Assessment, differential diagnosis, and initial clinical evaluation of the
pediatric patient with obesity: An Obesity Medical Association (OMA)
Clinical Practice Statement 2022
Suzanne E. Cuda
a
,
*
, Marisa Censani
b
a
Alamo City Healthy Kids and Families, 1919 Oakwell Farms Parkway, Ste 145, San Antonio, TX, 78218, USA
b
New York Presbyterian Hospital, Weill Cornell Medicine, Department of Pediatrics, Division of Pediatric Endocrinology; New York, NY, USA
ARTICLE INFO
Keywords:
Assessment
Clinical evaluation
Differential diagnosis
Obesity
Pediatric patients
ABSTRACT
Background: The Obesity Medical Association (OMA) Clinical Practice Statement (CPS) on the assessment, dif-
ferential diagnosis, and initial clinical evaluation of pediatric patients with obesity is intended to provide clini-
cians with an overview of clinical practices applicable to children and adolescents with body mass indexes greater
than or equal to the 95th percentile for their ages, particularly those with adverse consequences resulting from
increased body mass. The information in this CPS is based on scientific evidence, supported by the medical
literature, and derived from the clinical experiences of members of the OMA.
Methods: The scientific information and clinical guidance in this CPS is based upon referenced evidence and
derived from the clinical perspectives of the authors.
Results: This OMA Clinical Practice Statement on assessment, differential diagnosis, and initial clinical evaluation
of pediatric patients with obesity provides clinical information regarding classification of children and adolescents
with overweight or obesity, differential diagnoses to consider, and a roadmap for the initial clinical evaluation.
Conclusions: This OMA Clinical Practice Statement on assessment, differential diagnosis, and initial clinical
evaluation of pediatric patients with obesity is an overview of current recommendations. Assessment of pediatric
patients with obesity is the first step in determining treatments leading to the improvement of the health of
children and adolescents with obesity, especially those with metabolic, physiological, and psychological
complications.
1. Introduction
The purpose of the CPS on assessment, differential diagnosis, and
initial clinical evaluation of pediatric patients with obesity is to provide
clinicians with a tool to assess children with obesity. The OMA is an
organization of providers in the field of obesity medicine dedicated to the
comprehensive care of patients with obesity. OMA members are physi-
cians, nurse practitioners, physician assistants, and other healthcare
providers who take a comprehensive, evidence-based approach to
treating obesity. This approach is comprised of the four pillars of nutri-
tion, physical activity, behavior, and medication. While it is hoped many
clinicians may find the recommendations in this CPS helpful, the final
decision regarding the optimal care of the patient with overweight or
obesity is dependent upon the individual clinical presentation and the
judgment of the clinician who is tasked with directing a treatment plan
that is in the best interest of the patient.
2. Assessment of pediatric patients
