WEBINAR ON
NOVEL FOOD
APPLICATIONS
26 OCTOBER 2023
WEBINAR | 26/10/2023

WELCOME AND INTRODUCTION
• To help applicants better understand the
procedure for novel food applications and the
support activities provided by EFSA during the
pre-submission phase
• To present the most common issues identified
during the suitability check of a novel food
application, based on two years of experience
with implementing the Transparency
Regulation
• To address questions from participants
regarding EFSA’s procedures and common
issues related to novel food applications
2
OBJECTIVES
WHO WE ARE
• Presenters
Patricia Romero Fernandez, Silvia Federici
• Contributors
Federico Morreale, Irene Baratto, Ellen Van
Haver, Costanza Casiraghi, Catalina Manieu,
Sara De Berardis, Ilaria Mangerini, Oscar
Gonzalez, Maja Gorgieva
• Moderator: Goran Kumric
• Event organizer: Carolina Vastola
• Event producer: Marina Canale
• Technical support: Margherita Olivieri
AGENDA
Time
Topic
Speaker
10.30-10.35
Introduction and outline of the webinar
Goran Kumric
10.35-10.55
Overview of the procedure for novel food applications and EFSA
support activities
Silvia Federici
10.55-11.20
Most common issues identified during the suitability check
Patricia Romero Fernández
11.20-11.40
Q&A session
11.40-11.45
Conclusion
Goran Kumric
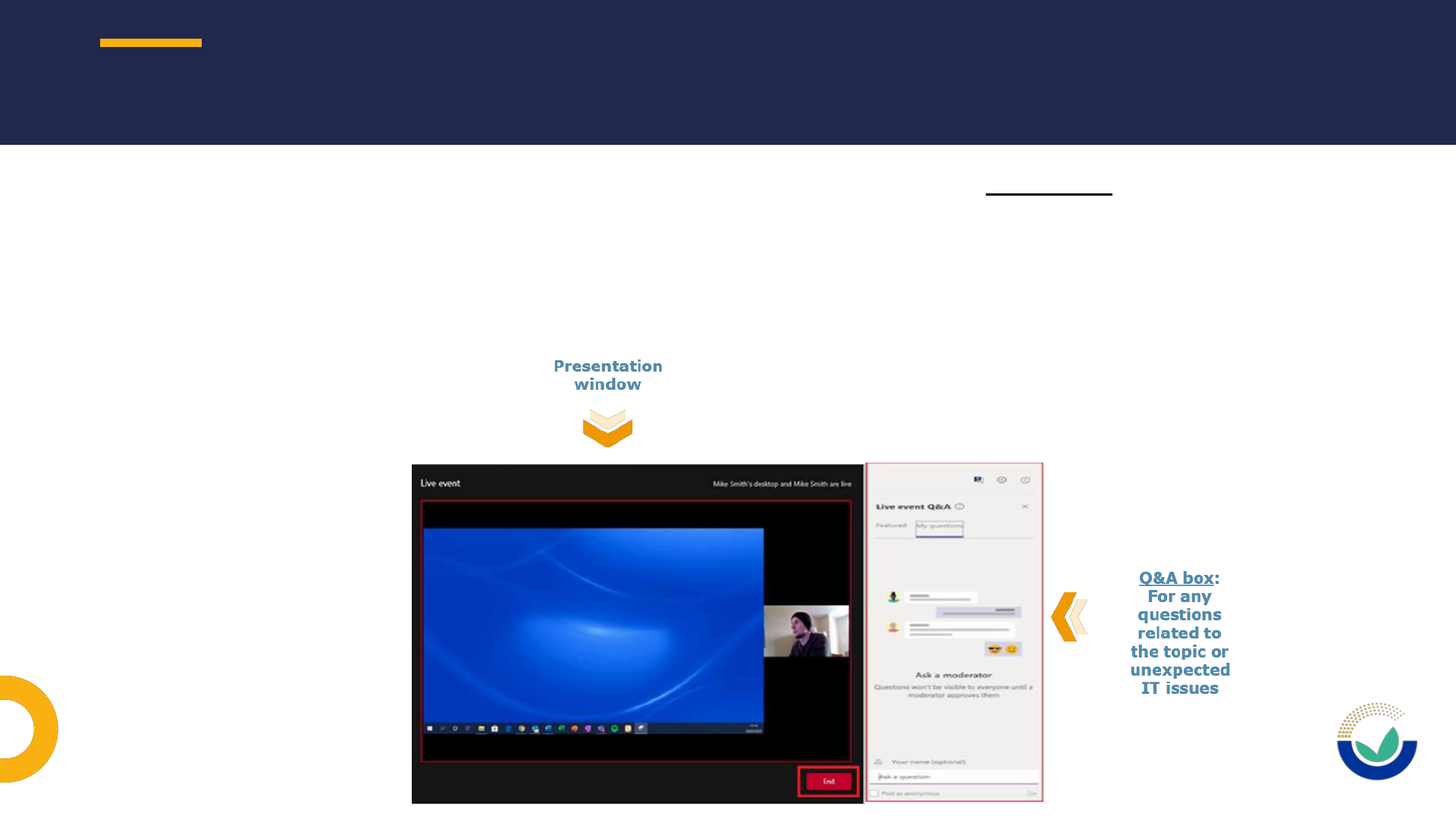
HOUSE KEEPING RULES
• You are automatically connected to the audio broadcast. One-way audio (listen only mode).
• The event is in English. Questions should be submitted in English via the Q&A chat;
• This event is being recorded and recordings will be published on EFSA’s website
• After the event, attendees will receive a link to a survey to evaluate EFSA’s event & services
4

