Manual for
Family Planning
Indemnity Scheme
Family Planning Division
Ministry of Health and Family Welfare
Government of India
March 2016
March 2016
Ministry of Health & Family Welfare
Government of India, Nirman Bhawan, New Delhi-110101
Any part of this document may be reproduced and excerpts from it may be quoted without
permission provided the material is distributed free of cost and the source is acknowledged.
1
st
Edition, October 2013
2
nd
Edition, March 2016
Prologue
Quality of services plays a major role in acceptance of any service.
Poor quality of services leads to unsatised clients resulting in
under-utilization of services. To build the condence of clients it
is necessary to provide them safeguards against adverse events.
Sterilization services are largely provided through public and
accredited Pvt/NGO health facilities. There is a continuing concern
about the number of adverse events following sterilization as well
as litigations faced by the facilities /doctors against such cases.
To mitigate this, the Government of India introduced the National
Family Planning Insurance Scheme which was later modied
as Family Planning Indemnity Scheme “FPIS”, now operational
through State NHM Program Implementation Plan instead of
private sector insurance company.
This updated manual is the second edition of the “Manual for Family
Planning Indemnity Scheme, October 2013” and is in accordance
with the “Standards and Quality Assurance in Sterilization
Services, Nov 2014”. This manual has been revised with a view to
provide guidance to the state, district health authorities and service
providers alike to process the payment of compensation for adverse
events following sterilization and provide indemnity coverage to
the service providers both in public and accredited private/NGO
facilities. In addition to this, it will also be of assistance to program
managers in assessing quality of sterilization services.

Manual for Family Planning Indemnity Scheme
5
CONTENTS
Background 9
Introduction 10
Target Audience 10
Chapter 1: Family Planning Indemnity Scheme-Overview 11
Chapter 2: Scheme Implementation 15
Chapter 3: Operational Procedure for Claim Selement 18
Chapter 4: Monitoring of the Scheme 23
Annexure-1: Claim Form for Family Planning Indemnity Scheme 27
Annexure-2: Application Cum Consent Form for Sterilization Operation 31
Annexure-3: Medical Record & Check List for Female / Male Sterilization 34
Annexure-4: Sterilization Certicate 40
Annexure-5: Post Operative Instruction Card 41
Annexure-6: Proforma for Conducting Audit of Death 43
Annexure-7: Report on Complications/Failures following Sterilization 45
Annexure-8: Checklist of FPIS Claim Documents 49
Annexure-9: Beneciary Wise Claim Status 51
Annexure-10: Quarterly Report Form 52
Annexure-11: Statewise Annual Claims Status 53
Annexure-12: Sterilization Death Audit Report 54

Manual for Family Planning Indemnity Scheme
7
ABBREVIATIONS
CMO Chief Medical Ocer
DBT Direct Benet Transfer
DISC District Indemnity Sub-Commiee
DQAC District Quality Assurance Commiee
EAG Empowered Action Group
FOGSI Federation of Obstetric and Gynaecological Societies of India
FPIS Family Planning Indemnity Scheme
HFS High Focus States
IAP Indian Academy of Pediatrics
IAPSM Indian Association of Preventive and Social Medicine
IMA Indian Medical Association
IUD Intra Uterine Device
MTP Medical Termination Of Pregnancy
NGO Non-Government Organization
NHM National Health Mission
NPCC National Program Coordination Commiee
PIP Program Implementation Plan
RCHO Reproductive & Child Health Ocer
RHFWTC Regional Health & Family Welfare Training Centre
SIHFW State Institute of Health & Family Welfare
SISC State Indemnity Sub-Commiee
SQAC State Quality Assurance Commiee
TFR Total Fertility Rate
USG Ultra Sonography
UT Union Territory

Manual for Family Planning Indemnity Scheme
9
Background
India was the rst country to launch a National Family Planning Programme way back in 1952,
emphasizing fertility regulation for reducing birth rates to the extent necessary to stabilize the
population at a level consistent with the socio-economic development and environment protection.
Since then the demographic and health proles of India have steadily improved.
With a view to encourage people to adopt permanent methods of Family Planning ,the Government has
been implementing a centrally sponsored scheme since 1981 to compensate sterilization beneciaries
for the loss of wages for the period they require for recuperation following sterilization. This
compensation scheme for beneciaries of sterilization services was revised with eect from 31.10.2006
and was further improved upon with eect from 07.09.2007. The scheme has now been enhanced for 11
high focus states namely Uar Pradesh, Bihar, Madhya Pradesh, Rajasthan, Jharkhand, Chhaisgarh,
Uarakhand, Odisha, Assam, Haryana and Gujarat where the TFR continues to be high. The scheme
has been modied in the light of rise in cost of living and transportation costs.
Previously, under the scheme, the Central Government released funds to States/UTs @ Rs.300/- per
Tubectomy, Rs.200/- per Vasectomy and Rs.20/- per IUD Insertion. The States/UTs had the exibility
to decide the amount of apportionment among various components, provided minimum amount of
Rs.150 was paid to the beneciaries of Tubectomy/Vasectomy and Rs.60 per Tubectomy, Rs.25 per
Vasectomy and Rs.20 per IUD insertion was used by the medical facility towards drugs and dressing.
This was intended to ensure quality of service in these procedures. Flexibility rested with the States for
determining sub components of the remaining amount, within the total package. In the case of EAG
States viz. Bihar, Chhaisgarh, Jharkhand, Madhya Pradesh, Orissa, Rajasthan, Uar Pradesh and
Uaranchal, the compensation package for sterilization was further raised from Rs.300/- to Rs.400/-
per Tubectomy, Rs.200/- to Rs.400/-per Vasectomy if conducted in a public health facility or accredited
private health facility and from Rs.20 to Rs.75 per IUD insertion, if conducted in an accredited private
health facility.
Apart from providing cash compensation to the beneciaries of sterilization for loss of wages,
transportation, diet, drugs, dressing etc out of the funds released to States/UTs under this scheme,
States/UTs apportioned some amount for creating a miscellaneous purpose fund. This fund was
utilized for payment of ex-gratia to the beneciary of sterilization or his/her nominee in the unlikely
event of his/her death or incapacitation or for treatment of post-operative complications aributable
to the procedure of sterilization, as under:
i) Rs. 50,000/- per case of death
ii) Rs. 30,000/- per case of incapacitation
iii) Rs. 20,000/- per case of cost of treatment of serious post operation complication
Any liability in excess of the above limit was to be borne by the State/UT/NGO/ Voluntary Organization
concerned from their own resources.
Under the then existing Government Scheme no compensation was payable for failure of sterilization,
and no indemnity cover was provided to Doctors/Health Facilities providing professional services for
conducting sterilization procedures etc. Moreover, no apportioning of the amount disbursed under
the revised compensation scheme (2007) was admissible for creating a miscellaneous purpose fund
for payment of compensation with respect to death/failure/complication aributable to sterilization
operations.
On the other hand, there was a great demand in the states for indemnity insurance cover to doctors/
health facilities, since many empanelled doctors/facilities were facing litigation on account of claims
led by the beneciaries for compensation following death/failure/complication. This led to reluctance
among the doctors/health facilities to conduct sterilization operations and the programme suered.

10
Manual for Family Planning Indemnity Scheme
To address this issue, the Government of India introduced the “National Family Planning Insurance
Scheme” since 25th November, 2005 which has now been modied into “Family Planning Indemnity
Scheme (FPIS)” with eect from 1st April, 2013.
The objective of the FPIS is to indemnify all beneciaries of sterilization, doctors and health facilities
(public and accredited private/NGO) conducting sterilization operation in the unlikely event of death/
failure/complication following sterilization operation.
Introduction
This updated manual on “Family Planning Indemnity Scheme” will serve as a guide for the process
of payment of compensation for death/failure/complication cases aributable to sterilization for
beneciaries and indemnity coverage for service providers both in the public and in the accredited
private/NGO facilities. In addition to this it will also serve as a guide to program managers for assessing
quality of sterilization services.
This updated manual is in accordance with the “Standards and Quality Assurance in Sterilization
Services, Nov 2014” and sets out the guidelines for indemnity coverage to beneciaries as well as
service providers.
Target Audience
This document is meant to be used universally all over the country, by all stakeholders comprising of
policy makers at the national and state levels, programme managers at the national, state, district and
block levels, faculty of medical colleges, trainers at the national and state level, service providers at all
levels as well as by the beneciaries who want to get acquainted with the nuances of the programme
and be aware of their rights and responsibilities.

Manual for Family Planning Indemnity Scheme
11
Chapter 1
Family Planning Indemnity Scheme-Overview
Sterilization is still the most popular family planning method adopted by the clients to limit their family
size. Family planning services are largely being provided through a network of public and private
accredited facilities. However, persistent high unmet need for limiting methods and lack of trained
providers at peripheral level leads to dependence on the camp approach. There has been growing
concern about the quality of sterilization services being oered, particularly at the camp facilities.
The continuing deaths, failures and complications following sterilizations also results in increased
litigation being faced by the providers, which is another barrier in scaling up the sterilization services.
Directives of Hon’ble Supreme Court
The Hon’ble Supreme Court of India in its Order dated 1.3.2005 in Civil Writ Petition No. 209/2003
(Ramakant Rai V/s Union of India) had, inter alia, directed the Union of India and States/UTs for
ensuring enforcement of Union Government’s Guidelines for conducting sterilization procedures and
norms for bringing out uniformity with regard of sterilization procedures by –
1. Introduce a system of having an approved panel of doctors and limiting the persons entitled to
carry on sterilization procedure in the State to those doctors whose names appear on the panel.
The panel may be prepared either State-wise, District-wise or Region-wise.
2. The State Government shall also prepare and circulate a checklist which every doctor will be
required to ll in before carrying out sterilization procedure in respect of each proposed patient.
The checklist must contain items relating to (a) the age of the patient, (b) the health of the patient,
(c) the number of children and (d) any further details that the State Government may require
on the basis of the guidelines circulated by the Union of India. The doctors should be strictly
informed that they should not perform any operation without lling in this check list.
3. The State Government shall also circulate uniform copies of the proforma of consent. Until the
Union Government certies such proforma, for the time being, the proforma as utilized in the
State of U.P. shall be followed by all the States ;and
4. Each States shall set up a Quality Assurance Commiee which should, as being followed by the
State of Goa, consist of the Director of Health Services, the Health Secretary and the Chief Medical
Ocer, for the purpose of not only ensuring that the guidelines are followed in respect of pre-
operative measures (for example, by way of pathological tests, etc), operational facilities (for
example, sucient number of necessary equipment and aseptic conditions) and post-operative
follow ups. It shall be the duty of the Quality Assurance Commiee to collect and publish six
monthly reports of the number of persons sterilized as well as the number of deaths or complications
arising out of the sterilization.
5. Each State shall also maintain overall statistics giving a breakup of the number of the sterilizations
carried out, particulars of the procedure followed(since we are given to understand that there are
dierent methods of sterilization), the age of the patients sterilized, the number of children of
the persons sterilized, the number of deaths of the persons sterilized either during the operation
or thereaer which is relatable to the sterilization , and the number of persons incapacitated by
reason of the sterilization programmes.
6. The State Government shall not only hold an enquiry into every case of breach of the Union of
India guidelines by any doctor or organization but also take punitive action against them. As
far as the doctors are concerned, their names shall, pending enquiry, be removed from the list of
empanelled doctors.
7. The state shall also bring into eect an insurance policy according to the format followed by the
state of Tamil Nadu until such time the Union of India prescribes a standard format.

