State of Texas
Assessments of
Academic Readiness
STAAR
®
Chemistry
Administered May 2013
TX-EOC-CHEM__Release-Form-May-2013__r3__052813.indd 1 6/26/13 8:39 AM
RELEASED
Copyright © 2013, Texas Education Agency. All rights reserved. Reproduction of all or portions of this work is prohibited without express
written permission from the Texas Education Agency.
5PUBMQSFTTVSFPGBHBT
=
(
TVNPGUIFQBSUJBMQSFTTVSFT
PGUIFDPNQPOFOUHBTFT
)
1 1 1 1
5
1SFTTVSFWPMVNF
=
NPMFTJEFBMHBTDPOTUBOUUFNQFSBUVSF
17 O35
.PMBSJUZ
NPMFT PG TPMVUF
MJUFS PG TPMVUJPO
.
NPM
-
*POJ[BUJPODPOTUBOUPGXBUFS
=
(
IZESPHFOJPO
DPODFOUSBUJPO
)(
IZESPYJEFJPO
DPODFOUSBUJPO
)
,
X
<) ><0) >
(
7PMVNFPG
TPMVUJPO
)(
NPMBSJUZPG
TPMVUJPO
)
=
(
WPMVNFPG
TPMVUJPO
)(
NPMBSJUZPG
TPMVUJPO
)
7 . 7 .
"50.*$4536$563&
#&)"7*030'("4&4
40-65*0/4
*OJUJBM WPMVNF
*OJUJBM UFNQFSBUVSF
GJOBM WP
MMVNF
GJOBM UFNQFSBUVSF
*OJUJBMQSFTTVSFJOJUJBMWPMVNF
=
GJOBMQSFTTVSFGJOBMWPMVNF
*OJUJBM QSFTTVSFJOJUJBM WPMVNF
*OJUJBM NPMFTTJOJUJBM UFNQFSBUVSF
GJOBM QSFTTVSFGJOBM
WPMVNF
GJOBM NPMFTGJOBM UFNQFSBUVSF
17
O 5
1 7
O 5
17 1 7
7
5
7
5
*OJUJBMWPMVNF GJOBMWPMVNF
*OJUJBMNPMFT GJOBMNPMFT
)FBUHBJOFEPSMPTU
=
NBTT
(
TQFDJGJD
IFBU
)(
DIBOHFJO
UFNQFSBUVSF
)
2 ND 5
Q
$
=
(
FOUIBMQZ
PGQSPEVDUT
)
−
(
FOUIBMQZ
PGSFBDUBOUT
)
$ $ $) ) )
G
P
G
P
QSPEVDUT SFBDUBOUT
5)&3.0$)&.*453:
7
O
7
O
&OUIBMQZPG
SFBDUJPO
Q)
=
−MPHBSJUINIZESPHFOJPODPODFOUSBUJPO
Q) MPH<) >
4QFFEPGMJHIU
=
GSFRVFODZXBWFMFOHUI
D G L
&OFSHZ
=
1MBODLµTDPOTUBOUGSFRVFODZ
&
QIPUPO
IG
&OFSHZ
=
1MBODLµTDPOTUBOUTQFFEPGMJHIU
XBWFMFOHUI
&
QIPUPO
ID
L
45""3$)&.*453:
3&'&3&/$&."5&3*"-4
State of Texas
Assessments of
Academic Readiness
STAAR
TM
TX-EOC-CHEM__Release-Form-May-2013__r3__052813.indd 2 5/28/2013 12:34:28 PM

( )
STAAR CHEMISTRY
REFERENCE MATERIALS
State of Texas
Assessments of
Academic Readiness
STAAR
TM
ATOMIC STRUCTURE
Speed of light
=
(frequency)(wavelength)
cfλ
=
Energy
=
(Planck’s constant)(frequency)
E
photon
hf
=
Energy
=
(Planck’s constant)(speed of light)
(wavelength)
E
photon
hc
=
λ
BEHAVIOR OF GASES
Total pressure of a gas
=
sum of the partial pressures
of the component gases
PPPP
T
...= +++
123
(Pressure)(volume)
=
(moles)(ideal gas constant)(temperature)
PV nRT=
(Initial pressure)(initial volume)
(Initial mole
ss)(initial temperature)
(final pressure)(final
=
volume)
(final moles)(final temperature)
PV
nT
PV
nT
11
11
22
22
=
(Initial pressure)(initial volume)
=
(final pressure)(final volume)
PV PV
11 22
=
(Initial volume)
(Initial temperature)
(final vo
=
llume)
(final temperature)
V
T
V
T
1
1
2
2
=
(Initial volume) (final volume)
(Initial moles) (final moles)
=
V
n
V
n
1
1
2
2
=
SOLUTIONS
Molarity
molesofsolute
liter of solution
=
M =
mol
L
Ionization constant of water
=
hydrogen ion
concentration
hydroxide ion
concentration
K
w
+
[H ][OH]=
−
( )( )
(
Volume of
solution 1
molarity of
solution 1
=
volume of
solution 2
molarity of
solution 2
VM VM
11 22
=
)( ) ( )( )
pH
=
−logarithm (hydrogen ion concentration)
pH log[H]=−
+
THERMOCHEMISTRY
Heat gained or lost
=
(mass)
specific
heat
change in
temperature
QmcT=
p
∆
( )( )
=
enthalpy
of products
−
enthalpy
of reactants
∆∆ ∆HH H=−
f
o
f
o
(products) (reactants)
Enthalpy of
reaction
( ) ( )
Page 3

STAAR CHEMISTRY
REFERENCE MATERIALS
OTHER FORMULAS
Density
mass
volume
=
D
m
V
=
Percenterror
=
accepted valueexperimentalvalue
accepted value
−
(100)
( )
Percentyield
=
actual yield
theoreticalyield
(100)
( )
CONSTANTS AND CONVERSIONS
Avogadro 6.02 10 particlesper mole
23
’s number =×
h Planck’s constant =×=
−
6.63 10
34
J ⋅ s
c ==×speed of light 3.00 10
m
s
8
K
w
14
ionization constant ofwater 1.00 10×
−
==
mol
L
2
alphaparticle( )Heα =
2
4
neutronn=
0
1
beta particle () eβ =
−1
0
standardtemperatureandpressure(STP)
=
0°Cand1atm
0°C
=
273K
volume of idealgas at STP22.4
L
mol
=
1cm1mL 1cc
3
==
1atm
=
760mmHg
=
101.3kPa
idealgas constant 0.0821
Latm
molK
8.31
L
=R =
⋅
⋅
=
⋅⋅
⋅
=
⋅
⋅
kPa
molK
62.4
Lmm Hg
molK
1calorie(cal)
=
4.18joules(J)
1000calories(cal)
=
1Calorie(Cal)
=
1kilocalorie(kcal)
RULES FOR SIGNIFICANT FIGURES
)
(
1. Non-zerodigitsandzerosbetweennon-zerodigitsarealwayssignificant.
2. Leadingzerosarenotsignificant.
3. Zerostotherightofallnon-zerodigitsareonlysignificantifadecimalpointisshown.
4. Forvalueswritteninscientificnotation,thedigitsinthecoefficientaresignificant.
5. Inacommonlogarithm,thereareasmanydigitsafterthedecimalpointasthereare
significantfiguresintheoriginalnumber.
Page 4

