
Species,
Interindividual,
and
Tissue
Specificity
in
Endocrine
Signaling
Cheryl
Walker,1
S.
Ansar
Ahmed,2
Terry
Brown,3
Shuk-Mei
Ho,4
Leslie
Hodges,1
George
Lucier,5
Jose
Russo,6
Nancy
Weigel,7
Tom
Weise,8
and
John
Vandenbergh9
'University
of
Texas,
MD
Anderson
Cancer
Center,
Smithville,
Texas
USA;
2Virginia
Polytechnic
Institute
and
State
University,
Blacksburg,
Virginia
USA;
3Johns
Hopkins
University
School
of
Medicine,
Baltimore,
Maryland
USA;
4Tufts
University,
Medford,
Massachusetts
USA;
5National
Institute
of
Environmental
Health
Sciences,
Research
Triangle
Park,
North
Carolina
USA;
6Fox
Chase
Cancer
Center,
Philadelphia,
Pennsylvania
USA;
7Baylor
College
of
Medicine,
Houston,
Texas
USA;
8U.S.
Environmental
Protection
Agency,
National
Health
and
Environmental
Effects
Research
Laboratory,
Research
Triangle
Park,
North
Carolina
USA;
9North
Carolina
State
University,
Raleigh,
North
Carolina
USA
The
activity
of
endocrine-active
agents
exhibits
specificity
at
many
levels.
Differential
responsiveness
to
these
agents
has
been
observed
between
different
species
and
extends
to
interindividual
differences
within
a
species
and
between
different
tissues
as
well.
In
cases
where
they
have
been
identified,
the
biologic
and
molecular
mechanisms
underlying
this
specificity
are
quite
diverse.
Determinants
of
species
specificity
include
differences
that
exist
in
receptor
binding,
gene
transcription,
and
cellular
responses
to
endocrine-active
compounds
between
species.
Interindividual
differences
in
responsiveness
may
be
determined
at
the
level
of
genetic
polymorphisms
in
hormone-
metabolizing
enzymes,
hormone
receptors,
and
in
those
genes
that
are
transactivated
by
these
receptors,
as
well
as
during
changing
windows
of
susceptibility
that
occur
as
a
function
of
age,
such
as
prenatal
and
postmenopausal
exposures.
Extrinsic
factors
such
as
diet
can
also
impact
individual
susceptibility
to
endocrine-active
agents.
Tissue-specific
determinants
of
susceptibility
are
well
documented,
but
little
is
known
regarding
the
mechanisms
underlying
these
different
responses.
Differences
in
the
expression
of
accessory
proteins
for
steroid
hormone
receptors
and
different
patterns
of
receptor
expression,
estrogen
receptor
a
and
estrogen
receptor
,B
for
example,
may
contribute
to
tissue
specificity,
as
may
differences
in
the
pattern
of
expression
of
other
genes
such
as
hormone-metabolizing
enzymes.
The
use
of
animal
model
systems
and
development
of
appropriate
mathematical
models
has
the
potential
to
yield
additional
valuable
information
for
elucidating
the
role
of
these
determinants
of
specificity
at
low-dose
exposures
and
for
improved
risk
assessments
for
the
adverse
health
effects
of
endocrine-active
compounds.
Key
words:
animal
models,
endocrine
disruptor,
metabolizing
enzymes,
p450,
polymorphisms,
reproductive
tract,
steroid
hormone
receptors,
susceptibility.
-
Environ
Health
Perspect
1
07(suppl
4):619-624
(1999).
http.//ehpnet1.
niehs.
nih.gov/docs/1999/suppl-4/619624walker/abstract.html
This
article
is
the
result
of
a
workshop
concerned
with
characterizing
the
effects
of
endocrine
disruptors
on
human
health
at
environmental
exposures.
This
workshop
pro-
vided
a
forum
for
the
discussion
of
methods
and
data
needed
to
improve
risk
assessments
of
endocrine
disruptors.
This
working
group
report
addresses
issues
related
to
the
physio-
logic
and
biochemical
basis
for
species,
interindividual,
and
tissue-specific
differences
in
response
to
an
endocrine-disrupting
chemi-
cal
at
environmentally
relevant
doses.
In
these
discussions,
group
members
addressed
what
factors
have
been
identified
that
may
underlie
differential
responsiveness
at
each
of
these
levels
and
where
questions
remain
to
be
answered
regarding
the
basis
for
differences
in
response
that
should
serve
to
direct
future
research
initiatives.
Included
in
this
report
are
issues
related
to
genetic
versus
epigenetic
phe-
nomena,
the
adequacy
of
in
vitro
and
in
vivo
models
for
predicting
variability,
and
how
this
body
of
information
could
be
used
to
improve
risk
assessments
for
sensitive
subpopulations.
Species-Specific
Factors
That
Can
Impact
Endocrine
Signaling
Three
levels
of
hormone
activity
at
which
species-specific
factors
may
have
an
impact
were
discussed:
receptor
binding,
gene
transcription,
and
cellular
response.
Recptor
Binding
Several
factors
were
identified
that
can
affect
receptor
binding
to
endogenous
and
poten-
tially
exogenous
hormonally
active
compounds
(1,2).
Such
factors
include
serum-binding
pro-
teins
(SBPs)
that
sequester
and/or
transport
hormones
to
target
cells.
SBPs
are
differentially
expressed
in
different
species.
Although
in
humans,
steroid
hormones
are
found
primarily
associated
with
SBPs
in
the
blood,
the
rat
does
not
express
this
protein.
Both
rats
and
humans
express
a-fetoprotein
during
fetal
develop-
ment,
but
this
expression
does
not
persist
in
the
adult
rat.
SBPs
increase/activate
cyclic
adenosine
monophosphate
(cAMP)
when
bound
to
steroid
hormones
in
hormonally
sen-
sitive
cells
such
as
the
prostate
(3,4).
Because
endocrine
disruptors
exhibit
differences
in
their
ability
to
bind
these
same
proteins,
it
would
be
important
to
assess
whether
they
may
similarly
initiate
this
activation
cascade
and
at
what
doses.
Differences
also
exist
in
the
ligand-
binding
domain
of
steroid
hormone
receptors
from
different
species.
Whereas
rodent
and
human
estrogen
receptors
(ER)
are
essentially
the
same,
fish
and
quail
receptors
exhibit
significant
variation
in
their
ligand-binding
domains
compared
to
humans.
In
fact,
in
some
species,
receptors
are
adapted
to
recog-
nize
different
hormones
(for
example,
trout
androgens
and
their
cognate
receptor).
However,
receptors
from
all
species
appear
to
recognize
the
same
consensus
sequence
in
the
DNA.
Ligand-independent
receptor
activation
also
exhibits
species
specificity
(5,6).
Ligand-
independent
progesterone
receptor
(PR)
acti-
vation
does
not
occur
in
humans
but
has
been
observed
for
rodents
and
chickens.
The
androgen
receptor
(AR)
appears
to
exhibit
ligand-independent
activation
in
humans
but
not
in
rats
(7).
However,
whether
this
differ-
ence
is
real
or
due
to
interlaboratory
experi-
mental
variation
is
unclear.
Similar
species
differences
could
also
exist
at
the
level
of
recep-
tor
crosstalk,
cAMP
activation,
and
AP1
signaling
(nontraditional
promoter
events),
and
as
these
differences
could
impact
on
the
activity
of
endocrine
disruptors
in
different
species,
this
area
warrants
further
exploration.
In
particular,
ligand-independent
activation
is
facilitated
by
low
levels
of
hormones
("prim-
ing
the
pump"),
suggesting
that
these
non-
traditional
means
of
receptor
activation
may
be
particularly
relevant
for
low-dose
exposures.
