
1
Molecular Machines Stimulate Intercellular Calcium Waves and
1
Cause Muscle Contraction
2
Jacob L. Beckham, Alexis R. van Venrooy, Soonyoung Kim, Gang Li, Bowen Li, Guillaume
3
Duret, Dallin Arnold, Xuan Zhao, Ana L. Santos, Gautam Chaudhry, Jacob T. Robinson,* and
4
James M. Tour*
5
6
J. L. Beckham, A. R. van Venrooy, G. Li, B. Li, D. Arnold, G. Chaudhry
7
Department of Chemistry, Rice University, 6100 Main Street MS 222, Houston, TX 77005 USA.
8
9
S. Kim, G. Duret, X. Zhao
10
Department of Electrical Engineering, Rice University, 6100 Main Street MS 222, Houston, TX
11
77005 USA.
12
13
A. L. Santos
14
Department of Chemistry, Rice University, 6100 Main Street, MS, 222, Houston, TX 77005
15
USA.
16
IdISBA - Fundación de Investigación Sanitaria de las Islas Baleares, Palma, Spain
17
18
Associate Professor J. T. Robinson
19
Department of Bioengineering, Department of Electrical Engineering, Rice University, 6500
20
Main Street, BRC 973, Houston, TX 77005 USA.
21
E-mail: [email protected]
22
23
Professor J. M. Tour
24
Department of Chemistry, Smalley-Curl Institute, NanoCarbon Center, Welch Institute for
25
Advanced Materials, Department of Materials Science and Nanoengineering, Department of
26
Computer Science, Rice University, 6100 Main Street MS 222, Houston, TX 77005 USA.
27
28
.CC-BY-ND 4.0 International licenseavailable under a
(which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprintthis version posted November 28, 2022. ; https://doi.org/10.1101/2022.11.04.515191doi: bioRxiv preprint
2
Abstract
29
Intercellular calcium waves (ICW) are complex signaling phenomena that control many
30
essential biological activities, including smooth muscle contraction, vesicle secretion, gene
31
expression, and changes in neuronal excitability. Accordingly, the remote stimulation of ICW
32
may result in versatile new biomodulation and therapeutic strategies. Here, we demonstrate that
33
light-activated molecular machines (MM), molecules that rotate and perform mechanical work
34
on the molecular scale, can remotely stimulate ICW. Live-cell calcium tracking and
35
pharmacological experiments reveal that MM-induced ICW are driven by the activation of
36
inositol triphosphate (IP
3
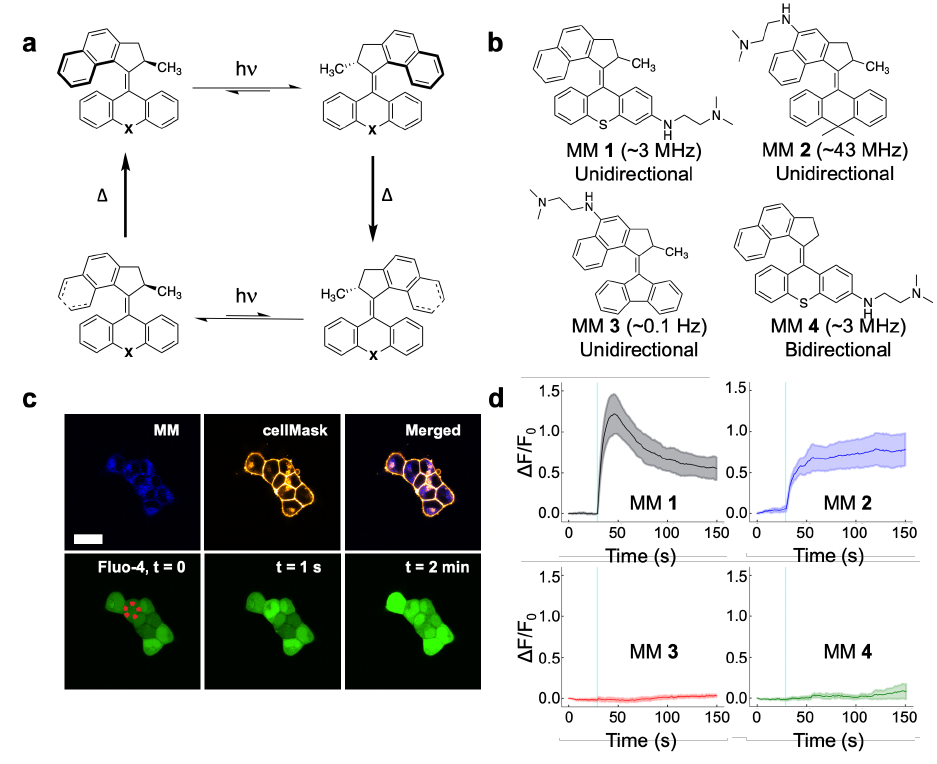
) mediated signaling pathways by unidirectional, fast-rotating MM. We
37
then demonstrated that MM-induced ICW can be used to control muscle contraction in vitro in
38
cardiomyocytes and animal behavior in vivo in Hydra vulgaris. Consequentially, this work
39
demonstrates a new strategy for the direct control of cell signaling and downstream biological
40
function using molecular-scale devices.
41
.CC-BY-ND 4.0 International licenseavailable under a
(which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprintthis version posted November 28, 2022. ; https://doi.org/10.1101/2022.11.04.515191doi: bioRxiv preprint
3
Main
42
Calcium signaling impacts nearly every process relevant to cellular life, and the ability of
43
calcium ions to alter protein shape and charge by reversible binding constitutes the most ubiquitous
44
signaling motif in receptor biology
1
. The localized nature of calcium signaling, as well as its ability
45
to activate downstream effector proteins, allows it to drive a vast array of biological activities. In
46
single cells, calcium directly controls cellular proliferation
2
, gene expression
3,4
, differentiation
5
,
47
movement
6
, and metabolism
7
. In organisms, calcium signals propagate through secondary
48
messengers to cause intercellular calcium waves (ICW) that coordinate concerted action in whole
49
tissues
8
. ICW play direct or indirect roles in processes ranging from muscle contraction
9
,
50
potentiation of neuronal firing
10
, blood vessel dilation
11
, digestion
12
, and breathing
13
.
51
Dysfunctional calcium signaling contributes to disease states ranging from cancer to
52
cardiovascular disease and neurodegenerative disorders
14-15
.
53
Due to the multiplexed nature of calcium signaling
16
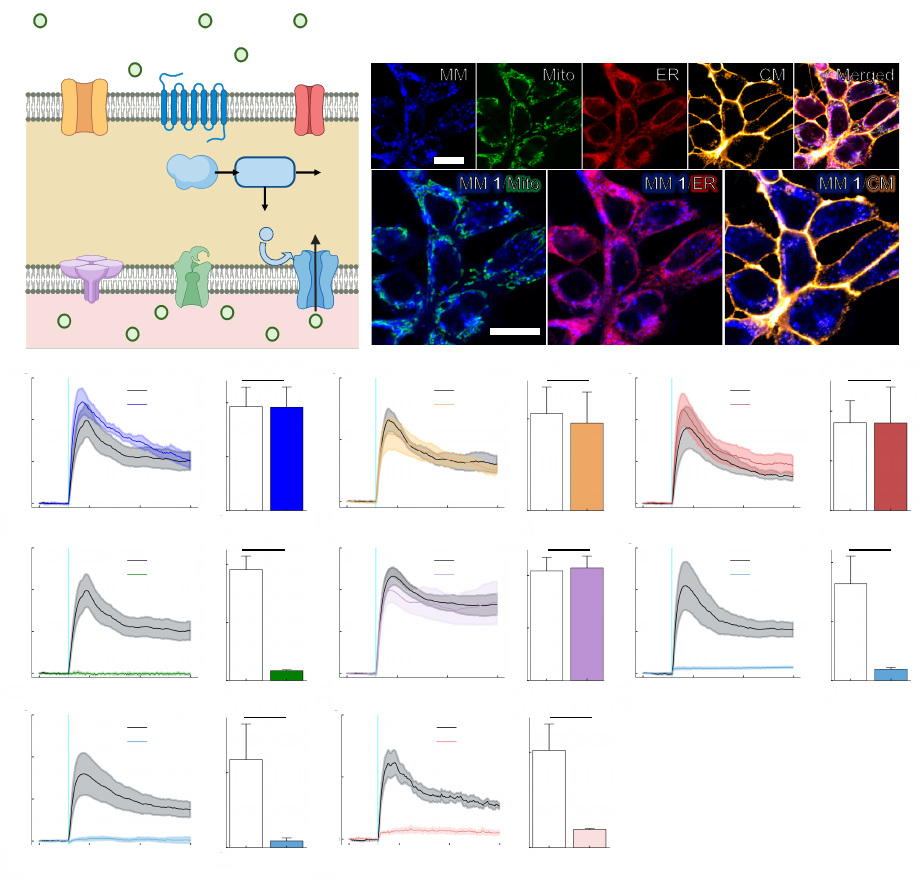
, the ability to remotely trigger ICW
54
with high spatiotemporal precision may permit access to numerous downstream signaling
55
pathways, offering a dynamic new strategy for the control of biological activity. Such advances
56
may also yield new therapeutic avenues for diseases characterized by calcium signaling
57
dysfunction. Currently, ICW are largely initiated experimentally by chemical methods
17
or by
58
applying mechanical stimuli using a micropipette attached to a microcontroller
2,8,18
.