Acetate
C H O
2 3 2
−
,
CH COO
3
−
Ammonium
NH
4
+
Carbonate
CO
3
2−
Chlorate
ClO
3
−
Chlorite
ClO
2
−
Chromate
CrO
4
2−
Cyanide
CN
−
Dichromate
Cr O
72
2−
Hydrogencarbonate
HCO
3
−
Hydroxide
OH
−
Hypochlorite
ClO
−
Nitrate
NO
3
−
Nitrite
NO
2
−
Perchlorate
ClO
4
−
Permanganate
MnO
4
−
Phosphate
PO
4
3−
Sulfate
SO
4
2−
Sulte
SO
3
2−
Lithium
Potassium
Barium
Calcium
Sodium
Magnesium
Aluminum
Manganese
Zinc
Chromium
Iron
Cobalt
Nickel
Tin
Lead
(Hydrogen)
Copper
Mercury
Silver
Platinum
Gold
C H O
2 3 2
−
,
CH COO
3
−
NH
4
+
NO
3
−
CN
−
ClO
−
ClO
2
−
ClO
3
−
ClO
4
−
Br
−
Cl
−
I
−
SO
4
2−
CO
3
2−
PO
4
3−
CrO
4
2−
Cr O
72
2−
OH
−
S
2−
None
None
None
None
None
None
None
None
Compoundsof
Ag
+
,
Pb
2+
,and
Hg
2
2+
Compoundsof
Ag
+
,
Pb
2+
,and
Hg
2
2+
Compoundsof
Ag
+
,
Pb
2+
,and
Hg
2
2+
Compoundsof
Sr
2+
,
Ba
2+
,
Pb
2+
,and
Hg
2
2+
Compoundsof
NH
4
+
andthealkalimetalcations
Compoundsof
NH
4
+
andthealkalimetalcations
Compoundsof
NH
4
+
andthealkalimetalcations
Compoundsof
NH
4
+
andthealkalimetalcations
Compoundsof
NH
4
+
,thealkalimetalcations,
Ca
2+
,
Sr
2+
,and
Ba
2+
Compoundsof
NH
4
+
,thealkalimetalcations,
Ca
2+
,
Sr
2+
,and
Ba
2+
Page 5
12
2B
13
3A
14
4A
15
5A
16
6A
17
7A
18
8A
10 11
1B
He
Hg
Cd
Zn
Au
Ag
Cu
Pt
Pd
Ni
Tl
Ga
Pb
Sn
Ge
Bi
Sb
As
Po
Te
Se
At
Br
Rn
Xe
Kr
Al Si P S Cl Ar
B C N O F Ne
Yb
No
Tm
Md
Er
Fm
Ho
Es
Dy
Cf
Tb
Bk
Gd
Cm
Eu
Am
Mass numbers in parentheses are those of
the most stable or most common isotope.
Si
Silicon
14
Symbol
Atomic number
Name
28.086Atomic mass
In I
K Ca
Na Mg
Li Be
2
2A
H
1
1A
Sc
3
3B
Ti
4
4B
V
5
5B
Cr
6
6B
Mn
7
7B
Fe
8
Co
9
Rb Sr Y Zr Nb Mo Tc Ru
Cs Ba Hf Ta W Re Os
Fr Ra Rf Db
Sg
Bh
Hs
Mt
Pr
Pa
Nd
U
Pm
Np
Sm
Pu
Rh
2
80
48
30
79
47
29
78
110
46
28
81
49
31
82
50
32
83
51
33
84
52
34
85
53
35
86
54
36
13 14 15 16 17 18
5 6 7 8 9 10
19 20
11 12
3 4
1
21 22 23 24 25 26 27
37 38 39 40 41 42 43 44
55 56 72 73 74 75 76
87 88 104 105 106 107 108 109
45
77
4.003
26.982 28.086
30.974
32.066 35.453 39.94822.990 24.305
10.812 12.011 14.007 15.999 18.998 20.1806.941 9.012
1.008
65.3863.54658.693 69.723 72.64 74.922
78.96
79.904
83.798
39.098
40.078
44.956 47.867
50.942 51.996
54.938 55.845
58.933
(281)
(223) (226)
(267)
(268) (271) (272) (270) (276)
112.412107.868
106.42
114.818 118.711
121.760
127.60 126.904 131.29485.468 87.62
88.906 91.224 92.906
95.96 (98)
101.07 102.906
200.59196.967
195.085
204.383 207.2 208.980
(209)
(210) (222)
132.905 137.328 178.49
180.948 183.84
186.207
190.23 192.217
2
3
4
5
6
7
1
Lanthanide Series
Actinide Series
Ce
Th
Ytterbium
Nobelium
Thulium
Mendelevium
Erbium
Fermium
Holmium
Einsteinium
Dysprosium
Californium
Terbium
Berkelium
Gadolinium
Curium
Europium
Americium
Praseodymium
Protactinium
Neodymium
Uranium
Promethium
Neptunium
Samarium
Plutonium
Cerium
Thorium
70
102
69
101
68
100
67
99
66
98
65
97
64
96
63
95
59
91
60
92
61
93
62
94
58
90
173.055
(259)
168.934
(258)
167.259
(257)
164.930
(252)
162.500
(251)
158.925
(247)
157.25
(247)
151.964
(243)
140.908
231.036
144.242
238.029
(145)
(237)
150.36
(244)
140.116
232.038
I r
Helium
Aluminum Silicon Phosphorus Sulfur Chlorine ArgonSodium Magnesium
Boron Carbon Nitrogen Oxygen Fluorine NeonLithium Beryllium
Hydrogen
ZincCopperNickel Gallium Germanium Arsenic Selenium Bromine KryptonPotassium Calcium Scandium Titanium Vanadium Chromium Manganese Iron Cobalt
CadmiumSilverPalladium Indium Tin Antimony Tellurium Iodine XenonRubidium Strontium Yttrium Zirconium Niobium Molybdenum Technetium Ruthenium Rhodium
MercuryGoldPlatinum Thallium Lead Bismuth Polonium Astatine Radon
Cesium Barium Hafnium
Tantalum Tungsten Rhenium
Osmium Iridium
Francium Radium
Rutherfordium
Dubnium
Seaborgium
Bohrium
Hassium Meitnerium
8B
Ds
Darmstadtium
Roentgenium
111
Rg
(280)
La
Ac
57
89
(227)
138.905
Lanthanum
Actinium
Lu
Lr
Lutetium
Lawrencium
71
103
174.967
(262)
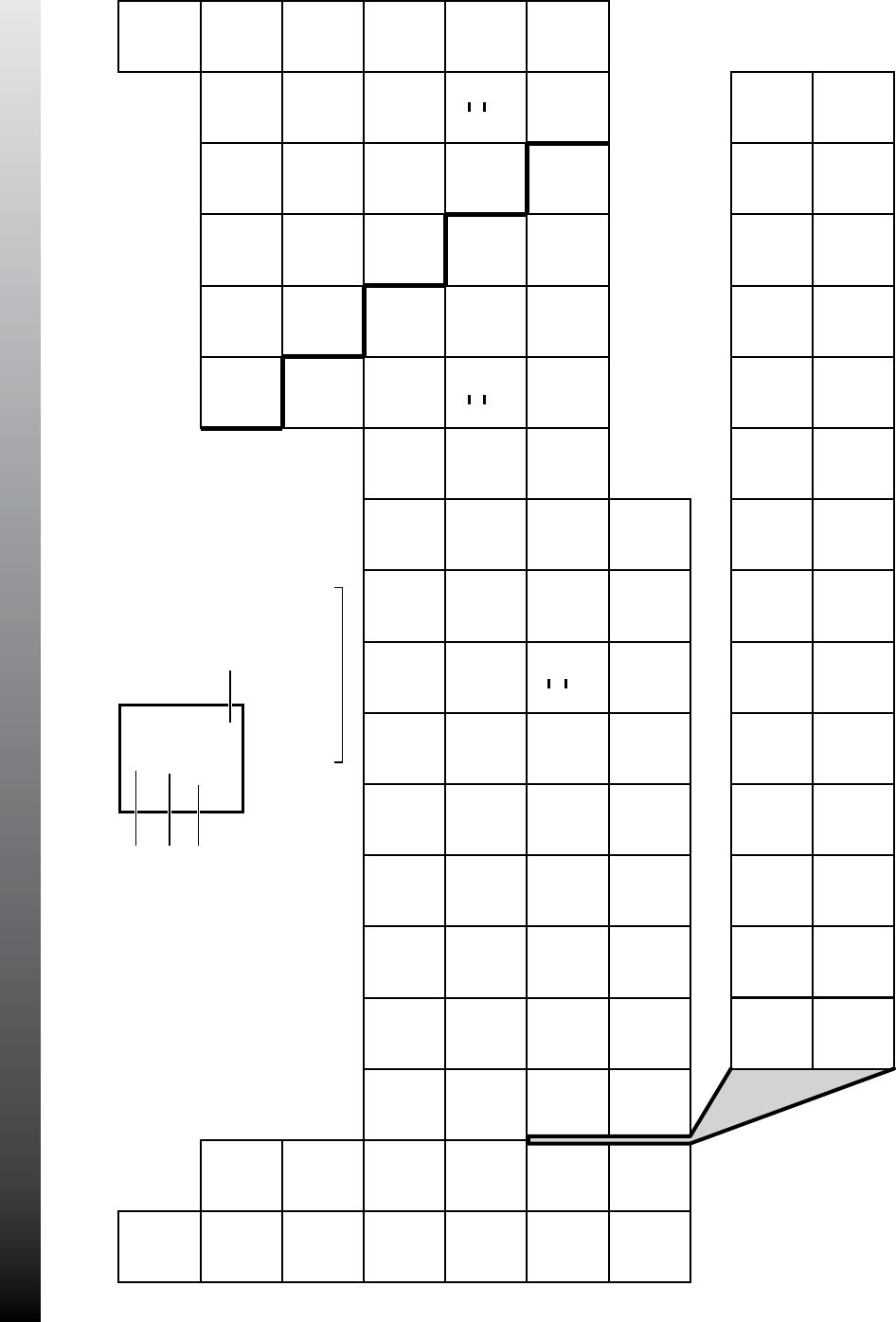
PERIODIC TABLE OF THE ELEMENTS
STAAR CHEMISTRY
REFERENCE MATERIALS
Updated Spring 2011
Page 6
Chemistry
Page 7
12
2B
13
3A
14
4A
15
5A
16
6A
17
7A
18
8A
10 11
1B
He
Hg
Cd
Zn
Au
Ag
Cu
Pt
Pd
Ni
Tl
Ga
Pb
Sn
Ge
Bi
Sb
As
Po
Te
Se
At
Br
Rn
Xe
Kr
Al Si P S Cl Ar
B C N O F Ne
Yb
No
Tm
Md
Er
Fm
Ho
Es
Dy
Cf
Tb
Bk
Gd
Cm
Eu
Am
Mass numbers in parentheses are those of
the most stable or most common isotope.
Si
Silicon
14
Symbol
Atomic number
Name
28.086Atomic mass
In I
K Ca
Na Mg
Li Be
2
2A
H
1
1A
Sc
3
3B
Ti
4
4B
V
5
5B
Cr
6
6B
Mn
7
7B
Fe
8
Co
9
Rb Sr Y Zr Nb Mo Tc Ru
Cs Ba Hf Ta W Re Os
Fr Ra Rf Db
Sg
Bh
Hs
Mt
Pr
Pa
Nd
U
Pm
Np
Sm
Pu
Rh
2
80
48
30
79
47
29
78
110
46
28
81
49
31
82
50
32
83
51
33
84
52
34
85
53
35
86
54
36
13 14 15 16 17 18
5 6 7 8 9 10
19 20
11 12
3 4
1
21 22 23 24 25 26 27
37 38 39 40 41 42 43 44
55 56 72 73 74 75 76
87 88 104 105 106 107 108 109
45
77
4.003
26.982 28.086
30.974
32.066 35.453 39.94822.990 24.305
10.812 12.011 14.007 15.999 18.998 20.1806.941 9.012
1.008
65.3863.54658.693 69.723 72.64 74.922
78.96
79.904
83.798
39.098
40.078
44.956 47.867
50.942 51.996
54.938 55.845
58.933
(281)
(223) (226)
(267)
(268) (271) (272) (270) (276)
112.412107.868
106.42
114.818 118.711
121.760
127.60 126.904 131.29485.468 87.62
88.906 91.224 92.906
95.96 (98)
101.07 102.906
200.59196.967
195.085
204.383 207.2 208.980
(209)
(210) (222)
132.905 137.328 178.49
180.948 183.84
186.207
190.23 192.217
2
3
4
5
6
7
1
Lanthanide Series
Actinide Series
Ce
Th
Ytterbium
Nobelium
Thulium
Mendelevium
Erbium
Fermium
Holmium
Einsteinium
Dysprosium
Californium
Terbium
Berkelium
Gadolinium
Curium
Europium
Americium
Praseodymium
Protactinium
Neodymium
Uranium
Promethium
Neptunium
Samarium
Plutonium
Cerium
Thorium
70
102
69
101
68
100
67
99
66
98
65
97
64
96
63
95
59
91
60
92
61
93
62
94
58
90
173.055
(259)
168.934
(258)
167.259
(257)
164.930
(252)
162.500
(251)
158.925
(247)
157.25
(247)
151.964
(243)
140.908
231.036
144.242
238.029
(145)
(237)
150.36
(244)
140.116
232.038
I r
Helium
Aluminum Silicon Phosphorus Sulfur Chlorine ArgonSodium Magnesium
Boron Carbon Nitrogen Oxygen Fluorine NeonLithium Beryllium
Hydrogen
ZincCopperNickel Gallium Germanium Arsenic Selenium Bromine KryptonPotassium Calcium Scandium Titanium Vanadium Chromium Manganese Iron Cobalt
CadmiumSilverPalladium Indium Tin Antimony Tellurium Iodine XenonRubidium Strontium Yttrium Zirconium Niobium Molybdenum Technetium Ruthenium Rhodium
MercuryGoldPlatinum Thallium Lead Bismuth Polonium Astatine Radon
Cesium Barium Hafnium
Tantalum Tungsten Rhenium
Osmium Iridium
Francium Radium
Rutherfordium
Dubnium
Seaborgium
Bohrium
Hassium Meitnerium
8B
Ds
Darmstadtium
Roentgenium
111
Rg
(280)
La
Ac
57
89
(227)
138.905
Lanthanum
Actinium
Lu
Lr
Lutetium
Lawrencium
71
103
174.967
(262)
1&3*0%*$5"#-&0'5)&&-&.&/54
45""3$)&.*453:
3&'&3&/$&."5&3*"-4
Updated Spring 2011
TX-EOC-CHEM__Release-Form-May-2013__r3__052813.indd 7 5/28/2013 12:34:30 PM
Page 8
TX-EOC-CHEM__Release-Form-May-2013__r3__052813.indd 8 5/28/2013 12:34:30 PM