Gene
Transription
In
terms
of
specific
gene
transcription,
the
work
group
identified
a
need
to
assess
the
available
literature
on
gene
transcription
in
different
species
in
response
to
steroid
hor-
mones.
This
discussion
led
further
to a
con-
sensus
that
a
hormone-responsive
gene
chip
would
be
very
useful
for
making
this
assess-
ment.
Such
a
chip,
containing
a
battery
of
hormone-responsive
genes,
could
be
used
to
quantitate
changes
in
the
expression
of
these
genes
in
different
species
in
response
to
This
report
was
developed
at
the
Workshop
on
Characterizing
the
Effects
of
Endocrine
Disruptors
on
Human
Health
at
Environmental
Exposure
Levels
held
1
1-13
May
1998
in
Raleigh,
North
Carolina.
Address
correspondence
to
C.
Walker,
University
of
Texas,
MD
Anderson
Cancer
Center,
Science
Park
Research
Division,
Park
Road
1
C,
Smithville,
TX
78957.
Telephone:
(512)
237-2403.
Fax:
(512)
237-2475.
E-mail:
Received
25
September
1998;
accepted
27
May
1999.
Environmental
Health
Perspectives
*
Vol
107,
Supplement
4
*
August
1999
619

WALKER
ET
AL.
endogenous
and
exogenous
hormones
in
human,
rat,
mouse,
and
fish
(fathead
min-
now).
Patterns
of
gene
expression
could
then
be
compared
across
multiple
species
to
iden-
tify
similarities
and
differences
in
hormonally
regulated
gene
expression
that
could
be
later
correlated
with
species-specific
responses.
Celular
Response
Differences
in
hormone
responses
have
been
observed
between
different
species
in
several
hormone-responsive
tissues
that
may
be
rele-
vant
to
low-dose
effects.
In
the
rat,
the
devel-
opment
of
mammary
gland
tumors
is
enhanced
by
pituitary
prolactin
production.
For
example,
estradiol-induced
tumors
in
AxC
and
Noble
rats
can
be
inhibited
by
hypo.
In
contrast,
secretion
of
pituitary
pro-
lactin
is
not
required
for
tumorigenesis
in
humans
but
may
in
fact
be
compensated
for
by
the
endogenous
production
of
prolactin
by
the
tumors
themselves.
Thus,
as
a
target
for
endocrine-disrupting
chemicals,
altered
pitu-
itary
function
may
have
quite
a
different
impact
in
rats
than
in
humans.
Other
species
differences
exist
in
the
timing
of
windows
of
susceptibility
to
the
effects
of
endocrine-disrupting
chemicals.
For
example,
in
the
mouse
a
critical
window
for
estrogen
exposure
in
terms
of
changes
in
prostate
weight
occurs
prenatally,
whereas
in
the
rat,
postnatal
exposures
have
the
most
dramatic
effects
on
the
prostate.
Treatment
of
rats
on
postnatal
day
3
with
either
diethyl-
stilbestrol
(DES)
or
estradiol
produces
a
decrease
in
prostate
size
and
increased
dys-
plasia
and
carcinoma
development
in
the
mature
prostate
gland.
The
existence
of
the
species-specific
differences
described
above
underscores
the
fact
that
mechanistic
information
will
be
nec-
essary
to
make
informed
choices
regarding
the
appropriateness
of
a
given
animal
model
for
modeling
and
testing
of
adverse
human
health
effects
as
a
result
of
exposure
to
endocrine
disruptors.
Intrinsic/Genetic
Factors
Responsible
for
Interindividual
Difflerences
Individuals
may
exhibit
differences
in
susceptibility
to
endocrine
disruptors
during
different
stages
of
their
life
cycle
relative
to
adult
exposures,
and
this
information
should
be
factored
into
human
risk
assessments.
Different
susceptibilities
may
exist
for
pre-
natal,
postnatal,
peripubertal,
adult,
and
aged
subpopulations
(8,9).
Prenatally,
uterine
position
effects
that
have
been
documented
for
rodents
suggest
that
very
low
levels
of
androgens,
and
by
inference
endocrine
disrup-
tors,
may
have
effects
on
the
organization
of
neural
and
other
tissues
and
may
have
perma-
nent
masculinizing
consequences.
Variability
in
anatomical,
physiologic
and
behavioral
characteristics
of
mouse,
rat,
and
gerbil
as
a
consequence
of
fetal
androgen
exposure
has
also
been
observed.
A
window
of
susceptibil-
ity
has
been
documented
for
postnatal
expo-
sures
to
polychlorinated
biphenyls
(PCBs)
and
mercury
in
terms
of
behavioral/neuro-
logic
effects
in
humans.
Experience
with
DES
exposures
in
both
humans
and
rodents
indi-
cates
that
similar
windows
of
susceptibility
exist
for
the
induction
of
reproductive
tract
abnormalities
and
cancer
during
pre-
and
postnatal
periods
of
development.
The
pro-
gressive
decrease
in
age
of
menarche
in
women
that
has
occurred
over
previous
decades
may
result
in
an
increased
time
until
first
pregnancy
if
maternal
age
at
conception
remains
the
same.
This
population
shift
toward
early
menarche-late
pregnancy
could
result
in
an
increase
in
breast
cancer
risk
within
the
population.
This
same
change
however,
could
also
prove
protective
for
other
endocrine-related
processes
such
as
osteo-
porosis.
In
aged
populations,
decreased
repair
enzyme
function
due
to
oxidative
inactivation
decreased
detoxification
capability,
and
changes
in
endogenous
hormone
levels
or
metabolites
may
place
this
subpopulation
at
increased
risk
for
the
adverse
health
effects
of
endocrine
disruptors.
Along
these
same
lines,
issues
were
raised
in
the
work
group
related
to
cyclical
versus
persistent
exposures.
All
steroids
are
released
in
a
rhythmic
fashion
and
some
receptor
sys-
tems
show
rhythmic
changes.
It
is
thus
possi-
ble
that
low-dose
effects
could
occur
if
they
are
persistent
in
a
system
in
which
endoge-
nous
hormone
levels
exhibit
peaks
and
valleys.
Although
the
amount
of
endocrine
disruptor
present
might
be
low
relative
to
peak
hor-
mone
levels,
it
could
have
a
biologic
impact
if
exposure
occurs
during
a
time
in
which
endogenous
hormones
themselves
are
at
very
low
levels
or
if
the
exposure
to
an
endocrine
disruptor
occurs
at
a
susceptible
period
during
cyclical
changes
in
hormone
levels.
In
a
simi-
lar
vein,
an
interesting
question
was
raised
regarding
circadian
rhythms
and
whether
there
were
any
data
to
suggest
that
hormones
could
have
different
effects
as
a
function
of
these
rhythms.
It
was
noted
that
there
is
an
evolutionary
link
between
transcription
fac-
tors
associated
with
dioxin
activity
(an
endocrine-disrupting
compound)
and
factors
involved
in
regulating
circadian
rhythms.
The
question
that
arises
as
a
natural
consideration
of
these
data
is
how
much
more
susceptible
might
individuals
be
during
these
different
life
stages?
Additional
analysis
of
available
data
may
give
some
indication
of
the
magnitude
of
this
increased
susceptibility,
much
as
earlier
analyses
for
dioxin
(in
which
good
quantitation
for
species
and
age
effects
was
available)
helped
quantify
dose-response
effects
for
this
compound.
