EPA/600/P–96/001F
September 1996
PCBs: Cancer Dose-Response Assessment
and Application to Environmental Mixtures
National Center for Environmental Assessment
Office of Research and Development
U.S. Environmental Protection Agency
Washington, DC
ii
DISCLAIMER
This document has been reviewed in accordance with U.S. Environmental
Protection Agency policy and approved for publication. Mention of trade names or
commercial products does not constitute endorsement or recommendation for use.
iii
CONTENTS
LIST OF TABLES .................................................... v
PREFACE ........................................................... vi
AUTHORS, CONTRIBUTORS, AND REVIEWERS .......................... viii
1. INTRODUCTION .................................................. 1
1.1. PCB MIXTURES ........................................... 1
1.2. CANCER POTENTIAL OF PCB MIXTURES ...................... 5
1.3. APPROACH TAKEN BY THIS ASSESSMENT .................... 6
2. SUMMARY OF STUDIES USED IN THE DOSE-RESPONSE ASSESSMENT . . . 8
2.1. CANCER STUDIES IN HUMANS .............................. 8
2.2. LIFETIME CANCER STUDIES IN ANIMALS ...................... 10
2.3. PARTIAL LIFETIME STUDIES IN ANIMALS ...................... 17
2.4. TUMOR INITIATING AND PROMOTING ACTIVITY ................ 19
2.5. ABSORPTION AND RETENTION .............................. 22
2.6. METABOLISM AND MODE OF ACTION IN THE LIVER ............. 25
2.7. MODE OF ACTION IN THE THYROID .......................... 26
3. DOSE-RESPONSE ASSESSMENT ................................... 28
3.1. APPROACHES TO DOSE-RESPONSE ASSESSMENT ............. 28
3.2. BIOLOGICALLY BASED MODELING OF TUMOR PROMOTION ...... 31
3.3. EMPIRICAL MODELING OF TUMOR INCIDENCE ................. 32
3.4. ANALYSES OF CONGENER TOXICITY ......................... 37
4. APPLICATION OF THE DOSE-RESPONSE ASSESSMENT ................ 39
4.1. APPLICATION TO PCB MIXTURES IN THE ENVIRONMENT ........ 39
4.2. APPLICATION TO DIFFERENT ROUTES OF EXPOSURE .......... 45
4.3. APPLICATION TO LESS-THAN-LIFETIME AND EARLY-LIFE
EXPOSURES ............................................ 46
4.4. APPLICATION WITH DIOXIN TOXIC EQUIVALENCE FACTORS ..... 49
5. CHARACTERIZATION AND GUIDANCE FOR RISK ASSESSORS ........... 50
5.1. DOSE-RESPONSE CHARACTERIZATION ....................... 50
5.2. INFLUENCE OF PROPOSED CANCER GUIDELINES .............. 55
5.3. RESEARCH NEEDS ........................................ 56
5.4. SUMMARY OF GUIDANCE FOR RISK ASSESSORS .............. 59
5.5. EXAMPLES ............................................... 61
6. REFERENCES ................................................... 65
iv
APPENDIX: EMPIRICAL MODELING RESULTS ........................... 73
v
LIST OF TABLES
1–1 Typical composition (%) of some commercial PCB mixtures ............... 2
1–2 Domestic production (%) of commercial PCB mixtures, 1957–1977 ......... 2
2–1 Liver tumor incidences in rats from lifetime exposure studies, 1975–1985 . . . 11
2–2 Liver tumor incidences in rats from 1996 lifetime exposure study .......... 14
2–3 Liver tumor incidences in female rats from 1996 stop study .............. 15
2–4 Thyroid gland tumor incidences in male rats from 1996 lifetime
exposure study ................................................ 16
2–5 Mixtures and congeners tested for tumor promoting activity .............. 21
3–1 Human potency and slope estimates derived from rat liver tumors ......... 34
3–2 Range of human potency and slope estimates ........................ 35
3–3 PCB congeners of highest concern ................................. 37
3–4 WHO interim TEFs for human intake of dioxin-like PCBs ................ 38
4–1 Tiers of human potency and slope estimates for environmental mixtures .... 44
5–1 Sample lifetime average daily dose calculations (examples 1 and 2) ....... 62
5–2 Sample risk calculations (example 1) ............................... 62
5–3 Sample risk calculations (example 2) ............................... 63
5–4 Sample congener concentrations and dioxin toxic equivalents (TEQs)
in edible portion of fish (example 3) ................................. 64
5–5 Sample lifetime average daily dose calculations (example 3) ............. 64
5–6 Sample risk calculations (example 3) ............................... 65
vi
PREFACE
This report updates the cancer dose-response assessment for PCBs and shows
how information on toxicity, disposition, and environmental processes can be
considered together to evaluate health risks from PCB mixtures in the environment.
Intended to be brief, it focuses on analysis and interpretation rather than a compilation
of study results. More detailed information on PCB toxicity has been compiled by the
Agency for Toxic Substances and Disease Registry (ATSDR, 1993, 1995), Safe (1994),
Silberhorn et al. (1990), and the U.S. Environmental Protection Agency (U.S.EPA)
(1988a).
Although not covered by this report, PCBs also have significant ecological and
human health effects other than cancer, including neurotoxicity, reproductive and
developmental toxicity, immune system suppression, liver damage, skin irritation, and
endocrine disruption. Toxic effects have been observed from acute and chronic
exposures to PCB mixtures with varying chlorine content. These toxic effects should be
included along with cancer in future assessments of PCBs.
This report is to be used to support risk-based decisions within the general
policy framework provided by applicable EPA statutes and does not alter such policies.
It does not imply that one kind of information or another is a prerequisite for action. Not
every risk assessment based on this dose-response assessment will have the same
scope or depth; the level of detail of an assessment is a matter of management policy.
This report is being made available to the public and the U.S. Congress,
responding to the report of the House of Representatives Appropriations Committee,
which specifies:
vii
By December 31, 1995, the Administrator shall submit to the Congress,
and make available to the public, a draft report providing an assessment
of the risk of each of the polychlorinated biphenyl (PCB) mixtures that has
been the subject of laboratory animal cancer bioassays, and a proposed
methodology for assigning cancer risk numbers to mixtures of PCB's
found in the environment. By September 1, 1996, the Committee directs
that EPA shall have completed, by a panel of independent experts on the
carcinogenicity of PCB's, a peer review of the draft report, and shall
submit a final report to the Congress and make it available to the public.
A new laboratory animal study of four commercial mixtures will soon be made
public. Because this study will provide the most comprehensive information for dose-
response modeling, this report makes use of preliminary information obtained through
July 1996. Although some of this information is still under review and additional
information may soon become available, updating the dose-response assessment at
this time allows current decisions to reflect current science and provides a framework
for incorporating new information.
viii
AUTHORS, CONTRIBUTORS, AND REVIEWERS
This report was written by Dr. Jim Cogliano of EPA's National Center for
Environmental Assessment. The author gratefully acknowledges the helpful and
insightful comments from many scientists in EPA's program, regional, and research
organizations:
Air and Radiation: Jane Caldwell.
Prevention, Pesticides and Toxic Substances: David Lai,
Elizabeth Margosches.
Solid Waste and Emergency Response: Dorothy Canter.
Water: Robert Cantilli, Michael Cox, Krishan Khanna, Edward Ohanian.
Region 1 (Boston): Mary Ballew, Andy Beliveau, Ann-Marie Burke,
Jui-Yu Hsieh, Ronnie Levin, Margaret McDonough, Marybeth Smuts.
Region 2 (New York): Marian Olsen, Anita Street, Doug Tomchuk.
Region 3 (Philadelphia): Debra Forman.
Region 5 (Chicago): Carole Braverman, Milton Clark, Stephen Johnson.
Region 6 (Dallas): Young-Moo Kim, Maria Martinez, Jeffrey Yurk.
Region 7 (Kansas City): Dave Crawford.
Region 8 (Denver): Bob Benson, Suzanne Wuerthele.
Region 9 (San Francisco): Arnold Den, Daniel Stralka.
Region 10 (Seattle): Dana Davoli, Roseanne Lorenzana.
Research and Development: Linda Birnbaum, Ruth Bleyler, William Farland,
Charli Hiremath, James Holder, Prasad Kodavanti, Aparna Koppikar,
Jim Lake, Susan Norton, Charles Ris, Cheryl Siegel Scott, Dharm Singh,
Jeanette Wiltse.
The author also gratefully acknowledges Dr. Stephen Hamilton of the General
Electric Company for providing preliminary results of the new cancer studies.
A draft report was made available to the public in January 1996. The following
people or organizations provided written comments:
Obaid Faroon, Agency for Toxic Substances and Disease Registry, Atlanta GA.
Richard W. Green, Delaware Department of Natural Resources and
Environmental Control, Dover DE.
Marion Harnois, Massachusetts Department of Environmental Protection,
Boston MA.
Greg Schweer, Versar Inc., Springfield VA.
Tracey Slayton, Gradient Corporation, Cambridge MA.
Chemical Land Holdings, Inc., Kearny NJ.
ix
The draft report was considered at a public, external peer review workshop in
May 1996. A workshop report was written by the review panel, which included the
following experts on the carcinogenicity of PCBs:
Lucy Anderson, National Cancer Institute, Frederick MD.
John Brown, Jr., General Electric Company, Schenectady NY.
Peter DeFur, Environmental Stewardship Concepts, Richmond VA.
Dale Hattis, Clark University, Worcester MA.
Kim Hooper, California Environmental Protection Agency, Berkeley CA.
Marty Kanarek, University of Wisconsin-Madison, Madison WI.
Nancy Kim, New York State Department of Health, Albany NY.
Loren Koller (chair), Oregon State University, Corvallis OR.
John Moore, Institute for Evaluating Health Risks, Washington DC.
Christopher Portier, National Institute of Environmental Health Sciences,
Research Triangle Park NC.
Paul Price, McLaren/Hart ChemRisk, Portland ME.
Larry Robertson, University of Kentucky, Lexington KY.
Stephen Safe, Texas A&M University, College Station TX.
Susan Velazquez, Toxicology Excellence for Risk Assessment, Cincinnati OH.
Gary Williams, American Health Foundation, Valhalla NY.
At the workshop, there was opportunity for public comment. The following
people provided oral comments:
Brent Finley, McLaren/Hart ChemRisk, Alameda CA.
John Schell, TERRA, Inc, Tallahassee FL.
Thomas Starr, ENVIRON Corporation, Arlington VA.
This report has been approved by EPA's consensus review panel for inclusion
on EPA's Integrated Risk Information System (IRIS).
The author is grateful for the contributions of the review panel and all who
commented on the draft report. Their efforts truly improved the final product.
Some notes on chemical structure and nomenclature: Each PCB molecule consists of two 6-carbon rings, with one
chemical bond joining a carbon from each ring (imagine sunglasses with hexagonal frames). Chlorine can attach to any
of the other 10 carbons; these positions are said to be substituted
. There are 209 possible arrangements, called
congeners
; congeners with the same number of chlorines are called isomers. The number and position of chlorines
determine a molecule's physical and chemical properties. The 10 positions are numbered 2–6 on one ring and 2'–6' on
the other. For example, the congener 2,4,2',5'-tetrachlorobiphenyl has chlorines in positions 2 and 4 of one ring and 2
and 5 of the other. (Standard chemical notation for this congener is 2,2',4,5'-tetrachlorobiphenyl; instead, this
assessment lists chlorines on one ring, then the other, to emphasize each ring's chlorination pattern.) Positions 2, 6, 2',
and 6', adjacent to the bond, are called ortho
positions; 3, 5, 3', and 5', meta positions; 4 and 4' (the outermost), para
positions. The International Union of Pure and Applied Chemists (IUPAC) has adopted an alternative system for
numbering congeners sequentially from 1 to 209; numbers assigned to congeners named in this assessment are listed
in table 3–3. A molecule's two rings can twist on the bond joining them; they are coplanar
if aligned in the same plane.
Chlorine in ortho positions inhibits a coplanar alignment. Coplanar molecules have dioxin-like properties (Safe, 1990,
1994; U.S. EPA, 1994b). PCB mixtures manufactured in the United States carried the trademark "Aroclor" followed by a
four-digit number; the first two digits are "12," and the last two digits indicate the percent chlorine content by weight. For
example, Aroclor 1260 contains approximately 60 percent chlorine by weight. Aroclor 1016 is an exception to this
scheme; it contains approximately 41 percent chlorine. "Clophens" and "Kanechlors" are PCB mixtures manufactured in
Germany and Japan, respectively; these series have their own numbering schemes.
1
1. INTRODUCTION
1.1. PCB MIXTURES
PCBs (polychlorinated biphenyls) are mixtures of synthetic organic chemicals.
1
Different mixtures can take on forms ranging from oily liquids to waxy solids. Although
their chemical properties vary widely, different mixtures can have many common
components. Table 1–1 shows the overlapping composition of some commercially
manufactured mixtures. Because of their inflammability, chemical stability, and
insulating properties, commercial PCB mixtures had been used in many industrial
applications, especially in capacitors, transformers, and other electrical equipment.
These chemical properties, however, also contribute to the persistence of PCBs after
they are released into the environment. Because of evidence that PCBs persist in the
environment and cause harmful effects, domestic manufacture of commercial mixtures
was stopped in 1977; existing PCBs, however, continue in use. Table 1–2 shows some
commercial mixtures as a percentage of domestic production.
2
Table 1–1. Typical composition (%) of some commercial PCB mixtures
Aroclor Clophen Kanechlor
1016
1242 1248 1254 1260 A 30 A 60 300 400 500
Mono-CBs 2 1 — — — — — — — —
Di-CBs 19 13 1 — — 20 — 17 3 —
Tri-CBs 57 45 21 1 — 52 — 60 33 5
Tetra-CBs 22 31 49 15 — 22 1 23 44 26
Penta-CBs — 10 27 53 12 3 16 1 16 55
Hexa-CBs — — 2 26 42 1 51 — 5 13
Hepta-CBs — — — 4 38 — 28 — — —
Octa-CBs ———— 7 — 4 ———
Nona-CBs ———— 1 —— ———
Deca-CB ————— —— ———
Columns may not total 100% due to rounding; "—" signifies less than 1%.
Lot-to-lot variability exists but has not been quantified.
Impurities include chlorinated dibenzofurans and naphthalenes; see World Health Organization
(WHO) (1993) for sample concentrations.
Sources: Adapted from Silberhorn et al. (1990), ATSDR (1995).
Table 1–2. Domestic production (%) of commercial PCB mixtures, 1957–1977
Percent of
Mixture
production
Aroclor 1016 13
Aroclor 1221 1
Aroclor 1232 < 1
Aroclor 1242 52
Aroclor 1248 7
Aroclor 1254 16
Aroclor 1260 11
Aroclor 1262 1
Aroclor 1268 < 1
Column does not total 100% due to rounding.
Source: Adapted from Brown (1994).
In the environment, PCBs also occur as mixtures of congeners, but their
composition differs from the commercial mixtures. This is because after release into
the environment, the composition of PCB mixtures changes over time, through
partitioning, chemical transformation, and preferential bioaccumulation.
3
Partitioning refers to processes by which different fractions of a mixture separate
into air, water, sediment, and soil. PCBs adsorb to organic materials, sediments, and
soils; adsorption tends to increase with chlorine content of the PCBs and organic
content of the other material (Callahan et al., 1979). PCBs can volatilize or disperse as
aerosols, providing an effective means of transport in the environment (Callahan et al.,
1979). Congeners with low chlorine content tend to be more volatile and also more
soluble in water (Callahan et al., 1979). Vaporization rates and water solubility of
different Aroclors and individual congeners vary over several orders of magnitude
(Hutzinger et al., 1974; Erickson, 1986).
Biodegradation transforms the chemical composition of PCB mixtures in the
environment. Anaerobic bacteria in sediments selectively remove chlorines from meta
and para positions, appearing to reduce the toxicity and bioaccumulation potential of
residues; the occurrence and extent of these dechlorinations can be limited by
sediment PCB concentrations (Abramowicz, 1990; Brown and Wagner, 1990; Lake
et al., 1992). (Dechlorination is not synonymous with detoxication, as congeners
having carcinogenic activity can be formed through dechlorination.) Aerobic bacteria
remove chlorines from PCBs with low chlorine content (1–4 chlorines) and break open
the carbon rings through oxidation (Abramowicz, 1990). PCBs with higher chlorine
content are extremely resistant to oxidation and hydrolysis (Callahan et al., 1979).
Photolysis can slowly break down congeners with high chlorine content (Callahan
et al., 1979). Overall, dechlorination processes are slow and altered PCB mixtures can
persist in the environment for many years.
PCBs can accumulate selectively in living organisms. PCBs are highly soluble
in lipids and are absorbed by fish and other animals. Rates of metabolism and
elimination are slow and vary by congener (Matthews and Anderson, 1975).
Bioaccumulation through the food chain tends to concentrate congeners of higher
chlorine content, producing residues that are considerably different from the original
Aroclors (Schwartz et al., 1987; Oliver and Niimi, 1988; Lake et al., 1995). PCB
residues in fish and turtles, changed through environmental or metabolic alteration,

4
could not be characterized by Aroclor 1242, 1248, 1254, or 1260 standards (Schwartz
et al., 1987). Congener distributions in several species, including humans, do not
resemble any Aroclor (McFarland and Clarke, 1989). Because, in general, some toxic
congeners are preferentially retained, bioaccumulated PCBs appear to be more toxic
than commercial PCBs (Aulerich et al., 1986; Hornshaw et al., 1983).
PCBs are widespread in the environment, and humans are exposed through
multiple pathways. Levels in air, water, sediment, soil, and foods vary over several
orders of magnitude, often depending on proximity to a source of release into the
environment (ATSDR, 1993; WHO, 1993). Average daily intake by humans via
ambient air is about 100 ng, and about an order of magnitude higher if indoor
concentrations are considered (ATSDR, 1993). Average daily intake via drinking water
is less than 200 ng (ATSDR, 1993). Estimates of average daily intake via diet vary
widely depending on geographic area, food habits, and sampling methodology;
5–15 g is considered a good estimate of average daily intake via diet in industrialized
countries (WHO, 1993). For nursing infants, average daily intake was estimated at
1.5–27 g/kg (ATSDR, 1993); another study estimated 3–11 g/kg (WHO, 1993). Using
the narrower range, average daily intake for a 5-kg nursing infant would be 15–55 g,
about triple the average adult intake, and approximately 50-fold higher when adjusted
for body weight. Nursing infants are, therefore, an important potentially highly exposed
population. Another is people whose diet is high in game fish, game animals, or
products of animals contaminated through the food chain.
Although environmental mixtures are often characterized in terms of Aroclors,
this can be both imprecise and inappropriate. Qualitative and quantitative errors can
arise from judgments in interpreting gas chromatography/mass spectrometry (GC/MS),
which reveals a spectrum of peaks that are compared with characteristic patterns for
different Aroclors. For environmentally altered mixtures, an absence of these
characteristic patterns can suggest the absence of Aroclors, even though some
congeners are present in high concentrations. Large differences have been found in
5
results reported by laboratories analyzing the same sediment samples (Alford-Stevens
et al., 1985; Alford-Stevens, 1986).
1.2. CANCER POTENTIAL OF PCB MIXTURES
Occupational studies show some increases in cancer mortality in workers
exposed to PCBs. Bertazzi et al. (1987) found significant excess cancer mortality at all
sites combined and in the gastrointestinal tract in workers exposed to PCBs containing
54 and 42 percent chlorine. Brown (1987) found significant excess mortality from
cancer of the liver, gall bladder, and biliary tract in capacitor manufacturing workers
exposed to Aroclors 1254, 1242, and 1016. Sinks et al. (1992) found significant excess
malignant melanoma mortality in workers exposed to Aroclors 1242 and 1016. Some
other studies, however, found no increases in cancer mortality attributable to PCB
exposure (ATSDR, 1993). The lack of consistency overall limits the ability to draw
definitive conclusions from these studies. Incidents in Japan and Taiwan where
humans consumed rice oil contaminated with PCBs showed some excesses of liver
cancer, but this has been attributed, at least in part, to heating of the PCBs and rice oil,
causing formation of chlorinated dibenzofurans (ATSDR, 1993; Safe, 1994).
A new study of rats fed diets containing Aroclors 1260, 1254, 1242, or 1016
found statistically significant, dose-related, increased incidences of liver tumors from
each mixture (Brunner et al., 1996). Earlier studies found high, statistically significant
incidences of liver tumors in rats ingesting Aroclor 1260 or Clophen A 60 (Kimbrough
et al., 1975; Norback and Weltman, 1985; Schaeffer et al., 1984). Partial lifetime
studies found precancerous liver lesions in rats and mice ingesting PCB mixtures of
high or low chlorine content.
Several mixtures and congeners test positive for tumor promotion (Silberhorn
et al., 1990). Toxicity of some PCB congeners is correlated with induction of mixed-
function oxidases; some congeners are phenobarbital-type inducers, some are
3-methylcholanthrene-type inducers, and some have mixed inducing properties
6
(McFarland and Clarke, 1989). The latter two groups most resemble 2,3,7,8-
tetrachlorodibenzo-p-dioxin in structure and toxicity.
Overall, the human studies have been considered to provide limited (IARC,
1987) to inadequate (U.S. EPA, 1988a) evidence of carcinogenicity. The animal
studies, however, have been considered to provide sufficient evidence of
carcinogenicity (IARC, 1987; U.S. EPA, 1988a). Based on these findings, some
commercial PCB mixtures have been characterized as probably carcinogenic to
humans (IARC, 1987; U.S. EPA, 1988a). There has been some controversy about how
this conclusion applies to PCB mixtures found in the environment.
1.3. APPROACH TAKEN BY THIS ASSESSMENT
Previous assessments developed a single dose-response slope (7.7 per
mg/kg-d average lifetime exposure) for evaluating PCB cancer risks (U.S. EPA, 1988a).
With no agreed-on basis for reflecting differences among environmental mixtures, this
slope was used by default for any mixture. Different alternatives have been suggested
that would make some distinctions about cancer risks from different PCB mixtures.
One alternative would assume there is no cancer hazard from environmental mixtures
unless the mixture is highly chlorinated, for example, an overall chlorine content of
approximately 60 percent or greater (Delaware Department of Natural Resources and
Environmental Control, 1994). Another alternative would develop a separate
assessment for each commercial mixture that has been studied. These alternatives
begin to distinguish among PCB mixtures, but they do not address how the
environmental processes of partitioning, transformation, and bioaccumulation diminish
the similarity of environmental mixtures to any of the commercial mixtures.
This new assessment adopts a related approach that distinguishes among PCB
mixtures by using information on environmental processes. Environmental processes
have profound effects that can decrease or increase toxicity, so toxicity of an
environmental mixture is only partly determined by the original commercial mixture.
This new assessment, therefore, considers all cancer studies (which used commercial

