
XAB2 Dynamics during DNA Damage-Dependent Transcription Inhibition
Lise-Marie DONNIO
1,#
, Elena CERUTTI
1,#
, Charlène MAGNANI
1
, Damien NEUILLET
1
, Pierre-Olivier MARI
1
and Giuseppina GIGLIA-MARI
1,*
1 : Institut NeuroMyogène (INMG), CNRS UMR 5310, INSERM U1217, Université Claude Bernard Lyon 1,
8 avenue de Rockefeller, 69008 LYON
* To whom correspondence and request for materials should be addressed.
G G-M : PHONE : 04 26 68 82 63
EMAIL : ambra.mari@univ-lyon1.fr
# L.-M.D. and E.C. contributed equally to this work
AUTHOR CONTRIBUTIONS: P.-O.M. and G.G.-M. designed research; L.-M.D.; E.C.; D.N.; C.M. performed
research; P.-O.M., E.C., L.-M.D. and G.G-M. analyzed data; and L.-M.D., E.C. and G.G.-M. wrote the paper.
COMPETING INTEREST STATEMENT: The authors declare no conflict of interest.
CLASSIFICATION: biological sciences; Cell Biology
KEYWORDS: XAB2; CSA; CSB; pre-mRNA splicing; TC-NER; RNA polymerase 2; R-loops
.CC-BY 4.0 International licenseperpetuity. It is made available under a
preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in
The copyright holder for thisthis version posted December 24, 2021. ; https://doi.org/10.1101/2021.12.23.473962doi: bioRxiv preprint
ABSTRACT
1
Xeroderma Pigmentosum group A (XPA)-binding protein 2 (XAB2) is a multi-functional protein
2
that plays a critical role in distinct cellular processes including transcription, splicing, DNA repair and
3
mRNA export. In this study, we detailed XAB2 involvement during Nucleotide Excision Repair (NER), a
4
repair pathway that guarantees genome integrity against UV light-induced DNA damage and that
5
specifically removes transcription-blocking damage in a dedicated process known as Transcription-
6
Coupled repair (TC-NER). Here, we demonstrated that XAB2 is involved specifically and exclusively in TC-
7
NER reaction and solely for RNA Polymerase 2 transcribed genes. Surprisingly, contrary to all the other
8
NER proteins studied so far, XAB2 does not accumulate on the local UV-C damage but on the contrary is
9
remobilized after damage induction. This fast change in mobility is restored when DNA repair reactions
10
are completed. By scrutinizing from which cellular complex/partner/structure XAB2 is released, we have
11
identified that XAB2 is detached after DNA damage induction from the DNA:RNA hybrids, commonly
12
known as R-loops, and from the CSA and XPG protein and this release is thought to contribute to the
13
DNA damage recognition step during TC-NER. Importantly, we have disclosed a role for XAB2 in retaining
14
RNAP2 on its substrate.
15
.CC-BY 4.0 International licenseperpetuity. It is made available under a
preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in
The copyright holder for thisthis version posted December 24, 2021. ; https://doi.org/10.1101/2021.12.23.473962doi: bioRxiv preprint
2
INTRODUCTION
16
The DNA molecule, contained in the nucleus of our cells, forms the instruction manual for proper
17
cellular functioning. Unfortunately, the integrity of our DNA is continuously challenged by a variety of
18
endogenous and exogenous agents (e.g. ultraviolet light, cigarette smoke, environmental pollution,
19
oxidative damage, etc …). These DNA lesions interfere with DNA replication, transcription and cell cycle
20
progression and lead to mutation and cell death, which may cause cancer, inborn disease or aging
21
(Chatterjee & Walker, 2017).
22
To prevent the deleterious consequences of persisting DNA lesions, all organisms are equipped
23
with a network of efficient DNA repair systems. One of these systems is the Nucleotide Excision Repair
24
(NER) which removes helix-distorting DNA adducts caused by ultraviolet light (UV) such as Cyclo-
25
Pyrimidine Dimers (CPD) and 6-4 Photoproducts (6-4PP) (Giglia-Mari et al, 2011).
26
In mammals, the different steps of NER require about thirty different proteins that are recruited
27
one by one into the DNA damage, as demonstrated by the different studies of NER proteins kinetics
28
(Moné et al, 2004; van den Boom et al, 2004; Politi et al, 2005; Zotter et al, 2006; Rademakers et al,
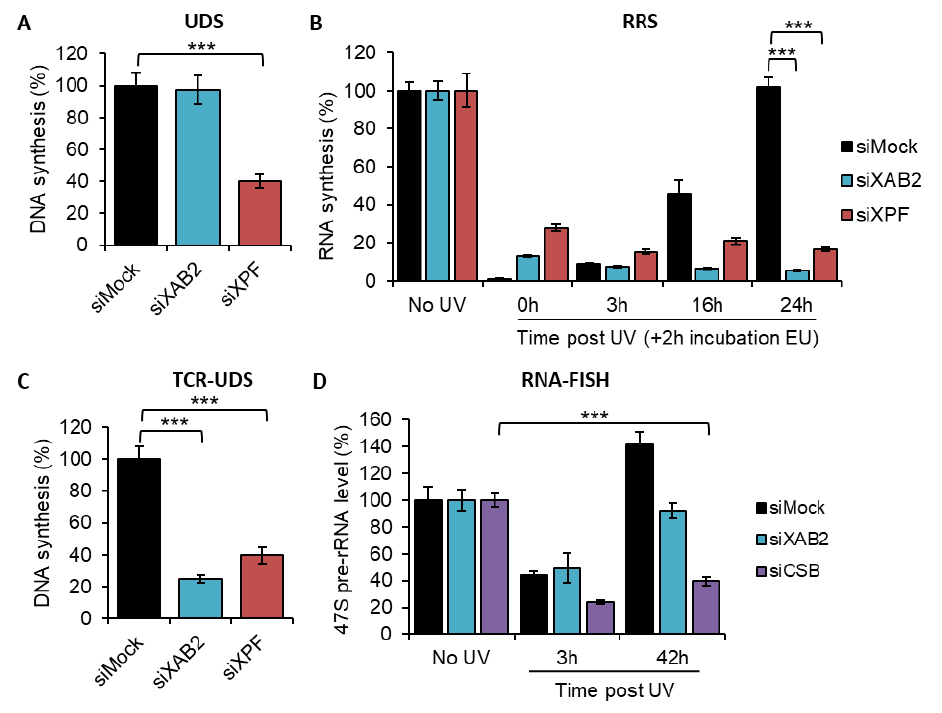
29
2003). The first step of NER consists of damage recognition, followed by the opening of the DNA duplex,
30
dual incisions on both sides of the damage, excision of 24–32 oligonucleotides containing the damage
31
and, finally, gap filling by repair DNA synthesis.
32
NER is divided into two sub-pathways depending on where DNA lesions are located within the
33
genome. The global genome repair (GG-NER) will detect and repair lesion throughout the genome,
34
whereas transcription-coupled repair (TC-NER) is associated with RNA polymerase II (RNAP2) to repair
35
lesions on the transcribed strand of active gene (Marteijn et al, 2014).
36
The NER system has been linked to rare human diseases classically grouped into three distinct
37
NER-related syndromes. These include the highly cancer prone disorder Xeroderma Pigmentosum (XP)
38
and the two progeroids diseases: Cockayne Syndrome (CS) and Trichothiodystrophy (TTD). Importantly,
39
CS and TTD patients are not cancer-prone but present severe neurological and developmental features
40
(Hanawalt, 1994).
41
.CC-BY 4.0 International licenseperpetuity. It is made available under a
preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in
The copyright holder for thisthis version posted December 24, 2021. ; https://doi.org/10.1101/2021.12.23.473962doi: bioRxiv preprint
3
Xeroderma Pigmentosum group A (XPA)-binding protein 2 (XAB2) is an evolutionarily highly
42
conserved protein of 100 kDa and consists of fifteen tetratricopeptide repeat (TPR) motifs that play a
43
role in protein-protein interactions. XAB2 protein was identified as a protein interacting with XPA, a NER
44
factor, using a yeast two-hybrid system (Nakatsu et al, 2000). Next, it has been shown that this protein
45
interacts also with the TC-NER-specific factors, CSA and CSB, as well as elongating RNAP2 (Nakatsu et al,
46
2000). XAB2 is also essential for early mouse embryogenesis as demonstrated by the preimplantation
47
lethality observed in XAB2 knockout mice (Yonemasu et al, 2005).
48
Downregulation of XAB2 using either anti-XAB2 or siRNA specifically inhibited normal RNA
49
synthesis and the recovery of RNA synthesis after UV irradiation (Nakatsu et al, 2000; Kuraoka et al,
50
2008). Furthermore, injection of anti-XAB2 in GG-NER deficient cells results in a significant reduction of
51
UV-induced Unscheduled DNA Synthesis during repair (Nakatsu et al, 2000). These results suggest the
52
involvement of XAB2 in transcription and in TC-NER.
53
Further studies have shown that XAB2 is a component of Prp19/XAB2 complex (Aquarius [AQR],
54
XAB2, Prp19, CCDC16, hISY1 and PPIE) or Prp19/CDC5L-related complex required for pre-mRNA splicing
55
(Kuraoka et al, 2008). XAB2, as well as PRP19 and AQR, has been involved in the DNA damage response
56
(Onyango et al, 2016; Maréchal et al, 2014; Sakasai et al, 2017). Indeed, XAB2 is important for
57
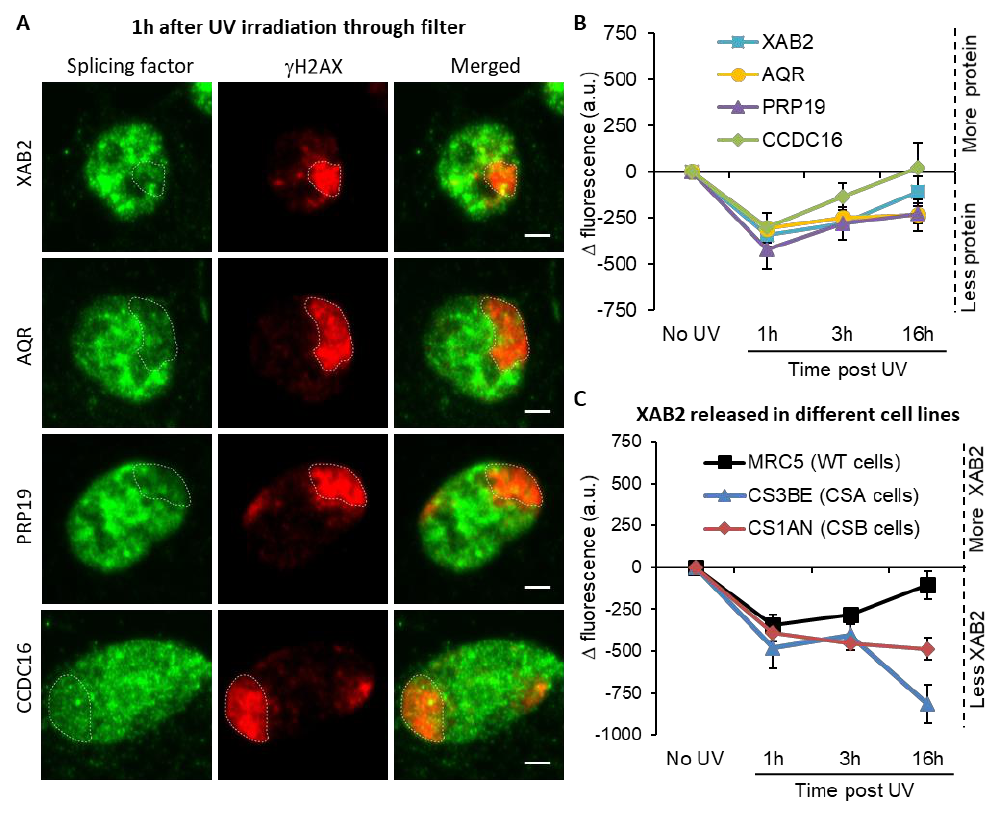
homologous recombination (HR) by promoting the end resection step (Onyango et al, 2016); PRP19 is a
58
sensor of RPA-ssDNA after DNA damage (Maréchal et al, 2014) and AQR contributes to the maintenance
59
of genomic stability via regulation of HR (Sakasai et al, 2017).