59
Here, we describe the generation of ICW via the nanomechanical action of a light-activated
60
molecular machine. Molecular machines (MM) are molecules that can be activated by external
61
stimuli, such as light, to perform mechanical work on the molecular scale
19
. Just as the mechanical
62
perturbation of a cell’s outer membrane causes intracellular calcium responses, the application of
63
mechanical force via a fast, unidirectionally rotating MM elicits calcium release from the
64
.CC-BY-ND 4.0 International licenseavailable under a
(which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprintthis version posted November 28, 2022. ; https://doi.org/10.1101/2022.11.04.515191doi: bioRxiv preprint
4
endoplasmic reticulum (ER). Slow-rotating and non-unidirectional MM do not elicit calcium flux
65
under the same conditions, implicating a mechanism of action that depends on rapid, unidirectional
66
molecular rotation. Calcium release is attenuated by the inhibition of inositol triphosphate (IP
3
)-
67
mediated signaling, suggesting the IP
3
signaling pathway as a primary driver of cellular responses.
68
Finally, we show that MM-induced ICW can be exploited to control downstream biological
69
processes, like muscle contraction, both in vitro and in vivo.
70
71
Results and Discussion
72
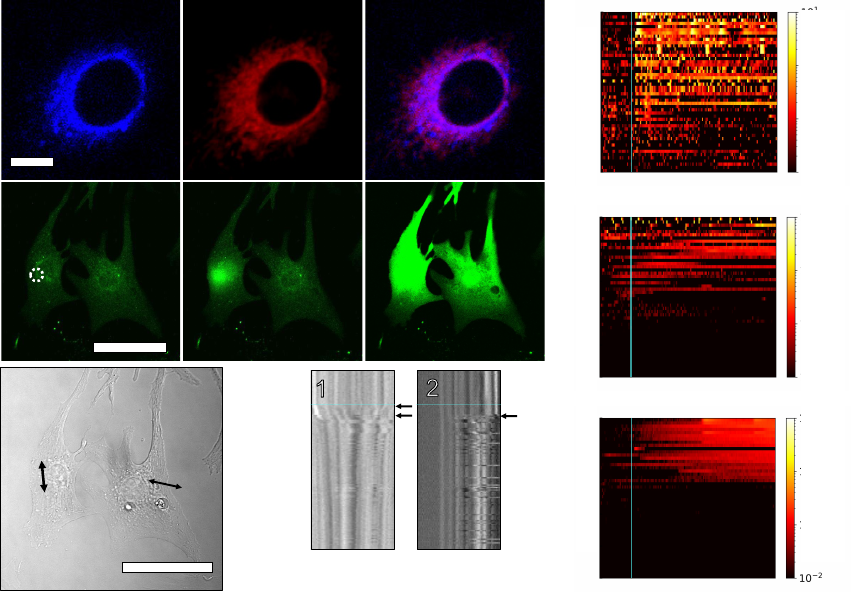
Fast, Unidirectional MM Induce Calcium Waves
73
MM employed in this study have overcrowded alkene motors based on the primary design
74
by Feringa et al
19
. Their typical structure consists of a rotor connected to a stator by an
75
atropisomeric alkene. When these MM are excited by incident photons, the rotor rotates
76
unidirectionally relative to the stator, undergoing two photoisomerization steps and two thermal
77
helix inversions before returning to the starting position (Fig. 1a). Overcrowded alkene motors
78
locomote in solution
20
, drill through synthetic lipid bilayers and cell membranes
21
, and have
79
previously been used to exert mechanical forces on individual cell-surface receptors via antibody
80
targeting
22
. Here, we study cellular responses to MM administered without the use of chemical
81
targeting or extracellular scaffolds, facilitating their use in vivo. The moiety “X” in Fig. 1a can be
82
interchanged to modulate MM rotation rate, which is determined by the favorability of the thermal
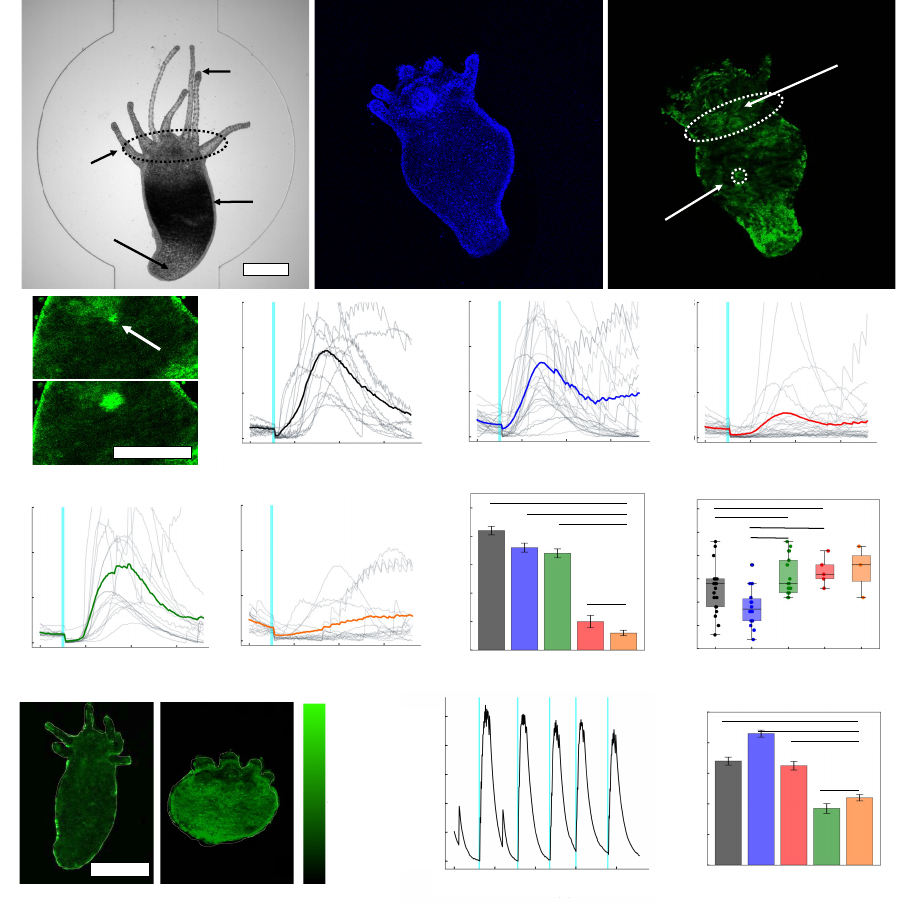
83
helix inversion step
23,24
.
84
To investigate cellular responses to the actuation of MM, we employed live-cell calcium
85
tracking. HEK293 cells were treated with the fluorescent intracellular calcium indicator Fluo-4
86
and loaded with MM. The structures of the MM employed in this study are shown in Fig. 1b, and
87
.CC-BY-ND 4.0 International licenseavailable under a
(which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprintthis version posted November 28, 2022. ; https://doi.org/10.1101/2022.11.04.515191doi: bioRxiv preprint
5
the calcium responses of cells treated with fast-rotating MM 1 are shown in Fig. 1c. Stimulation
88
of a single MM 1-treated cell with a 400 nm laser (3.2×10
2
W cm
-2
) increased Fluo-4 fluorescence
89
in the targeted cell, reflecting a spike in the intracellular calcium concentration similar to that
90
observed when cells are mechanically perturbed with a micropipette
8,18
. In the presence of the
91
vehicle only, no calcium responses were evoked by the same laser treatment (Supplementary Fig.
92
1). Calcium responses were observed to propagate to adjacent cells (Supplementary Fig. 2)
93
according to the degree of electrical connectivity between individual colonies. Similar responses
94
were observed when cells were treated with scavengers of reactive oxygen species (ROS,
95
Supplementary Fig. 3) and in experiments using X-Rhod-1 in place of Fluo-4 (Supplementary Fig.
96
4), suggesting that the observed responses do not depend on the production of ROS or fluorescence
97
resonant energy transfer between MM 1 and calcium tracking dyes.
98
Cellular responses to MM were repeatable (Supplementary Fig. 5), and their amplitude
99
could be controlled by the intensity of incident light (Supplementary Fig. 6). The strength of the
100
evoked response also determines the downstream effects of stimulation. At typical stimulation
101
irradiances (3.2×10
2
W cm
-2
for 250 ms), cells recovered from stimulation and showed no signs of
102
apoptosis or necrosis (see Supplementary Fig. 7-8 and the accompanying discussion). Cells
103
stimulated at higher intensities (6.4×10
2
W cm
-2
for 4 s) showed membrane blebbing and calcium
104
accumulation over a 30 min period, indicating cell death (Supplementary Fig. 9)
25
. Hence, MM-
105
induced ICW can be tuned between physiological, supraphysiological, and pathophysiological
106
response regimes by adjusting the stimulus intensity. Calcium responses induced by MM 1
107
actuation were also observed in other cell lines, including neuroblast (N2A; Supplementary Fig.
108
10) and HeLa cells (Supplementary Fig. 11).
