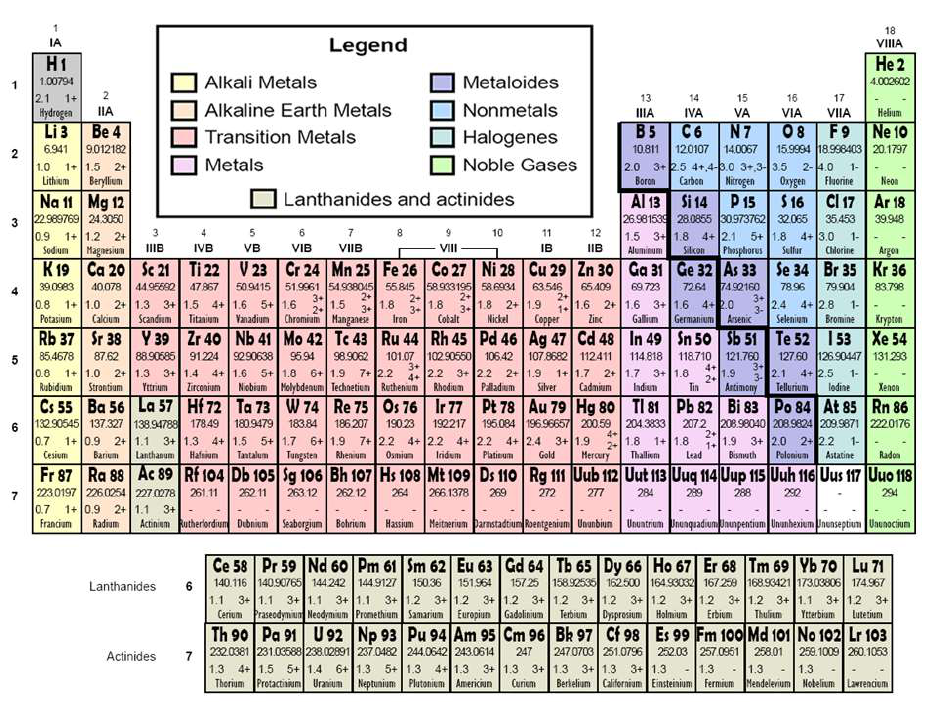
8U1 - 5(C) interpret the arrangement of the Periodic Table, including groups
and periods, to explain how properties are used to classify elements;
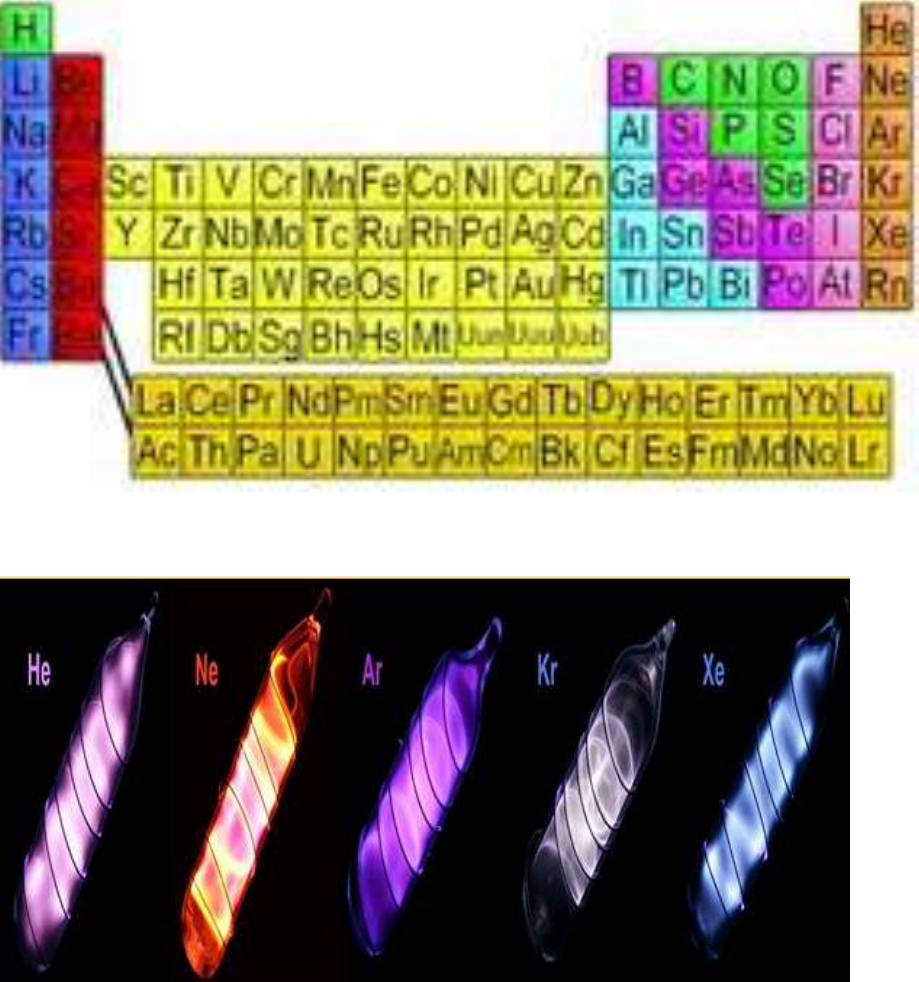
The Noble Gasses, Helium, Neon, Argon, Krypton, and Xenon glow when heated.
