
Centers for Disease Control and Prevention
Office of Readiness and Response
We Must Maintain Measles Elimination in the
United States: Measles Clinical Presentation,
Diagnosis, and Prevention
Clinician Outreach and Communication Activity (COCA) Call
Thursday, August 17, 2023
Free Continuing Education
▪ Free continuing education is offered for this webinar.
▪ Instructions on how to earn continuing education will be provided at the end of the
call.
Continuing Education Disclosure
▪ In compliance with continuing education requirements, all planners and presenters
must disclose all financial relationships, in any amount, with ineligible companies over
the previous 24 months as well as any use of unlabeled product(s) or products under
investigational use.
▪ CDC, our planners, and presenters wish to disclose they have no financial relationship(s)
with ineligible companies whose primary business is producing, marketing, selling, re-
selling, or distributing healthcare products used by or on patients. All of the relevant
financial relationships listed for these individuals have been mitigated.
▪ Content will not include any discussion of the unlabeled use of a product or a product
under investigational use.
▪ CDC did not accept financial or in-kind support from ineligible companies for this
continuing education activity.
At the conclusion of today’s session, the participant will be able to accomplish the
following:
1. Identify the clinical presentation of measles and other causes of febrile rash illness
which may mimic measles.
2. Diagnose measles infection with appropriate laboratory diagnostics.
3. Identify measles vaccine adverse reactions.
4. Implement measles prevention and public health control strategies.
Objectives
To Ask a Question
▪ Using the Zoom Webinar System
– Click on the “Q&A” button
– Type your question in the “Q&A” box
– Submit your question
▪ If you are a patient, please refer your question to your healthcare provider.
▪ If you are a member of the media, please direct your questions to CDC Media
Relations at 404-639-3286 or email media@cdc.gov.
Today’s Presenters
▪ Adria Mathis, MSPH
Epidemiologist
Division of Viral Diseases
National Center for Immunization and Respiratory Diseases
Centers for Disease Control and Prevention
▪ Dan Filardo, MD
Medical Officer
Division of Viral Diseases
National Center for Immunization and Respiratory Diseases
Centers for Disease Control and Prevention
▪ Stephen Crooke, PhD
Lead Research Microbiologist
Division of Viral Diseases
National Center for Immunization and Respiratory Diseases
Centers for Disease Control and Prevention

National Center for Immunization & Respiratory Diseases
We Must Maintain Measles Elimination in the United States:
Measles Clinical Presentation, Diagnosis, and Prevention
COCA Call
August 17, 2023

▪ Identify the clinical presentation of measles and
other causes of febrile rash illness which may mimic
measles.
▪ Diagnose measles infection with appropriate
laboratory diagnostics.
▪ Identify measles vaccine adverse reactions.
▪ Implement measles prevention and public health
control strategies.
Objectives

