
1
Introduction
CIGNA LIFESOURCE
TRANSPLANT NETWORK
PROVIDER REFERENCE GUIDE
Cigna LifeSOURCE is committed to providing access to
quality transplant care, improved health, and lower costs.
January 2024
PCOMM-2024-1940
2
Table of contents
Introduction ............................................................................................................ 4
Inside the guide .................................................................................................. 4
Our commitment and mission ................................................................................ 4
Notes ................................................................................................................ 5
The transplant process ............................................................................................ 6
Zone 1: Evaluation .............................................................................................. 6
Zone 2: Pre-transplant ......................................................................................... 6
Solid organs.............................................................................................. 6
Bone marrow, stem cell, and cord blood transplants ....................................... 6
Zone 3: Transplant event ......................................................................................7
Zone 4: Post-transplant follow up .......................................................................... 7
Quality information .................................................................................................. 8
Quality performance ............................................................................................ 8
Guidelines for network inclusion ............................................................................ 8
Ventricular assist device ........................................................................... 11
Chimeric Antigen Receptor T-Cell (CAR-T) Therapy Network ...........................11
Communicating staff and program changes ........................................................... 11
Appeal information ............................................................................................. 12
Case management ..................................................................................................13
Purpose ............................................................................................................
13
Goals and objectives .......................................................................................... 13
Managed services .............................................................................................. 13
Contact information ........................................................................................... 14
Coverage positions, clinical resource tools, and criteria ........................................... 14
Customer handbook ........................................................................................... 15
Referrals and appeals ............................................................................................. 16
Clinical coverage decisions and appeals ................................................................. 16
Prior authorization .................................................................................. 16
Authorization process ............................................................................... 16
Coverage determinations ......................................................................... 16
3
Appeals of coverage determinations .......................................................... 16
Clinical trial determinations ...................................................................... 16
Transplant case management referral process ....................................................... 17
Medical documentation checklists .......................................................................... 18
Medical documentation checklist for adult transplants ............................................. 19
Medical documentation checklist for pediatric transplants ....................................... 23
Medicare eligibility ................................................................................................. 26
Claim submissions ................................................................................................. 27
Cigna Healthcare customers ............................................................................... 27
Cigna Healthcare, Cigna Global Health Benefits
®
, and Shared Administration
Repricing (Taft-Hartley) plans ................................................................... 27
SAMBA account ...................................................................................... 27
Cigna Healthcare Medicare Advantage Arizona customers ............................. 28
Network Access Clients (NACs) ........................................................................... 28
Bundled billing ................................................................................................. 29
Sample claims cover sheet ................................................................................. 29
Required claim documentation ............................................................................ 30
Dispute resolution ................................................................................................. 31
Payment appeal process .................................................................................... 31
Travel benefit ........................................................................................................ 32
Air ambulance transport ........................................................................................ 33
Network Access Clients .......................................................................................... 34
Referral process ............................................................................................... 34
Claim process .................................................................................................. 34
Sample referral letter ........................................................................................ 36
Pharmacy, infusion services, and behavioral health services .................................. 37
Express Scripts ................................................................................................. 37
How to submit an order ........................................................................... 37
Accredo ........................................................................................................... 37
Coram
®
CVS Specialty
®
infusion services (Coram) ............................................... 37
Evernorth Behavioral Health ............................................................................... 38
Important contact information ................................................................................ 39
Legal statement ...................................................................................................... 40
4
Introduction
Welcome to Cigna LifeSOURCE Transplant Network
®
! For starters, we'd like you to
know that we're committed to giving all of our customers access to quality services
and benefits. That means working with you across all the aspects of today's health
care world. To help us stay on the same page, we have created this Reference
Guide for you. It highlights the programs and policies intended to keep our
relationship smooth and productive – for the sake of the people we serve together.
The Reference Guide contains Administrative Guidelines and Program Requirements
for the programs, policies, rules, and procedures pertaining to Cigna Healthcare
insured or administered benefit plans. We will give you advance notice of material
changes to our Administrative Guidelines and Program Requirements. Your Cigna
LifeSOURCE Participation Provider Agreement and this Reference Guide describe
many of the terms under which you agree to provide services to Cigna Healthcare
Plan Participants. Those terms include the reimbursement rates applicable to
Covered Services provided to Participants. However, the actual benefits payable by
a Payer for Covered Services provided to a Participant in all cases is determined by
the terms of the Payer’s Benefit Plan.
Inside the guide
Cigna LifeSOURCE Transplant Network is a specialized component of Cigna
Healthcare that offers access to high-performing transplant providers with an
enhanced benefit for customers with Cigna Healthcare-administered coverage. The
network is dedicated to managing and providing access to complex medical
services, including transplantation, cellular therapies, and the associated medical
and surgical services with the goal to reduce costs, reduce or eliminate unnecessary
procedures, maintain or improve the quality of the transplant procedure, and
improve the overall transplant experience for our customers and their families. The
Cigna LifeSOURCE Transplant Network Provider Reference Guide includes
information regarding case management, clinical documentation, network program
inclusion requirements, processes, administrative guidelines, contacts, claims,
terminology, and more.
This comprehensive guide provides the policies and procedures Cigna LifeSOURCE
employs to help you manage Cigna Healthcare customers who need a transplant.
We hope this guide helps you as we work together to ensure Cigna Healthcare
customers receive quality and affordable transplant care.
Our commitment and mission
The Cigna LifeSOURCE Transplant Network focuses on high-quality health care
providers to service our transplant customers and their families, which includes:
• A national quality program
• A comprehensive dedicated transplant case management team
• Coverage positions
• Specially trained claims personnel
• Dedicated contracting, medical directors, and clinical staff
• Travel benefits, when applicable

Introduction
5
Health care and transplant professionals head up the Cigna LifeSOURCE team. This
team of dedicated individuals work to uphold and improve all aspects of the Cigna
LifeSOURCE Transplant Network.
Notes
Please note that state law may supersede information provided in this manual.
Please check your facility’s contract for state-specific information. To check state-
specific information, please visit the Cigna for Health Care Professionals website
(CignaforHCP.com
). Registration is required (at no cost).
The transplant process
6
The transplant process
Cigna LifeSOURCE includes specialized, dedicated transplant case managers who
supervise services for transplant recipients through each phase of
the transplant process. Cigna LifeSOURCE identifies the phases of transplantation
as “zones.”
Zone 1: Evaluation
Please call Transplant Case Management at 800.668.9682 to get prior
authorization before you begin evaluation.
This is the candidacy period. The customer is evaluated by the transplant team to
determine if they are an acceptable candidate for a transplant.
This zone includes all diagnostic tests performed on the customer and a live donor,
if applicable. A Zone 1 authorization should be requested if human leukocyte
antigen (HLA) typing for potential liver donors will be performed as these services
are part of the evaluation period. Please notify the Transplant Care Manager if a
living donor will be receiving testing. Zone 1 should also be requested if an
unrelated search is conducted, as these services are also transplant services and
only intended to be covered if a customer is actively pursuing a transplant.
The transplant center is expected to provide all diagnostic tests. These
tests cannot be outsourced.
While some tests, such as colorectal testing and gynecological and dental exams,
may seem less critical to transplant, they are required to ascertain the health status
of Cigna LifeSOURCE customers facing transplant. If you are in doubt about
required testing, please consult the customer’s case manager.
Unless otherwise stated in your facility’s contract, the zone begins when the
customer starts the evaluation and must be authorized by Cigna Healthcare for the
evaluation. It ends on the date the patient is accepted into the hospital’s transplant
program, or deemed not acceptable as a transplant candidate.
Zone 2: Pre-transplant
Zone 2 is the pre-transplant period that occurs after Zone 1 and continues until the
day prior to the transplant procedure, or the beginning of the transplant event.
Non-transplant-related care (for the underlying disease condition) is typically
excluded from this zone. Please contact Transplant Case Management prior to
listing the patient for a transplant to ensure that correct authorizations are in place.
Solid organs
For solid organs, this zone includes transplant-related care only for routine
surveillance of the recipient as needed to maintain their candidacy status. This
includes any testing required to determine organ function, clinic visits, etc. Zone 2
does not include ongoing maintenance care such as renal dialysis.
Bone marrow, stem cell, and cord blood transplants
In typical contract language for autologous bone marrow and stem cell transplants
(BMT\SCT), the beginning of this zone is represented by the acceptance of the
The transplant process
7
participant into the hospital’s transplant program unless your facility’s contract
specifies otherwise.
For allogeneic BMT/SCT, the candidacy zone typically starts after the recipient has
been accepted into the program and ends the day prior to the transplant event.
Note: Approval is only given for one year at a time. When a patient approaches
one year in Zone 2, the case manager will contact your transplant program to find
out the status of the patient and to assess whether to extend Zone 2 approval.
Zone 3: Transplant event
For solid organ transplants, Zone 3 typically begins on the day of or the day prior to
the transplant procedure and ends when the recipient is discharged from the
hospital.
For autologous and allogeneic-related BMT/SCT, the transplant event zone typically
begins with mobilization.
For allogeneic unrelated BMT/SCT and cord blood transplants, Zone 3 typically
begins with the onset of preparative regimen. Please refer to your facility’s
contract.
Zone 3 includes living donor services for up to 30 days after the date of donation.
Please refer to your facility’s contract.
All transplant-related services for the recipient provided during this time are
included in the Zone 3 rate unless there is a specific exclusion referenced in the
contract. Please refer to your specific contract for terms related to the management
and treatment of the underlying disease.
The Zone 3 start date and end date is specific to each transplant facility’s contract.
Please consult your contract to determine the exact end date.
Note: The infusion of stem cells, including donor cells performed as a “boost” to
enhance cell recovery, is not considered a transplant and will be included in the
Zone 3 rate if performed during this period.
Zone 4: Post-transplant follow up
The time period typically included in this zone is one year.
Your facility’s contract should be reviewed for the specific amount of time and the
services included in this zone.
Zone 4 includes all transplant-related follow-up care for the recipient.

Quality information
8
Quality information
Quality performance
The foundation of the Cigna LifeSOURCE Transplant Network is our quality
performance program managed through the Network Performance Review
Committee (NPRC).
The quality process is essential in ensuring program performance and in providing
our customers and their families with access to excellence in transplant care. Each
transplant program under network consideration begins the quality process by
completing a Request for Information (RFI). The Cigna LifeSOURCE network uses
the United Network for Organ Sharing (UNOS) standardized RFI form for all solid
organ transplant procedures, and the American Society for Transplantation and
Cellular Therapy (ASTCT) standardized RFI form for all BMT\SCT procedures to
support consistency in the process.
The RFI provides program-specific data that is evaluated against the Cigna
LifeSOURCE performance guidelines for each transplant type. The published
guidelines are located at cignalifesource.com
. Critical components evaluated in the
RFI include, but are not limited to:
• Annual volumes of transplant procedures
• Patient survival
• Graft survival
• Transplant wait time
• Mortality on wait list
• Facility support
• Team stability
• Team training and experience
• Quality improvement program
• Protocols (patient selection, pre- and post-transplant management)
• Patient safety
• Communication systems
Guidelines for network inclusion
In addition to the RFI content, Cigna LifeSOURCE annually evaluates each
transplant program to determine continued compliance with established guidelines.
Facilities considered for the Cigna LifeSOURCE Transplant Network must be a Cigna
Healthcare -participating provider and maintain hospital accreditation (e.g., Joint
Commission of Hospital Accreditation or the National Integrated Accreditation for
Healthcare Organizations [NIAHO] by Det Norske Veritas [DNV] Healthcare).
