9 CFR 318.17.
• Cooked uncured meat patties must be processed to
meet or exceed the time-temperature combinations
listed in 9 CFR 318.23, which will achieve a 5-Log
reduction of Salmonella (and other pathogens
including STEC).
For products that are not subject to a performance standard,
FSIS recommends the following pathogen Log reductions
(i.e., targets) be achieved in order to support decisions in the
hazard analysis (9 CFR 417.5(a)(1)):
• For cooked meat products, FSIS recommends that
establishments achieve a target 6.5-Log or 5-Log
reduction of Salmonella in their process. To use a
target 5-Log reduction, establishments should provide
additional support for the safety of their process (see
Supporting an Alternative Lethality Target (e.g., 5-Log)
page 57).
• For shelf-stable meat products, FSIS recommends
that establishments achieve a target 5-Log reduction
of Salmonella (see How is Alternative 5-Log Lethality
Related to Risk of Foodborne Illness? page 57).
KEY DEFINITIONS
Performance standards
described in this guideline
are quantifiable pathogen
reduction levels or growth
limit requirements set by
FSIS for lethality and
stabilization of certain meat
and poultry products.
A Log reduction is a 90%
reduction of a pathogen.
For example, a 2-log
reduction is a 99%
reduction of a pathogen and
a 3-log reduction is a 99.9%
reduction of a pathogen in a
product.
Targets are quantifiable
pathogen reduction levels
or growth limits set by the
establishment to produce
safe products in the
absence of regulatory
performance standards.
An alternative lethality is a
treatment that achieves a
different (often lower) Log
reduction than what is
prescribed in the
regulations for certain
products, but still achieves
an equivalent probability
that no viable Salmonella
cells remain in the finished
product, nor other
pathogens and their toxins
or toxic metabolites. An
alternative lethality prevents
adulteration and must be
demonstrated to be
achieved throughout the
product (9 CFR
318.17(a)(1)).
20
An establishment should identify the performance standard or specific Log reduction
target its process is designed to achieve in its HACCP plan or supporting
documentation. If it does not, and FSIS cannot determine the pathogen reduction level
the process achieves, FSIS may determine the establishment lacks support for its
decisions related to Salmonella control (9 CFR 417.5(a)(1)). In addition, according to 9
CFR 417.2(c)(3), establishments must design their critical limits for Critical Control
Points (CCPs) to meet all applicable performance standards and targets.
NOTE: If an establishment uses the time-temperature tables provided in this guideline
or cooks beef patties according to 9 CFR 318.23, it does not need to indicate the
specific Log reduction that its process achieves. It would be sufficient for the
establishment to indicate that it uses time-temperature combinations from one of these
documents as these regulations were designed to achieve a 5-log reduction in
Salmonella and other pathogens including STEC.
Establishments are also required to validate that their HACCP system works as
intended to address these hazards (9 CFR 417.4(a)). For more information on
validation see the HACCP Systems Validation Guideline.
Key Question
Question: When a RTE meat food product is a mixture of meat and poultry such that the
product has a meat legend, and the establishment is following this cooking guideline, does the
RTE meat food product need to comply with the regulatory requirement found in 9 CFR
381.150(a)(1)?
Question: If a RTE meat food product has any amount of poultry in it, does it automatically
have to meet the poultry Log reduction in the FSIS Time-Temperature Tables?
Answer: Yes to both questions.
RTE meat or poultry food products consisting of any combination of meat and poultry must meet
the poultry lethality performance standard in 9 CFR 381.150(a)(1). Under the published final
rule "Performance Standards for the Production of Certain Meat and Poultry Products," cooked
product with any amount of poultry needs to meet the lethality requirements for the production of
fully cooked poultry products (9 CFR 381.150(a)(1)) which stipulate a 7-Log Salmonella
reduction or an alternative lethality that achieves an equivalent probability that no viable
Salmonella organisms remain in the finished product. This provision is based on the FSIS
national microbiological "baseline" survey of raw whole and ground meat and poultry products,
which found higher levels of Salmonella in poultry than in meat (USDA 1994, 1996a-f).
Consequently, FSIS established a higher lethality performance standard for RTE poultry
products than for meat (based on highest "worst case" levels).
21
Alternative Lethality
An alternative lethality is a treatment that achieves a different (often lower) Log
reduction than what is prescribed in the regulations but still achieves an equivalent
probability that no viable Salmonella cells remain in the finished product, as well as
ensures the reduction of other pathogens and their toxins or toxic metabolites (e.g.,
from S. aureus) necessary to prevent adulteration. Establishments may use alternative
lethality treatments to meet the performance standards (9 CFR 318.17(a)(1) and 9 CFR
381.150(a)(1)). When using an alternative lethality treatment (e.g., at least a 5-Log
reduction of Salmonella), the establishment must validate its HACCP system to ensure
that no viable Salmonella organisms (that is no organisms capable of causing human
illness) remain in the finished product. Risk assessments have demonstrated that
achieving a 5-Log reduction of Salmonella (instead of a 6.5-Log reduction) in cooked
meat and poultry products that are not shelf stable is less protective of public health
(Refer to text box: How is Alternative 5-Log Lethality Related to Risk of Foodborne
Illness? page 57). Therefore, to use these lower targets, the establishment must
provide additional support for its process as described in Attachment A1. Customized
Processes and Alternative Lethality Support: Supporting an Alternative Lethality Target
(e.g., 5-Log) on page 55. In contrast, risk assessments have shown that for shelf-stable
meat and poultry products, a 5-Log reduction of Salmonella (instead of a 6.5-Log or 7-
Log reduction) is sufficient. Therefore, no additional support is needed to use a 5-Log
reduction process in these shelf-stable products (9 CFR 417.5(a)(1) and 9 CFR
417.4(a)(1)).
Monitoring, Calibration, and Recordkeeping
The establishment’s cooking procedures should be designed to ensure all products in a
batch or lot achieve lethality, and the monitoring procedures should be designed to
detect a deviation when it occurs. To achieve these goals, establishments should
carefully consider the selection of the critical limit, as well as the design of their
monitoring procedures. Lessons learned from several recalls attributed, in part, to
insufficient monitoring procedures are shared on page 22.
Selection of the critical limit
Establishments producing cooked meat and poultry products should have sufficient
monitoring equipment, including recording devices, to assure that the time, temperature,
and relative humidity operating parameters of their processes are being met. With any
monitoring equipment, the establishment should take the normal variation of the
monitoring equipment into account when designing the critical limits. For example, if a
minimum internal temperature of 165°F is necessary to destroy pathogens in a product
and the thermometer has an accuracy of ± 1°F (plus or minus one degree), then the
critical limit should be set no lower than 166°F. The written reasoning and equipment
specification materials should be kept as part of the establishment’s supporting
documentation for its HACCP plan and the selection of its critical limit (9 CFR
417.5(a)(2)). All supporting documents and data from the recording devices must be
made available to FSIS employees upon request (9 CFR 417.5).
22
Selection of the monitoring procedures
Establishments are required to maintain documents supporting the selection of
monitoring procedures and associated monitoring frequencies (9 CFR 417.5(a)(2)). It is
important that establishments take into account variation within the cooking process
when developing monitoring procedures to ensure the procedures they develop can
identify any deviations.
In addition, to accurately measure the internal temperature of the meat or poultry
product, an establishment should understand the factors that can affect this
temperature. These factors include cold spots in the oven, as well as variations in oven
temperature during different seasons. Establishments should be aware that updated
smokehouses that contain alternating or rotating dampers that result in varying
breakpoints throughout the oven do reduce the temperature difference throughout the
oven, but they do not eliminate it. Although monitoring the internal product temperature
is strongly encouraged, an establishment can use the oven or smokehouse temperature
in place of the product temperature, provided that the establishment has a consistent
product and process and has sufficient data on file correlating the oven temperature
selected with the internal product temperature in the scientific support.
A disadvantage with monitoring oven temperature alone is that it may make supporting
product disposition after a cooking deviation more difficult. In many cases, FSIS
recommends using predictive microbial modeling programs to evaluate potential
hazards (see Attachment A2. Cooking Deviations on page 66). Microbial modeling
programs use product temperature to predict pathogen growth and potential Log
outgrowth or reductions achieved. Without product temperature records, the
establishment would need other support (e.g., product testing) to determine product
disposition.
Key Question:
Question: How does an establishment develop a monitoring procedure for measuring endpoint
temperature in meat or poultry products that are fried crispy such that a probe cannot be
inserted into the product to measure internal temperature (e.g., popped pork skins, and bacon
slices, pieces, or bits) because the product is too thin or hard or because the thin product cools
as soon as the product exits the cooking medium?
Answer: Depending on the product type, there are different recommendations. For example,
for a product such as bacon slices, it may be possible to cut a slice twice as thick as normal so
that the probe can be inserted. If this thicker piece reaches the lethality temperature, the thinner
pieces should as well. This procedure is also recommended for jerky. It is not recommended to
fold a piece of product over the thermometer, as this has been found to result in inaccurate
temperatures (Buege et al., 2006). For small products, such as bacon pieces or bits, it may be
possible to pile the pieces or bits around the thermometer for measurement. If none of these
procedures can be used, establishments may use other quantifiable measures such as a color
scale value that is correlated to crispiness or the number of pieces that pass as "fried until
crispy in all parts" based on a visual assessment as the critical limit for lethality for these
products due to the physical challenges in monitoring the internal temperature, and the lack of
outbreaks associated with them.

23
Lessons Learned from Undercooked Product Recalls
In 2016 and 2017, there were five recalls associated with under-cooked RTE poultry
products (RC-106-2016, RC-110-2016, RC-115-2016, RC-017-2017, and RC-037-
2017). For each of these recalls, FSIS determined that even though the establishments
had documentation showing the critical limit (either 160°F or 165°F) was met, there
were still pieces that may have entered commerce undercooked, indicating a loss of
process control and insufficient monitoring procedures to identify a process deviation.
Investigations revealed a variety of concerns related to monitoring procedures, including
taking temperatures from products not in the coldest spot, taking multiple product
temperatures, and averaging the results of multiple temperature measurements as
opposed to recording the lowest temperature.
Investigations also revealed a variety of contributing factors for inadequate cooking
including:
• Raw product was partially frozen.
• Belt speed was increased.
• Shorter dwell time and lower oven temperature than normal were used.
• Product was stacked during sous vide cooking, preventing full immersion of the
bags into the liquid cooking medium.
• Higher than normal product load overwhelmed the oven.
Each of these practices may have led to uneven or inadequate cooking. These findings
also highlight the importance of maintaining process control of critical operating factors,
such as oven temperature, product load, and belt-speed that affect the final product
temperature, dwell time, and relative humidity. The establishment is required to validate
that the entire HACCP system is operating as intended and to verify that it is producing
a safe and wholesome product on an ongoing basis.
Complete failure to document critical limit monitoring has also contributed to the recall of
cooked poultry products in the past due to a processing defect (RC-009-2017). Such a
failure highlights the importance of accurate records documenting the implementation of
the critical operating parameters to support the production of safe products.
Corrective Actions under HACCP Cooking Deviations
Cooking deviations occur when an establishment fails to meet its cooking CCP critical
limit or cooking humidity option. Common causes for cooking deviations include
product overlap, power failures, or breakdown of cooking equipment. The HACCP
regulations require establishments to take corrective actions in response to these
deviations, regardless of whether the cooking process is addressed through a CCP or
prerequisite program. Corrective actions include ensuring no product that is injurious to
health or otherwise adulterated because of the deviation enters commerce and
supporting product disposition decisions (9 CFR 417.3(a) and (b)).