109
.CC-BY-ND 4.0 International licenseavailable under a
(which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprintthis version posted November 28, 2022. ; https://doi.org/10.1101/2022.11.04.515191doi: bioRxiv preprint
6
We investigated the calcium responses elicited by a library of MM consisting of two fast-
110
rotating motors (MM 1-2) and two complementary motors (MM 3-4) used to test the effects of
111
rotation speed and directionality (Fig. 1b). MM 1, mimicking designs previously shown to kill
112
cancer cells and antibiotic-resistant bacteria
26-28
, was synthesized with a six-membered ring stator
113
containing a central sulfur atom. MM 1 rotates unidirectionally at ~3 MHz. MM 2 and MM 3 are
114
chemically similar to MM 1 but rotate at different rates. MM 2, which rotates at ~43 MHz, was
115
synthesized with a six-member ring stator with two methyl groups branching off the center carbon
116
atom
24
. MM 3, a slow-rotating motor that rotates at ~0.1 Hz
24
, was synthesized with a fluorene
117
stator. Finally, MM 4 is an analog to MM 1 that lacks a stereogenic center at the allylic methyl
118
site, which confers preference for unidirectional rotation. Without this stereogenic center, MM 4
119
“flaps” bidirectionally and switches stochastically between photoisomerization states. All four of
120
these motors possess an appended aniline that shifts their absorption into the visible spectrum,
121
enabling their observation by visible-light microscopy and the activation of their rotation with
122
visible light
28
. These terminal amines are protonated at physiological pH, promoting their
123
interaction with the hydrophilic heads of lipid bilayers in cell or organelle membranes.
124
Fig. 1d shows the calcium responses of cells treated with each MM and light. MM 3 was
125
used at 3x concentration because of its lower extinction coefficient relative to the other MM
126
(Supplementary Fig. 12, Supplementary Table 1). Fast-rotating MM 1 and MM 2 elicited rapid
127
increases in intracellular calcium. MM 1 elicited high-amplitude transients that peak ~10-20 s after
128
stimulation and then decay over the next minute, whereas MM 2 elicited a more stable increase in
129
intracellular calcium that does not decay as quickly as MM 1. The different calcium release kinetics
130
induced by MM 1 and MM 2 may be related to differences in their photoisomerization efficiency
131
(see the discussion in the Supplemental Information)
23
. Meanwhile, slow-rotating MM 3 elicited
132
.CC-BY-ND 4.0 International licenseavailable under a
(which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprintthis version posted November 28, 2022. ; https://doi.org/10.1101/2022.11.04.515191doi: bioRxiv preprint
7
no change in calcium activity upon irradiation, even at 3x the concentration and twice the stimulus
133
intensity used to activate MM 1 and MM 2 (Fig. 1d). MM 4, the fast-rotating motor with no
134
preference for unidirectional rotation, elicited only small changes in calcium concentration. This
135
experiment provides evidence to link MM rotation speed and directionality to their evoked cell
136
signaling behavior.
137
138
Mechanistic Study of MM-Induced ICW
139
Next, we studied the biological mechanism behind MM-driven calcium signaling. Calcium
140
equilibrium in the cytosol is regulated by both export across the plasma membrane and uptake into
141
the ER via membrane ATPases (Fig. 2a)
1
. Consequentially, the cytosolic concentration of calcium
142
is typically low (~100 nM) compared to those found inside the ER or extracellular medium (~1.5
143
mM). Cytosolic calcium spikes commonly involve the entry of calcium from one of these two
144
locations. Fluorescence microscopy showed that MM internalize within cells and interact with
145
subcellular organelles, including mitochondria and the ER (Fig. 2b; Supplementary Figs. 13-14).
146
Since MM are distributed primarily within cells, we hypothesized that MM-induced calcium
147
responses arise from the release of calcium from intracellular stores. To test this hypothesis, we
148
depleted calcium stores inside and outside of HEK293 cells and blocked various plasma membrane
149
or ER calcium channels prior to stimulation (see Supplementary Table 2 for a complete list of the
150
manipulations employed in these experiments).
151
Cells treated with MM 1 and stimulated by light pulses in the absence of extracellular
152
calcium (Fig. 2c) showed no differences in response amplitude compared to a positive control in
153
calcium-containing medium. Similar results were observed when plasma membrane calcium
154
channels were blocked. Treatment of cells with ruthenium red (RR), a pharmacological inhibitor
155
.CC-BY-ND 4.0 International licenseavailable under a
(which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprintthis version posted November 28, 2022. ; https://doi.org/10.1101/2022.11.04.515191doi: bioRxiv preprint
8
of temperature-sensitive vanilloid transient receptor potential (TRP) channels
29
, did not decrease
156
the magnitude of MM-induced calcium responses (Fig. 2d). Similarly, treatment of cells with Gd
3+
,
157
which is commonly used to block the effects of mechanosensitive plasma membrane channels such
158
as Piezo1, Piezo2, and TRPC4
30
, also did not affect the observed responses (Fig. 2e). In all, our
159
experiments show that plasma membrane TRP channels and extracellular calcium do not
160
appreciably contribute to MM-induced calcium responses.
161
On the other hand, cells treated with MM 1 and thapsigargin, a sarco-endoplasmic
162
reticulum calcium pump (SERCA) antagonist that depletes intracellular calcium
31
, did not show
163
any measurable calcium flux upon light stimulation (Fig. 2f; P = 0.00257 < 0.01). This result
164
implies that MM-evoked calcium responses arise from the release of ER-bound calcium stores by
165
MM 1.
166
Further mechanistic studies were conducted to determine how MM activation releases
167
calcium from the ER. The mammalian ER predominantly expresses two large tetrameric calcium
168
channels: IP
3
receptors (IP
3
Rs) and ryanodine receptors (RyRs)
1,32
. In IP
3
-mediated calcium
169
signaling, G-protein coupled receptors (commonly Gq/11 subtypes) expressed in the plasma
170
membrane activate phospholipase Cβ (PLCβ) and tyrosine kinase receptors activate PLCɣ to
171
cleave phosphatidylinositol 4,5-biphosphate into diacylglycerol and IP
3
32
. IP
3
can then diffuse to
172
the ER, where it binds to IP
3
R and causes calcium release. The activation of this network drives
173
such a wide array of cellular activities that it is often referred to as simply “calcium release” due
174
to its ubiquity in receptor biology
1
. RyRs are also expressed in many cell types and amplify
175
existing calcium signals through calcium-induced calcium release.
176
Treatment of cells with ryanodine (Ry; 100 µM) to block RyR signaling has no effect on
177
response amplitude (Fig. 2g) in HEK293 cells. However, treatment of cells with xestospongin C
178
.CC-BY-ND 4.0 International licenseavailable under a
(which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprintthis version posted November 28, 2022. ; https://doi.org/10.1101/2022.11.04.515191doi: bioRxiv preprint
9
(XeC; 25 µM), a known antagonist of IP
3
R
33
, significantly diminished the strength of cellular
179
responses to MM (Fig. 2h; P = 0.0167 < 0.05). Similar effects were observed when cells were
180
treated with the PLC antagonist U-73122 (U-73; 10 µM) as an alternate method of blocking IP
3
181
signaling (Fig. 2i; P = 0.0281 < 0.05). Furthermore, MM-induced responses were also inhibited
182
in cells treated with cytochalasin D (Cyto D; 2 µM), an inhibitor of F-actin polymerization that
183
disrupts PLC signaling by increasing the spatial distance between PLC and IP
3
R (Fig. 2j; P =
184
0.01762 < 0.05)
34
. These results implicate IP
3
signaling as a primary driver of MM-induced
185
calcium waves. The IP
3
pathway is known to contribute to mechanosensitive calcium currents
35
186
and is also involved in the induction of ICW in response to mechanical stimulation with a
187
micropipette
2,18
.
188
189
MM Cause Muscle Contraction In Cardiomyocytes
190
Next, we investigated whether MM-elicited ICW could be used to modulate calcium-
191
driven biological processes, such as the beating activity and contraction of cultured
192
cardiomyocytes. Fig. 3 shows the effects of MM stimulation on primary rat cardiomyocytes. MM
193
distribute to the sarcoplasmic reticulum (SR; Fig. 3a), where subsequent light activation triggers
194
localized calcium release (Fig. 3b, Supplementary Movie 1). MM-induced calcium release initially
195
leads to localized myocyte contraction at the site of stimulation (Fig. 3c, kymograph 1, top arrow)
196
likely due to the calcium-mediated activation of troponin and subsequent actin-myosin cross-
197
bridge formation
36
. Then, SR calcium release induces beating in quiescent cardiomyocytes and
198
accelerated beating in active cardiomyocytes (Fig. 3c, bottom arrow in kymograph 1 and arrow in
199
kymograph 2; Supplementary Movie 2).
200
.CC-BY-ND 4.0 International licenseavailable under a
(which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprintthis version posted November 28, 2022. ; https://doi.org/10.1101/2022.11.04.515191doi: bioRxiv preprint
10
We tracked the behavior of colonies of cardiomyocytes in contact with cells stimulated
201
with MM and light to determine whether we could use MM to drive biological behaviors
202
coordinated in networks of cells, such as contraction. Colonies of cardiomyocytes adjacent to
203
stimulated cells responded to stimulation by firing action potentials (APs) or participating in the
204
generated calcium wave (Fig. 3d; Supplementary Figs. 15-16). Activation of adjacent
205
cardiomyocytes in response to stimulation could be prevented by either inhibiting IP
3
-mediated
206
calcium release in the stimulated cell (Fig. 3e) or by preventing the influx of calcium from outside
207
the cell during AP firing (Fig. 3f). Cells stimulated with MM 1 and light in calcium-containing
208
medium exhibited firing rates of 5.1 spikes min
-1
cell
-1
, whereas cells stimulated with MM 1 and
209
light in the presence of PLC inhibitor (U-73; 10 µM) exhibited firing rates of ~0.8 spikes min
-1
210
cell
-1
(P < 0.0001 by a one-tailed Welch’s t-test). Meanwhile, cells stimulated in the absence of
211
extracellular calcium did not exhibit any spiking activity, suggesting that the increased activity of
212
cardiomyocyte colonies in response to MM may be due to the action of voltage-gated ion channels
213
in the plasma membrane of myocytes. These ion channels are likely triggered by local
214
depolarization of the membrane induced by SR calcium release
37
. These experiments show that
215
biological behaviors coordinated in networks of cells, such as contraction, can be controlled by
216
intracellular MM-induced calcium wave generation.