The following accreditations must be met for each program prior to network
consideration:
• Centers for Medicare & Medicaid Service (CMS) solid organ program
certification: Hospital Conditions of Participation: Requirements for Approval and
Re-Approval of Transplant Centers to Perform Organ Transplants
• The Foundation for the Accreditation of Cellular Therapy (FACT) for all bone
marrow transplant programs, the National Marrow Donor Program (NMDP) as a
transplant center, and the Centers for International Blood and Marrow Research
Quality information
9
(CIBMTR).
• Listing at the Designated network level will also be dependent on a market
check to confirm the rates for each program are competitively priced relative to
equivalent programs in the region and/or market.
Solid organ program annual volume guidelines
The Cigna LifeSOURCE Transplant Network is a two-tiered network comprised of:
• Top tier network level transplant programs, Cigna LifeSOURCE Designated
network
• Second-tier level transplant programs, Cigna LifeSOURCE Supplemental
network
Designated level programs must have three years of risk-adjusted performance
data published in the Scientific Registry of Transplant Recipients (SRTR) and
continue to meet the annual program volumes in the table below.
Supplemental level programs must have one year of risk-adjusted performance
data published by the SRTR.
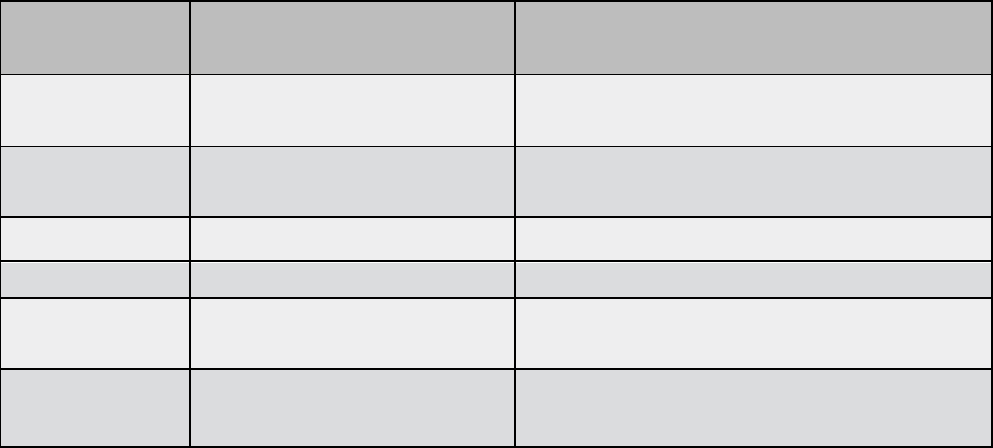
Solid organ annual Designated-level minimum volume guidelines
Transplant
program
Adult minimum volume Pediatric minimum volume
Heart 12 Average of five in the previous two years
Lung 12 Minimum of one in the previous two
years
Liver 12* Five
Kidney 30** Five
Intestinal Three
Minimum of three in the previous two
years
PTA/PAK/SPK**
*
Six – kidney program must
be approved
N/A
* Adult liver – 12 total combined deceased and living donors. Living liver programs must
demonstrate active, ongoing live donor transplants annually.
** Adult kidney – 30 total combined deceased and living donors. Kidney-only transplant
centers are considered on a case-by-case basis only.
*** Pancreas transplant alone (PTA), pancreas after kidney (PAK), simultaneous pancreas
kidney (SPK)
BMT/SCT program annual Designated level volume guidelines
• Adult programs must perform 50 total with at least 20 being allogeneic
• Pediatric programs must perform 15 total, combined autologous and
allogeneic
Quality information
10
Programs must meet the minimum volume requirements for two consecutive years
for Designated level consideration.
Performance guidelines for solid-organ programs
The following steps review the quality and performance of each transplant program
listed in the Cigna LifeSOURCE Transplant Network:
1. The most recent outcome and volume data from the SRTR and Organ
Procurement Transplant Network (OPTN) websites are used to determine if
the solid organ programs meet the Cigna LifeSOURCE transplant program
quality and performance guidelines.
The Cigna LifeSOURCE NPRC reviews program outcomes based on a
calculated Relative Performance Index (RPI). The RPI is consists of the
following statistical components: the waitlist transplant rate (getting a
deceased donor transplant faster), the pre-transplant mortality rate (adult
survival on waitlist), and the one-year graft survival and three-year graft
survival rates
.
• Pre-Transplant Mortality rate is defined as the number of patient deaths
that occurred while waiting for a transplant in a current year relative to
the national experience.
• Wait-list Transplant rate is defined as the number of patients on the
waitlist transplanted within a year relative to the national experience.
• Graft Failure Survival rate is a measure of actual transplant program
results compared with expected program results that are based on
modeling transplant outcomes from all programs in the United States.
These metrics create the program RPI. Programs in quintiles 2-5 of the RPI
will remain in the Designated network level.
2. Solid organ programs that fall into the lowest quintile of the RPI may move
to the Supplemental network level.
Performance guidelines for BMT/SCT programs
All adult and pediatric bone marrow/stem cell transplant programs must perform
both autologous and allogeneic programs for network consideration.
Designated
• Adult and pediatric programs must achieve FACT certification, hold NMDP
certification as a transplant center, and participate with the Center for
International Blood and Marrow Research (CIBMTR).
• Programs must be listed as “performing as predicted” (0) or
“overperforming” (+1) on the current CIBMTR Center Specific Survival
report. Designated network-level programs subsequently listed as
“underperforming” (–1) for ONE year will move to the Supplemental level.
• Demonstrate actual overpredicted survival ratios (actual/predicted) equal or
better than 0.90.
Quality information
11
Supplemental
• BMT/SCT adult and pediatric programs must achieve FACT and NMDP
transplant center certification, participate with the CIBMTR, and demonstrate
active program volumes each year.
• If programs move to the Supplemental level, they must achieve TWO
consecutive years of performing as predicted (0) or overperforming (1) to be
reinstated to the Designated level.
Additional network program requirements
• All new facility transplant programs approved for the Designated level must
agree to a site visit if a visit is requested.
• All network transplant programs must participate in the annual survey.
Programs that do not respond may be subject to administrative action under
the terms of their agreement with Cigna Healthcare. This may include
shifting the programs from the Designated to the Supplemental level.
• Solid organ transplant programs with live donor programs must continue to
demonstrate ongoing live donor transplants on a yearly basis to be included
as a live donor program.
• Designated and Supplemental-level programs must permit Cigna Healthcare
customers to multi-list.
• Programs that are at risk of losing accreditations or certifications will be
removed from the network program listings.
Ventricular assist device
Heart transplant programs must participate in the Cigna LifeSOURCE Transplant
Network for the ventricular assist device (VAD) program to be included. The VAD
program must obtain and maintain CMS certification for destination therapy and
hold accreditation by the Joint Commission or DNV Healthcare.
Chimeric Antigen Receptor T-Cell (CAR-T) Therapy Network
CAR-T programs considered for the network must be in conjunction with bone
marrow/stem cell transplant (BMT/SCT) programs in the Cigna LifeSOURCE
Transplant Network. CAR-T programs must obtain and maintain FACT accreditation
for both hematopoietic stem cell therapy and immune effector cell therapy.
For additional information on the network guidelines or RPI methodology, please
view the Network Inclusion Guidelines
(https://cignalifesource.com/assets/docs/cignalifesource/LifeSOURCEGuidelinesNet
workInclusion.pdf).
Communicating staff and program changes
A Cigna Healthcare quality director will ask annually if you have had any changes in
your transplant staff or operation of your transplant program. It is important that
you let us know when any changes happen. This is a requirement of most Cigna
LifeSOURCE contracts. Please email
LifeSOURCEProgramRequests@Cignahealthcare.com
with any personnel or program
status changes.
Quality information
12
Appeal information
If you are dissatisfied with our assessment of quality data (i.e., the RPI), please
submit an appeal in writing for reconsideration.
Cigna LifeSOURCE Transplant Network remains committed to continuing discussions
with the national transplant organizations and providers in the development of
network inclusion guideline updates. In addition, the network providers are
independent providers and are not employees or agents of Cigna Healthcare.
Case management
13
Case management
Purpose
The Transplant Case Management Unit includes utilization review and case
management services. It is designed to objectively monitor, evaluate, and
positively influence the provision and cost of medical care for customers referred for
transplant case management services in accordance with the terms of their benefit
plan.
Please call the Transplant Case Manager assigned to your patient prior to
providing any services.
Goals and objectives
The Transplant Case Management Unit’s goal is to effectively use available health
care resources to ensure and provide quality and appropriate care.
This is accomplished by:
• Consistently reviewing the medical necessity of procedures and treatments to
determine the appropriate level of care and setting in which the care will be
provided. This includes transplant-related and other non-transplant medical
services required by a transplant patient while their case is “open.”
• Providing and promoting access to appropriate and cost-efficient health care
services through appropriate referral to a Cigna LifeSOURCE facility,
providing customer education, and facilitating communication and developing
partnerships among customers, health care providers, and Cigna Healthcare
in an effort to enhance cooperation and appropriate use of health care
services.
• Managing all transplant participants, especially those who are considered at
risk of requiring extensive or ongoing health care services, or of developing
significant health care complications, and facilitating coordination and
continuity of care to assist providers in achieving optimal medical outcomes.
• Delivering our services in a customer service-focused platform. This includes
allowing reasonable access and timely communication of decisions made
during the transplant case management process.
• Providing services in compliance with requirements of regulatory and
accrediting bodies.
• Maintaining strict adherence to participant confidentiality.
• Partnering with the National Quality Review Council to identify and improve
transplant services, and provide effective monitoring and evaluation of
participant care and services.
• Promptly identifying and analyzing opportunities to improve service level,
and implementing action and follow up.
• Communicating quality of care concerns, as appropriate, to Cigna Healthcare
or Cigna LifeSOURCE medical director(s)
Managed services
The Transplant Case Management Unit manages services including, but not limited
to, the following:
• Solid organs
• Ventricular Assist Devices (VAD) and Total Artificial Hearts (TAH)
Case management
14
• Stem cell/bone marrow transplants
• Chimeric Antigen Receptor T-cell (CAR-T) Therapy
Please note that the Transplant Case Management Unit will not manage
participants who have Medicare as their primary insurance due to end-
stage renal disease or kidney transplant.
Contact information
General LifeSOURCE Case Management Transplant Unit contact information:
Telephone
Hours of operation:
8:00 a.m. to 6:00
p.m. ET
Monday through
Friday
800.668.9682
Prompts will direct the caller to the appropriate
confidential mailbox during non-operational hours.
Calls regarding urgent requests will be returned
within two hours. Other calls will be returned within
24 to 48 hours.
Note: All urgent requests require confirmation that
the physician responsible has specifically stated that
a determination is medically urgent.
Fax 877.598.2484
The Transplant Case Manager is your direct link to all prior authorizations for
transplant and non-transplant related services. Once your patient enters Transplant
Case Management, all services are handled by the assigned case manager for total
patient care.
Examples of when the case manager should be contacted include, but are not
limited to:
• Prior to the patient’s transplant consultation
• To obtain Zone 1 evaluation testing approval
• Prior to any movement between zones
• Prior to listing for the transplant
• Prior to any inpatient admission, whether transplant is related or not
• Prior to all specific tests, such as MRI, CT, and PET scans
• Prior to scheduling any home health care, durable medical equipment (DME),
and infusions (including intravenous immunoglobulin [IVIg])
• To obtain prior authorization per benefit plan specific requirements
Frequent contact with the assigned case manager is vital to ensuring that all care is
approved and authorized. If there is any question as to whether a service requires
prior authorization, the assigned case manager will be able to accurately advise
you. This partnership helps to provide the best care and coverage for our
customers.