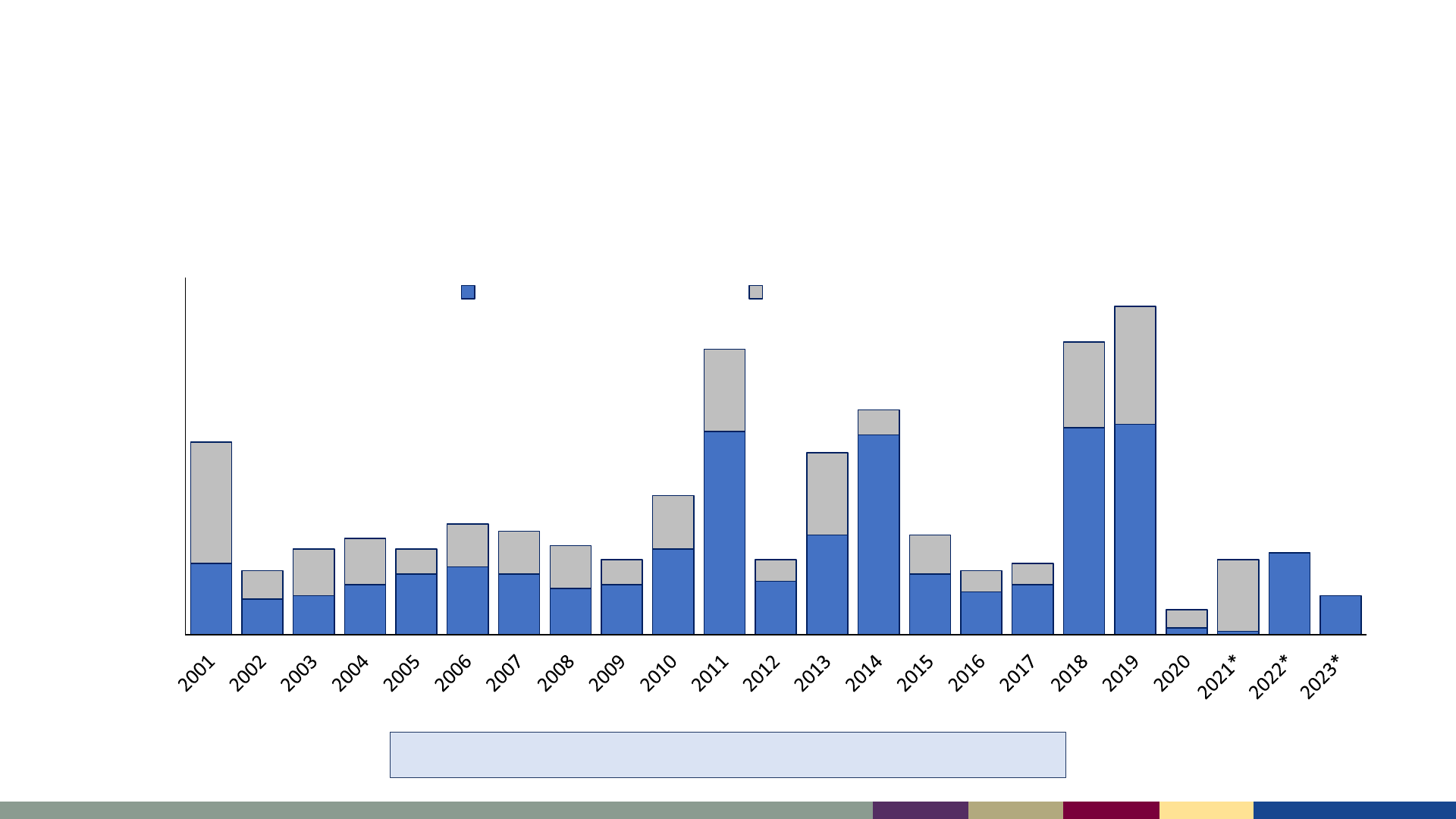
Measles Surveillance, 2023
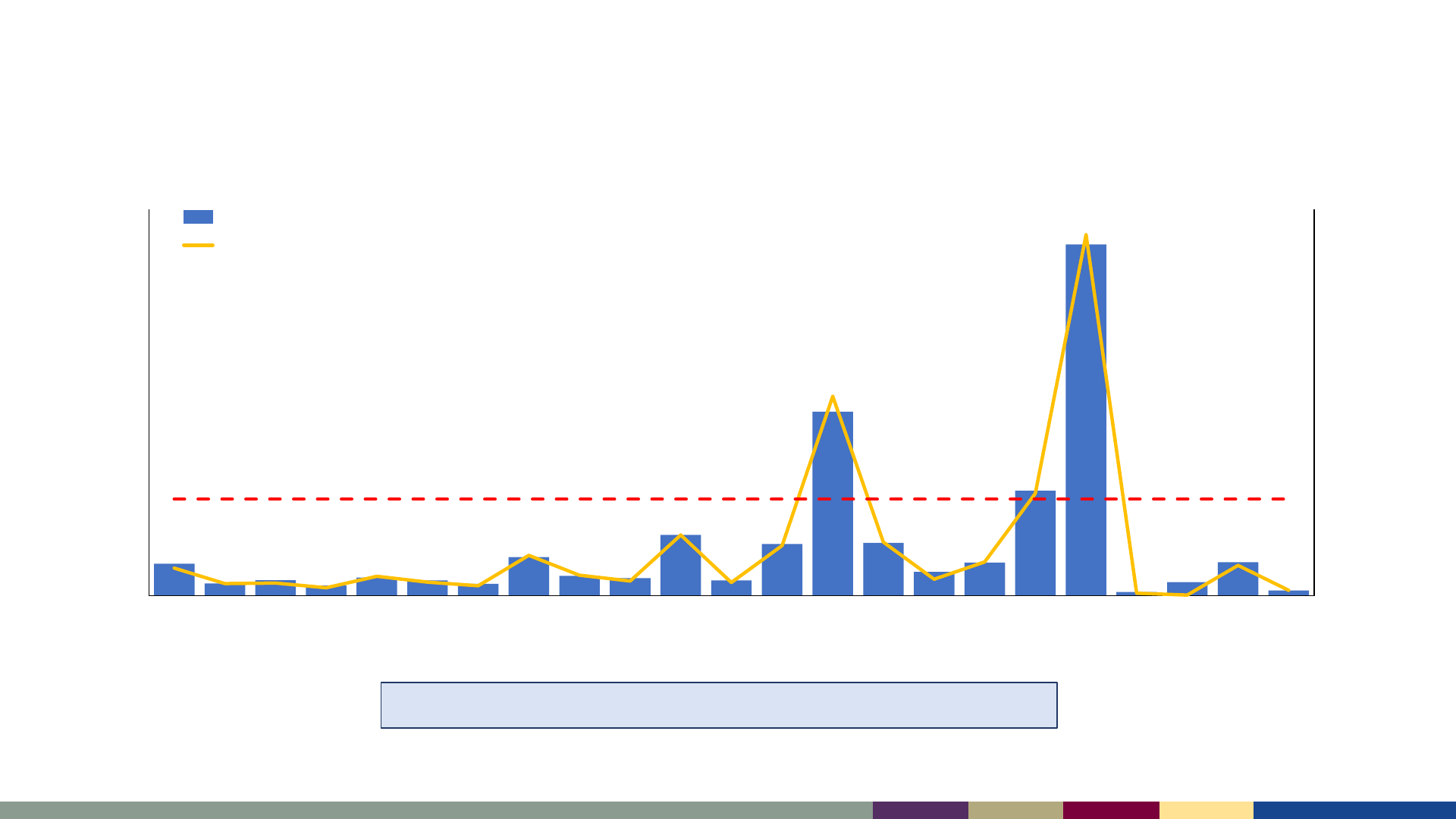
Number of reported measles cases, United States,
2001–2023
2001–2022: Median of 79 cases/year (range: 13–1,274)
*2023 data as of August 3, 2023. 2021–2023 data are preliminary and subject to change
0
0.5
1
1.5
2
2.5
3
3.5
4
0
200
400
600
800
1000
1200
1400
2001 2002 2003 2004 2005 2006 2007 2008 2009 2010 2011 2012 2013 2014 2015 2016 2017 2018 2019 2020 2021* 2022* 2023*
Incidence per 1,000,000 population
Number of Cases
Year
Number of Cases
Incidence
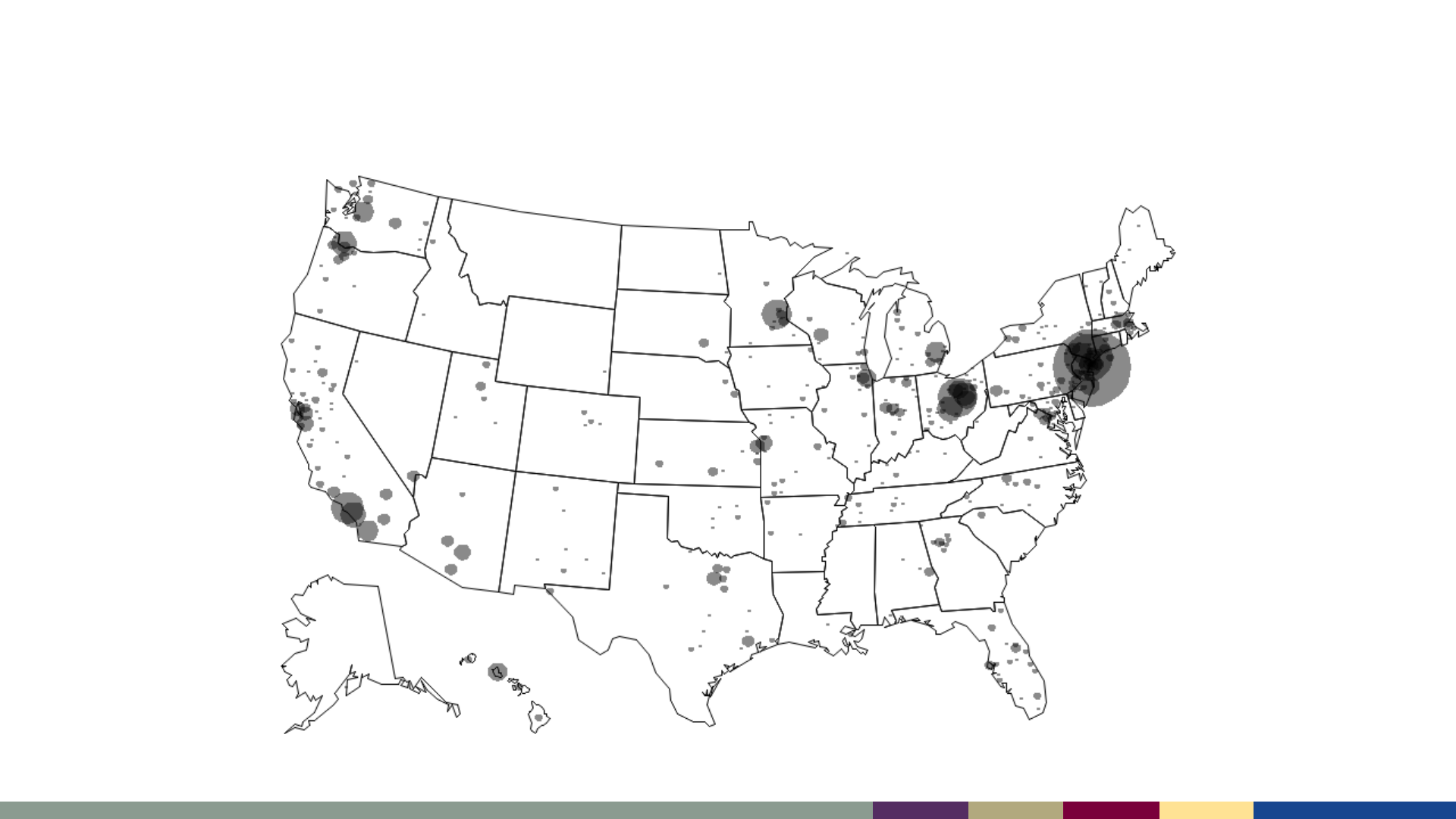
Measles has been reported from almost all U.S.
jurisdictions since 2001
Smallest gray dot = 1 case
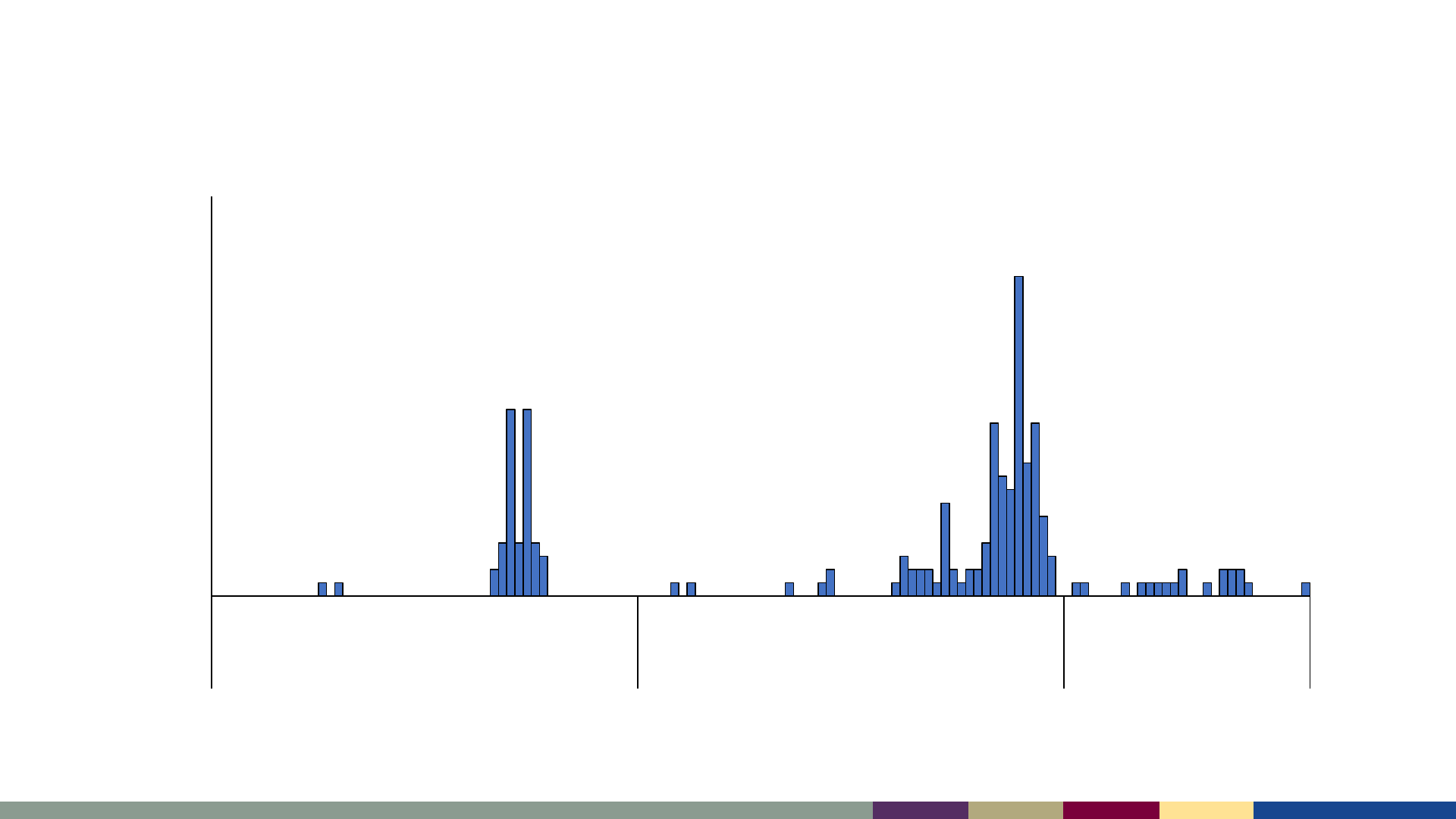
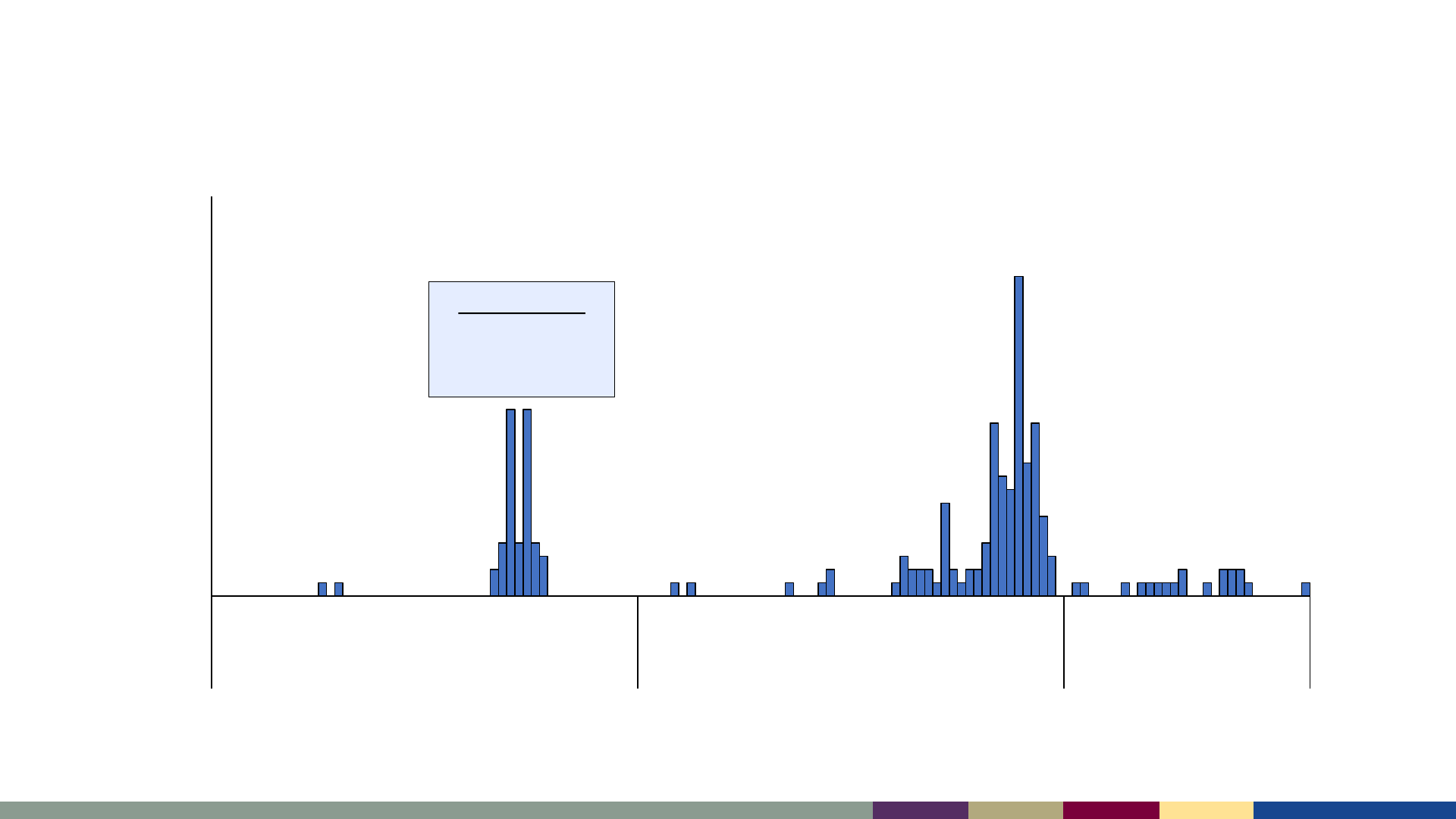
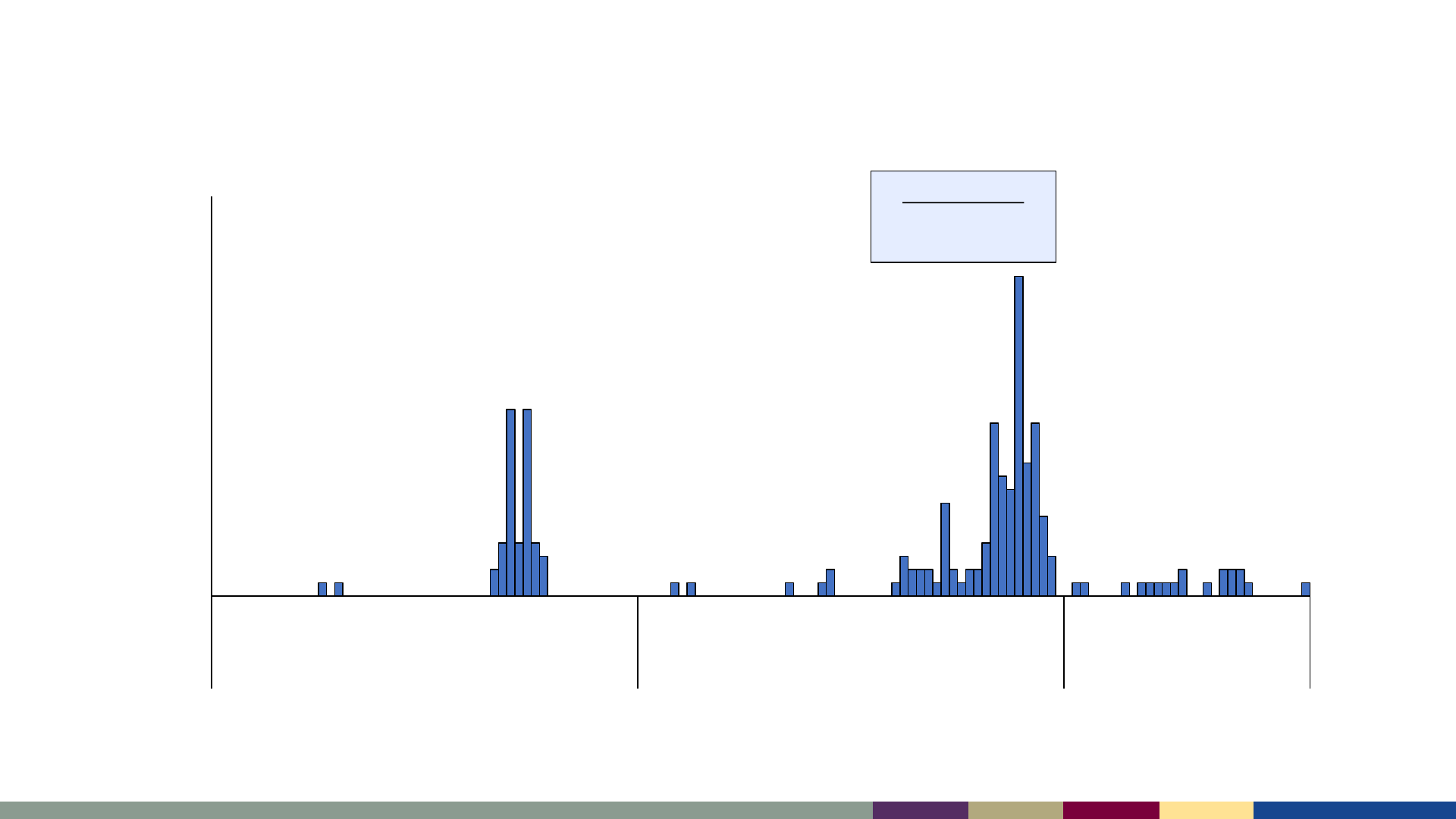
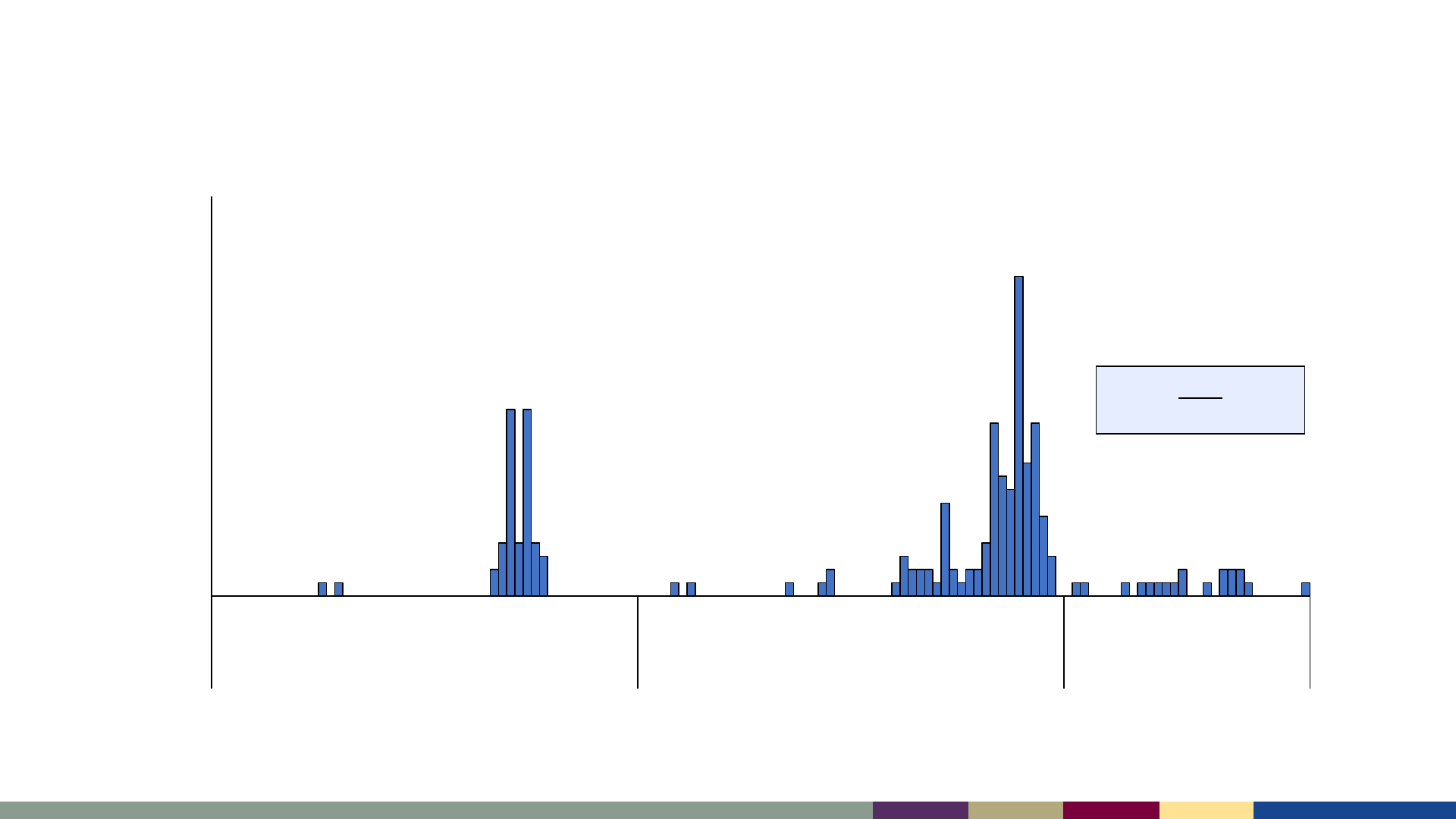
Measles cases by epidemiologic week,
January 2021–July 2023
*2023 data as of August 3, 2023. Data are preliminary and subject to change
0
5
10
15
20
25
30
1 5 9 13 17 21 25 29 33 37 41 45 49 1 5 9 13 17 21 25 29 33 37 41 45 49 1 5 9 13 17 21 25 29
2021 2022 2023
Number of Cases
Epidemiologic Week
0
5
10
15
20
25
30
1 5 9 13 17 21 25 29 33 37 41 45 49 1 5 9 13 17 21 25 29 33 37 41 45 49 1 5 9 13 17 21 25 29
2021 2022 2023
Number of Cases
Epidemiologic Week
Measles cases by epidemiologic week,
January 2021–July 2023
Sept-Oct 2021
47 cases during
Operation Allies
Welcome (OAW)
