Original Paper
Using Fitness Trackers and Smartwatches to Measure Physical
Activity in Research:Analysis of Consumer Wrist-Worn Wearables
André Henriksen
1
, MSc (Comp Sci), MBA; Martin Haugen Mikalsen
2
, BSc (Comp Sci); Ashenafi Zebene Woldaregay
2
,
MSc (Comp Eng), MSc (Telemed & e-Health); Miroslav Muzny
3,4
, MSc (Comp Sci); Gunnar Hartvigsen
2
, MSc
(Comp Sci), PhD; Laila Arnesdatter Hopstock
5
, RN, CRNA, MSc (Nursing), PhD; Sameline Grimsgaard
1
, MPH,
MD, PhD
1
Department of Community Medicine, University of Tromsø – The Arctic University of Norway, Tromsø, Norway
2
Department of Computer Science, University of Tromsø – The Arctic University of Norway, Tromsø, Norway
3
Norwegian Centre for E-health Research, University Hospital of North Norway, Tromsø, Norway
4
Spin-Off Company and Research Results Commercialization Center, 1st Faculty of Medicine, Charles University in Prague, Prague, Czech Republic
5
Department of Health and Care Sciences, University of Tromsø – The Arctic University of Norway, Tromsø, Norway
Corresponding Author:
André Henriksen, MSc (Comp Sci), MBA
Department of Community Medicine
University of Tromsø – The Arctic University of Norway
Postboks 6050 Langnes
Tromsø, 9037
Norway
Phone: 47 77644000
Email: [email protected]
Abstract
Background: New fitness trackers and smartwatches are released to the consumer market every year. These devices are equipped
with different sensors, algorithms, and accompanying mobile apps. With recent advances in mobile sensor technology, privately
collected physical activity data can be used as an addition to existing methods for health data collection in research. Furthermore,
data collected from these devices have possible applications in patient diagnostics and treatment. With an increasing number of
diverse brands, there is a need for an overview of device sensor support, as well as device applicability in research projects.
Objective: The objective of this study was to examine the availability of wrist-worn fitness wearables and analyze availability
of relevant fitness sensors from 2011 to 2017. Furthermore, the study was designed to assess brand usage in research projects,
compare common brands in terms of developer access to collected health data, and features to consider when deciding which
brand to use in future research.
Methods: We searched for devices and brand names in six wearable device databases. For each brand, we identified additional
devices on official brand websites. The search was limited to wrist-worn fitness wearables with accelerometers, for which we
mapped brand, release year, and supported sensors relevant for fitness tracking. In addition, we conducted a Medical Literature
Analysis and Retrieval System Online (MEDLINE) and ClinicalTrials search to determine brand usage in research projects.
Finally, we investigated developer accessibility to the health data collected by identified brands.
Results: We identified 423 unique devices from 132 different brands. Forty-seven percent of brands released only one device.
Introduction of new brands peaked in 2014, and the highest number of new devices was introduced in 2015. Sensor support
increased every year, and in addition to the accelerometer, a photoplethysmograph, for estimating heart rate, was the most common
sensor. Out of the brands currently available, the five most often used in research projects are Fitbit, Garmin, Misfit, Apple, and
Polar. Fitbit is used in twice as many validation studies as any other brands and is registered in ClinicalTrials studies 10 times as
often as other brands.
Conclusions: The wearable landscape is in constant change. New devices and brands are released every year, promising improved
measurements and user experience. At the same time, other brands disappear from the consumer market for various reasons.
Advances in device quality offer new opportunities for research. However, only a few well-established brands are frequently
used in research projects, and even less are thoroughly validated.
J Med Internet Res 2018 | vol. 20 | iss. 3 | e110 | p. 1http://www.jmir.org/2018/3/e110/
(page number not for citation purposes)
Henriksen et alJOURNAL OF MEDICAL INTERNET RESEARCH
XSL
•
FO
RenderX

(J Med Internet Res 2018;20(3):e110) doi: 10.2196/jmir.9157
KEYWORDS
motor activity; physical activity; fitness trackers; heart rate; photoplethysmography
Introduction
Background
The World Health Organization recommends 150 min of
moderate intensity physical activity (PA) each week for adults
and 60 min for children and adolescents [1]. However, 25% of
adults and more than 80% of adolescents do not achieve the
recommended PA targets [1]. Results from the Tromsø Study,
the longest running population study in Norway, shows that
only 30.4% of women and 22.0% of men reach the
recommended target [2].
Low PA is currently the fourth leading risk factor for mortality
worldwide [3]. Even though there is limited evidence that using
wearable fitness trackers will improve health [4,5], these devices
are still popular, and new fitness devices appear on the consumer
market regularly. In 2016, vendors shipped 102 million devices
worldwide, compared with 82 million in 2015 [6]. Fifty-seven
percent of these devices were sold by the top five brands: Fitbit,
Xiaomi, Apple, Garmin, and Samsung. The first quarter of 2017
shows an increase of 18% in devices sold, compared with the
same period in 2016 [7]. With a large number of available
devices and brands, it is difficult to navigate through an
ever-growing list of brands and devices with different
capabilities, price, and quality.
Available sensors and internal interpreting algorithms determine
device output. Sensor data are, in most devices, reduced to a
limited set of metrics before being transferred to the user’s
mobile phone. In addition, limited space affects how long the
device can collect data before such a transfer is needed. Data
are stored locally, and in many cases, uploaded to brand specific
or open cloud–based health repositories. Accessing these data
by third-party apps and comparing them is not always possible.
These interoperability challenges were recently identified in a
study by Arriba-Pérez et al [8]. They suggested ways to handle
these issues, but they did not make any brand or device
recommendations. Several studies have compared
activity-tracking wearables. As an example, Kaewkannate and
Kim [9] did a comparison of four popular fitness trackers in
2016. They compared devices objectively and subjectively.
Data were thoroughly collected, but because of the rapid release
of new devices, these four devices will be among the most
popular only for a relatively short time. A comparison of brands
is also of interest because brands from larger companies are,
compared with small start-ups and crowd funded brands, likely
to survive longer. In addition, it is of interest to know which
brands support the various available programming options.
Sanders et al [10] did a literature review on articles using
wearables for health self-monitoring and sedentary behavior
and PA detection. They reviewed various aspects of these
devices, but they gave no details about device sensor support
and suitability in research.
The objective of this study was to examine how the consumer
market for wearables has evolved, and analyze and summarize
available devices that can measure PA and heart rate (HR).
Moreover, we aim to identify brands that are used extensively
in research projects, and compare and consider their relevance
for future studies.
Sensors
A plethora of devices promises to measure PA in new and
improved ways. These devices use different sensors and
algorithms to calculate human readable metrics based on sensor
output. Traditional step counters use pedometers to detect daily
step counts. Although cheap and energy efficient, pedometers
are not as accurate as accelerometers, which is the current
standard for collecting PA data [11]. All modern fitness trackers
and smartwatches have an accelerometer. Compared with
research tools (eg, ActiGraph [12]), these devices are considered
less accurate for some measurements [13,14]. However, they
are generally less invasive, cheaper, have more functionality,
are more user-friendly, and are increasingly being used in
research. Most accelerometer-based fitness wearables measure
acceleration in three directions [15] and can be used to estimate
type of movement, count steps, calculate energy expenditure
(EE) and energy intensity, as well as estimate sleep patterns
and more. The validity and reliability of these metrics varies.
Evenson et al [14] did a review in 2015 and found high validity
for steps but low validity for EE and sleep. Furthermore, they
found reliability for steps, distance, EE, and sleep to be high
for some devices.
In addition, some wearables have gyroscopes, magnetometers,
barometers, and altimeters. A gyroscope can potentially increase
device accuracy by measuring gravitational acceleration, that
is, orientation and angular velocity, and better estimate which
activity type a person is performing [16]. A magnetometer is a
digital compass [15] and can improve motion tracking accuracy
by detecting the orientation of the device relative to magnetic
north. Magnetometers improve accuracy by compensating for
gyroscope drift, a problem with gyroscopes where the rotation
axis slowly drifts from the actual motion and must be restored
regularly. Accelerometers, gyroscopes, and magnetometers are
often combined into an inertial measurement unit (IMU). Most
mobile phones use IMUs to calculate orientation, and an
increasing number of fitness wearables include this unit to give
more accurate metrics. Barometers or altimeters detect changes
in altitude [15] and can be used to improve some metrics (eg,
EE), as well as report additional metrics (eg, climbed floors).
Photoplethysmography (PPG) is a relatively new technique in
wearables. PPG is an optical technique to estimate HR by
monitoring changes in blood volume beneath the skin [17]. A
light-emitting diode projects light onto the skin, which is
affected by the HR and reflected back to the sensor. However,
movement, ambient light, and tissue compression affect the
light, resulting in signal noise, and cleaning algorithms often
J Med Internet Res 2018 | vol. 20 | iss. 3 | e110 | p. 2http://www.jmir.org/2018/3/e110/
(page number not for citation purposes)
Henriksen et alJOURNAL OF MEDICAL INTERNET RESEARCH
XSL
•
FO
RenderX

use accelerometer data to assist HR estimation [18]. There is
some evidence that gyroscopes could be used [19] to reduce
PPG signal noise, so we are likely to see more devices in the
future equipped with PPG sensors. To further enrich the PA
data collection, some devices have a built in global positioning
system (GPS) receiver. This is especially true for high-end
fitness trackers and sports watches specifically targeting
physically active people. With a GPS, it is possible to track
more data, including position, speed, and altitude.
Algorithms and Mobile Apps
Raw data from sensors must be converted into readable metrics
to be meaningful for the user. Many devices only display a
limited set of metrics directly on the device (eg, today’s step
count or current HR) and rely on an accompanying mobile app
to show the full range of available metrics (eg, historic daily
step count and detailed HR data). Although the physical sensors
in these devices are very similar, the algorithms that interpret
sensor output are unique for most vendors. These algorithms
are often company secrets, and they can be changed without
notice. In addition, the quality and supported features of the
accompanying mobile apps varies, and the total user experience
will therefore differ. Each additional sensor included in a device
can be used to add additional types of metrics for the user or
supply internal algorithms with additional data to improve
accuracy of already available metric types. However, additional
sensors affect price and power consumption.
Device Types
There are many similarities between different types of devices,
and they may be difficult to categorize. We will use the term
wearable in this paper as a common term for wrist-worn devices
that can track and share PA data with a mobile phone.
A smartwatch is a wrist-worn device that, mostly, acts as an
extension to a mobile phone and can show notifications and
track PA and related metrics. Modern smartwatches often
include a touch screen and can support advanced features and
display high resolution activity trends [15]. Fitness trackers (ie,
smart band or fitness band), normally worn on the wrist or hip,
are devices more dedicated to PA tracking. A fitness tracker is
typically cheaper than a smartwatch because of less expensive
hardware and often fewer sensors. Due to this, it generally also
has better battery life and a limited interface for displaying
tracking results [15].
Other terms are also used, for example, sports watch and GPS
watch, which can be considered merges between smartwatches
and fitness trackers. In addition, there are hybrid watches (ie,
hybrid smartwatches) that have a traditional clockwork and
analogue display that have been fitted with an accelerometer.
An accompanying mobile app is needed to access most data,
but daily step counts are often represented as an analogue gauge
on the watch face.
Wearable Usage Scenario
Wearables come forward as a new alternative to tracking PA
in research (compared with, eg, ActiGraph), especially when it
is desired to collect measurements for a prolonged period of
time. In an intervention study, continuous data collecting from
wearables would allow researchers to better track changes in
PA and adjust the intervention accordingly. Wearables can also
be used in epidemiological research as a tool for tracking PA
for an extended period. This could reveal detailed PA changes
in a population over time. In both scenarios, there are several
potential important requirements to consider when choosing a
device for the study, including usability, battery life, price,
accuracy, durability, look and feel, and data access possibilities.
