
Immunology,
1966,
11,
499.
In
vitro
Studies
of
Cell-Bound
Immunity;
Cloning
Assay
of
the
Cytotoxic
Action
of
Sensitized
Lymphoid
Cells
on
Allogeneic
Target
Cells
K.
T.
BRUNNER,
J.
MAUEL
AND
R.
SCHINDLER
Swiss
Institute
for
Experimental
Cancer
Research,
Lausanne,
Switzerland
(Received
20th
April
1966)
Summary.
The
in
vitro
reaction
between
sensitized
lymphoid
cells
and
target
cells
has
been
studied
in
an
allogeneic
transplantation
system
in
mice.
Mastocytoma
cells
of
the
DBA/2
donor
strain
were
injected
into
C57BL
mice,
and
the
spleens
of
the
recipients
harvested
8
days
later.
The
immune
lymphoid
cells
thus
obtained
were
tested
for
their
ability
to
damage
in
vitro
cultures
of
target
mastocytoma
cells.
The
reaction
was
followed
by
two
methods:
(1)
microscopic
counts,
and
(2)
ability
of
target
cells
to
form
colonies
in
a
semi-solid
medium
after
various
periods
of
contact
with
immune
lymphoid
cells.
The
results
show:
(a)
that
the
effect
on
target
cells
is
an
exponential
function
of
the
number
of
immune
lymphoid
cells
employed,
and
(b)
that
the
reaction
becomes
noticeable
after
3
hours
and
reaches
completion
within
12
hours
when
evaluated
by
cloning
techniques,
while
it
takes
24-
48
hours
to
be
microscopically
clearly
demonstrable.
Problems
arising
from
use
of
both
methods
are
discussed.
INTRODUCTION
Immune
lymphocytes
are
thought
to
be
the
most
important
mediators
of
homograft
rejection,
and
have
been
shown
to
be
effective
in
the
passive
transfer
of
tumour
immunity.
The
actual
mechanism
by
which
immune
lymphocytes
attack
target
cells
is
but
little
understood,
especially
in
its
relationship
to
the
cytotoxic
and
enhancing
effect
of
humoral
antibody.
A
number
of
authors
(Rosenau
and
Moon,
1961;
Wilson,
1963,
1965;
Taylor
and
Culling,
1963;
Brondz,
1964)
have
described
in
vitro
systems
allowing
the
analysis
of
immune
lymphocyte-target
cell
interaction
in
the
absence
of
antibody
and
complement,
but
most
of
these
systems
lack
sensitivity
and
suggest
a
slow
inactivation
process
basically
different
from
antigen-antibody
reactions.
A
notable
exception
is
the
system
described
by
Friedman
(1964)
which
measures
the
inhibition
of
antibody
plaque
formation
by
sensitized
lymphoid
cells.
We
have
developed
an
assay
system
for
the
immune
lymphoid
cell
interaction
with
allogeneic
target
cells
which
appears
sensitive
and
has
allowed
the
analysis
of
some
of
the
mechanisms
involved
(Brunner,
Mauel
and
Schindler,
1966).
It
measures
the
effect
of
immune
lymphoid
cells
on
the
colony
forming
potential
of
the
target
cells.
Based
on
this
system,
the
kinetics
of
the
inactivation
of
target
cells
by
immune
lymphoid
cells
and
the
dose-effect
relationship
have
been
studied.
It
will
be
shown
that
the
number
of
colony
forming
target
cells
decreases
exponentially
with
increasing
numbers
of
immune
lymphoid
cells,
and
that
inactivation
takes
place
within
6-12
hours.
499

K.
T.
Brunner,
J.
Mauel
and
R.
Schindler
MATERIALS
AND
METHODS
Animals
and
tumour
A
homotransplantation
system
was
chosen
consisting
of
donor
cells
in
the
form
of
a
DBA/2
mastocytoma
(Dunn
and
Potter,
1957)
readily
transplantable
in
ascitic
form
in
inbred
DBA/2
mice,
and
of
C57BL
recipients
differing
in
major
histocompatibility
loci.
The
mastocytoma
cells,
originally
induced
with
methylcholanthrene,
can
be
grown
in
suspension
cultures,
and
cloned
with
an
efficiency
reaching
100
per
cent
(Schindler,
1963).
Donor
cells
DBA/2
mastocytoma
cells
maintained
in
ascitic
form
by
serial
transplantation
in
DBA/2
mice
were
harvested
by
aspiration.