Coverage positions, clinical resource tools, and criteria
When appropriate, the following guidelines are used for prior authorization,
concurrent and retrospective review of coverage for transplant-related services,
non-transplant services and procedures, inpatient admissions, and home care
services.

Case management
15
• Coverage positions are developed and maintained under the Cigna
Healthcare Clinical Review Unit (CRU) (medical management unit) under the
direction of Cigna LifeSOURCE Medical Director(s). These are developed,
maintained, and reviewed for all transplant procedures, including living
donors.
Cigna LifeSOURCE recognizes that transplant procedures are varied and
change as medicine advances. For up-to-date Cigna LifeSOURCE coverage
criteria for various transplant procedures, please register and log in to the
Cigna for Health Care Professionals website (CignaforHCP.co
m). Click on
Review coverage policies, go to View Documents under Medical
Administrative A-Z Index, and click on the procedure to find the appropriate
transplant criteria. Or, go to Medical and Administrative Categories >
Transplants.
• Milliman Care Guidelines for elective and emergent, inpatient, outpatient,
and home care services.
• Optimal Treatment Guidelines (OTG) for appropriateness of surgical
procedures and alternative diagnostic and treatment approaches.
• Tools to Administer Benefits (TABS) and Administrative Policies and
Procedures (APP).
Customer handbook
Cigna Healthcare customers who are identified as potential transplant patients will
receive a handbook, “By Your Side,” which includes extensive information on how
they can best use their benefits.
Patients can also request a handbook from their Transplant Case Manager.
Note: Cigna Healthcare Medicare Advantage customers (excluding Cigna Healthcare
Arizona Medicare Advantage) do not receive a customer handbook.
Referrals and appeals
16
Referrals and appeals
Clinical coverage decisions and appeals
Prior authorization
While Cigna Healthcare has eliminated some prior authorization requirements, it
continues to deliver value for you and your patients in situations such as transplant.
Prior authorization is required for each zone and all zone movement.
• Consultations for transplant should be pre-approved even if not asking for
evaluation approval
• Should be used to facilitate agreement to zone dates
• Serves as point of entry to validate eligibility and coverage
• Precertification required for all IVIg, cardiac assist devices, clinical trials, and
donor searches (related and unrelated)
Please note that prior authorization is required for additional surgical
procedures performed on the same day as the transplant procedure, or in
the same admission as the transplant.
Authorization process
1. Call 800.668.9682 and follow the prompts to reach a transplant referral
analyst, or fax to 877.598.2824
2. An analyst assigns the case to a Transplant Case Manager and benefit
specialist
3. The Transplant Case Manager will contact you and inform you of the
customer’s basic transplant coverage
4. If transplant is recommended following evaluation, contact the Transplant
Case Manager with evaluation results including:
• Results of evaluation testing
• Letter recommending transplant event approval
Coverage determinations
Only a Cigna Healthcare Medical Director can deny authorization for clinically based
services. A customer, their authorized representative, or a health care provider has
the right to appeal a denial of coverage for services.
Appeals of coverage determinations
This process generally includes provisions for expedited appeals (where
appropriate), at least one level of internal appeal and, in many instances, an
external review conducted by an independent review organization. The appeals
process may be adjusted to comply with state and federal guidelines.
Clinical trial determinations
Participation in a clinical trial depends on the customer’s specific benefit plan
language and on legislative mandates. Please discuss with the Transplant Case
Manager as soon as you think a customer might be a candidate for a clinical trial
prior to signing them up for the trial.
Referrals and appeals
17
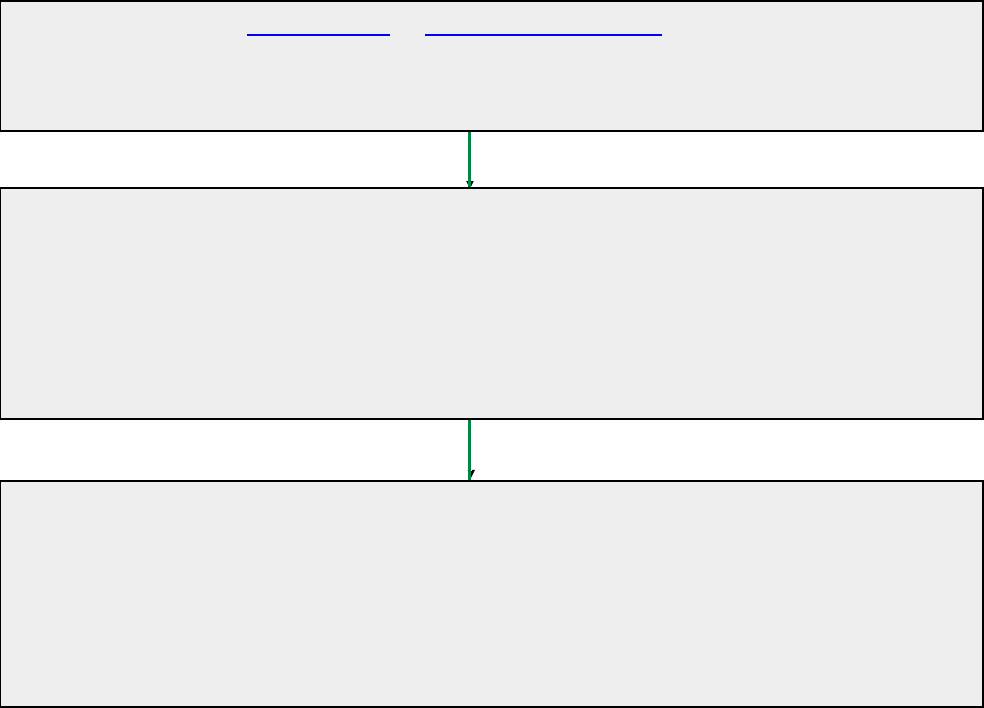
Transplant case management referral process
You must contact the Transplant Case Manager before providing any
services.
1.
Use the editable Referral Form at CignaLifeSOURCE.com under Health Care
Providers.
Download the form, add the information requested, and fax to 877.598.2484, or
2.
Call the Transplant Case Management Unit at 800.668.9682 (see below).
If you choose to call, please provide the Transplant Referral Analyst with the following:
•
Patient’s first name and last name, with spelling
•
Patient’s Cigna Healthcare ID number
•
Caller’s name and return telephone number
•
Transplant type requested and date of planned evaluation or procedure (if known)
Case is assigned to a Transplant Case Manager who will:
1.
Work with a benefits specialist to verify the eligibility of the customer
2.
Review coverage information
3.
Contact the health care provider and facility
4.
Contact the customer
Medical documentation checklists
18
Medical documentation checklists
Case managers in the Transplant Case Management Unit use transplant-specific
checklists to ensure all needed tests have been completed and submitted. To
perform the most complete review possible, Transplant Case Managers will typically
request the information in the checklists on the following pages. Please use these
checklists to assist us with managing your patient’s coverage. If a box has an X, we
ask that you provide the information for the corresponding transplant treatment.
Please include all documents that contain or refer to the information the physician
or hospital reviewed or relied upon in reaching the decision to transplant a patient.
Note that an authorization for coverage decision will NOT be made until these
records are received and reviewed.
Medical documentation necessary for referral for evaluation – obtain
from primary care provider (PCP) or specialty care provider ( SCP)
The following information should be requested of all participants:
1. Letter of medical necessity describing transplant procedure, or
2. Clinical data to support the request
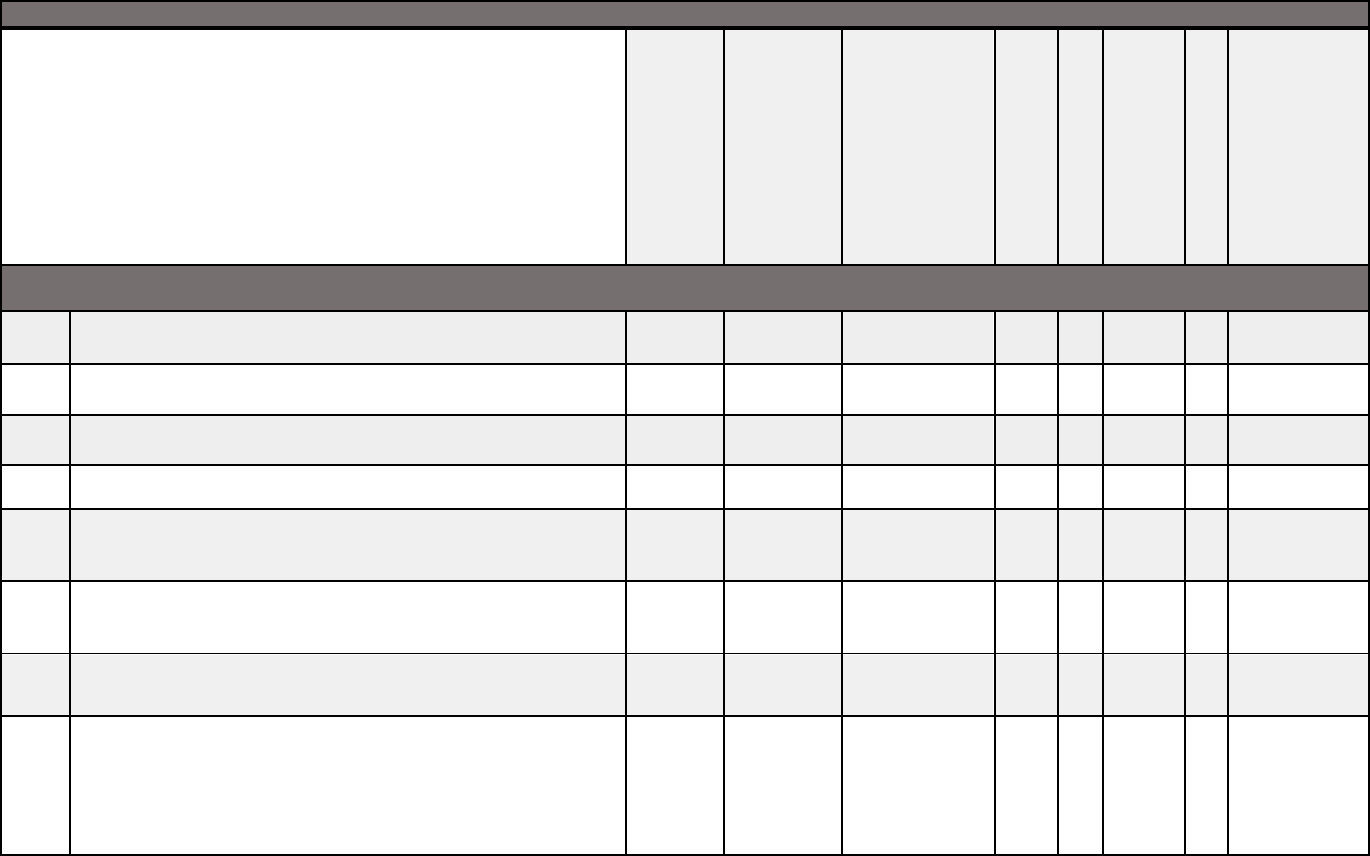
Medical documentation checklists
19
Medical documentation checklist for adult transplants
Adult Transplant
Allogenic
stem
cell/bone
marrow
Autologous
stem
cell/bone
marrow
Chimeric
Antigen
Receptor T
-cell
(CAR
-T) Therapy
Heart or
heart/lung
Kidney
Liver, intestine,
or multi-visceral
Lung
Pancreas or
kidney/pancre
as
Cardiopulmonary
Chest X-ray or CT
X X X X X X X X
MRI or CT of thorax
X
Electrocardiogram (EKG)
X X X X X X X X
Echocardiography or cardiac catheterization
X
X
Echocardiography or other cardiac functional test
MUGA/CATH that evaluates EF and valvular status
X X X
Cardiac clearance if abnormal from physical exam or
history of heart failure or heart disease
X X
X
Pulmonary function testing: spirometry, volumes,
DLCO, and room air arterial blood gas (ABG)
X
X
X
Pulmonary ventilation/perfusion (VQ) scan or CT
angiogram: for heart/lung transplants if history of
deep vein thrombosis (DVT) or pulmonary
embolism, or evidence of pulmonary hypertension
on echocardiography or cardiac catheterization
X
X
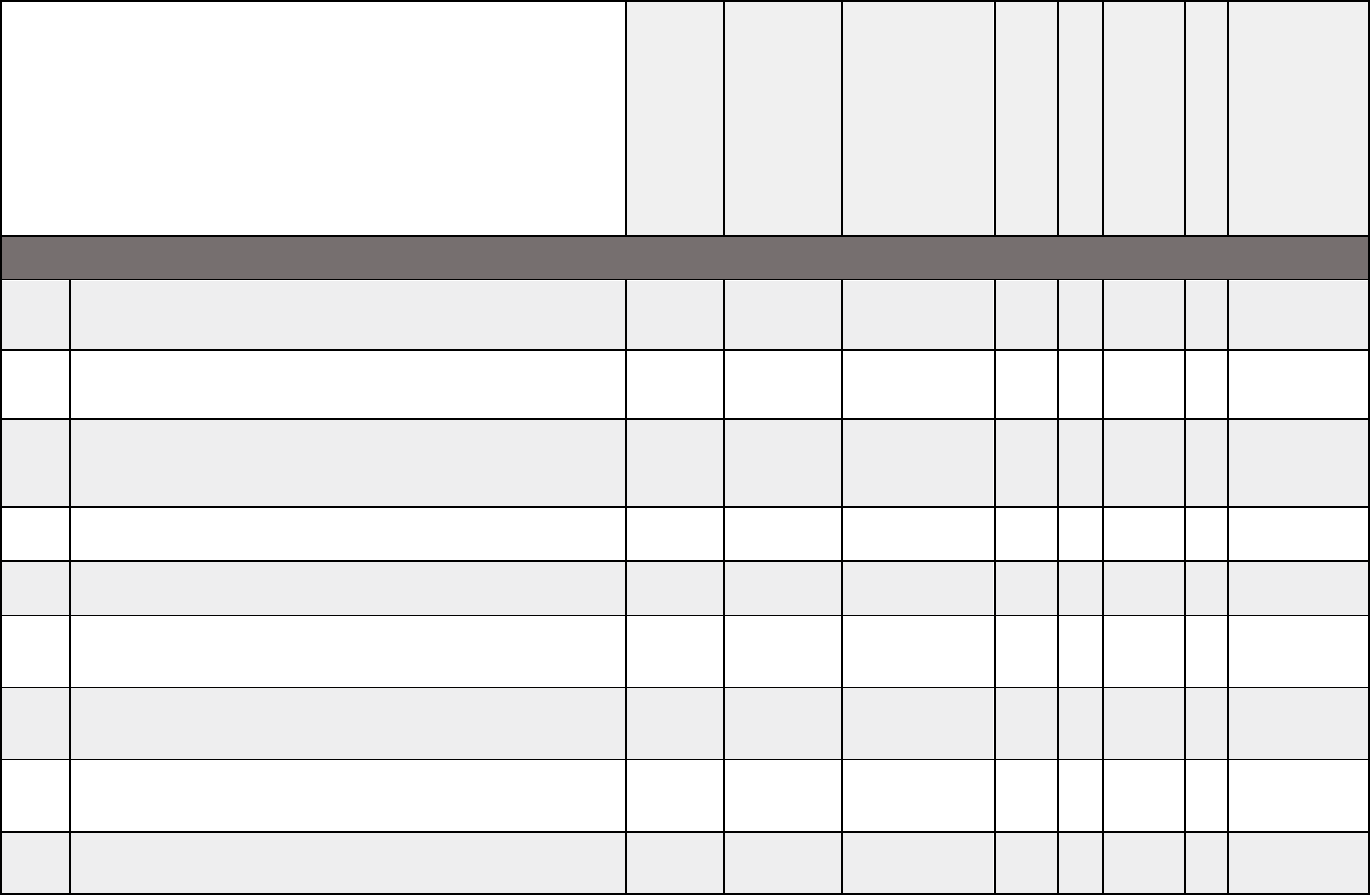
Medical documentation checklists
20
Allogenic
stem
cell/bone
marrow
Autologous
stem
cell/bone
marrow
Chimeric
Antigen
Receptor
T-cell
(CAR
-T) Therapy
Heart or
heart/lung
Kidney
Liver, intestine,
or multi-visceral
Lung
Pancreas or
kidney/pancre
as
Labs
Complete blood count (CBC), chemistry panel, liver
profile, and renal profile
X X X X X X X X
Estimated glomerular filtration rate (GFR) or
creatinine clearance if not on dialysis
X
Estimated glomerular filtration rate (GFR) or
creatinine clearance if level is greater than 2.0
X
X
X
Hgb A1c
X
ABO blood type
X X X X X X X X
Human leukocyte antigen (HLA) typing
X
HIV status
X X X X X X X X
Hepatitis serologies
X X X X X X X X
C-peptide level
X
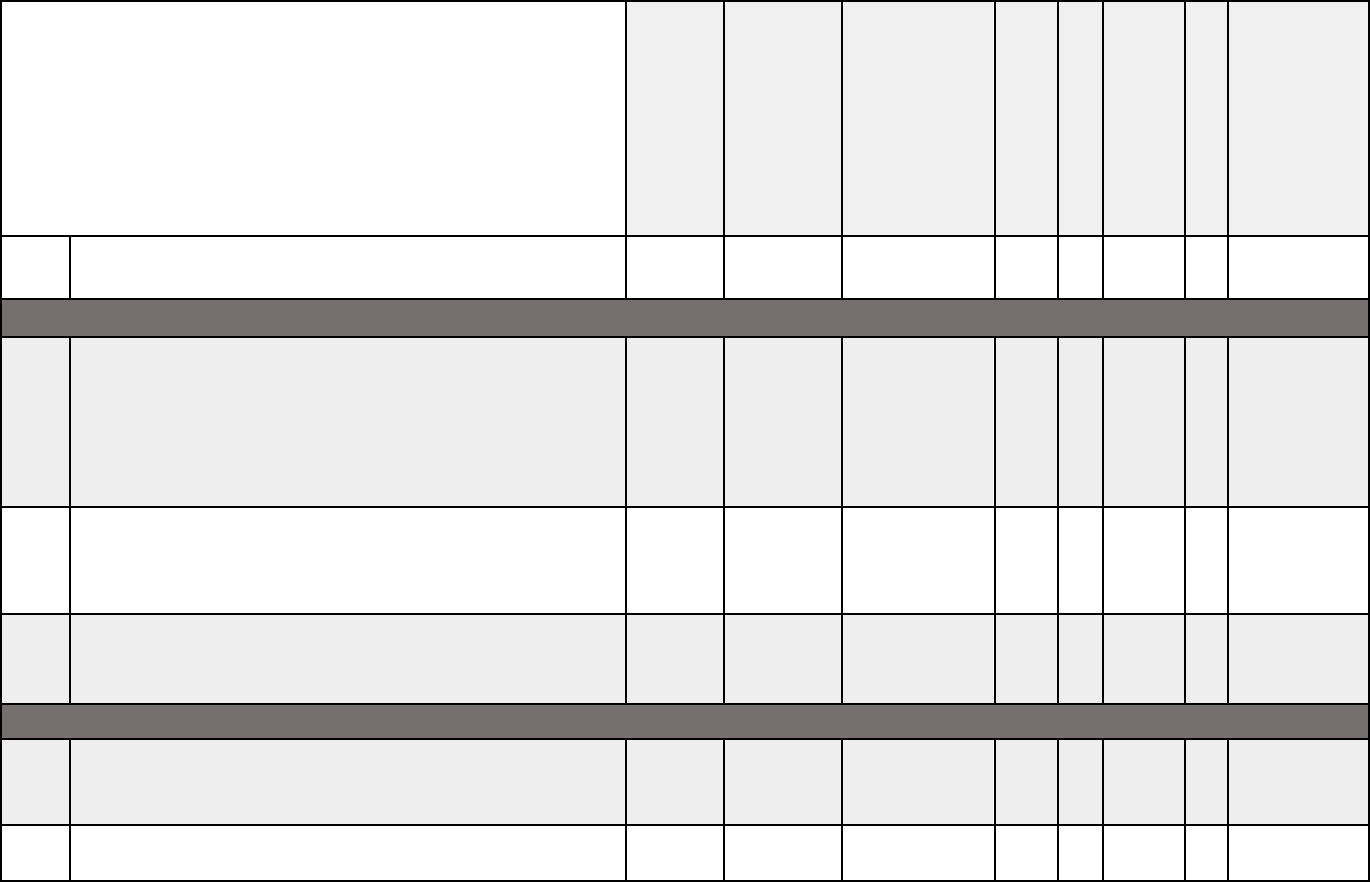
Medical documentation checklists
21
Allogenic
stem
cell/bone
marrow
Autologous
stem
cell/bone
marrow
Chimeric
Antigen
Receptor
T-cell
(CAR
-T) Therapy
Heart or
heart/lung
Kidney
Liver, intestine,
or multi-visceral
Lung
Pancreas or
kidney/pancre
as
Abdominal CT, MRI, or ultrasound
X
Cancer surveillance
Age 45 and older – age and condition appropriate
colon cancer screening (can include colonoscopy or
sigmoidoscopy or fecal occult blood test x3,
computed tomographic [CTC]/virtual colonoscopy,
stool-based deoxyribonucleic acid (DNA)
(Cologuard
®
)
X
X
X
X
X
X
X
X
Females age 21 and older – gynecological exam with
Pap smear within the past three years unless
absolute neutrophil count (ANC) < 1,000
X
X
X
X
X
X
X
X
Females age 50 and older – mammography within
the past three years
X
X
X
X
X
X
X
X
Other
requirements
Recent history and complete physical examination
(including rectal/pelvic, breast, and oral/dental,
unless contraindicated)
X
X
X
X
X
X
X
X
Psychosocial evaluation performed at the transplant
center
X X X X X X X X
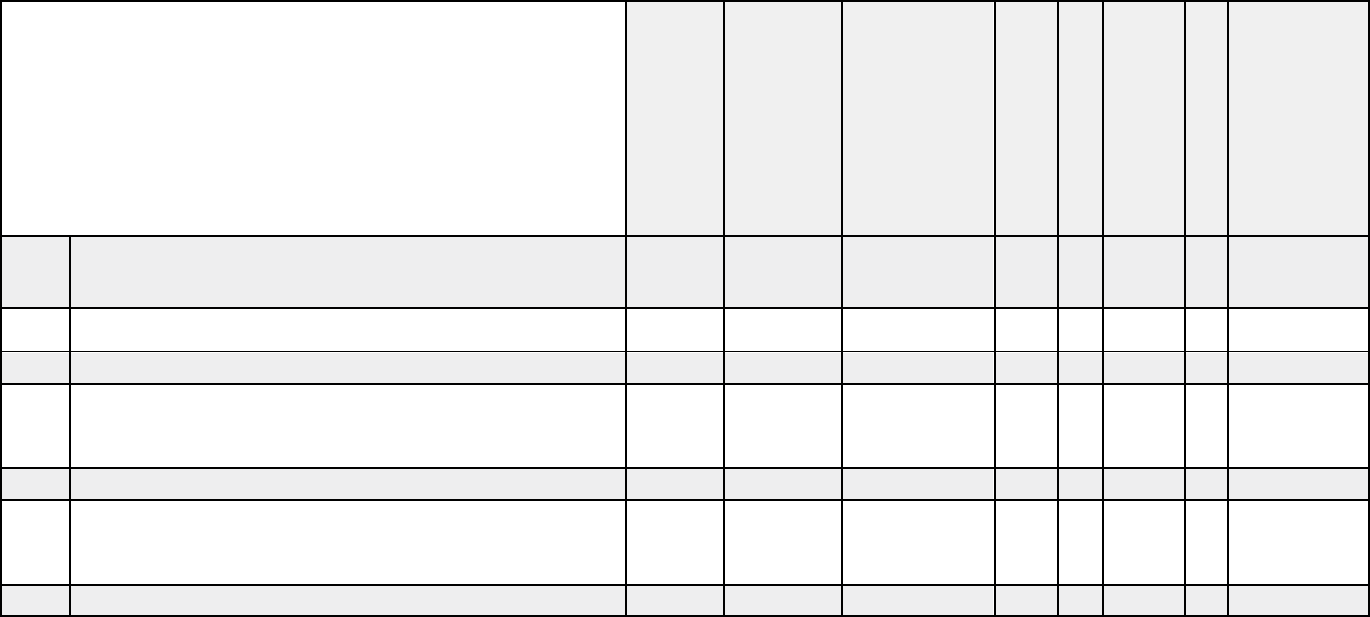
Medical documentation checklists
22
Allogenic
stem
cell/bone
marrow
Autologous
stem
cell/bone
marrow
Chimeric
Antigen
Receptor
T-cell
(CAR
-T) Therapy
Heart or
heart/lung
Kidney
Liver, intestine,
or multi-visceral
Lung
Pancreas or
kidney/pancre
as
Documentation of candidacy approval by facility
selection committee
X X X X X
Model for End-stage Liver Disease (MELD) score
X
Dental clearance, if abnormal physical exam
X
X
X
X
X
X
X
X
PPD testing for tuberculosis with history of
exposure, past history or family history of
tuberculosis, or abnormal chest X-ray
X
X
X
X
X
X
X
X
Protocol or written transplant treatment plan
X
X
X
Additional testing or clearance required by the
