Official address Domenico Scarlattilaan 6 ● 1083 HS Amsterdam ● The Netherlands
An agency of the European Union
Address for visits and deliveries Refer to www.ema.europa.eu/how-to-find-us
Send us a question Go to www.ema.europa.eu/contact
Telephone +31 (0)88 781 6000
© European Medicines Agency, 2024. Reproduction is authorised provided the source is acknowledged.
3 May 2024 Rev.4
EMA/37991/2019
Human Medicines Division
Questions & Answers for applicants, marketing
authorisation holders of medicinal products and notified
bodies with respect to the implementation of the
Regulations on medical devices and in vitro diagnostic
medical devices (Regulations (EU) 2017/745 and (EU)
2017/746)
This Question and Answer (Q&A) document provides practical considerations concerning the
implementation of the medical devices and the in vitro diagnostic medical devices regulations in the
context of combinations of medicinal products with medical devices.
This document has been produced to provide guidance to applicants, marketing authorisation holders
(MAH) and notified bodies (NB) as regards aspects falling within the scope of the European Medicines
Agency’s activities and should be read in conjunction with the medical devices Regulation (EU) 2017/745
(MDR), the in vitro diagnostic medical devices Regulation (EU) 2017/746 (IVDR) and the Medical Device
Coordination Group (MDCG)
1
guidance documents. The MDR and IVDR replace the three Directives
(90/385/EEC, 93/42/EEC and 98/79/EC) for medical devices. The MDR came into application on 26 May
2021 but provides for a transitional period for certain devices. The IVDR came into application on 26 May
2022 but also provides for a transitional period for certain devices. These regulations include provisions
concerning the responsibilities of the EMA, National Competent Authorities (NCA) for medicinal products
and medical devices and notified bodies as regards combinations of medicinal products with medical
devices as follows:
• For medical devices that form an integral product with a medicinal product (Regulation (EU)
2017/745, second subparagraph of Article 1(8) and 1(9)), new requirements to provide an EU
declaration of conformity issued by the medical device manufacturer, or a certificate of conformity
(so-called hereafter EU certificate) or an opinion from a notified body designated under Regulation
(EU) 2017/745
2
for the type of device in question are applicable in certain circumstances (Art. 117).
• For medical devices incorporating a medicinal substance with action ancillary to the device,
Regulation (EU) 2017/745 Article 1 (8), the notified body must seek a scientific opinion from either
an NCA or EMA
3
. The notified body must seek the opinion of EMA for medicinal products falling
1
The MDCG is the Member State group responsible for the oversight of implementation of the medical device Regulations.
It is set up according to Art. 103 of Regulation (EU) 2017/745 and Art. 98 of Regulation (EU) 2017/746.
2
Regulation 2017/745 Article 117
3
Regulation 2017/745 Annex IX 5.2

Questions & Answers for applicants, marketing authorisation holders of medicinal
products and notified bodies with respect to the implementation of the Regulations on
medical devices and in vitro diagnostic medical devices (Regulations (EU) 2017/745
and (E
EMA/37991/2019 Rev.4
Page 2/23
exclusively within the scope of centralised procedure
4
, or that incorporate human blood or plasma
derivatives.
• For medical devices that are composed of substances, or of combinations of substances, that are
systemically absorbed by the body in order to achieve their intended purpose, the notified body must
seek a scientific opinion from either an NCA or EMA (Regulation (EU) 2017/745 Article 52(11))
5
.
• For companion diagnostics, the notified body must seek a scientific opinion from either an NCA or
EMA (Regulation (EU) 2017/746, Article 48(3) and (4)
6
).
This document covers guidance for:
• Medical devices that form an integral product with a medicinal product,
• Medicinal products that include a medical device in the secondary packaging of the medicinal product
(co-packaged),
• Consultation procedure for ancillary medicinal substances that are integral part of medical devices,
• Consultation procedure for companion diagnostics.
This “questions and answers” document provides regulatory and procedural guidance and should be read
in conjunction with the EMA Guideline on quality documentation for medicinal products when used with
a medical device. This guideline describes the information that should be presented in the Quality part
of a marketing authorisation dossier for a medicinal product when it is used with a medical device, or
device part, and submitted in accordance with Directive 2001/83/EC and/or Regulation (EC) 726/2004.
This guideline focuses on product-specific quality aspects of a medical device, or device part, that may
have an impact on the quality, safety and/or efficacy (and hence overall benefit/risk determination) of a
medicinal product.
This “questions and answers” document is being updated continuously and will be marked by “New” or
“Rev.” with the relevant date upon publication.
4
Annex I, Regulation (EC) No 726/2004
5
Regulation 2017/745 Annex IX 5.4
6
Regulation 2017/746 Annex IX 5.2, Annex X 3(k)

Questions & Answers for applicants, marketing authorisation holders of medicinal
products and notified bodies with respect to the implementation of the Regulations on
medical devices and in vitro diagnostic medical devices (Regulations (EU) 2017/745
and (E
EMA/37991/2019 Rev.4
Page 3/23
Table of contents
1. Combinations of medicinal products and medical devices ....................... 5
1.1. What regulatory framework does a product incorporating both medicinal product and
medical device fall under? Rev. May 2024 .................................................................... 5
1.2. How do I choose a notified body for my integral drug-device combination? Rev. May
2024 ....................................................................................................................... 6
1.3 How to obtain advice on the qualification/classification of my drug-device combination,
especially for borderline products? New May 2024 ......................................................... 6
2. Integral Drug-Device Combinations ......................................................... 7
2.1. When is my medical device considered to form an integral product with a medicinal
product? Rev. May 2024 ............................................................................................ 7
2.2. What is Article 117 and what does it mean for medicinal products? Rev. May 2024 ...... 8
2.3. At what stage of the MAA do I need to submit the notified body opinion? Rev. May 2024
............................................................................................................................. 9
2.4. Can I provide a notified body opinion concluding on partial compliance with the GSPR?
What is the scope of the notified body opinion ? New May 2024 ...................................... 9
2.5. How does Article 117 of the MDR impact iDDCs authorised under MDD? Rev. May 2024
........................................................................................................................... 10
2.6. Will I need to provide a (new or updated) EU declaration of conformity/certificate of
conformity issued by a notified body/notified body opinion if there are changes to the device
(or device part) after the initial marketing authorisation of the integral DDC? Rev. May 2024
........................................................................................................................... 10
2.7. How should I submit minor changes to the terms of the Marketing Authorisation for
integral DDC following changes to the device (or device part)? New May 2024 ................ 12
2.8. Will I need to provide a new/updated notified body opinion for changes related to the
medicinal product (e.g. extension of indication, new strength, new pharmaceutical form) in
an integral drug-device combination? New May 2024 .................................................. 14
2.9. Is it possible to submit a notified body certificate issued under the Directives
(90/385/EEC or 93/42/EEC) to comply with Article 117? Rev. May 2024 ......................... 16
2.10. How will the notified body opinion be reflected in the European Public Assessment
Report (EPAR)? June 2021 ....................................................................................... 16
2.11. What is the impact of the MDR and Article 117 on marketing authorisation applications
of an iDDC on a Mutual Recognition Procedure submitted on or after the 26 May 2021? Rev.
May 2024 .............................................................................................................. 16
2.12. Do the requirements of MDR Article 117 also apply to an application for medicinal
products to be used outside of the European Union (Article 58 or EU-M4all)? Rev. June 2021
........................................................................................................................... 17
2.13. Are the requirements for UDI (unique device identifier) applicable to a medicinal
product that incorporates, as an integral part, a medical device? Rev. May 2024 ............. 17
3. Medicinal products that include a medical device in the secondary
packaging of the marketed medicinal product (co-packaged) ................... 17
3.1. How will the MDR affect the co-packaged medical device? Rev. May 2024 ................ 17
3.2. What requirements for medical device labelling are applicable to medical devices “co-
packaged” with medicinal products? Rev. May 2024 ..................................................... 18
3.2.1 If co-packaged medical devices class I and class IIa, are supplied without an individual
packaging and it is not technically feasible to implement the labelling requirements on the
device itself, what alternative solutions could be considered to display the labelling
requirements? New May 2024 .................................................................................. 19