24
When cooking is addressed through a CCP, establishments are required to determine
the cause of all cooking deviations, no matter how small (9 CFR 417.3(a)(1)), and
ensure measures are established to prevent recurrence (9 CFR 417.3(a)(3)). Continual
or repetitive process deviations from the critical limit demonstrate that the establishment
is unable to control its process.
When cooking is addressed through a prerequisite program, establishments are
required to reassess their HACCP system to determine whether the newly identified
deviation or unforeseen hazard should be addressed and incorporated into the HACCP
plan (9 CFR 417.3(b)(4)). Also, an establishment may not be able to continue to
support the decision in its hazard analysis that pathogens are not reasonably likely to
occur, if it has continual or repetitive deviations from its cooking prerequisite program (9
CFR 417.5(a)(1)). For more information on evaluating product disposition after a
cooking deviation see Corrective Actions to Perform When a Cooking Deviation Occurs
(page 66).
FSIS Critical Operating Parameters for Cooking
(Time-Temperature Tables)
Establishments that cook products to achieve lethality by applying the time-temperature
combinations from this guideline need to consider the critical operating parameters that
may affect pathogen Log reductions, specifically:
• Come-up-time (CUT),
• Relative Humidity, and
• Endpoint Time-Temperature.
Additionally, establishments cooking poultry products need to consider product species
composition and fat content if applying FSIS cooking lethality guidance in the tables on
pages 37 and 38. The FSIS Cooked Poultry Rolls Options (page 39) apply to all poultry
products regardless of poultry species or fat content. For information about why product
species should be considered when applying cooking lethality guidance on pages 37
and 38 and not when applying the FSIS Cooked Poultry Rolls Options see page 36.
Come-Up-Time (CUT)
When applying one of the time-temperature tables from this guideline, an establishment
must also consider the heating CUT to be a critical operating parameter unless the
establishment can provide a science-based rationale why heating CUT does not need to
be addressed. For example, products that are fermented and then cooked to lethality
may control S. aureus outgrowth by lowering the pH following the degree-hour concept
as recommended in the American Meat Institute’s Good Manufacturing Practices for
Fermented Dry & Semi-Dry Fermented Sausathge Products and therefore would not
address CUT.
FSIS has developed a CUT Option that establishments may use to support its process
control of S. aureus growth, specifically ≤ 2-Log that also
prevents enterotoxin formation:
Come-Up-Time Option: Total time product temperature is
between 50 and 130°F is 6 hours or less.
NOTE: This CUT Option is only for products that were cooked to
lethality (including those cooked to lethality but classified as
NRTE under a heat treated, not fully cooked, not shelf-stable
HACCP plan). Please refer to the FSIS Stabilization Guideline for
Meat and Poultry Products for the Agency’s recommendations
regarding CUT in partially cooked products that do not receive a
full lethality. Please also refer to the product reclassification
guidance in the Listeria Guideline, Attachment 1.2 on pages 22-
23 and Appendix 1.2 on pages 28-29.
FSIS is aware that establishments preparing some products (e.g.,
ham or beef brisket) may not be able to follow FSIS’s Come-Up-
Time Option above because of the thermodynamics of the
heating process. Therefore, FSIS identified long CUT as a
Scientific Gap since support does not exist for many common
processes (page 48). Additionally, alternative support for certain
long CUT processes have been included in Attachment A1.
Customized Processes and Alternative Lethality Support (page
55).
Temperatures referred to in FSIS’s Come-Up-Time Option above,
are internal temperatures. However, establishments may monitor
surface temperatures during CUT, if the establishment provides
support the product is intact and processed so pathogens have
not been introduced below the product surface. Non-intact
product temperatures should be taken internally at the center of
KEY DEFINITIONS
Come-up-time refers to
the
amount of time product
temperature is between 50-
130°F while heating.
Intact refers to products
where the interior remains
protected from pathogens
migrating below the
exterior/outside (such as
beef brisket or a picnic
shoulder that is not injected
or vacuum tumbled).
Non-Intact refers to
products where pathogens
may have been introduced
below the surface.
Examples include products
that have been
mechanically tenderized
(including those that have
been injected with
marinade or solution) or
vacuum tumbled.
the product (see Key Definitions panel to the right for an explanation of intact and non-
intact products). Establishments should also take temperatures at the center of the
product for products such as deboned and rolled hams where a portion of the product is
rolled or folded over and pathogens may be internalized.
NOTE: FSIS time-temp tables list internal endpoint temperatures during cooking. It is
not supportable to use surface temperature to address endpoint temperature. FSIS is
only making this recommendation for its CUT option.
24
Relative Humidity
FSIS time-temperature tables use relative humidity as a critical operating parameter to
ensure moist cooking and adequate surface lethality. An establishment that uses the
FSIS time-temperature tables to support its cooking process must address humidity,
unless it meets one of the criteria listed in Situations when Humidity is Not Needed
(page 31) or provides additional support for why
humidity would not be needed in its process to ensure
lethality on the product surface. FSIS has included
specific relative humidity options for use with the time-
temperature tables (page 26). Additional resources
for determining which relative humidity option to adopt
are included in Relative Humidity Resources (page
28).
NOTE: FSIS is aware that some establishments may
not be able to use FSIS’s humidity options because of
the nature of the cooking process. Examples include
products cooked for short times at high temperatures
(e.g., for meat balls or chicken tenders) or other
processes that do not allow the use of humidity (e.g.,
barbecue products cooked under dry heat including
those cooked in smokehouses or open pits). Please
refer to Scientific Gaps Identified by FSIS (page 41).
Selection of the proper relative humidity option
depends on the endpoint time-temperature. Products
cooked to endpoint time-temperatures of at least
145°F plus the dwell time, may apply any of the
relative humidity options in Table 1. Critical
Operating Parameters for FSIS Humidity Options.
25
Key Question
Question: An establishment cooks a brisket to full lethality but realizes the smoke
coloring is too light and wants to recook it to deepen the color. Can the establishment
apply a new 6 hour CUT for the second cook?
Answer: Yes. Once a product achieves a lethal time-temperature combination, the
allowed CUT is reset for the next cook. If the establishment chooses to recook the
product, it may apply a new 6 hour CUT limit (page 23). However, if the product did not
achieve a lethal time-temperature combination during the first cooking process, the CUT
does not start over. The establishment should support the total time product temperature
is between 50 and 130°F is 6 hours or less. Please review Attachment A2. Cooking
Deviations subsection Missed Time-Temperature Parameter (page 67) for additional
information.
KEY DEFINITIONS
Maintaining humidity means
keeping the humidity at the same
level throughout the cooking
process. If the humidity drops
during the cooking process, the
establishment will need to
provide additional support for the
safety of the product
A sealed oven is generally
defined as one in which the
smokehouse doors and oven
dampers are closed to prevent
moisture loss.
The cooking time includes the
time the product is placed in the
heated oven (including surface
preparation and color setting)
until the product reaches the
desired lethality time-
temperature combination (also
referred to as the “lethality
treatment”).
26
However, products cooked to an endpoint less than 145°F, should select Option 3 or 4
in Table 1. Critical Operating Parameters for FSIS Humidity Options depending on total
cooking time.
NOTE: To be most effective, humidity needs to be applied during the lethality
treatment, before drying. Using this guideline to support lethality processes in which
the drying step comes before the moist cooking step (e.g., country-cured ham)
creates a vulnerability in the establishment’s HACCP system. Establishments using
this guideline for these processes should read Attachment A6. Cooking Country-
Cured Hams (page 90) for recommendations to reduce this vulnerability, such as
measuring water activity after cooking to verify it increases and the product surface
was rehydrated during cooking.
To ensure that adequate humidity is attained, the establishment should monitor
the humidity throughout the lethality treatment. The process should be monitored
using wet and dry bulb thermometers (used to determine relative humidity) or a humidity
sensor. FSIS recommends that establishments monitor relative humidity for every lot or
batch of product produced.
Table 1. Critical Operating Parameters for FSIS Humidity Options
CRITICAL OPERATING PARAMETERS
Relative Humidity
Endpoint
Temperature
Cooking
Time
OPTION 1:
The relative humidity of the oven is
maintained by continuously introducing
steam for 50 percent of the cooking time,
or 1 hour, whichever is longer.
≥145°F +
dwell time
≥1 hour
OPTION 2:
The relative humidity of the oven is
maintained by a sealed oven for at least
50 percent of the total cooking time, or 1
hour, whichever is longer.
≥145°F +
dwell time
≥1 hour
OPTION 3: The relative humidity of the oven is
maintained at 90 percent or above for at
least 25 percent of the total cooking time,
or 1 hour, whichever is longer.
Any ≥1 hour
OPTION 4:
The relative humidity of the oven is
maintained at 90 percent for the entire
cooking time.
Any
Any
27
Key Question
Question: To follow the sealed oven or steam injection options, must establishments
achieve a specific relative humidity?
Answer: No. Establishments do not need to achieve a specific relative humidity level in
the oven if they are following the steam injection or sealed oven options in this guideline
as their scientific support. Based on expert opinion, the 2014 FSIS Jerky Guideline
recommended that establishments producing jerky that monitor relative humidity try to
achieve a wet bulb temperature of at least 125-130°F for 1 hour or more along with a
corresponding dry bulb temperature needed to achieve at least 27-32% relative humidity
or more. However, the Jerky Guideline also noted, achieving a wet bulb temperature of at
least 125-130°F and at least 27-32% relative humidity for 1 hour or more is not adequate
on its own to support that the process is being implemented consistently with FSIS
Humidity Options. Rather, establishments should ensure that all critical operating
parameters described in this guidance are met. Relative Humidity Resources (page 28
contains specific guidance for how to implement Option 1 steam injection and Option 2
sealed oven in a validated HACCP system. In addition, establishments should not apply
the wet-bulb and relative humidity recommendations in the Jerky Guideline to other
products without additional support.
Current Support for FSIS Relative Humidity Options
Although the research cited as the basis of FSIS guidance dates as far back as 1978,
newer research by McMinn et al., (2018) supports that the time-temperature parameters in
FSIS’s cooking guidance achieves sufficient reductions of Salmonella. This research by
McMinn et al. (2018) was conducted with product cooked in vacuum-sealed bags
supporting the importance of cooking in a high moisture environment. While newer
research has not been conducted to validate the sealed oven and steam injection relative
humidity options, research does continue to support the importance of moisture during
cooking. For example, Mann and Brashears (2007), support the need for at least 30%
relative humidity during cooking of roast beef. Based on FSIS knowledge of
establishments’ processes through its verification activities, the Agency believes when the
oven is sealed, or steam is introduced, at least 30% relative humidity is maintained,
suggesting that these practical recommendations result in adequate relative humidity.
The Agency is also not aware of any establishments that have had Salmonella positives or
been associated with a salmonellosis outbreak when following FSIS temperature, time,
and relative humidity guidance while using effective monitoring procedures.
2
*Relative humidity (RH) is 90% or higher for at least 25% of the total cooking
time, or 1 hour, whichever is longest.
**RH is maintained for
50% of the cooking time, or 1 hour, whichever is
longest
For more information,
refer to FSIS Relative Humidity Options on page 25.—
wouldn’t it be good to put the information together?
Additionally,
the following information in the FSIS Jerky Guideline can be
useful when deciding which humidity option to adopt:
Instructions for making your own wet bulb (reprinted with
permission from the University of Wisconsin, page 49); and
An example of a time-temperature recorder chart to support the
option of continuously injecting steam (page 53).
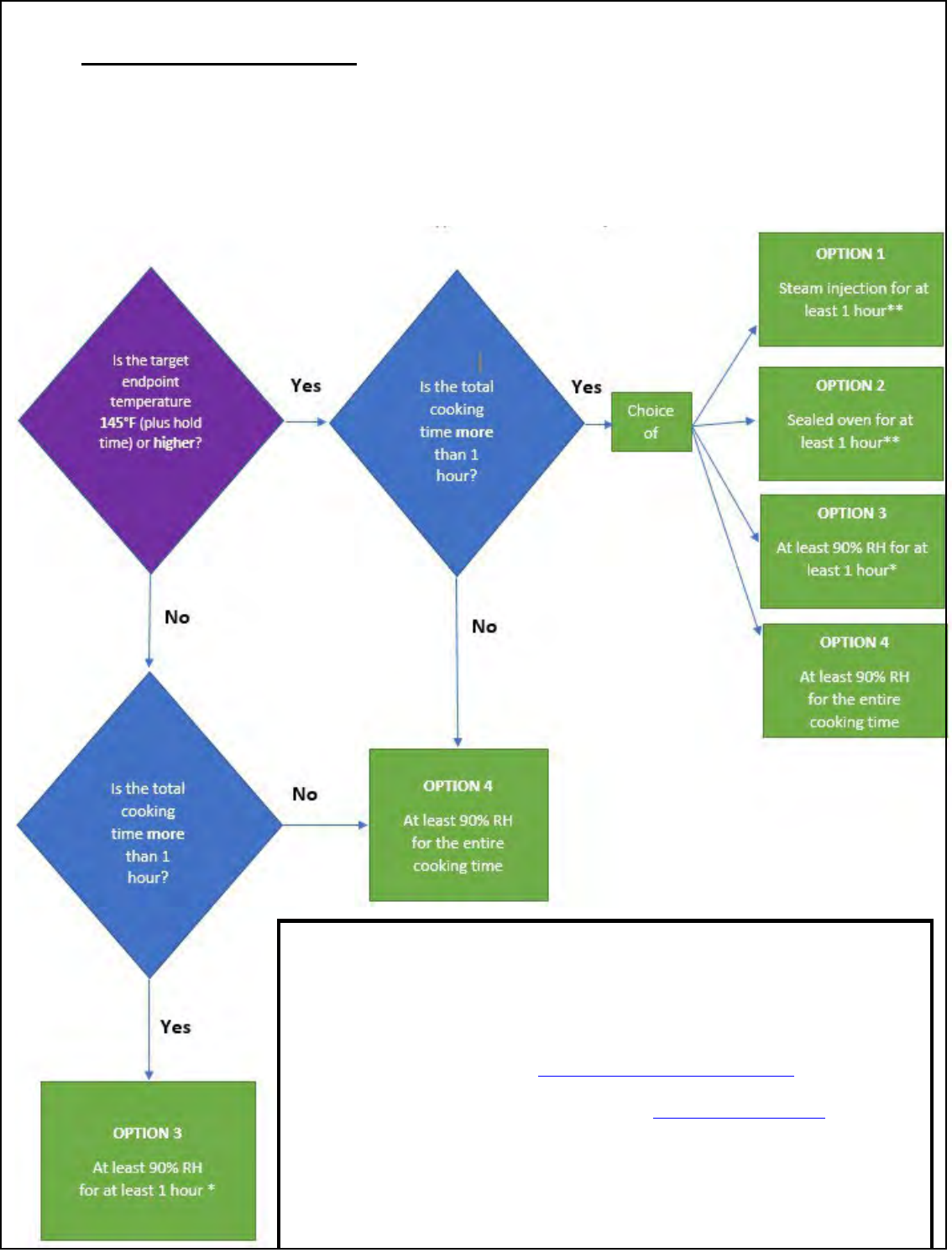
Relative Humidity Resources
The following flow chart contains specific guidance for how to choose a humidity option
and the resources on the next two pages are designed to help establishments
implement Option 1 steam injection and Option 2 sealed oven in a validated HACCP
system.
Flow Chart to Choose a Humidity Option
*Relative humidity (RH) is 90% or higher for at least 25% of the total cooking
time, or 1 hour, whichever is longest.
**RH is maintained for 50% of the cooking time, or 1 hour, whichever is
longest
For more information, refer to FSIS Relative Humidity Options on page 26.
Additionally, the following information in the FSIS Jerky Guideline can be
useful when deciding which humidity option to adopt:
• Instructions for making your own wet bulb (reprinted with
permission from the University of Wisconsin, page 49); and
• An example of a time-temperature recorder chart to support the
option of continuously injecting steam (page 53).
29
Specific Guidance for Using the “Sealed Oven” Option
To support the use of the sealed oven option for addressing relative humidity, FSIS
recommends establishments follow all 4 steps below:
1) Maintain documentation that supports that the product achieves an internal
product temperature equal to or greater than 145°F (plus the required dwell time)
from the FSIS time-temperature tables. Such documentation could include:
a. Records of internal product temperature and time held at that temperature, (if applicable); or
b. Records of the oven or smokehouse temperature in place of internal product temperature
provided that the establishment has a consistent product and process and has sufficient
data correlating the oven temperature selected with the internal product temperature in the
scientific support;
2) Maintain documentation that supports that the oven dampers are closed for at
least one hour or 50% of the cooking time, whichever is longer. Such
documentation could include:
a. Records from a computerized system that document the time at which the oven dampers
were open and were closed; or
b. Records, made manually, of the times at which the oven dampers were open and closed;
c. Records demonstrating that the relative humidity level in the oven is maintained for at least
one hour or 50% of the cooking time, whichever is longer (e.g., by use of dry and wet bulb
thermometers to calculate the relative humidity or use of a humidity sensor that provides a
direct measurement) with correlation data supporting a relationship between the relative
humidity level in the oven and the time at which the oven dampers were open and closed;
3) Maintain documentation that supports that when the oven dampers are closed,
humidity is maintained in the ovens. Such documentation could include:
a. Records demonstrating the relative humidity level in the ovens is maintained (e.g., by use of
dry and wet bulb thermometers to calculate the relative humidity or use of a humiditysensor
that provides a direct measurement), or
b. Data gathered during the initial validation period along with ongoing verification that
demonstrate that the relative humidity in the oven is maintained while the dampers are
closed; and
4) Perform routine checks to ensure the oven dampers are properly working along
with a maintenance program that includes periodic monitoring to ensure oven
seals are intact and functional, and that when the oven dampers are closed, a
tight seal is obtained.
A tight seal is one that prevents a significant loss of humidity. FSIS acknowledges
that a small amount of smoke or vapors might be seen escaping the smokehouse
even when a tight seal is obtained. FSIS also recommends establishments
consider whether there are other openings, particularly in older smokehouses, such
as drain valves or air intake valves that need to be closed to ensure that a seal is
obtained. Finally, some older ovens may have a stack or other opening that cannot
be closed. For those establishments with older ovens that cannot be completely
closed, the sealed oven method should not be used. However, the establishment
may choose to close the parts of the oven it can, then add moisture in the system
either by continuouslyintroducing steam, or by using another validated method.
30
Specific Guidance for Using the “Continuously Introducing Steam” Option
To support the use of the continuously introducing steam option for addressing relative
humidity, FSIS recommends establishments follow all 3 steps below:
1) Maintain documentation that supports that the product achieves an internal product
temperature equal to or greater than 145°F (plus the required dwell time) from the
FSIS time-temperature tables. Such documentation could include:
a. Records of internal product temperature and time held at that temperature, (if
applicable); or
b. Records of the oven or smokehouse temperature in place of internal product
temperature provided that the establishment has a consistent product and process and
has sufficient data correlating the oven temperature selected with the internal product
temperature in the scientific support;
2) Maintain documentation that supports that steam is continuously introduced for at
least one hour or 50% of the cooking time, whichever is longer. Such documentation
could include:
a. Records from a computerized system that contains the time at which the steam is turned
on and off; o r
b. Records, made manually, of the times at which the steam is turned on and off; or
c. Records demonstrating the relative humidity level in the oven is maintained for at least
one hour or 50% of the cooking time, whichever is longer (e.g., by use of dry and wet
bulb thermometers to calculate the relative humidity or use of a humidity sensor that
provides a direct measurement), along with correlation data supporting a relationship
between the relative humidity level in the oven and the time steam is turned on or a letter
from the manufacturer stating that when the relative humidity is rising, it is because of
live steam injection; and
3) Maintain documentation that supports that when steam is injected, humidity is
maintained in the ovens. Such documentation could include:
a. Records demonstrating the relative humidity level in the ovens is maintained (e.g., by
use of dry and wet bulb thermometers to calculate the relative humidity or use of a
humidity sensor that provides a direct measurement), or
b. Data gathered during the initial validation period along with ongoing verification which
demonstrates that the relative humidity in the oven is maintained while steam is being
injected.
NOTE: The “continuously introducing steam” option refers to the use of live steam.
This option may also apply to establishments that spray water onto hot heating
elements, which creates steam that in turn produces humidity in the smokehouse.
“Continuous” does not mean that the steam is injected for at least one hour during
one stage. Rather, steam could be injected during specific stages or time intervals
during the lethality (cooking) treatment as long as the total amount of time the steam
is introduced adds up to at least one hour or 50% of the cooking time, whichever is
longer. Furthermore, the establishment may turn the steam on and off throughout the
cooking time when the target humidity is reached.
31
Key definitions
Convective
heating
Conduction
Radiant heating
Conductive Heating:
Heat is transferred
directly into the food
product by physical
contact
with the heating
medium (e.g. heating
product on a
skillet).
Radiant Heating:
Heat
is transferred
directly
into the food product
by
radiant energy without
the movement of air or
physical
contact
between
the source
and
the food. Two common
examples:
Radiant
energy from the sun
warms Earth across
the
vacuum of space, or a
flame emits radiant
energy
to heat food
product in certain
rotisserie ovens.
Various
forms
of radiant
energy also include
gamma
rays, electron
beams, x
-rays, and
microwaves.
Establishments that use processes that match one of
these situations do not need to monitor relative
humidity as a critical operating parameter in their
cooking procedure.
Situations when Humidity is Not Needed
FSIS recognizes two situations when humidity does not need to be
addressed to ensure adequate lethality:
1. When moisture is inherently maintained; or
2. When product is cooked using direct heat.
Relative humidity does not need to be addressed when moisture is
inherently maintained around the product. Examples of these
types of processes include, but are not limited to:
• Completely immersing the meat or poultry product in a
liquid cooking medium throughout the entire cooking
process;
o E.g., unbagged, in water
• Cooking the product in a sealed, moisture impermeable bag
(e.g., cook-in-bag meat or poultry);
o Cook-in-bag products may be eligible to be labeled
as “pasteurized” (see Attachment A3. When can
Products be Labeled as Pasteurized? page 81).
• Cooking product in a casing that holds moisture (e.g.,
natural casings, cellulose casings, collagen casings, fibrous
casings and plastic casings (sometimes called "synthetic"
casings)).
o See the question box on page 33 for information on
cooking using natural casings.
• Heating meat or poultry products that weigh 10 pounds or
more in an oven maintained at 250°F (121°C) or higher
throughout a process achieving one of the time-
temperature combinations in this guideline.
NOTE: Humidity is not needed for products that weigh 10 pounds
or more in an oven maintained at 250°F (121°C) or higher
because they have a low surface to mass ratio (Goodfellow and
Brown, 1978). Therefore, the surface dries out slower than
smaller products and Salmonella is less likely to become heat
tolerant.
KEY DEFINITIONS
During
convective
heating
the food
product is indirectly
heated by the
movement of hot air.
This
type of heating
is
typical for
solid foods
cooked in a smoke
house oven.
Conductive Heating:
Heat is transferred
directly into the food
product by physical
contact with the
heating
medium (
e.g.,
heating product in a
skillet).
Radiant Heating:
Heat is transferred
directly into the food
product by radiant
energy without the
movement of air or
physical contact
between the source
and the food.
Two
common examples:
are
(1) broiling
where
food is exposed to
direct,
intense
radiant
heat or (2) certain
types of rotisserie
ovens where a flame
emits radiant energy
to heat food.
Forms of radiant
energy also include
gamma
rays,
electron
beams, and x-rays.
32
Relative humidity also does not need to be addressed for processes that apply direct
heat via conduction or radiant heating. Unlike convective heating, which uses moving
hot air or steam to heat the product (e.g., smoke house ovens, spiral ovens,
impingement ovens), direct heating (e.g., conductive heating, radiant heating) puts the
product in direct contact with the heating medium. Direct heat ensures the product
surface quickly reaches lethality temperatures before bacteria can develop heat
tolerance due to the product’s surface
quickly drying out.
Examples of direct heat include:
• Grill.
• Broil (exposure to direct, intense
radiant heat).
• Heating coil,
• Flame.
• Certain rotisserie ovens that cook the meat or poultry over the heat source
resulting in a product with a grilled quality.
NOTE: Direct heat cooking is rarely used in conjunction with rotisserie cooking. Indirect
heat cooking is most often used because it allows the meat or poultry to cook slowly
and evenly, which is the primary purpose for using a rotisserie for cooking. For indirect
heat cooking, the rotisserie is positioned in front of or next to the heat source and it is
the heated air that cooks the product (convection cooking).
Cooking meat patties per 9 CFR 318.23 does not include humidity considerations
because these products were assumed to be cooked with direct heat such as a grill,
heating coil, or flame. Meat patties cooked per 9 CFR 318.23 do not need to address
relative humidity. For the definition of a patty see 9 CFR 318.23.
NOTE: Products cooked using microwave cooking methods that are not designed to
control relative humidity is considered a Scientific Gap because these common cooking
processes can’t achieve the relative humidity options included in this guideline;
however, there is a lack of research to support alternative parameters. For the critical
operating parameters in this guideline that can be used for these processes, if using
FSIS guidance as scientific support, see Table 5. Scientific Gaps where Critical
Operating Parameters From Older Guidance May be Used page 44.
How is indirect heating identified?
Moving air or steam is a sign of convective (indirect)
heating. Ovens that use moving air to heat the
product need to address relative humidity to ensure
sufficient Log reductions for pathogens are achieved.
33
Do Products Cooked in Natural Casings Made from Animal Gastrointestinal
Tracts Need to Address Relative Humidity?
No, establishments using FSIS cooking guidance as support do not need to address
relative humidity for products that are cooked in a natural casing, including products
that are cooked and then dried.
1
Natural casings made from animal gastrointestinal tracts are typically considered
permeable and many establishments take advantage of their permeability to produce
dried products or smoked products. However, depending on how they are used, the
permeability of natural casings may be reduced. Most cooking processes likely reduce
the permeability of natural casings early in the process so that humidity around the
product is inherently maintained throughout the remainder of cooking and does not
have to be added or monitored. According to Sebranek, (2010), establishments will
apply smoke early in the process while the casing is still moist and permeable to the
smoke. Prior to smoke application, the casing surface should be "tacky" or "sticky."
After smoke deposition and color development, further cooking denatures the proteins
in the casing reducing permeability to the point that later cooking can be applied without
great moisture loss from the product. Proteins in natural casings begin denaturing at
126°F (Tornberg, 2005). However, most drying processes use lower temperatures and
address relative humidity to maintain casing permeability so that moisture can
evaporate from the product during drying.
Although most cooking processes likely result in reduced permeability of natural
casings early in the cooking process, little research has been performed to study the
critical operating parameters that impact the reduction of permeability such as the
length of the initial smoke application step, cooking temperature, total cooking time, use
of steam, size of casings, composition of sausage batter, etc. For this reason, FSIS
has posted a research study on its website to “Determine if natural casings maintain
sufficient moisture to ensure product lethality using Appendix A time and temperature
tables.” Without this additional research, the Log reduction of Salmonella is less
certain if meat products in natural casings are cooked using one of the time-
temperature parameters in this FSIS cooking guidance without following one of the
humidity options. So, while FSIS has indicated establishments using FSIS cooking
guidance as support do not need to address relative humidity for products that are
cooked in a natural casing, if an establishment uses one of the time-temperature
parameters in FSIS cooking guidance without addressing relative humidity has a
positive Salmonella test result through FSIS or its own testing, it should, as part of
corrective actions, provide evidence that lack of relative humidity was not the cause. In
addition, if research is completed and data becomes available that indicates relative
humidity needs to be addressed when products are cooked in a natural casing, FSIS
may change its recommendation.
1
NOTE: As described in the Products and Processes Not Covered by This Guidance,
this guideline is not appropriate support for lethality of a process that relies on drying
alone or to support a process where the drying step comes before a cooking step that
does not apply humidity or does not apply humidity during cooking at sufficient levels to
rehydrate the product surface during the cooking step under dry conditions.
34
Endpoint Time-Temperature
FSIS time-temperature tables in this guideline (Meat Table, the 5-Log Table, and the
Poultry Time-Temperature Tables) list internal product temperatures and the
corresponding dwell times needed to achieve specific Log reductions of Salmonella.
These tables may be used as scientific support to ensure that the process meets
regulatory requirements (see General Considerations for Designing HACCP Systems to
Achieve Lethality by Cooking, page 18).
NOTE: To apply an alternative lethality and use Table 6. Time-Temperature
Combinations for Meat Products to Achieve a 5-Log Reduction (page 59), an
establishment must provide additional documentation showing that the product meets
the performance standard (if applicable) and that potentially hazardous pathogens have
been controlled (see Attachment A1. Customized Processes and Alternative Lethality
Support subsection: Supporting an Alternative Lethality Target (e.g., 5-Log) on page
57). The support should demonstrate the incoming load of Salmonella is lower than
FSIS estimated based on its baseline studies, and therefore, a lower reduction from
cooking would result in no viable Salmonella in the finished product.
Key Question
Question: When an establishment decides to use a FSIS time-temperature table (i.e., the
5-Log Meat Table, 6.5-Log Meat Table, or the 7.0-Log Poultry Time-Temperature Tables)
from this guideline as its scientific support for its cooking/lethality step, can the
establishment use the entire table as its critical limit in its HACCP plan?
Answer: Yes, the establishment can use the entire table to comply with 9 CFR
417.2(c)(3). The establishment needs to make a sound determination and support its
decision in selecting and monitoring the time-temperature parameter(s) it uses for its
production (9 CFR 417.5(a)(2)). In addition, establishments must collect in-plant data for at
least one product from each HACCP category demonstrating the implementation of the
critical operational parameters of the scientific support (9 CFR 417.4(a)(1)). At a minimum,
the establishment will need to demonstrate that it is able to consistently meet a specific
time-temperature from the table identified during the initial validation period to support the
cooking/lethality process is validated (9 CFR 417.4(a)(1)).
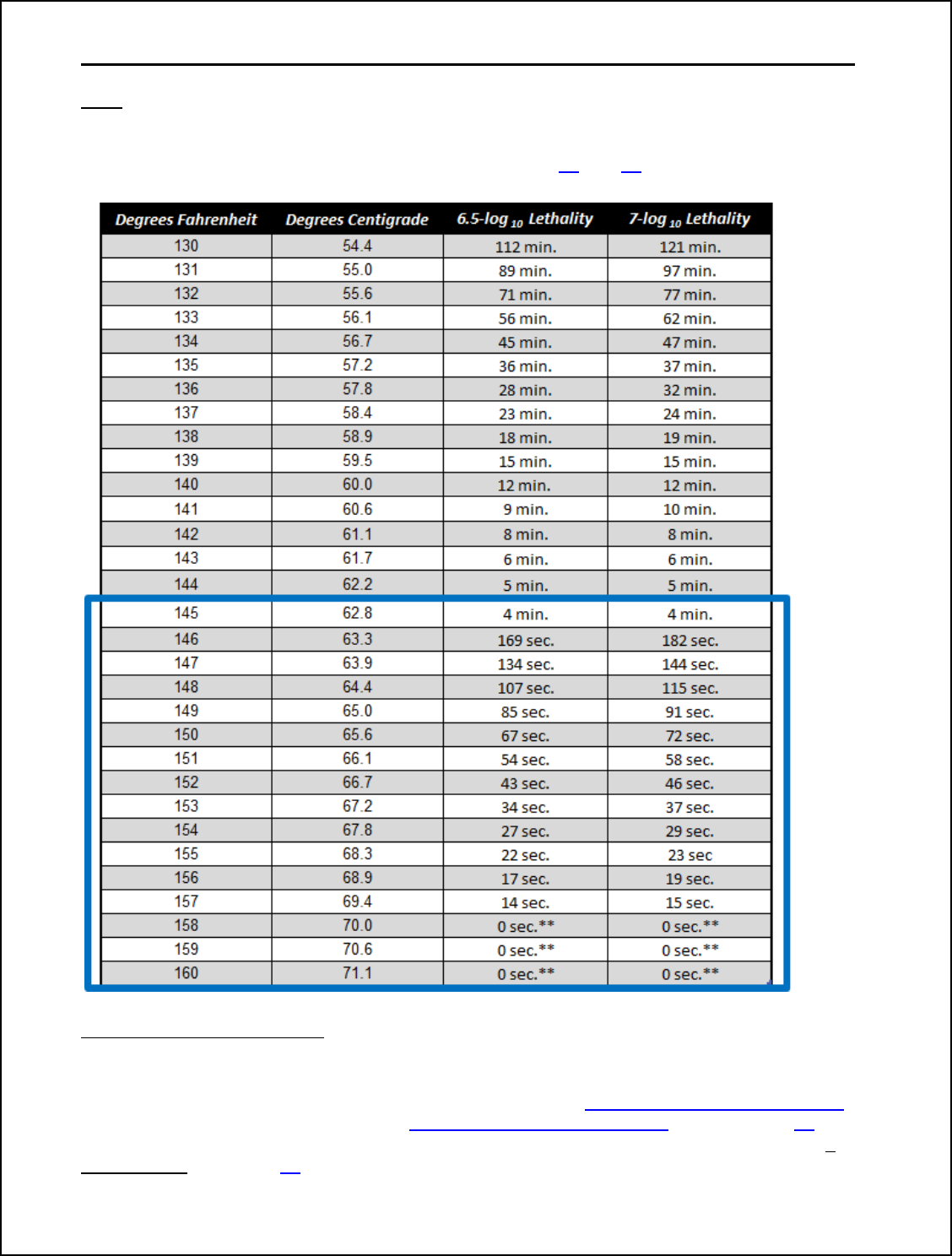
35
Table 2. Time-Temperature Combinations for Meat Products to Achieve Lethality
Temperatures stated are the minimum internal temperatures that must be met in all parts of the
meat product for the total dwell time listed.
5
An establishment must ensure both time and
temperature parameters are met to use this table to support its process achieves the Log
reduction target. Relative humidity
6
and heating come-up-time (CUT)
7
are also critical
operating parameters when using this table. (See pages 37 and 38 for poultry endpoint time-
temperature tables).
5
The required Log reductions are achieved instantly (0 seconds) when the internal temperature
of a cooked meat product reaches 158°F or above.
6
Time-Temperatures ≥ 145°F (in blue square) are eligible for FSIS Relative Humidity Options 1
and 2. All time-temperatures may apply FSIS Relative Humidity Options 3 and 4 (page 26).
7
FSIS recommends limiting the total time product temperature is between 50 and 130°F to 6
hours or less (see page 23).
36
Additional Critical Operating Parameters for Poultry Products
The following are additional critical operational parameters that should be considered
when cooking poultry products using FSIS newer guidance in the poultry time-
temperature tables.
Note: The older poultry recommendations for Cooked Poultry Rolls on page 39 apply
regardless of species or fat because these were not considered critical operating
parameters at the time the recommendation was developed. FSIS is not aware of any
outbreaks or food safety incidents as a result of applying these recommendations to
products of varying species or fat level.
Product Species
Generally, FSIS accepts that research for an intervention’s effectiveness on one
species of poultry (i.e., chickens, turkeys, ducks, geese, ratites, and squabs) can be
applied to another species of poultry without additional support (FSIS Directive 5000.6,
Performance of the Hazard Analysis Verification Task). However, research by Juneja et
al. (2001a) demonstrated that in cooking processes, Salmonella heat tolerance depends
on the poultry species. Therefore, when FSIS developed its time-temperature tables for
poultry it developed two unique poultry time-temperature tables: one for chicken (page
37), another for turkey (page 38).
When making poultry products containing poultry species other than chicken or
turkey, or products made with a mixture of poultry species, FSIS recommends
selecting an endpoint temperature, then using the longest dwell time recommended for
the product fat content and endpoint temperature in either the chicken or turkey table.
Comparing the tables and using the longest recommended dwell time ensures the
HACCP system is designed to address the worst-case scenario for Salmonella survival
in the product. Products that are a mixture of poultry and meat must achieve a 7-Log
reduction of Salmonella (see Key Question on page 19).
Fat Content
In the presence of fats, the heat tolerance of some microorganisms generally increases
(Jay et al., 2000). This is sometimes referred to as fat protection and is presumed to
increase heat tolerance by affecting cell moisture. Juneja et al., (2001b) showed that
higher fat levels in beef result in increased heat resistance of Salmonella, which is in
agreement with publications regarding other food borne pathogens (Line et al., 1991;
Ahmed et al., 1995). The Poultry Time-Temperature Tables (pages 37 and 38) provide
establishments with time-temperature combinations that can be used to cook chicken
and turkey products with different fat levels.
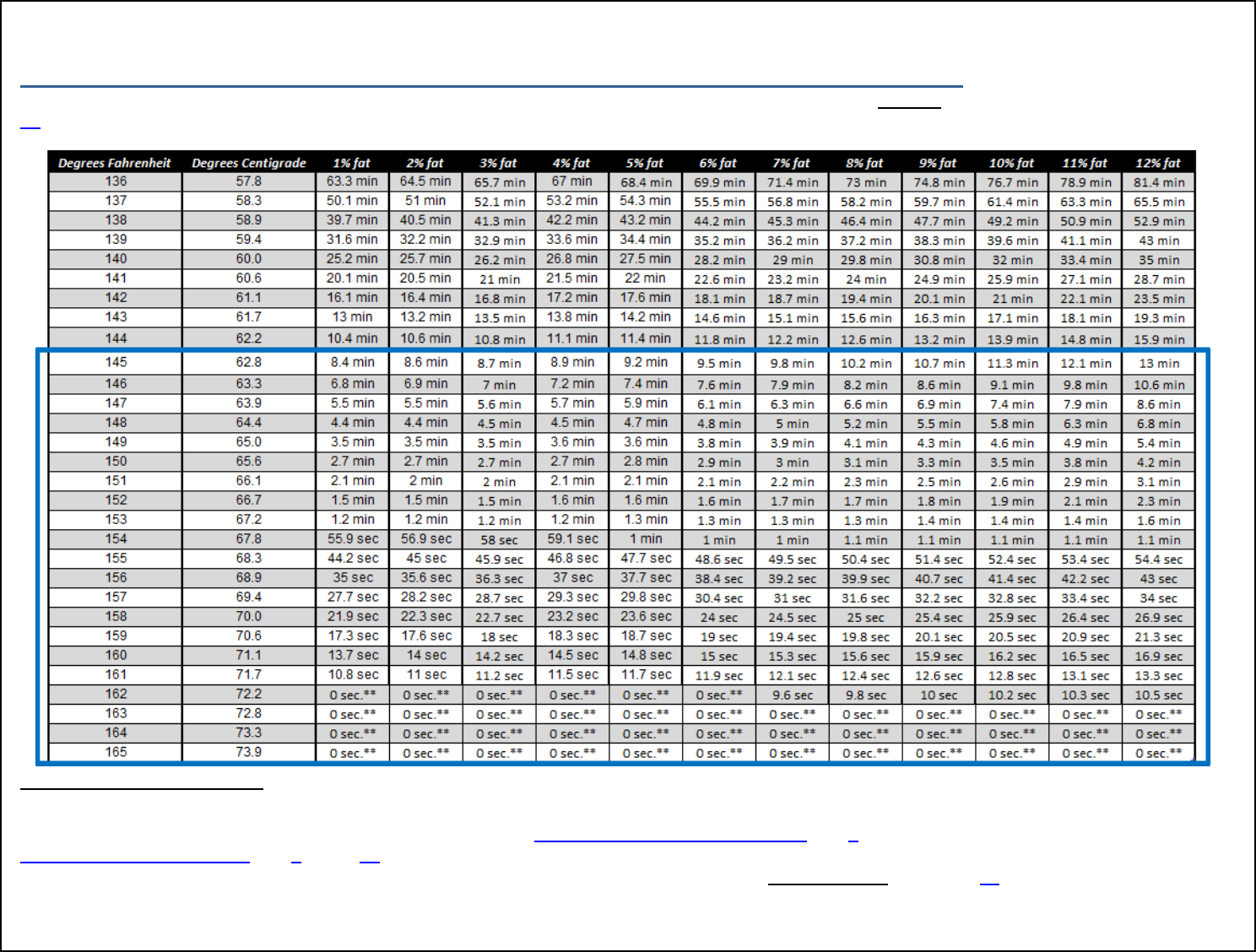
8
A 7-Log reduction of Salmonella is achieved instantly at internal temperatures in which the holding time is 0 seconds (0 sec.).
9
Time-Temperatures ≥ 145°°F (in blue square) are eligible for FSIS Relative Humidity Options 1 and 2. All time-temperatures may apply FSIS
Relative Humidity Options 3 and 4 (page 26).
10
FSIS recommends limiting the total time product temperature is between 50 and 130°F to 6 hours or less (see page 23).
37
Table 3. Time-Temperature Combinations for Chicken Products to Achieve Lethality
Times for given temperatures and fat levels that are needed to obtain a 7-Log reduction of Salmonella in chicken products.
8
As described on page
23, relative humidity
9
and heating come-up-time (CUT)
10
are critical operating parameters when using this table.
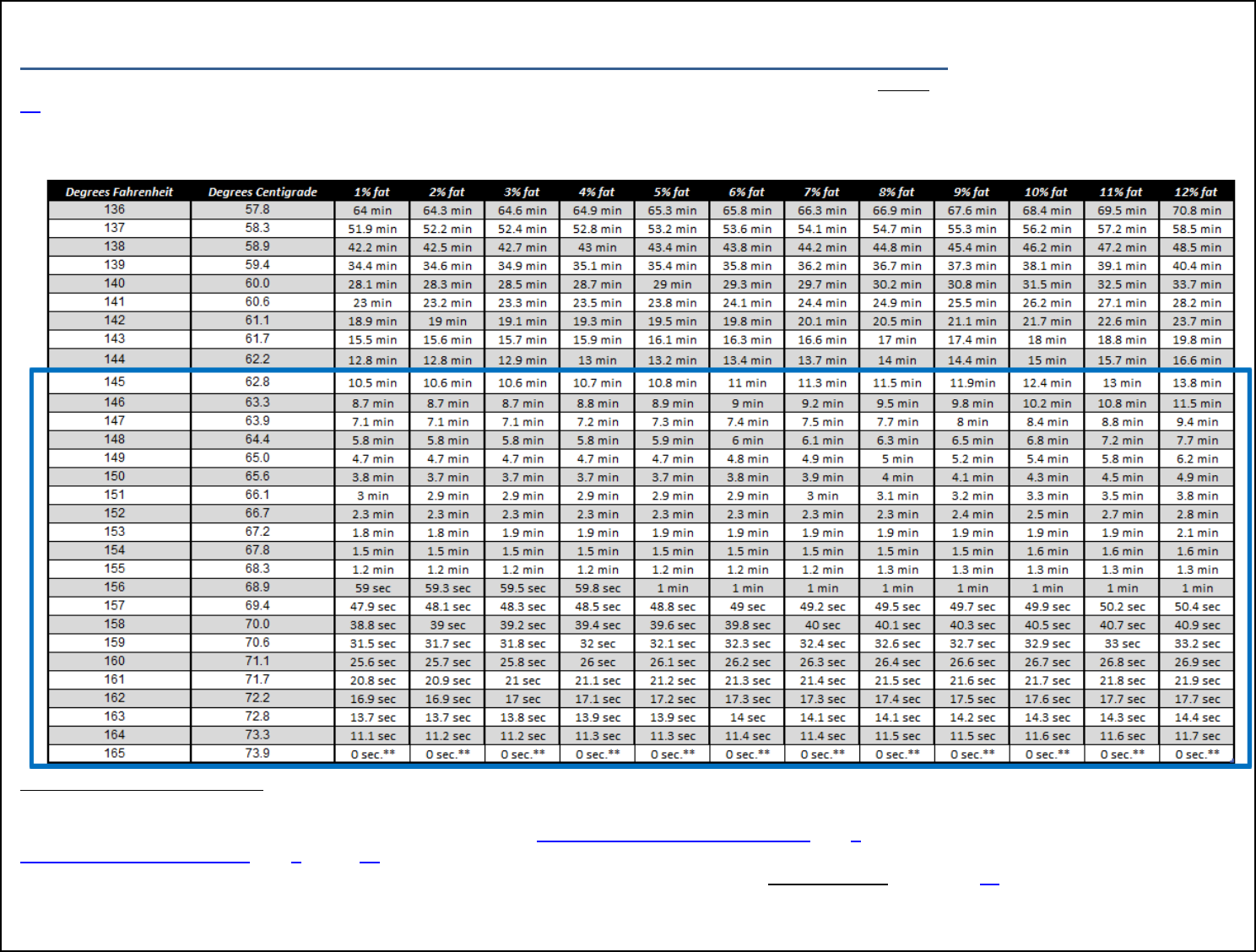
38
Table 4. Time-Temperature Combinations for Turkey Products to Achieve Lethality
Times for given temperatures and fat levels that are needed to obtain a 7-Log reduction of Salmonella in turkey products.
11
As described on page
23, relative humidity
12
and heating come-up-time (CUT)
13
are critical operating parameters when using this table.
11
A 7-Log reduction of Salmonella is achieved instantly at internal temperatures in which the holding time is 0 seconds (0 sec.).
12
Time-Temperatures ≥ 145°°F (in blue square) are eligible for FSIS Relative Humidity Options 1 and 2. All time-temperatures may apply FSIS
Relative Humidity Options 3 and 4 (page 26).
13
FSIS recommends limiting the total time product temperature is between 50 and 130°F to 6 hours or less (see page 23).