Methods
Search Strategies
Brands, Devices, and Sensors
We searched six databases to create a list of relevant wearable
devices: The Queen’s University’s Wearable Device Inventory
[20], The Vandrico Wearables database [21], GsmArena [22],
Wearables.com [23], SpecBucket [24], and PrisGuide [25,26].
We only used publicly available information when comparing
devices. We did the search from May 15, 2017 to July 1, 2017.
We identified wearables in two steps. In step one, we identified
and searched the six defined databases. In step two, we extracted
all brands from the list of devices identified in step one and
examined brand websites for additional devices. If we found
the same device in several databases with conflicting
information, we manually identified the correct information
from the device’s official website or other online sources (eg,
Wikipedia and Google search). We removed duplicates and
devices not fitting the inclusion criteria.
Brand Usage in Research
We searched Ovid MEDLINE on September 30, 2017 to
determine how often the most relevant brands were used in
previous studies. For each search, we performed a keyword
search with no limitations set. We divided our findings into
validation and reliability studies and data collection studies.
To decide which brand to consider most relevant, we did two
sets of searches. In the first set, we created a brand-specific
keyword search for brands that were (1) One of the five most
sold brands in 2015 or 2016 or (2) Had released 10 or more
unique devices. From the resulting list of articles, we screened
title, abstract, and the method section. This screening was done
to (1) Exclude articles out of scope and (2) To identify additional
brands used in these studies. We compiled a list of these brands
and performed a second set of searches, one for each new
identified brand. Eleven brands were finally included. The
specific keyword search used for each brand is given in the
Results section where we summarize our findings.
We also searched the US National Library of Medicine database
of clinical studies through the ClinicalTrials website, using the
same 11 keyword searches, to determine brand usage in ongoing
projects. One author did the articles screening, as well as the
projects description screening in ClinicalTrials.
Brand Developer Possibilities
To determine how relevant a specific brand is when planning
a new research project, we reviewed the 11 identified brands
and considered available developer options, supported mobile
J Med Internet Res 2018 | vol. 20 | iss. 3 | e110 | p. 3http://www.jmir.org/2018/3/e110/
(page number not for citation purposes)
Henriksen et alJOURNAL OF MEDICAL INTERNET RESEARCH
XSL
•
FO
RenderX

phone environments, and options for health data storage. We
especially reviewed availability of an application programming
interface (API) and a software development kit (SDK).
Information was collected from Google Play, Apple’s App
Store, and official brand websites. Information retrieval was
done in September 2017.
Inclusion and Exclusion Criteria
Brands, Devices, and Sensors
The study is limited to wrist-worn consumer devices that utilize
accelerometers to measure PA. Devices capable of collecting
HR from the wrist using an optical sensor were tagged as PPG
devices. Devices were tagged as GPS devices only if they had
a built-in GPS tracker. We only included devices meant for
personal use, designed to be worn continuously (24/7), and were
capable of sharing data with mobile phones through Bluetooth.
The wrist-worn limitation was added because hip-worn devices
are not normally worn during the night (ie, not 24/7). Only
devices released before July 1, 2017 were included. We excluded
hybrid watches because most hybrid vendors make a large
number of watch variations, with what seems to be the same
hardware. In addition, these watches are mostly available
through high-end suppliers of traditional watches, at a price
point that would prevent researchers from considering their use
in a large study.
Brand Usage in Research
Due to the large number of available brands, we limited our
search to include only the 11 brands already identified as
relevant. We excluded brands that are no longer available (ie,
company shut down). Review studies were also excluded.
Brand Developer Possibilities
When reviewing brand relevance in research, we only reviewed
developer capabilities for the 11 brands we had already included
in the list of relevant brands. We set the additional limitation
that the brand was used in at least one article in Ovid
MEDLINE.
Device Categorization, Data Collection, and Reporting
Categories
When collecting information about wearables, we categorized
them into three groups:
1.
Smartwatches: a device was tagged as a smartwatch if
•
It supported mobile phone notifications, and the vendor
described it as a smart watch, or if
•
It had a touch screen and was not explicitly described
as a fitness tracker by the vendor.
2.
Fitness trackers: we classified a device as a fitness tracker
if
•
Its main purpose was to track PA, or if
•
The vendor called it a fitness tracker, or if
•
The device did not support notifications from the
connected mobile phone (eg, incoming calls or texts).
3.
Hybrid watches: to be considered a hybrid watch, the device
had to have an analogue clockwork with a built-in digital
accelerometer.
We collected the following variables for each device: brand
name, device name, year of release, country of origin, device
type (eg, fitness tracker), and whether they had a built-in
accelerometer, gyroscope, magnetometer, barometer or altimeter,
GPS, and PPG.
We looked at three aspects of the devices we identified and
reported under three categories:
1.
Metrics and trends: in this category, we described the status
for available brands, devices, and sensors, as well as
reviewed trends in sensor availability over time.
2.
Brand usage in research: in this category, we searched Ovid
MEDLINE and ClinicalTrials and determined which brands
are most used in a research setting.
3.
Brand developer possibilities: in this category, we reviewed
software integration platforms and mobile platform support
for the most relevant brands.
Results
Relevant Devices
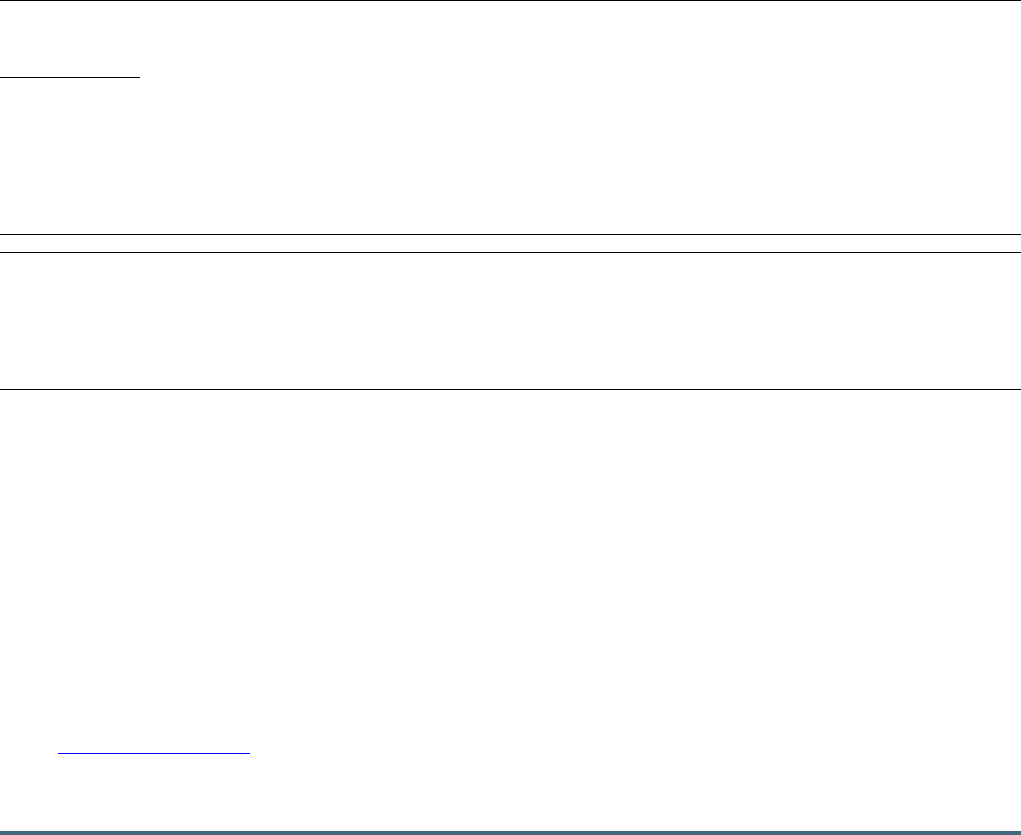
An overview of the device search process is given in Figure 1.
We found 572 devices by searching online and offline databases
and 131 additional devices by visiting the official websites for
each identified brand, totaling 703 devices. Removing duplicates
left 567 unique devices. These were screened for variation, that
is, the same device with different design. After excluding 41
because of variation, 526 remained and were screened for
eligibility. We removed 103 devices for not fitting the inclusion
criteria. The remaining 423 devices were included in the study.
Brands, Devices, and Sensors
Brands
We identified 423 unique wearables, distributed between 132
different brands. Almost half the brands (47.0%, 62/132) had
only one device. Moreover, 75.0% (99/132) of brands had three
or fewer devices, and 83.3% (110/132) had five or fewer
devices. Brands originated from 23 different countries, but the
United States (43.2%, 57/132) and China (16.7%, 22/132,
mainland China; 19.0%, 25/132, including Taiwan) represented
the largest number of brand origin. Each remaining country
represented between 0.8% (1/132) and 5.3% (7/132) of brands.
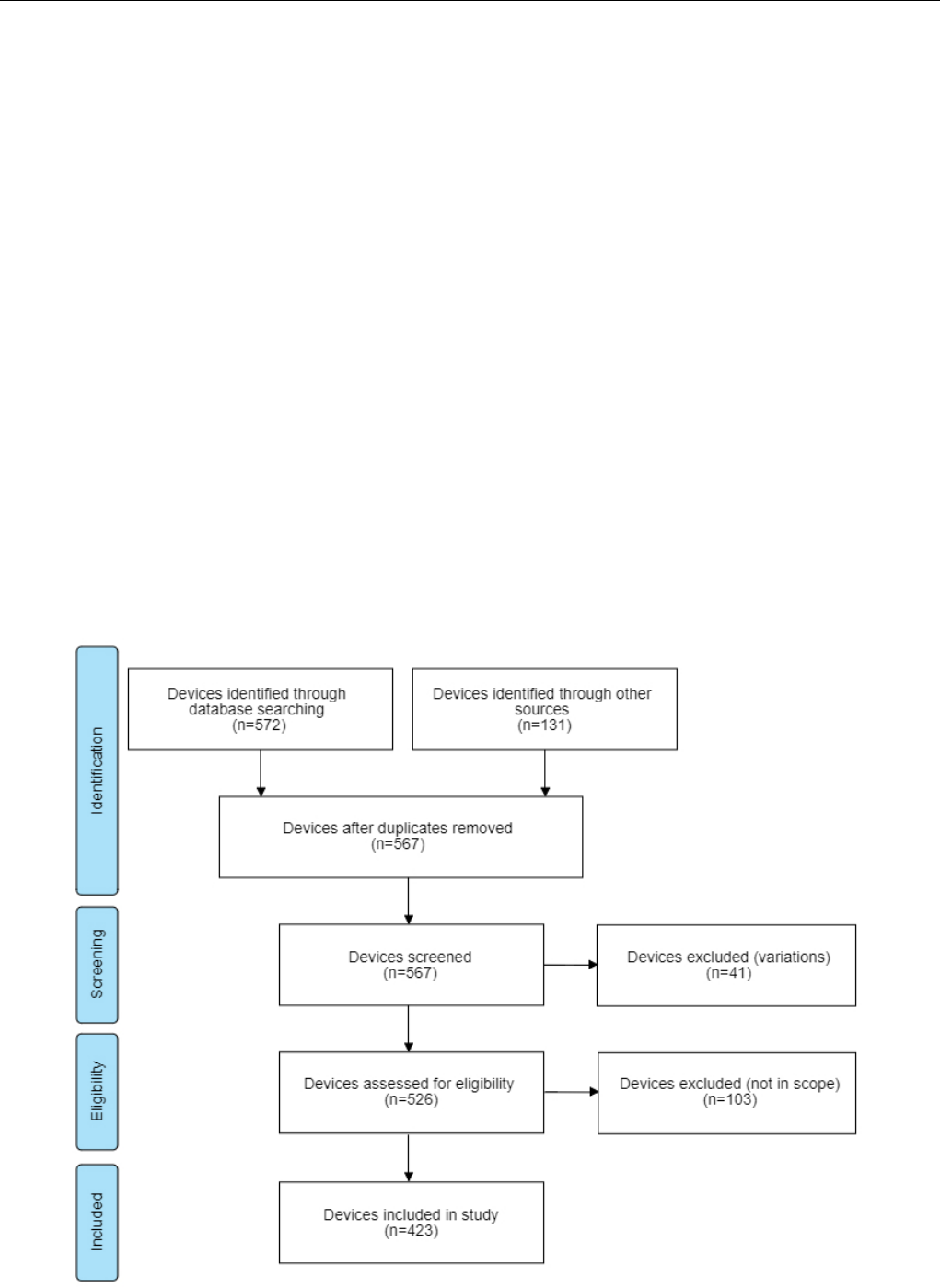
As the market has grown and wearable technology has become
increasingly popular, a number of new brands have appeared
on the market. In 2011, there were only three brands available.