217
218
MM Control Behavior In Vivo
219
Finally, using an in vivo model of muscle contraction, we sought to investigate whether
220
MM-induced ICW can control biological activity at the organism level. For this purpose, we chose
221
Hydra vulgaris as a model system. Hydra are radially symmetric, millimeter-sized freshwater
222
cnidarians containing tentacles, an oral region, and an aboral region connected by a long, tubular
223
.CC-BY-ND 4.0 International licenseavailable under a
(which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprintthis version posted November 28, 2022. ; https://doi.org/10.1101/2022.11.04.515191doi: bioRxiv preprint
11
body column. In the oral region, Hydra have a dome-shaped structure called a “hypostome”
224
surrounded by a ring of tentacles. At the other extremity, they have a foot called the “peduncle,”
225
which the animal uses to attach to substrates (Fig. 4a). Hydra were chosen as a model system
226
because of their small size, lack of chitin layer, excitable epitheliomuscular tissue, and simple
227
nervous system. In addition, Hydra exhibit spontaneous and stimulus-controlled contractions, both
228
driven by ICW
38
, which can be directly visualized due to their transparent body. In our
229
experiments, we used Hydra lines genetically engineered to express the calcium indicator
230
GCaMP7b in their endothelial epitheliomuscular tissue (see Methods).
231
Prior to stimulation experiments, Hydra were loaded with MM by incubating with solutions
232
containing 24 µM of MM for 24 h (Fig. 4b). Distinct stimulation protocols were employed to cause
233
either local ICW or whole-body contraction (Fig. 4c). First, treatment of MM-loaded Hydra with
234
pulses of laser light administered to a small region of the body column (Protocol I) caused ICW
235
emanating from the site of stimulation (Fig. 4d; Supplementary Fig. 17; Supplementary Movies
236
3-4). Similar to ICW evoked in vitro, these ICW propagated throughout the Hydra body column
237
according to the degree of electrical connectivity of the stimulated cells. Distinct propagation
238
kinetics were observed when stimulating different regions of Hydra simultaneously
239
(Supplementary Movie 5). Second, we attempted to use MM activation to drive whole-body Hydra
240
contractions by administering laser stimuli to the oral region (Protocol II). We decided to target
241
the oral region because mechanical stimulation of this region has been shown to stimulate burst
242
contraction, likely via the sensory neurons that cluster in this region
39-41
. When laser stimuli were
243
delivered to the oral region of Hydra treated with fast-rotating MM 1 and 2 (Protocol II), they
244
exhibited contraction bursts associated with whole-body calcium waves (Supplementary Movie 6)
245
similar to those observed with macro-mechanical stimulation
42
.
246
.CC-BY-ND 4.0 International licenseavailable under a
(which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprintthis version posted November 28, 2022. ; https://doi.org/10.1101/2022.11.04.515191doi: bioRxiv preprint
12
Fast-rotating MM were generally more successful in eliciting both regional ICW (Protocol
247
I) and whole-body contractions (Protocol II). Fig. 4e-i show responses from MM-treated Hydra
248
when stimulated via Protocol I to elicit regional ICW. Furthermore, Fig. 4j compares the response
249
rates of Hydra treated with each MM for exhibiting regional ICW. Fast-rotating MM, including
250
MM 1, MM 2, and even non-unidirectional fast motor MM 4, consistently elicited robust regional
251
ICW upon stimulation of the body column (Protocol I). Occasional responses were also observed
252
from Hydra treated with slow-rotating MM 3, but the response rate of these Hydra was not
253
significantly different from solvent-only controls (Fig. 4j). Hydra also demonstrated marked
254
differences in response kinetics depending on the type of MM employed. Fast, unidirectionally
255
rotating MM elicited appreciably quicker responses (12.76 s for MM 1 and 9.05 s for MM 2) than
256
slow or non-unidirectionally rotating MM (15.46 s for MM 3 and 16.6 s for MM 4; Fig. 4k).
257
Similar trends were observed for whole-body contractile response rates across Hydra
258
treated with different MM and light conditions (see Methods for a detailed description of the
259
response rate calculation). Fig. 4l-m show a representative whole-body contraction and GCaMP7b
260
fluorescence trace from a typical experiment using MM 2 and stimulation using Protocol II. In
261
these experiments, the fastest-rotating MM, MM 2, was most successful at inducing Hydra
262
contraction, demonstrating a response rate of 86% (Fig. 4n). This fast-rotating MM elicited
263
contraction of Hydra at a higher rate than light alone (P < 0.00001) or slower-rotating MM 3 (P <
264
0.00001) by Fisher’s Exact Test. MM 1 also elicited contraction at a significantly higher rate than
265
light alone (P = 0.0199 < 0.05), while the slow-rotating MM 3 (P = 0.5325 > 0.05) did not elicit
266
contraction at a significantly higher rate than light alone. Interestingly, MM 4, which rotates
267
quickly but non-unidirectionally, elicited Hydra contraction with a response rate of 65% and was
268
significantly (P = 0.0439 < 0.05) more effective than light alone. MM 4 also elicited regional ICW
269
.CC-BY-ND 4.0 International licenseavailable under a
(which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprintthis version posted November 28, 2022. ; https://doi.org/10.1101/2022.11.04.515191doi: bioRxiv preprint
13
in Hydra despite causing only weak responses in vitro. These results imply that even the smaller
270
responses elicited by MM 4 in vitro can be amplified in vivo across networks of cells.
271
Consequentially, rotor speed is a better indicator of the ability of MM to drive Hydra contraction
272
than rotor unidirectionality. Further experiments are needed to elucidate both the biological
273
machinery responsible for amplifying MM-induced signals and the factors influencing MM
274
propensity for causing ICW in Hydra (see the discussion in the Supplemental Information).
275
The contractile behavior of Hydra in relation to the presentation of stimulus is detailed in
276
Supplementary Figs. 18-22. Hydra appeared to exhibit a photic response to light in the absence of
277
MM (Supplementary Fig. 22; P = 0.0525 > 0.05) consistent with the photosensitivity of the
278
hypostome and tentacles described previously
43
. However, fast-rotating MM actuation was notably
279
superior at driving muscle contraction compared to light alone. Peak identification algorithms were
280
employed to track calcium spikes in the fluorescence data and contraction bursts across the
281
timescale of our experiments. In Hydra treated with MM 2 and light, contraction onset occurs
282
predominantly upon the presentation of the stimulus, and a high density of contraction bursts
283
appears 5-10 seconds later (Supplementary Fig. 19). In the absence of MM, this relationship
284
weakens dramatically. In the absence of light, it disappears completely (Supplementary Fig. 22).
285
These results suggest that the actuation of fast-rotating MM can control the behaviors exhibited by
286
Hydra over the time scale of our experiments.
287
288
Conclusion
289
This work demonstrated that the activation of MM triggers calcium waves in a fashion that
290
depends on their fast, unidirectional rotation. MM localized to the endoplasmic reticulum, where
291
their activation with sub-second pulses of visible light caused a rapid rise in intracellular calcium
292
.CC-BY-ND 4.0 International licenseavailable under a
(which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprintthis version posted November 28, 2022. ; https://doi.org/10.1101/2022.11.04.515191doi: bioRxiv preprint
14
that propagated to neighboring cells. These ICW were shown to arise from IP
3
signaling. The
293
amplitude of MM-induced ICW was tunable by the laser light intensity. MM-induced ICW
294
remotely triggered muscle contraction in vitro and in vivo, suggesting that MM can be used for
295
molecular-scale mechanical control of biological activity.
296
297
298
.CC-BY-ND 4.0 International licenseavailable under a
(which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprintthis version posted November 28, 2022. ; https://doi.org/10.1101/2022.11.04.515191doi: bioRxiv preprint
15
Experimental Methods.
299
Synthetic Chemistry
300
Details on the synthesis and characterization of MM 2-4 are provided in the Supplementary
301
Information (Materials & Methods; Supplementary Figs. 23-28). Synthesis and characterization
302
information on MM 1 is provided elsewhere
28
. MM were dissolved in DMSO at a concentration
303
of 8 mM and sonicated for 5 s prior to use. MM solutions were stored at -20
o
C in aluminum foil-
304
wrapped containers to avoid degradation. UV-Vis spectra of MM were taken in spectral-grade
305
water using a Shimadzu UV-3600 Plus spectrophotometer. Extinction coefficients were calculated
306
by constructing a Beer’s Law plot using concentrations between 8 and 32 µM.
307
308
Cell Culture and Preparation of Cells for Microscopy
309
HEK293 cells were chosen as the principal model system due to their widespread use in
310
electrophysiological studies. HEK cells, like most excitable or non-excitable cells, are known to
311
exhibit calcium responses
44
. HEK293 cells, HeLa cells, and N2A cells were cultured in DMEM
312
(Lonza) supplemented with 10% FBS (Gibco), and 1% penicillin/streptomycin (Lonza) at 37
o
C
313
in 5% CO
2
atmospheric conditions. Cells were passaged at <90% confluence. To grow
314
cardiomyocytes, a dissolved rat heart (TransnetYX
©
) was triturated, centrifuged, and resuspended
315
in cardiomyocyte growth medium (TransnetYX
©
catalog #SKU-NBCG) and seeded at a
316
concentration of 60,000 cells cm
-2
. Growth substrates were pre-treated with 1% gelatin solution
317
for 3 h, then washed with PBS. Cardiomyocytes were allowed to grow for 4 days, after which the
318
growth medium was exchanged for cardiomyocyte maintenance medium (TransnetYX
©
catalog
319
#SKU-NBCM). Experiments were conducted from day 4 to day 6.