39
Cooked Poultry Rolls Options
FSIS recommends establishments use the options in the Poultry Time-Temperature
Tables (page 37 and 38) (which include dwell times at 160°F that vary based on fat
content and species) because they have been validated with updated research to
address species and fat content as critical operating parameters to ensure adequate
Log reductions of Salmonella. However, FSIS is including the two older options below
for cooking poultry rolls and other poultry products because they still may be used by
some establishments. Applying the cooked poultry rolls options below may achieve the
same Log reductions as the time-temperature combinations in the Poultry Time-
Temperature Tables, particularly when applied to a lean product, because the product
may be maintained at 160°F for the recommended dwell times (between 13.7 to 26.9
seconds depending on species and fat) during the time it takes to complete temperature
monitoring. Regardless, FSIS recommends establishments monitor the dwell time in
the Poultry Time-Temperature Tables as opposed to relying on the older guidance for
cooked poultry rolls below to better assure safety.
The options below can be applied to any poultry product (not just cooked poultry rolls)
regardless of fat content or poultry species. However, if FSIS collects a RTE sample
that is positive for Salmonella or if the establishment is implicated in a food safety
investigation related to Salmonella (i.e., is associated with reports of illness or
outbreak), FSIS will review and determine the adequacy of the establishment’s required
corrective actions (taken under 9 CFR 417.3), to address process deviations. The
establishment will need to show FSIS that inadequate lethality was not the root cause of
the process deviation if it wants to continue to follow the cooked poultry rolls options.
To use a cooked poultry rolls option, the establishment must address all critical
operating parameters for cooking identified in this guideline (other than species or fat),
including relative humidity (page 26) and CUT (page 23).
1. Cooked poultry rolls and other cooked poultry products must reach an internal
temperature of at least 160°F (instantaneous) during the cooking process.
2. Cured and smoked poultry rolls and other cured and smoked poultry must
reach an internal temperature of at least 155°F (instantaneous) during the
cooking process.
Key Question
Question: Can establishments that produce poultry products with higher than 12% fat use values
for 12% fat in the Poultry Time-Temperature Tables?
Answer: Yes. The time-temperature combinations listed in the tables for poultry products with
12% fat can be used for products with higher percentages of fat and for products with unknown fat
content. These time-temperature combinations will achieve sufficient lethality as long as adequate
humidity (FSIS Relative Humidity Options page 26) is applied during the process.
40
Resources for Customized and Alternative Support
FSIS recognizes that not all meat and poultry products can be cooked using the FSIS
critical operating parameters (humidity, CUT and endpoint time-temperature) included in
this guideline. To assist establishments in cooking their products, FSIS has identified
additional resources which may provide scientific support for a specific process or part
of a process. Attachment A1. Customized Processes and Alternative Lethality Support
includes information on the following:
• Alternative Lethality Target: Under certain circumstances and with additional
support, establishments may be able to use an alternative lethality target (e.g. 5-
Log). See Attachment A1. Supporting an Alternative Lethality Target, page 57 of
this guideline.
• Journal Articles: Establishments could identify a published journal article which
shows a specific process meets the performance standard and use this as
scientific support. See Attachment A1. Common Topics and Journal Articles
Used for Alternative Support page 60 of this guideline.
• Customized Cooking Schedule: Establishments may design a customized
cooking plan using validated microbial models. See Attachment A1. Predictive
Microbial Modeling for Critical Operating Parameters, page 62 of this guideline.
• Challenge Studies: Establishments could conduct challenge studies to
determine if their proposed process would meet the performance standard. See
Attachment A1. Designing Challenge Studies for Cooking, page 63 of this
guideline.
In addition to information for developing customized critical operating parameters, this
guideline contains additional resources, listed below, to address common questions and
issues establishments may have regarding cooking of meat and poultry products.
• Pasteurized Label: Establishments may be able to label their cooked meat or
poultry product as “Pasteurized.” See Attachment A3. When can Products be
Labeled as Pasteurized?, page 81 of this guideline.
• Common Sources of Salmonella: Salmonella contamination may occur on
cooked products for a variety of reasons. For information on sources of
Salmonella contamination and Best Practices to implement to address it, see
Attachment A4. Sources of Salmonella Contamination in RTE Products and Best
Practices to Address It page 82 of this guideline.
• Ready-to-eat (RTE) Self-Assessment Tool: FSIS has included a self-
assessment tool that establishments can use to identify areas in their process
where they could improve Salmonella control. See Attachment A5. RTE
Salmonella Self-Assessment Tool page 87 of this guideline.

41
Scientific Gaps Identified by FSIS
FSIS has identified several common cooking processes that can’t achieve the critical
operating parameters included in this guideline. FSIS encourages establishments to
conduct challenge studies when other support is not available (page 63). However, the
Agency realizes it may not be cost effective for establishments to conduct individual
challenge studies for commonly produced meat and poultry products. To address these
common processes, which lack readily available scientific support, FSIS has identified
and communicated scientific gaps and is working to facilitate filling these gaps. FSIS
posted research priorities on its website to communicate clear research needs with
USDA Agricultural Research Service (ARS) and academic researchers. As additional
data becomes available, FSIS will update the recommendations for these scientific gaps
with the latest available scientific support.
An establishment producing products using processes that fall under an identified
scientific gap may use the critical operating parameters in this guideline as scientific
support (see Table 5. Scientific Gaps where Critical Operating Parameters From Older
Guidance May be Used page 43). Table 5 also describes specific vulnerabilities with
using the gaps as scientific support and recommends steps to reduce the vulnerabilities.
In addition to those specific vulnerabilities, FSIS has the following concerns with
establishments continuing to process products using the critical operating parameters in
Table 5:
• Use of these critical operating parameters represents a vulnerability because
these processes have not been validated to address all hazards of concern. The
original research used to develop these critical operating parameters was
performed on only the few products covered by the performance standard to be
included in the 1999 version of Appendix A [64 FR 732].
• If a process deviation occurs for a process that is included as a scientific gap, it is
unlikely an establishment would be able to identify adequate support for product
safety without performing product testing.
• If FSIS or the establishment collects a ready-to-eat (RTE) sample that is positive
for Salmonella, or the establishment is implicated in a food safety investigation
related to Salmonella (i.e., is associated with reports of illness or outbreak), FSIS
would verify, as part of the corrective actions (9 CFR 417.3), that the
establishment can support inadequate lethality was not the root cause, if it wants
to continue to use the older recommendation.
• As additional data becomes available, FSIS will change the recommendations for
processes that fall under one of these scientific gaps.