There was a small increase in brand count in 2012 and 2013,
but in 2014, we saw the largest increase with 41 new brands.
The number of new brands started to decrease in 2015, with 36
new brands in 2015 and 23 in 2016. Only three new brands have
been introduced in 2017, but this number only represents the
first 6 months of 2017. The final count for 2017 will likely be
higher. An overview of the number of new brands that appeared
on the market between 2011 and 2017 is given in Figure 2. Note
that some companies are no longer active and, for 17 devices,
we could not determine release year.
Most brands only had a small number of wearables, but some
produced a lot more. The brand with most unique wearables
J Med Internet Res 2018 | vol. 20 | iss. 3 | e110 | p. 4http://www.jmir.org/2018/3/e110/
(page number not for citation purposes)
Henriksen et alJOURNAL OF MEDICAL INTERNET RESEARCH
XSL
•
FO
RenderX
was Garmin (United States) with 40 different devices. No.1
(China) introduced the second highest number of wearables
with 19 devices. An overview of the release year of the 22 (out
of 132) brands that have released more than five devices is given
in Table 1. Seven out of these 22 brands originated in the United
States, five (six including Taiwan) originated in China, and two
originated in South Korea. All other countries are represented
only once. Some of these brands are no longer active (eg, Pebble
and Jawbone).
Devices
Three devices were released in 2011 (earliest year), seven in
2012, 30 in 2013, and 87 in 2014. The year with the highest
number of new wearables was 2015, with 121 new devices. In
2016, 120 new devices were released; the first year with a
decreasing number of new wearables. The number of new and
accumulated devices from 2011 to 2017 is summarized in Table
2. The last column (unknown) represents devices where we
could not identify the release year. The above numbers represent
the total number of new devices. If grouped into fitness trackers
and smartwatches, there is a small overrepresentation among
new smartwatches. Up until 2014, about half of devices were
smartwatches. In 2015 and 2016, smartwatches represented
59.3% (143/241) of new devices, whereas fitness trackers
represented 40.6% (98/241).
Sensors
The number of sensors included in new devices have increased
in the last few years. Since 2015, the order of the most common
sensors has consistently been PPG, GPS, gyroscope,
magnetometer, and barometer or altimeter. In addition, these
sensors have had a steady increase in availability in the same
period. For 2017, 71% (27/38) of new devices included a PPG
sensor, 50% (19/38) included a GPS, 39% (15/38) included a
gyroscope, 34% (13/38) included a magnetometer, and 32%
(12/38) included a barometer or altimeter. Figure 3 gives an
overview of the number of devices each year that includes each
sensor, in percent of total number of released devices that year.
Devices with more than one sensor are represented once for
each sensor it includes.
In total, since 2011, 38.5% (163/423) of wearables have only
been equipped with one sensor (accelerometer). Moreover,
29.8% (126/423) of devices had two sensors, 12.1% (51/423)
had three sensors, 11.1% (47/423) had four sensors, and 6.4%
(27/423) had five sensors. Only 2.1% (9/423) of devices had
all six sensors. In Table 3, these numbers are broken down by
sensor combination and year. Some sensor combinations do not
exist and are excluded.
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart.
J Med Internet Res 2018 | vol. 20 | iss. 3 | e110 | p. 5http://www.jmir.org/2018/3/e110/
(page number not for citation purposes)
Henriksen et alJOURNAL OF MEDICAL INTERNET RESEARCH
XSL
•
FO
RenderX
Figure 2. Number of new and aggregated available brands by year.
Table 1. Device count per year for brands with six or more wearables.
Total
a
Unknown2017201620152014201320122011CountryBrand
4041311651United StatesGarmin
914211United StatesFitbit
821311United StatesMisfit
7151United StatesLifeTrak
6141United StatesiFit
63111United StatesJawbone
61311United StatesPebble
19595ChinaNo. 1
9252ChinaOmate
9252ChinaZeblaze
81331ChinaHuawei
711221ChinaOumax
8422TaiwanMobile Action
124161South KoreaSamsung
72113South KoreaLG
7511EnglandWorldSim
1122421FinlandPolar
624GermanyTechnaxx
743ItalyAwatch
752JapanEpson
7412NetherlandsTomTom
181764SwitzerlandMyKronoz
a
Total brand count for the United States=7, China and Taiwan=6, and South Korea=2. All other countries are represented only once.
Table 2. Number of new and accumulated devices by year.
Unknown2017201620152014201320122011Devices
1738120121873073New
42340636824812740103Accumulated
J Med Internet Res 2018 | vol. 20 | iss. 3 | e110 | p. 6http://www.jmir.org/2018/3/e110/
(page number not for citation purposes)
Henriksen et alJOURNAL OF MEDICAL INTERNET RESEARCH
XSL
•
FO
RenderX
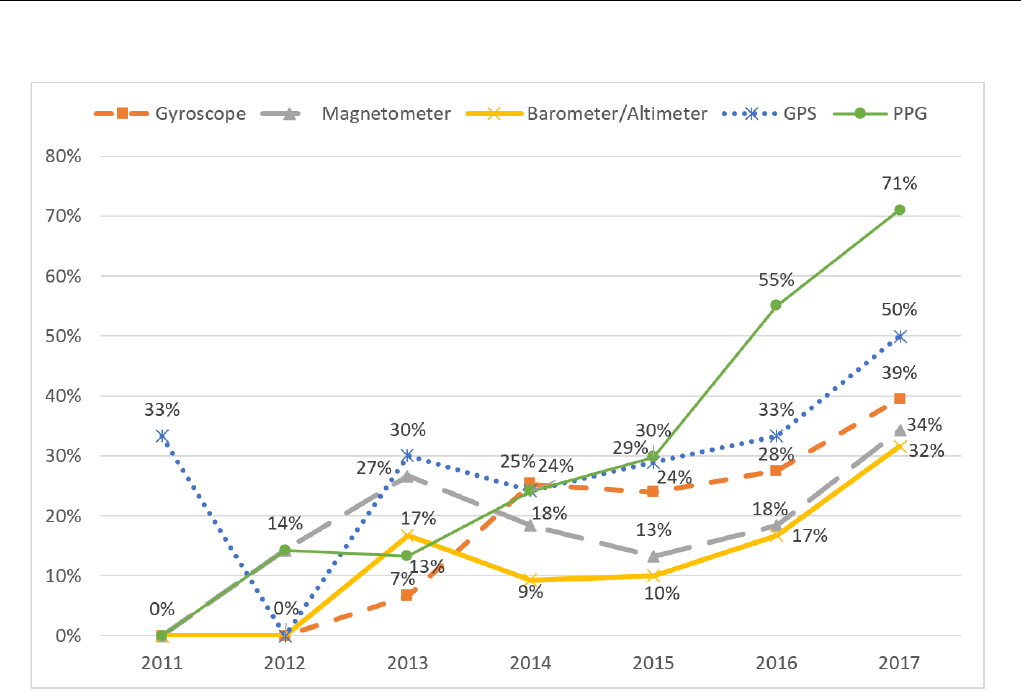
Figure 3. Percentage of devices released each year, supporting each sensor. GPS: global positioning system; PPG: photoplethysmography.
Brand Usage in Research
The top five vendors in 2015 [27] and 2016 [6], in sold units,
were Fitbit, Xiaomi, Apple, Garmin, and Samsung. Brands with
more than 10 unique wearables include Garmin, No.1,
MyKronoz, Samsung, and Polar. These eight, and additional
brands identified during the MEDLINE search and ClinicalTrials
search, were considered. We did not find any publications or
active clinical trials that used devices from No.1 or MyKronoz.
Devices from Basis, BodyMedia, Pebble, Jawbone, Microsoft,
and Nike were also used in some of the identified studies, but
these brands do no longer produce wearables within the scope
of this paper and were excluded from further analysis.
The MEDLINE search resulted in 81 included studies that we
divided into two groups: (1) validation and reliability studies
and (2) data collection studies. Studies where wearable output
was compared with existing research instruments known to give
accurate results (eg, ActiGraph) or with direct observation, as
well as studies where several wearables were compared with
each other for accuracy or reliability, were classified as
validation and reliability studies. Studies where wearables were
used as a tool for intervention or observation, to collect data on
PA, HR, EE, sleep, or other available metrics, were classified
as data collection studies. Out of these 81 studies, 61 were
classified as validation and reliability studies, whereas 20 were
classifies as data collection studies.
Fitbit devices were used in 54 studies [9,13,28-79]. Out of these,
40 studies were validation or reliability studies. In 22 of the
studies, one or more Garmin devices were used
[32,33,46,49,50,62,77-92]. Of these, 18 were validation or
reliability studies. Eight studies used Apple devices
[29,30,35,49,62,79,93,94]. Six of these were validation or
reliability studies. All studies using devices from Misfit, Polar,
Withings, Mio, Samsung, PulseOn, TomTom, and Xiaomi were
validation or reliability studies. Misfit devices were used in 12
studies [9,36,42,43,46,61-63,85,95-97]; Polar devices were used
in 6 studies [36,43,46,62,98,99]; Withings [63,85,89,100,101],
Mio [29,30,54,102,103], and Samsung [29,30,58,62,96] devices
were used in 5 studies; PulseOn devices were used in 4 studies
[29,104-106]; TomTom devices were used in 2 studies [54,79];
and Xiaomi devices were used in 1 study [96].
From ClinicalTrials, we found that the vast majority of ongoing
projects use, or are planning to use, Fitbit devices. All other
devices were mentioned in three or less projects, whereas Fitbit
devices were mentioned in 31 studies. A summary of these
studies and projects is given in Table 4. We further grouped the
validation and reliability studies into five categories. A total of
31 studies focused on step counts or distance, 15 studies
researched EE, 15 studies measured HR, 10 studies measured
sleep, and 7 studies collected other metrics. Multimedia
Appendix 1 gives an overview of articles found in MEDLINE,
which brands they included in the study, and which of the five
categories they are grouped into.
Brand Developer Possibilities
Next, we considered developer possibilities for the 11 brands
already identified as most relevant in research: Apple, Fitbit,
Garmin, Mio, Misfit, Polar, PulseOn, Samsung, TomTom,
Withings, and Xiaomi. All brands had an app in the Apple App
Store and could connect to the iPhone. Except for the Apple
Watch, all other brands had an app in Google Play and could
be used with Android phones.
J Med Internet Res 2018 | vol. 20 | iss. 3 | e110 | p. 7http://www.jmir.org/2018/3/e110/
(page number not for citation purposes)
Henriksen et alJOURNAL OF MEDICAL INTERNET RESEARCH
XSL
•
FO
RenderX

Table 3. Number and percentage of devices supporting a specific group of sensors, by year.