*2023 data as of August 3, 2023. Data are preliminary and subject to change
0
5
10
15
20
25
30
1 5 9 13 17 21 25 29 33 37 41 45 49 1 5 9 13 17 21 25 29 33 37 41 45 49 1 5 9 13 17 21 25 29
2021 2022 2023
Number of Cases
Epidemiologic Week
Measles cases by epidemiologic week,
January 2021–July 2023
Oct-Dec 2022
85 cases among
Ohio residents
*2023 data as of August 3, 2023. Data are preliminary and subject to change
0
5
10
15
20
25
30
1 5 9 13 17 21 25 29 33 37 41 45 49 1 5 9 13 17 21 25 29 33 37 41 45 49 1 5 9 13 17 21 25 29
2021 2022 2023
Number of Cases
Epidemiologic Week
Measles cases by epidemiologic week,
January 2021–July 2023
2023
No outbreaks reported
*2023 data as of August 3, 2023. Data are preliminary and subject to change
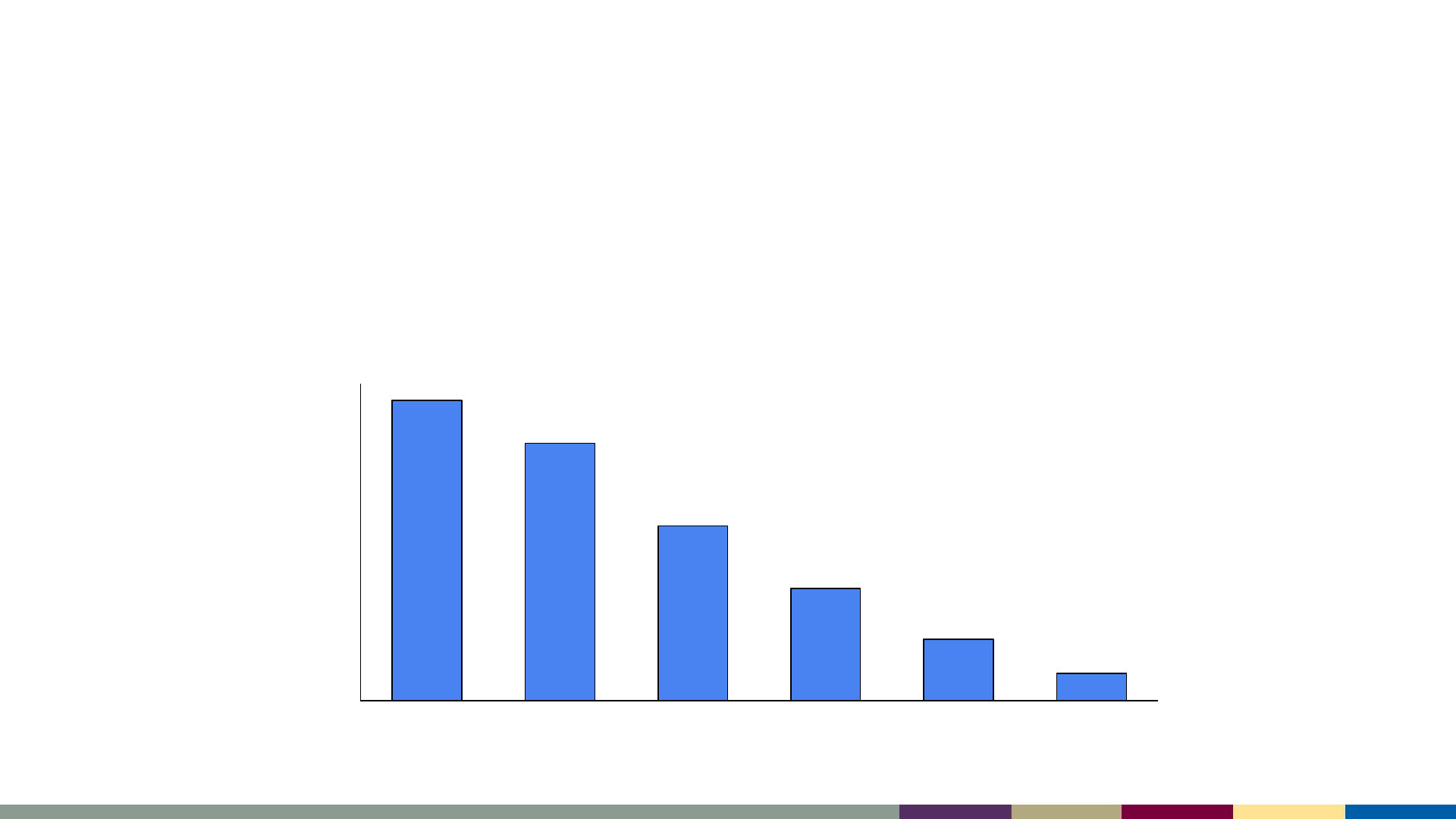
Measles cases are imported primarily by unvaccinated
U.S. residents traveling abroad
• Median 26 importations per year (range: 7–92)
512 (63%) importations among US residents
0
10
20
30
40
50
60
70
80
90
100
Number of Cases
Year
U.S. Resident (n = 512) Foreign Visitor (n = 297)
*2023 data as of August 3, 2023. Data are preliminary and subject to change
International importations
▪ Among imported cases, 62% of patients reported travel to countries in the
European and Western Pacific Regions during their exposure periods
▪ Top 5 source countries: India, the Philippines, China, Pakistan, and the UK
0%
5%
10%
15%
20%
25%
30%
35%
European Western
Pacific
South-East
Asian
Eastern
Mediterranean
African Americas
% Importations
WHO Region

