Contents lists available at ScienceDirect
Physiology & Behavior
journal homepage: www.elsevier.com/locate/physbeh
Effect of short- and long-term protein consumption on appetite and appetite-
regulating gastrointestinal hormones, a systematic review and meta-analysis
of randomized controlled trials
Ali Kohanmoo, Shiva Faghih, Masoumeh Akhlaghi
⁎
Department of Community Nutrition, School of Nutrition and Food Sciences, Shiraz University of Medical Sciences, Shiraz, Iran
ARTICLE INFO
Keywords:
Protein
Appetite
Hunger
Satiety
Ghrelin
Cholecystokinin
Glucagon-like peptide-1
ABSTRACT
Aim: High-protein diets are considered as useful diets for weight loss programs. We collected randomized
controlled trials that evaluated the effect of protein on appetite and gastrointestinal hormones involved in ap-
petite regulation.
Methods: Trials were included if participants were healthy adults and isocaloric treatments were used in
control and treatment arms. Random-effects model was used to calculate mean difference and 95% confidence
intervals.
Results: In total, 49 publications for acute and 19 articles for long-term effect of protein were included. In
acute interventions, protein decreased hunger (-7 mm visual analogue scale (VAS), P<0.001), desire to eat
(-5 mm, P = 0.045), and prospective food consumption (-5 mm, P = 0.001) and increased fullness (10 mm,
P<0.001) and satiety (4 mm, P<0.001). There was also a decrease in ghrelin (-20 pg/ml, P<0.001) and in-
crease in cholecystokinin (30 pg/ml, P<0.001) and glucagon-like peptide-1 (GLP-1) (21 ng/ml, P<0.001), but
no change in gastric inhibitory polypeptide and peptide YY was observed. Appetite markers were affected by
protein doses < 35 g but ghrelin, cholecystokinin, and GLP-1 changed significantly after doses ≥ 35 g. Long-
term ingestion of protein did not affect these outcomes, except for GLP-1 which showed a significant decrease.
Conclusion: Results of this meta-analysis showed that acute ingestion of protein suppresses appetite, decreases
ghrelin, and augments cholecystokinin and GLP-1. Results of long-term trials are inconclusive and further trials
are required before a clear and sound conclusion on these trials could be made.
1. Introduction
After decades of combat against obesity, obesity is still an important
health concern around the world [1]. Since a number of obesity cases
occur due to overeating, proper regulation of appetite may help in
weight management programs as demonstrated by a recent meta-ana-
lysis [2]. Appetite is the desire or motivation to eat food. Appetite is
determined by two contradictory feelings of satiety and hunger [3].
These feelings play important roles in controlling the amount of food
and energy consumption and thus managing body weight [4]. Hence,
appetite can be considered as a promising target for prevention and
treatment of obesity.
The gut-brain axis is a bidirectional communication route between
gastrointestinal tract and brain [5, 6]. One of the gut communications is
exerted by gut endocrine system which induces neural circuits in hy-
pothalamus and brainstem to regulate appetite and control feeding
behavior. These hormones are secreted following sensing the presence
(or absence) of macronutrients in the gastrointestinal tract. There are
two major types of gastrointestinal hormones: orexigenic such as
ghrelin and anorexigenic such as cholecystokinin (CCK) [5, 6].
For decades, high-protein diets have been used in weight loss pro-
grams [7, 8]. In fact, the role of protein in suppression of appetite has
been put forward as a potential explanation for the high prevalence of
obesity (especially among low-income populations) and also as a
strategy for its treatment [9]. In addition, there is a protein leverage
hypothesis which states that human body prioritizes protein over car-
bohydrate and fat [9]. According to this hypothesis, if a diet lacks
sufficient protein, then the consumption of food increases in an attempt
to obtain higher amount of protein from food, leading to overeating and
increased risk of obesity [10]. In contrast, high-protein foods meet body
protein needs and decline energy intake.
A number of clinical trials have investigated the effect of protein
https://doi.org/10.1016/j.physbeh.2020.113123
Received 8 April 2020; Received in revised form 2 August 2020; Accepted 3 August 2020
⁎
Corresponding author.
Physiology & Behavior 226 (2020) 113123
Available online 05 August 2020
0031-9384/ © 2020 Elsevier Inc. All rights reserved.
T
consumption on appetite sensations as well as appetite-regulating
hormones. Here, we evaluated the effect of protein ingestion on appe-
tite markers and a number of gastrointestinal hormones involved in
appetite regulation. In addition, we performed an extensive subgroup
analysis based on sex and BMI of participants as well of dose, protein
source, and placebo type. We further questioned if the effect of protein
on the assessed outcomes differs between short and longer term protein
intake.
2. Methods
2.1. Search
PubMed, Scopus, and Embase were searched to find articles related
to the effect of protein on appetite markers and gastrointestinal hor-
mones involved in appetite regulation. The search was performed from
the earliest available date until September 2019. No limitation on
language was made. Search terms included appetite, satiety, satiation,
fullness, hunger, ghrelin, cholecystokinin (CCK), glucagon-like peptide-
1 (GLP-1), gastric inhibitory polypeptide (GIP), incretins, and peptide
YY (PYY). These hormones are the mostly recognized gastrointestinal
hormones involved in appetite regulation [ 11, 12]. Screening the li-
brary, reading the articles, and extraction of the data was performed by
two independent investigators.
2.2. Eligibility criteria
Randomized controlled trials were included if the following criteria
were met: 1) healthy subjects; 2) adult ages; and 3) isocaloric treat-
ments in control and treatment arms. Both acute (i.e. short-term) and
long-term (3 days to 9 months) trials were included. Acute trials were
those in which the effect of protein was determined within few hours
(< 5.5 h) after protein consumption whereas in long-term trials the
intervention period was between 3 days to 9 months. Trials were ex-
cluded in the case of any of the following situations: 1) participants
involved in diseases or medical conditions such as diabetes, glucose
intolerance, kidney failure, cancer, protein-energy malnutrition, sar-
copenia, anorexia, bulimia, and carbohydrate craving; 2) ad libitum (or
uncontrolled) consumption of protein supplements or protein meals; 3)
high-protein ketogenic diets; 4) treatments combined with exercise; 5)
protein treatments mixed with fiber; 6) examining proteins with unu-
sual amino acid content, for instance, proteins enriched with specific
amino acids such as leucine; 7) non-isocaloric control or control with
the same amount of protein as treatment arm; 8) insufficient informa-
tion for the time of measurements in long-term interventions; 9) re-
porting mean change in appetite markers throughout a day or over a
number of days instead of reporting them at specific time points after a
test meal; 10) expressing data as area under the curve instead of linear
curve or instead of actual values at specific post-treatment time points;
11) insufficient information for the macronutrient composition of the
diets or test meals or mean and standard deviation (SD) of the data; 12)
repeated publications.
2.3. Outcomes
Investigated outcomes included hunger, fullness, satiety, desire to
eat, and prospective food consumption as markers of appetite, and
ghrelin, CCK, GLP-1, GIP, and PYY as gastrointestinal hormones in-
volved in appetite regulation.
2.4. Data extraction
Mean and SD (or SE) of the data were collected in Excel sheets. Most
articles reported the outcomes at different time points after ingestion of
a test meal in the form of linear graphs. The 3 h post-treatment was
recognized and used as the most common post-intervention time point
measured but in studies with shorter post-treatment duration, the
nearest time point to the 3 h was used. The values of linear graphs were
quantified by Plot Digitizer software version 2.6.6 (Free Software
Foundation Inc., USA).
2.5. Statistical analysis
Mean and SD of the difference between pre- and post-intervention
data was used to calculate pooled effects. The random-effects inverse-
variance model was used to obtain weighted mean di
fference
and 95%
confidence interval (CI). Between-study heterogeneity was evaluated
using Cochrane χ
2
test and I
2
. Publication bias was determined by
Egger's test [13]. Subgroup analysis was performed based on partici-
pants’ body mass index (BMI) (lean (< 25 kg/m
2
), overweight and
obese (≥ 25 kg/m
2
), or both), protein dose (< 35 g/day vs. ≥ 35 g/
day), protein source (whey, casein (or dairy, milk, yogurt), meats/egg
(veal, turkey, egg), vegetable (soy, wheat, gluten, pea), or mixed), and
control type (carbohydrate, fat, or both). The cutoff point for protein
dose was chosen according to the median of doses used in the trials.
STATA software version 12.0 (StataCorp, USA) was used for data ana-
lysis. The trim-and-fill analysis was used to adjust any significant
publication bias detected. P < 0.05 was considered statistically sig-
nificant.