320
.CC-BY-ND 4.0 International licenseavailable under a
(which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprintthis version posted November 28, 2022. ; https://doi.org/10.1101/2022.11.04.515191doi: bioRxiv preprint
16
Imaging was performed in imaging extracellular buffer (iECB; 119 mM NaCl, 5 mM KCl,
321
10 mM HEPES, 2 mM CaCl
2
, 1 mM MgCl
2
(pH 7.2); 320 mOsm). Cells were prepared for
322
imaging by seeding a 35 mm Ibidi imaging dish with ~50,000 cells in 1 mL of complete growth
323
medium, and grown for 2 days. Prior to imaging, cells were incubated with dyes and/or molecules
324
resuspended in complete growth medium at the appropriate concentration [MM 1 (8 µM), MM 2
325
(8 µM), MM 3 (24 µM), MM 4 (8 µM), Fluo-4 (2 µM)]. MM and Fluo-4 were incubated with cells
326
for 45 min. Unless otherwise specified, all experiments were performed in triplicate.
327
328
In vitro Imaging and Stimulation
329
Cells were imaged with a Nikon A1-Rsi confocal system mounted on a widefield Ti-E
330
fluorescence microscope. Imaging was performed using a 60x water immersion objective (NA of
331
1.27, 0.17 mm working distance). Green fluorophores (Fluo-4, MitoTracker Green) were excited
332
with a 488 nm photodiode laser. Red fluorophores (ER-Tracker-Red, PI) were excited with a 561
333
nm photodiode laser. Deep red fluorophores (CellMask plasma membrane stain) were excited with
334
a 630 nm photodiode laser. Laser stimulation for in vitro experiments was performed with a 400
335
nm photodiode laser (Coherent OBIS
TM
LX SF) operating in a fluorescence-recovery-after-
336
photobleaching (FRAP) experiment mode, delivering up to 6.4
×
10
2
W cm
-2
at sample level. In
337
vitro experiments with HEK293 cells were conducted using a stimulus irradiance of 3.2
×
10
2
W
338
cm
-2
except where otherwise indicated. Cardiomyocyte experiments were conducted using a
339
stimulus irradiance of 5.1
×
10
2
W cm
-2
. Power was calibrated using a Thor Labs S130C laser power
340
meter. Stimulation was targeted to a circular area of diameter 5 µm in a 250 ms pulse, during which
341
the laser rastered across the entire region of interest. An additional laser stimulation set-up was
342
used to verify the requisite stimulation power and demonstrate excitation in a non-scanning laser
343
.CC-BY-ND 4.0 International licenseavailable under a
(which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprintthis version posted November 28, 2022. ; https://doi.org/10.1101/2022.11.04.515191doi: bioRxiv preprint
17
mode (see Supplementary Fig. 29 and the accompanying discussion). Fluo-4 fluorescence was
344
collected for ~30 s before and 2 min after stimulation (since the recorded time of the first data
345
point is 0 s, stimulation occurs between 28.6 and 28.85 s in recorded traces). Images were collected
346
using a Galvano scanner operating at 0.94 fps. In some experiments, images were also collected
347
using a resonant scanner operating at 7.7 fps.
348
349
Colocalization Analysis
350
Cells were treated with MitoTracker Green (ThermoFisher, 400 nM), ER Tracker Red
351
(ThermoFisher, 500 nM), and CellMask Deep Red Plasma Membrane Stain (ThermoFisher, 500
352
nM). ER Tracker Red and MitoTracker Green were loaded into cells for 45 min in complete growth
353
medium. Then, CellMask Deep Red Plasma Membrane Stain was loaded into cells for 5 min and
354
cells were subsequently loaded into the microscope chamber. Images for co-localization analysis
355
were collected in a Nikon A1 confocal microscope using a 60x water immersion objective. Z-stack
356
images spanning a minimum 20 µm range were collected and processed using the Coloc-2 plugin
357
in Fiji. Colocalized pixel intensity maps were generated using the colocalization threshold function
358
in Fiji.
359
360
Pharmacological Experiments.
361
In pharmacological blocking experiments, cells were loaded with MM and Fluo-4 as
362
previously described. Typical imaging experiments were then performed on six cells in iECB alone
363
as a positive control. Then, the imaging buffer was replaced, and the cells were treated as required
364
for each experiment (see below). After a brief recovery period, six non-previously imaged cells
365
were stimulated and imaged. Phosphate-buffered saline (PBS) was used for experiments in
366
.CC-BY-ND 4.0 International licenseavailable under a
(which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprintthis version posted November 28, 2022. ; https://doi.org/10.1101/2022.11.04.515191doi: bioRxiv preprint
18
calcium-free buffer. Thapsigargin (Th; 1 µM) and cytochalasin D (Cyto D; 2 µM) were incubated
367
with cells in the incubator in complete growth medium for 1 h (Th) or 2 h (Cyto D) before imaging.
368
Ruthenium red (RR; 1 µM), gadolinium (Gd
3+
; 50 µM), ryanodine (Ry; 100 µM), xestospongin C
369
(XeC; 20 µM), and U-73122 (U-73; 10 µM) were administered directly into the iECB after control
370
imaging was finished. Cells were incubated in the medium for ~5 min prior to imaging. For
371
experiments using ROS scavengers, cells were incubated with either melatonin (Mel; 100 µM),
372
thiourea (thioU; 50 mM), or L-ascorbic acid (VitC; 2 mM) for 1 h prior to experiments. The traces
373
shown are representative results across at least six cells from individual experiments, while bar
374
graphs represent the average results across at least three distinct experiments. See Supplementary
375
Table 2 for more information.
376
377
Hydra Preparation
378
Hydra were grown in Hydra medium containing CaCl
2
⋅2H
2
O (1 mM), MgCl
2
⋅6H
2
O (0.1
379
mM), KNO
3
(0.03 mM), NaHCO
3
(0.5 mM), and MgSO
4
(0.08 mM) in deionized water at 18
o
C
380
in a light-cycled (12 h light, 12 h dark) incubator. Hydra were fed with an excess of freshly brined
381
Artemia Nauplii (Brine Shrimp Direct, Ogden, UT, # BSEP 8Z) three times a week. All
382
experiments were performed at room temperature after starving the animals for 2 days. The
383
transgenic line expressing calcium indicator GCaMP7b under the Ef1α promoter in endodermal
384
epitheliomuscular tissue was generated by embryonic microinjection in a collaboration by the
385
Robinson lab (Rice University) and the Juliano lab (University of California, Davis)
42
.
386
387
Hydra Imaging and Stimulation
388
.CC-BY-ND 4.0 International licenseavailable under a
(which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprintthis version posted November 28, 2022. ; https://doi.org/10.1101/2022.11.04.515191doi: bioRxiv preprint
19
Hydra were incubated with the selected MM for 24 h before imaging. Stimuli were applied
389
using an ROI-driven fluorescence-recovery-after-photobleaching (FRAP) mode in a Nikon A1
390
confocal microscope. All Hydra were able to recover after stimulation experiments, indicating
391
both MM administration and subsequent stimulation treatments were not toxic to the Hydra.
392
Distinct stimulation protocols were employed to elicit either regional ICW or whole-body
393
contraction. In Protocol I, stimulation was delivered using a 405-nm laser diode at 9.0
×
10
2
W cm
-
394
2
in 1 s pulses delivered to a 10 µm region of the Hydra body column. Protocol I resulted in regional
395
ICW. In Protocol II, stimulation was delivered using a 405-nm laser diode at 9.0
×
10
2
W cm
-2
in
396
a 2 s pulse delivered to the oral region of the Hydra (typical area of ~1,000-2,000 µm
2
). Protocol
397
II resulted in whole-body calcium waves and contraction. Note that despite the longer stimulation
398
time used in Protocol II, the Hydra still experiences less pixel dwell time and less incident light
399
per unit area compared to in vitro experiments and Protocol I due to the size of the stimulation
400
region (~50-100x larger depending on the size of the animal). GCaMP7b fluorescence were
401
recorded using a 456 nm laser diode for fluorophore excitation. In experiments using Protocol I,
402
stimuli were presented at 10 s. In experiments using Protocol II, stimuli were presented at irregular
403
intervals at least 60 s apart to prevent interference from periodic spontaneous contractions of
404
Hydra.
405
406
Data Analysis
407
Data were analyzed using custom-written Python scripts. Fluorescence traces of the
408
calcium indicator Fluo-4 from cells were imported and processed using the Pandas library. F
0
was
409
calculated as the average fluorescence intensity in the first 10 frames (~10 s) of the acquisition and
410
used to calculate
ΔF/𝐹
!
over the entire length of the acquisition. Since data was not collected
411
.CC-BY-ND 4.0 International licenseavailable under a
(which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprintthis version posted November 28, 2022. ; https://doi.org/10.1101/2022.11.04.515191doi: bioRxiv preprint
20
during stimulation, a “dead time” equivalent to the time of stimulation was manually added to each
412
recording. Spiking behavior of cardiomyocytes was calculated using the SciPy peak finder
413
function.
414
Fluorescence traces of calcium indicator GCaMP7b in Hydra vulgaris were processed
415
similarly to in vitro data and baseline-corrected to set the minimum measured
ΔF/𝐹
!
value as “0”
416
due to the spontaneous, periodic activity of the Hydra. For experiments analyzing regional ICW
417
(stimulating using Protocol I), responses reaching
ΔF/𝐹
!