Weight assessment of children is age dependent. For children less
than two years of age, weight percentiles and weight for length charts are
used. Clinicians can choose between growth charts provided by the
Centers for Disease Control and Prevention (CDC) or the World Health
Organization (WHO). The CDC charts are based on a cohort of mainly
white American children who were mostly non-breastfed. The WHO
charts are based on children from diverse racial and ethnic backgrounds,
mostly breastfed [1–3].
For children and adolescents ages 2–20, body mass index (BMI)
percentile is used. For children and adolescents ages 2–20 with BMIs
greater than the 95th percentile, use percent of the 95th percentile [4].
* Corresponding author. 1919 Oakwell Farms Parkway, Ste 145, San Antonio, TX, 78218, USA.
Contents lists available at ScienceDirect
Obesity Pillars
journal homepage: www.journals.elsevier.com/obesity-pillars
https://doi.org/10.1016/j.obpill.2022.100010
Received 23 December 2021; Received in revised form 26 December 2021; Accepted 2 January 2022
2667-3681/© 2022 The Author(s). Published by Elsevier Inc. on behalf of Obesity Medicine Association. This is an open access article under the CC BY-NC-ND license
(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Obesity Pillars 1 (2022) 100010
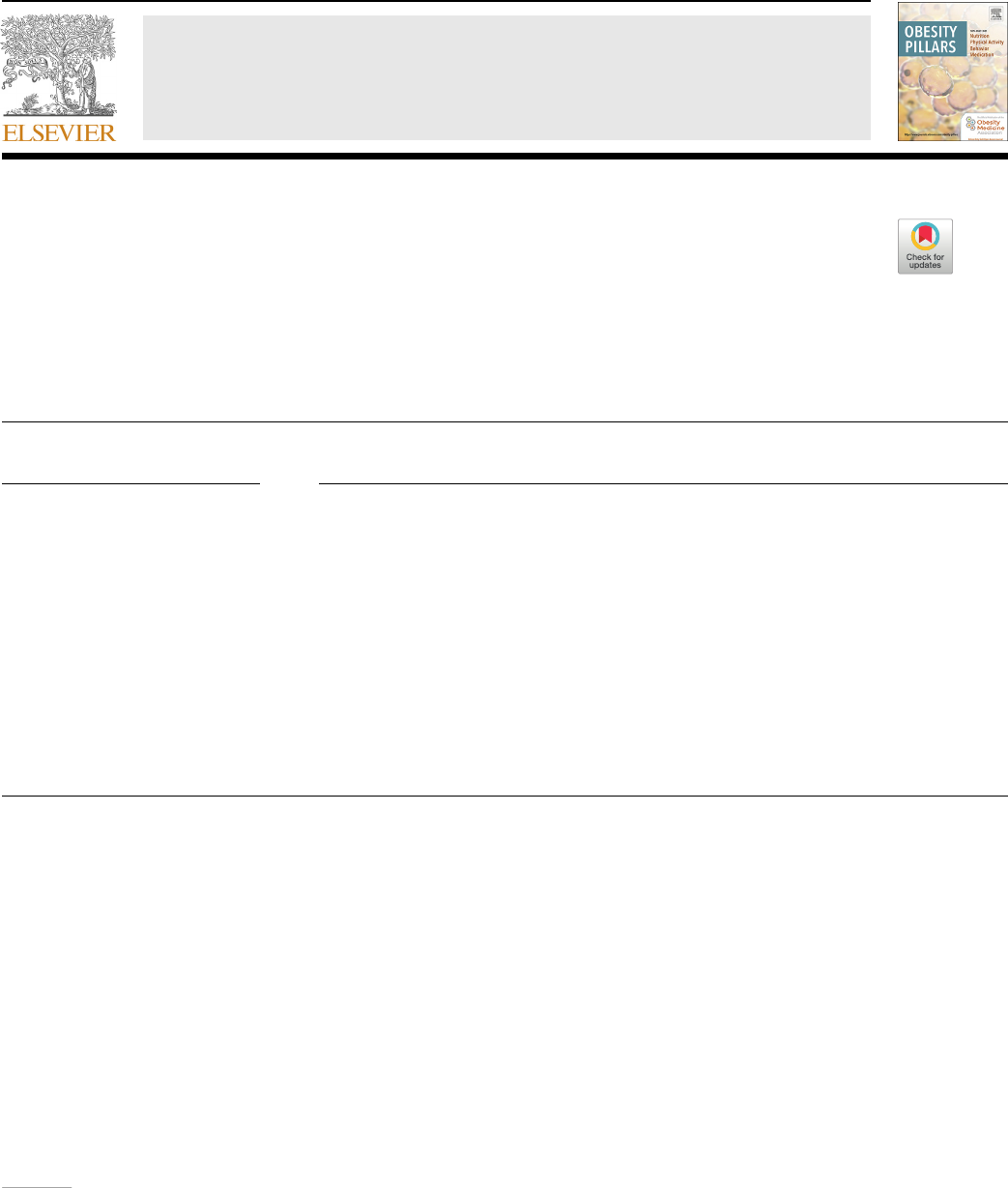
Categories of weight status are as follows: less than 5th percentile is
underweight, 5–84th percentile is healthy weight, 85–94th percentile is
overweight, 95th percentile to 119% of the 95th percentile is Class 1
obesity, 120–139% of the 95th percentile or a BMI greater than 35 kg/m
2
and less than 39 kg/m
2
is Class 2 obesity, and greater than 140% of the
95th percentile or BMI greater than 40 is Class 3 obesity [5,6]. Any BMI
greater than or equal to 120% of the 95th percentile is severe obesity.
Different charts are used to track BMI for up to the 95th percentile and
above the 95th percentile [4,6–8]. Body mass index categories and per-
centiles are shown in Fig. 1.
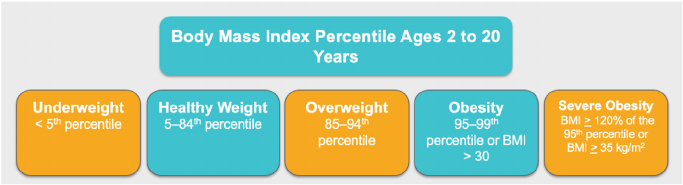
BMI z-scores, or BMI standard deviation scores, can be used to adjust
for child age and sex [9] but should not be used to assess BMI changes
among children and adolescents with BMIs greater than or equal to 120%
of the 95th percentile. High BMI z-scores are compressed into a narrow
range, which can result in a clinically significant reduction in BMI being
represented by a small reduction in BMI z-score. Change in the percent of
the 95th percentile is the preferred metric to use in the management of
children and adolescents with severe obesity [7,8]. BMI charts for chil-
dren and adolescents with severe obesity are shown in Fig. 2.
A BMI of 35 kg/m
2
is a higher threshold than a BMI greater than or
equal to 120% of the 95th percentile among most children, but it is a
somewhat lower threshold (and therefore expands the population that is
categorized as severely obese) among boys approximately 18 years of age
and older and girls approximately 16 years of age and older [7,10].
Overall, assessments should consider age as well as BMI status. The top
10 takeaway messages for assessing children and adolescents with
obesity are shown in Table 1.
