
Environmental Health Division, Environmental Surveillance and Assessment Section
PO Box 64975
St. Paul, MN 55164-0975
651-201-5000
www.health.state.mn.us
Guidance for Evaluating the Cancer Potency
of Polycyclic Aromatic Hydrocarbon (PAH)
Mixtures in Environmental Samples
Minnesota Department of Health
February 8, 2016

Guidance for Evaluating the Cancer Potency
of Polycyclic Aromatic Hydrocarbon (PAH)
Mixtures in Environmental Samples
February 8, 2016
For more information, contact:
Environmental Health Division, Environmental Surveillance and Assessment
Section
PO Box 64975
St. Paul, MN 55164-0975
Phone: 651-201-5000
Fax: 651-201-4606
T
his report describes guidance for conducting risk assessments. This work was conducted by the Site
Assessment and Consultation Unit, Carl Herbrandson, PhD, senior toxicologist, under the supervision of
Rita Messing, PhD, with the collaboration of the Health Risk Assessment Unit, Helen Goeden, PhD,
senior toxicologist, and Kate Sande, MS, Research Scientist, under the supervision of Pamela Shubat,
PhD.
Questions on the content of this report should be directed to the Health Risk Assessment Unit, 651-201-
4899 or by email to health.risk@state.mn.us
.
Information about this work is also posted on the MDH website at Guidance for PAHs
(http://www.health.state.mn.us/divs/eh/risk/guidance/pahmemo.html)
Upon request, this material will be made available in an alternative format such as large print, Braille or
audio recording. Printed on recycled paper.

1
Guidance for Evaluating the Cancer Potency
of Polycyclic Aromatic Hydrocarbon (PAH)
Mixtures in Environmental Samples
Executive Summary
The Minnesota Department of Health (MDH) offers guidance for a wide range of risk assessment
needs. Cancer potency evaluations of environmental mixtures are a necessary component of
cancer risk assessments. This guidance for estimating the cancer potency of mixtures of
polycyclic aromatic hydrocarbons (PAHs) is an update of 2013 guidance, which revised MDH
2001 PAH guidance on estimating health risks from carcinogenic PAHs (cPAHs).
MDH finds that the most accurate assessment of cancer risk from a mixture, one based on site-
specific whole mixture toxicity information, will not likely be possible. Therefore, other
approaches will be needed. Information about a similar mixture (“surrogate whole mixture
potency”) is preferred, but is rarely available. Summing the individual potencies of constituents
of the mixture (“relative potency method”) is a reasonable alternative. However, it is likely to be
the least accurate site-specific method for evaluating mixture cancer potency.
As a default surrogate whole mixture alternative for the most common types of cPAH mixtures
found close to an industrial or pyrogenic source MDH recommends multiplying the
benzo[a]pyrene (B[a]P) concentration in a mixture by seven to estimate the cancer potency of an
environmental sample. MDH recommends using a site-specific surrogate whole mixture or the
relative potency method for PAHs in air or water samples taken far from the source, as such
mixtures may have changed significantly in composition (fractionation). MDH makes specific
recommendations on cancer risk evaluation of PAH mixtures, relative potencies of individual
cPAHs, important cPAH analytes, and determining appropriate analytical detection limits in this
guidance.
MDH recommends that all cPAH cancer risk evaluations include the use of age-dependent
exposure estimates in combination with age-dependent adjustments to potency (as described in
MDH Risk Assessment Advice for Incorporating Early-Life Sensitivity into Cancer Risk
Assessments for Linear Carcinogens (
http://www.health.state.mn.us/divs/eh/risk/guidance/adafrecmd.pdf)).
Finally, it is important to maintain a state-wide database of site and source-type specific cPAH
concentrations so that different source-type default multipliers can be developed. This could
significantly decrease analytical and cleanup costs in the future for sites with significant cPAH
contamination

2
Introduction
Cancer potency evaluations of environmental mixtures are a necessary component of cancer risk
assessments. MDH has reviewed and relied on a large number of authoritative scientific analyses to
determine appropriately protective cancer risk assessments for polycyclic aromatic hydrocarbons (PAHs).
Extensive reviews of potential exposures and health effects of PAHs and PAH mixtures are available
from the U.S. Agency for Toxic Substances and Disease Registry (ATSDR, 1995
), the International
Agency for Research on Cancer (IARC, 2013, 1973) and the U.S. Environmental Protection Agency
(EPA, 1986, 2009). In addition, the European Food Safety Authority (EFSA)
(http://www.efsa.europa.eu/) is actively conducting research on PAH laboratory analyses and food
exposures to PAHs.
Environmental exposures to PAHs are always to mixtures of PAHs. Individual PAHs are not found
isolated in the environment. PAHs originate from three sources:
• diagenic - natural PAHs generated by biological processes (retene and perylene are examples of
PAHs that may be from diagenic or petrogenic sources)
• petrogenic - typically, petroleum and fossil fuels (these typically include many alkylated PAHs)
• pyrogenic - products of incomplete combustion (typically the biggest component of most urban
and industrial samples)
Mixtures of diagenic PAHs are generally not considered to have health impacts on people at
environmental exposures levels. Short-term environmental exposures to petrogenic and pyrogenic PAHs
can lead to tissue irritation (e.g., skin, respiratory, eyes, gastrointestinal). Dermal irritation can be
enhanced by exposure to sunlight, sometimes causing severe irritation and rash. MDH recommends
avoiding exposure of the skin and eyes, as well as short-term inhalation of large amounts of PAHs.