DIRECTIONS
Read each question carefully. For a multiple-choice question, determine the best
answer to the question from the four answer choices provided. For a griddable question,
determine the best answer to the question. Then fill in the answer on your answer
document.
1 What is the formula of the ion hydrogen sulfite, which has a charge of
−
1?
A SO
−1
3
B SO
−1
4
C HSO
−1
3
D HSO
−1
4
Page 9
2 What is the pH of a substance that has a hydrogen ion concentration of 1.2 × 10
−
2
M?
F 2.08
G 1.92
H 1.00
J 0.080
TX-EOC-CHEM__Release-Form-May-2013__r3__052813.indd 9 5/28/2013 12:34:30 PM
Page 10
3 Which of the following includes an example of a chemical property of an element?
A Aluminum is a solid at room temperature and is a poor thermal insulator.
B Sulfur is not shiny and is not malleable.
C Sodium is a solid at room temperature and reacts with other elements.
D Silicon is shiny and is a poor conductor of electricity.
4 Elements in a group of the periodic table are described below.
Some Properties of a Certain Group of Elements
• Soft silvery-white color
• Good conductor of thermal energy
• Good conductor of electricity
• Atoms contain a single valence electron
These elements most likely belong to which group?
F Alkali metals
G Alkaline earth metals
H Halogens
J Noble gases
TX-EOC-CHEM__Release-Form-May-2013__r3__052813.indd 10 5/28/2013 12:34:30 PM
Page 11
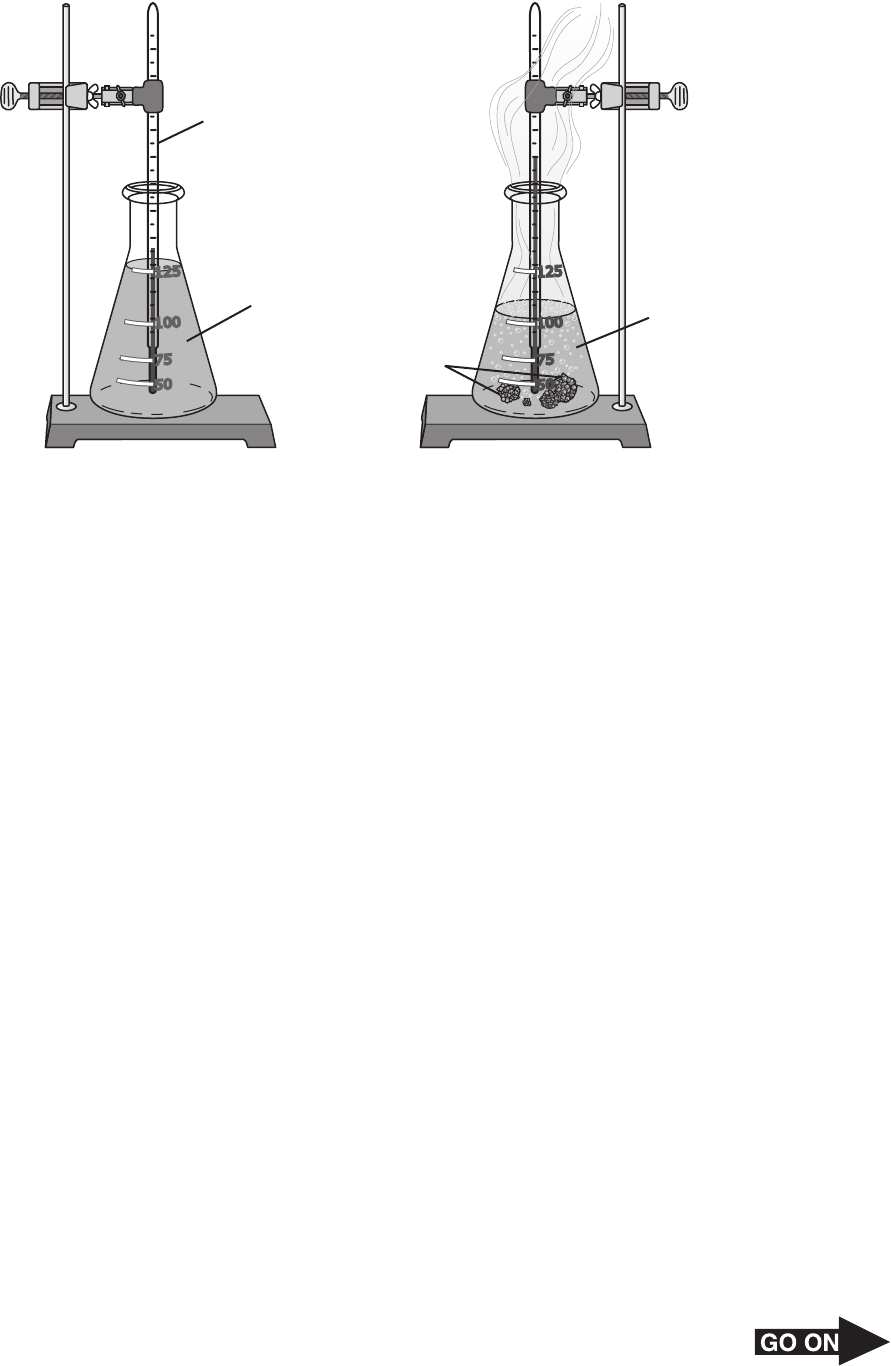
5 The diagram below shows what happens when zinc reacts with hydrochloric acid.
Initial Final
Hydrochloric
acid
Thermometer
Zinc pellets
Hydrogen
(H
2
) gas
75
100
125
50
50
75
100
125
Zn and HCl
Which of these best describes the energy transformation that occurs during this reaction?
A Thermal energy → kinetic energy C Chemical energy → thermal energy
B Kinetic energy → potential energy D Potential energy → chemical energy
TX-EOC-CHEM__Release-Form-May-2013__r3__052813.indd 11 5/28/2013 12:34:30 PM