5
Novel Food Application Procedure

6
• Under EU Regulations, a novel food is any food that was not consumed to a significant
degree within the Union prior to May 1997
• As a first step, you should first verify whether or not the product you want to place on
the EU market, falls under the scope of Regulation (EU) 2015/2283
• You can consult the Union list of novel foods on the European Commission website, to
see whether your product is an already authorised novel food
• If you are unsure whether your product is a novel food or not, you can contact the
Competent Authority of the Member State where you first intend to place the food on
the market. EFSA is not in charge of deciding whether a food is novel or not
IS YOUR PRODUCT A NOVEL FOOD?
7
• If your product qualifies as novel food, you must submit an application to the European
Commission, complying with Regulation (EU) 2015/2283 and Regulation (EU)
2017/2469
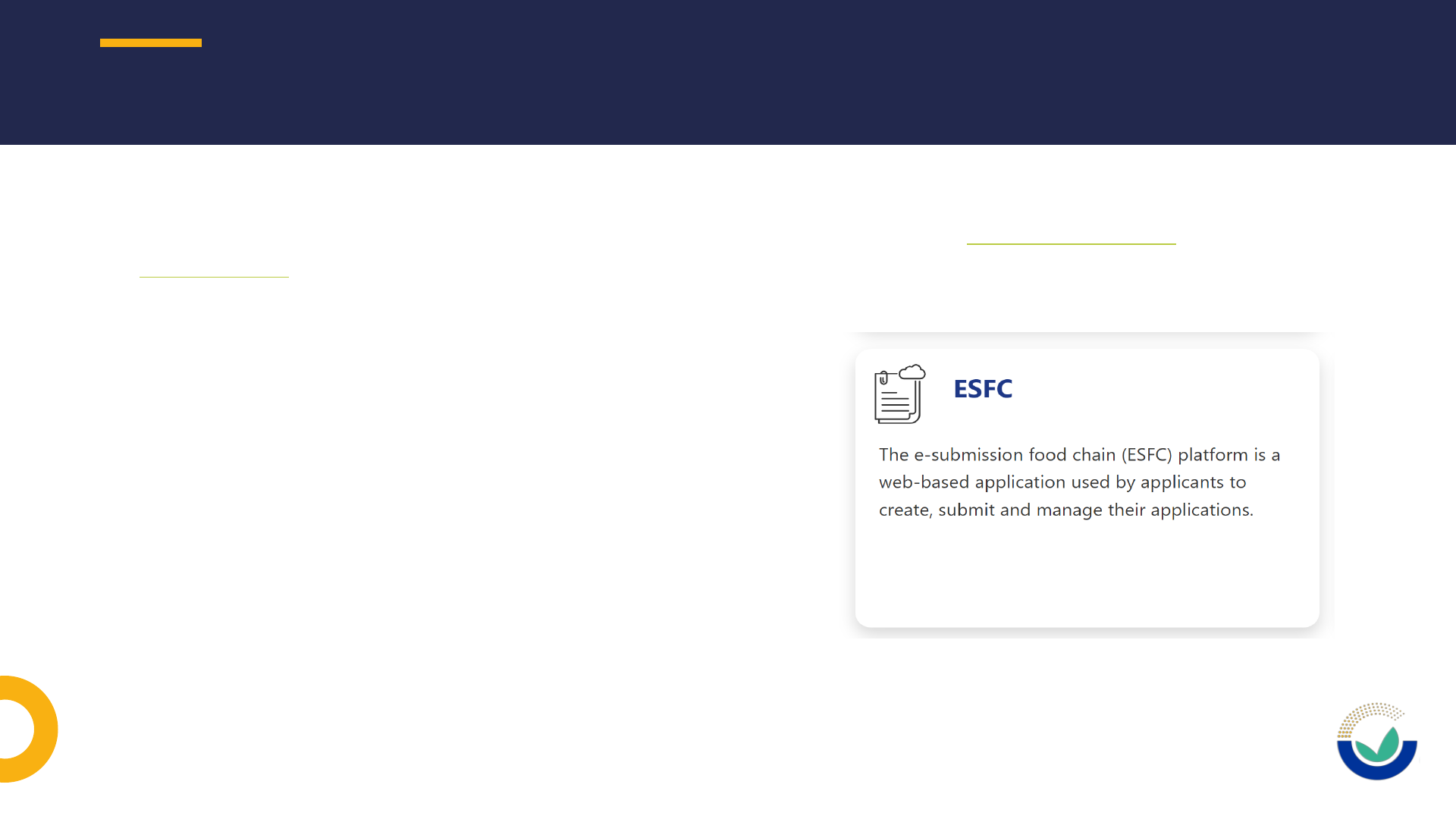
NOVEL FOOD APPLICATION
• The application must be submitted through
the e-submission food chain platform (ESFC)
• No fees will be charged for the submission
and the scientific assessment of an
application
• The application must fulfil the data
requirements described in the EFSA guidance
documents
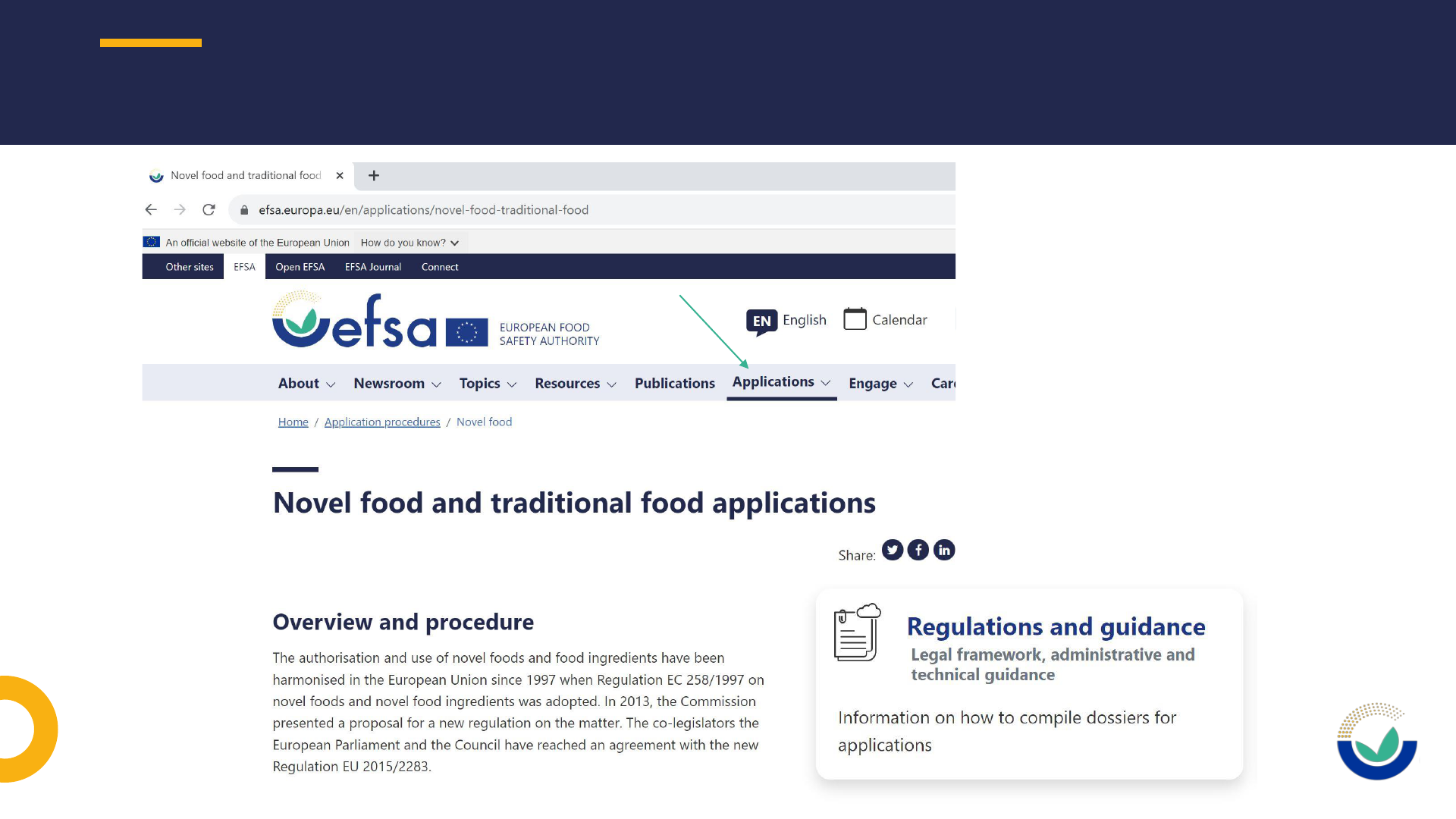
NOVEL FOOD OVERVIEW AND PROCEDURE
8
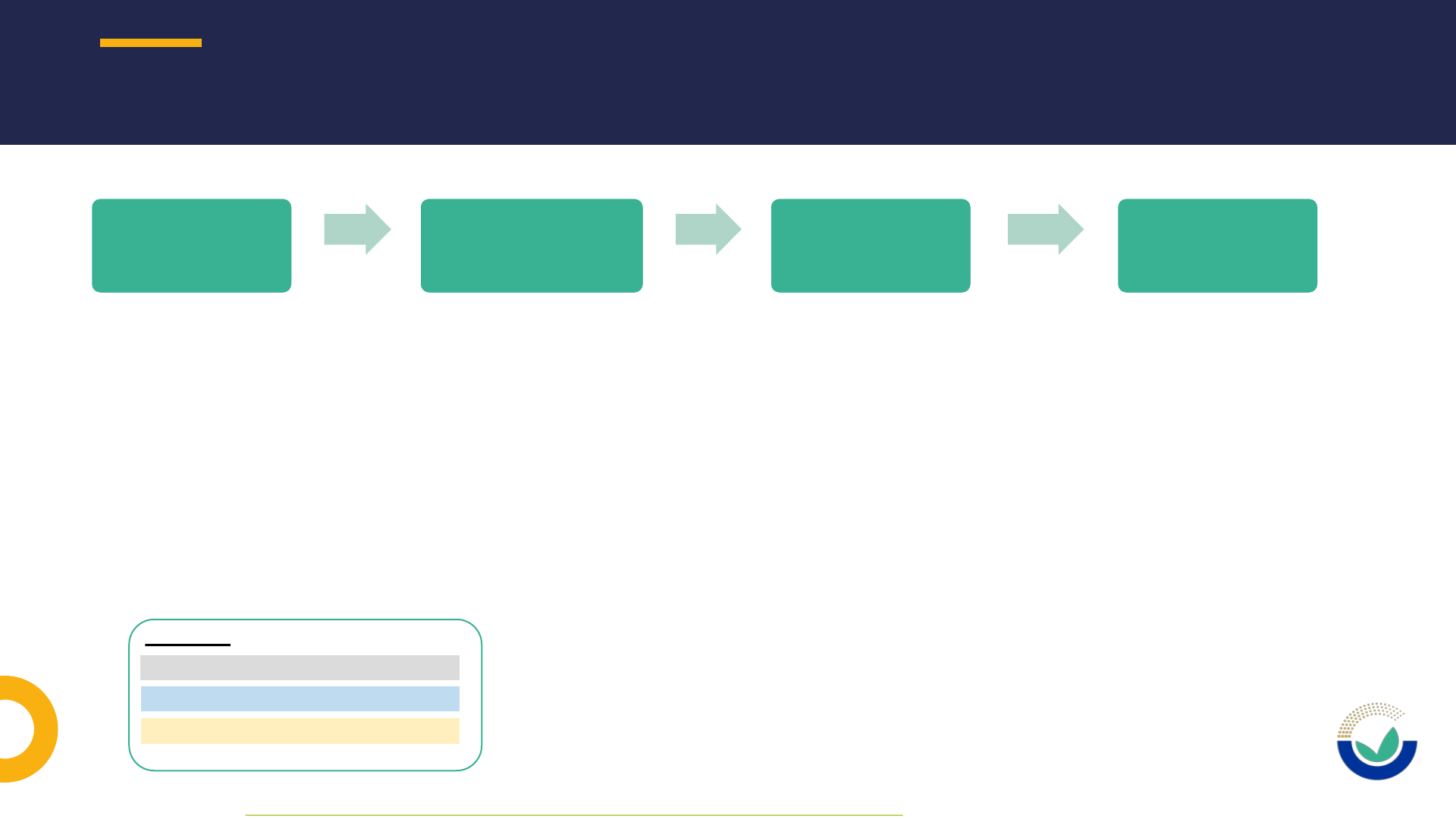
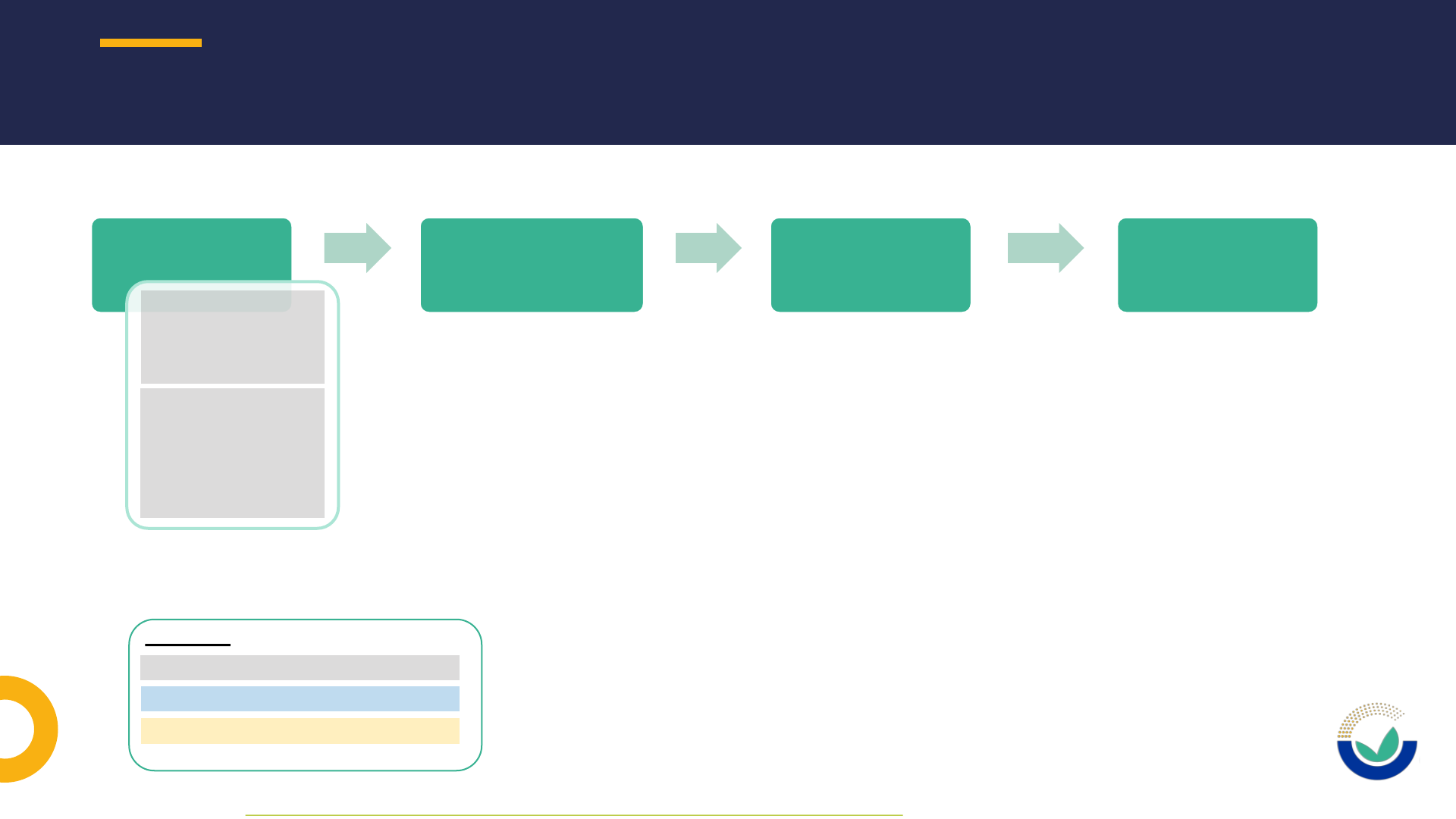
NOVEL FOOD APPLICATION PROCEDURE IN A NUTSHELL
1010
Pre-submission
phase*
Submission phase &
Suitability check
Risk assessment
phase
Post-adoption
phase
Legend:
Potential applicant / applicant
EC: European Commission
EFSA: European Food Safety Authority
* As defined in EFSA’s Practical Arrangements on pre-submission phase and public consultations
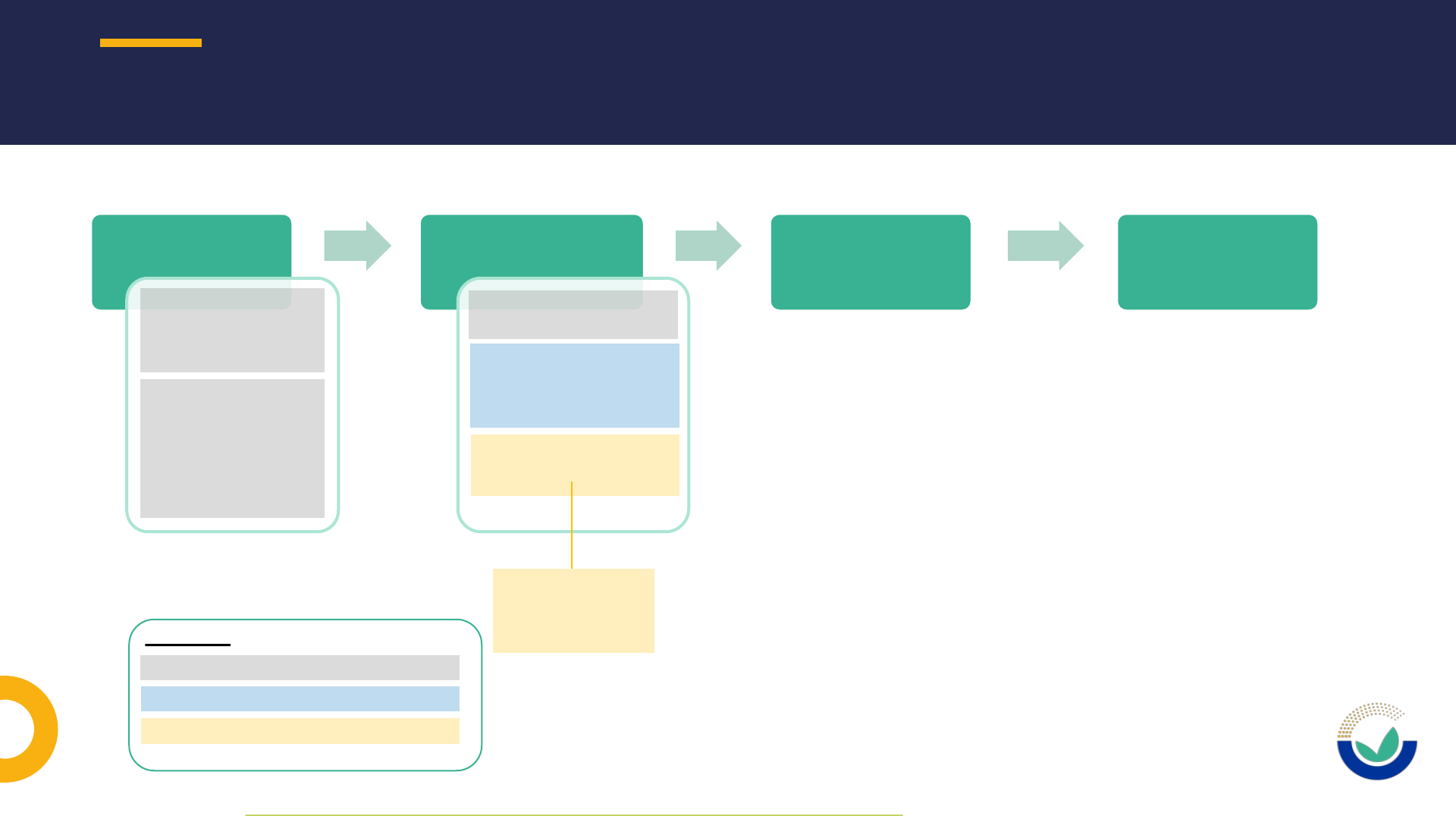
NOVEL FOOD APPLICATION PROCEDURE IN A NUTSHELL
1111
Pre-submission
phase*
• Potential applicant
requests general
pre submission
advice (optional)
• Potential applicant
notifies studies
commissioned or
carried out as of
27/03/21
(mandatory)
Submission phase &
Suitability check
Risk assessment
phase
Post-adoption
phase
Legend:
Potential applicant / applicant
EC: European Commission
EFSA: European Food Safety Authority
* As defined in EFSA’s Practical Arrangements on pre-submission phase and public consultations
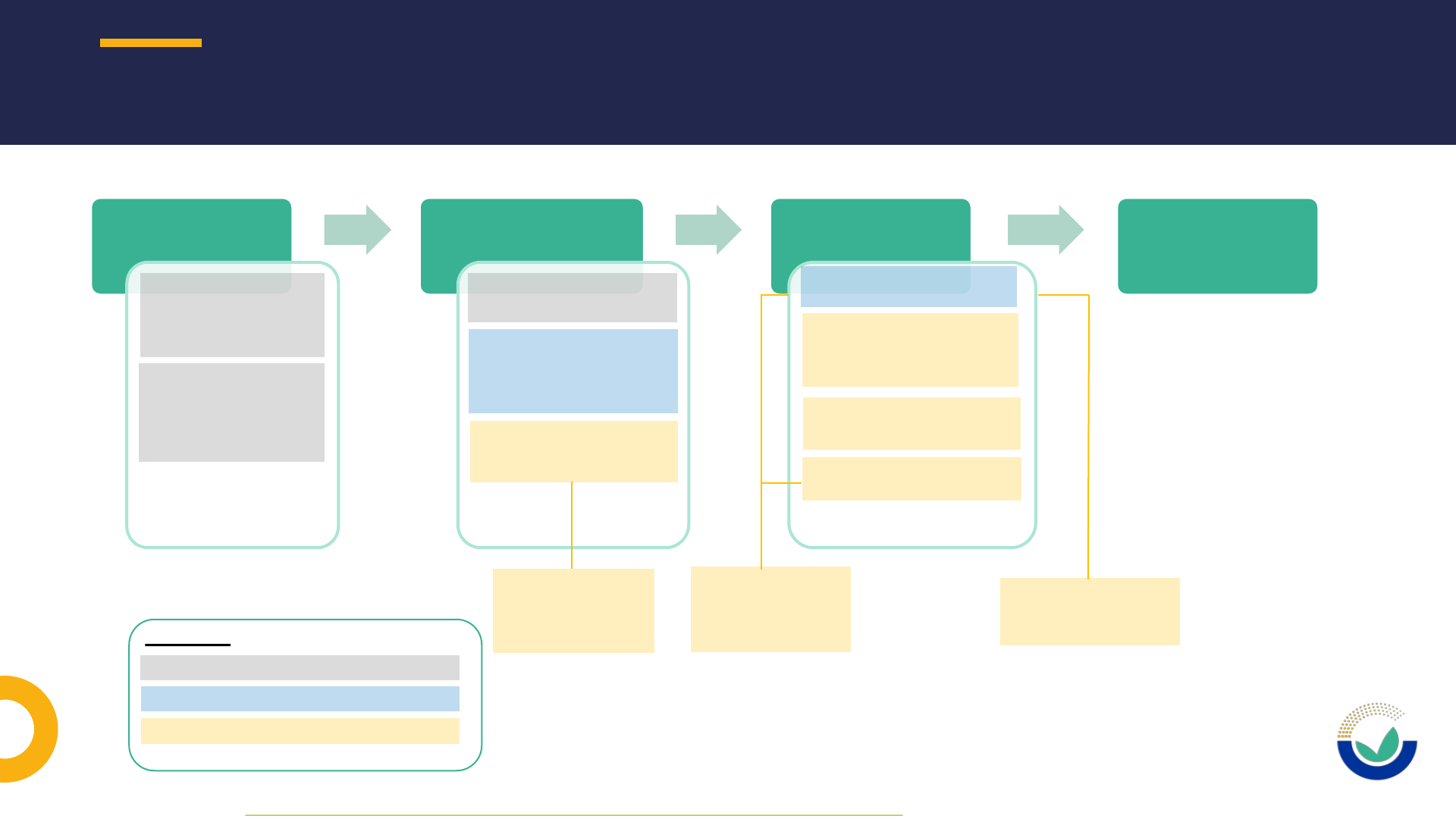
NOVEL FOOD APPLICATION PROCEDURE IN A NUTSHELL
1212
Pre-submission
phase*
•Potential applicant
requests general pre
submission advice
(optional)
•Potential applicant
notifies studies
commissioned or
carried out as of
27/03/21 (mandatory)
Submission phase &
Suitability check
•Applicant submits the
application to EC
•EC may consult EFSA for
the suitability check and
make the application
available to EFSA
•EFSA performs the
suitability check of the
application
Risk assessment
phase
Post-adoption
phase
Legend:
Potential applicant / applicant
EC: European Commission
EFSA: European Food Safety Authority
30 working days for
suitability check +
possible requests for
information
* As defined in EFSA’s Practical Arrangements on pre-submission phase and public consultations
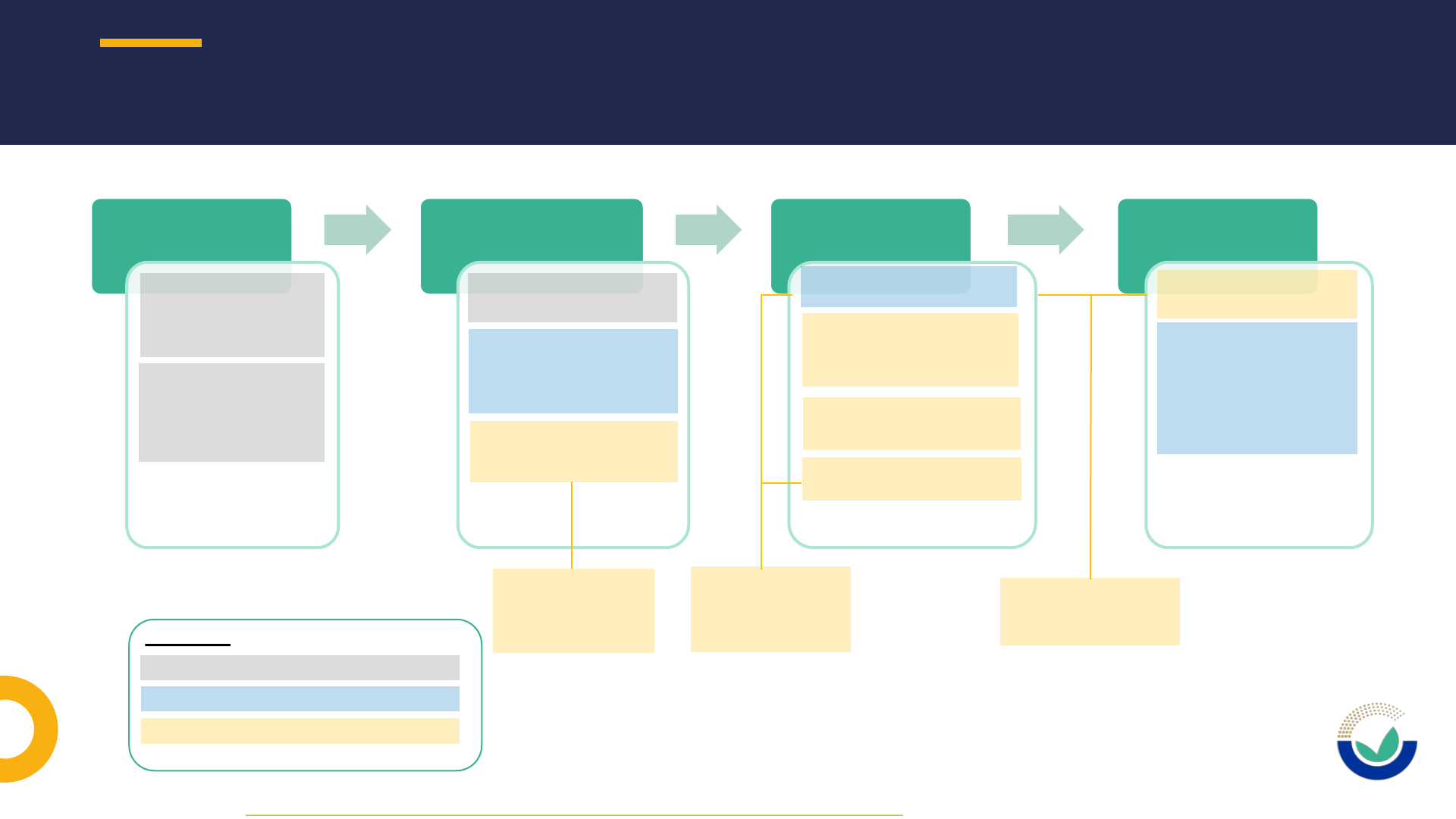
NOVEL FOOD APPLICATION PROCEDURE IN A NUTSHELL
1313
Pre-submission
phase*
•Potential applicant
requests general pre
submission advice
(optional)
•Potential applicant
notifies studies
commissioned or
carried out as of
27/03/21 (mandatory)
Submission phase &
Suitability check
•Applicant submits the
application to EC
•EC may consult EFSA for
the suitability check and
make the application
available to EFSA
•EFSA performs the
suitability check of the
application
Risk assessment
phase
•EC validates the application
•EFSA launches public
consultation on the non-
confidential application
dossier
•EFSA performs thorough
risk assessment
•EFSA Panel adopts the
scientific output
Post-adoption
phase
Legend:
Potential applicant / applicant
EC: European Commission
EFSA: European Food Safety Authority
9 months for risk
assessment +
requests for additional
information
30 working days for
suitability check +
possible requests for
information
* As defined in EFSA’s Practical Arrangements on pre-submission phase and public consultations
Confidentiality decision-
making and proactive
disclosure
NOVEL FOOD APPLICATION PROCEDURE IN A NUTSHELL
1414
Pre-submission
phase*
•Potential applicant
requests general pre
submission advice
(optional)
•Potential applicant
notifies studies
commissioned or
carried out as of
27/03/21 (mandatory)
Submission phase &
Suitability check
•Applicant submits the
application to EC
•EC may consult EFSA for
the suitability check and
make the application
available to EFSA
•EFSA performs the
suitability check of the
application
Risk assessment
phase
•EC validates the application
•EFSA launches public
consultation on the non-
confidential application
dossier
•EFSA performs thorough
risk assessment
•EFSA Panel adopts the
scientific output
Post-adoption
phase
•EFSA publishes the
scientific output
•Based on EFSA's advice
the EC takes the decision
on granting authorisation
of the novel food for
placing it on the EU
market
Confidentiality decision-
making and proactive
disclosure
Legend:
Potential applicant / applicant
EC: European Commission
EFSA: European Food Safety Authority
9 months for risk
assessment +
requests for additional
information
30 working days for
suitability check +
possible requests for
information
* As defined in EFSA’s Practical Arrangements on pre-submission phase and public consultations