7
mixtures only) to develop a range of dose-response slopes, then uses information on
environmental processes to provide guidance on choosing an appropriate slope for
representative classes of environmental mixtures and different exposure pathways.
Different kinds of information, many not typically considered in dose-response
assessments, are used in this approach. Other innovative features include:
A range of upper-bound potency estimates for PCB mixtures, plus a range
of central estimates, with guidance for choosing estimates from these
ranges to reflect the effect of environmental processes on a mixture's
toxicity. Sources of uncertainty in these estimates are identified and
discussed.
A tiered approach that can use site-specific congener information when
available, but can be adapted if information is limited to total PCBs
encountered through each exposure pathway.
Application of EPA's proposed cancer guidelines (U.S. EPA, 1996a) in the
quantitative dose-response assessment, including the interagency
consensus cross-species scaling factor (U.S. EPA, 1992b) and discussion
of circumstances affecting cancer risk.
A new rat study (Brunner et al., 1996), with parallel experiments for Aroclors
1260, 1254, 1242, and 1016, will soon be made public. Each experiment tested both
sexes at several dose levels. Because this study will provide the most comprehensive
information for dose-response modeling, this assessment makes use of preliminary
information that could be obtained through July 1996. To ensure the scientific quality
of this information, the laboratory report was reviewed by four members of the external
peer review panel (Koller, 1996).
Section 2 briefly summarizes the studies used in developing the dose-response
assessment and applying it to environmental mixtures. For a comprehensive
discussion of PCB toxicity, including many other studies, see ATSDR (1993), Safe
(1994), Silberhorn et al. (1990), or U.S. EPA (1988a). Section 3 uses the studies
summarized in section 2 to develop a new dose-response assessment. Section 4

Standardized mortality ratio (SMR) = 100 × observed / expected.
CI = 95% confidence interval.
8
discusses application of the dose-response assessment to environmental mixtures, to
different exposure routes, to less-than-lifetime and early-life exposure, and in
combination with dioxin toxic equivalence factors. Section 5 characterizes the results
of this assessment, lists research needs, and gives specific guidance for risk
assessors.
2. SUMMARY OF STUDIES USED IN THE DOSE-RESPONSE ASSESSMENT
2.1. CANCER STUDIES IN HUMANS
EPA's cancer guidelines (U.S. EPA, 1986a, 1996a) favor basing dose-response
assessments on human studies. This requires quantitative information on both
exposure and response. This limited review focuses on the suitability of the human
studies for dose-response assessment. More detailed information on these studies and
on other studies not amenable to dose-response assessment has been compiled by
ATSDR (1993).
Bertazzi et al. (1987). This cohort study analyzed cancer mortality among
workers at a capacitor manufacturing plant in Italy. PCB mixtures with 54, then
42 percent chlorine were used through 1980. The cohort included 2100 workers (544
males and 1556 females) employed at least 1 week. At the end of follow-up in 1982,
there were 64 deaths, 26 from cancer.
In males, there was a statistically significant increase in death from
gastrointestinal tract cancer, compared with national and local rates (6 observed, 1.7
expected using national rates, SMR=346, CI=141–721; 2.2 expected using local rates,
SMR=274, CI=112–572). In females, there was a statistically significant excess risk of
2
death from hematologic cancer compared with local, but not national, rates (4
observed, 1.1 expected, SMR=377, CI=115–877). Analyses by exposure duration,
latency, and year of first exposure revealed no trend; however, the numbers are small.

9
Brown (1987). This cohort study analyzed cancer mortality among workers at
two capacitor manufacturing plants in New York and Massachusetts. At both plants the
Aroclor mixture being used changed twice, from 1254 to 1242 to 1016. The cohort
included 2588 workers (1270 males and 1318 females) employed at least 3 months in
areas of the plants considered to have potential for heavy exposure to PCBs. At the
end of follow-up in 1982, there were 295 deaths, 62 from cancer.
Compared with national rates, there was a statistically significant increase in
death from cancer of the liver, gall bladder, and biliary tract (5 observed, 1.9 expected,
SMR=263,
p
<0.05). Four of these five occurred among females employed at the
Massachusetts plant. Analyses by time since first employment or length of employment
revealed no trend; however, the numbers are small.
Sinks et al. (1992). This cohort study analyzed cancer mortality among workers
at a capacitor manufacturing plant in Indiana. Aroclor 1242, then 1016, had been used.
The cohort included 3588 workers (2742 white males and 846 white females) employed
at least 1 day. At the end of follow-up in 1986, there were 192 deaths, 54 from cancer.
Workers were classified into five exposure zones based on distance from the
impregnation ovens.
Compared with national rates, there was a statistically significant excess risk of
death from skin cancer (8 observed, 2.0 expected, SMR=410; CI=180–800); all were
malignant melanomas. A proportional hazards analysis revealed no pattern of
association with exposure zone; however, the numbers are small.
Other occupational studies. Other studies (NIOSH, 1977; Gustavsson et al.,
1986; Shalat et al., 1989) looked for an association between occupational PCB
exposure and cancer mortality. Because of small sample sizes, brief follow-up periods,
and confounding exposures to other potential carcinogens, these studies are
inconclusive and not amenable to dose-response analysis.
Accidental ingestion. Serious adverse health effects, including liver cancer
and skin disorders, have been observed in humans who consumed rice oil
contaminated with PCBs in the "Yusho" incident in Japan or the "Yu-Cheng" incident in

10
Taiwan. These effects have been attributed, at least in part, to heating of the PCBs
and rice oil, causing formation of chlorinated dibenzofurans, which have the same
dioxin-like mode of action as some PCB congeners (ATSDR, 1993; Safe, 1994).
2.2. LIFETIME CANCER STUDIES IN ANIMALS
Because of their controlled exposures and absence of confounding factors,
animal studies are often used for dose-response analysis. A new study compared
several commercial mixtures over a range of dose levels; earlier studies had focused on
mixtures with high chlorine content. This limited review focuses on the information that
would be used in a dose-response assessment. More detailed information on these
studies has been compiled by ATSDR (1993).
Kimbrough et al. (1975). Groups of 200 female Sherman rats were fed diets
with 0 or 100 ppm Aroclor 1260 for about 21 months. Six weeks later the rats were
killed and their tissues were examined. Hepatocellular carcinomas and neoplastic
nodules were significantly increased in rats fed Aroclor 1260 (see table 2–1).

11
Table 2–1. Liver tumor incidences in rats from lifetime exposure studies, 1975–1985
Study, sex and strain, mixture
Dose Original Reevaluation
a a,b
Kimbrough et al. (1975) Control ** 1/173 ( 1%) ** 1/187 ( 1%)
F Sherman, 1260 100 ppm 170/184 (92%) 138/189 (73%)
NCI (1978) Control ** 0/24 ( 0%) ** 0/24 ( 0%)
M Fischer, 1254 25 ppm 0/24 ( 0%) 1/24 ( 4%)
50 ppm 1/24 ( 4%) 1/24 ( 4%)
100 ppm 3/24 (12%) 3/23 (13%)
NCI (1978) Control ** 0/23 ( 0%) 0/23 ( 0%)
F Fischer, 1254 25 ppm 0/24 ( 0%) 1/24 ( 4%)
50 ppm 1/22 ( 5%) 2/24 ( 8%)
100 ppm 2/24 ( 8%) 1/24 ( 4%)
Schaeffer et al. (1984) Control ** 2/120 ( 2%) 8/120 ( 7%)
c
M Wistar, Clophen A 30 100 ppm 42/130 (32%) 16/128 (12%)
Schaeffer et al. (1984) Control ** 2/120 ( 2%) ** 8/120 ( 7%)
c
M Wistar, Clophen A 60 100 ppm 123/129 (95%) 114/125 (91%)
Norback and Weltman (1985) Control ** 0/32 ( 0%) 0/31 ( 0%)
M Sprague-Dawley, 1260 100/50/0 ppm 7/46 (15%) 5/40 (12%)
d
Norback and Weltman (1985) Control ** 1/49 ( 2%) ** 1/45 ( 2%)
F Sprague-Dawley, 1260 100/50/0 ppm 45/47 (96%) 41/46 (89%)
d
**Statistically significant (
p
<0.05) by Cochran-Armitage trend test (for experiments with more than one
dosed group) or Fisher exact test (for experiments with one dosed group).
Hepatocellular adenomas or carcinomas
a
Decreases between original and reevaluated denominators are due to lost slides; increases, to slides that
b
were excluded originally but could not be specifically identified for exclusion in the reevaluation.
One control group supported both experiments.
c
Dosing was decreased twice during the study.
d
Source: Adapted from Moore et al. (1994).
National Cancer Institute (NCI, 1978). Groups of 24 male or female
Fischer 344 rats were fed diets with 0, 25, 50, or 100 ppm Aroclor 1254 for
104–105 weeks (24 months). Then the rats were killed and their tissues were
examined. The combined incidence of leukemia and lymphoma in males was
significantly increased by the Cochran-Armitage trend test; however, since Fisher exact
tests were not also significant, NCI did not consider this result clearly related to

12
Aroclor 1254. Hepatocellular adenomas and carcinomas were increased (see table 2–1).
Morgan et al. (1981) and Ward (1985) reevaluated gastric lesions from this
study and found 6 adenocarcinomas in 144 exposed rats. This result is statistically
significant, as gastric adenocarcinomas had occurred in only 1 of 3548 control male
and female Fischer 344 rats in the NCI testing program. Intestinal metaplasia in
exposed rats differed morphologically from controls, suggesting Aroclor 1254 can act
as a tumor initiator.
Schaeffer et al. (1984). Male weanling Wistar rats were fed a standard diet for
8 weeks, then were divided into three groups. One group was fed the basic diet; for the
other groups 100 ppm Clophen A 30 or A 60 was added. Rats were killed at
801–832 days (26.3–27.3 months) and were examined for lesions in the liver and some
other tissues. For both mixtures, preneoplastic liver lesions were observed after
500 days (16.4 months) and hepatocellular carcinomas after 700 days (23 months) in
rats dying before the end of the study (see table 2–1). The investigators concluded,
"Clophen A 60 had a definite, and Clophen A 30 a weak, carcinogenic effect on rat
liver."
Norback and Weltman (1985). Groups of male or female Sprague-Dawley rats
were fed diets with 0 or 100 ppm Aroclor 1260 for 16 months; the latter dose was
reduced to 50 ppm for 8 more months. After 5 additional months on the control diet, the
rats were killed and their livers were examined. Partial hepatectomy was performed on
some rats at 1, 3, 6, 9, 12, 15, 18, and 24 months to evaluate sequential morphologic
changes. In males and females fed Aroclor 1260, liver foci appeared at 3 months, area
lesions at 6 months, neoplastic nodules at 12 months, trabecular carcinomas at
15 months, and adenocarcinomas at 24 months, demonstrating progression of liver
lesions to carcinomas. By 29 months, 91 percent of females had liver carcinomas and
95 percent had carcinomas or neoplastic nodules; incidences in males were lower, 4
and 15 percent, respectively (see table 2–1).
Vater et al. (1995) obtained individual animal results to determine whether the
partial hepatectomies, which exert a strong proliferative effect on the remaining tissue,

13
affected the incidence of liver tumors. They reported that the hepatectomies did not
increase the tumor incidence. Among females fed Aroclor 1260, liver tumors
developed in 4 of 7 with hepatectomies and 37 of 39 without hepatectomies; no liver
tumors developed in controls or males with hepatectomies.
Moore et al. (1994); Institute for Evaluating Health Risks (IEHR) (1991). The
preceding rat liver findings were reevaluated using criteria and nomenclature that had
changed to reflect new understanding of mechanisms of toxicity and carcinogenesis.
The reevaluation found somewhat fewer tumors than did the original investigators. The
apparent increase for Clophen A 30 (Schaeffer et al., 1984) is no longer statistically
significant. Original and revised rat liver tumor incidences are given in table 2–1.
Brunner et al. (1996). This new study compared carcinogenicity across
different Aroclors, dose levels, and sexes. Groups of 50 male or female Sprague-
Dawley rats were fed diets with 25, 50, or 100 ppm Aroclor 1260 or 1254; 50 or 100
ppm Aroclor 1242; or 50, 100, or 200 ppm Aroclor 1016. There were 100 controls of
each sex. The animals were killed at 104 weeks, after which a complete
histopathologic evaluation was performed for control and high-dose groups;
histopathologic evaluations of liver, brain, mammary gland, and male thyroid gland
were also performed for low- and mid-dose groups.
Statistically significant increased incidences of liver adenomas or carcinomas
were found in female rats for all Aroclors and in male rats for Aroclor 1260 (see
table 2–2). Several of these tumors were hepatocholangiomas, a rare bile duct tumor
seldom seen in control rats. Hepatocholangiomas occurred in three females and two
males fed 100 ppm Aroclor 1260, in two, six, and one female fed Aroclor 1254 at 25,
50, and 100 ppm, respectively, and in one and two females fed Aroclor 1242 at 50 and
100 ppm, respectively; there was a hepatocholangiocarcinoma in one female fed
50 ppm Aroclor 1242.

14
Table 2–2. Liver tumor incidences in rats from 1996 lifetime exposure study
Mixture
Dose Females Males
aa
Aroclor 1260 Control ** 1/85 ( 1%) ** 7/98 ( 7%)
b
25 ppm 10/49 (20%) 3/50 ( 6%)
50 ppm 11/45 (24%) 6/49 (12%)
100 ppm 24/50 (48%) 10/49 (20%)
Aroclor 1254 Control ** 1/85 ( 1%) 7/98 ( 7%)
b
25 ppm 19/45 (42%) 4/48 ( 8%)
50 ppm 28/49 (57%) 4/49 ( 8%)
100 ppm 28/49 (57%) 6/47 (13%)
Aroclor 1242 Control ** 1/85 ( 1%) 7/98 ( 7%)
b
50 ppm 11/49 (24%) 1/50 ( 2%)
100 ppm 15/45 (33%) 4/46 ( 9%)
Aroclor 1016 Control ** 1/85 ( 1%) 7/98 ( 7%)
b
50 ppm 1/48 ( 2%) 2/48 ( 4%)
100 ppm 6/45 (13%) 2/50 ( 4%)
200 ppm 5/50 (10%) 4/49 ( 8%)
**Statistically significant (
p
<0.05) by Cochran-Armitage trend test.
Hepatocellular adenomas, carcinomas, cholangiomas, or cholangiocarcinomas in rats alive when the first
a
tumor was observed.
One control group supported all experiments.
b
Source: Adapted from Brunner et al. (1996), Keenan and Stickney (1996).
To investigate tumor progression after exposure stops, groups of 24 female rats
were exposed for 52 weeks, then exposure was discontinued for an additional
52 weeks before the rats were killed. For Aroclors 1254 and 1242, tumor incidences
from the stop study were approximately half those of the lifetime study; that is, nearly
proportional to exposure duration. In contrast, stop-study tumor incidences were zero
for Aroclor 1016, while for Aroclor 1260 they were generally greater than half those of
the lifetime study (see table 2–3). For 100 ppm Aroclor 1260, the stop study incidence
was greater than that of the lifetime study, 71 vs. 48 percent. (This 48 percent lifetime
study incidence was also low compared with incidences of 73, 91, and 89 percent from
the earlier studies of 100 ppm Aroclor 1260 or Clophen A 60.)

15
Table 2–3. Liver tumor incidences in female rats from 1996 stop study
Mixture
Dose Stop study Lifetime study
ab
Aroclor 1260 Control ** 1/85 ( 1%) ** 1/85 ( 1%)
c
25 ppm 4/24 (17%) 10/49 (20%)
50 ppm 3/24 (12%) 11/45 (24%)
100 ppm 17/24 (71%) 24/50 (48%)
Aroclor 1254 Control ** 1/85 ( 1%) ** 1/85 ( 1%)
c
25 ppm 5/24 (21%) 19/45 (42%)
50 ppm 7/24 (29%) 28/49 (57%)
100 ppm 6/24 (25%) 28/49 (57%)
Aroclor 1242 Control ** 1/85 ( 1%) ** 1/85 ( 1%)
c
50 ppm 3/24 (12%) 11/49 (22%)
100 ppm 6/24 (25%) 15/45 (33%)
Aroclor 1016 Control 1/85 ( 1%) ** 1/85 ( 1%)
c
50 ppm 0/24 ( 0%) 1/48 ( 2%)
100 ppm 0/24 ( 0%) 6/45 (13%)
200 ppm 0/24 ( 0%) 5/50 (10%)
**Statistically significant (
p
<0.05) by Cochran-Armitage trend test.
Hepatocellular adenomas, carcinomas, or cholangiomas in female rats dosed for 52 weeks and killed at
a
104 weeks.
Hepatocellular adenomas, carcinomas, cholangiomas, or cholangiocarcinomas in female rats dosed for
b
104 weeks and killed at 104 weeks (comparison from table 2–2).
One control group supported all experiments.
c
Source: Adapted from Brunner et al. (1996).
Thyroid gland follicular cell adenomas or carcinomas were increased in males
for all Aroclors (see table 2–4); significant dose trends were noted for Aroclors 1254
and 1242. The increases did not continue proportionately above the lowest dose. No
trends were apparent in females.

16
Table 2–4. Thyroid gland tumor incidences in male rats from 1996 lifetime exposure study
Mixture
Dose Males
a
Aroclor 1260 Control 2/100 ( 2%)
b
25 ppm 7/50 (14%)
50 ppm 5/50 (10%)
100 ppm 4/50 ( 8%)
Aroclor 1254 Control ** 2/100 ( 2%)
b
25 ppm 7/50 (14%)
50 ppm 7/50 (14%)
100 ppm 6/50 (12%)
Aroclor 1242 Control ** 2/100 ( 2%)
b
50 ppm 7/50 (14%)
100 ppm 6/50 (12%)
Aroclor 1016 Control 2/100 ( 2%)
b
50 ppm 4/50 ( 8%)
100 ppm 3/50 ( 6%)
200 ppm 1/50 ( 2%)
**Statistically significant (
p
<0.05) by Cochran-Armitage trend test.
Follicular cell adenomas or carcinomas in male rats dosed for 104 weeks.
a
One control group supported all experiments.
b
Source: Adapted from Brunner et al. (1996).
In female rats, the incidence of mammary tumors was decreased with lifetime
exposure to Aroclor 1254 and, to a lesser extent, to 1260 or 1242; this result was not
observed for Aroclor 1016. Decreases did not occur for any Aroclor in the stop study.
The first mammary tumor was observed at a later age in the dosed groups.
Studies of structurally related agents. Studies of 2,3,7,8-
tetrachlorodibenzo-p-dioxin and a polybrominated biphenyl (PBB) mixture are
summarized here because the pattern of tumors found by Brunner et al. (1996) mimics
the tumors induced in rats by these structurally related agents.
The National Toxicology Program (NTP, 1982) exposed groups of 50 male or
female Osborne-Mendel rats by gavage to 0, 1.4, 7.1, or 71 ng/kg-d 2,3,7,8-
tetrachlorodibenzo-p-dioxin for 2 years. Similar to the Brunner et al. (1996) study, liver
tumors were increased in female rats and thyroid gland follicular cell tumors were

17
increased in male rats. Mammary tumors were not, however, decreased in dosed
female rats.
NTP (1983) exposed groups of 51 male or female Fischer 344/N rats by gavage
to 0, 0.1, 0.3, 1, 3, or 10 mg/kg-d of a PBB mixture ("Firemaster FF–1") for 6 months,
then exposure was discontinued for 23 months before the animals were killed.
Statistically significant increased incidences of liver tumors were found in male and
female rats. Dose-related increased incidences of cholangiocarcinomas were found in
male and female rats. The Firemaster FF–1 mixture comprised an anticaking agent
blended with a PBB mixture containing 56 percent 2,4,5,2',4',5'-hexabromobiphenyl,
27 percent 2,3,4,5,2',4',5'-heptabromobiphenyl, and other unspecified penta-, hexa-,
and heptabromobiphenyls. The analogous PCB congeners are noted for their high
toxicity and abundance in environmental samples (McFarland and Clarke, 1989);
2,4,5,2',4',5'-hexachlorobiphenyl is highly persistent in the body (Matthews and
Anderson, 1975) and comprises 21.5 and 12.0 percent, respectively, of PCB residues
in human fat and milk (McFarland and Clarke, 1989).
2.3. PARTIAL LIFETIME STUDIES IN ANIMALS
Although lifetime studies are preferred for dose-response modeling, partial
lifetime studies often use experimental designs addressing specific issues in the
application of a dose-response assessment. Partial lifetime studies for PCBs have
compared different commercial mixtures and the relative sensitivity of the sexes. Some
studies examined early-life exposure, which is not covered by most lifetime cancer
studies, where exposure starts at age 2–3 months, when the animals are mature. This
limited review focuses on the information that pertains to issues in the dose-response
assessment. More detailed information on these studies has been compiled by ATSDR
(1993).
Kimbrough et al. (1972). Groups of 10 male or female Sherman rats were fed
diets with 0, 20, 100, 500, or 1000 ppm Aroclor 1254 or 1260, beginning at 3–4 weeks
of age and continuing for 8 months. Incidences of adenofibrosis reached 2/10 in males

