PETITIONS
Endocrine Disruptors:
from Scientific Evidence
to Human Health
Protection
Policy Department for Citizens' Rights and Constitutional Affairs
Directorate General for Internal Policies of the Union
PE 608.866 - March 2019
STUDY
Requested by the PETI committee
EN

Endocrine Disruptors:
from Scientific Evidence to
Human Health Protection
STUDY
Updated version, May 2019
Abstract
This study, commissioned by the PETI Committee of the European Parliament, presents
the scientific knowledge regarding the health effects of endocrine disruptors, a class of
hazards recognized in EU regulation since 1999. This report reviews the scientific evidence
regarding the concept of endocrine disruption, the extent of exposure, associated health
effects and costs. The existing relevant EU regulations are discussed and
recommendations made to better protect human health.
ABOUT THE PUBLICATION
This research paper was requested by the European Parliament's Committee on Petitions and
commissioned, overseen and published by the Policy Department for Citizen's Rights and
Constitutional Affairs.
Policy Departments provide independent expertise, both in-house and externally, to support European
Parliament committees and other parliamentary bodies in shaping legislation and exercising
democratic scrutiny over EU external and internal policies.
To contact the Policy Department for Citizens’ Rights and Constitutional Affairs or to subscribe to its
newsletter please write to: poldep-[email protected]
RESPONSIBLE RESEARCH ADMINISTRATOR
Martina SCHONARD
Policy Department for Citizens' Rights and Constitutional Affairs
European Parliament
B-1047 Brussels
E-mail: poldep-citizens@europarl.europa.eu
AUTHOR(S)
Barbara DEMENEIX, PhD, UMR 7221 CNRS/MNHN, Muséum National d’Histoire Naturelle, Paris, France.
Rémy SLAMA, PhD, Senior Investigator, INSERM (National Institute of Health and Medical Research), IAB
Research Center, Team of Environmental Epidemiology, Grenoble, France.
LINGUISTIC VERSION(S)
Original: EN
Manuscript completed in January 2019 and updated May 2019 (this version).
© European Union, 2019
This document is available on the internet at:
http://www.europarl.europa.eu/supporting-analyses
DISCLAIMER
The opinions expressed in this document are the sole responsibility of the author and do not
necessarily represent the official position of the European Parliament.
Reproduction and translation for non-commercial purposes are authorised, provided the source is
acknowledged and the publisher is given prior notice and sent a copy.
Endocrine Disruptors: from Scientific Evidence to Human Health Protection
____________________________________________________________________________________________
3
To repair is twenty times more difficult than to prevent.
Henri-Frédéric Amiel (1821-1881)
Truth is ever to be found in the simplicity, and not in the multiplicity and confusion of things.
Isaac Newton (1643-1727)
Policy Department for Citizens’ Rights and Constitutional Affairs
____________________________________________________________________________________________
4
CONTENTS
LIST OF ABBREVIATIONS 8
LIST OF TABLES 9
LIST OF FIGURES 9
EXECUTIVE SUMMARY 11
PART A: SCIENTIFIC KNOWLEDGE ON THE EFFECTS OF EDs 14
1 CONCEPTS OF ENDOCRINE DISRUPTION 15
1.1 A short history of the discovery of endocrine disruption 16
1.1.1 The insecticide DDT 16
1.1.2 The drug diethylstilboestrol (DES) 16
1.1.3 Endocrine disruption is defined in 1991 17
1.2 Definition of endocrine disruptors 17
1.3 Relevant knowledge from endocrinology 17
1.3.1 Endocrinology is the study of hormones and their controls 17
1.3.2 Hormone act at very low doses within three main endocrine axes 18
1.3.3 Non-linear responses are seen for endogenous responses and in endocrine disruption 18
1.3.4 The endocrine system plays essential roles from conception to aging 19
1.4 Fine scale evidence that exogenous substances can interfere with the endocrine system 20
1.4.1 Interference with the oestrogen or androgen binding to their receptors 20
1.4.2 EDs affecting aromatase action 20
1.4.3 ED Interference with thyroid hormone distribution in the blood 21
1.4.4 Interference with iodine uptake by the thyroid gland. 21
1.5 Evidence available at the scale of organisms and populations 21
1.5.1 Compounds affecting brain development through alteration of the endocrine system 22
1.5.2 Compounds altering diabetes risk and other metabolic disorders through alteration of the
endocrine system 22
1.5.3 Compounds inducing reproductive disorders through alteration of the endocrine system 23
1.5.4 Implication of oestrogen-like compounds in breast cancer 24
1.5.5 Implication of other potential EDs in cancer risk 24
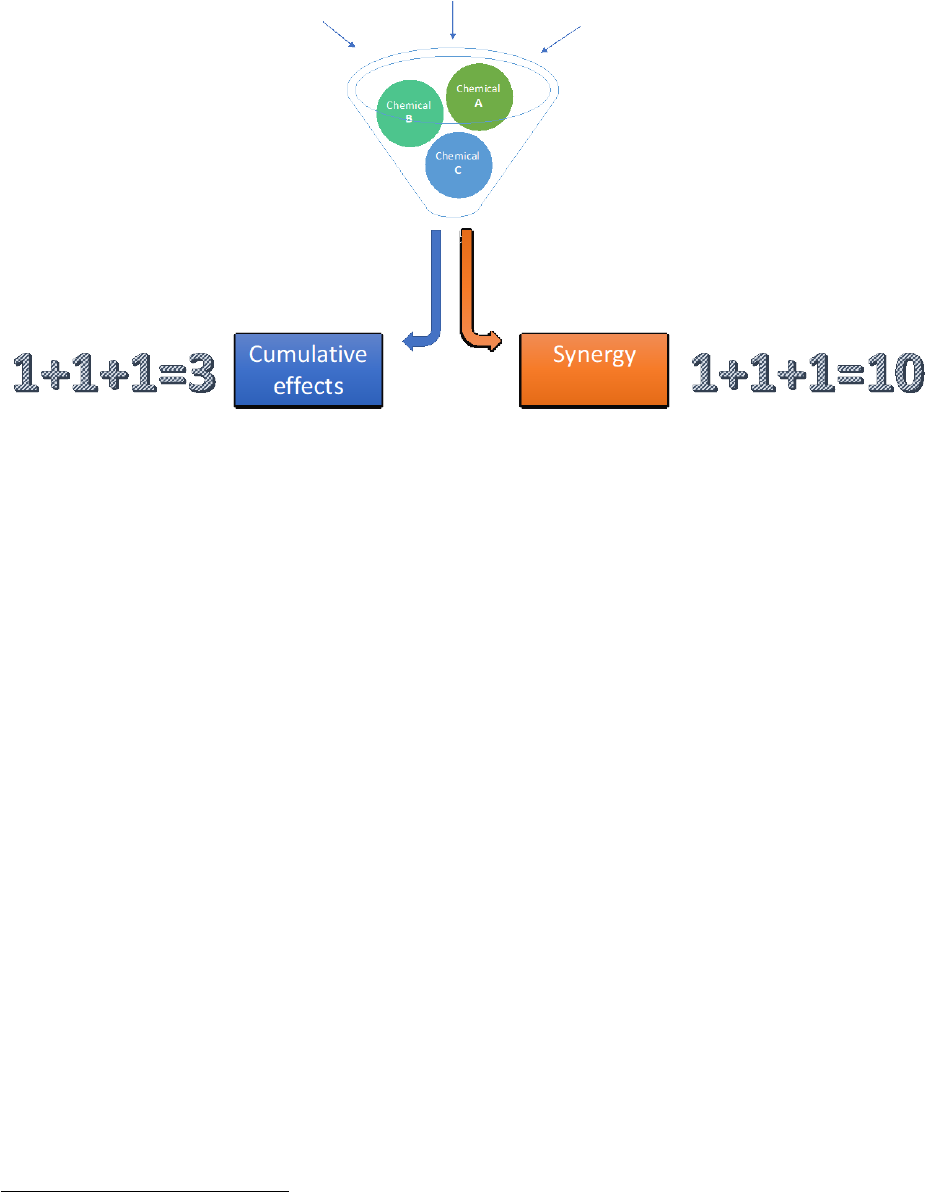
1.6 EDs can act additively and synergistically 25
1.6.1 Different types of effects of mixtures 25
1.6.2 Response addition (“independent action”) 26
1.6.3 Dose addition (cumulative effects) 27
1.6.4 Synergy, antagonism 27
1.6.5 Which of the different mixtures models is expected to be most valid? 29
1.6.6 How to handle cumulative effects and synergy in a regulatory context 30
1.7 Some populations are more vulnerable to EDs 30
1.8 Diseases and adverse effects suspected to be linked with ED exposure 31
1.9 Epigenetic effects and potential effects on successive generations 33
1.10 EDs’ specific characteristics are recognized by the scientific community at large 33
Endocrine Disruptors: from Scientific Evidence to Human Health Protection
____________________________________________________________________________________________
5
2 MAIN FAMILIES OF KNOWN AND SUSPECTED ENDOCRINE DISRUPTORS: EVIDENCE FOR
WIDESPREAD EXPOSURE AND ADVERSE HEALTH EFFECTS 35
2.1 Which methodologies have been used to demonstrate health effects of EDs? 35
2.1.1 Efficient methodologies to highlight the cause of multifactorial diseases exist 35
2.2 Some suspected EDs present in food contact materials and diet 37
2.2.1 Bisphenol A 37
2.2.2 Phthalates 38
2.2.3 Perfluoroalkyl substances (PFASs) 39
2.3 Some known and suspected EDs present in cosmetics and personal care products 39
2.3.1 Parabens 40
2.3.2 Triclosan 40
2.3.3 Benzophenones 40
2.4 Some suspected EDs present in drugs 41
2.5 Known and suspected EDs present in consumer goods and cleaning products 41
2.5.1 Brominated and phosphorylated flame retardants 41
2.5.2 Phthalates 42
2.5.3 Perfluoroalkyl substances (PFAS) 42
2.5.4 Cleaning products 42
2.5.5 Building materials 43
2.5.6 Medical Devices 43
2.5.7 Toys 43
2.6 Some known and suspected EDs present in pesticides 43
2.6.1 Organophosphate pesticides 44
2.6.2 Triazoles and other fungicides 44
2.6.3 Pyrethroids 44
2.6.4 Neonicotinoids 44
2.7 Biomonitoring data on EDs and suspected EDs 44
2.7.1 In diet 44
2.7.2 In human populations – data available at the EU level 45
2.7.3 In human populations – data available in specific countries 45
2.8 Available estimates of population impact 46
2.8.1 Results of Trasande et al. costs estimate 46
2.8.2 Published criticisms of Trasande et al. costs estimate 48
2.9 Limitations of the current regulatory risk assessment framework to minimize ED exposure
and efficiently protect health 49
2.9.1 Current regulatory framework for the management of chemicals 49
2.9.2 The “threshold” debate 50
2.9.3 Are existing test methods sufficiently sensitive? 51
PART B: MANAGING THE RISK INCURRED BY EDCs – WHAT IS CURRENTLY DONE 53
3 CURRENT REGULATION OF ENDOCRINE DISRUPTORS IN THE EU 54
3.1 Overarching regulations 55
3.1.1 The 7
th
environment action programme 55
3.1.2 1999 EU strategy on EDs 56
3.1.3 The 2018 Communication toward a comprehensive EU framework on EDs 57
3.2 The plant protection products (PPPR) and biocides (BPR) regulations 58
3.2.1 Management of plant protection products and biocides containing EDs 58
3.2.2 Definitions of EDs for plant protection products and biocides 59
3.2.3 Identification of EDs for plant protection products and biocides 60
Policy Department for Citizens’ Rights and Constitutional Affairs
____________________________________________________________________________________________
6
3.2.4 Test requirements to identify plant protection products and biocides with ED properties 61
3.3 REACH chemicals regulation 62
3.3.1 General principles and aims of REACH regulation 62
3.3.2 Management of EDs under REACH regulation 62
3.3.3 Definition and identification of EDs under REACH regulation 63
3.3.4 Test requirements to identify which REACH chemicals are EDs 64
3.3.5 Conclusion regarding REACH regulation 65
3.4 Regulation of EDs in cosmetics 66
3.4.1 General principles of the cosmetics regulation 66
3.4.2 Management of EDs present in cosmetics 66
3.5 Other sectors in which the regulation refers to EDs 67
3.5.1 Water 67
3.5.2 Medical devices regulation 67
3.6 Sectors in which the regulation does not explicitly refer to EDs 67
3.6.1 The toys’ safety directive 67
3.6.2 Workers’ protection regulations 68
3.6.3 Food and food contact materials 68
3.6.4 Food additives 70
3.7 Piecewise regulation of specific EDs 70
3.7.1 “Legacy” persistent chemicals suspected to be EDs (POPs) 70
3.7.2 Bisphenol A 70
3.7.3 Triclosan 70
3.7.4 Phthalates, including DEHP 71
3.7.5 Are piecewise regulations of specific EDs efficient? The example of bisphenol A 71
3.8 The Classification Labelling and Packaging (CLP) directive 71
3.9 Examples from specific Member States 72
3.9.1 Denmark 72
3.9.2 France 72
3.9.3 Sweden 72
PART C: MANAGING THE RISK INCURRED BY EDs – WHAT COULD BE FURTHER DONE IN THE EU? 76
4 IDENTIFICATION OF ENDOCRINE DISRUPTORS 77
4.1 Gaps in the current regulation and general considerations regarding possible
improvements 77
4.1.1 The regulation does not allow minimizing the health risks incurred by EDs 77
4.1.2 The heterogeneous regulation of EDs in different sectors is hard to justify scientifically 78
4.1.3 Even within specific sectors, management of EDs generally lacks coherence 78
4.1.4 In the past, important decisions have been delayed by lack of proper application of policies
on conflicts of interest 79
4.1.5 Translating scientific knowledge into regulation 79
4.2 Towards a cross-sectorial definition of EDs 81
4.2.1 Assessment of the approved criteria for ED Identification under EU Regulations on Plant
Protection Products and on Biocidal Products 81
4.2.2 Definition of EDs 81
4.2.3 Definition of presumed and suspected EDs 82
4.3 A guidance document is needed to explain how test results and literature should be used
to apply the ED definition 83
4.3.1 Assessment of the EU’s Guidance document for the implementation of the New Criteria on
Endocrine Disruptors for Plant protection products and Biocides 83
Endocrine Disruptors: from Scientific Evidence to Human Health Protection
____________________________________________________________________________________________
7
4.3.2 A cross-sectorial guidance document will be needed once a cross-sectorial definition of EDs
is enforced 84
4.4 Setting and enforcing coherent test requirements 85
4.5 Test development 85
4.6 Surveillance of ED production, use and exposure 87
4.6.1 Monitoring of ED production and use 87
4.6.2 Environmental monitoring of EDs 87
4.6.3 Human biomonitoring of EDs and hazardous chemicals 87
4.7 Consideration of potential conflicts of interests in events organised with scientists in EU
institutions 88
4.8 Key research initiatives to be supported 88
4.8.1 Epigenetics 88
4.8.2 Multi- and transgenerational effects 89
4.8.3 ED effects on the microbiome 89
4.8.4 Green chemistry 89
4.8.5 EDs acting on less studied modalities beyond E, A, T, S and metabolism 89
4.8.6 Characterization of dose-response functions for ED effects in humans 90
5 MANAGEMENT OF EDS ACROSS SECTORS TO PROTECT HEALTH 91
5.1 General strategy to manage the ED risk in all sectors and media 91
5.1.1 Management logic for products used by consumers as part of REACH regulation 92
5.1.2 Management logic as part of REACH regulation – towards a clearer “equivalent concern”
principle for CMRs and EDs 92
5.1.3 Management logic for plant protection products and biocides 92
5.1.4 Management logic for cosmetics 93
5.1.5 Review of the cosmetics regulation by the European Commission (2018) 93
5.1.6 Management logic in other sectors with likely widespread exposure 94
5.2 Management of EDs in specific media including water 94
5.2.1 Management of EDs in water 94
5.2.2 Concerns regarding the use of paracetamol during pregnancy 95
5.3 Labelling of products containing EDs 95
5.4 Conclusion 95
RECOMMENDATIONS 97
ANNEX 99
ANNEX 1: DEFINITIONS 100
ANNEX 2: DISCUSSION OF PAST CONTROVERSIES REGARDING EDS REGULATION IN THE EU 102
2013 “ED criteria” and Dietrich et al. 2013 letter 102
2018 Parliament hearing at the PETI commission: statements from dr. Dietrich 102
REFERENCES 107
CONFLICTS OF INTERESTS’ STATEMENT ACKNOWLEDGMENTS 125
INDEX 126
Policy Department for Citizens’ Rights and Constitutional Affairs
____________________________________________________________________________________________
8
LIST OF ABBREVIATIONS
ADHD
Attention Deficit and Hyperactivity Disorder
BBP
Benzyl butyl phthalate
BPR
Biocidal Products Regulation (2012)
CLP regulation
Regulation on Classification, Labelling and Packaging (2008)
DBP
Dibutyl phthalate
DES
Diethystilbestrol (drug)
DDE
Dichlorodiphenyltricholoenthylene (metabolite of DDT)
DDT
Dichlorodiphenyltrichloroethene (insecticide)
DEHP
Bis(2-Ethylhexyl) phthalate
DIBP
Diisobutyl phthalate
EAP
Environment Action Program
ED
Endocrine Disruptor
EFSA
European Food Safety Authority
FDA
Food and Drugs Administration (USA)
GHS
Globally Harmonised System
IQ
Intellectual Quotient
OECD
Organisation for Economic Co-operation and Development
PBDE
Polybrominated Diphenyl Ethers
PCB
Polychlorinated biphenyls
PPPR
Plant Protection Products Regulation (2009)
RIVM
Dutch Institute for Public Health and the Environment
SCCS
Scientific Committtee on Consumer Safety (European Commission)
WHO
World Health Organization
WoE
Weight of Evidence
Endocrine Disruptors: from Scientific Evidence to Human Health Protection
____________________________________________________________________________________________
9
LIST OF TABLES
TABLE 1: Overview of the existing EU framework regarding protection from the health effects of
endocrine disruptors. .............................................................................................................................. 13
TABLE 2: Some recognized or suspected adverse effects of potential EDs. ........................................... 32
TABLE 3: Distribution of urinary or blood concentrations of chemicals in 1300 EU children from
Helix exposome project. P25, P50, P75: 25
th
, 50
th
(median) and 75
th
percentiles [123]. ... 46
TABLE 4: stimated costs incurred yearly in the EU by various lifestyle or environmental factors. See
above for discussion. ............................................................................................................................... 48
TABLE 5: Tests required to identify EDs in various sectors of the EU regulation. ................................. 61
TABLE 6: List of legally recognized EDs in the EU. This corresponds to the substances added to the
REACH list of substances of very high concern because of their endocrine-disrupting
properties. .................................................................................................................................................... 64
TABLE 7: Current status of the regulatory provisions defining EDs in various regulatory areas, or
allowing the identification, and existence of explicit management logics. See also Table
1 for a simplified version. ....................................................................................................................... 69
TABLE 8: List of the main hazards legally recognized in the EU regulation. .......................................... 73
TABLE 9: Suggested hazard categories and criteria for endocrine disruptors and parallel with
carcinogens. ................................................................................................................................................ 83
LIST OF FIGURES
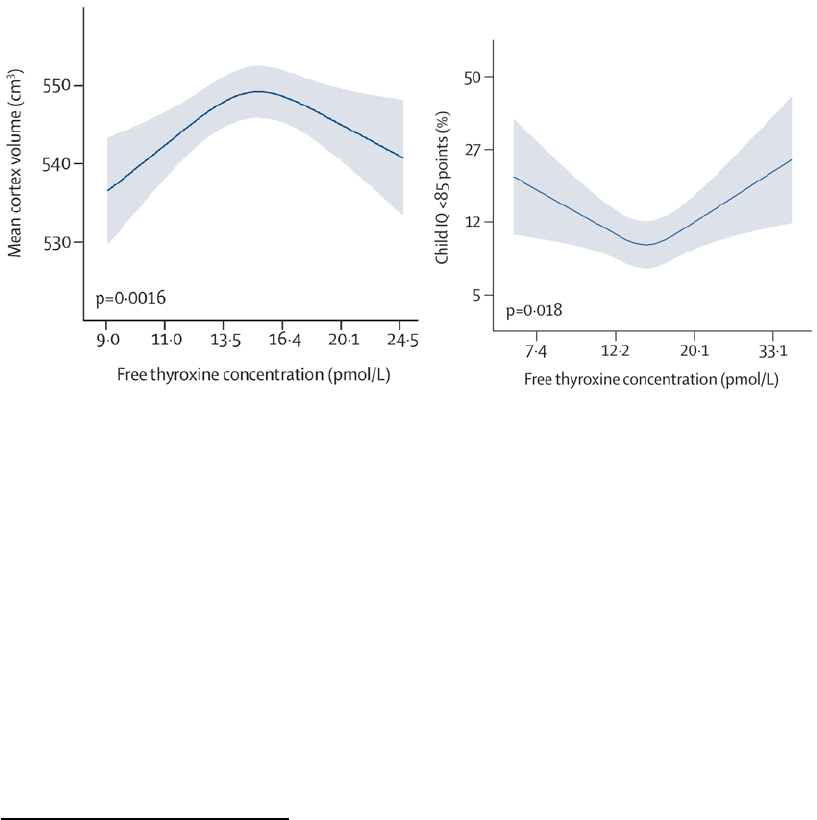
FIGURE 1: Relation between maternal thyroid hormone level (thyroxine) during pregnancy and (A)
offspring cortex volume at the age of 8 years; (B) the predicted probability of offspring
having an Intellectual Quotient (IQ) at the age of 6-8 years below 85 points. As women
with overt hyperthyroidism or hypothyroidism were excluded, the range of values
corresponds to those that can be considered within the normal limits for pregnancy, i.e.
7 to 34 picomole/l of free thyroxine [28]. ......................................................................................... 19
FIGURE 2: Schematic overview of the main modalities and health endpoints that can be affected by
endocrine disruptors. .............................................................................................................................. 20
FIGURE 3: Known and suspected EDs linked to adverse effects on neurodevelopment. Note that the
multiple levels by which EDs can interfere with thyroid hormone signalling complicate
the setting up of in vitro screening methods for each level of action. This is particularly
acute given the multiplicity of thyroid hormone actions during brain development [45,
46]. Different structures (cell, brain) are not drawn to scale. Adapted from [33]. .............. 22
FIGURE 4: Overview of the relations of suspected EDs with nuclear receptors implicated in the
development of adipogenesis and metabolic disorders such as insulin resistance. From
[44]. ................................................................................................................................................................. 23
FIGURE 5: Examples of compounds with oestrogenic or anti-oestrogenic activity that may influence
breast cancer risk. ...................................................................................................................................... 24
FIGURE 6: Different types of health effects of mixtures of hazardous chemicals. Note that in addition
to synergy, antagonism is also possible (omitted for simplicity). ............................................ 26
FIGURE 7: Illustration of the cumulative effect of a mixture of compounds each present in the body
below a dose that would be a concern in the case of a single exposure, but for which the
mixture constitutes a risk. From [78]. ................................................................................................. 27
Policy Department for Citizens’ Rights and Constitutional Affairs
____________________________________________________________________________________________
10
FIGURE 8: Synergy between EDs - Example of a supra-molecular ligand composed of trans-
nonachlor and 17α-ethinyl-oestradiol. a) Pregnane-X receptor (PXR) in which trans-
nonachlor (TNC) and 17α -EE (EE2) are bound. b) TNC and EE2 molecules. c) Visualization
of the interactions between the molecules TNC and EE2, creating a supra-molecular
ligand in the ligand-binding pocket of PXR [81]. ........................................................................... 28
FIGURE 9: A) Observed effect of a mixture of anti-androgenic compounds on testosterone
production in fetal testis explants (blue vertical lines) and effect predicted on the basis of
dose-addition (orange continuous curve) as a function of the mixture concentration (in
mol/l). B) Effect of bisphenol A on testosterone production when used alone (blue curve)
or in presence of a mixture with 7 other compounds (red dotted curve) [84]. .................. 29
FIGURE 10: Exposure in pregnancy touches three generations: mother (F0), foetus (F1) and the next
generation through the germinal cells growing in the foetus (F2). ....................................... 31
FIGURE 11: Schematic view of the steps between the identification of the hazard related to a
substance and its management by authorities. Adapted from [93]. ...................................... 33
FIGURE 12: Estimated costs associated with exposure to endocrine disruptors in the EU, following a
weight of evidence approach; from [240]. ....................................................................................... 47
FIGURE 13: Illustrations of the decreased sensitivity of regulatory tests for a given number of animals
in each compared group, in the hypothetical situation of a compound with a
monotonous effect. Generally, test methods do not require the number of animals
compared to increase as the tested dose decreases. As the tested exposure decreases,
the probability to detect an effect decreases, so the test could conclude the existence of
a threshold at exposures for which an effect may still exist. ..................................................... 51
FIGURE 14: EDs are expected to be present in different sectors; list of the main EU regulatory areas
with relevance to endocrine disruption. Adapted from [7]. ...................................................... 55
FIGURE 15: Schematic view of the authorization and restriction procedures of REACH, possibly
leading to the restriction of marketing of hazardous substances. .......................................... 63
FIGURE 16: Schematic fate of man-made chemicals from their production site to the human body
illustrating the possible different levels of monitoring of chemicals. .................................... 87
Endocrine Disruptors: from Scientific Evidence to Human Health Protection
____________________________________________________________________________________________
11
EXECUTIVE SUMMARY
Background
The endocrine system orchestrates our physiological functions from conception onwards. Endocrine
disruptors (EDs) were recognised by the scientific community in 1991. In 2002, the World Health
Organization defined EDs as “an exogenous substance or mixture that alters function(s) of the
endocrine system and consequently causes adverse health effects in an intact organism, or its progeny,
or (sub) populations.” Thousands of scientific publications have identified several modalities of ED
action, chronic diseases linked to ED exposure and provided a first estimate of population impact in
the EU. The EU identified this issue as early as 1996 and has since recognized EDs as a new type of health
and environmental hazard, together with carcinogens, mutagens, substances toxic for reproduction
(CMRs), persistent, bioaccumulative and toxic (PBTs) substances. With REACH (2006) and the Plant
Protection Products (2009) regulation, specific sectors of the regulation began considering EDs.
Aim
This report presents the scientific evidence regarding the concept of endocrine disruption, the extent
of exposure and of the health effects of EDs. The relevant EU regulations are reviewed and
recommendations made to change them for better protection of human health.
Main conclusions
Scientific knowledge on EDs
Hormones coordinate harmonious development and function of all organs and act at minute
concentrations (part per trillion to per billion range). Given the essential role of the endocrine system
during development, ED exposure during vulnerable periods can induce long-lasting changes, with
adverse effects in the short and long terms; some of these effects are expected at very low doses. Non-
monotonic dose responses can be observed. Hundreds of man-made and some natural chemicals can
disrupt the function of the endocrine system. Certain of them have been demonstrated to induce
adverse effects as a consequence of this disruption.
Although multifactorial, many chronic health disorders have been clearly linked by animal experiments
and epidemiology to EDs. These disorders include obesity and metabolic disorders, reproductive
disorders, reproductive cancers, thyroid disorders, neurodevelopmental disease and IQ loss.
EDs are present in food, food contact materials, cosmetics, consumer goods (including furnishings,
cleaning products), toys, as well as drinking water. Consequently, the EU population is widely exposed
to known and suspected EDs. This fact is confirmed by biomonitoring studies, including on susceptible
subgroups such as pregnant women and children. Annual costs related to ED exposure were estimated
to be €163 billion (above €22 billion with a 95% probability and above €196 billion with a 25%
probability).
Multiple exposures result in cumulative effects, a situation expected for compounds acting via similar
pathways, and that is also likely for compounds acting on similar health outcomes via different
pathways. Synergistic effects can also be observed. Currently, EU chemical regulations do not generally
consider these cumulative effects, notably for ED exposures.
Scientific consensus now exists for (1) the definition of endocrine disruptors; (2) the presence of
suspected or recognized EDs in the environment and in humans in the EU; (3) EDs as a serious concern
for the health of current and future generations and the environment; (4) the limitations of current
Policy Department for Citizens’ Rights and Constitutional Affairs
____________________________________________________________________________________________
12
regulatory approaches used to identify so-called safe thresholds and (5) the lack of consideration of
cumulative effects of combined exposures in regulations.
Regulation of EDs in the EU
Minimising overall exposure of humans and the environment to EDs is a relevant aim for the EU, as
expressed in the 7
th
Environmental Action Program and 2018 EU framework on EDs.
Attaining this goal requires a) a cross-sectorial (“horizontal”) definition of EDs, distinguishing three
categories according to the level of evidence; b) a guidance document explaining how to apply the
definition using test results and scientific literature; c) tests covering all ED modalities; d) legal
requirements to make these tests compulsory in application dossiers; e) a management logic, which
could distinguish sectors with likely human exposure (where EDs should be avoided) from those for
which exposure is rare.
Currently, a legal definition of EDs only exists for biocides and plant protection products (pesticides),
the sectors with the most advanced EDs regulation in the EU. The Guidance document for biocides and
plant protection products is thorough and, if correctly used, can help identifying EDs. However, even
for pesticides, the regulation is imperfect, in that a definition and a management logic exist (zero
exposure to EDs in pesticides) but without ED tests covering all ED endpoints being compulsory in
application dossiers, making ED identification very difficult in practice.
This lack of efficient consideration of EDs is more pronounced in other sectors where human ED
exposure is also very likely, such as food additives and food contact materials, cosmetics, toys,
consumer goods and workers’ protection.
Test development: There is an urgency to accelerate test development and validation, especially in
modalities beyond steroid hormones. Coverage is currently insufficient for the thyroid axis, metabolic
hormones and the corresponding endpoints. Regulators should rely more on academic publications
when assessing ED properties and request faster test validation.
Test requirements: The regulatory documents setting out the content of application dossiers for
authorization generally do not require tests that would allow to scientifically assess if the substance
under evaluation is an ED. Regulations setting test requirements in all sectors with possible ED use
should be modified to include provisions ensuring that dossiers contain test results allowing to
conclude if the evaluated substance or product is an ED.
Management of EDs across sectors: In order to minimize ED exposure among EU citizens, the EU should
move towards identical management of EDs across all sectors for which ED use is very likely to entail
population exposure, notably pesticides, food contact materials and additives, consumer goods,
cosmetics, medical devices and toys.
Specifically, given the widespread EU population exposure to many suspected EDs and the fact that
combined exposure to several EDs acting on similar or different pathways can have cumulative effects,
to minimize ED exposure and render EU regulation more coherent across sectors, a logic similar to that
already in use for pesticides (no human exposure) appears justified in sectors with likely human
exposure.
The oestrogenic, androgenic, thyroid, steroid loads, and that of other ED modalities, in consumer
products, food, cosmetics and drinking water should be evaluated and monitored, and the
implementation of limit values in such media considered.
Endocrine Disruptors: from Scientific Evidence to Human Health Protection
____________________________________________________________________________________________
13
Research needs
Besides test development, six research areas should be prioritised: (i) Epigenetic effects of EDs; (ii)
Effects across generations; (iii) ED effects on the microbiome, (iv) Green (safe) chemistry; (v) Novel ED
modalities and (vi) Characterization of dose-response functions for ED effects in humans.
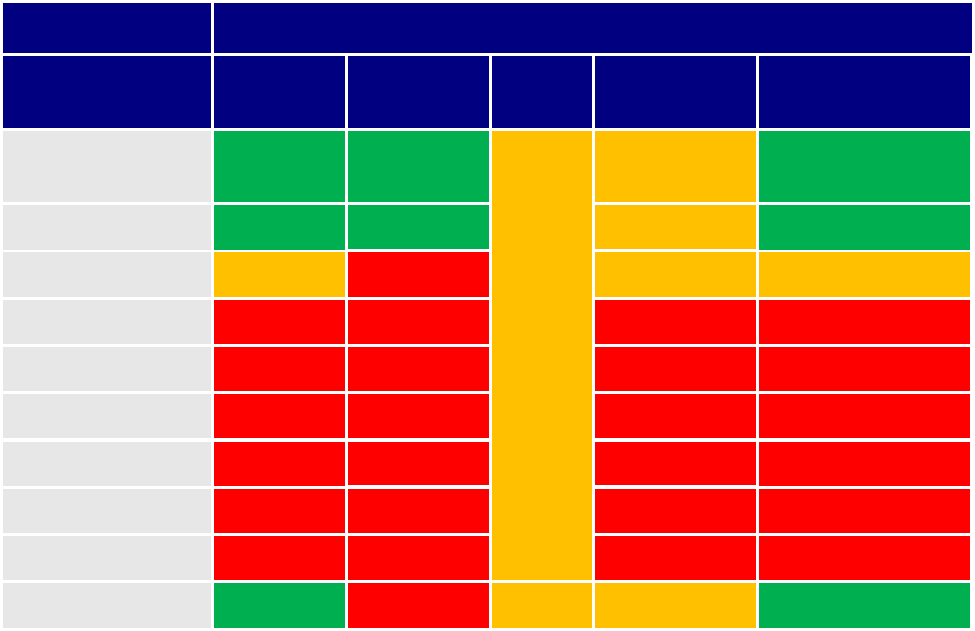
Table 1: Overview of the existing EU framework regarding protection from the health effects of endocrine
disruptors.
Regulatory steps to protect health
Sector/regulation
Definition of
EDs
Guidance
document
Tests
Test
requirements
Risk management
logic
Plant protection
products
Y
Y
I
I
Y
Biocides
Y
Y
I
Y
REACH chemicals
I
N
I
I
Cosmetics
N
N
N
N
Food additives
N
N
N
N
Food contact material
N
N
N
N
Drinking water
N
N
N
N
Toys
N
N
N
N
Workers’ regulations N N N N
Medical devices
Y
N
I
Y
I: Insufficient/needs reinforcement. N: None or very limited. Y: Yes, satisfying existing regulation.

Policy Department for Citizens’ Rights and Constitutional Affairs
____________________________________________________________________________________________
14
PART A: SCIENTIFIC KNOWLEDGE
ON THE EFFECTS OF EDs

Endocrine Disruptors: from Scientific Evidence to Human Health Protection
____________________________________________________________________________________________
15
1 CONCEPTS OF ENDOCRINE DISRUPTION
KEY FINDINGS
• The endocrine system controls our main physiological functions from conception
onwards.
• Hormones are present in body fluids and act at very low concentrations (usually in the
part per trillion to part per billion range).
• Certain man-made and natural chemicals can disrupt the function of the endocrine
system and consequently induce adverse effects. These substances are referred to as
endocrine disruptors (EDs).
• EDs act at low doses, with different effects during vulnerable exposure windows. Non-
monotonic dose responses can be observed.
• Given the essential role of the endocrine system during human development, ED
exposure in vulnerable periods can induce organizational changes, with adverse effects
occurring in the short and long terms, such as congenital malformations, altered
neurodevelopment and IQ loss, metabolic disorders (type-2 diabetes, obesity) and
specific “endocrine-related” cancers such as breast and prostate cancers. In adulthood, ED
exposure has been associated with reduced fecundity and thyroid disorders.
• Multiple exposures can result in cumulative effects, a situation that is expected for
compounds acting via similar pathways, and also probably for those acting on similar
health outcomes via different pathways. Synergistic effects can also be observed.
This report deals with the health impact of endocrine disruptors (EDs) and the corresponding regulatory
framework in the European Union (EU).
A number of thorough scientific reviews and reports on endocrine disruption have been published
since 2010, underlining the importance, the concern and the rapid growth of the field. At the end of
2011, the EU commissioned a “State of the Art Assessment of Endocrine Disrupters” [1]. In 2012 the
World Health Organization (WHO), with the United Nations Environment Programme (UNEP),
published their “State of the Science of Endocrine Disrupting Chemicals” [2]. That year WHO also
published “Endocrine Disrupters and Child Health”, underlining the overriding importance of
protecting vulnerable groups such as pregnant women, children and adolescents [3]. In 2015, the
Endocrine Society produced their second report on the question, with over 1300 references to different
aspects of endocrine disruption [4]. Then, in 2016 UNEP commissioned the International Panel on
Chemical Pollution (IPCP) to do three reports on EDs, each published in 2018 [5-7].
Here, our aim is not to systematically review the literature in these reports, but to provide an overview of
health impacts, current regulatory framework and suggestions for changes. The report is structured in
three parts; part A presents the scientific knowledge regarding the concept of endocrine disruption
(chapter 1) and the extent of the impact of EDs in the EU (chapter 2). Part B addresses the current EU
regulatory framework, while part C suggests improvement for this framework distinguishing the
identification of EDs (chapter 4) and the risk management logic (chapter 5). Though EDs also impact our
environment, on which our health depends, here, the discussion is limited to human health concerns.
Policy Department for Citizens’ Rights and Constitutional Affairs
____________________________________________________________________________________________
16
1.1 A short history of the discovery of endocrine disruption
1.1.1 The insecticide DDT
In 1962, Rachel Carson’s book Silent Spring [8] galvanised public attention on effects of excessive use of
the insecticide DDT on wildlife. The population losses in fish and birds observed then were not simply
due to reductions in a main food source, insects, but also to accumulation of DDT (and its metabolites) in
their organs. Astutely, Carson realised that this in turn was affecting their reproductive capacity. For the
emblematic “bald eagle” (the USA’s national symbol), the massive decrease in population was in large
part due to increased fragility of the egg shell that broke under the parents’ weight. Although Carson
never employed the term endocrine disruption, she was in fact describing this phenomenon, as DDT has
since been shown to reducing circulating levels of the sex hormone, oestradiol in birds [9, 10] and to alter
prostaglandin (a family of chemical messengers related to the hormonal system) levels, thereby
interfering with enzymes needed for shell mineralization. She also presciently realized that humans
would be contaminated through their food, and that effects would later be seen on human health. This
is unfortunately the case. Half a century later, it was reported that girls (female foetuses) who had been
exposed to high levels of DDT in utero during the 1960s have an increased risk of breast cancer in the
following 50 years [11].
1.1.2 The drug diethylstilboestrol (DES)
DES was developed as a synthetic oestrogen. It was prescribed from the 1940s onwards. Prescriptions
were based on the erroneous assumption that it could prevent miscarriage and other pregnancy
complications, which was shown to be wrong in 1953 [12]. In 1971, the USA Food and Drug
Administration (FDA) advised against its use due to vaginal cancers occurrence in girls born to mothers
who had used DES, while this cancer usually develops post-menopause. DES was banned in the
Netherlands in 1975 and in France and Spain in 1977. Women who took DES have a slightly higher risk of
breast cancer [13], but the most striking effects are seen on offspring exposed during pregnancy [13].
Epidemiology shows in utero DES exposure to be linked not only to vaginal cancer in daughters of
exposed women, but also to reproductive tract disorders, infertility and higher rates of spontaneous
abortion [13]. Sons display higher rates of genital abnormalities, and increased risks of prostate cancer; in
addition, an increased risk of testicular cancer has been suggested [14]. Importantly, effects such as
increased risk of malformations of the male genitalia and possibly attention deficit and hyperactivity
disorders (ADHD) are also observed in the grandchildren of DES-prescribed women [15, 16].
In contrast to DDT, which is persistent in the body, DES is quickly eliminated, showing that chemicals can
exert effects long after they disappeared from the organism, possibly on successive generations. There
are biological mechanisms whereby the organism could keep a memory of exposure. One possibility
relates to adverse effects that can be traced to epigenetic modifications. Work on animal models shows
that certain DES impacts could result from epigenetic effects on the germ cells (the sperm and egg cells)
forming in the in utero DES exposed foetuses (see 1.7 and [17]).
Both DDT and DES provide examples of compounds able to interact with the endocrine system in humans
or wildlife species (DES was designed to mimic a natural hormone, oestrogen; DDT and its metabolites
were found to alter hormone production, mimic oestrogen and block androgen actions) and to cause
adverse effects. They resonate with a concept developed in 1.7 and 1.9: the Developmental origin of
Health and Disease (DOHaD)[18], underlining foetal life as a determinant factor for child and adult health.

Endocrine Disruptors: from Scientific Evidence to Human Health Protection
____________________________________________________________________________________________
17
1.1.3 Endocrine disruption is defined in 1991
The Wingspread conference (1991) defined and detailed the global problem of endocrine disruption in
both wildlife and humans, emphasising that developmental exposure could lead to disease later in life
[19]. At the time, focus was on reproductive problems, so the principal concern was interference of
chemicals with the two main classes of receptors that control reproduction — the receptors for
oestrogens and androgens, two classes of steroid hormones
1
. In 1996, the EU piloted, with the OECD and
other organisations, a meeting of scientists and regulators at Weybridge in the UK
2
. The Weybridge
meeting presciently noted the need for research on effects of EDs on health and wildlife beyond those
principally studied, the reproductive steroids.
1.2 Definition of endocrine disruptors
The definition of EDs proposed by the World Health Organization (WHO) and International Programme
on Chemical Safety (IPCS) in 2002 [9] is now widely accepted scientifically:
“An endocrine disrupter is an exogenous substance or mixture that alters function(s) of the
endocrine system and consequently causes adverse health effects in an intact organism, or its
progeny or (sub)populations.” [9]
An adverse effect is defined as:
“a change in the morphology, physiology, growth, development, reproduction or life span of an
organism, system or (sub)population that results in an impairment of functional capacity, an
impairment of the capacity to compensate for additional stress or an increase in susceptibility to
other influences.”
3
Definitions of substances with endocrine-disrupting activities are now present in the EU regulation in the
context of plant protection products and biocides (see 3.2 and 4.2 below).
1.3 Relevant knowledge from endocrinology
1.3.1 Endocrinology is the study of hormones and their controls
Hormones are produced in endocrine glands, for example the ovaries, testes, thyroid, and pancreas,
amongst others (See Annex 1). Hormones are released into the blood stream. By acting on multiple
distant organs, the endocrine system ensures harmonious coordination of tissue function. Before the
foetal endocrine system is functional, maternal hormones ensure foetal development.
The endocrine system regulates all physiological systems: growth of the skeleton and muscles,
reproduction including puberty, digestion and metabolism, control of body temperature, brain
development and brain activity including mood and alertness. Hormones produced by the pancreas
control sugar levels, other hormones (glucocorticoids) govern stress responses. Hormones affect immune
responses, hence responses to vaccination and disease. The endocrine system has a continual dialogue
with the two other main communication systems of our body, the nervous system and the immune
system, so that any disruption of the endocrine system may also impact these other systems.
1
Steroids are organic molecules with four benzenic rings with (among others) a signaling role in the body. Steroid hormones include the
androgen class (those controlling the development of male reproductive function, with testosterone as the main androgen hormone) and
oestrogen class (with oestradiol as the major oestrogen hormone in humans).
2
http://www.iehconsulting.co.uk/IEH_Consulting/IEHCPubs/EndocrineDisrupters/WEYBRIDGE.pdf
3
REGULATION (EU) 2018/605 of 19 April 2018 amending Annex II to Regulation (EC) No 1107/2009 by setting out scientific criteria for the
determination of endocrine disrupting properties. Link.