> 1 in the stimulated area were
418
considered a “success” in the calculation of the ICW response rate. The “delay time” was the time
419
at which
ΔF/𝐹
!
reached 1. Peaks corresponding to whole-body contraction were not included in
420
the analysis of ICW amplitude. ICW response rate was calculated across n=25 experiments for
421
each condition across at least 5 Hydra.
422
In experiments analyzing whole-body contraction (stimulating using Protocol II), the
423
induction of a contractile “response” comprising a change in body GCaMP7b fluorescence of
424
ΔF/𝐹
!
' >
1.5x within 3 s of stimulus presentation was counted as a “success” in the calculation of
425
the contractile response rate. To identify contraction onset, a limit-of-detection peak finding
426
algorithm was used with a threshold value of 1.5. To identify individual calcium spikes in a
427
contraction burst, the SciPy peak finder function was employed. Induction of a contractile
428
"response" comprising a change in body GCaMP7b fluorescence of
ΔF/𝐹
!
> 1.5x within 3 s of
429
stimulus presentation was considered a "success" when calculating response rates. Response rates
430
were calculated over at least 50 presentations of stimuli. Stimuli presented at a time when the
431
Hydra were already contracting (
ΔF/𝐹
!
> 0.3) were discarded. Hydra that exhibited mouth-
432
opening behavior during the experiment were discarded. The effects of spontaneous and photic
433
.CC-BY-ND 4.0 International licenseavailable under a
(which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprintthis version posted November 28, 2022. ; https://doi.org/10.1101/2022.11.04.515191doi: bioRxiv preprint
21
responses were accounted for by comparison with DMSO and DMSO and light controls. For more
434
information on Hydra data processing, see Supplementary Table 3.
435
436
Statistical Analysis
437
One-tailed Welch’s t-tests were performed to assess differences in cellular responses after
438
pharmacological manipulations, or when treated with MM of different rotation speeds. Fisher’s
439
Exact Tests was used to assess differences in contractile response rates between Hydra vulgaris
440
treated with MM of variable rotation speed and light. * P-value < 0.05, ** P-value < 0.01, ***
441
P-value < 0.001, **** P-value < 0.0001.
442
443
Acknowledgements.
444
This project received funding from the Discovery Institute, the Robert A. Welch
445
Foundation (C-2017-20190330), the National Science Foundation Graduate Research Fellowship
446
Program (JLB), the DEVCOM Army Research Laboratory under Cooperative Agreement
447
W911NF-18-2-0234 (AVV), and the European Union's Horizon 2020 research and innovation
448
programme under the Marie Skłodowska-Curie grant agreement No. 843116 (ALS). The views
449
and conclusions contained in this document are those of the authors and should not be interpreted
450
as representing the official policies, either expressed or implied, of the Army Research Laboratory
451
or the U.S. Government. The U.S. Government is authorized to reproduce and distribute reprints
452
for Government purposes notwithstanding any copyright notation herein. The funders had no role
453
in the study design, data collection and analysis, decision to publish, or preparation of the
454
manuscript. This work was conducted in part using resources from the Light Microscopy Facility
455
and the Shared Equipment Authority at Rice University. The authors acknowledge Zachary C.
456
.CC-BY-ND 4.0 International licenseavailable under a
(which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprintthis version posted November 28, 2022. ; https://doi.org/10.1101/2022.11.04.515191doi: bioRxiv preprint
22
Sanchez (Vanderbilt University) for useful help and advice regarding growth and activity of
457
cardiomyocytes.
458
459
Author Contributions.
460
Conceptualization, JLB, ARV, JMT.
461
Methodology, JLB, GD, SK, JZ, ALS.
462
Organic synthesis: ARV, GL, BL, JMT.
463
Formal analysis, JLB.
464
Investigation, JLB, SK, GC, DA, JZ.
465
Resources, JTR, JMT.
466
Writing, Original draft, JLB.
467
Writing, Reviewing, and Editing, JLB, GD, SK, ALS, JTR, JMT.
468
Visualization, JLB.
469
Supervision, GD, JTR, JMT.
470
Funding acquisition, JMT.
471
Project oversight: JMT.
472
473
Declaration of interests
474
Rice University owns intellectual property on the use of electromagnetic (light) activation
475
of MM for the stimulation of intercellular calcium waves. Conflicts of interest are managed
476
through regular disclosure to the Rice University Office of Sponsored Projects and Research
477
Compliance. The authors declare no other competing interests.
478
Biological materials
479
.CC-BY-ND 4.0 International licenseavailable under a
(which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprintthis version posted November 28, 2022. ; https://doi.org/10.1101/2022.11.04.515191doi: bioRxiv preprint
23
All biological materials used in this work are available from commercial sources.
480
Data availability
481
All data supporting the findings of this study are available within the article and its supplementary
482
information files.
483
Code availability.
484
Data analysis scripts are available upon request to the author, JMT.
485
486
.CC-BY-ND 4.0 International licenseavailable under a
(which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprintthis version posted November 28, 2022. ; https://doi.org/10.1101/2022.11.04.515191doi: bioRxiv preprint
24
487
Fig. 1. Structures of MM used in this study, their mechanism of rotation, and their activation
488
to induce ICW in HEK293 cells. (a) The rotation cycle of a typical MM, showing two
489
photoisomerizations (hn) and two thermal (Δ) helix inversions to complete a unidirectional
490
rotation. The moiety in the “X” position can be changed to synthesize MM of different rotation
491
speeds. (b) Structures of the MM employed in this study. MM 1 and MM 2 complete fast (MHz-
492
scale) unidirectional rotation. MM 3 rotates at 0.1 Hz at room temperature. MM 4 lacks chirality
493
imparted by the allylic methyl and possesses no preference for unidirectional rotation. (c) Confocal
494
microscope images of cells treated with MM 1, calcium-tracking dye Fluo-4, and cell membrane
495
labeling dye CellMask (used to differentiate individual cells), showing a rapid increase in
496
.CC-BY-ND 4.0 International licenseavailable under a
(which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprintthis version posted November 28, 2022. ; https://doi.org/10.1101/2022.11.04.515191doi: bioRxiv preprint
25
intracellular calcium levels after stimulation with 400 nm light. The red dotted circle labels the
497
area of laser stimulation. The images in the top row were taken before stimulation, and the images
498
in the bottom row were taken immediately prior to, 1 s after, and 2 min after stimulation. The scale
499
bar applies to all images and is 20 µm. (d) Representative normalized fluorescence intensity traces
500
of Fluo-4 in HEK293 cells treated with each MM. The solid line represents the average responses
501
of n=6 independent cells, and the shaded area represents the standard error of the mean. MM 1,
502
MM 2, and MM 4 were administered to cells at 8 µM. MM 3 was administered to cells at 24 µM.
503
Stimuli for cells treated with MM 1, 2, and 4 used a 250 ms pulse width delivered to a circular area
504
of diameter 5 µm at 3.2
×
10
2
W cm
-2
. Stimuli for cells treated with MM 3 were administered at
505
6.4
×
10
2
W cm
-2
. For all plots, the cyan line indicates the time of stimulus presentation.
506
507
.CC-BY-ND 4.0 International licenseavailable under a
(which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprintthis version posted November 28, 2022. ; https://doi.org/10.1101/2022.11.04.515191doi: bioRxiv preprint
26
508
Fig. 2. Mechanistic study of MM-induced ICW. (a) Schematic of possible mechanisms by which
509
calcium can enter the cytoplasm. Prepared with Biorender.com. (b) Confocal microscope images
510
of HEK293 cells treated with MM 1, MitoTracker Green, ER Tracker Red, and CellMask Deep
511
Red. Scale bars are both 20 µm and apply to the images in the same row. (c-h) Calcium waves
512
elicited by MM 1-treated cells (c) in calcium-free PBS, (d) in RR (1 µM), (e) in Gd
3+
(50 µM), (f)
513
after treatment of cells with Th (1 µM), (g) in Ry (100 µM), (h) in XeC (25 µM), (i) in U-73 (10
514
µM), (j) after pre-treatment of cells with Cyto D (2 µM). The solid line represents the calcium
515
= Ca
2+
PIP
2
IP
3
IP
3
R
PLC
GPCR
RyR SERCA
TRPV1, 2, 4
Piezo1, 2,
TRPC4
DAG
a
Cytosol
ER
n.s. n.s. n.s.
**
n.s.