60
Moreover, AQR has also a role in the removal of R-loops, a three-stranded nucleic acid structure
61
composed of a DNA:RNA hybrid and the associated non-template single-stranded DNA (Sollier et al,
62
2014). These structures can form during transcription when an RNA molecule emerging from the
63
transcription machinery hybridizes with the DNA template. They are found abundantly in human gene
64
promoters and terminators where RNA processing takes place (Wang et al, 2018).
65
Despite the knowledge acquired in the last decades on XAB2 and its different cellular roles, little
66
is known on the exact crosstalk and dynamics between its diverse cellular functions, specifically between
67
the DNA repair function, the involvement in transcription and its splicing activity. In this work, we
68
.CC-BY 4.0 International licenseperpetuity. It is made available under a
preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in
The copyright holder for thisthis version posted December 24, 2021. ; https://doi.org/10.1101/2021.12.23.473962doi: bioRxiv preprint
4
described the molecular dynamics of XAB2 within the cell after UV-damage induction and during the TC-
69
NER repair process. We determine in vivo that, in absence of XAB2, the transcription-coupled repair
70
reaction is impaired and hence the transcription restart after UV damage is abolished. Surprisingly,
71
unlike all the other NER proteins studied so far, the mobility of XAB2 is increased after DNA damage and
72
XAB2 shows no accumulation on the local UV damage. This faster dynamic is not restored until DNA
73
repair is completed and in damaged TC-NER-deficient cells, XAB2 remains more mobile. Interestingly, we
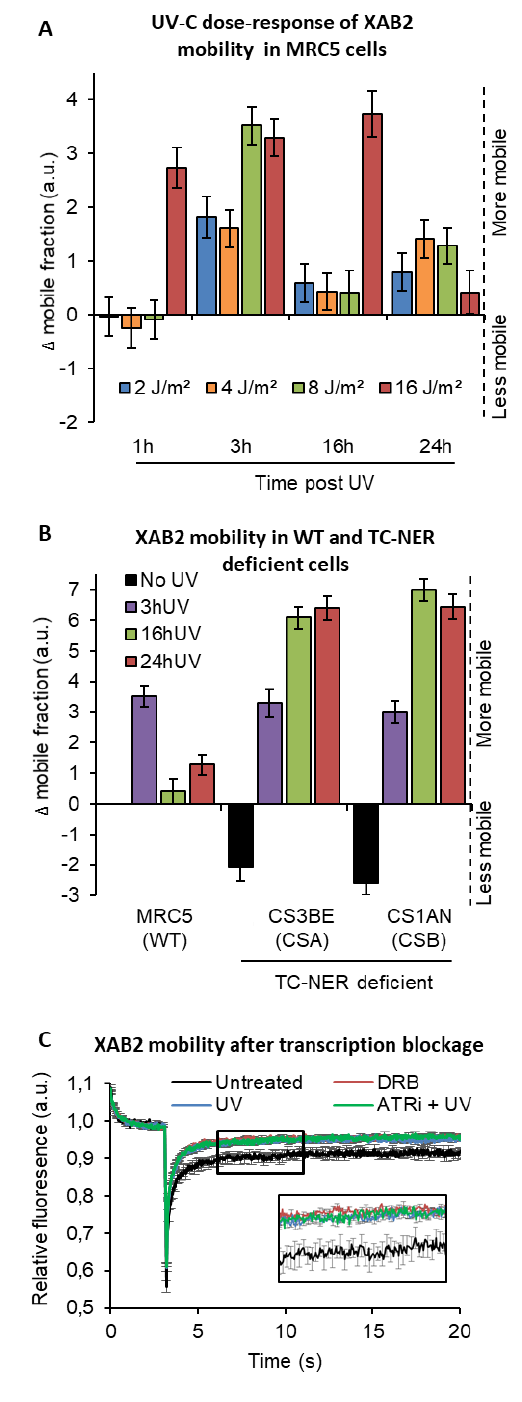
74
demonstrate that, after DNA damage induction, XAB2 is not released from the splicing complex but is
75
detached from R-loops, a recently identified substrate of XAB2 (Goulielmaki et al, 2021).
76
.CC-BY 4.0 International licenseperpetuity. It is made available under a
preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in
The copyright holder for thisthis version posted December 24, 2021. ; https://doi.org/10.1101/2021.12.23.473962doi: bioRxiv preprint
5
RESULTS
77
XAB2 is involved in TC-NER process
78
Two decades ago, Tanaka’s research group demonstrated the involvement of XAB2 in the NER
79
pathway (Kuraoka et al, 2008; Nakatsu et al, 2000). However, the dynamics of XAB2 during the DNA
80
Repair process remained to be elucidated. We aimed to study the molecular dynamics of XAB2 and the
81
shuttling between its different functions when DNA damage is induced. Firstly, we wanted to verify that
82
XAB2 is exclusively involved in the TC-NER reaction and not in other steps or pathways repairing UV-
83
lesions.
84
The well-known standard assay used to quantify NER activity is the Unscheduled DNA Synthesis
85
(UDS), which measure replication activity outside of the S-phase after UV treatment. This technique
86
quantifies the refilling of the single-strand DNA gap by the DNA replicative machinery. When we
87
performed UDS assay in XAB2 silenced cells, no decreased level of UDS was observed (as well as in mock-
88
treated cells) (Figure 1A, blue and black columns and Figure S1). As a positive control, when we silenced
89
the excision repair factor XPF, we observed a strong reduction in UDS level (Figure 1A, red column and
90
Figure S1). This result shows that XAB2 is not involved in GG-NER sub-pathway, but does not exclude an
91
involvement of XAB2 in TC-NER sub-pathway.
92
The commonly used assay measuring TC-NER activity is the RNA Recovery Synthesis (RRS). In this
93
assay, the newly transcribed RNA is measured via the incorporation of a nucleoside analog coupled to a
94
fluorophore. The experiment is conducted at different time point after UV irradiation (0, 3, 16 and 24h),
95
in order to quantify, 3h after UV damage, the decline in transcriptional activity and 16-24h after UV
96
irradiation, the restart of transcriptional activity after DNA repair. When we performed this assay in
97
XAB2 silenced cells no restart of transcription after UV damage was observed (Figure 1B, blue column
98
and Figure S2A), as well as in siXPF-treated cells due to the inability to repair DNA lesions (Figure 1B, red
99
column and Figure S2A) and in contrast with siMock-treated cells (Figure 1B, black columns and Figure
100
S2A). Surprisingly, contrary to Tanaka’s group which observed a decreased of nascent RNA synthesis in
101
absence of XAB2, we observed in our experiment an increase of transcription in siXAB2-treated cells
102
without irradiation (Figure S2B) (Kuraoka et al, 2008; Nakatsu et al, 2000). This result demonstrated an
103
.CC-BY 4.0 International licenseperpetuity. It is made available under a
preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in
The copyright holder for thisthis version posted December 24, 2021. ; https://doi.org/10.1101/2021.12.23.473962doi: bioRxiv preprint

6
involvement of XAB2 in the TC-NER sub pathway, but did not discriminate between a role in the repair
104
reaction per se or in the restart of transcription after repair (RTR) (Mourgues et al, 2013).