42
NOTE: Scientific gaps only affect very specific products and processes. Process
deviations and malfunctioning equipment are NOT scientific gaps. Additionally,
Products and Processes Not Covered by this Guideline would NOT be adequately
supported by the critical operating parameters listed in Table 5.
Scientific gaps are processes which have not been validated to achieve
sufficient lethality and to address all potential hazards during cooking, but
establishments may continue to use this guidance as support to allow
additional time for research to be conducted and gaps filled.
FSIS will update this guideline as more research becomes available and new options
can be developed.
43
NOTE: Scientific gaps only affect very specific products and processes. Process deviations and malfunctioning equipment
are NOT scientific gaps. Products and Processes Not Covered by this Guideline would NOT be adequately supported by
the critical parameters listed in scientific gaps (Table 5).
Table 5. Scientific Gaps where Critical Operating Parameters From Older Guidance May be Used
Gap
Examples of
Products
1999 Critical
Operating
Parameters
Vulnerability with Continuing to Follow 1999
Parameters
1. Products cooked for short
times at high temperatures that
cannot maintain 90% humidity per
Option 4 and do not meet the
Situations when Humidity is Not
Needed (page 31).
Processes that meet this gap
include those in which product
is:
• Cooked for less than 1 hour, at
dry bulb oven temperatures
above 212°F.
NOTE: Above 212°F the
maximum relative humidity
decreases as the temperature
increases making it impossible to
achieve 90% relative humidity in
the oven regardless of the amount
of moisture present.
Cooking
meatballs or
poultry tenders
using
impingement,
spiral, and
steam-injected
inline ovens.
NOTE: Jerky
products are not
included under
this gap. There
are many
validated
lethality
processes
available for
jerky products.
Apply FSIS time-
temperature tables
(pages 35, 37, 38),
addressing all critical
operating parameters
(page 23) except
relative humidity.
NOTE: Relative
humidity does not
need to be
addressed for
products cooked in
completely immersed
in water (page 31).
These parameters may allow the surface of the
product to dry out during cooking. Lack of humidity
can cause pathogens to develop heat tolerance
and allow them to survive the heating process. In
addition, shorter cooking processes allow for
limited additional lethality during the heating come-
up time (sometimes called cumulative or integrated
lethality) which reduces the safety margin the
process provides.
To minimize these vulnerabilities, an establishment
may choose to implement, validate, and monitor as
part of the HACCP system, any of the following to
ensure moist cooking and demonstrate surface
lethality:
o Wet-bulb temperature.
o Dew point temperature.
o Percent moisture by volume.
o Increase dwell time or endpoint temperature.
o Increase total cooking time to increase
integrated lethality.
Or perform a challenge study (page 63).
Or conduct finished product testing for Salmonella
as part of on-going verification.
44
NOTE: Scientific gaps only affect very specific products and processes. Process deviations and malfunctioning equipment
are NOT scientific gaps. Products and Processes Not Covered by this Guideline would NOT be adequately supported by
the critical parameters listed in scientific gaps (Table 5).
Gap
Examples of
Products
1999 Critical
Operating
Parameters
Vulnerability with Continuing to Follow 1999
Parameters
2. Products cooked using
microwave cooking methods
that are not designed to control
relative humidity.
Processes that meet this gap
include those in which a meat or
poultry product is cooked using
a continuous or non-continuous
microwave oven.
Sliced bacon or
bacon chips
cooked using
continuous
microwave
ovens.
Apply FSIS time-
temperature tables
(pages 35, 37, 38),
addressing all critical
operating parameters
(page 23) except
relative humidity.
These parameters may not ensure surface
lethality. In addition, shorter cooking processes
allow for limited additional lethality during the
heating come-up time (sometimes called
cumulative or integrated lethality) which reduces
the safety margin the process provides.
To minimize these vulnerabilities, an establishment
may choose to implement, validate, and monitor,
any of the following to ensure moist cooking and
demonstrate surface lethality:
o Increase dwell time or endpoint temperature.
o Increase total cooking time to increase
integrated lethality.
Or perform a challenge study (page 63).
Or conduct finished product testing for Salmonella
as part of on-going verification.
NOTE: There is an additional vulnerability with
microwave cooking that the microwave energy
may not result in lethality of pathogens on
continuous belt surfaces (Taormina et al., 2011).
45
NOTE: Scientific gaps only affect very specific products and processes. Process deviations and malfunctioning equipment
are NOT scientific gaps. Products and Processes Not Covered by this Guideline would NOT be adequately supported by
the critical parameters listed in scientific gaps (Table 5).
Gap
Examples of
Products
1999 Critical
Operating
Parameters
Vulnerability with Continuing to Follow 1999
Parameters
3. Products cooked using
cooking methods that are not
designed to control relative
humidity other than microwave
ovens.
Processes that meet this gap
include those where product is
either:
• Cooked in ovens that are not
designed to be sealed (e.g., no
dampers) and designed
without a mechanism to
introduce steam.
Or
• Barbecue products cooked
under dry heat to meet labeling
requirements (e.g., 9 CFR
319.80; and 9 CFR 381.164).
NOTE: This does not include
smokehouses where the gaskets
or dampers are broken or have
been removed.
Rotisserie
chicken
Products such
as pork butt or
beef brisket
cooked using
restaurant or
foodservice type
convection
ovens.
Barbecue
products cooked
under dry heat
including those
cooked in
smokehouses or
open pits.
NOTE: Jerky
products are not
included in this
gap. There are
many validated
lethality
processes
available for
jerky products.
Apply FSIS time-
temperature tables
(pages 35, 37, 38),
addressing all critical
operating parameters
(page 23) except
relative humidity.
NOTE: Relative
humidity does not
need to be
addressed for
products 10 pounds
or more cooked in an
oven at 250°F or
higher (page 31).
These parameters may allow the surface of the
product to dry out during cooking. Lack of humidity
can cause pathogens to develop heat tolerance
and allow them to survive the heating process.
To minimize this vulnerability, an establishment
may choose to implement, validate, and monitor,
any of the following to ensure moist cooking and
demonstrate surface lethality:
o Wet-bulb temperature.
o Dew point temperature.
o Percent moisture by volume.
Depending on the process, pans of water may be
added to increase moisture in the cooking
chamber.
Or perform a challenge study (page 63).
Or conduct finished product testing for Salmonella
as part of on-going verification.
46
NOTE: Scientific gaps only affect very specific products and processes. Process deviations and malfunctioning equipment
are NOT scientific gaps. Products and Processes Not Covered by this Guideline would NOT be adequately supported by
the critical parameters listed in scientific gaps (Table 5).
Gap
Examples of
Products
1999 Critical
Operating
Parameters
Vulnerability with Continuing to Follow 1999
Parameters
4. Other processes that may
inherently maintain relative
humidity around the meat and
poultry filling but cannot follow one
of the relative humidity options.
Processes that meet this gap
include those that involve:
• Use of an edible wrapping that
fully encloses a raw meat or
poultry filling before cooking.
Example wrappings include:
o dough,
o leaves, and
o edible rice paper.
NOTE: Products cooked in a
natural casing are not included in
this gap, since FSIS includes
natural casing in Situations when
Humidity is Not Needed (page 31).
Baked pasties,
empanadas,
pot-stickers, and
dumplings.
Apply FSIS time-
temperature tables
(pages 35, 37, 38),
addressing all critical
operating parameters
(page 23) except
relative humidity.
These parameters may allow the surface of the
filling or wrapping to dry during cooking. Lack of
humidity can cause pathogens to develop heat
tolerance and allow them to survive cooking.
To minimize this vulnerability, an establishment
may choose to implement, validate, and monitor as
part of the HACCP system, any of the following to
ensure sufficient lethality on the outside and inside
of the wrapped products:
o Cook filling first.
o Measure water activity of filling before and
after cooking to support moisture is inherently
maintained (water activity stays the same or
increases after cooking). FSIS recommends
establishments achieve the highest water
activity possible during cooking. Values ≥
0.96 have been shown to prevent pathogen
heat tolerance, but this water activity may not
be possible to achieve for all processes
(Kieboom, et al. 2006).
o Cook to a higher endpoint temperature than
the FSIS time-temperature tables, to
compensate for the low humidity conditions.
Or perform a challenge study (page 63).
Or conduct finished product testing for Salmonella
as part of on-going verification.
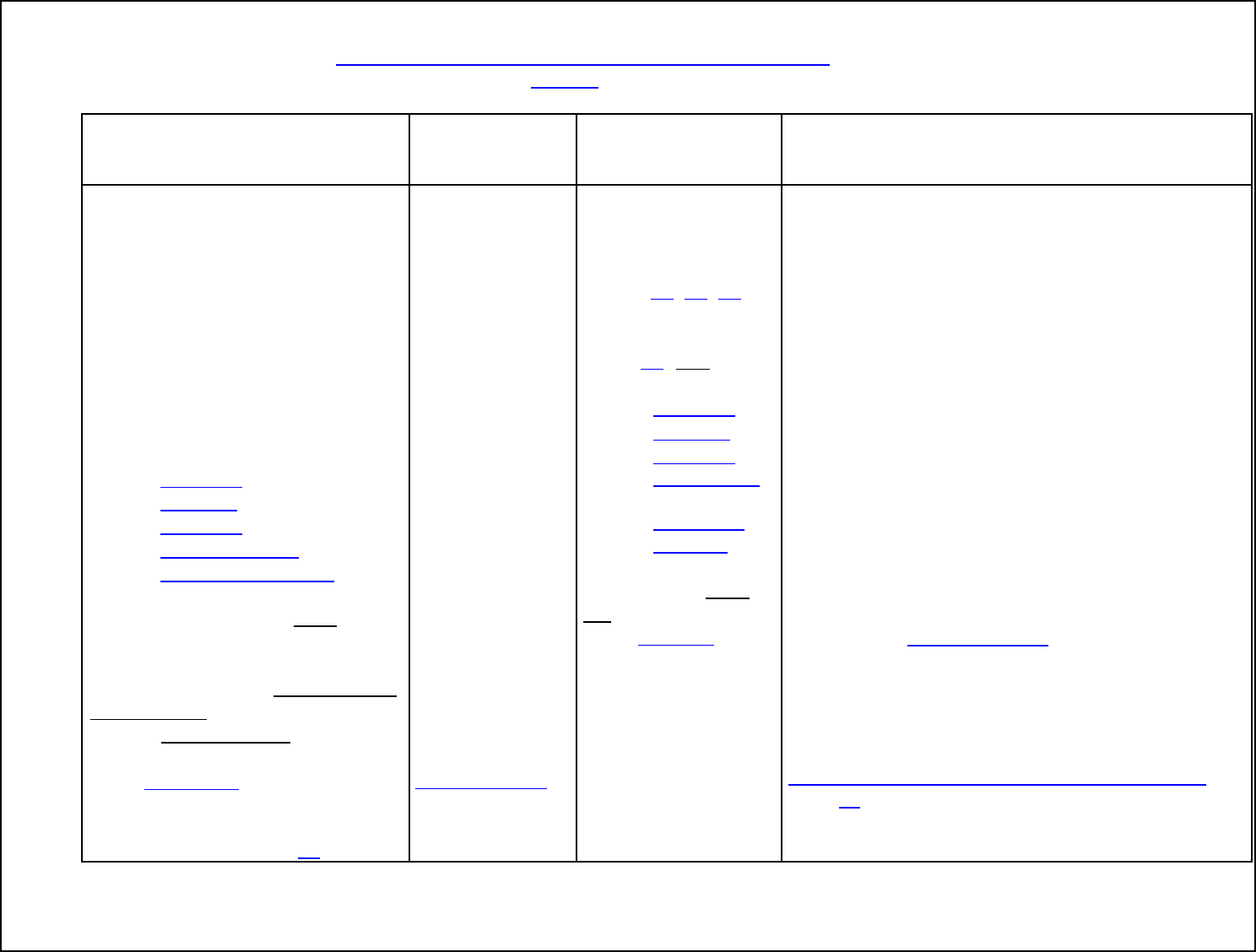
47
NOTE: Scientific gaps only affect very specific products and processes. Process deviations and malfunctioning equipment
are NOT scientific gaps. Products and Processes Not Covered by this Guideline would NOT be adequately supported by
the critical parameters listed in scientific gaps (Table 5).
Gap
Examples of
Products
1999 Critical
Operating
Parameters
Vulnerability with Continuing to Follow 1999
Parameters
5. Processes where the drying
step comes before cooking under
moist conditions.
Processes that meet this gap
include those in which products
are:
• Dried to reduce the water
activity and then cooked using
one of the following options
that ensures high relative
humidity
o Option 1, or
o Option 3, or
o Option 4, or
o Cook-in-bag, or
o Immersion cooking.
NOTE: This gap does NOT apply
products cooked after drying
without applying relative humidity
(e.g., cooking under dry conditions
or direct heat), or to dried products
cooked multiple times. It is not
supportable for dried products to
apply direct heat instead of
addressing relative humidity,
without additional support for
surface lethality (page 31).
Country-cured
hams that are
cooked-in-bag
one time.
Soups that have
a reduced water
activity due to a
high salt
concentration
but are a liquid
medium.
NOTE: Jerky
products are not
included in this
gap. There are
many validated
lethality
processes
available for
jerky products.
Apply:
FSIS time-
temperature tables
(pages 35, 37, 38),
addressing all critical
operating parameters
(page 23) and use
relative humidity:
o Option 1, or
o Option 3, or
o Option 4, or
o Cook-in-bag,
or
o Immersion
cooking.
NOTE: FSIS does
not consider a sealed
oven (Option 2) to be
adequate support
that the surface of
the product is
rehydrated during
cooking of reduced
water activity
products.
There is a vulnerability if pathogens develop heat
tolerance during drying which could allow them to
survive the cooking process.
To minimize this vulnerability, an establishment
may choose to implement, validate, and monitor as
part of the HACCP system, any of the following to
ensure sufficient moisture during cooking:
o Take water activity measurements of the
surface of the product before and after
cooking to support the surface is rehydrated
(water activity increases after cooking).
o Achieve the highest water activity possible
during cooking. Values ≥ 0.96 have been
shown to prevent pathogen heat tolerance,
but this water activity may not be possible to
achieve for all processes (Kieboom, et al.
2006).
Or perform a challenge study (page 63).
Or conduct finished product testing for Salmonella
and Lm as part of on-going verification.
Additional recommendations are included in
Attachment A6. Cooking Country-Cured Hams on
page 90.

48
NOTE: Scientific gaps only affect very specific products and processes. Process deviations and malfunctioning equipment
are NOT scientific gaps. Products and Processes Not Covered by this Guideline would NOT be adequately supported by
the critical parameters listed in scientific gaps (Table 5).
Gap
Examples of
Products
1999 Critical
Operating
Parameters
Vulnerability with Continuing to Follow 1999
Parameters
6. Products with long heating
come-up-times (CUTs).
This gap applies to processes
that require a:
• Heating come-up-time longer
than 6 hours (page 23).
NOTE: See page 62 for references
supporting longer CUTs for fully
cooked products formulated with
antimicrobials to inhibit S. aureus
that are cooked to lethality.
Ham and beef
brisket.
NOTE: Dry-
cured or
immersion cured
products
produced under
this Cooking
Guideline
Scientific Gap
may also be
produced under
a Stabilization
Guideline
Scientific Gap if
formulated
without
erythorbate or
ascorbate.
Apply any of FSIS’s
applicable time-
temperature
combinations (pages
35, 37, 38) and
relative humidity,
without considering
CUT as a critical
operating parameter.
NOTE: For intact
products,
establishment may
be able to monitor
the surface
temperature to allow
for longer CUTs,
instead of addressing
this gap (page 24).
A vulnerability exists in that S. aureus may grow
to levels that result in the production of a heat-
stable enterotoxin if CUTs are longer than 6 hours
without the use of antimicrobials.
To minimize this vulnerability, an establishment
may choose to implement, validate, and monitor as
part of the HACCP system, any of the following to
ensure S. aureus outgrowth is limited:
o Critical parameters from a
published journal
article that supports extending the come-up-
time in products and processes. (page 62).
o Reduce product diameter to reduce CUT.
o Conduct predictive pathogen modeling for a
particular product and process (page 55).
o Limit CUT between 50 – 130°F and set a
defined limit based on the shortest CUT
possible for the establishment’s specific
process.
o Apply smoke, which may inhibit S. aureus
and C. perfringens growth.
Or perform a challenge study (page 63).
Or conduct finished product verification testing for
S. aureus enterotoxins (page 77); or
49
References
Ahmed, M.N., Conner, D.E. and Huffman, D.L. 1995. Heat resistance of Escherichia coli
O157:H7 in meat and poultry as affected by product composition. Journal of Food
Science 60:606-610.
Ajene, A.N., Walker, C.L.F., Black, R.E. 2013. Enteric pathogens and reactive arthritis:
a systematic review of Campylobacter, Salmonella and Shigella-associated reactive
arthritis. Journal of Health, Population, and Nutrition. 31(3):299-307.
AMIF (American Meat Institute Foundation). 1997. Good Manufacturing Processes for
Fermented Dry & Semi‐Dry Sausage Products.
https://foodsafety.wisc.edu/meat-haccp/. Accessed 27 April 2020.
Blankenship L.C. 1978. Survival of a Salmonella typhimurium experimental contaminant
during cooking of beef roasts. Applied Environmental Microbiology. 35(6):1160-1165.
Boles, Neary, and Clawson. 2004. New intervention and validation for the control of
pathogens in the processing of jerky. Report available at:
https://www.fsis.usda.gov/sites/default/files/media_file/2021-08/C-
11_New_Technology_FY2004_Final_Report.pdf.
Borowski, A. G., Ingham, S. C., Ingham, B. H. 2009. Lethality of home-style dehydrator
processes against Escherichia coli O157: H7 and Salmonella serovars in the
manufacture of ground-and-formed beef jerky and the potential for using a pathogen
surrogate in process validation. Journal of Food Protection. 72(10): 2056-2064.
Buege, D.R., Searls, G., Ingham, S.C. 2006. Lethality of commercial whole-muscle beef
jerky manufacturing processes against Salmonella serovars and Escherichia coli O157:
H7. Journal of Food Protection. 69(9):2091-2099.
Center for Disease Control (CDC). 1971a. Staphylococcal gastroenteritis associated
with salami: United States. Morbidity and Mortality. 20(28): 253-258. Accessed 21 April
2020. https://www.jstor.org/stable/44070511.
Center for Disease Control (CDC). 1971b. Gastroenteritis associated with Genoa
salami: United States. Morbidity and Mortality. 20(29): 261-266 Accessed 21 April 2020.
www.jstor.org/stable/44070520.
Center for Disease Control (CDC). 1975. Staphylococcal food poisoning associated
with Italian dry Salami: California. Morbidity and Mortality. 24(44):374-379. Accessed
21 April 2020. www.jstor.org/stable/44074111.
Dierschke, S., Ingham, S.C., Ingham, B.H. 2010. Destruction of Escherichia coli O157:
H7, Salmonella, Listeria monocytogenes, and Staphylococcus aureus achieved during

50
manufacture of whole-muscle beef jerky in home-style dehydrators. Journal of Food
Protection. 73(11):2034-2042.
Doyle, M.P., Buchanan, R.L. (ed.). 2013. Food microbiology: fundamentals and
frontiers—4
th
ed. Washington (DC): ASM Press.
FDA (Food and Drug Administration). 2018. Hazard analysis and risk-based
preventative controls for human food: draft guidance for industry. Available at:
https://www.fda.gov/media/99572/download. Accessed: 7
th
July 2020.
Freier, T.A. 2001. Use of the AMI Process Lethality Spreadsheet to validate the safety
of cooking procedures. American Meat Science Association (AMSA) Proceedings of
the 54
th
Reciprocal Meat Conference. pp. 52-53. Accessed 26 November 2019.
https://meatscience.org/docs/default-source/publications-resources/rmc/2001/use-of-
the-ami-process-lethality-spreadsheet-to-validate-the-safety-of-cooking-
procedures.pdf?sfvrsn=115cbbb3_2.
Genigeorgis, C., Lindroth, S. 1984. The safety of Basturma, and Armenian-type dried
beef with respect to Salmonella. Proceedings of the 30
th
European Meeting of Meat
Research Workers, Bristol, United Kingdom. (30):217–224.
Goepfert J.M., Iskander I.K., Amundson C.H. 1970. Relation of the heat resistance of
salmonellae to the water activity of the environment. Applied Environmental
Microbiology. 19(3):429-433.
Goodfellow S.J., Brown W.L. 1978. Fate of Salmonella inoculated into beef for cooking.
Journal of Food Protection. 41(8):598-605.
Gunvig, A., Andresen, M.S., Jacobsen, T., Borggaard, C. 2018. Staphtox predictor-A
dynamic mathematical model to predict formation of Staphylococcus enterotoxin during
heating and fermentation of meat products. International Journal of Food Microbiology.
285:81-91.
ICMSF (International Commission on Microbiological Specifications for Foods). 1996.
Microorganisms in Foods 5: Characteristics of microbial pathogens. Springer Science &
Business Media.
ICMSF (International Commission on Microbiological Specifications for Foods). 2002.
Microorganisms in Foods 7: Microbiological Testing in Food Safety Management.
Springer Science & Business Media.
IFT (Institute of Food Technologists) 2003. Current and Proposed Definitions of
“Potentially Hazardous Foods”. Comprehensive Reviews in Food Science and Food
Safety. 2:17-20. doi:10.1111/j.1541-4337.2003.tb00047.x