The
ascitic
fluid
was
mixed
with
several
volumes
of
Eagle's
medium
without
serum
containing
5
units
of
heparin
per
ml.
The
cells
were
sedimented
at
1000
rev/min
for
5
minutes,
re-suspended
in
Eagle's
medium
without
serum,
counted
in
a
haemocytometer,
and
the
suspension
adjusted
to
30x
106
cells/ml.
Sensitization
of
mice
Female
C57BL
mice
were
sensitized
by
one
injection
of
30x
106
DBA/2
mastocytoma
ascites
cells
half
i.p.
and
half
i.v.
Spleen
cells
of
the
recipient
mice
were
harvested
8
days
later.
Spleen
cell
suspensions
Recipient
and
untreated
control
mice
were
anaesthetized
with
ether,
bled
from
the
retro-orbital
venous
plexus
to
obtain
serum,
and
killed
by
fracture
of
the
cervical
spine.
Using
aseptic
techniques
throughout,
the
spleens
were
removed,
cut
into
a
few
fragments,
and
homogenized
by
hand
in
a
glass
Ten-Broek
grinder
with
Eagle's
medium
added.
The
suspension
was
centrifuged
at
1500
rev/min
for
5
minutes,
the
sediment
re-suspended
in
the
same
medium,
and
left
standing
for
2
hours
at
4°.
The
sediment
was
then
discarded,
the
cells
in
the
supernatant
fluid
washed
1-2
times
with
Eagle's
medium,
and
finally
resuspended
in
an
assay
medium
described
below.
The
number
of
viable
cells
was
then
determined
by
haemocytometer
counts
using
the
Trypan-blue
exclusion
test,
and
the
suspension
was
adjusted
to
20x
106
viable
cells/ml.
The
number
of
viable
cells
usually
varied
between
80
and
95
per
cent
of
the
total
cell
population.
Spleen
cells
from
im-
munized
mice
will
be
described
as
'immune'
or
'sensitized'
spleen
or
lymphoid
cells.
Target
cells
DBA/2
mastocytoma
cells
maintained
in
suspension
cultures
were
used
as
target
cells.
The
cells,
growing
in
a
semi-synthetic
medium*
(Schindler,
1963)
supplemented
with
10
per
cent
horse
serum,
were
centrifuged
at
600
rev/min
for
2
minutes,
resuspended
in
the
assay
medium
described
below,
and
adjusted
to
2
x
I05
cells/ml.
Assay
medium
For
the
in
vitro
reactions,
an
assay
medium
was
prepared
consisting
of
the
semi-synthetic
growth
medium
for
mastocytoma
cells
in
which
the
horse
serum
was
replaced
either
by
bovine
serum
albumin
(BSA)
at
a
final
concentration
of
0-38
per
cent
and
'Fraction
Nlv't
from
human
plasma
at
a
final
concentration
of
0-001
per
cent
alone
or
together
with
*
Since
this
manuscript
was
prepared,
numerous
experiments
have
shown
that
Dulbecco's
modification
of
Eagle's
medium
[Vogt,
M.
and
Dulbecco,
R.
(1963).
Proc.
nat.
Acad.
Sci.
(Wash.),
49,
171]
supplemented
with
10
per
cent
commercial
calf
serum
(Microbiological
Associates,
Bethesda,
Maryland)
makes
an
exellent
growth,
assay
and
cloning
medium.
It
eliminates
the
reduced
cloning
efficiency
observed
in
the
control
target
cell
populations
(see
'Results',
p.
504),
and
is
now
used
exclusively.
t
Fraction
NIv,
from
the
Swiss
Red
Cross
Transfusion
Service,
Bern,
is
almost
identical
with
Cohn's
Fraction
IV
(Kistler
and
Nitschmann,
1962).
It
contains
mainly
a-
and
fl-globulins.
500

Cytotoxic
Action
of
Lymphoid
Cells
501
10
per
cent
heated
calf
serum.
BSA
and
Fraction
NIV
have been
shown
(Schaer
and
Schind-
ler,
1966)
to
replace
serum
in
mastocytoma
cultures.
Calf
serum
was
found
not
to
inhibit
the
immune
lymphoid
cell
reaction
described
below.
It
was
added
to
the
medium
in
most
of
the
experiments
since
it
supported
mastocytoma
cell
reproduction
up
to
higher
cell
densities
than
the
two
serum
protein
fractions
alone.
Technique
of
the
test
A
volume
of
target
cell
suspension
containing
2
x
105
cells/ml
was
added
to
an
equal
volume
of
the
spleen
cell
suspension
containing
20x
106
cells/ml.