105
In order to discriminate this point, we performed an assay designed previously in our group that
106
specifically measures repair replication during TC-NER: the TCR-UDS assay (Mourgues et al, 2013). For
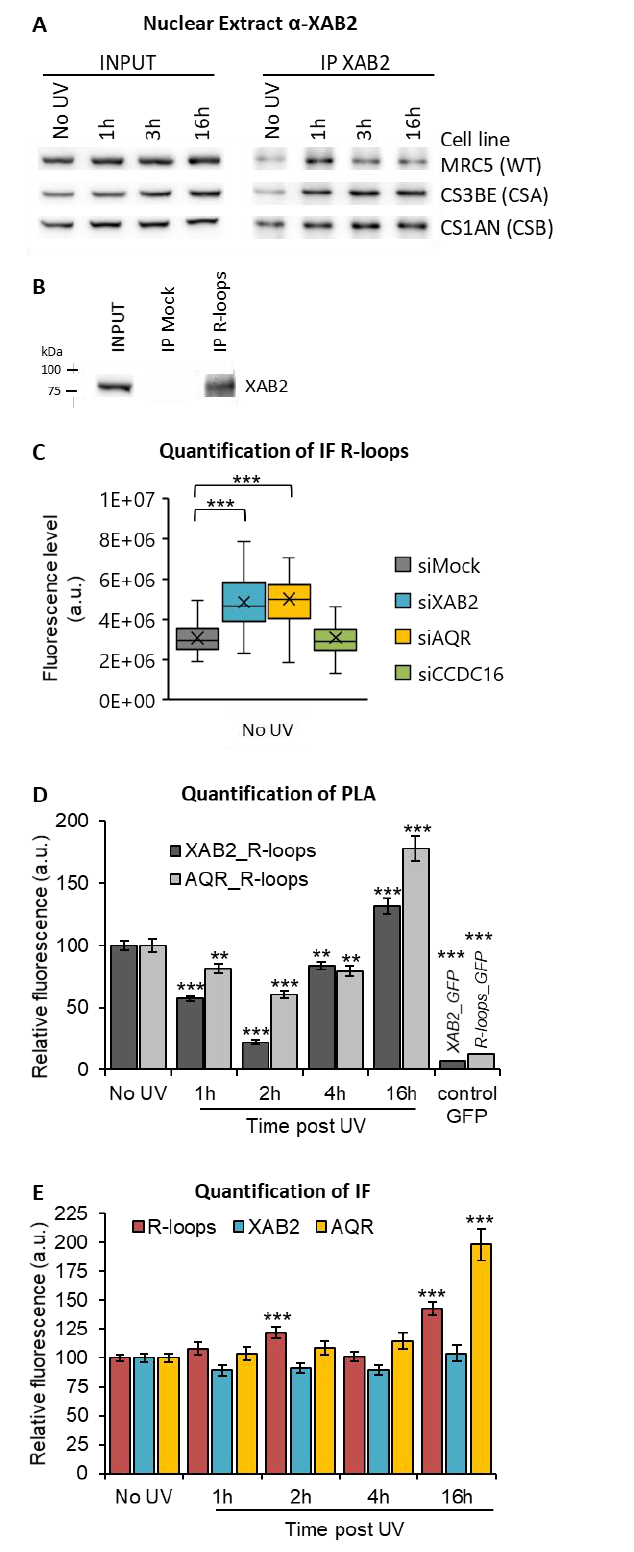
107
this assay, we performed UDS assay in GG-NER deficient cells using XPC (Xeroderma Pigmentosum
108
complementation group C) mutant cells (XP4PA-SV). The cells were transfected with siRNA and then
109
locally irradiated with UV-C through a filter. In order to precisely localize DNA-damaged areas, a yH2AX
110
co-immunofluorescence labeling was performed, and repair-replication was quantified. In siXPF-treated
111
XPC-negative cells, both the GG-NER and the TC-NER pathways are compromised and as expected low
112
TCR-UDS levels were observed compared to siMock-treated cells (Figure 1C, red and black column and
113
Figure S3). Knockdown of XAB2 results in a decrease of TCR-UDS levels (Figure 1C, blue column and
114
Figure S3). This result demonstrates a role of XAB2 in the repair reaction itself, its silencing preventing
115
the DNA-synthesis associated with the excision of UV lesions on actively transcribed genes.
116
Recently we demonstrate that a fully functional NER mechanism is necessary for repair of
117
ribosomal DNA (rDNA), genes transcribed by the RNA polymerase I (RNAP1) (Daniel et al, 2018). To
118
investigate the involvement of XAB2 in the repair of ribosomal genes, the level of RNAP1 transcription
119
was measured at different time points after UV irradiation by using a specific ribosomal RNA probe
120
coupled to a fluorophore, as described previously (Daniel et al, 2018). This probe recognizes the 5’ end of
121
the rDNA transcript, the 47S pre-rRNA (upstream from the first site cleaved rapidly during rRNA
122
processing) (a sketch of the 47S is depicted in figure S4A). In siMock treated cells, we observed a
123
decrease of 47S levels 3h after UV-C exposure and the restart of RNAP1 transcription 40h after
124
irradiation (Figure 1D, black column and Figure S4C). The TC-NER-deficient siCSB-treated cells presented
125
a low level of rRNA synthesis even 40h after UV-C exposure (Figure 1D, violet column and Figure S4B and
126
S4C). A restart of RNAP1 transcription was observed in the absence of XAB2, as for the control (Figure
127
1D, blue column and Figure S4C).
128
All these results demonstrated a function of XAB2 in the TC-NER repair reaction specifically and
129
exclusively for RNAP2 transcribed genes.
130
.CC-BY 4.0 International licenseperpetuity. It is made available under a
preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in
The copyright holder for thisthis version posted December 24, 2021. ; https://doi.org/10.1101/2021.12.23.473962doi: bioRxiv preprint
7
131
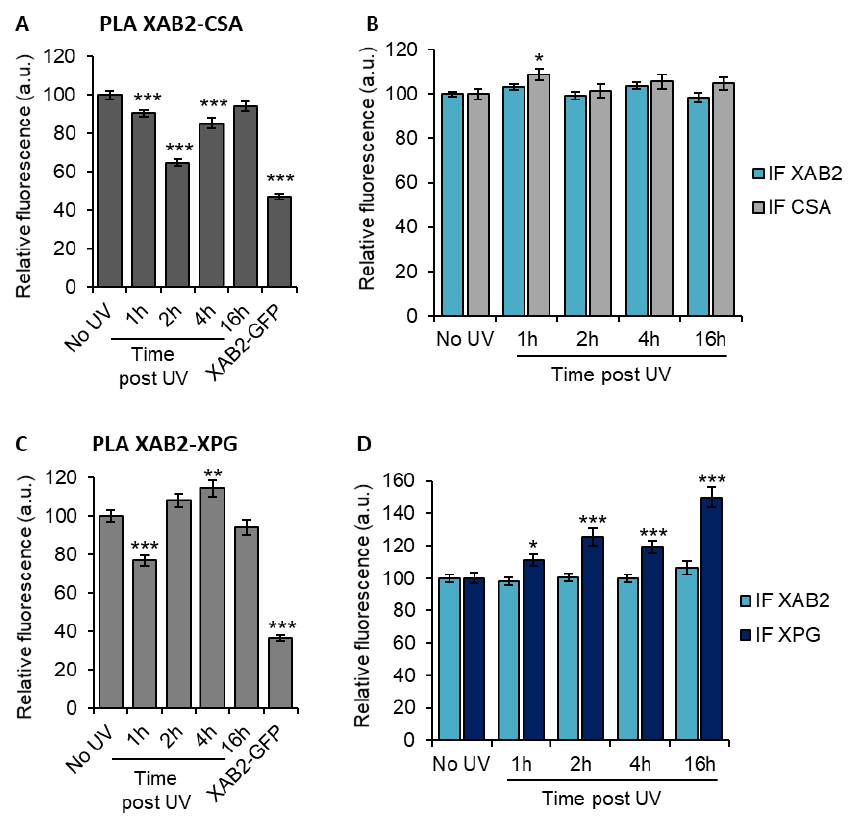
Figure 1. XAB2 involvement in DNA repair
132
A) Quantification of Unscheduled DNA Synthesis assay (UDS) determined by EdU incorporation after
133
local damage (LD) induction with UV-C (100J/m²) in WT cells (MRC5 cells) treated with siRNAs against
134
indicated factors. The graph is the average of two independent experiments and error bars represent the
135
SEM obtained from at least 30 LDs. B) Quantification of RNA Recovery Synthesis (RRS) assay determined
136
by EU incorporation after UV-C (10J/m²) exposure in WT cells treated with siRNAs against indicated
137
factors. Error bars represent the SEM obtained from at least 50 cells and data are representative of three
138
independent experiments. C) Quantification of TCR-UDS assay determined by EdU incorporation after LD
139
induction with UV-C (100J/m²) in GG-NER deficient cells (XPC-/- cells) treated with siRNAs against
140
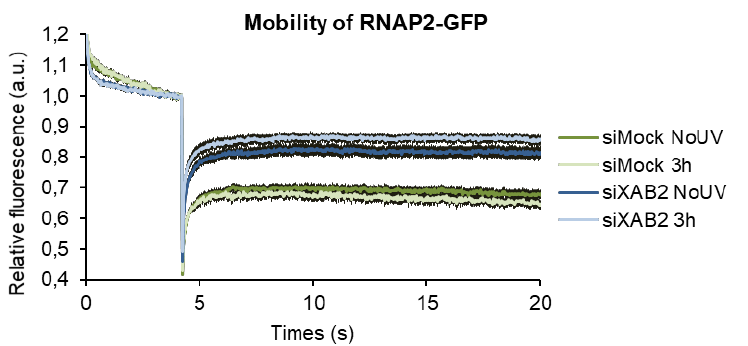
indicated factors. Error bars represent the SEM obtained from at least 15 LDs and data are
141
representative of two independent experiments. D) Quantification of RNA-FISH assay showing the 47S
142
pre-rRNA level after UV-C (16J/m²) exposure in WT cells treated with siRNAs against indicated factors.
143
Error bars represent the SEM obtained from at least 30 cells and data are representative of two
144
independent experiments. For all graph, p-value of student’s test compared to No UV or siMock
145
condition : ***<0.001.
146
147
148
.CC-BY 4.0 International licenseperpetuity. It is made available under a
preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in
The copyright holder for thisthis version posted December 24, 2021. ; https://doi.org/10.1101/2021.12.23.473962doi: bioRxiv preprint
8
XAB2-splicing complex mobility is released from the DNA damage area.
149
XAB2 is included in a splicing complex composed of five other proteins, namely Aquarius (AQR),
150
PRP19, CCDC16, PPIE and ISY1 (Kuraoka et al, 2008). In order to explore how the XAB2 splicing complex is
151
behaving after local damage induction, the localization of XAB2, AQR, PRP19 and CCDC16 was revealed
152
by immunofluorescence assays at different time points after local UV-irradiation of the cells. In this
153
assay, the fluorescence signal from each protein in the damaged area (visualized by a co-staining of
154
yH2AX) was compared to the rest of the nucleus. Unexpectedly, in contrast with all other NER proteins
155
studied so far, we observed a relatively rapid (1 hour after UV-irradiation) release of XAB2, AQR, PRP19
156
and CCDC16 from the damaged area (Figure 2A and 2B). The localization of the XAB2-splicing complex is
157
re-established after the completion of DNA repair reactions, when the transcription is fully restarted, 16h
158
after irradiation (Figure 2B).
159
We thus verified if the entire XAB2-splicing complex was involved in TC-NER or whether only
160
XAB2 played a role in this process. In order to measure the repair capacity of cells silenced for XAB2-
161
related proteins, we performed UDS, TCR-UDS and RRS experiments in AQR/PRP19/CCDC16/PPIE/ISY1-
162
siRNAs treated cells (Figure S5, S6, S7 and S8) and compared the results with XPF-siRNAs treated cell
163
lines. Our results clearly show that none of the cells silenced for XAB2-related proteins are deficient in
164
DNA Repair and both GG-NER (Figure S6) and TC-NER (Figure S7 and S8) are proficient in absence of
165
AQR/PRP19/CCDC16/PPIE/ISY1.
166
In order to investigate whether XAB2 release from damaged areas was dependent of the TC-NER
167
reaction, the localization of XAB2 was detected and quantified within locally damaged areas in TC-NER-
168
deficient cells, CSA (CS3BE) and CSB (CS1AN) mutant cells. Interestingly, the absence of CSA and CSB did
169
not hinder the release of XAB2 from locally damaged areas but this release continued 16h after UV-C
170
exposure (Figure 2C blue and red curve compared to black curve and figure S9), suggesting that the re-
171
establishment of the proper localization of XAB2 within the nucleus after the DNA repair process
172
depends either on the repair process per se or on the restart of transcription after completion of the
173
DNA repair reactions.