15
Pre-submission activities
CONNECT.EFSA PLATFORM
16
Dedicated IT platform for engaging with EFSA
and performing different activities, including:
- Notification of studies
- request General pre-submission advice
- Ask a Question to EFSA
- Participate in public consultations
• Important to register in advance to Connect.EFSA to be ready to perform
activities. Registration will require a verification step by EFSA and may
take a few days
• Important to get familiar with the user guides on how to perform pre-
submission activities: user guide on pre-application ID and user guide on
notification of studies
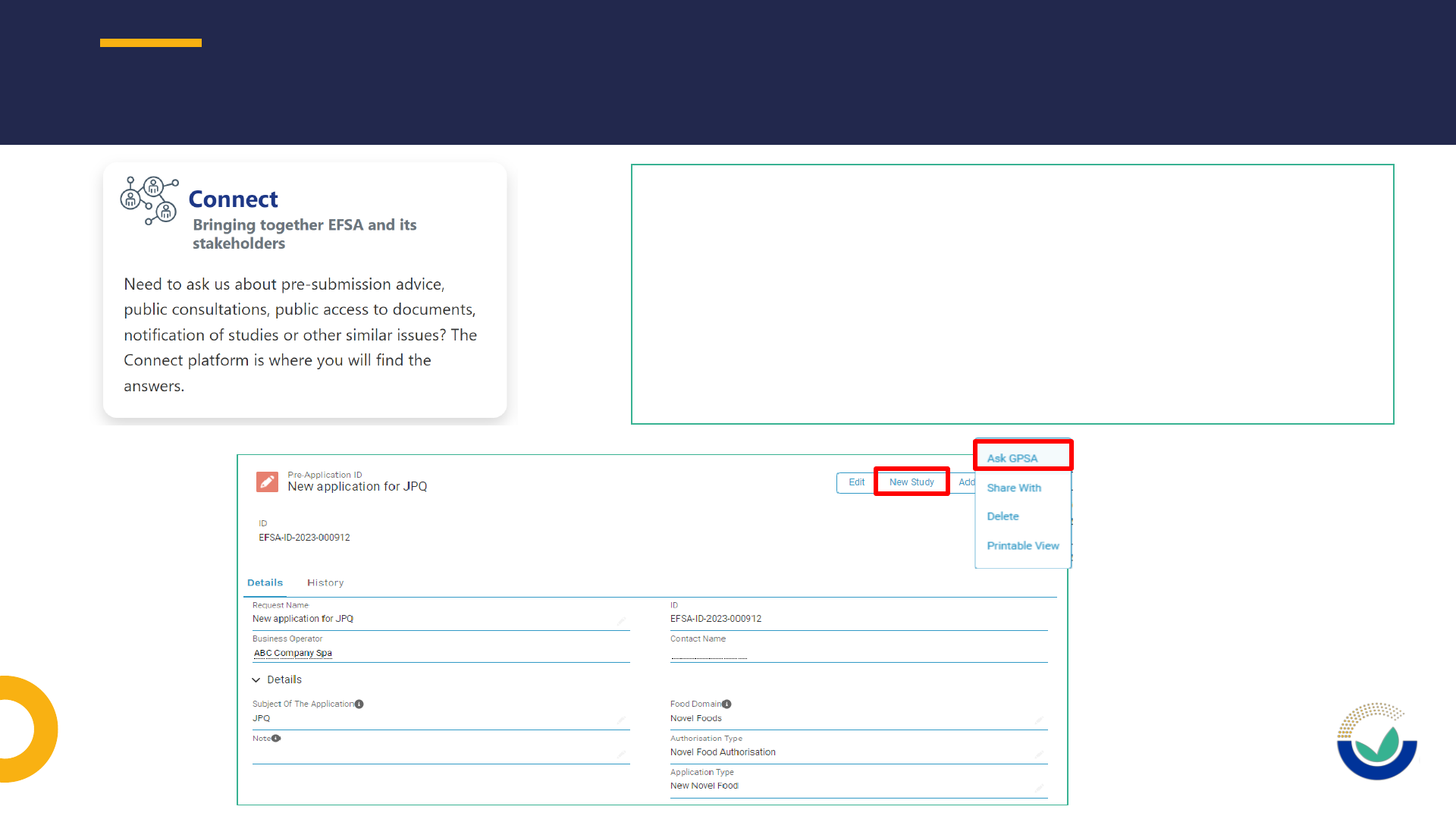
PRE-SUBMISSION ACTIVITIES
17
Prior to initiating any pre-submission activity,
applicants must request a pre-application ID.
Pre-submission activities can be performed from the
pre-application ID:
- Notification of studies
- Request for general pre-submission advice (GPSA)
NOTIFICATION OF STUDIES (NOS)
18
• Potential applicants must notify without delay the studies carried out or commissioned to
laboratories and other external testing facilities for regulatory purposes and for which,
pursuant to Union law, EFSA may or shall be requested to provide a scientific output,
including a scientific opinion
Article 32b of the General Food Law
• Studies must be notified before their starting date. Delayed notification or non-notification
must be justified
• All notified studies must be included in the application. Non-inclusion of notified studies
must be justified
• A justification is needed for studies that have been notified and then withdrawn from the
database
Obligations
• No response is foreseen from EFSA after the submission of a study notification. Compliance
is verified during suitability check
• In case of non-compliance with NoS obligations, the application is declared non-valid
• If the non-valid application is resubmitted, the suitability check will start after 6 months from
the resubmission
Compliance with NoS obligations
NOTIFICATION OF STUDIES (NOS)
19
• After two years’ experience of the notification obligation of Article 32b of the General Food
Law*, it is understood that some analytical measurements should be exempted from the
NoS obligations (Question 4, Part B of updated Q&A)
Update of the Q&A on EFSA Practical Arrangements
• Analysis to assess the identity/composition of a product, including the determination of its
impurities and whole genome sequencing
• Analysis to determine physico-chemical properties
• Method validation studies
Studies exempted with the updated Q&A
• Ongoing applications
• Future applications
Applicability
* Regulation (EC) No 178/2002
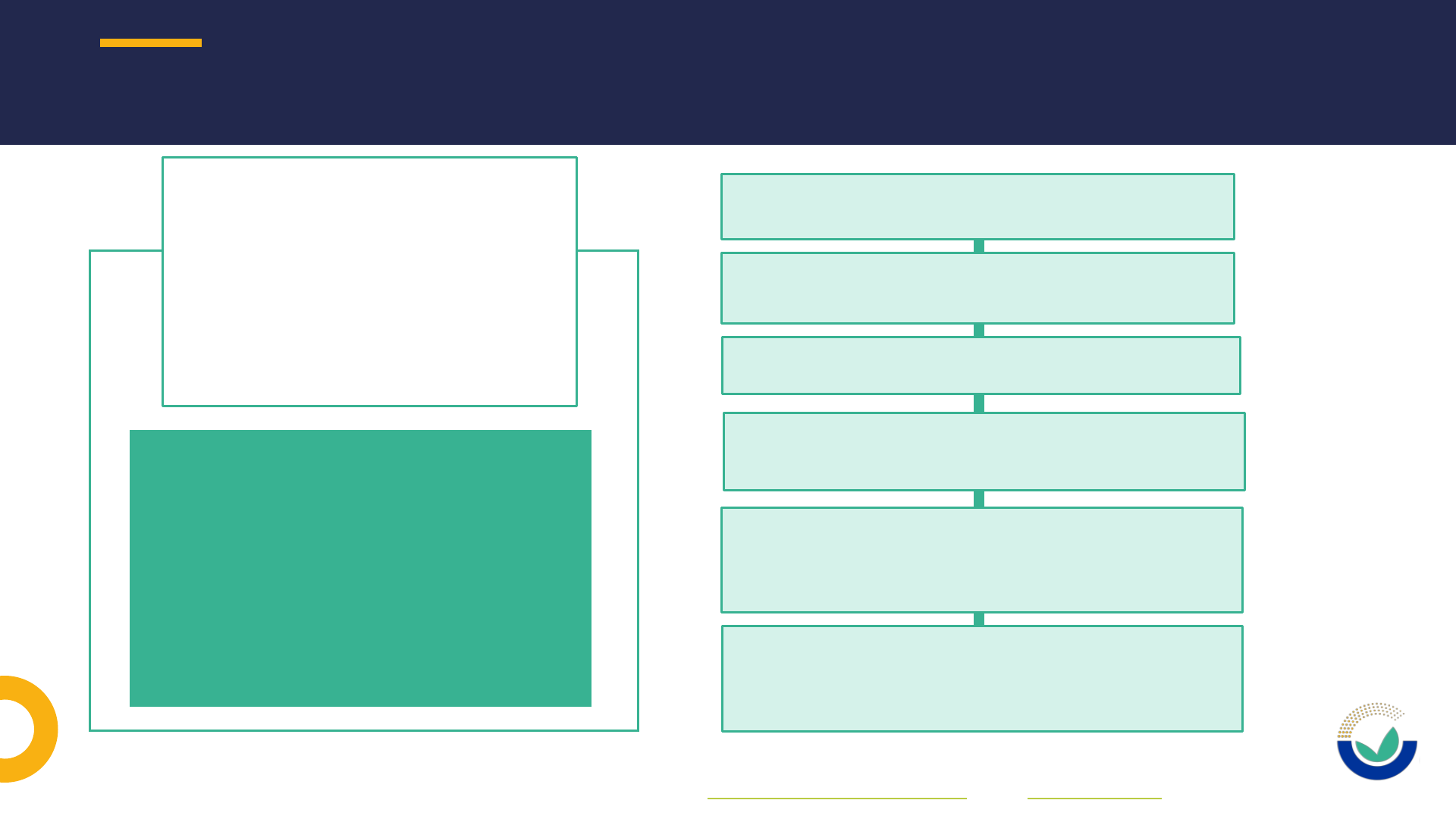
20
General Pre-
Submission
Advice (GPSA)
Non-mandatory (but highly
recommended)
Do you have questions,
while preparing your
application, regarding the
applicable rules and the
content in guidance
documents?
Non-committal for the applicants nor
for EFSA and its Scientific Panels
Available for all kind of applications
Can be requested any time before
sending the application
Only a succinct summary of the
advice is published together with
the application upon its validation
Written advice given within 30
working days (35, if the advice is
given in a telemeeting)
SUPPORT TO APPLICANTS – GENERAL PRE-SUBMISSION ADVICE
More information on how to request a GPSA can be found in section 3.11 of the User guide on pre-application ID and in this video tutorial
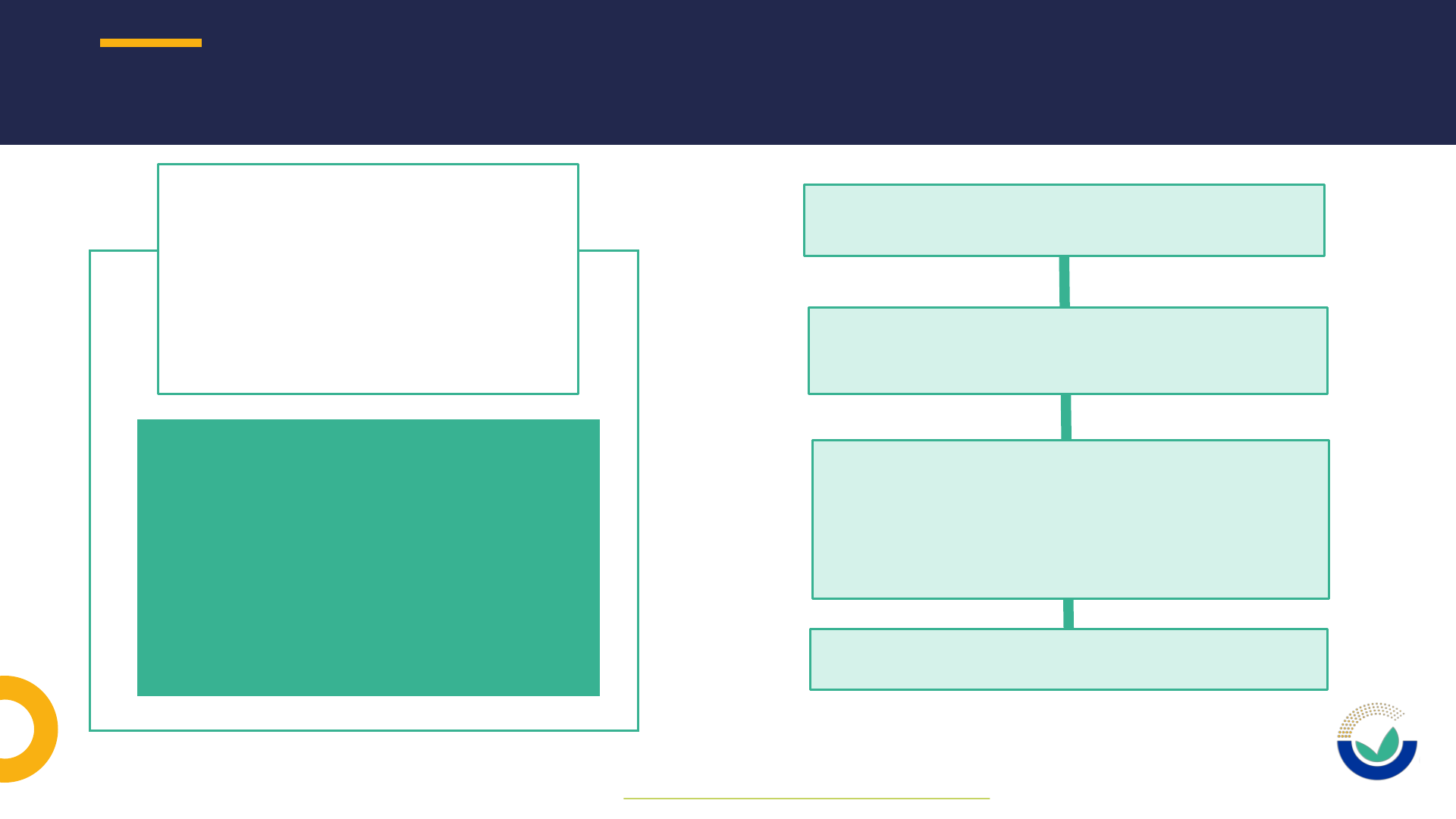
21
Ask a
Question
Not necessarily related to an
application
Do you have questions
regarding the status of
applications, procedural
steps, administrative/
scientific requirements
and/or IT tools*?
Can be submitted anytime, not only
during the pre-submission phase
Questions out of scope are those
related to rules and content for a
future application and to risk
management and interpretation of
EU legislation
Replies given within 15 working days
SUPPORT TO APPLICANTS – ASK A QUESTION
* Requests for technical assistance on ESFC should be addressed to sante-e-submission-food-chain@ec.europa.eu

22
Under development / pilot - General pre-submission advice:
• fast processing: 15 working days instead of 30
• responses in tele-meeting instead of in writing
Already in place - Ask a Question fast processing:
• responses within 7 working days instead of 15
Ask a
question
GPSA
GPSA
SUPPORT TO APPLICANTS – INITIATIVES FOR SMES