However,
this
is
clearly
one
area
in
which
additional
research
will
be
necessary
to
understand
which
adverse
health
effects
resulting
from
exposure
to
endocrine
disruptors
occur
as
quantitative
alterations
in
different
susceptible
subpopu-
lations
and
which
effects
are
qualitative
in
nature
and
specific
for
a
given
window
of
exposure.
Polymorphisms
in
steroid
hormone-
metabolizing
genes
also
represent
genetic
fac-
tors
that
can
predispose
to
adverse
health
effects
of
endocrine
disruptors
(10-12).
5ac-
Reductase
levels
can
be
altered
by
the
presence
of
TA
dinudeotide
repeats
in
the
3'
region
of
the
gene
and
changes
in
these
levels
can
impact
the
conversion
of
testosterone
to
dihy-
drotestosterone
(DHT),
the
active
form
of
this
hormone.
These
polymorphisms
have
also
been
linked
to
increased
risk
of
prostatic
carci-
noma,
although
this
is
somewhat
controversial
(13-15).
The
V89L
substitution
can
also
affect
5a-reductase
activity.
Polymorphisms
in
several
cytochrome
P450
genes,
including
CyplB1
and
Cyp
l7a
(aromatase)
as
well
as
catechol-OH-transferase
(COMT),
have
been
linked
to
increased
risk
of
hormone-
dependent
cancers
including
breast
cancer.
Similarly,
deletions
in
glutathione
transferase,
a
detoxifying
enzyme
for
xenobiotics
that
is
present
in
some
individuals,
put
them
at
increased
risk
for
breast
and
prostate
cancer,
possibly
as
a
result
of
increased
endogenous/
exogenous
hormone
levels
(16).
Receptor
polymorphisms
may
also
increase
susceptibility
to
endocrine
disruptors
via
changes
in
the
regulation
or
function
of
steroid
hormone
receptors
(10).
AR
hyper-
sensitivity
is
a
function
of
the
length
of
CAG
nucleotide
repeats,
with
individuals
carrying
shorter
length
repeats
expressing
AR
that
are
more
sensitive
to
androgens
(17).
Polymorphisms
in
the
PR
have
been
associated
with
increased
risk
for
ovarian
cancer,
possibly
due
to
increased
activity
or
stability
of
the
receptor.
Similar
polymor-
phisms
may
exist
for
ER-a
and
ER-p.
Target
gene
polymorphisms
can
also
predispose
to
hormonally
related
diseases
such
as
breast
cancer
(18).
Individuals
carry-
ing
BRCA1
mutations,
for
example,
are
refractory
to
the
protective
effects
of
preg-
nancy
on
breast
cancer
risk.
Whether
such
target
gene
mutations
would
predispose
indi-
viduals
to
the
adverse
health
effects
of
endocrine
disruptors
is
not
known
at
this
time.
Mutations
in
other
potential
target
genes
may
also
soon
be
identified
through
the
Environmental
Genome
Project,
and
these
will
need
to
be
investigated
to
determine
if
they
can
potentially
affect
susceptibility
to
low-dose
exposures.
Several
of
the
genetic
alterations
and
polymorphisms
noted
above
may
contribute
Environmental
Health
Perspectives
*
Vol
107,
Supplement
4
*
August
1999
620

DETERMINANTS
OF
SPECIFICITY
IN
ENDOCRINE
SIGNAUNG
to
the
observed
ethnic
differences
in
risk
for
hormonally
related
diseases
such
as
breast
cancer
and
prostate
cancer.
The
frequency
of
higher
activity
alleles
of
CyplB1
that
result
in
increased
4-OH-estradiol
(4-OH-E2)
levels,
which
in
turn
are
associated
with
increased
potential
for
free
radical
damage
to
the
DNA
and
more
potent
activation
of
the
ER
than
17[-estradiol
(1
7BE2),
are
more
prevalent
in
African
Americans
than
Caucasians
and
may
contribute
to
increased
breast
cancer
risk
(19).
Similarly,
alleles
of
COMT
with
decreased
activity
could
also
increase
4-OH-
E2
levels
and
increase
risk
for
developing
postmenopausal
breast
cancer
(11).
There
is
evidence
that
endocrine
disruptors
can
modu-
late
the
activity
of
estradiol-metabolizing
enzymes,
with
indole-3-carbinol
increasing
the extent
of
2-hydroxylation
of
estradiol
and
decreasing
mammary
tumor
incidence
and
multiplicity
in
mice
(20),
whereas
PCBs
can
increase
the
production
of
16a-OH
metabo-
lites
of
estrogen
that
can
bind
to
the
ER
and
form
a
protein-reactive
Schiff
base.
The
ratio
of
2-OH
to
16a-OH
metabolites
is
thought
to
be
one
determinant
of
breast
cancer
risk,
with
compounds
that
shift
the
balance
toward
2-OH
being
protective
and
those
that
produce
increases
in
16a-OH
increasing
cancer
risk.
Several
research
needs
were
identified
as
a
result
of
these
discussions
related
to
possible
genetic
determinants
of
susceptibility.
First,
many
of
the
studies
relating
genetic
polymor-
phisms
in
the
population
to
increased
risk
for
a
particular
disease
are
quite
controversial
and
conflicting
data
sets
have
been
reported.
Therefore,
a
primary
research
need
is
to
con-
firm
these
studies
and
resolve
conflicting
data
present
in
the
literature.
Second,
the
impact
of
receptor
polymorphisms
on
receptor
acti-
vation
by
endocrine
disruptors
should
be
investigated
to
determine
if
any
of
these
polymorphisms
may
predispose
individuals
to
the
adverse
health
effects
of
these
chemi-
cals.
Finally,
the
functionality
of
genetic
polymorphisms
in
metabolizing
genes
or
other
relevant
genes,
especially
in
terms
of
how
they
affect
the
dose-response
curves
for
endogenous
or
exogenous
compounds
resulting
in
shifts
in
sensitivity
or
response
levels,
needs
to
be
determined.
A
second
area
of
research
needs
relates
to
epigenetic
effects
on
responsivity
to
endocrine
disruptors.
Hormonal
exposure
early
in
devel-
opment
has
organizational
and
lasting
effects
on
later
sensitivity
to
hormones
that
activate
hormone-dependent
physiologic
and
behav-
ioral
functions.
For
example,
it
would
be
important
to
learn
whether
exposure
of
a
fetus
to
one
or
more
natural
or
xenobiotic
endocrine-active
substances
has
long-term
effects
on
susceptibility,
i.e.,
endocrine
imprinting
(10,12,21).
Extrinsic
Factors
Affecting
Susceptibility
Several
extrinsic
factors
such
as
diet,
socioeconomic
status,
and
obesity
affect
the
risk
for
hormonally
related
diseases
such
as
breast
cancer.
Dietary
history
and
previous
chemical
exposures
can
produce
a
biologic
imprint
that
can
persist
even
in
the
absence
of
the
continued
presence
of
the
causative
agent.
These
types
of
historical
exposures
may
be
very
difficult
to
assess
in
human
populations
but
must
be
considered
as
important
con-
tributing
risk
factors.
Obesity
can
have
dra-
matic
affects
on
the
hormonal
milieu,
especially
in
postmenopausal
women
in
which
aromatization
of
fat
stores
can
significantly
alter
estrogen
levels,
particularly
levels
of
estriol
and
estrone.
Similarly,
increased
weight
gain
in
adolescent
girls
appears
to
be
one
of
the
contributing
factors
to
early
onset
of
puberty.
Many
of
these
extrinsic
factors
are
not
evenly
distributed
across
ethnic
and
socioeconomic
populations
and
may
con-
tribute
to
the
observed
decreased
breast
cancer
risk
in
Asian
women
(caloric
restriction)
and
increased
risk
for
breast
and
uterine
cancer
in
African
American
women
(obesity
and
early
menarche).