3. Obesity as a disease
Obesity has been recognized as a disease by the American Medical
Association since 2013 [11]. Response to the disease of obesity manifests
differently in children and adolescents than in adults. A breakdown of
responses into categories of endocrine or immune response, physical
response, and psychological response is a useful way to work through the
clinical presentation [12].
Endocrine and immune responses are due to adiposopathy or “sick
fat.” Common phenomena include impaired fasting glucose, metabolic
syndrome, hypertension, menstrual dysfunction, early onset or a delay in
onset of puberty, nonalcoholic fatty liver disease, dyslipidemia, insulin
resistance, type 2 diabetes, increased uric acid, microalbuminuria, gy-
necomastia, cholecystitis, and accelerated growth [13,14].
Physical response is attributed to fat mass disease. Asthma, immo-
bility, lipomastia, tissue compression (obstructive sleep apnea, gastro-
esophageal reflux, hypertension), tissue friction (intertrigo), stress on
weight bearing joints, slipped capital femoral epiphysis, Blount's disease,
scoliosis, and orthopedic disorders are all possible findings [15].
Psychological responses can impact quality of life and include isola-
tion from peers, decreased ability to participate in normal childhood
activities, victimization (bullying, emotional/physical abuse, or neglect),
lack of social age-appropriate relationships, anxiety, depression, binge
eating disorder, night eating disorder, and bulimia [15,16].
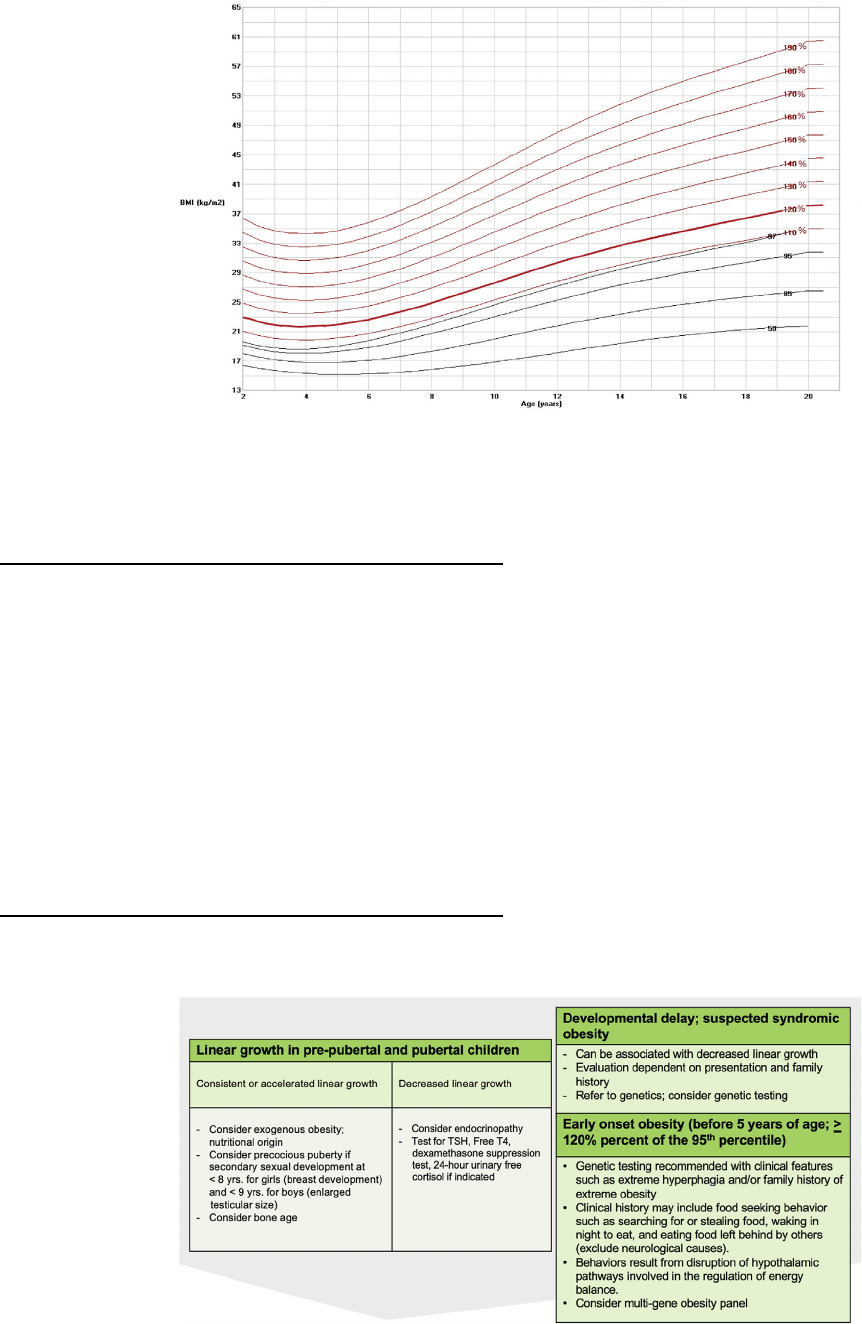
4. Differential diagnosis of children and adolescents with obesity
The diagnosis of exogenous obesity in children and adolescents due to
nutritional origin is a diagnosis of exclusion. Clinicians must consider
endocrinopathies and syndromal obesity as well as both monogenic and
polygenic etiologies.