• Measles cases in the U.S. are most commonly the result of virus importation by
unvaccinated foreign visitors
– A: True
– B: False
Knowledge Check #1

• Measles cases in the U.S. are most commonly the result of virus importation by
unvaccinated foreign visitors
– A: True
– B: False
Knowledge Check #1

Clinical Overview of Measles
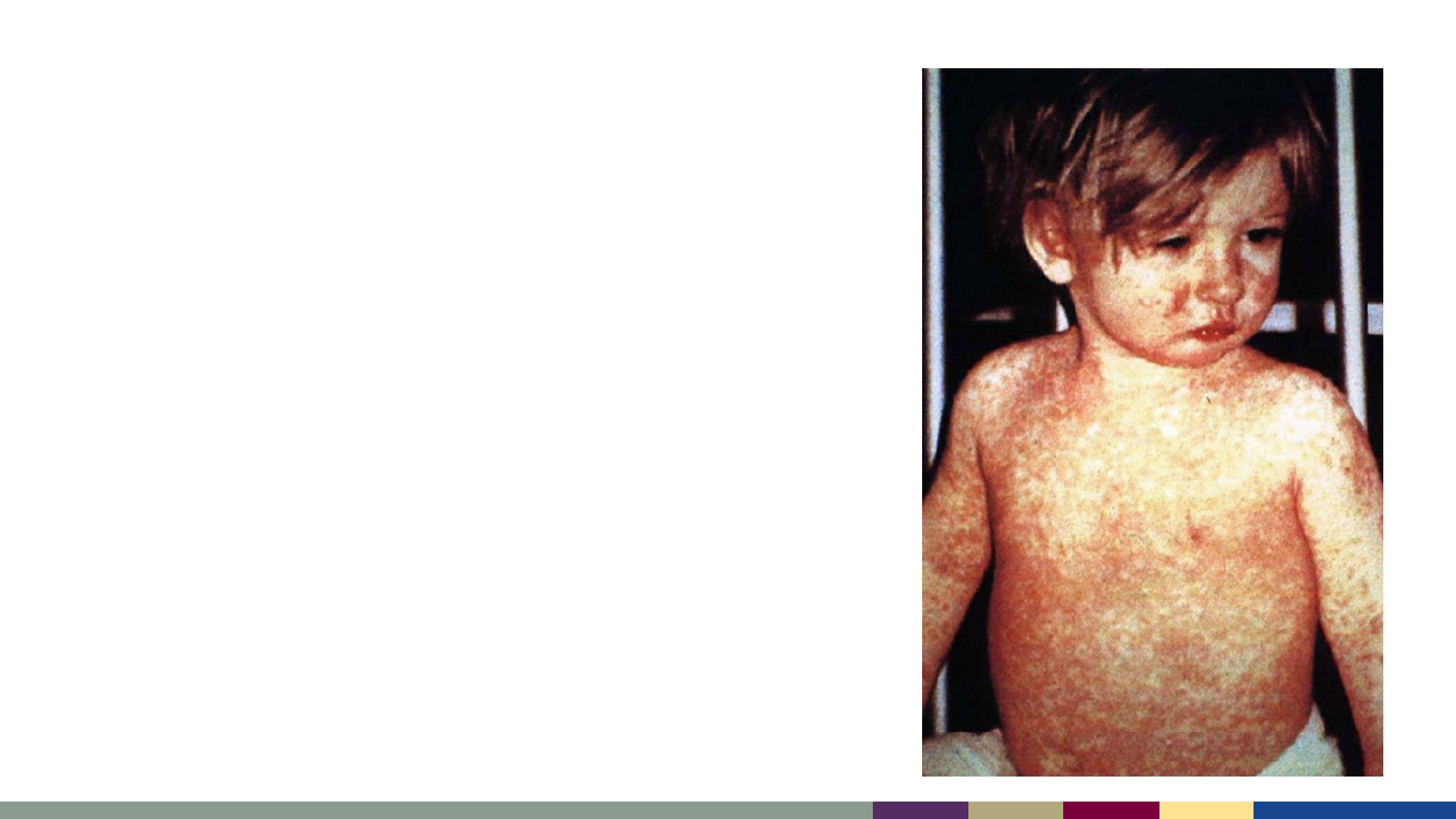
• An acute, febrile rash illness caused by the
measles virus
• Transmitted by direct contact with infectious
droplets or airborne route
• Measles is highly contagious
– 90% of susceptible household contacts will
develop illness
– R
o
(the number of people who are infected by a
single case) is estimated to be 12–16 in an
unvaccinated population
Measles

• Measles cases require a coordinated and robust
public health response
• Measles is nationally notifiable and cases should
be reported immediately to the appropriate
health department
– Health departments can assist with testing
when measles is suspected
Measles Notification
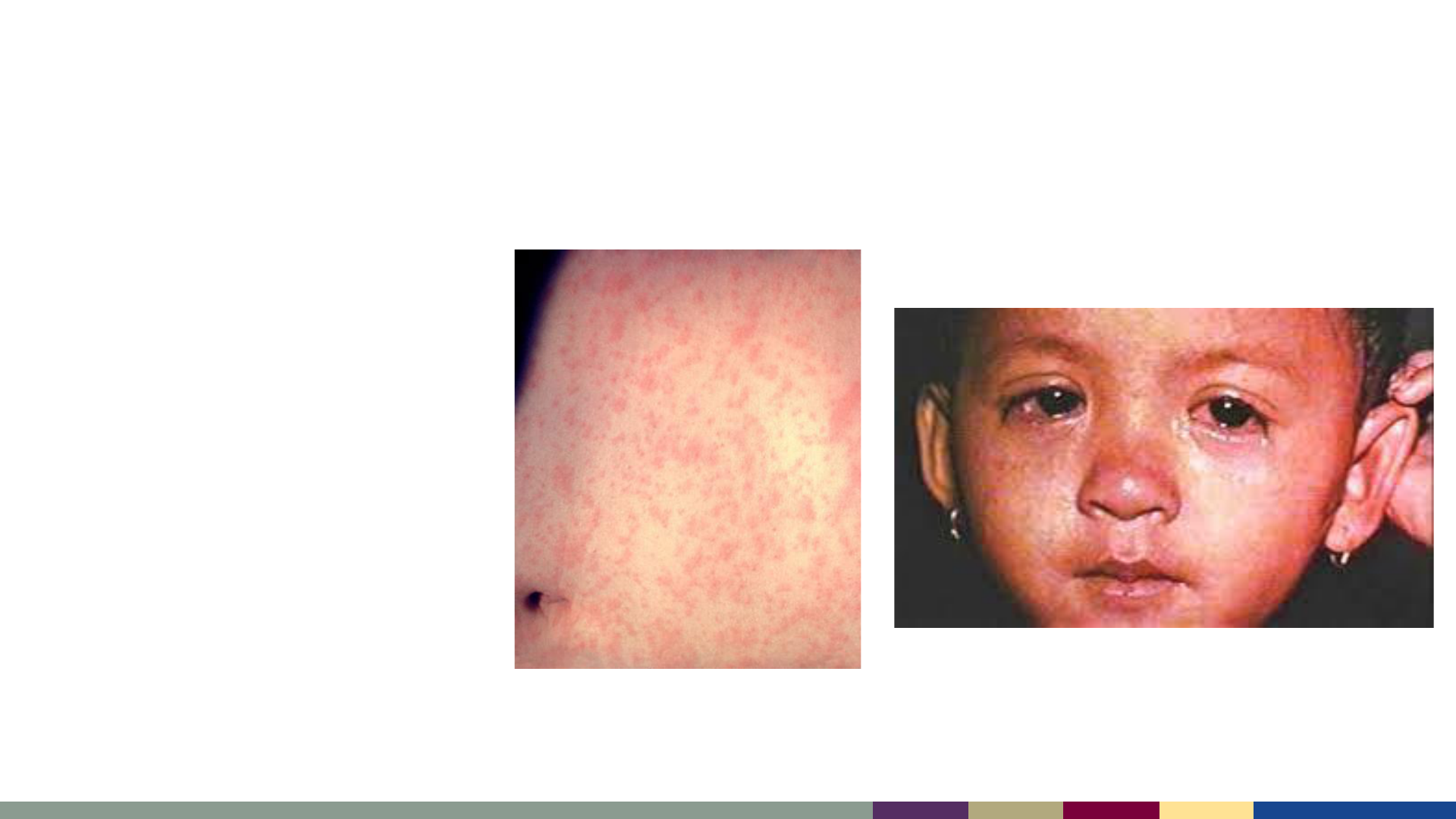
• Fever (up to 105
o
F)
AND
• Rash
AND
• At least 1 of “The 3 C’s”
– Cough
– Coryza (runny nose)
– Conjunctivitis
Measles – Clinical Case Definition
Measles conjunctivitis
Measles rash
https://healthjade.com/measles
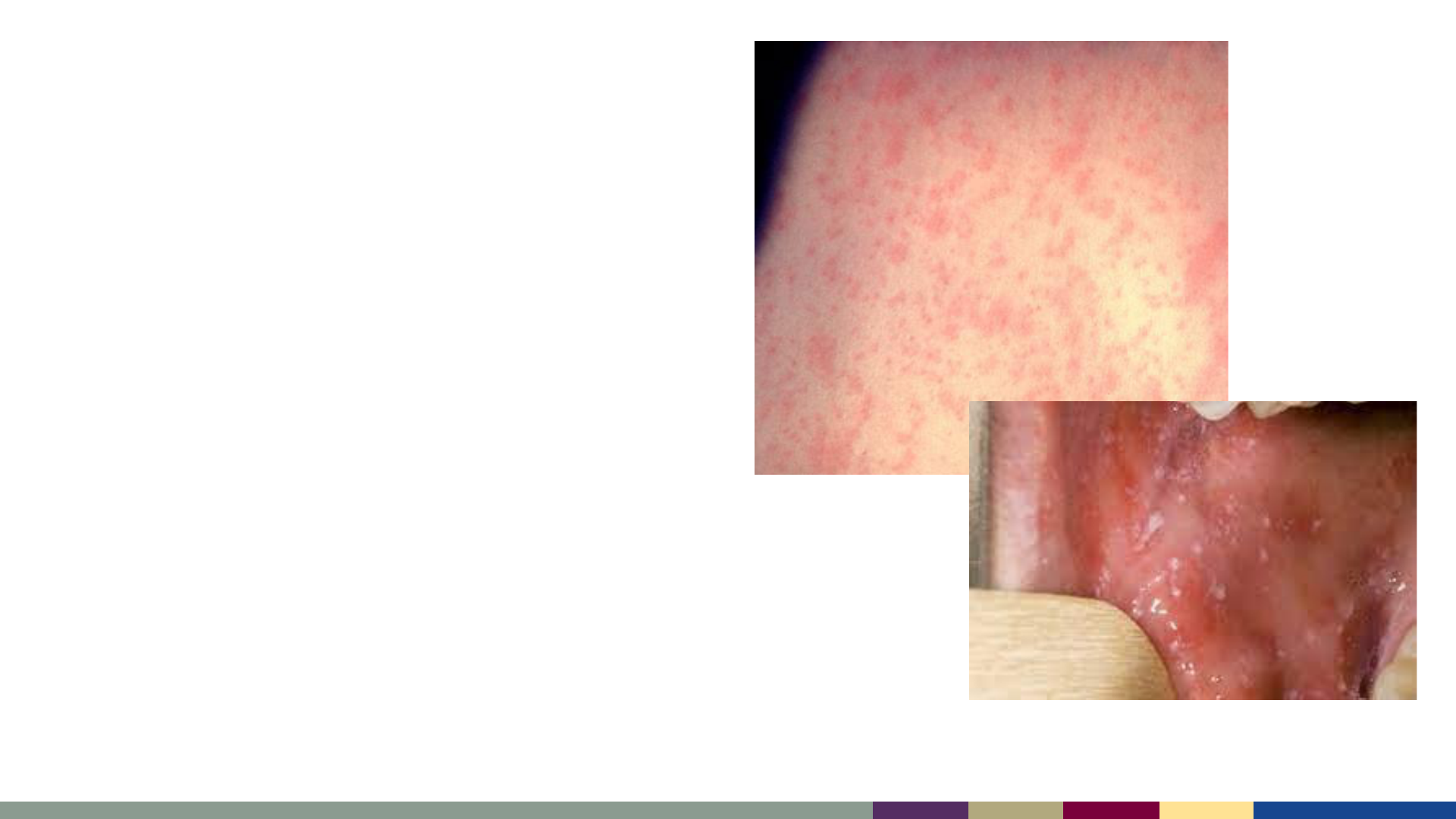
• Typical presentation:
– Starts on face, at hairline, or behind
ears
– Spreads downwards to neck, trunk,
extremities
– Maculopapular
› Small raised or flat red bumps
› Spots may join together as the rash
spreads
– Not usually itchy
– Koplik spots may be present on buccal
mucosa
Measles Rash
Koplik Spots
https://www.nhs.uk/conditions/measles
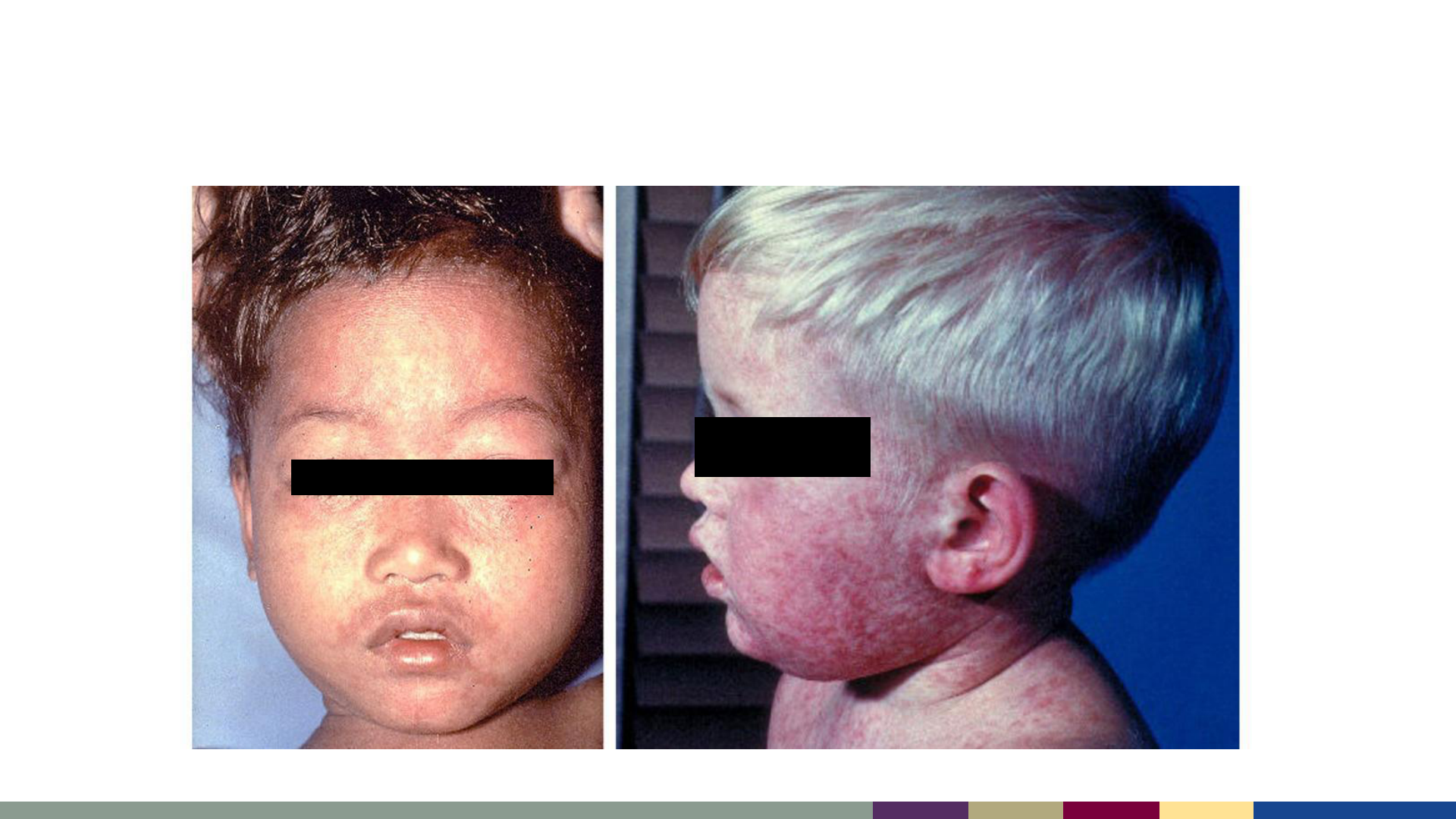
Measles Rash

