
OXFORD MEDICAL PUBLICATIONS
Oxford Handbook of
Clinical Surgery
Published and forthcoming Oxford Handbooks
Oxford Handbook for the Foundation Programme 3e
Oxford Handbook of Acute Medicine 3e
Oxford Handbook of Anaesthesia 3e
Oxford Handbook of Applied Dental Sciences
Oxford Handbook of Cardiology 2e
Oxford Handbook of Clinical and Laboratory Investigation 3e
Oxford Handbook of Clinical Dentistry 5e
Oxford Handbook of Clinical Diagnosis 2e
Oxford Handbook of Clinical Examination and Practical Skills
Oxford Handbook of Clinical Haematology 3e
Oxford Handbook of Clinical Immunology and Allergy 3e
Oxford Handbook of Clinical Medicine – Mini Edition 8e
Oxford Handbook of Clinical Medicine 8e
Oxford Handbook of Clinical Pathology
Oxford Handbook of Clinical Pharmacy 2e
Oxford Handbook of Clinical Rehabilitation 2e
Oxford Handbook of Clinical Specialties 9e
Oxford Handbook of Clinical Surgery 4e
Oxford Handbook of Complementary Medicine
Oxford Handbook of Critical Care 3e
Oxford Handbook of Dental Patient Care 2e
Oxford Handbook of Dialysis 3e
Oxford Handbook of Emergency Medicine 4e
Oxford Handbook of Endocrinology and Diabetes 2e
Oxford Handbook of ENT and Head and Neck Surgery
Oxford Handbook of Epidemiology for Clinicians
Oxford Handbook of Expedition and Wilderness Medicine
Oxford Handbook of Gastroenterology & Hepatology 2e
Oxford Handbook of General Practice 3e
Oxford Handbook of Genetics
Oxford Handbook of Genitourinary Medicine, HIV and AIDS 2e
Oxford Handbook of Geriatric Medicine
Oxford Handbook of Infectious Diseases and Microbiology
Oxford Handbook of Key Clinical Evidence
Oxford Handbook of Medical Dermatology
Oxford Handbook of Medical Imaging
Oxford Handbook of Medical Sciences 2e
Oxford Handbook of Medical Statistics
Oxford Handbook of Nephrology and Hypertension
Oxford Handbook of Neurology
Oxford Handbook of Nutrition and Dietetics 2e
Oxford Handbook of Obstetrics and Gynaecology 2e
Oxford Handbook of Occupational Health 2e
Oxford Handbook of Oncology 3e
Oxford Handbook of Ophthalmology 2e
Oxford Handbook of Oral and Maxillofacial Surgery
Oxford Handbook of Paediatrics 2e
Oxford Handbook of Pain Management
Oxford Handbook of Palliative Care 2e
Oxford Handbook of Practical Drug Therapy 2e
Oxford Handbook of Pre-Hospital Care
Oxford Handbook of Psychiatry 3e
Oxford Handbook of Public Health Practice 2e
Oxford Handbook of Reproductive Medicine & Family Planning
Oxford Handbook of Respiratory Medicine 2e
Oxford Handbook of Rheumatology 3e
Oxford Handbook of Sport and Exercise Medicine 2e
Oxford Handbook of Tropical Medicine 3e
Oxford Handbook of Urology 3e
1
Oxford Handbook of
Clinical
Surgery
Fourth edition
Edited by
Greg McLatchie
Consultant Surgeon,
Hartlepool General Hospital,
Hartlepool, UK
Neil Borley
Consultant Colorectal Surgeon,
Cheltenham General Hospital,
Cheltenham, UK
Joanna Chikwe
Associate Professor, Department of Cardiothoracic
Surgery, Mount Sinai Medical Center,
New York, United States
3
Great Clarendon Street, Oxford, OX2 6DP,
United Kingdom
Oxford University Press is a department of the University of Oxford.
It furthers the University’s objective of excellence in research, scholarship,
and education by publishing worldwide. Oxford is a registered trade mark of
Oxford University Press in the UK and in certain other countries
© Oxford University Press, 2013
The moral rights of the authors have been asserted
First Edition published 1990
Second Edition published 2002
Third Edition published 2007
Fourth Edition published 2013
Impression: 1
All rights reserved. No part of this publication may be reproduced,
stored in a retrieval system, or transmitted, in any form or by any means,
without the prior permission in writing of Oxford University Press,
or as expressly permitted by law, or under terms agreed with the appropriate
reprographics rights organization. Enquiries concerning reproduction
outside the scope of the above should be sent to the Rights Department,
Oxford University Press, at the address above
You must not circulate this book in any other binding or cover and you must
impose the same condition on any acquirer
British Library Cataloguing in Publication Data
Data available
ISBN 978–0–19–969947–6 (fl exicover: alk.paper)
Printed in China by
C&C Offset Printing Co. Ltd.
Oxford University Press makes no representation, express or implied, that the drug
dosages in this book are correct. Readers must therefore always check the product
information and clinical procedures with the most up-to-date published product
information and data sheets provided by the manufacturers, and the most recent
codes of conduct and safety regulations. The authors and the publishers do not
accept responsibility or legal liability for any errors in the text, or for the misuse or
misapplication of material in this work. Except where otherwise stated, drug dosages
and recommendations are for the non-pregnant adult who is not breastfeeding.

v
Preface to the fourth
edition
Sometimes we have to look backward to look forward. Since 1990, sur-
gery has witnessed cataclysmic changes. In our Trust, the fi rst laparoscopic
cholecystectomy was performed in 1992, and has now become the pro-
cedure of choice for most gall bladder disease and many other surgical
operations in the western world. With the expansion of laparoscopic
surgery, we have encountered a whole new range of complications with
an escalation in the demise of general surgery as the result of hyperspe-
cialization. There are many surgical trainees who have scant experience
of open surgery and who have, due to European directives, limited time
exposure to surgical procedures. In fact, most technical training is now
obtained from emergency on call such that a new speciality of emergency
surgery is developing. A recent British Medical Journal (BMJ) article recom-
mended a training programme for surgeons wishing to work in remote
and rural surgery—not only in the Developing World, but in remote and
isolated communities in the United Kingdom! General surgery may largely
have gone, but it should not be forgotten. Most countries in the world do
not have access to these recent innovations and there is still a case in the
developed world for experience in open and general surgery to be incor-
porated in the formal training programmes of junior surgeons.
G. R. McLatchie
Hartlepool, September 2012

vi
Preface to the third
edition
This, the third edition of the Oxford Handbook of Clinical Surgery, refl ects
the changes which have occurred in general surgery over the 17 years
since the fi rst edition was published.
Firstly, we have recruited the services of two new editors, a stark con-
trast to the original, which was written by a single author with the assis-
tance of a surgical registrar.
Secondly, each chapter has been written by a specialist consultant or
registrar in the subject and, therefore, presents a modern, state-of-the-art
treatise on each topic.
Again, each condition is covered in the original two-page format with
blank pages for accompanying notes.
I am particularly grateful for the commitment that Jo Chikwe and Neil
Borley have made, and also wish to thank staff at Oxford University Press
for their support and patience. I am also grateful for the contribution and
support given by many colleagues.
G. R. McLatchie
Hartlepool, March 2007

vii
Preface to the fi rst
edition
The idea of this book was fi rst suggested by Mr Gordon McBain, consult-
ant surgeon at the Southern General Hospital, Glasgow. We have received
considerable support from the staff of Oxford University Press, and are
also indebted to Mr J. Rhind and Dr J. Daniel for their contributions and
our surgical teachers, especially Mr J. S. F. Hutchison, Mr M. K. Browne,
Mr J. Neilson, Mr D. Young, Mr A. Young, and the late Mr I. McLennan
whose practical advice and anecdotes pepper the pages….
G. R. McLatchie
S. Parameswaran
1990

viii
Dedications
For Ross, Cameron, Ailidh, Claire, and Calum
GRM
For Alexander, Christopher, and Jennifer
NB
Acknowledgements
We are grateful to the support of our colleagues and Oxford University
Press and to Mrs Pamela Lines for her diligent support in the fi nal editing
of the manuscript.
ix
Contents
Detailed contents xi
Contributors xxiii
Symbols and abbreviations xxv
1 Good surgical practice
1
2 Principles of surgery
23
3 Surgical pathology
141
4 Practical procedures
185
5 Head and neck surgery
221
6 Breast and endocrine surgery
239
7 Upper gastrointestinal surgery
271
8 Liver, pancreatic, and biliary surgery
311
9 Abdominal wall
335
10 Urology
353
11 Colorectal surgery
391
12 Paediatric surgery
423
13 Paediatric orthopaedic
457
14 Major trauma
477
15 Orthopaedic surgery
489
16 Plastic surgery
589
17 Cardiothoracic surgery
619
18 Peripheral vascular disease
641
19 Transplantation
675
20 Surgery in tropical diseases
701
21 Common operations
729
22 Eponymous terms and rarities
757
Anatomy and physiology key revision points index 777
Index 779
This page intentionally left blank

xi
Detailed contents
Contributors xxiii
Symbols and abbreviations xxv
1 Good surgical practice 1
Duties of a doctor 2
Communication skills 4
Evidence-based surgery 6
Critical appraisal 10
Audit 12
Consent 14
Death 16
End-of-life issues 18
Clinical governance 20
2 Principles of surgery 23
Terminology in surgery 24
History taking and making notes 26
Common surgical symptoms 28
Examination and investigation of the patient:
Evaluation of breast disease 30
Evaluation of the neck 32
Evaluation of the abdomen 34
Abdominal investigations 36
Evaluation of pelvic disease 38
Evaluation of peripheral vascular disease 40
Evaluation of the skin and subcutaneous tissue disease 42
Surgery at the extremes of age 44
Day case and minimally invasive surgery 46
Preoperative care:
Surgery in pregnancy 48
Surgery and the contraceptive pill 50
Surgery in endocrine disease 52

xii
DETAILED CONTENTS
Surgery and heart disease 54
Surgery and respiratory disease 58
Surgery in renal and hepatic disease 60
Surgery in neurological disease 62
Pre-optimization of the patient:
Fluid optimization 64
Nutrition in surgical patients 66
Enhanced recovery after surgery 68
Perioperative care:
Getting the patient to theatre 70
Prophylaxis—antibiotics and thromboprophylaxis 72
In-theatre preparation 74
Positioning the patient 76
Sterilization, disinfection, and antisepsis 78
Scrubbing up 79
Surgical instruments 80
Incisions and closures 82
Drains 83
Stomas 84
Knots and sutures 86
Post-operative:
Post-operative management 88
Drain management 90
Fluid management 92
Acid–base balance 94
Blood products and procoagulants 96
Transfusion reactions 98
Shock 100
Post-operative haemorrhage 102
Wound emergencies 104
Cardiac complications 106
Respiratory complications 108
Renal complications 110
Urinary complications 112
Gastrointestinal complications 114
Neurological complications 116
Haematological complications 118
Deep venous thrombosis and pulmonary embolism 120
Risk scoring 122

xiii
DETAILED CONTENTS
Critical care 124
Commonly used terms in ITU 126
Invasive monitoring 128
Ventilation and respiratory support 130
Circulatory support 132
Renal support 134
Enteral support 136
Sepsis, SIRS, MODS, and ALI 138
3 Surgical pathology 141
Cellular injury 142
Infl ammation 144
Wound healing 146
Ulcers 148
Cysts, sinuses, and fi stulas 150
Atherosclerosis 152
Thromboembolic disease 154
Gangrene and capillary ischaemia 158
Tumours 160
Carcinogenesis 162
Screening 164
Grading and staging 168
Tumour markers 170
Surgical microbiology 172
Surgically important organisms 174
Soft tissue infections 176
Blood-borne viruses and surgery 178
Bleeding and coagulation 180
Anaemia and polycythaemia 182
4 Practical procedures 185
Endotracheal intubation 186
Cardioversion 188
Defi brillation 190
Venepuncture 192
Intravenous cannulation 194
Arterial puncture and lines 196
Insertion of central venous catheter 198

xiv
DETAILED CONTENTS
Chest drain insertion 200
Management of chest drains 202
Pericardiocentesis 204
Cricothyroidotomy 206
Nasogastric tube insertion 208
Urethral catheterization 210
Suprapubic catheterization 212
Paracentesis abdominis 214
Rigid sigmoidoscopy 216
Local anaesthesia 218
Intercostal nerve block 220
5 Head and neck surgery 221
Thyroglossal cyst, sinus, and fi stula 222
Branchial cyst, sinus, and fi stula 224
Salivary calculi 226
Acute parotitis 228
Salivary gland tumours 230
Head and neck cancer 232
Facial trauma 234
Neck space infections 236
6 Breast and endocrine surgery 239
Breast cancer 240
Surgical treatment of breast cancer 242
Breast cancer screening 244
Benign breast disease 246
Acute breast pain 248
Goitre 250
Thyrotoxicosis 252
Thyroid tumours—types and features 254
Thyroid tumours—diagnosis and treatment 256
Post-thyroid surgery emergencies 258
Primary hyperparathyroidism 260
Multiple endocrine neoplasia 262
Cushing’s syndrome 264
Conn’s syndrome 266
Phaeochromocytoma 268

xv
DETAILED CONTENTS
7 Upper gastrointestinal surgery 271
Upper gastrointestinal endoscopy 272
Oesophageal motility disorders 274
Pharyngeal pouch 276
Hiatus hernia 278
Gastro-oesophageal refl ux disease 280
Oesophageal tumours 282
Peptic ulcer disease 284
Gastric tumours 286
Chronic intestinal ischaemia 288
Surgery for morbid obesity 290
Small bowel tumours 292
Acute haematemesis 294
Acute upper GI perforation 296
Acute appendicitis 298
Acute peritonitis 300
Acute abdominal pain 302
Gynaecological causes of lower abdominal pain 306
Intra-abdominal abscess 308
8 Liver, pancreatic, and biliary surgery 311
Jaundice—causes and diagnosis 312
Jaundice—management 314
Gall bladder stones 316
Common bile duct stones 318
Chronic pancreatitis 320
Portal hypertension 322
Cirrhosis of the liver 324
Pancreatic cancer 326
Cancer of the liver, gall bladder, and biliary tree 328
Acute variceal haemorrhage 330
Acute pancreatitis 332
9 Abdominal wall 335
Abdominal wall hernias 336
Inguinal hernia 338
Femoral hernia 340
Umbilical and epigastric hernias 342

xvi
DETAILED CONTENTS
Incisional hernias 344
Other types of hernia 346
Rectus sheath haematoma 347
Groin disruption 348
Acute groin swelling 350
10 Urology 353
Symptoms and signs in urology 354
Investigations of urinary tract disease 356
Urinary tract stones 358
Obstruction of the ureter 360
Benign prostatic hyperplasia 362
Stricture of the urethra 364
Scrotal swellings 366
Disorders of the foreskin 368
Common conditions of the penis 370
Erectile dysfunction 372
Adenocarcinoma of the kidney 374
Transitional cell tumours 376
Adenocarcinoma of the prostate 378
Carcinoma of the penis 380
Testicular tumours 382
Haematuria 384
Acute urinary retention (AUR) 386
Acute testicular pain 388
11 Colorectal surgery 391
Ulcerative colitis 392
Crohn’s disease 394
Other forms of colitis 396
Colorectal polyps 398
Colorectal cancer 400
Restorative pelvic surgery 402
Minimally invasive colorectal surgery 403
Diverticular disease of the colon 404
Rectal prolapse 406
Pilonidal sinus disease 408
Fistula-in-ano 410
Haemorrhoids 412

xvii
DETAILED CONTENTS
Acute anorectal pain 414
Acute rectal bleeding 416
Acute severe colitis 418
Post-operative anastomotic leakage 420
12 Paediatric surgery 423
Principles of managing paediatric surgical cases 424
Acute abdominal emergencies—overview 426
Oesophageal atresia 428
Pyloric stenosis 430
Malrotation and volvulus 432
Intussusception 434
Hirschsprung’s disease 436
Rare causes of intestinal obstruction 438
Abdominal wall defects 440
Necrotizing enterocolitis (NEC) 442
Inguinal hernia and scrotal swellings 444
Other childhood hernias 446
Prepuce (foreskin) and circumcision 448
Undescended testis 450
Solid tumours of childhood 452
Neck swellings 454
13 Paediatric orthopaedic 457
Developmental dysplasia of the hip (DDH) 458
Slipped upper femoral epiphysis (SUFE) 460
The limping child 462
The child with a fracture 464
Non-accidental injury (NAI) 466
Legg–Calvé–Perthes disease 468
Motor development 470
Club foot or congenital talipes equinovarus (CTEV) 471
Flat feet (pes planus) 472
The osteochondritides 474
14 Major trauma 477
Management of major trauma 478
Thoracic injuries 480

xviii
DETAILED CONTENTS
Abdominal trauma 482
Vascular injuries 484
Head injuries 486
15 Orthopaedic surgery 489
Examination of a joint 490
Examination of the limbs and trunk 492
Fracture healing 494
Reduction and fi xation of fractures 498
The skeletal radiograph 502
Injuries of the phalanges and metacarpals 504
Wrist injuries 508
Fractures of the distal radius and ulna 510
Fractures of the radius and ulnar shaft 512
Fractures and dislocations around the elbow in children 514
Fractures of the humeral shaft and elbow in adults 518
Dislocations and fracture dislocations of the elbow 522
Fractures around the shoulder 524
Dislocations of the shoulder region 526
Fractures of the ribs and sternum 530
Fractures of the pelvis 532
Femoral neck fractures 536
Femoral shaft fractures 538
Fractures of the tibial shaft 540
Fractures of the ankle 544
Fractures of the tarsus and foot 546
Injuries and the spinal radiograph 550
Spinal injuries 554
Acute haematogenous osteomyelitis 558
Chronic osteomyelitis 560
Septic arthritis 562
Peripheral nerve injuries 564
Brachial plexus injuries 566
Osteoarthrosis (osteoarthritis) 568
Carpal tunnel syndrome 570
Ganglion 572
Bone tumours 574
Low back pain 578
Paget’s disease (osteitis deformans) 582
The great toe 584

xix
DETAILED CONTENTS
Arthroplasty 586
Useful reading 588
16 Plastic surgery 589
Suturing wounds 590
Skin grafts 594
Surgical fl aps 596
Management of scars 598
Excision of simple cutaneous lesions 600
Skin cancer 602
Burns: assessment 604
Burns: management 606
Soft tissue hand injuries 610
Hand infections 612
Dupuytren’s disease 614
Breast reduction 616
Breast augmentation 617
Breast reconstruction 618
17 Cardiothoracic surgery 619
Basics 620
Principles of cardiac surgery 622
Coronary artery disease 626
Valvular heart disease 628
Cardiothoracic ICU 630
Lung cancer 632
Pleural effusion 634
Pneumothorax 636
Mediastinal disease 638
18 Peripheral vascular disease 641
Acute limb ischaemia 642
Chronic upper limb ischaemia 644
Chronic lower limb ischaemia 647
Intermittent claudication 648
Critical limb ischaemia 650
Aneurysms 652
Ruptured abdominal aortic aneurysm 654

xx
DETAILED CONTENTS
Vascular developmental abnormalities 656
Carotid disease 658
The diabetic foot 660
Amputations 662
Vasospastic disorders 664
Varicose veins 666
Deep venous thrombosis 668
Thrombolysis 670
Complications in vascular surgery 672
19 Transplantation 675
Basic transplant immunology 676
Immunosuppression and rejection 678
Transplant recipients 682
Transplant donors 684
Heart and lung transplantation 690
Kidney transplantation 692
Pancreas and islet transplantation 694
Liver transplantation 696
Small bowel transplantation 698
20 Surgery in tropical diseases 701
Medicine in the tropics 702
Typhoid 704
Amoebiasis and amoebic liver abscess 706
Anaemias in the tropics 708
Malaria 710
Schistosomiasis (bilharziasis) 712
Filariasis 714
Hydatid disease 716
Ascariasis 718
Leishmaniasis 719
Trypanosomiasis 720
Tuberculosis in the tropics 722
Leprosy (‘Hansen’s disease’) 724
Guinea worm infestation 726
Threadworms 727
Mycetoma (madura foot) 728

xxi
DETAILED CONTENTS
21 Common operations 729
Diagnostic laparoscopy 730
Principles of laparotomy 732
Cholecystectomy 734
Appendicectomy 736
Inguinal hernia repair 738
Perforated peptic ulcer repair 740
Haemorrhoid surgery 742
Pilonidal sinus excision (Bascom II) 744
Femoral embolectomy 746
Right hemicolectomy 748
Stoma formation 750
Wide local excision—breast 752
Below knee amputation 754
22 Eponymous terms and rarities 757
Anatomy and physiology key revision points index 777
Index 779
This page intentionally left blank

xxiii
Contributors
Alex Acornley
Consultant Orthopaedic
Surgeon,
Airedale Hospital NHS
Foundation Trust, West
Yorkshire, UK
Anil Agarwal
Consultant General and Colorectal
Surgeon,
North Tees and Hartlepool NHS
Trust, University Hospital of
Hartlepool, UK
Khalid A. Al-Hureibi
Specialist Registrar,
Department of General Surgery,
Lister Hospital, Stevenage, UK
John Asher
Consultant Transplant Surgeon,
Transplant Unit, Western
Infi rmary, Glasgow, UK
David Chadwick
Consultant Urological Surgeon,
The James Cook University
Hospital, Middlesbrough, UK
Lucy Cogswell
Specialist Registrar,
Department of Plastic &
Reconstructive Surgery, John
Radcliffe Hospital, Oxford, UK
J. H. Dark
Consultant Cardiothoracic
Surgeon,
Freeman Hospital, Newcastle upon
Tyne, UK
Richard P. Jeavons
Specialist Registrar,
Trauma and Orthopaedics
(Northern Deanery), Department
of Trauma and Orthopaedics,
University Hospital of North Tees,
Stockton, UK
Vijay Kurup
Consultant Breast and Endocrine
Surgeon,
University Hospital of North Tees,
Stockton on Tees, UK
Jamie Lyall
Consultant Head and Neck
Surgeon (Maxillofacial),
Surgical Division, James Cook
University Hospital Trust,
Middlesbrough, Queen Margaret
Hospital, Dunfermline, UK
Alan Middleton
Consultant Orthopaedic Surgeon,
Department of Hand and Wrist
Surgery, University Hospital of
North Tees, Stockton, UK
Rob Milligan
ST3 General Surgery, Northern
Deanery, UK
Sandrasekeram
Parameswaran
General Surgeon,
Cold Lake Healthcare Centre,
Visiting Surgeon, Canadian forces
base, 4 Wing, Cold Lake, Alberta,
Canada

xxiv
CONTRIBUTORS
Lakshmi Parameswaran
Senior House Offi cer,
Mater Misericordiae University
Hospital, Dublin, Ireland
Saumitra Rawat
Consultant Surgeon,
Macclesfi eld District General
Hospital, UK
Andreas Rehm
Consultant Paediatric Orthopaedic
and Trauma Surgeon,
Depatment of Orthopaedic and
Trauma Surgery, Addenbrooke’s
Hospital, Cambridge University
Hospitals NHS Trust, Cambridge,
UK
David Talbot
Consultant Transplant and
Hepatobiliary Surgeon,
Transplant Institute, Freeman
Hospital, Newcastle. Visiting
Professor, University of Sunderland.
Reader, University of Newcastle
upon Tyne, UK
Mark Whyman
Consultant General and Vascular
Surgeon,
Department of Surgery,
Cheltenham General Hospital,
Cheltenham, UK

xxv
Symbols and abbreviations
d decreased
i increased
n normal
l leading to
warning
2 important
3 don’t dawdle
b cross reference
♀ female
♂ male
p primary
s secondary
< less than
> more than
t equal to or greater than
d equal to or less than
% per cent
7 approximately
8 approximately equals to
α alpha
β beta
°C degree Celsius
AAA abdominal aortic aneurysm
ABG arterial blood gas
A&E Accident and Emergency Department
ABPI ankle–brachial pressure index
ACAS Asymptomatic Carotid Artery Stenosis Study
ACE angiotensin-converting enzyme
ACh acetylcholine
AChE acetylcholinesterase
ACJ acromioclavicular joint
ACL anterior cruciate ligament
ACST Asymptomatic Carotid Surgery Trial
ACTH adrenocorticotropic hormone
ADH antidiuretic hormone
ADP adenosine diphosphate
xxvi
AF atrial fi brillation
AFP alpha-fetoprotein
AIDS acquired immunodefi ciency syndrome
AIN anal intraepithelial neoplasia
AKA above-knee amputation
ALI acute lung injury
ALS advanced life support
a.m. ante meridiem
amp ampere
AMPLE allergy/medication/past medical history/last meal/events
of the incident
ANDI abnormalities of normal development and involution
(of breast)
ANF antinuclear factor
APA aldosterone-producing adenoma
APACHE Acute Physiology And Chronic Health Evaluation
APC antigen-presenting cell or argon plasma coagulation
APER abdominoperineal resection
APTR activated partial thromboplastin time ratio
APTT activated partial thromboplastin time
AR aortic regurgitation
ARDS acute respiratory distress syndrome
ARR absolute risk reduction or aldosterone/renin ratio
5-ASA 5-aminosalicyclic acid
ASB assisted spontaneous breathing
ATG anti-thymocyte globulin
ATLS advanced trauma life support
ATP adenosine triphosphate
AUR acute urinary retention
AV arteriovenous or atrioventricular
AVM arteriovenous malformation
AVN avascular necrosis
AVS adrenal venous sampling
AXR abdominal X-ray
BCC basal cell carcinoma
BCG Bacillus Calmette–Guérin
BCR B-cell receptor
B-HCG beta-human chorionic gonadotrophin
BiPAP biphasic positive airway pressure
BKA below-knee amputation
SYMBOLS AND ABBREVIATIONS
xxvii
BMI body mass index
BMJ British Medical Journal
BNF British National Formulary
BP blood pressure
BPH benign prostatic hyperplasia
BS blood sugar
BSA body surface area
BXO balanitis xerotica obliterans
Ca calcium
CABG coronary artery bypass graft
CAD coronary artery disease
CAPD continuous ambulatory peritoneal dialysis
CAVH continuous arteriovenous haemofi ltration
CBP cardiopulmonary bypass
CCF congestive cardiac failure
CD cellular differentiation (molecule)
CDH congenital dysplasia of the hip
CDT Clostridium diffi cile toxin
CEA carcinoembryonic antigen or carotid endarterectomy
CF cystic fi brosis
CFU colony-forming unit
CI confi dence interval
Cl chloride
CLI critical limb ischaemia
cm centimetre
CMI cell-mediated immune (reaction)
CMV cytomegalovirus or controlled mechanical ventilation
CNI calcineurin inhibitor
CNS central nervous system
CNST criminal negligence scheme for Trusts
CO cardiac output
CO
2
carbon dioxide
COAD chronic obstructive airway disease
COC combined oral contraceptive
COPD chronic obstructive pulmonary disease
CPAP continuous positive airway pressure
CPB cardiopulmonary bypass
C,P,&O cysts, parasites, and ova
CPR cardiopulmonary resuscitation
Cr creatinine
SYMBOLS AND ABBREVIATIONS
xxviii
CRCa colorectal cancer
CRP C-reactive protein
CSF cerebrospinal fl uid
C-spine cervical spine
CT computerized tomography
CTA CT angiography
CTPA computerized tomography pulmonary angiography
Cu copper
CV central venous
CVA cerebrovascular accident
CVP central venous pressure
CVVH continuous venovenous haemofi ltration
Cx circumfl ex
CXR chest X-ray
2D two-dimensional
3D three-dimensional
DA dopamine
DALM dysplasia-associated lesion or mass
DBD donor after brainstem death
DC direct current
DCD donor after circulatory death
DCIS ductal carcinoma in situ
DDAVP 1-deamino-8-D-arginine vasopressin
DDH developmental dysplasia of the hip
DHS dynamic hip screw
DHT dihydrotestosterone
DIC disseminated intravascular coagulation
DIEP deep inferior epigastric perforator (fl ap)
DIPJ distal interphalangeal joint
dL decilitre
DM diabetes mellitus
DMSA dimercaptosuccinate
DNA deoxyribonucleic acid
DNR do not resuscitate
DOH Department of Health
DP distal phalanx or diastolic pressure
2,3-DPG 2,3-diphosphoglycerate
DPL diagnostic peritoneal lavage
DRUJ distal radioulnar joint
DSA digital subtraction angiography
SYMBOLS AND ABBREVIATIONS
xxix
DTPA diethylene triamine pentaacetic acid
DVT deep venous thrombosis
EBV Epstein–Barr virus
ECF extracelllular fl uid
ECG electrocardiogram
ECST European Cardiac Surgery Trial
ED erectile dysfunction
e.g. exempli gratia (for example)
ELISA enzyme-linked immunosorbent assay
EMD electromechanical delay
EMG electromyography
EMR endocospic mucosal resection
EOCP (o)estrogen-containing contraceptive pill
EPL extensor pollicis longus
EPO erythropoietin
ER (o)estrogen receptor
ERAS enhanced recovery after surgery
ERCP endoscopic retrograde cholangiopancreatography
ES endoscopic sphincterotomy
ESD endoscopic submucosal dissection
ESR erythrocyte sedimentation rate
ESWL extracorporeal shock wave lithotripsy
ET endotracheal tube
EUA examination under anaesthetic
EUS endoscopic ultrasound
EVAR endovascular aneurysm repair
EVLT endovenous laser therapy
FAP familial adenomatous polyposis
FAST focused abdominal sonography for trauma
FBC full blood count
FDP fl exor digitorum profundus
FDS fl exor digitorum superfi cialis
FEV
1 forced expiratory volume in 1 second
FFP fresh frozen plasma
FiO
2 fraction of oxygen in inspired air
FLC fi brolamellar carcinoma
FNAB fi ne needle aspiration biopsy
FNAC fi ne needle aspiration cytology
FPL fl exor pollicis longus
FSH follicle-stimulating hormone
SYMBOLS AND ABBREVIATIONS
xxx
5-FU 5-fl uorouracil
g gram
G gauge
GA general anaesthetic
GANT gastrointestinal autonomic nerve tumour
GCS Glasgow coma scale
GFR glomerular fi ltration rate
GGT gamma glutamyl transferase
GH growth hormone
GI gastrointestinal
GIP gastric inhibitory polypeptide
GIST gastrointestinal stromal tumour
GMC General Medical Council
GORD gastro-oesophageal refl ux
GP general practitioner
GTN glyceryl trinitrate
Gy gray
h hour
HAT hepatic artery thrombosis
Hb haemoglobin
HCC hepatocellular carcinoma
HCG human chorionic gonadotrophin
HCO
3
bicarbonate
HCV hepatitis C virus
HDU high dependency unit
HES hydroxyethyl starch
HGV heavy goods vehicle
HHD handheld Doppler
HIDA hepatobiliary iminodiacetic acid
HIT heparin-induced thrombocytopenia
HITT heparin-induced thrombocytopenia and thrombosis
HIV human immunodefi ciency virus
HLA human leucocyte antigen
HMMA 4-hydroxy-3-methoxymandelic acid
HNPCC hereditary non-polyposis colorectal cancer
H
2
O water
HPV human papilloma virus
HR heart rate
HRT hormone replacement therapy
HSV herpes simplex virus
SYMBOLS AND ABBREVIATIONS
xxxi
5-HT 5-hydroxytryptamine (serotonin)
HTLV human T-cell lymphocytotrophic virus
HVA homovanillic acid
IABP intra-aortic balloon pump
IC intermittent claudication
ICA internal carotid artery
ICD intracardiac defi brillator
ICP intracranial pressure
ICU intensive care unit
i.e. id est (that is)
IF intrinsic factor
IGF insulin growth factor
IHD ischaemic heart disease
IM intramuscular
IMA inferior mesenteric artery
IMHS intramedullary hip screw
in inch
INPV intermittent negative pressure ventilation
INR international normalized ratio
IPJ interphalangeal joint
IPPV intermittent positive pressure ventilation
IPSS international prostate symptom score
ITA internal thoracic artery
ITU intensive treatment unit
IU international unit
IV intravenous
IVU intravenous urogram
J joule
JCHST Joint Committee on Higher Surgical Training
JVP jugular venous pressure
K potassium
kcal kilocalorie
KCl potassium chloride
kg kilogram
kPa kilopascal
KUB kidneys/ureters/bladder
L litre
LA local anaesthetic or left atrium/atrial
LAD left anterior descending (artery)
LAP left atrial pressure
SYMBOLS AND ABBREVIATIONS
xxxii
LatexAT latex agglutination test
lb pound
LDH lactate dehydrogenase
LDL low density lipid
LESS laparoscopic and endoscopic single site (surgery)
LFT liver function test
LH luteinizing hormone
LHRH luteinizing hormone releasing hormone
Li lithium
LIF left iliac fossa
LITA left internal thoracic artery
LMS left main stem
LMWH low molecular weight heparin
LOS lower oesophageal sphincter
LSV long saphenous vein
LUQ left upper quadrant
LUTS lower urinary tract symptoms
LV left ventricle
LVEDP left ventricular end-diastolic pressure
LVEDV left ventricular end-diastolic volume
LVF left ventricular failure
m metre
MAG3
99m
Tc-mercaptoacetyltriglycine
MALT mucosa-associated lymphoid tissue
MAO monoamine oxidase
MAP mean arterial pressure
MCPJ metacarpophalangeal joint
MCRP magnetic resonance cholangiopancreatography
M,C,&S microscopy, culture, and sensitivity
MCV mean cell volume
MDT multidisciplinary team
MEN multiple endocrine neoplasia
mEq milliequivalent
mg milligram
Mg magnesium
MHC major histocompatibility complex
MHz megahertz
MI myocardial infarction
MIBG meta-iodo-benzyl-guanidine
min minute
SYMBOLS AND ABBREVIATIONS
xxxiii
MIP minimally invasive parathyroidectomy
MIST mechanism of injury/injuries identifi ed/(vital)signs at
scene/treatment administered
mL millilitre
MMF mycophenolate mofetil
mmHg millimetre mercury
mmol millimole
MMR mismatch repair (genes)
MMV mandatory minute ventilation
Mn manganese
MODS multiple organ dysfunction syndrome
mph mile per hour
MR mitral regurgitation
MRA magnetic resonance angiography
MRC Medical Research Council (scale)
MRCP magnetic resonance cholangiopancreatogram
MRI magnetic resonance imaging
ms millisecond
MRSA methicillin (or multiply) resistant Staphylococcus aureus
MSU midstream urine
MTC medullary thyroid carcinoma
mTOR mammalian target of rapamycin
MTP mid-thigh perforator
MTPJ metatarsophalangeal joint
MUA manipulation under anaesthesia
MV mitral valve
Na sodium
NA noradrenaline (norepinephrine)
NaHCO
3
sodium bicarbonate
NAI non-accidental injury
NASCET North American Symptomatic Carotid Endarterectomy Trial
NBM nil by mouth
NCEPOD National Confi dential Enquiry into Patient Outcomes
and Death
NEC necrotizing enterocolitis
ng nanogram
NG nasogastric
NGT nasogastric tube
NHS National Health Service
NICE National Institute for Health and Clinical Excellence
SYMBOLS AND ABBREVIATIONS
xxxiv
NIPPV non-invasive intermittent positive pressure ventilation
NK natural killer (cell)
NNT number needed to treat
N
2
O nitrous oxide
NSAID non-steroidal anti-infl ammatory drug
NSF National Service Framework
NSGCT non-seminomatous germ cell tumour
NSPCC National Society for the Prevention of Cruelty to Children
NSTEMI non-ST segment elevation myocardial infarction
NVB neurovascular bundle
nvCJD new variant Creutzfeldt–Jakob disease
NYHA New York Heart Association
O
2
oxygen
OCP oral contraceptive pill
od omne in die (once a day)
OGD oesophago-gastro-duodenoscopy
OM obtuse marginal
OPT orthopantomogram
ORIF open reduction with internal fi xation
PA pulmonary artery or posterior-anterior
PAC plasma aldosterone concentration
PaCO
2 arterial carbon dioxide tension
PAF platelet-activating factor
PAL primary hyperaldosteronism
PaO
2 arterial oxygen tension
PAP pulmonary artery pressure or placental alkaline phosphatase
PAS patient administration system
PAWP pulmonary artery wedge pressure
PCA patient-controlled analgesia
PCI percutaneous coronary intervention
PCNL percutaneous nephrolithotomy
PCO
2 carbon dioxide tension
PCR polymerase chain reaction
PCV packed cell volume or pressure control ventilation
PDA posterior descending artery
PDGF platelet-derived growth factor
PE pulmonary embolism
PEEP positive end-expiratory pressure
PEFR peak expiratory fl ow rate
PEG percutaneous endoscopic gastrostomy
SYMBOLS AND ABBREVIATIONS
xxxv
PEP post-exposure prophylaxis
PET positron emission tomography
PGME Postgraduate medical education
PH portal hypertension
PHPT primary hyperparathyroidism
PICC peripherally inserted central venous catheter
PID pelvic infl ammatory disease
PIPJ proximal interphalangeal joint
PLL posterior longitudinal ligament
PMETB Postgraduate Medical Education and Training Board
PMN polymorphonuclear neutrophil
PO orally (per os)
PO
2 oxygen tension
PO
4 phosphate
POSSUM Physiologic and Operative Severity Score for the
enumeration of Mortality and morbidity
PPH procedure for prolapse and haemorrhoids
PPI proton pump inhibitor
PPN peripheral parenteral nutrition
PR per rectum
prn pro re rata (as required)
PS pressure support
PSA prostate-specifi c antigen
PSARP posterior sagittal anorectoplasty
PT prothrombin time
PTC percutaneous transhepatic cholangiogram
PTE pulmonary thromboembolism
PTEF polytetrafl uoroethylene
PTH parathyroid hormone
PTLD post-transplant lymphoproliferative disorder
PTT partial prothrombin time
PUJ pelviureteric junction
PV per vagina
PVD peripheral vascular disease
PVR pulmonary vascular resistance
PVRI pulmonary vascular resistance index
qds quater die sumandus (four times a day)
RA right atrial or rheumatoid arthritis
RAP right atrial pressure
RCA right coronary artery
SYMBOLS AND ABBREVIATIONS
RCT randomized controlled trial
Rh rhesus
rhTSH recombinant human thyroid-stimulating hormone
RIF right iliac fossa
RLN recurrent laryngeal nerve
RNA ribonucleic acid
RR relative risk or risk ratio
RRR relative risk reduction
RSTL relaxed skin tension line
RTA road traffi c accident
RUQ right upper quadrant
RV right ventricle
s second
SA sinoatrial (node)
SAC specialist advisory committee
SaO
2 arterial oxygen saturation
SBE subacute bacterial endocarditis
SC subcutaneous
SCAT sheep cell agglutination test
SCC squamous cell carcinoma
SCI spinal cord injury
SCM sternocleidomastoid
SD standard deviation
SEMS self-expanding metal stenting
SEPL subfascial endoscopic perforator ligation
SFA superfi cial femoral artery
SFJ saphenofemoral junction
SILS single incision laparoscopic surgery
SIMV synchronized intermittent mandatory ventilation
SIRS systemic infl ammatory response syndrome
SL sublingual
SLE systemic lupus erythematosus
SMA superior mesenteric artery
SNP sodium nitroprusside
SPJ saphenopopliteal junction
spp species
STD sodium tetradecyl sulphate
STEMI ST segment elevation myocardial infarction
STI sexually transmitted infection
SUFE slipped upper femoral epiphysis
xxxvi
SYMBOLS AND ABBREVIATIONS
xxxvii
SV stroke volume
SVC superior vena cava
SVI stroke volume index
SvO2 percentage oxygen saturation of mixed venous haemoglobin
SVR systemic vascular resistance
SVRI systemic vascular resistance index
SVT supraventricular tachycardia
T
3 triiodothyronine
T
4 thyroxine
TAP transversus abdominis percutaneous
TAPS transabdominal pre-peritoneal surgery
TB tuberculosis
TBSA total body surface area
TCC transitional cell carcinoma
TCR T-cell receptor
TCT transitional cell tumour
tds ter die sumendus (three times a day)
TEDS thromboembolic deterrent stockings
TEMS transanal endoscopic microsurgery
TEPS totally extra-peritoneal surgery
TFCC triangular fi brocartilage complex
TFT thyroid function test
TGF transforming growth factor
THR total hip replacement
TIA transient ischaemic attack
TIBC total iron binding capacity
TIPS transjugular intraparenchymal portosystemic shunt/stent
TKA through-knee amputation
TKR total knee replacement
TLSO thoracolumbar spine orthosis
TMT tarsometatarsal
TNF tumour necrosis factor
TNM tumour nodes metastasis (cancer staging)
tPA tissue plasminogen activator
TPN total parenteral nutrition
TRAM transverse rectus abdominis myocutaneous (fl ap)
TRUS transrectal ultrasound
H thyroid-stimulating hormone
TT thrombin time or total thyroidectomy
TTE transthoracic echocardiogram
SYMBOLS AND ABBREVIATIONS

xxxviii
TUIP transurethral incision in the prostate
TURP transurethral resection of the prostate
TVF transversalis fascia
U (international) units
UADT upper aerodigestive tract
U&E urea and electrolytes
UC ulcerative colitis
UCL ulnar collateral ligament
UFH unfractionated heparin
UK United Kingdom
UOS upper oesophageal sphincter
USA United States of America
UTI urinary tract infection
UV ultraviolet
V volts
VACTERL vertebral defects/anorectal atresia/cardiac defects/
tracheo-oesophageal fi stula ± (o)esophageal atresia/
renal anomalies/limb defects
VAD ventricular assist device
VATS video-assisted thoracoscopic surgery
VF ventricular fi brillation
VHL von Hippel–Lindau (disease)
VIP vasoactive inhibitory polypeptide
VMA vanillylmandelic acid
VQ ventilation/perfusion (scan)
VRE vancomycin-resistant Enterococcus
VT ventricular tachycardia
VTE venous thromboembolism
VWF von Willebrand factor
WCC white cell count
WHO World Health Organization
y year
Symbols and abbreviat
SYMBOLS AND ABBREVIATIONS

CHAPTER 1 Good surgical practice
2
Duties of a doctor
The General Medical Council (GMC) lists the duties of a doctor in its docu-
ment Good medical practice.
1
The duties can be thought of under three head-
ings (the 3 Cs): competency, communication, correctness (or probity).
Competency
• Keep your professional knowledge and skills up to date.
• Recognize the limits of your professional competence.
Perform an adequate assessment of the patient’s conditions, based •
on the history and symptoms and, if necessary, an examination.
Arrange investigations or treatment where necessary.•
Take suitable and prompt action when necessary.•
Refer the patient to another practitioner when indicated.•
Be willing to consult colleagues.•
Keep clear, accurate, legible, and contemporaneous patient records •
that report relevant clinical fi ndings, decisions made, information
given to patients, and any drugs or other treatment prescribed.
Keep colleagues well informed when sharing the care of patients.•
Provide the necessary care to alleviate pain and distress whether or •
not curative treatment is possible.
Prescribe drugs or treatment, including repeat prescriptions, only •
where you have adequate knowledge of the patient’s health and
medical needs. You must neither give or recommend to patients
any investigation or treatment that you know is not in their best
interests, nor withhold appropriate treatments or referral.
Report adverse drug reactions as required under the relevant •
reporting scheme and cooperate with requests for information
from organizations monitoring the public health.
Take part in regular and systematic medical and clinical audit, •
recording data honestly, and respond to the results of audit to
improve your practice, e.g. by undertaking further training.
Communication
• Treat every patient politely and considerately.
• Respect patients’ dignity and privacy.
• Listen to patients and respect their views.
• Give patients information in a way they can understand.
Correctness (or probity)
• Make the care of your patient your fi rst concern.
• Respect the rights of patients to be involved in decisions.
• Be honest and trustworthy.
• Respect and protect confi dential information.
• Make sure your personal beliefs do not prejudice your patients’ care.
• Act quickly to protect patients from risk if you have good reason to
believe that you or a colleague may not be fi t to practise.
• Avoid abusing your position as a doctor.
• Work with colleagues in the ways that best serve patients’ interests.
• In an emergency, wherever it may arise, you must offer anyone at risk
the assistance you could reasonably be expected to provide.

DUTIES OF A DOCTOR
3
Confi dentiality
Patients have a right to expect that information about them will be held
in confi dence by their doctors. Confi dentiality is central to trust between
doctors and patients. Without assurances about confi dentiality, patients
may be reluctant to give doctors the information they need in order to
provide good care. The GMC states that if you are asked to provide infor-
mation about patients, you must:
• Inform patients about the disclosure or check that they have already
received information about it.
• Anonymize data where unidentifi able data will serve the purpose (this
includes your surgical logbook).
• Keep disclosures to the minimum necessary.
• Keep up to date with and observe the requirements of statute and
common law, including data protection legislation.
Daily practice
• When you are responsible for personal information about patients,
you must make sure that it is effectively protected against improper
disclosure at all times (e.g. password-protected electronic fi les).
• Many improper disclosures are unintentional. You should not discuss
patients where you can be overheard or leave patients’ records,
either on paper or on screen, where they can be seen by other
patients, unauthorized health care staff, or the public. You should take
all reasonable steps to ensure your consultations with patients are
private.
• Patients have a right to information about the health care services
available to them presented in a way that is easy to follow and use.
Special circumstances
If in any doubt, contact your medical defence union for advice.
• You must disclose information to satisfy a specifi c statutory
requirement, such as notifi cation of a known or suspected
communicable disease. Inform patients about such disclosures,
wherever that is practicable, but their consent is not required.
• You must also disclose information if ordered to do so by a judge or
presiding offi cer of a court. You should object if attempts are made to
compel you to disclose what appear to you to be irrelevant matters.
• You must not disclose personal information to a third party, such as
a solicitor, police offi cer, or offi cer of a court, without the patient’s
express consent, except when:
The patient is not competent to give consent.•
Reasonable efforts to trace patients are unlikely to be successful.•
The patient has been or may be violent, or obtaining consent •
would undermine the purpose of the disclosure (e.g. disclosures in
relation to crime).
Action must be taken quickly (e.g. in the detection or control of •
outbreaks of some communicable diseases) and there is insuffi cient
time to contact patients.
Reference
1 GMC (2012). Good medical practice. Available at: M http://www.gmcuk.org/guidance/good_
medical_practice.asp

CHAPTER 1 Good surgical practice
4
Communication skills
Communicating with patients and relatives
When
• During admission and before discharge.
• On ward rounds.
• During clinical examinations and procedures.
• When the results of treatments are known and management changes.
• In outpatient clinics.
Where
2 Maintain the patient’s privacy. This is particularly important on an open
ward. Knock on doors and close them after you. Draw the curtains round
the bed. Ask a nurse to accompany you, particularly if you are explain-
ing something complex or breaking bad news. They will have to answer
the patients’ and relatives’ questions when you have left the ward or
clinic room.
How
• Know your facts. Are you giving the right diagnosis to the right
patient? Are you equipped to consent a patient for the surgical
procedure?
• Sit at the same level as the person to whom you are talking, maintain
appropriate eye contact, and introduce yourself.
• Find out what the patient knows and what they are expecting.
• Listen. The patient’s own knowledge, state of mind, and ability to grasp
concepts will dictate both how and how much you explain.
• Tell the truth. Know your facts, be sensitive to what the patient may
not want to know at this stage, and do not lie.
• Avoid jargon. ‘Chronic’ may simply mean ‘longstanding’ to you; to
most patients, it means ‘severe’.
• Avoid vague terms. Try to describe risk quantitatively, ‘a 1 in a
hundred chance’, rather than qualitatively, ‘a small risk’.
• Check that the patient understands. Don’t assume that they do.
• Help the patient to remember. Use information booklets, draw
diagrams, write instructions down.
• Maintain a professional relationship. Never allow your personal likes,
dislikes, and prejudices to hamper your clinical skills.
Breaking bad news
• Is there a relative or friend whom the patient might wish to have with
them, who may be a source of emotional support as well as being
better able to retain information?
• Know what options, if any, are available. If a cancer is inoperable, is
chemotherapy planned? If an operation is cancelled, when is the next
date?
• Do not be afraid to stop to allow the patient time to gather their
thoughts and emotions, and recommence at a later time.
• Do not mistake numbness for calm acceptance and try not to take
anger personally unless the bad news is actually your fault.

COMMUNICATION SKILLS
5
Communicating with nurses
• Introduce yourself on arrival to the staff nurse in charge.
• Establish early on which nurses are experienced. The help you get
from them will be different from the questions you get from others.
• In theatre, scrub nurses are not the enemy. Your inexperience is.
• Try to remember all their names as they will remember yours.
• Do ward work effi ciently. Recognize how important it is for the
smooth running of the ward that your ward rounds, note-keeping,
prescriptions, and discharge letters are timely and accurate.
• Let the nurses know when you are going for lunch, teaching, or sleep.
If they can discuss problems now, it will save you being paged later.
• Do an evening ward round to check on problem patients and drug
requirements—your sleep is less likely to be constantly interrupted.
Communication with hospital doctors
• Don’t refer without fi rst asking your consultant or registrar.
• When making requests for clinical consultations, write a concise, but
clear letter in the notes to the appropriate clinician.
• When asked to see a patient, go the same day, write your opinion
in the case notes, stating clearly what you recommend, and always
discuss it with the seniors on your own fi rm.
• If a preoperative patient is complex or has signifi cant comorbidity,
contact the appropriate anaesthetist. They will help you ensure that
the patient is adequately prepared for surgery.
Communication with general practitioners (GPs)
The GP has usually looked after your patient for years and, however
inspired your diagnostic or operating skills, they will be there to sort out
all the complications that are hidden from you once the patient is dis-
charged. They often know your consultant well. So think!
• Telephone the GP in the case of a death of a patient, if you
unexpectedly admit a patient, or to help with a diffi cult discharge.
• Write useful, legible discharge summaries. What would you want
to know if you were going to have to wait 4 weeks for the typed
discharge letter to arrive—at an absolute minimum, the date and name
of the operation, post-operative complications, and plan.
• Keep clinic letters clear and concise.
Radiology and laboratory colleagues
• Know exactly how the investigation will change your management.
• If there is doubt about the correct investigation, telephone for advice.
• Complete request forms correctly and include clinical data. It can make
a big difference, particularly if you have requested the wrong test.
Administration
• Introduce yourself to your consultant’s secretary early, fi nd out how
they like things run, and then run things their way: they will usually
have more than typing input on your reference.
• Produce GMC, defence union, occupational health, holiday, and study
leave paperwork with good grace. They are mostly legal requirements
and being rude won’t change that.

CHAPTER 1 Good surgical practice
6
Evidence-based surgery
Summarizing simple data
This pattern of results is called a normal or Gaussian distribution: the curve
is a symmetrical bell-shaped curve. Height, weight, age, serum sodium,
and blood pressure (BP) are other examples of normally distributed data
(see Table 1.1).
• The mean is the same as the average: add up every result and divide by
the number of results. The average Hb here is 11.1g/dL.
• The standard deviation (SD) is a measure of how spread out the values
are: result – mean = its deviation.
√((sum of deviations
2
/(sample size – 1)) = SD. Here SD = 1.6g/dL.
• With normally distributed data, the mean ± 1 SD includes 68% of
observations; ± 2 SD includes 95%; ± 3 SD includes 99%.
This pattern of results is called a skewed distribution. Post-operative
blood loss (see Table 1.2), length of stay, and survival all show skewed
distributions.
• 2 Don’t use mean and SD to summarize skewed data.
• The mean blood requirement, which is skewed to 8U of blood
because of one outlier (*), is useful for planning budgets.
• The best summary statistic for skewed data is the median (2U of
blood) which is the value exactly halfway through the sample.
• The interquartile range is what the middle 50% of observations were
(1–2U here) and should be used instead of SD when summarizing
skewed data.
Table 1.1 Auditing preoperative Hb in 100 patients
Hb (g/dL) No. of patients Hb (g/dL) No. of patients
7–7.9 1 11–11.9 36
8–8.9 3 12–12.9 9
9–9.9 9 13–13.9 4
10–10.9 37 14–14.9 2
Table 1.2 Auditing post-operative blood transfusions in 100 patients
Units of blood No. of patients Units of blood No. of patients
0 1 45
1 34 5–10 1
2 41 10–20 0
3 17 20–30 1*
* Outlier.
EVIDENCE-BASED SURGERY
7
Tests (see Table 1.3)
Sensitivity (a/(a+c)) A measure of how good the test is at correctly iden-
tifying a positive result (>98% is very sensitive). If a very sensitive test is
negative, it rules the condition out (sign out).
Specifi city (d/(b+d)) A measure of how good the test is at correctly iden-
tifying a negative result (>98% is very sensitive). If a very specifi c test is
negative, it rules the condition in (spin).
Likelihood ratio This is the chance that a person testing positive has the
disease, divided by the chance that a person testing positive doesn’t have
the disease, or sensitivity/(1 – specifi city). A likelihood ratio >10 is large and
represents an almost conclusive increase in the likelihood of disease, <0.1
is an almost conclusive decrease, and 1 signifi es no change.
Treatments and hazards (see Table 1.4)
Absolute risk reduction (ARR) a/(a+b) – c/(c+d) This is the difference in
the event rate between the control and the exposed group. It refl ects the
prevalence of a disease and the potency of a treatment or hazard.
Relative risk or risk ratio (RR) a/(a+b)/c/(c+d) The event rate in the
exposed group divided by the event rate in the control group. Used in
randomized controlled trials (RCTs) and cohort studies. It is not affected
by the prevalence of a disease.
Relative risk reduction (RRR) (a/(a + b) – c/(c + d))/(c/c + d) ARR divided
by the control event rate. It refl ects disease prevalence.
Number needed to treat (NNT) 1/ARR The number of people who must
be treated to prevent one event.
Odds ratio This is the odds of an exposed person having the condition
divided by the odds of the control group having the condition. If the event
is rare, it approximates to relative risk. Odds ratios are less intuitive than
relative risk, but they are used because they are:
• Usually larger.
• Mathematically versatile.
Table 1.4 Risk Assessment
Outcome event No outcome event
Exposure a b
No exposure (control) c d
Table 1.3 Sensitivity
Disease present No disease
Test is positive a b
Test is negative c d

CHAPTER 1 Good surgical practice
8
• Always used in case control studies and appear in meta-analyses of
case control studies.
• The basis of logistic regression analysis.
Statistical signifi cance
• Studies are designed to disprove the null hypothesis that fi ndings are
due to chance.
• The p-value is the probability of a study rejecting the null hypothesis if
it were true (a type I error), i.e. fi nding a difference where none exists.
• Statistical signifi cance is commonly taken as a less than 1 in 20 chance
of this happening, i.e. p <0.05.
• Power is the probability of detecting an association if one exists.
Underpowered trials contain too few patients and may make type
II errors, accepting the null hypothesis when it is false, i.e. fi nding no
difference where one does exist.
• 95% confi dence intervals, derived from the mean and SD, are the range
of results predicted if the study were repeated 95 times.

EVIDENCE-BASED SURGERY
9
Other useful terms
Censored data Essentially incomplete data, usually due to variable lengths
of follow-up. Common in surgical studies because 1) some patients will
have been lost to follow-up and 2) patients will have shorter follow-up
where they had operations more recently in a study.
Actuarial and Kaplan–Meier survival Two methods used to calculate the
percentage of study patients that survive a specifi ed time after an opera-
tion when a study provides censored data.
Survival curves Usually not curves. A linear graph, with percentage sur-
vival (or freedom from a complication) on the x-axis and time on the
y-axis, which drops as each study patient dies (or gets the complication).
If there are thousands of patients in the study, the curve is smooth. If
there are very few, it is possible to see individual deaths/events as steps
in the graph. Ideally, these graphs should have confi dence intervals.
Confi dence intervals These refl ect the precision of the study results.
Narrow confi dence intervals are better than wide ones because the con-
fi dence interval provides a range of values for the percentage survival (or
odds ratio or other proportion) that has a specifi ed probability (usually
95%) of containing the true value for the entire population from which
the study patients were recruited. Always look for confi dence intervals;
they give you a ‘best case and worst case’ snapshot.
Regression analysis Essentially looking back from a group of patients with
a known outcome (e.g. dead/alive) to see whether there were any predic-
tors (e.g. age, recent myocardial infarction (MI)). Univariate analysis looks
at single variables in turn. Multivariate analysis looks at a group of variables
together; it is used to identify independent risk factors for an outcome. For
example, age may be found to be a risk factor for post-operative death
in univariate analysis, but that is because elderly patients are more likely
to have other risk factors for post-operative death (e.g. recent MI). If age
is not found to be an independent risk factor in multivariate analysis, it
suggests that elderly people without other risk factors (e.g. recent MI) are
not at higher risk of post-operative death.

CHAPTER 1 Good surgical practice
10
Critical appraisal
Types of study
Studies appraising treatments can take several forms.
Randomized controlled trial (RCT) Prospective study in which partici-
pants are allocated to control or treatment groups on a random basis.
Gold standard for assessing treatment effi cacy, but time-consuming and
expensive to run.
Cohort study Partly prospective study in which two cohorts of patients
are identifi ed, one of which was exposed to the treatment and one is the
control group. They are followed over time to see the outcome. Cheaper
and quicker than RCT and suitable for looking at prognosis, but prone to
bias or false associations.
Case control study Retrospective study in which patients with the out-
come of interest are identifi ed and paired with patients without the out-
come of interest, and the exposure rates are compared. Cheapest and
quickest way of looking for causation. Bias arises when patients are mis-
classifi ed as cases or controls.
Case series A collection of anecdotes or case reports.
Systematic review Differs from the traditional literature review by apply-
ing explicit, systematic, and reproducible methods to retrieve and appraise
literature to answer a clearly formulated question. Large amounts of data
are summarized and conclusions are more accurate.
Meta-analysis A mathematical synthesis of the results of two or more pri-
mary studies, increasing the statistical signifi cance of positive overall results.
However, it reduces the ability of studies to demonstrate local effects.
Levels of evidence
Studies of treatment/hazard can be arranged in order of decreasing sta-
tistical validity.
• Level 1a. Systematic review of RCTs.
• Level 1b. High quality RCT with narrow confi dence intervals.
• Level 1c. All-or-none case series (either all patients died before
treatment became available, but some now survive or some used to
die, but now with treatment, all survive).
• Level 2a. Systematic review with homogeneity of cohort studies.
• Level 2b. Cohort study or low quality RCT.
• Level 2c. ‘Outcomes’ research.
• Level 3a. Systematic review with homogeneity of case control studies.
• Level 3b. Individual case control study.
• Level 4. Case series and poor quality cohort and case control studies.
• Level 5. Expert opinion without explicit critical appraisal or based on
physiology, bench research, or fi rst principles.
How to appraise a paper
Answer these questions systematically. This information should all be
stated explicitly within the manuscript.

CRITICAL APPRAISAL
11
How relevant is the paper?
Does the paper address a clearly focused, important, and answerable clini-
cal question that is relevant to my patients?
How valid are the fi ndings?
• Was the paper published in an independent peer-reviewed journal?
• Does the paper defi ne the condition to be treated, the patients to be
included, the interventions to be compared, and the outcomes to be
examined?
• Was a power calculation performed and is the power adequate?
• Were all clinically relevant outcomes reported?
• Was follow-up adequate?
• Were all patients accounted for at the end of the study?
• Was the appropriate study type selected and was the design
appropriate?
• Were the statistical methods described and were they appropriate?
• Were the sources of error discussed?
Systematic reviews
• Is the clinical question clearly defi ned and an acceptable basis for
including or excluding papers?
• Was the literature search thorough and were other potentially
important sources explored?
• Were trials appropriately included and excluded?
• Was the methodological quality assessed and trials appropriately
weighted?
RCTs
• Were patients properly randomized?
• Were patients treated equally apart from the intervention being
studied?
• Was analysis on an intention-to-treat basis?
• Are confi dence intervals narrow and not overlapping?
Case control studies
• Were patients correctly classifi ed as case or control?
• Were all patients accounted for at the end of the study?
How important are the results?
• Were the results statistically signifi cant?
• Were the results expressed in terms of numbers needed to treat and
are they clinically important?
How applicable are the fi ndings?
• Were the study patients similar to mine?
• Is the treatment feasible within my practice: is information on safety,
tolerability, effi cacy, and price presented?

CHAPTER 1 Good surgical practice
12
Audit
What is audit?
National Institute for Health and Clinical Excellence (NICE) defi nes clinical
audit as ‘a quality improvement process that seeks to improve patient care
and outcomes through systematic review of care against explicit criteria
and the implementation of change. Aspects of the structure, processes,
and outcomes of care are selected and systematically evaluated against
explicit criteria. Where indicated, changes are implemented . . . and further
monitoring is used to confi rm improvement in health care delivery.’
Why do it?
Clinical audit is currently seen as the most effective way of assessing rou-
tine health care delivery and the basis of improving outcomes.
• All hospital doctors are required to fully participate in clinical audit
(NHS Plan, Department of Health, 2000).
1
• The GMC advises that all doctors ‘must take part in regular and
systematic medical and clinical audit . . . Where necessary, you must
respond to the results of audit to improve your practice’.
How to do it
Audit of outcome or process can be divided into fi ve stages: each stage
needs to be carefully planned to produce a clinically effective audit.
Preparing for audit Choose a topic and defi ne the purpose of the audit.
One option is to identify (by consulting patients and clinicians) a poten-
tial problem that may involve high costs or risks for which there is good
evidence to inform standards and that may be amenable to change. NICE
stresses the importance of identifying skills and resources to carry out
the audit.
Selecting audit criteria Audit can assess process or outcome.
• Defi ne the patients to be included.
• Criteria to assess performance should be derived from the available
evidence, e.g. trials, systematic reviews, society guidelines, or clinician
consensus.
• Benchmarking prevents unrealistically high or low targets.
Measuring performance This is about collecting data. Identify patients or
episodes from several sources (e.g. operating room logbooks and patient
administration system (PAS)) to avoid missing patients because of incom-
plete data. Electronic information systems can improve data collection.
Training dedicated audit personnel can improve the process further.
Making improvements Identify local barriers to change, develop a practi-
cal implementation plan, which should involve several interventions (prac-
tice guidelines, education, and training). Clinical governance programmes
should provide the structure.
Sustaining improvements Repeating the audit to assess improvements is
also called closing the audit loop. Alternatives such as critical incident
review may be effective.
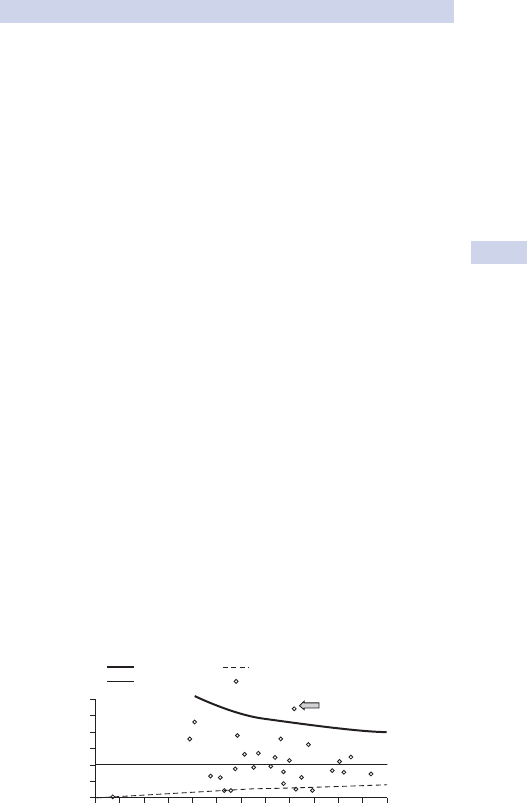
AUDIT
13
Measuring surgical performance
Rationale The Kennedy report on the enquiry into perioperative deaths in
paediatric cardiac surgery at Bristol Royal Infi rmary stated that ‘Patients
must be able to obtain information as to the relative performance of the
Trust . . . and consultant units within the Trust’. The idea that every patient
has the right to expect their surgery to be performed by a surgeon whose
results are not statistically worse than average is widely held.
• League tables ranking cardiothoracic surgeons on the basis of surgeon-
specifi c mortality data have been published by the government.
• Surgeons can be ranked using a number of other outcomes.
• Ranking should incorporate a system that accounts for differences in
case mix, i.e. risk stratifi cation, so that surgeons who operate on sicker
or more complicated patients are not unfairly penalized.
Risk scoring systems Examples include:
• Euroscore and Parsonnet scores for predicting operative mortality in
cardiac surgical patients.
• Apache II scores for intensive care patients.
• POSSUM (Physiologic and Operative Severity Score for the
enUmeration of Mortality and morbidity)—variants exist for vascular
and colorectal surgery.
Presenting results The aim of presenting performance data is to distin-
guish between normal variation between surgeons or institutions and sig-
nifi cant divergence. There are three main ways of doing this:
• Average outcome over a given time frame.
Ranking or league tables of surgical mortality or other •
complications; the data may be crude or risk-stratifi ed (i.e. taking
into account the case mix).
Survival plots which may also be crude or risk-stratifi ed.•
Standardized mortality ratio plots.•
• Volume and outcome control charts.
Funnel plots (see Fig. 1.1).•
Spectrum plots.•
• Performance trends over time.
Cumulative summation charts (CUSUM).•
Variable life-adjusted display charts (VLAD), risk-adjusted CUSUM.•
6
Upper 95% Cl Lower 95% Cl
%lanoitaNnoegruS
5
4
3
2
1
0
0 50 100 150 200 250 300
Number of cases
350 400 450 500 550 600
Crude mortality %
Fig. 1.1 Funnel plot of mortality data for 50 cardiac surgeons. The arrow marks
an outlier with mortality outside 95% confi dence intervals (CI).
Reference
1 Department of Health. (2000). The NHS Plan: a plan for investment, a plan for reform. Available at: M
http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/
DH_4002960

CHAPTER 1 Good surgical practice
14
Consent
Legal aspects
Successful surgery depends on a relationship of trust between the patient
and doctor. The patient’s right to autonomy must be respected, even if
their decision results in harm or death. This right is protected by law.
• A doctor performing a procedure on a patient without their consent
can be found guilty of battery.
• A doctor who has failed to give the patient adequate information to
allow them to give informed consent can be found guilty of negligence.
• No adult in the United Kingdom (UK) can legally consent to surgery on
behalf of another adult. It is important to involve relatives, particularly
where patients are unable to consent, but their wishes are not legally
binding and do not form part of the legal consent.
Obtaining consent
The key to good consenting is good communication (see b p. 4). It may
be necessary to use a translator and some Trusts will not accept consent
gained by using patients’ relatives as translators. GMC guidelines state ‘If
you are the doctor providing treatment or undertaking an investigation,
it is your responsibility to discuss it with the patient and obtain consent’.
In practice, this may be done verbally by the consultant or registrar in
clinic, or on a ward round with a house offi cer obtaining written con-
fi rmation later. Consent must be given freely: patients may not be put
under duress by clinicians, employers, police, or others to undergo tests
or treatment. Declare any potential confl icts of interest. The amount of
information should be suffi cient to allow a mentally competent patient
to make an informed decision. It will vary according to the individual, the
nature of the condition, the complexity of treatment, and risks involved. It
is unacceptable to limit the amount of information on the basis that it may
cause distress, but be sympathetic to the patients’ needs. Consent must
be obtained for taking photographs for teaching or publication, and taking
samples for research.
Informed consent
There are fi ve aspects that the patient must understand to give informed
consent:
• The reason for carrying out the procedure. The patient needs to
understand the nature of their illness and its prognosis.
• What the procedure involves. Where and how long is the scar; what is
being removed; what prosthesis will be implanted; will there be drains?
• The risks of the procedure. Specifi c to the procedure (e.g. stoma, limb
dysfunction) and in general (e.g. anaesthesia, bed rest, deep vein
thrombosis (DVT)).
• The benefi ts of the procedure. Improvement in symptoms or prognosis
or purely diagnostic.
• Alternatives. Including conservative treatment, with their advantages
and disadvantages.

CONSENT
15
Modes of consent
• Implied consent. The patient is presumed to consent to minor
procedures, e.g. X-rays, phlebotomy, by cooperating with ward
procedures.
• Express written consent. Whenever possible, this should be obtained for
all patients undergoing procedures involving an anaesthetic, complex
treatments with signifi cant risks and side effects, or as part of research.
Written consent is not legal proof that adequate consent was obtained at
the time the document was signed.
• Express verbal consent. Should be obtained when it is not possible
to get written consent, witnessed by an independent health care
professional, and documented in the notes accordingly, or for simple
procedures with minimal risk of harm.
Special considerations
Emergencies When consent cannot be obtained, you may provide emer-
gency medical treatment, provided it is limited to what is needed to pre-
serve life. However, you must respect any valid advance refusals that you
know about or that are drawn to your attention.
Mentally incapable patients No adult in the UK can legally consent to sur-
gery on behalf of another adult. Assess the patient’s competence to make
an informed decision. If unable to decide, and provided they comply,
treatment may be instigated that is judged to be in their best interests.
Otherwise, treatment may be carried out under the Mental Health Act
1989. Controversial and non-therapeutic treatments (e.g. sterilizations)
require court approval.
Advance statements/living wills Advance statements made by patients
before losing the capacity of informed consent must be respected, pro-
vided the decision is applicable to the present circumstances and there is
no reason to believe that they may have changed their minds. The known
wishes of the patient should be taken into consideration if an advance
statement is unavailable.
Children
• Over 16s are regarded as young adults and have capacity to decide.
• Under 16s may give their own consent if they are judged to
understand what is involved.
• Unlike adults, where a competent child refuses treatment, a person
with parental responsibility (except in Scotland) or a court may
authorize treatment if deemed in the child’s best interests.
• If the parents refuse treatment deemed in the child’s best interests,
you are not bound by this and may seek a ruling from the court.
• Emergency treatment may be instigated without consent in a similar
manner to that in adults.
Pregnancy The right to autonomy applies equally to pregnant women.
It includes the right to refuse treatment that is intended to benefi t the
unborn child.

CHAPTER 1 Good surgical practice
16
Death
Confi rming death
There is no legal defi nition of death in the UK. It is generally regarded as
the cessation of circulation and respiration.
• Clinically, there is:
No respiratory effort, denoted by the absence of breath sounds on •
auscultation over 1min.
Absence of a palpable pulse and heart sounds over 1min.•
No response to painful stimuli, e.g. sternal or supraorbital rub.•
Fixed dilated pupils (beware drugs such as atropine).•
• If there is doubt, perform an electrocardiogram (ECG).
• Hypothermia (core temperature <34°C) must have been corrected.
Brain death/brainstem death
The concept of brain death has arisen from the advances in intensive ther-
apy, and the ability to maintain cardiac and respiratory function artifi cially
in patients who have sustained severe irreversible brain damage. Brain
death is defi ned as the ‘irreversible cessation of all functions of the entire
brain, including the brainstem’.
1
This, alongside the traditional defi nition,
is taken to equate to death in the UK, United States of America (USA),
Australia, and many other countries. In order to diagnose brain death, a
number of strict criteria must be met.
• An identifi able cause for the brain death must be established, e.g.
severe head injury/intracerebral bleed, and
• Other causes, including central nervous system (CNS) depressants,
hypothermia, metabolic and endocrine disturbance, need to be
excluded, and
• The patient is unable to breathe spontaneously despite adequate CO
2
drive (i.e. PaCO
2
>6.7kPa), and
• The following brainstem refl ex tests, performed by the consultant in
charge (or deputy of 5y registration) and another suitably experienced
doctor, have been failed on two separate occasions, usually 24h apart:
Both pupils are fi xed and unresponsive to light (oculomotor nerve).•
Corneal refl exes are absent (trigeminal nerve).•
Vestibulo-ocular refl exes are absent (absent eye movements when •
20mL of ice-cold water is injected into each ear with tympanic
membranes visualized beforehand) (vestibulo-cochlear nerve).
Absent motor responses to painful stimuli in the distribution of the •
cranial nerves in the absence of neuromuscular blockade (spinal
cord injury may ablate peripheral motor responses).
Absence of respiratory effort when disconnected from the •
ventilator despite a PaCO
2
>6.7kPa (i in chronic obstructive
pulmonary disease (COPD)).
Absent gag and cough refl ex upon pharyngeal and endotracheal •
stimulation.
Coroners
It is always wise to discuss with the consultants involved if there is any rea-
son to discuss cases with the coroner’s offi cer. Poor quality information

DEATH
17
can lead to death certifi cates being returned or the coroner becoming
involved unnecessarily.
In-hospital deaths must be discussed with the coroner’s offi cer if:
• Death has occurred during an operation.
• Death occurred before recovery from anaesthetic.
• More than 14 days have elapsed since the patient last saw a doctor.
• There is doubt about the cause of death.
• Death is thought to be suspicious (e.g. caused by overdoses of
prescribed substances, medical error, suicide).
Certifying death
Documenting in the medical notes
If you are asked to ‘certify’ a patient, fi rst confi rm death (see b p. 16):
• Document the date and time that death was pronounced.
• Document your examination.
• Document the causes of death as they will appear on the death certifi cate
if these have been decided. 2 If in doubt, always speak to the consultant.
The death certifi cate
This can be issued by anyone with full medical qualifi cations who looked
after the patient during their last illness, or where referral to the coroner
has been made and permission to issue the certifi cate has been granted.
• Write legibly. The record is retained by the relatives and illegible or
incomplete certifi cates may be rejected by the funeral director.
• Part I. The cause of death. Events leading to Ia are listed in Ib and Ic.
• Part II. Conditions that contributed to, but did not directly cause death.
• General terms like heart failure and sepsis may not be accepted.
Cremation forms
These forms vary slightly between regions, but certain rules always apply.
• There are two parts. The fi rst is fi lled in by a doctor who attended
the patient during the illness leading up to death, the second by an
independent clinician who has been fully registered for at least 5y.
• They should not be issued if the cause of death is not established.
• It is the responsibility of the issuing doctor to ensure that they have
seen and identifi ed the person after death, and that there are no
radioactive implants or pacemakers present.
Post-mortems
• A coroner’s post-mortem is required for suspicious deaths, but is most
commonly performed where the Coroner’s Offi ce has ‘taken’ a case
where the cause of death is uncertain, or may be related to surgery or
interventions. The consent of relatives is not necessary to proceed.
• A hospital post-mortem may be carried out with the consent of relatives
to investigate other deaths. In 60% of post-mortems in one series, new
diagnoses that would have substantially changed management were
found—they are a vital part of audit.
Reference
1 (1981). Guidelines for the determination of death: report of the medical consultants on the
diagnosis of death to the president’s commission for the study of ethical problems in medicine
and biomedical and behavioral research. J Am Med Assoc 246, 2184–6.

CHAPTER 1 Good surgical practice
18
End-of-life issues
Do not resuscitate (DNR) orders
A DNR order should be considered when the frailty, comorbidity (e.g.
inoperable disseminated malignancy, multiple organ failure), maximal
medical treatment, or advanced age of a patient means that any attempt at
cardiopulmonary resuscitation (CPR) in the event of a cardiac or respira-
tory arrest will be futile. DNR decisions should be reached on a case by
case basis: a blanket ‘do not resuscitate’ policy based on a specifi c patient
group, such as elderly patients, is unacceptable. An 84-y-old patient who
was an appropriate candidate for cardiac surgery is an appropriate can-
didate for CPR post-operatively, whereas a 72-y-old patient undergoing
palliative care for end-stage hepatorenal failure is probably not.
• Never make a DNR decision without discussing it with a consultant.
• Patients and, where appropriate, their relatives must be involved.
• Document the clinical reasons for the DNR order and state explicitly
whether ‘full active medical management’ is to be continued: DNR
orders do not always include withdrawing treatment. Discuss each
case with the nurses involved.
• Complete the appropriate documentation and review process, which
varies from Trust to Trust, and make sure the nursing staff are fully
aware so that they do not call the arrest team when the patient dies.
Euthanasia
Euthanasia is the painless termination of life at the request of the patient
concerned. In the UK, it is illegal to administer any drug to accelerate death,
irrespective of how compassionate the motive may be. Withdrawing futile
treatment is not euthanasia. UK law states that the intention to kill is mali-
cious and such action would be classifi ed as murder. Terminally ill people
and the parents of terminally ill or severely disabled children may have
several reasons for requesting euthanasia. Effective palliative care, counsel-
ling, and multidisciplinary support should be able to address most of these
reasons, which include:
• Pain.
• Disability.
• Disfi gurement.
• Depression.
• Fear of being a burden, being unable to cope.
Palliative care
Palliative care is surgical, medical, and nursing care aimed specifi cally at
relieving the problems associated with terminal conditions when the pos-
sibility of cure has been abandoned. Palliative care is delivered by palliative
medicine and nursing specialists and can take place in the community or
in residential care settings. Refer early: palliative care beds are limited and
acute surgical wards are rarely the best places for dying patients. Palliative
care physicians specialize in:
• Control of symptoms, including pain, anorexia, nausea and vomiting,
confusion, dysphagia, dyspnoea, incontinence.

END-OF-LIFE ISSUES
19
• Psychological aspects of terminal illness.
• Bereavement.
Suicide The suicide rate in the UK is currently 12.5 per 100 000.
Patients at risk
• The recently bereaved.
• Cancer patients have a fi ve times increased risk.
• Men over 55y with oral cancer and a history of alcohol abuse.
• Women of any age, often suffering from gynaecological or breast
cancer. (In both of these latter groups, the treatment of the disease
involves disfi gurement and a change of body image.)
Action
Patients about to undergo disfi guring surgery for any reason should be
counselled carefully in the period after confi rmation of the diagnosis and
before surgery. Doctors should discuss all treatment options and implica-
tions clearly. The support of a ‘mastectomy counsellor’ or ‘stoma thera-
pist’ is invaluable. Post-operatively:
• Look for symptoms of depression, including low mood, tearfulness,
anorexia, early morning waking, suicidal thoughts, especially in long-
term patients.
• Do not discontinue antidepressant medication.
• Ensure that arrangements for discharge include community nursing
support and that the GP is aware of the patient’s state of mind.
Organ donation
• When brain death is established, organ donation should be considered
for all patients who are under 75y of age with no history of malignant
disease or major untreated sepsis.
• All donors should be tested for human immunodefi ciency virus (hiv),
hepatitis B and C, herpes simplex virus (HSV), and cytomegalovirus
(CMV).
• Organ donation is usually coordinated by regional transplant teams.
• The body should be identifi ed and next of kin contacted.
• If, despite reasonable attempts, the identity of the corpse or next of
kin remains unknown, the body becomes the property of the health
authority.
• If a donor card is present, it is reasonable to assume that the deceased
wished to donate his organs and the transplant team can proceed.
• If relatives are identifi ed and do not wish organ donation to proceed,
even though there is a donor card, their wishes must be respected.
• Relatives should be asked to act as agents in expressing what they
believe to be the wishes of the patient. Ideally, the person seeking
permission should be someone whom they already know. This may be
the consultant in charge, but, on occasion, a senior staff nurse, chaplain
(or other religious fi gure), or the family GP may be more appropriate.
• In the case of accidental deaths, the coroner’s permission should be
sought before proceeding.

CHAPTER 1 Good surgical practice
20
Clinical governance
Clinical governance is the system through which National Health Service
(NHS) organizations are accountable for continuously improving the qual-
ity of their services. Clinical governance involves setting standards, per-
formance monitoring, and reporting systems at national, institutional, and
personal levels. Risk management is an integral part of clinical governance:
it is the systematic identifi cation and avoidance of risks associated with
any procedure.
Setting standards
In addition to conventional clinical evidence and guidelines, the following
organizations have a responsibility for setting standards in health care.
National Service Frameworks (NSFs)
NSFs are long-term national strategies for improving specifi c areas of care,
produced by government after consultation with clinicians. They set stand-
ards and establish methods of delivering them. NSFs have been published
for coronary heart disease and cancer.
National Institute for Health and Clinical Excellence (NICE)
NICE is the governmental organization responsible for setting standards
by reviewing the best available evidence and publishing guidelines. Local
authorities are obliged to fund interventions recommended by NICE,
but NICE guidance does not overrule individual clinical decision-making.
Currently, NICE produces three kinds of guidance:
• Technology appraisals. Guidance on the use of new and existing
medicines and treatments within the NHS in England and Wales.
• Clinical guidelines. Guidance on the appropriate treatment and care of
people with specifi c conditions within the NHS in England and Wales.
• Guidance on whether interventional procedures used for diagnosis or
treatment are safe enough and work well enough for routine use in
England, Wales, and Scotland.
Postgraduate Medical Education and Training Board (PMETB) and
professional organizations
The PMETB was set up in 2003 to develop a single, unifying framework for
postgraduate medical education (PGME) and training across the UK.
• Medical ethics, undergraduate and pre-registration medical education,
and fi tness to practise remain the responsibility of the GMC.
• Accreditation and the approval of basic surgical training remain the
responsibility of the Royal Colleges of Surgeons.
• Higher surgical training is supervised by the Joint Committee on Higher
Surgical Training (JCHST) and the specialist advisory committees
(SACs).
Performance monitoring
Healthcare Commission (replaces CHI and the Audit Commission)
The Healthcare Commission is a new body that has been set up to help
improve the quality of health care. It will do this by providing an independ-
ent assessment of the standards of services, whether they are provided

CLINICAL GOVERNANCE
21
by the NHS, independent health services, or voluntary organizations. It
provides an independent second stage of complaints assessment, assesses
the arrangements in place to promote public health, and acts as the coor-
dinating inspectorate in relation to health care.
National Confi dential Enquiry into Patient Outcomes and Death
(NCEPOD)
NCEPOD (which used to be the National Confi dential Enquiry into
PeriOperative Deaths) is an organization independent of the Department
of Health (DOH) and the professional associations, although it receives
over 85% of its funding from the DOH via NICE. It stopped collecting
data on all deaths within 30 days of surgery in 2002 when its remit was
extended to cover all medical and surgical deaths. Data is now collected
by local reporters in response to specifi c areas of research.
National Patient and User Survey The Healthcare Commission has car-
ried out fi ve national surveys involving over 500 institutions and 300 000
patients. The results are disseminated to health care providers and gov-
ernmental agencies to inform strategy.
Audit See b p. 12.
Revalidation The purpose of revalidation will be to create public confi -
dence that all licensed doctors are up to date and fi t to practise. Currently,
consultants and general practitioners undergo regular appraisal: this will
be included within revalidation which is yet to be introduced.
Reporting systems
Critical incident reporting Critical incident reporting was initially volun-
tary and anonymous. Incidents perceived to have exposed patients or staff
to actual or potential risk were reported on forms that would be sent to
responsible individuals in each directorate, serious adverse events being
discussed at regular meetings with the clinical director. Now effective criti-
cal incident reporting systems are a requirement for CNST (criminal neg-
ligence scheme for Trusts) insurance and are assessed by the Healthcare
Commission on Trust visits.
Complaints Patients make formal complaints about treatment or clinicians
to the hospital concerned: the new NHS complaints procedure, which was
reformed in 2002 to address concerns that it was fragmented, complex,
and insuffi ciently independent, still stresses that wherever possible, issues
should be resolved locally. Effective use of the patient advice and liai-
son services (PALS) and independent complaints advocacy service (ICAS)
should mean that only serious complaints are referred to the second level,
which is handled by the Healthcare Commission which currently handles
3000–5000 complaints per annum.
Whistle-blowing Trusts are required to have a whistle-blowing policy to
enable individual staff members to express concerns about treatment and
to protect them from reprisals. The policy must include a mechanism for
investigating and acting on such claims.
This page intentionally left blank

23
Principles of surgery
Chapter 2
Terminology in surgery 24
History taking and making
notes 26
Common surgical symptoms 28
Examination and investigation of
the patient
Evaluation of breast disease 30
Evaluation of the neck 32
Evaluation of the abdomen 34
Abdominal investigations 36
Evaluation of pelvic disease 38
Evaluation of peripheral vascular
disease 40
Evaluation of the skin and
subcutaneous tissue disease 42
Surgery at the extremes of age 44
Day case and minimally invasive
surgery 46
Preoperative care
Surgery in pregnancy 48
Surgery and the contraceptive
pill 50
Surgery in endocrine disease 52
Surgery and heart disease 54
Surgery and respiratory
disease 58
Surgery in renal and hepatic
disease 60
Surgery in neurological disease 62
Pre-optimization of the patient
Fluid optimization 64
Nutrition in surgical patients 66
Enhanced recovery after
surgery 68
Perioperative care
Getting the patient to theatre 70
Prophylaxis—antibiotics and
thromboprophylaxis 72
In-theatre preparation 74
Positioning the patient 76
Sterilization, disinfection, and
antisepsis 78
Scrubbing up 79
Surgical instruments 80
Incisions and closures 82
Drains 83
Stomas 84
Knots and sutures 86
Post-operative
Post-operative management 88
Drain management 90
Fluid management 92
Acid–base balance 94
Blood products and
procoagulants 96
Transfusion reactions 98
Shock 100
Post-operative haemorrhage 102
Wound emergencies 104
Cardiac complications 106
Respiratory complications 108
Renal complications 110
Urinary complications 112
Gastrointestinal
complications
114
Neurological complications 116
Haematological complications 118
Deep venous thrombosis and
pulmonary embolism 120
Risk scoring 122
Critical care 124
Commonly used terms in
ITU 126
Invasive monitoring 128
Ventilation and respiratory
support 130
Circulatory support 132
Renal support 134
Enteral support 136
Sepsis, SIRS, MODS, and ALI 138

CHAPTER 2 Principles of surgery
24
Terminology in surgery
How to describe an operation
The terminology used to describe all operations is a composite of basic
Latin or Greek terms.
First describe the organ to be operated on
Examples:
lapar-, abdomen (• laparus = fl ank);
nephro-, kidney;•
pyelo-, renal pelvis;•
cysto-, bladder;•
chole-, bile/the biliary system;•
ileo-, small bowel (distal)•
col(on)-, large bowel;•
hystero-, uterus;•
thoraco-, chest;•
rhino-, nose;•
masto/mammo-, breast.•
Second describe any other organs or things involved in the procedure
Examples:
docho-, duct;
•
angio-, vessel (blood- or bile-carrying);•
litho-, stone.•
Third describe what is to be done
Examples:
-otomy, to cut (open);•
-ectomy, to remove;•
-plasty, to change shape or size;•
-pexy, to change position;•
-raphy, to sew together;•
-oscopy, to look into;•
-ostomy, to create an opening in (• stoma = mouth);
-paxy, to crush;•
-graphy/gram, image (of).•
Lastly add any terms to qualify how or where the procedure is done
Examples:
percutaneous, via the skin;
•
trans-, across;•
antegrade, forward;•
retrograde, backward;•
ventral, anterior surface of.•
Examples of terms
Choledochoduodenostomy• . An opening between the bile duct and the
duodenum.
Rhinoplasty• . Nose reshaping.
Pyelolithopaxy• . Destruction of pelvicalyceal stones.

TERMINOLOGY IN SURGERY
25
Bilateral mastopexy• . Breast lifts.
Percutaneous arteriogram• . Arterial tree imaging by direct puncture
injection.
Loop ileostomy• . External opening in the small bowel with two sides.
Flexible cystourethroscopy• . Internal bladder and urethral inspection.

CHAPTER 2 Principles of surgery
26
History taking and making notes
Making medical notes
All medical and paramedical professionals have a duty to record their
input and care of patients in the case notes. These form a permanent legal
and medical document. There are some basic rules.
Write in blue or black ink; other colours do not photocopy well.
•
Date, time, and sign all entries; always identify retrospective entries.•
Be accurate.•
Make it clear which diagnoses are provisional.•
Abbreviations are lazy and open to misinterpretation; avoid them.•
Clearly document information given to patients and relatives.•
Avoid non-medical judgements of patients or relatives.•
Basics
Always record name, age, occupation, and method of presentation.•
Cover all the principal areas of medical history:•
Presenting complaint and past history relevant to it.•
Other past medical history, drug history, and systematic enquiry.•
Previous operations/allergies/drugs.•
Family history, social history, and environment.•
Presenting complaint
This is a one- or two-word summary of the patient’s main symptoms, e.g.
abdominal pain, nausea and vomiting, swollen leg, PR bleeding.
In emergency admissions, do not write a diagnosis here (e.g. ischaemic •
leg). The diagnosis of referral may well turn out to be wrong.
In elective admissions, it is reasonable to write: ‘elective admission for •
varicose vein surgery’.
History of presenting complaint
This is a detailed description of the main symptom and should include •
the relevant systems enquiry.
Try to put the important positives fi rst, e.g. right-sided lower •
abdominal pain, sharp, worse with moving, and coughing, anorexia 24h.
Include the relevant negatives, e.g. no vomiting, no PR bleeding.•
Be very clear about the chronology of events.•
In a complicated history or with multiple symptoms, use headings, e.g. •
‘Current episode’, ‘Previous operations for this problem’, ‘Results of
investigations’.
Summarize the results of investigations performed prior to admission •
systematically: bedside tests, blood tests, histology or cytology, X-rays,
cross-sectional imaging, specialized tests.
Past medical history
Ask about thyroid problems, tuberculosis (TB), hypertension, •
rheumatic fever, epilepsy, asthma, diabetes, ischaemic heart disease,
stroke, and previous surgery, specifi cally.
List and date all previous operations.•
Ask about previous problems with an anaesthetic.•
Asking ‘Have you • ever had any medical problem or been to hospital for
anything?’ at the end often produces additional information.

HISTORY TAKING AND MAKING NOTES
27
Systematic enquiry
This is extremely important and often neglected. A genitourinary history
is highly relevant in young females with pelvic pain. A good cardiovascular
and respiratory systems enquiry will help avoid patients being cancelled
because they have undiagnosed anaesthetic risks. Older patients may have
pathology in other systems that may change management, e.g. the patient
with prostatism should be warned about urinary retention.
Cardiovascular.• Chest pain, effort dyspnoea, orthopnoea, nocturnal
dyspnoea (see b p. 58), palpitations, swollen ankles, strokes, transient
ischaemic attacks, claudication.
Respiratory.• Dyspnoea, cough, sputum, wheeze, haemoptysis.
Gastrointestinal.• Anorexia, change in appetite, weight loss (quantify how
much, over how long).
Genitourinary.• Sexual activity, dyspareunia (pain on intercourse),
abnormal discharge, last menstrual period.
Neurological.• 3 Fs: fi ts; faints; funny turns.
Social history
At what time did they last eat or drink?•
Ask who will look after the patient. Do they need help to mobilize?•
Smoking and alcohol history.•
2 Tips for case presentation
Practise• . Every case is a possible presentation to someone!
Always ‘set the scene’ properly• . Start with name, age, occupation, and
any key medical facts together with the main presenting complaint(s).
Be chronological• . Start at the beginning of any relevant prodrome or
associated symptoms; they are likely to be an important part of the
presenting history.
Be concise with the past medical history• . Only expand on things that you
really feel may be relevant either to the diagnosis or management, e.g.
risks of general anaesthesia.
For systematic examination techniques, see the relevant following •
pages.
Always summarize the general appearance and vital signs fi rst.•
Describe the most signifi cant systemic fi ndings fi rst, but be •
systematic—‘inspection, palpation, percussion, and auscultation’.
Briefl y summarize other systemic fi ndings• . Only expand on them if they
may be directly relevant to the diagnosis or management.
Finally, • summarize and synthesize—don’t repeat. Try to group
symptoms and signs together into clinical patterns and recognized
scenarios.
Finish with a proposed diagnosis or differential list and be prepared to •
discuss what diagnostic or further evaluation tests might be necessary.

CHAPTER 2 Principles of surgery
28
Common surgical symptoms
Dyspepsia (epigastric discomfort or pain, usually after eating) What is the
frequency? Is it always precipitated by food or is it spontaneous in onset? Is
there any relief, especially with milky drinks or food? Is it positional?
Dysphagia (diffi culty during swallowing) Is the symptom new or long-
standing? Is it rapidly worsening or relatively constant? Is it worse with
solid food or fl uids? (Worse with fl uids suggests a motility problem, rather
than a stenosis.) Can it be relieved by anything, e.g. warm drinks? Can
the patient point to a ‘level’ of hold-up on the surface (usually related to
the sternum)? This often accurately relates to the level of an obstructing
lesion. Is it associated with ‘spluttering’ (suggests tracheo-oesophageal fi s-
tula or inhalation of food/fl uid).
Oesophageal refl ux (bitter or acidic tasting fl uid in the pharynx or
mouth) How frequently? What colour is it? (Green suggests bile whereas
white suggests only stomach contents). When does it occur (lying only, on
bending, spontaneously when standing)? Is it associated with coughing?
Haematemesis (the presence of blood in vomit) What colour is the
blood (dark red-brown ‘coffee grounds’ is old or small-volume stomach
bleeding; dark red may be venous from the oesophagus; bright red is arte-
rial and often from major gastric or duodenal arterial bleeding)? What
volume has occurred over what period? Did the blood appear with the
initial vomits or only after a period of prolonged vomiting (suggests a
traumatic oesophageal cause).
Abdominal distension Symmetrical distension suggests one of the ‘5
Fs’ (fl uid ascites, fl atus due to ileus or obstruction, fetus of pregnancy, fat,
or a ‘fl ipping big mass’). Asymmetrical distension suggests a localized mass.
What is the time course? Does it vary? It is changed by vomiting, passing
stool/fl atus?
Pain
Pain anywhere should have the same features elicited. These can be
summarized by the acronym SOCRATES.
S• ite. Where is the pain, is it localized, in a region, or generalized?
O• nset. Gradual, rapid, or sudden? Intermittent or constant?
C• haracter. Sharp, stabbing, dull, aching, tight, sore?
R• adiation. Does it spread to other areas? (From loin to groin in
ureteric pain, to shoulder tip in diaphragmatic irritation, to back in
retroperitoneal pain, to jaw and neck in myocardial pain.)
A• ssociated symptoms. Nausea, vomiting, dysuria, jaundice?
T• iming. Does it occur at any particular time?
E• xacerbating or relieving factors. Worse with deep breathing,
moving, or coughing suggests irritation of somatic nerves either in
the pleura or peritoneum; relief with hot water bottles suggests
deep infl ammatory or infi ltrative pain.
S• urgical history. Does the pain relate to surgical interventions?

COMMON SURGICAL SYMPTOMS
29
Change in bowel habit May be change in frequency or looser or more
constipated stools. Increased frequency and looser stools is more likely
than isolated constipation to be due to a pathological cause. Is it a persist-
ent or transient? Are there associated symptoms? Is it variable?
Frequency and urgency of defecation New urgency of defecation
is almost always pathological. What is the degree of urgency—how long
can the patient delay? Is there associated discomfort? What is passed—is
the stool normal?
Bleeding per rectum What colour is the blood? Is it pink-red and only
on the paper when wiping? Does it splash in the pan? (Both suggest a case
from the anal canal.) Is it bright red on the surface of the stool (suggests
a lower rectal cause)? Is the blood darker with clots or marbled into the
stools (suggests a colonic cause)? Is the blood fully mixed with the stool
or altered (suggests a proximal colonic cause)?
Tenesmus (desire to pass stools with either no result or incomplete
satisfaction of defecation) Suggests rectal pathology.
Jaundice (yellow discoloration due to hyperbilirubinaemia; b p. 312)
How quickly did the jaundice develop? Is there associated pruritus? Are
there any symptoms of pain, fever, or malaise (suggests infection)?
Haemoptysis (the presence of blood in expectorate) What colour is
the blood? (Light pink froth suggests pulmonary oedema.) Are there clots
or dark blood (infection or endobronchial lesion)? How much blood?
Moderate bleeds quickly threaten airways: get help quickly.
Dyspnoea (diffi culty in or increased awareness of breathing) When does
the dyspnoea occur—quantify the amount of effort. Is it positional?
Orthopnoea.• Diffi culty in breathing that occurs on lying fl at; quantify
it by asking how many pillows the patient needs at night to remain
symptom-free.
Paroxysmal nocturnal dyspnoea.• Intermittent breathlessness at night.
Both orthopnoea and paroxysmal nocturnal dyspnoea suggest cardiac
failure.
Claudication (the presence of pain in the muscles of the calf, thigh, or
buttock precipitated by exercise and relieved by rest) After what degree
of exercise does the pain occur (both distance on the fl at and gradients)?
How quickly is the pain relieved by rest?
Rest pain (pain in a limb at rest without signifi cant exercise) How long
has the pain been present? Is it intermittent? Does it occur mainly at night?
Is it relieved by dependency of the limb involved?
Dysuria (pain on passing urine) When does the pain occur (beginning, end,
or throughout the stream)? Is if felt in the penis or suprapubically? Is it asso-
ciated with frequency? Is the urine discoloured or does it contain debris?
Haematuria (blood in the urine) Does the blood occur at the start
(suggests bladder origin), during, or end (suggests prostatic or penile ori-
gin) of the stream? Is there associated pain (suggests infection or stone
disease)?

CHAPTER 2 Principles of surgery
30
Evaluation of breast disease
Positioning and inspection
Breasts are best examined semi-recumbent and then sitting upright.
Initially, the arms are by the side, semi-recumbent. After initial inspection,
they should be positioned ‘hands on hips’, sitting upright (initially relaxed
and then with forced pressure on the hips to tense the pectoral mus-
cles), and fi nally abducted slowly above the head. For palpation, the hands
should return to the hips and the patient may lie back semi-recumbent
again.
Inspection is critical and should concentrate on the following.
Overall symmetry and position.
• Are the breasts the same size? Is there
deformity due to underlying disease? Is the position normal?
Skin appearance.• Is the skin erythematous or oedematous? Is there
fi xed lymphoedema of the skin (‘peau d’orange’)? Are there scars from
previous surgery?
Skin tethering.• Does the skin move freely as the arms are raised?
(Tethering is suggestive of underlying intraparenchymal scarring or
tumour.)
Nipples.• Are the nipples indrawn, deviated, or ulcerated (suggestive of
retroareolar tumour or infection)? Is there any evidence of discharge?
Palpation
Use the fl at of the fi ngers and use all four fi ngers at once. Palpate the
‘normal’ breast fi rst. Be methodical and don’t ‘knead’ the breast. A com-
mon routine is: upper outer quadrant; lower outer; lower inner; upper
inner; central (retroareolar); supraclavicular fossa; axilla. Features to look
for include the following:
Palpable mass.• Is it hard, irregular, and tethered (cancer) or smooth,
rounded, and mobile (cysts or fi broadenoma)?
Diffuse nodularity.
• Typical of benign disease.
Nipple discharge.• On palpation of the central area. Blood suggests
tumour; pus suggests infection; serous or milky may not be relevant.
Axillary and supraclavicular lymphadenopathy.• Is it multiple and tethered
(cancer)?
Investigations
Ultrasound
Easy to perform and painless—often done in breast outpatient clinic.
•
Avoids radiation dose in young women.•
Highly sensitive for differentiating between solid tumours and cysts.•
Mammography
Used both for population screening and diagnostic testing.
•
Uncomfortable for most women and involves a low radiation dose.•
Able to identify impalpable lesions.•
Able to identify premalignant lesions (e.g. ductal carcinoma • in situ).
Mammographic features of malignancy include: spiculated •
microcalcifi cation; irregularity; stellate outline.
EVALUATION OF BREAST DISEASE
31
Aspiration cytology
Well tolerated, easy to perform, and quick to report on—often done •
in one half day during breast outpatient clinic.
Does not provide histology: provides only cellular information and •
relies upon cellular atypia for a diagnosis of malignancy.
Does not differentiate between invasive and • in situ carcinoma.
Occasionally therapeutic for cysts.•
Good sensitivity and specifi city.•
Guided core biopsy
Performed under ultrasound or mammographic guidance using a
•
Trucut
®
needle or similar device.
Can be done under general or local anaesthetic.•
Provides actual histology information—allows cancers to be graded.•
Able to differentiate between invasive and carcinoma • in situ.
Highly sensitive and specifi c.•
Computerized tomography (CT) scanning
Relatively non-specifi c for local breast pathology.
•
Useful for assessment of extensive local invasion and regional and •
systemic staging.
CT positron emission tomography (PET) scanning
Occasionally used to assess indeterminate lesions identifi ed on plain •
CT and identify unsuspected metastatic disease.
Magnetic resonance imaging (MRI) scanning
Occasionally used for the assessment of local breast pathology.•
Key revision points—anatomy of the breast
Breast comprises epithelial ductal tissue, epithelial secretory lobules, •
fat, and connective tissue.
It is divided into four ‘quadrants’ and a peri/retroareaolar central
•
zone for clinical description of abnormalities.
The • areola is the pigmented area around each nipple.
The • arterial supply is from segmental perforators from the internal
thoracic artery (ITA).
Lymphatic drainage• —important in breast cancer management.
Non-pathological lymph drainage is almost entirely to the axillary •
nodes.
Medial half can occasionally drain to internal mammary nodes.•
Lymph nodes are divided into three levels (1, below; 2, behind; 3, •
above pectoralis minor).