Policy Department for Citizens’ Rights and Constitutional Affairs
____________________________________________________________________________________________
18
1.3.2 Hormone act at very low doses within three main endocrine axes
The main endocrine axes are the hypothalamo/pituitary/gonad axis controling reproduction and
puberty, the hypothalamo/pituitary/adrenal axis that controls many aspects of our stress responses and
the hypothalamo/pituitary/thyroid axis, that controls thyroid hormone, needed for brain development
and function, growth and control of energy metabolism.
Hormones are secreted into the blood and act on target tissues throughout the body at extremely low
concentrations (typically in the part per trillion to part per billion (ppb) range
4
). These effects are
explained by the high affinity of receptors and other proteins (transporters, metabolic enzymes) for their
endogenous hormones.
The endocrine system also includes the proteins in the blood system that distribute the hormones, the
enzymes involved in hormone synthesis, activation and inactivation, the membrane transporters
allowing hormone entry into target cells and the hormone receptors themselves (found on the cell
membrane, in the cytoplasm or in the nucleus).
Not surprisingly, disruption of the endocrine system can affect negatively each of these levels. Certain
EDs interfere with hormone synthesis and distribution or transport, others with hormone-receptor
interactions. The overall result can be adverse effects on development and growth, brain function and
behaviour, metabolism and energy balance.
Interference with the reproductive system can alter timing of puberty and increase the risk of certain
cancers (e.g. breast, prostate and testicular cancers). EDs that affect thyroid hormone production,
distribution or action can affect brain development and function. Hence, thyroid disruption has been
implicated as a possible cause of neurodevelopmental disease; other disease links are suspected in
relation to thyroid disruption, including certain forms of thyroid cancer [20-22].
Hormones act through nuclear receptors. Nuclear receptors behave as transcription factors that directly
control gene expression. In humans there are 48 nuclear receptors, including those that bind the sex
hormones (oestrogens and androgens) and thyroid hormone. ED research has largely focused on these,
but we know that many other receptors are also ED targets, including many nuclear receptors implicated
in metabolism, such as the peroxisome proliferator activated receptors (PPARs) [23, 24].
EDs that interfere with any level of hormone production, entry into cells, metabolism and action, will
affect the level of the endogenous hormone in the target cell. As such, EDs can directly modify gene
transcription responses within target cells, even though they may act upstream (i.e. by interfering with
distribution of hormone).
1.3.3 Non-linear responses are seen for endogenous responses and in endocrine disruption
A key characteristic of both endogenous hormone responses and ED action is their non-linearity and that
they display non-monotonic dose response curves [25] (see Figure 1). Both changes above or below the
optimal hormonal level can be detrimental, as seen in the case of thyroid hormone levels during
pregnancy (see Figure 1). When it comes to the effect of EDs, this implies that stronger physiological
effects can sometimes be seen at lower ED doses than at higher doses. This concept is contrary to
toxicological scenarios where effects increase monotonously as the dose increases. Thus, the principle
that “the dose makes the poison” (understood as “the effect of the poison increases with the dose”) is not
4
One part per billion corresponds to the dilution of a drop of water in an Olympic swimming pool. One part per trillion is a thousandth of a
drop diluted in the same volume.
Endocrine Disruptors: from Scientific Evidence to Human Health Protection
____________________________________________________________________________________________
19
generally valid for EDs
5
. For some EDs, low exposure levels can have stronger effects than higher
exposures. Hence, trying to characterize dose-response functions and identify safe thresholds by testing
a small number of doses (usually three in some regulatory tests) may be inefficient for EDs.
There are a number of physiological explanations to this phenomenon. In the case of endogenous
hormones, non-monotonic responses are often due to desensitisation and internalisation of receptors or
negative feedback effects. In the case of EDs, other factors also enter into play. One type of explanation
relates to differences in tissue sensitivity and response to a given ED, so that different tissues are affected
at different doses [26]. Second, different receptors can be implicated in the same tissue and activated at
different doses. An example is the pancreas, where differential dose-responses are observed to bisphenol
A, according to the receptor activated [27].
Figure 1: Relation between maternal thyroid hormone level (thyroxine) during pregnancy and (A)
offspring cortex volume at the age of 8 years; (B) the predicted probability of offspring having an
Intellectual Quotient (IQ) at the age of 6-8 years below 85 points. As women with overt hyperthyroidism or
hypothyroidism were excluded, the range of values corresponds to those that can be considered within the
normal limits for pregnancy, i.e. 7 to 34 picomole/l of free thyroxine [28].
(A) (B)
1.3.4 The endocrine system plays essential roles from conception to aging
Endocrine signalling is implicated in the fine control of every stage of development from the earliest
stages of organogenesis (organ formation), growth of all foetal structures through to timing of birth,
placental function, all aspects of early post-natal life and childhood, puberty and adolescence. It is also
implicated in reproduction (including control of egg and sperm production), other key functions in
adulthood (metabolism, temperature control, brain function) and ageing [29]. In consequence,
perturbation of the endocrine system can influence susceptibility to a large range of disease and
disorders, from congenital malformations, growth and metabolic disorders (overweight, type-2 diabetes),
fecundity, neurological disorders, cardiovascular disease and cancers in hormonally-sensitive tissues such
as the breast, prostate, testis... Some EDs likely to elicit such effects are listed in 1.8.
5
Note that this is also true for environmental exposures other than EDs. For example, the relation of temperature with mortality follows a U-
shape pattern, with high mortality both for the lowest and highest temperatures.
Policy Department for Citizens’ Rights and Constitutional Affairs
____________________________________________________________________________________________
20
1.4 Fine scale evidence that exogenous substances can interfere with the endocrine
system
Multiple sets of data demonstrate that EDs interfere with endogenous endocrine signalling at the
molecular and cellular levels. In many cases, this allows one to deduce the molecular target and hence
the ED mechanism of action. However, one must note that there are several adverse effects observed in
animal studies and at the level of populations (see 1.5 and 1.6) without the corresponding mechanism(s)
of action to be yet known. Here, four examples are chosen underlining the fact that in certain cases the
molecular events are known (see also Figure 2).
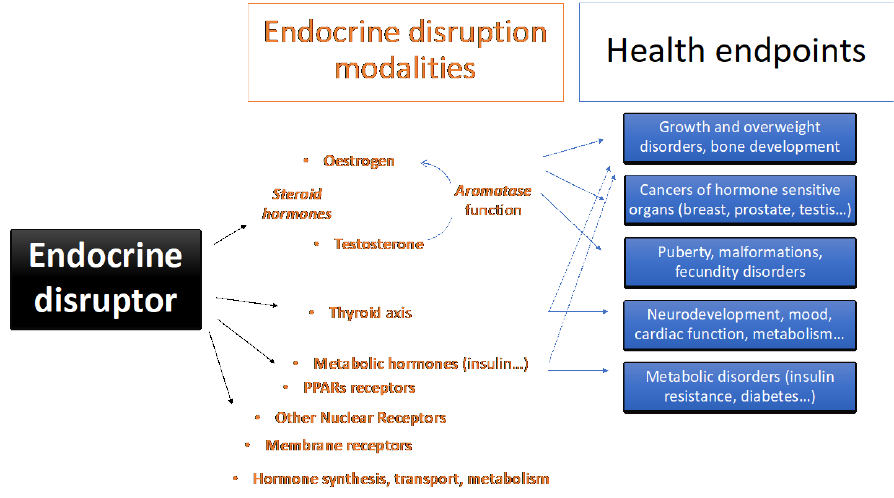
Figure 2: Schematic overview of the main modalities and health endpoints that can be affected by
endocrine disruptors.
1.4.1 Interference with the oestrogen or androgen binding to their receptors
Many EDs have the capacity to interfere with oestrogen binding to the oestrogen receptor (ER), providing
the basis for screening tools based on interactions of EDs with the two main types of oestrogen receptors
[30]. Similar cell-based or in vitro tools have been developed for the androgen receptor (AR) [31].
Additional screening tests have been developed for many other nuclear receptors, in human cells and
cells from model organisms [32]. However, the thyroid hormone receptor cannot readily be used for such
cell-based screening methods, as the ligand binding domain of the thyroid hormone receptor is highly
specific and as many other levels of thyroid disruption have been identified as more likely modes of action
for thyroid axis disruptors [33].
1.4.2 EDs affecting aromatase action
Many EDs have been identified as interfering with the enzyme aromatase, which converts androgen to
17β-oestradiol (an oestrogen) by demethylation. Aromatase is a cytochrome P450 enzyme, essential for
an astonishingly wide range of physiological functions, with roles in the placenta, ovarian follicle
development, bone mineralisation, glucose homeostasis and brain function [34]. One of the most striking
roles of aromatase includes post-natal masculinisation of different areas of the brain [35]. In humans,
Endocrine Disruptors: from Scientific Evidence to Human Health Protection
____________________________________________________________________________________________
21
aromatase is strongly expressed in brain areas associated with control of reproduction and behaviour
[36]. Not surprisingly, EDs modulating aromatase expression have been associated with multiple
reproductive [37], as well as neurodevelopmental and behavioural disorders [38]. As for the oestrogen
and androgen disruptors, cell-based screening methods have been developed to detect aromatase
disruption [39].
To date, there is a long list of EDs demonstrated to modulate aromatase activity at the level of gene
expression, including bisphenol A, polychlorinated biphenyls (PCBs), certain phthalates and various
pesticides [4].
1.4.3 ED Interference with thyroid hormone distribution in the blood
Hormones act as messengers at distance from their site of production and are carried to their target cells
in the bloodstream on distributor proteins. Three main distributor proteins convey thyroid hormone and
are targets for multiple EDs [40]. The physiological consequences of displacement of thyroid hormone
from its distributor proteins will ultimately be a decrease in circulating thyroid hormone levels due to
changes thyroid hormone metabolism by the liver. Such changes could be particularly adverse during
early pregnancy, when the foetal brain is entirely dependent on maternal thyroid hormone supply. EDs
that are known to alter the equilibrium between distributor proteins and thyroid hormone include certain
pesticides, flame-retardants and perfluorinated compounds.
1.4.4 Interference with iodine uptake by the thyroid gland.
Iodine is needed for thyroid hormone production by the thyroid gland. Both iodine deficiency and thyroid
hormone deficiency are known to adversely affect brain development, cause IQ loss and increase the risk
of neuro-developmental disease [33]. A number of EDs impede iodine uptake by the thyroid gland,
including perchlorate, nitrate and thiocyanate. Perchlorate exposure is widespread and can modulate
maternal thyroid hormone during pregnancy [41]. Perchlorate contamination has been reported in
amniotic fluid [42]. Mild iodine deficiency is present and is even increasing in various parts of Europe [43].
Iodine deficiency (due to perchlorate, thiocyanate or nitrate exposure or insufficient dietary sources of
iodine) could therefore exacerbate the effects of ED exposure, especially during pregnancy.
Beyond these examples related to the oestrogen, androgen and thyroid modalities, interactions of
xenobiotics with other key nuclear receptor such as PPARs (implied in lipid metabolism, and for which
disruption can entail impacts on metabolic disorders such as overweight or type-2 diabetes) have been
identified [44].
1.5 Evidence available at the scale of organisms and populations
Going now from the molecular and cellular levels to the organism, there is no shortage of animal studies
and human epidemiology demonstrating that EDs interfere with multiple physiological systems, even at
low doses. Here we have chosen recent examples on EDs and the links with male and female
reproduction, brain development and brain function, metabolic diseases such as obesity.
Policy Department for Citizens’ Rights and Constitutional Affairs
____________________________________________________________________________________________
22
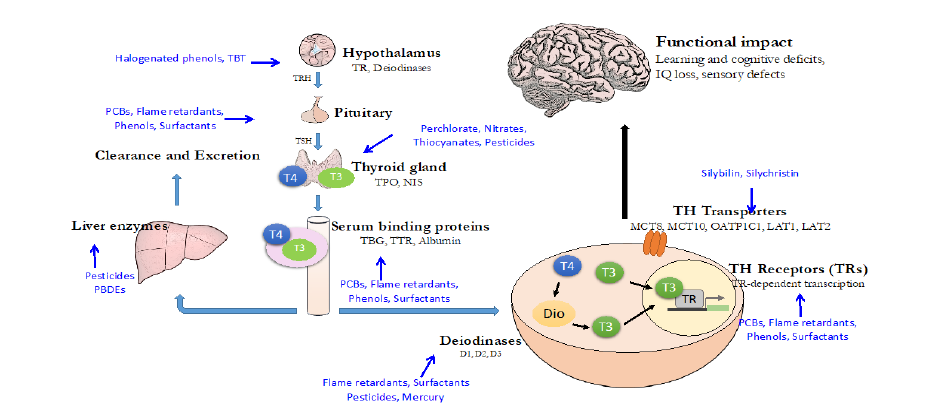
Figure 3: Known and suspected EDs linked to adverse effects on neurodevelopment. Note that the multiple
levels by which EDs can interfere with thyroid hormone signalling complicate the setting up of in vitro screening
methods for each level of action. This is particularly acute given the multiplicity of thyroid hormone actions during
brain development [45, 46]. Different structures (cell, brain) are not drawn to scale. Adapted from [33].
NIS: Sodium Iodide Symporter; PCB: Polychlorinated biphenyl; T3: tri-iodothyronine (most active form of thyroid hormone);
T4: thyroxine (less active form of thyroid hormone); TH: Thyroid Hormone; TBT: Tributyl tin; TPO: Thyroid peroxidase; TR:
Thyroid hormone Receptor.
1.5.1 Compounds affecting brain development through alteration of the endocrine system
Both epidemiological and experimental studies have shown that prenatal exposure to multiple EDs can
diminish IQ or increase risk of neurodevelopmental diseases. Many of the EDs act through altering thyroid
signalling (for recent reviews see: [33, 47]), but other players, including EDs affecting androgen and
oestrogen signalling, can be implicated in neurodevelopmental disorders, especially in the context of
sexual differentiation of the hypothalamus [48].
Some of the best studied EDs adversely affecting neurodevelopment include PCBs. Their production was
banned in the 1970s, but PCBs are still present in human fluids today due to their persistence. Reductions
in cognitive function and up to 5 IQ points loss have been observed for highest maternal PCBs exposures
[49, 50]. There is a long list of other known or suspected EDs that can affect brain development, including
both phosphorylated and brominated flame retardants, some phenols, phthalates, perchlorate and
mercury (Figure 2). Mercury is also an ED in that it interferes with the deiodinases that activate and
inactivate thyroid hormone. All deiodinase enzymes contain selenium which is chelated by mercury [51].
1.5.2 Compounds altering diabetes risk and other metabolic disorders through alteration of
the endocrine system
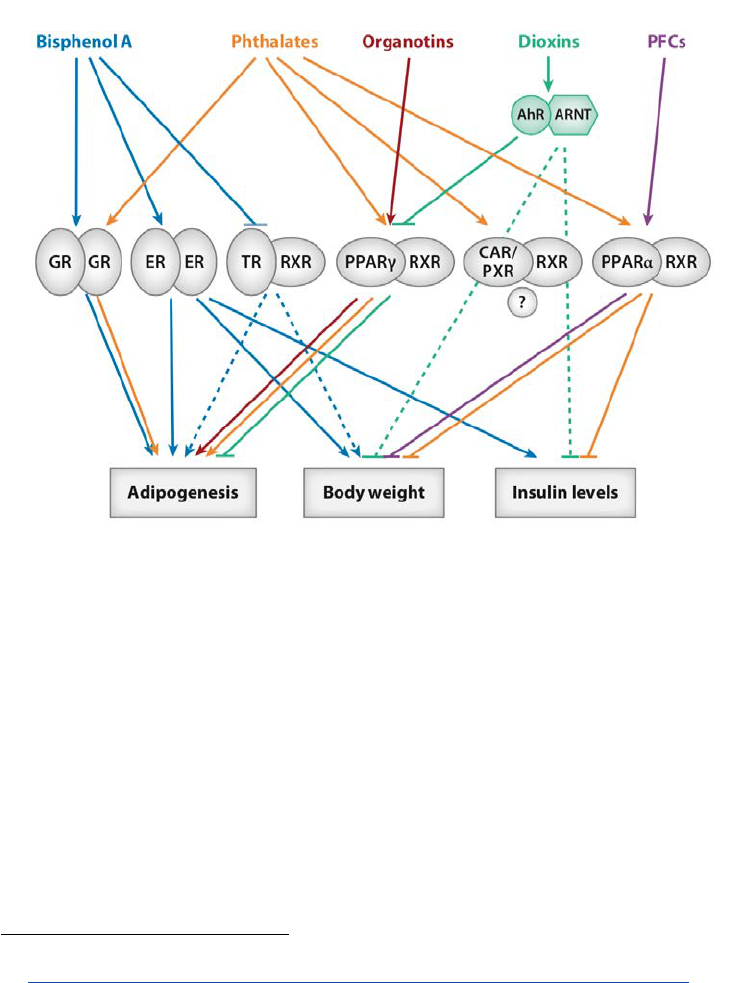
Overweight and obesity development is a huge health concern in the EU, including but not only because
of its influence on type-2 diabetes and life expectancy. Recognized risk factors of these multifactorial
conditions include limited physical activity, high energy intake and poor diet. In addition, it has been
demonstrated that environmental chemicals in generals, and some EDs in particular, can contribute to
the development of overweight and obesity (Figure 4). A clear example of such an environmental
obesogen is that of bisphenol A. A working group from the French environmental health agency (ANSES)
reviewed the scientific literature and concluded that “bisphenol A may increase metabolic disturbances
eventually leading to type-2 diabetes.” [52]. The mode of action has been shown to be endocrine
disruption and the effects were judged relevant for humans because of the similarity between the
Endocrine Disruptors: from Scientific Evidence to Human Health Protection
____________________________________________________________________________________________
23
considered animal models and humans in terms of insulin production, and because of in vitro evidence
based on human cells [52]. Another systematic review of the animal literature further indicated that
effects were observed below exposures of 50 µg/kg body weight/day [53], while the current tolerable
daily intake of 4 µg/kg body weight/day for bisphenol A in the EU assumes a lack of adverse effect below
600 µg/kg body weight/day
6
.
Additional examples of EDs implicated in obesity development include the case of tributyl tin (TBT, a
banned compound used as in anti-fouling paints), with studies on mice showing both effects on the first
generation and transgenerational effects [54]. Studies on other models have implicated triclosan and
benzo(a)pyrene in metabolic disruption [55].
Figure 4: Overview of the relations of suspected EDs with nuclear receptors implicated in the development
of adipogenesis and metabolic disorders such as insulin resistance. From [44].
AhR: Aryl hydrocarbon Receptor. ER: Oestrogen receptor. GR: Glucocorticoid receptor. PPARγ: Peroxisome Proliferator Activated
Receptor γ. PXR: Pregnane-X Receptor. RXR: Retinoid X Receptor. TR: Thyroid hormone Receptor.
1.5.3 Compounds inducing reproductive disorders through alteration of the endocrine
system
Besides reproductive cancers (e.g. of the testis, prostate or breast), many confirmed and suspected EDs
have been implicated in multiple reproductive disorders in men and women, from reduced fertility and
fecundity (longer time to pregnancy), to modified ovarian cyclicity [56], endometriosis and fibroids
7
[57].
Epidemiology and animal experiments have often focused on male reproduction, testicular dysgenesis
and decreased fertility [58, 59], with costs for male health effects estimated at around 15 billion euros per
annum in Europe [60]. Those EDs most clearly linked to male reproductive disorders include specific
phthalates (such as DEHP), which, in the case of prenatal exposures, have been linked to cryptorchidism,
6
Given that a safety factor of 150 was used to derive an authorized level from the lowest dose at which effects are deemed likely in humans.
See http://www.efsa.europa.eu/sites/default/files/corporate_publications/files/factsheetbpa150121.pdf
.
7
Fibroids are tumours of the uterus that are non-cancerous in their vast majority.
Policy Department for Citizens’ Rights and Constitutional Affairs
____________________________________________________________________________________________
24
hypospadias, reduced anogenital distance [61, 62]. Epidemiological data has linked endometriosis
incidence to phthalates [57] and animal models endometriosis to benzophenones, dioxins and
phthalates [63, 64].
Experimental work has shown maternal exposure to EDs (DES, vinclozolin, bisphenol A and PCBs) to
adversely affect hypothalamic controls on reproduction and to exert multigenerational effects through
epigenetic mechanisms, including altered DNA methylation patterns [65]. Similarly, work on animal
models has shown ED exposure to affect mating behaviour [66, 67].
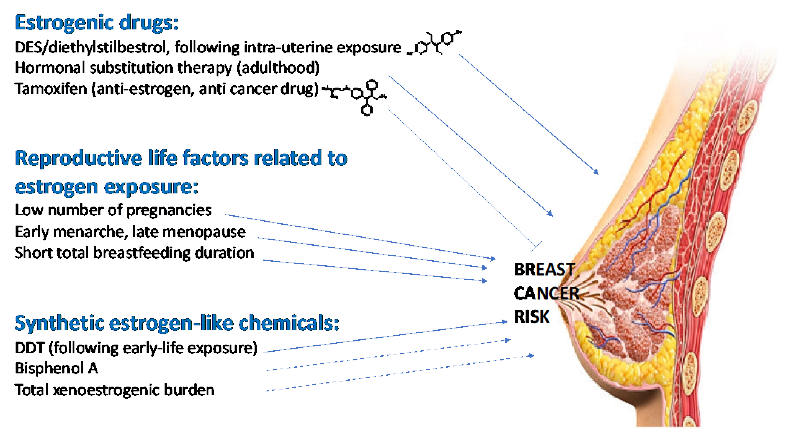
Figure 5: Examples of compounds with oestrogenic or anti-oestrogenic activity that may influence breast
cancer risk.
1.5.4 Implication of oestrogen-like compounds in breast cancer
Strong evidence has accumulated since the 1970s for an implication of oestrogens in the incidence of
breast cancer. First, reproductive life factors associated with an increased risk of breast cancer include a
small number of pregnancies or children, a short total duration of breastfeeding, an early menarche and
late menopause, all of which tending to indicate a role of exposure to natural hormones secreted during
the menstrual cycles as increasing breast cancer risk [68]. Second, drugs such as diethylstilboestrol (DES),
a synthetic oestrogen, has been shown to increase breast cancer risk following intra-uterine exposure
(see 1.1.2). Third, the first efficient drug against breast cancer is Tamoxifen, a potent anti-oestrogen [69].
Fourth, regarding industrial chemicals, intrauterine exposure to the insecticide DDT has been identified
as a probable risk factor of breast cancer incidence in a prospective cohort study with a 50 year follow-up
[70]. Bisphenol A, which interacts among others with the oestrogen receptors, is also a possible risk factor
for breast cancer, promoting mammary cell growth [71]. Fifth, epidemiological case-control studies
conducted in Spain documented the xeno-oestrogenic burden (corresponding to the overall oestrogen-
like activity from molecules stemming from outside the body) as being a predictor of breast cancer
incidence [72, 73]. This evidence of the implication of the oestrogen-like activity in breast cancer is
synthesized in Figure 5.
1.5.5 Implication of other potential EDs in cancer risk
Several potential EDs have been identified as influencing other types of cancer; this is in particular the
case of chlordecone, a chlorinated (now banned) pesticide that increases prostate cancer risk [74];
Endocrine Disruptors: from Scientific Evidence to Human Health Protection
____________________________________________________________________________________________
25
bisphenol A, for which experimental evidence shows effect on prostate cancer [71]; DES and clear cell
adenocarcinoma of the vagina [75]. Increased incidence of papillary thyroid cancer has also been linked
by epidemiology or experimental work to EDs, including flame-retardants and biocides [20-22].
1.6 EDs can act additively and synergistically
1.6.1 Different types of effects of mixtures
In an industrial, commercial or toxicological context, mixtures are sometimes restricted to intentional
mixtures, such as the list of active substances, adjuvants, stabilizers and excipients composing marketed
products like biocides, drugs or cosmetics. However, from a public health perspective, it is also relevant
to consider mixtures of other origins, such as discharge mixtures (e.g., the list of effluents of an industrial
site), coincidental and environmental mixtures (combination of substances in one environmental
compartment from different sources, e.g. the presence of metals, persistent pollutants, pesticides and
chemical additives in the diet) and, more generally, the situation of combined exposure. This expression
of combined exposure refers to exposure to multiple chemicals by multiple routes, from one or multiple
sources and/or use(s) [76].
In addition, (i) as there are mechanisms whereby the body may keep a memory of an exposure even if the
compound is not persistent and has been excreted (e.g. through epigenetic mechanisms), (ii) since the
effect of an environmental factor on health can be delayed, and (iii) given that many chronic diseases,
such as cancers, are assumed to occur as a result of a multi-hit multi-step process, the question of the
effect of combined exposure is also a concern for protractive exposures, i.e. exposures that have occurred
at different times rather than simultaneously. We will use here the term mixture in this broadest meaning
of combined exposure to chemicals that may or may not have the same source, route of exposure, mode
of action, type of effect and may even not be present in a given organism at the same time.
The question of the possible effects of the biological and health effects of several chemicals jointly
present in the organism is a classical topic for pharmacology as some patients need to take several drugs
[77]. Given the widespread exposure to many environmental contaminants, many of which with potential
health effects in the general population (see section 2.7), whether mixtures of chemicals affect health is
no longer a question limited to patients, but is of importance for all population groups from foetuses to
the elderly.
Generally, several situations can occur when the organism is exposed to a mixture:
a) Each chemical is present at a dose that would not have a detectable effect in the case of an
exposure to the chemical alone, and the mixture has no detectable effect either (lack of
observable effect);
b) Each chemical is present at a dose that may or not have an observable effect in the case of an
exposure to the chemical alone, and the mixture has an effect that can be predicted from the
dose-response function of each of the chemicals. Typically, the effect of the mixture might be
equivalent to the sum of the responses induced by each of the chemical alone at the dose at
which it is present in the mixture (response addition, see 1.6.2 below), or to the effect of one of
the chemicals at a dose corresponding to the sum (or a weighted sum) of the doses of all the
chemicals (dose addition, or cumulative effect, see 1.6.3 below);
c) Each chemical is present at a dose that may or not have an observable effect in the case of an
exposure to the chemical alone, and the mixture has an effect that cannot be predicted from the
dose-response function of each of the chemicals; for example, the effect of the mixture might be
stronger (synergy) or weaker (antagonism) than the sum of the effects of each chemical.
Policy Department for Citizens’ Rights and Constitutional Affairs
____________________________________________________________________________________________
26
The expression “cocktail effect” is sometimes used to refer to situation c) or jointly to situations b) and c).
Situations b) and c) are depicted in
Figure 6. Both situations b) and c) are, generally, ignored by regulatory
(with exceptions, see below) and common practice outside the situation of drug prescription to patients.
Figure 6: Different types of health effects of mixtures of hazardous chemicals. Note that in addition to
synergy, antagonism is also possible (omitted for simplicity).
1.6.2 Response addition (“independent action”)
Historically, regulatory toxicology (i.e., the regulatory approach to toxicology) has considered (i) that most
compounds act according to a threshold, i.e. that there is a safe threshold below which a given hazardous
compound does not elicit a biological response (see 2.9); this assumption is generally still applied in
regulatory toxicology for most hazardous compounds with the exception of carcinogenic and genotoxic
compounds; and (ii) that the response of a mixture of chemicals can, when the compounds act according
to different modes or mechanisms of action
8
, be predicted by combining (usually summing) the
responses of the population to each of the chemical at the considered exposure level. For example, if
animals are exposed to 15 chemicals, each at a dose able to induce a given same effect in the exposed
group for single-compound exposure, then the response addition model would predict that the effect is
equivalent to that of one of the chemicals present at 15 times this dose. If each of the 15 chemicals is
present below the estimated safe threshold, then the model predicts that the mixture will not induce an
adverse effect.
This situation of response addition is also referred to as “independent action”, because it is the response
classically assumed for compounds that have independent modes of action, i.e., through different
biological pathways (e.g., one through an oestrogenic action and another one through an anti-
androgenic mechanism).
Response addition is the general approach that will lead to the highest regulatory “safe levels” (compared
to dose addition), because this model assumes that one can be exposed to a large number of compounds,
each being below its “safe level”, without any adverse effect occurring (this is assuming that none of the
8
Mode of action and mechanism of action are defined in appendix I page 83.
Endocrine Disruptors: from Scientific Evidence to Human Health Protection
____________________________________________________________________________________________
27
compounds act via the same pathway, which is actually generally done by the current regulatory
approach to combined exposure).
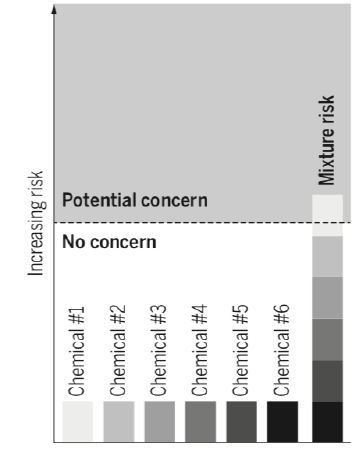
1.6.3 Dose addition (cumulative effects)
Another situation in which the effect of a mixture can be predicted from the independent effect of each
of its components is that of dose addition (or equivalently concentration addition). Cumulative effects
correspond to a situation in which the mixture effect corresponds to the effect expected from a single
chemical present in the mixture but at a dose corresponding to a weighted sum of the doses of all the
mixture components. The concentrations of each compound are weighted according to a factor
decreasing with the dose required to reach a certain effect (e.g., occurrence of the adverse effect
considered in 10% or 30% of the population). We will call this situation of dose addition a situation of
cumulative effects, to be distinguished from response addition described in 1.6.2.
For example, exposure to six compounds with identical dose-response slopes each present at 20% of the
dose expected to cause a given health effect (e.g., disease occurrence in 30% of the population) when
present alone may produce the same effect as the single exposure to one of these chemicals at
6x20% = 120% of the dose expected to cause this effect, as illustrated Figure 7. In this situation, the
response addition model would, in contrast, predict a lack of adverse effect.
Figure 7: Illustration of the cumulative effect of a mixture of compounds each present in the body below
a dose that would be a concern in the case of a single exposure, but for which the mixture constitutes a
risk. From [78].
1.6.4 Synergy, antagonism
Synergism is used to refer to the situation when the effect of two or more compounds on a specific
endpoint (e.g. the occurrence of a clinical effect) is stronger than that predicted by “adding” the
concentrations or the effects of each of the compounds considered alone. Well-known examples of
synergy include the effect of catalysts in chemistry (allowing a reaction to take place while no reaction
would occur without the catalyst) or, in pharmacology, those between alcohol (ethanol) and specific
drugs, whose effect is potentiated by ethanol, or between grapefruit juice and several drugs.
Policy Department for Citizens’ Rights and Constitutional Affairs
____________________________________________________________________________________________
28
Synergism or antagonism may occur because of metabolic effects of either compound in the mixture, or
any effect on a system that will modify the availability of the other compounds. For example, synergy of
xenobiotics with grapefruit juice is explained by grapefruit juice disrupting the action of enzymes of the
cytochrome P450 family, a group of enzymes implicated in the metabolism of many drugs and
xenobiotics in general [79]. Regarding EDs specifically, bisphenol A has been shown to alter the gut
permeability [80], which can be expected to modify the level or effect of other hazardous substances
present in diet.
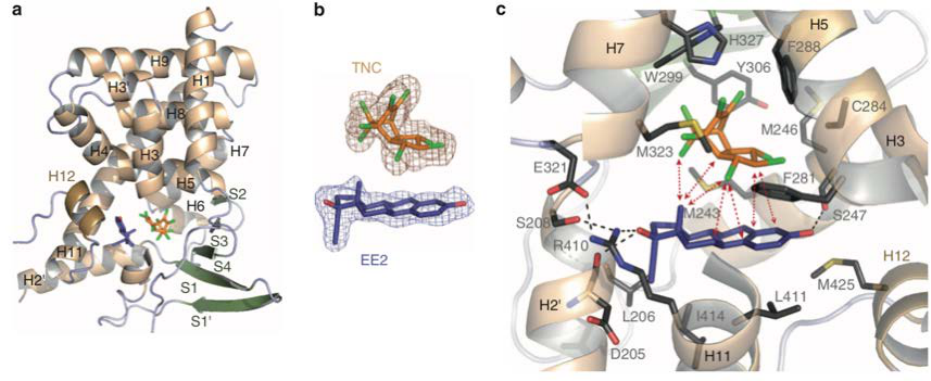
Another explanation of synergy at the molecular level and specific to EDs has recently been characterized
[81]. Forty chemicals were tested alone or in binary mixture for agonistic effects on pregnane-X-receptor
(PXR), a nuclear receptor regulating the body’s defence against xenobiotics. The authors observed
additive effects for most pairs of compounds, suggesting that the effect of most binary mixtures of these
chemicals on PXR could be predicted through a dose-additive (cumulative) effect. However, one pair of
compounds, trans-nonachlor (an organochlorine pesticide) and 17α-ethinyl-oestradiol (the active
component of the contraceptive pill), showed supra-additive effects. A similar supra-additive (synergistic)
effect was observed for other combinations of steroid and organochlorine compounds. Molecular studies
allowed to understand the mechanism of this supra-additive effect, in that the two chemicals could
simultaneously bind to PXR, with 10- to 30-fold increased avidity compared to each compound alone.
They further showed that trans-nonachlor and 17α-ethinyl-oestradiol built a supramolecular compound
interacting with PXR (Figure 8).
Figure 8: Synergy between EDs - Example of a supra-molecular ligand composed of trans-nonachlor and
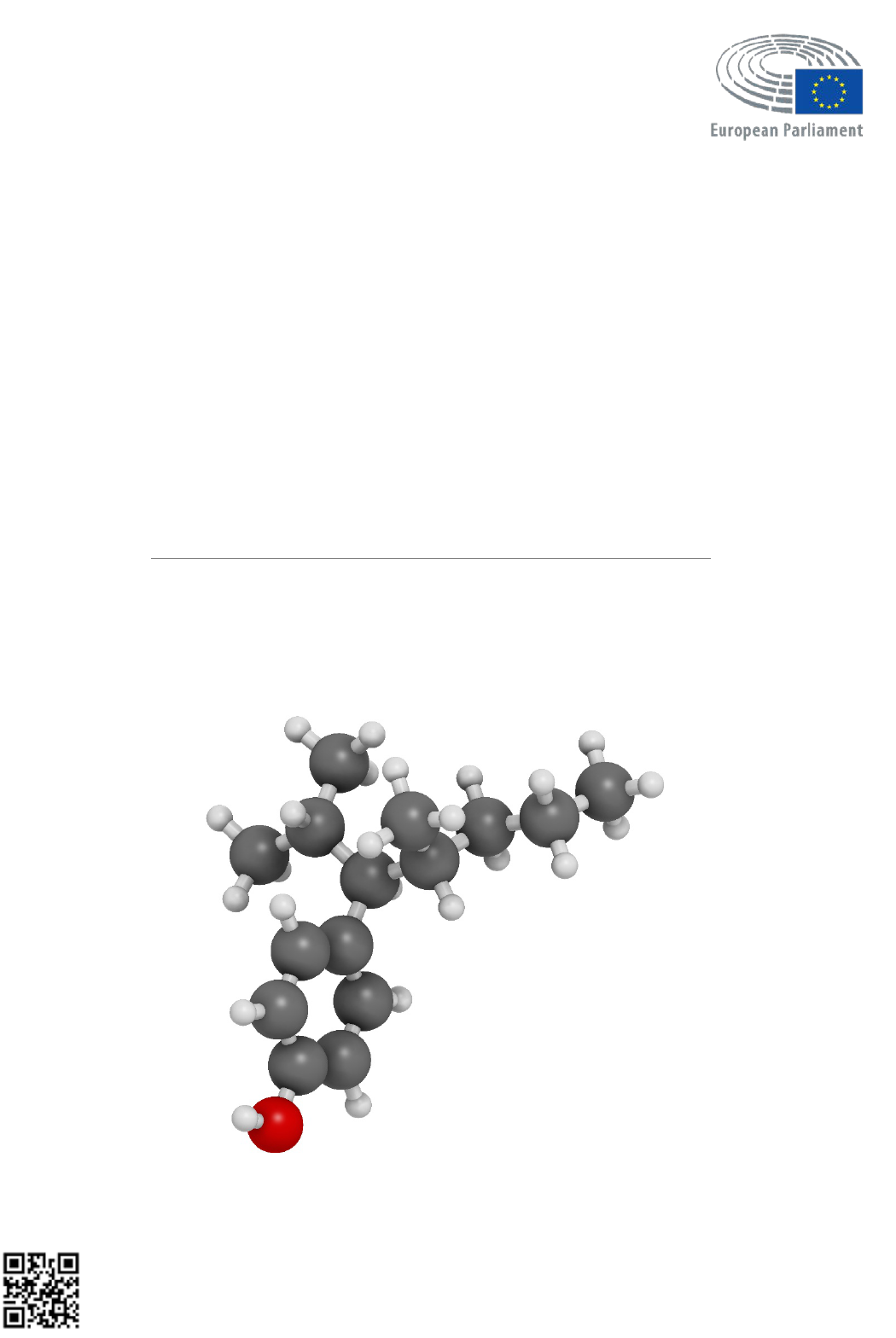
17
α-ethinyl-oestradiol. a) Pregnane-X receptor (PXR) in which trans-nonachlor (TNC) and 17α-EE (EE2) are
bound. b) TNC and EE2 molecules. c) Visualization of the interactions between the molecules TNC and EE2,
creating a supra-molecular ligand in the ligand-binding pocket of PXR [
81].
Another clear example of synergy between EDs is that of an experiment in which rats were
simultaneously exposed to a phthalate (DEHP), to two fungicides present in food and to finasteride, a
pharmaceutical; these four compounds are known to exert anti-androgenic effects, but according to
different mechanisms [82]. For some of the endpoints considered, such as changes in anogenital distance
(AGD) and sex organ weight, the effect of the mixture could be predicted by dose addition. However,
regarding the most adverse endpoint, namely malformations of external sex organ, the effect of the
mixture was synergistic.
Endocrine Disruptors: from Scientific Evidence to Human Health Protection
____________________________________________________________________________________________
29
1.6.5 Which of the different mixtures models is expected to be most valid?
Historically, regulatory toxicology assumed that the situation of dose addition will occur in the case of a
mixture of different compounds acting on a given biological pathway [76]; it was not expected to occur
in the case of synergy or antagonism (see 1.6.4), nor, again according to the original concepts of
regulatory toxicology, in the case of a mixture of compounds assumed to act according to different
pathways.
However, over the last twenty years, a large number of studies have challenged this assumption. In
academic toxicology, the view is increasingly that, for compounds acting on similar diseases, the mixture
effect can generally be predicted using a dose addition model, even if it is not certain that they share the
same mode of action. In a review on mixtures published in 2007, Kortenkamp [83] mentioned that
“…combinations of EDs with similar effects are able to act together at doses that when used alone do not
lead to observable effects. The experimental evidence is in line with the assumptions of dose addition.”
Since then, the evidence supporting the fact that response addition should not be seen as the default
model has continued to increase. We describe here a few examples.
Gaudriault et al, tested the effect of mixtures of anti-androgenic chemicals on testosterone production in
an organotypic model of human foetal testis explants [84]. They reported that the effect of chemicals
selected for their effects on testosterone production was higher in the case of a simultaneous exposure
to several of these compounds than when each compound was alone; the effect of the mixture was
generally compatible with a cumulative effect corresponding to dose addition (Figure 9A). As an example,
the bisphenol A dose causing a 50% reduction in testosterone production was divided by approximately
four in the presence of three other chemicals impacting testosterone, and by approximately 10 in the
presence of seven other chemicals (Figure 9B). Furthermore, several of the chemicals, when combined,
were able to act at levels that would not have produced observable effects had they been alone.
Figure 9: A) Observed effect of a mixture of anti-androgenic compounds on testosterone production in
fetal testis explants (blue vertical lines) and effect predicted on the basis of dose-addition (orange
continuous curve) as a function of the mixture concentration (in mol/l). B) Effect of bisphenol A on
testosterone production when used alone (blue curve) or in presence of a mixture with 7 other compounds
(red dotted curve) [84].
A) Effect of mixture B) Effect of bisphenol A
For phthalate exposure, it was concluded that concentration addition is the relevant approach to use for
the assessment of risk due to multiple sources [85].
To cite another example, Lukowicz et al. [86] fed rats a combination of common pesticides at non-toxic
doses that independently were without adverse effect. Their results showed that exposure increased the
Policy Department for Citizens’ Rights and Constitutional Affairs
____________________________________________________________________________________________
30
incidence of metabolic disease. The obesogenic and diabetogenic effects of the pesticide mix were
gender-specific, in that females were most significantly affected.
1.6.6 How to handle cumulative effects and synergy in a regulatory context
The question of handling of combined exposures is of huge practical importance for regulation. Indeed,
many hazardous chemicals are regulated under the logic of authorized level, that is estimated from
experiments in which a single exposure is present, and assuming that the compound, if present below
that authorized level, will not pose a strong risk, even if other compounds are simultaneously present.
This is in particular the case for many EDs in the context of REACH chemicals (bisphenol A is for example
regulated with a tolerable daily intake currently set at 4 µg/kg body weight per day), cosmetics, food
contact materials (see chapter 3).
Contrary to the situation of cumulative effect, which is expected to be very general, it is too early to state
at which frequency synergy between compounds might occur. In other words, it is currently unclear
which proportion of EDs can show synergy or antagonism with another chemical.
Currently, the regulatory framework of EDs little considers cumulative effects. In a few cases, when a limit
concentration is set for a compound in products, the limit applies to the sum of the concentrations of a
small group of compounds (for example, in plasticised toys and childcare articles, a limit of 0.1% in weight
is set for the sum of three phthalates: DEHP, DBP, BBP). However, this approach remains very rare, and is
far from considering all chemicals acting on the same endpoint, nor even according to the same
mechanism.
Generally, there are several options to manage the risk incurred by cumulative effects and synergy in
regulation [78]. These include:
a) Lowering the existing authorized levels estimated ignoring mixtures by dividing them by a factor
that takes into account exposure to other compounds that may act in a cumulative manner with
the chemical considered; for example, if there are 10 chemicals that have the same authorized
level and that are expected to act cumulatively on a given health endpoint, all authorized levels
could be divided by 10. Such an logic has been formulated as the Mixture Assessment Factor, or
MAF, put forward by RIVM [87];
b) Banning hazardous chemicals for which cumulative effects can be expected (i.e., get rid of
hazardous mixtures in order to get rid of mixture effects).
1.7 Some populations are more vulnerable to EDs
As mentioned in 1.7, pregnancy and early postnatal life (infants and toddlers) are exceedingly sensitive
periods for ED exposure [88]. This is not surprising, given the fact that it is during these periods (especially
early pregnancy) that all the organs (brain, liver, muscles, skeleton) are formed and that certain endocrine
feedback mechanisms are not yet mature [89]. It also explains why pregnant women are advised to avoid
medication, other than that prescribed.
Several lines of evidence show that many childhood and adult diseases, including cardiovascular disease,
obesity and metabolic disorders including type-2 diabetes, certain reproductive cancers,
neurodevelopmental disease and IQ loss, are consequences of ED exposure during pregnancy [89]. Even
though there is less data on childhood and adolescence, given their marked dependence on the
endocrine system, adverse ED effects are suspected, and have been documented on animal models [90]
and in epidemiological studies [91, 92].
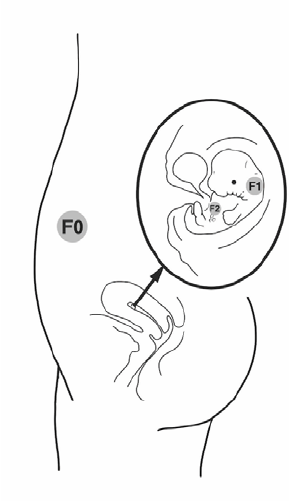
Endocrine Disruptors: from Scientific Evidence to Human Health Protection
____________________________________________________________________________________________
31
What is more, during pregnancy, not only are the mother and the foetus exposed, but also the next
generation via the germinal cells (the eggs and the sperm) that are forming in the unborn child (Figure
10). During foetal development, significant changes in methylation patterns in the germ cells occur, with
complex waves of DNA demethylation and re-methylation occurring modulating gene expression
through epigenetic changes. These may contribute to the extreme sensitivity of the developing foetus.
Figure 10: Exposure in pregnancy touches three generations: mother (F0), foetus (F1) and the next
generation through the germinal cells growing in the foetus (F2).
1.8 Diseases and adverse effects suspected to be linked with ED exposure
Table 2 provides an overview of some of the recognized or suspected effects of EDs on human health,
together with an indication of the level of evidence.
Of course, pointing out diseases whose occurrence can be influenced by ED exposure is not enough to
provide an estimate of the disease burden (or population impact) occurring because of ED exposure at the
population level. This is because in the context of multifactorial diseases, translating the health effects (the
hazards) of a substance into a population impact (the risk) requires knowledge on the frequency (or
distribution) of exposure in the population considered and on the dose-response function relating
exposure with disease occurrence.
Policy Department for Citizens’ Rights and Constitutional Affairs
____________________________________________________________________________________________
32
Table 2: Some recognized or suspected adverse effects of potential EDs.
Adverse effects
Compounds
Metabolic
disorders
Neurodevelopment,
thyroid function
Reproduction Cardiovascular
effects
Cancer and
other
Pesticides
DDT*
Thyroid homeostasis
(VL)
Menstrual function (P),
early foetal loss (S)
Breast cancer (P)
Environmental
impacts
Organophosphat
e pesticides
Neurodevelopmental changes,
cortex thickness, Lower IQ (VL)
Triclosan
Increased BMI
(S) and head
circumference (P)
Neurobehavioral changes (P)
(Environmental
impacts)
Drugs
Paracetamol
Cryptorchidism, altered
testicular function (VL)
DES*
(pregnancy)
Uterine malformation (C),
hypospadias (P)
Vaginal cancer (C),
breast cancer (VL)
Other chemicals
Bisphenol A
Growth and
overweight (VL)
Anxiety and hyperactivity
disorders (VL). Memory
performances (P)
Reproductive process (P)
Effects on cardiac
function (P)
Breast and prostate
cancer
susceptibility (P/VL)
Benzophenones
DEHP (phthalate)
Modified thyroid function, Lower
IQ (VL)
Cryptorchidism and
testicular function (VL)
Flame-retardants
Brominated
flame retardants
(PBDEs)
Lower IQ (VL)
Increased ADHD risk (VL)
Phosphorylated
flame retardants
Thyroid homeostasis (C)
Reduced male fertility (S)
Perfluoroalkyl
substances
Growth (S)
Thyroid homeostasis, for PFOA
and PFOS
(VL/C)
Testis and kidney
cancers, for PFOA
(S)
Mercury
Neurodevelopmental toxicity,
lower IQ (C)
PCBs*
Modified thyroid function (C),
Lower IQ (VL)
Reduced fertility (P)
PBDEs
Modified thyroid function, Lower
IQ (VL)
* Banned compound (at least for use during pregnancy for DES). The letter indicates the level of evidence: C: Certain or almost certain;
VL: Very likely; P: Probable; S: Suspected. PBDE: Polybrominated flame retardants.
Indeed, ideally, a transparent and relevant decision-making process in environmental health issues
requires an estimation of the overall impact of each option on human health and the environment (the
overall “risk” in the risk management terminology), and possibly of the associated costs. This overall risk
can be obtained by identifying the hazards potentially associated with each option (e.g., the use of a given
chemical substance), that is, the health effects that may occur in certain subgroups of the population,
under some exposure circumstances; by characterizing the dose-response function corresponding to
each of these hazards induced by the substance, and by assessing the distribution of exposure in the
population. By combining these parameters, and repeating the assessment for all possible hazards
related to a substance and all substances of a given group (e.g., endocrine disruptors), one can obtain an
estimate of the overall health impact of the group of substances (Figure 11). This approach has so far been
applied to EDs as a whole in one study (see 2.8). As a crucial step, it requires a good characterization of
the hazards associated with exposure to each ED, which we review here briefly.
Endocrine Disruptors: from Scientific Evidence to Human Health Protection
____________________________________________________________________________________________
33
These results (Table 2) illustrate the variety of adverse health effects that can be induced by exposure to
specific EDs, from metabolic disorders (overweight, type-2 diabetes), to adverse reproductive outcomes,
altered thyroid function, neurodevelopmental disease and certain cancers such as breast or prostate
cancer.
Figure 11: Schematic view of the steps between the identification of the hazard related to a substance and
its management by authorities. Adapted from [93].
1.9 Epigenetic effects and potential effects on successive generations
As explained in 1.7, the contact of a pregnant woman with EDs exposes three generations (see Figure 10).
Such multi-generational effects have been demonstrated for the grandchildren of women prescribed DES
during early pregnancy, including congenital malformations [16] and possibly neurodevelopmental
disorders such as Attention Deficit/Hyperactivity Disorder (ADHD) [15]. Reproductive disorders have been
recorded in both sons and daughters, with the latter being most affected, notably by vaginal cancer and
difficulties to conceive [94].
Transgenerational effects in this context refer to effects that are observed beyond the grandchildren (or F2
generation), i.e. in the generation(s), not directly exposed in utero (F3 or beyond, see Figure 10). To date,
only animal studies on models with short generation times (mainly rodents) have provided such
demonstrations. Transgenerational effects have been shown for a number of EDs. Examples include
transient DDT exposure of F0 rats and increased obesity in the F3 generation [95], as well as impaired
glucose tolerance, a factor linked to type-2 diabetes [96]. Similarly, for tributyl tin (TBT), exposure of
pregnant mice to this ED predisposes unexposed F4 males to obesity [54]. In this case, differential DNA
methylation (an epigenetic modification) was shown in sperm and adipose tissue of mice that were the
third and fourth generation descendants of the exposed mouse. Note that the existence of epigenetic
changes does not preclude alterations in the endocrine system. These mechanisms are not mutually
exclusive, in that a compound binding a nuclear receptor will ultimately entail changes in gene expression
in the nucleus of the cell, which can correspond to an epigenetic mechanism.
1.10 EDs’ specific characteristics are recognized by the scientific community at large
It is important to note that there is currently no longer any dispute on the definition of an ED nor on the
fact that this class of chemicals induces adverse effects on health and wildlife by interfering with the
hormonal system. At a scientific meeting in Berlin in 2016 between epidemiologists, endocrinologists and
toxicologists, consensus was reached on the fact that EDs can act at low doses and display non-monotonic
dose response curves (see 1.3.3) without evidence of thresholds below which no effect occurs [97]. In this
Policy Department for Citizens’ Rights and Constitutional Affairs
____________________________________________________________________________________________
34
meeting, the concept of the use of potency with respect to EDs was also discussed and determined as not
relevant for ED identification [97].
Another feature of EDs now recognised is the demonstrated vulnerable periods of exposure, often with
markedly different tissue sensitivities (see 1.7).
The questions of regulatory issues and requirements that derive from these features are developed in
chapters 4 and 5, after some elements on the extent of the impact of EDs on human health, which are
presented in the next chapter, and a discussion of the existing ED regulation in the EU (chapter 3).