3. Results
Following the search of the databases, 8862 articles were found, of
which 3805 were duplicates and excluded, the rest were screened, and
at last 325 full texts were assessed according to the eligibility criteria
described in the Methods (Supplemental Figure 1). Of these, 257 arti-
cles were excluded due to reasons described in Fig. 1 and 68 passed the
eligibility stage and entered in the meta-analysis: 49 publications in-
vestigated acute effect of protein and 19 articles conducted long-term
interventions. A total of 2740 and 1159 subjects participated in the
acute and long-term interventions, respectively. Except 2 trials which
had a parallel design, acute interventions had either a crossover or a
within-subject design, meaning that all their participants experienced
both treatment and control conditions. Long-term interventions were
conducted in both parallel and crossover design. Characteristics of the
short- and long-term trials are outlined in Supplemental Tables 1 and 2,
respectively.
Among acute interventions, 13 trials had multiple arms based on
various protein sources [14, 21, 28, 32, 36, 40 , 41], protein doses [16,
18, 19], or divergent participants [22, 23, 38]. These studies were cited
more than once. Likewise, among long-term interventions 6 trials were
cited more than once due to using different proteins [45, 48, 50, 51]
and different doses [44, 47].
In acute interventions, trials reported the outcomes at different
times following protein load but 3 h was the mostly used time point. In
longer trials, the length of the intervention varied between 3 days and 9
months. Parameters of interest were measured either in fasting state,
pre- and post- protein ingestion, or at a speci fic time point during the
day of measurement.
Whey protein was the mostly examined protein but there were also
reports on protein from other sources such as casein, milk, yogurt, soy,
beef, turkey, and egg. According to the inclusion criteria, it was ne-
cessary for the control group to be isocaloric with the treatment.
Although some control meals contained protein but extra protein in the
treatment group needed to be substituted with fat or carbohydrate in
control in order to have isocaloric intakes in both groups. The actual
dose of protein was calculated from subtraction of protein in the
treatment and control groups (Supplemental Tables 1 and 2). The actual
protein dose varied from 8.5 g to about 130 g per day.
Among 49 acute interventions, 28, 23, 18, 15, and 11 trials assessed
hunger, fullness, desire to eat, prospective food consumption, and sa-
tiety, and 25, 15, 25, 12, and 11 trials examined ghrelin, CCK, GLP-1,
A. Kohanmoo, et al.
Physiology & Behavior 226 (2020) 113123
2
GIP, and PYY respectively. Also, among 19 long interventions, 13, 13, 6,
4, and 8 studies assessed hunger, fullness, desire to eat, prospective
food consumption, and satiety, and 6, 1, 6, 1, and 6 trials determined
ghrelin, CCK, GLP-1, GIP, and PYY, respectively.
4. Acute interventions
Hunger, fullness, desire to eat, prospective food consumption, and
satiety were the commonly assessed markers of appetite. The method of
assessment was visual analogue scale (VAS) which is a tool that rates
the perception of a sensation or feeling on a 100-mm horizontal line.
This line is anchored at the ends by words that define bounds of the
sensation.
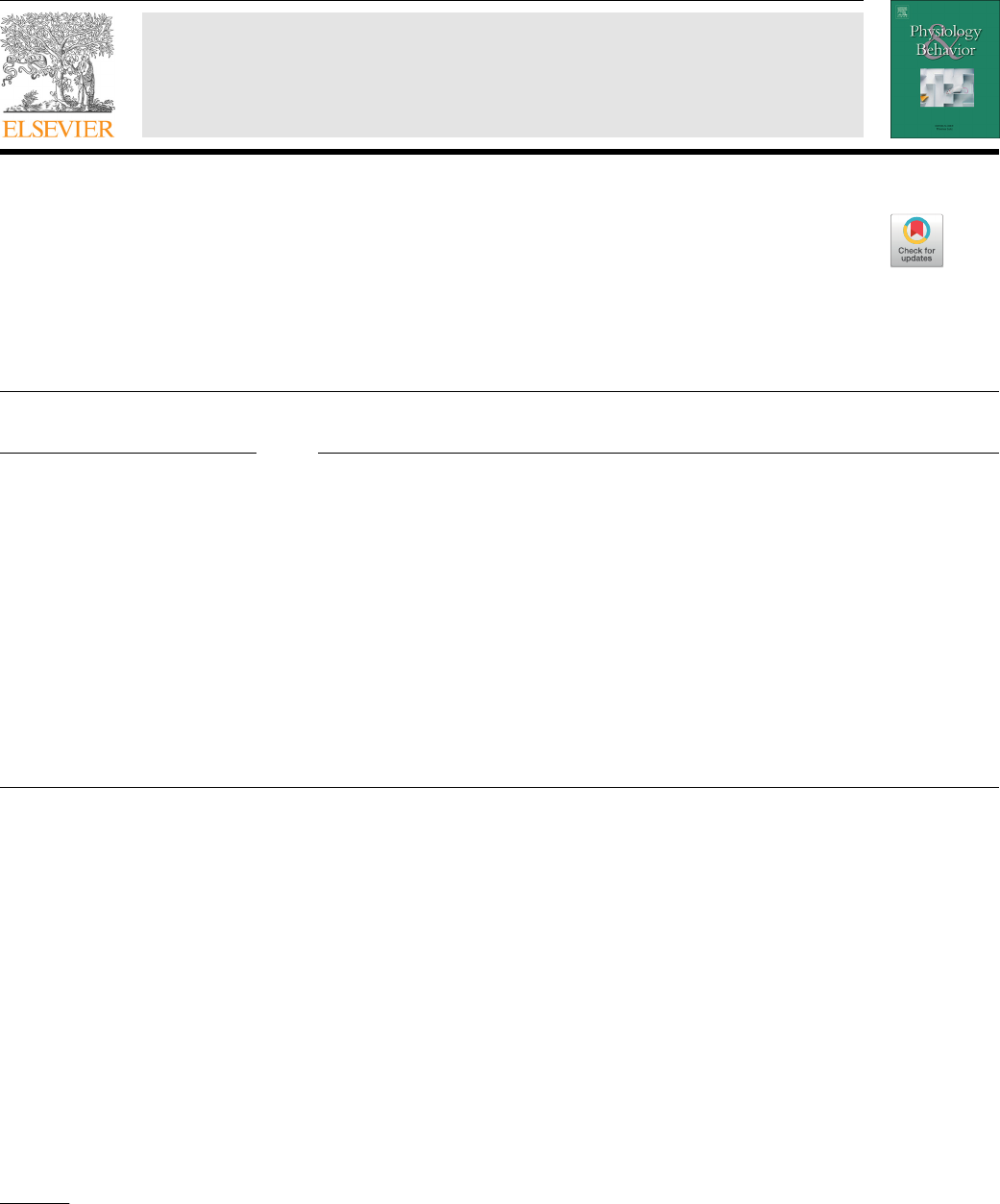
Estimated pooled effects showed significant decrease in hunger
(−7; 95% CI: −11, −3 mm; P < 0.001; n = 28) (Fig. 1), desire to eat
(−5; 95% CI: −11, −0.1 mm; P = 0.045; n = 18) (Table 1), and