18
and 4/7 in females fed 1000 ppm Aroclor 1260; in contrast, for 100 and 500 ppm
Aroclor 1254, incidences were 1/10 and 10/10 in males and 7/10 and 9/9 in females.
There was no adenofibrosis in 10 controls of each sex. With regard to differences
between sexes, the investigators concluded Aroclor 1260 is more toxic to female rats
than males, but such a difference could not be established for Aroclor 1254. With
regard to differences between mixtures, the investigators concluded the effect on the
liver "is more pronounced with Aroclor 1254 when all morphologic changes of
equivalent dietary levels of Aroclor 1254 and 1260 are compared."
Although adenofibromas are not carcinomas, these lesions, particularly in less-
than-lifetime studies, are sometimes regarded as indicating a potential for tumor
formation over a longer duration. For example, in a subsequent study, most female rats
of this strain fed 100 ppm Aroclor 1260 developed hepatocellular carcinomas or
neoplastic nodules after 23 months (Kimbrough et al., 1975).
Kimbrough and Linder (1974). Groups of 50 male BALB/cJ mice were fed
diets with 300 ppm Aroclor 1254 for 11 months, or for 6 months followed by 5 months
without exposure. Hepatomas were found in 9 of 22 surviving mice exposed for
11 months, in 1 of 24 mice exposed for 6 months, and in none of 58 controls.
Adenofibrosis was observed in all mice exposed for 11 months, but in none of the
others.
Kimura and Baba (1973). Groups of 10 male or female Donryu rats were fed
diets that increased from 38 to 462 ppm (time-weighted average, 330 ppm)
Kanechlor 400, beginning at 10 weeks of age and continuing for different durations of
up to 400 days (13 months). Multiple adenomatous liver nodules were found in the six
females exposed for the longest durations. No nodules were found in males or in five
controls of each sex.
Ito et al. (1973). Groups of 12 male dd mice were fed diets with 100, 250, or
500 ppm Kanechlor 300, 400, or 500, beginning at 8 weeks of age and continuing for
32 weeks (7.5 months). Among mice fed 500 ppm Kanechlor 500, five had

19
hepatocellular carcinomas and seven had nodular hyperplasia. No other groups,
including six controls, showed these effects.
Ito et al. (1974). Male Wistar rats were fed diets with 0, 100, 500, or 1000 ppm
Kanechlor 300, 400, or 500, beginning at 8 weeks of age and continuing for
28–52 weeks (6.5–12 months). Nodular hyperplasia was seen with all three mixtures,
highest for Kanechlor 500 and lowest for Kanechlor 300, but not in controls.
Histologically, the nodular hyperplasia was similar to that induced by other chemical
carcinogens, suggesting the nodular hyperplasia is preneoplastic. The investigators
concluded, "Hepatocellular carcinomas could be induced by administration of
Kanechlor-500, -400, or -300 for a longer period."
Rao and Banerji (1988). Groups of 32 male Wistar rats were fed diets with 0,
50, or 100 ppm Aroclor 1260, beginning at 5 weeks of age and continuing for 120 days
(4 months). Neoplastic nodules with adenofibrosis were found in 24 of 32 rats fed 50
ppm Aroclor 1260 and in 16 of 32 rats fed 100 ppm. None of 32 controls showed these
changes. The investigators concluded Aroclor 1260 induces liver tumors when fed to
young rats for a short time.
2.4. TUMOR INITIATING AND PROMOTING ACTIVITY
Studies of tumor initiating and promoting activity are available for a few
commercial mixtures and congeners. The congener studies are beginning to identify a
subset of mixture components that may be significant contributors to cancer induction.
As some of these congeners are present in environmental mixtures, these studies
provide information about the potential for environmental mixtures to cause cancer.
This limited review focuses on identifying congeners with tumor promoting activity to
help risk assessors know what to look for in a site-specific congener analysis. More
detailed information on these and other studies has been compiled by Silberhorn et al.
(1990).
Several commercial PCB mixtures and congeners show tumor promoting activity
(Silberhorn et al., 1990). Aroclor 1254 and Kanechlors 400 and 500 promote liver
20
tumors in initiation-promotion studies; Aroclor 1254 also promotes lung tumors
(Anderson et al., 1983, 1994; Beebe et al., 1992, 1993). Aroclor 1254, Clophens A 30
and A 50, four tetrachlorobiphenyls, three pentachlorobiphenyls, and one
hexachlorobiphenyl showed promoting activity in studies to identify alterations in
adenosine triphosphatase (ATPase), gamma-glutamyl transpeptidase (GGT), or
placental glutathione S-transferase (PGST) activity, markers of tumor promoting activity
in the liver. One study found the interaction of 2,5,2',5'- and 3,4,3',4'-
tetrachlorobiphenyl to produce more alterations than either alone (Sargent et al., 1991).
One monochlorobiphenyl and one dichlorobiphenyl showed no promoting activity. Lists
of mixtures and congeners tested for promoting activity (with either positive or negative
results) appear in table 2–5; references can be found in Silberhorn et al. (1990) and
later references cited in the table.
Table 2–5. Mixtures and congeners tested for tumor promoting activity
Mixture
Tumors Mixture or congener Altered foci
Aroclor 1254 Liver, lung Aroclor 1254 GGT+
Kanechlor 400 Liver Clophen A 30 Marker not reported
Kanechlor 500 Liver Clophen A 50 ATPase-, GGT+
4–MCB Negative
4,4'–DiCB Negative
2,4,2',4'–TeCB GGT+
2,4,2',5'–TeCB ATPase–, GGT+
2,5,2',5'–TeCB ATPase–, PGST+
3,4,3',4'–TeCB ATPase–, GGT+, PGST+
2,3,4,3',4'–PeCB GGT+, PGST+
2,4,5,3',4'–PeCB ATPase–, GGT+
3,4,5,3',4'–PeCB GGT+, PGST+
2,4,5,2',4',5'–HxCB ATPase–, GGT+, PGST+
Compiled from many studies; not all mixtures or congeners were tested in all systems.
Sources: Adapted from Silberhorn et al. (1990), Buchmann et al. (1991), Laib et al. (1991), Sargent et al.
(1991), Beebe et al. (1992, 1993), Hemming et al. (1993), Anderson et al. (1994).
Although PCBs are not generally described as tumor initiators, in some studies a
small number of ATPase-deficient or GGT-positive foci were initiated by treatment with
21
Clophen A 50 alone (Silberhorn et al., 1990). Weak initiating activity was found with
2,4,2',5'-tetrachlorobiphenyl, which induced ATPase-deficient, but not GGT-positive,
foci (Rose et al., 1985; Laib et al., 1991). Initiation potential had been suggested by
the different intestinal metaplasia morphology induced by Aroclor 1254 (Morgan et al.,
1981; Ward, 1985). Many other investigators, however, report negative results for
tumor initiation by PCB mixtures or congeners (Silberhorn et al., 1990).
The significance of the promotion studies is apparent, as all six ortho-substituted
congeners producing altered foci are abundant in commercial mixtures (Schulz et al.,
1989) and have been found in environmental samples (Lake et al., 1995; McFarland
and Clarke, 1989), though the tetrachlorobiphenyls are not particularly persistent in the
environment. Known for its bioaccumulation potential and abundance in environmental
samples, 2,4,5,2',4',5'-hexachlorobiphenyl has been found to comprise 21.5 and 12.0
percent, respectively, of PCB residues in human fat and milk; 2,4,5,3',4'-
pentachlorobiphenyl constitutes 5.4 and 6.5 percent, respectively, of these residues
(McFarland and Clarke, 1989). The coplanar congeners 3,4,3',4'-tetrachlorobiphenyl
and 3,4,5,3',4'-pentachlorobiphenyl have lower abundance in commercial mixtures
(Kannan et al., 1988) but have been found in a variety of organisms, including humans
(Safe, 1994).
2.5. ABSORPTION AND RETENTION
Cancer studies of lifetime and partial lifetime PCB exposure have been by
ingestion only. Pharmacokinetic studies provide information about the potential for
absorption and a risk of cancer by other exposure routes. Other studies have quantified
the retention and persistence of PCBs in the body. This limited review focuses on the
information that pertains to applying the dose-response assessment to dermal and
inhalation exposure. More detailed information on these studies and on ingestion
studies has been compiled by ATSDR (1993).
Humans absorb PCBs from ingestion, inhalation, and dermal exposure (ATSDR,
1993). Once absorbed, PCBs enter the circulation and are transported throughout the
22
body. Initial distribution is to liver and muscle, which are highly perfused;
subsequently, PCBs, being highly lipophilic, accumulate in fat and skin (Matthews and
Anderson, 1975).
Inhalation can be a principal absorption route for occupational PCB exposure
(Wolff, 1985). In animals, an inhaled PCB aerosol was rapidly absorbed, although
rates were not estimated (ATSDR, 1993).
PCBs can cross human skin and increase the body burden. Dermal exposure
can contribute significantly to body burdens of workers (Wolff, 1985) and can be a
major route of environmental exposure (ATSDR, 1993). In vivo dermal absorption by
rhesus monkeys exposed for 24 hours to soil containing 44 ppm Aroclor 1242 or
23 ppm Aroclor 1254 was 14 percent in each case (Wester et al., 1993). Earlier
studies found similar absorption rates for PCBs in mineral oil, trichlorobenzene, and
acetone (Wester et al., 1990). Subsequent washing did not remove all PCBs,
especially if time had elapsed after exposure (Wester et al., 1983). In vitro human skin
accumulation of Aroclor 1254 from water was 12 percent after a half hour (Wester
et al., 1987) and 44 percent after 24 hours (Wester et al., 1990), suggesting absorption
is rapid initially and continues at a slower rate with further contact.
PCBs are eliminated through metabolism, which occurs primarily in the liver
(Matthews and Anderson, 1975). Metabolism rates are generally lower with high
chlorine content, but chlorine position is also important (Hutzinger et al., 1974;
Matthews and Anderson, 1975). Absence of chlorine at two adjacent positions
facilitates metabolism (Matthews and Anderson, 1975). Metabolism and elimination
can be quite slow; for example, the biological half-life of 2,4,5,2',4',5'-
hexachlorobiphenyl exceeds the lifespan of rats (Matthews and Anderson, 1975).
In addition to variability by congener, there is human variability in PCB
metabolism and elimination. People with decreased liver function, including inefficient
glucuronidative mechanisms in infants, can have less capacity to metabolize and
eliminate PCBs (Calabrese and Sorenson, 1977). Additionally, approximately five
percent of nursing infants receive a steroid in human milk that inhibits the activity of

The workers came from the plant studied by Sinks et al. (1992), where Aroclor 1242, then 1016, had been used. The
3
quantitation of PCBs as Aroclors 1254 and 1242 illustrates both (1) selective retention of congeners with high chlorine
content and (2) the imprecision of characterizing altered PCB mixtures as if they were Aroclors.
Serum concentration was modeled as an exponentially decreasing function of time:
c
=
c
exp(–
bt
), where
c
is
4
t
0
t
concentration at time
t
,
c
is initial concentration, and
b
is the rate parameter, estimated by linear regression of ln(
c
/
c
) on
0 0
t
t
.
23
glucuronyl transferase, further reducing PCB metabolism and elimination (Calabrese
and Sorenson, 1977).
Persistent congeners can retain biological activity long after exposure stops;
residual liver enzyme induction was observed in mice 42 weeks after a single dose of
Aroclor 1254 (Anderson et al., 1991a). The majority of the retained mixture comprised
2,4,5,3'4'- and 2,3,4,3',4'-pentachlorobiphenyl and 2,4,5,2',4',5'- and 2,3,4,2',4',5'-
hexachlorobiphenyl (see tables 2–5, 3–3, and 3–4).
Analysis of 1977 and 1985 serum levels in 58 Indiana workers exposed to PCBs
yielded median half-lives of 2.6 years for Aroclor 1242 and 4.8 years for Aroclor 1254
3
(Phillips et al., 1989). Among workers with lowest concentrations (0–30 ppb), median
half-lives were higher, 3.1 years for Aroclor 1242 and 6.5 years for Aroclor 1254. In
another study in the same Indiana city, from 1977 to 1984 serum levels in five workers
exposed to PCBs decreased 89–94 percent (median, 92 percent) for Aroclor 1242 and
14–53 percent (median, 16 percent) for Aroclor 1260; among six others without current
occupational exposure, decreases were 23–71 percent (median, 39 percent) for total
PCBs (Steele et al., 1986). Analysis of serum levels in the exposed workers yields
half-lives of 2 years for Aroclor 1242 and 16 years for Aroclor 1260; in those without
current occupational exposure, a half-life of 8 years for total serum PCBs.
4
A study of people exposed through eating contaminated fish suggests that these
mixtures can be more persistent. From 1977 to 1985 mean serum levels (quantified
using Aroclor 1260 as a reference standard) from 111 Great Lakes fish eaters
decreased only slightly, from 20.5 to 19.0 ppb (Hovinga et al., 1992).

To illustrate, consider a mixture of two components in equal parts: one component has a half-life of 1 year; the other,
5
100 years. If the mixture concentration is sampled after 10 years, the half-life of the total mixture will appear to be
approximately 10 years: virtually all the first component will be gone, virtually none of the second, so about half the
original mixture will remain. This half-life, however, overestimates the slow rate of decrease in the more persistent
mixture fraction that remains.
24
It is important to recognize that ascribing a half-life to a mixture is problematic if
half-lives of its components differ widely; more specifically, half-life estimates for a
mixture can underestimate its long-term persistence.
5
2.6. METABOLISM AND MODE OF ACTION IN THE LIVER
Mechanistic information provides insight and understanding of the biological
activity of PCBs and their metabolites. The following discussion was contributed by
peer reviewers Drs. Larry Robertson and Lucy Anderson.
Although the rate of metabolism is slow (Mills et al., 1985), PCBs may be
converted by hepatic enzymes to hydroxylated metabolites. The relative rates of
conversion are dependent on the number and placement of the chlorine atoms present.
PCBs with fewer chlorines and with adjacent, unsubstituted carbon atoms are more
readily susceptible to metabolic attack. Cytochrome P–450 isozymes may catalyze
these hydroxylation reactions via an electrophilic arene oxide intermediate or via direct
insertion mechanisms. Evidence for the intermediacy of arene oxides during PCB
metabolism is found in the identification of (1) NIH-shift products, (2) dihydrodiol
metabolites, (3) mercapturic acid products, and (4) sulfone metabolites (Sipes and
Schnellman, 1987).
PCB metabolites with multiple hydroxyl groups also have been identified in
animals and in microsomal incubations (McLean et al., 1996a). Dihydroxy metabolites
may be oxidized in vitro to o- or p-quinones by peroxidases. In vitro studies have
demonstrated that adducts of PCBs and nucleotides (dGp and dAp) or exogenous DNA
may be formed during the hydroxylation step (from electrophilic arene oxides) and
during the peroxidase-catalyzed oxidation of PCB catechol and hydroquinone
metabolites to the respective o- and p-quinones (McLean et al., 1996b; Oakley et al.,
25
1996). Hydroxylated PCB metabolites may have estrogenic activity (Gierthy et al.,
1995).
Higher halogenated PCBs may be efficacious inducers of xenobiotic-
metabolizing enzymes, although they are poor substrates. Several PCBs, possessing
no or one ortho chlorine, bind the aryl hydrocarbon receptor with avidity (Bandiera
et al., 1982) and induce cytochrome P–450 1A. Several di-ortho substituted PCBs
induce cytochrome P–450s as does phenobarbital, while other congeneric PCBs may
induce cytochrome P–450s from both subfamilies. Many of these PCBs may also
induce epoxide hydrolase, glutathione transferases, and glucuronosyl transferases.
Induction of xenobiotic metabolites may be accompanied by an increase in hepatic cell
size and number and a proliferation of the endoplasmic reticulum. The persistent
induction of hepatic cytochrome P–450s, in the absence of an oxidizable xenobiotic
substrate, may provide suitable conditions for generation of reactive oxygen species.
Several PCBs tested as promoters in rat two-stage hepatocarcinogenesis were
efficacious when they were administered at doses that caused liver hypertrophy and
the induction of cytochrome P–450s (Silberhorn et al., 1990). Promoter activity has
been observed among groups of PCB congeners that have been characterized as
having widely different kinds of biological activity, including congeners that are aryl
hydrocarbon agonists, congeners that induce cytochrome P–450 1A and 2B isozymes,
and congeners that have a pattern of enzyme induction similar to that of phenobarbital.
This may indicate multiple mechanisms of action for promotion (Buchmann et al.,
1991). Congeneric PCBs may interfere with gap-junctional intercellular communication
via structure-specific mechanisms. Mono- and di-ortho chlorine substituted PCBs were
more active (Swierenga et al., 1990).
2.7. MODE OF ACTION IN THE THYROID
Recent mechanistic insights into thyroid carcinogenesis provide a rationale for
choosing a dose-response approach for thyroid tumors. The following discussion was
contributed by EPA consensus reviewer Dr. Richard Hill.
26
Thyroid tumors are noted for PCBs (Brunner et al., 1996) and for structurally
related 2,3,7,8-tetrachlorodibenzo-p-dioxin (NTP, 1982). These compounds are
accompanied by a lack of mutagenic activity in many different test systems. Liver
microsomal enzyme inducers of both the AHH and PB types, which include the PCBs,
commonly increase the metabolism and excretion of thyroid hormone (McClain, 1989).
Depending upon the compound, there may be increased clearance of thyroid hormone
from the blood, accentuated binding of the hormone in the liver, increased
glucuronidation of the hormone following induction of UDP-glucuronyl transferase
(Barter and Klaassen, 1992), increased bile flow and increased excretion of hormone in
the bile. Effects are usually more pronounced for thyroxine (T4) than triiodothyronine
(T3).
PCBs have effects on thyroid hormone status independent of their influence on
thyroid hormone metabolism and excretion. They cause damage to follicular cells
(Kasza et al., 1978; Byrne et al., 1987) and bind to and possibly displace thyroid
hormone from plasma protein carriers (Rickenbacher et al., 1986). Both of these may
contribute to a reduction in effective levels of circulating thyroid hormone.
Decreases in circulating thyroid hormone stimulate the pituitary by negative
feedback to increase the output of thyroid stimulating hormone (TSH). TSH is a trophic
hormone for the thyroid, resulting in the increased synthesis of thyroid hormone. When
thyroid hormone needs cannot be met by existing follicular cells, cells undergo
hypertrophy and diffuse hyperplasia. With continuing disruption in thyroid-pituitary
status, focal hyperplasia and then benign and malignant neoplasms develop (Hill et al.,
1987).
It is not totally clear whether hormonal derangement noted in rodents is a factor
in the development of thyroid tumors in humans. Some studies of persons with iodide
deficiency or inborn deficiency in the synthesis of thyroid hormone support the
contention, while others do not. At this time there is not enough information to dismiss
the animal model as not being relevant to human thyroid carcinogenesis. Even if
27
humans are susceptible to cancer from thyroid-pituitary disruption, existing information
indicates that humans are less sensitive than rodents (Hill et al., 1987).
Thyroid cancer risks in rodents exist under conditions of disruption in thyroid-
pituitary status. When, however, circulating levels of thyroid hormone and TSH pertain,
risks would be expected to be minimal. Such findings are best expressed by nonlinear
dose-response relationships. In assessing the risks from thyroid tumors, one would
want dose-response and time-action data from repeat dose studies on such things as
thyroid weight and morphology, UDP-glucuronyl transferase activity, and thyroid
hormone and TSH levels. Points of departure for evaluation of risks could be
determined from doses not associated with perturbations in thyroid status. Margin of
exposure—the ratio of the point of departure to expected human exposure
levels—could be used to express the nonlinear risks.
3. DOSE-RESPONSE ASSESSMENT
3.1. APPROACHES TO DOSE-RESPONSE ASSESSMENT
Dose-response assessment begins with consideration of developing a
biologically based model, that is, a model whose mathematical structure reflects the
ascertained mode of action and whose parameters are measured in experimental
studies. Biologically based models have been developed for 2,4,2',5'- and 3,4,3',4'-
tetrachlorobiphenyl; few congeners or mixtures, however, have been tested to measure
the rate parameters that would be used in a biologically based model. Further, PCBs
can cause cancer through multiple modes of action (Safe, 1990, 1994), indicating a
need for multiple models. Consequently, the information available at this time is more
suited to empirical modeling, where a flexible default model—allowing either linearity or
nonlinearity—is fitted to describe tumor incidence as a function of dose in the
experimental range.
Extrapolation to lower doses considers both linear and nonlinear approaches,
with a linear default if there is not sufficient information to support a sublinear model
28
(U.S. EPA, 1986a, 1996a). This policy rests, in part, on some general considerations.
Low-dose-linear models are appropriate for extrapolation to lower doses when a
carcinogen acts in concert with other exposures and processes that cause a
background incidence of cancer (Crump et al., 1976; Lutz, 1990). Further, even when
the mode of action indicates a nonlinear dose-response curve in homogeneous animal
populations, the presence of genetic and lifestyle factors in a heterogeneous human
population tends to make the dose-response curve more linear (Lutz, 1990). This is
because genetic and lifestyle factors contribute to a wider spread of human sensitivity,
which extends and straightens the dose-response curve over a wider range. Although
these considerations provide a reasonable argument for a model that is linear at low
doses, the relation of the low-dose slope to one from the experimental range is
uncertain; this uncertainty increases with the distance from the experimental range.
PCBs give generally negative results in tests of genetic activity (ATSDR, 1993),
implying that PCBs induce tumors primarily through modes of action that do not involve
gene mutation. This raises the possibility of a nonlinear dose-response curve. There
is, however, no dose-response information on either tumors or tumor precursors to
describe the dose range where the curve would be sublinear. At the low end of the
experimental range (25–100 ppm), dose-response curves are not sublinear, as tumor
incidence declines less than proportionately with dose for Aroclors 1260, 1254, and
1242 (Brunner et al., 1996). At much lower doses, some PCB congeners add to the
considerable background of human exposure to dioxin-like compounds and augment
processes associated with dioxin toxicity, providing a linear component to the dose-
response curve. Between these supralinear and linear dose ranges there is no dose-
response information; consequently, the information available at this time is more
suited to linear extrapolation.
Environmental PCBs occur as mixtures, prompting consideration of which agents
provide the most appropriate basis for an assessment. EPA's mixture guidelines (U.S.
EPA, 1986b) favor basing assessments on the effects of the mixture of interest; the
29
second choice is to use a sufficiently similar mixture; next, to assess the components of
the mixture. The guidelines further advise,
Attention should also be given to the persistence of the mixture in the
environment as well as to the variability of the mixture composition over
time or from different sources of emissions. If the components of the
mixture are known to partition into different environmental compartments
or to degrade or transform at different rates in the environment, then
those factors must also be taken into account, or the confidence in and
applicability of the risk assessment is diminished.
There are no cancer studies of PCB mixtures found in the environment. Studies
are available for some commercial mixtures, though their similarity to an environmental
mixture can be a matter of considerable uncertainty as mixtures are partitioned,
transformed, and bioaccumulated in the environment. Assessing mixture components
is not now a viable alternative, because only a few congeners have been tested, none
in long-term carcinogenesis studies. Thus assessments of environmental mixtures
must use information on commercial mixtures. Partitioning, transformation, and
bioaccumulation in the environment, however, must also be taken into account.
Risk estimates can be derived from either human or animal studies; each has
strengths and limitations. Estimates derived from human studies reflect an observed
association between human exposure and cancer; however, it is difficult to reconstruct
reliable estimates of past exposure and separate the effect of confounding exposures
to other carcinogens. Estimates derived from animal studies benefit from controlled
exposures and absence of confounding factors; however, there is uncertainty in
extrapolating dose and response rates across species. EPA's cancer guidelines (U.S.
EPA, 1986a, 1996a) favor basing dose-response assessments on human studies. In
the absence of adequate human information, assessments use animal species
responding most like humans. If this cannot be determined, assessments emphasize
long-term animal studies showing the greatest sensitivity, with due regard to biological
relevance, exposure route, and statistical considerations; this default is considered to
be conservative, tending toward public health protection.
30
For PCBs, the human studies involve relatively few cancer cases and lack
contemporaneous exposure estimates. Some studies report air concentrations, but
because skin contact is a major route of occupational exposure, air concentrations
would be a poor measure of exposure (Bertazzi et al., 1987; Brown, 1987). Some
studies report blood levels, but for relatively few workers at the end of exposure
(Bertazzi et al., 1987; Brown, 1987; Taylor, 1988; Sinks et al., 1992). Reconstruction of
past exposure is problematic because different mixtures had been in use over the
years, the distribution of exposure and absorption by route and congener is unknown,
and congener persistence in the body varies greatly from congener to congener
(Brown, 1994) and person to person (Steele et al., 1986). Similarly, adjustment for
confounding exposures to other potential carcinogens, many of them unidentified, is
also problematic. Because of these limitations in quantitative information, the human
studies are not well suited to dose-response assessment. Because of their controlled
exposures, absence of confounding factors, and ability to provide comparable
information on a range of different mixtures, the animal studies will be used for dose-
response modeling.
A biologically based model for two congeners is discussed in section 3.2.
Empirical models are developed and discussed in section 3.3. Analyses of congener
toxicity are discussed in section 3.4.
3.2. BIOLOGICALLY BASED MODELING OF TUMOR PROMOTION
Using a two-stage carcinogenesis model, Luebeck et al. (1991) modeled tumor
promoting activity of 2,4,2',5'- and 3,4,3',4'-tetrachlorobiphenyl, based on the study of
Buchmann et al. (1991). Female Wistar rats were initiated with 10 mg/kg-d
diethylnitrosamine for 10 days, followed by eight weekly injections of 10 or 150 mol/kg
of various compounds. The rats were killed 1 or 9 weeks later, and preneoplastic
activity was characterized by changes in ATPase and GGT activity.
Because results are available for two times after dosing stopped, modeling can
assess persistence of promoting activity. There was little or no promoting activity by

