
The Economic Impact and
Functional Applications of
Human Genetics and Genomics
Commissioned by the American Society of Human Genetics
Produced by TEConomy Partners, LLC.
Report Authors: Simon Tripp and Martin Grueber
May 2021

TEConomy Partners, LLC (TEConomy) endeavors at all times to produce work of the highest quality, consistent with our contract
commitments. However, because of the research and/or experimental nature of this work, the client undertakes the sole
responsibility for the consequence of any use or misuse of, or inability to use, any information or result obtained from TEConomy,
and TEConomy, its partners, or employees have no legal liability for the accuracy, adequacy, or efcacy thereof.
Acknowledgements
ASHG and the project authors wish to thank the following organizations for their generous support of this study.
Invitae Corporation, San Francisco, CA
Regeneron Pharmaceuticals, Inc., Tarrytown, NY
The project authors express their sincere appreciation to the following indi-
viduals who provided their advice and input to this project.
ASHG Government and Public Advocacy Committee
Lynn B. Jorde, PhD
ASHG Government and Public Advocacy Committee (GPAC) Chair, President (2011)
Professor and Chair of Human Genetics
George and Dolores Eccles Institute of Human Genetics
University of Utah School of Medicine
Katrina Goddard, PhD
ASHG GPAC Incoming Chair, Board of Directors (2018-2020)
Distinguished Investigator, Associate Director, Science Programs
Kaiser Permanente Northwest
Melinda Aldrich, PhD, MPH
Associate Professor, Department of Medicine, Division of Genetic Medicine
Vanderbilt University Medical Center
Wendy Chung, MD, PhD
Professor of Pediatrics in Medicine and
Director, Clinical Cancer Genetics
Columbia University
Mira Irons, MD
Chief Health and Science Officer
American Medical Association
Peng Jin, PhD
Professor and Chair, Department of Human Genetics
Emory University
Allison McCague, PhD
Science Policy Analyst, Policy and Program Analysis Branch
National Human Genome Research Institute
Rebecca Meyer-Schuman, MS
Human Genetics Ph.D. Candidate, Antonellis Lab
University of Michigan
Charles Schwartz, PhD
Senior Research Scientist Emeritus
Greenwood Genetic Center
Wendy Uhlmann, MS, CGC
Clinical Professor of Internal Medicine and Clinical Professor of Human Genetics
Genetic Counselor and Clinic Coordinator, Medical Genetics Clinic
University of Michigan
David Wheeler, PhD
Director of Precision Genomics
Department of Computational Biology
St Jude Children’s Research Hospital
Advisors
Lawrence Brody, PhD
Director, Division of Genomics and Society
National Human Genome Research Institute
Eric D. Green, MD, PhD
Director
National Human Genome Research Institute
Cristina Kapustij, MS
Chief, Policy and Program Analysis Branch
National Human Genome Research Institute
Cynthia Casson Morton, PhD
ASHG President (2014)
Professor of Obstetrics, Gynecology and Reproductive Biology and Professor of Pathology
Harvard Medical School
Chair, Obstetrics and Gynecology, and Director of Cytogenetics
Brigham and Women’s Hospital
Heather C. Mefford, MD, PhD
ASHG Program Committee Chair (2018), Board of Directors (2021)
Associate Professor
Genetics, Epilepsy Program—Center for Integrative Brain Research
University of Washington and Seattle Children’s
Kiran Musunuru, MD, PhD, MPH, ML
ASHG Program Committee Chair (2019), Board of Directors (2021)
Professor of Medicine
Director, Genetic and Epigenetic Origins of Disease Program, Cardiovascular Institute
Perelman School of Medicine at the University of Pennsylvania
David L. Nelson, PhD
ASHG President (2018)
Professor
Molecular and Human Genetics
Baylor College of Medicine
Telephone and Email Interview Participants
Todd R. Golub, MD
Director, Founding Core Institute Member, Broad Institute of MIT and Harvard, and
Founding Core Member and Director of the Gerstner Center for Cancer Diagnostics, Broad Institute
Leroy Hood, MD, PhD
Senior Vice President and Chief Science Officer
Providence St. Joseph Health, and
Chief Strategy Officer and Professor, Institute for Systems Biology
Kathryn A. Phillips, PhD
Professor of Health Economics and Health Services Research, and
Founding Director, UCSF Center for Translational and Policy Research on Personalized Medicine
University of California, San Francisco
Philip Reilly, MD, JD
Venture Partner, Third Rock Ventures
David Veenstra, PharmD, PhD
Professor and Associate Director, Comparative Health Outcomes, Policy & Economics (CHOICE) Institute
School of Pharmacy, University of Washington
Carrie D. Wolinetz, PhD
Associate Director for Science Policy
National Institutes of Health
American Society of Human Genetics Staff
Mona Miller, MPP
Chief Executive Officer
Derek Scholes, PhD
Senior Director, Policy and Advocacy
Lyly G. Luhachack, PhD
Policy and Advocacy Specialist

Contents
Executive Summary ......................................................................................................................................................ES-1
I. Introduction ........................................................................................................................................................................ 1
A. Science as a Driver of Economic and Social Advancement ................................................................................. 1
B. Funding and Supporting American Science ................................................................................................................ 2
C. Human Life Sciences .................................................................................................................................................................... 2
D. The Genome—Coding Life ....................................................................................................................................................... 3
E. Human Genetics and Genomics—Applications and Impacts............................................................................ 5
F. Purpose of the Study .................................................................................................................................................................... 7
G. Limitations of the Study ........................................................................................................................................................... 9
II. The Economic Impact of Human Genetics and Genomics ........................................................................... 11
A. Measuring Human Genetics and Genomics Economic Impacts .....................................................................11
B. Drivers of Economic Impacts: Methodology and Assumptions ......................................................................13
C. Economic Impact Analysis of Human Genetics and Genomics in the U.S. ............................................. 19
D. Healthcare Costs for Genetic Diseases .......................................................................................................................... 20
III. The Functional Impacts of Human Genetics and Genomics .................................................................... 23
A. The Structure of Functional Impacts (Application Domains of Human Genomics) .........................23
B. Fundamental Knowledge Advancement .................................................................................................................... 24
C. Functional Applications for Human Health .................................................................................................................26
D. Summary .......................................................................................................................................................................................... 65
IV. Into the Future ............................................................................................................................................................. 67
A. Ongoing Fundamental Discovery .................................................................................................................................... 68
B. Expanding the Clinical Application of Genomics ................................................................................................... 70
C. Educating and Updating Providers .................................................................................................................................. 71
D. Ethical Considerations .............................................................................................................................................................. 71
E. Conclusion .........................................................................................................................................................................................72
Glossary of Terms .............................................................................................................................................................. 75
Appendix—Additional Economic Impact Information ...................................................................................... 77
ES-1
Modern life sciences and associated advancements
in biopharmaceuticals, diagnostics, medical devices,
and healthcare services have enabled unprecedented
improvements in human health and longevity.
Executive Summary
Perhaps nowhere has life science research advanced
more in the modern age than through insights provid-
ed by genetics and genomics. This field is both funda-
mental in biological research—elucidating the basic
code of life, DNA, upon which our form and function
depend—and in enabling applied and translational
discoveries across most diseases and health disorders.
This report examines and describes the positive im-
pacts that are derived from modern human genetics
and genomics science and its associated commercial
and clinical applications on the nation’s economy,
society, and the health and well-being of individuals.
Twenty years after the completion of the Human
Genome Project, there has been widespread expan-
sion and application of human genetics and genomics
technologies. Technologies for sequencing and for
genome analysis have advanced quite spectacularly—
to the extent that genome sequencing is now both
fast and affordable. The technologies of genetics
and genomics, and the research advancements they
have enabled scientists to make, have now brought
human genetics and genomics to a visible inflection
point—a point in time where scientific discoveries are
rapidly translating into clinical insights and signifi-
cant human health and well-being advancements.
The Economic Impact
of the Human Genetics
and Genomics Sector
The U.S. economy has advanced on the back of sci-
entific progress—progress that has enabled national
leadership in diverse industries such as aerospace,
energy, agriculture, transportation, advanced ma-
terials, information technology, and biotechnology.
Continuing to strengthen the competitiveness of
the U.S. economy requires ongoing expansion of the
national capacity for innovation and the scientific and
technological research and development (R&D) upon
which innovation depends. Particularly important is
leveraging science and innovation to give rise to new,
fast-growing, advanced industries that spark econom-
ic growth and improved standards of living. Born out
of federal investment in the Human Genome Project,
the U.S. achieved early leadership in the genetics and
genomics industry—leadership that has resulted in the
growth of an important and dynamic economic sector.
Substantial U.S. economic activity, supporting a
large volume of high-paying jobs across the nation,
is generated from the performance of genetic and
genomic research, the development and manufactur-
ing of commercial genomic technologies, the broad
range of diagnostics products and therapeutics on the

ES-2
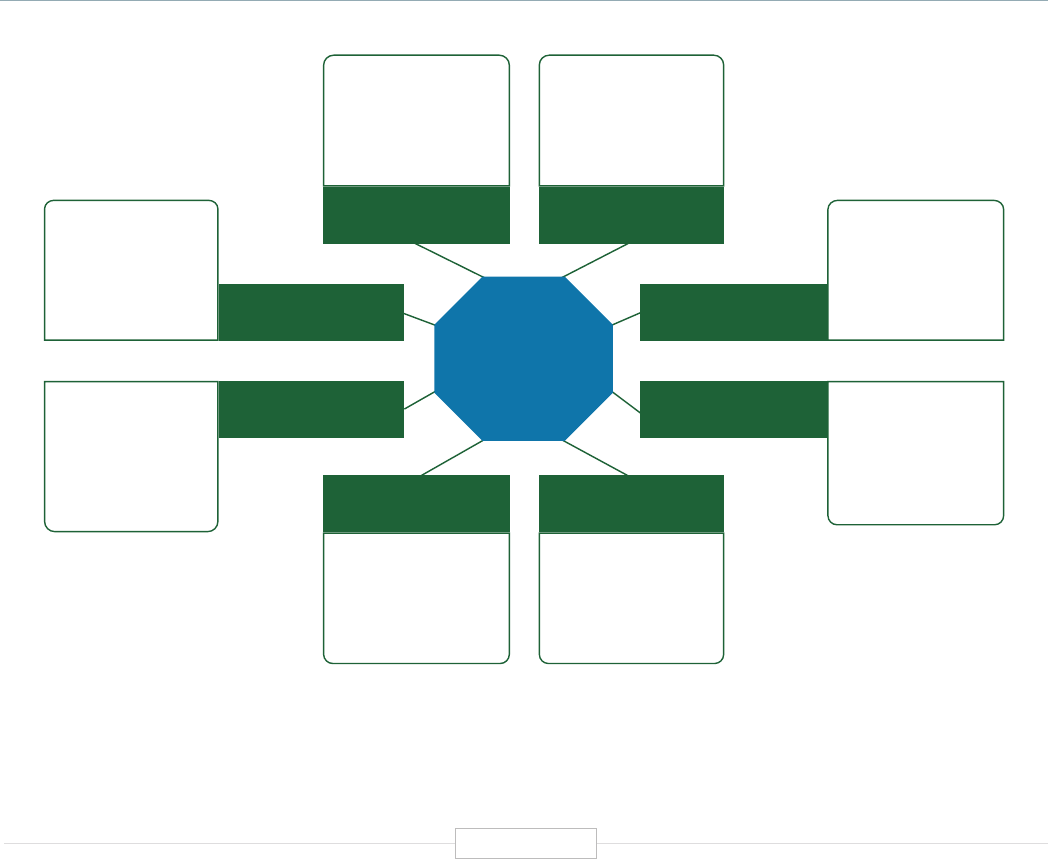
Figure ES-1: The Economic Impact of the Human Genetics
and Genomics Sector in the United States
Human Genetics &
Genomics Focused
Research Expenditures,
Services, and Corporate
Operations in the U.S.
Purchase of Secondary
Inputs & Services from U.S.
Suppliers and Vendors
Human Genetics &
Genomic Supported
Employees Spending
Disposable Income
in the U.S. Economy
Total Economic
Impacts of
Human Genetics
& Genomics
DIRECT EFFECT INDIRECT EFFECT INDUCED EFFECT
$3.3B
FEDERAL RESEARCH
Federal research funding, using a conservative definition of what constitutes human genetics
and genomics research, reached $3.3 billion in 2019, with most of this coming from NIH.
152,000
INDUSTRY JOBS
89,464 core private sector industry jobs and an estimated 62,710 additional extended
industry jobs (related employment share from major pharmaceutical and medical
testing/diagnostics companies).
850,000
TOTAL SUPPORTED JOBS
With a direct employment estimate of nearly 166,000 academic and industry jobs,
human genetics and genomics supports more than 850,000 total jobs. Each direct
human genetics and genomics job supports 4.12 additional jobs in the U.S. economy.
$
$265B
TOTAL ECONOMIC IMPACT
The direct economic activity generated by the human genetics and genomics industry
exceeds $108 billion in 2019 and ultimately supports a total of more than $265 billion across
the U.S. economy. Every $1.00 of direct human genetics and genomics activity generates
an additional $1.45 in the U.S. economy.
$5.2B
DIRECT FEDERAL TAX REVENUES
The federal tax revenues of $5.2 billion generated by the direct operations of the human
genetics and genomics domain alone surpasses the single year federal investment in human
genetics and genomics of approximately $3.3 billion across all federal agencies.
4.75:1.00
FEDERAL RETURN ON INVESTMENT
In the simplest of terms, from a federal investment and revenue perspective, the overall
economic impacts of U.S. human genetics and genomics generates a return on investment
(ROI) of more than 4.75 to 1.00 ($3.3 billion in federal investment in human genetics and
genomics – while the whole domain generates $15.5 billion in federal tax revenues).
Source: TEConomy Partners, LLC.
market that are derived from genomics knowledge
and have pharmacogenomic associations, and the
associated healthcare services that are delivered.
The economic impact of the human genetics and
genomics sector on the U.S. economy is assessed
using the standard regional economics methodology
of input-output analysis. The results demonstrate
the growth of a powerful economic sector across
the nation— a sector that has grown five-fold in
its annual economic impact since 2010. Even more
importantly, it is a sector that also generates robust
functional impacts in terms of human health and
well-being. Figure ES-1 summarizes some of the
topline findings from the economic impact analysis.
The sector also supports high wage jobs. Because it
requires a well-educated and technically skilled work-
force, direct jobs in the genetics and genomics sector
pay more than $130,000 in annual total compensation
(income and benefits) per worker, while the total jobs
supported by human genetics and genomics eco-
nomic activity (direct + induced) average greater than
$81,000 in compensation per employee.
ES-3
The Functional Impacts of
Human Genetics and Genomics
The speed and affordability of gene sequencing
and advanced genomic data analytics have helped
produce deep biomedical insights and innovations,
which are being combined with advancements in
biopharmaceuticals, diagnostics, and other medical
technologies that leverage genomic information. An
evident tipping point has been achieved where the
utility of genomics and wide-spread use of sequencing
is clearly advantageous for significantly enhancing
human health outcomes. As this report highlights,
the functional application of human genetics and
genomics to clinical healthcare is now a daily reality
in some medical fields (e.g., cancer diagnosis and
treatment) and is increasingly front-and-center in
neurological, psychiatric, gastrointestinal, immunolog-
ic, rheumatologic, dermatologic, pain management,
and other application areas of clinical medicine. It is
also fundamental to advancements being made in
the diagnosis and treatment of a wide range of rare
diseases and disorders—helping to end the diagnostic
odysseys of millions of patients afflicted with rare
diseases that have been difficult to diagnose and
sparsely served in terms of available treatments.
In reviewing the functional applications of human
genetics and genomics, the authors find that
the positive impacts being generated are highly
diverse—generated within eight major domains
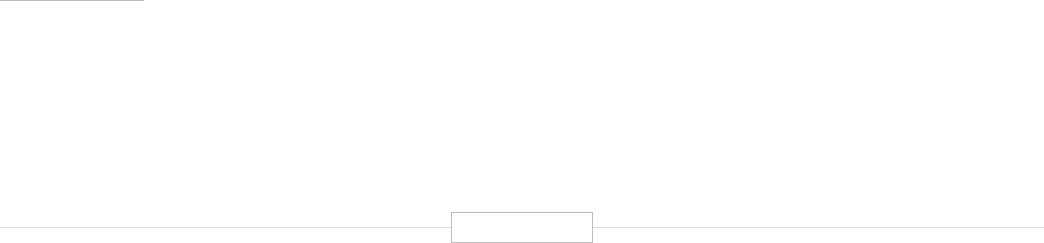
Figure ES-2: Functional Biomedical Impact Domains
(Applications) of Human Genetics and Genomics
Minable Big Data
(Discovery Science)
Identifying Predisposition
to Diseases and Disorders
Diagnosing Diseases
and Disorders
Rational Drug
Development
Pharmacogenomics
(Personalized Medicine)
Gene Editing
and Gene Therapy
Human-Microbe
Interaction
Environmental Genomics
and Metagenomics
Analyzing sequencing
data from large and
diverse populations to
provide deep insights
into disease biology and
identify characteristics
associated with health.
Genetic and genomic
testing to identify
carrier status, and
identify predisposition
for genetic disease via
prenatal, newborn and
adult screening.
Using biomarkers
and gene signatures
to diagnose the
presence of diseases
or disorders that are
associated with
specific genes or
gene products.
Using genetic
information and gene
associated biomarkers
to inform molecular
targeting in drug design.
Using sequencing data
to enable the
prescription of drugs
best suited to the
patient’s genotype
(increasing efficacy and
reducing adverse events)
Modifying the genes
associated with a
disease or disorder
to treat or cure the
disease
Biomedical
Application
Domains of
Human
Genetics
and
Genomics
Examining the human
genome’s impact
upon hosted microbial
populations, and
microbe impacts upon
the human genome
and gene expression
Examining the impact
of human interactions
with the environment
on the human genome,
gene regulation, muta-
tion, and disease
etiology.
1
2
3
4
8
7
6
5
Biomedical
Application
Domains of
Human Genetics
and Genomics
Source: TEConomy Partners, LLC.
ES-4
of activities impacting human health. These are
summarized and briefly described in Figure ES-2.
The eight domains identified in Figure ES-2 are
already having profound impacts in advancing
clinical health sciences and health outcomes.
Each of these areas is profiled briefly below and
detailed further in the full body of the report.
1. Minable Big Data (Discovery Science)
Advancements in high-speed gene sequencing
technologies have facilitated the assembly of exabytes
1
of genomic information that can be analyzed (assisted
by highly advanced and automated analytical systems)
for unique insights into genome structure and func-
tion and the association of gene variants with human
diseases and health disorders. It is anticipated that by
2025 more than 60 million patients will have had their
genome sequenced in a healthcare context.
2
Access to
extremely large volumes of sequenced individuals pro-
vides a rich platform for important scientific discovery
and for advancing the identification and classification
of genomic variant pathogenicity (variants associated
with causation of disease). Both science and techno-
logical capabilities are now at the point where the
analysis of genomic and phenomic big data provides
a powerful pathway forward for biomedical discovery
and clinical applications to improve human health.
2. Identifying Predisposition
to Diseases and Disorders
One of the primary research and clinical applications
of human genetics and genomics is identification of
the potential predisposition for individuals to develop
specific diseases or health disorders. Modern genetic
screening for such predispositions divides into three
key categories: 1) carrier screening, which tests a
prospective parent for the presence of gene variants
that have been shown to be associated with risk of
passing down a hereditary disorder (thereby helping
An exabyte = bytes (,,,,,, bytes).
Birney, Ewan. “Luminaries Share Their Thoughts on Advances in ‘Omics Over the Past Five Years.” Clinical Omics Magazine, vol. , no. , March-
April .
National Center for Advancing Translational Sciences, Genetic and Rare Diseases Information Center. “FAQs About Rare Diseases.” https://
rarediseases.info.nih.gov/diseases/pages//faqs-about-rare-diseases. Accessed May .
Ibid.
to inform family planning and associated decisions);
2) pre-natal and post-natal testing, which focuses on
testing for genetic predisposition to disease in the
fetus or in newborns; and, 3) child and adult testing.
Information provided by predisposition screening
enables patients and their physicians to make in-
formed healthcare decisions, plan follow-up health
monitoring strategies, and identify strategies for
care using evidence-based clinical best practices.
3. Diagnosing Disease,
Rare Diseases, and Disorders
Whole genome and whole exome sequencing are
increasingly being used in clinical practice to facilitate
the diagnosis of diseases or health disorders. In addi-
tion to the many common chronic diseases (such as
heart disease, diabetes, cancer, etc.), approximately
7,000 rare diseases have been recognized
3
and have
historically been a significant challenge to diagnose.
Rare diseases, by their inherent nature of being rare,
present diagnostic challenges because so few physi-
cians have encountered them. Often, these diseases
may present symptoms seen in other, more common
diseases, resulting in an understandable misdiagnosis
and inappropriate treatment strategies being adopt-
ed. Patients, and their families, may embark on long
“diagnostic odysseys”, seeing dozens of practitioners,
undergoing multiple tests and procedures, enduring
fruitless attempts at treatment over many years
without ever getting a definitive, accurate diagnosis.
Genetic and genomic testing provides a pathway
to solving this dilemma in multiple diseases and
disorders impacting many thousands of patients.
Collectively, rare diseases have a significant population
impact, with approximately 1 in 10 individuals having
a rare disease (estimated at between 25-30 million pa-
tients in the U.S. and 350 million worldwide).
4
Modern
genetic and genomic diagnostic tools, informed by
ES-5
scientific advancements in identifying gene variants
associated with specific diseases, are providing clear
diagnostic benefits. By deploying genetic and ge-
nomic testing, up to and including whole genome
sequencing, diagnostic odysseys may be ended for
many patients—not only providing a pathway to
appropriate treatment but also reducing significant
waste in the healthcare system and the associated
costs of incorrect diagnosis. Even if no treatment is
available, peace of mind can result through simply
having an “answer” and being able to end the costly
hunt for diagnosis. It has been noted that “this is
clearly the most powerful diagnostic tool ever devel-
oped for the millions of children with rare diseases.”
5
4. Rational Drug Development
Rational drug development uses genetic and genomic
information to advance the development of new
biopharmaceuticals to treat diseases. Biomarkers
(genes or gene products) are providing molecular
targets for purposefully designed drugs that are engi-
neered to bind to targets. The application of genetics
and genomics to drug development has resulted in
multiple clinical successes, with specific examples
highlighted in this report. Biopharmaceutical com-
panies are now able to use genetic and genomic
information to target the trials of their pharma-
ceutical and biologic molecules to patients who
have been preselected through the presence of
biomarkers (often genetic). This has the potential to
advance more drugs successfully to market since
Kingsmore, Stephen. “Luminaries Share Their Thoughts on Advances in ‘Omics Over the Past Five Years.” Clinical Omics Magazine, vol. , no. ,
March-April .
they are more likely to demonstrate efficacy in
their trials by virtue of being rationally targeted.
It is also notable that progress in genetics and
genomics has enabled pharmaceutical research
to increasingly address rare diseases—helping to
rebalance biopharmaceutical research in terms of
work on chronic diseases versus rare diseases.
5. Precision Medicine and Targeted
Therapeutics (Pharmacogenetics)
Having an ability to sequence a patient’s whole
genome rapidly and cost-effectively has opened
the door to a new paradigm in healthcare termed
“precision medicine” whereby an individual’s genetic
profile is used to guide decisions made in regard to
the prevention, diagnosis, and treatment of disease.
The discipline of “pharmacogenetics” (also “pharma-
cogenomics”) has developed as a field of research
and, increasingly, clinical practice, that addresses the
genetically determined variation in how individuals re-
spond to specific drugs in terms of differences in dose
requirement, efficacy, and the risk of adverse drug
reactions (ADRs). It is increasingly being employed to
help physicians select the “right drug and the right
dose” for a patient based on their genome (assuming
there is statistically significant clinical information
linking a drug to specific gene variants in terms of effi-
cacy and side effects). Currently, pharmacogenetics is
improving health outcomes along three primary paths:
The ability to tailor a drug regimen to a specific genetic code that is
truly personalized to that specific DNA double helix has been a dream of
researchers, physicians, and patients alike. Advances in precision medicine,
specifically around the genome…are making this dream a reality.”
Kristen Ciriello Pothier. Personalizing Precision Medicine. A Global Voyage from Vision to Reality. John Wiley & Sons, Inc., 2017.

ES-6
• Selection of the therapeutic (among multiple
choices) that is likely to prove most efficacious
based on the patient’s genome and a drug’s
proven efficacy for their specific genotype.
• Ruling-out a therapeutic (among multiple
choices) based on the patient’s genome and
a drug’s potential for unacceptable adverse
side effects given their specific genotype.
• Development of an optimized drug dosage for a
patient based on their genotype’s influence on
the rate at which they will metabolize the drug.
Cancer is perhaps the most well-recognized cluster
of disease for which genetic tests may impact drug
selection and dosing; however, analysis of U.S. Food
and Drug Administration (FDA) data shows that
pharmacogenetic associations are also in place for
multiple chronic diseases and conditions, covering
applications in major categories such as cardio-
vascular disease, gastroenterological diseases and
disorders, infectious diseases, neurological diseases
and disorders, psychiatric conditions, and rheuma-
tologic diseases. Pharmacogenetic associations
now span a range from relatively rare diseases, such
as Tourette’s syndrome and Tardive dyskinesia, to
common conditions, such as hypercholesterolemia
and depression. There are more than 100 drugs for
which the associations are now listed by the FDA.
6. Gene Editing and Gene Therapy
As noted above, genetic and genomic advancements
are elucidating gene variant associations with the
predisposition for disease, providing enhanced diag-
nosis of diseases, and providing increasingly effective
pathways for therapeutics and disease treatment.
Another developing approach is to use the expanding
knowledge of gene variants associated with disease
to provide targets for potential modification of a
patient’s genes themselves—modification that has
the goal of treating, and potentially curing, the target
It should be noted that the discussion of gene editing and gene therapy pertains to modifying non-hereditable (somatic) genes—changes
to an individual’s genes that will only affect the individual being treated but not the genes of future generations. There is ongoing discussion
and public debate about the potential use of gene editing to make heritable genetic changes (changes to the germline). Such genome edits
would result in changes to an individual’s DNA being passed to their progeny and subsequent generations. At the present time, the general
consensus of leading organizations in medical genetics, genetics research, and genetic counseling is that genome editing which culminates
in human pregnancy should not be undertaken, and that further research is required into the scientific, clinical, and ethical implications of
germline editing.
disease through what is termed gene editing or gene
therapy. Ultimately, gene editing and gene therapy
represent new pathways to the treatment and curing
of diseases, but these approaches are still in the early
stages of clinical application.
6
Part of the caution in
clinical application arises from a need for further study
of the potential for off-target gene edits (mutagenesis)
to occur in non-targeted genes and for unintended
mosaicism to occur. Despite these challenges, there
are several important gene therapies that have suc-
cessfully advanced through clinical trials, helping to
treat a series of previously untreatable rare diseases.
It is a promising field for ongoing advancement.
7. Human-Microbe Interactions
Each of us is host to communities of trillions of
microbes. Microbes serve important functions for
humans, for example aiding our digestion and the
breakdown of micronutrients, defending us from
pathogenetic microbes, and priming our immune
system. Recent research has shown that we have a
symbiotic two-way genetic interaction with microbes,
with microbes impacting our genes and gene expres-
sion, and human genotype impacting the make-up of
the microbial communities we host.
While microbes play an important positive role in
our health, many microbes are pathogenic, being
the causative agents for human infectious diseases.
Research is finding that individual genomes can
be associated with resistance or susceptibility to
certain infectious diseases, and the recent COVID-19
pandemic, coinciding with the current significant
volumes of patients for which genome sequences
are available, has enabled significant clinical study of
genome effects on viral susceptibility and resistance.

ES-7
8. Metagenomics and
Environmental Genomics
There exists a vast network of interactions between
individual genomes and other biological and environ-
mental systems. Each of us walks a slightly different
path through life, experiencing different influences
upon our physiology in terms of the food we eat, the
amount of sun we expose ourselves to, the environ-
ments we experience in our jobs, the pathogens that
we by chance encounter, etc. Any and all of these and
more may be subtly changing (mutating) letters in our
genome or periodically influencing gene regulation
or expression. Metagenomics is the field of genomics
that investigates these interactions and their effects.
Obviously, the human genome is highly complex. Add
to that all the genomes in the environment with which
one may come into contact, and the enormity of the
subject comes into focus. Large-scale sequencing
programs are, however, providing a rich resource of
data for scientists to mine in metagenomic studies.
Genomics in the
COVID-19 Pandemic
Genomics rapidly assumed crucial roles in
COVID-19 research and clinical care in areas such
as: (1) the deployment of DNA and RNA sequenc-
ing technologies for diagnostics, tracking of viral
isolates, and environmental monitoring; (2) the
use of synthetic nucleic acid technologies for
studying SAR- CoV-2 virulence and facilitating vac-
cine development; (3) examination of how human
genomic variation influences infectivity, disease
severity, vaccine efficacy, and treatment response;
(4) the adherence to principles and values relat-
ed to open science, data sharing, and consortia
based collaborations; and (5) the provision of
genomic data science tools to study COVID-19
pathophysiology. The growing adoption of ge-
nomic approaches and technologies into myriad
aspects of the global response to the COVID-19
pandemic serves as another important and highly
visible example of the integral and vital nature of
genomics in modern research and medicine.
Eric D. Green, et al. “Perspective: Strategic Vision for
Improving Human Health at the Forefront of Genomics.”
Nature, vol. 586, no. 29, October 2020.