By
definition,
these
extrinsic
factors
would
be
considered
epigenetic
contributors
to
increased
risk.
Factors
Affecting
Tissue
Specificity
These
factors
can
be
broadly
grouped
into
those
that
modulate
the
transcriptional
acti-
vation
function
of
steroid
hormone
receptors
and
those
that
occur
as
a
result
of
altered
pat-
terns
of
gene
expression
in
specific
tissues.
A
new
and
important
area
of
investigation
in
the
former
category
is
steroid
hormone
receptor
accessory
proteins
that
can
function
as
either
coactivators
or
corepressors
for
gene
transcription.
Very
little
information
is
presently
available
on
how
these
proteins
might
confer
tissue-specific
responsiveness,
and
more
research
is
needed
on
a)
whether
polymorphisms
in
these
accessory
proteins
exist
that
might
have
functional
conse-
quences
for
their
activity,
b)
whether
expres-
sion
levels
are
different
in
different
tissues
and
how
the
ratio
of
coactivators
to
corepres-
sors
affects
receptor
activation,
and
c)
whether
tissue-specific
modifications
such
as
splicing
variants
or
phosphorylation
might
affect
their
activity.
These
accessory
proteins
may
participate
in
nontraditional
receptor
activa-
tion
pathways,
which
as
mentioned
above,
may
be
particularly
sensitive
to
low-dose
exposures.
SRC-1
can
facilitate
ligand-inde-
pendent
activation
of
steroid
hormone
recep-
tors
and
in
combination
with
NCoR
can
act
as
a
determinant
of
agonist
or
antagonist
activity
for
ligands
such
as
tamoxifen
(ER)
and
RU486
(PR)
and
therefore,
possibly
for
endocrine
disruptors
as
well
(22-24).
Tissue-specific
receptor
distribution
or
number
may
also
influence
the
response
of
different
tissues
to
endocrine
disruptors
(25).
ER-a
and
ER-f
receptors
exhibit
tissue-
specific
patterns
of
expression,
with
ovary,
prostate,
testis,
brain
and
bone
being
primar-
ily
driven
by
ER-P
(25).
As
ER-a
and
ER-f
have
different
agonist
and
antagonist
activi-
ties
for
the
same
ligand,
this
expression
pat-
tern
could
ultimately
influence
whether
a
specific
ligand
acts
as
an
agonist
or
antago-
nist
in
a
given
tissue
(26).
The
mammary
gland
also
exhibits
changes
in
ER
and
PR
expression
as
a
function
of
age
and
differenti-
ation
status
(for
example,
during
pregnancy
or
neonatal
estrogen
exposure)
that
can
alter
its
susceptibility
to
induction
of
breast
cancer;
similar
changes
may
occur
in
the
uterus
as
well
(27).
It
should
also
be
recog-
nized
that
there
are
numerous
members
of
the
nuclear
receptor
family
that
are
termed
orphan
receptors
because
their
ligands
or
functions
are
unknown.
However,
it is
evi-
dent
from
those
ligands
that
have
been
iden-
tified
that
ligands
are
typically
small
hydrophobic
molecules.
The
identification
of
orphan
receptors
that
bind
progesterone
or
androgen
metabolites
suggests
that
some
of
these
receptors
(e.g.,
the
steroid
and
xenobi-
otic-sensing
nuclear
receptor)
may
also
be
targets
of
endocrine
disruptors
(28).
Further
research
to
determine
if
these
orphan
recep-
tors
contribute
to
the
adverse
health
effects
of
endocrine
disruptors
will
be
needed.
Along
similar
lines,
it
has
been
shown
that
the
activity
of
a
given
receptor
can
have
quite
different
effects
in
different
tissues
and
in
dif-
ferent
species.
An
example
of
this
would
be
endometrium
and
breast,
where
estrogen
priming
(or
possibly
low-dose
endocrine
dis-
ruptor
exposure)
followed
by
progesterone
results
in
a
mitogenic
stimulus,
whereas
in
the
ovary,
progesterone
induces
an
apoptotic
response.
Species-specific
behavioral
responses
mediated
by
the
PR
have
been
observed
at
the
level
of
the
brain.
In
rodents
for
example,
estrogen
priming
followed
by
progesterone
(Pg)
is
required
for
behavioral
sexual
estrus,
whereas
in
primates,
expression
of
sexual
behavior
is
inhibited
by
Pg.
Differential
gene
expression
in
various
target
tissues
should
also
be
considered
as
a
determinant
of
tissue-specific
response
(12).
Extrahepatic
estrogen-metabolizing
enzymes
display
tissue-specific
patterns
of
expression
resulting
in
different
profiles
or
activity
of
endogenous
hormones
in
different
tissues.
Exposure
to
endocrine
disruptors
that
impact
the
activity
of
these
metabolizing
enzymes
could
therefore
exhibit
a
tissue-specific
target
cell
pattern.
Another
example
of
tissue-
specific
protein
expression
affecting
response
Environmental
Health
Perspectives
*
Vol
107,
Supplement
4
*
August
1999
621

WALKER
ET
AL.
is
metallothionine
expression
in
the
testis,
where
expression
of
this
protein
is
very
low.
As
a
result
of
this
low
expression
level,
the
testis
displays
an
increased
sensitivity
to
Cd
that
results
from
a
cascade
of
events
initiated
by
decreased
blood
supply
to
this
tissue,
decreased
viability
of
Leydig
cells,
and
ulti-
mately
decreased
production
of
testosterone.
Membrane-bound
receptors
for
steroid
hor-
mones
are
also
differentially
expressed
and
are
found
primarily
on
sperm
and
neurons.
These
receptors
could
potentially
mediate
endocrine
disruptor
activity
in
these
cells,
although
research
addressing
this
point
is
lacking.
Finally,
the
testis
is
another
site
of
SBP
expression
in
addition
to
the
liver,
where
it
is
known
as
androgen-binding
protein
(ABP).
Here
it
primarily
binds
testosterone
and,
in
contrast
to
the
production
of
SBP
in
the
liver,
is
synthesized
in
both
rat
and
human
testis.
Different
effects
of
xenobiotics
have
been
observed
on
the
binding
of
5oc-
DHT
to
rat
ABP
or
to
human
sex
hormone-
binding
globulin
(29).
The
immune
system
is
another
potentially
important
but
understudied
target
tissue
for
endocrine
disruptors.
There
is
now
a
large
body
of
literature
supporting
the
concept
that
estrogens
are
potent
immunomodulators.
Gender
differences
exist
in
both
normal
phys-
iology
of
the
immune
system
as
well
as
the
elaboration
of
diverse
autoimmune
diseases.
Furthermore,
it
is
now
clear
that
there
are
bidirectional
interactions
between
the
immune
system,
central
nervous
system,
and
endocrine
system.
For
example,
castration
of
males
results
in
marked
hyperplasia
of
the
thymus,
whereas
administration
of
estrogens
or
androgens
induces
thymic
atrophy.
Conversely,
neonatal
thymectomy
has
been
shown
to
result
in
ovarian
dysgenesis,
auto-
immune
oophenitis,
and
autoimmune
thy-
roiditis.
Given
the
fact
that
estrogen
and
progesterone
modulate
inflammatory
activity
in
the
mouse
uterus
(30),
it
is
plausible
that
xenoestrogens
and
other
endocrine
disruptors
that
affect
the
endocrine
system
will
likely
impact
the
immune
system
as
well.