CHAPTER 2 Principles of surgery
32
Evaluation of the neck
Evaluation of carotid artery disease is described on b p. 658.
Positioning and inspection
Sit the patient upright at rest with the head looking straight ahead. •
Inspect the neck from the front, side, and, if necessary, behind.
Observe the neck at rest and during swallowing (a glass of water). If •
necessary, inspect rotation left and right.
Observe the neck while asking the patient to protrude the tongue.•
Inspection includes looking for the following.
Overall symmetry and lumps.• Are there obvious lumps? Are they single
or multiple? Is the lump lying in or close to the midline? Does the lump
move with swallowing (suggests thyroid-related lesion)?
Skin abnormalities.• Are there any ulcers of sinuses (suggests chronic
infection such as TB)?
Associated structures.• Is there evidence of venous engorgement or
collateral vessels visible?
Palpation
Be systematic; palpate the regions of the neck in order. Use both hands
with the fl ats of the fi ngers to compare each side, but move only one
hand at once to prevent ‘cross-palpation’. A typical sequence of palpation
is: anterior triangle (bottom to top); submental area; submandibular area;
posterior triangle (top to bottom); supraclavicular fossae; parotid, pre-
auricular and post-auricular areas. Repalpate the neck with the patient
swallowing a mouthful of water—particularly the anterior triangle (see
Fig. 2.1). Lastly, feel specifi cally for the carotid arteries.
Lumps.• Is it single or multiple (multiple strongly suggests
lymphadenopathy)? Is it strictly in the midline (likely to be related to
the thyroid)? Does it move with swallowing (almost always thyroid-
related)? What are the general features (see b p. 42)?
Thyroid lumps.• Is it unilateral or bilateral? Does it move with tongue
protrusion?
Carotid arteries.• Are they normal, ecstatic, or aneurysmal?
Supraclavicular fossae.• Is there associated lymphadenopathy (suggests
malignancy)?
Auscultation Listen to the carotid arteries and any large masses for bruits,
suggesting a hypervascular local circulation or stenosis.
Investigations
Ultrasound
Easy to perform and painless.
•
Avoids radiation dose.•
Highly sensitive for the differentiation between solid tumours and •
cysts.
Aspiration cytology
Easy to perform and quick to report on—often done in one half day
•
during outpatients.

EVALUATION OF THE NECK
33
Usually well tolerated in outpatients.•
Provides only cellular information and relies upon cellular atypia for a •
diagnosis of malignancy.
Does not provide histological information.•
Occasionally therapeutic for cysts.•
Good sensitivity and specifi city.•
Contraindicated where there is a suspicion the lesion may be vascular.•
CT scanning
Useful for assessment of extensive local invasion and regional and •
systemic staging of tumours.
Allows evaluation of the thorax in some thyroid tumours.•
CT PET scanning
Occasionally used to assess indeterminate lesions identifi ed on plain CT
and identify unsuspected metastatic disease.
MRI scanning
Useful for detailed assessment of local invasion of tumours.
Clavicle
Trapezius
Sternocleidomastoid
Midline
Mandible
Ant
Post
Fig. 2.1 Key revision points—triangle of the neck.

CHAPTER 2 Principles of surgery
34
Evaluation of the abdomen
Positioning
Lie the patient supine with the head slightly raised with adequate •
support for the head to ensure the abdominal muscles are relaxed.
Arms should be by the sides to relax the lateral abdominal muscles.•
The patient may be rolled into left or right lateral positions during •
palpation and percussion.
Ask the patient to cough during inspection; it may reveal hernias.•
Stand the patient up to examine the groin only if necessary; most •
hernias and groin pathology can be fully assessed in supine position.
Inspection
Perform during normal and deep respiration.•
General features.• Is there evidence of jaundice or signs of anaemia?
Does the patient looked underweight, malnourished, or cachectic?
Scars.• Where are they? How old do they appear? Is there evidence of
herniation on coughing?
Is there a stoma?• What type? Does it look healthy or abnormal? What
is the content in the stoma appliance?
Overall appearance.• Is the abdomen symmetrical? Is there evidence
of global distension (e.g. ascites, distended bowel)? Is there evidence
of local distortion (e.g. a local mass or organomegaly)? Does the
abdomen move well and symmetrically with deep respiration (reduced
in peritoneal irritation)? Is there any discoloration (periumbilical
bruising (Cullen’s sign) or fl ank bruising (Grey Turner’s sign), where
either suggests retroperitoneal haemorrhage or major infl ammation)?
Umbilicus.• Is it herniated? Is there discharge or ulceration suggestive of
infection or a malignant deposit?
Pulsation.• Is there visible pulsation? (Further assessment requires
palpation.)
Persistalsis.
• Is there visible peristalsis? (Identifi cation may take several
minutes of observation.) It is rarely possible to suggest a cause or level
of obstruction related to the pattern of visible peristalsis.
Palpation
Be methodical. Use the fl at of one hand (usually the right). It is usual to
examine and describe the abdomen in areas. It can be divided it into nine
regions or fi ve ‘quadrants’ (see Fig. 2.2). Examine the areas lightly at fi rst
in a set order. Identify any masses or areas of tenderness. Repeat the
examination with deeper palpation. Go back to any identifi ed masses and
try to ascertain their key features.
Signs of peritoneal irritation.• Are there signs of local visceral peritoneal
irritation (tenderness and pain on palpation)? Are there signs of mild
parietal peritoneal irritation (guarding) or signs of marked parietal
peritoneal irritation (rigidity)? Rigidity may be localized or generalized.
Rebound tenderness is an unnecessary test; it merely confi rms the
presence of guarding and is often excessively painful for the patient.
Masses.• Assess their surface, edge, consistency, movement with
respiration, and overall mobility.
EVALUATION OF THE ABDOMEN
35
Organs.•
Liver• . Palpate from right lower quadrant into right upper quadrant,
feeling for the liver edge during inspiration every few cm upwards
until it is found. Assess the edge. Is it smooth/nodular/craggy?
Assess any palpable surface. Is it smooth/nodular/craggy?
Spleen• . Palpate from right lower quadrant into left upper quadrant,
feeling for the spleen edge during inspiration as for the liver. Assess
the edge and any palpable surface.
Kidneys• . Palpate bimanually in each loin. ‘Ballotting’ (bouncing the
kidneys between each hand) is of little additional value.
Percussion
Percussion identifi es the presence of excessive amounts of gas or fl uid. It
is also useful, when done carefully, in the confi rmation of the presence of
mild to moderate parietal peritoneal irritation (‘percussion tenderness’).
Gas• (hyperresonance). Is it generalized or localized? Is there
evidence of loss of dullness over the liver (suggestive of copious free
intraperitoneal gas)?
Fluid• (ascites). Usually identifi ed as ‘shifting dullness’; dullness in
the fl anks in the supine position moves to the lower portion of the
abdomen on turning to the lateral position.
Auscultation
To fully assess bowel sounds, it is necessary to listen for at least 1min, but
they are a notoriously unreliable sign of either intra-abdominal pathology
or bowel function. If commented on, bowel sounds should broadly be
divided into: absent, normal, active, or obstructive (characterized by high-
pitched, frequent sounds often with crescendos of activity, e.g. ‘tinkling’,
‘bouncing marbles’).
Abdominal assessment should always include a rectal examination in
adults; this is very rarely useful and should usually be avoided in children.
RUQ
LUQ
LLQRLQ
Central
Fig. 2.2 The fi ve quadrants: RUQ, right upper quadrant; LUQ, left upper
quadrant; LLQ, left lower quadrant; RLQ, right lower quadrant.

CHAPTER 2 Principles of surgery
36
Abdominal investigations
Faecal occult blood testing
May be chemical or immunological.•
Commonest use is as the primary community test for colorectal •
carcinoma (see b p. 400) as part of the National Bowel Cancer
Screening Programme.
Rigid proctoscopy and sigmoidoscopy (see b p. 216).
Flexible sigmoidoscopy
Very low risk (perforation 1 in 5000) outpatient procedure, usually •
performed without sedation.
Should visualize up to the descending colon.•
Allows minor therapeutic procedures (polypectomy, biopsy, injection).•
Colonoscopy
Low risk (perforation 1 in 1000) outpatient procedure, usually •
performed with sedation; requires bowel preparation.
Should visualize the entire colon (>95% of the time).•
Allows minor therapeutic procedures (polypectomy, including •
‘advanced’ endoscopic mucosal resection (EMR) and endoscopic
submucosal dissection (ESD), injection, marking by tattoo, and biopsy).
Typically used for: assessment of (suspected) colitis, diagnosis and •
assessment of colonic neoplasia, investigation of rectal bleeding.
Transabdominal ultrasound
Easy, safe, non-invasive, and avoids radiation dose.•
Typical uses include:•
Identifi cation of ovarian disease, e.g. in suspected acute appendicitis.•
Primary investigation of the biliary tree for gallstones, bile duct size, •
and liver parenchymal texture.
Investigation of suspected subphrenic or pelvic collections.•
Assessment of the liver/splenic parenchyma.•
Identifying free fl uid in abdominal trauma.•
CT scanning
Easy, non-invasive; requires signifi cant radiation exposure and •
intravenous (IV)/oral (PO) contrast.
Typical uses include:•
Primary assessment of all intra-abdominal masses.•
Staging of intra-abdominal and pelvic malignancy.•
Investigation of acute abdominal pain of unknown origin.•
Investigation of suspected intestinal obstruction.•
May be specifi cally tailored for pancreatic, biliary, visceral vessel •
assessment.
Investigation of suspected post-operative complications.•
MRI scanning
Conventional body scanner with external coils.•
Avoids radiation dose.•

ABDOMINAL INVESTIGATIONS
37
May be performed with specialized ‘contrast’ agents (e.g. ferumoxides).•
Typically used for:•
Investigation of suspected bile duct disease.•
Assessment of liver disease/possible metastases.•
Assessment of pancreas.•
Assessment of pelvic and retroperitoneal soft tissue disease, e.g. •
pelvic cancers.
Plain abdominal radiograph
Limited use.•
May identify intestinal obstruction, urinary tract stones, free intra-•
abdominal air, intra-abdominal fl uid.
Barium enema (double contrast, single contrast)
May be single contrast (contrast material fi lling the colon) or double •
contrast (dilute contrast and air to coat the mucosal surface of the
colon).
Requires bowel preparation and relatively mobile patient.•
Single contrast used to identify strictures and obstructions (used to •
assess colorectal anastomoses in dilute or water-soluble form).
Double contrast typically used to identify colonic neoplasia, assess •
colonic anatomy.
Intestinal transit studies
Serial abdominal X-rays to identify the progress of ingested radio-•
opaque markers.
Used to assess intestinal motility and transit time.•
PET scanning
Injection of radioactive metabolic substrate to identify metabolically •
active tissue.
Combined with high resolution CT scanning to co-locate ‘hot spots’.•
Typically used to:•
Identify unsuspected metastatic tumour deposits.•
Differentiate fi brosis from tumour post-surgery.•
Physiological testing
Manometry testing of the oesophagus, including lower oesophageal •
sphincter and the anal canal.
Pressure sensitivities of the oesophagus and anal canal.•
pH testing of the contents of the oesophagus (isolated or continuously •
for 24h).
Used to assess anorectal function, oesophageal motility and function, •
and gastro-oesophageal refl ux.

CHAPTER 2 Principles of surgery
38
Evaluation of pelvic disease
Positioning and inspection
Examination is performed in up to three positions: supine (for transabdom-
inal palpation of the ‘false’ pelvis); supine with hips fl exed and abducted
(for vaginal and bimanual palpation which may be performed to help assess
rectal disease); and left lateral position with hips fl exed (for rectal palpa-
tion and rigid endoscopy). Any intimate examination should always have a
chaperone present and particularly so for pelvic examinations.
Anus.
• Is the anus deformed? Is there evidence of mucosal or rectal
prolapse? Does the vaginal introitus look normal? Is there vaginal
prolapse or evidence of a cystocele? Are there scars from previous
surgery, sinuses, or evidence of sepsis?
Look for additional or abnormal tissue.• Are there skin tags, external
haemorrhoids, warts, or abnormal areas of skin (such as anal
intraepithelial neoplasia (AIN))? Is there an external punctum (as may
be seen in a fi stula) or the outer limit of a fi ssure visible?
Palpation
Palpate the lower abdominal quadrants.•
Rectal examination.• Is anal tone normal and the sphincter symmetrical?
Is the prostate normal size with a normal central sulcus? Does the
rectal mucosa feel normal? Is there any mass or tenderness anterior
to the upper rectum (pouch of Douglas)? The latter may be due to
sigmoid disease, small bowel in the pelvis, a pelvic appendix, or ovarian
disease.
Vaginal examination• (often omitted unless there is a clear indication
that valuable information may be gained from it). Is the cervix
present and normal? Is the vagina of normal calibre and feel? Is there
tenderness in either vaginal fornix?
Investigations
Rigid proctoscopy (‘anoscopy’)
Performed in outpatients without sedation.
•
Only visualizes the very lowermost rectum and anal canal (referred to •
as anoscopy in USA). Views may not be good if done without enema
preparation.
May be combined with therapy (banding, injection, or cryotherapy) for •
anorectal disorders.
Rigid sigmoidoscopy
Performed in outpatients without sedation.
•
Aims to visualize the rectum to the recto-sigmoid junction. The •
sigmoid colon is NOT adequately seen with this (referred to as
proctoscopy in USA). Views may not be good if done without enema
preparation.
Flexible sigmoidoscopy
Low risk, outpatient procedure, usually performed without sedation.
•
Should visualize up to the descending colon.•
Allows minor therapeutic procedures (polypectomy, tattoo, injection).•
EVALUATION OF PELVIC DISEASE
39
Transabdominal/transvaginal ultrasound
Easy, safe, and avoids radiation dose.•
Good for identifi cation of ovarian disease (e.g. in right iliac fossa pain).•
Endoanal/transrectal ultrasound
A 360• ° scanning endoanal/endorectal probe without sedation.
Endoanal scans. For assessment of anal sphincter integrity.
•
Transrectal scans. For assessment of some rectal tumours, prostatic •
disease (including biopsy), pre-sacral lesions.
CT scanning
Easy, safe, but signifi cant radiation exposure and IV contrast.
•
Investigation of choice for undiagnosed pelvic symptoms and post-•
operative complications.
MRI scanning
Usually via conventional body scanner with external coils (occasionally •
performed with endorectal coil).
Investigation of choice for the assessment of advanced rectal, •
gynaecological, and urological cancer, or complex pelvic sepsis.
Investigation of choice for complex pelvic and anal sepsis.•
Key revision points—pelvic anatomy
The true pelvis lies between the pelvic inlet (sacral promontory, •
illiopectineal lines, symphisis pubis) and outlet (coccyx, ischial
tuberosities, pubic arch).
Pelvic fl oor muscles (such as levator ani) support and are integral •
to the function of the anorectum, vagina, and bladder. They are
innervated by anterior primary rami of S2, 3, 4.
Anterior relations of the rectum (palpable during PR exam) are
•
(from below up):
Women—vagina, cervix, pouch of Douglas.•
Men—prostate, seminal vesicals, recto-vesical pouch.•

CHAPTER 2 Principles of surgery
40
Evaluation of peripheral vascular
disease
Positioning and inspection
Ideally, the patient should be examined in a warm environment at rest.
Remember fi rst to take the pulse and blood pressure, and examine the
abdomen (aneurysm, scars). Inspect the limb in the supine position, then
elevated (passively), and fi nally dependent. Expose the entire limb, includ-
ing the foot or hand to allow thorough inspection. If necessary, take any
dressings down (or ask for them to be removed if you are not happy to).
For venous disease, the patient should also be examined standing.
During supine inspection, look for the following.
Appearance.
• Are there any areas of established skin necrosis (dry
gangrene, e.g. apex of digits, between digits, heel of the foot)? Are
there changes of chronic venous stasis (fl are veins, venous eczema,
lipodermatosclerosis, leg ulceration)?
Colour.• Waxy white suggests severe acute ischaemia; blue and mottled
suggests potentially irreversible acute ischaemia; dark red/purple
suggests chronic ischaemia.
Colour changes during position.• Note the angle at which the skin of
the limb blanches when passively elevated (Buerger’s test). Normal
limbs may not blanch at all. An angle of 15° or less suggests severe
ischaemia. Note the presence and delay in change in colour when the
limb is dependent. Ischaemic limbs slowly turn deep purple.
Ulcers.• What is the location (digital or foot suggests arterial disease)?
Be sure to inspect between the toes/fi ngers and on the plantar surface
of the foot (especially for diabetic disease).
Venous inspection.• Stand the patient up. Inspect for varicose veins. Are
they in the long saphenous or short saphenous distribution?
Palpation
Temperature.• Does the skin feel cold or warm? Is there a transition
level?
Skin capillary compression and refi ll.• Normal is 2s or less. A delay of
greater than 5s suggests signifi cant ischaemia.
Peripheral pulses.• Start with the most proximal (major) vessels and
work distally. Record if the pulse is normal, reduced, or absent. Record
if there are any thrills palpable.
In venous disease, tests of • venous competence may be performed (see
b p. 666).
Surgical grafts.• Palpate the course of any surgical grafts and record the
presence or absence of pulses.
Auscultation
Listen for bruits. Are there bruits in the proximal vessels (suggestive of
stenosis)?

EVALUATION OF PERIPHERAL VASCULAR DISEASE
41
Investigations
Doppler ultrasound
Straightforward and portable.•
May be used to confi rm or refute the presence of fl ow in a vessel or •
graft.
May be used to evaluate the relative fl ow in vessels by measuring the •
pressure at which detectable fl ow ceases using a compression cuff.
The commonest example is ankle–brachial pressure index (ABPI).
May be used to evaluate the presence of refl ux in veins.•
Colour fl ow duplex
Combined two-dimensional (2D) ultrasound image with Doppler-•
derived fl ow represented using colour, superimposed in real time.
May be used for assessment of stenosis/occlusion in vessels or grafts.•
May be used for assessment of refl ux or occlusion in deep and •
superfi cial veins.
Direct angiography
Most commonly, digital subtraction angiography (DSA; used to reduce •
background image ‘noise’ and convert the arterial images to black for
easier viewing).
Invasive, requiring direct arterial puncture with the associated risks.•
Requires IV contrast with the small risk of allergy (relatively •
contraindicated in renal dysfunction or where renal blood fl ow is
poor).
Gives direct views of arterial tree, but lumen only so not good for •
aneurysm sizing.
Magnetic resonance angiography
Provides images of an arterial tree based on the presence of arterial
•
fl ow during scanning.
Safe and non-invasive; requires no ‘contrast’, but commonly gadolinium •
used to highlight fl owing blood.
Tends to overestimate degree of stenosis due to very low fl ow being •
underrepresented.
CT angiography
Requires multislice rapid acquisition (‘helical’/’spiral’) scanner.
•
Images acquired in arterial phase after IV injection of contrast.•
Three-dimensional (3D) reconstruction allows ‘virtual angiogram’ •
images to be produced.
Fast and relatively safe, especially where direct angiogram is diffi cult, •
e.g. visceral vessels.
Requires dose of IV contrast, so caution with allergy and renal •
dysfunction.
CHAPTER 2 Principles of surgery
42
Evaluation of the skin and
subcutaneous tissue disease
Assessment and description of a lump
Key features in the history include the following:
Speed of development.
• Rapid increase in size is suspicious of malignancy
(primary or secondary).
Recent change in size.• Suggests malignant change or infection in a
previously benign lesion.
Associated symptoms.• Paraesthesia or weakness suggests involvement
of nerves; reduced movement suggests involvement of muscle.
History of local trauma.• May indicate a cause, although a previously
undiagnosed underlying lump should always be suspected.
The following features should all be considered when examining the lump.
Basic facts
Position.
•
Size.•
Shape.•
Features of infection or infl ammation
Temperature.
•
Tenderness.•
Colour.•
Features of malignancy
Surface (e.g. craggy).
•
Edge (e.g. irregular).•
Consistency (e.g. hard).•
Features of fl uid or vascular lesions
Fluctuant (fl uid-fi lled).
•
Presence of thrill (fl uid-fi lled connected to the vascular tree).•
Transilluminance (fl uid-fi lled).•
Pulsatile (arterial lesion).•
Presence of a bruit (arterial lesion).•
Presence of expansility (indicative of an arterial aneurysm).•
Presence of compressibility (e.g. venous lesion or arteriovenous •
malformation).
Features of locoregional invasion
Tethering to surrounding structures.
•
Involvement of surrounding structures (e.g. nerves).•
Regional lymphadenopathy.•
Lumps in detail
Superfi cial lumps, see • b pp. 600–603.
Neck lumps, see • b pp. 222, 224.
Abdominal lumps and herniae, see • b p. 336.
Scrotal lumps, see • b p. 366.
EVALUATION OF THE SKIN AND SUBCUTANEOUS TISSUE DISEASE
43
Assessment and description of an ulcer
Key features in the history include the following:
Is it painful (venous, diabetic, and neuropathic ulcers are painless)?•
Did it start as an ulcer or did a lump become ulcerated (suggests a •
malignancy in/of the skin)?
Is there a history of underlying infection, e.g. of bone?•
Describe the basic morphology of the ulcer:
Location.•
Over pressure points and bony prominences suggests pressure •
sore.
Medial shin suggests venous ulcer.•
Lateral shin, dorsum of foot, toes suggest arterial ulcer.•
Edge.•
Sloping edge suggests conventional ulcer (can be many aetiologies).•
Rolled edge is typical of basal cell or squamous carcinomas.•
Everted edge suggests squamous or metastatic carcinomas.•
Vertical edge (punched out) suggests syphilis or chronic infection.•
Base.•
Friable, red, and bleeding suggests venous or traumatic.•
Green slough suggests infected.•
Black hard eschar suggests chronic ischaemia.•
Discharge.• May suggest an underlying cause, e.g. intestinal fi stula with
enteric content, golden pus in chronic actinomycosis.
Surrounding tissue.• Erythema and swelling suggest secondary infection.
Ulcers in detail
Cutaneous malignancy, see • b p. 647.
Ischaemic ulcers, see • b p. 647.
Venous ulcers, see • b pp. 148, 666.
Fistulas, see • b p. 150.
Wound infections, see • b p. 104.

CHAPTER 2 Principles of surgery
44
Surgery at the extremes of age
Surgery is increasingly used in older and older patients and the range of
procedures available to surgeons for both the very elderly and the very
young and neonates is increasing. Minimally invasive surgery is increas-
ingly being offered to older patients at risk from open surgery. Both these
groups need particular attention and have specifi c potential problems.
Surgery and the elderly
Common misconceptions corrected
Elderly patients benefi t just as much from potentially curative cancer •
surgery as younger patients. Cancers demonstrate the same range of
behaviours in all ages and are neither more ‘benign’ nor less responsive
to treatment in the elderly.
Minimally invasive procedures in the elderly can offer all the benefi ts •
available to younger patients.
‘Palliative’ procedures for benign disease (e.g. cholecystectomy, joint •
surgery, eye surgery) are just as important in the elderly as they
may allow preservation of independence and offer just as much
improvement in quality of life as in the young.
Common problems in the elderly
Multiple comorbidities and polypharmacy increase the scope for •
potential complications and drug interactions.
Comorbidities are often ‘silent’, either due to atypical presentation or •
underreporting of symptoms (e.g. angina may not be manifest due to
reduced mobility).
Social, family, nursing, and medical support structures are often •
complex and easily lost during a hospital admission.
Reduced or acutely impaired mental faculties may make history taking •
and consent taking diffi cult.
Reduced or abnormal immune responses may reduce or impair some •
physical signs (e.g. clinically detectable peritonism may be absent).
The elderly are particularly prone to mild or moderate chronic •
malnutrition, increasing general complication rates, and the risk of
pressure sores, etc.
Strategies for the management of the elderly
Involve all the necessary specialities as soon as possible (prior to •
admission for elective surgery), e.g. elderly care, anaesthetists,
physicians.
Consider pre-optimization in critical care (high dependency unit •
(HDU)), especially in urgent or emergency surgery.
Start to plan for discharge on the day of admission and liaise with the •
GP and family, if necessary.
Consider nutrition as soon as possible after surgery. Is hyper-•
alimentation necessary?
Surgery and the young
Although most surgery undertaken in neonates and very young children is
done so by specialist paediatric surgical and nursing teams, most surgeons

45
SURGERY AT THE EXTREMES OF AGE
will care for young children at some time and the principles of care used
in paediatric surgery can be usefully applied to older children.
Common problems in children
Young children may not be able to accurately report symptoms and
•
illness behaviour is often non-specifi c.
Cardiovascular responses in the young are excellent. Tachycardia and •
particularly hypotension are (very) late signs of hypovolaemia.
2 Tips for managing children
Take the history from the parents or carers
• and the child.
Remember infections are common and often present with non-specifi c •
signs.
Consider non-surgical diagnoses at all times, e.g. meningitis, urinary •
sepsis, systemic viral infections.
Examine the child as much as possible while they are sitting on a •
parent’s lap. Use the same position for phlebotomy and siting cannulae.
Put local anaesthetic cream on phlebotomy sites 30min in advance.•
Some children are simply too young to cooperate with procedures •
under local anaesthetic and will require general anaesthesia for
relatively trivial procedures.
Make sure all prescriptions for drugs and fl uids are written according •
to weight to avoid inadvertent adult dosing—if in doubt, ask.
Fluid balance may be critical since small volume changes are highly •
signifi cant in small children. Pay close attention to fl uid resuscitation.
Paediatric surgery
Conversion tables, see • b p. 424.
Paediatric surgery, see • b pp. 424–474.
Consent and children, see • b p. 14.

CHAPTER 2 Principles of surgery
46
Day case and minimally invasive
surgery
Day surgery procedures
An increasing number of procedures in all aspects of surgery are being
performed as day surgery. The key features that make a procedure suit-
able include:
Low risk of major complications.
•
Predictable recovery period not requiring specialist post-operative •
therapy or treatment.
Post-operative analgesia that does not need routine opiates.•
Anaesthetic technique not requiring invasive monitoring, prolonged •
muscle relaxation, or epidural/spinal anaesthesia.
Low risk of diffi cult or unpredictable anaesthetic technique.•
Many areas of surgery are now performed routinely as day surgery, includ-
ing minor and intermediate anorectal surgery, hernia surgery, minor lapar-
oscopic surgery, arthroscopy, and minor endoscopic bladder surgery.
Selection of patients for day case surgery
Most hospitals have well defi ned protocols to select patients for suitability
for day surgery and most day surgery units conduct their own pre-admis-
sion assessment either by telephone or questionnaire. Typical criteria
might include:
Maximum age of 75y (this upper limit has gradually increased as •
familiarity with the procedures has grown).
Appropriate social support for the patient at home, including transport •
and a responsible adult to monitor progress.
No history of more than mild to moderate cardiac or respiratory •
disease (e.g. uncomplicated asthma or controlled angina).
Non-insulin dependent diabetes only (unless for local anaesthetic (LA) •
procedures).
Body mass index (BMI) below 35 (typically)—higher than this is •
associated with increased risk of anaesthetic and surgical complications.
Minimally invasive surgical procedures
Minimally invasive surgery is becoming more common in many areas of
surgery. It is a broad term that includes many types of procedure and
there is much overlap with conventional ‘open’ surgery and, at the other
end of the spectrum, interventional radiological procedures. A useful
defi nition of minimally invasive surgery is a procedure that can be per-
formed by a technique involving fewer or smaller incisions than alternative
‘conventional’ surgery or under less invasive anaesthetic techniques. This
includes most laparoscopic and thoracoscopic surgery (cholecystectomy,
gastric fundoplication, colectomy, lobectomy, nephrectomy, adrenalec-
tomy). It also includes fl exible and rigid endoscopic procedures (diagnostic
and therapeutic colonoscopy, cystoscopy, transurethral prostate surgery,
hysteroscopic surgery), and several procedures using specifi c techniques
or equipment (e.g. transanal endoscopic microsurgery, subfascial endo-
scopic venous surgery).

DAY CASE AND MINIMALLY INVASIVE SURGERY
47
Advantages of minimally invasive surgery
Many minimally invasive surgical techniques require specifi c training to
perform and utilize expensive equipment and consumables so surgeons
and managers look to minimally invasive surgery to provide benefi ts to
both patients and hospitals. Although some benefi ts can be achieved by
modern post-surgical management, there are demonstrable benefi ts in dif-
ferent areas.
Patient benefi ts
Smaller, fewer, or absent scars.
•
Reduced time in hospital.•
Fewer post-operative complications (particularly wound and •
respiratory-related).
Surgeon benefi ts
Reduced post-operative stay.
•
Possible avoidance of the need for interventional anaesthetic •
techniques such as epidurals.
Hospital benefi ts
Increased bed use effi ciency.•
Reduced post-operative complications.•
To whom should minimally invasive surgery be offered?
The advantages of minimally invasive surgery give it a wide application.
Young patients.• Small scars and short hospital stays are ideal.
Elderly. • Reduced post-operative complications and shortened hospital
stay are vital in patients who often have multiple comorbidities.
Unfi t patient.• Easier anaesthetic techniques and reduced surgical stress
may reduce the perioperative risk.

CHAPTER 2 Principles of surgery
48
Surgery in pregnancy
Changes in anatomy and physiology
Pregnancy results in several changes relevant to surgery.
First trimester
Drugs may have teratogenic effect (see Box 2.1).•
Reduced lower oesophageal sphincter tone, increasing the risk of •
gastro-oesophageal refl ux and aspiration when supine.
Second trimester
Drugs may have adverse effect in fetal development or metabolism •
without causing gross malformation.
Increased susceptibility to urinary tract infections, particularly •
ascending renal infections and pyelonephritis.
Increased risk of venous thromboembolism rises in the second •
trimester and remains constantly raised in the third.
Increased susceptibility to superfi cial infections.•
Third trimester
Drugs may induce labour.
•
Displacement of the mobile abdominal viscera superiorly and behind •
the enlarging uterus. In particular, the appendix comes to lie in the
right upper quadrant.
Risk of hypotension in the supine position due to inferior vena caval
•
compression by the gravid uterus: this can be avoided by positioning
the sedated or unconscious patient in slight lateral decubitus.
Risks of miscarriage
The risk of miscarriage related to surgical pathology and surgery varies
according to trimester. It is highest in the fi rst. The risk of a viable pre-
mature labour rises in the third trimester. The risk of miscarriage induced
by general anaesthetic (GA) is always balanced against the risk induced by
sepsis from untreated surgical pathology, particularly acute appendicitis. It
is a common dilemma in surgical practice. Ultrasound imaging may be less
useful due to poor views and CT scanning is contraindicated due to radia-
tion dose. Diagnostic laparoscopy is contraindicated due to the effects of
pneumoperitoneum on the pregnancy. The only way to a diagnosis may be
surgery, once important differential diagnoses have been excluded.
Pregnancy testing
Urinary dipstick • B-HCG is 91% sensitive (even lower for women self-
testing). Specifi city ranges from 61% to 100% if tested from the fi rst
day of the fi rst missed period (2 weeks after ovulation).
Blood
• B-HCG is almost 100% sensitive and specifi c and able to
detect pregnancy 6–8 days after ovulation.
False negatives and positives are most commonly due to user error.•

SURGERY IN PREGNANCY
49
Common differential diagnoses of appendicitis in pregnancy
Ectopic pregnancy complications.•
Pyelonephritis.•
Threatened miscarriage/placental abruption.•
Box 2.1 Prescribing drugs in pregnancy
It is clearly unethical to screen drugs for harmful effects on the human
fetus; many new and commonly used drugs have, therefore, never been
used in pregnancy. Some older drugs have been used in pregnancy and
are regarded as ‘safe’ in the absence of any reports of fetal harm. There
is an important balance to maintain between treating serious illness in
the mother and potentially harming the fetus. Generally:
Avoid prescribing drugs, if at all possible.•
Know the stage of the pregnancy; many drugs are only approved in •
particular trimesters.
2 Check every drug that you prescribe in Appendix 4 of the British
National Formulary (BNF).
If in doubt, seek specialist advice.•
Important teratogens include:•
Thalidomide (an antiemetic).•
Carbamazepine and sodium valproate.•
Isotretinoin (Roaccutane•
®
).
Tetracycline.•
Warfarin.•
Angiotensin-converting enzyme (ACE)-inhibitors.•
Lithium.•
Methotrexate, cyclophosphamide.•

CHAPTER 2 Principles of surgery
50
Surgery and the contraceptive pill
(O)estrogen-containing contraceptive pills (EOCP) increase the risk
of thromboembolic disease in women taking them prior to surgery.
Progesterone-only contraceptives appear to pose little or no additional
risk and may be continued during surgery. The increase in risk is related
to the size of the operative procedure and the existing comorbidity; the
advice is adjusted accordingly.
Low risk procedures.• Dental, day case, minor laparoscopic. EOCP may
be continued.
Medium risk. • Abdominal, orthopaedic, major breast surgery.
EOCP should be discontinued at least 1 month prior to elective •
surgery.
Urgent or emergency surgery should be conducted with full •
thromboprophylaxis (see b p. 72).
High risk.• Pelvic, lower limb orthopaedic surgery, cancer.
EOCP should be discontinued at least 1 month prior to elective •
surgery.
Urgent or emergency surgery should be conducted with extended •
thromboprophylaxis (see b p. 72).
This page intentionally left blank

CHAPTER 2 Principles of surgery
52
Surgery in endocrine disease
Diabetes
Specifi c perioperative risks
Hypoglycaemia, hyperglycaemia, or ketoacidosis.•
Underlying diabetes-related comorbidity is often unrecognized (e.g. •
mild renal impairment, small-vessel coronary and cerebrovascular
disease, mild autonomic neuropathy with associated reduced
cardiovascular homeostasis responses).
Increased susceptibility to infection, poor wound healing.•
Increased susceptibility to skin pressure necrosis.•
Management of the diabetic patient
Inform the anaesthetist, the diabetologist, and any specialists involved •
in the patient’s ongoing care, e.g. nephrologists.
Clarify if the patient is oral-controlled, insulin-dependent (low or •
high requirement), or brittle insulin-dependent since the risk of
perioperative problems increases with each group.
Diabetics should be fi rst on operating lists to ensure timings can be as •
predictable as possible for blood sugar management.
Check preoperative investigations for signs of underlying comorbidity.•
Ketoacidosis in the perioperative period is associated with a very high •
morbidity and mortality and should be avoided at all costs.
Minor surgery
Oral-controlled.• Give normal regimen.
Insulin-controlled. • Omit preoperative insulin on day of surgery; monitor
blood sugar (BS) every 4h; restart normal insulin once oral diet is
established.
Major surgery
Oral-controlled.
• Omit long-acting hypoglycaemics preoperatively.
Monitor BS every 4h. If BS exceeds 15mmol/L, start IV insulin regimen.
Insulin-controlled.• Commence on IV insulin sliding scale preoperatively
once nil by mouth (NBM) and continue until normal diet is
re-established. Check BS every 4h. Restart normal insulin regimen
(initially at half dose) once oral diet is established.
Emergency surgery
Check for existing ketoacidosis. If present, use medical treatment
•
algorithm to control BS and postpone surgery until BS <20mmol/L
unless the condition is life-threatening.
Use IV insulin sliding scale for all patients to optimize BS control. A •
typical IV sliding scale (Actrapid
®
with 5% dextrose) is:
BS <4mmol/L, infusion 0.5U/h + consider medical review.•
BS 4–15mmol/L, infusion 2.0U/h.•
BS 15–20mmol/L, infusion 4.0U/h.•
BS >20mmol/L, infusion 4.0U/h + consult diabetology team and •
consider treatment as for ketoacidosis.

SURGERY IN ENDOCRINE DISEASE
53
Steroids
Specifi c perioperative risks
Oral steroids are used to treat a number of common illnesses, including
rheumatoid arthritis, severe asthma, and COPD, temporal arteritis, and
polymyalgia rheumatica. Steroids reduce neutrophil and fi broblast func-
tion and immune response and lead to irreversible changes in connective
tissue. Long-term use of systemic steroids results in adrenal suppression.
The following problems are associated with chronic steroid use.
Addisonian (hypoadrenal) crisis (see Box 2.2)
Increased susceptibility to infection.
•
Poor wound healing, including anastomotic leaks, pressure areas.•
Osteoporosis.•
Patients on long-term inhaled steroids, e.g. for asthma and COPD, are •
not high risk as there is minimal systemic absorption.
Management of the patient on steroids
If the steroid dose can be weaned preoperatively, this should be done.
•
Prescribe IV hydrocortisone 25–100mg qds (roughly corresponding to •
2.5–20mg od of prednisolone) to start on the morning of surgery and
continuing until the patient is able to go back to their oral steroids.
Thyroid disease (see b pp. 250–256).
Box 2.2 Addisonian (hypoadrenal) crisis
Stresses such as surgery and sepsis require increased adrenal secre-
tion of corticosteroids; failure to mount this response can result in an
Addisonian (hypoadrenal) crisis. The following groups of patients are
at high risk:
Any patient currently taking >5mg prednisolone for >2 weeks.•
Any patient who reduced their long-term steroids within 2–4 weeks.•
Patients who have undergone adrenalectomy.•
Clinical features
Lethargy and malaise.•
Abdominal pain, often poorly localized (may present as an acute •
abdomen).
Nausea and vomiting.•
Hypotension.•
Hypoglycaemia, hyponatraemia.•
Coma, death.•
Management
Treat with IV hydrocortisone 100mg qds or 400mg infusion over 24h
•
as long as the patient is NBM.
Fluid resuscitation with normal saline.•
50% dextrose IV to treat hypoglycaemia (titrate against BS).•

CHAPTER 2 Principles of surgery
54
Surgery and heart disease
Ischaemic heart disease
Risk factors include age (♂ >45y; ♀ >55y), family history of early MI,
current or treated i BP, smoking, diabetes, i cholesterol.
Assess severity: quantify exercise tolerance; enquire about palpitations, •
orthopnoea, use of anti-anginals, previous MI, percutaneous coronary
intervention (PCI), or coronary artery bypass graft (CABG).
The ECG is the most important routine screening test, but it is normal •
in about one-third of patients with proven ischaemia.
Symptomatic patients undergoing major surgery should be •
discussed with a cardiologist with a view to optimizing anti-anginal
medications.
Myocardial infarction
The risk of a perioperative MI relates to past history and risk factors.
Overall population incidence after abdominal surgery, 0.5%.•
Incidence with pre-existing cardiovascular symptoms, 2%.•
Incidence with previous MI (old), 5–10%.•
Incidence after recent MI, 25% (70% will die with re-infarction).•
Strategies to reduce risk
Non-urgent surgery should be delayed for at least 6 months following
•
acute MI and, possibly, acute ischaemia. Cancer surgery may be
undertaken if the risk of disease progression is felt to outweigh the
increased perioperative mortality rate.
Ensure all normal cardiovascular medication is continued up to and •
through surgery. Control any new symptoms of angina if surgery is
urgent.
Continue antiplatelet medication if not contraindicated.•
Consider involving the critical care services (HDU) for the •
perioperative period.
Valvular heart disease
Cardiac murmurs are common. Request a transthoracic echo to evaluate
the lesion and discuss abnormalities with a cardiologist.
Severe • aortic stenosis carries a high risk of mortality (see b p. 630).
Elective surgery should be postponed: high gradient aortic stenosis
carries an associated mortality of 10% with non-cardiac surgery.
Severe • mitral stenosis can lead to pulmonary oedema and heart failure.
Major elective surgery should be postponed until lesion corrected.
Aortic regurgitation• requires attention to fl uid and rate control.
Antibiotic prophylaxis should be given, but surgery can go ahead.
Mitral regurgitation• (MR) should be managed with diuretics
and vasodilators. Beware: left ventricular function is frequently
overestimated in MR.
Prosthetic valves
• have several associated issues.
Mechanical valves• require anticoagulation. Stop warfarin 5 days
preoperatively and admit early for IV heparinization.

SURGERY AND HEART DISEASE
55
Do not heparinize if the international normalized ratio (INR) will be •
only briefl y subtherapeutic.
Stop IV heparin 6h pre-surgery and resume as soon as surgical •
bleeding is no longer a problem until INR therapeutic.
Thrombosis is most likely in mechanical mitral valves, atrial fi brillation •
(AF), poor left ventricle (LV), previous embolus, ball-and-cage valves.
In surgery for life-threatening bleeding, e.g. bleeding peptic •
ulcer, intracranial haemorrhage, it may be necessary to reverse
anticoagulation for several days. Liaise closely with cardiology.
2 Prosthetic valves no longer require antibiotic prophylaxis for
procedures that cause bacteraemias
1
; if in doubt, discuss with
cardiology.
Arterial hypertension
Control of BP preoperatively may reduce the tendency to perioperative
ischaemia. Always note BP and, if severe (>180mmHg), surgery should be
delayed until control is obtained.
Review existing antihypertensive management or start treatment:•
Beta-blockers (e.g. metoprolol 25–50mg PO tds) reduce BP and •
perioperative ischaemia and mortality.
Calcium channel blockers are often used, e.g. nifedipine 10mg •
sublingual (SL).
Look for evidence of end-organ damage and associated heart disease.•
Look for rare, but important causes: phaeochromocytoma, •
hyperaldosteronism, coarctation of the aorta, renal artery stenosis.
Congestive cardiac failure
Heart failure is associated with a poorer outcome in non-cardiac surgery.
Risk factors include ischaemic and valvular heart disease.
Listen for S•
3
as well as pedal oedema, raised jugular venous pressure
(JVP), bibasal crepitations.
Chest X-ray may show cardiomegaly or pulmonary oedema.•
Cardiac arrhythmias
Arrhythmias and conduction defects are common. Asymptomatic arrhyth-
mias are not associated with an increase in cardiac complications, but look
for underlying problems, e.g. ischaemic heart disease, drug toxicity, meta-
bolic derangements.
High grade conduction abnormalities, e.g. complete heart block, should •
be discussed with a cardiologist. Pacing may be indicated.
Patients with known AF and either a history of embolic stroke or •
associated structural cardiac defect normally take warfarin.
Request a cardiology review preoperatively if rate control is poor.•
Beware of the patient with the • permanent pacemaker or intracardiac
defi brillator (ICD). Diathermy may cause the pacemaker to reset or
completely inhibit pacing and trigger ICD discharge.
Pacemakers and ICDs should be evaluated by a cardiac technician •
preoperatively and post-operatively.
Pacemakers should be changed to fi xed-rate pacing for surgery and •
then reprogrammed after surgery.

CHAPTER 2 Principles of surgery
56
ICDs should be switched off to prevent discharge and external •
fi brillator pads positioned on the patient.
If defi brillation or synchronized cardioversion is required, place the •
paddles as far from the pacemaker or ICD as possible.
Type of diathermy used should be considered. Monopolar is not •
absolutely contraindicated, but bipolar may be preferable.
Reference
1 M http://publications.nice.org.uk/prophylaxis-against-infective-endocarditis-cg64
This page intentionally left blank

CHAPTER 2 Principles of surgery
58
Surgery and respiratory disease
Surgery and smoking
Smoking tobacco increases the risks of anaesthesia and many of the risks
of surgery. There is a six-fold increase in post-operative respiratory com-
plications among patients smoking in excess of ten cigarettes per day.
Effects of smoking
Reduction in general and specifi c immune function via reduced •
neutrophil chemotaxis and reduced natural killer (NK) cell effi cacy.
Increased platelet aggregation (probably explaining the increased risk •
of perioperative acute MI and cerebrovascular accident in smokers).
Reduced oxygen-carrying capacity of blood per unit volume due to the •
presence of carboxyhaemoglobin increasing the risk of tissue hypoxia
in susceptible organs.
Increased upper aerodigestive mucosal secretions. This worsens •
initially after stopping smoking until the chronic effects on the mucosa
wear off.
Reduced mucociliary escalator function.•
Reduced lung compliance and increased ‘closing volume’ of the small •
airways, increasing the risk of air trapping, especially whilst supine in
the post-operative period.
Stopping smoking
Within 48h: carboxyhaemoglobin is cleared from the blood, platelet •
aggregation begins to return to normal.
Within 7 days: neutrophil, macrophage, and NK cell function improve. •
Mucus production temporarily increases, but mucociliary escalator
function takes up to 6 weeks to recover, leading to a ‘rebound’ effect.
Within 6 weeks: upper aerodigestive function returns to underlying •
level, lung dynamics improve to ‘normal’ levels (depending on the
extent of fi xed parenchymal disease).
The optimal time for stopping smoking is at least 6 weeks prior to surgery,
but a minimum of 7 days is required to reduce the ‘rebound’ effects of
stopping on upper aerodigestive tract function.
2 Mitigating the effects of smoking in the post-operative period
Active and recently stopped smokers should receive extra attention to
prevent the risks associated with smoking and surgery.
Ensure patients remain well hydrated until oral intake is restored.
•
Use thromboembolic prophylaxis in most cases.•
Use preoperative chest physiotherapy and education on breathing and •
coughing techniques.
Mobilize as soon as possible post-operatively.•
Consider the use of epidural anaesthesia to improve compliance with •
post-operative physiotherapy.
Use preoperative and post-operative saline nebulizers 5mL qds.
•
Ensure post-operative analgesia is effective.•

SURGERY AND RESPIRATORY DISEASE
59
Respiratory conditions
Respiratory tract infection
An active respiratory tract infection may be suffi cient reason to cancel
elective patients, so ask about cough, fevers, and sputum, but minor colds
and nasal discharge may not prevent GA.
If you suspect the patient has a respiratory tract infection, check their •
temperature, C-reactive protein (CRP), and white cell count (WCC)
early.
Elective patients should be cancelled and asked to return in 2 weeks if •
their symptoms are better.
Reserve antibiotics for patients with suspected bacterial infections; •
most acute respiratory tract infections are viral.
Asthma
Assess severity of asthma by asking about hospital admissions, inhalers, •
nebulizers, peak expiratory fl ow rates (PEFR), and home oxygen.
Elective surgery should ideally coincide with remission of symptoms.•
Identify patients on long-term steroid therapy.•
Sometimes it is possible to time surgery to coincide with a •
reduction in steroids, but this requires several weeks’ notice.
Any patient taking more than 5mg daily prednisolone and •
undergoing inpatient surgery or presenting with sepsis should
be started on an equivalent dose of IV hydrocortisone; adrenal
suppression may otherwise result in an Addisonian crisis (see b
p. 52)
Patients receiving a general anaesthetic generally experience •
deterioration in their lung function (see b p. 108). Prophylactically
increase their normal therapy by converting inhalers to nebulizers and
increasing frequency.
Chronic obstructive pulmonary disease (COPD)
If dyspnoea is the prominent symptom and the patient has COPD, get
•
lung function tests, including blood gases.
Admitting these patients a few days early for physiotherapy, education, •
and nebulizers can reduce the length of hospital stay.
Patients receiving a general anaesthetic generally experience •
deterioration in their lung function (see b p. 108). Prophylactically
increase their normal therapy by converting inhalers to nebulizers and
increasing the frequency.
Prescribe 6-hourly 5mL nebulized saline and give humidifi ed oxygen •
wherever possible (to prevent mucus plugging).
Ensure the patient gets twice daily chest physiotherapy.•
Ensure the patient is on their usual inhalers and consider converting •
these to nebulizers for major surgery (see b p. 108).

CHAPTER 2 Principles of surgery
60
Surgery in renal and hepatic disease
Renal impairment
Renal impairment covers a spectrum, ranging from patients with subclinical
dysfunction (normal serum creatinine and urea, but borderline creatinine
clearance) to patients with end-stage renal failure. It is helpful to consider
these patients in two main groups: patients with chronic renal impairment
and dialysis-dependent patients. Post-operative management of renal
impairment is discussed on b p. 110; dialysis is discussed on b p. 134.
Chronic renal impairment
Surgery may precipitate acute renal failure in patients with chronic renal
impairment.
Avoid hypovolaemia and hypotension. Ensure these patients receive •
adequate IV hydration if they are to be NBM for any length of time.
Avoid nephrotoxic drugs wherever possible, including non-steroidal •
anti-infl ammatory drugs (NSAIDs), aminoglycosides, ACE-inhibitors,
and radiological contrast.
Reduce doses of drugs with renal elimination, e.g. morphine, low •
molecular weight heparin (LMWH), digoxin, and request appropriate
levels frequently.
Patients with established renal failure, on dialysis
Discuss post-operative management of patients undergoing major •
surgery with the anaesthetist and intensive care unit (ICU) as early as
possible.
Dialysis should be performed the day before surgery.•
Patients must have full blood count (FBC) and urea and electrolytes •
(U&E) on admission, pre- and post-dialysis, and twice daily U&E post-
major surgery until the patient is stabilized on their normal dialysis
regime.
Reduce doses of drugs with renal elimination, e.g. morphine, LMWH,
•
digoxin, and request appropriate levels frequently.
If the patient is normally anuric, there is little point in inserting a •
urinary catheter which exposes them to unnecessary infection risk.
Note the sites of arteriovenous fi stulas. • Never use them for
phlebotomy or cannulation and avoid using BP cuffs on that side.
These patients are prone to several problems. • 2 Hyperkalaemia,
acidosis, and pulmonary oedema are potential life-threatening
emergencies. Management is described on b p. 110.
Infection.•
Anaemia and coagulopathy.•
Fluid and electrolyte disturbances.•
Metabolic acidosis.•
Systemic hypertension, pericarditis.•
Hepatic impairment
The risk posed by liver disease to patients undergoing general surgery was
graded by Child and Turcotte (see Box 2.3). Child grade C is associated
with high perioperative mortality.

SURGERY IN RENAL AND HEPATIC DISEASE
61
Liver failure causes the following problems:•
Hypoglycaemia.•
Hepatic encephalopathy.•
Coagulopathy.•
Ascites.•
Infection.•
Several factors may cause acute decompensation of mild hepatic •
impairment and should be avoided or treated aggressively in this
group:
Infection, especially bacterial peritonitis.•
Sedation.•
Diuretics.•
Constipation.•
Electrolyte imbalance.•
Dehydration and hypotension.•
Preoperatively:•
Check hepatitis serology.•
Request liver ultrasound in newly diagnosed hepatic impairment.•
Discuss requesting additional blood products with haematology.•
Discuss doses of standard medication with a specialist.•
Jaundice
Patients with obstructive jaundice are at risk of developing renal failure
post-operatively (hepatorenal syndrome). This is thought to be due to
the nephrotoxic effect of toxins normally eliminated by the liver as well
as circulatory changes.
Ensure adequate hydration. When the patient is NBM, prescribe IV
•
normal saline 1L over 6–8h.
Insert a urinary catheter and start an hourly fl uid balance chart.•
Measure U&E and liver function tests (LFTs) daily.•
Coagulopathy in longstanding cholestatic jaundice may be improved •
with vitamin K 1mg IV: discuss with haematology.
Avoid or reduce the doses of hepatotoxic drugs and drugs with •
hepatic elimination.
Box 2.3 Child’s classifi cation of surgical risk in hepatic
dysfunction
A (minimal risk) B (moderate risk) C (advanced risk)
Serum bilirubin
•
<20mg/L
Serum bilirubin •
20–30mg/L
Serum bilirubin •
>30mg/L
Serum albumin •
>35g/L
Serum albumin •
30–35g/L
Serum albumin •
<30g/L
No ascites• Controlled ascites• Uncontrolled ascites•
No focal neurology• Minimal neurological •
dysfunction
Coma•
Excellent nutrition• Good nutrition• Cachexia•

CHAPTER 2 Principles of surgery
62
Surgery in neurological disease
Cerebrovascular accidents (stroke)
Ischaemic events are associated with a risk of re-infarction or •
extension of the infarct area due to interference with cerebrovascular
autoregulation by anaesthetic agents. Autoregulation is re-established
in around 6 weeks.
Haemorrhagic infarcts are associated with a small increased risk of
•
further bleeding, especially if the patient is given thromboprophylaxis.
Penumbral oedema also interferes with autoregulation as for ischaemic
events.
Strategies to reduce risk
Delay all non-essential surgery for 6 weeks following infarcts, especially •
ischaemic ones.
Consider omitting thromboprophylaxis in patients with a recent •
haemorrhagic event.
Ensure BP is well controlled (the prevention of both hypotension and •
hypertension) in the perioperative period to reduce fl uctuations in
cerebral blood fl ow.
Avoid positioning the patient head down on the operating table as this •
increases cerebral venous pressure.
Epilepsy
Paroxysmal neuronal discharge from various areas of the brain causes a
range of disturbances that may affect consciousness (grand mal seizures,
and petit mal or absences), movement, or sensory perception. In addition
to epilepsy, cerebral space-occupying lesions, uraemia, cerebral oedema,
drug toxicity, and hypercalcaemia may cause the same problems. In
patients with known epilepsy, the following measures are advised.
Try to establish the normal frequency and severity of seizures, what •
form they take, and features, if any, of the prodrome.
Ensure that normal anticonvulsant medication is continued while the •
patient is NBM preoperatively and immediately post-operatively.
If this is not possible, phenytoin and sodium valproate may be given IV: •
check the BNF for equivalent dosing regimes.
Phenytoin interacts with a number of drugs used perioperatively.•
Phenytoin induces monoamine oxidase, increasing elimination •
of the following commonly used drugs: prednisolone, warfarin,
lignocaine.
The following drugs increase oral absorption of phenytoin: •
amiodarone, fl uconazole, omeprazole, paroxetine.
The following drugs reduce oral absorption of phenytoin: antacids •
containing magnesium, calcium carbonate, or aluminium, and
enteral tube feeding.
Management of status epilepticus (uncontrolled fi tting).•
Airway.• Remove dentures; insert Guedel or nasophayngeal airway if
unable to open jaw.
Breathing.• Give high fl ow oxygen (O
2
); get Yankauer sucker and
wall suction.

SURGERY IN NEUROLOGICAL DISEASE
63
Give diazepam 10mg IV over 2min (PR if no IV access); repeat once •
if seizures do not stop.
Check blood sugar and give 50mL 50% dextrose IV if BS <5mmol/L.•
If seizures persist start, phenytoin 18mg/kg IV at a maximum rate of •
50mg/min in a separate line to the diazepam.
Seizures should resolve quickly. If they do not:•
Get expert help. —
Discuss with anaesthetist. —
Consider pseudoseizures (pelvic thrusts, fl ailing limbs, resistance —
to attempts to open eyes).
Myasthenia gravis
An antibody-mediated autoimmune disease with insuffi cient muscle ace-
tylcholine receptors, leading to muscle weakness. Usually found in young
adults, the disease presents with extraocular (ptosis, diplopia), bulbar
(weak voice), neck, limb girdle, distal limb, and fi nally trunk weakness.
Patients may present for thymectomy as specifi c treatment or for inciden-
tal surgery. Management includes the following:
Continue normal medication.•
Elective post-operative ventilation is indicated in major thoracic or •
upper abdominal surgery or if the patient’s vital capacity <2L. Discuss
with an anaesthetist and ICU.
If ventilation is prolonged post-operatively, a tracheostomy may be •
required. This should be discussed with the patient at time of consent.
Post-operative respiratory failure may occur as a result of muscle •
weakness. Precipitants include:
Hypokalaemia.•
Infection.•
Over- or undertreatment.•
Emotion, exertion.•

CHAPTER 2 Principles of surgery
64
Fluid optimization
2 Identifying patients in need of fl uid optimization
Any patient may be in need of preoperative fl uid resuscitation, but several
groups are typically affected. Remember to think of less obvious cases of
fl uid depletion—there are typically more patients who would benefi t from
fl uid optimization than get it.
Acute presentations with vomiting or diarrhoea, including intestinal
•
obstruction, biliary colic, gastroenteritis.
Acute presentations where the patient has been immobile or •
debilitated for a period before presentation, causing reduced fl uid
intake, e.g. pancreatitis, chest infections, acute-on-chronic vascular
insuffi ciency, prolonged sepsis with pyrexia.
Elderly patients in whom reduced renal reserve makes fl uid balance •
control less effective.
Drugs that impair renal responses to fl uid changes, e.g. diuretics.•
Patients with low body weight with overall lower total body fl uid •
volume in whom similar losses have a greater effect.
Children are more susceptible to fl uid depletion and may not show •
such obvious physical signs.
Fluids used for optimization
The most important aspects of fl uid optimization are using the correct vol-
umes at the correct rate. Other than exceptional circumstances, isotonic
crystalloids are the fl uid of choice to correct imbalances.
Isotonic (‘normal’) 0.9% saline—the most widely used fl uid. Provided •
there is adequate renal function, isotonic saline prevents rapid cellular
fl uid shifts during rehydration and excess Na
+
is excreted via the
kidney. K
+
should usually be added only if d K
+
is present or likely (e.g.
prolonged vomiting, pancreatic or small bowel fi stula).
Dextrose (4%) saline (0.18%).
•
Hartmann’s solution.•
Ringer’s lactate solution—technically, the closest fl uid to serum •
composition although theoretical advantages are of limited practical
value.
Fluids that should only be used in very specifi c circumstances include
hypertonic (1.8%) or hypotonic (0.45%) saline since they risk causing sig-
nifi cant fl uid shifts in and out of cells, which can cause cellular injury, par-
ticularly to neurons. If there is a signifi cant disorder of sodium balance that
may require non-isotonic fl uid optimization, the patient is likely to require
optimization in HDU.
How to give the fl uids
Before giving fl uid, it is important to assess the volume of depletion. It is
rarely possible to use estimates of losses due to vomiting or diarrhoea as
these are wholly inaccurate. Useful calculations include the following:
Body weight on admission (provided a recent, accurate body weight •
during normal health is known) since acute weight loss is mostly water.

FLUID OPTIMIZATION
65
Haematocrit on admission (provided a recent haematocrit during •
normal health is known) since the degree of haemoconcentration is
due to fl uid depletion. An approximate calculation is given by:
Fluid depletion (L) = ((PCV
1
– PCV
2
)/PCV
1
) x 0.7 x weight in kg
PCV
1
= normal haematocrit; PCV
2
= current haematocrit
Serum urea is raised disproportionately more than serum creatinine
•
in dehydration, renal disease, gastrointestinal (GI) bleeds, and acute
proteolysis.
Signs of extracellular fl uid depletion (lax skin tone, reduced sweating, •
dry mucosae) are often misleading and can be affected by age and
underlying diseases, including pyrexia and tachypnoea.
Signs of intravascular volume depletion (hypotension, tachycardia) •
may be unreliable and usually only occur with loss of 10–15% of body
water.
Once the volume of fl uid required is assessed, it can be given. There are
some broad rules on how to give fl uid resuscitation.
Young, fi t patients with normal renal and cardiac function can usually •
be given up to 15% of body fl uid volume by rapid infusion. A typical
regimen might include: 1000mL 0.9% saline over 2h, further 1000mL
infusions of 0.9% saline over 4h each until corrected.
Elderly patients and patients with renal or cardiac impairment should •
have infusions more slowly to prevent acute intravascular volume
overload. A typical regimen might include: 1000mL 0.9% saline over 4h,
500mL infusion of 0.9% saline over 3–4h with regular review of vital
signs, including chest auscultation. Patients requiring more fl uid volume
more rapidly than this should be monitored closely in critical care
during resuscitation.
Monitoring fl uid optimization
Methods of assessing the progress of fl uid optimization include the
following:
Skin turgor and mucosal hydration change slowly after optimization •
and are unreliable guides.
1-hourly urine output measurement is a good guide to renal blood •
fl ow, which indirectly relates to intravascular fl uid volume and cardiac
output. It is an easy and reliable indicator of adequate blood volume
repletion. It is not a good indicator of total body water and there may
be signifi cant intra- and extracellular depletion in the presence of an
acceptable urine output. A commonly used minimum is 0.5mL/kg/h.
Monitoring of serum urea is an approximate guide provided renal •
function is adequate and there is no acute GI bleeding or proteolysis.
In the emergency situation, particularly where patients require urgent
surgery and require fl uid optimization prior to anaesthesia, more rapid
fl uid infusions may be required and it may be appropriate for this to be
monitored on HDU.

CHAPTER 2 Principles of surgery
66
Nutrition in surgical patients
Nutrition is critical to the well-being of surgical patients and timely nutri-
tional support helps reduce acute catabolism and resultant skeletal muscle
weakness due to increased metabolic demands.
It is common and amenable to effective intervention that infl uences •
the outcome of surgical patients.
Incidence of pre-existing malnutrition is substantial and rises with age.•
Anticipate those patients with higher than normal nutritional •
requirements (e.g. severe burns, severe sepsis, intestinal fi stulas,
advanced malignancy, immunosuppression); even if they are well
nourished prior to illness, they may need nutritional support to prevent
excessive acute catabolism due to increased metabolic demands.
Assessment of nutritional status
All patients should be considered for nutritional assessment. Many meth-
ods can be used:
BMI• (weight/height
2
in kg/m
2
). Relatively insensitive for all but major
malnutrition, but easy to perform. A BMI of 18–25 is normal, <18 is
underweight, >30 is obese.
Triceps skinfold thickness.• Easy to perform and a good measure of body
fat as a marker of chronic nutritional status.
Grip strength.• Easy repeatable index of lean skeletal muscle.
Serum albumin.• Poor indicator of acute nutritional status. Responds
slowly to nutritional supplementation and is affected by many other
diseases and conditions.
Serum transferrin. • Accurate responsive indicator of acute status and
response to treatment. Not commonly used.
Effects of protein–calorie malnutrition
Reduced neutrophil and lymphocyte function.•
Impaired albumin production.•
Impaired wound healing and collagen deposition.•
Skeletal muscle weakness (‘critical illness myopathy’) with resultant •
increase in respiratory and abdominal complications.
Micronutrient defi ciencies may cause specifi c clinical syndromes.•
Types of nutritional support
Oral supplementation.• High calorie, high protein nutritional
supplements (e.g. Fortisip
®
, Calshakes
®
, Ensures
®
/Enlives
®
). May be
used in addition to promotion of conventional oral intake.
Nasogastric (NG)/nasojejunal feeding.• Often used in addition to oral
supplementation. Sometimes given overnight to reduce the impact on
appetite suppression during the day.
Feeding gastrostomy/jejunostomy• (via a surgically implanted tube). Not
routinely used. Reserved for patients where the GI tract is functioning,
but food cannot be taken via the oropharyngeal route.
Parenteral nutrition.• May be central or peripheral. (See b p. 67.)
The oral route is always to be preferred in nutritional supplementation. It
promotes the normal health of the GI fl ora and has been shown to reduce
the risk of complications after GI surgery.

NUTRITION IN SURGICAL PATIENTS
67
Total parenteral nutrition (TPN)
TPN is commonly encountered in surgical wards. It is a major advance
in the treatment of surgical malnutrition, but has serious side effects and
both long- and short-term potential complications.
Routes of administration for TPN
Peripheral (PPN).
• Given via a medium calibre cannula in a peripheral
vein. Maximum calorie input limited by the maximum osmolarity of the
solution. Avoids the risks of central venous cannulation. Usually used
for short-term supplementation.
Central (TPN). • Given into a central vein (superior vena cava (SVC) or
brachiocephalic). May be via a dedicated tunnelled line (e.g. Hickman
line), a conventional central venous (CV) cannula, or a peripherally
inserted central venous catheter (PICC line). Maximum calorie input
only limited by volume of fl uid that can be infused. Carries risks of
central venous catheterization.
General risks of TPN/PPN
Hyperosmolarity.
•
Lack of glycaemic control.•
Micronutrient defi ciencies.•
Liver cell dysfunction, cholestasis, and pancreatic atrophy.•
Fluid volume overload.•
Specifi c catheter-related risks of TPN
Complications of insertion (air embolism, pneumothorax, vascular •
injury, dysrhythmias).
Catheter thrombosis and thromboembolism.•
Central line infection, infective endocarditis, and bacteraemia.•
Care of TPN patients
Patients on TPN require regular review and monitoring, including:
U&E (initially daily, then twice weekly once established).•
Glucose (initially od, then twice weekly unless signs of abnormal •
glucose levels).
LFTs (twice weekly).•
Micronutrients, including magnesium (Mg), phosphate (PO•
4
),
manganese (Mn), copper (Cu) (weekly).
CV catheters require specifi c attention. They should not be used for non-
TPN infusions and/or phlebotomy unless in exceptional circumstances as
this increases the risk of catheter sepsis dramatically. Dressings should be
dated, changed regularly, and the catheter entry site kept clean.
Common indications for TPN
Short term
Prolonged post-operative ‘ileus’ (unresolved GI tract dysfunction).•
Acute abdominal sepsis with ITU likely (possibly slow oral route).•
Long term
Inability for GI tract to absorb adequate nutrition (e.g. extensive resection,
extensive radiotherapy damage, extensive disease such as Crohn’s).

CHAPTER 2 Principles of surgery
68
Enhanced recovery after surgery
Enhanced recovery after surgery (ERAS) is one of many similar approaches
to patient recovery after major surgery, which aims to coordinate many
aspects of patient care to minimize the perioperative physiological
derangement of the patient, reduce the stress response to surgery, opti-
mize speed of recovery and reduce complication rates. It is usually applied
to otherwise healthy individuals who do not need particular preoperative
correction or adjustments.
The major areas of consideration are the following.
Nutrition
Preoperative carbohydrate loading.• Often given as oral solutions over
the 24h prior to surgery, including up to 4h prior to anaesthetic. Has
been shown to reduce the early catabolic response to major surgery.
Early re-introduction of carbohydrate rich fl uids. • Given from 6h post-
surgery and encouraged from fi rst post-operative day.
Early re-introduction of full nutrition.• Nutritional supplements and ‘light
diet’ components from 48h post-op to encourage ‘immediate’ return
of GI tract function.
Anaesthetic technique
Avoidance of the use of opiates • (e.g. IV/PO morphine, opiate patient-
controlled analgesia (PCA), opiate epidurals). Aims to prevent
reduction in GI tract motility and nausea associated with opiates.
Avoidance of the use of epidurals • (some regimens use LA epidurals).
Aims to improve early mobilization and reduce cardiovascular and GI
effects of autonomic spinal blockade.
Use of regional LA-based techniques • (e.g. transversus abdominis
percutaneous (TAP) block, regional LA infi ltration, regional LA
infusional catheters). Aims to minimize central nociceptor input, which
may enhance the systemic stress response to surgery.
Surgical technique
Laparoscopic or other minimally invasive techniques • are often, but
not always a feature of ERAS type programmes. Aim to reduce the
metabolic response to surgery, aid early mobilization, and reduce
GI tract exposure (and associated impact on function) in abdominal
surgery.
Avoidance of bowel preparation• for abdominal surgery where possible.
Reduces the risk of fl uid and electrolyte imbalances, reduces disruption
to GI tract fl ora and has been associated with lower GI tract-related
complications (e.g. anastomotic leakage).
Physiotherapy
Early mobilization. • Specifi c exercises, including sitting out within 12h of
surgery, walking within 48h of surgery.
Perioperative respiratory exercises.•

ENHANCED RECOVERY AFTER SURGERY
69
Nursing
Intensive patient preparation.• Preoperative leafl ets and education on
what to expect.
Intensive perioperative and post-operative nursing.• Encouraging early
re-establishment of diet, early mobilization and early self-care.
Although intensive and demanding, ERAS type protocols are as effective
in the elderly as the young.
They are not suitable for:
Some insulin-dependent diabetics.
•
Patients with pre-existing signifi cant nutritional compromise.•
Patients with cognitive impairment.•

CHAPTER 2 Principles of surgery
70
Getting the patient to theatre
Preparing the patient for a surgical procedure is all about organization and
routine. If the preparation is not good, much can go wrong with major
consequences for the patient.
Background paperwork
Ensure the theatre or endoscopy list is correctly fi lled in and avail-
able well in advance. The list should specify the patient’s name, hospital
number, location, operation details as well as the operating surgeon and
anaesthetist.
Patient paperwork
Make sure the medical notes are available and contain the most up-to-•
date history and examination for this admission.
Check the blood results are up to date and specifi c blood results, e.g. •
clotting function in anticoagulated patients, K
+
in patients with renal
failure, Ca
2+
in parathyroidectomy patients, have been collected.
If they are not available in digital format, ensure imaging results •
that might be needed in theatre are available with the notes (e.g.
arteriograms, staging CT scans, barium enema fi lms).
Check that the consent form has been completed and is on the •
medical notes even though you may not be responsible for seeing the
form signed.
The drug chart should be completed and include any specifi c •
prescriptions for drugs to be administered in theatre or the
anaesthetic room. It is the job of the surgical team to prescribe
prophylactic antibiotics, etc.
Patient preparation
Check that the side is marked on the patient for any operation that •
might involve an organ or tissue that is bilateral. This must be done
with the patient awake and verifi ed by the nursing staff.
If necessary, check that the patient has been assessed and marked by •
any relevant specialists (stoma care if a stoma is possible; prosthetist
for amputees).
Ensure that if the patient requires any blood or blood products, these •
are available or requested from the transfusion department. Most
hospitals have protocols to ensure that the correct number of units of
blood is requested for major surgery.
Find out well in advance if any specifi c preparation is required. •
Examples follow.
Bowel preparation
Bowel preparation is sometimes used for operations on or around the
large bowel. Types of preparation include the following.
Stimulant mechanical bowel preparation, e.g. Picolax
•
®
(piccosulphate),
two sachets taken with plenty of fl uid at least 8h prior to surgery.
Should be avoided whenever there is a risk of obstruction present.
Commonly used for barium enemas.

GETTING THE PATIENT TO THEATRE
71
Osmotic mechanical bowel preparation, e.g. Citramag•
®
(Mg citrate),
Kleen Prep
®
, two or four sachets taken dissolved in water up to 8h
prior to surgery. Used for most colonic operations where bowel
preparation is required, colonoscopy, and CT colonography.
Stimulant left colon preparation, e.g. phosphate enema. Used for •
operations on the rectum/anus or for fl exible sigmoidoscopy.
Mechanical bowel preparation is less commonly used than previously.
•
There is evidence that it may actually increase the septic complication
rates following bowel surgery and has well recognized side effects.
These include the risks of:
Electrolyte imbalances.•
Hypovolaemia, especially in the elderly.•
Nausea and vomiting (particularly with the large volume osmotic •
preparations).
Anaesthetic premedication
Principles
Anaesthetic premedication is used for the following reasons:
Relaxing the patient to reduce anxiety during preparation for
•
anaesthesia.
Relaxation of the patient decreases the amount of anaesthetic agent •
required for induction of anaesthesia.
Typical agents used for premedication
Benzodiazepines.• Often given 1–2h preoperatively on the ward (e.g.
diazepam PO). May be given shortly before some procedures (e.g.
midazolam 5mg IV).
Buscopan•
®
. Rarely given, but sometimes used to reduce upper
aerodigestive tract secretions.

CHAPTER 2 Principles of surgery
72
Prophylaxis—antibiotics and
thromboprophylaxis
Antibiotic prophylaxis
Prophylactic antibiotics are used to reduce the risk of surgical site •
infection and are usually of very short course (one to three doses).
Active antibiotic treatment for established infections encountered •
during surgery may be for 5 or more days.
Most prophylaxis is directed at prevention of infection of the surgical •
wound or to counter the effect of potential spillage of organisms from
colonized organs such as the bowel once it has been opened.
The broad principles of prophylaxis are the following:
It is most important to have a high circulating serum level of antibiotics •
at the time of potential tissue contamination, i.e. administered around
the time of induction of anaesthesia.
Prophylaxis rarely needs to continue beyond the time of the •
procedure unless there are high risk factors or specifi c indications,
i.e. most prophylaxis is one or occasionally three doses on the day of
surgery.
Many clean wounds, e.g. skin lesion excision, do not require •
prophylaxis.
High risk patients who may warrant an extended prophylactic course •
(e.g. up to 3 days) or specifi c prophylaxis include:
Neutropenic or immunosuppressed patients.•
Severely malnourished patients.•
Prophylactic regimens
Most hospitals have established guidelines for prophylaxis and these should
be consulted for prescribing. See Table 2.1 for an example of a regimen.
Thromboprophylaxis
Types of thromboprophylaxis
Mechanical devices
Thromboembolic deterrent stockings (TEDS).
• Reduce stasis in
infrapopliteal veins by continuous direct compression.
Pneumatic compression boots.• Reduce stasis in infrapopliteal veins
by intermittent compression emptying of foot and lower leg veins,
promoting venous fl ow.
Drugs acting on the clotting cascade
Heparin.
• Activates antithrombin III.
Prophylaxis: 5000U SC od.•
Treatment: IV 2000U loading dose, 2000U/h (usually 1000U/mL of •
normal saline). Check APTT 6h after starting and 6–12h thereafter.
Titrate to maintain APTT 50–70.
LMWH.
• Activates antithrombin III. Given by SC injection.
Prophylaxis: 20–40mg SC Clexane•
®
od.
Treatment: 2mg/kg SC in two divided doses.•
PROPHYLAXIS—ANTIBIOTICS AND THROMBOPROPHYLAXIS
73
Antiplatelet drugs (not used as venous thromboembolism (VTE)
prophylaxis)
Aspirin (300mg PO od).
•
Dipyridamole (75mg PO qds).•
Clopidogrel (75mg PO od).•
Drugs indirectly affecting clot formation
Dextran 70.
• Interferes with clot formation. Given as regular bolus
infusions (500mL over 1h od).
At risk groups
2 All patients are ‘at risk’ of developing deep vein thrombosis just as is the
general population. National requirements for VTE prophylaxis require
all patients to be assessed for risk factors on admission and after 24h in
hospital.
1
Risk is judged according to:
Procedure factors. • Prolonged anaesthetic time, lower limb or pelvic
surgery.
Patient factors. • Immobility, malignancy, age, dehydration, obesity,
diabetes, cardiorespiratory disease, infl ammatory pathologies, oral
contraceptive pill or hormone replacement therapy (HRT), past or
family history of thromboembolic disease.
Balanced against:
Bleeding risks.• Active bleeding, stroke, invasive procedures, bleeding
disorders (liver disease, thrombocytopenia, inherited disorders).
Risks of compression devices.• Peripheral vascular disease (PVD).
The risks should be recorded on the patient’s drug chart or VTE docu-
mentation and mechanical devices (e.g. TEDS) and/or chemical thrombo-
prophylaxis (e.g. LMWH) prescribed according to local policy.
Reference
1 M http://publications.nice.org.uk/venous-thromboembolism-reducing-the-risk-cg92
Table 2.1 An example of an antibiotic prophylactic regimen
Indication
Typical prophylaxis (single dose
on induction)
‘Clean’ bowel surgery, e.g. acute
non-perforated appendicitis,
elective colonic resection
Cefuroxime, 1.5g IV + metronidazole
500mg IV or gentamicin 120mg
IV Amoxicillin 500mg IV +
metronidazole 500mg IV
‘Clean’ hepatobiliary surgery, e.g.
ERCP, open biliary surgery
Gentamicin 120mg IV + amoxicillin
500mg IV + metronidazole 500mg
‘Clean’ gynaecological surgery Metronidazole 500mg IV or 1g PR
Elective orthopaedic surgery Flucloxacillin 1g IV
‘Clean’ vascular surgery Flucloxacillin 500mg IV + gentamicin
120mg IV + amoxicillin 500mg IV

CHAPTER 2 Principles of surgery
74
In-theatre preparation
Theatre is a potentially dangerous place for patients; many of these dan-
gers arise directly as a result of poor preparation and checking of basic
facts. For example:
Wrong side surgery (e.g. healthy kidney removed, wrong hip joint •
replaced).
Wrong site surgery (e.g. inguinal, not femoral, hernia repaired; hip, not •
knee, arthroscoped).
Allergic reaction to medication.•
Vital material not available, if required (e.g. blood not cross-matched).•
Vital equipment required for surgery not available (e.g. image •
intensifi er, specialist joint replacement jig).
Retained swabs or instruments.•
Experience from other high risk industries (e.g. the airline industry) has
shown that strict adherence to a checklist can minimize the risk of these
‘never events’ happening. The World Health Organization (WHO) rec-
ommends a standardized checklist approach.
WHO checklist
1
The WHO checklist is a basic template that sets out a series of steps,
which can be modifi ed or adapted in different organizations, but has four
areas of focus.
Before commencement of the list
Confi rm surgical, anaesthetic, and nursing team present and identifi ed.
•
Confi rm patients on the list and the order of the procedures to be •
performed.
Check anaesthetic requirements are correct and functioning (machine, •
medication, monitoring).
Confi rm vital imaging/equipment required for the list.•
Before induction of anaesthesia
Check patient identity and consent valid.•
Check site and side marked, if appropriate.•
Check anaesthetic requirements are correct and functioning (machine, •
medication, monitoring).
Check allergies, anticipated blood loss.•
Before skin incision
Check all team members present and known.•
Check the procedure to be performed.•
Confi rm any surgical/anaesthetic/nursing concerns.•
Confi rm vital imaging/equipment available.•
Before the patient leaves the theatre
Check the correct name for the procedure actually performed is •
known and recorded.
Check the swab and instrument count correct.•

IN-THEATRE PREPARATION
75
Confi rm any surgical specimens collected, ‘potted’, and labelled •
correctly.
Confi rm any specifi c instructions, either surgical or anaesthetic, which •
apply to the patient in recovery or on transfer to the ward.
Reference
1 M http://www.who.int/patientsafety/safesurgery/ss_checklist/en/index.html

CHAPTER 2 Principles of surgery
76
Positioning the patient
Getting the patient on to the operating table
The surgical team is partly responsible for the safety of the patient all
the way onto and off the operating table. Be sure the basic rules of
safety are being observed. See Fig. 2.3 for some typical patient positions
in surgery.
The anaesthetist is responsible for the patient’s airway and should •
coordinate all moves of the patient to ensure it is maintained.
Be sure not to dislodge IV cannulae, epidural sites, or existing drains.•
Use approved manual handling techniques (e.g. a ‘Patslide’ or similar •
device, rather than lifting the patient).
Be aware if extra care needs to be taken, e.g. prosthetic joints that •
may dislocate once protective muscle tone is lost during relaxation,
unstable fractures, potentially unstable joints due to rheumatoid
arthritis, existing ulcers or skin lesions.
Once in position
Ensure that no points on the patient are in contact with the metal of •
the operating table to prevent diathermy exit point burns.
Make sure bony prominences and areas of thin skin are well padded, •
e.g. the neck of the fi bula in leg stirrups.
Ensure any diathermy pad is correctly applied and not liable to be •
affected by skin preparation.
Ensure there are appropriate supports for the patient to secure the •
position, particularly if the table may be moved, tilted, or rotated
during the procedure (e.g. arm, thoracic, and abdominal supports
for lateral positions; shoulder bolsters if the patient will be head
down).
For procedures requiring access to the perineum, be sure that the •
pelvis is properly supported, but that the perineum is exposed over
the end of the operating table.
Consider the positioning of ancillary equipment (e.g. where will video •
stacks be positioned? Is more than one energy source required and
where will the generators be located? Is there access of mobile imaging
equipment or on-table radiography?). All equipment positioning needs
to allow enough access to the patient for the surgical team.
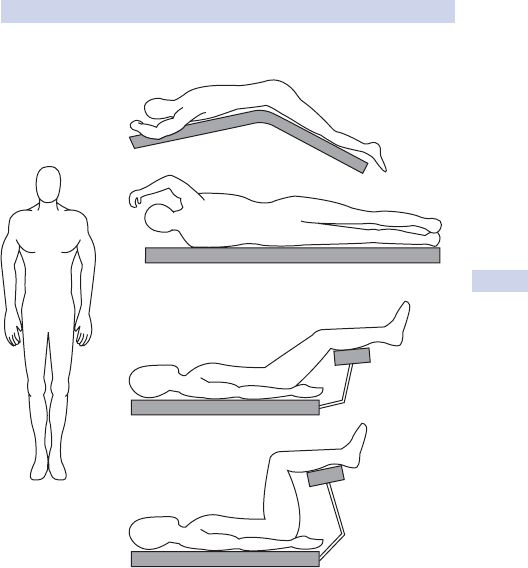
POSITIONING THE PATIENT
77
Supine
Prone jack-knifed
Lateral
Lloyd–Davis
Lithotomy
Fig. 2.3 Typical positions for surgery. Supine (most abdominal surgery); prone
jackknifed (some rectal or vaginal procedures); lateral (thoracotomy); Lloyd–Davis
(pelvic surgery); lithotomy (most perineal procedures).

CHAPTER 2 Principles of surgery
78
Sterilization, disinfection, and
antisepsis
Sterilization
Heat
Dry heat (e.g. incineration, fl aming to red hot) is effective, but rarely
•
useful. Dry heat requires temperatures of 160°C for at least 60min.
Moist heat (e.g. autoclave heating using pressurized steam 121• °C at
15lb/in
2
, 15min) is effective and useful, especially in operating theatres.
Irradiation
Gamma radiation. Effective for inorganic materials.
Filtration
Air or fl uids can be sterilized by ultrafi ne membrane fi lters, but are rarely
useful in hospital practice.
Disinfection
Chemical
Acids/alkalis, e.g. bleach. Effective for non-human contact use.
•
Alcohols/phenols, e.g.•
Ethyl alcohol—skin swabs.•
Alcohol solutions (Aqagel•
®
)—hand disinfection.
Carbolic.•
Chloroxylenols (Dettol•
®
).
Phenol (Clearsol•
®
).
Oxidizers, e.g.•
Povidone–iodine (Betadine•
®
)—skin disinfection/surgical scrubbing.
Hydrogen peroxide (H•
2
O
2
)—superfi cial wound cleansing.
Aldehydes (Cidex•
®
)—surgical instruments such as endoscopes.
Cationic solutions, e.g. Chlorhexidine•
®
—antiseptic washes.
Organic dyes, e.g. Profl avine•
®
.
Antisepsis
Principles of antisepsis include the following.
Always remove gross contamination with simple soap fi rst.•
Use high potency acid/alkali disinfection on inert surfaces.•
Use less corrosive oxidizers on delicate inert materials.•
Use weak alcohols, oxidizers for skin cleansing.•
Defi nitions
Sterilization• is removal of all viable microorganisms, vegetative, and
spores.
Disinfection• is the removal of actively dividing vegetative
microorganisms.
Antisepsis• is the process whereby the risk of medical cross-infection
by microorganisms is reduced.

SCRUBBING UP
79
Scrubbing up
Scrubbing up is designed to reduce the risk of infection from the sur-
geon to the patient. A thorough clean with bactericidal soaps reduces the
number of organisms that can be cultured from skin swabs, but the skin
(particularly sweat glands and hair follicles) cannot be sterilized. Moisture
and heat occurring under surgical gloves quickly raise the bacterial count
again and despite modern cleaning agents, signifi cant growth can be
achieved within 2h. Common bactericidal soaps include:
Hibiscrub•
®
(chlorhexidine).
Betadine•
®
(povidone–iodine).
Protocol
The fi rst time you come to theatre, ensure that the senior scrub nurses
know who you are. It is polite and safe. Everybody should know who the
people in theatre are and what they are there for/can do.
How to scrub
Wet your hands and arms fi rst.•
Lather well with disinfectant soap and wash off.•
‘Scrub’ under the nails and heavily soiled areas with a sterile brush and •
more disinfectant soap. Don’t scrub too vigorously, especially on clean
skin as this causes irritation without any increased bactericidal effect.
Wash again with soap, being sure to cover the commonly missed •
areas—between the fi ngers, back of the hands, under fi ngernails, base
of the thumbs.
Rinse thoroughly to remove all soap to reduce the chance of skin •
irritation.
Rinse off, trying to ensure the water runs off the arms at the elbows.•
Dry the hands completely fi rst and do not go back to them once •
drying the arms. Be sure your hands are completely dry before trying
to put on gloves—there’s nothing worse than gloves that aren’t on
properly due to wet hands!
How to gown and glove
Be sure to open the gown without touching the outer ‘face’.•
Don’t push your hands through the cuffs.•
Pick up the right glove with your right hand ‘through’ the cuff of the •
gown, holding it by the edge of the glove on the palm side with the
fi ngers pointing down your forearm.
With your left hand, fold the other side of the edge of the glove ‘over’ •
your right hand.
Slide your right hand into the glove.•
Once on, pick up the left glove, holding it by the edge and pull it over •
the cuff of the left hand.
Slide your left hand into the glove and adjust glove positions.•
It is becoming common practice to wear eye protection and two pairs of
gloves to reduce the risk of exposure to infectious agents (see b p. 178).

CHAPTER 2 Principles of surgery
80
Surgical instruments
See Fig. 2.4.
‘Sharps’
Scalpels. • Two sizes of handle (4 and 6). Types of blade and uses
include: no. 11 (used for stab incisions), no. 10 (most skin incisions),
no. 15 (fi ne incisions), no. 22 (adhesiolysis).
Scissors.
• May be dissecting or stitch cutting. Dissecting scissors
may be straight (e.g. Mayo) or curved (e.g. curved Mayo, McIndoe,
Metzenbaum, Nelson’s).
Forceps
Non-toothed. • Fine non-toothed (e.g. DeBakey, Adson’s forceps) used
for handling delicate tissues such as vessels, bowel. Heavy non-toothed
used for general handling, including specimens and sutures.
Toothed.• Fine toothed (e.g. Gillie’s, McIndoe’s forceps) used for
handling skin, fi ne fascia, and occasionally for precise holds on delicate
tissues. Heavy toothed (e.g. Lane’s forceps) used for holding heavy
tissues such as fascia and scar tissue.
Ring-tipped and microforceps.• Used in microvascular anastomoses.
Clips and clamps
Artery clips (e.g. Spencer–Wells, Robert’s (large), Dunhill’s, Mosquito •
(small)) have serrated jaws. Used for vascular clamps and tissue/suture
holding.
Tissue clamps, for example:•
Lahey clamp• . Similar to a curved arterial clip.
Doyen bowel clamp• . Non-crushing atraumatic.
Babcock/Duval clamp• . Non-toothed semi-atraumatic tissue-holding
clamp.
Lane’s/Allis’s/’Littlewood’ clamp• . Heavy toothed traumatic tissue-
holding clamps.
Retractors
Self-retaining retractors:•
Large (e.g. Goligher retractor for abdominal incisions, Finichetto •
retractor for thoracic incisions).
Small (e.g. Travers/Norfolk and Norwich retractors for small skin •
and abdominal incisions).
Handheld retractors:•
Large (e.g. Deaver, Kelly, Morris).•
Small (e.g. Kilner ‘Catspaw’, Langenbeck).•
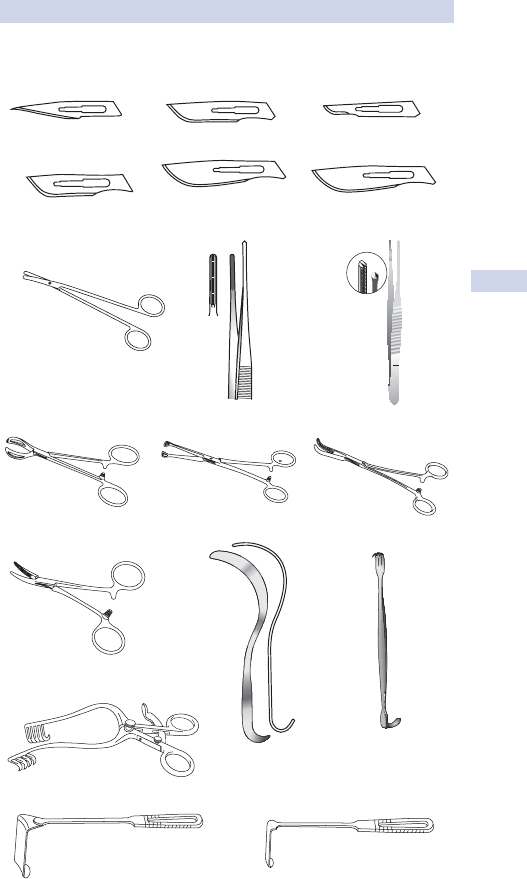
SURGICAL INSTRUMENTS
81
11
10
15
20
22
Metzenbaum scissors
Lane’s forceps
Dunhill’s forceps
Travers retractor
Morris retractor Langenbeck retractor
Deaver retractor Kilner retractor
Babcock forceps Lahey clamp
DeBakey forceps Gillie’s forceps
No. 11
Blades:
No. 10 No. 15
No. 20 No. 22
23
No. 23
Fig. 2.4 Commonly used surgical instruments.

CHAPTER 2 Principles of surgery
82
Incisions and closures
Body cavity incisions
General terms are applied to the incisions of access to each body cavity.
Laparotomy.• Any incision accessing the peritoneal cavity or
retroperitoneal space. Separate types of laparotomy are described
according to their location in the abdomen, tissues that are crossed,
or, occasionally, the individual who described them (see Fig. 2.5).
Thoracotomy.• Accessing the chest cavity, typically the pleural space
or posterior mediastinum. Median sternotomy is a particular type of
thoracotomy for access to the anterior and middle mediastinum.
Craniotomy.• Accessing the compartments of the skull.
Incision closures
Incisions in body cavities are generally closed according to some basic
principles.
Fascial layers offer the best tissue to bear the strength of apposition •
and form the main closure in the abdomen. Closure is usually made
with heavyweight non-permanent sutures.
Bony defects, such as in a craniotomy, should be apposed to allow •
minimal movement.
Defects in fascial or bony tissues should be replaced either with •
transposed tissues (e.g. skin, fascial, muscle fl aps) or with inserted
tissues (e.g. synthetic products such as polypropylene mesh)
(see b p. 596).
Large cavities and potential spaces between tissues should be avoided •
to reduce the risk of fl uid collections which run the risk of becoming
infected.
A
B
1. Kocher
2. Midline
3. Muscle splitting loin (ureter)
4. Pfannenstiel
5. Thoraco-abdominal
(oesophagogastrectomy
9th or 10th intercostal
space)
Paramedian 1.
McBurney 3.
Lanz 4.
Muscle-cutting traverse 5.
Roof-Top 6.
McEvedy (femoral hernia) 7.
Inguinal hernia incision 8.
1
2
5
3
4
6
5
4
21
7
3
8
Fig. 2.5 Incisions. Reproduced with permission from Longmore, M. et al. (2007).
Oxford Handbook of Clinical Medicine, 7th edn. Oxford University Press, Oxford.

DRAINS
83
Drains
Uses and complications
Drains may be used for several reasons (see also b p. 93).
To remove existing abnormal collections of fl uid, blood, pus, air (e.g. •
drainage of a subphrenic abscess, removal of a pneumothorax).
To prevent the build-up of either normal bodily fl uids (e.g. bile after •
surgery to the bile duct) or potential abnormal fl uids or air (e.g.
bloody fl uid in the pelvis after rectal surgery).
Occasionally used to prevent or ‘warn’ of potentially serious or life-•
threatening complications (e.g. neck drains after thyroid surgery, chest
drains after chest trauma in patients undergoing GA).
Potential complications should be balanced against drain use.
Damage to structures during insertion, even if under CT or ultrasound •
guidance (e.g. risk of injury to spleen in subphrenic abscess drainage,
haemorrhage from abdominal vessels in operative drains).
Drains provide a potential route of introduction of infection, especially •
external drains that remain for longer than a few days.
Damage to structures close to the drain, e.g. pressure injury to bowel •
if subjected to high pressure suction drainage.
Drains do not always drain the substance expected and may give a •
false ‘sense of security’, e.g. failure to drain bleeding after thyroid
surgery or failure to drain faecal fl uid after anastomotic leakage.
There is no place for ‘routine’ use of drains after surgery unless there is a
clear indication—‘Better no drainage than ignorant use of it.’ (Halstead).
Types of drain (see Box 2.4)
Materials used include latex rubber (e.g. T tubes), silastic rubber (e.g.
long-term urinary catheters), polypropylene (e.g. abdominal drains), poly-
urethane (e.g. NGT).
Box 2.4 Types of drains
Open passive drains
These provide a conduit around which secretions may fl ow.
Yates corrugated drain (after subcutaneous abscess drainage).
•
Penrose tube drain.•
Drainage setons placed in anal fi stulas.•
Closed passive drains
These drain fl uid by gravity (‘siphon effect’) or by capillary fl ow.
Robinson tube drain (after intra-abdominal abscess drainage).
•
NGT.•
Ventriculoperitoneal shunt.•
Chest drain (tube thoracostomy).•
Closed active drains
These generate active suction (low or high pressure).
Exudrains
•
®
, Redivac drains
®
, Minivac
®
, Jackson Pratt drains (after
pelvic or breast surgery).

CHAPTER 2 Principles of surgery
84
Stomas
Terminology and types
The term stoma is usually applied to an external opening (temporary or
permanent) in a lumenated organ.
Ileostomy.• Formed from any part of the mid- or distal small bowel. May
be loop (often to ‘rest’ the distal bowel) or end (usually as a result of
surgical removal of distal bowel).
Colostomy.
• Formed from any part of the large bowel. May be loop (to
rest distally) or end (because of surgical resection).
Urostomy.• Formed from a short length of disconnected ileum into
which one or both ureters are diverted (usually after radical lower
urinary tract surgery).
Gastrostomy.• Either a surgically created or endoscopically formed
connection between anterior stomach and anterior abdominal wall.
Often for stomach drainage or direct feeding.
Jejunostomy.• Either a surgically created or endoscopically formed
connection between proximal jejunum and anterior abdominal wall.
Often for direct feeding.
Identifying stomas
Any stoma may have many different appearances. Typical features that
help in identifying stomas are the following:
Ileostomies (loop or end) are usually spouted, have prominent •
mucosal folds, tend to be dark pink/red in colour, and are most
common in the right side of the abdomen.
Colostomies (loop or end) are usually fl ush, have fl at mucosal folds, •
tend to be light pink in colour, and are most common in the left side
of the abdomen.
Urostomies (end) are usually spouted, have prominent mucosal folds, •
tend to be dark pink/red in colour, and are most common in the right
side of the abdomen. They are indistinguishable from end ileostomies
unless the output can be seen.
Gastrostomies and jejunostomies are usually narrow calibre, fl ush with •
little visible mucosa, and are most common in the left upper quadrant
of the abdomen. They are usually fi tted with indwelling tubes or access
devices.
This page intentionally left blank

CHAPTER 2 Principles of surgery
86
Knots and sutures
Types of suture
See Fig. 2.6 and Table 2.2.
Non-absorbable sutures tend to be used where any loss of strength •
might compromise the future integrity of the tissues being joined, e.g.
vascular anastomoses, hernia mesh fi xation, tendon repairs, sternal
wiring.
Absorbable sutures tend to be used where the persistence of foreign •
material would cause unnecessary tissue reaction or increased risk of
infection, e.g. bowel anastomoses, skin and subcutaneous tissues.
Monofi laments have the advantage of smooth tissue passage and •
minimal tissue reaction, but tend to have a crystalline structure that
increases the ‘memory’ effect of the suture, making knotting less
secure and increasing the risk of suture ‘fracture’.
Braided polyfi laments exert more tissue friction during the passage •
through, but are intrinsically more fl exible and knot securely more
easily.
Sizes of suture
Size is denoted by imperial sizes from 10/0 (smallest—invisible to naked
eye) through 4/0 (typical size for fi ne vascular anastomoses), 2/0 (typical
size for bowel anastomoses), and 1 (typical size for abdominal closure), to
4 (largest available—size of sternal wires).
Types of needle
Needles may be curved (anything from half round to shallow curved) or
straight.
Blunt-ended, round-bodied• (often used for closing major incisions).
Relatively safe as it has low tissue penetrance.
Round-bodied• (sharp-pointed, but smooth, round, cross-sectional
profi le). ‘Pushes’ tissue apart so often used for delicate tissues such as
bowel, blood vessels, etc.
Cutting point • (sharp point with triangular cross-section giving a specifi c
cutting edge). Slices through tissues so often used for dense structures
such as fascia and tendons (also reverse cutting point).
Table 2.2 Types of suture material
Natural fi bre Synthetic fi bre
Non-absorbable
monofi lament
Silver wire Steel wire Polypropylene nylon
Non-absorbable
braided polyfi lament
Silk (eventually does
decay)
Ethilon
®
Absorbable
monofi lament
Monocryl
®
PDS
®
Absorbable braided
polyfi lament
Vicryl
®
Dexon
®
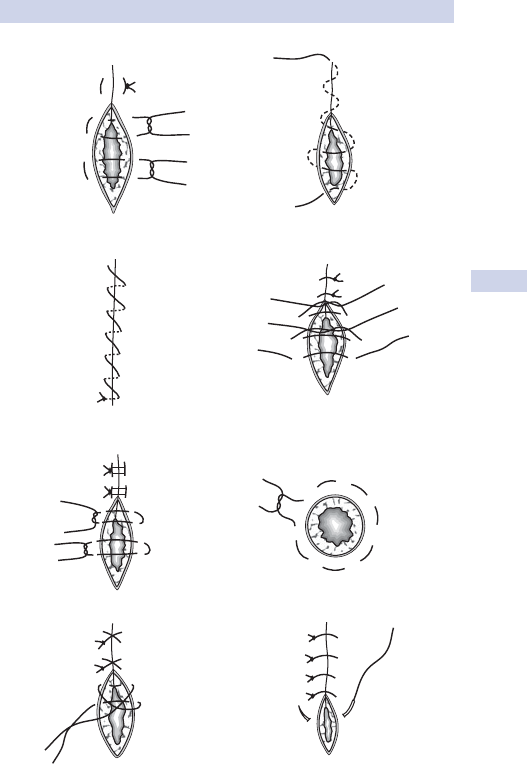
KNOTS AND SUTURES
87
Figure-of-eight Interrupted
Halsted
Purse-string
dnahrevo suounitnoCtrebmeL detpurretnI
Horizontal mattress Subcuticular continuous
Fig. 2.6 Types of suturing used (illustrated as skin sutures).