Endocrine Disruptors: from Scientific Evidence to Human Health Protection
____________________________________________________________________________________________
35
2 MAIN FAMILIES OF KNOWN AND SUSPECTED ENDOCRINE DISRUPTORS:
EVIDENCE FOR WIDESPREAD EXPOSURE AND ADVERSE HEALTH
EFFECTS
KEY FINDINGS
•
Non-infectious pathologies are causing a huge burden on our societies.
• The diseases and disorders associated with ED exposure are generally multifactorial.
However, by using randomization and statistical analysis, toxicological and epidemiological
approaches allow disentangling the effects of specific factors. Such approaches have
provided demonstrations of actions of specific EDs on different chronic disease outcomes.
• The diseases include obesity and metabolic disorders, male and female reproductive
disorders, reproductive cancers as well as thyroid disorders, neurodevelopmental disease
and IQ loss.
• Numerous consumer products from cosmetics, toys, furnishings, building materials,
cleaning products and food contact materials contain proven and suspected EDs. Human
population exposure is widespread, as documented by biomonitoring studies.
• Population impacts and annual costs for seven categories of EDs have been estimated to be
over €22 billion with a 95% probability and over €196 billion with a 25% probability in
Europe. The largest proportion of costs is related to IQ loss and neurodevelopmental
disease.
This chapter deals principally with EDs documented in different products (toys, cosmetics, consumer
goods etc). The main suspected or proven health effects of key well-studied EDs are summarized in Table
2 above. Regulations concerning these substances are considered in chapter 3.
2.1 Which methodologies have been used to demonstrate health effects of EDs?
The OECD report updated in 2018 [98] provides a methodological review of the experimental methods
(from in vitro and in silico methods to whole animal tests) that can be used to determine effects of EDs.
An overview of the epidemiological approaches used to characterize the effect of EDs on health has also
been published recently [99]
9
.
The Endocrine Society’s second statement on ED effects describes the epidemiological and experimental
methods that link human and animal exposures, notably during development, to later disease through
endocrine disruption [4]. The effects discussed range from obesity and metabolic disorders, male and
female reproductive disorders, reproductive cancers as well as thyroid disorders, neurodevelopment
disease and IQ loss, as well as modified neuroendocrine function
.
2.1.1 Efficient methodologies to highlight the cause of multifactorial diseases exist
It has sometimes been stated that the multifactorial nature of the diseases and environmental impacts
that can be caused by EDs makes the identification of these effects scientifically difficult. As an example,
9
Available here:
https://www.sciencedirect.com/science/article/pii/S1631069117301294/pdfft?md5=14c2b9f9d6843a7ebed927eb4c009a86&pid=1-s2.0-
S1631069117301294-main.pdf

Policy Department for Citizens’ Rights and Constitutional Affairs
____________________________________________________________________________________________
36
the recent communication towards a comprehensive ED framework [100] mentions that “…there is
limited understanding of the specific contribution of chemical exposure and the way to separate it from
other possible causes of the negative impacts being investigated. Other factors are indeed also at play in
the development of such endocrine-related disorders (such as genetics, nutrition, lifestyle, or other
environmental factors) or impacts on wildlife (e.g. overexploitation, climate change);”.
However, such general statements misrepresent the nature of the scientific approaches used. It is in the
very nature of science to tackle multifactorial phenomena, from the movement of clouds to the
occurrence of diabetes or cancer; this is true for physics, economic science, environmental health sciences
in general and ED research in particular. Specifically, toxicology tackles complex diseases in several ways,
including a controlled approach that randomizes animals in the exposed and unexposed groups so as to
make sure that the groups are identical with respect to all factors other than the exposure, allowing to
discard the role of any other factor. Ecotoxicology combines observations and experiments (e.g. as in the
historical example of the DDT effects on egg shell thinning, with, first, observations in wildlife suggesting
a role of DDT, and then, experiments showing that the thinning of egg shells could be reproduced with
controlled application of DDT) and also allows to study efficiently the web of causation of multifactorial
environmental impacts.
For human studies, a randomized controlled approach can generally not be undertaken for proven EDs –
although this has in a few cases been done, e.g. in a randomized control trial with low dose bisphenol A
exposure due to drinking from cans [101] or in the case of the effects of a phthalate on male health [102,
103]. Here, the main approach used to tackle multifactorial diseases is that the other factors influencing
the disease and also possibly associated with ED exposure (the potential confounders in the
epidemiological terminology) are measured and controlled for by statistical tools such as multiple
regression models
10
, which can, with some assumptions, provide estimates of the association between
the exposure and the health outcome as if exposure had been randomized. See e.g. [99] or the literature
on structural causal modeling for more details.
This is not to claim that science has unravelled everything regarding the effects of EDs – for example the
exact number of EDs in currently marketed products is not known; neither are all the fine-scale
mechanisms implicated in ED effects. Nor is the overall population impact of EDs accurately characterized
– just like the overall impact of all carcinogens on health is currently not known. However, these
knowledge gaps do not pertain to the lack of efficient methodologies to demonstrate the role of a specific
ED in the etiology of a given disease.
There is no lack of tools here – support to higher education and environmental health research is
probably one of the main drivers of the pace of discoveries and of the progress towards a better
understanding of whether a given substance is an ED. If decision makers need stronger evidence on
whether to classify a substance as an ED, one way to achieve this would be stronger support of research
(which could be achieved e.g. by finding a mechanism that would make the support to the research on
the effects of chemicals dependent on the number of substances marketed). Another option is to enforce
stronger test requirements in the application dossiers filed in by the industry (see 3.3.4 and 4.4). This is
precisely the logic of the precautionary principle, that calls for action if a strong threat is likely, even in the
absence of certainty, with more knowledge generation subsequently confirming or not the threat.
The approaches highlighted above have allowed identifying EDs in various media and sectors.
10
Incidentally, this explains why it would be wrong to state that epidemiology only produces “correlations”. In a prospective cohort setting,
confounders can be efficiently controlled for and the study allows estimating regression parameters (not coefficients of correlation) that are
adjusted for the confounders. Other approaches such as propensity scores can be used to increase the validity of inferences based on
epidemiological results.

Endocrine Disruptors: from Scientific Evidence to Human Health Protection
____________________________________________________________________________________________
37
2.2 Some suspected EDs present in food contact materials and diet
EDs reported in food packaging (or food contact materials) include bisphenol A, benzophenones and
organotins [104]. A data based has been compiled (Chemicals associated with Plastic Packaging, or CPPdb
[105]) that provides a list of over 906 chemicals associated with plastic packaging and another 3377 likely
associated. Food packaging components liable to contain EDs include paper, glue, inks and cardboard.
Here we focus on bisphenol A (an ED of particularly high concern for human health), phthalates and
perfluoroalkyl substances (PFASs).
2.2.1 Bisphenol A
Bisphenol A was synthesized in 1891 but interest grew after the discovery of its oestrogenic properties in
the 1930s, which led to its consideration for use as an oestrogenic drug, until DES, which was more potent
in terms of oestrogenicity, was discovered. Instead, bisphenol A was used in the plastics industry. The
polymerization of bisphenol A produces polycarbonate, a hard and transparent plastic that is or has been
used in various food containers such as water tanks, baby bottles, goblets or other food contact materials.
It is also used in the synthesis of polysulfone and polyacrylate. Bisphenol A is also used (at least in EU
countries where this use is not banned by national regulations) as an additive in epoxy resins used in the
inner coating of food and beverage cans. Bisphenol A is also used as an antioxidant and inhibitor of end
of polymerization in polyvinyl chloride plastics (PVC). It has been demonstrated in 1992 that in specific
but not unusual conditions (e.g., heating, detergent use…), the polycarbonate polymer molecule can
break down into smaller molecules, including bisphenol A monomer, that can migrate to the content of
the polycarbonate container. Similarly, bisphenol A from epoxy resins is able to migrate to the food,
which constitutes one source of exposure for the general population. In a review written in 2012, it was
estimated that diet is the main source of bisphenol A exposure in humans by an order of magnitude [106],
a situation that may have changed since then.
Bisphenol A generates a lot of interest and concern in the scientific community, with over 800 articles
published each year in 2014-2018, including 300 per year on the topic “bisphenol A AND health” (source,
PUBMED
database). We provide here only an overview of the main concerns regarding the health effects
of bisphenol A.
At the molecular level, bisphenol A has been shown to be able to interact with an impressive number of
nuclear receptors, such as the oestrogen receptors, the orphan receptor human oestrogen-related
receptor gamma, ERRγ, the androgen receptor, the glucocorticoid receptor, the PPARγ receptor and to
interfere with the thyroid axis. It can also interact with GPR30 (G-protein coupled receptor 30) cellular
receptors [107]. Although the strength of the binding of bisphenol A with the oestrogen receptor is much
weaker than that of natural (endogenous) oestrogen, this multiplicity of the receptors (some binding to
bisphenol A with strong affinity) and signalling pathways that may be activated or influenced by
bisphenol A may explain the large number of biological and health parameters likely to be influenced by
bisphenol A at very low doses [108].
The strongest evidence for adverse effects of bisphenol A exposure comes from animal studies. These
show or strongly suggest effects of bisphenol A on fat weight, metabolic disorders leading to type-2
diabetes [52, 53], neurodevelopment and behaviour such as hyperactivity [109], reproductive processes
[110, 111] and memory performance [112]. Associations with several other outcomes, including gut
permeability and learning and memory performance [112], are likely or suspected [108] (Table 2).
Regarding cancer, the main concerns relate to breast and prostate cancers. In a review, Seachrist et al.
concluded that “Based on the definitions of “carcinogen” put forth by the International Agency for
Research on Cancer and the National Toxicology Program, we propose that BPA [bisphenol A] may be
Policy Department for Citizens’ Rights and Constitutional Affairs
____________________________________________________________________________________________
38
reasonably anticipated to be a human carcinogen in the breast and prostate due to its tumour promoting
properties” [71].
Because of its very strong variability in the body over time, due to a short biological half-life and repeated
exposures throughout the day, bisphenol A is one of the compounds whose health effects are the most
difficult to characterize directly in humans, at least with approaches relying on a single biospecimen
collected in each subject, a design used so far in most epidemiological cohorts. This strong temporal
variability will, on average, lead to a strong under-estimation of the slope of dose-response functions and
a decrease in the ability of studies to highlight any effect of the compound, but no increased risk of
generating false-positive studies [113]. In spite of this limitation, there is increasing evidence for health
effects on bisphenol A directly from human studies, in particular for outcomes such as anxiety in
childhood [114-116] and alterations of cardiac or cardiovascular function [101, 117].
Several studies documented effects of bisphenol A at doses assumed safe by regulatory thresholds valid
in the EU. Examples include the hypothalamic and hippocampal effects on gene transcription in rats
[118], in vitro work on mouse and human pancreas showing environmentally relevant levels (exposures
in the 1-20 µg/kg body weight/day range) to alter insulin signals [27] and other organ systems [119, 120].
Currently, bisphenol A is banned from food contact materials used for children under 3 years old in the
EU; it is not banned for food contact materials in general, so exposure of pregnant women and foetuses
(since bisphenol A crosses the placenta) through diet is still present.
2.2.2 Phthalates
Phthalate esters are a major category of industrial chemicals, with an annual production of probably
about 1 million tons per year in Europe. The principal use of phthalates is to increase the flexibility of
plastics (e.g. of polyvinylchloride, or PVC). They are found in a plethora of situations and appliances.
Examples include toys, medical devices including catheters and blood/saline bags, pharmaceuticals,
perfumes and personal care products, paints, printing inks, building materials, detergents, shower
curtains and building materials. As phthalates are not bound to their matrix, they can be easily transferred
or leach into other substrates. This is notably the case for DEHP [121]. Unsurprisingly, human exposure to
phthalates is ubiquitous in EU populations [122-124].
Even though it is not entirely understood how phthalates enter the food chain, a part of human exposure
is thought to come from food contact materials [125].
Numerous adverse health effects have been reported for many phthalates. Here we focus mainly on those
associated with one of the most used phthalates, DEHP. At the molecular level, DEHP is able to interact
with the androgen receptor, eliciting anti-androgenic effects [126]. It can also interact with PPAR
receptors and the aryl-hydrocarbon receptor (AhR) [127]. Effects on thyroid signalling are also observed,
most likely through effects on the thyroid hormone entry into cells or on thyroid hormone distribution
(see references in [128]). No interaction with the thyroid hormone receptor is expected, as the ligand
binding domain is highly specific.
Adverse health effects for DEHP have been shown for prenatal exposure and for exposure in childhood.
As summarized in Table 2, multiple studies have demonstrated effects on various reproductive disorders.
In animals and humans, prenatal DEHP exposure is associated with reduced anogenital distance in males,
an effect consistent with the anti-androgenic properties of DEHP [129](for review of epidemiological
studies on phthalate effects on male reproduction see [130]). One of the few prospective human studies
with repeated phthalate exposure assessment reported an increased risk of preterm birth in relation to
pregnancy DEHP concentration [131]. Another study showed significant association of prenatal exposure
with raised oestrogen levels in the mother [
132].

Endocrine Disruptors: from Scientific Evidence to Human Health Protection
____________________________________________________________________________________________
39
Both animal studies and epidemiology have linked phthalates to the developmental neurotoxicity of
DEHP (see for instance [133, 134]). Regarding DBP, in adults, a recent experiment reported a possible
disruption of the thyroid axis in men following DBP exposure [103].
Some phthalates found in food packaging [105] are classified as EDs within REACH (see Table 6).
Consequently, use of four phthalates (DEHP, BBP, DBP and DIBP) will be restricted in consumer products
(i.e., authorized only up to a proportion of 0.1% of the weight of the product) as of June 2020
11
, a
restriction that does not cover use in food.
With increasing restrictions on certain phthalates, those restricted are gradually being replaced by non-
phthalate plasticizers. These include Di(isononyl)cyclohexane-1,2-dicarboxylate (DINCH), for which
concern regarding possible adverse health effects has been reported [135].
2.2.3 Perfluoroalkyl substances (PFASs)
PFASs are primarily used in food contact materials for their water and oil repellent characteristics. That
they can migrate into food [136] and increase blood PFAS levels is known and has been underlined in the
Madrid statement on PFASs [137]. Intake of fast food was associated with increased levels of different
PFASs, including perfluorononanoic acid (PFNA), whereas increased use of canned food was positively
associated with perfluorohexane sulphonic acid (PFHxS) [138]. Different PFASs have been reported in
pizza boxes and in pre-prepared bags for popcorn [139]. Consumption of fast food has been associated
with higher PFAS levels and decreased circulating thyroid hormones [140].
The complexity and the continually changing profile of PFAS production makes it difficult to have an
exhaustive characterization of their effects, in addition to the very different metabolism of PFOA between
humans and rodents. In spite of this, effects of several PFASs have been identified. These include effects
of PFASs on thyroid hormone levels during pregnancy and in childhood [141, 142] and on the thyroid axis
[141, 143, 144]
12
. With respect to neurodevelopmental disorders, certain longitudinal studies have
reported effects of prenatal PFAS exposure on increased hyperactivity, conduct problems and a
composite score for autism screening [145] whereas other studies have been less conclusive [146, 147].
In many studies, most of the PFASs measured (up to 16) were present in above 90 % of samples.
Immune responses have also been documented as associated with PFAS levels [148, 149], but whether
this implicates an endocrine mechanism remains to be investigated. However, many nuclear receptors
are expressed in different immune cells [150]. Hence, the possibility that endocrine mechanisms are
implicated should be investigated. Effects on weight gain are also plausible for some PFAS [151], as is an
effect of PFOA on ulcerative colitis, an autoimmune disease [152]. Associations of PFOA exposure with
kidney and testis cancer incidence in human populations exposed from drinking water following an
industrial contamination have also been reported [153]. Effects on liver function have also been reported
in humans [154].
2.3 Some known and suspected EDs present in cosmetics and personal care products
As underlined by the SSCS in their SCCS/1544/14 memorandum, the regulation of EDs in cosmetics is
limited by the restriction on animal testing for cosmetic products, which in turn limits identification of
EDs in this sector. Hence it may be particularly difficult to distinguish between a potential ED and a certain
ED, if the substance is only registered for use in cosmetic products.
11
https://ec.europa.eu/growth/sectors/chemicals/reach/restrictions_en
12
See also the evaluation from C8 project regarding thyroid disease:
http://www.c8sciencepanel.org/pdfs/Probable_Link_C8_Thyroid_30Jul2012.pdf
Policy Department for Citizens’ Rights and Constitutional Affairs
____________________________________________________________________________________________
40
Despite this, there are a number of recognised EDs used in cosmetics and personal care products. Three
main classes are briefly covered below.
2.3.1 Parabens
Parabens are a large class of chemicals commonly used as preservatives in many consumer products,
including food additives, cosmetics and sunscreens. They have been shown to be exert estrogenic and
antiandrogenic activities [155]. Effects on fecundity in rodents have also been reported [156, 157].
Human studies show paraben exposure to be very frequent, with certain parabens also found in amniotic
fluid [158]. Few studies on their effects have been conducted in humans so far, which have a limited
sensitivity because of the strong within-subject variability of paraben concentrations. Effects on the
postnatal growth of boys have been suggested, with maternal levels of parabens being associated with
an increased weight at three years in boys in two studies [159, 160]. Other epidemiological studies have
examined the main types of parabens in pregnant women and their associations with thyroid and
reproductive hormones, suggesting changes for methyl and butylparabens [161].
Currently a few parabens have been banned from cosmetics, and this specifically for use on the nappy
area of babies and toddlers under the age of three. Other parabens, such as the methyl-, ethyl-, propyl-
and butyl-parabens, are considered safe as long as the total paraben content does not exceed 0.4 %
content for a single paraben and 0.8 % for mixtures of all parabens in cosmetics. Parabens can modify
bisphenol A pharmacokinetics [162].
2.3.2 Triclosan
Like parabens [163] and benzophenones [164], triclosan can be found in amniotic fluid and is found in
98% or more of pregnant women in some populations [123, 165], raising the question of effects arising
during pregnancy. Triclosan was first characterized as an ED affecting thyroid homeostasis in 2007 [166].
Triclosan activates hepatic nuclear receptors [167] affecting metabolism and hormone sensitive
pathways, notably thyroid hormone levels [168, 169]. High triclosan concentrations have been associated
with lower thyroid hormone levels [165, 168].
Numerous studies in animal models have detailed adverse effects, including metabolic effects [55, 167].
In humans, a cross-sectional study associated triclosan exposure with increased body mass index [170].
Associations of triclosan levels during pregnancy with reduced head circumference, at least in the male
offspring, have been documented in several epidemiological cohorts [160, 171, 172], an association
coherent with the ability of triclosan to disrupt the thyroid axis. As for bisphenol A, estimates of
association of triclosan with health from human studies based on a spot biospecimen are expected to be
underestimated.
In 2016, the FDA banned triclosan use (and that of triclocarban) in consumer soaps, but not in other
products. In the EU, the substance is authorized in cosmetics (with a concentration limit according to
product, see sections 3.4 and 3.7.3).
2.3.3 Benzophenones
As for the previous chemical categories, human exposure to UV filters is ubiquitous, as they are used not
only in sunscreens but also as UV-absorbers in a broad range of products, including in plastics to prevent
friability [
173]. Benzophenones (e.g. benzophenone-1, benzophenone-2, benzophenone-3) are used in
sunscreens, with certain being known EDs. For instance, Schlumpf and collaborators screened 9 UV filters
for in vitro oestrogenic activity. All but one displayed estrogenic activity [174]. As regards the thyroid axis,
in vitro studies showed five benzophenones to significantly to decrease expression of thyroid-responsive

Endocrine Disruptors: from Scientific Evidence to Human Health Protection
____________________________________________________________________________________________
41
genes [175]. The same study showed benzophenone-1, benzophenone-3 and benzophenone-8 to
decrease thyroid hormone levels in an animal model.
Benzophenone-1, benzophenone-3, 4-methyl-benzophenone (4-MBP) and 4-hydroxy-benzophenone
(4-HBP) have been detected in amniotic fluid and cord blood samples from Danish populations [164].
Here again, multiple sources of exposure and interactions between chemical classes need to be
considered. For instance, both benzophenone-3 and a phthalate (DEHP) were associated with decreased
thyroid hormone levels in the NHANES study [176] and in the Danish study cited previously [173]. Given
the demonstrated endocrine-disrupting effects of many benzophenones, the risk/benefit of UV filters has
been questioned [177].
2.4 Some suspected EDs present in drugs
DES provides a first example of a drug with endocrine-disrupting properties able to induce severe adverse
health effects (including congenital malformations and cancer) following intra-uterine exposure [178].
Another relevant example is paracetamol (acetaminophen), which is widely used. Analgesics, including
paracetamol and aspirin, are the most widely sold over-the-counter drugs, with increasing trends in the
EU since the 1990s [179]. Paracetamol is the drug most often used by pregnant women, who tend to
consider that paracetamol is not a drug and is safe for use during pregnancy.
Recent evidence disputes this belief. Paracetamol crosses the placenta, reaching the foetus. Toxicological
studies show that it has antiandrogenic properties. In animal models, gestational paracetamol exposure
is associated with reduced anogenital distance in male offspring and inhibition of testosterone
production as well as interference with prostaglandin production. Some epidemiological studies
reported a possible increase in the risk of undescended testes at birth [179, 180]. Adverse effects on girls’
development have been reported, including on language development [181, 182].
2.5 Known and suspected EDs present in consumer goods and cleaning products
Today our homes are a major and constant source of EDs [183]. Numerous consumer products such as
electronic devices (computers, TV screens, telephones), furniture, upholstery and cleaning products may
contain suspected or confirmed EDs. Given these multiple sources, it is not surprising to find that
household dust is a source of ED exposure. This is a concern for infants who crawl on the floor and
toddlers who tend to put their hands in their mouths.
We discuss specific classes of chemicals and then address issues by sector.
2.5.1 Brominated and phosphorylated flame retardants
To limit fire risk, electronic equipment, furniture covers and mousse and most mattress materials are
treated with flame-retardants, many of which are confirmed EDs [184, 185]. Examples include the
polybrominated flame retardants, such as polybrominated diethyl ethers (PBDEs) that have been banned
under the Stockholm convention
13
but are still present in household dust [186].
Others still in use include tetrabrominated bisphenol A (TBBPA) and the more recently introduced
phosphorylated flame-retardants. Members of each group have been shown in experimental and
epidemiological studies to interfere with thyroid hormone signalling and, in many cases, as a
consequence, with brain development and neurodevelopmental disease. More specifically, PBDEs have
been linked in longitudinal studies to IQ loss and increased risk of Autism Spectrum Disorders and ADHD
[187-189]. PBDEs have also been associated with reproductive disorders [190, 191], TBBPA with adverse
13
http://chm.pops.int/Implementation/NIPs/Guidance/GuidancefortheinventoryofPBDEs/tabid/3171/Default.aspx

Policy Department for Citizens’ Rights and Constitutional Affairs
____________________________________________________________________________________________
42
thyroid hormone and neurodevelopmental effects in rats [192] and phosphorylated forms with thyroid
homeostasis [193, 194].
2.5.2 Phthalates
Besides their use in perfumes and personal care products, phthalates are commonly used in cleaning
agents [195]. As the ED properties of this chemical class have been developed above (see 2.2.2), they are
not reiterated here.
2.5.3 Perfluoroalkyl substances (PFAS)
Besides often being treated with flame-retardants, carpeting and upholstery (e.g. curtains) can also be
treated with polyfluoroalkyl substances (PFASs) or surfactants to waterproof them and render them “spill-
resistant”. Two categories of PFASs, perfluorooctanoic acid (PFOA, also known as C8) and perfluorooctane
sulfonic acid (PFOS), are of particular concern as they are exceptionally persistent and can bioaccumulate.
These compounds are found in human blood, cross the placenta and are found in amniotic fluid. Their
half-life in humans (the time to eliminate 50% of the load) is four to five years. The health effects of PFASs
were developed in 2.2.3.
The application of REACH regulation introduced restrictions on PFAS in 2017 and included several
derogations for different industrial sectors and uses. It will apply from 2020
14
.
The EPA has instigated a number of actions on PFAS
15
. In 2010/2015 they asked manufacturers of PFASs
to complete of the phaseout of one of the major compounds of the family, PFOA. However, as changes
in production resulted, serum concentrations of others PFASs has increased, including perfluorohexane
sulfonic acid (PFHxS), perfluorononanoic acid (PFNA), and perfluorodecanoic acid (PFDA), with some
estimates placing the number of PFASs at several thousands
16
. Independently of household use, PFAS
surfactants are widely used in food contact materials (see section 2.2.3) and firefighting foams. Significant,
health-endangering PFAS contamination of water supplies near military bases using PFAS-based foams
was reported in Sweden [196].
2.5.4 Cleaning products
Many cleaning products intended for use in bathrooms and kitchens can also contain known and
potential EDs, e.g. nonylphenols, parabens, phthalates and glycol ethers.
The France-based PELAGIE cohort (see [197-200]) has highlighted groups of ethyl glycols as ED
candidates. These epidemiological studies have demonstrated associations between maternal levels of
certain glycol ethers, notably methoxyacetic acid (MAA) and risk of cryptorchidism and hypospadias
[198]. Other studies on this cohort showed that phenoxyacetic acid (PhAA) levels in cord blood were
associated with lower sex steroid and steroid hormone binding globulin (SHBG) in boys and with higher
SHBG levels and certain steroids in girls. Two other glycol ethers displayed associations with oestradiol
levels. As the authors conclude, given the data on glycol ether exposure and time to pregnancy [200] and
neurocognitive performance [199], maternal exposure to these compounds is cause for concern.
Nonylphenols were amongst the first oestrogenic EDs identified [201]. They are commonly found in
detergents and other consumer products. Although weak, the oestrogenic activity of nonylphenol has
been associated with various ED related adverse outcomes including breast cancer [201] and fertility
14
https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32017R1000&from=EN
15
https://www.epa.gov/pfas/epa-actions-address-pfas
16
https://www.epa.gov/sites/production/files/2018-11/documents/cecs-pfcs-factsheet.pdf

Endocrine Disruptors: from Scientific Evidence to Human Health Protection
____________________________________________________________________________________________
43
problems in men [202] and women [203]. Exposure to nonylphenol during pregnancy has been
associated with smaller gestational weight and shorter offspring length [204].
2.5.5 Building materials
Other than the flame-retardants and surfactants described in 2.5.1 and 2.5.3, different construction
materials contain significant levels of known or suspected EDs [205]. Examples include polyvinylchloride
(PVC), used in floor coverings is associated with increased phthalate exposure [206, 207], PCBs widely
used, prior to their ban, for electrical and general building insulation [205].
2.5.6 Medical Devices
Some medical devices contain EDs or suspected EDs, which is of particular concern if the EDs are on a
part of the device in contact or within the body. One of the most difficult situations is care of premature
babies. This exceedingly vulnerable population is highly exposed in intensive care situations, notably to
phthalates from polyvinylchloride (PVC) tubing. Estimates have placed exposures up to 160,000 times
above safe levels [208].
2.5.7 Toys
Phthalates are well-documented EDs found in many flexible plastic toys [209]. Banned phthalates have
been reported to be present above permitted levels in 20% of toys inspected
17
. Other EDs including
bisphenol A [210] can also be present. Exposure can occur directly or indirectly through household dust
[211]. See 3.6.1 for discussions about the regulation.
2.6 Some known and suspected EDs present in pesticides
In 2013, EFSA published a report showing that of 287 currently-marketed pesticides, 101 affected thyroid
signaling at some level [212], displaying potential ED properties. Another 97 showed neurotoxic effects.
This latter finding raised questions as to the efficacy of the plant protection products and biocide
evaluations. In the same vein, in late 2018, it was revealed that the chlorpyrifos documents submitted by
the manufacturer to the regulatory authorities had underestimated the effects of exposure on brain
parameters [213]. These discrepancies were ignored by the agencies evaluating the dossier in the USA
and in the EU. These facts underline the importance of thorough review of pesticide data. They also
contribute to explaining the large cost in terms of IQ and neurodevelopmental disease risk associated
with prenatal chlorpyrifos and other organophosphate pesticides exposures [214] (see section 2.8).
These currently insufficiently tested plant protection products and biocides add to the effects of banned
ED pesticides, or “legacy pesticides” still present in human fluids, long after production has ceased due
to their persistence. The list includes lindane, chlordane, DDT and hexachlorobenzene (HCB). Numerous
other plant protection products or biocides have been described as known or suspected EDs; see for
instance [215-217] and The Endocrine Disruption Exchange (TEDX) list which includes pesticides
18
, as
does the EU Commission’s Impact Review for the implementation of the plant protection products
regulation and biocidal products regulation
19
.
Below we focus on four categories of pesticides of major current concern.
17
https://echa.europa.eu/fr/-/inspectors-find-phthalates-in-toys-and-asbestos-in-second-hand-products
18
https://endocrinedisruption.org/interactive-tools/tedx-list-of-potential-endocrine-disruptors/updates
19
https://ec.europa.eu/health/sites/health/files/endocrine_disruptors/docs/2016_impact_assessment_en.pdf

Policy Department for Citizens’ Rights and Constitutional Affairs
____________________________________________________________________________________________
44
2.6.1 Organophosphate pesticides
A recent publication [218] underlined how the widespread use of this category of pesticides has led to
ubiquitous human contamination that are compromising children’s mental health through effects on
brain development. Of particular concern, is chlorpyrifos, exposure during pregnancy, which has been
associated with IQ loss, thinner brain cortex and increased neurodevelopmental disease risk including
ADHD and autism spectrum disorders. The ED effects of chlorpyrifos have been shown multiple times,
but recent evidence of its thyroid disrupting potential on developing coral fish (and hence on other
vertebrates including humans) is among the most detailed and disquieting [219].
2.6.2 Triazoles and other fungicides
Many fungicides exert ED effects, including bifonazole, imazalil, and flusilazole, that have been shown to
inhibit aromatase action purified from human placenta [220]. Other mechanisms of action include
inhibition of thyroid peroxidase [169], a factor that will affect thyroid hormone production.
Other fungicides have been shown to affect multiple aspects of endocrine signalling (see for example
[221-225]). Examples of fungicides with anti-androgenic properties include prochloraz (an imidazole)
[226] and procymidone [227].
2.6.3 Pyrethroids
Pyrethroids are increasingly applied as alternatives to organophosphate pesticides and are now the
fourth most used pesticide category world-wide [228]. As a result, there is wide scale contamination of
rivers, and as a consequence, fish. Work on fish and mammalian models, as well as human epidemiology
studies show multiple endocrine disrupting effects of different pyrethroids [228-231].
2.6.4 Neonicotinoids
Much current concern focuses on the effects of neonicotinoids on insect, notably bee, populations [232,
233], with obvious socio-economic consequences. However, many mammalian studies have linked this
pesticide class with ED activity, notably on placental aromatase activity [234]. A recent report using an in
vitro cell-based assay showed significant ED effects of one neonicotinoid on adipose cells [235].
2.7 Biomonitoring data on EDs and suspected EDs
As a result of the past or current use of suspected EDs in the above-mentioned sectors, ED presence is
documented in the environment, in various media including diet and in humans. For a large proportion
of the suspected EDs that have been assayed in EU population, most subjects are exposed, as
documented by biomonitoring studies.
2.7.1 In diet
In a few EU countries, detailed monitoring of the contamination of food by chemical substances
(including suspected or proven EDs) has been performed using a rigorous sampling design and assessing
a large number of chemicals. For example, for France, Anses performed studies of the total diet (TDS, total
diet studies
20
) in 2000-2004, 2006 and 2011; the latter survey focused on the diet of children up to 3 years
of age. In this survey, the concentration of 670 chemicals was assessed in almost 5500 food samples
covering most of the diet of children under 3 years and collected in all French regions. In addition to the
assessment of exposure levels (based on actual food patterns in children), Anses performed a risk
20
https://www.anses.fr/en/content/total-diet-studies-tdss
Endocrine Disruptors: from Scientific Evidence to Human Health Protection
____________________________________________________________________________________________
45
assessment for each of the chemical considered separately, taking into account any existing dose-
response function relating exposures to adverse effects, when available. The study found detectable
levels in the food of children under 3 for several proven or suspected EDs, such as PCBs, methylmercury,
genistein (for children using soy-based products), benzophenone, bisphenol A (at a time when it was still
authorized in food contact materials for young children), perfluoroalkyl substances including PFOA and
PFOS, PBDEs, and a large number of pesticide residues. For some of these substances, a health risk could
not be discounted, based on dose-response functions from the literature.
2.7.2 In human populations – data available at the EU level
In 2017, the first results of one of the main “exposome” projects funded by the EU, Helix early-life
exposome projects, provided an assessment of the levels of 45 chemicals circulating in the bodies of
pregnant women from six EU countries (pregnant in 1999-2010) and their children. The assessment of
the levels of chemicals in blood and urine was done in a central laboratory ensuring high quality. Among
the 45 chemicals assessed, several were either officially recognized EDs (bisphenol A, DEHP...) or
suspected EDs such as other phthalates, perfluorinated compounds, mercury and metabolites of
organophosphate pesticides. In 90% of children and of their mothers, at least two thirds of the
investigated substances were found at detectable levels (see Table 3). Over 90% of children, for whom
samples were collected in 2013-2016, had detectable levels of several PCBs, of DDE (a DDT metabolite),
PBDE-47, several perfluoroalkyl substances including PFOA and PFOS, several phthalates including DEHP
metabolites, parabens such as methyl- and ethyl-parabens, triclosan…
Such data are informative and confirm the widespread exposure, to varying concentrations, to a large
number of EDs or suspected EDs in children and pregnant women. However, because of the focus that is
limited to a few EU cities and the lack of a random sampling frame, they cannot be used to provide a
representative estimate of exposures of the EU population. To our knowledge, there is currently no
biomonitoring study available with EU-wide implementation of a harmonized population sampling on a
specific population (e.g., pregnant women or children from a specific age-group) covering a large number
of EDs.
2.7.3 In human populations – data available in specific countries
Detailed information about exposure to chemicals, including EDs, exist in specific countries such as
Belgium [236], France [237], Germany, where compounds such as phthalates have been monitored for a
long time [124] and Portugal [238]…
As already mentioned, it is by combining the dose response functions controlled for confounders issued
from epidemiological studies for EDs with the highest likelihood of an effect with the type of
biomonitoring data that we just described that an estimate of the population impact of EDs can be
obtained.
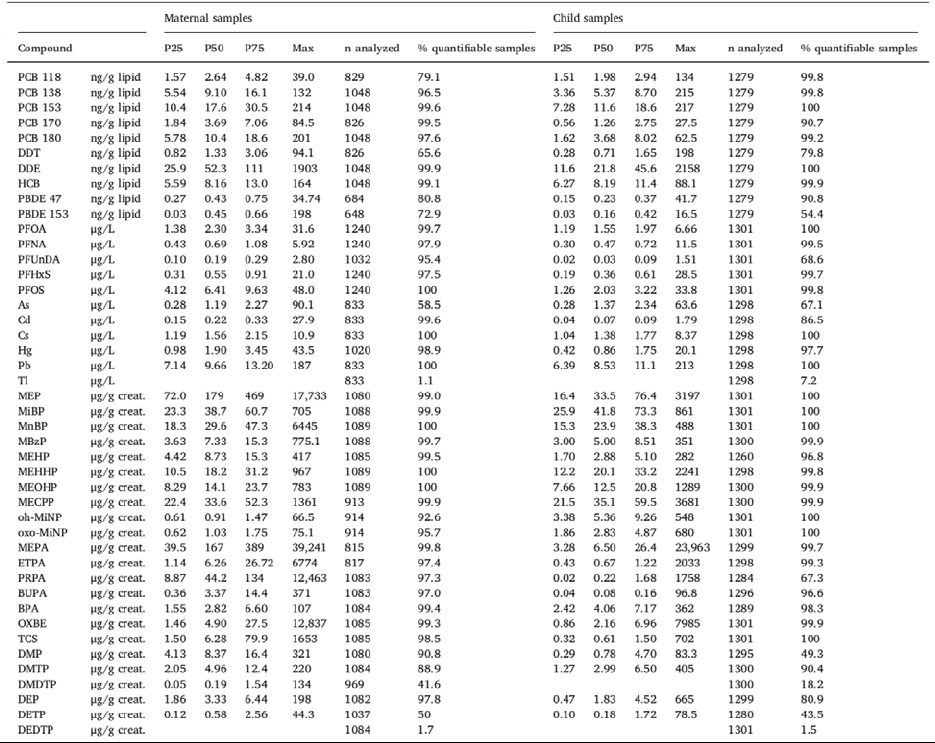
Policy Department for Citizens’ Rights and Constitutional Affairs
____________________________________________________________________________________________
46
Table 3: Distribution of urinary or blood concentrations of chemicals in 1300 EU children from Helix
exposome project.
P25, P50, P75: 25
th
, 50
th
(median) and 75
th
percentiles [123].
BPA: Bisphenol A. BUPA: Butylparaben. DDE: metabolite of the DDT insecticide. DMP, DMTP… DEDTP are organophosphate pesticide metabolites.
HCB: Hexachlorobenzene (organochlorine pesticide). ETPA: Ethylparaben. MEP, MiBP, MnBP, MBzP, MEHP…oxo-MiNP are phthalates metabolites,
with MEHP, MEHHP, MEOHP and MECPP being DEHP metabolites. OXBE: Oxybenzone. PCB: polychlorinated biphenyls. MEPA: Methylparaben.
PBDE: Polybrominated flame retardants. PFOA, PFNA, PFUnDA, PFHxS, PFOS are perfluoroalkyl substances (PFASs). PRPA: Propylparaben. TCS:
Triclosan.
2.8 Available estimates of population impact
2.8.1 Results of Trasande et al. costs estimate
A series of recent studies have attempted to provide an impact of the risk associated with exposure to
EDs, following the logic of Figure 11. Generally, such studies are limited by the facts that a) efforts to
identify EDs have been so far limited, relative to the total number of substances present in the
environment or marketed in the EU; b) by the limited availability of dose-response functions relevant for
human populations; c) by the limited amount of harmonized data on ED population exposure at the scale
of the EU (see above); d) by the limited availability of data on the healthy (or disability-adjusted) life-years
lost (DALYs) and tangible and intangible costs relative to diseases that may be induced by ED exposure.
This does not preclude commenting the few available studies, since in some cases it is possible to
anticipate the direction of the impact of these uncertainties on the results.