In addition to irritation, decreased fertility, developmental neurological effects and renal toxicity have
been demonstrated in laboratory animals exposed to relatively high levels of PAHs. MDH recommends
evaluating the non-carcinogenic health effects from exposure to PAHs using criteria developed for
individual PAHs or mixtures (e.g., total petroleum hydrocarbons). These criteria and guidance on
evaluating the potency of non-carcinogenic mixtures are not discussed in this guidance.
The most studied endpoint for long term PAH exposure, and the endpoint exclusively addressed in this
guidance, is cancer. Long term occupational exposures to PAH mixtures (often confounded by exposure
to other materials as well) have been associated with increased incidence of lung, skin, gastrointestinal
tract, bladder, and scrotal cancer. Individual carcinogenic PAHs (cPAHs) have been shown to have
different cancer potencies and may induce different types of cancer in laboratory animals (e.g., oral
exposure to benzo[a]pyrene or dibenzo[a,l]pyrene predominantly result in gastrointestinal tract cancers or
lung cancer, respectively).
Early life exposures to a number of cPAHs (e.g., benzo[a]pyrene, dibenzo[a,h]anthracene, 7,12-
dimethylbenz[a]anthracene) have demonstrated that younger animals are more sensitive than older
animals, and cPAHs are generally assumed to be more potent when exposure occurs early in life (
US
EPA, 2005). The amount of exposure to environmental contaminants will also change over a lifetime.
Therefore, MDH recommends applying age-dependent potency adjustment factors (ADAFs) in
conjunction with age-specific exposure parameters when conducting cancer risk assessments. MDH offers
guidance on the incorporation of ADAFs (
MDH, 2010).
While there may be hundreds of different PAHs and analogues in a mixture, only a few compounds are
typically analyzed when evaluating an environmental mixture. Table 1 is a list of many of the PAHs that
are recommended for analysis by different environmental and health agencies. The lists were compiled
3
for different purposes and with different chemical structure inclusion restrictions. Analyte lists are
typically limited to the fewest number of compounds necessary to economically, yet accurately evaluate a
mixture for a specific purpose.
This guidance document describes and recommends three approaches for estimating the cancer potency of
mixtures of polycyclic aromatic hydrocarbons (PAHs). While each method can be used to evaluate
exposures to different types of environmental media, different types of data (e.g., site specific whole
mixture toxicity data, surrogate whole mixture toxicity data, or individual cPAH sample data) are needed
for each method of evaluation.
Evaluating Cancer Potency
Evaluating the cancer potency of a PAH mixture can be difficult. Three methods are recommended. Each
method has advantages depending on: the availability or expense of compiling biological data on the
cancer potency of a site-related or surrogate PAH mixture; and the availability or expense of sample
analysis for a relatively long list of cPAHs.
• Site specific whole mixture potency evaluation:
A site specific whole mixture evaluation uses known or experimentally determined cancer potency data
on environmental samples collected from the site being evaluated to estimate the potency of all site
samples. This is the method with the least uncertainty and the best choice for evaluating the potential
health impacts of an environmental PAH mixture. However, mixture cancer potency data are not readily
available for most sites, and obtaining the data experimentally can be expensive. Therefore, this method is
typically impractical. Limited analytical data of site-specific samples are needed to evaluate a site using
this method.
• Surrogate whole mixture potency evaluation:
A surrogate whole mixture evaluation uses available mixture potency data from a similar source
(surrogate) to estimate the potency of all site samples. This method can be reasonably accurate and may
be the easiest and cheapest option for evaluating mixture potency. However, determining whether the
surrogate mixture and the site mixture are similar with respect to cancer potency can be problematic: what
constituents should be used to evaluate the similarity of the potencies; how similar do the mixtures need
to be to consider one a surrogate for the other’s cancer potency? Generally, limited analytical data of site-
specific samples are needed to evaluate a site using this method.
• cPAH relative potency evaluation:
A cPAH relative potency evaluation relies on analytical data for site samples to establish a cPAH mixture
potency. Detection limits for each cPAH must be at or below a pre-determined concentration to assure
that each cPAH is appropriately evaluated. The accuracy of this method is less certain because it makes
assumptions about the impact that interactions between PAHs and other mixture constituents may have on
the cancer potency of the whole mixture. This method is likely the best method for evaluating the cancer
potency of an environmental PAH mixture when there are no whole mixture potency data available and
no reasonable surrogate potency data. While this method relies heavily on analytical data, if the PAH
mixture is homogeneous over a site, a cPAH fingerprint can be developed with analytical results from a
limited number of samples. This fingerprint can then be applied across the site by indexing the cPAH
potency to the concentration of a commonly analyzed PAH (or PAHs).
A brief technical discussion of the methods for conducting each type of cancer potency evaluation for
PAH mixtures follows:
4
1. Site-specific whole mixture potency evaluation
While site-specific whole mixture potency evaluations have been incorporated in ecological assessments,
MDH is not aware of sites where these have been used to evaluate a mixture’s cancer potency to people.
In a site-specific whole mixture potency evaluation, the potency of the mixture of interest is tested in
laboratory animals. Study design will affect the usefulness of the data.
The site-specific whole mixture potency from the study should be reported as a function of the
concentration of an index PAH in the site-specific mixture. This potency can be age-adjusted for exposure
and sensitivity to calculate site-specific criteria, or to calculate cancer risk based on exposure to site
material. Equations are similar to Equations 1 and 2, below. While this method of evaluating cancer risk
will likely be the most accurate method, the cost of conducting animal toxicity studies with site-associated
materials typically excludes this option from consideration for site work.