ES-8
Conclusion
The fields of human genetics and genomics are
having profound positive impacts not only in terms
of biomedical discovery, but also in terms of the
clinical practice of medicine—working to improve
the lives for millions of patients and demonstrating
great promise for future highly positive contribu-
tions to human health and well-being worldwide.
As the eight functional domains for human health
application of genetics and genomics illustrate,
this field of science (and the expanding industry
associated with it) generates a profound impact
on biomedical research and the practice of clinical
healthcare. In addition to applications in human
medicine and wellness, there are also several
non-medical human applications of genetics and
genomics, including forensic science, anthropology
and genealogy, evolutionary biology, and paternity
testing. These are also highlighted in the report.
It is readily evident that, as fundamental genomic
knowledge has expanded, the enhanced understand-
ing of genetic mechanisms generated, in concert with
access to rich whole exome and genome datasets
(and associated reference compendia of human gene
variants), has opened the door to a new era of discov-
ery and progress in medicine. The impacts of these
advancements are now increasingly reverberating
across medicine, a fact highlighted by Eric Green, the
Director of the National Human Genome Research
Institute (NHGRI), and colleagues who note that:”
With insights about the structure and function
of the human genome, and ever improving
laboratory and computational technologies,
genomics has become increasingly woven
into the fabric of biomedical research, medical
practice, and society. The scope, scale, and
pace of genomic advances so far were nearly
unimaginable when the human genome
project began; Even today, opportunities
Green, Eric D., et al. “Perspective: Strategic Vision for Improving Human Health at the Forefront of Genomics.” Nature, vol. , Oct. .
beyond their initial expectations, with many
more anticipated in the next decade.
7
While generating these positive functional impacts
is the raison d’etre for pursuing the advancement of
human genetics and genomics, it has also had the very
positive spillover effect of building a powerful science-
and technology-based economic sector for the U.S.—a
sector that supports over 850,000 jobs across the nation
and generates a $265 billion economic impact in terms
of U.S. economic output. The continued innovation in
human genetics and genomics is expanding the stock
of knowledge upon which our continued advancement
depends and shows great promise to continue to do
so long into the future. The field represents a particu-
larly strong example of how investing in fundamental
and applied science generates robust economic,
social, and individual benefits for humankind.
1
I. Introduction
A. Science as a Driver of Economic
and Social Advancement
Scientific research is of high importance not only be-
cause it reveals fundamental truths but also because it
increases the stock of human knowledge upon which
economic, societal, and technological progress de-
pends. The U.S. economy, in particular, has advanced
on the back of scientific progress that has enabled
national leadership in diverse industries such as aero-
space, energy, agriculture, transportation, materials,
digital technology, communications, and healthcare.
In the past two decades, scientific advancements
have seen new industries advanced, typically with the
U.S. being an early innovator in commercial applica-
tions of both science and associated technologies.
Developments in physics, chemistry, biology, and
mathematical and computational sciences have paved
the way for new industries in nanotechnology and
advanced materials, renewable energy, AI-powered
autonomous systems, biotechnology, and genetic
engineering. There has also been an observable trend
of “convergence,” whereby multiple science and
technological disciplines combine to advance new
opportunities. Advancements in computational and
digital analytics converging with large-scale data from
other sciences have helped accelerate this trend.
What has become clear is that continuing to strength-
en the competitiveness of the U.S. economy requires
ongoing expansion of national innovation capacity
and the scientific and technological research and
development (R&D) upon which that innovation
From Fundamental
to Applied Research
Public/Private and Market/
Non-Market Returns
Scientific research may produce both private and so-
cial returns. Research that leads to improved knowl-
edge, national security, public health, enhanced food
security, etc. provides social (public) returns. Research
generating a patented technology or improving the
productivity of a production process provides private
returns to those inventing and using the research-
based tool or knowledge for commerce. Often, re-
search leads to both forms of return at the same time.
For example, the development of a vaccine for an
infectious disease provides private monetary returns
to the vaccine developer and social returns through
enhanced public health. Both types of returns mo-
tivate investment in research. Research finds that
the rate of social return on R&D exceeds the rate of
private return (although both are strong). Despite the
large U.S. national investment in research, analysis
shows that optimal investment in research would be
more than four times actual investment.
The robust levels of social return on research un-
derpin the core rationale for public investment in
research. Indeed, without public support for research,
a wide-ranging and important suite of research topics
would go unaddressed. Because so much important
research is non-market (focused on phenomena or
subject matter without an immediate line-of-sight to
a market application) or focused on the generation of
social returns (benefiting society overall, but perhaps
not able to realize private returns to investment),
the public sector plays a critically important fund-
ing function in the R&D ecosystem. Further, private
sector investment in basic science is relatively scarce
because of the speculative nature of early fundamen-
tal research, the long time-horizons involved in the
performance of basic inquiry, the risk of experiment
failures, and, most importantly, the lack of immediate
line-of-sight to market.
What is critically important to understand is that the
U.S. economy, and the innovations and technologies
upon which it depends, is built upon a bedrock of
fundamental scientific advancements and an overly-
ing strata of applied and translational discoveries that
leverage fundamental knowledge.
2
depends. This requires funding for research and the
institutions that perform research, together with
funding for the education of the scientists, technol-
ogists, engineers, mathematicians, and other skilled
intellectual talent that innovates and produces the
products and services that result from innovation.
B. Funding and Supporting
American Science
The federal government has been and continues
to be an essential component of the U.S. research
ecosystem, funding research performed at univer-
sities, independent research institutions, and other
organizations and conducting research within na-
tional institutes and laboratories. Federal funding for
research has been especially important in supporting
fundamental science, which in turn represents the
platform upon which applied research may advance.
Federal funding also plays an important role in trans-
lating research into early-stage commercialization,
through translational research funding and dedicated
early-stage venture support through the federal
Small Business Innovation Research (SBIR) and Small
Business Technology Transfer (STTR) programs.
Support for scientific research in the U.S. comes from
both public and private sources. As noted in the prior
sidebar textbox, public resources focus primarily on
“ Global R&D Funding Forecast”, R&D World, February , WTWH Media, LLC.
Kochanek, Kenneth D., et al. “Mortality in the United States, .” NCHS Data Brief, No. , Dec. .
Ibid.
supporting basic through applied research while
private (commercial) funding focuses primarily on
powering applied and translational research that
advances innovations into commercial application. In
total, the U.S. R&D enterprise spent $596.6 billion in
2019, representing 2.84% of GDP.
8
However, the extent
to which the economy is driven by and built upon the
innovative output of R&D makes the impact of R&D
on overall GDP many times larger and a dominant
factor in U.S. economic success. The economic future
of the U.S. hinges upon research and development.
C. Human Life Sciences
Scientific research produces many benefits and
returns for society, but perhaps none are as important
as the preservation and extension of human life itself.
Modern life sciences and associated advancements
in biopharmaceuticals, diagnostics, medical devices,
and healthcare services have enabled unprecedented
improvements in human health and longevity. Today,
the average life expectancy for a newborn female
and male in the U.S. is 81.4 years and 76.3 years,
respectively.
9
In 1950, those same metrics were 71.1
and 65.6 years.
10
Average lifespans have expanded
by more than a decade in less than two generations
through advancements in health, hygiene, and safety.
Today, a powerful argument can be made for substantially
increased investment in research in the United States. Equally
great is the need to train the next generation of scientists and
citizens for what will be a very different world.”
National Academy of Sciences. 2021. “The Endless Frontier. The Next 75 Years in Science.”
“

3
The impact and importance of human life science
advancement have come sharply into focus during the
COVID-19 pandemic, where R&D-based innovation of
diagnostic tests, advanced therapeutics, and the rapid
development of vaccines has proven crucial in forg-
ing a path for a return to normal life and commerce.
As noted in a recent report: “the COVID-19 crisis has
vividly illustrated the critical importance of life science
research and innovation systems and the ecosystems
that support the advancement of innovations through
commercial deployment to address health needs.”
11
Scientific advancements in biology and medicine
contribute to our daily lives and are also unveiling the
incredibly complex physical mechanisms and human/
environment interactions that govern our develop-
ment and health. The Russian doll analogy appears
to hold, with the life sciences uncovering level upon
level of complexity and interrelationships in biological
structures, mechanisms of influence, and associated
health outcomes. While unveiled complexity may
confound easy solutions to questions and problems,
it also provides an expanding universe of potential for
discoveries, applications, and functional possibilities.
Tripp, Simon, et al. Response and Resilience: Lessons Learned from Global Life Sciences Ecosystems in the COVID- Pandemic. TEConomy
Partners, LLC for Pfizer, Inc., Jan. .
Perhaps nowhere has life science research advanced
more in the modern age than through insights
provided by genetics and genomics. This field is both
fundamental in biological research—elucidating the
basic code of life, DNA, upon which our form and func-
tion depend—and in enabling applied and translational
discoveries across most diseases and health disorders.
D. The Genome—Coding Life
Humans are complex. That is true on many levels,
and it is certainly true in terms of our biology. The
more biologists learn of our biological structure
and function, the more complex and intricate
the machinery of our biology is revealed to be.
We each comprise approximately 30 trillion indi-
vidual cells and over 200 different cell types. Our
development and ongoing biological function are
orchestrated by our DNA (deoxyribonucleic acid), a
linear molecule arranged in a double helix (a spiraling
ladder) comprising linked base pairs of the nucleo-
tides adenine (A) and cytosine (T), and guanine (G)
and thymine (T). Our DNA contains six billion base
pairs of these nucleotides, the sum of which is called
Investing in People
and Infrastructure
Science relies on an educated base of sci-
entists who make discoveries and is also
advanced through the development of new
tools and technologies that power experi-
ments and enable new insights into biolog-
ical processes. For example, advancements
in ultra-high resolution imaging technology,
functional imaging of real-time processes,
analytical chemistry instruments, computa-
tional systems, and gene sequencing equip-
ment have facilitated leaps forward in funda-
mental and applied scientific insights.

4
our “genome,” which is effectively the governing
instruction set and a regulatory “code” for our bodies.
The six billion base pairs of our genome are organized
into 46 chromosomes (23 inherited from our mother
and 23 from our father). A chromosome consists of
a long section of DNA containing up to 500 million
base pairs of DNA with thousands of genes. An
individual “gene” is defined as a grouping of base
pairs that together perform a function, encoding the
synthesis of a gene product (either RNA or a protein).
We each have approximately 20,000 protein-coding
genes, which comprise circa 2% of our genome.
As genetics and genomics developed as scientific
disciplines, for a long time the standard hypothesis
was that the part of our genome that “mattered” is
the 2% comprising our protein-coding genes, because
proteins are the functional biomolecules performing
the vast majority of biological activities. It was com-
mon to consider the 98% majority of our DNA as “junk
DNA”, legacy base pairs accumulated over the huge
span of time in our evolution, but no longer relevant or
“functional” to our development and predominant bio-
logical functioning. We now know that is not the case.
Parrington, John. The Deeper Genome: Why There Is More to the Human Genome than Meets the Eye. Oxford University Press, .
After the publishing of the draft human genome by
the Human Genome Project and Celera, an inter-
national team of 442 scientists from 32 institutions
embarked on a large-scale team research project
called ENCODE (the ENCyclopedia of DNA Elements),
which used leading edge approaches to measure
biochemical activity across the entire human genome,
not just protein coding genes. ENCODE revealed that
the non-protein coding regions are far from being
“junk” and primarily contain DNA with an active
biochemistry, even if the preliminary findings did not
elucidate the function of that activity. The results
indicate a far more complex and multifaceted func-
tionality to our genome than previously thought.
12
Complicating matters further is that while the genome
codes for our fundamental life processes it is not
deterministic. Other factors also influence our biology
and health, including a multitude of interactions
with external environmental factors and stimuli,
such as the foods we consume and the microbial
communities that inhabit us over our life journey.
We are all the same species, homo sapiens, but we
are all different. None of us have exactly the same
genome sequence nor the same environmental
interactions. A large number of the diseases and
health disorders we will face across our lifespan will
be similarly diverse and complex, many being found
to be associated with multiple genes and gene
variants in combination with wide-ranging external
factors—for example, prior infection with pathogens
or differential exposure to mutagenic factors such as
chemicals or radiation (causing genotoxic injury).
Numerous diseases and health disorders result
from single gene changes (known as monogenic
or Mendelian diseases), where a variant within a
single gene may code the wrong protein or a dys-
functional gene product. However, many diseases
with genetic engagement involve multiple genes
and interactions between various genes and ad-
ditional factors. Many diseases, including most of
We also are each host to trillions of
microorganisms found in our gut and
other parts of our anatomy, many of
which play an important symbiotic role
for us in digestion and immune system
function. Each of these microorganisms
has its own DNA. Collectively, all of
those microorganisms and their DNA
comprise our “microbiome.” Research in
epigenetics has found that microbiome
structure can influence and impact
human gene expression and regulation.
5
our large-scale chronic diseases, turn out to be a
complex soup of genetic, epigenetic, and envi-
ronmental factors interfacing with one another.
As individuals, our incredible complexity, and the
biologically influential environmental factors we
each uniquely encounter across our lives, explain
why the development of diagnostics and drugs
is such an intense, difficult, and expensive chal-
lenge. Finding generic solutions to individually
variable disease causations and expressions is no
small task. It is further complicated by the fact
that genetic, and other factors, can influence how
we each respond to and metabolize a drug.
Biomarkers are one of the pathways by which the
complexity problem is resolved. At the highest level, a
“biomarker” is a measurable substance in an organism
whose presence is indicative of some phenomenon
such as disease, infection, or environmental exposure.
When a biomarker is discovered related to a disease,
it provides a target for further research, a potential
measure to be used in achieving diagnosis, and, if
found to be “druggable,” a target for a therapeutic.
In 1998, the National Institutes of Health Biomarkers
Definitions Working Group defined a biomarker
as “a characteristic that is objectively measured
and evaluated as an indicator of normal biological
processes, pathogenic processes, or pharmacologic
responses to a therapeutic intervention.”
13
Genetics
and genomics advancements, including technolog-
ical advancements such as genome sequencing,
have provided an important modern pathway for
identifying genetic biomarkers, including diagnostic
and therapeutic targets. As extensive collections of
whole-genome or partial genome sequences build,
scientists can mine these sequences to identify
Strimbu, Kyle, and Jorge A Tavel. “What are Biomarkers?” Current Opinion in HIV and AIDS vol. , (): -. doi:./
COH.beed
National Human Genome Research Institute. “The Cost of Sequencing a Human Genome.” www.genome.gov/about-genomics/fact-sheets/
Sequencing-Human-Genome-cost. Accessed May .
Whole Genome Sequencing (WGS) is a term used to describe sequencing that at present does not provide full coverage of the entire
genome, where repeat sections in the genome still remain a challenge to resolve. As defined by the National Cancer Institute, whole genome
sequencing is a laboratory process that is used to determine nearly all of the approximately billion nucleotides of an individual’s complete
DNA sequence, including non-coding sequence.
Nebula Genomics. “Did You Know that most DNA Tests Decode Only .% of Your DNA?” www.nebula.org/whole-genome-sequencing-dna-
test/. Accessed May .
National Human Genome Research Institute. “DNA Sequencing Costs: Data.” www.genome.gov/about-genomics/fact-sheets/DNA-
Sequencing-Costs-Data. Accessed April .
genes and gene variants that stand out differently in
people with a disease or condition of interest. These
identified genes and variants can then be studied
to identify their gene products and the biochem-
istry involved in their regulation and expression.
E. Human Genetics and
Genomics—Applications
and Impacts
The applications and impacts of genetics and ge-
nomics in human biology and medicine have grown
in parallel with advancements in gene sequencing
technologies and digital analytics platforms for de-
riving meaning within the resulting large sequence
datasets. The Human Genome Project cost approx-
imately $2.7 billion resulting in publishing of the
reference genome.
14
The Human Genome Project
and subsequent genomics initiatives sparked the
advancement of commercial sequencing instruments
and processes that have dramatically increased the
speed of sequencing and decreased the price of
each sequence. In March 2021, Nebula Genetics was
providing whole genome sequencing (WGS)
15
with
30x resolution (meaning that each position is read
30 times to enhance accuracy) for less than $300, a
nearly 10,000 fold decrease in price versus the first
sequenced human genomes.
16
NHGRI tracks costs
associated with DNA sequencing performed at the
sequencing centers funded by the Institute, and the
most recent NHGRI data (August 2020) place the cost
per genome at $689.
17
The pace of advancement in
gene sequencing has exceeded even the much-vaunt-
ed pace of Moore’s Law in computer processors.

6
The current speed and price of sequencing a hu-
man’s genome has led to the sequencing of patients
increasingly becoming a clinical reality in modern
healthcare systems. Barriers are less a factor of se-
quencing cost, and instead relate more to building
the capacity needed to analyze the huge volume
of data generated, to interpret the meaning of that
genetic code for the individual patient, and counsel
the patient as to implications for their health.
A developmental tipping point has been achieved in
which the utility of genomics and wide-spread use of
sequencing is clearly advantageous for significantly
enhancing human health outcomes. As discussed
below, and in further detail within Chapter III, the
functional application of human genetics and ge-
nomics to clinical healthcare is now a daily reality
in some medical fields (e.g., cancer diagnosis and
treatment) and is increasingly front-and-center in
neurological, psychiatric, gastrointestinal, immunolog-
ic, rheumatologic, dermatologic, pain management,
and other application areas of clinical medicine. It is
Note: the average cost of an MRI in the U.S. is $,. Source: Vanvuren, Christina. “What Can Affect the Cost of an MRI?” New Choice Health,
Inc., www.newchoicehealth.com/mri/cost. Accessed May .
also fundamental to advancements being made in
the diagnosis and treatment of a wide range of rare
diseases and disorders—helping to end the diagnostic
odysseys of millions of patients afflicted with rare
diseases that have been difficult to diagnose and
sparsely served in terms of available treatments.
TEConomy has divided this study of the impacts of
human genetics and genomics into two macro classes
of impacts: economic impacts (examining the impact
of human genetics and genomics on the U.S. econo-
my) and the functional (application) impact domains
in which genetics and genomics are affecting human
health and the clinical practice of medicine.
1. Economic Impacts
The performance of research, the development and
manufacturing of commercial genomics technology
platforms, the multitude of diagnostics products
and therapeutics on the market that is derived from
genomics knowledge, and the associated healthcare
services provided generate economic activity and
support a large volume of jobs across the nation—
these are economic impacts, that positively ripple
through the U.S. economy. Other economic impacts
are associated with lives saved, lives improved, and
impacts on people who would otherwise have to be
caregivers to loved ones. It is also the case that the
application of genetics and genomics to individual
healthcare needs costs money, and there is con-
siderable complexity in assessing the comparative
costs and benefits of one therapy versus another, or
whole-genome sequencing (and what it may eluci-
date) versus other diagnostic tools and technologies.
Because genetics and genomics are such a rapidly
advancing field of application and technology in
healthcare, cost/benefit equations are continually
changing. No health insurer was going to pay $3 billion
to sequence a whole genome for a patient, but $689
(less than the average cost of a single MRI)
18
to gener-
ate a dataset with lifelong, and increasingly expanding
utility for the patient opens up a whole new ballgame.
Genomics in the
Mainstream of Human
Biological Research
The movement of genetics and genomics from a
niche in biomedical science into the mainstream
underpinning research across most biomedical
disciplines is evident in the expansion of genetics
and genomics content across the research sphere
funded by the National Institutes of Health (NIH).
In 1990, greater than 95% of human genomics
research funding flowed, in a concentrated way,
through the National Human Genome Research
Institute (NHGRI) at NIH. By 2020, the vast majority
of human genomics research funding is provided
through twenty other individual NIH institutes,
indicative of the relevance of genomics across al-
most every domain of medical science and human
life science research.
7
2. Functional Applications
The functional impacts of human genetics and ge-
nomics are the impacts resulting from discoveries
via the advancement of research and the clinical
applications of genomics to benefit human health.
The decoding of the human genome was a signature
inflection point for science and has been widely
acknowledged as a towering achievement for life sci-
ences. What it sparked, however, has been an ongoing
expanding universe of advancement in genetics and
genomics and incredibly wide-ranging elucidation
of biological processes, systems, and outcomes with
genetic connectivity that is now fundamental to
advancing human health and clinical medicine.
In reviewing applications of human genetics and ge-
nomics, functional impacts are generated within eight
major domains of activities impacting human health.
These are illustrated in Figure 1, and this structure
Tripp, Simon, and Martin Grueber. Economic Impact of the Human Genome Project. Battelle Memorial Institute, May .
forms the foundation for discussion of functional im-
pacts of human genetics and genomics contained in
Chapter III. The study also briefly describes non-med-
ical applications of human genetics and genomics.
F. Purpose of the Study
This study seeks to provide an accessible reporting of
the positive impacts for the economy, society, and in-
dividual health that are derived from modern human
genetics and genomics science and the associated
commercial and clinical application of advancements.
In 2011, the authors of this report (while at Battelle
Memorial Institute) conducted a detailed impact
analysis of the Human Genome Project. The result-
ing Battelle report
19
highlighted the growth of an
emerging industry in the application of genomics
that was born from the scientific and technological
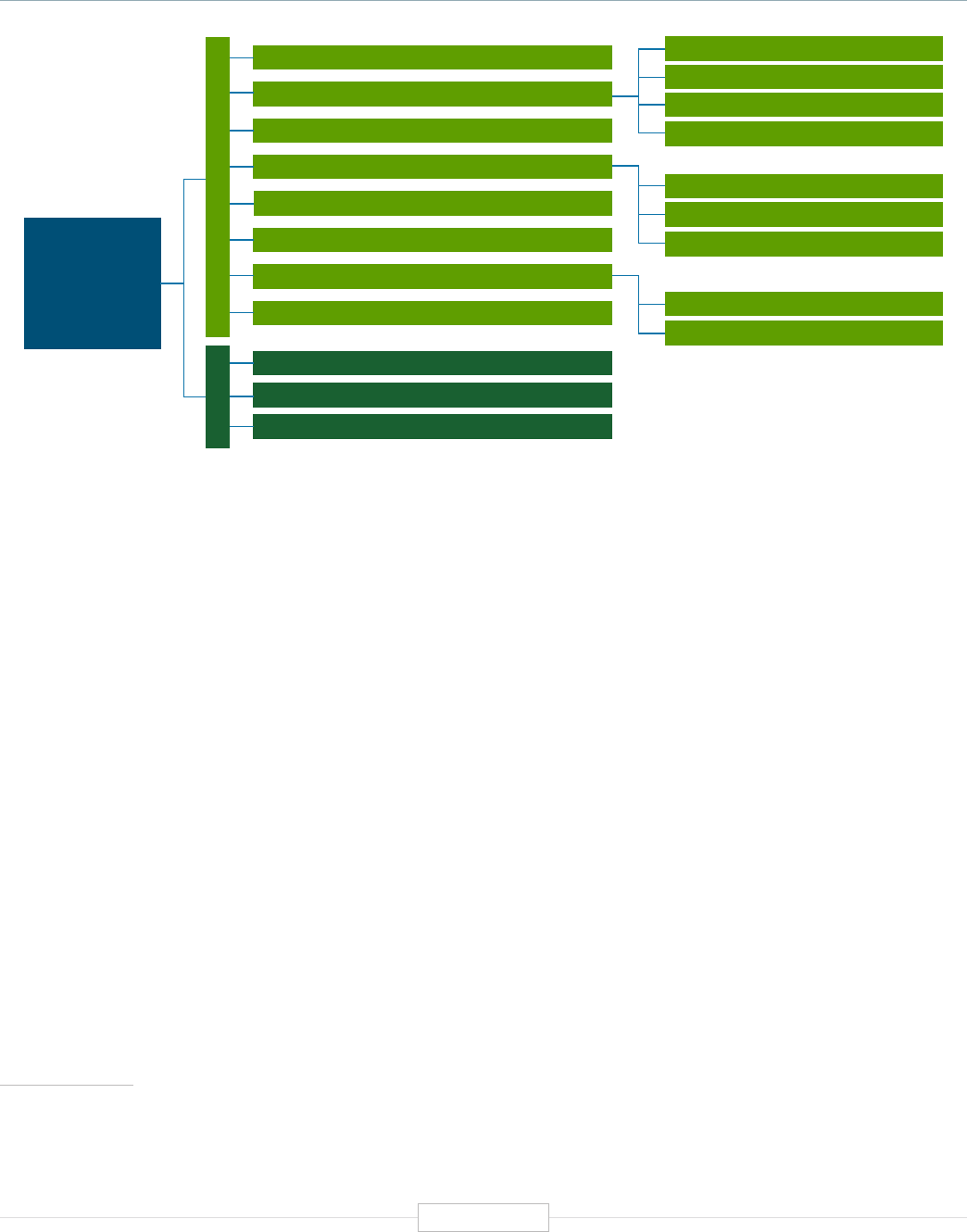
Figure 1: Current Functional Impact Domains (Applications)
of Human Genetics and Genomics
Minable Big Data (Discovery Science)
Predisposition to Diseases and Disorders
Medical
Diagnosing Diseases and Disorders
Rational Drug Development
Pharmacogenomics (Personalized Medicine)
Gene Editing and Gene Therapy
Human-Microbe Interaction
Environmental Genomics, Metagenomics, & OneHealth
Carrier Screening
Pre-Natal Screening
Newborn Screening
Child and Adult Testing
Single Gene (Mendelian) Diseases & Disorders
Resolving Mystery Diseases
Polygenic Diseases & Disorders
Targeting to Increase Effectiveness
Reducing Adverse Events
FUNCTIONAL
IMPACT DOMAINS
APPLICATIONS OF
HUMAN GENETICS
AND GENOMICS
Forensic Science
Evolutionary Biology and Anthropology
Paternity Testing
Non-Medical
Source: TEConomy Partners, LLC.

8
momentum driven by the Human Genome Project
and Celera’s work to sequence a reference human
genome. It also highlighted some of the early appli-
cations occurring through genetics and genomics
advancements in healthcare and other life science-re-
lated challenges and needs. The 2011 report played a
role in highlighting not only the important scientific
impacts of federal government investment in “big
science” and genomics in particular, but also demon-
strated the robust return on public investment that
had occurred through economic growth in genom-
ics technologies and emerging applied genomics
application domains. The report continues to be
linked on the website of NHGRI at genome.gov.
20
Now, 20 years after the publication of the draft se-
quence, there has been a significant and large-scale
expansion of the human genetics and genomics
universe. Technologies for sequencing and for genome
analysis have advanced significantly—to the extent
that whole-genome sequencing is quite affordable
(certainly in comparison to many common medical
procedures and tests) and an entire genome may be
sequenced in less than one day.
21
Advanced analytics
and artificial intelligence systems are now available
that can simplify deriving actionable insights from
Ibid.
Genomics England. “Sequencing a Genome.” www.genomicsengland.co.uk/understanding-genomics/genome-sequencing/#:~:text=One%
human%genome%can%be,pieces %C%around%%letters%long. Accessed May .
the sequencing data. Research discoveries in hu-
man genetics and genomics have compounded,
building upon one another in a virtuous network of
expanding information, knowledge, and application.
Today, this expansion has brought human genetics
and genomics to a rather visible inflection point.
The speed and affordability of gene sequencing,
in combination with deep insights into genomics
and -omic sciences more broadly, together with
advancing biopharmaceutical, diagnostics, and
other medical technologies that can leverage ge-
nomic information, have now made genomics a
part of the clinical practice of medicine across many
medical specializations and medical conditions.
This study seeks to provide a generalized overview
of human genetics and genomics achievements
and scan the current status of human genetics and
genomics in answering human health questions
and advancing clinical applications. The study also
seeks to highlight the economic contribution of the
expanding genetics and genomics sector. The U.S.
has been a pioneer in genomic sciences, leveraging
both public and private sector investment to build
an advanced industry that provides economic ex-
pansion and opportunities for further growth while

9
at the same time advancing human health and
well-being. This report characterizes those positive
impacts using quantitative analytics in conjunction
with a qualitative description of identified func-
tional impact domains and associated benefits.
It is anticipated that this report will be useful to public
policymakers and those seeking an understanding of
the public and private, and monetary and non-mon-
etary, returns to investments in science broadly,
and specific to genomics. It may also be useful for
those seeking to gain a broad introduction into the
multi-faceted ways genetics and genomics are being
used to improve human health and clinical health
outcomes—helping to illustrate the power of a rapidly
expanding biomedical field that is poised to advance
and transform many avenues of clinical medicine. It is
also hoped that the reader will be encouraged by the
promise of genomic medicine and the evident hope it
provides for improved health outcomes for humanity.
G. Limitations of the Study
The economic analysis deployed in this study provides
a one-year, point-in-time quantification of the nation’s
genetics and genomics sector. One of the challenges
in measuring genetics and genomics impacts in the
economy is that the U.S. industry classification sys-
tem does not contain a NAICS
22
code that covers the
industry specifically. Instead, it is a partial component
of many different industry sectors. Without having
access to data through NAICS codes, establishing a
baseline measure of the genetics and genomics indus-
try in aggregate within the U.S. economy requires the
development of a custom dataset (comprising data
on individual establishments and companies engaged
in human genetics and genomics research, technol-
ogy development, and application) that effectively
builds the data from the ground-up, establishment
by establishment (rather than relying on generally
available government summary sectoral statistics).
One of the principal challenges in developing estab-
lishment-level data in genetics and genomics is that
NAICS is an abbreviation for the North American Industrial Classification System.
while for some enterprises or organizations genetics
and genomics comprises the preponderance of their
business or institutional work (for example, gene
sequencer manufacturers, genetic testing companies,
genetic counselors), for many active in the sector, it is
only part of their business or work (for example drug
companies, large national diagnostic laboratories,
clinical care providers, etc.). Informed estimations have
to be made of the portion of revenues, expenditures,
and employment at organizations that are related
to the application of genetics and genomics. The
fact that estimates must be used this way to build
the overall dataset that drives the direct effect in the
input-output models used is a limitation (discussed
further in Chapter II). The study has endeavored to
err on the side of being conservative in developing
portioning estimates, and thus the resulting mea-
sures of impact are likely low rather than high.
The examination of functional impacts is, in many
respects, an even greater challenge. Part of this is evi-
dent in the very large volume of academic and indus-
try life science research studies in which genetics and
genomics are a component or focus. As a fast-moving
field, there is ongoing evolution and expansion in
the applications of genomics in human healthcare,
and it is a significant challenge to do justice to such a
wide-ranging field. With thousands of diseases, many
hundreds of drugs, and a broad compendium of diag-
nostics tests having genetic associations, providing full
coverage of every application of genomics in health-
care would be an extremely challenging task and out-
of-date immediately upon completion, not to mention
a rather daunting read. This is not attempted in this
study, but rather the functional impact assessment
herein works to classify human genetics and genomics
advancements by broad application domain (disease
diagnostics, pharmacogenomics, gene therapy, etc.)
and then uses specific narrative examples of genetics
and genomics in action within these domains (to-
gether with some measures indicative of scale where
readily available). As such, the functional impact
section of this report should be viewed as providing

10
an overview of the broad areas in which genetics
and genomics are providing benefits to human
health, not a formal quantification of these impacts.
This report is also limited in that it focuses on human
genetics and genomics only, and this certainly under-
counts the wide economic and societal benefits that
accrue to advancements in genetics and genomics
more broadly. While the application of genomics to
medicine is certainly an important area of use, the
ubiquity of DNA as the basis for all life on Earth means
that genomics finds application in many more fields of
science and commerce. Both the science of genomics
and the tools and technologies of genomics find ap-
plication in multiple additional endeavors, including:
• Veterinary medicine
• Agriculture (in applications such as
crop improvement, crop protection,
animal science, and nutrition)
• Industrial biotechnology (in applications
using microbes with engineered genomes
to produce biochemical products), and
• Environmental and ecological services (in
applications using engineered microbes
in industrial waste cleanup, wastewater
treatment, and other applications).
These additional areas of genetics and ge-
nomics application have significant impacts
that are not addressed in this report.
This report is primarily intended to highlight the
current status of human genetics and genomics
impacts, but it does contain a chapter that briefly
discusses the frontiers and potential future advance-
ments that may occur in the foreseeable future. The
discussion in that chapter is, of course, speculative,
and thus subject to the usual limitations involved
when looking towards an uncertain future.
Readers should consider the benefits of genomics
from both the economic and functional perspectives,
not just one or the other. Examining the field through
the hard lens of economics must be tempered by the
fact that much that matters in life may not be readily
broken down into dollars and cents. The alleviation
of pain and discomfort from a medical condition, the
ending of a diagnostic odyssey of a patient with a
mystery disease, or the lifelong experiences of a child
and their parent made possible through that child
being effectively treated for a rare genetic condition,
are principally humanitarian benefits. The other side
of the coin is that novel genetic tests, customized
medicines, and highly specialized care can come at
a significant cost to individuals and those who pay
for healthcare, and it is important to understand the
economic implications of emerging clinical frontiers.
In this regard, we caution that because medical
genomics is an emerging field, there is relatively
sparse literature on the monetary impacts and costs/
benefits associated with genomic medicine. It is
anticipated that genomic medicine may increase
costs in many of its early applications, but these early
applications are part of a path that will lead to overall
cost savings as medicines are targeted and used
more effectively, chronic diseases better managed
(or perhaps cured), adverse reactions to medications
curbed, and the costs of lifelong care potentially
avoided by addressing genetic components in
diseases that may be attended to through gene
therapies and effective personalized therapeutics.
Chapter II provides an assessment of the eco-
nomic impact of sectors engaged in human
genetics and genomics R&D, the provision of
genetics and genomics tools, technologies, and
services, and associated economic activity.
Chapter III provides discussion of the multifaceted
functional impacts allocable to the key application do-
mains of human genetics and genomics in healthcare.
This follows the structure shown previously in Figure 1,
and also briefly touches upon some of the non-med-
ical applications of human genetics and genomics.
Chapter IV looks to the future of human ge-
netics and genomics and introduces some of
the factors that need to be addressed to in-
crease the positive impacts of the sector.
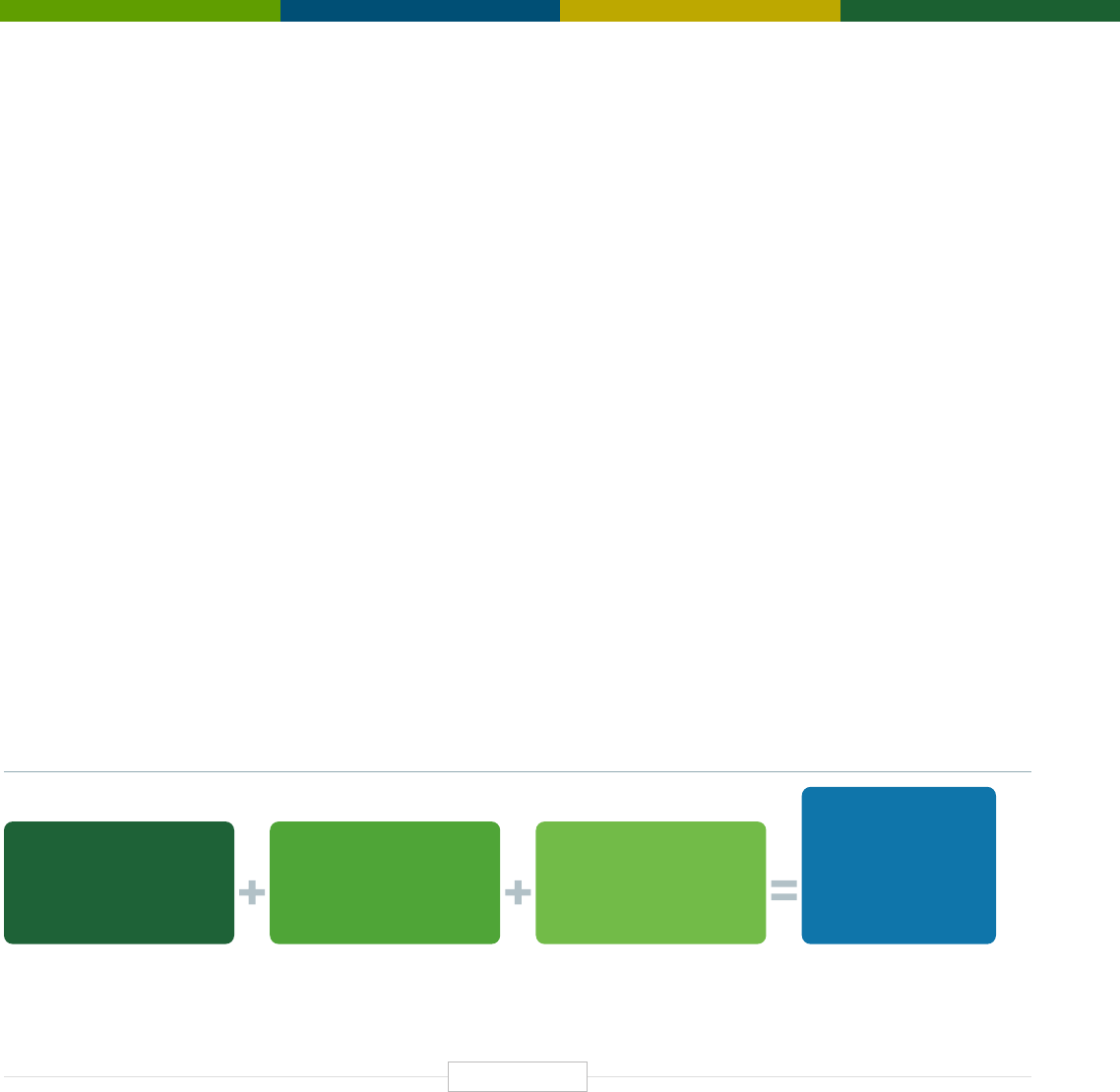
11
A. Measuring Human Genetics and
Genomics Economic Impacts
As described in the previous chapter, the develop-
ment of the economic (expenditure) impacts within
this study is focused on and limited to estimating
those impacts stemming from the use of genetics
and genomics for human biomedical purposes.
1. Basics of Impact Modeling
The estimation of economic impact makes use of an
input-output (I-O) model to represent the interrela-
tionships among economic actors and sectors. Within
these models, the flow of commodities (or the value
they represent) between industries, consumers, and
institutions in an economy are modeled through
a matrix representation allowing for the impact of
changes in employment, expenditures, or economic
output in one sector of the economy to be projected
onto other sectors of the economy. These transac-
tions continue under the premise that every dollar
spent in the economy (the direct effect) is re-spent
on the purchase of additional inputs, goods, or ser-
vices generating additional economic activity and
impacts. I-O analysis is the generally accepted “gold
standard” in economic impact measurement and
examines the relationships among economic sectors
(e.g., institutions or industries) and final consumers.
For the purposes of this study, the I-O analysis
models the “ripple effect” that originates from the
expenditures made for human genetics and genom-
ics research and direct company operations in the
economy, flows through suppliers and vendors as
additional inputs are purchased, and through employ-
ees, faculty, staff, and related supplier workers who
spend their wages in the U.S. economy (Figure 2).
For modeling and estimating expenditure impacts,
TEConomy used a 2019 professional IMPLAN I-O
economic impact model of the U.S. Originally de-
veloped in 1976 by the U.S. Forest Service, IMPLAN
II. The Economic Impact of
Human Genetics and Genomics
Figure 2: Measuring Economic Impacts of Human Genetics and Genomics
Human Genetics &
Genomics Focused
Research Expenditures,
Services, and Corporate
Operations in the U.S.
Purchase of Secondary
Inputs & Services from U.S.
Suppliers and Vendors
Human Genetics &
Genomic Supported
Employees Spending
Disposable Income
in the U.S. Economy
Total Economic
Impacts of
Human Genetics
& Genomics
DIRECT EFFECT INDIRECT EFFECT INDUCED EFFECT
Source: TEConomy Partners, LLC.