Endocrine Disruptors: from Scientific Evidence to Human Health Protection
____________________________________________________________________________________________
47
In 2015-2017, Trasande et al. published a series of studies aiming at estimating the health and economic
costs “that can reasonably be attributed to endocrine disrupting chemicals exposure in the EU” [239, 240].
With some adaptations, the methodology relied on the classical methodology of attributable fraction,
which requires information on a measure of exposure level to each compound of interest and an estimate
of the dose-response function(s) for all health outcomes influenced by each compound. To the extent
that the dose-response functions issued from epidemiological studies are adjusted for the main
confounders, such an approach does not suffer from bias due to the existence of other causes of the
diseases considered, including genetic factors, which are very unlikely confounders in such studies.
Figure 12: Estimated costs associated with exposure to endocrine disruptors in the EU, following a weight
of evidence approach; from [240].
Costs were estimated by multiplying the number of disease cases attributable to ED exposure with the
unitary cost associated with each disease. An important feature of the study was the approaches used to
account for uncertainties. Authors relied on a weight of evidence approach allowing to weigh the cases
and costs associated with the health events attributed to a given exposure by the level of evidence of the
dose-response function relating the exposure and the outcome. The framework for evaluating the level
of evidence consisted in combining the probability of causation based on toxicological and
epidemiological studies. To illustrate this, if there is some evidence that ten different EDs cause specific
diseases, with a cost, considering the dose-response functions and exposure levels to each ED, evaluated
to €100M for each endocrine disruptor and disease, but that the level of evidence for these effects was
about 50% (that is, the level of scientific evidence is far below certainty), then the total cost considered
for these 10 EDs and diseases in the study would have been €500M. Such a weight of evidence approach
allows avoiding the pitfall of considering only effects with a very high level of evidence (which is expected
to underestimate impacts) without falling into the opposite pitfall of taking for granted all associations
that have been reported in the literature, sometimes with poor designs.
The estimated costs related to the effects of exposure to the considered EDs was €163 billion per year
(Figure 12), with a 95% probability that costs were above €22 billion and a 25% probability of costs at
least €196 billion/year [240].
An important additional conclusion to draw from this study is the limited availability of data on the body
burden of EDs in the EU population. This, and the lack of consideration of EDs effects whose plausibility
Neurological
impacts
89%
Obesity and diabetes
6%
Cancer
0%
Reproductive disorders
5%
COSTS ASSOCIATED WITH EDS EXPOSURE (B€)
Total estimated cost
most probably
above €22 billion.
Most likely
estimate: €163
0.5%
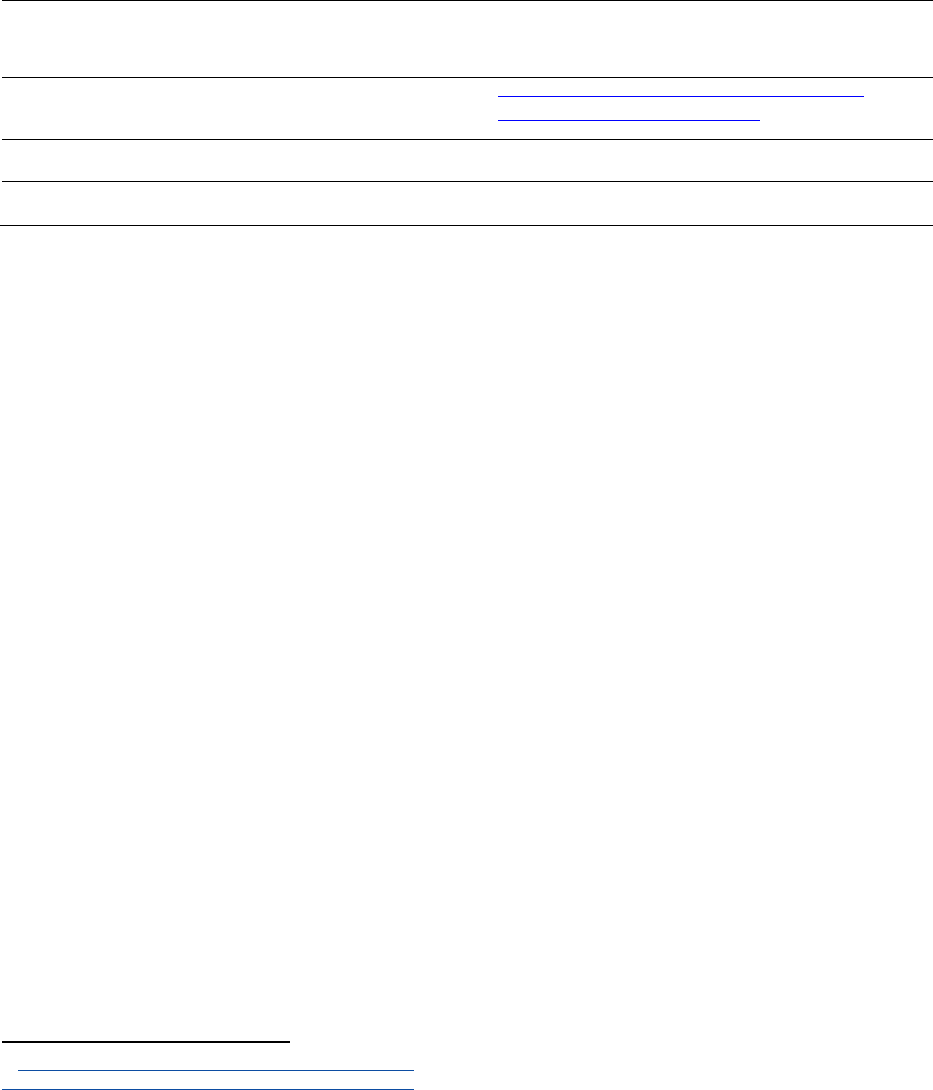
Policy Department for Citizens’ Rights and Constitutional Affairs
____________________________________________________________________________________________
48
increased since these costs were estimated (see below) argue, if anything, for underestimation of costs,
although extensive EU-wide biomonitoring, as well as additional cohort studies would be warranted to
precisely confirm all assumptions of the Trasande et al. studies.
Table 4 provides a comparison of these estimates with available estimates of the effect of other
environmental and lifestyle factors, showing that the current ED cost estimates, even though they are
possibly underestimations, would correspond to approximately 30% of the costs incurred by smoking
the EU.
Table 4: Estimated costs incurred yearly in the EU by various lifestyle or environmental factors. See above
for discussion.
Factor
Estimated cost, EU
(Billion €)
Year of estimate and reference
Smoking
544
https://www.erswhitebook.org/chapters/tobacco-
smoking/societal-costs-of-smoking/
Atmospheric pollution (PM
2.5
)
704
(WHO)
Endocrine disruptors*
163
2015 [240]
2.8.2 Published criticisms of Trasande et al. costs estimate
To our knowledge these studies have not been criticized by independent researchers and no
contradicting cost estimates from independent researchers have been published.
The Trasande et al studies were criticized in a commentary first authored by a consultant with ties to the
American Chemistry Council [241] and in two succinct letters addressed to the editor of Journal of Clinical
Endocrinology and Metabolism. The letters criticized the paper on IQ loss and neurodevelopmental
disease (Attention deficit/hyperactivity disorders and Autism), which found the highest costs [214], and
the female reproduction study [57]. As to the actual letters, in each case, one of the two authors had ties
to industry. Trasande and colleagues published argued replies to each letter
21
.
In the paper by Bond and Dietrich [241], several concerns were voiced using terms such as “alleged costs”
and insufficiently “adequate scientific scrutiny”. The criticisms seem to have been undertaken with the
assumption, “given the aggressive media campaign that accompanied the Trasande et al. (2015)
publication”, that the study “may have biased the results toward exaggerating costs”. This a priori
assumption by Bond and Dietrich might explain why specific limitations that might have led to an
underestimation of costs were not addressed in the criticisms.
Another criticism related to the strength of evidence for the effects of polybrominated flame retardants
(PBDE) and of organophosphate pesticides on child IQ, and the lack of reliance on a systematic review
methodology in assessing the overall epidemiological evidence on the topic.
Similarly, Bond and Dietrich [241], questioned the strength of evidence for organophosphate pesticides
effects on IQ. They examined the data on chlorpyrifos and cite a series of articles most of which are (co)-
authored by consulting groups linked to industry. The only one that is authored by academics concluded
in 2008 that “ further investigation is needed” , as are “more rigorous measures of exposure” [242].
21
https://academic.oup.com/jcem/article/100/6/L54/2829568
https://academic.oup.com/jcem/article/101/11/L110/2765052
Endocrine Disruptors: from Scientific Evidence to Human Health Protection
____________________________________________________________________________________________
49
Trasande et al. considered longitudinal cohort studies available for prenatal exposure to chlorpyrifos in
relation to IQ. Since then, further experimental animal evidence as to the ED effects of chlorpyrifos on
thyroid hormone, a hormone essential for brain development, has appeared [219]. In addition, a
publication has shown [213] that the manufacturers’ file submitted for chlorpyrifos authorisation was
deliberately misleading, notably for exposure effects on brain development, which were significant.
Effects of prenatal organophosphate exposure on IQ loss have been further documented in a longitudinal
human study [243]. Similarly, a recent review of exposure during pregnancy to organophosphate
pesticide use and brain disorders has been published [218]. Importantly, a natural experiment occurred
when a ban on household use of chlorpyrifos was introduced. Before the ban, decreases in birth weight
and length in relationship to levels of chlorpyrifos were seen in newborn cord blood, associations no
longer seen after the ban [244].
As to the effects of PBDE on IQ, since the publication of the Trasande et al. studies, a systematic review of
the evidence regarding PBDE exposure and IQ was published. It concluded that the level of evidence for
an effect of PBDE on IQ, based on epidemiological studies, was sufficient [245], which can be seen as
consistent with the assumption by Trasande et al. who proposed that the strength of the human evidence
regarding this association was moderate to high.
Regarding dose-response functions in general, it can further be stated that, for the non-persistent
chemicals considered (such as organophosphate pesticides), the epidemiological studies used, which so
far almost exclusively rely on spot biospecimens, are expected to provide under-estimates of dose
response functions for the least persistent compounds [113, 246].
One general criticism from Bond and Dietrich [241] related to the fact that the study was overseen by a
“steering committee that consisted of self-appointed group of eight scientists who have published
research and actively engaged in public policy advocacy on the topic of [EDs].” However, each of the
individual studies were written by experts in the different fields, principally academic researchers who
stated if they held conflicts of interest and each of whom had clear publication and funding track records
on the relevant topics under study. Despite the criticism suggesting a collusion by scientists, Bond and
Dietrich suggest no alternative method to the rigorous selection criteria for participation used by
Trasande et al. Also, in their critique, Bond and Dietrich cite evidence that mixes adult exposure with
prenatal exposure, thus ignoring the well documented windows of vulnerability for exposure and
underestimating effects.
In addition, one can note that the level of evidence for several dose-response functions not included in
the Trasande et al. evaluations, and corresponding to chemicals with widespread exposure, was
strengthened since this publication, e.g. regarding the implication of bisphenol A in prostate cancer or
breast cancer [71] and of prenatal DDT exposure for breast cancer [11] and childhood obesity [247].
Overall, Bond and Dietrich [241] offered no alternative method for cost estimate, and their arguments
confounded prenatal and adult exposures. Further, since the Trasande et al publications, multiple studies
show documented levels of prenatal ED exposure to affect IQ and other adverse health outcomes (see
references above). For a further discussion of cost criticisms see Annexe 3 page 102.
2.9 Limitations of the current regulatory risk assessment framework to minimize ED
exposure and efficiently protect health
2.9.1 Current regulatory framework for the management of chemicals
In some sectors for which human exposure can be expected, chemicals in general and EDs in particular
are managed under a logic that requires identification of thresholds. These refer to the concepts of
Policy Department for Citizens’ Rights and Constitutional Affairs
____________________________________________________________________________________________
50
predicted no effect concentrations (PNEC), derived no effect level (DNEC, both used in the context of REACH
regulation) or no observed adverse effect levels (NOAEL). This logic is currently applied e.g. in the sectors of
REACH chemicals, at least for substances not recognized as carcinogens, mutagens or reprotoxicants.
It is important, following Slob et al. (cited in [248]), to distinguish several types of thresholds, and in
particular:
• Biological thresholds: The dose below which the organism does not suffer from any [adverse]
effects from the compound considered.
• Experimental thresholds: The dose below which no effects are observed using specific tests.
These are in addition to regulatory thresholds, e.g., the maximum exposure tolerated in a population.
Biological thresholds are expected to depend on many parameters, such as existence of subjects with
heightened sensitivity to the compound in the population, e.g. because of a sensitive stage of
development, of specific tissue sensitivities, of existing diseases or altered detoxification of reparation
mechanisms.
As noted in 2013 following a meeting convened by the JRC [248], experimental approaches cannot
demonstrate the existence of (biological) thresholds. Indeed, it is generally scientifically not possible to
demonstrate that something does not exist (in this case, that there is no difference in disease occurrence
between a group not exposed to the chemical of interest and a group exposed to a very low dose). It is
by biological considerations that one may dispute the plausibility of the substance acting at extremely
low doses, which requires very detailed knowledge on its mechanisms of action.
When it comes to the risk management of the chemicals, as recalled by the JRC:
“The current risk assessment paradigm follows one of two approaches; either assuming a biological
threshold exists and taking the experimental NOAEL/NOEC or benchmark dose from the critical
study as being a dose level at which there is a small response level and incorporating a number of
uncertainty or variability factors to derive an acceptable (by society) exposure level; or in the case of
genotoxic carcinogens and germ cell mutagens, being unsure about whether or not a biological
threshold exists and that even if an experimental NOAEL were to be available, it has to be considered
inappropriate to derive an acceptable exposure level by applying the same methodology used for
threshold effects. The latter approach leads in many regulatory domains to risk management
measures by removing the substance from the market or, if not possible, by reducing exposure to as
low as achievable. In the case of genotoxic carcinogens this so-called non-threshold approach has
as its historical origins the premise that even one molecule could cause one irreversible mutation
which could be the starting point of an eventual malignant tumour.”
2.9.2 The “threshold” debate
The previous paragraph provides insight as to why a part of the debate between some toxicologists from
the chemical industry and academic toxicologists focused on the issue of whether EDs effects on health
follow a dose-response function with a threshold. If the existence of a biological threshold is unlikely, as
for carcinogens, then EDs should be managed according to a logic of strong restrictions or ban (no
exposure or exposure minimization). If the existence of a biological threshold is plausible and deemed
identifiable by tests, then there would be no need to lay down specific management procedures for this
class of hazard, but simply to identify the threshold using relevant tests as is done for the other hazard
classes. This logic assumes that, if a biological threshold exists, the tests commonly used allow to estimate
them accurately.
Endocrine Disruptors: from Scientific Evidence to Human Health Protection
____________________________________________________________________________________________
51
2.9.3 Are existing test methods sufficiently sensitive?
Available evidence suggests that currently used tests are, in general, inefficient to identify effects of the
test substance occurring at environmentally-relevant doses (i.e., poor sensitivity of tests).
The current approach to threshold identification in regulatory toxicology does not equate experimental
thresholds with regulatory thresholds, in that a number of safety factors (also termed uncertainty factors)
are applied to the experimental threshold to obtain the regulatory threshold. Thus, a factor of 1/100 may
be applied to the experimental threshold to obtain the regulatory threshold (the regulatory threshold
being set to a hundredth of the identified experimental threshold). These safety factors are meant to take
into account uncertainties related to the interspecies extrapolation, and to the variability of sensitivity
within human populations, but are not meant to compensate other technical limitations of the test, such
as limited power, interpretation of lack of observed effect as evidence of lack of effect, insensitive
outcome or animal strain, etc.
Figure 13: Illustrations of the decreased sensitivity of regulatory tests for a given number of animals in
each compared group,
in the hypothetical situation of a compound with a monotonous effect.
Generally, test methods do not require the number of animals compared to increase as the tested dose
decreases. As the tested exposure decreases, the probability to detect an effect decreases, so the test
could conclude the existence of a threshold at exposures for which an effect may still exist.
As recalled in the JRC report [248], “It was also highlighted that the experimental NOAEL/NOEC is not
equivalent to the true biological threshold but rather reflects the limit of detection of the method for that
endpoint, regarding statistical power to detect an effect as well as the inclusion of relevant sensitive
endpoints.” The issue of statistical power (defined as the probability to highlight an effect if there is one)
refers to issues such as (i) the habit to interpret a “non-significant” test (corresponding to a p-value above
a threshold of usually 0.05) as evidence of a lack of effect (while it generally should only be seen as lack of
evidence of an effect), and (ii) reliance on a rather small number of animals in the compared groups. We
illustrate the issue of statistical power in Figure 13. Even assuming a linear dose-response function, these
issues related to statistical power would warrant an increase in the number of animals or observation
units as the dose decreases (to magnify the sensitivity of the test at this dose), an approach which is to
our knowledge not generally used. Such an approach would go against the aim of limiting animal
experiments.
Thus, generally, in spite of the use of “safety factors”, experimental thresholds are probably not
conservative estimates (i.e., they are not very protective). This is illustrated by the fact that, over the years,
as newer more sensitive tests are developed and academic research is conducted, the regulatory
thresholds of compounds tend to decrease. For example, the EU tolerated daily intake of bisphenol A was
Policy Department for Citizens’ Rights and Constitutional Affairs
____________________________________________________________________________________________
52
decreased from 50 µg/kg body weight/day in 2011 to 4 µg/kg/day in 2015 (a value considered to be too
high by some regulatory agencies and many scientists). For this reason, this “safe thresholds” approach
may be deemed acceptable for rather limited hazards, that is, for health effects leading to rather minor or
curable diseases. However, this approach is less advisable for more serious health effects with fewer
therapeutic options, such as cancer or neurodevelopmental troubles. It would also be in agreement with
the precautionary principle not to accept an approach that is not totally efficient in identifying safe
thresholds for carcinogens or EDs. Such a logic prevailed e.g. in the plant protection products (PPPR) and
biocides (BPR) regulations, that do not rely on the identification of “safe thresholds” for the hazards of
highest concerns, namely CMR substances and EDs (see 3.2.1).
In the case of EDs specifically, further limitations to the “safe threshold” approach exist. These relate (i) to
the sensitivity of the biological outcomes considered by the tests used in regulatory toxicology for EDs,
such as those validated by OECD guidelines. One of these tests, the uterotrophic assay, for example
focuses on the organ weight; although a change of the weight of an organ may be the sign of toxicity,
many toxic effects are exerted by subtle mechanisms that do not entail any change in the overall organ
weight (see [249] for an example); (ii) to the variability of some of the main tests used; regarding again
the main test used to detect oestrogenic effects, the uterotrophic assay, which relies on changes in uterus
weight in mice or rats models, its results have been shown to depend on variations in protocol within the
guidelines, such as the choice of rats or mice, or the exposure route (injection versus gavage), which are
all permitted by the guidelines [250]; (iii) to the likelihood of a monotonic dose response function
generally assumed in regulatory testing, which is an unlikely default assumption for most EDs [25]; (iv) to
the lack of consideration of combined exposure to many other possible EDs in the identification of safe
thresholds (see 1.6.2), some of which are expected to influence the same outcome or act on the same
pathway as that influenced by the considered chemical; and most importantly (v) to the biological
knowledge about the mode of actions and EDs, that are expected to act at very low doses (see 1.3.2).
In other words, these methods will, because of the methodological limitations outlined above, generally
identify experimental thresholds, but these will in no way constitute strong evidence for the existence of
a biological threshold. These test thresholds are expected to provide a poor identification of the dose
range where any effect would be very rare for EDs.
These considerations on biological and regulatory thresholds, at the frontier between science and
regulation, lead us to the description of the current regulatory framework of EDs in Europe.

Endocrine Disruptors: from Scientific Evidence to Human Health Protection
____________________________________________________________________________________________
53
PART B: MANAGING THE RISK INCURRED BY EDCs – WHAT IS
CURRENTLY DONE

Policy Department for Citizens’ Rights and Constitutional Affairs
____________________________________________________________________________________________
54
3 CURRENT REGULATION OF ENDOCRINE DISRUPTORS IN THE EU
KEY FINDINGS
• In principle, the regulation of hazardous substances requires five main components: (1)
hazard definition, (2) validated tests; (3) guidance to explain how to apply the definition
based on test results and scientific publications; (4) test requirements (steps 1-4 allow
hazard identification); and (5) a management logic.
• A legal definition of EDs exists for the sector of biocides and plant protection products
(pesticides) but not in other key sectors (REACH chemicals, cosmetics, food additives and
food contact material, toys…).
• Even for biocides and plant protection products, the regulation lacks coherence, in that a
definition and a management logic exist (zero exposure to EDs) but without tests covering
the main ED modalities and endpoints being compulsory in application dossiers for
product authorization, in particular for biocides, making identification of EDs very difficult
in practice.
• Currently, only 13 REACH chemicals have been classified as EDs, in part due to limited
information in the application dossiers. Two compounds on the list of substances of very
high concern have been added to the REACH list of substances requiring authorization
because of their endocrine-disrupting properties.
• For sectors other than plant protection products and biocides, although regulations in
some cases required definitions to be put forward (e.g. for cosmetics), no definition
(“identification criteria”) has been proposed and generally no management logic specific
to EDs exist. A partial exception is that of REACH regulation, which puts EDs on the same
level of concern as carcinogenic, mutagenic substances and reprotoxicants, but without
making ED testing compulsory in application dossiers for product authorization.
• Some compound- and sector-specific regulations for EDs also exist (e.g., ban of bisphenol
A in food contact materials for children up to 3 years old), but given that they apply to
compounds present in multiple sources and sectors, such provisions are not expected to
guarantee exposure minimization.
• Thus, despite the significant progress that REACH, the plant protection products and
biocides regulations represent, neither the current regulatory framework nor its
implementation are sufficient to minimize exposure to EDs and protect human health and
the environment from the impact of EDs. Given the limitations for ED identification noted
above, it is very unlikely that the aim of having all EDs recognized as substances of very
high concern by 2020 will be achieved, as promised by the 7
th
Environment Action
Programme.
A succinct overview of the sectors and regulations dealing with health and environmental hazards is
provided Figure 14. These hazards in general, and EDs specifically, are mentioned in some of these
regulations, be they media-oriented or use-oriented. Currently, they are considered in the regulations
relative to pesticides (plant protection products and biocides, see 3.2 below), in REACH regulation (3.3),
and (more succinctly) mentioned in the cosmetics regulation (3.4), all of which are presented below. We
also describe regulations central for the protection of health and the environment or the management
of chemicals in which EDs are not mentioned, such as the toys’ safety directive (see 3.6 below) and the
CLP (Classification, Labelling, Packaging) directive, an important regulation defining the main categories
of hazards, including hazards for health and the environment, but not EDs (3.8). A summary of the current
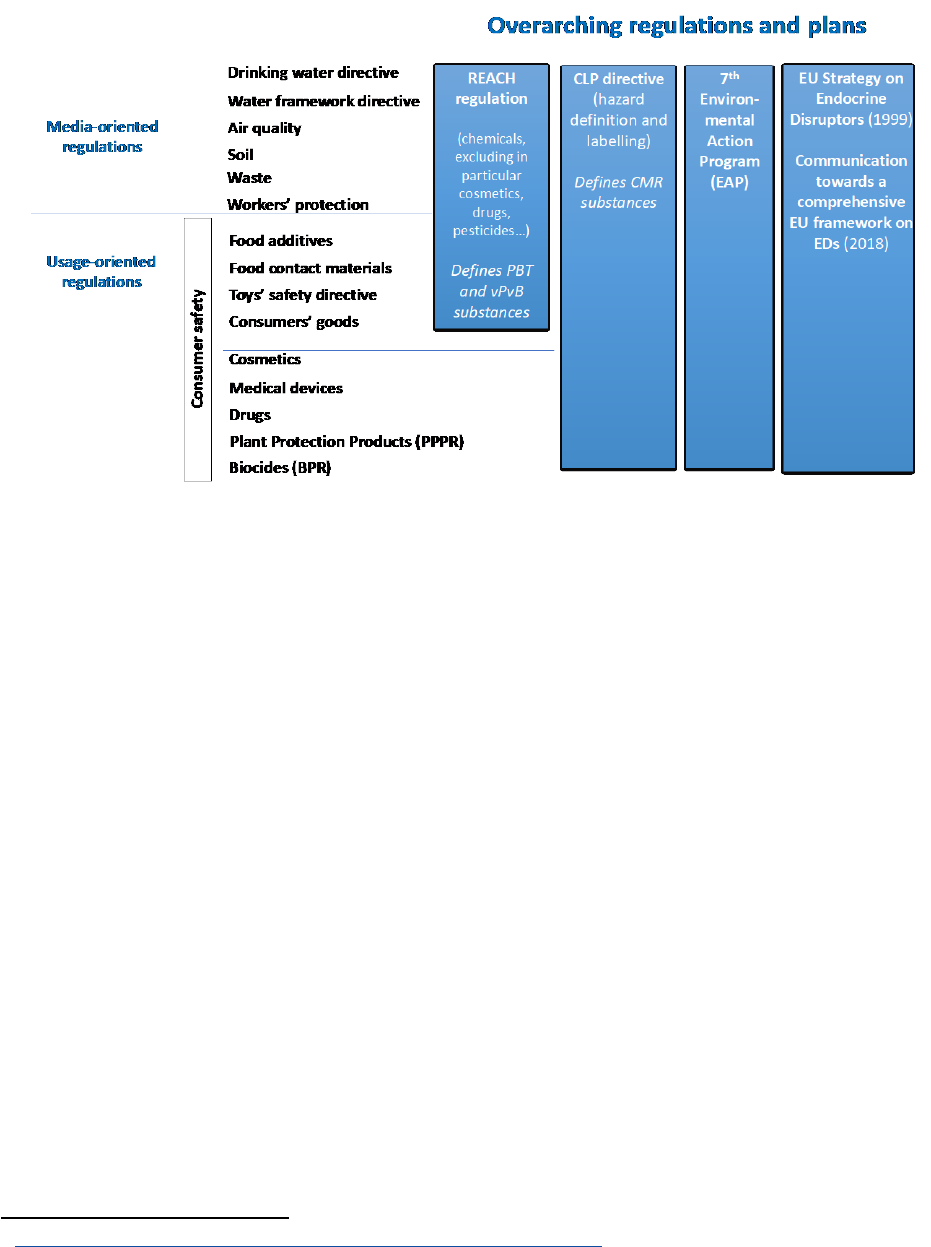
Endocrine Disruptors: from Scientific Evidence to Human Health Protection
____________________________________________________________________________________________
55
regulation is provided in Table 7, and in a simplified version in Table 1. For size limitation reasons, this
review does not constitute an exhaustive presentation of the EU chemicals regulation.
Figure 14: EDs are expected to be present in different sectors; list of the main EU regulatory areas with
relevance to endocrine disruption. Adapted from [7]
.
3.1 Overarching regulations
3.1.1 The 7
th
environment action programme
The EU policy on environment and health is framed in its Environment Action Programmes (EAPs). The
7
th
EAP (decision 1386/2013/EU
22
), encompassing the 2013-2020 period, recalls that:
The Union has agreed to achieve, by 2020, the objective that chemicals are produced and used in
ways that lead to the minimisation of significant adverse effects on human health and the
environment
23
.
And that:
The Union has agreed to stimulate the transition to a green economy and to strive towards an
absolute decoupling of economic growth and environmental degradation.
This programme states notably that:
efforts need to be stepped up to ensure that, by 2020, all relevant substances of very high concern,
including substances with endocrine-disrupting properties, are placed on the REACH candidate list.
(Article 50)
It is also recalled that:
Pursuant to Article 191(2) of the Treaty on the Functioning of the European Union (TFEU), Union
policy on the environment aims at a high level of protection taking into account the diversity of
situations in the various regions of the Union, and is based on the precautionary principle and on the
22
https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32013D1386&from=EN
23
Decision No 1600/2002/EC; Johannesburg Plan of Implementation (WSSD 2002).

Policy Department for Citizens’ Rights and Constitutional Affairs
____________________________________________________________________________________________
56
principles that preventive action should be taken, that environmental damage should, as a priority,
be rectified at source and that the polluter should pay.
Note that the Precautionary principle is, in the EU framework, meant to be applied to environment,
health, animal or plant health:
Although the precautionary principle is not explicitly mentioned in the Treaty except in the
environmental field, its scope is far wider and covers those specific circumstances where scientific
evidence is insufficient, inconclusive or uncertain and there are indications through preliminary
objective scientific evaluation that there are reasonable grounds for concern that the potentially
dangerous effects on the environment, human, animal or plant health may be inconsistent with the
chosen level of protection.
24
More specifically, legislation on the Circular Economy Package was introduced recently, with member
states having 24 months to enact the law in their national equivalents. Notably, by 2030 all plastic
packaging should be recyclable. The case of EDs within the circular economy has recently been addressed
by the EU Parliament in its resolution (2018/2589(RSP)
tabled in September 2018. The resolution raised
the twin problems of EDs in recycled products and the need to consider EDs in the design stage of the
product.
3.1.2 1999 EU strategy on EDs
Historically, the first reference to EDs by the European Parliament dates back to 1998, with a resolution
calling upon the European Commission to take action on the issue of EDs, aiming for an improvement of
the legislative framework, reinforcement of research efforts and an increased effort to make information
available to the public. This resolution was followed by a “Community strategy for EDs – a range of
substances suspected of interfering with the hormone systems of humans and wildlife” in 1999
25
. The
strategy listed short to long-term actions, as summarized elsewhere
26
:
Short-term actions (1-2 years) included:
- Establish a priority list of substances for further evaluation of their role in endocrine disruption
including the identification of a) substances for priority testing b) substances
addressed/regulated under existing Community legislation, c) gaps in knowledge d) specific
cases of consumer use e.g. by vulnerable groups such as children
- Establish monitoring programmes to estimate exposure to and the effects of the substances on
the ED priority list
- Collect, exchange, assess and provide information on EDs to the public
Medium-term actions (2-4 years):
- Identify and assess EDs: include harmonisation of the development and validation of new
improved testing methods
- Research and development to provide greater understanding of the mechanisms of endocrine
disruption, causal links between exposure to substances and adverse effects in humans and
wildlife, investigation of risk assessment concepts, exposure assessment and the development of
environmental monitoring tools
24
European Commission, COM(2000)1 of 2 February 2000, see https://eur-lex.europa.eu/legal-
content/EN/TXT/PDF/?uri=CELEX:52000DC0001&from=EN
25
https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:51999DC0706
26
http://ec.europa.eu/environment/chemicals/endocrine/documents/index_en.htm#SubThemes2
Endocrine Disruptors: from Scientific Evidence to Human Health Protection
____________________________________________________________________________________________
57
Long-term actions (4 years or more):
- Adapt and/or amend present EU legislative instruments which cover chemical as well as
consumer, health and environmental protection to take account of ED effects
3.1.3 The 2018 Communication toward a comprehensive EU framework on EDs
In 2018, the EU Commission published a “Communication toward a comprehensive EU framework on
EDs” [100]. This Communication states that:
The implementation of the Community Strategy of 1999 has put the EU at the forefront in
understanding and regulating these hazardous chemicals. But in order to further progress and
maintain the expected high level of protection, it is important to ensure that the EU framework
continues to coherently address endocrine disruptors across different areas.
The main aims to follow in this sector for the years to come are listed:
The EU strategic approach on endocrine disruptors for the years to come should be based on the
application of the precautionary principle and aim at:
- minimising overall exposure of humans and the environment to endocrine disruptors, paying
particular attention to exposures during important periods of development of an organism, such
as foetal development and puberty;
- accelerating the development of a thorough research basis for effective and forward-looking
decision-making;
- and promoting an active dialogue allowing all stakeholders to be heard and to work together.
Thus, the main concrete goal lies in the minimisation of overall exposure of humans and the environment
to EDs.
To move in this direction, the communication acknowledges that
The legislative measures constituting the EU legal framework regulating chemicals have been
developed at different points in time and have, in certain cases, different objectives. This has resulted
in different approaches to endocrine disruptors, depending on the sector being regulated, and has
raised questions as to whether the EU legal framework regulating endocrine disruptors is sufficiently
coherent.
(these different approaches to the regulation of EDs in different sectors are discussed below in 4.1.2 and
4.1.3).
Specifically, the need for a horizontal identification of EDs across sectors is acknowledged:
Horizontal approach to the identification of endocrine disruptors: the Commission considers that
there should be a coherent approach to the identification of endocrine disruptors across all relevant
Union legislation, based on the broadly accepted definition of the World Health Organisation.
(see 4.2 below for a discussion).
Importantly, beyond the lack of homogeneous identification of EDs across sectors, the heterogeneous
management of EDs in different sectors is also acknowledged:
Regulatory consequences for endocrine disruptors: different regulatory approaches exist in different
pieces of legislation for substances identified as endocrine disruptors.

Policy Department for Citizens’ Rights and Constitutional Affairs
____________________________________________________________________________________________
58
The main actions planned in this regard consist in supporting scientific research, the organization of a
yearly forum on EDs, the launch of a web portal on EDs, the support for the recognition of the definition
of EDs in the international system for classification of chemicals, and the launch of a “fitness check”.
However, no detailed plan regarding changes in the regulation are laid down to tackle this issue:
Some stakeholders have argued that, in some areas, EU legislation does not provide adequate
regulatory approaches to address endocrine disruptors effectively. This matter deserves further
examination.
We discuss this issue in chapter 5. Comments on this Framework have also been provided by scientific
societies including the Endocrine Society
27
and the European Society for Endocrinology
28
. Both societies
underlined that the strategy described in the communication will fail to fully protect human health and
the environment. Notably, the European Society of Endocrinology deplores the absence of concrete
measures and time-points to achieve them.
3.2 The plant protection products (PPPR) and biocides (BPR) regulations
3.2.1 Management of plant protection products and biocides containing EDs
The management of plant protection products follows the logic that the plant protection products
should not be authorized if they have endocrine disrupting properties. More specifically, the plant
protection products regulation (1107/2009) states that:
The residues of the plant protection products… (a) shall not have any harmful effects on human
health, including that of vulnerable groups, or animal health, taking into account known cumulative
and synergistic effects where the scientific methods accepted by the Authority to assess such effects
are available, or on groundwater; (b) they shall not have any unacceptable effect on the
environment. (article 4)
Annex II lists the Procedure and criteria for approval of active substances, safeners and synergists in plant
protection products, specifically stating that these shall only be approved if they are not considered to
have ED properties, unless the exposure of humans is negligible:
An active substance, safener or synergist shall only be approved if, on the basis of the assessment of
Community or internationally agreed test guidelines or other available data and information,
including a review of the scientific literature, reviewed by the Authority, it is not considered to have
endocrine disrupting properties that may cause adverse effect in humans, unless the exposure of
humans to that active substance, safener or synergist in a plant protection product, under realistic
proposed conditions of use, is negligible, that is, the product is used in closed systems or in other
conditions excluding contact with humans and where residues of the active substance, safener or
synergist concerned on food and feed do not exceed the default value set in accordance with point
(b) of Article 18(1) of Regulation (EC) No 396/2005. (Annex II, 3.6.5)
Note that a similar provision exists for plant protection products recognized as carcinogens (level of
evidence 1A or 1B, that is, known or presumed carcinogens, annex II, 3.6.3), mutagens (1A and 1B) and
toxic for reproduction (annex II, 3.6.4). Thus, the PPPR regulation follows a similar management logic for
CMRs and substances with ED properties. However, unlike CMRs which had a legally valid definition in
27
https://www.endocrine.org/news-room/2018/european-commission-communication-falls-short-of-protecting-public-from-edc-exposure
28
ESE statement commenting on EC Communication from November 07: https://www.ese-hormones.org/media/1600/ese-statement-on-
ec-com_final.pdf

Endocrine Disruptors: from Scientific Evidence to Human Health Protection
____________________________________________________________________________________________
59
2009, substances with ED properties did not, so this logic was not implemented then. Article 3.6.5 (annex
II) of the PPPR further requested a draft definition to be presented by the EU Commission by December
2013. This was not done at this time, and a definition (the so-called “ED criteria”, see 3.2.2) was eventually
adopted and applied as of October 2018 for plant protection products. As yet (January 2019), to our
knowledge, no plant protection product or biocide has been banned due to its ED properties
29
. Given the
existence of a scientifically recognized definition of EDs in the World Health Organization 2002 report
(and of earlier definitions internationally agreed upon by scientists), there is no scientific justification for
the lack of definition of EDs between 2009 and 2018.
The BPR, or biocidal product regulation (528/2012), follows a logic similar to that laid down for biocides,
i.e. that “active substances which…are considered as having endocrine-disrupting properties that may
cause adverse effects in humans or which are identified in accordance with [REACH] regulation as having
endocrine disrupting properties” shall not be approved (article 5). It may however be approved for an
initial period not exceeding five years if “the risk to humans, animals or the environment from exposure
to the active substance in a biocidal product, under realistic worst case conditions of use, is negligible, in
particular where the product is used in closed systems or under other conditions which aim at excluding
contact with humans and release into the environment” (article 5(2)). Identical provisions exist for active
substances classified as CMRs (categories 1A or 1B), PBT or vPvB. A distinction with the plant protection
products regulation relates to the replacement of the term “negligible exposure…” in the plant
protection products (2009) regulation by the term “negligible risk…”
30
in the biocides (2012) regulation,
although the context (exclusion of contact with humans and release into the environment) remains the
same. The clause of authorization of active substances for which risk is negligible does not apply to
biocidal products for use by the general public (Article 19(4)). The Commissions’ interpretation [100],
which seems a logical interpretation of the biocide law, is that quantitative risk assessment is not required
for biocides containing EDs or CMRs and that a no exposure logic is to be applied, like for pesticides.
3.2.2 Definitions of EDs for plant protection products and biocides
The WHO-IPCS [9] definition of ED served as the basis of the definition of substances with endocrine
disrupting properties in the context of the 2009 EU Plant Protection Products Regulation (PPPR)
31
, which
rephrases the definition the following way:
- an active substance, safener or synergist shall be considered as having endocrine disrupting
properties that may cause adverse effect in humans if [...] it is a substance that meets all of the
following criteria, unless there is evidence demonstrating that the adverse effects identified are
not relevant to humans:
- it shows an adverse effect in an intact organism or its progeny, which is a change in the
morphology, physiology, growth, development, reproduction or life span of an organism, system
or (sub)population that results in an impairment of functional capacity, an impairment of the
capacity to compensate for additional stress or an increase in susceptibility to other influences;
- it has an endocrine mode of action, i.e. it alters the function(s) of the endocrine system;
- the adverse effect is a consequence of the endocrine mode of action.
29
Triclosan has been banned as a biocide, due to its toxicity for aquatic life.
30
Risk is defined as the probability of an adverse effect in an organism, system, or (sub)population caused under specified circumstances by
exposure to an agent.
31
Regulation (EU) 2018/605 of 19 April 2018 amending Annex II to Regulation (EC) No 1107/2009 by setting out scientific criteria for the
determination of endocrine disrupting properties. Link.

Policy Department for Citizens’ Rights and Constitutional Affairs
____________________________________________________________________________________________
60
The comparison with the WHO definition notably shows:
- the reference to active substances, safeners or synergist, which is specific to the area of plant
protection products and biocides;
- the use of the expression “mode of action” in the EU ED criteria for plant protection products and
biocides.
We discuss the relevance of these modifications in 4.2.2.
3.2.3 Identification of EDs for plant protection products and biocides
The Commission regulations appending the annex II of the PPPR and BPR [251, 252], or so-called “ED
criteria”, additionally specify that:
- A weight of evidence (WoE
32
) approach should be used for the assessment of the available data;
- All available relevant scientific data (in vivo studies or adequately validated alternative test
systems predictive of adverse effects in humans or animals; as well as in vivo, in vitro, or, if
applicable, in silico studies informing about endocrine modes of action) must be considered in
applying this WoE approach: (a) scientific data generated in accordance with internationally
agreed study protocols, in particular those listed in the Commission Communications in the
framework of setting out the data requirements for active substances and plant protection
products, in accordance with this Regulation; (b) other scientific data selected applying a
systematic review methodology […];
- The application of the WoE approach should consider: both negative and positive results; the
relevance of study designs; the quality and consistency of the data […] within and between
studies of a similar design and across different species”; the route of exposure, toxicokinetic and
metabolism studies;
- “The link between the adverse effect(s) and the endocrine mode of action shall be established
based on biological plausibility”;
- “Adverse effects that are non-specific secondary consequences of other toxic effects shall not be
considered for the identification of the substance as endocrine disruptor”.
It can be noted that, in accordance with the PPPR and BPR, two definitions are actually provided, one for
substances with endocrine disrupting properties that may cause adverse effects in humans and one for
substances with endocrine disrupting properties that may cause adverse effects on non-target organisms.
No further categories of substances with endocrine disrupting properties (e.g., distinguishing according
to the level of evidence) are defined in the context of the EU regulation.
Further, a guidance document from ECHA and EFSA [253] stipulates how to perform hazard identification
for endocrine-disrupting properties in the context of the PPPR and BPR on the basis of these ED criteria
and of test results from the authorization dossiers. Specifically, this guidance document “describes how
to gather, evaluate and consider all relevant information for the assessment, conduct a mode of action
(MoA) analysis, and apply a weight of evidence (WoE) approach, in order to establish whether the ED
criteria are fulfilled.” [253].
We discuss the ED criteria in 4.2.1 and the ECHA-EFSA guidance document in 4.3.1.
32 “Weight of evidence assessment is defined … as a process in which evidence is integrated to determine the relative support for possible
answers to a question. [The weight of evidence assessment comprises] three basic steps: (1) assembling the evidence into lines of
evidence of similar type, (2) weighing the evidence, (3) integrating the evidence.” (EFSA guidance on weight of evidence, EFSA Journal,
2017, https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2017.4971
).
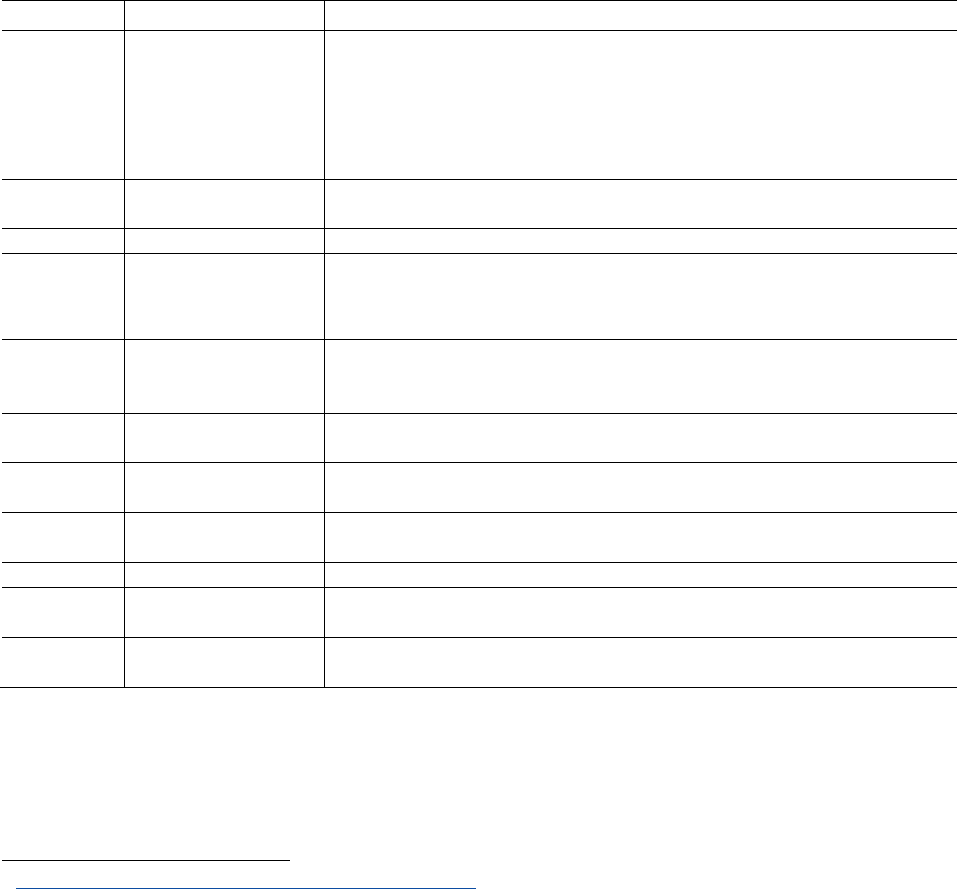
Endocrine Disruptors: from Scientific Evidence to Human Health Protection
____________________________________________________________________________________________
61
3.2.4 Test requirements to identify plant protection products and biocides with ED
properties
The test requirements regarding plant protection products and biocides are listed in regulations 283 and
284/2013 (EU) for plant protection products and 528/2012 for biocides and reiterated in the Guidance
document [253]. The requirements are summarized in Table 5. For active substances used in plant
protection products, there are explicit test requirements relative to the identification of oestrogenicity,
anti-androgenicity, thyroid disruption. The requirements for the identification of compounds toxic for
reproduction additionally requires tests on anogenital distance and nipple retention, which are also
sensitive to anti-androgenic substances. Overall, many of these tests have a low sensitivity and
sometimes high variability [249, 250]. No in vitro screening of the product by available OECD validated
tests with nuclear receptors is required if an in vivo test of oestrogenicity is performed. If this in vivo test is
not performed, data for oestrogen receptor activity can come from the US ToxCast ER models.
For biocides, the requirement regarding the testing of EDs corresponds to that laid down in REACH
regulation, which is rather limited (see 3.3.4).
Table 5: Tests required to identify EDs in various sectors of the EU regulation.
Area
Regulation
Test requirement
Plant
protection
products
Active substances:
283/2013 (EU) and
2013/C 95/01;
Plant protection
products: 284/2013
(EU)
For active substances:
Oestrogenicity: ToxCast ER models or uterotrophic assay (OECD TG 440)
Anti-androgenicity: Hershberger assay (OECD TG 441)
Thyroid disruption: OCSPP Guideline 890.1450: Pubertal Development and Thyroid Function
in Intact Juvenile/Peripubertal female rats’ assay.
Steroidogenesis: H295R assay (OECD TG 456)
No mention to ED nor ED tests required specifically in regulation 284/2013.
Biocides
528/2012 (BPR, annex
II)
Testing requirements for EDs are those requested by REACH regulation. However, the
requirements specific to EDs in REACH are very limited (see below).
Cosmetics
1223/2009 (EC)
No identification of ED required.
REACH
chemicals
1907/2006 (EC);
440/2008 (EC)
Extended One-Generation Reproductive Toxicity Study (OECD 421 or 422) with the extension
of cohort 1B to include the F2 generation if 1) significant exposure and 2) there are
indications of one or more relevant modes of action related to endocrine disruption from
available in vivo studies or non-animal approaches.
Food
additives
1333/2008/,
234/2011/EU, EFSA
(2009)
33
No specific test for ED identification required.
Food contact
materials
1935/2004/EC
No specific test for ED identification required.
Consumer
goods
1999/44/EC
No specific test for ED identification required.
Drinking
water
98/83/EC
No specific test for ED identification required.
Toys
2009/48
No specific test for ED identification required.
Workers’
protection
89/391/EEC
No specific test for ED identification required.
Medical
devices
2017/745/EU
Same requirements as in REACH regulation (see above)
33
https://efsa.onlinelibrary.wiley.com/doi/abs/10.2903/j.efsa.2009.1188
Policy Department for Citizens’ Rights and Constitutional Affairs
____________________________________________________________________________________________
62
3.3 REACH chemicals regulation
3.3.1 General principles and aims of REACH regulation
REACH regulation (2006R1907 (EC)) deals with the registration, evaluation, authorization and restriction
of chemicals in the EU. This regulation does not deal with plant protection products, biocides, cosmetics,
drugs and certain other sectors, that are regulated distinctly.
REACH regulation aims at achieving that “by 2020, chemicals are produced and used in ways that lead to
the minimization of significant adverse effects on human health and the environment” (recital 4); it aims
“to ensure the good functioning of the internal market while assuring that the risks from substances of
very high concern are properly controlled and that these substances are progressively replaced by
suitable alternative substances or technologies where these are economically and technically viable”
(article 55).
3.3.2 Management of EDs under REACH regulation
Substances with endocrine disrupting properties may be included in annex XIV (article 57 of REACH
regulation), together with carcinogens, mutagens, substances toxic for reproduction substances with
persistent, bioaccumulative and toxic (PBT) or very persistent and very bioaccumulative properties (vPvB),
if they are shown to be of equivalent concern (Figure 15). This annex XIV of REACH contains substances
requiring authorization. In practice, placing a compound in annex XIV means that the EU Commission
shall specifically grant an authorization for the marketing of the substance – this shall happen “if the risk
to human health or the environment from the use of a substance arising from the intrinsic properties
specified in Annex XIV is adequately controlled”. If the authorization cannot be granted under this logic,
then “an authorization may only be granted if it is shown that socio-economic benefits outweigh the risk
to human health or the environment arising from the use of the substance and if there are no suitable
alternative substances or technologies.” (commonly referred to as the “socio-economic route”) (Article 60
of REACH regulation). In no way does the placement on the authorization list constitute a ban of the
substance or a guarantee that human exposure will cease.
Many years can pass between the substance being placed on this list and any restriction of use being
officially decided by the Commission. Placement in annex XIV may imply limitations of use, such as a
presence of the compound in the manufactured product that shall not exceed 0.1% of the product
weight; this may happen after some duration, if no authorization is eventually granted. As of 14 Jan 2019,
43 substances had been placed in annex XIV of REACH, two of which are recognized EDs.
Another route that can lead to limitations of use of a compound is the restriction route (Figure 15). A
simplified restriction procedure can be taken for articles that meet the criteria for CMR substances and
that could be used by consumers, for which placement in annex XVII (restricting manufacture, marketing
and use) is planned (article 68.2). While articles that could be used by consumers are those representing
the highest likelihood of human exposure, this article does not mention EDs.
One can note that, in its article 57(f), REACH regulation actually does not exactly handle EDs the same way
as it does CMR substances. For CMR substances, REACH regulation states that
The following substances may be included in Annex XIV…:
(a) substances meeting the criteria for classification in the hazard class carcinogenicity category 1A or
1B in accordance with section 3.6 of Annex I to Regulation (EC) No 1272/2008; (article 57a)
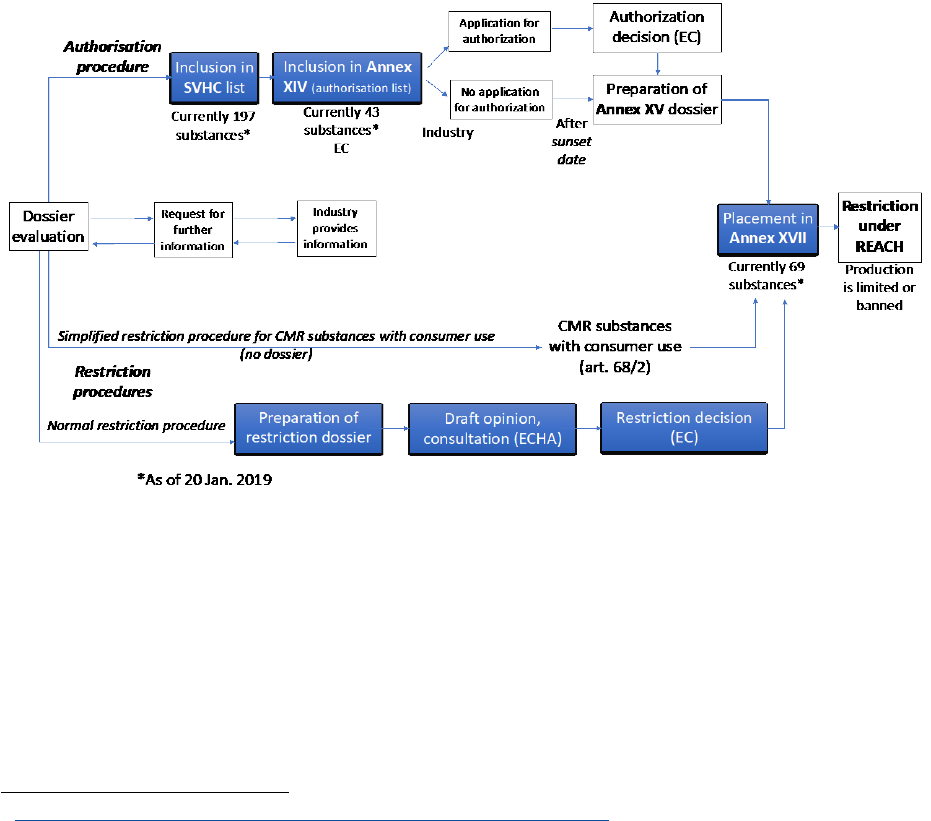
Endocrine Disruptors: from Scientific Evidence to Human Health Protection
____________________________________________________________________________________________
63
For EDs, the phrasing is that “substances…having endocrine disrupting properties… for which there is
evidence of probable serious effects to human health or the environment which give rise to an equivalent
concern to those of other substances listed in points (a) to (e) and which are identified on a case by case
basis…”. Regarding this specific point, the regulation called for a review and possible revision of the law
by the European Commission so that EDs are exactly handled as CMR, PBT and vPvB substances (article
138.7). This review was due in June 2013, and was published in 2016
34
. It concluded that, since it was not
possible to assume that all EDs acted without a threshold effect, no change in REACH regulation
regarding EDs was required. More specifically, the current practice is that the applicant for authorisation
of an ED shall demonstrate in their application file that a threshold for the health or environmental effect
exists. If not, or if this demonstration is not accepted by ECHA, then the candidate substance will be
subject to authorization via the so-called “socio-economic route”
35
.
Figure 15: Schematic view of the authorization and restriction procedures of REACH, possibly leading to
the restriction of marketing of hazardous substances.
3.3.3 Definition and identification of EDs under REACH regulation
Despite the provisions regarding the management of substances with ED properties within REACH, EDs
are not defined as part of REACH or any related regulation. Notwithstanding this lack of definition, REACH
regulation allows Member States to submit dossiers to put substances on a candidate list of substances
to be subject to authorisation. So far, 13 chemicals (including bisphenol A, DEHP, nonylphenol) have been
put on the list of substances of very high concern (SVHC) because of their endocrine-disrupting properties
in the context of REACH regulation (Table 6). This is likely to represent a small proportion of all marketed
34
https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52016DC0814&from=en
35
“…Consequently, Article 60(3) of REACH will continue to be applicable to those EDs for which it is not possible to determine a threshold. It
remains the responsibility of applicants for authorization to demonstrate that a threshold exists and to determine that threshold in
accordance with Annex I to REACH. Even though this might be particularly difficult for EDs, it cannot be excluded on the basis of current
knowledge that it will be possible. It is up to RAC to assess the validity of the assessment and ultimately decide on the possible
existence or not of this threshold. Furthermore, as for other substances, RAC may on a case- by-case basis set reference DNELs, or
reference dose-response curves, which industry can use when applying for authorization. Therefore, as under REACH as it stands today
only the “Socio-Economic Route” can be used when a threshold cannot be determined…”
Policy Department for Citizens’ Rights and Constitutional Affairs
____________________________________________________________________________________________
64
suspected and known EDs. So far two substances have been put in REACH Annex XIV of substances
requiring authorization, and an additional four phthalates may enter this annex in 2019 due to their
endocrine-disrupting properties (DEHP, DBP, BBP and DIBP).
Table 6: List of legally recognized EDs in the EU. This corresponds to the substances added to the REACH
list of substances of very high concern because of their endocrine-disrupting properties.
Substance acronym and name
CAS
number
Date of
Inclusion
Reason for inclusion
3-BC
- 1,7,7-trimethyl-3-
(phenylmethylene)bicyclo[2.2.1]heptan-2-one. 3-
benzylidene camphor
15087-24-8
2019
ED properties – environment
DCHP
- Dicyclohexyl phthalate
84-61-7
2018
Toxic for reproduction (Article 57c)
ED properties - human health
RP-HP
- Reaction products of 1,3,4-
thiadiazolidine-2,5-dithione, formaldehyde and 4-
heptylphenol, branched and linear
2018
ED properties – environment
BPA
- 4,4'-isopropylidenediphenol
Bisphenol A
80-05-7
2017
Toxic for reproduction (Article 57c)
ED properties - environment.
ED properties - human health
4-HP
- 4-heptylphenol
-
2017
ED properties – environment
PTAP
- p-(1,1-dimethylpropyl)phenol
80-46-6
2017
ED properties – environment
4-Nonylphenol
2013
ED properties – environment
4-(1,1,3,3-tetramethylbutyl)phenol,
ethoxylated
2012
ED properties – environment
4-(1,1,3,3-tetramethylbutyl)phenol
140-66-9
2011
ED properties – environment
DIBP
- Diisobutyl phthalate
84-69-5
2010
Toxic for reproduction
ED properties - human health
BBP
- Benzyl butyl phthalate
85-68-7
2008
Toxic for reproduction
ED properties - human health
DEHP
- Bis (2-ethylhexyl)phthalate
117-81-7
-
2008
Toxic for reproduction
ED properties - environment
ED properties - human health
DBP -
Dibutyl phthalate
84-74-2
-
2008
Toxic for reproduction
ED properties - human health
Source: ECHA
36
3.3.4 Test requirements to identify which REACH chemicals are EDs
When it comes to test requirements, REACH and the related regulations appear very limited (see Table 5).
Specifically, REACH regulation states that
Information on any other adverse effects on the environment shall be included where available, such
as environmental fate (exposure), photochemical ozone creation potential, ozone depletion
potential, endocrine-disrupting potential and/or global warming potential. (section II, 12.6)
But this seems restricted to “available” information (not an information that the applicant should
generate if it is not already available).
Thus, although the principles laid down in REACH regulation call for identification of EDs, the current
regulation does not compel application dossiers for products authorization about existing or new
chemicals or products to be put on the EU market to contain enough information to ensure that agencies
and other relevant bodies can identify if the substance is an ED. Only if a substance has been on the
36
https://echa.europa.eu/candidate-list-table
Endocrine Disruptors: from Scientific Evidence to Human Health Protection
____________________________________________________________________________________________
65
market for some time and thoroughly studied by academic research (as is the case of bisphenol A or DEHP
for example), can it be expected to have generated enough information to decide if the substance has
endocrine-disrupting properties.
Another illustration of the fact that most information relevant for the identification of EDs is not
regulatorily requested or available in REACH dossiers was provided through an effort of the French
environmental health agency ANSES to evaluate 15 REACH chemicals, as part of the French Strategy on
Endocrine Disruptors (SNPE1): ANSES evaluated the dossiers of 15 substances of concern. For 10 of them
(two thirds), it was not possible to conclude if the compound had endocrine properties, showing that the
majority of dossiers for substances with some a priori concern do not contain the relevant information.
It should be noted that, at least for substances already on the market, the fact that a substance cannot be
classified in terms of ED properties will generally have no immediate consequence regarding the
marketing of the substance, which will stay on the market. It is our understanding that, if for some reason,
the Member State in charge of the evaluation of the substance has grounds to believe that the substance
might be an ED, then additional information may be requested to the industry marketing the substance.
This procedure, according to the kind of test required, may take some time, and whilst awaiting the results
the product will remain on the market. Thus, it seems that, in the case an applicant has some doubt that
a substance he wishes to continue selling might be an ED, there is little incentive or requirement for him
to perform all the relevant tests that could lead to the identification of the substance as an ED, and little
legal risk, if any, in not doing these tests.
3.3.5 Conclusion regarding REACH regulation
REACH regulation provides an overall framework for the regulation of chemicals in many areas in the EU,
allowed recognition of the hazards incurred by many substances, and as such represents a clear progress
towards a safe and sustainable use of chemicals.
REACH regulation aims at minimizing significant adverse health effects on human health and the
environment, recognizes EDs as substances of very high concern and, as such, puts them on the same
level of concern as carcinogens, mutagens and reprotoxicants. However, beyond this (not very specific
and explicit) management logic, REACH regulation does not rely on any definition of EDs; it does not
recognize criteria about EDs nor is there any guidance document explicating how to identify EDs. The
test requirements planned in relation to REACH regulation are very limited, not providing enough
evidence allowing to conclude if a chemical is an ED. Given that ECHA, the Commission and Member
States managed to identify some substances as EDs even without a legal definition, the regulatory efforts
regarding REACH should focus on the test requirements in application dossiers and on the management
of substances identified as EDs.
Given the limited information present in dossiers, ECHA and the national evaluation agencies should be
complimented for having managed to include compounds on the list of substances of very high concern
because of their endocrine-disrupting properties. However, so far, only two compounds present on the
list of substances of very high concern have been added to REACH Annex XIV authorization list because
of their endocrine-disrupting properties. Even assuming that this figure increases to 6 in 2019, as can be
expected, this would correspond to a ratio of less than 1 out of 3,500 REACH chemicals subject to
authorization because of its endocrine-disrupting properties. This figure suggests that, in spite of the
progress it represents, when it comes to the protection of health and the environment from the impact
of EDs, REACH regulation is currently doing stoo little too slowly. At this stage, it seems unlikely that the
aim of the 7
th
Environment Action Programme to have all EDs recognized as substances of very high
concern by 2020 will be achieved.