Measles – Typical Timeline
Incubation Period
10-14 days
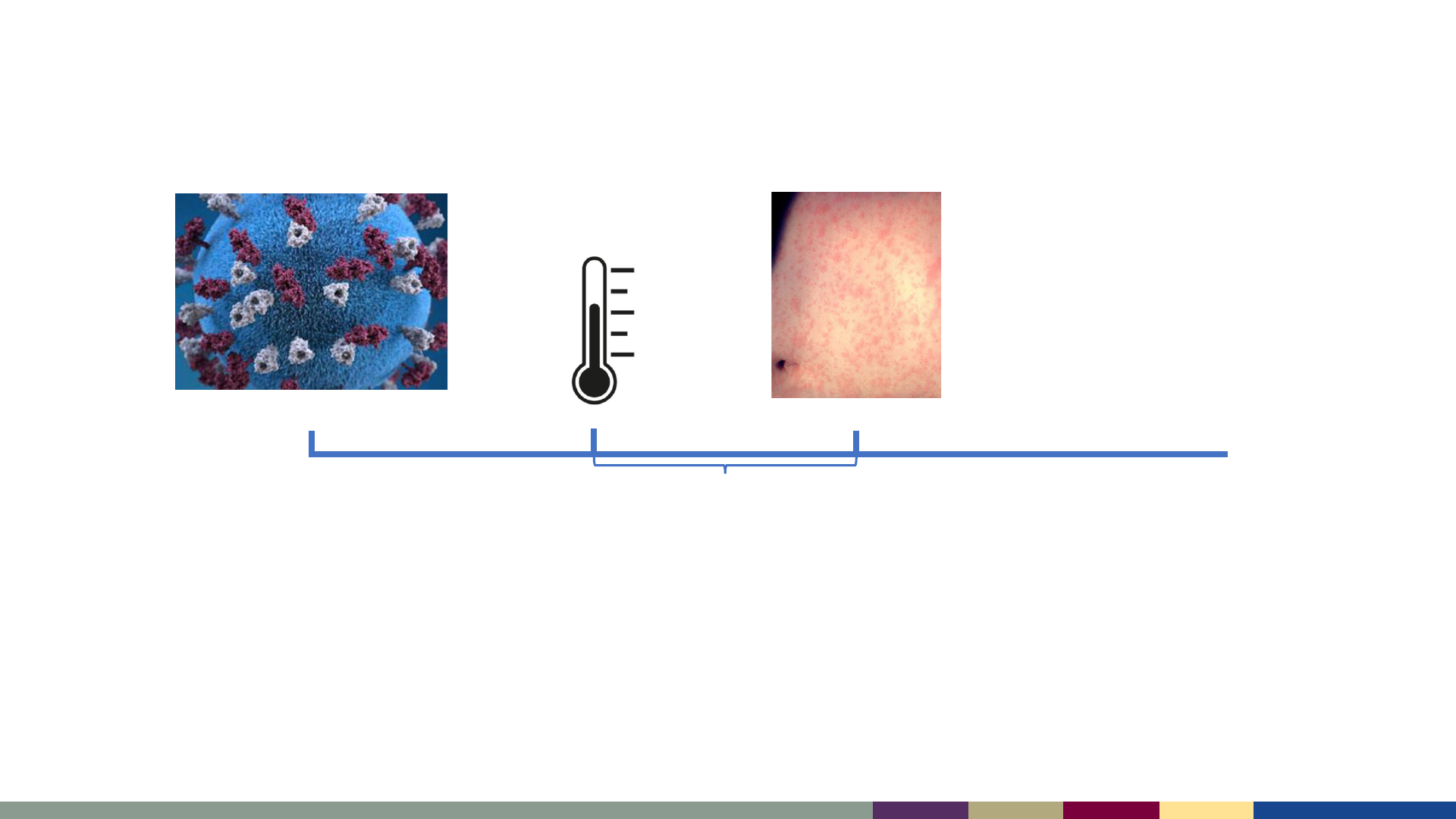
Measles – Typical Timeline
Day 0
Prodrome
Day -4 to -2
Measles – Typical Timeline
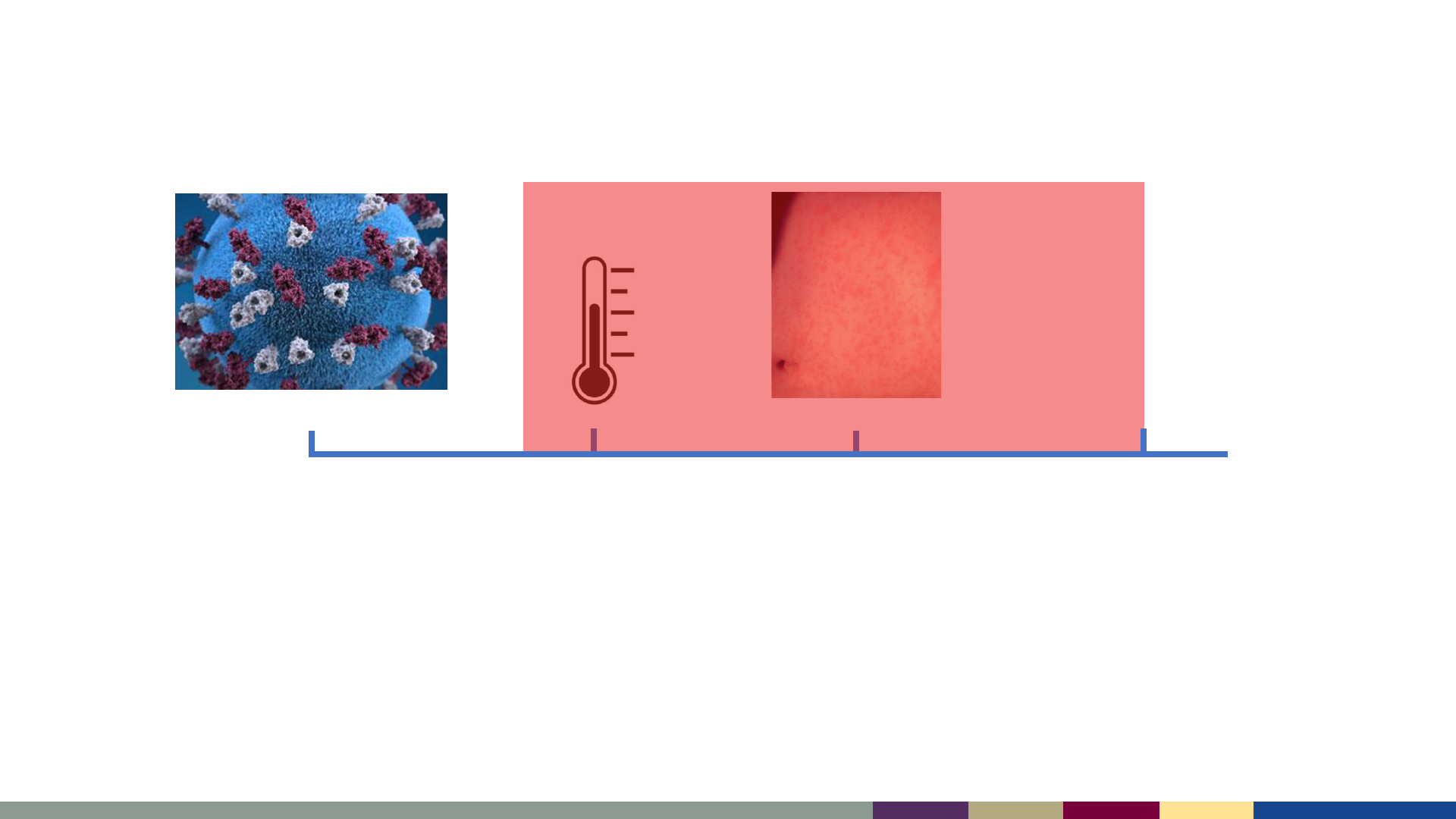
Day 0
Infectious Period:
4 days before rash to 4 days after rash
Day -4 to -2
Contagious
Day 4