Questions & Answers for applicants, marketing authorisation holders of medicinal
products and notified bodies with respect to the implementation of the Regulations on
medical devices and in vitro diagnostic medical devices (Regulations (EU) 2017/745
and (E
EMA/37991/2019 Rev.4
Page 4/23
3.3. Do I need to submit a declaration of conformity / EU certificate as part of the dossier for
a co-packaged medical device? Rev. May 2024 ........................................................... 20
3.4. What actions, if any, do I need to take if my co-packaged device is up-classified and
requires to be certified by a notified body for the first time? Rev. May 2024 .................... 20
4. Consultation procedure for ancillary medicinal substances in medical
devices (Art 1(8)) ..................................................................................... 20
4.1. What type of consultation procedure needs to be carried out for an ancillary medicinal
substance that has already been consulted under the medical device Directive 93/42/EEC?
Rev. May 2024 ....................................................................................................... 20
5. Consultation procedure for companion diagnostics ............................... 21
5.1. What type of consultation procedure needs to be carried out for a companion diagnostic
New May 2024 ....................................................................................................... 21

Questions & Answers for applicants, marketing authorisation holders of medicinal
products and notified bodies with respect to the implementation of the Regulations on
medical devices and in vitro diagnostic medical devices (Regulations (EU) 2017/745
and (E
EMA/37991/2019 Rev.4
Page 5/23
1. Combinations of medicinal products and medical devices
1.1. What regulatory framework does a product incorporating both medicinal product and
medical device fall under? Rev. May 2024
Products which combine a medicinal product (or substance) and a medical device are regulated either
by Regulation (EU) 2017/745 on medical devices or Directive 2001/83/EC relating to medicinal products
for human use, depending on their principal mode of action. Certain combinations of medicinal products
and medical devices are governed by or require consultation of EMA or a national competent authority
for medicinal products (NCA), as laid down in Articles 1(8) and 1(9) of the MDR.
The regulatory framework for devices incorporating medicinal substances as an integral part is laid down
in Article 1(8) of MDR:
• Where the action of the medicinal substance is ancillary to the action of the medical device, the
product is regulated as a medical device and must be CE marked. For these combinations, a
scientific opinion on the quality and safety of the ancillary substance including the benefit or risk
of its incorporation in the device must be provided from one of the national competent authorities
for medicinal products or from the EMA (referred to collectively as medicines authority) before a
notified body can issue a EU certificate. For more information on the consultation procedure by
EMA and for a list of products previously reviewed by EMA, please see EMA webpage on Ancillary
medicinal substances in medical devices.
• Where the action of the medicinal substance is principal and not ancillary to the action of the
medical device, the integral drug device combination (iDDC) is governed under the medicinal
products framework and are referred to as iDDCs in the Q&A hereafter
7
. In that case, the relevant
general safety and performance requirements of the Annex I of the MDR apply to the device part
of the iDDC.
The regulatory framework for medical devices intended to administer medicinal products is laid down in
Article 1(9) MDR:
• If the medicinal product and administration device are marketed as a single integral product
intended exclusively for use in the given combination and is not reusable, the iDDC is
governed under the medicinal products framework. In that case, the relevant general safety and
performance requirements of Annex I of the MDR apply to the device part.
• In all other cases (e.g. where the medical device is co-packaged with the medicinal product or
when the product information of the medicinal product refers to a specific device to be used and
the device is obtained separately), the administration device is governed by the medical device
framework. These administration devices must meet the requirements of the MDR and will need
to be CE marked.
For the purpose of this document, integral products falling within MDR Article 1(8) second subparagraph
and 1(9) second subparagraph, and for which the principal mode of action is pharmacological, metabolic
or immunological, are regulated under the medicinal products framework.
7
so-called ‘integral medicinal products’ in the EMA Guideline on quality documentation for medicinal products when used
with a medical device (EMA/CHMP/QWP/BWP/259165/2019)
Questions & Answers for applicants, marketing authorisation holders of medicinal
products and notified bodies with respect to the implementation of the Regulations on
medical devices and in vitro diagnostic medical devices (Regulations (EU) 2017/745
and (E
EMA/37991/2019 Rev.4
Page 6/23
There are cases where a medicinal product and a medical device are placed on the market in the same
secondary packaging but do not form an integral product, for example, a vial containing a medicinal
product solution with an (empty) CE marked sterile syringe. These are referred as a medicine with a co-
packaged device. This product is not considered an iDCC as the medical device falls under the first
subparagraph of Article 1(9) of the MDR. Furthermore, when a medical device is referenced in the product
information of a medicinal product, for a use in combination with the medicinal product, but the two
products are not placed on the EU market within the same secondary packaging, requirements for these
products are covered in section 3 of this Q&A.
Of note, IVD kits may not include medicinal products. If an IVD or an IVD kit is intended to be used with
a medicinal product and the products are co-packaged, the combination with the medicinal product may
not be qualified as an IVD kit. Each product must comply with its corresponding legislation.
1.2. How do I choose a notified body for my integral drug-device combination? Rev. May
2024
A notified body within the European Union (EU) is a third-party conformity assessment body designated,
by an EU Member State authority, to carry out conformity assessment activities regarding medical
devices and/or in-vitro diagnostics (IVDs) in accordance with the MDR and/or IVDR before being placed
on the Union market. Companies are free to choose any notified body; the only criterion is that the
notified body must be designated to carry out the conformity assessment procedure for the particular
medical device for which a certification or notified body opinion is sought; this is defined by the notified
body’s designation codes. Applicants can check the NANDO (New Approach Notified and Designated
Organisations) website, by clicking on ‘Legislation’ and select the relevant Directive/Regulation to search
for a designated notified body according to the codes/scope needed.
1.3 How to obtain advice on the qualification/classification of my drug-device combination,
especially for borderline products? New May 2024
Borderline products are those where it is not clear which regulatory framework applies, i.e., whether
they fall under the medical device framework or the pharmaceutical framework.
The demarcation between the MDR on one hand and the pharmaceutical legislation on the other hand
is crucial for applying the appropriate set of requirements.
The qualification/classification of a borderline product (e.g. whether it should be regulated as a
medicinal product or as a medical device) lies with the national competent authorities for medicinal
products and/or medical devices.
Several provisions to establish the demarcation between the two legal frameworks have been laid
down in the MDR and the Directive 2001/83/EC and explanations and examples clarifying these
provisions can be found in MDCG 2022 – 5 Guidance on borderline between medical devices and
medicinal products under Regulation (EU) 2017/745 on medical devices. In addition, the MDCG Manual
on borderline and classification for medical devices under Regulation (EU) 2017/745 on medical
devices and Regulation (EU) 2017/746 on in vitro diagnostic medical devices records the views of the
Member State members of the MDCG Borderline and Classification Working Group regarding the
regulatory status of a product (see Section 1.1.2 regarding the borderline between medical devices and
medicinal products).
Qualification and risk classification of medical devices should be agreed with the notified body, if
applicable. Any dispute between the manufacturer and the notified body regarding the classification of
a device is to be referred for a decision to the national competent authority for medical devices in the