One
millilitre
of
the
reaction
mixtures
thus
obtained
was
placed
in
each
of
several
Leighton
tubes
and
the
cells
were
incubated
at
370
for
the
specified
time
period.
When
calf
serum
had
been
added
to
the
assay
medium,
the
mastocytoma
cells
showed
a
tendency
to
attach
to
the
glass.
For
the
microscopic
counts
or
cloning
assays,
the
target
cells
were
harvested
by
flushing
the
tubes
and
homogenizing
the
cell
suspension
with
a
pipette,
or
by
scraping
the
tubes
with
a
small
silicone
rubber
'policeman'
if
necessary.
The
contents
of
two
tubes
per
reaction
mixture
were
pooled
and
the
number
of
surviving
target
cells
determined.
Cloning
assays
and
microscopic
counts
of
target
cells
The
cloning
assays
were
performed
following
a
technique
described
in
detail
elsewhere
(Schindler,
1964).
The
reaction
mixtures
were
diluted
appropriately
with
'cloning
medium'.
Volumes
of
12
ml
of
the
diluted
suspensions
were
mixed
with
3
ml
of
a
0-5
per
cent
fibrinogen
solution,
placed
in
15
ml
screw
cap
tubes,
and
the
gels
thus
obtained
incubated
at
370.
Colonies
developing
from
surviving
mastocytoma
cells
were
counted
5-10
days
later.
For
microscopic
counts,
the
cell
suspensions
were
mixed
with
equal
volumes
of
a
0'05
per
cent
trypan
blue
solution,
and
the
unstained
mastocytoma
cells
counted
in
a
haemocytometer.
A
small
proportion
of
the
spleen
cells
proved
to
be
similar
in
appearance
to
the
usually
larger
and
refractile
mastocytoma
cells.
Since
all
reaction
mixtures
contained
equal
numbers
of
spleen
cells,
the
error
thus
introduced
was
not
considered
to
be
significant.
RESULTS
EFFECT
OF
INCREASING
NUMBERS
OF
IMMUNE
LYMPHOID
CELLS
A
first
series
of
experiments
was
designed
to
analyse
the
dose-effect
relationship
of
the
immune
lymphoid
cell-target
cell
interaction.
Increasing
numbers
of
'immune
lymphoid
cells'
(as
defined
in
'Methods')
were
mixed
with
decreasing
numbers
of
normal
lymphoid
cells,
the
final
mixture
in
each
case
containing
a
total
of
20x
106
viable
spleen
cells.
To
each
of
these
suspensions,
an
equal
volume
of
target
cell
suspension
containing
2
x
105
cells/
ml
was
added,
and
the
reaction
mixtures
incubated.