CHAPTER 2 Principles of surgery
88
Post-operative management
Routine tests
Protocols vary widely according to the complexity of surgery and the age
of the patient: this is a rough guide to management of the older patient
following major abdominal, cardiac, or reconstructive surgery.
Blood tests FBC and U&E on days 1, 2, and 5. Look for the following.
Anaemia: consider haemodilution or slow surgical bleeding.•
Raised WCC: look for other signs of sepsis (see • b pp. 104, 138).
Monitor INR/clotting daily if the patient is anticoagulated and before •
insertion of drains or central lines.
Check Na•
+
and K
+
to guide choice of crystalloid (see b p. 92).
Monitor urea and creatinine, especially in preoperative renal •
dysfunction, cardiac and aortic surgery, nephrotoxic drugs (e.g.
NSAIDs, ACE-inhibitors, vancomycin/gentamicin, fl uid restriction).
ECG Very rarely used routinely outside post-cardiac surgery. Look for:
rhythm disturbances such as AF or evidence of ischaemia.
CXR
Request daily if chest drains are present on suction, after drain removal,
and to check position of newly placed central lines. Look for:
Position of indwelling lines;
•
Consolidation, pneumothorax (on side of central line), pulmonary •
oedema, pleural effusion.
Ward rounds
See patients once a day (twice if unwell, not progressing as expected, or
undergoing investigations or treatment). In the evening, review the blood
results and other investigations from that day. For formal ward rounds,
do the following:
Make sure you have a nurse with you—the nursing record.
•
Make sure someone writes a summary in the patient’s notes.•
Ask the patient if they are experiencing any problems: establish •
whether they are mobilizing appropriately, eating and drinking, have
adequate pain control, passing urine, and opening bowels.
Check the obs chart for ‘ABCDE’ (oxygen saturations, pulse, and •
BP trends), as well as temperature, fl uid balance, and drainage if
monitored.
Look at exposed wounds for evidence of infection or seromas, but do •
not expose covered wounds.
Check diabetic charts for BS control.•
Review the drug chart. Restart regular oral medication as soon as •
possible. Convert IV to PO where appropriate. Look actively for drugs
that can be discontinued to minimize polypharmacy.
Review the most recent blood results.•
Review the nutritional status of the patient—what is the best route?•
Make a clear problem list and a clear plan.•

POST-OPERATIVE MANAGEMENT
89
Special cases
Surgery for malignancy
Liaise with specialist nurses; they will know the overall plan from the multi-
disciplinary team (MDT). Don’t discuss results without consulting them
fi rst. Breaking bad news is discussed on b pp. 4 and 16.
Plastic and reconstructive surgery
Check perfusion of fl aps daily.
•
Check take of split-skin grafts on day 5.•
Book post-operative medical illustration for photos prior to discharge.•
Orthopaedic surgery
Check X-rays of prosthesis to assess position, fraction reduction.
Vascular surgery
Check distal pulses and capillary refi ll in patients after reconstructive
•
surgery.
Perform neurological examination to evaluate patients post-carotid •
endarterectomy.
Arrange prosthesis fi tting for amputees.•
Cardiac surgery
Check sternal stability daily (ask the patient to cough while you feel for •
abnormal sternal movement).
Request transthoracic echo for day 4–5 post-operatively in valve repair •
patients and auscultate daily.
Discharge
Plan discharge from the day of admission. Use the hospital discharge •
team and identify patients requiring special facilities, such as
rehabilitation as soon as possible.
Make sure the patient understands what operation they have had.•
Tell them how to look out for common problems like wound •
infections, what is normal, and who to contact if they are worried.
Tell them when they will be seen in clinic.•
Tell them when they can expect to go back to work.•
Ensure an informative, but concise, discharge summary for the GP •
(see b p. 5).
Rehabilitation
The average times for patients to be fi t to go back to work are as
follows:
Two to six weeks after abdominal or thoracic surgery, depending on
•
size and approach (e.g. laparoscopic or open).
Weight bearing takes up to 2 months after lower limb arthroplasties •
and 3 months after lower limb fractures.
Patients can usually drive once they are fully mobile as long as they •
have not experienced blackouts or fi ts.
There are detailed rules for heavy goods vehicle (HGV) drivers and •
pilots available online.
1
Reference
1 M http://www.dvla.gov.uk/medical.aspx

CHAPTER 2 Principles of surgery
90
Drain management
Uses and types of abdominal drains are covered on b p. 83. A summary
follows:
Drains may remove collections of fl uid or gas from body cavities.•
They can be inserted as • prophylaxis or treatment.
Effl uent can be collected in • closed or open containers.
Closed containers may have a • simple or an underwater seal.
Drainage can be • suction or non-suction.
Drains may be made of latex, PVC, polyurethane, or silicone.•
Chest drains
Indications for chest drains and insertion are described on b p. 194.
Chest drainage should always be into an underwater sealed container.
Management of chest drains inserted for pneumothorax
Put the drain on low pressure, high volume wall suction (–3–5kPa) •
initially (not the high pressure wall suction used for tracheal toilet).
Request and review CXRs daily.•
Bubbling in the underwater seal, either continuously or only when •
the patient coughs, indicates an air leak and implies that the lung
parenchyma has not healed. 2 You can only remove the drain when
there is no air leak; otherwise a pneumothorax will rapidly re-form.
When the air leak stops, take the drain off suction for 12h and repeat •
the CXR: if the lung is fully up, the drain can be removed.
Get a CXR after drain removal to check for a pneumothorax.•
Management of chest drains inserted to drain collections
Management of chest trauma is described on b p. 480.
There is no evidence that suction improves outcome.•
Make sure that the nursing staff measures the drainage: hourly in the •
post-operative patient, every 24h in longer-term drains.
A haemodynamically unstable post-operative patient or one who is
•
draining more than 200mL blood/h should be discussed urgently with
the thoracic surgeons.
Post-operative thoracic drains are normally removed when they drain •
nothing for 2 consecutive hours unless there is an air leak.
Drains for pleural effusions can be removed when they drain less than •
250mL in 24h.
Drains for empyemas can be removed when they stop draining.•
Always request and review a CXR after drain removal to check for •
pneumothorax.

DRAIN MANAGEMENT
91
Clamping chest drains
Clamping drains when transferring patients stems from the days of TB
treatment with caustic solutions and was aimed to prevent drain effl uent
draining back into the chest. In modern practice, the only indications to
clamp a drain are: (1) in the trauma setting if the patient is exsanguinating
through it; (2) under specialist supervision in patients with chronic air
leaks or pneumonectomy.
Clamping a thoracic drain in a patient with an air leak may rapidly
•
result in a tension pneumothorax.
Clamping a mediastinal drain in a patient who is bleeding may rapidly •
result in cardiac tamponade.
The safest mode is an • unclamped drain connected to an underwater
seal that is kept below the level of the patient at all times.
If you connect the drain to wall suction, but do not put the wall •
suction on, this is effectively clamping the drain; if you and the nurses
do not know what you are doing, ask for help.
If you press in the one-way valve on the top of the underwater seal •
too tightly, this effectively clamps the drain. If in doubt, leave it alone!

CHAPTER 2 Principles of surgery
92
Fluid management
Fluid management is aimed at making sure the patient is neither fl uid
depleted nor fl uid overloaded. The principle is to replace whatever is lost.
Success can make the difference between a short, uncomplicated post-
operative course and the patient ending up on ICU.
Maintenance crystalloid
The principle is to replace Na
+
, K
+
, and water loss from urine, vomit-
ing, diarrhoea, high output fi stulae and stomas, and fl uid ‘third-spaced’
(e.g. ascites, tissue oedema). For fl uid management in burns, see b
pp. 604–7.
Approximate average daily loss of fl uid and electrolytes
Water loss 2500mL/day• (insensible loss from skin, respiratory and GI
tract, and in urine). i loss in sepsis, ventilation, diarrhoea, vomiting,
high output fi stulas, polyuric renal failure.
Na+ 100mmol/day• (in urine). i loss in pyrexia, diarrhoea, vomiting,
high output fi stulas. Urine concentration is less effective in elderly
patients and dextrose infusions may cause hyponatraemia.
K+ 80mmol/day• (in urine). i loss in pyrexia, diarrhoea, vomiting, high
output fi stulas.
Regime 1
This gives 3L of fl uid, 200mmol Na, and 80mmol KCl in 24h. It is only
suited to adult patients with no signifi cant comorbidity. It takes no account
of patient age, size, cardiac function, or fl uid loss.
1L normal saline with 40mmol KCl over 8h.
•
1L normal saline with 40mmol KCl over 8 h.•
1L 5% dextrose over 8h.•
Regime 2
This adjusts fl uid given to fl uid lost, but needs the nursing resources to
run an hourly fl uid balance chart and infusion pumps. It is more suited to
patients on HDU.
1mL/kg/h normal saline.
•
Gelofusine or 5% dextrose fl uid challenges to maintain central venous •
pressure (CVP) 8–12cmH
2
O or BP >120mmHg.
10–40mmol KCl in 100mL 5% dextrose via central line if K•
+
<3.0.
Regime 3—Alder Hey paediatric regimen
Useful in all ages and all body weights, except extreme starvation.
Fluid volume/24h
• . 100mL/kg for each kg 0–10kg; 50mL/kg for each kg
10–20kg; 20mL/kg for each kg over 20kg.
Electrolytes• . Na
+
2mmol/kg/24h; K
+
1mmol/kg/24h.
Colloid
Colloids (especially blood) produce a more lasting expansion of intravas-
cular volume than crystalloid which rapidly enters the interstitial tissues.
Gelofusine is succinylated gelatin (a bovine collagen) which has a •
half-life of about 2h in plasma and is associated with increased bleeding
times in post-operative patients.

FLUID MANAGEMENT
93
Dextran is a glucose polymer mixture that has a plasma half-life of •
about 2h; it has been associated with anaphylactic reactions and
profound coagulopathy.
HES preparations are derived from hydroxyethyl starch; they have •
widely differing plasma half-lives and effects on plasma expansion.
Albumin is a naturally occurring plasma protein, sterilized by •
ultrafi ltration: 5% albumin is isotonic; 20% albumin is hypertonic.
Indications for use of albumin as a volume expander are very limited.
Blood, platelets, fresh frozen plasma (FFP), and cryoprecipitate •
(see b p. 96).
Assessing volume status This is usually straightforward, but in the
HDU patient 24h post-complex surgery, you need information from sev-
eral sources.
History and examination
The dry patient.• May have been NBM several days preoperatively and
feels thirsty, complains of a dry mouth, may be dehydrated because
of diarrhoea or vomiting, has a low JVP, dry mucous membranes, and
reduced skin turgor.
The overfi lled patient.• Usually doesn’t feel thirsty, has a raised JVP,
normal skin turgor, and may have dependent oedema and evidence of
pulmonary oedema on auscultation.
Observations chart
The dry patient.
• May have falling BP, rising pulse rate, low CVP
that does not rise with fl uid challenges, weight is several kg below
preoperative weight.
The overfi lled patient.• Is not usually tachycardic and has a high CVP
that rises and plateaus with fl uid challenges. BP may fall with fl uid
challenges; weight is several kg above preoperative weight.
Fluid balance chart In sick patients, ask the nurses to start an hourly fl uid
balance chart. Add up all fl uid loss (urine output, wound, stoma, and fi stula
drainage) and subtract from all IV, NG, and oral fl uids given.
The dry patient.
• Will usually be in several litres of negative fl uid
balance, possibly over a few days, with a urine output <1mL/kg/h.
The overfi lled patient.• Will be in several litres of positive fl uid balance,
possibly over a few days. Urine output may be low because of heart
failure or renal dysfunction.
Blood results
The dry patient.
• Has high Na, K, creatinine, and urea, with the urea
often disproportionately raised.
The overfi lled patient.• May have low Na.
CXR
The dry patient.
• Has no evidence of pulmonary oedema or effusions.
The overfi lled patient.• May have both pulmonary oedema and effusions.

CHAPTER 2 Principles of surgery
94
Acid–base balance
The pH of arterial blood is maintained at 7.35–7.45. Normal function-
ing of the body’s complex enzyme systems depends on this stability.
Derrangements may be primarily due to respiratory or metabolic dysfunc-
tion (see Box 2.5). Compensatory mechanisms are also divided into meta-
bolic and respiratory. The true clinical picture is mixed.
Box 2.5 Diagnosing acid–base abnormalities
pH <7.35 is an acidaemia; pH >7.45 is an alkalaemia
Look at the pH—is there an acidaemia or an alkalaemia?•
Look at the PaCO•
2
—is there a change in keeping with the pH
derangement? If so, the derangement is a respiratory one.
Look at the base defi cit (or anion gap). This will tell you if there is a •
metabolic derangement; if pH is normal, it is fully compensated.
The Flenley nomogram (see Fig. 2.7) is a useful diagnostic aid where
mixed metabolic and respiratory derangements are present.
Base excess, base defi cit, and anion gap
These are derived numbers, calculated by blood gas analysers, quantifying
changes in metabolic or fi xed acids, but because they depend on several
assumptions, they do not always refl ect the true acid–base balance.
Base excess• is defi ned as the mmol/L of acid that would be required to
titrate the blood pH back to 7.4 if the pCO
2
were normal.
Base defi cit• (negative base excess) is defi ned as the mmol/L of base to
titrate the blood pH back to 7.4 if the pCO
2
were normal.
A base defi cit is negative and a base excess is positive by convention. •
Normal values are –2mmol/L to +2mmol/L. A base defi cit greater than
this (e.g. –6mmol/L) indicates a metabolic acidosis.
The
• anion gap is the difference between measured cations and
measured anions (= K
+
+ Na
+
– Cl
–
+ HCO
3
–
). This is made up of
metabolic acids: ketones, lactate, and phosphates. The anion gap is
normally 8–16mmol/L; an increase in anion gap indicates a metabolic
acidosis.
Metabolic acidosis
Uncompensated: • dpH, npCO
2
, dHCO
3
–
.
Compensated: • dnpH, dpCO
2
, dHCO
3
–
.
Metabolic acidosis due to increased metabolic acids (i anion gap)
Lactic acid (global and/or regional hypoperfusion, hypoxia, sepsis,
•
hepatic failure as the liver normally metabolizes lactate).
Uric acid (renal failure).•
Ketones (diabetic ketoacidosis, alcoholic and starvation ketoacidosis).•
Drugs/toxins (salicylates, sodium nitroprusside overdose).•
Due to loss of bicarbonate or hyperchloraemia (normal anion gap)
Renal tubular acidosis (loss of bicarbonate).•
Diarrhoea, high output ileostomy (loss of bicarbonate).•
ACID–BASE BALANCE
95
Pancreatic fi stulas (loss of bicarbonate).•
Hyperchloraemic acidosis (excessive saline administration).•
Metabolic alkalosis
Uncompensated: • i pH, n pCO
2
, i HCO
3
–
.
Compensated: • in pH, i pCO
2
, i HCO
3
–
.
Loss of H
•
+
from gut (vomiting, NG tube suction).
Renal loss of H•
+
(diuretics), i reabsorption of
HCO
3
–
(hypochloraemia).
Administration of base (NaHCO•
3
, citrate in blood transfusions).
Respiratory acidosis
Uncompensated: • d pH, i pCO
2
, n HCO
3
–
.
Compensated: • dn pH, i pCO
2
, i HCO
3
–
.
Any cause of respiratory failure or hypoventilation.•
Increased production of CO•
2
, e.g. sepsis, malignant hyperpyrexia.
Rebreathing CO•
2
(circuit misconnections, soda lime exhaustion).
Respiratory alkalosis
Uncompensated: • i pH, d pCO
2
, n HCO
3
–
.
Compensated: • ni pH, d pCO
2
, d HCO
3
–
.
Hyperventilation: deliberate, inadvertent, or in non-ventilated patients •
caused by stroke, anxiety, pulmonary embolism (PE), pneumonia,
asthma, pulmonary oedema.
110
100
90
80
70
[H
+
]
a
nmol/L
60
50
40
30
20
0
024681012
10
Respiratory
alkalaemia
Acute respiratory acidaemia
Chronic respiratory acidaemia
Metabolic
acidosis
20 30 40 50 60 70 80 90 mmHg
P
a
CO
2
Metabolic alkalosis
7.6
7.5
7.4
7.3
7.2
pH
a
7.1
7.0
Fig. 2.7 Flenley nonogram. Reproduced with permission from Longmore M. et al.
(2007). Oxford Handbook of Clinical Medicine, 7th edn. Oxford University Press,
Oxford.

CHAPTER 2 Principles of surgery
96
Blood products and procoagulants
Blood
One unit of blood increases Hb by about 1g/dL in a 70kg adult.•
Blood is normally provided as packed red cells (1U • 8 350mL).
Cross-matched blood can normally be provided within 20min; it •
contains blood from a single donor.
In dire emergencies, O-negative blood (universal donor) can be given •
to recipients of any ABO Rh group without incompatibility reaction.
Autologous blood transfusion may be used with up to 2U of blood •
withdrawn from patients preoperatively, which may be stored for up
to 5 weeks.
Cell salvage reduces the need for allogeneic blood. Shed blood is •
collected intraoperatively, heparinized, spun with normal saline to
remove all material, including residual heparin, platelets, and clotting
products, and repackaged as red blood cells suspended in saline for
transfusion.
Simple measures to reduce the need for blood transfusion include:•
Treating anaemia and coagulopathies preoperatively.•
Stopping warfarin, heparin, aspirin, and clopidogrel appropriately.•
Special methods to reduce homologous blood transfusion include:•
Autologous blood transfusion.•
Cell salvage.•
Procoagulants (see • b p. 97).
Erythropoietin (EPO) stimulates erythrocyte production.•
Platelets
One unit of platelets increases platelet count by 10•
9
/L in a 70kg adult.
Platelets are provided as units (1U • 8 50mL).
Platelets do not need to be cross-matched, but they should be ABO-•
compatible (and rhesus-matched in females of childbearing age).
One unit contains platelets from a single donor.•
They are stored at 22• °C and have a shelf life of 5 days.
Indications for blood products
Young, fi t patients tolerate haemodilution much better than elderly •
patients with multiple comorbidities (particularly cardiovascular and
respiratory disease).
There is a higher threshold for giving blood products in the patient •
who is not actively bleeding or about to undergo a procedure.
Blood.• In general, aim to maintain Hb at 7–9.0g/dL; in older patients,
in those with cardiorespiratory disease, maintain Hb >9.0g/dL.
FFP.• Patient’s circulating blood volume replaced or activated partial
thromboplastin time ratio (APTR) >1.5 with active bleeding.
Platelets.• Platelets <50 x 10
9
/L or <100 x 10
9
/L with active bleeding
(lower threshold if patient was on aspirin or clopidogrel within 5
days and is actively bleeding).
Cryoprecipitate.• Patient’s circulating blood volume replaced or
fi brinogen <1g/L with active bleeding.

BLOOD PRODUCTS AND PROCOAGULANTS
97
Fresh frozen plasma (FFP)
One unit of FFP contains all the coagulation factors except platelets.•
1mL of FFP per kg will raise most clotting factors by 1% in a 70kg adult.•
One unit of FFP = 150–250mL and 5–10mL/kg is normally given.•
One unit of FFP usually contains product from a single donor, but is •
sometimes pooled, in which case it may be from several donors.
FFP does not need to be cross-matched, but should be ABO •
compatible (and rhesus-matched in females of childbearing age).
FFP must be stored at <–18• °C. It must be thawed, usually over 20min,
before giving and discarded if not used within 2h.
Cryoprecipitate
One bag of ‘cryo’ contains 150–250mg fi brinogen and factors VII and •
VIII.
If cryopreciptate is unavailable, 5U of FFP contain the same amount of •
fi brinogen as 10U of cryoprecipitate.
Ten bags of ‘cryo’ raises the fi brinogen 0.6–0.7mg/L in a 70kg adult.•
One bag of cryoprecipitate = 20mL; 5–10 pooled bags are normally •
given.
ABO and rhesus compatibility are not relevant.•
Antifi brinolytics (e.g. aprotinin and tranexamic acid)
Action.• Inhibit plasminogen and plasmin; reduce active fi brinolysis.
Indications.• Prophylaxis against bleeding in cardiovascular surgery,
especially high risk (e.g. redo surgery). Some studies suggest they
are useful in ‘high risk’ orthopaedic surgery. Treatment of excessive
bleeding post-operatively (liver surgery; very rarely, radical pelvic
resections).
Key revision points—physiology of blood groups
ABO antigens • pre-exist on red cells. ABO antibodies pre-exist in
the circulation and will cause immediate reaction if incompatible.
Anti-rhesus (D/E) antibodies• only develop following exposure to
the RhD antigen, e.g. during blood transfusion or delivery.
O Rh negative are • universal donors (red cells carry no ABO/Rh
antigens).
AB Rh positive are • universal recipients (serum contains no A, B,
RhD antibodies).
Blood grouping• involves adding A, B, RhD agglutinins to donated
blood to determine blood type; it takes less than 5min.
Cross-matching• involves mixing donated blood with the intended
recipient serum; assessing compatibility takes about 20min.

CHAPTER 2 Principles of surgery
98
Transfusion reactions
3 Acute haemolytic reaction
ABO incompatibility as a result of clerical, bedside, sampling, or laboratory
error is the most common cause. It may also be caused by incompatibility
within other antigen systems (Duffy/Kidd). Donor erythrocytes carrying
either A and/or B erythrocyte antigens bind to the recipient’s anti-A or
anti-B antibodies, resulting in complement formation, membrane attack
complex, and immediate haemolysis. Cytokine and chemokine release
mediates sympathetic infl ammatory response characterized by sudden
onset of hypotension, tachycardia, pyrexia, breathlessness, tachypnoea,
and back pain. Bilirubinaemia, anaemia, and haemoglobinuria as a result
of haemolysis ensue.
Stop the transfusion immediately and give basic life support if required.•
Keep the bag and giving set for analysis; inform haematology.•
Give crystalloid and furosemide to encourage diuresis.•
Dialysis may be required.•
3 Anaphylaxis and allergic reactions
Normally, IgE-mediated histamine release reactions to plasma, platelets,
and red blood cells. Mild allergic reactions are relatively common and
are characterized by erythematous papular rashes, wheals, pruritus, and
pyrexia. These are treated by stopping the transfusion and administering
chlorpheniramine (10mg IV). Anaphylaxis characterized by hypotension,
bronchospasm, and angioedema occasionally occurs.
Stop the transfusion immediately and disconnect connection tubing.•
Basic life support may be required.•
Treat bronchospasm and angioedema with adrenaline (1mL of 1:10 000 •
IV), chlorpheniramine (10mg IV), and hydrocortisone (100mg IV).
Non-haemolytic febrile reaction
These common and normally mild reactions are caused by recipient
antibodies directed against donor human leucocyte antigen (HLA) and
leucocyte-specifi c antigen on leucocytes and platelets. Cytokine release
mediates mild pyrexia, typically over an hour after transfusion is started.
Antipyrogens such as paracetamol 1g PO/PR limit pyrexia, but antihista-
mines are not helpful. Severe reactions feature high grade fever, rigors,
nausea, and vomiting. The severity of symptoms is proportional to the
number of leucocytes in the transfused blood and the rate of transfusion.
Leucocyte-depleted blood helps prevent these reactions.
Delayed extravascular haemolytic reaction
Although pre-transfusion antibody testing is negative (a satisfactory cross-
match), these patients experience accelerated destruction of transfused
red blood cells 7–10 days following transfusion. This is an antibody-medi-
ated reaction, usually by a patient’s antibody (commonly Rh E, Kell, Duffy,
and Kidd), present in levels too low to be detected clinically until pro-
duced in larger amounts on exposure to circulating antigen. As haemolysis
is extravascular, haemoglobinaemia and haemoglobinuria are uncommon:

TRANSFUSION REACTIONS
99
it is characterized by an unexpected fall in haematocrit a few days post-
transfusion, hyperbilirubinaemia, and positive Coombs’ test.
Transfusion-related acute lung injury
Non-cardiogenic pulmonary oedema, typically within 6h of transfusion, is
mediated by recipient antibodies against donor HLA. Activated recipient
leucocytes migrate to the lung, releasing proteolytic enzymes that cause a
localized capillary leak syndrome and pulmonary oedema.
Infection
Bacterial
Serious bacterial contamination of stored blood may occur although
platelets, which are usually stored at room temperature, are at greater
risk of this. Common organisms include Staphylococcus spp., Enterobacter,
Yersinia, and Pseudomonas spp. Contamination is diffi cult to detect. The
recipient becomes pyrexial at >40°C and hypotensive. This may occur
during the transfusion or hours after completion and unlike febrile transfu-
sion reactions, is not self-limiting.
Volume resuscitation.•
Culture the patient and send bag and giving sets to microbiology.•
Start empirical broad-spectrum antibiotics.•
Non-bacterial
Pre-transfusion testing includes screening for hepatitis B (HbsAg, anti-
HBc), hepatitis C (anti-HCV), HIV (anti-HIV-1/2, HIV-1 p24 antigen),
HTLV (anti-TLC-1/2), and syphilis. HIV can be transmitted by an infective,
but seronegative, donor for about 15 days after infection. The HCV win-
dow is 20 days. CMV is common in the donor population (40–60%) and
immunocompromised donors must receive leucocyte-depleted or CMV-
negative blood. Malaria may be transmitted by blood transfusion as may
new variant Creutzfeldt–Jakob disease (nvCJD).
Fluid overload
Characterized by hypotension, acute dyspnoea, high CVP, and hypoxia.
Stop the transfusion.•
Give high fl ow O•
2
and loop diuretics (40mg furosemide IV).
Massive transfusion
Replacement of the patient’s circulating volume within 24h.•
Stored red cells are depleted of ATP and 2,3-DPG and leak K•
+
and the
fl uid contains citrate.
Large volumes of this blood lead to a blood volume that has poor O•
2
carrying capacity, i K
+
, hypothermic (if blood is not warmed), and
coagulopathic due to Ca
2+
sequestration.
Reduce the effect of massive transfusion by the following.
Use infusion warmers and a warming blanket.•
Monitor central circulation and respiratory function closely.•
Consider giving Ca•
2+
supplements (with care!).
Check platelets, APTT, and fi brinogen: replace if needed.•
Check potassium regularly.•

CHAPTER 2 Principles of surgery
100
Shock
Defi nition
Inadequate end-organ perfusion and tissue oxygenation. ‘Cellular shock’
is a term describing failure of normal cellular processes, including oxygen
processing.
Emergency management
Assess the airway. If patent, give high fl ow O•
2
by non-rebreathing mask.
Check carotid or femoral pulse.•
Secure • IV access and start giving 500mL crystalloid rapidly.
Recheck BP. If low and falling fast, call crash team.•
Take a rapid history and examine patient to differentiate between the •
following types of shock.
Hypovolaemic shock
Causes.• Trauma, ruptured abdominal aortic aneurysm (AAA), ruptured
ectopic, post-operative haemorrhage, profound dehydration, burns,
pancreatitis.
Clinical features.• As above with history of trauma/surgery/illness.
Treatment.•
Lie patient fl at; high fl ow O•
2
; lift legs to autotransfuse if no IV
access.
Repeat fl uid infusion 500mL IV rapidly: you should see rise in BP.•
Take blood and send for FBC, U&E, clotting, and cross-match.•
Take arterial blood gas (ABG): estimate Hb, K•
+
as well as ABG.
Treat • i K
+
.
If no rapid improvement in BP, look for other causes.•
Anaphylactic shock
Causes.
• Drug allergy, blood product reaction, latex allergy.
Clinical features.• History of sudden onset after administration of drug.
Stridor or bronchospasm, angioedema, urticaria, pruritus, rash.
Treatment.•
Sit patient up; give high fl ow O•
2
; call anaesthetist if stridor.
If IV access: give 1mL of 1:10 000 adrenaline bolus; fl ush; then •
100mg hydrocortisone bolus; fl ush; then 10mg chlorpheniramine IV.
Repeat again in 5–10min if no improvement.•
If no IV access: give 1mL 1:1000 adrenaline IM. Then secure IV •
access.
If wheezy, give 5mL nebulized salbutamol.•
Septic shock
Cause.• Overwhelming sepsis (see b p. 138).
3 Recognizing shock
d• BP, i pulse, and usually cold, clammy, pale, sweating.
Confused—may be agitated • or drowsy.
Young patients will compensate, with the only signs being decreased •
pulse pressure, tachycardia, and decreased urine output.

SHOCK
101
Clinical features.• May be the same as hypovolaemic shock or, if
established, with circulatory collapse. Earlier in the evolution, the
patient may look ‘septic’—pyrexial, fl ushed, bounding pulses.
Treatment.•
As for hypovolaemic shock.•
Take blood cultures; then give IV cefuroxime 750mg tds.•
Cardiogenic shock
Rapidly reversible causes.• cardiac tamponade (trauma, post-cardiac
surgery), arrhythmias, tension pneumothorax.
Other causes.• Fluid overload and congestive cardiac failure (CCF),
MI, PE, subacute bacterial endocarditis (SBE), aortic dissection,
decompensated valvular heart disease.
Clinical features.• History of recent surgery/trauma, chest pain,
dyspnoea, palpitations.
Treatment.•
Give high fl ow O•
2
.
Give 2.5mg morphine IV (anxiolytic, venodilator, analgesic, anti-•
arrhythmic).
Put patient on cardiac and sats monitors; request 12-lead ECG.•
Treat arrhythmias (see advanced life support (ALS) algorithm on •
inside back cover).
Treat myocardial ischaemia with 0.1mg GTN, 300mg aspirin.•
Auscultate heart sounds and lung fi elds.•
Treat tension pneumothorax (see • b p. 636), cardiac tamponade
(see b p. 638).
Discuss with ITU.•
Consider central venous and peripheral arterial monitoring.•
Send blood for ABGs, FBC, U&E, clotting, troponin.•
Catheterize the patient.•
Request CXR—look for pulmonary oedema.•
Treat fl uid overload with diuretics: furosemide 40mg IV.•
Consider transthoracic echo to exclude pericardial effusion and •
valvular lesions, and to assess LV function.

CHAPTER 2 Principles of surgery
Post-operative haemorrhage
Post-operative haemorrhage may be arterial or venous. Signifi cant arterial
haemorrhage is rare and usually occurs from vascular anastomoses. Very
rarely, it arises from solid organ injury or loosening of arterial ties. It is
rapid, bright red in colour, and often pulsatile. Venous bleeding is a more
common cause of post-operative haemorrhage and is usually due to the
opening up of unsecured venous channels, or from damage to the liver
or spleen at surgery. Although it is non-pulsatile, low pressure, and dark
in colour, it can be very large volume and is every bit as life-threatening
as arterial bleeding. Most post-operative bleeding is not overt and is con-
tained within body cavities. Drains, even correctly placed, are an unreli-
able sign of bleeding. Rely on your clinical instincts even if the drains are
empty.
Causes and features
Primary haemorrhage.• Occurs immediately after surgery or as a
continuation of intraoperative bleeding. Usually due to unsecured
blood vessels (e.g. liver bleeding following trauma).
Reactionary haemorrhage.• Occurs within the fi rst 24h. Usually due to
venous bleeding and is commonly thought to be due to improved
post-operative circulation and fl uid volume, exposing unsecured
vessels that bleed (e.g. delayed splenic bleeding following minor
trauma at laparotomy).
Secondary haemorrhage.• Occurs up to 10 days post-operatively. Usually
due to infection of operative wounds or raw surfaces, causing clot
disintegration and bleeding from exposed tissue.
Symptoms
Confusion and agitation (due to cerebral hypoxia secondary to
hypotension).
Signs
Soaked dressings, acute wound swelling, blood in drains.•
Pallor, sweaty, tachypnoea, tachycardia, hypotension (a late sign in •
children and young adults).
Emergency management
Resuscitation
Establish large calibre IV access. Give crystalloid fl uid up to 1000mL •
bolus if tachycardic or hypotensive. Do not waste time trying to insert
a CV catheter. They are unreliable measures of CVP at the bedside in
acute situations and are too long and too fi ne calibre to be of use for
rapid volume resuscitation.
Attempt to control superfi cial bleeding with direct compression. Do •
not use tourniquets on limb wounds.
Take blood for emergency cross-match if serum is not already •
available. Detail an assistant to telephone blood transfusion for
emergency cross-match of a minimum of 2U of blood.
Inform senior help immediately if signifi cant blood loss. Consider •
alerting theatres and/or ITU.
102

POST-OPERATIVE HAEMORRHAGE
Do not use O Rh negative blood for resuscitation unless the patient •
is in extremis. In these cases, the patient should already be being
transferred to theatre or ITU.
Catheterize and place on a fl uid balance chart if hypotensive, but •
stable.
Establish a diagnosis
The cause may be obvious from the bleeding or the operation.
Read the operation notes. Is there any potential cause mentioned?
•
If the bleeding is severe, the only way to establish a diagnosis may be •
at re-operation.
If the patient is stable and re-operation is undesirable, consider imaging.
CT scanning may reveal intra-abdominal or intrathoracic blood.•
Angiography may reveal active bleeding sites and may be therapeutic •
(coil embolization).
Defi nitive management
Most post-operative bleeding does not require re-operation, but if it does,
it should always be done by a senior surgeon. If this is not the surgeon who
performed the original surgery, it may be wise to try to contact them in
case they can give useful information about the original procedure.
If re-operation is highly undesirable, e.g. rebleeding after solid organ
trauma, then defi nitive conservative management might include:
Radiologically guided embolization;•
FFP infusions (see • b p. 96);
Controlled, permissive hypotension;•
Monitoring on ITU.•
Wound haematoma
A localized collection of blood beneath the wound or at the site of sur-
gery, usually characterized by swelling and discoloration.
2
• If this occurs after vascular surgery, fl ap surgery, or procedures
on the limbs or neck, get senior help as urgent surgical exploration
and evacuation may be indicated to avoid ischaemia, compartment
syndromes, airway obstruction, fl ap failure, or ongoing haemorrhage.
Apply fi rm pressure followed by a pressure dressing.•
Check clotting and FBC and treat appropriately (see • b p. 96).
Withhold heparin.•
Surgical management is the same as for haemorrhage.•
103

CHAPTER 2 Principles of surgery
Wound emergencies
Infection
Causes
Most wound infections are acquired from the patient’s own fl ora. The
majority are skin organisms (e.g. Staphylococcus aureus, Staphylococcus
epidermidis), although the second commonest cause is contamination
from opened viscera during surgery (e.g. Escherichia coli from the GI tract,
Pseudomonas from the biliary tree).
Symptoms
Pain and discharge in the wound.
•
Malaise, anorexia, and fever (systemic infl ammatory features).•
Signs
Fever, tachycardia.•
Red, swollen, tender wound (may be discharging pus or fl uctuant due •
to contained pus).
Complications
Bacteraemia is common, but rarely signifi cant.
•
Septicaemia is rare unless the organism is resistant or the patient is •
immunosuppressed.
Emergency management
Resuscitation
Ensure there is IV access. Give crystalloid fl uid up to 1000mL if tachycardic
or hypotensive.
Establish a diagnosis
Send any discharging pus for microscopy, culture and sensitivities •
(M,C,&S).
Send blood for FBC (Hb, WCC) and blood cultures.•
Early treatment
Give IV antibiotics if there are any systemic features. If there is no pre-
•
existing infection, use anti-staphylococcals: fl ucloxacillin 1g + 500mg
qds. If the patient is immunosuppressed or very unwell, add in broad-
spectrum cover to include anaerobic cover: metronidazole 500mg IV +
500mg IV tds and cefuroxime 1.5g IV + 500mg IV tds.
If there is real concern about MRSA infection, consult microbiology •
and consider adding IV vancomycin 500mg. If so, monitor drug plasma
concentration.
Open or aspirate the wound if there is contained pus. Wash the •
wound.
Dehiscence
Wound dehiscence may be superfi cial (including skin and subcutaneous
tissue) or full thickness/deep (involving fascial closures or bony closures).
Full thickness dehiscence may expose deep structures. In the abdo-
men, this includes the viscera which may protrude through the wound
(evisceration).
104

WOUND EMERGENCIES
Causes
Most wound dehiscences are secondary to wound infection. Contributary
factors include immunosuppression, malnutrition, steroid use, poor surgi-
cal technique, previous surgery or procedures. Occasionally, the dehis-
cence is due to intracavity pathology causing wound breakdown from
within (e.g. anastomotic leakage causing enteric fi stulation).
Symptoms
Usually painless.
Signs
Open wound, visible fat and fascia if superfi cial; visible viscera if full
•
thickness.
Occasionally associated organ dysfunction if involved by the •
accompanying wound infection (e.g. pericarditis/anterior mediastinitis
in sternal dehiscence).
Emergency management
Resuscitation
Ensure there is IV access.
•
Calm the patient, particularly if there is any degree of evisceration.•
Early treatment
If there are exposed viscera, cover these with saline soaked dressings.•
Give IV antibiotics if there features of wound infection (see • b p. 104).
If the dehiscence is superfi cial, ensure the wound is open and any pus •
is fully drained. Lightly pack the wound with absorbent dressing (e.g.
Sorbsan
®
).
Defi nitive management
Superfi cial
Continue regular wound lavage and dressings.
•
For large defects, consider vacuum-assisted closure.•
Full thickness
Resuturing/closure of the defect in theatre may be appropriate.
•
For some deep defects, re-closure may be inappropriate (e.g. •
the presence of infection, intestinal contents/fi stulas, severe
immunocompromise, physiologically unstable, intracavity pathology
causing the dehiscence). In these cases, the wound should be allowed
to form a chronic wound and close by secondary intention (e.g. called
a laparostomy in the abdomen). This may be assisted by vacuum
closure devices.
Bleeding (see b p. 102).
105

CHAPTER 2 Principles of surgery
Cardiac complications
Chest pain
Taking a careful pain history should help differentiate between the causes
of chest discomfort listed in Box 2.6 below.
Diagnosis
Take a careful history and examine the patient.•
A CXR will demonstrate most lung pathology.•
12-lead ECG should help exclude myocardial ischaemia.•
Recent WCC and CRP help identify sepsis.•
Review previous medical history for peptic ulcer disease and the drug •
chart for NSAID use.
Myocardial ischaemia
Patients, particularly in vascular surgery, may have pre-existing ischaemic
heart disease. Surgery can precipitate ischaemia through:
Stress response to major surgery (endogenous catecholamine release •
triggered by anxiety, pain).
Fluid overload post-operatively.•
Profound hypotension.•
Failing to restart anti-anginal medication post-operatively.•
Diagnosis
Take a history, particularly of chest discomfort brought on by exertion and
relieved by GTN. Check that the patient is back on any regular cardiac
medication. The physiotherapists may report bradycardia on exercising.
A 12-lead ECG will confi rm the presence of myocardial ischaemia (see
Box 2.7). Cardiac enzymes (troponin T (and I) or CK
MB
) may be slightly
raised post-operatively, but a high level or serial measurements showing a
continued rise would suggest ongoing myocardial damage.
Box 2.6 Causes of post-operative chest pain
Dull, central ache
Myocardial ischaemia.•
Gastric distension.•
Central pain radiating through to back
Thoracic aneurysm or dissection.•
Peptic ulcer disease, oesophagitis, rarely pancreatitis.•
Pain on movement
Musculoskeletal pain.
•
Chest drains.•
Pleuritic pain
Chest infection.•
Pneumothorax.•
Haemothorax, pleural effusion, empyema.•
Chest drain • in situ.
PE.•
106

CARDIAC COMPLICATIONS
Management
Sit patient up; give high fl ow O•
2
.
Ensure the patient is on aspirin 75mg od PO and LMWH, e.g. 40mg •
enoxaparin (Clexane
®
) SC.
Give GTN sublingually.•
Restart preoperative anti-anginal medication.•
Discuss urgently with a cardiologist.•
Perioperative MI
Perioperative MI may be diffi cult to diagnose because the patient may be
unable to give a good history or to distinguish between chest and upper
abdominal pain.
The presentation is similar to that of myocardial ischaemia, but •
the duration is longer (>20min) and may be associated with
haemodynamic instability, nausea, vomiting, confusion, and distress.
The patient will often be cold, clammy, and may be hypoxic.•
Management
Attach an ECG monitor and a sats probe and get a 12-lead ECG.
•
Make sure the defi brillator trolley is close at hand.•
Give high fl ow O•
2
.
Get IV access.•
Give morphine 5mg IV and metoclopramide 10mg IV.•
Give aspirin 300mg • PO/PR and GTN 0.5mg SL.
Contact cardiologists urgently for consideration of acute intervention.•
Box 2.7 Diagnostic criteria for MI
In the setting of symptoms suggestive of acute coronary syndrome:
ECG shows ST segment elevation—ST segment elevation MI •
(STEMI).
No ST elevation, but elevated CK•
MB
(2 x normal) or troponin
T-positive—non-Q wave or non-ST segment elevation MI (NSTEMI).
Key revision points—physiology of coronary blood fl ow
Myocardial cells extract up to 70% of O•
2
from blood.
Coronary blood fl ow occurs during diastole.•
Tachycardia reduces diastolic interval and increases O•
2
demand,
which may reveal occult ischaemia.
Coronary vasodilatation is mediated by adenosine, K•
+
, hypoxia, and
B
2
stimulants and the N
2
O pathway.
107

CHAPTER 2 Principles of surgery
Respiratory complications
These are common after surgery as a result of the effect of general anaes-
thetic, post-operative pain, and immobility.
Respiratory failure
Basic assessment and management
Sit the patient up and give high fl ow O•
2
through a tight fi tting mask.
Assess the airway. Is chest expansion asymmetrical?•
Auscultate the chest. Listen for bilateral breath sounds, poor air entry, •
wheeze, bronchial breathing, crepitations.
Assess circulation and treat shock which causes hypoxaemia (see • b
p. 100).
Treat bronchospasm with nebulized salbutamol 5mg.•
Get a CXR. Look for consolidation, oedema, effusions, and •
pneumothoraces.
Chest infection
Diagnosis
Cough with purulent sputum.•
Pyrexia.•
Bronchial breath sounds and reduced air entry on auscultation.•
Leucocyte neutrophilia, raised CRP.•
Consolidation on CXR.•
Culture of sputum may yield sensitivities of causative organisms.•
In the dyspnoeic, hypoxic patient perform ABGs to guide immediate •
management.
Prevention
There is no good evidence that prophylactic physiotherapy helps to pre-
vent chest infection after surgery. The single most important intervention
is to prevent patients with active chest infections undergoing surgery. Any
elective patient with a current cough (dry or productive), temperature,
clinical signs of chest infection, neutrophilia, or suspicious CXR should be
deferred for a fortnight and then reassessed. Other risk factors include
active smokers or those who have stopped smoking within the last 6
weeks; patients with COPD, obesity; patients requiring prolonged ventila-
tion post-operatively; and patients who aspirate.
Management
Physiotherapy helps the patient with a cough to expectorate sputum •
and prevent mucus plugging.
Effective analgesia is important to allow patients to cough.•
Defi nitions of respiratory failure
Hypoxia. • PaO
2
<10.5kPa.
Hypercapnia.• PaCO
2
>6.5kPa. Hypocapnia. PaCO
2
<3.5kPa.
Type I respiratory failure. • PaO
2
<8.0kPa on air.
Type II respiratory failure.• PaO
2
<8kPa and PaCO
2
>6.0kPa.
108

RESPIRATORY COMPLICATIONS
Defi nitive treatment is antibiotics: ciprofl oxacin 500mg bd • PO provides
good Gram –ve and +ve cover until organism sensitivities are known.
Suspected aspiration pneumonia should be treated (e.g. • IV cefuroxime
1g tds and IV metronidazole 500mg tds).
If the patient requires oxygen (PaO•
2
<8.0kPa on room air), humidifying
it reduces the risk of mucus plugs and makes secretions easier to shift.
Continuous positive airway pressure (CPAP) can be used to improve •
basal collapse.
The hypoxic, tachypnoeic, tiring patient on respiratory support should •
be reviewed urgently by the critical care team.
Exacerbation of COPD
The incidence of moderate to severe COPD in surgical patients is 5%.
Most studies show that moderate COPD is not associated with an •
increase in post-operative complications, mortality, or length of stay.
Severe COPD and preoperative steroid use are associated with •
increased morbidity and mortality after surgery.
Ensure that all patients on preoperative • β-agonist inhalers are
routinely prescribed regular post-operative nebulizers (saline 5mL prn,
salbutamol 2.5–5mg qds prn, and becotide 500 micrograms qds prn).
In hypoxic patients with COPD, give maximal O•
2
by CPAP and titrate
against PaCO
2
and PO
2
: do not restrict O
2
empirically.
Key revision points—monitoring/measuring lung function
Pulse oximetry estimates the percentage of saturated Hb present in •
capillary blood by the change in wavelength ratios of absorbed red
light. It is inaccurate in carbon monoxide poisoning, cold peripheries,
low fl ow states, and tachydysrhythmias.
PaO•
2
can be approximately estimated from the SaO
2
.
95%, >12kPa.•
85%, • 710kPa.
75%, <6kPa.•
Capnography works on similar principles; different gases (e.g. CO•
2
)
absorb different amounts of infrared light.
109

CHAPTER 2 Principles of surgery
110
Renal complications
Aetiology of renal failure
Preoperative risk factors.• Age >75y; creatinine >150μmol/L; LV
dysfunction; hypertension; diabetes; peripheral vascular disease;
hypoperfusion as a result of diuretic therapy and vasodilators;
sepsis; CCF; intrinsic renal damage caused by NSAIDs, contrast,
aminoglycosides, diuretics; endocarditis; obstructive uropathy.
Intraoperative risk factors.• Cardiac surgery, aortic surgery.
Post-operative risk factors.•
Pre-renal• . Shock, e.g. hypovolaemic, septic, cardiogenic (see b
p. 106).
Renal• . Sepsis, hypoxia, drugs (NSAIDs, gentamicin, vancomycin,
teicoplanin), haemoglobinuria, myoglobinuria.
Post-renal• . Obstructive uropathy, obstructed Foley catheter,
prostatic hypertrophy.
Preventing renal failure
There are a number of measures that reduce the risk of renal
dysfunction.
Preoperatively, • ensure adequate hydration, particularly before
undergoing procedures involving contrast.
Identify and • eliminate nephrotoxic medications where possible,
particularly NSAIDs and ACE-inhibitors.
Consider whether the patient would benefi t from HDU •
preoperatively.
Avoid intraoperative hypotension.•
Post-operatively maintaining satisfactory cardiac output and optimizing •
intravascular volume are the most important factors in avoiding renal
dysfunction.
Management of renal failure
The management of oliguria is described on b pp. 112–3. This section
deals with the management of established oliguric renal failure. The aim is
fi rstly to avoid the potentially lethal complications of renal failure (hyper-
kalaemia, acidosis, pulmonary and cerebral oedema, severe uraemia,
and drug toxicity) and secondly to avoid exacerbating the renal insult.
Investigation of the underlying causes of renal failure is also important.
Aim for higher BP (stop antihypertensives; optimize fl uid balance), •
except in established anuria.
Treat hypoxia aggressively.
•
Aim for daily fl uid balance of even to negative 500mL to avoid •
pulmonary oedema in anuric patients.
Renal dysfunction
Creatinine: >126μmol/L in males; >102μmol/L in females.•
Urea: >7.0mmol/L.•
Creatinine clearance: <90mL/min.•

RENAL COMPLICATIONS
111
Monitor electrolytes daily, and potassium and acid–base balance •
every few hours. Avoid potassium supplements and medication that
increases potassium levels (ACE-inhibitors).
Avoid nephrotoxic drugs (aminoglycosides, NSAIDs, ACE-inhibitors) •
and monitor serum levels of drugs dependent on renal excretion
(digoxin, antibiotics such as vancomycin and gentamicin).
Essential amino acid diets are recommended for patients who are able
•
to eat. Patients on dialysis require high protein content (1.5g/kg/day) as
dialysis results in negative nitrogen balance.
Enteral and parenteral feeds can be similarly adjusted.•
Renal ultrasound, renal angiography may be indicated.•
Hyperkalaemia
Hyperkalaemia (K
+
>5.0) is seen in the setting of renal failure, tissue necro-
sis, and potassium-sparing diuretics and supplements. Acute hyperkalemia
(K
+
>6.0) can cause life-threatening ventricular arrhythmias. ECG changes
that herald myocardial dysfunction are fl attened P waves, wide QRS com-
plexes, tenting of T waves, and, in peri-arrest, a sine wave.
2• Treat the patient with ECG changes as an emergency.
Treat the underlying cause.•
Give • 50mL of 50% dextrose containing 15U of Actrapid
®
as an IV
infusion over 10–20min, repeating as necessary, monitoring BS after
each infusion. If inadequate response:
Give 10mL calcium gluconate 10% IV over 2min• ; repeat as necessary.
Calcium resonium enema binds K•
+
and removes it from the body.
Dialysis • should be urgently considered in patients with refractory
hyperkalemia despite these measures, irrespective of renal function.
Hypokalaemia
Hypokalaemia (K
+
<3.0) is common. It predisposes patients to dysrhythmias.
It is normally related to diuretic therapy, insulin sliding scales, diarrhoea
and vomiting, steroids, and poor nutrition. Acute severe hypokalaemia (K
+
<2.5) may result in life-threatening arrhythmias. It can be recognized by
small or inverted T waves, depressed ST segments, prolonged PR interval,
and U waves on the ECG.
Educate the patient about which foods are rich in potassium (bananas, •
prunes, apricots, tomatoes, orange juice) and ensure availability.
Change furosemide to co-amilofruse 5/40 or 2.5/20 which contains •
furosemide (either 40mg or 20mg) and amiloride (5mg or 2.5mg).
Add oral potassium supplements up to 160mmol daily (1 tablet of •
Sando K
+
contains 20mmol of K
+
, 1 tablet of Slow K
+
, which is better
tolerated by most patients, contains 12.5mmol KCl).
If a central line is in place, give 20mmol KCl in 50–100mL of 5% •
dextrose over 20min to 1h.
If it is necessary to use a peripheral line, place a maximum of 40mmol •
of potassium in 1L 5% dextrose running at a maximum of 125mL/h.
Monitor K•
+
daily and avoid discharging the patient home on a
combination of potassium supplements and potassium-sparing
diuretics.

CHAPTER 2 Principles of surgery
112
Urinary complications
Oliguria
Urine ouput is an indicator of glomerular fi ltration rate which is an indica-
tor of renal plasma fl ow and renal perfusion. Hence, urine output is an
indirect measure of renal (and hence systemic) blood fl ow as well as renal
function. Patients with normal renal function usually maintain a urine out-
put of at least 0.5mL/kg/h.
Management of oliguria
Check that the Foley catheter is not the problem.•
The urine catheter may be obstructed, bypassing, or malpositioned. Is •
the bed wet? Flush with 60mL saline—can you draw this amount back
without diffi culty? If not, or if the urine is bypassing the catheter, or if
the bladder is palpable, change the catheter.
Optimize cardiac function
Patients who were markedly hypertensive (see
• b pp. 92, 132)
preoperatively may require high BPs to maintain a satisfactory urine
output.
Is the patient overfi lled or underfi lled• ?
Make sure the patient is adequately fi lled • by giving careful fl uid
challenges to achieve CVP of 14–16mmHg or raise the JVP moderately.
But not too fi lled. • If the CVP rises to >16mmHg and stays up with a
fl uid challenge or if the BP falls, the patient may be overfi lled and need
diuretics (see below).
Invasive monitoring.
• If the patient does not rapidly respond to basic
measures, need CVP line insertion and monitoring (see b p. 198).
Loop diuretics
Furosemide will not prevent acute tubular necrosis, but it does have a use-
ful role in offl oading fl uid from the overfi lled patient. It converts oliguric
renal failure to polyuric renal failure.
If the patient is adequately fi lled and mean arterial pressures are •
satisfactory, give a loop diuretic: 20mg of furosemide IV. If there is no
response, give a further 40mg furosemide IV.
If the urine produced in response to diuretic challenges is •
concentrated, the patient is probably inadequately fi lled.
Important problems associated with oliguria of any cause
Pulmonary and cerebral oedema.•
CCF (see • b p. 106).
Hyperkalaemia (see • b p. 110).
Acidosis.•
Drug toxicity.•
Terminology
Oliguria—urine output <0.5mL/kg/h.•
Anuria—no measurable urine output.•

URINARY COMPLICATIONS
113
Further assessment and management
Haemodialysis is indicated in the oliguric patient to avoid pulmonary
oedema indicated by deteriorating blood gases despite increasing respira-
tory support, hyperkalaemia, and acidosis. It is not indicated purely for
rising serum creatinine and urea in the fi rst instance (b p. 134).
Acute urinary retention
Common post-operatively, especially in elderly males, after abdomino-
pelvic or groin surgery and after anticholinergics.
Clinical features
Suprapubic discomfort, inability to initiate micturition, or dribbling.
•
History of prostatic disease or symptoms preoperatively (see • b p.
362).
Percussable bladder on examination.•
Management
Conservative. Improve analgesia, treat constipation, mobilize, warm
•
bath to encourage micturition, restart preoperative tamsulosin.
Insert urethral catheter if conservative measures fail and patient in •
great discomfort or renal dysfunction is suspected.
Urinary tract infection
Common in females and patients catheterized for prolonged periods.
Clinical features
Dysuria, frequency, dribbling, offensive smell, pyrexia, • i WCC.
Dipstick urine to confi rm (dipstick should test nitrites and leucocytes).•
Send specimen for microbiology to identify organism and sensitivities.•
Management
Remove catheters as soon as possible.
•
Encourage drinking or increase fl uid infusion if safe to increase urine •
fl ow.
Treat empirically with trimethoprim 400mg bd until sensitivities •
known.
Key revision points—physiology of diuretics
Osmotic diuretics• , e.g. mannitol, are not well reabsorbed in
the distal tubules. Increased osmotic pressure reduces H
2
O
reabsorption.
Loop diuretics• inhibit Na
+
K
+
Cl
–
exchange in the ascending loop of
Henle, decreasing osmolality in the medulla and water reabsorption.
Aldosterone antagonists• , e.g. spironalactone, and sodium channel
blockers, e.g. amiloride, reduce Na
+
reabsorption and K
+
and H
+
secretion in the distal tubule.
Alcohol• inhibits antidiuretic hormone (ADH) release.

CHAPTER 2 Principles of surgery
114
Gastrointestinal complications
Paralytic ileus
This is the cessation of GI tract motility.
Causes
Prolonged surgery, exposure and handling of the bowel.
•
Peritonitis and abdominal trauma.•
Electrolyte disturbances (most can affect GI function!!).•
Anticholinergics or opiates.•
Prolonged hypotension or hypoxia.•
Immobilization.•
Clinical features
Nausea, vomiting and hiccoughs.•
Abdominal distension, tympanic or dull on percussion.•
Air-/fl uid-fi lled loops of small and/or large bowel on abdominal X-ray •
(AXR).
Prognosis
Intestinal ileus usually settles with appropriate treatment.
Treatment
Pass an NGT to empty the stomach of fl uid and gas if the patient is •
nauseated or vomiting. Small volumes of tolerated oral intake may help
mild ileus to resolve.
Ensure adequate hydration by • IV infusion (‘drip and suck’).
Maintain the electrolyte balance.•
Reduce opiate analgesia and encourage the patient to mobilize.•
Consider other causes (e.g. occult intra-abdominal sepsis) and consider •
nutritional status.
Post-operative mechanical small bowel obstruction
It is important to distinguish between mechanical obstruction and ileus
since management may be different.
Causes
Early adhesions (usually self-limiting).•
Internal, external, parastomal, or wound herniation.•
Intra-abdominal sepsis (usually slightly later presentation).•
Clinical features
Nausea and vomiting.
•
Colicky abdominal pain.•
Abdominal distension, tympanic on percussion.•
Examine hernial orifi ces and stoma, if any, for incarcerated hernias.•
High-pitched ‘tinkling’ bowel sounds MAY be present.•
Dilated loops of small bowel (relative paucity of gas in colon).•
Treatment
As for paralytic ileus with strict bowel rest.
•
Consider CT scan to defi ne diagnosis and level of the obstruction.•
GASTROINTESTINAL COMPLICATIONS
115
Prognosis
Surgery is rarely indicated (for suspected herniation or complications or,
very occasionally, adhesional obstruction that fails to resolve).
Nausea and vomiting
This affects up to 75% of patients. It predisposes to increased bleeding,
incisional hernias, aspiration pneumonia, d absorption of oral medication,
poor nutrition, and d K
+
. Causes include:
Prolonged surgery; anaesthetic agents, e.g. etomidate, ketamine, N•
2
O,
opioids; spinal anaesthesia; gastric dilatation from CPAP;
Post-operative ileus; bowel obstruction; constipation; gastric refl ux; •
peptic ulceration or bleeding; medications, including many antibiotics,
NSAIDs, opiates, statins; pancreatitis; sepsis; and hyponatraemia.
Constipation
Failure to pass stool is common. Caused by lack of privacy, immobility,
pain from wounds or anal fi ssures, dehydration, poor nutrition, d dietary
fi bre, opiates, iron supplements, and spinal anaesthesia. Treat with:
Bulking agents• , e.g. Fybogel one sachet PO bd.
Stool softeners• , e.g. sodium docusate 30–60mg od PO.
Osmotic agents• , e.g. lactulose 5–10mL bd.
Stimulants• , e.g. senna one tablet bd PO, bisacodyl 5–20mg nocte PO.
Diarrhoea
Common causes in post-operative patients:
Resolving ileus or obstruction.•
Related to underlying disease or surgery (e.g. ileal pouch or Crohn’s).•
Antibiotic-related diarrhoea (send for M,C,&S).•
Clostridium (C.) diffi cile• diarrhoea (send stool for C. diffi cile toxin) and
pseudomembranous colitis (see b p. 396).
Anastomotic leakage (see b p. 420).
Classifi cation of antiemetics
Combining two different types of antiemetic increases effi ciency.
Antidopaminergic agents
Good against opioid nausea and vomiting, sedative, extrapyramidal •
side effects.
For example, prochlorperazine 12.5mg IM, metoclopramide 10mg • IV/
IM/PO tds.
Antihistamines
Sedation, tachycardias, hypotension with • IV injection.
For example, cyclizine 50mg • IM/IV/PO tds.
Anticholinergics
Active against emetic effect of opioids, sedation, confusion, dry mouth.
•
For example, hyoscine (scopolamine) 0.3–0.6mg • IM.
Antiserotonergics
Lowest side effect profi le of all antiemetics.•
Ondansetron 1–8mg • PO/IV/IM tds, granisetron 1mg PO/IV tds.

CHAPTER 2 Principles of surgery
116
Neurological complications
Confusion
Confusion is common post-operatively. It is often obvious with a disori-
ented, uncooperative, or hallucinating patient. Frequently, it is more sub-
tle, consisting of inactivity, quietness, slowed thinking, and labile mood,
and it is only spotted by relatives or nursing staff. Actively assess whether
the patient is oriented in time, person, and place. Perform a quick mini-
mental state examination if you are still unsure.
Management
If the patient’s behaviour poses a physical danger to themselves •
or others, it may be necessary to sedate as fi rst-line management.
Haloperidol 2.5mg may be given up to a total of 10mg in 24h PO,
IM, or IV, but if the patient remains disturbed, 2.5–5mg of midazolam
should be given IV and the patient placed under close observation.
2 Beware of sedating the hypoxic or hypotensive patient as this may
trigger a cardiorespiratory arrest: confusion is a common symptom of
shock and profound hypoxia.
Assess and treat hypoxia (see • b p. 130) and hypotension (see b p. 132).
Reassess the drug chart: stop opiates and benzodiazepines.•
Correct abnormalities, e.g. • d glucose, d Na.
Alcohol withdrawal is diagnosed from a history of chronically •
high alcohol consumption often with raised GGT, combined with
psychomotor agitation post-operatively. It can be treated with either
diazepam 5–10mg tds PO/PR, haloperidol 2.5–5mg tds PO/IM/IV, or
allowing the patient alcohol 1U orally.
Perform a neurological examination to look for focal neurological •
defi cit and consider head CT to exclude stroke.
Reassure patient and relatives: confusion is common, almost always •
reversible, and it is not a sign that the patient is ‘going mad’.
Stroke
Stroke is most common in vascular and cardiac surgical patients (2%), but
elderly patients undergoing other major surgery are at risk.
Risk factors for stroke
Increasing age (>80y risk of cerebrovascular accident (CVA) 5–10%).•
Diabetes.•
Common causes of confusion
Medication (particularly benzodiazepines, opiates, anticonvulsants).•
Stroke.•
Hypoxia, hypercapnia.•
Shock.•
Sepsis.•
Alcohol withdrawal.•
Metabolic disturbances (• d glucose, Na
+
, pH; i Ca
2+
, creatinine, urea,
bilirubin).
Post-ictal.•
Preoperative dementia.•

NEUROLOGICAL COMPLICATIONS
117
Previous history of stroke or transient ischaemic attack (TIA) •
(increases risk three-fold).
Carotid artery atherosclerosis.•
Perioperative hypotension.•
Left-sided mural thrombus.•
Mechanical heart valve.•
Post-operative AF.•
Aetiology
Embolic.• Carotid stenosis/atheroma, thrombus from AF.
Haemorrhagic.• Post-operative warfarinization.
Cerebral hypoperfusion.• Profound hypotension, raised intracranial
pressure (ICP).
Hypoxia.•
Clinical features
Any defi cit resolving within 24h is called a TIA. Clinical features of periop-
erative stroke include:
Failure to regain consciousness once sedation has been weaned.•
Hemiplegia (middle cerebral artery or total carotid artery occlusion).•
Initial arefl exia becoming hyperrefl exia and rigidity after a few days.•
Aphasia, dysarthria, ataxia (gait or truncal), inadequate gag refl ex.•
Visual defi cits, unilateral neglect, confusion, seizures.•
Persistent, marked hypertension.•
Hypercapnia.•
Diagnosis
The aim is to establish a defi nitive diagnosis, establish a cause to guide
appropriate secondary prevention, and establish a baseline of function to
help plan long-term rehabilitation or withdrawal of therapy.
Carry out a full neurological examination (cognitive function, cranial
•
nerves, and tone, power, refl exes, and sensation in all four limbs).
Modern contrast head CT will show infarcts within 2h (older scanners
•
may not pick up lesions until they are 2–3 days old. You must
distinguish between haemorrhagic and ischaemic CVAs (1 in 10 are
haemorrhagic). MRI is necessary to image brainstem lesions.
Initial management
Assess the airway, breathing, and circulation.
•
If the patient is unable to maintain their airway, insert a Guedel airway, •
bag and mask, ventilate with high fl ow O
2
, and call an anaesthetist.
Monitor BP, but do not attempt to correct high pressures as these are •
critical for adequate cerebral perfusion.
Monitor oxygen saturations.•
Secure • IV access and give colloid if indicated.
If the patient is able to maintain their own airway and is not •
haemodynamically compromised, explain what has happened and
reassure them.
Perform a full neurological examination.•
Put the patient NBM if there is no gag refl ex.•
Send FBC, U&E, glucose, and clotting.•
Send blood cultures if there is any history of endocarditis, pyrexia.•
Request a CT head and consider a transthoracic echo.•

CHAPTER 2 Principles of surgery
118
Haematological complications
Heparin-induced thrombocytopenia (HIT)
HIT occurs in about 5% of patients receiving heparin (5.5% of patients
on bovine heparin, 1.0% with porcine heparin). It is characterized by the
formation of complement-mediated heparin-dependent IgG platelet anti-
body. It occurs 5–10 days after initiation of heparin therapy or after the
fi rst dose of heparin in patients with previous exposure to heparin within
the last 3 months.
Diagnosis
Fall in platelet count by over 30% to <150
• x 10
9
/L or by over 50%.
And• positive serology for HIT antibodies.
Heparin-induced thrombocytopenia and thrombosis (HITT) occurs •
in about 20% of patients with HIT and is characterized by major
thrombotic episodes. It has a mortality of about 30%.
Patients may show tachyphylaxis to heparin as well as bleeding •
complications.
Treatment
Discontinue all heparin therapy, including heparinized saline fl ushes.•
If it is at all possible, delay any surgery requiring bypass until HIT •
antibodies are undetectable and then follow standard heparinization,
but do not use heparin in the post-operative period.
If it is impossible to delay bypass surgery, then danaparoid and iloprost •
are alternatives to heparin with the major disadvantages that they
cannot be reversed after bypass and require specialized assays.
Hirudin, iloprost, danaparoid, and warfarin are alternative •
anticoagulants to heparin in the post-operative period.
Discuss with haematologist.•
Disseminated intravascular coagulation (DIC)
DIC may occur as a complication of sepsis, transfusion reaction, drug
reaction, transplant rejection, and aortic aneurysm surgery. It is character-
ized by widespread activation of coagulation, resulting in the formation
of intravascular fi brin, fi brin degradation products, consumption plate-
lets, and clotting factors, and ultimately, thrombotic occlusion of vessels.
Patients may present with bleeding from indwelling venous lines, wounds,
and minor abrasions.
Diagnosis
There is no single diagnostic test. The following fi ndings suggest DIC:
Sudden fall in platelet count to <100
• x 10
9
/L.
Bleeding and/or thrombotic complications.•
i• APTT, PT, INR.
i• fi brin degradation products.
d• fi brinogen in severe DIC.
HAEMATOLOGICAL COMPLICATIONS
119
Management
The key is to treat the underlying disorder. Bleeding patients should
receive FFP, platelets, blood, and cryoprecipitate as indicated by coagula-
tion screens. Patients with thrombosis should be heparinized.
Excessive warfarinization
Warfarin inhibits carboxylation of vitamin K, inhibiting the synthesis of
vitamin K dependent factors (V, VII, XI). Management depends on whether
the patient is bleeding, why they are warfarinized and if urgent/emergency
surgery is indicated.
If the INR is <5.0 and the patient is not bleeding, simply omit warfarin •
and recheck INR daily, restarting warfarin once the INR is within range.
If the INR is >5.0, give 10mg vitamin K • PO.
If INR is elevated and emergency reversal to normal clotting is •
required (e.g. for high risk surgery), IV Beriplex (mixed FII/VII/IX/X
protein C/S) may be used.
If the patient is bleeding, give FFP and up to 5mg vitamin K • PO.
If the patient has a mechanical valve, the risk of thromboembolic •
events when anticoagulation is reversed is <0.1% per day.
Anticoagulation with heparin or warfarin should be recommenced
within 1–2 weeks once any bleeding complications have resolved.
Key revision points—physiology of haemostasis
Vascular phase• . Vasospasm, local oedema, and haematoma.
Platelet phase• . Adherence of platelets activated by ADP, collagen,
von Willebrand factor and mediated by IIb/IIIa, fi brinogen, 5-HT,
thromboxane A2.
Clotting phase• :
Intrinsic• (kallikrein, XII, XI, and IX).
Extrinsic• (thromboplastin, VII) pathways converge on common
pathway (X, thrombin, fi brinogen).
Fibrinolysis depends on plasmin, antithrombin III, proteins C and S, tissue
factor pathway inhibitor.

CHAPTER 2 Principles of surgery
Deep venous thrombosis and
pulmonary embolism
Deep venous thrombosis (DVT) is most common in patients over 40
years of age who undergo major surgery. A post-operative increase in
platelets coupled with venous endothelial trauma and stasis all contribute
(Virchow’s triad). If no prophylaxis is given, 30% of these patients will
develop DVT and 0.1–0.2% will die from pulmonary thromboembolism
(PTE).
High-risk groups (see Prophylaxis b p. 72)
Patients undergoing pelvic or hip surgery.•
Patients with malignant disease.•
Patients on the contraceptive pill (pregnancy).•
Previous history of • DVT or PTE.
Older patients. The increase in • DVT is almost linear with advancing
age.
Other factors: obesity, diabetes mellitus, polycythaemia, varicose veins, •
cardiac and respiratory disease, thrombophilia (e.g. factor V Leiden
protein C or S defi ciency).
Diagnosis
Clinical
Systemic pyrexia at 7–8 days post-operatively.•
May present with embolism (pulmonary).•
Pain, swelling of the leg, and a rise in local skin temperature.•
Investigations
DVT may be diagnosed using:
•
Duplex Doppler: less sensitive for calf DVT than thigh DVT.•
Ascending venography: invasive, but more sensitive than duplex.•
PE may be diagnosed using:•
CTPA (almost universally favoured now over VQ lung scans).•
VQ lung scanning with radioactive technetium-labelled •
microaggregates of albumin (
99
Tcm MAA).
Treatment
Confi rm the site and the extent of the DVT by Doppler and lung scan.
Calf vein thrombosis may be treated by compression alone.•
All other thrombi should be treated with heparin for 4–7 days (40 •
000U/24h), checked by bleeding times (5000–10 000U by bolus, then
1000–1500U/h IV alone), or followed by oral anticoagulation for 6–12
weeks (warfarin, checked by PT/INR).
Lytic therapy—urokinase, streptokinase—is most effective within •
24–36h of onset of DVT. This may be effective in life-threatening PE.
Surgical thromboectomy or embolectomy is used when there is •
massive PTE. In bilateral ileofemoral DVT, embolism may be prevented
by inserting an ‘umbrella’ fi lter into the vena cava at a level below the
renal vessels.
Prevention (see b p. 72).
120

DEEP VENOUS THROMBOSIS AND PULMONARY EMBOLISM
Pulmonary embolism
Diagnosis of pulmonary embolism
PE is incorrectly diagnosed in almost 75% of patients. The differential diag-
nosis includes acute MI, aortic dissection, septic shock, chest infection,
haemothorax, and pneumothorax. Massive PE is PE resulting in haemo-
dynamic compromise or where >30% of the pulmonary vasculature is
compromised.
Outcome
The 30-day mortality of acute massive PE is about 50%.•
About 10% of mortality occurs within the fi rst hour.•
Up to 80% of mortality occurs within the fi rst 2h.•
The mortality of surgical intervention is up to 70% for patients •
requiring CPR or mechanical circulatory support preoperatively.
The operative mortality of stable patients is about 30%.•
Management
If the patient is haemodynamically unstable, emergency pulmonary
embolectomy should be considered. In patients with large PE and no con-
traindications (i.e. surgery within previous 30 days), thrombolysis is the
defi nitive management. Otherwise do the following:
Sit the patient up and give 100% O
•
2
.
Patient may require intubation.•
Get • IV access and give heparin 5000U bolus IV.
Start a heparin infusion (50 000U heparin in 50mL of normal saline) at •
1000–2000U/h.
Check APTT after 6h (target APTT 70–90), adjust infusion rate •
accordingly, and recheck APTT after 6h.
Once APTT is stabilized, APTT should be checked every 12–24h.•
Low molecular weight tinzaparin is licensed for use in acute PE. It has •
the same effi cacy as unfractionated heparin without the requirement
for repeated APTT checks.
Begin warfarinization.•
Look for causes of PE.•
Clinical features of PE
Symptoms• . Dyspnoea, pleuritic or dull chest pain.
Signs• . Tachypnoea, tachycardia, hypotension, elevated JVP.
Risk factors for or clinical evidence of DVT.•
ECG shows RV strain pattern (S1, Q3, T3), but this is neither a •
specifi c nor a sensitive test.
d• PaO
2
; PaCO
2
may be low.
CXR may show consolidation and effusion early on.•
Echo may show right ventricular (RV) dilatation, tricuspid •
regurgitation, and right atrial or RV thrombus.
Pulmonary angiography, CT-angio, and VQ scanning are diagnostic.•
121

CHAPTER 2 Principles of surgery
122
Risk scoring
Scoring systems attempt to quantify the severity of illness so that:
Different interventions, clinicians, or centres can be compared, •
adjusting for differences in case mix.
Clinicians can predict prognosis more accurately.•
APACHE III scoring system (Acute Physiology and Chronic
Health Evaluation)
This predicts an individual’s risk of dying in hospital. Twenty-seven patient
variables (physiological variables such as core temperature, heart rate, BP,
creatinine, age, and chronic illness variables) are entered into a programme
that gives a score which can be compared against previous performance
to give a risk of dying in the hospital. There are approximately another 60
hospitals worldwide where the APACHE III methodology is used to gener-
ate reports that compare their actual average ICU outcomes with those
predicted by the APACHE III methodology.
EuroSCORE (European System for Cardiac Risk Evaluation)
This is a weighted additive score, based on a European sample of cardiac
surgical patients. Variables such as age, renal function, and comorbidity
are given points that add up to an approximate percentage of predicted
perioperative mortality. Scoring systems like this are useful when consent-
ing patients for surgery and in risk-stratifying operative outcomes so that
surgeons and hospitals can be compared with each other.
POSSUM (Physiologic and Operative Severity Score for the
enUmeration of Mortality and morbidity)
This is a weighted additive score. Eighteen variables are combined to pro-
duce a physiological score and an operative score, which in turn are com-
bined to produce an estimate of the percentage risk of defi ned morbidity
and mortality. (Variants exist for vascular, orthopaedic, and colorectal
surgery.)
Examples of risk scoring systems
Predicting risk of dying in hospital (APACHE III).•
Quantifying morbidity (ASA score, Apgar score).•
Quantifying symptoms (NYHA angina classifi cation).•
Predicting operative mortality (EUROscore, Parsonnet score in •
cardiac surgery, POSSUM).
Predicting risk of dying on waiting list (New Zealand score in cardiac •
surgery).
Predicting risk of dying for specifi c illnesses (Glasgow and Ranson •
criteria in pancreatitis).
This page intentionally left blank

CHAPTER 2 Principles of surgery
124
Critical care
Recognizing the critically ill surgical patient (see Box 2.8)
It may be obvious that a patient needs a critical care bed, e.g. the patient
needing ventilation, inotropes, or dialysis. But anticipating, and maybe
avoiding, this is more diffi cult. The fi rst step is recognizing compensated
critical illness (e.g. shock compensated by tachycardia and peripheral shut-
down or respiratory failure compensated by unsustainable respiratory
effort).
Immediate management
First identify and treat potentially life-threatening conditions. Then quantify
the problem (important for referring patients to other clinicians and for
establishing a baseline by which to guide treatment and monitor progress).
Finally start looking for the underlying problem. Some of these tasks over-
lap. Keep reassessing the patient and adjust your management.
Quickly assess airway, breathing, and circulation: ALS algorithms are •
printed on the inside back cover; management of shock is described on
b p. 100; management of haemorrhage is described on b p. 102.
Sit patient up and give high fl ow O•
2
.
Secure • IV access and take blood for FBC, U&E, amylase, glucose, LFTs,
cardiac enzymes, clotting, group and save, and blood cultures.
Take ABG: good O•
2
saturations do not rule out respiratory failure and
ABGs will also show acidosis and electrolyte abnormalities.
Give 500mL gelofusine if patient not obviously fl uid overloaded.•
Request a 12-lead ECG.•
Review drug, diabetic, and fl uid balance charts.•
Perform a focused history and examination: ask about symptoms that •
have changed recently and focus your examination on that.
Review recent bloods and X-rays and request appropriate radiology.•
If the patient needs HDU or ITU, talk to your registrar or consultant.•
A patient may need to be discharged from HDU or surgery need to be •
postponed: think ahead.
Box 2.8 Signs that should ring alarm bells
History• . ‘I feel like I’m going to die.’ Timor mortis (fear of dying) may
accompany MI, hypovolaemic shock, respiratory failure. Never ignore
the patient who thinks they are dying; they are often right.
Nurses• . ‘Mr Smith just doesn’t look right.’ Experienced nurses
quickly recognize the patterns of critical illness; listen to them.
General• . Hypothermia or hyperpyrexia, sweating.
Cardiovascular• . d BP, id pulse, arrhythmias, peripheral shutdown.
Respiratory• . Tachypnoea, diffi culty getting full sentences out.
Renal• . Oliguria <0.5mL/kg/h.
Gastrointestinal• . New anorexia, nausea, and vomiting.
Neurological• . Confusion, agitation or drowsiness, fi ts.

CRITICAL CARE
125
High dependency unit
The HDU allows a level of care between ICU and the general ward.
Invasive monitoring and inotropic support are routine, but ventilation and
renal support are not. Nurse:patient ratio is 1:2. Patients with single organ
failure requiring basic respiratory support, including non-invasive mask
ventilation with CPAP, should be admitted to HDU.
Guidelines for admission to HDU
Need for monitored bed.•
Need for invasive monitoring.•
Need for inotropes.•
Need for CPAP or other respiratory support.•
Need for 1:2 nursing.•
Intensive care unit
The ICU offers advanced ventilatory and inotropic support, renal replace-
ment therapy, full invasive monitoring, and 1:1 nursing care.
How to use critical care
The surgical team should consider using critical care services for both
elective and emergency surgical patients. Some guides follow:
Is the elective patient in need of intensive infusional treatment prior to
•
surgery (e.g. IV anticoagulation, IV clotting factors)? l HDU.
Has the patient undergone major surgery with signifi cant transfusion •
requirements that might lead to haemodynamic and clotting
abnormalities (e.g. elective extensive pelvic surgery, aortic surgery,
extensive burns surgery)? l HDU.
Is the patient over 80, having had major abdominal, thoracic, or limb •
surgery? l HDU.
Does the patient have known signifi cant respiratory disease making •
intensive respiratory therapy likely? l ITU.
Would a patient due for emergency surgery benefi t from aggressive, •
closely monitored fl uid resuscitation prior to anaesthesia? l HDU.
Does the post-op patient need infusional inotropic support, renal •
replacement therapy, or invasive monitoring? l ITU/HDU.
2 The golden rule is, ‘If in doubt, ask for the advice of the critical care
team—more patients can benefi t than do.’
Guidelines for admission to ITU
Need for mechanical ventilation.•
Failure of two or more organ systems.•
Need for advanced monitoring, e.g. pulmonary artery catheter.•
Need for escalating or additional inotropes.•
Primary pathology should be reversible.•
Consultant involvement from both surgery and ITU is essential.•
Patient’s stated or written preference against intensive care should •
be taken into account and documented.

CHAPTER 2 Principles of surgery
126
Commonly used terms in ITU
Cardiac function (see b p. 620—cardiothoracic surgery).
Oxygenation and ventilation
Oxygenation• , the amount of oxygen in arterial blood, is described in
terms of the partial pressure of oxygen in arterial blood (PaO
2
) and the
percentage saturation of arterial haemoglobin with oxygen (SaO
2
).
Ventilation• , the movement of air in and out of the lungs, is described
in terms of minute volume, and assessed by measuring the partial
pressure of carbon dioxide in arterial blood (PaCO
2
).
Oxygenation is independent of minute volumes until they are very low.•
In post-operative patients, the primary cause of hypoxia is atelectasis •
and this must be reversed before the patient can benefi t from
increasing the fraction of oxygen in inspired air (FiO
2
).
Positive end expiratory pressure• (PEEP) and CPAP treat and prevent
atelectasis.
Oxygen consumption is assessed indirectly by measuring the •
percentage saturation of mixed venous haemoglobin with oxygen (SvO
2
;
indirect measurement of oxygen uptake by peripheral tissues).
Pulmonary ventilation is described in terms of four ‘volumes’ (• tidal
volume, inspiratory reserve volume, expiratory reserve volume, and
residual volume) that may be combined to give four ‘capacities’
(inspiratory capacity, functional residual capacity, vital capacity, and
total lung capacity). Tidal volume is the only measurement used on
ITU.
Loss of functional residual capacity through atelectasis, supine position, •
lobar consolidation and collapse, effusions, and obesity results in
hypoxia. CPAP, PEEP, and physiotherapy are aimed at limiting this loss.
This page intentionally left blank

CHAPTER 2 Principles of surgery
128
Invasive monitoring
Invasive monitoring is used on ITU and HDU because it provides accurate
and sensitive real-time measurements, routes for sampling blood, routes for
administration of drugs.
Arterial monitoring
Insertion technique and complications are described on b p. 196.
Indications
Precise measurement where inotropic or mechanical support used.•
Frequent sampling of ABGs.•
Contraindications
Absolute.
• Infection at the site of insertion, distal limb ischaemia.
Relative.• Coagulopathy, proximal obstruction, surgical considerations.
Central venous pressure lines
Insertion technique and complications are described on b p. 198.
Indications
Continual RA pressure (RAP) measurements in patients requiring •
circulatory support.
Infusion port for some drugs that cannot be given peripherally.•
Insertion pulmonary artery catheter or transvenous pacing wires.•
Infusion port for TPN.•
Contraindications
Absolute.
• SVC syndrome, infection at the site of insertion.
Relative.• Coagulopathy, undrained contralateral pneumothorax,
uncooperative patient. DVT of the head and neck vessels may make
insertion diffi cult. Patients with septal defects are at risk of CVA from
air emboli caused by poor technique.
Pulmonary artery catheter
Complications are as for CVP lines, additionally arrhythmias and pulmo-
nary artery infarction or perforation. The catheter has four lumens: a
proximal lumen 25cm from the tip that sits in the RA; a distal lumen con-
nected to a pressure transducer sitting in the pulmonary artery; a balloon
lumen allowing balloon infl ation; a thermistor lumen.
Indications
These are indicated in patients with hypoperfusion states refractory to
fi rst- and second-line inotropic support.
Pressure monitoring: RAP, RV pressure (RVP), PAP, pulmonary artery
•
wedge pressure (PAWP).
Flow monitoring: cardiac output.•
Mixed venous oxygen saturations.•
Derived parameters: systemic vascular resistance (SVR), SVR index (SVRI), •
pulmonary vascular resistance (PVR), PVR index (PVRI), LVSV, VO
2
, DO
2
.
Temporary atrial and ventricular pacing.•
Contraindications As for CV catheters (see b p. 198). Those specifi c to
pulmonary artery catheterization include tricuspid or pulmonic valvular
stenosis, RA and RV masses that may embolize, tetralogy of Fallot, severe
arrhythmias, coagulopathy.
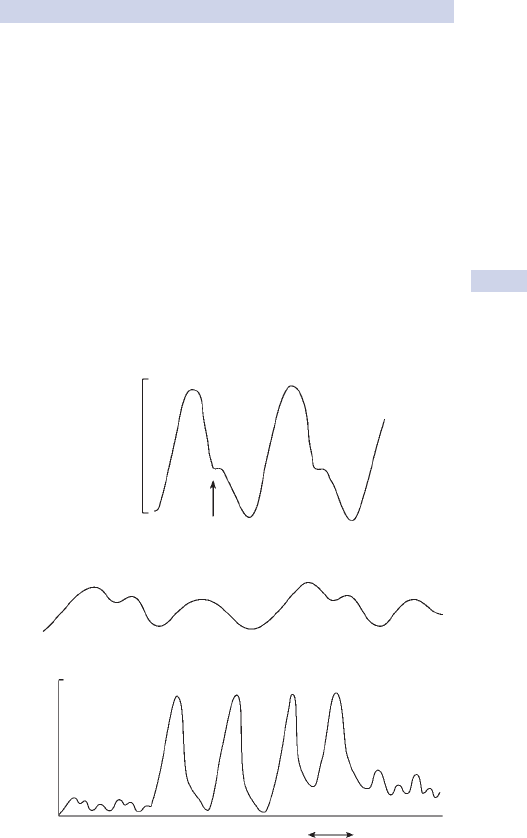
INVASIVE MONITORING
129
Waveforms See Fig. 2.8.
The arterial waveform
The arterial waveform has a fast upstroke and slower downstroke with a
notch that represents aortic valve closure.
The CVP waveform
The waveform is composed of three upstrokes (the ‘a’, ‘c’, and ‘v’ waves)
and two descents (the ‘x’ and ‘y’ descent). The ‘a’ wave—atrial systole;
‘v’ wave—venous return fi lling the RA; ‘c’ wave—bulging of the closed
tricuspid valve cusps into the RA. The ‘x’ descent occurs in atrial diastole.
The ‘y’ descent occurs in ventricular diastole.
The pulmonary artery pressure waveform
Waveform progression during correct insertion of the pulmonary artery
catheter shows a sudden increase in systolic pressure as the catheter
enters the RV. As the catheter enters the pulmonary artery, the diastolic
pressure increases. There is a decrease in mean pressure as the catheter
enters the wedge position.
120
60
Dicrotic notch
Pressure (mmHg)
a
c
x
v
y
40
(c)
(b)
(a)
0
RA RV PA PAWP
Ballon
inflated
Ballon
deflated
Fig. 2.8 (a) Arterial waveform. (b) Central venous pressure waveform and
responses to fl uid boluses. (c) Pulmonary artery pressure waveform.

CHAPTER 2 Principles of surgery
130
Ventilation and respiratory support
Invasive methods
Intermittent positive pressure ventilation (IPPV); controlled mechanical
ventilation (CMV); pressure control ventilation (PCV)
IPPV or CMV is the mode commonly used during routine surgery. Raising
airway pressure forces air into the lungs via an endotracheal or tracheos-
tomy tube. Expiration occurs when airway pressures are allowed to fall to
zero. This mode is poorly tolerated by the awake patient.
Positive end-expiratory pressure (PEEP)
If, instead of allowing airway pressures to fall to zero, a small positive air-
ways pressure is maintained throughout expiration (positive end-expiratory
pressure or PEEP), the collapse of small airways and alveoli that occurs at
the end of expiration is prevented.
Functional residual capacity, intrapulmonary shunts, lung compliance,
•
and PaO
2
are improved and the work of breathing is reduced.
High levels of PEEP (>15cmH•
2
O) may be necessary to reverse
established atelectasis, but increase intrathoracic pressure, reducing
venous return and cardiac output, which may reduce PaO
2
.
Barotrauma is a complication of high PEEP. PEEP as low as 5cmH•
2
O
may cause haemodynamic compromise in the patient with poor LV.
Physiological PEEP is provided by an intact glottis: patients with COPD •
purse their lips during expiration to increase physiological PEEP.
Synchronized intermittent mandatory ventilation (SIMV)
One variation of IPPV is SIMV, where positive airway pressure may be
synchronized with patient-initiated breaths. Mandatory (machine-initi-
ated) breaths are given if no spontaneous breaths occur in a preset time.
Machine-initiated breaths do not occur at the same time as patient-initi-
ated breaths. This mode is used in patients that are awake, but any patient
may safely be ventilated on SIMV.
Mandatory minute ventilation (MMV)
Another variation of IPPV is MMV where the ventilator initiates breaths
only if patient-initiated ventilation falls below a preset minute volume.
Pressure support (PS) and assisted spontaneous breathing (ASB)
Patient-initiated breaths can be supported with a preset positive airway
pressure of 6–20cmH
2
O. The ventilator detects the drop in airway pres-
sures as the patient begins inspiration and assists air infl ow with a positive
airway pressure. This is lower than the preset infl ationary pressures in
IPPV as the patient is making some inspiratory effort.
At high levels of pressure support (>20cmH
2
O), the patient, although
controlling the timing and frequency of respiration, is on IPPV.
Continuous positive airway pressure (CPAP)
In CPAP, a standing airway pressure, continuous throughout all phases of
respiration, is applied via the endotracheal tube. It is more commonly used
in the extubated patient via a tight fi tting facemask (see b p. 131).

VENTILATION AND RESPIRATORY SUPPORT
131
Non-invasive methods
Intermittent negative pressure ventilation (INPV) Virtually never used
today, INPV involves placing the patient inside a tank ventilator sealed at
the neck. Tank pressure is intermittently lowered, expanding the chest and
lowering intrapleural pressure.
Non-invasive intermittent positive pressure ventilation (NIPPV) This is
IPPV delivered by face or, more commonly, nasal mask. The patient must
be cooperative in order to understand how to synchronize breaths with
the ventilator. It may be used in the tiring COPD patient.
Continuous positive airway pressure (CPAP) In CPAP, a standing airway
pressure, continuous throughout all phases of respiration, is applied via an
entotracheal tube, tracheostomy, nasal, or, more commonly, face mask to
a spontaneously breathing patient.
This results in a kind of non-invasive PEEP, where additional alveoli are •
recruited with the benefi ts described (see b p. 130).
CPAP is a useful adjunct in the extubated patient with COPD, •
atelectasis, pulmonary oedema, or acute respiratory distress syndrome
(ARDS).
CPAP cannot produce ventilation by itself.•
Biphasic positive pressure ventilation (BiPAP) is a solution to the problem
of air trapping that can occur in patients, particularly those with COPD,
on CPAP. Airway pressure is cycled at preset rates between high and
low levels.
High fl ow (Venturi) face mask (fi xed performance) The key part of the
Venturi facemask is the Venturi valve which draws in an amount of air
through calibrated inlets, which is mixed with O
2
fl owing into the valve
before entering the mask.
More mixed air (up to 30L/min) is delivered to the mask than the •
patient can use: the excess escapes though holes in the mask.
The FiO
•
2
is set by the choice of valve, not by the patient’s breathing
pattern (hence the term fi xed performance).
The maximum FiO•
2
that can be delivered by a Venturi mask is about
60%. There is a minimum fl ow rate of O
2
for each Venturi.
Low fl ow (Hudson) face mask (variable performance) O
2
fl ows at a set
rate (e.g. 2L/min) into the mask. It is diluted by air drawn into the mask,
which depends on the patient’s minute volumes, ranging 5–30L/min.
The FiO•
2
achieved depends primarily on the patient and the delivery
system should not be used when accurate control of FiO
2
is required.
The maximum FiO•
2
that can reliably be delivered is about 30%.
Use of a non-rebreathing mask and reservoir bag, into which high fl ow •
O
2
is drawn during expiration and then inhaled, increases FiO
2
to up to
60%: the reservoir bag must be fi lled with O
2
before the patient uses it.
Nasal prongs Nasal prongs deliver an FiO
2
that is determined primarily
by the patient, as in a low fl ow mask, but they are less obtrusive, allowing
the patient to expectorate and eat. They increase tracheal FiO
2
to barely
more than room air levels, particularly if the patient breathes through
their mouth.
CHAPTER 2 Principles of surgery
132
Circulatory support
Principles
Improving cardiac function and end-organ perfusion involves:
Careful fl uid balance to optimize preload or ‘fi lling’ (see • b p. 64);
Using vasoconstrictors and vasodilators to optimize afterload;•
Using inotropes and chronotropes to improve cardiac output (CO);•
Mechanical support in selected cases.•
Inotropes
Adrenaline (epinephrine) 0.03–0.5micrograms/kg/min or bolus
Catecholamine produced by the adrenal medulla.
Action.• Direct agonist at α, β
1
, and β
2
receptors.
Pharmacodynamics.• Instant onset, half-life 2min. Metabolized by MAO.
Indications.• Cardiac arrest (asystole, ventricular fi brillation (VF),
electromechanical delay (EMD)); anaphylaxis; low CO states;
bronchospasm.
Dopamine 3–10micrograms/kg/min
A catecholamine precursor to noradrenaline (NA) and adrenaline.
Action.
• α, β
1
, β
2
, and DA
1
agonist, and release of stored neuronal
NA. At the lower doses, B and DA effects (increased heart rate
(HR), contractility) predominate. At >10micrograms/kg/min, A effects
predominate (i SVR, dysrhythmias).
Pharmacodynamics.
• Fast onset, slow offset. Metabolized by monoamine
oxidase (MAO).
Indications.• Low CO states; renal insuffi ciency.
Milrinone 0.3–0.8micrograms/kg/min
Bipyridine derivative that inhibits phosphodiesterase.
Action.
• Potent inotropic and vasodilator effects.
Pharmacodynamics.• Half-life is 4h. Metabolism is hepatic.
Indication.• Low CO state in the setting of i SVR.
Vasopressors
Inotropes increase inotropy (contractility)
A• -adrenergic receptor stimulation leads to i SVR and PVR.
B•
1
stimulation leads to i contractility, heart rate, and conduction.
B•
2
stimulation causes peripheral dilatation and bronchodilation.
Dopamine (DA)• stimulation causes coronary, renal, and mesenteric
vasodilatation.
Vasopressors cause vasoconstriction
A•
2
-adrenergic stimulation increases SVR and PVR.
A
•
1
-adrenergic stimulation i contractility without i HR.

CIRCULATORY SUPPORT
133
Noradrenaline (norepinephrine) 0.03–1.0micrograms/kg/min
Catecholamine produced by the adrenal medulla.
Action.• Direct α agonist: potent vasoconstriction + β
1
effect = i BP.
Pharmacodynamics.• Immediate onset; half-life 2min.
Indication.• Hypotension because of low SVR in good to high CO states.
Chronotropes
Atropine (0.3–1mg IV bolus)
Atropine is a belladonna alkaloid.
Action.• A competitive antagonist at muscarinic cholinergic receptors,
reducing parasympathetic tone to ‘reveal’ underlying sympathetic tone.
Pharmacodynamics.• Almost instant onset. When given IV, offset is
15–30min. When given IM, SC, or PO, 4h. Renal elimination.
Indication.• Bradydysrhythmias, reduction of oral secretions.
Isoprenaline (0.01–0.3micrograms/kg/min)
Synthetic catecholamine.
Action.• Direct β
1
(i contractility, conductivity, and HR) and β
2
i
(vasodilatation and bronchodilatation) effect. No α activity.
Pharmacodynamics.• Plasma half-life 2min. Hepatic metabolism by MAO,
40% conjugated, 60% excreted unchanged.
Indication.• Bradycardia unresponsive to atropine.
Mechanical support
Intra-aortic balloon counterpulsation
Mechanism
The intra-aortic balloon pump (IABP) is a polyethylene balloon fi lled with
helium, ranging from 2 to 50cm
3
in size, that sits in the descending aorta
just distal to the left subclavian artery. The balloon infl ates in early dias-
tole, improving coronary perfusion, and defl ates just before systole, reduc-
ing afterload. The myocardial oxygen supply:demand ratio is improved and
CO may be increased by up to 40%.
Indications
Weaning from cardiopulmonary bypass, refractory ischaemia, cardiogenic
shock.
Contraindications
Aortic regurgitation (AR) (balloon infl ation during diastole will worsen
•
AR).
Aortic dissection.•
Ventricular assist devices (VADs) These are pumps that are anastomosed
to the great vessels or cardiac chambers to support failing right, left, or
both ventricles.
Chronotropes increase heart rate
B•
1
stimulation leads to i contractility, HR, and conduction.
Muscarinic cholinergic• activity is predominantly parasympathetic.

CHAPTER 2 Principles of surgery
134
Renal support
The main aims of treatment of renal failure are:
Maintain renal perfusion.•
Optimize fl uid balance.•
Provide adequate enteral support.•
Correct hyperkalaemia and acidosis.•
Identify and treat sepsis.•
Target therapy to underlying pathology.•
Provide renal replacement therapy.•
Haemodialysis and haemofi ltration
There are two main techniques of renal replacement therapy used on car-
diac ICUs: haemodialysis and haemofi ltration. In both techniques, access
to the circulation is required and blood passes through an extracorporeal
circuit that includes either a dialyser or a haemofi lter.
In haemodialysis, blood fl ows along one side of a • semipermeable
membrane as a solution of crystalloids is pumped against the other side
of the membrane, against the direction of blood fl ow.
In haemofi ltration, • blood under pressure passes down one side of a
highly permeable membrane on the other side of which is a static
crystalloid solution.
In haemodialysis, removal of solutes depends on diffusion: molecules •
move from high to low concentration and smaller molecules move
faster so the amount of solute removed depends on its concentration
in the dialysis fl uid and on the size of the molecule.
In haemofi ltration, removal of solutes with a molecular weight up •
to 20 000 depends on convective fl ow as in glomerular fi ltration:
all molecules are removed at a similar rate and virtually all ions are
removed.
In haemodialysis, large molecules are not effi ciently removed.•
In haemofi ltration, large molecules are so effectively removed that •
drugs such as heparin, insulin, and vancomycin may need to be
replaced and reduction in circulating infl ammation mediators and
pyrogens leads to a reduction in pyrexia and systemic infl ammation.
In haemodialysis, controlled amounts of sodium and water are •
removed by creation of a transmembrane pressure gradient.
In haemofi ltration, large amounts of salt and water are removed
•
and must be replaced by an infusion of an appropriate amount of
physiologic crystalloid into the distal port of the haemofi lter.
Indications for renal replacement therapy
Refractory hyperkalemia (see • b p. 111).
Refractory acidosis.•
Pulmonary oedema.•
Drug toxicity.•
Progressive uraemia or uraemia associated with pericarditis, •
encephalopathy, seizures, or coagulopathy.

RENAL SUPPORT
135
Types of haemofi ltration
Haemofi ltration has become an increasingly popular form of renal replace-
ment therapy for acute renal failure post-operatively, even though it is
several times more expensive than dialysis. This is because the continuous
nature of the process avoids the big swings in fl uid balance and electro-
lytes that characterize dialysis. There are several variants.
Continuous arteriovenous haemofi ltration (CAVH) This is the original and
simplest form of fi ltration. The femoral artery and vein are cannulated and
blood passes through the haemofi lter under arterial pressure alone. It is
therefore less appropriate for patients with low CO states. Prolonged
arterial cannulation carries complications (see b p. 197).
Continuous arteriovenous haemodialysis with fi ltration Because clearance
rates are as low as 10mL/h with CAVH, a dialysis circuit is added to the
equipment, improving clearance rates at considerable cost.
Continuous venovenous haemofi ltration (CVVH) An occlusive pump is
incorporated into the circuit to drive blood so that venous cannulation
is all that is required. This also allows control of blood fl ow and fi ltration
rate. Clearance of up to 100mL/min can be achieved. This is the most com-
monly used system in ICUs.
Continuous venovenous haemodialysis with fi ltration Some systems add
haemodialysis to the CVVH circuit for additional fi ne control.
Management of the haemofi ltered patient
A double lumen catheter (Vascath) is inserted into a central vein. The
blood pump is set at 125mL/min and the haemofi ltration rate to 25mL/
min. The replacement fl uid pump is programmed to balance the infl ow
and outfl ow of fl uid to achieve a preset rate of fl uid loss. The circuit
is heparinized with an infusion of 200–1600U/h. Lactate is the common-
est replacement anion, but in lactic acidosis, a lactate-free replacement
solution must be employed. Mg
2+
, Ca
2+
, PO
4
, and HCO
3
are replaced.
Hypotension is common when commencing haemofi ltration.

CHAPTER 2 Principles of surgery
136
Enteral support
(See also b p. 66.) Neglecting the patient’s nutritional needs increases
morbidity and mortality.
Routine management
Patients may be started on oral intake directly after most forms of •
surgery unless contraindicated. There is good evidence of reduced
pulmonary, septic, and anastomotic complications in patients fed early.
Patients with a history of dyspepsia or peptic ulceration should be •
commenced electively on an IV proton pump inhibitor (PPI).
Analgesia should be downscaled from IV and oral opiates to non-•
opiate analgesia such as paracetamol 1g qds PO/PR as soon as possible
to avoid ileus, constipation, anorexia, nausea, and vomiting.
Avoid NSAID analgesia in patients at risk of peptic ulceration.•
Give regular • IV antiemetics to treat persistent nausea and vomiting.
Enteral nutrition
This is nutrition using the GI tract. It is superior to, safer, and cheaper
than TPN.
Routes/methods for supplemented enteral nutrition
Sip feeds.
•
Fine bore NG tube (NGT) with use of infusion pump.•
Nasojejunal tube (needs radiographic imaging).•
Percutaneous endoscopic gastrostomy (• PEG) and jejunostomy.
Open surgical gastrostomy or jejunostomy.•
Enteral tube feeds come in sterile, ready to hang 500mL and 1000mL packs.
Standard feeds are nutritionally complete and provide 1kcal/mL. They are
based on whole protein. Fibre-enriched feeds also provide 1kcal/mL. They
contain soy polysaccharides that are soluble, but remain undigested pass-
ing to the colon where they are fermented by bacteria to produce short
chain fatty acids, promoting absorption of sodium and water and reducing
diarrhoea. They are also probiotic. High energy feeds provide 1.5kcal/mlL
and are used in patients requiring reduced fl uid input.
Renal failure patients require high energy, low volume, and electrolyte •
feeds. Nepro
®
provides 2kcal/mL and high protein content.
Drugs can be given by the NGT being used for enteral feeding. Liquid •
preparations designed for oral administration should ideally be used as
crushed tablets may block the tube. The feed should be stopped and
the NGT fl ushed with saline before and after administration of drugs.
The therapeutic effect of warfarin is reduced by the vitamin K content •
in feeds; it is frequently necessary to increase the dose of warfarin.
Examples of enteral feeds
Standard feeds—Osmolite•
®
, Nutrison
®
standard.
Fibre enriched—Jevity•
®
, Nutrison
®
Multi-Fibre.
High energy—Nutrison•
®
Energy, Ensure
®
Plus.

ENTERAL SUPPORT
137
Phenytoin interacts with enteral feeds, which should be stopped for 2h •
before and after phenytoin administration.
Indications for supplementary enteral feeding
Inadequate oral intake due to anorexia, practical diffi culties with
•
feeding, being on ITU/HDU, drug-induced nausea, poor oral health or
dentition.
Hypercatabolism exceeding normal intake (e.g. chronic major sepsis, •
malignancy, trauma, burns).
Gut intact, but absorption impaired excessive losses; chronic •
diarrhoea, high output stoma or fi stula (usually low residue or
elemental feeds to maximize absorption).
Complications of supplementary enteral feeding
Complications of feeding tube. • Malposition, blockage (wound infection
around jejunostomy tubes).
Complications of administration. • Pulmonary aspiration, regurgitations,
diarrhoea, bloating, nausea, cramps.
Complications of contents. • Vitamin and trace mineral defi ciencies,
electrolyte imbalances, drug interactions.
Diarrhoea is the most common complication. If feed-related, reducing the
osmotic load is effective (using half-strength feed or slowing the infusion).
Omeprazole, Lomotil
®
, and erythromycin may be tried. It is mandatory to
exclude other causes of diarrhoea such as subacute obstruction and infec-
tious causes (particularly Clostridium diffi cile).
Total and peripheral parenteral nutrition (TPN, PPN)
TPN consists of specially formulated feed given IV. Because TPN solutions
have high osmolality, they cause thrombophlebitis if infused into periph-
eral veins. They are given via central lines (subclavian or internal jugular)
which may be tunnelled for long-term use. PPN uses heparin, in-line fi ltra-
tion, buffering, fi ne bore cannulas, and reduced osmolality feeds to avoid
thrombophlebitis.
Indications
Failure of bowel to absorb food, e.g. radiation damage, severe acute •
enteritis, or malabsorption syndromes.
Failure of adequate length of bowel for absorption (short bowel •
syndrome due to Crohn’s disease or after massive intestinal resection).
GI tract not accessible for enteral route, e.g. acute severe pancreatitis, •
oesophagogastric surgery, or disease where tube feeding not possible.
Failure of enteral feeding to accomplish nutritional targets (rare).•
Complications
Complications of CV catheters (see
• b p. 199).
Late complications: sepsis, migration, erosion, DVT, occlusion.•
Metabolic complications: • id glucose, K
+
, Na
+
, i Ca
2+
, d PO
4
, d Zn,
Mg, folate.