12
Manual for Family Planning Indemnity Scheme
8. The Union of India shall lay down within a period of four weeks from date uniform standards
to be followed by the State Governments with regard to the health of the proposed patients, the
age, the norms for compensation, the format of the statistics, check list and consent proforma and
insurance.
9. The Union of India shall also lay down the norms of compensation which should be followed
uniformally by all the states. For the time being until the Union Government formulates the norms
of compensation, the States shall follow the practice of the State of Andhra Pradesh and shall pay
Rs 1 lakh in case of death of the patient sterilized, Rs 30,000/- in case of incapacity and in the case of
post- operative complications, the actual cost of treatment being limited to the sum of Rs 20,000/-
The Union Government complied with the orders of the Supreme Court as enumerated below:
1. Creation of panel of doctors/health facilities for conducting sterilization procedures and laying
down criteria for empanelment of doctors for conducting sterilization procedures.
2. Laying down of medical record and checklist to be followed by every doctor before carrying out
sterilization procedures.
3. Laying down of uniform proforma for obtaining ‘consent’ of persons undergoing sterilization.
4. Seing up of Quality Assurance Commiees at State and District level for ensuring enforcement
of pre and postoperative guidelines regarding sterilization procedures.
5. The Union of India brought into eect an Insurance Policy for all States/UTs with eect from
29
th
Nov, 2005 .
Against the backdrop of the directions of the Hon’ble Supreme Court, the “NFPIS” was introduced
from 29th Nov, 2005 so as to do away with the complicated process of payment of ex-gratia to the
beneciaries of sterilization for treatment of post-operative complications, failure of sterilization or
death aributable to the procedure of sterilization. Since then, the scheme has witnessed changes in
the insurers and modications in limits and payment procedures.
Initially the scheme was operated by The Oriental Insurance Company Limited from 29th Nov, 2005
and renewed w.e.f. 29-11-2006 with modication in the limits and payment procedures.
Later, the scheme was operated by ICICI Lombard General Insurance company w.e.f. 01-01-08 up
to 31-03-2013 with yearly renewals. The scheme thereaer has been modied as “Family Planning
Indemnity Scheme” and is operational from 01.04.2013.
Settlement of cases not covered under Family Planning Insurance Scheme (FPIS):
There might be cases not covered by the Family Planning Insurance Scheme, viz. cases of sterilization
operations conducted before coming into force of Insurance Scheme, i.e. prior to 29
th
November,2005
or the cases already pending in courts etc.
Liability in respect of such cases has to be met aer the due clearance from SISC/DISC by the State
Government/UTs Administration from the Miscellaneous Purpose Fund created in respective State/
UTs by apportioning some amount from the grants released to them by the Union Government under
the Scheme of Compensation.
1.1. Family Planning Indeminty Scheme
(Under NHM State Programme Implementation Plans (PIPs) w.e.f. 1st April ,2013)
Under the Family Planning Indemnity Scheme it has been decided that States/UTs would
process and make payment of claims to beneciaries of sterilization in the event of death/
failure/complication and indemnity cover to doctors/health facilities. It is envisaged that
States/UTs would make suitable budget provisions for implementation of the scheme through
their respective Program Implementation Plans (PIPs) in the relevant head under the National
Health Mission (NHM). The scheme is uniformly applicable for all States/UTs.

Manual for Family Planning Indemnity Scheme
13
It will be the responsibility of the SISC/DISC to ensure timely ling and processing of eligible
claims. With eect from 1st April 2013, liability in respect of such cases would be met by the
State Government/UT Administration from funds released by Government of India, under
the National Health Mission (NHM), as per the approval in NPCC of respective State PIPs.
The maximum fund allocated by Government of India to the States /UTs would be on the
basis of average number of sterilization cases in the last three years multiplied by a premium
amount of Rs. 50/- per sterilization case. However, if the State wishes to provide more or
spends more than the allocation, the state may make payment of claims, from their state
budget. States whose claims are less would also be free to allocate lesser funds than their due,
so that resources within the approved envelope for their PIP could be beer utilized for other
activities. In smaller States and UTs where the average number of beneciaries of sterilization
is very low, a minimum amount to the extent of Rs 5 lakhs may be proposed.
The available benets under the Family Planning Indemnity Scheme are as under
Section Coverage Limits
SECTION I (A-D) : For Beneciaries
I A Death following sterilization (inclusive of death during process
of sterilization operation) in hospital or within 7 days from the
date of discharge from the hospital
Rs. 2 lakh
I B Death following sterilization within 8 - 30 days from the date of
discharge from the hospital
Rs. 50,000/-
I C Failure of sterilization Rs 30,000/-
I D Cost of treatment in hospital and up to 60 days arising out of
complication following sterilization operation (inclusive of
complication during process of sterilization operation) from the
date of discharge
Actual not
exceeding
Rs. 25,000/-
SECTION II: Empanelled Doctors under Public and Accredited Private/NGO Sector and
Health Facilities under Public and Accredited Private/NGO Sector
II* Indemnity coverage up to 4 cases of litigations per doctor and
per health facility in a year
Upto Rs. 2 Lakh
per case of
litigation
*Indemnity coverage for service providers/health facilities has been detailed in Chapter 3.
1.2. Salient Features of the Scheme
1. The Family Planning Indemnity Scheme has all India coverage.
2. All persons undergoing/undergone sterilization operations in public health facility or
private/NGO facilities accredited by SQAC/DQAC for sterilization services are covered
under Section- I–A, I-B, I-C and I-D of the scheme.
3. The Consent Form duly lled in by the beneciary at the time of enrolling himself/herself
for sterilization operation duly countersigned at the medical facility shall be a proof of
coverage under the scheme (Annexure 2).
4. The medical records and checklist for female/male sterilization should also be duly lled
in by the Doctors/Health Facilities (Annexure 3).
5. All the doctors/health facilities in public sector and private/NGO facilities empanelled/
accredited with SQAC/DQACs conducting such operations are covered under Section-II
of the scheme. There is a stipulated criterion for empanelment of doctors/accreditations
of health facilities for sterilization which can be referred from “Standard and Quality
Assurance in sterilization services, Nov 2014”
6. All claims arising under Section I and Section II shall be admissible from 1
st
April 2013,
under the scheme.

14
Manual for Family Planning Indemnity Scheme
7. Claims arising out of cases of sterilization operations which were detected and reported
aer 1st April, 2013, will come under the purview of State Programme Implementation Plans
(PIPs). Claims arising out of cases of sterilization operations detected and reported before
1st April, 2013, will not come under the purview of State Programme Implementation
Plans (PIPs). Such claims would be covered and processed as per the respective guidelines
of expired policies from 29th November 2005 to 31st March, 2013 and the convener of DISC
(CMO or Equivalent) designated for this purpose at district level would be responsible for
unpaid/time barred claims above. No provision will be made for unpaid claims in the State
PIPs.
8. The claims will fall within the “Family Planning Indemnity Scheme” only if the beneciary
les the claim with the DISC within 90 days from the occurrence of the event of death/
failure/complication.
9. Every claim, writ and summons related to the event of death/failure/complication should
be forwarded to SISC/DISC by the doctors/health facilities under Section II.

Manual for Family Planning Indemnity Scheme
15
Scheme Implementation
Chapter 2
2.1. Quality Assurance Committee
Quality Assurance Commiees (QACs) have been formed at the State and Districts level
to ensure that the Standards for female and male sterilization as laid down by the GoI are
followed in respect of pre-operative measures, operational facilities etc. The composition of the
Commiee would be as follows:
2.1.1 AT STATE LEVEL: State Quality Assurance Committee (SQAC)
2.1.1.1 Composition:
1. Secretary, Medical and Health (Chairperson)
2. Mission Director –NRHM (Vice Chairperson)
3. Director Family Welfare/Director Health Services/Director Public Health
Equivalent (Convener): to be nominated by the Chairperson.
4. Additional/Joint Director (FW)/Deputy Director (FW)/Equivalent,
designated by the state government as the nodal ocer for the Quality
Assurance (QA) Cell (Member Secretary)
5. Director, Medical Education
6. Director/Principal of state training institution e.g. SIHFW/CTI/RHFWTC
7. One Empanelled Gynaecologist (from public institutions)
8. One Empanelled Surgeon (from public institutions)
9. One Anaesthetist (from public institutions)
10. One Paediatrician (from public institutions)
11. State Nursing Adviser/ Equivalent
12. One member from an accredited private sector hospital/ NGO (health care
sector)
13. One representative from the legal cell
14. One representative from medical professional bodies e.g. FOGSI/ IMA/
IAP/IAPSM/ Association of Public Health
15. Any other member or representatives of public health organisations of
eminence as nominated by the state government
Note: The Quality Assurance Commiee as laid down in the ‘Standards
& Quality Assurance In Sterilization Services’, Nov 2014 shall stand
subsumed within the QAC mentioned above.
However a 5 member “State Indemnity Sub-Commiee (SISC)” from within
the SQAC would redress, dispose and disburse claims/complaints received
through the DQAC, to the district health society as per procedure and time
frame laid down in this manual.

16
Manual for Family Planning Indemnity Scheme
The subcommiee would comprise of the following:
1. Mission Director –NRHM (Chairperson)
2. Director Family Welfare/Director Health Services/Director Public Health Equivalent
(Convener)
3. Additional/Joint Director (FW)/Deputy Director (FW)/Equivalent
(Member Secretary)
4. Empanelled Gynaecologist (from public institutions)
5. Empanelled Surgeon (from public institutions)
2.1.1.2 Terms of Reference of the Committee
y Visit both public and private facilities providing family planning services
in the state to ensure implementation of national standards.
y Review and report deaths/complications following Sterilization in the
state.
y Review and report conception due to failure of sterilization in the state
y Give directions on implementation of measures to improve quality of
sterilization services.
y Review the implementation of the National Family Planning Indemnity
Scheme / payment of compensation in the state.
y Share review report with all district commiees and other stakeholders.
y Send the regular reports on sterilization related indicators (Death,
Failure,Complication) to the Centre aer ratication of the same by the
Chairperson of the SQAC.
y The “State Indemnity Sub-Commiee(SISC)” would meet every six
months or sooner if warranted.
y At least three members would constitute the quorum of this sub-
commiee.
2.1.2 AT DISTRICT LEVEL: District Quality Assurance Committee (DQAC)
2.1.2.1 Composition
1. District Collector, Chairperson
2. Chief Medical Ocer/District Health Ocer (Convener)
3. District Family Welfare Ocer/RCHO/ACMO/equivalent (Member
Secretary)
4. Nodal Ocers of Programme Divisions at districts
5. One empanelled gynaecologist (from public institutions)
6. One empanelled surgeon(from public institutions)
7. One anaesthetist (from public institutions)
8. One paediatrician (from public institutions)
9. One representative from the nursing cadre
10. One representative from the legal cell
11. One member from an accredited private sector hospital/ NGO (health care
sector)
12. One representative from medical professional bodies e.g. FOGSI/IMA/
IAP/IAPSM/ Association of Public Health

Manual for Family Planning Indemnity Scheme
17
However a 5 member “District Indemnity Sub-Commiee (DISC)” from
within the DQAC would review and process claims received from the
clients and complaints/ claims lodged against the surgeons and accredited
facilities, as per procedures and time frame laid down in this manual.
The subcommiee would comprise of the following:
1. District Collector, (Chairperson)
2. Chief Medical Ocer/District Health Ocer (convener)
3. District Family Welfare Ocer/RCHO/ ACMO/ equivalent (member
secretary)
4. Empanelled gynaecologist (from public institutions)
5. Empanelled surgeon (from public institutions)
2.1.2.2 Terms of Reference of the committee
y Conducting medical audit of all deaths related to Sterilization and sending
reports to the State QA commiee oce.
y Collecting information on all hospitalization cases related to complications
following sterilization, as well as sterilization failure.
y Reviewing all static institutions i.e. Government and accredited Private/
NGOs and selected camps providing sterilization services for quality of
care as per the standards and recommend remedial actions for institutions
not adhering with standards.
y Review, report and process compensation claims for onward submission
to the SISC under the National Family Planning Indemnity Scheme for
cases of deaths, failure and complication following male and female
sterilization procedures.
y In case facility reports sterilization related death, the convenor of the DISC
should inform the convenor of the SISC within 24 hours.
y Death audit needs to be undertaken mandatorily for each case of death by
the DISC.
y The “District Indemnity Sub-Commiee (DISC)” would meet every
three months or sooner if warranted.
y At least three members would constitute the quorum of this sub-
commiee.