Formerly, EPA guidelines (U.S. EPA, 1986a) called for using the linearized multistage procedure, which fits models
6
where risk is a function of dose
d
,
Risk(
d
) = 1 – exp(–
qd
–
qd
– . . . –
qd
);
q
0,
i
=1,...,
k
12
ki
2
k
The linearized multistage procedure fits several such models, through degree
k
=6. The peer review panel objected to
the polynomial degree exceeding the number of dosed groups (U.S. EPA, 1996b). Consequently, this assessment uses
linear-quadratic models (that is,
k
=2) unless there is only one dosed group, in which case it uses a linear model (
k
=1).
Equivalent human dose = animal dose × (animal weight / 70 kg human weight) .
7 1/4
The tables compiled by Brunner et al. (1996) combined hepatocholangiomas with hepatocellular adenomas and
8
carcinomas, a combination not recommended by McConnell et al. (1986). Individual animal results needed to remove
the hepatocholangiomas from the incidences used for modeling were not provided to EPA. The effect, however, is
expected to be negligible, as few rats had a hepatocholangioma.
31
2,4,2',5'-tetrachlorobiphenyl after dosing stopped, but 3,4,3',4'-tetrachlorobiphenyl
continued to promote vigorously (Luebeck et al., 1991).
Modeling can also estimate the probability of altered foci becoming extinct; that
is, disappearing after dosing stops. After dosing stops, the probability of extinction is
high, as most altered foci do not develop into observable tumors. Those that become
large, however, tend to persist, as the probability of extinction decreases as size
increases (Luebeck et al., 1991).
3.3. EMPIRICAL MODELING OF TUMOR INCIDENCE
The new feeding study by Brunner et al. (1996), with parallel experiments for
four commercial mixtures in both sexes of rats, provides the most comprehensive
information for empirical modeling. Each experiment tested several dose levels,
providing information about the shape of the dose-response curve in the experimental
range. For each mixture and sex, a linear-quadratic multistage model (Howe et al.,
1986) was fitted to experimental results. Dose was expressed as a lifetime daily
6
average (U.S. EPA, 1986a, 1992a), calculated from weekly body weight measurements
and food consumption estimates (Keenan and Stickney, 1996). Doses were scaled to
humans using a factor based on the 3/4 power of relative body weight (U.S. EPA,
7
1992b). Response was taken as the incidence of hepatocellular adenomas or
carcinomas ; combining adenomas and carcinomas reflects guidance of the National
8

The slope is the change in response divided by the change in dose. Relative to the origin (an increased response of 0
9
at a dose of 0), the change in response is 0.10 at the dose ED10; thus the slope is 0.10/ED10.
32
Toxicology Program (McConnell et al., 1986) and the observed progression of
hepatocellular adenomas to carcinomas (Norback and Weltman, 1985).
Cancer potency is described by an ED10 (estimated dose associated with
10 percent increased incidence) and its lower bound, LED10. ED10s have been used
both for potency ranking and as a starting point for low-dose extrapolation (Cogliano,
1986; U.S. EPA, 1988b, 1994a; National Research Council, 1993). These measures
are expressed as equivalent human doses.
For extrapolation to lower doses, an ED10 can be converted to a slope by
computing 0.10/ED10. (Note that slopes are inversely proportional to ED10s; high
9
potency is indicated by high slopes, but low ED10s.) Similarly, an upper-bound slope
can be obtained by computing 0.10/LED10. Formerly, upper-bound slopes were
calculated by the linearized multistage procedure (U.S. EPA, 1980, 1986a); these are
reported in the appendix as "
q
*"s. The LED10 method and the linearized multistage
1
procedure give similar upper-bound slopes; for example, for female rats fed
Aroclor 1254, the LED10 method and the linearized multistage procedure give upper-
bound slopes of 1.5 and 1.6 per mg/kg-d, respectively. Potency and slope estimates
are compiled in table 3–1; details supporting the calculations appear in appendix tables
A–1 through A–8.