Tissue-specific
differences
in
response
to
steroid
hormones
underscore
the
fact
that
endocrine
disruptors
may
use
multiple
cellular
mechanisms
to
produce
an
adverse
cellular
response,
and
these
mechanisms
may
be
dif-
ferent
at
low-
versus
high-dose
exposures.
Examples
of
this
would
be
high-
versus
low-
dose
effects
of
genistein,
which
has
been
shown
to
have
agonist
effects
mediated
via
the
ER
but
can
also
have
growth
inhibitory
effects
that
are
not
receptor
mediated,
such
as
inhibi-
tion
of
protein
tyrosine
kinase
activity
at
high
concentrations
of
this
compound
(31).
Thus
it
will
be
important
to
achieve
an
understand-
ing
of
these
and
other
tissue-specific
determi-
nants
of
responsiveness
to
various
endocrine
disruptors
and
to
identify
which
pathways
are
used
at
different
dose
levels.
Utility
of
Available
Model
Systems
Short-term
in
vitro
assays
that
utilize
reporter
genes
may
be
useful
tools
for
determining
a)
the
functional
consequences
of
receptor
polymorphisms,
interactions,
and
number;
b)
the
functional
impact
of
polymorphisms
in
metabolic
enzymes;
and
c)
the
dose-response
relationships
between
promoter
structure
and
gene
expression.
The
development
of
in
vitro
assays
that
can
address
these
questions
would
be
particularly
useful
for
studying
how
these
parameters
affect
response
to
low
doses
of
endocrine
disruptors,
and
should
be
a
research
priority.
Such
assays
may
also
prove
useful
for
assigning
functionality
to
gene
polymor-
phisms
identified
through
the
Environmental
Genome
Project,
which
might
impact
responsiveness
to
endocrine
disruptors.
Several
in
vitro
and
in
vivo
model
systems
that
focus
primarily
on
cellular
responses
such
as
cell
proliferation
are
currently
avail-
able
and
these
may
also
be
useful
for
study-
ing
the
effects
of
endocrine
disruptors
on
susceptible
populations.
Breast
cell
lines
from
individuals
with
inherited
cancer
susceptibili-
ties
such
as
Li-Fraumeni
syndrome
(p53)
and
BRCA1
mutations
are
available
that
display
differential
responsiveness
to
chemical
car-
cinogens.
These
cell
lines
may
be
useful
for
studying
the
impact
of
low-dose
exposures
on
susceptible
populations
at
increased
risk
for
adverse
effects
of
endocrine
disruptors
due
to
inherited
mutations
in
relevant
target
genes.
However,
the
use
of
both
short-term
assays
and
cell
lines
will
have
limited
utility
for
extrapolating
how
these
susceptibility
fac-
tors
contribute
to
the
variability
observed
in
heterogeneous
human
populations
and
for
understanding
the
biologic
basis
of
adverse
health
effects
observed
in
individuals
exposed
to
endocrine
disruptors.
In
this
regard,
in
vivo
models
of
cancer
susceptibility
with
relevance
to
adverse
health
effects
of
endocrine
disruptors
are
also
available
and
may
be
used
to
address
some
of
these
questions.
These
include
the
Noble
rat
for
prostate
and
breast
cancer,
Sprague-Dawley
rat
for
breast
cancer,
F344
rat
for
pituitary
tumors,
and
the
Eker
rat
model
for
uterine
fibroids
(32-35).
These
models
have
been
well
characterized
for
their
sensitivity
to
steroid
hormones,
and
research
opportunities
exist
for
investigating
the
effects
of
endocrine
disruptors
on
the
specific
target
tissues
that
are
susceptible
to
endocrine
mod-
ulation
in
these
animal
models.
Some
mouse
models
are
also
available
that
may
be
useful
for
this
purpose,
such
as
the
T-ramp
murine
prostatic
carcinoma
model
and
the
mouse
mammary
tumor
virus
(MMTV)-aromatase
transgenic
mouse
model
for
breast
and
testicular
cancer.
The
sensitivity
of
these
models
to
hormone-induced
tumor
develop-
ment
may
make
them
particularly
useful
for
studying
low-dose
effects
of
endocrine
disrup-
tors.
More
research
in
this
area
is
recom-
mended.
In
particular,
these
in
vivo
models
may
provide
additional
dosimetry
data
that
could
be
useful
for
modeling
low-dose
expo-
sures
to
these
compounds.
An
additional
area
for
consideration
of
model
development
is
one
or
more
behavioral
tests
for
endocrine
disruptors.
Alterations
in
behavior
are
the
outcome
of
a
cascade
of
effects
at
the
molecular,
cellular,
and
organ
levels.
This
is
both
a
blessing
in
that
it
sums
across
many
effects
and
a
curse
in
that
it
is
hard
to
attribute
changes
to
specific
internal
effects.
Another
issue
to
be
addressed
in
the
development
of
behavioral
models
would
be
when
one
considers
the
behavioral
alteration
to
be
adverse.
This
topic
may
be
more
suit-
able
to
a
small
workshop
of
its
own
rather
than
a
workshop
on
a
specific
model.
Incorporation
of
This
Information
into
Improved
Risk
Assessments
Three
paradigms
for
translating
relevant
information
from
the
discussion
above
into
risk
assessments
for
sensitive
populations
were
discussed:
a)
the
use
of
quantitative
informa-
tion
related
to
a
receptor
polymorphism
that
affects
receptor
activity,
b)
use
of
mechanistic
information
to
identify
the
critical
rate-limit-
ing
step
for
a
model
of
endocrine
disruption,
and
c)
use
of
a
quantitative
structure-activity
relationship
(QSAR)
approach
for
modeling
the
activity
of
endocrine
disruptors.
The
example
of
AR
polymorphisms
asso-
ciated
with
increased
risk
for
prostatic
cancer
was
discussed
as
an
example
of
how
this
type
of
information
would
be
incorporated
into
a
risk
assessment
model.
Decreases
in
the
polyglutamine
repeat
length
in
this
receptor
are
associated
with
increased
cancer
risk,
with
loss
of
each
repeat
contributing
an
additional
3%
increase
in
relative
risk.
Mechanistic
data
suggest
that
the
Kj
of
these
receptor
variants
is
unchanged
but
that
transactivation
func-
tion
of
the
receptor
is
functionally
different.