For approaching the differential diagnosis of childhood obesity, it is
important to assess linear growth. Prepubertal and pubertal children with
exogenous obesity (i.e., related to unhealthful nutrition and physical
inactivity) usually have accelerated or consistent linear growth, in
contrast to those with an endocrinopathy, who frequently present with a
decrease in linear growth [17]. Precocious puberty can occur in both
those with exogenous obesity and those with an endocrinopathy. The
diagnosis of precocious puberty is made in females younger than 8 years
of age with Tanner II breast development and in boys younger than 9
years of age with testicular enlargement greater than 4 cc [18]. In these
children, a bone age assessment may show an advancement of bone age
as compared to chronological age of 1–2 years or more. If a child with
obesity presents with a decrease in linear growth, the clinician should
consider possible hypothalamic/pituitary dysfunction (with growth
hormone deficiency), hypercortisolism (consider a dexamethasone sup-
pression test or 24-h urinary free cortisol), hypothyroidism [check thy-
roid-stimulating hormone (TSH), conduct a free thyroxine (T4) test], or
genetic syndromes [19,20].
Syndromal obesity is obesity accompanied by other behavior, func-
tional, or anatomic abnormalities, such as hyperphagia, cognitive delay,
dysmorphic features, and organ-specific disorders. The clinician should
consider syndromal obesity in children with obesity who present with a
developmental delay. Developmental delay can be associated with
decreased linear growth, and the evaluation of these children is depen-
dent on presentation and family history. A child presenting with severe
obesity (>120% of the 95th percentile) before 5 years of age may indi-
cate a genetic etiology. If hyperphagia and/or a family history of extreme
obesity is present, genetic testing is recommended [21]. The clinical
history may include food-seeking behavior such as searching for or
stealing food, waking at night to eat, and eating food left behind by
others. Neurological causes should be excluded. These dysfunctional
behaviors result from disruption of the hypothalamic pathways involved
in the regulation of energy balance. Genetic testing for multi-gene panels
and referral to a geneticist should be considered [19,22–24]. A summary
of differential diagnoses regarding childhood obesity is shown in Fig. 3.
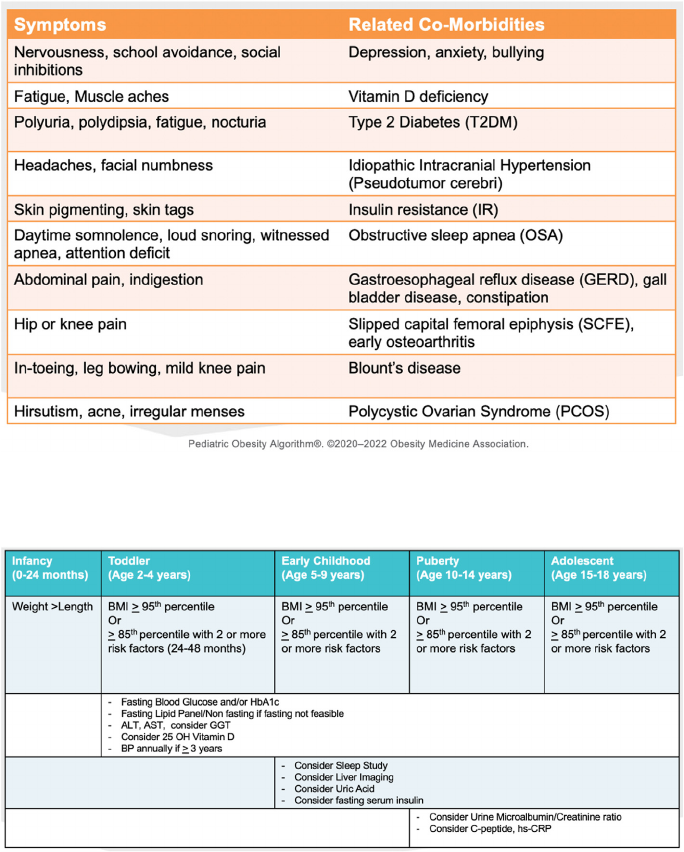
5. Focused Review of Systems
Children with obesity are commonly seen in primary care for a
problem other than obesity. Therefore, specific symptoms secondary to
obesity must be carefully assessed. Children may or may not complain of
symptoms, as many have been living with symptoms for years and are not
aware of what life is like without symptoms. Fig. 4 identifies symptoms
with accompanying related co-morbidities.
6. Diagnostic workup
The diagnostic workup is determined by the age at presentation of the
Fig. 1. Body Mass Index Categories in Children
and Adolescents Ages 2–20. BMI categories are
shown for children ages 2–20 who are underweight, at
a healthy weight, overweight, obese, and severely
obese [6]. Note that not all patients with BMI in the
85th percentile or above have excess adiposity, and
many children and adolescents with BMIs below the
5th percentile are healthy and do not need treatment.