CHAPTER 2 Principles of surgery
138
Sepsis, SIRS, MODS, and ALI
Systemic infl ammatory response syndrome (SIRS) is a pro-infl ammatory
state that does not include a documented source of infection. It may lead
to multiple organ dysfunction syndrome (MODS).
Pathophysiology
The pathophysiology involves systems involved in infl ammation, immunity,
ischaemia, and homeostasis, including complement, clotting and cytokine
cascades, cell-mediated immunity, and humoral immunity.
Metabolic acidosis is a frequent accompaniment to SIRS and it is
•
derived principally from lactate.
SIRS may affect all organ systems and may lead to MODS.•
Defi nitions
SIRS
Any two or more of the four following signs:
•
Tachycardia >90 beats/min.•
Tachypnoea >20 breaths/min.•
Pyrexia >38• °C (or hypothermia <36°C).
White blood count >12 • × 10
9
/L (or <4 × 10
9
/L).
Without • identifi able bacteraemia or need for organ support and in
the setting of a known cause of endothelial infl ammation such as:
Suspected infection.•
Pancreatitis.•
Ischaemia.•
Multiple trauma and tissue injury.•
Haemorrhagic shock.•
Immune-mediated organ injury.•
Sepsis
Systemic response to infection manifested by two or more of:
Tachycardia >90 beats/min.
•
Tachypnoea >20 breaths/min.•
Pyrexia >38• °C (or hypothermia <36°C).
White blood count >12 • × 10
9
/L (or <4 × 10
9
/L).
Multiple organ failure Presence of altered organ function in an acutely
ill patient such that homeostasis cannot be maintained without
intervention.
Acute lung injury (ALI)
Acute onset.•
PaO•
2
/FiO
2
<300.
Bilateral infi ltrates on CXR.•
PAWP <18mmHg or no clinical evidence of raised left atrial •
pressure.
Acute/adult respiratory distress syndrome (ARDS) As for ALI, except PaO
2
/
FiO
2
<200.

SEPSIS, SIRS, MODS, AND ALI
139
Cell signalling molecules involved include interleukins IL-1, IL-5, and •
IL-6; chemokines; tumour necrosis factor (TNF).
Theories explaining why SIRS develops include:•
Immunologically mediated infl ammation.•
i• intestinal permeability and colonization with Gram –ve anaerobes
that produce endotoxins that migrate across the mucosa to drive
the infl ammatory and immune response.
‘Second hit’ hypothesis.•
Acute lung injury (ALI)
Thirty per cent of patients with SIRS develop ALI. It is the pulmonary
manifestation of the global physiological insult (rarely occurs in isola-
tion). ARDS is one end of the continuum of ALI. Management of ALI
is supportive, including prone ventilation and aggressive diuresis or early
ultrafi ltration.
Investigations
These are aimed at excluding differential diagnoses such as overwhelming
sepsis, cardiogenic shock, hypovolaemic shock, and hypersensitivity reac-
tions as well as identifying an underlying cause.
U&E, LFTs, serum amylase, lactate and cardiac enzymes.•
FBC, APTT, PT, fi brinogen, D-dimers.•
Cultures of blood, urine, sputum, stool.•
ABGs.•
Pulmonary artery catheter measurements of cardiac function and •
mixed venous oxygen saturations.
Cytokine assays can be used as markers of severity.•
Management
Volume resuscitation:•
Aims to achieve normovolaemia: increases CO and oxygen delivery •
without large increase in oxygen consumption.
Hypervolaemia appropriate in selected cases.•
Albumin probably benefi cial in selected cases.•
Pulmonary artery catheter may guide management.•
Maintenance of oxygen delivery:•
Airway protection and ventilatory support usually necessary.•
Inotropic support of cardiac function has important role.•
Vasoactive drugs selected so that BP is not maintained at the •
expense of splanchnic vasoconstriction and hypoperfusion.
Organ support:•
In addition to cardiovascular and respiratory support, these patients •
frequently need renal, hepatic, and enteral support.
Attempts to reduce translocation, e.g. introduction of competing •
bacteria (e.g. lactobacillus of yoghurt), early enteral nutrition,
particularly with the use of immunostimulating enteral diets.
Prognosis Mortality ranges from 25% to100%, depending on the number
and type of organ failures involved, age, and pre-existing comorbidity.
This page intentionally left blank

141
Surgical pathology
Cellular injury 142
Infl ammation 144
Wound healing 146
Ulcers 148
Cysts, sinuses, and fi stulas 150
Atherosclerosis 152
Thromboembolic disease 154
Gangrene and capillary ischaemia 158
Tumours 160
Carcinogenesis 162
Screening 164
Grading and staging 168
Tumour markers 170
Surgical microbiology 172
Surgically important organisms 174
Soft tissue infections 176
Blood-borne viruses and surgery 178
Bleeding and coagulation 180
Anaemia and polycythaemia 182
Chapter 3

CHAPTER 3 Surgical pathology
142
Cellular injury
Causative agents
Cellular injury is caused by:
• Trauma.
• Thermal injury.
• Chemicals, including drugs.
• Infectious organisms.
• Ionizing radiation.
Mechanisms of injury
These causative agents cause cell damage via a number of mechanisms.
• Mechanical disruption. Trauma, freezing, osmotic imbalance.
• Failure of membrane integrity. Failure of ion pumps, cytolysis, trauma.
• Blockage of metabolic pathways. Cellular respiration (e.g. cyanide),
protein synthesis (e.g. streptomycin), DNA damage or loss (e.g.
X-rays).
• Defi ciency of essential metabolites. Oxygen (ischaemia), glucose
(diabetic ketoacidosis), hormones (d trophic hormones results in
apoptosis).
• Free radicals. Toxins (e.g. carbon tetrachloride), ischaemia-reperfusion
injury, intracellular killing of bacteria.
Necrosis
Coagulative necrosis
This is the most common form of necrosis and occurs in all organs.
Cells retain their shape as cell proteins coagulate and metabolic activ-
ity stops. Digestion by macrophages may cause the tissue to become
soft. Histologically, there is progressive loss of staining. The presence of
necrotic material normally provokes an infl ammatory response.
Colliquative necrosis
This occurs in the brain because of the lack of tissue architecture provided
by substantial surrounding stroma.
Caseous necrosis
Dead tissue lacks any structure and is characterized by a white, soft, or
liquid ‘cheesy’ appearance. This is common in TB.
Gangrene
Necrosis with dessication or putrefaction (see b p. 158).
Fibrinoid necrosis
In malignant hypertension, necrosis of smooth muscle vessel walls allows
seepage of plasma into the media and deposition of fi brin.
Fat necrosis
• Direct trauma. Release of extracellular fat produces an infl ammatory
response, fi brosis and eventually, in some cases, a palpable mass.
• Acute pancreatitis. Fat is digested by pancreatic lipase to produce fatty
acids which precipitate with calcium in the process of saponifi cation.
Necrosis is death of tissue or cells.

CELLULAR INJURY
143
Apoptosis
Apoptosis is a physiological process requiring energy. It is the normal
means of maintaining the size of an organ in the face of continuing cell
turnover or a reduction in size during atrophy. It is mediated by endog-
enous endonucleases. The cell shrinks and fragments into apoptotic bod-
ies. Examples include:
• Physiological. Epithelium of GI tract, bone marrow, clonal selection in
immune system, targets of cytotoxic T cells.
• Pathological. After exposure to ionizing radiation, chemotherapy,
smooth muscle cells around atherosclerotic plaque, viral hepatitis.
Apoptosis (programmed cell death) is the cell-mediated, controlled
elimination of individual cells.

CHAPTER 3 Surgical pathology
144
Infl ammation
Acute infl ammation
This is the initial tissue reaction to a wide range of agents: accumulation of
neutrophil polymorphs in the extracellular space is diagnostic. It lasts hours
to days and is usually described with the suffi x ‘-itis’.
Causes
• Physical and chemical, e.g. mechanical trauma, X-rays, acid, alkali.
• Infection: bacteria, viruses, parasites, fungi, or protozoa.
• Ischaemia.
• Hypersensitivity.
Macroscopic appearance
Calor, rubor, tumor, dolor, and functio laesa (heat, redness, swelling, pain,
and impaired function). Special macroscopic appearances include:
• Serous. Infl ammation + abundant fl uid-rich exudates, e.g. peritonitis.
• Catarrhal. Infl ammation + mucus hypersecretion, e.g. common cold.
• Haemorrhagic. Infl ammation + vascular injury, e.g. pancreatitis.
• Suppurative. Infl ammation + pus produced to form abscess or
empyema.
• Fibrinous. Exudates contain fi brin which forms coating, e.g. pericarditis.
• Membranous. Coating of fi brin and epithelial cells, e.g. laryngitis.
• Pseudomembranous. Superfi cial mucosal ulceration with slough, e.g.
pseudomembranous colitis secondary to C. diffi cile (see b p. 396).
• Necrotizing (gangrenous). Infl ammation + tissue necrosis (see b
p. 158).
Microscopic changes
Mediated by endogenous chemicals released by cells (histamine, prostaglan-
dins, leukotrienes, serotonin, and lymphokines) and plasma factors (com-
plement, kinin, coagulation, and fi brinolytic cascades). Changes are:
• Changes in vessel calibre and fl ow.
Immediate and transient smooth muscle vasoconstriction.•
Vasodilation (active hyperaemia) lasting 15min to hours.•
Capillaries, then arterioles dilate to increase blood fl ow.•
• Increased vascular permeability and fl uid exudates.
Capillary hydrostatic pressure is increased.•
Endothelial cells contract, creating gaps.•
Plasma proteins escape into extracellular space.•
Increase in colloid osmotic pressure draws more fl uid.•
• Formation of cellular exudates.
Accumulation of neutrophil polymorphs in extracellular space.•
Begins with margination of neutrophils (fl ow next to vessel walls).•
Neutrophils then adhere to vessel walls: mechanism unknown.•
Migrate by amoeboid movement through gaps between cells.•
Infl ammation is the local physiological response to tissue injury. It can
be acute or chronic.
INFLAMMATION
145
Neutrophil polymorphs phagocytose debris and kill microbes •
intracellularly using oxygen-dependent (hydrogen peroxide and
hydroxyl radicals) and -independent (lysosymes) means.
Sequelae of acute infl ammation
• Resolution. Restoration of tissue to normal; likely if minimal tissue
damage, rapid destruction of causal agent, rapid removal of exudates
by good vascular drainage, and organ with restorative capacity, e.g.
liver.
• Suppuration. Formation of pus (see b p. 144).
• Organization. Replacement by granulation tissue.
• Chronic infl ammation.
Chronic infl ammation
This is an infl ammation where lymphocytes, plasma cells, and macrophages
predominate, granulation tissue often accompanies.
Causes
• Resistance of infective agent to phagocytosis (TB, viral infections).
• Foreign body (endogenous, e.g. urate or exogenous, e.g. asbestos).
• Autoimmune (e.g. contact hypersensitivity, RA, organ-specifi c).
• Primary granulomatous disease (e.g. Crohn’s, sarcoidosis).
• Unkown aetiology (e.g. ulcerative colitis).
Macroscopic appearances
The commonest appearances are:
• Chronic ulcer, e.g. peptic ulcer;
• Chronic abscess cavity, e.g. empyema;
• Thickening of wall of hollow viscus, e.g. Crohn’s disease;
• Granulomatous infl ammation, e.g. TB;
• Fibrosis, e.g. chronic cholecystitis.
Microscopic changes
Lymphocytes, plasma cells, and macrophages (see b p. 145) predominate;
neutrophil polymorphs are scarce, eosinophil polymorphs are present.
Fluid exudate is not prominent.
Granuloma
This is different to granulation tissue (see b p. 147).
Causes
• Specifi c infections.
Endogenous. necrotic bone or fat, keratin, urate.•
Exogenous. Talc, silicone, asbestos, sutures.•
• Drugs. Sulphonamides, allopurinol.
• Unknown. Crohn’s, sarcoidosis, Wegener’s granulomatosis.
A granuloma is an aggregate of epithelioid histiocytes.

CHAPTER 3 Surgical pathology
146
Wound healing
General principles of healing
Tissue healing in any organ follows some basic principles:
• Cells may be labile (good capacity to regenerate, e.g. surface epithelial
cells), stable (capacity to regenerate slowly, e.g. hepatocytes), or
permanent (no capacity to regenerate, e.g. nerve and striated muscle
cells).
• Tissue architecture is important: complex arrangements cannot be
reconstructed if destroyed, e.g. renal glomeruli.
• Complete restitution occurs when part of a labile population of cells is
damaged, e.g. a minor skin abrasion.
Classifi cation of wounds
• Clean. Non-traumatic wounds with no break in surgical technique,
no septic focus, and no viscus opened (e.g. hernia repair).
• Clean contaminated. Non-traumatic wounds with contaminated
entry into a viscus, but with minimal spillage (e.g. elective
cholecystectomy).
• Contaminated. Clean, traumatic wounds or signifi cant spillage from
a viscus or acute infl ammation (e.g. emergency appendectomy).
• Dirty. Includes traumatic wounds from a dirty source or when
signifi cant bacterial contamination or release of pus is encountered.
Key revision points—the four stages of wound healing
When specialized tissue is destroyed, it cannot be replaced and a stere-
otyped response called repair then follows in four stages:
• Haemostasis (immediate). In response to exposed collagen, platelets
aggregate at the wound and degranulate, releasing infl ammatory
mediators. Clotting and complement cascades activated. Thrombus
formation and reactive vasospasm achieve haemostasis.
• Infl ammation (0–3 days). Vasodilation and increased capillary
permeability allow infl ammatory cells to enter wound and cause
swelling. Neutrophils amplify infl ammatory response by release of
cytokines, reduce infection by bacterial killing, and debride damaged
tissue. Macrophages follow and secrete cytokines, growth factors,
and collagenases. They phagocytose bacteria and dead tissue
and orchestrate fi broblast migration, proliferation, and collagen
production.
• Proliferation (3 days–3 weeks). Fibroblasts migrate into the wound
and synthesize collagen. Specialized myofi broblasts containing actin
cause wound contraction. Angiogenesis is stimulated by hypoxia and
cytokines and granulation tissue forms.
• Remodelling (3 weeks–1y). Reorientation and maturation of collagen
fi bres increases wound strength.

WOUND HEALING
147
• Granulation tissue is the combination of capillary loops and
myofi broblasts. This is unrelated to a granuloma (see b p. 145).
• Organization is the process where specialized tissues are repaired by
formation of mature connective tissue, e.g. pneumonia or infarcts.
• Wound contraction mediated by myofi broblasts; can reduce the tissue
defect by up to 80%, but can lead to problems, e.g. burns contractures.
• Collagen is secreted at the same time to form a scar.
Factors affecting wound healing
• Impaired arterial supply or venous drainage (global or local).
• Excessive movement, local distension, or distal obstruction.
• Infection, malignancy, foreign body, necrotic tissue, smoking.
• Malnutrition. Obesity, recent weight loss, nutrient defi ciency.
• Immunosuppressive. Cancer, steroids, immunosuppressants, HIV.
• Anticancer therapies. Radiotherapy and chemotherapy.
• Metabolic. Diabetes, jaundice, uraemia, musculoskeletal diseases, age.
Wound healing in specifi c tissues
Skin: fi rst intention healing
This takes place where there is close apposition of clean wound edges.
• Thrombosis in cut blood vessels prevents haematoma formation.
• Coagulated blood forms a surface scab which keeps the wound clean.
• Fibrin precipitates to form a weak framework between the two edges.
• Capillaries proliferate to bridge the gap.
• Fibroblasts secrete collagen into the fi brin network.
• Basal epidermal cells bridge the gap and are eventually resorbed.
• The elastic network in the dermis cannot be replaced.
Skin: second intention healing
This takes place in wounds where skin edges cannot be cleanly apposed.
• There is phagocytosis to remove debris.
• Granulation tissue to fi ll in defects.
• Epithelial regeneration covers the surface.
Gastrointestinal tract
• Erosion is loss of part of the thickness of the mucosa.
Adjacent epithelial cells proliferate to regenerate the mucosa.•
Healing may take place this way in a matter of hours.•
• Ulceration is loss of the full thickness of the mucosa.
Mucosa is replaced from the margins.•
The muscularis propria cannot be regenerated: it is replaced by scar.•
Damaged blood vessels bleed, fi brin covers the raw surfaces.•
Macrophages migrate in and phagocytose dead tissue.•
Granulation tissue is produced in the base.•
If the cause persists, the ulcer becomes chronic.•
Fibrous scar tissue may result in contractions.•

CHAPTER 3 Surgical pathology
148
Ulcers
Classifi cation
Venous, arterial, diabetic, neuropathic, malignant, traumatic.
Features to note on examination
• Site. Neck, groin, and axilla (TB); legs and feet (vascular); anywhere
(malignant).
• Surface. Usually depressed. Elevated in malignancy, vascular
granulations.
• Size. Measure the ulcer. Is it large by comparison to the length of
history?
• Shape. Oval, circular, serpiginous, straight edges.
• Edge. Eroded (actively spreading), shelved (healing), punched out
(syphilitic), rolled or everted (malignant).
• Base. Fixed to underlying structures? Mobile? Indurated? Penetrating?
• Discharge. Purulent (infection), watery (TB), bleeding (granulation or
malignancy).
• Pain. Usually occurs during the extension phase of non-specifi c ulcers.
In diabetic patients, ulcers are relatively painless.
• Number. Widespread locally (local infection such as cellulitis),
widespread generally (constitutional upset).
• Progress. Short history (pyogenic), chronic (vascular or trophic, e.g.
post-phlebitic syndrome, decubitus ulceration of paraplegia).
• Lymph nodes. In the region of an ulcer may indicate secondary infection
or malignant change.
Natural history
• Extension. There is discharge, thickened base, infl amed margin. Slough
and exudates cover the surface.
• Transition. Slough separates and the base becomes clean. The discharge
becomes scanty, the margins less infl amed.
• Repair. Granulation becomes fi brous tissue and forms a scar after
re-epithelialization.
Investigations
History, biopsy and histology, serology, as indicated by presentation.
Chronic leg ulcers
Their aetiology is diverse, but can usually be diagnosed clinically.
Venous ulcers
Part of post-phlebitic limb syndrome where there may be a history of past
DVT. The ulcer is associated with oedema, lipodermatosclerosis (woody
thickening of soft tissues around the calf), and venous congestion with
secondary calf perforators and varicose veins. The ulcer is usually over
the medial malleolus, but can be large, involving the whole of the gaiter
region.
An ulcer is a breach in an epithelial surface.

ULCERS
149
• If pulses are absent in the foot, there may be an arterial element which
can be excluded by measurement of the ankle to brachial pressure
index (ABPI).
• If any doubt persists, a vascular referral for arterial reconstruction
should be considered, but four-layer bandaging must be avoided when
there is arterial insuffi ciency.
Arterial ulcers
These are often multiple and occur distally over and between the toes or
at pressure points such as heels or malleoli. They may occur elsewhere on
the leg, usually when there is an associated diabetic or venous element.
There is usually a history of arterial disease, particularly peripheral vascular
disease with claudication.
• Unlike venous ulcers where bacterial colonization is common, the
presence of organisms suggests infection, particularly when there is
moisture around the ulcers: wet gangrene (caused by staphylococci
and streptococci, not clostridia, see later) may ensue with cellulitis.
• If the leg is kept dry, infection is minimized and a line of demarcation
may aid in decision-making for the level of amputation.
• Arterial reconstruction should be considered before this stage.
Diabetic ulcers
These commonly occur in conjunction with arterial disease. They repre-
sent large- and small-vessel disease with an impaired ability to heal and
increased susceptibility to infection. Ulcers may occur in the arterial distri-
bution, particularly at pressure points, and involve deep tissue infections
(such as plantar abscesses) and osteomyelitis. The associated diabetic neu-
ropathy with Charcot’s joints presents with deformed feet and joints, which
are susceptible to ulceration.
• Management involves good diabetic and ulcer care, which includes
orthotist help with shoes and gait.
• Surgery aims to avoid major amputation, but requires debridement
of necrotic tissue, drainage of abscesses, and excision of dead tissue,
often involving bone as ‘ray’ excisions of toes.
Other causes
Include pressure, vasculitic, lymphatic, infective, and artefactual causes. Leg
ulcer clinics have emphasized the value of a team approach.

CHAPTER 3 Surgical pathology
150
Cysts, sinuses, and fi stulas
Cysts
Classifi cation
Congenital
• Sequestration dermoid. Due to displacement of epithelium along
embryonic fi ssures during closure, e.g. skin. Sites include outer and
inner borders of orbit, midline of the body, anterior triangle of neck
(brachial cyst), (cf. implantation dermoid due to skin implantation from
injury).
• Tubulo-dermoid/tubulo-embryonic. Abnormal budding of tubular
structures, e.g. enteric cysts, post-anal dermoid, thyroglossal cyst.
• Dilatation of vestigial remnants. For example, urachal, vitellointestinal,
paradental and branchial cleft cysts, hydatid of Morgagni, Rathke’s
pouch.
Acquired
• Retention cysts. Due to the blocking of a glandular or excretory duct,
e.g. sebaceous cyst (sweat gland); ranula (salivary gland); and cysts
of the pancreas, gall bladder, parotid, breast, epididymis, Bartholin’s
glands, hydronephrosis, hydrosalpinx.
• Distension cysts. Due to the distension of closed cavities as a result
of exudation or secretion, e.g. thyroid or ovarian cysts; hygroma
(lymphatic cysts), hydrocoele, ganglia, bursas (false cysts).
• Cystic tumours. For example, cystadenoma, cystadenocarcinoma of
ovary.
• Parasitic cysts. For example, hydatid cysts (Taenia echinococcus).
• Pseudocysts. Due to necrosis of haemorrhage with liquefaction and
encapsulation, e.g. necrotic tumours, cerebral softening, or coalescence
of infl ammatory fl uid collections, e.g. pseudocyst of pancreas.
Clinical features
Subcutaneous/superfi cial
Smooth, spherical, soft, and fl uctuant when palpated in two planes with
the fi ngers at right angles to each other. If tense contents, may produce
pain in the cyst or surrounding tissue. If the fl uid is clear, the swelling
will transilluminate. Ultrasound and aspiration of contents are methods
of determining whether a given swelling is cystic and may differentiate a
cyst from a lipoma. May compress surrounding tissues. May produce pain
if complications supervene. They are also subject to infection, torsion if on
a pedicle, haemorrhage, and calcifi cation.
A cyst is a collection of fl uid in a sac lined by endothelium or epithelium
which usually secretes the fl uid.
• True cysts are lined by endo- or epithelium.
• False cysts are the result of exudation or degeneration, e.g.
pseudocyst of pancreas, cystic degeneration in a tumour.

CYSTS, SINUSES, AND FISTULAS
151
Treatment
• Excision. Only if symptomatic, cosmetic, or concern over diagnosis.
• Marsupialization (deroofi ng and suture of the lining to skin). If chronic
or infected.
• Drainage (deep site). If symptomatic or complicated. Not if concern
over malignancy.
Sinuses/fi stulas
Causes
• Specifi c disease, e.g. Crohn’s.
• Abscess formation and spontaneous drainage, e.g. diverticular abscess
discharging into vagina with fi stula formation.
• Penetrating wounds.
• Iatrogenic (e.g. anastomotic leak discharging via wound).
• Neoplastic.
Persistence of a fi stula is due to the following
• Presence of foreign material, e.g. suture/bone in a sinus.
• Distal obstruction of the viscus of origin.
• Continuing active sepsis, e.g. TB, actinomycosis.
• Epithelialization of the track.
• Chronic infl ammation, e.g. Crohn’s.
• Malignancy in the track.
Investigation
Establish the extent by sinography/fi stulogram. MRI scan is often helpful.
Treatment
Principles of sinus treatment:
• Ensure adequate drainage, laying it open and remove granulations.
• Remove septic material, foreign bodies.
• Biopsy sinus wall if concern over underlying diagnosis.
• Loose packs may be used to help drainage.
Principles of fi stula treatment:
• Treat any sepsis, fl uid imbalances, and poor nutrition if associated.
• Ensure good drainage to prevent fi stula extension.
• Identify the anatomy, use examination under anaesthetic (EUA) or
imaging if required.
• Biopsy the fi stula if concern over underlying diagnosis.
• Defi nitive treatment requires:
Excision of the organ of origin or closure of the site of origin.•
Removal of chronic fi stula track and surrounding infl amed tissue.•
Closure of ‘recipient’ organ if internal or drainage of external site •
if to skin.
• A sinus is a blind epithelial track, lined by granulation tissue which
extends from a free surface into the tissues, e.g. pilonidal sinus.
• A fi stula is an abnormal communication between two epithelial
surfaces. It is lined by granulation tissue and colonized by bacteria,
e.g. fi stula-in-ano, pancreaticocutaneous, colovesical, vesicovaginal.

CHAPTER 3 Surgical pathology
152
Atherosclerosis
Aetiology
Reversible risk factors include smoking, hypercholesterolaemia, obesity,
and hypertension. Irreversible risk factors include diabetes, male sex, age,
and family history.
Pathological features
• There are three stages of atheromatous lesion; fatty streaks are linear
lesions on the artery lumen, composed of lipid-fi lled macrophages, and
which progress to fi brolipid plaques and fi nally, complex lesions.
• In sites predisposed to atherosclerosis (sites of vessel bifurcation,
turbulent fl ow, post-stenotic areas, areas denuded of endothelial
cells), lipid-laden macrophages enter the vessel wall via gaps between
endothelial cells.
• A fi brolipid plaque contains a mixture of macrophages and smooth
muscle cells which migrate into the plaque, capped by a layer of
fi brous tissue.
• Growth factors, particularly platelet-derived growth factor (PDGF),
stimulate the proliferation of intimal smooth muscle cells and the
synthesis of collagen, elastin, and mucopolysaccharide.
• Lipid accumulates within the plaque extracellularly and in the
myocytes, ultimately producing foam cells.
• Cell death eventually ensues with the release of intracellular lipids,
calcifi cation, and a chronic infl ammatory reaction.
• High levels of circulating LDL-cholesterol are thought to lead to
atherosclerosis by damaging endothelium, both directly by increasing
membrane viscosity and indirectly through free radical formation, and
by inducing secretion of PDGF.
• In larger vessels such as the aorta, atherosclerotic plaques may release
atheroemboli and mural thrombus or impinge on the vessel media,
causing tissue atrophy resulting in aneurysm formation or dissection.
• Acute MI is caused by three processes in coronary vessels: progressive
atherosclerosis, disruption of unstable plaque with acute thrombosis,
and acute haemorrhage into the intima around the plaque.
Atherosclerosis is a degenerative disease of large and medium-sized
arteries characterized by lipid deposition and fi brosis.
This page intentionally left blank

CHAPTER 3 Surgical pathology
154
Thromboembolic disease
Thrombus
The formation, structure, and appearance of thrombus and clot are com-
pletely different. Clinical features of thrombus—see b p. 120.
Aetiology
Described by Virchow, the three types of risk factors for thrombus are
called Virchow’s triad (see Box 3.1). Not all three are needed: any one
of them may result in thrombus formation in arteries or veins. Arterial
thrombus is most commonly associated with atheroma, venous thrombus
with stasis.
Thrombus formation
Wherever thrombus forms, the principal mechanisms are similar.
• Initial trigger is one or more of Virchow’s triad.
• Fibrin deposition on vessel wall and formation of platelet layer.
• Red cells trapped in fi brin meshwork on top of platelet layer.
• Mass projects into lumen, causing turbulent blood fl ow.
• Thrombus grows in direction of blood fl ow: propagation.
• In veins, alternating patterns of white platelets and red blood cells may
be seen: lines of Zahn.
• Thrombophlebitis is infl ammation of veins secondary to thrombus.
• Phlebothrombosis is thrombus formation secondary to phlebitis.
• Phlegmasia alba dolens (white painful leg) occurs after slow thrombosis
formation in the ileofemoral veins and is a chronic condition.
• Phlegmasia cerulean dolens (blue painful leg) is due to acute massive
ileofemoral venous thrombosis and can result in shock and gangrene.
• Thrombophlebitis migrans are transient thromboses in previously
healthy veins anywhere in the body, suggesting visceral cancer.
A thrombus is a solid mass of blood constituents formed within the
vascular system.
Box 3.1 Virchow’s triad
• Disruption in the blood vessel endothelium.
Atheromatous plaque, e.g. acute MI.•
Thrombophlebitis, e.g. DVT.•
Trauma, e.g. from pressure, surgery, fractures, previous thrombus.•
• Disruption in the pattern of blood fl ow.
Stasis, e.g. immobilization, surgery, low CO states.•
Turbulence, e.g. post-stenotic, atherosclerotic plaques.•
• Changes in blood constituents.
Age.•
Smoking.•
Malignancy.•
DIC, HITT.•
Pregnancy, oral contraceptive pill.•

THROMBOEMBOLIC DISEASE
155
Embolism
Types of embolism
The aetiology is very different, depending on the cause of embolism.
The clinical effects depend on the territory supplied by the vessel that is
blocked. Emboli can be divided into:
• Systemic emboli (see b p. 642) (cause stroke, end-organ ischaemia, MI);
• PE (see b p. 120).
Both types of embolism can be further classifi ed by the substance
involved:
Thrombus.• Most emboli are derived from thrombi.
Gas.• Injection or entraining of air, decompression sickness.
Fat.• Long bone fractures, severe burns, extensive soft tissue
trauma.
Amniotic fl uid.• i Intrauterine pressure forces fl uid into uterine veins
and into systemic circulation.
Septic emboli.• Vegetations from heart valves.
Atheromatous plaque.• Peripheral vascular disease, iatrogenic.
Tumour.• Common route of metastasis.
Foreign bodies.• IV drug users, medically inserted catheters.
Clot
Clot forms in vessels after death or outside the body as part of the
response to trauma. Activation of the clotting cascade (see b p. 119)
results in formation of fi brin from fi brinogen, resulting in the formation of
fi brin meshwork that enmeshes cells in a solid, elastic clot.
Ischaemia and infarction
Oxygen supply-demand mismatch is caused by:
• Vascular narrowing (atherosclerosis, thrombus, embolus, spasm).
• Global hypoperfusion (shock, cardiopulmonary bypass).
• Hypoxaemia (anaemia, hypoxia).
• Vascular compression (ventricular distension, venous occlusion).
• Increased oxygen demand (exercise, pregnancy, hyperthyroidism).
Pathological features
The shape of the infarct depends on the territory and perfusion of the
occluded vessel.
• Seconds. Change from aerobic to anaerobic metabolism.
• Minutes. d Contractility of muscle, cell and mitochondria swell.
An embolism is a mobile mass of material in the vascular system capable
of blocking its lumen.
A clot is a solid collection of blood cells within a fi brin network.
Ischaemia is tissue effect due to insuffi cient oxygen delivery.
Infarction is tissue death due to insuffi cient oxygen delivery.

CHAPTER 3 Surgical pathology
156
• Hours. Myocyte death, coagulation necrosis, muscle pale, oedematous.
• Days. Infl ammatory exudates with polymorphonuclear leucocytes, then
fi broblast infi ltration beginning scar formation; macroscopically, the
infarcted area appears yellow and rubbery with haemorrhagic border.
• Weeks. Neovascularization and margins.
• Months. Scar maturation—tough, white, contracted area.
This page intentionally left blank

CHAPTER 3 Surgical pathology
158
Gangrene and capillary ischaemia
Gangrene
Aetiology
• Thrombosis, e.g. appendiceal artery secondary to infl ammation.
• Embolus, e.g. atherosclerotic emboli in peripheral vascular disease.
• Extrinsic compression, e.g. fracture, organ torsion, tourniquet.
Clinical appearances
Dry gangrene
The affected limb, digit, or organ is black because of breakdown of hae-
moglobin, dry, and shrivelled. Dry gangrene shows little or no tendency
to spread. A zone of demarcation appears between the dead and viable
tissue and separation begins to take place by aseptic ulceration in a few
days.
Wet gangrene
Veins as well as arteries are blocked. Pain is initially severe, but lessens as
the patient becomes more septic. There is always infection (putrefaction).
The skin and superfi cial tissues become blistered. There is a broad zone
of ulceration which separates it from normal tissue. Proximal spread is a
feature, leading to septicaemia and death.
Gas gangrene
Gangrene complicated by infection with gas-producing anaerobic bacteria,
e.g. Clostridium perfringens. Gases elaborated from putrefaction lead to
surgical emphysema and crepitus (see b p. 175).
Principles of treatment
• Systemic treatment.
Aggressive fl uid resuscitation is often necessary.•
Pain relief (IV morphine 5–10mg).•
IV antibiotics—broad-spectrum (e.g. benzylpenicillin, metronidazole, •
piperacillin/tazobactam, or according to microbiological advice).
• Conservative treatment. Only possible for non-vital organs affected
by dry gangrene (e.g. toes/forefoot). Aim is to let the affected areas
mummify and spontaneously separate.
• Surgical salvage procedures. Conservative excision possibly combined
with reconstruction or restoration of blood supply (e.g. foot
amputation and bypass surgery for distal lower limb gangrene).
• Radical surgical excision. Only possible where affected organ is
completely resectable (e.g. limbs, perineal tissues)—excision must
be radical in spreading or gas gangrene. Ensure all pus is released, all
affected tissue (not just the necrosed area) is excised back to bleeding
healthy tissue. Often requires ‘relook’ surgery to ensure adequate
excision of infected tissue.
Gangrene is ischaemic tissue necrosis with dessication (dry gangrene)
or putrefaction (wet gangrene).

GANGRENE AND CAPILLARY ISCHAEMIA
159
• Palliative care. Consider for unresectable gangrene (e.g. retroperitoneal
gangrene, very extensive intestinal gangrene) or for elderly sick
patients where surgery is inappropriate.
Capillary ischaemia
This is ischaemia mediated by injury to capillaries.
• Frostbite. Exposure to cold with freezing results in fi xed capillary
contraction, ischaemia, and infarction.
• Trenchfoot. Exposure to cold without freezing results in capillary
contraction followed by fi xed dilation.
• DIC (see b p. 116).
• Cryoglobulinaemia, sickle cell, parasites.

CHAPTER 3 Surgical pathology
160
Tumours
Metaplasia
This represents an adaptive response of a tissue to environmental stress.
It is mediated by changes in expression of genes involved in cellular differ-
entiation. It does not progress to malignancy: if the environmental changes
persist, dysplasia may result and progress to malignancy. For example:
• Change from ciliated to squamous cells in the respiratory epithelium of
the trachea and bronchi in smokers;
• Change from squamous to columnar cells in the oesophageal
epithelium of patients with gastro-oesophageal refl ux disease
(Barrett’s) (see b p. 280).
Dysplasia
Potentially premalignant condition. May be a response to chronic infl am-
mation or exposure to carcinogens. Early forms may be reversible: severe
dysplasia has a high risk of progression to malignancy, for example:
• Dysplasia arising in colonic epithelium due to chronic ulcerative colitis.
• Squamous dysplasia in the bronchi of smokers (sputum cytology).
Classifi cation of tumours
Use this classifi cation to give a differential diagnosis for any neoplasm.
• Tissue of origin. Organ and tissue type (see Table 3.1).
• Behaviour. Benign or malignant.
• Primary or secondary.
Benign tumours
• Slow growing, usually encapsulated, do not metastasize, do not recur
if completely excised, rarely endanger life. Effects are due to size and
site.
• Histology: well differentiated, low mitotic rate, resemble tissue of
origin.
Malignant tumours
• These expand and infi ltrate locally, encapsulation is rare, metastasize
to other organs via blood, lymphatics or body spaces, endanger life if
untreated.
• Histology: varying degrees of differentiation from tissue of origin,
pleomorphic (variable cell shapes), high mitotic rate.
Defi nitions
• Metaplasia. Reversible transformation of one type of terminally
differentiated cell into another fully differentiated cell type.
• Dysplasia. Potentially premalignant condition characterized
by increased cell growth, atypical morphology, and altered
differentiation.
• Neoplasia. Autonomous abnormal growth of cells which persists
after the initiating stimulus has been removed.
• A neoplasm is a lesion resulting from neoplasia.

TUMOURS
161
Invasion
Invasion is the most important single criterion for malignancy and is also
responsible for clinical signs and prognosis as well as dictating surgical
management. Factors that enable tumours to invade tissues include:
• Increased cellular motility.
• Loss of contact inhibition of migration and growth.
• Secretion of proteolytic enzymes, such as collagenase, which weakens
normal connective tissue bonds.
• Decreased cellular adhesion.
Metastasis
Metastasis is a consequence of these invasive properties: it is the proc-
ess by which malignant tumours spread from their site of origin (primary
tumour) to form secondary tumours at distant sites. Carcinomatosis
denotes extensive metastatic disease. The routes of metastasis are:
• Haematogenous. Via the bloodstream.
Five tumours—breast, bronchus, kidney, thyroid, prostate—•
classically metastasize via haematogenous spread to bone.
Lung, liver, and brain are common sites for secondaries.•
• Lymphatic. To local, regional, and systemic nodes.
• Transcoelomic. Across pleural, pericardial, and peritoneal cavities.
• Implantation. During surgery or along biopsy tracks.
Table 3.1 Structural classifi cation
Tissue of origin Tumour type
Epithelium Benign: papilloma, adenoma (glandular epithelium)
Malignant: carcinoma (adenocarcinoma, squamous cell
carcinoma indicate cell types)
Connective tissue Benign: fi broma (fi brous tissue), lipoma (fat),
chondroma (cartilage), osteoma (bone), leiomyoma
(smooth muscle), rhabdomyoma (striated muscle)
Malignant: sarcoma, e.g. fi brosarcoma, osteosarcoma,
etc. (if well differentiated); spindle cell sarcoma, etc.
(if poorly differentiated)
Neural tissue These arise from nerve cells, nerve sheaths, and
supporting tissues, e.g. astrocytoma, medulloblastoma,
neurilemmoma, neuroma, etc.
Haemopoietic The leukaemias, Hodgkin’s disease, multiple myeloma,
lymphosarcoma, reticulosarcoma
Melanocytes Melanoma
Mixed origins E.g. fi broadenoma, nephroblastoma, teratoma (all three
germ layers), choriocarcinoma
Developmental
blastomas
E.g. neuroblastoma (adrenal medulla), nephroblastoma
(kidney), retinoblastoma (eye)

CHAPTER 3 Surgical pathology
162
Carcinogenesis
• Initiators produce a permanent change in the cells, but do not
themselves cause cancer, e.g. ionizing radiation: this change may be in
the form of gene mutation.
• Promoters stimulate clonal proliferation of initiated cells, e.g. dietary
factors and hormones: they are not mutagenic.
• Latency is the time between exposure to carcinogen and clinical
recognition of tumour due to:
Time taken for clonal proliferation to produce a signifi cant cell •
mass.
Time taken for exposure to multiple necessary carcinogens.•
• Persistence is when clonal proliferation no longer requires the presence
of initiators or promoters and the tumour cells exhibit autonomous
growth.
See Table 3.2 for a list of common risk factors for cancer.
Tumour growth
Tumour doubling time depends on cell cycle time, growth function, and cell
loss fraction. In tumours such as leukaemias, the doubling time remains
remarkably constant: the cell mass increases proportionally with time.
This is exponential growth. In solid tumours, doubling time slows as size
increases. This is referred to as Gompertzian growth.
Genetic abnormalities in tumours
Two genetic mechanisms of carcinogenesis are proposed:
• Oncogenes. Enhanced expression of stimulatory dominant genes.
• Tumour suppressor genes. Inactivation of recessive inhibitory genes.
Oncogenes
At least 60 oncogenes have been identifi ed. They can be classifi ed accord-
ing to the function of the gene product (e.g. growth factors, cell signalling
agents). The proteins produced (oncoproteins) can be produced in abnor-
mal quantities or be abnormally active forms and cause:
• Independence from extrinsic growth factors.
• Production of tumours in immunotolerant animals.
• Production of proteases to assist in invasion of normal tissues.
• Reduced cell cohesiveness assisting metastasis.
• Growth to higher cell densities and abnormal cellular orientation.
Examples include BRCA1, p53, k-ras, APC, DCC.
Carcinogenesis is the process that results in malignant neoplasm for-
mation. Usually more than one carcinogen is necessary to produce
a tumour, a process which may occur in several steps—multistep
hypothesis.

CARCINOGENESIS
163
Table 3.2 Common risk factors for cancer
Known carcinogen Type of cancer
Chemicals
Polyaromatic hydrocarbons Lung cancer (smoking), skin cancers
Aromatic amines Bladder cancer (rubber and dye workers)
Alkylating agents Leukaemia
Viruses
HIV Kaposi’s sarcoma, lymphoma
Epstein–Barr virus Burkitt’s lymphoma, nasopharyngeal cancer
Human papillomavirus Squamous papilloma (wart), cervical cancer
Hepatitis B virus Liver cell carcinoma
Radiation
UV light (UVB>UVA) Malignant melanoma, basal cell carcinoma
Ionizing radiation Particularly breast, bone, thyroid, marrow
Biological agents
Hormones, e.g. oestrogens Breast and endometrial cancer
Mycotoxins, e.g. afl atoxins Liver cell carcinoma
Parasites, e.g. schistosoma Bladder cancer
Miscellaneous
Asbestos Mesothelioma and lung cancer
Nickel Nasal and lung cancer
Host factors Type of cancer
Race
Caucasians Malignant melanoma, stomach cancer
Diet
High dietary fat Breast, colorectal cancer
Alcohol Breast cancer
Gender, inherited risks
Female sex Breast cancer
Familial polyposis coli Colorectal cancer
Multiple endocrine neoplasia Phaeo, parathyroid, medullary cancer thyroid
BRCA1–17q21 Breast, ovarian and prostate cancer
Premalignant lesions and conditions
Adenomatous rectal polyp Colorectal adenocarcinoma
Mammary ductal hyperplasia Breast carcinoma
Ulcerative colitis Colorectal adenocarcinoma
Transplacental exposure
Diethylstiboesterol Vaginal adenocarcinoma

CHAPTER 3 Surgical pathology
164
Screening
The aim is reduction in morbidity and mortality from screened diseases.
Requirements for successful screening
• Screening test must be:
Sensitive (see • b p. 7).
Specifi c (see • b p. 7).
Safe.•
Inexpensive.•
Acceptable.•
• The population screened must be:
Easily identifi ed and contactable.•
Compliant.•
• The disease screened must be:
Detectable in a treatable, premalignant form or earlier stage.•
Preventable or more amenable to successful or curative •
treatment.
A suffi cient burden on the population to justify cost of screening.•
Chronic or of suitable evolution for sporadic testing to detect it.•
Disadvantages of screening
• Cost (time and resources).
• The benefi t may be small.
• False positive tests may be physically or psychologically detrimental.
Examples of screening programmes
Abdominal aortic aneurysm (4000 deaths/y)
The UK multicentre aneurysm screening study of 68 000 men showed
screening halves aneurysm-related deaths by reducing risk of rupture. The
conclusion was that aneurysm screening should be offered in the UK. The
MASS study showed a benefi t where other studies failed because:
• It was adequately powered (see b p. 8).
• Screening compliance was higher:
GP-based ultrasound had a better compliance than specialist •
clinics.
Participants unlikely to attend were excluded from the study.•
Breast cancer (14 000 deaths/y)
A meta-analysis of thirteen breast cancer screening trials concluded that
screening mammography signifi cantly reduced breast cancer mortality in
women aged 50–74. A BMJ analysis concluded that:
• For every 1000 women screened over 10y, around 200 (depending on
age) are recalled because of an abnormal result and of these:
Around 60 will have at least one biopsy.•
About 15 will have invasive cancer and 5 will have ductal carcinoma •
in situ (DCIS).
Screening is testing any population for a disease.

SCREENING
165
• About 0.5, 2, 3, and 2 fewer deaths from breast cancer occur over 10y
per 1000 women aged 40, 50, 60, and 70y, respectively, who choose to
be screened.
• Ten per cent of invasive carcinoma is not radiologically detectable.
• Risk of a false positive screen is approximately 25% over 10y.
• Studies suggest up to a 30% reduction in mortality from screen-
detected early breast cancer.
• Features looked for on screening mammography include: spiculated
calcifi cation, microcalcifi cation.
What is offered?
• Since 1988, population-based screening offered.
• Starts age 50 and continues to age 70 (covers peak ages of incidence
of new diagnoses and excludes low risk younger women—prevents
‘psychological morbidity of screening the well’).
• Seventy per cent of women offered it accept screening (lowest take-up
in lower socio-economic groups and those diffi cult to contact, e.g.
rapidly changing addresses or no fi xed address).
• Two-view (lateral and oblique) mammography of both breasts.
• Suspicious or malignant-looking lesions invited for clinical assessment
by standard triple assessment.
Cervical cancer (1500 deaths/y)
Since the mid-1980s, incidence of and mortality from cervical cancer in
women under 70 in England and Wales has fallen. Screening is thought to
be the most likely explanation. A BMJ analysis concluded that:
• In the NHS cervical screening programme, 1000 women need to be
screened for 35 years to prevent one death:
150 have an abnormal result and 75 need repeat for inadequate test.•
80 undergo biopsy.•
55 have an abnormal biopsy result.•
2 have carcinoma, the rest have dysplasia.•
• At least one woman dies within the 35y despite being screened.
Prostate cancer (9000 deaths/y)
A third of men over 50y have evidence of prostate cancer at post-mortem,
but less than 1% of these have clinically active disease. Screening is con-
troversial because:
• Prostate-specifi c antigen (PSA), rectal examination, and transrectal
ultrasound have low specifi city and sensitivity alone or in combination.
• Treatment of prostate cancer is controversial (see b p. 374).
• No randomized trial has shown a survival benefi t in screened
populations: screening may cause more harm than good.
• Screening can be carried out on request despite the evidence above.
Colorectal cancer (16 000 deaths/y)
The lifetime risk of colorectal cancer is about 1 in 20. A nationwide screen-
ing programme is likely, following current pilot centres:
• Several possible screening tests exist:
Faecal occult blood (low sensitivity, 90% specifi city)—requires •
colonoscopy for positive results (false positives common).

CHAPTER 3 Surgical pathology
166
Colonoscopy (sensitivity and specifi city near 100%).•
Flexible sigmoidoscopy (sensitivity 80%, specifi city near 100%).•
• Colorectal cancer is suited to screening:
It has a detectable premalignant phase.•
It is detectable at an earlier and potentially highly treatable stage.•
Screening has been shown to be cost-effective and acceptable.•
This page intentionally left blank

CHAPTER 3 Surgical pathology
168
Grading and staging
Key facts
The objectives of staging and grading a tumour are:
• To plan appropriate (treatment) for the individual patient.
• To give an estimate of the prognosis.
• To compare similar cases when assessing outcomes or designing
clinical trials.
Staging and grading methods
Staging
The commonest system is the internationally agreed TNM classifi ca-
tion (see Table 3.3). It is not appropriate for leukaemia, lymphomas, or
myeloma. A four-stage classifi cation (I. II, III, IV) is also often used and is
compatible with TNM. Specifi c staging systems also exist for some tumour
sites (e.g. Duke’s stage in colorectal cancer, see b p. 400).
Staging may be:
• Radiological (often performed preoperatively): indicated by the prefi x
‘r’ before the letter (e.g. rT3, rM1). If different radiological modalities
are used, separate prefi xes can be used, e.g. ‘u’ for ultrasound (uT2).
Radiological staging is used to plan treatment (e.g. neoadjuvant
therapy, selection for surgery, planning of surgery).
• Pathological (performed on surgical specimens): indicated by the
prefi x ‘p’ before the letter (e.g. pT3, pN2, pM1). If there has
preoperative radiotherapy used, the prefi x ‘y’ is used to denoted that
the pathological stage may have been modifi ed by this (e.g. ypT2).
Pathological staging is used to plan adjuvant treatment (chemotherapy
or radiotherapy) and for informing prognosis.
An example of lung cancer staging is:
• Stage I (T1N0, T2N0), 85% 5y survival with surgery.
• Stage II (T1N1, T2N1, T3N0), 60% 5y survival with surgery.
• Stage IIIa (T3N1 or any N2), 20% 5y survival with surgery.
• Stage IIIb (any T4, any N3), <20% 5y survival, no benefi t with surgery.
• Stage IV (M1), <10% 5y survival, no benefi t with surgery.
Other pathological features may be included with the TNM system for
some tumours, for example:
• Presence of extratumoural vascular invasion V0 or V1.
• Presence of extratumoural lymphatic invasion Ly0 or Ly1.
• Presence of viable tumour cells at or within 1mm of the surgical
margin of excision R0, R1 (microscopic), R2 (macroscopic).
• Staging is the process of assessing the extent of local and systemic
spread of a malignant tumour or the identifi cation of features which
are risk factors for spread.
• Grading is the process of assessing the degree of differentiation of a
malignant tumour.

GRADING AND STAGING
169
Histological grading
Gives a guide to the behaviour of a cancer by describing the degree of
differentiation of the tumour (e.g. breast cancer).
• Grade 1, represents the least malignant tumours.
• Grade 2, 25–50% of the cells are undifferentiated.
• Grade 3, 50–75% of the cells are undifferentiated.
• Grade 4, >75% of the cells are undifferentiated.
Other methods of describing tumours
• Depth of invasion (e.g. Breslow thickness in malignant melanoma).
• Tumour type (e.g. small cell versus non-small cell lung cancer).
Table 3.3 Basic form of TNM classifi cation*
Classifi cation Interpretation
Primary tumour (T)
TX Primary tumour cannot be
evaluated
T0 No evidence of primary tumour
Tis Tumour in situ
T1, T2, T3, T4 Size and extent of primary tumour
Regional lymph nodes (N)
NX Regional lymph nodes cannot be
evaluated
N0 No regional lymph node
involvement
N1, N2, N3 Number and location of involved
lymph nodes
Distant metastasis (M)
MX Distant metastasis cannot be
evaluated
M0 No distant metastasis
M1 Distant metastasis
* Additional codes used with the TNM: pul, pulmonary; hep, hepatic; V, vascular; Ly, lymphatic
vessels; R, radial margin. Prefi xes used with the TNM: u, ultrasound; r, radiological; p,
pathological.

CHAPTER 3 Surgical pathology
170
Tumour markers
Key facts
• Tumour markers (see Table 3.4) are complex molecules, often
proteins that can be detected by a variety of techniques, including
chemical, immunological, or bioactivity testing.
• Most are molecules normally produced by normal cells in small
amounts, but which may be produced in increased amounts by tumour
cells due to changes in cellular function (e.g. increased production,
increased gene expression, decreased degradation, increased release).
Testing
Testing is most commonly in vitro via serum measurements or testing tis-
sue specimens. Common uses include:
• Screening (detection of subclinical disease).
• Diagnosis (including differentiation of tumour origin in metastatic
disease).
• Monitoring response to treatment.
• Monitoring for development of recurrence.
Non-tumour related elevations in tumour marker levels (reducing the spe-
cifi city of these tests for tumours) may occur due to:
• Increased production/release due to infl ammation, infection, trauma,
or surgery.
• Decreased removal/destruction due to renal or liver disease.
Abbreviations for some tumour markers
• AFP (alpha-fetoprotein).
• β-HCG (beta-human chorionic gonadotrophin).
• PAP (placental alkaline phosphatase).
• CEA (carcinoembryonic antigen).
• LDH (lactic dehydrogenase).
• PSA (prostate-specifi c antigen).

TUMOUR MARKERS
171
Table 3.4 Commonly used tumour markers
Marker Useful in Notes/use
AFP Hepatoma; teratoma
(75% of cases); pancreatic
carcinoma some patients)
Elevated in liver disease,
e.g. hepatitis, cirrhosis, and
pregnancy
β-HCG Choriocarcinoma (almost
all cases); testicular
tumours/teratoma (75%);
other germ cell tumours
Measured both in blood
and urine
PAP Seminoma; ovarian
adenocarcinoma
CEA Colonic adenocarcinoma;
ovarian adenocarcinoma;
advanced breast cancer;
pancreatic cancer
Not useful for diagnosis or
screening. Used to monitor
response to treatment and
identify relapse in tumours
showing raised CEA at
diagnosis. May be elevated
in pancreatitis, ulcerative
colitis, gastritis, and heavy
smokers
CA 19–9 Pancreatic cancer (80%);
advanced colorectal cancer
(75%)
A polysialated antigen
(Lewis blood group antigen).
Ratio of CA 19–9:CEA most
sensitive for pancreatic
cancer diagnosis
LDH Lymphoma
Thyroglobulin Thyroid cancer Used to monitor and
identify relapse after
treatment
Calcitonin Medullary carcinoma of the
thyroid
Used to monitor and
identify relapse after
treatment
PSA Prostate cancer May be measured in
serum and tissue by
immunohistochemistry.
Serum level closely relates
to disease status
Alkaline
phosphatase
Osteosarcoma Also raised in bony
metastases, osteitis, Paget’s
disease

CHAPTER 3 Surgical pathology
172
Surgical microbiology
Key facts
• Three-quarters of nosocomial infection occur in surgical patients who
account for 40% of hospital inpatients.
• Risk factors for wound infection include the type of surgery, patient age,
malnutrition, immunosuppression, obesity, lack of appropriate antibiotic
prophylaxis, foreign bodies, and residual malignancy or necrotic tissue.
Nosocomial infections are acquired in hospital.
Community acquired infections are acquired outside hospital.
Sources of surgical infection
Three-quarters of nosocomial infections occur in surgical patients (see
Table 3.5) who account for 40% of hospital inpatients. Sources of infection
include:
• Patient’s own body fl ora:
Failure of correct aseptic technique.•
Contaminated surgery (see • b p. 146).
• Indirect contact:
Contact from hands of doctors, nursing staff, patients, visitors.•
Contaminated surfaces, e.g. door handles, cups.•
• Direct inoculation:
Surgeon or environmental fl ora through failure of aseptic technique.•
Contaminated instruments or dressings.•
Colonization of indwelling drains, catheters, intravenous lines.•
• Airborne contamination:
Skin and clothing of staff, patients, and visitors.•
Air fl ow in operating theatre or ward.•
• Haematogenous spread:
Intravenous and intra-arterial lines.•
Contaminated infusions.•
Sepsis at other anatomical sites.•
• Food and waterborne.
• Faecal-oral.
• Insect borne.
Risk factors for wound infection
• General:
Age.•
Malnutrition, obesity, malnutrition.•
Immunosuppression, including steroid therapy, chemotherapy.•
Endocrine and metabolic disorders, e.g. diabetes, jaundice, uraemia.•
Hypoxia and anaemia.•
• Local:
Type of surgery (see Table 3.5), lengthy procedures.•
Necrotic tissue.•
Residual local malignancy.•
Foreign bodies, including prosthetic implants.•
Ischaemia, haematoma.•

SURGICAL MICROBIOLOGY
173
• Microbiology:
Lack of antibiotic prophylaxis.•
Type and virulence of organism, size of inoculate.•
Modes of occupational infections of health workers
• Direct percutaneous inoculation of infected blood (e.g. needle-stick
injury, scalpel wounds).
• Entry of infection through minute skin abrasions after contact with
spilled infectious bodily fl uids (e.g. blood, saliva, semen, urine, faeces).
• Entry of infection via mucosal surfaces after exposure to contaminated
infectious bodily fl uids (e.g. eye splashes in theatre, faecal-oral route).
• Transfer of infection by fomites (e.g. via contaminated equipment—
prions transferred by neurosurgical equipment).
Procedures designed to minimize transmission
See also b p. 174 for HIV and hepatitis.
• Identify infected (infectious) patients by serology.
• Identify potentially infected (infectious) patients by risk factors (e.g. IV
drug users at risk from hepatitis B carriage).
• Specifi c procedures for the care of infected (infectious) patients (e.g.
barrier nursing for C. diffi cile diarrhoea).
• Careful disposal of disposable items related to patient care.
• Specifi c treatment and sterilization of non-disposable equipment.
• Additional/specifi c precautions for theatre staff:
Make all procedures ‘safe’ procedures by having the highest •
standards of safety and care using instruments and sharps:
remember ALL patients may be infected/infectious.
Wearing of plastic aprons in procedures with expected soiling with •
urine/faeces/ascites.
Wearing of two pairs of gloves to reduce the risk of skin exposure •
when gloves tear.
Wearing of re-enforced gloves for procedures with a high risk of •
penetrating injury (e.g. fragmented fractures).
Wearing of glasses, goggles, or visors for eye protection.•
Handle all sharps using a transfer container: never pass them hand •
to hand.
Don’t allow unnecessary blood or fl uid spillage.•
Table 3.5 Expected wound infection rates after surgical procedures
Type of surgery
Rate of post-operative
infection (%)
Clean (no viscus opened), e.g. hernia repair <2
Clean contaminated (viscus opened minimal
spillage), e.g. cholecystectomy
<10
Contaminated (open viscus with spillage or
infl ammatory disease), e.g. simple appendicectomy
15–20
Dirty (pus or perforation or incision through
abscess), e.g. perforated appendicectomy
>40

CHAPTER 3 Surgical pathology
174
Surgically important organisms
Normal body fl ora
These are usually involved in infections in surgical patients and include:
Staphylococci
• Normal fl ora of skin, oropharynx, and nasopharynx.
• S. aureus is an important pathogen in many surgical infections.
• S. aureus is the only staphylococcus that can coagulate plasma.
• ‘Coagulase negative’ effectively means non S. aureus staph, e.g. S.
epidermis: they are usually dismissed as contaminants, but they are
an increasingly common cause of line and prosthesis infections,
particularly in immunocompromised patients.
• Antibiotic sensitivities: cephalosporins especially cefuroxime, gentamicin,
fusidic acid, vancomycin, rifampicin, teicoplanin.
• Antiseptic sensitivities: chlorhexidine, povidone-iodine.
• Methicillin-resistant S. aureus (MRSA) is resistant to all cephalosporins;
vancomycin, teicoplanin, fusidic acid should be reserved to treat this.
Streptococci
• Normal fl ora of skin, oropharynx, and nasopharynx.
• ‘α-haemolytic’ streptococci haemolyse blood agar, e.g. S. pyogenes.
• ‘B-haemolytic’ streptococci also haemolyse erythrocytes, e.g. S. viridens.
• Pneumococci and enterococci (below) are subtypes of streptococci.
• S. pyogenes has been called ‘the most important human pathogen’: it
causes ‘strep throat’, a range of skin infections, septicaemia, necrotizing
fasciitis, toxic shock syndrome, and valvular disease in rheumatic fever.
• Antibiotic sensitivities: penicillin, erythromycin, cephalosporins,
clindamycin, fusidic acid, mupirocin.
• Antiseptic sensitivities: chlorhexidine, povidone-iodine.
Enterococci
• Normal fl ora of large intestine.
• These are an increasingly important cause of nosocomial infections.
• Involved in wound infections, intra-abdominal sepsis, urinary tract
infections, intravascular line infections, and dialysis-related infections.
• Antibiotic sensitivities: enterococci are intrinsically resistant to many
antibiotics, including all cephalosporins, and must usually be treated by
a combination drug regime, e.g. ampicillin plus glycoside.
• Vancomycin-resistant enterococcus (VRE) is resistant to all
cephalosporins and vancomycin, and sometimes teicoplanin.
‘The Gram-negative rods’
• Normal fl ora of large intestine.
• Gram-negative bacilli (also known as coliforms) include Escherichia coli,
Salmonella, Klebsiella, Enterobacter, and Proteus.
• Pseudomonas and Actinobacter are non-coliform Gram-negatives.
•
Antibiotic sensitivities: most are intrinsically resistant to penicillin
and there is increasing resistance to amoxicillin and ampicillin;
cephalosporins are the commonest fi rst-line treatment for non-
resistant forms or ‘extended range’ penicillins (e.g. piperacillin/

SURGICALLY IMPORTANT ORGANISMS
175
tazobactam), aminoglycosides (e.g. gentamicin, streptomycin, amikacin,
tobramycin), alone or in combination with cephalosporins, offer good
bactericidal action.
Anaerobes
• Normal fl ora of skin, oropharynx, large bowel, terminal ileum, and
genitourinary tract.
• Include Bacteroides and clostridia (bowel).
• C. diffi cile causes pseudomembranous colitis (see b p. 396).
• Cause anaerobic infections, including cellulitis, gas gangrene,
empyemas, and colonize diabetic foot ulcers.
• Act usually with aerobes to produce ‘synergistic’ necrotizing infections
of skin, fascia, and muscle spontaneously or after trauma or surgery.
• Antibiotic sensitivities: metronidazole is only active against anaerobes
and resistance is rare; most anaerobes are also sensitive to penicillins,
cephalosporins, clindamycin, erythromycin, and co-trimoxazole.
Specifi c infections
Gas gangrene
Gas gangrene is caused by Clostridium perfringens, a Gram-positive bacillus,
found in soil or faeces. Injury may be trivial. More common in immuno-
compromised patients. There is exudate and gas in the tissues; skeletal
muscle is affected. Oedema, spreading gangrene, and systemic signs follow.
Aggressive debridement and fasciotomies are required, with resuscitation,
organ support, and penicillin (2g 4-hourly IV) and metronidazole.
Synergistic spreading gangrene (Meleney’s or Fournier’s gangrene)
Also known as necrotizing fasciitis. The organisms involved are not clostrid-
ial, but rather aerobes and synergistic microaerophilic/anaerobes. Patients
may be immunocompromised. The initial wound might have been minor
or an uneventful operation. Severe wound pain and gas in the tissues
(crepitus) may be seen: the extent of subdermal gangrene may not be
apparent. Systemic support and antibiotics are required, with excision of
involved tissues.
Tetanus
This is a rare infection in the UK, but is common in many parts of the
world, with a mortality rate of about 60%. The causal organism, Clostridium
tetani, produces a powerful exotoxin which is neurotoxic. It enters the
spinal cord via peripheral nerves where it blocks inhibitory spinal refl exes.
It is found widely: infection often follows a trivial puncture wound.
Treatment is benzylpenicillin, 1g every 6h IV, metronidazole, and human
anti-tetanus immunoglobulin 30U/kg IM. If a wound is present, it is excised
and left open to heal by secondary intention. Immunization with 10-yearly
boosters is protective.

CHAPTER 3 Surgical pathology
176
Soft tissue infections
Cellulitis is the presence of actively dividing infectious bacteria within
the skin tissues.
Abscess is a liquid collection of pus lined by granulation tissue and fi bro-
sis (if chronic).
Lymphangitis is the presence of actively dividing infectious bacteria in
the lymphatic vessels of an area of the body.
Fasciitis is infl ammation of connective tissue that may be infective.
Myositis is infection or infl ammation of muscle tissue.
Cellulitis
Pathological features
• Skin entry by pathogenic bacteria (scratch, ulcer, hair follicle).
• Usually Gram +ve cocci (e.g. Streptococcus pyogenes, S. aureus).
• Usually heals by resolution if treated promptly.
• Spread may result in lymphangitis, suppuration results in a furuncle (skin
gland), carbuncle (upper dermis), or an abscess (deep skin tissue).
Clinical features
• Skin widely involved: warm, red (usually blanches with pressure),
swollen (often pitting), and exquisitely painful.
• Crepitus indicates the development of gas-forming tissue necrosis
which may be an emergency (see b p. 175).
Treatment
• IV antibiotics: benzylpenicillin (1g qds) and fl ucloxacillin (500mg qds).
• Always assess with microscopy and culture, if possible.
Abscess
Pathological features
• Contain polymorphonuclear neutrophils (PMNs)/macrophages,
lymphocytes (live and dead), bacteria (dead and viable), and liquefi ed
tissue products.
• May rupture (‘pointing’), discharge into another organ (forming a
fi stula eventually), or open onto another epithelial surface (sinus) (see
b p. 151).
• Incomplete treatment due to resistant organisms (mycobacteria) or
poor treatment may lead to chronic abscesses.
• Complete elimination of the organisms in a chronic abscess without
drainage can lead to a ‘sterile abscess’ (‘anti-bioma’).
Typical causes
• Suppuration of tissue infection (e.g. renal abscess from pyelonephritis).
• Contained infected collections (e.g. subphrenic abscesses).
• Haematogenous spread during bacteraemias (e.g. cerebral abscesses).
Diagnosis
• Deep abscesses are characterized by swing fever, rigors, high WCC,
and high CRP. Untreated, they lead to catabolism, weight loss, and a

SOFT TISSUE INFECTIONS
177
falling serum albumin. Ultrasound, CT, MRI, or isotope studies may be
necessary to confi rm the diagnosis.
Treatment
• Drain the pus, e.g. incision and drainage (perianal abscess), CT-guided
drain (renal abscess), closed surgical drainage (chest empyema), or
surgical drainage and debridement (intra-abdominal abscess).
• IV antibiotics (may require course of several weeks, indicating PICC
line insertion).
Fasciitis
Suspect when pain, oedema, and skin necrosis appear within 24–48h of
injury or operation.
Signs
The skin may be normal, oedematous, or mottled. There may be spiking
fever, hypotension, mental confusion.
Treatment
• IV fl uids; extensive excision may be indicated.
• Antibiotics on the basis of Gram stain.
• Dress incisions with moist gauze impregnated with dilute aqueous
antiseptics. Change these frequently under general anaesthetic.
Myositis
The bacterial infection has penetrated and destroyed muscle bundles.
Cause
Usually Clostridium perfringens, a Gram-positive, anaerobic, spore-forming
rod.
Management
• Recognize the condition: there is oedema and serosanguinous exudate;
exposed muscle is swollen and ranges from salmon pink to
• deep green/black in appearance. Crepitation may be present. There is
a ‘sickly sweet’ smell.
• Excise dead tissue until healthy, contractile, bleeding muscle is
encountered.
• Carry out Gram staining immediately. Streptococcal myositis rarely
needs amputation, clostridial may.
• Replace extracellular fl uid (ECF) defi cit. Transfuse if needed.
• High-dose penicillin (IM or IV, 0.6–1.2g every 2h). May need
antianaerobic therapy.
• Excision of all infected tissue may necessitate full thickness excision of
abdominal wall with prosthetic mesh replacement. Amputations are
performed by guillotine technique and the wounds packed open.
• There is no proven advantage to hydrogen peroxide dressings or
the use of hyperbaric oxygen, but passive immunization in proven
clostridial gangrene has been suggested, using a variety of bacterially
derived products.
Osteomyelitis Covered on b pp. 558–62.

CHAPTER 3 Surgical pathology
178
Blood-borne viruses and surgery
Viral hepatitis
• Commonest liver disease in the world.
• May cause acute liver failure or chronic active hepatitis.
Hepatitis A
Formerly known as infectious hepatitis. This is the most common form of
jaundice in children and young adults.
• Spread is by the faecal-oral route; the incubation period is 1 month.
• The antibody to the virus is anti-HAV.
• There is no vaccine and health care workers do not have to be tested.
Hepatitis B
• Double-shelled DNA virus: 565 new cases in UK in 2000. Ten per
cent of adults fail to clear the virus after infection. Up to 5% people
worldwide are carriers.
• Infection is largely blood-borne and is transmitted by blood transfusion,
inoculation, sharing syringes (drug addicts), sexual intercourse during
menstruation (with an infected partner), and anal intercourse.
• Transmission from a contaminated sharps injury is 30%.
• Antigens appear in the serum: HBsAg, the surface antigen; HBcAg,
the hepatitis core antigen; HBeAg, the ‘e’ antigen; the Dane particle;
double-stranded DNA; and DNA polymerase activity.
• Antibodies formed against these antigens (anti-HBs, anti-HBe) can be
detected in the peripheral blood:
HBsAg +ve.• Failure to clear infection, residual infectivity.
HBsAb +ve.• Protection marker from immunization or infection.
HBeAg +ve.• Close correlation of infectivity.
• Hospital staff are routinely offered vaccination for hepatitis B:
Infectious carriers may not perform exposure-prone procedures.•
Vaccination against hepatitis B is not compulsory. The alternatives •
are frequent testing to check infectivity or limited clinical practice.
The NHS Injury Benefi ts Scheme provides some benefi ts where •
hepatitis B has been occupationally acquired.
• Treatment. Any health care worker who remains HBeAg +ve may
undergo antiviral therapy under supervision by a hepatologist including:
Immunomodulation with interferon.•
Viral suppression with nucleoside analogues.•
Hepatitis C
RNA virus which causes cirrhosis of the liver and primary liver cancer.
There is no vaccination. Detected in 1 in 150 screened blood donations:
0.4% of the UK population are chronically infected. Transmission from a
contaminated sharps injury is 2–3%. Surgeons must be tested for hepatitis C
and may not carry out exposure-prone procedures if hepatitis C +ve.
Human immunodefi ciency virus (HIV)
Double-stranded RNA retrovirus transmitted by passage of infected body
fl uids from one person to another by several methods: anal and vaginal
sexual intercourse, peripartum, sharps, and infected blood products.

BLOOD-BORNE VIRUSES AND SURGERY
179
• HIV infection results in widespread immunological dysfunction,
manifested by a fall in CD4 +ve lymphocytes, monocytes, and antigen-
presenting cells (APCs).
• There is usually a 3-month asymptomatic, but infective viraemia.
• During this period, ELISA tests for HIV antibodies are negative.
• At seroconversion, an acute illness can occur.
• This is followed by generalized lymphadenopathy.
• Acquired immunodefi ciency syndrome (AIDS) develops in 5–10y.
• Median survival with untreated AIDS is 2y, treated is >20y.
High risk populations (2004 WHO statistics)
• Prevalence in adult population of southern Africa is 15–30%.
• Prevalence in adult population of sub-Saharan Africa is 5–15%.
• Prevalence in homosexual men at London genitourinary clinics is 10%.
• Prevalence of HIV in injecting drug users in UK is about 1%.
High risk procedures
The UK General Medical Council (GMC) has made it clear that surgeons
are obliged, if required, to operate on patients with AIDS or HIV infection.
Always use universal precautions. High risk procedures include:
• Any invasive procedure in HIV +ve patients.
• Invasive procedures in at-risk populations (see above).
• Biopsies for the diagnosis of opportunistic infection or suspected HIV.
• Procedures to deal with malignancies, e.g. Kaposi’s sarcoma, B-cell and
non-Hodgkin’s lymphoma, squamous oral carcinoma.
Precautions and post-exposure prophylaxis (PEP)
• The HIV risk from an HIV contaminated hollow needle is 0.3%.
• The risk from splashes on broken skin or mucous membranes is 0.1%.
• PEP 2 Go to occupational health or A&E out of hours. PEP
reduces the risk of seroconversion by over 80% if started within
1h of exposure; PEP is continued for 4 weeks. Side effects include
diarrhoea and vomiting.
Universal precautions
These are designed to protect workers from exposure to diseases
spread by blood and body fl uids. 2 All patients are assumed to be infec-
tious for blood-borne diseases, including HIV.
• They apply to blood; amniotic, synovial, pleural, peritoneal and
pericardial fl uid; semen, vaginal secretions; and CSF.
• They do not apply to faeces, sputum, urine, vomit, or saliva.
• Universal precautions include:
Use of protective clothing, e.g. gloves, gowns, masks, eye-guards.•
Removing hazards from work place, e.g. sharps bins, ventilation.•
Work practice, e.g. hand washing, handling of sharps, transport of •
soiled goods, reducing unnecessary procedures.
Single use disposable injection equipment.•
Hospital policy for all sharps injuries: squeeze, wash, and report.•

CHAPTER 3 Surgical pathology
180
Bleeding and coagulation
Blood products and transfusion reactions are described on (b
pp. 96–9).
Haemostasis
This is the physiological process by which bleeding is controlled. It has
four components:
• Vessel wall response, primarily vasoconstriction due to smooth muscle
contraction, is the fi rst response.
• Platelet activity results in formation of a platelet plug.
Platelets • adhere to exposed endothelial collagen, a process which
required von Willebrand factor (VWF) (factor VIII).
Release of adenosine diphosphate (ADP), arachidonic acid, •
prostaglandin, and thromboxane A2 promotes platelet aggregation.
Aggregated platelets react with thrombin and fi brin, forming a • plug.
Aspirin• irreversibly inhibits the cyclooxygenase-mediated formation of
prostaglandin, lasting for the life of the platelet (7–10 days); clopidogrel
irreversibly inhibits ADP-mediated aggregation (7–10 days).
• The coagulation cascade converts prothrombin to thrombin to
produce a fi brin clot: two interacting pathways are involved.
The • intrinsic pathway involves only normal blood components and
starts when factor XII (Hageman factor) is activated by binding to a
damaged vessel, resulting in the sequential activation of factors XI,
IX, VIII, and X.
The • extrinsic pathway requires thromboplastin (a tissue
phospholipid) which forms a complex with calcium and factor VII,
which activates factor X.
Both these pathways converge at the activation of factor X, which •
converts prothrombin to thrombin: thrombin converts soluble
fi brinogen to fi brin to produce a stable clot.
All of the soluble factors are manufactured by the liver except •
factor VIII (made by endothelium).
Warfarin• inhibits the manufacture of vitamin K-dependent clotting
factors (prothrombin, VII, IX, and X): taking 3–4 days to have effect.
• The fi brinolytic system terminates thrombus propagation to maintain
circulating blood in a fl uid state; it depends on four proteins.
Plasmin• , a serine protease which is produced by the action of
thrombin on plasminogen and attacks unstable bonds between
fi brin molecules to generate fi brin degradation products.
Antithrombin• III which deactivates thrombin, XIIa, IXa, and Xa.
Proteins• C and S which prevent thrombin generation by binding
factors Va and VIIIa. Tissue factor pathway inhibitor, produced by
platelets, inhibits factors Xa and VIIa.
Heparin• potentiates antithrombin III with immediate effect;
protamine binds heparin, reversing its effect almost immediately.
Disorders of haemostasis
These can be thought of in terms of the four components below:
• Vessel wall abnormalities, e.g. Henoch–Schonlein purpura, Cushing’s
syndrome, steroid use, vitamin C defi ciency (scurvy).

BLEEDING AND COAGULATION
181
• Platelet abnormalities.
Thrombocytopaenia• (<100 x 10
9
/L
3
) caused by reduced production
(bone marrow failure, radiotherapy, chemotherapy, infi ltrative
disease, e.g. neoplasia, leukaemia); faulty maturation (e.g. folate and
B12 defi ciency); abnormal distribution (splenomegaly); increased
destruction (autoimmune disorders, drugs, DIC, haemorrhage);
dilutional thrombocytopaenia in massive banked blood transfusion.
Abnormal function• , e.g. von Willebrand’s disease, uraemia, idiopathic
causes, drug effects, especially aspirin and clopidogrel.
• Coagulation abnormalities.
Congenital• , e.g. haemophilia A (d factor VIII), haemophilia B or
Christmas disease (d factor IX), von Willebrand’s disease (d VWF).
Acquired• , e.g. DIC (see b p. 118); d vitamin K (which is produced
by gut fl ora) secondary to poor nutrition, antibiotic therapy,
obstructive jaundice; liver disease; exogenous anticoagulants.
Preoperative evaluation of haemostasis
NICE guidelines
• Routine testing not recommended unless patient is ASA 3 as a result
of renal disease.
• For at-risk patients undergoing ‘major +’ cardiac or neurosurgery,
testing may be considered.
History and examination
• Ask about bleeding problems, e.g. menorrhagia, bruising, family history
of bleeding, and medication (specifi cally aspirin, clopidogrel, and
warfarin which should all be stopped 5 days before elective surgery)
(see b p. 72).
• Look for petechiae and purpura, jaundice, and hepatosplenomegaly.
Laboratory investigation
• Platelet count. Normally 200–400 x 10
9
/L; 70 x 10
9
/L is needed for
surgical haemostasis, <20 x 10
9
/L results in spontaneous bleeding.
• Blood fi lm. Estimate of platelet count and indicates morphology.
• Bleeding time. Useful as normal bleeding time indicates normal
platelets, normal function, and normal vascular response to injury, but
uncommonly used as a screening test.
• Prothrombin time (PT). Refl ects the extrinsic pathway (I, II, V, VII, X).
• Partial thromboplastin time (PTT). Refl ects the intrinsic pathway (all
factors except VII).
• Individual clotting factor assays.
• Thrombin time (TT). Rate of conversion from fi brin to fi brinogen.
• Fibrin degradation products. These are released by the action of plasmin
and are raised in DIC.
Principles of management
• Indications for FFP, platelets, and anti-fi brinolytics are listed on b p. 96.
• IV heparin may be reversed with protamine and/or FFP.
• Warfarin may be reversed over 12–24h with 1mg vitamin K SC or
acutely with FFP.

CHAPTER 3 Surgical pathology
182
Anaemia and polycythaemia
Anaemia
This is a reduction in haemoglobin concentration below normal (approxi-
mately 13–16g/dL in men, 11.5–15g/dL in females). It is classifi ed as
follows:
• Decreased red cell production.
Haematinic defi ciency (• d Fe, B12
,
folic acid).
Bone marrow failure (congenital, chemotherapy, radiotherapy, •
infi ltrative disease).
• Abnormal red cell maturation.
Myelodysplasia.•
Sideroblastic anaemia.•
• Increased red cell destruction (haemolytic anaemias).
Inherited (e.g. sickle cell, thalassaemia).•
Acquired (e.g. autoimmune, DIC—see • b p. 118).
• Chronic disease (common cause of anaemia in surgical patients).
Renal failure (• d production of erythropoietin).
Endocrine, liver disease.•
Iron defi ciency anaemia
Commonest cause of anaemia in surgical patients. Causes include:
• Menstruation (in 15% of females).
• GI losses (peptic ulcer, oesophagitis, gastric carcinoma, colorectal
carcinoma).
• Reduced iron uptake (poor diet, coeliac disease, malabsorption).
Sickle cell anaemia
Single base substitution gene defect causing an amino acid substitution in
haemoglobin, making HbS instead of HbA. Deoxygenated HbS polymer-
izes and causes red blood cells to sickle, resulting in occlusion of small
blood vessels and infarction. Common in black Africans. Homozygotes
have high levels of HbS and are prone to crises. Heterozygotes (‘sickle
trait’) are only symptomatic in hypoxic conditions, e.g. unpressurized air-
craft, limb ischaemia.
• Most patients are diagnosed: screening for sickle cell is widespread.
• The patient typically has an Hb of 6–8g/dL, reticulocytes 10–20%.
• There are three types of sickle cell ‘crisis’:
Thrombotic crises.• Precipitate by cold, dehydration, infection,
ischaemia, may mimic acute abdomen or pneumonia, priapism;
Aplastic crises.• Due to parvoviruses and require urgent transfusion;
Sequestration crises.• Spleen and liver enlarge rapidly from trapped
erythrocytes, resulting in right upper quadrant (RUQ) pain, i INR,
i LFT, dd Hb.
• Treatment involves removing causes and decreasing percentage of
HbS.
Keep warm and well hydrated, if necessary with IV fl uids.•
Give O•
2
.

ANAEMIA AND POLYCYTHAEMIA
183
Give opiate analgesia.•
Empirical antibiotics if any evidence of sepsis.•
If Hb <6g/L, give blood; if Hb >9g/dL, exchange transfusion.•
• Exchange transfusion to maintain HbA >60% before cardiac surgery.
Thalassaemia
Thalassaemias are genetic diseases of Hb synthesis, resulting in underpro-
duction of one chain which results in destruction of red cells while they
are still in the bone marrow. α-thalassaemia leads to d α chain production
with unbalanced β chain production and β-thalassaemia leads to d β chain
production. Common in the Mediterranean to Far East.
• Severity correlates with the genetic defi cit.
• Death may result by 1y of age without transfusion.
• Symptoms of iron overload after 10y: endocrine failure, liver disease,
and cardiac toxicity.
• Death at 20–30y due to cardiac siderosis.
Preoperative screening of anaemia: NICE guidelines
• FBC ‘should be considered’ for all surgery in adults >60y of age.
• FBC is recommended for ‘intermediate’ surgery (e.g. primary repair of
inguinal hernia, varicose vein surgery) in adults >60y of age.
• FBC is recommended in any adult undergoing major surgery.
• Sickle cell screening is recommended in any patient of African descent
undergoing a general anaesthetic: consent should be obtained.
Preoperative evaluation of anaemia
d Mean cell volume (MCV) or microcytic anaemia
• Iron defi ciency (blood loss, dietary): d serum ferritin and iron, i total
iron binding capacity (TIBC).
• Thalassaemia (i serum iron and ferritin, d TIBC).
• Hyperthyroidism.
i MCV or macrocytic anaemia
• B12 or folate defi ciency (dietary, pernicious anaemia, anti-folate drugs).
• Alcohol.
• Liver disease.
• Myelodysplasia and bone marrow infi ltration.
• Hypothyroidism.
Normal MCV or normocytic anaemia
Anaemia of chronic disease, renal failure, bone marrow failure, haemolysis,
pregnancy, dilutional.
Management of anaemia
• Elective patients should be investigated and treated appropriately.
• Blood transfusion (see b p. 96) is indicated in patients with Hb <8g/
dL undergoing emergency or elective surgery.
• Evidence suggests that maintaining Hb 7–9g/dL has a better outcome
than maintaining Hb 10–12g/dL, except in patients with unstable
angina.

CHAPTER 3 Surgical pathology
184
Polycythaemia
• Relative (d plasma volume). Dehydration from alcohol or diuretics).
• Absolute (i red cell mass).
Primary• (polycythaemia rubra vera).
Secondary• (altitude, smoking, COPD, tumours, e.g. fi broids).
• Treat underlying cause; consider venesection.

185
Practical procedures
Endotracheal intubation 186
Cardioversion 188
Defi brillation 190
Venepuncture 192
Intravenous cannulation 194
Arterial puncture and lines 196
Insertion of central venous catheter 198
Chest drain insertion 200
Management of chest drains 202
Pericardiocentesis 204
Cricothyroidotomy 206
Nasogastric tube insertion 208
Urethral catheterization 210
Suprapubic catheterization 212
Paracentesis abdominis 214
Rigid sigmoidoscopy 216
Local anaesthesia 218
Intercostal nerve block 220
Chapter 4

CHAPTER 4 Practical procedures
186
Endotracheal intubation
Key facts
Indicated in cardiac arrest, serious head injury, certain acute respiratory
and trauma settings, and prior to many surgical operations.
• 2 Effective bag-and-mask ventilation is better than ineffective attempts
at endotracheal intubation in the arrest setting.
• 2 Except in a dire emergency, this procedure should not be
performed without expert supervision.
Equipment
• Empty 10mL syringe.
• Endotracheal tube (ET; size 8–9 for females and 9–11 for males).
• Laryngoscope.
• Ribbon to secure tube, lubricating jelly.
• Connection tubing, Ambubag, and O2 (cylinder or wall connection).
• Working wall suction, tubing, and Yankauer.
Preparation
• Move bed forward so that you can stand behind patient’s head and
raise it so that you are working a comfortable height. Put on gloves.
• Elective setting. Pre-oxygenate the patient; attach pulse oximeter to
patient, connect Ambubag to 100% O
2
, use effective bag-and-mask
ventilation for 2–3min to achieve O
2
saturations >95%.
• Emergency setting. Suction mouth (aspiration is major risk, bag-and-
mask ventilation with Ambubag, and 100% O
2
).
• Check laryngoscope light works and blade opens and ET tube cuff
infl ates and defl ates with 10mL syringe.
• Remove any dentures and suction again any saliva and secretions.
• Extend the neck.
• Insert the laryngoscope, pushing the tongue to the left.
• Advance the scope anterior to the epiglottis and pull gently, but fi rmly,
upwards to expose the vocal cords.
• Insert the lubricated ET tube between the cords into the trachea.
• Confi rm correct positioning of the tube by observing chest
movements and listening over lung bases and stomach.
• Progressively infl ate the cuff and attach ventilation equipment.
• Confi rm correct cuff infl ation by listening for whistling or bubbling in
the larynx suggesting air leak and secure the tube in place with ribbon.
• 2 Patients not in cardiac arrest or who maintain a gag refl ex will need
anaesthetizing prior to oropharyngeal intubation, i.e. administration of
inducing agent plus muscle relaxant: this should be done only under
supervision of a trained anaesthetist.
• 2 The best setting to learn intubation is preoperatively in the anaesthetic
room of a theatre with good supervision in controlled conditions.
Tips and pitfalls
• Oesophageal intubation. Potentially fatal if not recognized. Always check
for bilateral breath sounds, chest movement, absence of stomach
sounds, pulse oximetry, blood gas, and capnography, if available. Bag-
ENDOTRACHEAL INTUBATION
187
and-mask ventilation is the safest and most effective way to oxygenate
a patient if you are not experienced at endotracheal intubation.
• Cannot visualize vocal cords. Ask for ‘cricoid pressure’—fi rm
downwards pressure from an assistant over the cricoid can help bring
cords into view. Some patients are ‘diffi cult intubations’ just because
of their particular anatomy and build; the safest way to maintain their
airway is to avoid repeat attempts at intubation, resume bag-and-mask
ventilation, and wait for senior help.
• Inadequate cuff pressure. Too little and airway not protected from
aspiration, too much and pressure injury can result in erosion or
stenosis. The balloon should feel as fi rm as your fi ngertip.
Key revision points—anatomy of the lower pharynx and larynx
(Fig. 4.1)
Trachea
Uvula
Vocal cords
Epiglottis
Catheter mount for connecting
endotracheal tube to inflation bag
Cuff
inflated
Fig. 4.1 (a) Diagram of the larynx as seen at intubation. (b) Correct position of
infl ated endotracheal tube cuff.

CHAPTER 4 Practical procedures
188
Cardioversion
Key facts
• Synchronized direct current (DC) cardioversion is the treatment of
choice for tachyarrhythmias compromising cardiac output (CO), such
as AF and supraventricular tachycardia (SVT) and for AF refractory to
chemical cardioversion.
Checklist for elective DC cardioversion
• Is it indicated? Is the patient still in AF?
• Is it safe (see b p. 189)?
Either AF has lasted <24h; or•
The patient must have had at least 6 weeks of formal •
anticoagulation; or
The patient must have a TOE excluding intracardiac thrombus.•
• Is the patient ready?
The potassium should be 4.5–5.0 (otherwise, repeat AF likely).•
The INR, if anticoagulated, should be >2.0.•
The patient should have a valid consent form.•
• The patient should be starved for 6h.
DC cardioversion for AF and SVT
Patient should be anaesthetized: some anaesthesiologists prefer not to
intubate, managing the airway with a bag and mask. You can either use
adhesive external defi brillator pads which remain fi xed to the patient until
the procedure is completed or handheld paddles and gel pads. As soon as
the anaesthesiologist is happy:
• Expose chest.
• Place pads on chest in position shown in Fig. 4.2: the aim is to direct as
much of the current as possible through the heart.
• Place three ECG electrodes on the patient as shown and connect to
the defi brillator so that an ECG trace is visible.
• Switch defi brillator on and turn dial on to appropriate power setting
(100J, 200J, 360J).
• 2 Press the ‘SYNC’ button and ensure that each R wave is accented on
the ECG: failure to do this can mean that a DC shock is delivered while
the myocardium is repolarizing, resulting in VF. Check that the ‘SYNC’
button is on before every shock for AF.
• If you are using handheld paddles, hold them fi rmly on the gel pads.
• Perform a visual sweep to check that no one is in contact with the
patient at the same time as saying clearly, ‘Charging. Stand clear’.
• Press the ‘CHARGE’ button.
• Press the ‘SHOCK’ button when the machine is charged.
• If the shock has been delivered successfully, the patient’s muscles
will contract violently: anyone in contact with the patient risks
experiencing an electric shock.
• Check the rhythm.
• If still AF, press the ‘CHARGE’ button and repeat the sequence.
CARDIOVERSION
189
Complications of DC cardioversion
• Complications of general anaesthesia(see b p. 218).
• Systemic embolization (see b pp. 155, 642.)
• Failure to cardiovert.
• Burns from incorrect application of gel pads.
• Muscle pain from involuntary contraction.
• Arrhythmias, including asystole and ventricular fi brillation (VF).
Common pitfalls
Failure to deliver a shock
Check that the defi brillator is switched on and adequately charged. Check
that the correct power setting has been selected. Change the machine.
Failure to cardiovert
Check the latest available serum K
+
was 4.5–5.0. Check that the cor-
rect power setting has been selected. Replace gel pads with fresh ones.
Reposition the patient on their side and the pads as shown and try two
further shocks at 200J (see Fig. 4.2). Don’t start at too low a power set-
ting: each shock leaves the myocardium less sensitive to further shocks.
There is some evidence that 360J as the fi rst power setting results in less
myocardial damage and a better conversion rate than multiple shocks at
lower power settings.
(a)
(b)
Fig. 4.2 Using defi brillators. (a) Correct positioning for defi brillation and
cardioversion. (b) Alternative positioning for synchronized DC cardioversion.

CHAPTER 4 Practical procedures
190
Defi brillation
Key facts
• Defi brillation is the treatment of choice for VF and pulseless VT.
• Biphasic defi brillators cycle current direction every 10ms: the same
amount of current (roughly 12amp and 1500V) is delivered, but with
less energy (200J compared to 360J in older monophasic models),
reducing the risk of burns and myocardial damage.
External defi brillation for VF and pulseless VT
Do not delay defi brillation for manoeuvres such as intubation, massage, or
administration of drugs.
• Expose chest.
• Place gel pads on chest in position shown in Fig. 4.2: the aim is to
direct as much of the current as possible through the heart.
• Switch defi brillator on and turn dial on to appropriate power setting
(200J for external defi brillation).
• Press ‘CHARGE’ button.
• If you are using handheld paddles instead of adhesive external
defi brillator pads, place them fi rmly on gel electrodes and hold.
• Perform a visual sweep to check that no one is in contact with the
patient at the same time as saying clearly, ‘Charging. Stand clear’.
• Press the red/orange ‘SHOCK’ button on the paddles.
• If the shock has been delivered successfully, the patient’s muscles
will contract violently: personnel in contact with the patient may
experience an electric shock.
• Check the rhythm: if VF, charge again and repeat the sequence.
• If the rhythm changes to one compatible with an output, check the
pulse before proceeding further.
Common pitfalls
Failure to deliver a shock
Check that the defi brillator is switched on and adequately charged. Check
that the correct power setting has been selected. Check that the ‘SYNC’
button is off if you are trying to defi brillate VF. Change the machine and
paddles.
Failure to defi brillate
Exclude causes of intractable VF, i.e. failure to effect rhythm changes com-
patible with an output despite repeated attempts. In internal defi brilla-
tion, decompress the heart using massage (with the fl ows down if you’re
on cardiopulmonary bypass). Double-check that the rhythm is not in fact
asystole. Epinephrine, lidocaine, or amiodarone may improve chances of
converting VF and maintaining rhythm.
This page intentionally left blank

CHAPTER 4 Practical procedures
192
Venepuncture
Key facts
This is a mandatory skill to learn for all doctors, but many patients will
have ‘diffi cult’ veins and regular practice is needed.
Indications
Obtaining venous blood samples for laboratory analysis, venesection.
Equipment
• Tourniquet.
• 23G or 21G needle, vacutainer holder.
• Syringe (appropriate size: 10–20mL).
• Alcohol swabs.
• Appropriate laboratory sample tubes.
• Cotton wool ball and tape.
• Pillow if the vein looks diffi cult.
Most hospitals now have vacuum tube systems as an alternative to the
‘needle and syringe’ approach for obtaining blood samples.
Preparation
Apply tourniquet above the elbow and inspect the arm for suitable
engorged veins. Place arm on a pillow, especially if you may be a while.
Method
• Clean the skin thoroughly with alcohol at the site of access.
• Tether the skin distal to the site with the thumb of your left hand.
• Pass the needle obliquely through the skin at a point approximately
2mm distal to the point of planned entry to the vein.
• Advance the needle slowly until a ‘give’ is felt as the vein is entered
and a ‘fl ashback’ is seen in the needle: push vacutainer onto holder.
• Aspirate the desired amount of blood while holding the barrel of the
syringe fi rmly.
• Release the tourniquet before gently withdrawing needle and syringe.
• Apply pressure to the site to arrest any bleeding. Do not assume the
patient can help with this, e.g. stroke patients.
Tips and pitfalls
• Poor veins. If the patient is cold and the samples non-urgent, place the
arm in warm water as this may aid venodilation. Veins on the dorsum
of the hand may be the only ones readily available; try using a smaller
or butterfl y needle to obtain samples. Aspirate gently using a syringe
on a butterfl y needle, not a vacutainer as this may collapse veins.
• Obese patients. Try the dorsum of the hand or the radial aspect of the
wrist, access may be easier here.
• Failed attempts. Repeated failed attempts will distress the patient
and demoralize the doctor! Ask someone to help. If the samples are
extremely urgent, a femoral stab may be the best option for obtaining
blood samples, e.g. during cardiac arrest.
VENEPUNCTURE
193
• IV cannulae. If blood samples and IV access are needed, obtain samples
through the cannula—simple and saves the patient another needle,
although be careful to draw blood slowly as haemolysis is more
common via a cannula.
• Sample bottles and request forms. Ensure these are labelled correctly
and the appropriate tests are ordered. If in doubt about a particular
investigation, seek advice from a senior or the laboratory.
• Blood cultures. Ensure that the skin is swabbed thoroughly. Do not
touch the skin again unless sterile gloves are worn. Once the sample
is taken, change the needle before transferring the sample to the
appropriate culture bottle. Document whether the patient was on
antibiotics at the time of the sample and ensure the sample is not
placed in the fridge during transfer to the lab.
Key revision points—venous drainage of the upper limb
• Superfi cial venous system.
Cephalic vein.• Commences from the lateral end of the dorsal
venous network overlying the anatomical snuffbox, ascending the
lateral and anterolateral aspect of the arm to the deltopectoral
groove, piercing the clavipectoral fascia to join the axillary vein.
Basilic vein.• Commences from medial end of the dorsal venous
network, ascending along medial and anteromedial aspect of
forearm, piercing the deep fascia to join the venae comitantes of
the brachial artery which eventually join the axillary vein.
Median cubital vein.• Connects these two veins in the cubital
fossa.
• Deep system: venae comitantes of ulnar, radial, and brachial artery,
which fl ow into the axillary vein.
• Most common sites for phlebotomy and cannulation are:
Dorsal venous network.•
Median cubital vein.•
• Cephalic vein in the forearm.

CHAPTER 4 Practical procedures
194
Intravenous cannulation
Key facts
A similar skill to that of simple venepuncture, but needs plenty of practice
to become competent. If having diffi culty, observe a few experts in action;
an ideal setting is in the anaesthetic room of theatres.
Indications
Venous access for administration of IV fl uids, blood, or IV drugs.
Equipment
• Tourniquet.
• Cannula (20G or 18G) (see Table 4.1).
• Adhesive dressing/tape.
• Alcohol swabs.
• 5mL syringe containing 0.9% saline or heparinized saline.
• IV fl uid bag with giving set, if necessary.
Preparation
Apply tourniquet above or below the elbow and inspect the arm for suit-
able engorged veins.
Method
• Clean the skin thoroughly at the site of access; put on sterile gloves.
• Identify a suitable vein.
• Tether the skin distal to the proposed site of puncture.
• Pass the cannula obliquely through the skin at a point approximately
2mm distal to the point you wish to enter the vein.
• Advance the cannula smoothly until the vein is entered: a ‘give’ will be
felt and a ‘fl ashback’ seen in the hub of the cannula.
• Hold the hub of the needle with one hand and advance the cannula
into the vein, while maintaining skin fi xation until the cannula is well
into the vein.
• Remove the tourniquet and press on the vein proximal to the cannula as
the needle is removed. Apply the screw cap to the end of the cannula.
Table 4.1 Size and function of different cannulae
Colour Size Flow (mL/min) Use
Blue 22G 31 Small veins,
paediatrics
Pink 20G 55 Slow infusions
Green 18G 90 IV fl uids, drugs,
transfusions
White 17G 135
Grey 16G 170 Rapid IV fl uids,
emergencies
Brown 14G 265

INTRAVENOUS CANNULATION
195
• Secure the cannula in place with a dressing.
• If the cannula is not going to be used immediately, fl ush with
heparinized saline.
Tips and pitfalls
• Poor veins, obese patients, and failed attempts. See ‘Venepuncture’
section (see b p. 192).
• Agitated or fi tting patients. Try not to place the cannula over a joint as
these tend to become easily dislodged or ‘tissued’.
• Secure the cannula. Cannulae are all too easily dislodged because of
poor fi xation to the skin. Use of two cannula dressings (one placed
above and one below) and a bandage is often needed.
• Hairy arm. Shaving the skin at the planned cannula site seems tedious,
but will allow the cannula to be secured adequately.
• Non-dominant hand. Placing the cannula in the non-dominant hand,
if possible, will allow the patient a little bit more freedom and may
prevent the cannula becoming dislodged easily.
• Fragile veins. Tends to be a problem in elderly or debilitated patients.
Try using a smaller cannula; the dorsum of the hand is often ideal site.
• Poor peripheral access. In some patients with multiple collapsed or
damaged veins, alternative cannula sites may have to be considered,
e.g. feet. If peripheral cannulation becomes impossible, a central line
will have to be considered.
• Blood transfusion. If blood is being given IV, then an 18G or 16G
cannula will be needed.
Complete failure to cannulate
• Is a cannula necessary?
Can IV medication or fl uids be omitted until elective central/long •
line insertion is possible?
Can medication or fl uids be given orally or via NGT?•
Discuss with microbiology if antibiotics are involved: changing route •
of administration often requires appropriate changes in antibiotic.
Fluid and insulin regimes can be modifi ed to be given •
subcutaneously if desperate.
Many painkillers and antiemetics can be given PR or IM.•
• Ask another member of your team to try: sometimes a ‘fresh’ pair of
hands is all that is needed.
• If no one in your team can site the cannula, ask the on-call anaesthetist
if they can help, but remember they are not a cannulation service and
do not ask them until you have asked every member of your team
unless it is an emergency!
• If peripheral access is impossible or required for a long time (e.g. IV
antibiotic regimes for infected prostheses), consider:
Elective PICC line insertion (long-term line inserted electively by •
specialist nurse into the basilic vein);
Elective central line insertion: this should be done in an anaesthetic •
room rather than on ward and during daytime hours (b p. 198).
Femoral line insertion (less ideal as this site is more prone to line •
sepsis).

CHAPTER 4 Practical procedures
196
Arterial puncture and lines
Key facts
Arterial puncture is needed to sample ABGs; if serial measurements are
required or continuous monitoring of arterial blood pressure is needed,
then an arterial line should be sited (see b p. 128).
Equipment
• Arterial puncture only requires a 22G needle on the green 5mL blood
gas syringe, an alcohol swab, and cotton wool.
• Arterial line insertion equipment generally comes prepacked in sterile
kits, but if unavailable, you will need:
Two 20G arterial cannulas with guidewire.•
Connectors, transducer, and three-way tap.•
2mL 1% lidocaine.•
5mL syringe and blue needle.•
10mL saline.•
Skin prep, sterile gloves, small drape.•
Gauze swabs.•
Preparation
• Explain the procedure to the patient if appropriate.
• It is good practice to perform Allen’s test (see below) to demonstrate
that the ulnar arterial supply to the hand arcades is intact.
• For radial artery cannula insertion, place the forearm on a pillow so
that the wrist is dorsifl exed; for femoral artery insertion, abduct and
fl ex the hip slightly.
Landmarks
• Radial artery. Lies between tendon of fl exor carpi radialis and head of
radius.
• Femoral artery. Lies midway between the anterior superior iliac spine
and the symphysis pubis.
Technique
• Prepare and check equipment and prep skin; put on sterile gloves.
• Infi ltrate local anaesthetic in the skin, but avoid distorting the anatomy.
• Palpate pulse between two fi ngers for 2–3cm.
• Pass cannula at 45° into skin.
• Once the cannula is in situ, aspirate and fl ush via the three-way tap.
Transfi xion technique (see Fig. 4.3)
The cannula is passed through both artery walls, the needle completely
withdrawn, and the cannula then withdrawn slowly until fl ashback occurs,
at which point it is advanced into the artery.
Partial transfi xion technique
The cannula is advanced until fl ashback stops and the needle withdrawn
while holding the cannula steady which is then advanced into the artery.
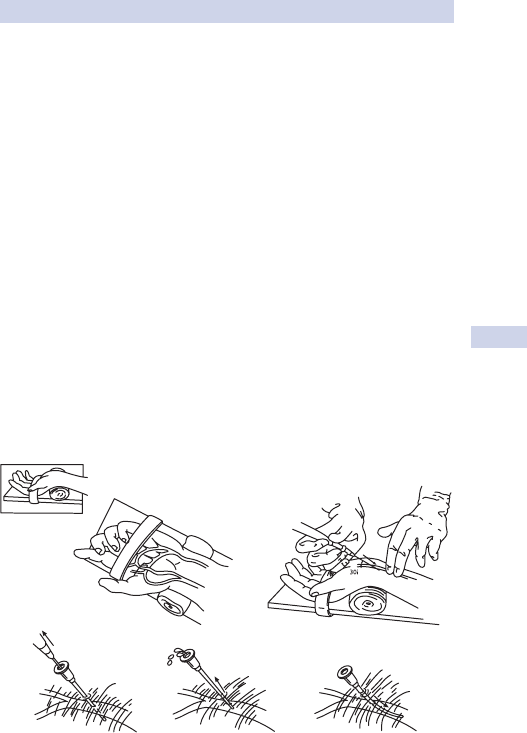
ARTERIAL PUNCTURE AND LINES
197
Artery not transfi xed
The cannula is advanced carefully in 0.5mm increments until fl ashback is
seen, at which point the catheter is slid off the needle in the artery.
Guidewire
A guidewire is useful where it is possible to get fl ashback, but diffi cult to
advance the catheter up the artery.
Complications
Ischaemia, thrombosis, bleeding, damage to radial and median nerve.
Inadvertent intra-arterial injection of drugs.
Allen’s test
Allen’s test demonstrates a patent palmar collateral circulation: the patient
clenches his fi st to exclude blood from palm and the doctor fi rmly com-
presses both ulnar and radial pulses while patient opens his palm, which
should be blanched. The doctor releases the ulnar compression whilst still
occluding the radial pulse: the palm becomes pink in <5s if there is good
collateral supply from the ulnar artery (see b p. 647). About 3% of people
do not have a collateral palmar supply and hand ischaemia is a real risk if
the radial artery is cannulated.
Fig. 4.3 Transfi xion technique of arterial cannula insertion.