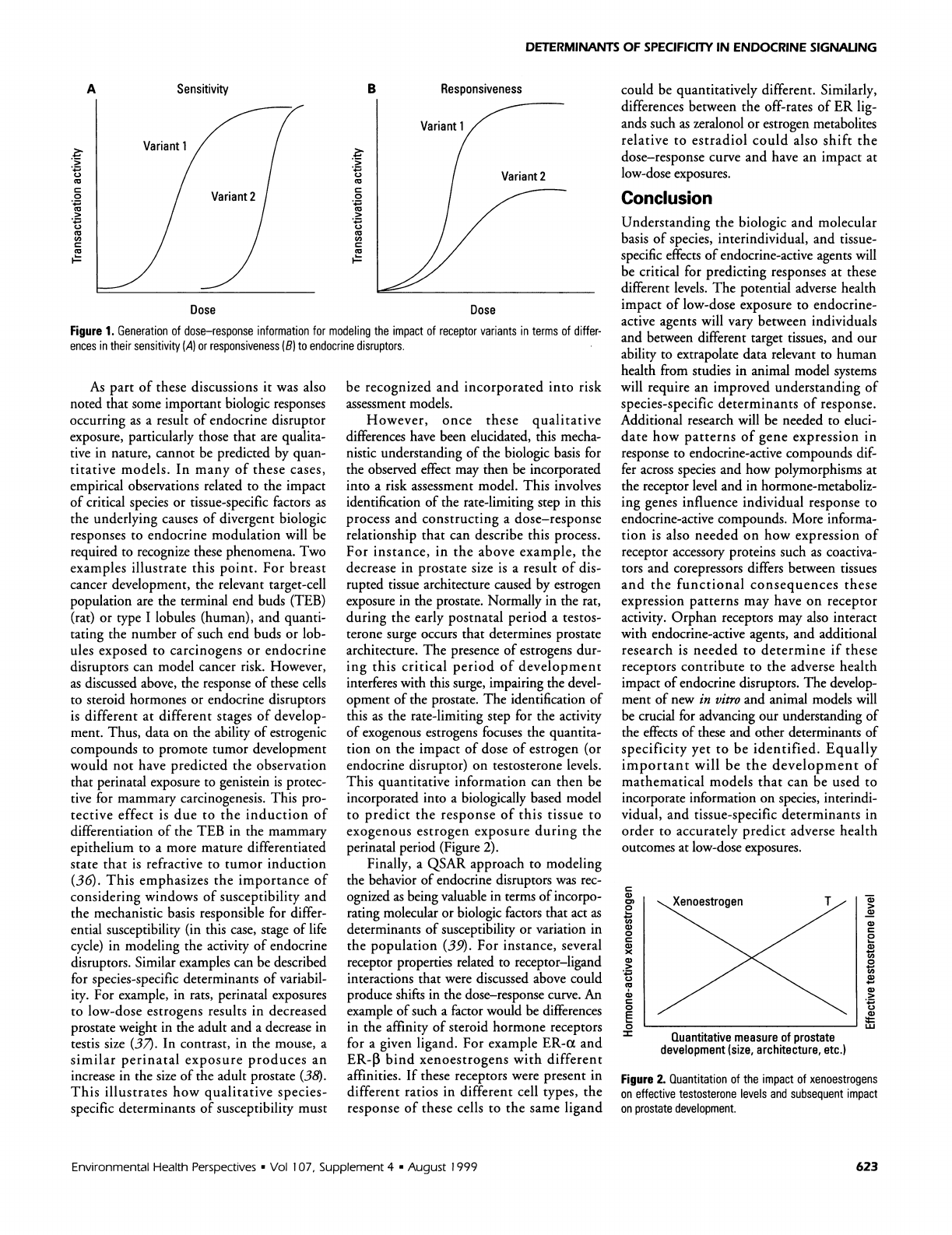
Information
needed
for
modeling
this
poly-
morphism
could
be
acquired
by
establishing
a
quantitative
dose-response
relationship
for
these
receptor
variants
(Figure
1)
to
deter-
mine
if
transactivation
activity
by
these
vari-
ants
differed
in
sensitivity
or
responsiveness
to
an
endocrine
disruptor
and
the
magnitude
of
these
changes.
This
information
could
then
be
translated
to a
population
in
which
these
receptor
variants
were
distributed
with
a
given
frequency
to
model
the
effect
of
these
polymorphisms
on
the
response
of
an
exposed
population.
Environmental
Health
Perspectives
*
Vol
107,
Supplement
4
*
August
1999
622
DETERMINANTS
OF
SPECIFICITY
IN
ENDOCRINE
SIGNALING
Sensitivity
Cu
C.)
cc
0
cc
.,_
C)
c
c
ca
B
Responsiveness
Dose
Dose
Figure
1.
Generation
of
dose-response
information
for
modeling
the
impact
of
receptor
variants
in
terms
of
differ-
ences
in
their
sensitivity
(A)
or
responsiveness
(B)
to
endocrine
disruptors.
As
part
of
these
discussions
it
was
also
noted
that
some
important
biologic
responses
occurring
as
a
result
of
endocrine
disruptor
exposure,
particularly
those
that
are
qualita-
tive
in
nature,
cannot
be
predicted
by
quan-
titative
models.
In
many
of
these
cases,
empirical
observations
related
to
the
impact
of
critical
species
or
tissue-specific
factors
as
the
underlying
causes
of
divergent
biologic
responses
to
endocrine
modulation
will
be
required
to
recognize
these
phenomena.
Two
examples
illustrate
this
point.
For
breast
cancer
development,
the
relevant
target-cell
population
are
the
terminal
end
buds
(TEB)
(rat)
or
type
I
lobules
(human),
and
quanti-
tating
the
number
of
such
end
buds
or
lob-
ules
exposed
to
carcinogens
or
endocrine
disruptors
can
model
cancer
risk.
However,
as
discussed
above,
the
response
of
these
cells
to
steroid
hormones
or
endocrine
disruptors
is
different
at
different
stages
of
develop-
ment.
Thus,
data
on
the
ability
of
estrogenic
compounds
to
promote
tumor
development
would
not
have
predicted
the
observation
that
perinatal
exposure
to
genistein
is
protec-
tive
for
mammary
carcinogenesis.
This
pro-
tective
effect
is
due
to
the
induction
of
differentiation
of
the
TEB
in
the
mammary
epithelium
to
a
more
mature
differentiated
state
that
is
refractive
to
tumor
induction
(36).
This
emphasizes
the
importance
of
considering
windows
of
susceptibility
and
the
mechanistic
basis
responsible
for
differ-
ential
susceptibility
(in
this
case,
stage
of
life
cycle)
in
modeling
the
activity
of
endocrine
disruptors.
Similar
examples
can
be
described
for
species-specific
determinants
of
variabil-
ity.
For
example,
in
rats,
perinatal
exposures
to
low-dose
estrogens
results
in
decreased
prostate
weight
in
the
adult
and
a
decrease
in
testis
size
(37).
In
contrast,
in
the
mouse,
a
similar
perinatal
exposure
produces
an
increase
in
the
size
of
the
adult
prostate
(38).
This
illustrates
how
qualitative
species-
specific
determinants
of
susceptibility
must
be
recognized
and
incorporated
into
risk
assessment
models.
However,
once
these
qualitative
differences
have
been
elucidated,
this
mecha-
nistic
understanding
of
the
biologic
basis
for
the
observed
effect
may
then
be
incorporated
into
a
risk
assessment
model.
This
involves
identification
of
the
rate-limiting
step
in
this
process
and
constructing
a
dose-response
relationship
that
can
describe
this
process.
For
instance,
in
the
above
example,
the
decrease
in
prostate
size
is
a
result
of
dis-
rupted
tissue
architecture
caused
by
estrogen
exposure
in
the
prostate.
Normally
in
the
rat,
during
the
early
postnatal
period
a
testos-
terone
surge
occurs
that
determines
prostate
architecture.
The
presence
of
estrogens
dur-
ing
this
critical
period
of
development
interferes
with
this
surge,
impairing
the
devel-
opment
of
the
prostate.
The
identification
of
this
as
the
rate-limiting
step
for
the
activity
of
exogenous
estrogens
focuses
the
quantita-
tion
on
the
impact
of
dose of
estrogen
(or
endocrine
disruptor)
on
testosterone
levels.
This
quantitative
information
can
then be
incorporated
into
a
biologically
based
model
to
predict
the
response
of
this
tissue
to
exogenous
estrogen
exposure
during
the
perinatal
period
(Figure
2).
Finally,
a
QSAR
approach
to
modeling
the
behavior
of
endocrine
disruptors
was
rec-
ognized
as
being
valuable
in
terms
of
incorpo-
rating
molecular
or
biologic
factors
that
act
as
determinants
of
susceptibility
or
variation
in
the
population
(39).