Equivalent human dose (mg/kg-d) = (ppm in diet) × 0.05 × (animal weight / 70 kg human weight) .
10 1/4
In the studies by Kimbrough et al. (1975) and Norback and Weltman (1985) initial dose levels were later decreased or
discontinued. It is likely, however, that tumor development had already begun and internal exposure remained high with
release of PCBs stored in fat (Vater et al., 1995). Thus, for these studies, initial dose levels were used without averaging
over the study duration; this reduces these potency estimates by up to one-third compared to the default of averaging
dose over the study duration.
33
Table 3–1. Human potency and slope estimates derived from rat liver tumors
Central Upper-bound See
Study, sex and strain, mixture
ED10 LED10 slope slope table
abcd
Brunner, F Sprague-Dawley, 1260 0.24 0.19 0.4 0.5 A–1
Brunner, F Sprague-Dawley, 1254 0.086 0.067 1.2 1.5 A–2
Brunner, F Sprague-Dawley, 1242 0.38 0.27 0.3 0.4 A–3
Brunner, F Sprague-Dawley, 1016 2.4 1.4 0.04 0.07 A–4
Brunner, M Sprague-Dawley, 1260 1.0 0.55 0.1 0.2 A–5
Brunner, M Sprague-Dawley, 1254 1.7 0.87 0.06 0.1 A–6
e
Brunner, M Sprague-Dawley, 1242 2.9 1.2 0.03 0.08 A–7
e
Brunner, M Sprague-Dawley, 1016 5.9 2.5 0.02 0.04 A–8
e
Kimbrough, F Sherman, 1260 0.10 0.091 1.0 1.1 A–9
NCI, M Fischer, 1254 1.0 0.55 0.1 0.2 A–10
NCI, F Fischer, 1254 1.2 0.61 0.08 0.2 A–11
e
Schaeffer, M Wistar, A 30 2.1 1.0 0.05 0.1 A–12
e
Schaeffer, M Wistar, A 60 0.058 0.047 1.7 2.1 A–13
Norback, M Sprague-Dawley, 1260 1.0 0.53 0.1 0.2 A–14
e
Norback, F Sprague-Dawley, 1260 0.062 0.046 1.6 2.2 A–15
Estimated dose associated with 10% increased incidence, in mg/kg-d.
a
95% lower bound on ED10, in mg/kg-d.
b
Per mg/kg-d, computed as 0.10/ED10.
c
Per mg/kg-d, computed as 0.10/LED10.
d
No significant increase; quantities indicate sensitivity of study.
e
In conjunction with the Brunner et al. (1996) study, the earlier studies provide
useful information on the potential for lot-to-lot and strain-to-strain differences.
Because these studies did not report body weight and food consumption, administered
doses were converted from ppm in the diet to mg/kg-d using default factors based on
rats weighing 350 grams and consuming food equal to 5 percent of body weight daily
(U.S. EPA, 1980). Potency and slope estimates from the earlier studies are included
10
in table 3–1; details, in tables A–9 through A–15.
34
The range of potency values in table 3–1 is summarized in table 3–2. It is based
primarily on the range for Aroclors 1260, 1254, 1242, and 1016 in female Sprague-
Dawley rats (Brunner et al., 1996), but considers the other studies, too. For example,
the two studies of female Sprague-Dawley rats fed Aroclor 1260 (Brunner et al., 1996;
Norback and Weltman, 1985) could reflect, in part, lot-to-lot differences that are
pertinent to mixtures altered in the environment; thus the range of upper-bound slopes
includes those from the Brunner et al. (1996) study (0.07–1.5 per mg/kg-d) and the
Norback and Weltman (1985) study (2.2 per mg/kg-d). The earlier gastric tumors and
leukemias and lymphomas in male rats fed Aroclor 1254 (NCI, 1978) are not included
in this range, because they were not confirmed by the Brunner study; they would
contribute little to the overall estimates since incidences are several-fold less than
those of the liver tumors.
Table 3–2. Range of human potency and slope estimates
Central Upper-bound See
ED10
LED10 slope slope table
abcd
Highest observed potency 0.086 0.046 1.2 2.2 A–2,15
ee
Lowest observed potency 2.4 1.4 0.04 0.07 A–4
Estimated dose associated with 10% increased incidence, in mg/kg-d.
a
95% lower bound on ED10, in mg/kg-d.
b
Per mg/kg-d, computed as 0.10/ED10.
c
Per mg/kg-d, computed as 0.10/LED10.
d
Bound from Norback and Weltman (1985).
e
These ranges reflect experimental uncertainty and variability of commercial
mixtures, but not human heterogeneity and differences between commercial and
environmental mixtures. Environmental processes have profound effects that can
increase or decrease toxicity, so toxicity of an environmental mixture is only partly
determined by the original commercial mixture. Potency estimates for an Aroclor tested
in the laboratory may not be the best surrogate for assessing that Aroclor as altered in
35
the environment. Sections 4 and 5 develop specific guidance for applying these
potency ranges to environmental mixtures.
The new upper-bound slopes are lower than the previous estimate of 7.7 per
mg/kg-d average lifetime exposure (U.S. EPA, 1988a). The previous estimate was
derived from female rats in the Norback and Weltman (1985) study; the new estimate
from the same study is 2.2 per mg/kg-d. This difference is attributable to three factors,
each responsible for reducing the slope by approximately one-third: the rat liver tumor
reevaluation (Moore et al., 1994), use of the new cross-species scaling factor (U.S.
EPA, 1992b), and not using a time-weighted average dose (see previous footnote).
The difference between the highest observed new upper-bound slope (2.2 per mg/kg-d)
and the lowest (0.07 per mg/kg-d) is entirely attributable to the available of tests on
several commercial mixtures (Brunner et al., 1996). This 30-fold range in potency
reflects differences in commercial mixture composition.
The different responses for male and female rats (Brunner et al., 1996) suggest
the possibility of developing different potency values for males and females. In view of
the 91 percent response in male Wistar rats (Schaeffer et al., 1984), as well as the
sensitivity of male mice (Kimbrough and Linder, 1974; Ito et al., 1973), it is premature
to conclude that females are always more sensitive.
For the thyroid tumors, no meaningful ED10 or LED10 can be computed. The
dose-response curves for each Aroclor are virtually horizontal across the experimental
range, so mathematical models cannot determine whether a dose-response curve
begins to be sublinear immediately below the experimental range or whether it remains
horizontal for several orders of magnitude below the experimental range before
becoming sublinear. This difficulty transcends PCBs and thyroid tumors; in general,
there would be an unacceptable level of uncertainty in using a sublinear extrapolation
approach when study results in the experimental range are not at all sublinear.
36
3.4. ANALYSES OF CONGENER TOXICITY
McFarland and Clarke (1989) explain how toxicity of some PCB congeners is
correlated with induction of mixed-function oxidases. Some congeners are described
as phenobarbital-type inducers, others as 3-methylcholanthrene-type inducers, and
some as having mixed inducing properties. The latter two groups most resemble
2,3,7,8-tetrachlorodibenzo-p-dioxin in structure and toxicity. Based on potential for
toxicity (some forms of toxicity, for example, neurotoxicity, may not be well represented)
and frequency of occurrence in environmental samples, 36 congeners of highest
concern were identified and classified (see table 3–3).
Table 3–3. PCB congeners of highest concern
Highest toxicity High toxicity Abundant in Potential
and abundance
and abundance environment for toxicity
ab c d
3–MC-type inducers: PB-type inducers: 18: 2,5,2'–TrCB 37: 3,4,4'–TrCB
77: 3,4,3',4'–TeCB 87: 2,3,4,2',5'–PeCB 44: 2,3,2',5'–TeCB 81: 3,4,5,4'–TeCB
126: 3,4,5,3',4'–PeCB 99: 2,4,5,2',4'–PeCB 49: 2,4,2',5'–TeCB 114: 2,3,4,5,4'–PeCB
169: 3,4,5,3',4',5'–HxCB 101: 2,4,5,2',5'–PeCB 52: 2,5,2',5'–TeCB 119: 2,4,6,3',4'–PeCB
153: 2,4,5,2',4',5'–HxCB 70: 2,5,3',4'–TeCB 123: 3,4,5,2',4'–PeCB
Mixed-type inducers: 180: 2,3,4,5,2',4',5'–HpCB 74: 2,4,5,4'–TeCB 157: 2,3,4,3',4',5'–HxCB
105: 2,3,4,3',4'–PeCB 183: 2,3,4,6,2',4',5'–HpCB 151: 2,3,5,6,2',5'–HxCB 158: 2,3,4,3',4',6'–HxCB
118: 2,4,5,3',4'–PeCB 194: 2,3,4,5,2',3',4',5'–OCB 177: 2,3,5,6,2',3',4'–HpCB 167: 2,4,5,3',4',5'–HxCB
128: 2,3,4,2',3',4'–HxCB 187: 2,3,5,6,2',4',5'–HpCB 168: 2,4,6,3',4',5'–HxCB
138: 2,3,4,2',4',5'–HxCB 201: 2,3,4,5,2',3',5',6'–OCB 189: 2,3,4,5,3',4',5'–HpCB
156: 2,3,4,5,3',4'–HxCB
170: 2,3,4,5,2',3',4'–HpCB
Pure 3-methylcholanthrene-type inducers and mixed-type inducers reported frequently in environmental samples.
a
Phenobarbital-type inducers reported frequently in environmental samples.
b
Weak inducers or noninducers reported frequently in environmental samples.
c
Mixed-type inducers not reported frequently in environmental samples, but toxicologically active.
d
Source: Adapted from McFarland and Clarke (1989).
U.S. EPA (1991) examined toxic effects, including cancer, of four structural
classes: dioxin-like PCBs, ortho-substituted PCBs, hydroxylated metabolites, and
sulfonated metabolites. Different mechanisms were discussed for dioxin-like and other
PCBs. It was concluded that congener toxicity could not be characterized by chlorine
content alone. Before adopting toxic equivalence factors (TEFs) for PCB congeners, it
was recommended to define other classes of PCBs and identify the mechanisms
involved. Criteria for developing TEFs were listed as (1) a demonstrated need, (2) a
well defined group of chemicals, (3) a broad base of toxicological data, (4) consistency
37
in the relative toxicity of congeners across toxicological endpoints, (5) demonstrated
additivity between the toxicity of individual congeners, (6) a mechanistic rationale, and
(7) consensus.
Safe (1990, 1994) characterized dioxin-like PCBs as eliciting a spectrum of
biochemical and toxic responses similar to chlorinated dibenzo-p-dioxins and
dibenzofurans, all acting through the aryl hydrocarbon receptor. Based on quantitative
structure-activity studies, the first conservative TEFs for dioxin-like PCBs were
proposed and refined. Use of these TEFs is limited to responses mediated through the
aryl hydrocarbon receptor.
Subsequently, WHO derived TEFs for dioxin-like PCBs (Ahlborg et al., 1994).
Included were congeners that show structural similarity to chlorinated
dibenzo-p-dioxins and dibenzofurans, bind to the aryl hydrocarbon receptor, elicit
dioxin-specific biochemical and toxic responses, and persist and accumulate in the
food chain. On the basis of these criteria, 13 PCB congeners were assigned TEFs,
expressed as a fraction of the toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin (see
table 3–4). As new information is developed, these TEFs could change, or TEFs for
additional congeners could be developed. Section 4 gives guidance, and section 5 an
example, for applying these TEFs where a congener analysis is available.
Table 3–4. WHO interim TEFs for human intake of dioxin-like PCBs
Non-ortho congeners TEF Mono-ortho congeners TEF Di-ortho congeners TEF
77: 3,4,3',4'–TeCB 0.0005 105: 2,3,4,3',4'–PeCB 0.0001 170: 2,3,4,5,2',3',4'–HpCB 0.0001
126: 3,4,5,3',4'–PeCB 0.1 114: 2,3,4,5,4'–PeCB 0.0005 180: 2,3,4,5,2',4',5'–HpCB 0.00001
169: 3,4,5,3',4',5'–HxCB 0.01 118: 2,4,5,3',4'–PeCB 0.0001
123: 3,4,5,2',4'–PeCB 0.0001
156: 2,3,4,5,3',4'–HxCB 0.0005
157: 2,3,4,3',4',5'–HxCB 0.0005
167: 2,4,5,3',4',5'–HxCB 0.00001
189: 2,3,4,5,3',4',5'–HpCB 0.0001
Source: Adapted from Ahlborg et al. (1994).
Brown (1994) calculated relative metabolic rates for 146 congeners and
combined them into a measure of "relative human accumulability" for each Aroclor,
based on congener concentrations in the Aroclors. Assuming a correlation between
38
relative human accumulability and chronic toxicity or cancer, it was suggested to
calculate relative human accumulability (relative to Aroclor 1260) for each
environmental mixture. Because environmental processes can alter the relative
congener concentrations, this approach can yield cancer slopes outside the range
determined previously for commercial mixtures. In premeeting comments on this
assessment (U.S. EPA, 1996b), Brown refined this approach to express cancer slope
as a function of both relative human accumulability and dioxin toxic equivalents.
Congener concentrations for the commercial mixtures in the Brunner et al. (1996) study
will be used to evaluate this suggested approach.
4. APPLICATION OF THE DOSE-RESPONSE ASSESSMENT
4.1. APPLICATION TO PCB MIXTURES IN THE ENVIRONMENT
After release into the environment, PCB mixtures change through partitioning,
transformation, and bioaccumulation, differing considerably from commercial mixtures.
How can toxicity values for commercial mixtures be applied to mixtures in the
environment?
A consensus is emerging on the difficulty of assessing environmental mixtures
by reference to Aroclors. Safe (1994) wrote, "Regulatory agencies and environmental
scientists have recognized that the composition of PCBs in most environmental extracts
does not resemble the composition of the commercial products." Along similar lines,
ATSDR (1993) advised,
It is important to recognize that the PCBs to which people may be
exposed are likely to be different from the original PCB source because of
changes in congener and impurity composition resulting from differential
partitioning and transformation in the environment and differential
biological metabolism and retention. Because of this concern, current
data are considered inadequate to differentiate between the toxicity and
carcinogenicity of environmental PCB mixtures with any reasonable
degree of confidence.
39
For these reasons, risks from environmental mixtures are not assessed by
characterizing environmental mixtures as if they were Aroclors. This does not mean
that all environmental mixtures are regarded as equally potent; environmental mixtures
differ from commercial mixtures and from each other. To make distinctions about risks
from environmental mixtures, the range of potency observed for commercial mixtures
can be considered along with factors that increase or decrease risk.
First among these is persistence and bioaccumulation through the food chain.
Each species, in turn, retains persistent congeners that prove resistant to metabolism
and elimination (Oliver and Niimi, 1988). Bioaccumulated PCBs appear to be more
toxic than commercial PCBs (Aulerich et al., 1986). Mink fed Great Lakes fish
contaminated with PCBs showed liver and reproductive toxicity comparable to mink fed
Aroclor 1254 at quantities three times greater (Hornshaw et al., 1983). It is crucial to
recognize that commercial PCBs tested in laboratory animals were not subject to prior
selective retention of persistent congeners through the food chain. For exposure
through the food chain, risks can be higher than those estimated in this assessment.
Also important is the presence or absence of congeners and metabolites that
contribute to cancer induction. Mechanistic studies are beginning to identify these
congeners and describe their modes of action. Several congeners have dioxin-like
activity (Ahlborg et al., 1994; Safe, 1994), some promote tumors through different
modes of action (Buchmann et al., 1991). Because concentrations of these congeners
are altered by partitioning, transformation, and bioaccumulation in the environment,
risks from environmental mixtures can differ from those of commercial mixtures.
Congener analyses of environmental samples can provide information on the extent of
this difference and can be an important tool in risk assessment, particularly when fish
consumption is an issue.
Chlorine content was formerly regarded by some scientists as correlated with
cancer risk. Recently, however, Aroclor 1254 was found to be more potent than 1260,
which was only slightly more potent than 1242 (Brunner et al., 1996). This casts doubt
on chlorine content being a useful indicator of cancer potency in this range of chlorine
40
content; both the number and position of chlorines are important. It is instructive to
compare how the Aroclors rank by other measures. With respect to resistance to
metabolism and persistence in the body, there is an association with chlorine content,
which partially explains the greater experimental potency of commercial mixtures with
higher chlorine content. With respect to dioxin toxic equivalents (TEQs), however,
several studies have ranked Aroclor 1254, 1248, and 1242 as more potent than 1260
(Harper et al., 1995; Safe, 1994; Harris et al., 1993; Hong et al., 1993; Schulz et al.,
1989). The combined effect is difficult to predict, as Aroclor 1260 and mixtures with
higher chlorine content have lower dioxin TEQs but persist longer in the environment
and in the body.
A key finding is the several-fold lower potency of Aroclor 1016 compared with
1242 (Brunner et al., 1996). Though these mixtures are similar in average chlorine
content (41 and 42 percent, respectively), Aroclor 1016 has virtually no congeners with
more than four chlorines. This suggests that one way to differentiate less potent
mixtures is to verify the absence of congeners with more than four chlorines.
Since mixtures of congeners with more than four chlorines cannot be assessed
using chlorine content alone, other determinants of toxicity are used. Two important
determinants, persistence and bioaccumulation, can be related to exposure pathway.
(Persistence is not synonymous with toxicity; however, in the absence of testing on
most congeners, it is reasonable to suppose some correlation between persistence and
toxicity.) Evaporated or dissolved congeners tend to be lower in chlorine content than
the original mixture; they tend also to be more inclined to metabolism and elimination
and lower in persistence and toxicity. On the other hand, congeners adsorbed to
sediment or soil tend to be higher in chlorine content and persistence, and
bioaccumulated congeners ingested through the food chain tend to be highest of all.
Rates of these processes vary over several orders of magnitude (Hutzinger et al.,
1974; Erickson, 1986), thus the effect of environmental processes can be greater than
the spread in potency or slope estimated from commercial mixtures.
41
For these reasons, a tiered approach is recommended. The default tier uses
exposure pathway to choose appropriate potency values from the ranges described in
table 3–2. The highest observed potency from these ranges is appropriate for food
chain exposure, sediment or soil ingestion, and dust or aerosol inhalation, pathways
where environmental processes tend to increase risk. Lower potencies are appropriate
for ingestion of water-soluble congeners or inhalation of evaporated congeners,
pathways where environmental processes tend to decrease risk. To the extent that
drinking water or ambient air contains contaminated sediment or dust, the higher
potency values would be appropriate, as congeners adsorbed to sediment or dust tend
to be of high chlorine content and persistence, especially for sediment or dust with high
organic content. Since the lowest observed potency, based on studies to date, is
derived from Aroclor 1016, its use is most appropriate in the absence of congeners with
more than four chlorines. When congeners with more than four chlorines are present,
but exposure is by drinking water ingestion or vapor inhalation, potency values derived
from the next lowest tested mixture, Aroclor 1242, can be more appropriate.
42
Thus three reference points can be designated for each range (see table 4–1):
a "high risk" point based on studies of Aroclor 1260 and 1254, which give the highest
observed potencies; a "low risk" point, based on the study of Aroclor 1242; and a
"lowest risk" point, based on the study of Aroclor 1016. The "high risk" point is used for
exposure pathways associated with environmental processes that tend to increase risk;
the "low risk" point, for exposure pathways that tend to decrease risk; and the "lowest
risk" point, for cases where congener or isomer analyses verify that congeners with
more than four chlorines comprise less than one-half percent of total PCBs, suggesting
that potency is best represented by the least potent tested mixture. This demonstrates
a potential use of congener analysis of environmental samples. Section 5 provides a
series of examples illustrating this tiered approach.
43
Table 4–1. Tiers of human potency and slope estimates for environmental mixtures
HIGH RISK AND PERSISTENCE
Central Upper-bound
ED10
LED10 slope slope Criteria for use
abcd
0.086 0.067 1. 2. Food chain exposure
Sediment or soil ingestion
Dust or aerosol inhalation
Dermal exposure, if an absorption factor has
been applied to reduce the external dose
Presence of dioxin-like, tumor-promoting, or
persistent congeners in other media
Early-life exposure (all pathways and mixtures)
LOW RISK AND PERSISTENCE
Central Upper-bound
ED10
LED10 slope slope Criteria for use
abcd
0.38 0.27 0.3 0.4 Ingestion of water-soluble congeners
Inhalation of evaporated congeners
Dermal exposure, if no absorption factor has
been applied to reduce the external dose
LOWEST RISK AND PERSISTENCE
Central Upper-bound
ED10
LED10 slope slope Criteria for use
abcd
2.4 1.4 0.04 0.07 Congener or isomer analyses verify that
congeners with more than 4 chlorines comprise
less than 1/2% of total PCBs
Estimated dose associated with 10% increased incidence, in mg/kg-d.
a
95% lower bound on ED10, in mg/kg-d.
b
Per mg/kg-d, computed as 0.10/ED10 and rounded to one significant digit.
c
Per mg/kg-d, computed as 0.10/LED10 and rounded to one significant digit.
d
This reasoning assumes that the PCB mixture has undergone alteration in the
environment for many years and that partitioning into different environmental media has
approached equilibrium. Congener or isomer analyses are important to verifying
equilibrium. For example, if drinking water samples contain high concentrations of
congeners with high chlorine content, this could indicate a recent release of PCBs that
has not had sufficient time to partition as expected, a continuing release of PCBs, or
44
the presence of stirred-up sediment and the subsequent release of adsorbed
congeners with high chlorine content. Judgment should be used in assessing these
situations, which are most likely not best represented by the "lowest risk" point derived
from Aroclor 1016.
4.2. APPLICATION TO DIFFERENT ROUTES OF EXPOSURE
What inferences can be made about dermal or inhalation exposures, two routes
for which there are no lifetime cancer studies?
PCBs are absorbed through ingestion, inhalation, and dermal exposure, after
which they are transported similarly through the circulation. This provides a
reasonable basis for expecting similar internal effects from different routes of
environmental exposure. In addition, the capacity of Aroclor 1254 to promote mouse
lung tumors (Anderson et al., 1983, 1994; Beebe et al., 1992, 1993) and the
observation of skin cancer from occupational exposure (Sinks et al. 1992) suggest a
potential for cancer at the point of entry.
Dermal absorption through human skin is rapid initially and continues at a slower
rate with further contact (Wester et al., 1987, 1990). Rhesus monkeys exposed to
Aroclor 1242 or 1254 in soil for 24 hours absorbed 14 percent through the skin (Wester
et al., 1993). Absorption of PCBs from soil involves competition between the lipophilic
attraction of PCBs to skin and adsorption to organic soil material. Absorption increases
with duration of skin contact and moisture content of soil, but these relationships have
not been quantified. Overall, use of the low end of the potency ranges for dermal
exposure appears appropriate in light of the substantial but incomplete absorption
through the skin. If, however, an estimate of dermal exposure has been reduced by an
absorption factor, then the high end of the potency ranges would be appropriate, as the
use of an absorption factor converts an external dose to an internal dose.
The lung is also a potential target for cancer risk following PCB exposure.
Aroclor 1254 is active as a promoter in the mouse lung initiated with methylating
nitrosamines. Relevant mechanistic information includes: (1) persistent induction of
45
cytochrome P–450 1A in the lung (up to several months after a single PCB dose),
(2) promotion by 2,3,4,2',4',5'–HxCB, not by 2,4,5,2',4',5'–HxCB, and partial abrogation
of 2,3,4,2',4',5'–HxCB's effects by 2,4,5,2',4',5'–HxCB, (3) correlation of the promotion
effect with body burden of 2,4,5,2',4'–PeCB, and (4) selective retention of PCB
congeners, especially 2,3,4,3',4'–PeCB, in mouse lung (Anderson, 1991b).
Inhaled PCBs can be rapidly absorbed, although rates have not been quantified
(ATSDR, 1993). Rapid absorption, however, suggests potency by inhalation is
comparable to potency by ingestion. Because PCBs are slowly metabolized, little
uncertainty results from the first-pass effect, where ingested toxicants are subject to
metabolism in the liver before entering the circulation, while inhaled toxicants enter the
circulation before reaching the liver. As with ingested mixtures, the composition of an
inhaled mixture influences its toxicity. Evaporated mixtures tend to have low chlorine
content and persistence, mixtures adsorbed to dust and soil tend to be high in this
regard, and mixtures suspended in an aerosol can be more diverse.
4.3. APPLICATION TO LESS-THAN-LIFETIME AND EARLY-LIFE EXPOSURES
In assessing cancer risks from less-than-lifetime exposure, the common practice
is to prorate cumulative exposure over the lifespan (U.S. EPA, 1986a, 1992a). For
example, exposure lasting 7 years of a 70-year lifespan would be assumed to have
one-tenth the effect of lifetime exposure. Does the information available for PCBs
support this default or suggest an alternative?
Less-than-lifetime exposure induced statistically significant increased incidences
of liver tumors in female rats fed Aroclors 1260, 1254, and 1242 (Brunner et al., 1996).
This result was most pronounced for Aroclor 1260, where tumor incidences at the
highest dose were higher for a 12-month exposure than for a 24-month lifetime
exposure. Only Aroclor 1016 showed no significant increases from less-than-lifetime
exposure. The earlier less-than-lifetime studies in rats and mice suggest that less-
than-lifetime exposure can quickly induce high incidences of early stages of tumor
development (Kimbrough et al., 1972; Ito et al., 1973, 1974). With further exposure,
46
these can progress to malignancy (Kimbrough et al., 1975; Norback and Weltman,
1985). Tumor incidences from less-than-lifetime exposure were sometimes lower
(Kimbrough and Linder, 1974), and sometimes similar (Rao and Banerji, 1988), to those
from full lifetime exposure.
The strong response for Aroclor 1260, coupled with the lack of response for
Aroclor 1016, suggests that these findings may be related to persistence in the body.
PCBs entering the body are transported by the circulation to internal organs and fat,
where they are stored (Matthews and Anderson, 1975). Equilibrium is maintained
among external exposure levels, concentrations in blood, and concentrations in fat and
other tissues. When external exposure is reduced, to maintain equilibrium, stored
PCBs reenter the circulation and provide a continuing internal source of exposure
(Matthews and Anderson, 1975). Thus PCBs from short-term exposure can be stored
in the body and emerge as a source of exposure much later.
Persistence in the body can enhance the opportunity for PCB congeners to
express tumor promoting activity (Safe, 1994). Persistent congeners can retain
biological activity long after exposure stops (Anderson et al., 1991a); some persistent
congeners are tumor promoters. The congener 3,4,3',4'-tetrachlorobiphenyl continues
to promote tumors vigorously after dosing stops, but not 2,4,2',5'-tetrachlorobiphenyl
(Buchmann et al., 1991; Luebeck et al., 1991). Although the probability of liver focus
extinction is high after dosing stops, those that become large tend to persist (Luebeck
et al., 1991). This would allay concern for short-term exposure but increase concern as
exposure duration increases. Further studies of less-than-lifetime exposure, as well as
methods for quantifying the differential effects of less-than-lifetime exposure, are
needed.
Regarding early-life exposure, infants can be highly exposed to PCBs during
pregnancy and lactation (Dewailly et al., 1991, 1994). The accumulation of PCBs in
human adipose tissue creates a store for subsequent release of PCBs into the
bloodstream and then into the fetal circulation. During the postpartum period, PCBs
are mobilized from adipose stores, transferred into human milk, and delivered to the
47
neonate via nursing (Dewailly et al., 1991). This source of exposure may account for a
substantial fraction of chlorinated dibenzo-p-dioxins and dibenzofurans, and the same
may be true for dioxin-like and other PCBs. It is, therefore, important to assess the
extent of exposure through the human milk pathway; if direct measurement of
concentrations in milk are not available, estimates can be derived from maternal
exposures (Smith, 1987).
Normal fetal development depends on the timing and rate of release of T3 and
T4. Some evidence indicates that PCBs can alter normal T3 and T4 metabolism,
thereby disturbing thyroid function and provoking secondary impacts on organogenesis
during development. Any estrogenic/anti-estrogenic, androgenic/anti-androgenic, or
other hormonal activity of PCB mixtures has the possibility of altering the development
of reproductive organs or the urogenital tract, potentially causing cancer or other
adverse effects through a mechanism different from those causing liver cancer (U.S.
EPA, 1996b).
Few studies, however, have investigated early-life sensitivity. In human infants,
glucuronidative mechanisms are not fully developed; additionally, some nursing infants
receive a steroid in human milk that inhibits the activity of glucuronyl transferase,
further reducing PCB metabolism and elimination (Calabrese and Sorenson, 1977). In
animals, Aroclor 1260 induced high incidences of liver tumors when fed to 5-week-old
rats for a short time (Rao and Banerji, 1988). On the other hand, acute perinatal
dosing with Aroclor 1254 promoted nitrosamine-initiated lung and liver tumors in mice
but did not induce cancer in the offspring when administered alone (Anderson et al.,
1983, 1986, 1994). A study of polybrominated biphenyls (PBBs) found that perinatal
exposure enhanced susceptibility to liver tumors for female rats also exposed as adults
and increased the incidence of liver tumors in male and female mice not further
exposed as adults (NTP, 1993). Because of the potential magnitude of early-life
exposures, the possibility of greater perinatal sensitivity, and the likelihood of
interactions among thyroid and hormonal development, it is reasonable to conclude
that early-life exposures may be associated with increased risks; this would indicate
48
using the "high-risk" potency estimates for early-life exposure. A method for
quantifying the differential effects of early-life exposure is needed.
4.4. APPLICATION WITH DIOXIN TOXIC EQUIVALENCE FACTORS
TEFs have been proposed for some dioxin-like PCB congeners, while this
assessment develops potency values for overall concentrations of mixtures. How can
the TEF approach supplement the mixture-based approach?
When assessing PCB mixtures, it is important to recognize that both dioxin-like
and nondioxin-like modes of action contribute to overall PCB toxicity (Safe, 1994;
McFarland and Clarke, 1989; Birnbaum and DeVito, in press). Because relatively few
PCB congeners are dioxin-like, dioxin equivalence explains only part of a PCB
mixture's toxicity. (This applies to cancer and other forms of toxicity, for example,
neurotoxicity and endocrine disruption; Birnbaum and DeVito, in press.) Hence, PCB
assessments should begin with the mixture-based approach developed in this report.
At the same time, it is possible that concentrations of dioxin-like congeners are
increased in an environmental mixture. When congener concentrations are available,
the mixture-based approach can be supplemented by analysis of dioxin TEQs to
evaluate dioxin-like toxicity. Section 5 gives an example for calculating dioxin TEQs
when a congener analysis is available.
When assessing mixtures of dioxin and related compounds, it is important to
consider the contribution of dioxin-like PCBs to total dioxin equivalents (U.S. EPA,
1994b). TEQs for dioxin-like PCBs (Ahlborg et al., 1994) can be added to those for
other dioxin-like compounds. In some situations, PCBs can contribute more dioxin-like
toxicity than chlorinated dibenzo-p-dioxins and dibenzofurans (Schecter et al., 1994;
Dewailly et al., 1991, 1994). The congener 2,4,5,3',4'-pentachlorobiphenyl, shown to
have tumor-promoting activity, is a major contributor to total dioxin equivalents in the
United States (Patterson et al., 1994) and maritime Quebec (Dewailly et al., 1994).
Exposure to dioxin-like PCBs adds to background exposure of dioxin-like
compounds and augments processes associated with dioxin toxicity. There is support
49
for using low-dose-linear dose-response models for incremental doses that add to
existing background exposure (Crump et al., 1976; Lutz, 1990). Thus confidence in
this assessment's use of low-dose-linear models is enhanced for the dioxin-like portion
of a PCB mixture.
5. CHARACTERIZATION AND GUIDANCE FOR RISK ASSESSORS
5.1. DOSE-RESPONSE CHARACTERIZATION
Joint consideration of cancer studies and environmental processes leads to a
conclusion that environmental PCB mixtures are highly likely to pose a risk of cancer to
humans. Although environmental mixtures have not been tested in cancer assays, this
conclusion is supported by several complementary sources of information. Statistically
significant, dose-related, increased incidences of liver tumors were induced in female
rats by Aroclors 1260, 1254, 1242, and 1016 (Brunner et al., 1996). These mixtures
contain overlapping groups of congeners that, together, span the range of congeners
most frequently found in environmental mixtures. Several congeners promote tumors
or have dioxin-like activity; these congeners are found in environmental samples and in
a variety of organisms, including humans.
The range of potency observed for commercial mixtures is used to represent the
potency of environmental mixtures. The range reflects experimental uncertainty and
variability of commercial mixtures, but not human heterogeneity or differences between
commercial and environmental mixtures. Environmental processes alter mixtures
through partitioning, transformation, and bioaccumulation, thereby decreasing or
increasing toxicity. The overall effect can be considerable, and the range observed for
commercial mixtures may underestimate the true range for environmental mixtures.
Limiting the potency of environmental mixtures to the range observed for commercial
mixtures reflects a decision to base potency estimates on experimental results,
however uncertain, rather than apply safety factors to compensate for lack of
information (U.S. EPA, 1996b).
50
A tiered approach allows use of different kinds of information in estimating the
potency of environmental mixtures. When congener information is limited, exposure
pathway is used to indicate whether environmental processes have decreased or
increased a mixture's potency. Partitioning, transformation, and bioaccumulation have
been extensively studied and can be associated with exposure pathway, thus the use
of exposure pathway to represent environmental processes increases confidence in the
risks inferred for environmental mixtures. When available, congener information is an
important tool for refining a potency estimate that was based on exposure pathway.
Extrapolation to environmental levels is based on models that are linear at low
doses. Low-dose-linear models are appropriate when a carcinogen acts in concert with
other exposures and processes that cause a background incidence of cancer. Even
when the mode of action indicates a nonlinear dose-response curve in homogeneous
animal populations, the presence of genetic and lifestyle factors in a heterogeneous
human population tends to make the dose-response curve more linear.
Depending on the specific application, either central estimates or upper bounds
can be appropriate. Central estimates describe a typical individual's risk, while upper
bounds provide assurance that this risk is not likely to be underestimated if the
underlying model is correct. The upper bounds calculated in this assessment reflect
study design and provide no information about sensitive individuals or groups. Central
estimates are useful for estimating aggregate risk across a population. Central
estimates are used for comparing or ranking environmental hazards, while upper
bounds provide information about the precision of the comparison or ranking.
Comparing a central estimate with its upper bound indicates whether the central
estimate is stable enough to support credible risk estimates. In this assessment, the
less-than-twofold difference between central estimates and upper bounds indicates that
these estimates are stable.
Uncertainty around these estimates extends in both directions. The slope factor
ranges primarily reflect mixture variability, and so are not necessarily appropriate for
probabilistic analyses that attempt to describe model uncertainty and parameter