174
.CC-BY 4.0 International licenseperpetuity. It is made available under a
preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in
The copyright holder for thisthis version posted December 24, 2021. ; https://doi.org/10.1101/2021.12.23.473962doi: bioRxiv preprint
9
175
Figure 2. Splicing complex released from DNA damage
176
A) Representative confocal images of immunofluorescence against XAB2, AQR, PRP19 or CCDC16 (green)
177
and γH2AX (red) one hour after local damage (LD) induction with UV-C (60J/m²). LD are indicated by
178
dashed lines. Scale bar 3µm. B) Quantification of the immunofluorescence signal of the different splicing
179
proteins on the LD after different times of recovery. C) Quantification of XAB2 signal on LD in different
180
cell lines after different times of recovery. For both graph, the signal from the local damage has been
181
subtracted from the background of each cell. Error bars represent the SEM obtained from at least 20
182
cells and data are representative of two independent experiments.
183
184
185
XAB2 dynamic during TC-NER
186
To further analyze the mobility of XAB2 within the nuclei, we performed SPOT-FRAP (Fluorescent
187
Recovery After Photo-Bleaching) experiments. In this technique, fluorescence molecules are photo-
188
.CC-BY 4.0 International licenseperpetuity. It is made available under a
preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in
The copyright holder for thisthis version posted December 24, 2021. ; https://doi.org/10.1101/2021.12.23.473962doi: bioRxiv preprint
10
bleached in a small spot by a high intensity laser pulse; then the recovery of fluorescence within the
189
bleached area is monitored over time (Figure S10A). With no treatment, this measure of fluorescence
190
recovery corresponds to the protein mobility within the living cells (Figure S10A black curve). After
191
perturbation of the nuclear environment (e.g. DNA damage), a protein can become less mobile if it
192
physically interacts with a new substrate or a bigger complex (Figure S10A, green curve); more mobile if
193
the protein is released from its substrate (Figure S10A, blue curve); or eventually will not change its
194
mobility (Figure S10A red curve).
195
We stably transfected a vector expressing a fluorescently version of XAB2 (XAB2-GFP, Figure
196
S10B) in different SV40-immortalized human fibroblast: wild-type cells (MRC5, Figure S10C), CSA
197
deficient cells (CS3BE) and CSB deficient cells (CS1AN). In order to determine the minimum dose of UV-C
198
needed to detect a significant difference in XAB2 mobility, MRC5 XAB2-GFP cell were irradiated with
199
doses of UV-C ranging from 2 to 16 J/m² and SPOT-FRAP experiments were performed at different time
200
point after irradiation (Figure 3A). Interestingly, we observed a dose-dependent increase in mobility of
201
XAB2 (Figure 3A). Doses of UV-C as weak as 2 and 4 J/m² induced a moderate increase in mobility 3
202
hours post-irradiation and a re-establishment of the basal XAB2 mobility within 16 hours post-irradiation
203
(Figure 3A, blue and yellow bar). High UV-C doses (16 J/m²) induced an increased mobility of XAB2 more
204
rapidly (1-hour post-irradiation) and a re-establishment of the control mobility 24 hours post-irradiation
205
(Figure 3A, red bar). At intermediate doses of 8 J/m² of UV-C, we observed a significant increase in XAB2
206
mobility during repair (3h after UV-C exposure) and the following return to the normal condition once
207
repair is completed and transcription restarted (16h after irradiation) (Figure 3A, green bar).
208
Interestingly, in CSA and CSB mutant cells, the increase in XAB2 mobility is also observed after 8 J/m²
209
irradiation but lasted until 24h after UV-C exposure (Figure 3B) witnessing the fact that in these cells
210
DNA damage is not repaired and hence initial XAB2 mobility is not restored. Interestingly, without
211
damage, XAB2 mobility is reduced in TC-NER deficient cells compared to wild-type cells for still unknown
212
reasons (Figure 3B, black histogram).
213
The results of these experiments directed us to explore the possibility that the change in XAB2
214
mobility was due to transcription inhibition and not really to the repair process itself. In order to verify
215
.CC-BY 4.0 International licenseperpetuity. It is made available under a
preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in
The copyright holder for thisthis version posted December 24, 2021. ; https://doi.org/10.1101/2021.12.23.473962doi: bioRxiv preprint
11
this hypothesis XAB2 mobility was measured after DRB (transcription inhibitor) treatment. The results
216
show that XAB2 increased mobility in transcription inhibition conditions is very similar to the one
217
measured upon UV treatment (Figure 3C).
218
As XAB2, the mobility of the late-stage spliceosomes change after UV irradiation and this
219
mobilization depends on DNA damage response (DDR) signaling pathways (Tresini et al, 2015). Key
220
mediators of DDR are the ATM and ATR kinases, which induced cell cycle arrest and facilitate DNA repair.
221
To demonstrate that the change of XAB2 mobility is not due to the UV-Damage Response, we realized
222
the same measures in presence of ATR and ATM inhibitors. Both drugs did not modify the increase of
223
XAB2 mobility after UV irradiation (Figure 3C and Figure S10D).
224
Taken together, these results demonstrated that XAB2 mobility changes after DNA damage is
225
triggered and sustained by the transcriptional inhibition.
226
.CC-BY 4.0 International licenseperpetuity. It is made available under a
preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in
The copyright holder for thisthis version posted December 24, 2021. ; https://doi.org/10.1101/2021.12.23.473962doi: bioRxiv preprint
12
227
Figure 3. XAB2 dynamic during TC-NER
228
.CC-BY 4.0 International licenseperpetuity. It is made available under a
preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in
The copyright holder for thisthis version posted December 24, 2021. ; https://doi.org/10.1101/2021.12.23.473962doi: bioRxiv preprint
13
A) FRAP analysis of XAB2-GFP mobility in WT cells. Cells were treated or not with different doses of UV-C
229
(2 to 16J/m²) and XAB2 mobility was measured at different time points after UV-C exposure. The No UV
230
condition was used to calculate the change in bound fraction. B) FRAP analysis of XAB2-GFP expressed in
231
WT cells (MRC5-SV) and TC-NER deficient cells (CSA -/- and CSB -/-). Cells were treated or not with 8J/m²
232
of UV-C. The No UV condition of the WT cell lines was used to calculate the change in bound fraction. C)
233
FRAP analysis of XAB2-GFP mobility in WT cells. Cells were either untreated (dark line) or treated with
234
100µg/ml of DRB for 2h (red line) or with 10J/m² of UV-C for 3h (blue line). Inhibitor of ATR pathway was
235
added at 10µM in the medium 1h before irradiation (purple line). For all graph, error bars represent the
236
SEM obtained from at least 10 cells and data are representative of two independent experiments.
237
238
239
XAB2 is not released from the splicing complex during DNA repair reactions.
240
The increase of XAB2 mobility after UV-induced transcription inhibition could be explained by
241
either the release of XAB2 from a bigger complex and/or the release from an immobile (or nearly
242
immobile) substrate such as the chromatin or a DNA-related substrate.
243
In order to distinguish between these two possibilities, we firstly investigated whether XAB2
244
dissociates after DNA damage induction from the splicing complex described earlier. XAB2 was
245
immunoprecipitated together with AQR (Figure S11A). Interestingly, XAB2 was strongly and consistently
246
immunoprecipitated 1-hour post-irradiation which corresponded to the time in which XAB2 mobility is
247
increased and at the same time point more AQR is also immunoprecipitated. No clear release of XAB2
248
from AQR was observed at different time points.
249
In parallel, we also verified by PLA (Proximity Ligation Assay) whether the binding of XAB2 to
250
AQR was modified after UV-C irradiation (Figure S11B). 2 hours after UV-C irradiation, instead of a
251
release of XAB2 from AQR, we measured a stronger interaction (Figure S11B). However, this stronger
252
interaction could very well result from the increase of AQR concentration 1h after UV irradiation (Figure
253
S11C).
254
In conclusion, immunoprecipitation or PLA experiment could not explain the increased mobility
255
of XAB2 after DNA damage, indeed XAB2 is not released from splicing complex during DNA repair
256
reactions.
257
.CC-BY 4.0 International licenseperpetuity. It is made available under a
preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in
The copyright holder for thisthis version posted December 24, 2021. ; https://doi.org/10.1101/2021.12.23.473962doi: bioRxiv preprint
14
258
259
XAB2 is released from R-loops during DNA repair reactions.
260
Interestingly, while trying to immunoprecipitate XAB2 interacting partners during DNA Repair
261
reactions, we could observe that systematically and consistently more XAB2 could be immuno-
262
precipitated from nuclear extracts 1 to 3 hours after UV-C irradiation in WT cells (Figure S11A and 4A). At
263
16 hours post irradiation, we observed that the amount of XAB2 immunoprecipitated was comparable to
264
the level observed in non-irradiated cells. Moreover, in CSA-/- and CSB-/- cell lines, in which UV-lesions
265
on transcribed strands of genes are not repaired, the amount of XAB2 immunoprecipitated was
266
remained high all along the time course of the experiment (3 and 16 hours) (Figure 4A). These results
267
suggest that in TCR-deficient cells the binding between XAB2 and its substrate is not restored.
268
AQR has a role in the removal of DNA:RNA hybrids, commonly known as R-loops structure
269
(Sollier et al, 2014) and recently XAB2 was found to be involved in the R-loops resolution (Goulielmaki et
270
al, 2021). We thus decided to investigate if XAB2 has also a link with R-loops, a DNA-related substrate.
271
Indeed, we could find by immunoprecipitation that XAB2 directly interacts with R-loops (Figure 4B) and
272
that in cells silenced for XAB2 or AQR compared to control cells, we observed an accumulation of R-loops
273
(Figure 4C and Figure S12A). We thus decided to examine whether XAB2 is released from R-loops after
274
DNA damage induction by performing a PLA assay using a specific R-loops antibody (S9.6). We measured
275
a strong and consistent reduction, more than 40% reduction, of the interactions between R-loops and
276
XAB2 and between R-loops and AQR (Figure 4D and Figure S12B). This reduced interaction is not caused
277
by a reduction in either R-loops, XAB2 or AQR concentration during DNA repair (Figure 4E and figure
278
S12B). To verify that this result is specific for RNA-loops and not for a non-specific signal coming from the
279
interaction of XAB2 with messenger RNAs, we performed the same assays (PLA and IF) in presence of
280
RNAse H which specifically degrades R-loops structures and not the RNA (Figure S13A and S13B). Our
281
results show that RNAse H decreases the quantity of RNA-loops (Figure S13C-F) and in doing so it
282
decreases also the interaction with both XAB2 (Figure S13C and S13D) and AQR (Figure S13E and S13F).