2. Surrogate whole mixture potency evaluation
If site-specific whole mixture potency data are not available, potency of a similar, surrogate mixture may
provide the best estimate for the potency of the site media (e.g., sediment, soils). If the source of the
surrogate mixture and its composition are determined to be similar to site materials, available published or
reviewed potency data from the surrogate mixture may be used as a substitute for site-specific mixture
potency data. An example of this is using mixture potency data from available studies of coal tar mixtures
to approximate potency for mixtures from another site where the primary source was known to have been
coal tar. In this example, the coal tar mixture with available data would be presumed to be a reasonable
surrogate for the mixture of concern. The quality of available potency studies, as well as the match
between the surrogate and the site materials will affect the usefulness of a surrogate.
Similar sources (e.g., two different manufactured gas plant residues, or two different diesel exhausts) may
be anticipated to have similar potencies. Data on the absolute potency of the surrogate mixture and,
minimally, surrogate mixture concentrations of a standard list of commonly measured, semi-volatile and
nonvolatile PAHs (e.g., 15 carcinogenic and noncarcinogenic PAHs included on the EPA List of Priority
Pollutants, Table 1, below) should be available. The surrogate mixture potency data must be of sufficient
quality to allow calculation, with reasonable confidence, of a surrogate mixture cancer slope factor (CSF).
Site samples should also be analyzed for the standard list of PAHs. Ratios of constituent PAHs from both
site and surrogate mixtures should be similar. Upon request, MDH may be able to review the
appropriateness of potency data developed from site-specific or surrogate whole mixture studies.
Because the individual cPAHs responsible for the potency of the surrogate mixture are not determined in
mixture potency studies, the concentrations are typically reported as the concentration of a specific index
PAH found in the environmental mixture.
Benzo[a]pyrene (B[a]P) potency equivalence is typically used as the index of the cPAH concentration in
the surrogate and in analyzed environmental samples. The relative potency of the surrogate, expressed as
the mixture B[a]P potency equivalence (B[a]P PEQ), is calculated using Equation 1:
B[a]P PEQ
surrogate
{unitless} = CSF
surrogate
/ CSF
bap
Equation 1.
Where:
CSF
surrogate
{(mg B[a]P / kg body weight / d)
-1
} = the cancer slope factor of the surrogate mixture in
relationship to the mixture B[a]P concentration
CSF
bap
{(mg B[a]P / kg body weight / d)
-1
} = the cancer slope factor for B[a]P
The surrogate mixture B[a]P PEQ and individual sample B[a]P concentration data can be used to determine a
sample B[a]P PEQ (C
bap peq
) using Equation 2:

5
C
bap peq
{mg B[a]P PEQ / kg media} = C
bap
* B[a]P PEQ
surrogate
Equation 2.
Where:
C
bap
{mg/kg} = the concentration of B[a]P in individual samples
Data from a US National Toxicology Program (NTP) rat feeding study described in Culp et al. (1998
)
have been used to calculate CSFs for B[a]P and for coal tar mixtures. Gaylor et al. (2000) used these data
to calculate a (human adjusted) CSF of 1.2 (mg B[a]P/kg/d)
-1
for B[a]P in the most sensitive tissue
(forestomach). The California Office of Environmental Health Hazard Assessment (
CA, OEHHA, 2010)
performed a similar analysis using data from the forestomach as well as oral cavity data, and developed a
human-adjusted B[a]P CSF of 1.7 (mg B[a]P/kg/d)
-1
. Schneider et al. (2002) used the NTP data for the
coal tar mixtures to calculate a human-adjusted CSF for all tissues of 11.5 (mg B[a]P/kg/d)
-1
based on
ingestion of B[a]P in the coal tar mixtures. Coal tar and to a lesser extent B[a]P have been shown to be
oral carcinogens in multiple tissues. For developing a B[a]P PEQ for coal tar (B[a]P PEQ
coal
tar
) MDH
recommends using CSFs developed from data on cancer in all sensitive tissues. Therefore, the CA
OEHHA B[a]P CSF and the Schneider et al. coal tar CSF are used in calculating a B[a]P PEQ
coal tar
.
Using CSFs developed from the NTP data:
CSF
coal tar
= 11.5 (mg B[a]P / kg / d)
-1
(Schneider et al., 2002);
CSF
bap
= 1.7 (mg B[a]P / kg / d)
-1
(CA OEHHA, 2010)
B[a]P PEQ
coal tar
{unitless} = CSF
coal tar
/ CSF
bap
B[a]P PEQ
coal tar
= 11.5 / 1.7 = 6.76 rounded to 7
Site-specific sample concentrations can be converted to B[a]P PEQs (C
bap peq
) using Equation 2:
C
bap peq
{typically, mg/kg environ. media} = B[a]P PEQ
coal tar
* C
bap
{mg/kg environ. media}
C
bap peq
{mg/kg environ. media} = 7 * C
bap
{mg/kg environ. media} Equation 3.
C
bap peq
for each sample can be directly compared to media-specific criterion for B[a]P. Criteria should be
exposure and sensitivity adjusted by age (see MDH 2010 guidance)
While a surrogate whole mixture potency evaluation of an environmental PAH mixture is not as desirable
as a site-specific whole mixture potency evaluation of the site-specific mixture, it can be a reasonable
method for estimating the potency and risk from exposure to some PAH mixtures.