2017201620152014201320122011Sensors
4 (11)37 (30.8)50 (41.3)40 (46)16 (53)5 (71)2 (67)Accelerometer (Acc), n (%)
Acc + 1 sensor, n (%)
10 (26)27 (22.5)11 (9.1)9 (10)1 (3)1 (14)
PPG
a
3 (2.5)15 (12.4)9 (10)2 (7)1 (33)
GPS
b
1 (3)4 (3.3)9 (7.4)3 (3)1 (3)Gyroscope (Gyro)
3 (2.5)1 (1)2 (7)1 (14)Magnetometer (Mag)
2 (5)1 (0.8)1 (1)Barometer (Bar)
Acc + 2 sensors, n (%)
3 (8)6 (5)7 (5.8)1 (3)GPS + PPG
1 (3)5 (4.2)5 (4.1)4 (5)Gyro + PPG
2 (1.7)2 (1.7)1 (1)Gyro + GPS
2 (1.7)1 (0.8)1 (3)Bar + PPG
1 (0.8)2 (2)Gyro + Mag
1 (0.8)1 (1)1 (3)Mag + GPS
1 (0.8)Mag + PPG
1 (1)Gyro + Bar
2 (2)Bar + GPS
Acc + 3 sensors, n (%)
1 (3)2 (1.7)3 (2.5)3 (3)1 (3)Gyro + Mag + GPS
1 (3)3 (2.5)2 (1.7)4 (5)Gyro + Mag + PPG
1 (3)4 (3.3)2 (2)3 (10)Mag + Bar + GPS
1 (3)6 (5)1 (1)Gyro + GPS + PPG
2 (5)2 (1.7)Bar + GPS + PPG
1 (3)1 (0.8)Mag + GPS + PPG
2 (1.7)Gyro + Bar + PPG
1 (0.8)Gyro + Mag + Bar
Acc + 4 sensors, n (%)
4 (3.3)3 (2.5)1 (3)Mag + Bar + GPS + PPG
3 (8)3 (2.5)1 (1)Gyro + Mag + GPS + PPG
1 (3)4 (3.3)2 (1.7)Gyro + Bar + GPS + PPG
2 (5)1 (0.8)Gyro + Mag + Bar + GPS
1 (0.8)1 (1)Gyro + Mag + Bar + PPG
Acc + 5 sensors, n (%)
4 (11)2 (1.7)2 (1.7)1 (1)All sensors
38120121873073Total, n
a
PPG: photoplethysmography.
b
GPS: global positioning system.
J Med Internet Res 2018 | vol. 20 | iss. 3 | e110 | p. 8http://www.jmir.org/2018/3/e110/
(page number not for citation purposes)
Henriksen et alJOURNAL OF MEDICAL INTERNET RESEARCH
XSL
•
FO
RenderX

Table 4. Number of identified articles in Medical Literature Analysis and Retrieval System Online (MEDLINE) and ClinicalTrials.
ClinicalTrialsMEDLINE
MEDLINE
a
search term
Brand
Data collection
studies
e
Validation or reliability
studies
d
Data collection studies
c
(total article count=20)
Validation or reliability
studies
b
(total article
count=61)
3011440Fitbit AND (Alta OR Blaze OR
Charge OR Flex OR Surge)
Fitbit
21418Garmin AND (Approach OR D2
OR Epix OR Fenix OR Forerunner
OR Quatix OR Swim OR Tactix
OR Vivo*)
Garmin
10012Misfit AND (Flare OR Flash OR
Link OR Ray OR Shine OR Va-
por)
Misfit
1126Apple watchApple
3106Polar AND (“Polar Loop” OR
M200 OR M4?0 OR M600 OR
V800 OR A3?0)
Polar
2005WithingsWithings
2105Mio Alpha OR Mio Fuse OR Mio
Slice
Mio
2005Samsung Gear NOT “Gear VR”
NOT Oculus
Samsung
1004PulseOnPulseOn
102TomTomTomTom
1001XiaomiXiaomi
a
MEDLINE: Medical Literature Analysis and Retrieval System Online.
b
Number of validation or reliability studies in MEDLINE.
c
Number of data collection studies in MEDLINE.
d
Number of validation or reliability studies in ClinicalTrials.
e
Number of data collection studies in ClinicalTrials.
Three brands supported Windows Phone: Fitbit, Garmin, and
Misfit. Apple Health and Google Fit are the two most common
open cloud health repositories. Mio, Misfit, Polar, Withings,
and Xiaomi, were the only brands that automatically
synchronized fitness data to both of these repositories through
these open APIs. The Apple Watch only synchronized
automatically to the Apple Health repository. Seven out of 11
brands had a private cloud repository with an accompanying
API, which allows third-party apps to access these data. Five
brands had an SDK, which makes it possible to create custom
programs to communicate with the device or create watch faces
that can run on the device.
The Apple Watch was the only device running on watchOS.
Three brands had at least one device running on Android Wear.
The remaining seven brands used a custom system. A summary
of all attributes for each brand is given in Table 5. Not all
devices for a specific brand support all features. In addition,
this is a snapshot of the status of these attributes, which are
likely to change over time as new devices and brands expand
their capabilities. The Apple Watch development environment
is called WatchKit SDK and can be used to write apps for the
Apple Watch [107]. Apple’s health storage solution is called
Apple Health. A variety of different data types can be stored
here and accessed by third-party developers through the
HealthKit API [108]. Access to any of these services requires
enrollment in the Apple Developer Program, which currently
costs US $99 per year.
Fitbit offers three major SDKs (Device API, Companion API,
and Settings API) for developing apps for Fitbit devices. In
addition, Fitbit offers the Web API that can be used to access
Fitbit cloud-stored fitness data. The Web API exposes six types
of data: PA, HR, location, nutrition, sleep, and weight [109].
Fitbit also has a solution for accessing high-resolution step and
HR data (ie, intraday data), granted on a case by case basis.
There is no cost for developing with the Fitbit SDKs or API.
There are two generations of programmable Garmin wearables
[110]. The Connect IQ SDK can be used by both generations,
but devices using the newer Connect IQ 2 generation support
more features. Development with this SDK is free. Garmin also
offers a cloud-based Web API, Garmin Connect, which allows
third-party apps to access users’cloud-based fitness data. Access
to this API costs US $5000 (one-time license). In addition,
Garmin maintains a separate Health API intended to be used
by companies for wellness improvement of their employees.
This API is free but requires a manual approval from Garmin.
J Med Internet Res 2018 | vol. 20 | iss. 3 | e110 | p. 9http://www.jmir.org/2018/3/e110/
(page number not for citation purposes)
Henriksen et alJOURNAL OF MEDICAL INTERNET RESEARCH
XSL
•
FO
RenderX

Table 5. Brand environment, integration, and development support.
XiaomiWithingsTomTomSamsungPulseOnPolarMisfitMioGarminFitbitAppleFeature
Supported platform
✓✓✓✓✓✓✓✓✓✓Android
✓✓✓✓✓✓✓✓✓✓✓iPhone
✓✓✓Windows phone
Integration
✓✓✓✓✓✓Automatic synchronization
to Apple Health
✓✓✓✓✓Automatic synchronization
to Google Fit
✓✓✓✓✓✓✓Private cloud storage
✓✓✓✓✓✓✓✓
Cloud storage API
a
✓✓✓✓✓
Developer SDK
b
Watch system
✓✓✓Android Wear
✓watchOS (Apple)
✓✓✓✓✓✓✓Custom
a
API: application programming interface.
b
SDK: software development kit.
The Misfit developer ecosystem consists of three SDKs (Sleep
SDK, Link SDK, and Device SDK) [111]. The Misfit Device
SDK is the major SDK for developing apps for and
communication with Misfit devices. This SDK is only available
on request. Misfit also offers the Misfit Scientific Library that
can be used to access Misfits proprietary sensor algorithms
directly. This library is also only available on request. In
addition, the Misfit Cloud API is used to access users’data from
the Misfit cloud server. All SDKs and the API are free.
Polar does not offer a separate SDK. Polar devices can integrate
with Google Fit and Apple Health and deposits collected data
there [112]. This data are accessed using Google Fit APIs and
Apple HealthKit APIs. In addition, data are uploaded to Polar’s
cloud storage, which is accessible by third-party developers
through the AccessLink API. Besides PA data (steps, EE, and
sleep), basic training data are also stored here. Access to
AccessLink is free.
Development for a Samsung smartwatch is done using the Tizen
SDK (Samsung smartwatch operating system is called Tizen).
The Samsung Health SDK platform consists of two parts: Data
SDK and Service SDK. Together these can be used to store and
access health data collected from internal and external sensors,
as well as third-party apps running on a Samsung watch or a
mobile phone. Development using any of these services is free
[113].
TomTom offers the Sports Cloud API for accessing data
collected from TomTom devices. The API provides four types
of data: PA (eg, exercises bouts), HR, tracking (eg, steps and
EE), and physiology (eg, weight). Access to the API is free
[114].
Nokia acquired Withings in 2016, and the original Withings
API is now available as the Nokia Health API. Besides PA and
sleep measurements, the API also gives access to intraday PA
data. Nokia must manually approve access to this high-resolution
activity API. The API is free [115].
Summarizing Results
Which features are most important when considering devices
for a research project will depend on the purpose and design of
the study. It is therefore not possible to identify one brand as
the best brand in all circumstances. However, we have tried to
quantify various aspects of a brand to identify and summarize
their benefits.
J Med Internet Res 2018 | vol. 20 | iss. 3 | e110 | p. 10http://www.jmir.org/2018/3/e110/
(page number not for citation purposes)
Henriksen et alJOURNAL OF MEDICAL INTERNET RESEARCH
XSL
•
FO
RenderX

Table 6. Brand summary.
No. 1MyKronozXiaomiTomTomPulseOnMioWithingsSamsungPolarAppleMisfitGarminFitbitBrand
191837132121138409
Devices
a
12455568122254
MEDLINE
b
12455566121840Validation or
reliability
c
1422161021Steps
221343410Energy ex-
penditure
1245214147Heart rate
21418Sleep
1243Other
111322421331
ClinicalTrials
d
✓✓✓✓✓✓
SDK
e
✓✓✓✓✓✓✓✓
API
f
✓✓✓✓✓✓
Apple Health
g
✓✓✓✓✓
Google Fit
h
a
Number of unique devices.
b
MEDLINE: Medical Literature Analysis and Retrieval System Online. Number of articles in MEDLINE.
c
Number of validation or reliability studies in MEDLINE, grouped by metric (step, EE, HR, sleep, and others).
d
Number of active projects in ClinicalTrials.
e
Supports an SDK for third-party software implementation.
f
API: application programming interface. Supports an API for developer access to data cloud.
g
Supports automatic synchronization to Apple Health data cloud.
h
Supports automatic synchronization to Google Fit data cloud.
We used eight categories in this custom comparison, which we
suggest to consider before deciding on a brand for any research
project:
1.
Device count: a higher number of available devices make
it possible to pick a device that is more tailored to the study.
2.
Article count: a higher number of articles in Ovid
MEDLINE indicate usage in previous studies.
3.
Validation or reliability count: a high number of validation
or reliability studies provides knowledge about device and
brand accuracy.
4.
ClinicalTrials count: a high number of active projects in
ClinicalTrials indicate brand relevance.
5.
SDK support: brands that allows third-party programs to
run on their devices or communicate directly with the
device, by offering an SDK, adds more possibilities for
customization.
6.
API support: brands that allows third-party programs to
access the data cloud repository, by offering API access,
adds more possibilities for health data collection and
retrieval.
7.
Apple Health: brands supporting automatic synchronization
to Apple Health allow usage of Apple HealthKit API.
8.
Google Fit: brands supporting automatic synchronization
to Google Fit allow usage of Google Fit API.
A consensus between authors was reached to include these
specific categories because we think together they indicate how
often a specific brand has been used in the past and will be used
in the future, and they show which options are available for data
extraction. These are not the only possible categories, and each
category will not be equally important for all studies.
Table 6 gives a summary of these categories for each brand. A
transposed Excel (Microsoft) version for dynamic sorting is
given in Multimedia Appendix 2. We have divided MEDLINE
validation and reliability studies into subgroups, making it easier
to compare brands for specific study purposes.