18
Manual for Family Planning Indemnity Scheme
Operational Procedure for Claim Settlement
Chapter 3
3.1. Claims Procedure for Section I (Death/Failure/Complication)
1. In the event of death/failure/complication following sterilization, the beneciary shall
immediately ll up claim form (Annexure 1).
If such covered cause is detected “during examination of the beneciary in health facility”,
the health facility shall ensure to get the claim form lled from the beneciary on the
spot without loss of time. The health facility shall forward the claim papers along with
necessary documents to the DISC.
2. On receiving the claim papers, proper acknowledgement must be made by the DISC, for
further processing and payment of the claims. Based on the submied documents, claims
shall be processed by the DISC under dierent sections of the scheme.
3. The claims processing under Section-I (death, failure and complication) following
sterilization operation will be processed by the DISC and forwarded to SISC. The SISC can
scrutinize the documents and ask for any new and relevant piece of information missing
from the recommendation of the DISC. However, if the district is reporting suspiciously
large number of claims for failure/complication, the SISC has the right to verify the claims
and recommend the release of funds to the district accordingly.
4. The SISC should review and examine every single case of death before endorsing.
5. Verication and medical evaluation of the claim lodged by the beneciary would be done
by the DISC and for all purpose the authority shall be with the convenor of DISC (CMO or
Equivalent) designated for this purpose at district level.
6. The Claims processing together with all the required documents should preferably be
completed within 30 days of ling of claims by the beneciary.
7. Stipulated time limit for selement of claims under Section-I of the scheme would
be 15 working days in case of death and 21 days in case of others, aer completion of
documentation.
3.1.1. Death Following Sterilization (Section I-A & B)
Claims under Section-I-A (death following Sterilization (inclusive of death during
process of sterilization operation) in hospital or within 7 days from the date of discharge
from the hospital) and under Section I-B (death following sterilization within 8-30
days from the date of discharge from the hospital) shall be paid equally in favor of the
spouse and unmarried dependent children whose names are entered in the relevant
rows of Consent Form. In case there is no surviving spouse, the payment shall be made
to the unmarried dependent children only.
If the death occurs within 7 days of discharge or during the process of sterilization
operation the amount of Rs 50,000/- (in cash only if there is no accounts/Jan Dhan
accounts) should be released immediately from the State or District Health Society
funds. The balance amount of Rs 1.5 lakhs will be released through account payee
cheque/DBT(wherever account number /Jan Dhan account is available) later only
when the DISC recommends compensation under “Death aributable to Sterilization”
to the SISC and the SISC endorses (Please refer D.O No. Y.11013/1/2016-FP dated 29
th
January, 2016 for further clarication).
If the death occurs beyond 7 days of discharge to one month, the DISC should examine
the case and establish the cause. If death is aributed to sterilization and subsequently

Manual for Family Planning Indemnity Scheme
19
endorsed by SISC, Rs 50,000/- (through account payee cheque/DBT, wherever account
number /Jan Dhan account is available) should be paid to the kin of the deceased whose
names are entered in the relevant rows of the Consent Form.
If the dependent children are minor, the payment shall be made by the District Health
Society in the name of minor children. The cheques, in this case would be issued by the
District Health Society in the name of minor beneciary with the following endorsement
(overleaf);
“Amount to be deposited as FDR in the name of minor Sh/Ku ................. till the minor
aains the maturity. No premature payment of FDR is allowed. Quarterly interest
may be paid to the guardian”.
In case, there are no surviving spouse/unmarried dependent children, the claim shall
then be payable to the legal heir of the deceased beneciary subject to production of
legal heir certicate.
In case of ling claims for death under Section-I (IA & IB) following sterilization
operation (inclusive of death during process of sterilization operation), following
documents are required to be submied by the kin of the deceased beneciary:
a) Claim Form Cum Medical Certicate in original,
b) Copy of death certicate issued by hospital/ municipality or any other authority
c) Copy of Post-Operative Instruction Card/Discharge Certicate
Documents required for processing of claims for death following sterilization under
(Section I-A & B)
a) Claim Form cum Medical Certicate in original duly signed and stamped by the
convener of DISC (CMO or Equivalent ) designated for this purpose at district level
(Annexure 1).
b) Copy of Consent Form & Medical Record and Checklist duly aested by the
convener of DISC (CMO or Equivalent )designated for this purpose at district level
(Annexure 2 & Annexure 3).
c) Copy of Post-Operative Instruction Card /Discharge Certicate duly aested by the
convener of DISC (CMO or Equivalent) designated for this purpose at district level
(Annexure 5).
d) Copy of Death certicate issued by Hospital/Municipality or authority designated
duly aested by the convener of DISC (CMO or equivalent) designated for this
purpose at district level.
e) Proforma for Conducting Audit of Death by DISC (Annexure 6).
3.1.2. Failure of Sterilization (Section I-C)
In case of ling claims for failure of sterilization under Section I-C, following
documents are required to be submied by claimant.
a) Claim Form cum Medical Certicate in original
b) Copy of Sterilization Certicate
c) Copy of any Diagnostic Report conrming Failure of Sterilization

20
Manual for Family Planning Indemnity Scheme
Documents required for processing of claims for failure of sterilization under
(Section I-C)
a) Claim Form cum Medical Certicate in original duly signed and stamped by the
convener of DISC (CMO or Equivalent) designated for this purpose at district level
(Annexure 1).
b) Copy of Consent Form & Medical Record & Checklist duly aested by the convener
of DISC (CMO or Equivalent) designated for this purpose at district level (Annexure
2 & 3).
c) Copy of Sterilization Certicate duly aested by the convener of DISC (CMO or
Equivalent) designated for this purpose at district level (Annexure 4).
d) Copy of any of the Diagnostic Reports (as given in Section 3.1.2.1, 3.1.2.2) conrming
failure of sterilization duly aested by the convener of DISC (CMO or Equivalent)
designated for this purpose at district level.
e) Report on complication/failure following sterilization by District Quality Assurance
Commiee (Annexure 7).
3.1.2.1 In Case of Failure of Tubectomy following Documents can be Submitted
1. Urine test report supported by Physical Examination report/ANC Card/
USG report
2. Physical examination report
3. USG report
4. Certicate of MTP /MTP report
3.1.2.2 In Case of Failure of Vasectomy
1. Semen Examination Report
NOTE: Any one of the diagnostic reports detecting failure of sterilization would be
sucient for processing the claim under this section.
3.1.3. Complications Arising Following Sterilization (Section I-D)
For claims arising due to complications following sterilization operation (inclusive of
complication during process of sterilization operation) as per Section I-D, following
documents are required to be submied by claimant.
a) Claim Form cum Medical Certicate in original
b) Copy of Post-Operative Instruction Card/Discharge Certicate
c) Original Bills/Receipts/Cash Memos along with Original Prescription and Case
Sheet conrming treatment taken for complication due to sterilization.
Documents required for processing claims of complications arising due to
Sterilization under (Section I-D)
a) Claim Form cum Medical Certicate in original duly signed and stamped by the
convener of DISC ( CMO or Equivalent )designated for this purpose at district level
(Annexure 1).
b) Copy of Consent Form & Medical Record & Checklist duly aested by the convener
of DISC (CMO or equivalent ) designated for this purpose at district level (Annexure
2 & 3).
Manual for Family Planning Indemnity Scheme
21
c) Copy of Post-Operative Instruction Card /Discharge Certicate duly aested by the
convener of DISC (CMO or Equivalent) designated for this purpose at district level
(Annexure 5).
d) Original Bills/Receipts/Cash Memos along with Original Prescription and Case
Sheet conrming treatment taken for complication following sterilization. Convener
of DISC (CMO or Equivalent) designated for this purpose at district level shall
certify the cost of treatment of such complications incurred by the beneciary and
or hospital.
e) Report on complication/failure following sterilization by District Quality Assurance
Commiee (Annexure 7).
NOTE: No additional document should be solicited by the designated district level
ocer
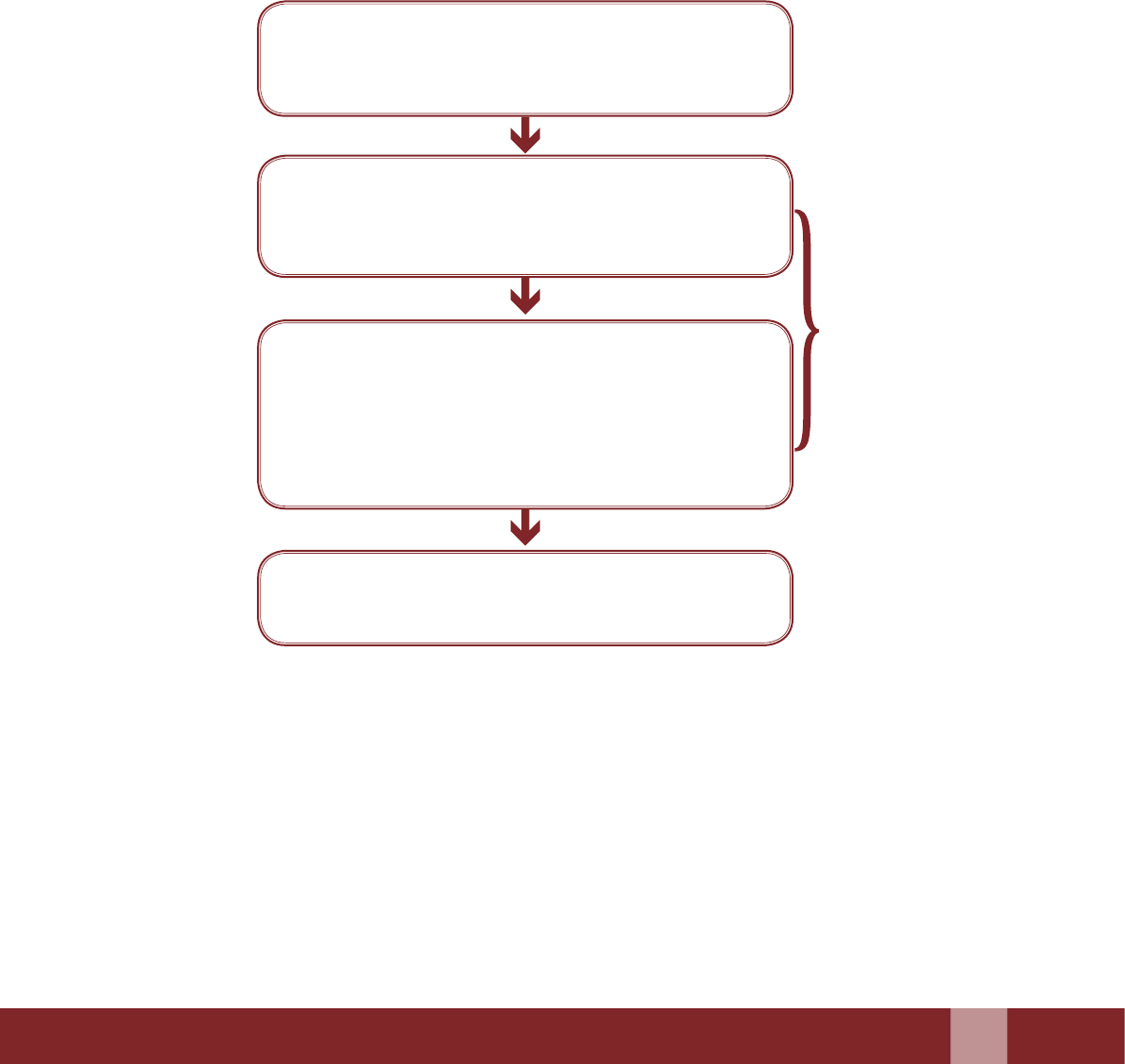
Flowchart Illustrating the Steps of the Claims Process
Beneciary to le the claims document (within 90
days from the occurrence of event of Death/Failure/
Complication).
Stipulated time limit
for selement of
claims would be 15
working days in case
of death and 21 days
in case of others,
aer completion of
processing.
DISC to process (Signing, Stamping and
Authenticating the documents) the claims arising
out of death, complication and failure aributable to
sterilization and put up to SISC.
SISC to scrutinize the documents and if required, call
for any new and relevant material missing from the
recommendation of DISC. SISC to endorse the cases
and release funds to the district, wherever applicable.
Mandatory : SISC TO REVIEW EVERY SINGLE
CASE OF DEATH BEFORE ENDORSING
The Claims processing together with all the required
documents should preferably be completed within 30
days from the date of receipt of claim.
3.2 Claims Procedure for Section II ( Indemnity Coverage to Doctors/Facilities)
1. For claims under Section II of the scheme, it will be the responsibility of the doctor/health
facility on receiving any Legal Notice/ Summons from the Court to immediately inform,
in writing, to SISC/DISC, who would thereaer, take over entire defense process of the
case, including engagement of advocate and payment of legal expenses which would be
paid later by State Health Society/ District Health Society. However, State Health Society/
District Health Society shall not be liable to pay more than the amount mentioned in the
Section - II in any case, under all heads.