Measles Complications
Diarrhea 8%
Otitis media 7 – 9%
Pneumonia 1 – 6%
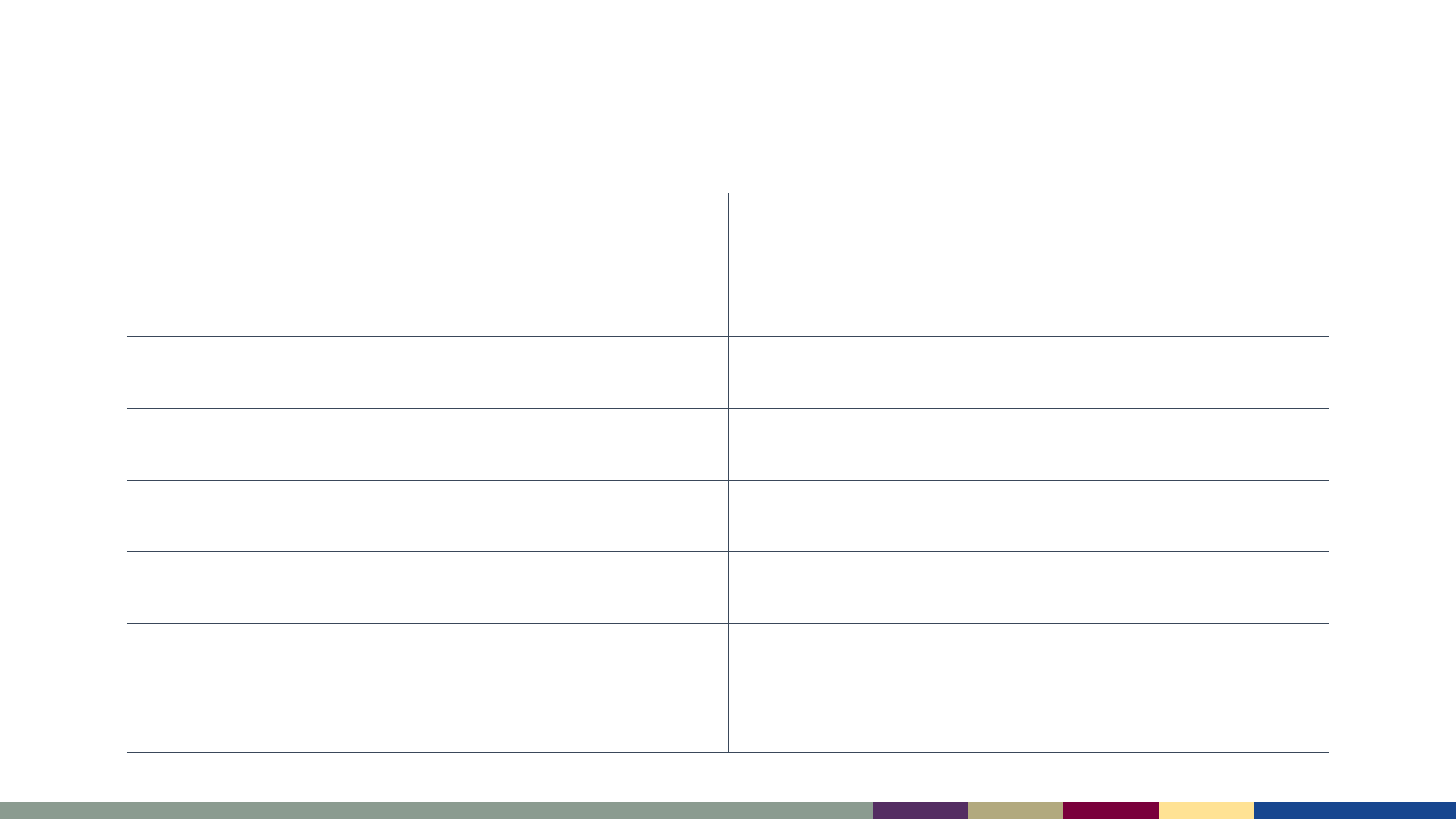
Measles Complications
Diarrhea 8%
Otitis media 7 – 9%
Pneumonia 1 – 6%
Hospitalization 1 in 4 cases
Encephalitis 1 per 1,000 cases
Death 1–3 per 1,000 cases
Subacute Sclerosing
Panencephalitis (SSPE)
7–11 per 100,000 cases*
*Bellini WJ et al. J Infect Dis 2005;192:1686–93.
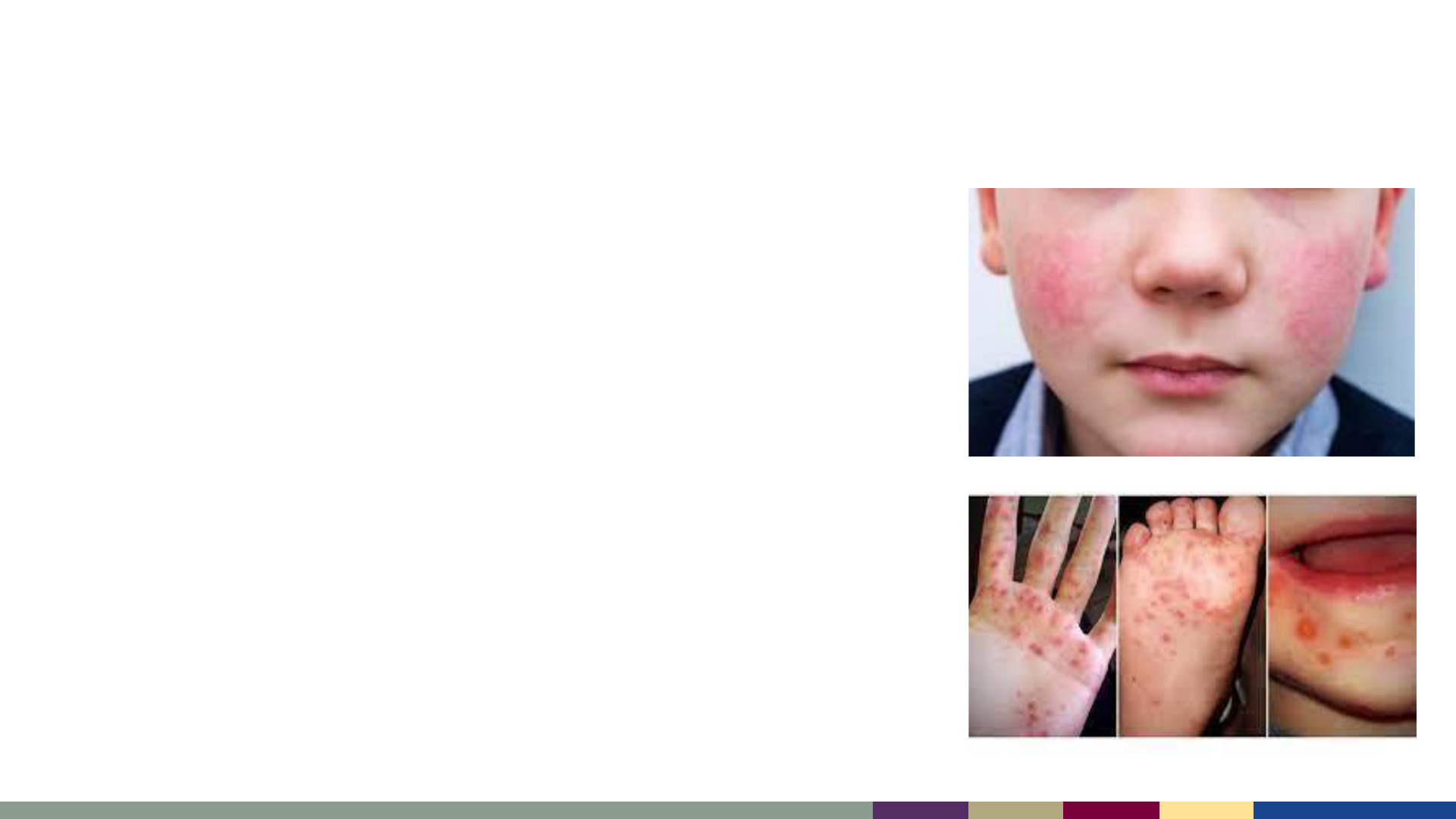
• Parvovirus B-19 (Fifth Disease)
– “Slapped cheek” rash (photo) which can spread to
trunk and extremities; more common in school-
aged children than infants
• Human Herpesvirus 6 (Roseola, Sixth Disease)
– Common cause of fever and rash in children; fever
often resolves, and rash appears the next day
starting on trunk and spreading outwards
• Enteroviruses
– Rash can be maculopapular or urticarial
– Often on hands/feet (Hand Foot Mouth, photo)
• Antibiotic sensitivity reactions or allergies
Other Common Causes of Maculopapular Febrile Rash
HFM
Slapped Cheek rash

• Measles is a highly infectious acute febrile rash illness
• Measles cases should be reported to health departments immediately
• Clinical definition includes:
– Fever + Rash + 3 C’s (cough, conjunctivitis, coryza)
• Measles can cause severe complications, including pneumonia and encephalitis
• Measles has multiple mimickers (e.g., parvovirus, HHV-6)
Clinical Presentation of Measles

• What is the typical presentation of a measles rash?
A. Starts on the trunk then spreads up to the hairline and extremities
B. Starts on the extremities and spreads everywhere on the body, including the
palms and soles
C. Starts at the hairline or face then moves down to the trunk and extremities
Knowledge Check #2

• What is the typical presentation of a measles rash?
A. Starts on the trunk then spreads up to the hairline and extremities
B. Starts on the extremities and spreads everywhere on the body, including the
palms and soles
C. Starts at the hairline or face then moves down to the trunk and extremities
Knowledge Check #2

Laboratory Diagnosis of Measles
• Clinical, epidemiologic, and laboratory data should all be considered when
diagnosing measles infection
• Using serology (IgM) alone to test patients with low pre-test probability of having
measles will result primarily in false positive results
• Both serum and NP/OP swabs should be collected for all suspect cases
Bottom Line Up Front

• Detection of measles antibodies is useful to help confirm the diagnosis
– Serology can increase the window in which measles can be diagnosed, if diagnostic or
reporting delays are encountered
– IgM detection starts 1–3 days after rash onset and can be detected for up to 6–8 weeks
› May disappear rapidly, be delayed, or not appear at all in vaccinated persons
– IgM testing alone can be problematic in settings with low measles incidence
› Cross-reactivity with other causes of febrile rash illness has been documented*
› False positive results are relatively common when the likelihood of measles is low:
» There isn’t local active transmission and patients have not traveled
†
» Patients have been fully vaccinated and have no known exposure
Measles Serology
*Jenkerson SA et al.N Engl J Med. 1995;332(16):1103-1104.
†
Ciccone FH et al. Rev Soc Bras Med Trop. 2010;43(3):234-239.
Hiebert J et al. J Clin Microbiol. 2021;59(6):e03161-20.

• rRT-PCR testing can be performed on nasopharyngeal and throat swabs, as well as urine
– Specimens are ideally collected within 3 days of rash onset, but can be positive up to 10
days after rash onset
› It is best to collect specimens for rRT-PCR as soon as possible after rash onset
– Proper specimen collection, storage, and processing is critical to maintain the stability of
viral nucleic acids. Most rRT-PCR assays include a control for specimen integrity
(reference gene).
– rRT-PCR has much higher sensitivity and specificity than serology
› False positive results can occur, but are MUCH less common
– CDC and state public health labs can perform rRT-PCR
Measles PCR
rRT-PCR: real-time reverse transcriptase polymerase chain reaction

• Some large commercial laboratories (e.g., Quest, Labcorp) are offering measles rRT-
PCR testing, and others are in the process of onboarding tests
• Some issues that arise with commercial testing:
– Loss of integration with public health departments
– Specimens are not maintained appropriately or for long enough to allow for
genotyping or additional testing if necessary
Commercial PCR Testing

• Serology
– Paired (acute and convalescent) IgG Testing
› Can provide additional evidence of measles infection if other data are
inconclusive
– Avidity Testing (IgG)
› Can provide information about breakthrough measles cases among previously
vaccinated people
Additional Testing is Available

• Genotyping
– Assists with outbreak detection and tracking, and should be performed ideally
on all rRT-PCR positive specimens
– Important to document sustained elimination of measles in the U.S.
• MeVA
– A specialized rRT-PCR assay which can determine if detected measles virus is
vaccine-derived or from community transmission
– Among people recently exposed but also recently vaccinated, can differentiate a
vaccine reaction from a measles case
• Both are performed at CDC and vaccine-preventable disease reference centers
(CA, MN, NY, WI)
Additional Testing is Available

• Measles serology is a useful piece of diagnostic testing but is limited by:
– Cross-reactivity with other causes of febrile rash
– High dependence on disease prevalence
• Diagnostic evaluation of measles should include:
– Both molecular testing (rRT-PCR) and serology
– Consideration of the clinical and epidemiologic context (e.g., travel history,
vaccination status)
• Additional testing is available at CDC and VPD reference centers, in coordination with
state or local public health laboratories
Key Laboratory Diagnostic Points