Schneider et al. (2002
) reviewed the carcinogenicity PAHs in environmental mixtures and concluded that
a reasonable potency for most PAH mixtures from industrial sources in soil (CSF
ind soil
) is 11.5 (mg
B[a]P/kg/d)
-1
(all tissues and adjusted for humans). This is about 7 times the potency of B[a]P in the
mixture (see calculation, above). In 2002, the European Commission (EC) approved the use of 10 times
B[a]P potency alone for evaluating cPAH potency in food (
European Commission – SCF 2002). In
addition, the EC also recommended chemical analysis of an extended list of cPAHs so that fingerprints of
contamination and the presence of potent cPAHs can be noted and possibly associated with various food
manufacturing processes. The EC has since retracted the mixture potency guidance, in part because some
food samples with apparent PAH cancer potency did not contain detectable levels of B[a]P (
European
Commission - EFSA, 2008). Required detection limits for cPAHs (including B[a]P) in food are lower
than required detection limits in environmental media such as soil and sediments. Furthermore,
environmental samples typically offer a range of concentrations over which detection of low
concentrations of B[a]P may be possible. Inability to detect B[a]P at sites with environmental PAH
contamination should not be an issue if appropriate methods are used.
Given that cancer potency screening of environmental samples is not very precise, and that the screening
criteria are not “bright lines”, MDH recommends using a B[a]P-PEQ of 7 times the B[a]P concentration
6
as a policy option for default evaluation of most environmental pyrogenic PAH samples. While the use of
this criterion is likely to be protective for diagenic and petrogenic PAH mixtures as well, this guidance is
only intended for application to pyrogenic PAH mixtures.
The default value for surrogate whole mixture evaluation (i.e., 7 times the B[a]P concentration) is not
recommended when the original PAH mixture has been substantially changed in the environment
(fractionation). Significant fractionation is likely to occur in ambient air or in groundwater down gradient
from a PAH source and is dependent on the chemical/physical properties of the individual constituent
PAHs, distance and speed traveled, and other factors. Therefore, the policy of using 7 times the B[a]P
concentration as a default method for evaluating whole mixture cPAH potency should be evaluated
whenever groundwater plumes or ambient air are the media of interest. It may be possible in some
circumstances to develop temporally or spatially restricted default B[a]P-PEQs for some mixtures or at
some sites.
Even if a site-specific or surrogate whole mixture potency method is used to evaluate PAH cancer
potency at a site, MDH recommends analysis of an extended list of MDH Priority cPAHs (described in
the relative potency evaluation section below) in a few samples so that cPAH fingerprints can be
developed for different PAH source-types. These data could be used to develop protective default
multipliers for different source-types, and potentially could lead to a subsequent reduction in analytical
and cleanup costs at similar sites.
3. cPAH relative potency evaluation
In this approach, each individual cPAH is presumed to contribute to the potency of a mixture in
proportion to its own potency and its concentration in the mixture. The potencies of individual cPAHs can
be very different. The easiest way to add the potencies of individual cPAHs in an environmental sample is
to convert the potency of each cPAH into a unitless relative potency factor (RPF). Previous MDH
guidance referred to these factors as potency equivalence factors (PEFs). The concentration of each cPAH
times the RPF can then be considered as a concentration of the index cPAH.
B[a]P is used, by convention, as the index PAH, and relative potencies of individual cPAHs are
determined relative to the potency of B[a]P. As a result, cPAHs that have been experimentally determined
to be more potent than B[a]P have RPFs greater than 1, and cPAHs that are less potent than B[a]P have
RPFs less than 1. For example: a cPAH ten-fold more potent than B[a]P has an RPF of 10.
The amount of an individual cPAH found in an environmental sample, as well as the relative potency of
the cPAH, determines its presumed contribution to the total cancer potency of the sample. Environmental
PAH mixture composition can vary greatly. For some mixtures it may be important to measure the
concentration of the most potent cPAHs, while for other mixtures, high concentrations of less potent
cPAHs may drive the mixture potency calculations. Therefore a fingerprint of the concentrations of most
cPAHs in an environmental mixture should be determined. If the mixture across the site or across an area
of the site is homogeneous, a cPAH fingerprint from a couple of samples can be used to calculate the
estimated potency of additional samples using data from only a small number of analytes. Using this
method, cPAH fingerprints can be determined by analyzing samples with optimum PAH concentrations,
and the ratios of constituents can then be applied to samples that may be below analytical detection limits
for some cPAHs.

7
The RPFs for individual MDH Priority cPAHs are listed in Table 2. RPFs cited in Table 2 are (1) average
RPFs from draft EPA PAH Mixture Guidance (US EPA, 2010
); (2) calculated from MDH-reviewed
CSFs; and (3) California Office of Environmental Health Hazard Assessment potency equivalents (CA
OEHHA, 2010). MDH currently recommends an adult (not age-adjusted) cancer slope factor for B[a]P of
1.7 (mg/kg/d)
-1
and an inhalation exposure adult (not age-adjusted) cancer slope factor of 3.9 (mg/kg/d)
-1
[equivalent to an adult unit risk of 1.1x10
-3
(µg/m
3
)
-1
]. If children are likely to be exposed, cancer potency
should be age-adjusted in conjunction with age-dependent exposures. Typically, these adjustments are
coordinated during the development of environmental media exposure criteria (
MDH, 2010).
The cPAH relative potency evaluation method requires analysis of many cPAHs in an environmental
mixture. Chemical analysis of these cPAHs is difficult and achieving required detection limits can be
challenging. MDH recommends analysis of MDH Priority cPAHs in Table 2 for evaluating sample
potency or developing a site and source-specific fingerprint. The results of Equation 4 (C
bap peq
: B[a]P
potency equivalence concentration) can be directly compared to a media-specific B[a]P criterion (e.g.,
Minnesota Pollution Control Agency (MPCA) B[a]P Soil Reference Value, MPCA B[a]P Inhalation
Screening Value, MDH health-based guidance for drinking water):
C
bap peq
{typically, mg B[a]P PEQ / kg media} = ∑ (C
i
* RPF
i
) Equation 4.