22
Manual for Family Planning Indemnity Scheme
2. In emergent situation the defence costs incurred by the doctor/health facility shall be
reimbursable, if incurred in consultation with the SISC/DISC; the defence costs shall be
limited to Rs. 5,000 per incidence for such cases under section II.
3. Liability of the State Health Society under Section -II would be limited to four cases of
litigation in respect to every doctor and health facility in a year. Empanelled Doctors*
under Public and accredited Private/NGO Sector and Health Facilities** under Public and
Private/NGO Sector accredited with SQAC/DQACs for rendering sterilization services shall
stand indemnied against the litigation cases arising out of death, failure or complication
resulting therefrom, up to a maximum amount of Rs. 2 lakh per doctor and per facility upto
4 cases of litigation per year.
*For Empanelled Public/Private Providers: A maximum of 4 cases of litigation
y Irrespective of the number of sterilization cases he/she performs.
y Irrespective of the number of health facilities where he/she conduct surgeries.
**For Public and Accredited Private/NGO Facilities: A maximum of 4 cases of litigation
y Irrespective of the number of sterilization cases performed in that facility
y Irrespective of the number of empanelled providers conducting surgeries at that
facility.
3.2.1 Documents Required Under Indemnity Cover (Section II)
a) Intimation in writing
b) Copy of summons/FIR
c) Copy of Sterilization Certicate (Annexure 4)
d) Copy of Consent Form (Annexure 2)
e) Certicate from the convener of DISC (CMO or Equivalent) designated for this
purpose at district level conrming that the sterilization operation was conducted
by the doctor etc.
f) Copy of the award given by the court along with the original receipts for which
payment is made to the lawyer
In case any claim is found untenable, the reason for rejection of the claim will be
communicated to the beneciary/kin by the convener of DISC (CMO or Equivalent) of
the district with a copy to the State Nodal Ocer.
District Health Society shall not be liable under this scheme for compensation
under more than one Section in respect of the same eventuality except under section
(I-C & D).

Manual for Family Planning Indemnity Scheme
23
Monitoring of the Scheme
Chapter 4
4.1 Mechanism of Monitoring of the Scheme
4.1.1 At State/District Level-
y District Quality Assurance Commiee (DQAC) shall review quarterly the status
of accredited facilities, empaneled providers, claim status, period of pendency of
claims and advice the district ocials to respond/comply with the deciencies, if
any. In case the numbers of pending claims are high, the commiees can meet sooner
if warranted. Moreover, SISC may intervene to fast track the claim disbursement
process.
y In case of death aributable to sterilization DISC should audit every single case as
per procedure laid in Quality Assurance Guidelines issued by Ministry of Health
and Family Welfare, GOI in compliance with the Hon’ble Supreme Court directions
(Annexure 6).
y The claims aer due diligence by the DISC should be put up to the SISC who would
be the nal arbiter for the same.
y SQAC/DQAC shall ensure that each district and health facility is provided with
FPIS Manual and mandatory documents required for claims.
y SISC/DISC shall ensure that District Ocials are ling the FPIS Claims well within
the stipulated period as per the scheme.
y Convener of SISC (Director Family Welfare or Equivalent) designated for this
purpose at state level shall review all pending maers including pending claims on
monthly basis.
y State shall organize review meetings on biannual basis to review all pending maers
including pending claims under the chairmanship of Mission Director (NHM) with
the designated district machinery.
y State shall monitor the low/high reporting trend of FPIS claims from the districts;
review the performance of the empanelled providers and issues necessary guidelines
for corrective measures.
y The MOHFW, GOI shall conduct annual review on all maers relating to FPIS.
4.1.2 Reporting Mechanism
y State will collate the district wise beneciary claim status as per the prescribed
format and will furnish the details to the Government of India on quarterly basis
(Annexure 9).
y District will submit Quarterly Report to the State showing claims pertaining to
death, complication, failure of sterilization, including claims under Section II
(Annexure 10).
y Copy of all death audit reports will be analysed by the state in a periodic manner
and the same will be shared quarterly with FP Division at Central Level (Annexure
6 & Annexure 12).
y States/UTs will share the annual claim status pertaining to death, complication,
failure of sterilization and the amount paid as compensation under each category
(Annexure 11).

24
Manual for Family Planning Indemnity Scheme

ANNEXURES
Manual for Family Planning Indemnity Scheme
27
Claim Form for Family Planning Indemnity
Scheme
Annexure-1
1. This form “Claim Form cum Medical Certicate” is required to be completed for lodging claim
under Section-I of the scheme.
2. This form is issued without admission of liability and must be completed and returned to the
District Health Society/State Health Society for processing of claim.
3. No claim can be admied unless certied by the convener of DISC (CMO or Equivalent )
designated for this purpose at district level by the State Government.
Claim no. : _________________
PART A: Beneciary/Claimant Information (To be Submied by Claimant)
1. Details of the Claimant:
Name in full: ___________________________________________________ Present Age: ______ Years
Relationship with the beneciary of Sterilization: __________________________________________
Residential Address: ___________________________________________________________________
_________________________________________ Telephone no. ________________________________
2. Details of the person undergone sterilization operation:
Name in Full: ______________________________________________ Age: __________________ Years
Son /daughter of: ______________________________________________________________________
Name of the Spouse: _______________________________________ Age of the Spouse: _______Years
Address: ______________________________________________________________________________
3. Occupation/Business: __________________________________________________________________
4. Details of Dependent children:
S. No. Name Age
(Yrs)
Sex
(M/F)
Whether
Unmarried
If unmarried,
Whether
dependent
1
2
3
5. (a) Date of Sterilization Operation:______________________________________________________
(b) Nature of Sterilization operation:
(i) Interval Tubectomy: ____________________________________________________________
(ii) Vasectomy: ____________________________________________________________________
(iii) MTP followed by sterilization: ___________________________________________________

28
Manual for Family Planning Indemnity Scheme
(iv) Post Partum Sterilization (Caesarean/ Normal Delivery): ______________________________
(v) Any other surgery followed by sterilization: _______________________________________
6. (a) Name and address of the doctor who conducted the operation:
__________________________________________________________________________________
(b) Name and address of the hospital where operation was conducted:
__________________________________________________________________________________
(c) Nature of claim:
1) Failure of sterilization :
2) Complication due to Sterilization (state exact nature of complication):
a. Date: _______________
b. Details of Complication:__________________________
c. Doctor /Health facility: ___________________________
3) Death aributable to sterilization:
a. Date of Admission: _______________ Time:___________
b. Date of Discharge :________________ Time:___________
c. Date of Death:____________________ Time:___________
7. Give details of any disease suered by beneciary prior to undergoing sterilization operation:
______________________________________________________________________________________
______________________________________________________________________________________
I HEREBY DECLARE that the particulars are true to the best of my knowledge and warrant the
truth of the foregoing particulars in every respect, and I agree that if I have made, or shall make any
false of untrue statement, suppression or concealment of fact, my right to the compensation shall
be absolutely forfeited.
I hereby claim a sum of Rs._________________________/- under the scheme, which I agree in full
selement of my claim and shall have no further right whatsoever to claim under the scheme.
Date: _______________ Name of Client/Claimant: _____________________________________
Place: ______________ Signature (in full) or thumb impression

Manual for Family Planning Indemnity Scheme
29
PART B: MEDICAL CERTIFICATE
(To be issued by CMO or Equivalent designated for this purpose at district level)
It is certied that Smt/Shri. _________________________________________________________________
S/o/W/o: _________________________________________________________________________________
R/o______________________________________________________________________________________
had undergone ________________________________(Specify which procedure was done) sterilization
operation on_____________ at _______________________________________ (hospital) and conducted
by Dr.__________________________________Qualications _______________________empanelled for
____________________procedure posted at ___________________________________________________
____________________________________________________
Nature of Sterilization operation done:
(i) Interval Tubectomy: ___________________________________________________________________
(ii) Vasectomy: __________________________________________________________________________
(iii) MTP followed by Sterilization: __________________________________________________________
(iv) Post Partum Sterilization (Caesarean/ Normal Delivery): ___________________________________
(v) Any other surgery followed by Sterilization: ______________________________________________
I have examined all the medical records and documents and hereby conclude that the sterilization
operation is the antecedent cause of:
(a) Failure of Sterilization (Aach documentary evidence)
(b) Complication: (please give the details as under)
(i) Nature of Complication:______________________________ ____________
(ii) Period: ________________________________________________________
(iii) Expenses incurred for treatment of complication Rs. ___________ (Aach Original Bills/
Receipts/Prescriptions)
(c) Death of Person (cause): ___________________________________________________
a. Date of Admission: ______ Time: _____
b. Date of Discharge:______ Time: ______
c. Date of Death: ______________ Time: __________ (Aach death certicate)
I have further examined all the particulars stated in the claim form and are in conformity with my
ndings and is eligible for a compensation of Rs…………………… due to……………………… (Cause).

30
Manual for Family Planning Indemnity Scheme
Please pay Rs_________________________ to the beneciary.
Documents Enclosed:
(a) Original Claim cum Medical certicate ( )
(b) Aested copy of sterilization certicate ( If applicable) ( )
(c) Aested copy of consent form ( )
(d) _________________________________ ( )
(e) _________________________________ ( )
Date:______________________________ Seal:
Name____________________________ Designation___________________
Tel/Mob. No. _____________________ Signature _____________________

Manual for Family Planning Indemnity Scheme
31
Application Cum Consent Form for
Sterilization Operation
Annexure-2
An informed consent is to be taken from all clients of sterilization before the performance of the surgery
as per the consent form placed below
Name of Health Facility………….……………….............................…………………………………………..
Client Hospital Registration Number: …...........................……………………………………………………
Date: ………/……./20…..…
1. Name of the Client: Shri/Smt. ….………………..………........................…………………………………
2. Name of Husband/Wife: Shri/Smt. ………….………….........................………………………………....
3. Address …………………..………………………………........................…………………………………..
4. Contact No: ……………………....................................................................................................………..…
5. Names of all living, unmarried dependent Children
i) ……..………………………........…………… Age……………………………………..............………….
ii)……………………….........………………… Age………………………………...............……………....
iii)……………………….........………………… Age…………………………..............…………………....
iv)………………………………..........………… Age…………………….............…………………………
6. Father’s Name of beneciary: Shri……………………………........................………………..…………..
7. Address: ………………………………………………........................……………………………………...
8. Religion/Nationality: ………………………………..........................……………………….……………...
9. Caste- SC/ST/General………………........................……………………………………………………….
10. Status- APL/BPL
11. Educational Qualications…….............................…………………………………………………………
12. Business/Occupation: …………………………………............................…………….……………………
13. Operating Centre: …………………………………...........................……………………………………….

32
Manual for Family Planning Indemnity Scheme
I, Smt/Shri …………………………………… (client) hereby give consent for my sterilization operation.
I am ever married. My age is ……years and my husband/wife’s age is …… years. I have …(Nos.) male
and …. (Nos.) female living children. The age of my youngest living child is …… years.
a) I have decided to undergo the sterilization / re-sterilization operation on my own without any
outside pressure, inducement or force. I declare that I / my spouse has not been sterilized previously
(not applicable in case of re-sterilization).
b) I am aware that other methods of contraception are available to me. I know that for all practical
purposes this operation is permanent and I also know that there are still some chances of failure of
the operation for which the operating doctor and health facility will not be held responsible by me
or by my relatives or any other person whomsoever
c) I am aware that I am undergoing an operation, which carries an element of risk.
d) The eligibility criteria for the operation have been explained to me and I arm that I am eligible to
undergo the operation according to the criteria.
e) I agree to undergo the operation under any type of anaesthesia, which the doctor/health facility
thinks suitable for me and to be given other medicines as considered appropriate by the doctor/
health facility concerned. I also give consent for any additional life-saving procedure, if required
f) I agree to come for follow-up visits to the Hospital/Institution/Doctor/health facility as instructed,
failing which I shall be responsible for the consequences, if any.
g) If, aer the sterilization operation, I experience a missed menstrual cycle, then I shall report within
two weeks of the missed menstrual cycle to the doctor/health facility and may avail of the facility
to get an MTP done free of cost. I shall be responsible for the consequences, if any.
h) I understand that Vasectomy does not result in immediate sterilization. *I agree to come for
semen examination 3 months aer the operation to conrm the success of sterilization surgery
(Azoospermia) failing which I shall be responsible for the consequences, if any.
*Applicable for male sterilization cases)
i) In case of complications, failure and the unlikely event of death aributable to sterilization, I/
my spouse and dependent unmarried children will accept the compensation as per the existing
provisions of the Government of India “Family Planning Indemnity Scheme” as full and nal
selement and will not be entitled to claim any other compensation including compensation for
upbringing of the child, if any, born on account of failure of sterilization, over and above the
one oered, from any court of law in this regard.
I have read the above information or the above information has been read out and explained to me in
my own language and that this form has the authority of a legal document.
I am aware that I have the option of deciding against the sterilization procedure at any time without
sacricing my rights to other reproductive health services.
Date: …….....………… Signature or Thumb Impression of the Client
Name of client: ….....................………………………….
Signature of Witness (Clients side):
Full Name: ……………..........……………………….……
Full Address………………………………………........….