283
To verify whether XAB2 increase in mobility is due to a decreased interaction with the mRNA we
284
.CC-BY 4.0 International licenseperpetuity. It is made available under a
preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in
The copyright holder for thisthis version posted December 24, 2021. ; https://doi.org/10.1101/2021.12.23.473962doi: bioRxiv preprint
15
performed PLA assays in UV-irradiated cells (Figure S14A and S14C). A decrease in the interaction
285
between XAB2 and mRNA was observed (Figure S14A and S14C) but this decrease corresponded to the
286
decrease of mRNA caused by UV-dependent transcription inhibition (Figure S14B and S14C) invalidating
287
the results observed in the PLA XAB2-mRNAs (Figure S14A).
288
These results clearly demonstrate that XAB2 is released from R-loops during DNA repair
289
reactions.
290
291
.CC-BY 4.0 International licenseperpetuity. It is made available under a
preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in
The copyright holder for thisthis version posted December 24, 2021. ; https://doi.org/10.1101/2021.12.23.473962doi: bioRxiv preprint
16
292
.CC-BY 4.0 International licenseperpetuity. It is made available under a
preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in
The copyright holder for thisthis version posted December 24, 2021. ; https://doi.org/10.1101/2021.12.23.473962doi: bioRxiv preprint
17
Figure 4. XAB2 and AQR are released from R-loop during DNA repair
293
A) Immunoprecipitation of XAB2 in nuclear extract from different cell lines treated with 10J/m² of UV-C
294
at different times. Bound proteins were revealed by Western blotting with antibodies against XAB2.
295
INPUT, 10% of the lysate used for immunoprecipitation (IP) reaction. B) Immunoprecipitation of R-loops
296
in non-crosslinked chromatin extract from WT cells. XAB2 bound to R-loops was revealed by Western
297
blotting. INPUT, 10% of the lysate used for immunoprecipitation (IP) reaction. C) Quantification of
298
immunofluorescence (from at least 50 cells) against R-loops in WT cells treated with siRNAs against
299
indicated factors. D-E) Quantification of fluorescent signal in the nucleus against the couple XAB2_R-
300
loops or AQR_R-loops from PLA experiment (D) or from the immunofluorescence done in parallel of PLA
301
assay (E). Error bars represent the SEM obtained from at least 50 cells.
302
For all graph, data are representative of two independent experiments. For R-loops quantification, the
303
nucleolar signal was subtracted from the nuclear signal. P-value of student’s test compared to No UV or
304
siMock condition: **<0.01;***<0.001.
305
306
307
XAB2 is released from CSA during DNA repair.
308
Because XAB2 has been found to participate specifically in TCR-NER repair reactions (Figure 1C),
309
we wanted to investigate whether part of the increased mobility of XAB2 observed after UV-induction
310
was due to a release from repair complexes. We measured, by PLA, the amount of interactions between
311
XAB2 and CSA, CSB, XPB and XPG proteins, observed during TC-NER. Amongst all the proteins tested, we
312
could observe a clear and consistent release from the CSA protein 2h after UV irradiation (Figure 5A) and
313
from the XPG protein 1h after UV irradiation (Figure 5C). The corresponding IF did not show a decreased
314
quantity of CSA or XPG (Figure 5B and 5D) which validated the specificity of the XAB2-CSA and XAB2-XPG
315
decreased interaction at those time points. On the contrary, no clear decrease in interaction was
316
observed between XAB2 and CSB (Figure S15A and S15B) or XPB (figure S15C and S15D).
317
.CC-BY 4.0 International licenseperpetuity. It is made available under a
preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in
The copyright holder for thisthis version posted December 24, 2021. ; https://doi.org/10.1101/2021.12.23.473962doi: bioRxiv preprint
18
318
Figure 5: XAB2 are released from CSA and XPG during DNA repair
319
A to D) Quantification of fluorescent signal in the nucleus against the couple XAB2-CSA (A and B) and
320
XAB2-XPG (C and D) from PLA experiment (A and C) or from the immunofluorescence done in parallel of
321
PLA assay (B and D). Data are the average of at least two independent experiments and error bars
322
represent the SEM obtained from at least 80 cells. P-value of student’s test compared to No UV
323
condition: *<0.05; **<0.01; ***<0.001.
324
325
326
XAB2 depletion modify the mobility of the RNAP2.
327
Because XAB2 was found to interact with RNAP2 (Nakatsu et al, 2000; Kuraoka et al, 2008) and
328
because the increased mobility observed is dependent on the UV-dependent transcription inhibition
329
step, we wanted to explore whether the depletion of XAB2 might play a role in the overall mobility of
330
RNAP2. In order to investigate this point, we performed FRAP experiments on RNAP2-GFP expressing
331
.CC-BY 4.0 International licenseperpetuity. It is made available under a
preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in
The copyright holder for thisthis version posted December 24, 2021. ; https://doi.org/10.1101/2021.12.23.473962doi: bioRxiv preprint
19
cells in presence and absence (depletion) of XAB2 (Figure 6) (Donnio et al, 2019). Interestingly, depletion
332
of XAB2 greatly increase the mobility of RNAP2 (Figure 6, dark green curve versus dark blue curve).
333
Moreover, UV-irradiation intensify this remobilization (Figure 6, light green curve versus light blue
334
curve), demonstrating that XAB2 might play a role in maintaining the RNAP2 bound to the substrate in
335
absence or presence of UV-lesions on the DNA.
336
337
Figure 6: Pol2 behavior with XAB2
338
Strip-FRAP analysis of RNAP2-GFP expressing WT cells treated or not with UV-C (10J/m²) after siRNA
339
mediated knockdown of the indicated factors. Error bars represent the SEM obtained from at least 10
340
cells and data are representative of two independent experiments.
341
342
.CC-BY 4.0 International licenseperpetuity. It is made available under a
preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in
The copyright holder for thisthis version posted December 24, 2021. ; https://doi.org/10.1101/2021.12.23.473962doi: bioRxiv preprint
20
DISCUSSION
343
Helix distorting lesions continuously challenge the cell survival by interfering with and blocking
344
fundamental cellular functions, such as transcription and replication. In order to prevent the deleterious
345
effects of these events, cells have developed different mechanisms to restore an undamaged DNA
346
molecule and allow the restart of cellular processes. The importance of rapidly reestablishing perturbed
347
cellular functions is underlined by the presence of a repair mechanism directly coupled with
348
transcription, like the Transcription-Coupled Nucleotide Excision Repair (TC-NER).
349
Two phases can be distinguished during TC-NER events: (i) the actual repair reaction of the
350
damaged strand via the TC-NER sub-pathway and (ii) the resumption of transcription after repair. The
351
experiment known as ‘RNA Recovery Synthesis’ (RRS) allow measuring the restart of transcription after
352
DNA repair completion and thus the involvement of a protein in the TC-NER process. However, this assay
353
does not discriminate between the two phases of the TC-NER. By consequence, proteins that are fully
354
proficient in the repair reaction but fail in the transcription restart after DNA repair completion might
355
have the same defect in RRS as proteins that are fully deficient in the DNA repair reaction.
356
Up to now, all studies have demonstrated the involvement of XAB2 in TC-NER process using only
357
RRS experiments (Nakatsu et al, 2000; Kuraoka et al, 2008). In this study, we used a specific test called
358
TCR-UDS, developed in house (Mourgues et al, 2013), and demonstrated that indeed XAB2 is solely
359
needed for the repair reaction per se (Figure 1B and 1C).
360
XAB2 has been found as part of the pre-mRNA splicing complex composed of AQR, PRP19,
361
CCDC16, PPIE and ISY1 (Kuraoka et al, 2008). However, none of these proteins seems to take part in the
362
repair process, underlying the peculiar function of the splicing factor XAB2 in TC-NER repair (Figure S5).
363
We also demonstrated that, unlike all the other NER protein studied so far, XAB2 protein is
364
released from the damage induced by UV-C exposure (Figure 3). Concomitantly, we also observed an
365
increased XAB2 cellular mobile fraction after UV irradiation (Figure 4D). This release from transcription-
366
blocking DNA lesions and increased mobility is surprising and atypical for a repair protein. However, this
367
behavior has also been observed for late-stage spliceosomes (Tresini et al, 2015) and could be explained
368
by the importance for the cell to rapidly provide access to the repair machinery.
369
.CC-BY 4.0 International licenseperpetuity. It is made available under a
preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in
The copyright holder for thisthis version posted December 24, 2021. ; https://doi.org/10.1101/2021.12.23.473962doi: bioRxiv preprint
21
Surprisingly, the increased mobility of XAB2 after UV irradiation occurs also in absence of CSA
370
and CSB protein, in presence of transcription inhibitors and in presence of ATR and ATM inhibitors
371
(Figure 2, 3 and Figure S6). These results strongly suggest that XAB2 remobilization is independent from
372
the repair process but it is more a result of the damage-dependent transcription inhibition. Accordingly
373
with this result, the return of XAB2 normal mobility is CSA and CSB dependent, suggesting that a proper
374
repair and re-establishment of the transcription process is needed to restore the mobility of XAB2.
375
Previously, we reported that a complete TC-NER mechanism is required to repair the UV-lesions
376
present on active ribosomal DNA, genes transcribed by RNA Polymerase 1 (RNAP1) (Daniel et al. 2018).
377
Notably, both Cockayne syndrome proteins (CSA and CSB) are implicated in this specific repair reaction,
378
as well as the UV-stimulated scaffold protein A (UVSSA), a protein required for the stabilization of CSB
379
specifically after UV irradiation (Higa et al, 2016). Interestingly, XAB2 is involved only in repair of RNAP2-
380
transcribed genes and not in repair of RNAP1-transcribed genes (Figure 1D), confirming a probable
381
specific interaction with RNAP2 and reinforcing the idea that RNAP1 and RNAP2 repair processes are
382
distinct although they share common proteins.
383
FRAP experiments in CSA and CSB mutant cells show a more immobile XAB2 fraction than in WT
384
cells (Figure 3B) already without any damage induction. Tanaka’s group found that XAB2 interacts in vitro
385
with CSA and CSB protein in the absence of DNA damage (Nakatsu et al, 2000). In addition, several
386
studies demonstrated an involvement of CSB in transcription regulation (Boetefuer et al, 2018). As both
387
CSB and XAB2 are necessary during the transcription process, it is therefore possible that the absence of
388
CSB will modify the mobility of XAB2. However, it is not excluded that in CSA and CSB mutant cells, a low
389
level of unrepaired oxidative damage (de Waard et al, 2004) might interfere with the proper XAB2
390
mobility, eventually modifying the amount of R-loops within these cells. Nevertheless, it is difficult to
391
precisely estimate the exact number of R-loops between different cell types and this hypothesis is
392
difficult to clearly assess.