12
is now the most widely used economic impact
modeling data and analysis tool in the nation.
23
IMPLAN models are built upon underlying federal
information including the U.S. Bureau of Economic
Analysis (BEA) national accounts data, supplement-
ed with state level employment data from the U.S.
Bureau of Labor Statistics (BLS) and other economic
data from the U.S. Bureau of the Census. Currently, the
IMPLAN model reflects and represents 546 sectors of
the U.S. economy. The core data and structures devel-
oped within an IMPLAN model can be used to analyze
the economic impacts of institutions, projects, or
entire industries. Employment and expenditure data
developed and estimated as part of this study provide
the direct impacts to drive the overall economic im-
pact models and estimations. Ultimately, the impact
model generates estimates of the additional indirect
Note, the authors followed the same I-O methodology by using a prior year’s IMPLAN model to estimate the economic impacts of the Human
Genome Project.
and induced impacts (also known as the multiplier
effects) for employment, personal income, value add-
ed, output, and federal and state/local tax revenues.
2. Prior Human Genome Project
Economic Impact Results
For context both in terms of size and breadth,
values from the authors’ prior work on the
Economic Impact of the Human Genome Project
are provided in Table 2. These values reflect the
full breadth of genetic and genomic research and
the nascent involvement of industry as of 2010.
By comparison, the current study, to be described
and discussed in the following pages, focuses
solely on the human genetics and genomics
domain for a period one decade later, 2019.
Table 1: Impact Measures Included in the Analysis
Impact Measure Definition
Output
Also known as production, sales, or business volume, is the total value of goods
and services produced in the economy. For public/non-profit entities, such as
universities, expenditures are often the truest measure of this economic activity.
Employment
The total number of jobs created; Includes the direct jobs paid for through salary
and benefit expenditures and indirect/induced jobs generated through purchase
expenditures.
Labor Income
Also known as total compensation, is the total amount of income, including
salaries, wages and benefits, received by employees, proprietors, and other
supplier workers in the economy;
Value Added
The difference between an industry’s total output and the cost of its intermediate
inputs; sometimes referred to as the industry’s “Contribution to GDP”.
Federal and State/Local
Government Tax
Revenues
Includes the estimated revenues to federal and state/local governments from all
sources as a result of the impacts estimated.

13
B. Drivers of Economic Impacts:
Methodology and Assumptions
Three types of economic inputs or “drivers” are used
to develop and estimate the impacts of human
genetics and genomics—research expenditures,
core industry employment, and extended industry
sales or employment. For this analysis, 2019 data
was captured to the extent practicable using the
fiscal year or calendar year. The following specifies
the conservative methodology and assumptions
used in developing these economic impact drivers.
1. Research Drivers—
Investments in Research
A significant driver of human genetics and genomics
economic impacts comes in the form of focused
research funding from federal agencies and other
non-profit organizations. This section describes and
captures this funding mechanism for use in driving
the economic impact model. It captures the value
of human genetics and genomics research funded
by these organizations and ultimately performed
by universities, research institutes, federal agencies,
and other non-profit research organizations.
National Institutes of Health
With the focus of this study specifically on human ge-
netics and genomics impacts, the National Institutes
For the purposes of this study “NIH research funding” was limited to research-oriented grants or contracts (external and intramural) to U.S.
researchers. Grants to non-U.S. research performers, U.S. firms (via SBIR/STTR awards), or construction and training awards were excluded from
the analysis. For FY , this NIH total research funding reached $. billion. Note: grants to U.S. firms were excluded as individual firms
are typically captured in the core industry drivers analysis.
of Health (NIH) becomes the principal governmental
agency funding research in this domain. However,
even within NIH, the role of human genetics and
genomics in its research portfolio is subject to inter-
pretation and perspective—ranging from fundamental
research into human genetics and genomics to the
use of genetics and genomics as an enabling “tool” in
research focused on specific diseases or conditions.
To account for this, TEConomy established three
funding scenarios for NIH, each differentiated by
size and breadth of funding, developed through an
analytical approach that eliminates double count-
ing of funded research efforts and builds upon the
previous scenario. The structure of these scenarios
and the research funding that is captured within
each scenario is built using internal NIH classifica-
tions and keywords.
24
These scenarios include:
• Core NIH Human Genetics and
Genomics Funding
• Includes all research funding from
NHGRI (524 projects, $416.3 million)
• Research reviewed and approved by spe-
cific genetic and genomic-related proposal
review study sections (e.g., Genetics of
Health and Disease Study Section and
others) (1,434 projects, $611.1 million)
Table 2. Core Metrics from Economic Impact of HGP Study
Impact Employment (Jobs) Output ($M)
Direct Effect 51,655 $22,627.5
Total Impact 310,360 $67,146.0
Source: Economic Impact of the Human Genome Project, Battelle Memorial Institute, May .
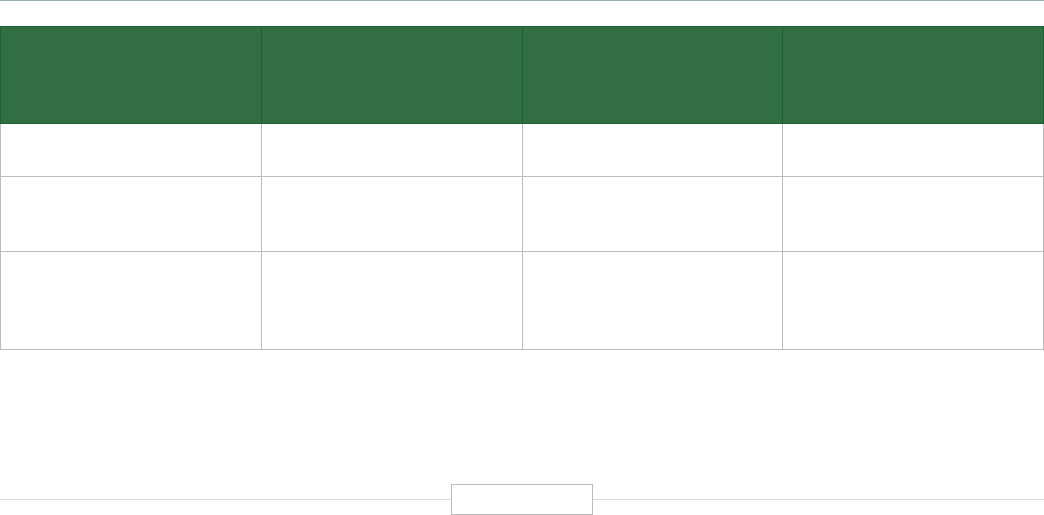
14
• Research classified in specific and relat-
ed NIH Research, Condition, and Disease
Categories (RCDC) EXCEPT the broadest
Genetics category (e.g., Cancer Genomics,
Gene Therapy, Gene Therapy Clinical Trials,
Genetic Testing, and Human Genome)
(5,424 projects, $2,107.3 million)
• Core + Additional Expanded NIH Funding
• Additional research within the specific
Genetics RCDC funding category, not cap-
tured above (10,493 projects, $3.884 billion)
• Core + Additional Expanded +
Additional Use as Tool Funding
• Additional research listing genetics or ge-
nomics as a Principal Investigator-provided
keyword in the funding information not cap-
tured above. A review of these awards showed
that most were non-genetics and genomics
focused yet were using genetics or genomics
as a key analytical approach or tool enabling
the research. (13,448 projects; $7.184 billion)
Table 3 shows the value and incremental additions
to each scenario and the share of total NIH research
funding.
A striking finding from this assessment of NIH
research funding is the pervasive nature of
genetics and genomics in human biomedical
research. Nearly half of all NIH research fund-
ing specifies some connections with human
genetics and genomics, at least as an inves-
tigative tool to support other research.
To maintain a conservative perspective on the role
NIH research funding plays in the overall econom-
ic impact of human genetics and genomics, the
remainder of this chapter focuses on the impacts
generated with the inclusion of the smallest, core
funding scenario. Economic impact results estimated
using the additional more expansive NIH research
funding scenarios are included in the Appendix.
Other Federal Agencies
Beyond NIH, other federal agencies pro-
vide significant human genetics and ge-
nomics-related research funding.
Department of Veterans Affairs
In FY 2019, the Department of Veterans Affairs (VA)
Office of Research and Development invested a total
of $107.0 million in genomics research and infra-
structure. Of this, $26.0 million was towards funding
genomics research projects, $44.0 million towards the
Table 3: NIH Research Funding Captured by Each Scenario
NIH Human Genetics
and Genomics Funding
Scenario
2019 NIH Research
Investment
Component ($B)
2019 NIH Research
Scenario Value ($B)
Scenario Cumulative
Share of 2019 NIH
Research Funding
Core Funding $3.135 $3.135 10.8%
Core + Additional
Expanded Funding
$3.884 $7.018 24.2%
Core + Additional
Expanded + Additional
Use as Tool Funding
$7.184 $14.202 49.1%
Source: TEConomy analysis of NIH research awards using the RePORT website’s ExPORTER Project file for FY

15
Million Veteran Program (MVP) core infrastructure
for recruitment, enrollment as well as clinical and
genomic data curation, and $37.0 million towards
genotyping and whole genome sequencing DNA
samples from the MVP. With over 830,000 Veterans
enrolled to date, MVP is one of the largest healthcare
system-based genomic cohorts in the world.
25
National Science Foundation
The National Science Foundation (NSF) funds substan-
tial research efforts within the broad context of genet-
ics and genomics through research programs in ge-
netic mechanisms, phylogenetic systematics, and the
large-scale Plant Genome Research Project. For the
purposes of this study, NSF awards that were active at
any time within FY 2019 were considered and exam-
ined to find those that met the requirement of fund-
ing research primarily aimed at human genetics and
genomics understanding. Included in these awards
are significant research funding for big data and/or
bioinformatics research that was primarily aimed at fa-
cilitating or enhancing the ability to manage and ana-
lyze human genetics and genomics data. The included
research reflects 212 awards funded at $19.3 million.
26
For additional information on the Million Veteran Program see: www.mvp.va.gov.
Multi-year awards that extended into FY were weighted by the number of FY days
as a share of the total project’s estimated duration.
Other Health and Human Services (HHS)
Undoubtedly, other HHS agencies beyond NIH in-
clude some level of human genetics and genomics
research and/or research funding. However, given
the limited detailed information upon which to
assess the connections to human genetics and
genomics, only within the National Institute for
Occupational Safety and Health (NIOSH) was this
information as well as funding information available.
For FY 2019, TEConomy identified nearly $384,000
in human genetics and genomics-related funding.
Voluntary Health Associations and
Other Non-Profit Funding
An important funding stream for human genetics
and genomics research is provided by a wide variety
of voluntary health associations, patient groups,
and other non-profit funders. Likely included with-
in this set of funders are many of the members of
National Organization for Rare Disorders that are
funding research efforts to understand the genet-
ic and genomic traits of these rare disorders.
The difficulty in including funding from these asso-
ciations and non-profits is the limited information
on human genetics and genomics research within
16
their specific research grants and the requirement
to gather this information, if it exists, from liter-
ally hundreds of organizations across the U.S.
To reflect at least some level of funding from these
groups, TEConomy worked to develop a conservative
estimate. Building off of prior work, TEConomy first
estimated a 2019 value for total research funding from
voluntary health associations.
27
An assumption is then
made that, at a minimum, these organizations fund
human genetics and genomics research at approxi-
mately the same “share” that NIH does. To stay most
conservative, the “core” percentage of NIH funding,
10.8% (see Table 3), is applied to the estimated FY 2019
voluntary health association total funding level of $1.56
billion to generate a human genetics and genomics
research funding estimate of $169.1 million in 2019.
The actual level of funding from these organizations
is likely to be considerably larger. Anecdotally and via
website information, it appears the cutting-edge re-
search funded by these groups is becoming more and
more engaged with genetic and genomic exploration.
Research Funding Summary
Combined, human genetics and genomics research
funding as captured from funding organizations
reaches $3.4 billion under the most conservative
estimate of NIH funding. Using the IMPLAN model
this level of research funding is estimated to directly
employ 13,800 researchers throughout the U.S.
2. Core Industry Drivers—Employment of
Core Human Genetics and Genomics Firms
The previous section captures the level of human
genetics and genomics research activity stem-
ming from federal and other sources of funding.
This section develops an employment-based
estimate of the size and scope of the core firms
operating primarily, if not exclusively, within
the human genetics and genomics domain.
U.S. Investments in Medical and Health Research and Development – . Research!America, Fall .
Developing true employment values from these sources can be difficult due to reasons such as outdated data, self-reported data, and the
effects of M&A activity. Conservative employment estimates were made, if required.
Following a similar approach to the authors’ prior
work analyzing the genetics and genomics industry,
a database of firms with their total employment was
developed. The database was initiated by starting with
the previous work’s 2010 database of firms, exclud-
ing those that were primarily in the plant/animal/
agricultural domain and determining whether these
firms were still in business in 2019, and correcting
for considerable mergers and acquisition activity
that has occurred within the industry over the past
decade. For the purposes of this study, firms that are
part of the important instrumentation (e.g., Illumina,
ThermoFisher) or bioinformatics subsectors are
included in this database even though their prod-
ucts and services may also be used outside of the
specific human genetics and genomics domain.
To supplement this existing firm database, lists of
firms from a variety of organizations, websites, and
market research publications were curated to gen-
erate an additional set of U.S. firms for review and
inclusion. These firms were then evaluated using
web-based research to determine their fit within the
human genetics and genomics domain and whether
they were still in business. If they met these criteria, an
employment value was developed using third-party
databases such as Dun and Bradstreet and PitchBook
(a provider of angel and venture capital information)
and, at times, the firm’s website or LinkedIn pages.
28
The impacts are modeled as an aggregation of
IMPLAN sectors, as appropriate, to capture the extent
and variety of research and corporate activities by
using employment to drive the direct impacts of
the economic impact model. These firms and their
employment were classified into one of six human
genetics and genomics core industry subsectors
(Table 4). The employment figures reflect the total
employment of firms in each industry subsector.
Within this employment of more than 89,000, some
specific caveats and specifications are warranted. The
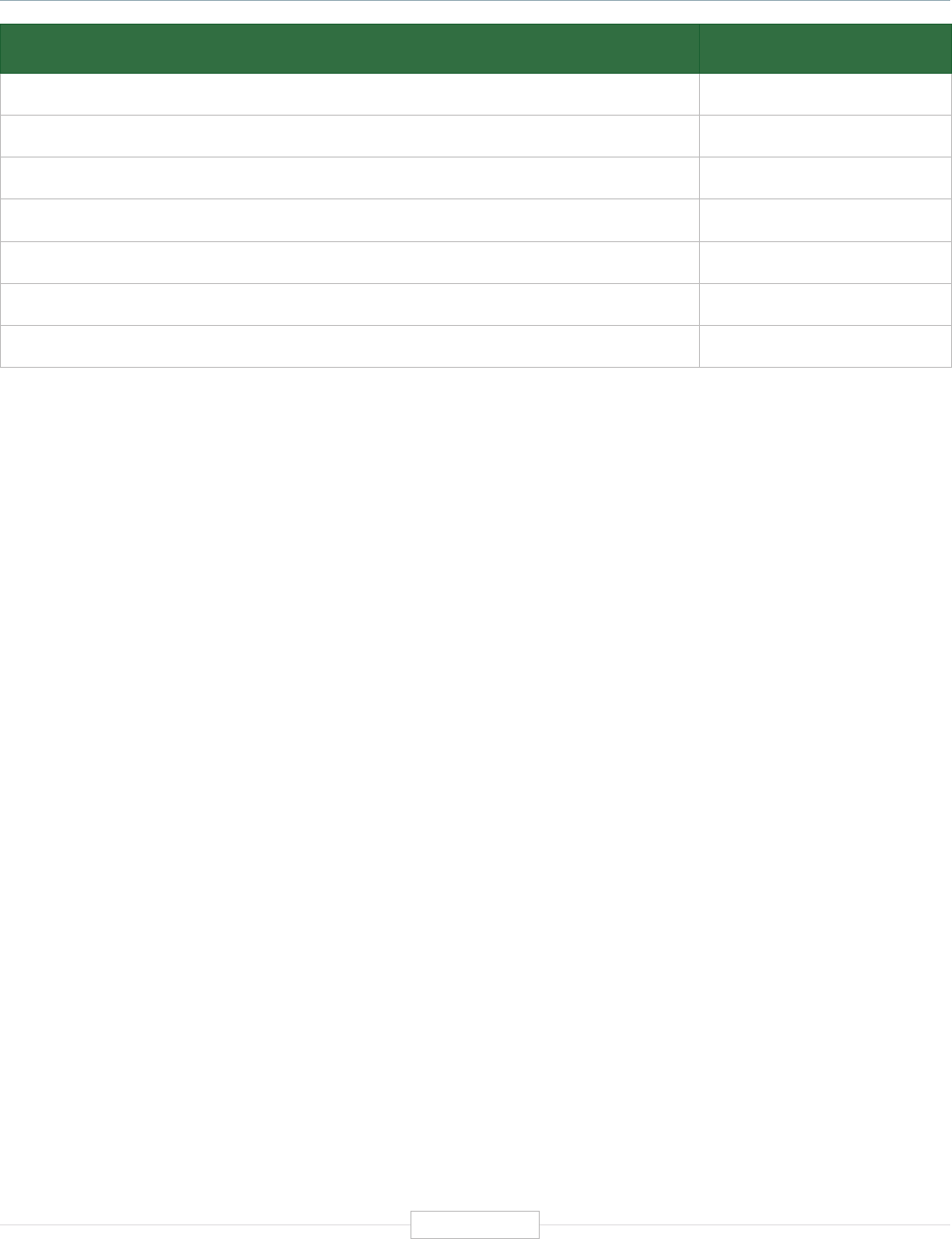
17
data only include small/mid-sized biopharmaceutical
focused firms’ employment—the impact of large bio-
pharmaceutical firms is captured via sales estimates
in the next section. Dun & Bradstreet and corporate
website information was used to determine whether
to include firms within the R&D/biotech firms’ catego-
ry versus small biopharma category. The core medical/
diagnostic laboratories category does not include
employment for Quest Diagnostics and Laboratory
Corporation of America (LabCorp); rather their role is
estimated in the next section. An assessment of the
list of genetic counselors available from the National
Society of Genetic Counselors was used to identify U.S.
firms. These firms were then classified into medical/
diagnostic laboratories or genetic counselor practices
as warranted as many medical/diagnostic laboratories
include genetic counselor employees. The specific
category for genetic counselor and other related
services was used to capture, as best as possible,
the employment of those firms operating without a
direct testing capability. It should be noted that the
final economic impact assessment also included
1,752 individual counselors, including those practicing
within the VA’s Million Veteran Program, that are not
captured within the employment figures in Table 4.
Core Industry Drivers Summary
Across six industry subsectors, the economic im-
pact model is driven by direct employment within
the core industry drivers of 89,464 employees.
3. Extended Industry Drivers
Two subcomponents of the industry analysis ef-
forts required different approaches to estimating
their size and importance in driving the human
genetics and genomics economic impacts.
Pharmaceutical Manufacturing
The core industry employment analysis captured
small and/or nascent biopharmaceutical firms whose
sole focus is the development of pharmacogenetic/
genomics drugs (drugs associated with a genetic test)
and other genetic and genomic therapies. However,
larger pharmaceutical manufacturers with develop-
ment efforts ranging from small molecule chemistries
to large molecule biologics also are a significant
component of human genetics and genomics-related
economic activities. For these firms, a different ap-
proach is used to apportion part of their operations
(sales) to the human genetics and genomics domain.
Table 4: Employment by Human Genetics and Genomics Core Industry Subsectors
Industry Subsector Est. of 2019 Employment
Core Analytical/Biomedical Instruments and Equipment Manufacturers 26,758
Core Small Biopharma Manufacturers 22,383
Core R&D/Biotech Firms 21,797
Core Medical/Diagnostic Laboratories 14,639
Core Software/IT/Bioinformatics Service Firms 3,729
Core Genetic Counselor and Other Related Services 158
Total Core Firms’ Employment 89,464
Sources: Lists of firms developed from various industry and professional websites. Employment estimates from corporate websites and Dun &
Bradstreet data. Additionally, LinkedIn information was used, at times, to update and correct some firms’ employment levels.

18
The original Economic Impact of the Human Genome
Project study also treated these large pharmaceutical
firms in a slightly different manner from core indus-
try employment. In the previous study, an estimate
of genetic and genomic research expenditures was
used to reflect these firms’ involvement in the human
genetics and genomics domain—one that was just
beginning to see the opportunities that genetics
and genomics would provide in the development of
modern medicines. In the decade since these esti-
mates were developed, the role of human genetics
and genomics in biopharmaceutical development
is still evolving. However, its use has extended its
impact on these large biopharmaceutical firms
beyond simply research and further into targeted drug
development and the labeling, use, and efficacy of
existing products ultimately driving corporate sales.
Lists of the U.S.-based members of the Pharmaceutical
Research and Manufacturers Association (PhRMA)
29
and pharmaceuticals by sales for 2019 were exam-
ined.
30
Using a sales cut-off value of $1.5 billion to
represent the most active biopharmaceutical products
generates a list of 102 medicines. Of these, 26 are
currently listed in the FDA Table of Pharmacogenomic
PhRMA. “Members.” www.phrma.org/en/About/Members. Accessed May .
PhRMALive and Outcomes LLC. “Top Medicines Annual Report: Climbing Mount Humira.”
U.S. Food & Drug Administration. “Table of Pharmacogenomic Biomarkers in Drug Labeling.”
www.fda.gov/media//download. December .
Spitzer, Dan. Brand Name Pharmaceutical Manufacturing in the U.S. IBISWorld Industry Report a, August .
Data obtained and calculated from corporate -K filings reflecting performance.
Curran, Jack. Diagnostic & Medical Laboratories in the U.S. IBISWorld Industry Report , November .
Biomarkers in Drug Labeling.
31
This subset of biomark-
er-labeled medicines account for 28% of global sales
of these 102 medicines. To generate a U.S. specific
estimate, this 28% was then applied to a market
study value of 2019 total U.S. prescription drug sales
of $177.7 billion yielding an estimated $49.5 billion in
U.S. prescription drug sales with one or more genetic
biomarkers.
32
This estimate is considered conservative
due to the higher usage rate of more expensive phar-
maceuticals within the U.S. Total global sales of these
26 medicines reached $96.5 billion, nearly twice the
value used to drive the U.S. economic impact estimate.
National Medical Testing Laboratories
Two medical/diagnostic laboratories, Laboratory
Corporation of America (LabCorp) and Quest
Diagnostics, together account for more than $16.5
billion in total U.S. sales and more than 90,000 U.S.
workers.
33
While human genetic and genomic testing
is becoming more common in U.S. healthcare, it is still
a smaller share of overall medical and diagnostic test-
ing. Using corporate websites and reporting as well
as third party market studies, TEConomy developed
estimates for these two firms’ human genetic and
genomic-related sales and employment.
34
Together,
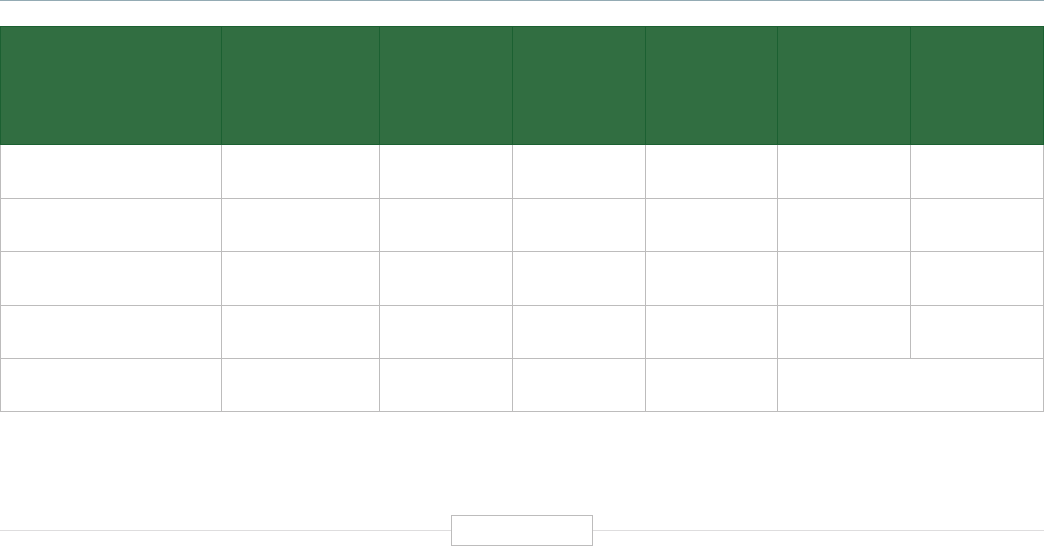
19
these two firms are estimated to account for just un-
der 22,000 workers and more than $3.77 billion in sales
within the human genetics and genomics domain.
Extended Industry Drivers Summary
The revenue and employment size of the firms cap-
tured within the Extended Industry Drivers warrant
special and distinct attention. For both subcompo-
nents, the use of human genetics and genomics
technologies and techniques constitutes a relatively
small share of overall operations. However, due to
the sheer size of these corporate operations, these
two subcomponents directly add more than $53.3
billion and nearly 61,000 jobs to the U.S. economy.
C. Economic Impact Analysis
of Human Genetics and
Genomics in the U.S.
The combination of the three economic impact drivers
presented above—Research Drivers, Core Industry
Drivers, and Extended Industry Drivers—yields a signif-
icant direct economic presence in the U.S. economy.
Using the most conservative NIH research funding
levels and using the IMPLAN model to estimate
employment from sales (or research funding levels)
values, it is estimated that the U.S. human genetics
and genomics research and industrial domain
employs nearly 166,000 workers as a direct result
of these operations (Table 5). This number includes
human genetics researchers, medical geneticists,
and genetic counselors, as well as a large number of
workers in adjacent, corporate, or operational roles
in firms developing lab equipment and software,
performing clinical genetics and genomics testing,
or manufacturing pharmacogenomic drugs. Overall,
this combined set of drivers directly generate over
$108 billion within the U.S. economy. With this
set of direct drivers, the IMPLAN model is used to
estimate the total impacts of human genetics and
genomics domain on the U.S. economy (Table 5).
From an employment perspective, these impacts
show the U.S. human genetics and genomics do-
main supporting an additional 684,000 jobs (indirect
and induced effects) within the U.S. economy for
a total employment impact of more than 850,000
workers, reflecting an employment multiplier of
5.12. For every direct job in the human genetics and
genomics domain, 4.12 additional jobs are generated
throughout the U.S. economy. On average, due to
the higher technical and educational requirements,
Table 5: Economic (Expenditure) Impacts —
Core NIH Human Genetics and Genomics Funding Scenario (2019)
Impact Type Employment
Labor
Income
($B)
Value
Added
($B)
Output
($B)
State/
Local Tax
Revenues
($B)
Federal
Tax Reve-
nues ($B)
Direct Effect 165,973 $21.66 $52.59 $108.16 $2.90 $5.18
Indirect Effect 288,866 $24.81 $43.59 $87.04 $2.90 $5.38
Induced Effect 395,425 $22.40 $39.44 $70.15 $3.67 $4.95
Total Impacts 850,263 $68.88 $135.62 $265.35 $9.47 $15.51
Multiplier 5.12 3.18 2.58 2.45
Source: TEConomy analysis of Human Genetics and Genomics Input Dataset; IMPLAN U.S. Impact Model.

20
the direct jobs generate more than $130,000 in
annual compensation (income and benefits) per
worker while overall, the total jobs supported by the
human genetics and genomics domain still aver-
age over $81,000 in compensation per employee.
In terms of value added, or the contribution to U.S.
GDP, the human genetics and genomics domain
directly adds more than $50 billion to U.S. GDP, and
through the economic ripple effects these efforts
support, in total, nearly $136 billion of U.S. GDP.
The direct output also leads to considerable additional
economic activity in the U.S. For every $1 of output
(e.g., sales or research expenditures), $1.45 of additional
sales are generated in the U.S. economy leading to
an overall economic impact of U.S. human genetics
and genomics of more than $265 billion in 2019.
The federal tax revenues of $5.18 billion generated
by the direct operations of the human genetics and
genomics domain alone surpasses the single year fed-
eral investment in human genetics and genomics of
approximately $3.26 billion across all federal agencies.
In the simplest of terms, from a federal investment
perspective, the overall economic impacts of U.S.
human genetics and genomics generates a return
on investment (ROI) of more than 4.75 to 1.00.
Progress Over the Past Decade
At the outset of this chapter, the 2010 impact
estimates for the Human Genome Project were
provided. By comparison, this current analy-
sis shows the dramatic increase in impact over
the past decade that human genetics and
genomics has had on the U.S. economy.
Even though this current effort is focused solely on
“human” genetics and genomics, the size of the
workforce directly employed in these endeavors has
more than tripled over the decade from just under
52,000 workers in 2010 to nearly 166,000 in 2019.
Similarly, direct output has dramatically surpassed the
previous estimate as human genetics and genomics
developments are leading to actual and significant
sales in 2019. Direct output was estimated to be $22.6
billion in 2010 compared to $108.2 billion in 2019.
The economic importance of U.S. human genet-
ics and genomics cannot be denied. The HGP
impact study found that the broader genetics
and genomics field, for which the human do-
main is just one component, had an economic
impact of $67 billion in 2010. The growth of the
human genetics and genomics field over the
past decade has been substantial, with this one
domain area now representing a total econom-
ic impact of more than $265 billion in 2019.
D. Healthcare Costs
for Genetic Diseases
The application of human genetics and genomics,
as discussed in the next chapter, is providing a wide
range of functional benefits in healthcare in terms
of identification of patient predisposition to genetic
disease, diagnosis of diseases, identification of targets
for new drugs, precision drug dosing and limitation
of adverse drug events, and development of new
approaches to treatments and cures through gene
therapy and gene editing. As will be shown, human
genetics and genomics are very much at an inflection
point where many benefits are now occurring as
research discoveries translate into clinical innovations.
Because the large-scale clinical application of
human genetics and genomics is a relatively new
phenomenon, there is relatively limited literature on
the economic impacts of its applications to clinical
care. Longitudinal studies tracking impacts over time
are particularly scarce and indeed are challenging
to interpret given that the cost of gene sequencing,
up to and including whole-genome sequencing,
has plummeted. Sequencing the genome was, just
a few years ago, prohibitively expensive. However,
advancements in technology mean that cost is
no longer a primary barrier to use in the clinic.
One way to evaluate the economic impacts of human
genetics and genomics is to examine the cost bur-
den of disease imposed on the economy by diseases
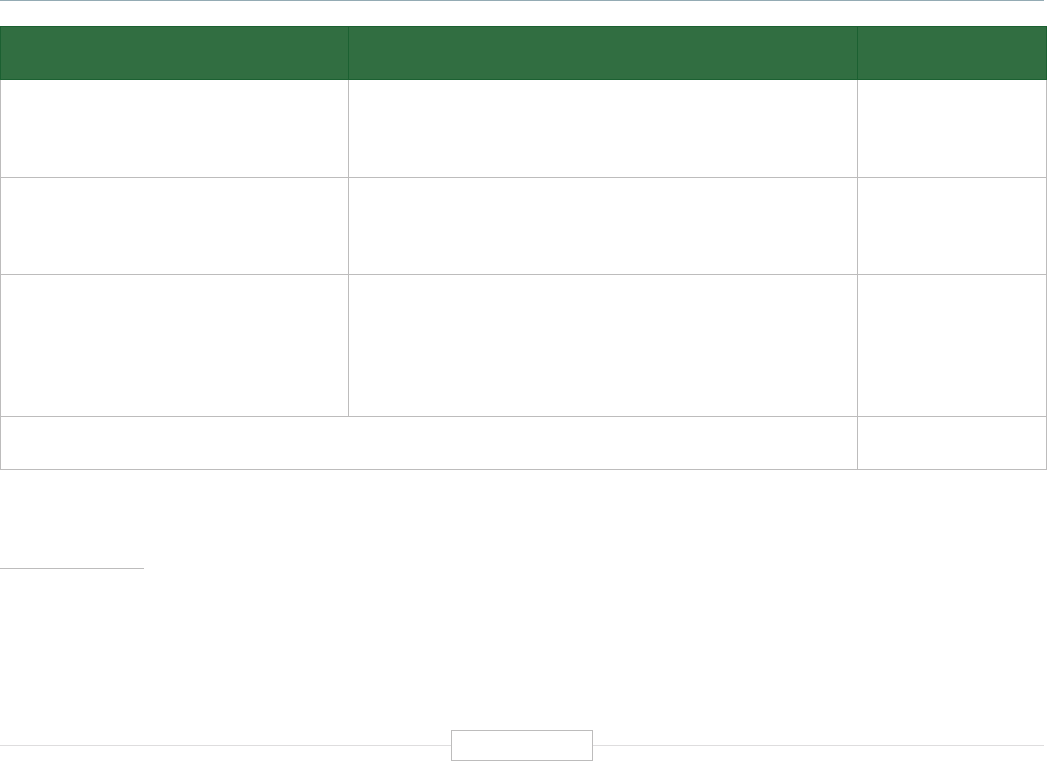
21
that are predominantly genetic in their etiology. The
majority of what is labelled “rare diseases and disor-
ders” falls into this category, often being single-gene
disorders. As such, the cost of these diseases can serve
as a surrogate for at least understanding the scale of
disease burden related to rare diseases, and by exten-
sion, provide intelligence on the kinds of economic
costs that may be ameliorated through advancements
in genetic and genomic diagnosis and treatments. A
recent study conducted by the Lewin Group for the
EveryLife Foundation for Rare Diseases makes an
important contribution to the literature and provides
robust evidence for the very substantial burden im-
posed by predominantly genetic rare disease.
35
The re-
search program, titled “The National Economic Burden
of Rare Disease Study,” uses data on medical care
costs, together with a detailed survey of rare disease
patients and caregivers, to derive estimates for the
burden of 379 rare diseases measured in terms of their
one year impact (2019). The study is conservative and
. Yang, Grace, et al. The National Economic Burden of Rare Disease Study. Lewin Group for EveryLife Foundation for Rare Disease, Feb. .
Liu, Zhichao, et al. “Toward Clinical Implementation of Next-Generation Sequencing-Based Genetic testing in Rare Diseases: Where Are We?”
Trends in Genetics, vol. , no. , Nov. .
only estimates the burden for 379 diseases for which
survey data were available (versus in excess of 7,000
rare diseases identified
36
)—but the results speak to the
large-scale costs of such disease to society, to patients,
and to patients’ families. The study finds the total
cost of the 379 assessed diseases to be $966 billion
for 2019. This total cost burden is shown in Table 6.
The Lewin authors do not choose to extrapolate their
findings for 379 rare diseases to the more than 7,000
such diseases that exist because they were unsure of
whether the 379 comprise a representative sample of
rare diseases extant. The measures shown in Table 6
are thus conservative. What they do provide, however,
is a window into the very large-scale cost of these (pre-
dominantly genetic) diseases—a cost that in just one
year in the U.S. imposed a burden of almost $1trillion.
The direct cost of the 379 rare disease impacts studied
by Lewin ($418 billion) can be put in context by exam-
ining total healthcare expenditure data maintained
Table 6: Economic Burden of 379 Rare Diseases in the United States (2019)
Cost Element Description 2019 Impact
Direct medical costs
Includes inpatient hospital or outpatient care,
physician visits, Rx medications, durable medical
equipment.
$418 billion
Indirect costs: productivity loss
Includes forced retirement, absenteeism,
presenteeism, and a reduction in community
participation and volunteer service.
$437 billion
Non-medical and uncovered
healthcare costs
Includes paid daily care, necessary home and vehicle
modifications, and transportation and education
costs. Also includes health care services not covered
by insurance: experimental treatments, medical
foods, and more.
$111 billion
$966 billion
Source: Lewin Group. “The National Economic Burden of Rare Disease Study.” Prepared for: EveryLife Foundation for Rare Diseases. February , .