51
Ingham, S.C., Ingham, B.H., Borneman, D., Jaussaud, E., Schoeller, E.L., Hoftiezer, N.,
Schwartzburg, L., Burnham, G.M., Norback, J.P. 2009a. Predicting pathogen growth
during short-term temperature abuse of raw sausage. Journal of Food Protection.
72(1):75-84.
Ingham, S.C., Vang, S., Levey, B., Fahey, L., Norback, J.P., Fanslau, M.A., Senecal,
A.G., Burnham, G.M., Ingham, B.H. 2009. Predicting behavior of Staphylococcus
aureus, Salmonella Serovars, and Escherichia coli O157: H7 in pork products during
single and repeated temperature abuse periods. Journal of Food Protection.
72(10):2114-2124.
Jay, J. M. 2000. Food Microbiology 6
th
Edition. Gaithersburg, Maryland (US).
Jofré, A., Garriga, M., Aymerich, T. 2008. Inhibition of Salmonella sp., Listeria
monocytogenes and Staphylococcus aureus in cooked ham by combining
antimicrobials, high hydrostatic pressure and refrigeration. Meat Science. 78(1-2):53-
59.
Juneja, V.K., Eblen, B.S., Ransom, G.M. 2001a. Thermal inactivation of Salmonella
spp. in chicken broth, beef, pork, turkey, and chicken: Determination of D‐and Z‐values.
Journal of Food Science. 66(1):146-152.
Juneja, V.K., Eblen, B.S., Marks, H.M., 2001b. Modeling non-linear survival curves to
calculate thermal inactivation of Salmonella in poultry of different fat levels. International
Journal of Food Microbiology. 70(1-2):37-51.
Kadariya, J., Smith, T.C. and Thapaliya, D., 2014. Staphylococcus aureus and
staphylococcal food-borne disease: an ongoing challenge in public health. BioMed
Research International, (2014).
Kieboom, J., Kusumaningrum, H.D., Tempelaars, M.H., Hazeleger, W.C., Abee, T.,
Beumer, R.R. 2006. Survival, elongation, and elevated tolerance of Salmonella enterica
serovar Enteritidis at reduced water activity. Journal of Food Protection. 69(11):2681-
2686.
Leistner, L. 1987. Shelf-stable products and intermediate moisture foods based on
meat products. In Rockland, L.B., Beuchat, L.R. (eds.), Water activity: Theory and
applications to food. New York (NY): Marcel Dekker.
Line, J.E., Fain J.R., A.R., Moran, A.B., Martin, L.M., Lechowich, R.V., Carosella, J.M.,
Brown, W.L. 1991. Lethality of heat to Escherichia coli 0157: H7: D-value and z-value
determinations in ground beef. Journal of Food Protection. 54(10):762-766.
Ma, L., Kornacki, J.L., Lin, C.M., Doyle, M.P. 2007. Development of thermal surrogate
microorganisms in ground beef for in-plant critical control point validation studies. J.
Food Prot. 70: 952-957.
52
Mann, J.E., Brashears, M.M. 2007. Contribution of humidity to the lethality of surface-
attached heat-resistant Salmonella during the thermal processing of cooked ready-to-
eat roast beef. Journal of Food Protection. 70(3):762-765.
Mbandi, E., Shelef, L.A. 2002. Enhanced antimicrobial effects of combination of lactate
and diacetate on Listeria monocytogenes and Salmonella spp. in beef bologna.
International Journal of Food Microbiology. 76(3):191-198.
McMinn, R.P., King, A.M., Milkowski, A.L., Hanson R., Glass K.A., Sindelar JJ. 2018.
Processed meat thermal processing food safety-generating D-Values for Salmonella,
Listeria monocytogenes, and Escherichia coli. Meat and Muscle Biology. 2(1):168-179.
Mikel, W.M and Newman, M.C. 2003. Development of appropriate intervention
methods to reduce the occurrence of pathogenic bacteria on country-cured hams.
Available at: https://www.fsis.usda.gov/news-events/publications/listeria-interventions-
country-hams. Accessed: 9th August 2021.
Murphy, R.Y., Duncan, L.K., Beard, B.L., Driscoll, K.H. 2003. D and z values of
Salmonella, Listeria innocua, and Listeria monocytogenes in fully cooked poultry
products. Journal of Food Science. 68(4):1443-1447.
Murphy R.Y., Osaili T., Duncan L.K., Marcy J.A. 2004. Thermal inactivation of
Salmonella and Listeria monocytogenes in ground chicken thigh/leg meat and skin.
Poultry science. 83(7):1218-25.
NACMCF (National Advisory Committee on Microbiological Criteria for Foods). 2006.
Requisite scientific parameters for establishing the equivalence of alternative methods
of pasteurization. Journal of Food Protection. 69(5):1190-1216.
NACMF (National Advisory Committee on Microbiological Criteria for Foods). 2010.
Parameters for determining inoculated pack/challenge study protocols. Journal of Food
Protection 73(1):140-202.
Porto-Fett, A.C., Call, J.E., Luchansky, J.B. 2008. Validation of a commercial process
for inactivation of Escherichia coli O157: H7, Salmonella Typhimurium, and Listeria
monocytogenes on the surface of whole muscle beef jerky. Journal of Food Protection.
71(5):918-926.
Ramirez-Hernandez, A., Inestroza, B., Parks, A., Brashears, M.M., Sanchez-Plata,
M.X., Echeverry, A. 2018. Thermal inactivation of Salmonella in high-fat rendering meat
products. Journal of Food Protection. 81(1):54-58.
Reynolds, A.E., Harrison, M.A., Rose‐Morrow, R., Lyon, C.E. 2001. Validation of dry
cured ham process for control of pathogens. Journal of Food Science. 66(9):1373-1379.
53
Scallan, E., Hoekstra, R.M., Angulo, F.J., Tauxe, R.V., Widdowson, M.A., Roy, S.L.,
Jones, J.L., and P.M. Griffin, P.M.. 2011. Foodborne illness acquired in the United
States—major pathogens. Emerging Infectious Diseases. 17(1): 7-15.
Scott, J., Weddig, L. 1998. Principles of integrated time-temperature processing. In
Proceedings of the Meat Industry Research Conference. (September).
Sebranek, J.G. 2010. Natural vs. artificial casings: Evaluating which is best for your
product. Meatingplace, In Print Online. American Association of Meat Processors.
Sindelar, J.J., Glass, K., Hanson, R. 2016. Investigating the development of thermal
processing tools to improve the safety of Ready-To-Eat meat and poultry products.
Foundation for Meat and Poultry Research and Education Final Report.
https://meatpoultryfoundation.org/research/investigating-development-thermal-
processing-tools-improve-safety-ready-eat-meat-and-poultry Accessed 19 December
2018.
Taormina, P. J., Anthony, M., Bartholomew, G., Dorsa, W.J. 2011. Validation of lethality
during an industrial microwave bacon cooking process. Prog. Intl. Assoc. Food Prot.
98th Annual Meeting, Jul 31st-Aug 3rd, Milwaukee, WI.
Tornberg, E. 2005. Effects of heat on meat proteins – Implications on structure and
quality of meat products. Meat Science. 70: 493-508.
U.S. Department of Agriculture, Food Safety and Inspection Service. Risk Assessment
of the Impact of Lethality Standards on Salmonellosis from Ready-to-Eat Meat and
Poultry Products. 2007a. Food Safety and Inspection Service, U.S. Department of
Agriculture, Washington, D.C.
U.S. Department of Agriculture, Food Safety and Inspection Service. 1994. Nationwide
Beef Microbiological Baseline Data Collection Program: Steers and Heifers. Available
at: https://www.fsis.usda.gov/science-data/data-sets-
visualizations/microbiology/baseline-microbiology-data-reports. Accessed: 9th August
2021.
U.S. Department of Agriculture, Food Safety and Inspection Service. 1996a.
Nationwide Beef Microbiological Baseline Data Collection Program: Cows and Bulls.
Available at: https://www.fsis.usda.gov/science-data/data-sets-
visualizations/microbiology/baseline-microbiology-data-reports. Accessed: 9th August
2021.
U.S. Department of Agriculture, Food Safety and Inspection Service. 1996b.
Nationwide Broiler Chicken Microbiological Baseline Data Collection Program.
Available at: https://www.fsis.usda.gov/science-data/data-sets-
visualizations/microbiology/baseline-microbiology-data-reports. Accessed: 9th August
2021.
54
U.S. Department of Agriculture, Food Safety and Inspection Service. 1996c.
Nationwide Federal Plant Raw Ground Beef Microbiological Survey. Available at:
https://www.fsis.usda.gov/science-data/data-sets-visualizations/microbiology/baseline-
microbiology-data-reports. Accessed: 9th August 2021.
U.S. Department of Agriculture, Food Safety and Inspection Service. 1996d.
Nationwide Pork Microbiological Baseline Data Collection Program: Market Hogs.
Available at: https://www.fsis.usda.gov/science-data/data-sets-
visualizations/microbiology/baseline-microbiology-data-reports. Accessed: 9th August
2021.
U.S. Department of Agriculture, Food Safety and Inspection Service. 1996e.
Nationwide Raw Ground Chicken Microbiological Survey. Available at:
https://www.fsis.usda.gov/science-data/data-sets-visualizations/microbiology/baseline-
microbiology-data-reports. Accessed: 9th August 2021.
U.S. Department of Agriculture, Food Safety and Inspection Service. 1996f. Nationwide
Raw Ground Turkey Microbiological Survey. Available at:
https://www.fsis.usda.gov/science-data/data-sets-visualizations/microbiology/baseline-
microbiology-data-reports. Accessed: 9th August 2021.
U.S. Department of Agriculture, Food Safety and Inspection Service. 2020. Listeria
monocytogenes illness outbreak associated with ready-to-eat, country-cured-ham 2017-
2018: After-Action Review Report 2018-16. Available at:
https://www.fsis.usda.gov/sites/default/files/media_file/2020-
11/Listeria%20monocytogenes%20Illness%20Outbreak%20Associated%20with%20Re
ady-to-Eat%2C%20Country-Cured%20Ham%2C%202017%E2%80%932018.pdf.
Accessed: 9th August 2021.
Veeramuthu, G.J., Price, J.F., Davis. price, C.E., David, A.M. Booren, A and D.M., Smith,
D.M. 1998. Thermal inactivation of Escherichia coli O157: H7, Salmonella senftenberg,
and enzymes with potential as time-temperature indicators in ground turkey thigh
meat. Journal of Food Protection. 61(2): 171-175.
Waldroup, A. L. 1996. Contamination of raw poultry with pathogens. World’s Poultry
Science Journal. 52(1): 7-25.
Williams, M. S., Y. Cao, Y., Ebel, and E. D. Ebel. 2013. Sample size guidelines for
fitting a lognormal probability distribution to censored most probable number data with a
Markov chain Monte Carlo method. International Journal of Int. J. Food Microbiology.
Micro. 165(2): 89-96.
55
Attachment A1. Customized Processes and
Alternative Lethality Support
Following FSIS Critical Operating Parameters for Cooking (Time-Temperature Tables)
(page 23) will yield product that meets the lethality performance standards and targets.
However, some establishments may want to develop customized processing
procedures to achieve lethality. Establishments or their process authorities may
develop customized processes or an alternative lethality that meets the performance
standards or targets by using information obtained from the literature or by comparing
their processes with established processes. However, all processes must achieve a
supported Log reduction of pathogens and prevent the production of toxins or toxic
metabolites (e.g., Staphylococcus aureus) to meet HACCP requirements and produce
safe food (General Considerations for Designing HACCP Systems to Achieve Lethality
by Cooking, page 18). Regardless of the scientific support used, the establishment’s
actual process must match the critical operating parameters in the scientific support in
order to achieve adequate lethality and meet validation requirements.
In addition to the recommendations provided in the HACCP Systems Validation
Guideline, FSIS recommends that establishments and processing authorities address
the following questions when evaluating how journal articles and other sources of
alternative support may apply to a cooking process:
1. Does the scientific support (e.g., book chapters, journal articles) demonstrate that
sufficient lethality of Salmonella (or a supported surrogate) is achieved in the
product?
o Negative results obtained from finished product sampling alone (without
inoculation) are not sufficient to demonstrate that the product meets the
performance standards or targets because they do not support any
particular reduction in pathogens is achieved by the process.
o Studies should evaluate the survival of a mixture (cocktail) of Salmonella,
including strains associated with human illness and strains isolated from
meat and poultry products. Ideally, some of the strains selected should be
those with known heat-tolerance properties.
2. Does the scientific support identify all critical operating parameters used to
achieve lethality (e.g., relative humidity)?
o Many research studies designed to determine D-values of pathogens in
different food matrices use enclosed systems that maintain moisture, such
as sealed glass tubes, or impermeable bags immersed in hot water.
These studies, as published in journal articles, may not specifically list
controlling moisture during cooking as a critical operating parameter, but
the methods used inherently maintain moisture in the system. To achieve
56
the same result as the study, an establishment would need to consider
how its process will apply moisture to ensure lethality on the product
surface during cooking (see page 16).
Acceptability of Challenge Study Results
There are different ways to evaluate the results of challenge studies and scientific
literature, such as journal articles. The National Advisory Committee on Microbiological
Criteria for Foods (NACMCF), in its 2010 article “Parameters for Determining
Inoculated Pack/Challenge Study Protocols” recommends a statistical analysis be
performed on results or, if not, a clear justification be provided.
Below are three acceptable ways to determine if the results of the research are
sufficient to support an establishment’s lethality process:
1. The mean (average) is ≥ performance standard or target log reduction.
2. Results for all replicates are ≥ performance standard or target.
3. The lower 95% confidence limit for the results from the study is ≥ performance
standard or target.
o What this means is the reduction is calculated based on the mean log
reduction minus 1.94 times the standard deviation. The recommendation
to subtract 1.94 times the standard deviation from the mean log reduction
is based on a study with an n of 6 (i.e., three replicates and two samples
per replicate or two replicates and three samples per replicate).
The approaches are listed in order of increasing confidence the results support an
acceptable lethality process. The first approach (using the mean or average result)
provides the least confidence the lethality process will consistently achieve the
performance standard or target because it does not take into account variation found in
the results. The third approach (using the lower 95% confidence limit) provides the
greatest confidence but is also the most conservative because it takes into account a
confidence interval based on variation found during the study.

57
Supporting an Alternative Lethality Target (e.g., 5-Log)
Establishments that use an alternative lethality (e.g., FSIS 5-Log Table) need to
consider a number of factors that were identified in the Salmonella risk assessment,
specifically:
• Product categorization (shelf-stable or not shelf-stable).
• Pathogen load in raw materials.
• Storage and growth.
• Consumer reheating.
How is Alternative 5-Log Lethality Related to Risk of Foodborne Illness?
Historically, FSIS has recommended that establishments achieve at least a 6.5-Log
reduction of Salmonella in cooked meat products (other than beef patties which
require a 5-Log reduction). The previous recommendations were due to the Risk
Assessment of the Impact of Lethality Standards on Salmonellosis from RTE Meat
and Poultry Products, 2005 (Salmonella Risk Assessment), which showed that a 5-
Log reduction of Salmonella (instead of a 6.5-Log reduction) would result in a
greater risk of illness in cooked meat products.
The regulations for cooked beef, corned beef, and roast beef in 9 CFR 318.17(a)(1)
allow for the use of alternative lethality, provided it provides equivalent probability
that no viable Salmonella cells remain in the finished product, as well as ensures the
reduction of other pathogens and their toxins or toxic metabolites necessary to
prevent adulteration. FSIS is providing guidance to establishments regarding how to
validate the alternative lethality option of achieving at least a 5-Log reduction of
Salmonella in cooked meat products other than beef patties to ensure the lower
reduction does not result in a greater risk of illness. For shelf-stable products, that
primarily rely on means other than cooking to achieve lethality, the Salmonella Risk
Assessment did not show a substantially higher risk of illness for product with a 5-
Log reduction compared to a 6.5-Log reduction, so FSIS continues to recommend a
5-Log reduction of Salmonella for shelf-stable products. Therefore, establishments
do not need to provide additional support for decisions in the hazard analysis (9
CFR 417.5(a)(1)) if they identify a 5-Log reduction of Salmonella as the lethality
target for shelf-stable.
58
An establishment can use the following bulleted options to support an alternative
lethality target. The alternative lethality target may be from alternative supporting
documentation (Attachment A1. Customized Processes and Alternative Lethality
Support page 55) or with the time-temperature combinations in Table 6. Time-
Temperature Combinations for Meat Products to Achieve a 5-Log Reduction (page 59).
• Use source materials that have been tested or treated to reduce pathogens. The
establishment can use a cooking process that achieves a 5-Log lethality of
Salmonella if it uses source materials that have been tested or treated to reduce
pathogens. The establishment should maintain support (e.g., Letters of
Guarantee (LOG), Certificates of Analysis (COA), or sampling information) for
each lot demonstrating that the levels of Salmonella are low enough to be
controlled by a process achieving 5-Log reduction with an appropriate safety
margin (e.g., 2-Log). For example, an establishment may provide a LOG
indicating that a certain Log reduction (e.g., 1.5-Log or 2-Log) is achieved in the
source materials using a validated antimicrobial intervention.
• Conduct a Salmonella baseline study on the raw source material. The baseline
study should be designed such that the establishment can demonstrate, with
reasonable confidence, that less than 0.01% of the raw, formulated product
contains concentrations > 10 CFU/gram of Salmonella before cooking. This is
based on the premise that a 5-Log lethality step would reduce a Salmonella level
of < 10 CFU/gram to < 1 CFU/ 100 grams and provide a 2-Log margin of safety
(NACMCF, 2010).
Key Question
Question: Do establishments that want to use the 6.5-Log Time-Temperature Tables need to
perform raw product testing or provide other support?
Answer: No. The times and temperatures listed in the tables for 6.5-Log or 7.0-Log reductions
can be used without any additional support or testing. These time-temperature combinations
will achieve sufficient lethality as long as adequate humidity (page 26) is applied during the
process.
Challenges Supporting a 5-Log Alternative Lethality for Cooked Beef Products
FSIS recognizes that extensive baseline sampling and testing needed to apply a 5-Log lethality
may be cost prohibitive for small and very small establishments. However, this document
provides multiple options for meeting the performance standards for certain RTE products. As
noted in the question box above, establishments do not need additional testing or support to
apply the 6.5-Log Meat Table, or the 7.0-Log Poultry Time-Temperature Tables in their
process.
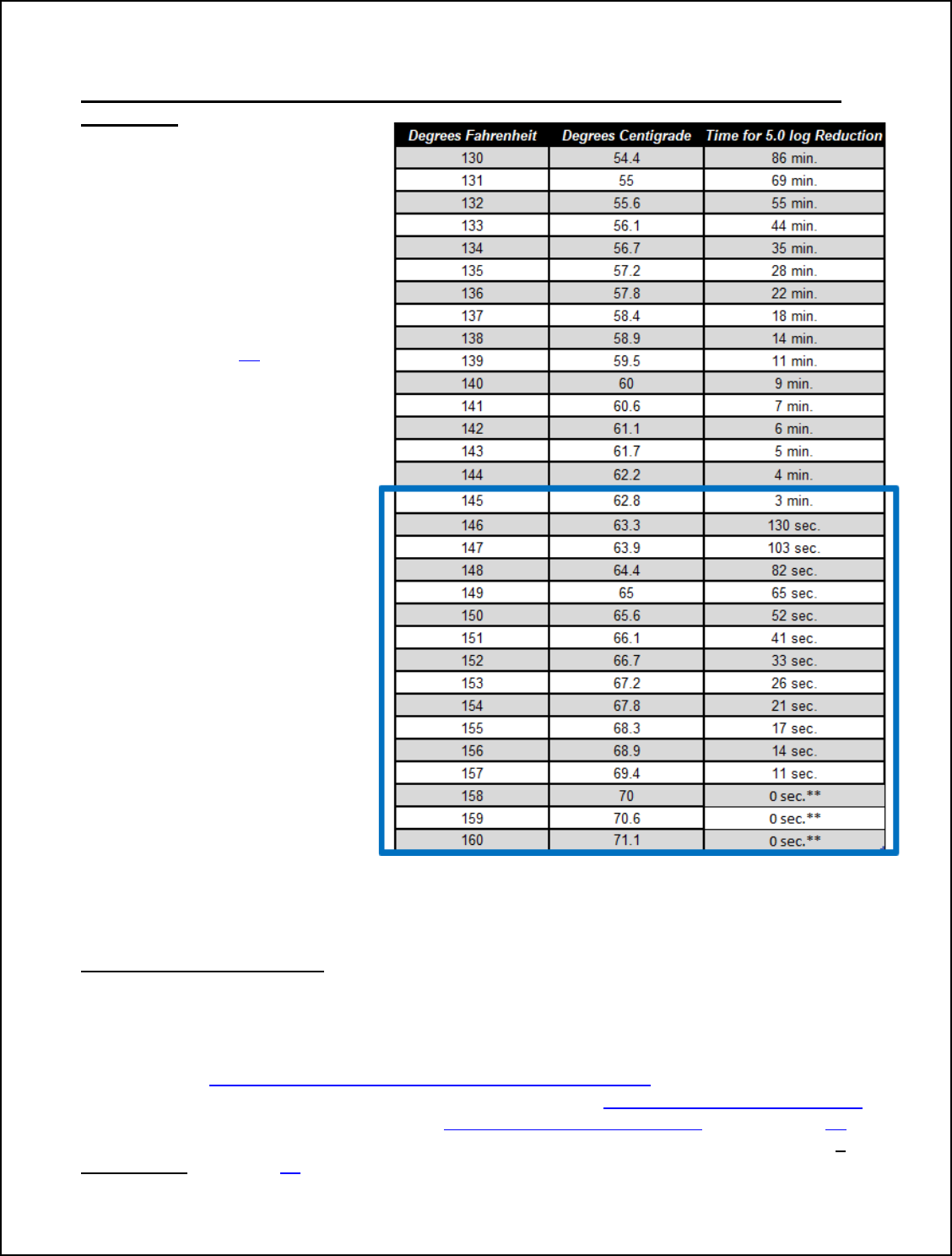
59
Table 6. Time-Temperature Combinations for Meat Products to Achieve a 5-Log
Reduction
Temperatures stated are the
minimum internal temperatures
that must be met in all parts of
the product for the total dwell
time listed
14, 15
. An establishment
must ensure both time and
temperature parameters are met
to use this table to support that
its process achieves a 5-Log
reduction of Salmonella. As
described on page 23, relative
humidity
16
and heating come-
up-time (CUT)
17
are critical
operating parameters when
using this table.
14
A 5-Log reduction of Salmonella is achieved instantly (0 seconds) when the internal
temperature of a cooked meat product reaches 158°F or above.
15
When using this table for not shelf-stable products other than meat patties, establishments
must provide additional support to show why a 5-Log reduction is sufficient to ensure pathogens
are eliminated (Supporting an Alternative Lethality Target (e.g., 5-Log) page 50).
16
Time-Temperatures ≥ 145°°F (in blue square) are eligible for FSIS Relative Humidity Options
1 and 2. All time-temperatures may apply to FSIS Relative Humidity Options 3 and 4 (page 26).
17
FSIS recommends limiting the total time product temperature is between 50 and 130°F to 6
hours or less (see page 23).
60
Common Topics and Journal Articles Used for Alternative Support
Many journal articles have been published that have increased scientific understanding
of the critical role of certain operating parameters during cooking including relative
humidity. FSIS recognizes that many of these journal articles, including that by Buege
et al., (2006), support the use of less than 90% relative humidity (FSIS Relative
Humidity Option 4; page 26). Establishments may use these journal articles as
scientific support as long as establishments ensure the published critical operating
parameters match the critical operating parameters being used in the establishment’s
process. FSIS agrees that wet-bulb temperature is a good indicator of surface lethality
during cooking but does not believe there is enough information at this time to make a
general recommendation that a single wet-bulb temperature can be used in place of the
FSIS relative humidity options for all products. For more information see FSIS’ wet-bulb
video available at: https://youtu.be/as-c2bCsoHQ.
Other commonly used alternatives to relative humidity include dew point temperature
and percent moisture by volume. Alternative measures are particularly valuable in
products cooked at high dry bulb temperatures. However, at this time, there is no
consensus or scientifically supported recommendation for how to use those parameters
or a targeted value to reach for each parameter. Consequently, FSIS has posted an
FSIS research priority on its website and is aware that researchers are actively
investigating this issue (Scientific Gaps Identified by FSIS page 41).
Journal articles or reports establishments may consider using as scientific support,
grouped by topic area, include:
• Validated cook schedules for making beef jerky by controlling dry bulb and wet
bulb temperatures.
o Buege, D.R., Searls, G., Ingham, S.C. 2006. Lethality of commercial
whole-muscle beef jerky manufacturing processes against Salmonella
serovars and Escherichia coli O157: H7. Journal of Food Protection.
69(9):2091-2099.
o Porto-Fett, A.C., Call, J.E., Luchansky, J.B. 2008. Validation of a
commercial process for inactivation of Escherichia coli O157: H7,
Salmonella Typhimurium, and Listeria monocytogenes on the surface of
whole muscle beef jerky. Journal of Food Protection. 71(5):918-926.
o Borowski, A. G., Ingham, S. C., Ingham, B. H. 2009. Lethality of home-
style dehydrator processes against Escherichia coli O157: H7 and
Salmonella serovars in the manufacture of ground-and-formed beef jerky
and the potential for using a pathogen surrogate in process validation.
Journal of Food Protection. 72(10): 2056-2064.
o Dierschke, S., Ingham, S.C., Ingham, B.H. 2010. Destruction of
Escherichia coli O157: H7, Salmonella, Listeria monocytogenes, and
Staphylococcus aureus achieved during manufacture of whole-muscle
beef jerky in home-style dehydrators. Journal of Food Protection.
73(11):2034-2042.
61
• Validated cook schedules for making turkey jerky by controlling dry bulb and wet
bulb temperatures.
o Porto-Fett, A.C.S., Call, J.E., Hwang, C.A., Juneja, V., Ingham, S.,
Ingham, B., Luchansky, J.B. 2009. Validation of commercial processes for
inactivation of Escherichia coli O157: H7, Salmonella Typhimurium, and
Listeria monocytogenes on the surface of whole-muscle turkey jerky.
Poultry Science, 88(6):1275-1281.
• Use of high temperature, short time cooking procedures and monitoring a wet
bulb temperature target. The research provides scientific support for alternative
processes including use of a wet-bulb temperature target.
o Sindelar, J.J., Glass, K., Hanson, R. 2016. Investigating the development
of thermal processing tools to improve the safety of Ready-To-Eat meat
and poultry products. Foundation for Meat and Poultry Research and
Education Final Report.
https://meatpoultryfoundation.org/research/investigating-development-
thermal-processing-tools-improve-safety-ready-eat-meat-and-poultry
Accessed 19 December 2018.
NOTE: Establishments may use this final report as scientific support until
a peer-reviewed journal article is published.
CUT Option
FSIS’s CUT option (page 23) was developed to support a wide variety of products. It is
designed to use product characteristics that would allow the most S. aureus growth
(worst-case scenario). Using worst-case conditions ensures that the option prevents S.
aureus from being a hazard in all products. Establishments may be able to identify
Why do some journal articles support using different critical operating parameters for
cooking than those recommended by FSIS?
FSIS guidance is designed to ensure lethality for a large number of meat and poultry products
across broad product categories. Research on specific processes and product types may
support adequate lethality can be achieved using different critical operating parameters for
certain products (e.g., shorter dwell time or lower endpoint temperature), but research is not
always available to support using those parameters across the many product categories and
product types that this guidance covers. Establishments may choose to follow journal articles
or other peer-reviewed scientific data instead of FSIS guidance, provided the same critical
operating parameters are met (e.g., product type, dry-bulb temperature, wet-bulb
temperature, internal product temperature, and intrinsic factors) and the process achieves
sufficient reductions for Salmonella based on the establishment’s desired target.
62
journal articles with longer CUT for products with specific characteristics that inhibit
pathogen growth (e.g., formulated with antimicrobials like sodium lactate).
Example:
• This following journal article provides critical limits for the brine injection and the
thermal process that control S. aureus growth and enterotoxin production during
a 14-hour CUT.
o Ingham, S.C., Losinski, J.A., Dropp, B.K., Vivio, L.L., Buege, D.R.
2004. Evaluation of Staphylococcus aureus growth potential in ham
during a slow-cooking process: use of predictions derived from the US
Department of Agriculture Pathogen Modeling Program 6.1 predictive
model and an inoculation study. Journal of food protection, 67(7):1512-
1516. https://foodsafety.wisc.edu/meat-haccp/.
• This following journal article provides critical operating parameters for hams
formulated with phosphate and cooked to lethality while applying a long CUT.
o Sindelar, J., Glass, K., Hanson, R., Sebranek, J.G., Cordray, J.,
Dickson, J.S. 2019. Validation for lethality processes for products with
slow CUT: Bacon and bone-in-ham. Food Control. 104:147-151.
NOTE: Although Sindelar et al. (2019) contains information on the growth of pathogens
during the heating CUT for partially heat-treated bacon, the article is not adequate sole
support for controlling the growth of C. perfringens and C. botulinum. Please review the
FSIS Stabilization Guideline for Meat and Poultry Products for additional details.
Predictive Microbial Modeling to Support CUT
Alternatively, establishments may use predictive microbiology modeling to develop
custom critical operating parameters. Predictive food microbiology uses models (i.e.,
mathematical equations) to describe the growth, survival, or inactivation of microbes in
food systems from knowledge of the intrinsic and extrinsic factors of the food over time.
There are many free predictive microbial models available to establishments either
online or through a download. Please refer to Predictive Microbial Modeling (page 72)
for FSIS recommendations on using predictive microbial models to evaluate S. aureus
growth during heating CUT deviations. These same recommendations can be applied
when validating a custom CUT for a HACCP system.
63
Designing Challenge Studies for Cooking
One of the most definitive tools at the disposal of an establishment or processing
authority for validating a process is the challenge study.
As stated in the HACCP Systems Validation Guideline, establishments may perform
challenge (or inoculated pack) studies to provide scientific support for their processes.
These studies are performed in a laboratory or pilot plant by a processing authority or
expert. The documentation on file should specify the level of pathogen reduction,
elimination, or growth control; describe the process, including all critical operating
parameters affecting the reduction or elimination of the pathogen of concern; and give
the source of the documentation. Such studies are often not published in peer-reviewed
journal articles but should contain the same level of detail as is provided for peer-
reviewed studies.
Challenge studies should be designed and conducted to accurately simulate the
commercial process. Challenge studies should be undertaken by individuals who have
a thorough knowledge of laboratory methods used in Salmonella research. Challenge
studies should be based on a sound statistical design (i.e., a statistical design that
ensures confidence in the data) and should also employ positive and negative controls.
The statistical design should include the number of samples collected at each time
interval and the number of study replicates needed to ensure the validity of the study.
There are quantitative methods for assessing the statistical quality of a study (e.g.,
power analysis). As per the National Advisory Committee on Microbiological Criteria for
Foods (NACMCF), the minimum number of samples to be analyzed initially and at each
time interval during processing or storage should be at least two. However, NACMCF
highly recommends analysis of three or more samples at each time interval. According
to NACMCF, challenge studies should include replicates. Replicates should be
independent trials using different lots of product and inoculum to account for variations
in product, process, inoculum, and other factors. When the number of samples
analyzed at each time interval is only two, NACMCF suggests it is better for the study to
be repeated (replicated) more than two times. In studies with three or more samples
tested at each time interval, two replicates are usually adequate. A cocktail of various
serotypes of Salmonella should be used in an inoculated pack study to demonstrate that
the lethality performance standard or target is met. At least five strains of the pathogen
should be used in the inoculum. Relatively heat tolerant pathogenic strains should be
included in the cocktail to develop a worst case. The serotypes/strains selected should
be among those that have been historically implicated in an appreciable number of
outbreaks.
FSIS does not require establishments to validate that their process achieves a specific
reduction of STEC or Lm in cooked product if they achieve sufficient reductions of
Salmonella because FSIS considers Salmonella an indicator of lethality for cooked
products. Without further scientific support, establishments should not use pathogens
other than Salmonella as indicators of lethality. For example, establishments should not
64
use reductions in Lm to support similar reductions in Salmonella without support that Lm
is at least equally as heat tolerant as Salmonella under the conditions being studied.
If an establishment chooses to conduct a challenge study in a testing laboratory, the
study should use at least five strains of Salmonella, including strains associated with
human illness and strains isolated from meat and poultry products. Ideally, some of the
Salmonella strains selected should be those with known heat-tolerance properties.
FSIS recommends that establishments and their laboratories include a justification for
the strains chosen (e.g., associated with human illness or isolated from meat or poultry
products) in the challenge study report.
In addition, the inoculum level should be at least 2-Log greater than the Log reduction to
be demonstrated. FSIS recommends that establishments use Salmonella as an
indicator of lethality (Goodfellow and Brown, 1978; Line et al., 1991) or an appropriate
surrogate of Salmonella that has similar heat and drying-tolerance properties. For
example, Enterococcus faecium has been validated as a suitable surrogate for
Salmonella during cooking of ground beef (Ma et al., 2007). FSIS considers all
Salmonella serotypes to be pathogens of public health concern. At a minimum, a study
for a microbiological food safety hazard should identify:
• The hazard (including the specific strains studied).
• The expected level of hazard reduction or prevention to be achieved.
• The processing steps that will achieve the specified reduction.
• All critical operating parameters or conditions (e.g., time, temperature, and
humidity) necessary to achieve the reduction.
• Procedures to monitor the critical operating parameters or conditions.
• The critical ingredients (e.g., concentration of salt, sugar, and cure).
• The critical product characteristics (e.g., pH, water activity, moisture level, and fat
content).
NOTE: For more information on conducting challenge studies, please review the
article, “Parameters for Determining Inoculated Pack/Challenge Study Protocols,”
Key Question
Question: Should a Challenge Study use S. Senftenberg 775W?
Answer: Not necessarily. FSIS would not require that. The FSIS Jerky Guideline states,
“One good [strain] choice, for example, might be Salmonella enterica serovar Senftenberg
strain 775W, which displays heat resistance properties (Ng et al., 1969). Salmonella enterica
serovar Senftenberg occurs in the top 10 serotypes seen in FSIS testing for both cow/bull
carcass testing and ground beef, as well as in turkeys (carcass and ground) (FSIS testing
data, 2012), so it would also be an appropriate choice for what might be seen in these
products being tested.” However, additional studies have determined that Salmonella
Senftenberg has much higher heat tolerance than other pathogens (McMinn, et al., 2018;
Veeramuthu, et al., 1998). In addition, more recent data does not continue to identify it in the
top 10 serotypes seen in FSIS testing.