Questions & Answers for applicants, marketing authorisation holders of medicinal
products and notified bodies with respect to the implementation of the Regulations on
medical devices and in vitro diagnostic medical devices (Regulations (EU) 2017/745
and (E
EMA/37991/2019 Rev.4
Page 7/23
Member States in which the manufacturer has its registered place of business (Article 51(2) MDR and
47(2) IVDR).
In order to facilitate the development of innovative drug-device combinations and foster an early
dialogue, informal input can be requested from the Innovative Task Force (ITF) of the European
Medicines Agency. While the ITF may express scientific views on borderline products classification, it is
the responsibility of the Applicant/MAH to contact a national competent authority for possible formal
advice on the qualification of combination or borderline products and/or risk classification of a medical
device, when needed and before submitting an application.
References
• EU National Competent Authorities for human medicinal products
• EU National Competent Authorities for medical devices
• EMA guidance on how to request an ITF meeting
• MDCG borderline guidance and manual
2. Integral Drug-Device Combinations
2.1. When is my medical device considered to form an integral product with a medicinal
product? Rev. May 2024
There are two types of iDDCs
8
according to MDR Articles 1(8) and 1(9) (see also Question 1.1).
1. A medical device that incorporates, as an integral part, a substance which, if used separately, would
be considered a medicinal product and where the action of that substance is principal, the integral
product will be regulated as a medicinal product (second subparagraph of Article 1(8)). Examples
include an ingestible sensor that is incorporated into a medicinal product.
2. If a medical device used to administer a medicinal product is placed on the market in such a way
that the two constituents parts (the medical device and medicinal product) form a single integral
product which is intended exclusively for use in the given combination and which is not reusable,
that single integral product shall be governed by Directive 2001/83/EC or Regulation (EC) No
726/2004, as applicable. The second paragraph of Article 1(9) of the MDR sets out three cumulative
conditions that need to be satisfied at the moment of placing on the market:
i. the device and the medicinal product form a single integral product when placed on the market,
ii. the single integral product is intended exclusively for use in the given combination,
iii. the single integral product is not reusable.
Below are provided some examples.
For medicinal products meeting either one or both of the above definitions, the integral product shall
be governed by Directive 2001/83/EC or Regulation (EC) No 726/2004, as applicable. However, the
relevant general safety and performance requirements set out in Annex I to Regulation (EU) 2017/745
shall apply as far as the safety and performance of the device part of the integral product is concerned.
8
so-called ‘integral medicinal products’ in the EMA Guideline on quality documentation for medicinal products when used
with a medical device (EMA/CHMP/QWP/BWP/259165/2019)

Questions & Answers for applicants, marketing authorisation holders of medicinal
products and notified bodies with respect to the implementation of the Regulations on
medical devices and in vitro diagnostic medical devices (Regulations (EU) 2017/745
and (E
EMA/37991/2019 Rev.4
Page 8/23
Examples of integral products which are not reusable are pre-filled syringes, pre-filled pens, nebulisers
pre-charged with a specific medicinal product, nasal and oromucosal sprays, patches for transdermal
drug delivery, pre-filled inhalers and co-packaged pre-filled syringe with solvent (e.g. water for
injections) used for administration of the reconstituted medicinal product.
In certain cases, the following components would be assessed by competent authorities in accordance
with the requirements of :
- a container closure system e.g. eye drops nozzle without measuring function, syringe for
reconstitution (without purpose for administration of the medicinal product) or syringe without
measuring function specifically intended to transfer into an IV bag or
- excipients used in the manufacture of the finished medicinal product e.g. in some implants and
some transdermal patches (using passive diffusion),
Note: the above examples are provided as a guide, however some devices could be classified differently
due to additional features and functionality. If in doubt over the qualification of your product as a
medicinal product, device or iDDC, it is recommended that you consult a national competent authority.
References
• National Competent Authorities for human medicinal products
• National Competent Authorities for medical devices
• MDCG borderline guidance and manual
2.2. What is Article 117 and what does it mean for medicinal products? Rev. May 2024
Applications for a marketing authorisation of an iDDC submitted as of 26 May 2021 must comply with
the requirements of Annex I to Directive 2001/83/EC, point 12 of section 3.2, as amended by Article 117
of Regulation (EU) 2017/745.
Article 117 of Regulation (EU) 2017/745 sets out that the DDC marketing authorisation dossier must
include, where available, the results of the assessment of conformity for the device part (i.e. the
declaration of conformity or the relevant EU certificate issued by a notified body).
If the dossier does not include the results of the assessment of conformity, and an EU certificate from a
notified body would be required if the device was used separately, then the applicant will be required to
provide an opinion from a notified body on the conformity of the device part with relevant requirements
of Annex I to Regulation (EU) 2017/745 as part of the Marketing Authorisation Application.
Article 117 applies to iDDCs referred to under the second subparagraphs of MDR Articles 1(8) and 1(9).
Article 117 does not apply in the case of combined advanced therapy medicinal products as defined
under Article 2(1)(d) of Regulation (EC) No 1394/2007.
The submission of evidence of compliance with the general safety and performance requirements of the
MDR in the marketing authorisation dossiers of integral DDCs did not apply to MAAs submitted before
26 May 2021, i.e. the date of application of the MDR.
Questions & Answers for applicants, marketing authorisation holders of medicinal
products and notified bodies with respect to the implementation of the Regulations on
medical devices and in vitro diagnostic medical devices (Regulations (EU) 2017/745
and (E
EMA/37991/2019 Rev.4
Page 9/23
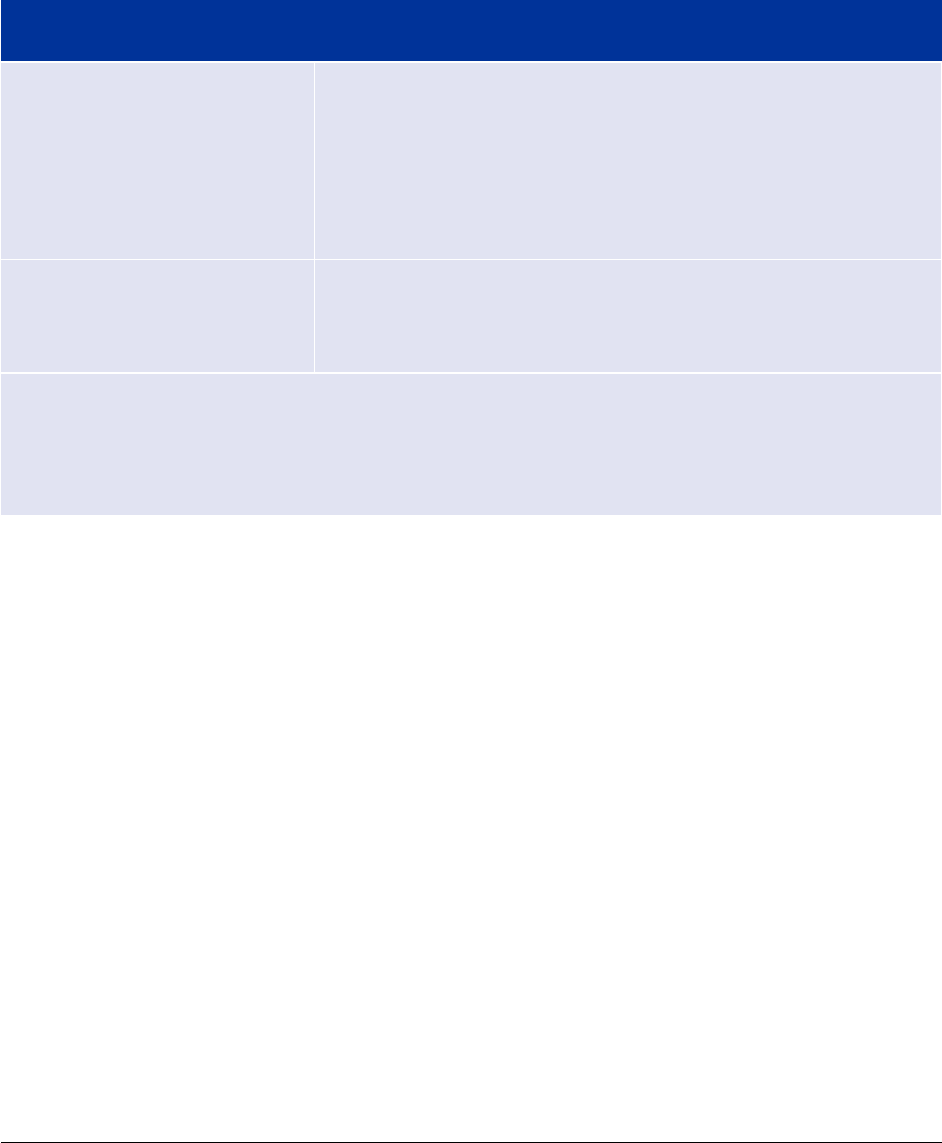
In summary, marketing authorisation applications for a iDDC submitted as of 26 May 2021, must
demonstrate that the device part meets the relevant requirements of Annex I of Regulation (EU)
2017/745 as presented in the below table.
Additional information may be requested to support the review of the benefit/risk assessment of the
medicinal product in order to ensure a safe and effective use of the iDDC.
Table 1. Summary of changes for Marketing Authorisation Applications involving iDDCs
Risk class of medical device
New submissions as of 26
th
May 2021
Class I sterile, measuring or
reusable surgical instrument*
Class IIa
Class IIb
Class III
The marketing authorisation dossier must include an EU a
certificate issued by a notified body designated for the type
of device part in question.
If the abovementioned documentation is not available, then an
opinion** from a notified body must be provided for the medical
device.
Class I non-sterile, non-
measuring, or non-reusable
surgical instrument
The marketing authorisation dossier must include a Declaration
of Conformity for the medical device.
* the reader should note that iDDC as referred to in second subparagraph of Regulation 2017/745
Article 1(9) are not reusable
**opinion on the conformity of the device part with the relevant general safety and performance
requirements set out in Annex I to Regulation 2017/745
2.3. At what stage of the MAA do I need to submit the notified body opinion? Rev. May 2024
EMA/NCAs strongly recommend submitting the declaration of conformity / EU certificate / notified body
opinion at the time of submission of the dossier of the initial marketing authorisation application for the
medicinal product to facilitate a smooth running of the procedure. In case the applicant cannot provide
the required documentation at the time of MAA submission, the relevant documents must be provided
before an opinion on the medicinal product application can be issued. Applicants should discuss their
plans to provide the required documentation during the EMA/NCA pre-submission meeting. The
absence of the required documentation may result in additional clock stops during the procedure.
2.4. Can I provide a notified body opinion concluding on partial compliance with the GSPR?
What is the scope of the notified body opinion ? New May 2024
A notified body opinion concluding on partial compliance cannot be accepted as the CHMP and the
NCAs do not have the remit of assessing compliance of the device part with the General Safety and
Performance requirements (GSPR). This can hence lead to an evaluation issue and an updated notified
body opinion confirming full compliance with the relevant GSPRs will need to be provided prior to the
issuance of an opinion on the medicinal product.