transplant team to address any comorbidities not
included above
X
X
X
X
X
X
X
X
New York Heart Association Functional Class
X
X
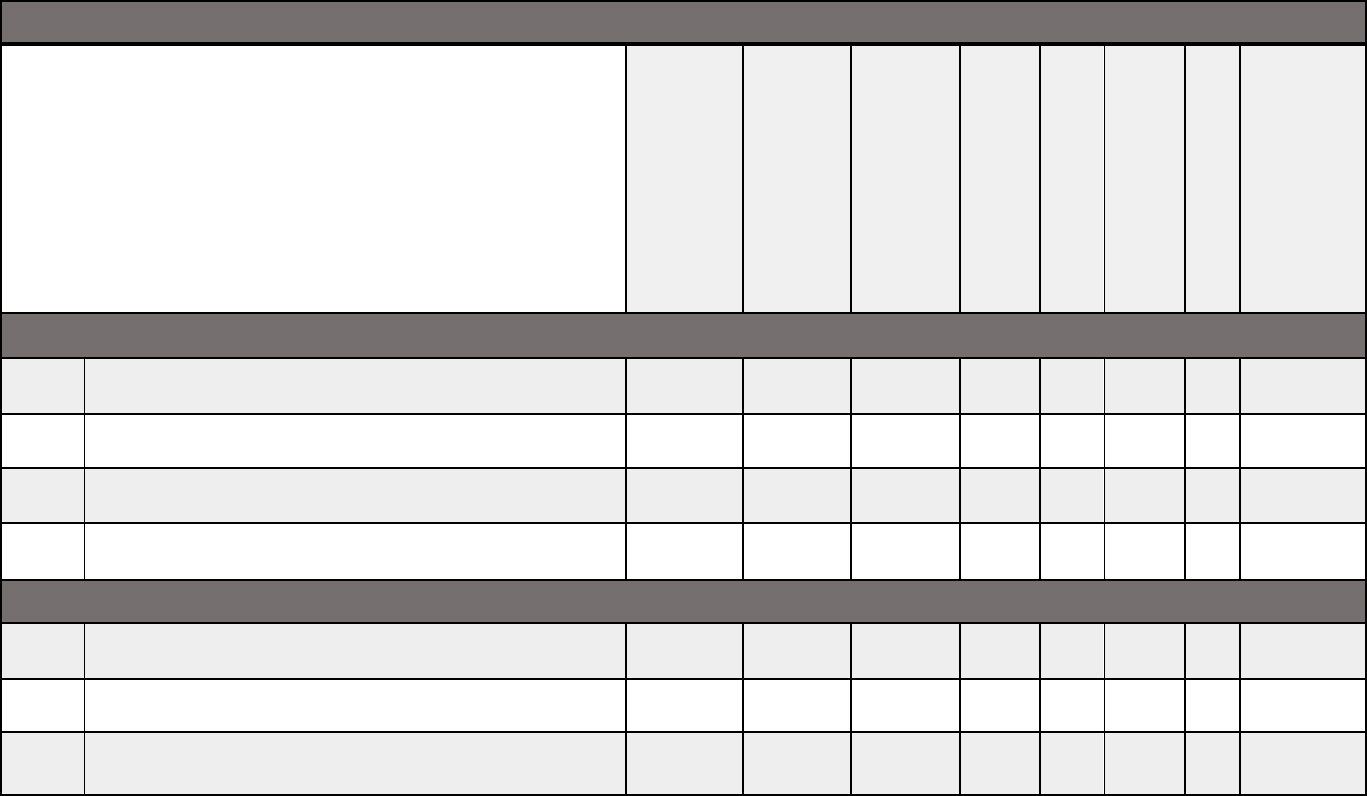
Medical documentation checklists
23
Medical documentation checklist for pediatric transplants
Pediatric
Transplant
Allogenic stem
cell/bone
marrow
Autologous stem
cell/bone
marrow
Chimeric
Antigen
Receptor T
-cell
(CAR
-
T) Therapy
Heart or
heart/lung
Kidney
Liver,
intestine,
or
multi-visceral
Lung
Pancreas or
kidney/pancreas
Cardiopulmonary
Chest x-ray or CT X X X X X X X X
Electrocardiogram (EKG) X X X X X X X X
Echocardiography or cardiac catheterization
X
X
Echocardiography or other cardiac functional test
MUGA/ CATH that evaluates EF and valvular status
X X
X
Labs
Complete blood count (CBC), chemistry panel,
liver profile, and renal profile
X X X X X X X X
Hgb A1c
X
Estimated glomerular filtration rate (GFR) or
creatinine clearance if not on dialysis
X
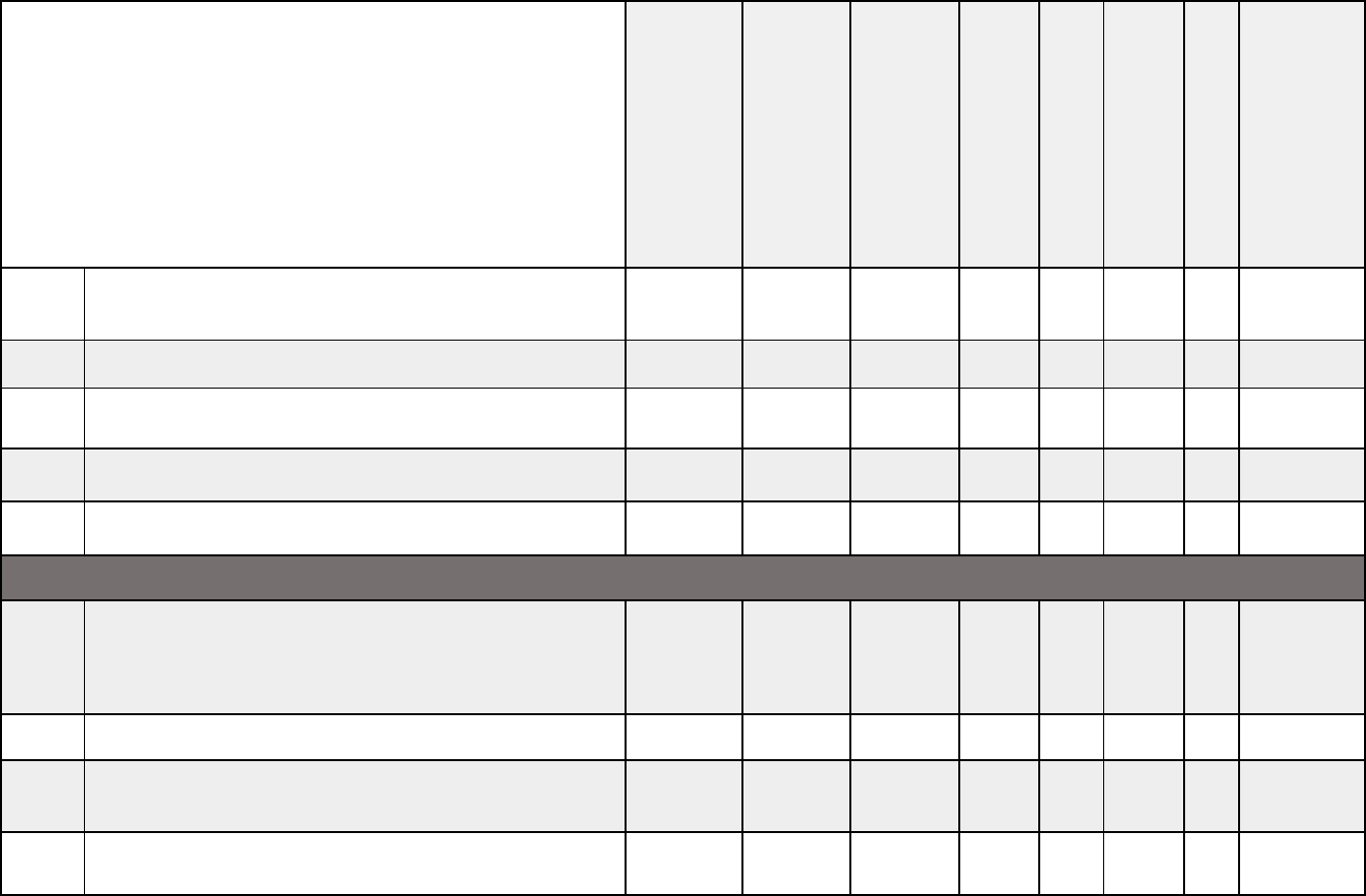
Medical documentation checklists
24
Allogenic stem
cell/bone
marrow
Autologous stem
cell/bone
marrow
Chimeric
Antigen
Receptor T
-cell
(CAR
-
T) Therapy
Heart or
heart/lung
Kidney
Liver,
intestine,
or
multi-visceral
Lung
Pancreas or
kidney/pancreas
Estimated glomerular filtration rate (GFR) or
creatinine clearance if level is greater than 2.0
X
X
ABO blood type X X X X X X X X
Human leukocyte antigen HLA typing X
HIV status X X X X X X X X
Hepatitis serologies X X X X X X X X
Other
requirements
Recent history and complete physical examination
(including oral/dental component and
breast/rectal/pelvis examination as age and
condition appropriate)
X
X
X
X
X
X
X
X
Abdominal CT, MRI, or ultrasound
X
Psychosocial evaluation of caregivers performed at
the transplant center
X X X X X X X X
Documentation of candidacy approval by facility
selection committee
X X X X X
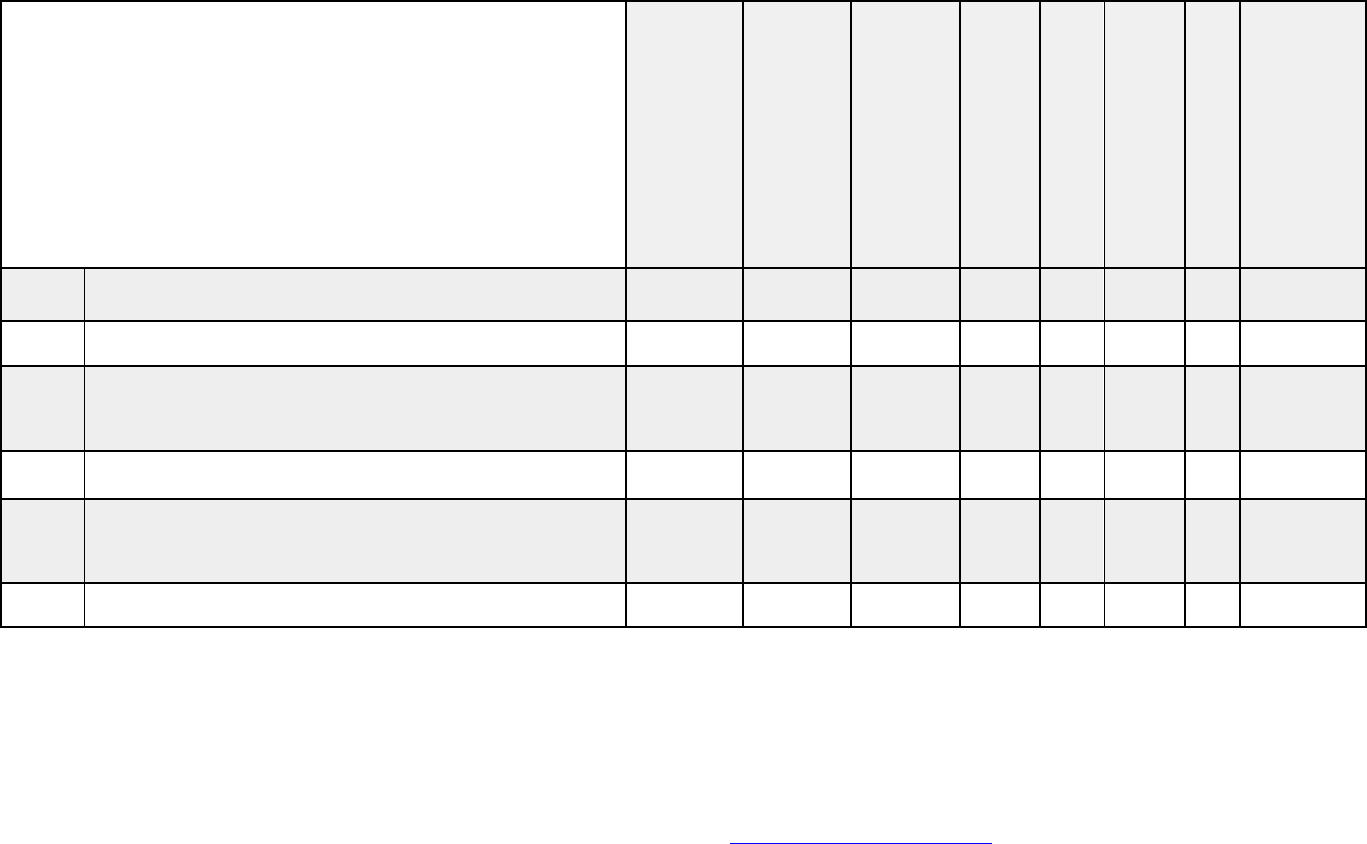
Medical documentation checklists
25
Allogenic stem
cell/bone
marrow
Autologous stem
cell/bone
marrow
Chimeric
Antigen
Receptor T
-cell
(CAR
-
T) Therapy
Heart or
heart/lung
Kidney
Liver,
intestine,
or
multi-visceral
Lung
Pancreas or
kidney/pancreas
Pediatric End-stage Liver Disease (PELD) score
X
Protocol or written transplant treatment plan X X X
PPD testing for tuberculosis with history of
exposure, past history, family history of
tuberculosis, or abnormal chest X-ray
X
X
X
X
X
X
X
X
Dental clearance, if abnormal physical exam X X X X X X X X
Additional testing or clearance required by the
transplant team to address any comorbidities not
included above
X
X
X
X
X
X
X
X
New York Heart Association Functional Class
X
X
Note: If a living donor is being considered for a patient, information required for review per UNOS and OPTN policies:
Verbal or written confirmation of: Psychosocial evaluation, completion of independent living donor advocate (ILDA),
donor medical evaluation (physical evaluation and lab work), and donor informed consent.
These checklists are a guide and are not absolute. Please contact your patient’s Transplant Case Manager if you have
questions or concerns. For more information please refer to our medical documentation
checklist.
Medicare eligibility
26
Medicare eligibility
For end-stage renal disease (ESRD) and kidney transplants
• Does the patient have end-stage renal disease and is on dialysis?
• Will the patient be receiving a kidney transplant without having started
dialysis prior to the transplant?
If you answered yes to either of the above questions, the patient may be eligible for
Medicare Part A and Part B, regardless of age.
Follow up with the Transplant Case Manager to review the patient’s benefit plan for
coverage limitations that may apply when they are eligible for Medicare. The benefit
plan may assume enrollment in BOTH MEDICARE PART A&B and pay as
SECONDARY even if the patient is not enrolled in Medicare.
Follow up with the Transplant Case Manager to discuss the patient’s particular
benefit plan.

Claim submission
27
Submit claims to the following address:
Cigna LifeSOURCE SAMBA
PO Box 188007
Chattanooga, TN 37422
Claim submissions
We have several lines of business that account for the transplant referrals that
Cigna LifeSOURCE submits to your transplant programs. To ensure accurate and
timely claim processing and repricing, please follow these instructions.
• Submit claims to the correct address noted below.
• For questions about where to send a transplant claim for all business lines
noted below, please call 800.287.0539.
• Please do not submit claims electronically.
• Do not submit claims to the address on the customer’s ID card.
To check on the status of transplant claims, please call the applicable customer
service number below or send an email, if applicable, and provide the following
information:
1. Patient’s name
2. Patient’s ID number
3. Dates of service for claims in question
4. Billed charges amount
Cigna Healthcare customers