393
The UV-induced remobilization of XAB2 is not explained by its release from the splicing complex,
394
as demonstrated by co-immunoprecipitation and PLA experiment, but from both a chromatin-specific
395
structures: R-loops and from repair proteins, CSA and XPG (Figure 4 and 5). An R-loop is a three-stranded
396
.CC-BY 4.0 International licenseperpetuity. It is made available under a
preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in
The copyright holder for thisthis version posted December 24, 2021. ; https://doi.org/10.1101/2021.12.23.473962doi: bioRxiv preprint
22
nucleic acid structure, composed of a DNA:RNA hybrid and the associated non-template single-stranded
397
DNA. This structure arises naturally in organisms from bacteria to humans, and have a multitude of roles
398
in the cell (Belotserkovskii et al, 2018). In this work, we show that R-loops are a substrate for XAB2 and
399
after DNA damage induction the interaction between XAB2 and R-loops are strongly reduced, this might
400
explain the increase mobility of XAB2 during the TC-NER reaction. However, we also show that XAB2
401
interactions with CSA and with XPG are reduced after UV irradiation and during repair reactions (Figure
402
5). So probably, a combination of reduced interactions of XAB2 results in an increased cellular mobility.
403
A study demonstrates that absence of AQR induces R-loops formation which are actively
404
processed into DNA double-strand breaks by XPF and XPG, the nucleotide excision repair endonucleases
405
(Sollier et al, 2014). Without any damage, we also observe an increased level of cellular R-loops in
406
absence of AQR, additionally we observed the same increase in cells silenced for XAB2 (Figure 4B),
407
suggesting an involvement of these two proteins in R-loops resolution, as recently also demonstrated by
408
Goulielmaki et al, 2021.
409
Transcription process and R-loops formation are finely interconnected. Indeed, R-loops
410
formation can cause transcription blockage but transcription blockage due to DNA damage appears to
411
result in R-loop formation (Steurer & Marteijn, 2017; Mullenders, 2015). Moreover, after UV irradiation,
412
transcription activity declines and mRNAs levels are drastically reduced (Figure S9). PLA assays show that,
413
after UV irradiation, XAB2 and total RNAs interaction is reduced, however, because the total amount of
414
RNAs is diminished after transcription block, we assume that XAB2_RNAs interaction is lessened because
415
of the intrinsic reduced amount of RNAs. Differently from total RNAs, in our study, we observe that R-
416
loops do not decrease in number after UV-damage and transcription inhibition (Figure 4). Therefore, the
417
interaction XAB2_R-loops and AQR_R-loops is specifically hindered after UV-damage (Figure 4).
418
Because XAB2 interacts in vitro with CSA and CSB protein (Nakatsu et al, 2000) and recently
419
studies have shown an interaction with XPG (Goulielmaki et al, 2021), we decided to verify whether
420
these interactions were modified during the DNA repair process and the concomitant transcription
421
inhibition. Our results show a clear and consistent reduction of interaction between XAB2 and CSA
422
(Figure 5A and 5B), and between XAB2 and XPG (Figure 5C and 5D) but not between XAB2 and CSB or
423
.CC-BY 4.0 International licenseperpetuity. It is made available under a
preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in
The copyright holder for thisthis version posted December 24, 2021. ; https://doi.org/10.1101/2021.12.23.473962doi: bioRxiv preprint
23
XAB2 and XPB (Figure S10). A working hypothesis would then be that XAB2 needs to be released from
424
CSA and XPG to allow a proper repair reaction.
425
The physical interaction between XAB2 and RNAP2 has already been established (Nakatsu et al,
426
2000; Kuraoka et al, 2008), however the exact relation between XAB2 and RNAP2 has not been
427
disclosed. In this study, we have clearly demonstrated by FRAP experiments that the mobility of RNAP2 is
428
severely affected in the absence of XAB2, namely, RNAP2 is released from its substrate and its mobility is
429
strongly increased (Figure 6). When cells are subjected to UV-damage, a small RNAP2 immobile fraction
430
can be observed, probably reflecting paused RNAP2 molecules on DNA damaged sites. However, in the
431
absence of XAB2 and in presence of a DNA damage, RNAP2 mobility is even more increased than without
432
damage, confirming that XAB2 might stabilize RNAP2 onto the transcribed strands of active genes in
433
presence of damage. This result leads to the hypothesis that XAB2 might collaborate with RNAP2 in the
434
detection of damage during TC-NER and hence be implicated in the first step of this process.
435
In conclusion, we described here an increased mobility of the protein XAB2 during the DNA
436
damage dependent transcription inhibition. This increased mobility might partly be explained by the
437
release of XAB2 from its substrate R-loops and its partner CSA and XPG. Importantly, we demonstrated
438
that XAB2 plays a role of anchoring RNAP2 to its substrate with and without DNA damage. The absence
439
of XAB2 might hinder the overall transcription activity of the cells as well as it severely affects the TC-NER
440
capacity.
441
.CC-BY 4.0 International licenseperpetuity. It is made available under a
preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in
The copyright holder for thisthis version posted December 24, 2021. ; https://doi.org/10.1101/2021.12.23.473962doi: bioRxiv preprint
24
MATERIAL AND METHODS
442
Cell culture and treatments
443
The cells used in this study were: (i) wild-type SV40-immortalized human fibroblasts (MRC5); (ii) XPC–
444
deficient SV40-immortalized human fibroblast (XP4PA, GG-NER deficient); (iii) CSA–deficient SV40-
445
immortalized human fibroblast (CS3BE, TC-NER-deficient); (iv) CSB-deficient SV40-immortalized human
446
fibroblast (CS1AN, TC-NER-deficient); (v) MRC5 stably expressing XAB2-GFP; (vi) CS3BE stably expressing
447
XAB2-GFP (vii) CS1AN stably expressing XAB2-GFP and (vii) MRC5 stably expressing RNAP2-GFP.
448
Immortalized human fibroblasts were cultured in DMEM (sigma) supplemented with 1% of penicillin and
449
streptomycin (Gibco) and 10% fetal bovine serum (Corning) and incubated at 37°C with 5% CO
2
.
450
XAB2-GFP and RNAP2-GFP stably expressing cell lines were produced by transfecting XAB2-GFP or
451
RNAP2-GFP using FuGENE® 6 Transfection Reagent (Promega) according to the manufacturer’ protocol.
452
Selection was performed with G418 at 2 mg/ml.
453
DNA damage was inflicted by UV-C light (254 nm, 6-Watt lamp). Cells were globally irradiated with a UV-
454
C dose of 2, 4, 8, 10 or 16 J/m
2
or locally irradiated with a UV-C dose of 60 or 100 J/m
2
through a filter
455
with holes of 5 µm of diameter (Millipore). After irradiation, cells were incubated at 37°C with 5% CO
2
for
456
different periods of time. Inhibitor of ATR pathway (VE821) was added at 10µM in the medium 1h before
457
irradiation.
458
459
Transfection of small interfering RNAs (siRNAs)
460
The small interfering RNA (siRNAs) used in this study are : siMock, Horizon, D-001206-14 (10nM); siXAB2,
461
Horizon, L-004914-01 (20nM); siXPF, Horizon, M-019946-00 (10nM); siCSB, Horizon, L-004888-00
462
(10nM). The final concentration used for each siRNA is indicated in parentheses. All siRNA from Horizon
463
are a pool of four different siRNA.
464
Cells were seeded in six-well plates and allowed to attach for at least 24h. Coverslip were added inside
465
the well if needed for experiment. 24h and 48h after seeding, cells were transfected with siRNA using
466
Lipofectamine® RNAiMAX reagent (Invitrogen; 13778150) or GenJet (Tebu-Bio), according to the
467
.CC-BY 4.0 International licenseperpetuity. It is made available under a
preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in
The copyright holder for thisthis version posted December 24, 2021. ; https://doi.org/10.1101/2021.12.23.473962doi: bioRxiv preprint
25
manufacturer’ protocol. Experiments were performed between 24h or 72h after the second transfection.
468
Protein knockdown was confirmed for each experiment by western blot.
469
470
Recovery of RNA synthesis (RRS) assay
471
MRC5 cells were grown on 18 mm coverslips. siRNA transfections were performed 24h and 48h before
472
RRS assay. RNA detection was done using a Click-iT RNA Alexa Fluor Imaging kit (Invitrogen), according to
473
the manufacturer’s instructions. Briefly, cells were UV-C irradiated (10 J/m²) and incubated for 0, 3, 16
474
and 24 h at 37°C. Then, cells were incubated for 2 hours with 5-ethynyl uridine. After fixation and
475
permeabilization, cells were incubated for 30 min with the Click-iT reaction cocktail containing Alexa
476
Fluor Azide 594. After washing, the coverslips were mounted with Vectashield (Vector). The average
477
fluorescence intensity per nucleus was estimated after background subtraction using ImageJ and
478
normalized to not treated cells.
479
480
RNA Fluorescence In Situ Hybridization (RNA FISH)
481
Cells were grown on 18 mm coverslips, washed with warm PBS and fixed with 4% paraformaldehyde for
482
15 min at 37° C. After two washes with PBS, cells were permeabilized with PBS + 0.4 % Triton X-100 for 7
483
min at 4° C. Cells were washed rapidly with PBS before incubation (at least 30 min) with pre-
484
hybridization buffer : 15% formamide in 2X SSPE pH8.0 (0.3M NaCl, 15.7mM NaH
2
PO
4
.H
2
O and 2.5mM
485
EDTA). 35 ng of probe was diluted in 70 µl of hybridization mix (2X SSPE, 15% formamide, 10% dextran
486
sulphate and 0.5 mg/ml tRNA). Hybridization of the probe was conducted overnight at 37° C in a
487
humidified environment. Subsequently, cells were washed twice for 20 min with pre-hybridization buffer
488
and once for 20 min with 1X SSPE. After several washing with PBS, the coverslips were mounted with
489
Vectashield containing DAPI (Vector). The probe sequence (5’ to 3’) is: Cy5-
490
AGACGAGAACGCCTGACACGCACGGCAC. Images of the cells were obtained using a Zeiss LSM 780 NLO
491
confocal laser scanning microscope and the following objective: Plan-Apochromat 63X/1.4 oil DIC M27.