Cloning
assays
were
performed
after
24
hours,
and
microscopic
counts
made
after
24
and
48
hours.
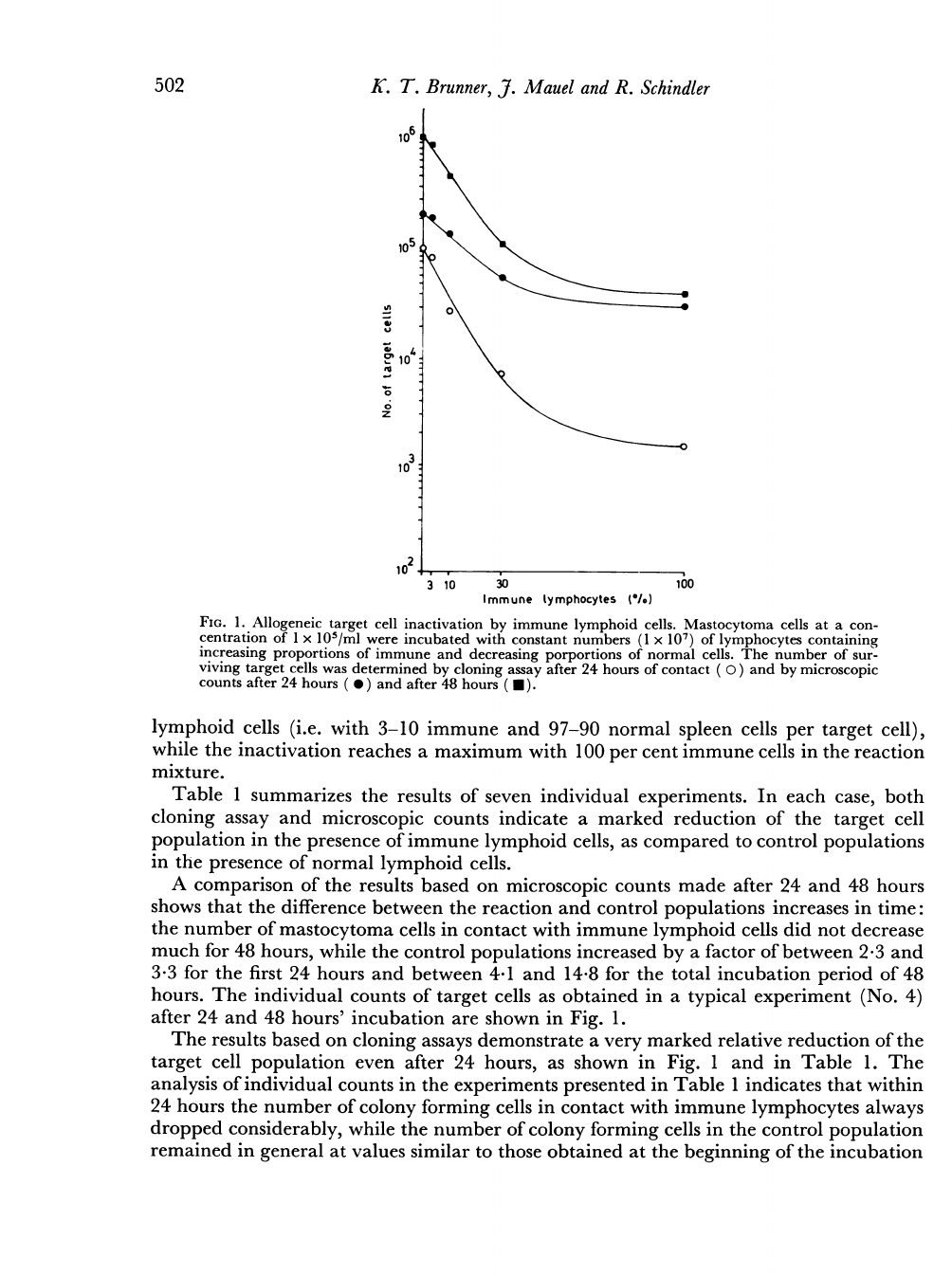
Fig.
1
shows
the
typical
inactivation
curves
obtained
in
a
representative
experiment.
The
logarithms
of
the
number
of
surviving
target
cells
as
determined
either
by
micro-
scopic
counts
or
by
cloning
assay
were
plotted
against
the
relative
number
of
immune
lymphoid
cells.
The
upper
portion
of
the
curves
approaches
a
straight
line,
indicating
an
exponential
decrease
in
number
of
surviving
target
cells
with
increasing
numbers
of
immune
cells.
A
cytotoxic
effect
can
thus
be
demonstrated
with
3-10
per
cent
immune
K.
T.
Brunner,
J.
Mauel
and
R.
Schindler
156
_ok
0
10
Z
\
3
10
102
3
10
30
100
Immune
lymphocytes
11/o)
FIG.
1.
Allogeneic
target
cell
inactivation
by
immune
lymphoid
cells.
Mastocytoma
cells
at
a
con-
centration
of
1
x
105/ml
were
incubated
with
constant
numbers
(1
x
10)
of
lymphocytes
containing
increasing
proportions
of
immune
and
decreasing
porportions
of
normal
cells.
The
number
of
sur-
viving
target
cells
was
determined
by
cloning
assay
after
24
hours
of
contact
(
0)
and
by
microscopic
counts
after
24
hours
(
0)
and
after
48
hours
(
U).
lymphoid
cells
(i.e.
with
3-10
immune
and
97-90
normal
spleen
cells
per
target
cell),
while
the
inactivation
reaches
a
maximum
with
100
per
cent
immune
cells
in
the
reaction
mixture.
Table
1
summarizes
the
results
of
seven
individual
experiments.
In
each
case,
both
cloning
assay
and
microscopic
counts
indicate
a
marked
reduction
of
the
target
cell
population
in
the
presence
of
immune
lymphoid
cells,
as
compared
to
control
populations
in
the
presence
of
normal
lymphoid
cells.
A
comparison
of
the
results
based
on
microscopic
counts
made
after
24
and
48
hours
shows
that
the
difference
between
the
reaction
and
control
populations
increases
in
time:
the
number
of
mastocytoma
cells
in
contact
with
immune
lymphoid
cells
did
not
decrease
much
for
48
hours,
while
the
control
populations
increased
by
a
factor
of
between
2-3
and
3-3
for
the
first
24
hours
and
between
4*1
and
14-8
for
the
total
incubation
period
of
48
hours.