prospective food consumption (−5; 95% CI: −8, −2 mm; P = 0.001;
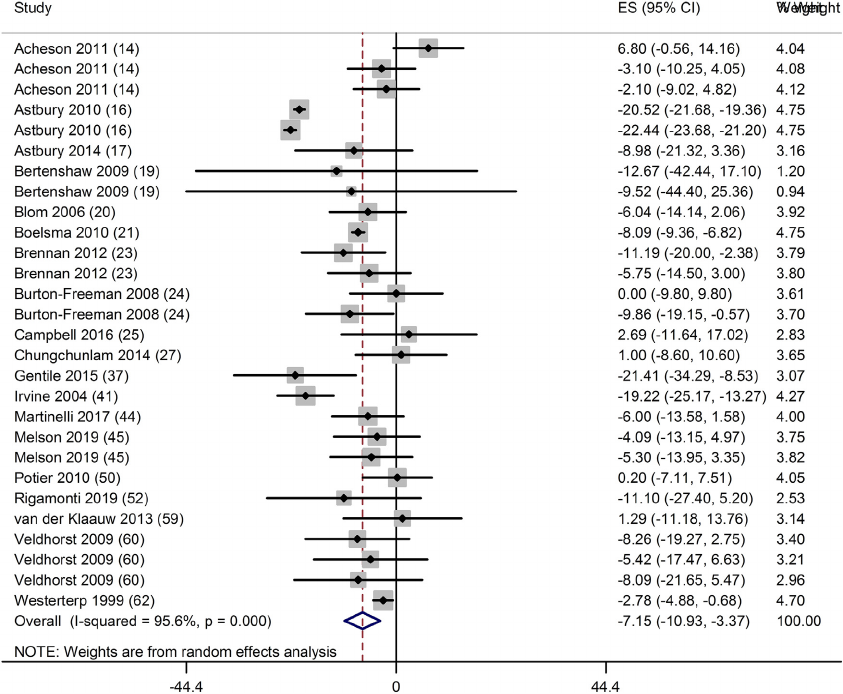
n = 15) (Table 1) and significant increase in fullness (10; 95% CI: 5,
14 mm; P < 0.001; n = 23) (Fig. 2) and satiety (4; 95% CI: 2, 6 mm; P
< 0.001; n = 11) (Table 1) following consumption of protein. Also,
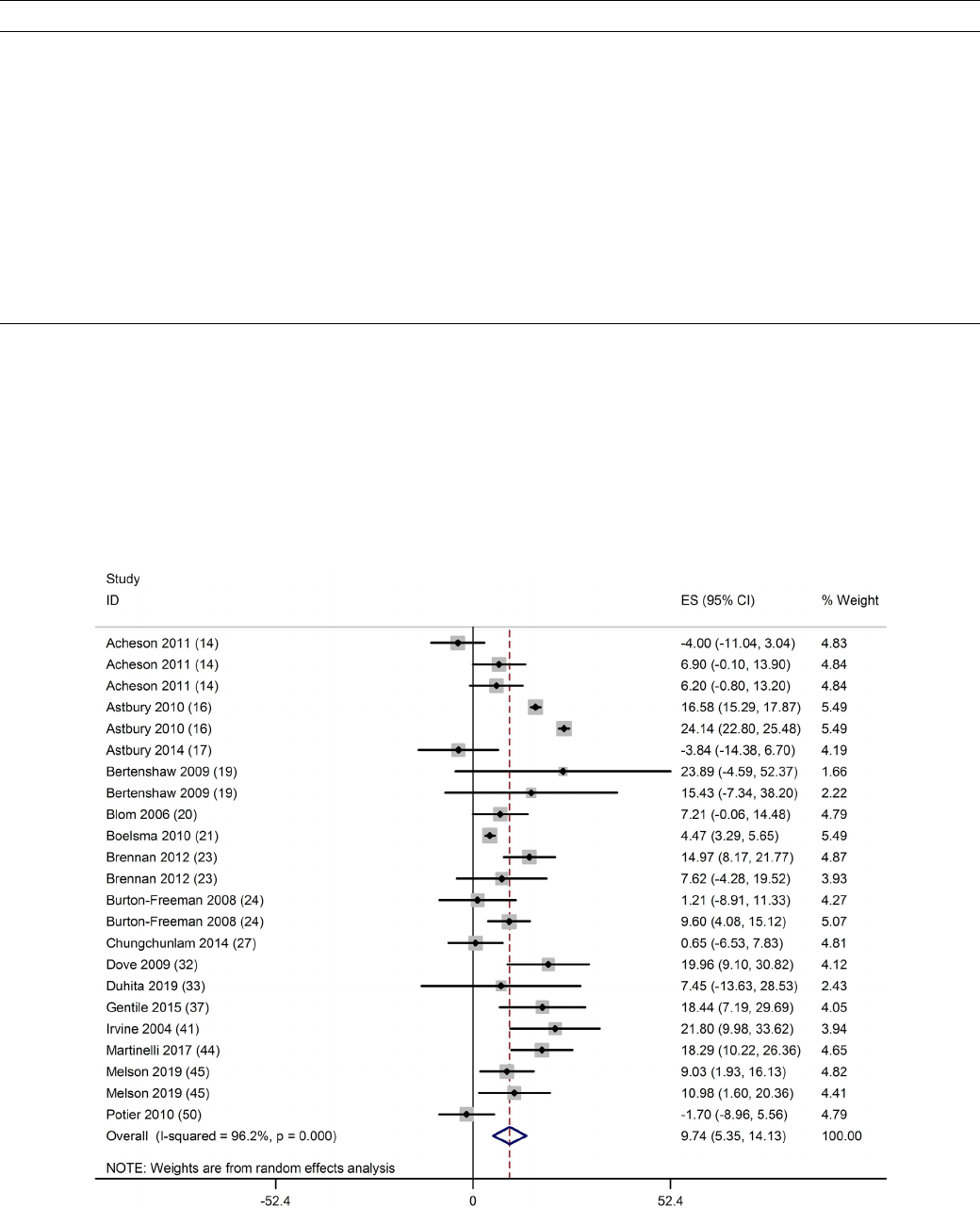
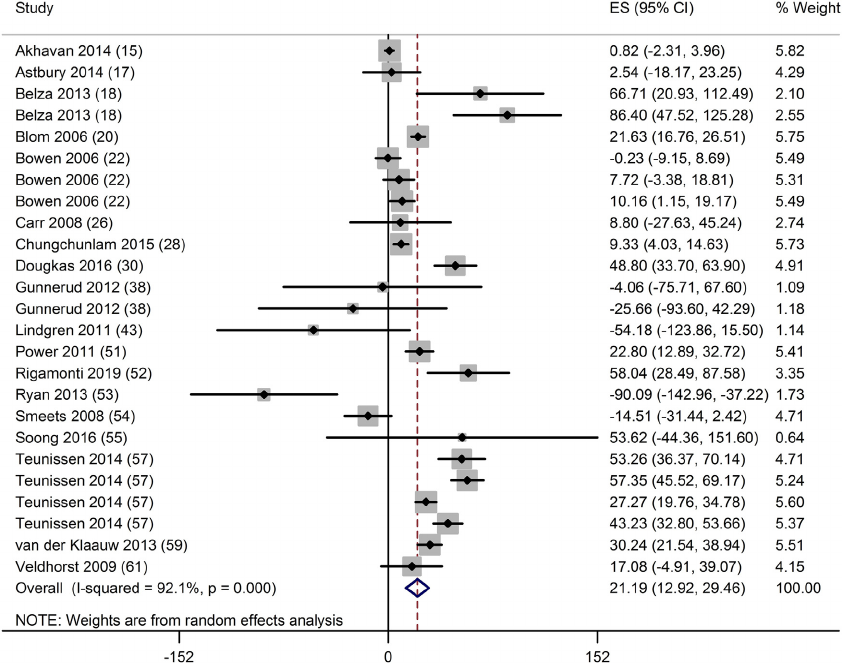
there was a significant decrease in ghrelin (−20; 95% CI: −29, −12
pg/ml; P < 0.001; n = 25) (Fig. 3), significant increase in CCK (30;
95% CI: 17, 43 pg/ml; P < 0.001; n = 15) (Fig. 4) and GLP-1 (21; 95%
CI: 13, 29 ng/ml; P < 0.001; n
= 25) (Fig.
5), and no change in GIP
(−2; 95% CI: −32, 28 ng/ml; P = 0.891; n = 12) (Table 1) and PYY
(3; 95% CI: −24, 30 ng/ml; P = 0.817; n = 11). There was a high
heterogeneity in the findings in all of the outcomes except for satiety
(I
2
= 0), ranging from 69.7% to 98.4% (P < 0.001) (Table 1).
5. Long-term interventions
In long-term interventions, protein did not have a significant effect
on hunger (P = 0.077; n = 13), fullness (P = 0.165; n = 13), desire to
eat (P = 0.676; n = 6), prospective food consumption (P = 0.210;
n = 4), satiety (P = 0.213; n = 8), ghrelin (P = 0.535; n = 6), and PYY
(P = 0.256; n = 6), but GLP-1 decreased significantly (−7; 95% CI:
−1.2, −0.02 ng/ml; P = 0.008; n =6)(Table 1). CCK and GIP were
assessed in only one trial. Except for ghrelin (I
2
= 12.5%; P = 0.335),
the results for other parameters had high heterogeneity ranging from
70.6% to 95.5% (P < 0.05).
6. Subgroup analysis for acute interventions
Subgroup analysis based on participants’ BMI and sex, source and
dose of protein, and placebo type is shown in Table 2 (for briefness only
data of hunger, fullness, ghrelin, CCK, GLP-1, and GIP have been
shown). In the dose subgroups, appetite markers were affected by
protein doses < 35 g but ghrelin, CCK, and GLP-1 changed significantly
by doses ≥ 35 g; although less substantial but still significant alteration
was also observed in ghrelin in doses of < 35 g protein (Table 2). In the
placebo subgroups, protein decreased hunger and ghrelin and increased
fullness, CCK, GLP-1, and PYY compared to carbohydrate (data not
shown for PYY). In some outcomes (fullness, CCK, GIP, and PYY), the
number of trials with fat placebo was insufficient to allow making an
accurate conclusion. Similarly, subgroup analysis based on protein
source was not useful because of limited number of trials in protein
sources other than whey. Whey was the most frequently examined
protein for appetite investigations. In the whey subgroup, a significant
Fig. 1. Forest plot of clinical trials examining the effect of protein intake on the sensation of hunger in acute interventions. Data are presented as mean difference
between treatment and control groups with 95% CIs.
A. Kohanmoo, et al.
Physiology & Behavior 226 (2020) 113123
3
reduction was observed in hunger and ghrelin and a significant increase
was observed in fullness and GLP-1 following protein consumption, but
no effect was observed on CCK, GIP, and PYY. Results of subgroup
analysis based on sex showed that protein intake reduced hunger and
increased fullness in both males and females but ghrelin was decreased
and CCK was increased only in males. The number of trials with females
was merely 2 for CCK and GLP-1 and no significant effect was observed
for females in these outcomes. Likewise, subgroup analysis based on
BMI was not successful because there were not enough trials in over-
weight/obese subgroup (Table 2).