23
Most common issues in suitability check
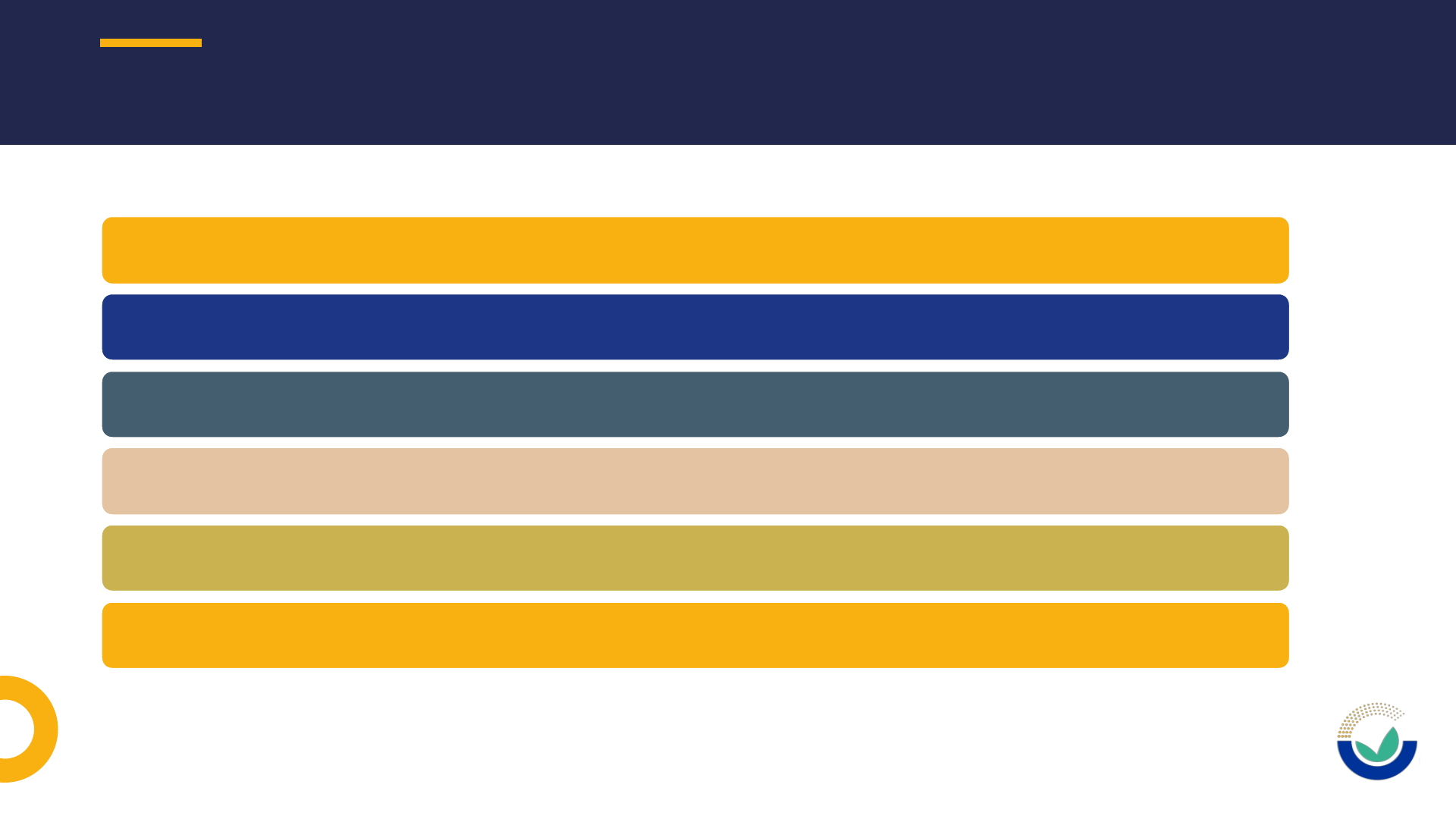
MOST COMMON ISSUES IDENTIFIED IN SUITABILITY CHECK
Missing or incomplete information
Notification of studies (NOS)
Metadata of files in support of an application
Confidentiality and Sanitization
Replying to Request For Information (RFI)
Administrative issues
24
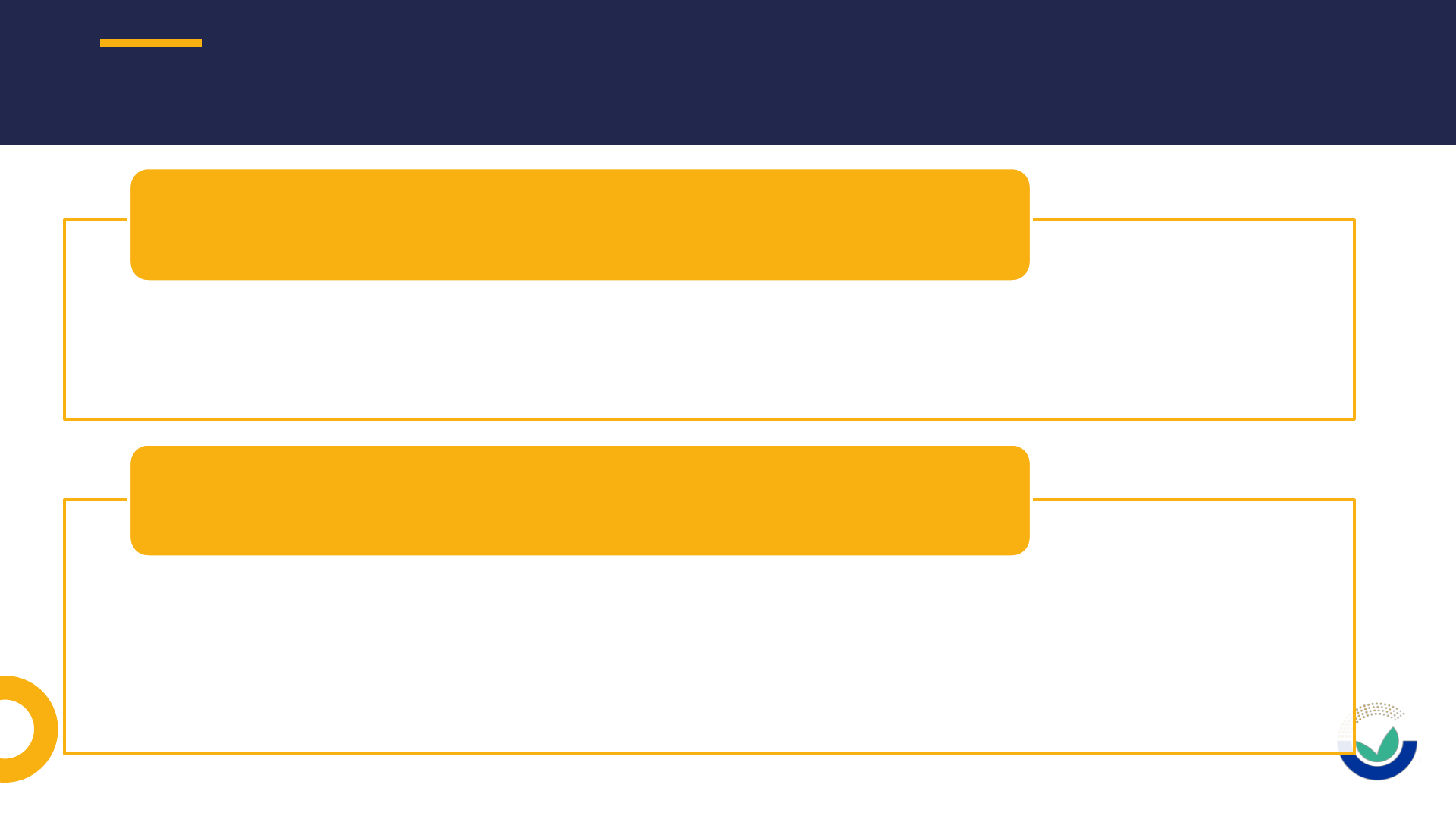
• Description of steps are not detailed enough
• When different productions process are used not all of them are completely described
• Quality control and safety assurance are not properly described
Production process
• Number of batches analysed is not enough (according to the EFSA GD should be
preferably at least 5 batches)
• Information on batches in main text does not match with the certificates of analysis
included or certificates are not provided
• Description and validation of “in house” methods are often not provided
Compositional data
MISSING OR INCOMPLETE INFORMATION
• Literature review not provided
• Search strategy of literature review not provided
History of use of the novel food
• Information does not match with what is included in Cover letter and/or in section
“Proposed entry in the Union list”
Proposed uses and use levels
• Laboratory accreditation or justifications are missing
• GLP certifications are not properly signed or not included
Quality and certifications
MISSING OR INCOMPLETE INFORMATION

NOTIFICATION OF STUDIES (NOS)
27
• Notification should be done before the starting date of the study
• Studies commissioned/performed before 27 March 2021 do not require a
notification, still they can be used in support of an application with simple
justification referring to the commission date
• Notification can be done even if the laboratory is not registered in Connect.EFSA
General information on NoS and tools
• Timepoints part of a study do not need a separate notification (E.g. In Stability
studies no need to notify each timepoint separately)
One study – One notification
• Justifications for non-notification, non-inclusion, withdrawal, delayed notification of
studies or any deviation should be provided as detailed as possible
• Provide all the supporting evidences for any justification as part of the submission
of the application (E.g. proof that EU was not the original market by providing
evidence of the submission of the specific study to another regulatory agency)
Justifications
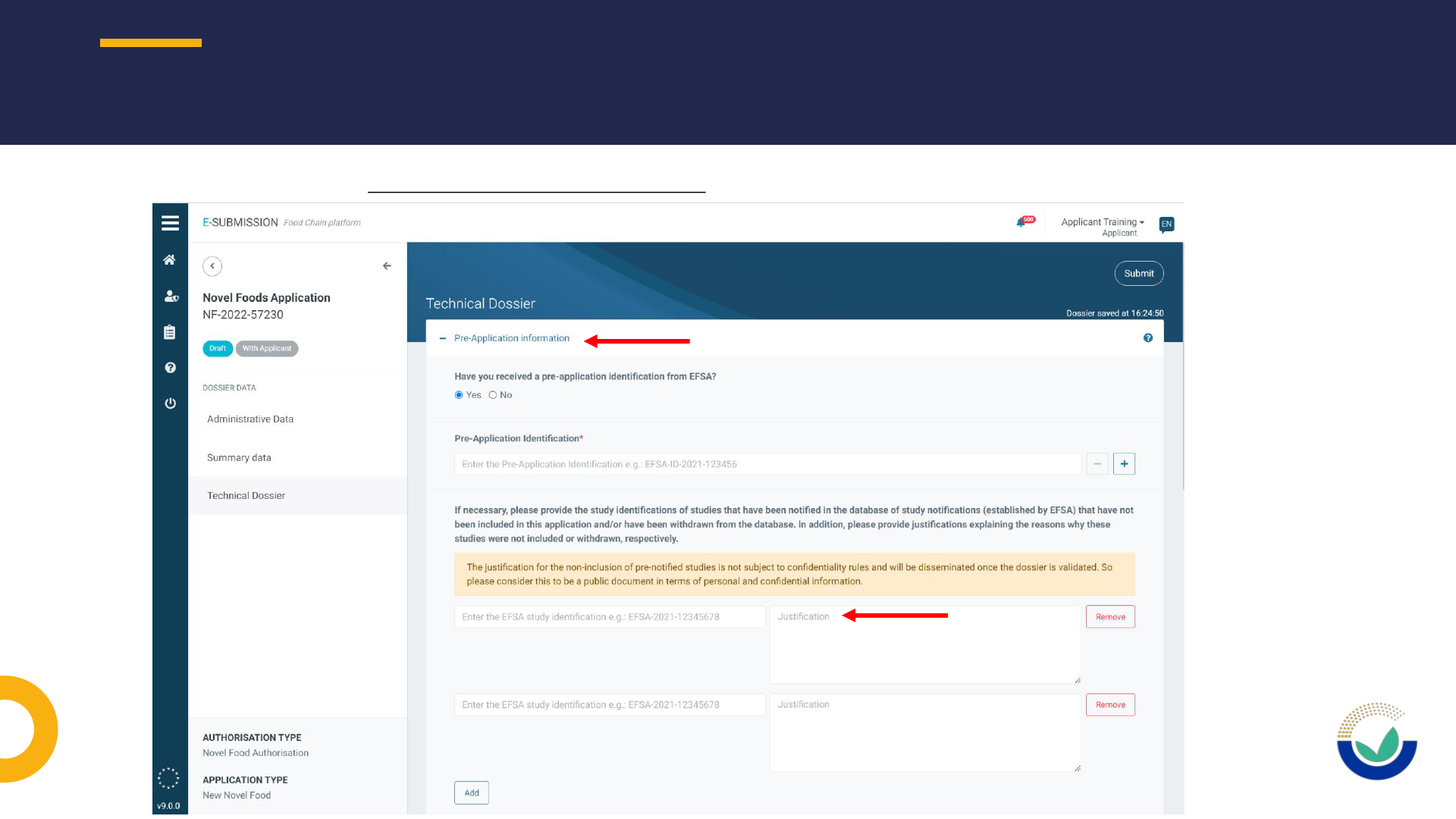
NOTIFICATION OF STUDIES (NOS)
28
• Justifications for non-inclusion of studies notified or justifications for study withdrawn
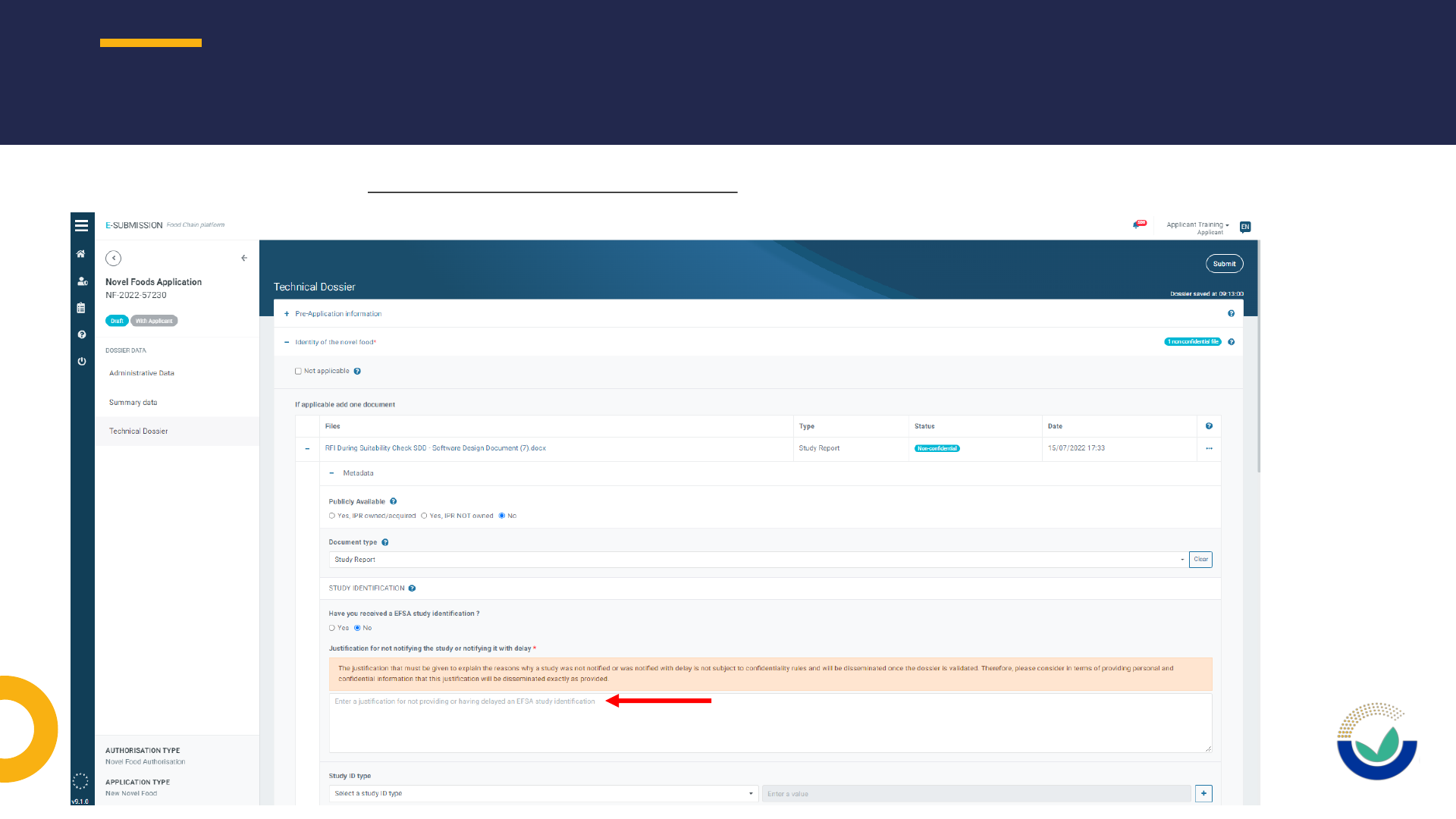
NOTIFICATION OF STUDIES (NOS)
29
• Justifications for non-notification of studies submitted in the dossier
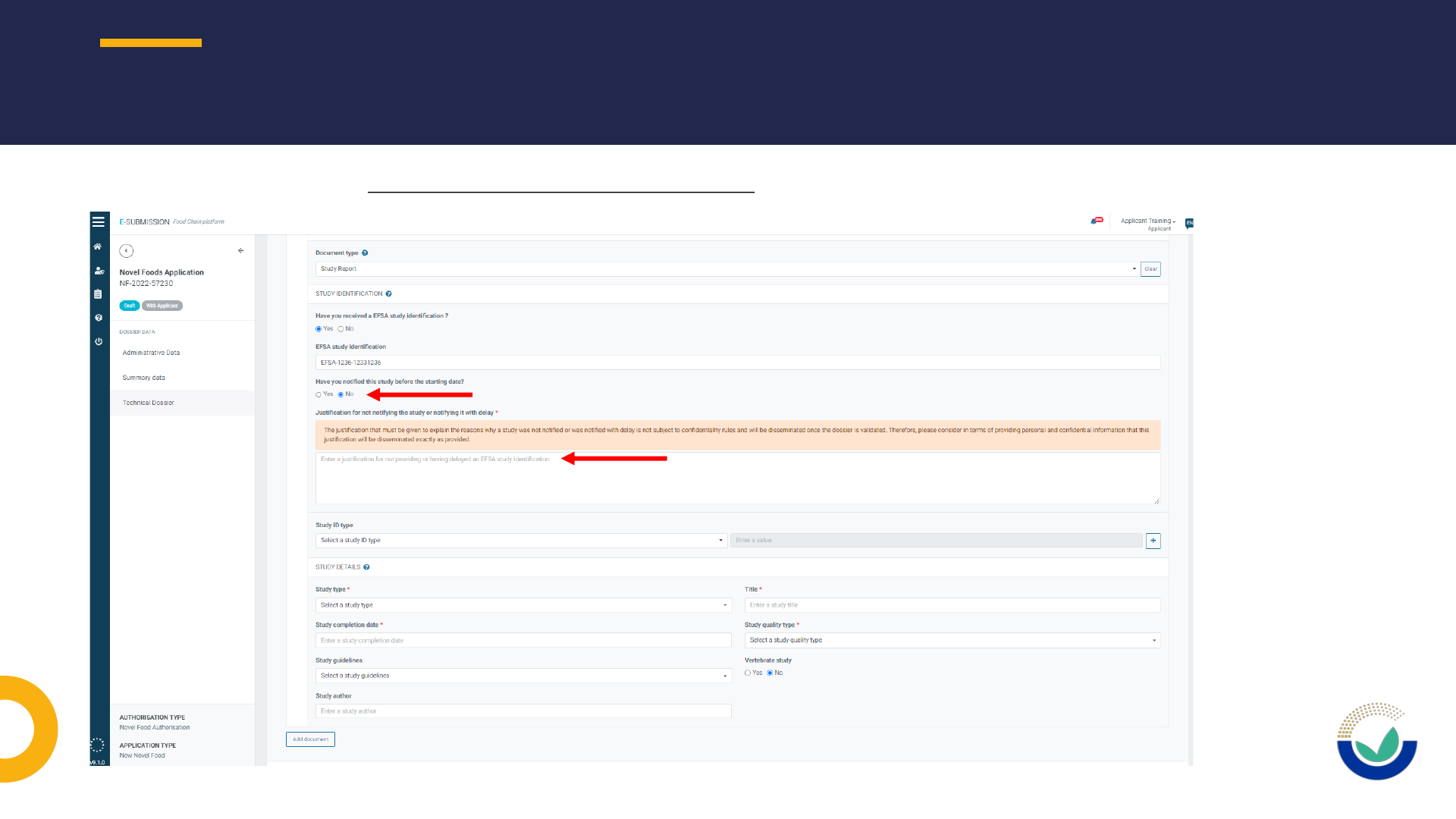
NOTIFICATION OF STUDIES (NOS)
30
• Justifications for delay notification of studies submitted in the dossier

