Theoretical Minimum Energies
To Produce Steel
for Selected Conditions
March 2000
R.J. Fruehan
O. Fortini
H.W. Paxton
R. Brindle
Prepared under contract to
Energetics, Inc.
Columbia, MD
For the
U.S. Department of Energy
Office of Industrial Technologies
Washington., DC
Carnegie Mellon University
Pittsburgh, PA
THEORETICAL MINIMUM ENERGIES TO PRODUCE STEEL
FOR SELECTED CONDITIONS
R. J. Fruehan
O. Fortini
H.W. Paxton
R. Brindle
Carnegie Mellon University
Pittsburgh, PA
May 2000
Prepared under contract to
Energetics, Inc.
Columbia, MD
for the
U.S. Department of Energy
Office of Industrial Technologies
Washington, DC
DISCLAIMER
This report was prepared as an account of work sponsored by an agency of the United States
Government. Neither the United States Government nor any agency thereof, nor any of their
employees, makes any warranty, expressed or implied, or assumes any legal liability or responsibility
for the accuracy, completeness, or usefulness of any information, apparatus, product, or process
disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any
specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise
does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United
States Government or any agency thereof. The views and opinions of authors expressed herein do not
necessarily state or reflect those of the United States Government or any agency thereof.

TABLE OF CONTENTS
EXECUTIVE SUMMARY ..................................................... iii
1. INTRODUCTION ..........................................................1
2. BASIC ASSUMPTIONS AND CASES CONSIDERED ...........................1
3. METHODOLOGY ..........................................................2
4. ABSOLUTE THEORETICAL MINIMUM ENERGY VALUES ....................3
5. CASES APPROACHING ACTUAL CONDITIONS ..............................4
6. PRODUCTION OF STAINLESS STEEL .......................................9
7. ROLLING ...............................................................11
8. EFFECT OF YIELD LOSSES ON ENERGY ...................................13
9. OTHER MAJOR ENERGY-CONSUMING ACTIVITIES ........................15
10. TOTAL THEORETICAL MINIMUM ENERGY TO PRODUCE STEEL ........... 16
11. SPECIAL CONSIDERATIONS .............................................16
12. COMPARISON OF TYPICAL ACTUAL AND
THEORETICAL MINIMUM ENERGY ...................................19
13. SUMMARY AND CONCLUSIONS .........................................20
REFERENCES ..............................................................23
APPENDIX A: THEORETICAL CO
2
EMISSIONS FROM
STEELMAKING PROCESSES ..........................................25
Theoretical Minimum Energies to Produce Steel for Selected Conditions i

ii Theoretical Minimum Energies to Produce Steel for Selected Conditions
-- -- --
EXECUTIVE SUMMARY
The energy used to produce liquid steel in today’s integrated and electric arc furnace (EAF)
facilities is significantly higher than the theoretical minimum energy requirements. This study presents the
absolute minimum energy required to produce steel from ore and mixtures of scrap and scrap
alternatives. Additional cases in which the assumptions are changed to more closely approximate actual
operating conditions are also analyzed. The results, summarized in Table E-1, should give insight into
the theoretical and practical potentials for reducing steelmaking energy requirements. The energy values
have also been converted to carbon dioxide (CO
2
) emissions in order to indicate the potential for
reduction in emissions of this greenhouse gas (Table E-2).
The study showed that increasing scrap melting has the largest impact on energy consumption.
However, scrap should be viewed as having "invested" energy since at one time it was produced by
reducing ore. Increasing scrap melting in the BOF may or may not decrease energy if the "invested"
energy in scrap is considered.
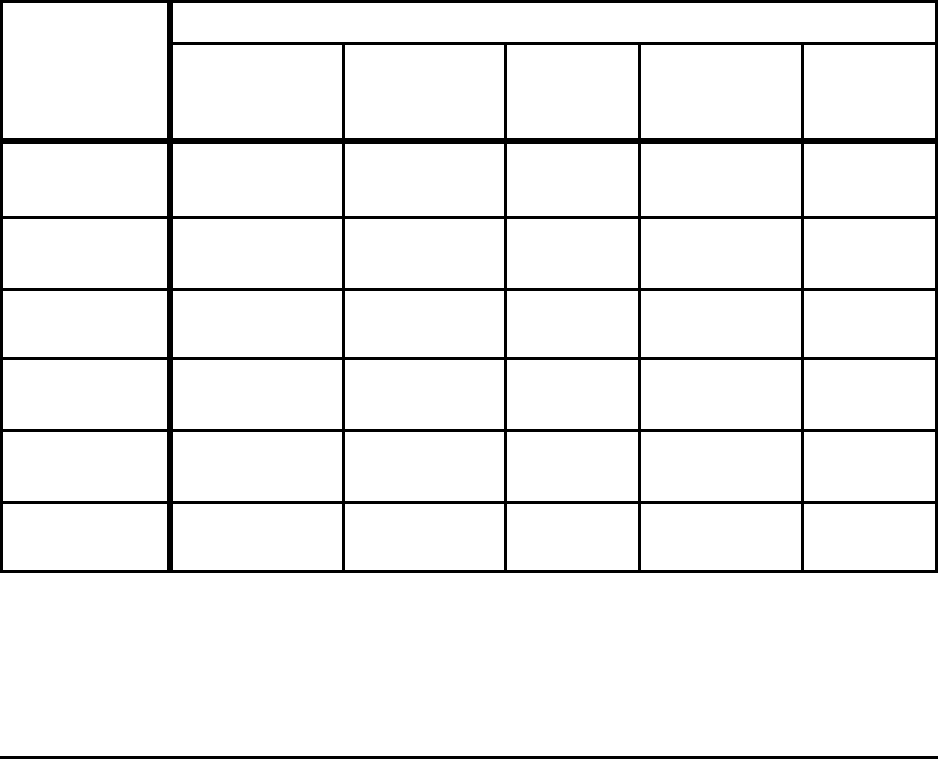
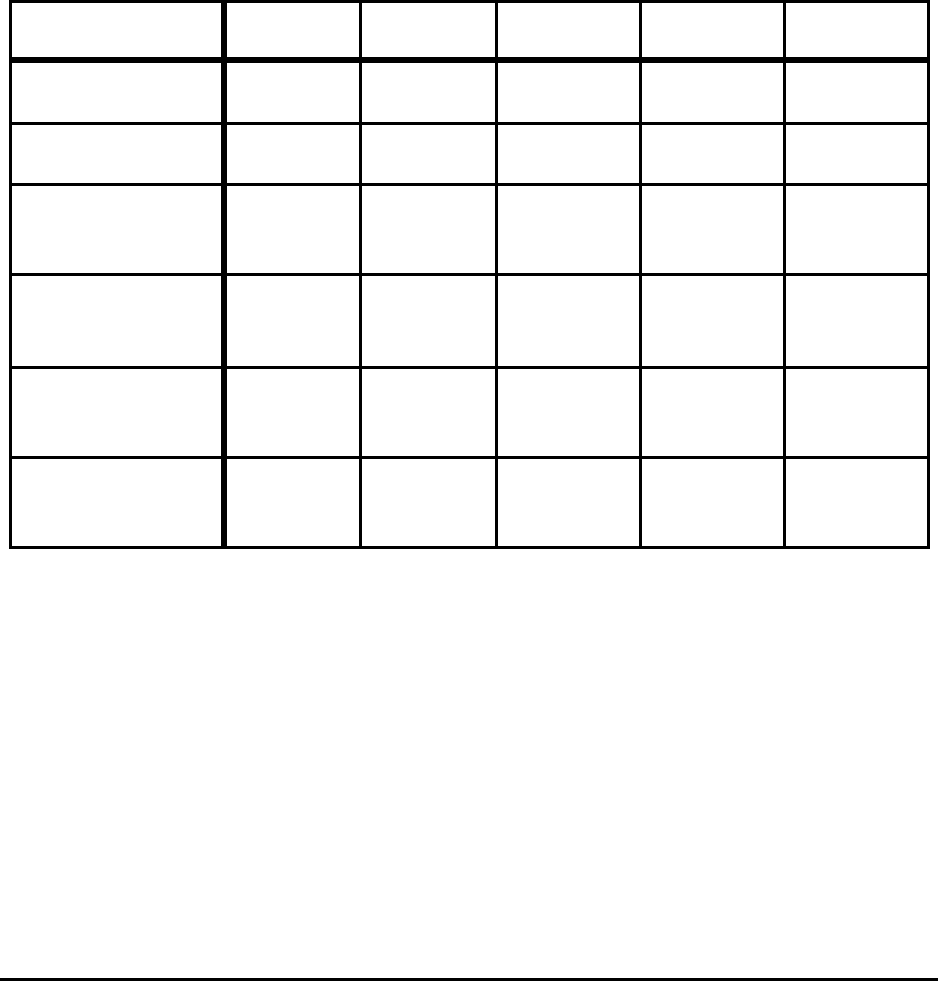
Table E-1. Comparison of Theoretical Minimum Energy
and Actual Energy Requirements for Selected Processes
Process
Energy (GJ/metric ton product)
Actual
Requirements
[10
6
Btu/ton]
Absolute
Minimum
[10
6
Btu/ton]
%
Difference
Practical
Minimum
[10
6
Btu/ton]
%
Difference
Liquid Hot
Metal (5%C)
13 - 14
[11.2 - 12.1]
9.8
[8.5]
25 - 30 10.4
[9.0]
20 - 26
Liquid Steel
(BOF)
10.5 - 11.5
[9.1 - 9.9]
7.9
[6.8]
25 - 31 8.2
[7.1]
22 - 29
Liquid Steel
(EAF)
2.1 - 2.4
[1.8 - 2.1]
1.3
[1.1]
38 - 46 1.6
[1.4]
24 - 33
Hot Rolling Flat 2.0 - 2.4
[1.7 - 2.1]
0.03
[0.03]
99 0.9
[0.8]
55 - 63
Cold Rolling
Flat
1.0 - 1.4
[0.9 - 1.2]
0.02
[0.02]
98 - 99 0.02
[0.02]
98 - 99
18-8 Stainless
Melting
1.2
[1.0]
1.5
[1.3]
Notes:
Actual includes yield losses and is the average of state-of-art and less-efficient operations for the United
States, Japan, and Europe. All of the values exclude electrical generation and transmission losses.
BOF energy is primarily from hot metal; actual process consumes 0.2 to 0.4 GJ/t and, if CO is oxidized to
CO
2
, could theoretically produce 0.5 GJ.
For 18-8 stainless no estimates are available, in particular for HCFeCr.
In all cases, full credit is taken for the energy in off gas.
Theoretical Minimum Energies to Produce Steel for Selected Conditions iii
-- --
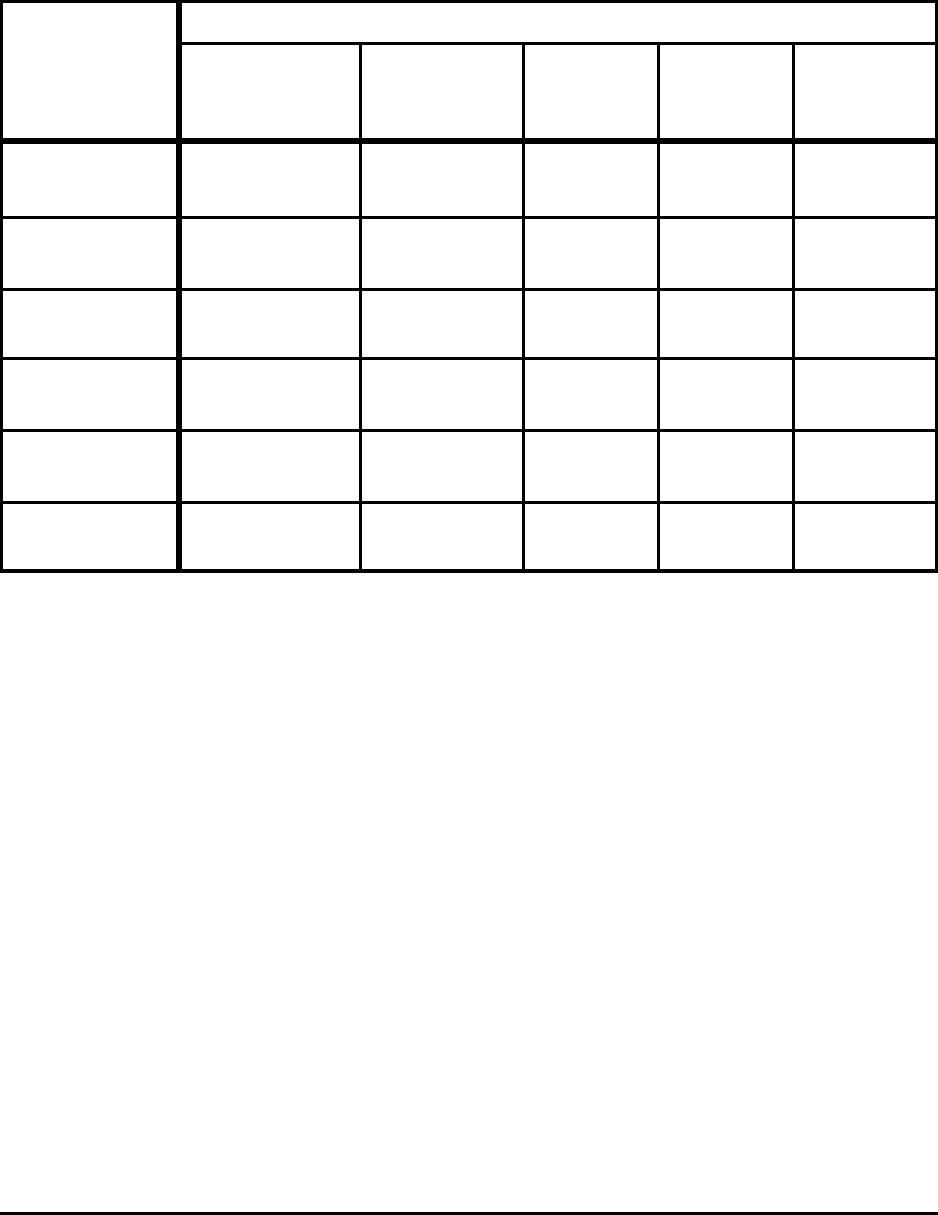
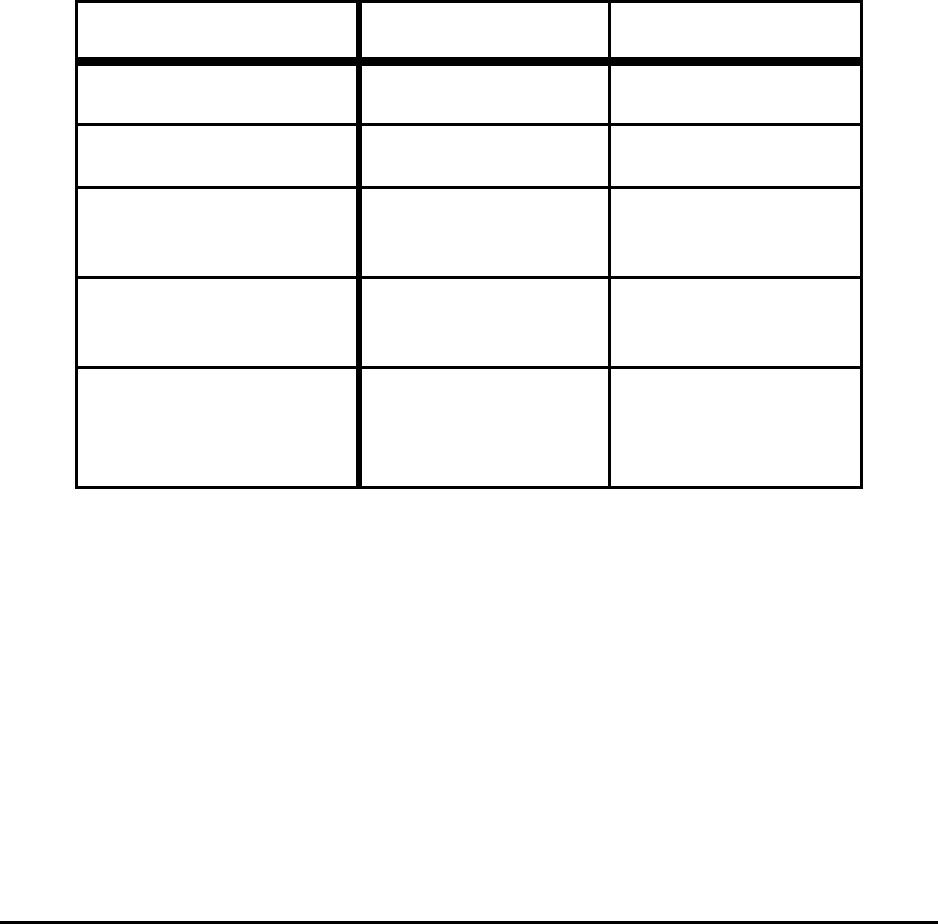
Table E-2. Comparison of Theoretical Minimum
and Actual CO
2
Emissions for Selected Processes
Process
CO
2
Emissions (kg/metric ton product)
Actual
Emissions
[lbs/ton]
Absolute
Minimum
[lbs/ton]
%
Difference
Practical
Minimum
[lbs/ton]
%
Difference
Liquid Hot
Metal
1,447 - 1,559
[2,894 - 3,118]
1,091
[2,182]
25 - 30 1,158
[2,316]
20 - 26
Liquid Steel
(BOF)
189 - 207
[378 - 414]
144
[288]
24 - 30 144
[288]
24 - 30
Liquid Steel
(EAF)
364 - 416
[728 - 832]
225
[450]
38 - 46
[76 - 92]
277
[554]
24 - 33
Hot Rolling
110 - 132
[220 - 264]
2
[4]
98
[196]
50
[100] 55 - 62
Cold Rolling
173 - 243
[346 - 486]
4
[8]
98
[196]
4
[8] 98
18-8 Stainless
Melting
– 208
[416]
260
[520]
Note:
Based on theoretical minimum energy consumption shown in Table E-1.