XeC
Control
*
b
*
*
0 50 100 150
2
1
0
3
Time (s)
ΔF/F
0
Ca
2+
(-)
Control
Control Ca
2+
(-)
1
0
2
Max ΔF/F
0
Control Gd
3+
1
0
Max ΔF/F
0
0 50 100 150
1
0
2
Time (s)
ΔF/F
0
Gd
3+
Control
d
0 50 100 150
2
1
0
3
Time (s)
ΔF/F
0
RR
Control
e
Control RR
1
0
2
Max ΔF/F
0
0 50 100 150
2
1
0
3
Time (s)
ΔF/F
0
Th
Control
f
Control Th
1
0
2
Max ΔF/F
0
0 50 100 150
2
1
0
3
Time (s)
ΔF/F
0
Ry
Control
g
Control
Ry
1
0
2
Max ΔF/F
0
0 50 100 150
2
1
0
3
Time (s)
ΔF/F
0
XeC
Control
h
Control XeC
1
0
2
Max ΔF/F
0
0 50 100 150
2
1
0
3
Time (s)
ΔF/F
0
U-73
Control
i
Control U-73
1
0
Max ΔF/F
0
0 50 100 150
1
0
2
Time (s)
ΔF/F
0
Cyto D
Control
j
Control
U-73
1
0
Max ΔF/F
0
c
.CC-BY-ND 4.0 International licenseavailable under a
(which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprintthis version posted November 28, 2022. ; https://doi.org/10.1101/2022.11.04.515191doi: bioRxiv preprint
27
profiles averaged from six independent cells. The shaded region represents the standard error of
516
the mean (n=6). Stimuli were presented after 30 s of imaging in each case, and the time of stimulus
517
presentation is indicated by the vertical cyan line. For all plots, the black trace shows a positive
518
control consisting of MM 1-treated cells in typical imaging buffer recorded on the same day. All
519
stimuli were delivered with a pulse width of 250 ms to a circular area of diameter 5 µm at a power
520
ranging from 3.2
×
10
2
W cm
-2
to 5.1
×
10
2
W cm
-2
. For all plots, the cyan line indicates the time of
521
stimulus presentation. Controls and experimental groups were imaged and stimulated on the same
522
day using the same conditions and with the same batch of cells. Error bars in the bar graphs
523
represent the standard deviation of the mean of n=3 experiments (at least 6 stimulated cells per
524
experiment). Statistical analyses were performed using a one-tailed Welch’s t-test. * P-value <
525
0.05, ** P-value < 0.01, *** P-value < 0.001, **** P-value < 0.0001.
526
527
.CC-BY-ND 4.0 International licenseavailable under a
(which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprintthis version posted November 28, 2022. ; https://doi.org/10.1101/2022.11.04.515191doi: bioRxiv preprint
28
528
Fig. 3. MM-induced calcium waves cause localized calcium release, contraction, and beating
529
in cardiomyocytes. (a) Confocal microscope images of a single myocyte treated with MM 1 and
530
ER Tracker Red. The scale bar is 10 µm and applies to all images. (b) Confocal microscope images
531
showing Fluo-4 fluorescence, revealing calcium activity in cardiomyocytes before and after
532
stimulation. The scale bar is 50 µm and applies to all images. The white circle represents the
533
stimulation region. (c) Kymographs representative of cardiac myocyte contractile responses from
534
the same cells shown in (b). The line profiles from which kymographs 1 and 2 were taken are
535
shown as double-sided arrows in the bright field image. Kymographs 1 and 2 were taken from the
536
stimulated cell and an adjacent cell, respectively. The scale bar in the bright field image is 50 µm.
537
Kymograph 1 shows local contraction in the stimulated cell (top arrow in 1), indicated by a
538
convergence of the edges in the kymograph plot. Both kymographs 1 and 2 show periodic beating
539
a
MM 1
Fluo-4 t = -1 s t = 5 s t = 10 s
b
SR Merge
f
e
d
0
50
40
30
20
10
Cell #
0
50
40
30
20
10
Cell #
0
50
40
30
20
10
Cell #
<10
-2
10
-1
10
0
>10
1
ΔF/F
0
<10
-2
10
-1
10
0
>10
1
ΔF/F
0
<10
-2
10
-1
10
0
>10
1
ΔF/F
0
30 60 12090
Time (s)
0
30 60 120900
30 60 120900
c
0 5 10 15 0 5 10
0
30
60
90
120
Time (s)
Distance (µm)
1
2
15
Movement
MM 1
U-73
Ca
2+
(-)
.CC-BY-ND 4.0 International licenseavailable under a
(which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprintthis version posted November 28, 2022. ; https://doi.org/10.1101/2022.11.04.515191doi: bioRxiv preprint
29
in the stimulated cell and surrounding cell (bottom arrow in 1 and arrow in 2). (d-f) Normalized
540
fluorescence intensity change of Fluo-4 in cardiomyocytes adjacent to stimulated cardiomyocytes
541
that were treated with (d) MM 1 alone, (e) MM 1 and U-73 (10 µM), and (f) MM 1 in calcium-
542
free PBS. For each condition, n=50 cells were used. The x-axis label in (f) also applies to (d-e).
543
All stimulation experiments were performed with a stimulation time of 250 ms at 5.1×10
2
W cm
-2
544
in a circular region of 5 µm diameter using a 400 nm laser. In all plots, the cyan line indicates the
545
time of stimulus presentation.
546
547
.CC-BY-ND 4.0 International licenseavailable under a
(which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprintthis version posted November 28, 2022. ; https://doi.org/10.1101/2022.11.04.515191doi: bioRxiv preprint
30
548
Fig. 4. MM induce ICW and muscle contraction in vivo. (a) Image of a Hydra loaded into a
549
microfluidic chamber with anatomical regions marked. Scale bar is 100 µm and applies to (a-c).
550
(b) MM 1 (24 µM) loaded into Hydra for 24 h. (c) A Hydra expressing GCaMP7b in
551
endodermal epitheliomuscular cells. The stimulation regions used for Protocols I and II are
552
marked. Protocol I was used for body column stimulation (1 s pulse to a 10 µm diameter circular
553
area) to trigger regional ICW. Protocol II was used for oral region stimulation (2 s pulse to the
554
MM 1
Peduncle
Oral Region
Body
ba
Tentacles
c
n
21 4 3
Rest Contracting
l
0
255
e
Group
0
0.2
ΔF/F
0
0.4
0.6
0.8
1.0
100
Time (s)
0 200 300 400
0
20
Resp. Rate (%)
40
60
80
100
Fluor. Int
m
Oral Region
(Contraction)
Body Column
(ICW)
n.s.
*
****
*
20 6040
Time (s)
0
0
1
2
3
ΔF/F
0
20 6040
Time (s)
0
0
1
2
3
f
20
6040
Time (s)
0
0
1
2
3
ΔF/F
0
20 6040
Time (s)
0
0
1
2
3
20 6040
Time (s)
0
0
1
2
3h
i
j
Group
2 4 31 C
5
10
15
20
Resp. Delay (s)
30
*
**
****
****
25
0
Protocol I
Protocol II
C
21 4 3
0
20
40
60
80
100
Resp. Rate (%)
Group
n.s.
****
****
****
C
d
t = 10 s
t = 30 s
Protocol I
Protocol II
k
g
ΔF/F
0
ΔF/F
0
ΔF/F
0
.CC-BY-ND 4.0 International licenseavailable under a
(which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprintthis version posted November 28, 2022. ; https://doi.org/10.1101/2022.11.04.515191doi: bioRxiv preprint

31
oral region, ~1000-2000 µm
2
) to trigger whole-body contraction. (d) A regional ICW in a Hydra
555
treated with MM 1 and stimulated with 405-nm light via Protocol I. The indicated times
556
represent the time after stimulation at which the image was taken. Scale bar applies to both
557
images and is 100 µm. (e-i) Responses observed from Hydra treated with (e) MM 1, (f) MM 2,
558
(g) MM 3, (h) MM 4, or (i) DMSO control (“C”) and stimulated for 1 s using Protocol I. Bold,
559
colored traces represent the average trace (n=25 across 5 Hydra). Gray traces represent
560
individual experiments. (j) Bar graph showing ICW response rate and (k) box-and-whisker plot
561
showing ICW delay time of Hydra stimulated using Protocol I across MM treatment conditions
562
(n=25 experiments across at least 5 Hydra per condition). ICW responses were defined as
ΔF/𝐹
!
563
> 1. (l) Representative contraction in a Hydra treated with MM 2 and stimulated via Protocol II.
564
Scale bar applies to both images and is 100 µm. (m) Representative GCaMP7b fluorescence
565
trace in a Hydra treated with MM 2 and stimulated using Protocol II. (n) Bar graph showing
566
contractile response rates across treatment conditions for Hydra stimulated for 2 s using Protocol
567
II (at least 50 experiments across >5 Hydra per condition). Error bars in bar graphs represent the
568
standard error of the mean. See Methods for more info on the calculation of ICW and contractile
569
response rates. For all plots, cyan lines indicate the time of stimulus with 405-nm light (9.0×10
2
570
W cm
-2
), during which no data was collected. * P-value < 0.05, ** P-value < 0.01, *** P-value <
571
0.001, **** P-value < 0.0001.
572
.CC-BY-ND 4.0 International licenseavailable under a
(which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprintthis version posted November 28, 2022. ; https://doi.org/10.1101/2022.11.04.515191doi: bioRxiv preprint
32
References.
573
1. Clapham, D. E. Calcium Signaling. Cell 131, 1047–1058 (2007).
574
2. Tsutsumi, M. et al. Mechanical-stimulation-evoked calcium waves in proliferating and
575
differentiated human keratinocytes. Cell Tissue Res. 338, 99–106 (2009).
576
3. Screaton, R. A. et al. The CREB Coactivator TORC2 Functions as a Calcium- and cAMP-
577
Sensitive Coincidence Detector. Cell 119, 61–74 (2004).
578
4. Carrasco, M. A. & Hidalgo, C. Calcium microdomains and gene expression in neurons and
579
skeletal muscle cells. Cell Calcium 40, 575–583 (2006).
580
5. Glaser, T. et al. ATP and spontaneous calcium oscillations control neural stem cell fate
581
determination in Huntington’s disease: a novel approach for cell clock research. Mol. Psychiatry
582
26, 2633–2650 (2021).
583
6. Tada, M. & Concha, M. L. Vertebrate gastrulation: Calcium waves orchestrate cell
584
movements. Curr. Biol., 11, R470–R472 (2001).