Discussion
Availability and Trends
The number of new brands increased every year from 2011 to
2014, but from 2015 to 2016, we saw a decrease in the number
of new brands. The number of new devices also increased from
2011 to 2015, with a slight reduction in 2016. Many new and
existing companies have tried to enter the wearable market
during these years. Some have become popular, whereas others
are no longer available. The number of new devices in the first
two quarters of 2017 seems low, and there is a small indication
that the number of new brands and devices released each year
is declining. During the data collection phase, we also identified
J Med Internet Res 2018 | vol. 20 | iss. 3 | e110 | p. 11http://www.jmir.org/2018/3/e110/
(page number not for citation purposes)
Henriksen et alJOURNAL OF MEDICAL INTERNET RESEARCH
XSL
•
FO
RenderX
a large number of hybrid watches. Although we did not report
on these, this relatively new branch of wearables has grown in
popularity. The Fossil group, representing 19 brands, recently
announced they would launch more than 300 hybrid watches
and smartwatches in 2017 [116]. Most of these will be hybrids,
and 2017 may see the highest number of new hybrids released
to date.
We only found nine devices that support all five sensors
considered in this study. Among the 11 most relevant brands,
only Fitbit Surge, Garmin Forerunner 935, Garmin Quatix 5,
Samsung Gear S, and TomTom Adventure fall in this category.
Most devices (68%) support only one sensor, in addition to the
accelerometer. These numbers indicate that sensor count is not
the main argument when choosing a device for personal use. In
addition to the accelerometer, the most common sensors are
PPG and GPS, regardless of sensor count. One reason for this
may be that the added benefit of having these sensors, in a
fitness setting, is very clear. Accelerometers can be used for
step counting, PA intensity, exercise detection, and other
well-understood metrics, whereas the added benefit of a
gyroscope may be less intuitive. The added convenience of
using a PPG compared with a pulse chest strap, or no HR
detection at all, is also easy to understand. Adding a GPS also
adds some easy-to-understand benefits, where tracking progress
on a map and the possibility to detect speed is the most obvious.
Magnetometers and barometers or altimeters may not be sensors
that most people consider relevant for PA, although they can
be used to enhance accuracy of EE and other metrics.
Brand Usage in Research
In the MEDLINE literature search, we found 81 studies that
used one or more of the 11 brands we identified as most relevant
in research. Out of these, 61 were validation or reliability
studies. The remaining 20 studies used wearable devices as data
collection instruments to measure PA, HR, EE, sleep, or other
metrics. Fitbit was used in twice as many validation or reliability
studies as any other brand. This has likely contributed to the
high number of studies where Fitbit was used as the only
instrument for health data collection. The same trend will likely
continue in future publications because numbers from
ClinicalTrials for active projects shows an overrepresentation
of Fitbit-enabled projects. Of the brands currently available, the
five most often used in research projects are Fitbit, Garmin,
Misfit, Apple, and Polar. In addition, these brands have all
existed for several years and have either released a large number
of unique devices or shipped a large number of total devices.
As such, they are likely to stay on the market for the near future.
A high article count, high number of validation or reliability
studies, or high number of studies in ClinicalTrials for a specific
brand does not automatically imply validity or reliability. It
does, however, show researcher interest in these brands.
Implication for Practice
Table 6 is a good starting point when considering brands for a
new research project. Article count, validation or reliability
study count, and ClinicalTrials count together indicate brand
dependability. Larger numbers indicate how relevant, usable,
and valid previous researchers have found each brand to be. In
projects where it is relevant, SDK support allows programmatic
interaction directly with the device. API support allows storage
in, and access to, a brand-specific cloud-based health data
repository. Apple Health and Google Fit support are alternative
solutions for storing and accessing health data in an open cloud
repository. For projects that require multiple brand support,
using open solutions reduces the need to implement specific
software for each brand. SDK, API, Apple Health, and Google
Fit must be supported on both the brand and device level,
however.
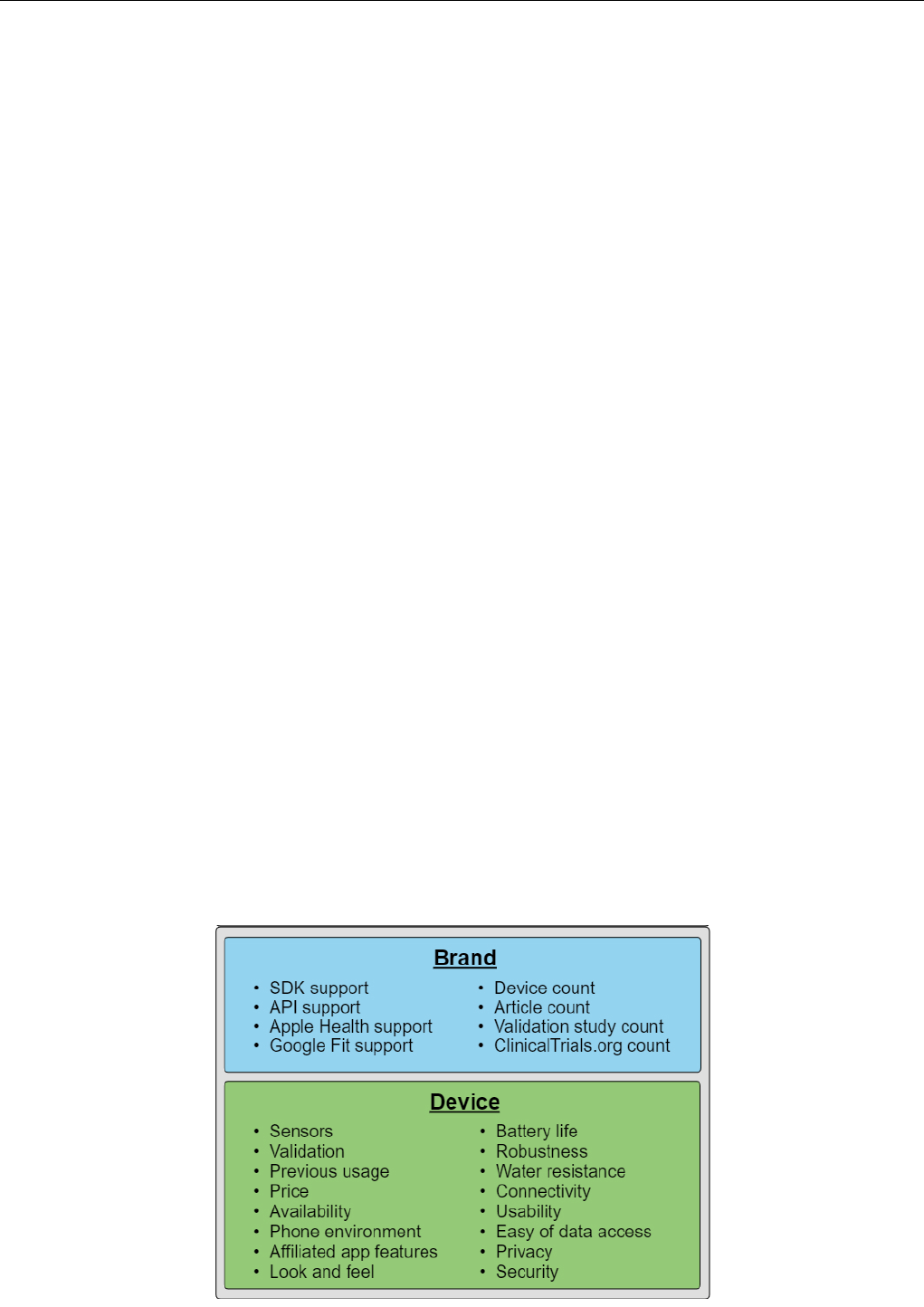
A high brand device count makes it easier to find a device that
best supports the study needs. In addition to available sensors
(ie, metrics), validation, and previous usage in research, several
other potential relevant criteria exist, including price,
availability, phone environment support, affiliated app features,
look and feel, battery life, build quality or robustness, water
resistance, connectivity, and usability.
Figure 4. Criteria to consider when choosing brand or device. API: application programming interface; SDK: software development kit.
J Med Internet Res 2018 | vol. 20 | iss. 3 | e110 | p. 12http://www.jmir.org/2018/3/e110/
(page number not for citation purposes)
Henriksen et alJOURNAL OF MEDICAL INTERNET RESEARCH
XSL
•
FO
RenderX
Furthermore, projects that need programmatic access to the
wearable or stored health data should especially consider SDK
or API features and ease of use, as well as privacy and security.
Figure 4 gives a summary of criteria to consider when selecting
brand and device.
Limitations
We visited all the brands’ websites to find additional devices,
but several sites did not contain any information about
discontinued devices. The release year of a device was rarely
available on device webpages, and we had to search for reviews
and other sources to find this information. The level of detail
in device hardware specifications varied. Some vendors did not
specify which sensor they included in their devices and only
mentioned which features the device had. In some cases, the
sensor could be derived from this information, but in other cases,
we had to find this information elsewhere. Wikipedia was also
used to collect sensor support and release year for some devices.
This open editable encyclopedia is not necessarily always
updated with correct information. For these reasons, there may
be some inaccuracies in reported sensor support and release
year. We did not collect information about device
discontinuation. Reported numbers for total available devices
does, therefore, not reflect the numbers of devices that currently
can be store bought but rather the number of unique devices
that have existed at some point.
Conclusions
In the last few years, we have seen a large increase in available
brands and wearable devices, and more devices are released
with additional sensors. However, for activity tracking, some
sensors are more relevant than others are. In this study, we have
focused on sensor support, health data cloud integration, and
developer possibilities; because we find these to be most relevant
for collection of PA data in research. However, deciding which
wearable to use will depend on several additional factors.
The wearable landscape is constantly changing as new devices
are released and as new vendors enter or leave the market, or
are acquired by larger vendors. What currently are considered
relevant devices and brands will therefore change over time,
and each research project should carefully consider which brand
and device to use. As a tool for future research, we have defined
a checklist of elements to consider when making this decision.
Acknowledgments
The authors would like to thank Vandrico Solutions Inc for giving them API access to their database. They would also like to
thank Steven Richardson and Debra Mackinnon at Queen’s University for giving them an excerpt from their offline wearable
database. The publication charges for this study have been funded by a grant from the publication fund of the University of
Tromsø – The Arctic University of Norway.
Conflicts of Interest
None declared.
Multimedia Appendix 1
List of MEDLINE articles included in the results for "Brand usage in research".
[XLSX File (Microsoft Excel File), 24KB-Multimedia Appendix 1]
Multimedia Appendix 2
Summary of the most important categories to consider when selecting a wearable brand for research.