Other conclusions that can be drawn from the findings are as follows:
• Elimination of ore agglomeration and cokemaking, which are not theoretically necessary to
produce steel, would yield significant energy savings.
• Effective utilization of the energy contained in the off gases from the blast furnace, coke plant,
and direction reduction process is essential.
• The best scrap substitute with respect to energy usage is liquid pig iron or hot metal.
• Gangue and ash in ore and coal increase minimum ironmaking energy by about 6%.
• In BOF steelmaking, the energy to produce steel is less than the energy to produce iron
because scrap is melted in the process.
• Dirt in scrap and air entrainment in an EAF increase the energy required by about 25%.
• When carbon is used in the EAF, about 38% of the CO must be post combusted to CO
2
to
equal electrical energy, even taking into account the production and transmission losses
associated with electrical energy.
• The melting energy for stainless steel is similar to the melting energy for carbon steel, but is
much greater if the energy associated with ferrochrome is included.
• Direct charging of slabs or billets reduces total rolling energy by about 80%.
iv Theoretical Minimum Energies to Produce Steel for Selected Conditions
1. INTRODUCTION
The absolute theoretical minimum energies to produce liquid steel from idealized scrap (100%
Fe) and ore (100% Fe
2
O
3
) are much lower than consumed in practice, as are the theoretical minimum
energies to roll the steel into its final shape. This study presents the absolute minimum energy
required to produce steel from ore and mixtures of scrap and scrap alternatives, clearly
defining the necessary assumptions involved. The energy calculations are then repeated for a
series of cases in which the assumptions are changed one by one to more closely approximate
the actual conditions.
The results should give insight as to what is theoretically and practically possible in terms of
reducing the energy required to produce steel and will also indicate what factors cause significant
increases in energy consumption. The theoretical minimum energies will be compared to typical actual
energy consumptions to determine the potential energy savings for each process. In addition, the
theoretical minimum energies will be converted to CO
2
emissions (detailed in Appendix A), using
assumptions as to the type of energy used and national averages for CO
2
for the type of energy used.
It is not the objective of this study to
examine actual energy consumptions or CO
2
emissions. However, it is useful to compare
approximate industry averages to the minimums
to see what the potential energy savings are for
each process. These comparisons also indicate
the relative energy intensities of the different
steelmaking processes. Particular attention is
given to ironmaking, which consumes 70-80% of
the energy in integrated steel production, and the
electric furnace, which consumes the largest
portion of the overall energy in scrap-based
production.
Objectives
• Compute the theoretical energy required in
the major steelmaking processes for a
number of conditions
• Compare the theoretical minimum energies to
typical actual energy consumptions to
determine the energy saving potential for
each process considered
• Convert the theoretical minimum energies to
CO
2
emissions using the national averages
for the type of energy used in the process
2. BASIC ASSUMPTIONS AND CASES CONSIDERED
The calculations performed are theoretical; the basic assumptions are given on the next page.
The baseline calculations are the absolute minimum energy to produce liquid iron from pure ore and
scrap. The theoretical minimum energy is then computed for a series of cases, including
•
blast furnace ironmaking,
•
direct reduction,
• oxygen steelmaking, and
• electric arc furnace (EAF) steelmaking.
Also calculated is the energy required for production of hot and cold rolled sheet from conventional
slab casters (254 mm or 10 inch) and thin slabs (50 mm or 1.97 inch) and for the production of bars
Theoretical Minimum Energies to Produce Steel for Selected Conditions 1
(25.4 mm, or 1 inch) from a billet (10.16 mm sq, or 4 inch sq). A number of specific issues are also
addressed, including the energy and CO
2
emissions for calcining limestone, using carbon in EAF, the
effect of yield losses, and the effect of air entrainment in the EAF.
Assumptions Used in the Calculations
• Only energy directly used in the process is considered.
• Energy to produce materials such as alloys, oxygen, refractories, and electrodes is excluded.
• Energy loss in producing and transmitting electrical energy is excluded in initial energy calculations. It
is considered for CO
2
emissions.
• Energy and heat losses are excluded. For example, heat losses from furnaces and energy losses from
mechanical and electrical equipment are not considered.
• Energy credits are allowed but noted. For example, the chemical and thermal energy in the off gas from
the blast furnace is credited to the process.
• Energy used in ore agglomeration and cokemaking are excluded; these are treated separately.
• Energy loss due to yield loss is not considered in the theoretical minimum calculations. However, the
minimum energy loss resulting from yield losses is discussed in Section 8.
3. METHODOLOGY
Several different types of energy were considered in the calculations of theoretical minimum
energy requirements, including
• sensible or heating energy,
• melting and phase transformation energy,
• heats of reactions, and
• heats of solution.
The required thermodynamic data was taken from several sources.
(1-6)
Computer programs
were developed that incorporated the energy and materials balance for the process being considered.
The accuracy of the calculation depends on the accuracy of the data used. In general, the uncertainties
of the calculated energies is 1 to 3%.
Sensible or Heating Energy
In all cases considered, input materials such as ore, carbon, oxygen, and scrap must be heated.
The heat ∆H is given by:
T
2
C
p
dT∆Η =
∫
T
1
C
p
= Heat capacity which is a function of temperature
T
1
, T
2
= the initial and final temperatures
2 Theoretical Minimum Energies to Produce Steel for Selected Conditions

Melting and Phase Transformation
The energies associated with materials melted in the process or going through phase transformations are
included.
Heats of Reactions
Chemical reactions either require energy (endothermic) or give off energy (exothermic). An
example of an endothermic reaction is the dissociation or reduction by carbon of FeO:
FeO = Fe + 1/2 O
2
FeO + C = Fe + CO
Examples of exothermic reactions are the oxidation of carbon to CO or CO
2
and of iron:
C + 1/2 O
2
= CO
C + O
2
= CO
2
Fe + 1/2 O
2
= FeO
Heats of Solution
When two or more components go into solution, heat can be given off or absorbed. These
heats compared to the other types considered are usually small. Examples include the heat of mixing of
CaO and SiO
2
and of Fe and carbon.
4. ABSOLUTE THEORETICAL MINIMUM ENERGY VALUES
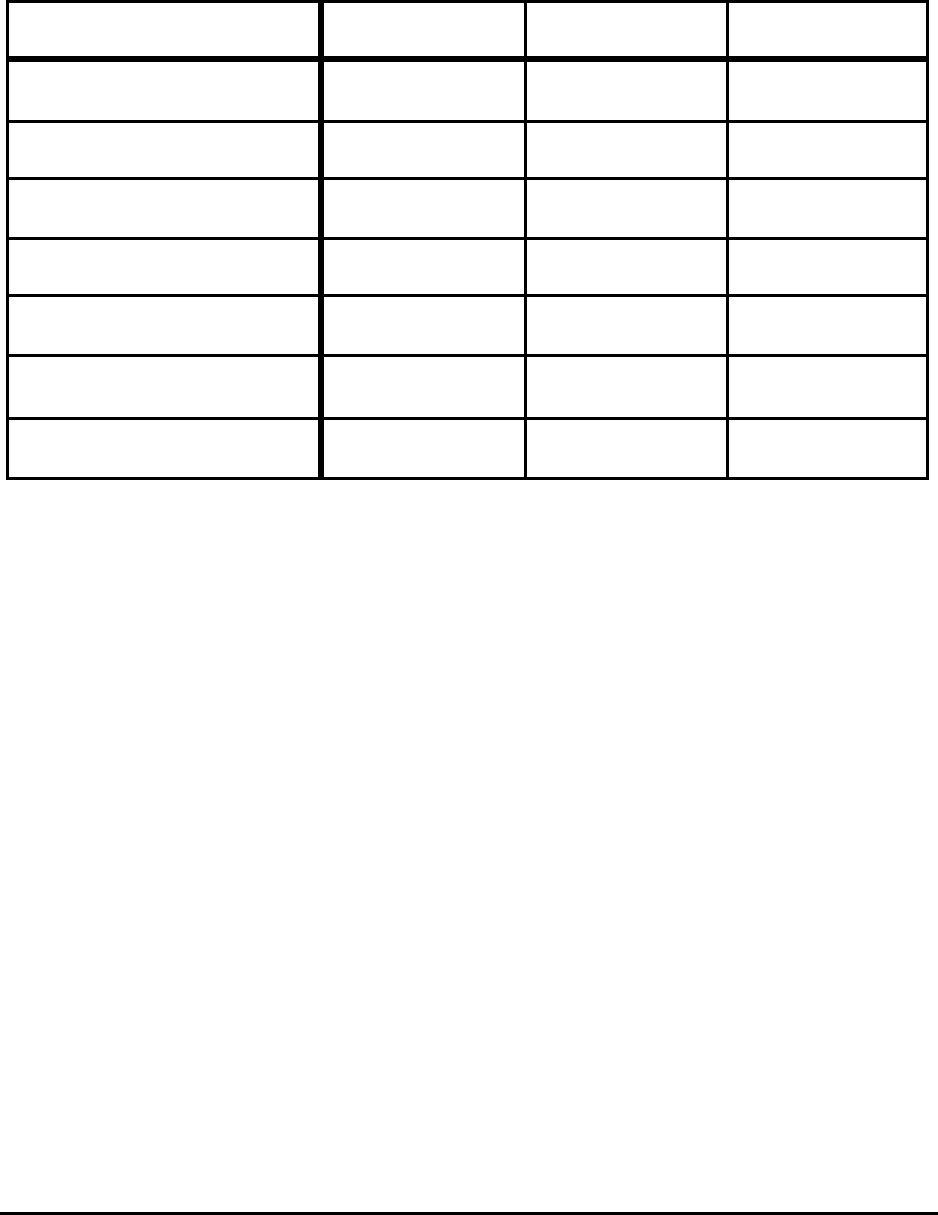
The absolute theoretical minimum energy values for ore reduction and scrap melting are shown
in Table 1 and discussed below.
Ore Reduction
The theoretical minimum energy to produce steel from ore was computed by assuming ore is
pure Fe
2
O
3
and is reduced at 298 K, with the resulting iron heated to its melting point and melted. Iron
goes through a number of phase changes whose energies are included. For the theoretical minimum, no
super heat is required and the product is cast into its final shape without finishing. For this case the
energy is 8620 MJ/tonne. For moderate super heat to 1873 K the energy is 8673 MJ/tonne.
Scrap Melting
The theoretical minimum in this case is simply heated and melted. The energy is 1,274
MJ/tonne. For super heating to 1873 K the energy increases to 1327 MJ/tonne. As in the previous
case, it is assumed the product is cast into its final shape.
Theoretical Minimum Energies to Produce Steel for Selected Conditions 3
Table 1. Absolute Theoretical Minimum Energy to Produce Steel
from Pure Ore (Fe
2
O
3
) and Pure Scrap (Fe)
Raw Material
Absolute Theoretical Minimum Energy
as a Function of Tap Temperature
(MJ/t)
1813 K 1873 K
Ore (Fe
2
O
3
) 8,620 8,673
Scrap (Fe) 1,274 1,327
5. CASES APPROACHING ACTUAL CONDITIONS
Blast Furnace Ironmaking
Blast furnace ironmaking or any ironmaking process requires more energy and releases more
CO
2
than any other process to produce steel. The reduction of Fe
2
O
3
to Fe is by far the most energy-
intensive step. The theoretical energy requirements to produce liquid hot metal for selected conditions
along are presented below, with the specific assumptions. Liquid hot metal or pig iron is defined as iron
containing 4% to 5% carbon. Full credit is given for the energy in the off gas. The ironmaking results
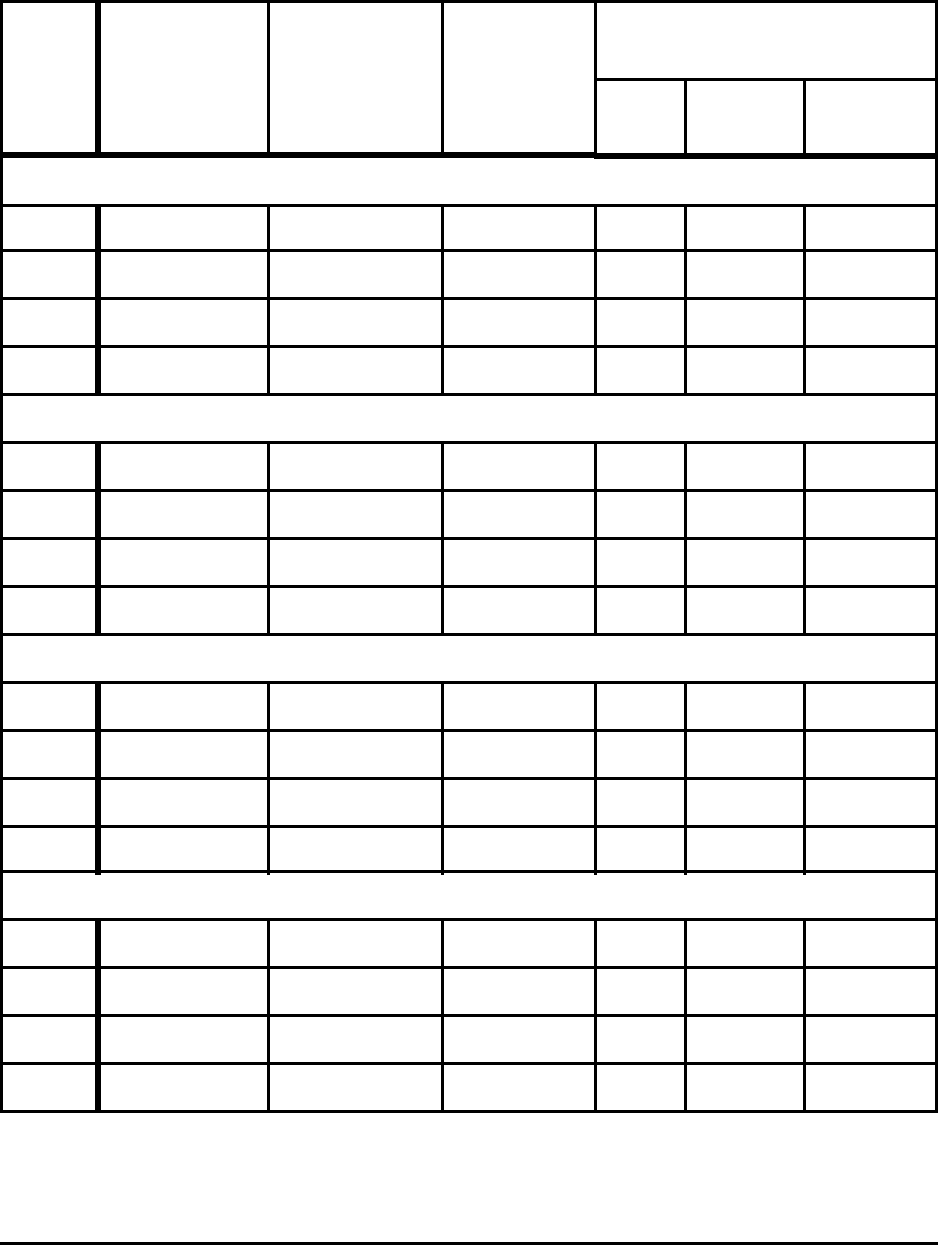
are summarized in Table 2.