CHAPTER 4 Practical procedures
198
Insertion of central venous catheter
Key facts
Indications are listed on b p. 128. Cannulae can be single or multi-lumen,
sheaths (for insertion of pulmonary artery (PA) catheters and pacing
wires), tunnelled, or long lines.
Equipment
• Appropriate central venous (CV) catheter.
• Ultrasound probe and condom if ultrasound is to be utilized.
• Enough three-way taps for all individual lumens.
• 10mL 1% lidocaine.
• 10mL syringe.
• Blue needle and a green needle.
• 20mL saline.
• 2 or 3/0 silk on a large handheld needle.
• 11-blade scalpel.
• Skin prep, sterile drape, sterile gloves, and gown.
• Gauze swabs, dressing.
Preparation
• Explain the procedure to the patient if appropriate.
• Ask a nurse to be present.
• Patient’s ECG and pulse oximetry should be continually monitored.
• Ensure that there is adequate light, a space behind the bed which you
can work in, and that it is possible to place the bed in Trendelenberg.
Landmarks
Internal jugular vein
• Central approach. Apex of triangle formed by clavicular and sternal
heads of sternocleidomastoid (SCM) muscle, aiming the needle
towards the opposite nipple.
• Posterior approach. Point where line drawn horizontally from the
cricoid cartilage to the lateral border of the clavicular head of SCM,
aiming the needle towards the sternal notch.
• Anterior approach. Medial border of the sternal head of SCM, aiming
needle towards ipsilateral needle.
Subclavian vein
Advance the needle at 45° to the junction of the outer and middle third of
the clavicle 1–2cm, then direct needle towards sternal groove.
Technique
There are numerous techniques: only one is described below.
• Prep the patient, gown, and glove.
• Drape so that all landmarks are exposed.
• Palpate the carotid pulse.
• Infi ltrate local anaesthetic around the planned puncture site.
• Spend 2–3min laying out the equipment in the order of use, secure
three-way taps to central line, and turn to closed position.
• Ask the nurse to place the bed in 10–20° of Trendelenberg.

INSERTION OF CENTRAL VENOUS CATHETER
199
• Ballot the internal jugular vein.
• Using aseptic technique and a 20G catheter on a 10mL syringe, enter
the skin at 45° as described in ‘Landmarks’ section (see b p. 196).
• On aspirating venous blood, remove the syringe and needle, but leave
the catheter in situ: check that the puncture is venous, not arterial, by
attaching manometry tubing, letting it fi ll with blood, and holding it
up—level should fall if venous.
• Pass the guidewire down the catheter, keeping hold of it at all times.
• Once an adequate length of wire is in place, remove the catheter over
the wire, and apply pressure to the vein.
• Make a 3mm nick in the skin over the wire with a scalpel.
• Pass the dilators over the wire through the skin, but not into the vein.
• Remove the dilators, apply pressure, and pass the CV cannula over the
wire into the vein up to an appropriate length.
• The wire normally protrudes through the brown (proximal) lumen of a
triple lumen line which should therefore be left open.
• Aspirate, fl ush, and close all lumens and suture the catheter to the skin.
• Check that there is a satisfactory pressure trace if a transducer is used.
• Chest X-ray (CXR) to identify pneumothorax.
Complications
Immediate
Damage to nearby structures (carotid artery puncture, pneumothorax,
haemothorax, chylothorax, brachial plexus injury, arrhythmias), air embo-
lism, loss of guidewire into right side of heart, haematoma.
Late
Sepsis, thromboembolism, arteriovenous (AV) fi stula formation.
Key revision points—anatomy of the internal jugular vein
• In the upper neck, the internal jugular vein may be cannulated as it
lies within the carotid sheath. The important relations here are:
Sheath is just anterior to the anterior border of SCM.•
Carotid artery is anteromedial.•
Vagus nerve lies between the two.•
• In the lower neck, the internal jugular vein may be cannulated as it
lies behind the SCM. The important relations are:
Vein lies 45• ° lateral and 45°
inferior to the junction of the sternal
and clavicular heads of the SCM.
The pleural lies inferomedial.•
The subclavian artery lies lateral.•
• The internal jugular drains into the brachiocephalic vein; on the right,
this is shorter and drains more vertically into the superior vena cava
(SVC), making the internal jugular vein cannulae easier to pass into
the SVC.

CHAPTER 4 Practical procedures
200
Chest drain insertion
Key facts
There are three main options for most patients with pleural effusions or
pneumothoraces that need intervention:
• Needle thoracentesis. Used for fi rst-time treatment of simple effusions
or pneumothoraces with low likelihood of recurrence.
• Pigtail thoracostomy. A 16G tube inserted using modifi ed Seldinger
technique; good for simple pneumothorax or effusion.
• Chest tube. Large bore tube inserted, either blunt (recommended) or
using trocar to treat tension pneumothorax, recurrent pneumothorax,
haemothorax, or empyema. Indications are listed below.
Indications (British Thoracic Society guidelines)
• Pneumothorax.
In any ventilated patient.•
Tension pneumothorax after initial needle relief.•
Persistent or recurrent pneumothorax after simple aspiration.•
Large secondary spontaneous pneumothorax in patients over 50y.•
• Malignant pleural effusion.
• Empyema and complicated parapneumonic pleural effusion.
• Traumatic haemopneumothorax.
• Post-operative, e.g. thoracotomy, oesophagectomy, cardiac surgery.
Equipment
• 28G intercostal drain.
• Underwater seal containing water to up to mark.
• Connection tubing.
• Line clamp.
• Roberts or other instrument for blunt dissection.
• 20mL 1% lidocaine.
• 10mL syringe.
• Blue needle and a green needle.
• 20mL saline.
• 2 or 3/0 silk on a large handheld needle.
• 11-blade scalpel.
• Skin prep, sterile drape, gloves and gown, gauze swabs.
Preparation
• Explain the procedure to the patient if appropriate; recheck side on
X-ray and sign consent form.
• Ensure continual monitoring of pulse oximetry.
• Position the patient at 45° with the arm abducted.
Technique
• Usual insertion site is the 5th intercostal space in the mid-axillary line.
• It may extend anteriorly to the anterior axillary line.
• Prep and drape the skin, gown, and glove.
• Infi ltrate site for tube insertion with local anaesthetic, ensuring
anaesthesia at all layers down to and including parietal pleura and the
periostem of the ribs posterior to the line of the incision.
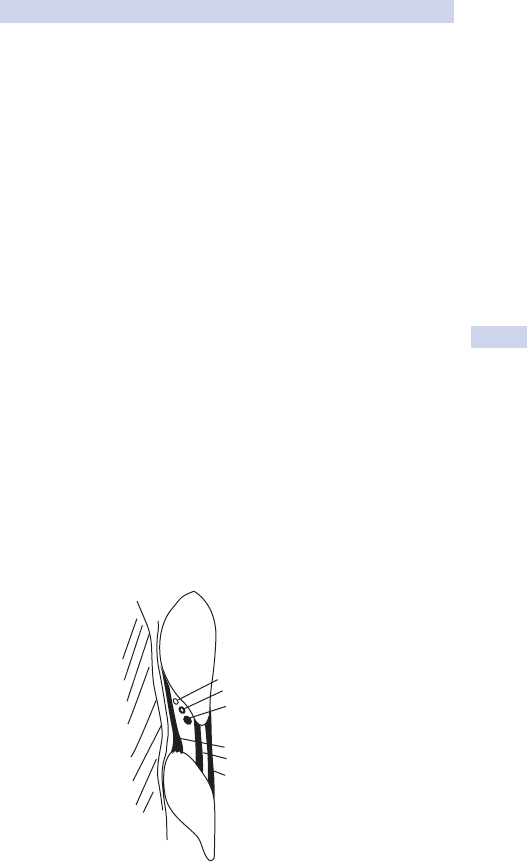
CHEST DRAIN INSERTION
201
• A 2cm transverse skin incision is made and the intercostal space (see
Fig. 4.4) is dissected bluntly.
• Place purse string suture and suture to secure drain now.
• Firmly and carefully pass a blunt-ended clamp over the lower rib
through the pleura (you will feel a pop as the tissue gives) and spread
to widen the hole.
• Place a fi nger into the pleural space to ensure there are no adhesions.
• Pass a chest tube without a trocar into the pleural space, guiding it
superiorly for a pneumothorax and basally for a haemothorax.
• Secure drain with at least one strong suture and connect immediately
to an underwater seal and place on –20mmH
2
O suction.
Tips and pitfalls
• Misplacement. Subcutaneous (more common in obese patients),
intraparenchymal; always check for an air leak on coughing and a swing
to confi rm that the chest tube is in the pleural space, particularly if no
effusion draining.
• Trauma to other structures (diaphragm, spleen, liver, heart, aorta, lung
parenchyma, intercostal arteries). Entry sites too low (common mistake,
remember you are much less likely to cause damage if you are too
high than if you are too low), too posterior or trocar used instead of
blunt dissection. Stay on the top of the lower rib to avoid injuring the
intercostal artery and causing a haemothorax.
• Surgical emphysema. Implies there is massive air leak not being drained
effectively by the chest tube. Is the tube blocked, kinked, pulled out so
that holes are communicating with skin, in too far so that it is wedged
in fi ssure, in the subcutaneous tissue rather than the pleura?
• Wound infection, empyema.
• Pain.
Rib
Vein
Artery
Nerve
Innermost
Internal
Intercostal
muscles
External
Visceral
pleura
Lung
Parietal
p
leura
Fig. 4.4 Anatomy of the intercostal space.

CHAPTER 4 Practical procedures
202
Management of chest drains
Technique of insertion is described on b p. 200. This section describes
the types of chest drainage systems available and basic protocols for man-
aging chest drains.
Types of drainage system
Underwater seal
Underwater seal drains used to consist of three bottles connected by
tubing, the third bottle providing suction control determined by the depth
the connection tube penetrated below the water level in the bottle. Now
most hospital wards have reliable high volume, low pressure wall suction,
which means that simple, lightweight, single underwater seal bottles can
be used instead of the cumbersome three-bottle systems. The system has
to be kept upright.
• Underwater seals are suitable for any condition requiring chest
drainage.
• They can be used with or without suction.
• Suction is usually –2–5kPa.
Heimlich valves
The Heimlich valve is a one-way fl utter valve within rigid tubing. It can be
connected to a standard chest drain. The system allows air and fl uid out
of the chest cavity, but prevents both from entering. The system has to be
open to air, which makes collecting liquid effl uent more diffi cult.
Heimlich valves are usually considered in patients with a permanent air
leak for whom surgery is not appropriate and for whom the main goal of
therapy is discharge to home or palliative care.
Portex bag
• The Portex bag was designed as an ambulatory chest drainage system.
It consists of a Heimlich valve within a drainage bag which has a
capacity of about 1500mL and can be emptied intermittently. This
drainage system cannot be connected to suction.
• These drains are indicated in patients with chronic pleural collections,
in whom surgery is not appropriate.
• As the systems are airtight, an air leak is a contraindication.
Suction
Almost all conditions can be safely managed by an underwater seal system
without suction, but suction helps to reinfl ate the acutely collapsed lung
and improves drainage of fl uid. There is a huge range in surgeons’ prefer-
ences for suction protocols: the points below represent commonly used
protocols.
• 1kPa = 7.5mmHg = 10cmH
2
O.
• Suction should be high volume, low pressure: approximately 2–3kPa.
• Blocked suction tubing or a blocked fi lter at the wall is the equivalent
of clamping the drain; have a low threshold for suspecting either.
• Most patients with chest drains should be on suction; the exceptions
to this are patients with pneumonectomies who are not placed on
suction.

MANAGEMENT OF CHEST DRAINS
203
• Ventilated patients cannot generate their own negative intrapleural
pressures and therefore all chest drains in these patients, with the
exception of post-pneumonectomy drains, should be placed on
suction.
• It is usually safe for a patient with an underwater seal on wall suction
to mobilize off suction for brief periods.
• Discontinue suction in extubated patients after 24–48h when the lung
is fully infl ated on CXR and there is no air leak (the drain does not
bubble when the patient coughs).
• Suction is unlikely to secure expansion in the lung that has been
collapsed chronically; it is most effective in the immediate post-
operative period.
Clamping drains
More patients have died as a result of clamped drains than unclamped
drains. The practice of clamping chest drains during transfer is a danger-
ous one. It reveals a failure to understand how a modern underwater seal
drain works as well as refl ecting outmoded practice that dates back to the
time of TB when drain bottles contained caustic sterilizing fl uid that could
drain back into the patient if lifted above the level of the chest during
transfer. The only indications for clamping a chest drain are below.
Post-pneumonectomy
The post-pneumonectomy chest drain is usually clamped for an hour at
a time and unclamped briefl y to allow blood to drain. Leaving the drain
unclamped risks causing mediastinal shift towards the pneumonectomy
side and cardiovascular compromise. The drain is usually removed on day
1 post-operatively.
Massive haemothorax or effusion
If more than 1500mL of fl uid is drained immediately on insertion of a
chest tube and/or the patient appears haemodynamically compromised as
a result of drainage, it is appropriate to clamp the drain for a brief period.
In massive haemothorax, the effect is to attempt to tamponade the bleed,
buying a little time to organize surgical exploration. In a massive effusion,
this allows time for the lung to expand without re-expansion pulmonary
oedema and to reduce mediastinal shift caused by rapid drainage.
Decision making in long-term drains
Occasionally, a surgeon may decide to see if a patient with a chronic
effusion or air leak can manage without a drain by clamping the drain.
Tension pneumothorax may result from doing this in a patient with an air
leak. Such patients must be observed frequently for any sign of respira-
tory or haemodynamic compromise, surgical emphysema, or radiological
evidence of lung collapse, and if any of the above occur, the drain must
be unclamped and placed on underwater seal. If the patient tolerates the
clamp for 24h, it is usually possible to remove the drain.

CHAPTER 4 Practical procedures
204
Pericardiocentesis
Key facts
• Occasionally used, usually by cardiologists under fl uoroscopic
guidance, to relieve acute pericardial tamponade.
• There is almost no indication, outside emergencies in an under-
equipped setting, to perform this procedure blindly without
fl uoroscopic or echo guidance.
• In an emergency due to trauma where tamponade is due to
active bleeding, clots will prevent effective needle aspiration and a
thoracotomy, sternotomy, or subxipoid incision, depending on the
circumstances, should be performed.
Equipment
• Pericardiocentesis needle or catheter.
• 10mL 1% lidocaine.
• 10mL syringe.
• 18G catheter.
• 20mL saline.
• 2 or 3/0 silk on a large handheld needle.
• 11-blade scalpel.
• Skin prep.
• Sterile drape.
• Sterile gloves and gown.
• Gauze swabs.
Preparation
• Explain the procedure to the patient where appropriate.
• Ensure patient has continual ECG monitoring.
Landmarks
One half centimetre below and to the left of the xiphoid, aiming at 45° to
skin, pointing at left shoulder or nipple.
Technique (see Fig. 4.5)
• Prep and drape the skin, gown, and glove.
• Infi ltrate 5mL 1% subcutaneous lidocaine and make a nick in the skin.
• To begin, identify the needle entry site 0.5cm immediately to the left
of the xiphoid tip.
• Insert the catheter, applying continuous aspiration in the direction
described above.
• After needle entry into the skin and sc tissue, watch the ECG monitor
(or echo/fl uoroscopic screening monitor if available) as the needle is
slowly advanced; if there are ectopics or changes in the ST segments,
stop and withdraw the needle a few mm.
• When in contact with the pericardium, advance the needle a few cm
into the pericardial space.
• If ST segment elevation is present, this indicates contact with the
myocardium and the needle should be withdrawn slightly into the
pericardial space where no ST segment elevation should be seen.
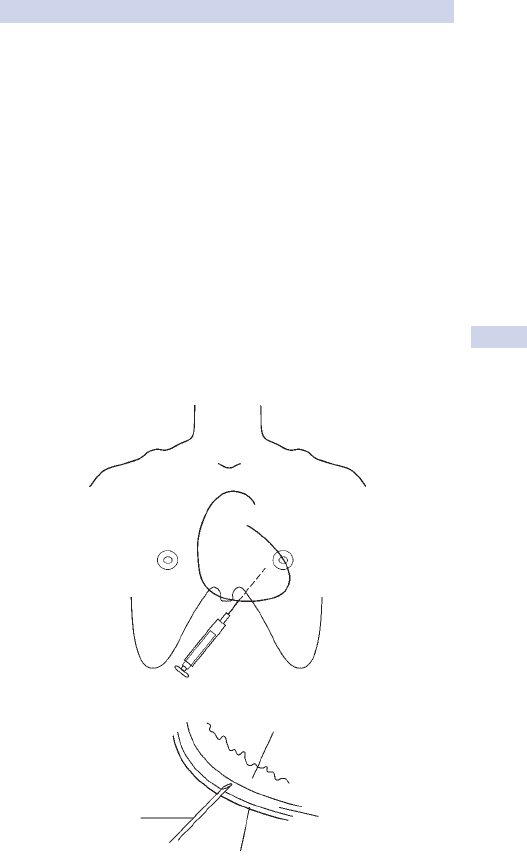
PERICARDIOCENTESIS
205
• When in the pericardial space, withdraw needle from catheter and
aspirate fl uid.
• If the tamponade is successfully reduced, right atrial pressures should
be decreased, cardiac output should increase and pulsus paradoxus
should disappear.
• Echocardiography, normally transoesophageal, is required to show
reduction in the size of the collection and improvement in the signs of
tamponade such as compression of right atrium and ventricle.
• Clotted blood cannot be evacuated in this way; a patient with
tamponade from a haemopericardium needs emergency surgical
evacuation, usually via a sternotomy if trauma is suspected.
Complications
• Cardiac puncture.
• Laceration of a coronary artery.
• Air emboli.
• Cardiac arrhythmias.
• Haemothorax.
• Pneumothorax.
• Infection.
(a)
(b)
Myocardium
Fluid
Pericardum
Needle
Fig. 4.5 Technique of pericardiocentesis. (a) Landmarks for needle.
(b) Pericardiocentesis.

CHAPTER 4 Practical procedures
206
Cricothyroidotomy
Indications
Emergency need for a surgical airway
• Major maxillofacial injury.
• Oral burns.
• Fractured larynx.
• Need for tracheal toilet in the extubated patient.
Needle cricothyroidotomy
• Patient peri-arrest.
• Use the landmarks described below.
• Omit local anaesthetic infi ltration, cut-down, and dissection.
• Pass a 12G (brown or larger) needle directly though the cricoid
membrane.
• Oxygenate using jet insuffl ation until a formal airway can be established.
Equipment
• Minitracheostomy, size 6.0 ET tube or 12G cannula in emergencies.
• Artery forceps.
• 10mL 1% lidocaine.
• 10mL syringe.
• Blue needle and a green needle.
• 20mL saline.
• 2 or 3/0 silk on a large handheld needle.
• 11-blade scalpel.
• Skin prep.
• Sterile drape.
• Sterile gloves and gown.
• Gauze swabs.
Preparation
• Explain procedure to the patient where appropriate.
• The trauma patient’s C-spine should be immobilized in the neutral
position.
Landmarks
The cricoid membrane is a small diamond-shaped membrane, palpable just
below the prominence of the thyroid cartilage.
Technique (see Fig. 4.6)
• Prep and drape, put on sterile gloves.
• If the patient is conscious and maintaining their own airway, infi ltrate
local anaesthetic using aseptic technique.
• Stabilize the thyroid cartilage with the left hand.
• With your right hand, make a 2cm transverse incision (smaller for
minitracheostomy) through the skin overlying the cricothyroid
membrane and then straight through the cricothyroid membrane.
• Now turn the scalpel blade 90° within the airway so that it acts as a
temporary retractor.
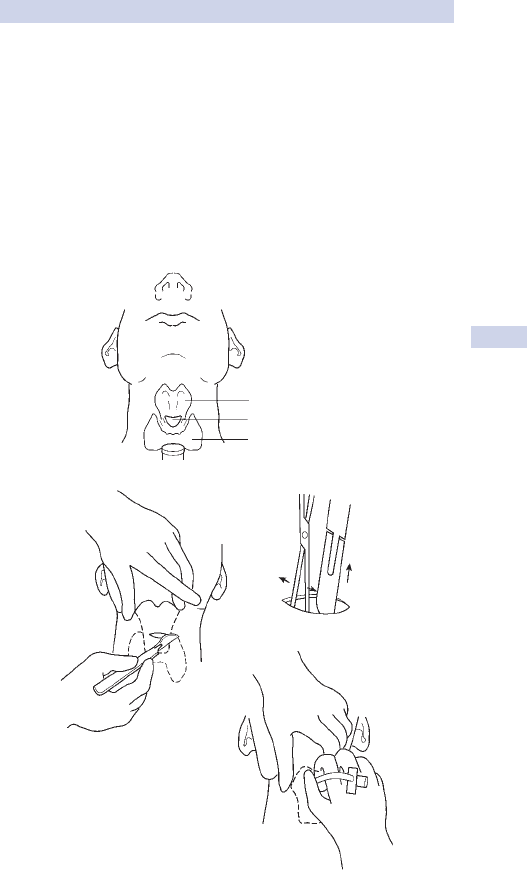
CRICOTHYROIDOTOMY
207
• Place an artery forceps through the incision and open it, remove the
scalpel, and insert a size 6.0 ET tube.
• Suction the tube, secure, and connect to a source of oxygen.
• Some minitracheostomy kits use the Seldinger technique; aspirating air
freely is a sign that the needle is in the trachea and that a guidewire
can be gently passed down the lumen.
Complications
• Bleeding.
• Loss of airway.
• Recurrent laryngeal nerve injury.
• Vocal cord injury.
(a)
)c()b(
(d)
Cricoid cartilage
Cricoid membrane
Thyroid gland
Fig. 4.6 Technique of cricothyroidotomy. (a) Structures involved. (b) Incision.
(c) Keeping cricothyroidotomy patent. (d) Inserting mini-thyroidostomy.

CHAPTER 4 Practical procedures
208
Nasogastric tube insertion
Key facts
• Nasogastric tubes (NGT) are used to decompress the stomach and to
administer enteral feeding and drugs in patients that cannot manage
oral intake. Enteral feeding is covered on b p. 136.
• Inadvertent placement of an NGT into the bronchial tree can cause
aspiration pneumonia or even respiratory arrest if it is then used
to administer feeds or other fl uids; placement must always be
systematically checked (see b p. 209) on a CXR prior to use.
• Never replace an NGT in an oesophagectomy patient without
discussing it with a senior; you risk pushing the tube through the fresh
anastomosis.
• It is worth learning how to place these; usually you will get a call when
the person who normally places them has failed.
Indications
• Intestinal obstruction (wide bore or Ryle’s tube).
• Paralytic ileus.
• Perioperative gastric decompression.
• Enteral feeding (fi ne bore tube).
Equipment
• NGT (sizes 10–12 French).
• Gloves.
• Lubricating gel.
• Lignocaine throat spray.
• NG collection bag.
• Litmus paper.
• Stethoscope.
• Sticky tape.
Preparation
• Chill NGT in fridge prior to passing. This stiffens the tube and makes it
easier to pass.
• Explain the procedure to the patient where appropriate.
• Position the patient, preferably in a sitting position, with the head tilted
slightly forward.
Method
• Wash hands and put on sterile gloves.
• Lubricate the tip of the NGT with gel.
• Pass the tube horizontally along the fl oor of the nasal cavity, aiming
towards the occiput.
• As the tube engages in the pharynx, ask the patient to swallow and the
tube should pass into the oesophagus.
• Some advise getting the patient to take a sip of water, hold it in their
mount while you introduce the tube and then swallow; this introduces
an aspiration risk and many patients are not able to cooperate to this
extent because of pain, nausea, confusion, etc.

NASOGASTRIC TUBE INSERTION
209
• Advance the tube approximately 40–60cm.
• Check the position of the tube as follows:
Aspirating gastric contents which will turn blue litmus red; • and
Insuffl ate 20mL air down the tube; if in stomach, should produce •
bubbling which can be heard on auscultation over the stomach; and
All feeding tubes • must be X-rayed prior to use to exclude
inadvertent bronchial intubation; you must be able to follow the
NGT all the way down to the fundus of the stomach on the CXR.
Always double-check you cannot see the tube in the bronchial
tree or pleura. This is the only true confi rmation that NGT is in
stomach.
• Tape tube securely to nostril and attach end to bag/suction.
Tips and pitfalls
• Patient has problems swallowing. Ask the patient to swallow sips of
water as the tube is passed.
• Constant coiling in the mouth. Tube may be soft; cool in the fridge.
• Resistance to passing. There may be an anatomical reason for this, e.g.
oesophageal stricture. The tube may need to be passed under X–ray
control.
• Tube migration. Just because the tube was in the correct position
yesterday does not mean it is today; patients pull at these, work them
out of the oesophagus with their tongue into a coil at the back of
the throat. If called to assess, always look at the back of the pharynx
(‘Open your mouth and say Ah’) and get a CXR.
• Aspiration of tube feeds. In the hypoxic or obtunded patient on NGT
feeds, think of aspiration. Stop the feeds. Sit the patient up and give O
2
.
Assess the tube position. If you suspect aspiration (tube feeds visible in
mouth, coughing up feeds, tube in bronchus on CXR), call for senior
help; the patient may need a bronchoscopy and/or intubation.

CHAPTER 4 Practical procedures
210
Urethral catheterization
Key facts
• Foley catheters are useful to monitor urine output hourly (renal
failure, fl uid balance) and in immobile patients.
• Catheterization of female patients is usually performed by nursing staff;
it is useful to learn the technique as you will be asked to try if they fail!
Indications
• Perioperative monitoring of urinary output.
• Acute urinary retention.
• Chronic urinary retention.
• Aid to abdominal or pelvic surgery.
• Incontinence.
Male catheterization
Equipment
• Foley catheter (size 12–20G, 14G most commonly used).
• Dressing/catheter pack containing drapes.
• Cleansing solution, sterile gloves (two pairs).
• Lidocaine gel.
• Gauze swabs, drainage bag and/or universal specimen pot for
midstream urine (MSU).
Preparation
• Consent the patient, explaining the procedure.
• Lay patient supine.
• Expose the genital area and cover with a sterile drape with a hole in it.
Method
• Clean hands and put on sterile gloves.
• Pick up the glans penis with your non-dominant ‘dirty’ hand through
the hole in the drape; the other hand will be your ‘clean’ hand.
• Holding a swab soaked in sterile saline with your clean hand, retract
the foreskin and clean the urethral orifi ce and glans thoroughly so your
gloved fi ngers only touch the swab, not the glans penis.
• Without letting go of the penis, discard the swab and pick up the
sterile lidocaine gel with your clean hand and inject into the urethra.
• Still holding the penis in a vertical position, introduce the catheter with
the clean hand and advance gently for approximately 10cm.
• Lower the penis to lie horizontally and advance the catheter fully
(through the prostatic urethra) up to the hilt.
• Infl ate the balloon now in the bladder via the smaller catheter channel
with the 10mL sterile water; some catheters have an integral bulb of
air which, when squeezed, infl ates the balloon.
• 2 NEVER infl ate the balloon until the catheter is fully inserted as this risks
infl ating the balloon within the prostatic urethra, causing urethral rupture;
ideally you should see urine before infl ating the balloon.
• Attach a catheter bag fi rmly to the catheter.
• 2 Replace the foreskin to avoid paraphimosis.

URETHRAL CATHETERIZATION
211
Tips and pitfalls
• Diffi culty identifying urethral orifi ce. Sometimes orifi ce is located in the
glans penis. If just diffi culty retracting foreskin, use plenty of gel.
• No urine immediately.
The bladder has just been emptied; insert a 2mL syringe into the •
end of the catheter and aspirate any residual urine.
The catheter tip may be blocked with lidocaine gel; try gently •
instilling 15–20mL of sterile water and gently aspirating.
• Still no urine. The patient may be anuric or a false passage may have
been created; palpate to see if the bladder is empty or if you can feel
the catheter balloon (which should not normally be palpable).
Treat anuria appropriately (see • b p. 112).
Consult a senior colleague if a false passage may have been created.•
• Inability to insert. Try a smaller catheter or a silastic (fi rmer). If
unsuccessful, ask a senior for help; suprapubic catheterization may be
needed (see b p. 212).
• Decompression of grossly distended bladder. Rapid decompression of a
distended bladder (e.g. from chronic retention) may result in mucosal
haemorrhage. Empty the bladder by 250–500mL every 30min until
empty. Then monitor urine output closely as a brisk diuresis and
dehydration may follow.
• Bypassing catheter. Usually due to catheter blockage. Check urine output,
fl ush the catheter, and observe. If urine is fl owing down the catheter and
bypassing it, the catheter may be too small; try a slightly larger size.
• Catheter stops draining. The catheter may be kinked or blocked. Flush
as above; if unsuccessful, try inserting a new catheter. Is the patient
oliguric or anuric? Treat appropriately (see b p. 64).
Female catheterization
2 In many hospitals, males are not allowed to catheterize awake females.
Check before doing do and request a female chaperone.
Equipment As for male catheterization.
Preparation Lie patient on back with knees bent. Ask the patient to place
heels together and allow knees to fall apart as far as possible.
Method
A similar technique is employed here to male catheterization, but note
the following:
• Separate the labia minora with the left hand and ensure the whole
genital area is adequately cleaned using the right hand.
• Identify the external urethral orifi ce. If this proves diffi cult in obese
patients, an assistant may help by retracting the dependent fat from
the pubic area.
• Lubricate the tip of the catheter with sterile water or lidocaine gel and
pass gently into the urethra.
Tips and pitfalls
• Diffi culty identifying urethral orifi ce. After warning the patient, place
an index fi nger in the vagina to elevate the anterior vulva. Guide the
catheter along the fi nger into the urethra.

CHAPTER 4 Practical procedures
212
Suprapubic catheterization
Indications
Urinary retention with failed or contraindicated urethral catheterization.
Cautions
• Do not perform suprapubic catheterization on a patient with known
bladder tumour or previous bladder surgery; seek expert advice.
• Ensure by clinical examination (and if available, ultrasound bladder
scanning) that the bladder is full and distended.
Equipment
• Dressing pack.
• Gloves.
• Cleansing solution.
• Two 10mL syringes.
• 25G and 21G needle.
• 10mL 1% lidocaine.
• Prepacked suprapubic catheter set (usually containing catheter, trocar,
and scalpel).
• 1/0 silk suture.
• Catheter bag.
Preparation
• Explain the procedure and consent the patient.
• Lie patient supine and expose abdomen.
• Confi rm clinically an enlarged, tense bladder.
• Identify catheterization site, 3–4cm (two fi nger breadths) above the
symphysis pubis (see Fig. 4.7).
Method
• Clean the skin thoroughly around the site and apply drapes.
• Inject lignocaine into skin and subcutaneous tissues, injecting and
aspirating in turn until urine is withdrawn.
• Two systems for introducing a suprapubic catheter are available.
‘Nottingham’ introducer (uses trocar)
• Make a 5mm incision at the identifi ed site.
• Advance the catheter, with trocar in place, through the incision and
subcutaneous tissues. A ‘give’ will be felt as the bladder is entered.
• Withdraw the trocar and ensure that there is free fl ow of urine from
the catheter.
• Infl ate the catheter balloon and suture the fl ange of the catheter to
the skin.
• Attach a catheter bag.
Bonnano (modifi ed Seldinger technique)
• Make a 5mm nick in the skin.
• Take the introducer needle and advance it, aspirating until urine is
withdrawn.
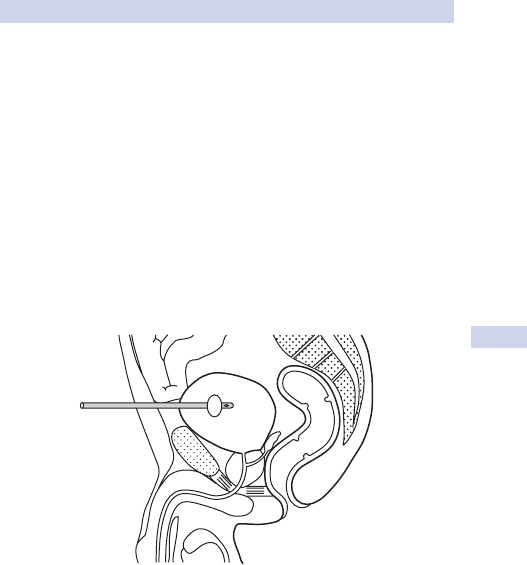
SUPRAPUBIC CATHETERIZATION
213
• Remove the syringe and pass the guidewire down the needle into the
bladder, then remove the needle, holding the guidewire in place.
• Pass the dilator fi rmly over the wire into the bladder.
• Remove the dilator and pass the catheter into the bladder, securing it
as above.
Tips and pitfalls
• Bypassing urine. With some types of catheter and trocar, urine may
initially bypass the catheter. This will cease with full advancement of
the catheter and decompression of the bladder.
• No urine or faeculent matter in catheter. Obtain help; you may have
entered the peritoneum or bowel.
Fig. 4.7 Site of typical suprapubic catheter insertion.

CHAPTER 4 Practical procedures
214
Paracentesis abdominis
Key facts
This is a useful technique in some patients for the diagnosis and manage-
ment of ascites, often in a patient with malignancy.
Indications
• Diagnostic evaluation of ascites.
• Therapeutic drainage of ascites.
Equipment
• Dressing pack.
• Gloves.
• Cleansing solution.
• 10mL syringe and 21G and 25G needles.
• 10mL 1% lidocaine.
• 60mL syringe with 16G aspiration needle for diagnostic ‘tap’.
• Bonano catheter or paracentesis catheter, three-way tap, and
collecting bag for therapeutic drainage.
• Specimen container if appropriate.
• Dressing.
Preparation
• Explain the procedure and consent the patient.
• Position the patient supine and expose the abdomen.
• Percuss out and identify the position of ascites.
• Identify a suitable tap site; the right lower quadrant is the commonest
with the patient turned semilateral to ensure the ascites fi lls this area
(see Fig. 4.8).
Method
• Prepare the skin at the appropriate site and place sterile drapes.
• Infi ltrate local anaesthetic into skin and subcutaneous tissues down to
the peritoneum. Aspirate as the needle is advanced to avoid accidental
vessel puncture.
Diagnostic tap
• Introduce the aspiration needle through the skin and subcutaneous
tissues while aspirating. A ‘give’ should be felt and fl uid freely aspirated
as the peritoneal cavity is entered.
• Withdraw 15–20mL of fl uid for a diagnostic evaluation.
• Remove the aspiration needle carefully and apply an occlusive dressing.
Therapeutic drainage
• Introduce catheter into abdominal wall until a ‘give’ is felt. Trial
aspirate with a syringe to ensure ascites returned.
• Slide catheter over the needle into the peritoneal cavity. Stop if
resistance is encountered.
• Allow up to 1000mL of ascites slowly over 1–2h.
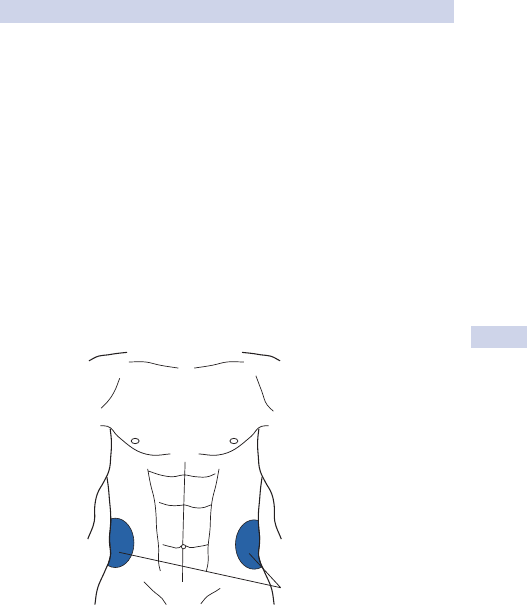
PARACENTESIS ABDOMINIS
215
Tips and pitfalls
• Unable to aspirate adequate quantity of fl uid. The ascites may be
loculated. Drainage under ultrasound guidance may be helpful.
• Blood or faeculent material. Continual staining of the ascitic fl uid
with fresh blood or any staining with faeculent material may indicate
puncture of a vessel or viscus. This is potentially serious; inform a
senior colleague.
• Peritoneal catheter. Some patients who require repeated ascitic taps
might benefi t from placement of a temporary intraperitoneal catheter
to allow daily drainage of ascites for symptomatic relief. There is a
risk of peritonitis with these devices and only a short period of use is
usually recommended, e.g. 2–3 days.
The volume of ascites drained should be closely monitored along with the
patient’s serum albumin and overall fl uid balance. A maximum drainage of
2L/day is usually advised.
Target areas
Fig. 4.8 Target areas for ascitic tap at the level of the umbilicus, 3–4cm lateral to
the mid-inguinal line.

CHAPTER 4 Practical procedures
216
Rigid sigmoidoscopy
Key facts
This is a useful skill to learn. It is usually performed in the outpatient depart-
ment as part of the investigation of lower GI complaints, but may have to be
performed on the ward, e.g. acute admissions with rectal bleeding.
Indications
• Investigation of anorectal symptoms.
• Visualization of the rectum.
Equipment
• Rigid sigmoidoscope with obturator and light source.
• Lubricating jelly.
• Gloves.
• Gauze swabs.
Preparation
• Explain the procedure and consent the patient.
• Position the patient in the left lateral position with the hips fl exed as
fully as possible and knees partially extended.
• Carry out a digital examination of the rectum to identify low-placed
lesions or faecal loading, which may prevent safe insertion or obscure
a useful view.
Method
• Lubricate the sigmoidoscope with jelly.
• With the obturator in place, introduce the scope gently through the
anal sphincter in the direction of the umbilicus for approximately 5cm.
• Remove the obturator; attach light source, insuffl ator, and eyepiece.
• Introduce small amounts of air to open up the lumen.
• Advance the instrument slowly under direct vision, ensuring that a
patent lumen is identifi ed prior to advancing the scope further.
• Note the appearance of the mucosa and the presence of any mucosal
lesions. The level of any lesion should also be noted using the marked
scale on the outer casing of the sigmoidoscope.
• If the patient experiences signifi cant discomfort, do not persist.
• Withdraw the scope slowly, again under direct vision.
• Clean the area around the patient’s anus.
Tips and pitfalls
• Biopsy. Unless experienced in the skill, do not attempt biopsy of
lesions. Note and document their position and inform a senior
colleague.
• Unable to see the upper rectum. Remember that the rectum has a
sacral curvature, often pronounced in women; GENTLY use the tip of
the scope as a ‘lever’ to push the anterior wall of the rectum forward
to open to lumen. If this isn’t easy and painless, don’t persist; it may
represent pathology.

RIGID SIGMOIDOSCOPY
217
• Rectosigmoid junction. Negotiation of the rectosigmoid junction can be
diffi cult. The best view that can be hoped for is to see the last sigmoid
fold above the junction. Do not attempt to pass the scope into the
distal sigmoid; this is the role of fl exible sigmoidoscopy.
Key revision points—anatomy of the rectum
• The rectum is said to start at the level of S2, but a distance of 15cm
from the anorectal junction is used to defi ne pathology which is
termed ‘rectal’.
• The rectum has two main angles.
The fi rst is the • acute anorectal angle which slopes posteriorly
and is formed in part by the pull of the sling of levator ani.
The second is the • sacral curvature which runs throughout the
rectum, sloping progressively anteriorly up to the level of the
rectosigmoid junction.
• Three ‘lateral valves’ are commonly described, but are only the
mucosal folds of the rectum equivalent to the colonic folds.
• The peritoneal-lined ‘pouch of Douglas’ (or rectovesical pouch in
males) extends a variable distance down the anterior wall of the
rectum. Its contents (e.g. sigmoid colon) may be easily palpable,
particularly in elderly females.
• The upper third is covered by peritoneum anterolaterally, the middle
third just anteriorly, and the lower third is entirely extraperitoneal.
• The rectum has a complete outer longitudinal muscle coat (thus
diverticular disease does not occur in the rectum).
• The rectum and associated mesorectal fat, blood vessels, and lymph
nodes are enclosed and separated from the ‘true’ pelvic organs by a
fascial sheet—the mesorectum.

CHAPTER 4 Practical procedures
218
Local anaesthesia
Local anaesthesia is used in a variety of settings and is easy to deliver. It is
essential to become familiar with the different agents, their relative merits,
and potential dangers.
Indications
• Minor procedures requiring anaesthesia, e.g. insertion of a chest drain,
CV access, suprapubic catheterization, etc.
• Excision of skin or subcutaneous lesions.
• Infi ltration of surgical wounds post-operatively.
Cautions
• Allergy. Do not use local anaesthesia if there is a history of allergy to
local anaesthetic.
• Infection at site of infi ltration. Injection may spread infection. The
effect of the local anaesthetic will be diminished (due to an acidic
environment) and injection may be more painful.
• Increased risk of toxicity. Heart block, low cardiac output, epilepsy,
myasthenia gravis, hepatic impairment, porphyria, B-blocker, or
cimetidine therapy.
• Epinephrine. Causes vasoconstriction, reducing bleeding locally and
prolonging anaesthetic effect. It should not be used for injections into
fi ngers, toes, ears, or penis (all supplied by end arteries) or where skin
fl aps are involved to reduce the chance of fl ap necrosis.
Agents The two most commonly used agents are lidocaine and bupi-
vacaine. Other agents, e.g. prilocaine, are less commonly used.
Lidocaine (previously known as lignocaine)
Used for local infi ltration for minor procedures.
• Concentrations. 0.5%, 1%, and 2%. Plain solutions (with no added
adrenaline) or solutions containing adrenaline.
• Duration of action. Rapid onset (2–3min), lasts 30–90min.
• Maximum dose.
Plain solutions• . 3mg/kg, 20mL 1% or 10mL 2% for 70kg adult.
Solutions with adrenaline• . 7mg/kg as systemic absorption is much
slower, 50mL 1% or 25mL 2% for 70kg adult.
Bupivacaine
Useful in some prolonged procedures, wound infi ltration, and regional
blocks as it has a longer duration of action than lidocaine.
• Concentrations. 0.25–0.75% plain solutions or with adrenaline.
• Duration of action. Slower onset than lidocaine; effects last 3–8h.
• Maximum dose. 3mg/kg for an adult, 2mg/kg for a child.
Equipment
• Syringe.
• Needles 21G–25G.
• Alcohol swabs.

LOCAL ANAESTHESIA
219
Preparation
• Identify site of infi ltration and check for any sign of infection or
obvious subcutaneous blood vessels.
• Calculate maximum dose of anaesthetic for each individual patient.
• Draw up anaesthetic and check details of drug and dose.
Method
• Clean area with alcohol swabs.
• Inject anaesthetic slowly with a fi ne needle to area required, aspirating
before each delivery to prevent accidental IV injection.
• Injecting local anaesthetic in a fan-shaped area subcutaneously from a
single injection is often more comfortable for the patient.
• Field block. Injecting anaesthetic into the tissues surrounding the
area which is to be anaesthetized (e.g. a cutaneous lesion) will often
produce a fi eld block, including the area itself.
Toxicity
This is caused by an overdose of local anaesthetic with systemic absorp-
tion or by accidental IV injection.
Symptoms and signs
• Neurological. Drowsiness, confusion, slurred speech, light-headedness,
tinnitus, numbness of tongue or mouth, convulsions, and coma.
• Cardiovascular. Early tachycardia and hypertension, late bradycardia,
hypotension, cardiac arrhythmias, and cardiac arrest may ensue.
• These features usually will occur at a peak of 10–25min after
subcutaneous injection, but occur immediately with IV injection.
Treatment
• Stop procedure.
• Maintain the patient’s airway and provide oxygen.
• Ensure IV access.
• Perform an ECG.
• Convulsions. Diazepam 5–10mg IV, slowly.
• Hypotension. Raise end of bed and initiate IV fl uids.
• Bradycardia. Usually resolves, atropine is rarely needed.
Tips and pitfalls
• 2 You are more likely to achieve good anaesthetic block with a
large volume of less concentrated local than a small volume of more
concentrated local anaesthetic; generally use 1%, rather than 2%.
• Allow 2–3min for the local to take effect; spend this time setting up
your instruments and draping the patient.
• Accidental IV injection. See toxicity section above.
• Inadequate analgesia. Infi ltrate more anaesthetic up to the patient’s
maximum calculated dose. If the patient is still not tolerating
the procedure, alternative anaesthetic methods may have to be
considered, e.g. regional anaesthesia (note maximum local anaesthetic
dose), sedation, or general anaesthetic.
• The smaller the needle and the more slowly you inject initially, the less
painful it is for the patient.
CHAPTER 4 Practical procedures
220
Intercostal nerve block
This may be a useful skill to learn, although it is usually performed by
anaesthetists.
Indications
• Pain due to fractured ribs.
• Post-thoracotomy pain relief.
Equipment
• Dressing pack.
• Skin antiseptic.
• Gloves.
• 20mL syringe and needle.
• 20mL of local anaesthetic, e.g. bupivacaine.
Preparation
The patient is positioned as for pleural aspiration (see ‘Pleural aspiration’,
b p. 635) and the site of infi ltration is identifi ed.
• Broken ribs. Medial to the site of fracture on the posterior aspect of
the chest wall.
• Post-thoracotomy. Medial to the posterior edge of the scar on the
posterior chest wall.
Method
• Ensure that the skin is prepared thoroughly with antiseptic. Drapes are
placed appropriately.
• Insert the needle and syringe containing anaesthetic through the skin,
inferior to the rib (unlike pleural aspiration) associated with the nerve
to be blocked.
• Aspirate the syringe to ensure that the needle has not entered a blood
vessel or the pleural space. If no blood or air is withdrawn, the site is
infi ltrated with 4–5mL of anaesthetic.
• This is repeated at various sites.
• Obtain a CXR to ensure a pneumothorax has not complicated the
procedure.
Note
• Multiple blocks. Ensure that the patient does not receive a toxic dose
of local anaesthetic.
• Air or blood is aspirated. Withdraw the needle slowly, get a CXR.
Mechanism of action of local anaesthetics
Local anaesthetic works by blocking Na channels in the nerve mem-
brane, preventing propagation of the action potential. Small, non-myeli-
nated pain fi bres are blocked fi rst. Large, myelinated fi bres that conduct
impulses from pressure senses are the last to be blocked.

CHAPTER 5 Head and neck surgery
222
Thyroglossal cyst, sinus, and fi stula
Key facts
• Thyroglossal cyst is a fl uid-fi lled sac resulting from incomplete closure
of the thyroglossal duct.
• Thyroglossal sinus results from persistence of the whole duct.
• Incidence <1%; ♂:♀, 1:1.
Anatomy (see Fig. 5.1)
The thyroglossal duct arises embryologically between the fi rst and sec-
ond pharyngeal pouches. It runs as a hollow tube from the foramen
caecum on the dorsal surface of the tongue, becoming a solid cord of
cells migrating through the tongue and into the midline of the neck. The
tract usually passes in front of the hyoid bone and then loops up behind
it before descending in the midline of the neck where the cells divide to
form the two lobes of the thyroid gland either side of the midline. The
duct normally atrophies in the sixth week of gestation.
Clinical features
• Usually presents in children or young adults.
• Ninety per cent present as a painless midline cyst.
• Ten per cent appear on one side of the midline, usually the left.
• Seventy-fi ve per cent appear in front of the hyoid bone and the
majority of the rest at any point to the root of the neck.
• The cyst elevates on protruding tongue if attached to hyoid or if
attached to isthmus of thyroid elevates on swallowing.
• Five per cent become infected presenting as a painful, red neck
swelling.
• Fifteen per cent have a fi stula to the skin (due to infection or
incomplete excision).
• Papillary carcinoma of the thyroglossal ductal cells is rare. Treatment is
by excision.
Diagnosis and investigations
• Ultrasound scan is investigation of choice.
• CT scan will often reveal a well circumscribed cyst related to the
midline of the hyoid bone.
• Fine needle aspiration may reveal a cloudy infected fl uid or a straw-
coloured fl uid.
Treatment
Infected thyroglossal cyst
• Majority respond to antibiotics.
• Surgical drainage if abscess formed or failure to respond to antibiotics.
• Elective excision of the cyst once acute infection has resolved.
Surgery
• Excision is recommended for most cysts.
• Remove through a transverse midline incision in a skin crease.
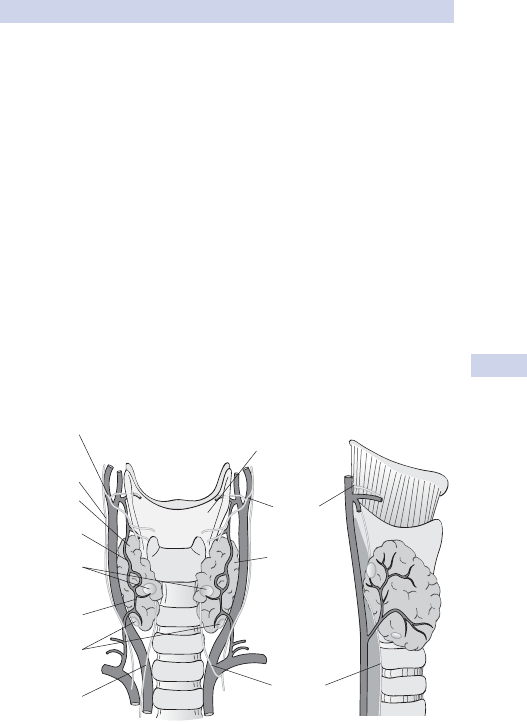
THYROGLOSSAL CYST, SINUS, AND FISTULA
223
• Divide the platysma muscle and excise the cyst using sharp and blunt
dissection.
• On the deep surface, it is attached to the hyoid bone; excise
approximately 1cm of the bone in midline, removing any underlying
thyroglossal duct epithelium. This is Sistrunk’s procedure.
• Close the wound in layers with a suction drain.
• If there is a fi stula or sinus in the neck, excise it through a transverse
elliptical incision. Again use blunt dissection and remove the middle
part of the hyoid bone (‘Sistrunk procedure’).
Complications These are usually very few. Remove the drain the next
day and discharge the patient.
The important structures that must be considered when operating on
the thyroid gland include:
• Recurrent laryngeal nerve.
• Superior laryngeal nerve.
• Parathyroid glands.
• Trachea.
• Common carotid artery.
• Internal jugular vein (not depicted).
Posterior
Lateral
Superior
Thyroid
cartilage
laryngeal
nerve
Superior
laryngeal
nerve
Vagus nerve
Recurrent
laryngeal
nerve
Recurrent
laryngeal
nerve
Superior
parathyroid
glands
Superior
t
hyroid artery
Inferior
thyroid artery
Inferior
parathyroid
glands
Right lobe of
thyroid gland
Left lobe of
thyroid gland
Fig. 5.1 The anatomy of the region of the thyroid gland. Reproduced with
permission from Longmore, M. et al. (2007). Oxford Handbook of Clinical Medicine,
7th edn. Oxford University Press, Oxford.

CHAPTER 5 Head and neck surgery
224
Branchial cyst, sinus, and fi stula
Key facts
• Disputed aetiology. Theories include:
Cystic degeneration of epithelial derivatives of the fi rst, second, or •
third branchial clefts.
Cystic degeneration of epithelial elements in a cervical lymph node.•
• A branchial fi stula is a tract running from the neck skin through to the
posterior pillar of the fauces; these are very rare.
• A branchial sinus occurs when the lower part of this tract remains
open on to the neck skin surface.
• A branchial abscess is an infected branchial cyst.
Clinical features
• Presents as a neck lump, usually painless.
• They typically present in early adulthood.
• Sixty to seventy per cent are anterior to the upper third of the
sternomastoid muscle with the posterior border lying beneath the
sternomastoid. Other sites include:
Parotid gland.•
Anterior to the lower two-thirds of the sternomastoid.•
Anterior to the pharynx.•
In the posterior triangle of the neck.•
• Two-thirds occur on the left side; 2% are bilateral.
• May present with an acute branchial cyst abscess causing pain,
increased swelling, and occasionally, pressure symptoms (diffi culty
swallowing or breathing).
Diagnosis and investigation
For branchial cyst or abscess
• Ultrasound scan is fi rst investigation of choice. CT/MRI for complex
cases.
• Fine needle aspiration biopsy:
Abscesses• . Purulent fl uid is obtained that may culture organisms.
Cysts• . Straw-coloured fl uid containing cholesterol crystals.
Treatment
Branchial abscess
• Drain via a transverse incision in the neck at the point of maximum
convexity.
• Suture a Yeates type drain.
• Give antibiotics and make no attempt to remove the cyst until the
infection has resolved completely.
Branchial cyst
• Most cysts are excised to achieve a diagnosis and prevent symptoms
or complications.
• Place a transverse incision over the cyst, preferably in a transverse skin
crease, long enough to match the size of the cyst.

BRANCHIAL CYST, SINUS, AND FISTULA
225
• Divide the platysma and the deep fascia over the anterior border of
the sternomastoid and retract the muscle posteriorly.
• Remove the cyst, usually by blunt/sharp dissection.
• Use suction drainage and close the wound in layers.
• If the cystic lesion is in the parotid gland and cannot be distinguished
from any other parotid lesion, extend a preauricular incision into the
neck as for a superfi cial parotidectomy.
Branchial fi stula
• Excise a sinus of fi stula through a horizontal elliptical incision around
the neck opening.
• Blunt and sharp dissection of sinus tract as far as possible.
• If the upper end of the tract cannot be reached, make a further
transverse incision at a higher level (‘stepladder’ incisions).
• Sometimes the tract runs between the internal and external carotid
arteries and sometimes up to the pharyngeal wall in the region of the
middle constrictor.
• Close the wounds in layers with suction drainage.
Complications
A branchial cyst at any site often lies near important nerves. Previous
infections causing fi brosis will increase the risk of damaging them. The
following nerves are at risk:
• Hypoglossal nerve (tongue deviates to affected side on protrusion).
• Mandibular branch of the facial nerve (movement of lower lip).
• Great auricular nerve (numb ear).
• Accessory nerve (paralysis of trapezius: weakness of arm abduction,
asymmetry, and chronic pain).

CHAPTER 5 Head and neck surgery
226
Salivary calculi
Key facts
• Salivary gland calculi occur most commonly within the submandibular
ductal tree (80%), 20% in the parotid.
• Composed of calcium phosphate and carbonate; may be related to
sialadenitis (infl ammation of a salivary gland).
• Most common in adults.
• No proven relationship with other calculi, e.g. renal.
Clinical features
• Pain and swelling of the affected gland on eating and drinking.
• If there is partial obstruction of the duct, the swelling can last minutes
to several hours.
• Complete obstruction leads to persistent swelling and infection.
• The patient may also experience colicky pain in the duct when eating.
Points in the examination of the submandibular gland
• Examine the gland from behind and feel the swelling by running the
fi nger backwards under the jaw. If you cannot feel a lump, ask the
patient to suck a sour sweet and re-examine them
• Examine the duct orifi ce from the front. Ask the patient to open
their mouth wide and point their tongue upwards. The ducts lie near
the midline at the root of the tongue. Are they red? Is there pus?
Can you see an impacted stone?
• Examine the gland bimanually from the front. Wear gloves and place
the fi nger of one hand over the gland. The index of the other hand is
placed in the mucosal surface of the mandible and the gland palpated
between the two.
Diagnosis and investigations
• Radiographs of the submandibular gland, parotid gland, and ducts are
helpful. Twenty per cent of submandibular and 80% of parotid calculi
are radiolucent.
Lower occlusal X-ray of the teeth will show a stone in the distal •
portion of submandibular duct.
A lateral oblique X-ray or orthopantomogram (OPT) of the •
mandible will show a calculus in the submandibular gland.
• Submandibular duct radiography (sialography) is technically diffi cult
and rarely done.
• Parotid sialography may show a fi lling defect. Sialectasis is often seen.
May provide therapeutic benefi t due to fl ushing out of debris in the
ductal tree.
• Ultrasound scanning of parotid and submandibular glands is often the
choice of investigation by head and neck radiologists.
SALIVARY CALCULI
227
Treatment
• Stones in the intra-oral part of the ducts can be removed under local
anaesthesia. Steady the stone with a Babcock’s forceps and incise
directly over it. Remove the stone; leave the duct marsupialized.
• Stones within the submandibular gland require removal of the gland
itself.
• Removal of a calculus from the parotid gland is a rare operation. Most
calculi are at the distal end of parotid duct (as it does an ‘S’ bend
through buccinator muscle) and can be released by intra-oral incision
of parotid duct papilla.
• Most parotid gland obstructive/infl ammatory disease is treated
conservatively with sialogogues and intermittent massage of the gland
towards the duct. Duct dilation using lacrimal probes is useful as most
strictures/obstruction occur at the ‘S’ portion noted above.
Key revision points—anatomy and physiology of
salivary glands
• Salivary glands produce: saliva-containing water; electrolytes
(especially K
+
and HCO
3
–
); varying amounts of mucus and enzymes.
• The parotid is a pure serous gland. It responds to salivary stimuli, e.g.
food in mouth, smell. There is little resting fl ow. The submandibular
is mixed with serous and mucous acini, responds to salivary stimuli,
and has a resting fl ow, which contributes along with sublingual and
minor glands to maintain mouth moisture.
• Saliva functions to lubricate, aid mastication, aid taste, suppress oral
bacteria, initiate starch digestion.
• Submandibular duct is palpable in the fl oor of the mouth and enters
mouth from gland on the sublingual papilla near the midline.
• Parotid duct is palpable over the anterior border of masseter and
enters the mouth on the medial wall of the cheek after passing
through buccinator muscle via an ‘S’ bend.
• The facial nerve trunk lies between the deep and superfi cial parts of
the parotid gland and divides into fi ve branches (pes anseris) within
the superfi cial portion.

CHAPTER 5 Head and neck surgery
228
Acute parotitis
Key facts
• Parotitis is infl ammation of the parotid gland. Causes include:
Acute or chronic obstruction (now commonest cause).•
Bacterial (ascending parotitis), less common.•
Viral infection, e.g. paramyxovirus (mumps), HIV.•
Infl ammatory disorders, e.g. Sjögren’s syndrome, sarcoidosis.•
Any cause of infl ammation of lymph nodes within the parotid gland.•
• Most patients develop this condition as an acute episode of a chronic
obstructive sialadenitis.
Clinical features
• Obstructive parotitis occurs more commonly in adults.
• Presents as an acutely painful preauricular swelling.
• There is often a history of recurrent, intermittent swelling of the gland.
• The gland is usually tender on palpation.
• The patient may be toxic with fever and raised WCC, and pus may
exude from the opening of the parotid duct opposite the crown of the
second upper molar tooth.
• Elderly, debilitated, dehydrated patients with poor oral hygiene or who
are on anticholinergic drugs are at greatest risk.
Diagnosis and investigations
• Plain X-rays to determine whether radio-opaque calculi are present in
the duct or gland.
• Ultrasound or CT scanning may help differentiate between stones,
infl ammation, and tumour.
• If pus is present, take a bacteriology swab and send it to the lab. The
commonest infecting organism is Staphylococcus aureus.
Treatment
Acute parotitis
• Most patients respond to antibiotics:
Give amoxicillin 500mg tds, IV if necessary.•
Rehydrate dehydrated and debilitated patients.•
Good oral nursing care with chlorhexadine mouth rinses.•
• Review patients by clinical examination after the infection has subsided
to make sure that the obstruction was not due to a parotid tumour.
• If a parotid abscess develops, it should be drained surgically:
Make an incision over the abscess under general anaesthetic where •
it appears to be pointing, parallel to the branches of the facial nerve
to avoid damaging them.
Open the abscess with sinus forceps and place a Yeates drain in the •
wound.
Recurrent parotitis
• Teach patients with recurrent parotitis to massage the gland in order
to express saliva from the duct.
• Dilatation of the duct with lacrimal probes can assist drainage.

ACUTE PAROTITIS
229
• Remove radio-opaque calculi, if possible.
• Advise the patient to keep an emergency supply of antibiotics at home.
• If recurrent parotitis persists for months or years, a total
parotidectomy is curative.

CHAPTER 5 Head and neck surgery
230
Salivary gland tumours
Key facts Salivary gland tumours are rare, accounting for 0.4% of all
malignant tumours; 80% arise in the parotid gland.
Clinical features Most patients present with a slow-growing lump in
the affected gland. Pain, paraesthesia (e.g. lingual nerve in submandibular
gland), facial palsy (parotid gland) imply malignancy. Salivary tumours of
minor glands in upper aerodigestive tract (UADT) present as a lump. Fifty
per cent of these are malignant.
Clinicopathological features
Pleomorphic adenoma
• Eighty per cent of benign parotid tumours.
• ♂:♀, 1:1.
• Peak incidence 30–50y.
• Composed of epithelial and mesothelial cells that form a mucous
matrix, often with chondromatous components.
• The tumour grows slowly and has no true capsule so that strands of
tumour cells protrude into normal surrounding tissue. Local extension
may be widespread with recurrence if excision is incomplete.
• Malignant change (adenocarcinoma) occurs in 20% after 10y and is
seen in asymptomatic deep lobe parotid tumours.
Warthin’s tumour (adenolymphoma)
• Usually affects men >50y; 10% are bilateral.
• Benign and presents as a slow-growing soft swelling.
• Successfully treated by wide local excision.
Malignant tumours
Mucoepidermoid tumour
• Low grade malignancy, though variable behaviour.
• Most grow slowly, invading locally and eventually metastasizing to neck
lymph nodes, lung, and skin.
Adenoid cystic carcinoma
• A slow growing malignant tumour with indolent behaviour.
• Perineural invasion propensity and facial palsy common with extension
through stylomastoid foramen. Lung metastasis common.
• Often regarded as incurable, but individuals can lead a normal life over
20–30y before succumbing.
• Treatment is extensive wide local excision, with nerve/organ
preservation where possible. Post-operative radiotherapy has a role.
Radiotherapy also has a role in controlling lung symptoms if they arise.
Acinic cell carcinoma
♀ > ♂; slow-growing, but may metastasize unexpectedly. Surgery is the
treatment of choice.
Squamous cell carcinoma, adenocarcinomas, and undifferentiated
carcinomas
• Generally high grade malignant tumours.

SALIVARY GLAND TUMOURS
231
• Often rapid local invasion into extraparotid tissues and infratemporal
fossa, leading to pain and trismus.
• There may be skin fi xation or ulceration with facial nerve palsy
and invasion of the external auditory canal; incurable; palliative
radiotherapy.
Diagnosis and investigations
• Clinical examination is still of great importance in assessing extent.
• CT scanning may help differentiate between stones, infl ammation, and
tumour.
• MRI scanning offers the most sensitive investigation for assessment of
local invasion and involvement of surrounding structures.
• PET CT is useful for assessing metastases.
Treatment
Benign parotid tumours
• Excise the parotid gland superfi cial to the facial nerve (superfi cial
parotidectomy). Deep lobe tumours should have a facial nerve-sparing
total parotidectomy.
• Enucleation is inadequate and often leads to local recurrence that is
diffi cult to manage.
Benign tumours in other salivary glands
Excision of the entire gland (e.g. simple submandibulectomy).
Malignant tumours
• Radical local excision (to sacrifi ce or preserve the facial nerve in
parotid tumours is controversial).
• May be accompanied by neck dissection, especially in parotid tumours.
Complications of parotid surgery
• Facial nerve injury (risk varies according to procedure: lowest in
primary surgery for benign tumours < redo surgery < surgery for
malignancy). Seventy-fi ve per cent neurapraxia with complete or
extensive recovery of function; 25% neurolysis with little or no
recovery (may be treated by nerve interposition grafting).
• Frey’s syndrome:
Late complication of surgery in up to 25% of patients.•
Facial fl ushing and sweating of the skin innervated by the •
auriculotemporal nerve when the patient salivates.
Caused in this case by division of the parasympathetic •
secretomotor fi bres that innervate the parotid gland: they may
regenerate erratically to control cutaneous secretomotor functions.
Subcutaneous botox injection is useful.•
Prognosis
• Recurrence of benign tumours. May develop 20y after surgery,
especially in the patient where enucleation, rather than superfi cial
parotidectomy has been performed.
• Five-year survival rate for all malignancies approximately 60%.
CHAPTER 5 Head and neck surgery
232
Head and neck cancer
Key facts
• Head and neck cancer refers to cancer of UADT; 90% are squamous
cell carcinomas (SCC).
• UK incidence 8–15 in 100 000 and rising. Wide geographical variation,
e.g. Indian subcontinent: 40% of all cancers.
• ♂:♀, 2:1, female incidence rising.
• Predisposing factors:
Carcinogens• . Tobacco, alcohol, betel nut chewing;
Infection• . Hyperplastic candidiasis, human papilloma virus (HPV) 16;
Extrinsic factors• . UV light in lip cancer;
Intrinsic factors• . Diet poor in fruit, vegetables, and fi sh oils,
immunodefi ciency/suppression.
Clinical features
• Peak incidence 40+y (increasing incidence in younger patients).
• Persistent oral ulcer with induration, bleeding, often painful.
• Persistent oral swelling, e.g. large tonsil, unexplained loose teeth.
• Unexplained earache: common in tongue, oropharyngeal tumours.
• Dysphagia, odynophagia occur in oro/hypopharyngeal cancer.
• Hoarseness lasting >3 weeks.
• Persistent unilateral serosanguineous nasal discharge.
• Unresolved head or neck swellings of >3 weeks.
• Examination of the neck is mandatory and should include all levels of
neck lymph nodes. Bilateral nodal spread common.
• Six per cent of patients have a synchronous SCC present in the
aerodigestive tract (mouth, larynx, lungs, oesophagus).
Diagnosis, investigations, staging, assessment
• Fibre optic nasendoscopy to examine nasopharynx, base of tongue,
hypopharynx, larynx.
• Fine needle cytology for neck mass.
• Imaging. CT of head and neck and chest with MRI in selected cases.
PET CT for unknown primary tumours, metastatic disease assessment.
• Haematology, biochemistry, ECG, lung function tests as patients
usually have high comorbidities.
• Examination under anaesthetic (EUA). Measure tumour size, biopsy.
Panendoscopy to exclude synchronous tumours of UADT.
• Extraction of any diseased teeth, especially if in possible radiotherapy
treatment fi eld to prevent osteoradionecrosis.
• All patients should be seen by dietician, speech and language therapist,
clinical nurse specialist, and restorative dentist.
Treatment
• Surgery, radiotherapy ± chemotherapy, or combination of all and may
be done with curative intent or palliation.
• In TNM system, T1–4 stage is complex and depends on anatomical
site; N1–3 stage applies to all sites.

HEAD AND NECK CANCER
233
• Function and quality of life are important outcomes. Gastrostomy/
NGT feeding often required during treatment.
Treatment of primary tumour
• Approximately equal cure rate for T1, T2 tumours with surgery
or primary radiotherapy. Surgery is usually offered for oral
cancer, sometimes for T1 larynx (laser surgery). Radiotherapy ±
chemotherapy have better functional outcome in pharyngeal, posterior
one-third tongue cancers.
• Larger T3, T4 tumours involving bone/cartilage are best managed
surgically, e.g. laryngectomy, and often require adjuvant radiotherapy.
Treatment of the neck
• N0 necks may have occult nodal metastases, depending on tumour
site, e.g. >50% for pharynx, and should have either a selective neck
dissection or radiotherapy.
• Single node disease (N1) should have either a neck dissection or
radical radiotherapy.
• Bulky nodal disease (N2, N3) should have a comprehensive neck
dissection followed by radiotherapy or vice versa.
Neck dissections
These are either comprehensive or selective. Selective dissection removes
groups of nodes likely to have occult metastases. Comprehensive includes
radical neck dissection (removal of all fi ve levels of lymph nodes, acces-
sory nerve, internal jugular vein, and sternomastoid muscle) and modifi ed
or functional neck dissection:
• Type 1 preserves the accessory nerve.
• Type 2 preserves the accessory nerve and internal jugular vein.
• Type 3 preserves the accessory nerve, internal jugular vein, and
sternomastoid muscle.
Reconstruction of surgical defect
• Good functional outcome (speech, eating, swallowing) is aim of
reconstruction of surgical defect in the UADT.
• Options include:
Primary closure, e.g. small tongue tumour.•
Local fl ap, e.g. nasolabial to fl oor of mouth.•
Regional fl ap, e.g. pectoralis major to retromolar region.•
Free microvascular transfer fl aps offer great versatility, e.g. radial •
forearm for lining, fi bula for bone, anterior thigh for bulk.
Prosthesis, e.g. obturator for palatal defect.•
Prognosis
• Crude overall 5y survival is 30–40% and of those deaths, 50% die from
other causes, usually tobacco-related.
• HPV 16 positive cancers appear to have better outcome.

CHAPTER 5 Head and neck surgery
234
Facial trauma
Key facts
• Eighty-fi ve per cent of facial injuries are from assault, often with
alcohol/drugs involved; the remaining from falls, sports, road accidents,
industrial injuries.
• Ten to twenty per cent have associated head injury, 2% cervical spine
injury.
• Fracture incidence: nose > zygoma > mandible > maxilla. Panfacial
fractures indicate high energy impact or multiple blows.
Emergency situations in facial injuries
As part of 1° and 2° survey, pay special attention to:
• Airway. Severely displaced fractures, tissue swelling (which may get
worse), blood, dislodged teeth can compromise airway, especially with
associated head injury; intubate if in doubt.
• Bleeding. Profuse bleeding can occur in midface fractures or deep
tongue wounds, requiring early theatre for suturing, nasal packing/
fracture stabilization. Swallowed blood is often vomited.
• Retrobulbar bleed. May follow even minor injury. Orbital swelling can
mask it. Cardinal signs are pain, proptosis, and falling visual acuity.
Treatment is lateral canthotomy under LA, then theatre for orbital
drainage via infra-orbital incision to open ocular muscle cone; 90min
window before blindness sets in.
Key clinical examination points
• Examine the eye even if it means opening swollen eyelids: check visual
acuity. Any diplopia indicates orbital fat/muscle entrapment in orbital
complex fracture. Orbital blow-out fracture may have enophthalmos.
• Dental occlusion (bite): ask patient if bite feels normal. If not, then a
fracture is likely. Manually check continuity of mandible. Fractures in
teeth-bearing segment are compound fractures. In maxilla, grasp upper
incisor teeth and any movement suggests maxillary fracture.
• Mental nerve or infra-orbital nerve paraesthesia indicates mandibular
or orbital fl oor/zygoma fracture, respectively.
• Look for deformity, e.g. nose deviation, fl attened cheek, forehead
hollow.
Investigations
• Imaging. Plain X-rays, OPT, and PA skull for fractured mandible;
occipitomental 30°, 45° views for zygoma fracture. For complex
fractures, CT with 3D reconstruction. Coronal CT/MRI is useful in
orbital complex injuries.
• Clinical photographs as a record which may be used in court.
• Other tests, e.g. ECG, Hb, U&Es for falls in the elderly.
Treatment
• Head injuries, soft tissue lacerations, and direct trauma to the eye take
precedence.

FACIAL TRAUMA
235
• Fractures involving the teeth are compound and antibiotics are
required, e.g. amoxicillin or erythromycin if allergic to penicillin.
• Timing: mandibular fractures involving tooth-bearing segments and any
soft tissue lacerations should be treated within 24h. Uncomplicated
fractures of orbit/malar/frontal bone/nose/maxilla are best treated
when facial swelling has settled. Optimum time is 5–10 days.
• All patients with orbital/malar/maxilla fractures must not blow their
nose for 10 days to prevent surgical emphysema of soft tissues.
• Undisplaced fractures may be treated conservatively. Advise soft diet if
tooth-bearing fragments involved.
• The aim of active treatment is to restore function and correct any
deformity, e.g. diplopia from orbital complex fracture; decompression
of any nerves involved in fracture line (infra-orbital, inferior dental,
frontal nerves); restoration of dental occlusion to correct bite
(mandibular/maxillary fractures); correct deformity (fractured nose/
zygoma).
• Fractures may be treated by closed reduction, e.g. intermaxillary
fi xation with wires or open reduction using mini-fracture plates.
Surgical access to fractures may be intra-oral, incisions around the eye
for orbit, submandibular for mandible, bicoronal to frontal bone.
• Patients who have had an unprovoked assault may experience
post-traumatic stress disorder and benefi t from referral to clinical
psychologist.

CHAPTER 5 Head and neck surgery
236
Neck space infections
Key facts
• Ninety per cent of neck space infections are of dental origin, especially
lower molar teeth.
• Ten per cent are from tonsils and infected epidermoid, branchial, and
thyroglossal cysts.
• Their importance is risk of airway obstruction, septicaemia, and
mediastinitis; mortality risk from overwhelming sepsis.
Anatomy
The investing layer of cervical fascia is attached to mastoid, superior
nuchal line, lower border of mandible, hyoid and descends to the clavicle.
It splits to enclose sternomastoid and trapezius muscles and thus forms a
structural collar to the neck. Medially lie the pharynx, larynx, trachea, and
upper oesophagus which is in direct continuity with the mediastinum. As it
splits to enclose parotid gland, a deep layer is formed attached to base of
skull, merging with the upper end of the carotid sheath and pharyngobasi-
lar fascia posteriorly. It also splits to enclose the submandibular gland with
deep layer attached to mylohyoid line. As a result, a number of important
anatomical compartments or potential spaces exist (see Fig. 5.2).
• Sublingual. Floor of mouth above mylohyoid.
• Submental. Anterior upper neck below mylohyoid.
• Submandibular. Below mylohyoid around submandibular gland.
• Parapharyngeal. Deep to parotid, lateral to pharynx.
• Pterygoid. Pterygomaxillary fi ssure.
These are all interconnected and continue inferiorly down the neck fol-
lowing outside the tough carotid sheath into the mediastinum. Related are
buccal and submasseteric spaces that are not connected.
Clinical features
• Infection may present as a localized fl uctuant swelling or it may
present as a spreading cellulitis with a brawny, hard, tender, hot,
erythematous mass. Often it is a mixture of both. Necrotizing faciitis is
rare and has high mortality.
• There is usually a history of toothache, sore throat, previous neck
swelling, e.g. branchial cyst.
• Cardinal signs of severity include: fever, trismus, hot potato speech,
dysphagia, stridor, tachycardia, and respiratory rate increase.
• Bilateral sublingual/submental/submandibular swelling (Ludwig’s angina)
is particularly aggressive.
Investigations
• Temperature, HR, BP, respiratory rate.
• WCC.
• Imaging. OPT if dental cause expected. Ultrasound scan can localize
any deep space collection. CT, including chest, is useful in severe cases.

NECK SPACE INFECTIONS
237
Treatment
• Admit if systemically unwell or any cardinal signs of severity as above.
• IV antibiotics. Co-amoxiclav or clindamycin if allergic to penicillin.
• Contact anaesthetist as may need fi bre optic intubation.
• Theatre before sunset if systemic sepsis.
Surgical management
• Remove cause of infection, e.g. extract offending teeth, incise quinsy of
tonsil.
• Incise and drain at dependent point any localized abscess.
• Send pus sample for culture and sensitivity.
Exploration of neck spaces
Use a submandibular incision, incise platysma and cervical fascia. Using
Hilton’s method, fi nd lower border of mandible, then explore medially;
this is the submandibular space; go anteriorly to open up sublingual space.
To open parapharyngeal space and pterygoid space, push forceps up
medial ramus of mandible and open forceps. If there is swelling extend-
ing to root of neck, make a second incision above clavicle and medial to
sternomastoid. Suture in a corrugated type drain.
If intubation diffi cult or airway compromised, e.g. unrelieved trismus
on induction, do a tracheostomy. The swelling often gets worse before
it gets better. You may need to re-explore the neck. Book ITU bed in
severe cases.
Posterior auricular
nodes
Parotid nodes
Submental nodes
Inferior deep
cervical nodes
Sternohyoidmuscle
Internal jugular
vein
Submandibular
nodes
Buccal nodes
Superior deep
cervical nodes
Posterior belly of
digastric muscle
Omohyoid muscle
Superficial cervical
nodes
Tonsillar node
Occipital nodes
Sternocleidomastoid
muscle
Fig. 5.2 The distribution of lymph nodes in the neck. Reproduced with permission
from Longmore, M. et al. (2007). Oxford Handbook of Clinical Medicine, 7th edn.
Oxford University Press, Oxford.
This page intentionally left blank

239
Breast and endocrine
surgery
Breast cancer 240
Surgical treatment of breast cancer 242
Breast cancer screening 244
Benign breast disease 246
Acute breast pain 248
Goitre 250
Thyrotoxicosis 252
Thyroid tumours—types and features 254
Thyroid tumours—diagnosis and treatment 256
Post-thyroid surgery emergencies 258
Primary hyperparathyroidism 260
Multiple endocrine neoplasia 262
Cushing’s syndrome 264
Conn’s syndrome 266
Phaeochromocytoma 268
Chapter 6

CHAPTER 6 Breast and endocrine surgery
240
Breast cancer
Key facts
• Total of 35 000 new cases per year; 1 in 9 lifetime risk for women.
• Commonest in Western Europe; least common in Japan and Africa.
• Incidence increases with age.
• One per cent occurs in men.
• Five per cent related to identifi able genetic abnormality (BRAC1,
BRAC2, ataxia–telangectasia genes.)
• Sixty per cent present as symptomatic disease; 40% during screening.
Pathological features
Eighty per cent ductal adenocarcinoma; 20% lobular, mucinous tubular or
medullary adenocarcinoma. Most carcinomas believed to originate as in
situ carcinoma before becoming invasive; 70% express oestrogen or pro-
gesterone receptors.
Clinical features
Breast lump
• Commonest presenting symptom.
• Usually painless (unless infl ammatory carcinoma).
• Hard and gritty feeling.
• May be immobile (held within breast tissue), tethered (attached to
surrounding breast tissue or skin), or fi xed (attached to chest wall).
• Ill-defi ned; irregular with poorly defi ned edges.
Nipple abnormalities
• Nipple may be the prime site of disease (Bowen’s disease), presenting
as an eczema-like change.
• Nipples may be affected by an underlying cancer:
Destroyed.•
Inverted.•
Deviated.•
Associated bloody discharge.•
Skin changes
• Carcinoma beneath skin causes dimpling, puckering, or colour changes.
• Late presentation may be with skin ulceration or fungation of the
carcinoma through the skin.
• Lymphoedema of the skin (peau d’orange) suggests local lymph node
involvement or locally advanced cancer.
• Extensive infl ammatory changes of the skin are associated with
infl ammatory carcinoma (aggressive form).
Systemic features
• Systemic features include weight loss, anorexia, bone pain, jaundice,
malignant pleural, pericardial effusions, and anaemia.

BREAST CANCER
241
Diagnosis and investigation
Diagnostic tests
Tissue diagnosis
• Core biopsy or fi ne needle aspiration cytology (FNAC) of the breast
lesion 9 axillary nodes.
• Core biopsy also fi nds oestrogen receptor status, differentiates between
invasive carcinomas and in situ carcinoma (ductal carcinoma in situ, DCIS).
Staging investigations
Systemic staging is usually reserved for patients following surgical treat-
ment with a tumour who are at risk of systemic disease.
• Staging CT scan (chest, abdomen, and pelvis).
• Liver ultrasound.
• Chest X-ray.
• Bone scan.
• LFTs, serum calcium.
• Specifi c investigations for organ-specifi c suspected metastases.
Treatment Surgical treatment is described on b p. 242.
Medical treatment
In non-metastatic disease, medical therapy is adjuvant to reduce the risk of
systemic relapse, usually after primary surgery. It is occasionally used as a
treatment of choice of elderly or those unfi t/inappropriate for surgery.
• Endocrine therapy.
Used in (o)estrogen receptor (ER) +ve patients.•
Anti-oestrogens like tamoxifen or aromatase inhibitors (letrozole).•
Post-menopausal patients—letrozole (caution osteoporosis).•
Premenopausal patients—tamoxifen.•
Herceptin—given in Her-2 receptor +ve patients.•
• Chemotherapy (e.g. anthracyclines, cyclophosphamide, 5-FU,
methotrexate). Offered to patients with high risk features (+ve nodes,
poor grade, young patients).
In metastatic disease, medical therapy is palliative to increase survival time
and includes:
• Endocrine therapy. As above.
• Chemotherapy (e.g. anthracyclines, taxanes, herceptin).
• Radiotherapy. To reduce pain of bony metastases or symptoms from
cerebral or liver disease.
• All breast lumps or suspected carcinomas are investigated with triple
assessment.
• Clinical examination (as above).
• Radiological assessment:
Mammography usual• , particularly over age 35y.
Ultrasound scan• used to assess the presence of involved lymph
nodes; sometimes used under age 35 because increased tissue
density reduces sensitivity and specifi city of mammography.
MRI • used in lobular carcinoma to assess the extent of the disease,
multifocality, and the opposite breast.
• Younger women with dense breast tissue. For screening purpose in
patients with strong family history.

CHAPTER 6 Breast and endocrine surgery
242
Surgical treatment of breast cancer
Surgery is the mainstay of non-metastatic disease. Options for treatment
of the primary tumour are as follows.
Wide local excision
• To ensure clear margins.
• Commonest procedure.
• Breast-conserving, provided breast is adequate size and tumour
location appropriate (not central/retro-areolar).
• Usually combined with local radiotherapy to residual breast to reduce
risk of local recurrence.
Simple mastectomy
• Best local treatment and cosmetic result for large tumours (especially
in small breast), central location, late presentation with complications
such as ulceration.
• Also used for multifocal tumours or where there is evidence of
widespread in situ changes.
• Adjuvant breast radiotherapy is very rarely necessary.
• Performed with reconstruction at the same time or later stage
including:
Latissimus dorsi fl ap;•
TRAM fl ap;•
Prosthesis (see • b p. 618).
Surgical management of regional lymph nodes
Axillary node sampling
• Minimum of four nodes should be retrieved.
• Avoids complete disruption to axillary lymph drainage, reducing risk of
lymphoedema.
• Is inadequate for treatment of the axilla. If nodes are +ve, they require
adjuvant radiotherapy to axilla or axillary node clearance.
Axillary node clearance
• Optimizes diagnosis and treatment of axilla.
• Increases risk of lymphoedema greatly.
Sentinel node biopsy
• One or two nodes primarily draining tumour identifi ed by radioactive
tracer or dye injected around tumour and node(s).
• Identify positive nodes, then require a full axillary clearance.
• Avoids major axillary surgery where not necessary.
Surgery for metastatic disease
Surgery in metastatic disease is limited to procedures for symptomatic
control of local disease (e.g. mastectomy to remove fungating tumour).
Ductal carcinoma in situ (DCIS)
• Precancerous condition.
• Ten to fi fty per cent develop invasive ductal cancers.

SURGICAL TREATMENT OF BREAST CANCER
243
• Mammograms show microcalcifi cation.
• Pathologically graded to low grade, intermediate grade, and high grade.
• DCIS is treated with wide local excision with clear margin.
• Mastectomy needed in larger breast lesions or multifocal disease.
• High grade DCIS treated by post-operative radiotherapy after wide
local excision.
• Axillary surgery is not needed as there is no potential for lymph node
metastasis.

CHAPTER 6 Breast and endocrine surgery
244
Breast cancer screening
Aims
• To identify asymptomatic (hopefully early) invasive breast cancer.
• To identify asymptomatic carcinoma in situ.
• Features looked for on screening mammography include: spiculated
calcifi cation; microcalcifi cation.
What is offered?
• Since 1988, population-based screening has been offered.
• Arranged regionally with centrally activated postal invitation.
• Starts age 50 and continues to age 70 (cover peak ages of incidence
of new diagnoses and excludes low risk younger women—‘prevents
psychological morbidity of screening the well’); plans to extend screening
age group from 47–74y.
• Two view (lateral and oblique) mammography of both breasts.
• Suspicious or malignant-looking lesions invited for clinical assessment
by standard triple assessment.
Results
• Seventy per cent of women offered it will accept screening (lowest
take-up in socio-economic groups and those diffi cult to contact, e.g.
rapidly changing addresses or no fi xed address).
• Ten per cent of invasive carcinoma is not radiologically detectable
(false negative rate).
• Risk of a false positive screening is approximately 25% over 10y of
screening.
• For every 1000 women screened over 10y, around 200 are recalled
because of an abnormal result.
Sixty (6%) will have at least one biopsy.•
Fifteen (1.5%) will have invasive cancer.•
Five (0.5%) will have DCIS.•
• Absolute reduction in cancer deaths due to screening over 10y are:
0.5 per 1000 at age 40.•
2 per 1000 at age 50.•
3 per 1000 at age 60.•
2 per 1000 at age 70.•
• Studies suggest up to a 30% reduction in mortality from screen-
detected early breast cancer.
This page intentionally left blank

CHAPTER 6 Breast and endocrine surgery
246
Benign breast disease
Most benign breast conditions arise from pathology related to abnor-
malities of the normal development and involution of the breast (ANDI).
Other benign diseases are related to infection or trauma.
Fibroadenoma
Benign overgrowth of one lobule of the breast. Usually isolated, may be
multiple or giant, especially in Afro-Caribbeans. Commonest under age 30,
but may occur at any age up to menopause.
• Features. Painless, mobile, discrete lump.
• Diagnosis. Ultrasound usually conclusive.
• Treatment. Excision if concern over diagnosis, cosmesis, or symptoms.
Cysts
Almost always benign, fi lled with green-yellow fl uid. Often associated with
fi brocystic disease (below).
• Features. Round, symmetrical lump(s); may be discrete or multiple.
Occasionally painful.
• Diagnosis. Aspiration—typical fl uid returned; residual mass or
recurrent cysts—mammography to exclude associated tumour.
• Treatment. Repeated aspiration; hormone manipulation occasionally
useful for multiple recurrent cysts.
Fibrocystic disease
Combination of localized fi brosis, infl ammation, cyst formation, and hor-
mone-driven breast pain. Occurs almost exclusively between menarche
and menopause (15–55y).
• Features. Cyclical pain and swelling, ‘lumpy’ breasts, multiple breast
cysts.
• Diagnosis. Lumps usually require triple assessment (even once a
diagnosis of fi brocystic disease is made—any woman may develop a
carcinoma).
• Treatment. Reassurance, anti-infl ammatories, hormone or ‘cellular’
manipulation (e.g. G-linoleic acid/evening primrose oil, combined oral
contraceptive (COC) pill, cyst aspiration).
Breast infections
Lactational mastitis
Due to acute staphylococcal infection of mammary ducts. May degenerate
into an acute lactational abscess. Treat with oral antibiotics and (repeated)
aspiration if abscess occurs. No need to stop lactating.
Recurrent mastitis/mammary duct ectasia
Due to dilated, scarred, chronically infl amed subareolar mammary ducts.
Associated with smoking. Present with recurrent yellow-green nip-
ple discharge or recurrent breast abscesses. Infection is usually mixed
anaerobic based. Treatment with metronidazole and drainage of acute
abscesses. Surgery is rarely necessary.

BENIGN BREAST DISEASE
247
Traumatic fat necrosis
Post-traumatic disorder of breast tissue caused by the organization of
acute traumatic injury by:
• Fibrosis.
• Organized local haematoma.
• Occasionally calcifi cation.
Presents with new, painless or painful breast lump, often poorly defi ned.
History of trauma is often absent.
Diagnosis may be diffi cult even on triple assessment. Failure to resolve
or doubt about diagnosis after assessment is an indication for excision
biopsy.

CHAPTER 6 Breast and endocrine surgery
Acute breast pain
Causes and features
Breast origin
• Breast abscess. Acute severe, localized pain in the breast, associated
with swelling, redness, and sometimes purulent nipple discharge. Most
common in breastfeeding women. May be due to chronic mastitis/
mammary duct ectasia (see b p. 246)—occasionally recurrent.
• Mastitis. Recurrent intermittent breast pain with swelling, tenderness,
seropurulent nipple discharge. Most common in smokers; associated
with mammary duct ectasia (see b p. 246).
• Fibrocystic disease (see b p. 246). Usually recurrent or chronic
breast pain, but may be acute isolated episode. Often multifocal and
associated with tender vague swelling or ‘lumpiness’.
Non-breast origin
• Musculoskeletal. Often onset after exercise, coughing, or straining,
but not always. No associated breast symptoms. Pain usually sharp
and precipitated by movement or breathing. Often tender deep to
breast tissue and over other chest wall areas (e.g. costochondral
junctions in costochondritis (Tietze’s disease)). May be due to pleural
disease (post-pneumonic, post-pulmonary embolism, viral pleurodynia
(Bornholm’s disease)).
• Visceral. May be due to atypical angina or acute coronary syndrome.
• Skin pathology. Such as infected sebaceous cysts, cellulitis, skin abscess.
Emergency management
Establish a diagnosis
• Good inspection and careful history taking is usually all that is required.
• Imaging is rarely necessary and is often painful if the pathology is
primary breast. Mammography should be avoided due to the breast
compression required. Breast ultrasound may help, particularly in the
diagnosis of breast abscess.
• Consider specialist referral or opinion if PE, cardiac ischaemia, or
pneumonia suspected. CXR is simple, but often unhelpful.
Early treatment
• Give adequate analgesia. NSAIDs (diclofenac (Voltarol
®
) 50mg PO or
100mg PR) are effective in most causes. Opiates may be necessary.
• Breast abscesses may be effectively aspirated for relief of pressure
symptoms under local anaesthetic. Formal incision and drainage is
often avoided, especially in lactational abscesses.
248

ACUTE BREAST PAIN
Defi nitive management
• Breast abscess. If lactational, oral antibiotics (including fl ucloxacillin
500mg tds) and aspirational drainage (often repeated several times on
a daily or alternate day basis). If associated with chronic mastitis, oral
antibiotics (to include metronidazole 400mg PO tds or co-amoxiclav
750mg tds PO).
• Fibrocystic disease. NSAIDS (e.g. ibuprofen 400mg prn), G-linoleic acid,
danazol, occasionally tamoxifen.
249

CHAPTER 6 Breast and endocrine surgery
250
Goitre
Key facts
• Goitre refers to an enlarged thyroid gland (from the Latin guttur,
meaning throat).
• For clinical practice, ‘enlargement’ is taken to mean a thyroid gland
that is easily visible or palpable with the neck in neutral position.
Pathological features
• Goitres result from follicular cell hyperplasia at one or multiple sites
within the thyroid gland.
• The mechanism is multifactorial—genetic, environmental, dietary,
endocrine, and other factors.
On the basis of clinical and pathological features, goitre can be subclassi-
fi ed as follows.
• Epidemiology.
Endemic.•
Sporadic.•
Familial.•
• Morphology.
Diffuse.•
Nodular.•
Multinodular. —
Solitary nodules. —
• Thyroid function status.
Toxic.•
Non-toxic.•
• Location.
Cervical.•
Retrosternal.•
Intrathoracic.•
Clinical features
Sporadic nodular goitre
• Commonest surgical presentation of thyroid disease.
• Generally asymptomatic and usually present with a neck mass or
compressive symptoms.
• Present as a small, diffuse, or nodular goitre and is generally euthyroid.
Compressive symptoms
• More likely to occur in patients with a retrosternal extension (at the
thoracic inlet, the bony structures create a limited space that cannot
expand).
• Growth of the goitre may cause:
Dyspnoea (worse when lying fl at) due to tracheal displacement.•
Dysphagia due to oesophageal compression.•
Voice changes due to recurrent laryngeal nerve (RLN) pressure.•

GOITRE
251
Distended neck veins, facial plethora, swelling, and stridor due to •
superior vena caval compression (worse with arms raised above
the head—‘Pemberton’s sign’).
Cosmesis May or may not be a signifi cant problem—varies widely.
Hyperthyroidism or hypothyroidism
• The vast majority of patients with goitre will be euthyroid.
• May be apparent clinically or biochemically (hyper = i free T
4
, d TSH;
hypo = d free T
4
, i TSH).
Diagnosis and investigations
• Thyroid function tests ((TFTs) for TSH and free T
4
). Usually normal,
especially outside endemic areas.
• CXR. Look for tracheal deviation and a retrosternal shadow.
• Thoracic CT. Used to defi ne the anatomy in patients with large
intrathoracic extension.
• Preoperative laryngoscopy. To assess the possibility of pre-existing RLN
palsy.
Treatment
Surgical treatment
• Indications include:
Relief of local compressive symptoms.•
Cosmetic deformity.•
Prevention of progressive thyroid enlargement.•
• Thyroid lobectomy is feasible if there is asymmetric enlargement with
only the one lobe creating the obstructive symptoms. This avoids the
need for long-term thyroxine replacement (important mainly in areas
where medical facilities are limited).
• Total thyroidectomy offers immediate improvement of obstructive
symptoms, minimal morbidity in experienced hands, less risk of
recurrent symptoms, particularly in large or retrosternal goitres.
Medical treatment
• Oral levothyroxine (
l
T
4
). Used to reduce the size of goitres in patients
with iodine defi ciency or subclinical hypothyroidism (i.e. when a raised
TSH stimulates the enlargement of the thyroid gland).
• Radioactive iodine (
131
I). Induces a gradual destruction of thyroid tissue,
with a decrease in goitre volume up to 50% in 2y. Large (or repeated)
doses of
131
I are needed. Used for non-toxic goitres (more in Europe
than UK or USA).
• The risks of radioactive iodine are:
Radiation thyroiditis (acute thyroid swelling can potentially be •
dangerous in patients with large substernal goitres).
Temporary thyrotoxicosis (due to rapid release of preformed •
hormones from the destroyed follicles).
Late hypothyroidism due to over-destruction of the gland.•
CHAPTER 6 Breast and endocrine surgery
252
Thyrotoxicosis
Key facts
• Hyperthyroidism occurs in 27 in 1000 women and 3 in 1000 men in the
UK.
• Graves’ disease is the most common cause of hyperthyroidism.
Causes and pathological features
• TSH-secreting pituitary adenoma.
• Autoimmune stimulation (Graves’ disease) (Fig. 6.1).
Thyroid-stimulating antibodies (IgG) bind to TSH receptors and •
stimulate the thyroid cells to produce and secrete excessive
amounts of thyroid hormones.
Thyroid gland hypertrophies and becomes diffusely enlarged.•
The autoimmune process leads to mucopolysaccharide infi ltration •
of the extra-ocular muscles and may lead to exophthalmos.
• T
3
, T
4
secreting site in the thyroid.
Nodule in a multinodular goitre (‘Plummer’s syndrome’).•
Adenoma or (very rarely) carcinoma.•
• Thyroiditis (large amount of preformed hormones are released
after the destruction of follicles, with transient thyrotoxicosis).
• Exogenous intake of thyroid hormones (factitious thyrotoxicosis).
Ant pituitary Blood
AA
TSH
del
del
del
Thyroid cell
Globulin
Follicle centre
Thyroglobulin
MIT
MIT
DIT
DIT
DIT
DIT
+
+
+
+
T
4
T
3
T
4
T
2
T
3
T
3
T
3
I
–
I
–
T
4
r
–
Fig. 6.1 Physiology of thyroid hormone secretion. AA, amino acids; del,
deiodination (especially of liver and kidney); DIT, di-iodotyrosine; MIT, mono-
iodotyrosine; TSH, thyroid-stimulating hormone.

THYROTOXICOSIS
253
Clinical features (any cause)
• Weight loss, heat intolerance, sweating (due to stimulated metabolism
and heat production).
• Tremor, nervousness, irritability, emotional disturbance, tiredness,
and lethargy (due to CNS overactivity).
• Cardiac features are caused by beta-adrenergic sympathetic activity:
Palpitations, tachycardia, and arrhythmias.
• Eye signs can be:
Minimal/mild (soft tissue oedema, chemosis).•
Very prominent (severe exophthalmos, corneal ulcers, diplopia).•
Ophthalmopathy is usually bilateral, but may only involve one eye.•
• Pretibial myxoedema, thyroid acropachy, vitiligo, and alopecia are rare.
Thyroid storm (thyrotoxic crisis)
• Rare presentation of extreme signs of thyrotoxicosis and severe
metabolic disturbances.
• Precipitated by non-thyroid surgery, major trauma, infection, imaging
studies with iodinated contrast medium in patients with unrecognized
thyrotoxicosis.
• Features are insomnia, anorexia, vomiting, diarrhoea, marked sweating,
fever, marked tachycardia.
• Early clinical diagnosis of the condition and immediate treatment
decrease the risk of fatal outcome.
Diagnosis and investigations
• TFTs. d TSH level, i free T
4
, and free T
3
(in all causes, but pituitary).
• Positive serology for thyroid autoantibodies.
• Radioactive iodine scan (or technetium scan).Helpful in distinguishing the
diagnosis of Graves’ disease, thyroiditis, toxic nodule (unilateral uptake
with negative scan on the contralateral side), or toxic multinodular goitre.
Treatment
Medical treatment
• Antithyroid drugs block hormone synthesis:
Carbimazole 20mg bd, then reducing dose (especially in UK).•
Propylthiouracil 200mg bd (especially in USA): blocks the peripheral •
conversion of T
4
to T
3
.
• Beta-blockers (propranolol 40–120mg/day) are used to control
tachycardia and tremor.
• Radioactive iodine (
131
I; see b p. 251 for risks). Contraindicated in
severe eye disease (could worsen after
131
I treatment), young women
(risk of teratogenicity in pregnancy), patients who are main carers of
small children.
Surgical treatment
• Total thyroidectomy (for Graves’ disease). Indicated in patients who are
not candidates for
131
I therapy. It is the treatment of choice in those with
eye disease and patients where control of symptoms has been diffi cult
on medication. Slightly higher risk of RLN injury and hypoparathyroidism
(due to increased vascularity of the gland and the local fi brosis).
• Thyroid lobectomy. For isolated nodules or adenomas.