7. Publication bias
Publication bias was detected for hunger (Egger's test P = 0.02),
ghrelin (Egger's test P = 0.01), and CCK (Egger's test P = 0.04) in
short-term trials but there was no bias in long-term interventions. Trim-
Table. 1
Pooled effect of protein on markers and hormones involved in appetite regulation in trials with short- and long-term interventions
1
.
Outcomes Studies (n) Mean difference (95% CI)* P value Heterogeneity P for heterogeneity
Acute effects
Desire to eat (mm) 18 −5(−11, −0.1) 0.045 97.7% < 0.001
Prospective food consumption (mm) 15 −5(−8, −2) 0.001 67.4% < 0.001
Satiety (mm) 11 4 ([2], [6]) < 0.001 0% 0.54
GIP (ng/ml) 12 −2(−32, 28) 0.891 95.2% < 0.001
PYY (ng/ml) 11 3 (−24, 30) 0.817 95.6% < 0.001
Long-term effects
Hunger (mm) 13 5 (−0.5, 10) 0.077 85.8% < 0.001
Fullness (mm) 13 3 (−1, 8) 0.165 87.9% < 0.001
Desire to eat (mm) 6 −2(−9, 6) 0.676 78.1% < 0.001
Prospective food consumption (mm) 4 −11 (−28, 6) 0.210 95.5% < 0.001
Satiety (mm) 8 3 (−2, 8) 0.213 79.5% < 0.001
Ghrelin (pg/ml) 6 0.4 (−1, 2) 0.535 12.5% 0.335
CCK (pg/ml) 1 −0.03 (−0.2, 0.1) 0.540 ––
GLP-1 (ng/ml) 6 −0.7 (−1.2, −0.02) 0.008 77.4% <0.001
GIP (ng/ml) 1 13 (−23, 48) 0.494 ––
PYY (ng/ml) 6 −3(−8, 2) 0.256 70.6% 0.004
1 Mean difference and its standard deviation (SD) of control and intervention groups were used to calculate pooled effects (expressed as mean difference and 95%
confidence interval). Statistical heterogeneity was assessed by I
2
test using random inverse-variance heterogeneity. CI: confidence interval.
Fig. 2. Forest plot of clinical trials examining the effect of protein intake on the sensation of fullness in acute interventions. Data are presented as mean difference
between treatment and control groups with 95% CIs.
A. Kohanmoo, et al.
Physiology & Behavior 226 (2020) 113123
4
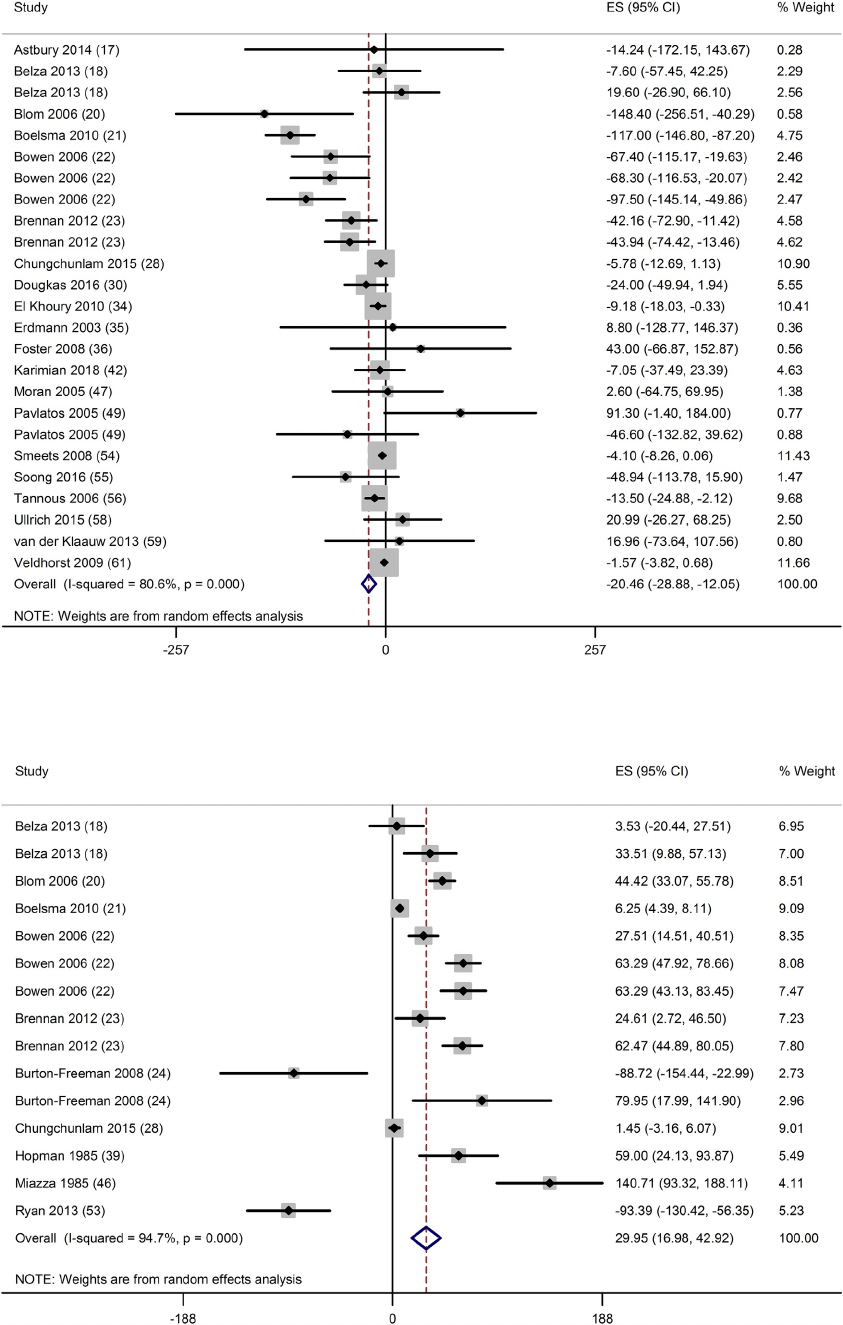
Fig. 3. Forest plot of clinical trials examining the effect of protein on ghrelin concentration in acute interventions. Data are presented as mean difference between
treatment and control groups with 95% CIs.
Fig. 4. Forest plot of clinical trials examining the effect of protein on CCK in acute interventions. Data are presented as mean difference between treatment and
control groups with 95% CIs.
A. Kohanmoo, et al.
Physiology & Behavior 226 (2020) 113123
5
and-fill analysis did not change the results, suggesting that the pub-
lication bias did not remarkably affect the results.
8. Discussion
Results of this meta-analysis showed that acute ingestion of protein
suppressed appetite as evidenced by decreased sensation of hunger,
desire to eat, and prospective food consumption, and increased fullness
and satiety. Protein intake also decreased ghrelin and increased CCK
and GLP-1 concentrations without affecting GIP and PYY. Long-term
ingestion of protein did not significantly a ffect these outcomes, except
for GLP-1 which showed a significant decrease.
9. Acute interventions
Overall, appetite is estimated by questioning five feelings of hunger,
fullness, satiety, desire to eat, and prospective food consumption. Short-
term interventions, where appetite was evaluated during hours after
protein consumption, demonstrated suppression of appetite in all five
types of feeling, providing a strong evidence for appetite-suppressing
effect of protein shortly after consumption. A number of mechanisms
have been suggested for this appetite suppression. Gut hormones in-
cluding ghrelin, CCK, GLP-1, GIP, and PYY may play a role in this
suppression [12]. Except ghrelin which is an orexigenic peptide that
promotes hunger, the other mentioned gastrointestinal hormones are
suggested to induce satiety. Cell culture studies have shown that pro-
ducts of protein digestion may induce signaling pathways involved in
synthesis or secretion of the aforementioned gastrointestinal hormones
[52]. For instance, CCK arouses vagus nerve to convey signals to the
brain, activating noradrenergic satiety neurons in the solitary nucleus
while decreasing mRNA expression of the vagal receptor of orexin-1 in
nodose ganglion, inducing satiety while inhibiting the antagonist orexin
signaling [53, 54]. Of incretin hormones, GLP-1 may render satiating
effect by delaying gastric emptying and stimulating insulin synthesis
and secretion [53] but GIP has not shown to delay gastric emptying
[55]. The satiating effect of protein may also be mediated by me-
chanisms independent of the gut hormones. For instance, it has been
suggested that high blood concentration of amino acids, particularly
those that are not utilized for protein synthesis, may provoke satiety
signals [54] .
Results of this meta-analysis did not show the effect of protein on
GIP and PYY. Previous studies using isocaloric meals have shown that
meals containing carbohydrate and fat induced substantial rises in in-
cretin hormones but protein had no effect [56]. In this regard, Elliott
and colleagues studied circulating levels of GLP-1 and GIP following
consumption of isocaloric meals containing carbohydrate, fat, or pro-
tein [56]. They found that both GLP-1 and GIP were secreted following
consumption of carbohydrates and fat; although secretion occurred at
slower rate after fat than after carbohydrates. Protein also stimulated
GLP-1 section but GIP was not affected by protein meals [56].