Ironmaking Case I - Absolute minimum. The iron blast furnace does not produce pure iron
but rather iron nearly saturated with carbon (typically about 5% C). The tap temperature is usually
about 1723 K (1450°C). In this case the energy associated with the carbon is significant and there is a
small positive heat of solution. The energy required to produce hot metal for this case is 9,805 MJ.
For a tap temperature of 1773 K the energy increases to 9,918 MJ. The energy of carbon associated
with the product (50 kg/t) at this point does not release any CO
2
; the CO
2
is released during
steelmaking. Also, the small heat of solution is given back during steelmaking as the carbon is burned.
Ironmaking Case II - Effect of slag resulting from gangue in ore. Ore is not pure Fe
2
O
3
but contains gangue or impurities, primarily SiO
2
and Al
2
O
3
. An ore with 6% gangue and an assumed
consumption of 4% SiO
2
, 1% MnO, and 1% Al
2
O
3
was considered. To form a blast furnace slag, lime
(CaO) is added. A typical slag will have a basicity (B) of 1.2, where B is defined as
B
=
(%CaO)
(%SiO
2
)
4 Theoretical Minimum Energies to Produce Steel for Selected Conditions
--
--
--
--
Table 2. Minimum Energies to Produce Liquid Hot Metal at 1723 K
(1450°C or 2642°F) for Selected Conditions
Metal Composition Gangue Ash
Energy
(MJ/t HM)
Fe - 5%C No No 9,805
Fe - 5%C Yes No 10,216
Fe - 5%C - 0.5%Si - 0.5%Mn Yes No 10,268
Fe - 5%C - 0.5%Si - 0.5%Mn Yes Yes 10,421
Notes: Gangue - 4% SiO
2
- 1% Al
2
O
3
- 1% MnO of ore
Ash - 5% of 500 kg of coal and coke or 25 kg/t HM
Slag Basicity
%CaO
=
1.2
%SiO
2
Energy credit for off gas approximately 5,400 - 5,600 MJ/t HM in each case
In this case all the gangue is assumed to enter the slag. In the calculation, the energy to heat and
melt the gangue and CaO are included. There is a significant negative heat of solution or heat release of
the slag components. Because of the presence of gangue, the energy increases to 10,216 MJ per
tonne of hot metal.
Ironmaking Case III - Effect of slag and reduction of SiO
2
and MnO in slag. During
ironmaking some of the SiO
2
and MnO is reduced. A typical metal composition is as follows:
Fe 94%
C 5%
Si 0.5%
Mn 0.5%
When the energy for slag formation and reduction of SiO
2
and MnO is included, the energy is 10,268
MJ per tonne of hot metal for a tap temperature of 1773 K.
Ironmaking Case IV - Effect of coke ash. Coke and injected coal contain ash. For this case
500 kg of coke plus coal per tonne of hot metal is assumed, with an average ash content of 5% (the ash
is assumed to contain 4% SiO
2
, 1% Al
2
O
3
, and 1% MnO). The assumed 500 kg of coke plus
coal/tonne hot metal is not the theoretical minimum but reflects typical actual values. The other
conditions for slag and reduction are similar to Case III, and the energy increases to 10,421 MJ per
tonne of hot metal.
Theoretical Minimum Energies to Produce Steel for Selected Conditions 5
Direct Reduction
The production of direct reduced iron by a number of processes has increased dramatically in the
past five years. For the purposes of this report direct reduction is defined as any process that converts
ore into a solid product containing primarily metallic iron. The product will also contain gangue from the
ore, along with reductant, carbon, and unreduced iron oxide (FeO). In most cases, natural gas (CH
4
)
supplies the energy. Examples of such processes are Midrex, HyL, FINMET, CIRCORED, and Iron
Carbide.
The general assumptions for direct reduction are the same as for ironmaking in a blast furnace. The
gangue is assumed to be entirely SiO
2
. It is also assumed that the ore is heated to 900°C and reduced.
Full credit for the energy in the off gas is taken. In these processes the off gas is usually recycled back
into the process, and the chemical energy is used but some thermal energy is lost.
As in the reduction of liquid hot metal, the simplest case is considered first and then realistic
computations such as gangue in the ore and carbon in the product are added. All energy values given
are per tonne of product. Some products require more energy when used to produce liquid steel in an
EAF, which is discussed in a later section of this report. The results of the five cases considered are
given in Table 3.
Table 3. Minimum Energies to Produce Direct Reduced Iron at 1173 K
(900°C or 1620°F) Reduction Temperature for Selected Conditions
Ore Product
Energy
(MJ/tonne)
Pure Fe
2
O
3
Fe 8,360
Fe
2
O
3
- 1.4% SiO
2
Fe - 2% SiO
2
8,380
Fe
2
O
3
- 1.4% SiO
2
Fe - 2% SiO
2
- 8% FeO 7,900
Fe
2
O
3
- 1.5% SiO
2
Fe - 2% SiO
2
- 8% FeO - 2% C 8,427
Fe
2
O
3
- 1.5% SiO
2
Fe - 2% SiO
2
- 7.7% FeO - 6% C 9,432
Note: Full credit is assumed for off gas
It is important to note that the energy per tonne of iron is different in each case and the energy to
melt the materials varies. Later in this section, the total energy to produce steel is given for a 50%
scrap - 50% DRI charge.
Oxygen Steelmaking
Oxygen steelmaking (OSM) does not in itself consume energy but instead generates it. In the
process, the carbon, silicon, and other elements in the hot metal are oxidized, producing energy
6 Theoretical Minimum Energies to Produce Steel for Selected Conditions
--
--
primarily used to melt scrap. The energy can also be used to melt and smelt (reduce) waste oxide
briquettes or smelt ore. The amounts of these oxide-containing materials that can be used are
significantly less than scrap because of the energy required to reduce the iron oxides. In the United
States scrap is almost exclusively used as a coolant and is included in this analysis.
Table 4 lists the amounts of scrap that can be melted per tonne of hot metal of selected
compositions. Also given is the amount of liquid steel produced and the energy per tonne of liquid
steel, computed by dividing the energy required to produce the hot metal by the amount of steel
produced.
Table 4. Energy to Produce Steel from Hot Metal and Scrap for Selected Conditions
Hot Metal Slag
Tap T
p
(K)
Scrap
(kg/t HM)
Steel
(kg/t HM)
Energy
(MJ/t steel)
Fe - 5% C 1873 290 1,240 7,907
Fe - 5% C 1923 244 1,194 8,212
Fe - 5%C - 0.5% Si
(coke ash)
B=3
30% FeO
1873 401 1,328 7,853
Fe - 5%C - 0.5%Si
(coke ash)
B=3
30% FeO
1923 353 1,278 8,154
Fe - 5%C - 0.5% Si
(coke ash)
B=3
20% FeO
1873 389 1,320 7,894
Fe - 5%C - 0.5%Si
(coke ash)
B=3
20% FeO
1923 341 1,270 8,205
Note: The decrease in energy per tonne of steel for oxidizing Si and Fe is due to increased scrap melting.
The calculations assume CO to be the off gas no post combustion. For 5% C in the hot metal the
potential energy in the off gas ranges from 950 MJ/t (steel) to1,000 MJ/t if the CO is oxidized to CO
2
.
The results in Table 4 indicate that less energy per tonne of steel is obtained when silicon is present
in the hot metal and iron is oxidized. This is somewhat misleading because the reduced energy per
tonne of steel is from melting scrap using the additional energy from the oxidation of Si and Fe. Since it
takes considerably more energy to produce hot metal from ore than to melt scrap, the energy per tonne
of steel produced is reduced when melting more scrap in OSM. This important fact can be illustrated
by considering a case in which the additional scrap melted by oxidizing Si and Fe was simply melted in
another process. For the case when there is no silicon of iron oxidized to the slag, 290 kg of scrap is
melted and the energy per tonne of steel is 7,907 MJ while for 30% FeO and 0.5% Si, 401 kg of scrap
is melted and the energy is 7,853 MJ per tonne of steel. If the additional 111 kg of scrap was simply
Theoretical Minimum Energies to Produce Steel for Selected Conditions 7
--
melted separately, it would require 146 MJ. This reduces the average energy per tonne of steel to
7,200 MJ for no FeO or SiO
2
in the slag if the additional 111 kg of scrap was simply melted.
Electric Furnace Steelmaking with Scrap
A number of cases were considered for scrap and scrap alternative melting in an EAF. The results
for 100% scrap melting are summarized in Table 5. The energy loss in producing electricity or the
energy required to process the scrap is not considered. Only the energy consumed in the process is
included, not the energy to produce other materials, such as electrodes and refractories.
Table 5. Theoretical Minimum Energies to Produce Steel from Scrap for
Selected Conditions, Tap Temperature 1873 K (1600°C or 2912°F)
Scrap Slag
Energy
(MJ/t)
Fe 1,327
Fe - 0.1%C - 0.2%Si
B = 2.5
%FeO = 25% 1,289
Fe - 0.1%C - 0.2%Si
1% dirt
B = 2.5
%FeO = 25%
1,352
Fe - 0.1%C - 0.2% Si
1% dirt
B = 2.5
%FeO = 35%
1,325
Fe - 0.1%C - 0.2%Si
1% dirt
100 Nm
3
air entrainment
B = 2.5
%FeO = 25%
1,577
Note: The decrease in melting energy with FeO in slag is due to oxidation of Fe
EAF Case I - Absolute Minimum to Melt Iron. The absolute minimum energy is 1,327 MJ/t,
the amount of energy required to heat and melt scrap and super heat the metal to 1873 K.
EAF Case II - Scrap Containing 0.1%C - 0.2%Si. In this case, it is assumed that the carbon
remains in the steel and the silicon is oxidized to SiO
2
. Flux (CaO) is also heated and melted, yielding a
slag with a basicity of 2.5 and 25% FeO. The energy requirement is 1,289 MJ/t. The reduction in
energy from Case I is due to the oxidation of Si and Fe to the slag, which results in a yield loss of 0.6%.
EAF Case III - Scrap Containing 0.1%C - 0.2%Si + 1.0% (10 kg) "Dirt." Scrap also has
other materials with it such as water, soil or dirt, and oil. Based on the final slag formed, considerable
amounts of materials containing SiO
2
and Al
2
O
3
(e.g., dirt) enter the furnace.
8 Theoretical Minimum Energies to Produce Steel for Selected Conditions

--
For 10 kg per tonne of scrap of SiO
2
plus Al
2
O
3
and maintaining a slag basicity of 2.5, the energy
required is increased to 1,352 MJ/t, with a yield loss to the slag of 2.5%
EAF Case IV - Air Entrainment. During EAF steelmaking large quantities of air can be
entrained into the furnace through the furnace door and other openings. It is estimated that 100
nm
3
/tonne of air can be entrained. If the N
2
leaves at 1473 K or 1873 K, the heat associated is 165
MJ and 225 MJ, respectively, increasing the energy from Case III to 1,517 MJ and 1,577 MJ/t,
respectively.
Electric Furnace Steelmaking with DRI and Scrap
The energy required to produce steel with 50% scrap and 50% scrap substitute was calculated.
The scrap was assumed to contain 0.2% Si and 10 kg dirt per tonne. There is no post combustion or
air entrainment in the process. The slag is assumed to have basicity of 2.5 and 25% FeO. The energy
required to produce steel (0.05%C) for several cases is given in Table 6. This table also shows the
energy required to produce steel with solid and liquid pig iron as 50% of the charge. The total energy
given in Table 6 includes the energy to produce the DRI (shown in Table 3).
There are numerous other combinations of scrap and DRI and other assumptions with regards
to slag and post combustion, which were covered in the AD Little report to AISI
(7)
.
6. PRODUCTION OF STAINLESS STEEL
There are numerous grades of stainless steel produced in the United States. The most common is
18-8 type stainless, which contains 18% Cr and 8% Ni. This analysis assumes the production of a
generic 18-8 type stainless steel in an EAF-AOD (Argon Oxygen Decarburization) sequence, which
accounts for over 90% of U.S. stainless steel production.
In the EAF, a mixture of stainless steel scrap, steel scrap, high carbon ferrochrome (HCFeCr), and
nickel are melted and the carbon content is reduced to about 1%. The metal is then transferred to an
AOD vessel where a mixture of O
2
- Ar or O
2
- N
2
gases oxidizes the carbon to its desired level. The
AOD itself does not theoretically require energy and is therefore not considered. After the AOD, a
reductant containing either silicon or aluminum is added to the slag to recover chromium, which is
oxidized to slag. For the assumptions of the present study, this stage also need not be considered.
A typical charge to the EAF per tonne of steel is as follows:
Steel Scrap 350 kg
Stainless Scrap -- 400 kg
The carbon is combusted with oxygen down to 1.0%. The absolute minimum energy for melting
the above charge is 1,213 MJ, which is less than melting Fe because of carbon oxidation. If the scrap
contains 1% dirt and there is no air entrainment, the melting energy is 1,275 MJ/t.
Theoretical Minimum Energies to Produce Steel for Selected Conditions 9
--
--
--
Table 6. Theoretical Minimum Energies to Produce Liquid Steel at 1873 K (1600°C or
2912°F) from 50% Scrap and 50% Scrap Substitute
Scrap Substitute
Melting Energy
(MJ/t)
Charge Energy
(MJ/t)
Total Energy
(MJ/t)
None (!00% Scrap Case) 1,289 1,289
Fe - 2% SiO
2
1,403 4,296 5,698
Fe - 2%SiO
2
- 8%FeO 1,559 4,233 5,792
Fe - 2%SiO
2
- 8%FeO -2%C 1,483 4,565 6,048
Fe - 2%SiO
2
- 7.7%FeO - 6%C 1,328 5,223 6,551
Liquid Pig Iron 487 5,388 5,875
Solid Pig Iron 1,145 5,388 6,533
Notes: Liquid Pig (Hot Metal) charged at 1723 K, all others 298 K
If air entrainment is included, it will add 238 MJ/t in each
Slag has B = 2.5 and 25% FeO
HCFeCr 250 kg
Nickel 48 kg
According to the initial assumptions and boundaries of the present calculations the energy to
produce the high carbon ferrochrome is not included. The amount of alloys for carbon steel production
is much smaller than it is for stainless steel. Since the energy in ferrochrome is so high, it is useful to
estimate the theoretical minimum for its production. Only the very simplest case of the absolute
minimum for the production of HCFeCr is considered.