Cigna Healthcare, Cigna Global Health Benefits
®
, and Shared
Administration Repricing (Taft-Hartley) plans. Please refer to the
Network Access Client (NAC) section below regarding claim submission
for Strategic Alliance customers.
U.S. mail
Submit claims to:
Cigna LifeSOURCE Transplant Claims
PO Box 182203
Chattanooga, TN 37422
FedEx only
Submit claims to:
Cigna LifeSOURCE Transplant Claims
5810 Brainerd Road
Chattanooga, TN 37411
Customer service:
• Cigna Healthcare, Cigna Global Health Benefits, and Shared Administration
Repricing: 800.287.0539
SAMBA account
Claim submission
28
Cigna Healthcare Medicare Advantage Arizona customers
Submit claims to the following address:
Cigna Healthcare of Arizona, Inc.
PO Box 38639
Phoenix, AZ 85069
Customer service: 800.627.7534
Network Access Clients (NAC)
Including Cigna Payer Solutions and Strategic Alliance relationships (HealthPartners,
Priority Health or MVP HealthCare issued ID card)
Referral letters are typically sent to the transplant program financial coordinator
and the managed care office, identifying these individuals and instructing that
claims should be submitted to the following address.
Please do not submit claims to the Chattanooga address for these
individuals.
Submit claims to the appropriate address below.
Effective December 1, 2021
U.S. mail
Cigna LifeSOURCE NAC Transplant Claims
PO Box 6471
Indianapolis, IN 46206
FedEx only
Attn: Trevor Evans
Cigna LifeSOURCE NAC Transplant Claims
11595 N. Meridian Street, Suite 600
Carmel, IN 46032
Alternatively, you may email claims to NACClaims@evernorth.com.
If you have questions, please email LifeSourceNACInquiries@evernorth.co
m and
provide the information below:
1. Patient’s name
2. Patient’s date of birth
3. Patient’s ID number
4. Dates of service for claims in question
5. Billed charges amount
6. Provider’s name
Customer service: 800.287.0539

Claim submission
29
Email claims submissions
All LifeSOURCE claims (except Medicare) can now be emailed to
LifeSOURCETransplantClaims@Cignahealthcare.com
.
Send secure emails through your Cigna-established TLS connection. If you do not
have a TLS connection established, please send an email to
LifeSOURCETranspl[email protected]
to request one.
The following coversheet is required for every email submitted with claims.
Please follow this process for each email:
• One email per member. Each email should contain 2 or 3 separate
attachments/files.
• One attachment/file for claims. Start the file name with prefix “cl_” (you can
attach more than 1 claim per file, but there has to be at least 1 claim file
attached and it needs to be named with prefix “cl_”.)
• One attachment/file for the required coversheet. Start the file name with
prefix “cs_” (please make sure all the required information marked with an
asterisk is filled out on the coversheet).
• One attachment/file for itemization. Start the file name with prefix “it_”
(itemizations are required for zone 3).
Bundled billing
If the hospital and physicians are reimbursed under a single Zone 3 case rate, the
hospital must bill all hospital and physician claims for services rendered during the
transplant admission, or transplant period, as one packet with a cover sheet.
Interim bills for Zone 3 will be accepted as long as a cover sheet is included with
each submission. The Zone 3 case rate payment will be made on the first packet
submission. Contract terms and provisions will continue to apply to processing of
any subsequent Zone 3 claims. For BMT/SCTs, a global packet after each cell
infusion may be submitted, but the global packet must include all applicable claims
for that transplant period.
If the hospital and physician group have separate Zone 3 case rates, the hospital
and physician group must bundle bill their respective claims as outlined above.
Note: Shared Administration Repricing (SAR) and Network Access Clients (NACs)
will not process a global case rate zone payment unless all hospital and physician
claims are included in the packet, even if there are no inlier or outlier provisions
that would apply.
Claim submissions
30
Required claim documentation
• Zone Authorizations (Prior Authorizations) for each zone should be
included on the UB-04 claim form (box 63) and submitted on separate claim
forms for each zone.
• Multiple zones should not be billed on the same UB-04 claim form, unless
specifically identified otherwise in your contract. Typically, the charges for
each zone should be on a separate UB-04 claim form.
• Non-transplant-related care charges should be submitted on a separate
UB-04 claim form from the transplant-related care charges.
• Split years claims must either be submitted with separate UB-04 claim
forms for each year, or with an itemization that includes revenue codes and
dates of service for each charge.
• Itemized bills may be required to process your claim. It should always
include the revenue code and date of service for each charge.