10. Subgroup analysis of acute interventions
10.1. Dose subgroups
Trials on the dose-dependent effects of protein on appetite sensa-
tions and gastrointestinal hormones are quite conflicting. A number of
trials have supported a positive dose-response relationship between
dietary protein and appetite sensations and/or hormones [
27, 18]
while
others have denied such relationship [19, 16]. Results of this meta-
Fig. 5. Forest plot of clinical trials examining the effect of protein on GLP-1 in acute interventions. Data are presented as mean difference between treatment and
control groups with 95% CIs.
A. Kohanmoo, et al.
Physiology & Behavior 226 (2020) 113123
6

Table. 2
Subgroup analysis based on participants’ BMI and sex, protein source and dose, and placebo for the effect of protein on hunger, fullness, and concentrations of
appetite-regulating hormones in short-term interventions.
Subgroup
categorization
Studies (n) Mean difference (95%
CI)
P value I
2
P forI
2
Studies (n) Mean difference (95%
CI)
P value I
2
P for I
2
Hunger (mm) Fullness (mm)
BMI BMI
< 25 kg/m
2
22 −7(−11, −3) 0.001 96.4% < 0.001 < 25 kg/m
2
16 9 ([4], [15]) 0.001 97.4% < 0.001
≥ 25 kg/m
2
4 −7(−14, 1) 0.08 57.9% 0.07 ≥ 25 kg/m
2
410([4], [16]) 0.002 41.4% 0.16
Both 2 −7(−15, 1) 0.08 – 0.57 Both 2 14 ([2], [24] ) 0.02 55.6% 0.13
Sex Sex
Male 8 −8(−9, −7) < 0.001 0 0.82 Male 8 7 ([2], [11]) 0.003 53.9% 0.03
Female 5 −7(−14, −1) 0.03 64.8% 0.02 Female 3 9 (0.1, 17) 0.047 73.9% 0.02
Male/female 15 −7(−12, −3) 0.001 93.4% < 0.001 Male/female 11 12 ([7], [17] ) < 0.001 94.3% < 0.001
Protein source Protein source
Whey 18 −8(−12, −3) 0.001 95.9% < 0.001 Whey 15 9 ([3], [14]) 0.002 97.5% < 0.001
Casein/dairy 3 −10 (−21, 2) 0.10 84.3% 0.002 Casein/dairy 3 15 ([5], [24] ) 0.003 69.8% 0.04
Meat/egg 2 −8(−15, −2) 0.008 0 0.39 Meat/egg 2 13 ([7], [19] ) < 0.001 9.5% 0.29
Vegetable 3 −4(−9, 1) 0.12 0 0.70 Vegetable 2 8 ([2], [14]) 0.006 0 0.42
Mixed 2 −3(−5, −1) 0.01 0 0.53 Mixed –– –––
Protein dose Protein dose
<35g 16 −
9(−15, −3)
0.002 96.9% < 0.001 < 35 g 12 13 ([9], [17]) < 0.001 65.5% 0.001
≥ 35 g 12 −5(−11, 2) 0.16 94.0% < 0.001 ≥ 35 g 10 6 (−2, 15) 0.11 98.3% < 0.001
Placebo type Placebo type
CHO 23 −7(−11, −3) 0.001 95.3% < 0.001 CHO 21 9 ([5], [14]) < 0.001 96.5% < 0.001
Fat 4 −12 (−19, −4) 0.003 55.0% 0.08 Fat 1 22 ([10], [30]) < 0.001 ––
CHO/fat 1 −3(−5, −1) 0.01 –– CHO/fat –– –––
Ghrelin (pg/ml) CCK (pg/ml)
BMI BMI
< 25 kg/m
2
13 −20 (−33, −7) 0.002 85.7% < 0.001 < 25 kg/m
2
715([1], [26]) 0.04 93.5% < 0.001
≥ 25 kg/m
2
6 −4(−8, −0.4) 0.03 0 0.55 ≥ 25 kg/m
2
425(−36, 86) 0.42 92.1% < 0.001
Both 6 −57 (−81, −33) < 0.001 27.9% 0.22 Both 4 54 ([30], [47]) < 0.001 83.3% < 0.001
Sex Sex
Male 14 −42 (−62, −21) < 0.001 84.1% < 0.001 Male 11 21 ([2], [33]) 0.04 95.2% < 0.001
Female 3 6 (−51, 64) 0.84 60.5% < 0.001 Female 2 34 (−42, 110) 0.38 83.7% 0.01
Male/female 8 −2(−4, − 0.3) 0.02 0 0.69 Male/female 2 98 ([18], 178) 0.02 86.5% 0.006
Protein source Protein source
Whey 8 −29 (−48, −10) 0.003 90.5% < 0.001 Whey 7 7 (−7, 22) 0.30 93.9% < 0.001
Casein/dairy 1 −24 (−50, 2) 0.07 –– Casein/dairy 2 98 ([18], 178) 0.02 86.5% 0.006
Meat/egg 4 −39 (−60, −18) < 0.001 0 0.43 Meat/egg 2 44 ([7], [53]) 0.02 85.7% 0.008
Vegetable 3 −76 (−11, −46) < 0.001 0 0.46 Vegetable 2 63 ([39], [49]) < 0.001 0 1
Mixed 9 −7(−12, −1) 0.02 15.6% 0.30 Mixed 2 19 (−11, 48) 0.22 67.2% 0.08
Protein dose Protein dose
<35g 9 −7(−13, −2) 0.01 57.7% 0.01 < 35 g 5 21 (
−16,
58) 0.27 87.7% < 0.001
≥ 35 g 16 −29 (−50, −9) 0.006 83.5% < 0.001 ≥ 35 g 10 32 ([17], [37]) < 0.001 95.7% < 0.001
Placebo type Placebo type
CHO 17 −32 (−45, −18) < 0.001 84.3% < 0.001 CHO 14 37 ([23], [61]) < 0.001 94.4% < 0.001
Fat 7 −4(−14, 6) 0.42 43.0% 0.10 Fat 1 −93 (−130, −56) < 0.001 ––
CHO/fat 1 9 (−129, 146) 0.90 –– CHO/fat –– –––
GLP-1 (ng/ml) GIP (ng/ml)
BMI BMI
< 25 kg/m
2
12 11 ([2], [20]) 0.02 88.8% < 0.001 < 25 kg/m
2
89(−29, 47) 0.64 94.8% < 0.001
≥ 25 kg/m
2
444(−1, 90) 0.06 93.0% < 0.001 ≥ 25 kg/m
2
3 −15 (−60, 29) 0.50 90.2% < 0.001
Both 9 28 ([13], [35]) < 0.001 92.8% < 0.001 Both 1 −33 (−50, −17) < 0.001 ––
Sex Sex
Male 12 10 (−1, 20) 0.06 88.1% < 0.001 Male 7 −4(−44, 36) 0.84 97.0% < 0.001
Female 2 31 (−16, 79) 0.20 90.1% 0.001 Female 1 −25 (−145, 94) 0.68 ––
Male/female 11 30 ([19], [34]) < 0.001 86.2% < 0.001 Male/female 4 4 (−40, 48) 0.86 84.7% < 0.001
Protein source Protein source
Whey 11 10 (0.2, 19) 0.045 89.0% < 0.001 Whey 5 4 (−57, 65) 0.90 97.6% < 0.001
Casein/dairy 2 51 ([33], [43]) < 0.001 0 0.70 Casein/dairy 1 −36 (−55, −17) < 0.001 ––
Meat/egg 5 36 (−11, 83) 0.13 83.8% 0.01 Meat/egg 3 −8(−52, 37) 0.74 32.9 0.80
Vegetable 2 15 ([2], [25]) 0.02 71.7% 0.007 Vegetable 1 37 ([3], [46]) 0.03 ––
Mixed 5 37 ([13], [42]) 0.003 90.7% < 0.001 Mixed 2 −4(−86, 78) 0.93 94.4% < 0.001
Protein dose Protein dose
<35g 9 9(−4, 22) 0.17 75.0% < 0.001 < 35 g 5 12 (−14, 38) 0.37 86.6% < 0.001
≥ 35 g 16 26 ([16], [31]) < 0.001 91.6% < 0.001 ≥ 35 g 7 −18 (
−74,
38) 0.52 97.0% < 0.001
Placebo type Placebo type
CHO 20 25 ([16], [29]) < 0.001 93.3% < 0.001 CHO 10 6 (−27, 38) 0.73 95.4% < 0.001
Fat 5 −14 (−58, 29) 0.52 76.7% 0.002 Fat 2 −34 (−51, −18) < 0.001 0 0.44
CHO/fat –– –––CHO/fat –– –––
I
2
indicates between-study heterogeneity. CHO, carbohydrate.