Policy Department for Citizens’ Rights and Constitutional Affairs
____________________________________________________________________________________________
66
3.4 Regulation of EDs in cosmetics
3.4.1 General principles of the cosmetics regulation
The cosmetic regulation (1223/2009 (EC)) aims at ensuring a high level of protection of human health
(recital 4) and states that “cosmetics should be safe under normal or reasonably foreseeable conditions
of use. In particular, a risk-benefit analysis should not justify a risk to human health.” (recital 9; a similar
provision exists in article 3). The regulation also requires that “the responsible person shall, prior to
placing a cosmetic product on the market, ensure that the cosmetic product has undergone a safety
assessment … and that a cosmetic product safety report is set up…” (article 10). It is also recalled that
“action by the Commission and Member States relating to the protection of human health should be
based on the precautionary principle” (recital 36).
This regulation includes a list of substances prohibited in cosmetic products (annex II), a list of colorants
(annex IV), preservatives (annex V) and UV filters (annex VI) allowed in cosmetics.
The cosmetic regulation also mentions (recital 5) that the environmental concerns of substances present
in cosmetics on the environment are considered through the application of REACH regulation (see 3.3
above).
3.4.2 Management of EDs present in cosmetics
The cosmetic regulation further states that “Given the hazardous properties of substances classified as
carcinogenic, mutagenic or toxic for reproduction (CMR), category 1A, 1B and 2… their use in cosmetic
products should be prohibited. However, as a hazardous property of a substance does not necessarily
always entail a risk, there should be a possibility to allow the use of substances classified as CMR 2
substances where, in view of exposure and concentration, they have been found safe for use in cosmetic
products by the SCCS…” (recital 32). Coherently, article 15 (“Substances classified as CMR substances”)
mentions that “The use in cosmetic products of substances classified as CMR substances of category 1A
or 1B… shall be prohibited” (15.2, with some derogatory provisions for CMR substances complying with
the food safety requirements for which there are no suitable alternative substances available and that
have been evaluated and found safe by the SCCS for use in cosmetic products...). Article 15.1 similarly
prohibits the use of substances classified as CMR substances of category 2, with a possible derogation if
the SCCS finds the substance safe for use in cosmetic products.
The same article refers to EDs, stating that “When Community or internationally agreed criteria for
identifying substances with endocrine-disrupting properties are available, or at the latest on 11 January
2015, the Commission shall review this Regulation with regard to substances with endocrine-disrupting
properties” (article 15.4). Consequently, there is no generic provision stating how EDs present in
cosmetics should be treated, inferring that in practice they are handled on a compound-by-compound
basis, unlike chemicals of specific concerns such as those with CMR properties.
Currently, as examples of suspected EDs, the list of authorized preservatives
37
includes parabens with a
linear chain of 1 to 4 carbon atoms (while other parabens have been banned previously) and triclosan.
Triclosan is authorized with a maximum concentration of 0.3%.
37
http://ec.europa.eu/growth/tools-databases/cosing/pdf/COSING_Annex%20V_v2.pdf

Endocrine Disruptors: from Scientific Evidence to Human Health Protection
____________________________________________________________________________________________
67
3.5 Other sectors in which the regulation refers to EDs
3.5.1 Water
The Council Directive on the quality of water intended for human consumption (98/83/EC) dates from
1998. It states that “Whereas there is at present insufficient evidence on which to base parametric values
for endocrine-disrupting chemicals at Community level, yet there is increasing concern regarding the
potential impact on humans and wildlife of the effects of substances harmful to health.”
Drinking water standards underwent a “REFIT” procedure in 2015 and an impact assessment was
presented to the EU parliament in February 2018. It proposed an updated list of parameters to be
monitored and regulated, including two perfluorinated compounds (PFOS and PFOA, with suggested
limit concentrations in drinking water of 0.4 µg/l and 4 µg/l, respectively), the sum of PFAS and three
compounds included because of their endocrine-disrupting properties: bisphenol A, nonylphenol and
beta-oestradiol. It was also recommended to lower the total authorized concentration for lead and
chromium.
As concerns materials that come into contact with drinking water (pipes, joints etc.), technical standards
are currently being developed within the Construction Product Regulations (No 305/2011), with limits for
certain EDs (bisphenol A and PFASs).
3.5.2 Medical devices regulation
The medical devices regulation
38
(2017/745 (EU)) allows the presence of CMRs and EDs in the parts of the
medical devices that come in contact with the body or with body fluids in a proportion above 0.1% of the
weight only upon certain conditions, such as justification why substitution by another less hazardous
substance would be inappropriate (article 10.4). Specific guidelines regarding the use of phthalates are
to be prepared by the Commission scientific committees.
The identification of EDs in medical devices refers to the ED criteria specified as part of the biocidal
products regulation (528/2012), with the test requirements related to REACH regulation. As already
discussed (see 3.3.4), these are too limited to allow proper identification of EDs.
3.6 Sectors in which the regulation does not explicitly refer to EDs
Many sectors of the EU regulation, including regulations of substances for which human exposure is likely
or very likely, do not include any specific provisions regarding EDs. Among several relevant areas, we
briefly discuss below the specific cases of the directives on the safety of toys (2009/48 (EC)), on workers’
protection and on food contact materials (1935/2004 (EC)).
3.6.1 The toys’ safety directive
As stated in the Commission ED framework published in 2018 [100
], legislation “on food contact
materials, cosmetics, toys or protecting workers at the workplace, does not contain specific provisions
for endocrine disruptors. However, substances with endocrine disrupting properties are subject to
case-by-case regulatory action on the basis of the general requirements of the legislation.”
The toys directive (2009/48 (EC) mentions (Article 10.2) that “Toys, including the chemicals they
contain, shall not jeopardise the safety or health of users or third parties when they are used as
intended or in a foreseeable way, bearing in mind the behaviour of children.”
38
https://ec.europa.eu/growth/sectors/medical-devices/regulatory-framework_en

Policy Department for Citizens’ Rights and Constitutional Affairs
____________________________________________________________________________________________
68
Article 11(2) deals with labels and the need to “draw the attention of users (..) to the inherent hazards
and risks of harm involved in using the toys, and to the ways of avoiding such hazards and risks.”
The Annex II (chemical properties) of the toys safety directive lists provisions for CMRs of category 1A, 1B
or 2, stating that they “shall not be used in toys, in components of toys or in micro-structurally distinct
parts of toys”. The generic category of EDs is not restricted.
Limit values for chemicals used in toys for children under 36 months or in toys intended to be placed in
the mouth are specified (Annex C); the list includes EDs such as bisphenol A (migration limit of 0.04 mg/l).
The toy directive also refers to the Restriction of Hazardous Substances (ROHS) directive (2011/65/EU),
which as of 2019 will add four ED phthalates at concentrations of up to 1000 ppm to the list: BBP, DEHP,
DIBP, and DBP. The list currently puts restrictions on lead, mercury, cadmium, hexavalent chromium,
polybrominated biphenyls (PBB), PBDEs (authorized below 1000 ppm). Note that PBDEs are known EDs
[187-189]. Other EDs such as PFOS are regulated in the toy directive with reference to the POPs regulation
(EC) (850/2004).
3.6.2 Workers’ protection regulations
Certain work environments are associated with higher exposures to toxic chemicals including EDs, e.g.
hairdressing and nail bar workers, those working in the cleaning sector, and in industries producing
pharmaceutics, plant protection products and biocides. Note that many working in these sectors will be
women of reproductive age.
The lack of consideration of EDs in general in the 1998 workers’ regulation has been recognized in the
2018 Commission ED framework [100]. Note that there is a specific directive on the protection of workers
from carcinogens and mutagens (2004/37/EC).
3.6.3 Food and food contact materials
The food regulation mentions that “The Community has chosen a high level of health protection as
appropriate in the development of food law” (178/2002)
39
. This food directive refers to the precautionary
principle (article 7). It also mentions that “…where there are reasonable grounds to suspect that a food
or feed may present… a risk for human or animal health, then, depending on the nature, seriousness and
extent of that risk, public authorities shall take appropriate steps to inform the general public of the
nature of the risk to health, identifying to the fullest extent possible the food or feed, or type of food or
feed, the risk that it may present, and the measures which are taken or about to be taken to prevent,
reduce or eliminate that risk.” (article 10).
In addition, there is a specific directive on food packaging (EC, 1935/2004), aiming to ensure the effective
functioning of the internal market, and provide the basis for securing a high level of protection of human
health and the interests of consumers. Its principles are that “any material or article intended to come into
contact directly or indirectly with food must be sufficiently inert to preclude substances from being
transferred to food in quantities large enough to endanger human health or to bring about an
unacceptable change in the composition of the food or a deterioration in its organoleptic properties”.
This regulation does not identify any type of health hazard that requires specific consideration.
39
https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32002R0178&from=FR
Endocrine Disruptors: from Scientific Evidence to Human Health Protection
____________________________________________________________________________________________
69
Table 7: Current status of the regulatory provisions defining EDs in various regulatory areas, or allowing
the identification, and existence of explicit management logics. See also Table 1 for a simplified version.
Steps required to manage health risks incurred by EDs
Sector
Definition of ED
Identification tools
ED test requirements*
Risk management logic of EDs
Plant
protection
products
Existing definition
Existing guidance document
Limited
Not authorized
, unless exposure is
negligible.
Biocides
Existing definition
Existing guidance document
Very limited
(requirements of REACH
regulation)
Not authorized
in biocidal products
for the general public. Not authorized
unless risk is negligible, in
particular
…under conditions which aim
at excluding contact with humans and
release into the environment for the
other biocides.
REACH
chemicals
No legally valid
definition
No guidance document on
identification
Very limited
Recognized EDs can be put on
“authorization list” (Annex XIV)
unless threshold can be demonstrated.
Otherwise: “authorized dose” logic.
Cosmetics
None
No generic management logic for
EDs (compound by compound
approach)
Food
additives
Food
contact
material
Drinking
water
Toys
Workers’
regulations
Medical
devices
Refers to the ED
definition used
for biocides
Very limited
(requirements of REACH
regulation)
Limited to a proportion of 0.1%
in
weight in parts of the device in contact
with the body or body fluids.
*Requirement of test allowing to identify EDs. Conditions relative to the identification of a specific compound that is an ED or a suspected
ED are not mentioned here.
Moreover, the commission regulation 10/2011
40
deals specifically with plastic materials and articles
intended to come into contact with food. It stipulates that “monomers, other starting substances and
additives should be risk assessed and authorised before their use in the manufacture of plastic materials
and articles.” This regulation precludes the use of CMR substances without authorization
41
. It does not
mention EDs and therefore does not provide specific provision to protect human health from the hazard
class of EDs present in plastics used as food contact materials. See section 2.2, for publications and data
bases on EDs in food contact materials.
40
https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32011R0010&from=FR
41
Specifically, “…a maximum level of 0.01 mg/kg in food should be established for the migration of a non-authorized substance through a
functional barrier. Substances that are mutagenic, carcinogenic or toxic to reproduction should not be used in food contact materials or
articles without previous authorization and should therefore not be covered by the functional barrier concept.” (regulation 10/2011).

Policy Department for Citizens’ Rights and Constitutional Affairs
____________________________________________________________________________________________
70
3.6.4 Food additives
The food additives regulation (1333/2008/EC) mentions that “Food additives must be safe when used,
there must be a technological need for their use, and their use must not mislead the consumer and must
be of benefit to the consumer.” It also requires decisions regarding food additives to be based on the
precautionary principle. The test requirements are laid down in EFSA scientific opinions relative to food
additives
42
, enzymes and flavours, which do not require application dossiers to contain any test
specifically relevant to EDs.
3.7 Piecewise regulation of specific EDs
3.7.1 “Legacy” persistent chemicals suspected to be EDs (POPs)
Although this ban took place before the birth of the ED concept, one should mention the ban on the
production and sale of various persistent substances considered by the scientific community to be
possible, likely or very likely EDs, as part of the international Stockholm convention on Persistent Organic
Pollutants (POP), ratified by the European Community in 2004. This includes a ban of the production and
use of DDT, PCBs, chlordecone. A polybrominated flame retardant (PBDE) (commercial mixture, c-deca-
BDE) is also restricted. The Stockholm convention proposed a ban on production of octa-BDE and that of
penta-BDE
43
, the bans becoming effective in the EU in 2004 and 1997 respectively. However, the deca-
BDE can break down into the banned penta-BDE and octa-BDE forms, explaining the persistence of PBDEs
in the environment and in the body of most EU citizens [254].
3.7.2 Bisphenol A
In 2011, following a 2010 decision from the French parliament that banned polycarbonate, a polymer
made out of bisphenol A from baby bottles in France, a similar decision has been taken at the scale of the
EU (regulation 321/2011). Bisphenol A has also been banned from food containers for infants and young
children. Regarding the general population, a migration limit of bisphenol A from varnishes or coatings
applied to materials and articles onto food shall not exceed 0.05 mg of bisphenol A per kg of food
(Commission regulation 2018/213).
As of 2020, a maximum concentration of bisphenol A of 0.02% by weight shall be authorized in thermal
papers in the EU, to limit the risk in people handling thermal paper receipts
44
. In toys, where EDs are not
subject to specific restrictions, a migration limit of 0.04 mg/l has been set for bisphenol A
45
. In 2017,
bisphenol A has been recognized as a substance of very high concern (SVHC) for its endocrine disrupting
properties by ECHA. It has not (yet) been added on REACH list of substances subject to authorization
(annex XIV).
3.7.3 Triclosan
Triclosan use cannot be used in the manufacture of plastics intended to come in contact with food. In
cosmetics, it is authorized up to a concentration of 0.3% in soaps and toothpastes, and 0.2% in
mouthwashes.
42
https://efsa.onlinelibrary.wiley.com/doi/abs/10.2903/j.efsa.2009.1188
43
http://chm.pops.int/Implementation/NIPs/Guidance/GuidancefortheinventoryofPBDEs/tabid/3171/Default.aspx
44
https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32016R2235
45
https://eur-lex.europa.eu/legal-content/EN/TXT/?toc=OJ:L:2017:138:TOC&uri=uriserv:OJ.L_.2017.138.01.0128.01.ENG
Endocrine Disruptors: from Scientific Evidence to Human Health Protection
____________________________________________________________________________________________
71
3.7.4 Phthalates, including DEHP
Four phthalates (BBP, DEHP, DIBP and DBP) have been recognized as EDs under REACH and they have
been added in REACH annex XIV. The use of DEHP, DBP and BBP is limited in plasticized materials used in
toys and childcare articles; this limitation is expected to be extended to DIBP, and to consumer products
in general.
3.7.5 Are piecewise regulations of specific EDs efficient? The example of bisphenol A
Taking the example of the case of bisphenol A, exposure is expected to start long before birth, via the
contamination of the foetus by bisphenol A contained in the diet and possibly cosmetics of the mother,
given that bisphenol A readily passes the placental barrier. It is our opinion that certain regulations (e.g.,
reduction of early-life exposure to endocrine disruptors, in the case of the bisphenol A ban from baby
bottles and food containers intended for infants and young children) can be seen as a first regulatory step
for minimizing exposure of susceptible populations. However, given that bisphenol A crosses the
placenta and the authorization of bisphenol A in food and consumer goods used by pregnant women, in
utero exposure is expected. From a public health perspective, given the reported effects of bisphenol A
at very low doses, trying to avoid bisphenol A exposure in the first years of life but not in the prenatal
period does not constitute a coherent and sufficient approach. We further discuss the gaps in the current
regulation in 4.1.
3.8 The Classification Labelling and Packaging (CLP) directive
Before turning to examples of actions on EDs in some Member States, we briefly discuss the important
CLP directive. The CLP directive (regulation (EC) No 1272/2008 on classification, labelling and packaging
of substances and mixtures) aims at ensuring “a high level of protection of human health and the
environment as well as the free movement of chemical substances, mixtures and certain specific articles,
while enhancing competitiveness and innovation” (recital 1); its objective is to define the main classes of
hazards, including physical hazards, health hazards, hazards to the environment, so that these hazards
can be properly identified and communicated. The CLP directive provides definitions for carcinogenic,
mutagenic substances and substances toxic for reproduction (see Table 8), but not for EDs.
The Directive does not apply to sectors such as cosmetics, animal nutrition, animal or human drugs,
medical devices, food or feeding stuffs for humans or animals. It calls on reliance on the Globally
Harmonised System (GHS) of classification and criteria developed internationally in the context of the
United Nations. The responsibility for the identification of hazards of substances and mixtures and for
deciding on their classification lies mainly with manufacturers, importers and downstream users of these
substances or mixtures (…) (Recital 16).
CLP mentions that the resources of the authorities should be focused on substances of the highest
concern with regard to health and the environment, and explicitly lists substances classified for
carcinogenicity, germ cell mutagenicity or reproductive toxicity (categories 1A, 1B or 2), for respiratory
sensitisation or in respect of other effects on a case by case basis (recital 52 and article 36). According to
international developments, the classification and labelling of persistent, bioaccumulative and toxic (PBT)
substances and very persistent very bio-accumulative (vPvB) substances should also be included in the
directive (recital 56), but in no instance are EDs mentioned.

Policy Department for Citizens’ Rights and Constitutional Affairs
____________________________________________________________________________________________
72
3.9 Examples from specific Member States
3.9.1 Denmark
The Danish population and government first became aware of the problems of ED when the first studies
on the temporal decline in sperm concentration in men from the EU and USA, authored by Danish
researchers in the Department led by Professor Niels-Erik Skakkebaek, were published in 1992 [255, 256].
Comprehensive studies later documented an increase in testis cancer in the Danish population; a high
frequency of sub-optimal semen quality in young men and of malformations of the male reproductive
organs (cryptorchidism, hypospadias) [257
]. Since then the Danish government, through its
Environmental Protection Agency has been very active on EDs, increasing the knowledge base on EDs,
setting up screening tests and enforcing regulations. It maintains a list of potential EDs, which currently
lists 432 chemicals
46
. The list categorises them according to their level of evidence. All chemicals classed
within category 1 (which includes many substances that are already prohibited or restricted at the EU
level) are included in the Danish Government’s list of undesirable substances
(in Danish).
3.9.2 France
Public concern led France to be one of the first countries, after Canada, to ban bisphenol A in babies’
bottles in 2010. As of 2015, it also banned bisphenol A from all food contact materials, first in those aimed
at children until the age of three years (a similar decision has now been taken at the EU level) and then in
all food contact materials, irrespective of the user’s age, and thus including women of reproductive age.
The 2019 project of French national strategy on EDs (SNPE2
47
) highlights several actions to be enacted
over the next four years with a view to protecting the population and the environment by reducing
exposure to EDs. Targets include publishing a list of potential EDs by 2021 (classified by level of proof
and/or need for further investigation), better informing the population, in particular through the labelling
of consumer goods, improving knowledge on PE effects on wildlife and reducing environmental
contamination by EDs.
3.9.3 Sweden
In 2015, the Swedish government renewed its four year plan for a “Non-toxic environment”. This plan,
elaborated in collaboration with the Swedish Chemicals agency, refers specifically to reducing chemical
risks in everyday life and has a focus on reproductive and child health, notably in the pre-school
environment. There is also an action plan for highly fluorinated compounds, in which fire-fighting
products are given high priority. As of January 2019, all companies (above a certain yearly turnover) based
in Sweden have to detail information on perfluorinated substances in their notifications to the national
registry and to provide information (by end 2020) on quantities produced or sold. The plan makes specific
mention of the need to develop legislation for EDs within the EU legal framework.
46
https://eng.mst.dk/chemicals/chemicals-in-products/endocrine-disruptors/the-eu-list-of-potential-endocrine-disruptors/
47
http://www.consultations-publiques.developpement-durable.gouv.fr/strategie-nationale-sur-les-perturbateurs-a1916.html (link available
until Feb. 8, 2019)
Endocrine disruptors: from scientific evidence to human health protection
____________________________________________________________________________________________
73
Table 8: List of the main hazards legally recognized in the EU regulation.
Type of hazard
Hazard definition and regulation
Management logic
Physical hazards
Various categories (e.g., explosives), not
detailed*
Not detailed here. (EC No 1272/2008)
Health hazards
Radioactivity
Not detailed here (96/29/Euratom, (EC))
Acute toxicity
Acute toxicity means those adverse effects occurring following oral or dermal administration of a single dose of a substance
or a mixture, or multiple doses given within 24 hours, or an inhalation exposure of 4 hours. (1272/2008)
Carcinogens
A substance or a mixture of substances which induce cancer or increase its incidence. Substances which have induced benign
and malignant tumours in well performed experimental studies on animals are considered also to be presumed or suspected
human carcinogens unless there is strong evidence that the mechanism of tumour formation is not relevant for humans.
Category 1A: known to have carcinogenic potential for humans, classification is largely based on human evidence;
Category 1B: presumed to have carcinogenic potential for humans, classification is largely based on animal evidence.
Category 2: Suspected human carcinogens. ((EC) No 1272/2008)
Banned from plant protection
products, biocides (categories
1A, 1B), cosmetics, toys (levels
1A, 1B, 2).
Subject to authorization for
REACH chemicals
, leading to
specific bans.
Specific regulation for
carcinogens and mutagens in the
occupational setting.
Germ cell mutagenicity
A mutation means a permanent change in the amount or structure of the genetic material in a cell. The term ‘mutation’ applies
both to heritable genetic changes that may be manifested at the phenotypic level and to the underlying DNA modifications
when known (including specific base pair changes and chromosomal translocations). The term ‘mutagenic’ and ‘mutagen’ will
be used for agents giving rise to an increased occurrence of mutations in populations of cells and/or organisms.
Category 1: Substances known to induce heritable mutations or to be regarded as if they induce heritable mutations in the
germ cells of humans.
Substances known to induce heritable mutations in the germ cells of humans. (categories 1A and 1B are defined).
Category 2: Substances which cause concern for humans owing to the possibility that they may induce heritable mutations in
the germ cells of humans.
Reproductive toxicity
Reproductive toxicity includes adverse effects on sexual function and fertility in adult males and females, as well as
developmental toxicity in the offspring.
Category 1A (resp. B, C): Known (presumed, suspected) human reprotoxicant.
((EC) No 1272/2008)
Specific target organ toxicity (STOT) –
single exposure
Specific, non-lethal target organ toxicity arising from a single exposure to a substance or mixture. All significant health effects
that can impair function, both reversible and irreversible, immediate and/or delayed (…). Excludes other above-mentioned
health hazards.
Category 1: Substances that have produced significant toxicity in humans or that, on the basis of evidence from studies in
experimental animals, can be presumed to have the potential to produce significant toxicity in humans following single
exposure
Category 2: Substances that, on the basis of evidence from studies in experimental animals can be presumed to have the
potential to be harmful to human health following single exposure
Category 3: Transient organ effects.
(not detailed)
Specific target organ toxicity (STOT) –
repeated exposure
Similar definitions as STOT-single exposure, replacing “single exposure” by “repeated exposure” and without category 3.
(not detailed)
Policy Department for Citizens’ Rights and Constitutional Affairs
__________________________________________________________________________________________
74
Type of hazard
Hazard definition and regulation
Management logic
Other hazards: Aspiration hazard, skin
corrosion/irritation, serious eye
damage/irritation…
Not detailed here
(not detailed)
Endocrine Disrupting properties that may
cause adverse effects in humans
An active substance, safener or synergist shall be considered as having endocrine disrupting properties that may cause
adverse effect in humans if [...] it is a substance that meets all of the following criteria, unless there is evidence demonstrating
that the adverse effects identified are not relevant to humans:
• it shows an adverse effect in an intact organism or its progeny, which is a change in the morphology, physiology,
growth, development, reproduction or life span of an organism, system or (sub)population that results in an
impairment of functional capacity, an impairment of the capacity to compensate for additional stress or an increase
in susceptibility to other influences;
• it has an endocrine mode of action, i.e. it alters the function(s) of the endocrine system;
• the adverse effect is a consequence of the endocrine mode of action.
No exposure (plant protection
products; biocides
for the
general public); no risk and use in
conditions excluding contact with
humans
and release into the
environment (other biocides);
can be subject to authorization
(REACH). Limit weight of 0.1%
(medical devices).
No general
restriction in other areas (see
Table 7).
Environmental hazards
Endocrine Disrupting properties
that may cause adverse effects in
non-target organisms
An active substance, safener or synergist shall be considered as having endocrine disrupting properties that may cause
adverse effects on non-target organisms if it (…) meets all of the following criteria, unless there is evidence demonstrating
that the adverse effects identified are not relevant at the (sub)population level for non-target organisms:
• it shows an adverse effect in non-target organisms, which is a change in the morphology, physiology, growth,
development, reproduction or life span of an organism, system or (sub)population that results in an impairment of
functional capacity, an impairment of the capacity to compensate for additional stress or an increase in sus-
ceptibility to other influences;
• (2) it has an endocrine mode of action, i.e. it alters the function(s) of the endocrine system;
• (3) the adverse effect is a consequence of the endocrine mode of action.
Hazardous to the aquatic
environment
Hazardous to the Aquatic Environment is differentiated into acute aquatic toxicity and chronic aquatic toxicity.
Acute aquatic toxicity means the intrinsic property of a substance to be injurious to an organism in a short-term exposure to
that substance.
Chronic aquatic toxicity means the intrinsic property of a substance to cause adverse effects to aquatic organisms during
exposures which are determined in relation to the life-cycle of the organism.
(not detailed)
Hazardous to the ozone layer
Not detailed here
(not detailed)
Very persistent, and very
bioaccumulative (vPvB) substances
A substance that fulfils the persistence and bioaccumulation criteria below shall be considered to be a vPvB substance.
Persistence - A substance fulfils the ‘very persistent’ criterion (vP) in any of the following situations:
(a) the degradation half-life in marine, fresh or estuarine water is higher than 60 days;
(b) the degradation half-life in marine, fresh or estuarine water sediment is higher than 180 days;
(c) the degradation half-life in soil is higher than 180 days.
Bioaccumulation - A substance fulfils the ‘very bioaccumulative’ criterion (vB) when the bioconcentration factor in aquatic
species is higher than 5,000. (REACH regulation)

Endocrine disruptors: from scientific evidence to human health protection
____________________________________________________________________________________________
75
Type of hazard
Hazard definition and regulation
Management logic
Health and environmental
hazards
Persistent, bioaccumulative and toxic
(PBT) substances
A substance that fulfils the persistence, bioaccumulation and toxicity criteria below shall be considered to be a PBT
substance.
Persistence - A substance fulfils the persistence criterion (P) in any of the following situations:
(a) the degradation half-life in marine water is higher than 60 days;
(b) the degradation half-life in fresh or estuarine water is higher than 40 days;
(c) the degradation half-life in marine sediment is higher than 180 days;
(d) the degradation half-life in fresh or estuarine water sediment is higher than 120 days;
(e) the degradation half-life in soil is higher than 120 days.
Bioaccumulation -A substance fulfils the bioaccumulation criterion when the bioconcentration factor in aquatic species is
higher than 2 000.
Toxicity - A substance fulfils the toxicity criterion (T) in any of the following situations:
(a) the long-term no-observed effect concentration (NOEC) or EC10 for marine or freshwater organisms is less than 0,01
mg/l;
(b) the substance meets the criteria for classification as carcinogenic (category 1A or 1B), germ cell mutagenic (category 1A
or 1B), or toxic for reproduction (category 1A, 1B, or 2);
(c) there is other evidence of chronic toxicity, as identified by the substance meeting the criteria for classification: specific
target organ toxicity after repeated exposure (STOT RE category 1 or 2). (REACH regulation)

Policy Department for Citizens’ Rights and Constitutional Affairs
__________________________________________________________________________________________
76
PART C: MANAGING THE RISK INCURRED BY EDs –
WHAT COULD BE FURTHER DONE IN THE EU?