For:
i = all MDH Priority cPAHs (Table 2)
Where:
C
i
{typically, mg i / kg media} = individual cPAH concentrations
RPF
i
= individual cPAH relative potency factors
Prior to analyzing environmental samples for MDH Priority cPAHs, laboratory detection limits should
be evaluated. Method detection limits (MDLs) for each MDH Priority cPAH should be less than or equal
to five percent of the cPAH (B[a]P) criterion:
MDL
x
{mg / kg media} = 0.05 * Health Criterion
B[a]P
/ RPF
x
. Equation 5.
Where: x = each MDH Priority cPAH
If one or more MDH Priority cPAHs are analyzed at higher detection limits, the potency of the mixture
may be underestimated. Therefore, prior to deciding to evaluate a site using the cPAH relative potency
approach, detection limits should be reviewed, and a statistical analysis of the uncertainties that may be
incurred as a result of elevated detection limits is recommended.
Chemical analysis of cPAHs can be difficult and expensive. In addition there is limited availability of
some cPAH analytical standards. MDH has restricted the recommended list of analytes to cPAHs that can
be analyzed in a competent laboratory. However, care and experience will be necessary to assure proper
quantification of a number of cPAHs; especially cPAHs with a molecular weight of 302 (which include
the benzofluoranthrenes), benzo[c]fluorene, dibenz[a,h]anthracene, chrysene, cyclopenta[c,d]pyrene, 5-
methylchrysene, and 6-nitrochrysene.
The EPA is expected to finalize their cPAH relative potency guidance in the near future. MDH anticipates
that the release of EPA guidance will not impact the MDH Priority cPAH list (Table 2, below). However,
it is expected that the EPA Guidance will contain newer and better RPFs for a number of the MDH
Priority cPAHs. MDH will review and update cPAH RPFs as soon as possible following release of
updated information from the EPA.

8
Summary and Conclusions
• While site-specific whole PAH mixture cancer potency data are rarely available, this type of an
analysis is likely to provide the most accurate assessment of the cancer potency of a PAH
mixture.
• If a site-specific or similar surrogate whole mixture evaluation is available, it is doubtful that a
summation of individual cPAH potencies (cPAH relative potency evaluation) will result in a
more accurate potency estimate.
• When data are not available to allow use of a whole mixture potency evaluation (site-specific or a
similar surrogate), a relative potency evaluation can provide a reasonable estimate of the cancer
potency of an environmental PAH mixture.
• It is important that laboratory detection limits for individual cPAHs should be less than or equal
to concentrations of interest (typically, five percent of the media-specific B[a]P criterion divided
by the relative potency factor (RPF)).
• It is important that site-specific cPAH criteria and all cancer risk evaluations should include
coordinated age-dependent exposure and sensitivity adjustments
(MDH, 2010).
• For initial, screening or default evaluation of a site it is reasonable to assume that a PAH mixture
(that has not undergone significant fractionation in the environment) is about seven (7) times
more potent than suggested by the concentration of B[a]P in that media.
• Even when a whole mixture potency or default evaluation is used to evaluate mixture potency, it
is beneficial to analyze a few samples for the MDH Priority cPAHs so that cPAH fingerprints and
default multipliers can be developed over time for different PAH source-types.
MDH Recommendations
• Determine the availability of site-specific or surrogate whole mixture cancer potency data and use
these data to characterize the potency of site samples.
• If there are no site-specific or appropriate surrogate whole mixture cancer potency data, use the
relative potency method or the default method to estimate sample cancer potency.
• Relative potency factors for MDH Priority cPAHs in Table 2 and Equation 4 should be used to
calculate sample cPAH cancer potencies and the result should be compared with the appropriate
media-specific, age-adjusted B[a]P criterion. Use the relative potency factor approach for
environmental mixtures that have undergone significant fractionation in the environment.
• For calculation of PAH mixture default cancer potency multiply the B[a]P concentration of a
sample by seven (7) using Equation 3 and compare the value to the appropriate media-specific,
age-adjusted B[a]P criterion.
• Use an adult cancer slope factor for B[a]P of 1.7 (mg/kg/d)
-1
and an inhalation exposure adult
cancer slope factor of 3.9 (mg/kg/d)
-1
[equivalent to an adult unit risk of 1.1x10
-3
(µg/m
3
)
-1
]. Use
of age-dependent exposure estimates in combination with age-dependent adjustments to potency
for all cPAH risk calculations.
• Prior to chemical analysis, confirm that the laboratory detection limits for cPAHs are equal to or
below levels of interest using Equation 5. If detection limits are above levels of interest conduct
an uncertainty analysis of the impact that this could have on potency evaluations.
• If sufficient cPAH or surrogate mixture potency data are available, MDH may support the use of
source-type specific default cancer potency factors or multipliers to use at sites with similar
sources.
• Individual cPAHs frequently occur as part of an environmental mixture. The individual potency
factors are not intended to be used as the basis of cancer slope factor calculations, rather their
purpose is to be used as part of a cumulative risk assessment to estimate the cancer risk from a
mixture of cPAHs.