HCFeCr is produced from chromite ore whose composition depends on the specific mine
location. The major component is FeCr
2
O
4
. For the absolute minimum, the ore is considered to be
pure FeCr
2
O
4
and the product 61% Cr - 33% Fe - 6% C. The absolute minimum energy is 11.7
GJ/tonne of product. For the 250 kg used in the present example, the absolute minimum is 2,925 MJ
per tonne of steel; the actual energy will be over 4,000 MJ. It should be noted that HCFeCr is not
produced domestically and therefore no energy is used in the United States. If the minimum energy to
produce ferrochrome is included, the total energy is increased to 3,200 MJ/tonne of 18-8 stainless
steel.
10 Theoretical Minimum Energies to Produce Steel for Selected Conditions

7. ROLLING
There are two general types of rolling -- hot and cold. With hot rolling, the steel either is
reheated or directly charged after casting. Reheating is the major energy consumer in the rolling
process. For both hot and cold rolling, energy is also consumed in deformation, where the deformation
energy (E) is given by
E=sec
where
σ = the yield stress
ε = strain or deformation
c = a constant describing the shape of the stress strain curve, assumed to be an average of 0.7
The strain is defined in terms of the initial and final dimensions, t
o
and t
f
, respectively.
e
= ln
t
o
t
f
The yield strengths used in the following calculations were taken from the Making, Shaping
and Treating of Steel.
(8)
Cases included were hot and cold rolling for flat rolled carbon steel and 18-8 type stainless
(starting from both normal slabs and thin slabs), as well as hot rolling of bars from billet. The effects of
direct charging and billet splitting are also included in the analysis.
For hot rolling, the deformation energy is low because the yield stress is low at high
temperatures. For stainless steel, the yield stress is about three times higher than for carbon steel, while
for bars it is slightly higher than for flat steel because the carbon content is generally higher. The energy
used for heating is fossil fuel and for rolling electrical. Table 7 lists the minimum energies for rolling,
assuming no energy or yield losses.
The results in Table 7 indicate that reheating the slab or billet accounts for the vast majority of
the rolling energy. If slabs of normal thickness are directly charged, as is the case for most thin slabs,
the energy for rolling these slabs is similar to that for thin slabs. Strip casting a 2-mm strip does not
significantly reduce the total energy if the normal slab is directly charged. The total energy for a thin
slab with hot and cold rolling with direct charging is 33 MJ, compared to cold rolling a 2-mm strip to 1
mm, which requires 17 MJ.
During hot rolling the metal being processed cools and must be reheated or deformed at a
lower temperature. These effects require more energy but are not considered.
Theoretical Minimum Energies to Produce Steel for Selected Conditions 11
--
--
--
--
--
--
--
--
Table 7. Theoretical Minimum Energies to Roll Steel for Selected Products and Conditions
Bar Carbon (10 cm billet to 2 cm bar)
a
Slab temperature prior to rolling
b Billet split into 4 pieces prior to rolling
Rolling
Type
Rolling
Temperature
(K)
Slab
Temperature
a
(K)
Reduction
(mm)
Energy
(MJ/t)
Heat
Deform-
ation Total
Flat Carbon Slab (25.4 cm or 10 inch)
Hot 1473 298 254 to 2 825 25 850
Hot 1473 1173 254 to 2 270 25 295
Hot 1473 1473 254 to 2 25 25
Cold 298 298 2 to 1 17 17
Flat Carbon Slab (5.0 cm or 1.97 inch)
Hot 1473 298 50 to 2 825 16 841
Hot 1473 1173 50 to 2 270 16 286
Hot 1473 1473 50 to 2 15 16
Cold 298 298 2 to 1 17 17
Flat 18-8 Stainless Steel Slab (25.4 cm or 10 inch)
Hot 1473 298 254 to 2 825 72 897
Hot 1473 1173 254 to 2 271 72 342
Hot 1473 1473 254 to 2 72 72
Cold 298 298 2 to 1 51 51
Hot 1473 298 10 sq to 2 sq 825 20 845
Hot 1473 1473 10 sq to 2 sq 20 20
Hot 1473 1173 10 sq to 2 sq 270 20 290
Hot
b
1473 1473 10 sq to 2 sq 11 11
12 Theoretical Minimum Energies to Produce Steel for Selected Conditions

8. EFFECT OF YIELD LOSSES ON ENERGY
The calculation of minimum energies assumed no process yield losses. However, yield losses
occur in all processes, and their effect on energy depends on where the yield loss occurs and how much
energy is "invested" in the iron or steel to that point. Some forms of yield loss have been taken into
account and the energy reclaimed in the calculation. For example, in steelmaking Fe is oxidized to the
slag, releasing energy that is used in the process. This energy release has been included in the
steelmaking energy calculation. For oxygen steelmaking, this added energy increased the hot metal
requirement and, therefore, the energy per tonne of steel. For scrap-based EAF, the "invested energy"
in the scrap was not included.
The following section addresses the impact of yield losses related to the various processes. In
all cases, the energy invested in the product is assumed to be that for the most likely case for
production. For example, for oxygen steelmaking the most likely case for production is the case for hot
metal containing silicon. The most likely case for production in the EAF is assumed to include slag and
air entrainment. A summary of the effect of yield loss on the minimum theoretical energy requirements
to produce steel is summarized in Table 8.
Ironmaking and Hot Metal Treatment
Significant yield losses can occur during tapping, handling, and treatment of hot metal. In
particular, iron is extracted with the slag during slag removal after hot metal desulfurization. Yield losses
of 1% to 3% are typical; for a 1% yield loss of liquid pig iron the energy loss would be 104 MJ. If the
iron is recovered from the slag and reused, only the sensible and melting energy (about 14 MJ) is lost.
Steelmaking
Yield losses in both oxygen steelmaking and EAF steelmaking can be significant. The highest
yield loss is FeO to the slag, which can be 5% to 10%. This yield loss releases energy, which was
considered, but more iron must be charged. Both of these effects were taken into account in the results
given for OSM and the EAF. However, there is no energy charge for the increased scrap usage.
Other yield losses can be vaporization of iron (which is oxidized to the dust), other iron losses to the
dust, and losses during tapping and handling.
Vaporization of iron consumes a considerable amount of energy in steelmaking. The energy is
not recovered when the vaporized iron is oxidized in the off gas. For 1.0% iron vaporizing in the BOF
and no energy recovery from iron oxidation, the energy loss is the energy invested in the steel (80 MJ)
and the heat of vaporization (64 MJ), or a total of 144 MJ/t of steel. Typical losses by vaporization
are about 0.5% to 1.0%. In the case of 1.0% iron vaporizing in the EAF, the invested energy in the
steel is less (13 MJ) and the total energy loss is 77 MJ.
The direct energy lost by vaporization, 64 MJ, can actually cause a larger decrease in energy
for oxygen steelmaking. The energy loss will result in less scrap melting, which will cause a shift from
scrap to hot metal. Since hot metal requires considerably more energy than melting scrap, the resulting
increase in energy could be as high as 500 MJ.
Iron is also lost to the dust by simple metal ejection. In this case, only the energy invested is
lost. Again, the iron is oxidized in the off gas and little is recovered. For a 1.0% yield
Theoretical Minimum Energies to Produce Steel for Selected Conditions 13
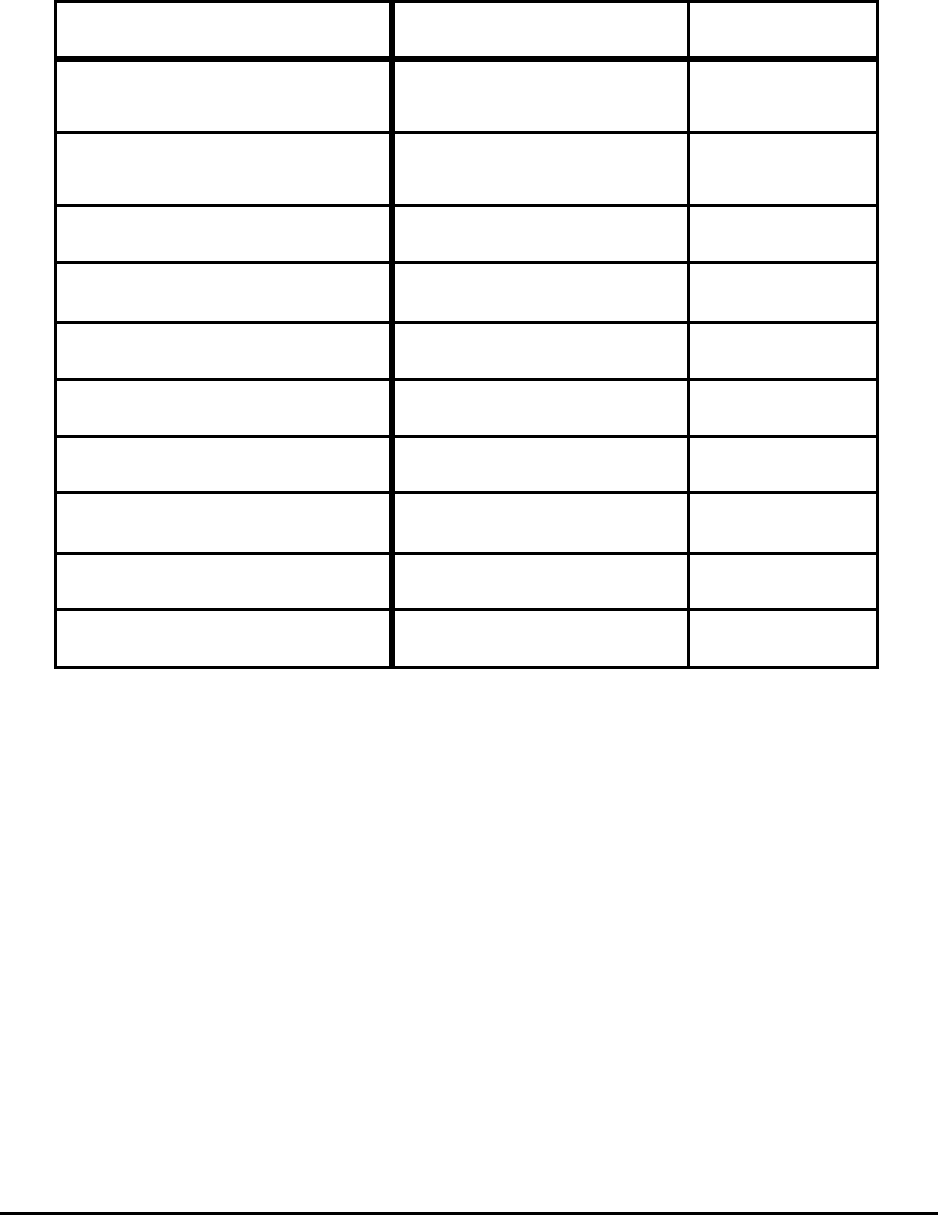
Table 8. Effect of Yield Loss on Minimum Theoretical Energies
to Produce Steel for 1% Yield Loss
Process Type of Loss Energy (MJ/t)
Hot metal loss in pretreatment
Hot metal loss to slag
but recovered 14
Hot metal loss in pretreatment
Hot metal loss to slag
not recovered 104
Direct Reduction Fines lost 60
Direct Reduction Fines recycled 6
BOF Fe vaporized to dust 144
EAF Fe vaporized to dust 102
BOF Fe ejected to dust 80
EAF Fe ejected to dust 16
Hot rolling Fe lost but recovered 13
Cold rolling Fe lost after reheating for 34
Notes: Losses to slag in BOF and EAF were accounted for in the process.
For rolling, if the metal is not recovered or oxidized, the energy invested in the steel is lost (about
80 MJ for BOF and 16 MJ for EAF).
The energy consumed by Fe vaporization reduces scrap melting, thus requiring more hot metal per
tonne of steel. This could represent 500 MJ due to the higher energy associated with hot metal.
loss by metal ejected, the energy loss is 80 MJ for OSM and 16 MJ for EAF steelmaking. The
vaporization or loss of other metals, such as zinc, are possible but not considered.
Direct Reduction
Some material is lost as fines during the production of DRI/HBI. This material is often
recovered and used to form briquettes or injected into an EAF, in which case there is no energy loss.
However, if the material is lost or more likely recycled back into the direct reduction furnace, some
energy is lost. Assuming the material does not reoxidize and is recycled into the process, the energy
loss is 6.2 MJ/t for 1% material recycle. If the material is reoxidized, the energy loss per 1% is 84
MJ/t.
14 Theoretical Minimum Energies to Produce Steel for Selected Conditions

Rolling
One of the largest cost and energy consumers in rolling is yield loss. The yield loss is usually
remelted, requiring about 13 MJ/t for a 1% loss of Fe. However, the yield loss often occurs later in the
process when additional energy has been invested into the steel. For example, if the slab is reheated
and hot rolled, the yield loss after cold rolling results in a 34 MJ/t loss. If the iron is oxidized or not
recovered, the invested energy in the steel is lost. A 1% yield loss applied to the most likely production
cases represents an additional loss of 80 MJ/t for BOF steel and 16 MJ/t for EAF steel.
9. OTHER MAJOR ENERGY-CONSUMING ACTIVITIES
Several significant sources of energy consumption were neglected in computing the theoretical
minimum energies to produce steel for selected conditions. In some cases, estimates of the energy for
these sources can be made while for others the conditions vary greatly and reasonable estimates are
difficult.
In theory, ore agglomeration and cokemaking are not necessary for integrated steel production
and were not considered. However, these processes are virtually always part of actual integrated
steelmaking. Ore agglomeration consists of taking ore fines or concentrate and sintering them into sinter
or pellets. Cokemaking is achieved by heating coal under pressure, driving off volatiles and increasing
the strength of the material. The theoretical minimum energies for these processes were determined by
calculating the energy to heat idealized coal and ore to 1100°C and 1350°C, respectively. In
cokemaking the heat of devolatilization is also required but is usually accounted for in computing the
energy value of coal. The coke output was taken as 0.768 per tonne of the coal input. The volatiles
represent the remainder, which is used as a fuel or for chemicals. The approximate minimum energies
for these processes, along with typical actual consumptions, are given in Table 9. The actual energies
are given as a range because the values vary according to the actual process and its efficiency.
During cokemaking the volatiles are either used for producing chemicals with energy values and
credits or as a fuel in the process itself. The process also produces a relatively rich off gas for which an
energy credit is taken. The total credits are about 4.0 to 5.0 GJ/t steel or 11.8 to 14.5 GJ/t coke. The
values given in Table 9 have taken into account all the energy credits.
The actual and theoretical minimum energy requirements for ore agglomeration are reasonably
close because of the simplicity of the process. For cokemaking the differences are greater because of
process complications and yield losses.
There are other energy-consuming process steps or materials used in steelmaking that were
beyond the scope of this study. Often the energy consumption is very site-specific; the energy is
consumed in another area or even country and depends on process details. These other steps include
• mining of ore, coal, and limestone (integrated production),
• scrap preparation (in scrap-based production),
• transportation of materials to and within the plant, and
• production of alloys, refractories, electrodes, special fluxes, and other materials
consumed in steelmaking.