CHAPTER 6 Breast and endocrine surgery
254
Thyroid tumours—types and features
Key facts
• Solitary thyroid nodule is the most common thyroid disorder.
• Ultrasound studies show that up to 50% of patients have thyroid
nodules by the age of 50.
• Although thyroid nodules are common, malignant nodules are rare
(incidence of 4 in 100 000 individuals per year).
Pathological features
• Colloid nodule.
The most commonly encountered solitary thyroid nodule.•
Ultrasound examination may reveal numerous other small nodules •
as part of a multinodular gland.
Nodules are formed mainly of collagenous material interspersed •
with benign thyroid cells with little or no malignant potential.
• Follicullar adenoma.
Benign tumour that grows in a glandular or follicular pattern.•
Tends to develop slowly with a pseudocapsule of compressed •
normal thyroid tissue.
• Papillary carcinoma.
Most common malignant neoplasm of the thyroid.•
Malignant cells show typical cytological features (nuclear ‘grooves’, •
intranuclear inclusions, or ‘optically clear nuclei’—‘Orphan Annie
cells’).
Spread tends to be via lymphatics to local lymph nodes.•
• Follicular carcinomas. Malignant tumours divided into two histologically
distinct groups.
Minimally invasive• . Usually small, encapsulated neoplasms that
show invasion only into the tumour capsule; vascular and lymphatic
invasion is normally absent; associated with an excellent prognosis.
Widely invasive• . Invasion through the capsule into the surrounding
thyroid tissue; they can replace the entire thyroid, invade local
structures, and display haematogenous metastases.
• Medullary thyroid cancer. Rare, derived from calcitonin-secreting C-cells
of the thyroid.
Sporadic• . Single, unilateral, and presenting in isolated patients with a
neck mass and often cervical lymphadenopathy.
Familial• . Either as part of the multiple endocrine neoplasia (MEN)
type 2 (see b p. 262) or non-MEN familial tumours when cancers
may be multiple and multifocal, arising in a background of diffuse
C-cell hyperplasia.
• Anaplastic thyroid cancer. Very rare and extremely aggressive tumour,
characteristically occurring in older women.
• Thyroid lymphoma.
Tumour of mucosa-associated lymphoid tissue (MALToma).•
Classifi ed as diffuse B-cell non-Hodgkin’s lymphomas.•
Rarely associated with longstanding Hashimoto’s thyroiditis.•

THYROID TUMOURS—TYPES AND FEATURES
255
Clinical features
Most thyroid nodules are asymptomatic, presenting as a chance fi nding by the
patient or during a routine general examination. Clinical assessment should
include an assessment of risk factors related to malignancy (see Table 6.1).
Retrosternal extension should be assessed.
Differential diagnosis of neck swellings (see Table 6.2)
Table 6.1 Clinical features
Sex Thyroid nodules—females > males.
A solitary nodule in a man is more likely to
represent a cancer.
Age Nodules in children and old patients are
more likely to represent a cancer.
Family history MEN2A and MEN2B (medullary Ca).
Geographic
Previous neck irradiation
Solitary versus multiple
nodules
Nodule characteristics Firm/hard or fi xed nodules are more likely to
be a cancer. Rapid increase in size of a
previously static longstanding nodule is
worrying (particularly in an elderly patient).
Local lymphadenopathy
Voice changes RLN palsy is a sign of invasive cancer.
Table 6.2 Differential diagnosis
Congenital conditions Thyroglossal tract abnormalities
Branchial cyst
Cystic hygroma
Cervical rib
Tumours Thyroid
Salivary glands
Chemodectoma (carotid body tumour)
Sarcoma
Lipoma, fi broma
Lymph nodes Primary malignancy (lymphomas, leukaemias)
Secondary malignancy (skin, nasopharynx, mouth,
oesophagus, thyroid, breast, or occult)
Infl ammatory conditions (tonsillitis, dental,
mononucleosis, toxoplasma, HIV, cat scratch fever)
Diverticulae Oesophagus
Traumatic Sternocleidomastoid ‘tumour’

CHAPTER 6 Breast and endocrine surgery
256
Thyroid tumours—diagnosis and
treatment
Diagnosis and investigation
• TFTs (free T
4
, TSH levels).
• Thyroid autoantibodies.
• Fine needle aspiration biopsy (FNAB). Mandatory for all thyroid nodules.
An 18G needle is used to obtain a sample for cytological analysis. The
results are presented on a 5-point scale.
Thy1, non-diagnostic sample (though this may be expected if the •
nodule contains cystic fl uid).
Thy2, benign colloid nodule.•
Thy3, follicular lesion (i.e. either an adenoma or a carcinoma, the •
distinction being possible only after excision biopsy and histological
analysis).
Thy4, suspicious, but not diagnostic of papillary cancer.•
Thy5, diagnostic for thyroid cancer.•
• Neck ultrasound. Sometimes used to assess the size and characteristics
of a nodule and to determine whether the nodule is solitary or part of
multinodular goitre.
Treatment
Surgical treatment
• Thyroid lobectomy, including the isthmus and pyramidal lobe (if
present), is the minimum operation for thyroid tumours. It is curative
for colloid nodule (alleviating pressure symptoms), enables full
histological diagnosis in suspicious (Thy3) follicular lesions, whilst
being considered curative for minimal papillary cancers (<1cm) and for
minimally invasive follicular cancers.
• Total thyroidectomy at initial operation is indicated for cytologically
proven cancers. Completion total thyroidectomy (following thyroid
lobectomy) is deemed necessary for papillary thyroid cancers larger
than 2cm in diameter or histologically proven widely invasive follicular
cancer after initial lobectomy.
• Total thyroidectomy plus cervical nodal dissection. A modifi ed
(selective) functional neck dissection is performed in patients
presenting with palpable lymphadenopathy and in patients with
medullary thyroid cancer.
Medical treatment for patients with thyroid cancer
• T
3
substitution (levothyronine, 20 micrograms tds) is used in the
immediate post-operative period in patients due to undergo
131
I-whole
body scan. The shorter half-life of T
3
means it can be stopped for only
2 weeks to allow a rise in TSH that would favour uptake of
131
I in any
remaining thyroid cells.
•
131
I is administered to patients with thyroid cancer following total
thyroidectomy. The
131
I is extremely effective in killing any residual
thyroid cells or metastatic cells that may be present.

THYROID TUMOURS—DIAGNOSIS AND TREATMENT
257
• T
4
replacement in slightly higher doses (thyroxine, 100–200 micrograms
od) is used to maintain a suppressed TSH. This has been shown to
decrease the possibility of contralateral disease in patients undergoing
lobectomy for thyroid cancer and to reduce the risk of local
recurrence or metastatic disease in patients who underwent total
thyroidectomy.
• Recombinant human TSH (rhTSH) has recently become available as a
means of inducing
131
I uptake without having to stop thyroid hormone
replacement therapy (therefore avoiding the distressing symptoms of
hypothyroidism in the weeks before and after the
131
I scan).
Key revision points—anatomy of the thyroid gland
• The thyroid consists of two lateral lobes that make up 90% of the
gland substance and a central midline isthmus with a small pyramidal
lobe.
• Each lobe contains lobules that comprise follicles containing colloid
and lined by thyroid epithelial cells with parafollicular C (calcitonin-
secreting) cells.
• The arterial supply is from superior thyroid arteries (2) from the
external carotid (related to the external laryngeal nerves in their
course), and the inferior thyroid arteries (2) from the subclavian
artery (related to the recurrent laryngeal nerves).
• Four parathyroid glands are usually found posteromedial to the mid-
upper and inferior poles of the lateral lobes.

CHAPTER 6 Breast and endocrine surgery
258
Post-thyroid surgery emergencies
Neck bleeding
May occur immediately (in recovery) or late (on the ward, sometimes due
to infection).
• Symptoms. Usually due to the pressure of a haematoma on neck
structures: dyspnoea, pain, sensation of neck swelling.
• Signs. Stridor, neck swelling, bleeding from wound, cyanosis (if high
pressure compression of neck).
Resuscitation
• If the patient is at all unwell, call for senior help—acute bleeding can be
rapidly life-threatening.
• If possible, establish large calibre IV access. Give crystalloid fl uid up to
1000mL if tachycardic or hypotensive.
• Give high fl ow O
2
(8L/min via non-rebreathing mask).
• Consider opening the wound immediately. If the patient is cyanosed
or unconscious, cardiorespiratory arrest may be imminent and loss of
blood from opening the wound will be trivial in comparison.
Early treatment Returning to theatre to deal with the cause is the defi ni-
tive treatment and the patient may be transferred while resuscitation and
emergency treatment are continuing.
Acute bilateral recurrent laryngeal nerve injury
• Extremely rare; due to surgical technique.
• Causes acute paralysis (and therefore adduction) of both vocal cords,
leading to acute airway obstruction.
• Usually noticed immediately after extubation.
• Signs. Acute severe stridor, falling O
2
saturations, and cyanosis.
Resuscitation
• Usually conducted by the anaesthetist.
• Reintubation or, if not possible, immediate cricothyroidotomy.
• Usually recovers as the nerve injuries are rarely both complete.
Acute thyrotoxic crisis
• Rare due to improved medical pre-conditioning of patients prior to
surgery for thyrotoxic conditions.
• May occur due to handling of the gland.
• Has features similar to those of acute severe thyrotoxicosis (see b
p. 252).
• Features. Sweating, fever, tachycardia (may include tachydysrrhythmias
such as AF or atrial fl utter), hypertension.
Resuscitation
• Ensure the patient has large calibre IV access. Crystalloid may be
required if there is marked vasodilatation with hypotension, but
tachycardia may not represent fl uid depletion.
• Give high fl ow O
2
(8L/min via non-rebreathing mask).

POST-THYROID SURGERY EMERGENCIES
259
• Catheterize and monitor urine output.
• Severely ill patients may need transfer to critical care due to the
need for control of adrenal amine release and the cardiac effects of
excessive thyroid hormones.

CHAPTER 6 Breast and endocrine surgery
260
Primary hyperparathyroidism
Key facts
• Primary hyperparathyroidism (PHPT) is a common endocrine disease.
• Prevalence is highest among post-menopausal women, with 1 in 500
possibly being affected.
• Most patients are identifi ed by an incidental fi nding of raised serum
calcium during investigations for another condition.
Pathological features
• Eighty-fi ve per cent have a single parathyroid adenoma. Most of these
tumours are small, less than 1g (normal glands are 30–50mg).
• Ten to fi fteen per cent have multigland hyperplasia, either as a
sporadic disease or in association with familial disease (e.g. MEN
syndromes, see b p. 262).
• Parathyroid cancer is rare, representing less than 1% of patients.
Clinical features
• Classical symptoms are described as:
Moans.• Psychological/psychiatric symptoms (lethargy, depressed
mood).
Groans.• Non-specifi c GI symptoms (abdominal pain, constipation).
Bones.• Aches/pains localized in large joints.
Stones.• Calcium-based renal stones.
• Polyuria, polydipsia, and nocturia are also common features.
• More than half of patients report no specifi c symptoms and accept
most of the symptoms as part of ‘generally getting older’.
• Hypercalcaemic crisis can occur in patients with PHPT exposed to
severe dehydration (e.g. diarrhoea/vomiting). In severe cases, patients
can present in coma.
Diagnosis and investigations
• i Corrected serum calcium is highly suggestive if unexplained, but not
diagnostic.
• i Serum parathyroid hormone concentration (PTH) in the presence
of hypercalcaemia confi rms the diagnosis (e.g. bone metastases
(breast, renal, thyroid carcinoma) have a low (i.e. inhibited) PTH
concentration).
• High resolution neck ultrasound may identify tumours.
• Sestamibi (radioisotope) scanning used to localize adenomas
(accurate in 50%) and allows a focused approach (minimally invasive
parathyroidectomy).
Treatment
Surgical treatment
• Bilateral neck exploration, visualization of all four parathyroid glands
with excision of the enlarged one(s), has, for many years, been the
standard treatment. It remains the treatment for those with negative
localization scans.

PRIMARY HYPERPARATHYROIDISM
261
• When imaging studies identify reliably the position of the adenoma,
patients can undergo minimally invasive parathyroidectomy (MIP). This
is a focused neck exploration through a lateral cervical scar, aiming to
remove the adenoma visualized on scanning and not to explore the
other parathyroid glands.
Medical treatment
• Hypercalcaemic crisis needs aggressive rehydration.
• Establish large calibre IV access. Give 1L in fi rst hour, further 4–6L in
fi rst 24h.
• Monitor urine output and CVP until normalized.
• Furosemide can be added to increase urinary excretion of calcium
once rehydration is adequate.
• Bisphosphonates (e.g. IV pamidronate) should be avoided in PHPT
when parathyroidectomy is anticipated since they impair the ability
to maintain normocalcaemia after the excision of an overactive
parathyroid adenoma.

CHAPTER 6 Breast and endocrine surgery
262
Multiple endocrine neoplasia
Key facts
• Familial endocrine diseases constitute a group of rare conditions.
• Familial syndromes are autosomal dominant diseases involving tumours
of several endocrine glands in a synchronous or metachronous pattern.
Clinicopathological features
Multiple endocrine neoplasia type I (MEN-1)
A syndrome of the ‘3Ps’.
• Parathyroid gland tumours. By age 40, 95% of patients have
hypercalcaemia which is the commonest manifestation.
• Pancreatic islet cell tumours.
Prevalence of 30–75%.•
Usually multicentric, slow-growing.•
Secrete multiple polypeptides (insulin and gastrin commonest).•
Gastrinoma leads to Zollinger–Ellison syndrome (recurrent and •
multiple peptic ulcers, severe refl ux oesophagitis, and diarrhoea).
Rarer tumours are VIPoma, glucagonoma, somatostatinoma.•
• Anterior pituitary tumours.
Detected in 15–40%.•
Commonest is prolactinoma.•
Rarer are GH- (causes acromegaly) or ACTH- (causes Cushing’s •
disease) secreting tumours.
Carcinoid tumours (thymus, lungs, foregut), adrenal tumours, lipomas, and
pinealomas have also been reported to appear in MEN-1 patients.
MEN-1 gene, Chr11, encodes a nuclear protein, menin (role unclear).
Multiple endocrine neoplasia type II (MEN-2) Has two forms.
MEN-2A Syndrome with the following features.
• Medullary thyroid carcinoma (MTC).
Originates in the calcitonin-secreting parafollicullar C-cells •
(derivatives of the neuroectodermal tube);
Commonly multicentric and bilateral and appear on a background •
of C-cell hyperplasia;
Presents as unilateral or bilateral thyroid nodules with/without •
associated cervical lymphadenopathy;
Associated secretion of other (some unidentifi ed) peptides can •
lead to severe diarrhoea.
• Phaeochromocytoma (in 50% of patients; see b p. 268 for features).
• Primary hyperparathyroidism (15% of patients).
MEN-2B Syndrome with the following features.
• MTC.
• Phaeochromocytoma.
• ‘Marfanoid-specifi c body habitus’ (tall, slender, high arched palate, and
long extremities), 90% of patients.
MEN-2B is associated with mucosal neuromas and intestinal ganglioneuro-
matosis and characteristic facial appearance.

MULTIPLE ENDOCRINE NEOPLASIA
263
MEN-2 gene, Chr10, encodes a cell surface glycoprotein member of
receptor tyrosine kinases (RET proto-oncogene). Point mutations in spe-
cifi c parts of the RET gene lead to specifi c clinical syndromes (genotype–
phenotype correlation). Because of near complete penetrance, all gene
carriers are likely to be affected.
Familial MTC A syndrome of isolated familial with MTC.
Diagnosis and investigations
MEN-1
• Biochemical screening from second decade in known families (serum
calcium, PTH, prolactin, and insulin growth factor-1 (IGF-1) for
pituitary lesions, and serum glucose, insulin, gastrin, and chromogranin
for pancreatic tumours).
• Genetic screening can be used for offspring of known index cases.
Because 10% of menin mutations are de novo, siblings of an index case
are not necessarily at risk.
MEN-2
• Genetic screening for point mutations of the RET gene has 100%
accuracy for identifying carriers (before biochemical abnormalities).
• Affected children are offered total thyroidectomy at an age related
to the individual risk of each mutation (as early as 3y old for some
aggressive mutations).
• Biochemical screening with 24h urine excretion of catecholamines and
metanephrines and serum calcium and PTH are measured annually.
Treatment
Surgical treatment
MEN-1
• Parathyroidectomy.
• Pancreatic tumours. Enucleation of individual tumours in the head of
the pancreas and distal pancreatectomy for tumours in the tail/body.
• Hypophysectomy and external beam irradiation are considered for
pituitary tumours.
MEN-2
• Total thyroidectomy (TT) indicated in patients identifi ed by genetic
screening. Symptomatic patients need TT and cervical nodal dissection
for the lymph nodes on the involved side.
• Laparoscopic adrenalectomy for phaeochromocytoma.
• Parathyroidectomy for MTC in patients belonging to families in which
hyperparathyroidism is frequently associated.
Medical treatment
MEN-1 Prolactinomas can be treated with dopamine agonists (bromo-
criptine/cabergoline).

CHAPTER 6 Breast and endocrine surgery
264
Cushing’s syndrome
Key facts
A syndrome of excess levels of plasma cortisol and associated clinical
features.
Causes
• Commonest cause is iatrogenic administration of steroids.
• Primary adrenal disease (50% of patients).
Unilateral. Cortical adenoma or cortical carcinoma.•
Bilateral. ACTH-independent macronodular adrenal hyperplasia or •
pigmented nodular adrenal cortical disease.
• Secondary adrenal disease.
ACTH-secreting pituitary adenoma (Cushing’s disease, 25%).•
Ectopic ACTH secretion (25%) from other malignant tumours (e.g. •
small cell lung carcinoma).
Clinical features
• Weight gain. Obesity is predominantly truncal with a protuberant
abdomen and a ‘buffalo hump’.
• Muscle weakness, especially thigh and upper arms (add to the overall
appearance, likened to a ‘lemon on sticks’).
• Menstrual irregularities, headache, and backache are common
presenting symptoms.
• Psychological changes are commonly overlooked: lethargy/depression,
paranoid ideas, hallucinations, and a tendency to suicide.
• Plethora, acne, striae, and multiple bruising are common, as is
hirsutism.
• Hypertension, osteoporosis, and impaired glucose tolerance/diabetes.
All these symptoms and signs are non-specifi c and not exclusively related
to Cushing syndrome.
Diagnosis and investigations
Diagnosis is by proving cortisol excess and then by establishing the cause.
• Loss of normal circadian rhythm of cortisol secretion. Samples taken
at 9 a.m. and midnight demonstrate a loss of the normal morning peak
and night nadir.
• Persistent increase in cortisol levels. 24h urine cortisol levels are
elevated, but false positive results can appear in obese patients,
athletes, and patients suffering stress.
• Overnight dexamethasone test. After administration of 1mg
dexamethasone in the evening, the morning cortisol is inhibited in
normal patients, but not in Cushing’s syndrome. It is a very valuable
outpatient screening test.
• Low dose dexamethasone test. Administration of 0.5mg dexamethasone
qds for 48h fails to inhibit plasma cortisol and urine cortisol and
metabolites.
• ACTH levels are inhibited in primary adrenal disease (see above)
and are increased in patients with pituitary adenomas and ectopic
secretion.

CUSHING’S SYNDROME
265
• High dose dexamethasone test. Administration of 2mg dexamethasone
qds for 48h inhibits ACTH secretion from pituitary tumours and leads
to a drop in cortisol levels in such patients. The test is negative in
primary adrenal disease and in ectopic ACTH secretion.
• Imaging.
Abdominal CT or MRI scanning demonstrates whether there is a •
solitary adrenal tumour (with an atrophic contralateral gland) or
whether both adrenals are enlarged. Cancer should be strongly
suspected in tumours greater than 7cm.
Pituitary MRI usually demonstrates tumours over 10mm; small •
microadenomas may need confi rmation by measuring ACTH
concentrations in the inferior petrosal sinuses (to demonstrate
laterality of the tumour).
Treatment
Surgical treatment
• Unilateral adrenalectomy (may be laparoscopic). For patients with
primary adrenal disease.
• Bilateral adrenalectomy. For patients with pituitary ACTH-secreting
adenomas who failed pituitary surgery or gamma-knife treatment. It
is also needed for the very rare patients with ACTH-independent
bilateral adrenal hyperplasia.
Medical treatment
• Metyrapone and ketoconazole can be used preoperatively to decrease
cortisol synthesis, but their effi cacy is limited.
• Cortisol replacement after unilateral or bilateral adrenalectomy is vital.
Patients with solitary adrenal tumours have the contralateral •
adrenal gland atrophied and it may take up to 1y for a return
to normal function. Start on 50–100mg IV tds hydrocortisone
post-operatively. Maintenance dose is usually prednisolone orally
long-term.
Patients should be informed about the possibility of an Addisonian •
crisis triggered by any illness that could impair their ability to
continue medication (e.g. severe diarrhoea/vomiting episodes).
They should wear a bracelet and carry a card with details of their
condition.
• Mineralocorticoid replacement (fl udrocortisone 0.1mg) is also
necessary after bilateral adrenalectomy.

CHAPTER 6 Breast and endocrine surgery
266
Conn’s syndrome
Key facts
• Syndrome of hypertension, severe hypokalaemia, and aldosterone
hypersecretion with suppression of plasma renin activity.
• Originally described in 1954 by Dr Jerome Conn; caused by a benign
adrenocortical tumour.
Causes and pathological features
• Aldosterone-producing adenomas are usually solitary tumours
involving only one adrenal gland. Most adenomas are small (<2cm in
diameter). Aldosterone-producing adenomas (APA) account for about
50–75% of cases of primary hyperaldosteronism (PAL).
• Other causes are idiopathic bilateral adrenal hyperplasia (25–30% of
cases) and familial hyperaldosteronism (very rare cases).
Type I familial hyperaldosteronism.• Autonomous aldosterone
hypersecretion that is suppressible by dexamethasone (mutation
in the ACTH-responsive regulatory portion of the 11b-hydroxylase
gene).
Type II familial hyperaldosteronism.• Autosomal dominant
autonomous aldosterone hypersecretion that is not suppressible by
dexamethasone.
Clinical features
PAL is characterized by:
• Hypertension. Moderate to severe and indistinguishable from other
forms of hypertension (up to 10% of new diagnoses of hypertension);
• Hypokalaemia. Signs include muscle weakness, cramping, intermittent
paralysis, headaches, polydipsia, polyuria, and nocturia.
Diagnosis and investigations
• Serum and urinary K
+
levels. PAL suspected if serum K
+
<3mmol/L
and urinary K
+
excretion >40mmol/L per day. (Spironolactone or
ACE inhibitors should be stopped prior to testing and any K
+
defi cit
corrected).
• Ratio of plasma aldosterone concentration to plasma renin activity,
PAC/PRA (i.e. aldosterone/renin ratio, ARR).
Aldosterone is elevated in all cases (normal 2.2–15ng/dL).•
In PAL, plasma renin activity is suppressed.•
PAC:PRA ratio of >50 is diagnostic for PAL.•
False positive due to beta-blockers, clonidine, NSAIDS, renal •
impairment, and the contraceptive pill.
False negative due to diuretics, ACE inhibitors, renovascular •
hypertension, malignant hypertension, calcium blockers, and very
low Na
+
diets.
• Aldosterone suppression test.
Inability to suppress aldosterone with a high Na•
+
diet.
Oral Na•
+
(9g/day for 3 days) and 0.5mg of fl udrocortisone are given
and a 24h urine sample obtained.

CONN’S SYNDROME
267
Na+ values >200mEq with aldosterone levels >12micrograms/L are •
diagnostic.
Normokalaemia should be ensured prior to testing as the test •
may precipitate hypokalaemia. The test is positive in only 3 of 10
patients with Conn’s syndrome.
• Posture test.
• i PAC after standing for 4h in bilateral adrenal hyperplasia.
• d PAC after standing for 4h in unilateral disease (i.e. adrenocortical
adenoma, Conn’s syndrome).
APAs are unresponsive to angiotensin, but still follow the circadian •
rhythm of ACTH/cortisol.
• Adrenal imaging.
CT scan. To localize the cause.•
If a solitary unilateral macroadenoma (>1cm), no other localization •
studies are necessary and treatment is unilateral adrenalectomy.
• Adrenal venous sampling (AVS) is useful when CT localization has
failed.
Patients in whom localization is not achieved may have bilateral adrenal
hyperplasia and should be treated medically.
Treatment
Surgical treatment
Laparoscopic adrenalectomy for aldosterone-secreting adenomas.
Hypokalaemia should be corrected before the operation by the use
of spironolactone, oral potassium, or both. Normalization of BP after
treatment with spironolactone is a good predictor of the successful treat-
ment of hypertension after unilateral adrenalectomy.
Medical treatment Spironolactone can control hypertension and correct
K
+
levels in the preparation for surgical treatment.

CHAPTER 6 Breast and endocrine surgery
268
Phaeochromocytoma
Key facts
• Rare—incidence of 2–8 cases per million population/year.
• Many cases probably remain undiagnosed.
Clinicopathological features
• Said to follow the ‘10% rule’:
10% are multifocal.•
10% are bilateral.•
10% are extra-adrenal.•
10% are malignant.•
10% occur in children.•
• Originate from the neural crest tissue that forms the adrenal medulla,
sympathetic chain, and visceral autonomic tissue.
• Most common active products are catecholamines (adrenaline,
dopamine, and noradrenaline), but vasopression, somatostatin, ACTH,
and oxytocin may also be secreted.
• Excess catecholamine secretion leads to characteristic episodes
(‘attacks’) of:
Headache.•
Sweating.•
Palpitations.•
Paroxysmal hypertension, tachydysrrhythmias, and a feeling of •
‘impending doom or death’ may also occur.
• Attacks can be triggered by activities causing mechanical pressure
on the tumour (e.g. physical exercise, defecation, intercourse),
by ingestion of alcohol, labour, general anaesthesia, and surgical
procedures.
• Only 50% of patients have persistent hypertension. The other 50%
have normal BP or are hypotensive between the acute episodes.
Diagnosis and investigations
Consider the diagnosis in patients with characteristic paroxysmal episo-
des, those with unusually labile or intermitted hypertension, those with
a family history of phaeochromocytoma or related conditions (see MEN
syndromes), and in hypertensive children.
• 24h urine collection and assessment for vanillylmandelic acid (VMA)
and noradrenaline is most accurate for diagnosis (97% sensitive).
• Clonidine suppression test (failure of urine levels to fall after clonidine
dose) confi rms the diagnosis where urine levels are borderline.
• Provocative testing (e.g. stimulation with bolus IV glucagon) is rarely
necessary and not without risk.
Localizing studies
• Thoraco-abdominal CT or MRI scanning. First-line test, especially for
adrenal and sympathetic chain tumours.
• MIBG (meta-iodo-benzyl-guanidine) scanning localizes extra-adrenal
sites not seen on CT or MRI.
PHAEOCHROMOCYTOMA
269
Treatment
Medical treatment
• It is imperative to control BP prior to contemplating any surgical
intervention.
• Alpha-blockade (e.g. phenoxybenzamine 10mg bd/tds up to the
maximum dose tolerated) until hypertension controlled.
• Beta-blockade (e.g. propranolol) can be added after hypertension
controlled to control the beta-adrenergic effects (tachycardia).
• Alternative treatments with doxazosin (alpha-/beta-blocker) or calcium
channel blockers have been described, but are not widely used.
Surgical treatment
• The principle of surgery is complete resection of the tumour (with
clear negative margins if suspected of malignancy).
• Laparoscopic adrenalectomy is the treatment of choice for smaller
adrenal tumours (<8cm); open adrenalectomy for larger tumours.
• Local or radical excision is appropriate for extra-adrenal tumours.
Key revision points—anatomy of the adrenal gland
• Two main regions—cortex (steroid producing) and medulla
(catecholamine-producing).
• Three cortical zones.
Glomerulosa (outer)—mineralocorticoids (aldosterone).•
Fasciculata (middle)—glucocorticoids (cortisol).•
Reticularis (inner)—sex hormones (andosterone).•
• Blood supply.
Arterial supply is triple—mainly suprarenal artery, some from •
renal artery and inferior phrenic artery.
Venous drainage—usually single vein to the vena cava (right •
suprarenal vein is very short).
• Innervation.
Autonomic—sympathetic from greater splanchnic nerve (T12), •
parasympathetic from vagus (X) via coeliac plexus.
Somatic—from pudendal nerve (S2, 3, 4) to supply external •
urethral sphincter.
This page intentionally left blank

271
Upper gastrointestinal
surgery
Upper gastrointestinal endoscopy 272
Oesophageal motility disorders 274
Pharyngeal pouch 276
Hiatus hernia 278
Gastro-oesophageal refl ux disease 280
Oesophageal tumours 282
Peptic ulcer disease 284
Gastric tumours 286
Chronic intestinal ischaemia 288
Surgery for morbid obesity 290
Small bowel tumours 292
Acute haematemesis 294
Acute upper GI perforation 296
Acute appendicitis 298
Acute peritonitis 300
Acute abdominal pain 302
Gynaecological causes of lower abdominal pain 306
Intra-abdominal abscess 308
Chapter 7

CHAPTER 7 Upper gastrointestinal surgery
272
Upper gastrointestinal endoscopy
There are four types of endoscopy looking at the upper gastrointestinal
(GI) and pancreaticobiliary tracts.
Gastroscopy
Correctly termed oesophago-gastro-duodenoscopy (OGD). Allows direct
visualization of pathology, small channel biopsies to be taken, and minor
interventions (e.g. injection).
Indications
• Investigation of dysphagia.
• Investigation of dyspepsia, refl ux disease, upper abdominal pain.
• Investigation of acute or chronic upper GI bleeding.
• Investigation of iron defi ciency anaemia (with colonoscopy).
• Therapeutic interventions for upper GI pathology:
Balloon dilatation of benign strictures.•
Endoluminal stenting of malignant strictures.•
Injection, coagulation, or banding of bleeding sources, including •
ulcers, varices, tumours, and vascular malformations.
Resection of early neoplastic lesions in stomach and oesophagus •
(endoscopic mucosal resection (EMR)).
Preparation and procedure
• Patient should be starved for 4h (except in emergency indications).
• IV access may be used.
• Always performed with local anaesthetic throat spray (lidocaine).
• Often performed with IV sedation (e.g. midazolam 3mg).
Risks and complications
• Perforation (usually of the oesophagus). Median risk approximately
1 in 3000; highest in elderly, with oesophageal pathology, during
therapeutic interventions.
• Bleeding. Commonest after biopsies or therapeutic procedures.
• Respiratory depression and arrest. Related to overmedication with
sedative; commonest in frail, low body weight, elderly patients.
Endoscopic ultrasound (EUS)
Utilizes a video endoscope with an ultrasound scanner in its tip.
Indications
• Staging of oesophageal, gastric, or pancreatic cancers.
• Investigation of pancreatic cysts/tumours.
• Investigation of possible distal common bile duct stones/sludge.
• Guided biopsy of pancreatic, peri-oesophageal, or perigastric masses/
lymph nodes.
• Guided drainage of pancreatic (pseudo)cysts.
• Guided coeliac plexus blockade.

UPPER GASTROINTESTINAL ENDOSCOPY
273
Endoscopic retrograde cholangiopancreatography (ERCP)
Indications
• Investigation of possible biliary disease (common bile duct stones,
biliary strictures, biliary tumours, biliary injuries, intrahepatic biliary
disease) only where non-interventional imaging (e.g. magnetic
resonance cholangiopancreatogram (MRCP), CT cholangiography) is
not possible.
• Investigation of pancreatic disease (pancreatic duct strictures,
pancreatic duct abnormalities).
• Therapeutic interventions for pancreatico-biliary disease:
Stenting for common bile duct stones, strictures, and tumours, •
post-operative bile leak.
Sphincterotomy for the extraction of biliary stones.•
Preparation and procedure
• Patient should be starved for 4h (except in emergency indications).
• IV access required.
• Always performed with local anaesthetic throat spray (lidocaine).
• Always performed with IV sedation (e.g. midazolam 5mg) and
occasionally, analgesia (pethidine 50mg, fentanyl).
• Performed under X-ray screening guidance, often in X-ray department.
• May be performed under GA.
• LFTs and INR needed prior to procedure.
Risks and complications
• Perforation (of the oesophagus or of the duodenum). Median risk
approximately 1 in 1000; highest in elderly, with pathology, during
therapeutic interventions, especially sphincterotomy.
• Bleeding. Commonest after biopsies or therapeutic procedures,
especially sphincterotomy; usually controlled by balloon pressure, may
require open surgery.
• Post-ERCP pancreatitis.
• Post-ERCP cholangitis. Particularly in jaundiced patient in whom
procedure has been unsuccessful.
• Respiratory depression and arrest. Related to overmedication with
sedative; commonest in frail, low body weight, elderly patients.
Ileoscopy
Often termed ‘push endoscopy’. Performed with long length thin calibre
endoscope, aiming to intubate past the duodenojejunal junction and visual-
ize the fi rst loops of the upper small bowel.
Indications
• Investigation of undiagnosed upper GI bleeding (possibly due to
proximal small bowel pathology).
• Investigation of abdominal pain.
• Investigation of upper small bowel Crohn’s disease.
Preparation and procedure As for gastroscopy.
Risks and complications As for gastroscopy.

CHAPTER 7 Upper gastrointestinal surgery
274
Oesophageal motility disorders
Key facts A spectrum of diseases involving failure of coordination or
contraction of the oesophagus and its related muscular structures.
Pathological features In some cases, degeneration of the inner and
outer myenteric plexuses can be demonstrated, but often no structural
abnormality is seen.
Clinical features
Achalasia
• Peak ages of incidence in young adulthood (idiopathic) and old age
(mostly degenerational).
• Slowly progressive dysphagia. Initially worse for fl uids than solids.
• Frequent regurgitation of undigested food common late in the disease.
• Secondary recurrent respiratory infections due to aspiration.
Diffuse oesophageal spasm
• Commonest in young adults; ♂ > ♀.
• Characterized by acute pain along the length of the oesophagus
induced by ingestion, especially of hot or cold substances
(odynophagia).
Diagnosis and investigations
Achalasia
• Video barium swallow. A characteristic failure of relaxation of the lower
oesophagus with a smooth outline ‘rat’s tail’ or ‘bird beak’.
• Oesophageal manometry. Hypertonic lower oesophageal high pressure
zone with failure of relaxation normally induced by swallowing; in
chronic cases, the proximal oesophagus may be adynamic.
• Oesophagoscopy. To exclude benign and malignant strictures.
Diffuse oesophageal spasm
• Video barium swallow. ‘Corkscrew’ appearance of the oesophagus
caused by discoordinated diffuse contractions.
• Oesophageal manometry. Diffuse hypertonicity and failure of relaxation;
little or no evidence of coordinated progressive peristalsis during
episodes, but normal peristalsis when asymptomatic.
• Oesophagoscopy. Required to exclude underlying associated
malignancy.
Treatment
Achalasia
• Endoscopically guided controlled balloon dilatation (fi xed pressure).
Successful in up to 80% of patients; low complication rates
(perforation); may need multiple procedures over time.
• Botulinum toxin injections. Success in some patients failing dilatation.

OESOPHAGEAL MOTILITY DISORDERS
275
• Surgical myotomy (Heller’s cardiomyotomy). Usually performed
laparoscopically with division of the lower oesophageal circular muscle
fi bres; highly successful in resistant cases; mostly applicable to young
patients. Specifi c complications include refl ux, obstruction of gastro-
oesophageal junction, oesophageal perforation.
Diffuse oesophageal spasm
• Oral calcium channel blockers or relaxants, e.g. benzodiazepines.
• Long-acting nitric oxide donors (smooth muscle relaxant).
• Widespread oesophageal pneumatic dilatations (often repeated).
• Long surgical open myotomy, rarely undertaken.
Key revision points—anatomy and physiology of the
oesophagus
• Upper two-thirds. Stratifi ed squamous epithelial-lined (develops
squamous carcinoma), striated skeletal muscle, lymphatic drainage
to neck and mediastinal nodes, somatic innervation of sensation (e.g.
moderately accurate location of level of pathology).
• Lower third. Transition to columnar epithelium (develops
adenocarcinoma), transition to smooth muscle, lymphatic drainage to
gastric and para-aortic nodes, visceral innervation (poor localization
of pathology).
• Gastro-oesophageal junction is site of portosystemic anastomosis
(between left gastric and (hemi)azygous veins)—may develop gastric
or oesophageal varices.
• Upper oesophageal sphincter (UOS) = cricopharyngeus.
• Lower oesophageal sphincter (LOS) = functional zone of high
pressure above the gastro-oesophageal junction. Relaxants include
alcohol.
• Swallowing requires intact and coordinated innervation from vagus
(UOS, oesophagus, LOS) and intramural myenteric plexus.

CHAPTER 7 Upper gastrointestinal surgery
276
Pharyngeal pouch
Key facts
• An acquired ‘pulsion’ diverticulum arising in the relatively fi brous tissue
between the inferior constrictor and cricopharyngeus muscle—
‘Killian’s dehiscence’.
• Arises primarily as a result of failure of appropriate coordinated
relaxation of the cricopharyngeus, causing increased pressure on the
tissues directly above during swallowing.
• Typically occurs in the elderly.
• Associated with lower cranial nerve dysfunction (e.g. motor neuron
disease, previous CVA).
Pathological features
• Acquired diverticulum (fi brous tissue and serosa without muscle fi bres
in most of the wall).
• Tends to lie to one side of the midline due to the cervical spine
directly behind.
Clinical features
• Upper cervical dysphagia.
• Intermittent ‘lump’ appearing to the side of the neck on swallowing.
• Regurgitation of food—undigested.
• Nocturnal aspiration—‘waking up coughing’.
Diagnosis and investigations
• Diagnosis may be made on observed swallowing with a transient neck
swelling appearing.
• Video barium swallow will show fi lling of pouch.
2 Gastroscopy should be avoided unless there is a question of associated
pathology since the pouch is easily missed and easily damaged or perfo-
rated by inadvertent intubation.
Treatment
Endoscopic stapled pharyngoplasty—side-to-side stapling of pouch to the
upper oesophagus, which also divides the cricopharyngeus muscle.
This page intentionally left blank

CHAPTER 7 Upper gastrointestinal surgery
278
Hiatus hernia
Key facts
The presence of part or all of the stomach within the thoracic cavity,
usually by protrusion through the oesophageal hiatus in the diaphragm
(see Fig. 7.1).
• Very common; ♀ > ♂; majority are asymptomatic.
• May or may not be associated with gastro-oesophageal refl ux disease
(GORD).
• Predisposing factors include obesity, previous surgery.
Clinico-pathological features
Sliding hernia
• Results from axial displacement of upper stomach through the
oesophageal hiatus, usually with stretching of the phrenico-
oesophageal membrane.
• By far, the commonest form; may result in GORD.
Rolling (para-oesophageal) hernia
Results from the displacement of part or all of the fundus and body of the
stomach through a defect in the phrenico-oesophageal membrane such
that it comes to lie alongside the normal oesophagus.
• Much less common.
• Symptoms include hiccough, ‘pressure’ in the chest, odynophagia.
• May result in volvulus or become incarcerated and cause obstruction.
Diagnosis and investigations
• Upper GI endoscopy (OGD). To exclude oesophageal mucosal
pathology
• Video barium swallow. Usually identifi es the type and extent.
• CT scanning of the thorax. Investigation of choice in acute presentations.
Treatment
Medical (mainly for GORD symptoms)
• Reduce acid production. Stop smoking, lose weight, reduce alcohol
consumption.
• Counteract acid secretion. Proton pump inhibitors (PPIs), symptomatic
relief with antacids, mucosal protectants.
• Promote oesophageal and gastric emptying. Promotilants, e.g.
metoclopramide.
Surgical
Rarely required. Indicated for:
• Persistent symptoms despite maximal medical therapy;
• Established complications of rolling hernia such as volvulus or
obstruction.
HIATUS HERNIA
279
Elective procedure of choice is laparoscopic (or occasionally open) reduc-
tion of the hernia and fi xation (gastropexy), usually with plication of the
oesophageal opening (cural plication), occasionally with a fundoplication
(e.g. Nissen’s operation) if GORD symptoms predominate. Acute presen-
tations may rarely require a partial gastrectomy.
Sliding hernia
Peritoneal sac
Diaphragm
Rolling hernia
Fig. 7.1 Hiatus hernia—sliding and rolling. Reproduced with permission from
Longmore, M. et al. (2007). Oxford Handbook of Clinical Medicine, 7th edn.
Oxford University Press, Oxford.

CHAPTER 7 Upper gastrointestinal surgery
280
Gastro-oesophageal refl ux disease
Key facts
• Pathologically excessive entry of gastric contents into the oesophagus.
• Refl ux occurs in ‘normals’ up to 5% of the time.
• Commonest in middle-aged adults.
• Usually due to gastric acid, but also due to bile refl ux.
• Contributory factors include:
Reduced tone in the lower oesophageal sphincter. • Idiopathic, alcohol,
drugs, previous surgery, secondary to existing peptic stricture.
Increased intragastric pressure. • Coughing, delayed gastric emptying,
large meal.
Pathological features
Oesophagitis
• Results in infl ammatory changes in the squamous-lined oesophagus.
• Varies in severity from minor mucosal erythema and erosions to
extensive circumferential ulceration and stricturing (graded I to IV).
Stricture
• Chronic fi brosis and epithelial destruction may result in stricturing.
• Eventually shortening and narrowing of the lower oesophagus.
• May lead to fi xation and susceptibility to further refl ux.
Oesophageal metaplasia (‘Barrett’s oesophagus’)
• May develop as a result of gastro-oesophageal refl ux; possibly more
commonly in biliary refl ux.
• Normal squamous epithelium is replaced by columnar epithelium
(metaplasia).
• Dysplasia and premalignant change may occur in the columnar
epithelium.
Clinical features
• Dyspepsia may be the only feature; may radiate to back and left of
neck.
• True refl ux may occur with acid in the pharynx.
• Commonly worse at night, after large meals, and when recumbent.
• Dysphagia may occur if there is associated ulceration or a stricture.
Diagnosis and investigations
Under the age of 45 Symptoms are relatively common and can be treated
empirically. Investigation is only required if symptoms fail to respond to
treatment.
Over the age of 45 Refl ux can be confi rmed by 24h continuous pH moni-
toring. Peaks of pH change must correspond to symptoms. OGD should
be performed in all new cases over the age of 45 to exclude oesophageal
malignancy.

GASTRO-OESOPHAGEAL REFLUX DISEASE
281
Treatment
Medical
• Reduce acid refl ux. d Smoking, d weight, d alcohol consumption.
• Counteract acid secretion. PPI (e.g. omeprazole 20mg od), symptomatic
relief with antacids (e.g. Gaviscon
®
10mL PO od).
• i Gastric and oesophageal emptying. Promotilants, e.g. metoclopramide
10mg tds PO.
Surgical
Procedure of choice is laparoscopic fundoplication, ‘Nissen’s operation’
(wrapping fundus of the stomach around the intra-abdominal oesophagus
to augment high pressure zone).
Rarely required. Indicated for:
• Persistent symptoms despite maximal medical therapy.
• Large volume refl ux with risk of aspiration pneumonia.
• Complications of refl ux, including stricture and severe ulceration.
Uncertain role in the prevention of progressive dysplasia in Barrett’s
oesophageal metaplasia in the absence of symptoms.

CHAPTER 7 Upper gastrointestinal surgery
282
Oesophageal tumours
Key facts and pathological features
There are several types of oesophageal tumours.
Adenocarcinoma
• Rapidly increasing incidence in western world; ♂:♀, 5:1.
• Commonest in Western Europe.
• Associated with dietary nitrosamines, GORD, and Barrett’s metaplasia.
• Most commonly occurs in the lower third of the oesophagus.
Squamous carcinoma
• Incidence slightly reducing in western world. Commonest in Japan,
northern China, and South Africa; ♂:♀, 3:1.
• Associated with smoking, alcohol intake, diet poor in fresh fruit and
vegetables, chronic achalasia, chronic caustic strictures.
• May occur anywhere in the oesophagus.
Rhabdomyo(sarco)ma Malignant tumour of skeletal muscle wall of the
oesophagus; very rare.
Lipoma and gastrointestinal stromal tumours (GIST, see b p. 292) Rare.
Clinical features
• Dysphagia. Any new symptoms of dysphagia, especially over the age
of 45, should be assumed to be due to tumour until proven otherwise.
• Haematemesis. Rarely the presenting symptom.
• Incidental/screening. Occasionally identifi ed as a result of follow-up/
screening for Barrett’s metaplasia, achalasia, or refl ux disease. Presence
of high grade dysplasia in Barrett’s is associated with the presence of
an occult adenocarcinoma in 30%.
• Features of disseminated disease. Cervical lymphadenopathy,
hepatomegaly due to metastases, epigastric mass due to para-aortic
lymphadenopathy.
• Symptoms of local invasion. Dysphonia in recurrent laryngeal nerve
palsy, cough and haemoptysis in tracheal invasion, neck swelling
in superior vena cava (SVC) obstruction, Horner’s syndrome in
sympathetic chain invasion.
Diagnosis and investigations
• Diagnosis usually by fl exible oesophagoscopy and biopsy.
• Barium swallow only indicated for failed intubation or suspected post-
cricoid carcinoma (often missed by endoscopy).
Staging investigations
• Local staging. Endoluminal ultrasound scan to assess depth of invasion.
• Regional staging. CT scanning to evaluate local invasion, locoregional
lymphadenopathy, liver disease; laparoscopy to assess for peritoneal
disease in junctional tumours.
• Disseminated disease. PET scanning may be used to exclude occult
disseminated disease in patients otherwise considered for potentially
curative treatment.

OESOPHAGEAL TUMOURS
283
Treatment
Palliative
Most patients present with incurable disease and require palliation.
• Dysphagia can be treated by endoluminal self-expanding metal stenting
(SEMS), external beam radiotherapy; surgery is very rarely indicated
for palliation.
• Metastases. Systemic chemotherapy if symptomatic.
Potentially curative
• Squamous carcinoma. Radical external beam chemoradiotherapy or
neoadjuvant chemotherapy followed by surgery (radical resection).
• Adenocarcinoma (large). Neoadjuvant chemotherapy followed by
surgery (radical resection).
• Adenocarcinoma (small) or high grade dysplasia in Barrett’s. Surgical
resection/EMR/ablation.

CHAPTER 7 Upper gastrointestinal surgery
284
Peptic ulcer disease
Key facts
• Peptic ulceration develops when a breakdown in the mucosal defence
of the stomach or duodenum leads to a mucosal breach.
• May be acute and transient (e.g. stress ulceration after surgery, in
acutely unwell ITU patients).
• If the repair system fails to deal with the breakdown of the mucosa, it
may become chronic.
• The term ‘peptic’ refers to ulcers in columnar mucosa in the lower
oesophagus, stomach, duodenum, or small bowel, usually due to the
action of acid.
Classifi cation
Peptic ulcers can be broadly classifi ed into:
• Gastric ulcers (type I, body and fundal).
• Duodenal and gastric ulcers (type II, prepyloric).
• Atypical ulceration.
Gastric ulceration
• ♂:♀, 3:1; peak age of incidence 50y.
• Associated with Helicobacter (H.) pylori in 45% of cases and with high
alcohol intake, smoking, NSAID use, normal or low acid secretion.
Duodenal and type II gastric ulceration
• ♂:♀, 5:1; peak age of incidence 25–30y.
• Associated with H. pylori in 85% of cases and with high acid secretion,
smoking, NSAID use.
Atypical ulceration
• Usually due to either atypical sites of gastric acid secretion (e.g.
ectopic gastric mucosa in a Meckel’s diverticulum) or abnormally high
levels of acid secretion (e.g. Zollinger–Ellison syndrome; see b p. 285).
• Associated with ulceration that fails to respond to maximal medical
therapy, multiple ulcers, ulcers in abnormal locations (e.g. distal
duodenum or small bowel).
Clinical features
• Nausea and epigastric pain.
• Duodenal ulceration typifi ed by hunger pains with central back pain
relieved by food; pain is often cyclical and occurs in the early hours of
the morning.
• Gastric ulceration typifi ed by pain precipitated by food with associated
weight loss and anorexia; pain less cyclical.
• Vomiting and upper abdominal distension suggest gastric outlet
obstruction.
Diagnosis and investigations
• Gastroscopy. Commonest diagnostic test.
• Barium meal. May be used if gastroscopy contraindicated.

PEPTIC ULCER DISEASE
285
• Urease testing. To assess for presence of H. pylori can be performed on
antral biopsies from gastroscopy or as a CO
2
breath test.
• Fasting serum gastrin levels. If hypergastrinaemia suspected.
Complications
• Acute upper GI bleeding (see b p. 294).
• Iron defi ciency anaemia due to chronic low level bleeding.
• Perforation (see b p. 296).
• Gastric outlet obstruction due to chronic scarring at or around the
pylorus.
Treatment
Medical
• Advice to reduce alcohol intake, stop smoking, avoidance of NSAIDs.
• PPIs (e.g. omeprazole 20mg PO od, lansoprazole) or H
2
blockers (e.g.
ranitidine 150mg PO bd, cimetidine 400mg PO bd) if intolerant to PPI.
• Topical antacids (e.g. Gaviscon
®
, sucralfate, colloidal bismuth),
especially for acute ulceration post-operatively or in ITU patients.
• H. pylori eradication therapy (usually triple therapy of metronidazole,
PPI, and clarithromycin).
Surgical
Rarely necessary with the very highly effective acid-reducing drugs and
eradication therapy. Indications include the following.
• Gastric outlet obstruction not responsive or suitable for endoscopic
dilatation. Usual procedure is pyloroplasty (with or without highly
selective vagotomy) or type II partial gastrectomy (Bilroth II or polya).
• Failure to respond to maximal medical treatment with severe
symptoms or due to habitual recidivism. Procedure is type I partial
gastrectomy for type I gastric ulcer or type II partial gastrectomy for
duodenal ulcer.
• Emergency indications include:
Perforation (see • b p. 296);
Bleeding (see • b p. 294).
Zollinger–Ellison syndrome
• Due to hypergastrinaemia causing extensive, persistent, or typical
ulceration.
• Commonest cause is benign secretory gastrinoma (usually
intrapancreatic); occasionally cause is malignant gastrinoma (associated
with MEN syndromes).
• Diagnosed by raised serum gastrin level, tumour located by CT
scanning, angiography, selective pancreatic venous cannulation at
surgery.
• Treatment. Resection of pancreatic tissue containing tumour.

CHAPTER 7 Upper gastrointestinal surgery
286
Gastric tumours
May arise from the tissues of the mucosa (adenocarcinoma), connective
tissue of the stomach wall (previously known as leiomyoma or leiomyosa-
rcoma, but part of the spectrum of disease called gastrointestinal stromal
tumours (GISTs); see b p. 292), the neuroendocrine tissue (carcinoid
tumours; see b p. 292), or the lymphoid tissue (lymphomas).
Key facts
Adenocarcinoma; commonest age of incidence >50y; ♂:♀, 3:1. Predis-
posing factors include:
• Diet rich in nitrosamines (smoked or fresh fi sh, pickled fruit);
• Chronic atrophic gastritis;
• Blood group A;
• Chronic gastric ulceration related to H. pylori.
Clinical features
Symptoms
• Dyspepsia (any new onset of dyspepsia over the age of 45 should be
considered to be due to adenocarcinoma until proven otherwise).
• Weight loss, anorexia, and lethargy.
• Anaemia (iron defi ciency due to chronic blood loss).
• Occasionally presents as acute upper GI bleeding (see b p. 294).
• Dysphagia uncommon unless involving the proximal fundus and
gastro-oesophageal junction.
Signs
• Weight loss.
• Palpable epigastric mass.
• Palpable supraclavicular lymph node (Troisier’s sign) suggests
disseminated disease.
Diagnosis and investigation
• Diagnosis usually by gastroscopy (barium meal may be required if
gastroscopy contraindicated).
• Staging (see Table 7.1) investigations include:
Thoraco-abdominal CT scan to assess for distant metastases and •
local lymphadenopathy.
Endoluminal ultrasound to assess for local disease.•
Laparoscopy (for patients considered for potential resection) to •
exclude small volume peritoneal metastases.
Treatment
Unfortunately, in the UK, the majority of tumours are metastatic or unre-
sectable due to local extension and so not suitable for consideration of
surgical treatment for cure. The majority of treatment is directed at symp-
toms and palliation. When a tumour is considered potentially curable, the
treatment offered is based on the extent of disease at staging.

GASTRIC TUMOURS
287
Early gastric cancer (T1 or 2, N0/1, P0, H0)
• Suitable for attempted curative resection if patient medically fi t enough.
• Surgery. Radical gastrectomy usually preceded by neoadjuvant
chemotherapy in patients fi t.
• Patients now frequently offered preoperative and post-operative
chemotherapy.
• Local resection or ablation has an uncertain place in treatment.
Advanced gastric cancer (T3 or more or any of N2/P1+/H1+)
• Patients will be offered pre- and post-operative chemotherapy.
• Surgical intervention unlikely to be curative.
• May be undertaken for palliative treatment.
• Local ablation for symptom control occasionally possible.
• Palliative chemotherapy occasionally effective for disseminated disease.
Key revision points—anatomy and physiology of the
stomach
• The fundus is predominantly a storage zone with few active cells.
• The body contains mostly chief cells (secrete pepsinogen; stimulated
by gastrin and local ACh release) and oxyntic cells (secrete H
+
;
stimulated by gastrin, histamine, and ACh; inhibited by H
+
, secretin,
and GIP).
• The antrum contains G cells (secrete gastrin; stimulated by ACh
from vagus, stretch; inhibited by VIP, secretin, H
+
).
• The pyloric sphincter is a functional sphincter of circular muscle.
• Arterial supply is profuse (gastric ischaemia is rare) via coeliac
axis—left gastric, splenic, and common hepatic arteries.
• Lymphatic drainage follows arteries and is profuse (signifi cant lymph
node metastases are usually fatal).
Table 7.1 TNM staging of gastric cancers
T (tumour) N (nodes) M (metastases)
Tis, in situ within
mucosa
N0, no lymph nodes P (peritoneal metastases)
T1, confi ned to
submucosa
N1, involved nodes
within 3cm of primary
P0, no peritoneal
metastases
T2, confi ned to
muscle wall
N2, involved nodes
more than 3cm from
primary
P1/2/3, peritoneal
metastases in increasing
extent
T3, involvement of
serosal surface
H (hepatic metastases)
T4, involvement of
other organs
H0, no hepatic metastases
H1/2/3, hepatic metastases
in increasing extent

CHAPTER 7 Upper gastrointestinal surgery
288
Chronic intestinal ischaemia
Caused by chronic reduction in blood supply to the intestine without
acute threat to the viability of the bowel.
Key facts
Chronic intestinal ischaemia is uncommon. Usually presents with vague
symptoms and diagnosis is often prolonged. Causes include:
• Progressive atherosclerosis affecting the visceral vessels; usually
requires more than one vessel to be affected (e.g. superior mesenteric
artery occlusion and coeliac artery stenosis).
• Obliterative small vessel disease (e.g. thromboangiitis obliterans,
systemic sclerosis, severe diabetic vasculopathy).
Clinical features
Symptoms
• Commonest symptom is mesenteric angina, chronic central abdominal
pain brought on by eating; associated with nausea and vomiting.
• May present with weight loss, general anorexia, and malnutrition.
• Often associated with other features of extensive vascular disease such
as renal impairment, coronary disease, claudication.
Signs
Weight loss, central abdominal tenderness.
Diagnosis and investigations
• Usually diagnosed by imaging of the visceral arteries in combination
with clinical symptoms. Methods of imaging visceral vessels include:
CT angiogram.•
MR angiogram.•
Transfemoral digital subtraction angiogram/aortogram.•
• Other investigations should include:
Assessment of renal function.•
Assessment of coronary circulation.•
Exclusion of aneurysmal disease.•
• Small vessel disease may require autoimmune screen.
Complications
• Acute intestinal ischaemia is less common due to the development of
collaterals although, if undiagnosed, loss of all visceral vessels results in
eventual pan-intestinal infarction.
• Chronic ischaemic strictures due to focally severe ischaemia.
Treatment
Medical
• Stop smoking.
• Control of hypertension; treatment of any hyperlipidaemia.
• Aspirin 75mg od to prevent thromboembolic events.
• Control diabetes if present.
• Treatment of autoimmune disease if present.

CHRONIC INTESTINAL ISCHAEMIA
289
Interventional
Commonest treatment for large vessel stenosis is radiologically guided
stenting. Risks converting stenosis to acute occlusion with the risk of pre-
cipitating emergency surgery. Not possible if the stenosis is at the aortic
ostium of the vessel affected.
Surgical
• Rarely indicated. Commonest procedure is external iliac to ileocolic
artery side-to-side bypass.
• Overall prognosis is poor as the underlying disease process is often
widespread and progressive.
Key revision points—anatomy and physiology of the
small intestine
• Duodenum is a secretory and digestive organ; described in four parts
(second part admits the common bile and pancreatic ducts via the
ampulla of Vater on the medial wall).
• Jejunum is a secretory and digestive organ. Typical features include:
thick, red-purple wall; prominent plicae circulares; single arterial
arcades with long mesenteric vessels.
• Ileum is predominantly an absorptive organ. Typical features include:
thin blue-purple wall; prominent lymphoid aggregates; multilayered
mesenteric arterial arcades.
• Terminal ileum is a specialized area of ileum, particularly concerned
with absorption of bile salts, vitamin B12/IF complex.

CHAPTER 7 Upper gastrointestinal surgery
290
Surgery for morbid obesity
Key facts
• Approximately 25% of UK adult population are classifi ed as obese, a
body mass index (BMI) of greater than 30kg/m
2
.
• Admissions to hospital due to obesity-related disease have increased
eightfold in the last 10y.
• Obese patients are exposed to an increased risk of chronic diseases,
particularly type 2 diabetes, hypertension, hyperlipidaemia, and
arthritis.
• Increased risk of some forms of cancer (colon and endometrial).
Clinical features
Symptoms
• The symptoms of obesity are generally related to the underlying
condition that develops in association with it.
• Commonly, patients fi nd their physical capacity is reduced and they
become short of breath more easily on exertion
Signs
• Examination of obese patients can be challenging as their size can
reduce the ability to elicit clinical signs.
• Assess for signs of respiratory disease, dyspnoea at rest.
• Investigation of possible biliary disease (common bile duct stones,
biliary strictures, biliary tumours, biliary injuries, intrahepatic biliary
disease).
• Investigation of pancreatic disease (pancreatic duct strictures,
pancreatic duct abnormalities).
• Therapeutic interventions for pancreatico-biliary disease:
Stenting for common bile duct stones, strictures, tumours.•
Sphincterotomy for the extraction of biliary stones.•
Diagnosis and investigations
Exclude underlying endocrine disorders—Cushing’s disease, hypothy-
roidism, polycystic ovarian syndrome.
Treatment
Medical
• Diabetic control. Oral hypoglycaemics and insulin, if necessary.
• Medication for anti-hypertensive and cardiovascular disease.
• Psychological and dietetic support for weight loss programme.
• Anti-obesity medication. Orlistat (reduces fat absorption) is the
commonest.
Surgical
• Should only be considered after non-surgical treatments have failed
and patient has been through a preoperative assessment to ensure
they are able to make the necessary post-operative dietary and
lifestyle changes.
• Procedures have either a restrictive or malabsorptive effect.

SURGERY FOR MORBID OBESITY
291
• Adjustable gastric banding is the commonest restrictive operation
(the band is placed around the cardia of the stomach and restricts the
volume of food, but not liquid that can be ingested at one time).
• Gastric bypass involves a restrictive element (division of the stomach
to create a small remnant) and a malabsorptive element (division and
re-anastomosis of small bowel to reduce its ability to absorb food).
• These procedures are most commonly performed laparoscopically.

CHAPTER 7 Upper gastrointestinal surgery
292
Small bowel tumours
Key facts
The small bowel is a rare location for tumours. Tumours may arise from:
• Mucosa small bowel—adenocarcinoma (<5% of all GI malignancies).
• Neuroendocrine tissue, e.g. carcinoid tumours.
• Connective tissue of the bowel wall, e.g. GIST, lipoma.
• Lymphoid tissue (lymphoma).
Adenocarcinoma of small bowel
• Forty per cent of all small bowel tumours.
• Commonest in the duodenojejunal junction and proximal jejunum;
least common in the mid- and distal ileum.
• Associations:
Familial adenomatous polyposis (third commonest location of •
adenocarcinoma after colorectum and duodenum).
Peutz–Jegher’s syndrome.•
Crohn’s disease.•
Untreated longstanding coeliac disease.•
Carcinoid tumours
• Twenty-fi ve per cent of all small bowel tumours.
• Commonest in the distal small bowel; may arise in the appendix or
Meckel’s diverticulum.
• Majority are benign (non-metastatic).
• May produce enteric hormones (e.g. 5-HT, kallikrein, substance P);
hormone effects only occur when the primary is able to secrete
hormones into the systemic circulation or when hepatitic metastases
secrete into the caval circulation (carcinoid syndrome; see b p. 293).
GIST (gastrointestinal stromal tumours)
• Ten per cent of small bowel tumours.
• Arise from the mesenchymal tissues of the bowel wall and mesentery
(smooth muscle cells, fi broblasts, lipocytes).
• Previously called variously leiomyo(sarc)oma, lipo(sarco)ma.
• Tumours of myenteric plexus tissues are a variant called GANT
(gastrointestinal autonomic nerve tumours).
Primary lymphoma
• Twenty per cent of small bowel tumours.
• Arise from the lymphoid tissue of the small bowel wall.
• Almost always non-Hodgkin’s; commonest are B-cell lymphomas
arising from the mucosa-associated lymphoid tissue (MALTomas).
Clinical features
• Adenocarcinoma of small bowel. Often presents late with metastases;
may present with small bowel obstruction, recurrent abdominal pain,
or recurrent/occult GI bleeding.
• Carcinoid tumours. Commonest presentation is incidental fi nding after
appendicectomy or Meckel’s diverticulectomy.

SMALL BOWEL TUMOURS
293
• Carcinoid syndrome. Rare (typifi ed by fl ushing, tachycardia, colicky
abdominal pain, diarrhoea, wheezing).
• GIST. Often present with slow-growing abdominal mass, vague
abdominal pain; may present with occult GI bleeding due to tumour
ulceration.
• Primary lymphoma. Presents with malaise, abdominal pain, diarrhoea;
may present with acute perforation or small bowel obstruction.
Diagnosis and investigations
Common diagnostic tests for all are:
• CT chest and abdomen. Identifi es primary tumour, assesses extent of
involvement of other tissues, assesses possible metastatic disease.
• CT angiography. For assessment of particularly vascular tumours or
GISTs lying close to major visceral vessels to establish resectability.
• Small bowel contrast study. Rarely required for identifi cation of primary
tumours.
• Ileoscopy. May demonstrate proximal lesions presenting with occult,
recurrent upper GI bleeding.
Complications
• Bleeding. Common with adenocarcinoma and some GISTs.
• Obstruction. Especially adenocarcinoma, GISTs, GANTs, and lymphoma.
• Perforation. Commonest with lymphoma, especially shortly after
starting chemotherapy (due to bowel wall replacement by tumour);
also occurs with adenocarcinoma.
• Malabsorption. Often with lymphoma if widespread.
Treatment
Surgery
• Primary surgical resection with macroscopic clearance of tumour for
adenocarcinoma, carcinoid, GISTs, GANTs.
Potentially curative for non-metastatic disease.•
Palliative to prevent complications if disease is metastatic.•
• Surgical resection may be indicated for primary lymphoma prior to
chemotherapy if there is a high risk of perforation of primary.
• Surgical resection of metastases is uncommon.
Medical
• Chemotherapy. Lymphoma and metastatic adencarcinoma.
• Hepatic abalation/embolization. Used to treat carcinoid metastases to
treat symptoms of carcinoid syndrome.
• Imatinib (Glivec
®
) (anti-CD113). Treatment for GISTs that are positive
for c-Kit.

CHAPTER 7 Upper gastrointestinal surgery
294294
Acute haematemesis
Key facts
See Table 7.2 for predicting mortality of acute upper GI bleeding.
• Incidence 1 in 1000 adults per year in UK.
• Twenty per cent require intervention because of ongoing bleeding or
rebleeding.
• Haematemesis is vomiting of blood, usually due to bleeding proximal
to the duodenojejunal junction.
• Melaena is the passage of altered blood (dark purple, pitch black, or
‘tarry’, usually due to bleeding below the gastro-oesophageal junction.
Causes and features
• Peptic (gastric or duodenal) ulceration (benign, 50%). Fresh red blood
with clots, occasionally mixed with food.
• Oesophageal varices. Copious dark red venous blood with little mixing
with food; features of portal hypertension (e.g. caput medusa).
• Oesophageal ulceration. Small volumes of bright red blood/streaks
typical.
• Oesophageal trauma (‘Mallory–Weiss tear’). Small volumes of fresh
bright blood preceded by violent or prolonged vomiting or retching.
• Vascular malformations/lesions (e.g. ‘Dieu la Foy’).
• Gastric carcinoma, leiomyoma.
• Aortoenteric fi stula. Copious bright red blood (often rapidly fatal).
Associated or predisposing conditions
• Agents affecting mucosal health—NSAIDs, steroids, alcohol, major
trauma, or massive burns.
• Agents worsening risk of bleeding—anticoagulants.
Emergency management
Resuscitation
• Establish large calibre IV access; give crystalloid fl uid up to 1000mL if
tachycardic or hypotensive. Only use O –ve blood if the patient is in
extremis; otherwise wait for cross-matched blood if transfusion needed.
• Catheterize and place on a fl uid balance chart if hypotensive.
• Send blood for FBC (Hb, WCC), U&E (Na, K), LFTs (albumin), cross-
match (at least 3U if haematemesis large), clotting.
• Always consider alerting HDU/ITU if very unwell.
• Monitor pulse rate, BP, and urine output (urinary catheter).
• Insertion of a Sengstaken–Blakemore gastro-oesophageal tube may be
a life-saving resuscitation manoeuvre.
Establish a diagnosis
• Urgent OGD is the investigation of choice (at least within 24h).
May require ongoing resuscitation with anaesthetist present.•
Allows diagnosis and biopsy if appropriate.•
May allow therapeutic interventions including adrenaline injection, •
heater probe coagulation, banding of varices, clipping vessel.

ACUTE HAEMATEMESIS
295295
• Angiography.
Occasionally suitable for active bleeding due to invasive •
intervention done in radiology department.
May allow selective embolization in some patients with recurrent •
bleeding.
Early treatment
• Give IV PPI (e.g. omeprazole 40mg IV); stop all NSAIDs.
• Blood transfusion if large volume haematemesis or drop in Hb.
• Ensure the appropriate surgical team knows of the patient in case
surgical intervention is required.
• Known or suspected liver disease—consider FFP to correct clotting.
• Surgery may be required if:
Massive haemorrhage requiring ongoing resuscitation.•
Failed initial endoscopic treatment with ongoing bleeding.•
Rebleeding not suitable for repeated endoscopic treatment.•
Defi nitive management
Varices (see b p. 322)
• Endoscopy coagulation or banding/interventional radiology/surgery.
• IV vasopressin or analogues.
Gastric/duodenal ulcer
• Endoscopic coagulation or injection; may be repeated if suitable ulcer.
• Surgery for failed primary management or rebleeding that is unsuitable
for attempted repeat endoscopic treatment.
• Surgical options—local excision of gastric ulcer or partial gastrectomy;
under-running of duodenal ulcer.
• H. pylori eradication (triple therapy).
Gastric carcinoma
• Endoscopic treatment often not effective.
• Partial or subtotal gastrectomy (often palliative, rarely curative).
Oesophageal trauma Oral antacids.
Aortoenteric fi stula Surgery if the patient survives beyond diagnosis.
Table 7.2 Rockall score for predicting mortality of acute upper GI
bleeding*
Rockall score
0 1 2
Age (y) <60 60–79 >80
Shock None HR
>100bpm
Systolic BP
<100mmHg
Comorbidities None Cardiac Hepatorenal
disease
Carcinoma
* Percentage mortality for scores: 0, <1%; 1, 3%; 2, 6%; 3, 11%; 4, 25%; 5, 40%; 6, >80%.

CHAPTER 7 Upper gastrointestinal surgery
296296
Acute upper GI perforation
Causes and features
• Duodenal ulceration.
• Gastric ulceration (usually anterior prepyloric; less commonly anterior
body).
• Gastric carcinoma.
• Traumatic, e.g. fi sh bone perforation.
• Ischaemic (usually secondary to gastric volvulus).
Symptoms
• Acute onset upper abdominal pain. Severe, constant, worse with
breathing and moving; may radiate to back or shoulders.
• Prodrome of upper abdominal pain (in benign or malignant ulceration).
• Copious vomiting and upper abdominal distension suggest volvulus.
• Prodrome of weight loss, dyspepsia, and anorexia suggests carcinoma.
Signs
• Generalized peritonism common (‘board-like’ generalized rigidity with
marked guarding and tenderness).
• Localized upper abdominal peritonism may occur, especially
with previous surgery where adhesions may act to contain the
contamination.
• Mild fever, pallor, tachycardia, and hypotension (often profound due
to autonomic reaction); typically respond quickly to modest fl uid
resuscitation.
Emergency management
Resuscitation
• Establish large calibre IV access; give crystalloid fl uid up to 1000mL if
tachycardic or hypotensive.
• Catheterize and place on a fl uid balance chart.
• Send blood for FBC (Hb, WCC), U&E (Na, K), LFTs (albumin), group
and save, clotting.
Establish a diagnosis
• Erect CXR (looking for free gas). If the CXR is non-diagnostic, a lateral
decubitus abdominal fi lm can be performed, although a CT is more
common.
• CT scan if diagnosis unclear on CXR; may demonstrate presence of
gastric carcinoma.
Early treatment
• Once the diagnosis of perforation is confi rmed on clinical or
radiological grounds, the treatment is surgical unless:
The patient declines.•
The patient is considered unlikely to survive and supportive care is •
deemed more appropriate.
• Conservative management. IV PPI, limited oral intake, active
physiotherapy—has a very limited role in management; it offers an
outcome similar to that of surgery only in cases where the perforation

ACUTE UPPER GI PERFORATION
297297
has sealed at the time of presentation, there is no haemodynamic
instability, and there are no signs of peritonism.
Defi nitive management
Duodenal ulcer
• Sutured closure with omental patch.
• Empirical oral triple therapy for H. pylori.
• Defi nitive surgery (partial gastrectomy, vagotomy, and drainage
procedure) should only be performed for recurrent perforation, failed
fully compliant medical therapy, recidivist non-compliant patient.
Gastric ulcer
• Sutured closure with omental patch if prepyloric.
• Local excision and sutured closure if body.
Gastric carcinoma Partial gastrectomy (usually palliative).
Traumatic Sutured closure.
Volvulus with ischaemia Usually subtotal gastrectomy.

CHAPTER 7 Upper gastrointestinal surgery
298298
Acute appendicitis
Causes and features
2 Commonest cause of urgent abdominal surgery and the common provi-
sional diagnosis of all emergency surgical admissions in the UK.
• Can affect any age, but uncommon under the age of 4 and over the age
of 80. Peak age of incidence is early teens to early twenties. Three types:
Mucosal.• Mildest form usually diagnosed by pathology reporting.
Phlegmonous.• Typifi ed by slow onset and relatively slow progression.
Necrotic.• Often due to acute bacterial infection with ischaemic
necrosis; leads to perforation unless treated by surgery.
Differential diagnosis
• Children.
Non-specifi c abdominal pain, including ‘mesenteric adenitis’.•
Meckel’s diverticulitis.•
Ovarian cyst/menstrual symptoms (peri-menarchal girls).•
• Adults.
Terminal ileal pathology• . Crohn’s, Meckel’s diverticulitis,
gastroenteritis.
Retroperitoneal pathology• . Pancreatitis, renal colic.
Ovarian pathology• . Ectopic pregnancy, cyst, infection, menstrual pain.
• Older adults.
Ileocaecal pathology• . Caecal diverticulitis, caecal tumours.
Colonic pathology• . Sigmoid diverticulitis.
Ovarian pathology• . Cysts, infection, tumours.
Clinical features
• Symptoms.
Malaise, anorexia, and fever.•
Diarrhoea common and may be mistaken for acute (gastro)•
enteritis.
Abdominal pain starts centrally and localizes to the right iliac fossa •
(RIF).
Abdominal pain caused by coughing and moving.•
• Signs.
Fever, tachycardia.•
Abdominal tenderness. Peritonism suggests perforation (local or •
generalized). Often maximal over ‘McBurney’s point’ (see opposite),
but only if appendix is in conventional anatomical position.
Palpation of left iliac fossa (LIF) causes pain worse in RIF (• Rovsing’s
sign).
• Investigations may be normal and none are diagnostic or exclusive (see
b p. 299).
Complications
• Perforation (localized or generalized).
• RIF ‘appendix’ mass (usually appendicitis with densely adherent caecum
and omentum, forming a ‘mass’).
• RIF abscess (usually 2* to perforated retrocaecal appendicitis).
• Pelvic abscess (usually 2* to perforated pelvic appendicitis).

ACUTE APPENDICITIS
299299
Emergency management
Resuscitation
• Establish IV access.
• Catheterize and place on a fl uid balance chart only if d BP or septic.
• Request FBC (Hb, WCC), U&E (Na, K), CRP (usually i WCC, CRP).
Establish a diagnosis
• The diagnosis is a clinical one in all but exceptional cases and
investigations are usually unnecessary.
• CT is appropriate in adults, especially over the age of 65 or if the
diagnosis is unclear since the differential diagnosis is much wider and
appendicitis relatively less likely above this age.
• CT is the best investigation in suspected appendix mass or abscess.
• Ultrasound scan (pelvic) is indicated in young women of childbearing
age if ovarian pathology is suspected.
• Laparoscopy is a useful surgical diagnostic manoeuvre, allowing
diagnosis of pelvic pathology, e.g. pelvic infl ammatory disease (PID)
without a major abdominal incision.
Early treatment Avoid giving IV antibiotics without a clear diagnosis.
Defi nitive management
2 Acute appendicitis
• Open or laparoscopic appendicectomy.
• IV antibiotics on induction; continued antibiotics only indicated for
perforation.
Appendix mass or appendix abscess
• IV antibiotics (e.g. cefuroxime 750mg tds + metronidazole 500mg tds).
• If symptoms settle, delayed (interval) appendicectomy after 6 weeks.
• If symptoms fail to settle, may need acute appendicectomy.
• Appendix abscess may be amenable to CT-guided drainage.
Key revision points—anatomy of appendicectomy
• Commonly retrocaecal, but may be pelvic, retroileal, or retrocolic.
• Taenia coli of caecum converge at base of appendix and aid
location, especially in diffi cult locations.
• Small mesentery with sole blood supply from appendicular artery
(a terminal branch ileocolic) which may thrombose causing gangrene.
• Principles of appendicectomy are as follows:
Muscle splitting gridiron• incision centred at McBurney’s point.
Lapararoscopic approach• increasingly popular.
Appendix is carefully located and delivered into the wound.•
The mesentery of the appendix is divided and ligated.•
The appendix is clamped and tied at the base and excised.•
Some surgeons invaginate the stump using a purse-string in the •
wall of the caecum round the base of the appendix.

CHAPTER 7 Upper gastrointestinal surgery
300300
Acute peritonitis
Defi ned as acute infl ammation in the peritoneal cavity.
Causes
May be primary (rare) or secondary (common).
• Primary peritonitis. Typically streptococcal with probable portal of
entry via bloodstream rather than intra-abdominal organs.
• Commonest causes of secondary peritonitis are:
Acute perforated appendicitis • (see b p. 298)—commonest cause of
peritonitis especially in under 45s).
Acute perforated diverticular disease • (see b p. 404)—commonest
cause in elderly.
Upper GI perforation • (see b p. 296).
Perforated tumours (colonic or gastric).•
Perforated ischaemic bowel, e.g. due to adhesions.•
Acute pancreatitis (usually infl ammatory rather than infective).•
Peritoneal dialysis-related—often atypical or cutaneous organisms •
gaining entry via contaminated dialysate bags or catheter.
Post-surgical intervention, e.g. anastomotic leak, enteric injury.•
Clinical features
There are features common to all causes. Additional features suggestive of
an underlying cause should also be sought, particularly in the history.
Symptoms
• Anorexia and fever.
• Severe generalized abdominal pain radiating to shoulders and back.
• Abdominal pain worse with movement, coughing, sneezing.
Signs
• Fever, tachycardia.
• Generalized abdominal tenderness with guarding and rigidity.
• Differential maximal tenderness may indicate the possible underlying
cause.
• Gentle palpation may allow identifi cation of an underlying mass.
Emergency management
Resuscitation
• Establish large calibre IV access.
• Catheterize and place on a fl uid balance chart.
• Send blood for FBC (Hb, WCC), U&E (Na, K), CRP, amylase, group
and save.
• ABGs if shocked or ischaemic bowel/pancreatitis suspected.
Establish a diagnosis
Most causes of acute peritonitis require surgery to correct them, but sur-
gery is contraindicated in most cases of acute pancreatitis.
Diagnostic investigations are indicated if the patient would otherwise be
a candidate for surgical intervention.

ACUTE PERITONITIS
301301
• Blood investigations may show neutrophilia, i CRP.
• Raised amylase may suggest pancreatitis.
• Abdominal CT scanning is the investigation of choice for diagnosis.
It should reliably exclude acute pancreatitis and often locate the
probable source of the pathology.
• Laparoscopy is occasionally useful in patients where a formal
laparotomy should be avoided if possible.
Early treatment
IV antibiotics are appropriate without a clear diagnosis, especially if surgery
is likely (e.g. metronidazole 500mg IV tds + cefuroxime 750mg IV tds).
Defi nitive management
Acute appendicitis See b p. 298.
Upper GI perforation See b p. 296.
Perforated diverticular disease
• IV antibiotics (e.g. cefuroxime 750mg tds + metronidazole 500mg tds).
• Surgical treatment involves resection of the affected segment.
Depending on the length of time from perforation, extent of the
contamination, and extent of infl ammation in the affected segment, the
bowel may be anastomosed (primary anastomosis) or the proximal
end brought out on to the abdominal wall as an end colostomy
(‘Hartmann’s type’ resection).
Perforated tumour Surgical resection is required even if palliative. Ends of
bowel may be re-anastomosed or exteriorized as stomas, depending on
the circumstances (degree of contamination, underlying pathology).
Primary peritonitis or continuous ambulatory peritoneal dialysis
(CAPD)-related peritonitis
• A diligent and systematic search is necessary to ensure there is no
occult source of perforation as a cause.
• Primary treatment is extensive lavage of all quadrants and treatment
with appropriate antibiotics (guided by culture results from peritoneal
fl uid).

CHAPTER 7 Upper gastrointestinal surgery
302302
Acute abdominal pain
Acute abdominal pain is the commonest emergency presentation to hos-
pitals in the UK. It is often a daunting challenge to the admitting team
because of the huge differential diagnosis possible, and the wide range
of tests available to try and establish a diagnosis. Be methodical and
remember some simple rules.
• Take a proper history and examination; do not work to the diagnosis
given to you by the referring doctor.
• Resuscitate the patient properly and give adequate analgesia; this often
helps to clarify the diagnosis. There is no reason to withhold analgesia
prior to ‘senior’ clinical examination.
• Try to clarify if you think the patient has signs of peritonitis (localized
or generalized); this will narrow the differential and may require
surgery as part of the diagnostic work-up.
Causes
Causes approximate to the fact that pathology of underlying structures
in each region tend to give rise to abdominal pain maximal in that
region (see Box 7.1). Although a good guide, it is wise to remember that
viscera are often mobile and pain often radiates to adjacent sections of
the abdomen.
Clinical features
Each condition has its own clinical features, but here are some rules to
follow.
Symptoms and signs
• Constant pain, gradual in onset, but progressive worsening suggests an
underlying infl ammatory cause.
• Intermittent pain that is poorly localized suggests colic arising from a
visceral structure.
• Central and lower abdominal pain in children (under the age of 12) is
self-limiting (non-specifi c) in 70%, from benign gynaecological causes in
25% (girls), and only pathological in 10–20%.
• Severe pain out of proportion to the clinical signs suggests ischaemic
bowel until proven otherwise.
• Pain in the loin or back arises from (at least partially) retroperitoneal
structures; consider the pancreas, renal tract, and abdominal aorta.
Emergency management
Resuscitation
• Establish IV access.
• Catheterize and place on a fl uid balance chart only if hypotensive.
• Give adequate analgesia. If renal pathology is suspected, diclofenac
(Voltarol
®
) 100mg pr is very effective (avoid in asthma and renal
disease). If intra-abdominal pathology is suspected, 5–10mg morphine
iv is reasonable. Morphine IV never hides established clinical signs; it
often helps to clarify the diagnosis by its anxiolytic effect on patients.
• Send blood for FBC (Hb, WCC), U&E (Na, K), amylase, LFTs, CRP,
group and save.

ACUTE ABDOMINAL PAIN
303303
Establish a diagnosis
The time frame for the diagnosis of acute abdominal pain varies according
to the presentation. It is not uncommon for 12–24h of ‘masterful inac-
tivity’ to be used to allow the diagnosis to be clarifi ed. Young patients
with central and mild RIF pain are typical of this sort of management. Do
not assume this is normal. Some causes of acute abdominal pain require
diagnosis and management immediately upon admission or within 6–8h or
less. Try to be thoughtful in diagnostic tests; many may be requested, but
usually only one or two are really useful.
• Blood investigations are very rarely diagnostic. Serum amylase more than
3x normal maximum is very highly suggestive of acute pancreatitis.
• Plain abdominal radiographs are very rarely diagnostic.
• Always request a plain erect chest radiograph; it is the fi rst-line test of
choice for free abdominal air.
• Upper abdominal ultrasound is an excellent investigation for suspected
hepatobiliary pathology.
• Pelvic ultrasound (transabdominal or transvaginal) is a good test for
suspected tubo-ovarian disease.
• CT scanning may well be indicated and is a good ‘general survey ‘
of the abdomen, but exposes the patient to signifi cant radiation and
should not be routinely requested.
Box 7.1 Causes of acute abdominal pain arranged
according to abdominal region
Right hypochondriac Epigastric Left hypochondriac
• Right lower lobe
pneumonia/
embolism
• Cholecystitis
• Biliary colic
• Hepatitis
• Pancreatitis
• Gastritis
• Peptic ulcer
• Myocardial
infarction
• Left lower lobe
pneumonia/
embolism
• Large bowel
obstruction
Right lumbar Umbilical Left lumbar
• Renal colic
• Appendicitis
• Intestinal
obstruction
• Intestinal ischaemia
• Aortic aneurysm
• Gastroenteritis
• Crohn’s disease
• Renal colic
• Large bowel
obstruction
Right iliac Hypogastric Left iliac
• Appendicitis
• Crohn’s disease
• Right tubo-ovarian
pathology
• Cystitis
• Urinary retention
• Dysmenorrhoea
• Endometriosis
• Sigmoid
diverticulitis
• Left tubo-ovarian
pathology

CHAPTER 7 Upper gastrointestinal surgery
304304
Early treatment
• IV antibiotics are inappropriate without a clear diagnosis; they will
suppress, but may not adequately treat, developing infection.
• Until a defi nitive management plan is established, concentrate on fl uid
balance, analgesia, and monitoring vital signs.
This page intentionally left blank

CHAPTER 7 Upper gastrointestinal surgery
306306
Gynaecological causes of lower
abdominal pain
Gynaecological pathologies are a common cause of lower abdominal
pain, not only in women of childbearing age, but they can also affect
post-menopausal women.
Causes
• Complications of menstruation. Retrograde menstruation, mid-cycle
ovulation pain (‘Mittelschmerz’).
• Ovarian cyst. Acute swelling, rupture, torsion.
• Tubo-ovarian infection, including PID, abscess.
• Ectopic pregnancy, including rupture.
Clinicopathological features
Complications of menstruation
• Commonest during development of regular periods.
• Typically cyclical pains, often sharp and sudden in onset.
• May have marked tenderness bordering on peritonitis.
• Normal blood investigations; self-limiting.
Ovarian cyst complications
• Commonest in mid-childbearing years.
• May have severe pain with few clinical signs.
• Normal blood investigations.
Tubo-ovarian infection
• Commonly caused by Escherichia coli, Bacteroides fragilis,
Streptococcus sp.
• Associated with cervical disease or instrumentation.
• Sexually-transmitted infections can cause tubo-ovarian sepsis, which
may be more chronic and recurrent (Neisseria gonorrhoeae,
Chlamydia trachomatis).
• Associated with multiple sexual partners and unprotected intercourse.
• Pyrexia, mild tachycardia, occasional purulent vaginal discharge.
• Often affects both sides causing bilateral pain and tenderness.
Ectopic pregnancy
• May occur at any age.
• Commonest site is the Fallopian tube (ampulla, tube, or isthmus).
• Associated with previous tubal disease or surgery.
• Menstrual irregularity or a ‘late’ period is common, but not uniform.
• May give rise to symptoms whilst enlarging with unilateral pelvic pain.
• Symptoms increase with complications (bleeding into site of
pregnancy, free rupture with bleeding into pelvis and peritoneal cavity).
• Typifi ed by lower abdominal pain without fever.
• Hypotension with tachycardia suggests active intra-abdominal bleeding,
but is fortunately rare at presentation.

GYNAECOLOGICAL CAUSES OF LOWER ABDOMINAL PAIN
307307
Emergency management
Resuscitation
• Establish large calibre IV access if an ectopic pregnancy is suspected.
• Catheterize and place on a fl uid balance chart only if hypotensive.
• Give adequate analgesia (5–10mg morphine IV is reasonable).
• Send blood for FBC (Hb, WCC), U&E (Na, K), CRP, group and save.
Establish a diagnosis
• Urine B-HCG (and serum B-HCG where urine test is positive since
this is more reliable). All women of childbearing age should be assumed
to be pregnant until proven otherwise. Pregnancy testing may be
negative in ectopic pregnancy if the fetus is already dead by the time of
presentation.
• High vaginal swabs should be taken if tubo-ovarian sepsis is suspected.
• Pelvic ultrasound (transabdominal or transvaginal) is the diagnostic
investigation of choice unless the patient is acutely unstable. It has a
high sensitivity and good specifi city.
• Laparoscopy is a very common diagnostic investigation. It allows a fi rm
diagnosis of most gynaecological pathology and may be therapeutic
(e.g. pelvic lavage, cyst treatment).
Early treatment
• IV antibiotics for a clear diagnosis of pelvic infection.
• If ruptured or bleeding ectopic pregnancy is seriously considered,
make sure the surgical and gynaecological teams are aware. Direct
transfer to theatre may be necessary.
Defi nitive management
• Complications of menstruation. Conservative management—pelvic
lavage if laparoscopy is performed.
• Ovarian cyst complications. Ovarian preservation if below the age of
menopause; cystectomy or drainage where possible.
• Tubo-ovarian infection. Cephradine 500mg tds PO and metronidazole
400m PO tds for non-sexually transmitted infections; metronidazole
400mg PO for chlamydia; IV penicillin for neisserial infections.
• Ectopic pregnancy. Conservation or reconstruction of the affected
tube/ovary wherever possible. If not salvageable, unilateral salpingo-
oophrectomy.

CHAPTER 7 Upper gastrointestinal surgery
308308
Intra-abdominal abscess
Key facts
Intra-abdominal sepsis can present as an intra-abdominal abscess if the
sepsis is contained by tissues or anatomy. Common locations are:
• Alongside the organ of origin (e.g. paracolic in diverticulitis,
parapancreatic after infected pancreatitis).
• Pelvic (especially after pelvic sepsis such as appendicitis or after
generalized peritoneal infection).
• Subphrenic (e.g. after upper GI perforation).
Causes
• Sigmoid diverticulitis (see b p. 404).
• Acute appendicitis (see b p. 298).
• Severe acute cholecystitis (see b p. 316).
• Upper GI perforation (see b p. 296).
• Post-anastomotic leakage (see b p. 420).
• Infected acute pancreatitis (see b p. 332).
• Post-trauma.
Clinical features
Depending on the source, the preceding pathology may have specifi c clini-
cal features, but the development of an abscess gives rise to certain com-
mon features independent of the origin.
Symptoms
• Malaise, anorexia.
• Localized abdominal pain—constant.
Signs
• Swinging fever, typically peaks in excess of 38.5*C occurring twice a
day.
• Tachycardia tends to follow the temperature.
• Localized abdominal tenderness with a possible mass if abscess in an
accessible position (e.g. paracolic).
Emergency management
Resuscitation
• Establish large calibre IV access if the patient is unwell.
• Catheterize and place on a fl uid balance chart only if hypotensive.
• Give adequate analgesia (e.g. 5–10mg morphine IV).
• Send blood for FBC (Hb, WCC), U&E (Na, K), CRP, group and save.
Establish a diagnosis
• Helical CT scanning is the diagnostic investigation of choice.
• Pelvic ultrasound (transabdominal or transvaginal) is occasionally
useful if a pelvic abscess is suspected and CT scanning is to be avoided
due to age.

INTRA-ABDOMINAL ABSCESS
309309
Early treatment IV antibiotics are appropriate if the patient is septic and
should be given according to the most likely underlying diagnosis and
organisms.
Defi nitive management
• Radiologically guided drainage by ultrasound or CT scanning wherever
possible. Limitations include retroperitoneal or intermesenteric
abscesses with dangerous access or complex multiloculated abscesses.
• Open surgical drainage usually only indicated if:
Radiological drainage not possible or safe.•
Radiological drainage fails to deal with the clinical symptoms or •
abscess recurs.
Surgical treatment is required for the primary underlying pathology.•
This page intentionally left blank

311
Liver, pancreatic, and
biliary surgery
Jaundice—causes and diagnosis 312
Jaundice—management 314
Gall bladder stones 316
Common bile duct stones 318
Chronic pancreatitis 320
Portal hypertension 322
Cirrhosis of the liver 324
Pancreatic cancer 326
Cancer of the liver, gall bladder, and biliary tree 328
Acute variceal haemorrhage 330
Acute pancreatitis 332
Chapter 8

CHAPTER 8 Liver, pancreatic, and biliary surgery
312
Jaundice—causes and diagnosis
Key facts
Jaundice is clinically apparent at serum bilirubin levels above 40mmol.
Key revision points—physiology of bile
• Unconjugated bilirubin formed mainly in spleen by the breakdown
of haemoglobin.
• It is insoluble and is transported in the plasma bound to albumin.
• Taken up by the liver by active transport, it is converted in the
hepatocytes into conjugated bilirubin (water-soluble).
• It is excreted into the bile canaliculi and via the main bile ducts into
the duodenum.
• Ten per cent of the unconjugated bilirubin is reduced to
urobilinogen by small intestinal bacteria, reabsorbed in the terminal
ileum, and then excreted in the urine (enterohepatic circulation).
• Ninety per cent is converted by colonic bacteria to stercobilinogen
which is excreted in faeces.
Causes and features
Pre-hepatic jaundice (haemolytic)
• Congenital abnormalities of red cell structure or content (e.g.
hereditary spherocytosis, sickle cell disease).
• Autoimmune haemolytic anaemia.
• Transfusion reactions.
• Drug toxicity.
Hepatic jaundice (hepatocellular)
• Hepatic unconjugated hyperbilirubinaemia.
Gilbert’s syndrome. Defi ciency or abnormalities of unconjugated •
bilirubin uptake system.
Crigler–Najjar syndrome. Abnormality of conjugation process •
enzymes.
• Hepatic conjugated hyperbilirubinaemia.
Infection. Viral (e.g. hepatitis A, B, C, EBV, CMV); bacterial (e.g. liver •
abscess, leptospirosis); parasitic (e.g. amoebic).
Drugs, e.g. paracetamol overdose, antipsychotics, antibiotics.•
Non-infective hepatitis, e.g. chronic active hepatitis, alcohol-related.•
Post-hepatic jaundice (obstructive)
• Intraluminal abnormalities of bile ducts.
Gallstones.•
Blood clot.•
Parasites (e.g. fl ukes).•
• Mural abnormalities of bile ducts.
Cholangiocarcinoma.•
Congenital atresia.•
Sclerosing cholangitis.•

JAUNDICE—CAUSES AND DIAGNOSIS
313
Biliary cirrhosis (primary (autoimmune) or secondary to sepsis).•
Traumatic/post-surgical stricture.•
• Extrinsic compression of bile ducts.
Pancreatitis.•
Tumours, e.g. head of pancreas, ampulla of Vater.•
Lymphadenopathy of porta hepatis nodes.•
Diagnosis and investigations
History
Common aspects overlooked in the clinical history of jaundiced patients.
• Family history of blood disorders.
• Recent foreign travel and work (exposure to infective agents).
• Recent drugs or changes in medications.
• Recent surgery or anaesthesia.
• History of gallstones.
• Alcohol intake, cholangitis (pain, fever, rigors), and carcinoma,
especially the head of the pancreas.
Basic tests
• Reticulocytosis, abnormal blood fi lm (haemolysis).
• i Prothrombin time.
• ‘Hepatitis screen’ (viral titres for hepatitis A, B, C, CMV, EBV).
• Immunology (anti-smooth muscle antibodies (chronic active hepatitis)
and anti-mitochondrial antibodies (primary biliary cirrhosis)).
• LFTs (see Table 8.1).
Advanced tests
• Ultrasound scan (liver, gall bladder, bile ducts, and pancreas).
Excludes the presence of extrahepatic obstruction (dilated •
common bile duct).
May locate cause and site of obstruction.•
Examines hepatic parenchyma in possible hepatitis.•
• Magnetic resonance cholangiopancreatography (MRCP) for suspected
extrahepatic obstruction with no cause seen on ultrasound.
• Liver biopsy (ultrasound-guided) for suspected hepatitis.
Table 8.1 Liver function tests in jaundice
Haemolytic Hepatocellular Obstructive
Unconjugated
bilirubin
Increased Increased Normal
Alkaline
phosphatase
Normal Normal Much increased
γ glutamyl
transferase
Normal Increased Much increased
Transaminases Normal Increased Normal
Lactate
dehydrogenase
Normal Increased Normal

CHAPTER 8 Liver, pancreatic, and biliary surgery
314
Jaundice—management
Complications of jaundice
• Renal failure (hepatorenal syndrome). Caused by a combination of
infection, dehydration, and a direct effect of high levels of bilirubin and
other toxic products of metabolism on the kidney; mortality is highest
when the patient is over 65 with an elevated blood urea.
• Biliary infection (cholangitis). Commonest in obstructive jaundice or
with previously damaged biliary tree; commonly due to Gram –ve
bacteria (e.g. Escherichia coli, Pseudomonas).
• Deranged coagulation. Due to decreased synthesis of vitamin K
dependent clotting factors (III, VII, IX, X) and impaired platelet
function.
• Relative immunosuppression. Predisposes to systemic infections (e.g.
chest infection) and reduces wound healing due to combinations of
jaundice, infection, and reduced proteosynthesis.
Acute presentation—general treatment
Fluid balance
• Correct dehydration. Give up to 1000mL IV crystalloid if there is no
pre-existing liver disease; sodium input should be carefully monitored
in pre-exisisting liver disease—ask for senior advice.
• Monitor hourly urine output—urethral catheter.
• Treat infection. Take blood cultures if the patient is pyrexial. Give IV
antibiotics according to local protocol (e.g. cefuroxime 750mg IV tds,
IV gentamicin, PO or IV ciprofl oxacin (500mg IV)); treatment of bile
duct obstruction may be required urgently (e.g. radiologically-guided
drainage, ERCP, or rarely, surgery); consider prophylactic antibiotics.
• Check clotting times (APTT, PT). Give vitamin K 10mg IV stat if PT is
prolonged.
• Ensure adequate nutrition. Ensure the patient has a dietetic review;
enteral feeding is optimum, but may require a fi ne bore NGT or, very
occasionally, a surgical gastrostomy or jejunostomy.
• Preoperative biliary decompression has not been proven to reduce
post-operative complications.
Acute presentation—specifi c treatments
• Endoscopic procedures (ERCP).
Sphincterotomy. Used for common bile duct stone extraction, •
treatment of ampullary strictures due to tumours or infl ammation.
Stent insertion (plastic or expanding metal). Used for bile duct •
stones that cannot be removed easily, post-operative or benign
strictures, malignant strictures, external compression of bile duct.
• Percutaneous transhepatic cholangiogram (PTC). Used for stent
insertion (often in combination with ERCP), temporary external
drainage of obstructed biliary system.
• Surgical drainage (e.g. choledochoduodenostomy). Very rarely used
if other interventions failed due to very high morbidity and mortality.

JAUNDICE—MANAGEMENT
315
Elective presentation—specifi c treatments
• Haemolytic jaundice.
Steroids for autoimmune case.•
Splenectomy (laparoscopic). Rarely used for hereditary causes and •
failed medical treatment.
• Obstructive jaundice.
ERCP and PTC may be used as above for stones, strictures, •
compression.
Surgical drainage (e.g. choledochoduodenostomy or •
cholecystojejunostomy) used for failed interventional treatments.
Surgical resection, e.g. Whipple’s pancreaticoduodenectomy. Used •
for very selected cases where pancreatic or distal bile duct tumours
are benign or malignant, but potentially curable on staging; staging
of potentially suitable patients may include endoscopic ultrasound,
CT scan, ERCP or MRCP, visceral arteriography, laparoscopy.
• Hepatocellular jaundice.
Remove causative agent and support liver function.•
Consider transplantation in specifi c circumstances.•
• Selective arteriography of the hepatic, coeliac, and superior mesenteric
arteries gives information about anatomical variants, vessel invasion,
tumour operability.
Prognosis in acute jaundice
Adverse risk factors include:
• Age >65y.
• Elevated plasma urea.
• Elevated plasma bilirubin (>200g/L).
• Uncontrolled sepsis and multiple organ dysfunction (typically acute
tubular necrosis).
• Underlying malignant disease.

CHAPTER 8 Liver, pancreatic, and biliary surgery
316
Gall bladder stones
Key facts Present in 10% of people >50y in the UK.
Pathological features
Bile has three major constituents:
• Bile salts (primary—cholic and chenodeoxycholic acids; secondary—
deoxycholic and lithocholic acids).
• Phospholipids (90% lecithin).
• Cholesterol.
Bile containing excess cholesterol relative to bile salts and lecithin predis-
poses to gallstone formation.
Types of gallstones
• Pure cholesterol (10%). Often solitary, large (>2.5cm), round.
• Pure pigment (bile salts 10%). Pigment stones are of two types:
Black (associated with haemolytic disease).•
Brown (associated with chronic cholangitis and biliary parasites).•
• Mixed (80%). Most common; usually multiple.
Predisposing conditions
• Increasing age.
• Female (pregnancy and use of the oral contraceptive).
• Obesity.
• Multiparity.
• Chronic haemolytic disorders (only for pigment stones).
• Long-term parenteral nutrition (alteration of bile constituents).
• Previous surgery (e.g. vagotomy or resection of the terminal ileum)
or disease involving the distal small bowel (e.g. Crohn’s disease)—
alteration of bile constituents.
Clinical features (common presentations)
Biliary colic Intermittent severe epigastric and right upper quadrant pain;
usually associated with nausea and vomiting. Resolves after few hours;
tenderness over gall bladder during acute episodes.
Acute cholecystitis
Severe continuous right upper quadrant pain; often radiates to right fl ank
and back associated with anorexia and pyrexia. Tenderness over gall blad-
der during inspiration (Murphy’s sign).
Complications of acute cholecystitis include:
• Formation of an empyema or abscess of the gall bladder (rare).
Indicated by high swinging fever and severe localized pain;
• Perforation with biliary peritonitis (very rare).
• Cholecystoenteric fi stula formation (may lead to a gallstone entering
and obstructing the distal ileum (‘gallstone ileus’; see b p. 302);
• Jaundice due to compression of the adjacent common bile duct by
pressure (‘Mirizzi syndrome’).
Chronic cholecystitis Repeated episode of infection causes thickening and
fi brosis of gall bladder.

GALL BLADDER STONES
317
Mucocele Stone in neck of gall bladder; bile is absorbed, but mucus secre-
tion continues, producing a large, tense globular mass in right upper
quadrant.
Empyema Abscess of gall bladder.
Diagnosis and investigations
• FBC, U&E, LFTs, blood culture, serum amylase—in acute
presentations
• Abdominal X-ray. Only 10% of calculi are radio-opaque.
• Oral cholecystogram (Graham–Cole test). Rarely used.
• Ultrasound. Procedure of choice; identifi es stones, determines wall
thickness, and assesses ductal dilatation.
• Hepatobiliary iminodiacetic acid (HIDA) scan. Useful when ultrasound
fi ndings are equivocal.
Surgical treatment
Cholecystectomy
Majority done laparoscopically; often done as a day case. This is the treat-
ment of choice for all patients fi t for GA. Indicated for:
• Patients with symptoms deemed to be due to gall bladder stones.
• Asymptomatic patients with gall bladder stones at risk of complications
(diabetics, porcelain gall bladder (15–20% associated with carcinoma),
history of pancreatitis, long-term immunosuppressed).
Risks of laparoscopic cholecystectomy
• Conversion to open operation, 5–10%.
• Bile duct injury, <1%.
• Bleeding, 2%.
• Bile leak, 1%.
Non-surgical treatments
Percutaneous drainage of gall bladder
• Done under ultrasound or CT guidance.
• Used for empyema of the gall bladder in patients unsuitable for
emergency cholecystectomy.
• After resolution of the infection, the calculi may be removed
percutaneously.
Dissolution therapy
• Rarely used. Requires a functioning gall bladder, small stones.
• Problems—requires prolonged treatment, <70% response, high rate of
recurrence of stones, side effects of medication (diarrhoea, pruritus).
Extracorporeal shock wave lithotripsy Hardly ever used; risk of visceral
injury and high risk of stone recurrence.