Endocrine disruptors: from scientific evidence to human health protection
____________________________________________________________________________________________
77
4 IDENTIFICATION OF ENDOCRINE DISRUPTORS
KEY FINDINGS
•
Currently for EDs, the EU regulation lacks coherence, both within sectors and between
sectors. It ignores several key scientific facts, including the widespread exposure to known
and suspected EDs in the European population. It is therefore inefficient to protect human
health.
• The general objective of the minimization of ED exposure and body burden in the EU
population is a pertinent central goal of the EU ED regulation. Such an aim has been put
forward by the 2018 Commission’s communication towards a comprehensive EU
framework on ED and is coherent with the 7
th
Environmental Action Programme.
• Attaining this goal requires a) a cross-sectorial definition of EDs (currently it only exists for
plant protection products and biocides); b) a guidance document (further to that for plant
protection products and biocides) explaining how to apply the definition on the basis of
tests results and scientific literature to identify EDs; c) tests covering all ED modalities; d)
legal requirements to make these tests compulsory in application dossiers; e) a
management logic, which may distinguish sectors with very likely human exposure from
those for which exposure is not systematic.
• Test development: There is an urgent need, not only to accelerate test development and
validation, especially in areas beyond E, A, T, S (which are currently insufficiently covered,
in particular for the thyroid axis), but also for regulators to use academic publications when
assessing ED properties.
• Test requirements: As noted in the previous chapter, the regulatory texts setting out the
content of application dossiers generally do not require tests that would allow to assess
scientifically if the substance under evaluation is an ED. A logical and essential step would
be to modify all regulations setting test requirements in sectors for which specific
conditions apply to EDs, and include provisions allowing to make sure that dossiers will
contain test results allowing to conclude if the evaluated substance or product is an ED.
• Research: In addition to the needs related to test development, six research areas should
be considered as priorities: (i) epigenetics; (ii) effects across generations; (iii) ED effects on
the microbiome, (iv) Green (safe) chemistry; (v) ED modalities beyond E, A, T, S and
metabolism, (vi) Characterization of dose-response functions for ED effects in humans.
4.1 Gaps in the current regulation and general considerations regarding possible
improvements
4.1.1 The regulation does not allow minimizing the health risks incurred by EDs
The review of compounds with suspected or very likely ED properties (chapter 2) has highlighted that
recognized or suspected EDs are found in all media (diet, air, water), sectors (plant protection products,
biocides, drugs, food, cosmetics, REACH chemicals…), in the general population as well as in
occupational settings. The production, importation and marketing of several EDs have been forbidden in
the past, mainly for persistent EDs such as DDT or PCBs, but precisely due to their persistence, the
contamination of the diet and of the bodies of EU citizens is still widespread more than thirty years after
their ban ([42] and references therein). The use of DES in pregnant women was banned over 40 years ago;
Policy Department for Citizens’ Rights and Constitutional Affairs
__________________________________________________________________________________________
78
DES is not a persistent compound and is quickly eliminated by the body. However, due to its long-term
effects in the subjects exposed in utero and in their offspring, today many “DES daughters” and DES
“grand-children” suffer from the use of the drug [15, 16]. This illustrates how crucial the step of
identification of hazards before the marketing of substances is, and that, however important, any system
of identification of hazards of already marketed substances shall be seen as a second line of defence. As
outlined in the 7
th
Environmental Action Programme, efficient identification of hazardous substances
before their being put on the market should be the main approach, in particular in the context of possible
irreparable health effects, limited means for post-authorization controls and complex compensation
judiciary procedures.
In addition, many substances still authorized today represent a risk for the EU population and the
environment because of their endocrine-disrupting properties.
As can be seen from Table 2 (section 1.8), some potential EDs are also possibly carcinogenic or toxic for
the reproduction, so that they would also belong to the CMR hazard classes. However, this does not imply
that a specific regulation for the ED hazard category is superfluous; indeed, first, the regulation of CMR
substances in the EU does not always provide a relevant protection of the health of population, as can be
seen from the ongoing population impacts of recognized carcinogenic substances such as particulate
matter or benzene [258]; second, more than half of the substances currently recognized as substances of
very high concern because of their endocrine-disrupting properties as part of REACH regulation are not
simultaneously recognised as carcinogen, mutagen or toxic for the reproduction.
Finally, “safe thresholds” are unlikely to generally exist for EDs. Current regulatory tests may identify
experimental thresholds for EDs, but these are expected to represent the limit of sensitivity of the test
rather than constitute a biological threshold valid in human populations in their diversity (see 2.9 above).
4.1.2 The heterogeneous regulation of EDs in different sectors is hard to justify scientifically
Up to now, EDs have generally been managed heterogeneously. For instance, the EU has banned
bisphenol A from food containers for infants and young children with the aim of reducing the exposure
of this sensitive population. However, since bisphenol A crosses the placenta, exposure to bisphenol A
starts at conception, nine months or more before any exposure through postnatal diet may occur, e.g. via
the contamination of the diet of the pregnant woman or of consumer products used by her. In this
context, the regulation that applies is the tolerable daily intake of bisphenol A, currently set at 4 µg/kg
body weight per day, which assumes a lack of effect of bisphenol A in animal experiments below 600
µg/kg body weight per day. This tolerable daily intake is most probably too high to guarantee protection
of health, given that many studies documented effects of bisphenol A on sensitive endpoints in the 5-10
µg/kg body weight per day range [119, 120], a range of doses where currently internationally agreed tests
used in application dossiers for authorization are apparently not sensitive enough to detect adverse
effects of bisphenol A.
Another example is that EDs are banned in plant protection products and biocides, but not generically
banned in cosmetics. Their presence in cosmetics can entail exposure of pregnant women and foetuses.
4.1.3 Even within specific sectors, management of EDs generally lacks coherence
The regulatory sector with the most advanced regulation on EDs is that of pesticides; here, a management
logic (no exposure to pesticides containing EDs) has been put forward as early as 2009 (PPPR); scientific
criteria defining EDs have been set (with validity as of 2018) and a guidance document explaining how to
apply these criteria on the basis of test results has been developed by the relevant agencies in 2018 [253],
quickly after the scientific criteria were made available. However, it is unclear that this is enough for the
Endocrine disruptors: from scientific evidence to human health protection
____________________________________________________________________________________________
79
spirit of the law to be satisfied. Specifically, given that the specific regulations listing the tests required in
pesticides application dossiers for authorization do not make all the tests listed in the 2018 guidance
document compulsory, it will be difficult to assess if the substance is an ED (see 3.2.4). This means that a
key element of the chain required for the regulatory logic set out in the 2009 PPPR regulation to be
applied is missing (see 3.2 for details).
The situation is equivalent or worse in other key sectors mentioning EDs, such as REACH chemicals or
cosmetics (see Table 7 for an overview). In several sectors with high potential for widespread human
exposure, the hazard category of EDs is not even considered: this includes food additives, food contact
materials, toys and workers’ protection.
There are surely historical or political reasons for this lack of coherence, but the situation seems hard to
justify from scientific and public health standpoints, especially when considering the core principles of
the EU such as the Precautionary principle and the 7
th
Environment Action Programme.
4.1.4 In the past, important decisions have been delayed by lack of proper application of
policies on conflicts of interest
In several instances, important regulatory decisions have been postponed or modified by what had been
described as a “scientific debate”, but that appears to be more similar to what the environmental
humanities literature [259] refers to as “manufactured doubts”, most often by scientists with probable
conflicts of interests. This is in particular the case of the postponement of the publication of draft criteria
defining substances with endocrine disrupting properties prepared by DG Environment in 2013, as
requested by the plant protection products regulation of 2009. This planned publication has been
cancelled following several events, including an open letter to the Chief scientific advisor of the President
of the European Commission, that was later published in an editorial in 14 scientific journals [260]. Many
of the authors were the editors of the journals where the editorial was published and turned out to have
conflicts of interest (such as support from industries producing substances likely to be EDs), which were
not reported. Similarly, on several occasions, scientists were invited to talk about EDs in EU institutions
without being asked to report any potential conflict of interest. It has been scientifically demonstrated in
several areas that scientists with potential conflicts of interest or studies on environmental factors
supported by actors who may benefit from the use of the factor are far less likely to conclude about the
existence of a hazard link to this factor than independent studies [261]. See also Annex 3 page 102.
It is striking to note that, during meetings, hearing or debates organized by or in EU institutions such as
the European Parliament or the European Commission, scientists invited to talk are not always obliged to
fill in conflicts of interest forms nor to declare any potential conflict of interest at the start of their talks or
statements. This would not be the case in similar institutions in other parts of the world, such as the USA.
4.1.5 Translating scientific knowledge into regulation
The key scientific facts (see chapters 1 and 2) and regulation of EDs in the EU reviewed in this report
demonstrate a need for regulation to be more coherent and consistent with scientific knowledge,
thereby facilitating the overarching aim of minimizing the health impacts of EDs among EU citizens (as
set out in the 7
th
Environment Action Programme and reiterated in the Commission communication
towards a comprehensive EU framework on ED [100]). We suggest this aim to be made concrete by
explicitly setting the general objective of the minimization of ED exposure and body burden in the
EU population as a central goal of the EU regulation.
Several regulatory decisions, listed below, should trickle down from this aim:
Policy Department for Citizens’ Rights and Constitutional Affairs
__________________________________________________________________________________________
80
a) The recognition of endocrine disruption as a hazard of similar concern to that of CMR
(carcinogens, mutagens, toxics for reproduction) substances;
b) The requirement to avoid or minimize exposure to (known or presumed) EDs.
Point a) is a principle of REACH regulation, in the context of identifying substances of very high concern
requiring authorization, while b) is explicitly written in the PPPR and currently only applies to plant
protection products and biocides recognized as EDs, and should be extended to sectors where consumer
exposure is most expected, i.e. on cosmetics, food additives, food contact materials and toys.
c) To ensure there is a unique (cross-sectorial) definition of EDs, distinguishing known EDs (level
1A), presumed EDs (1B) and suspected EDs (2). This definition could be written down in the CLP
regulation, which defines the main categories of health hazards (see Table 8);
d) To provide a guidance document explaining how to check if a substance or a mixture is an ED on
the basis of the scientific definition and of test results; ideally, such a document should also be
cross-sectorial, but sector specific provisions are to be expected (e.g., in the case of cosmetics, for
which animal testing is banned);
e) To make sure that all tests required in the guidance document are made compulsory for any
application dossier for authorization, so as to facilitate ED identification;
f) In the case of sector-specific assessment, to ensure that the recognition of a substance as an ED
in a sector automatically entails its recognition as an ED with the same level of evidence in all
other sectors;
g) To extend the “no exposure to EDs” logic present in the pesticides regulations to all other use
sectors for which human exposure is likely, in particular for industrial REACH chemicals with
consumer uses, cosmetics, food additives, food packaging, toys, with the exception of drugs for
which a specific logic is required.
Indeed, given the scientific knowledge on specific actions of EDs (low dose effects, possible non-
monotonic dose responses, cumulative effects often expected from combined exposure and vulnerable
periods of exposure) that it is unlikely that safe levels can be set.
h) To define a management logic for EDs in all media-oriented regulations (such as the water
directive, food contact materials) following the principle of minimization of exposure to EDs
outlined for all sectors for which human exposure is very likely (see 5.2 below);
i) To inform EU citizens about the presence of recognized or suspected EDs in all types of consumer
goods (in particular through labelling) and possibly environmental media;
j) To ensure that any potential conflict of interest is declared by anyone communicating with an EU
institution.
The issues related to the management of EDs are discussed at greater length in chapter 5; we discuss in
this chapter those related to the identification of EDs.
Endocrine disruptors: from scientific evidence to human health protection
____________________________________________________________________________________________
81
4.2 Towards a cross-sectorial definition of EDs
4.2.1 Assessment of the approved criteria for ED Identification under EU Regulations on Plant
Protection Products and on Biocidal Products
In November 2017 and April 2018, the European Commission published regulations setting out scientific
criteria for the determination of endocrine-disrupting properties in the areas of plant protection products
and biocides [251, 252].
These publications result from provisions of the 2009 Plant Protection Product Regulation (PPPR) and the
2012 Biocide Products Regulation (BPR) that specified provisions regarding the way plant protection
products and biocides containing substances with endocrine-disrupting properties should be managed,
without explaining how such substances should be defined and identified. The PPPR and BPR stipulated
that “No later than 13 December 2013, the Commission shall adopt delegated acts in accordance with
Article 83 specifying scientific criteria for the determination of endocrine-disrupting properties” [262].
With four years of delay, these criteria were published as a Commission Delegated Regulation for the BPR
in November 2017 [251] and a Commission regulation in April 2018 for the PPPR [252]. In 2018, ECHA,
EFSA and the JRC provided a guidance document [253] describing how to perform hazard identification
for endocrine-disrupting properties following the scientific criteria adopted in 2017 (this guidance
document is discussed in 4.3.1 below).
The ED criteria for plant protection products and biocides is a document containing two sections. Our
short discussion specifically refers to the text agreed for the PPPR.
In accordance with the PPPR and BPR, two definitions are actually provided, one for substances with
endocrine disrupting properties that may cause adverse effects in humans (see 3.2.2 p.59) and one for
substances with endocrine disrupting properties that may cause adverse effects on non-target organisms.
Although the recitals of the ED criteria acknowledge the need to identification of known and presumed
EDs [251, 252], no categories of substances with endocrine disrupting properties distinguished according
to the level of evidence are defined.
The criteria acknowledge the relevance of WHO definition of EDs (see 1.2). The criteria still deviate to some
extent from this definition, e.g. by referring to the concept of “endocrine mode of action”.
The criteria require the adverse effect to be a consequence of the endocrine mode of action, which reads
as a very strong requirement. However, it is indicated below this statement that “the link between the
adverse effect(s) and the endocrine mode of action shall be established based on biological plausibility”,
which seems more relevant.
When it comes to the assessment of the existing evidence to determine if the criteria are fulfilled, the text
calls for reliance on a weight of evidence approach, which is relevant to avoid selective consideration of
a part of the literature (e.g., lack of consideration of academic studies).
The criteria further call for reliance on the concept of “limit dose”. This should not be used by applicants
to limit testing to “low” doses, as focusing only on very low doses may require increased numbers of
animals, in order to have sufficient statistical power (see 2.9 and Figure 13).
4.2.2 Definition of EDs
We suggest that a definition of EDs be adopted as part of the EU regulation, with validity in all sectors.
This definition could be written down in the CLP regulation, that already defines CMR substances and
other hazards.
Policy Department for Citizens’ Rights and Constitutional Affairs
__________________________________________________________________________________________
82
A simple option would be to use the widely accepted WHO definition of EDs.
An alternative would be to rely on the phrasing in use for plant protection products and biocides [251],
with the following adaption that relies on the interpretation provided in section 3 of ECHA-EFSA guidance
document [253]:
A substance or mixture shall be considered as an endocrine disruptor if it meets all of the following
criteria:
(1) it shows an adverse effect in an intact organism or its progeny or in (sub)populations, which is
a change in the morphology, physiology, growth, development, reproduction or life span of
an organism, system or (sub)population that results in an impairment of functional capacity,
an impairment of the capacity to compensate for additional stress or an increase in
susceptibility to other influences;
(2) it shows endocrine activity;
(3) there is a biologically plausible link between the adverse effect and the endocrine activity.
Point (1) is from the biocide ED criteria [251]; point (2) is inspired from ECHA-EFSA guidance document
[253], which rightly suggests to delete the reference to the concept of mode of action before referring to
endocrine activity. Point (3) is taken from ECHA-EFSA guidance document [253] without repeating the
explanation “i.e. it has the potential to alter the function(s) of the endocrine system”.
Before moving to the issue of the identification of EDs (see 4.3), we discuss the relevance of additional
definitions for substances for which the level of scientific evidence is lower.
4.2.3 Definition of presumed and suspected EDs
From a scientific perspective, it is common and relevant in environmental and health sciences to
categorize the level of evidence regarding an effect or a phenomenon. For example, IARC, the
International Agency for Research on Cancer, classifies possible carcinogens in four categories (plus a
category corresponding to substances that are probably not carcinogenic), while in the EU, there are
three categories of carcinogens (see Table 9).
It would therefore be relevant to define presumed and suspected EDs. We make the following suggestions:
Presumed endocrine disruptor:
A presumed endocrine disruptor is an exogenous substance or mixture that possesses properties
that are likely to lead to endocrine disruption in an intact organism or its progeny, or
(sub)populations.
Suspected endocrine disruptor:
A suspected endocrine disruptor is an exogenous substance or mixture that possesses properties
that might lead to endocrine disruption in an intact organism or its progeny, or (sub)populations.
Note that this latter definition (suspected EDs) corresponds to the definition of a potential endocrine
disruptor from WHO [9] and from the Scientific Committee on Consumer Safety SCCS (SCCS/1544/14),
but we suggest to use suspected ED instead of potential for consistency with the EU categories of
carcinogens.
Endocrine disruptors: from scientific evidence to human health protection
____________________________________________________________________________________________
83
Table 9: Suggested hazard categories and criteria for endocrine disruptors and parallel with carcinogens.
Hazard
category Criteria for endocrine disruptors Criteria for carcinogens*
1A
Known endocrine disruptors
Substances known to have endocrine-
disrupting properties
, i.e.
Showing an adverse effect in an intact organism or its
progeny or (sub) populations;
Showing endocrine activity;
there is a biologically plausible link between the adverse
effect and the endocrine activity
Known carcinogens
Substances known to have carcinogenic
potential for humans
A substance or a mixture of substances which induce
cancer or increase its incidence. Substances which
have induced benign and malignant tumours in well
performed experimental studies on animals are
considered also to be presumed or suspected human
carcinogens unless there is strong evidence that the
mechanism of tumour formation is not relevant for
humans.
Category 1A: Classification is largely based on
human evidence.
1B
Presumed endocrine disruptors
Substances presumed to have endocrine-
disrupting properties (lower level of
evidence than 1A)
Presumed carcinogens
Substances presumed
to have carcinogenic
potential for humans (lower level of
evidence than 1A)
2
Suspected endocrine disruptors
Substances suspected to have endocrine-
disrupting properties (lower level of
evidence than 1B)
Suspected human carcinogens
Substances suspected
to have carcinogenic
potential for humans (lower level of
evidence than 1B)
*Taken from CLP regulation.
4.3 A guidance document is needed to explain how test results and literature should
be used to apply the ED definition
A guidance document based on the criteria defined in Regulations (EU) 2017/2100 and Commission
Regulation (EU) 2018/605 for biocidal products and plant protection products, respectively, was
published in 2018 [253]. We discuss this document and then explain why and how a guidance document
covering most or all sectors will be needed once a cross-sectorial definition of EDs has been adopted.
4.3.1 Assessment of the EU’s Guidance document for the implementation of the New Criteria
on Endocrine Disruptors for Plant protection products and Biocides
In the authors’ opinion, ECHA, EFSA and the JRC are to be commended for their compiling of this
Guidance document. The guidance document provided (in its section 3) interpretations of the original
ED criteria, which are relevant and that we suggest to use in any future (cross-sectorial) definition of EDs
(see 4.2.1 above).
There are many excellent aspects to the document, if it is used and applied correctly. Most problems can
be expected to arise from data gaps in testing and testing requirements (which are beyond the scope of
the guidance document). It should be noted that the ED criteria state that the identification should be
done mostly on the basis of existing data. This means that there is very little encouragement to generate
new data, which could represent a major drawback. The identification of ED properties cannot be done
on the basis of existing data for most chemicals, not even with systematic review methodology. Different
national regulatory bodies have already underlined this fact. The Danish EPA and their French equivalent
(ANSES) noted that failures to identify EDs most often arose from incomplete files due to unclear testing
requirements.
Policy Department for Citizens’ Rights and Constitutional Affairs
__________________________________________________________________________________________
84
Further points need to be made:
(i) That the guidance document is currently restricted to Oestrogen, Androgen, Thyroid and Steroids
(E, A, T, S or EATS). However, it should be noted that thyroid disruption is the focus of an appendix
(Appendix 1). This reflects the difficulty of assessing not only thyroid disruption but also the
potential effects of brain development that can ensue from disruption of thyroid signalling. That
the main text focuses on steroid hormones is understandable given that current data and research
are largely focused on these areas and that the discipline first evolved from work on reproduction.
However there, as research progresses in non-EAS fields, results will need consideration and
incorporation. There are 48 nuclear receptors in humans, many of which are poorly investigated.
Progress can be expected on each.
(ii) Certain recommendations are highlighted (boxed) in the text (e.g. pp 20, 37, 40 and 42). Notably,
on page 40, the box highlights the need to consider biological plausibility in the analysis,
potentially obviating the need for identifying with certainty a mode of action, which is relevant as
already mentioned.
(iii) Appendix 1 (Additional consideration on how to assess potential thyroid disruption for human
health) is a critical and integral part of the document. Given the increase in neurodevelopmental
disease and even taking different multifactorial drivers into account, the fact that maternal thyroid
hormone is determinant of the foetus’ brain development and the child’s IQ is established [28].
Thus, a number of recommendations in the Appendix need to be underlined. Page 102 states that
even though thyroid signalling is conserved across vertebrates, rats and mice (the most
commonly used toxicological models) do display certain species-specific differences, so the
document emphasises the need to interpret data by applying three recommendations including
the idea that in the absence of specific proof to the contrary “humans and rodents are considered
to be equally sensitive to thyroid disruption”. Similarly, the section referring (page 103) to hazard
assessment of decreases in T
4
(thyroid hormone) in adults, “in the absence of adverse histological
changes should act as a trigger for further studies”.
Given the recommendations on developing new tests (see 4.5), there will be a need to revise the current
guidance document for plant protection products and biocides regularly.
4.3.2 A cross-sectorial guidance document will be needed once a cross-sectorial definition of
EDs is enforced
In general, subsequent requirements to update and adapt this document will be required on a number
of fronts:
- On non-EATS mechanisms
- For applications to other regulatory scenarios. This will be particularly difficult for cosmetics
where animal testing is not permitted. Adverse effects are most often identified in animal tests
(and human epidemiology). Given that adverse effects need to be demonstrated and related to
the endocrine mode of action for EDs, in cosmetics this conundrum needs to be resolved. One
approach is to use free-living embryos at the in vitro / in vivo interface. This approach is exploited
in one of the 8 research proposals currently funded in the ED screening call (see 4.5).
Once definitions of known EDs, presumed and suspected EDs have been published, the guidance
document should be updated and expanded to cover all regulatory sectors. Specific issues will need to
be considered, such as the identification of EDs for substances only used in cosmetics, a sector in which
animal testing is not permitted.
Endocrine disruptors: from scientific evidence to human health protection
____________________________________________________________________________________________
85
4.4 Setting and enforcing coherent test requirements
One of the key conclusions of the description of the current EU regulation regarding EDs is the lack of
coherence between and also within sectors (see chapter 3). In particular, as already mentioned, even for
sectors where some management logic specific to EDs is specified by the regulation (plant protection
products, biocides, REACH chemicals), it appears that the regulatory texts setting out the content of
application dossiers generally do not require tests that would allow to scientifically assess if the substance
under evaluation is an ED.
It is our perception that, in many cases, in particular in the situation of the re-evaluation of a substance
that is already on the market, even if additional information are requested to the applicant, not having
the information allowing to assess if a substance is an ED will, at least in the short term, have it handled
as a substance that is not an ED (that is, unclassifiable substances will, at least for some duration, be
handled as non-hazardous substances, which will result in increased population exposure and possible
health impacts if the substance turns out to be hazardous).
A logical and essential step would be to modify all regulations setting test requirements in sectors for
which specific conditions apply to EDs, and include provisions allowing to make sure that dossiers will
contain test results allowing to conclude if the evaluated substance or product is an ED. Of course,
evaluation dossier from any sector need to be easily made available to assessors from all other sectors.
If EDs become regulated in additional sectors (see chapter 5), then as the regulations setting up the
management logic of EDs, the specific directives listing the test requirements for the authorization of
products or substances in this sector should be updated to make sure that all application dossiers contain
the information allowing to classify the substance in one of the ED categories. In particular, the specific
regulations listing test requirements for REACH chemicals, cosmetics, food additives and packaging, toys,
should be expanded to take (more) efficient consideration of EDs and allow their identification in each
sector. Identification of a substance in one sector should trigger automatically its recognition as an ED in
all other sectors.
Finally, there should be controls on application dossiers to identify misconducts in the preparation of
dossiers (e.g., lack of reporting of a test that had turned out positive, selective reporting of test results or
falsification of test results) and sanctions should be planned and implemented to discourage such
misconduct.
The test requirements should be defined by ECHA, EFSA and any other relevant authority keeping in mind
the above-mentioned issues and making sure that all ED modalities and all potential ED endpoints
(including those related to alterations of brain development) are well covered.
4.5 Test development
There are many identified ED modalities (i.e., ways whereby a substance can alter the activity of the
endocrine system), including the endocrine, androgen, thyroid, steroid (E, A, T, S) modalities, as well as
modalities related to other nuclear receptors (see chapter 1). Currently, internationally validated
guidelines related to EDs in the context of mammalian toxicology focus on oestrogen, androgen,
aromatase activity and, to a lesser extent, thyroid modalities. These tests are not perfectly sensitive and
some provide variable results with variations in protocols within the guidelines (e.g., depending on the
animal model used or route of exposure [250]). As mentioned, in the case of identification of oestrogenic

Policy Department for Citizens’ Rights and Constitutional Affairs
__________________________________________________________________________________________
86
activity, the test relies on changes in uterine weight of exposed animals, while oestrogenic effects can
occur in the absence of modification of uterine weight [249].
Thus, better and more efficient test methods are required, especially in a number of under-investigated
areas, including thyroid disruption and disruption of hormone synthesis and metabolism, beyond
aromatase activity.
The lack of specific tests for thyroid and related neurodevelopmental adverse effects led to a series of
OECD scoping meetings on the topic, resulting in two reports in 2014 and 2017
48
. These OECD reports
focused on in vitro and ex vivo (cell-based screens) that could eventually be used for high throughput
assays. In 2017/18, DG Environment spearheaded a thyroid Expert Group focused on improving
endpoints in the currently validated OECD in vivo assays. The meetings underlined the urgency of the
need for better tests (and validation of those tests) to assess the multiple levels through which EDs could
affect thyroid signalling and brain development. Many of the conclusions of the thyroid expert group are
reiterated in the thyroid appendix of ECHA/EFSA Guidance document [253].
In 2017-2018 the EU Commission launched a research call (SC1-BHC-27-2018: New testing and screening
methods to identify endocrine disrupting chemicals). Researchers were asked to develop novel, faster and
more effective screening methods using both in vivo, in vitro and in silico approaches. Proposals were
invited to focus on the “most urgent regulatory needs” in five main areas: thyroid disruption,
developmental neurotoxicity, female reproduction, metabolism and non-genomic carcinogenicity. The
eight successful projects started in early 2019 for five years. Further, it was specified that the tests
developed in the proposals should be taken through to validation by the OECD.
However, the process of test guideline validation by the OECD is long, often being in the order of ten
years. Most often it involves a succession of ring tests, i.e. a series of tests on a selected number of active
and inactive controls (at different doses) carried out in independent or industrial (often industry contract
laboratories) in different OECD countries. The results are subjected to statistical analysis and then the
process repeated up to three times, often extending the number of substances tested. One of the main
problems is that the country proposing the test has to find the financial and infrastructural resources to
carry out the tests, which in the current economic climate can be challenging. France for instance, being
aware of this problem, has proposed a national centre for ED testing and validation in its ED strategy on
EDs.
Hence, there is an urgent need, not only to accelerate test development and validation, especially in areas
beyond E, A, T, S (which are currently insufficiently covered, in particular for the thyroid axis), but also for
regulators to use academic publications when assessing ED properties as clearly stated in the ECHA-EFSA
Guidance Document [253].
A full guide to the tests currently validated by the OECD is given in their recently updated document [98];
see also Table 5.
48
http://www.oecd.org/publications/new-scoping-document-on-in-vitro-and-ex-vivo-assays-for-the-identification-of-modulators-of-
thyroid-hormone-signalling-9789264274716-en.htm
https://doi.org/10.2779/921523

Endocrine disruptors: from scientific evidence to human health protection
____________________________________________________________________________________________
87
4.6 Surveillance of ED production, use and exposure
Monitoring of suspected and proven EDs and of hazardous substances can generally be done at different
levels, from their production, to the environment and the human body (Figure 16).
Figure 16: Schematic fate of man-made chemicals from their production site to the human body
illustrating the possible different levels of monitoring of chemicals.
4.6.1 Monitoring of ED production and use
Monitoring of production and use of EDs and suspected EDs is of central importance. The implementation
of REACH regulation has provided an estimate of the number of chemicals currently marketed in the EU,
outside specific sectors such as plant protection products, biocides or cosmetics. Currently, about 22,000
compounds have been registered by REACH, but other substances still lack information for registration
49
.
Overall, the EU institutions lack detailed information on the volume of chemicals produced or used,
including proven or suspected EDs or CMRs. Such information should be collected and made available
by the relevant industry and activity sectors, with a fine level of detail (that is, on a compound by
compound basis, on a time scale of a year or less, and at a spatial scale finer than the country). For
example, datasets on pesticides production, sales and use, distinguishing between each pesticide and at
a fine spatial scale regarding the use, should be developed and made available (such databases exist, e.g.
in California
50
).
4.6.2 Environmental monitoring of EDs
Environmental monitoring of selected EDs should also be performed, in a harmonized way at the EU scale.
This should include monitoring of drinking water, which is currently conducted but without targeting
EDs specifically and without data being centralized, standardized, cleaned and made accessible at the EU
level. It may not be relevant or possible to assess a large number of EDs on a regular basis in all water
networks, so that the monitoring of the overall oestrogenic, androgenic, thyroid… activities could be
monitored, instead of providing a list of substances.
4.6.3 Human biomonitoring of EDs and hazardous chemicals
Crucially, human biomonitoring should be conducted at the EU level for a large list of hazardous or
possibly hazardous substances, including EDs. This information is an essential complement of the former
approaches since the levels of chemicals in body fluids, such as urine or blood, constitute an integrated
49
https://echa.europa.eu/fr/registration-statistics-infograph# and
https://echa.europa.eu/documents/10162/13628/evaluation_report_recommendations_2017_en.pdf/c2cb9cd3-e2b5-662a-c359-
c5d998444853
50
https://calpip.cdpr.ca.gov/main.cfm

Policy Department for Citizens’ Rights and Constitutional Affairs
__________________________________________________________________________________________
88
measure of exposures from various environmental compartments (drinking water, diet, air, the
occupational setting…). Standardized approaches, both in terms of recruitment of the population and
analytical approaches, should be used, taking example from NHANES, the biomonitoring survey
conducted in the USA by the American Centers for Disease Controls
51
and drawing all required lessons
from attempts to conduct harmonized biomonitoring in the EU, such as the current HBM4EU H2020
project
52
. The volume of biospecimens collected in each subject and sensitivity of the analytical methods
used should allow simultaneous assessment of a large number of chemicals in order to characterize
combined exposure. A fraction of each sample should be kept frozen to allow future assessment of new
substances as knowledge evolves.
4.7 Consideration of potential conflicts of interests in events organised with
scientists in EU institutions
Any scientist invited to present his opinions in an oral or written form in an EU institution or to a member
of such an institution should declare any potential conflict of interest. This is not intended to discourage
scientists working for the industry to bring in their expertise, but aims at allowing to better distinguish
true scientific controversies from disagreements between academic and regulatory science, or academic
science and industry. This would be merely the translation at the level of the highest institutions of rules
that now apply to any scientist working or bringing expertise to an EU agency. In practice, this would
imply to request to fill in conflicts of interest form before any meeting, to start any scientific presentation
by a statement of the potential conflicts of interest, or a lack thereof, and to mention any potential conflict
of interest in reports or documents sent to an EU institution.
4.8 Key research initiatives to be supported
In addition to the needs related to test development, five research areas should be considered as
priorities: (i) epigenetics; (ii) effects across generations; (iii) ED effects on the microbiome, (iv) Green (safe)
chemistry; (v) novel ED modalities and (vi) characterization of dose-response functions for ED effects in
humans.
4.8.1 Epigenetics
Many EDs act through modulation of epigenetic processes. Epigenetics means literally “above the gene”,
i.e. changing when and where genes are expressed in different tissues without modifying the sequence
of DNA. Epigenetic effects most often implicated either DNA methylation or changes in chromatin
structure (chromatin being the proteins surrounding the actual DNA sequence) and can be referred to as
nongenomic.
Epigenetic effects can be limited to a given cell or maintained as the cell divides and differentiates, thus,
nongenomic or epigenetic effects are to be expected in situations where a foetus, child or adult is
exposed. Similarly, in the case of exposure during pregnancy the foetal sperm or eggs cells can also
undergo epigenetic changes that will result in adverse effects in the grandchildren of exposed mothers.
Epigenetic regulatory mechanisms characterise many nuclear receptor responses, including the most
studied, the E, A, T, S. A key example can be found in thyroid hormone action. Besides its role in human
brain development, thyroid hormone also orchestrates transformation of a tadpole into a frog. Tadpoles
and frogs share the same genome, the same genetic information, but the way the information is
expressed is different in the frog and the tadpole, giving them a different phenotype (morphology and
51
https://www.cdc.gov/exposurereport/index.html
52
https://www.hbm4eu.eu/the-project/
Endocrine disruptors: from scientific evidence to human health protection
____________________________________________________________________________________________
89
physiology). The changes induced by thyroid hormone are principally epigenetic modifications. The field
of epigenetics has exploded over the last 20 years and how EDs (alone and in mixtures) affect these gene
regulatory mechanisms needs to be better examined.
4.8.2 Multi- and transgenerational effects
As explained above, adverse effects have been demonstrated for three generations subsequent to
exposure during pregnancy. However, whether exposure to EDs can affect subsequent generations,
demonstrating transgenerational effects beyond the population directly exposed requires more
research, both in terms of the potential mechanisms implicated and in terms of disease categories. In
some cases, as mentioned in section 1.9, animal studies have demonstrated clear transgenerational
effects of EDs beyond the “grandchildren” of mice studied [54]. Transgenerational effects of a number of
EDs have been shown in animal studies (including bisphenol A, PCBs, vinclozolin and DES), arguing for
better understanding of the phenomenon. Potential mechanisms for transgenerational effects include
different nongenomic mechanisms, mainly DNA methylation changes (see [65] for review of mechanisms
implicated). The urgency of research in this area has recently been emphasised both in terms of human
health [263] and for environmental concerns [264].
4.8.3 ED effects on the microbiome
The microbiome represents an essential component of all our physiological responses, especially our
immune responses [265, 266]. The genome of the multiple species that compose our microbiome is more
complex than our own and is an integral part of our endocrine responses [267]. That certain EDs can affect
the microbiome is known. One example is the biocide triclosan [268].
4.8.4 Green chemistry
Green chemistry is a broad subject, with many journals already devoted to the topic. In the current
context, it can be defined as producing chemicals for which production methods require less energy and
more importantly are readily biodegradable and less toxic to the environment and to human health than
those in use today.
Replacing known EDs with others that can be equally harmful is often referred to as “regrettable
substitution”. An example is the possible replacement of part of the bisphenol A by bisphenol S or F, both
of the latter having similar or worse ED properties (see for example [269]).
Hence, carrying out research for chemical substitutes for known, presumed or suspected EDs can fulfil
two purposes each with strong socio-economic benefits. In the first place, finding less harmful, well-
tested substitutes will enhance public health and reduce environmental risks. Second, the potential for
innovation by the chemical industry will be a strong economic driver, similar to that seen for
development of alternative energy sources that can create employment whilst encouraging
sustainability [270].
4.8.5 EDs acting on less studied modalities beyond E, A, T, S and metabolism
As mentioned above (see for example chapter 1 and section 4.5) most current research is directed to
adverse ED effects on reproduction through disruption of actions of the sex steroids (e.g. oestrogen and
androgen) and effects on brain development and metabolism, whether through thyroid hormone or
other related hormones. Hence, the focus in the current guidance document is on steroid hormones
(mainly oestrogens and androgens). However, as amply emphasized, there at least 40 other nuclear
receptors for hormones other than these, and their signalling pathways can also be implicated in
Policy Department for Citizens’ Rights and Constitutional Affairs
__________________________________________________________________________________________
90
reproductive, metabolic, neurodevelopmental diseases as well as in other forms of non-communicable
disease such as immune disorders and allergies [271]. Examples include the Vitamin D receptor [272] and
the vitamin A receptor [273].
Although nuclear receptors, with their capacity for ligand binding and control of gene transcription, are
classically considered as the principle pathways for endocrine disruption, many other signalling pathways
can be implicated even in classical endocrine signalling systems such as thyroid hormone (see for
instance [274]). Potential research domains include non-canonical nuclear receptor signalling through
membrane located receptors, diverse second messenger systems [275, 276] and microRNAs [277].
4.8.6 Characterization of dose-response functions for ED effects in humans
Dose response functions characterizing the effect of exposure to specific EDs or suspected EDs in humans
are relevant to allow better identification of EDs. The accurate assessment of exposures in the relevant
exposure windows, before occurrence of any disease, and control for potential confounding factors,
which is important in the context of multifactorial diseases, is best obtained in longitudinal
epidemiological cohorts.
This will require new types of cohorts with deep and accurate characterization of exposure to EDs, which,
in the case of non-persistent EDs that are to be assessed via biomarkers, shall imply collection of repeated
biospecimens in each subject [246].

Endocrine disruptors: from scientific evidence to human health protection
____________________________________________________________________________________________
91
5 MANAGEMENT OF EDs ACROSS SECTORS TO PROTECT HEALTH
KEY FINDINGS
•
Better health protection could be achieved by recognizing EDs as a class of hazard of
equivalent concern to carcinogens, mutagens and reprotoxicants in all sectors and not only
for plant protection products, biocides and REACH chemicals.
• In order to minimize ED exposure among EU citizens, the EU should move towards an
identical management of EDs across all sectors for which ED use is very likely to entail
population exposure. This includes in particular, plant protection products, biocides, food
contact materials, food additives, consumer goods, cosmetics and toys. Established
scientific facts show that (a) hormones act at extremely low doses; (b) some recognized EDs
also act at low doses; (c) there are methodological limitations to the approaches often used
to identify so-called safe thresholds/ tolerable daily intakes in regulatory toxicology and (d)
the approaches generally used to identify these safe thresholds generally do not consider
cumulative effects of combined exposure. Hence, one option to protect human health and
make the EU regulation more coherent across sectors would be to apply a logic similar to
that already in use for pesticides, i.e. that substances identified as EDs or presumed EDs
should not be authorized (“no exposure” logic).
• The oestrogenic, androgenic, thyroid, steroid (E, A, T, S) load of specific media such as
consumer products, food and drinking water should be evaluated and monitored, and the
implementation of limit values for E, A, T, S activities (e.g. in drinking water) should be
considered.
5.1 General strategy to manage the ED risk in all sectors and media
In complement to the development of a horizontal ED definition, an accompanying guidance document
and implementation of relevant test requirements (see 4.4), a risk management logic needs to be defined
for EDs in each sector.
The need to have a unique identification of EDs across sectors (“One chemical, one assessment”) does not
imply that the logic of management of EDs be the same in all sectors.
Our suggestion is to distinguish sectors on the basis of two main factors:
- The expected benefit of the family of products;
- The potential for widespread exposure in the general population or in sensitive populations.
The first factor would lead to much more stringent use of EDs in areas such as toys, food additives and
food contact materials, than for drugs, which have a proven benefit.
The second factor would lead to distinguish the sectors for which human exposure to the chemicals
present in the marketed products is very likely (e.g., food contact materials, food additives, cosmetics,
toys, consumer goods) from sectors for which exposure is much less frequent. Similarly, media and places
for which exposure is very likely (water, work place…) deserve specific consideration.
Here, we provide some suggestions for changes in the regulations reviewed in chapter 3.

Policy Department for Citizens’ Rights and Constitutional Affairs
__________________________________________________________________________________________
92
5.1.1 Management logic for products used by consumers as part of REACH regulation
Currently, REACH regulation follows a logic according to which the substances belonging to specific
hazard classes are to be put on an authorization list (the so-called Annex XIV of REACH, as well as Annex
XVII), following which management decisions about the substance may occur. Annex XIV currently only
includes 43 substances
53
, which represents about one substance added to the authorization list every 500
substances
54
. The list of substances of very high concern currently includes 13 EDs, which is much lower
than the expected number of known or presumed EDs. It is unlikely that the aim set forth in the 7
th
Environmental Action Programme, namely that “efforts need to be stepped up to ensure that, by 2020,
all relevant substances of very high concern, including substances with endocrine-disrupting properties,
are placed on the REACH”, can be attained at this rate.
One suggestion for improvement toward minimization of ED exposure would be to revert the logic of
entry in REACH authorization list for EDs in products with possible human exposure: that is, as soon as a
substance or a product with potential exposure of the general population is identified as an ED or
suspected ED (as a result of an evaluation conducted in any sector), the substance would not be
authorized. Following requests from applicants, derogations could be evaluated and granted if deemed
justified by the relevant authority. Note that, in order to follow the logic of equivalent concern for EDs
and CMRs , such provisions would have to be also set up for products with potential general population
exposure containing CMRs.
5.1.2 Management logic as part of REACH regulation – towards a clearer “equivalent
concern” principle for CMRs and EDs
REACH regulation tends to set EDs on a similar level of concern to CMRs, but this logic is currently not
followed because of 1) a lack of efficient tests requirements for EDs in REACH regulation (see chapters 3
and 4), and 2) a slightly different handling of EDs in the text of REACH regulation. A final consideration is
that there is no official definition of EDs for this sector (or for chemicals in general, see 4.2)
Regarding point 2, the REACH regulation could be amended so that article 57 includes a specific bullet
point setting EDs at exactly the same level as CMR, PBT substances, without the need to show that the
specific ED is of similar concern to CMR, PBT or vPvB substances. In practice, this could be done by adding
a bullet point after article 57.e stating:
- substances meeting the criteria for classification in the hazard class endocrine disruptor 1A or 1B
in accordance with section … of Regulation (EC) No 1272/2008 [or any other relevant regulation
where the definition of ED would be located];
and to delete reference to EDs in the current article 57(f).
5.1.3 Management logic for plant protection products and biocides
Although the recitals of the ED criteria for plant protection products and biocides call for the identification
of known and presumed endocrine disrupting substances (as for known and presumed CMR substances,
which are not authorized), currently only known endocrine disrupting substances are defined in the
53
https://echa.europa.eu/fr/authorisation-list
54
This calculation is based on the figure of about 22,000 substances registered in the context of REACH regulation reported by ECHA as of 8
May 2019 (https://echa.europa.eu/fr/registration-statistics-infograph#
)

Endocrine disruptors: from scientific evidence to human health protection
____________________________________________________________________________________________
93
context of the plant protection products and biocides regulation. One should either make sure that the
current definition and guidance document allows to cover known and presumed EDs, or that the plant
protection products and biocides regulations, their annexes and the ECHA-EFSA guidance document to
identify EDs in plant protection products and biocides, are updated to distinguish known and presumed
EDs, thereby not authorizing plant protection products and biocides containing known and presumed
EDs.
5.1.4 Management logic for cosmetics
In cosmetics, substances recognized as CMRs (categories 1A, 1B or 2) are banned. Following the principle
of equivalent concern of EDs, a similar logic should be enacted in the cosmetics regulation for proven,
presumed and suspected EDs. The inclusion of suspected EDs is important here, given that for substances
only used in cosmetics, in vivo tests on animals are not authorized. As such tests could improve the level
of evidence regarding ED properties, all evidence from other sectors must be made available and used.
5.1.5 Review of the cosmetics regulation by the European Commission (2018)
A review of the cosmetics regulation was published in November 2018 by the Commission
55
. It mentions
that “Although these criteria [defining substances with endocrine disrupting properties in the context of
the plant protection products and biocides regulations] do not have direct legal consequences for other
areas of EU law than the areas of plant protection products and biocides, they should be taken into
account, as far as possible, for the purposes of the present review of the Cosmetics Regulation.”
The EC review further mentions that “substances identified as endocrine disruptors are currently subject
to the general safety assessment of the SCCS. They are treated like substances of concern for human
health and are subject to case-by-case regulatory action”.
This approach is deemed relevant by the Commission: “Mindful of the different approaches taken in
relevant pieces of EU legislation to address endocrine disruptors in different sectors, the experience
collected since the entry into application of the Cosmetics Regulation has not revealed elements which
would justify deviating from the regime designed by the legislator to address the safety concerns related
to the use of endocrine disruptors in cosmetics.”
The review concludes that “The Cosmetics Regulation provides the adequate tools to regulate the use of
cosmetic substances that present a potential risk for human health and to take the appropriate regulatory
measures based on a scientific assessment of available data concerning human health”.
As already mentioned, a number of substances used for cosmetic and personal care products are known
or suspected EDs. Examples include parabens, triclosan and benzophenones (see 2.3), which are
authorized up to specific concentrations. This case-by-case logic deemed relevant in the November 2018
statement from the Commission stands in clear contrast with the logic in place for CMR substances
present in cosmetics (known, presumed and suspected CMR categories), which is that of a ban on the
hazard classes as a whole, case by case exemptions being possible. In the perspective of protecting health
and the environment, it is not clear why CMRs and EDs are put on a similar level of concern in the plant
protection products, biocides and REACH regulations and why this should not also be the case in the
cosmetics regulation, which deals with a sector with expected human exposure, especially for vulnerable
populations.
55
https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52018DC0739&rid=2

Policy Department for Citizens’ Rights and Constitutional Affairs
__________________________________________________________________________________________
94
In the same perspective, it is also unclear why EDs should be managed mostly on an “authorized level”
logic in the cosmetics sector, while EDs are treated under a no exposure logic for plant protection
products and biocides sold to the general public.
5.1.6 Management logic in other sectors with likely widespread exposure
In other key sectors with widespread consumer exposure, minimization of risk could be attained through
adopting a no-exposure logic, as for pesticides. This would be particularly relevant for toys, for which
there should be no justification of use of any substance of very high concern, for food additives, food
contact material, and possibly for consumer goods in general. For the latter sector, distinctions could be
made between goods for which the potential for human and environmental exposure is very unlikely, for
which strong restrictions may not be warranted, and goods for which there is potential for human or
environmental exposure, e.g. through contact or migration.
In addition, we outline below some sector-specific provisions, together with a discussion regarding the
labelling of products containing EDs.
5.2 Management of EDs in specific media including water
5.2.1 Management of EDs in water
In specific media such as drinking water, options include (i) the monitoring of all suspected EDs; (ii) the
monitoring of a list of specific EDs and (iii) the monitoring of endocrine disrupting activity for specific ED
modalities. The options are not mutually exclusive and can either be implemented in all drinking water
networks or in a subset of networks deemed representative of the EU region considered.
In its 2018 proposal for a revised drinking water directive, the Commission suggested an approach
leaning towards option (ii), limited to three recognized EDs (beta-oestradiol, nonylphenol, bisphenol A,
see 3.5.1 above), termed as “representative EDs”. Although it is relevant to monitor and limit their
occurrence in drinking water, it would be optimistic to assume that three EDs could be representative of
all EDs, that have very diverse sources, chemical nature, properties in water, and that this would be
enough to quantify or efficiently limit the impact of ED from drinking water exposure.
A relevant additional step would be to also follow option (iii) above, consisting in monitoring oestrogenic,
androgenic, thyroid disrupting activities (and possibly activity related to other ED modalities) in drinking
water. This option is expected to cover a larger range of possible ED effects and to be much cheaper than
monitoring a long list of EDs. If evidence of a very high activity of any ED modality (e.g., a high oestrogenic
activity) is found in a given network, then more detailed investigations could be undertaken to identify
the specific compound(s) that may cause this increased activity. Maximum levels for the main ED
modalities and for the sum of all ED modalities could be set, as a way to minimize ED exposure from
drinking water taking into account effects of combined exposures.
Such a logic is also worth considering for surface and ground waters.
Given the presence of EDs in the air, a reflexion on their monitoring and limitation, possibly considering
the logic outlined here for drinking water, could be undertaken.
Drugs constitute a source of contamination of drinking water by EDs and other hazardous chemicals
56
.
The options to limit the presence of EDs from drugs in water (treatment of water, specific collection and
treatment of the urine of patients using specific drugs…) should be outlined and considered.
56
See e.g. https://www.who.int/water_sanitation_health/diseases-risks/risks/info_sheet_pharmaceuticals/en/
Endocrine disruptors: from scientific evidence to human health protection
____________________________________________________________________________________________
95
5.2.2 Concerns regarding the use of paracetamol during pregnancy
Emerging scientific evidence raises concerns regarding the biological and possible health effects of use
of paracetamol (acetaminophen) and other mild analgesics (e.g. aspirin, ibuprofen) during pregnancy,
including early pregnancy (see 2.4). Paracetamol seems to be widely used during pregnancy in many EU
countries, so that even a small effect at the individual level could have a large impact at the population
level. The topic should be thoroughly expertized, then communication and possibly management
measures considered to limit use in pregnant women.
5.3 Labelling of products containing EDs
Currently, it is only for cosmetics that consumers are informed about the chemicals present in the goods
that they buy. Someone buying a garbage bag or a plastic bottle is generally not able to know if any
biocide has been added to the bag or if the bottle has been manufactured using an ED. Even in cosmetics,
the only information available corresponds to the list of chemicals used, which implies that probably few
consumers are able to sort out if one of the many chemicals listed is present on a documentation listing
all presumed EDs; such ability is not expected to be widely present in the population, and to be less
frequent in specific (possibly sensitive) subgroups with little time such as parents with young children or
socially disadvantaged subjects.
Until regulations such as the ones outlined in this chapter allowing to avoid or minimize the presence of
EDs in consumer goods are implemented, the possibility of defining a label indicating the presence of
EDs in a consumer good, and of making the use of such a label in all manufactured goods in which an ED
or presumed ED is present should be considered.
5.4 Conclusion
After several decades of multidisciplinary research in endocrinology, ecotoxicology, toxicology,
epidemiology, clinical research, epigenetics, environmental sciences and other disciplines, endocrine
disruption is now a strong and validated scientific concept. Several key mechanisms have been identified
whereby exogenous compounds or mixtures can induce adverse effects in humans or the environment
through an alteration of the endocrine system. Hormones act at extremely low doses, and EDs are
expected to also act at very low doses. The key organisational role of hormones during development
makes it very unlikely for any threshold of action to exist for EDs. The multiple functions of the endocrine
system and its interactions with the immune and nervous systems explain why EDs can be implicated in
such a wide range of diseases (see chapter 1).
Our review of the existing data on the presence of EDs in the environment and in the organism shows
that EDs or suspected EDs are currently present in all media (water, diet, food contact materials,
cosmetics…) and that the body of a large majority of EU citizens contains dozens of suspected EDs. This
figure is expected to strongly increase if more detailed information on the use of chemicals in each sector
are collected and EU-wide biomonitoring studies encompassing large numbers of chemicals conducted.
The probable range of the corresponding societal costs are exceptionally high, as is the price of inaction.
This evidence justifies to consider EDs as a specific class of hazard. The principle of assigning this new
hazard category a level of concern equivalent to that of CMRs, currently present in parts of the EU
regulation but not clearly enshrined, is totally well-founded.
Many areas of scientific uncertainty remain. These include the identification of additional modes of
actions of EDs; the exact characterization of hazards and dose-response functions in human populations;
the identification of EDs among marketed and natural substances; the exact number and list of EDs in our
environment. Given the already proven effects of EDs and the number of substances that may turn out
Policy Department for Citizens’ Rights and Constitutional Affairs
__________________________________________________________________________________________
96
to be EDs, these uncertainties are incentive for further research. Similar types of uncertainties exist for
other hazard categories such as carcinogens.
Although claims to the contrary have been made in the past due to loopholes in the procedures to limit
conflicts of interest, the existing uncertainties are in no way reasons to postpone regulatory action, at
least not for the substances that are most likely to be EDs.
The current regulatory procedures do not provide an efficient filtering of EDs allowing their identification
and limitations of their use before they are marketed, nor is there any safety net to do so once they are
on the market, which is anyway generally more expensive for society and less efficient. The current
situation is clearly detrimental for the environment, human health, society, sustainability and most
probably for our economy. The aim set forth in the 7
th
Environment Action Program to have all EDs placed
on REACH SVHC candidate list by 2020 is unlikely to be fulfilled.
We have listed several possible regulatory actions, many stemming from the identification of
incoherencies in the current EU chemicals regulation. In key sectors with strong potential for human
exposure to the products marketed, the regulation appears inconsistent. For example, in the case of the
plant protection products, biocides and REACH regulations, the current regulatory test requirements are
not sufficient to permit correct identification of EDs. Between-sector comparisons also point to striking
inconsistencies. Indeed, while in some sectors for which there is strong potential for human exposure,
EDs exposure is intended to be banned or strongly limited, the regulation of other sectors with very
strong or certain likelihood of human exposure (such as that of food additives or food contact material or
workers’ protection) does not even mention EDs as a generic concern.
Regarding ED identification, making the regulation simpler and coherent (without reducing its efficacy),
and closer to the aim of the 7
th
Environment Action Program, would require providing a (horizontal)
definition of EDs based on the current scientific consensus that would be valid for all sectors. A second
step would be to make sure that the provisions making it compulsory for all application dossiers for
marketed substances to include information allowing to identify EDs are laid down in regulations. For
transparency, coherence with the CMR regulation, and to account for the acknowledgment of
uncertainties as well as the limitation of animal testing, the regulation should distinguish three categories
of EDs, according to the level of scientific evidence (such as known, presumed and suspected EDs).
When it comes to risk management, the general aim of minimizing ED exposure has recently been put
forward by the European Commission in its 2018 communication towards a comprehensive EU
framework on EDs. This aim should not only be strongly integrated at several levels of regulations, but
also be developed into concrete risk management decisions in most sectors relevant to chemicals,
environment and health. We suggest specifically to distinguish on the one hand all sectors for which there
is a high likelihood of human exposure (cosmetics, food, food additives, food packaging, cosmetics,
biocides, REACH chemicals, consumer goods) from those with less likely human exposure. In the former
sectors, an option would be not to authorize the substance.
Our recommendations will not lead to a ban of a large number of poorly characterized substances.
However, they should strongly increase the level of knowledge on the hazards and safety of many
substances, and would only lead to decreased use or ban for substances with evidence of an adverse
effect used in products entailing exposure of the general population. Ultimately, this will improve health,
better protect the environment and help to develop a sustainable economy.