Page 12
6 The diagram below represents a nuclear reaction.
Energy
NeutronNeutron
Nuclear
fragment 2
Nuclear
fragment 1
Target
nucleus
Neutron
Neutron
Which of the following best describes this reaction?
F Nuclear fusion is occurring because many smaller nuclei are being fused.
G Nuclear fission is occurring because large amounts of energy are being absorbed.
H Nuclear fusion is occurring because many energetic neutrons are being emitted.
J Nuclear fission is occurring because a nucleus is being split into smaller nuclei.
TX-EOC-CHEM__Release-Form-May-2013__r3__052813.indd 12 5/28/2013 12:34:30 PM

Page 13
7 A detail from a label on a bottle of a water-softening agent is shown below.
(2.00 kg)
Which inference about the contents of the bottle can best be drawn?
A They consist of barium chloride anhydrous.
B They consist of barium chloride dihydrate.
C They consist of barium chloride hexahydrate.
D They consist of barium chloride heptahydrate.
8 Heart cells require a certain balance of sodium and potassium ions to function. The blood,
which is approximately 83% water, carries these two types of ions to the heart. The property
of water that allows it to carry ions to the heart is its —
F molecular mass
G specific heat
H polarity
J density
TX-EOC-CHEM__Release-Form-May-2013__r3__052813.indd 13 5/28/2013 12:34:30 PM

Page 14
9 The equation below represents a chemical reaction that produces a gas.
2Na(s) + 2H O(l) → 2NaOH(a q) + H (g)
2 2
What is the theoretical yield in liters of H gas if 5.00 g of Na are completely reacted and the
2
H gas is collected at STP?
2
A 0.109 L
B 2.44 L
C 4.88 L
D 5.09 L
10 Which of these is the electron-dot diagram for Br (l)?
2
F
BrBr
G
BrBr
H
−
Br
−
Br
J
−
Br
−
Br
TX-EOC-CHEM__Release-Form-May-2013__r3__052813.indd 14 5/28/2013 12:34:30 PM
Page 15
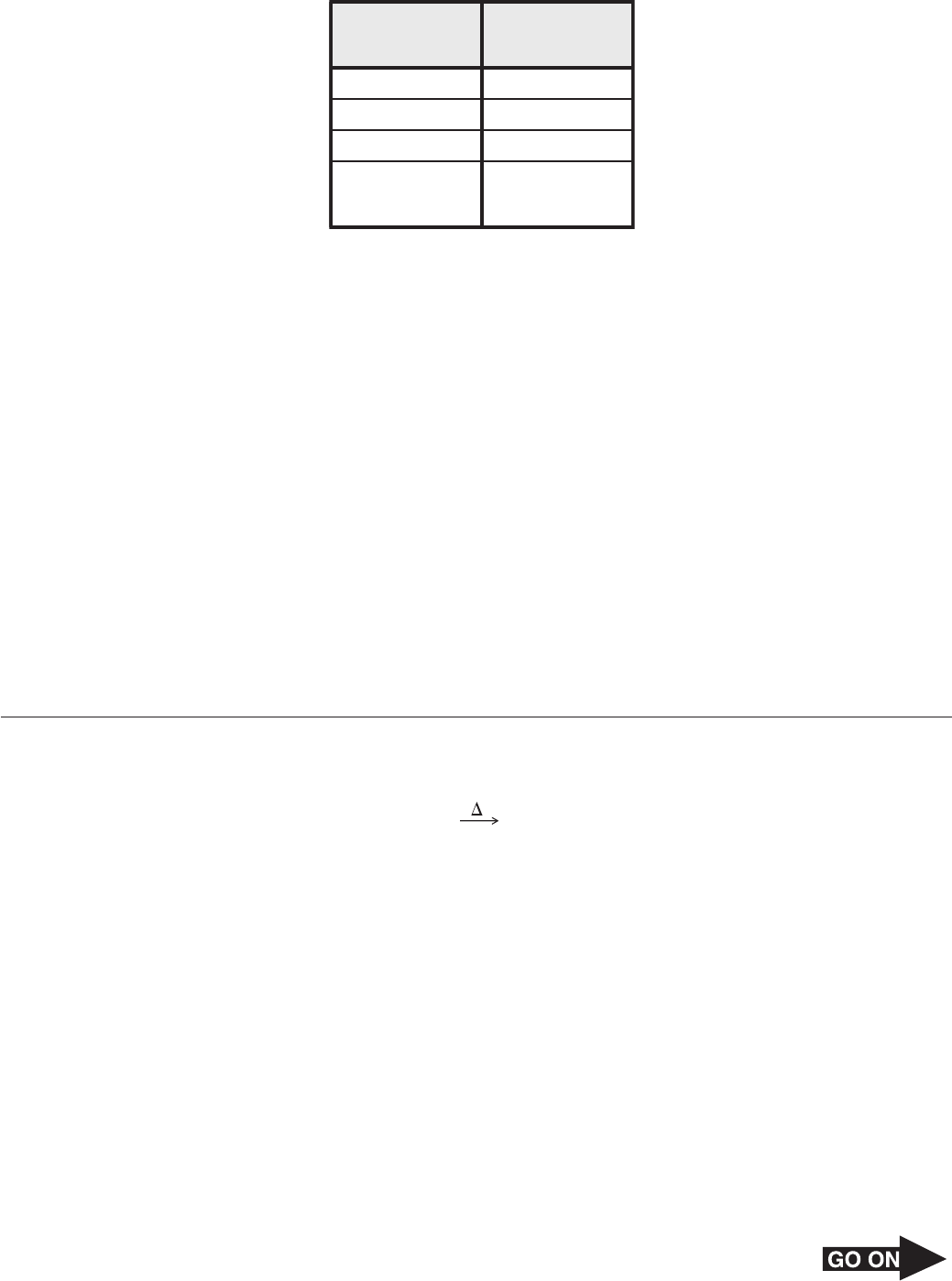
11 The table below lists some properties of a sample of lauric acid.
Property Value
Volume
Mass
Boiling point
Number of
moles
79.6 mL
70.022 g
222°C
0.350
Which of these is an intensive property of this sample?
A Volume
B Mass
C Boiling point
D Number of moles
12 When the equation below is balanced, what is the coefficient for oxygen?
____C
3
H
8
(g) + ____O
2
(g) ____CO
2
(g) + ____H
2
O(g)
Record your answer and fill in the bubbles on your answer document.
TX-EOC-CHEM__Release-Form-May-2013__r3__052813.indd 15 5/28/2013 12:34:30 PM
Page 16
13 Which mixture can be separated through filtration because one of the substances is insoluble
in water?
A NaC lO and Pb(ClO )
3 3 2
B Na SO and SrSO
2 4 4
C NaNO and Pb(NO )
3 3 2
D NaC H O and Pb(C H O )
2 3 2 2 3 2 2
14 Some students used a variety of procedures to investigate four liquid samples. The students
recorded the following information.
• When Sample W was cooled, solid particles settled out of the liquid.
• The mass and volume of Sample X were measured, and the density of Sample X was
calculated to be 1.6 g/mL.
• Sample Y was heated, and the temperature was recorded. All the liquid boiled away at
the same temperature and left no residue in the container.
• When a dilute acid was added to Sample Z, gas bubbles formed and rapidly rose to the
surface of the liquid.
Based on these observations, which sample was clearly identifiable as a pure substance?
F Sample W
G Sample X
H Sample Y
J Sample Z
TX-EOC-CHEM__Release-Form-May-2013__r3__052813.indd 16 5/28/2013 12:34:31 PM