Manual for Family Planning Indemnity Scheme
33
I am aware that client is ever married and has 1 living child over one year of age
Signature of ASHA/ Counsellor/ Motivator:……………………………………………...........................……
Full Name: …………………………………….……………………………………............................………….
Full Address…………………………………………………………………...........................………………….
I certify that I have satised myself that -
a. Shri/Smt……………………………………is within the eligible age-group and is medically t for the
sterilization operation.
b. I have explained all clauses to the client and that this form has the authority of a legal document.
c. I have lled the Medical record–cum-checklist and followed the standards for sterilization
procedures laid down by the Government of India.
Signature of Operating Doctor Signature of Medical Ocer in-charge of the Facility
(Name of Operating Doctor) (Name of Medical Ocer in-charge of the Facility)
Date: ………..............……… Date: ………………………
Seal Seal
DENIAL OF STERILIzATION
I certify that Shri/Smt………………………………………………………………..is not a suitable client for
sterilization/re-sterilization for the following reasons:
1. ……………………………………………………………..................................……………………………….
2. ………………………………………..................................…………………………………………………….
He/She has been advised the following alternative methods of contraception.
1. ……………………………….................................……………………………………………………………..
2. ………………………………………...............................………………………………………………………
Signature of the Doctor making the decision
Date: ……........……
Name and full Address: …………………........…............................…………………………………………

34
Manual for Family Planning Indemnity Scheme
Medical Record & Check List for Female /
Male Sterilization
Annexure-3
A checklist is to be lled by the doctor before conducting sterilization procedure for ensuring the
eligibility and tness of the client for sterilization.
Name of Health Facility: ………………………...............................................…………………………………
Beneciary Registration Number: ……….....................................................…………………………………..
Date………………….
A. Eligibility Checklist
Client is within eligible age Yes…………….... No……..………….
Client is ever married Yes…………….... No……..………….
Client has at least one child over one year of age Yes…………….... No……..………….
Lab investigations (Hb, urine) undertaken are within
normal limits (7.0 gms or more)
Yes…………….... No……..………….
Medical status as per clinical observation is within normal
limits
Yes…………….... No……..………….
Mental status as per clinical observation is normal Yes…………….... No……..………….
Local examination done is normal Yes…………….... No……..………….
Informed consent given by the client Yes…………….... No……..………….
Explained to the client that consent form has authority of
a legal document
Yes…………….... No……..………….
Abdominal/Pelvic examination has been done in the
female and is within normal limits
Yes…………….... No……..………….
Infection prevention practices as per laid down standards Yes…………….... No……..………….
B. Menstrual History (for female clients)
Cycle Days
Length
Regularity Regular………........…………………...
Irregular………........………………….
Date of LMP (DD/MM/YYYY) …………/……../………………
C. Obstetric History (for female clients)
Number of Spontaneous Abortions
Number of Induced Abortions
Currently Lactating Yes………….......…. No…....…………
Amenorrheic Yes………….......…. No…....…………
Whether Pregnant Yes………….......…. No…....…………
If Yes (No. of weeks pregnancy)
……………………….....................
No. of Children Total No…........................................…..
Date of Birth of Last Child (dd/mm/yyyy) ……../………../………..
Manual for Family Planning Indemnity Scheme
35
D. Contraceptive History
Have you or your spouse ever used contraception? Yes..........…………. No….......……….
Are you or your spouse currently using any contraception
or have you or your spouse used contraception during the
last six months?
() Tick the option
None……..........…………..………….
IUCD………..........…………………..
Condoms…........…………………….
Oral Pills……........…………….…….
Any Other (specify)………......…….
E. Medical History
Recent medical Illness Yes…………….... No……..………….
Previous Surgery Yes…………….... No……..………….
Allergies to medication Yes…………….... No……..………….
Bleeding Disorder Yes…………….... No……..………….
Anemia Yes…………….... No……..………….
Diabetes Yes…………….... No……..………….
Jaundice or liver disorder Yes…………….... No……..………….
RTI/STI/PID Yes…………….... No……..………….
Convulsive disorder Yes…………….... No……..………….
Tuberculosis Yes…………….... No……..………….
Malaria Yes…………….... No……..………….
Asthma Yes…………….... No……..………….
Heart Disease Yes…………….... No……..………….
Hypertension Yes…………….... No……..………….
Mental Illness Yes…………….... No……..………….
Sexual Problems Yes…………….... No……..………….
Prostatitis (Male sterilization) Yes…………….... No……..………….
Epididymitis (Male Sterilization) Yes…………….... No……..………….
H/O Blood Transfusion Yes…………….... No……..………….
Gynecological problems (Female Sterilization) Yes…………….... No……..………….
Currently on medication (if yes specify) Yes…………….... No……..………….
Comments ........................................................................................................................................................
............................................................................................................................................................................
............................................................................................................................................................................
F. Physical Examination
BP…………....................……………….Pulse…………….................………..Temperature……..………..
Lungs Normal……….. Abnormal……...……
Heart Normal……….. Abnormal……...……
Abdomen Normal……….. Abnormal……...……
36
Manual for Family Planning Indemnity Scheme
G. Local Examination (Strikeout whichever is not applicable)
1. Male Sterilization
Skin of Scrotum Normal……....................….. Abnormal…..........…...……
Testis Normal……....................….. Abnormal…..........…...……
Epididymis Normal……....................….. Abnormal…..........…...……
Hydrocele Yes…..........…....…….…..…. No………....................…….
Varicocele Yes…..........…....…….…..…. No………....................…….
Hernia Yes…..........…....…….…..…. No………....................…….
Vas Deferens Normal……....................….. Abnormal…..........…...……
Both Vas Palpable Yes…..........…....…….…..…. No………....................…….
2. Female Sterilization
External Genitalia Normal……....................….. Abnormal…..........…...……
PS Examination Normal……....................….. Abnormal…..........…...……
PV Examination Normal……....................….. Abnormal…..........…...……
Uterus Position A/V……….................………R/V……...............………….
Mid position…….................Not determined…...............
Uterus size Normal……….. Abnormal……...……Size…………
Uterus Mobility Yes…..................…..No………………. Restricted / Fixed
Cervical Erosion Yes…..........…....…….…..…. No………....................…….
Adnexa Normal……....................….. Abnormal…..........…...……
Comments ........................................................................................................................................................
............................................................................................................................................................................
............................................................................................................................................................................
H. Laboratory Investigations
Hemoglobin level ………...……..............................………………….Gms%
Urine: Albumin Yes…..........…....…….…..…. No………....................…….
Urine- Sugar Present…….......................... Absent…..............................
Urine test for Pregnancy Positive: ……........................ Negative: …….....................
Any Other (specify) ...............................................................................................
Name: Signature of the Examining Doctor
Date: HOSPITAL SEAL
Manual for Family Planning Indemnity Scheme
37
I. Preoperative Preparation
Fasting Yes ..................duration…….... hrs. No…................……
Passed urine Yes…..........…....…….…..…. No………....................…….
Any other (specify)
J. Anaesthesia/Analgesia
Type of anesthesia given
() Tick the option
y Local only
y Local and analgesia
y General, no intubation
y General, intubation
y Any other (specify)
Time
Drug name
Dosage
Route
..............................................................................................
..............................................................................................
..............................................................................................
..............................................................................................
*Signature of anaesthetist in case of regional or general anaesthesia
K. Surgical Approach
Male sterilization
Local anaesthesia Lignocaine 2% ............................cc/Other...............................
Technique Conventional.............................. NSV................................
Type of incision Conventional NSV Single vertical....................... Double vertical........................
Single puncture .......................................................................
Material for occlusion of vas 2-0 Silk ................................... 2-0 Catgut...............................
Fascial interposition Yes …….........................…… No……….............…………
If no, give reasons...............................................................
……………………………..................……………………..
Length of vas resected .......................................................................................... Cm
Suture of skin for conventional
vasectomy
Silk ................................... Other ...................................
Surgical notes
Any other surgery done at time of
sterilization?
Yes …….........................…… No……….............…………
If yes give details...................................................................
Specify details of complications and
management
Name…………………………………….. Signature of the Operating Surgeon
Date……………………………………

38
Manual for Family Planning Indemnity Scheme
Female sterilization
Local anaesthesia Lignocaine %
Other
Timing of procedure
() Tick the option used
y Within 7 days post-partum...............................................
y Interval (42 days or more aer delivery or abortion) ..
y With abortion, induced or spontaneous.........................
y Less than 12 weeks .......................................................
y More than 12 weeks
y Any other (specify)
Technique
() Tick the option used
y Minilap Tubectomy
y With C section
y With other surgery .........................................................
y Laparoscopy Tubal Occlusion
y SPL/DPL ..........................................................................
Method of occlusion of fallopian
tubes
() Tick the option used
y Modied Pomeroy Laparoscopy:
y Ring
y Clip
Details of gas insuation
pneumoperitoneum created (CO2/
Air)
Yes ………...................................….. No………....…………..
Insuator used Yes ......................................... No............................................
Specify details of complications and
management
Name ………………………………………… Signature Of The Operating Surgeon
Date…………………………………………..
L. Vital Signs: Monitoring Chart (For Female Sterilization)
*Sedation: 0—Alert 1—Drowsy 2—Sleeping/arousable 3—Not arousable
Event Time Sedation* Pulse Blood
Pressure
Respiratory
Rate
Bleeding Comments
(Treatment)
Preoperative (every 15
in aer premedication)
Intra-operative
(continuous)
Post-operative
1. Every 15 min for
rst hour and longer
if the patient is not
stable/awake
2. Every 1 hour until 4
hours aer surgery
15 min
30min
45 min
1 hr
2 hrs
3 hrs
4 hrs
Name: .................................................................... Signature of the Aending Sta Nurse
Date: .......................................
Manual for Family Planning Indemnity Scheme
39
M. Post-Operative Information
Passed urine Yes .............................. No........................................
Abdominal distension Yes .............................. No........................................
Patient feeling well Yes .............................. No........................................
If no, please specify
N. Instructions For Discharge
Male sterilization client observed for half an
hour aer surgery
Yes .............................. No........................................
Female sterilization client observed for four
hours aer surgery
Yes .............................. No........................................
Post-operative instructions given verbally Yes .............................. No........................................
Post-operative instructions given in writing Yes .............................. No........................................
Patient counselled for postoperative
instructions
Yes .............................. No........................................
Comments
Name…………………………………… Signature of the Discharging Doctor
Date: .......................................

40
Manual for Family Planning Indemnity Scheme
Sterilization Certificate
Annexure–4
Hospital Registration No. (IPD/OPD) _______________
1. This is to certify that Smt/ Shri………................................................................................ S/O; W/O Shri
............................................................................. working as .......................................residing at ..............
....................... ........................................................ has undergone Minilap Tubectomy – (Interval/Post-
Partum/Post Abortion/Concurrent with other procedures)/Laparoscopic Tubal Occulsion (Interval/
Post Abortion/Concurrent with other surgeries)/Vasectomy Conventional/NSV) in this facility/
hospital ....................................................................(Name of facility/Hospital) on .................................
by Dr..............................................................................
For Female Sterilization:
2. She has resumed her menstrual Cycle (LMP_____) or she has not resumed her menses within the
month of sterilization but pregnancy test is negative.
For Male Sterilization:
3. His semen examination undertaken on (Date)_________________ revealed no sperm (azoospermia)
*Strike out whichever is not applicable
She/ He is therefore certied to be sterile
Signature of Medical Ocer I/c
Name…………….....…………….
Date ............................ Seal .............................................
Note :
• Client should acknowledge ‘received’ on the duplicate copy before receiving the original copy. The
duplicate to be maintained as a record in the facility as per state norms.