Endocrine disruptors: from scientific evidence to human health protection
____________________________________________________________________________________________
97
RECOMMENDATIONS
1. Policy goal: Endocrine disruptors (EDs) are one of the main classes of health hazards and are of
similar concern to carcinogens, mutagens, substances toxic to reproduction (CMRs), PBT
(persistent, bioaccumulative and toxic) and vPvB (very persistent and very bioaccumulative)
substances. Consequently, in order to better protect human health and the environment, on
which our health depends, the European Union should develop a set of trans-sectorial and
harmonized regulations to minimize human and environmental exposure to endocrine
disruptors.
2. Attaining this goal requires a) a cross-sectorial (“horizontal”) definition of EDs with three categories
according to the level of evidence; b) a guidance document explaining how to apply the definition
on the basis of tests results and scientific literature, and identify EDs; c) tests covering all ED
modalities; d) legal requirements to make these tests compulsory in application dossiers; e) risk
management measures aiming to minimize ED exposure, which may distinguish sectors with very
likely human exposure from those for which exposure is rare.
3. ED definition: Currently EDs are only defined in the plant protection products and biocides
regulations context. EU regulation should include a definition of EDs valid for all sectors. We
suggest using the WHO definition, or the current EU definition with slight modifications:
“Endocrine disruptors are defined as a substance or mixture that meets all of the following criteria:
1) It shows an adverse effect in an intact organism or its progeny or (sub)populations, which is a
change in the morphology, physiology, growth, development, reproduction or life span of an
organism, system or (sub)population that results in an impairment of functional capacity, an
impairment of the capacity to compensate for additional stress or an increase in susceptibility to
other influences; 2) it shows endocrine activity; 3) there is a biologically plausible link between the
adverse effect and the endocrine activity.” Similarly to other hazards, such as CMRs, this cross-
sectorial definition requires regulation to distinguish three categories according to the level
of evidence: known (category 1A), presumed (1B) and suspected (2) EDs.
4. Guidance document: A guidance document, based on that currently existing for plant protection
products and biocides, should be developed to explain how EDs should be identified on the basis
of this definition across sectors, including REACH chemicals, cosmetics, food contact materials and
food additives. The currently existing logic requiring a weight of evidence approach that
establishes the link between the adverse effect and the endocrine activity based on biological
plausibility should be maintained.
5. Test development: There is an urgent need to accelerate test development and validation,
especially for modalities beyond reproductive steroids, such as disruptors of the thyroid axis, and
the related adverse effects. There is also a need to enhance use of academic publications when
assessing ED properties.
6. Test requirements: Currently, there are insufficient data requirements in the regulation to be able
to efficiently identify EDs in any sector, including for pesticides and REACH chemicals. Regulations
should explicitly require data that would allow for the identification of known, presumed and
suspected EDs used or to be used in any sector. The use of ED tests covering all ED modalities and
endpoints, should be made compulsory in all application dossiers submitted by the industry.
Increased means should be allocated for the control of substance application dossiers. More

Policy Department for Citizens’ Rights and Constitutional Affairs
__________________________________________________________________________________________
98
ambitious objectives should be set for the numbers of chemicals examined each year for their ED
properties in national and EU agencies.
7. Management of EDs across sectors (1): EDs should be recognized as a class of hazard of
equivalent concern to carcinogens, mutagens and reprotoxicants in all sectors and not only for
pesticides and REACH chemicals, but also cosmetics, toys, food additives and food contact
materials.
8. Management of EDs across sectors (2): In order to minimize ED exposure among EU citizens, the
EU should move towards an identical management of EDs across all sectors for which ED use is
very likely to entail population exposure. This includes in particular plant protection products,
biocides, food contact materials and additives, consumer goods, cosmetics and toys. Established
scientific facts show that (a) hormones act at extremely low doses; (b) EDs are expected to also act
at low doses, and this is proven for the most studied EDs; (c) there are methodological limitations
to the approaches typically used to identify so-called safe thresholds in regulatory toxicology and
(d) the approaches commonly used to identify these safe thresholds generally do not consider
cumulative effects of combined exposures. Hence, one option to protect human health and make
the EU regulation more coherent across sectors would be to apply a logic similar to that already in
use for pesticides, i.e. that substances identified as known or presumed EDs should not be
authorized (“no exposure” logic) in products with general population exposure. For the cosmetic
sector specifically, a logic similar to that applied for CMRs should be used for EDs, consisting in
banning known, presumed and suspected EDs in cosmetics.
9. Management of EDs in specific sectors (1) - Media-oriented regulations: The oestrogenic,
androgenic, thyroid, steroid (E, A, T, S) loads of food and drinking water should be evaluated and
monitored, and the implementation of limit values for E, A, T, S activities should be considered,
bearing in mind that such endocrine activity may be indicative of adverse effects.
10. Management of EDs in specific sectors (2) - Occupational exposures: Occupational exposure
limits should be set for EDs.
11. Surveillance of production, use and exposure to EDs: Data on ED production, use of EDs across
sectors should be gathered by the industry and relevant actors and made available at fine
geographic and temporal scales. Monitoring known, presumed and suspected EDs (banned or still
in use) in human populations (human biomonitoring) should be implemented in a harmonized
way at the EU scale, including among pregnant women and children.
12. Research priorities: The current scientific knowledge, accumulated over the last 30 years, is
sufficient to justify the above-mentioned recommendations. However, in order to identify new
EDs, develop new tests and better quantify their population impacts, six areas of research are
flagged: (i) Epigenetic effects of EDs; (ii) Concern beyond the current generation; (iii) ED effects on
the microbiome, an essential component of physiological and immune responses (iv) Green (safe)
chemistry; (v) Novel ED modalities (vi) Characterization of dose-response functions for ED effects
in human.

Endocrine disruptors: from scientific evidence to human health protection
____________________________________________________________________________________________
99
ANNEX
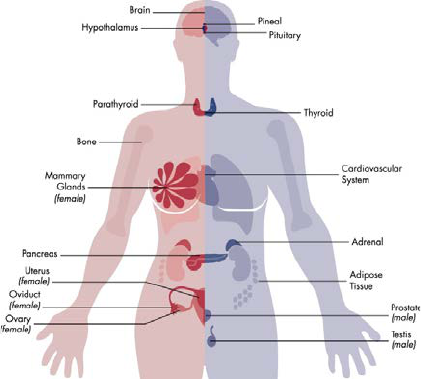
Policy Department for Citizens’ Rights and Constitutional Affairs
__________________________________________________________________________________________
100
ANNEX 1: DEFINITIONS
Adverse effect: A change in the morphology, physiology, growth, development, reproduction or life span
of an organism, system or (sub)population that results in an impairment of functional capacity, an
impairment of the capacity to compensate for additional stress or an increase in susceptibility to other
influences
Adverse outcome: According to the OECD[76] an adverse outcome is a specialised type of key event that
is of regulatory significance on the basis of correspondence to an established protection goal or
equivalence to an apical endpoint in an accepted regulatory guideline toxicity test. Depending on
whether the protection goal is for human health or ecological health the endpoints considered may
differ.
Combined exposure: This concept refers to exposure to multiple chemicals by multiple routes, from one
or multiple sources and/or use(s).
Endocrine disrupter (or disruptor): According to the WHO definition “an endocrine disrupter is an
exogenous substance or mixture that alters function(s) of the endocrine system and consequently causes
adverse health effects in an intact organism, or its progeny or (sub)populations.” [9]
Endocrine system: Multiple glands compose the endocrine system. All produce hormones that are
secreted in low doses into the blood system from whence they act on other tissues see Section 1.3. Figure
reproduced from [4] (No permission required).
Epigenetic: This term means literally “above the gene” and refers to changes in transcription that occur
without modifying or mutating the DNA sequence, but changing when and where genes are expressed
in different tissues and at different time points. Epigenetic effects most often implicated either DNA
methylation or changes in chromatin structure (chromatin being the proteins surrounding the actual
DNA sequence).
Mechanism of Action: According to the OECD [76], a mechanism of action for toxicity is the detailed
molecular description of key events in the induction of cancer or other health endpoints. Mechanism
of action represents a more detailed understanding and description of events that is meant by mode of
action (see below).

Endocrine disruptors: from scientific evidence to human health protection
____________________________________________________________________________________________
101
Mode of Action: This is defined by WHO as a “Biologically plausible sequence of key events leading to
an observed effect supported by robust experimental observations and mechanistic data.”
Risk: The probability of an adverse effect in an organism, system, or (sub)population caused under
specified circumstances by exposure to an agent [278].
Weight of Evidence (WoE): Weight of evidence assessment is defined … as a process in which evidence
is integrated to determine the relative support for possible answers to a question. [The weight of
evidence assessment comprises] three basic steps: (1) assembling the evidence into lines of evidence
of similar type, (2) weighing the evidence, (3) integrating the evidence.” (From EFSA guidance on
weight of evidence, EFSA Journal, 2017,
https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2017.4971
).

Policy Department for Citizens’ Rights and Constitutional Affairs
__________________________________________________________________________________________
102
ANNEX 2: DISCUSSION OF PAST CONTROVERSIES REGARDING EDs
REGULATION IN THE EU
2013 “ED criteria” and Dietrich et al. 2013 letter
In 2013, Dr. Daniel Dietrich and 18 colleagues published a letter in a series of journals [260]. The large
majority of these authors did not declare their conflicts of interest, notably with the chemical
industry. They had previously addressed the same letter to Dr. Glover, then Chief Scientific Advisor to
Mr. Barroso (at that time EU Commission President) stating among other points that:
“…we also think that the identification and regulation of such substances [EDs] should depend on a)
the definition of adverse effects that are relevant to whole human or animal organisms and not to
isolated test systems of unknown homeostatic significance, and b) on a characterization of real-life
potency and therefore of thresholds of concern.”
This commentary was countered by 30 clinical and basic endocrinologists (see for instance [279]),
underlining the innumerable published studies that refuted the main arguments. Regarding specifically
point a) it can be noted that the WHO definition of EDs and the current ED criteria applied in the EU for
plant protection products and biocides do refer to adverse effects relevant to organisms and populations,
which is in agreement with the letter [260]
The second point regarding the potency concept was clearly refuted in several publications (see e.g. [93]);
the point was debated and agreed upon by a group of scientists (several of which had signed the 2013
letter, including Dr. Dietrich) in a meeting convened by the German Institute for Risk Evaluation (BfR) in
Berlin [97], who concluded in a consensus statement that:
“This enabled the workshop participants to conclude that differences in opinion regarding the
existence of thresholds and non-monotonic dose–response curves, although relevant to the risk
characterization of EDs, are not a hindrance for defining scientific criteria for their identification.” and
that “…potency is not relevant for identification of a compound as an endocrine disruptor” [97].
A detailed account of the role of the chemical industry in inflecting and delaying the decision making
process on EDs in 2013 was published by a non-governmental organisation [280].
2018 Parliament hearing at the PETI commission: statements from dr. Dietrich
In addition to the criticisms raised in Bond and Dietrich [241], in a hearing in the EU parliament
in March
2018, Dr. Dietrich made several statements in a talk titled “Synthetic EDC [endocrine disrupting
compounds] at the present human exposure ARE NO RISK for human health”. Dr. Dietrich is a toxicologist
working on cyanobacteria and mycotoxins, i.e. toxins of natural origin, but with very few original
publications on the effects of man-made chemicals nor on endocrinology.
The recorded talk made several assertions. We discuss briefly the main ones.
1) Margin of exposure and naturally occurring EDs:
• Margin of exposures for environmental exposures are above 10,000 for most compounds.
• “Daidzein, genistein, BPA, DDE are GR [glucocorticoid receptor] agonists, but how potent are
they?”
• “Daidzein and genistein levels were 10-100-fold lower than endogenous oestrogen levels. BPA
[bisphenol A] levels were 100,000-fold lower than endogenous oestrogen levels”
Endocrine disruptors: from scientific evidence to human health protection
____________________________________________________________________________________________
103
These three comments do not take into account (i) the demonstrated cumulative effects of mixtures (see
section 1.6) nor (ii) vulnerable periods of exposure ( see section 1.7) nor non-linear responses and low
dose effects (see section 1.3.3). The specific question of genistein and daidzein is dealt with in point 3
below.
Note also, with respect to the reference to potency in the second quote, that the Berlin consensus
statement included this point stating (point 22) that:
“We agree that a chemical’s potency to induce an adverse effect is an important factor for
consideration during the characterization of the hazards of endocrine disruptors. However, potency
is not relevant for identification of a compound as an endocrine disruptor.”
2) Pregnancy: “Endogenous hormone levels vary dramatically during pregnancy” (citing
Teeguarden et al, March 2018).
This statement is obviously correct. Indeed, hundreds of million years of mammalian evolution have fine-
tuned circulating and placental hormone levels in pregnancy to ensure appropriate homeostasis at
different times and within appropriate compartments in pregnancy. (For a review of neuroendocrine
mechanisms and pregnancy see [281]). Interference from EDs during this period, particularly the first
trimester, represents a major threat (see section 1.7). Notably, shifting the oestrogenic, androgenic or
thyroid hormone levels during pregnancy away from the normal level, even by a very small amount, can
have dramatic effects (see Figure 1).
3) Bisphenol F in mustard: “Intake of naturally occurring BPF [bisphenol F] from yellow mustard is
similar if not greater than daily BPA [bisphenol A] exposure”
The fact that some people eating mustard may have higher exposure to bisphenol F than the average
population exposure to bisphenol A bears no consequence at all regarding the innocuity of bisphenol A
exposure (if this is what is meant by this statement). The two compounds are different, and there is no
guarantee at all that bisphenol F exposure is safe; on the contrary, published evidence shows that it does
exert ED effects [269].
It is well accepted that a small number of naturally occurring compounds can exert endocrine disrupting
activity. Examples include genistein and daidzein found in soy products. These substances inhibit iodine
uptake by the thyroid [282, 283] and displace thyroid hormone from one of its main transporter proteins
[284]. Just as acknowledging the health impact of tobacco smoke and the need for decreased exposure
does not diminish the impact of ambient air pollution on health, we cannot fathom why acknowledging
bisphenol F exposure from natural sources would make the need for minimisation of bisphenol A
exposure irrelevant.
4) BPA and the CLARITY Study: “The NTP [US-national toxicology program] Research Report on the
CLARITY-BPA Core Study with rats February 2018 shows: “BPA produced minimal effects that
were distinguishable from background in this study, particularly below 25,000 µg/kg body
weight.day”
(note: distinguishable probably used for undistinguishable).
It is important to note that numerous publications arising from the Clarity-BPA study do show significant
effects, notably on brain gene expression (see for instance [118], the recent review by Prins et al. [119]
and references in section 2.2.1). Contrary to the statement above, there is evidence of effects of bisphenol
A in the 1-10 µg/kg body weight.day range (reviewed e.g. in [119, 120]), that is, 2500 times below the dose
quoted by Dr. Dietrich in his statement to the EU Parliament.

Policy Department for Citizens’ Rights and Constitutional Affairs
__________________________________________________________________________________________
104
5) Criticism of the alleged link between DDT exposure and obesity, considered by Trasande et al. [285]:
Dr. Dietrich highlighted correlations between adult obesity and current DDT exposures (as opposed to
prenatal exposures and childhood obesity), citing WHO figures on adult obesity from 2008, where
Indonesia (which banned DDT use in 1994) and India (“which opposes a 2020 DDT ban”) both show very
low obesity levels at the country scale. One should note that such data at the region or country level
correspond to so-called “ecological” studies, which provide a very low level of evidence. In particular,
such an approach does not allow to control for potential confounders at the individual level (such as
caloric intake and physical activity) as in epidemiological cohort studies, which generally provide a much
stronger level of evidence. The ecological data provided by Dr. Dietrich do not even include a direct
estimate of exposure to DDT, but a very indirect one related to the year when DDT was banned (which is
a very imperfect indicator given the persistence of the compound and its main metabolite, DDE).
Therefore, this does not constitute clear evidence that DDT exposure is not a risk factor for overweight or
obesity.
Furthermore, data from the US National Health and Nutrition Examination Study (NHANES) showed that
“for a given amount of caloric intake, macronutrient intake or leisure time physical activity, the predicted
BMI was up to 2.3 kg.m
−2
higher in 2006 than it was in 1988” [286]. Similarly, since the Trasande cost study
in 2015 [285], further evidence for a link between prenatal DDT exposure and childhood obesity has been
published [247].
6) Temporal trends in DDT in human breast milk and adipose tissue. “The results support a
continuing decrease in human body burdens of PCBs, DDE and HCB during the 1990s”
That the body burden of certain persistent organic pollutants has decreased over time in many countries
in the last decades is not debated. However, it is also not informative with respect to these compounds
influencing obesity risk. Just like comparing countries (the so-called spatial “ecological approach”
discussed above) generally provides very limited evidence regarding the effect of environmental factors,
comparing temporal trends in exposure (e.g., to DDT) and health outcomes (e.g., obesity, that are on the
rise in several countries) corresponds to a temporal ecological study, and is a very weak study design. See
discussion on 5) above regarding the evidence of DDT affecting obesity.
7) “Current exposure levels of EDCs e.g. BPA and DDE are too low to have any effect on the foetus
or the developing child.”
This conclusion is not accompanied by any reference so that it is not easy to see what would justify it. The
fact is that there are many solid demonstrations of increased risk of disease following prenatal exposure
to either bisphenol A or DDE (see sections 2.8.2 and 2.2.1).
8) “Current exposures to isoflavones could have an added-on endocrine effect”
See response to point 3) above.
9) “Current exposures to known EDCs such as sugar will definitely have an adverse health effect.”
This is undoubtedly the case, hence the need for better dietary advice and the need to argue for and even
recommend lower sugar levels in certain foodstuffs (see also following point).
10) “Caloric intake will definitely have an adverse health effect”
The impact of high caloric intake on health is not debated. As for any other multifactorial disease, it does
not exclude the existence of other (e.g., chemical) risk factors. As presented in section 2.8.2, data from the
US National Health and Nutrition Examination Study (NHANES) showed that “for a given amount of caloric
Endocrine disruptors: from scientific evidence to human health protection
____________________________________________________________________________________________
105
intake, macronutrient intake or leisure time physical activity, the predicted BMI was up to 2.3 kg.m
−2
higher in 2006 than it was in 1988” [286]. This finding argues for the presence of other obesogenic factors
in our environment, as developed by Blumberg and colleagues [54].
11) “Our review of the Trasande et al human cost burden analyses uncovered substantial flaws in
approach taken and conclusions drawn and therefore are highly speculative and should not be
considered in weight of evidence approach.”
The principle of weight of evidence approaches is precisely not to discard any study on the considered
topic, but to include them all, with a weight depending on its quality.
See above (2.8.2) for a more detailed discussion of the Trasande et al. studies.
12) “EDCs follow a concentration response principle, with a threshold.”
See response to point 1 above.
13) “With the exception of natural EDCs (sugar, isoflavones, BPF), prominent human diseases, e.g.
prevalence of T2D [type-2 diabetes], are impossible to associate or causally relate or to synthetic
EDC exposure based on the actual low concentrations found in exposed persons.”
Numerous sections of this report refute this unsubstantiated claim (see 1.7, 1.8, chapter 2). Besides, there
is no general scientific reason why only naturally-occurring EDs would have an effect and an impact, and
not man-made EDs.
14) “Any regulation of EDCs should embrace in language and foreseen procedure:
• A. “causality” and not “plausibility” of the hazards determined in the in vivo, in vitro and in silico
test systems used”
• B. Must consider potency of the compounds in question
• C. Must consider true human exposure (several age groups)
• D. Must consider the “more likely explanations” in a human disease, before an association of an
EDC (or any other mechanism) with the specific disease is considered.
A: The ECHA/EFSA Guidance document for ED identification in plant protection products and biocides
[253] emphasizes that “...the link between the adverse effect(s) and the endocrine mode of action shall
be established based on biological plausibility”. Causality is not a scientifically defined concept in
environmental health, the current approach relies on statement about the overall level of evidence,
established using weight of evidence methods, as enshrined in several parts of the EU regulation; this is
what “plausibility” refers to. Further regulations could define several categories of EDs, distinguishing
those with a high level of evidence (possibly corresponding to what is referred to by Dr. Dietrich as
“causal”) from those for which evidence is lower (e.g., presumed and suspected EDs, which may have
different regulatory implications).
B: As concluded in a meeting of scientists held in Berlin in 2016, “…potency is not relevant for
identification of a compound as an endocrine disruptor.” [97]. Note that potency is an ill-defined concept
that pharmacologists recommend not to use [287] and that Dr. Dietrich is a co-author of the Berlin
consensus article that was published following the meeting [97].
C: This statement can have several meanings. First, it can be noted that existing regulation of EDs already
considers human exposure. For example, the 2009 plant protection products regulation states that plant
protection products recognized as EDs shall not be authorized unless human exposure is negligible. In its
Policy Department for Citizens’ Rights and Constitutional Affairs
__________________________________________________________________________________________
106
recent communication on EDs (from November 2018), the European Commission called for a
minimization of ED exposure for humans and the environment.
D. is a very simplistic view of environmental health sciences, that have over decades, developed
various approaches to multifactorial health outcomes (see chapter 2, 2.1).

Endocrine disruptors: from scientific evidence to human health protection
____________________________________________________________________________________________
107
REFERENCES
1. Kortenkamp, A., O. Martin, et al., State of the art assessment of Endocrine Disruptors. Final report.
2011.
2. WHO/UNEP, State of the science of endocrine disrupting chemicals - 2012, A. Bergman, et al., Editors.
2012. Available from:
http://www.who.int/iris/bitstream/10665/78101/1/9789241505031_eng.pdf
.
3. WHO, Endocrine disrupters and child health - Possible developmental early effects of endocrine
disrupters on child health, J. Toppari, et al., Editors. 2002, World Health Organization. Available
from: https://www.who.int/iris/bitstream/10665/75342/1/9789241503761_eng.pdf
.
4. Gore, A.C., V.A. Chappell, et al., EDC-2: The Endocrine Society's Second Scientific Statement on
Endocrine-Disrupting Chemicals. Endocr Rev, 2015. 36(6): p. E1-E150.
5. UNEP/IPCP, Overview Report I: Worldwide initiatives to identify endocrine disrupting chemicals
(EDCs) and potential EDCs. 2017, United Nations Environment Program. Available from:
https://wedocs.unep.org/bitstream/handle/20.500.11822/25633/EDC_report1.pdf?sequence=1
&isAllowed=y.
6. UNEP/IPCP, Overview Report II: An Overview of Current Scientific Knowledge on the Life Cycles,
Environmental Exposures, and Environmental Effects of Select Endocrine Disrupting Chemicals
(EDCs) and Potential EDCs. 2017, United Nations Environment Programme. Available from:
http://wedocs.unep.org/handle/20.500.11822/25634
.
7. UNEP/IPCP, Overview Report III: Existing national, regional, and global regulatory frameworks
addressing Endocrine Disrupting Chemicals (EDCs). 2017. Available from:
http://wedocs.unep.org/bitstream/handle/20.500.11822/25636/edc_report3.pdf?sequence=1&
isAllowed=y.
8. Carson, R.L., Silent spring. 1962, Boston: Houghton Mifflin Co. x, 368 p.
9. WHO/IPCS, Global assessment of the state-of-the-science of endocrine disruptors. 2002. Available
from: http://www.who.int/ipcs/publications/new_issues/endocrine_disruptors/en/
.
10. Peakall, D.B., p,p'-DDT: effect on calcium metabolism and concentration of estradiol in the blood.
Science, 1970. 168(3931): p. 592-4.
11. Cohn, B.A., M. La Merrill, et al., DDT Exposure in Utero and Breast Cancer. J Clin Endocrinol Metab,
2015: p. jc20151841.
12. Dieckmann, W.J., M.E. Davis, L.M. Rynkiewicz, and R.E. Pottinger, Does the administration of
diethylstilbestrol during pregnancy have therapeutic value? Am J Obstet Gynecol, 1953. 66(5): p.
1062-81.
13. Newbold, R.R., Prenatal exposure to diethylstilbestrol (DES). Fertil Steril, 2008. 89(2 Suppl): p. e55-
6.
14. Schrager, S. and B.E. Potter, Diethylstilbestrol exposure. Am Fam Physician, 2004. 69(10): p. 2395-
400.
15. Kioumourtzoglou, M.A., B.A. Coull, E.J. O'Reilly, A. Ascherio, and M.G. Weisskopf, Association of
Exposure to Diethylstilbestrol During Pregnancy With Multigenerational Neurodevelopmental
Deficits. JAMA Pediatr, 2018. 172(7): p. 670-677.
Policy Department for Citizens’ Rights and Constitutional Affairs
__________________________________________________________________________________________
108
16. Klip, H., J. Verloop, et al., Hypospadias in sons of women exposed to diethylstilbestrol in utero: a
cohort study. Lancet, 2002. 359(9312): p. 1102-7.
17. Li, Y., K.J. Hamilton, et al., Diethylstilbestrol (DES)-stimulated hormonal toxicity is mediated by
ERalpha alteration of target gene methylation patterns and epigenetic modifiers (DNMT3A, MBD2,
and HDAC2) in the mouse seminal vesicle. Environ Health Perspect, 2014. 122(3): p. 262-8.
18. Barker, D.J., The origins of the developmental origins theory. J Intern Med, 2007. 261(5): p. 412-7.
19. Markey, C.M., B.S. Rubin, A.M. Soto, and C. Sonnenschein, Endocrine disruptors: from Wingspread
to environmental developmental biology. J Steroid Biochem Mol Biol, 2002. 83(1-5): p. 235-44.
20. Kohrle, J., Environment and endocrinology: the case of thyroidology. Ann Endocrinol (Paris), 2008.
69(2): p. 116-22.
21. Perdichizzi, S., M.G. Mascolo, et al., Cancer-related genes transcriptionally induced by the fungicide
penconazole. Toxicol In Vitro, 2014. 28(1): p. 125-30.
22. Hoffman, K., J.A. Sosa, and H.M. Stapleton, Do flame retardant chemicals increase the risk for thyroid
dysregulation and cancer? Curr Opin Oncol, 2017. 29(1): p. 7-13.
23. Evans, R.M. and D.J. Mangelsdorf, Nuclear Receptors, RXR, and the Big Bang. Cell, 2014. 157(1): p.
255-66.
24. Ahmadian, M., J.M. Suh, et al., PPARgamma signaling and metabolism: the good, the bad and the
future. Nat Med, 2013. 19(5): p. 557-66.
25. Vandenberg, L.N., T. Colborn, et al., Hormones and endocrine-disrupting chemicals: low-dose effects
and nonmonotonic dose responses. Endocr Rev, 2012. 33(3): p. 378-455.
26. Ropero, A.B., P. Alonso-Magdalena, et al., Bisphenol-A disruption of the endocrine pancreas and
blood glucose homeostasis. Int J Androl, 2008. 31(2): p. 194-200.
27. Soriano, S., P. Alonso-Magdalena, et al., Rapid insulinotropic action of low doses of bisphenol-A on
mouse and human islets of Langerhans: role of estrogen receptor beta. PLoS One, 2012. 7(2): p.
e31109.
28. Korevaar, T.I., R. Muetzel, et al., Association of maternal thyroid function during early pregnancy with
offspring IQ and brain morphology in childhood: a population-based prospective cohort study.
Lancet Diabetes Endocrinol, 2016. 4(1): p. 35-43.
29. Bowers, J., J. Terrien, et al., Thyroid Hormone Signaling and Homeostasis During Aging. Endocr Rev,
2013.
30. Judson, R.S., K.A. Houck, E.D. Watt, and R.S. Thomas, On selecting a minimal set of in vitro assays to
reliably determine estrogen agonist activity. Regul Toxicol Pharmacol, 2017. 91: p. 39-49.
31. Bagchi Bhattacharjee, G. and S.M. Paul Khurana, In vitro reporter assays for screening of chemicals
that disrupt androgen signaling. J Toxicol, 2014. 2014: p. 701752.
32. Simon, C., M. Onghena, et al., Screening of endocrine activity of compounds migrating from plastic
baby bottles using a multi-receptor panel of in vitro bioassays. Toxicol In Vitro, 2016. 37: p. 121-133.
33. Mughal, B.B., J.B. Fini, and B.A. Demeneix, Thyroid-disrupting chemicals and brain development: an
update. Endocr Connect, 2018. 7(4): p. R160-R186.
34. Patel, S., Disruption of aromatase homeostasis as the cause of a multiplicity of ailments: A
comprehensive review. J Steroid Biochem Mol Biol, 2017. 168: p. 19-25.
Endocrine disruptors: from scientific evidence to human health protection
____________________________________________________________________________________________
109
35. Roselli, C.E., M. Liu, and P.D. Hurn, Brain aromatization: classic roles and new perspectives. Semin
Reprod Med, 2009. 27(3): p. 207-17.
36. Sasano, H., K. Takashashi, F. Satoh, H. Nagura, and N. Harada, Aromatase in the human central
nervous system. Clin Endocrinol (Oxf), 1998. 48(3): p. 325-9.
37. Gore, A.C., Developmental programming and endocrine disruptor effects on reproductive
neuroendocrine systems. Front Neuroendocrinol, 2008. 29(3): p. 358-74.
38. Gore, A.C., K.M. Martien, K. Gagnidze, and D. Pfaff, Implications of prenatal steroid perturbations for
neurodevelopment, behavior, and autism. Endocr Rev, 2014. 35(6): p. 961-91.
39. Chen, S., J.H. Hsieh, et al., Cell-Based High-Throughput Screening for Aromatase Inhibitors in the
Tox21 10K Library. Toxicol Sci, 2015. 147(2): p. 446-57.
40. ENV.A.3/FRA/2014/0029, F.c., Supporting the organisation of a workshop on thyroid disruption –
Final Report. 2017.
41. Steinmaus, C., M. Pearl, et al., Thyroid Hormones and Moderate Exposure to Perchlorate during
Pregnancy in Women in Southern California. Environ Health Perspect, 2016. 124(6): p. 861-7.
42. Fini, J.B., B.B. Mughal, et al., Human amniotic fluid contaminants alter thyroid hormone signalling
and early brain development in Xenopus embryos. Sci Rep, 2017. 7: p. 43786.
43. Henjum, S., I. Aakre, et al., Suboptimal Iodine Status among Pregnant Women in the Oslo Area,
Norway. Nutrients, 2018. 10(3).
44. Casals-Casas, C. and B. Desvergne, Endocrine disruptors: from endocrine to metabolic disruption.
Annu Rev Physiol, 2011. 73: p. 135-62.
45. Bernal, J., Thyroid hormones and brain development. Vitam Horm, 2005. 71: p. 95-122.
46. Bernal, J., Thyroid hormone regulated genes in cerebral cortex development. J Endocrinol, 2017.
232(2): p. R83-R97.
47. Ghassabian, A. and L. Trasande, Disruption in Thyroid Signaling Pathway: A Mechanism for the Effect
of Endocrine-Disrupting Chemicals on Child Neurodevelopment. Front Endocrinol (Lausanne), 2018.
9: p. 204.
48. Dickerson, S.M., S.L. Cunningham, H.B. Patisaul, M.J. Woller, and A.C. Gore, Endocrine disruption of
brain sexual differentiation by developmental PCB exposure. Endocrinology, 2011. 152(2): p. 581-
94.
49. Jacobson, J.L., S.W. Jacobson, and H.E. Humphrey, Effects of in utero exposure to polychlorinated
biphenyls and related contaminants on cognitive functioning in young children. J Pediatr, 1990.
116(1): p. 38-45.
50. Jacobson, J.L. and S.W. Jacobson, Intellectual impairment in children exposed to polychlorinated
biphenyls in utero. N Engl J Med, 1996. 335(11): p. 783-9.
51. Demeneix, B., Losing our minds: how environmental pollution impairs human intelligence and
mental health. Oxford series in behavioral neuroendocrinology. 2014, Oxford ; New York: Oxford
University Press. xxiii, 284 pages.
52. Le Magueresse-Battistoni, B., L. Multigner, C. Beausoleil, and C. Rousselle, Effect of bisphenol A on
metabolism and evidences of a mode of action mediated through endocrine disruption. Molecular
and Cellular Endocrinology, 2018. 475: p. 74-91.
Policy Department for Citizens’ Rights and Constitutional Affairs
__________________________________________________________________________________________
110
53. Wassenaar, P.N.H., L. Trasande, and J. Legler, Systematic Review and Meta-Analysis of Early-Life
Exposure to Bisphenol A and Obesity-Related Outcomes in Rodents. Environmental Health
Perspectives, 2018. 125(10).
54. Chamorro-Garcia, R., C. Diaz-Castillo, et al., Ancestral perinatal obesogen exposure results in a
transgenerational thrifty phenotype in mice. Nat Commun, 2017. 8(1): p. 2012.
55. Regnault, C., M. Usal, et al., Unexpected metabolic disorders induced by endocrine disruptors in
Xenopus tropicalis provide new lead for understanding amphibian decline. Proc Natl Acad Sci U S A,
2018. 115(19): p. E4416-E4425.
56. Viguie, C., S. Mhaouty-Kodja, et al., Evidence-based adverse outcome pathway approach for the
identification of BPA as en endocrine disruptor in relation to its effect on the estrous cycle. Mol Cell
Endocrinol, 2018. 475: p. 10-28.
57. Hunt, P.A., S. Sathyanarayana, P.A. Fowler, and L. Trasande, Female Reproductive Disorders,
Diseases, and Costs of Exposure to Endocrine Disrupting Chemicals in the European Union. J Clin
Endocrinol Metab, 2016. 101(4): p. 1562-70.
58. Schwartz, C.L., S. Christiansen, et al., Anogenital distance as a toxicological or clinical marker for
fetal androgen action and risk for reproductive disorders. Arch Toxicol, 2018.
59. Wohlfahrt-Veje, C., K.M. Main, and N.E. Skakkebaek, Testicular dysgenesis syndrome: foetal origin of
adult reproductive problems. Clin Endocrinol (Oxf), 2009. 71(4): p. 459-65.
60. Hauser, R., N.E. Skakkebaek, et al., Male reproductive disorders, diseases, and costs of exposure to
endocrine-disrupting chemicals in the European Union. J Clin Endocrinol Metab, 2015. 100(4): p.
1267-77.
61. Toppari, J., H.E. Virtanen, K.M. Main, and N.E. Skakkebaek, Cryptorchidism and hypospadias as a
sign of testicular dysgenesis syndrome (TDS): environmental connection. Birth Defects Res A Clin Mol
Teratol, 2010. 88(10): p. 910-9.
62. Lioy, P.J., R. Hauser, et al., Assessment of phthalates/phthalate alternatives in children's toys and
childcare articles: Review of the report including conclusions and recommendation of the Chronic
Hazard Advisory Panel of the Consumer Product Safety Commission. J Expo Sci Environ Epidemiol,
2015. 25(4): p. 343-53.
63. Smarr, M.M., K. Kannan, and G.M. Buck Louis, Endocrine disrupting chemicals and endometriosis.
Fertil Steril, 2016. 106(4): p. 959-66.
64. Bruner-Tran, K.L., J. Gnecco, et al., Exposure to the environmental endocrine disruptor TCDD and
human reproductive dysfunction: Translating lessons from murine models. Reprod Toxicol, 2017. 68:
p. 59-71.
65. Walker, D.M. and A.C. Gore, Transgenerational neuroendocrine disruption of reproduction. Nat Rev
Endocrinol, 2011. 7(4): p. 197-207.
66. Krishnan, K., N. Mittal, et al., Effects of the Endocrine-Disrupting Chemicals, Vinclozolin and
Polychlorinated Biphenyls, on Physiological and Sociosexual Phenotypes in F2 Generation Sprague-
Dawley Rats. Environ Health Perspect, 2018. 126(9): p. 97005.
67. Mhaouty-Kodja, S., L. Naule, and D. Capela, Sexual behavior: From hormonal regulation to
endocrine disruption. Neuroendocrinology, 2018.
Endocrine disruptors: from scientific evidence to human health protection
____________________________________________________________________________________________
111
68. Duarte, E., B. de Sousa, C. Cadarso-Suarez, V. Rodrigues, and T. Kneib, Structured additive
regression modeling of age of menarche and menopause in a breast cancer screening program. Biom
J, 2014. 56(3): p. 416-27.
69. Yang, G., S. Nowsheen, K. Aziz, and A.G. Georgakilas, Toxicity and adverse effects of Tamoxifen and
other anti-estrogen drugs. Pharmacol Ther, 2013. 139(3): p. 392-404.
70. Cohn, B.A., M. La Merrill, et al., DDT Exposure in Utero and Breast Cancer. J Clin Endocrinol Metab,
2015. 100(8): p. 2865-72.
71. Seachrist, D.D., K.W. Bonk, et al., A review of the carcinogenic potential of bisphenol A. Reprod
Toxicol, 2016. 59: p. 167-82.
72. Pastor-Barriuso, R., M.F. Fernandez, et al., Total Effective Xenoestrogen Burden in Serum Samples
and Risk for Breast Cancer in a Population-Based Multicase-Control Study in Spain. Environ Health
Perspect, 2016. 124(10): p. 1575-1582.
73. Ibarluzea, J., M.F. Fernandez, et al., Breast cancer risk and the combined effect of environmental
estrogens. Cancer Causes Control, 2004. 15: p. 591-600.
74. Multigner, L., Chlordecone exposure and risk of prostate cancer. Journal of Clinical Oncology, 2010.
28(21): p. 3457-3462.
75. Herbst, A.L., H. Ulfelder, and D.C. Poskanzer, Adenocarcinoma of the vagina. Association of
maternal stilbestrol therapy with tumor appearance in young women. N Engl J Med, 1971. 284(15):
p. 878-81.
76. OECD, Considerations for assessing the risks of combined exposure to chemicals. 2018. 296.
77. Dumbreck, S., A. Flynn, et al., Drug-disease and drug-drug interactions: systematic examination of
recommendations in 12 UK national clinical guidelines. BMJ, 2015. 350: p. h949.
78. Kortenkamp, A. and M. Faust, Regulate to reduce chemical mixture risk. Science, 2018. 361(6399):
p. 224-226.
79. Uno, T., A. Nakao, et al., Modification of small molecules by using cytochrome P450 expressed in
Escherichia coli. J Ind Microbiol Biotechnol, 2006. 33(12): p. 1043-50.
80. Braniste, V., A. Jouault, et al., Impact of oral bisphenol A at reference doses on intestinal barrier
function and sex differences after perinatal exposure in rats. Proc Natl Acad Sci U S A, 2010. 107(1):
p. 448-53.
81. Delfosse, V., B. Dendele, et al., Synergistic activation of human pregnane X receptor by binary
cocktails of pharmaceutical and environmental compounds. Nat Commun, 2015. 6: p. 8089.
82. Christiansen, S., M. Scholze, et al., Synergistic disruption of external male sex organ development by
a mixture of four antiandrogens. Environ Health Perspect, 2009. 117(12): p. 1839-46.
83. Kortenkamp, A., Ten years of mixing cocktails: a review of combination effects of endocrine-
disrupting chemicals. Environ Health Perspect, 2007. 115 Suppl 1: p. 98-105.
84. Gaudriault, P., S. Mazaud-Guittot, et al., Endocrine Disruption in Human Fetal Testis Explants by
Individual and Combined Exposures to Selected Pharmaceuticals, Pesticides, and Environmental
Pollutants. Environ Health Perspect, 2017. 125(8): p. 087004.
85. Christen, V., P. Crettaz, A. Oberli-Schrammli, and K. Fent, Antiandrogenic activity of phthalate
mixtures: validity of concentration addition. Toxicol Appl Pharmacol, 2012. 259(2): p. 169-76.