Elements have characteristic colors when burned.
periodic
table
An arrangement of the elements in order
of their atomic numbers. elements with
similar properties fall in the same column,
or group.
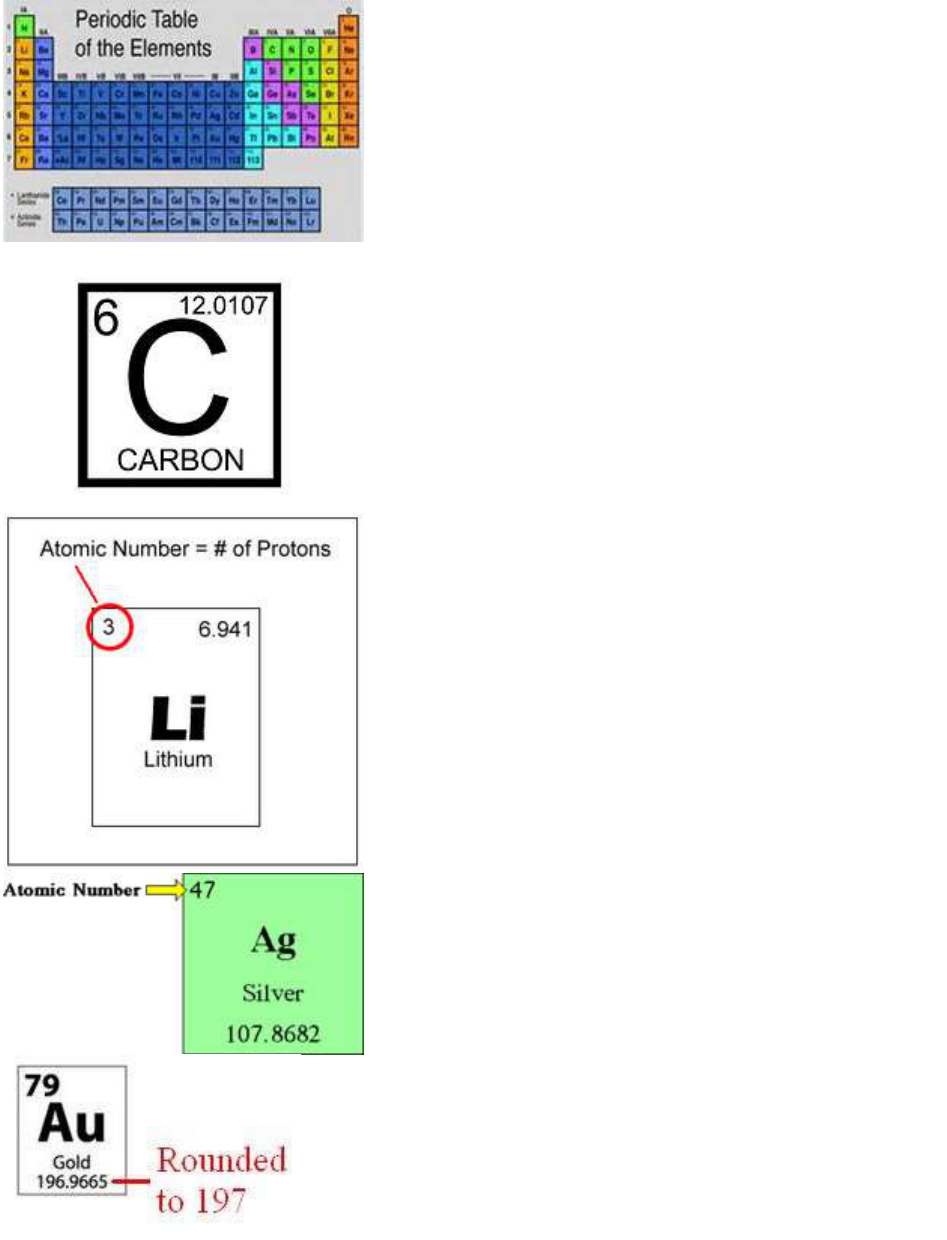
Periodic Table
Square
Information
On the periodic table, the
number in the left corner
is the number of protons,
the number in the right
corner is the atomic
weight, the Symbol for the
element is in the center,
and the name is on the
bottom.