The
individual
counts
of
target
cells
as
obtained
in
a
typical
experiment
(No.
4)
after
24
and
48
hours'
incubation
are
shown
in
Fig.
1.
The
results
based
on
cloning
assays
demonstrate
a
very
marked
relative
reduction
of
the
target
cell
population
even
after
24
hours,
as
shown
in
Fig.
1
and
in
Table
1.
The
analysis
of
individual
counts
in
the
experiments
presented
in
Table
1
indicates
that
within
24
hours
the
number
of
colony
forming
cells
in
contact
with
immune
lymphocytes
always
dropped
considerably,
while
the
number
of
colony
forming
cells
in
the
control
population
remained
in
general
at
values
similar
to
those
obtained
at
the
beginning
of
the
incubation
502
503
Cytotoxic
Action
of
Lymphoid
Cells
TABLE
1
PERCENTAGE
OF
TARGET
CELL
REDUCTION
BY
IMMUNE
LYMPHOID
CELLS
Percentage
target
cell
reduction
*
in
the
presence
of
100
immune
lymphocytes
per
target
cell
as
judged
by:
No.
of
experiment
Cloning
assay
Microscopic
counts
after:
after
24
hours
24
hours
of
48
hours
of
of
incubation
incubation
incubation
1
94
72
91
2
76
48
67
3
99*4
76
4
99
86
96
5
99-2
88
6
87 66
74
7
99-3
69
89
*The
percentage
of
reduction
is
expressed
as:
100
-
No.
of
surviving
target
cells
in
the
presence
of
100
per
cent
immune
lymphocytes
X
100
No.
of
surviving
target
cells
in
the
presence
of
100
per
cent
normal
lymphocytes
10b:
z,
10
a#
:
z
0
3
10
1
3
6
12
24
Time
of
contact
(hours)
FIG.
2.
Relative
decrease
in
time
of
the
cloning
efficiency
of
target
cells
after
contact
with
immune
lymphoid
cells.
Mixtures
of
1
x
105
mastocytoma
cells
and
(a)
1
x
107
immune
lymphoid
cells
(
0)
or
(b)
1
x
107
normal
lymphoid
cells
(
0)
were
incubated
for
various
periods
of
time.
Each
point
rep-
resents
the
number
of
target
cells
still
able
to
form
clones.
period.
In
experiment
No.
4
(presented
in
Fig.
1),
the
number
of
colony
forming
cells
in
the
control
population
increased
from
88,000
to
106,000/ml
during
24
hours,
while
it
dropped
to
1460/ml
in
the
reaction
mixture.
The
differences
between
microscopic
counts
and
cloning
assays
indicate
that
some
of
the
microscopically
visible
cells
have
undergone
alterations
only
measurable
by
their
cloning

K.
T.
Brunner,
J.
Mauel
and
R.
Schindler
efficiency.
Since
such
differences
are
found
to
some
extent
also
in
the
control
populations,
it
appears
that
not only
an
interaction
with
immune
lymphoid
cells,
but
also
other
factors
such
as
possible
effects
of
normal
lymphoid
cells,
deficiencies
of
the
medium
used
or
manipulations
of
target
cells
in
the
course
of
the
experiments
have
led
to
decreased
cloning
efficiencies
without
corresponding
lysis
of
cells,
and
without
affecting
the
rate
of
cellular
multiplication
as
measured
by
microscopic
counts.
TIME
COURSE
OF
THE
INTERACTION
In
a
second
series
of
experiments,
the
response
of
the
target
cells
was
followed
in
time.
The
cloning
assay
was
chosen,
since
it
allows
a
critical
evaluation
of
cell
damage
as
expressed
by
loss
of
cloning
efficiency
after
short
periods
of
incubation
with
immune
lymphoid
cells.