A. Kohanmoo, et al.
Physiology & Behavior 226 (2020) 113123
7
analysis showed that appetite may be induced more effectively by lower
doses of protein while appetite hormones including ghrelin, CCK, and
GLP-1 might be stimulated by higher doses. In agreement with our
results, King et al. reported that ingestion of a small dose of whey
protein immediately before meal increased satiety but could not induce
GLP-1, GIP, and PYY responses [57]. Also, Veldhorst and colleagues
reported that breakfasts with 25% energy from protein affected ghrelin
and GLP-1 more effectively than breakfasts with 10% energy from
protein [42].
10.2. Placebo subgroups
Protein decreased hunger and ghrelin and increased fullness, CCK,
GLP-1, and PYY compared to carbohydrate. We could not compare the
satiating effect of protein with fat because there were only a few trials
with fat placebo. However, previous studies have found that protein has
a more pronounced effect on suppressing appetite than fat [27]. In fact,
fat has been found the least satiating macronutrient which suppresses
hunger to a less extent than carbohydrate and particularly compared to
protein [27, 58 ].
10.3. Other subgroups
The effect of various proteins on appetite markers have been com-
pared in a number trials. However, the results have been conflicting.
Some trials have found comparable subjective appetite ratings for
proteins from different sources, for instance, from animal and plant
sources [28, 59], or from casein, soy and whey sources [41, 36]. But
there is also evidence for different satiating effect from various proteins.
For instance, Acheson et al. observed a higher satiating effect from
casein and soy compared to whey [14]. Also, in a trial by Teunissen
et al. pea and milk proteins increased GLP-1 more than egg white
protein [40]. However, due to the paucity of data in some protein types,
subgroup analysis based on protein source did not give us much in-
formation for comparison of different proteins. Whey was the mostly
examined protein which affected appetite markers as well as ghrelin
and GLP-1. More trials need to be conducted on other protein sources,
including casein, meat, egg, and plant proteins.
Most of the trials were conducted on males; so subgroup analysis
produced unequal number of trials in sex subgroups. In spite of small
number of trials in females, protein demonstrated significant effect on
appetite markers in both males and females but the effect of protein on
the gastrointestinal outcomes in women remained inconclusive.
Moreover, unequal number of trials did not allow us to estimate the
extent of the effects on males and females [60]. In this regard, Gieze-
naar et al. reported that consumption of whey protein suppressed
hunger and increased CCK and GLP-1 concentrations in men more than
that in women [60],
suggesting that the satiating effect of protein may
be stronger in men than in females.
Similar to protein source subgroups, subgroup analysis for BMI did
not produce useful results because most of the trials had been per-
formed on normal weight participants. Nevertheless, the few trials that
have compared the effect of protein on appetite feelings found no dif-
ference in appetite suppression following protein intake between
normal weight and obese individuals [61, 59].
10.4. Long-term interventions
GLP-1 was the only outcome with significant change over long-term
interventions. The lack of protein effect on other outcomes including
appetite markers may be explained by diversity in the study design and
time of measurements in these trials compared to acute interventions.
For instance, in acute trials the parameters were measured pre- and
post- meal ingestion while in long-term interventions the time of
measurements differed between trials, with some measuring the out-
comes pre- and post- protein ingestion and others measuring them at a
specific time point (for instance in fasting state or pre-lunch). Moreover,
the form of administered protein in long-term trials varied from diet to
snack or meals whereas in acute interventions protein was always ad-
ministered in the form of snacks, beverages, or meals. When protein is
administered in the form of diet, the satiating effect of protein may not
be appropriately appeared. In this regard, Stubbs et al. reported that
breakfasts with high protein content led to detectable change in hunger
during hours between breakfast and lunch but the effect was not of
sufficient magnitude to influence lunch-time intake [62] . In addition, in
long-term trials higher doses of protein were given with an average of
33.6 g/day compared to 9.3 g/day in acute interventions. Comparably,
higher doses have weaker effect on appetite feelings but stronger effect
on gastrointestinal hormones [57, 42]. The number of long-term trials
in each outcome was almost half of that of acute interventions and this
reason may also contribute to the lack of protein effect in long-term
trials. These differences may explain the difference in the results of
short- and long-term trials.
10.5. Limitations
This was the first meta-analysis investigating the effect of protein on
gastrointestinal hormones involved in appetite regulation. However,
during meta-analysis we encountered several limitations. The number
of reports on some of the gastrointestinal hormones especially in long-
term investigations were limited. Likewise, there was insufficient
number of trials in subgroups of protein source (e.g., meat, egg, and
vegetable protein), fat placebo, and subjects with BMI ≥ 25 kg/m
2
. Not
all studies specified the form of hormones that they examined or had
reported data on active form of hormones. The variability in the form of
hormones that were measured as well as the variability in the method of
measurement could also have influenced the results. The form of car-
bohydrates in studies that gave carbohydrates as placebo is also im-
portant. Moreover, the rate of absorption macronutrients can also affect
secretion of gastrointestinal hormones like GLP-1 [56]. Long-term trials
encountered more diversities, for instance, in the time of measurements
and the form of protein administration (diet vs. supplement/meal).
10.6. Concluding remarks
According to the results of this meta-analysis acute ingestion of
protein suppresses appetite, decreases ghrelin, and augments CCK and
GLP-1. Due to numerous limitations, results of long-term trials are in-
conclusive and further well-designed and targeted trials are required
before a clear and sound conclusion could be made.
Supplementary materials
Supplementary material associated with this article can be found, in
the online version, at doi:10.1016/j.physbeh.2020.113123 .
References
[1] M.E. Gluck, P. Viswanath, E.J Stinson, Obesity, appetite, and the prefrontal cortex,
Curr. Obes. Rep. 6 (Dec(4)) (2017) 380–388, https://doi.org/10.1007/s13679-017-
0289-0.
[2] T.T. Hansen, S.V. Andersen, A. Astrup, J. Blundell, A Sjödin, Is reducing appetite
beneficial for body weight management in the context of overweight and obesity? A
systematic review and meta-analysis from clinical trials assessing body weight
management after exposure to satiety enhancing and/or hunger reducing products,
Obes. Rev. 20 (Jul(7)) (2019) 983–997, https://doi.org/10.1111/obr.12854.
[3] C. Gibbons, M. Hopkins, K. Beaulieu, P. Oustric, J.E Blundell, Issues in measuring
and interpreting human appetite (satiety/satiation) and its contribution to obesity,
Curr. Obes. Rep. 8 (Jun(2)) (2019) 77–87, https://doi.org/10.1007/s13679-019-
00340-6.
[4] J.C. Halford, J.A. Harrold, Satiety-enhancing products for appetite control: science
and regulation of functional foods for weight management, Proc. Nutr. Soc. 71 (May
(2)) (2012) 350–362, https://doi.org/10.1017/S0029665112000134.
[5] N. Weltens, J. Iven, L. Van Oudenhove, M Kano, The gut-brain axis in health
neuroscience: implications for functional gastrointestinal disorders and appetite
regulation, Ann. N Y Acad. Sci. 1428 (Sep(1)) (2018) 129–150, https://doi.org/10.
A. Kohanmoo, et al.
Physiology & Behavior 226 (2020) 113123
8
1111/nyas.13969.
[6] D. Zanchi, A. Depoorter, L. Egloff, S. Haller, L. Mählmann, U.E. Lang, J. Drewe,
C. Beglinger, A. Schmidt, S Borgwardt, The impact of gut hormones on the neural
circuit of appetite and satiety: a systematic review, Neurosci. Biobehav. Rev. 80
(Sep) (2017) 457–475, https://doi.org/10.1016/j.neubiorev.2017.06.013.
[7] K.J. Petzke, A. Freudenberg, S Klaus, Beyond the role of dietary protein and amino
acids in the prevention of diet-induced obesity, Int. J. Mol. Sci. 15 (Jan 20(1))
(2014) 1374–1391, https://doi.org/10.3390/ijms15011374.
[8] P.M. Clifton, D. Condo, J.B Keogh, Long term weight maintenance after advice to
consume low carbohydrate, higher protein diets–a systematic review and meta-
analysis, Nutr. Metab. Cardiovasc. Dis. 24 (Mar(3)) (2014) 224–235, https://doi.
org/10.1016/j.numecd.2013.11.006.
[9] S.J. Simpson, Raubenheimer D. obesity: the protein leverage hypothesis, Obes. Rev.
6 (May(2)) (2005) 133–142.
[10] A.K. Gosby, A.D. Conigrave, D. Raubenheimer, S.J Simpson, Protein leverage and
energy intake, Obes. Rev. 15 (Mar(3)) (2014) 183–191, https://doi.org/10.1111/
obr.12131.
[11] Z. Ye, V. Arumugam, E. Haugabrooks, P. Williamson, S Hendrich, Soluble dietary
fiber (Fibersol-2) decreased hunger and increased satiety hormones in humans
when ingested with a meal, Nutr. Res. 35 (May(5)) (2015) 393–400, https://doi.
org/10.1016/j.nutres.2015.03.004.