• Donor claims should be billed under the recipient’s name and benefit plan
ID number and clearly marked as a donor claim. The donor’s name can be
included as a remark and the donor’s authorization number should be
included on the claim.
Once a patient enters Transplant Case Management, all claims for that patient are
handled by our dedicated transplant claims teams – including non-transplant-
related services. This dedicated claims service continues for one-year post-
transplant to ensure that all related claims are reimbursed accurately.

Dispute resolution
31
Dispute resolution
Payment appeal process
Payment disputes should be submitted to the Cigna National Appeals Organization.
All appeals must be initiated in writing within 180 calendar days of the date of the
initial payment or denial decision. If the appeal relates to a payment that Cigna
Healthcare adjusted, the appeal must be initiated within 180 calendar days from
the date of the last payment adjustment.
The appeal process aims to resolve contractual disputes about post-service
payment denials (or partial denials) and other payment disputes. If the issue is not
resolved to the provider’s satisfaction, a dispute resolution may be requested,
including arbitration, as the final resolution step.
For additional information on how to submit an appeal, review and follow the claim
adjustment and appeals guidelines on the Cigna for Health Care Professionals
website (CignaforHCP.com). For Claim Appeals Policies and Procedures: Cigna
HealthCare Appeal Policy and Procedures.
Please contact the patient’s Transplant Case Manager or the customer service
number on the back of the patient’s ID card for further assistance.
Arbitration of disputes
If the dispute is not resolved through the appeal processes described above, either
party can initiate arbitration by providing written notice to the other. Please see
facility’s contract for specifics. The appeal processes must be followed in its entirety
before initiating arbitration.
Note: The terms and conditions of the arbitration provision in the provider contract
apply.
If the parties are unable to agree upon an arbitrator within 30 days after one of the
parties has notified the other of the desire to submit a dispute for arbitration, then
the parties will prepare a Request for a Dispute Resolution List and submit it to the
American Health Lawyers Association Alternative Dispute Resolution Service (AHLA
ADR Service), along with the appropriate administration fee.
If a hospital fails to request an appeal or arbitration for a payment dispute within
the applicable time frames, the last determination from Cigna Healthcare regarding
the dispute shall be binding on the hospital. Customers cannot be billed for
payments that are denied if the hospital failed to submit the request for review or
arbitration within the required time frames.
For more information on how to appeal a denial, please contact the patient’s
Transplant Case Manager.
Travel benefit
32
Travel benefit
Cigna Healthcare offers a transplant travel benefit to eligible participants up to
$10,000 when they choose a Cigna LifeSOURCE Designated Program.
This benefit covers eligible transportation and lodging for the transplant recipient
and one caregiver (up to two caregivers for a dependent minor) when traveling to
and from their home and the Cigna LifeSOURCE transplant facility. The recipient
must participate in the Transplant Case Management Program to be eligible for this
benefit.
The travel program is available only when Cigna Healthcare has authorized
coverage for a transplant at a Cigna LifeSOURCE Transplant Network facility that is
Designated for the specific transplant being requested, and when the facility is
located more than 60 miles (one-way) from the recipient’s home. The customer
must receive approval from their case manager to use this benefit. This benefit may
not be the same for all customers.

Air ambulance transport
33
Cigna Healthcare has contracted with several air ambulance companies
at competitive rates.
Please work with the Cigna LifeSOURCE Case Manager to contact a
contracted air ambulance provider when appropriate and medically
necessary. If an urgent situation arises after business hours, please
contact the Health Information Line at 800.856.9286 to arrange for
these services.
Air ambulance transport
Air ambulance transport is covered when medically necessary only if:
• The individual’s medical condition is such that transportation by basic or
advanced life support is required and ground ambulance is not clinically
appropriate due to the medical condition, or
• ground ambulance transportation is not available or feasible.
Important points to note:
1. Coverage for air ambulance transport is limited to the closest appropriate
Cigna LifeSOURCE Transplant Network Center.
2. All air ambulance transport, if covered, for a transplant event or related
service is a core medical benefit and is not eligible for reimbursement under
the Transplant Travel Benefit. The standard air ambulance benefit applies.
3. Air ambulance transport provided solely for the convenience of the individual
is not a covered benefit. Air ambulance is considered a convenience for an
individual who chooses to remain outside a reasonable driving distance from
the transplant facility while on the waiting list. An individual is expected to
remain within a reasonable driving distance from the transplant facility to
allow safe transport to the transplant facility within the time frame specified
by the facility when an organ becomes available for transplantation.

Network Access Clients
34
Cigna LifeSOURCE NAC Transplant Claims
PO Box 6471
Indianapolis, IN 46206
Network Access Clients
Network Access Clients (NACs) contract with us specifically to access the Cigna
LifeSOURCE Transplant Network for their payers needing transplantation benefit
management and support. These clients do not have benefit plans for which Cigna
is the claims administrator, including Cigna Payer Solutions. Cigna currently has
this arrangement with approximately 400 different payors.
Referral process
The customer initiates the referral process by filling out an online referral form and
forwarding it to Cigna LifeSOURCE. One of our Transplant Care Coordinators (TCC)
will review the referral form to ensure that the benefits are adequate and that the
form is complete. The TCC will then forward a referral letter via fax, unless an email
is requested, to the appropriate contacts at your organization – typically the
financial coordinator at the transplant program and the managed care office. A
sample referral letter is included on page 37.
The TCC will follow up on a regular basis with the transplant program and the
payer’s case manager to check on the status of the case – whether the patient
continues to be a candidate for transplant – to confirm zone dates and the date that
the case is closed. This helps to keep all parties informed and ensures that
everyone is in agreement on the status of the customer, whether pre-transplant
admissions are transplant related or not, and to ensure accuracy in repricing of
claims.
Claim process
A dedicated post office box has been set up for the NAC claims. Each referral letter
will note this address to ensure that you submit NAC claims to the correct address:
Please note that this is a separate mailing address for NAC claims for each referral.
If NAC claims are mailed to the Cigna LifeSOURCE address for Cigna customers,
they will most likely be denied and returned to your billing department.
A dedicated Cigna LifeSOURCE claims representative reprices claims and the
average turnaround time is five business days. The payer is instructed to pay within
30 days of the date the claim is received and repriced, and a specific due date is
provided on the claims cover sheet. The claims and claims cover sheet are sent to
the appropriate payer for processing. If there are any questions or concerns about
an amount that the payer has paid on a particular claim (or batch of claims) or if
there is nonpayment of a claim, please contact the NAC claims team. (Refer to the
contact information and sample claims cover sheet on pages 29 and 30.)
Cigna LifeSOURCE will reprice claims for all four zones. At the time we find out that
a case is ready to be closed under the terms of our agreement, we will fax a letter,

Network Access Clients
35
unless an email is requested, stating that Cigna LifeSOURCE is preparing to close
the case, provide the case closure date, and request final submission of claims.
For questions concerning referrals, please contact Sarah Shafer at 860.902.9604,
or Sarah.Shafer@evernorth.com
.
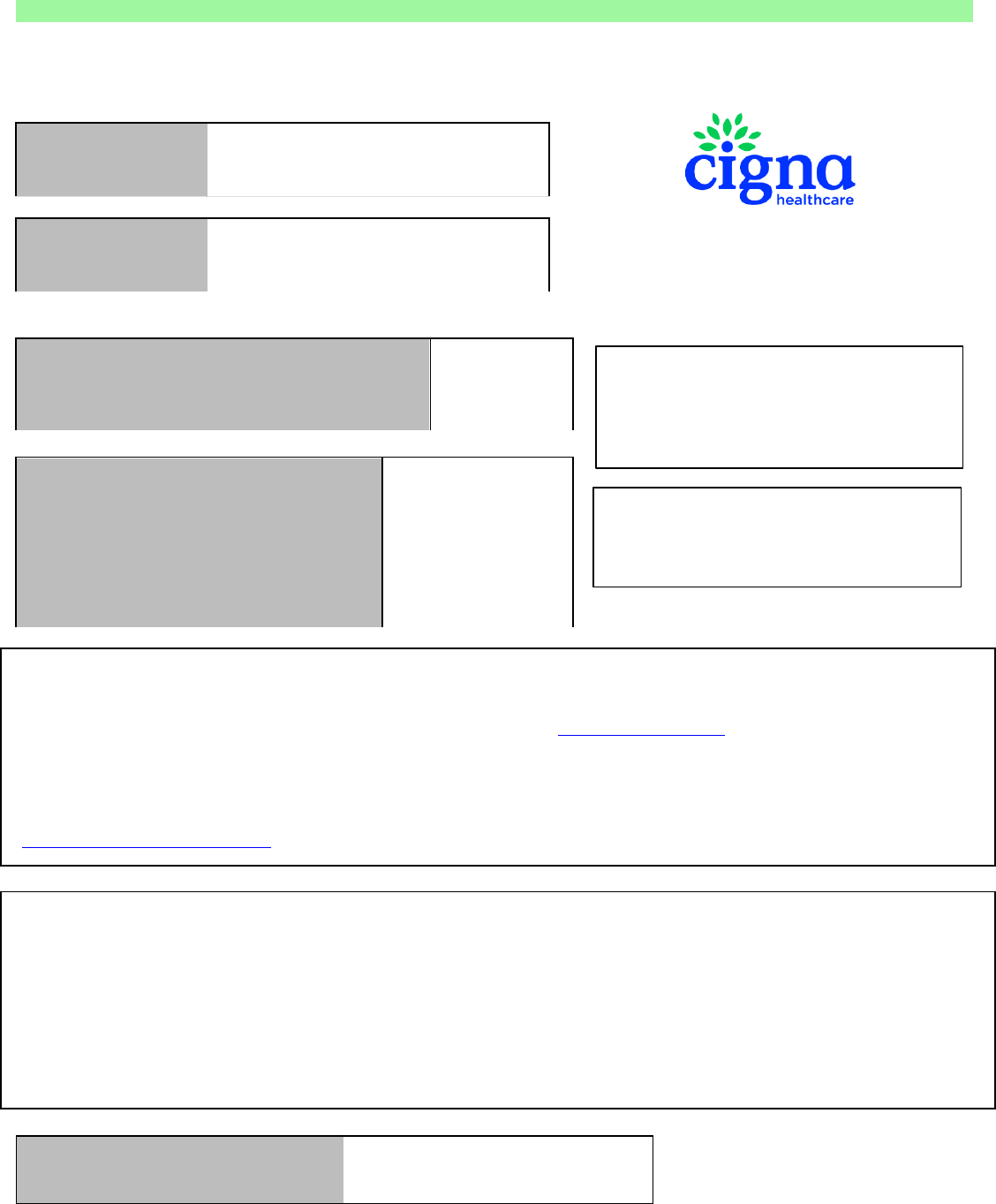
Network Access Clients
36
Today’s date January 31, 2022
Transplant Facility University Hospital
Transplant Type
Liver
Sample referral letter
Cigna LifeSOURCE Transplant Network Referral Notification
This notification is NOT an authorization for services.
Please contact the case manager below for authorizations.
ALL questions regarding benefits and eligibility MUST be
directed to the payor listed below.
Please use the number provided on the
member's insurance ID card when submitting
claims.
Please do NOT use the member's social
security number as the member's ID number.
Claims should be submitted to:
Cigna LifeSOURCE NAC Claims
PO Box 6471
Indianapolis, IN 46206
Claims sent via FedEx ONLY should be submitted to:
Cigna LifeSOURCE NAC Claims
11595 N. Meridian Street, Suite 600
Carmel, IN 46032
Claims can also be submitted via email to NACClaims@evernorth.com
Please do not submit the claims to the Cigna LifeSOURCE address in Chattanooga as they will be denied due to member not being a Cigna Healthcare
member.