Questions & Answers for applicants, marketing authorisation holders of medicinal
products and notified bodies with respect to the implementation of the Regulations on
medical devices and in vitro diagnostic medical devices (Regulations (EU) 2017/745
and (E
EMA/37991/2019 Rev.4
Page 10/23
Furthermore, the scope of the notified body opinion should correspond to the intended purpose of the
device for the particular marketing authorisation application.
2.5. How does Article 117 of the MDR impact iDDCs authorised under MDD? Rev. May 2024
Annex I to Directive 2001/83/EC, point 12 of section 3.2, as amended by Article 117 of MDR, is not
intended to apply retrospectively to iDDCs already authorised or to those MAAs that have been already
submitted prior to 26 May 2021.
However, if after the granting of the marketing authorisation there is a major change to a device such
as in its design, performance, safety or intended purpose of the device (part) that may have a significant
impact on the delivery or the quality, safety, or efficacy of the medicinal product, or a new device is
introduced, any required declaration of conformity / EU certificate / notified body opinion should be
submitted as part of the appropriate regulatory procedure to EMA/NCA (see also Q2.6).
As for any other changes, the MAH should determine whether there is a potential impact on the delivery,
quality, safety and/or efficacy of the iDDC. If the MAH determines that the change impacts the registered
information, a variation application according to the variation guideline will be required. If the change
does not impact the registered information but the MAH concludes that there is an impact on the quality,
safety and/or efficacy of the iDDC, a variation application must also be submitted.
In case of change to the design, performance or intended purpose of the device (part) or other changes
to the medicinal product that may affect the medical device, the MAH should assess and provide a
justification whether the change has no significant impact on the safety or performance of the device to
justify the absence of a notified body opinion, EU certificate or declaration of conformity in accordance
with the MDR, as appropriate. Otherwise, an updated or new proof of compliance with the MDR should
be provided.
In cases where the need for a variation and/or the category of the change is unclear, it is recommended
that the national competent authority for medicinal products that issued the marketing authorisation is
consulted.
In line with the advice provided in the EMA Q&A for Post-authorisation procedural advice for users of the
centralised procedure, given the relatively short timelines for variations procedures, the (new/updated)
EU declaration of conformity / EU certificate issued by a designated notified body / notified body opinion
for medical devices should be provided at the time of submission of the application to avoid any delays
of the procedure.
2.6. Will I need to provide a (new or updated) EU declaration of conformity/certificate of
conformity issued by a notified body/notified body opinion if there are changes to the device
(or device part) after the initial marketing authorisation of the integral DDC? Rev. May 2024
Article 117 requirement applies post-authorisation to all marketing authorisations, irrespective whether
already compliant with Annex I to Directive 2001/83/EC, point 12 of section 3.2, as amended by Article
117 MDR at the time of the initial MAA, in case of major changes to a device that may affect significantly
the safety or performance of the device part or the intended use of the device. Contractual agreements
between the MAH and the medical device manufacturer should ensure appropriate level of
communication and action as regards changes to the device part. There are two situations where a (new

Questions & Answers for applicants, marketing authorisation holders of medicinal
products and notified bodies with respect to the implementation of the Regulations on
medical devices and in vitro diagnostic medical devices (Regulations (EU) 2017/745
and (E
EMA/37991/2019 Rev.4
Page 11/23
or updated) EU declaration of conformity / EU certificate issued by a notified body / notified body opinion
must be submitted in a post-authorisation setting of the medicinal product.
a) Addition or full replacement of the device or device part
Where a device (or device part) is replaced or a new device is added, a new EU declaration of conformity/
EU certificate issued by a notified body / notified body opinion must be provided as part of a variation or
extension application.
b) Changes to the device or to a device part
Where the medical device manufacturer plans to introduce changes that may affect the safety and
performance of the device part or the conditions prescribed for the intended use of the device part, there
are three possible situations:
• For devices covered under a manufacturer’s EU declaration of conformity only (no
involvement of a notified body): the device (part) manufacturer is responsible to ensure
compliance with the MDR, including changes to the device (part). The EU declaration of
conformity should be updated accordingly, if necessary.
• For devices covered under an MDR EU certificate issued by a notified body: if the
assessment of changes leads to the issuance of a new/supplemented EU certificate according to
the requirements established in the relevant annexes (Annexes IX, X, XI) of the MDR, the EU
certificate must be provided as part of an appropriate post-authorisation regulatory procedure.
• For devices holding a notified body opinion: if the assessment of changes lead to the
issuance of a new notified body opinion, the new notified body opinion must be provided as part
of an appropriate post-authorisation regulatory procedure.
Contractual arrangements between the notified body and the device manufacturer address the
notification obligation and the oversight of changes to the device in the context of the life cycle
management of a EU certificate.
For a notified body opinion in the absence of a contractual arrangement between the notified body and
the MAH, it is the responsibility of the MAH to determine when a new or updated NB opinion is needed
in support of a change to the device or its intended use. The MAH is responsible for liaising with the EMA
or the NCA for the iDDC.
As for any other changes, the MAH should determine whether there is a potential impact on, the delivery,
quality, safety and/or efficacy of the iDDC. If the MAH determines that the change impacts the registered
information, the MAH will have to submit a variation application according to the variation guideline. If
the change does not impact the registered information but the MAH concludes that there is an impact
on the delivery, quality, safety and/or efficacy of the iDDC, the MAH must also submit a variation
application.
In order to support identification of situations where a new or revised notified body opinion for the
device part of an iDDC is anticipated, the following guidance is provided, however this is without
prejudice of the specificities of the concerned product case and the fact that assessment of compliance
with the general safety and performance requirements (GSPRs) of the device remains the remit of the
notified body.