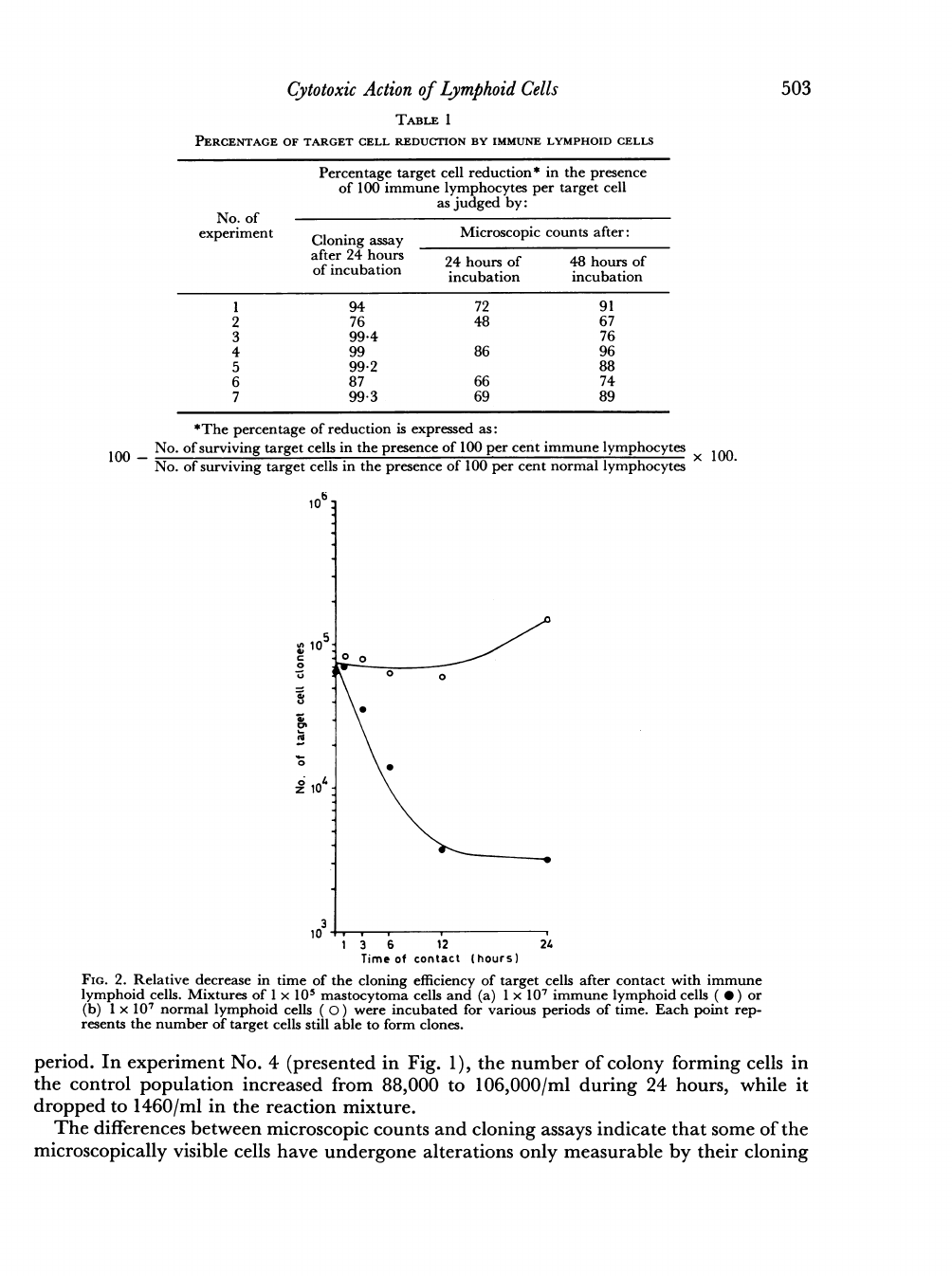
As
shown
in
Fig.
2,
there
was
a
rapid
reduction
of
the
clone
forming
potential
of
the
target
cells
exposed
to
immune
lymphoid
cells,
reaching
significant
proportions
after
3
hours
incubation.
The
inactivation
progressed,
and
arrived
at
a
maximum
after
12
hours,
leaving
a
markedly
reduced
surviving
cell
population.
In
three
of
five
experiments,
similar
rates
of
inactivation
were
observed,
with
a
76-97
per
cent
reduction
of
clone-forming
cells
in
6
hours
as
compared
to
the
initial
count,
and
reaching
a
maximum
in
12
hours
with
95-99f6
per
cent
inactivation.
In
two
of
the
five
experiments,
the
inactivation
was
somewhat
retarded,
leading
to
83
and
97
per
cent
reduction
in
24
hours.
DISCUSSION
The
interaction
between
sensitized
spleen
cells
and
homologous
target
cells
in
vitro
resulted
in
inhibition
of
target
cell
multiplication
as
determined
by
microscopic
counts,
and
in
a
rapid
decrease
of
cloning
efficiency.