The CDC recommends using the WHO growth charts
to monitor growth for infants and children ages 0–2
years of age in the U.S. and using the CDC growth
charts for children ages 2 years and older [2].
S.E. Cuda, M. Censani Obesity Pillars 1 (2022) 100010
2
child with obesity and the classification of obesity presented by the child.
Children with obesity are at risk for glycemic dysregulation [25]. A
fasting blood glucose or a hemoglobin A1c (HbA1c) test will determine
whether glycemic dysregulation is present. Dyslipidemia is the most
common laboratory abnormality found in children with obesity [26].
Either a fasting lipid profile or a non-fasting lipid profile, if fasting is not
feasible, can assess dyslipidemia. Liver function tests, specifically alanine
aminotransferase (ALT) and aspartate aminotransferase (AST), can
screen for non-alcoholic fatty liver disease. Many children are at risk for
vitamin D deficiency, which is detected with a 25-hydroxy (25 OH)
vitamin D test [27]. Blood pressure is checked if the child is older than
three years of age. Other studies are indicated based on history: a sleep
study for any history of snoring, daytime sleepiness, disrupted sleep cycle
or even hyperactivity may be indicated. Liver imaging can be done if the
liver function tests (ALT and AST) are high. Uric acid can be obtained in
those children with diabetes or prediabetes. Uric acid can be elevated
with high intake of high fructose corn syrup and even table sugar. Fig. 5
shows recommended diagnostics based on age ranges, BMIs, and risk
factors [ 19,22–24].
7. Special populations
Turner syndrome (i.e., absence or dysfunction of an X chromosome),
achondroplasia (i.e., mutation of fibroblast growth factor receptor 3
Fig. 2. Body Mass Index Charts for Children and Adolescents Ages 2–20 Years with Severe Obesity. BMI vs. age is shown for children ages 2–20 years.
Table 1
Top 10 Takeaway Messages: Assessment of the Child with Obesity. Shown
are the top 10 messages from the OMA regarding weight assessment of children
[1–8].
1. For children less than two years of age, weight for length percentile is used to
assess weight status.
2. For children 2–20 years of age, BMI is used to assess weight status.
3. Overweight is de fined as a BMI percentile between the 85th percentile and the
94th percentile.
4. Obese is defined as a BMI percentile between the 95th percentile and 119% of
the 95th percentile.
5. Severely obese de fined as is a BMI percentile greater than or equal to 120% of
the 95th percentile.
6. Class 1 obesity is defined as a BMI percentile between the 95th percentile and
119% of the 95th percentile.
7. Class 2 obesity is defined as a BMI percentile that is 120–139% of the 95th
percentile.
8. Class 3 obesity is defined as a BMI percentile greater than or equal to 140% of
the 95th percentile.
9. BMI z-scores should not be used to assess BMI change among children and
adolescents with BMI greater than 120% of the 95th percentile.
10. A BMI of 35 kg/m
2
is used for adolescent boys at approximately 18 years of age
and adolescent girls at approximately 16 years of age as the threshold for
severely obese and is somewhat lower than 120% of the 95th percentile.
Fig. 3. Childhood Obesity: Differential Diagnosis. Considerations and associations are shown regarding linear growth, developmental delays, and early onset
obesity in children. Abbreviations: TSH: thyroid-stimulating hormone; T4: thyroxine.
S.E. Cuda, M. Censani Obesity Pillars 1 (2022) 100010
3
gene), and Down syndrome (i.e., trisomy 21 or three copies of chromo-
some 21) are commonly seen populations of children at risk for obesity.
These populations require additional considerations regarding health
impacts and screening tools for obesity.
Children with Turner syndrome have restricted height growth (i.e.,
short stature) and increased adiposity, with an increase in total and
visceral fat mass and a decrease in lean body mass. In addition, 70% of
girls with Turner syndrome have abnormal glucose metabolism leading
to an increased prevalence of both Type 1 and Type 2 diabetes [30,31].
Children with Down syndrome typically have short stature and have
shorter limbs and poor coordination, leading to less activity and a pro-
pensity to gain weight. Growth charts for height and weight are used to
follow children with Down Syndrome, but the American Academy of
Pediatrics (AAP) recommends that clinicians use BMI guidelines from the
CDC for normally developing children to classify BMI status [32–34].
Children with achondroplasia, the most common form of inherited
disproportionate short stature, have no pubertal growth spurt and a
relatively flat growth curve for height from infancy on. They do not have
adiposity rebound and are at high risk for early cardiovascular disease,
obstructive sleep apnea, and difficulty with mobility [35,36]. Fig. 6
shows a summary of considerations for children with Turner syndrome,
Down syndrome, and achondroplasia. Table 2 shows the top eight
takeaway messages from the OMA regarding differential diagnoses and
diagnostic workups for special populations.