9
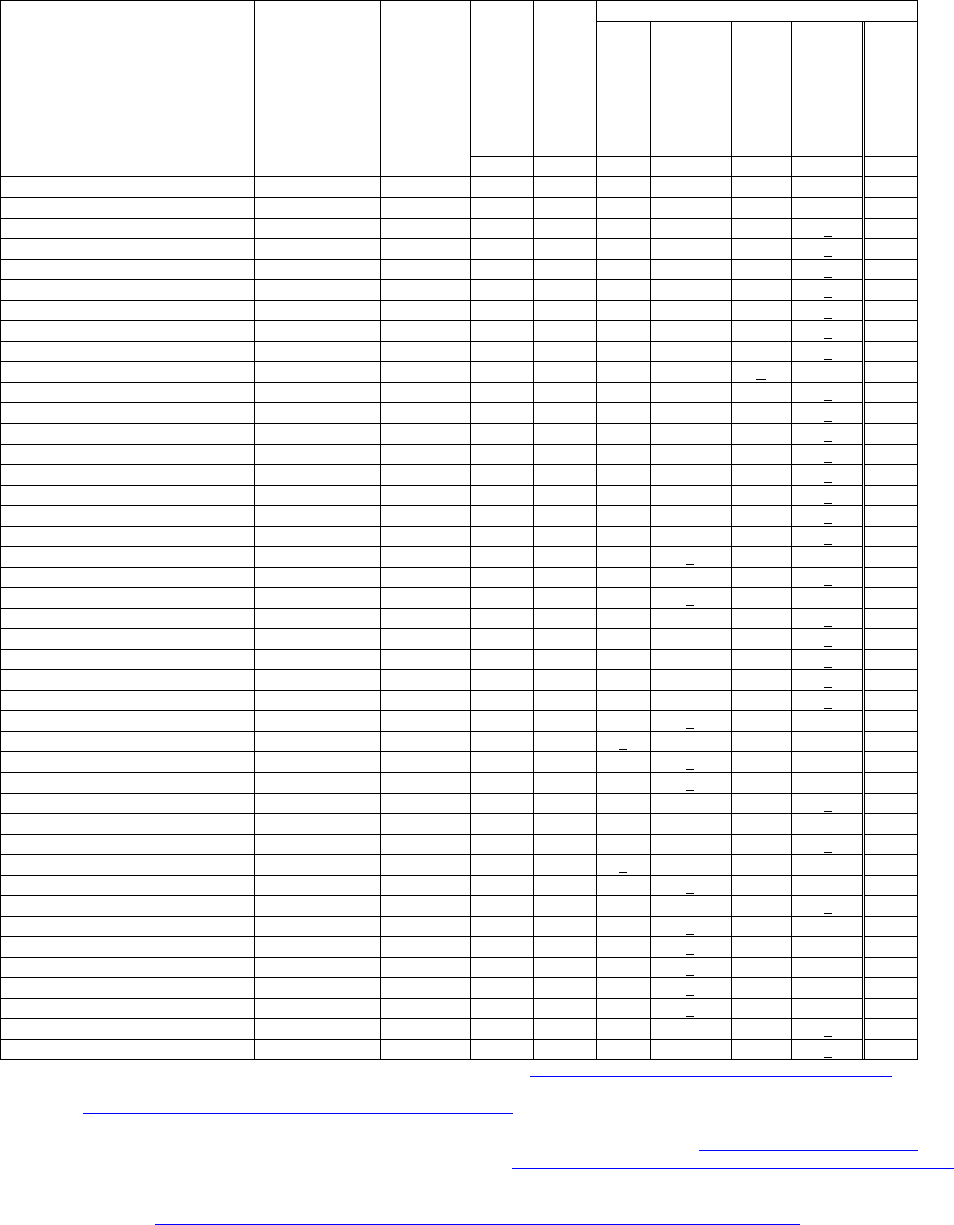
Table 1: PAH Lists
Polycyclic Aromatic Hydrocarbons
from selected Environmental and
Public Health Agency Lists
CAS #
Molecular
Weight
* - EPA - 15
‡- EPA – RTK,TRI
cPAH Lists
¥ - OEHHA CSF
§- OEHHA 2009
(MDH 2001)
¶ EC “15+1”
£ - EPA Draft RPF
# - MDH Priority
Total # PAHs = 43
15
22
7
24
16
26
19
Acenaphthene
83-32-9
154.21
*
Acenaphthylene
208-96-8
152.2
*
Anthanthrene
191-26-4
276.34
£
#
Anthracene
120-12-7
178.23
*
£
Benz[a]anthracene
56-55-3
228.29
*
ǂ
§
¶
£
#
11H-Benz[b,c]aceanthrylene
202-94-8
240.3
£
Benz[e]aceanthrylene
199-54-2
252.32
£
Benz[j]aceanthryIene
202-33-5
252.32
£
Benz[l]aceanthrylene
211-91-6
252.32
£
Benzo[a]pyrene
50-32-8
252.31
*
ǂ
¥
¶
#
Benzo[b]fluoranthene
205-99-2
252.32
*
ǂ
§
¶
£
#
Benzo[c]fluorene
205-12-9
216.28
¶
£
#
Benzo[g,h,i]perylene
191-24-2
276.34
*
ǂ
¶
£
#
Benzo[j]fluoranthene
205-82-3
252.32
ǂ
§
¶
£
#
Benzo[k]fluoranthene
207-08-9
252.32
*
ǂ
§
¶
£
#
Chrysene
218-01-9
228.29
*
ǂ
§
¶
£
#
Cyclopenta[c,d]pyrene
27208-37-3
226.28
¶
£
#
4H-Cyclopenta[d,e,f]chrysene
202-98-2
240.3
£
Dibenz[a,h]acridine
226-36-8
279.33
ǂ
§
Dibenz[a,h]anthracene
53-70-3
278.35
*
ǂ
¥
§
¶
£
#
Dibenz[a,j]acridine
224-42-0
279.33
ǂ
§
Dibenzo[a,e]fluoranthene
5385-75-1
302.38
ǂ
£
Dibenzo[a,e]pyrene
192-65-4
302.38
ǂ
§
¶
£
#
Dibenzo[a,h]pyrene
189-64-0
302.38
ǂ
§
¶
£
#
Dibenzo[a,i]pyrene
189-55-9
302.38
ǂ
§
¶
£
#
Dibenzo[a,l]pyrene
191-30-0
302.38
ǂ
§
¶
£
#
7H-Dibenzo[c,g]carbazole
194-59-2
267.32
ǂ
§
7,12-Dimethylbenz[a]anthracene
57-97-6
256.34
ǂ
¥
§
1,6-Dinitropyrene
42397-64-8
292.25
§
1,8-Dinitropyrene
42397-65-9
292.25
§
Fluoranthene
206-44-0
202.26
*
ǂ
£
#
Fluorene
86-73-7
166.22
*
Indeno[1,2,3-cd]pyrene
193-39-5
276.34
*
ǂ
§
¶
£
#
3-Methylcholanthrene
56-49-5
268.35
ǂ
¥
§
5-Methylchrysene
3697-24-3
242.31
ǂ
¥
§
¶
#
Naphtho[2,3-e]pyrene
193-09-9
302.38
£
5-Nitroacenaphthene
602-87-9
199.21
¥
§
6-Nitrochrysene
7496-02-8
273.29
¥
§
#
2-Nitrofluorene
607-57-8
211.22
§
1-Nitropyrene
5522-43-0
247.25
ǂ
§
4-Nitropyrene
57835-92-4
247.25
§
Phenanthrene
85-01-8
178.23
*
£
Pyrene
129-00-0
202.26
*
£
*- EPA-15. US EPA - PAHs on the Clean Water Act List of Priority Pollutants - http://water.epa.gov/scitech/methods/cwa/pollutants.cfm
ǂ - EPA-RTK,TRI. US EPA (2001) - Right-To-Know Act: Polycyclic Aromatic Compounds Category.
http://www.epa.gov/sites/production/files/documents/2001pacs.pdf
.
cPAH Lists Agency carcinogenic PAH Lists:
¥ - OEHHA CSF. California Office of Health Hazard Assessment PAH Cancer Slope Factors - http://oehha.ca.gov/tcdb/index.asp
§ - OEHHA 2009. California Office of Health Hazard Assessment http://www.oehha.ca.gov/air/hot_spots/2009/TSDCancerPotency.pdf