Questions & Answers for applicants, marketing authorisation holders of medicinal
products and notified bodies with respect to the implementation of the Regulations on
medical devices and in vitro diagnostic medical devices (Regulations (EU) 2017/745
and (E
EMA/37991/2019 Rev.4
Page 12/23
In case of a EU certificate or device manufacturer’s declaration of conformity (DoC), the MAH should
follow the MDR requirements for the maintenance of the EU certificate or DoC.
As a guiding approach, a new or revised notified body opinion for the device part of an iDDC is:
1) required when a new device is introduced with a line extension or variation;
2) expected when major changes are introduced to an existing device, such as:
- change to its design ;
- Addition or replacement of an integral device (part)
- change to its performance characteristics;
- change to its intended purpose such as a different patient population and/or new user (e.g.
home versus hospital setting) and/or new usability study, and/or significantly different
instructions for use.
which may have a significant impact on the delivery or the quality, safety, or efficacy of the medicinal
product.
3) expected in case of changes to the medicinal product which may impact the performance or safety
of a device, for example the introduction of a new finished product formulation resulting in different
viscosity significantly affecting device performance.
In case a change or combination of changes to the device part of an iDDC and/or its intended use is
(are) not considered to require a new or revised notified body opinion, applicants are expected to
provide a justification and risk assessment in Module 3.2.R. Depending on the device change(s) and
considering safe and accurate administration and performance, the impact on the Quality Target
Product Profile (QTPP), Critical Quality Attributes (CQAs) and control strategy for the iDDC should be
considered.
In case of doubt regarding the need to provide a new or revised notified body opinion (or DoC or EU
Certificate) to support a variation or line extension and where the need for a variation and/or the
category of the change is unclear, applicants are strongly recommended to liaise with the national
competent authorities for medicinal products well in advance during the pre-submission phase.
In line with the advice provided in the EMA Q&A for Post-authorisation procedural advice for users of the
centralised procedure, given the relatively short timelines for variations procedures, the (new/updated)
EU declaration of conformity /EU certificate issued by a designated notified body / notified body opinion
for medical devices should be provided at the time of submission of the application to avoid any delays
of the procedure. If not available at submission, it will be legally required before CHMP Opinion (line
extension and Type II variations) or Notification (Type IB variations). The notified body opinion should
confirm full compliance with the relevant GSPRs and correspond to the intended use of the device part
of an iDDC as claimed in the line extension or variation.
Please read this question in conjunction with questions 2.7 and 2.8.
2.7. How should I submit minor changes to the terms of the Marketing Authorisation for
integral DDC following changes to the device (or device part)? New May 2024
On the basis of the general principles explained in 2.5 and 2.6 which are as follows:

Questions & Answers for applicants, marketing authorisation holders of medicinal
products and notified bodies with respect to the implementation of the Regulations on
medical devices and in vitro diagnostic medical devices (Regulations (EU) 2017/745
and (E
EMA/37991/2019 Rev.4
Page 13/23
• As for any other changes, the MAH should determine whether there is a potential impact on the
delivery, quality, safety and/or efficacy of the iDDC.
• If the MAH determines that the change impacts the registered information, a variation
application according to the EC variation classification guideline will be required.
• If the change does not impact the registered information but the MAH concludes that there is
an impact on the delivery, quality, safety and/or efficacy of the iDDC, a variation application
must also be submitted.
The following guidance is provided without prejudice that assessment of compliance with the GSPRs of
the device remain the remit of the notified body. The MAH of the integral combination has the overall
responsibility to ensure that the device used in the integral combination is supported by an up-to-date
notified body opinion or EU certificate in case of major changes to the device, read also in conjunction
with guidance under Q n° 2.6.
Minor changes to the device (or device part) that do not impact the safety or performance of the
device (part) or the intended use of the device but that still require an update of the registered
information in the MAA should be submitted with the corresponding variation application according to
the variation guideline. The MAH should assess and provide a justification whether the change has no
significant impact on the device to justify the absence of a NB opinion. Otherwise, proof of compliance
with the MDR should be provided.
Some examples of minor changes to the MA are listed below. The list is not exhaustive and is provided
as a guidance only. In the below examples which would result in minor variations, a justification for not
including a new or revised NB opinion should be provided based on the risk assessment performed by
the MAH. An assessment of the proposed change will be performed case by case upon submission. In
cases where the need for a variation and/or the category of the change is unclear, it is recommended
to consult the national competent authority for medicinal products that issued the MA.
• Change in suppliers of a device (part):
Suppliers of the device (part) for iDDC should be stated in section 3.2.P.7 according to the QWP-BWP
Guideline on medicinal products used with a medical device (europa.eu).
A variation under category B.II.e.7 Change in supplier of packaging components or devices should be
submitted to add/delete a supplier of a medical device (part).
In case of addition or replacement of the supplier of the device (or device part), if the risk assessment
performed by the MAH has concluded that the change is non-significant and there are no other
changes to the device beyond the change in suppliers, a statement can be included in the submission
to justify the absence of an NB opinion / EU certificate/ EU declaration of conformity.
If the device manufacturer is also performing sterilisation and the device (part) is supplied as sterile
ready-to-use, the change should be submitted under B.II.b.1 in line with Question 3.26 of the CMDh
Q/A-List for the submission of variations according to Comm.Reg. (EC) 1234/2008 (also applicable to
CAPs).
• Change in dimensions to a device (part):

Questions & Answers for applicants, marketing authorisation holders of medicinal
products and notified bodies with respect to the implementation of the Regulations on
medical devices and in vitro diagnostic medical devices (Regulations (EU) 2017/745
and (E
EMA/37991/2019 Rev.4
Page 14/23
Changes in dimensions to a device (part) not in contact with the medicinal product (i.e. finger grip,
plunger rod) that do not impact the functionality or performance of the device but still require an
update of the registered information can be submitted as a IA notification under B.II.e.6 Change in any
part of the (primary) packaging material not in contact with the finished product formulation (such as
colour of flip-off caps, colour code rings on ampoules, change of needle shield (different plastic used)),
as long as all conditions stated in the EC Variation classification guideline are met.
Changes in dimensions to a device (part) in contact with the medicinal product (i.e. needle, syringe
barrel, plunger…) that require an update of the registered information should be submitted as a IB or
type II variation under B.IV.1 depending on the criticality of the change.
• Change in qualitative and/or quantitative composition of a device (part):
The replacement of a material (change in qualitative and/or quantitative composition) of a device
(part) by an equivalent one for a medical device (part) that is not in contact with the medicinal product
but require an update of the registered information can be submitted as IA notification under B.II.e.6
Change in any part of the (primary) packaging material not in contact with the finished product
formulation (such as colour of flip-off caps, colour code rings on ampoules, change of needle shield
(different plastic used)) as long as all conditions stated in the Variations guideline are met.
The replacement of a material (change in qualitative or quantitative composition) by an equivalent one
for a medical device (part) in contact with the medicinal product should be submitted under B.IV.1
classification.
If the change in composition includes also a change in manufacturer, both should be submitted in a
single variation B.IV.1.z, type IB or type II depending on the criticality.
• Change in the sterilisation method for a device (part):
Changes to the sterilisation method for the device part of an iDDC with no change in sterilisation site
should be submitted under B.II.b.3.
If there is a change in manufacturer, the addition of a new sterilisation site with the corresponding
sterilisation method should be submitted under B.II.b.1.
Of note, in case of several changes to the existing device resulting into the addition or replacement of
a new syringe e.g. change not limited to the supplier of some device parts of the existing syringe but
other changes are introduced as well such as an alternative syringe with changes to the design and
materials of the existing device parts, both the change in composition including the change in
manufacturer, should be submitted in a single variation B.IV.1.z, type IB or type II depending on the
criticality.
2.8. Will I need to provide a new/updated notified body opinion for changes related to the
medicinal product (e.g. extension of indication, new strength, new pharmaceutical form) in
an integral drug-device combination? New May 2024
Changes to the medicinal product may have an impact on the safety or performance of the device or
on its intended purpose.