Theoretical Minimum Energies to Produce Steel for Selected Conditions 15
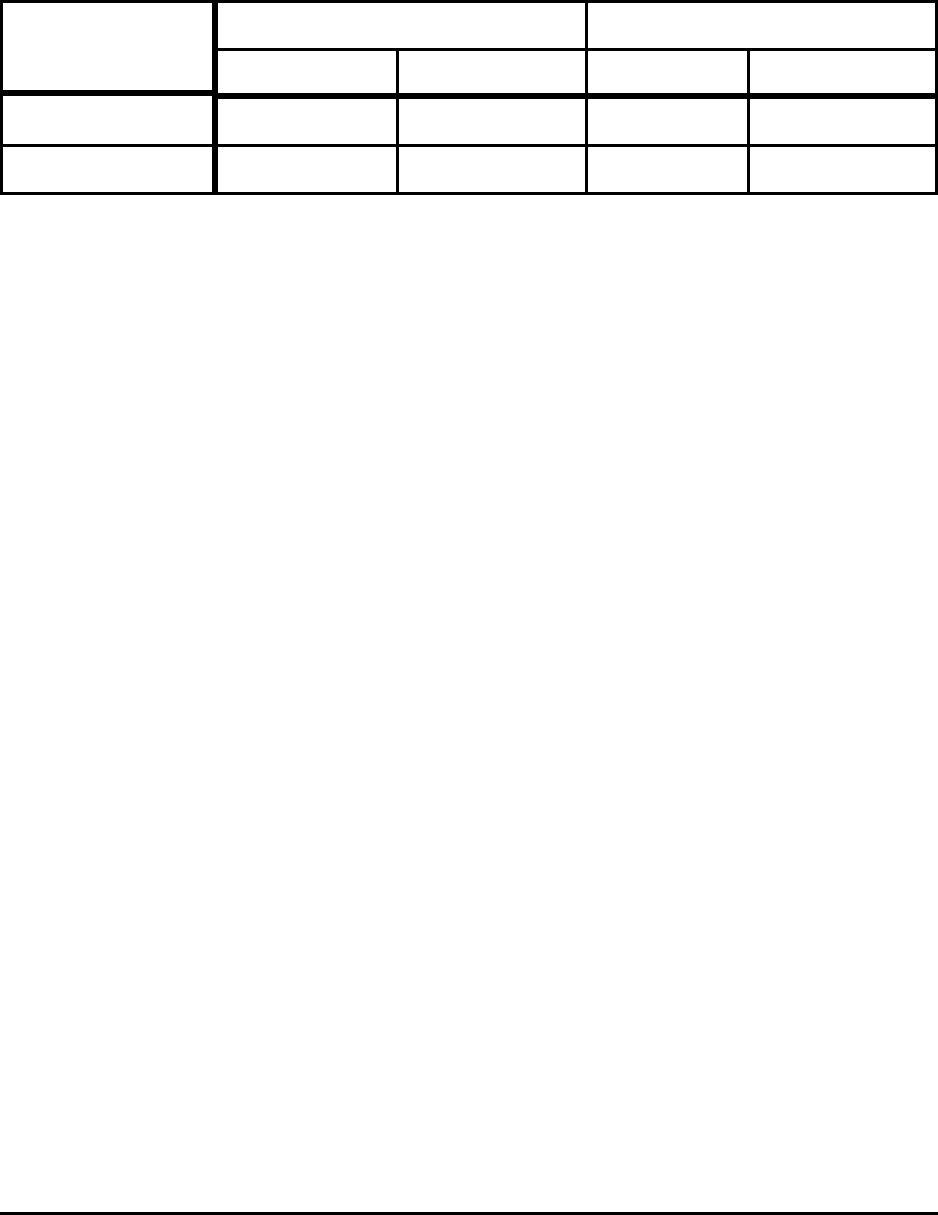
Table 9. Theoretical Minimum and Typical Energy Consumption
in Cokemaking and Ore Agglomeration
Process
Theoretical Energy Actual Energy
GJ/t (output) GJ/t (steel) GJ/t(output) GJ/t (steel)
Cokemaking 2.0 0.8 5.4 - 6.2 2.2 - 2.6
Ore Agglomeration 1.2 1.6 1.5 - 1.7 2.1 - 2.4
Note: For cokemaking, full credit is taken for off gas and by-product energy, approximately 4-6 GJ.
10. TOTAL THEORETICAL MINIMUM ENERGY TO PRODUCE STEEL
It is difficult to adequately describe the total energy to produce final steel products because it
depends on the process used (EAF or integrated), the raw or charge materials, and the conditions of
rolling. Furthermore, the total does not give any insight as to where and how energy can be conserved.
However, it is somewhat useful to compare process routes and theoretical to actual energy
consumption requirements.
Table 10 summarizes the total energy requirements for producing selected steel products for
selected conditions. The most realistic minimum values are about 8,150 MJ/tonne for integrated
production and 1,600 MJ/tonne for scrap-based production. Yield losses and other additional losses
are not considered.
11. SPECIAL CONSIDERATIONS
This section discusses information regarding theoretical minimum energies that is beyond the
primary scope of this report. Whereas the process primarily uses lime (CaO), the raw material is
limestone (CaCO
3
). The conversion of limestone to lime requires energy and produces CO
2
. The
impact of SiO
2
on gangue materials in the ore and coke on energy is followed through the process.
The impact of post combustion and carbon in the BOF and EAF are also considered.
Energy and CO
2
in Producing CaO
The major flux used in iron and steelmaking is CaO. CaO is obtained from limestone (CaCO
3
)
by heating it and driving off the CO
2
according to the following reaction:
CaCO
3
= CaO + CO
2
The reaction will only occur when the equilibrium pressure reaches one atmosphere. Therefore, the
reactants must be heated to 1155 K and the energy of the reaction supplied.
16 Theoretical Minimum Energies to Produce Steel for Selected Conditions
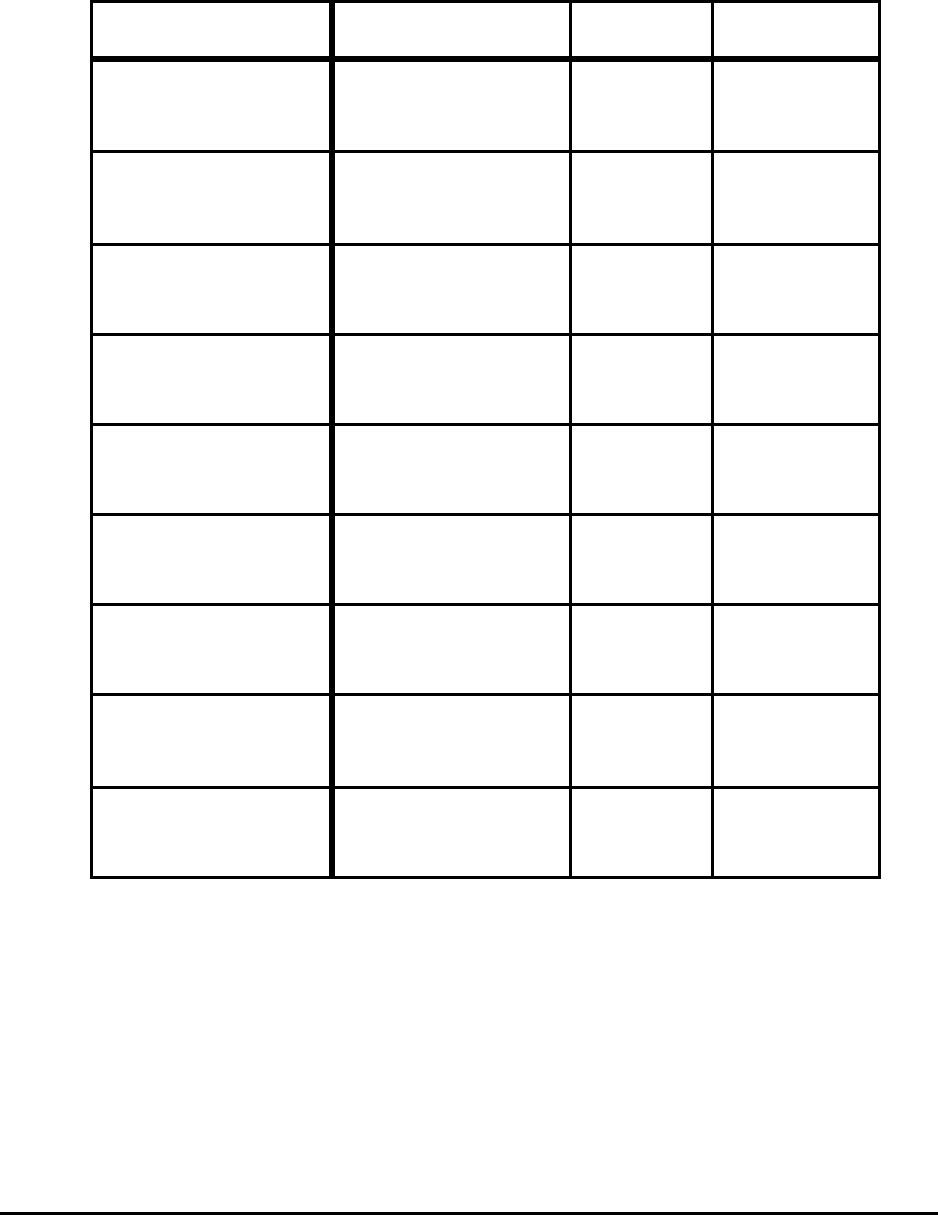
Table 10. Total Theoretical Minimum Energies to Produce
Selected Products for Selected Conditions
Process Product Rolling Energy (MJ/t)
BF-BOF
(Conventional Slab)
Hot Rolled Sheet Direct
Reheat
7,878
8,703
BF-BOF
(Conventional Slab)
Cold Rolled Sheet Direct
Reheat
7,895
8,720
EAF (Scrap)
(Thin Slab)
Hot Rolled Sheet Direct
Reheat
1,341
2,166
EAF Scrap
(Thin Slab)
Cold Rolled Sheet Direct
Reheat
1,358
2,183
EAF (50% DRI)
(Thin Slab)
Cold Rolled Sheet Direct
Reheat
6,081
6,906
EAF (Scrap)
(Billet)
Bar Direct
Reheat
1,345
2,170
EAF (50% DRI)
Billet
Bar Direct
Reheat
6,068
6,893
EAF-AOD
Slab
Cold Rolled
18-8 Stainless
Direct
Reheat
1,397
2,223
EAF-AOD-(HCFeCr)
Slab
Cold Rolled
18-8 Stainless
Direct
Reheat
4,323
5,148
Notes: Does not include ore preparation, cokemaking, or yield losses.
Stainless for melting only and for production of required HCFeCr.
Liquid steel energy:
BF-BOF 7853 MJ/t
EAF-scrap 1355 MJ/t
EAF-DRI 6048 MJ/t
EAF-AOD 1275 MJ/t
EAF-AOD-HCFeCr 4200 MJ/t
Theoretical Minimum Energies to Produce Steel for Selected Conditions 17

To produce one tonne of CaO from CaCO
3
requires a theoretical minimum energy of 4,670
MJ. The product is CaO and CO
2
at 1155 K. It is theoretically possible but highly impractical to
utilize the heat contained in the CaO (1,471 MJ). In addition to the energy required, 0.79 tonnes of
CO
2
is released per tonne of CaO.
Effect of SiO
2
Silica enters the steelmaking process primarily as gangue in the ore or coke ash. Silicon is also
present in scrap. The silica requires energy in ironmaking and steelmaking because it must be heated
and melted along with the appropriate amount of lime. The energy in ironmaking increases by about
110 MJ for 1% extra silica in the gangue or ash. However, this SiO
2
requires CaO. Whereas the
heating and melting is included in the 110 MJ, the production of the CaO is not for a slag basicity of1.2,
which requires 12 kg CaO per 1% SiO
2
. This requires an additional 5.5 MJ, and 0.008 tonnes of CO
2
is released from the limestone.
Energy Credits in Off Gases
Full energy credit was taken for the off gas for the processes of blast furnace ironmaking,
cokemaking, and direct reduction. These credits are a large percentage of the total energy. This
energy must be efficiently utilized for the processes to be energy-efficient. The off gases can be used in
the process itself such as in blast furnace air preheating stoves, in reheating furnaces, or producing
steam for electricity. For integrated steel production, including cokemaking, the total energy in off
gases can be as high as 6-10 GJ per tonne of steel. Therefore, the efficient use of this energy is critical.
Carbon in Steelmaking
Carbon is usually used as a fuel in the EAF and is sometimes used to melt more scrap in a
BOF. The carbon used in an EAF usually contains ash, which reduces its energy value by 5% to15%,
depending on the amount. For these theoretical calculations the material is assumed to be pure carbon.
The reaction product in the presence of carbon or iron is primarily CO. If the carbon is only oxidized
to CO, it is much less efficient than using the same carbon to produce electrical energy. For the carbon
to be as effective as electricity generation and transmission with an efficiency of 36% (which
corresponds to 10 MJ/kWh), it must be post combusted (PC) to 37.6% with 100% of the energy
going to the steel. The equation below represents the post-combustion percentage:
CO
PC
=
2
(100)
CO
+
CO
2
These conditions are difficult to achieve in practice, and in the actual case the required PC
would be higher because of the ash in the carbon. Therefore, using carbon does not reduce energy use
in the EAF, even if the inefficiency of electric power production is considered. However, carbon
oxidation does improve productivity and refining of the steel, which is its primary purpose.
18 Theoretical Minimum Energies to Produce Steel for Selected Conditions

Post Combustion
In steelmaking processes, the equilibrium product for the oxidation of carbon is primarily CO,
with less than 10% CO
2
. It has been recognized for decades that significant quantities of energy are
available if the CO can be combusted to CO
2
in such a way that the CO
2
does not react with iron or
carbon, and the energy can be transferred to the system.
The post combustion reaction is
CO + 1/2 O
2
= CO
2
After accounting for the heating of the oxygen, the heat released at 1873 K is 255 KJ per mole
of CO. For example, if 10% of the CO can be post combusted in OSM with 100% heat- transfer
efficiency, the energy released is 106 MJ per tonne of hot metal. This energy can melt 80 kg of scrap
per tonne of metal, which can significantly reduce the energy to produce steel because of the shift from
ore to scrap. For OSM with a slag basicity of 3 and 30% FeO and a tap temperature of 1873 K, the
energy per tonne of steel is reduced from 7,853 MJ to 7,401 MJ. The major reason for the energy
savings is the use of more scrap.
In the EAF, large amounts of charge carbon are initially oxidized to CO. About 16 MJ/t of
energy is released for 10 kg carbon for each 10% post combustion to CO
2
with 100% heat transfer.
12. COMPARISON OF TYPICAL ACTUAL AND
THEORETICAL MINIMUM ENERGY
Table 11 shows the theoretical minimum energy for the most realistic case and the typical range
of actual energy consumption for selected processes. The low value for the actual consumptions
represents the lowest values for any country as reported by the International Iron and Steel Institute
(IISI), not necessarily best practice.
(9)
For BOF steelmaking, the energies represent the energy of the
hot metal; the steelmaking process itself does not consume significant energy. For hot rolling, the
energy values represent a mixture of direct charging and reheating of conventional slabs. Most likely
the majority of the slabs are reheated and the theoretical values include reheating.