• What testing is recommended for measles diagnosis?
A. Measles IgM only
B. Swab from nasopharynx / oropharynx (NP/OP) for rRT-PCR only
C. Both serology and a swab for rRT-PCR
D. None – measles is a clinical diagnosis
Knowledge Check #3

• What testing is recommended for measles diagnosis?
A. Measles IgM only
B. Swab from nasopharynx / oropharynx (NP/OP) for rRT-PCR only
C. Both serology and a NP/OP swab for rRT-PCR
D. None – measles is a clinical diagnosis
Knowledge Check #3

Measles Vaccination

• Measles vaccine was licensed in the U.S. in 1963, and
combination MMR vaccine was licensed in 1971
• MMR is an attenuated (weakened) live virus vaccine
– The vaccine cannot cause measles, mumps, or rubella
and cannot be transmitted from person to person
– However, transient side effects can occur which can
mimic these diseases
• Vaccine is highly effective
– 1 dose: ~93% protection
– 2 dose: ~97% protection
• MMR vaccination has an excellent safety record
Measles Vaccine

• Pediatric vaccination schedule
– First dose at 12–15 months
– Second dose at 4–6 years of age
– A dose between 6-11 months of age can be given for travel or
outbreak response
› If a “zero” dose is given between 6–11 months of age, two
more doses should be given on the usual schedule
MMR Vaccine Routine Recommendations

• Adults who have presumptive evidence of immunity* include:
– Birth before 1957
– Laboratory evidence of immunity (positive IgG)
– Prior laboratory confirmed measles diagnosis
• Adults without evidence of immunity generally should get one dose of MMR
– Two doses are required/recommended for high-risk adults
› Healthcare personnel
› International travelers
› Postsecondary school students
MMR Vaccine Recommendations for Adults
2013 ACIP recommendations: http://www.cdc.gov/mmwr/pdf/rr/rr6204.pdf
2019 Adult Immunization schedule: http://www.cdc.gov/vaccines/schedules/hcp/adult.html
* Apart from written documentation of age-appropriate vaccination

• CDC recommends that all U.S. residents older than age 6 months who will travel
internationally receive MMR vaccine prior to departure if they are without evidence
of immunity:
– Infants 6–11 months of age: 1 dose of MMR vaccine
› Followed by two more doses on the typical pediatric schedule
– Children 12 months of age or older: 2 doses of MMR vaccine, separated by at
least 28 days
– Teenagers or adults without evidence of immunity: 2 doses of MMR vaccine
separated by at least 28 days
MMR Recommendations for International Travelers
https://emergency.cdc.gov/han/2023/han00493.asp

• Severe immunocompromising conditions (e.g., hematologic malignancy, receipt of
chemotherapy, long-term immunosuppressive therapy)
– HIV if CD4 % < 15% or absolute CD4 <200
• Family history suggestive of a congenital immunocompromising condition, unless
assessed to be immunocompetent by a clinician or laboratory testing
• History of severe allergic reaction to MMR or to an MMR vaccine component (e.g.,
gelatin)
• Pregnancy
MMR Vaccine Contraindications
https://www.cdc.gov/vaccines/vpd/mmr/public/index.html

• MMR vaccine is generally very well tolerated
• Common side effects include:
– Fever (<15%)
– Brief rash (5%)
– Lymphadenopathy (5% of children, 20% of adults)
• Rare serious adverse events include:
– Anaphylaxis (2–14 events per million doses)
– Febrile seizures (1 event per 3–4,000 doses)
– Low platelet count (1 event per 40,000 doses; may be higher risk if known ITP)
MMR Vaccine Adverse Events

• MMR can cause a short-lived febrile rash syndrome
that is not contagious to others
• Differentiating measles from an MMR reaction in the
setting of an outbreak can be challenging, especially
if MMR was given to prevent measles after an
exposure
– Serology cannot differentiate measles infection
from measles vaccination
– Molecular testing (MeVA) can differentiate
measles from an MMR reaction
MMR Can Cause a Self-limited Rash

• Some commercial labs nationally have begun
offering measles rRT-PCR as part of a viral exanthem
or upper respiratory infection panel
• Positive results for measles have so far only detected
vaccine reactions
• Clinicians should be aware that measles PCR can
detect vaccine-derived measles virus 14+ days after
MMR vaccination
Rash or URI Panels May Detect Vaccine Reactions

• MMR is a safe and effective vaccine
• MMR is contraindicated for pregnant people and people who are severely
immunocompromised
• International travelers should be assessed for prior vaccination or evidence of
immunity, and provided MMR if appropriate for protection
• MMR can cause a self-limited febrile rash, but molecular testing can distinguish
between measles and a vaccine reaction if necessary
MMR Review

Measles Prevention
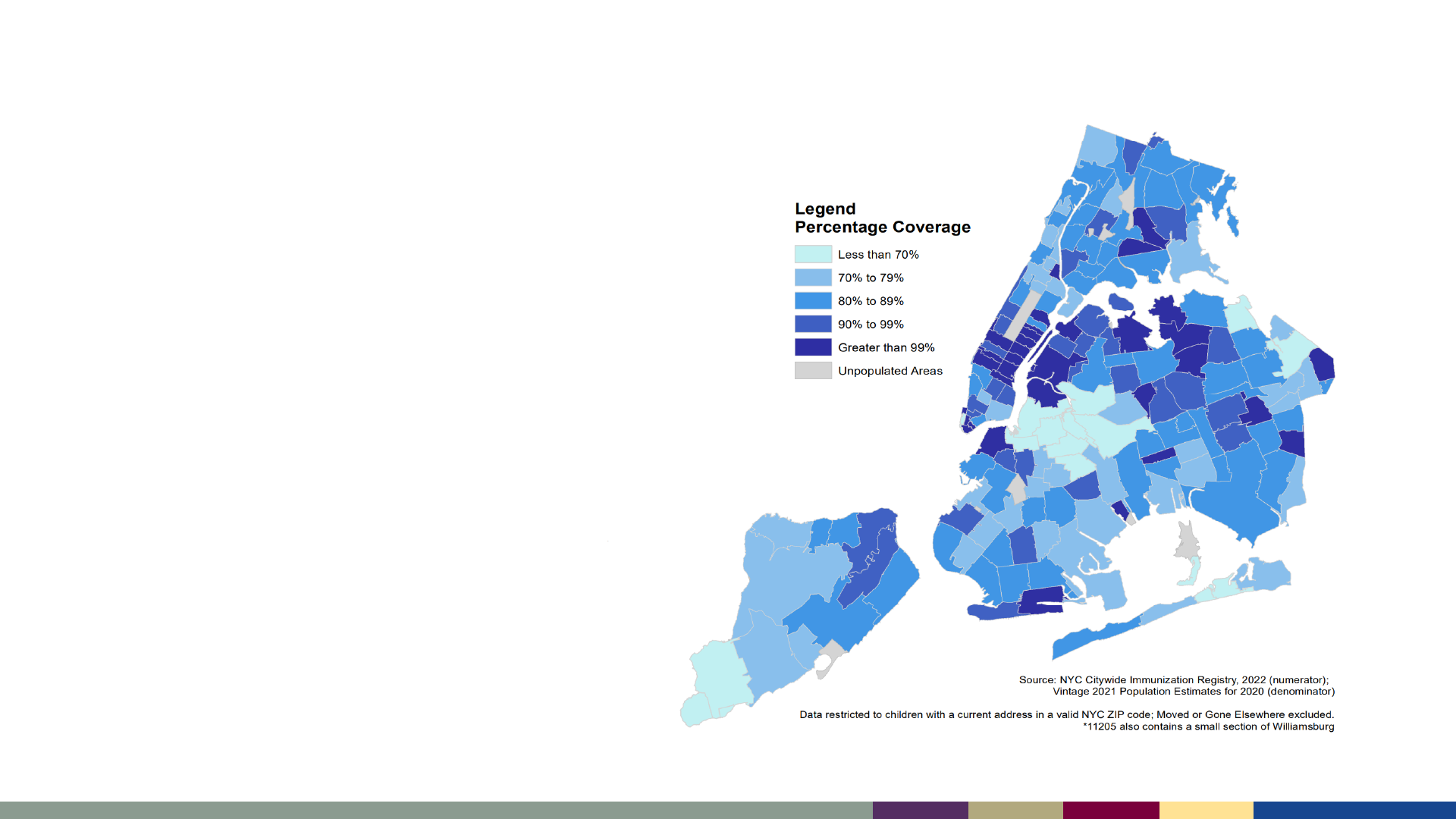
Regional Differences in Coverage Can Hinder Measles
Control
• CDC is working to estimate fine-
area coverage for measles
vaccination to identify regions
where measles may spread more
easily
https://www.nyc.gov/assets/doh/downloads/pdf/cd/polio-vaccination-coverage-by-zip.pdf
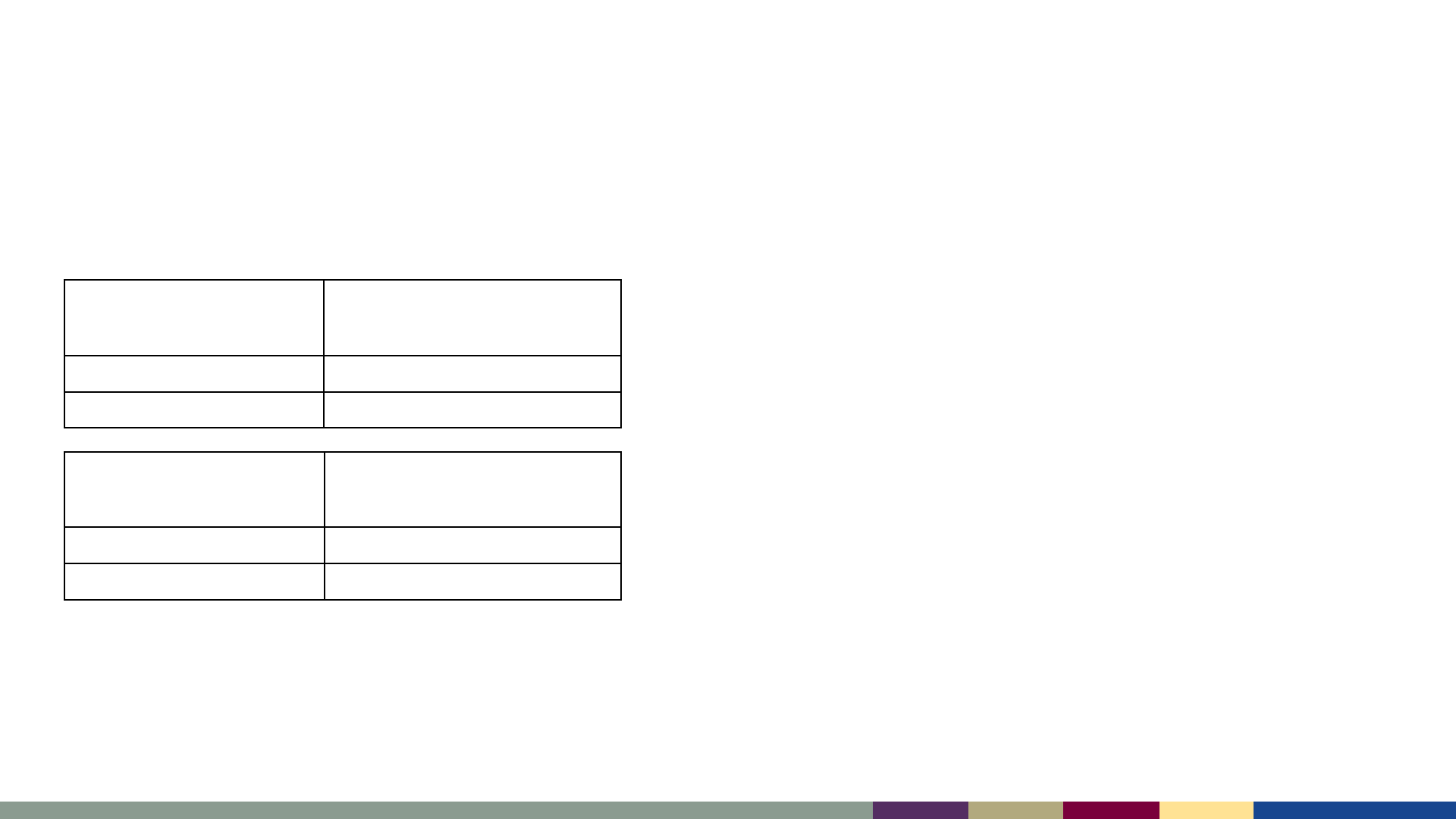
National Coverage with 2 Doses of MMR Fell <95%,
and Disparities Persist
Birth Year
MMR1 coverage
(by age 24 months)*
2017–2018 91.6%
2018–2019 91.6%
School Year
MMR2
(~5–6 years of age)
§
2019–2020 95.2 %
2020–2021 93.9 %
*Estimates for MMR1 are from the National Immunization Survey–Child (NIS–Child)
and represent the estimated national percent of children born in 2017 and 2018 with
provider–reported receipt of at least one dose of MMR by age 24 months.
§
MMR2 column includes the estimated national percent of children entering
kindergarten who have received two MMR doses, as reported by state health
departments. The 2020–21 data represent estimates from the 2020–21 school year
(see https://www.cdc.gov/mmwr/volumes/71/wr/mm7116a1.htm).
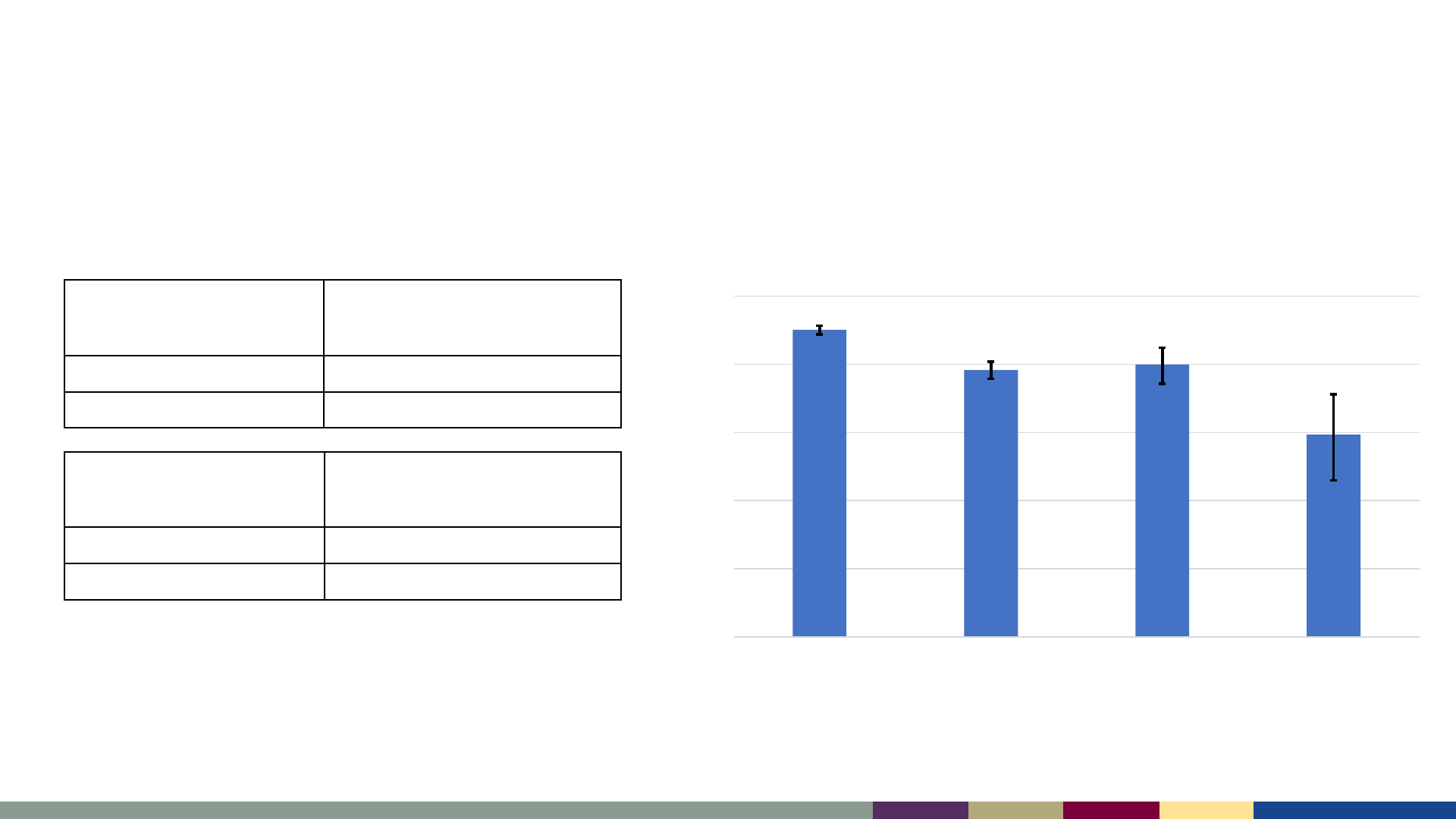
National Coverage with 2 Doses of MMR Fell <95%,
and Disparities Persist
50%
60%
70%
80%
90%
100%
Private Insurance Any Medicaid Other Insurance Uninsured
MMR1 Coverage, 2019–2021
Adapted from: https://www.cdc.gov/mmwr/volumes/72/wr/mm7202a3.htm
*Estimates for MMR1 are from the National Immunization Survey–Child (NIS–Child)
and represent the estimated national percent of children born in 2017 and 2018 with
provider–reported receipt of at least one dose of MMR by age 24 months.
§
MMR2 column includes the estimated national percent of children entering
kindergarten who have received two MMR doses, as reported by state health
departments. The 2020–21 data represent estimates from the 2020–21 school year
(see https://www.cdc.gov/mmwr/volumes/71/wr/mm7116a1.htm).
Birth Year
MMR1 coverage
(by age 24 months)*
2017–2018 91.6%
2018–2019 91.6%
School Year
MMR2
(~5–6 years of age)
§
2019–2020 95.2 %
2020–2021 93.9 %