8. Conclusions
This Clinical Practice Statement on the assessment, differential
diagnosis, and initial clinical evaluation of pediatric patients with obesity
provides clinicians with assessment tools and recommendations
regarding their pediatric patients. Assessment of pediatric patients with
obesity is the first step in determining treatments that may lead to im-
provements in the health and wellbeing of children and adolescents with
obesity, especially those with metabolic, physiological, and psychologi-
cal complications.
8.1. Writing Process and ethics statement [38]
8.1.1. Transparency
This manuscript was largely derived and edited from the 2020–2022
Fig. 4. Focused Review of Systems. Symptoms and associated co-morbidities for children with obesity are shown. Children may or may not complain of symptoms,
so a careful assessment of symptoms associated with obesity is necessary.
Fig. 5. Diagnostic Workup: Labs and Studies. Shown are recommended diagnostics based on age ranges, BMIs, and risk factors for pediatric patients [28,29].
Abbreviations: BMI: body mass index; HbA1c: hemoglobin A1c; ALT: alanine aminotransferase; AST: aspartate aminotransferase; GGT: gamma-glutamyl transferase;
25 OH: 25-hydroxy; hs-CRP: high-sensitivity C-reactive protein.
S.E. Cuda, M. Censani Obesity Pillars 1 (2022) 100010
4

Obesity Medicine Association (OMA) Pediatric Obesity Algorithm.
Beginning in 2016, the OMA created and maintained an online Pediatric
“Obesity Algorithm” (i.e., educational slides and eBook) that underwent
updates approximately every two years by OMA authors and was
reviewed and approved annually by the OMA Board of Trustees. Authors
of prior years’ versions are included in Supplement #1. This manuscript
is the first published version of the applicable chapter/s of the
2020–2022 OMA Pediatric Obesity Algorithm.
8.1.2. Group composition
Over the years, the authors of the OMA Pediatric Obesity Algorithm
have represented a diverse range of clinicians, allied health professionals,
clinical researchers, and academicians. (Supplement #1) The authors
reflect a multidisciplinary and balanced group of experts in obesity sci-
ence, patient evaluation, and clinical treatment.
8.1.3. Author contributions
SEC transcribed the first draft from the 2020–2022 OMA Pediatric
Obesity Algorithm. MC and SEC then reviewed, edited, and approved the
document for pre-peer review submission and post-peer review
publication.
8.1.4. Disclosures (declaration of potential competing interest)
Potential dualities or conflicts of interest of the authors are listed in
the Individual Disclosure section. Assistance of a medical writer paid by
the Obesity Medicine Association is noted in the Acknowledgements
section. Neither the prior OMA Pediatric Algorithms nor the publishing
of this Clinical Practice Statement received outside funding. The authors
of prior OMA Pediatric Obesity Algorithms never received payment for
their writing, editing, and publishing work. Authors of this Clinical
Practice Statement likewise received no payment for their writing,
editing, and publishing work. While listed journal Editors received pay-
ment for their roles as Editors, they did not receive payment for their
participation as authors.
8.1.5. Individual Disclosures
SEC declares a relationship with Novo Nordisk as a member of an
Advisory Board and a relationship with Rhythm Pharmaceuticals as a
member of their Gold Panel.
MC reports no disclosures pertaining to this project.
8.1.6. Evidence
The content of the OMA Pediatric Obesity Algorithm and this
manuscript is supported by citations, which are listed in the References
section.
8.1.7. Ethics review
After approval by the authors, a draft manuscript was peer-reviewed
and approved by the OMA Board of Trustees prior to publication. This
submission did not involve human test subjects or volunteers.
8.1.7.1. Conclusions and recommendations. This Clinical Practice State-
ment is intended to be an educational tool that incorporates the current
medical science and the clinical experiences of obesity specialists. The
intent is to better facilitate and improve the clinical care and manage-
ment of patients with pre-obesity and obesity. This Clinical Practice
Statement should not be interpreted as “rules” and/or directives
regarding the medical care of an individual patient. The decision
regarding the optimal care of the patient with overweight and obesity is
best reliant upon a patient-centered approach, managed by the clinician
tasked with directing an individual treatment plan that is in the best
interest of the individual patient.
8.1.7.2. Updating. It is anticipated that sections of this Clinical Practice
Statement may require future updates. The timing of such an update will
depend on decisions made by Obesity Pillars Editorial team, with input
from the OMA members and OMA Board of Trustees.
8.1.7.3. Disclaimer and limitations. Both the OMA Obesity Algorithms
and this Clinical Practice Statement were developed to assist health care
professionals in providing care for patients with pre-obesity and obesity
Fig. 6. Special Populations: Turner Syndrome, Down Syndrome, and Achondroplasia. Children with Turner syndrome, Down syndrome, and achondroplasia
require special considerations regarding health risks and obesity. Takeaways for each condition are shown [30–37].
Table 2
Top Eight Takeaway Messages: Differential Diagnoses and Diagnostic
Workups for Special Populations. The top eight messages from the OMA
regarding differential diagnosis, diagnostic workups, and special populations are
shown [30–37].