The
immunological
mechanism
responsible
for
the
cytotoxi
ceffect
observed
is
apparently
based
on
a
direct
action
of
immune
lymphoid
cells,
and
is
not
mediated
by
cytotoxic
antibody.
This
conclusion
is
supported
by
the
observations
that
the
reaction
takes
place
in
the
absence
of
complement,
that
only
minimal
haemagglutination
titres
(1:
16
or
less)
were
found
in
the
serum
of
the
recipient
mice
at
the
time
of
lymphoid
cell
harvest
(8
days
after
allograft),
and
that
addition
of
a
specific
high-titre
cytotoxic
isoantiserum
to
control
reaction
mixtures
containing
normal
spleen
and
target
cells
had
no
inhibitory
effect
(Brunner
et
al.,
1966).
On
the
other
hand,
no
effort
was
made
to
differentiate
between
lymphocytes
and
antibody
producing
cells
in
the
spleen-cell
suspensions
used.
No
inhibitory
effect
of
normal
inactivated
calf
serum
on
the
reaction
was
noted.
The
reports
of
Brondz
(1964)
indicating
such
an
inhibition
in
an
in
vitro
assay
system
of
immune
lymphoid
cell
reaction
with
target
cells
grown
as
monolayers
were
confirmed
by
our
own
unpublished
observations
using
monolayer
cultures.
Morphological
observation
of
the
spleen
cell-mastocytoma
cell
interaction
showed
that
both
immune
and
normal
spleen
cells
aggregate
on
some
of
the
target
cells.
No
specific
'immune-adsorption'
as
described
by
several
authors
(Rosenau
and
Moon,
1961;
Koprowski
and
Fernandes,
1962;
Taylor
and
Culling,
1963;
Wilson,
1963)
was
noted.
Normal
spleen
cells,
as
well
as
spleen
cells
of
mice
injected
8
days
previously
with
com-
plete
Freund's
adjuvant,
never
showed
any
noticeable
cytotoxic
action,
but
stimulated
the
growth
rate
of
the
target
cells
if
added
to
the
assay
medium
not
containing
calf
serum.
504

Cytotoxic
Action
of
Lymphoid
Cells
505
The
findings
of
Wilson
(1965)
demonstrating
dose
dependence
of
target
cell
inactivation
using
similar
multiplicities
of
immune
lymphoid
cells
agree
with
our
results.
When
plotting
the
logarithm
of
the
percentage
of
surviving
target
cells
against
the
numbers
of
attacking
lymphoid
cells,
Wilson
obtained
a
straight
line.
This
suggested
a
single
hit
phenomenon,
with
one
lymphoid
cell
sufficient
to
destroy
or
detectably
damage
one
target
cell.
In
most
of
our
experiments,
the
corresponding
inactivation
curves
approached
a
straight
line
in
their
upper
portion,
but
then
deviated.
This
phenomenon
might
be
explained
by
a
resistant
fraction
of
the
target
cell
population,
but
results
obtained
by
Wilson
(1963)
make
this
hypothesis
unlikely.
On
the
other
hand,
unknown
factors
might
increasingly
inhibit
the
reaction,
as
for
instance
antigen
liberated
by
lysed
target
cells.
Wilson
(1965)
has
reported
evidence
suggesting
that
the
cytotoxic
action
of
immune
lymphoid
cells
is
preceded
by
a
latent
period
of
approximately
20
hours.
This
agrees
with
the
traditional
concept
that
the
reaction
is
slow,
and
therefore
basically
different
from
antigen-antibody
reactions.
On
the
other
hand,
the
observations
of
several
authors
(Taylor
and
Culling,
1963;
Friedman,
1964)
had
suggested
that
damage
to
target
cells
might
occur
within
relatively
short
periods
of
time.
Taylor
and
Culling
(1963)
detected
morphological
alterations
after
6
hours,
while
target
cell
counts
declined
after
24
hours.
In
addition,
they
concluded
that
owing
to
the
rapid
death
of
the
lymphoid
cells
(30
per
cent
viable
cells
after
12-16
hours),
the
injury
must
have
been
initiated
within
a
short
period
of
contact.
Most
of
the
studies
on
immune
lymphocyte
reactions
have
not
included
quanti-
tative
data
on
the
dynamics
of
target
cell
inactivation
during
the
first
hours
of
contact
with
immune
lymphocytes.
An
exception
is
the
work
of
Friedman
(1964)
showing
a
50-80
per
cent
reduction
of
antibody
plaque
forming
cells
within
8
hours.