[XLSX File (Microsoft Excel File), 13KB-Multimedia Appendix 2]
References
1. World Health Organization. Physical activity URL: http://www.who.int/mediacentre/factsheets/fs385/en [accessed
2017-09-11] [WebCite Cache ID 6tOYSnXaX]
2. Emaus A, Degerstrøm J, Wilsgaard T, Hansen BH, Dieli-Conwright CM, Furberg AS, et al. Does a variation in self-reported
physical activity reflect variation in objectively measured physical activity, resting heart rate, and physical fitness? Results
from the Tromso study. Scand J Public Health 2010 Nov;38(5 Suppl):105-118. [doi: 10.1177/1403494810378919] [Medline:
21062845]
3. World Health Organization. 2017. Global Strategy on Diet, Physical Activity and Health URL: http://www.who.int/
dietphysicalactivity/pa/en [accessed 2017-09-11] [WebCite Cache ID 6tOXoFxbF]
4. Finkelstein EA, Haaland BA, Bilger M, Sahasranaman A, Sloan RA, Nang EE, et al. Effectiveness of activity trackers with
and without incentives to increase physical activity (TRIPPA): a randomised controlled trial. Lancet Diabetes Endocrinol
2016 Dec;4(12):983-995. [doi: 10.1016/S2213-8587(16)30284-4] [Medline: 27717766]
5. Jakicic JM, Davis KK, Rogers RJ, King WC, Marcus MD, Helsel D, et al. Effect of wearable technology combined with
a lifestyle intervention on long-term weight loss: the IDEA randomized clinical trial. J Am Med Assoc 2016 Sep
20;316(11):1161-1171. [doi: 10.1001/jama.2016.12858] [Medline: 27654602]
J Med Internet Res 2018 | vol. 20 | iss. 3 | e110 | p. 13http://www.jmir.org/2018/3/e110/
(page number not for citation purposes)
Henriksen et alJOURNAL OF MEDICAL INTERNET RESEARCH
XSL
•
FO
RenderX
6. IDC. Wearables Aren't Dead, They're Just Shifting Focus as the Market Grows 16.9% in the Fourth Quarter, According to
IDC URL: https://www.idc.com/getdoc.jsp?containerId=prUS42342317 [accessed 2018-03-01] [WebCite Cache ID
6xZyWGaxT]
7. IDC. Xiaomi and Apple Tie for the Top Position as the Wearables Market Swells 17.9% During the First Quarter URL:
https://www.idc.com/getdoc.jsp?containerId=prUS42707517 [accessed 2018-03-01] [WebCite Cache ID 6xZypbatQ]
8. de Arriba-Perez F, Caeiro-Rodríguez M, Santos-Gago JM. Collection and processing of data from wrist wearable devices
in heterogeneous and multiple-user scenarios. Sensors (Basel) 2016 Sep 21;16(9) [FREE Full text] [doi: 10.3390/s16091538]
[Medline: 27657081]
9. Kaewkannate K, Kim S. A comparison of wearable fitness devices. BMC Public Health 2016 May 24;16:433 [FREE Full
text] [doi: 10.1186/s12889-016-3059-0] [Medline: 27220855]
10. Sanders JP, Loveday A, Pearson N, Edwardson C, Yates T, Biddle SJ, et al. Devices for self-monitoring sedentary time or
physical activity: a scoping review. J Med Internet Res 2016 May 04;18(5):e90 [FREE Full text] [doi: 10.2196/jmir.5373]
[Medline: 27145905]
11. Corder K, Brage S, Ekelund U. Accelerometers and pedometers: methodology and clinical application. Curr Opin Clin
Nutr Metab Care 2007 Sep;10(5):597-603. [doi: 10.1097/MCO.0b013e328285d883] [Medline: 17693743]
12. ActiGraph Corp. ActiGraph URL: http://actigraphcorp.com/ [accessed 2017-09-11] [WebCite Cache ID 6tOYrA9Ke]
13. Reid RE, Insogna JA, Carver TE, Comptour AM, Bewski NA, Sciortino C, et al. Validity and reliability of Fitbit activity
monitors compared to ActiGraph GT3X+ with female adults in a free-living environment. J Sci Med Sport 2017
Jun;20(6):578-582. [doi: 10.1016/j.jsams.2016.10.015] [Medline: 27887786]
14. Evenson KR, Goto MM, Furberg RD. Systematic review of the validity and reliability of consumer-wearable activity
trackers. Int J Behav Nutr Phys Act 2015 Dec 18;12:159 [FREE Full text] [doi: 10.1186/s12966-015-0314-1] [Medline:
26684758]
15. Richardson S, Mackinnon D. Left to their own devices? Privacy implications of wearable technology in Canadian workplaces
URL: http://www.sscqueens.org/publications/left-to-their-own-devices [accessed 2018-03-01] [WebCite Cache ID
6xZzwLAN6]
16. Wagenaar RC, Sapir I, Zhang Y, Markovic S, Vaina LM, Little TD. Continuous monitoring of functional activities using
wearable, wireless gyroscope and accelerometer technology. Conf Proc IEEE Eng Med Biol Soc 2011;2011:4844-4847.
[doi: 10.1109/IEMBS.2011.6091200] [Medline: 22255423]
17. Allen J. Photoplethysmography and its application in clinical physiological measurement. Physiol Meas 2007
Mar;28(3):R1-39. [doi: 10.1088/0967-3334/28/3/R01] [Medline: 17322588]
18. Kim SH, Ryoo DW, Bae C. Adaptive noise cancellation Using accelerometers for the PPG signal from forehead. Conf Proc
IEEE Eng Med Biol Soc 2007;2007:2564-2567. [doi: 10.1109/IEMBS.2007.4352852] [Medline: 18002518]
19. Casson AJ, Vazquez Galvez A, Jarchi D. Gyroscope vs. accelerometer measurements of motion from wrist PPG during
physical exercise. ICT Express 2016 Dec;2(4):175-179. [doi: 10.1016/j.icte.2016.11.003]
20. Richardson SM, Mackinnon D. Queen's University. 2017. Wearable Device Inventory, Queen's University.
21. Vandrico. 2017. The wearables database URL: http://vandrico.com/wearables/ [accessed 2017-07-08] [WebCite Cache ID
6roNvEjdE]
22. GSM Arena. 2017. URL: http://www.gsmarena.com/results.php3?sFormFactors=8 [accessed 2017-07-08] [WebCite Cache
ID 6roO1WCdv]
23. Wearables. 2017. Wearables.Com: Helping you make sense of wearable tech URL: http://www.wearables.com/devices/
[accessed 2017-07-08] [WebCite Cache ID 6roO94rOu]
24. Smartwatches. 2017. SpecBucket: Smart Watches URL: https://smartwatches.specbucket.com/ [accessed 2017-07-08]
[WebCite Cache ID 6roOBKrxr]
25. Prisguiden. 2017. Smartklokke URL: https://prisguiden.no/kategorier/smartklokke [accessed 2017-07-08] [WebCite Cache
ID 6roOHtXzf]
26. Prisguiden. 2017. Aktivitetsarmband URL: https://prisguiden.no/kategorier/aktivitetsarmband [accessed 2017-07-08]
[WebCite Cache ID 6roOJt6HJ]
27. Business Wire. 2016. The Worldwide Wearables Market Leaps 126.9% in the Fourth Quarter and 171.6% in 2015 URL:
https://www.businesswire.com/news/home/20160223005496/en/Worldwide-Wearables-Market-Leaps-126.9-Fourth-Quarter
[accessed 2008-03-01] [WebCite Cache ID 6xa0wRrvV]
28. Balto JM, Kinnett-Hopkins DL, Motl RW. Accuracy and precision of smartphone applications and commercially available
motion sensors in multiple sclerosis. Mult Scler J Exp Transl Clin 2016;2:2055217316634754 [FREE Full text] [doi:
10.1177/2055217316634754] [Medline: 28607720]
29. Shcherbina A, Mattsson CM, Waggott D, Salisbury H, Christle JW, Hastie T, et al. Accuracy in wrist-worn, sensor-based
measurements of heart rate and energy expenditure in a diverse cohort. J Pers Med 2017 May 24;7(2) [FREE Full text]
[doi: 10.3390/jpm7020003] [Medline: 28538708]
30. Wallen MP, Gomersall SR, Keating SE, Wisløff U, Coombes JS. Accuracy of heart rate watches: implications for weight
management. PLoS One 2016;11(5):e0154420 [FREE Full text] [doi: 10.1371/journal.pone.0154420] [Medline: 27232714]
J Med Internet Res 2018 | vol. 20 | iss. 3 | e110 | p. 14http://www.jmir.org/2018/3/e110/
(page number not for citation purposes)
Henriksen et alJOURNAL OF MEDICAL INTERNET RESEARCH
XSL
•
FO
RenderX
31. Chow JJ, Thom JM, Wewege MA, Ward RE, Parmenter BJ. Accuracy of step count measured by physical activity monitors:
The effect of gait speed and anatomical placement site. Gait Posture 2017 Sep;57:199-203. [doi:
10.1016/j.gaitpost.2017.06.012] [Medline: 28666177]
32. Chen MD, Kuo CC, Pellegrini CA, Hsu MJ. Accuracy of wristband activity monitors during ambulation and activities.
Med Sci Sports Exerc 2016 Dec;48(10):1942-1949. [doi: 10.1249/MSS.0000000000000984] [Medline: 27183123]
33. Duncan M, Murawski B, Short CE, Rebar AL, Schoeppe S, Alley S, et al. Activity trackers implement different behavior
change techniques for activity, sleep, and sedentary behaviors. Interact J Med Res 2017 Aug 14;6(2):e13 [FREE Full text]
[doi: 10.2196/ijmr.6685] [Medline: 28807889]
34. Sprint G, Cook D, Weeks D, Dahmen J, La Fleur A. Analyzing sensor-based time series data to track changes in physical
activity during inpatient rehabilitation. Sensors (Basel) 2017 Sep 27;17(10) [FREE Full text] [doi: 10.3390/s17102219]
[Medline: 28953257]
35. Chowdhury EA, Western MJ, Nightingale TE, Peacock OJ, Thompson D. Assessment of laboratory and daily energy
expenditure estimates from consumer multi-sensor physical activity monitors. PLoS One 2017;12(2):e0171720 [FREE Full
text] [doi: 10.1371/journal.pone.0171720] [Medline: 28234979]
36. Mercer K, Li M, Giangregorio L, Burns C, Grindrod K. Behavior change techniques present in wearable activity trackers:
a critical analysis. JMIR Mhealth Uhealth 2016;4(2):e40 [FREE Full text] [doi: 10.2196/mhealth.4461] [Medline: 27122452]
37. Gaudet J, Gallant F, Bélanger M. A bit of fit: minimalist intervention in adolescents based on a physical activity tracker.
JMIR Mhealth Uhealth 2017 Jul 06;5(7):e92 [FREE Full text] [doi: 10.2196/mhealth.7647] [Medline: 28684384]
38. Jacobsen RM, Ginde S, Mussatto K, Neubauer J, Earing M, Danduran M. Can a home-based cardiac physical activity
program improve the physical function quality of life in children with fontan circulation? Congenit Heart Dis
2016;11(2):175-182. [doi: 10.1111/chd.12330] [Medline: 26879633]
39. Ridgers ND, Timperio A, Brown H, Ball K, Macfarlane S, Lai SK, et al. A cluster-randomised controlled trial to promote
physical activity in adolescents: the Raising Awareness of Physical Activity (RAW-PA) Study. BMC Public Health 2017
Jan 04;17(1):6 [FREE Full text] [doi: 10.1186/s12889-016-3945-5] [Medline: 28052773]
40. Li LC, Sayre EC, Xie H, Clayton C, Feehan LM. A community-based physical activity counselling program for people
with knee osteoarthritis: feasibility and preliminary efficacy of the track-OA study. JMIR Mhealth Uhealth 2017 Jun
26;5(6):e86 [FREE Full text] [doi: 10.2196/mhealth.7863] [Medline: 28652228]
41. Dondzila C, Garner D. Comparative accuracy of fitness tracking modalities in quantifying energy expenditure. J Med Eng
Technol 2016 Aug;40(6):325-329. [doi: 10.1080/03091902.2016.1197978] [Medline: 27280592]
42. Bellone GJ, Plano SA, Cardinali DP, Chada DP, Vigo DE, Golombek DA. Comparative analysis of actigraphy performance
in healthy young subjects. Sleep Sci 2016;9(4):272-279 [FREE Full text] [doi: 10.1016/j.slsci.2016.05.004] [Medline:
28154740]
43. Bai Y, Welk GJ, Nam YH, Lee JA, Lee JM, Kim Y, et al. Comparison of consumer and research monitors under
semistructured settings. Med Sci Sports Exerc 2016;48(1):151-158. [doi: 10.1249/MSS.0000000000000727] [Medline:
26154336]
44. Lee HA, Lee HJ, Moon JH, Lee T, Kim MG, In H, et al. Comparison of wearable activity tracker with actigraphy for sleep
evaluation and circadian rest-activity rhythm measurement in healthy young adults. Psychiatry Investig 2017
Mar;14(2):179-185 [FREE Full text] [doi: 10.4306/pi.2017.14.2.179] [Medline: 28326116]
45. Chu AH, Ng SH, Paknezhad M, Gauterin A, Koh D, Brown MS, et al. Comparison of wrist-worn Fitbit Flex and waist-worn
ActiGraph for measuring steps in free-living adults. PLoS One 2017;12(2):e0172535 [FREE Full text] [doi:
10.1371/journal.pone.0172535] [Medline: 28234953]
46. Brooke SM, An HS, Kang SK, Noble JM, Berg KE, Lee JM. Concurrent validity of wearable activity trackers under
free-living conditions. J Strength Cond Res 2017 Apr;31(4):1097-1106. [doi: 10.1519/JSC.0000000000001571] [Medline:
27465631]
47. Block VJ, Lizée A, Crabtree-Hartman E, Bevan CJ, Graves JS, Bove R, et al. Continuous daily assessment of multiple
sclerosis disability using remote step count monitoring. J Neurol 2017 Feb;264(2):316-326. [doi: 10.1007/s00415-016-8334-6]
[Medline: 27896433]
48. Morhardt DR, Luckenbaugh A, Goldstein C, Faerber GJ. Determining resident sleep during and after call with commercial
sleep monitoring devices. Urology 2017 Aug;106:39-44. [doi: 10.1016/j.urology.2017.03.059] [Medline: 28502597]
49. Dooley EE, Golaszewski NM, Bartholomew JB. Estimating accuracy at exercise intensities: a comparative study of
self-monitoring heart rate and physical activity wearable devices. JMIR Mhealth Uhealth 2017 Mar 16;5(3):e34 [FREE
Full text] [doi: 10.2196/mhealth.7043] [Medline: 28302596]
50. Leth S, Hansen J, Nielsen OW, Dinesen B. Evaluation of commercial self-monitoring devices for clinical purposes: results
from the Future Patient Trial, Phase I. Sensors (Basel, Switzerland) 2017;17(1):1-11.