Questions & Answers for applicants, marketing authorisation holders of medicinal
products and notified bodies with respect to the implementation of the Regulations on
medical devices and in vitro diagnostic medical devices (Regulations (EU) 2017/745
and (E
EMA/37991/2019 Rev.4
Page 15/23
While the change to the medicinal product can be the trigger of the variation or line extension, this
procedure might need to include a new or updated EU declaration of conformity/ EU certificate /notified
body opinion in case of impact to the safety or performance of the device or to its intended purpose.
GSPR compliance entails that "[…] if the devices are intended to administer medicinal products they
shall be designed and manufactured in such a way as to be compatible with the medicinal products
concerned in accordance with the provisions and restrictions governing those medicinal products and
that the performance of both the medicinal products and of the devices is maintained in accordance
with their respective indications and intended use’.
Regarding the need for providing a new / updated notified body opinion for iDDC, please refer to the
guiding principles provided under question 2.6.
In case of an EU certificate or device manufacturer’s EU declaration of conformity, the MAH should
follow the MDR requirements for the maintenance of the EU certificate or EU declaration of conformity.
The following guidance is provided without prejudice that assessment of compliance with the general
safety and performance requirements of the device remain the remit of the notified body.
The following is provided as a guidance only, without prejudice to that the assessment of the proposed
change will be performed case by case upon submission.
Of note, in the case of medicinal products authorised nationally and through MRP/DCP, a line extension
can result in the authorisation of a new marketing authorisation, hence it should be confirmed with the
concerned member states whether this triggers the need for a new notified body opinion per se.
• Extension of indication
In case of extension of indication e.g. to another condition and if there is no change to the device or
user, the MAH should assess and provide a justification whether the new indication has no significant
impact on the safety and performance of the device. In such case a new or updated notified body
opinion might not be needed in support to the variation to the MA.
• New strength
In case of a new strength to an existing iDDC and if there is no change to the device, the MAH should
assess and provide a justification whether the new strength has no significant impact on the safety and
performance of the device. In such case a new or updated NB opinion might not be needed in support
to the regulatory procedure to change the MA.
• New pharmaceutical form
In case the new pharmaceutical form is combined with the introduction of a device, depending whether
the medical device forms an integral part with the medicinal product or is co-packaged, the appropriate
proof of compliance with the MDR will need to be provided.
In case the new pharmaceutical form is combined with a device already authorised for the medicinal
product, the MAH should assess whether the new pharmaceutical form may have a significant impact
on the safety and performance of the device. In such case a new or updated NB opinion is expected to
be provided. In case it is considered not to have significant impact on the safety and performance of
the device, a justification should be provided in support to the regulatory procedure to change the MA.
• New route of administration
In case of a new route of administration, it may impact on the safety or performance of the device. In
such case a new or updated NB opinion is likely to be expected in support to the variation to the MA.

Questions & Answers for applicants, marketing authorisation holders of medicinal
products and notified bodies with respect to the implementation of the Regulations on
medical devices and in vitro diagnostic medical devices (Regulations (EU) 2017/745
and (E
EMA/37991/2019 Rev.4
Page 16/23
2.9. Is it possible to submit a notified body certificate issued under the Directives
(90/385/EEC or 93/42/EEC) to comply with Article 117? Rev. May 2024
A medical device with a certificate that was issued in accordance with the Medical Device Directives
90/385/EEC or 93/42/EEC, and which remains valid under the transitional provisions of Article 120(2),
(3a) and (3c) of the MDR, can still be submitted to support requirements of Annex I to Directive
2001/83/EC, point 12 of section 3.2, as amended by Article 117 of the MDR.
Article 120(3b) of the MDR provides for a transition period also for class I devices having a declaration
of conformity that was drawn up prior to 26 May 2021 under the Directive 93/42/EEC and for which
the conformity assessment procedure under the Regulation (EU) 2017/745 requires involvement of a
notified body for the first time. If the conditions set out in Article 120(3c) of the MDR are met, the
declaration of conformity can be accepted until 31 December 2028 to satisfy requirements of Annex I
to Directive 2001/83/EC, point 12 of section 3.2, as amended by Article 117.
2.10. How will the notified body opinion be reflected in the European Public Assessment
Report (EPAR)? June 2021
An EPAR provides public information on a medicinal product, including how it was assessed, and
reflects the scientific conclusions of the EMA / NCA.
The EPAR will summarise information on the medical device part, relevant to the use with/of the
medicinal product, whether a declaration of conformity or, where applicable, an EU certificate or a
notified body opinion was submitted as part of the marketing authorisation application for the
medicinal product. The applicant/MAH will be requested to identify information in the EPAR that is
considered to be commercially confidential and to make a proposal including justifications for
deletions/alternative wording. The notified body opinion itself will not be published separately.
2.11. What is the impact of the MDR and Article 117 on marketing authorisation applications
of an iDDC on a Mutual Recognition Procedure submitted on or after the 26 May 2021? Rev.
May 2024
As the Mutual Recognition Procedure (MRP)/Repeat Use Procedure (RUP) is a new application for a
marketing authorisation in the concerned Member States, the dossier must comply with the regulatory
requirements applicable at the time of the MRP/RUP application. If the requirements have changed since
the original National, Decentralised or Mutual Recognition procedure, then the dossier will need to be
updated. However, if an application for a iDDC is submitted in the MRP/RUP on or after 26 May 2021
then the General Safety and Performance Requirements (GSPR) of the MDR and Annex I to Directive
2001/83/EC, point 12 of section 3.2, as amended by Article 117 of the MDR apply and the applicable
supporting documentation such as the declaration of conformity, certificate of conformity or notified body
opinion must be included in the dossier only in case of a significant change to the design or intended
purpose of the device (part), or in case of a new device.
In the absence of device related major changes of the integral DDC authorised in the RMS since the
entry into application of the MDR, the previously assessed documentation according to MDD for legacy
devices can be accepted.

Questions & Answers for applicants, marketing authorisation holders of medicinal
products and notified bodies with respect to the implementation of the Regulations on
medical devices and in vitro diagnostic medical devices (Regulations (EU) 2017/745
and (E
EMA/37991/2019 Rev.4
Page 17/23
In case of device changes triggering the need to provide a NB opinion or EU certificate or DoC, prior to
the commencement of the MRP/RUP, a variation application is required to add a declaration of conformity,
certificate of conformity or notified body opinion, to formally update the original dossier in the reference
Member State and, where applicable, existing concerned Member States.
Prior to the start of the MRP/RUP, the applicant should discuss their plans to provide the required
documentation and, as applicable to submit a variation for the existing MAs.
2.12. Do the requirements of MDR Article 117 also apply to an application for medicinal
products to be used outside of the European Union (Article 58 or EU-M4all)? Rev. June 2021
EMA's Committee for Medicinal Products for Human Use (CHMP) assesses medicines and vaccines under
Article 58 of Regulation (EC) No 726/2004 to the same rigorous standards as medicines intended for use
in the European Union. Therefore, the requirements of Annex I to Directive 2001/83/EC, point 12 of
section 3.2, as amended by MDR Article 117 also apply by analogy to Article 58 applications (called EU-
M4all).
2.13. Are the requirements for UDI (unique device identifier) applicable to a medicinal
product that incorporates, as an integral part, a medical device? Rev. May 2024
A iDDC falling under the medicinal products legislation does not have to meet MDR obligations related
to UDI. A device part related UDI should therefore not be applied to the package of such a iDDC.
Additional information is provided in the MDCG 2019-2 guidance on application of UDI rules to device-
part of products referred to in Article 1(8), 1(9) and 1(10) of Regulation 2017/745.
In cases where the device part of a iDDC is CE marked, the product labelling for the iDDC should follow
the labelling requirements for medicinal products as outlined in the QRD (working group on Quality
Review of Documents) templates. Where an UDI is already directly marked on the device part, it does
not need to be removed. The UDI should not appear on the labelling or outer package of the medicinal
product.
3. Medicinal products that include a medical device in the
secondary packaging of the marketed medicinal product (co-
packaged)
3.1. How will the MDR affect the co-packaged medical device? Rev. May 2024
Applicants for marketing authorisations of medicinal products where a medical device (e.g. spoons,
measuring cups, inhalers, spacers) is provided within the secondary packaging of the marketed medicinal
product (i.e. co-packaged) and does not form an integral product with the medicinal product will need
to ensure that their co-packaged medical device is CE marked in accordance with the relevant legislation
on medical devices to continue placing the product on the market.
• Certain medical devices can benefit from a transitional period provided for in MDR Article 120(3a) to
(3e), which allows devices with a valid certificate or declaration of conformity issued under the
Directives 93/42/EEC or 90/385/EEC to be placed on the market in accordance with the extended
transitional periods until 31 December 2027 or 31 December 2028, depending on the risk class of
the device and provided the relevant conditions are met. The medical device manufacturer will need
Questions & Answers for applicants, marketing authorisation holders of medicinal
products and notified bodies with respect to the implementation of the Regulations on
medical devices and in vitro diagnostic medical devices (Regulations (EU) 2017/745
and (E
EMA/37991/2019 Rev.4
Page 18/23
to comply with certain requirements of the MDR from 26 May 2021 (see FAQ’s published by the
CAMD MDR/IVDR transition subgroup: FAQs – MDR Transitional provisions; the European
Commission services' Q&A on practical aspects related to the implementation of Regulation (EU)
2023/607 and relevant MDCG guidance available on the Commission webpage).
• Self-CE marked Class I devices must be in compliance with the MDR by 26 May 2021. If your self-
CE marked Class I device is up-classified by the MDR then the transition period provided for in MDR
Article 120(3b) is applicable.
3.2. What requirements for medical device labelling are applicable to medical devices “co-
packaged” with medicinal products? Rev. May 2024
Co-packaged products need to be distinguished from integral drug-device combinations that form a
single integral product governed either by Directive 2001/83/EC or Regulation (EC) No 726/2004 or by
Regulation (EU) No 2017/745 (see also Q2.1).
In the case of co-packaged products, the medical device must be in conformity with the MDR
9
. This
includes requirements regarding the information to be supplied with the device, which are part of the
general safety and performance requirements laid down in Annex I of the MDR.
In accordance with Annex I, Chapter III, 23.1 (b) of Regulation (EU) 2017/745, the information required
on the label of the medical device (e.g. CE marking, identification of the device, identification of the
manufacturer (and, if applicable of the authorised representative), lot/serial number, UDI carrier etc.)
should be provided on the device itself or on its own packaging.
The product information annexes (SmPC, labelling and package leaflet) of the medicinal product which
is co-packaged with a medical device, should follow the requirements of Directive 2001/83/EC (see QRD
(Quality Review of Documents) templates) and should not include any administrative information such
as device manufacturer/ authorised representative, CE mark (incl. NB number), device symbols, UDI or
references to device vigilance reporting that is provided on or with the medical device.
In accordance with Annex I, Chapter III, Section 23.1 (d) of Regulation (EU) 2017/745, instructions for
use (IFU) for class I and class IIa are not required if such devices can be used safely without any such
instructions and unless IFU are required by another provision in Section 23. If IFU are required, they
should be provided together with the device.
Relevant information for the use of the co-packaged device, especially if necessary for the intended use
of the medicinal product with the device should be included in the appropriate sections of the medicinal
product package leaflet and SmPC, as applicable (for details please refer to the SmPC guideline & the
QRD (Quality Review of Documents) template).
The MAH may itself be the manufacturer of the co-packaged medical device or assume the responsibility
of the device manufacturer in accordance with Article 16(1)(a) of the MDR. In this case, the MAH is
responsible for compliance with the MDR during the lifecycle of the device and only the contact details
of the MAH need to be provided.
Where the MAH is not the manufacturer of the co-packaged medical device, the medical device
manufacturer remains responsible for compliance with the MDR during the lifecycle of the device and
needs to be identified on the device label and/or IFU.
9
In accordance with Article 120(3) to (3e) of the MDR, certain devices that are covered by a valid declaration of
conformity or certificate issued in accordance with Directive 90/385/EEC or Directive 93/42/EEC may be placed on the
market during a transitional period ending on 31 December 2027 or 31 December 2028, depending on the device’s risk.