22
by the Centers for Medicare and Medicaid Services
(CMS). CMS data for 2019 show total healthcare costs
for hospital, physician and other provider services,
medications, and durable medical equipment being
$2.4 trillion in 2019.
37
It is evident, therefore, that the
high amount of care required in diagnosing and
treating rare diseases comprises a significant portion
of overall healthcare costs. At $418 billion (again,
just for 379 rare diseases), the direct care costs of
these diseases equate to 17.4% of total national direct
spending across equivalent expenditure categories.
Of course, many common diseases also have genetic
involvement. Cancer is fundamentally a genetic dis-
ease, whereby gene mutations cause uncontrolled cell
growth. Many more diseases, including cardiovascular
diseases, gastrointestinal diseases, autoimmune disor-
ders, psychiatric and neurological disorders, musculo-
skeletal disorders, etc., have genetic involvement, typi-
cally involving many genes (i.e., polygenic). Quantifying
the overall cost burden of these diseases, where
genetics is part of the equation, is particularly chal-
lenging. Still, just referencing cancer, the American
Cancer Society reports that “the Agency for Healthcare
Centers for Medicare & Medicaid Services. “National Health Expenditures Highlights.” www.cms.gov/files/document/highlights.pdf.
Accessed May .
American Cancer Society. “Economic Impact of Cancer.”
www.cancer.org/cancer/cancer-basics/economic-impact-of-cancer.html. Accessed May .
Research and Quality (AHRQ) estimates that the direct
medical costs (total of all health care costs) for cancer
in the U.S. in 2015 were $80.2 billion”
38
—with 52% of
this cost being for hospital outpatient or doctor office
visits, and 38% of the cost for inpatient hospital stays.
While a definitive total cannot be calculated with
available data, there should be little doubt that the
economic burden of genetic and genomic-related
diseases in the U.S. reaches into the trillions of dollars
on an annual basis. However, as examined in the
next chapter, researchers and clinicians in research
institutes, universities, industry, and government
labs are making far-reaching contributions to re-
ducing the many burdens associated with genetic
and genomic diseases and disorders—making deep
progress in the clinical application of genetics sci-
ence and technology advancements to make a very
real difference in the lives of millions of patients.
23
The application of genetics and genomics advance-
ments to healthcare can certainly be viewed through
an economic lens, and the economic impact of this
dynamic science and technology-driven sector is
extremely large. However, the generation of busi-
ness income and jobs is not the raison d’etre for
medical genomics. Human genetics and genomics
are pursued for the scientific and clinical insights
they enable, and for their functional impacts,
these being the domains of application of the sci-
ence and technologies of genetics and genomics
to preserving and improving human health.
Genomics has become fundamental to advancement
of biomedical research, and the insights, tools, and
technologies provided by genetics and genomics are
now seeing increasingly widespread deployment in
clinical healthcare. This chapter seeks to provide a
broad overview of the key areas (domains) in which
human genetics and genomics are being applied
in clinical research and healthcare. The functional
impacts are divided into eight medical domains
(Figure 3): minable big data, whereby large data sets
of multi-patient data are providing deep insights
into disease biology (and also identifying charac-
teristics associated with health); identification of
genetic predisposition to diseases and disorders;
diagnosis of diseases and disorders though genetic
signatures; rational drug development, whereby
genetics information informs molecular targeting
in drug design; pharmacogenomics, which enables
the personalized prescription of drugs best suited
to the person’s genetics (with a goal of increasing
effectiveness and reducing adverse events); gene
editing and gene therapy, whereby genes associ-
ated with disease are modified to treat or cure the
disease; and two more “emerging” areas in which
human-microbe genetic interactions and hu-
man-environmental metagenome interactions are
being examined for association with human gene
expression, regulation, mutation, and disease.
In addition to applications to human medicine,
there are also several additional human applica-
tion domains relevant for non-medical uses. These
are briefly discussed herein, covering applications
in forensic science, anthropology and genealogy,
evolutionary biology, and paternity testing.
III. The Functional Impacts of
Human Genetics and Genomics
A. The Structure of Functional Impacts
(Application Domains of Human Genomics)
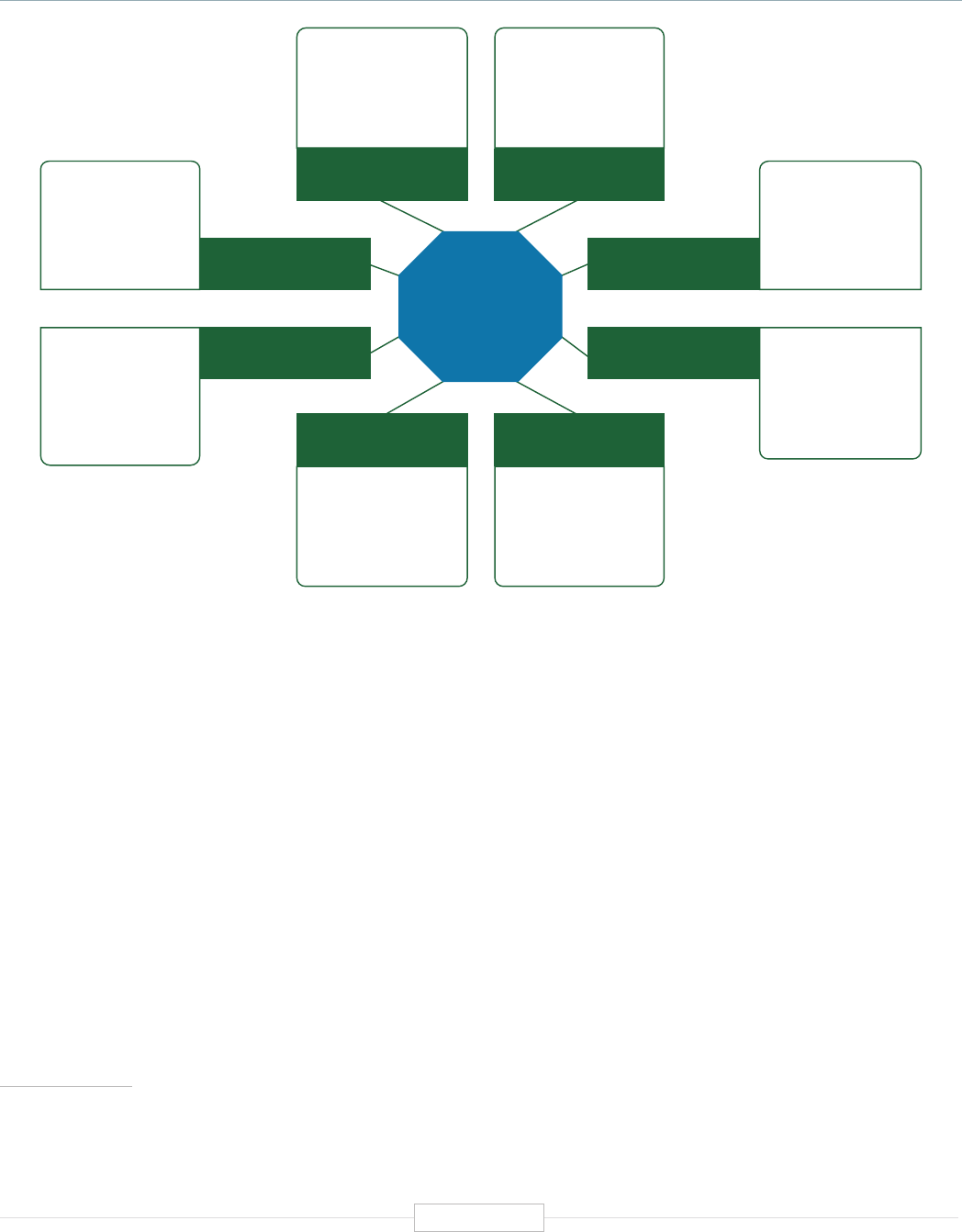
24
B. Fundamental Knowledge
Advancement
Before discussing the clinical impact domains
of human genetics and genomics, it is import-
ant to note that applied and clinical research
depends upon a healthy base of discoveries and
fundamental insights that are derived through
basic research, which may be defined as:
Systematic study directed toward greater
knowledge or understanding of the
fundamental aspects of phenomena and of
observable facts without specific applications
towards processes or products in mind.
39
Cornell Law School. “ CFR § . - Definition of Basic Research.” www.law.cornell.edu/cfr/text//.. Accessed May .
Genetics can inform us of our past evolution, and
genetics and genomics as disciplines have themselves
been evolving. Fundamental scientific research,
facilitated by advancements in genomics technolo-
gies and analytics, has provided a stream of notable
discoveries, with just some highlighted below:
• The ENCODE project (ENCyclopedia of DNA
Elements) revealed that noncoding DNA, previ-
ously termed “junk DNA” because it was thought
to be a relic of evolution with little biological
function, instead has specific functionality in
transcription and translational regulation of
protein-coding. In other words, most of it is not
Figure 3: Functional Biomedical Impact Domains
(Applications) of Human Genetics and Genomics
Minable Big Data
(Discovery Science)
Identifying Predisposition
to Diseases and Disorders
Diagnosing Diseases
and Disorders
Rational Drug
Development
Pharmacogenomics
(Personalized Medicine)
Gene Editing
and Gene Therapy
Human-Microbe
Interaction
Environmental Genomics
and Metagenomics
Analyzing sequencing
data from large and
diverse populations to
provide deep insights
into disease biology and
identify characteristics
associated with health.
Genetic and genomic
testing to identify
carrier status, and
identify predisposition
for genetic disease via
prenatal, newborn and
adult screening.
Using biomarkers
and gene signatures
to diagnose the
presence of diseases
or disorders that are
associated with
specific genes or
gene products.
Using genetic
information and gene
associated biomarkers
to inform molecular
targeting in drug design.
Using sequencing data
to enable the
prescription of drugs
best suited to the
patient’s genotype
(increasing efficacy and
reducing adverse events)
Modifying the genes
associated with a
disease or disorder
to treat or cure the
disease
Biomedical
Application
Domains of
Human
Genetics
and
Genomics
Examining the human
genome’s impact
upon hosted microbial
populations, and
microbe impacts upon
the human genome
and gene expression
Examining the impact
of human interactions
with the environment
on the human genome,
gene regulation, muta-
tion, and disease
etiology.
1
2
3
4
8
7
6
5
Biomedical
Application
Domains of
Human Genetics
and Genomics
Source: TEConomy Partners, LLC.
25
junk at all; it is central to life functions (although
not fully understood in terms of functionality).
• Basic research into gene silencing led to funda-
mental discoveries regarding RNA, messenger
RNA (mRNA), and development of techniques
for RNA interference that enabled human
genes to be disabled in a very precise man-
ner to better study their effect and function.
Further research has elucidated the presence
of multiple types of non-coding RNAs and
their impacts on gene expression, one class
of which, microRNAs (mnRNAs), may be reg-
ulating more than half of all human genes.
• Ongoing refinement to the reference human
genome has occurred, closing many of the gaps
in the original sequences and uncovering previ-
ously uncharacterized parts of the human ge-
nome with potential for significant discoveries.
40
• Recent studies have been elucidating “mo-
saicism”, which is the term used to describe
genomic variation among cells (both germline
and somatic) within an individual. Much still
Miga, Karen H. “Human Genome: Bridging the Gaps.” Nature, vol. , no. , Feb. .
Green, Eric D., et al. “Perspective: Strategic Vision for Improving Human Health at the Forefront of Genomics.” Nature, vol. , Oct. .
Collins, Francis S., et al. “Perspective: Human Molecular Genetics and Genomics – Important Advances and Exciting Possibilities.” The New
England Journal of Medicine, vol., no. , Jan. .
Pharmaphorum Connect. “The Future of Genomic Medicine: Can it Fulfil its Promises?” www.pharmaphorum.com/views-analysis-patients/the-
future-of-genomic-medicine-can-it-fulfil-its-promises/. Accessed May .
remains to be studied in this area to better
understand “mosaic variation in both nuclear
and mitochondrial DNA, the mechanisms that
generate mosaicism, and the roles of mosa-
icism in physiology and human disease.”
41
• Recent work is focused on “studying patterns of
gene expression in individual cells, a step that
has been driven by new methods for single-cell
RNA sequencing and chromatin analysis. Tens
of millions of cells have been characterized
thus far on route to a complete cell Atlas of the
human body. This effort is revealing hundreds
of new cell types and characterizing the ways
in which cell types differ between healthy
people and people with various diseases.”
42
• Basic research has found that “not all genes
are expressed in all tissues and that not all
genes are expressed during all developmen-
tal stages.”
43
This has important downstream
implications in drug development, where, for
example, a research team developing a drug
for infantile epilepsy would need to know
The importance of basic science derives from its contribution to knowledge deeper within the tree
of information and, consequently, its greater potential for integration with other facts. In contrast,
the importance of translational science lies in its practicality. Hence, we do not view basic and
translational science as one being more important than the other but rather as complementary
areas of human endeavor, with the important distinction that basic science findings often precede
advances in translational science. We also note that observations in translational or applied science
can generate new questions for fundamental research, as illustrated from the fact that vaccination
preceded the field of immunology. Hence, the epistemological flow is bidirectional, and investments
in both types of science are needed.”
Ferric C. Fang and Arturo Casadevall. “Lost in Translation—Basic Science in the Era of Translational
Research.” Infection and Immunity, vol. 78, no. 2, Feb. 2010.
26
whether a drug target is “expressed in the
brain and also during early development.”
44
It should also be noted that the Human Genome
Project, and the ongoing development of genomic
tools and datasets, generated a rather seismic shift
in the way in which fundamental research is per-
formed. The big data, information mining approach
that gene sequencing enables has “transformed
the nature of medical discovery, enabling scien-
tists to undertake comprehensive and powerful
explorations rather than being confined to testing
hypothesis focused on candidate pathways.”
45
What is clear is that the human genome is immensely
complex, and there is much still to be discovered
through fundamental research into its structure,
mechanisms of action, and its interface with biochem-
ical signals from non-genomic origin. In some diseas-
es, hundreds of individual genes are being found to
have an effect on disease development and progres-
sion, often in concert with multiple environmental
and physiological factors. Solving such multigenic
and multifactorial challenges is no small task, but
distinct progress is being made, aided by tremendous
technological advancements in sequencing and data
analytics platforms. Any scientist, or group of scien-
tists, embarking on finding solutions to individual
human diseases will typically recognize that the path
from question to discovery to therapy or cure is long
(sometimes spanning their career, if successful at all).
Modern genetics and genomics are, however, provid-
ing new, more brightly lit paths informed by quantita-
tive datasets that can be mined for insights and ther-
apeutic targets. As noted in NHGRI’s strategic vision:
…the past decade has brought the initial
realization of genomic medicine, as
research successes have been converted
into powerful tools for use in health care,
including somatic genome analysis for
Ibid.
Collins, Francis S., et al. “Perspective: Human Molecular Genetics and Genomics – Important Advances and Exciting Possibilities.” The New
England Journal of Medicine, vol., no. , Jan. .
Green, Eric D., et al. “Perspective: Strategic Vision for Improving Human Health at the Forefront of Genomics.” Nature, vol. , Oct. .
Ibid.
cancer (enabling development of targeted
therapeutic agents), noninvasive prenatal
genetic screening, and genomics-based
tests for a growing set of pediatric conditions
and rare disorders, among others.
46
For some diseases, especially those where a sin-
gle or only a few genes are involved, real break-
throughs are occurring. The section that follows
describes the domains of health sciences and
clinical care where these impacts are being felt.
C. Functional Applications
for Human Health
As fundamental genomic knowledge has expanded,
the enhanced understanding of genetic mecha-
nisms, in concert with access to rich whole exome
and genome datasets (and associated reference
compendia of human gene variants), has opened
the door to a new era of discovery and progress
in medicine. The impacts of these advancements
are now increasingly reverberating across medi-
cine, a fact highlighted by Eric Green, the Director
of the NHGRI, and colleagues who note that:
With insights about the structure and function
of the human genome, and ever improving
laboratory and computational technologies,
genomics has become increasingly woven
into the fabric of biomedical research, medical
practice, and society. The scope, scale, and
pace of genomic advances so far were nearly
unimaginable when the human genome
project began. Even today, such advances are
yielding scientific and clinical opportunities
beyond their initial expectations, with many
more anticipated in the next decade.
47
Much of the advancement being seen is enabled by
dramatic progress in genome sequencing technology
performance and cost effectiveness. A virtuous cycle
27
has occurred, whereby the speed increases and cost
decreases in sequencing have facilitated the assembly
of exabytes
48
of genomic information that can be
mined (assisted by highly advanced and automated
analytical systems) for unique insights into genome
structure and function.
49
As highlighted by Green:
Leading the signature advances has been
a greater than one million-fold reduction in
the cost of DNA sequencing. This decrease
has allowed the generation of innumerable
genome sequences, including hundreds of
thousands of human genome sequences (both
in research and clinical settings), and the
continuous development of assays to identify
and characterize functional genomic elements.
These new tools, together with increasingly
sophisticated statistical and computational
methods, have enabled researchers to create
rich catalogs of human genomic variants,
to gain an ever deepening understanding
of the functional complexities of the human
genome, and to determine the genomic
bases of thousands of human diseases.
50
This leads us to the first functional impact domain of
genetics and genomics in human medicine, minable
big data, and what it enables, discovery science.
1. Minable Big Data (Discovery Science)
Laurence Hurst highlights the central role
that data are playing in advancing function-
al applications of genomics, noting that:
Genomics is in an age of exploration and
discovery. Whether we are discovering the
genomes of more species, the genomes
of more individuals in a species, or more
genomes within an individual (at single-cell
An exabyte = bytes (,,,,,, bytes).
Stephens, Zachary D., et al. “Big Data: Astronomical or Genomical?” PLOS Biology, vol. , July . doi:./journal.pbio..
Green, Eric D., et al. “Perspective: Strategic Vision for Improving Human Health at the Forefront of Genomics.” Nature, vol. , Oct. .
Cheifet, Barbara. “Editorial: Where is genomics going next?” Genome Biology, vol. , no. , Jan. . doi:./s---.
Zielinski, Dina and Janiv Erlich. “Genetic Privacy in the Post-Covid Word.” Science, vol. , no. , Feb. .
Birney, Ewan. “Luminaries Share Their Thoughts on Advances in ‘Omics Over the Past Five Years.” Clinical Omics Magazine, vol. , no. , March-
April .
National Human Genome Research Institute. “Genetics vs. Genomics Fact Sheet.” www.genome.gov/about-genomics/fact-sheets/Genetics-vs-
Genomics#:~:text=All%human% beings%are%.,about%the%causes%of%diseases. Accessed May .
resolution), we are very much in a phase
where we are letting the data lead.
51
Studying one person’s genome (or in the case of the
“reference” human genome, a composite of a few peo-
ple) has provided valuable information regarding the
structure of the human genome and the number of
protein-coding genes. It has also allowed for compar-
ison to an expanding library of reference genomes for
other organisms, helping to identify regions of similar-
ity and difference that could help illuminate function-
ality. At a time when whole-genome sequencing cost
many millions of dollars per genome, the field was
limited to small volumes of sequenced genomes to
work with. As sequencing costs declined and sequenc-
ing speed increased, the ability to generate data from
a large number of individuals started to become real-
istic, and this has opened new horizons for research.
Currently, whole genome sequencing and whole
exome (the part of the genome formed from exons
that code proteins) sequencing is fast and affordable
(requiring just a day and $689 for whole genome
sequencing), and as affordability and speed have
increased, the number of sequenced individuals
has expanded exponentially. In a recent edition of
Science, it is noted that “today, more than 30 million
individuals have access to their detailed genomic
datasets,”
52
while Ewan Birney of the European
Bioinformatics Institute notes that “estimates show
that over 60 million patients will have their genome
sequenced in a healthcare context by 2025.”
53
Having access to an extremely large volume of se-
quenced individuals creates a dramatically enlarged
platform for discovery. Each of us has a unique
genome. While 99.9% of the genome between
individuals will be the same,
54
the 0.1% differenc-
es can add up to profoundly dissimilar physical
characteristics and differential predisposition to
28
disease, rates of metabolizing drugs, and other
factors. Identifying and understanding these differ-
ences becomes more feasible the more sequences
and data are available. The devil is in the details,
and more sequences provide more details.
Large-scale sequencing implementation is enabling
the ongoing assembly of robust, evidence-based
resources for the identification and classification of
genomic variant pathogenicity (variants associated
with causation of disease). To-date, the vast majority
of larger-scale whole genome sequences have been
produced in Western nations, with the result that
the available data skew quite significantly in terms
of individuals of European ancestry. This bias in the
data is being addressed through multiple initiatives
worldwide that will contribute greatly to a broader
base of represented humanity. Recent research by
Table 7: Large Population Precision Medicine Initiatives
Country Project/program name Expected size
Common
diseases
Rare diseases
(and cancers)
Australia Genomics Health Futures Mission 200,000
Canada
Canadian Genomics Partnership
for Rare Diseases and Canadian
Longitudinal Study on Aging
Nationwide
China Precision Medicine Initiative
100,000–
100 million
Denmark Danish National Genome Center 60,000
Dubai Dubai Genomics Nationwide
Estonia Personalised Medicine Programme 150,000
European Union 1+ Million Genomes Initiative 1,000,000+
Finland FinnGen 500,000
France Genomic Medicine France 2025
235,000
each year
Hong Kong Hong Kong Genome Project 50,000
Italy SardiNIA Project 60,000
Japan GEnome Medical alliance Japan Nationwide
Saudi Arabia Saudi Human Genome Program 100,000
Singapore (And
International)
Genome Asia 100 K 100,000
Thailand Genomics Thailand 50,000
Turkey Turkish Genome Project
100,000–
1,000,000
United Kingdom 100,000 Genomes Project 100,000
United Kingdom Accelerating Detection of Disease 5,000,000
United States NHGRI Genomic-Medicine Nationwide
United States All of Us Research Program 1,000,000+
Source: Identified in Chung, B.H.Y., Chau, J.F.T. & Wong, G.KS. “Rare versus common diseases: a false dichotomy in precision medicine.” npj Genom.
Med. , ().
29
Brian Hon Yin Chung, Jeffrey Fong Ting Chau, and
Gane Ka-Shu Wong summarizes many of the larg-
er (>20,000 subject genomes) precision medicine
projects (for which sequencing is primarily a major
element), showing how in forthcoming years, the
richness of sequenced populations will be enhanced
significantly.
55
The global distribution of these studies
(Table 7) holds promise for the development of reliable
genomic data on many different populations and
sub-populations, helping to build a more inclusive
atlas of genome variability across the human species.
The expanding diversity in the base of human
genome sequence data is further highlighted by
Rotimi, Callier, and Bentley who recently note that:
Growing prioritization of diverse populations
in genomics research has begun to respond
to these gaps. Programs, such as TOPMed,
All of Us, International Common Disease
Alliance, Human Heredity and Health in
Africa (H3Africa), Million Veteran Program,
GenomeAsia, and the COVID global consortium,
contribute to advances in diversity and
inclusion among research participants.
56
In the U.S., several large-scale sequencing initiatives
are ongoing. Among the largest is the NIH’s “All of Us”
program, which began in 2018 and is consolidating
Chung, B.H.Y., et al. “Rare versus Common Diseases: a False Dichotomy in Precision Medicine.” npj Genomic Medicine, vol. , no. , Feb.
. doi.org/./s---x.
Rotimi, Charles N., et al. “Lack of Diversity Hinders the Promise of Genome Science.” Science, vol. , no. , Feb. .
genetic, health, and environmental data for more than
one-million participants, providing a robust resource
for evaluating genotype-to-phenotype associations.
Another federally initiated program is the “Million Vets
Program” (MVP) that, similar to the NIH program, is
collecting deep data to allow the study of links be-
tween genes, lifestyle, and military exposures and their
associated impacts on health and illness. Since launch-
ing in 2011, over 825,000 veterans have signed up to
participate. The VA notes that “in addition to common
health conditions that affect everyone, such as cancer,
cardiovascular disease, and diabetes, MVP researchers
are also looking at conditions specific to Veterans. This
Increasing Sequenced
Population Diversity to Enhance
Studies of Genetic Variation.
Up until relatively recently the participants in-
volved in genomic research have largely been of
European ancestry.
Multiple initiatives are now underway to substan-
tially increase diversity in genomic datasets. For
example, the Human Heredity and Health in Africa
(H3Africa) initiative has enrolled more than 60,000
research participants and engaged more than 500
African scientists.
Research in genome biology is often descriptive in nature, sequencing genomes and metagenomes,
profiling epigenomes and transcriptomes, charting evolutionary history, and cataloging disease
linked risk loci. Thanks to major technological advances, we can now generate such descriptive
datasets using high throughput platforms.”
Christoph Bock, CeMM Research Center for Molecular Medicine of the Austrian Academy of Sciences, quoted in Barbara
Cheifet. “Editorial: Where is Genomics Going Next?” Genome Biology, vol. 20, no. 17, 22 Jan. 2019.
30
includes PTSD [post-traumatic stress disorder], suicide
prevention, TBI [traumatic brain injury], and tinnitus.”
57
While advances in the technologies for gene se-
quencing are at the forefront in generating large
and deep datasets from diverse populations, equally
important, have been advancements in advanced
data analytics comprising the use of analytical
computer algorithms and statistical techniques
acting upon large-scale sets of structured and
unstructured data to derive actionable insight
(see sidebar definition for advanced analytics).
Genetic and genomic data, health record data, and
environmental data each provide important insights
on their own but promise far greater intelligence when
examined together. This presents the challenge of
analyzing extremely large-scale heterogenous data
compiled from multiple sources. Fortunately, as these
big data resources have been built, there has been
parallel advancement in the science and technology of
advanced data analytics, up to and including artificial
intelligence (AI) based systems. Advanced analytics
provides a pathway forward in terms of mining big
genome and genome-phenome datasets to provide
functional insights and impacts in broad areas such as:
biomarker discovery and identification of druggable
VA Million Veteran Program. “About the Million Veteran Program.” www.mvp.va.gov/webapp/mvp-web-participant/#/public/about. Accessed
May .
Liu, Zhichao, et al. “Toward Clinical Implementation of Next-Generation Sequencing-Based Genetic testing in Rare Diseases: Where Are We?”
Trends in Genetics, vol. , no. , Nov. .
Tripp, Simon, et al. Artificial Intelligence and Advanced Analytics in Indiana: An Initial Discussion of Industry Needs and University
Capabilities. TEConomy Partners, LLC for BioCrossroads, Jan. .
targets; multi-gene to disease associations; environ-
mental effects on gene regulation and expression;
gene expression effects of prior infectious diseases,
etc. The combination of genomics and phenomics
big data and advanced analytics provides a powerful
pathway forward for modern life science discovery
and healthcare improvement. This is highlighted
by Liu, Zhu, Roberts, and Tong who note that: “AI
is starting to realize its potential in augmenting
phenome-wide and genome-wide data profiles to
improve clinical utility and diagnostic power.”
58
Evidence for this technological convergence of bio-
medical big data and AI is seen in evident clusters
of new business ventures forming to pursue com-
mercialization of associated opportunities. Research
by TEConomy recently used machine learning to
identify clusters of U.S. activity focused on advanced
analytics applications.
59
Figure 4 illustrates three
distinct clusters of venture capital funded enter-
prises forming in this space, comprising: 1) drug
discovery and precision medicine, 2) healthcare
analytics, and 3) wearable health monitoring de-
vice analytics. The growth and interaction of these
clusters builds upon the promise of big data ana-
lytics using genome and phenome information to
derive clinical health insights and improvements.
Advanced Analytics is the autonomous or semi-autonomous examination of data or content using
sophisticated techniques and tools, typically beyond those of traditional business intelligence (BI),
to discover deeper insights, make predictions, or generate recommendations. Advanced analytic
techniques include those such as data/text mining, machine learning, pattern matching, forecasting,
visualization, semantic analysis, sentiment analysis, network and cluster analysis, multivariate
statistics, graph analysis, simulation, complex event processing, neural networks.”
Gartner. “Gartner Glossary.” www.gartner.com/en/information-technology/glossary/advanced-analytics.
Accessed 12 May 2021.

31
Figure 4: Biomedical-Related Clusters Identified in the Innovation Landscape Net-
work of U.S. AI Companies Receiving Significant VC Investment, 2014-2018
Source: TEConomy Partners, LLC
New genetic and genomic analytical tools are also
in development and coming online, which promise
additional high-throughput analytical capabilities
and assessment. It is anticipated that new analytical
techniques such as CRISPR single cell sequencing
and single-cell RNA-seq (scRNAseq) will generate data
that provides new insights into biological function.