Page 17
15 What is the electron configuration for an atom of germanium at ground state?
A [Ar]4s
2
3d
10
4p
2
B [Ar]4s
2
4d
10
4p
2
C [Kr]4s
2
3d
10
4p
2
D [Kr]4s
2
4d
10
4p
2
16 Which of the following correctly matches a compound with its molecular geometry?
F Water (H O): linear
2
G Carbon dioxide (CO ): tetrahedral
2
H Ammonia (NH ): trigonal planar
3
J Methane (CH ): tetrahedral
4
17 A sample of a compound is added to distilled water in a clean beaker. A reaction occurs, and
the water temperature drops rapidly. Which of the following statements is best supported by
this observation?
A An endothermic reaction occurred.
B A dehydration reaction occurred.
C The water was originally warmer than the compound.
D The beaker was contaminated by another compound.
TX-EOC-CHEM__Release-Form-May-2013__r3__052813.indd 17 5/28/2013 12:34:31 PM

Page 18
18 Use the equation below to answer the following question.
BaCl (aq) + Na SO (aq) 2NaCl(aq) BaSO (s)
2 2 4
→ +
4
The theoretical yield of BaSO is 58.35 g. If 44.34 g of BaSO are produced from the reaction
4 4
shown above, what is the percent yield of BaSO ?
4
F 31.67%
G 52.03%
H 75.99%
J 85.17%
19 When zinc is exposed to air, zinc oxide is produced. What happens in this reaction?
A Zinc is oxidized, and oxygen is reduced.
B Zinc is reduced, and oxygen is oxidized.
C Both zinc and oxygen are oxidized.
D Both zinc and oxygen are reduced.
20 The equation below represents a nuclear reaction.
n
1
0
Al +
? +
27
13
He
4
2
What is the mass number of the missing particle in this reaction?
Record your answer and fill in the bubbles on your answer document.
TX-EOC-CHEM__Release-Form-May-2013__r3__052813.indd 18 5/28/2013 12:34:31 PM
Page 19
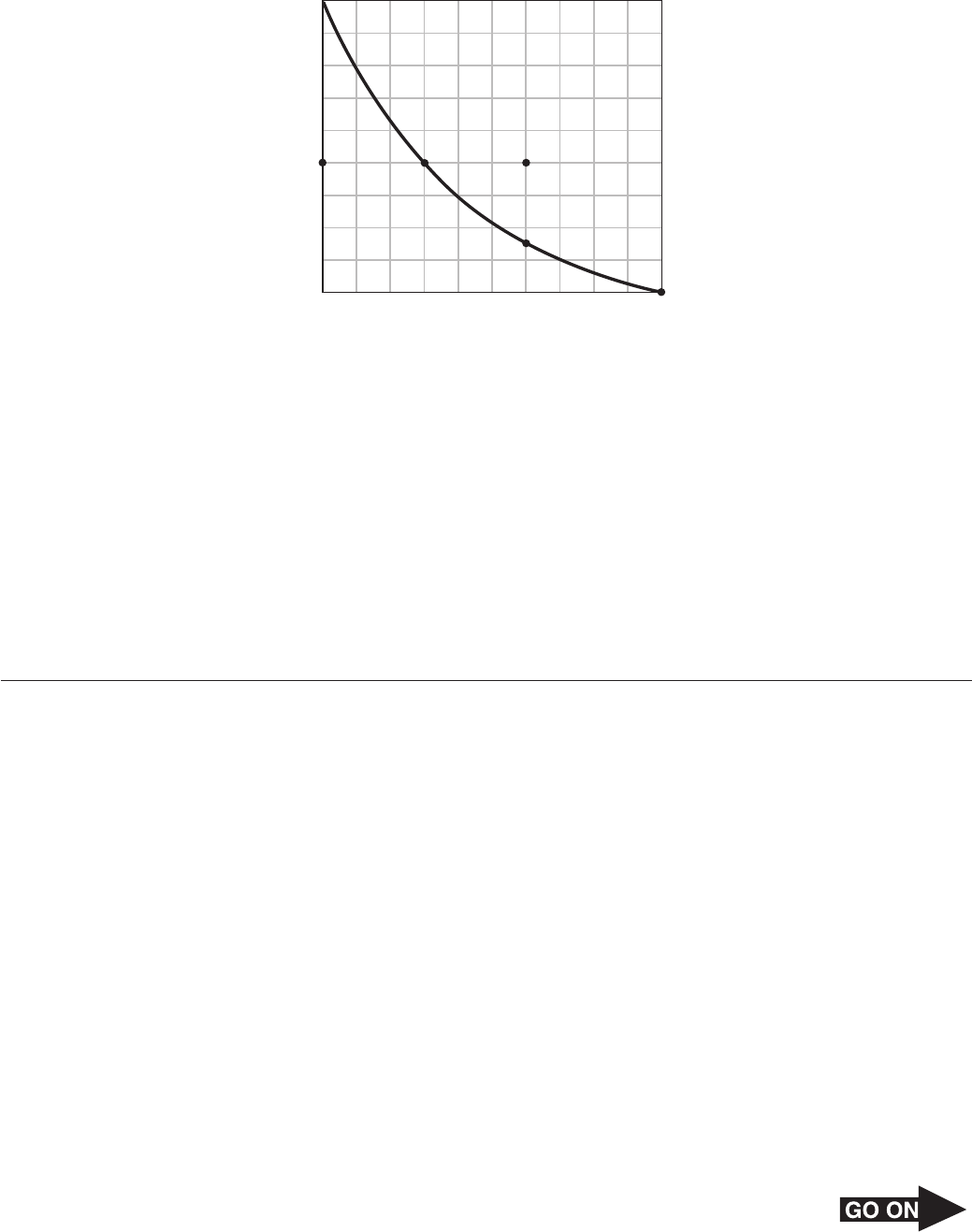
21 The graph below shows a solubility curve for ammonia gas and solubility measurements
taken at different temperatures.
90
80
70
60
50
40
30
20
10
Temperature (°C)
Solubility of Ammonia (NH
3
) Gas vs.
Water Temperature
Solubility (g NH
3
/100 g H
2
O)
Z
Y
V XW
0 10 20 30 40 50 60 70 80 90 100