51
uncertainty. Several sources of uncertainty are inherent in the experimental
information used in this assessment:
Experimental design and conduct: The new rat study (Brunner et al., 1996) is
quite extensive in design and conduct, going beyond standard designs for
cancer studies in many respects.
Variability in commercial mixture composition: For the four Aroclors tested
in female Sprague-Dawley rats (Brunner et al., 1996; Norback and Weltman,
1985), there is a 30-fold range in potency. This whole range is used to
represent environmental mixtures.
Variability across strains: In the four rat strains tested, sensitivity varies up to
15-fold. Potency and slope estimates were derived from a strain covering the
middle of this range.
Variability between sexes: Potency and slope estimates were derived from
female rats, whose liver response was usually greater than that of males. The
greatest response in the liver, however, was in male rats. Greater sensitivity of
females was not seen in mice, nor in the thyroid.
Variability across experiments: For the same Aroclor, sex, and strain,
differences up to four-fold were observed. To reflect this lot-to-lot variability,
both estimates were included.
Experimental uncertainty (sample size): Central and upper-bound potency
estimates differ by no more than about two-fold. This is a minor source of
uncertainty.
Potential for other carcinogenic effects: The new rat study reported small
increases in thyroid tumor incidence for male rats, suggesting a potential for a
hormonal mode of action. These results have not yet been publicly discussed or
peer reviewed.
Other sources of uncertainty arise in the methods for assessing this
experimental information and applying it to human environmental exposure:
52
Animal-to-human extrapolation: The use of default cross-species scaling
factors is intended as an unbiased projection not expected to provide
conservatism (U.S. EPA, 1992b). Information is lacking to evaluate whether
humans are more or less sensitive than rats.
High-to-low-dose extrapolation: The use of models that are linear at low
doses can potentially overestimate potency by an unknown amount. The rat
studies, however, show no evidence of sublinearity in the experimental range.
Route-to-route extrapolation: Information on relative absorption rates
suggests that differences in toxicity across exposure routes are small.
Difference between commercial and environmental mixtures: Commercial
mixtures released into the environment are altered by environmental processes.
Qualitatively, exposure pathway is a reasonably good indicator of whether
potency has been decreased or increased. Quantitatively, the percentage
change in toxicity is unknown, though the 30-fold range in potency observed for
commercial mixtures likely underestimates the range for environmental mixtures.
Persistence and exposure duration: Some PCBs persist in the body and
retain biological activity after exposure stops (Anderson et al., 1991a).
Compared with the current default practice of assuming that less-than-lifetime
effects are proportional to exposure duration, rats exposed to the persistent
mixture Aroclor 1260 had more tumors, while rats exposed to the less persistent
Aroclor 1016 had fewer tumors (Brunner et al., 1996). Thus the current default
practice can underestimate risks for persistent mixtures.
Human variability in sensitivity: People with decreased liver function can
have less capacity to metabolize and eliminate PCBs. Approximately five
percent of nursing infants receive a steroid in human milk that further inhibits
PCB metabolism and elimination (Calabrese and Sorenson, 1977).
Human variability in exposure: Blood concentrations vary over a 100-fold
range (ATSDR, 1993). Highly exposed populations include nursing infants,
consumers of game animals contaminated through the food chain, and workers
53
with occupational exposure. There is greater confidence in risk estimates for
highly exposed groups.
When exposure involves the food chain, uncertainty extends principally in one
direction: through the food chain, living organisms selectively bioaccumulate persistent
congeners, but commercial mixtures tested in laboratory animals were not subject to
prior selective retention of persistent congeners. Bioaccumulated PCBs appear to be
more toxic than commercial PCBs (Aulerich et al., 1986; Hornshaw et al., 1983) and
appear to be more persistent in the body (Hovinga et al., 1992). For exposure through
the food chain, risks can be higher than those estimated in this assessment. Two
highly exposed populations, nursing infants and consumers of contaminated game
animals, are exposed through food.
The dioxin-like nature of some PCBs raises a concern for cumulative exposure,
as dioxin-like congeners add to background exposure of other dioxin-like compounds
and augment processes associated with dioxin toxicity. This weighs against
considering PCB exposure in isolation or as an increment to a background exposure of
zero. Confidence in this assessment's use of low-dose-linear models is enhanced
when there is additivity to background exposures and processes.
To gauge the distance of an extrapolation, human exposures can be compared
with the LED10. For mixtures that are altered in the environment, however, this simple
comparison of relative exposure is of limited value as some congeners increase in
concentration while others decrease. Additionally, for exposures that augment
processes leading to a background incidence of cancer, identifying and quantifying
background human exposures are necessary for a true measure of total exposure.
5.2. INFLUENCE OF PROPOSED CANCER GUIDELINES
This assessment demonstrates several ideas from EPA's proposed cancer
guidelines (U.S. EPA, 1996a). Most prominent is development of a range of potency
estimates, using studies for a range of mixtures, instead of focusing on the highest-
potency mixture. For low-dose extrapolation, an LED10 approach replaces the

54
linearized multistage procedure. An ED10 approach provides a statistically stable
method for deriving central estimates of low-dose slopes. Dose calculations use the
interagency consensus cross-species scaling factor, based on the 3/4 power of relative
body weight (U.S. EPA, 1992b).
Also evident is the proposed guidelines' encouragement to use different kinds of
information. It is reflected in the tiered approach that uses site-specific congener or
isomer analyses when available, but can differentiate among environmental mixtures
using exposure pathway when mixture information is limited. It is also found in this
assessment itself, which combines information on toxicity and environmental
processes.
The proposed guidelines' emphasis on discussing circumstances that affect
cancer risks, especially exposure route considerations, is found throughout this
assessment. There is extensive discussion of how environmental processes alter the
composition and toxicity of PCB mixtures. Exposure circumstances are addressed in a
framework that distinguishes different exposure pathways as lower risk or higher risk.
None of these features, however, is inconsistent with previous guidelines (U.S.
EPA, 1986a), whose intent is "to permit sufficient flexibility to accommodate new
knowledge and new assessment methods as they emerge." Each new feature of this
assessment can be viewed in this spirit.
5.3. RESEARCH NEEDS
This dose-response assessment has tried to make the best use of the available
information. For some questions it has stopped short because information or methods
are needed to resolve an important issue. Research that would enable this
assessment to proceed further includes:
Cancer studies comparing commercial and environmental mixtures, especially
those found in the food chain. This assessment warns that food chain risks can
be underestimated, but the extent is not quantified.

55
A method for using lifetime studies to assess less-than-lifetime exposure to
persistent agents. This assessment warns that assuming risk and exposure
duration are proportional can underestimate risks for persistent mixtures, but the
extent is not quantified.
Relative cancer potency factors for congeners identified by McFarland and
Clarke (1989). This will improve evaluations of mixtures where carcinogenic
potential has been increased or decreased beyond the range of tested
commercial mixtures. To help determine such factors, 2-year studies in female
rats are recommended for 3,4,5,3',4'-pentachlorobiphenyl and 3,4,5,3',4',5'-
hexachlorobiphenyl, the two congeners with the highest dioxin TEFs, and
2,4,5,2',4',5'-hexachlorobiphenyl and 2,3,4,5,2',4',5'-heptachlorobiphenyl, the
two persistent congeners whose PBB analogues constitute over 80 percent of
the PBB mixture that caused hepatocellular adenomas, carcinomas, and
cholangiocarcinomas in rats.
Studies to test the hypothesis that the carcinogenic activity of Aroclor 1016 is
due to its tetrachlorobiphenyls only, with no contribution from congeners with
1–3 chlorines. Current information is inadequate to evaluate this hypothesis.
At the peer review workshop, the panel identified other areas where research
could help resolve important questions. These can be grouped broadly into exposure
methods research, effects research, and risk assessment methods development.
Research needs in exposure methods include:
Standard analytical methods, including sample preparation, for measuring PCB
congeners in environmental samples.
Database of congener levels in environmental samples.
Research needs on effects include:
Epidemiologic studies focused on tumor promotion. If PCBs act as tumor
promoters, they would increase cancer mainly in humans with already-initiated
cancer cells. For common cancers with complex etiologies, promotional effects
will be seen only if specifically looked for.

56
Mechanism-oriented dose-response data for environmental mixtures, including
promotional, hormonal, sex-specific effects.
Mechanisms of PCB-induced liver cancer in rats, its similarity to the
mechanism(s) of rat liver cancer induced by "nongenotoxic" carcinogens, its
activities at low dose levels, and its relevance to humans.
Identifying the most significant congeners in commercial and environmental
mixtures, describing their modes of action, and conducting studies to quantify
their slope factors.
Dose-response studies in a broader range of test animals.
Determine sensitivity of fetuses and newborns to the carcinogenic effects of
environmental mixtures.
Determine risks for thyroid and urogenital/reproductive tract cancers in
newborns and adults.
Methods development needs for risk assessment include:
Developing an appropriate dose metric for PCBs.
Exploring structure-activity methods for predicting pharmacokinetic parameters
for PCB mixtures.
Verifying the appropriateness, for PCBs, of the new cross-species scaling factor.
Evaluating the consistency of human and animal studies.
Developing quantitative uncertainty distributions for key sources of uncertainty.
Work in progress includes using congener compositions of the tested Aroclors
from the Brunner et al. (1996) study to evaluate the "relative human accumulability"
approach (Brown, 1994; U.S. EPA, 1996b). Congener compositions of the Aroclors
and the rat tissues can also be used in a factor analysis that would identify a subset of
congeners most associated with tumor induction. Field analyses can then reduce
uncertainty by quantifying a small number of critical congeners.
Elsewhere, new epidemiologic information is being analyzed. The National
Institute for Occupational Safety and Health is updating its study of the Indiana cohort,
and is expanding and updating its studies of the cohorts in Massachusetts and New
57
York. Additionally, the females in these three cohorts are being pooled for a study of
breast cancer.
Finally, future risk assessments of PCBs in the environment should consider
more than the risk of cancer. Although the purpose of this report was to evaluate
cancer risks, the emerging scientific literature indicates that toxicological endpoints
other than cancer may also be important to human health. These toxic effects should
be included along with cancer in future assessments of PCBs.
5.4. SUMMARY OF GUIDANCE FOR RISK ASSESSORS
Joint consideration of cancer studies and environmental processes leads to a
conclusion that environmental PCB mixtures are highly likely to pose a risk of cancer to
humans. The cancer potency of PCB mixtures is determined using a tiered approach
that depends on the information available (see table 4–1).
Upper-bound slope factors, derived by linear extrapolation from LED10s, are
described by a range of estimates with three reference points.
Upper-bound slope factors: 0.07 – 0.4 – 2 per mg/kg-d
Slope factors are multiplied by lifetime average exposure levels to estimate the risk of
cancer. The upper bounds reflect study design and provide no information about
sensitive individuals or groups. Although PCB exposures are often characterized in
terms of Aroclors, this can be both imprecise and inappropriate. Total PCBs or
congener or isomer analyses are recommended.
The first (default) tier is invoked when information on the mixture of interest is
limited. The upper reference point (2 per mg/kg-d) is appropriate for food chain
exposure, sediment or soil ingestion, and dust or aerosol inhalation; these are
exposure pathways for which environmental processes are likely to increase risk. Due
to potential for higher sensitivity early in life, the upper reference point is also used for
all early-life exposure. The middle reference point (0.4 per mg/kg-d) is appropriate for
drinking water ingestion and vapor inhalation; these are exposure pathways for which

58
environmental processes are likely to decrease risk. The lowest reference point (0.07
per mg/kg-d) should not be used without specific information on the congener
composition of the mixture.
The second tier is invoked when there are congener or isomer analyses for the
mixture of interest. The lowest reference point (0.07 per mg/kg-d) can be used if these
analyses verify that congeners with more than four chlorines comprise less than one-
half percent of total PCBs, as well as the absence of dioxin-like, tumor-promoting, and
persistent congeners. When congener concentrations are available, the slope-factor
approach can be supplemented by analysis of dioxin TEQs to evaluate dioxin-like
toxicity.
Central-estimate slope factors, derived by linear extrapolation from ED10s, can
be described by a similar range with three reference points.
Central-estimate slope factors: 0.04 – 0.3 – 1 per mg/kg-d
Central estimates describe a typical individual's risk, while upper bounds provide
assurance that this risk is not likely to be underestimated if the underlying model is
correct. Central estimates are useful for estimating aggregate risk across a population.
Highly exposed populations include nursing infants and consumers of game fish,
game animals, or products of animals contaminated through the food chain. Highly
sensitive populations include people with decreased liver function and infants.
A few limitations of this assessment should be noted:
It is crucial to recognize that commercial PCBs tested in laboratory animals were
not subject to prior selective retention of persistent congeners through the food
chain. Bioaccumulated PCBs appear to be more toxic than commercial PCBs
and appear to be more persistent in the body. For exposure through the food
chain, risks can be higher than those estimated in this assessment.
PCBs persist in the body, providing a continuing source of internal exposure
after external exposure stops. There may be greater-than-proportional effects