32
Case Study: Example of Minable Big Data (Discovery Science)
Geisinger is an integrated healthcare system headquartered in Pennsylvania integrating “primary care
and specialists, hospitals and trauma centers, insurance, medical education and research.”
60
Geising-
er has been at the forefront in terms of recognizing the clinical insights and utility that genomic data
and health record data that are mined together provide. Begun in 2007, Geisinger’s MyCode Commu-
nity Health Initiative has seen over 250,000 patients consent to participate in a research program that
has been performed in collaboration with Regeneron’s genetics research center. More than 100,000
whole exomes had been sequenced (as of reporting in April 2019). Motivated by the MyCode experience,
Geisinger launched a “clinical whole-exome population screening program in mid-2018 as part of rou-
tine clinical care in a variety of Geisinger clinics, developing an end to end implementation platform—
from patient engagement and consenting, to whole exome sequencing in a certified clinical laboratory,
to physician education, to genetic counseling at scale, and to integration of clinical results into the
electronic health record.”
61
The main focus of MyCode is finding and confirming new disease-causing
variants (changes) in patient genes, with research programs directed at:
• Searching for changes in genes that protect against disease.
• Targeting new drug development.
• Researching and learning what are the best ways to share medically-actionable genetic results with
patient-participants, and then how to facilitate the sharing of that information with other potential-
ly affected family members.
• Translating the results into clinical care.
The CEO of Geisinger, Jaewon Ryu, notes that:
About 90 percent of the patients we ask let us look at their entire genomes. That’s huge trust; a typ-
ical rate is 15 percent. Our patients come from an unusually stable population—giving us volumes
of data from more than 15 years of electronic health records. MyCode therefore provides unprece-
dented opportunity for early diagnosis and developing new and tailored treatments, or precision
medicine. MyCode is already letting us help participants and their families prevent or mitigate the
impacts of some identified genetic risk factors, including cancer and heart disease.
62
Some of the results emanating from MyCode in terms of advancements in breast cancer genetics
have been called out as among the most significant applications of medical genetics in the “Genomic
Medicine Year in Review.” BRCA1 and BRCA2 are genes associated with hereditary breast/ovarian cancer
syndrome. They have a high degree of sequence variation, but the population prevalence of “pathogen-
ic” and “more likely pathogenic” variance (P/LP) has not been known. The research team used 50,000+
whole exome sequences from MyCode to examine the frequency of P/LP variants, finding a frequency
significantly higher than other estimates. Importantly, it was found that “almost half of all variant car-
riers did not meet current criteria for clinical testing, and of those meeting testing criteria, nearly half
had not undergone clinical testing. Thus, 3/4 of at-risk women were not identified as such and are not
benefiting from evidence based interventions; this is a significant care gap with implications for popu-
lation health.”
63
This represents a prime example of how the assembly and analysis of big data enables
robust functional impacts to be generated in clinical care.
Geisinger. “About Geisinger.” www.geisinger.org/about-geisinger. Accessed May .
Willard, Huntington. “Luminaries Share Their Thoughts on Advances in ‘Omics Over the Past Five Years.” Clinical Omics Magazine,
vol. , no. , March-April .
Geisinger. “About Geisinger.” www.geisinger.org/about-geisinger. Accessed May .
Manolio, Teri A., et al. “Genomic Medicine Year in Review: .” The American Journal of Human Genetics, vol. . December
. Reporting on findings from Manickam, K. et al. “What We’re Missing: Most BRCA and BRCA Variant Carriers are
Undetected.” JAMA Network Open.
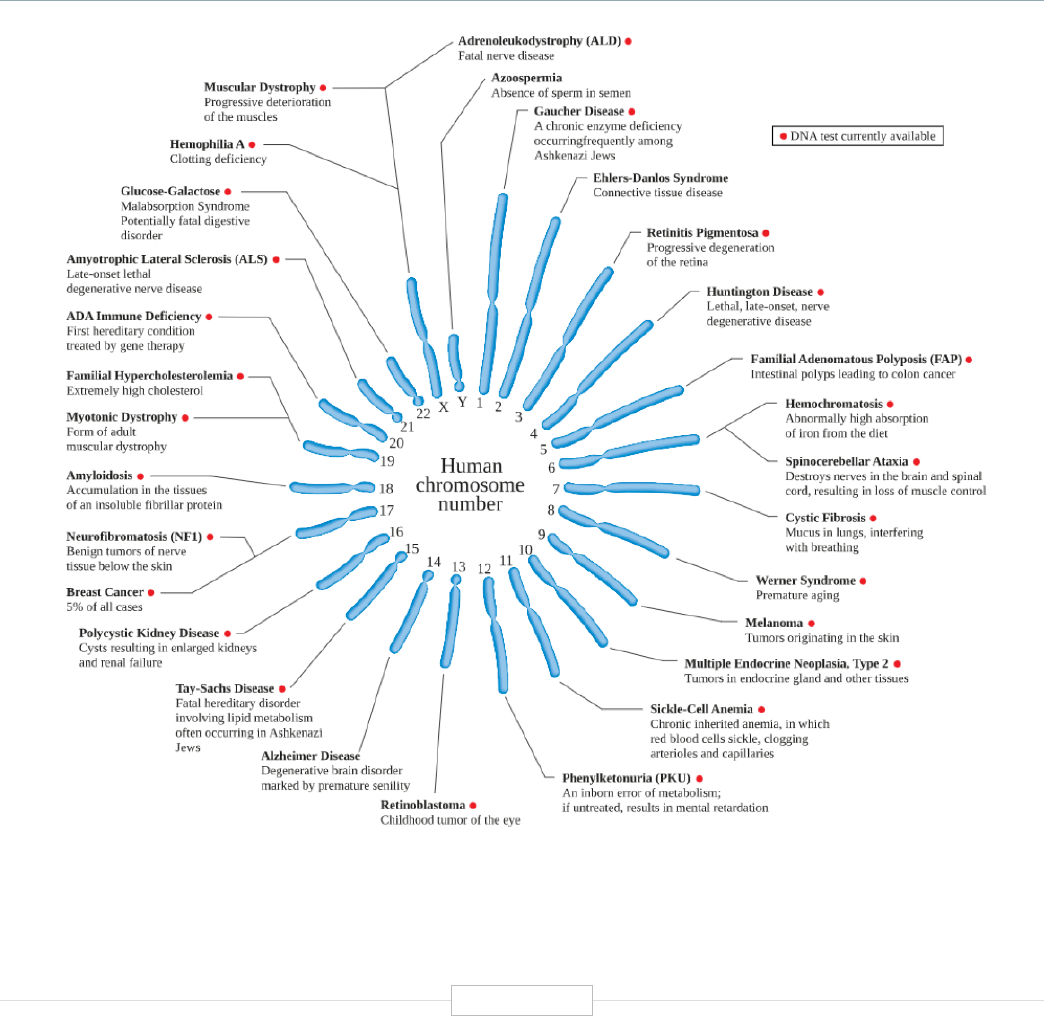
33
2. Identifying Predisposition
to Diseases and Disorders
One of the primary research and clinical applica-
tions of human genetics and genomics is its use in
identifying potential predisposition to developing
diseases and health disorders. The BRCA gene ex-
ample from Geisinger is an example of this, where
the identification of these genes in the patient
identifies risk for breast cancer and guides clinical
decision making. The library of gene-disease as-
sociations has expanded rapidly, and as Figure 7
illustrates, there are genes within every human
chromosome associated with disease. Notably, all
but three of the diseases or disorders listed in Figure
5 now have a genetic test associated with them.
Figure 5: The human chromosome set, indicating examples of locations for patho-
genic gene variants causing hereditary diseases
Note: Conditions that can be diagnosed using DNA analysis are indicated by a red dot.
Source: By - Own work, CC, https://commons.wikimedia.org/w/index.php?curid=
34
Figure 5 illustrates only a small sampling of the
diseases and health disorders that have genetic
associations. The number identified is growing, and
at the time of writing 4,395 genes are noted by the
Online Mendelian Inheritance in Man (OMIM) project
as being identified with a disease or disorder-causing
mutation (phenotype-causing mutation), and 6,828
diseases or disorders have a known genetic basis.
64
One of the key applications of this knowl-
edge, and an extremely valuable one, is the
development of genetic testing for predis-
position to disease. Genetic testing is:
The use of a laboratory test to look for genetic
variations associated with a disease. The
results of a genetic test can be used to confirm
or rule out a suspected genetic disease or to
determine the likelihood of a person passing on
a mutation to their offspring. Genetic testing
may be performed prenatally or after birth.
65
The above definition from NHGRI implies two principal
uses for genetic tests: 1) diagnosis of present disease
(which was discuss previously), and 2) identification
of the presence of a gene variant that may predispose
an individual or their offspring to the development
of a disease associated with that gene. This latter
use of genetic testing is becoming increasingly
deployed as more gene-disease associations are
established. Genetic testing for predisposition to
diseases or health disorders may be divided into
three categories: 1) carrier screening, which tests a
prospective parent for the presence of gene variants
that have been shown to be associated with risk of
passing down a hereditary disorder (thereby helping
to inform family planning and associated decisions);
2) pre-natal and post-natal testing, which focuses
on testing for genetic predisposition to disease in
the fetus or in newborns; and, 3) child and adult
testing. Each application is introduced below.
OMIM®, Online Mendelian Inheritance in Man®. OMIM is a comprehensive, authoritative compendium of human genes and genetic
phenotypes that is freely available and updated daily. OMIM is authored and edited at the Johns Hopkins University School of Medicine. For
more information see: www.omim.org/statistics/geneMap.
National Human Genome Research Institute. “Genetic Testing.” www.genome.gov/genetics-glossary/Genetic-Testing. Accessed May .
National Human Genome Research Institute. “Carrier Screening.” www.genome.gov/genetics-glossary/Carrier-Screening. Accessed May
.
a. Carrier Screening
As noted by NHGRI:
Carrier screening is a type of genetic testing
performed on people who display no
symptoms for a genetic disorder but may
be at risk for passing it on to their children.
A carrier for a genetic disorder has inherited
one normal and one abnormal allele for a
gene associated with the disorder. A child
must inherit two abnormal alleles in order
for symptoms to appear. Prospective parents
with a family history of a genetic disorder
are candidates for carrier screening.
66
Carrier screening has generated significant impacts
on decision making for potential parents. This particu-
larly comes into play when two individuals each carry
a single copy of a disease allele that, while harmless to
them, has a serious risk of manifesting into a serious
genetic disease in their offspring. Carrier screening
can be used by individuals, in advance of marriage or
long-term partnering, to assess genetic risks, and by
couples contemplating starting a family. The appli-
cation of carrier screening in some communities and
populations has had profound effects, limiting, for
example, the birth of children with devastating, often
fatal, diseases such as Tay-Sachs disease and highly
debilitating disorders such as Sickle Cell Anemia and
Beta-thalassemia. The rise of low cost, high accuracy
whole genome sequencing is greatly expanding the
potential to perform DNA-based carrier screening
across a broad range of autosomal recessive, sin-
gle-gene disorders. Carrier screening may also be used
by at-risk couples pursuing in vitro fertilization (IVF),
allowing their clinician to test for potential genetic
abnormalities before implantation of fertilized eggs
(this is termed “preimplantation genetic diagnosis”)
or to select embryos with normal chromosomes for
implantation (“preimplantation genetic screening”).

35
b. Pre-natal and Post-natal Testing
Not every potential parent is in a position to access
carrier screening and, of course, a great many preg-
nancies are unplanned. As a result, many (indeed
most) pregnancies occur without prior carrier screen-
ing. Once a pregnancy is underway, pre-natal screen-
ing provides the ability for clinicians to evaluate the
healthy development of the baby (through established
diagnostics such as ultrasounds and maternal blood
tests) and can also include genetic tests to screen for
whether the baby may be born with certain genetic
conditions and chromosomal disorders (such as
Down’s syndrome). Such genetic testing has tradition-
ally been reserved for mothers with a certain risk pro-
file (such as family history of genetic disease, mothers
who are older, or persons who know they carry certain
monogenic alleles that confer risk). Such genetic
prenatal testing has required invasive procedures,
such as amniocentesis, that carry a measure of risk
to the pregnancy. Increasingly, however, physicians
are able to order non-invasive prenatal screening (or
“cell-free DNA screening”) that uses cell-free placental
A “trisomy” is a condition in which an extra copy of a chromosome is present in the cell nuclei, causing developmental abnormalities.
Dondorp, Wybo, et.al. “Non-invasive Prenatal Testing for Aneuploidy and Beyond: Challenges of Responsible Innovation in Prenatal Screening.”
European Journal of Human Genetics, vol. , no. , Mar. .
DNA fragments in maternal blood to screen for fetal
genetic conditions, such as the common trisomies
67
(e.g., Down syndrome) and deletion or duplication
syndromes. A non-invasive prenatal screening (NIPS)
is more accurate, with fewer false positives for the
most common trisomies, than other screening
tests—leading to fewer invasive procedures.
68
NIPS can now test for:
• Trisomy 21 (Down’s syndrome)
• Trisomy 18 (Edwards syndrome)
• Trisomy 13 (Patau syndrome)
• XXY chromosome (Klinefelter syndrome)
• XO chromosome (Turner syndrome)
• Microdeletions in chromosomes
• Rh factor (positive or negative determination)
• Multiple other less common triso-
mies and single-gene disorders.
At the present time, these NIPS tests are not con-
sidered fully diagnostic, and follow-up testing is
recommended using other procedures if a posi-
tive result is achieved through NIPS. Ultimately,

36
pre-natal diagnostics can help mothers make
informed decisions regarding their pregnancy
and discuss options for care with their health-
care provider, help families prepare for a poten-
tial challenge to their baby’s health, and help
ensure that clinicians are ready to support any
special health needs of the resulting newborn.
Finally, post-natal testing is a suite of genetic and ge-
nomic tests that are employed to evaluate newborn
health and diagnose present or emerging health is-
sues. As noted by Holm, “the greatest opportunity for
lifelong impact of genomic sequencing is during the
newborn period.”
69
Having a whole-genome sequence,
or at least a whole-exome sequence, completed as
soon as possible after birth provides a broad spectrum
of genetic information for significant immediate use
and expanding clinical utility across the lifespan. As
noted above, 6,828 diseases or disorders currently have
a known genetic basis, and a whole-genome se-
quence provides an increasingly accessible pathway to
evaluating common or rare genetic mutations associ-
ated with immediate health challenges or the devel-
opment or emergent health issues over a life span.
The most immediate benefit of newborn screening is
as a contributor to achieving a diagnosis of a genetic
disease or disorder in the newborn—the advantages
of which are discussed in the diagnosis section.
Holm, Ingrid A., et al. “The BabySeq Project: Implementing Genomic Sequencing in Newborns.” BMC Pediatrics, vol. , no. , July .
doi:./s---.
Newborn genetic screening represents a
highly visible and successful approach to
identification of inherited health conditions.
c. Child and Adult Testing
The clinical reality of whole genome, or whole exome,
sequencing is a relatively new phenomenon. Thus,
the vast majority of the present U.S. population did
not benefit from access to this valuable screening
and diagnostic tool at birth. Having sequencing
performed at any stage in life will, however, still
have potentially significant clinical utility, with utility
obviously maximized the earlier in life the sequencing
is performed. With the expanding library of identified
gene-disease linkages and the assurance that this
library will continue to grow as more research findings
accumulate, it is only a matter of time before full
sequencing of everyone is a clinical reality and con-
sidered standard of care—with our genome sequence
ideally connected to a lifelong electronic health record.
The cost/benefit ratio of such a data structure has
been consistently shifting in its favor as the cost of
sequencing dramatically declines and information
on gene-disease associations increases. Indeed, the
promise of universal sequencing in clinical application
is being realized in some health systems, as shown in
the Geisinger MyCode example. Multiple other health
systems have large-scale pilot projects underway, in-
cluding examples such as Mayo Clinic, Intermountain
37
Healthcare, Mount Sinai Healthcare System, Kaiser
Permanente, and the Veterans Administration. It is
also increasingly the case that “centers for person-
alized medicine” have been established at leading
healthcare centers that offer whole-genome or
whole-exome sequencing to selected patients, often
with an initial focus on cancers or rare disorders.
Today, with thousands of genes associated with
thousands of diseases, it is perhaps not surprising that
genetic and genomic tests are becoming increas-
ingly applied in medicine across the lifespan. A key
application is in determining the “risk” for patients in
developing a disease that is associated with particular
alleles. The previously mentioned BRCA gene tests
for risk of hereditary breast cancers are one example,
with the BRCA1 and BRCA2 genes accounting for
20-25% of hereditary breast cancers. Testing positive
for these gene variants allows a patient to enter into
informed discussions with their physicians regarding
potential risk reduction approaches, such as increased
screening frequency or prophylactic breast removal
surgery. Multiple cancers now have genetic tests
associated with them for risk evaluation, including
breast, colorectal, cutaneous melanoma, gastric,
ovarian, pancreatic, prostate, renal cell, thyroid, and
uterine cancers. Cardiovascular-related tests are also
available to evaluate risks for developing aortopathies,
arrhythmias, cardiomyopathies, genetic forms of high
blood pressure and high cholesterol, and thrombophil-
ia. The above are just some of the areas of disease in
which genetic testing for risk is seeing application, and
the library of available tests will continue to expand.
Because most common diseases have been found
to have associations with many individual genes (i.e.,
they are polygenic, as opposed to monogenic), a
new area of science and practice is in development
focused on determining potential risk based on
the presence of multiple gene variants. This testing
results in the generation of “polygenic risk scores”
Ossorio, Pilar N. “Polygenic Risk in a Diverse World.” Science, vol. , no. , Feb. .
Ibid.
Worthy, Liz. “Luminaries Share Their Thoughts on Advances in ‘Omics Over the Past Five Years.” Clinical Omics Magazine, vol. , no. , March-
April .
(PRS), which are an “emerging technology for aggre-
gating the small effects of multiple polymorphisms
across a person’s genome into a single score.”
70
It has
been noted, “In medicine and public health, PRSs
could, in the future, be used for initiating additional
risk screening or motivating behavior change.”
71
It
is an area of research interest and potential prom-
ise. Writing in early 2019, Liz Worthy notes that:
Over the last 18 months we are seeing increased
application of polygenic risk score analysis
making use of large GWAS [genome wide
association studies] and WGS [whole genome
sequencing] data. PRS seeks to estimate an
individual’s propensity towards particular
phenotype… These methods have a variety of
uses including human disease risk assessment
in research settings and there is increased focus
on their application within healthcare settings.
72
For the patient, the advantages afforded through
the application of genetic tests for disease pre-
disposition are potentially significant—providing
a pathway for adopting risk reduction lifestyle or
medicinal approaches, more frequent use of early
disease detection screenings, and prophylactic
surgeries in selected instances. It is important to
note that genetic and genomic testing for the
predisposition of disease is best performed in
consultation with a patient’s physician and with
genetic counselors—skilled personnel who can
interpret the results and provide recommendations
for health strategies rooted in evidence-based clinical
practice. While there are direct-to-consumer tests,
there is risk attached to non-professional test result
interpretation that could lead to unnecessary anxiety
or pursuit of unnecessary/unproven interventions.
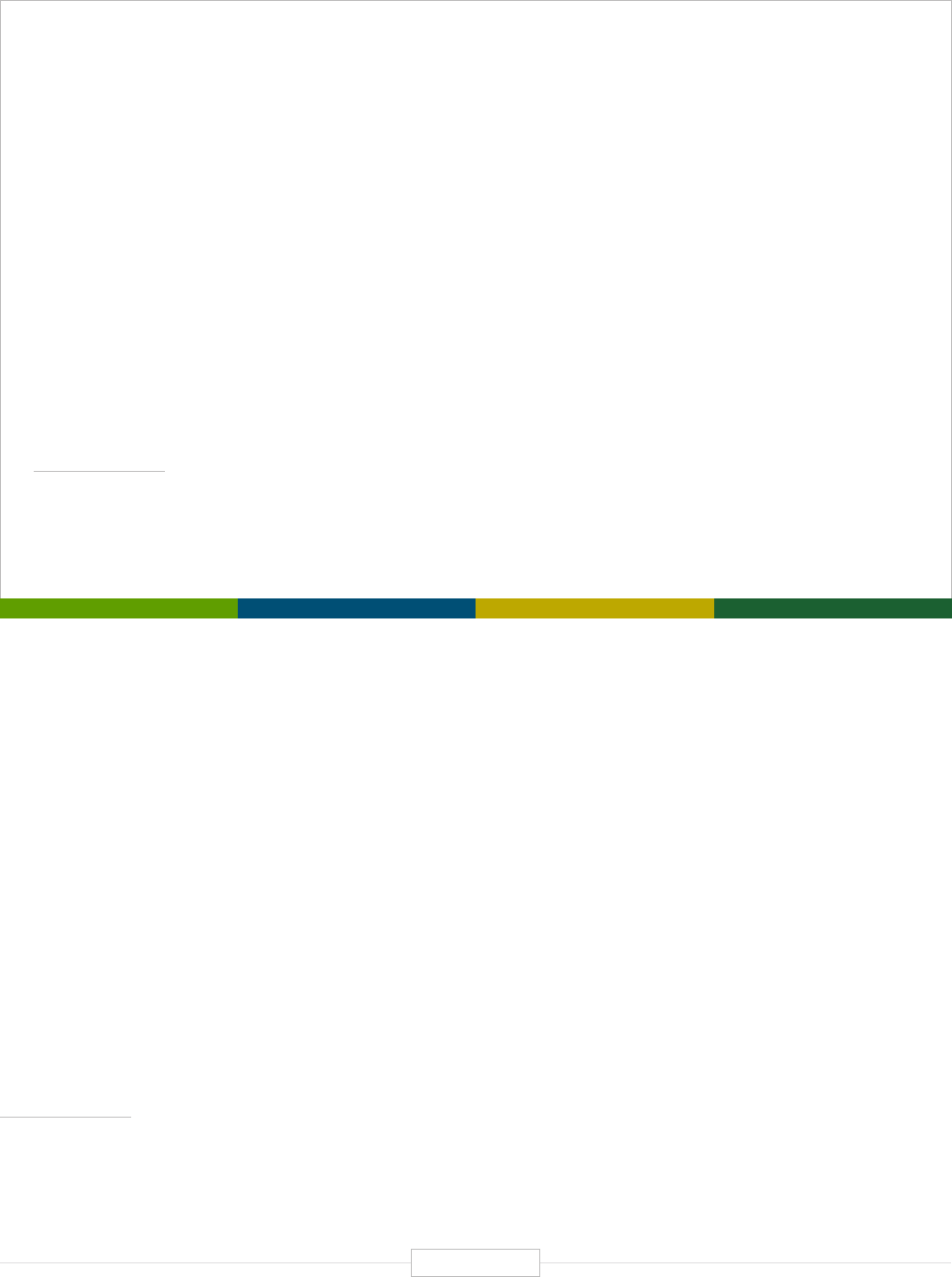
38
3. Diagnosing Diseases,
Rare Diseases, and Disorders
Genetic or genomic tests can not only determine
potential risk for developing a disease, they can
also be highly informative in guiding the diagnosis
of a present disease or disorder. As noted by the
precision medicine program at Duke University:
Whole genome and whole exome sequencing
are increasingly being used in the clinic to aid
in the diagnosis of rare congenital disorders
and solve diagnostic dilemmas. One of the first
and most high-profile examples of using clinical
sequencing to end a diagnostic dilemma is
the case of 6 year old Nic Volker at the Medical
College of Wisconsin, who suffered from a
Duke Center for Applied Genomics and Precision Medicine. “Clinical Whole Genome Sequencing.” https://precisionmedicine.duke.edu/
researchers/precision-medicine-programs/clinical-whole-genome-sequencing. Accessed May .
mysterious severe bowel disease of unknown
origin. Searching for an explanation and
treatment, doctors turned to next-generation
DNA sequencing technology. They identified a
mutation in the XIAP gene as the likely cause of
his illness, knowledge that suggested a course
of treatment that led to his recovery, ending
his diagnostic odyssey. Early results from some
clinical sequencing programs estimate the
success rate of disease gene identification at
about 25-30%, offering hope to thousands of
individuals with previously undiagnosed or
untreated rare disorders, while recognizing that
sequencing will not provide all of the answers.
75
Case Study in Genetic Screening: Detecting
At-Risk Carriers Not Detected Through Other Methods
Results from a large cohort study conducted at Renown Health in Nevada show how genetic carrier
testing results in the identification of risk in patients where it was not previously suspected. Describing
the study, Manolio noted that:
The value of genetic screening in an unselected population for identifying individuals carrying P/
LP genomic variants for HBOC [Hereditary Breast and Ovarian Cancer], Lynch syndrome, and FH
[Familial Hypercholesterolemia] has not been widely explored. The Healthy Nevada Project at Re-
nown Health performed exome sequencing in 26,906 participants with available electronic med-
ical records and analyzed genomic variants in nine risk genes for these conditions. Roughly 1.3%,
90% of whom had not been previously identified, carried P/LP variance. Among carriers, 22%, 70%
of whom were diagnosed before age 65, were diagnosed with clinically relevant disease. Less than
20% of carriers had medical record documentation of inherited genetic disease risk or relevant
family history.
73
The researchers conclude that: “this suggests that genomic screening for inherited cancer and cardio-
vascular risk conditions can identify a significant number of at-risk carriers who are not detected by
standard medical practice and who may benefit from earlier clinical risk screening.”
74
Manolio, Teri A., et al. “Genomic Medicine Year in Review: .” The American Journal of Human Genetics, vol. , December
. Reporting on findings from Grzymski, J.J. et al. “Population Genetic Screening Efficiently Identifies Carriers of Autosomal
Dominant Diseases.” Nature Medicine, vol. , July .
Ibid.
39
Genetic and genomic tests for disease diagnosis
are being deployed across a broad range of rare
and more common diseases and disorders.
a. Diagnosis of Rare Diseases and Disorders
Rare diseases, by their inherent nature, present
diagnostic challenges because so few physicians have
encountered them. Often these diseases may present
symptoms seen in other, more common diseases
resulting in an understandable misdiagnosis and
inappropriate treatment strategies being adopted.
Patients and their families may embark on long
“diagnostic odysseys”, seeing dozens of practitioners,
undergoing multiple tests and procedures, enduring
fruitless attempts at treatment over many years
without ever getting a definitive, accurate diagnosis.
Genetic and genomic testing has been a pathway
to solving this dilemma in multiple diseases and
disorders impacting many thousands of patients.
While individual rare diseases are, by definition, rare,
they collectively impact a large global and domestic
population. Liu, Zhu, Roberts, and Tong estimate that:
Approximately 7000 rare diseases have been
recognized, a substantial number of which
are life threatening or chronically debilitating.
Around 80% of rare diseases are genetic in
origin. A single rare disease affects a small
number of the population (defined as <1/15,000
in the US and <1/2000 in Europe)… Most rare
disease patients (50 to 75%) show onset at
birth or in childhood. As many as 30% of
rare diseases patients die before the age of
five years. Furthermore, each rare disease
patient has been estimated to cost a total
of $5 million throughout their lifespan.
76
Liu, Zhichao, et al. “Toward Clinical Implementation of Next-Generation Sequencing-Based Genetic testing in Rare Diseases: Where Are We?”
Trends in Genetics, vol. , no. , Nov. .
National Center for Advancing Translational Sciences, Genetic and Rare Diseases Information Center. “FAQs About Rare Diseases.” https://
rarediseases.info.nih.gov/diseases/pages//faqs-about-rare-diseases. Accessed May .
Liu, Zhichao, et al. “Toward Clinical Implementation of Next-Generation Sequencing-Based Genetic testing in Rare Diseases: Where Are We?”
Trends in Genetics, vol. , no. , Nov. .
Chung, B.H.Y., et al. “Rare versus Common Diseases: a False Dichotomy in Precision Medicine.” npj Genomic Medicine, vol. , no. , Feb.
. doi:./s---x.
The Genetic and Rare Diseases Information Center
reports that 25-30 million people in the U.S. have a
rare disease, and over 350 million people worldwide
are afflicted.
77
Approximately 1 in 10 individuals
has a rare disease, so collectively rare diseas-
es have a significant population impact.
Liu, Zhu, Roberts, and Tong further report that:
An incomplete knowledge of the natural history
of each rare disease can make a substantial
proportion (~60%) of rare diseases intractable
and undiagnosable. Panel-based NGS or
targeted sequencing tests are designed to
reveal causal mutations for genes known to
be associated with a specific rare disease.
Since the NGS gene panel is predesigned or
expert-selected, ultradeep, uniform coverage
allows for high sensitivity and also for specific
variant calling for rare genetic variants.
78
By deploying genetic and genomic testing, up to
and including whole genome sequencing, diagnos-
tic odysseys may be ended for many patients—not
only providing a pathway to appropriate treatment
but also reducing significant waste in the health-
care system and the associated costs of incorrect
diagnosis. Even if no treatment is available, peace of
mind can result through simply having an “answer”
and being able to end the costly hunt for diagnosis.
In discussing rare diseases and the application of
sequencing, Chung, Chau, and Wong report on
impressive results from sequencing adoption:
79
Affected individuals often endure years of
diagnostic odyssey, which is not only fruitless
but more expensive than sequencing their
40
genomes upfront
80,81
. For infants admitted
to intensive care within the first 100 days of
life, sequencing produced diagnostic yields
of 36.7%; and in 52.0% of the diagnosed,
medical management was affected.
82
Results
improved to 50.8% and 71.9%, respectively,
when trio sequencing was conducted. Other
studies have given similar results.
83
Ranging from individual genetic tests through to
complete whole-genome sequencing, the full range
of genetic and genomic tools and technologies are
now being deployed in clinical diagnostic testing.
“Besides targeted sequencing, there are increasing
applications of whole-genome sequencing/whole-ex-
ome sequencing (WGS/WES) to detect complex
genetic variants and provide complete genetic
information in support of rare disease diagnosis.”
84
This statement recognizes that while many rare
diseases may be associated with a single gene (i.e.,
Mendelian), there are also many challenging diseases
where multiple genes come into play (polygenic).
Tan, T. Y., et al. “Diagnostic Impact and Cost-effectiveness of Whole-exome Sequencing for Ambulant Children with Suspected Monogenic
Conditions.” JAMA Pediatrics, vol. , Sept. .
doi:./jamapediatrics...
Farnaes, L., et al. “Rapid whole-genome sequencing decreases infant morbidity and cost of hospitalization.” npj Genomic Medicine, vol. , no.
, Apr. . doi:./s---.
Meng, L., et al. “Use of Exome Sequencing for Infants in Intensive Care Units: Ascertainment of Severe Single-gene Disorders and Effect on
Medical Management.” JAMA Pediatrics, vol. , no. , Dec. . doi:./jamapediatrics...
Wright, C. F., et al. “Pediatric Genomics: Diagnosing Rare Disease in Children.” Nature Reviews Genetics, vol. , no. , Feb. .
doi:./nrg...
Liu, Zichao, et al. “Editorial: Advancing Genomics for Rare Disease Diagnosis and Therapy Development.” Frontiers in Pharmacology, vol. , no.
, September .
Collins, Francis S., et al. “Perspective: Human Molecular Genetics and Genomics – Important Advances and Exciting Possibilities.” The New
England Journal of Medicine, vol., no. , Jan. .
Centers for Disease Control and Prevention. “Sickle Cell Disease.” www.cdc.gov/ncbddd/ sicklecell/data.html#:~:text=In%the%United%
States&text=It%is%estimated%that%A,every%%C%Hispanic%DAmerican%births. Accessed May .
b. Diagnosing Single Gene (Mendelian)
Disease and Disorders
Mendelian, single-gene diseases represent an ex-
tremely large compendium of diseases and disorders.
As noted by Collins, Doudna, Lander, and Rotimi, “The
discovery of genes responsible for more than 5000 rare
mendelian diseases has facilitated genetic diagnostics
for many patients, pregnancy-related counseling, new
drug treatments, and in some cases, gene therapies.”
85
A number of these single gene-associated diseas-
es and disorders have a substantial impact across
populations and within certain sub-populations.
Some of the more widely known examples include:
• Cystic Fibrosis (CF)—a progressive, genetic
disease that causes persistent lung infections and
limits the ability to breathe over time. In people
with CF, mutations in the cystic fibrosis trans-
membrane conductance regulator (CFTR) gene
cause the CFTR protein to become dysfunctional.
CF affects approximately 30,000 Americans.
86
• Alpha- and beta-thalassemias—inherited
genetic blood disorders causing the body to
Whole genomic sequencing and whole exome sequencing are eliminating the phenomenon of the
diagnostic odyssey for rare genetic disease: it’s realistic today to have a genome or exome test ordered at
first subspecialist outpatient visit and to have a diagnosis by the time of the second visit . This is clearly
the most powerful diagnostic tool ever developed for the millions of children with rare diseases.”
Stephen Kingsmore. “Luminaries Share Their Thoughts on Advances in ‘Omics Over the Past Five Years.” Clinical Omics
Magazine, vol. 6, no. 2, March-April 2019.
41
make fewer healthy red blood cells and less
hemoglobin than normal. Beta-thalassemia
affects at least 1,000 people in the U.S.; how-
ever, the exact prevalence is not known.
87
• Sickle cell disease—an inherited group of dis-
orders in which red blood cells are misshapen
into a sickle shape. The cells die early, leaving
a shortage of healthy red blood cells (sickle
cell anemia), and can block blood flow. The
Centers for Centers for Disease Control and
Prevention (CDC) estimate that the disease
affects approximately 100,000 Americans.
88
• Fragile X Syndrome (FXS)—a genetic disease
that causes mild to severe intellectual disability,
with typical associated symptoms including
delays in talking, anxiety, and hyperactive be-
havior. The exact number of people who have
FXS is unknown, but a review of research studies
estimated that about 1 in 7,000 males and about 1
in 11,000 females have been diagnosed with FXS.
89
• Huntington’s Disease—a fatal genetic disorder
that leads to progressive breakdown of nerve
Challenge TDT. “Beta-Thalassemia Overview.” www.challengetdt.com/beta-thalassemia-overview. Accessed May .
Centers for Disease Control and Prevention. “Sickle Cell Disease.” www.cdc.gov/ncbddd/ sicklecell/data.html#:~:text=In%the%United%
States&text=It%is%estimated%that%A,every%%C%Hispanic%DAmerican%births. Accessed May .
Centers for Disease Control and Prevention. “Fragile X Syndrome.” www.cdc.gov/ncbddd/fxs/ data.html. Accessed May .
Yohrling, George, et al. “Prevalence of Huntington’s Disease in the US.” Neurology, vol. , no. , , Apr. .
cells in the brain. Huntington’s Disease is esti-
mated to affect more than 40,000 Americans.
90
The undiagnosed diseases network (UDN), a multidisciplinary collaboration evaluating patients
who have with complex presentations and have remained undiagnosed despite extensive clinical
investigation, performed in depth clinical evaluations along with exome and whole genome
sequencing, metabolomics testing, and studies in model organisms. From 2015 to 2017, the UDN
accepted 601 of 1519 patients referred for evaluation. Of the first 382 patients with a completed
evaluation, 132 (35%) received a diagnosis; these included 31 (11%) with new syndromes. Among
diagnosed patients, the majority (58%) had medical care changes, such as changes in therapy or
shortening of the diagnostic odyssey.”
Teri A. Manolio et al. 2019. “Genomic Medicine Year in Review: 2019.” The American Journal of Human Genetics, vol. 105, 5
Dec. 2019. Reporting on original research by Splinter, K., et al. The New England Journal of Medicine, vol. 379, 2131-2139, 2018.