585
7. McCormack, J. G., Halestrap, A. P., & Denton, R. M. Role of calcium ions in regulation
586
of mammalian intramitochondrial metabolism. Phys. Rev., 70, 391-425 (1990).
587
8. Leybaert, L. & Sanderson, M. J. Intercellular Ca
2+
Waves: Mechanisms and Function.
588
Phys. Rev. 92, 1359–1392 (2012).
589
9. G., Eng, et al., Autonomous beating rate adaptation in human stem cell-derived
590
cardiomyocytes. Nat Commun 7, 10312 (2016).
591
10. Llano, I. et al., Presynaptic calcium stores underlie large-amplitude miniature IPSCs and
592
spontaneous calcium transients. Nat Neurosci 3, 1256–1265 (2000).
593
11. Takano, T. et al., Astrocyte-mediated control of cerebral blood flow. Nat Neurosci 9, 260–
594
267 (2006).
595
.CC-BY-ND 4.0 International licenseavailable under a
(which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprintthis version posted November 28, 2022. ; https://doi.org/10.1101/2022.11.04.515191doi: bioRxiv preprint
33
12. Drumm, B. T. et al., The effects of mitochondrial inhibitors on Ca2+ signalling and
596
electrical conductances required for pacemaking in interstitial cells of Cajal in the mouse small
597
intestine. Cell Calcium 72, 1–17 (2018).
598
13. Gourine, A. V. et al., Astrocytes Control Breathing Through pH-Dependent Release of
599
ATP. Science 329, 571–575 (2010).
600
14. Berridge, M. J. Calcium signaling remodeling and disease. Biochem. Soc. Trans., 40, 297-
601
309 (2012).
602
15. Stewart, T. A., Yapa, K. T. D. S., & Monteith, G. R. Altered calcium signaling in cancer
603
cells. Biochim Biophys Acta Biomembr., 1848, 2502-2511 (2015).
604
16. Berridge, M. J., Lipp, P., Bootman, M. D. The versatility and universality of calcium
605
signaling. Nat. Rev. Molec. Cell Biol., 1, 11-21 (2000).
606
17. Cornell-Bell, A. H. et al., Glutamate Induces Calcium Waves in Cultured Astrocytes:
607
Long-Range Glial Signaling. Science 247, 470–473 (1990).
608
18. Sanderson, M. J., Charles, A. C. & Dirksen, E. R. Mechanical stimulation and intercellular
609
communication increases intracellular Ca
2+
in epithelial cells. Cell Regul 1, 585–596 (1990).
610
19. Klok, M. et al. MHz Unidirectional Rotation of Molecular Rotary Motors. J. Am. Chem.
611
Soc. 130, 10484–10485 (2008).
612
20. García-López, V. et al. Unimolecular Submersible Nanomachines. Synthesis, Actuation,
613
and Monitoring. Nano Lett., 15, 8229-8239 (2015).
614
21. García-López, V. et al. Molecular machines open cell membranes. Nature 548, 567–572
615
(2017).
616
22. Zheng, Y. et al. Optoregulated force application to cellular receptors using molecular
617
motors. Nat. Commun., 12, 3580 (2021).
618
.CC-BY-ND 4.0 International licenseavailable under a
(which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprintthis version posted November 28, 2022. ; https://doi.org/10.1101/2022.11.04.515191doi: bioRxiv preprint
34
23. García-López, V., Liu, D., & Tour, J. M. Light-activated organic molecular motors and
619
their applications. Chem. Rev., 120, 79-124 (2020).
620
24. Pollard, M. M., Klok, M., Pijper, D. & Feringa, B. L. Rate Acceleration of Light-Driven
621
Rotary Molecular Motors. Adv. Func. Mater., 17, 718–729 (2007).
622
25. Kepp, O., Galluzzi, L., Lipinski, M., Yuan, J. & Kroemer, G. Cell death assays for drug
623
discovery. Nat Rev Drug Discov., 10, 221–237 (2011).
624
26. Galbadage, T. et al. Molecular nanomachines disrupt bacterial cell wall, Increasing
625
sensitivity of extensively drug-resistant Klebsiella pneumoniae to meropenem. ACS Nano 13,
626
14377–14387 (2019).
627
27. Santos, A. L. et al. Light-activated molecular machines are fast-acting broad-spectrum
628
antibacterials that target the membrane. Sci. Adv., 8, eabm2055 (2022).
629
28. Alaya-Orozco, C. et al. Visible-light-activated molecular nanomachines kill pancreatic
630
cancer cells. ACS Appl. Mater. Int., 12, 410-417 (2020).
631
29. Vriens, J. ,Appendino, G., & Nilius, B. Pharmacology of vanilloid transient receptor
632
potential cation channels. Mol. Pharmacol., 75, 1262-1279 (2009).
633
30. Hamill, O. P. & McBride Jr., D. W. The pharmacology of mechanogated membrane ion
634
channels. Pharmacol. Rev., 48, 231-252 (1996).
635
31. Thastrup, O., Cullen, P. J., Drøbak, B. K., Hanley, M. R. & Dawson, A. P. Thapsigargin,
636
a tumor promoter, discharges intracellular Ca
2+
stores by specific inhibition of the endoplasmic
637
reticulum Ca
2+
-ATPase. PNAS 87, 2466–2470 (1990).
638
32. Bock, G. R. & Ackrill, K. Calcium Waves, Gradients and Oscillations. (John Wiley &
639
Sons, 2008).
640
.CC-BY-ND 4.0 International licenseavailable under a
(which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprintthis version posted November 28, 2022. ; https://doi.org/10.1101/2022.11.04.515191doi: bioRxiv preprint
35
33. Gafni, J. et al. Xestospongins: potent membrane permeable blockers of the inositol 1,4,5-
641
trisphosphate receptor. Neuron, 19, 723-733 (1997).
642
34. Ribeiro, C. M. P., Reece, J. & Putney, J. W. Role of the Cytoskeleton in Calcium Signaling
643
in NIH 3T3 Cells. J. Biol. Chem., 272, 26555–26561 (1997).
644
35. Xu, J. et al. GPR68 senses flow and is essential for vascular physiology. Cell, 173, 762-
645
775.e16 (2018).
646
36. Feher, J. Quantitative Human Physiology: An Introduction. (Elsevier, 2007).
647
37. Stuyvers, B. D., Boyden, P. A. & ter Keurs, H. E. D. J. Calcium waves. Circ. Res., 86,
648
1016–1018 (2000).
649
38. Wang, H. et al. From neuron to muscle to movement: a complete biomechanical model of
650
Hydra contractile behaviors. bioRxiv (2020). doi:10.1101/2020.12.14.422784.
651
39. Kinnamon, J. C. & Westfall, J. A. A three dimensional serial reconstruction of neuronal
652
distributions in the hypostome of a Hydra. J. Morphol., 168, 321–329 (1981).
653
40. Kinnamon, J. C. & Westfall, J. A. Types of neurons and synaptic connections at
654
hypostome-tentacle junctions in Hydra. J. Morphol., 173, 119–128 (1982).
655
41. Dupre, C. & Yuste, R. Non-overlapping neural networks in Hydra vulgaris. Curr. Biol.,
656
27, 1085–1097 (2017).
657
42. Badhiwala, K. N., Primack, A. S., Juliano, C. E., & Robinson, J. T. Multiple neuronal
658
networks coordinate Hydra mechanosensory behavior. eLife, 10:e64108 (2020).
659
43. Guertin, S. & Kass-Simon, G. Extraocular spectral photosensitivity in the tentacles of
660
Hydra vulgaris. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 184, 163–170 (2015).
661
.CC-BY-ND 4.0 International licenseavailable under a
(which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprintthis version posted November 28, 2022. ; https://doi.org/10.1101/2022.11.04.515191doi: bioRxiv preprint
36
44. Sinnecker, D. & Schaefer, M. Real-time analysis of phospholipase C activity during
662
different patterns of receptor-induced Ca
2+
responses in HEK293 cells. Cell Calcium 35, 29–38
663
(2004).
664
45. Roke, D. et al. Light-gated rotation in a molecular motor functionalized with a
665
dithienylethene switch. Angew. Chem., Int. Ed., 57, 10515–10519 (2018).
666
46. Saywell, A. et al. Light-induced translation of motorized molecules on a surface. ACS Nano
667
10, 10945–10952 (2016).
668
47. Zhao, X., Fan, B., Hassan, S., Veeraraghavan, A., & Robinson, J. T. Near field optical
669
sensing of single cell activity with integrated micro-ring resonators. Biophotonics Congress 2021,
670
OSA Technical Digest (Optical Society of America, 2021), paper BTu3B.4.
671
48. Gunasekera, R. S. et al. Molecular nanomachines can destroy tissue or kill multicellular
672
eukaryotes. ACS Appl. Mater. Int., 12, 13657-13670 (2020).
673
49. Hajnoczky, G., Davies, E., & Madesh, M. Calcium signaling and apoptosis. Biochem.
674
Biophys. Ref. Commun., 304, 445-454 (2003).
675
50. Cnossen, A., Kistemaker, J. C. M., Kojima, T., & Feringa, B. L. Structural dynamics of
676
overcrowded alkene-based molecular motors during thermal isomerization. J. Org. Chem., 79,
677
927-935 (2014).
678
51. Grigoryan, B. et al. Development, characterization, and applications of multi-material
679
stereolithography bioprinting. Sci. Rep., 11, 3171 (2021).
680
.CC-BY-ND 4.0 International licenseavailable under a
(which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made
The copyright holder for this preprintthis version posted November 28, 2022. ; https://doi.org/10.1101/2022.11.04.515191doi: bioRxiv preprint