MDH, 2001 - Previous Minnesota Department of Health cPAH Guidance Memo
¶ - EC "15+1". European Commission - Commission Regulation (EC) No 1881/2006 -
http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CONSLEG:2006R1881:20100701:EN:PDF
£ - EPA Draft RPF - PAHs with Relative Potency Factors from US EPA Draft Document (potency =0 included) - (US EPA, 2010)
# - MDH Priority – List of MDH Priority Carcinogenic Polycyclic Aromatic Hydrocarbons and Relative Potency Factors, 2014
10
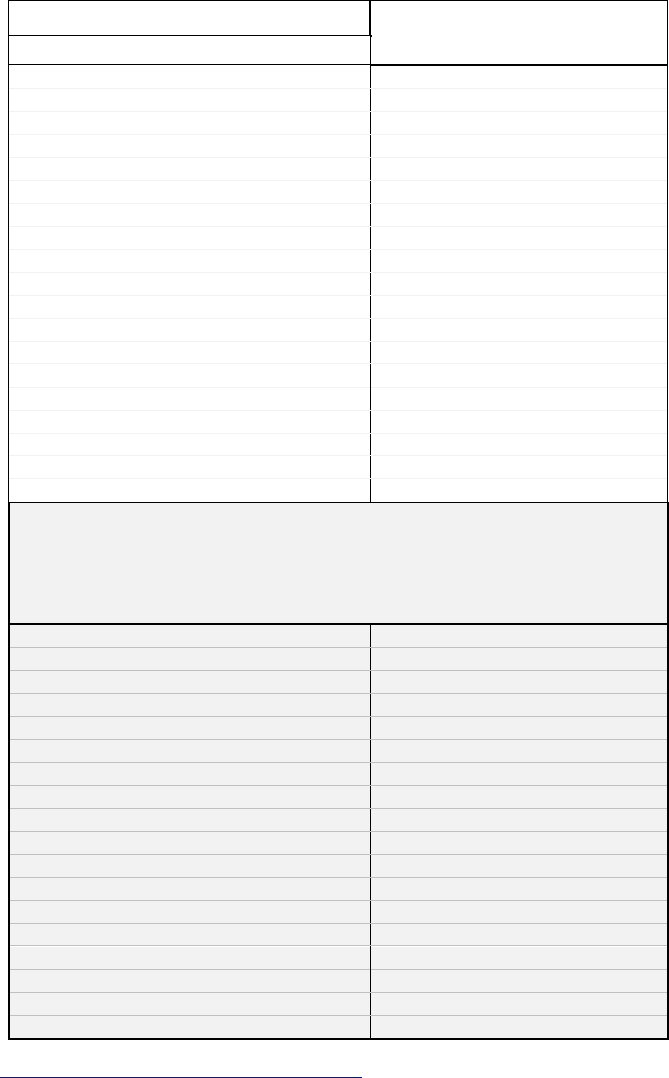
Table 2: MDH Priority Carcinogenic Polycyclic Aromatic
Hydrocarbons and Relative Potency Factors
MDH Priority cPAHs
Relative Potency Factors
n = 19
Anthanthrene
0.4
Benz[a]anthracene
0.2
Benzo[a]pyrene
1
Benzo[b]fluoranthene
0.8
Benzo[c]fluorene
20
Benzo[g,h,i]perylene
0.009
Benzo[j]fluoranthene
0.3
Benzo[k]fluoranthene
0.03
Chrysene
0.1
Cyclopenta[c,d]pyrene
0.4
Dibenz[a,h]anthracene
10
Dibenzo[a,e]pyrene
0.4
Dibenzo[a,h]pyrene
0.9
Dibenzo[a,i]pyrene
0.6
Dibenzo[a,l]pyrene
30
Fluoranthene
0.08
Indeno[1,2,3-cd]pyrene
0.07
5-Methylchrysene
1
6-Nitrochrysene
10
Secondary cPAHs
(MDH guidance does not consider these Priority PAHs at this time:
analytical issues, toxicological or environmental database
uncertainties, or low projected impact on mixture potency)
11H-Benz[b,c]aceanthrylene
0.05
Benz[e]aceanthrylene
0.8
Benz[j]aceanthryIene
60
Benz[l]aceanthrylene
5
4H-Cyclopenta[d,e,f]chrysene
0.3
Dibenz[a,h]acridine
0.1
Dibenz[a,j]acridine
0.1
Dibenzo[a,e]fluoranthene
0.9
7H-Dibenzo[c,g]carbazole
1
7,12-Dimethylbenz[a]anthracene
64 (air only) 150 (oral/derm)
1,6-Dinitropyrene
10
1,8-Dinitropyrene
1
3-Methylcholanthrene
5.6 (air only) 13 (oral/derm)
Naphtho[2,3-e]pyrene
0.3
5-Nitroacenaphthene
0.02
2-Nitrofluorene
0.01
1-Nitropyrene
0.1
4-Nitropyrene
0.1
Bolded PAHs from US EPA Clean Water Act List of Priority Pollutants -
http://water.epa.gov/scitech/methods/cwa/pollutants.cfm
Italicized Potency Equivalence calculated from specific compound cancer slope factors (OEHHA Toxicity Criteria
Database), not based on a direct relative potency comparison from a single study.
11
References
ATSDR (1995) Toxicological Profile for Polycyclic Aromatic Hydrocarbons (PAHs).
http://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=122&tid=25
CA OEHHA (2009). Appendix B. Chemical-specific summaries of the information used to derive unit risk and
cancer potency values., Air Toxics Hot Spots Risk Assessment Guidelines Part II: Technical Support Document
for Cancer Potency Factors, California Office of Environmental Health Hazard Assessment, Sacramento, CA.
May 2009. http://www.oehha.ca.gov/air/hot_spots/2009/AppendixB.pdf