492
493
Unscheduled DNA synthesis (UDS or TCR-UDS)
494
.CC-BY 4.0 International licenseperpetuity. It is made available under a
preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in
The copyright holder for thisthis version posted December 24, 2021. ; https://doi.org/10.1101/2021.12.23.473962doi: bioRxiv preprint
26
MRC5 or XP4PA cells, WT or GG-NER-deficient cells respectively, were grown on 18 mm coverslips. siRNA
495
transfections were performed 24h and 48h before UDS assays. After local irradiation at 100 J/m
2
with
496
UV-C through a 5 µm pore polycarbonate membrane filter, cells were incubated for 3 or 8 hours (UDS
497
and TCR-UDS respectively) with 5-ethynyl-2’-deoxyuridine (EdU), fixed and permeabilized with PBS and
498
0.5% triton X-100. Then, cells were blocked with PBS+ solution (PBS containing 0.15% glycine and 0.5%
499
bovine serum albumin) for 30 min and subsequently incubated for 1h at room temperature with mouse
500
monoclonal anti-yH2AX antibody (Ser139 [Upstate, clone JBW301]) 1:500 diluted in PBS+. After extensive
501
washes with PBS containing 0.5% Triton X100, cells were incubated for 45min at room temperature with
502
secondary antibodies conjugated with Alexa Fluor 594 fluorescent dyes (Molecular Probes, 1:400 dilution
503
in PBS+). Next, cells were washed several times and then incubated for 30 min with the Click-iT reaction
504
cocktail containing Alexa Fluor Azide 488. After washing, the coverslips were mounted with Vectashield
505
containing DAPI (Vector). Images of the cells were obtained with the same microscopy system and
506
constant acquisition parameters. Images were analyzed as follows using ImageJ and a circle of constant
507
size for all images: (i) the background signal was estimated in the nucleus (avoiding the damage, nucleoli
508
and other non-specific signal) and subtracted, (ii) the locally damaged area was defined by using the
509
yH2AX staining, (iii) the average fluorescence correlated to the EdU incorporation was then measured
510
and thus an estimate of DNA synthesis after repair was obtained.
511
512
Immunofluorescence
513
Cells were plated on 12 or 18 mm coverslips in order to reach 70% confluence on the day of the staining.
514
After two washes with PBS, cells were fixed with 2% paraformaldehyde for 15 min at 37° C. Cells were
515
permeabilized by three short washes followed by two washes of 10min with PBS + 0.1 % Triton X-100.
516
Blocking of non-specific signal was performed with PBS+ (PBS, 0.5 % BSA, 0.15 % glycine) for at least 30
517
min. Then, coverslips were incubated with primary antibody diluted in PBS+ for 2 h at RT or overnight at
518
4°C in a moist chamber. After several washes with PBS + 0.1 % Triton X-100 (three short washes and two
519
of 10min) and a short wash with PBS+, cells were incubated for 1h at RT in a moist chamber with
520
secondary antibody coupled to fluorochrome (Goat anti-mouse Alexa Fluor® 488 [A11001, Invitrogen] or
521
.CC-BY 4.0 International licenseperpetuity. It is made available under a
preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in
The copyright holder for thisthis version posted December 24, 2021. ; https://doi.org/10.1101/2021.12.23.473962doi: bioRxiv preprint
27
594 [A11005] and Goat anti-rabbit Alexa Fluor® 488 [A11008] or 594 [A11012], 1/400 dilution in PBS+).
522
After the same washing procedure with PBS instead of PBS + 0.1 % Triton X-100, coverslips were finally
523
mounted using Vectashield with DAPI (Vector Laboratories).
524
The variation of fluorescence in the locally irradiated zone has been calculated as for the UDS
525
experiment.
526
527
Proximity Ligation Assay (PLA)
528
PLA experiments were done using Duolink™ II secondary antibodies and detection kits (Sigma-Aldrich,
529
#DUO92002, #DUO92004, and #DUO92008) according to the manufacturer’s instruction. The cells were
530
fixed and permeabilized with the same procedure as immunofluorescence. After blocking 1h at 37°C with
531
the Blocking Solution from the kit, incubation of the primary antibodies diluted in Antibody Diluent was
532
performed at 4°C overnight. Duolink™ secondary antibodies were added and incubated for 1h at 37°C. If
533
secondary antibodies were in close proximity (<40 nm), they were ligated together to make a closed
534
circle by the Duolink™ ligation solution. The DNA is then amplified (rolling circle amplification) and
535
detected by fluorescence 594 (red dot). Coverslips were mounted using Vectashield with DAPI (Vector
536
Laboratories).
537
538
Cytostripping
539
To remove the background generated by some antibodies or EdU incorporation, the cytoplasm of the
540
cells was removed before fixation. After two washes with cold PBS, cells were incubated on ice 5 min
541
with cold cytoskeleton buffer (10mM PIPES pH6,8; 100mM NaCl; 300mM Sucrose; 3mM MgCl2; 1mM
542
EGTA; 0,5% Triton X100) followed by 5mn with cold cytostripping buffer (10mM Tris HCL pH7,4; 10mM
543
NaCl; 3mM MgCl2; 1% Tween 40; 0,5% sodium deoxycholate). After 3 gently washes with cold PBS, cells
544
were fixed.
545
546
Images acquisition
547
.CC-BY 4.0 International licenseperpetuity. It is made available under a
preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in
The copyright holder for thisthis version posted December 24, 2021. ; https://doi.org/10.1101/2021.12.23.473962doi: bioRxiv preprint
28
For RRS, images of the cells were obtained using an Andor spinning disk : Olympus IX 83 inverted
548
microscope, equipped with a Yokaga CSU-X1 Spinning disk Unit and BOREALIS technology for
549
homogeneous illumination. The acquisition software is IQ3.
550
For RNAFish, UDS, TCR-UDS and IF of splicing complex after local damage, images of the cells were
551
obtained using a Zeiss LSM 780 NLO confocal laser scanning microscope and the following objective:
552
Plan-Apochromat 63X/1.4 oil DIC M27 or 40X/1.3 oil DIC. The acquisition software is ZEN.
553
PLA and some IF has been performed on a Zeiss Z1 imager right using a 40x/0.75 dry objective. The
554
acquisition software is Metavue.
555
All images were analyzed with ImageJ software.
556
557
Primary antibodies used for IF and PLA
558
Primary antibodies used for Immunofluorescence and PLA experiments were anti-XAB2 (mouse, sc-
559
271037 [Santa Cruz Biotechnology], 1/1000 dilution and rabbit, A303-638A [Béthyl], 1/500 dilution), anti-
560
AQR (IPB160 rabbit, A302-547A [Béthyl], 1/500 dilution), anti-CCDC16 (rabbit, HPA027211 [atlas
561
antibodies], 1/250 dilution), anti-PRP19 (rabbit, ab27699 [abcam], 1/500 dilution), anti-DNA:RNA hybrid
562
clone S9.6 (mouse, MABE1095 [Merck Millipore], 1/100 dilution and rabbit, Ab01137-23.0 [Absolute
563
antibody], 1/100 dilution), anti-CSA (rabbit, GTX100145 [genetex], 1/400 dilution), anti-XPG (rabbit,
564
sc84663 [santa-cruz], 1/1000 dilution), anti-Pol2tot (rabbit, A300-653A [Béthyl] 1/400 dilution, anti-
565
Pol2Ser2P (rabbit, ab5095 [abcam] 1/100 dilution) and anti-Pol2Ser5P (rabbit, 13523S [cell signaling]
566
1/1000 dilution).
567
568
Fluorescence Recovery after Photo-Bleaching (FRAP)
569
FRAP experiments were performed on a Zeiss LSM 780 NLO confocal laser scanning microscope (Zeiss),
570
using a 40x/1.3 oil objective, under a controlled environment (37°C, 5% CO2). A narrow region of interest
571
(ROI) centered across the nucleus of a living cell was monitored every 20 ms (1% laser intensity of the
572
488 nm line of a 25 mW Argon laser) until the fluorescence signal reached a steady state level (after ≈ 2
573
s). The same region was then photo-bleached for 20 ms at 100% laser intensity. Recovery of fluorescence
574
in the bleached ROI was then monitored (1% laser intensity) every 20 ms for about 20 seconds. Analysis
575
.CC-BY 4.0 International licenseperpetuity. It is made available under a
preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in
The copyright holder for thisthis version posted December 24, 2021. ; https://doi.org/10.1101/2021.12.23.473962doi: bioRxiv preprint
29
of raw data was performed with the ImageJ software. All FRAP data were normalized to the average pre-
576
bleached fluorescence after background removal.
577
Analysis of SPOT FRAP data was performed as follows (Figure S6). The average fluorescence (over all
578
cells) of the No UV condition was subtracted from the average fluorescence of the UV treated conditions.
579
The obtained difference between the two FRAP curves was then summed point by point, starting from
580
the bleach up to the following 100 measurements i.e. the area between the curve of interest and the
581
untreated condition curve.
582
583
Protein extraction
584
Cells cultured in 10-cm dishes were harvested by trypsinization and the pellet was washed once with PBS
585
supplemented with the Protease Inhibitor Cocktail (PIC, Roche). The extraction of nuclear proteins has
586
been performed using the CelLytic™ NuCLEAR™ Extraction kit (Sigma-Aldrich) complemented with PIC.
587
Protein concentration was determined using the Bradford method. The samples were diluted with
588
Laemmli buffer (10% glycerol, 5% β-mercaptoethanol, 3% sodium dodecyl sulfate, 100mM Tris-HCL [pH -
589
.8], bromophenol blue), and heated 95°C before loading on an SDS-PAGE gel.
590
591
Co-immunoprecipitation
592
For co-immunoprecipitation, 10 µl of protein G magnetic beads (Bio-adembead, Ademtech) were used
593
per IP. 1µg of anti-XAB2 antibody (rabbit, A303-638A, Bethyl) were bound to the beads in PBS with BSA
594
(3%) during 2h at 4°C with rotation. 100 µg of nuclear extracts were then incubated with beads-
595
antibodies complex for 2h at 4°C with rotation. After two washes at 100 mM salt, two washes at 150mM
596
and one wash at 100mM, beads were boiled in 2x Laemmli buffer and eluted samples loaded on a SDS
597
PAGE gel.
598
599
RNA/DNA Hybrid IP
600
Non-crosslinked MRC5 cells were lysed in 85 mM KCl, 5 mM PIPES (pH 8.0), and 0.5% NP-40 for 10 min
601
on ice. Pelleted nuclei were resuspended in RSB buffer (10 mM Tris-HCl pH 7.5, 200 mM NaCl, 2.5 mM
602
.CC-BY 4.0 International licenseperpetuity. It is made available under a
preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in
The copyright holder for thisthis version posted December 24, 2021. ; https://doi.org/10.1101/2021.12.23.473962doi: bioRxiv preprint
30
MgCl2) with 0.2% sodium deoxycholate, 0.1% SDS, 0.05% sodium lauroyl sarcosinate and 0.5% Triton X-
603
100, and extracts were sonicated for 10 min (Diagenode Bioruptor, 60 cycles high power, 10sec ON and
604
20 sec OFF). Extracts were then diluted 1:4 in RSB with 0.5% Triton X-100 (RSB + T) and subjected to IP
605
with the S9.6 antibody overnight at 4°C. RNaseA was added during IP at 0.1 ng RNaseA per microgram
606
genomic DNA. Then protein G dynabeads (Invitrogen) washed with RSB + T was added and incubated for
607
3h. Beads were washed 4x with RSB + T; 2x with RSB; and eluted in 2x Laemmli buffer for 10 min at 95°C
608
before loading on SDS-PAGE.