Studies are not notified/notified with delay due to lack of knowledge of applicable NoS
obligation requirements (Article 32b(2) of the General Food Law)
Studies are not notified/notified with delay because they were performed after EC/EFSA
request for information
Self-declaration for non-notification/notified with delay of a study as EU was initially not
considered as a potential market (additional supporting evidence should be provided)
Studies not notified/notified with delay as initially performed for research purposes only
when conducted according to OECD test guidelines and/or GLP
EXAMPLES OF JUSTIFICATIONS NOT ACCEPTED
NOTIFICATION OF STUDIES (NOS)
32
• Resubmit a new application in ESFC as soon as all issues have been solved
providing the dossier/question number of the previous application declared non-
valid
• 6 months penalty starts from the moment of the resubmission of the
application and not the declaration of non-validity
Resubmission of an application after non-validity due to NoS

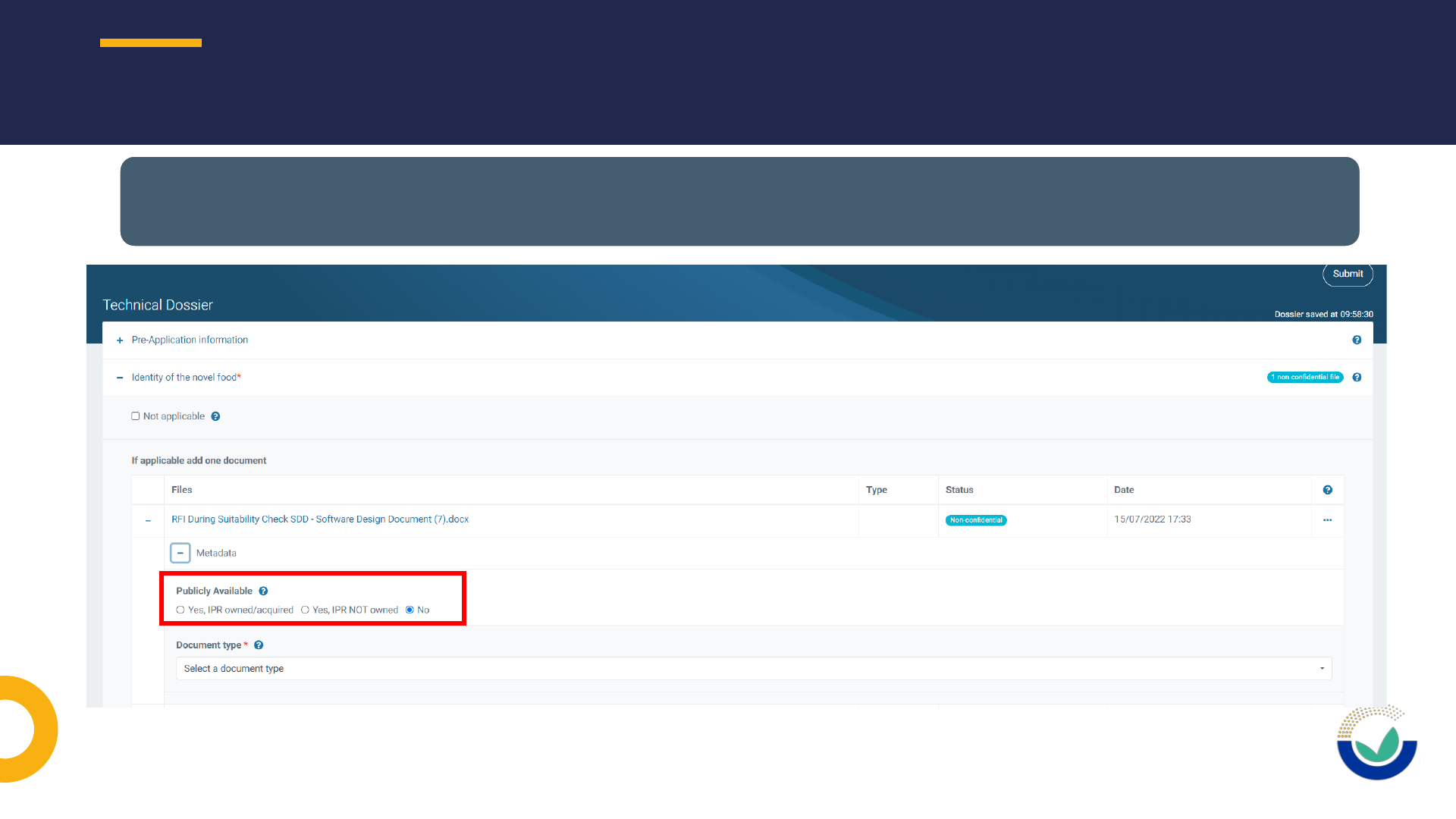
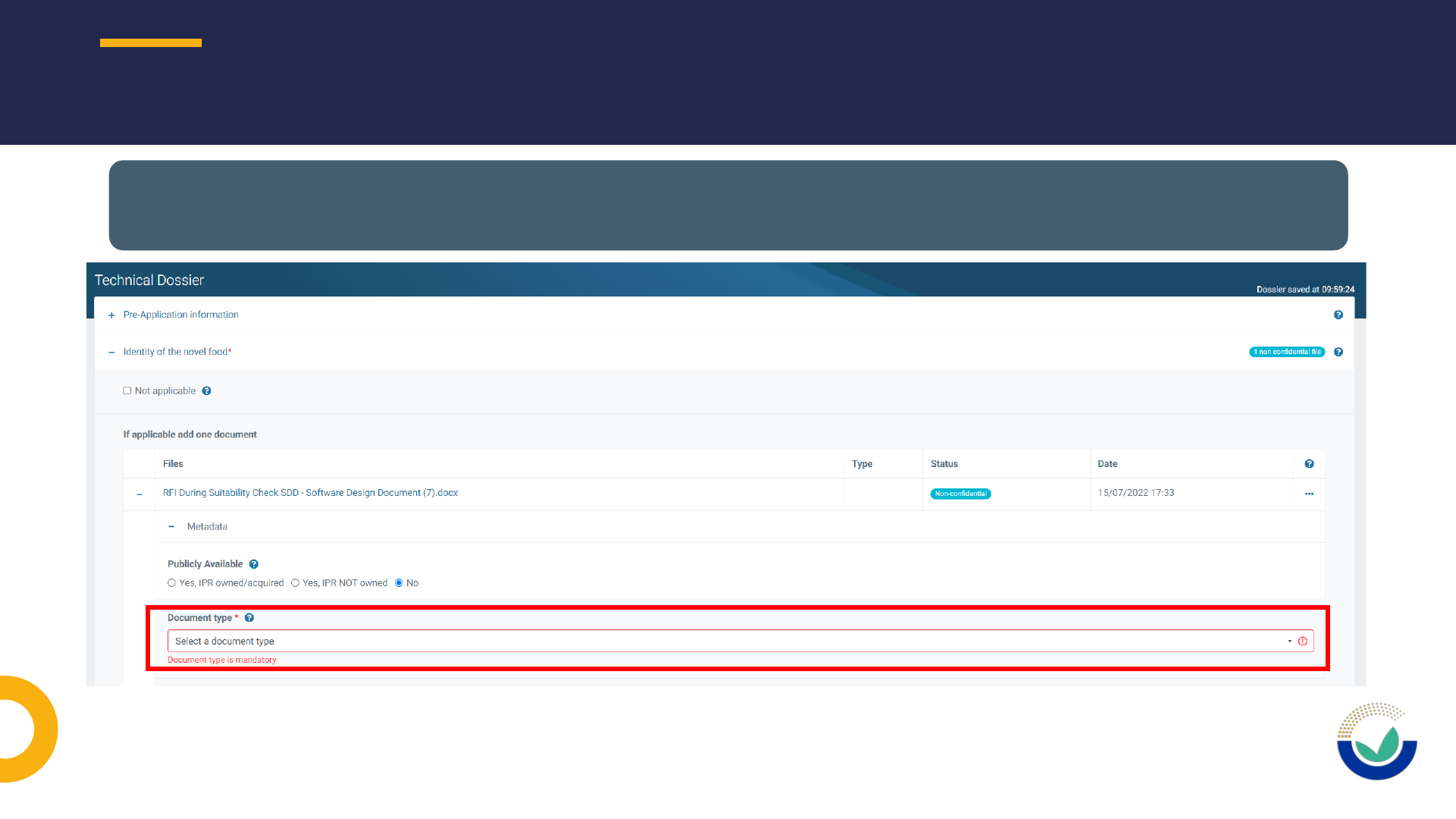
Publicly available
Document type
Study identification
FILES METADATA
FILES METADATA: PUBLICLY AVAILABLE
34
Publicly available
• YES (for all published documents as e.g. publicly available reports, bibliographic references)
• Yes, IPR owned/acquired – applicant has the rights to disseminate the content
➢ Include a full text copy that will be made available in Open EFSA after validation
• Yes, IPR NOT owned – applicant does not have the rights to disseminate the content
➢Include a full text copy that will be used for assessment purposes only
➢Include the bibliographic citation in the free-text box that will be made available in Open EFSA after
validation
• NO (when documents are not published)
Publicly available
Document type
Study identification
FILES METADATA: PUBLICLY AVAILABLE
IPR - Intellectual Property Rights
FILES METADATA: DOCUMENT TYPE
36
Document type
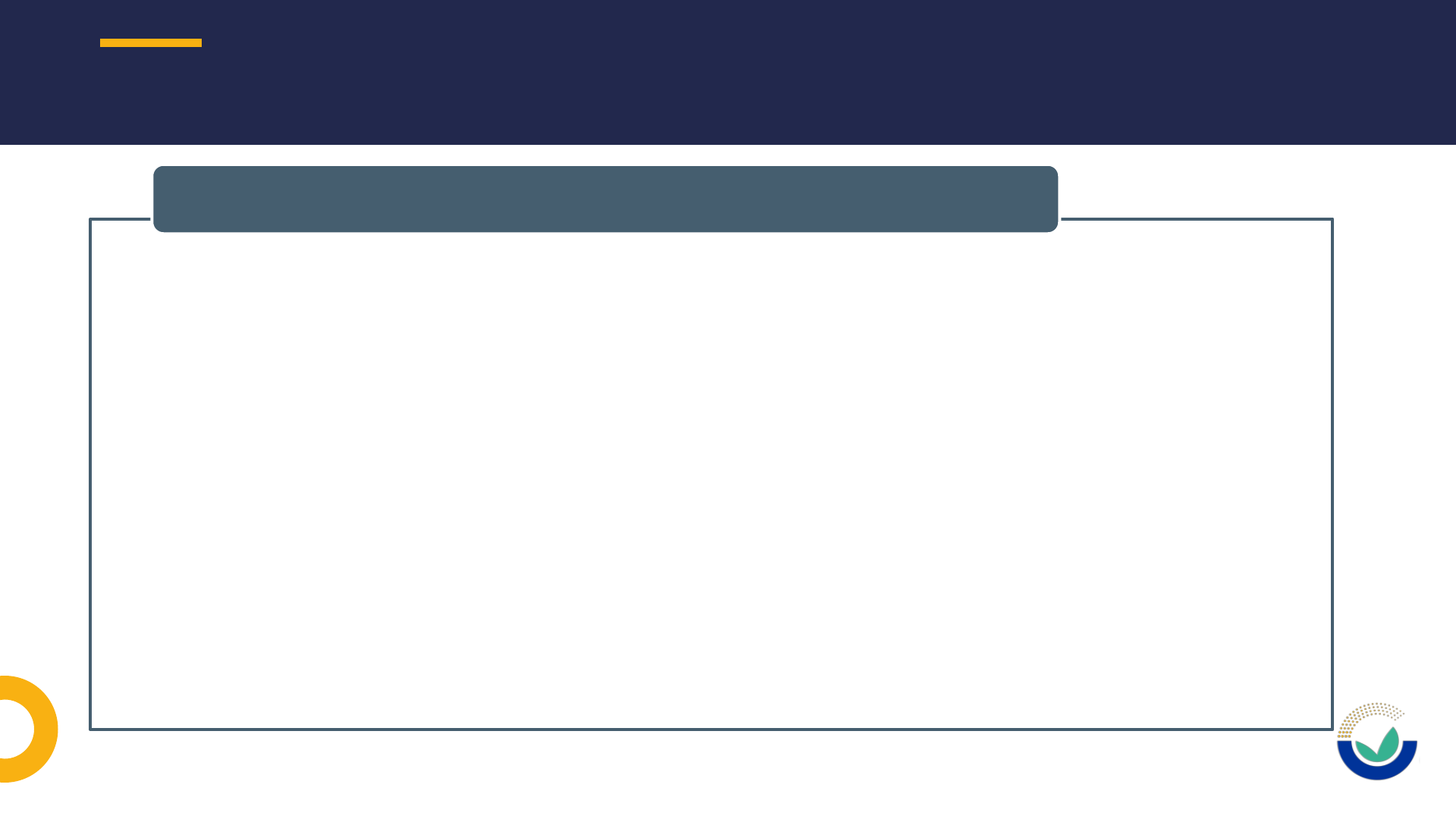
1.Technical dossier
2.Study report
3.Publication
4.Certificate of analysis
5.Laboratory accreditation certificate
6.Scientific summary
7.Raw data
8.Literature search
9.Code for statistical analysis
10.Data sharing agreement/Access letter
11.Copyright licenses
12.Flow charts
13.Graphs/Images
14.Cover letter
15.List of annexes
16.List of references
17.Check list
18.Other supporting documents
Document type
FILES METADATA: DOCUMENT TYPE
Study identification
Publicly available
Document type
FILES METADATA: STUDY IDENTIFICATION
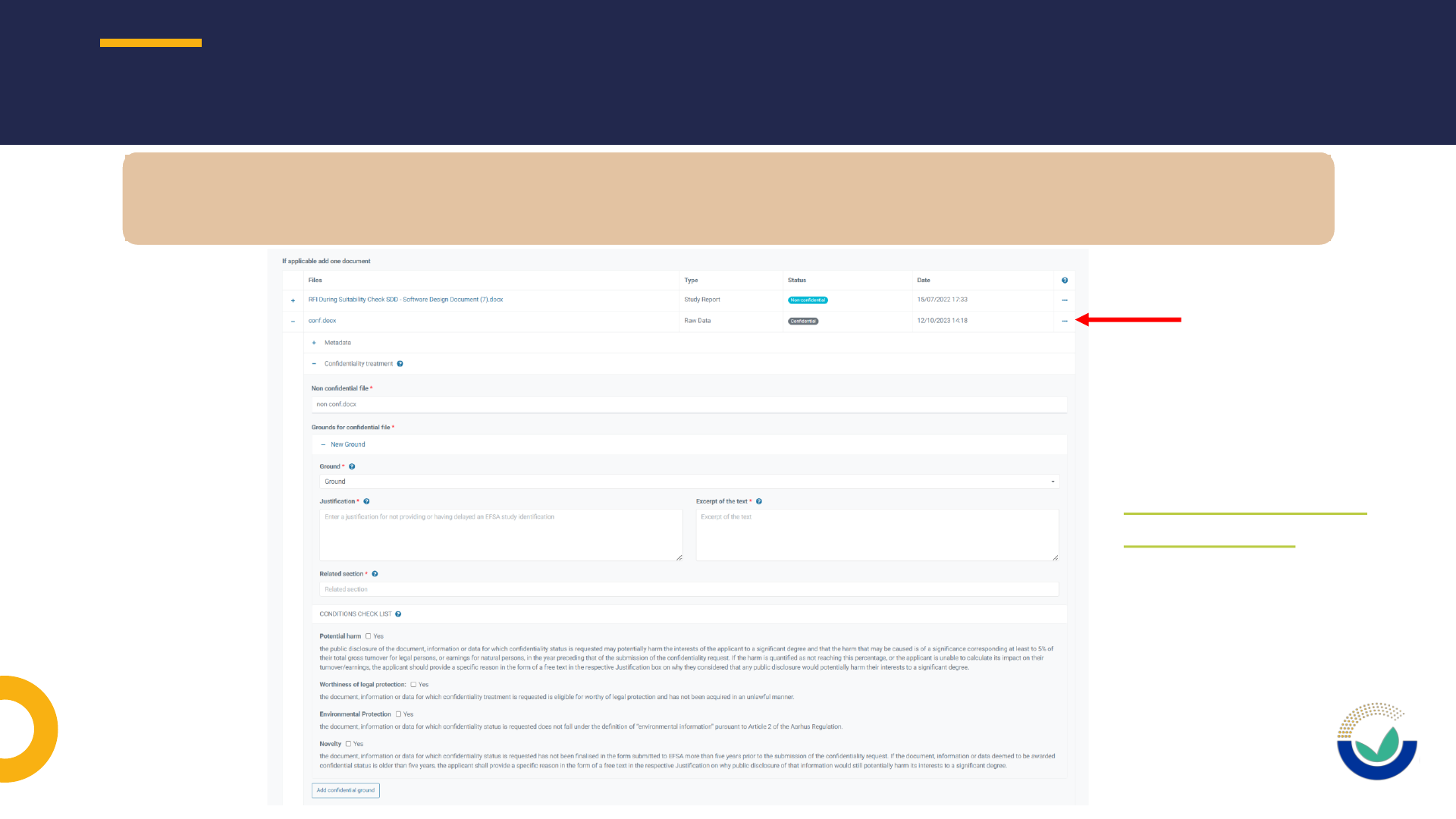
General information for submission of confidential information
Sanitization of confidential information and personal data
CONFIDENTIALITY AND SANITIZATION
• Identify parts on which confidentiality is requested in a clear and consistent manner
•Confidential version and public version must always be submitted and should match
• Ensure information claimed confidential in one part is not visible in another part of the
document
• Do not include watermark ‘confidential’ on parts of documents that you do not claim
confidential
• Properly name documents to distinguish between confidential and non-confidential version
• Refer to correct legal basis. If qualification is not self-evident, justify why the element falls
under that legal basis
• No unfounded confidentiality requests or requests on publicly available information
General information for submission of confidential information
CONFIDENTIALITY AND SANITIZATION
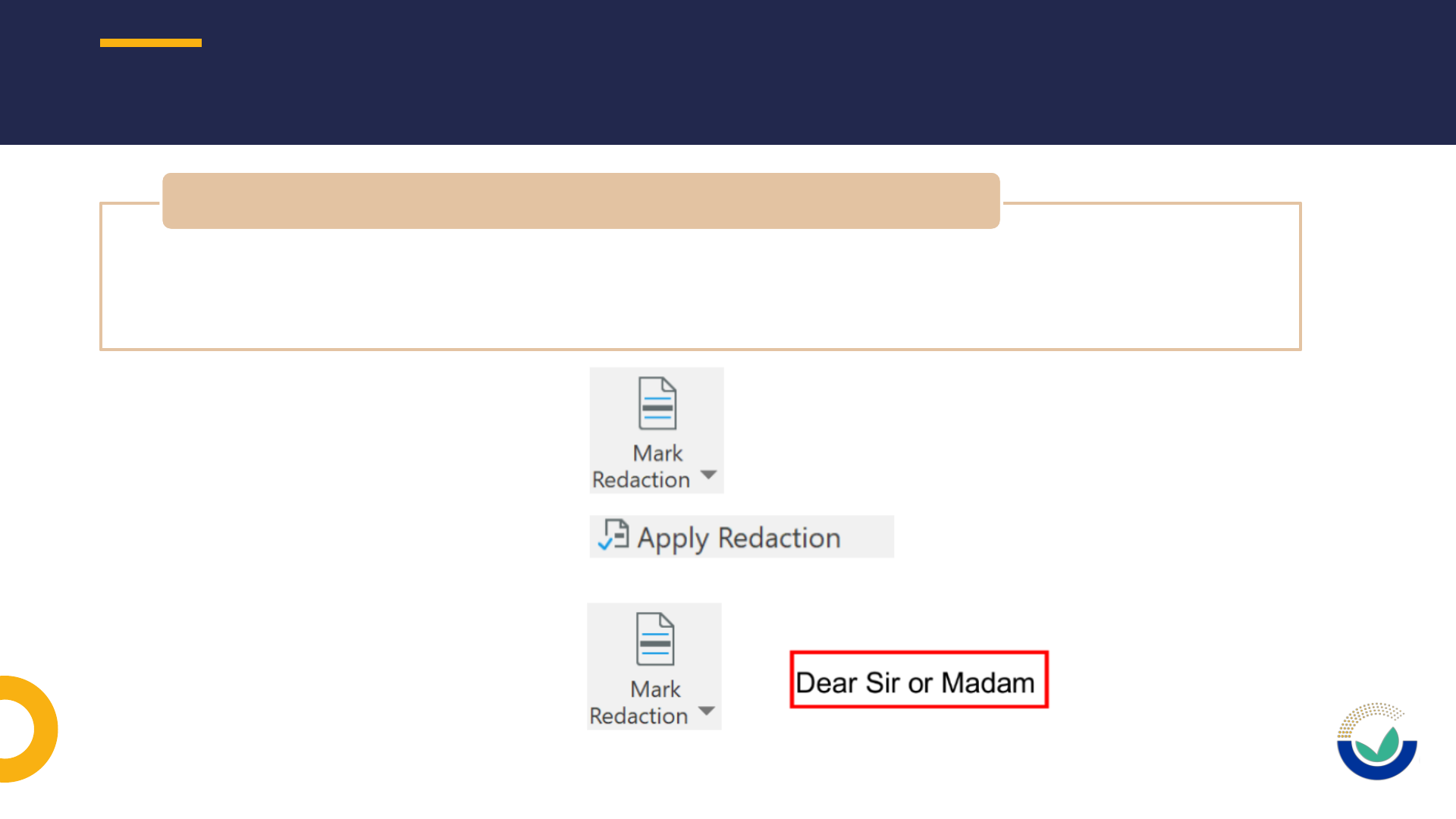
• Use permanent sanitization in non-confidential version of documents for masking
confidential information and for personal data
• Earmarking/boxing of confidential information in confidential version of documents
Sanitization of confidential information and personal data
• Identify parts on which confidentiality is requested in a clear and consistent manner
• Confidential version and public version must always be submitted and should match
• Do not include watermark ‘confidential’ on parts of documents that you do not claim
confidential
• Properly name documents to distinguish between confidential and non-confidential version
• No unfounded confidentiality requests or requests on publicly available information
• EFSA user guide on confidentiality
General issues on submission of Confidential information
CONFIDENTIALITY AND SANITIZATION
• Confidential version
• Non – confidential version