Manual for Family Planning Indemnity Scheme
41
Post Operative Instruction Card
Annexure–5
Name and type of hospital/facility
Client's name
Father's name
Husband's name/Wife’s Name
Address
Contact number (if available)
Date of operation (dd/mm/yyyy) ....................../ ....................../ ......................
Type of operation Minilap/Post-partum/Laparoscopic (SP/DP)/
Conventional Vasectomy/NSV................................
1. Follow-up:
a) Aer 48 hours, rst contact is established
b) On the 7th day for stitch removal
c) For Female Sterilization: Aer one month or aer rst menstrual period, whichever is earlier
For Male Sterilization: Aer 3 months, for semen examination for sperm count
d) In an emergency, as and when required to the nearest health facility
2. Medication as prescribed
3. Return home and rest for the remainder of the day
4. For Female Sterilization: - Resume only light work aer 48 hours and gradually return to full
activity in two weeks following surgery.
5. For Male Sterilization: - Scrotal support or snug undergarment for 48 hours.
- Resume normal work aer 48 hours and return to full activity,
including cycling, within one week following surgery.
6. Resume normal diet as soon as possible.
7. Keep the incision area clean and dry. Do not disturb or open the dressing.
8. Bathe aer 24 hours following the surgery. If the dressing becomes wet, it should be changed so
that the incision area is kept dry until the stitches are removed.
9. Sexual intercourse:
Vasectomy/ Tubectomy does not interfere with sexual pleasure, ability, or performance
Female Sterilization: In the case of interval sterilization (Minilap and Laparoscopic), the
client may have intercourse one week aer surgery or whenever she
feels comfortable thereaer.
In case of post partum sterilization (aer caesarian or normal delivery)
client may have intercourse 2 weeks aer sterilization or whenever she
feels comfortable.
Male Sterilization: The client may have intercourse whenever he is comfortable aer the
surgery but must ensure use of condom if his wife/partner is not using
any method of contraception.

42
Manual for Family Planning Indemnity Scheme
10. Report to the doctor or clinic if there is excessive pain, fainting, fever, bleeding or pus discharge
from the incision, or if the client has not passed urine, not passed atus and experiences bloating
of the abdomen.
11. Contact health personnel or a doctor in case of any doubt.
12. Female Sterilization: Return to the facility if there is any missed period/suspected pregnancy,
within two weeks to rule out pregnancy.
13 Male Sterilization: Return to the facility aer three months for semen examination to see
if azoospermia has been achieved. If semen still shows sperm return to
facility every month till 6 months.
Follow-up report
Follow up Time aer surgery Date of follow-up Complications,
if any
Action
Taken
1
st
48 hours
2
nd
7
th
day
3
rd
1 month aer surgery or aer the
rst menstrual period, whichever is
earlier (Female Sterilization)
Aer 3 months for semen examination
(Male Sterilization)
Emergency
Comment ................................................................................................................................................................
...................................................................................................................................................................................
...................................................................................................................................................................................
Result of Semen Examination:
Name……………………….. Designation…………………
Date .......................... Signature of the Person Filling Out the Report
Manual for Family Planning Indemnity Scheme
43
Proforma for Conducting Audit of Death
Annexure-6
To be submied within one month of sterilization by DISC and forwarded to SISC
Copy of this report has to be sent to the MOHFW Mandatorily
Name of the State/District/Union Territory: ......................................................................................................
Details of the Deceased
1 Full name
2 Age
3 Sex Female……...............…….Male………................
4 Name of spouse ( his/her age) ......................................................................................
5 Address of the deceased ......................................................................................
......................................................................................
6 Number of living children (with details
concerning age and sex)
......................................................................................
......................................................................................
......................................................................................
......................................................................................
......................................................................................
7 Whether the operation was performed aer
delivery or otherwise
......................................................................................
......................................................................................
8 If aer delivery:
Date of delivery
Place of delivery
Type of delivery
Person who conducted the delivery
......................................................................................
......................................................................................
......................................................................................
......................................................................................
......................................................................................
......................................................................................
9 Whether tubectomy operation was done
along with MTP
......................................................................................
......................................................................................
10 Whether wrien consent was obtained
before the operation
......................................................................................
......................................................................................
11 Whether the operation was done at a camp
or as a xed day static procedure at the
institution.
......................................................................................
......................................................................................
......................................................................................
......................................................................................
Details of Operations
12 Place of operation ......................................................................................
13 Date and time of operation (D/M/Y)
14 Date and time of death (D/M/Y)
15 Name of surgeon ......................................................................................
16 Whether surgeon was empanelled or not Yes ................................... No ....................................
......................................................................................
......................................................................................
17 If the operation was performed at a camp,
who primarily screened the client clinically?
......................................................................................
......................................................................................
18 Was the centre fully equipped to handle
any emergency complications during the
procedure?
Yes ................................... No ....................................
......................................................................................
......................................................................................
44
Manual for Family Planning Indemnity Scheme
19 Number of clients admied and number of
clients operated upon on the day of surgery
......................................................................................
......................................................................................
20 Did any other clients develop complications?
If so, give details of complications.
......................................................................................
......................................................................................
Anaesthesia/Analgesia/Sedation
21 Name of anaesthetist, if present
22 Details of anaesthesia drugs used
23 Type of anaesthesia/analgesia /sedation ......................................................................................
24 Post-operative complications(according to
sequence of events)
A. Details of symptoms and signs ......................................................................................
B. Details of laboratory and other
investigations done
......................................................................................
C. Details of treatment given, with
timings, dates, etc. from time of
admission until the death of the patient
......................................................................................
......................................................................................
......................................................................................
Details of Death Audit
25 Cause of death (Primary cause) ......................................................................................
26 Has post-mortem been done? If yes, aach
the post-mortem report
......................................................................................
......................................................................................
27 Whether rst notication of death was sent
within 24 hours.
Yes .................................... No ...................................
If not, give reasons
28 Details of the ocers from the District
Quality Assurance Commiee (QAC) who
conducted the enquiry
......................................................................................
......................................................................................
29 In the opinion of the chairman of the DQAC,
was death aributable to the sterilization
procedure?
Yes .................................... No ...................................
......................................................................................
30 What factors could have helped to prevent
the death?
......................................................................................
31 Were the sterilization standards established
by GOI followed?
Yes .................................... No ...................................
32 Did the facility meet and follow up the
sterilization standards established by GOI?
If no, list the deviation[s].
Yes .................................... No ...................................
......................................................................................
......................................................................................
33 Additional information ......................................................................................
......................................................................................
......................................................................................
34 Recommendations made ......................................................................................
......................................................................................
......................................................................................
35 Action proposed to be taken ......................................................................................
......................................................................................
......................................................................................
Date: Signature
Name Designation
Note: If any member of the SQAC/DQAC has performed the operation, he/she should recuse himself/
herself from the proceedings of this audit.

Manual for Family Planning Indemnity Scheme
45
Report on Complications/Failures following
Sterilization
Annexure-7
(To be lled in by the DISC)
Name of the State/ District/Union Territory……...........................................…………………………………
Date of this report (D/M/Y)………...................…………/………….............…………/………….............……
1 Name and address of client
2 Name of spouse
3 Date of sterilization (D/M/Y) ......................../.............................../............................
4 Place where surgery was performed Camp……………………...............…………………
PP Centre………………….............………………...
PHC/CHC…………………………..............………
District Hospital…………………............…………
Medical College Hospital………...........…………..
Accredited private/NGO facility……............…….
(Also specify the name of the facility)….........…....
…………………………………………..............……
5 Type of surgical approach Minilap……………............…………………………
Laparoscopy………………….........……………….
Post-Partum Tubectomy…….........………………..
Conventional Vasectomy……….........…………….
NSV……………………………….............………….
Any other specify……………..........……………….
6 Level of experience of the person who
performed the sterilization procedure
Trainee……………………………....................…….
Empanelled surgeon……………................………..
7 Type of anaesthesia Local without sedation……...................……………
Local with sedation……………...................……….
Spinal/Epidural/General……..................………….
Part A: Complications aributable to sterilization requiring hospitalization
8 Date when complication was rst
reported(D/M/Y)
Types of complication(s)
......................../.............................../............................
A. If complications were related to
anaesthesia, list all anaesthetic agents,
analgesics, sedatives and muscle
relaxants
46
Manual for Family Planning Indemnity Scheme
B Injury/Trauma
y Injury to bladder
y Injury to fallopian tube
y Mesosalpinx
y Injury to bowel
y Uterine perforation
y Testicular artery
y Spermatic cord
y Any other (specify)
Yes ............................. No ...........................................
Yes ............................. No ...........................................
Yes ............................. No ...........................................
Yes ............................. No ...........................................
Yes ............................. No ...........................................
Yes ............................. No ...........................................
Yes ............................. No ...........................................
Yes ............................. No ...........................................
i. What factors contributed to injury/
trauma?
C Haemaorrhage
y Epigatric vessel
y Fallopian Tube
y Haematoma requiring intervention/
hospitalization
y Any other(specify)
Yes ............................. No ...........................................
Yes ............................. No ...........................................
Yes ............................. No ...........................................
Yes ............................. No ...........................................
i. What factors contributed to the
haemorrhage?
ii. Did the client have a blood transfusion? Yes ............................. No ...........................................
D Infection
y Wound Infection
y Pelvic Infection
y Epididymoorchitis
y Generalized peritonitis
y Any other (specify)
Yes ............................. No ...........................................
Yes ............................. No ...........................................
Yes ............................. No ...........................................
Yes ............................. No ...........................................
Yes ............................. No ...........................................
E. Complications not mentioned in 8A-D
(specify)** including need to abandon
the procedure or adopt a change in
approach
F. Was sterilization done postpartum or
with MTP?
If Yes, was hospitalization a result of
complications arising from those
procedures and not from sterilization?
Yes ............................. No ...........................................
Yes ............................. No ...........................................
G. Describe the procedure leading to the
complication
9 Describe the type of treatment administered
aributable to complication Medical/
surgical
Yes ............................. No ...........................................
10 Date of recovery(D/M/Y) ............................./............................../........................
11 Number of days of hospitalization
Part B: Pregnancy or Failure aributable to male sterilization ( Pregnancy aer certication of
vasectomy)
12 If Pregnant
A. Estimate date of conception (D/M/Y) ............................./............................../........................
B. Was semen examination done Yes/No…………..............………………………….
C. If yes, give date
Result of analysis
............................./............................../........................

Manual for Family Planning Indemnity Scheme
47
D. In the opinion of Medical Ocer
y Pregnancy was due to unprotected
intercourse before azoospermia was
achieved
y Pregnancy existed before vasectomy
y Cause of pregnancy could not be
determined
y Any Other (Specify)
Yes/No…………..............………………………….
Yes/No…………..............………………………….
Yes/No…………..............………………………….
Yes/No…………..............………………………….
Part C: Pregnancy or failure aributable to female sterilization
13 A. Date pregnancy was detected
(D/M/Y)
…………./……………/………
B. Estimated date of conception …………./……………/………
C. Conrmation of pregnancy test done
USG
Yes/No…………..............………………………….
Yes/No…………..............………………………….
D. Location of pregnancy
() Tick the option
y Intrauterine
y Ectopic
y Undetermined
E. Was the women already pregnant at
time of sterilization
Yes/No…………..............………………………….
F. In opinion of the Commiee Members,
the pregnancy was due to:
Names, designations and signatures of the Commiee Members
…………………………………………… ……………………………………………
…………………………………………… ……………………………………………
…………………………………………… ……………………………………………
…………………………………………… ……………………………………………
Comments by QAC
In the opinion of the QAC:
(a) Were the sterilization standards established by GOI followed? Yes/No
(b) Was the complication/failure aributable to the sterilization procedure? Yes/No
(c) What factors contributed to the complication/failure………………............................…………………
…………………………………………………………………………………………………………………
…………………………………………………………………………………………………………………
…………………………………………………...........................................………
(e) Was the woman already pregnant at the time of sterilization? Yes/No
(f) Does the facility meet all the physical and other requirements as laid down in the GOI Standards
for Sterilization? Yes/No

48
Manual for Family Planning Indemnity Scheme
If no, list the deviation[s]:………………………………………………………………......................…….
…………………………………………………………………………………………………………………
……………………………………………………………….......................................................…………….
Additional information discussed, not presented in the report:…................…………………………
…………………………………………………………………………………………………………………
………………………………………………………………………….......................................................….
Based on the investigation report, the following recommendations are made:……................…....….
…………………………………………………………………………………………………………………
…………………………………………………………........................................................……………….
Reviewed by Signatures Designation
…………………………....... …………………………....... ………………………….......
…………………………....... …………………………....... ………………………….......
…………………………....... …………………………....... ………………………….......
Note: If any member of the QAC has performed the operation, he/she should not act as a chairman/
member for this report.