42
Case Study in Whole Genome Sequencing for Diagnosis: Project
Baby Bear, California
As noted in ClinicalOmics, a pilot program in California funded by the state “showed that precision
medicine for critically ill babies enrolled in California’s Medicaid program reduced their suffering and
yielded better health outcomes, while decreasing the cost of their healthcare, saving the Golden State
$2.5 million.”
91
The initiative, named Project Baby Bear, deployed rapid Whole Genome Sequencing
(rWGS) as the core approach. Stephen Kingsmore, the President and CEO of Rady Children’s Institute
for Genomic Medicine, which led the project, notes that:
For seriously ill children who are hospitalized in intensive care units, the most significant advance
has been ultra-rapid whole-genome sequencing. It’s routinely possible now to examine nearly
every genetic disease and either make a diagnosis or rule out genetic disease, in 36 hours. That’s
fast enough to guide weighty management decisions in even the most seriously ill children. Where
rapid whole-genome sequencing is absolutely transformative is in seriously ill children in whom a
genetic disease was not suspected at test order. Those children were, with the best intentions in
the world, being treated for the wrong diagnosis.
92
In describing the results of the pilot project, Kingsmore reports that the project (which used WGS for Medi-
Cal-enrolled infants in intensive care units at five California children’s hospitals) had compelling results:
In 720 infants in intensive care units tested so far, one in three received the genetic disease di-
agnosis, and in about 1/3, we are able to exclude genetic disease as the course of illness. One in
four infants has a change in care as a result of rapid whole genome sequencing. One in five has a
change in outcome.
93
A recent review report on the results of Project Baby Bear shows that over 23 months, the project:
• Completed rWGS on 178 babies and families.
• Provided diagnoses for 76 babies (43%).
• Led to a change in the management of 55 babies (31%) that resulted in fewer hospital days, fewer
procedures or new therapies.
• Diagnosed 35 rare conditions that occur in less than one in one million births.
• Achieved a three-day turnaround time for provisional results.
94
The study also demonstrated that this clinical application of whole genome sequencing “reduced
healthcare costs and downstream spending, primarily by empowering doctors to eliminate unneces-
sary procedures and discharge babies sooner.”
95
In a retrospective analysis of the program’s economic
impacts, it is concluded that:
By introducing Medi-Cal babies into a coordinated system of care that included physicians trained
in identifying babies likely to benefit from whole genome sequencing, lab interpretation scien-
tists, genetic counselors and others, the state of California saved millions of dollars in healthcare
expenses due to avoided procedures and shorter hospital stays… The avoided procedures and
reduced hospital time amounted to $2.5 million in cost savings. These cost savings stemmed from
changes in the medical management of just 29 babies who received significant benefit from ge-
nome sequencing.
96
“Rady Children’s Helps California’s Project Baby Bear Improve Outcomes, Save $.M.” Clinical Omics Magazine, June .
Kingsmore, Stephen. “Luminaries Share Their Thoughts on Advances in ‘Omics Over the Past Five Years.” Clinical Omics Magazine,
vol. , no. , March-April .
Ibid.
Rady Children’s Hospital – San Diego. “Project Baby Bear Final Report: Period Covering July , – June , .” Report to the
State of California.
Ibid.
Ibid.
43
c. Diagnosing Complex (Polygenic)
Diseases and Disorders
Most common debilitating diseases are not caused by
mutation of a single gene, rather they are influenced
by combinations of mutations in many genes each
having a small effect (but combining to have the
potential for large effects). Complicating the situation
is that the expression, regulation, or products of these
genes may be a result of interactions with a multiplic-
ity of environmental factors. There is thus a complex
“soup” of genetic and environmental factors at play in
many common diseases. While these diseases have
a complex etiology that does not mean that progress
cannot be made. Indeed, with the sophisticated tools
of next generation sequencing and advanced compu-
tational analytics, significant biological and mechanis-
tic insights are being produced. As noted in a recent
perspective in the New England Journal of Medicine:
The discovery of more than 100,000 robust
associations between genomic regions
and common diseases has pointed to new
biologic mechanisms, such as the role of
microglia in Alzheimer’s disease, autophagy
in inflammatory bowel disease, and synaptic
pruning in schizophrenia. It has also enabled
the development of polygenic risk scores
to identify patients at increased risk for
heart disease, breast cancer, and other
Collins, Francis S., et al. “Perspective: Human Molecular Genetics and Genomics – Important Advances and Exciting Possibilities.” The New
England Journal of Medicine, vol., no. , Jan. .
Helbig, I. et al. “The ClinGen Epilepsy Gene Curation Expert Panel—Bridging the Divide Between Clinical Domain Knowledge and Formal Gene
Curation Criteria”. Human Mutation, vol. , no. , Nov. .
conditions, although additional rigorous
testing of such scores is needed, including
evaluation of clinical outcomes.
97
For many common diseases, the challenge of
identifying and characterizing genetic effects
is not insignificant. In the case of epilepsy, for
example, analysis of the ClinGen Epilepsy Gene
Curation Expert Panel indicates 2,702 genes as-
sociated with epilepsy, a disorder that affects
approximately 50 million people worldwide.
98
Multifactorial inheritance disorders are caused by a
combination of environmental factors and mu-
tations in multiple genes. For example, different
genes that influence breast cancer susceptibility
have been found on chromosomes 6, 11, 13, 14, 15, 17,
and 22. Some common chronic diseases are multi-
factorial disorders, with examples including:
• heart disease
• high blood pressure
• Alzheimer’s disease
• arthritis
• diabetes
• cancer, and
• obesity.
Eric D. Green, et al. “Perspective: Strategic Vision Medi-
cineNet. “Genetic Diseases (Disorder Definition, Types, and
Examples). www.medicinenet.com/genetic_disease/article.
htm. Accessed 12 May 2021.
Building on the recent successes of unraveling the genetic underpinnings of rare and undiagnosed
diseases, the field is poised to gain a more comprehensive understanding of the genetic
architecture of all human diseases and traits. However, myriad complexities can be anticipated. For
example, any given genomic variant may affect more than one disease or trait; can confer disease
risk or reduce it; and connect additively, synergistically, and/or through intermediates.”
Eric D. Green, et al. “Perspective: Strategic Vision for Improving Human Health at the Forefront of Genomics.” Nature,
vol. 586, 29 October 2020.
44
4. Rational Drug Development
Pharmaceuticals (drugs) are a fundamental part of the
armamentarium of medicine, providing the means
to treat and ameliorate the symptoms of disease, and
in some cases, cure the disease. Pharmaceuticals
bring relief to millions worldwide and have greatly
extended the average human lifespan and quality of
life across that lifespan. Traditionally, drug discovery
has used a trial-and-error approach whereby a library
of chemical substances is tested on cultured cells or
animals and evaluated for its effects. Molecules gen-
erating an apparent positive effect are then brought
forward into clinical trials to evaluate effectiveness
on a disease in humans. Rational drug development,
however, takes a different approach, one in which
biomarkers or preidentified druggable targets that
are present in, or generated by, a disease may serve
as molecular targets for purposefully designed drugs
designed to bind to the target. Human genetics and
genomics assist in this approach in multiple ways:
• Helping to identify biomarkers, protein targets,
etc. through comparative analysis of disease
affected patients versus healthy individuals.
• Identifying genetic variations across indi-
viduals impacted by the disease that may
influence the effectiveness of a designed
drug (often related to differences in metab-
olism) and potential adverse side effects.
• As noted by Dugger, Platt and Goldstein,
99
sequencing can inform understanding of “the
phenotypic effects of a spectrum of rare muta-
tions ranging from loss-of-function to gain-of-
function mutations within a single gene” and can
provide “information on the putative efficacy and/
or toxic effects resulting from the modulation
of that particular gene product in humans. This
knowledge thereby builds confidence in the
rationale for targeting that gene product for the
Dugger, Sarah A., et al. “Drug Development in the Era of Precision Medicine.” Nature Reviews Drug Discovery, vol. , no. , Dec. .
Ibid.
The PCSK gene provides instructions for making a protein that helps regulate the amount of cholesterol in the bloodstream.
Dugger, Sarah A., et al. “Drug Development in the Era of Precision Medicine.” Nature Reviews Drug Discovery, vol. , no. , Dec. .
Ciriello Pothier, Kristen. Personalizing Precision Medicine. A Global Voyage from Vision to Reality. John Wiley & Sons, Inc., .
treatment of a more common human disease,
rather than relying on information gained from
less predictive animal or cellular models.”
100
The application of genetics and genomics to drug
development has resulted in multiple clinical success-
es. Monoclonal antibodies for the treatment of various
immune-mediated conditions (targeting the protein
interleukin-23 for example, produced by the IL23R
gene) are already used clinically. Similarly, mutations
in the PCSK9
101
gene were identified in families with
autosomal dominant hypercholesterolemia, and
pursuing PCSK9 as a drug target resulted in the FDA
“approving two monoclonal antibodies (alirocumab
and evolocumab) for the treatment of high cholesterol
not adequately controlled by statins or diet.”
102
Put
simply, since genes code for proteins, and proteins
(and nucleic acids) are typical biomolecular targets
for drugs, understanding the relationship between
genes and disease provides potential for rationally
identifying drug targets. Kalydeco, a targeted drug for
cystic fibrosis, approved by FDA in 2012, resulted from
rational drug development informed by genomics.
Cystic fibrosis is characterized by physical responses
to the abnormal flow of salt and fluids in and out
of the cells in different parts of the body. Kalydeco
specifically “acts on the gating defect associated
with the CFTR protein [coded by the CFTR gene],
helping to open up the blocked chloride channels.”
103
It is interesting to note that, in some regards, ge-
netics and genomics advancements have helped
to rebalance pharmaceutical research in terms of
work on chronic diseases versus rare diseases. Given
the prior trial-and-error model, it was in the best
interests of the industry to concentrate resources
on major chronic conditions in search of blockbust-
er drugs. Costly and with a high failure rate, drug
companies had to triage their funds towards areas
with the most promise for financial return. Modern
genomics, however, and the large-scale identification
45
of gene-disease associations has provided a more
target-rich environment to address rarer, typically
monogenic, disorders
104
—while at the same time
showing that many of the more common diseases are
highly complex genetically, with sometimes hundreds
and even thousands of genes engaged. The result is
noted in a recent 2021 study by the Biotechnology
Innovation Organization (BIO), which reports that:
Throughout the last decade, industry investment
and drug development have pivoted towards
rare, congenital diseases. Specific examples
of clinical and commercial successes have
encouraged this transition. Drivers of these
successes include targeting molecularly defined
causes of disease, regulatory incentives, and
favorable reimbursement environments.
105
The BIO report provides a review of two longitudinal
datasets on drug development, and notes that:
One large difference between this 2011–2020
dataset and the previous 2006–2015 iteration
is the intensifying focus on rare diseases. Our
latest analysis includes 1,256 phase transitions
within rare diseases, a considerable increase over
the 521 noted in the previous study. This spans
685 different lead developers (not including
those listed solely as partners). This indicates
that companies view pivoting to rare disease
clinical development as a sound strategy.
106
The BIO authors note that drug development for
rare disorders has had “notably more successful
than industry averages—and in particular chronic,
highly prevalent diseases.” They also note that:
A greater understanding of human disease—
whether at the molecular or genomic
level—ultimately leads to the investigation
Rare diseases have often been termed “orphan diseases” in that they represent a class of disease that had not been “adopted” by the
pharmaceutical industry.
Thomas, David. Clinical Development Success Rates and Contributing Factors –. BIO, Informa Pharma Intelligence, and QLS, Feb.
.
Ibid.
Ibid.
Ibid.
of personalized medicine. Indications are
increasingly segmented by biomarkers in
order to match patients with the treatments
most likely to show the greatest benefit,
according to the underlying drug mechanism
and disease pathophysiology. We identified
767 phase transitions out of 12,728 (6%) that
incorporated patient preselection biomarkers
in their corresponding clinical trial design.
This was accomplished by mapping Informa
Pharma Intelligence’s Biomedtracker
and Trialtrove databases, to provide the
supplemental level of clinical trial detail.
107
This later statement is important—showing that phar-
maceutical companies are now able to use genetic
and genomic information to target the trials of their
biopharmaceutical molecules to patients who have
been preselected through the presence of biomarkers
(often genetic). This has the potential to advance
more drugs successfully towards market since they
are more likely to demonstrate efficacy in their trials
by virtue of being rationally targeted. The BIO authors
conclude that the data they have reviewed “builds
confidence in the pursuit of drug development
programs targeted at biomarker-enriched patient
populations. Such assets are likely to advance through
clinical development with lower levels of attrition and
should in theory improve patient outcomes via the
advent of increasingly personalized medicine.”
108
What
is evident is that advancements in human genetics
and genomics are not only delivering definitive
diagnoses for patients, guiding their care; they
are also highly contributory to the development of
new therapeutics to treat identified conditions.

46
Case Study: Genomics and Rational Drug Development in Cancer
As noted by Collins, Doudna, Lander, and Rotimi: “Studies of cancer genomes have revealed hundreds of genes in
which somatic mutations propelled tumor initiation and growth, information that has fueled the development of
new drugs.”
109
Two long-standing cancer drugs, developed with the help of genetics and genomics, are illustrative:
• Approved by the FDA in 1998, Herceptin is a drug that targets metastatic breast cancer cells that overproduce
the HER2 protein (a product of the HER2 gene). It was the first approved targeted drug based on an individu-
al’s genetics, and also came with development of an FDA approved companion diagnostic called HercepTest.
Herceptin has had robust results in achieving improved outcomes in patients. Pothier reports that an October
2014 study showed the overall 10-year survival rate for patients to be 84% for those treated with Herceptin and
chemotherapy, versus 75% for patients treated with chemotherapy only.
110
• Another example of rational drug development for cancer is Gleevec (imatinib). Chronic myeloid leukemia
(CML) is fundamentally a genetic disease caused by mutations in a patient’s DNA that translocate genes be-
tween chromosomes 9 and 22. The translocated genes generate a hybrid gene that produces a novel protein
that causes greatly accelerated production of white blood cells in bone marrow (i.e., a cancer, as defined by
uncontrolled cell proliferation). According to the American Cancer Society, CML comprises circa 15% of leuke-
mias in adults. Without a cure, a diagnosis of CML used to mean a best-case survival of 5 years post diagnosis
through aggressive cytotoxic chemotherapy with severe side effects. The discovery of the gene translocation
causation of CML provided scientists with a target for therapeutic development. Oncologist Brian Druker de-
veloped a targeted drug, Gleevec (imatinib) and collaborated with Novartis to bring the drug forward through
clinical trials. The drug was a success, effectively blocking the effect of the translocated genes. For the great
majority of CML patients, Gleevec is a highly effective treatment—providing a pathway to normal life expec-
tancy through a pill taken once a day with only mild side effects. Gleevec is not a “cure”, because the mutated
genes are still present, but the drug is highly effective at stopping these genes from causing leukemia. In up to
30% of cases, patients start to develop resistance to Gleevec, but ongoing research has resulted in the develop-
ment of two alternative drugs aimed at the hybrid gene, Sprycel (dasatinib) and Tasigna (nilotinib) enabling
a patient to switch between drugs if resistance develops. Through increasingly sensitive diagnostic tests of
BCR-ABL gene expression and blood analysis, clinicians are able to monitor the effect of the administered drug,
modify dosage, and switch drugs if resistance development is detected. In effect, the treatment of CML became
one of the pioneers in a personalized approach to genetic medicine.
Other examples are Tarceva (erlotinib) and Iressa (gefitinib) both of which restrict activation of a protein (epidermal
growth factor, or EGFR) which is abnormally active in a subset of lung cancers due to mutations in the protein.
111
The National Cancer Institute (NCI) reports that “as a result of research into the genomic changes associated with
cancer, drugs have been developed to fight the disease in several ways:
• Inhibiting enzymes that trigger the abnormal growth and survival of cancer cells.
• Blocking aberrant gene expression characteristic of cancer cells.
• Halting molecular signaling pathways that are in overdrive in cancer cells.
112
The NCI notes that “these “targeted therapies” specifically combat characteristics of cancer cells that are different
from normal cells of the body. This makes them less likely to be toxic for patients compared to other treatments
such as chemotherapy and radiation that can kill normal cells.”
113
Collins, Francis S., et al. “Perspective: Human Molecular Genetics and Genomics – Important Advances and Exciting Possibilities.” The
New England Journal of Medicine, vol., no. , Jan. .
Ciriello Pothier, Kristen. Personalizing Precision Medicine. A Global Voyage from Vision to Reality. John Wiley & Sons, Inc., .
National Cancer Institute. “Cancer Genomics Overview.” www.cancer.gov/about-nci/organization/ccg/cancer-genomics-overview.
Accessed May .
Ibid.
Ibid.
47
5. Precision Medicine and Targeted
Therapeutics (Pharmacogenetics)
Having an ability to sequence a patient’s whole ge-
nome rapidly and cost-effectively has opened the door
to a new paradigm in healthcare termed “precision
medicine” whereby an individual’s genetic profile is
used to guide decisions made in regard to the preven-
tion, diagnosis, and treatment of disease. As noted by
NHGRI, “knowledge of a patient’s genetic profile can
help doctors select the proper medication or therapy
and administer it using the proper dose or regimen.”
114
The discipline of “pharmacogenomics” (also “phar-
macogenetics”) has grown to be able to deploy
genetic and genomic knowledge and tools to help
physicians select the “right drug and the right dose”
for a patient based on their genome (assuming
there is statistically significant clinical information
linking a drug to specific gene variants in terms of
efficacy and side effects). Pharmacogenetics is an
area of research and, increasingly, clinical practice,
that addresses the genetically determined varia-
tion in how individuals respond to specific drugs in
terms of differences in dose requirement, efficacy,
and the risk of adverse drug reactions (ADRs).
David Khan, in the Journal of Allergy and
Clinical Immunology, notes that:
At its most basic, the term pharmacogenetics
describes any influence that genetics
can have on drug therapy. The newer
National Human Genome Research Institute. “ Glossary of Genetic Terms: Personalized Medicine.” www.genome.gov/genetics-glossary/
Personalized-Medicine. Accessed May .
Khan, David A. “Pharmacogenomics and Adverse Drug Reactions: Primetime and Not Ready for Primetime Tests.” Journal of Allergy and
Clinical Immunology, vol. , no. , Oct. . doi:./j.jaci....
term pharmacogenomics is often used
interchangeably with pharmacogenetics,
but there are some subtle differences.
Pharmacogenetics mainly deals with
single drug-gene interactions. In contrast,
pharmacogenomics incorporates genomics
and epigenetics to look at the effect of multiple
genes on drug responses. Pharmacogenomics
is considered the future of drug therapy
and is a rapidly growing field in the area
of precision (personalized) medicine.
115
Pharmacogenetics and pharmacogenom-
ics enable three principal pathways to the
improvement of clinical outcomes:
1. Selection of the therapeutic (among multiple
choices) that is likely to prove most efficacious
based on the patient’s genome and a drug’s
proven efficacy for their specific genotype.
2. Ruling-out a therapeutic (among multiple
choices) based on the patient’s genome and
a drug’s potential for unacceptable adverse
side effects given their specific genotype.
3. Development of an optimized drug dosage for a
patient based on their genotype’s influence on
the rate at which they will metabolize the drug.
In terms of this last benefit, Namandie
Bumpus notes that:
Owing to genetics, people can be categorized
as poor, intermediate, extensive, or ultrarapid
The ability to tailor a drug regimen to a specific genetic code that is truly personalized to that
specific DNA double helix has been a dream of researchers, physicians, and patients alike. Advances
in precision medicine, specifically around the genome…are making this dream a reality.”
Kristen Ciriello Pothier. 2017. Personalizing Precision Medicine. A Global Voyage from Vision to Reality. John Wiley &
Sons, Inc., 2017.

48
metabolizers of certain drugs. For example, in
the case of a drug that is pharmacologically
active and its metabolites inactive, a drug may
accumulate in the body of a poor metabolizer
and toxicity could occur as a result. By contrast,
someone who is an ultrarapid metabolizer of
the same drug may not achieve concentrations
of the drug in their blood that are high enough
to be effective. For prodrugs, where the parent
drug is inactive or substantially less active
than its metabolite, genetically encoded
variation in drug metabolism could affect the
ability of a person to activate the drug.
116
In regard to adverse drug reactions,
David Khan points out that:
Medications are a cornerstone of the therapeutic
armamentarium for most clinicians. The goal of
pharmacotherapy is to cure or control a specific
condition or disease without causing adverse
effects. Unfortunately, adverse drug effects are
common and not always predictable. Adverse
drug reactions (ADRs) have been defined as
reactions that are noxious and unintended
Bumpus, Namandje N. “For Better Drugs, Diversity Clinical Trials.” Science, vol. , no. , Feb. . doi:./science.abe.
Khan, David A. “Pharmacogenomics and Adverse Drug Reactions: Primetime and Not Ready for Primetime Tests.” Journal of Allergy and
Clinical Immunology, vol. , no. , Oct. . doi:./j.jaci....
Ibid.
Relling, Mary. “Luminaries Share Their Thoughts on Advances in ‘Omics Over the Past Five Years.” Clinical Omics Magazine, vol. , no. , March-
April .
and occur at doses normally used in human
subjects. ADRs can be related to a number of
factors, including known pharmacologic activity
of a drug, drug interactions, drug toxicity, and
drug hypersensitivity. ADRs are a relatively
common cause of morbidity and mortality.
117
He further notes that “genetic factors can play a role
in pharmacokinetics, pharmacodynamics, and sus-
ceptibility to hypersensitivity responses. The degree
to which genetics contributes to ADRs is not entirely
clear and varies by drug, as well as the type of ADR.”
118
Keeping up with the research literature in regard to
pharmacogenetics and pharmacogenomics findings
regarding specific drugs has been an expanding
challenge for clinicians. However, several leading
organizations have been collaborating, and reliable
peer-reviewed compendia resources for recom-
mendations have come online. This is highlighted
by Mary Relling, who reports that the application of
pharmacogenomics to improving healthcare is being
codified through “a number of users converging
on key peer reviewed, nonprofit curated genomic
medicine resources to guide clinical actions, such
as ClinVar, ClinGen, the Clinical Pharmacogenetics
Implementation Consortium (CPIC), and PharmGKB.”
119
a. Targeting to Increase Effectiveness
When a healthcare provider is considering prescribing
a drug the knowledge of the patient’s genotype can
now be used in many cases to guide the therapeutic
strategy, identify the most effective drug, determine
appropriate dosing, and assess the risk of toxicity or
other negative side effects. The U.S. Food and Drug
Administration (FDA) provides a list of pharmaco-
genetic associations that it has evaluated and for
which it considers there to be “sufficient evidence
to suggest that subgroups of patients with certain
genetic variants, or genetic variant-inferred pheno-
types, are likely to have altered drug metabolism,
In cancer, tumor profiling is typically
performed for patients: with cancer with
an unknown primary; with cancer that has
not responded to standard treatments;
and, to help guide decision-making when
there are multiple treatment options. De-
pending on the clinical situation, the test-
ing method may be a single gene, a gene
panel, whole exome, or less commonly,
whole genome sequencing.
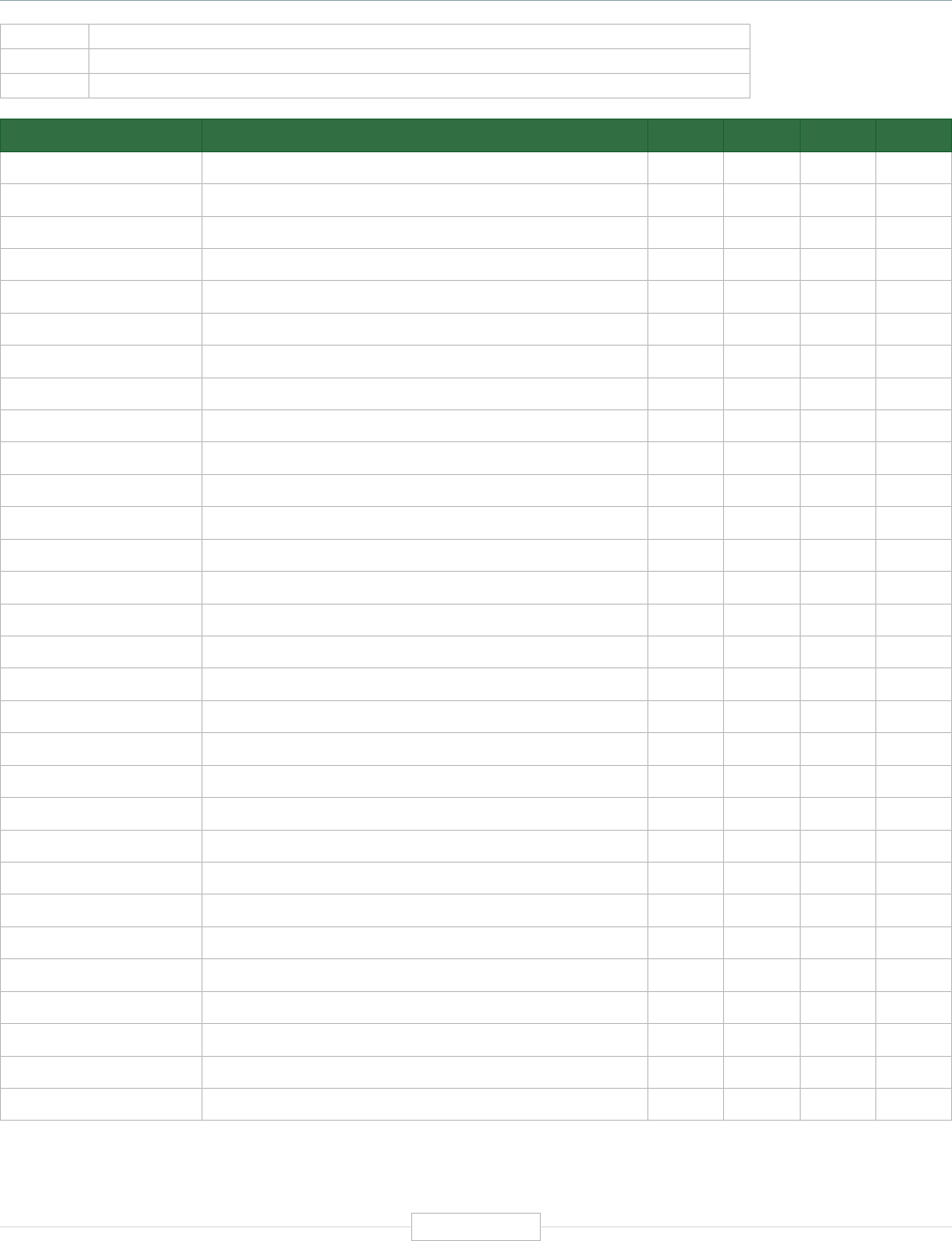
49
Table 8: Analysis of FDA Recognized Pharmacogenetic Associations
A1 = Data support therapeutic management recommendations
A2 = Data indicate a potential impact on safety or response
A3 = Data demonstrate a potential impact on pharmacokinetic properties only
Macro Category Disease or Condition A1 A2 A3 Sum
Cancer Cancer - Breast
2 1 3
Cancer Cancer - Leukemia
2 1 3
Cancer Cancer - Colon
2 2
Cancer Cancer - Lung
2 2
Cancer Cancer - Peripheral T-Cell Lymphoma
1 1
Cancer Cancer - Rectal
1 1
Cancer Cancer - Bladder
1 1
Cancer Cancer - Skin
1 1
Cancer Cancer - Kidney
1 1
Cancer Cancer - Soft Tissue Sarcoma
1 1
Cardiovascular Heart Attack or Stroke (blood thinners)
2 2
Cardiovascular Hypertension (high blood pressure)
1 1 2
Cardiovascular Cardiac Arrythmia
1 1 2
Cardiovascular Hypercholesterolemia
1 1
Dermatologic Eczema
1 1
Gastroenterological Nausea and Vomiting (antiemetics)
3 3
Gastroenterological GERD/Acid Reflux
2 1 3
Gastroenterological Ulcerative Colitis
1 1 2
Hematologic Thrombocytopenia
1 1
Immunologic Transplant Rejection (immune suppression)
1 1
Infectious Disease HIV/AIDS
2 1 1 4
Infectious Disease Tuberculosis
1 1
Infectious Disease Bacterial Infections
1 1
Metabolic Obesity
1 1
Metabolic Gaucher’s Disease
1 1
Muscle Rare Muscle Disorders
2 2
Muscle Muscle Relaxation for Surgery/Intubation
2 2
Neurologic Convulsions/Seizures
3 2 1 6
Neurologic Pain (treated by anti-inflammatories, narcotics, etc.)
3 1 1 5
Neurologic Nerve Pain (treated by antidepressants)
4 4
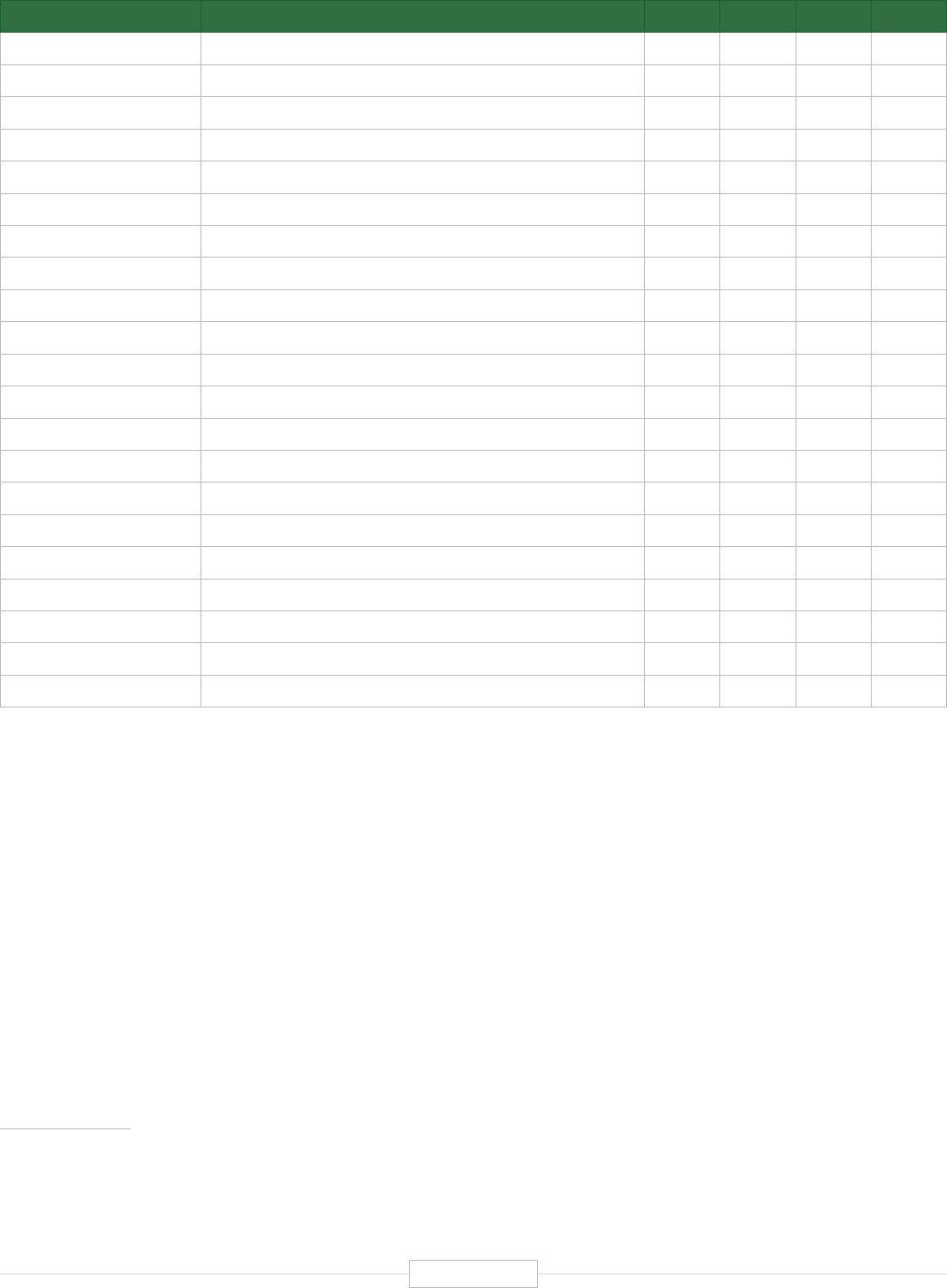
50
Macro Category Disease or Condition A1 A2 A3 Sum
Neurologic Tourette’s Syndrome
2 2
Neurologic Huntington’s Disease (Chorea)
2 2
Neurologic Narcolepsy
1 1
Neurologic Opioid Withdrawal
1 1
Neurologic Multiple Sclerosis
1 1
Neurologic Tardive Dyskinesia
1 1
Neurologic Alzheimer’s Disease/Dementia
1 1
Neurologic Insomnia
1 1
Psychiatric Depression
5 5 10
Psychiatric Schizophrenia
6 1 7
Psychiatric ADHD
2 2
Psychiatric Bipolar Disorder
2 2
Psychiatric Obsessive Compulsive Disorder
1 1
Psychiatric Anxiety
1 1
Psychiatric Female Hypoactive Sexual Desire Disorder
1 1
Rheumatologic Rheumatoid Arthritis
2 1 3
Rheumatologic Osteoarthritis
2 2
Rheumatologic Gout
1 1
Rheumatologic Dry Mouth in Sjogren’s Syndrome
1 1
Urologic Overactive Bladder/Incontinence
1 1 2
Urologic Kidney Stones
1 1
Note: An individual drug may have more than one disease or condition for which it has application. For example, the drug Irinotecan is used for both
colon cancer and small cell lung cancer, and the UGTA gene is associated with the metabolism of the drug and thus is of effect for both types of
cancers for which Irinotecan may be prescribed.
Source: TEConomy Partners analysis of FDA Table of Pharmacogenetic Associations.
and in certain cases, differential therapeutic effects,
including differences in risk of adverse events.”
120
While cancer is perhaps the most well recognized
cluster of disease for which genetic tests may impact
drug selection and dosing, analysis of FDA data
(Table 8) shows that pharmacogenetic associations
are in place for multiple chronic diseases and con-
ditions, covering applications in major categories
such as cardiovascular disease, gastroenterological
U.S. Food & Drug Administration. “Table of Pharmacogenomic Associations.” www.fda.gov/ medical-devices/precision-medicine/table-
pharmacogenetic-associations. Feb. .
diseases and disorders, infectious diseases, neuro-
logical diseases and disorders, psychiatric conditions,
and rheumatologic disease. Pharmacogenetic
associations now span a range from relatively rare
diseases such as Tourette’s syndrome and Tardive
dyskinesia to common conditions such as hy-
percholesterolemia and depression (with over 50
listed in Table 8). There are more than 100 drugs for
which the associations are now listed by the FDA.