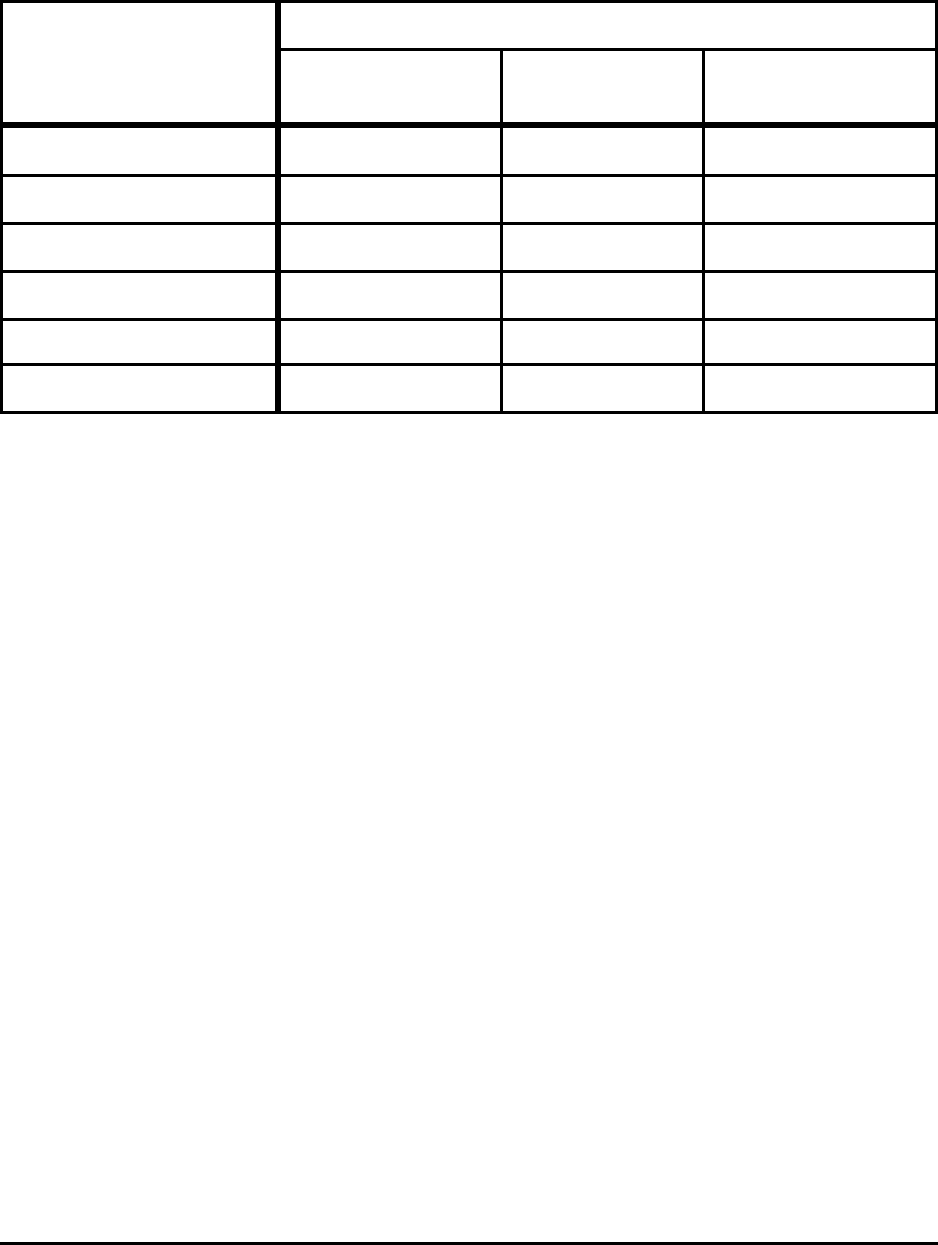
The results indicate that for ironmaking the absolute maximum energy savings available are
about 20% to 25%. Similarly for EAF steelmaking, the absolute maximum savings are about 23% to
33%.
The potential energy savings for rolling are lower simply because the processes use less energy.
Although it is not clear from Table 11, the potential for the greatest energy savings in finishing lies in
direct charging of slabs and billets. This would require major equipment changes and improved clean
steel practices in order to avoid inspection and grinding of slabs and billets.
Table 8 showed the total energy in cokemaking and ore agglomeration to be about 5 GJ/t
(steel). If an ironmaking process can be developed that uses coal and ore fines or concentrates, this
energy could be eliminated.
Theoretical Minimum Energies to Produce Steel for Selected Conditions 19
--
Table 11. Practical Theoretical Minimum and Energy for Selected Processes
Process
Energy (GJ/t product)
Absolute
Minimum
Practical
Minimum
Reported
Consumption
Liquid Hot Metal (5%C) 9.8 10.4 13 - 14
Liquid Steel (BOF) 7.9 8.2 10.5 - 11.5
Liquid Steel (EAF) 1.3 1.6 2.1 - 2.4
Hot Rolling Flat 0.03 0.9 2.0 - 2.4
Cold Rolling Flat 0.02 0.02 1.0 - 1.4
18-8 Stainless Melting 1.2 1.5
Notes:
Actual includes yield losses and is the average of state-of-art and less-efficient operations for the United
States, Japan, and Europe.
BOF energy is primarily from hot metal; actual process consumes 0.2 to 0.4 GJ/t and, if CO is oxidized to
CO
2
, could theoretically produce 0.5 GJ.
For 18-8 stainless no estimates are available, in particular for HCFeCr
In all cases, full credit is taken for the energy in off gas.
13. SUMMARY AND CONCLUSIONS
The theoretical minimum energies to produce several steel products for ore- and scrap-based
processes were computed. In general, only the energy for the process itself was considered and not
the energy used to produce input materials or electricity. The cases included ironmaking, direct
reduction, oxygen steelmaking, scrap and scrap substitute, EAF steelmaking, production of 18-8 type
stainless, and rolling of cast material to final shape. In addition, the effects of yield loss, gangue, and use
of carbon post combustion (as well as other special cases) on energy consumption were estimated.
Table 12 presents a comparison of the theoretical minimums to actual typical energy consumption
values. Some additional findings are shown in the summary box.
Increasing scrap melting has the largest impact on energy consumption. However, scrap should be
viewed as having "invested" energy since at one time it was produced by reducing ore. Therefore, it
should be used wisely. For example, oxidizing it to the slag may reduce energy in the process but the
"invested" energy is lost. Also, increasing scrap melting in the BOF may or may not decrease energy if
the "invested" energy in scrap is considered. Scrap supplies are not unlimited, and new iron from ore
will always be required.
20 Theoretical Minimum Energies to Produce Steel for Selected Conditions
-- -- --
Table 12. Comparison of Theoretical Minimum Energy
and Actual Energy Requirements for Selected Processes
Process
Energy (GJ/t product)
Actual Energy
Requirements
Absolute
Minimum
%
Difference
Practical
Minimum
%
Difference
Liquid Hot Metal
(5%C)
13 - 14 9.8 25 - 30 10.4 20 - 26
Liquid Steel (BOF) 10.5 - 11.5 7.9 25 - 31 8.2 22 - 29
Liquid Steel (EAF) 2.1 - 2.4 1.3 38 - 46 1.6 24 - 33
Hot Rolling Flat 2.0 - 2.4 0.03 99 0.9 55 - 63
Cold Rolling Flat 1.0 - 1.4 0.02 98 - 99 0.02 98 - 99
18-8 Stainless
Melting
1.2 1.5
Notes: Actual includes yield losses and is the average of state-of-art and less-efficient operations for the United
States, Japan, and Europe.
BOF energy is primarily from hot metal; actual process consumes 0.2 to 0.4 GJ/t and, if CO is oxidized to
CO
2
, could theoretically produce 0.5 GJ.
For 18-8 stainless no estimates are available, in particular for HCFeCr.
In all cases, full credit is taken for the energy in off gas.
Other conclusions that can be drawn from the findings are as follows:
• Elimination of ore agglomeration and cokemaking, which are not theoretically necessary to produce
steel, would yield significant energy savings.
•
Effective utilization of the energy contained in the off gases from the blast furnace, coke plant, and
direction reduction process is essential.
• The best scrap substitute with respect to energy usage is liquid pig iron or hot metal.
Theoretical Minimum Energies to Produce Steel for Selected Conditions 21
Additional Findings
Effect of Gangue and Ash
•
Gangue and ash in ore and coal increase minimum energies by 0.6 GJ (about 6%) for ironmaking.
Direct Reduction
•
Energy requirements for direct reduction per tonne of iron are similar to those for the blast furnace.
Scrap in the BOF
•
In BOF (OSM) steelmaking, the energy to produce steel is less than the energy to produce iron
because scrap is melted in the process.
•
Due to the "invested" energy in scrap any process that increases scrap melting lowers the total energy
because of the substitution of scrap for ore.
Variables in the EAF
•
Dirt in scrap and air entrainment in an EAF increase the energy required by 0.3 GJ/t (about 25% of the
total energy).
• Total energy increases dramatically when using scrap substitute such as DRI or pig iron with scrap in
an EAF. The best scrap substitute with respect to energy usage is liquid pig iron or hot metal.
Stainless Steel
• The melting energy for stainless steel is similar to the melting energy for carbon steel. However, the
energy is much greater if the energy associated with ferrochrome is included.
Direct Charging
• Direct charging of slabs or billets reduces total rolling energy by about 80%.
Ore Agglomeration and Cokemaking
• Ore agglomeration and cokemaking, which are not theoretically necessary to produce steel, have
significant theoretical minimum energies (1.6 and 0.8 GJ/t steel, respectively) and actual energies (2.2
and 2.4 GJ/t, respectively).
Limestone Calcination
• Calcination of limestone requires 4.7 GJ/t CaO and releases 0.79 tonnes of CO
2
.
Post Combustion
•
Post combustion can significantly increase scrap melting in the BOF and decrease energy required in
the EAF.
Carbon in the EAF
•
When carbon is used in the EAF, about 38% of the CO must be post combusted to CO
2
to equal
electrical energy, even taking into account the production and transmission losses associated with
electrical energy.
Off-Gas Energies
•
Off-gas energies from the blast furnace, coke plant, and direct reduction were significant and were
credited to the processes. For example, the total energy credit for coke and ironmaking is 6-10 GJ.
22 Theoretical Minimum Energies to Produce Steel for Selected Conditions
REFERENCES
1. V. F. Campos and R. M. Figueira, Dados Termodinamicos para Metalurgistas, EEUFMG, Belo
Horizonte, Minas Gerais, Brazil, p. 392, 1977.
2. O. Kubaschewski, E. Evans and C. B. Alcock, Metallurgical Thermochemistry, Pergamon Press,
New York, NY 1967.
3. S. Ban-ya, M. Hino, “Chemical Properties of Molten Slags,” ISIJ, p. 278, 1991.
4. E. T. Turkdogan, “Physicochemical Properties of Molten Slags and Glasses,” The Metals Society, 1
Carlton House Terrace, London, p. 516, 1983.
5. M. W. Chase, Jr. et al. Ed: JANAF Thermochemical Tables, 3rd Ed., American Chemical Society,
1985.
6. J. F. Elliott, M. Gleiser and V. Ramakreshna: Thermochemistry for Steelmaking, Addison-Wesley
Publishing, Reading, MA.
7. “New and Emerging Sources of Iron,” R.J. Fruehan, Prepared for the American Iron and Steel
Institute, 19xx?
8. The Making, Shaping and Treating of Steel, 10th Edition, AISE, Pittsburgh, PA, 1985.
9. “Energy Use in the Steel Industry, International Iron and Steel Institute, September 1998.
Theoretical Minimum Energies to Produce Steel for Selected Conditions 23

24 Theoretical Minimum Energies to Produce Steel for Selected Conditions

APPENDIX A
THEORETICAL CO
2
EMISSIONS
FROM STEELMAKING PROCESSES
Theoretical Minimum Energies to Produce Steel for Selected Conditions 25

In this section, the CO
2
emissions associated with the energies required to produce steel are calculated.
The following tables present theoretical CO
2
emissions from various steelmaking processes for a
number of conditions. These conditions are based on those used in the preceding analysis of theoretical
energy consumption for steel production.
Emission factors were calculated by considering the heats of reaction for the following reactions:
C + ½ O
2
� CO
2
- “H
rxn
= 395.3 kJ/mol (1)
H
2
+ ½ O
2
� H
2
O - “H
rxn
= 247.3 kJ/mol
(2)
C + 2H
2
� CH
4
- “H
rxn
= 91.0 kJ/mol (3)
CH
4
+ 2O
2
� CO
2
+ H
2
O - “H
rxn
= 798.9 kJ/mol
(4)
These reactions represent the basic reactions that occur when fuel (carbon or methane) is combusted.
By using these heats of reaction and the energy requirements for each process (shown in the report),
the necessary amount of moles of fuel can be calculated. Once the amount of fuel is known,
stoichiometry dictates the amount of CO
2
generated by the combustion of that fuel. Because the
production of electricity is not a matter of simple chemical reaction, the emission factor for electricity
(U.S. grid) accepted by the American Iron and Steel Institute (AISI) was used in this analysis (AISI
1996). Table A-1 shows the emission factors used in this analysis according to the assumptions noted
and compares them to the AISI emission factors for each fuel source considered. Some assumptions
have been made to simplify calculations and are noted below:
• The energy for ironmaking is assumed to come from 100% carbon and converted entirely to
CO
2
when consumed.
•
Natural gas is assumed to be 100% methane and converted entirely to CO
2
and H
2
O when
consumed.
• Electricity emissions include efficiency and transmission losses.
• Emissions for BOF steelmaking are a result of 50 kg C/tonne HM that remains in the liquid pig
iron and is used as fuel during oxygen steelmaking. This carbon is not combusted to CO
2
during the ironmaking step and thus is not included in the emissions for ironmaking. Since
oxygen steelmaking generates rather than consumes energy, this process has no additional
combustion emissions beyond the 50 kg C/tonne HM remaining in the liquid pig iron.
The minimum theoretical energy consumption data presented in the body of this report are not estimates
but rather actual physical quantities. When those energy data are converted to CO
2
emissions, a
number of assumptions and approximations are necessary. Particularly, while the energy data are
simply energy (no fuel type is specified), fuel types must be specified when calculating carbon
emissions. Additionally, carbon emission factors have been estimated based on the composition of
common fuel sources. The distinction between the theoretical minimum energy consumption as a
physical quantity and the minimum CO
2
as an estimate must be made clear.
26 Theoretical Minimum Energies to Produce Steel for Selected Conditions
Table A-1. Emission Factors Used to Convert Theoretical Energy Consumption
to CO
2
Emissions
Fuel Source
Theoretical Emission Factor
(kg CO
2
/MJ)
AISI Emission Factor
(kg CO
2
/MJ)
Difference
(percent)
Coke 0.111 0.109 1.8
Natural Gas 0.055 0.050 9.1
Hydrogen 0 0 0.0
Electricity N/A 0.173 N/A
Coke Oven Gas 0.055 0.046 16.4
Source: Survey of Consumption of Energy in the U.S. Iron and Steel Industry, American Iron and Steel Institute,
1996.
Notes:
Coke is assumed to be 100% carbon combusted to 100% CO
2
.
Natural gas is assumed to be 100% methane and combusted to 100% CO
2
and H
2
O.
Electricity emission factor accounts for transmission and efficiency losses at the power plant (based on IISI
value for the U.S. grid).
Hydrogen used as fuel does not result in any CO
2
emissions.
Coke oven gas used as fuel is considered as natural gas when considering CO
2
emissions.
The remainder of this appendix consists of the following tables:
Table A-2. Absolute Theoretical Minimum CO
2
Emissions for Producing Steel from Pure Ore
(Fe
2
O
3
) and Pure Scrap (Fe)
Table A-3.
Minimum CO
2
Emissions to Produce Liquid Hot Metal at 1723 K for Selected
Conditions
Table A-4. Minimum CO
2
Emissions to Produce Direct Reduced Iron at 1173 K Reduction
Temperature for Selected Conditions
Table A-5.
Minimum CO
2
Emissions to Produce Steel from Hot Metal and Scrap for Selected
Conditions
Table A-6. Minimum CO
2
Emissions to Produce Steel from Scrap for Selected Conditions, Tap
Temperature = 1873 K
Table A-7.
Minimum CO
2
Emissions to Produce Liquid Steel at 1873 K from 50% Scrap and
50% Scrap Substitute
Table A-8. Minimum CO
2
Emissions to Roll Steel for Selected Products and Conditions
Table A-9. Effect of Yield Loss on Minimum CO
2
Emissions to Produce Steel for 1% Loss
Table A-10.
Minimum and Typical CO
2
Emissions in Cokemaking and Ore Agglomeration
Table A-11.