Claims will be repriced and forwarded to Payor within 5 business days of receipt by Cigna Healthcare.
Please allow 45 days for processing of the claim before contacting the Payor at the phone number provided above.
If you have not received resolution after contacting the payor, please email the claims repricing team at
LifeSourceNACInquiries@evernorth.com Please provid
e the name of the person you contacted, the date and time you contacted them, and the
information you were provided.
The above-named Payor has signed a direct Service Agreement with Cigna Healthcare for access to the Cigna LifeSOURCE Participation Agreement between
Cigna Health Corporation, Inc. and the above named Facility for the above-named Patient. The Service Agreement provides the following terms:
•
Payor is responsible for verifying Patient’s benefits and eligibility for transplant services. Payor is not responsible for covering transplant services that it has
not authorized. Any dispute about coverage is solely between the patient and the Payor.
•
The Payor will pay for the transplant services and supplies that are covered under the Patient’s benefit contract and provided by the Hospital and Group
pursuant to Hospital and Group’s Participation Agreement with Cigna LifeSOURCE. The Service Agreement creates direct obligations of Payor to Hospital
and Group, and if Payor fails to perform its obligation to Hospital or Group, Hospital and Group will have a direct cause of action against Payor.
•
The Payor agrees to have the claim processed promptly so that payment is received by the applicable provider within 30 calendar days of receipt of claims
by the designated payor designee, and in accordance to required criteria of a "clean" claim (a "clean" claim is completed in compliance with UB92 and HCFA
1500 requirements or its successors and includes a claims coversheet from Cigna LifeSOURCE Transplant Network.) Prompt payment state laws will
apply.
•
The Payor will reimburse Hospital per the terms of the Cigna LifeSOURCE Transplant Network agreement between Hospital and Cigna Healthcare for all
hospital and professional transplant related services for zones 1 – 4. All exclusions and terms of the Cigna LifeSOURCE agreement apply.
Transplant Care Coordinator Name:
Mary Smith, RN
Telephone Number:
999-999-9999
Fax Number:
777-777-7777
Email Address:
Mary.Smith@company.com
Claims are to be submitted directly to Cigna
LifeSOURCE Transplant Network.
Claims submitted directly to the payor will be
denied.
All claims beginning with the referral effective
date are to be submitted directly to Cigna
LifeSOURCE Transplant Network.
Claims repricing will begin on the first date of
the evaluation.
Member Name John Smith
Policy Number 12345678
Date of Birth
1/1/1960
Referral Date
1/31/2022
Evaluation Start Date 2/2/2022
Member Accessing Non-Transplant Related Rates
Yes
Medicare Advantage Member? Yes
Payor ABC Health Plan
Employer Group XYZ Corporation
Case Manager Name Sue Smith
Case Manager’s Phone Number 222-333-4444
Utilization Review Nurse's Name John Jones
Utilization Review Nurse's Phone Number 222-333-5555
Payor's Customer Service Phone Number
222-333-6666
Pharmacy, infusion services, and behavioral health services
37
• Call 800.351.3606, option 101 to speak with a specialist
• Send a fax to 800.351.3616
Pharmacy, infusion services, and behavioral health
services
Express Scripts
Express Scripts Pharmacy
®
, a Cigna Healthcare company, is our home delivery
pharmacy. We deliver specialized care that puts patients first, through a smarter
approach to pharmacy services. Express Scripts makes prescription medications
safer and more affordable for customers.
Express Scripts provides the following for initial discharge orders post-transplant:
• Prescription-only medications associated with transplants
• Some transplant-related over the counter (OTC) medication
• Diabetic supplies, blood pressure, and cholesterol medications
• Express Scripts can bill Cigna Healthcare directly for the medications. They
can also bill Medicare as either a primary or secondary payer for covered
immunosuppressive medications for eligible customers
• 90-day supplies available
• Free, overnight shipping directly to hospital or patient
How to submit an order
Accredo
Accredo, a Cigna Healthcare company, is the national preferred source for specialty
medications, and is in the pharmacy network for many of your patients with Cigna
Healthcare-administered plans. By using an in-network pharmacy, your patients
with Cigna Healthcare coverage will be able to take full advantage of their specialty
drug coverage options.
Through Accredo, you and your patients have access to a team of pharmacists and
nurses with extensive training and experience. You will be able to obtain specialty
products, including many limited distribution drugs (LDDs) or those with exclusive
distribution, and your eligible patients will be able to obtain financial assistance
coordination.
Information on Accredo as well as medication-specific order forms can be found on
Accredo.com > Prescribers >
Referral Form
s.
Contact Accredo for specialty medication prescriptions and renewals at
800.294.6012.
Coram
®
CVS Specialty
®
infusion services (Coram)
Coram, a CVS Health company, is a leading provider of specialty infusion and
enteral nutrition services nationally, and acute infusion services in many markets.
Their experienced clinicians coordinate care and education for patients and help
ensure a smooth transition home. They are dedicated to increasing access to
quality care for patients, while delivering excellent outcomes and lowering health

Pharmacy, infusion services, and behavioral health services
38
care costs. With more than 35 years of experience, they are the only national home
infusion provider accredited by The Joint Commission.
As a preferred infusion provider for Cigna LifeSOURCE Transplant Network, Coram
delivers high-quality, personalized care to your transplant patients in their home or
alternate-care setting. They provide comprehensive pre- and post-transplant
therapies, including:
• Anti-infectives (antibiotics, antivirals, antifungals)
• Enteral nutrition (tube feeding)
• Immunoglobulins (intravenous and subcutaneous)
• Immunosuppressive
• Parenteral nutrition
• Inotropes (cardiology)
Call 800.423.1411 or the appropriate number listed below to make a referral.
Coram accepts referrals seven days a week.
• Infusion services for specialty medications, including immunoglobulins
Telephone: 866.899.1661
Fax: 866.843.3221
• Enteral nutrition services
Telephone: 877.936.6874
Fax: 800.693.7322 or 866.202.7319
• Acute infusion services, including infused antibiotics, parenteral nutrition, and
inotropes
Telephone: 800.423.1411
Fax: 949.639.5606
Evernorth Behavioral Health
Cigna LifeSOURCE customers may have access to behavioral health coverage. The
psychological impact of serious illness often requires treatment. Many of your
patients have access to benefits including crisis counseling by phone, one-on-one
counseling, inpatient psychiatric counseling, and more.
Remember, for the entire time your patient is in Transplant Case Management
through Cigna LifeSOURCE, they must still work with their Transplant Case Manager
for behavioral health coverage. The case manager will work with Evernorth
Behavioral Health.
Your patient must confirm their behavioral health coverage through their Transplant
Case Manager. For more information about behavioral health benefits and eligibility,
please visit the Evernorth provider website (Provider.Evernorth.com
) or call
Behavioral Provider Services at 800.926.2273.
Important contact information
39
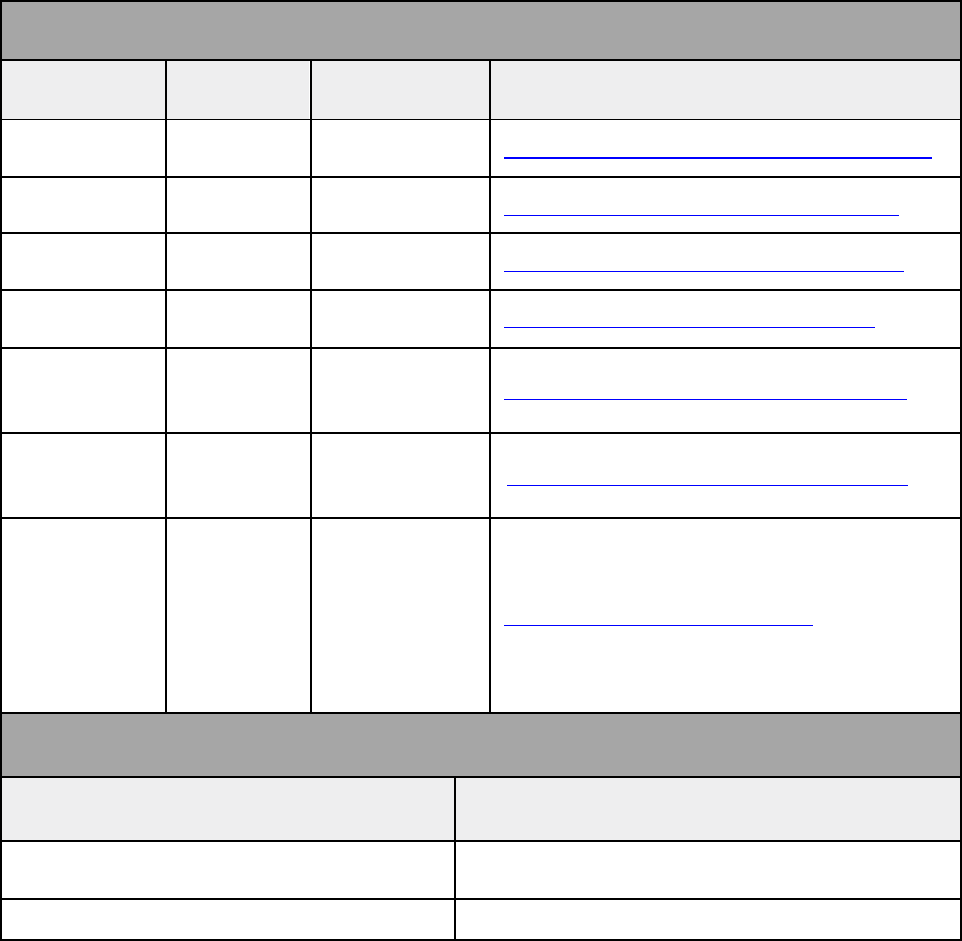
Important contact information
Below you will find Cigna LifeSOURCE Unit important contacts. Cigna LifeSOURCE
Unit hours of operation are from 8:00 a.m. to 5:00 p.m. EST.
Important contacts
Contact Position Telephone Email
Dr. Stephen
Crawford
Medical
Director
770.261.3485 Stephen.Crawford@CignaHealthcare.co
m
Dr. Janet
Campion
Medical
Director
Janet.Campion@CignaHealthcare.com
Dr. Maryann
Payne
Medical
Director
Maryann.Payne@CignaHealthcare.com
Dr. Jeff
Schriber
Medical
Director
480.244.2142
Jeff.Schriber@CignaHealthcare.co
m
Adriana
Mariani
National
Quality
Director
860.902.2973
Arianna
Graves
LifeSOURCE
Provider
Relations
770.261.7899
Arianna.Graves@CignaHealthcare.com
Sarah Shafer
Network
Access
Clients
Transplant
Care
Coordinator
Supervisor
860.902.9604
Sarah.Shafer@evernorth.com
Other important contacts
Contact Telephone
Cigna LifeSOURCE Transplant Case
Management and Referral Line
800.668.9682
Cigna LifeSOURCE Customer Service* 800.287.0539
*Please see claims submission page for additional customer service contact
information.

Legal statement
Legal statement
All Cigna Healthcare products and services are provided exclusively by or through
operating subsidiaries of The Cigna Group, including Cigna Health and Life
Insurance Company (CHLIC), Connecticut General Life Insurance Company,
Evernorth Behavioral Health, Inc., Evernorth Care Solutions, Inc., Express Scripts,
Inc., or their affiliates.
PCOMM-2024-1940. © 2024 Cigna. Some content provided under license.
40