51
Case Study: Pharmacogenetics/Pharmacogenomics
and Cancer Treatment Efficacy
Cancer is a genetic disease. As noted by the National Cancer Institute (NCI):
Cancer is a group of diseases caused by changes in DNA that alter cell behavior, causing uncon-
trolled growth and malignancy. These abnormalities can take many forms including DNA muta-
tions, rearrangements, deletions, amplifications, and the additional removal of chemical marks.
These changes can cause cells to produce abnormal amounts of particular proteins or make
misshapen proteins that do not work as they should. Oftentimes a combination of several genomic
alterations work together to promote cancer . Genetic alterations can be inherited from one’s par-
ents, caused by environmental factors, or occur during natural processes such as cell division. The
changes that accumulate over one’s lifetime are called acquired or somatic changes and account
for 90 to 95% of all cases of cancer. The field of cancer genomics is a relatively new research area
that takes advantage of recent technological advances to study the human genome, meaning our
full set of DNA. By sequencing the DNA and RNA of cancer cells and comparing the sequences to
normal tissues such as blood, scientists identify genetic differences that may cause cancer. This
approach, called structural genomics, may also measure the activity of genes encoded in our DNA
in order to understand which proteins are abnormally active or silenced in cancer cells, contribut-
ing to their uncontrolled growth.
121
The NCI reports that “genomic information about cancer is leading to better diagnosis and treatment
strategies that are tailored to patients’ tumors, an approach called precision medicine. As a result of
research into the genomic changes associated with cancer, drugs have been developed to fight the
disease in several ways:
• inhibiting enzymes that trigger the abnormal growth and survival of cancer cells
• blocking aberrant gene expression characteristics of cancer cells, and
• halting molecular signaling pathways that are in overdrive in cancer cells.”
122
It should also be noted that terms such as breast cancer, pancreatic cancer, or lung cancer, for example,
denote the location in which the cancer presents in the body, but that does not mean that every occur-
rence of these cancers is the same. Each form of cancer is typically characterized by there being many
cancer subtypes that can vary considerably between patients. The NCI notes that genetics and genom-
ics are proving to be an effective tool in characterizing cancer types and subtypes and elucidating their
comparative response to different therapeutic approaches. The NCI reports, for example, that:
Cancer genomics research also contributes to precision medicine by defining cancer types and
subtypes based on their genetics. This molecular taxonomy of cancer can provide patients with
more precise diagnosis, and therefore a more personalized treatment strategy. There are several
ways in which the molecular definition of cancer already benefits patients:
• Breast cancer is classified based on molecular characteristics into distinct subgroups—Lumi-
nal A, Luminal B, triple-negative/basal-like, and HER2 type—that vary in their aggressiveness
and respond differently to therapies. Breast cancer patients may benefit from diagnosis and
treatment guided by knowledge of their tumor’s molecular subtype.
• Diffuse large B cell lymphoma can be divided into the ABC and GCB subtypes by genomic
profiling, identifying patients who respond differently to current chemotherapy regimens and
molecularly targeted therapies.
National Cancer Institute. “Cancer Genomics Overview.” www.cancer.gov/about-nci/organization/ccg/cancer-genomics-overview.
Accessed May .
Ibid.
52
• In 2013, The Cancer Genome Atlas project identified four subtypes of endometrial cancer—
POLE ultramutated, microsatellite instability (MSI) hypermutated, copy-number (CN) low,
and CN high—that correlate with patient survival. This research has already given rise to new
clinical trials and investigation of how these subtypes can improve the future of endometrial
cancer care.
• Lung cancer patients who have a gene fusion involving the ROS1 gene often respond well to
treatment with a targeted therapy called crizotinib. In these cases the disease is best defined and
treated based on its unique genetic change.
123
Elaine Mardis reviewing major advancements in omics sciences notes that:
In my opinion, the most significant advances are the increasing numbers of FDA-approved tar-
geted and immunotherapies in cancer, most of which can be correlated to genomic aspects of
cancers including specific genes/mutations of known cancer driver genes, and immunogenomic
metrics such as increased neoantigen load, microsatellite instability in the setting of mismatch
repair defects that predict sensitivity to immune checkpoint blockade inhibitors, or sensitivity to
PARP inhibitors in the setting of homologous repair defects.
124
Because of its nature as a “genetic disease”, cancer has long been on the frontlines in the clinical appli-
cation of advanced genetics and genomics. This has paid off considerably, such that “today, biomarkers
directly connected to drugs, or to crucial outcomes in the human body, allow physicians to identify
drugs that are most likely to help a patient, and those drugs can be used to target cancerous cells only,
which reduces the side effects that the patient experiences.”
125
Ibid.
Mardis, Elaine. “Luminaries Share Their Thoughts on Advances in ‘Omics Over the Past Five Years.” Clinical Omics Magazine, vol. ,
no. , March-April .
Ciriello Pothier, Kristen. Personalizing Precision Medicine. A Global Voyage from Vision to Reality. John Wiley & Sons, Inc., .
b. Reducing Drug Side Effects
According to the U.S. Department of Health and
Human Services (DHHS), an “adverse drug event (ADE)
is an injury resulting from medical intervention related
to a drug. This includes medication errors, adverse
drug reactions, allergic reactions, and overdoses.”
126
In
inpatient settings, the DHHS reports that adverse drug
events account for an estimated 1 in 3 of all hospital
adverse events, affecting about 2 million hospital stays
each year and prolonging hospital stays by 1.7 to 4.6
days. In outpatient settings, adverse drug events each
year account for over 3.5 million physician office visits,
U.S. Department of Health and Human Services. “Adverse Drug Events.” https://health.gov/our-work/health-care-quality/adverse-drug-events.
Accessed May .
Ibid.
an estimated 1 million emergency department visits,
and approximately 125,000 hospital admissions.
127
Variations in individual genomes have been found
to have significant impact on risk for adverse drug
reactions, and there is increasing evidence helping
to guide physician decisions regarding which drugs
to prescribe, which to avoid, and what dosing should
be used to lessen the risk of an adverse event. The
previously cited data for FDA pharmacogenetic
associations lists several drugs for which genetic tests
can identify patients at higher risk for adverse drug
reactions. Examples are shown on Tables 9 and 10.

53
Table 9: Examples of Drugs with Pharmacogenetic Associations with Adverse Reactions
Drug Disease or Disorder Treated Gene Affected Subgroups
Abacavir HIV/AIDS HLA-B *57:01 allele positive
Carbamazepine
Seizures (anticonvulsant), also used in
peripheral neuropathy and bipolar disorder
HLA-B *15:02 allele positive
Lapatinib Breast cancer HLA-DRB1 *07:01 allele positive
Simvastatin Hypercholesterolemia and high triglycerides SLC01B1
521 TC or 521 CC
(intermediate or poor
function transporters)
Warfarin
Blood thinner used in patients with risk of
heart attack or stroke
CYP4F2 V433M variant carriers
Source: TEConomy Partners analysis of FDA Table of Pharmacogenetic Associations.
Table 10: Examples of Drugs with Pharmacogenetic Associations
with Poor Drug Metabolism which may Result in High Systemic
Concentration and Associated Adverse Reactions
Drug Disease or Disorder Treated Gene Affected Subgroups
Amifampridine Rare muscle diseases NAT2 Poor metabolizers
Amphetamine ADHD, Narcolepsy, Obesity CYP2D6 Poor metabolizers
Fluorouracil Skin cancer and actinic keratosis DPYD
Intermediate and
poor metabolizers
Iloperidone Schizophrenia CYP2D6 Poor metabolizers
Tolterodine Overactive bladder CYP2D6 Poor metabolizers
Source: TEConomy Partners analysis of FDA Table of Pharmacogenetic Associations.

54
Case Study: Avoiding Adverse Drug Events in HIV Treatment
Ziagen (abacavir) is a frequently prescribed antiviral for HIV patients. It is an example of an important
medication that, unfortunately, has significant adverse effects for a proportion of patients taking it. Ap-
proximately 3-5% of patients taking Ziagen are hypersensitive to it and may have significant reactions
(including potentially fatal reactions). In the early 2000s, a family of genes were discovered to be asso-
ciated with Ziagen hypersensitivity (those with the HLA-B*57:01 gene variant). Genetic testing defini-
tively identifies whether an HIV positive patient has the gene variant, and it has been found that those
patients without the variant will be free of the hypersensitivity.
As noted by David Khan:
Approximately 3% to 5% of patients treated with abacavir have a hypersensitivity syndrome that
typically appears within the first 6 weeks of therapy and rarely can be fatal. Multiorgan symptoms,
including fever, rash, gastrointestinal symptoms, respiratory symptoms, and hypotension, can occur
along with liver and renal involvement.”… “A landmark study was performed to determine whether
screening patients with HIV-1 for HLA-B*5701 before treatment with abacavir would reduce the inci-
dence of the hypersensitivity reaction. This study was the first to show in a very rigorous manner the
benefits of pharmacogenetic testing in reducing the risk of hypersensitivity reactions. In 2008, the US
Food and Drug Administration issued an alert recommending that all patients should be screened
for the HLA-B*5701 allele before starting or restarting therapy with abacavir or abacavir-containing
medications.
128
Khan, David A. “Pharmacogenomics and Adverse Drug Reactions: Primetime and Not Ready for Primetime Tests.” Journal of Allergy
and Clinical Immunology, vol. , no. , Oct. . doi:./j.jaci....

55
6. Gene Editing and Gene Therapy
Much of the important work of genetics and genomics
has come in the form of identifying gene variants that
are associated with a disease. The identification of a
gene variant that codes for a malformed protein, or
fails to produce an important protein, or otherwise
effects disease etiology, provides potential biomarkers,
or targets, for developing diagnostics and, hopefully,
therapeutic drugs—very useful tools in the clinical
toolkit. But what if instead of treating the effects of
a miscoded gene, we could instead correct (edit) the
gene itself? If we could do that, then the application
of genetics and genomics would not just be to treat
the symptoms of a disease, it would potentially cure
it (particularly in the case of a monogenic disease or
disorder). This is the basis of the development of the
field of gene therapy, which the FDA describes as:
A technique that modifies a person’s genes
to treat or cure disease. Gene therapies can
work by several mechanisms: replacing a
disease-causing gene with a healthy copy
of the gene; inactivating a disease-causing
gene that is not functioning properly, [or]
introducing a new or modified gene into
the body to help treat a disease.
129
Ultimately, gene editing and gene therapy repre-
sent new pathways to the treatment and curing of
diseases, but these approaches are still in the early
stages of clinical application. Part of the caution in
clinical application currently arises from a need for
further study of the potential for off-target gene
edits (mutagenesis) to occur in non-targeted genes
and for unintended mosaicism to occur. Researchers
note, however, that the utility of gene editing is not
only in its potential clinical application, but also
as a powerful tool for investigating gene variation,
function, and linkages to diseases and disorders:
Advances in DNA synthesis and genome
editing allow the field of genomics to progress
U.S. Food & Drug Administration. “What is Gene Therapy.” www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/what-gene-
therapy. Accessed May .
Green, Eric D., et al. “Perspective: Strategic Vision for Improving Human Health at the Forefront of Genomics.” Nature, vol. , Oct. .
from a largely observational (‘reading DNA’)
to more experimental (‘writing’ and ‘editing’
DNA) approaches. Enabling true synthetic
genomics (that is the synthesis modification and
perturbation of nucleic acid sequences at any
scale) will allow for more powerful experimental
testing of hypothesis about genome variation
and function and improve opportunities for
linking genotype to phenotypes. Genome
editing is increasingly being used for practical
applications in medicine such as in gene
therapy, biotechnology and agriculture.
130
There are a variety of types
of gene therapy products,
including:
• Plasmid DNA: Circular DNA molecules can be
genetically engineered to carry therapeutic
genes into human cells.
• Viral vectors: Viruses have a natural ability to
deliver genetic material into cells, and therefore
some gene therapy products are derived from vi-
ruses. Once viruses have been modified to remove
their ability to cause infectious disease, these
modified viruses can be used as vectors (vehicles)
to carry therapeutic genes into human cells.
• Bacterial vectors: Bacteria can be modified to
prevent them from causing infectious disease
and then used as vectors (vehicles) to carry ther-
apeutic genes into human tissues.
• Human gene editing technology: The goals of
gene editing are to disrupt harmful genes or to
repair mutated genes.
• Patient-derived cellular gene therapy prod-
ucts: Cells are removed from the patient, genet-
ically modified (often using a viral vector) and
then returned to the patient.
U.S. Food & Drug Administration. “What is Gene Therapy.”
www.fda.gov/vaccines-blood-biologics/cellular-gene-thera-
py-products/what-gene-therapy. Accessed 12 May 2021.

56
For research, the key advantage of modern gene
editing is the ability to precisely change a gene to
experimentally examine the effect of the change.
Modern technology using CRISPR-Cas9 provides
a highly refined way to silence genes and insert
genes, and even modify a single letter in the ge-
nome. In describing it, Nessa Carey highlights that:
A breakthrough in 2012 ripped open the
genetic fabric of every organism on this
planet, from humans to ants and from rice
to butterflies. It’s giving every biologist in the
world the tools to answer in a few months
questions that some scientists have spent
half their careers trying to address.
131
As a result of both technological and scientific
advancement, the application of gene editing has
progressed from the benchtop, through animal
models, and onwards into human clinical trials and
approved clinical therapeutics. Gene editing is still
in its early days in terms of clinical use, with issues
remaining to be resolved in terms of risk of unintend-
ed off-target changes that may happen along with
intended changes. More work needs to be done before
the full promise of this technology can be realized,
but emerging application areas point to gene editing
and gene therapies’ potential to become import-
ant additional clinical tools for addressing genetic
diseases and disorders. Collins, Doudna, Lander,
and Rotimi capture this optimism, writing that:
After years of ups and downs, some dramatic
successes of gene therapy are emerging, such
as for spinal muscular atrophy and hemophilia.
The pace of this research could increase
dramatically in the future; precisely targeted
genome editing technologies (e.g., CRSPR-Cas9)
now provide new avenues to therapeutics…
as these technologies continue to mature,
it will become increasingly possible to alter
cellular genomes efficiently and accurately.
132
Carey, Nessa. Hacking the Code of Life. How Gene Editing Will Rewrite our Futures. Icon Books, Ltd., .
Collins, Francis S., et al. “Perspective: Human Molecular Genetics and Genomics – Important Advances and Exciting Possibilities.” The New
England Journal of Medicine, vol., no. , Jan. .
The thousands of rare monogenic diseases and
disorders may particularly lend themselves to gene
therapy approaches. However, the development of
such therapies will, at least early on, be limited to
those diseases and disorders that meet some fairly
stringent criteria, which are noted by Carey as follows:
There is a whole list of key factors. Can you be
100% certain that patients you have diagnosed
with the condition all have the same disease?
This rules out disorders like schizophrenia where
there are probably many different forms of the
illness. Do you know exactly how the disease
is caused in your patients? This rules out type
2 diabetes where it isn’t clear which is the key
step in the development of the condition. Do you
know what genetic change you need to create?
This rules out multiple sclerosis, where we think
multiple minor genetic variations interact with
the environment to trigger the condition. Can
you be sure that making the specific edit you
have in mind will prevent or reverse pathology?
This rules out Alzheimer’s disease. Drug trials
A Key Consideration
It should be noted that the discussion of gene edit-
ing and gene therapy pertains to changing non-he-
reditable (somatic) genes—that is changes to an
individual’s genes that will impact the individual but
not then be passed to any children they may subse-
quently have.
There is also ongoing discussion and public debate
about the potential use of gene editing for making
heritable genetic changes (changes to the germ-
line). Such genome edits would result in changes to
an individual’s DNA being passed to their progeny
and subsequent generations. At the present time,
the general consensus of leading organizations in
medical genetics, genetics research, and genetic
counseling is that genome editing that culminates
in human pregnancy should not be currently un-
dertaken and that further research is required into
the scientific, clinical, and ethical implications of
germline editing.
57
targeting what we thought was the key pathway
failed spectacularly recently, and the companies
involved have probably lost billions of dollars as
a consequence. Can you get the gene editing
reagents to the tissues where they are most
needed, in high enough levels? This probably
excludes Parkinson’s disease as the brain is
quite a difficult tissue to access. … Many of the
most common and debilitating conditions aren’t
likely to be good candidates for gene editing
anytime soon, because they are too challenging
in one or more of these problem areas.
133
Even with the above challenges, biomedical research-
ers and clinicians have identified many diseases and
disorders that meet the criteria. Significant prog-
ress has been made in advancing gene therapies
through clinical trials, with examples including:
• Metachromatic leukodystrophy (MLD)—a rare
storage disorder caused by mutations in the
gene coding for arylsulfatase A that results in
affected children failing to develop motor skills,
typically leading to their death by age 10.
• Lipoprotein lipase deficiency—a rare disor-
der in which “patients as young as two have
extremely high levels of cholesterol and suffer
recurring, life-threatening bouts of pancreati-
tis.”
134
The drug Glybera resulted and is the first
approved gene therapy product in clinical use.
• Childhood X-linked adrenoleukodystro-
phy (CCALD)—is a genetically determined
metabolic disorder. Those affected typically
experience “normal development until they
reach 4–10 years of age, at which time behav-
ioral changes including memory impairment
and emotional instability manifest to varying
Carey, Nessa. Hacking the Code of Life. How Gene Editing Will Rewrite our Futures. Icon Books, Ltd., .
Reilly, Philip R. Orphan: The Quest to Save Children with Rare Genetic Disorders. Cold Spring Harbor Laboratory Press, .
Kim, Ji Hyung and Hyon J. Kim. “Childhood X-linked Adrenoleukodystrophy: Clinical-Pathologic Overview and MR Imaging Manifestations at
Initial Evaluation and Follow-up.” RadioGraphics, vol. , no. , May .
National Organization for Rare Disorders. “Rare Disease Database.” www.rarediseases.org/rare-diseases/leber-congenital-amaurosis/. Accessed
May .
The American Society of Hematology. “Gene Therapy for Hemophilia B Found Safe and Effective in First Phase III Trial.” American Society of
Hematology, Dec. , www.hematology.org/newsroom/press-releases//gene-therapy-for-hemophilia-b-found-safe-and-effective-in-
first-phase-iii-trial. Accessed May .
degrees, followed by progressive deterioration
of vision, hearing, and motor function.”
135
• Leber congenital amaurosis (LCA)—is a rare
genetic eye disorder. Affected infants are of-
ten blind at birth, and other symptoms may
include crossed eyes; rapid, involuntary eye
movements; unusual light sensitivity; cataracts;
and/or, keratoconus. In 2017, the gene therapy
Luxturna (voretigene neparvovec-rzyl) was
approved by the FDA to treat children and
adults with two mutations in the RPE65 gene
which includes a type of LCA called LCA2.
136
• Beta-thalassemia—is a rare genetic blood
disorder that reduces the production
of hemoglobin, leading to severe ane-
mia and the need for transfusions.
• Spinal muscular atrophy—is a genetic de-
generative disorder that starts in the central
nervous system and progressively affects all the
muscles in the body. The therapy Spinraza is an
FDA-approved synthetic antisense oligonucle-
otide that binds to RNA, which corrects splicing
errors in the causative genes. Gene therapy with
Zolgensma adds a functional version of the gene.
• Hemophilia B—is a rare genetic bleeding
disorder in which affected individuals have
insufficient levels of a blood protein called factor
IX. “The gene therapy etranacogene dezapar-
vovec substantially increased production of
the blood-clotting protein factor IX among
52 patients in the largest and most inclusive
hemophilia B gene therapy trial to date.”
137
Gene-based therapies are also being successfully
applied in the treatment of selected cancers. An
approach proving successful is CAR-T therapy, which

58
is an abbreviation for chimeric antigen receptor (CAR)
T-cell therapy. The process is described as follows:
First, T cells, a type of immune cell, are taken
from a person’s blood. Then, in the laboratory,
gene replacement therapy is used to add a new
gene to T cells. This new gene adds a special
receptor, called a chimeric antigen receptor
(CAR), to T cells to make CAR-T cells. CAR-T cells
are able to bind to and attack certain cancer
cells. Large numbers of the CAR-T cells are
made in the laboratory, and once a sufficient
amount has been produced, the cells are put
back into the body to fight certain cancers.
138
At its heart, CAR-T cell therapy uses genet-
ics to change a person’s own immune cells
to recognize and fight cancer cells inside the
body. Currently, there are two FDA approved
CAR-T therapies used in clinical oncology:
• CAR-T Lentiviral vector, ex vivo, used
for acute lymphoblastic leukemia.
Explore Gene Therapy. “Get to Know the Different Types of Gene-Based Therapies.” AveXis, Inc. www.exploregenetherapy.com/how-gene-
replacement-therapy-is-different. Accessed Feb. .
Yong, Ed. I Contain Multitudes: The Microbes Within us and a Grander View of Life. Ecco, .
• CAR-T Retroviral vector, ex vivo, used for re-
lapsed or refractory large B-cell lymphoma.
7. Human-Microbe Interactions
None of us go through life truly alone. Each of us
is host to communities of trillions of microbes,
an amount that is considerably larger than the
total count of human cells in our bodies. Ed Yong
highlights this fact quite effectively in the title of
his book I Contain Multitudes and notes: “how
ubiquitous and vital microbes are: they sculpt our
organs, defend us from disease, break down our
food, educate our immune systems, guide our
behavior, bombard our genomes with their genes,
and grant us incredible abilities.”
139
As Yong’s quote
highlights, there is significant biological interaction
between the human genome and microbes.
a. The Human Microbiome
Even though our microbiome has a considerable effect
on our health, for a report focused on human genetics
and genomics it might be asked why one would con-
sider microbes to be within the bounds of this study.

59
Each microbe has its own unique genome, but this re-
port is focused on the human genome. It is a fair point,
but two very interesting findings from recent research
into our microbial passengers points to a distinct hu-
man genome effect—with impacts going in two direc-
tions (microbes impacting our genes and gene expres-
sion, and human genotype impacting the make-up of
the microbial communities humans’ host). In effect,
it has been found that humans are, loosely speaking,
genetic symbiotes. It is a difficult subject matter to
research, requiring access to human sequencing and
metagenomic sequencing of the human microbiome,
but the data collected by the Human Microbiome
Project (HMP) can now be referenced to completed
human genome sequences to make important find-
ings. Providing a signpost to potentially interesting
genetic interactions is the fact that the gut microbi-
omes of monozygotic (“identical”) twins are found to
be significantly more similar than those of dizygotic
(non-identical) twins. The findings in human twins
confirm findings in mouse models that show that
the host (the mouse) genome influences microbiota
composition and that host genotype explains a signif-
icant proportion of variation in the gut microbiome.
140
Additional research on whole genome sequencing and
microbiome metagenome sequencing of participants
in the HMP are compelling, showing that “most mi-
crobes are correlated to genetic principal components,
especially in stool, but also in oral samples.”
141
The au-
thors note that they “identified associations between
high level genetic features and various microbiome
features; however, the mechanistic forces of those
associations remain unclear.”
142
It is a very interesting,
albeit nascent area of human genomics research, but
given the sheer complexity of microbiota and the im-
pact on health of human microbiomes, interesting and
clinically relevant future findings are to be anticipated.
Benson, Andrew K., et al. “Individuality in Gut Microbiota Composition is a Complex Polygenic Trait Shaped by Multiple Environmental and Host
Genetic Factors.” Proceedings of the National Academy of Sciences, vol. , no. , Oct. . doi:./pnas..
Kolde, Raivo, et al. “Host Genetic Variation and its Microbiome Interactions within the Human Microbiome Project.” Genome Medicine, vol. ,
no. , Jan. . doi: ./s---.
Ibid.
b. Infectious Diseases
While many microbes serve important positive life
functions for humans, for example aiding digestion
and the breakdown of micronutrients, many are
pathogenic—the viruses and bacteria that cause
infectious diseases. For 2020 and into 2021, COVID-19
has been very much on the minds of all. Clearly,
understanding the genetic structure of the SARS-
CoV-2 virus has been highly important in the global
mission to control the pandemic, and genetics and
genomics as disciplines have been on the front-
lines contributing to many areas (see sidebar).
In terms of advancing understanding of the effect of
the human genome on infectious disease response
and susceptibility, it has been serendipitous that the
Genomics in the
COVID-19 Pandemic
“Genomics rapidly assumed crucial roles in COVID-19
research and clinical care in areas such as: (1) the
deployment of DNA and RNA sequencing technol-
ogies for diagnostics, tracking of viral isolates, and
environmental monitoring; (2) the use of synthetic
nucleic acid technologies for studying SARS-CoV-2
virulence and facilitating vaccine development;
(3) examination of how human genomic variation
influences infectivity, disease severity, vaccine effi-
cacy, and treatment response; (4) the adherence to
principles and values related to open science, data
sharing, and consortia based collaborations; and (5)
the provision of genomic data science tools to study
COVID-19 pathophysiology. The growing adoption of
genomic approaches and technologies into myriad
aspects of the global response to the COVID-19 pan-
demic serves as another important and highly visible
example of the integral and vital nature of genomics
in modern research and medicine.”
Eric D. Green, et al. “Perspective: Strategic Vision for Improv-
ing Human Health at the Forefront of Genomics.” Nature,
vol. 586, 29 Oct. 2020.
60
pandemic hit at a time when widespread human
genetic sequencing is relatively inexpensive. While se-
quencing the virus itself is not “human” genomics, the
discipline has been contributing important research
that may influence future approaches to diagnosis and
treatment of infectious disease. The COVID-19 Host
Genetics Initiative, for example, has brought together
multiple stakeholders in the human genetics’ commu-
nity to “generate, share, and analyze data to learn the
genetic determinants of COVID-19 susceptibility, sever-
ity, and outcomes.”
143
The Initiative formed to help ad-
vance research that could lead to potential discoveries
that “could help to generate hypotheses for drug re-
purposing, identify individuals at unusually high or low
risk, and contribute to global knowledge of the biology
of SARS-CoV-2 infection and disease.”
144
Worldwide
engagement in the Initiative has been considerable,
with participation of “over 2000 scientists from over
54 countries working collaboratively to share data,
ideas, recruit patients and disseminate findings.”
145
Another example is the COVID Human Genetic
Effort, which is an international consortium aiming
to “discover the human genetic and immunological
bases of the various clinical forms of SARS-CoV-2
infection.”
146
In particular, the COVID Human
Genetic Effort is directing work to search for:
• “Monogenic or digenic inborn errors of im-
munity (IEI), rare or common, underlying
severe forms of COVID-19 in previously healthy
individuals, including severe pneumonia,
multisystem inflammatory syndrome in chil-
dren (MIS-C), Long COVID, COVID Toes, etc.
• Phenocopies of these monogenic IEI, such
as auto-antibodies neutralizing gene prod-
ucts of loci whose variants underlie these
IEI (e.g., auto-antibodies to type I IFNs
mimicking inborn errors of type I IFNs).
COVID- Host Genetics Initiative. “About.” www.covidhg.org/about/. Accessed May .
Ibid.
Ibid.
COVID Human Genetic Effort. “Our Mission.” www.covidhge.com/. Accessed May .
Ibid.
Supriya, Lakshmi, Ph. D. “Study Finds Protective Genetic Associations with Mild COVID-.” News Medical, Jan. , Life Sciences sec.
Ibid.
• Single-gene variants, rare or common, which
make certain individuals resistant to the infec-
tion by the SARS-CoV2 itself, despite repeated
exposure, or resistant to the development of
clinical manifestations despite infection.”
147
Multiple U.S. research centers and labs are
active participants in both of the above
referenced international consortia.
Some interesting research contributions have
also been made as a result of the growth of di-
rect-to-consumer genetic testing, where the service
of AncestryDNA has built a deep repository of DNA
information for its participants. There have been
separate genome-wide association studies (GWAS),
performed in hospitals with COVID-19 patients that
indicated hereditary genetic associations with higher
levels of disease impact and infection. However, as
noted in a news article by Lakshmi Supriya,
148
these
studies have the inherent bias of examining those who
are infected, typically with a more severe case (since
they are hospitalized). AncestryDNA took a different
approach. Recognizing that their deep resource of
customer DNA may contain clues to COVID-19 ge-
netic protection or susceptibility associations, they
surveyed their customers to capture self-reported
information on “exposure, risk factors, symptoms and
demographics, most of whom had mild disease.”
149
With a large sample of over 700,000 respondents, the
AncestryDNA research team had a deep resource to
work with, ultimately finding gene associations related
to immunity and others associated with susceptibil-
ity and disease severity—some of which represent
new findings, and some confirming associations
identified by other researchers. As Supriya notes, “the
study provides a complementary analysis to stud-
ies focusing on severe disease, which is promising
61
for finding new genetic associations, in particular
those that provide protection against the virus.”
150
The degree of susceptibility or immunity to various
infectious disease organisms has shaped the human
genome throughout our evolution as a species.
Historic pandemics, involving large-scale deaths due
to smallpox, plague, or influenza, for example, have
been “natural selection” events, favoring ongoing
reproduction of genotypes with disease resistance
traits and down selecting genotypes with high
susceptibility to the disease. It is also the case that
some of the protective genotypes may serve to help
an individual resist certain pathogens, but then be
related to negative repercussions also.
151
This has been
found to be the case with sickle cell disease where the
genes that are associated with the disease also appear
to be associated with a positive immunity to Malaria.
Another area of important research at the inter-
face between the human genome and infectious
disease is the affect that infection can have on
the human genome and upon gene regula-
tion and expression. As noted by the National
Institute of General Medical Sciences (NIGMS):
When viruses infect us, they can embed
small chunks of their genetic material in our
DNA. Although infrequent, the incorporation
of this material into the human genome
has been occurring for millions of years.
As a result of this ongoing process, viral
genetic material comprises nearly 10 percent
of the modern human genome.
152
Interestingly, recent research at the University of
Colorado Cancer Center by Sharon Kuss-Duerkop and
Dohun Pyeon finds that viruses are not just cutting
and pasting code within DNA; they are also engaged
in “suppressing gene expression via DNA methylation,
specifically by targeting DNA methyltransferases
Ibid.
Pittman, Kelly J., et al. “The Legacy of Past Pandemics: Common Human Mutations That Protect against Infectious Disease.” PLOS Pathogens,
vol. , no. , July .
NIH, National Institute of General Medical Sciences (NIGMS). “Our Complicated Relationship with Viruses.” ScienceDaily, November .
University of Colorado Anschutz Medical Campus. “Here’s How Viruses Inactivate the Immune System, Causing Cancer.” ScienceDaily, March
.
Ibid.
(DNMTs).”
153
They note that “viruses elect to turn off
genes like interferon-b that the immune system
regularly enlist to fight foreign pathogens which
allows them to replicate quickly and run rampant.
This could lead to cancer development.”
154
Human genetics and genomics as a discipline has
much more to discover regarding the complex relation-
ship between the human genome and the pathogenic,
and potentially positive, effects of microbial infections.
8. Metagenomics and
Environmental Genomics
All the above discussion of genetics and genomics
research, clinical application, and impacts will have
hopefully resulted in an appreciation for the incred-
ible complexity of not just the human genome but
the vast network of interfaces between the genome
and other biological and environmental systems.
Each of us walks a slightly different path through
life, experiencing different influences upon our
physiology in terms of the food we eat, the amount
of sun we expose ourselves to, the environments we
are exposed to in our jobs, the pathogens that we
by chance encounter, etc. Any and all of these and
more may be subtly changing (mutating) letters
in our genome or periodically influencing gene
regulation or expression. If you want a challenging
career, working on unravelling genome-environment
interactions and effects would have to be high on
the list. There is a specific area of research inquiry
in genetics and genomics that provides perspective
on the subject—it is termed “metagenomics”
Rob Knight, in considering the future of
human genetics and genomics, notes that:
Genomics is a key underpinning for
metagenomics. This is the case because
reference-based approaches are dramatically
62
faster and more accurate than reference-free
approaches whenever the reference database
is complete and correct. However, with a few
exceptions (such as bacteria in the human
gut of healthy western adults), we are far from
having adequate reference data. Sequencing
efforts such as the Genomic Encyclopedia of
Bacteria and Archaea (GEBA) projects have
been extremely valuable in filling in missing
branches of the tree of life, but projects such as
microbial earth which seeks to sequence all type
strains, and 1000 fungal genomes project remain
under-resourced. Building these references and
augmenting them with new clinical isolates and
with isolates from remote human populations
and from a panel of diverse environmental
samples, such as those provided by the Earth
Microbiome Project, could dramatically
accelerate progress in all metagenomic studies,
whether targeted at human or animal health or
at the environment. The benefits that could be
achieved would greatly outweigh the modest
investment required to complete these studies.
155
There are countless research questions that
metagenomics will be used to pursue, and each
will benefit from the speed, accuracy, and afford-
ability of gene sequencing in combination with
ongoing advancement in bioinformatics and
artificial intelligence-based approaches to the
mining of genomic and metagenomic big data.
Cheifet, Barbara. “Editorial: Where is genomics going next?” Genome Biology, vol. , no. , Jan. . doi:./s---.
Phillips, Melissa Lee. “Crime Scene Genetics: Transforming Forensic Science through Molecular Technologies.” BioScience, vol. , no. , June
. doi:./B.
9. Non-Medical Applications
of Human Genomics
Each of the functional impact domains of human
genetics and genomics discussed above (domains 1
through 8) have been viewed through the primary
lens of medical science—the application of genetics
and genomics to understanding genomic struc-
tures and mechanisms and their effect on human
health and pathology. There are, however, multiple
other areas of scientific research and functional
application of human genetics and genomics that
are not principally directed at medical questions.
Three such applications are highlighted briefly
below, focused on: forensic science, anthropology
and evolutionary biology, and paternity testing.
a. Forensic Science
Genetics, and more recently genomics, has become
an essential tool for forensic scientists in criminal
justice systems. In the late 1980s, genetic analysis
entered forensic use through examination of non-cod-
ing region repeats in DNA that are highly variable
among individuals. This became known as DNA
“fingerprinting,” helping to identify crime suspects.
The technology was revolutionary for forensic science
in that, as noted by Melissa Lee Phillips, “for the first
time, forensic scientists could create genetic profiles
so specific that the only people who share them are
identical twins.”
156
Phillips notes that “DNA fingerprint
techniques evolved subtly over the next several years,
until the polymerase chain reaction (PCR), developed
by Kary Mullis, was introduced into forensic work. By
Metagenomics. Also known as environmental genomics or community genomics, metagenomics
investigates the communal genome contained within an environmental sample. It enables
the study of the symbiosis and interactions of organismal genomes and genetic products as a
biological system.”
Simon Tripp and Martin Grueber. Economic Impact of the Human Genome Project. Battelle Memorial Institute, May 2011.