• Use permanent sanitization in non-confidential version of documents for masking
confidential information and for personal data
• Earmarking/boxing of confidential information in confidential version of documents
Sanitization of confidential information and personal data
• Identify parts on which confidentiality is requested in a clear and consistent manner
• Confidential version and public version must always be submitted and should match
• Do not include watermark ‘confidential’ on parts of documents that you do not claim
confidential
• Properly name documents to distinguish between confidential and non-confidential version
• No unfounded confidentiality requests or requests on publicly available information
• EFSA user guide on confidentiality
General issues on submission of Confidential information
CONFIDENTIALITY AND SANITIZATION
➢ Permanent sanitization does not mean highlighting in different colours, nor masking with
a black/white box
➢ Personal data includes names and addresses of natural persons involved in toxicological
studies and any other personal data including names, addresses, and/or signatures of
natural persons, contained in the different documents (e.g. certificates, studies)
Where to include the information requested by EFSA
How to request to have a section unlocked in ESFC
How to request an extension of deadline
REPLYING TO REQUEST FOR INFORMATION (RFI)
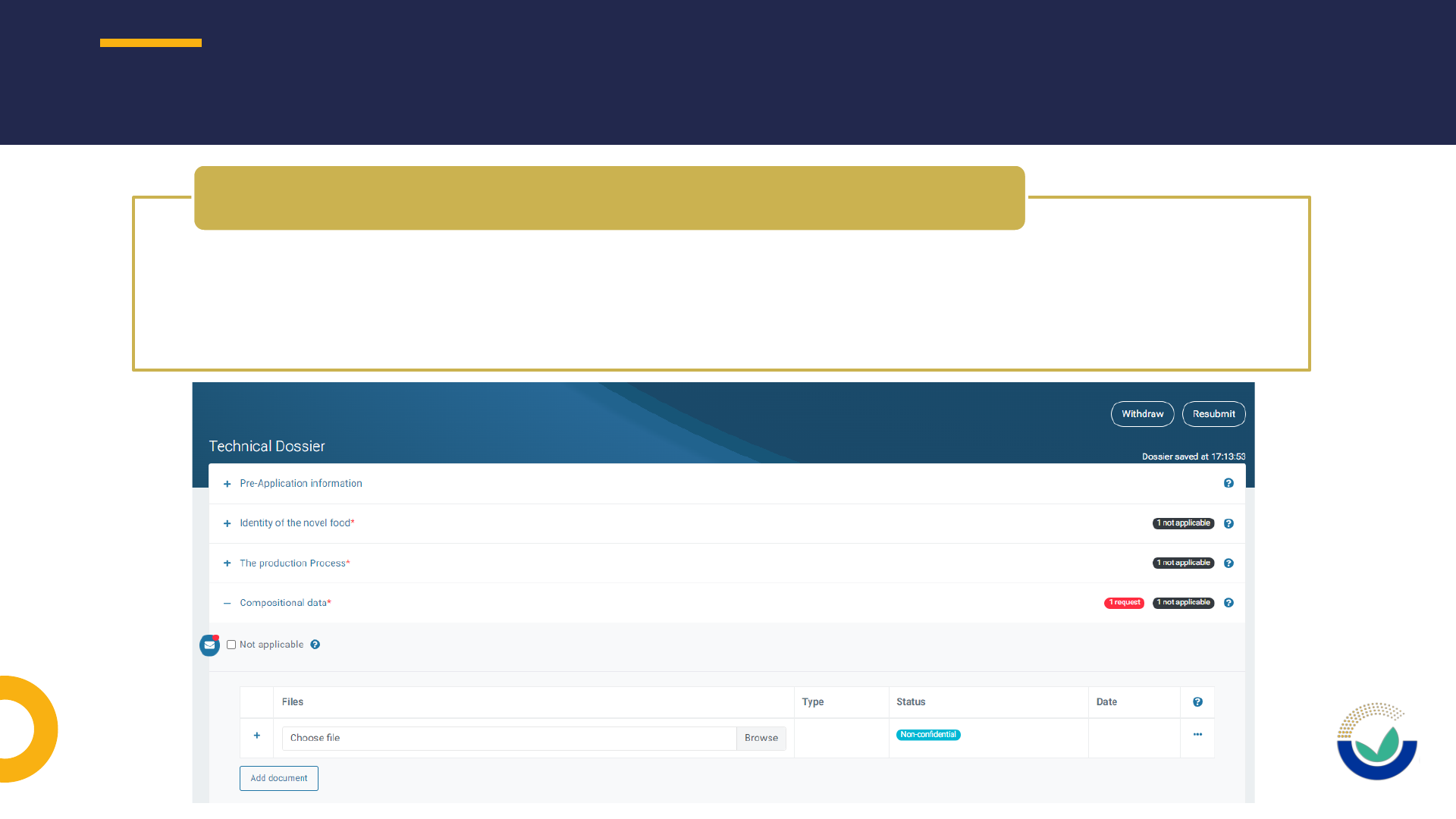
• An updated document should be submitted including the requested information
• Do not use the text box to provide replies to the request for information as it will not
allow the publication as part of the dossier as required by the Transparency
Regulation and will not be considered for the risk assessment
Where to include the information requested by EFSA
How to request to have a section open
• Extension of DL should be requested using ESFC
How to request an extension of DL
REPLYING TO REQUEST FOR INFORMATION (RFI)
• An updated document should be submitted including the requested information
• Do not use the text box to provide replies to the request for information as it will not
allow the publication as part of the dossier as required by the Transparency
Regulation and will not be considered for the risk assessment
Where to include the information requested by EFSA
How to request to have a section open
• Extension of DL should be requested using ESFC
How to request an extension of DL
REPLYING TO REQUEST FOR INFORMATION (RFI)
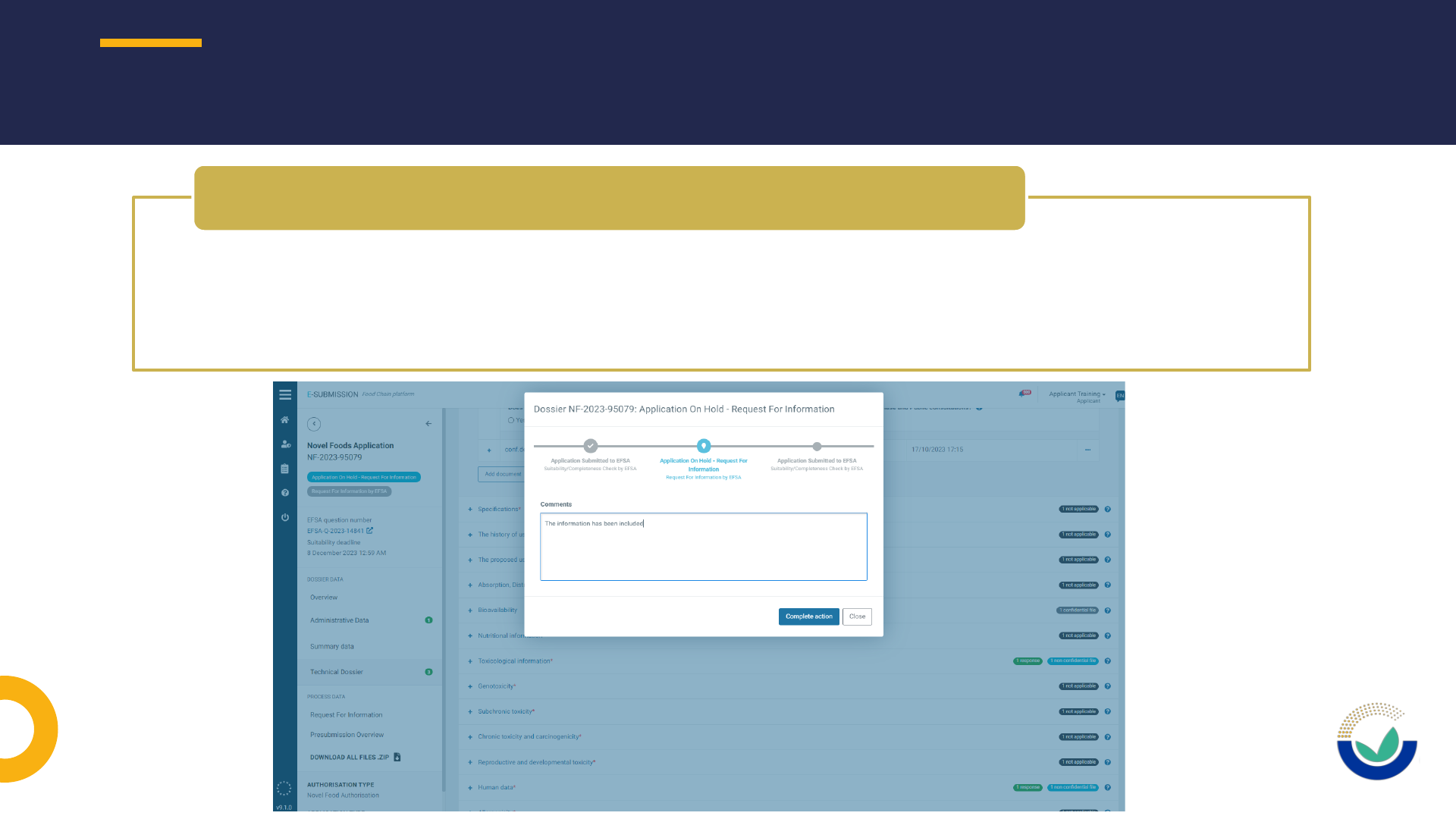
• An updated document should be submitted including the requested information
• Do not use the text box to provide replies to the request of information as it will not
allow the publication as part of the dossier as required by the Transparency
Regulation and will not be considered for the risk assessment
Where to include the information requested by EFSA
How to request to have a section open
• Extension of DL should be requested using ESFC
How to request an extension of DL
REPLYING TO REQUEST FOR INFORMATION (RFI)