Questions & Answers for applicants, marketing authorisation holders of medicinal
products and notified bodies with respect to the implementation of the Regulations on
medical devices and in vitro diagnostic medical devices (Regulations (EU) 2017/745
and (E
EMA/37991/2019 Rev.4
Page 19/23
3.2.1 If co-packaged medical devices class I and class IIa, are supplied without an individual
packaging and it is not technically feasible to implement the labelling requirements on the
device itself, what alternative solutions could be considered to display the labelling
requirements? New May 2024
In accordance with Annex I, Chapter III, 23.1 (b) of Regulation (EU) 2017/745, the information required
on the label of the medical device (e.g. identification of the manufacturer, lot/serial number, etc.) should
be provided on the device itself or on its own packaging. However, it is understood that co-packaged
medical devices, in particular class I and class IIa (such as dosing devices like measuring spoons,
measuring cups, or measuring syringes…), can be provided in bulk by the device manufacturer without
an individual packaging and will not contain their own packaging or IFU, pursuant to Annex I, section
23.1(d). These devices are by nature small and direct marking of any information on the device itself
can be challenging or technically not feasible.
Considering that the product information annexes (which includes the SmPC, labelling and package
leaflet) of the medicinal product must not include the required labelling information of the medical device
(see Q&A 3.2), the aim of the below proposed solutions is to provide for an acceptable way to include
the latter information.
A. Provide a separate, additional, leaflet within the packaging of the medicinal product, to provide
medical device administrative information as per the MDR. This option will result in two separate
leaflets included in the secondary packaging, i.e. the package leaflet (PL) of the medicinal product,
as well as the leaflet containing the medical device administrative information. It is recommended to
include a cross-reference to the other leaflet to avoid that one of the two leaflets is overlooked.
The above option is not preferred where several devices are co-packaged together with the medicinal
product. Having several leaflets for different devices in the same package could be confusing for the
end-user.
B. Attach the leaflet containing the medical device administrative information to the package leaflet of
the medicinal product and place it within the secondary package of the medicinal product as one
single folded component. To implement this option manufacturers shall consider the following:
• the leaflet containing the medical device administrative information should be clearly
differentiated from the package leaflet of the medicinal product. This can be achieved by adding
the leaflet containing the medical device administrative information as a tear-off section at the
end of the printed package leaflet.
• the product information annexes (SmPC, labelling and package leaflet) of the medicinal product,
which is co-packaged with a medical device, should follow the requirements of Directive
2001/83/EC and should not include any administrative information of the device as laid down by
MDR.
• the purpose of the section containing administrative information of the device should be clearly
indicated by using a relevant subheading, e.g. entitled ”<Device name > specific information”.
C. Affix a fold out vignette/sticker containing device-specific information directly onto the device itself,
or on the packaging of each device, when available.
The following points should be considered when including a fold out vignette/sticker:

Questions & Answers for applicants, marketing authorisation holders of medicinal
products and notified bodies with respect to the implementation of the Regulations on
medical devices and in vitro diagnostic medical devices (Regulations (EU) 2017/745
and (E
EMA/37991/2019 Rev.4
Page 20/23
• The information on the fold out vignette should be indelible, easily legible, and clearly
comprehensible to the intended user or patient; (Article 10(11) MDR);
• The risk of possible loss of information (the sticker can become loose) should be addressed;
• The adhesive should be functional throughout the life cycle of the product (e.g. during shipping,
storage in a refrigerator or in a freezer).
3.3. Do I need to submit a declaration of conformity / EU certificate as part of the dossier for
a co-packaged medical device? Rev. May 2024
It is the responsibility of the applicant/MAH to ensure that the medical devices co-packaged with the
medicinal product meet the applicable general safety and performance requirements set out in MDR
Annex I and are in compliance with the entirety of the MDR before the combined product is placed on
the market. For marketing authorisations of medicinal products co-packaged with a medical device
submitted after 26 May 2021, evidence for the device should be provided that relevant requirements
have been met e.g. EU Declaration of Conformity or, where applicable, EU certificate, or other
appropriate documentation such as summary information confirming compliance with relevant GSPRs.
For marketing authorisations of medicinal products co-packaged with a medical device submitted or
approved prior to 26 May 2021, and where the dossier contains a certificate of conformity or declaration
of conformity, it is not necessary to submit a variation to the marketing authorisation to replace the
previous evidence of conformity with a new EU certificate or declaration of conformity in compliance with
the MDR.
3.4. What actions, if any, do I need to take if my co-packaged device is up-classified and
requires to be certified by a notified body for the first time? Rev. May 2024
If the medicinal product is co-packaged with a medical device that did not require a notified body
assessment under the Medical Device Directive (MDD) but will now require a notified body assessment
under the MDR, there is a transition phase up until 31 December 2028 where the device may continue
to be placed on the market if it continues to comply with the MDD, provided there are no significant
changes in the design and intended purpose of the medical device and other conditions set out in Article
120(3c) of the MDR are met. However, the requirements of the MDR with respect to post-market
surveillance, market surveillance, vigilance and registration of economic operators of devices will apply.
4. Consultation procedure for ancillary medicinal substances
in medical devices (Art 1(8))
4.1. What type of consultation procedure needs to be carried out for an ancillary medicinal
substance that has already been consulted under the medical device Directive 93/42/EEC?
Rev. May 2024
According to Article 52(9) MDR, as clarified by MDCG Guidance 2020-12 notified bodies are required to
request a consultation with a competent authority for medicinal products as part of the conformity
assessment under the MDR for ancillary medicinal substances already consulted under the medical device
Directives 93/42/EEC or 90/385/EEC.
Questions & Answers for applicants, marketing authorisation holders of medicinal
products and notified bodies with respect to the implementation of the Regulations on
medical devices and in vitro diagnostic medical devices (Regulations (EU) 2017/745
and (E
EMA/37991/2019 Rev.4
Page 21/23
It is possible to take the opportunity of an upcoming variation (i.e. change to the ancillary medicinal
substance triggering the need for a follow-up consultation opinion) to request an opinion in accordance
with the MDR. Please consult the table below to determine whether a full initial consultation or a follow-
up (variation) consultation should be submitted.
This guidance is only applicable to the EMA consultation. For nationally competent authorities for
medicinal products consultation, please refer to their national guidance or liaise with the NCA.
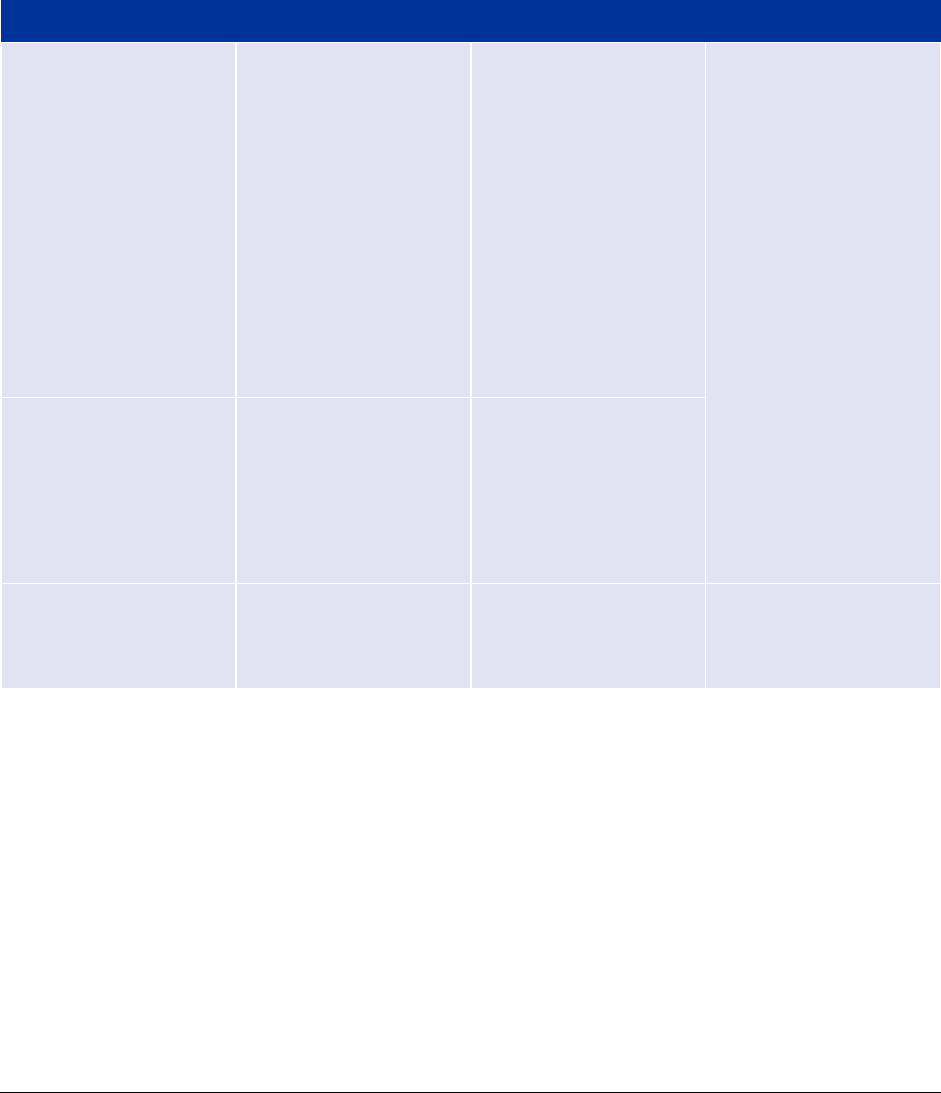
Table 1. EMA consultation for ancillary medicinal substances under MDR where a consultation already
took place under the Directive 93/42/EEC or Directive 90/385/EEC
Procedure type
Timetable
Conditions
Documentation
Type IB variation
First phase 30 days
(with possibility to RSI
and assessment of
responses up to 60
days)
Previous opinion
issued by EMA
Minor variation (as
classified by analogy to
Commission Regulation
(EC) No 1234/2008)
or / and
change to NB’s
assessment of
conformity (this
includes where the NB
is different to MDD
consultation)
• Full package
including
description of the
manufacturing
process and the
data relating to the
usefulness of
incorporation of
the substance into
the device
(according to
section 5.2 Annex
IX of the MDR)
• Declaration from
manufacturer and
NB detailing which
elements are
changed, if
applicable
Type II variation
First phase 60 or 90
days (with possibility
to RSI and assessment
of responses up to 210
days)
Previous opinion
issued by EMA
Major variation (as
classified by analogy to
Commission Regulation
(EC) No 1234/2008)
Full initial
consultation
up to 210 days
A different CA has
issued the previous
opinion
In addition to above:
• NCA opinion from
previous
consultation
5. Consultation procedure for companion diagnostics
5.1. What type of consultation procedure needs to be carried out for a companion diagnostic
New May 2024
A companion diagnostic is an in vitro diagnostic test that supports the safe and effective use of
a specific medicinal product, by identifying patients that are suitable or unsuitable for treatment.
A companion diagnostic is defined in Article 2(7) of Regulation (EU) 2017/746 as follows: ‘companion
diagnostic’ means a device which is essential for the safe and effective use of a corresponding medicinal
product to:
Questions & Answers for applicants, marketing authorisation holders of medicinal
products and notified bodies with respect to the implementation of the Regulations on
medical devices and in vitro diagnostic medical devices (Regulations (EU) 2017/745
and (E
EMA/37991/2019 Rev.4
Page 22/23
(a) identify, before and/or during treatment, patients who are most likely to benefit from the
corresponding medicinal product; or
(b) identify, before and/or during treatment, patients likely to be at increased risk of serious
adverse reactions as a result of treatment with the corresponding medicinal product;
Annex II of MDCG 2020-16 provides a flowchart to determine whether an in vitro diagnostic test fulfils
the definition of a companion diagnostic under Regulation (EU) 2017/746.
Regulation (EU) 2017/746 makes companion diagnostics subject to conformity assessment by a notified
body, as well as to a consultation of a medicinal products authority regarding the suitability of the device
in relation to the medicinal product concerned.
Before it can issue a EU certificate, the notified body must seek a scientific opinion from EMA on
the suitability of the companion diagnostic to the medicinal product concerned if:
• the medicinal product falls exclusively within the scope of the centralised procedure for the
authorisation of medicines, or
• the medicinal product is already authorised through the centralised procedure, or
• a marketing authorisation application for the medicinal product has been submitted through
the centralised procedure.
In other instances, the notified body can seek the opinion either from a national competent authority for
medicinal products or from the EMA.
A procedural guidance is available on the consultation procedure whereby notified bodies seek a
scientific opinion from EMA. This is accompanied by a question-and-answer (Q&A) document on
practical arrangements and a frequently asked questions on medicinal products development and
assessment involving companion diagnostic (CDx).

Questions & Answers for applicants, marketing authorisation holders of medicinal
products and notified bodies with respect to the implementation of the Regulations on
medical devices and in vitro diagnostic medical devices (Regulations (EU) 2017/745
and (E
EMA/37991/2019 Rev.4
Page 23/23