59
from less-than-lifetime exposure, especially for persistent mixtures and for early-
life exposures.
5.5. EXAMPLES
Example 1. Consider a release of PCBs onto the ground near a river or lake.
Potential pathways of human exposure include vapor inhalation, drinking water, fish
ingestion, and skin contact with ambient water and contaminated soil. The population
of interest includes anglers who consume an average of two 105-g portions of local fish
each week. They spend most of their time in the area, breathing 20 m air and drinking
3
2 L water, on average, each day. Skin contact with ambient water and soil is negligible
for this population. A 30-year exposure duration is to be considered, with a
representative lifespan of 70 years and body weight of 70 kg. Environmental samples
indicate long-term average concentrations of 0.01 g/m in ambient air, 5 g/L in
3
drinking water, and 110 g/kg in the edible portion of local fish. Dust in ambient air and
sediment in drinking water are negligible.
Because of partitioning, transformation, and bioaccumulation, different fractions
of the original mixture are encountered through these pathways, hence different
potency values are appropriate. Vapor inhalation is associated with "low risk" in
table 4–1 (evaporating congeners tend to have low chlorine content and be inclined to
metabolism and elimination), so the low end of the range (upper-bound slope of 0.4 per
mg/kg-d) is used for vapor inhalation. Similarly, ingestion of water-soluble congeners is
associated with "low risk" (dissolved congeners tend to have low chlorine content and
be inclined to metabolism and elimination), so the low end is also used for drinking
water. (If ambient air or drinking water had contained significant amounts of
contaminated dust or sediment, the high-end potency values would be appropriate, as
adsorbed congeners tend to be of high chlorine content and persistence.) Food chain
exposure appears is associated with "high risk" (aquatic organisms and fish selectively
accumulate congeners of high chlorine content and persistence that are resistant to
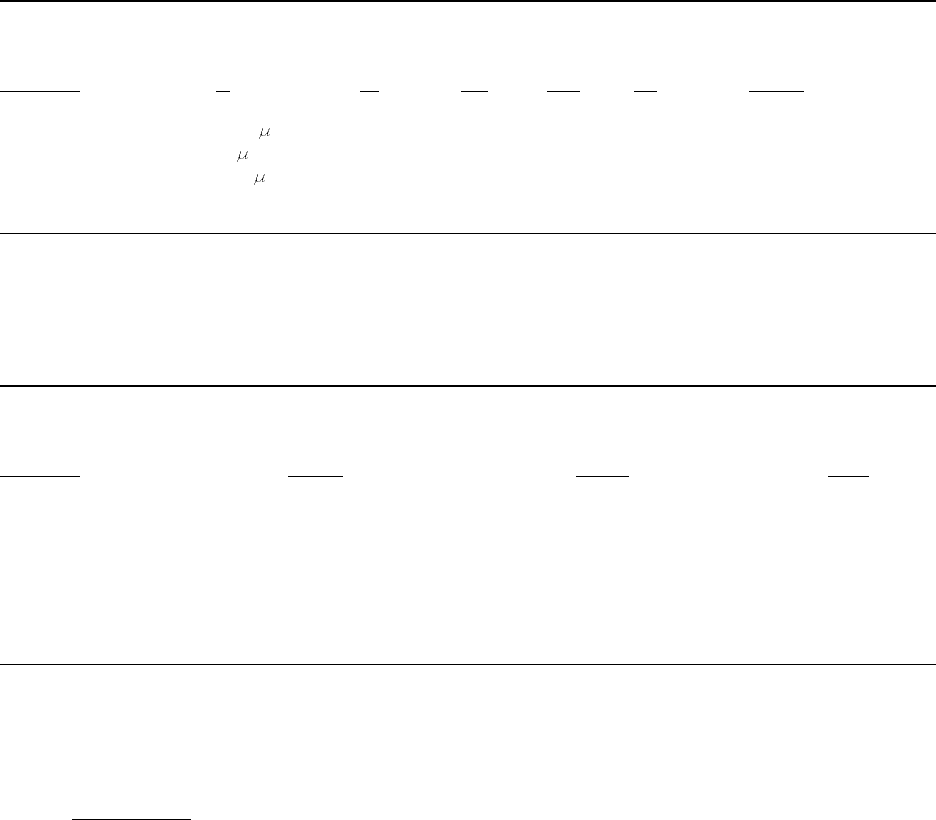
60
metabolism and elimination), so the high end of the range (upper-bound slope of 2 per
mg/kg-d) is used for fish ingestion.
The lifetime average daily dose (
LADD
) is calculated as the product of
concentration
C
, intake rate
IR
, and exposure duration
ED
divided by body weight
BW
and lifetime
LT
(U.S. EPA, 1992a)(table 5-1):
Table 5–1. Sample lifetime average daily dose calculations (examples 1 and 2)
Pathway
C IR ED BW LT LADD
a
Vapor inhalation 0.01 g/m 20 m /d 30 yr 70 kg 70 yr 1.2×10 mg/kg-d
33 –6
Drinking water 5. g/L 2 L/d 30 yr 70 kg 70 yr 6.1×10 mg/kg-d
–5
Fish ingestion 110 g/kg 30 g/d 30 yr 70 kg 70 yr 2.0×10 mg/kg-d
–5
LADD
=
C
×
IR
×
ED
/ (
BW
×
LT
)
a
For each pathway, the lifetime average daily dose is multiplied by the
appropriate slope to estimate risk (table 5-2):
Table 5–2. Sample risk calculations (example 1)
Pathway
LADD
Slope Risk
a
Vapor inhalation 1.2×10 mg/kg-d 0.4 per mg/kg-d 4.8×10
–6 –7
Drinking water 6.1×10 mg/kg-d 0.4 per mg/kg-d 2.4×10
–5 –5
Fish ingestion 2.0×10 mg/kg-d 2 per mg/kg-d 4.0×10
–5 –5
Sum 8.2×10 mg/kg-d 6.4×10
–5 –5
Risk =
LADD
× Slope
a
It is important to remember that this specific site exposure adds to a background
level of exposure from other sources.
Example 2. To show how additional, better information can improve the risk
assessment, suppose an analysis of PCB congeners in drinking water is performed in
the previous example. Suppose this analysis confirms that congeners with more than
four chlorines comprise less than a half-percent of total PCBs. Then it would be
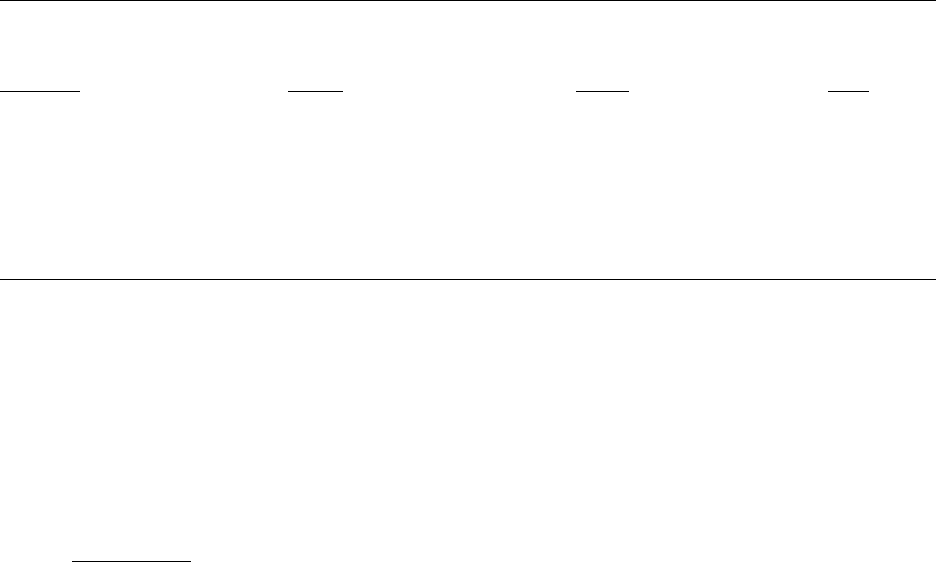
61
plausible to treat PCBs in drinking water as similar to Aroclor 1016. The slope derived
from the Aroclor 1016 study could be used for this pathway(see table 5-3):
Table 5–3. Sample risk calculations (example 2)
Pathway
LADD
Slope Risk
a
Vapor inhalation 1.2×10 mg/kg-d 0.4 per mg/kg-d 4.8×10
–6 –7
Drinking water 6.1×10 mg/kg-d 0.07 per mg/kg-d 4.3×10
–5 –6
Fish ingestion 2.0×10 mg/kg-d 2 per mg/kg-d 4.0×10
–5 –5
Sum 8.2×10 mg/kg-d 4.5×10
–5 –5
Risk =
LADD
× Slope
a
The additional information leads to a conclusion that fish ingestion is the
principal pathway contributing to risk, and that drinking water and vapor inhalation are
of lesser consequence. It would be advisable to examine variability in fish consumption
rates and fish tissue concentrations to determine whether some individuals are at much
higher risk.
Example 3. Next, suppose an analysis of PCB congeners in the edible portion
of the fish is performed in the previous example, and it shows that concentrations of
dioxin-like congeners are greatly enhanced (see table 5-4):
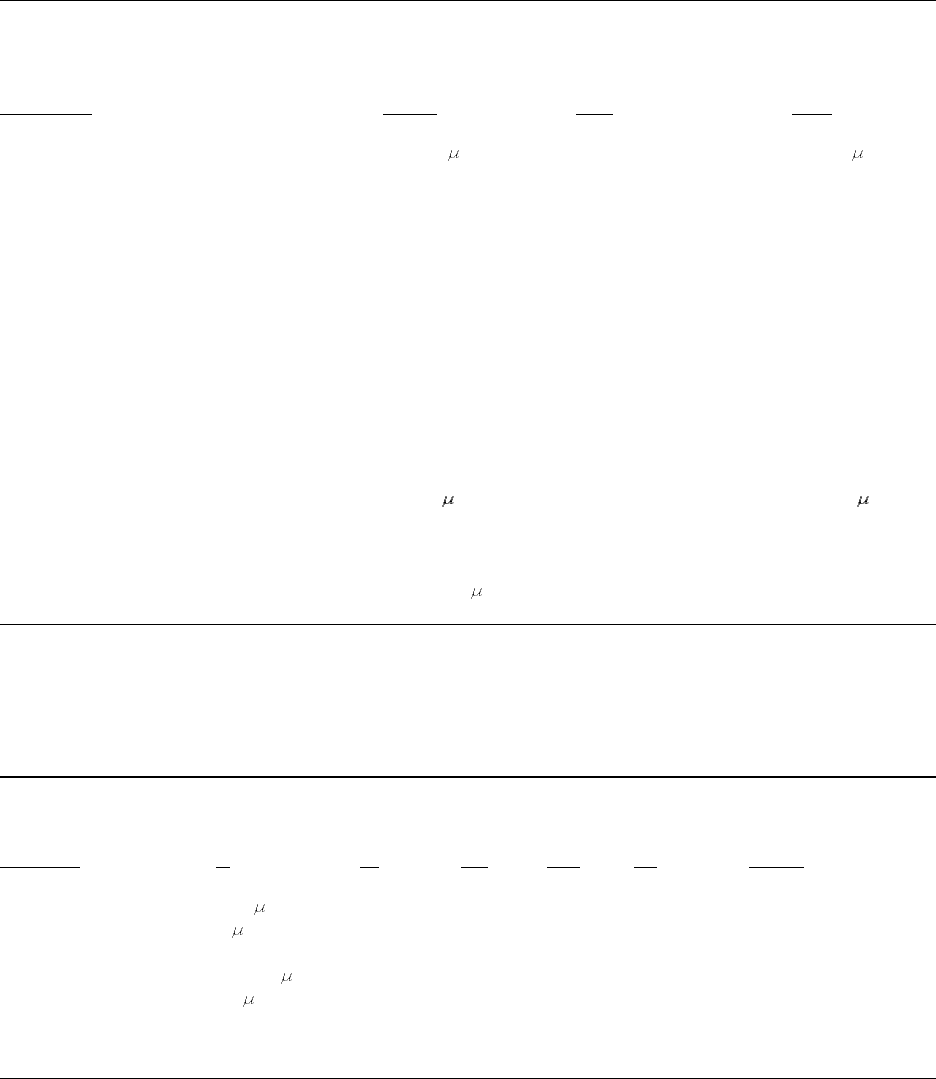
62
Table 5–4. Sample congener concentrations and dioxin toxic equivalents (TEQs) in edible portion
of fish (example 3)
Congener
Conc. TEF TEQ
ab
77: 3,4,3',4'–TeCB 2.1 g/kg 0.0005 0.0011 g/kg
105: 2,3,4,3',4'–PeCB 14. 0.0001 0.0014
114: 2,3,4,5,4'–PeCB 1.8 0.0005 0.0009
118: 2,4,5,3',4'–PeCB 54. 0.0001 0.0054
123: 3,4,5,2',4'–PeCB 1.4 0.0001 0.0001
126: 3,4,5,3',4'–PeCB 0.14 0.1 0.0140
156: 2,3,4,5,3',4'–HxCB 5.0 0.0005 0.0025
157: 2,3,4,3',4',5'–HxCB 1.1 0.0005 0.0006
167: 2,4,5,3',4',5'–HxCB 7.7 0.00001 0.0001
169: 3,4,5,3',4',5'–HxCB 0.0068 0.01 0.0001
170: 2,3,4,5,2',3',4'–HpCB <4.2 0.0001 0.0002
c
180: 2,3,4,5,2',4',5'–HpCB <4.2 0.00001 0.0000
c
189: 2,3,4,5,3',4',5'–HpCB 0.28 0.0001 0.0000
Others 18.85 0. 0.0000
d
Sum 111. g/kg 0.0264 g/kg
From Ahlborg et al. (1994)
a
TEQ = Conc. × TEF
b
Not detected, treated as half the detection limit of 4.2 g/kg
c
Nondioxin-like PCB congeners
d
The lifetime average daily dose would be recalculated separately for the dioxin-
like and nondioxin-like portions of the mixture (table 5-5):
Table 5–5. Sample lifetime average daily dose calculations (example 3)
Pathway
C IR ED BW LT LADD
a
Vapor inhalation 0.01 g/m 20 m /d 30 yr 70 kg 70 yr 1.2×10 mg/kg-d
33 –6
Drinking water 5. g/L 2 L/d 30 yr 70 kg 70 yr 6.1×10 mg/kg-d
–5
Fish ingestion
Dioxin TEQ 0.0264 g/kg 30 g/d 30 yr 70 kg 70 yr 4.8×10 mg/kg-d
–9
Nondioxin-like 19 g/kg 30 g/d 30 yr 70 kg 70 yr 3.5×10 mg/kg-d
–6
LADD
=
C
×
IR
×
ED
/ (
BW
×
LT
)
a
Using 150,000. per mg/kg-d as the slope for dioxin, the cancer risk would be
calculated as follows (table 5-6):
63
Table 5–6. Sample risk calculations (example 3)
Pathway
LADD
Slope Risk
a
Vapor inhalation 1.2×10 mg/kg-d 0.4 per mg/kg-d 4.8×10
–6 –7
Drinking water 6.1×10 mg/kg-d 0.07 per mg/kg-d 4.3×10
–5 –6
Fish ingestion
Dioxin TEQ 4.8×10 mg/kg-d 150,000 per mg/kg-d 7.2×10
–9 –4
Nondioxin-like PCBs 3.5×10 mg/kg-d 2 per mg/kg-d 7.0×10
–6 –6
Sum 7.3×10
–4
Risk =
LADD
× Slope
a
This example, although perhaps extreme, shows how it is possible for a total-
PCB approach to underestimate the toxicity of a mixture when concentrations of a few
dioxin-like or highly toxic congeners are enhanced through environmental and
metabolic processes. This shows the importance of obtaining congener analyses and
of continuing to develop quantitative methods for incorporating congener information
into risk assessments.
6. REFERENCES
Abramowicz, D.A. (1990) Aerobic and anaerobic biodegradation of PCBs: a review. Biotechnology 10(3):241–251.
Ahlborg, U.G.; Becking, G.C.; Birnbaum, L.S.; Brouwer, A.; Derks, H.J.G.M.; Feeley, M.; Golor, G.; Hanberg, A.;
Larsen, J.C.; Liem, A.K.D.; Safe, S.H.; Schlatter, C.; Wærn, F.; Younes, M.; Yrjånheikki, E. (1994) Toxic
equivalency factors for dioxin-like PCBs. Chemosphere, 28(6):1049–1067.
Alford-Stevens, A.L.; Budde, W.L.; Bellar, T.A. (1985) Interlaboratory study on determination of polychlorinated
biphenyls in environmentally contaminated sediments. Anal. Chem. 57:2452–2457.
Alford-Stevens, A.L. (1986) Analyzing PCBs. Environ. Sci. Technol. 20(12):1194–1199.
Anderson, L.M.; van Havere, K.; Budinger, J.M. (1983) Effects of polychlorinated biphenyls on lung and liver tumors
initiated in suckling mice by
N
-nitrosodimethylamine. J. Natl. Cancer Inst. 71(1):157–163.
Anderson, L.M.; Ward, J.M.; Fox, S.D. et al. (1986) Effects of a single dose of polychlorinated biphenyls to infant mice
on N-nitrosodimethylamine-initiated lung and liver tumors. Int. J. Cancer 18:109–116.
Anderson, L.M.; Fox, S.D.; Dixon, D.; Beebe, L.E.; Issaq, H.J. (1991a) Long-term persistence of polychlorinated
biphenyl congeners in blood and liver and elevation of liver aminopyrine demethylase activity after a single high
dose of Aroclor 1254 to mice. Environ. Toxicol. Chem. 10:681–690.
Anderson, L.M.; Beebe, L.E.; Fox, S.D.; Issaq, H.J.; Kovatch, R.M. (1991b) Promotion of mouse lung tumors by
bioaccumulated polychlorinated aromatic hydrocarbons. Exp. Lung Res. 17:455–471.
64
Anderson, L.M.; Logsdon, D.; Ruskie, S.; Fox, S.D.; Issaq, H.J.; Kovatch, R.M.; Riggs, C.M. (1994) Promotion by
polychlorinated biphenyls of lung and liver tumors in mice. Carcinogenesis 15(10):2245–2248.
ATSDR (Agency for Toxic Substances and Disease Registry) (1993) Toxicological profile for polychlorinated biphenyls.
Atlanta: ATSDR, TP–92/16, update.
ATSDR (Agency for Toxic Substances and Disease Registry) (1995) Toxicological profile for polychlorinated biphenyls.
Atlanta: ATSDR, draft for public comment.
Aulerich, R.J.; Ringer, R.K.; Safronoff, J. (1986) Assessment of primary vs. secondary toxicity of Aroclor 1254 to mink.
Arch. Environ. Contam. Toxicol. 15:393–399.
Bandiera, S.; Safe, S.; Okey, A.B. (1982) Binding of polychlorinated biphenyls classified as either phenobarbitone-,
3-methylcholanthrene- or mixed-type inducers to cytosolic
Ah
receptor. Chem.-Biol. Interactions 39:259–277.
Barter, R.A.; Klaassen, C.D. (1992) UDP-glucuronosyltransferase inducers reduce thyroid hormone levels in rats by an
extrathyroidal mechanism. Toxicol. Appl. Pharmacol. 113:36–42.
Beebe, L.; Fox, S.D.; Riggs, C.W.; Park, S.S.; Gelboin, H.V.; Issaq, H.J.; Anderson, L.M. (1992) Persistent effects of a
single dose of Aroclor 1254 on cytochromes P450IA1 and IIB1 in mouse lung. Toxicol. Appl. Pharmacol.
114:16–24.
Beebe, L.E.; Kim, Y.E.; Amin, S.; Riggs, C.W.; Kovatch, R.M.; Anderson, L.M. (1993) Comparison of transplacental and
neonatal initiation of mouse lung and liver tumors by
N
-nitrosodimethylamine (NDMA) and
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and promotability by a polychlorinated biphenyls mixture
(Aroclor 1254). Carcinogenesis 14(8):1545–1548.
Bertazzi, P.A.; Riboldi, L.; Pesatori, A.; Radice, L.; Zocchetti, C. (1987) Cancer mortality of capacitor manufacturing
workers. Am. J. Ind. Med. 11:165–176.
Birnbaum, L.S.; DeVito, M.J. (in press) Use of toxic equivalency factors for risk assessment for dioxins and related
compounds. Toxicology.
Brown, D.P. (1987) Mortality of workers exposed to polychlorinated biphenyls—an update. Arch. Environ. Health
42(6):333–339.
Brown, J.F., Jr.; Wagner, R.E. (1990) PCB movement, dechlorination, and detoxication in the Acushnet Estuary.
Environ. Toxicol. Chem. 9:1215–1233.
Brown, J.F., Jr. (1994) Determination of PCB metabolic, excretion, and accumulation rates for use as indicators of
biological response and relative risk. Environ. Sci. Technol. 28(13):2295–2305.
Brunner, M.J.; Sullivan, T.M.; Singer, A.W.; Ryan, M.J.; Toft, II, J.D.; Menton, R.S.; Graves, S.W.; Peters, A.C. (1996)
An assessment of the chronic toxicity and oncogenicity of Aroclor-1016, Aroclor-1242, Aroclor-1254, and
Aroclor-1260 administered in diet to rats. Columbus, OH: Battelle Study No. SC920192., Chronic toxicity and
oncogenicity report.
Buchmann, A.; Ziegler, S.; Wolf, A.; Robertson, L.W.; Durham, S.K.; Schwarz, M. (1991) Effects of polychlorinated
biphenyls in rat liver: correlation between primary subcellular effects and promoting activity. Toxicol. Appl.
Pharmacol. 111:454–468.
Byrne, J.J.; Carbone, J.P.; Hanson, E.A. (1987) Hypothyroidism and abnormalities in the kinetics of thyroid hormone
metabolism in rats treated chronically with polychlorinated biphenyl and polybrominated biphenyl.
Endocrinology 121:520–527.
Calabrese, E.J.; Sorenson, A.J. (1977) The health effects of PCBs with particular emphasis on human high risk groups.
Rev. Environ. Health 2:285–304.
65
Callahan, M.A.; Slimak, M.W.; Gabel, N.W.; May, I.P.; Fowler, C.F.; Freed, J.R.; Jennings, P.; Durfee, R.L.;
Whitmore, F.C.; Maestri, B.; Mabey, W.R.; Holt, B.R.; Gould, C. (1979) Water-related environmental fate of
129 priority pollutants, vol. I, ch. 36. Washington: U.S. EPA, Report No. EPA–440/4–79–029a.
Cogliano, V.J. (1986) The U.S. EPA's methodology for adjusting the reportable quantities of potential carcinogens.
Proceedings of the 7th National Conference on Management of Uncontrolled Hazardous Wastes
(Superfund '86). Washington: Hazardous Materials Control Research Institute, pp. 182–185.
Crump, K.S.; Hoel, D.G.; Langley, C.H.; Peto, R. (1976) Fundamental carcinogenic processes and their implications for
low dose risk assessment. Cancer Res. 36:2973–2979.
Delaware Department of Natural Resources and Environmental Control (1994) Summary and assessment of
polychlorinated biphenyls and selected pesticides in striped bass from the Delaware Estuary. Delaware, Project
No. AFC–5; Grant No. NA26FA0148–01.
Dewailly, É.; Weber, J.-P.; Gingras, S.; Laliberté, C. (1991) Coplanar PCBs in human milk in the province of Québec,
Canada: are they more toxic than dioxin for breast fed infants? Bull. Environ. Contam. Toxicol. 47:491–498.
Dewailly, É.; Ryan, J.J.; Laliberté, C.; Bruneau, S.; Weber, J.-P.; Gingras, S.; Carrier, G. (1994) Exposure of remote
maritime populations to coplanar PCBs. Environ. Health Perspect. 102(Suppl. 1):205–209.
Erickson, M.D. (1986) Analytical chemistry of PCBs. Boston: Butterworth Publishers.
General Electric Company (1995) Letter from Stephen B. Hamilton, Jr., to U.S. Environmental Protection Agency
Section 8(e) Coordinator, October 10, 1995.
Gierthy, J.F.; Arcaro, K.F.; Floyd, M. (1995) Assessment and implications of PCB estrogenicity. Organo-
Halogen Compounds 25:419-423
Gustavsson, P.; Hogstedt, C.; Rappe, C. (1986) Short-term mortality and cancer incidence in capacitor manufacturing
workers exposed to polychlorinated biphenyls (PCBs). Am. J. Ind. Med. 10:341–344.
Harper, N.; Connor, K.; Steinberg, M.; Safe, S. (1995) Immunosuppressive activity of polychlorinated biphenyl mixtures
and congeners: nonadditive (antagonistic) interactions. Fund. Appl. Toxicol. 27:131–139.
Harris, M.; Zacharewski, T.; Safe, S. (1993) Comparative potencies of Aroclors 1232, 1242, 1248, 1254, and 1260 in
male Wistar rats—assessment of the toxic equivalency factor (TEF) approach for polychlorinated biphenyls
(PCBs). Fund. Appl. Toxicol. 20:456–463.
Hemming, H.; Flodström, S.; Wärngard, L.; Bergman, Å.; Kronevi, T.; Nordgren, I.; Ahlborg, U. (1993) Relative tumour
promoting activity of three polychlorinated biphenyls in rat liver. Eur. J. Pharmacol. 248:163–174.
Hill, R.N.; Erdreich, L.S.; Paynter, O.E.; Roberts, P.A.; Rosenthal, S.L.; Wilkinson, C.F. (1987) Thyroid follicular cell
carcinogenesis. Fund. Appl. Toxicol. 12:627–697.
Hong, C.-S.; Bush, B.; Xiao, J.; Qiao, H. (1993) Toxic potential of non-ortho and mono-ortho coplanar polychlorinated
biphenyls in Aroclors, seals, and humans. Arch. Environ. Contam. Toxicol. 25:118–123.
Hornshaw, T.C.; Aulerich, R.J.; Johnson, H.E. (1983) Feeding Great Lakes fish to mink: effects on mink and
accumulation and elimination of PCBs by mink. J. Toxicol. Environ. Health 11:933–946.
Hovinga, M.E.; Sowers, M.; Humphrey, H.E.B. (1992) Historical changes in serum PCB and DDT levels in an
environmentally-exposed cohort. Arch. Environ. Contam. Toxicol. 22:362–366.
Howe, R.B.; Crump, K.S.; Van Landingham, C. (1986) Global 86: a computer program to extrapolate quantal animal
toxicity data to low doses. Prepared for U.S. EPA under contract 68–01–6826.
Hutzinger, O.; Safe, S.; Zitko, V. (1974) The chemistry of PCB's. Boca Raton, FL: CRC Press.
66
IARC (International Agency for Research on Cancer) (1987) IARC Monographs on the Evaluation of Carcinogenic Risks
to Humans, Supplement 7, Overall Evaluations of Carcinogenicity: An Updating of IARC Monographs
Volumes 1-42. Lyon, France.
Institute for Evaluating Health Risks (1991) Reassessment of liver findings in five PCB studies in rats. Washington, DC,
dated July 1. Report submitted to U.S. EPA.
Ito, N.; Nagasaki, H.; Arai, M.; Makiura, S.; Sugihara, S.; Hirao, K. (1973) Histopathologic studies on liver tumorigenesis
induced in mice by technical polychlorinated biphenyls and its promoting effect on liver tumors induced by
benzene hexachloride. J. Natl. Cancer Inst. 51(5):1637–1646.
Ito, N.; Nagasaki, H.; Makiura, S.; Arai, M. (1974) Histopathological studies on liver tumorigenesis in rats treated with
polychlorinated biphenyls. Gann 65:545–549.
Kannan, N.; Tanabe, S.; Tatsukawa, R. (1988) Toxic potential of non-
ortho
and mono-
ortho
coplanar PCBs in
commercial PCB preparations: "2,3,7,8–T CDD toxicity equivalence factors approach." Bull. Environ. Contam.
4
Toxicol. 41:267–276.
Kasza, L.; Weinberger, M.A.; Hinton, D.E.; Trump, B.J.; Patel, C.; Friedman, L.; Garthoff, L.H. (1978) Comparative
toxicity of polychlorinated biphenyl and polybrominated biphenyl in the rat liver: Light and electron microscopic
alterations after subacute dietary exposures. J. Envir. Pathol. Toxicol. 1:241-257.
Keenan, R.E.; Stickney, J.A. (1996) Letter dated June 24 to J. Cogliano, U.S. EPA, Washington.
Kimbrough, R.D.; Linder, R.E.; Gaines, T.B. (1972) Morphological changes in livers of rats fed polychlorinated biphenyls:
light microscopy and ultrastructure. Arch. Environ. Health 25:354–364.
Kimbrough, R.D.; Linder, R.E. (1974) Induction of adenofibrosis and hepatomas of the liver in BALB/cJ mice by
polychlorinated biphenyls (Aroclor 1254). J. Natl. Cancer Inst. 53(2):547–552.
Kimbrough, R.D.; Squire, R.A.; Linder, R.E.; Strandberg, J.D.; Montali, R.J.; Burse, V.W. (1975) Induction of liver
tumors in Sherman strain female rats by polychlorinated biphenyls Aroclor 1260. J. Natl. Cancer Inst.
55:1453–1459.
Kimura, N.T.; Baba, T. (1973) Neoplastic changes in the rat liver induced by polychlorinated biphenyl. Gann
64:105–108.
Koller, L.D. (1996) Letter dated July 3 to E.V. Ohanian, U.S. EPA, Washington.
Laib, R.J.; Rose, N.; Brunn, H. (1991) Hepatocarcinogenicity of PCB congeners. Toxicol. Environ. Chem. 34:19–22.
Lake, J.L.; Pruell, R.J.; Osterman, F.A. (1992) An examination of dechlorination processes and pathways in New
Bedford Harbor sediments. Marine Environ. Res. 33:31-47.
Lake, J.L.; McKinney, R.; Lake, C.A.; Osterman, F.A.; Heltshe, J. (1995) Comparisons of patterns of polychlorinated
biphenyl congeners in water, sediment, and indigenous organisms from New Bedford Harbor, Massachusetts.
Arch. Contam. Toxicol. 29:207–220.
Luebeck, E.G.; Moolgavkar, S.H.; Buchmann, A.; Schwarz, M. (1991) Effects of polychlorinated biphenyls in rat liver:
quantitative analysis of enzyme-altered foci. Toxicol. Appl. Pharmacol. 111:469–484.
Lutz, W.K. (1990) Dose-response relationship and low dose extrapolation in chemical carcinogenesis. Carcinogenesis
11(8):1243–1247.
Matthews, H.B.; Anderson, M.W. (1975) Effect of chlorination on the distribution and excretion of polychlorinated
biphenyls. Drug Metab. Dispos. 3(5):371–380.
McClain, R.M. (1989) The significance of hepatic microsomal enzyme induction and altered thyroid function in rats:
implications for thyroid gland neoplasia. Toxicol. Pathol. 17:294–306.
67
McConnell, E.E.; Solleveld, H.A.; Swenberg, J.A.; Boorman, G.A. (1986) Guidelines for combining neoplasms for
evaluation of rodent carcinogenesis studies. J. Natl. Cancer Inst. 76(2):283–289.
McFarland, V.A.; Clarke, J.U. (1989) Environmental occurrence, abundance, and potential toxicity of polychlorinated
biphenyl congeners: considerations for a congener-specific analysis. Environ. Health Perspect. 81:225–239.
McLean, M.R.; Bauer, U.; Amaro, A.R.; Robertson, L.W. (1996a) Identification of catechol and hydroquinone
metabolites of 4-monochlorobiphenyl. Chem. Res. Toxicol. 9:158–164.
McLean, M.R.; Robertson, L.W.; Gupta, R.C. (1996b) Detection of PCB adducts by the P-postlabeling technique.
32
Chem. Res. Toxicol. 9:165–171.
Mills, R.A.; Millis, C.D.; Dannan, G.A.; Guengerich, F.P.; Aust, S.D. (1985) Studies on the structure-activity relationships
for the metabolism of polybrominated biphenyls by rat liver microsomes. Toxicol. Appl. Pharmacol. 78:96–104.
Moore, J.A.; Hardisty, J.F.; Banas, D.A.; Smith, M.A. (1994) A comparison of liver tumor diagnoses from seven PCB
studies in rats. Regul. Toxicol. Pharmacol. 20:362–370.
Morgan, R.W.; Ward, J.M.; Hartman, P.E. (1981) Aroclor 1254-induced intestinal metaplasia and adenocarcinoma in
the glandular stomach of F344 rats. Cancer Res. 41:5052–5059.
National Cancer Institute (1978) Bioassay of Aroclor 1254 for possible carcinogenicity. Carcinogenesis Tech. Rep. Ser.
No. 38.
National Institute for Occupational Safety and Health (1977) Criteria for a recommended standard.
Occupational exposure to polychlorinated biphenyls (PCBs). Rockville, Md: U.S. Department of Health,
Education and Welfare, Public Health Sevice, Centers for Disease Control. NIOSH Publ 77-225.
National Research Council (1993) Issues in risk assessment. Washington: National Academy Press.
National Toxicology Program (1982) Carcinogenesis bioassay of 2,3,7,8-tetrachlorodibenzo-p-dioxin (CAS
no. 1746–01–6) in Osborne-Mendel rats and B6C3F mice (gavage study). Research Triangle Park NC: NTP
1
Tech. Rep. Ser. No. 209.
National Toxicology Program (1983) Carcinogenesis studies of polybrominated biphenyl mixture (Firemaster FF–1)
(CAS no. 67774–32–7) in F344/N rats and B6C3F mice (gavage studies). Research Triangle Park NC: NTP
1
Tech. Rep. Ser. No. 244.
National Toxicology Program (1993) Toxicology and carcinogenesis studies of polybrominated biphenyls
(Firemaster FF–1) (CAS no. 67774–32–7) in F344/N rats and B6C3F mice (feed studies). Research Triangle
1
Park NC: NTP Tech. Rep. Ser. No. 398.
Norback, D.H.; Weltman, R.H. (1985) Polychlorinated biphenyl induction of hepatocellular carcinoma in the Sprague-
Dawley rat. Environ. Health Perspect. 60:97–105.
Oakley, G.G.; Robertson, L.W.; Gupta, R.C. (1996) Analysis of polychlorinated biphenyl-DNA adducts by
P-postlabeling. Carcinogenesis 17(1):109–114.
32
Oliver, B.G.; Niimi, A.J. (1988) Trophodynamic analysis of polychlorinated biphenyl congeners and other chlorinated
hydrocarbons in the Lake Ontario ecosystem. Environ. Sci. Technol. 22:388–397.
Patterson, D.G.; Todd, G.D.; Turner, W.E.; Maggio, V.; Alexander, L.R.; Needham, L.L. (1994) Levels of non-ortho-
substituted (coplanar), mono-, and di-ortho-substituted polychlorinated biphenyls, dibenzo-p-dioxins, and
dibenzofurans in human serum and adipose tissue. Environ. Health Perspect. 102(Suppl. 1):195–204.
Phillips, D.L.; Smith, A.B.; Burse, V.W.; Steele, G.K.; Needham, L.L.; Hannon, W.H. (1989) Half-life of polychlorinated
biphenyls in occupationally exposed workers. Arch. Environ. Health 44(6):351–354.
Rao, C.V.; Banerji, A.S. (1988) Induction of liver tumors in male Wistar rats by feeding polychlorinated biphenyls
(Aroclor 1260). Cancer Lett. 39:59–67.
68
Rickenbacher, U.; McKinney, J.D.; Oatley, S.J.; Blake, C.C.F. (1986) Structurally specific binding of halogenated
biphenyls to thyroxine transport protein. J. Med. Chem. 29:641–648.
Rose, N.; Laib, R.J.; Brunn, H.; Bolt, M.M. (1985) Biotransformation and toxicity of polychlorinated biphenyls (PCBs):
investigation of initiating and promoting activities of 2,2',4,5'-tetra- and 2,2',4,4',5,5'-hexachlorobiphenyl.
Naunyn-Schmiedeberg's Arch. Pharmacol. Suppl. 330 (Abstr. 87), R21.
Safe, S. (1990) Polychlorinated biphenyls (PCBs), dibenzo-p-dioxins (PCDDs), dibenzofurans (PCDFs), and related
compounds: environmental and mechanistic consideration which support the development of toxic equivalency
factors (TEFs). Crit. Rev. Toxicol. 21(1):51–88.
Safe, S. (1994) Polychlorinated biphenyls (PCBs): environmental impact, biochemical and toxic responses, and
implications for risk assessment. Crit. Rev. Toxicol. 24(2):87–149.
Sargent, L.; Dragan, Y.P.; Erickson, C.; Laufer, C.J.; Pitot, H.C. (1991) Study of the separate and combined effects of
the non-planar 2,5,2',5'- and 3,4,3',4'-tetrachlorobiphenyl in liver and lymphocytes
in vivo
. Carcinogenesis
12(5):793–800.
Schaeffer, E.; Greim, H.; Goessner, W. (1984) Pathology of chronic polychlorinated biphenyl (PCB) feeding in rats.
Toxicol. Appl. Pharmacol. 75:278–288.
Schecter, A.; Stanley, J.; Boggess, K.; Masuda, Y.; Mes, J.; Wolff, M.; Fürst, P.; Fürst, C.; Wilson-Yang, K.; Chisholm, B.
(1994) Polychlorinated biphenyl levels in the tissues of exposed and nonexposed humans. Environ. Health
Perspect. 102(Suppl. 1):149–158.
Schulz, D.E.; Petrick, G.; Duinker, J.C. (1989) Complete characterization of polychlorinated biphenyl congeners in
commercial Aroclor and Clophen mixtures by multidimensional gas chromatography—electron capture
detection. Environ. Sci. Technol. 23(7):852–859.
Schwartz, T.R.; Stalling, D.L.; Rice, C.L. (1987) Are polychlorinated biphenyl residues adequately described by Aroclor
mixture equivalents? Isomer-specific principal components analysis of such residues in fish and turtles. Environ.
Sci. Technol. 21:72–76.
Shalat, S.L.; True, L.D.; Fleming, L.E.; Pace, P.E. (1989) Kidney cancer in utility workers exposed to polychlorinated
biphenyls (PCBs). Br. J. Ind. Med. 46(11):823–824.
Silberhorn, E.M.; Glauert, H.P.; Robertson, L.W. (1990) Carcinogenicity of polyhalogenated biphenyls: PCBs and PBBs.
Crit. Rev. Toxicol. 20(6):439–496.
Sinks, T.; Steele, G.; Smith, A.B.; Watkins, K.; Shults, R.A. (1992) Mortality among workers exposed to polychlorinated
biphenyls. Am. J. Epidemiol. 136(4):389–398.
Sipes and Schnellman (1987) Biotransformation of PCBs: metabolic pathways and mechanisms. In: Safe and Hutzinger,
eds., Polychlorinated biphenyls (PCBs): mammalian and environmental toxicology. Heidelberg: Springer Verlag,
pp. 97–110.
Smith, A.H. (1987) Infant exposure assessment for breast milk dioxins and furans derived from waste incineration
emissions. Risk Analysis 7(3):347–353.
Steele, G.; Stehr-Green, P.; Welty, E. (1986) Estimates of the biologic half-life of polychlorinated biphenyls in human
serum. New Engl. J. Med. 314(14):926–927.
Swierenga, S.H.H.; Yamasaki, H.; Piccoli, C.; Robertson, L.; Bourgon, L.; Marceau, N.,; Fitzgerald, D.J. (1990) Effects
on intercellular communication in human keratinocytes and liver-derived cells of polychlorinated biphenyl
congeners with differing
in vivo
promotion activities. Carcinogenesis 11(6):921–926.
Taylor, P. (1988) The health effects of polychlorinated biphenyls. Boston: Harvard School of Public Health, unpublished
thesis.
U.S. Environmental Protection Agency (1980) Water quality criteria documents; availability. Federal Register
45(231):79318–79379.
U.S. Environmental Protection Agency (1986a) Guidelines for carcinogen risk assessment. Federal Register
51(185):33992–34003.
69
U.S. Environmental Protection Agency (1986b) Guidelines for the health risk assessment of chemical mixtures. Federal
Register 51(185):34014–34025.
U.S. Environmental Protection Agency (1988a) Drinking water criteria document for polychlorinated biphenyls (PCBs).
Cincinnati: U.S. EPA, ECAO–CIN–414.
U.S. Environmental Protection Agency (1988b) Methodology for evaluating potential carcinogenicity in support of
reportable quantity adjustments pursuant to CERCLA section 102. Washington, DC. Report No.
EPA/600/8–89/053.
U.S. Environmental Protection Agency (1991) Workshop report on toxicity equivalency factors for polychlorinated
biphenyl congeners. Risk Assessment Forum, Washington, DC. Report No. EPA/625/3–91/020.
U.S. Environmental Protection Agency (1992a) Guidelines for exposure assessment. Federal Register
57(104):22888–22938.
U.S. Environmental Protection Agency (1992b) Draft report: a cross-species scaling factor for carcinogen risk
assessment based on equivalence of mg/kg /day; notice. Federal Register 57(109):24152–24173.
3/4
U.S. Environmental Protection Agency (1994a) Technical background document to support rulemaking pursuant to the
Clean Air Act—section 112(g): ranking of pollutants with respect to hazard to human health. Research
Triangle Park, NC. Report No. EPA–450/3–92–010.
U.S. Environmental Protection Agency (1994b) Health assessment document for 2,3,7,8-tetrachlorodibenzo-p-dioxin
(TCDD) and related compounds. Prepared by the Office of Health and Environmental Assessment, Office of
Research and Development, Washington, DC. External Review Draft, 3 vol. Report No.
EPA/600/BP–92/001c, Available from the National Technical Information Service, Springfield, VA; PB 94-
205457.
U.S. Environmental Protection Agency (1996a) Proposed guidelines for carcinogen risk assessment; notice. Federal
Register 61(79):17960–18011.
U.S. Environmental Protection Agency (1996b) Report on peer review workshop on
PCBs: cancer-dose response
assessment and application to environmental mixtures
. Washington, DC, National Center for Environmental
Assessment.
Vater, S.T.; Velazquez, S.F.; Cogliano, V.J. (1995) A case study of cancer data set combinations for PCBs. Regul.
Toxicol. Pharmacol. 22:2–10.
Ward, J.M. (1985) Proliferative lesions of the glandular stomach and liver in F344 rats fed diets containing Aroclor 1254.
Environ. Health Perspect. 60:89–95.
Wester, R.C.; Bucks, D.A.W.; Maibach, H.I.; Anderson, J. (1983) Polychlorinated biphenyls (PCBs): dermal absorption,
systemic elimination, and dermal wash efficiency. J. Toxicol. Environ. Health 12:511–519.
Wester, R.C.; Mobayen, M.; Maibach, H.I. (1987) In vivo and
in vitro
absorption and binding to powdered stratum
corneum as methods to evaluate skin absorption of environmental chemical contaminants from ground and
surface water. J. Toxicol. Environ. Health 21:367–374.
Wester, R.C.; Maibach, H.I.; Bucks, D.A.W.; McMaster, J.; Mobayen, M. (1990) Percutaneous absorption and skin
decontamination of PCBs:
in vitro
studies with human skin and
in vivo
studies in the rhesus monkey. J. Toxicol.
Environ. Health 31:235–246.
Wester, R.C.; Maibach, H.I.; Sedik, L.; Melendres, J.; Wade, M. (1993) Percutaneous absorption of PCBs from soil:
in vivo
rhesus monkey,
in vitro
human skin, and binding to powdered human stratum corneum. J. Toxicol.
Environ. Health 39:375–382.
WHO (World Health Organization) (1993) Polychlorinated biphenyls and terphenyls. Geneva: WHO, Environmental
Health Criteria 140, second ed.
Wolff, M.S. (1985) Occupational exposure to polychlorinated biphenyls (PCBs). Environ. Health Perspect. 60:133–138.