Between which two points did the ammonia solution change from being unsaturated to
saturated?
A V → W
B W → X
C X → Y
D Y → Z
22 How many molecules are in 0.500 mole of N O ?
2 5
F 1.20 × 10
23
molecules
G 3.01 × 10
23
molecules
H 6.02 × 10
23
molecules
J 3.01 × 10
24
molecules
TX-EOC-CHEM__Release-Form-May-2013__r3__052813.indd 19 5/28/2013 12:34:31 PM
Page 20
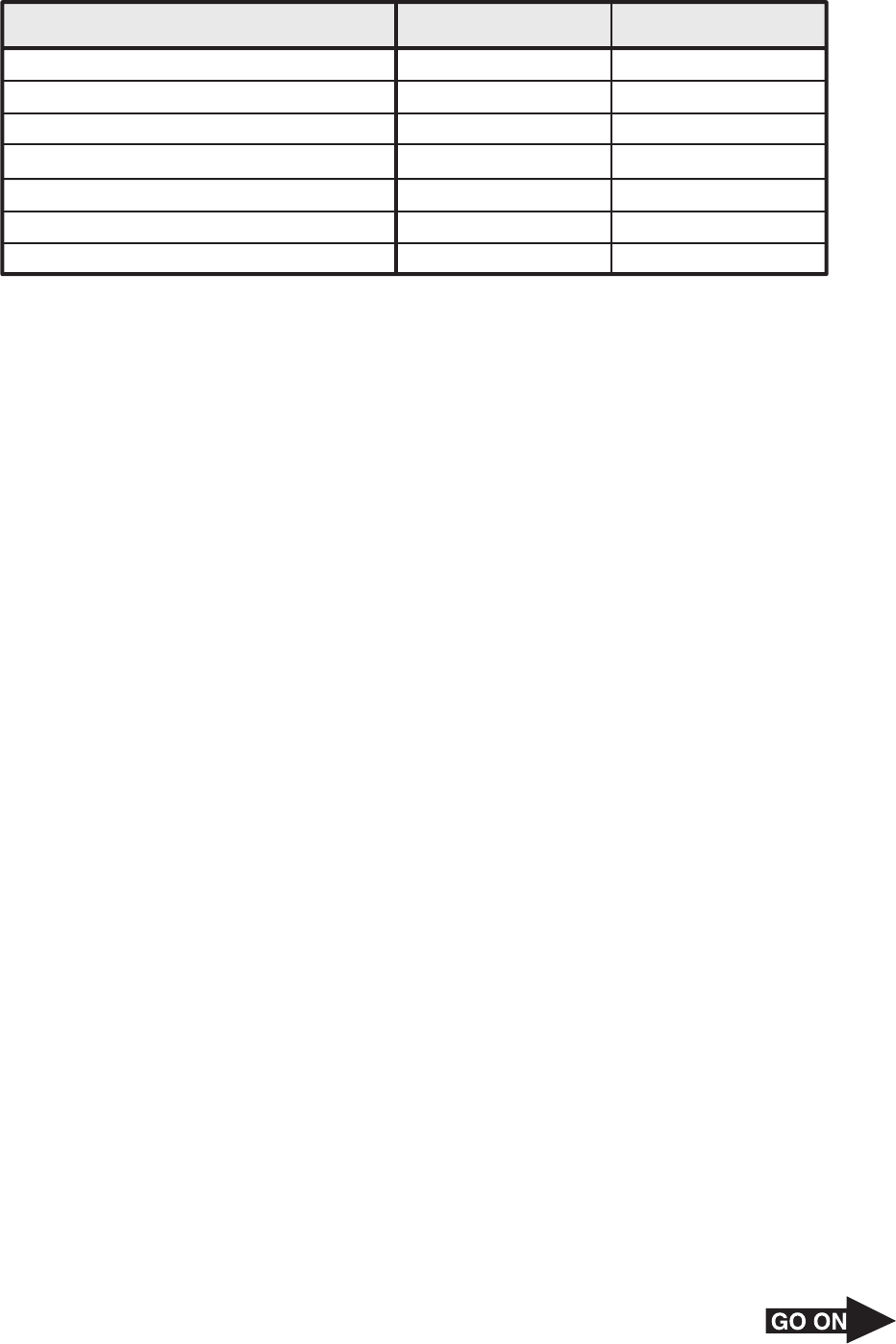
23 The following table lists some properties of copper and sulfur.
Property Copper
Color Reddish
Conductor of electricity Yes
State of matter at room temperature Malleable solid
Metal or nonmetal Metal
Sulfur
Pale yellow
No
Brittle solid
Luster Metallic
Ductile Yes
Density (g/cm
3
)
Dull
No
8.96 2.07
Nonmetal
Samples of copper metal and sulfur powder are placed in the same test tube and heated over
a Bunsen burner. The resulting substance has the following properties.
• Does not conduct electricity
• Has a density of 5.6 g/cm
3
• Has a metallic luster
• Is a black brittle crystalline solid
This black substance is classified as —
A a heterogeneous mixture C a compound
B an element D a homogeneous mixture
TX-EOC-CHEM__Release-Form-May-2013__r3__052813.indd 20 5/28/2013 12:34:31 PM
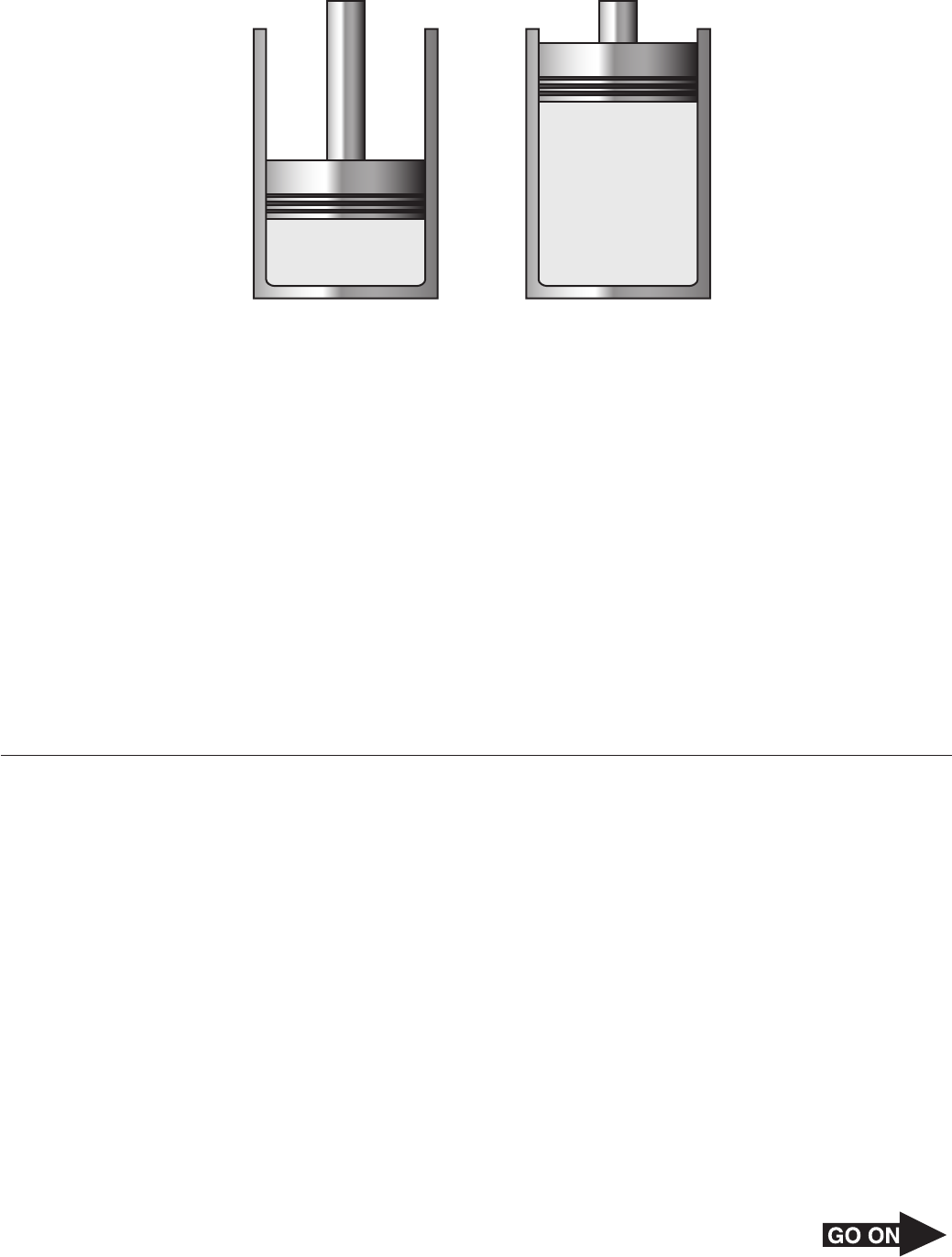
Page 21
24 The diagram below shows a gas with an initial pressure of 3060 mm Hg in a cylinder at a
constant temperature. The gas expands inside the cylinder and pushes the piston up.
V = 2.03 L
Final
Initial
V = 0.520 L
What is the final pressure of the gas after the expansion?
F 544 mm Hg
G 784 mm Hg
H 1830 mm Hg
J 6212 mm Hg
25 Which of these statements is an accurate description of the ionization energies of elements in
the periodic table?
A The ionization energy of lithium is greater than that of potassium.
B The ionization energy of iodine is greater than that of fluorine.
C The ionization energy of magnesium is greater than that of sulfur.
D The ionization energy of krypton is greater than that of neon.
TX-EOC-CHEM__Release-Form-May-2013__r3__052813.indd 21 5/28/2013 12:34:31 PM