[12] P. Prinz, A. Stengel, Control of food intake by gastrointestinal peptides: mechanisms
of action and possible modulation in the treatment of obesity, J.
Neurogastroenterol. Motil. 23 (Apr. 30(2)) (2017) 180–196, https://doi.org/10.
5056/jnm16194.
[13] P. Sedgwick, What is publication bias in a meta-analysis? BMJ 351 (2015) h4419.
[14] K.J. Acheson, A. Blondel-Lubrano, S. Oguey-Araymon, M. Beaumont, S. Emady-
Azar, C. Ammon-Zufferey, I. Monnard, S. Pinaud, C. Nielsen-Moennoz, L Bovetto,
Protein choices targeting thermogenesis and metabolism, Am. J. Clin. Nutr. 93 (Mar
(3)) (2011) 525–534, https://doi.org/10.3945/ajcn.110.005850.
[15] T. Akhavan, B.L. Luhovyy, S. Panahi, R. Kubant, P.H. Brown, G.H Anderson,
Mechanism of action of pre-meal consumption of whey protein on glycemic control
in young adults, J. Nutr. Biochem. 25 (Jan(1)) (2014) 36–43, https://doi.org/10.
1016/j.jnutbio.2013.08.012.
[16] N.M. Astbury, E.J. Stevenson, P. Morris, M.A. Taylor, I.A Macdonald, Dose-response
effect of a whey protein preload on within-day energy intake in lean subjects, Br. J.
Nutr. 104 (Dec(12)) (2010) 1858–1867, https://doi.org/10.1017/
S000711451000293X.
[17] N.M. Astbury, M.A. Taylor, S.J. French, I.A Macdonald, Snacks containing whey
protein and polydextrose induce a sustained reduction in daily energy intake over 2
wk under free-living conditions, Am. J. Clin. Nutr. 99 (May(5)) (2014) 1131–1140,
https://doi.org/10.3945/ajcn.113.075978.
[18] A. Belza, C. Ritz, M.Q. Sørensen, J.J. Holst, J.F. Rehfeld, A Astrup, Contribution of
gastroenteropancreatic appetite hormones to protein-induced satiety, Am. J. Clin.
Nutr. 97 (May(5)) (2013) 980–989,
https://doi.org/10.3945/ajcn.112.047563.
[19] E.J. Bertenshaw, A. Lluch, M.R Yeomans, Dose-dependent effects of beverage pro-
tein content upon short-term intake, Appetite 52 (Jun(3)) (2009) 580–587, https://
doi.org/10.1016/j.appet.2009.01.010.
[20] E. Boelsma, E.J. Brink, A. Stafleu, H.F Hendriks, Measures of postprandial wellness
after single intake of two protein-carbohydrate meals, Appetite 54 (Jun(3)) (2010)
456–464, https://doi.org/10.1016/j.appet.2009.12.014.
[21] J. Bowen, M. Noakes, P.M Clifton, Appetite regulatory hormone responses to var-
ious dietary proteins differ by body mass index status despite similar reductions in
ad libitum energy intake, J. Clin. Endocrinol. Metab. 91 (Aug(8)) (2006)
2913–2919.
[22] I.M. Brennan, N.D. Luscombe-Marsh, R.V. Seimon, B. Otto, M. Horowitz,
J.M. Wishart, C Feinle-Bisset, Effects of fat, protein, and carbohydrate and protein
load on appetite, plasma cholecystokinin, peptide YY, and ghrelin, and energy in-
take in lean and obese men, Am. J. Physiol. Gastrointest. Liver Physiol. 303 (Jul(1))
(2012) G129–G140, https://doi.org/10.1152/ajpgi.00478.2011.
[23] B.M. Burton-Freeman, Glycomacropeptide (GMP) is not critical to whey-induced
satiety, but may have a unique role in energy intake regulation through cholecys-
tokinin (CCK), Physiol. Behav. 93 (Jan 28(1–2)) (2008) 379–387.
[24] R.D. Carr, M.O. Larsen, M.S. Winzell, K. Jelic, O. Lindgren, C.F. Deacon, B Ahrén,
Incretin and islet hormonal responses to fat and protein ingestion in healthy men,
Am. J. Physiol. Endocrinol. Metab. 295 (Oct(4)) (2008) E779–E784, https://doi.
org/10.1152/ajpendo.90233.2008.
[25] S.M. Chungchunlam, S.J. Henare, S. Ganesh, P.J Moughan, Dietary whey protein
influences plasma satiety-related hormones and plasma amino acids in normal-
weight adult women, Eur. J. Clin. Nutr. 69 (Feb(2)) (2015) 179–186, https://doi.
org/10.1038/ejcn.2014.266.
[26] S.M. Chungchunlam, S.J. Henare, S. Ganesh, P.J Moughan, Effect of whey protein
and glycomacropeptide on measures of satiety in normal-weight adult women,
Appetite 78 (Jul) (2014) 172–178, https://doi.org/10.1016/j.appet.2014.03.027.
[27] A. Dougkas, E. Östman, Protein-enriched liquid preloads varying in macronutrient
content modulate appetite and appetite-regulating hormones in healthy adults, J.
Nutr. 146 (Mar(3)) (2016) 637–645, https://doi.org/10.3945/jn.115.217224.
[28] A. Dougkas, E. Östman, Comparable effects of breakfast meals varying in protein
source on appetite and subsequent energy intake in healthy males, Eur. J. Nutr. 57
(Apr(3)) (2018) 1097–1108, https://doi.org/10.1007/s00394-017-1392-4.
[29] M.R. Duhita, Y. Schutz, J.P. Montani, A.G. Dulloo, J.L Miles-Chan, Assessment of
the dose-response relationship between meal protein content and postprandial
thermogenesis: effect of sex and the oral contraceptive pill, Nutrients 11 (Jul 15(7))
(2019), https://doi.org/10.3390/nu11071599 pii: E1599.
[30] D. El Khoury, R. El-Rassi, S. Azar, N Hwalla, Postprandial ghrelin and PYY responses
of male subjects on low carbohydrate meals to varied balancing proportions of
proteins and fats, Eur. J. Nutr. 49 (Dec(8)) (2010) 493–500, https://doi.org/10.
1007/s00394-010-0108-9.
[31] K.E. Foster-Schubert, J. Overduin, C.E. Prudom, J. Liu, H.S. Callahan, B.D. Gaylinn,
M.O. Thorner, D.E Cummings, Acyl and total ghrelin are suppressed strongly by
ingested proteins, weakly by lipids, and biphasically by carbohydrates, J. Clin.
Endocrinol. Metab. 93 (May(5)) (2008) 1971–1979, https://doi.org/10.1210/jc.
2007-2289.
[32] U.J. Gunnerud, C. Heinzle, J.J. Holst, E.M. Östman, I.M Björck, Effects of pre-meal
drinks with protein and amino acids on glycemic and metabolic responses at a
subsequent composite meal, PLoS ONE 7 (9) (2012) e44731, https://doi.org/10.
1371/journal.pone.0044731.
[33] R. Hursel, L. van der Zee, M.S Westerterp-Plantenga, Effects of a breakfast yoghurt,
with additional total whey protein or caseinomacropeptide-depleted alpha-lactal-
bumin-enriched whey protein, on diet-induced thermogenesis and appetite sup-
pression, Br. J. Nutr. 103 (Mar(5)) (2010) 775–780, https://doi.org/10.1017/
S0007114509992352.
[34] N. Karimian, M. Moustafa, J. Mata, A.K. Al-Saffar, P.M. Hellström, L.S. Feldman,
F Carli, The effects of added whey protein to a pre-operative carbohydrate drink on
glucose and insulin response, Acta Anaesthesiol. Scand. 62 (May(5)) (2018)
620–627, https://doi.org/10.1111/aas.13069.
[35] O. Lindgren, R.D. Carr, J.J. Holst, C.F. Deacon, B Ahrén, Dissociated incretin hor-
mone response to protein versus fat ingestion in obese subjects, Diabetes. Obes.
Metab. 13 (Sep(9)) (2011) 863–865, https://doi.org/10.1111/j.1463-1326.2011.
01420.x.
[36] C.E. Melson, S. Nepocatych, T.A Madzima, The effects of whey and soy liquid
breakfast on appetite response, energy metabolism, and subsequent energy intake,
Nutrition 61 (May) (2019) 179–186, https://doi.org/10.1016/j.nut.2018.11.007.
[37] B. Miazza, R. Palma, J.R. Lachance, J.A. Chayvialle, P.P. Jonard, R Modigliani,
Jejunal secretory effect of intraduodenal food in humans. A comparison of mixed
nutrients, proteins, lipids, and carbohydrates, Gastroenterology 88 (May(5 Pt 1))
(1985) 1215–1222.