70
APPENDIX: EMPIRICAL MODELING RESULTS
Table A–1. Empirical modeling of liver tumors in female Sprague-Dawley rats fed Aroclor 1260
Tumors Liver hepatocellular adenomas and carcinomas
Animal Female Sprague-Dawley rats
Reference Brunner et al. (1996), doses from Keenan and Stickney (1996)
Exposure duration 24 mo
Study duration 24 mo (assumed animal lifespan)
Animal weight 0.403 0.413 0.399 0.391 kg
Administered dose 0 25 50 100 ppm diet
Equivalent human dose 0 0.35 0.72 1.52 mg/kg-d
Tumor incidence 1/85 10/49 11/45 24/50
Model Risk(
d
) = 1 – exp(–0.013–0.44
d
) in experimental range
Potency, slope estimates ED10=0.24, LED10=0.19, ED01=0.023, LED01=0.018,
q
*=0.56
1
Table A–2. Empirical modeling of liver tumors in female Sprague-Dawley rats fed Aroclor 1254
Tumors Liver hepatocellular adenomas and carcinomas
Animal Female Sprague-Dawley rats
Reference Brunner et al. (1996), doses from Keenan and Stickney (1996)
Exposure duration 24 mo
Study duration 24 mo (assumed animal lifespan)
Animal weight 0.403 0.375 0.355 0.321 kg
Administered dose 0 25 50 100 ppm diet
Equivalent human dose 0 0.36 0.76 1.59 mg/kg-d
Tumor incidence 1/85 19/45 28/49 28/49
Model Risk(
d
) = 1 – exp(–0.012–1.2
d
) in experimental range
Potency, slope estimates ED10=0.086, LED10=0.067, ED01=0.0082, LED01=0.0064,
q
*=1.6
1
Table A–3. Empirical modeling of liver tumors in female Sprague-Dawley rats fed Aroclor 1242
Tumors Liver hepatocellular adenomas and carcinomas
Animal Female Sprague-Dawley rats
Reference Brunner et al. (1996), doses from Keenan and Stickney (1996)
Exposure duration 24 mo
Study duration 24 mo (assumed animal lifespan)
Animal weight 0.403 0.398 0.372 kg
Administered dose 0 50 100 ppm diet
Equivalent human dose 0 0.75 1.53 mg/kg-d
Tumor incidence 1/85 11/49 15/45
Model Risk(
d
) = 1 – exp(–0.012–0.28
d
) in experimental range
Potency, slope estimates ED10=0.38, LED10=0.27, ED01=0.036, LED01=0.026,
q
*=0.39
1

71
Table A–4. Empirical modeling of liver tumors in female Sprague-Dawley rats fed Aroclor 1016
Tumors Liver hepatocellular adenomas and carcinomas
Animal Female Sprague-Dawley rats
Reference Brunner et al. (1996), doses from Keenan and Stickney (1996)
Exposure duration 24 mo
Study duration 24 mo (assumed animal lifespan)
Animal weight 0.403 0.414 0.421 0.394 kg
Administered dose 0 50 100 200 ppm diet
Equivalent human dose 0 0.72 1.43 2.99 mg/kg-d
Tumor incidence 1/85 1/48 7/45 6/50
Model Risk(
d
) = 1 – exp(–0.012–0.044
d
) in experimental range
Potency, slope estimates ED10=2.4, LED10=1.4, ED01=0.23, LED01=0.14
q
*=0.073
1
Table A–5. Empirical modeling of liver tumors in male Sprague-Dawley rats fed Aroclor 1260
Tumors Liver hepatocellular adenomas and carcinomas
Animal Male Sprague-Dawley rats
Reference Brunner et al. (1996), doses from Keenan and Stickney (1996)
Exposure duration 24 mo
Study duration 24 mo (assumed animal lifespan)
Animal weight 0.697 0.690 0.703 0.695 kg
Administered dose 0 25 50 100 ppm diet
Equivalent human dose 0 0.31 0.62 1.25 mg/kg-d
Tumor incidence 7/98 3/50 6/49 10/49
Model Risk(
d
) = 1 – exp(–0.071–0.11
d
) in experimental range
2
Potency, slope estimates ED10=1.0, LED10=0.55, ED01=0.31, LED01=0.053
q
*=0.19
1
Table A–6. Empirical modeling of liver tumors in male Sprague-Dawley rats fed Aroclor 1254
Tumors Liver hepatocellular adenomas and carcinomas
Animal Male Sprague-Dawley rats
Reference Brunner et al. (1996), doses from Keenan and Stickney (1996)
Exposure duration 24 mo
Study duration 24 mo (assumed animal lifespan)
Animal weight 0.697 0.694 0.659 0.601 kg
Administered dose 0 25 50 100 ppm diet
Equivalent human dose 0 0.31 0.62 1.29 mg/kg-d
Tumor incidence 7/98 4/48 4/49 6/47
Model Risk(
d
) = 1 – exp(–0.075–0.0052
d
–0.032
d
) in experimental range
2
Potency, slope estimates ED10=1.7, LED10=0.87, ED01=0.49, LED01=0.083
q
*=0.12
1

72
Table A–7. Empirical modeling of liver tumors in male Sprague-Dawley rats fed Aroclor 1242
Tumors Liver hepatocellular adenomas and carcinomas
Animal Male Sprague-Dawley rats
Reference Brunner et al. (1996), doses from Keenan and Stickney (1996)
Exposure duration 24 mo
Study duration 24 mo (assumed animal lifespan)
Animal weight 0.697 0.711 0.681 kg
Administered dose 0 50 100 ppm diet
Equivalent human dose 0 0.60 1.25 mg/kg-d
Tumor incidence 7/98 1/50 5/46
Model Risk(
d
) = 1 – exp(–0.058–0.012
d
) in experimental range
2
Potency, slope estimates ED10=2.9, LED10=1.2, ED01=0.91, LED01=0.16
q
*=0.061
1
Table A–8. Empirical modeling of liver tumors in male Sprague-Dawley rats fed Aroclor 1016
Tumors Liver hepatocellular adenomas and carcinomas
Animal Male Sprague-Dawley rats
Reference Brunner et al. (1996), doses from Keenan and Stickney (1996)
Exposure duration 24 mo
Study duration 24 mo (assumed animal lifespan)
Animal weight 0.697 0.723 0.699 0.712 kg
Administered dose 0 50 100 200 ppm diet
Equivalent human dose 0 0.61 1.23 2.44 mg/kg-d
Tumor incidence 7/98 2/48 2/50 4/49
Model Risk(
d
) = 1 – exp(–0.058–0.0031
d
) in experimental range
2
Potency, slope estimates ED10=5.9, LED10=2.5, ED01=1.8, LED01=0.32
q
*=0.032
1
Table A–9. Empirical modeling of liver tumors in female Sherman rats fed Aroclor 1260
Tumors Liver hepatocellular adenomas and carcinomas
Animal Female Sherman rats
Reference Kimbrough et al. (1975), reevaluated by Moore et al. (1994)
Exposure duration 21 mo
Study duration 23 mo (assumed animal lifespan)
Animal weight 0.35 kg (assumed)
Administered dose 0 100 ppm diet
Equivalent human dose 0 1.3 mg/kg-d
Tumor incidence 1/187 138/189
Model Risk(
d
) = 1 – exp(–0.0054–1.0
d
) in experimental range
Potency, slope estimates ED10=0.10, LED10=0.091, ED01=0.010, LED01=0.0086
q
*=1.2
1

73
Table A–10. Empirical modeling of liver tumors in male Fischer 344 rats fed Aroclor 1254
Tumors Liver hepatocellular adenomas and carcinomas
Animal Male Fischer 344 rats
Reference NCI (1978), reevaluated by Moore et al. (1994)
Exposure duration 104–105 wk
Study duration 104–105 wk (assumed animal lifespan)
Animal weight 0.3 kg
Administered dose 0 25 50 100 ppm diet
Equivalent human dose 0 0.32 0.64 1.28 mg/kg-d
Tumor incidence 0/24 1/24 1/24 3/23
Model Risk(
d
) = 1 – exp(–0.091
d
–0.0099
d
) in experimental range
2
Potency, slope estimates ED10=1.0, LED10=0.55, ED01=0.11, LED01=0.052,
q
*=0.19
1
Table A–11. Empirical modeling of liver tumors in female Fischer 344 rats fed Aroclor 1254
Tumors Liver hepatocellular adenomas and carcinomas
Animal Female Fischer 344 rats
Reference NCI (1978), reevaluated by Moore et al. (1994)
Exposure duration 104–105 wk
Study duration 104–105 wk (assumed animal lifespan)
Animal weight 0.2 kg
Administered dose 0 25 50 100 ppm diet
Equivalent human dose 0 0.29 0.58 1.16 mg/kg-d
Tumor incidence 0/23 1/24 2/24 1/24
Model Risk(
d
) = 1 – exp(–0.084
d
) in experimental range
Potency, slope estimates ED10=1.2, LED10=0.61, ED01=0.12, LED01=0.058,
q
*=0.17
1
Table A–12. Empirical modeling of liver tumors in male Wistar rats fed Clophen A 30
Tumors Liver hepatocellular adenomas and carcinomas
Animal Male Wistar rats
Reference Schaeffer et al. (1984), reevaluated by Moore et al. (1994)
Exposure duration 24 mo
Study duration 24 mo (assumed animal lifespan)
Animal weight 0.35 kg (assumed)
Administered dose 0 100 ppm diet
Equivalent human dose 0 1.3 mg/kg-d
Tumor incidence 8/120 16/128
Model Risk(
d
) = 1 – exp(–0.069–0.050
d
) in experimental range
Potency, slope estimates ED10=2.1, LED10=1.0, ED01=0.20, LED01=0.096,
q
*=0.10
1

74
Table A–13. Empirical modeling of liver tumors in male Wistar rats fed Clophen A 60
Tumors Liver hepatocellular adenomas and carcinomas
Animal Male Wistar rats
Reference Schaeffer et al. (1984), reevaluated by Moore et al. (1994)
Exposure duration 24 mo
Study duration 24 mo (assumed animal lifespan)
Animal weight 0.35 kg (assumed)
Administered dose 0 100 ppm diet
Equivalent human dose 0 1.3 mg/kg-d
Tumor incidence 8/120 114/125
Model Risk(
d
) = 1 – exp(–0.069–1.8
d
) in experimental range
Potency, slope estimates ED10=0.058, LED10=0.047, ED01=0.0055, LED01=0.0045,
q
*=2.2
1
Table A–14. Empirical modeling of liver tumors in male Sprague-Dawley rats fed Aroclor 1260
Tumors Liver hepatocellular adenomas and carcinomas
Animal Male Sprague-Dawley rats
Reference Norback and Weltman (1985), reevaluated by Moore et al. (1994)
Exposure duration 24 mo
Study duration 29 mo (assumed animal lifespan)
Animal weight 0.35 kg (assumed)
Administered dose 0 100 ppm diet
Equivalent human dose 0 1.3 mg/kg-d
Tumor incidence 0/31 5/40
Model Risk(
d
) = 1 – exp(–0.10
d
) in experimental range
Potency, slope estimates ED10=1.0, LED10=0.53, ED01=0.098, LED01=0.051,
q
*=0.20
1
Table A–15. Empirical modeling of liver tumors in female Sprague-Dawley rats fed Aroclor 1260
Tumors Liver hepatocellular adenomas and carcinomas
Animal Female Sprague-Dawley rats
Reference Norback and Weltman (1985), reevaluated by Moore et al. (1994)
Exposure duration 24 mo
Study duration 29 mo (assumed animal lifespan)
Animal weight 0.35 kg (assumed)
Administered dose 0 100 ppm diet
Equivalent human dose 0 1.3 mg/kg-d
Tumor incidence 1/45 41/46
Model Risk(
d
) = 1 – exp(–0.022–1.7
d
) in experimental range
Potency, slope estimates ED10=0.062, LED10=0.046, ED01=0.0059, LED01=0.0044,
q
*=2.3
1