Practical Minimum CO
2
Emissions for Selected Processes
Theoretical Minimum Energies to Produce Steel for Selected Conditions 27
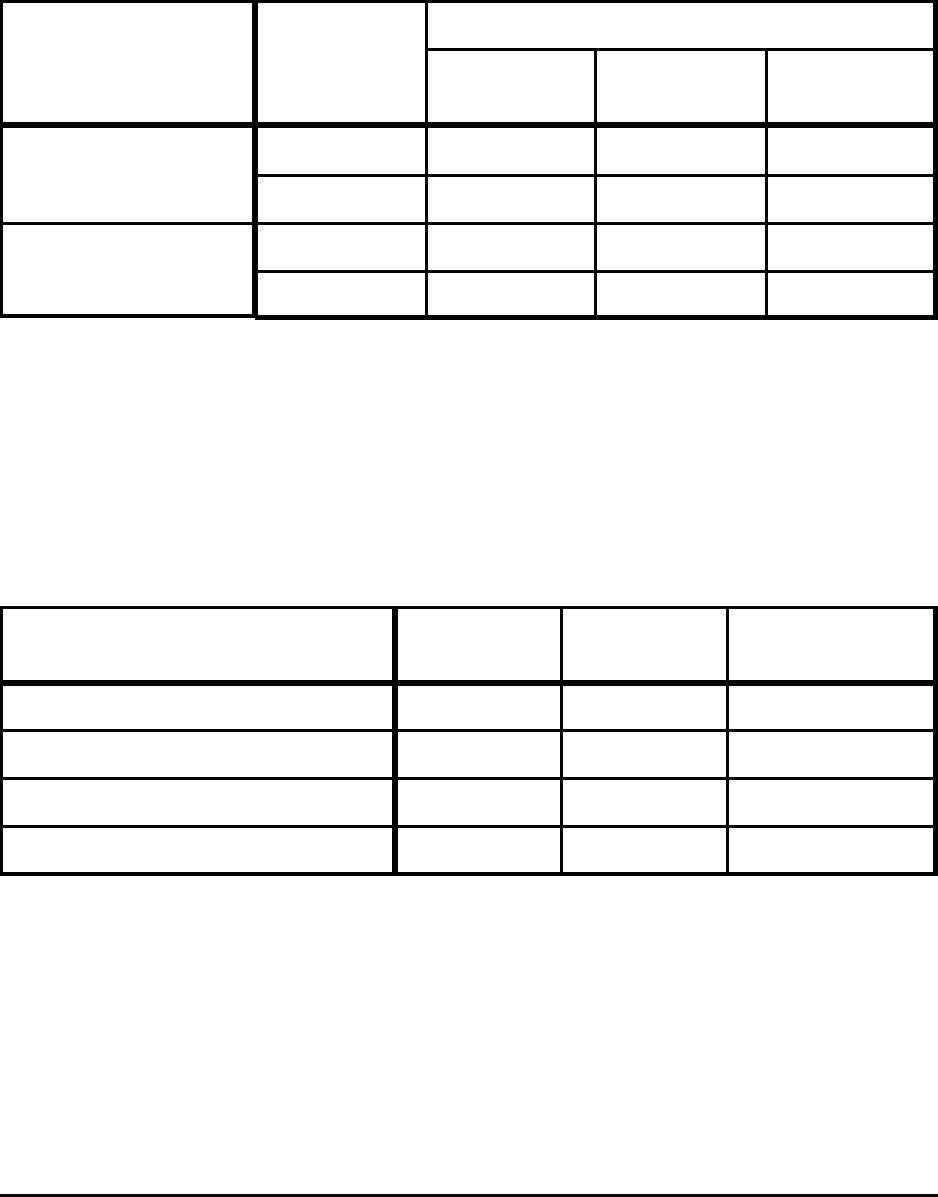
Table A-2. Absolute Theoretical Minimum CO
2
Emissions
for Producing Steel from Pure Ore (Fe
2
O
3
) and Pure Scrap (Fe)
Raw Material
Temperature
(degrees K)
Carbon Emissions (kg CO
2
/tonne)
Fuel = Carbon
Fuel = Natural
Gas
Fuel =
Electricity
Ore (Fe
2
O
3
)
1813 960 475 1,494
1873 966 478 1,503
Scrap (Fe)
1813 142 70 221
1873 148 73 230
Notes: Based on theoretical minimum energy consumption shown in Table 1.
Absolute theoretical minimum energy consumption is independent of fuel type and not based on any actual
production process. Therefore, the three main sources of fuel have been used to calculate theoretical
carbon emissions for each.
Table A-3. Minimum CO
2
Emissions to Produce Liquid Hot Metal
at 1723 K for Selected Conditions
Material Consumption Gangue Ash
CO
2
(kg/
tonne HM)
Fe - 5% C No No 908
Fe - 5% C Yes No 954
Fe - 5% C - 0.5% Si - 0.5% Mn Yes No 960
Fe - 5% C - 0.5% Si - 0.5% Mn0 Yes Yes 977
Notes: Based on theoretical minimum energy consumption shown in Table 2.
Gangue contains 4% SiO
2
, 1% Al
2
O
3
, and 1% MnO. Ash content is 25 kg/tonne hm (5% of coal and coke
used).
Fuel used in ironmaking is assumed to be 100% coke.
Carbon that remains in the hot metal (5% C, or 50 kg C/tonne HM) is subtracted from the overall carbon
emissions for liquid hot metal production shown in this table. Those carbon emissions are attributed to the
process in which that carbon is used as fuel (i.e., raw steel production from hot metal, see Table A-5).
28 Theoretical Minimum Energies to Produce Steel for Selected Conditions
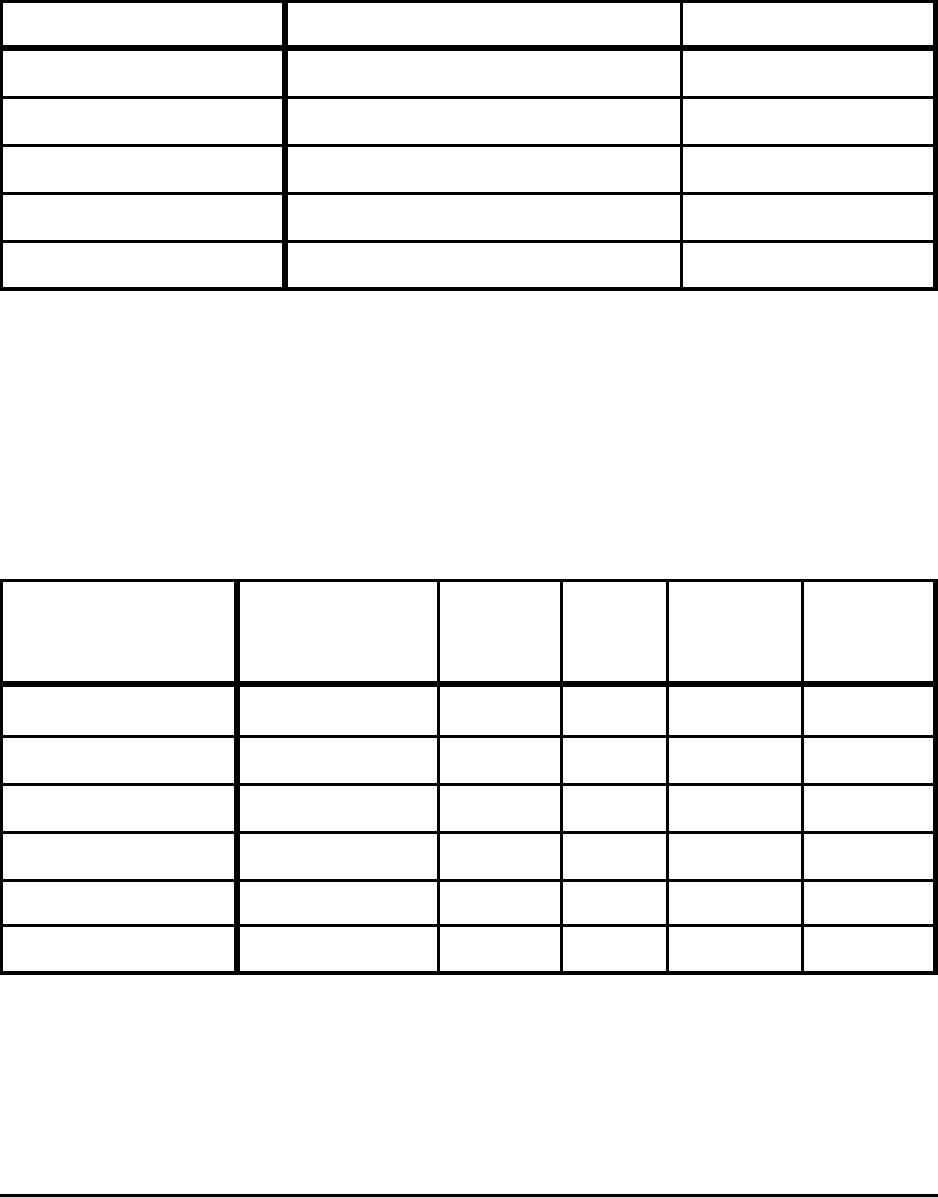
Table A-4. Minimum CO
2
Emissions to Produce Direct Reduced Iron
at 1173 K Reduction Temperature for Selected Conditions
Ore Product CO
2
(kg/tonne)
Pure Fe
2
O3 Fe 461
Fe
2
O
3
- 1.4% SiO
2
Fe - 2% SiO
2
462
Fe
2
O
3
- 1.4% SiO
2
Fe - 2% SiO
2
- 8% FeO 435
Fe
2
O
3
- 1.5% SiO
2
Fe - 2% SiO
2
- 8% FeO - 2% C 391
Fe
2
O
3
- 1.5% SiO
2
Fe - 2% SiO
2
- 7.7% FeO - 6% C 300
Notes:
Based on theoretical minimum energy consumption shown in Table 3.
The energy used to produce direct reduced iron is assumed to be 100% natural gas.
When carbon remains in the final product (the final two rows in the table above), that carbon is subtracted
from the overall CO
2
emissions of the process. The subtracted CO
2
is attributed to the process in which the
carbon is used as fuel (i.e., liquid steel production from scrap and scrap substitute, Table A-7).
Table A-5. Minimum CO
2
Emissions to Produce Steel
from Hot Metal and Scrap for Selected Conditions
Hot Metal Slag
Tap
Temp.
(K)
Scrap
(kg)
Steel
(kg/tonne
HM)
CO
2
(kg/tonne)
Fe - 5% C – 1873 290 1,240 148
Fe - 5% C – 1923 244 1,194 135
Fe - 5% C - 0.5% Si B = 3; 30% FeO 1873 401 1,328 138
Fe - 5% C - 0.5% Si B = 3; 30% FeO 1923 353 1,278 143
Fe - 5% C - 0.5% Si B = 3; 20% FeO 1873 389 1,320 139
Fe - 5% C - 0.5% Si B = 3; 20% FeO 1923 341 1,270 144
Notes: Based on theoretical energy consumption shown in Table 4.
Oxygen steelmaking is theoretically an energy-producing process due to the exothermic nature of the
reactions taking place. Therefore, fuel consumption is theoretically not needed, and carbon emissions do
not result from the combustion of fuels.
The CO
2
emissions shown are the result of the 5% carbon (50 kg/tonne HM) in the hot metal feed that is
consumed during steelmaking. The carbon is actually used as a fuel source for melting scrap and is
converted to CO
2
in this process.
Theoretical Minimum Energies to Produce Steel for Selected Conditions 29
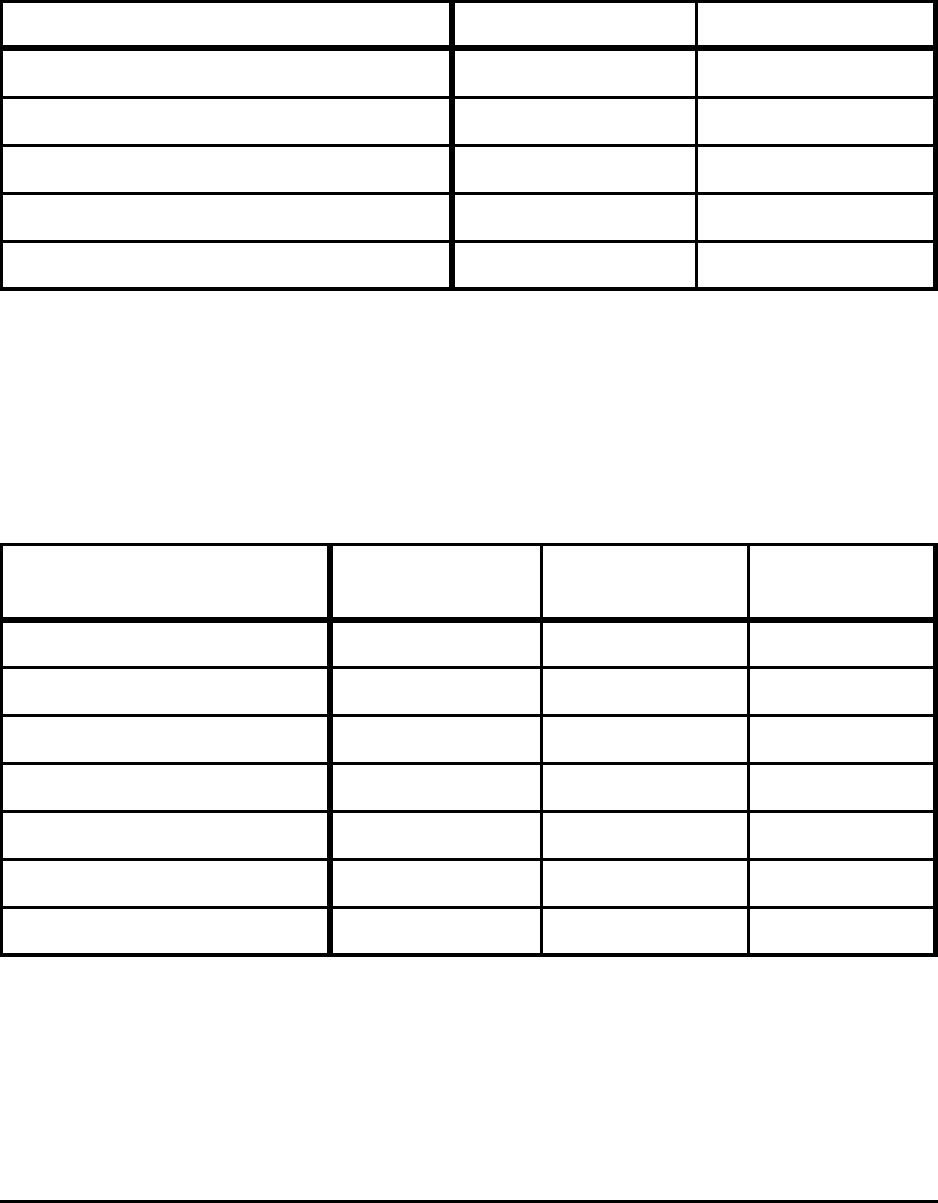
Table A-6. Minimum CO
2
Emissions to Produce Steel from
Scrap for Selected Conditions, Tap Temperature = 1873 K
Scrap Slag CO
2
(kg/tonne steel)
Fe – 230
Fe - 0.1%C - 0.2% Si B=2.5; 25% FeO 223
Fe - 0.1%C - 0.2% Si -1% dirt B=2.5; 25% FeO 234
Fe - 0.1%C - 0.2% Si -1% dirt B=2.5; 35% FeO 230
Fe - 0.1%C - 0.2% Si -1% dirt -100 Nm
3
air B=2.5; 25% FeO 275
Notes:
Based on theoretical energy consumption shown in Table 5.
Energy is assumed to be 100% electricity, using a conversion factor of 10,500 Btu/kWh to include
transmission and efficiency losses.
Table A-7. Minimum CO
2
Emissions to Produce Liquid Steel
at 1873 K from 50% Scrap and 50% Scrap Substitute
Input
Melting Energy
(MJ/tonne steel)
Charge Energy
(MJ/tonne steel)
CO
2
(kg/tonne
steel)
None (100% Scrap Case) 1,289 – 234
Fe - 2% SiO
2
1,403 4,296 480
Fe - 2% SiO
2
- 8% FeO 1,559 4,233 503
Fe - 2% SiO
2
- 8% FeO - 2% C 1,483 4,565 548
Fe - 2% SiO
2
- 8% FeO - 6% C 1,328 5,223 640
Liquid Pig Iron 487 5,388 779
Solid Pig Iron 1,145 5,388 893
Notes:
Based on theoretical energy consumption shown in Table 6.