[38] S. Pavlatos, A. Kokkinos, N. Tentolouris, J. Doupis, D. Kyriaki, N Katsilambros,
Acute effects of high-protein and high-fat isoenergetic meals on total ghrelin plasma
concentrations in lean and obese women, Horm. Metab. Res. 37 (Dec(12)) (2005)
773–775.
[39] O. Power, C. Conway, W. McCormack, F. Oharte, D. Fitzgerald, P Jakeman,
Comparison of the insulinotropic and enterogastric response to ingestion of an
equivalent quantity of maltodextran and whey protein, Proc. Nutr. Soc. 70 (OCE6)
(2011) E357.
[40] K.F. Teunissen-Beekman, J. Dopheide, J.M. Geleijnse, S.J. Bakker, E.J. Brink,
P.W. de Leeuw, J. Serroyen, M.A van Baak, Differential effects of proteins and
carbohydrates on postprandial blood pressure-related responses, Br. J. Nutr. 112
(Aug 28(4)) (2014) 600–608, https://doi.org/10.1017/S0007114514001251.
[41] M.A. Veldhorst, A.G. Nieuwenhuizen, A. Hochstenbach-Waelen, K.R. Westerterp,
M.P.
Engelen, R.J. Brummer, N.E. Deutz, M.S Westerterp-Plantenga, A breakfast
with alpha-lactalbumin, gelatin, or gelatin + TRP lowers energy intake at lunch
compared with a breakfast with casein, soy, whey, or whey-GMP, Clin. Nutr. 28
(Apr(2)) (2009) 147–155, https://doi.org/10.1016/j.clnu.2008.12.003.
[42] M.A. Veldhorst, A.G. Nieuwenhuizen, A. Hochstenbach-Waelen, K.R. Westerterp,
M.P. Engelen, R.J. Brummer, N.E. Deutz, M.S Westerterp-Plantenga, Effects of
complete whey-protein breakfasts versus whey without GMP-breakfasts on energy
intake and satiety, Appetite 52 (Apr(2)) (2009) 388–395, https://doi.org/10.1016/
j.appet.2008.11.014.
[43] M.S. Westerterp-Plantenga, V. Rolland, S.A. Wilson, K.R Westerterp, Satiety related
to 24 h diet-induced thermogenesis during high protein/carbohydrate vs high fat
diets measured in a respiration chamber, Eur. J. Clin. Nutr. 53 (Jun(6)) (1999)
495–502.
[44] S.M. Douglas, L.C. Ortinau, H.A. Hoertel, H.J Leidy, Low, moderate, or high protein
yogurt snacks on appetite control and subsequent eating in healthy women,
Appetite 60 (Jan(1)) (2013) 117–122, https://doi.org/10.1016/j.appet.2012.09.
012.
[45] L. Kjølbæk, L.B. Sørensen, N.B. Søndertoft, C.K. Rasmussen, J.K. Lorenzen,
A. Serena, A. Astrup, L.H Larsen, Protein supplements after weight loss do not
improve weight maintenance compared with recommended dietary protein intake
despite beneficial effects on appetite sensation and energy expenditure: a rando-
mized, controlled, double-blinded trial, Am. J. Clin. Nutr. 106 (Aug(2)) (2017)
684–697, https://doi.org/10.3945/ajcn.115.129528.
[46] H.J. Leidy, N.S. Carnell, R.D. Mattes, W.W Campbell, Higher protein intake pre-
serves lean mass and satiety with weight loss in pre-obese and obese women, Obes.
(Silver Spring) 15 (Feb(2)) (2007) 421–429.
[47] J. Li, C.L. Armstrong, W.W Campbell, Effects of dietary protein source and quantity
during weight loss on appetite, energy expenditure, and cardio-metabolic re-
sponses, Nutrients 8 (Jan 26(2)) (2016) 63, https://doi.org/10.3390/nu8020063.
[48] A. Marsset-Baglieri, G. Fromentin, G. Airinei, C. Pedersen, J. Léonil, J. Piedcoq,
D. Rémond, R. Benamouzig, D. Tomé, C Gaudichon, Milk protein fractions mod-
erately extend the duration of satiety compared with carbohydrates independently
of their digestive kinetics in overweight subjects, Br. J. Nutr. 112 (Aug 28(4))
(2014) 557–564, https://doi.org/10.1017/S0007114514001470.
[49] L.C. Ortinau, J.M. Culp, H.A. Hoertel, S.M. Douglas, H.J Leidy, The effects of in-
creased dietary protein yogurt snack in the afternoon on appetite control and eating
initiation in healthy women, Nutr. J. 12 (Jun 6) (2013) 71, https://doi.org/10.
1186/1475-2891-12-71.
[50] S. Pal, S. Radavelli-Bagatini, M. Hagger, V Ellis, Comparative effects of whey and
casein proteins on satiety in overweight and obese individuals: a randomized
controlled trial, Eur. J. Clin. Nutr. 68 (Sep(9)) (2014) 980–986, https://doi.org/10.
1038/ejcn.2014.84.
A. Kohanmoo, et al.
Physiology & Behavior 226 (2020) 113123
9
[51] N. Reyna, R. Moreno-Rojas, L. Mendoza, K. Parra, S. Linares, E. Reyna, F Cámara-
Martos, Using whey proteins and caseins as dietetic supplements in regulation of
satiating effect of overweight women, Nutr. Hosp. 33 (1) (2016) 47–53. Article in
Spanish.
[52] J. Caron, D. Domenger, P. Dhulster, R. Ravallec, B Cudennec, Protein digestion-
derived peptides and the peripheral regulation of food intake, Front. Endocrinol.
(Lausanne) 8 (Apr 24) (2017) 85, https://doi.org/10.3389/fendo.2017.00085.
[53] M. Cuenca-Sánchez, D. Navas-Carrillo, E Orenes-Piñero, Controversies surrounding
high-protein diet intake: satiating effect and kidney and bone health, Adv. Nutr. 6
(May 15(3)) (2015) 260–266, https://doi.org/10.3945/an.114.007716.
[54] O. Davidenko, N. Darcel, G. Fromentin, D Tomé, Control of protein and energy
intake - brain mechanisms, Eur. J. Clin. Nutr. 67 (May(5)) (2013) 455–461, https://
doi.org/10.1038/ejcn.2013.73.
[55] J.J. Meier, O. Goetze, J. Anstipp, D. Hagemann, J.J. Holst, W.E. Schmidt,
B. Gallwitz, M.A Nauck, Gastric inhibitory polypeptide does not inhibit gastric
emptying in humans, Am. J. Physiol. Endocrinol. Metab. 286 (Apr(4)) (2004)
E621–E625.
[56] R.M. Elliott, L.M. Morgan, J.A. Tredger, S. Deacon, J. Wright, V Marks, Glucagon-
like peptide-1 (7-36) amide and glucose-dependent insulinotropic polypeptide se-
cretion in response to nutrient ingestion in man: acute post-prandial and 24-h se-
cretion patterns, J. Endocrinol. 138 (Jul(1)) (1993) 159–166.
[57] D.G. King, M. Walker, M.D. Campbell, L. Breen, E.J. Stevenson, D.J West, A small
dose of whey protein co-ingested with mixed-macronutrient breakfast and lunch
meals improves postprandial glycemia and suppresses appetite in men with type 2
diabetes: a randomized controlled trial, Am. J. Clin. Nutr. 107 (Apr 1(4)) (2018)
550–557, https://doi.org/10.1093/ajcn/nqy019.
[58] J. Johnson, Z. Vickers, Effects of flavor and macronutrient composition of food
servings on liking, hunger and subsequent intake, Appetite 21 (Aug(1)) (1993)
25–39.
[59] C.M. Crowder, B.L. Neumann, J.I Baum, Breakfast protein source does not influence
postprandial appetite response and food intake in normal weight and overweight
young women, J. Nutr. Metab. 2016 (2016) 6265789, , https://doi.org/10.1155/
2016/6265789.
[60] C. Giezenaar, N.D. Luscombe-Marsh, A.T. Hutchison, K. Lange, T. Hausken,
K.L. Jones, M. Horowitz, I. Chapman, S Soenen, Effect of gender on the acute effects
of whey protein ingestion on energy intake, appetite, gastric emptying and gut
hormone responses in healthy young adults, Nutr. Diabet. 8 (Jul 13(1)) (2018) 40,
https://doi.org/10.1038/s41387-018-0048-7.
[61] N. Bellissimo, M.V. Desantadina, P.B. Pencharz, G.B. Berall, S.G. Thomas,
G.H Anderson, A comparison of short-term appetite and energy intakes in normal
weight and obese boys following glucose and whey-protein drinks, Int. J. Obes.
(Lond.) 32 (Feb(2)) (2008) 362–371.
[62] R.J. Stubbs, M.C. van Wyk, A.M. Johnstone, C.G Harbron, Breakfasts high in pro-
tein, fat or carbohydrate: e ffect on within-day appetite and energy balance, Eur. J.
Clin. Nutr. 50 (Jul(7)) (1996) 409 –417.
A. Kohanmoo, et al.
Physiology & Behavior 226 (2020) 113123
10