1. Although most children with obesity have exogenous obesity of nutritional
origin, endocrinopathies and syndromal obesity must be considered.
2. Not all children with syndromal obesity have developmental delays and/or a
decrease in linear growth, but, if either is present, consider syndromal obesity.
3. Children with severe early-onset obesity and hyperphagia are at increased risk
for genetic etiologies for obesity.
4. The review of systems and initial work up is determined by the age at
presentation and degree of obesity of the child. Glycemic dysregulation,
dyslipidemia, and hepatic function are generally assessed.
5. Other testing is directed by presenting symptoms/physical findings.
6. Turner syndrome is a special population at high risk for abnormal glucose
metabolism.
7. Children with Down syndrome are at high risk for obesity. BMI is assessed using
the CDC charts for normally developing children; height and weight are followed
using specific charts for Down syndrome.
8. Achondroplasia is a form of inherited disproportionate short stature in which
there is no pubertal growth spurt, no typical “J” shaped BMI curve, and high rates
of obesity. Specific height, weight, and BMI curves should be used.
S.E. Cuda, M. Censani Obesity Pillars 1 (2022) 100010
5
based upon the best available evidence. In areas regarding inconclusive
or insufficient scientific evidence, the authors used their professional
judgment. This Clinical Practice Statement is intended to represent the
state of obesity medicine at the time of publication. Thus, this Clinical
Practice Statement is not a substitute for maintaining awareness of
emerging new science. Finally, decisions by practitioners to apply the
principles in this Clinical Practice Statement are best made by consid-
ering local resources, individual patient circumstances, patient agree-
ment, and knowledge of federal, state, and local laws and guidance.
Declaration of competing interest
The authors declare the following financial interests/personal re-
lationships which may be considered as potential competing interests:
Suzanne Elizabeth Cuda reports financial support and travel were pro-
vided by Novo Nordisk Inc. Suzanne Elizabeth Cuda reports a relation-
ship with Novo Nordisk Inc that includes: consulting or advisory and
travel reimbursement.
Acknowledgements
Medical writing support (funded by the Obesity Medicine Associa-
tion) was provided by Savannah Logan, who helped implement author
revisions while adhering to Good Publication Practice (GPP3) guidelines
and International Committee of Medical Journal Editors (ICMJE) rec-
ommendations. Otherwise, this manuscript received no funding.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https
://doi.org/10.1016/j.obpill.2022.100010.
References
[1] Grummer-Strawn LM, Reinold C, Krebs NF, Centers for Disease C Prevention. Use of
World Health Organization and CDC growth charts for children aged 0-59 months
in the United States. MMWR Recomm Rep (Morb Mortal Wkly Rep) 2010;59:1–15.
[2] WHO growth standards are recommended for use in the U.S. For infants and
children 0 to 2 Years of age. https://www.cdc.gov/growthcharts/who_charts.htm.
[Accessed 8 November 2021].
[3] Ogden CL, Kuczmarski RJ, Flegal KM, Mei Z, Guo S, Wei R, et al. Centers for disease
Control and prevention 2000 growth charts for the United States: improvements to
the 1977 national center for health statistics version. Pediatrics 2002;109:45–60.
[4] Gulati AK, Kaplan DW, Daniels SR. Clinical tracking of severely obese children: a
new growth chart. Pediatrics 2012;130:1136–40.
[5] Kumar S, Kelly AS. Review of childhood obesity: from epidemiology, etiology, and
comorbidities to clinical assessment and treatment. Mayo Clin Proc 2017;92:
251–65.
[6] Body mass index-for-age percentiles. http://www.health.ny.gov/publications/
4949.pdf. [Accessed 8 November 2021].
[7] Freedman DS, Berenson GS. Tracking of BMI z scores for severe obesity. Pediatrics
2017;140.
[8] Freedman DS, Butte NF, Taveras EM, Goodman AB, Ogden CL, Blanck HM. The
limitations of transforming very high body mass indexes into z-scores among 8.7
million 2- to 4-year-old children. J Pediatr 2017;188. 50-6.e1.
[9] Must A, Anderson SE. Body mass index in children and adolescents: considerations
for population-based applications. Int J Obes 2006;30:590–4.
[10] Dietz WH. Time to adopt new measures of severe obesity in children and
adolescents. Pediatrics 2017:140.
[11] Kyle TK, Dhurandhar EJ, Allison DB. Regarding obesity as a disease: evolving
policies and their implications. Endocrinol Metab Clin N Am 2016;45:511–20.
[12] Katzmarzyk PT, Barlow S, Bouchard C, Catalano PM, Hsia DS, Inge TH, et al. An
evolving scientific basis for the prevention and treatment of pediatric obesity. Int J
Obes 2014;38:887–905.
[13] Bays HE. Sick fat," metabolic disease, and atherosclerosis. Am J Med 2009;122:
S26–37.
[14] Bays HE, Gonz
alez-Campoy JM, Henry RR, Bergman DA, Kitabchi AE, Schorr AB,
et al. Is adiposopathy (sick fat) an endocrine disease? Int J Clin Pract 2008;62:
1474–83.