CA OEHHA (2010). Public Health Goals for Chemicals in Drinking Water: Benzo(a)pyrene. Technical Support
Document, California Office of Environmental Health Hazard Assessment, Sacramento, CA. September 2010.
http://oehha.ca.gov/water/phg/pdf/091610Benzopyrene.pdf
Culp, S., D. Gaylor, W. Sheldon, L. Goldstein and F. Beland (1998). A comparison of the tumors induced by coal
tar and benzo [a] pyrene in a 2-year bioassay. Carcinogenesis 19(1): 117.
European Commission - European Food Safety Authority (2008). Findings of the EFSA Data Collection on
Polycyclic Aromatic Hydrocarbons in Food. A Report from the Unit of Data Collection and Exposure on a
Request from the European Commission. First issued on 29 June 2007 and revised on 31 July 2008.
European Commission - Scientific Committee on Food (2002). Opinion of the Scientific Committee on Food on
the risks to human health of Polycyclic Aromatic Hydrocarbons in food. Report, Health And Consumer Protection
Directorate-General, Scientific Committee on Food, Brussels, Belgium. SCF/CS/CNTM/PAH/29 Final,
December 4, 2002.
Gaylor, D., S. Culp, L. Goldstein and F. Beland (2000). Cancer Risk Estimation for Mixtures of Coal Tars. Risk
Analysis 20(1): 81-86.
IARC (1973). Certain Polycyclic Aromatic Hydrocarbons and Heterocyclic Compounds,
http://monographs.iarc.fr/ENG/Monographs/vol1-42/mono3.pdf
IARC (2013). Bitumens and Bitumen Emissions, and Some N- and S-Heterocyclic Polycyclic Aromatic
Hydrocarbons http://monographs.iarc.fr/ENG/Monographs/vol103/mono103.pdf
MDH (2010). Risk Assessment Advice for Incorporating Early-Life Sensitivity into Cancer Risk Assessments for
Linear Carcinogens. http://www.health.state.mn.us/divs/eh/risk/guidance/adafrecmd.pdf
Schneider, K., M. Roller, F. Kalberlah and U. Schuhmacher-Wolz (2002). Cancer risk assessment for oral
exposure to PAH mixtures. Journal of Applied Toxicology 22(1): 73-83. http://dx.doi.org/10.1002/jat.828
U.S. Environmental Protection Agency (2010). [DRAFT] Development Of A Relative Potency Factor (RPF)
Approach For Polycyclic Aromatic Hydrocarbon (PAH) Mixtures. Office of Research and Development, National
Center for Environmental Assessment. EPA/635/R-08/012A, February, 2010.
http://ofmpub.epa.gov/eims/eimscomm.getfile?p_download_id=494851
US Environmental Protection Agency (2005). Supplemental Guidance for Assessing Susceptibility from Early-
Life Exposures to Carcinogens. Risk Assessment Forum. EPA/630/R-03/003F, March, 2005.
http://www3.epa.gov/ttn/atw/childrens_supplement_final.pdf
US Environmental Protection Agency (2009). Polycyclic Aromatic Hydrocarbons.
http://www.epa.gov/sites/production/files/2014-03/documents/pahs_factsheet_cdc_2013.pdf