Our
results
demonstrate
loss
of
cloning
efficiency
of
target
cells
within
similar
time
periods,
and
are
thus
in
full
agreement
with
those
observed
by
Friedman.
The
experimental
system
described
in
this
paper
is
shown
to
be
a
highly
sensitive
indicator
of
cell
bound
immunity.
The
growth-inhibiting
effect
of
immune
lymphoid
cells
on
the
multiplying
allogeneic
target
cells
in
suspension
cultures
can
be
evaluated
by
microscopic
counts
within
24-48
hours.
Quantitative
data
on
the
kinetics
of
target
cell
inactivation
are
provided
by
the
cloning
assay.
This
has
allowed
the
demonstration
that
target
cell
injury
begins
at
or
near
the
time
of
contact
with
immune
lymphoid
cells,
and
progresses
to
completion
within
6-12
hours.
ACKNOWLEDGMENTS
We
are
grateful
to
Professor
F.
W.
Fitch
and
Dr
J.
Ch.
Cerottini
for
their
help
and
for
their
critical
discussion
of
the
manuscript.
RREFER
ENlCES
BRONDZ,
B.
D.
(1964).
'Interaction
of
immune
lympho-
cytes
in
vitro
with
normal
and
neoplastic
tissue
cells.'
Folia
biol.
(Praha),
10,
164.
BRUNNER,
K.
T.,
MAUEL,
J.
and
SCHINDLER,
R.
(1966).
'In
vitro
studies
of
cell-bound
immunity;
Inhibitory
effect
of
isoantibody
on
in
vivo
sensitiza-
tion
and
on
the
in
vitro
cytotoxic
action
of
immune
lymphocytes.'
Nature
(Lond.),
(In
press).
DUNN,
T.
B.
and
POrrER,
M.
(1957).
'A
transplant-
able
mast-cell
neoplasm
in
the
mouse.'
J.
Cancer
Inst.,
18,
587.
FRIEDMAN,
H.
(1964).
'Inhibition
of
antibody
plaque
formation
by
sensitized
lymphoid
cells:
rapid
in-
dicator
of
transplantation
immunity.'
Science,
145,
607.
KISTLER,
P.
and
NITSCHMANN,
Hs.
(1962).
'Large
scale
production
of
human
plasma
fractions.'
Vox
Sang.
(Basel),
7,
414.
KOPROWSKI,
H.
and
FERNANDES,
M.
V.
(1962).
'Autosensitization
reaction
in
vitro.
Contactual
agglutination
of
sensitized
lymph
node
cells
in
brain
tissue
culture
accompanied
by
destruction
of
glial
elements.'_J.
exp.
Med.,
116,
467.

506
K.
T.
Brunner,
J.
Mauel
and
R.
Schindler
ROSENAU,
W.
and
MOON,
H.
D.
(196
1).
'Lysis
of
homologous
cells
by
sensitized
lymphocytes
in
tissue
culture.'
J.
nat.
Cancer
Inst.,
27,
471.
SCHAER,
J.
C.
and
SCHINDLER,
R.
(1966).
Unpublished
data.
SCHINDLER,
R.
(1963).
'Biochemical
studies
of
the
divi-
sion
cycle
of
mammalian
cells:
evidence
for
the
premitotic
period.'
Biochem.
Pharmacol.,
12,
533.
SCHINDLER,
R.
(1964).
'Quantitative
colonial
growth
of
mammalian
cells
in
fibrin
gels.'
Exp.
Cell
Res.,
34,
595.
TAYLOR,
H.
E.
and
CULLING,
C.
F.
A.
(1963).
'Cyto-
pathic
effect
in
vitro
of
sensitized
homologous
and
heterologous
spleen
cells
on
fibroblasts.'
Lab.
Invest.,
12,
884.
WILSON,
D.
B.
(1963).
'The
reaction
of
immuno-
logically
activated
lymphoid
cells
against
homo-
logous
target
tissue
cells
in
vitro.'
J.
cell.
Comp.
Physiol.,
62,
273.
WILSON,
D.
B.
(1965).
'Quantitative
studies
on
the
behaviour
of
sensitized
lymphocytes
in
vitro.
I.
Relationship
of
the
degree
of
destruction
of
homo-
logous
target
cells
to
the
number
of
lymphocytes
and
to
the
time
of
contact
in
culture
and
consideration
of
the
effect
of
isoimmune
serum.'J.
exp.
Med.,
122,
143.