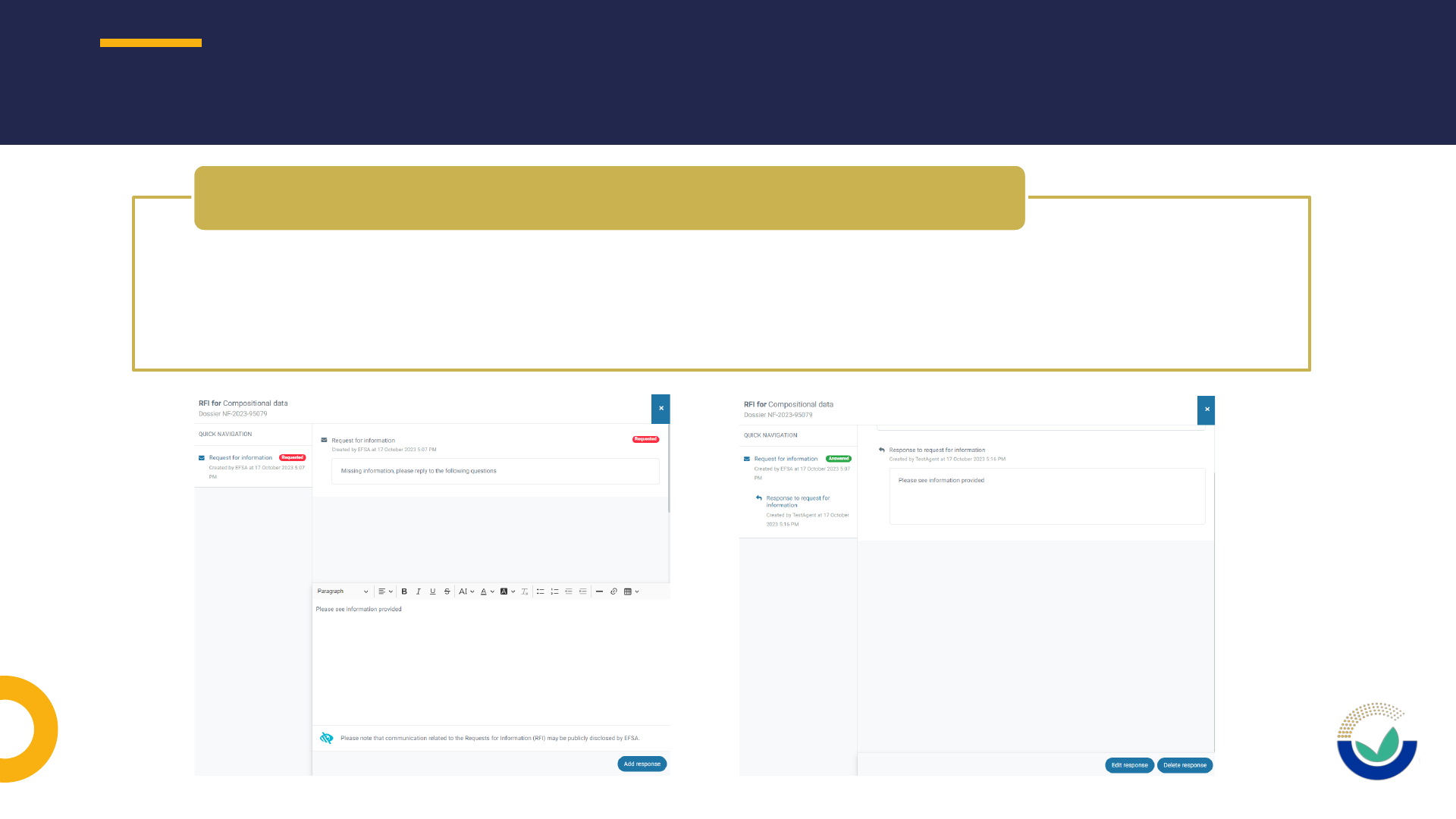
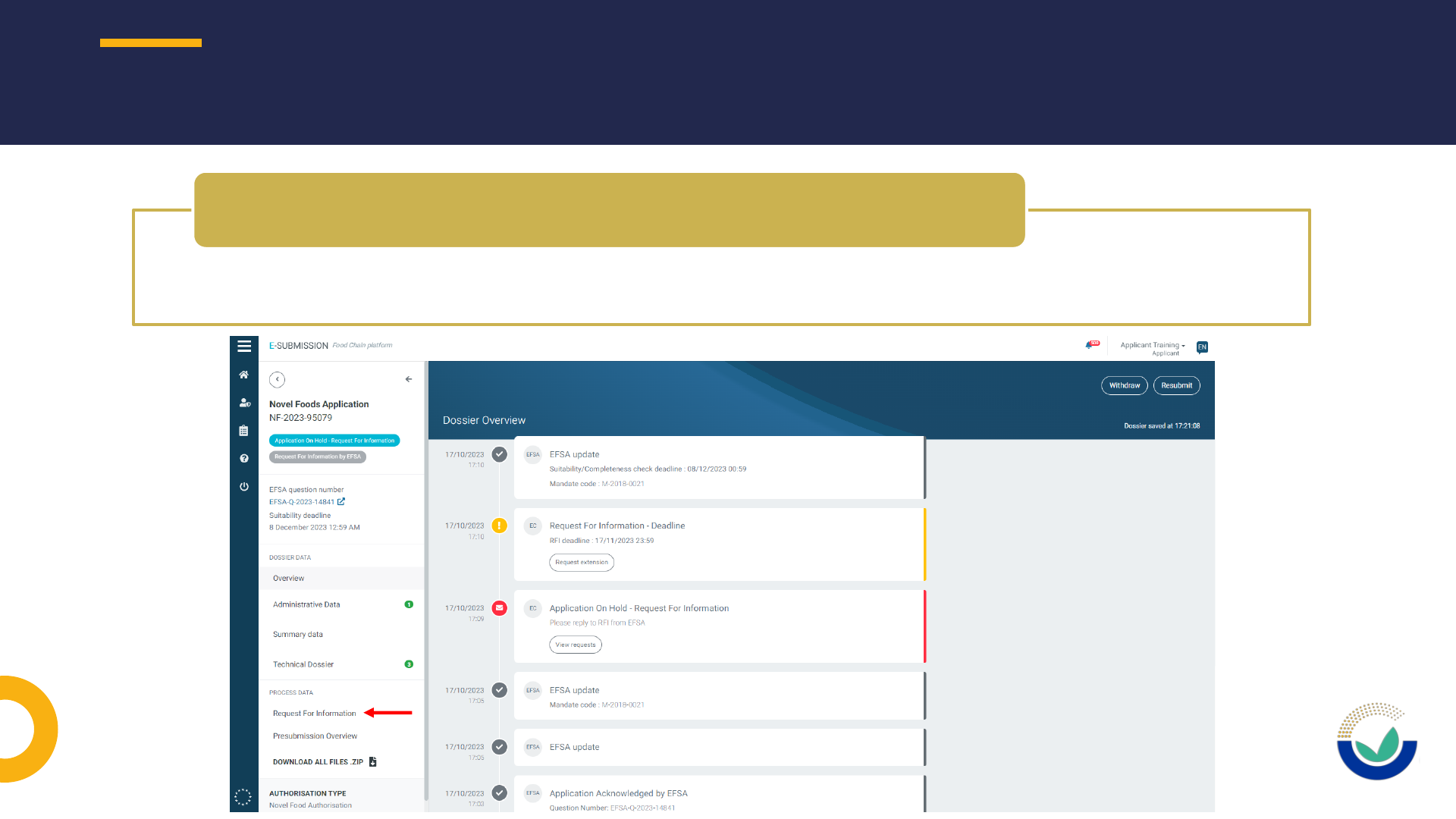
• If the application is with the applicant after a request for information
• Reply to the request for information and include the sections to be opened
• A new request for information will follow opening the relevant sections
• If the application is with EFSA after received replies of Request for information
• Contact EFSA staff by email at FDP@efsa.europa.eu indicating the sections to be
unlocked
• A new request for information will follow opening the relevant sections
• Do not contact: SANTE-E-SUBMISSION-FOOD-CHAIN@ec.europa.eu
How to request to have a section unlocked in ESFC
REPLYING TO REQUEST FOR INFORMATION (RFI)
REPLYING TO REQUEST FOR INFORMATION (RFI)
49
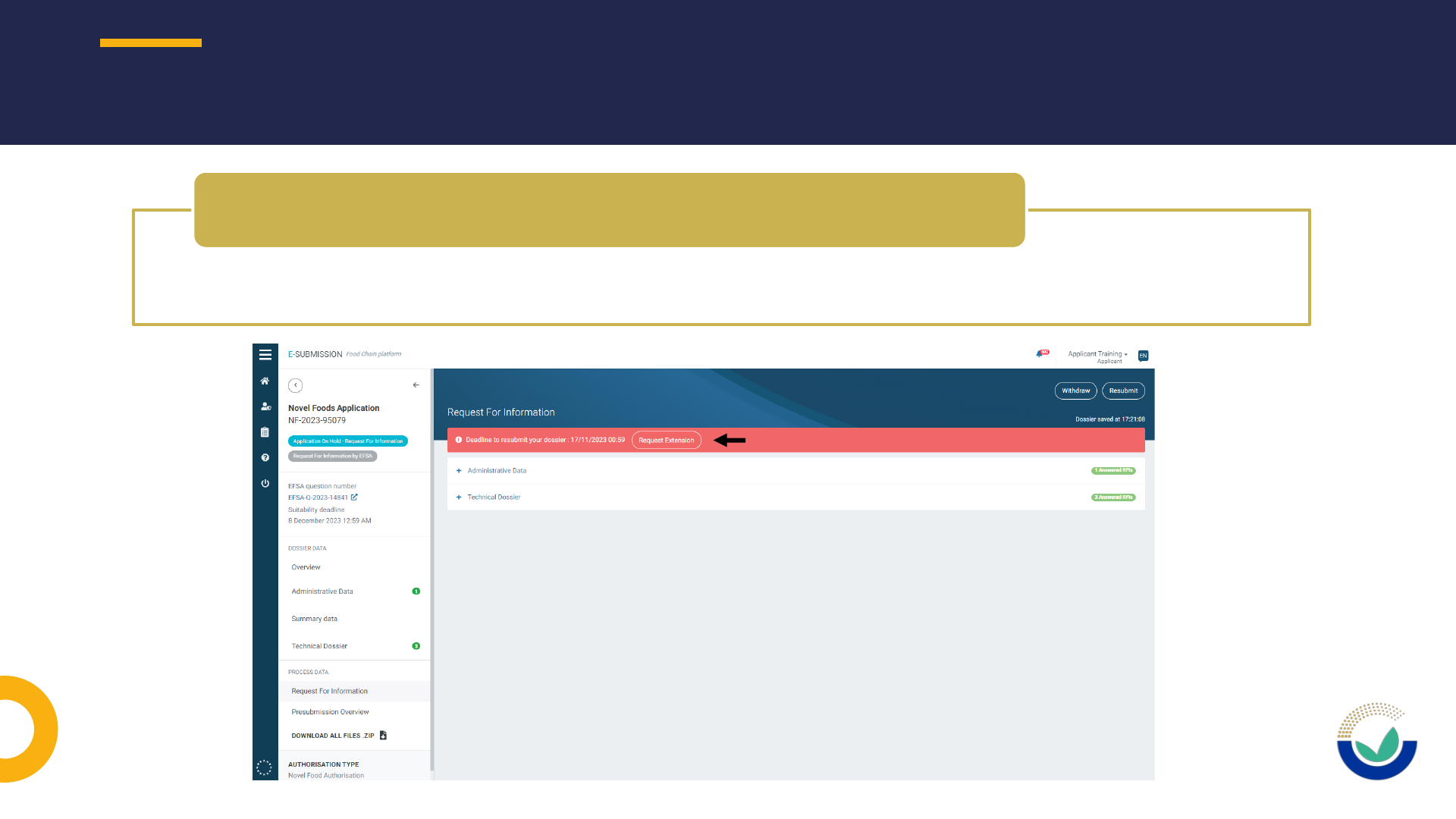
• Use ESFC tool to request an extension of use to provide requested information
How to request an extension of deadline
How to request to have a section open
• Extension of DL should be requested using ESFC
How to request an extension of DL
REPLYING TO REQUEST FOR INFORMATION (RFI)
50
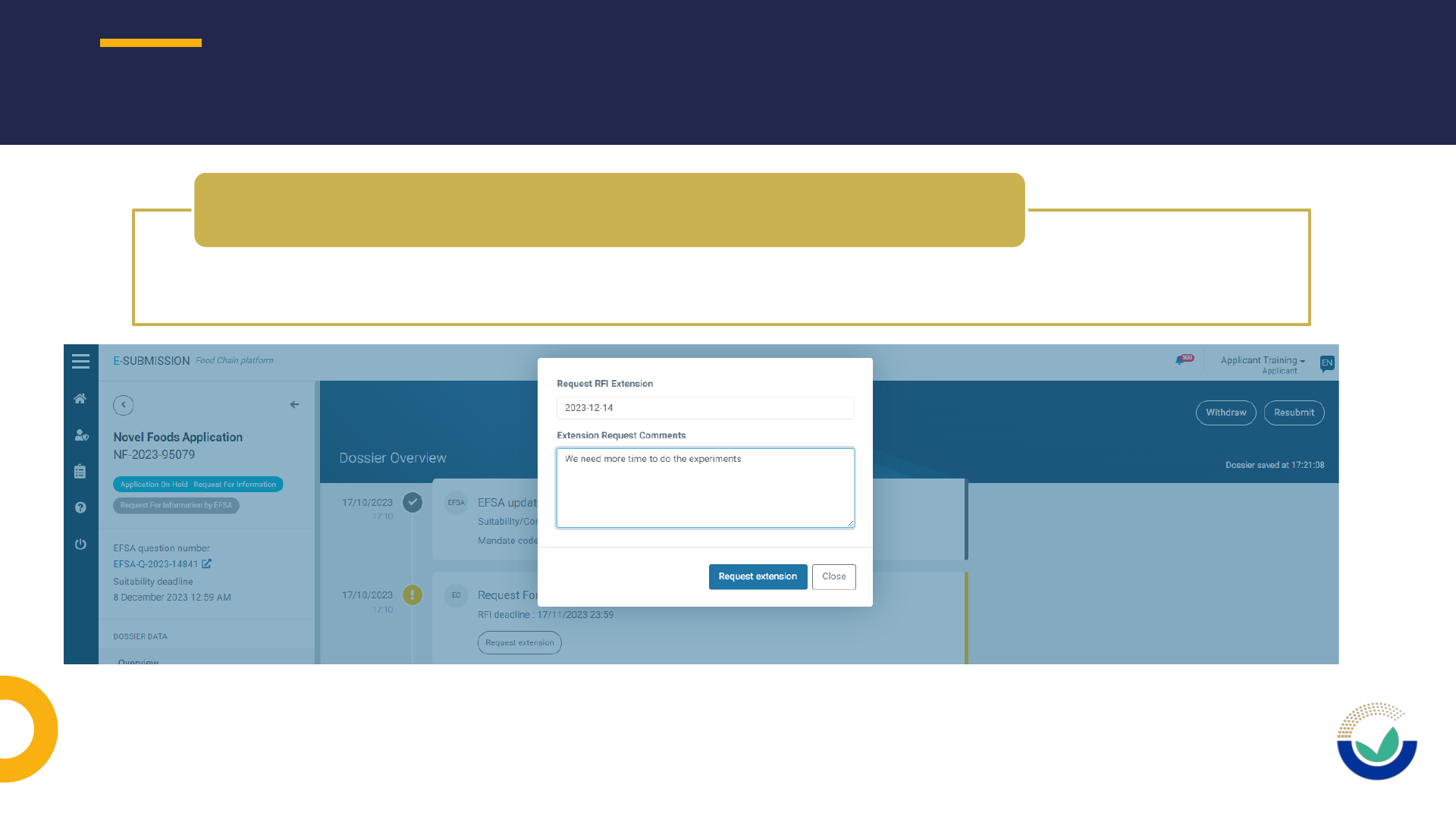
• Use ESFC tool to request an extension of use to provide requested information
How to request an extension of deadline
How to request to have a section open
• Extension of DL should be requested using ESFC
How to request an extension of DL
REPLYING TO REQUEST FOR INFORMATION (RFI)
51
• Use ESFC tool to request an extension of use to provide requested information
How to request an extension of deadline
How to request to have a section open
• Extension of DL should be requested using ESFC
How to request an extension of DL
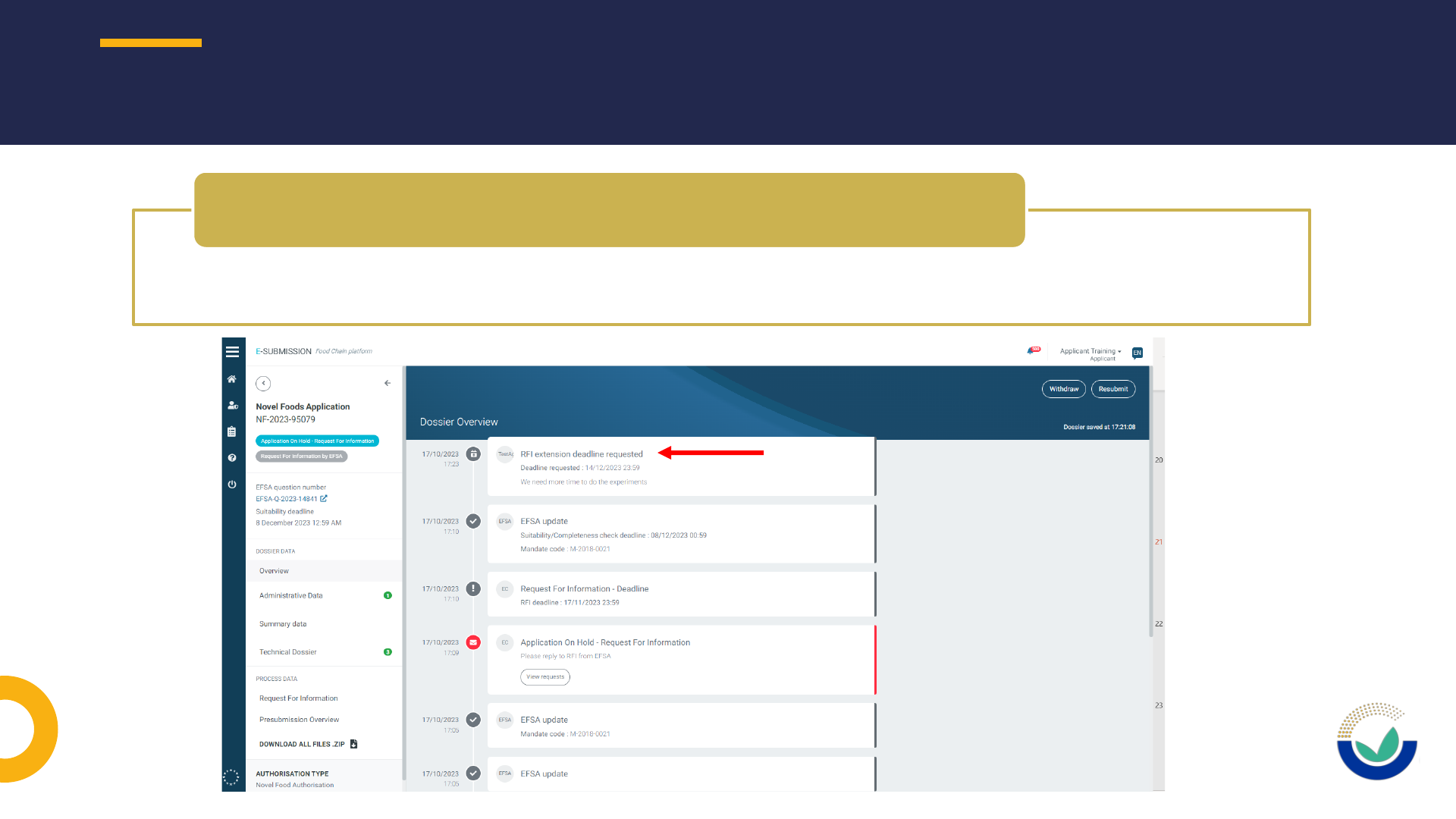
REPLYING TO REQUEST FOR INFORMATION (RFI)
52
• Use ESFC tool to request an extension of use to provide requested information
How to request an extension of deadline
How to request to have a section open
• Extension of DL should be requested using ESFC
How to request an extension of DL
ADMINISTRATIVE ISSUES
Lack of signature, starting date in study reports
Documents submitted in languages different from English
Annexes with wrong document type
Documents electronically non-searchable
53

54
Q&A Session

Q&A
55
• How is the review process organized? Can we expect
multiple rounds of questions or will all questions be
addressed in a single round (unless questions arise of
course from the answers provided)?

Q&A
56
• Notified studies that are now exempt from notification,
can/should be withdrawn from Connect.EFSA? under
the new interpretation, which studies should be notified?