For
instance,
several
receptor
properties
related
to
receptor-ligand
interactions
that
were
discussed
above
could
produce
shifts
in
the
dose-response
curve.
An
example
of
such
a
factor
would
be
differences
in
the
affinity
of
steroid
hormone
receptors
for
a
given
ligand.
For
example
ER-a
and
ER-,
bind
xenoestrogens
with
different
affinities.
If
these
receptors
were
present
in
different
ratios
in
different
cell
types,
the
response
of
these
cells
to
the
same
ligand
could be
quantitatively
different.
Similarly,
differences
between
the
off-rates
of
ER
lig-
ands
such
as
zeralonol
or estrogen
metabolites
relative
to
estradiol
could
also
shift
the
dose-response
curve
and
have
an
impact
at
low-dose
exposures.
Conclusion
Understanding
the
biologic
and
molecular
basis
of
species,
interindividual,
and
tissue-
specific
effects
of
endocrine-active
agents
will
be
critical
for
predicting
responses
at
these
different
levels.
The
potential
adverse
health
impact
of
low-dose
exposure
to
endocrine-
active
agents
will
vary
between
individuals
and
between
different
target
tissues,
and
our
ability
to
extrapolate
data
relevant
to
human
health
from
studies
in
animal
model
systems
will
require
an
improved
understanding
of
species-specific
determinants
of
response.
Additional
research
will
be
needed
to
eluci-
date
how
patterns
of
gene
expression
in
response
to
endocrine-active
compounds
dif-
fer
across
species
and
how
polymorphisms
at
the
receptor
level
and
in
hormone-metaboliz-
ing
genes
influence
individual
response
to
endocrine-active
compounds.
More
informa-
tion
is
also
needed
on
how
expression
of
receptor
accessory
proteins
such
as
coactiva-
tors
and
corepressors
differs
between
tissues
and
the
functional
consequences
these
expression
patterns
may
have
on
receptor
activity.
Orphan
receptors
may
also
interact
with
endocrine-active
agents,
and
additional
research
is
needed
to
determine
if
these
receptors
contribute
to
the
adverse
health
impact
of
endocrine
disruptors.
The
develop-
ment
of
new
in
vitro
and
animal
models
will
be
crucial
for
advancing
our
understanding
of
the
effects
of
these
and
other
determinants
of
specificity
yet
to
be
identified.
Equally
important
will
be
the
development
of
mathematical
models
that
can
be
used
to
incorporate
information
on
species,
interindi-
vidual,
and
tissue-specific
determinants
in
order
to
accurately
predict
adverse
health
outcomes
at
low-dose
exposures.
c
0Y)
0
4-
C,,
0
c
X
C.)
0
0
Quantitative
measure
of
prostate
development
(size,
architecture,
etc.)
C)
C)
CD
,0
Cu
0
Cu
Cu
.,_
Cu
LU
Figure
2.
Quantitation
of
the
impact
of
xenoestrogens
on
effective
testosterone
levels
and
subsequent
impact
on
prostate
development.
Environmental
Health
Perspectives
*
Vol
107,
Supplement
4
*
August
1999
A
C4
c
0
.4
cc
CO
623

WALKER
ET
AL.
REFERENCES
AND
NOTES
1.
Zacharewski
T.
Identification
and
assessment
of
endocrine
disruptors:
imitations
of
in
vivo
and
in
vitro
assays.
Environ
Health
Perspect
106(suppl
2):577-582
(1998).
2.
Steinmetz
R,
Brown
NG,
Allen
DL,
Bigsby
RM,
Ben-Jonathan
N.
The
environmental
estrogen
bisphenol
A
stimulates
pro-
lactin
release
in
vitro
and
in
vivo.
Endocrinology
138:1780-1786
(1997).
3.
Ding
VD,
Moller
DE,
Feeney
WP,
Didolkar
V,
Nakhla
AM,
Rhodes
L,
Rosner
W,
Smith
RG.
Sex
hormone-binding
globu-
lin
mediates
prostate
androgen
receptor
action
via
a
novel
signaling
pathway.
Endocrinology
139:213-218
(1998).
4.
Nakhla
AM,
Romas
NA,
Rosner
W.
Estradiol
activates
the
prostate
androgen
receptor
and
prostate-specific
antigen
secretion
through
the
intermediacy
of
sex
hormone-binding
globulin.
J
Biol
Chem
272:6838-6841
(1997).
5.
Picard
D,
Bunone
G,
liu
JW,
Donze
0.
Steroid-independent
activation
of
steroid
receptors
in
mammalian
and
yeast
cells
and
in
breast
cancer.
Biochem
Soc
Trans
25:597-602
(1997).
6.
Weigel
NL.
Steroid
hormone
receptors
and
their
regulation
by
phosphorylation.
Biochem
J
319:657-667
(1996).
7.
Darne
C,
Veyssiere
G,
Jean
C.
Phorbol
ester
causes
ligand-
independent
activation
of
the
androgen
receptor.
Eur
J
Biochem
256:541-549
(1998).
8.
Russo
IH,
Russo
J.
Developmental
stage
of
the
rat
mammary
gland
as
determinant
of
its
susceptibility
to
7,12-dimethyl-
benz[a]anthracene.
J
NatI
Cancer
Inst
61:1439-1449
(1978).
9.
Russo
J,
Tay
LK,
Russo
IH.
Differentiation
of
the
mammary
gland
and
susceptibility
to
carcinogenesis.
Breast
Cancer
Res
Treat
2:5-73
(1982).
10.
Feigelson
HS,
Ross
RK,
Yu
MC,
Coetzee
GA,
Reichardt
JK,
Henderson
BE.
Genetic
susceptibility
to
cancer
from
exoge-
nous
and
endogenous
exposures.
J
Cell
Biochem
Suppl
25:15-22
(1996).
11.
Lavigne
JA,
Heizisouer
KJ,
Huang
HY,
Strickland
PT,
Bell
DA,
Selmin
0,
Watson
MA,
Hoffman
S,
Comstock
GW,
Yager
JD.
An
association
between
the
allele
coding
for
a
low
activity
variant
of
catechol-D-methyltransferase
and
the
risk
for
breast
cancer.
Cancer
Res
57:5493-5497
(1997).
12.
Zhu
BT,
Conney
AH.
Functional
role
of
estrogen
metabolism
in
target
cells:
review
and
perspectives.
Carcinogenesis
19:1-27
(1998).
13.
Kantoff
PW,
Febbo
PG,
Giovannucci
E,
Krithivas
K,
Dahl
DM,
Chang
G,
Hennekens
CH,
Brown
M,
Stampfer
MJ.
A
poly-
morphism
of
the
5ax-reductase
gene
and
its
association
with
prostate
cancer:
a
case-control
analysis.
Cancer
Epidemiol
Biomarkers
Prev
6:189-192
(1997).
14.
Reichardt
JK,
Makridakis
N,
Henderson
BE,
Yu
MC,
Pike
MC,
Ross
RK.
Genetic
variability
of
the
human
SRD5A2
gene:
implications
for
prostate
cancer
risk.
Cancer
Res
55:3973-3975
(1995).
15.
Ross
RK,
Coetzee
GA,
Reichardt
J,
Skinner
E,
Henderson
BE.
Does
the
racial-ethnic
variation
in
prostate
cancer
risk
have
a
hormonal
basis?
Cancer
(Phila.)
75:1778-1782
(1995).
16.
Hengstler
JG,
Arand
M,
Herrero
ME,
Oesch
F.
Polymorphisms
of
N-acetyltransferases,
glutathione
S-
transferases,
microsomal
epoxide
hydrolase
and
sulfotrans-
ferases:
influence
on
cancer
susceptibility.