63
allowing the selective amplification of any desired
stretch of DNA, PCR ushered in unprecedented sen-
sitivity in low-level DNA detection at crime scenes.”
157
Analysis of DNA provides a pathway to definitively
identify the individual associated with DNA evidence
at a crime scene, and also may be used to establish
the identity of human remains. Forensic genetics is
an evolving discipline, with new technologies en-
abling varied use in criminal justice applications:
Ibid.
Forensic genetics is slowly transitioning into
forensic genomics… Genomic, transcriptomic,
and epigenomic principles, data, and
technologies are applied to identify and analyze
useful DNA and RNA markers to address
various forensic questions that cannot be
answered, or only in a limited way, via genetic
or other approaches. Human genome data
produced with SNP microarray technologies,
and increasingly whole exome and whole
genome data established via massively
parallel sequencing (MPS) technologies, are
used to identify DNA markers for individual
identification, as well as for appearance and
ancestry prediction. The latter is forensically
relevant for finding unknown perpetrators of
crime who are unidentifiable with standard DNA
profiling. Human transcriptome data of various
tissues generated with expression microarray
technologies, and increasingly with whole
transcriptome sequencing via MPS technologies,
are used to identify RNA markers to determine
the cellular source of crime scene sample. This
is forensically relevant for reconstructing the
course of events that may have happened at the
Identifying the
“Golden State Killer”
For decades, police sought to identify the individ-
ual responsible for 12 murders and 45 rapes across
California between 1976 and 1986. The police had
DNA evidence from crime scenes, but the DNA did
not match any individuals in existing criminal DNA
databases, and without a suspect there was no way
to identify the offender.
The recent growth of ancestral DNA databases
provided a pathway to a breakthrough in the case.
Police analyzed one of the databases and were able
to narrow the DNA to a particular family. Standard
investigative procedures were then able to be used
to narrow the family members down to one suspect
The U.S. Department of Justice – resulting in a con-
fession and conviction.
The U.S. Department of Justice
on Advancing Justice Through
DNA Technology
DNA can be used to identify criminals with incredi-
ble accuracy when biological evidence exists. By the
same token, DNA can be used to clear suspects and
exonerate persons mistakenly accused or convicted
of crimes. In all, DNA technology is increasingly vital
to ensuring accuracy and fairness in the criminal
justice system.
For example, in 1999, New York authorities linked
a man through DNA evidence to at least 22 sexual
assaults and robberies that had terrorized the city.
In 2002, authorities in Philadelphia, Pennsylvania,
and Fort Collins, Colorado, used DNA evidence
to link and solve a series of crimes (rapes and a
murder) perpetrated by the same individual. In the
2001 “Green River” killings, DNA evidence provided
a major breakthrough in a series of crimes that had
remained unsolved for years despite a large law en-
forcement task force and a $15 million investigation.
DNA is generally used to solve crimes in one of
two ways. In cases where a suspect is identified, a
sample of that person’s DNA can be compared to
evidence from the crime scene. The results of this
comparison may help establish whether the sus-
pect committed the crime. In cases where a suspect
has not yet been identified, biological evidence
from the crime scene can be analyzed and com-
pared to offender profiles in DNA databases to help
identify the perpetrator. Crime scene evidence can
also be linked to other crime scenes through the
use of DNA databases.
The United States Department of Justice. “Advancing Justice
Through DNA Technology: Using DNA to Solve Crimes.”
www.justice.gov/archives/ag/advancing-justicethrough- dna-
technology-using-dna-solve-crimes. Updated 7 March 2017.

64
scene of crime and to support the use of DNA at
the activity level of evidence interpretation.
158
Genetics and genomics are also being used by
researchers in the field of criminology to examine
genetic correlates to offenders, drawing upon ad-
vancements in understanding genetic factors that
influence human behavior. An example of this is the
work of Eric Connolly and Kevin Beaver that incorpo-
rated behavioral genetic methods to “assess gene-en-
vironment interplay between anger, family conflict,
and violence using a subsample of kinship pairs
drawn from the Child and Young Adult Supplement
of the National Longitudinal Survey of Youth.”
159
Their
analysis reveals “a significant shared genetic liability
for anger and exposure to family conflict indicating
gene-environment correlation” and they conclude
that findings from the study “underscore the impor-
tance of using genetically informed methodologies
to identify underlying risk factors involved in both
exposure and response to different forms of strain.”
160
b. Anthropology and Evolutionary Biology
Our DNA codes for us as individuals in the present,
but it is also a molecular historical record of our
ancestry—providing coded documentation of our
lineage (ancestry) and our evolution as a species.
Advancements in human genetics and genomics
have provided important scientific capabilities that
Kayser, Manfred and Walther Parson (Editors). “Special Issue: Forensic Genomics.” Gene, .
Connolly, Eric and Kevin Beaver. “Assessing the Salience of Gene–Environment Interplay in the Development of Anger, Family Conflict, and
Fhysical Violence: A Biosocial Test of General Strain Theory.” Journal of Criminal Justice, vol. , no. , November–December .
Ibid.
FridovichKeil, Judith. “Human Genome Project.” Encyclopedia Britannica Scientific Project.
andMe. “Your DNA is Amazing!” www.andme.com/about. Accessed May .
have enabled researchers in evolutionary biology,
physical anthropology, archaeology, and associated
disciplines to answer many questions regarding
our evolutionary biology, our genetic linkages to
other species, our population migrations, and our
genealogy. Modern genetics and genomics have,
for example, enabled substantial advancement in
anthropology such that Judith FridovichKeil writes:
“comparative DNA sequence analyses of samples
representing distinct modern populations of humans
have revolutionized the field of anthropology.”
161
While genetics and genomics are proving funda-
mental to advancements in the above-cited aca-
demic research disciplines, they have also enabled
the development of commercial services that offer
genetic ancestry testing (genetic genealogy) to the
general population—providing insights regarding
ancestry that supplement traditional methods of
review of family records and historical documentation.
Interest levels have been high, to the extent that two
private companies 23andMe and Ancestry now have
among the largest repositories of human genetic
data in the world. 23andMe, for example, reports
that it has “more than 12,000,000 customers.”
162
Consumer interest in these services has created a
rather rich resource of genetic data that is being
used now to advance research, with 23andMe, for
Information about the relationships amongst species or populations within species and the time
of their divergence from each other can be found in the DNA. It is the job of the evolutionary
geneticist to interpret this information from DNA. A subset of anthropology—anthropological
genetics—uses the evolutionary geneticist’s tool kit to infer human evolutionary history from our
and our closest relative’s DNA.”
Jason Hodgson & Todd Disotell. “Anthropological Genetics: Inferring the History of Our Species Through the Analysis of
DNA.” Evolution: Education and Outreach, vol. 3, Sept. 2010.

65
example, noting that it has published more than
150 peer-reviewed studies in scientific journals.
163
c. Paternity testing
While connecting a baby to his or her mother is rather
easily accomplished, by the obvious nature of birth—
the question of paternity is less obvious. Before genet-
ic tests were available, blood tests and other methods
were deployed, but they were less than fully conclu-
sive. Today, however, as noted by the Cleveland Clinic:
A DNA paternity test is nearly 100% accurate at
determining whether a man is another person’s
biological father. DNA tests can use cheek
swabs or blood tests. You must have the test
done in a medical setting if you need results
for legal reasons. Prenatal paternity tests can
determine fatherhood during pregnancy.
164
Determining paternity may be performed simply to
inform a father and parenting decisions, but it may
also be required as part of a legal process—providing a
determinative path to legal rights in child support cas-
es and child custody cases, and also for determining
Ibid.
The Cleveland Clinic. “DNA Paternity Test.” https://my.clevelandclinic.org/health/diagnostics/ -dna-paternity-test. Accessed May .
legal rights to Social Security benefits and inheritance.
Some applications of paternity testing could also
be listed under the previous discussion of medical
applications of human genomics because there
is utility in establishing paternity for identification
of links to genetic conditions and for determining
potential compatibility for organ or tissue donation.
D. Summary
Whether for medical or non-medical applications,
it is clear that human genetics and genomics
advancements provide extremely large-scale ben-
efits across a broad variety of functional impact
domains. Genetics and genomics are considered
fundamental within modern biological science,
providing answers to basic biological research
questions, and they underpin a diverse range
of applied innovations and applications that are
greatly enhancing human health and well-being.

66
67
Since the authors first wrote the impact study for
the Human Genome Project on its 10th anniver-
sary, genetics and genomics technologies, and
the cost-effectiveness of their application, have
advanced astonishingly quickly. The literature of
fundamental and applied research discoveries
in genetics and genomics has expanded equally
rapidly, and the application of human genetics
and genomics is evident in almost every branch of
human medicine and modern biological science.
While the accomplishments of genetics and ge-
nomics scientists have been many and varied, with
key categories of application highlighted herein, an
overused analogy still applies. We are probably just
looking at the “tip of the iceberg” in terms of the
future of human genomics. There are still multiple
large outstanding areas to pursue, including:
• Understanding of the full structure of
the human genome and the biologi-
cal activity it produces or influences.
• Developing additional knowledge regarding
the functional relationships between genotype
and phenotype and the influence that environ-
mental interactions have on gene expression,
regulation, and mutation over lifespans.
• Advancing knowledge of gene-disease
relationships, especially (but not exclu-
sively) in regard to common complex,
polygenic diseases and disorders.
• Overcoming the current skewing of genomic
data towards northern European genotypes by
supporting the concerted effort to build more
diversity of data across humanity worldwide.
This is an important effort to help ensure health
disparities are better understood and addressed,
and an equitable future secured in the appli-
cation of genetic medicine advancements.
• There is significant need to translate the research
and innovation advancements already being
made into much more widespread clinical appli-
cation, and a distinct need to connect patient ge-
nome data to medical records and family history.
Generating predictions for the future of fields of
science, technology, and their application is no
easy task, especially in areas as large and diverse
as those driven by human genetics and genomics.
The frontiers of genetics knowledge are constantly
expanding, and it is impossible to predict in advance
the breakthroughs that may occur that will open-up
new pathways to discovery, innovation, and applica-
tion. CRISPR is a recent example that quite suddenly
IV. Into the Future
The primary purpose of this report is to provide a current overview, or
point-in-time snapshot, of the economic impact of human genetics
and genomics in the U.S., and to provide a useful overview of the
principal application domains of human genetics and genomics that are
generating positive advancements in human health and well-being.

68
and unexpectedly is making available to scientists an
exquisitely precise and flexible tool for gene editing
that is greatly accelerating progress in both research
inquiry and practical application. Similarly, the con-
tinued convergence of genetics and genomics with
the rapidly advancing field of advanced analytics and
artificial intelligence promises a dynamic future.
Researchers have regularly unveiled increasing
complexity in human genome functionality and
its interactions with other biological systems, and
that tendency makes it somewhat challenging
to fully predict future status. That said, there are
certain observable trends—in sequencing speed,
in emerging areas of inquiry, in expanding clinical
applications—that point to near-term directionality
with some degree of confidence. Several anticipated
future areas of advancement are highlighted below.
A. Ongoing Fundamental
Discovery
The population of fully sequenced human genomes
already encompasses millions of individuals, and
further rapid expansion of this universe is to be an-
ticipated. As this report highlights, there are many
large-scale sequencing projects presently being
conducted around the world. As these datasets are
built, they increase opportunities to identify inter-
esting variation across human genomes enabling
fundamental questions to be pursued with higher
resolution regarding gene functions and the impact of
genetic variation on diseases and disorders (many of
which are particularly endemic in certain geographic
regions and associated regional population subtypes).
One of the key advances to be anticipated is devel-
opment of an enhanced understanding of genomic
variation among diverse population groups spread
across the world. The limitations imposed by current
data being skewed to genotypes associated with
European ancestry will be overcome as sequencing
programs in Africa (where the most genetically
diverse population is located) and Asia, for example,
expand significantly. It may be anticipated that more
diversity in the data will reveal interesting findings,
as was the case in genes for sickle cell disease being
associated with protection against malaria. More
diversity in sequenced populations will also be im-
portant for further advancing precision medicine,
likely unveiling many more mutations associated
with drug efficacy and adverse drug reactions.
Computational data sciences are also progressing
rapidly, with significant progress being made in
advancing automated analysis tools rooted in artificial
intelligence advancements. It is likely that computa-
tional and analytical sciences will be as important as
biological sciences in contributing to a rich discovery
environment. A challenge area that will need to be
69
addressed, given the importance of data analytics, is
data access and data interoperability. The ability to
analyze data and make discoveries can only occur if
scientists have access to data, and there is still much
that needs to be done to provide access to health
records and other non-genomic data that can be
matched to genomic data for analysis. There are
also issues in terms of data needing to be format-
ted and archived in ways that facilitate analysis.
It should also be anticipated that significant ad-
vancements in fundamental knowledge will be
advanced though studies at a single-cell resolution,
assisted by recent advancements in technology
and techniques. As noted by Olivier Harismendy:
DNA sequencing at deep coverage or at
single-cell resolution is revealing a vast genetic
heterogeneity of normal or dysplastic tissues.
At present these insights are mostly at the
stage of observations, but future studies will
address the consequences of such heterogeneity
in tissue homeostasis and function. The new
information that is provided will provide a better
understanding of diseases and conditions
Cheifet, Barbara. “Editorial: Where is genomics going next?” Genome Biology, vol. , no. , Jan. . doi:./s---.
Ibid.
associated with aging, genotoxic injuries, and
the accumulation of such mosaic mutations.
165
This is also echoed by Jernej Ule, noting that “we
will be able to move beyond the static picture of
genomic data towards studies of the dynamic
transitions that cells make on a genomic scale
in response to external and internal cues.”
166
It should also be anticipated that fundamental and ap-
plied research in genetics and genomics will increas-
ingly uncover linkages not just between gene varia-
tions and disease but also gene variations and health.
Some institutions are already focusing on this oppor-
tunity. For example, the Institute for Systems Biology
(ISB) in Seattle is a proponent of medicine that can be
predictive, preventive, personalized, and participatory
(termed P4 medicine), and is working on quantifying
wellness—integrating genomics as a component in
helping individuals improve their health, longevity, and
quality of life. As Lee Hood, the cofounder of ISB notes:
Systems biology will revolutionize the practice
of health care in the coming decades. Today,
medicine is largely reactive. It waits until a
person is sick and then treats a disease with
One of the biggest challenges is most health care systems are not built to prevent adverse
events, but mostly to treat adverse events. Another is the lack of a centralized, organized health
care system designed to support life-long results such as genomic testing. For example, we can
conduct a preemptive screen for pharmacogenetic tests in a single test, and these results are
more likely to be applicable as the patient grows older and is exposed to more high-risk drugs. But
we don’t have a good system for making these results available when needed. There is no uniform
electronic health record. At any one time, patients may have multiple prescribers and pharmacies
with little to no coordination, much less with common access to genetic test results that can
inform prescribing and capitalize on the availability of clinical decision support. The lack of logical
prescribing based on laboratory tests is just one small example of the disconnectedness and lack
of computationally informed medicine that impacts all levels of our healthcare.”
Mary Relling. “Luminaries Share Their Thoughts on Advances in ‘Omics Over the Past Five Years.” Clinical Omics
Magazine, vol. 6, no. 2, March-April 2019.
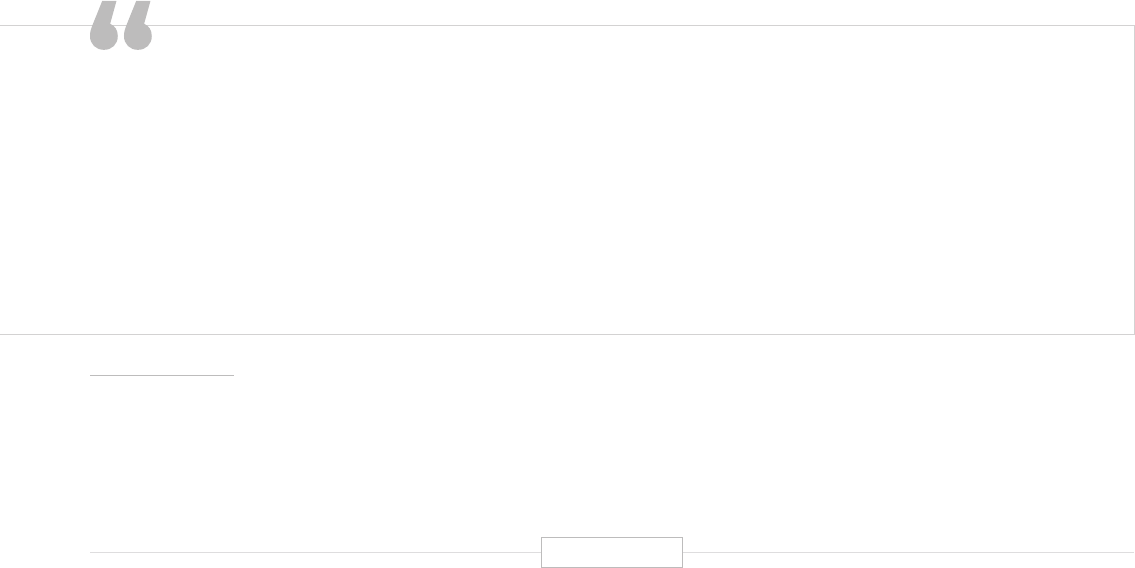
70
varying levels of success. The revolution will
emerge from the convergence of systems
biology and the digital revolution’s ability to
create consumer devices, generate and analyze
“big data” sets and deploy this information
through business and social networks. By
providing an understanding of disease at
the molecular level, systems medicine will
eventually be able to predict when an organ will
become diseased or when a perturbation in a
biological network could progress to disease.
167
B. Expanding the Clinical
Application of Genomics
It is safe to predict that the application of genetics
and genomics in clinical medicine will continue
to expand substantially. Currently, there is a large
observable difference between genetics and ge-
nomics having ubiquitous use in biological research
versus a far less uniform application of discoveries
and advancements into actual clinical practice.
In the U.S., a key challenge is imposed by the hetero-
geneous structure of the nation’s healthcare system.
Rather than a single system, the U.S. comprises a
patchwork quilt of individual health systems, to-
gether with intermediate insurance organizations
and third-party payers, that influence the adoption
Institute for Systems Biology. “Scientific Wellness.” https://isbscience.org/research/ scientificwellness/. Accessed May .
National Academies of Sciences, Engineering, and Medicine. Implementing and Evaluating Genomic Screening Programs in Health Care
Systems: Proceedings of a Workshop. Washington, DC: The National Academies Press, . doi:./.
of established genomics tools and practice ad-
vancements. In some locations (for example, central
Pennsylvania within the Geisinger health system),
genomics is becoming integral to the management of
the healthcare of covered patient populations; how-
ever, this is far from the norm. There is a long way to
go before all patients across the nation have access to
state-of-the-art genomics and the personalized med-
icine and the improved outcomes they enable. The dis-
connected nature of the U.S. health system structure
also hampers effective cascade screening since pa-
tients move and their records do not follow-them, and
family members may reside in different health systems
across the nation. As the National Academies note
“the benefits of screening will be multiplied if systems
for affective cascade screening can be implemented,
but there is currently no roadmap for such testing.”
168
It should also be anticipated that new classes of
medicines, developed through synthetic biology
and gene editing, will expand in clinical use. At the
present time, gene therapies and gene editing see
limited clinical application. However, the degree of
editing precision provided by CRISPR technology will
lead to more widespread therapeutic applications
developed using gene editing procedures, which
themselves cause editing within the patient’s genome.
Now that the sequencing technology has advanced to the point where delivering high quality,
cheap, and rapid genomes is a commodity – and where robust, reproducible, and accessible
methods exist for analysis and interpretation of these datasets – it is clear that the largest hurdle
relates to the integration of these types of methods into clinical practice. Tools, methods, and
processes need to be developed in order to deliver this information in ways that care providers can
digest it and use it for the treatment of their patients.”
Liz Worthy. “Luminaries Share Their Thoughts on Advances in ‘Omics Over the Past Five Years.” Clinical Omics Maga-
zine, vol. 6, no. 2, March-April 2019.

71
C. Educating and
Updating Providers
Part of the widespread clinical adoption challenge
with genomic medicine relates to the education of
medical professionals. Genetics and genomics are
complex and fast-moving fields, rendering it difficult to
keep physicians up-to-speed in the latest findings and
clinical practice implications and recommendations.
It remains to be seen what model for the practice of
genomic medicine will predominate. For example:
• There may be development and adoption of
computational clinical decision support tools
that assist primary care and other physicians in
interpreting the results of genetic and genomic
tests and guide clinical decision making.
• Physicians may simply be expected to adapt and
to educate themselves regarding genomics simi-
lar to how they have to access information on new
drugs and new practice procedures. Education
here would occur via continuing professional
education courses, through visits by company
representatives, and other traditional pathways.
• Genetics and genomics counselors may become
increasingly embedded in large clinical prac-
tices as a localized resource, working to keep
pace with expanding genetics and genomics
advancements and to provide consultation
with physicians and other clinical providers.
Because the field is moving quite fast, there will be a
need to not only relay new discoveries and advance-
ments to clinicians, but also to revise their knowledge
since it is likely that reinterpretation of variant results
will occur under evolving evidence and study.
More widespread use of testing for predisposition
for disease, and the growth in polygenic risk score
systems and other tools, will require physicians to
become comfortable in working with patients to in-
terpret results and develop preventive care regimens.
Center for Genetics and Society. “Other Countries.” www.geneticsandsociety.org/topics/other-countries. Accessed May .
D. Ethical Considerations
Particularly in biological sciences, the frontiers of sci-
ence may raise ethical considerations. Such is certainly
the case in human genetics and genomics where
abilities to edit the genome, up to and including
hereditary germline DNA, present challenges requir-
ing ethical debate. Should we edit carrier genomes to
prevent passing down of genetic disease to progeny,
and if so, which diseases should qualify? Some dis-
eases are relatively easily managed with medicines,
while some have no treatments at all. Should we
reserve gene editing as a last resort for these cur-
rently intractable diseases, or should it only be used
in diseases that dramatically shorten lives, involve
great pain in those afflicted, or impose large-scale
economic burdens on society? We will not presume to
guess how such issues will be resolved, but it is clear
that they are presenting and will require addressing.
Since germline editing impacts our evolution as a
species, it is a particularly contentious issue, and
it needs to be globally addressed. Currently, more
than 40 countries ban germline editing, but there
are 195 countries in the world.
169
Somatic gene
editing holds significant promise for helping peo-
ple who are sick and presents less of an ethical
challenge (although there are still issues relating
to the potential for unforeseen consequences
in editing a not fully understood genome).
The widespread collection of individual genetic
data also presents privacy issues and potential for
genetic discrimination (for example, the risk of a
person being treated differently by their employer or
insurance company because they have a gene vari-
ant that causes or increases the risk of an inherited
disorder). The NHGRI sought to identify potential
ethical and legal issues pertaining to human genet-
ics as a component of the original Human Genome
Project, establishing the Ethical, Legal, and Social
Implications of Human Genetics Research (ELSI)
program to “examine these issues and assist in
the development of policy recommendations and
72
guidelines to ensure that genetic information is used
appropriately.”
170
The ELSI program continues to study
and address these issues, and NHGRI notes that:
As the ELSI program has evolved, four high pri-
ority areas have emerged from its work that
serve to categorize domains of ethical, legal,
public policy, and societal education that will
need to be further addressed. These include:
1. Privacy and Fairness in the Use and
Interpretation of Genetic Information
• Privacy
• Discrimination/Stigmatization
• Philosophical/Conceptual Assumptions
• Public Policy Issues
2. Clinical Integration of Genetic Technologies
• Clinical Ethical Issues
• Genetic Testing/Counseling
• Professional Issues and Standards
3. Issues Surrounding Genetics Research
• Informed Consent
• Other Philosophical and Ethical Issues
• Legal Issues
• Ethnocultural Issues
• Other
4. Education
• Professional-Health
• Professional-Other
• Public-K through 12
• Public-College
• Public-Consumer
• Combination Professional/Public.
Equitable access to the benefits of modern human
genetics and genomics advancements also rep-
resents a challenge to be addressed. As noted in
this report, there are concerted efforts underway
to increase the diversity of sequencing human
National Human Genome Research Institute. “Review of the Ethical, Legal and Social Implications Research Program and Related Activities
(-).” www.genome.gov/ /elsi-program-review-#:~:text=The%original%issues% identified %
in,impact%of%genetic%information%on. Accessed May .
ASHG Survey Finds Americans Strongly Support Human Genetics Research. Research!America and American Society of Human Genetics,
Jan. .
sub-populations with multiple large-scale genome
sequencing projects taking place in areas of the globe
whose populations have been underrepresented
in current genomic data. Increasing diversity in the
data is important to advancing equitable research
and, ultimately, for equity in the clinical applica-
tions of genetic and genomic medical innovations
that can benefit the full spectrum of humanity.
E. Conclusion
What is absolutely clear is that human genetics
and genomics will be in the vanguard in terms of
contributing to advancements in medical science
and enhancing the practice of clinical medicine.
Expanding understanding of the human genome,
variations in genomes, external factors that interface
with the genome, and genetic relationships to health
and disease will provide improved health, quality of
life, and large-scale benefits to society (both economic
and social). Indeed, as this report finds, it already has.
It is heartening to note that a survey of the general
public in the U.S. found that people generally share
an optimistic view of genetics and genomics and
the promise it holds for a better future. A study re-
leased by the American Society of Human Genetics
in partnership with Research!America found that
the “large majority of Americans agree that genetic
knowledge will be important to their own health
and their families’ health.”
171
The study also found
that “a strong majority of Americans (77%) indicate
positive feelings about human genetic research”.
Figure 6 summarizes other topline survey findings.
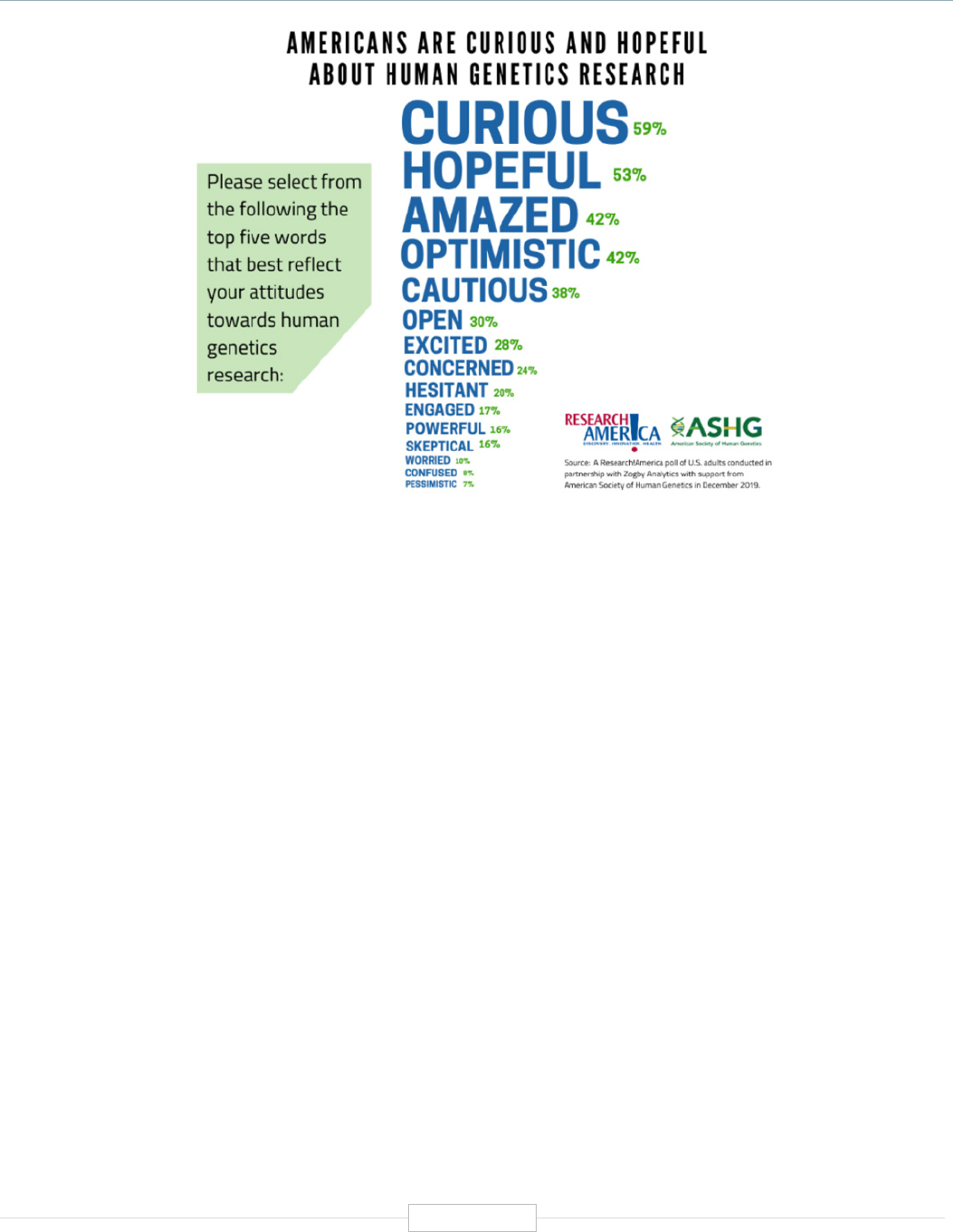
73
Based on the economic and functional impacts
of human genetics and genomics detailed herein,
the public is right to feel optimistic about human
genetics research. The fields of human genetics
and genomics are having profound positive impacts
both in terms of biomedical discovery as well as
within the clinical practice of medicine—working
to improve outcomes for millions of patients and
demonstrating great promise for future highly
positive contributions to human health and well-be-
ing worldwide. While generating these positive
functional impacts is the raison d’etre for pursuing
the advancement of human genetics and genomics,
the fields have also had the very positive spillover
effect of building a powerful science- and technol-
ogy-based economic sector for the U.S.—a sector
that supports 850,263 jobs across the nation and
generates $265.4 billion in economic output. Human
genetics and genomics innovation is expanding
the stock of knowledge upon which the nation’s
continued advancement depends and shows great
promise to continue to do so long into the future.
Figure 6: U.S. Adults Attitudes Regarding Human Genetics Research – Survey Findings
Source: American Society of Human Genetics and Research!America . “ASHG Survey Finds Americans Strongly Support Human Genetics Research.”

74

75
Glossary of Terms
Term Definition
Allele
A variant of a gene inherited from one parent. An allele’s frequency in a population
can change due to four forces: mutation, natural selection, random genetic drift,
and gene flow.
Biomarker
Any substance, structure, or process that can be measured in the body or its
products that influence or predict the incidence of outcome or disease
Cancer Genomics
The study of the DNA sequence and gene expression in tumor cells as they compare
to normal host cells.
Clinical
The observation and treatment of actual patients rather than theoretical or
laboratory studies.
DNA
Deoxyribonucleic acid, the molecule that carries genetic instructions in all living
things. The DNA molecule consists of two strands that wind around one another
to form a double helix. Each strand has a backbone made of alternating sugar
(deoxyribose) and phosphate groups. Attached to each sugar is one of four bases:
adenine (A), cytosine (C), guanine (G), and thymine (T). The sequence of the bases
along the backbones serves as instructions for assembling protein and RNA
molecules.
Functional Genomics The study of how elements in the genome contribute to biological processes.
Gene
The basic physical and functional unit of heredity. Technically a distinct sequence
of nucleotides forming part of a chromosome, the order of which determines the
order of monomers in a polypeptide or nucleic acid molecule which a cell (or virus)
may synthesize.
Genotype The genetic constitution of an individual organism.
Germline Cells
Germline cells pass on their genetic material to the progeny. These include sperm
and egg cells.
Microbiome
The collective genomes of the microbes (composed of bacteria, bacteriophage,
fungi, protozoa, and viruses) that live inside and on the human body
Mosaicism
Mosaicism is when a person has two or more genetically different sets of cells in his
or her body
Pharmacogenomics
The study of the role of the genome, or multiple genes, in predicting drug
metabolism and response.
76
Term Definition
Protein
A molecule made up of amino acids. Proteins are needed for the body to function
properly. They are the basis of body structures and of other substances such as
enzymes, cytokines, and antibodies.
Somatic Cells All cells in the body that are not germline cells
Synthetic Biology
An interdisciplinary field that involves the application of engineering principles to
biology. It aims at the (re-)design and fabrication of biological components and
systems that do not already exist in the natural world.
Systems Biology
The holistic study of the interactions and behavior of the components of biological
entities, including molecules, cells, organs, and organisms.
Whole Exome
Sequencing (WES)
Identification of the sequence of base-pairs in the protein-coding regions of the
genome.
Whole Genome
Sequencing
Identification of the sequence of base-pairs across the full genome, including
protein-coding and regulatory regions.
77
Appendix—
Additional Economic Impact Information
Table 11: Listing of NIH Genetic and Genomic-Related Proposal Review Study Sections
• Behavioral Genetics and Epidemiology Study Section [BGES]
• Cancer Genetics Study Section [CG]
• Gene and Drug Delivery Systems Study Section [GDD]
• Genes, Genomes, and Genetics [F08]
• Genetic Variation and Evolution Study Section [GVE]
• Genetics of Health and Disease Study Section [GHD]
• Genomics, Computational Biology and Technology Study Section [GCAT]
• Molecular Genetics A Study Section [MGA]
• Molecular Genetics B Study Section [MGB]
• Molecular Neurogenetics Study Section [MNG]
• Therapeutic Approaches to Genetic Diseases Study Section [TAG]
Table 12: Economic (Expenditure) Impacts—
Additional Expanded NIH Funding Scenario ($7.018 billion)
Impact Type Employment
Labor
Income
($B)
Value
Added
($B)
Output
($B)
State/
Local Tax
Revenues
($B)
Federal
Tax Reve-
nues ($B)
Direct Effect 181,595 $23.31 $54.78 $112.04 $2.95 $5.48
Indirect Effect 303,139 $25.83 $45.15 $89.86 $3.00 $5.59
Induced Effect 418,167 $23.69 $41.71 $74.18 $3.88 $5.23
Total Effect 902,902 $72.84 $141.64 $276.08 $9.83 $16.31
Multiplier 4.97 3.12 2.59 2.46
Source: TEConomy Partners analysis of Human Genetics and Genomics Input Dataset; IMPLAN U.S. Impact Model
78
Table 13: Economic (Expenditure) Impacts—
Additional Use as Tool NIH Funding Scenario ($14.202 billion)
Impact Type Employment
Labor
Income
($B)
Value
Added
($B)
Output
($B)
State/
Local Tax
Revenues
($B)
Federal
Tax Reve-
nues ($B)
Direct Effect 210,493 $26.37 $58.85 $119.23 $3.05 $6.04
Indirect Effect 329,541 $27.72 $48.03 $95.07 $3.16 $5.98
Induced Effect 460,234 $26.07 $45.91 $81.64 $4.27 $5.76
Total Effect 1,000,268 $80.16 $152.78 $295.93 $10.48 $17.78
Multiplier 4.75 3.04 2.60 2.48
Source: TEConomy Partners analysis of Human Genetics and Genomics Input Dataset; IMPLAN U.S. Impact Model

ASHG and the project authors wish to thank the following organizations
for their generous support of this study.