Policy Department for Citizens’ Rights and Constitutional Affairs
__________________________________________________________________________________________
112
86. Lukowicz, C., S. Ellero-Simatos, et al., Metabolic Effects of a Chronic Dietary Exposure to a Low-Dose
Pesticide Cocktail in Mice: Sexual Dimorphism and Role of the Constitutive Androstane Receptor.
Environ Health Perspect, 2018. 126(6): p. 067007.
87. RIVM, N.i.f.P.H.a.t.E., Addressing combined effects if chemicals in environmental safety under REACH
- a thought starter. 2016.
88. Heindel, J.J. and L.N. Vandenberg, Developmental origins of health and disease: a paradigm for
understanding disease cause and prevention. Curr Opin Pediatr, 2015. 27(2): p. 248-53.
89. Bourguignon, J.P., A.S. Parent, et al., Rationale for Environmental Hygiene towards global protection
of fetuses and young children from adverse lifestyle factors. Environ Health, 2018. 17(1): p. 42.
90. Crews, D., R. Gillette, I. Miller-Crews, A.C. Gore, and M.K. Skinner, Nature, nurture and epigenetics.
Mol Cell Endocrinol, 2014. 398(1-2): p. 42-52.
91. Cohn, B.A., M.S. Wolff, P.M. Cirillo, and R.I. Sholtz, DDT and breast cancer in young women: new data
on the significance of age at exposure. Environ Health Perspect, 2007. 115(10): p. 1406-14.
92. Sergeyev, O., J.S. Burns, et al., The association of peripubertal serum concentrations of
organochlorine chemicals and blood lead with growth and pubertal development in a longitudinal
cohort of boys: a review of published results from the Russian Children's Study. Rev Environ Health,
2017. 32(1-2): p. 83-92.
93. Slama, R., J.P. Bourguignon, et al., Scientific Issues Relevant to Setting Regulatory Criteria to Identify
Endocrine Disrupting Substances in the European Union. Environ Health Perspect, 2016.
94. Swan, S.H., Intrauterine exposure to diethylstilbestrol: long-term effects in humans. APMIS, 2000.
108(12): p. 793-804.
95. Skinner, M.K., M. Manikkam, et al., Ancestral dichlorodiphenyltrichloroethane (DDT) exposure
promotes epigenetic transgenerational inheritance of obesity. BMC Med, 2013. 11: p. 228.
96. Song, Y. and L. Yang, Transgenerational pancreatic impairment with Igf2/H19 epigenetic alteration
induced by p,p'-DDE exposure in early life. Toxicol Lett, 2017. 280: p. 222-231.
97. Solecki, R., A. Kortenkamp, et al., Scientific principles for the identification of endocrine-disrupting
chemicals: a consensus statement. Arch Toxicol, 2017. 91(2): p. 1001-1006.
98. OECD, Revised Guidance Document 150 on Standardised Test Guidelines for Evaluating Chemicals
for Endocrine Disruption. 2018.
https://www.oecd-ilibrary.org/docserver/9789264304741-
en.pdf?expires=1544870770&id=id&accname=guest&checksum=DDD08DC7B6BDBB58F93735
2A4716AFF7.
99. Slama, R., C. Vernet, F.L. Nassan, R. Hauser, and C. Philippat, Characterizing the effect of endocrine
disruptors on human health: The role of epidemiological cohorts. C R Biol, 2017.
https://www.sciencedirect.com/science/article/pii/S1631069117301294?via%3Dihub
100. EU Commission, Communication from the commission to the EU parliament, the Council, the EU
economic and social committe and the committee of the regions. Towards a comprehensive
European Union framework on endocrine disruptors. 2018. Available from:
http://ec.europa.eu/transparency/regdoc/rep/1/2018/EN/COM-2018-734-F1-EN-MAIN-PART-
1.PDF.
101. Bae, S. and Y.C. Hong, Exposure to bisphenol A from drinking canned beverages increases blood
pressure: randomized crossover trial. Hypertension, 2015. 65(2): p. 313-9.
Endocrine disruptors: from scientific evidence to human health protection
____________________________________________________________________________________________
113
102. Nassan, F.L., B.A. Coull, et al., A crossover-crossback prospective study of dibutyl-phthalate exposure
from mesalamine medications and semen quality in men with inflammatory bowel disease. Environ
Int, 2016. 95: p. 120-30.
103. Nassan, F.L., T.I.M. Korevaar, et al., Dibutyl-phthalate exposure from mesalamine medications and
serum thyroid hormones in men. International Journal of Hygiene and Environmental Health,
2019. 222(1): p. 101-110.
104. Muncke, J., Endocrine disrupting chemicals and other substances of concern in food contact
materials: an updated review of exposure, effect and risk assessment. J Steroid Biochem Mol Biol,
2011. 127(1-2): p. 118-27.
105. Groh, K.J., T. Backhaus, et al., Overview of known plastic packaging-associated chemicals and their
hazards. Sci Total Environ, 2019. 651(Pt 2): p. 3253-3268.
106. Geens, T., D. Aerts, et al., A review of dietary and non-dietary exposure to bisphenol-A. Food Chem
Toxicol, 2012. 50(10): p. 3725-40.
107. Murata, M. and J.H. Kang, Bisphenol A (BPA) and cell signaling pathways. Biotechnol Adv, 2018.
36(1): p. 311-327.
108. Vandenberg, L.N., S. Ehrlich, et al., Low dose effects of bisphenol A. Endocrine Disruptors, 2014. 1(1):
p. e26490.
109. Rebuli, M.E., L. Camacho, et al., Impact of Low-Dose Oral Exposure to Bisphenol A (BPA) on Juvenile
and Adult Rat Exploratory and Anxiety Behavior: A CLARITY-BPA Consortium Study. Toxicol Sci, 2015.
148(2): p. 341-54.
110. Tomza-Marciniak, A., P. Stepkowska, J. Kuba, and B. Pilarczyk, Effect of bisphenol A on reproductive
processes: A review of in vitro, in vivo and epidemiological studies. J Appl Toxicol, 2018. 38(1): p. 51-
80.
111. Peretz, J., L. Vrooman, et al., Bisphenol a and reproductive health: update of experimental and
human evidence, 2007-2013. Environ Health Perspect, 2014. 122(8): p. 775-86.
112. Mhaouty-Kodja, S., L.P. Belzunces, et al., Impairment of learning and memory performances induced
by BPA: Evidences from the literature of a MoA mediated through an ED. Mol Cell Endocrinol, 2018.
475: p. 54-73.
113. Perrier, F., L. Giorgis Allemand, R. Slama, and C. Philippat, Within-subject pooling of biological
samples as a way to reduce exposure misclassification in biomarker-based studies of chemicals with
high temporal variability. Epidemiology, 2016. 27(3): p. 378-388.
114. Perera, F., E.L.R. Nolte, et al., Bisphenol A exposure and symptoms of anxiety and depression among
inner city children at 10-12 years of age. Environ Res, 2016. 151: p. 195-202.
115. Rochester, J.R., A.L. Bolden, and C.F. Kwiatkowski, Prenatal exposure to bisphenol A and
hyperactivity in children: a systematic review and meta-analysis. Environ Int, 2018. 114: p. 343-356.
116. Mustieles, V., R. Perez-Lobato, N. Olea, and M.F. Fernandez, Bisphenol A: Human exposure and
neurobehavior. Neurotoxicology, 2015. 49: p. 174-84.
117. Melzer, D., N.J. Osborne, et al., Urinary bisphenol A concentration and risk of future coronary artery
disease in apparently healthy men and women. Circulation, 2012. 125(12): p. 1482-90.
Policy Department for Citizens’ Rights and Constitutional Affairs
__________________________________________________________________________________________
114
118. Arambula, S.E., S.M. Belcher, A. Planchart, S.D. Turner, and H.B. Patisaul, Impact of Low Dose Oral
Exposure to Bisphenol A (BPA) on the Neonatal Rat Hypothalamic and Hippocampal Transcriptome:
A CLARITY-BPA Consortium Study. Endocrinology, 2016. 157(10): p. 3856-3872.
119. Prins, G.S., H.B. Patisaul, S.M. Belcher, and L.N. Vandenberg, CLARITY-BPA academic laboratory
studies identify consistent low-dose Bisphenol A effects on multiple organ systems. Basic Clin
Pharmacol Toxicol, 2018.
120. Hessel, E.V.S., J. Ezendam, et al., Assessment of recent developmental immunotoxicity studies with
bisphenol A in the context of the 2015 EFSA t-TDI. Reproductive Toxicology, 2016. 65: p. 448-456.
121. Faessler, D., G. McCombie, M. Biedermann, F. Felder, and U. Subotic, Leaching of plasticizers from
polyvinylchloride perfusion lines by different lipid emulsions for premature infants under clinical
conditions. International Journal of Pharmaceutics, 2017. 520(1-2): p. 119-125.
122. Casas, M., C. Chevrier, et al., Exposure to brominated flame retardants, perfluorinated compounds,
phthalates and phenols in European birth cohorts: ENRIECO evaluation, first human biomonitoring
results, and recommendations. International journal of hygiene and environmental health, 2013.
216(3): p. 230-42.
123. Haug, L.S., A.K. Sakhi, et al., In-utero and childhood chemical exposome in six European mother-child
cohorts. Environ Int, 2018. 121(Pt 1): p. 751-763.
124. Koch, H.M., M. Ruther, et al., Phthalate metabolites in 24-h urine samples of the German
Environmental Specimen Bank (ESB) from 1988 to 2015 and a comparison with US NHANES data from
1999 to 2012. Int J Hyg Environ Health, 2017. 220(2 Pt A): p. 130-141.
125. Varshavsky, J.R., R. Morello-Frosch, T.J. Woodruff, and A.R. Zota, Dietary sources of cumulative
phthalates exposure among the U.S. general population in NHANES 2005-2014. Environ Int, 2018.
115: p. 417-429.
126. Stroheker, T., N. Cabaton, et al., Evaluation of anti-androgenic activity of di-(2-ethylhexyl)phthalate.
Toxicology, 2005. 208(1): p. 115-21.
127. Wojtowicz, A.K., A.M. Sitarz-Glownia, M. Szczesna, and K.A. Szychowski, The Action of Di-(2-
Ethylhexyl) Phthalate (DEHP) in Mouse Cerebral Cells Involves an Impairment in Aryl Hydrocarbon
Receptor (AhR) Signaling. Neurotox Res, 2019. 35(1): p. 183-195.
128. Boas, M., H. Frederiksen, et al., Childhood exposure to phthalates: associations with thyroid function,
insulin-like growth factor I, and growth. Environ Health Perspect, 2010. 118(10): p. 1458-64.
129. Swan, S.H., S. Sathyanarayana, et al., First trimester phthalate exposure and anogenital distance in
newborns. Hum Reprod, 2015. 30(4): p. 963-72.
130. Radke, E.G., J.M. Braun, J.D. Meeker, and G.S. Cooper, Phthalate exposure and male reproductive
outcomes: A systematic review of the human epidemiological evidence. Environment International,
2018. 121: p. 764-793.
131. Ferguson, K.K., T.F. McElrath, and J.D. Meeker, Environmental phthalate exposure and preterm birth.
JAMA Pediatr, 2014. 168(1): p. 61-7.
132. Sathyanarayana, S., S. Butts, et al., Early Prenatal Phthalate Exposure, Sex Steroid Hormones, and
Birth Outcomes. J Clin Endocrinol Metab, 2017. 102(6): p. 1870-1878.
Endocrine disruptors: from scientific evidence to human health protection
____________________________________________________________________________________________
115
133. Miodovnik, A., A. Edwards, D.C. Bellinger, and R. Hauser, Developmental neurotoxicity of ortho-
phthalate diesters: Review of human and experimental evidence. NeuroToxicology, 2014. 41: p. 112-
122.
134. Olesen, T.S., D. Bleses, et al., Prenatal phthalate exposure and language development in toddlers
from the Odense Child Cohort. Neurotoxicol Teratol, 2018. 65: p. 34-41.
135. Minguez-Alarcon, L., I. Souter, et al., Urinary concentrations of cyclohexane-1,2-dicarboxylic acid
monohydroxy isononyl ester, a metabolite of the non-phthalate plasticizer di(isononyl)cyclohexane-
1,2-dicarboxylate (DINCH), and markers of ovarian response among women attending a fertility
center. Environ Res, 2016. 151: p. 595-600.
136. Trier, X., K. Granby, and J.H. Christensen, Polyfluorinated surfactants (PFS) in paper and board
coatings for food packaging. Environ Sci Pollut Res Int, 2011. 18(7): p. 1108-20.
137. Blum, A., S.A. Balan, et al., The Madrid Statement on Poly- and Perfluoroalkyl Substances (PFASs).
Environ Health Perspect, 2015. 123(5): p. A107-11.
138. Averina, M., J. Brox, S. Huber, and A.S. Furberg, Perfluoroalkyl substances in adolescents in northern
Norway: Lifestyle and dietary predictors. The Tromso study, Fit Futures 1. Environ Int, 2018. 114: p.
123-130.
139. Zabaleta, I., E. Bizkarguenaga, et al., Fast and simple determination of perfluorinated compounds
and their potential precursors in different packaging materials. Talanta, 2016. 152: p. 353-63.
140. Ji, K., S. Kim, et al., Serum concentrations of major perfluorinated compounds among the general
population in Korea: dietary sources and potential impact on thyroid hormones. Environ Int, 2012.
45: p. 78-85.
141. Shah-Kulkarni, S., B.M. Kim, et al., Prenatal exposure to perfluorinated compounds affects thyroid
hormone levels in newborn girls. Environ Int, 2016. 94: p. 607-613.
142. Dufour, P., C. Pirard, M.C. Seghaye, and C. Charlier, Association between organohalogenated
pollutants in cord blood and thyroid function in newborns and mothers from Belgian population.
Environ Pollut, 2018. 238: p. 389-396.
143. Kar, S., M.S. Sepulveda, K. Roy, and J. Leszczynski, Endocrine-disrupting activity of per- and
polyfluoroalkyl substances: Exploring combined approaches of ligand and structure based modeling.
Chemosphere, 2017. 184: p. 514-523.
144. Long, M., M. Ghisari, and E.C. Bonefeld-Jorgensen, Effects of perfluoroalkyl acids on the function of
the thyroid hormone and the aryl hydrocarbon receptor. Environ Sci Pollut Res Int, 2013. 20(11): p.
8045-56.
145. Oulhote, Y., U. Steuerwald, F. Debes, P. Weihe, and P. Grandjean, Behavioral difficulties in 7-year
old children in relation to developmental exposure to perfluorinated alkyl substances. Environ Int,
2016. 97: p. 237-245.
146. Braun, J.M., A.E. Kalkbrenner, et al., Gestational exposure to endocrine-disrupting chemicals and
reciprocal social, repetitive, and stereotypic behaviors in 4- and 5-year-old children: the HOME study.
Environ Health Perspect, 2014. 122(5): p. 513-20.
147. Liew, Z., B. Ritz, et al., Attention deficit/hyperactivity disorder and childhood autism in association
with prenatal exposure to perfluoroalkyl substances: a nested case-control study in the Danish
National Birth Cohort. Environ Health Perspect, 2015. 123(4): p. 367-73.
Policy Department for Citizens’ Rights and Constitutional Affairs
__________________________________________________________________________________________
116
148. Timmermann, C.A., E. Budtz-Jorgensen, et al., Association between perfluoroalkyl substance
exposure and asthma and allergic disease in children as modified by MMR vaccination. J
Immunotoxicol, 2017. 14(1): p. 39-49.
149. Dalsager, L., N. Christensen, et al., Association between prenatal exposure to perfluorinated
compounds and symptoms of infections at age 1-4years among 359 children in the Odense Child
Cohort. Environ Int, 2016. 96: p. 58-64.
150. Howie, D., A. Ten Bokum, A.S. Necula, S.P. Cobbold, and H. Waldmann, The Role of Lipid
Metabolism in T Lymphocyte Differentiation and Survival. Front Immunol, 2017. 8: p. 1949.
151. Halldorsson, T.I., D. Rytter, et al., Prenatal exposure to perfluorooctanoate and risk of overweight at
20 years of age: a prospective cohort study. Environ Health Perspect, 2012. 120(5): p. 668-73.
152. Steenland, K., L. Zhao, A. Winquist, and C. Parks, Ulcerative Colitis and Perfluorooctanoic Acid
(PFOA) in a Highly Exposed Population of Community Residents and Workers in the Mid-Ohio Valley.
Environmental Health Perspectives, 2013. 121(8): p. 900-905.
153. Barry, V., A. Winquist, and K. Steenland, Perfluorooctanoic acid (PFOA) exposures and incident
cancers among adults living near a chemical plant. Environ Health Perspect, 2013. 121(11-12): p.
1313-8.
154. Darrow, L.A., A.C. Groth, et al., Modeled Perfluorooctanoic Acid (PFOA) Exposure and Liver Function
in a Mid-Ohio Valley Community. Environ Health Perspect, 2016. 124(8): p. 1227-33.
155. Karpuzoglu, E., S.D. Holladay, and R.M. Gogal, Jr., Parabens: potential impact of low-affinity
estrogen receptor binding chemicals on human health. J Toxicol Environ Health B Crit Rev, 2013.
16(5): p. 321-35.
156. Maske, P., V. Dighe, and G. Vanage, n-butylparaben exposure during perinatal period impairs fertility
of the F1 generation female rats. Chemosphere, 2018. 213: p. 114-123.
157. Pollock, T., R.E. Weaver, R. Ghasemi, and D. deCatanzaro, Butyl paraben and propyl paraben
modulate bisphenol A and estradiol concentrations in female and male mice. Toxicol Appl
Pharmacol, 2017. 325: p. 18-24.
158. Philippat, C., M.S. Wolff, et al., Prenatal exposure to environmental phenols: concentrations in
amniotic fluid and variability in urinary concentrations during pregnancy. Environ Health Perspect,
2013. 121(10): p. 1225-31.
159. Guo, J., C. Wu, et al., Urinary paraben concentrations and their associations with anthropometric
measures of children aged 3 years. Environ Pollut, 2017. 222: p. 307-314.
160. Philippat, C., J. Botton, et al., Prenatal Exposure to Phenols and Growth in Boys. Epidemiology, 2014.
25(5): p. 625-635.
161. Aker, A.M., D.J. Watkins, et al., Phenols and parabens in relation to reproductive and thyroid
hormones in pregnant women. Environ Res, 2016. 151: p. 30-37.
162. Kolatorova, L., M. Duskova, J. Vitku, and L. Starka, Prenatal exposure to bisphenols and parabens
and impacts on human physiology. Physiol Res, 2017. 66(Supplementum 3): p. S305-S315.
163. Shekhar, S., S. Sood, et al., Detection of phenolic endocrine disrupting chemicals (EDCs) from
maternal blood plasma and amniotic fluid in Indian population. Gen Comp Endocrinol, 2017. 241:
p. 100-107.
Endocrine disruptors: from scientific evidence to human health protection
____________________________________________________________________________________________
117
164. Krause, M., H. Frederiksen, et al., Presence of benzophenones commonly used as UV filters and
absorbers in paired maternal and fetal samples. Environ Int, 2018. 110: p. 51-60.
165. Wang, X., F. Ouyang, et al., Maternal Urinary Triclosan Concentration in Relation to Maternal and
Neonatal Thyroid Hormone Levels: A Prospective Study. Environ Health Perspect, 2017. 125(6): p.
067017.
166. Crofton, K.M., K.B. Paul, M.J. Devito, and J.M. Hedge, Short-term in vivo exposure to the water
contaminant triclosan: Evidence for disruption of thyroxine. Environ Toxicol Pharmacol, 2007. 24(2):
p. 194-7.
167. Paul, K.B., J.T. Thompson, S.O. Simmons, J.P. Vanden Heuvel, and K.M. Crofton, Evidence for
triclosan-induced activation of human and rodent xenobiotic nuclear receptors. Toxicol In Vitro,
2013. 27(7): p. 2049-60.
168. Paul, K.B., J.M. Hedge, et al., Developmental triclosan exposure decreases maternal, fetal, and early
neonatal thyroxine: a dynamic and kinetic evaluation of a putative mode-of-action. Toxicology,
2012. 300(1-2): p. 31-45.
169. Paul, K.B., J.M. Hedge, et al., Development of a thyroperoxidase inhibition assay for high-throughput
screening. Chem Res Toxicol, 2014. 27(3): p. 387-99.
170. Lankester, J., C. Patel, M.R. Cullen, C. Ley, and J. Parsonnet, Urinary triclosan is associated with
elevated body mass index in NHANES. PLoS One, 2013. 8(11): p. e80057.
171. Lassen, T.H., H. Frederiksen, et al., Prenatal Triclosan Exposure and Anthropometric Measures
including Anogenital Distance in Danish Infants. Environ Health Perspect, 2016.
172. Etzel, T.M., A.M. Calafat, et al., Urinary triclosan concentrations during pregnancy and birth
outcomes. Environ Res, 2017. 156: p. 505-511.
173. Krause, M., H. Frederiksen, et al., Maternal exposure to UV filters: associations with maternal thyroid
hormones, IGF-I/IGFBP3 and birth outcomes. Endocr Connect, 2018. 7(2): p. 334-346.
174. Schlumpf, M., P. Schmid, et al., Endocrine activity and developmental toxicity of cosmetic UV filters-
-an update. Toxicology, 2004. 205(1-2): p. 113-22.
175. Lee, J., S. Kim, Y.J. Park, H.B. Moon, and K. Choi, Thyroid Hormone-Disrupting Potentials of Major
Benzophenones in Two Cell Lines (GH3 and FRTL-5) and Embryo-Larval Zebrafish. Environ Sci
Technol, 2018. 52(15): p. 8858-8865.
176. Kim, S., S. Kim, S. Won, and K. Choi, Considering common sources of exposure in association studies
- Urinary benzophenone-3 and DEHP metabolites are associated with altered thyroid hormone
balance in the NHANES 2007-2008. Environ Int, 2017. 107: p. 25-32.
177. Krause, M., A. Klit, et al., Sunscreens: are they beneficial for health? An overview of endocrine
disrupting properties of UV-filters. Int J Androl, 2012. 35(3): p. 424-36.
178. Swan, S.H., Intrauterine exposure to diethylstilbestrol: long-term effects in humans. APMIS : acta
pathologica, microbiologica, et immunologica Scandinavica, 2000. 108(12): p. 793-804.
179. Kristensen, D.M., S. Mazaud-Guittot, et al., Analgesic use - prevalence, biomonitoring and endocrine
and reproductive effects. Nat Rev Endocrinol, 2016. 12(7): p. 381-93.
180. Jegou, B., Reproductive endocrinology: Paracetamol-induced endocrine disruption in human fetal
testes. Nat Rev Endocrinol, 2015. 11(8): p. 453-4.
Policy Department for Citizens’ Rights and Constitutional Affairs
__________________________________________________________________________________________
118
181. Bauer, A.Z., D. Kriebel, M.R. Herbert, C.G. Bornehag, and S.H. Swan, Prenatal paracetamol exposure
and child neurodevelopment: A review. Horm Behav, 2018. 101: p. 125-147.
182. Bornehag, C.G., A. Reichenberg, et al., Prenatal exposure to acetaminophen and children's language
development at 30 months. Eur Psychiatry, 2018. 51: p. 98-103.
183. Dunagan, S.C., R.E. Dodson, R.A. Rudel, and J.G. Brody, Toxics Use Reduction in the Home: Lessons
Learned from Household Exposure Studies. J Clean Prod, 2011. 19(5): p. 438-444.
184. Lema, S.C., J.T. Dickey, I.R. Schultz, and P. Swanson, Dietary exposure to 2,2',4,4'-
tetrabromodiphenyl ether (PBDE-47) alters thyroid status and thyroid hormone-regulated gene
transcription in the pituitary and brain. Environ Health Perspect, 2008. 116(12): p. 1694-9.
185. Stapleton, H.M., S. Eagle, R. Anthopolos, A. Wolkin, and M.L. Miranda, Associations between
polybrominated diphenyl ether (PBDE) flame retardants, phenolic metabolites, and thyroid
hormones during pregnancy. Environ Health Perspect, 2011. 119(10): p. 1454-9.
186. Krol, S., J. Namiesnik, and B. Zabiegala, Occurrence and levels of polybrominated diphenyl ethers
(PBDEs) in house dust and hair samples from Northern Poland; an assessment of human exposure.
Chemosphere, 2014. 110: p. 91-6.
187. Lam, J., B.P. Lanphear, et al., Developmental PBDE Exposure and IQ/ADHD in Childhood: A
Systematic Review and Meta-analysis. Environ Health Perspect, 2017. 125(8): p. 086001.
188. Eskenazi, B., J. Chevrier, et al., In utero and childhood polybrominated diphenyl ether (PBDE)
exposures and neurodevelopment in the CHAMACOS study. Environ Health Perspect, 2013. 121(2):
p. 257-62.
189. Chen, A., K. Yolton, et al., Prenatal Polybrominated Diphenyl Ether Exposures and
Neurodevelopment in U.S. Children through 5 Years of Age: The HOME Study. Environ Health
Perspect, 2014. 122(8): p. 856-62.
190. Choi, G., Y.B. Wang, et al., Polybrominated diphenyl ethers and incident pregnancy loss: The LIFE
Study. Environ Res, 2019. 168: p. 375-381.
191. Suvorov, A., A. Shershebnev, et al., Perinatal exposure to low dose 2,2',4,4'-tetrabromodiphenyl ether
(BDE-47) alters sperm DNA methylation in adult rats. Reprod Toxicol, 2018. 75: p. 136-143.
192. Lilienthal, H., C.M. Verwer, L.T. van der Ven, A.H. Piersma, and J.G. Vos, Exposure to
tetrabromobisphenol A (TBBPA) in Wistar rats: neurobehavioral effects in offspring from a one-
generation reproduction study. Toxicology, 2008. 246(1): p. 45-54.
193. Hill, K.L., T. Hamers, J.H. Kamstra, W.G. Willmore, and R.J. Letcher, Organophosphate triesters and
selected metabolites enhance binding of thyroxine to human transthyretin in vitro. Toxicol Lett,
2018. 285: p. 87-93.
194. Meeker, J.D. and H.M. Stapleton, House dust concentrations of organophosphate flame retardants
in relation to hormone levels and semen quality parameters. Environ Health Perspect, 2010. 118(3):
p. 318-23.
195. Vinas, P., N. Campillo, M. Pastor-Belda, A. Oller, and M. Hernandez-Cordoba, Determination of
phthalate esters in cleaning and personal care products by dispersive liquid-liquid microextraction
and liquid chromatography-tandem mass spectrometry. J Chromatogr A, 2015. 1376: p. 18-25.
Endocrine disruptors: from scientific evidence to human health protection
____________________________________________________________________________________________
119
196. Filipovic, M., A. Woldegiorgis, et al., Historical usage of aqueous film forming foam: a case study of
the widespread distribution of perfluoroalkyl acids from a military airport to groundwater, lakes, soils
and fish. Chemosphere, 2015. 129: p. 39-45.
197. Warembourg, C., A.C. Binter, et al., Prenatal exposure to glycol ethers and sex steroid hormones at
birth. Environ Int, 2018. 113: p. 66-73.
198. Warembourg, C., J. Botton, et al., Prenatal exposure to glycol ethers and cryptorchidism and
hypospadias: a nested case-control study. Occup Environ Med, 2018. 75(1): p. 59-65.
199. Beranger, R., R. Garlantezec, et al., Prenatal Exposure to Glycol Ethers and Neurocognitive Abilities in
6-Year-Old Children: The PELAGIE Cohort Study. Environ Health Perspect, 2017. 125(4): p. 684-690.
200. Garlantezec, R., C. Warembourg, et al., Urinary glycol ether metabolites in women and time to
pregnancy: the PELAGIE cohort. Environ Health Perspect, 2013. 121(10): p. 1167-73.
201. Sonnenschein, C. and A.M. Soto, An updated review of environmental estrogen and androgen
mimics and antagonists. J Steroid Biochem Mol Biol, 1998. 65(1-6): p. 143-50.
202. Noorimotlagh, Z., N.J. Haghighi, M. Ahmadimoghadam, and F. Rahim, An updated systematic
review on the possible effect of nonylphenol on male fertility. Environ Sci Pollut Res Int, 2017. 24(4):
p. 3298-3314.
203. Rattan, S., C. Zhou, et al., Exposure to endocrine disruptors during adulthood: consequences for
female fertility. J Endocrinol, 2017. 233(3): p. R109-R129.
204. Tsai, M.S., C.H. Chang, et al., Neonatal outcomes of intrauterine nonylphenol exposure--a
longitudinal cohort study in Taiwan. Sci Total Environ, 2013. 458-460: p. 367-73.
205. Rudel, R.A. and L.J. Perovich, Endocrine disrupting chemicals in indoor and outdoor air. Atmos
Environ (1994), 2009. 43(1): p. 170-181.
206. Jeon, S., K.T. Kim, and K. Choi, Migration of DEHP and DINP into dust from PVC flooring products at
different surface temperature. Sci Total Environ, 2016. 547: p. 441-446.
207. Carlstedt, F., B.A. Jonsson, and C.G. Bornehag, PVC flooring is related to human uptake of phthalates
in infants. Indoor Air, 2013. 23(1): p. 32-9.
208. Mallow, E.B. and M.A. Fox, Phthalates and critically ill neonates: device-related exposures and non-
endocrine toxic risks. J Perinatol, 2014. 34(12): p. 892-7.
209. Shea, K.M. and H. American Academy of Pediatrics Committee on Environmental, Pediatric
exposure and potential toxicity of phthalate plasticizers. Pediatrics, 2003. 111(6 Pt 1): p. 1467-74.
210. Andaluri, G., M. Manickavachagam, and R. Suri, Plastic toys as a source of exposure to bisphenol-A
and phthalates at childcare facilities. Environ Monit Assess, 2018. 190(2): p. 65.
211. Schettler, T., Human exposure to phthalates via consumer products. Int J Androl, 2006. 29(1): p. 134-
9; discussion 181-5.
212. EFSA, Scientific Opinion on the identification of pesticides to be included in cumulative assessment
groups on the basis of their toxicological profile1, in EFSA Journal 2013;11(7):3293. 2013.
213. Mie, A., C. Ruden, and P. Grandjean, Safety of Safety Evaluation of Pesticides: developmental
neurotoxicity of chlorpyrifos and chlorpyrifos-methyl. Environ Health, 2018. 17(1): p. 77.
Policy Department for Citizens’ Rights and Constitutional Affairs
__________________________________________________________________________________________
120
214. Bellanger, M., B. Demeneix, P. Grandjean, R.T. Zoeller, and L. Trasande, Neurobehavioral deficits,
diseases, and associated costs of exposure to endocrine-disrupting chemicals in the European Union.
J Clin Endocrinol Metab, 2015. 100(4): p. 1256-66.
215. Kugathas, S., K. Audouze, et al., Effects of Common Pesticides on Prostaglandin D2 (PGD2) Inhibition
in SC5 Mouse Sertoli Cells, Evidence of Binding at the COX-2 Active Site, and Implications for Endocrine
Disruption. Environ Health Perspect, 2016. 124(4): p. 452-9.
216. Brucker-Davis, F., Effects of environmental synthetic chemicals on thyroid function. Thyroid, 1998.
8(9): p. 827-56.
217. Mantovani, A., Endocrine Disrupters and the Safety of Food Chains. Horm Res Paediatr, 2016. 86(4):
p. 279-288.
218. Hertz-Picciotto, I., J.B. Sass, et al., Organophosphate exposures during pregnancy and child
neurodevelopment: Recommendations for essential policy reforms. PLoS Med, 2018. 15(10): p.
e1002671.
219. Holzer, G., M. Besson, et al., Fish larval recruitment to reefs is a thyroid hormone-mediated
metamorphosis sensitive to the pesticide chlorpyrifos. Elife, 2017. 6.
220. Egbuta, C., J. Lo, and D. Ghosh, Mechanism of inhibition of estrogen biosynthesis by azole fungicides.
Endocrinology, 2014. 155(12): p. 4622-8.
221. Bernabo, I., A. Guardia, R. Macirella, S. Tripepi, and E. Brunelli, Chronic exposures to fungicide
pyrimethanil: multi-organ effects on Italian tree frog (Hyla intermedia). Sci Rep, 2017. 7(1): p. 6869.
222. Rieke, S., T. Heise, et al., Mixture effects of azole fungicides on the adrenal gland in a broad dose
range. Toxicology, 2017. 385: p. 28-37.
223. Lv, X., L. Pan, et al., Effects of triazole fungicides on androgenic disruption and CYP3A4 enzyme
activity. Environ Pollut, 2017. 222: p. 504-512.
224. Costa, N.O., M.L. Vieira, et al., Evaluation of the reproductive toxicity of fungicide propiconazole in
male rats. Toxicology, 2015. 335: p. 55-61.
225. Axelstad, M., J. Boberg, et al., Exposure to the widely used fungicide mancozeb causes thyroid
hormone disruption in rat dams but no behavioral effects in the offspring. Toxicol Sci, 2011. 120(2):
p. 439-46.
226. Vinggaard, A.M., U. Hass, et al., Prochloraz: an imidazole fungicide with multiple mechanisms of
action. Int J Androl, 2006. 29(1): p. 186-92.
227. Svechnikov, K., V. Supornsilchai, et al., Influence of long-term dietary administration of
procymidone, a fungicide with anti-androgenic effects, or the phytoestrogen genistein to rats on the
pituitary-gonadal axis and Leydig cell steroidogenesis. J Endocrinol, 2005. 187(1): p. 117-24.
228. Brander, S.M., M.K. Gabler, N.L. Fowler, R.E. Connon, and D. Schlenk, Pyrethroid Pesticides as
Endocrine Disruptors: Molecular Mechanisms in Vertebrates with a Focus on Fishes. Environ Sci
Technol, 2016. 50(17): p. 8977-92.
229. Tu, W., C. Xu, et al., Acute exposure to synthetic pyrethroids causes bioconcentration and disruption
of the hypothalamus-pituitary-thyroid axis in zebrafish embryos. Sci Total Environ, 2016. 542(Pt A):
p. 876-85.
Endocrine disruptors: from scientific evidence to human health protection
____________________________________________________________________________________________
121
230. Ye, X., W. Pan, et al., Association of pyrethroids exposure with onset of puberty in Chinese girls.
Environ Pollut, 2017. 227: p. 606-612.
231. Saillenfait, A.M., D. Ndiaye, and J.P. Sabate, The estrogenic and androgenic potential of pyrethroids
in vitro. Review. Toxicol In Vitro, 2016. 34: p. 321-32.
232. Crall, J.D., C.M. Switzer, et al., Neonicotinoid exposure disrupts bumblebee nest behavior, social
networks, and thermoregulation. Science, 2018. 362(6415): p. 683-686.
233. Manjon, C., B.J. Troczka, et al., Unravelling the Molecular Determinants of Bee Sensitivity to
Neonicotinoid Insecticides. Curr Biol, 2018. 28(7): p. 1137-1143 e5.
234. Caron-Beaudoin, E., R. Viau, and J.T. Sanderson, Effects of Neonicotinoid Pesticides on Promoter-
Specific Aromatase (CYP19) Expression in Hs578t Breast Cancer Cells and the Role of the VEGF
Pathway. Environ Health Perspect, 2018. 126(4): p. 047014.
235. Mesnage, R., M. Biserni, D. Genkova, L. Wesolowski, and M.N. Antoniou, Evaluation of
neonicotinoid insecticides for oestrogenic, thyroidogenic and adipogenic activity reveals
imidacloprid causes lipid accumulation. J Appl Toxicol, 2018. 38(12): p. 1483-1491.
236. Reynders, H., A. Colles, et al., The added value of a surveillance human biomonitoring program: The
case of FLEHS in Flanders (Belgium). International Journal of Hygiene and Environmental Health,
2017. 220(2): p. 46-54.
237. Dereumeaux, C., C. Fillol, M.A. Charles, and S. Denys, The French human biomonitoring program:
First lessons from the perinatal component and future needs. Int J Hyg Environ Health, 2017. 220(2
Pt A): p. 64-70.
238. Correia-Sa, L., M. Kasper-Sonnenberg, et al., Exposure assessment to bisphenol A (BPA) in
Portuguese children by human biomonitoring. Environ Sci Pollut Res Int, 2017. 24(35): p. 27502-
27514.
239. Trasande, L., R.T. Zoeller, et al., Estimating burden and disease costs of exposure to endocrine-
disrupting chemicals in the European union. J Clin Endocrinol Metab, 2015. 100(4): p. 1245-55.
240. Trasande, L., R.T. Zoeller, et al., Burden of disease and costs of exposure to endocrine disrupting
chemicals in the European Union: an updated analysis. Andrology, 2016. 4(4): p. 565-72.
241. Bond, G.G. and D.R. Dietrich, Human cost burden of exposure to endocrine disrupting chemicals. A
critical review. Arch Toxicol, 2017. 91(8): p. 2745-2762.
242. Eaton, D.L., R.B. Daroff, et al., Review of the toxicology of chlorpyrifos with an emphasis on human
exposure and neurodevelopment. Crit Rev Toxicol, 2008. 38 Suppl 2: p. 1-125.
243. Gunier, R.B., A. Bradman, K.G. Harley, K. Kogut, and B. Eskenazi, Prenatal Residential Proximity to
Agricultural Pesticide Use and IQ in 7-Year-Old Children. Environ Health Perspect, 2017. 125(5): p.
057002.
244. Whyatt, R.M., V. Rauh, et al., Prenatal insecticide exposures and birth weight and length among an
urban minority cohort. Environ Health Perspect, 2004. 112(10): p. 1125-32.
245. Lam, J., B.P. Lanphear, et al., Developmental PBDE Exposure and IQ/ADHD in childhood: A Systematic
Review and Meta-analysis. Environmental Health Perspectives, 2017.
246. Casas, M., X. Basagana, et al., Variability of urinary concentrations of non-persistent chemicals in
pregnant women and school-aged children. Environ Int, 2018. 121(Pt 1): p. 561-573.

Policy Department for Citizens’ Rights and Constitutional Affairs
__________________________________________________________________________________________
122
247. Warner, M., M. Ye, et al., Prenatal DDT exposure and child adiposity at age 12: The CHAMACOS study.
Environ Res, 2017. 159: p. 606-612.
248. Joint Research Center (JRC), Thresholds for Endocrine Disrupters and Related Uncertainties, in JRC
scientific and policy reports. 2013. Available from:
https://ec.europa.eu/jrc/en/publication/eur-
scientific-and-technical-research-reports/thresholds-endocrine-disrupters-and-related-
uncertainties.
249. Ceccatelli, R., O. Faass, M. Schlumpf, and W. Lichtensteiger, Gene expression and estrogen
sensitivity in rat uterus after developmental exposure to the polybrominated diphenylether PBDE 99
and PCB. Toxicology, 2006. 220(2-3): p. 104-16.
250. Kleinstreuer, N.C., P.C. Ceger, et al., A Curated Database of Rodent Uterotrophic Bioactivity. Environ
Health Perspect, 2016. 124(5): p. 556-62.
251. European Commission, Commission Delegated Regulation (EU) 2017/2100 of 4 September 2017
setting out scientific criteria for the determination of endocrine-disrupting properties pursuant to
Regulation (EU) No 528/2012 of the European Parliament and Council., European commission,
Editor. 2017: OJ L 301, 17.11.2017. p. 1-5. Available from:
http://data.europa.eu/eli/reg_del/2017/2100/oj
.
252. European Commission, Commission Regulation (EU) 2018/605 of 19 April 2018 setting out scientific
criteria for the determination of endocrine- disrupting and amending Annex II to Regulation (EC)
1107/2009. , European Commission, Editor. 2018: OJ L 101, 20.4.2018. p. 33-36. Available from:
http://data.europa.eu/eli/reg/2018/605/oj
.
253. ECHA, EFSA, et al., Guidance for the identification of endocrine disruptors in the contect of
regulations (EU) No 528/2012 and (EC) 1107/2009. EFSA Journal, 2018. 16(6): p. 1-135.
254. Law, R.J., A. Covaci, et al., Levels and trends of PBDEs and HBCDs in the global environment: status at
the end of 2012. Environ Int, 2014. 65: p. 147-58.
255. Carlsen, E., A. Giwercman, N. Keiding, and N.E. Skakkebaek, Evidence for decreasing quality of
semen during past 50 years. British Medical Journal, 1992. 305(6854): p. 609-13.
256. Carlsen, E., A. Giwercman, N. Keiding, and N.E. Skakkebaek, Declining semen quality and increasing
incidence of testicular cancer: is there a common cause? Environ Health Perspect, 1995. 103 Suppl
7: p. 137-9.
257. Skakkebaek, N.E., E. Rajpert-De Meyts, and K.M. Main, Testicular dysgenesis syndrome: an
increasingly common developmental disorder with environmental aspects. Hum Reprod, 2001.
16(5): p. 972-8.
258. Hänninen, O., A.B. Knol, et al., Environmental Burden of Disease in Europe: Assessing Nine Risk
Factors in Six Countries. Environmental Health Perspectives, 2014. 122(5): p. 439-446.
259. Michaels, D. and C. Monforton, Manufacturing uncertainty: contested science and the protection of
the public's health and environment. American Journal of Public Health, 2005. 95 Suppl 1: p. S39-
48.
260. Dietrich, D.R., S. Aulock, et al., Scientifically unfounded precaution drives European Commission's
recommendations on EDC regulation, while defying common sense, well-established science and risk
assessment principles. Chem Biol Interact, 2013. 205(1): p. A1-5.

Endocrine disruptors: from scientific evidence to human health protection
____________________________________________________________________________________________
123
261. Benbrook, C.M., How did the US EPA and IARC reach diametrically opposed conclusions on the
genotoxicity of glyphosate-based herbicides? Environmental Sciences Europe, 2019. 31(1).
262. European Parliament, Regulation (EU) No 528/2012 of 22 May 2012 concerning the making available
on the market and use of biocidal products (BPR). 2012. Available from:
http://eur-
lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02012R0528-20140425&from=EN.
263. Grandjean, P., L. Abdennebi-Najar, et al., Timescales of developmental toxicity impacting on
research and needs for intervention. Basic Clin Pharmacol Toxicol, 2018.
264. Windsor, F.M., S.J. Ormerod, and C.R. Tyler, Endocrine disruption in aquatic systems: up-scaling
research to address ecological consequences. Biol Rev Camb Philos Soc, 2018. 93(1): p. 626-641.
265. Gilbert, J.A., R.A. Quinn, et al., Microbiome-wide association studies link dynamic microbial consortia
to disease. Nature, 2016. 535(7610): p. 94-103.
266. Thaiss, C.A., N. Zmora, M. Levy, and E. Elinav, The microbiome and innate immunity. Nature, 2016.
535(7610): p. 65-74.
267. Evans, J.M., L.S. Morris, and J.R. Marchesi, The gut microbiome: the role of a virtual organ in the
endocrinology of the host. J Endocrinol, 2013. 218(3): p. R37-47.
268. Ribado, J.V., C. Ley, et al., Household triclosan and triclocarban effects on the infant and maternal
microbiome. EMBO Mol Med, 2017. 9(12): p. 1732-1741.
269. Karrer, C., T. Roiss, et al., Physiologically Based Pharmacokinetic (PBPK) Modeling of the Bisphenols
BPA, BPS, BPF, and BPAF with New Experimental Metabolic Parameters: Comparing the
Pharmacokinetic Behavior of BPA with Its Substitutes. Environ Health Perspect, 2018. 126(7): p.
077002.
270. Connolly, D., , Lund, H., Mathiesen, B.V. , Smart Energy Europe: The technical and economic impact
of one potential 100% renewable energy scenario for the European Union. Renewable and
Sustainable Energy Reviews, 2016. 60(July): p. 1634-1653.
271. Berger, K., B. Eskenazi, et al., Prenatal high molecular weight phthalates and bisphenol A, and
childhood respiratory and allergic outcomes. Pediatr Allergy Immunol, 2018.
272. Bouillon, R., G. Carmeliet, et al., Vitamin D and energy homeostasis: of mice and men. Nat Rev
Endocrinol, 2014. 10(2): p. 79-87.
273. Davey, J.C., A.P. Nomikos, et al., Arsenic as an endocrine disruptor: arsenic disrupts retinoic acid
receptor-and thyroid hormone receptor-mediated gene regulation and thyroid hormone-mediated
amphibian tail metamorphosis. Environ Health Perspect, 2008. 116(2): p. 165-72.
274. Hones, G.S., H. Rakov, et al., Noncanonical thyroid hormone signaling mediates cardiometabolic
effects in vivo. Proc Natl Acad Sci U S A, 2017. 114(52): p. E11323-E11332.
275. Zhu, X., L. Gao, C. Yan, and Y. He, A novel role and mechanism of cystic fibrosis transmembrane
conductance regulator in bisphenol A-induced prostate cancer. J Cell Biochem, 2018.
276. Rosenfeld, C.S. and P.S. Cooke, Endocrine disruption through membrane estrogen receptors and
novel pathways leading to rapid toxicological and epigenetic effects. J Steroid Biochem Mol Biol,
2018.
277. Derghal, A., M. Djelloul, J. Trouslard, and L. Mounien, An Emerging Role of micro-RNA in the Effect
of the Endocrine Disruptors. Front Neurosci, 2016. 10: p. 318.

Policy Department for Citizens’ Rights and Constitutional Affairs
__________________________________________________________________________________________
124
278. WHO/IPCS, IPCS Risk AssessmentTerminology. 2004, World Health Organization. Available from:
https://www.who.int/ipcs/methods/harmonization/areas/terminology/en/
.
279. Gore, A.C., J. Balthazart, et al., Policy decisions on endocrine disruptors should be based on science
across disciplines: a response to Dietrich et al. Eur J Endocrinol, 2013. 169(6): p. E1-4.
280. Corporate Europe Observatory and Horel S., A Toxic Affair. How the Chemical Lobby Blocked Action
on Hormone-Disrupting Chemicals.
http://corporateeurope.org/sites/default/files/toxic_lobby_edc.pdf
, 2015.
281. Petraglia, F., A. Imperatore, and J.R. Challis, Neuroendocrine mechanisms in pregnancy and
parturition. Endocr Rev, 2010. 31(6): p. 783-816.
282. Schmutzler, C., I. Gotthardt, et al., Endocrine disruptors and the thyroid gland--a combined in vitro
and in vivo analysis of potential new biomarkers. Environ Health Perspect, 2007. 115 Suppl 1: p.
77-83.
283. Schmutzler, C., I. Hamann, et al., Endocrine active compounds affect thyrotropin and thyroid
hormone levels in serum as well as endpoints of thyroid hormone action in liver, heart and kidney.
Toxicology, 2004. 205(1-2): p. 95-102.
284. Radovic, B., B. Mentrup, and J. Kohrle, Genistein and other soya isoflavones are potent ligands for
transthyretin in serum and cerebrospinal fluid. Br J Nutr, 2006. 95(6): p. 1171-6.
285. Legler, J., T. Fletcher, et al., Obesity, diabetes, and associated costs of exposure to endocrine-
disrupting chemicals in the European Union. J Clin Endocrinol Metab, 2015. 100(4): p. 1278-88.
286. Brown, R.E., A.M. Sharma, et al., Secular differences in the association between caloric intake,
macronutrient intake, and physical activity with obesity. Obes Res Clin Pract, 2016. 10(3): p. 243-55.
287. Neubig, R.R., M. Spedding, et al., International Union of Pharmacology Committee on Receptor
Nomenclature and Drug Classification. XXXVIII. Update on terms and symbols in quantitative
pharmacology. Pharmacol Rev, 2003. 55(4): p. 597-606.
Endocrine disruptors: from scientific evidence to human health protection
____________________________________________________________________________________________
125
CONFLICT OF INTERESTS STATEMENT AND ACKNOWLEDGEMENTS
Conflicts of interests’ statement
The authors declare no financial conflict of interests.
Barbara Demeneix is a cofounder of Watchfrog S.A., a company involved in the development of test to
identify EDs. She receives no financial compensation for any activity related to her work with the
company.
Acknowledgments
The authors would like to thank Professor Andreas Kortenkamp (Brunel University, UK), Dr. Jean-Pierre
Cravédi (INRA) and Professor Laura Vandenberg (University of Massachusetts, USA) for useful comments
on an earlier version of this report.
Policy Department for Citizens’ Rights and Constitutional Affairs
__________________________________________________________________________________________
126
INDEX
A
ADHD (attention deficit and hyperactivity
disorders), 17, 33, 34, 42, 45
adverse effect, 16, 17, 18, 19, 21, 22, 24, 27, 28,
30, 32, 33, 34, 38, 41, 42, 46, 50, 56, 57, 59,
60, 98
androgen, 18, 19, 21, 22, 23, 27, 29, 30, 38, 39,
41, 42, 45, 62, 85, 86, 88, 90, 92, 95, 104
anogenital distance (AGD), 24, 29, 42, 62
Anses (French environmental health agency),
23, 45, 66, 85
aromatase, 21, 45, 86
attributable fraction, 47
Autism Spectrum Disorders (ASD), 42, 45
B
baby bottle, 72
BBP (phthalate), 8, 31, 40, 69, 71
benzene, 79
benzo(a)pyrene, 24
BfR (German Institute for Risk Evaluation), 103
biocide, 44, 68
biomonitoring, 45, 46, 88, 89, 96
bisphenol A (BPA), 23, 25, 29, 30, 31, 33, 39, 41,
44, 46, 47, 50, 55, 68, 69, 71, 72, 79, 90, 95,
104
BPR. See biocide
C
cancer, 17, 19, 24, 25, 26, 31, 33, 36, 40, 42, 43,
50, 72, 74, 84, 101
chlorpyrifos (pesticide), 44, 45, 49, 50
circular economy, 57
Clarity-BPA study, 104
CLP regulation (1272/2008 EC) on
Classification, Labelling and Packaging, 55,
72, 74, 81, 82
CMR (carcinogens, mutagens, toxics for
reproduction) substances, 53, 56, 59, 63, 64,
67, 68, 69, 70, 79, 81, 88, 93, 94, 96
cohort, 25, 39, 41, 43, 91, 105
combined exposure, 89, 95, 101
conflict of interest, 80
congenital malformation, 17, 20, 24, 29, 33, 34,
42, 43, 72
cosmetics, 31, 40, 41, 62, 67, 70, 71, 72, 74, 81,
85, 86, 94, 96, 99
costs (economic), 48
cumulative effect, 28
D
DBP (phthalate), 31, 40, 65, 69, 71
DDT, 25, 71
definition, 18
DEHP, 24, 29, 31, 39, 40, 46, 47, 65, 69, 71
DES, 17, 25, 33, 34, 38, 78, 90
diabetes, 20, 22, 23, 31, 34, 38, 106
drug, 25, 28, 29, 42, 95, 96
E
environment action programme (EAP), 56, 79,
80, 93, 97
epigenetic, 17, 25, 26, 31, 34, 89
exposome, 46
F
food additives regulation (1333/2008/EC), 70
food contact materials, 69
food contact materials regulation (1935/2004
(EC)), 68
G
genistein, 46
Globally Harmonised System (GHS), 72
GPR30 (G-protein coupled receptor 30)
receptor, 38
Endocrine disruptors: from scientific evidence to human health protection
____________________________________________________________________________________________
127
H
hazard, 31, 33, 34, 51, 53, 55, 61, 64, 66, 67, 69,
70, 72, 74, 99
I
IQ (Intellectual Quotient), 49, 50, 85
L
labelling, 69, 73, 81, 96
M
medical devices, 68
medical devices regulation (2017/745 (EU)), 68
mercury, 23, 46
microbiome, 90
migration limit, 69
mixture, 26
Mixture Assessment Factor, 31
N
neurodevelopmental, 34
NHANES study (USA), 88
non-monotonic dose response, 19
nonylphenol, 43, 64, 68, 79, 95
nuclear receptor, 19, 24, 29, 41
O
oestradiol, 18, 95
oestrogen, 21, 22, 23, 24, 25, 27, 38, 39, 41, 53,
62, 85, 88, 90, 92, 95, 103, 104
organophosphate pesticides, 33, 44, 45, 46, 47,
49, 50
P
paraben, 41, 46, 47, 67
paracetamol, 42
particulate matter (PM), 79
PBDE (polybrominated flame retardants), 23,
42, 46, 49, 50, 69, 71
PCB (polychlorinated biphenyl), 22, 23, 25, 33,
44, 46, 47, 71, 78, 90, 105
perfluoroalkyl substances (PFAS), 33, 40, 43,
46, 47
Persistent Organic Pollutant (POP), 71
pesticide, 22, 29, 33, 44, 45, 46, 90
PFOA (perfluorooctanoic acid), 33, 40, 43, 46,
68
PFOS (perfluorooctanesulfonic acid), 33, 43, 46,
68, 69
phosphorylated flame-retardants, 42
phthalate, 24, 30, 39, 65, 68, 69
placenta, 72
Plant Protection Product Regulation (PPPR),
59, 60, 61, 80, 81, 82
polycarbonate, 38, 71, see also bisphenol A
potency, 103, 104
precautionary principle, 67, 69, 70, 80
pregnane-X-receptor (PXR), 29
prostaglandin, 17, 42
pyrethroid pesticides, 45
R
REACH regulation (2006R1907), 31, 56, 62, 63,
64, 65, 66, 67, 68, 70, 71, 74, 78, 79, 81, 86, 93
regrettable substitution, 90
restriction, 40, 43, 63, 69, 71, 95
risk, 33, 34
S
strategy on EDs, 57
French strategy on EDs, 66, 73, 87
SVHC (substance of very high concern), 63, 66,
79, 97
synergy, 26, 27, 28, 29, 31
T
Tamoxifen, 25
TBBPA (tetrabromobisphenol A), 42
testosterone, 18, 21, 30, 42
thyroid, 18, 19, 20, 21, 23, 24, 25, 33, 34, 36, 38,
41, 44, 62, 87, 89, 104
thyroid hormone, 19, 20, 21, 22, 41, 42, 43, 45,
49, 85, 90, 91
Policy Department for Citizens’ Rights and Constitutional Affairs
__________________________________________________________________________________________
128
thyroid peroxidase, 45
tolerable daily intake (TDI), 24, 31, 79
toys, 44, 68
transgenerational, 90
tributyl tin (TBT), 23, 24, 34
triclosan, 24, 33, 41, 46, 47, 67, 71, 94
U
uterotrophic assay, 53, 62
W
water, 14, 40, 43, 59, 62, 68, 70, 75, 78, 88, 95
Weight of Evidence (WoE), 48, 61, 82, 102, 106
workers’ protection, 62, 68, 69, 80, 97

PE 608.866
Print ISBN 978-92-846-4672-2 | doi: 10.2861/742 | QA-01-19-272-EN-C
PDF ISBN 978-92-846-4671-5 | doi: 10.2861/802173 | QA-01-19-272-EN-N
This study, commissioned by the PETI Committee of the European Parliament,
presents the scientific knowledge regarding the health effects of endocrine
disruptors, a class of hazards recognized in EU regulation since 1999. This report
reviews the scientific evidence regarding the concept of endocrine disruption,
the extent of exposure, associated health effects and costs. The existing
relevant EU regulations are discussed and recommendations made to better
protect human health.