51. Bian J, Guo Y, Xie M, Parish AE, Wardlaw I, Brown R, et al. Exploring the association between self-reported asthma
impact and Fitbit-derived sleep quality and physical activity measures in adolescents. JMIR Mhealth Uhealth 2017 Jul
25;5(7):e105 [FREE Full text] [doi: 10.2196/mhealth.7346] [Medline: 28743679]
52. Coughlin SS, Hatzigeorgiou C, Anglin J, Xie D, Besenyi GM, De Leo G, et al. Healthy lifestyle intervention for adult clinic
patients with type 2 diabetes mellitus. Diabetes Manag (Lond) 2017;7(2):197-204 [FREE Full text] [Medline: 28794802]
J Med Internet Res 2018 | vol. 20 | iss. 3 | e110 | p. 15http://www.jmir.org/2018/3/e110/
(page number not for citation purposes)
Henriksen et alJOURNAL OF MEDICAL INTERNET RESEARCH
XSL
•
FO
RenderX
53. Åkerberg A, Koshmak G, Johansson A, Lindén M. Heart rate measurement as a tool to quantify sedentary behavior. Stud
Health Technol Inform 2015;211:105-110. [Medline: 25980855]
54. Stahl SE, An HS, Dinkel DM, Noble JM, Lee JM. How accurate are the wrist-based heart rate monitors during walking
and running activities? Are they accurate enough? BMJ Open Sport Exerc Med 2016;2(1):1-7 [FREE Full text] [doi:
10.1136/bmjsem-2015-000106]
55. Alinia P, Cain C, Fallahzadeh R, Shahrokni A, Cook D, Ghasemzadeh H. How accurate is your activity tracker? A
comparative study of step counts in low-intensity physical activities. JMIR Mhealth Uhealth 2017 Aug 11;5(8):e106 [FREE
Full text] [doi: 10.2196/mhealth.6321] [Medline: 28801304]
56. Winslow BD, Nguyen N, Venta KE. Improved mental acuity forecasting with an individualized quantitative sleep model.
Front Neurol 2017;8:160 [FREE Full text] [doi: 10.3389/fneur.2017.00160] [Medline: 28487671]
57. Spiotta AM, Fargen KM, Denham SL, Fulton ME, Kellogg R, Young E, et al. Incorporation of a physical education and
nutrition program into neurosurgery: a proof of concept pilot program. Neurosurgery 2016 Oct;79(4):613-619. [doi:
10.1227/NEU.0000000000001358] [Medline: 27465847]
58. Modave F, Guo Y, Bian J, Gurka MJ, Parish A, Smith MD, et al. Mobile device accuracy for step counting across age
groups. JMIR Mhealth Uhealth 2017 Jun 28;5(6):e88 [FREE Full text] [doi: 10.2196/mhealth.7870] [Medline: 28659255]
59. Dominick GM, Winfree KN, Pohlig RT, Papas MA. Physical activity assessment between consumer- and research-grade
accelerometers: a comparative study in free-living conditions. JMIR Mhealth Uhealth 2016 Sep 19;4(3):e110 [FREE Full
text] [doi: 10.2196/mhealth.6281] [Medline: 27644334]
60. Schoenfelder E, Moreno M, Wilner M, Whitlock KB, Mendoza JA. Piloting a mobile health intervention to increase physical
activity for adolescents with ADHD. Prev Med Rep 2017 Jun;6:210-213 [FREE Full text] [doi: 10.1016/j.pmedr.2017.03.003]
[Medline: 28373931]
61. Kooiman TJ, Dontje ML, Sprenger SR, Krijnen WP, van der Schans CP, de Groot M. Reliability and validity of ten consumer
activity trackers. BMC Sports Sci Med Rehabil 2015;7:24 [FREE Full text] [doi: 10.1186/s13102-015-0018-5] [Medline:
26464801]
62. Fokkema T, Kooiman TJ, Krijnen WP, van der Schans CP, de Groot M. Reliability and validity of ten consumer activity
trackers depend on walking speed. Med Sci Sports Exerc 2017 Apr;49(4):793-800. [doi: 10.1249/MSS.0000000000001146]
[Medline: 28319983]
63. Mantua J, Gravel N, Spencer RM. Reliability of sleep measures from four personal health monitoring devices compared
to research-based actigraphy and polysomnography. Sensors (Basel) 2016 Dec 05;16(5) [FREE Full text] [doi:
10.3390/s16050646] [Medline: 27164110]
64. Chen JL, Guedes CM, Cooper BA, Lung AE. Short-term efficacy of an innovative mobile phone technology-based
intervention for weight management for overweight and obese adolescents: pilot study. Interact J Med Res 2017 Aug
02;6(2):e12 [FREE Full text] [doi: 10.2196/ijmr.7860] [Medline: 28768612]
65. Reichardt LA, Aarden JJ, van Seben R, van der Schaaf M, Engelbert RH, Bosch JA, Hospital-ADL study group. Unravelling
the potential mechanisms behind hospitalization-associated disability in older patients; the Hospital-Associated Disability
and impact on daily Life (Hospital-ADL) cohort study protocol. BMC Geriatr 2016 Mar 05;16:59 [FREE Full text] [doi:
10.1186/s12877-016-0232-3] [Medline: 26945587]
66. Laranjo L, Lau AY, Martin P, Tong HL, Coiera E. Use of a mobile social networking intervention for weight management:
a mixed-methods study protocol. Br Med J Open 2017 Jul 12;7(7):e016665 [FREE Full text] [doi:
10.1136/bmjopen-2017-016665] [Medline: 28706104]
67. Pumper MA, Mendoza JA, Arseniev-Koehler A, Holm M, Waite A, Moreno MA. Using a Facebook group as an adjunct
to a pilot mHealth physical activity intervention: a mixed methods approach. Stud Health Technol Inform 2015;219:97-101.
[Medline: 26799887]
68. Cook JD, Prairie ML, Plante DT. Utility of the Fitbit Flex to evaluate sleep in major depressive disorder: a comparison
against polysomnography and wrist-worn actigraphy. J Affect Disord 2017 Aug 01;217:299-305. [doi:
10.1016/j.jad.2017.04.030] [Medline: 28448949]
69. Jo E, Lewis K, Directo D, Kim MJ, Dolezal BA. Validation of biofeedback wearables for photoplethysmographic heart
rate tracking. J Sports Sci Med 2016 Sep;15(3):540-547 [FREE Full text] [Medline: 27803634]
70. Floegel TA, Florez-Pregonero A, Hekler EB, Buman MP. Validation of consumer-based hip and wrist activity monitors in
older adults with varied ambulatory abilities. J Gerontol A Biol Sci Med Sci 2016 Jun 02;72(2):229-236. [doi:
10.1093/gerona/glw098] [Medline: 27257217]
71. Alharbi M, Bauman A, Neubeck L, Gallagher R. Validation of Fitbit-Flex as a measure of free-living physical activity in
a community-based phase III cardiac rehabilitation population. Eur J Prev Cardiol 2016 Feb 23;23(14):1476-1485. [doi:
10.1177/2047487316634883] [Medline: 26907794]
72. Diaz KM, Krupka DJ, Chang MJ, Shaffer JA, Ma Y, Goldsmith J, et al. Validation of the Fitbit One® for physical activity
measurement at an upper torso attachment site. BMC Res Notes 2016 Apr 12;9:213 [FREE Full text] [doi:
10.1186/s13104-016-2020-8] [Medline: 27068022]
J Med Internet Res 2018 | vol. 20 | iss. 3 | e110 | p. 16http://www.jmir.org/2018/3/e110/
(page number not for citation purposes)
Henriksen et alJOURNAL OF MEDICAL INTERNET RESEARCH
XSL
•
FO
RenderX

73. Sushames A, Edwards A, Thompson F, McDermott R, Gebel K. Validity and reliability of Fitbit Flex for step count,
moderate to vigorous physical activity and activity energy expenditure. PLoS One 2016;11(9):e0161224 [FREE Full text]
[doi: 10.1371/journal.pone.0161224] [Medline: 27589592]
74. Kang SG, Kang JM, Ko KP, Park SC, Mariani S, Weng J. Validity of a commercial wearable sleep tracker in adult insomnia
disorder patients and good sleepers. J Psychosom Res 2017 Jun;97:38-44. [doi: 10.1016/j.jpsychores.2017.03.009] [Medline:
28606497]
75. Voss C, Gardner RF, Dean PH, Harris KC. Validity of commercial activity trackers in children with congenital heart disease.
Can J Cardiol 2017 Jun;33(6):799-805. [doi: 10.1016/j.cjca.2016.11.024] [Medline: 28347581]
76. Nelson MB, Kaminsky LA, Dickin DC, Montoye AH. Validity of consumer-based physical activity monitors for specific
activity types. Med Sci Sports Exerc 2016 Aug;48(8):1619-1628. [doi: 10.1249/MSS.0000000000000933] [Medline:
27015387]
77. Treacy D, Hassett L, Schurr K, Chagpar S, Paul S, Sherrington C. Validity of different activity monitors to count steps in
an inpatient rehabilitation setting. Phys Ther 2017;97(5):581-588.