Page 22
26 Which of the following shows a correct Lewis dot structure?
F
Si
G
Y
H
Al
J
N
27 What is the volume of 2.00 moles of chlorine (Cl ) at STP, to the nearest tenth of a liter?
2
Record your answer and fill in the bubbles on your answer document.
28 In a famous experiment conducted by Ernest Rutherford, positively charged alpha particles
were scattered by a thin gold foil. Which of the following is a conclusion that resulted from
this experiment?
F The nucleus is negatively charged.
G The atom is a dense solid and is indivisible.
H The mass is conserved when atoms react chemically.
J The nucleus is very small and the atom is mostly empty space.
TX-EOC-CHEM__Release-Form-May-2013__r3__052813.indd 22 5/28/2013 12:34:31 PM
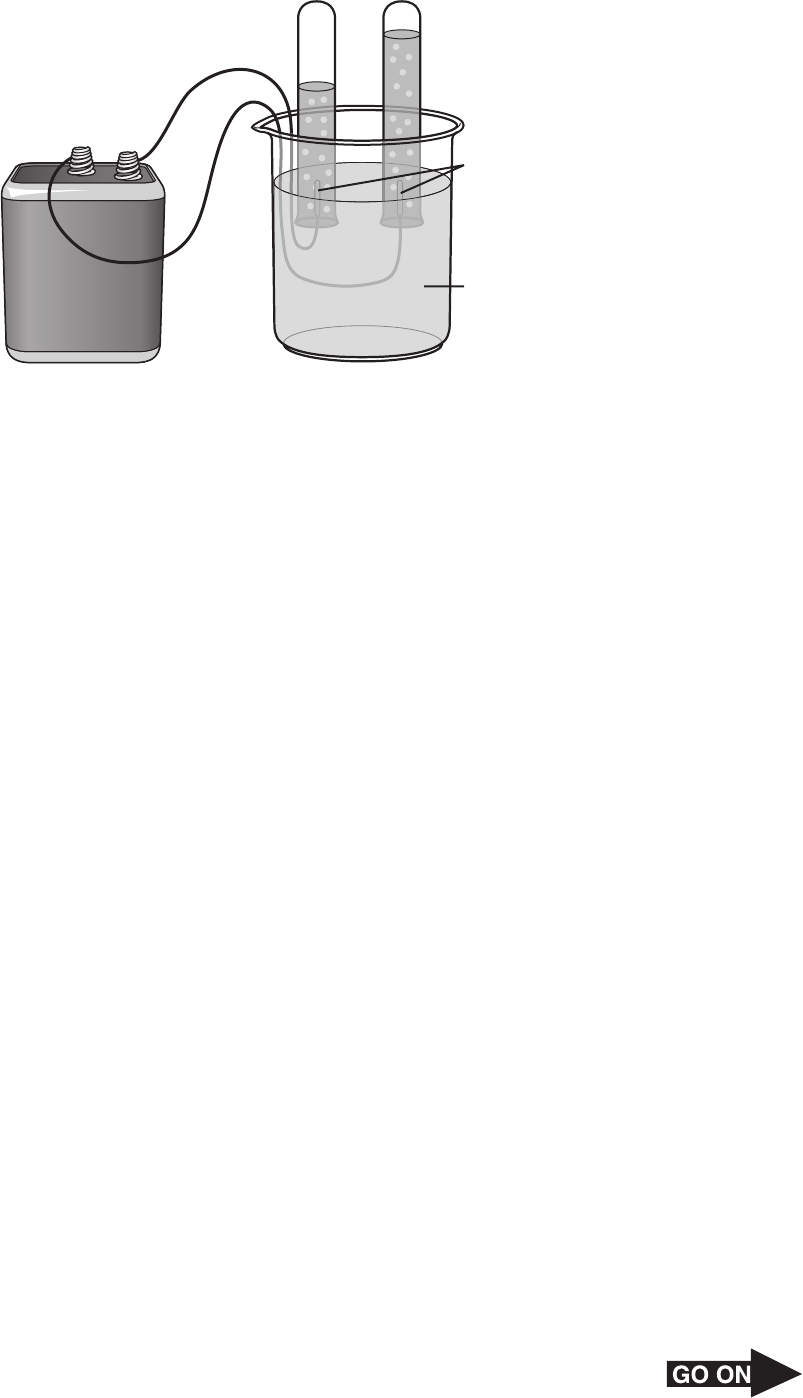
Page 23
29 The diagram below shows a battery giving off a current producing bubbles in two test tubes.
Battery
Test tubes
Electrodes
Na
2
SO
4
(aq)
+
–
Which of the following best shows that the investigation results in a chemical change?
A Liquid condenses on a cold glass rod when gas from the test tube on the left is
released.
B A gas probe indicates that the water in the beaker contains dissolved nitrogen and
oxygen.
C A burning wood splint placed above the mouth of the test tube on the right glows
brighter when some gas is released from the test tube.
D The temperature of the wire connected to the battery increases.
TX-EOC-CHEM__Release-Form-May-2013__r3__052813.indd 23 5/28/2013 12:34:31 PM

Page 24
30 A material safety data sheet (MSDS) for a chemical is shown below.
MSDS
H
3
PO
4
(aq)
Section 9: Physical and Chemical Properties
Physical state and appearance: Viscous liquid
Odor: Odorless
Color: Clear, colorless
Boiling point: 158°C
Melting point: 21°C
Specific gravity: 1.685 at 25°C
Which of these is the IUPAC name for H PO (aq)?
3 4
F Trihydrogen phosphite
G Phosphoric acid
H Phosphorous hydroxide
J Phosphorous acid
TX-EOC-CHEM__Release-Form-May-2013__r3__052813.indd 24 5/28/2013 12:34:31 PM

Page 25
31 The diagram below shows part of Dmitri Mendeleev’s original periodic table, with symbols of
known elements and their atomic masses.
Be = 9.4
B = 11
C = 12
N = 14
O = 16
F = 19
Mg = 24
Al = 27.1
Si = 28
P = 31
S = 32
Cl = 35.5
Cu = 63.4
Zn = 65.2
X
Z
As = 75
Se = 79.4
Br = 80
H = 1
Mendeleev’s arrangement of elements is different than that of the modern periodic table.
Based on Mendeleev’s arrangement, which elements should be placed in the shaded boxes
labeled X and Z respectively?
A Indium (In), because it has a slightly higher atomic mass than aluminum (Al), and tin
(Sn), because it has a slightly higher atomic mass than silicon (Si)
B Cadmium (Cd), because it has chemical properties similar to those of zinc (Zn), and
mercury (Hg), because it has chemical properties similar to those of arsenic (As)
C Antimony (Sb), because it has a slightly higher atomic mass than zinc (Zn), and
bismuth (Bi), because it also has a higher atomic mass than zinc (Zn)
D Gallium (Ga), because it has chemical properties similar to those of aluminum (Al), and
germanium (Ge), because it has chemical properties similar to those of silicon (Si)
32 Which particle has the lightest mass?
F
2
4
He
G
−
1
0
e
H
1
1
H
J
0
1
n
TX-EOC-CHEM__Release-Form-May-2013__r3__052813.indd 25 5/28/2013 12:34:31 PM

Page 26
33 A 5.0 g sample of aluminum with a specific heat of 0.90 J/(g ⋅ °C) was heated from 22.1°C to
32.1°C. How much heat, to the nearest joule, did the aluminum gain?
Record your answer and fill in the bubbles on your answer document.
34 Chemists can identify the composition of some unknown salts by conducting a flame test.
When potassium salts are heated in a flame, a purple color is observed. This is due to
the movement of electrons between energy levels. What is the electron configuration of a
potassium atom at ground state?
F 1s
2
2s
2
2p
6
3s
2
3p
6
4d
1
G 1s
2
2s
2
2p
6
3s
2
3p
6
3d
1
H 1s
2
2s
2
2d
6
3s
2
3d
6
4s
1
J 1s
2
2s
2
2p
6
3s
2
3p
6
4s
1
35 Which product balances the chemical equation below?
3AgNO (aq) + FeCl (aq) 3AgCl(s)
3 3
→ +
A FeCl(aq)
B FeCl (aq)
2
C FeNO (aq)
3
D Fe(NO ) (aq)
3 3
TX-EOC-CHEM__Release-Form-May-2013__r3__052813.indd 26 5/28/2013 12:34:31 PM
Page 27
36 Sodium, mercury, argon, and neon are used in the production of lamps. There are fewer
safety guidelines regarding the handling of neon and argon than for mercury and sodium.
Which of the following best describes the elements within the group of the periodic table that
contains neon and argon gas?
F Gaseous at room temperature and highly reactive with metals
G Solid at room temperature and mildly reactive with strong acids
H Gaseous at room temperature and mostly unreactive with metals
J Solid at room temperature and mostly unreactive with strong acids
37 The table below shows the standard enthalpy of formation for each of three substances.
CaCO
3
(s)
CaO(s)
CO
2
(g)
Compound
−1206.9
−635.1
−393.5
H
f
(kJ/mol)
o
CaCO decomposes according to the equation
CaCO (s) CaO(s) + CO (g).
What is the
3
3 2
enthalpy of reaction?
A 178.3 kJ
B 571.8 kJ
C
−
1029 kJ
D
−
2236 kJ
TX-EOC-CHEM__Release-Form-May-2013__r3__052813.indd 27 5/28/2013 12:34:32 PM

Page 28
38 What is the percentage by mass of sodium (Na) in a formula unit of sodium hydrogen
carbonate (NaHCO )?
3
F 44.2%
G 37.7%
H 27.4%
J 16.7%
39 A form of technetium-99 has a half-life of approximately 6 hours.
43
99
1
0
Tc e→ + +
−
γ
Which substance correctly completes the equation above?
A
42
99
Mo
B
43
94
Te
C
43
99
Es
D
44
99
Ru
40 How many atoms are present in 179.0 g of iridium?
F 5.606 × 10
23
atoms
G 6.464 × 10
23
atoms
H 1.078 × 10
26
atoms
J 1.157 × 10
26
atoms
TX-EOC-CHEM__Release-Form-May-2013__r3__052813.indd 28 5/28/2013 12:34:32 PM