609
610
Western-blot
611
Proteins were separated on a SDS-PAGE gel composed of bisacrylamide (37:5:1), blotted onto a
612
polyvinylidene difluoride membrane (PVDF, 0.45μm Millipore) The membrane was blocked in PBS-T (PBS
613
and 0.1 % Tween 20) with 5 % milk and incubated for 2 h at RT or overnight at 4° C with the following
614
primary antibodies diluted in milk PBS-T : Rabbit anti-XAB2, A303-638A (Bethyl) 1/1000. Subsequently,
615
membrane was washed repeatedly with PBS-T and incubated 1 h at RT with the following secondary
616
antibody diluted 1/5000 in milk PBS-T: Goat anti-rabbit IgG HRP conjugate (170-6515; BioRad). After the
617
same washing procedure, protein bands were visualized via chemiluminescence (ECL Enhanced
618
Chemiluminescence; Pierce ECL Western Blotting Substrate) using the ChemiDoc MP system (BioRad).
619
620
Statistical analysis
621
Error bars represent the Standard Error of the Mean (SEM) of the biological replicates. Microsoft Excel
622
was used for statistical analysis and plotting of all the numerical data. Statistics were performed using a
623
test of student to compare two different conditions (siMock vs siRNA X or No UV irradiation vs after
624
irradiation) with the following parameters: two-tailed distribution and two-sample unequal variance
625
(heteroscedastic).
626
627
628
.CC-BY 4.0 International licenseperpetuity. It is made available under a
preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in
The copyright holder for thisthis version posted December 24, 2021. ; https://doi.org/10.1101/2021.12.23.473962doi: bioRxiv preprint
31
ACKNOWLEDGMENTS AND FUNDING SOURCES
629
This work has been funded by Agence Nationale de la Recherche (ANR-14-CE10-0009), Institut National
630
du Cancer (PLBIO17-043 et PLBIO19-126), La Ligue contre le Cancer (218398) et Électricité de France
631
(contrat 218398).
632
633
.CC-BY 4.0 International licenseperpetuity. It is made available under a
preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in
The copyright holder for thisthis version posted December 24, 2021. ; https://doi.org/10.1101/2021.12.23.473962doi: bioRxiv preprint
32
REFERENCES :
634
Belotserkovskii BP, Tornaletti S, D’Souza AD & Hanawalt PC (2018) R-loop generation during
635
transcription: Formation, processing and cellular outcomes. DNA Repair 71: 69–81
636
Boetefuer EL, Lake RJ & Fan H-Y (2018) Mechanistic insights into the regulation of transcription and
637
transcription-coupled DNA repair by Cockayne syndrome protein B. Nucleic Acids Res 46: 7471–
638
7479
639
van den Boom V, Citterio E, Hoogstraten D, Zotter A, Egly J-M, van Cappellen WA, Hoeijmakers JHJ,
640
Houtsmuller AB & Vermeulen W (2004) DNA damage stabilizes interaction of CSB with the
641
transcription elongation machinery. J Cell Biol 166: 27–36
642
Chatterjee N & Walker GC (2017) Mechanisms of DNA damage, repair and mutagenesis. Environ Mol
643
Mutagen 58: 235–263
644
Daniel L, Cerutti E, Donnio L-M, Nonnekens J, Carrat C, Zahova S, Mari P-O & Giglia-Mari G (2018)
645
Mechanistic insights in transcription-coupled nucleotide excision repair of ribosomal DNA. Proc
646
Natl Acad Sci U S A 115: E6770–E6779
647
Donnio L-M, Lagarou A, Sueur G, Mari P-O & Giglia-Mari G (2019) CSB-Dependent Cyclin-Dependent
648
Kinase 9 Degradation and RNA Polymerase II Phosphorylation during Transcription-Coupled
649
Repair. Mol Cell Biol 39: e00225-18
650
Giglia-Mari G, Zotter A & Vermeulen W (2011) DNA Damage Response. Cold Spring Harb Perspect
651
Biol 3: a000745
652
Goulielmaki E, Tsekrekou M, Batsiotos N, Ascensão-Ferreira M, Ledaki E, Stratigi K, Chatzinikolaou G,
653
Topalis P, Kosteas T, Altmüller J, et al (2021) The splicing factor XAB2 interacts with ERCC1-
654
XPF and XPG for R-loop processing. Nat Commun 12: 3153
655
Hanawalt PC (1994) Transcription-coupled repair and human disease. Science 266: 1957–1958
656
Higa M, Zhang X, Tanaka K & Saijo M (2016) Stabilization of Ultraviolet (UV)-stimulated Scaffold
657
Protein A by Interaction with Ubiquitin-specific Peptidase 7 Is Essential for Transcription-coupled
658
Nucleotide Excision Repair. J Biol Chem 291: 13771–13779
659
Kuraoka I, Ito S, Wada T, Hayashida M, Lee L, Saijo M, Nakatsu Y, Matsumoto M, Matsunaga T, Handa
660
H, et al (2008) Isolation of XAB2 complex involved in pre-mRNA splicing, transcription, and
661
transcription-coupled repair. J Biol Chem 283: 940–950
662
Maréchal A, Li J-M, Ji XY, Wu C-S, Yazinski SA, Nguyen HD, Liu S, Jiménez AE, Jin J & Zou L (2014)
663
PRP19 transforms into a sensor of RPA-ssDNA after DNA damage and drives ATR activation via
664
a ubiquitin-mediated circuitry. Mol Cell 53: 235–246
665
Marteijn JA, Lans H, Vermeulen W & Hoeijmakers JHJ (2014) Understanding nucleotide excision repair
666
and its roles in cancer and ageing. Nat Rev Mol Cell Biol 15: 465–481
667
Moné MJ, Bernas T, Dinant C, Goedvree FA, Manders EMM, Volker M, Houtsmuller AB, Hoeijmakers
668
JHJ, Vermeulen W & van Driel R (2004) In vivo dynamics of chromatin-associated complex
669
formation in mammalian nucleotide excision repair. Proc Natl Acad Sci U S A 101: 15933–15937
670
Mourgues S, Gautier V, Lagarou A, Bordier C, Mourcet A, Slingerland J, Kaddoum L, Coin F, Vermeulen
671
W, Gonzales de Peredo A, et al (2013) ELL, a novel TFIIH partner, is involved in transcription
672
restart after DNA repair. Proc Natl Acad Sci 110: 17927–17932
673
Mullenders L (2015) DNA damage mediated transcription arrest: Step back to go forward. DNA Repair
674
36: 28–35
675
.CC-BY 4.0 International licenseperpetuity. It is made available under a
preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in
The copyright holder for thisthis version posted December 24, 2021. ; https://doi.org/10.1101/2021.12.23.473962doi: bioRxiv preprint
33
Nakatsu Y, Asahina H, Citterio E, Rademakers S, Vermeulen W, Kamiuchi S, Yeo JP, Khaw MC, Saijo
676
M, Kodo N, et al (2000) XAB2, a novel tetratricopeptide repeat protein involved in transcription-
677
coupled DNA repair and transcription. J Biol Chem 275: 34931–34937
678
Onyango DO, Howard SM, Neherin K, Yanez DA & Stark JM (2016) Tetratricopeptide repeat factor
679
XAB2 mediates the end resection step of homologous recombination. Nucleic Acids Res 44:
680
5702–5716
681
Politi A, Moné MJ, Houtsmuller AB, Hoogstraten D, Vermeulen W, Heinrich R & Driel R van (2005)
682
Mathematical Modeling of Nucleotide Excision Repair Reveals Efficiency of Sequential
683
Assembly Strategies. Mol Cell 19: 679–690
684
Rademakers S, Volker M, Hoogstraten D, Nigg AL, Moné MJ, Zeeland AA van, Hoeijmakers JHJ,
685
Houtsmuller AB & Vermeulen W (2003) Xeroderma Pigmentosum Group A Protein Loads as a
686
Separate Factor onto DNA Lesions. Mol Cell Biol 23: 5755–5767
687
Sakasai R, Isono M, Wakasugi M, Hashimoto M, Sunatani Y, Matsui T, Shibata A, Matsunaga T &
688
Iwabuchi K (2017) Aquarius is required for proper CtIP expression and homologous
689
recombination repair. Sci Rep 7: 13808
690
Sollier J, Stork CT, García-Rubio ML, Paulsen RD, Aguilera A & Cimprich KA (2014) Transcription-
691
coupled nucleotide excision repair factors promote R-loop-induced genome instability. Mol Cell
692
56: 777–785
693
Steurer B & Marteijn JA (2017) Traveling Rocky Roads: The Consequences of Transcription-Blocking
694
DNA Lesions on RNA Polymerase II. J Mol Biol 429: 3146–3155
695
Tresini M, Warmerdam DO, Kolovos P, Snijder L, Vrouwe MG, Demmers JAA, van IJcken WFJ,
696
Grosveld FG, Medema RH, Hoeijmakers JHJ, et al (2015) The core spliceosome as target and
697
effector of non-canonical ATM signaling. Nature 523: 53–58
698
de Waard H, de Wit J, Andressoo J-O, van Oostrom CTM, Riis B, Weimann A, Poulsen HE, van Steeg H,
699
Hoeijmakers JHJ & van der Horst GTJ (2004) Different Effects of CSA and CSB Deficiency on
700
Sensitivity to Oxidative DNA Damage. Mol Cell Biol 24: 7941–7948
701
Wang IX, Grunseich C, Fox J, Burdick J, Zhu Z, Ravazian N, Hafner M & Cheung VG (2018) Human
702
proteins that interact with RNA/DNA hybrids. Genome Res 28: 1405–1414
703
Yonemasu R, Minami M, Nakatsu Y, Takeuchi M, Kuraoka I, Matsuda Y, Higashi Y, Kondoh H &
704
Tanaka K (2005) Disruption of mouse XAB2 gene involved in pre-mRNA splicing, transcription
705
and transcription-coupled DNA repair results in preimplantation lethality. DNA Repair 4: 479–
706
491
707
Zotter A, Luijsterburg MS, Warmerdam DO, Ibrahim S, Nigg A, van Cappellen WA, Hoeijmakers JHJ,
708
van Driel R, Vermeulen W & Houtsmuller AB (2006) Recruitment of the nucleotide excision
709
repair endonuclease XPG to sites of UV-induced dna damage depends on functional TFIIH. Mol
710
Cell Biol 26: 8868–8879
711
712
.CC-BY 4.0 International licenseperpetuity. It is made available under a
preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in
The copyright holder for thisthis version posted December 24, 2021. ; https://doi.org/10.1101/2021.12.23.473962doi: bioRxiv preprint