Q&A
57
• Will a detailed database be available where all the novel
food applications and dossier are collected and freely
consultable?
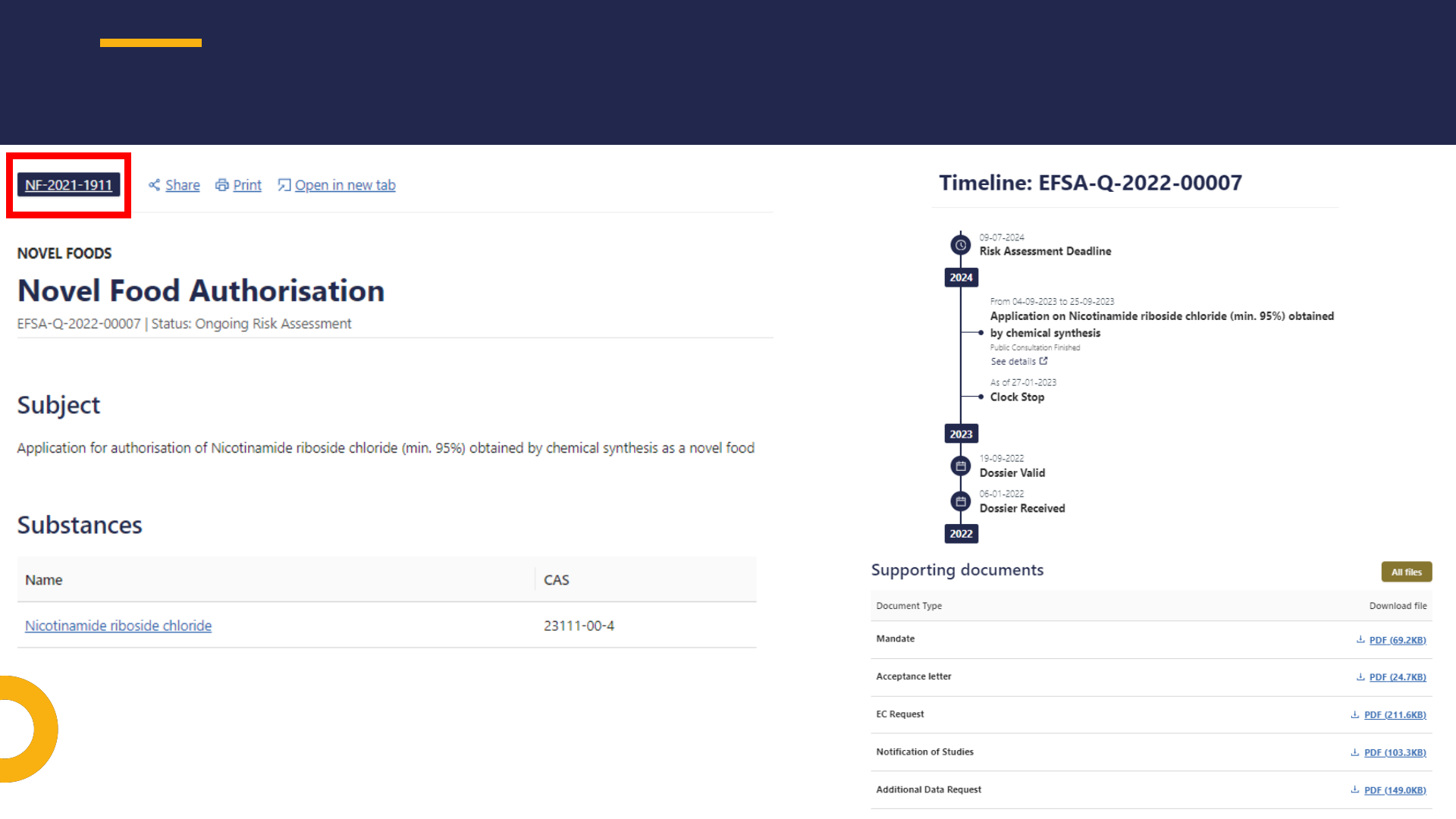
TIMELINE & SUPPORTING DOCUMENTS
59
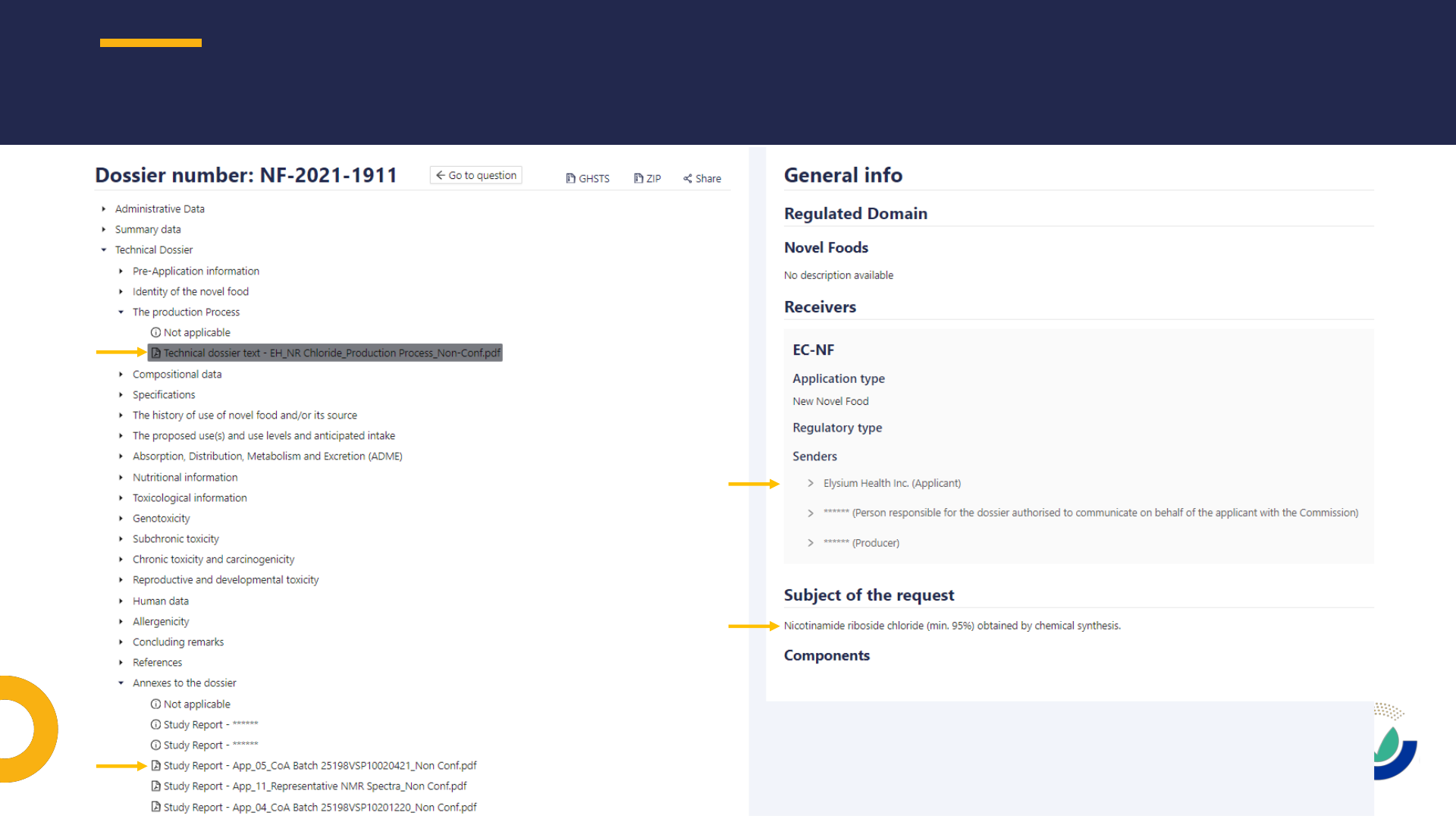
PUBLIC VERSION OF NOVEL FOOD DOSSIERS
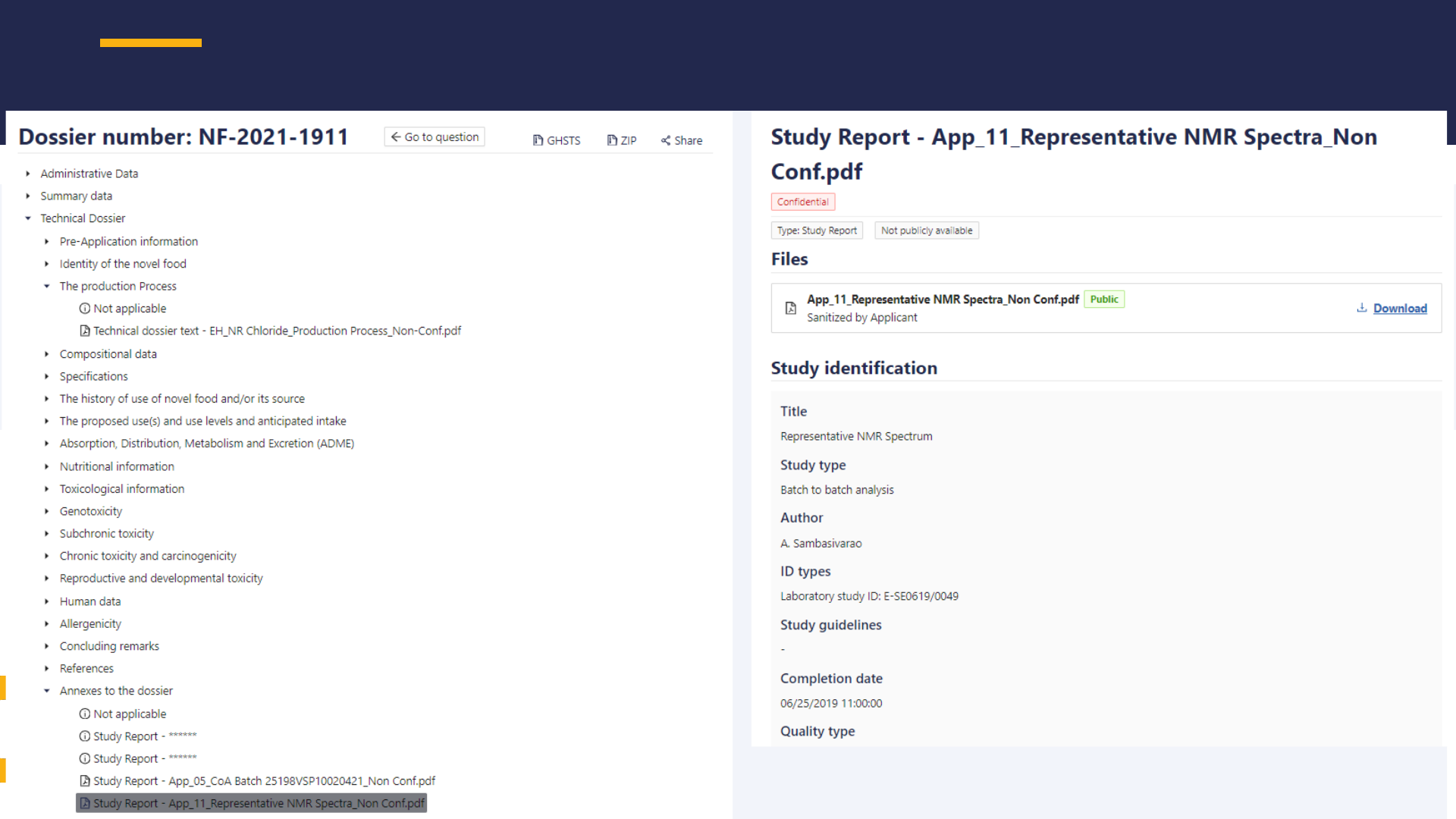
PUBLIC VERSION OF NOVEL FOOD DOSSIERS
61

Q&A
62
• Does the obligation to notify stability studies imply that
such studies need to be performed by an external and
certified lab?

Q&A
63
• What advice would you offer to applicants on how to
prepare a thorough and robust submission?
HOW TO ADDRESS QUESTIONS DIRECTLY TO EFSA
64
Register in the Connect.EFSA platform and then:
• Request a General Pre-Submission
Advice by following the instructions in
section 3.11 of the User guide on pre-
application ID and this video tutorial
OR
• Send a query to Ask a Question
via the Ask a Question service

65
THANK YOU FOR ATTENDING OUR EVENT
• In case we did not manage to answer all your questions, please feel free to re-submit
them via EFSA Ask a question webform (EFSA.Connect at:
https://connect.efsa.europa.eu/RM/s/askefsa)
• The recording of today’s webinar will be available on the EFSA website in coming days.
• Please take few minutes to fill out the satisfaction survey that you will receive shortly in
your inbox. Your feedback is essential to improve our future webinars
• We hope that this webinar will help you to better understand the procedure and
requirements for novel food product applications.

66
A space where you will find:
• Information and support materials
• Updates on the developments and progress of IT tools and platforms
• Alerts on new training material and upcoming events
• Clarifications to the most frequently asked questions received by
applicants
• A space for interaction with your peers with more than 1000 members.
https://www.linkedin.com/groups/9083910/
“EFSA support to applicants”
JOIN OUR LINKEDIN GROUP

USEFUL LINKS
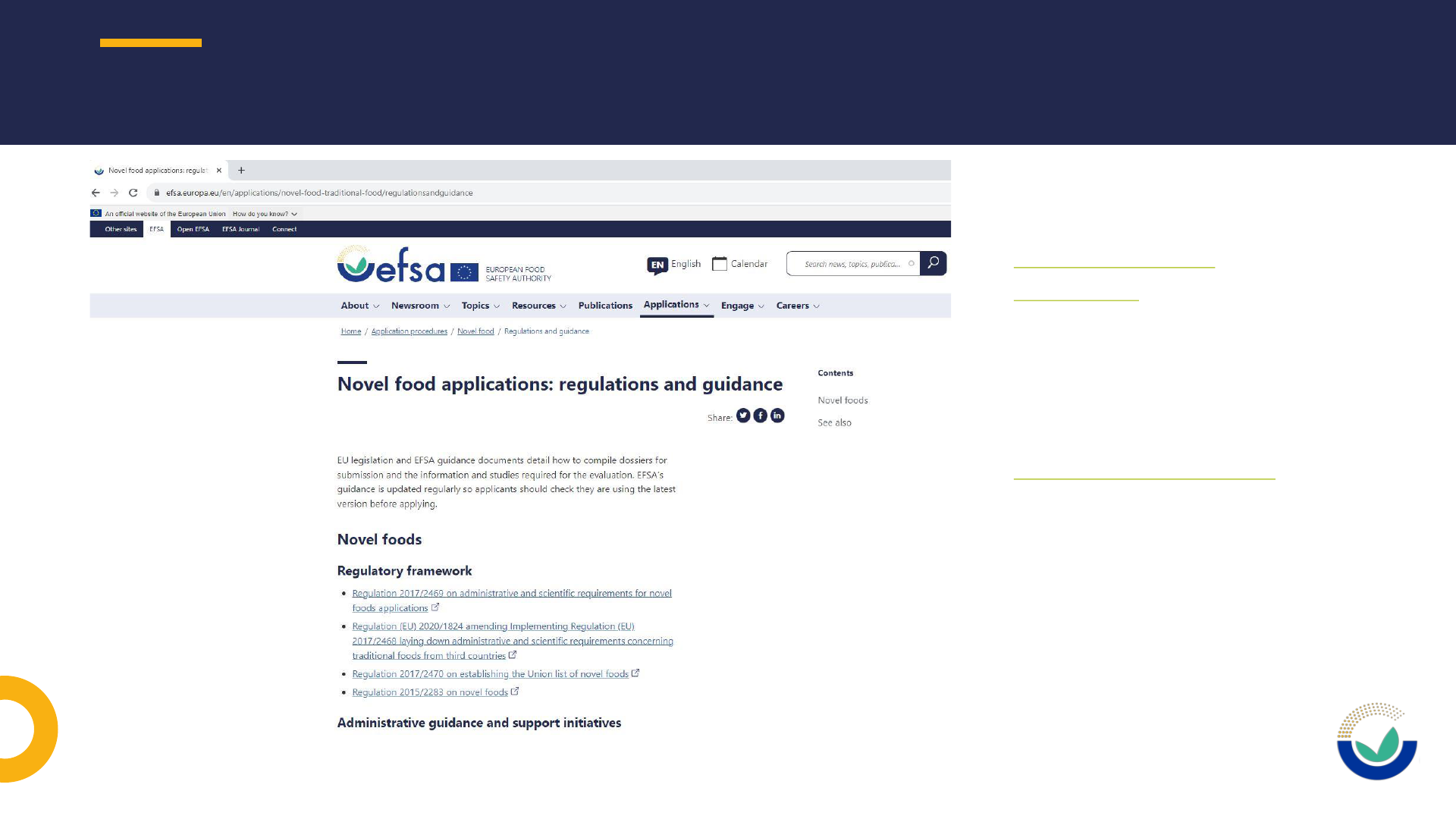
• Novel food and traditional food applications
https://www.efsa.europa.eu/en/applications/novel-food-traditional-food
• Applicants Toolkit
https://www.efsa.europa.eu/en/applications/toolkit
• Services for applicants
https://www.efsa.europa.eu/en/applications/about/services
• Webinar on novel food applications 2021
https://www.efsa.europa.eu/en/events/webinar-novel-food-applications
• Last webinar on notification of studies
https://www.youtube.com/watch?v=NB2Ajn_2R74
67
STAY CONNECTED
SUBSCRIBE TO
efsa.europa.eu/en/news/newsletters
efsa.europa.eu/en/rss
Careers.efsa.europa.eu – job alerts
FOLLOW US ON TWITTER
@efsa_eu @methods_efsa
@plants_efsa @animals_efsa
FOLLOW US ON INSTAGRAM
@one_healthenv_eu
CONTACT US
efsa.europa.eu/en/contact/askefsa
FOLLOW US ON LINKEDIN
Linkedin.com/company/efsa
https://www.linkedin.com/groups/9083910/
LISTEN TO OUR PODCAST
Science on the Menu –Spotify, Apple Podcast and YouTube