Page 29
41 What volume of 1.0 M sodium phosphate, to the nearest tenth of a liter, must be used to
make 4.0 L of 0.80 M sodium phosphate?
Record your answer and fill in the bubbles on your answer document.
42 X-ray crystallography is a technique that allows scientists to determine the ionic and atomic
radii of elements. Which of these statements correctly describes a trend in ionic or atomic
radii in the periodic table?
F The ionic radius decreases from top to bottom in a group.
G The atomic radius increases from left to right across a period.
H The ionic radius remains constant from right to left across a period.
J The atomic radius increases from top to bottom in a group.
43 Which of the following substances is a strong electrolyte when dissolved in water?
A NaNO
3
B C H OH
2 5
C S Cl
2 2
D C H O
12 22 11
TX-EOC-CHEM__Release-Form-May-2013__r3__052813.indd 29 5/28/2013 12:34:32 PM
Page 30
44 Which of the following best explains why doubling the temperature of an ideal gas in a closed
vessel doubles the pressure?
F Increasing the temperature increases the size of the gas molecules, which then can put
more pressure on the vessel walls.
G Increasing the temperature decreases the volume, causing molecules to strike the
vessel walls more frequently.
H Increasing the temperature causes gas molecules to collide more often and with
enough force to displace electrons.
J Increasing the temperature causes gas molecules to move more rapidly, striking the
vessel walls more frequently and with greater force.
45 Which of the following diagrams correctly represents the formation of a compound consisting
of magnesium and fluorine?
A
F
Mg
F
B
FMg
C
F
F
Mg
D
FMg
TX-EOC-CHEM__Release-Form-May-2013__r3__052813.indd 30 5/28/2013 12:34:32 PM
Page 31
46 Which equation represents a neutralization reaction?
F H CO (aq) CO (g) H O(l)
2 3
→
2
+
2
G H SO (aq) + 2NaOH(aq) → Na SO (aq) + 2H O(l)
2 4 2 4 2
H 2H (g) + O (g) → 2H O(l)
2 2 2
J 2Al(OH) (s) → Al O (s) + 3H O(l)
3 2 3 2
47 A scientist filters a sample of river water. The data from this process are listed below.
Trial
Initial mass of dry
filter paper (g)
Mass of filter paper
after filtering river
water and drying
sample (g)
2.05 2.06 2.04
2.64 2.53 2.61
2 31
These data support which of the following descriptions of the sample?
A It is a pure substance because solid particles cannot pass through the filter paper.
B It is a pure substance because the river water is composed only of free elements.
C It is a mixture because dissolved ions in the water pass through the filter paper.
D It is a mixture because solid particles are separated from the river water.
TX-EOC-CHEM__Release-Form-May-2013__r3__052813.indd 31 5/28/2013 12:34:32 PM

Page 32
48 As a distant star moves away from Earth, the light given off by the star has a measurably
lower frequency. What happens to the wavelength and energy of the photons of light when
the frequency becomes lower?
F The wavelength becomes longer, and the energy decreases.
G The wavelength becomes shorter, and the energy decreases.
H The wavelength becomes longer, and the energy increases.
J The wavelength becomes shorter, and the energy increases.
49 Which of these is a postulate of kinetic molecular theory?
A Molecules of gases have a finite volume.
B Molecules of gases attract and repel one another.
C Collisions between gas molecules are inelastic.
D The kinetic energy of gas molecules depends on temperature.
TX-EOC-CHEM__Release-Form-May-2013__r3__052813.indd 32 5/28/2013 12:34:32 PM
Page 33
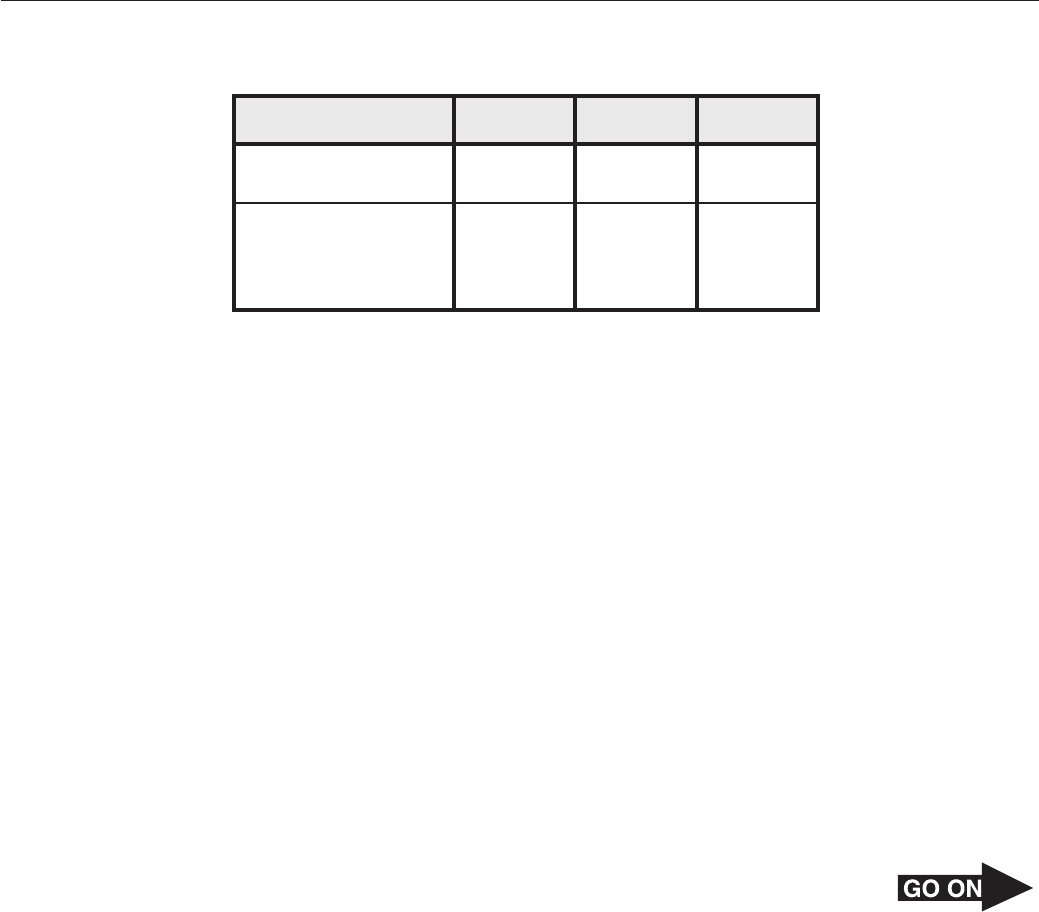
50 The solubility of an unknown substance was tested during an experiment.
Solubility (grams of solute/100 g H
2
O)
120
110
100
90
80
70
60
50
40
30
20
10
10080 9060 7040 502010 300
Temperature (°C)
KNO
3
NaNO
3
KCl
NaCl
Mass of Unknown Solute That Dissolves
in 100 g Water
Trial
1
2
3
Average
80.2 g
81.3 g
79.8 g
80.4 g
63.2 g
61.4 g
62.9 g
62.5 g
46.0 g
45.9 g
44.3 g
45.4 g
30°C 40°C 50°C
Based on the solubility curve information and the results of the experiment, what is most
likely the identity of this unknown solute?
F NaC l
G KCl
H KNO
3
J NaNO
3
TX-EOC-CHEM__Release-Form-May-2013__r3__052813.indd 33 5/28/2013 12:34:32 PM
STOP
Page 34
51 Which of the following best explains why CO gas is easily compressible but solid CO
2 2
(dry ice) is incompressible?
A The molecules of CO gas are much closer together than the molecules in dry ice.
2
B The molecules of solid CO are much closer together than the molecules of CO gas.
2 2
C The molecules of CO gas are much smaller than the molecules of solid CO .
2 2
D The molecules of CO gas attract one another, while the molecules of the solid CO
2 2
repel one another.
52 What is the chemical formula for disulfur decafluoride?
F S F
10 2
G S F
3 9
H S F
2 10
J S F
2 8
TX-EOC-CHEM__Release-Form-May-2013__r3__052813.indd 34 5/28/2013 12:34:32 PM
BE SURE YOU HAVE RECORDED ALL OF YOUR ANSWERS
ON THE ANSWER DOCUMENT.
TX-EOC-CHEM__Release-Form-May-2013__r3__052813.indd 35 5/28/2013 12:34:32 PM
STAAR
Chemistry
May 2013
TX546295 1 2 3 4 5 A B C D E Printed in the USA DPSS/ISD6192
TX-EOC-CHEM__Release-Form-May-2013__r3__052813.indd 36 5/28/2013 12:34:32 PM