[15] Balasundaram P, Krishna S. Obesity effects on child health. Treasure Island (FL):
StatPearls; 2021.
[16] Pont SJ, Puhl R, Cook SR, Slusser W, Section On O, Obesity S. Stigma experienced
by children and adolescents with obesity. Pediatrics 2017;140.
[17] Shalitin S, Kiess W. Putative effects of obesity on linear growth and puberty. Horm
Res Paediatr 2017;88:101–10.
[18] Li W, Liu Q, Deng X, Chen Y, Liu S, Story M. Association between obesity and
puberty timing: a systematic review and meta-analysis. Int J Environ Res Publ
Health 2017;14.
[19] Barlow SE. Expert committee recommendations regarding the prevention,
assessment, and treatment of child and adolescent overweight and obesity:
summary report. Pediatrics 2007;120(Suppl 4):S164–92.
[20] Styne DM, Arslanian SA, Connor EL, Farooqi IS, Murad MH, Silverstein JH, et al.
Pediatric obesity-assessment, treatment, and prevention: an endocrine society
clinical practice guideline. J Clin Endocrinol Metab 2017;102:709–57.
[21] Farooqi IS. Genetic and hereditary aspects of childhood obesity. Best Pract Res Clin
Endocrinol Metabol 2005;19:359–74.
[22] Davis MM, Gance-Cleveland B, Hassink S, Johnson R, Paradis G, Resnicow K.
Recommendations for prevention of childhood obesity. Pediatrics 2007;120(Suppl
4):S229–53.
[23] Krebs NF, Himes JH, Jacobson D, Nicklas TA, Guilday P, Styne D. Assessment of
child and adolescent overweight and obesity. Pediatrics 2007;120(Suppl 4):
S193–228.
[24] Spear BA, Barlow SE, Ervin C, Ludwig DS, Saelens BE, Schetzina KE, et al.
Recommendations for treatment of child and adolescent overweight and obesity.
Pediatrics 2007;120(Suppl 4):S254–88.
[25] Sinha R, Fisch G, Teague B, Tamborlane WV, Banyas B, Allen K, et al. Prevalence of
impaired glucose tolerance among children and adolescents with marked obesity.
N Engl J Med 2002;346:802–10.
[26] Cook S, Kavey RE. Dyslipidemia and pediatric obesity. Pediatr Clin 2011;58:
1363–73. ix.
[27] Peterson CA, Belenchia AM. Vitamin D deficiency & childhood obesity: a tale of two
epidemics. Mo Med 2014;111:49–53.
[28] Agirbasli M, Tanrikulu A, Acar Sevim B, Azizy M, Bekiroglu N. Total cholesterol-to-
high-density lipoprotein cholesterol ratio predicts high-sensitivity C-reactive
protein levels in Turkish children. J Clin Lipidol 2015;9:195–200 .
[29] Sakuno T, Tomita LM, Tomita CM, Giuliano Ide C, Ibagy A, Perin NM, et al.
Sonographic evaluation of visceral and subcutaneous fat in obese children. Radiol
Bras 2014;47:149–53.
[30] Sun L, Wang Y, Zhou T, Zhao X, Wang Y, Wang G, et al. Glucose metabolism in
turner syndrome. Front Endocrinol 2019;10:49.
[31] Davis SM, Geffner ME. Cardiometabolic health in Turner syndrome. Am J Med
Genet C Semin Med Genet 2019;181:52–8.
[32] Hatch-Stein JA, Zemel BS, Prasad D, Kalkwarf HJ, Pipan M, Magge SN, et al. Body
composition and BMI growth charts in children with Down syndrome. Pediatrics
2016;138.
[33] M OS, C OS, Gibson L, Leo J, Carty C. The prevalence of obesity in children and
young people with Down syndrome. J Appl Res Intellect Disabil 2018;31:1225–9.
[34]
Basil JS, Santoro SL, Martin LJ, Healy KW, Chini BA, Saal HM. Retrospective study
of obesity in children with Down syndrome. J Pediatr 2016;173:143–8.
[35] Saint-Laurent C, Garcia S, Sarrazy V, Dumas K, Authier F, Sore S, et al. Early
postnatal soluble FGFR3 therapy prevents the atypical development of obesity in
achondroplasia. PLoS One 2018;13:e0195876.
[36] Saint-Laurent C, Garde-Etayo L, Gouze E. Obesity in achondroplasia patients: from
evidence to medical monitoring. Orphanet J Rare Dis 2019;14:253.
[37] Bertapelli F, Machado MR, Roso RD, Guerra-Júnior G. Body mass index reference
charts for individuals with Down syndrome aged 2-18 years. J Pediatr 2017;93:
94–9.
[38] Institute of Medicine (U.S.). Committee on standards for developing trustworthy
clinical practice guidelines., Graham R. Clinical practice guidelines we can trust.
Washington, DC: National Academies Press; 2011.
S.E. Cuda, M. Censani Obesity Pillars 1 (2022) 100010
6