CHAPTER 8 Liver, pancreatic, and biliary surgery
318
Common bile duct stones
Key facts
• Types of stones as per gall bladder stones (see b p. 316).
• Common bile duct (CBD) stones present in 10% of patients with
gallstones.
• Most pass from the gall bladder into the CBD (secondary duct stones).
• Rarely form within the CBD (primary duct stones); almost always
associated with partial duct obstruction.
Clinicopathological features
Asymptomatic Usually found incidentally on ultrasound for gall bladder
stones.
Obstructive jaundice
• Usually due to CBD stone causing obstruction; rarely due to stone-
induced CBD stricture.
• Anorexia, nausea, itching.
• Dark urine and pale stools.
• Epigastric pain and fever more common with CBD stones than other
cause; due to associated low grade bile infection.
• A palpable, distended gall bladder is rare with CBD stones.
Courvoisier’s law
‘If in the presence of jaundice, the gall bladder is palpable, then the cause
of the jaundice is unlikely to be due to stone.’
This is due to the fact that CBD stones originate in the gall bladder
which is usually scarred and fi brotic, preventing distension.
Ascending cholangitis Constant severe right upper quadrant pain, obstruc-
tive jaundice, and high swinging fever (‘Charcot’s triad’).
Acute pancreatitis Sixty per cent of acute pancreatitis in adults in the UK
is due to gallstones (see b p. 316).
Diagnosis and investigations
Basic tests
FBC (i WCC in cholangitis and pancreatitis), U&E, creatinine, LFTs
(i conjugated bilirubin and alkaline phosphatase), serum amylase
(i in pancreatitis), clotting studies.
Advanced tests
Ultrasound (transabdominal)
• Best fi rst-line investigation.
• Accuracy low for distal CBD stones, in acute presentations, obesity,
with extensive overlying bowel gas.

COMMON BILE DUCT STONES
319
MRCP
• Investigation of choice for inconclusive ultrasound result.
• Non-invasive, avoids radiation exposure, highly accurate.
ERCP
• Used diagnostically for patients unable to tolerate MRCP
(claustrophobia).
• Mainly reserved for therapeutic interventions:
Endoscopic sphincterotomy (ES) and stone extraction or •
destruction (lithotrypsy).
Stent insertion for unextractable stones.•
• Risks of ERCP (i with ES):
Haemorrhage.•
Acute pancreatitis.•
Ascending infection.•
Perforation (usually retroduodenal, may cause peritonitis).•
PTC
• Used for failure of ERCP as therapeutic procedure (often in
combination with ERCP).
• Risks include sepsis, tube movement, leakage around the tube, and
dehydration.
Treatment
Principles of treatment of CBD stones are as follows.
Emergency treatment of CBD stones
• Indicated in unresolving gallstone pancreatitis, unresolving ascending
cholangitis.
• Usually ERCP with stone extraction or stent insertion.
• Occasionally PTC required.
Elective treatment of CBD stones
• Indicated for:
All patients having had complications (pancreatitis, cholangitis, •
obstructive jaundice).
All patients with gall bladder stones due for cholecystectomy.•
• Usually by ERCP or combined ERCP/PTC.
• Common bile duct exploration required at time of cholecystectomy
(laparoscopic or open) if ERCP/PTC fail or impossible or as surgeon’s
preference. Open CBD exploration may require a T-tube to be left in
the CBD.
Treatment of persistent CBD stones after cholecystectomy
• Rarely necessary with more accurate preoperative diagnosis and more
effective preoperative treatments.
• Stones can be extracted via a T-tube track if present (6 weeks after
surgery with radiologically guided basket extraction).
• Post-operative ERCP is rarely required.

CHAPTER 8 Liver, pancreatic, and biliary surgery
320
Chronic pancreatitis
Key facts
• Characterized by recurrent or persistent abdominal pain arising from
the pancreas.
• Often associated with exocrine or endocrine pancreatic insuffi ciency.
• Characterized by irreversible destruction and fi brosis of pancreatic
parenchyma.
• May arise following one or more episodes of acute pancreatitis or may
be a chronic progressive process de novo.
Pathological features
• The process may affect the whole or part of the gland (focal).
• The head tends to be the most severely involved part in chronic
alcohol disease.
• Features of acute pancreatitis may occur—oedema, acute
infl ammatory infi ltrate, focal necrosis, intraparenchymal haemorrhage.
• Chronic infl ammatory changes cause progressive disorganization of the
pancreas:
Glandular atrophy and duct ectasia.•
Microcalcifi cation and intraductal stone formation with cystic •
changes secondary to duct occlusion.
Causes and clinical features
Causes
• Recurrent acute pancreatitis of any cause, especially alcohol.
• Secondary to pancreatic ductal obstruction:
Pancreatic head cysts, tumours.•
Pancreatic duct strictures—post-surgery, ERCP, parasitic infestation.•
Congenital pancreatic abnormalities (pancreas divisum, annular •
pancreas).
Cystic fi brosis.•
• Associated with autoimmune diseases (primary biliary cirrhosis,
primary sclerosing cholangitis).
• Congenital idiopathic chronic pancreatitis.
Features of chronic infl ammation
• Recurrent or chronic abdominal pain:
Typically epigastric, radiating to the back and requiring opiates.•
Worse with food, alcohol.•
Features of exocrine failure
• Anorexia and weight loss (due to protein malabsorption).
• Steatorrhoea (due to fat malabsorption); soft, greasy, foul-smelling
stools that typically fl oat on water.
Features of endocrine failure Insulin-dependent diabetes mellitus (due to
loss of β islet cells).

CHRONIC PANCREATITIS
321
Diagnosis and investigations
Basic tests
• Plain abdominal X-ray may show pancreatic calcifi cation.
• Abdominal ultrasound may show cystic change and duct dilatation
within the pancreas.
Advanced tests
• Pancreatic CT scan.
May identify a cause, e.g. anatomical variants, tumours, cysts.•
May show extent of disease. Pancreatic atrophy, disorganization of •
pancreatic ducts, altered acinar pattern with fi brosis, calcifi cation,
and cystic change.
• MRI scan. May show the same changes as CT.
• ERCP. Demonstrates irregularity of the pancreatic duct strictures,
calculi, dilated segments (‘chain of lakes’), and changes in fi rst and
second order branches and cyst formation; a secondary effect from
involvement of the head is stricture of the bile duct, leading to an
‘obstructive’ pattern of LFTs.
Treatment
• Prevention of cause/progressive damage.
Stop alcohol, deal with gallstones, treat autoimmune disease.•
Encourage a diet rich in antioxidants (vitamins A, C, E, selenium).•
• Control symptoms/complications.
Dietary modifi cations. Adequate carbohydrates and protein, •
reduced fat.
Pancreatic exocrine enzyme supplements (e.g. Creon•
®
).
Analgesia. May require opiates (e.g. MST) or coeliac plexus block.•
Control of diabetes mellitus often requires insulin; control is often •
diffi cult due to variable pancreatic function.
Surgical treatment Indications include the following.
• Treatment of reversible cause (anatomical abnormalities, tumours,
cysts, ductal strictures and stones). Operations used include those to
remove causes and those to drain an obstructed pancreatic duct:
Pancreaticoduodenectomy (Whipple procedure).•
Partial pancreatectomy of the head (Frey procedure) or tail (distal •
pancreatectomy).
Pancreaticojejunostomy (Peustow or Duval procedure).•
• Treatment of severe intractable pain or multiple relapses. Operations
are usually to resect affected portion:
Partial pancreatectomy of the head (Frey procedure) or tail (distal •
pancreatectomy).
Total pancreatectomy.•
• Complications (pseudocyst, obstruction, fi stula, infections, portal
hypertension).
Resectional surgery is associated with increasing risk of exocrine and
endocrine pancreatic failure and high risk of complications. All surgery
is associated with a risk of symptom recurrence due to recurrent or pro-
gressive disease.

CHAPTER 8 Liver, pancreatic, and biliary surgery
322
Portal hypertension
Key facts Normal portal vein pressure is 5–10mmHg. Portal hyperten-
sion (PH) develops when the portal pressure is greater than 12mmHg.
Causes and pathological features
Causes
• Prehepatic. Congenital portal vein atresia or portal vein thrombosis
due to neonatal umbilical sepsis, phlebitis of the portal vein from
abdominal infection (e.g. acute appendicitis or diverticulitis), trauma, or
a thrombosed portocaval shunt.
• Hepatic. Cirrhosis (e.g. alcoholic most frequently in the UK), chronic
active hepatitis, and parasitic diseases (e.g. schistosomiasis).
• Post-hepatic. Budd–Chiari syndrome (hepatic vein thrombosis),
constrictive pericarditis, or tricuspid valve incompetence (rare).
Features and complications
• Decreased or reversed portal blood fl ow to the liver promotes the
development of portosystemic anastomosis between the portal system
and systemic circulation:
Left gastric vein into the oesophageal veins at the gastro-•
oesophageal junction—oesophageal and gastric varices.
Superior rectal into inferior rectal veins at the lower rectum—•
rectal varices.
Obliterated umbilical vein into the epigastric veins—‘caput •
medusae’.
• Oesophageal or gastric varices may bleed torrentially.
• Liver cell dysfunction/liver failure occurs in hepatic and post-hepatic
causes.
• Ascites. In part due to portal hypertension, but may be due to
associated liver dysfunction.
• Splenomegaly (hypersplenism may result).
• The Child–Pugh classifi cation is used to assess the severity of portal
hypertension (see Table 8.2).
Diagnosis and investigations
Many investigations may be used at different times in PH.
• FBC, U&Es, LFTs, and clotting.
• Screening tests for causes of cirrhosis (see b p. 324).
• CT and ultrasound scan to assess liver morphology, diagnose PH, and
assess cause.
• Transabdominal Doppler ultrasound to assess blood fl ow in the portal
vein and hepatic artery.
• Gastroscopy in acute variceal bleeding (see b p. 272).
Treatment
Cause
• Anticoagulation for Budd–Chiari syndrome.
• Treatment for hepatic causes.

PORTAL HYPERTENSION
323
Chronic complications
• Oesphago-gastric varices.
Beta-blockers (e.g. propranolol or nadolol) reduce portal venous •
pressure.
Repeated injection sclerotherapy or variceal ligation.•
Elective portosystemic shunts (e.g. splenorenal anastomosis).•
Liver transplant may be considered for treatment if associated with •
severe liver disease.
• Rectal varices. Injection sclerotherapy.
• Symptomatic splenomegaly or hypersplenism. Splenectomy (laparoscopic
or open).
• Ascites. Oral spironolactone; in cases of tense ascites, paracentesis may
be required with IV albumin replacement.
Acute complications Bleeding oesophago-gastric varices.
Key revision points—anatomy of portal circulation
• The hepatic portal circulation carries blood from the GI tract (from
the distal oesophagus to the anorectal junction) to the liver.
• Portosystemic anastomoses occur in ‘junctional’ areas of venous
drainage.
Left gastric veins (portal) and oesophageal veins (hemi/azygous •
veins) at the gastro-oesophageal junction.
Superior rectal veins (portal) and inferior rectal veins (pudendal •
veins) in the lower rectum.
Pancreatic and duodenal veins (portal) and retroperitoneal (hemi/•
azygous) veins in the upper retroperitoneum.
Umbilical vein (portal) into the epigastric veins at the umbilicus.•
• Portal venous blood drains into liver venous sinusoids and hence
into the hepatic veins.
Table 8.2 The Child–Pugh classifi cation of portal hypertension
1 point 2 points 3 points
Bilirubin (μmol/L) <34 34–51 >51
Albumin (g/L) >35 28–35 <28
PT (s) <3 3–10 >10
Ascites None Moderate Moderate–severe
Encephalopathy None Moderate Moderate–severe
Grade A: 5–6 points; Grade B: 7–9 points; Grade C: 10–15 points.

CHAPTER 8 Liver, pancreatic, and biliary surgery
324
Cirrhosis of the liver
Key facts
• Commonest cause of liver failure in the UK.
• Commonest cause is alcohol-related.
Causes
• Congenital.
Haemochromatosis.•
Wilson’s disease.•
Other metabolic disorders (e.g. • α1-anti-trypsin defi ciency).
• Acquired.
Alcohol intake.•
Chronic hepatitis (autoimmune, infective types B, C, and D, •
drug-induced).
Primary biliary cirrhosis.•
Secondary biliary cirrhosis (gallstones, strictures, cholangitis).•
Hepatic vein obstruction, e.g. Budd–Chiari syndrome.•
Idiopathic.•
Pathological features
Cirrhosis is characterized by fi brosis of the liver parenchyma, nodular
regeneration, and hepatocellular necrosis.
• Micronodular form. Small and uniform nodules (<4mm in diameter),
separated by thin fi brous septa uniformly throughout the liver.
• Macronodular form. Larger nodules separated by wider scars and
irregularly distributed throughout the liver.
• Mixed.
Clinical features
• One-third of cirrhosis patients are compensated, i.e. do not produce
any clinical symptoms, and are incidentally discovered during a medical
examination, at operation, or at autopsy.
• Two-thirds are decompensated, i.e. have features of liver cell
dysfunction or complications.
Features fall into three broad groups.
Portal hypertension See b p. 322.
Hepatocellular failure
• Jaundice.
• Spider naevi.
• Ascites.
• Hypoalbuminaemia.
• Clotting disorders.
• Encephalopathy.
• Gynaecomastia and testicular atrophy.
• Hepatorenal syndrome (renal failure in the setting of hepatic failure
due to renal vasoconstriction of unknown aetiology).

CIRRHOSIS OF THE LIVER
325
Malignant change
• Hepatatocellular carcinoma (particularly chronic hepatitis B infection).
• Often indicated by sudden rapid decrease in hepatocellular function.
Diagnosis and investigation
Diagnosis of cause
• Metabolic screen (e.g. serum copper).
• Hepatitis screen (A, B, C, D, E; EBV, CMV).
• Autoimmune screen (anti-mitochondrial antibodies, anti-smooth
muscle antibodies).
• Abdominal ultrasound and CT may show type of cirrhosis, intra- or
extra-hepatic biliary dilatation, extrahepatic obstructive causes.
• Liver biopsy to confi rm diagnosis and establish type, activity, evolution,
and cause.
Investigation of severity or complications
• LFTs (transaminases, γGT, albumin, bilirubin).
• Clotting studies (PT).
• Transabdominal ultrasound or CT scan (splenomegaly and ascites).
Treatment
Removal of the cause/prevent progression
• Abstinence from alcohol.
• Interferon α. Chronic hepatitis B, response rate <50%.
• Combination therapy (interferon A and ribavirin for 6 months.
Moderate to severe hepatitis C.
• Immunosuppression for autoimmune causes.
Treatment of complications
• PH (see b p. 322).
• Encephalopathy. Treatment aims to lower the amount of nitrogen
absorbed from the gut.
Administration of oral lactulose.•
Oral, non-absorbable antibiotics.•
Careful IV fl uid replacement to prevent sodium overload.•
Diet of high carbohydrate, low salt, moderate protein.•
• Ascites. Oral spironolactone; in cases of tense ascites, paracentesis may
be required with IV albumin replacement.
• Decompensated hepatocellular failure. Consider liver transplant.

CHAPTER 8 Liver, pancreatic, and biliary surgery
326
Pancreatic cancer
Key facts
• Fourth commonest solid organ cancer in the UK.
• Incidence is increasing rapidly.
• Eighty per cent of cases occur between the sixth and seventh decades.
• Risk factors include cigarette smoking, increasing age, high fat diet,
diabetes mellitus, excessive alcoholism, and chronic pancreatitis.
• Occupational hazards, e.g. exposure to naphthylene and benzidine.
• There may be hereditary factors involved as 1 in 20 patients with
pancreatic cancer have a family history of pancreatic cancer.
Pathological features
• Ninety per cent ductal adenocarcinoma.
• Seven per cent mucinous cystic neoplasms (mucinous cystadenoma/
cystadenocarcinoma), serous cystadenoma, and papillary cystic tumour.
• Three per cent islet cell tumours.
Clinical features
Carcinoma of the head of pancreas (65%)
• Obstructive jaundice (90%). Due to compression or invasion of the
CBD. Gall bladder is typically palpable.
• Pain (70%). Epigastric or left upper quadrant, often vague and radiates
to the back.
• Hepatomegaly. Due to metastases.
• Anorexia, nausea and vomiting, fatigue, malaise, dyspepsia, and pruritus.
• Acute pancreatitis. Occasionally the fi rst presenting feature.
• Thrombophlebitis migrans (10%). Presents as emboli; splenic vein
thrombosis may lead to splenomegaly in 10% of patients.
Carcinoma of the body (25%) and tail (10%)
• Usually asymptomatic in the early stages.
• Weight loss and back pain (60%).
• Epigastric mass.
• Jaundice suggests spread to hepatic hilar lymph nodes or metastases.
• Thrombophlebitis migrans (7%).
• Diabetes mellitus (15%).
Diagnosis and investigations
• FBC, LFTs, blood sugar.
• Elevated serum CA 19–9 (sensitivity 90%; specifi city 70% for diagnosis).
Level correlates with the tumour volume.
• Transabdominal ultrasound scan (sensitivity 70%; 30% in lesions <2cm).
• Doppler ultrasound images blood fl ow in the portal vein and superior
mesenteric vessels.
• Helical CT scan of pancreas with dual phase IV contrast assesses size
of the primary lesion, vascular invasion, and distant metastasis.

PANCREATIC CANCER
327
• FNAC. Usually CT or ultrasound-guided (specifi city 99%; sensitivity
50–70%).
• EUS more accurate than CT in detecting pancreatic lesions <3cm in
diameter and peripancreatic lymph node involvement.
• PET may help differentiate neoplastic from non-neoplastic lesions and
may be used to exclude extra-pancreatic spread that would preclude
surgical resection.
• ERCP is 85% accurate; can provide cytology as well as achieving biliary
drainage via insertion of a stent.
• Selective angiography or CT angiography used to assess resectability
based on encasement of the major vessels.
• Laparoscopy used to rule out peritoneal disease and liver metastasis
<2cm prior to offering surgical resection.
Treatment
Palliative treatment
The majority of tumours (95%) are not suitable for surgical resection due
to presence of metastases, local invasion, involved lymph glands, age, or
comorbidity of patient.
Relief of jaundice
Obstructive jaundice is associated with pruritus, coagulopathy, immuno-
logical and nutritional derangement, deterioration in liver function, risk of
acute renal failure (hepatorenal syndrome), and increased susceptibility to
infection. Relief of jaundice is achieved by:
• Endoscopic biliary stenting by ERCP.
• Percutaneous biliary drainage by PTC and internal stenting or insertion
of an internal-external drainage catheter.
• Surgical biliary drainage by cholecystojejunostomy or choledoco-
jejunostomy.
Relief of duodenal obstruction Surgical gastric bypass (gastrojeju nostomy).
Relief of pain
• Oral morphine (oramorph or MST).
• Chemical ablation of the coeliac ganglia (percutaneous coeliac
nerve block or thoracoscopic division of the splanchnic nerves are
alternatives).
Curative treatment Radical surgical resection is the only hope of cure if
patient is suitable.
• Pancreatoduodenectomy (Whipple’s operation) for periampullary
tumours and cancer of the pancreas confi ned to the head.
• Total pancreatectomy for extensive tumour.
• Distal pancreatectomy for tumours in the tail.
Adjuvant therapy For advanced disease, adjuvant chemotherapy (e.g. 5-fl u-
orouracil) improves prognosis.
Prognosis Poor; even in patients with resectable disease, the 5y survival
is 12%.

CHAPTER 8 Liver, pancreatic, and biliary surgery
328
Cancer of the liver, gall bladder,
and biliary tree
Key facts
• Commonest tumours of the liver are metastatic (pancreas, colon,
stomach, oesophagus, and breast).
• Thirty-fi ve per cent of patients who die of malignant disease have
hepatic metastases.
Clinicopathological features
Hepatocellular carcinoma (HCC)
• Ninety per cent of primary liver tumours, but <1% of all new cancers
in UK.
• Common in Africa and Asia; commoner in men than women.
• Risk factors:
Cirrhosis, especially due to chronic viral hepatitis (HBV/HCV) or •
alcohol.
Afl atoxin exposure, contraceptives, and androgens.•
• Arises from liver parenchymal cells, spreads via local invasion, via
portal vein invasion to other sites in the liver, or via hepatic vein
invasion to distant metastases (e.g. lung).
• Commonest presentation—rapid deterioration in pre-existing
cirrhosis.
Cholangiocarcinoma
• Usually arises in the extrahepatic biliary tree, but may be intrahepatic.
• Typical sites are distal CBD, common hepatic duct, confl uence of
hepatic ducts (‘Klatskin tumour’).
Adenocarcinoma of the gall bladder
• Gallstones are found in 70% of cases.
• Associated with ulcerative colitis and primary sclerosing cholangitis.
• Often diagnosed incidentally as unexpected fi nding during or after
cholecystectomy for ‘benign’ disease causing right upper quadrant pain.
• May present as a gall bladder mass or obstructive jaundice due to local
invasion of the common hepatic duct.
• Spread is direct into liver tissue (possibly resectable), to hilar lymph
nodes, or blood-borne (incurable).
Ampullary carcinoma
• Typically small and presents relatively early due to the early onset of
painless obstructive jaundice.
• Best prognosis of all hepatobiliary cancers due to early presentation
before local or lymphatic spread.
Other primary liver cancers
• Fibrolamellar carcinoma (FLC).
Usually affects younger patients (3rd and 4th decades).•
Does not occur on a background of liver disease.•
Presents as a large vascular mass.•

CANCER OF THE LIVER, GALL BLADDER, AND BILIARY TREE
329
• Angiosarcoma.
Less than 1% of liver tumours (most common sarcoma of the liver).•
Associated with exposure to arsenicals, vinyl chloride, anabolic •
steroids, and contraceptives.
Diagnosis and investigations
• AFP >500ng/mL highly suggestive of HCC, even in cirrhosis.
• Ultrasound scan often identifi es site and cause of biliary obstruction;
good assessment of liver parenchyma.
• Needle biopsy to confi rm diagnosis of HCC.
• ERCP. Diagnosis of ampullary and bile duct carcinoma; allows biopsy
or brush cytology of distal tumours; allows therapeutic stenting.
• MRCP. Diagnosis of proximal tumours or where ERCP not possible.
• PTC. Diagnosis of intrahepatic biliary tumours, therapeutic stenting, or
external drainage of proximal biliary tumours.
• CT scan. Assessment of local spread (including blood vessels), lymph
nodes, metastases.
Treatment
Curative
• Surgery offers the only cure for primary liver or biliary cancers.
• Patients suitable for resection must:
Be fi t for major surgery.•
No evidence of metastases or involved lymph nodes (rare).•
Tumours technically suitable for complete resection (rare).•
• Surgical options for resection include:
Partial hepatectomy (HCC).•
Liver transplantation (HCC associated with chronic hepatitis).•
Radical cholecystectomy (adenocarcinoma of gall bladder).•
Radical excision of bile duct with reconstruction •
(cholangiocarcinoma).
Pancreaticoduodenectomy (Whipple procedure) (distal •
cholangiocarcinoma or ampullary carcinoma).
Palliative
• Endoscopic or percutaneous stenting for unresectable
cholangiocarcinoma or ampullary carcinoma.
• Chemotherapy is of minimal benefi t in any primary liver or biliary
cancers.
• Embolization. Percutaneous thermal or radiofrequency ablation
(HCC).
Prognosis
• HCC. 5y survival 44% if surgically resectable.
• Cholangiocarcinoma. Median survival 9 months.
• Adenocarcinoma of gall bladder. 5y survival <5%.
About 20% of these tumours are resectable at the time of diagnosis.

CHAPTER 8 Liver, pancreatic, and biliary surgery
330330
Acute variceal haemorrhage
Key facts Mortality rate of fi rst variceal bleed with established PH
is 30%.
Causes and features
See b p. 294 for differential diagnosis. Typical variceal bleeding is:
• Rapid onset, copious dark red venous blood with little mixing with
food.
• Features of established PH, e.g. caput medusa.
• Features of developing hepatic encephalopathy (ingested blood
provides an extremely protein-rich ‘meal’).
Emergency management
Resuscitation (see Fig. 8.1)
• Establish large calibre IV access. Give crystalloid fl uid up to 1000mL
if tachycardic or hypotensive. Only use O –ve blood if the patient
is in extremis; otherwise wait for cross-matched blood if transfusion
needed.
• Catheterize and place on a fl uid balance chart.
• Send blood for FBC (Hb, WCC), U&E (Na, K), LFTs (albumin), cross-
match (at least 3U if haematemesis large), clotting.
• Always consider alerting HDU/ITU; variceal bleeds can deteriorate
extremely rapidly.
• Monitor pulse rate, BP, and urine output (urinary catheter).
• Insertion of a Sengstaken–Blakemore gastro-oesophageal tube may be
a life-saving resuscitation manoeuvre. Usually only inserted without a
prior gastroscopy if the patient is known to have varices and has life-
threatening bleeding. Key points are the following.
If the patient needs a ‘Sengstaken’ tube, they need to be on ITU.•
Most patients need sedation or a GA for the tube to be inserted.•
The tube is inserted and the gastric balloon blown up fi rst and •
traction applied gently until the tube becomes fi xed; this alone may
stop the bleeding if the varices are gastric.
If bleeding continues, the oesophageal balloon is blown up to a •
pressure around 20–30mmHg.
The oesophageal balloon must be defl ated regularly to prevent •
oesophageal necrosis.
Establish a diagnosis
• Urgent OGD is the investigation of choice (at least within 24h).
May require ongoing resuscitation with anaesthetist present.•
Never• biopsy suspected varices.
Therapeutic interventions, including sclerotherapy and banding, are •
up to 90% successful at controlling acute bleeds and preventing
further interventions.

ACUTE VARICEAL HAEMORRHAGE
331331
Early treatment
• Give IV PPI (e.g. omeprazole 40mg); stop all NSAIDs.
• Give IV vasopression, somatostatin, or octreotide to lower
oesophageal variceal pressure.
• Blood transfusion if large volume haematemesis or drop in Hb.
• Ensure that the appropriate surgical team knows of the patient in case
surgical intervention is required.
• Consider giving FFP to correct clotting abnormalities.
Defi nitive management
Considered for failed endoscopic treatment and ongoing bleeding.
• Transjugular intrahepatic portosystemic shunt formation (TIPS)
(intrahepatic shunt). May be performed to rapidly reduce the portal
pressure, but has the risk of inducing portal encephalopathy.
• Extrahepatic shunt. In portacaval shunts, encephalopathy occurs in 50%
of survivors and the procedure is now seldom performed.
• Oesophageal transaction.
Left gastric vein devascularization.•
Extremely high mortality.•
Low incidence of encephalopathy, but high incidence of recurrent •
bleeding.
Variceal bleed (Dx by Hx & Ex)
Active bleeding
Emergency OGD ± endotherapy
Active bleeding
despite endotherapy
Insert Sengstaken tube
Continue terilipressdin IV
Failure to control bleeding
Consider repeated endotherapy
Consider TIPS
Bleeding controlled
by endotherapy
Grossly unstable
Insert CVL
Admit to critical care
Consider Sengstaken tube
If INR > 1.4 give 2U FFP & Vit K 10mg IV
Start IV terilipressin 4mg stat IV bolus & 1mg IV qds
Give ciprofloxacin 1g IV
Fig. 8.1 Management of acute variceal haemorrhage.

CHAPTER 8 Liver, pancreatic, and biliary surgery
332332
Acute pancreatitis
Causes and features
Infl ammatory process with cascade of release of infl ammatory cytokines
(TNFA, IL2, IL6, platelet-activating factor (PAF)) and pancreatic enzymes
(trypsin, lipases, co-lipases) initiated by pancreatic injury, but which may
develop into full blown MODS or SIRS (see b p. 138).
Causes
• Gallstones (60%).
• Alcohol (30%).
• Hyperlipidaemia.
• Hypercalcaemia (hyperparathyroidism, multiple myeloma).
• Direct damage (trauma, ERCP, post-surgery, cardiopulmonary bypass).
• Toxins:
Drugs, e.g. azathioprine, oestrogens, thiazides, isoniazid, steroids, •
NSAIDs.
Infection, e.g. viral (mumps, CMV, hepatitis B), mycoplasma.•
Venom (scorpion, snake bites).•
• Idiopathic.
Classifi cation/complications
• Oedematous (70%). May be simple or associated with phlegmon
formation; transient fl uid collections common.
• Severe/necrotizing (25%). Necrosis may be sterile or infected.
Persistent large peripancreatic fl uid collections may form
(‘pseudocyst’), which may become infected.
• Haemorrhagic (5%).
See b p. 138 for complications of SIRS.
Clinical features
• Severe epigastric pain radiating to the back.
• Severe nausea and vomiting.
• Fever, dehydration, hypotension, tachycardia (may be frankly shocked).
• Epigastric tenderness associated with guarding and in severe cases,
rigidity which may be generalized.
• Left fl ank ecchymosis (Grey–Turner’s sign) and periumbilical
ecchymosis (Cullen’s sign), 1–3% of cases haemorrhagic pancreatitis.
Emergency management
Resuscitation
• Establish large calibre IV access. Give crystalloid fl uid up to 1000mL
if tachycardic or hypotensive; may require ongoing fl uids IV.
• Catheterize and place on a fl uid balance chart.
• Send blood for FBC (Hb, WCC), U&E (Na, K), LFTs (bilirubin,
albumin), amylase, group and save, clotting.
• Monitor pulse rate, BP, and urine output (urinary catheter).
• Consider insertion of a central line and manage patient in HDU if
haemodynamically unstable or fails to respond to early resuscitation.
• Assess the severity of the attack by the Glasgow Imrie criteria
(see Box 8.1).

ACUTE PANCREATITIS
333333
Establish a diagnosis
• Serum amylase >1000U. Diagnostic, but may be normal even in
severe cases; elevated amylase may occur in a wide range of other
acute abdominal events (intestinal ischaemia, leaking aneurysm,
perforated ulcer, cholecystitis).
• Serum lipase. Remains elevated longer than serum amylase; more
specifi c, but less sensitive.
• AXR (non-specifi c fi ndings). Absent psoas shadows, ‘sentinel loop sign’
(dilated proximal jejunal loop adjacent to pancreas because of local
ileus’), ‘colon cut-off sign’ (distended colon to mid-transverse colon
with no air distally); may show gallstone, pancreatic calcifi cation.
• CT may be required. Shows pancreatic oedema, swelling, loss of fat
planes; may show haemorrhagic or necrotic complications.
• Ultrasound scan. Must be done within 48h of admission to identify
gallstones in the bile duct.
1
Early treatment Urgent ERCP and stone extraction are indicated for
proven bile duct stones causing obstruction and pancreatitis.
Defi nitive management
Identify/prevent complications
• IV antibiotics (e.g. IV imipenem tds), sometimes started in
moderate to severe cases even without evidence of infected necrosis.
• CT scan identifi es development of pancreatic phlegmon, early fl uid
collections, necrosis, or haemorrhage.
• CT-guided pancreatic aspiration to identify infected necrosis.
• Early low volume enteral feeding is increasingly used to reduce the risk
of stress ulceration and bacterial translocation causing sepsis.
Treatment of early complications
• Consider treating all severe cases on HDU/ITU for optimized fl uid
balance, respiratory, cardiovascular, and renal support.
• Proven infected necrosis. Surgical debridement may be required, but is
associated with a poor prognosis.
• Acute pseudocysts rarely need drainage unless very large.
Box 8.1 Glasgow Imrie criteria
Three or more positive criteria within 48h of admission = severe attack
(mnemonic: PANCREAS):
- PaO
2
<8kPa.
- Age >55y.
- Neutrophils/WCC >15 000
× 10
9
/L.
- Corrected calcium <2mmol/L.
- Raised blood urea >16mmol/L.
- Elevated Enzymes, AST>200U/L, LDH >600U/L.
- Albumin <32g/L.
- Sugar, blood glucose >10mmol/L.

CHAPTER 8 Liver, pancreatic, and biliary surgery
334
Overall outcome Mortality is associated with pancreatic necrosis and
the presence of sepsis, including MODS.
Reference
1 UK Working Party on Acute Pancreatitis (2005). UK guidelines for the management of acute
pancreatitis. Gut 54, iii1–iii9.

CHAPTER 9 Abdominal wall
336
Abdominal wall hernias
No disease of the human body, belonging to the province of the surgeon,
requires in its treatment a better combination of accurate anatomical knowl-
edge with surgical skill than hernia in all its varieties.
Sir Astley Paston Cooper (1804)
Defi nition of a hernia The abnormal protrusion of a viscus or part
of a viscus through a weakness in its containing wall.
Aetiology
• Congenital. Associated with a developmental disorder, such as
persistent processus vaginalis (infantile inguinal hernia) or failure of
complete obliteration of umbilical opening (infantile umbilical hernia).
• Acquired. Weakness of the abdominal wall due to ageing or previous
surgery; risk increases in conditions where there is i intra-abdominal
pressure, such as heavy lifting, chronic cough, straining on urination or
defecation, abdominal distension, ascites, pregnancy, etc.
Composition of a hernia
• Sac. Peritoneal lining of a hernia; may be complete or incomplete as in
sliding hernia (b p. 346).
• Neck of the sack. At the level of the defect in the abdominal wall
where the hernia emerges.
• Contents. Bowel or omentum.
Behaviour
• Reducible. Contents can be fully restored to the abdominal cavity,
spontaneously or with manipulation.
• Incarcerated. Part or all of the contents cannot be reduced due to a
narrow neck and/or adhesions; there is a risk of strangulation.
• Obstructed. Contains an obstructed bowel loop due to kinking; usually
goes on to strangulation.
Groin hernias rank third, after adhesive obstruction and cancer, as
the most common cause of bowel obstruction in the west. In tropical
Africa, strangulated external hernia is the commonest cause of intestinal
obstruction.
• Strangulated. Blood supply to the contents of the sac is cut off; the
tight neck of the peritoneal sac is the usual site of strangulation.
Pathological sequence Venous and lymphatic occlusion l oedema and
i venous pressure l impeding arterial fl ow l bowel necrosis and
perforation.
2 If not diagnosed and managed early, bowel infarction can result and lead
to serious complications like peritonitis and septic shock.

ABDOMINAL WALL HERNIAS
337
Key revision points—general considerations in assessing
a patient with a hernia
History
- Is it a hernia? (history of reducibility)
- Site?
- Simple or complicated? Complicated if:
Incarcerated: patient can’t reduce it anymore.
-
Obstructed: symptoms of bowel obstruction.-
Strangulated: acute and severe pain, bowel obstruction, and -
patient is generally unwell.
- Any risk factors? (heavy lifting, COPD, constipation, BPH, previous
surgery).
Physical examination
- Confi rm the diagnosis and type (reducibility, cough impulse,
anatomical location).
- Always examine both sides in suspected groin hernias.
- Any scars? (recurrent or incisional hernia)
- General examination is essential to look for predisposing factors like
bowel pathology and BPH.
Decision making
Is surgery warranted? (symptomatic, i risk of strangulation as with nar-
row neck, patient mobility and fi tness for surgery).

CHAPTER 9 Abdominal wall
338
Inguinal hernia
Key facts
• It has been estimated that worldwide, >20 million repairs of inguinal
hernia are carried each year and in the UK 100 000.
• Commonest type of abdominal hernia; ♂:♀, 8:1.
• Abdominal contents protrude through the inguinal canal.
• Classifi ed to indirect and direct according to its (surgically determined)
relationship to the inferior epigastric artery (see Table 9.1).
• Coexistence of direct and indirect hernias descending either side of
the epigastric artery produces a ‘pantaloon hernia’.
Clinical features
• Most have no symptoms until a lump is noticed in the groin.
• Ache or dragging sensation, especially towards the end of the day.
• Some can relate the onset of the pain and bulge to a specifi c activity
(e.g. lifting).
Diagnosis and investigations
If the diagnosis is uncertain, investigations are of some help.
• Ultrasound. Least invasive and cheap, but may lead to false results.
• CT and MRI. Highly accurate, but CT involves substantial radiation.
• Herniography (intraperitoneal contrast injection and subsequent X-ray).
Aids in the diagnosis in cases of groin pain when no hernia can be felt;
rarely performed.
Treatment
• Patients with symptoms or have had episodes of irreducibility or bowel
obstruction documented should be offered repair.
• Elderly, immobile patients or those with high morbidity for operation
may be safely observed if asymptomatic or mildly symptomatic (annual
risk of incarceration is 2–3 per 1000 patients per year).
• A groin truss is of limited symptomatic benefi t for non-surgical
patients.
Technical aspects
• Repair may be performed by open surgery or via the laparoscopic
approach (either transperitoneal or in the pre-peritoneal space).
• General or local anaesthesia if done via the open approach.
• Tension-free reinforcement of the transversalis fascia (TVF) layer
(usually with non-absorbable mesh); in open repairs, this lies in front of
the TVF and in laparoscopic, behind it).
• Mesh may be fi xed in place by sutures (open) or ‘tacking’ devices
(laparoscopic approach).
• Avoid heavy lifting and straining for fi rst to second week post-op.
• Lifetime recurrence of combined mesh repairs is approximately
1–2%.
• Laparoscopic approach is recommended for recurrent and bilateral
hernias and should be carried out by experienced surgeons in well-
equipped units (NICE guidelines).

INGUINAL HERNIA
339
Table 9.1 Comparison between indirect and direct inguinal hernias
Indirect Direct
Age Any age, but usually
young
Uncommon in children
and young adult
Aetiology Congenital (patent
processus vaginalis)
Acquired weakness in
abdominal wall
Relationship to inferior
epigastric artery
Lateral Medial
Descending to
scrotum
Often Rarely
Occluding the internal
ring
Controls it Does not control it
Neck Narrow Wide
Strangulation More likely Rare
Treatment Infant—herniotomy
(ligation and excision of
the sac)
Adult—open mesh repair,
laparoscopic repair
Open mesh repair,
laparoscopic repair
Key revision points—anatomy of the inguinal canal
- The inguinal canal is the oblique passage through the lower
abdominal wall. It runs from deep to superfi cial, from the internal to
the external inguinal rings.
- The inguinal canal transmits the spermatic cord (round ligament in
the female) and the ilioinguinal nerve.
- Contents of the spermatic cord are:
Three vessels (testicular artery, cremasteric artery, artery to the
-
vas).
Three nerves (genital branch of genitofemoral, autonomic supply
-
to the testicle, ilioinguinal nerve).
Three structures (vas, pampiniform venous plexus, testicular
-
lymphatics).
Three coverings (external spermatic fascia, cremasteric fascia,
-
internal spermatic fascia).
- The deep ring is formed through the transversalis fascia and lies
1–2cm above the inguinal ligament, midway between the symphysis
pubis and the anterior superior iliac spine.
- The superfi cial ring is a V-shaped defect in the aponeurosis of
external oblique, above and medial to the pubic tubercle.
- Direct inguinal hernias pass through a weakness in the transversalis
fascia in the Hesselbach’s triangle area (bounded by inguinal
ligament inferiorly, inferior epigastric artery laterally, and lateral
border of the rectus muscle medially).

CHAPTER 9 Abdominal wall
340
Femoral hernia
Key facts
• Commoner in women than men.
• Occurs through tissues of femoral canal.
• Has a high risk of strangulation due to the neck of the sac having bony
and ligamentous structures limiting it on three sides.
• Approximately 30% of femoral hernias present as emergencies; 50% of
these require bowel resection for strangulation and ischaemia.
Clinical features
• Appears below and lateral to pubic tubercle, medial to femoral pulse.
• May be asymptomatic until incarceration or strangulation occurs.
• May be mistaken for an upper medial thigh swelling.
Diagnosis and investigations
• Differential diagnosis includes:
Low presentation of inguinal hernia.•
Femoral canal lipoma.•
Femoral lymph node.•
Saphena varix (compressible, disappears when lying fl at, palpable •
thrill on coughing or percussion of the saphenous vein).
Femoral artery aneurysm (pulsatile).•
Psoas abscess (fl uctuant and lateral to femoral artery).•
• Ultrasound scanning may help with the differential diagnosis. If there is
signifi cant doubt, exploration is usually indicated due to the high risk of
complications in untreated femoral hernia.
Treatment
• All should be repaired because of great risk of strangulation; truss has
no place in the management.
• Once the hernia is reduced, the femoral canal should be narrowed by
interrupted sutures to prevent recurrence; care must be taken not to
narrow the adjacent femoral vein. There are two main approaches.
Low approach (infrainguinal)
• Incision below inguinal ligament approaching femoral canal from below.
• Has the advantage of not interfering with the inguinal structures, but
provides little or no scope for resecting any compromised small bowel
and so is best reserved for elective surgery.
High approach (inguinal)
• Incision above the inguinal ligament approaching femoral canal from
above by dissecting through the posterior wall of the inguinal canal.
• Requires repair of the inguinal canal on closure, but offers excellent
access to the peritoneal cavity should small bowel surgery be required
and is the usual approach in emergency presentations.

FEMORAL HERNIA
341
Key revision points—anatomy of the femoral canal
- Lies medial to femoral vein within femoral sheath.
- Contains loose areolar tissue and a lymph node known as the lymph
node of Cloquet.
- The femoral ring is the abdominal opening of the femoral canal.
The increased diameter of the true pelvis in females proportionally
widens the femoral canal.
- Boundaries to the femoral ring are:
Anteriorly, inguinal ligament.
-
Medially, lacunar ligament.-
Posteriorly, pectineal ligament.-
Laterally, femoral vein.-
- An aberrant obturator artery branch of inferior epigastric may cross
the lacunar ligament and can cause haemorrhage during surgical
repair.
- Femoral hernia repair (open) involves suture-plication of the inguinal
and pectineal ligaments or placement of a mesh plug that is fi xed in
position in the defect with non-absorbable sutures.

CHAPTER 9 Abdominal wall
342
Umbilical and epigastric hernias
Key facts
These hernias are sometimes referred to collectively as ventral hernias.
• Umbilical hernias can be divided into:
True umbilical hernias• . Occur through the umbilical cicatrix and
are almost always congenital in origin; those present at birth may
close spontaneously before the age of 3; more common in Afro-
Caribbean races.
Paraumbilical hernias• . Occur through the periumbilical tissues and
are always acquired; common in obese and parous women.
• Epigastric hernias are defects in the linea alba somewhere between the
xiphisternum and umbilicus at sites of penetration of the linea alba by
nerves and vessels; they may be small or extensive.
Clinical features
Umbilical hernia
• Small, centrally placed within the umbilicus.
• Often contains pre-peritoneal fat and rarely contains bowel/omentum.
• May be painful, but rarely strangulates.
Paraumbilical hernia
• Variable in size, up to moderate.
• Paced eccentrically and distorts the shape of the umbilicus.
• May contain bowel or omentum.
• Often painful and occasionally strangulate.
Epigastric hernia
• Variable, up to large defects.
• Always placed in the midline although when large, may lie to one side.
• Most frequently contains only pre-peritoneal fat.
• Moderate risk of strangulation.
Diagnosis and investigations The diagnosis is rarely in doubt. If there
is concern that a palpable lump may be a lipoma or subcutaneous tissue
growth, then a CT scan can usually confi rm the diagnosis.
Treatment
• Congenital umbilical hernias should only be repaired if they persist
beyond the age of 2–3y.
• Surgical repair is offered for symptomatic hernias or those with a high
risk of complications.
Principles of repair
• Identify edges of hernial sac and reduce hernia.
• Small defects are usually repaired by an overlapping sutured repair
using non-absorbable suture, e.g. 0 Prolene, without reinforcements;
larger defects or recurrent hernias may be repaired with mesh (usually
polypropylene-based, e.g. Prolene).
• Laparoscopic repair is increasingly being used.
This page intentionally left blank

CHAPTER 9 Abdominal wall
344
Incisional hernias
Key facts
• Incisional hernias are very uncommon outside the abdomen.
• Up to 10% of midline laparotomy wounds suffer herniation to some
degree. Factors that predispose to incisional herniation include:
Wound infection.•
Steroid use, anaemia, or malnutrition at the time of original surgery.•
Incisional hernias are probably slightly less common after muscle •
splitting or transverse incisions, compared to midline laparotomies.
Poor surgical techniques in abdominal closure increase the risk.•
• The peak time of presentation is up to 5y after surgery.
Pathological features
The hernia occurs through the tissues in which the incision is made.
Typically, the sac is made up of peritoneum, eventrated scar tissue, and
subcutaneous scar tissue.
Clinical features
• The hernia may vary from a few cm to a near complete defect in the
anterior abdominal wall through which all the mobile viscera regularly
protrude.
• The risk of strangulation is maximal in small- to medium-sized defects.
In large and very large defects, the viscera are often permanently herni-
ated and if this has been the case for a long period, they ‘lose the right of
abode’ within the true abdominal cavity. This means that the remaining
lateral abdominal wall tissues chronically retract and there may be insuf-
fi cient room for all the viscera within the revised abdominal cavity when
the tissues are re-approximated.
Diagnosis and investigations
When assessing incisional hernias, ask yourself the following.
• What is the risk of complications/strangulation?
• Is it likely that the contents of the hernia can be reduced fully?
• Is the patient able to undergo the anaesthesia necessary for the
surgery required?
• Is there a risk of compromise to respiratory function if a very large
incisional hernia is reduced and repaired?
Treatment
• Small defects (<4cm). Simple sutured repair.
• Medium and large defects (>4cm). A mesh is placed between the
posterior rectus sheath and the rectus muscle fi bres. If below the
umbilicus, the mesh is placed in the pre-peritoneal space. After the
mesh is fi xated, the anterior rectus sheath is closed.
• Laparoscopic repair is increasingly being used. The use of an
‘underlay’ intraperitoneal mesh enhances the repair, but also
creates the potential for bowel adhesions or fi stula formation.

INCISIONAL HERNIAS
345
Polytetrafl uoroethylene (PTEF) mesh is recommended to reduce
adhesive complications.
• Unfi t patients or patients unwilling to have surgery. A custom-made
support corset is often useful.

CHAPTER 9 Abdominal wall
346
Other types of hernia
These types are rare, but clinically signifi cant.
Spigelian
• Occurs under the lower edge of the linea semilunaris and protrudes
along the lateral border of the rectus sheath.
• Typically diffi cult to diagnose in the supine position.
• Ultrasound and CT scan may help to confi rm the diagnosis.
• Has a high risk of complications.
• Repair is via direct sutured repair of the rectus sheath.
Obturator
• Occurs through the obturator canal from the lateral wall of the pelvis
with the sac protruding into the medial upper thigh.
• Symptoms include pain or abnormal sensations in the distribution of
the obturator nerve in the skin of the inner medial thigh.
• Diagnosis is often very diffi cult and is usually made by CT scan.
• A high proportion present with complications of bowel obstruction
due to the sac being hidden within the adductor muscles
compartment.
• The neck is narrow and prone to strangulation.
• Repair can be via exposure of the sac in the medial thigh or, more
commonly, at laparotomy for complications.
Lumbar
• Occurs through either the inferior or superior lumbar triangles
(bounded by the lumbar muscles, lumbosacral fascia, and bony features
of the posterior abdominal wall) or, rarely, through lumbar incisions.
• Usually contain retroperitoneal fat and rarely bowel.
• May be repaired by direct suture or mesh repair for larger defects.
Perineal
• Spontaneous perineal hernias occur through the greater or lesser
sciatic foramina and are exceptionally rare.
• Present with acute complications and are diagnosed only at surgery.
• Post-operative perineal hernias occur through the pelvic fl oor muscles
and do so usually as a result of surgical procedures (particularly after
abdominoperineal resection of the rectum).
• Repair may be via sutured closure of the defect or, more commonly,
fi lling of the defect with prosthetic material (mesh) or biological tissue
(muscle fl ap).
Sliding
• The sac is formed partly by a retroperitoneal structure.
• It is thought that the structure slides down the canal pulling its
overlying peritoneum with it hence the name ‘hernia-en-glissade’.
Littre’s hernia The hernia sac contains a Meckel’s diverticulum.
Richter’s hernia Only part of the circumference of the bowel is
strangulated.

RECTUS SHEATH HAEMATOMA
347
Rectus sheath haematoma
Key facts
Result from haemorrhage from any of the vascular network within the
rectus sheath that is formed by terminal branches of the superior and
inferior epigastric arteries.
Clinical features
• Presents with a sudden localized abdominal pain ± a tender mass.
• A history of major or minor blunt trauma may be elicited.
• Some patients report events that cause sudden contraction of the
rectus muscle, such as coughing, sneezing, or any vigorous physical
activity; it may also develop spontaneously in anticoagulated patients.
• Pain typically increases with contraction of the rectus muscles and a
tender mass may be palpated and remains unchanged when the rectus
muscle is contracted.
Diagnosis and investigations
• Can be confused with conditions that present with unilateral
abdominal pain like appendicitis.
• Hb level and coagulation profi le should be checked.
• Ultrasonography may help in the diagnosis; CT is diagnostic.
Treatment
• Small and stable haematomas may be observed without patient
hospitalization.
• Bilateral or large haematomas need hospitalization; blood transfusion
and reversal of coagulation may be needed.
• Angiographic embolization is required infrequently, but may be
necessary in case of haematoma enlargement, free bleeding, or clinical
deterioration.
• Surgical ligation of bleeding vessels is indicated if angiographic
treatment fails or the patient becomes haemodynamic unstable.

CHAPTER 9 Abdominal wall
348
Groin disruption
Key facts
• Also called ‘Gilmore’s groin’ or ‘sports hernia’ (although hernia is
rarely present).
• An overuse syndrome that results in muscular imbalances of the pelvis
and abdominal wall muscles.
• Common in male athletes, especially in sports that require repetitive
twisting and turning at high speeds (e.g. soccer, tennis, ice hockey).
Pathological features
It has distinctive features that include a torn external oblique aponeurosis,
a torn conjoined tendon, a dilated superfi cial inguinal ring, and a dehis-
cence between inguinal ligament and conjoined tendon.
Clinical features
• Unilateral groin pain that is often insidious and gradually worsens and
is felt ‘deep’ in the groin area.
• Some patients may identify a distinct provocative event like kicking or
a sudden change in direction while playing.
• Pain is almost always absent at rest.
• Physical fi ndings are non-specifi c. The external inguinal ring may be
dilated and tender. Valsalva manoeuvres and resisted adduction may
elicit pain and are useful provocative tests.
Diagnosis and investigations
• The differential diagnosis includes osteitis pubis, adductor
tendinopathy, and pubic instability or fracture.
• Radiographic fi ndings are usually normal, but helpful in ruling out other
conditions. Therefore, it is a clinical diagnosis that is confi rmed intra-
operatively.
Treatment
• These patients are managed conservatively initially due to the diffi culty
in making a diagnosis.
• If symptoms persist, surgical repair is indicated and should be carried
out by a surgeon who is experienced in managing groin disruption.
• Mesh reinforcement is often utilized during the repair.
• This is followed by 2–4-week rehabilitation programme.
This page intentionally left blank

CHAPTER 9 Abdominal wall
350350
Acute groin swelling
Causes of chronic testicular swelling are discussed on b p. 366.
Causes and features
• Incarcerated groin hernia (inguinal or femoral). May or may not be
associated with intestinal obstruction; often red, hot, and tender.
• Acute epididymo-orchitis (in males). Tenderness is particularly over the
spermatic cord and the epididymis.
• Torsion of the testis. May present with pain in the groin, but unless the
testicle is undescended, the tenderness is primarily over the scrotum
(and testis). Testicle is tender, swollen, and high riding. Elevation of the
scrotum, unlike epididymitis, makes the pain worse.
• Iliopsoas abscess. Tenderness primarily below the inguinal ligament;
may be fl uctuant, associated tenderness in the RIF due to underlying
pathology.
• Acute iliofemoral lymphadenopathy (e.g. from infected toenail). Tender
diffuse swelling; often multiple palpable lumps (nodes).
• Acute saphena varix. Compressible, cough thrill.
• Acute complications of femoral artery aneurysm.
Emergency management
Resuscitation
• Establish IV access; consider giving crystalloid fl uid if there is a
suspicion of a complicated hernia or an iliopsoas abscess.
• Catheterize and place on a fl uid balance chart if hypotensive.
• Send blood for FBC (Hb, WCC), U&E (Na, K).
• Group and save and clotting if arterial disease or abscess formation is
suspected.
Establish a diagnosis
2 Torsion of the testis is a true surgical emergency and should not wait
for a diagnosis short of exploration.
Colour fl ow Doppler assessment may be able to confi rm the presence
of a hyperaemic testis, but unless it is immediately available, it should not
delay operation.
2 If there is a strong clinical history in a young male, immediate operation
remains the diagnostic investigation of choice.
• If an iliopsoas abscess is suspected, abdominopelvic CT scanning is the
investigation of choice.
• Rigid sigmoidoscopy and biopsy only if not unstable.
• Flexible endoscopy may be indicated, but carries a risk of perforation.
Defi nitive management
Incarcerated groin hernia
• Repair is indicated for all patients except those considered unfi t for
any surgical procedure or those declining treatment.
• It may not be possible to establish if the hernia is inguinal or femoral
preoperatively; if so, it is safest to approach as if for an inguinal hernia.

ACUTE GROIN SWELLING
351351
• Femoral hernias may be approached via an infrainguinal or transinguinal
dissection (see b p. 342).
Infrainguinal approaches may be limited in exposure if there is •
necrotic bowel within the hernia requiring resection.
A transinguinal approach will involve repair of the inguinal canal as •
well, but offers an almost unlimited exposure of the femoral canal
from above and allows plenty of exposure for bowel resection.
• Inguinal hernias should be approached through a conventional incision.
Repair may require a mesh although there is an increased risk of
infection if there is an associated bowel resection.
• Anaesthesia may be general or local with sedation.
Psoas abscess The underlying cause should be identifi ed as a matter or
priority. Incision and drainage of the groin collection may be indicated, but
only as part of the overall treatment.
Torsion of the testis
• Once identifi ed, the affected testis should assessed; if non-viable, a
simple orchidectomy is performed and if viable, an orchidopexy.
• If the diagnosis is confi rmed, the contralateral testicle should be fi xed
by orchidopexy to prevent subsequent torsion.
Epididymo-orchitis Antibiotics PO (e.g. ciprofl oxacin 500mg od) for 14 days
or IV if severe.
This page intentionally left blank

353
Urology
Symptoms and signs in urology 354
Investigations of urinary tract disease 356
Urinary tract stones 358
Obstruction of the ureter 360
Benign prostatic hyperplasia 362
Stricture of the urethra 364
Scrotal swellings 366
Disorders of the foreskin 368
Common conditions of the penis 370
Erectile dysfunction 372
Adenocarcinoma of the kidney 374
Transitional cell tumours 376
Adenocarcinoma of the prostate 378
Carcinoma of the penis 380
Testicular tumours 382
Haematuria 384
Acute urinary retention (AUR) 386
Acute testicular pain 388
Chapter 10

CHAPTER 10 Urology
354
Symptoms and signs in urology
Symptoms
Pain
• May be located over the site of pathology, e.g. testes.
• May radiate in accordance with innervation of the structure involved.
Kidney pain• . In the renal angle (between the lower border of the
12th rib and the spine).
Ureteric pain• . Between the renal angle and the groin.
Bladder pain• . In the suprapubic region.
Prostatic pain• . In the perineum, but may radiate along the urethra to
the tip of the penis.
• May be related to function, e.g. suprapubic pain exacerbated by
bladder fi lling.
Haematuria (macroscopic)
• Frequently a sinister symptom of malignant disease, especially the
bladder when it is normally painless.
• When associated with painful voiding, it is usually due to bladder
infection or stones.
Lower urinary tract symptoms
• Refers to a group of symptoms that typically affect the ageing male.
• Often caused by bladder outfl ow obstruction related to prostatic
enlargement.
• Includes symptoms related to both voiding and storage.
• Voiding symptoms. Poor urine fl ow, hesitancy, post-micturition
dribbling.
• Storage symptoms. Frequency, nocturia, urgency, urge incontinence.
• International prostate symptom score (IPSS) is a validated
questionnaire to estimate the patient’s perception of severity of
symptoms.
Urinary incontinence
• Affects women more commonly than men.
• Stress incontinence. Urine leakage that occurs at times of increased
intravesical pressure, e.g. during coughing, sneezing, lifting.
Results from incompetence of urethral sphincter and bladder neck •
mechanism; usually related to pregnancy and childbirth.
• Urge incontinence. Urine leakage that occurs in association with a
strong desire to void.
Urine leaks from the bladder before the patient is able to reach a •
toilet.
Usual cause is overactivity of the detrusor muscle.•
May be idiopathic or secondary to other bladder disease.•
Stress and urge incontinence frequently coexist.•
• Insensible urine leakage. Occurs without any associated symptoms.
Urine leaks from the bladder continuously and the patient is •
sometimes unaware.

SYMPTOMS AND SIGNS IN UROLOGY
355
Causes• . Overfl ow incontinence from chronic retention, fi stulation
(commonly between bladder and vagina), gross sphincter
disturbance resulting from surgery or neurological disease.
Male sexual dysfunction
• Erectile dysfunction (ED), commonly known as impotence.
Inability to attain and maintain an erection adequate for satisfactory •
sexual intercourse.
There are degrees of ED.•
Men with incomplete ED respond more satisfactorily to treatment.•
The majority of cases have an organic basis.•
All cases have a degree of psychogenic involvement.•
Twenty per cent of cases are primarily psychogenic.•
• Premature ejaculation.
More common in younger men.•
Invariably has a psychogenic basis.•
Often associated with performance anxiety.•
• Loss of libido.
Loss of normal sex drive.•
Either psychogenic or related to hypogonadal states.•
Haemospermia
• Presence of blood in ejaculate.
• Rarely associated with signifi cant pathology.
Signs
Inspection
Examination of the penis must include retraction of the foreskin (if pos-
sible) and inspection of the glans and external meatus for signs of infection,
infl ammation, or tumour.
Palpation
• Tenderness in the renal angle or a palpable loin mass may indicate
renal pathology.
• Suprapubic dullness to percussion is an indication of a bladder mass or
full of urine.
• Check for testicular asymmetry, masses, or tenderness
(underdevelopment, tumours, and infection).
• Intrascrotal mass may include the testis (e.g. hydrocele) or be separate
from it (e.g. epididymal cyst).
• Do a digital rectal examination to determine the size, consistency,
regularity, and symmetry of the prostate.

CHAPTER 10 Urology
356
Investigations of urinary tract disease
Laboratory investigations
Urinalysis
• Dipstick analysis for blood, leucocytes, protein, nitrites, and glucose.
Nitrites, blood, and leucocytes—infection.•
Blood—microscopic haematuria.•
Protein, leucocytes—intrinsic renal disease.•
• Microbiology, cytology for presence of urinary infection or
malignant cells. Midstream urine (MSU) specimens are required for
bacteriological culture; take care to avoid contamination
particularly in women.
• Matched urine and serum biochemistry to assess glomerular function,
e.g. matched osmolarities, sodiums, and potassiums.
Blood
• Serum creatinine levels. Provides a crude assessment of overall renal
function.
• Creatinine (Cr) clearance (requires 24h urine collection and
measurement of serum creatinine).
Cr clearance (mL/min) = u x v/p
where u is urine Cr concentration, v is 24h urine volume, p is plasma Cr
concentration.
• Serum prostate-specifi c antigen (PSA). Indicator of prostate disease.
Interpreted according to age-specifi c reference range.•
High levels are found in benign prostatic hyperplasia, prostate •
cancer, acute retention, and urinary infection.
• Sex hormone measurements. Occasionally useful in the assessment of
male sexual dysfunction and infertility.
Radiology investigations
Ultrasound
• Renal and bladder scans. For haematuria and urinary tract infection
(UTI).
• Transrectal ultrasound scan. Measures prostate accurately and allows
systematic biopsy for detection of cancer.
• Scrotal ultrasound. Evaluates acute scrotum from a suspected testicular
cancer.
Intravenous urogram (IVU)
• Provides greater functional information than ultrasound.
• Provides superior imaging of the ureter.
CT
• Pre- and post-contrast scans provide some functional information with
regard to arterial and venous blood fl ow and excretory function of the
kidneys.
• Vital for the staging of renal, bladder, and testicular cancers.

INVESTIGATIONS OF URINARY TRACT DISEASE
357
MRI
• Provides greater accuracy than CT in assessment of the prostate
capsule and seminal vesicles.
• Sensitive test for the presence of bone metastases.
Isotope bone scan
• Demonstrates abnormal area of bone turnover.
• A useful screening test for the presence of bone metastases.
• Plain fi lms are taken to aid interpretation if the site or pattern of hot
spots is indeterminate.
Isotope renography
• Provides anatomical and functional information about the kidneys.
• Dimercaptosuccinate (DMSA) scan provides an image of functioning
renal parenchymal tissue.
• 99m
Tc-mercaptoacetyltriglycine (MAG3) renogram provides dynamic
information regarding excretion from the kidneys and determines
whether or not obstruction is present.
Endoscopy
Flexible cystoscopy
• Examines urethra and bladder.
• Performed using local anaesthetic gel.
• There is limited potential for intervention.
Rigid cystoscopy Under GA, permits biopsy and resectoscope allows
resection of tissue.
Ureteroscopy
• Rigid and fl exible ureteroscopes provide access to the ureter and
pelvicalyceal system.
• Allows the passage of instruments and laser fi bres for the treatment of
stones and upper tract tumours.

CHAPTER 10 Urology
358
Urinary tract stones
Key facts
• Prevalence of stones in the population is around 3%; ♂ > ♀.
• The commonest reason for emergency urological admissions.
• Peak presentation in the summer months.
• Most common age of presentation of urinary calculi is 20–50y.
• Ninety per cent of urinary calculi are radio-opaque.
Aetiology
• Metabolic. Hyperparathyroidism, idiopathic hypercalciuria,
disseminated malignancy, sarcoidosis, hypervitaminosis D.
• Familial metabolic causes. Cystinuria, errors of purine metabolism,
hyperoxaluria, hyperuricuria, xanthinuria.
• Infection.
• Impaired urinary drainage, e.g. medullary sponge kidney, pelviureteric
junction (PUJ) obstruction, ureteric stricture, extrinsic obstruction.
Pathological features
Calcium stones
• Seventy-fi ve per cent of all urinary calculi.
• Usually combined with oxalate or phosphate, are sharp, and may cause
symptoms, even when small.
Triple phosphate stones (‘struvite stones’)
• Compounds of magnesium, ammonium, and calcium phosphate.
• Fifteen per cent of all calculi.
• Commonly occur against a background of chronic urinary infection and
may grow rapidly.
• ‘Staghorn’ calculi (fi ll the calyceal system) are a form of struvite.
Uric acid stones
• As a consequence of high levels of uric acid in the urine.
• Five per cent of all urinary stones; radiolucent.
Cystine stones
• Relatively rare; 1–2% of all cases.
• Diffi cult to treat due to extremely hard consistency.
Other stones Xanthine, pyruvate, and other stones; 1% of all calculi.
Clinical features
• ‘Ureteric/renal colic’. Severe, intermittent, stabbing pain radiating from
loin to groin.
• Microscopic or, rarely, frank haematuria.
• Systemic symptoms such as nausea, vomiting, tachycardia, pyrexia.
• Loin or renal angle tenderness due to infection or infl ammation.
• Iliac fossa tenderness if the calculus has passed into the distal ureter.
Investigations
• Basic tests.
Raised WCC and CRP suggest superadded infection (should be •
confi rmed by MSU); raised Cr suggests renal impairment.

URINARY TRACT STONES
359
Stones often visible on plain abdominal X-ray (‘kidneys/ureters/•
bladder’ (KUB)).
Serum calcium, phosphate, and uric acid.•
24h urine for calcium, phosphate, oxalate, urate, cystine, and •
xanthine.
• Advanced tests.
Non-contrast spiral CT is the gold standard for locating stones and •
assessing evidence of complications.
IVU will locate stones and show any proximal obstruction.•
Renal ultrasound scan for hydronephrosis.•
Treatment
Acute presentations (renal colic, ureteric obstruction)
• Analgesia, e.g. diclofenac 100mg PR; antiemetic, e.g. metoclopramide
10mg IV; IV fl uids.
• Small stones (<0.5cm) may be managed expectantly as most will pass
spontaneously.
• Emergency treatment with percutaneous nephrostomy and/or ureteric
stent insertion is necessary if either pain or obstruction is persistent.
Elective presentations
• Extracorporeal shock wave lithotripsy (ESWL).
Focused, externally generated electrohydraulic or ultrasonic shock •
waves.
Targeted onto the calculus using ultrasound, X-ray, or a •
combination.
Causes stone disintegration and the fragments are then voided.•
• Percutaneous nephrolithotomy (PCNL).
For stones in the renal pelvis or calyces and occasionally for stones •
in the upper ureter.
Percutaneous track into the renal pelvis using fl uoroscopic •
guidance.
Nephroscope is inserted and the calculus visualized.•
Removed either in total or, if large, following fragmentation.•
• Endoscopic treatment.
Ureteroscope is inserted and the stone visualized.•
Stone is fragmented using ultrasound, electrohydraulic •
intracorporeal lithotripsy, or laser.
• Open nephrolithotomy/ureterolithotomy. For large staghorn calculi or
complex stones, e.g. above ureteric stricture.
Prevention of recurrence
• Increase oral fl uid intake and reduce calcium intake.
• Correct metabolic abnormalities.
• Treat infection promptly.
• Urinary alkalization, e.g. sodium bicarbonate 5–10g/24h PO in water
(mainly for cystine and urate stones).
• Thiazide diuretics (for idiopathic hypercalciuria).

CHAPTER 10 Urology
360
Obstruction of the ureter
(See Fig. 10.1)
Key facts
Ureteric obstruction leads to hydronephrosis (ureteric and
pelvicalyceal dilatation).
Pathological features Hydronephrosis can be unilateral or bilateral.
Unilateral
• Extramural.
Aberrant vessels at the PUJ.•
Extrinsic tumour. Carcinoma of the cervix, prostate, large bowel, or •
retroperitoneal endometriosis.
Idiopathic retroperitoneal fi brosis.•
Post-radiation fi brosis.•
Retrocaval ureter.•
Abdominal aortic aneurysm.•
• Intramural.
Transitional cell carcinoma of the renal pelvis or ureter.•
Urinary calculi.•
Ureteric stricture.•
Aperistaltic segment. Almost always congenital.•
Bilateral
• All causes of unilateral obstruction may cause bilateral hydronephrosis.
• Congenital posterior urethral valve.
• Congenital or acquired urethral stricture.
• Benign enlargement of the prostate.
• Locally advanced prostate cancer.
• Large bladder tumours.
• Gravid uterus.
Clinical features
• Loin pain.
• Fever and/or rigors (if complicated by infection).
• Symptoms and signs of renal failure (if obstruction longstanding).
Investigation and diagnosis
• Serum biochemistry and haematology.
• MSU.
• KUB X-ray/IVU.
• Ultrasound scan and/or CT scan.
• Isotope renogram.
• Retrograde pyelogram.
Complications
• Infection, pyonephrosis.
• Hypertension.
• Renal failure.
OBSTRUCTION OF THE URETER
361
Treatment
3 Emergency presentation
• Emergency treatment is indicated if there are signs of infection,
established renal failure, uncontrollable symptoms.
• Treatment is drainage of the kidney via a percutaneous nephrostomy
or retrograde ureteric stent.
Elective presentation
• Defi nitive treatment is directed at the underlying cause. Possible
interventions include:
Treatments of calculi (see • b p. 358).
Ureteric stenting (unilateral or bilateral).•
Ureterolysis and ureteric transfer (for retroperitoneal fi brosis).•
Prostatic resection.•
Bladder drainage using a urethral or suprapubic catheter.•
• In cases where renal function cannot be restored, a nephrectomy is
performed to avoid infective complications.
1. Pelviureteric junction
2. Mid ureter (lower retroperitoneum
and pelvic brim)
3. Vesicoureteric junction
4. Bladder neck
Fig. 10.1 Commonest sites of renal stone impaction. 1, pelviureteric junction;
2, pelvic brim; 3, vesicoureteric junction.

CHAPTER 10 Urology
362
Benign prostatic hyperplasia
Key facts
• Benign prostatic hyperplasia (BPH) is a non-malignant enlargement of
the prostate gland. There is an increase in both stromal and glandular
components.
• Incidence of BPH is about 25% in age 40–60y, 40% in over 60s.
• Commonest cause of lower urinary tract symptoms (LUTS) in
middle-aged and elderly men.
Pathological features
Aetiology is largely unknown. Possible factors include:
• Androgens. No BPH in those who have had early castration.
• Oestrogens. Increased oestrogen:testosterone ratio with age.
• Growth factors, e.g. high concentration of TGFA in BPH.
Clinical features
Symptoms
• Storage symptoms, such as frequency, urgency, nocturia, and
incontinence.
• Voiding symptoms, including hesitancy, poor stream, intermittency,
terminal dribble, and abdominal straining.
• Superimposed infection may cause dysuria and haematuria.
• Incomplete emptying and chronic or acute retention of urine.
Signs
• Smooth enlargement of the prostate detected by digital rectal
examination.
• Possible palpable bladder if chronic retention.
• Always examine for neurological signs in those with LUTS.
Complications of BPH
• Intractable LUTS.
• Haematuria.
• UTI.
• Stone formation.
• Acute retention of urine.
• Chronic retention of urine.
• Overfl ow incontinence.
• Obstructive renal failure.
Diagnosis and investigations
• Prostate symptom score to assess severity.
• Digital rectal examination and serum PSA measurement to assess for
features of malignancy.
Basic investigations
• Serum creatinine, urinalysis in all patients.
• Urine fl owmetry and residual volume estimation in those considered
for intervention.
BENIGN PROSTATIC HYPERPLASIA
363
Advanced investigations
• Cystoscopy. To exclude bladder disease.
• Transrectal ultrasound 9 guided biopsy. If concern over underlying
malignancy.
• Renal ultrasound, invasive urodynamic studies.
Treatment
Recommended for those with LUTS that are impacting on quality of life or
those with complications.
Medical treatment
• Patients with mild symptoms and no complications may be observed
(watchful waiting).
• A-adrenergic antagonists. Relax smooth muscle of prostatic urethra
to decrease outlet resistance; side effects include dizziness and
hypotension (especially postural).
• 5A-reductase inhibitors. Block conversion of testosterone to
dihydrotestosterone (DHT) and shown to cause involution of BPH;
side effects include loss of libido and erectile dysfunction.
• Combination drug therapy with both the above agents. May reduce
the clinical progression and decrease the need for surgery.
Surgical treatment
• Reserved for those with any of the complications or symptoms not
responding to medical therapy.
• Surgical options include:
Transurethral resection of the prostate (TURP), the most •
commonly performed procedure for BPH in the UK.
Open retropubic prostatectomy.•
Transurethral incision in the prostate (TUIP).•
Bladder neck incision.•
Laser ‘prostatectomy’.•
Microwave thermotherapy ablation of the prostate.•
Key revision points—anatomy of the prostate
• Three glandular zones:
Peripheral (70%), most prone to carcinoma formation.•
Central (25%), most prone to BPH.•
Transitional (5%), most prone to BPH.•
• Blood supply:
Arterial supply is triple, mainly inferior vesical, some from inferior •
rectal and internal pudendal.
Venous drainage, extensive plexus beneath capsule.•
• Innervation:
Autonomic, extensive from inferior hypogastric plexus as a •
capsular plexus supplying prostate, seminal vesicles, and urethra,
which also supplies the penile structures (glands, corpora, and
urethra).
Somatic, from pudendal nerve (S2, 3, 4) to supply external •
urethral sphincter.

CHAPTER 10 Urology
364
Stricture of the urethra
Key facts
• Classifi ed according to site and aetiology, e.g. post-infl ammatory
bulbar stricture or traumatic membranous stricture.
• Graded according to length and (in the anterior urethra) degree of
fi brosis of corpus spongiosum (spongiofi brosis).
• Any part of the urethra may be involved.
• The propensity for stricture recurrence parallels the severity (grade).
Aetiology
• Trauma.
Pelvic fracture.•
Falls astride, e.g. on bicycle crossbar.•
Urinary tract instrumentation/surgery.•
• Infection.
Neisseria gonorrhoea • and Chlamydia trachomatis.
Catheter-associated UTI.•
• Lichen sclerosis et atrophicus.
Pathological features
• Annular narrowing by scar tissue composed of dense collagen and
fi broblasts, which may extend into corpus spongiosum.
• Lichen sclerosus (balanitis xerotica obliterans (BXO)) consists of
dermal sclerosis with epidermal atrophy; urethral lesions are usually
confi ned to the meatus and fossa navicularis.
Clinical features
• History of urethritis, trauma, or urinary tract instrumentation.
• LUTS, divergent or diminished stream, straining to void, urgency,
frequency.
• Haematuria (‘initial’ or ‘terminal’, i.e. at the beginning or end of the
stream).
• UTI (often recurrent).
• Urinary retention, acute or chronic.
• Overfl ow incontinence.
Complications
• Urinary tract calculi formation.
• Infection, including UTI, prostatitis, epididymitis, and (rarely) Fournier’s
necrotizing fasciitis.
• Renal failure secondary to chronic obstruction.
Diagnosis and investigations
• Urinalysis (microbiology, biochemistry, and cytology).
• U&E.
• Urofl owmetry and measurement of post-void residual bladder volume.
• Voiding cystourethrogram with or without retrograde urethrogram.
• Endoscopy.

STRICTURE OF THE URETHRA
365
• Ultrasound scan (transperineal or endoluminal) to assess extent of
spongiofi brosis.
• Magnetic resonance tomography.
Treatment
Initial
• Treat infection before surgical treatment.
• For acute urinary retention, severe symptoms, or renal failure,
temporary suprapubic catheterization.
Defi nitive
• All urethral reconstructive surgery has an ‘attrition rate’.
• Initial internal urethrotomy may cure up to 30%, but repeat
procedures are rarely successful and may cause progression.
• Urethroplasty may be used for some cases.
Short strictures are excised and the urethra primarily •
re-anastomosed.
For longer or complex strictures, pedicled fl ap reconstruction or •
graft reconstruction is used; the graft or fl ap may be applied as an
onlay (to augment the native urethra) or as a tube.
For free grafts, buccal mucosa is currently favoured.•
More complicated repairs are best managed with staged repairs.•
• Perineal urethrostomy may be required.

CHAPTER 10 Urology
366
Scrotal swellings
Major causes
Intratesticular lesions
• Malignant testicular tumours.
• Benign intratesticular lesions. Simple intratesticular cyst or epidermoid
cysts and benign teratoma (especially in the prepubertal testis).
Infl ammatory lesions
• Acute epididymo-orchitis or viral orchitis.
• Chronic tuberculous epididymo-orchitis, schistosomal epididymitis,
sperm granuloma.
Traumatic lesions
• Scrotal haematoma.
• Haematocele (haematoma within tunica vaginalis).
• Testicular haematoma (within tunica albuginea testis).
Derangement of testicular, adnexal, or cord anatomy
• Epididymal cysts or spermatocele of the epididymis.
• Varicocele (varicosities of the pampiniform plexus).
• Inguinal hernia (patent processus vaginalis in children).
• Hydrocele (vagina = within tunica vaginalis or cordal).
• Late (missed) or prenatal torsion of the spermatic cord.
• Persistence of embryological vestigial structures.
Müllerian duct remnant (appendix testis).•
Wolffi an duct remnants (appendix epididymis, vas aberrans of •
Haller, paradidymis).
Miscellaneous
• Acute idiopathic scrotal oedema.
• Cutaneous lesions, e.g. sebaceous cysts.
• Henoch–Schönlein purpura.
Clinical features/diagnosis
• 2 Testicular tumours:
Firm intratesticular or progressively enlarging lesions are tumours •
until proven otherwise.
Do not be misled by painful testicular swelling following relatively •
trivial trauma; tumours may present this way.
• Tuberculous epididymitis may occur with or without evidence of
pulmonary, systemic, or other genitourinary involvement.
• Hydroceles, patent processus vaginalis, and large spermatoceles are
transilluminable, may be fl uctuant, and are usually confi ned to the
scrotum.
• Varicocele. Often associated with subfertility or dragging discomfort
that worsens on standing and settles when recumbent. More obvious
as a fl uctuant swelling with the patient standing. A cough impulse may
be felt. Ipsilateral testis may be atrophied. Examine the abdomen to
exclude an associated renal tumour.

SCROTAL SWELLINGS
367
• Inguinal hernias tend to be intermittent, associated with groin
discomfort, and when ‘out’, the examining hand cannot ‘get above’
the swelling in the cord/inguinal canal. A history of longstanding
enlargement is typical.
Investigations
• Urinalysis for M,C,&S.
• Blood tests. Consider tumour markers (AFP, β-HCG), infl ammatory
markers (CRP, WCC).
• Ultrasound scanning of the scrotal contents has several important uses.
Distinguishes intratesticular from paratesticular swellings, solid from •
cystic lesions, cellulitis from abscess, etc.
Can examine impalpable testes, e.g. within large hydroceles.•
Can identify rupture of the tunica albuginea testis.•
Examination of the abdomen can identify renal mass associated with •
varicocele or ascites with hydrocele/scrotal oedema, etc.
Colour fl ow Doppler ultrasound can identify hyperaemia, •
underperfusion, and varicocele.
Treatment
Testicular tumours, acute epididymo-orchitis (see b pp. 382, 388).
• Suspected paratesticular tumours are approached surgically in the
same way as testis tumours. Surgery may be conservative with benign
testicular and paratesticular tumours.
• Sperm granuloma may be excised or epididymectomy may be
performed. Reassurance may be all that is required in many cases.
Recurrence rates after surgery are high.
• Epididymal cysts/spermatocele may be aspirated, but recurrence is
common. Excision risks loss of epididymal patency, is associated with
risk of recurrence, and should probably be discouraged in men who
have not completed their family.
• Hydroceles. Treat if symptomatic. Procedures usually reconfi gure the
serosal remnant of tunica vaginalis so as to allow lymphatic drainage
via scrotal lymphatics. Reduction, inversion (Jaboulay), or imbrication
(Lord’s) of the tunica vaginalis is used.
• Embryological remnants. No treatment if asymptomatic.
• Varicocele. Treat if symptomatic, associated with infertility, or with
failure of testicular growth. Venous embolization and retroperitoneal
ligation (laparoscopic or open) of the testicular vein have similar
results. Minimally invasive treatments are preferable. Gubernacular
veins and other collaterals may account for failures. Open surgical
ligations via an inguinal incision can deal with these.
• Acute idiopathic penoscrotal oedema of childhood usually settles with
conservative treatment. Antihistamines and antibiotics are frequently
prescribed, but there is little evidence to support this.

CHAPTER 10 Urology
368
Disorders of the foreskin
Phimosis (see Fig. 10.2a)
Key facts
• Narrowness of the preputial opening, preventing retraction and
exposure of the glans.
• May be physiological in infants and young children and will resolve.
• Pathological phimosis is secondary to scarring such as BXO or
following monilial balanoposthitis.
• Pathological phimosis may be associated with discomfort, UTI,
balanoposthitis, and (perhaps) carcinogenesis.
Clinical features
• In childhood, physiological phimosis may be identifi ed by absence of
scarring of the preputial tip and by pouting of the inner layer of the
prepuce when a gentle attempt is made to retract it.
• A whitish sclerotic preputial tip and no pouting characterize BXO.
• Parents may complain of ballooning of their child’s foreskin or of
recurrent balanoposthitis. In adults, the diagnosis is obvious.
Treatment
• Physiological phimosis needs no treatment.
• Early BXO may be treated by application of topical steroid cream.
• Surgical treatment of phimosis includes circumcision and dorsal slit.
Paraphimosis (see Fig. 10.2b)
Key facts/diagnosis
• The retracted foreskin acts as a constricting ring, reducing lymphatic
and venous drainage of tissues distal to the ring (glans, inner layer of
foreskin). Subsequent oedema makes reduction more diffi cult.
• Involved tissues typically appear odematous and infl amed with
progression to infection, ulceration, and necrosis if left untreated. Can
occur in normal penises.
3 Treatment
• Early cases. Gentle manual compression of the oedematous tissues
within a saline-soaked swab will allow reduction of the foreskin after a
few minutes without anaesthesia.
• Established cases. LA penile block or anaesthesia may be necessary.
Hyaluronidase can be injected under the constriction.
• Neglected/severe cases. A relaxing dorsal incision may be made through
the constriction and the tissues immediately proximal and distal to it.
Subsequent circumcision is often offered.
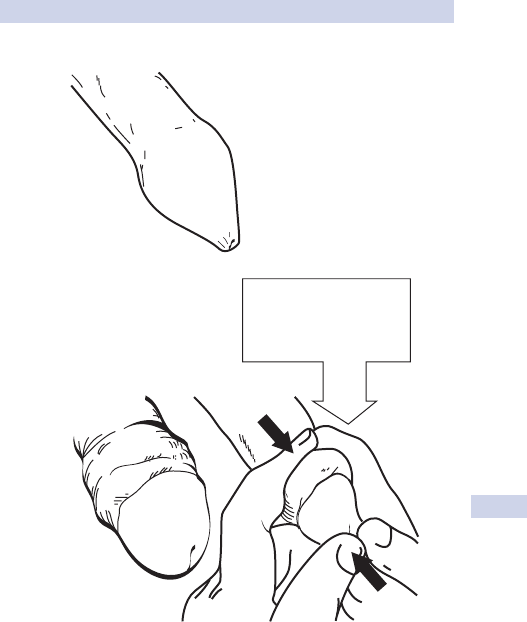
DISORDERS OF THE FORESKIN
369
Paraphimosis
Phimosis
(a)
(b)
Manual reduction of
paraphimosis by counter
pressure between
thumbs and fingers
Fig. 10.2 (a) Phimosis. (b) Reduction of an acute paraphimosis.

CHAPTER 10 Urology
370
Common conditions of the penis
Peyronie’s disease
Key facts
• Damage to tunica albuginea penis, forming inelastic penile plaques
mostly in the dorsal midline, causing local pain and deformity.
• Associated with Dupuytren’s contractures, plantar fascial contractures,
and tympanosclerosis.
• One-third of patients have erectile dysfunction.
Clinical features
History and examination
• Gradual or sudden development of palpable penile plaques with
painful distortion of the erect penis in two-thirds of patients.
• Occasional history of penile trauma, urethral instrumentation.
• Erectile dysfunction may involve the whole or part of the penis.
• Pain usually resolves, but deformity does not.
• Penetrative sexual intercourse may be more diffi cult or impossible
because of pain, angulation, or buckling.
• The plaque is palpable with the fl accid penis stretched.
• Erection is induced pharmacologically and a photo record kept.
Investigations Plain fi lm soft tissue radiography or grey-scale ultrasound
may demonstrate calcifi cation, an indicator of plaque maturity.
Treatment
Medical
Indicated for those able to engage in intercourse and whose disease is
still evolving.
• Oral treatment options. Colchicine, vitamin E, or Potaba
®
.
• Intralesional injections. Verapamil or collagenase (investigational).
• Treatment of associated erectile dysfunction.
Surgical
Indicated for those with stable lesions, severe deformity.
• Plication techniques. Plication of the tunica opposite the deforming
plaque.
• Grafting techniques. The plaque is excised and the defect grafted.
• Prosthesis insertion within corpora cavernosa. Generally reserved for
patients with severe erectile dysfunction refractory to medical therapy.
Priapism
Key facts Abnormally sustained erection unrelated to sexual stimulation. It
is classifi ed as high fl ow (arterial) or low fl ow (veno-occlusive).
Low fl ow priapism
• Congestion secondarily reduces arterial blood fl ow, leading to local
hypoxia, acidosis, and hypercapnoea.
• Causes include:
Drugs • (intracavernosal prostaglandin and papaverine, psychotropics,
e.g. trazodone, chlorpromazine).

COMMON CONDITIONS OF THE PENIS
371
Abnormal blood viscosity (sickle cell, myeloma, leukaemia, •
thalassaemia, total parenteral nutrition).
Neurological disease, e.g. spinal or cerebrovascular disease.•
Miscellaneous, e.g. infi ltration by solid tumour.•
• Characterized by painful persistent erection, not involving the glans
and corpus spongiosum.
High fl ow priapism
• Most commonly follows blunt trauma to penis or perineum, but has
been caused by intracavernosal injection or revascularization.
• The mechanism is arterio-cavernosal fi stula with unregulated arterial
infl ow and increased venous outfl ow.
• Characterized by partial, painless swelling of the glans and corpus
spongiosum.
Investigation
• Colour Doppler ultrasonography or cavernosal blood gases.
• pH <7.25, pO
2
<30mmHg, pCO
2
>60mmHg suggest low fl ow.
• FBC and differentials; Hb electrophoresis.
Treatment—low fl ow
• Correct underlying abnormalities, e.g. sickle cell (rehydration, O
2
,
transfusion), myeloma (plasmapheresis).
• Oral B-agonists, e.g. terbutaline 5mg + further 5mg 15min later.
• Intracavernosal phenylephrine.
• Corpora cavernosa aspiration (up to 50mL) + manual pressure.
• Surgical techniques augment venous drainage of the corpora
cavernosa.
Treatment—high fl ow
Selective pudendal arteriography and embolization.

CHAPTER 10 Urology
372
Erectile dysfunction
Key facts
• Erectile dysfunction (ED) is the inability to achieve or maintain an
erection satisfactory for sexual intercourse.
• Distinct from premature, retrograde, or delayed ejaculation.
• Causes include:
Psychogenic. • Anxiety, depression.
Drugs.• Antihypertensives, recreational drugs, tobacco, alcohol.
Vascular. • Hypercholesterolaemia, atheroma, diabetes mellitus (DM).
Metabolic/endrocrine. • Azotaemia, hypercholesterolaemia,
hypogonadism, hyperthyroidism, hyperprolactinaemia, DM.
Neurological.• Parkinson’s disease, CVA, spinal injury, neurological
damage following pelvic surgery, pelvic fracture, autonomic
neuropathies.
Penile. • Cavernositis, Peyronie’s disease, previous priapism.
Clinical features
• Specifi c validated questionnaires have been developed as
investigational tools and can be used in practice.
• The presence of morning erections strongly suggests psychogenic
cause.
• Small testicles, lack of secondary sexual characteristics suggest an
endocrine cause.
• Lack of lower limb pulses suggests possible vascular cause.
• Neurological defi cits in S2, 3, 4 distributions suggest neurological
cause.
Investigations and diagnosis
• Check blood glucose, lipids and serum electrolytes, hormone profi les
(testosterone, FSH/LH, prolactin), thyroid function for underlying
cause.
• Dynamic cavernosometry confi rms if there is a true vasculogenic cause
(venous or arterial).
• Angiography demonstrates arterial anatomy if revascularization is
contemplated.
Treatment
• Psychotherapy or specialist sexual counselling for psychogenic causes.
• Oral phosphodiesterase-5 inhibitors—sildenafi l (Viagra
TM
), vardenafi l,
tadalafi l.
• Apomorphine sublingual.
• Intracavernosal—prostaglandins, A-blockers, papaverine.
• Intraurethral—prostaglandin.
• Vacuum devices-induced pseudoerection.
• Prosthesis.
This page intentionally left blank

CHAPTER 10 Urology
374
Adenocarcinoma of the kidney
Key facts
• Accounts for 2% of all cancers.
• Incidence is 2–5 per 100 population.
• ♂:♀, 3:1.
Clinical features
• May be asymptomatic at presentation, the tumour being detected
during imaging of the abdomen for an unrelated condition (e.g. CT
scan or ultrasound scan).
• Symptoms include painless haematuria, groin pain, awareness of a mass
arising from the fl ank.
• Chest symptoms and bone pain may be present with metastases to
these sites.
• Positive family history or clinical evidence of neurological or ocular
disease should raise the possibility of von Hippel–Lindau disease (VHL).
• Renal carcinomas are often small and may be multiple.
• Local spread often includes spread via intravascular invasion into the
renal vein and inferior vena cava.
Diagnosis and investigations
• Blood tests. Hb and ferritin to check for anaemia (iron-defi cient);
electrolytes and Cr to check for overall renal function. Raised
corrected calcium and alkaline phosphatase suggest possible bony
metastases.
• Diagnostic and staging investigation of choice is a pre- and post-IV
contrast-enhanced CT scan of abdomen and chest (delineates size,
local extent, local invasion, likely sites of possible metastases).
• Isotope bone scan if there is clinical or biochemical evidence of bony
metastases.
Treatment
Surgery
• Recommended as the only curative treatment except in the very
elderly, extensive (inoperable) local invasion, and the presence of
metastases.
• May be via open or laparoscopic approach.
• Radical nephrectomy is recommended for large tumours.
• Partial nephrectomy may be suitable for peripheral tumours <4cm in size.
• Resection of the primary cancer is occasionally appropriate with
the presence of metastasis (the deposit must be solitary and itself
amenable to complete local resection, e.g. in the liver or lungs).
Medical therapy
• Used for metastatic disease.
• Biological therapy can be with immune modulators such as interferons
and interleukins. Partial response rates of 15–20% can be achieved, but
the treatment carries signifi cant morbidity. This is reserved for patients
with a good performance status.

375
ADENOCARCINOMA OF THE KIDNEY
• Chemotherapy is rarely used as the tumours are not chemosensitive.
• Hormonal therapy (androgens and tamoxifen) may have some benefi t.
• Radiotherapy is useful to palliate painful bony metastases.
Prognosis
• The outcome following nephrectomy is unpredictable.
• Tumours that are pathologically confi ned to the kidney confer a good
prognosis. Adverse risk factors include extracapsular spread, invasion
of the renal vein, lymph node involvement.
• Cure is likely if the tumour is <4cm in diameter and if there are no
adverse pathological features.
• Periodic radiological follow-up is recommended in most cases so that
locally recurrent or metastatic disease can be detected at an early
stage.
Key revision points—anatomy of the kidney
Usually fi ve segments (apical, anterior superior, anterior inferior, poste-
rior and inferior), each supplied by its own artery.
- Fascial coverings.
Perirenal, anterior and posterior layers, enclosing kidney and
-
perirenal fat.
Lateral conal fascia, formed from anterior and posterior perirenal
-
fascia; fused with transversalis and iliac fascia laterally.
- Blood supply.
Arterial supply, direct from the aorta; renal arteries also give
-
supply to the renal pelvis and upper ureter and adrenal gland.
Venous drainage, direct to inferior vena cava; left renal vein also
-
drains the left gonadal vein.
- Common anatomical variants.
Unilaterally absent kidney (1 in 1200).
-
Pelvic kidney (1 in 2500).-
Joined (horseshoe) kidney (1 in 400).-

CHAPTER 10 Urology
376
Transitional cell tumours
Key facts
• Transitional cell tumours (TCT) may affect any part of the urinary
epithelium (renal pelvis, ureter, bladder, or very rarely, urethra).
• TCT have a spectrum of disease from benign superfi cial ‘papilliferous’
growths to frankly invasive transitional cell carcinoma (TCC);
progression may occur from benign to more malignant forms with
time.
• TCC of the bladder. Fifth commonest cause of cancer deaths and the
commonest form of bladder cancer in the UK; ♂:♀,, 3:1.
• TCC of the upper urinary tract is similar in spectrum of disease and
management to bladder tumours, but much less common.
• TCT is associated with:
Exposure to aromatic hydrocarbons, e.g. workers in the •
petrochemical, industrial dye, rubber industries, chimney sweeps.
Smoking (especially in women).•
• Risk is probably due to excretion of carcinogenic products excreted
and concentrated in the urine and tumours are more likely in locations
exposed to urine for the longest periods, i.e. bladder.
Pathological features
• The majority (70%) are superfi cial in nature at diagnosis, being
confi ned to the mucosa.
• Invasion into the lamina propria, muscle, and perivesical fat can occur
with lymphatic and distant spread occurring in advanced cases.
• TCC in situ is pre-invasive and associated with a high risk of muscle-
invasive disease if not adequately treated.
• TCC must be differentiated from other forms of bladder cancer.
Squamous cell carcinoma. • Usually caused by chronic irritation due to
schistosomiasis infestation (bilharzia), indwelling catheter, repeated
previous surgical interventions.
Adenocarcinoma. • Rare; presents in middle age and is usually located
in the dome of the bladder in association with the urachus.
Clinical features
• The majority of cases present with painless haematuria.
• Other features are painful micturition, renal colic due to blood clot,
disturbance of urinary stream, retention of urine.
Diagnosis and investigation
Urine cytology May reveal malignant cells; if there are malignant cells, TCC
or carcinoma in situ will probably be present.
Cystoscopy
• Usually carried out using a fi bre optic fl exible cystoscope and local
anaesthetic gel.
• Images the bladder and urethra; suspect lesions usually require
transurethral resection under GA for diagnosis.

TRANSITIONAL CELL TUMOURS
377
Transurethral resection
• Usually carried out using a rigid endoresectoscope under GA.
• Permits resection of all or part of the tumour using a diathermy ‘loop’,
with the tumour resected piecemeal; resection may be carried out into
deep tissue (the muscle wall of the bladder beneath the tumour).
• Subsequent pathological examination will determine the histological
grade and the pathological stage, e.g. depth of invasion.
• Following resection, bimanual examination determines whether or not
a residual mass is present.
Upper tract imaging
• Used to identify and assess pelviureteric tumours.
• IVU or ultrasound scan.
• Ultrasound scan permits examination of the renal cortex and will
detect tumours of 1cm diameter in the pelvicalyceal system, ureter,
and bladder.
• Bladder tumour may show as a fi lling defect in the cystogram phase.
Local staging MRI and CT scanning to detect local or systemic spread.
Treatment
Superfi cial TCT
• Remove; completed by endoscopic resection.
• Recurrence is common and regular endoscopic surveillance with check
cystoscopy is performed.
• Intravesical chemotherapy reduces the risk of tumour recurrence
(single dose of mitomycin C instilled after resection of the tumour).
• For multiple or recurrent TCC, six intravesical treatments are given.
Carcinoma in situ
• Requires thorough therapy to prevent invasive TCC.
• Immunotherapy with intravesical BCG is effective in 60% of cases.
• Needs close endoscopic surveillance with regular bladder biopsy.
Invasive TCC
• Muscle-invasive tumours are of high grade and the prognosis is poor.
• Curative therapy can be offered with radical cystectomy (combined
with a urinary diversion via an ileal conduit) or radical radiotherapy.
Squamous cell and adenocarcinoma
• Radical cystectomy provided general condition allows.
• Usually resistant to radiotherapy and chemotherapy.
Prognosis
• Approximately 30% develop muscle-invasive disease.
• The 5y survival rate for muscle-invasive bladder cancer is 40–50%.
• Metastatic TCC is poor prognosis with a median survival of 13 months.
• Systemic chemotherapy with cis-platinum-containing regimes provides
a long-term response in 15% of cases.

CHAPTER 10 Urology
378
Adenocarcinoma of the prostate
Key facts
• Most commonly diagnosed cancer affecting men in the western world.
• Approximately 30 000 new cases diagnosed annually in the UK with
10 000 deaths.
• Peak incidence in eighth decade.
• Approximately 40% of cases present with early disease; 20% of cases
have metastases at presentation.
Clinical features
• The majority of men present with LUTS (b p. 354).
• Bone pain, pathological factures, and features of hypercalcaemia are
occasional presenting features due to metastases.
• May be diagnosed by digital rectal examination; areas of fi rmness or
palpable nodules are suggestive of malignant change.
Diagnosis and investigations
• Serum PSA. Can be used as a screening test; high sensitivity, but low
specifi city; elevated age-specifi c levels are an indication to consider
prostate biopsy.
• Transrectal ultrasound (TRUS). Permits detailed imaging of the prostate.
Systematic needle biopsy is performed guided by the ultrasound
images with antibiotic prophylaxis; graded using the Gleason grading
system which assigns a numerical score to adverse features from a
minimum of 2 to a maximum of 10.
• Pelvic MRI. Used to detect the presence of extracapsular extension or
the presence of pelvic lymphadenopathy (suggests spread).
• Laparoscopic node biopsy. May be performed to sample enlarged nodes
prior to considering radical treatment.
• Isotope bone scan. Will detect the presence of bone metastases.
Treatment
Localized disease (confi ned to prostate)
Patients with a life expectancy of <10y
• Active monitoring with treatment deferred until there is evidence of
disease progression (rising serum PSA).
• Hormonal therapy or A-blocker treatment offered for troublesome
LUTS.
• TURP is considered for severe symptoms with features of obstruction.
Life expectancy of >10y
• Counselled in detail about radical treatment aimed at cure.
• The options are as follows:
Radical prostatectomy• . Operation to remove the prostate and
seminal vesicles; complications include incontinence (severe in 3%)
and ED (40–50% of cases).
External beam radiotherapy• . Radiation is delivered at a radical
dose of 55–70Gy in 20–25 fractions over a 4–5-week period;
complications include cystitis, proctitis, and ED.

ADENOCARCINOMA OF THE PROSTATE
379
Brachytherapy• . Radioactive seeds placed into the prostate using
TRUS guidance; a relatively new technique and long-term follow-up
data are lacking.
Locally advanced disease (spread beyond the prostate)
• Incurable and treatment is therefore palliative.
• Eighty per cent are androgen-dependent. Hormone therapy reduces
androgenic drive to the prostate cancer cell using two methods:
Luteinizing hormone• -releasing hormone (LHRH) agonists. Given by
3-monthly depot injections, suppresses testosterone production by
the testes; side effects include hot fl ushes, lethargy, loss of sexual
function.
Anti-androgens• . Given orally, act as competitive inhibitors at the
level of the androgen receptor, reduce androgenic stimulus to the
prostate cancer cell without reducing serum testosterone levels;
side effects include gynaecomastia and nipple tenderness (60%),
potency is sometimes preserved.
Metastatic disease
Treated with hormonal therapy using LHRH analogues. Addition of an
anti-androgen provides a secondary response in some cases of PSA
relapse. Pain from bone metastases usually responds to radiotherapy.
Hormone-resistant disease
• All prostate cancers will eventually become hormone-resistant.
• Chemotherapy is appropriate for patients who have a good
performance status.
• Palliative radiotherapy and bisphosphonates are used for bony
metastases.
Prognosis
• Localized prostate cancer. Excellent prognosis with 70–90% 10y
disease-specifi c survival fi gures.
• Locally advanced, non-metastatic disease. Median survival of 7y.
• Metastatic disease. Median survival of 2–3y.
• Once the state of hormone-resistant disease has been reached, the
median survival is 6–12 months.

CHAPTER 10 Urology
380
Carcinoma of the penis
Key facts
• Rarest of the urological cancers.
• Occurs primarily in older men.
Clinicopathological features
• Over 95% are squamous cell carcinoma.
• Usually affects the glans, but may involve the shaft.
• Associated with chronic infection of the penis, particularly in the
presence of phimosis.
• Early cases present with a painless ulcer, nodule, or ‘warty’ outgrowth
on the penis that may also involve the foreskin.
• Advanced disease presents with a fungating mass, usually ulcerated.
Inguinal lymphadenopathy may be present on examination. Nodes are
often reactive rather than being metastatic and antibiotics should be
given prior to further assessment.
Diagnosis and investigations
• Biopsy lesion to confi rm the diagnosis.
• Pelvic and abdominal CT scanning provide further evidence of nodular
involvement in cases with positive inguinal nodes.
Treatment
• If primary tumour confi ned to glans, treatment involves either partial
amputation or radiotherapy.
• Superfi cial lesions can be treated by excision of the glans followed by
glans reconstruction.
• More advanced carcinomas require total penectomy. Inguinal and iliac
lymph node dissections are considered.
Prognosis
• Early stage penile cancer has a high cure rate with either surgery or
radiotherapy.
• Long-term survival is sometimes seen even in cases of lymph node
involvement.
This page intentionally left blank

CHAPTER 10 Urology
382
Testicular tumours
Key facts
• Commonest malignancy in men between the ages of 18 and 40.
• Annual incidence 6 per 100 000 males per year.
• Associated with testicular maldescent.
• Increased risk is associated with higher levels of exogenous oestrogens,
either prenatally or in childhood.
• Increased level of awareness has led to more tumours being detected
on self-examination, particularly amongst younger men.
Pathological features
• Common types are seminoma and non-seminomatous germ cell
tumours (NSGCT; previously called ‘teratoma’). Lymphoma is a rare
testicular tumour.
• Seminoma.
Peak incidence 30–40y.•
Lymphatic spread more common than haematogenous.•
Lymphatic spreads to iliac and para-aortic nodes.•
• NSGCTs.
Peak incidence 20–30y.•
Haematogeneous spread most common to lungs, brain, and liver.•
• Lymphomas. Peak incidence over 60y.
• Marsden staging (after investigations and treatment).
Stage 1, confi ned to testis.•
Stage 2, abdominal nodal spread.•
Stage 3, nodal disease outside the abdomen.•
Stage 4, extralymphatic spread.•
Clinical features
• The usual presentation is with a painless testicular mass.
• Typical features are irregular, fi rm, fi xed, and does not transilluminate.
• Palpate the abdomen for intra-abdominal masses (either para-aortic
node masses or hepatomegaly).
• Check for supraclavicular lymphadenopathy and signs of lung or
neurological disease.
Diagnosis and investigation
2 Any clinically suspicious mass requires urgent testicular ultrasound scan.
Typical features are a non-homogeneous mass with increased vascularity.
• Serum tumour markers, B-HCG and AFP. Increased levels suggest
metastatic disease in NSGCTs, but may be normal in localized or
metastatic disease; very rarely elevated in seminoma even if metastatic.
• CT scan of abdomen and chest. To assess presence of metastases.
• CT brain, bone scan. Only if clinically indicated.