78. Huang Y, Xu J, Yu B, Shull PB. Validity of FitBit, Jawbone UP, Nike+ and other wearable devices for level and stair
walking. Gait Posture 2016;48:36-41. [doi: 10.1016/j.gaitpost.2016.04.025]
79. Gillinov S, Etiwy M, Wang R, Blackburn G, Phelan D, Gillinov AM, et al. Variable accuracy of wearable heart rate monitors
during aerobic exercise. Med Sci Sports Exerc 2017 Aug;49(8):1697-1703. [doi: 10.1249/MSS.0000000000001284]
[Medline: 28709155]
80. Woodman JA, Crouter SE, Bassett Jr DR, Fitzhugh EC, Boyer WR. Accuracy of consumer monitors for estimating energy
expenditure and activity type. Med Sci Sports Exerc 2017;49(2):371-377. [Medline: 27580155]
81. Bronikowski M, Bronikowska M, Glapa A. Do they need goals or support? A report from a goal-setting intervention using
physical activity monitors in youth. Int J Environ Res and Public Health 2016;13(9):1-12. [Medline: 27649219]
82. Jones AP, Coombes EG, Griffin SJ, van Sluijs EM. Environmental supportiveness for physical activity in English
schoolchildren: a study using Global Positioning Systems. Int J Behav Nutr Phys Act 2009;6:42. [Medline: 19615073]
83. Mooney R, Quinlan LR, Corley G, Godfrey A, Osborough C, Olaighin G. Evaluation of the Finis Swimsense® and the
Garmin SwimTM activity monitors for swimming performance and stroke kinematics analysis. PloS One
2017;12(2):e0170902. [Medline: 28178301]
84. An HS, Jones GC, Kang SK, Welk GJ, Lee JM. How valid are wearable physical activity trackers for measuring steps?
Eur J Sport Sci 2017 Apr;17(3):360-368. [doi: 10.1080/17461391.2016.1255261] [Medline: 27912681]
85. Ehrler F, Weber C, Lovis C. Influence of pedometer position on pedometer accuracy at various walking speeds: a comparative
study. J Med Internet Res 2016 Oct 06;18(10):e268 [FREE Full text] [doi: 10.2196/jmir.5916] [Medline: 27713114]
86. Ammann R, Taube W, Neuhaus M, Wyss T. The influence of the gait-related arm swing on elevation gain measured by
sport watches. J Hum Kinet 2016;51:53-60. [doi: 10.1515/hukin-2015-0170]
87. Eyre EL, Duncan MJ, Birch SL, Cox V, Blackett M. Physical activity patterns of ethnic children from low socio-economic
environments within the UK. J Sports Sci 2015;33(3):232-242. [Medline: 24998418]
88. Schaffer SD, Holzapfel SD, Fulk G, Bosch PR. Step count accuracy and reliability of two activity tracking devices in people
after stroke. Physiother Theory Pract 2017;33(10):788-796. [Medline: 28777710]
89. O'Connell S, ÓLaighin G, Kelly L, Murphy E, Beirne S, Burke N, et al. These shoes are made for walking: sensitivity
performance evaluation of commercial activity monitors under the expected conditions and circumstances required to
achieve the international daily step goal of 10,000 steps. PLoS One 2016;11(5):e0154956 [FREE Full text] [doi:
10.1371/journal.pone.0154956] [Medline: 27167121]
90. Jennings CA, Berry TR, Carson V, Culos-Reed SN, Duncan MJ, Loitz CC, et al. UWALK: the development of a
multi-strategy, community-wide physical activity program. Transl Behav Med 2017;7(1):16-27. [doi:
10.1007/s13142-016-0417-5]
91. Price K, Bird SR, Lythgo N, Raj IS, Wong JY, Lynch C. Validation of the Fitbit One, Garmin Vivofit and Jawbone UP
activity tracker in estimation of energy expenditure during treadmill walking and running. J Med Eng Technol
2017;41(3):208-215. [Medline: 27919170]
92. Claes J, Buys R, Avila A, Finlay D, Kennedy A, Guldenring D, et al. Validity of heart rate measurements by the Garmin
Forerunner 225 at different walking intensities. J Med Eng Technol 2017;41(6):480-485. [doi:
10.1080/03091902.2017.1333166]
93. O'Brien A, Schlosser RW, Shane HC, Abramson J, Allen AA, Flynn S, et al. Brief report: just-in-time visual supports to
children with autism via the Apple watch®: a pilot feasibility study. J Autism Dev Disord 2016 Dec;46(12):3818-3823.
[doi: 10.1007/s10803-016-2891-5] [Medline: 27573856]
94. Cook S, Stauffer JC, Goy JJ, Graf D, Puricel S, Frobert A, et al. Heart rate never lies: interventional cardiologist and Braude's
quote revised. Open Heart 2016;3(1):e000373 [FREE Full text] [doi: 10.1136/openhrt-2015-000373] [Medline: 26835145]
95. Mercer K, Giangregorio L, Schneider E, Chilana P, Li M, Grindrod K. Acceptance of commercially available wearable
activity trackers among adults aged over 50 and with chronic illness: a mixed-methods evaluation. JMIR mHealth uHealth
2016;4(1):e7 [FREE Full text] [doi: 10.2196/mhealth.4225] [Medline: 26818775]
J Med Internet Res 2018 | vol. 20 | iss. 3 | e110 | p. 17http://www.jmir.org/2018/3/e110/
(page number not for citation purposes)
Henriksen et alJOURNAL OF MEDICAL INTERNET RESEARCH
XSL
•
FO
RenderX
96. El-Amrawy F, Nounou MI. Are currently available wearable devices for activity tracking and heart rate monitoring accurate,
precise, and medically beneficial? Healthc Inform Res 2015 Oct;21(4):315-320 [FREE Full text] [doi:
10.4258/hir.2015.21.4.315] [Medline: 26618039]
97. Ferguson T, Rowlands AV, Olds T, Maher C. The validity of consumer-level, activity monitors in healthy adults worn in
free-living conditions: a cross-sectional study. Int J Behav Nutr Phys Act 2015;12:42 [FREE Full text] [doi:
10.1186/s12966-015-0201-9] [Medline: 25890168]
98. Hernández-Vicente A, Santos-Lozano A, De Cocker K, Garatachea N. Validation study of Polar V800 accelerometer. Ann
Transl Med 2016 Aug;4(15):278 [FREE Full text] [doi: 10.21037/atm.2016.07.16] [Medline: 27570772]
99. Giles D, Draper N, Neil W. Validity of the Polar V800 heart rate monitor to measure RR intervals at rest. Eur J Appl Physiol
2016 Mar;116(3):563-571 [FREE Full text] [doi: 10.1007/s00421-015-3303-9] [Medline: 26708360]
100. Gruwez A, Libert W, Ameye L, Bruyneel M. Reliability of commercially available sleep and activity trackers with manual
switch-to-sleep mode activation in free-living healthy individuals. Int J Med Inform 2017 Jun;102:87-92. [doi:
10.1016/j.ijmedinf.2017.03.008] [Medline: 28495352]
101. O'Connell S, ÓLaighin G, Quinlan LR. When a step is not a step! Specificity analysis of five physical activity monitors.
PLoS One 2017;12(1):e0169616 [FREE Full text] [doi: 10.1371/journal.pone.0169616] [Medline: 28085918]
102. Parak J, Korhonen I. Evaluation of wearable consumer heart rate monitors based on photopletysmography. Conf Proc IEEE
Eng Med Biol Soc 2014;2014:3670-3673. [doi: 10.1109/EMBC.2014.6944419] [Medline: 25570787]
103. Spierer DK, Rosen Z, Litman LL, Fujii K. Validation of photoplethysmography as a method to detect heart rate during rest
and exercise. J Med Eng Technol 2015;39(5):264-271. [doi: 10.3109/03091902.2015.1047536] [Medline: 26112379]
104. Parak J, Uuskoski M, Machek J, Korhonen I. Estimating heart rate, energy expenditure, and physical performance with a
wrist photoplethysmographic device during running. JMIR mHealth uHealth 2017 Jul 25;5(7):e97 [FREE Full text] [doi:
10.2196/mhealth.7437] [Medline: 28743682]
105. Delgado-Gonzalo R, Parak J, Tarniceriu A, Renevey P, Bertschi M, Korhonen I. Evaluation of accuracy and reliability of
PulseOn optical heart rate monitoring device. Conf Proc IEEE Eng Med Biol Soc 2015 Aug;2015:430-433. [doi:
10.1109/EMBC.2015.7318391] [Medline: 26736291]
106. Parak J, Tarniceriu A, Renevey P, Bertschi M, Delgado-Gonzalo R, Korhonen I. Evaluation of the beat-to-beat detection
accuracy of PulseOn wearable optical heart rate monitor. Conf Proc IEEE Eng Med Biol Soc 2015 Aug;2015:8099-8102.
[doi: 10.1109/EMBC.2015.7320273] [Medline: 26738173]
107. Apple. 2017. WatchOS - Apple Developer URL: https://developer.apple.com/watchos [accessed 2017-09-19] [WebCite
Cache ID 6tamfJinU]
108. Apple. 2017. HealthKit - Apple Developer URL: https://developer.apple.com/healthkit/ [accessed 2017-09-13] [WebCite
Cache ID 6tRfiSKVw]
109. Fitbit. 2017. Fitbit Developer URL: https://dev.fitbit.com/ [accessed 2017-09-22] [WebCite Cache ID 6tfGRUrmR]
110. Garmin. Garmin Developers URL: https://developer.garmin.com/ [accessed 2017-09-13] [WebCite Cache ID 6tRfk6phg]
111. Misfit. Misfit Developer Toolkit URL: https://build.misfit.com/ [accessed 2017-09-13] [WebCite Cache ID 6tRgRKOAN]
112. Polar. Polar AccessLink URL: https://www.polar.com/en/connect_with_polar/polar_accesslink [accessed 2017-09-19]
[WebCite Cache ID 6tamdLu6Q]
113. Samsung. Samsung Health - Samsung Developers URL: http://developer.samsung.com/health [accessed 2017-09-13]
[WebCite Cache ID 6tRftLAHj]
114. TomTom. TomTom Developer Portal URL: https://developer.tomtom.com/ [accessed 2017-09-13] [WebCite Cache ID
6tRgHGW0A]
115. Nokia. Nokia Health API Developer Documentation URL: https://developer.health.nokia.com/api [accessed 2017-09-13]
[WebCite Cache ID 6tRgHmHhR]
116. PR Newswire Association. Fossil Group Reimagines the Watch URL: https://www.prnewswire.com/news-releases/
fossil-group-reimagines-the-watch-300427943.html [accessed 2018-03-01] [WebCite Cache ID 6xa1Z8fI6]
Abbreviations
API: application programming interface
EE: energy expenditure
GPS: global positioning system
HR: heart rate
IMU: inertial measurement unit
MEDLINE: Medical Literature Analysis and Retrieval System Online
PA: physical activity
PPG: photoplethysmography
SDK: software development kit
J Med Internet Res 2018 | vol. 20 | iss. 3 | e110 | p. 18http://www.jmir.org/2018/3/e110/
(page number not for citation purposes)
Henriksen et alJOURNAL OF MEDICAL INTERNET RESEARCH
XSL
•
FO
RenderX

Edited by G Eysenbach; submitted 17.11.17; peer-reviewed by J Sanders, P Wark, K Winfree, R Fallahzadeh, C Fernández; comments
to author 07.12.17; revised version received 18.12.17; accepted 06.01.18; published 22.03.18
Please cite as:
Henriksen A, Haugen Mikalsen M, Woldaregay AZ, Muzny M, Hartvigsen G, Hopstock LA, Grimsgaard S
Using Fitness Trackers and Smartwatches to Measure Physical Activity in Research: Analysis of Consumer Wrist-Worn Wearables
J Med Internet Res 2018;20(3):e110
URL: http://www.jmir.org/2018/3/e110/
doi: 10.2196/jmir.9157
PMID: 29567635
©André Henriksen, Martin Haugen Mikalsen, Ashenafi Zebene Woldaregay, Miroslav Muzny, Gunnar Hartvigsen, Laila
Arnesdatter Hopstock, Sameline Grimsgaard. Originally published in the Journal of Medical Internet Research (http://www.jmir.org),
22.03.2018. This is an open-access article distributed under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium,
provided the original work, first published in the Journal of Medical Internet Research, is properly cited. The complete bibliographic
information, a link to the original publication on http://www.jmir.org/, as well as this copyright and license information must be
included.
J Med Internet Res 2018 | vol. 20 | iss. 3 | e110 | p. 19http://www.jmir.org/2018/3/e110/
(page number not for citation purposes)
Henriksen et alJOURNAL OF MEDICAL INTERNET RESEARCH
XSL
•
FO
RenderX