Routine Immunizations on Schedule for Everyone (RISE)
https://www.cdc.gov/vaccines/partners/routine-immunizations-lets-rise.html
Initiative to get all Americans back on-schedule with their routine immunizations
Understand the size, scope
and cause of declines in
routine vaccinations
resulting from COVID-19
pandemic
Devise an evidence-based
strategy and operations
plan to better direct CDC
routine vaccination catch-
up activities
Equip partners with
evidence-based strategies
and resources to get
vaccination back on
schedule
Share data and insights on
trends in routine vaccination
rates to find and protect
communicates that have fallen
behind on vaccinations

Measles Outbreak Response

• Identify cases and establish the diagnosis
• Perform case and contact investigations
– Obtain accurate and complete immunization and travel histories
– Identify and prioritize contacts without presumptive evidence of immunity
• Implement Control Measures
– Community vaccination
– Consider PEP for susceptible contacts
– Isolation of cases and exclusion of susceptible contacts
– Implement specific precautions in healthcare settings
Overview of Measles Outbreak Response
Identify cases and establish the diagnosis
Clinical Case Definition Vaccination History Travel or Exposure
History in Prior 21 days

• Public health departments can help advise on the need for testing and on the
appropriate routing for specimens
– Early involvement of public health departments can help prevent measles
outbreaks
Identify cases and establish the diagnosis
• Contacts without presumptive evidence of immunity are at high risk to develop
measles
• Exposed persons who are at high risk for serious disease include:
Identify and Prioritize Susceptible Contacts
Infants aged <1 year Pregnant people
People with
immunocompromising
conditions or medications

• Providers can ensure patients are up to date with MMR vaccine requirements
• If preschool-aged children are at risk due to outbreak location and transmission
settings:
– A 2
nd
dose early between age 1 and 4 years could be considered*
• If infants <12 months of age are at risk:
– A “zero-dose” between age 6 and 11 months could be considered
– Repeat vaccination should be pursued according to the routine schedule
Control Measures: Community Vaccination
*Any MMR dose should be given at least 28 days after a prior dose. 2 MMR doses is considered fully protective, but
some states or territories may require an additional dose between ages 4–6 years in accordance with the usual schedule

Control Measures: Post Exposure Prophylaxis (PEP)
MMR
• Should be given within 72 hours (3
days) of initial measles exposure
• Vaccination can be given after this
window, but would only be expected
to protect from future exposures and
is not considered “adequate PEP”
Immunoglobulin
• Needs to be given within 6 days of
initial exposure
• Can be given intramuscularly (IMIG) or
intravenously (IVIG)
- IVIG should be prioritized for
adults at high risk of severe
disease
PEP within the target window may provide measles protection or modify the
clinical course of disease among susceptible people

• Case-patients should be isolated for four days
after rash onset
– People with immunocompromising
conditions with measles may require more
prolonged isolation
• Susceptible contacts without evidence of
immunity should be offered PEP or otherwise
excluded from congregate settings
Control Measures: Isolation
https://www.cdc.gov/vaccines/pubs/surv-manual/chpt07-measles.html

Control Measures: Health care settings
Encourage patients to
contact health care facility
before arrival, if known
(e.g., if they contact health
department prior to
seeking care)
Provide facemask to
patient and
promptly isolate the
patient in a room
with the door closed
Use standard and airborne
precautions including patient
placement in an airborne infection
isolation room (AIIR)
(if unavailable, a single room with
closed door may be used pending
transfer to an AIIR)
https://www.cdc.gov/infectioncontrol/guidelines/measles/index.html
• MMR Vaccine Information for Parents | CDC
– https://www.cdc.gov/vaccines/parents/diseases/measles.html
• Healthcare Professionals Patient Education | CDC
– https://www.cdc.gov/vaccines/hcp/patient-ed/index.html
• Measles Outbreak Toolkit for Healthcare Providers | CDC
– https://www.cdc.gov/measles/toolkit/healthcare-providers.html
• Vaccinate with Confidence | CDC
– https://www.cdc.gov/vaccines/covid-19/vaccinate-with-confidence/community.html
Resources for Clinicians

For more information, contact CDC
1-800-CDC-INFO (232-4636)
TTY: 1-888-232-6348 www.cdc.gov
The findings and conclusions in this report are those of the authors and do not necessarily represent the
official position of the Centers for Disease Control and Prevention.
Thank you!
Joining the Q&A Session
David Sugerman, MD, MPH
Medical Officer
CAPT, U.S. Public Health Service
National Center for Immunization and Respiratory
Diseases
Centers for Disease Control and Prevention
James T. Lee, MD, MSc
Medical Officer
National Center for Immunization and Respiratory
Diseases
Centers for Disease Control and Prevention
Andrew Beck, PhD
Microbiologist
National Center for Immunization and Respiratory
Diseases
Centers for Disease Control and Prevention
Raydel Anderson, MS
Microbiologist
National Center for Immunization and Respiratory
Diseases
Centers for Disease Control and Prevention
To Ask a Question
▪ Using the Zoom Webinar System
– Click on the “Q&A” button
– Type your question in the “Q&A” box
– Submit your question
▪ If you are a patient, please refer your question to your healthcare provider.
▪ If you are a member of the media, please direct your questions to CDC Media
Relations at 404-639-3286 or email media@cdc.gov.
Continuing Education
▪ All continuing education for COCA Calls is issued online through the CDC Training &
Continuing Education Online system at https://tceols.cdc.gov/.
▪ Those who participate in today’s COCA Call and wish to receive continuing education please
complete the online evaluation by September 18, 2023, with the course code WC4520-
081723. The access code is COCA081723.
▪ Those who will participate in the on-demand activity and wish to receive continuing
education should complete the online evaluation between September 19, 2023, and
September 19, 2025, and use course code WD4520-081723. The access code is COCA081723.
▪ Continuing education certificates can be printed immediately upon completion of your online
evaluation. A cumulative transcript of all CDC/ATSDR CEs obtained through the CDC Training
& Continuing Education Online System will be maintained for each user.
Upcoming COCA Calls & Additional Resources
▪ Join us for our next COCA Call, Thursday, August 31 at 2:00 P.M. ET.
▪ Topic: 2023-2024 Recommendations for Influenza Prevention and Treatment in
Children: An Update for Pediatric Providers
▪ Continue to visit https://emergency.cdc.gov/coca/ to get more details about
upcoming COCA Calls.
▪ Subscribe to receive notifications about upcoming COCA calls and other COCA
products and services at emergency.cdc.gov/coca/subscribe.asp.