Manual for Family Planning Indemnity Scheme
49
Checklist of FPIS Claim Documents
Annexure–8
Section IA-IB Section IC Section ID Section II
Death Failure Complication Indemnity cover for
Doctor/Facility
Original & duly
Completed” Claim
Form Cum Medical
certicate”
Original & duly
Completed” Claim
Form Cum Medical
certicate”
Original & duly
Completed” Claim
Form Cum Medical
certicate”
Intimation in writing
Copy of Consent Form
& Medical record and
Checklist
Copy of Consent Form
& Medical record and
Checklist
Copy of Consent Form
& Medical record and
Checklist Copy
Copy of Consent Form
Copy of Post-
Operative Card/
Discharge certicate
Copy of Sterilization
Certicate
Copy of Post-
Operative Card/
Discharge Certicate
Copy of Sterilization
Certicate
Copy of Death
certicate issued by
hospital/municipality
or any other
designated authority.
Copy of any of the
diagnostic Report
conrming failure of
Sterilization
Original Bills/
Receipts/Cash Memos
along with Original
Prescription and Case
Sheet
Certicate from the
convener of DISC
(CMO or equivalent)
designated for this
purpose at district
level conrming
that the Sterilization
Operation was
conducted by the
doctor etc.
Copy of Summon/FIR
Proforma for
Conducting Audit of
Death by DISC
Report on Failure
following sterilization
by DQAC
Report on
Complication
following sterilization
by DQAC
Copy of the Award
given by the court
along with the
original receipts for
which payment is
made to the lawyer
Note: All the documents should be duly aested by the Convener of DISC (CMO or equivalent) designated for
this purpose at district level
Before forwarding the Claim Form and other Required Document, it has to be checked that:
A. Consent Form
1. Registration number of the beneciary, date, and signature or thumb impression of the
beneciary are properly placed in respective columns.
2. Examination of patient record is lled in properly and doctor has put his signature and date.
3. Details of dependents of a beneciary are lled in.