Recent
Results
Cancer
Res
154:47-85
(1998).
17.
Kantoff
P,
Giovannucci
E,
Brown
M.
The
androgen
receptor
CAG
repeat
polymorphism
and
its
relationship
to
prostate
cancer.
Biochim
Biophys
Acta
1378:C1-5
(1998).
18.
Hu
YF,
Russo
IH,
Zalipsky
U,
Lynch
HT,
Russo
J.
Environmental
chemical
carcinogens
induce
transformation
of
breast
epithelial
cells
from
women
with
familial
history
of
breast
cancer.
In
Vitro
Cell
Dev
Biol
Anim
33:495-498
(1997).
19.
Bailey
LR,
Roodi
N,
Dupont
WD,
ParI
FF.
Association
of
cytochrome
P450
1B1
(CYP1B1)
polymorphism
with
steroid
receptor
status
in
breast
cancer.
Cancer
Res
58:5038-5041
(1998).
20.
Bradlow
HL,
Michnovicz
J,
Telang
NT,
Osborne
MP.
Effects
of
dietary
indole-3-carbinol
on
estradiol
metabolism
and
sponta-
neous
mammary
tumors
in
mice.
Carcinogenesis
12:1571-1574
(1991).
21.
Kao
YC,
Zhou
C,
Sherman
M,
Laughton
CA,
Chen
S.
Molecular
basis
of
the
Inhibition
of
human
aromatase
(estrogen
syn-
thetase)
by
flavone
and
isoflavone
phytoestrogens:
a
site-
directed
mutagenesis
study.
Environ
Health
Perspect
106:85-92
(1998).
22.
Wagner
BL,
Norris
JD,
Knotts
TA,
Weigel
NL,
McDonnell
DP.
The
nuclear
corepressors
NCoR
and
SMRT
are
key
regulators
of
both
ligand-
and
8-bromo-cyclic
AMP-dependent
transcrip-
tional
activity
of
the
human
progesterone
receptor.
Mol
Cell
Biol
18:1369-1378
(1998).
23.
Jackson
TA,
Richer
JK,
Bain
DL,
Takimoto
GS,
Tung
L,
Horwitz
KB.
The
partial
agonist
activity
of
antagonist-occupied
steroid
receptors
is
controlled
by
a
novel
hinge
domain-binding
coac-
tivator
L7/SPA
and
the
corepressors
N-CoR
or
SMRT.
Mol
Endocrinol
1
1:693-705
(1997).
24.
Zhang
X,
Jeyakumar
M,
Petukhov
S,
Bagchi
MK.
A
nuclear
receptor
corepressor
modulates
transcriptional
activity
of
antagonist-occupied
steroid
hormone
receptor.
Mol
Endocrinol
12:513-524
(1998).
25.
Kuiper
GG,
Carlsson
B,
Grandien
K,
Enmark
E,
Haggblad
J,
Nilsson
S,
Gustafsson
JA.
Comparison
of
the
ligand
binding
specificity
and
transcript
tissue
distribution
of
estrogen
receptors
a
and
3.
Endocrinology
138:863-870
(1997).
26.
McDonnell
DP.
Definition
of
the
molecular
mechanism
of
action
of
tissue-selective
oestrogen-receptor
modulators.
Biochem
Soc
Trans
26:997-1003
(1998).
27.
Mangal
RK,
Wiehle
RD,
Poindexter
AN
3rd,
Weigel
NL.
Differential
expression
of
uterine
progesterone
receptor
forms
A
and
B
during
the
menstrual
cycle.
J
Steroid
Biochem
Mol
Biol
63:195-202
(1997).
28.
Blumberg
B,
Sabbagh
W
Jr,
Juguilon
H,
Bolado
J
Jr,
van
Meter
CM,
Ong
ES,
Evans
RM.
SXR,
a
novel
steroid
and
xenobiotic-sensing
nuclear
receptor.
Genes
Dev
12:3195-3205
(1998).
29.
Danzo
BJ.
Environmental
xenobiotics
may
disrupt
normal
endocrine
function
by
interfering
with
the
binding
of
physio-
logical
ligands
to
steroid
receptors
and
binding
proteins.
Environ
Health
Perspect
105:294-301
(1997).
30.
Tibbetts
TA,
Conneely
OM,
O'Malley
BW.
Progesterone
via
its
receptor
antagonizes
the
pro-inflammatory
activity
of
estrogen
in
the
mouse
uterus.
Biol
Reprod
60:1158-65
(1999).
31.
Wang
TT,
Sathyamoorthy
N,
Phang
JM.
Molecular
effects
of
genistein
on
estrogen
receptor
mediated
pathways.
Carcinogenesis
17:271-275
(1996).
32.
Everitt
J,
Wolf
D,
Howe
S,
Goldsworthy
T,
Walker
C.
Rodent
model
of
reproductive
tract
leiomyomata:
clinical
and
patho-
logical
features.
Am
J Pathol
146:1556-1567
(1995).
33.
Howe
S,
Gottardis
M,
Everitt
J,
Goldsworthy
T,
Wolf
D,
Walker
C.
Rodent
model
of
reproductive
tract
leiomyomata:
establishment
and
characterization
of
tumor-derived
cell
lines.
Am
J
Pathol
146:1568-1579
(1995).
34.
Howe
S,
Gottardis
M,
Everitt
J,
Walker
C.
Estrogen
stimula-
tion
and
tamoxifen
inhibition
of
leiomyoma
cell
growth
in
vitro
and
in
vivo.
Endocrinology
136:4996-5003
(1995).
35.
Howe
SR,
Everitt
Jl,
Gottardis
MM,
Walker
C.
Rodent
model
of
reproductive
tract
leiomyomata:
characterization
and
use
in
preclinical
therapeutic
studies.
In:
Rodent
Model
of
Reproductive
Tract
Leiomyomata:
Characterization
and
Use
in
Preclinical
Therapeutic
Studies,
Vol
396
(McLachlan
J,
Slaga
TJ,
eds).
New
York:Wiley
Liss,
1997;205-215.
36.
Fritz
WA,
Coward
L,
Wang
J,
Lamartiniere
CA.
Dietary
genistein:
perinatal
mammary
cancer
prevention,
bloavail-
ability
and
toxicity
testing
in
the
rat.
Carcinogenesis
19:2151-2158
(1998).
37.
Sharpe
RM,
Fisher
JS,
Millar
MM,
Jobling
S,
Sumpter
JP.
Gestational
and
lactational
exposure
of
rats
to
xenoestro-
gens
results
in
reduced
testicular
size
and
sperm
production.
Environ
Health
Perspect
103:1136-1143
(1995).
38.
vom
Saal
FS,
Timms
BG,
Montano
MM,
Palanza
P,
Thayer
KA,
Nagel
SC,
Dhar
MD,
Ganjam
VK,
Parmigiani
S,
Welshons
WV.
Prostate
enlargement
in
mice
due
to
fetal
exposure
to
low
doses
of
estradiol
or
diethylstilbestrol
and
opposite
effects
at
high
doses.
Proc
Nati
Acad
Sci
USA
94:2056-2061
(1997).
39.
Tong
W,
Perkins
R,
Strelitz
R,
Collantes
ER,
Keenan
S,
Welsh
WJ,
Branham
WS,
Sheehan
DM.
Quantitative
struc-
ture-activity
relationships
(OSARs)
for
estrogen
binding
to
the
estrogen
receptor:
predictions
across
species.
Environ
Health
Perspect
105:1116-1124
(1997).
624
Environmental
Health
Perspectives
*
Vol
107,
Supplement
4
*
August
1999