65
published by the NACMCF in the Journal of Food Protection in 2010. For more
information on the use of positive and negative controls in challenge studies as well as
general guidance on how to select a microbiological testing laboratory please review
FSIS’ Establishment Guidance for the Selection of a Commercial or Private
Microbiological Testing Laboratory.

66
Attachment A2. Cooking Deviations
Corrective Actions to Perform When a Cooking Deviation Occurs
Cooking deviations occur when an establishment fails to meet its cooking CCP critical
limit for endpoint time-temperature, cooking humidity option, or heating come-up time
option. Common causes for cooking deviations include product overlap, power failures,
or breakdown of cooking equipment. Establishments are required to take corrective
actions, as required by the HACCP regulations, regardless of whether the cooking
process is addressed through a CCP or prerequisite program. This includes ensuring
no product that is injurious to health or otherwise adulterated because of the deviation
enters commerce (9 CFR 417.3(a) or (b)).
• When cooking is addressed through a CCP, establishments are required to
determine the cause of all cooking deviations, no matter how small (9 CFR
417.3(a)(1)), and ensure measures are established to prevent recurrence (9 CFR
417.3(a)(3)). If the cause of each small cooking deviation is not traced and
corrected when first noticed, the problem will likely recur and become more
frequent and more severe. The establishment should consider an occasional
small process deviation to be an opportunity to find and correct a process control
problem. Large process deviations or continual small ones always constitute
unacceptable risk. Also, continual or repetitive process deviations from the
critical limit demonstrate that the establishment is unable to control its process
and that its corrective actions are not preventing recurrence as intended.
• When cooking is addressed through a prerequisite program and a deviation
occurs, establishments are required to reassess their HACCP system to
determine whether the newly identified deviation or unforeseen hazard should be
addressed and incorporated into the HACCP plan (9 CFR 417.3(b)(4)). Also, an
establishment may not be able to continue to support the decision in its hazard
analysis that pathogens are not reasonably likely to occur, if it has continual or
repetitive deviations from its cooking prerequisite program (9 CFR 417.5(a)(1)).
To assist establishments in determining and supporting product disposition as required
9 CFR 417.3(a) or (b), FSIS is including information regarding potential pathogens of
concern during different types of cooking deviations and recommendations for using
pathogen modeling and sampling. Establishments should carefully evaluate each
deviation as each situation is unique and needs to be evaluated individually. Ultimately,
the establishment should rely on the expertise of a processing authority to determine
the severity of cooking deviations and subsequent appropriate disposition of the product
in question. Knowledge of the specific product and factors that would favor or inhibit the
growth of various bacterial pathogens is essential to determine product safety. As
stated in the HACCP Systems Validation Guideline, the advice of processing authorities
should include reference to established scientific principles as well as reference to peer-
reviewed scientific data.

67
Pathogens of Concern During Cooking Deviations
Cooking deviations can allow pathogens that are controlled under normal cooking
procedures to become a hazard, depending on the type of cooking deviation (described
below) that occurs. Specific pathogens of concern may include:
• Salmonella, STEC (in beef products), and Lm, which could grow as vegetative
cells to levels that overwhelm the Log reductions achieved by cooking.
• S. aureus, if allowed to grow to high levels, may produce heat-stable enterotoxins
in the food.
• Bacillus cereus (B. cereus) (in rare cases), if allowed to grow to high levels in the
food, may produce a heat-stable emetic toxin in the food or enterotoxins in the
small intestine.
• Clostridium perfringens (C. perfringens) and Clostridium botulinum (C. botulinum)
spore-forming pathogens that can germinate and grow in product held at higher
temperatures (e.g., > 80°F).
Again, it is important someone knowledgeable such as a processing authority evaluates
each deviation to determine the pathogens of concern.
Three Common Types of Cooking Deviations
When cooking products to lethality, deviations may occur due to three main reasons:
1. The establishment fails to meet a time--temperature parameter in its lethality
CCP for meat or poultry products.
2. The establishment fails to maintain sufficient humidity during the cooking step.
3. Slow heating CUT allows product to remain at temperatures that allow pathogen
growth (e.g., product remains at temperatures 50°F to 130°F for more than 6
hours; see FSIS Critical Operating Parameters for Cooking Come-Up-Time
(CUT), page 23).
Specific recommendations for evaluating each type of cooking deviation, including the
pathogens of concern, are provided below. Alternatively, the establishment can provide
additional support for the safety of the product (e.g., a journal article, or support from a
processing authority). These are general recommendations; the specific responses will
vary based on the unique factors of each deviation.
Type 1. Missed Endpoint Time-Temperature
When evaluating product disposition after the process fails to meet an endpoint time or
temperature parameter, the first step is to assess whether the process met a different
time-temperature combination in the reference table. In some cases, the process may
not have achieved an instantaneous lethality temperature (e.g., 158°F for meat)
identified in the CCP but may have achieved the dwell time needed for a lower
68
temperature in the same table (e.g., 154°F for 27 seconds) when considering the total
time product temperature was at or above the lower temperature.
Did the process meet a different validated time-temperature combination?
• If yes, then product is safe to release.
• If no, then FSIS recommends contacting a processing authority who may help
you identify proper D and z values to calculate integrated process lethality
considering the product come-up -time and come-down-time. One common tool
for calculating integrated lethality is the AMI Process Lethality Determination
Spreadsheet. If properly conducted, the AMI lethality spreadsheet is a sound
scientific approach for determining the overall lethality of a cooking process
(Scott and Wedding, 1998). The D-values at the reference temperature for the
three main pathogens of concern (Salmonella spp. E. coli O157:H7, and Lm) are
generally conservative values and should be valid for most cooked meat ready-
to-eat (RTE) processes provided that the product is moist when cooked (high
relative humidity). However, if the product is not moist when cooked and the
product surface is allowed to dry out during the lethality step, the D-values
referenced in the AMI lethality spreadsheet are not valid.
NOTE: There are many complexities involved in identifying appropriate D and z
values needed as inputs for calculating integrated pathogen lethality. FSIS
advises establishments to work with a processing authority or someone
knowledgeable in thermal death-time values, to ensure they select appropriate
values and are properly using the lethality calculator.
• Establishments may consider recooking the product, but only if all critical
operating parameters (including relative humidity and CUT time) were met during
the initial heating and during recook.
o If the relative humidity option in the scientific support was not applied,
the establishment should also follow recommendations for evaluating a
Type 2 Deviation: Insufficient Humidity During Cooking described on
page 69, or
o If the CUT parameter was not met, the establishment should also
follow recommendations for evaluating a Type 3 Deviation: Long
Heating CUT described on page 70, and contact a processing
authority for assistance.
NOTE: Cooking deviations that combine a missed time-temperature
parameter with a long CUT are complex situations which may require
considering C. perfringens and C. botulinum as described in the
Stabilization Guideline, in addition to the other pathogens of concern.

69
• If establishments cannot recook the product, they should consider the following
alternative actions:
o Provide alternative support (page 55) (e.g., information from a
processing authority that includes scientific citations that product is
safe to release);
o Sample and test the product (see Product Testing recommendations
for Type 1 deviations, page 77); or
o Destroy the product (renderer or landfill).
Type 2. Insufficient Humidity During Cooking
As described on page 16, some bacteria can become more heat tolerant when they are
exposed to moderate levels of heat, drying, and other factors. Bacteria can then survive
at higher temperatures than they normally would. Below are general recommendations
for an establishment to consider when evaluating products after a Type 2 cooking
deviation resulting from insufficient humidity (i.e., the relative humidity option in the
scientific support was not followed) during cooking.
• Consider sampling and testing product for Salmonella, Lm, and E. coli
O157:H7 (if a beef product), using a statistically based sampling program as
described in Product Testing on page 77.
• If recooking, apply a higher time-temperature combination validated to achieve
lethality in a product with similar intrinsic factors (e.g., water activity).
o It would not be appropriate to recook the product following FSIS
Relative Humidity Options (page 26) without additional support that
recooking conditions adequately rehydrate the product surface (see
Attachment A6. Cooking Country-Cured Hams, page 90).
o Under these circumstances, FSIS would need to verify that such
scientific support is adequate in the context of the specific product,
process, and situation. Examples of acceptable support may include
support that:
Demonstrates that a validated wet bulb temperature target has
been met to ensure lethality. To show that the surface has been
rehydrated, the wet bulb target should be higher than the product
surface temperature.
Includes water activity testing: A water activity increase after
recooking (compared to water activity before recooking), may
indicate that the surface has been rehydrated.
70
NOTE: FSIS is not aware of any research validating recooking procedures for
products that may have heat tolerant Salmonella because of a lack of relative
humidity during the initial cook. However, FSIS plans to update these
recommendations as more research becomes available.
Type 3. Long Heating CUT
If the total time between 50 and 130°F is longer than hours 6, recooking alone may not
be sufficient to ensure the safety of the product. That is because during the extended
CUT toxigenic pathogens could grow rapidly (e.g., S. aureus), allowing enterotoxins to
form. Some enterotoxins are extremely heat-stable and are not inactivated by normal
cooking temperatures. Therefore, it is not always possible to recook the product alone
to ensure its safety. The establishment should continue to recook the product to
address vegetative pathogens (e.g., STEC, Lm, and Salmonella). It should also provide
additional support that heat-stable enterotoxins do not present a hazard in the product
after the recooking step.
As noted in Type 1. Missed Endpoint Time-Temperature, cooking deviations that
combine a missed time-temperature parameter with a long CUT are complex situations
that may require considering C. perfringens and C. botulinum as described in the
Stabilization Guideline, in addition to the other pathogens of concern. The
establishment may want to contact a processing authority for assistance.
To determine product disposition after a long heating CUT deviation, the establishment
should:
1. Address growth of vegetative pathogens that do not produce toxins, AND
2. Address the potential enterotoxin formation as described below.
If either hazard is not controlled to safe levels, then product should be destroyed.
Further guidance on these two recommendations is provided below:
1. Address growth of vegetative pathogens: (e.g., STEC, Lm, and Salmonella).
o FSIS recommends that establishments use microbial modeling (page
72) and other information (e.g., scientific journal articles, book
chapters, and processing authorities) to estimate growth of E. coli, Lm,
and Salmonella.
If modeling estimates the growth of vegetative pathogens to be 1-
Log or less, provided the predictive microbial modeling program is
validated, modeling is adequate to show that the process prevented
vegetative pathogen outgrowth and the establishment can address
the potential for enterotoxin formation (see 2 on the next page).
If modeling estimates more than 1-Log growth of any vegetative
pathogen, establishments should recook product OR sample and
71
test for vegetative pathogens to determine the safety of the product
(see Type 3 deviation recommendations in Product Testing, page
77).
• Many establishments avoid the cost of sampling and testing
by recooking the product or consulting a processing authority
to identify alternative support that vegetative pathogens are
addressed.
• If product is recooked, it should be done to a higher time
and temperature that has been shown to achieve enough
additional Log reductions to address the amount of
vegetative cell growth the model predicted. Using a recook
procedure that achieves the correct additional Log reduction
is important to ensure increased pathogen load will not
overwhelm the Log reductions achieved during the recook
procedure (see page 72). For example, if predictive
microbial modeling showed a 2.5-Log and 3.0-Log increase
for Salmonella and E. coli O157:H7, respectively, in a roast
beef product, the recook step should be adjusted so that the
cooking time-temperature combination can achieve at least a
9.5-Log reduction of Salmonella instead of a 6.5-Log
reduction. The AMI Process Lethality Determination
Spreadsheet discussed on page 68 may be used to support
the cooking time-temperature combination can achieve
sufficient Log reductions.
2. Address potential enterotoxin formation: (e.g., S. aureus) by demonstrating
that toxigenic pathogens did not grow to levels of public health concern or
produce enterotoxin.
o FSIS recommends that establishments use microbial modeling (page
72) and other information (e.g., scientific journal articles, book chapters,
and processing authorities) to provide additional information to
determine product safety.
If predictive microbial modeling estimates a < 3-Log growth of S.
aureus, modeling is adequate to show that the process prevented
enterotoxin formation provided the predictive microbial modeling
program is validated. If growth of vegetative pathogens is also
addressed the product can be released.
NOTE: Due to the rapid growth of S. aureus in meat and poultry
products, modeling for B. cereus (which grows slower) is not
needed when S. aureus growth is controlled (< 3-Log).
If microbial modeling estimates a ≥ 3-Log growth of S. aureus,
then product should be tested for S. aureus enterotoxins A, B, C,
72
D, and E using a statistically representative sampling procedure. If
the product contains non-meat ingredients previously associated
with B. cereus associated illnesses (e.g., rice, or pasta) and
microbial modeling estimates > 3-Log growth of S. aureus, then
establishments may also want to consider testing for B. cereus
emetic toxin (Product Testing page 77).
NOTE: As stated previously, conditions that allow for 3-Log or
higher growth of S. aureus are a public health concern (ICMSF,
1996). Furthermore, this level of growth (i.e., 3-Log) for S. aureus
is consistent with the pass/fail criteria developed by the Institute of
Food Technologists (IFT) for the FDA to control for this food safety
hazard (IFT, 2003).
To support safe release of the product, both the vegetative pathogens and
enterotoxin formation must be addressed with supporting documentation. If
either hazard is not controlled to safe levels, then product should be destroyed.
Predictive Microbial Modeling
Establishments may use predictive microbial modeling to estimate the relative growth of
bacteria during a long heating CUT deviation (Type 3). As explained above for Type 3
heating deviations, modeling results can be used to support various product disposition
options including release, recooking, sampling and testing, or destruction provided the
model used has been validated. Predictive microbial modeling tools may be used to
evaluate product disposition in the event of other types of deviations (e.g., for Type 1
deviations establishments may use the AMI Process Lethality Determination
Spreadsheet). However, this section is focused on evaluating product disposition
during Type 3 heating deviations due to their complexity.
When performing predictive microbial modeling, it is important that establishments:
1. Use validated models (see examples below):
o It is not appropriate to rely solely on one model unless the model has been
validated for the particular food of interest. A validated cooking model is a
model whose predictions have been found to agree with or are more
conservative than actual observed results. If a model has not been
validated for a particular food of interest, the establishment should provide
additional supporting documentation to support the results from the model
(e.g., sampling data or comparison with other model results).
2. Enter accurate product formulation information:

73
o FSIS recommends entering the raw product formulation values for Type 3
Deviations: Long CUT, since the high moisture values at the start of
cooking will support faster pathogen growth and therefore represents the
worst-case scenario. If using finished product values, establishments
should provide reasoning for how that represents the product matrix
during CUT.
3. Enter accurate time and temperature information in the model:
o When entering time and temperatures into the model, the establishment
should include all parts of the process, including cooking and recooking
CUTs after a Type 1 or 3 cooking deviation. If the establishment does not
include all parts of the process, it may underestimate pathogen growth.
o When determining the temperature, the establishment should take into
account both the temperature at the coldest internal area (center) of the
product and at the surface of the product.
o It is important to obtain an internal time and temperature profile of the
product, and a wet bulb time and temperature profile of product since wet
bulb can be used to describe the product’s surface temperature. If an
establishment does not have wet bulb temperature data, it can conduct
predictive microbial modeling using the internal time-temperature profile of
the product, provided that sufficient humidity was used during cooking.
However, the establishment should take into account that the product
surface temperature will be higher than the center of the product under
high relative humidity conditions.
o For cases with large time gaps between known temperature observations,
establishments may consider interpolating to estimate additional time-
temperature data points between known observations assuming linear
heating. However, if the product temperature dwells or holds between 90
and 120°F (the optimal growth range of S. aureus) for an extended period
of time, excess S. aureus growth could result in a potential hazard in the
product being uncontrolled. The establishment should consider the likely
accuracy of the predicted growth when making a product disposition
determination using linear interpolation.
o Assume no S. aureus growth above 120°F.
NOTE: FSIS has included the time that product remains from 120 to
130°F in the heating CUT option (page 23) to reduce the risk B. cereus (a
spore-former) could germinate and then grow at these higher
temperatures, potentially producing a heat-stable emetic toxin.
74
4. Address model limitations in a conservative manner:
o
If product characteristics or other conditions are outside the range of the
model, accuracy is not guaranteed. Establishments should support how
the model results represent the product or the worst-case scenario for the
hazard in the product or should compare the results to several other
pathogen models and should make decisions based off the model that
shows the worst-case scenario (i.e., for S. aureus that is the model that
estimates the most outgrowth).
NOTE: This guidance contains recommendations for addressing certain limitations
in two recommended models at the time the guidance was written. Neither modeling
program is controlled by USDA-FSIS and may change. FSIS will update its
modeling recommendations in future revisions to be consistent with any changes
made to the modeling programs.
Recommended Models
• Therm 2.0 model (S. aureus, Salmonella, and E. coli O157:H7).
The University of Wisconsin Therm 2.0 model is designed to allow processors to
input the product’s time-temperature profile and it has been validated for
estimating the growth of S. aureus, Salmonella, and E. coli O157:H7.
The three input variables and their ranges for entering into the growth model are
provided below (Ingham et al., 2009):
o Input variables and ranges:
Temperature profile: 50°F to 110°F (10ºC to 43.33ºC)
Date/time: the model allows for entry of calendar date and time
Meats:
• In meat and poultry products containing salt (≤ 2.5%),
establishments should use the Therm 2.0 model for
Bratwurst for predicting pathogen growth. This model
should be used because it was designed to take into account
the bacterial pathogen’s behavior in pork sausage and
related products that contain higher fat levels, sodium
chloride, and spices. For example, adding salt to product
will inhibit the competing microorganisms, but allow for
greater growth of salt tolerant S. aureus; the Therm 2.0
model will predict this. Because the Therm 2.0 model for
Bratwurst was developed with data from a pork product,
establishments should compare the results with another
model, such as the DMRI Staphtox Predictor when
evaluating deviations involving poultry products.
75
• In meat and poultry products without any added salt,
establishments should use the Therm 2.0 model for Beef,
Pork, or Poultry based on the product type (Ingham et al.,
2009).
o Overcoming model temperature limitations: (maximum 110°F)
The Therm 2.0 model does not automatically interpolate
(estimate a linear change) between time-temperature data points
entered by the user. Therefore, FSIS recommends establishments
enter temperature observations for at least every 30 minutes, or at
the lowest time interval available.
For temperatures >110°F, substitute 110°F for any temperature
above 110°F up to 120°F. S. aureus grows fastest at 110°F. The
growth rate slows as temperatures increase from 110 to 120°F.
Modeling using 110°F for temperatures observed from 110 to 120°F
will slightly overestimate the growth of S. aureus.
For temperatures between 120 and 130°F assume no growth of S.
aureus (leave this out of the model).
• DMRI Staphtox Predictor (Version 1.0) (S. aureus)
The Danish Meat Research Institute’s (DMRI) Staphtox predictor (Version 1.0)
may also be used to predict the growth of S. aureus in meat and poultry
products, with added salt (i.e., 1.8% to 4.2%). This model has been validated
and was specifically designed to predict the growth of S. aureus in different meat
product processes based on product composition and changes in temperature.
The six input variables and their ranges for entering product composition
information into the growth model are provided below (Gunvig et al., 2018):
o Input variables and ranges:
Temperature profile: 32°F to 105.6°F (0ºC to 40.9ºC)
pH: 4.4 – 6.1
% sodium chloride (NaCl) in product (based on the total weight of
the product formulation): 1.8 – 4.2%.
NOTE: The model converts % sodium chloride (NaCl) into the %
water-phase salt.
% potassium chloride (KCl) in product (based on the total weight of
the product formulation): 0.0 – 4.2%
Sodium nitrite added to product: 0 – 150 ppm
% water in final product (as determined through laboratory
analysis): 62 – 78%

76
o Worst Case Scenario: FSIS recommends using the values listed below
as model inputs for any products where the values are unknown. These
values represent a worst-case scenario for the growth of S. aureus based
on product composition:
pH: 6.1
% NaCl in product: 1.8%
% KCl in product: 0.0
Sodium nitrite added to product: 0 ppm
% water in final product: 78% (highest allowed in model)
Initial level S. aureus: 100 CFU/g
o Overcoming model temperature limitations: (maximum 105.6°F)
For temperatures > 105.6°F (40.9°C), substitute 105.6°F for any
temperature above 105.6°F (40.9°C), up to 120°F (48.9°C). The
fastest growth in this model is at 105.6°F. As described above, S.
aureus continues growing at higher temperatures, but the growth
rate slows as temperature increases up to 120°F (48.9°C). For
modeling, use 105.6°F for temperatures observed from 105.8°F
(41°C) up to 120°F (48.9°C), which will slightly overestimate the
growth of S. aureus (fail-safe).
For temperatures between 120°F (48.9°C) and 130°F (54.4°C)
assume no growth of S. aureus (leave out of the model).
NOTE: Establishments may use the ComBase S. aureus model as support. However,
this model has not been validated and establishments should follow the
recommendation for using models that are not validated (i.e., compare the results of
several models and make decisions using the worst-case results) as described above.

77
Product Testing
As described in the cooking deviation and the microbial modeling recommendations
(pages 67-72), if the establishment is unable to support the product disposition through
predictive microbial modeling or some other means, the establishment can test a
statistically-based number of samples of the product to support its safety. Table 7
identifies the hazards to be tested for according to the type of cooking deviation that
took place. These are general recommendations; it is important that someone
knowledgeable such as a processing authority evaluate each deviation to determine the
appropriate sampling and testing plan.
Table 7. FSIS Recommendations for Product Sampling and Testing After Each
Type of Cooking Deviation to Determine Product Disposition
Type of Heating
Deviation*
Vegetative Pathogens
Heat-stable
Enterotoxins
Salmonella
Lm
E. coli
O157:H7
**
S. aureus
Enterotoxins
A, B, C, D, and E
1 - Missed Time-Temperature
X X
X
2 - Insufficient Humidity
X X X
3 - Long CUT
X
Multiple Types in Combination
(i.e., missed time-temperature
AND long CUT)
Contact a processing authority for assistance evaluating
product disposition in a complex deviation which combined
multiple types of heating deviation. May need to consider C.
perfringens and C. botulinum in addition to the hazards listed
in this table
.
*Cooking deviation Types 1-3 are described on page 66.
**E. coli O157:H7 testing recommended only for products containing beef. Establishments may also
choose to test for other STEC; however, testing for E. coli O157:H7 alone is sufficient.
Sampling in Response to a Cooking Deviation
• The establishment should test a statistically representative number of samples
per lot depending on the bacterial pathogen. FSIS recommends testing at least
10-15 products per lot as outlined by the two-class sampling plan (Case 11 and
Case 13, respectively) per the International Commission on Microbiological
Specifications for Foods (ICMSF, 2002).
• If the product contains non-meat ingredients previously associated with B. cereus
associated illnesses (e.g., rice, or pasta) and microbial modeling estimates >3-
Log growth of S. aureus, then establishments may also want to consider testing
for B. cereus emetic toxin.
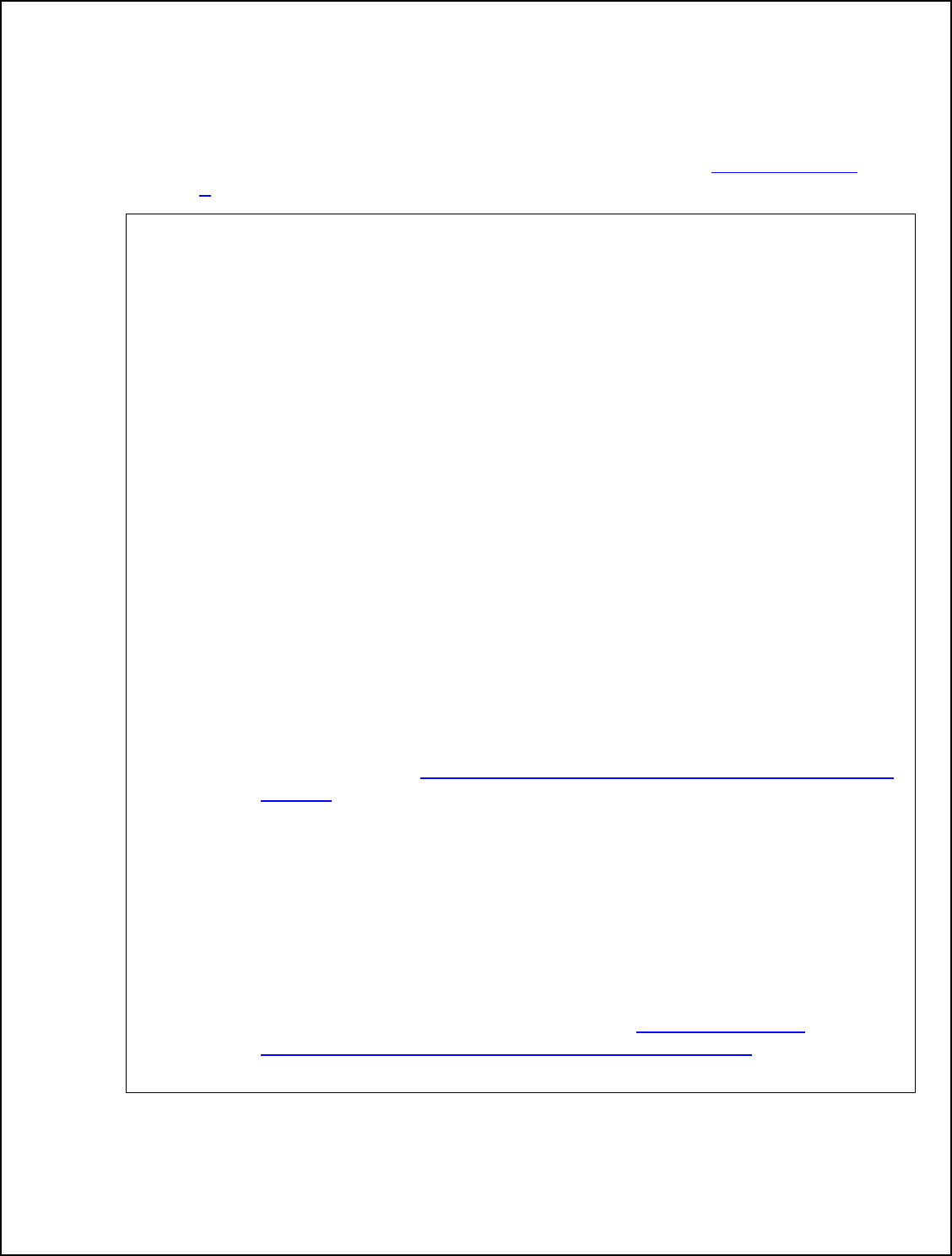
78
NOTE: FSIS does not recommend all products to be tested for B. cereus emetic
toxin due to the low incidence of B. cereus in raw meat and poultry. If you are
uncertain if the formulation of product affected by a cooking deviation may need
to address B. cereus emetic toxin as a potential hazard, please contact askFSIS
(page 9).
Key Question
Question: Can samples be composited for lab testing?
Answer: It depends on what the sample is being tested for:
• Enterotoxins? No. FSIS does not recommend compositing samples to be
tested for enterotoxins. Combining multiple samples for a single test (i.e.,
compositing) could prevent the test from detecting enterotoxins in the product.
• Vegetative pathogens? Yes. However, the number of samples that can be
combined depends on the pathogen. Additionally, establishments should
ensure the lab method has been validated for the larger test portion.
o Salmonella and E. coli O157:H7: FSIS recommends compositing up to
3 samples (total 75g) for a total of 5 analyses although establishments
may also be able to support compositing up to 15 – 25-g samples (total
375 grams). The establishment would collect 15 samples from different
pieces of product. The lab would combine the 25g sample from each of
3 different pieces, to make a 75g composited sample for analysis. The
lab analyzes 5 composited samples. When compositing,
establishments should ensure the method has been validated for the
larger test portion. FSIS has validated a 325g test portion size for its
analysis of RTE product samples collected under the RTEPROD
program (see the FSIS’ Microbiology Laboratory Guideline Salmonella
Chapter).
o Lm: FSIS recommends compositing up to 5 samples (total 125g) and 3
lab tests total. The establishment would collect 15 samples from
different pieces of product. The lab would combine the 25g test sample
from each of 5 different pieces, to make a 125g composited sample for
analysis. The lab analyzes 3 composited samples. When compositing,
establishments should ensure the method has been validated for the
larger test portion. FSIS has validated a 25g and 125g test portion size
for its analysis of RTE product samples collected under the RTEPROD
and RLm programs, respectively (see the FSIS’ Microbiology
Laboratory Guideline Listeria monocytogenes Chapter).

79
Disposition after Testing Results:
To support the safe release of the product, every hazard associated with the type of
heating deviation identified (see Table 7) must be controlled for the safe release of
product. If any single hazard is not controlled, then product should be destroyed
(renderer or landfill).
• Enterotoxins:
o If the product tests negative for enterotoxins, product can be released,
unless insanitary (or other) conditions exist that could adulterate the
product (e.g., vegetative pathogens).
o If any enterotoxin is found, the lot is adulterated, and product should be
destroyed (renderer or landfill).)
• Vegetative Pathogens:
o If the product tests negative for vegetative pathogens, product can be
released, unless insanitary (or other) conditions exist that could adulterate
the product (e.g., enterotoxins).
NOTE: It would be inappropriate to test for live S. aureus instead of
enterotoxin because it is possible for S. aureus to produce
enterotoxins prior to the death of the bacteria (e.g., during cooking).
The food product would still cause illness even though no vegetative
bacteria were found.
o If any vegetative pathogens are found, the lot is adulterated. Product may
be:
Recooked per Type 1 or Type 2 recommendations (pages 67-69);
or
Destroyed (rendered or denatured per 9 CFR 314.3(a), 9 CFR
325.11(a), 9 CFR 325.13(a)(1) through 325.13(a)(7), or 9 CFR
381.95 and sent to a landfill).

80
Common Mistakes made by Establishments when Evaluating
Heating Deviations—and the Recommended Solutions
1) The establishment did not input an accurate internal time-temperature profile into
the model. The establishment should be using a data logger or collecting time
and temperature data at regular intervals during cooking. The establishment
should take into account all parts of the process and temperatures at both the
center and surface of the product (Monitoring Endpoint Temperature page 21 and
Monitoring Surface Temperature page 24).
2) In Type 1 or 3 deviations with a missed time-temperature parameter, the
establishment failed to take into consideration the amount of bacterial growth that
could occur during the cooking come-up-time when the cooking cycle was
restarted. To address this issue, the establishment should consider both the
original come-up-time, the initial cooling, and second come-up-time when the
cooking is restarted as part of its modeling.
3) The establishment did not address whether additional growth of Salmonella, E.
coli O157:H7 and Lm could have occurred during the Type 1 heating deviation
and whether heat tolerance could have developed. To address this issue, when
recooking the product, the establishment should increase endpoint time-
temperature and apply sufficient humidity (FSIS Relative Humidity Options page
26).
4) The establishment failed to address the amount of growth of S. aureus and other
bacterial pathogens that could occur on the product’s surface. Measuring the
temperature both at the product center and at the surface (wet bulb) temperature
would address this issue.
5) The establishment failed to take into account the initial levels of S. aureus
commonly found in raw meat and poultry. Levels of pathogens in raw product are
approximately 2-Log. Increases of 3-Log or more could result in conditions where
enterotoxin could be formed. Establishments should limit S. aureus growth to 2-
Log or less, to support safe release of product based on microbial modeling. See
Biological Hazards of Concern During Cooking subsection: Staphylococcus
aureus (page 14) for more information.
81
Attachment A3. When can Products be Labeled as
Pasteurized?
FSIS defines pasteurization as any process, treatment, or combination thereof, that
eliminates or reduces the number of pathogenic microorganisms to achieve at least a 5-
Log reduction of either Salmonella or Lm, on or in ready-to-eat (RTE) meat or poultry
products in the final finished package.
With adequate validation, pasteurization processes may include alternative
technologies other than traditional cooking (e.g., high pressure processing (HPP)).
FSIS considers products with a raw appearance that have been treated with a lethality
process that renders the product RTE, and that are not post-lethality exposed (e.g.,
“steak tartare” subjected to a HPP treatment) as pasteurized.
For the product to be labeled “pasteurized,”
the treatment needs to:
1) Be applied in the final package (product
is not post-lethality exposed);
2) Be sufficient to eliminate the number of
pathogenic microorganisms to make the product safe for human consumption (so
there are no detectable pathogens; RTE), and
3) Be effective for at least as long as the product shelf life.
Establishments may label products as “pasteurized.” However, the term “pasteurized”
is a special statement and claim that needs to be submitted to the Agency for label
approval under 9 CFR 412.1(c)(3). The request for label approval needs to include
supporting documentation providing evidence that the process achieves a 5-Log
reduction of Salmonella or Lm. For more information see the FSIS Compliance
Guidance for Label Approval.
Irradiation is not a pasteurization process.
Although the effect is similar to
pasteurization, FSIS considers ionizing
radiation a food additive under 9 CFR 424.22.

82
Attachment A4. Sources of
Salmonella
Contamination in RTE Products and Best Practices
to Address It
Although the Salmonella percent positive found in ready-to-eat (RTE) products is low,
the presence of Salmonella in RTE products may indicate a serious processing and
public health problem. Common sources of Salmonella in RTE products include:
• Under processing.
• Cross-contamination.
o Product contact surfaces that are contaminated with Salmonella; or,
o Raw product contact with RTE product.
• Ingredients added to the product or the sauce after the cooking step.
• Improper handling by establishment employees.
• Insect or animal vectors.
Each common source of Salmonella contamination on RTE products and best practices
to prevent the hazard are discussed in detail below.
Under-Processing
Under-processing occurs when the lethality treatment is not adequate to eliminate the
pathogens of concern. For heat-treated product, under-processing may result from
inadequate cooking or the development of bacterial heat tolerance due to drying of the
product’s surface before completion of the lethality step because of inadequate humidity
(see FSIS Critical Operating Parameters for Cooking (Time-Temperature Tables) page
23).
Cross-Contamination
Cross-contamination of product can occur from situations such as the following:
• Using the same equipment (e.g., slicers) for both raw and cooked products
without complete cleaning and sanitizing of the equipment (as should be
addressed in the establishment’s Sanitation Standard Operating Procedure
(SOP)) after raw production and prior to RTE production.
o In a for-cause Food Safety Assessment (FSA) in response to a
Salmonella positive in a RTE head cheese product, FSIS identified
equipment used to grind both raw and cooked ingredients for head cheese
was not cleaned and sanitized between use for raw and cooked meat
potentially resulting in Salmonella cross-contamination.
• Placing cooked product on the same surface (e.g., cutting table) as raw product
without complete cleaning and sanitizing of the surface before reuse.

83
• Using the same utensils or containers (e.g., scoops or buckets) for both raw and
cooked product.
o In two FSAs, popped pork skins were most likely contaminated with
Salmonella when the same buckets and tongs were used for handling
both raw and RTE product.
• Condensation or aerosolization in the processing environment.
Best Practices to Prevent Cross-Contamination
Under the HACCP regulations, establishments are required to prevent contamination of
product with pathogens after the lethality step. Establishments are required to maintain
sanitation in the RTE area to ensure that food contact surfaces are free of
contamination from pathogens such as Lm and Salmonella. Best practices include:
• Completely separating the processing areas by time or space (e.g., scheduling
raw and RTE processing on different days).
• Installing separate air ventilation systems that are designed to prevent or
minimize condensation and other potential air contaminants. If separate
ventilation systems are not feasible, ensure that airflow is directed from the RTE
areas to the raw areas.
• Using separate equipment for RTE and raw processing. If this is not possible,
schedule use of equipment first for RTE processing and then for raw processing.
• Restricting travel of personnel from the non-RTE area to the RTE area during
processing.
• Establishing proper sanitation procedures for equipment that is moved from a
non-processing area to an RTE processing area to prevent product
contamination from the equipment during operation.
• Avoiding passing raw product through RTE areas and passing RTE product
through raw production areas.
• Not allowing RTE product in coolers to come into contact with raw products or
surfaces that may be contaminated.
• Discarding products that touch environmental surfaces (e.g., product that has
fallen on the floor) if the product cannot be properly reconditioned to ensure that
any possible contamination is eliminated.
• During cleaning and sanitizing, following proper sanitation procedures to ensure
that no food residue is left on the equipment.