50
Manual for Family Planning Indemnity Scheme
4. All columns of Consent form and Medical Record & Check List for female / male sterilization
are lled properly
B. Claim Form
1. Claim is submied in a prescribed Claim Form in original.
2. Claim forwarded through Medical Ocer/Health Facility conducting sterilization procedures.
3. Name and address of the beneciary are same mentioned on Consent form.
4. Signature or thumb impression of beneciary is same as mentioned on Consent form.
5. Date of sterilization is same as mentioned in the Sterilization Certicate and Consent form.
6. Other details lled in are tallied with other relevant documents which are becoming part of
claim form.
7. All columns of Medical Certicate which is a part of Claim Form are lled in and date, signature
and seal of CMO or Equivalent designated for this purpose at district level has been placed.
C. Sterilization Certicate
1. Name of beneciary is same as lled in on Consent form.
2. Date of sterilization is mentioned under specic column.
3. Certicate issued have signature and date of issuing authority.
4. Sterilization Certicate is in proper format as prescribed and having Registration Number and
date.
D. Diagnostic Report Issued for Failure of Sterilization
1. Report issued should be in a proper document i.e. hospital case sheet/ proper diagnostic report.
2. It should have registration number and date.
3. Cause detected for failure has been properly recorded by the issuing authority on the document.
4. First diagnostic report by which a failure is detected is aached.
E For Claims under Complication
1. The case sheet / prescription have the name of beneciary.
2. Case sheet/ prescription have proper hospital registration number and date.
3. Case sheet/ prescription have a date of sterilization.
4. Nature of post-operative complication has been recorded.
5. Medicines prescribed should tally with cash memo.
6. Case sheet/prescription and bills/cash memo are in original.
F. Death Certicate
1. Death certicate has been issued by the proper authority.
2. Name of deceased, date of death etc. are rightly lled in on the certicate.
3. Certicate should have registration number and date of issue and signature of issuing authority.
G. Post-Operative Discharge Card
1. Name of beneciary is same as lled in the Consent form.
2. Date and type of of sterilization is mentioned under specic column.
3. All columns of the post –operative discharge card should be properly lled.
4. Card should bear the name, designation, signature of the person lling the card.
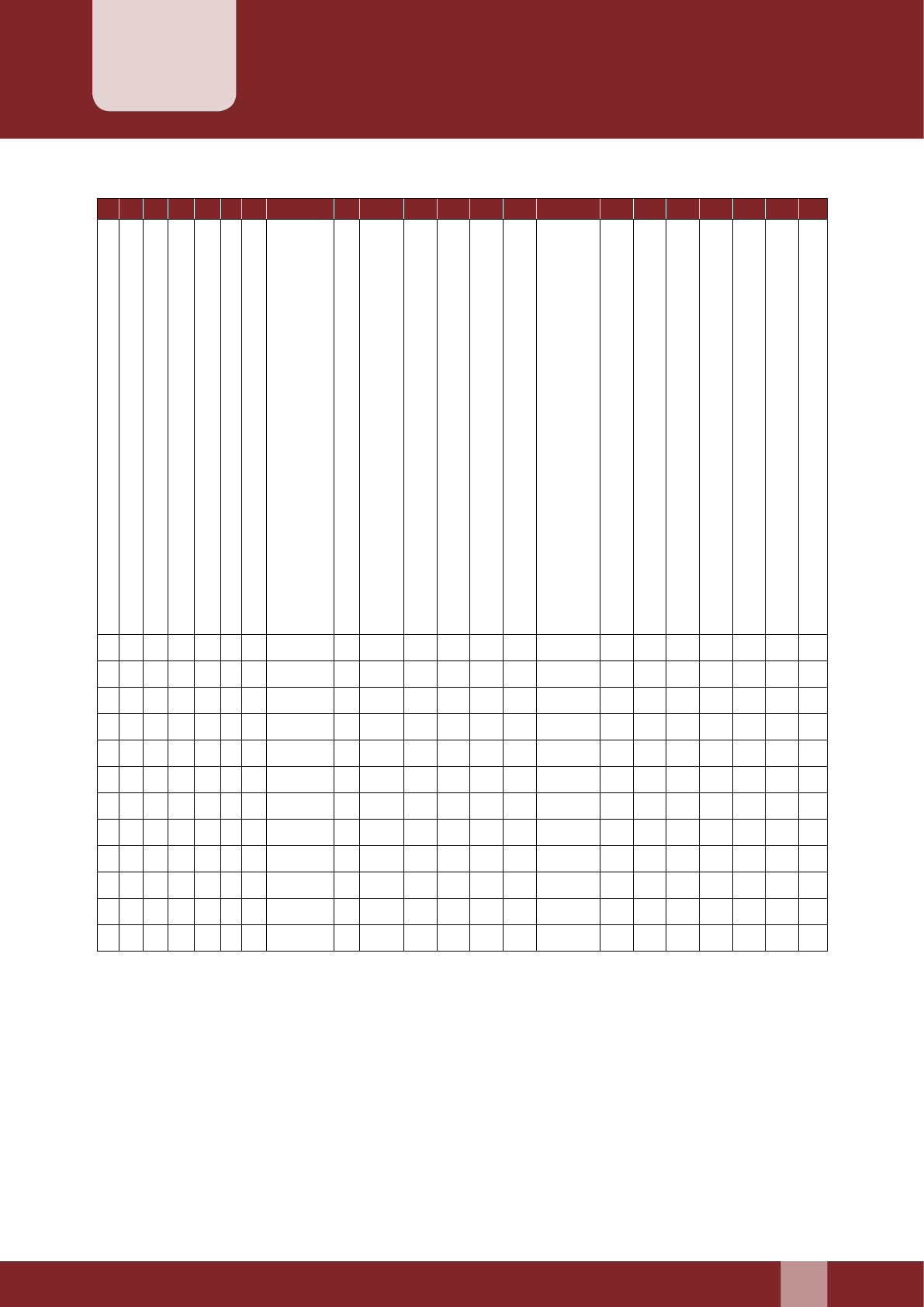
Manual for Family Planning Indemnity Scheme
51
Beneficiary Wise Claim Status
Annexure–9
A B C D E F G H I J K L M N O P Q R S T U V
S. No
Name of the District
S. No
Name of Beneciary /Claimant
Sex& Age
Address
Date of operation
Type of procedure
(Minilap/Post-Partum/Laparoscopic/ Conventional
Vasectomy/ NVSV)
Facility Name where operation conducted
Facility Type (PHC/CHC/DH/Medical college/
Accredited PVT/NGO Facility)
Operation done in Camp/ Fixed Day Static
Name of Surgeon/doctor who operated
Type of claim (Death/Complication/Failure)
Date Of Claim Submission
Diagnostic Report conrming Failure of
sterilization(Urine Test Report/ Usg/ Per Abdominal
Examination/ Mtp/ Semen Test Report)
Amount Claimed(in Rs)
Claim Approved / Rejected/Pending
Amount Paid
Mode of payment (Cheque/DBT)
Date of Payment
Outstanding Amount if any
If Rejected Reasons for Rejection
State needs to submit this list on quarterly basis to GoI
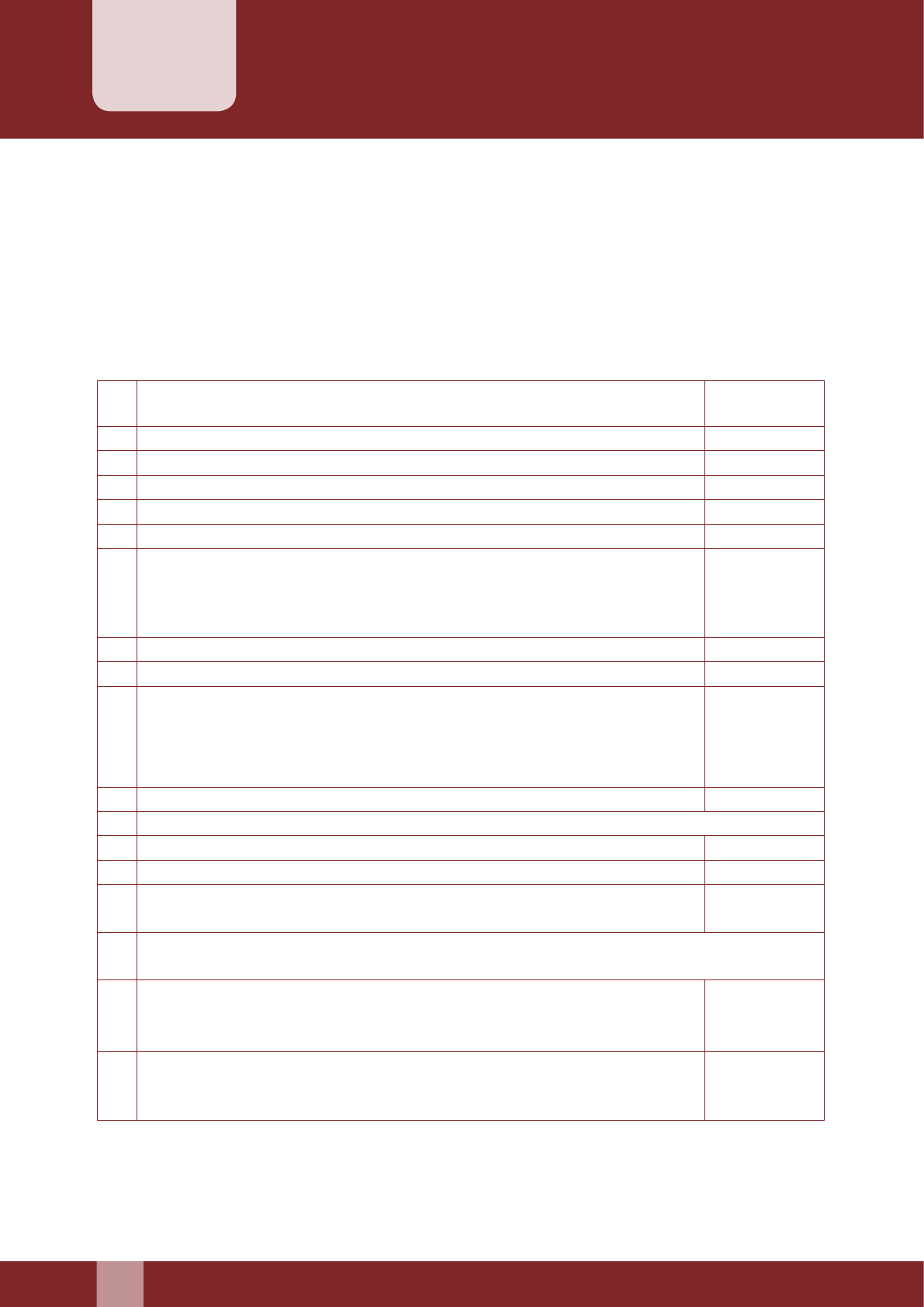
52
Manual for Family Planning Indemnity Scheme
Quarterly Report Form
Annexure–10
Quarterly report on maintenance of quality, failure of sterilizations, complications or deaths aributable
to sterilizations is to be sent by the Convener of DISC (CMO or equivalent) designated for this purpose
at district level to the SISC in the format given below.
Name of the District / Name of the State: ………….....................................................................…………..
To be submied by DISC to SISC.
REPORTING QUARTER: ………………………
1 Number of sterilization conducted in the districts/States in the reporting
quarter
(i) In Government Hospitals.
(ii) In Accredited Private/NGO Facility
2 Death reported in hospital or within 7 days from discharge.
3 No of cases where Rs. 50,000 paid from State/ District Health Society
4 Death reported between 8 – 30 days from discharge.
5 Number of claims accepted by District Health Society
Failure.......................................................................................................................
Complications .........................................................................................................
Death .........................................................................................................................
6 Number of cases where payment released by District Health Society
7 Number of claims pending for selement by District Health Society
Period of pendency: Number of Claims
30 days: ......................................................................................................................
31-90 days: .................................................................................................................
More than 90 days: ....................................................................................................
8 No. of Court cases against doctor/ health facility, if any.
(i) Action taken on court cases against doctor/ health facility:
(ii) Court cases for non-selement of claims in consumer courts etc.
9 Number of private doctors / health facilities empanelled/ accredited:
10 Whether prescribed consent forms are available in local languages with all
Doctors/ Health facilities in sucient number (as per manual).
11 Problem, if any, with general public reporting failures/ Complications / deaths etc. following
sterilization:
12 Details of enquiries held into each case of breach of guidelines by doctor or
health facility, punitive action taken against them including names of doctors
and health facilities removed from the panel.
(To be given
on separated
Sheet)
13 Any other information (To be given
on separated
Sheet)
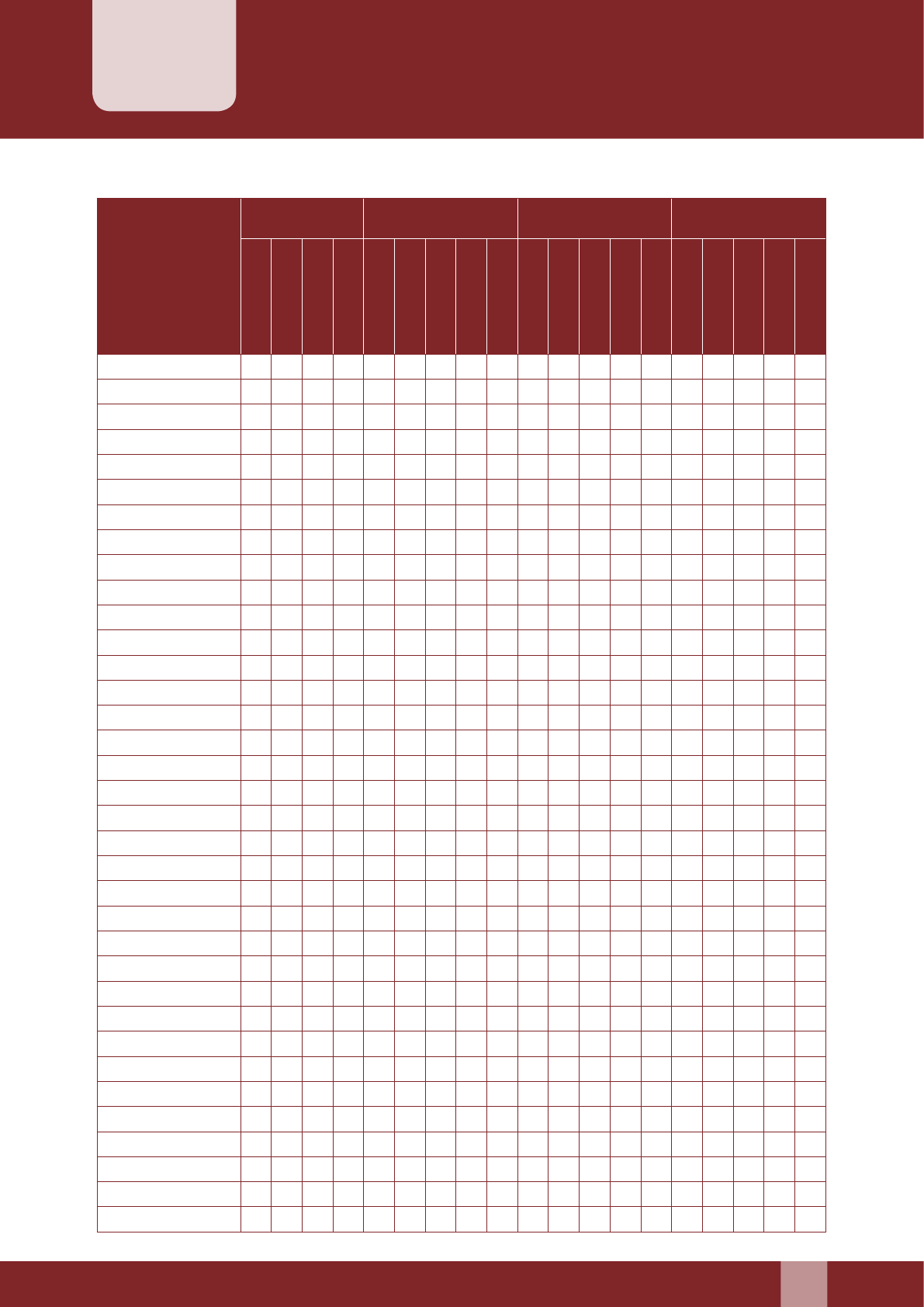
Manual for Family Planning Indemnity Scheme
53
Statewise Annual Claims Status
Annexure–11
State
Claim
Intimation
Paid Rejected Out
Standing
Complication
Death
Failure
Grand Total
Complication
Death
Failure
Total
Amount
Complication
Death
Failure
Total
Amount
Complication
Death
Failure
Total
Amount
Bihar
Chaisgarh
Himachal Pradesh
Jammu & Kashmir
Jharkhand
Madhya Pradesh
Orissa
Rajasthan
Uar Pradesh
Uarakhand
Arunachal Pradesh
Assam
Manipur
Meghalaya
Mizoram
Nagaland
Sikkim
Tripura
Andhra Pradesh
Goa
Gujarat
Haryana
Karnataka
Kerala
Maharashtra
Punjab
Tamil Nadu
West Bengal
A & N Islands
Chandigarh
D & N Haveli
Daman & Diu
Delhi
Lakshadweep
Puducherry
State needs to submit this on annual basis to GoI
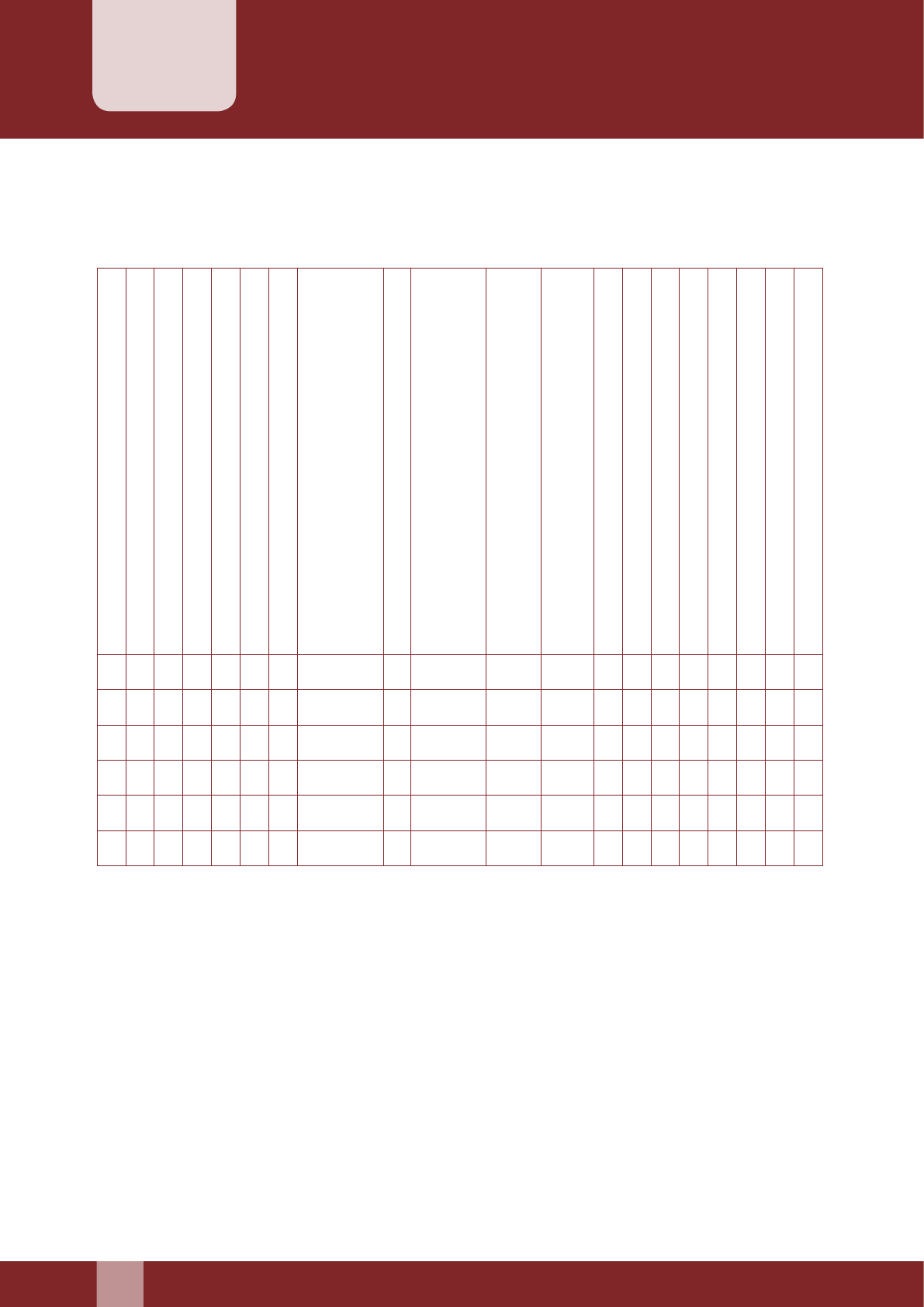
54
Manual for Family Planning Indemnity Scheme
Sterilization Death Audit Report
Annexure–12
Name of the state/union territory……...........................………………………………………………………
Report for the quarter ending …………………………..........................…………………………………….
S.No
District
S.No
Name of the Deceased Beneciary
Age
Sex
Date of operation
Type of Facility where operation was conducted
((PHC/CHC/DH/Medical college/Accredited PVT/
NGO Facility)
Camp of Fixed Static
Type of Procedure
(Interval Minilap/Post-Partum Minilap/
Laparoscopic/Conventional Vasectomy/ NSV)
If Post Partum
(With Cesarean/Normal delivery/MTP)
Atropine used in
preanaesthetic medication (Y/N)
Surgery under Anesthesia(LA/GA)
Empanelled Provider (Y/N)
Date of death
Time of death
Place of Death
Under-lying cause of death
Death audited By DISC (Y/N)
Action Taken
Medical death audit report must be annexed for each case.
Name of State FP Nodal Ocer ……………… Signature
State needs to submit this report on quarterly basis to GoI

Manual for Family Planning Indemnity Scheme
55

56
Manual for Family Planning Indemnity Scheme