Liquid pig iron is charged at 1723 K, all others at 298 K; melting energy is assumed to be 100% electricity.
Charge energy is assumed to be 100% natural gas for DRI and 100% coke for pig iron.
In addition to the CO
2
emissions generated from fuel consumption, the carbon contained in the input
materials is converted to CO
2
in this process (10.8 kg C/tonne, 2%C DRI; 33.2 kg C/tonne, 6%C DRI; 25.8 kg
C/tonne pig iron).
30 Theoretical Minimum Energies to Produce Steel for Selected Conditions
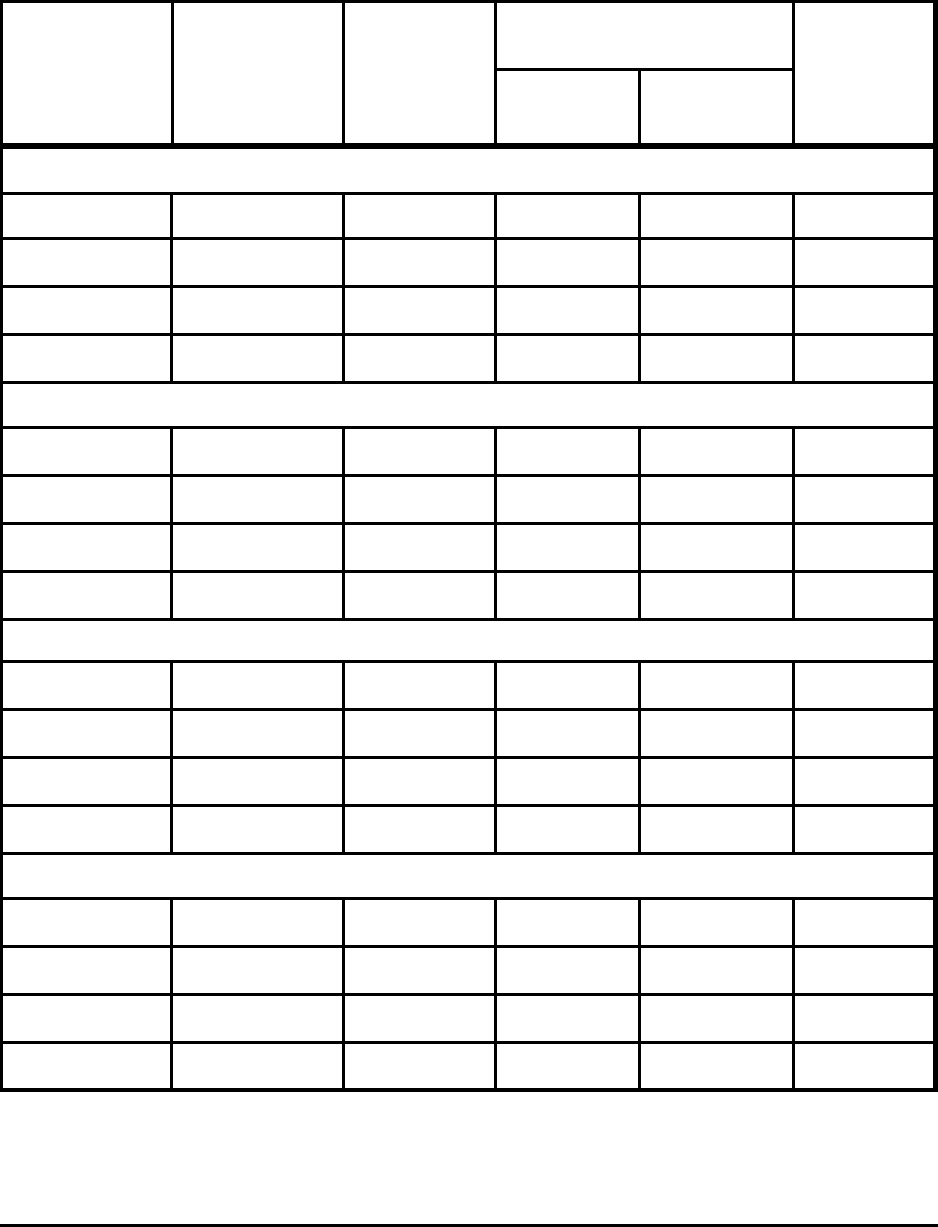
Table A-8. Minimum CO
2
Emissions to Roll Steel for Selected Products and Conditions
Rolling
Temperature
(K)
Slab
Temperature
(K)
Reduction
(mm)
Energy (MJ/tonne)
CO
2
(kg/tonne)Heat
Deforma-
tion
Flat Carbon (25.4 cm slab)
1473 298 254 - 2 825 25 50
1473 1173 254 - 2 270 25 19
1473 1473 254 - 2 – 25 4
298 298 2 - 1 – 17 3
Flat Carbon (5.0 cm slab)
1473 298 50 - 2 825 16 48
1473 1173 50 - 2 270 16 18
1473 1473 50 - 2 – 16 3
298 298 2 - 1 – 17 3
Flat 18-8 Stainless (25.4 cm slab)
1473 298 254 - 2 825 72 58
1473 1173 254 - 2 270 72 27
1473 1473 254 - 2 – 72 12
298 298 2 - 1 – 51 9
Bar Carbon (10 cm billet to 2 cm bar)
1473 298 10 sq - 2 sq 825 20 49
1473 1473 10 sq - 2 sq – 20 4
1473 1173 10 sq - 2 sq 270 20 18
1473 1473 10 sq - 2 sq – 11 2
Notes: Based on theoretical energy consumption shown in Table 7.
Heating energy is assumed to be 100% natural gas.
Deformation energy is assumed to be 100% electricity.
Theoretical Minimum Energies to Produce Steel for Selected Conditions 31
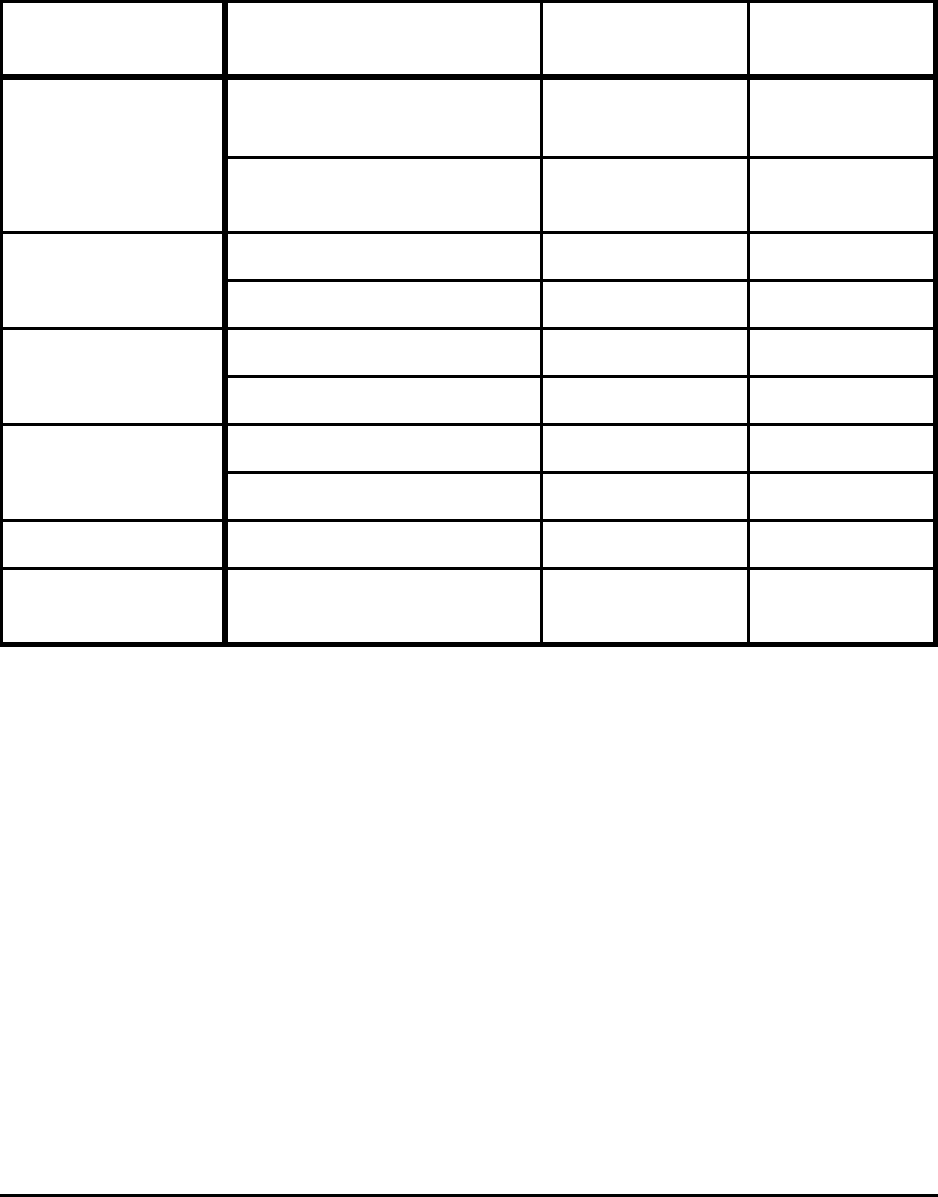
Table A-9. Effect of Yield Loss on Minimum CO
2
Emissions to Produce Steel
for 1% Loss
Process Type of Loss
Energy
(MJ/tonne) CO
2
(kg/tonne)
Hot metal loss in
pretreatment
Hot metal loss to slag but
recovered 14 1.6
Hot metal loss to slag, not
recovered 104 11.6
Direct Reduction
Fines lost 60 3.3
Fines recycled 6 0.3
BOF
Fe vaporized to dust 144 2.6
Fe ejected to dust 80 1.4
EAF
Fe vaporized to dust 102 17.7
Fe ejected to dust 16 2.8
Hot Rolling Fe lost but recovered 13 2.3
Cold Rolling
Reheating for hot rolling, but
recovered 34 5.9
Notes:
Based on theoretical energy consumption shown in Table 8.
Energy lost in hot metal loss is assumed to be 100% coke. Energy lost in direct reduction fines is assumed
to be 100% natural gas.
Energy lost in EAF and rolling operations is assumed to be 100% electricity.
For BOF steelmaking, emissions are calculated using the average emission rate for the BOF steelmaking
calculations (0.018 kg CO
2
/tonne/MJ).
32 Theoretical Minimum Energies to Produce Steel for Selected Conditions
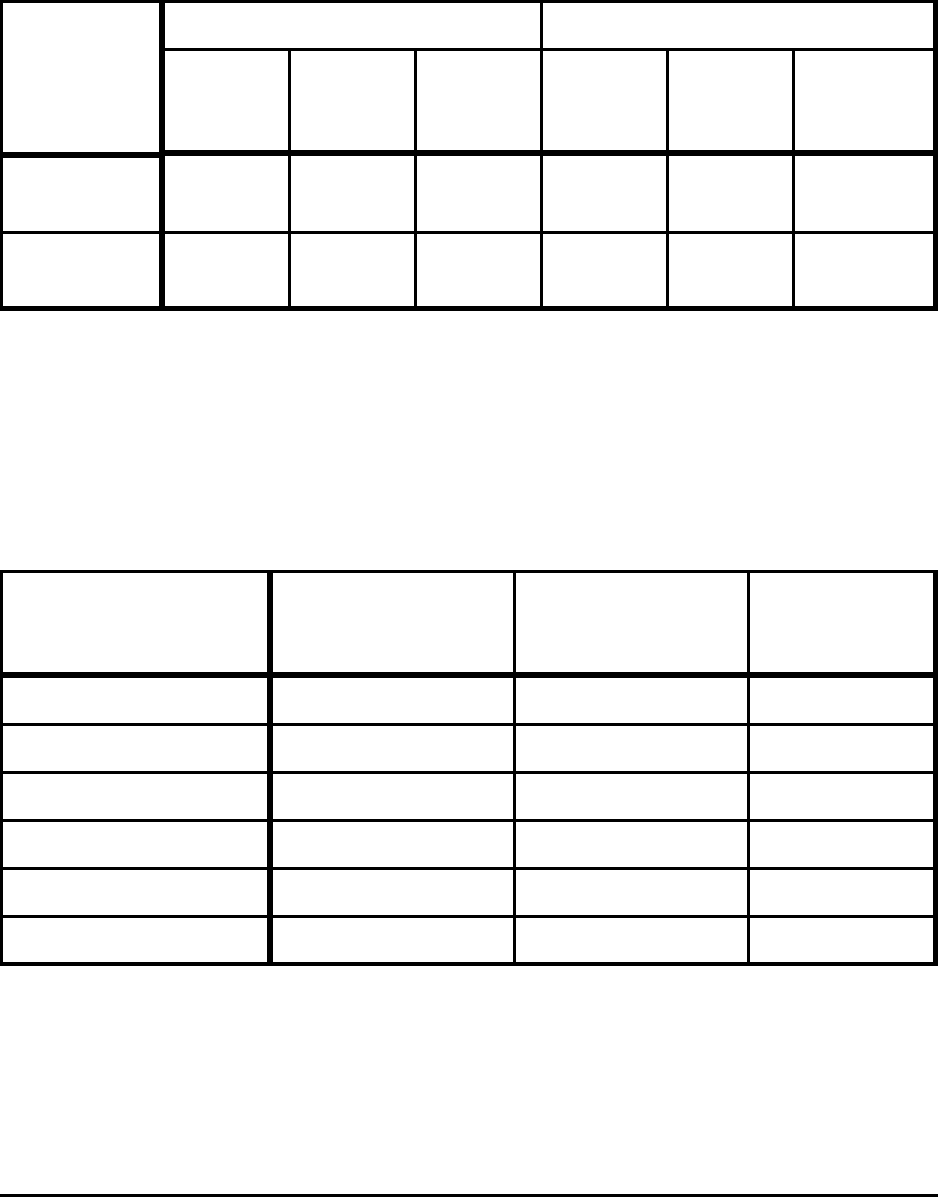
Table A-10. Minimum and Typical CO
2
Emissions
in Cokemaking and Ore Agglomeration
Process
Theoretical Actual
GJ/tonne
output
GJ/tonne
steel
CO
2
(kg/tonne
output)
GJ/tonne
output
GJ/tonne
steel
CO
2
(kg/tonne
output)
Cokemaking 2 0.8 0.11 5.4 - 6.2 2.2 - 2.6 0.30 - 0.34
Ore Agglom-
eration 1.2 1.6 0.07 1.5 - 1.7 2.1 - 2.4 0.17 - 0.19
Notes: Based on theoretical minimum energy consumption shown in Table 9.
Energy used in cokemaking is assumed to be 100% natural gas.
Energy used in ore agglomeration is assumed to be 100% coke breeze.
Table A-11. Comparison of Theoretical Minimum and Actual CO
2
Emissions
for Selected Processes
Process
Absolute Minimum
CO
2
(kg/tonne
product)
Practical Minimum
CO
2
(kg/tonne
product)
Actual CO
2
(kg/tonne
product)
Liquid Hot Metal 1,091 1,158 1,447 - 1,559
Liquid Steel (BOF) 144 144 189 - 207
Liquid Steel (EAF) 225 277 364 - 416
Hot Rolling 2 50 110 - 132
Cold Rolling 4 4 173 - 243
18-8 Stainless Melting 208 260 –
Notes:
Based on theoretical minimum energy consumption shown in Table 11.
Actual includes yield losses and is the average of state-of-the-art and less-efficient operations for the
United States, Japan, and Europe.
BOF energy is primarily from hot metal; the actual process consumes 0.2 to 0.4 GJ/tonne. Theoretically if
CO is oxidized, it could produce 0.5 GJ/tonne.
For 18-8 stainless melting no estimates of actual emissions were available.
Theoretical Minimum Energies to Produce Steel for Selected Conditions 33

34 Theoretical Minimum Energies to Produce Steel for Selected Conditions