84
• When adding ingredients to a second container, avoiding any contact between
the ingredient container and the interior of the second container.
Ingredients Added After the Lethality Treatment
Salmonella contamination may occur from the addition of uncooked vegetables (e.g.,
tomatoes and onions), fresh herbs, eggs, spices (that may or may not have been
treated to eliminate Salmonella), or other ingredients (e.g., nuts, hydrolyzed vegetable
protein (HVP)) to processed meat and poultry products after the primary lethality
treatment. Sauce that has not undergone a lethality treatment may also be a source of
contamination of the finished product, even if the pH is low. The safety of all ingredients
added to the product after the lethality step should be considered, even if they are
normally considered RTE. In some cases, FSAs determined the addition of seasonings
or other ingredients after the cooking step resulted in the contamination of RTE product
with Salmonella. Failure to identify all steps in a process, including the addition of
contaminated ingredients and sauces, can result in an inadequate food safety system.
Outbreaks related to ingredients added after lethality treatment
An outbreak and several recalls of meat and poultry products that were prepared using
Salmonella-contaminated ingredients exemplify the need to ensure the safety of all
ingredients added to the product after the lethality treatment. Examples include a 2010
outbreak-related recall of salami products coated with contaminated pepper (RC-006-
2010) and recalls involving products containing HVP that was the subject of an FDA
recall (i.e., bacon base, RC-015-2010; beef tornados, RC-016-2010, and beef taquitos
and chicken quesadillas, RC-017-2010). RC-055-2010 may have been due to
contaminated sauce added to the product after the lethality step. There have also
been two recalls of meat and poultry salads containing Salmonella contaminated
tomatoes recalled by the supplier (RC-033-2011 and RC-79-2011), and Caesar salad
containing contaminated cilantro that was the subject of an FDA recall (RC-059-2012).
In 2018, there were 12 recalls due to potential vegetable contamination with
Salmonella and Lm that were triggered by an FDA investigation and subsequent recall
from the same supplier (RC-092-2018, RC-093-2018, RC-094-2018, RC-095-2018,
RC-096-2018, RC-097-2018, RC-098-2018, RC-099-2018, RC-100-2018, RC-101-
2018, RC-102-2018, and RC-103-2018).
85
Requirements and Best Practices to Prevent Hazards from Ingredients Added
Post-Lethality
Establishments are required to:
• Ensure all ingredients and other articles used in the preparation of any meat or
poultry product are clean, sound, healthful, wholesome and otherwise such as
will not result in the product being adulterated (9 CFR 318.6 and 9 CFR
424.21).
• Consider any potential food safety hazards at the step in the process where the
non-meat ingredient is ‘received’ into the food safety system (9 CFR 417.2(a)(1))
and document any controls it needs to support its decisions (9 CFR 417.5(a)(1))
about those hazards.
o Establishments may choose to use COAs that include negative test results
for each lot of the non-meat ingredient as support or may test each lot of
non-meat ingredients upon receipt; however, establishments have
flexibility and do not have to only rely on testing.
o Alternatively, establishments may maintain supporting documentation
demonstrating that the ingredients such as spices, have been treated by
processes to kill pathogens (e.g., irradiation, ethylene dioxide, steam
treatment of spices), or they can apply a lethality treatment to the
ingredients (e.g., cook the sauce of a pork BBQ).
o In most cases, a LOG alone would not be sufficient to support the safety
of non-meat ingredients added to a product unless they indicate how each
lot of ingredients is processed, tested, treated, or otherwise processed to
ensure its safety as described in the bullet above.
o A LOG can be used to support the safety of pre-packaged ingredients
(e.g., ketchup or mustard) that have not been associated with previous
outbreaks or recalls.
NOTE: Many frozen vegetables are considered NRTE by the producing facility. FSIS
recommends establishments that do not receive a COA or LOG as described in the
bullets above, treat all frozen vegetables as NRTE and address potential hazards from
this ingredient (e.g., by testing each lot of non-meat ingredients upon receipt or applying
a validated lethality treatment). Additionally, any vegetables labeled with cooking
instructions are to be treated as NRTE.
• Developing procedures to ensure that spices or other source materials are
maintained under sanitary conditions and are not contaminated by the
introduction of pathogens during repeated opening of the container and removal
of the ingredient for use in multiple production lots.
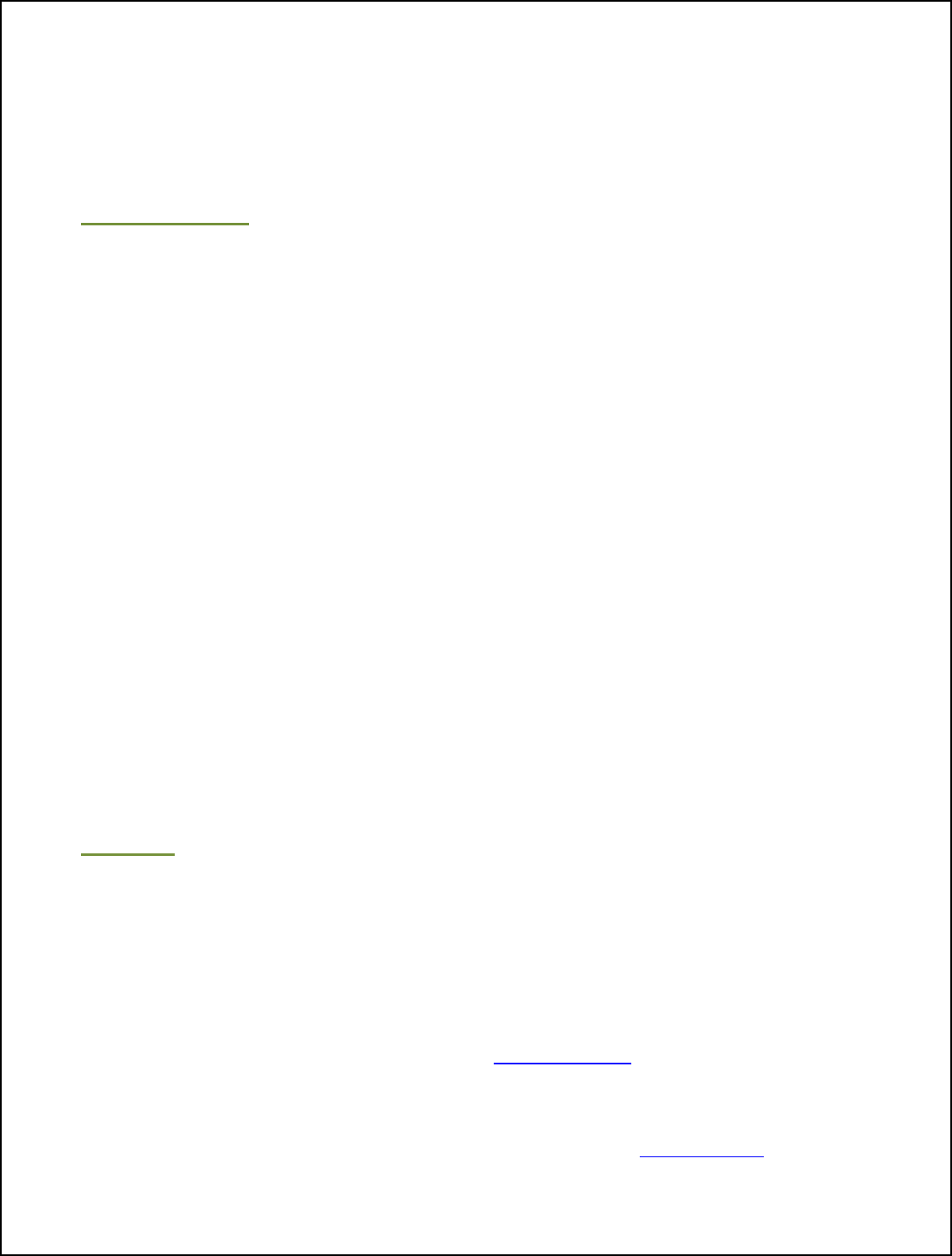
86
• Taking steps to ensure sauce used for RTE products is also not contaminated by
exposure to unclean surfaces, untreated ingredients, or contact with raw
products.
Food Handlers
There is a high incidence of salmonellosis in the US. Additionally, some people can be
asymptomatic carriers that spread Salmonella without appearing ill. Establishment
employees that are asymptomatic carriers may be a source of Salmonella in RTE
products.
Best Practices to Prevent Hazards from Food Handlers
Food handlers, employees, and supervisors at food preparation facilities should:
• Stay home from work when having symptoms of vomiting or diarrhea and wait to
resume work until at least 24 hours have passed since the vomiting and diarrhea
symptoms ended.
• Wash hands upon resuming duties after breaks and before putting on gloves.
• Wear separate or color-coded frocks in RTE areas of the establishment and
control employee traffic between raw and RTE production areas.
• Train employees in proper hygiene practices, and regularly monitor those
practices, and retrain employees at least annually.
• Develop and maintain procedures to ensure that sanitizer concentrations in
footbaths are monitored and maintained adequately.
Animals
Animals (e.g., birds and rodents) and insects may also contaminate food products with
Salmonella. It is possible for animal fecal contamination within and outside the
establishment to be introduced into the RTE production area.
Best Practices to Prevent Hazards from Animals
• Maintaining an effective pest control program to maintain sanitary conditions and
ensure that product is not adulterated (9 CFR 416.2(a)). Rats, mice, birds, and
insects are sources of pathogen contamination.
• Product and ingredients should always be protected from contamination and
adulteration during processing, handling, and storage (9 CFR 416.14).
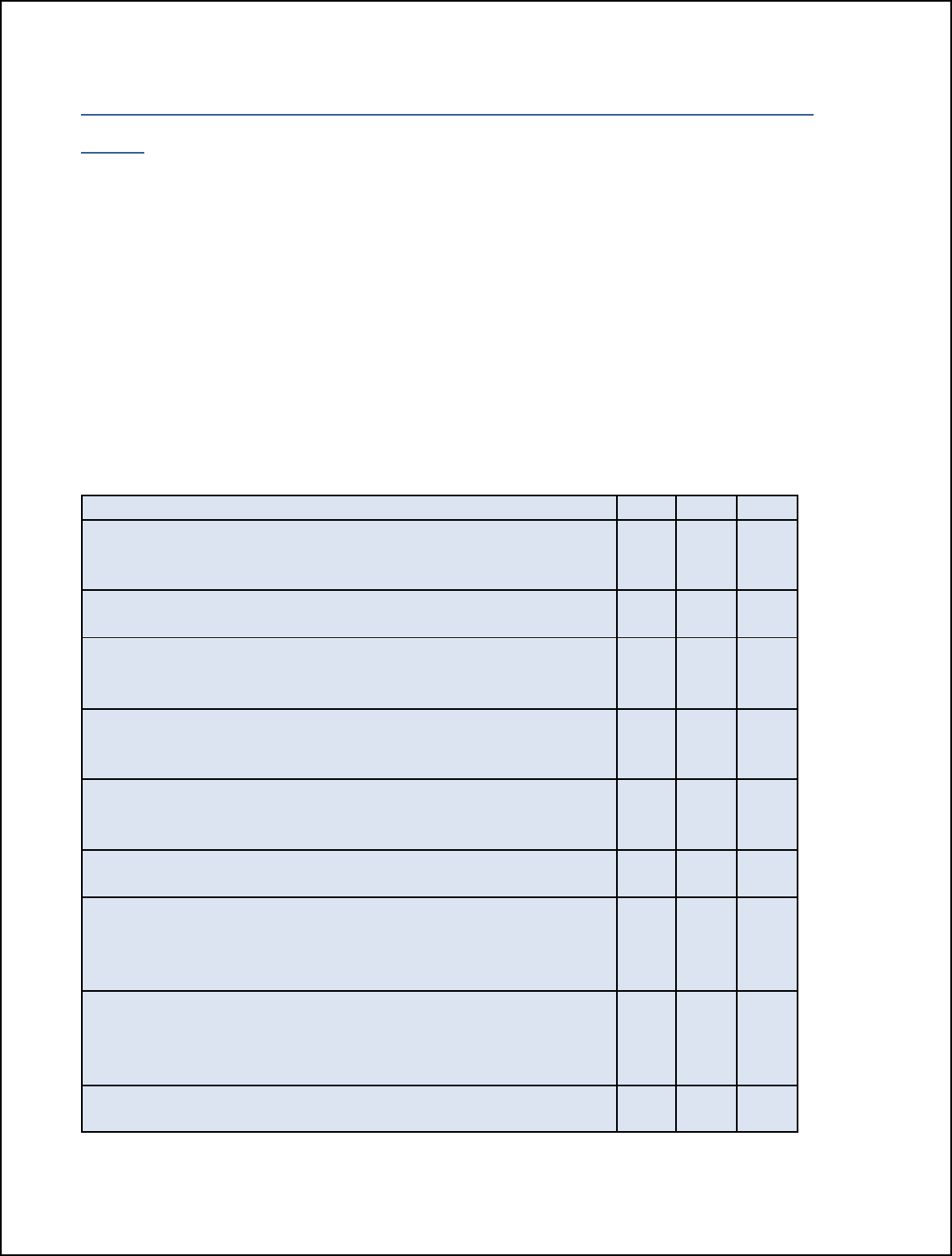
87
Attachment A5. RTE
Salmonella
Self-Assessment
Tool
FSIS recommends that establishments use this tool to determine whether they have
adopted the appropriate procedures to control Salmonella, or whether they should adopt
new procedures. If establishments find that they are not meeting the recommendations
in this guideline, FSIS recommends they consider changing practices to better control
Salmonella in the product.
The questions are related to evaluating the following:
• Hazard Analysis/HACCP Plan
• Ingredients
• Corrective Actions in Response to Salmonella Positives
Hazard Analysis/HACCP Plan
YES
NO
N/A
1. Have you considered whether Salmonella is a
hazard reasonably likely to occur (RLTO) in your
Hazard Analysis?
□
□
□
2. If you determined that Salmonella was RLTO, did
you establish CCPs to control or prevent it?
□
□
□
3. If you established CCPs, do you have sufficient
supporting documentation to support the
effectiveness of the measures you are taking?
□
□
□
4. If you produce roast, cooked, or corned beef, does
your process achieve at least a 6.5-Log or other
supportable (e.g., 5-Log) reduction of Salmonella?
□
□
□
5. If you produce cooked uncured meat patties, does
your process achieve at least a 5-Log reduction of
Salmonella?
□
□
□
6. If you produce cooked poultry, does your process
achieve at least a 7-Log reduction of Salmonella?
□
□
□
7. If you produce other cooked RTE meat products,
does your process achieve at least a 6.5-Log or
other supportable (e.g., 5-Log) reduction of
Salmonella in the product?
□
□
□
8. If you are using an alternative lethality Log
reduction target (e.g., 5-Log reduction) do you
have additional support such as COA, LOG,
combined interventions, or baseline testing?
□
□
□
9. As part of your critical limits, have you identified
the target or performance standard that your
□
□
□
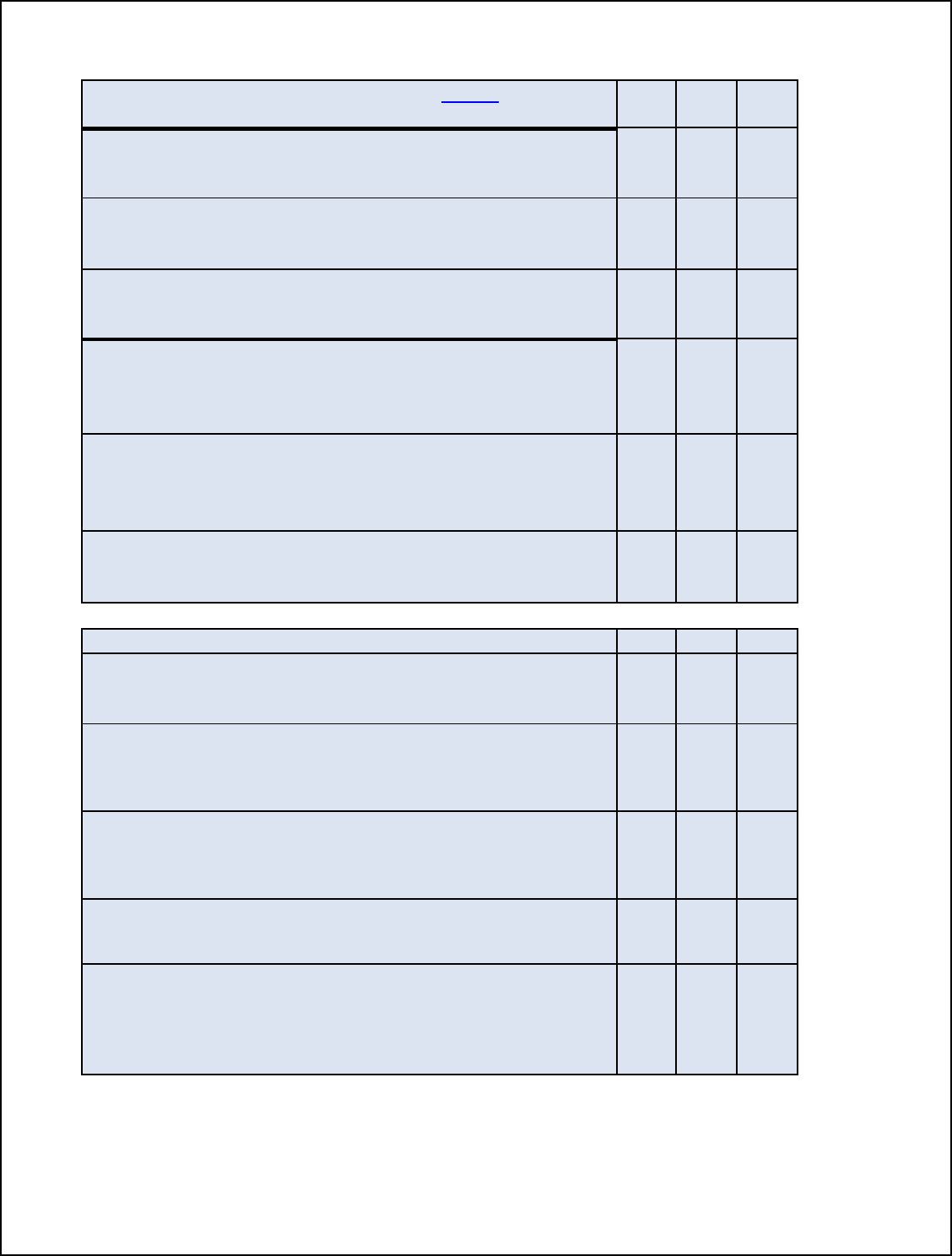
88
process is designed to achieve (9 CFR
417.2(c)(3))?
10. If you produce cooked products and use a time-
temperature table, are you applying humidity
during the cooking process?
□
□
□
11. If “no” to the question above, do you have support
for why relative humidity is not a critical operating
parameter?
□
□
□
12. If “no” to the question above, are you applying a
scientific gap for lack of relative humidity? Which
one? (fill in here)
□
□
□
13. If you produce cooked products and use a FSIS
time-temperature table, have you limited product
heating come-up-time (50 to 130°F) to 6 hours or
less?
□
□
□
14. If “no” to the question above, do you have
alternative support for applying a long come-up-
time?
□
□
□
15. If “no” to the question above, are you applying a
scientific gap for long come-up-time?
□
□
□
Ingredients
YES
NO
N/A
16. Do you add ingredients to the product after the
lethality treatment? (if “no,” move to the next
section)
□
□
□
17. Do you maintain COAs, LOGs, or other
information (e.g., sampling data) to support the
safety of the ingredients?
□
□
□
18. If you use LOGs, do they indicate how each lot of
ingredients is processed, tested, or otherwise
treated to ensure its safety?
□
□
□
19. Are the ingredients that you add to the product
included in your flow chart or hazard analysis?
□
□
□
20. If you use pre-packaged ingredients that are
included in the final package with the finished
product do you have LOGs or other information to
support their safety?
□
□
□

89
Corrective Actions in Response to Salmonella Positives
YES
NO
N/A
21. Has a RTE product sample tested positive for
Salmonella from FSIS or establishment testing? (If
“no” the assessment is complete).
□
□
□
22. If you control Salmonella in your HACCP plan, did
you take corrective actions according to 9 CFR
417.3(a)? (If you prevent Salmonella through a
Sanitation SOP or other prerequisite program, skip
to #26).
□
□
□
23. Did you take steps to identify and eliminate the
cause of the deviation, according to 9 CFR
417.3(a)(1)?
□
□
□
24. If the cause of the positive result is under-
processing, did you immediately review your
processing system and bring the process back
into compliance?
□
□
□
25. If the cause of the positive result is lack of support
for your lethality process, did you change your
process or provide additional support for the
safety of the process, in light of the positive result?
□
□
□
26. If you prevent Salmonella through a Sanitation
SOP or another prerequisite program, did you take
corrective actions according to 9 CFR 417.3(b)?
□
□
□
27. As part of your corrective actions, did you
reassess your HACCP plan according to 9 CFR
417.3(b)(4)?
□
□
□
28. As a result of your reassessment, did you address
the pathogen in a CCP or make substantive
changes to your prerequisite program?
□
□
□
90
Attachment A6. Cooking Country-Cured Hams
In October 2018, an establishment recalled cooked country-cured ham product that was
associated with a listeriosis outbreak (Recall 084-2018; CDC: Outbreak of Listeria
Infections Linked to Deli Ham). FSIS’s investigation at the establishment found that the
country-cured hams were cooked in a sealed bag multiple times. Before being cooked
multiple times, the ham was salt-cured and dried, thus reducing its water activity.
Additionally, after an initial cooking step in a sealed bag, the ham was removed, drained
of its juices, and placed into a second bag; during this process, the ham may have been
cross-contaminated from the processing environment. Additionally, the draining of
juices may have resulted in drier conditions during cooking. The establishment used
FSIS cooking guidance (Appendix A) as scientific support that cooking achieved
lethality of pathogens, including Lm. However, as discussed on page 12, Appendix A
guidance was not intended for lower water activity products cooked under dry conditions
or for dried products cooked multiple times. Hence the process may not have been
lethal to Lm (USDA/FSIS, 2020). Establishments that apply these types of processes
must identify other support for their HACCP System (9 CFR 417.5(a)(1) and 9 CFR
417.4(a)(1)).
During the outbreak investigation, FSIS also discovered that several establishments
cook country-cured hams once under moist conditions using FSIS cooking guidance as
support. FSIS cooking guidance was also not intended for lower water activity products
cooked even under moist conditions; however, FSIS is not aware of any imminent food
safety issues with this practice. Therefore, page 47 (Table 5), includes critical operating
parameters that may be applied to cook dried products like country cured hams if they
are cooked once under moist conditions to rehydrate the surface. While cooking under
moist conditions should rehydrate the surface, there is no research validating this
process so it is considered a scientific gap. As with other scientific gaps, there is a
vulnerability because FSIS’s lethality guidance is not designed for processes where the
drying step comes before the moist cooking step. This is because cooking under low
moisture conditions results in product with a lower water activity. These conditions lead
to pathogens, such as Lm, becoming more heat-tolerant and the organism could survive
the cooking process. To minimize this vulnerability, FSIS recommends:
If the product is cooked once:
• Establishments should gather support such as water activity measurements
after drying (before cooking), then again after cooking to demonstrate that the
water activity increased, and product surface was rehydrated during cooking.
This recommendation applies even if the product is cooked-in bag, because
the water activity may not be high enough to ensure that pathogens are killed
on the product without addition of moisture.
• Establishments should achieve the highest water activity possible during
cooking. Values ≥ 0.96 have been shown to prevent bacterial heat tolerance

91
(Kieboom, et al. 2006), but this water activity may not be possible for all
processes to achieve.
• Establishments conduct finished product testing for Salmonella and Lm as part of
on-going verification.
Establishments should also ensure that the cooking bag is completely sealed, so that
moisture is contained in the bag and the product is not exposed to the environment or
contaminants. Cooking bags may be compromised during steps such as molding or
shaping. The establishment should have a process to verify the package integrity, and
if leaks are observed, the establishment should reprocess/recook the product, using a
supported process.