Atomic
number
The number of protons,
unique to each element, in
an atom
Atomic Number
The number of protons in
the nucleus of an atom.
Atomic Mass
total weight of protons
and neutrons
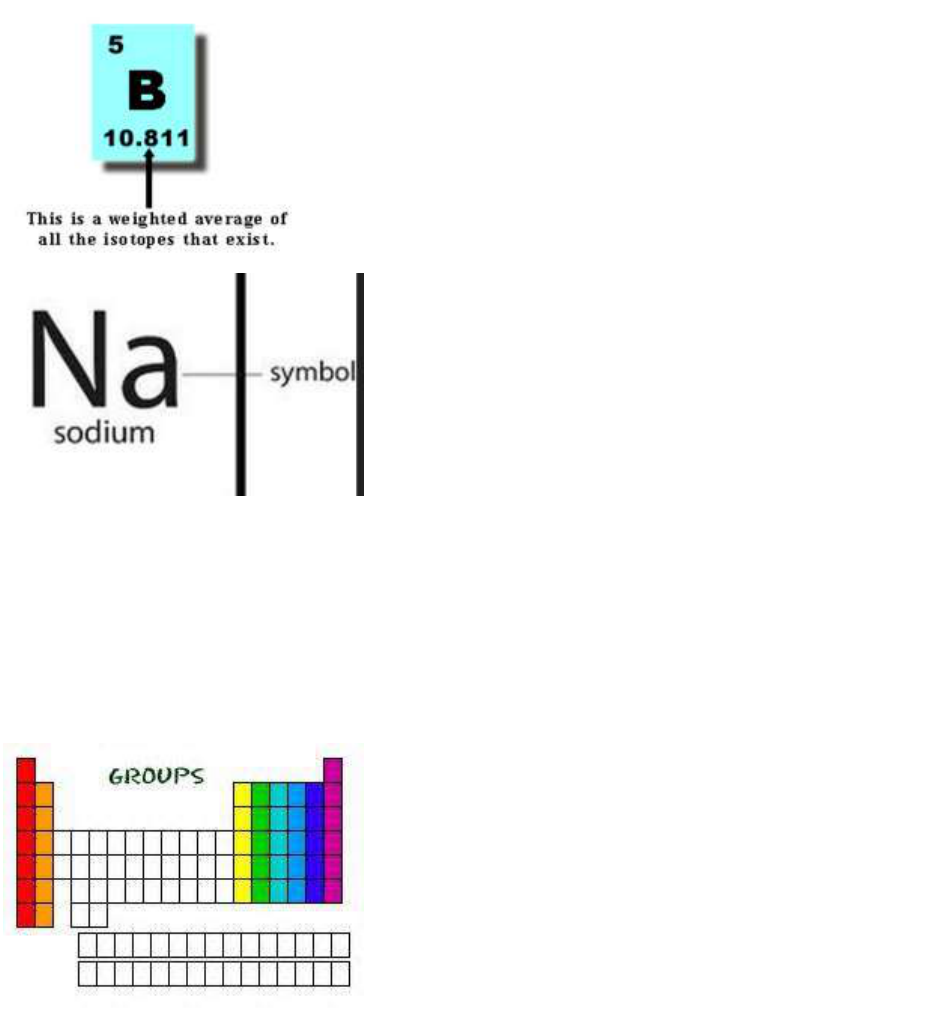
Atomic Mass
The average mass of one
atom of an element.
Chemical
symbol
One or two letter code that
stands for an element;
many symbols are
abbreviations of the
elements named, which
may be English, Latin or
Greek in origin
•
Finding the
Number of
Neutrons in an
Atom
round off the atomic mass
to the nearest whole
number and subtract the
atomic number from it.
•
Finding the
Number of
Protons or
Electrons in an
Atom
look at the atomic number
on the periodic table
family/group
A vertical column of
elements in the periodic
table; elements in a
group share chemical
properties. Because they
have the same number of
valence electrons, they
have similar properties
and reactivities

period
Horizontal row in
periodic table.
Properties are NOT
similar across periods!
Reactive
Elements like oxygen, hydrogen, and sodium
are very reactive. They can explode easily
and must be handled with care in their pure
form.
Inert
Elements such as Helium, Neon, Argon,
Krypton, and Xenon are known as the “noble”
gasses because they are inert – they do not
react with other elements. Nitrogen is a fairly
inert element.