TESTICULAR TUMOURS
383
Treatment
• Orchidectomy is carried out at the earliest opportunity; this is
performed via an inguinal approach so that the spermatic cord can be
clamped prior to mobilization of testis.
• Seminoma is radiosensitive and even widespread local disease
responds well to radiotherapy.
Stage• 1. May be treated by orchidectomy only, orchidectomy +
prophylactic iliac and para-aortic radiotherapy, or orchidectomy +
prophylactic chemotherapy.
Stages 2/3/4• . Orchidectomy + radiotherapy to involved node
groups 9 chemotherapy.
Visceral metastases are treated with a combination of •
chemotherapy and radiotherapy.
• NSGCT is chemosensitive and even widespread metastatic disease
responds well.
Stage 1• . Orchidectomy.
Stages 2/3/4• . Orchidectomy + chemotherapy; if lymphadenopathy is
still present following chemotherapy, a retroperitoneal lymph node
dissection is performed.
Prognosis
• Cure rates >95% for stage 1 tumours.
• Metastatic disease also has excellent long-time survival rates with
combination therapy.

CHAPTER 10 Urology
384384
Haematuria
Causes and features
May be microscopic or macroscopic.
• UTI. Commonest cause; usually associated with LUTS, particularly
cystitis.
• Renal stones. Often associated with pain (renal colic).
• Malignancy (TCT, renal adenocarcinoma, prostate adenocarcinoma).
Most likely to be macroscopic, often with few other acute symptoms.
• Post-interventional. For example, post-TURP, post-cystoscopy, post-
catheterization.
• Renal disease. For example, glomerulonephritis, vasculitis; usually
causes microscopic haematuria, is often asymptomatic, and rarely
presents as an emergency.
Complications
• Suprapubic colicky pain or acute retention of urine suggests clots in
the bladder/urethra.
• Cardiovascular collapse is rare.
Emergency management
Resuscitation
• Establish large calibre IV access if the bleed is large; give crystalloid
fl uid up to 1000mL if tachycardic or hypotensive.
• Do not catheterize without seeking senior advice if there is any
suggestion of lower urinary tract pathology or post-interventional
bleeding.
• Irrigation (‘3-way’) catheters may be used to relieve acute symptoms
of clot colic or clot retention, but should be placed by experienced
staff.
• Send blood for FBC (Hb, WCC), U&E (Na, K), group and save,
clotting.
Establish a diagnosis
• Full clinical examination. Particularly check the prostate on PR exam.
• Ultrasound kidney. To identify renal tumours, cysts.
• Cystoscopy (usually rigid, may be fl exible). To identify bladder tumours.
• CT scan abdomen. If renal tumour is suspected.
Early treatment
• Ensure all clotting abnormalities are corrected.
• Ensure fl uid balance is correct; promote an active diuresis to prevent
clot formation and retention.
• Transfuse blood only if Hb <8g/dL or the patient is symptomatic or
high risk.
• Start antibiotics according to local protocol if infection is suspected
(before cultures are available).

HAEMATURIA
385385
Defi nitive management
Transitional cell tumours Transurethral resection will control symptoms,
establish a diagnosis, and start treatment (see b p. 382).
Renal stones (see b p. 358)
• May pass spontaneously.
• May need endoscopic removal, lithotripsy, or percutaneous treatment.
Post-interventional Flexible cystoscopy may be required.

CHAPTER 10 Urology
386386
Acute urinary retention (AUR)
Causes and features
Defi ned as a painful inability to pass urine.
Local causes
• Prostatic enlargement (BPH or carcinoma) (see b p. 362). Often
acute-on-chronic retention.
• Post-urological surgery, e.g. post-TURP, clot impaction.
• Bladder or urethral stone impaction.
• Pressure on bladder, e.g. late pregnancy, faecal impaction.
• UTI.
General causes
• Pharmacological, for example:
Anticholinergic side effects of many drugs.•
Anaesthetic drugs.•
Alcohol intoxication.•
A• -sympatheticomimetics.
• Post-non-urological surgery:
Precipitated by recent catheterization.•
Abdominal surgery with lower abdominal pain.•
Epidural or spinal anaesthesia.•
• Loss of normal neurological control:
Spinal injury (trauma, ‘slipped disc’, neurological disease).•
Epidural/spinal anaesthesia.•
Symptoms
• Suprapubic pain, inability to pass urine despite desire.
• May dribble urine in small volumes, especially if there is underlying
chronic retention.
• Palpable/percussible bladder strongly suggests pre-existing chronic
retention/lower urinary tract disease.
Signs
• Prostatic enlargement on PR examination.
• Check for signs of neurological disease.
Emergency management
Resuscitation
• Give analgesia (e.g. morphine 5–10mg IV); it will also help relaxation
and may aid spontaneous micturition.
• A warm bath may aid micturition in drug-induced retention.
• Catheterize if retention persists. 2 Seek senior advice before starting
if there are concerns about local pathology as a cause or if there is a
history of previous surgical instrumentation of the urethra.
• Suprapubic catheterization may be required for known or suspected
urethral disease or failed urethral catheterization (see b p. 210).
• Document initial urine volume passed after catheter inserted; large
volumes suggest underlying chronic retention.

ACUTE URINARY RETENTION (AUR)
387387
• Send urine for M,C,&S.
• Send blood for FBC (Hb, WCC), U&E (Na, K), Cr.
Establish a diagnosis
• Check medications, especially recent changes.
• Cystoscopy may be required.
• Review full clinical examination, including neurological fi ndings and
rectal examination.
Early treatment
• Monitor renal function, especially if there is underlying chronic
retention; renal function may deteriorate even after relief of the
obstruction.
• Monitor fl uid balance in fi rst 48h if there is associated chronic
retention; a secondary diuresis may occur.
• Start antibiotics according to local protocols if there is evidence
of a UTI.
Defi nitive management
Prostatic disease (see b p. 362)
• TURP may be required.
• A-blocker may enable successful trial of voiding.

CHAPTER 10 Urology
388388
Acute testicular pain
Causes and features
This is an acute emergency in men of childbearing age. Torsion of the
testicle must be dealt with immediately to preserve testicular function. It
is the commonest cause for referral for acute testicular pain.
Torsion of the testicle
Key facts
• Occurs due to anatomical variants in testicular anatomy, e.g. ‘bell
clapper’ testicle with pronounced meso-orchium allowing rotation
within the tunica vaginalis.
• Peak age of incidence 12–18y.
• Torsion initially causes venous obstruction, but with prolonged
increased venous pressure, arterial compression occurs and the
testicle rapidly develops irreversible ischaemia and necrosis.
• Testicular salvage depends on the degree of torsion and time spent
torted. Speed of presentation, diagnosis, and treatment are all
important. Torsion greater than 360* lasting longer than 24h results in
near universal complete or severe atrophy.
• Spermatogenic cells are more susceptible to ischaemia than Leydig
cells. Subfertility may occur even if the testicle is macroscopically
normal after treatment.
Features
• Sudden onset of moderate to severe, constant, unilateral scrotal pain,
often with nausea, vomiting, and abdominal pain.
• May have been preceding episodes of intermittent pain that suddenly
resolved.
• The testis is globally tender, high in the scrotum, may have a transverse
axis, and be slightly enlarged. If it is infarcted, scrotal wall oedema and
tenderness may be present. Absence of ipsilateral cremasteric refl ex is
the most reliable sign.
Torsion of the testicular appendages
• Occurs in testicular appendix ‘hydatid of Morgagni’ or epididymal
appendages (e.g. cysts, ductal remnants).
• Similar features and symptoms to testicular torsion.
• The ‘blue dot sign’ is said to be pathognomonic when present.
• The testis and epididymis may be non-tender and the cremasteric
refl ex should be preserved.
Acute epididymo-orchitis
• Peak incidences vary according to cause, ages 35y and >55y.
• Common organisms include Chlamydia trachomatis, Neisseria
gonorrhoea in the young (sexually-transmitted infections (STI)).
• Escherichia coli and Proteus occur in chronic bladder outfl ow
obstruction or urinary tract instrumentation.
• One-third of male adolescents with mumps develop orchitis, which is
unilateral in 80%; a third of these testes atrophy.

ACUTE TESTICULAR PAIN
389389
Features
• Gradual onset of pain (hours or days).
• Dysuria, urethral discharge, and pyrexia are common.
• Tenderness and induration are localized to the epididymis and
spermatic cord in epididymitis.
• Cremasteric refl ex is preserved.
• Prehn’s sign (relief of pain with scrotal elevation).
Idiopathic scrotal oedema
• Often less painful and tender than appears.
• Swelling is mostly cutaneous and normal size and texture testicle may
be palpable with care.
Acute inguinal lymphadenopathy
• May occur secondary to lower limb, buttock, or perineal infections.
• Rarely part of systemic infection of lymphatic disorder.
Emergency management
Resuscitation Give analgesia (e.g. morphine 5–10mg IV).
Establish a diagnosis
3 Immediate surgical exploration is indicated for all cases where
the diagnosis of torsion is considered possible and the history is short (i.e.
testicular viability is still at issue).
• Testicular colour duplex ultrasound. May be used if immediately
available or where symptoms have been present for days and testicular
viability is unlikely if torted.
• Send MSU, urethral swab, chlamydia serology if suspected infection.
Defi nitive management
If a torted testicle is found at surgery:
• A viable testicle is detorted and fi xed.
• A clearly non-viable testicle is excised.
• The opposite testicle is fi xed (orchidopexy) to prevent the opposite
side torting in future.
Torsion of testicular or epididymal appendage Excise appendage.
Epididymo-orchitis
• Suspected STI, e.g. ceftriaxone 250mg IM single dose, doxycycline
100mg PO bd 7 days.
• Suspected UTI-related—ciprofl oxacin 500mg PO bd 10–14 days.
• Scrotal elevation, local ice therapy, and oral NSAIDs may help.
• Abscess formation may require drainage or orchidectomy.
• Treatment of acute viral orchitis is symptomatic.
This page intentionally left blank

391
Colorectal surgery
Ulcerative colitis 392
Crohn’s disease 394
Other forms of colitis 396
Colorectal polyps 398
Colorectal cancer 400
Restorative pelvic surgery 402
Minimally-invasive colorectal surgery 403
Diverticular disease of the colon 404
Rectal prolapse 406
Pilonidal sinus disease 408
Fistula-in-ano 410
Haemorrhoids 412
Acute anorectal pain 414
Acute rectal bleeding 416
Acute severe colitis 418
Post-operative anastomotic leakage 420
Chapter 11

CHAPTER 11 Colorectal surgery
392
Ulcerative colitis
Key facts
• An acute and chronic infl ammatory disease originating in the colonic
columnar mucosa.
• Precise aetiology is unknown, but an environmental trigger combined
with a genetic predisposition (family history) are factors.
• Often precipitated by an apparent acute GI infection; peak age of
diagnosis is the late teens and twenties, but may present in late
adulthood.
• Commonest in white Anglo-Saxon Caucasians.
Pathological features
• Granular, hypervascular, and mildly oedematous mucosa with loss of
vascular pattern seen at endoscopy.
• Acute neutrophil infi ltration of the colonic mucosa and submucosa;
mucosal crypt abscesses with goblet cell mucin depletion.
• With more severe infl ammation, there are multiple aphthous ulcers,
which may become confl uent with only islands of infl amed mucosa and
granulation tissue remaining (‘pseudopolyposis’).
• Transmural infl ammation may occur in severe disease secondary to the
widespread loss of mucosa and subsequent severe infl ammation.
• Chronic ‘burnt-out’ disease leads to a pale, featureless, ahaustral
pattern to the colon.
• Disease tends to be present in the distal colon and rectum and spread
proximally with increasing extent of disease.
Clinical features
• Proctitis. Commonest presentation. Rectum ‘always’ involved unless
already on topical treatment. Symptoms of urgency and frequency of
defecation due to rectal irritability; bloody mucus mixed with loose
stools (frank bloody diarrhoea rare).
• Left-sided colitis. Disease up to the splenic fl exure. Symptoms of rectal
irritation plus extensive bloody mucus in stools, often leading to
bloody diarrhoea; mild associated systemic features.
• Pancolitis. Disease involving the entire colon. May be associated with
mild secondary infl ammation of the terminal ileum (‘backwash ileitis’).
Diarrhoea predominant feature; systemic features common (fever,
malaise, anorexia, tachycardia). May be associated with anaemia (due to
blood loss), hypoalbuminaemia, and hypokalaemia (due to mucus loss).
Diagnosis and investigations
Basic tests
i WCC and CRP; d Hb and albumin, especially during episodes of infl am-
mation. AXR may show oedematous colonic mucosa (‘thumbprinting’),
but is unreliable for diagnosis or extent of disease. Proctosigmoidoscopy
usually shows erythematous, granular, or frankly ulcerated rectal mucosa
with mucus and blood. Biopsies should be taken before starting treatment.
Always send stool M,C,&S and test for parasites and cysts in any acute
presentation to exclude infectious causes.

ULCERATIVE COLITIS
393
Advanced tests
Extent of disease is best assessed with colonoscopy and biopsies; will also
usually exclude colonic Crohn’s disease.
Treatment
See b p. 418 for management of acute severe colitis.
Medical treatment
Principles are to reduce infl ammation and prevent complications. Acute
derangements in blood results should be corrected (e.g. blood transfusion
for severe anaemia, potassium supplementation, nutritional support for
hypoalbuminaemia).
Proctitis
• Topical steroids—Predsol
®
suppositories.
• Topical 5-aminosalicyclic acid (5-ASA) suppositories.
Left-sided colitis
• Topical steroids—Predsol
®
foam enemas (penetrate up as far as the
splenic fl exure).
• Topical 5-ASA foam enemas.
• May require systemic steroid treatment (prednisolone).
Pancolitis
• Topical steroids or 5-ASA treatments for local symptoms.
• Usually need systemic treatment, e.g. oral steroids (prednisolone),
5-ASA treatment.
• Oral immunosuppressives, e.g. azathioprine, 6-mercaptopurine.
• Systemic affectors of lymphocyte function, e.g. cyclosporin A,
anti-TNFα (infl iximab)
Surgical treatment
Surgery is indicated for acute colitis that fails to respond to treatment (see
b p. 418) and for chronic colitis when:
• Chronically symptomatic despite maximal medical therapy.
• Medical therapy controlling symptoms, but associated with
unacceptable side effects, e.g. osteoporosis, immunosuppression.
• Recurrent exacerbations affecting growth or development in
adolescents.
• Confi rmed diagnosis of either high grade dysplasia or dysplasia-
associated lesion or mass (DALM), or carcinoma of colon.
Surgical treatment may be:
• Proctocolectomy (removal of colon and rectum) with ileoanal pouch
formation (see b p. 402).
• Panproctocolectomy (removal of colon, rectum, and anus) with end
ileostomy formation (permanent).
• Total abdominal colectomy (removal of colon) with ileostomy (used
when the patient is too unwell for major pelvic surgery, e.g. for acute
severe colitis).

CHAPTER 11 Colorectal surgery
394
Crohn’s disease
Key facts
• A chronic infl ammatory non-caseating, granulomatous disease affecting
any part of the GI tract.
• Associated with several extraintestinal disorders (see Box 11.1).
• Precise aetiology is unknown, but products from the bacterial fl ora
combined with a genetic predisposition (family history) are factors.
• Peak age of onset of symptoms is the teens and early twenties, but
diagnosis is often several years later.
• Commonest in white Anglo-Saxon Caucasians.
Pathological features
• Commonly focused in the terminal ileum and caecum, but may affect
the anus, colon, or entire small bowel.
• Anal Crohn’s disease is not common, but may be severe and
associated with active small bowel disease.
• Colonic Crohn’s disease is a long-term risk factor for colorectal cancer
formation.
• Affected bowel looks blue-grey, thickened, with spiral surface vessels
and encroachment of the mesenteric fat around the bowel (‘fat
wrapping’).
• Transmural infl ammation in the form of lymphoid aggregates,
particularly in the subserosal tissues (‘Crohn’s rosary’), mucosal crypt
ulceration, and fi ssuring ulceration.
• Mucosal thickening and serpiginous longitudinal ulceration combine to
give the appearance of ‘cobblestoning’.
• Perforation, fi stulation, and abscess formation are occasional
‘fi stulizing’ sequelae of transmural infl ammation.
• Extensive fi brosis and smooth muscle hyperplasia may occur, giving
rise to stenosis.
Clinical features
• Infl ammatory features. Fever, malaise, abdominal pain (often RIF),
change in bowel habit (usually diarrhoea without blood), and weight
loss. Children and adolescents may have failure to thrive or have
retarded growth. Rectal bleeding is rare except in Crohn’s colitis.
• Fistulizing features. Para-enteric abscess formation often with a tender
abdominal mass, fi stula formation (ileocolic, ileoileal, ileocutaneous);
rarely free perforation with features of peritonitis.
• Stenosing features. Colicky abdominal pain, weight loss due to poor
food intake (‘food fear’), palpable or visible distended small bowel
loops.
• Anal disease. Atypical severe anal fi ssures, fi stula in ano, anal mucosal
thickening, and discoloration.
CROHN’S DISEASE
395
Diagnosis and investigations
Basic tests
i WCC and CRP; d Hb and albumin, especially during episodes of
infl ammation.
Advanced tests
• In acute presentations, an abdominal CT may show an infl ammatory
mass, abscess formation, localized or free perforation.
• In subacute or chronic presentations, small bowel disease may be
shown by a small bowel contrast study (shows mucosal irregularity and
narrowing) or a white cell scan showing ileal ‘hot spots’.
• Crohn’s colitis is diagnosed by endoscopy and biopsy.
• Anal disease may require, EUA, anal ultrasound, or MRI scanning for
assessment.
• OGD and biopsies may show features of Crohn’s in gastric mucosa.
Treatment
Medical treatment
Principles are to reduce infl ammation and control complications. Acute
derangements in blood results should be corrected.
• Systemic (5-ASA) drugs are fi rst-line acute and long-term treatment.
• Systemic steroids (hydrocortisone, prednisolone) control acute
exacerbations of infl ammation and steroids with very high fi rst pass
metabolism (budesonide) can be used chronically.
• Immunosuppressives (azathioprine, 6-mercaptopurine) are used as
maintenance therapy and anti-TNFA antibodies (infl iximab) may be
effective in fi stulizing complications.
• Dietary manipulation (elemental diet) may reduce infl ammatory
factors.
Surgical treatment
Principles are to deal with septic complications, relieve signifi cant bowel
obstruction, and remove as little bowel as possible. Indications for
surgery include the following.
• Acute. Free perforation, severe haemorrhage, acute severe colitis,
complete intestinal obstruction.
• Subacute. Infl ammatory mass, subacute obstruction, abscess formation,
symptomatic fi stulation.
• Chronic. Steroid dependency or complications, growth retardation,
cancer treatment or prevention.
Box 11.1 Extraintestinal manifestations of Crohn’s
disease
Associated with disease activity Independent of disease activity
• Pyoderma gangrenosum • Ankylosing spondylitis
• Erythema nodosum • Polyarthritis
• Primary biliary cirrhosis • Chronic active hepatitis

CHAPTER 11 Colorectal surgery
396
Other forms of colitis
Key facts and pathological features
Various insults of widely differing origin may give rise to colitis other then
idiopathic infl ammatory bowel disease.
Acute infective colitis
• Typically caused by pathological variants of normal enteric organisms,
e.g. enteropathogenic E. coli; only rarely progresses to acute severe
colitis.
• Typhoid colitis (Salmonella typhi) (rare in the UK). Typifi ed by acute
bloody diarrhoea, but few if any colonic mucosal neutrophils on biopsy
due to bone marrow suppression.
Clostridium (C.) diffi cile-related colitis
Caused by C. diffi cile infestation. Associated with antibiotic use, particularly
third generation cephalosporins (even a single dose), prolonged inpatient
stay. Toxin A produced by the organism causes acute severe infl ammation
in the mucosa.
Clinical picture may be varied.
• C. diffi cile diarrhoea. Foul green liquid without bloody stools.
• Acute C. diffi cile colitis. Caused by progressive rapid mucosal loss, acute
neutrophil infi ltration, and infl ammation
• Pseudomembranous colitis. Exudate and slough forms grey-white
‘plaques’ of material on the denuded colonic surface called
pseudomembranes. May rapidly progress to acute severe ‘invasive’
colitis, especially in the immunocompromised or acutely unwell.
Typifi ed by secondary infections associated with mucosal loss.
Neutropenic colitis
Occurs in the severely immunocompromised with neutropenia and/or
neutrophil dysfunction. Caused by multiple, normally non-pathogenic,
enteric organisms colonizing the colonic mucosa.
Radiation colitis
Acute, transient colitis caused by mucosal injury secondary to external
beam radiotherapy. May progress to chronic mucosal damage, haemor-
rhagic telangectasia, and possible stricturing after months or years.
Ischaemic colitis
Commonest in the upper left colon where the collateral blood supply
between the middle and inferior colic arteries is poorest. Usually pre-
cipitated by an acute occlusion of part or all of the inferior mesenteric
artery. May progress to infarction, but often presents with acute onset
bloody diarrhoea and abdominal pain; may settle spontaneously although
occasionally forms an ischaemic stricture.
Clinical features
Broadly similar, independently of the underlying cause. Typical features are
vague abdominal pain, mild fever (absent in neutropenic colitis), diarrhoea
(may be bloody, especially in ischaemic, radiation, severe pseudomembra-
nous, and typhoid colitis). Cessation of diarrhoea, except with treatment,

OTHER FORMS OF COLITIS
397
suggests acute severe colitis is developing and should be investigated
urgently.
Diagnosis and investigations
Depending on the suspected cause:
• Stool sent for C. diffi cile toxin (CDT), three samples.
• Stool for M,C,&S (if atypical infective causes possible, also send for
cysts, parasites, and ova (C,P,&O)).
• Plain abdominal radiograph may show thickened colonic haustrae.
• CT abdomen often shows typical mucosal thickening in colitis and may
be diagnostic for pseudomembranous colitis.
• Flexible endoscopy (usually fl exible sigmoidoscopy) with biopsy.
Treatment
Medical treatment
• Acute infective colitis. Antibiotics only if severely symptomatic.
• C. diffi cile diarrhoea/colitis. Oral vancomycin up to 200mg PO
daily or metronidazole 400mg PO tds; treatment may be as for
pseudomembranous colitis if severe.
• Pseudomembranous colitis. Oral vancomycin up to 200mg PO daily or
metronidazole 400mg PO tds; adjuvant systemic treatment may be
added in severe cases, e.g. IV vancomycin or tigicycline.
• Neutropenic colitis. Broad-spectrum antibiotics, bone marrow support.
• Radiation colitis. Symptomatic treatment only; anti-diarrhoeals.
• Ischaemic colitis. Supportive treatment; anticoagulation may be
appropriate if the underlying cause is thromboembolic.
Surgical treatment
Rarely indicated. Any form of colitis may progress to acute severe colitis
and require emergency colectomy (see b p. 418). Indications are:
• Failure to respond to maximal medical therapy with life-threatening
colitis (usually requires perioperative ITU support).
• Complications of colitis. Uncontrollable bleeding, perforation
(especially in ischaemic or neutropenic colitis).
Key revision points—colorectal resections
Anastomosis of the colon/rectum may be in several ways.
• Hand sewn. Either end to end or end to side, usually single layer of
sutures (dissolvable), either interrupted or continuous.
• Stapled colonic. By mechanical stapler (‘linear stapler’), usually side
to side.
• Stapled colorectal. By mechanical stapler (‘endoluminal stapler’), end
to end.
• Defunctioning (loop) ileostomy typically for rectal anastomosis
when:
Below the peritoneal refl ection.•
Comorbidities (diabetes, age, previous DXT, acute illness).•

CHAPTER 11 Colorectal surgery
398
Colorectal polyps
‘Polyp’ is a purely descriptive term and any growth from the lining of the
large bowel can be described as a polyp. Polyps may be predominantly
raised with a stalk attachment (pedunculated), fl at and spreading over
the surface of the bowel wall (sessile), or occasionally a combination of
the two.
Key facts and pathological features
Polyps may arise for many different reasons.
Juvenile polyps
Mucin-fi lled cystic swellings of the lower rectal mucosa. Rarely part of
a hereditary syndrome (juvenile polyposis) with multiple juvenile polyps
throughout the colon; small increased risk of colorectal cancer.
Hamartomatous polyps
Polyps containing excessive amounts of the normal architectural com-
ponents of the bowel wall, usually isolated. May be part of a hereditary
syndrome (Peutz–Jeghers syndrome, with polyps characterized by exten-
sive branched growth of the muscularis mucosa); small increased risk of
colorectal and other GI cancers.
Hyperplastic polyps
Small sessile polyps formed from normal elongated mucosal crypts.
Only associated with risk of colorectal cancer if numerous (‘hyperplastic
polyposis’).
Adenomatous polyps
• True neoplastic polyps formed by excessive growth of the colorectal
epithelium; divided by the morphology of the glandular tissue into
tubular, tubulovillous, and villous types.
• May be sessile, pedunculated, or mixed.
• Thought to be the precursor of most colorectal cancers; the risk of
cancerous change within an adenomatous polyp increases with size
(particularly >1cm), villous morphology, and sessile form.
• Majority are sporadic (either isolated or in small numbers), although
occasionally part of a hereditary syndrome.
Familial adenomatous polyposis (FAP)
Caused by an autosomal dominant defect in the APC gene on chromo-
some 5. Characterized by between dozens and thousands of adenomatous
polyps in the colorectum and an increased risk of polyp formation in the
stomach and duodenum. The risk of cancerous transformation in any given
polyp is similar to that in normal polyps, but the overall risk is very high
due to the vastly increased number present.
Associated with:
• Desmoid formation, particularly in the abdominal tissues.
• Multiple osteomata, fi bromata, and thyroid infl ammation (called
Gardner’s syndrome).

COLORECTAL POLYPS
399
Hereditary non-polyposis colorectal cancer (HNPCC)
A range of abnormalities of the mismatch repair (MMR) genes that
predispose adenomas to acquire multiple genetic defects and so progress
more rapidly than normal to cancer, although the overall rate of
adenoma formation is similar to that in normals.
Clinical features
Most polyps are asymptomatic, although symptoms may occur with increas-
ing size and with proximity to the anus. Typical symptoms are:
• Bleeding. Usually low volume, dark red, often fl ecks or mixed with
stool.
• Mucus discharge. White, clear, or watery; commonest with large villous
adenomas and may cause hypokalaemia and hypoproteinaemia if the
villous adenoma is large with copious mucus discharge.
• Prolapse. If pedunculated and low in the rectum polyps, may prolapse
out of the anus.
Diagnosis and investigations
• Most polyps are diagnosed by colonoscopy.
• Most patients with polyps require further follow-up investigations
to keep them under surveillance for future polyp formation; the
frequency and length of follow-up depends on the number, size, and
histology of the polyp.
1
• Hereditary polyposis syndromes may be investigated by genetic
mutation analysis.
Treatment
Medical treatment
• Colonoscopic polypectomy (see b p. 36). Simple, is carried out for
pedunculated polyps larger than 1–2mm; others may be removed by
EMR/ESD (see b p. 403).
• Patients with FAP require regular gastroscopy and upper GI
surveillance to identify premalignant polyps.
Surgical treatment
• Surgical excision is required for polyps that are too large or unsuitable
for colonoscopic removal and in which there is a risk of current or
future malignant change; for colonic polyps this means either resection
or open excision.
• Rectal polyps may be removed by transanal microsurgery (see b p. 403).
• FAP is usually treated by proctocolectomy (usually with ileoanal
pouch formation) or colectomy and ileorectal anastomosis before
early adulthood; other polyposis syndromes may also be treated by
prophylactic colectomy.
Reference
1 Atkin WS, Saunders BP (2002). Surveillance guidelines after removal of colorectal adenomatous
polyps. Gut 51 (Suppl. V), v6–v9.

CHAPTER 11 Colorectal surgery
400
Colorectal cancer
Key facts
Colorectal cancer (CRCa) is the second commonest tumour and com-
monest GI malignancy. One in 18 of the population will suffer CRCa; ♂:♀
8 3:1. Peak age of incidence 55–75y, but is increasing in younger ages.
Pathological features
The predominant type is adenocarcinoma (mucinous, signet ring cell, and
anaplastic subtypes). Classifi ed as well, moderately, or poorly differenti-
ated. Predisposing factors include:
• Polyposis syndromes (including FAP, HNPCC, juvenile polyposis).
• Strong family history of colorectal carcinoma.
• Previous history of polyps or CRCa.
• Chronic ulcerative colitis or colonic Crohn’s disease.
• Diet poor in fruit and vegetables.
Morphology
CRCa may occur as a polypoid, ulcerating, stenosing, or infi ltrative tumour
mass. The majority (75%) lie on the left side of the colon and rectum
(rectum, 45%; descending-sigmoid, 30%; transverse, 5%; right-sided,
20%). Three to fi ve per cent have a synchronous carcinoma at time of
diagnosis.
Clinical features
Rectal location
• PR bleeding. Deep red on the surface of stools.
• Change in bowel habit. Diffi culty with defecation, sensation of
incomplete evacuation, and painful defecation (tenesmus).
Descending-sigmoid location
• PR bleeding. Typically dark red, mixed with stool, sometimes clotted.
• Change in bowel habit. Typically increased frequency, variable
consistency, mucus PR, bloating, and fl atulence.
Right-sided location
Iron defi ciency anaemia may be the only elective presentation.
Emergency presentations
Up to 40% of colorectal carcinomas will present as emergencies.
• Large bowel obstruction (colicky pain, bloating, bowels not open).
• Perforation with peritonitis.
• Acute PR bleeding.
Diagnosis and investigations
Elective diagnosis By PR examination or rigid sigmoidoscopy for rectal
carcinoma. Colonoscopy is the preferred diagnostic investigation (alterna-
tives are barium enema and CT colonography).
Emergency presentations Commonly diagnosed by abdominal CT scan.
Single contrast enema may be used when the diagnosis of large bowel
obstruction is possible and CT scanning is unavailable. Acute PR bleeding
is sometimes investigated by urgent colonoscopy.

COLORECTAL CANCER
401
Staging investigations
• Assessment of the presence of metastases (liver, lung, or para-aortic).
Thoracoabdominopelvic CT scanning is gold standard; CT PET scan
may be used to evaluate equivocal lesions.
• Assessment of local extent. For colonic carcinoma, CT scanning is
adequate; for rectal cancer, pelvic MRI and TRUS are commonly used.
• Assessment of synchronous tumours. If not diagnosed by colonoscopy
or barium enema, one of these two tests is usually performed to
identify synchronous tumours.
• Tumour marker (CEA) is of no use for diagnosis or staging, but can
be used to monitor disease relapse if raised at diagnosis and falls to
normal after resection.
Treatment
Potentially curative treatment
Suitable for technically resectable tumours with no evidence of metastases
(or metastases potentially curable by liver or lung resection).
• Surgical resection (with lymphadenectomy) is the only curative
treatment. Typical operations:
Right/transverse. Right/extended right hemicolectomy.•
Left. Left hemicolectomy.•
Sigmoid/upper rectum. High anterior resection.•
Lower rectum. Low anterior resection/abdominoperineal resection •
(APER).
Anorectal. APER.•
• Preoperative (neoadjuvant) chemoradiotherapy may be used in rectal
cancer to increase the chance of curative resection.
• Adjuvant chemotherapy (5-FU based) is offered for tumours with
positive lymph nodes or evidence of vascular invasion.
• Hepatic or lung resection may be offered to patients with suitable
metastases and a clear resected/resectable primary tumour.
Palliative treatment
For unresectable metastases or unresectable tumours.
• Chemotherapy may effectively extend life expectancy with a good
quality of life.
• Obstructing tumours may be endoluminally stented with self-
expanding metal stents or transanally ablated if rectal.
• Surgery reserved for untreatable obstruction, bleeding, or severe
symptoms.
Pathological staging
Duke’s (approx. % 5y survival) TNM
• A, confi ned to bowel wall only
(75–90)
• B, through bowel wall (55–70)
• C, any with +ve lymph nodes
(30–60)
• D, any with metastases (5–10)
• T1–4, stages of invasion of
bowel wall
• N0/1/2, no/up to 4/more than
4 lymph nodes involved
• M0/1, metastases not present/
present

CHAPTER 11 Colorectal surgery
402
Restorative pelvic surgery
Low/ultralow anterior resection
Anterior resection = removal of part or all of the rectum and anastomosis
of the left colon to the remaining stump of tissue.
• Low anterior resection refers to a join that takes place below the level
of the peritoneal refl ection. i.e. to a short stump of rectum.
• Ultralow anterior resection refers to a join that takes place on to the
top of the anal canal, i.e. no native rectum remains. The anastomosis
may be stapled or sewn by hand.
The lower the level of the anastomosis, the higher the risk of complica-
tions of anastomosis, particularly anastomotic leakage (see b p. 420).
Most low and almost every ultralow anastomosis will have a temporary
loop ileostomy formed to reduce the risk of major septic complications
and consequences of leakage, but cannot prevent them.
Indications
• Rectal carcinoma.
• Rectal adenoma untreatable by other means (very rare with transanal
endoscopic microsurgery (TEMS).
• Severe or complex anorectal sepsis (including rectovaginal fi stula).
Ileoanal pouch formation
For operations that remove all the colon and rectum, but do not require
removal of the anus, a permanent stoma can be avoided by the formation of
an ileal pouch. Formed from a side-to-side double fold of ileum (‘J’ pouch) or
three folds sewn together (‘W’ pouch), joined either by hand or by staples to
the upper anal canal (ileoanal anastomosis). A temporary loop ileostomy is
often formed for the same reasons as for a low anterior resection (above).
Indications
• Ulcerative colitis not responding to medical management.
• FAP or multiple colorectal polyposis.
• Crohn’s disease of the colon (controversial).
• Multiple colonic tumours including the rectum.
Complications of pelvic anastomosis (anterior resection
and ileoanal pouch)
• Leakage. Occurs in up to 15% of cases; highest in the lowest
anastomosis. Typically presents as fever, abdominal pain, and
tachycardia (see b p. 420).
• Bleeding. Uncommon; usually settles with supportive treatment.
• Ischaemia. The proximal bowel involved in the anastomosis may
become ischaemic. This may present as a leak, bleeding PR, or fever
and tachycardia. Diagnosis is by careful fl exible sigmoidoscopy. May
resolve spontaneously; progressive ischaemia results in perforation and
death if not corrected by surgery.
• Stenosis. Narrowing of the anastomosis or bowel used to form it is
an occasional late complication. Presents with diffi culty in defecation
and small volume frequent stools. Treatment is dilatation under
anaesthetic; very rarely requires re-operation.

MINIMALLY-INVASIVE COLORECTAL SURGERY
403
Minimally-invasive colorectal surgery
Transanal endoscopic microsurgery (TEMS)
Large calibre operating protoscope with operating microscope allows
microsurgery within the rectum.
Indications
• Excision of large adenomas of the rectum (up to the rectosigmoid
junction) in a single specimen either by mucosectomy (or occasionally
full thickness).
• Excision of early rectal carcinoma (only if <3cm size, early tumour
(T1), no adverse features or in elderly/comorbid patients) by single
specimen full thickness excision.
• Repair of a rectovaginal fi stula.
Advantages Include entirely endoscopic technique, very low risk of parar-
ectal/pelvic sepsis, single complete specimen for histological assessment,
may avoid more radical surgery.
Complications Include bleeding (rarely requires active treatment), infec-
tion in the pelvic tissues (rare, presents with deep pelvic pain, fever, tachy-
cardia, and disturbance of bowel habit; treat with IV antibiotics).
Laparoscopic surgery
Several variants now exist. All use the same principles: minimal incisions,
avoidance of exposure of viscera, light anaesthetic techniques with mini-
mal opiates, and often enhanced recovery post-operatively.
Typical Indications
• Any colorectal resection can be performed by laparoscopic surgery.
• Rectopexy for prolapse.
• Combined treatment of large/extensive colonic polyps.
• Formation of some stomas.
Two newer variants have been used:
• SILS. Single incision laparoscopic surgery; one larger port for camera
and instruments used at the umbilicus.
• LESS. Combined laparoscopic and endoscopic single site surgery;
combines laparoscopic mobilization and handling with endoluminal
endoscopic techniques to remove very large lesions.
Advanced polypectomy
Several advanced endoscopic techniques are used to remove large and
often sessile colonic polyps.
• EMR. Endoscopic mucosal resection; excision (usually piecemeal)
of (presumed) adenoma with use of submucosal fl uid injection to
facilitate snaring of the polyp.
• ESD. Endoscopic submucosal dissection; attempted complete excision
of (presumed) adenoma using submucosal injection and endoscopic
diathermy ‘knife’.

CHAPTER 11 Colorectal surgery
404
Diverticular disease of the colon
Key facts
Colonic diverticula are acquired outpouchings of colonic mucosa and
overlying connective tissue through the colonic wall.
• Tend to occur along the lines where the penetrating colonic arteries
traverse the colonic wall between the taenia coli.
• Associated with hypertrophy of the surrounding colonic muscle
with thickening of the colonic mucosa. This is probably due to the
underlying pathological process, which is high pressure contractions of
the colon, causing chronic pressure on the colonic wall.
• Peak age of presentation is 50–70y, but diverticular disease is
increasing in frequency and occurring at a progressively younger age.
Clinical and pathological features
Asymptomatic
The majority of diverticular disease is found incidentally on barium enema
examination.
Painful diverticular disease
Intermittent LIF pain may be due to diverticular disease, but irritable bowel
syndrome commonly coexists and may be the cause of symptoms.
Acute diverticulitis
Rapid onset of LIF pain, nausea, fever, frequently with loose stools. Usually
febrile with moderate tachycardia and LIF tenderness. Colonic wall shows
acute neutrophil infi ltration around the infl amed diverticulum and in the
subserosal tissues.
Bleeding diverticular disease
Usually spontaneous in onset with no prodromal symptoms. Presenting
with large volume dark red, clotted rectal blood. Due to rupture of a
peridiverticular submucosal blood vessel. Not typically associated with
infl ammation.
Complications
Pericolic/paracolic mass/abscess
Acute diverticulitis may progress to persistent pericolic infection with
thickening of surrounding tissues and the formation of a mass. If this sup-
purates, a pericolic abscess forms. Enlargement and extension of this into
the paracolic area leads to a paracolic abscess. The features are those of
acute diverticulitis with a swinging fever, fl uctuating tachycardia, unresolv-
ing abdominal pain, and a tender LIF mass.
Peritonitis
Perforation of a pericolic or paracolic abscess usually leads to purulent
peritonitis. Direct perforation of the acute diverticular segment leads to
faeculent peritonitis. The features are of acute diverticulitis with high fever,
severe abdominal pain, and generalized guarding and rigidity.

DIVERTICULAR DISEASE OF THE COLON
405
Diverticular fi stula
Acute infection with paracolic sepsis may drain by perforation into
adjacent structures. This is typically the posterior vaginal vault in women or
the bladder in either sex. Colovesical fi stula leads to recurrent UTI caused
by enteric organisms with bubbles and debris in the urine. Colovaginal
fi stula leads to faeculent per vagina (PV) discharge.
Stricture formation
Chronic or repetitive infl ammatory episodes may lead to fi brosis and nar-
rowing of the colon. A history of recurrent diverticulitis with recurrent col-
icky abdominal pain, distension, and bloating suggests stricture formation.
Diagnosis and investigations
• Elective diagnosis is usually by double contrast barium enema.
Colonoscopy is a relatively poor investigation to assess number and
extent of diverticula.
• Hb, WCC, CRP during acute episodes of infl ammation.
• CT scanning is the test of choice to identify complications, including
abscess formation and perforation.
• Double contrast barium enema is used to assess for possible stricture
formation.
• Colonoscopy is indicated if there is any suggestion of coexistent
malignancy.
Treatment
Medical treatment
• High fi bre diet, high fl uid intake, and stool softeners to reduce
intracolonic pressure.
• IV antibiotics (amoxycillin 500mg IV tds, metronidazole 500mg IV tds,
gentamicin IV od) during acute infective exacerbations.
• Recurrent infective episodes may be prevented by a 6-week course of
oral antibiotics (e.g. ciprofl oxacin 500mg PO od).
• Signifi cant paracolic abscesses may be drained by radiological guidance.
Surgical treatment
• Resection is indicated for:
Acute infl ammation failing to respond to medical management.•
Undrainable paracolic sepsis.•
Free perforation.•
The affected region should be resected (segmental colectomy). The ends
may be re-anastomosed if they are healthy and the patient’s general condi-
tion is suitable. If not, a proximal end colostomy and oversewing of the
distal end is usual (Hartmann’s type resection).
• Stricture may be treated by elective resection or balloon dilatation.
• Diverticular fi stula may be treated by elective resection to prevent
recurrent infections.

CHAPTER 11 Colorectal surgery
406
Rectal prolapse
Key facts
Rectal prolapse may be partial thickness (usually just mucosa) or full
thickness involving all the layers of the rectal wall. Full thickness may
be contained within the rectum (internal prolapse also called intussuscep-
tion). Commonest in post-menopausal women, multiple vaginal deliver-
ies, associated with chronic straining and chronic disorders of defecation
(which cause weakness of the pelvic fl oor and sphincter complex), and
slow transit constipation. Occasionally occurs in children suffering consti-
pation (usually self-limiting).
Pathological features
Mucosa involved in prolapse undergoes chronic changes.
• Typically glandular branching and occasional gland misplacement.
• Thickening of the muscularis mucosae and excess submucosal collagen
deposition.
• Mucosal infl ammation and focal ulceration may also occur. Extensive
mucosal ulceration associated with mucosal prolapse may result in an
appearance called ‘solitary rectal ulcer’.
Clinical features
• Mucosal prolapse. Discharge of mucus and small volume faecal staining,
pruritus ani, and occasionally small volume bright red rectal bleeding.
• Internal full thickness prolapse. Sensation of rectal fullness/mass,
incomplete defecation, dissatisfaction after defecation and repeated
defecation.
• External full thickness prolapse. External prolapsing mass after
defecation (usually requiring manual reduction), mucus and faecal
soiling, occasional bright red rectal bleeding (may be large volume if
prolapse becomes ulcerated).
Diagnosis and investigations
• Rigid sigmoidoscopy may show features of mucosal infl ammation,
particularly the anterior rectal mucosa.
• Prolapse may be demonstrable on straining in clinic.
• Defecating proctogram may be performed to confi rm the diagnosis
if it is unclear and surgery is contemplated. Proctogram required
to confi rm the diagnosis of internal prolapse if suspected. May also
demonstrate associated problems of pelvic fl oor and rectocele.
• Colonic transit studies may be used if there is suspected slow transit
constipation and resection is possible.
Treatment
Medical treatment
• Avoidance of straining and adaptation of defecatory habit (bio-
feedback).
• Avoidance of constipation (stool softeners and bulking agents, rather
than stimulants).
RECTAL PROLAPSE
407
Surgical treatment
Mucosal prolapse
• Recurrent banding or dilute phenol injection of excess mucosa.
• Mucosal excision.
• Stapled anopexy (also called procedure for prolapse and haemorrhoids
(PPH)) sometimes used.
Full thickness prolapse (internal or external)
Surgery indicated for failure of control of symptoms. Choice of operation
depends on age and extent of prolapse.
• Delorme’s perineal rectopexy (mucosal excision with sutured plication
of the excessively long rectal muscle tube in an effort to shorten it to
prevent prolapse). Ideal for very frail and elderly, but least successful
with highest recurrence rate of all surgical procedures.
• Altmeier’s perineal rectal resection (mucosal and rectal muscle
tube excision with sutured perineal anastomosis). Avoids abdominal
operation, but has increased morbidity due to perineal anastomosis.
• Transabdominal rectopexy (mobilization of the rectum and suturing to
the presacral fascia). May be done via a laparotomy or laparoscopically.
May be just to ventral surface of the rectum with suspensory mesh
(ventral mesh rectopexy). Highest success rate for prevention of
recurrence of prolapse. May be combined with a sigmoid resection if
there is marked associated constipation on transit studies.
Key revision points—anorectal physiology
• The internal anal sphincter is smooth muscle and under involuntary
control of the pelvic autonomic system. Relaxants include nitric
oxide donors (e.g. GTN) and calcium antagonists (e.g. diltiazem).
• The external anal sphincter is skeletal muscle and under voluntary
control of the pudendal nerve (S2, 3, 4). Relaxation (by temporary
partial paralysis) may be achieved by botulinum toxin injection.
• Defecation is a complex sensorimotor process that requires intact
pelvic autonomics, sacral spinal nerves, and pelvic fl oor muscle
function.

CHAPTER 11 Colorectal surgery
408
Pilonidal sinus disease
Key facts
Single or multiple sinuses (‘pits’) that exist in the midline of the buttock
clefts. Usually contain hair, inspissated secretions, and debris. Commonest
in men, dark-haired, hirsute people, especially eastern Mediterranean
races. Probably caused by local trauma, causing retention of hairs within
initially normal midline pits. May be precipitated by long periods seated,
e.g. lorry drivers, computer operators.
Pathological features
Typifi ed by chronic infl ammation. Once infl ammation has started, sinuses
often extend and may become interlinked. Lateral tracks may run out into
the neighbouring buttock tissue.
Clinical features
• Irritative features. Intermittent discharge and infl ammation with pain
and swelling.
• Acute sepsis. Acute abscess formation is common with swelling, pain,
and erythema; may discharge spontaneously or may cause fi stulation
with sinuses appearing in the lateral buttock tissue.
• Chronic sepsis. Usually follows unresolved acute sepsis either after
spontaneous discharge or surgical drainage.
Diagnosis and investigations
• Ensure the patient is tested for occult diabetes mellitus.
• Very extensive sinus formation and fi stulation may be assessed by MRI
scanning of the natal cleft and buttocks.
Treatment
Medical/non-surgical
• Shaving of local hairs and washing of accessible cavities (usually by a
partner or family member) may control local symptoms.
• Intermittent courses of antibiotics may be required for septic episodes.
• Formed pilonidal abscess or collection requires surgical drainage
(under local or general anaesthetic).
• Recurrent acute sepsis or persistently symptomatic chronic sepsis
usually requires surgical treatment.
Surgical
Principles of surgical treatments are:
• Excision of all sinus openings.
• Obliteration of all infected or chronically infl amed tissue.
• Obliteration of the natal cleft by fl attening (thought to be most
important in the prevention of recurrence by reducing the risk of
further hair implantation).

PILONIDAL SINUS DISEASE
409
Surgical options are:
• Primary excision with laying open of wound and closure by secondary
intention is very rarely used, except in extensive recurrent disease; it
requires daily dressings for many weeks or months.
• Tension-free apposition of the skin edges (may be by lateral fl aps,
e.g. Karyadakis or Bascom procedures, or by plastic surgical fl aps, e.g.
rhomboid, rotational, or Z plasty fl aps).
Key revision points—anatomy of the large bowel
• Main features of large intestine structure:
Complete layer of circular smooth muscle throughout, but •
incomplete bands of longitudinal muscle (taeniae coli) in colon
(complete in rectum).
Fatty appendages along taeniae (appendices epiploicae).•
Folded internal mucosal appearances (haustrations).•
‘Segmented’ external appearances (sacculations).•
• Four main arterial (and lymph node) territories (used for resections):
Ileocolic and right colic arteries (from SMA): last terminal ileal •
loop, caecum, and ascending colon.
Middle colic artery (from SMA): transverse colon up to the •
splenic fl exure.
Left colic (from IMA): splenic fl exure and descending colon.•
Superior rectal artery (from IMA): rectum and upper anal canal.•
• Autonomic nerve supply:
Sympathetic, mainly from greater splanchnic nerves via SMA and •
IMA plexuses.
Parasympathetic, from vagus via SMA and IMA plexus from •
caecum to splenic fl exure and from pelvic parasympathetics (S2,
3, 4) via hypogastric plexuses and retroperitoneal nerves from
splenic fl exure to upper anal canal.

CHAPTER 11 Colorectal surgery
410
Fistula-in-ano
Key facts
A fi stula is an abnormal connection of two epithelial surfaces and the two
surfaces joined in fi stula in ano are the anorectal lining and the perineal or
vaginal skin. Very common, especially in otherwise fi t young adults. May
occur in the presence of Crohn’s disease; minor association with obesity
and diabetes mellitus, very rarely due to trauma or ulceration of anorectal
tumours.
Pathological features
Commonest cause is sepsis arising in an anal gland that forces its way out
through the anal tissues to appear in the perianal or in women, vaginal
skin (cryptoglandular theory of fi stula in ano). Often presents initially as
an acute perianal abscess. The tissues through which the track pushes
determines the classifi cation of fi stulas (see Fig. 11.1).
Clinical features
• Acute perianal abscess. Rapid onset of severe perianal or perineal pain.
Swelling and erythema of the perianal skin with fever and tachycardia.
• Recurrent perianal sepsis. Recurrent intermittent sepsis typifi ed by
gradual build-up of ‘pressure’ sensation and swelling in the perianal skin
and eventual discharge of bloodstained purulent fl uid.
• Chronic perianal discharge. Persistent low grade sepsis of the track with
chronic discharge of seropurulent fl uid via a punctum that is usually
clearly identifi ed by the patient.
Diagnosis and investigations
Diagnosis and investigation should aim to confi rm the presence of
a fi stula and identify the course of the track to determine the type of
fi stula:
• Examination of the perineum and rectal examination may reveal a
palpable fi brous track.
• EUA with probing of any external opening to aid identifi cation of the
course of the track.
• Endoanal ultrasound (sometimes with hydrogen peroxide injected into
the track) identifi es the course of the track.
• MRI scanning is probably the most sensitive method of determining the
course of the track and identifying any occult perianal or pelvic sepsis.
• Flexible sigmoidoscopy if associated colorectal disease, e.g. Crohn’s
disease, is suspected.
Treatment
Medical treatment
• Antibiotics may reduce symptoms from recurrent sepsis, but cannot
treat the underlying fi stula.
• Medical treatment of infl ammatory bowel disease may dramatically
reduce symptoms from associated fi stulas.
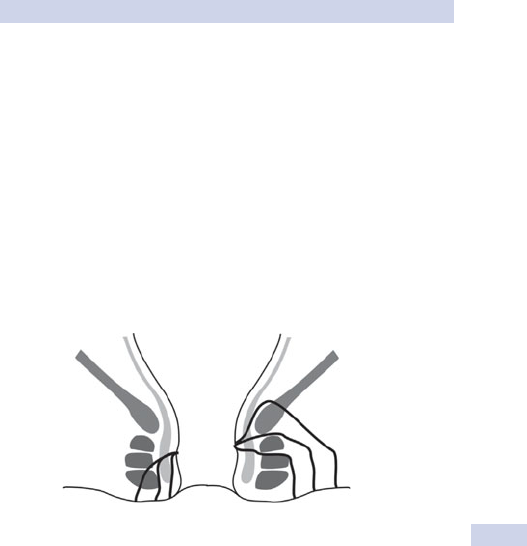
FISTULA-IN-ANO
411
Surgical treatment
Principles of surgical treatment are as follows:
• Drainage of any acute sepsis if present.
• Prevention of recurrent sepsis. Usually by insertion of a loose seton
suture, e.g. silastic sling.
• Low fi stula in ano. Lay open track, remove all chronic granulation
tissue, and allow to heal spontaneously (fi stulotomy); little risk of
impairment of continence due to minimal division of sphincter tissues.
• High fi stula in ano:
Remove fi stula track and close the internal opening (core •
fi stulectomy and endorectal fl ap advancement).
Slowly divide the sphincter tissue between the fi stula and the •
perianal skin (cutting seton); low risk of incontinence.
Fill the fi stula with fi brin glue.•
Rectum
Pelvic
floor
Anus
Low
fistulae
High
fistulae
Sphincter
muscle
Low
transphincteric
Submucosal
Intersphincteric
Extrasphincteric
Suprasphincteric
High
transphincteric
Fig. 11.1 Classifi cation of fi stula-in-ano.

CHAPTER 11 Colorectal surgery
412
Haemorrhoids
Key facts
Broad term, often incorrectly used to refer any perianal excess tissue.
True haemorrhoids are excessive amounts of the normal endoanal cush-
ions that comprise anorectal mucosa, submucosal tissue, and submucosal
blood vessels (small arterioles and veins). Commonest age of onset is
in young adulthood. Associated with constipation, chronic straining, obes-
ity, and previous childbirth. May become ulcerated and infl amed if recur-
rently prolapsing.
• If confi ned to the tissue of the upper anal canal, they are referred to
as ‘internal’.
• If extend to the tissues of the lower anal canal, they are referred to
as ‘external’.
• Typically occur in the same location as the main anal blood vessel
pedicles (described as 3, 7, and 11 o’clock positions as seen in the
supine position).
Clinical features
• Features of irritation. Pruritus ani, mucus discharge, and perianal
discomfort.
• Features of damage to mucosal lining. Recurrent post-defecatory
bleeding—bright red, not mixed with stools, on paper or splashing in
the toilet pan.
• Features of prolapse. Intermittent lump appearing at anal margin,
usually after defecation, may spontaneously reduce or require manual
reduction.
Diagnosis and investigations
• Diagnosis is usually by rigid sigmoidoscopy and proctoscopy.
• Flexible sigmoidoscopy or colonoscopy may be appropriate if there
is concern about the cause of symptoms; remember—haemorrhoids
rarely start over the age of 55y and it is often best to assume another
cause until proven otherwise in these cases.
Treatment
Medical treatment Avoidance of constipation and straining; bulking or
softener laxatives.
Surgical treatment
• Banding or excessive tissue. Best for prolapse symptoms (not possible
for external components due to excellent nerve supply of lower anal
canal).
• Dilute phenol injections (5% in almond oil). Best for bleeding
symptoms.
• Haemorrhoidal devascularization procedures (e.g. arterial ligation
‘HALO’ either Doppler ultrasound-guided or blind). For failed topical
treatments and surgery not indicated/desired.
• Stapled anopexy (also called PPH). Sometimes used for circumferential
prolapsing haemorrhoids.

HAEMORRHOIDS
413
• Haemorrhoidectomy for large external haemorrhoids or haemorrhoids
failing to respond to conservative treatment. Associated with a small
risk of impaired continence and anal stenosis.
Key revision points—anatomy of the anus
• Lower third of the anal canal is somatic tissue in origin—stratifi ed
squamous epithelium; very sensitive (pudendal and distal sacral
nerves); relatively poor blood supply and healing.
• Upper third of the canal is visceral tissue in origin—columnar
epithelium; insensitive; excellent blood supply and healing.

CHAPTER 11 Colorectal surgery
414414
Acute anorectal pain
Causes and features
Fissure-in-ano Acute severe, localized, ‘knife-like’ pain in the anus dur-
ing defecation. Often associated with deep throbbing pain for minutes
or hours afterwards due to pelvic fl oor spasm. Blood on the paper when
wiping (small volume, red-pink streaks or spots).
Haemorrhoids Usually acutely prolapsed and infl amed with associated peri-
anal lump, soreness, and irritation. May bleed, often profuse, bright red.
Perianal abscess Gradual onset, constant localized perianal pain. Associated
swelling with tenderness and possible discharge. May have associated sys-
temic features of fever, malaise, anorexia.
Perianal haematoma Usually sudden onset; acutely painful with associated
perianal swelling (dark red coloured).
Rectal prolapse Acute full thickness rectal prolapse, occasionally causes
pain. Obvious large perineal lump, dark red blue with surface mucus and
occasionally some surface ulceration.
Emergency management
Establish a diagnosis
• Good inspection and careful digital rectal examination is usually all that
is required.
• Rigid sigmoidoscopy may be painful and is often unnecessary.
• Flexible sigmoidoscopy is rarely indicated.
Early treatment
• Give adequate analgesia; opiates may be necessary.
• Topical treatment is highly effective; cool pads, topical local
anaesthetic gels.
Defi nitive management
Fissure-in-ano Mainstay of acute treatment is analgesia and anal sphincter
muscle relaxants, e.g. topical GTN 0.2% ointment, diltiazem 2% ointment.
Local anaesthetics are helpful early in treatment.
Haemorrhoids May require bed rest with continued topical treatment
until swelling resolves and spontaneous reduction begins. Acute haemor-
rhoidectomy is almost always best avoided due to the risk of over-excision
of anal tissue. Minimal anal dilatation under GA is rarely necessary.
Perianal abscess Incision and drainage is a surgical emergency, particularly
if the patient is diabetic or immunosuppressed.
Perianal haematoma Incision to allow decompression of acute haematoma
may be necessary, often done under topical LA.
Rectal prolapse Swelling may be reduced by cool packs, elevation, and
sometimes icing sugar applied to the swollen mucosa as a dessicant! Very
rarely requires emergency surgery.
This page intentionally left blank

CHAPTER 11 Colorectal surgery
416416
Acute rectal bleeding
Causes and features
Acute rectal bleeding is broadly divided into regions of the colon from
which it comes and the blood is typically different according to the origin.
Anorectal
Bright red blood, on the surface of the stool and paper, after defecation.
• Haemorrhoids.
• Acute anal fi ssure.
• Distal proctitis.
• Rectal prolapse.
Rectosigmoid
Darker red blood, with clots, in surface of stool and mixed.
• Rectal tumours (benign or malignant).
• Proctocolitis.
• Diverticular disease.
Proximal colonic
Dark red blood mixed into stool or altered blood.
• Colonic tumours (benign or malignant).
• Colitis.
• Angiodysplasia.
• NSAID-induced ulceration.
Upper GI bleeding occasionally produces dark red rectal bleeding, but it
is usually associated with signifi cant haemodynamic instability when
suffi ciently large.
Features (signs)
• Tachycardia and hypotension suggests substantial loss.
• LIF tenderness suggests diverticular infl ammation with bleeding.
Emergency management
Resuscitation
• Establish large calibre IV access; give crystalloid fl uid up to 1000mL
if tachycardic or hypotensive.
• Catheterize and place on a fl uid balance chart if hypotensive.
• Send blood for FBC (Hb, WCC), U&E (Na, K), LFTs (albumin), group
and save, clotting.
Establish a diagnosis
• Rigid proctosigmoidoscopy should be performed in all cases to
exclude a simple anorectal cause.
• Urgent fl exible sigmoidoscopy and colonoscopy may be undertaken. It
is higher risk than elective endoscopy, but may confi rm an origin and
may allow therapeutic intervention (adrenaline injection, heater probe
coagulation, argon plasma coagulation (APC)).
• Urgent selective mesenteric arteriography for obscure or persistent
bleeding; needs active bleeding of 0.5mL/min.

ACUTE RECTAL BLEEDING
417417
• Urgent gastroscopy should be used to exclude massive upper GI
bleeding if suspected.
Early treatment Consider blood transfusion if major bleed (persisting
haemodynamic instability despite resuscitation, Hb <8g/dL).
Defi nitive management
Anorectal causes Most can be controlled by local measures, such as
injection, coagulation, or packing.
Acute colitis
• IV or PO metronidazole if thought to be infective until organism
identifi ed.
• IV hydrocortisone 100mg qds if thought to be ulcerative or Crohn’s
colitis.
• Surgery may be necessary whatever the aetiology if bleeding persists
(subtotal colectomy and ileostomy formation).
Diverticular disease
• IV antibiotics (cefuroxime 750mg tds + metronidazole 500mg tds).
• Angiographic embolization if bleeding fails to stop and patient not
critically unstable for time in radiology.
• Surgery is high risk, but may be unavoidable. If the location is known,
a directed hemicolectomy may be performed (on-table colonoscopy
may be used). If not, a subtotal colectomy is safest.
Angiodysplasia
• Colonoscopic therapy (injection, heater probe, APC) is ideal.
• Angiographic embolization may be possible.
• Right hemicolectomy is occasionally unavoidable.
Undiagnosed source
Rarely, the patient remains unstable with active bleeding and no cause can
be reliably confi rmed. Surgical options to deal with this include:
• On-table colonoscopy with washout via colostomy to locate bleeding
source.
• Formation of mid-transverse loop colostomy and subsequent targeted
hemicolectomy.
• ‘Blind’ hemicolectomy (left if signifi cant diverticular disease present;
right if no other cause obvious and angiodysplasia is likely).

CHAPTER 11 Colorectal surgery
418418
Acute severe colitis
Causes and features
Any cause of colitis may progress to acute severity. Common causes
include:
• Severe ulcerative colitis (UC; usually pancolitis); occasionally, acute
severe colitis is the presentation of UC with no prior history;
• Acute infective colitis (e.g. Salmonella, C. diffi cile, amoebae, parasites);
• Neutropenic colitis;
• Pseudomembranous colitis (C. diffi cile-related);
• Progressive Crohn’s colitis (usually with a clear prior history).
Symptoms
• Diarrhoea (usually bloody with urgency and frequency). ‘Constipation’
may be an ominous feature, suggesting acute colonic dilatation.
• Abdominal pain (generalized).
• Malaise, anorexia, and fever (systemic infl ammatory features).
Signs
• Fever, tachycardia, possible hypotension.
• Abdominal tenderness; peritonism suggests perforation.
Complications
Any acute severe colitis may develop any of these complications.
• Haemorrhage.
• Hypokalaemia.
• Hypoalbuminaemia;.
• Perforation (localized or generalized).
‘Toxic dilatation’ is a term used to describe the situation of acute severe
colitis with colonic dilatation usually associated with reduced bowel fre-
quency and impending perforation.
Fulminant severe colitis is defi ned as: tachycardia >120bpm or stool fre-
quency >10 times/24h or albumin <25g/dL.
Emergency management
Resuscitation
• Establish large calibre IV access; give crystalloid fl uid up to 1000mL if
tachycardic or hypotensive.
• Catheterize and place on a fl uid balance chart if hypotensive.
• Send blood for FBC (Hb, WCC), U&E (Na, K), LFTs (albumin), group
and save, clotting.
Establish a diagnosis
• Send ‘hot’ stools for M,C,&S as well as microscopy for C,P,&O. Even
known colitics may catch acute infective colitis.
• Plain AXR (looking for colonic dilatation) and erect CXR (looking for
free gas).
• Rigid sigmoidoscopy and biopsy only if not unstable.
• Flexible endoscopy may be indicated, but carries a risk of perforation.

ACUTE SEVERE COLITIS
419419
Early treatment
• Give IV hydrocortisone if the diagnosis of UC is likely; if infective colitis
is suspected, consider withholding steroids until the M,C,&S results are
back.
• Blood transfusion if anaemic.
• Surgery if peritonitis or free gas on CXR (the operation for any
acute colitis is usually total abdominal colectomy and end ileostomy
formation).
Defi nitive management
Acute ulcerative colitis
• IV hydrocortisone 100mg qds, converted to oral prednisolone if
responding to treatment.
• IV cyclosporin if fulminant/not responding to IV steroids.
• Surgery for failure to respond to medical treatment or acute
complications of haemorrhage or perforation.
• Regular blood investigations and plain abdominal radiography to
monitor treatment.
Infective colitis
• IV or PO metronidazole until organism identifi ed.
• Salmonella, C. diffi cile—metronidazole.
• Surgery for failure to respond to medical treatment or acute
complications of haemorrhage or perforation.
Neutropenic colitis
• IV antibiotics.
• Bone marrow support.
• Surgery for failure to respond to medical treatment or acute
complications of haemorrhage or perforation.

CHAPTER 11 Colorectal surgery
420
Post-operative anastomotic leakage
Causes and features
Any intra-abdominal anastomosis may leak. Highest risk of leak occurs
with oesophageal and rectal anastomosis and lowest with small bowel
anastomosis (see Table 11.1).
Anastomotic leakage may present as one of several clinical pictures.
3 Peritonitis
Acute severe generalized abdominal pain with generalized guarding and
rigidity. Fever, tachycardia, and tachypnoea are common. Diagnosis is usu-
ally clinical, but may require CT scanning if unsure.
Intra-abdominal abscess (see b p. 308)
Swinging fever and tachycardia, commonly around 5–7 days post-opera-
tively. Localized tenderness related to the anastomosis may be present.
Diagnosis should be sought by CT scanning.
Enteric fi stula
A fi stula between the anastomosis and the wound or another organ may
occur. Usually occurs as a result of a subclinical leak and abscess forma-
tion that discharges through a pathway of low resistance. Often presents
late as an apparent wound infection that discharges with enteric content.
Diagnosis made by CT scanning or occasionally, fi stulography if presents
very late.
2 Cardiovascular complications
Sepsis originating from an initially subclinical leak may present with
apparent cardiovascular complications, e.g. AF, SVT, chest pain, and sinus
tachycardia. A wise precautionary rule is, ‘Any acute post-operative dis-
turbance of physiology in a patient with an intra-abdominal anastomosis is
due to leak until proven otherwise.’
Emergency management
Resuscitation
• Establish large calibre IV access; give crystalloid fl uid up to 1000mL if
tachycardic or hypotensive.
• Catheterize and place on a fl uid balance chart if hypotensive.
• Send blood for FBC (Hb, WCC), U&E (Na, K), LFTs (albumin), group
and save, clotting.
• Give appropriate analgesia if not on an epidural or PCA.
Establish a diagnosis
• Acute peritonitis needs no diagnostic investigation. Emergency re-look
laparotomy should be organized immediately.
• CT scanning with IV and PO contrast is the investigation of choice for
all other suspected leaks.
• For rectal anastomoses, a water-soluble contrast study may delineate a
leak.

POST-OPERATIVE ANASTOMOTIC LEAKAGE
421
Early treatment
• Give IV antibiotics (e.g. IV cefuroxime 750mg tds + metronidazole
500mg tds).
• Monitor fl uid balance hourly.
Defi nitive management
3 Peritonitis
Always requires surgical intervention unless the patient is deemed unfi t.
Prepare for theatre. Ensure blood results from resuscitation are avail-
able. Stoma care review is not always necessary and often not practical
or useful.
Once the leak has been identifi ed, options for management include:
• Dividing the anastomosis, closing the distal end, and forming the
proximal end into a stoma;
• Emptying the bowel (lavage) and forming a proximal defunctioning
stoma;
• Re-forming or repairing the anastomosis (only suitable for fi t patients
with minimal contamination and an otherwise healthy anastomosis);
• Placing a large drain(s) next to the anastomosis.
Intra-abdominal abscess
• Radiologically guided drainage and antibiotics, provided patient does
not become peritonitic or show signs of secondary complications.
• Open surgical drainage if inaccessible or unresponsive to radiological
drainage.
Enteric fi stula
• Usually managed by antibiotics. May close spontaneously; if fails to
close, surgical repair may be required.
• Treat as for abscess or peritonitis if either develops.
Table 11.1 Risk factors associated with increased risk of leak
Patient factors Disease factors Operative factors
Chronic malnutrition
Immunosuppression
Unprepared bowel, e.g.
obstruction
Poor blood supply or
bowel ends
High dose steroid use
Diabetes mellitus
Local or generalized sepsis
Metastatic malignancy
Tension on bowel ends
This page intentionally left blank

423
Paediatric surgery
Principles of managing paediatric surgical cases 424
Acute abdominal emergencies—overview 426
Oesophageal atresia 428
Pyloric stenosis 430
Malrotation and volvulus 432
Intussusception 434
Hirschsprung’s disease 436
Rare causes of intestinal obstruction 438
Abdominal wall defects 440
Necrotizing enterocolitis (NEC) 442
Inguinal hernia and scrotal swellings 444
Other childhood hernias 446
Prepuce (foreskin) and circumcision 448
Undescended testis 450
Solid tumours of childhood 452
Neck swellings 454
Chapter 12

CHAPTER 12 Paediatric surgery
424424
Principles of managing paediatric
surgical cases
Key facts
• Children are not small adults.
• Children come in different sizes; always obtain a weight before starting
treatment.
• Fluids and drug doses depend on body weight.
• Babies and children have different differential diagnoses from adults.
• Children often differ from adults in physiology and anatomy (see
Table 12.1).
• Babies and young children have diffi culty communicating symptoms.
Special problems with babies
• Thermoregulation is impaired (immature sweating, high surface area to
body weight increases rate of heat loss). Prone to hypothermia.
• Minimal glycogen stores. Prone to acute hypoglycaemia.
• Principal breathing pattern is diaphragmatic. Prone to breathing
diffi culties with abdominal distension.
• Immature body physiology. Much less biological functional reserve than
adults; acute disturbances of physiology more serious with less room
for error.
• May not metabolize drugs as expected.
Intravenous fl uids
Maintenance fl uids (see Box 12.1)
Box 12.1 Paediatric fl uid regimen
• 4mL/kg/h total fl uid for each of the fi rst 10kg of weight (0–10kg).
• 2mL/kg/h total fl uid for each of the next 10kg of weight (11–20kg).
• 1mL/kg/h total fl uid for each subsequent kg of weight (over 21kg)
• Always calculate fl uid and sodium requirements according to weight.
• Remember to add glucose, especially for neonates.
• Adjust the fl uid regimen according to clinical setting for neonates and
premature babies.
Resuscitation fl uids
• Crystalloids or colloids can be given as resuscitation fl uids.
• Mild dehydration—10mL/kg bolus; repeat as necessary.
• Moderate dehydration—20mL/kg bolus; repeat as necessary.

PRINCIPLES OF MANAGING PAEDIATRIC SURGICAL CASES
425
Table 12.1 Basic physiological parameters in children
Neonates Adolescents
Blood volume (mL/kg) 80 30–40
Oral fl uid intake (mL/kg/day) 150 30–40
Daily Na
+
intake (mmol/kg) 2–3 1–2
Daily K
+
intake (mmol/kg) 2–3 1
Systolic BP (mmHg) 40–50 100–120
Resting pulse rate (bpm) 120–160 70–80

CHAPTER 12 Paediatric surgery
426426
Acute abdominal emergencies—
overview
Key facts
• Whatever the cause (see Fig. 12.1), these typically present with signs
and symptoms of either peritonitis or intestinal obstruction.
• Features of peritonitis are often diffi cult to elicit in babies.
• Cardinal features of obstruction are:
Vomiting.•
Abdominal distension.•
Failure to pass meconium.•
Pain.•
Vomiting in children
• Vomiting is common in newborns and is often entirely benign. May be
due to:
Overfeeding.•
Rapid feeding.•
Air swallowing (inadequate winding).•
• Vomiting may be due to metabolic causes (inborn errors of
metabolism, acidosis) or infections (UTI, chest infection, meningitis).
• Bile-stained vomiting should never be ignored.
Abdominal distension Most pronounced in distal obstruction and less so in
proximal causes of obstruction.
Failure to pass meconium
• Term babies should pass meconium within 36h.
• Babies with proximal obstruction or atresia may still pass meconium.
Pain May be diffi cult to assess in babies. Typical features are going off
feeds, lethargy, erratic heart rate.
Diagnostic features
History
• Family/genetic history for cystic fi brosis (CF). Meconium ileus.
• Premature birth. Necrotizing enterocolitis.
• Time of onset related to birth. The more proximal the obstruction,
the earlier the presentation.
Examination
• Blood in vomit or stool may indicate necrotic bowel.
• Degree of distension. Most pronounced with distal obstruction.
Investigations
• Plain AXR may show diagnostic features.
‘Double bubble sign’• . Duodenal atresia or malrotation.
‘Ground glass’• . Meconium ileus.
Multiple loops of small bowel• . Distal obstruction.
Intramural gas• (‘pneumatosis intestinalis’). Necrotizing enterocolitis.
Free air• . Intestinal perforation.
• Abdominal ultrasound. Abdominal mass. Intussusception, tumour,
duplication cyst.
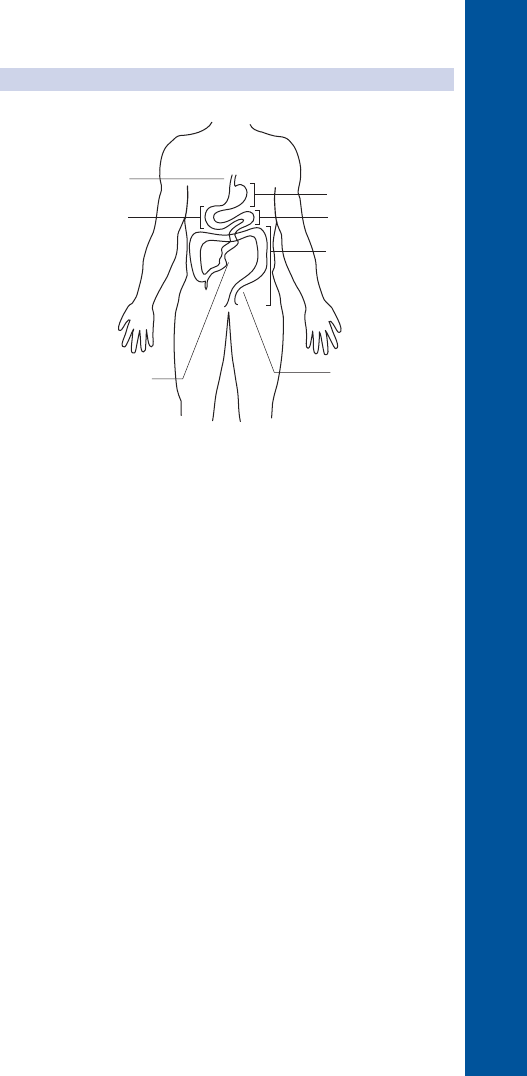
ACUTE ABDOMINAL EMERGENCIES—OVERVIEW
427427
Antral web
Pyloric stenosis (>3wks)
Jejunal atresia
Anorectal
malformation
Ileal adhesion
Meconium ileus
Incarcerated inguinal hernia
Exomphalos
Gastnoschisis
Duodenal atresia
Volvulus
Congenital oesophageal
problems
Hirschsprung’s disease
Meconium plug
Small left colon
syndrome
Fig. 12.1 Causes of acute abdominal emergencies in babies and infants.

CHAPTER 12 Paediatric surgery
428
Oesophageal atresia
Key facts
• Congenital abnormality of the formation of the upper aerodigestive
tract (UADT), resulting in partial or complete interruption of the
oesophageal lumen (see Fig. 12.2).
• Often associated with other congenital abnormalities (VACTERL).
Clinicopathological features
• May be diagnosed on prenatal ultrasound. Features include maternal
polyhydramnios, absent stomach bubble, associated abnormalities.
• Post-natal diagnosis relies on features of persistent salivary drooling,
regurgitation of all feeds, and cyanosis with feeding.
• Failure to pass NGT into stomach.
• Tracheo-oesophageal fi stula in isolation unusual. Presents with
recurrent aspiration/chest infections.
Diagnosis and treatment
• Plain AXR and thorax. NGT coiled in oesophagus.
• Presence of stomach gas suggests tracheo-distal oesophageal fi stula.
• Plain X-ray spine. Associated congenital abnormalities.
• Echocardiography. Associated cardiac abnormalities.
Medical treatment
• Nurse head-up.
• NBM and continuous Replogle (oro-oesophageal) tube.
• Antibiotics for possible aspiration pneumonia.
Surgical treatment
• Isolated atresia.
Gastrostomy for feeding + continuous drainage of upper pouch.•
Delayed closure of defect (may require interposition graft if a long •
segment involved).
• Atresia with tracheo-oesophageal fi stula. Ligation of fi stula and primary
closure of oesophageal defect.
• Isolated tracheo-oesophageal fi stula. Ligation of fi stula (through neck).
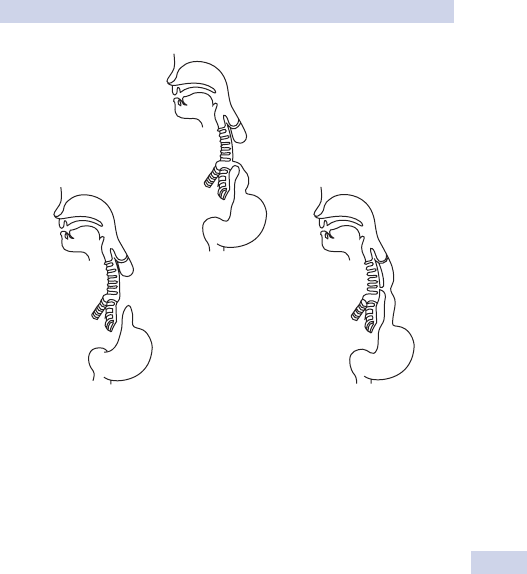
OESOPHAGEAL ATRESIA
429
Type C
87%
Type A
8%
Type E
4%
Fig. 12.2 Classifi cation of oesophageal atresia. Type C (III), distal fi stula; type A
(I), atresia without fi stula; type E (V), H-type fi stula.

CHAPTER 12 Paediatric surgery
430
Pyloric stenosis
Key facts (children)
• Incidence 3 in 1000 live births.
• Increased familial risk (family history).
• It typically occurs in fi rst born boys (♂:♀, 4:1).
Pathological features There is hypertrophy of the pyloric smooth
muscle in early infancy, which occurs at about 3–6 weeks.
Clinical features
• Classical projectile, forceful vomiting (secondary gastritis may cause
bloodstaining).
• Persisting vomiting.
• Sometimes a history of excessive positing and gastro-oesophageal
refl ux.
• Baby appears active and hungry, especially after vomiting.
• Small, green, starvation stools passed infrequently.
• Weight gain is poor.
• Dehydration with hypochloraemic alkalosis gradually supervenes in
untreated, established condition.
• Baby usually looks well in early state.
• Dehydration, pallor, underweight only in advanced condition.
• Epigastric fullness with left-to-right gastric peristaltic wave.
• Test feed is usually performed to palpate a pyloric ‘tumour’ (see
Box 12.2).
Diagnosis and investigations
• If pyloric ‘tumour’ is felt, no radiological investigations are necessary
prior to surgery.
• Ultrasound shows thickened (>4mm), elongated (>16mm) pyloric
muscle and increased muscle to lumen ratio with decreased movement
of fl uid through narrow canal (term infants).
• Barium meal (rarely necessary) shows an enlarged stomach, increased
gastric peristalsis, and elongated, narrowed pyloric canal.
• Electrolytes and capillary blood gases (d Na
+
, d K
+
, d Cl
–
, base excess,
and pH).
Treatment
• Resuscitation with IV rehydration.
• Correct hypovolaemia with 10mL/kg 0.9% saline.
• Correct hypochloraemic alkalosis and hypokalaemia (may take
24–48h)—0.45% sodium chloride/5% dextrose with added potassium
chloride at a rate of 120–150mL/kg/24h.
• NGT drainage to prevent aspiration of vomited secretions.
Surgical treatment
• Pyloromyotomy (division of pyloric muscle fi bres without opening of
bowel lumen).
Done via right upper quadrant incision, periumbilical, or •
laparoscopically.

PYLORIC STENOSIS
431
Caution not to open mucosa and avoid the prepyloric vein (‘of •
Mayo’).
Start feeding within 4–6h post-operatively and increase to full •
volume by 24h.
Box 12.2 How to perform a test feed
• Undress baby (leaving loose nappy) and place on carer’s lap with
head elevated.
• Sit opposite baby and carer; position baby so head is to examiner’s
right.
• Begin feeding (breast or bottle). With active suckling, the abdominal
wall relaxes.
• Palpate with left hand (middle fi nger).
• Begin above umbilicus and feel into RUQ under liver edge.
• Wait to feel appearance of olive-sized, fi rm, mobile lump in angle
between liver edge and upper right rectus muscle (contracted
pyloric muscles).
• As stomach infl ates with air and milk, pylorus becomes more
diffi cult to feel—aspiration of NGT may help.

CHAPTER 12 Paediatric surgery
432
Malrotation and volvulus
Key facts and clinical features (neonates)
Malrotation
• This can present at birth or soon after and symptoms are due to
rotation of the small bowel, leading to duodenal obstruction.
• ‘Ladd’s bands’ are occasionally the cause of the obstruction.
• Proximal duodenal distension leads to bile-stained vomiting.
• The caecum may be in an abnormally high or midline position.
Volvulus
• Twisting, in clockwise direction, of malrotated, non-fi xed midgut loop
on its narrow-based mesentery through 360* or more.
• Results in obstruction of superior mesenteric blood vessels.
• Signs. Sudden onset of abdominal pain, bile vomiting, progression to
shock, passage of blood per rectum.
• May be less dramatic; most dangerous in newborn period because of
delay in diagnosis and rapid development of gut ischaemia.
• Older children may present insidiously or as rapid onset of shock with
less prominent other symptoms.
Diagnosis and investigations
3 If in doubt, operate. Viability of twisted bowel is very time-
dependent—delays in diagnosis can be very serious.
• Plain AXR. ‘Double bubble’ sign with some distal gas.
• Barium meal. Obstruction of second part duodenum, non-rotation
of duodenum/jejunum, and corkscrew appearance of proximal small
bowel loops; absent C loop of duodenum.
• Ultrasound scan. Reversed relation of superior mesenteric artery
and vein.
• Doppler ultrasound. Absent or abnormal small bowel blood.
Treatment
• Resuscitation, including decompression with NGT.
• Prompt surgery to avoid irreversible bowel damage.
• Laparotomy may reveal:
Obstructed, but viable bowel.•
Patchy ischaemic changes.•
Established necrosis.•
• Resection of ischaemic gut may risk ‘short gut syndrome’.
• ‘Second look’ laparotomy (24–48h) allows reassessment prior to
resection.
This page intentionally left blank

CHAPTER 12 Paediatric surgery
434
Intussusception
Key facts (children)
• Incidence approximately 2 per 1000 live births.
• Peak age of presentation at 3–10 months.
• ♂:♀, 2:1.
• Fewer than 10% have a clear focal pathological cause that starts the
intussusception (‘apex’; older children are more likely to have an
apex).
Clinicopathological features
• Invagination/telescoping of the proximal bowel (called the
intussusceptum, e.g. terminal ileum/ileocaecal valve) into the distal
bowel (called the intussuscepiens, e.g. caecum/ascending colon).
• May be due to enlargement of lymphatic patches of Peyer (‘idiopathic’).
• Pathology at the apex may be:
Meckel’s diverticulum.•
Polyp.•
Lymphoma.•
Clinical features
• Classic triad of features is:
Abdominal pain (associated with pallor, screaming, and •
restlessness).
Palpable sausage-shaped mass (mid-abdominal or right upper •
quadrant).
Passage of ‘redcurrant jelly’ stool (rectal examination may reveal •
bloody mucus and the lead point may rarely be palpable).
• Typically, the infant is relatively settled between bouts of pain.
• Signs of shock (lethargy, poor feeding, hypotonia) require urgent fl uid
resuscitation.
• Features of obstruction (distension and vomiting) may occur.
Diagnosis and investigations
• Ultrasound (diagnostic test of choice). Intussusception in cross-section
(‘doughnut’ or ‘target’ sign).
• Plain X-ray. May show soft tissue mass, small bowel obstruction, free
air indicating perforation.
• Air (or rarely gastrograffi n) contrast enema. Diagnostic and may be
therapeutic (see below).
Treatment
• Immediate IV fl uid resuscitation to correct fl uid losses and to restore
fl uid, electrolyte, and acid/base balance.
• Maintenance fl uid replacement and replacement of continued losses
(vomiting or nasogastric losses). Reduction only attempted once fl uid
balance restored.
• Analgesia and sedation (morphine 0.2mg/kg) will aid process of
reduction.
• Antibiotics and NGT.

INTUSSUSCEPTION
435
Methods of reduction
Radiological reduction
• Air enema therapeutic in 75% of cases.
• Usually performed in radiology department under screening control.
• Surgeon should be present.
• Evidence of irreducible obstruction or perforation mandates
immediate halt.
• Partial or incomplete reduction may warrant repeat attempt after
4–6h.
• Informed consent includes risk of perforation.
Surgical reduction
• Laparotomy indicated without enema if evidence of peritonitis or
perforation.
• Manual reduction by retrograde squeezing and gentle proximal
traction.
• Resection and anastomosis if bowel viability is in doubt (710% require
resection).
• Post-reduction septic shock may occur with release of bacterial
products from viable, but damaged bowel segment.
• Most recover rapidly with resumption of oral feeding in 24–48h
and discharge home in 4–5 days.
Complications
• Recurrence rate is 5–7% in non-operative cases and about 3% for
operative reduction.
• Morbidity is low, but delayed diagnosis, inadequate resuscitation, and
failure to recognize ischaemic or perforated bowel account for
1% mortality.

CHAPTER 12 Paediatric surgery
436
Hirschsprung’s disease
Key facts (neonates/children)
• Incidence 1 in 5000 live births.
• Commoner in males.
Pathological features
• Due to incomplete migration of neural crest cells into the hindgut,
resulting in distal aganglionosis and failure of coordinated peristaltic
waves, abnormal anorectal relaxation, and loss of recto-anal inhibitory
refl exes.
• May involve:
Just the anorectal junction (ultrashort segment), presents in adult •
life.
The rectum and recto-sigmoid (short segment. 70%), presents in •
infancy or early childhood.
Extensive colonic involvement (long segment), rare.•
• The proximal (normal) bowel becomes progressively distended due to
build-up of faecal matter.
Clinical features
• There is failure to pass meconium within 24–48h, abdominal
distension, and bile vomiting.
• It may be associated with Down’s syndrome.
• It may present late with poor weight gain, offensive diarrhoea, or
enterocolitis.
Diagnosis and investigations
• Radiograph shows dilated colon to level of ‘transition zone’ to
aganglionic bowel.
• Contrast enema shows less distensible rectum and may indicate
transition zone.
• Suction rectal biopsy confi rms diagnosis; thickened nerve fi bres and
aganglionisis (i AChE).
• Anorectal manometry (in older children) shows failure of anal relaxation
on rectal balloon distension (loss of recto-anal inhibitory refl ex).
Treatment
• Resuscitation and analgesia.
• Decompression of the colon with regular saline rectal wash-outs.
• If decompression is not achieved or there is total colonic involvement,
a defunctioning stoma is necessary.
• Defi nitive surgery is to remove the aganglionic bowel and bring
normally innervated bowel to the anus (pull-through technique—
Soave, Swenson, or Duhamel types).
It is usually performed as a one-stage procedure without a covering •
stoma.
The pull-through can be performed transanally or abdominally.•
Laparoscopy assists in establishing a level and mobilizing the •
colon/rectum.

HIRSCHSPRUNG’S DISEASE
437
Complications
• Constipation.
• Enterocolitis can affect 20–50% of children pre- and post-operatively
(uncommon >5y of age unless an obstructive component exists).
Further reading
Langer JC (2004). Hirschsprung’s disease. Curr Probl Surg 41: 949–88.
Swenson O (2002). Hirschsprung’s disease: a review. Pediatrics 109(5): 914–8.
Teitelbaum DH, Coran AG (2003). Primary pull-through for Hirschsprung’s disease. Sem Neonatol
8(3): 233–41.

CHAPTER 12 Paediatric surgery
438
Rare causes of intestinal obstruction
Duodenal atresia
• Caused by failure of development or canalization of the duodenal
canal.
• May be complete (i.e. entirely separate proximal and distal duodenum)
or partial (e.g. an hourglass narrowing or web obstruction in the
second part of the duodenum).
Diagnostic features
• Bile-stained vomiting occurs from birth.
• Epigastric fullness on examination.
• Look for features of associated Down’s syndrome.
• Plain AXR. ‘Double bubble sign’ with no distal gas.
Management
• Resuscitation.
• Surgical bypass (duodenoduodenostomy bypass).
Jejuno-ileal atresia
• Caused by probable in utero vascular insult to mesenteric vessels.
• May occur in a single or multiple segments and may be short segments
or long stretches of small bowel involved.
Diagnostic features
• Bile-stained vomiting from birth.
• Prominent abdominal distension, especially with distal atresia.
• Features of obstruction.
Investigation and management
• Resuscitation.
• Contrast enema may be helpful to exclude other diagnoses.
• Surgical anastomosis between atretic ends.
Meconium ileus
• Caused by the presence of impacted, abnormally thick meconium
within the normal lumen of the small bowel.
• Pathognomonic of CF, but only 15% of CF present as meconium ileus.
Diagnostic features
• May be identifi ed during antenatal ultrasound examination (‘bright
spots’ in bowel) or family history with antenatal testing.
• Presents in neonatal period with features of distal obstruction:
Vomiting, distension, failure to pass meconium, mass in RIF
(meconium-obstructed bowel loops).
Investigation and management
• Resuscitation, IV fl uids, NGT.
• Plain AXR.
• Contrast enema may be diagnostic and therapeutic.
• Surgical removal of meconium (may involve a temporary ileostomy).
• Immunoreactive trypsin and commonly associated CF genes (δF508).

RARE CAUSES OF INTESTINAL OBSTRUCTION
439
Anorectal malformations
Incidence, approximately 1 in 5000 live births. Caused by failure of the cor-
rect septation of the hindgut cloaca or failure of formation of the anorectal
canal (and associated pelvic fl oor structures).
• Low anomalies traverse a normal levator muscle.
• High anomalies end above the levator and are commonly associated
with a fi stula (bladder, urethra, vagina).
• Malformations may be part of a syndrome or linked to chromosomal
abnormalities (VACTERL).
Diagnostic features
Condition should present at the neonatal check with recognition of an
absent or abnormally placed anus and the failure to pass meconium with
associated abdominal distension if a diagnosis has been missed. It may take
up to 24h before meconium passes through the fi stula.
Investigations and management
• Lateral prone X-ray of pelvis at 24h (assists in level assessment and
sacrum).
• Perineal, renal ultrasound, and echocardiography (for associated
abnormalities).
• Contrast loopogram 1 week after stoma formation (position of
fi stula/renal anomalies).
• Prophylactic antibiotics if vesicoureteric refl ux is demonstrated.
Surgical treatment
• Low lesions (perineal fi stula). Single stage perineal approach (anoplasty
or dilatation).
• All other lesions. Defunctioning colostomy.
• Posterior sagittal anorectoplasty (PSARP). At 1–6 months and
colostomy closure thereafter.
Prognosis
• Low anomalies often have relatively good function with a tendency to
constipation in later life.
• High anomalies often have impaired function with up to 80% lifetime
chance of soiling/incontinence.
CHAPTER 12 Paediatric surgery
440
Abdominal wall defects
Exomphalos (omphalocele)
Key facts
• Incidence 1 in 7000 births.
Clinicopathological features
• Herniation of the abdominal viscera through an umbilical defect that is
covered by a membrane (unless ruptured).
• Exomphalos minor. The defect is <5cm and only the bowel is herniated.
• Exomphalos major. The defect is >5cm and bowel, liver, and other
abdominal organs lie in the hernial sac.
• May present antenatally with an abnormal scan or raised maternal
serum alpha-fetoprotein (AFP); in post-natal presentation, there is an
obvious defect.
Diagnosis and investigations
• Investigations directed at identifying the associations (see Box 12.3).
Check blood sugar.
• All newborn babies should have cardiac imaging prior to further
management.
Treatment
• Parents may opt for termination in antenatally detected defects with
associated major cardiac or chromosomal anomaly (mortality 780%).
• Post-natal management involves protection of sac, insertion of NGT,
IV access, and fl uid management.
• Minor exomphalos should be suitable for reduction and primary
closure of umbilical defect.
• Major exomphalos may be associated with underdeveloped abdominal
cavity, precluding primary reduction. Epithelialization of the sac can be
encouraged with application of silver sulphadiazine paste, resulting in a
large ventral hernia that is suitable for delayed closure at 71y of age.
Surgical treatment
• Primary reduction of smaller defects. Excision of sac, closure of
umbilical defect (linear or purse string), and closure of umbilical skin.
• If the sac is ruptured in a larger defect. Application of silo or tissue
fl ap.
Box 12.3 Associations of exomphalos
• Chromosomal abnormality (trisomy 18, 13, 21).
• Cardiac and renal anomalies found in up to 40%.
• Pulmonary hypoplasia caused by abnormal diaphragm function.
• Beckwith–Wiedemann syndrome: exomphalos, macroglossia,
gigantism hyperinsulinism in infancy, renal/hepatic tumours.
• Pentalogy of Cantrell: exomphalos, sternal cleft, ectopia cordis,
anterior diaphragmatic hernia, ventricular septal defect.

ABDOMINAL WALL DEFECTS
441
Gastroschisis
Key facts
• Incidence 1 in 7000 births (increasing).
Clinicopathological features
• There is a defect to the right of the umbilicus with protrusion of the
stomach, small bowel, and large bowel.
• Associated with young maternal age and antenatal smoking or
recreational drug use.
• Most present antenatally with an abnormal scan or raised maternal
serum AFP.
• Antenatal diagnosis allows planned delivery (no evidence to
recommend Caesarean section).
• Extraintestinal associated anomalies are uncommon.
• Intestinal atresia found in 10–20%.
Diagnosis and investigations Associated anomalies are rare. No formal
investigations are required.
Treatment
Management of gastroschisis at birth
• Planned vaginal delivery as close as possible to neonatal surgical unit.
• Standard neonatal resuscitation (clean, dry, stimulate, facial O
2
, etc.).
• Cling fi lm wrap to protect herniated bowel against trauma,
contamination, heat loss, drying, and fl uid loss (ensure mesentery not
on tension).
• Insertion of NGT to decompress stomach.
• Fluid balance must include considerable evaporative losses from gut.
• Broad-spectrum antibiotics.
Non-surgical treatment Manual reduction and non-sutured closure of
defect.
Surgical treatment
• If possible, the defect is delineated and closed.
• If herniated contents are unable to be reduced, the application of
a ‘silo’ to cover gut and delayed closure once the gut is reduced
(7–10 days).

CHAPTER 12 Paediatric surgery
442
Necrotizing enterocolitis (NEC)
Key facts
• Range of intestinal infl ammation, ranging from mild mucosal injury to
full thickness necrosis and perforation.
• Perforated NEC associated with 40% mortality in neonates.
Clinicopathological features
• Associated with:
Premature delivery.•
Formula milk feeds.•
Hypoxia.•
Systemic sepsis.•
‘Micro-epidemic’ outbreaks in neonatal units.•
• Typically affects premature babies on ventilatory support.
• Features of vomiting, distension, bloody mucus passing PR.
• May shows signs of severe sepsis/shock (tachypnoea, poor perfusion,
temperature instability).
Diagnosis and investigations Plain AXR. Pneumatosis intestinalis,
portal venous gas, free gas if perforation, dilated, thick-walled (oedema-
tous) bowel.
Treatment
Medical
• Fluid resuscitation.
• IV antibiotics.
• Bowel rest and TPN.
Surgical treatment
• Indicated by complications (perforation, failure to respond to medical
treatment, abdominal mass, systemic sepsis).
• May include:
Peritoneal drainage.•
Bowel resection (usually with stoma formation).•
Complications
• Septicaemia.
• Enteric fi stulation.
• Peritonitis.
• Adhesions.
• Enteric stricture.
• Short gut syndrome.
• Death.
This page intentionally left blank

CHAPTER 12 Paediatric surgery
444
Inguinal hernia and scrotal swellings
Inguinal hernia
Key facts
• Childhood inguinal hernias derive from a persistent processus vaginalis
and are invariably indirect.
• ♂:♀, 7:1.
• Right-sided hernias (60%) are commoner than the left (25%); 15% are
bilateral.
• Higher incidence of complications (incarceration) than adult hernias.
Clinical features
• Usually noticed as a painless swelling, variable in size in the
inguinoscrotal or labial area.
• More prominent when the baby cries and may disappear
intermittently.
• Bowel entrapment causes pain and irreducibility leads to strangulation,
intestinal obstruction, perforation, and peritonitis.
• Ovarian entrapment may occur in females.
• Bile vomiting in a young infant should always prompt examination of
the inguinoscrotal area.
• Cardinal feature is a swelling in the groin above which the examining
fi ngers cannot defi ne the inguinal canal (‘cannot get above’).
• Asymmetrical thickening of the spermatic cord in the presence of a
history compatible with a hernia is strongly suggestive of the diagnosis.
Treatment
• Prompt surgical treatment is important in premature/young infants to
avoid risks of complications.
• Herniotomy alone is adequate. No need to repair the walls of the
canal; usually a simple, straightforward day case procedure.
• Acute surgery can be very diffi cult when it is irreducible or
strangulated or in very young infants.
Hydrocele
• Congenital fl uid-fi lled processus vaginalis and tunica vaginalis.
• Communicates with the peritoneal cavity in children.
• Scrotum is usually smoothly enlarged and sometimes bluish in colour
and the testis is often surrounded by the hydrocele.
• Occasionally acquired due to trauma, infection, or testicular tumour.
• Simple hydroceles may resolve spontaneously up to age of 18 months;
surgical intervention is deferred until 18 months.
• At operation, ligation of the patent processus vaginalis and drainage of
the fl uid are adequate; there is no need to excise the hydrocele wall.
Varicocele
• Due to a dilated pampiniform venous plexus of the spermatic cord.
• Onset usually after puberty.
• Has the feel of a ‘bag of worms’ during palpation of the cord.

INGUINAL HERNIA AND SCROTAL SWELLINGS
445
• Indications for treatment include discomfort (aching), cosmesis, and
concern about fertility. The procedure is carried out by high ligation of
the plexus, either by open or laparoscopic surgery.
• Beware an acute left varicocele in childhood due to obstruction of the
left renal vein by tumour (nephroblastoma).
• Treatment may be surgical ligation or radiologically-guided
embolization.
Idiopathic scrotal oedema
• Aetiology unknown; possibly due to an acute allergic reaction.
• Characterized by painless, red, unilateral scrotal swelling extending to
the groin and the perineum.
• Rapidly resolves spontaneously; the clinical diagnosis precludes the
need for investigation.

CHAPTER 12 Paediatric surgery
446
Other childhood hernias
Umbilical hernia
Key facts
• Persistence of the physiological umbilical defect beyond birth.
• Usually close spontaneously (especially in premature infants).
• Have a low incidence of complications (incarceration/strangulation).
Clinical features Usually noticed as a painless, intermittent swelling at the
umbilicus.
Treatment
• Delay repair beyond age 4 in Caucasian children; beyond age 8 in
Afro-Caribbean children.
• Simple sutured closure of defect in surgery required.
Epigastric hernia
Key facts
• Defect in the midline linea alba between the umbilicus and the xiphoid
process.
• Very rarely closes spontaneously.
• Have a low incidence of complications (incarceration/strangulation).
Clinical features Usually noticed as a painless, intermittent swelling above
the umbilicus.
Treatment Simple sutured closure of defect required.
This page intentionally left blank

CHAPTER 12 Paediatric surgery
448
Prepuce (foreskin) and circumcision
Key facts
• One of the commonest reasons for referral to a paediatric surgical
clinic.
• Prepuce (foreskin) is initially fused to the glans penis. Preputial
‘adhesions’ lyse spontaneously as part of normal development.
• Separation of the prepuce from the glans is gradual; 80% of newborns,
50% of 1y-olds, and 10% of 5y-olds will have a non-retractable
prepuce.
Non-retractable foreskin
Clinical features
• Only rarely causes problems which include dysuria, frequency, spots
of blood, ballooning, and spraying.
• Very occasionally causes recurrent balanitis with redness, soreness,
and cellulitis. Preputial ‘cysts’ are often present—these are collections
of subpreputial smegma and are part of normal development.
Treatment
• Often only reassurance and advice are needed.
• Leave the foreskin alone if asymptomatic.
• Frequent bathing and hygiene and gentle attempts at retraction.
• Hydrocortisone 1% topically relieves symptoms and may speed
separation.
• Topical or rarely, oral antibiotics only for recurrent balanitis.
• Persisting symptoms warrant retraction and separation using LA or
under GA.
Phimosis
Defi ned as a non-retractable foreskin with associated scarring that will not
resolve spontaneously.
Clinicopathological features
• May be congenital (uncommon) or acquired (usually age 5+) secondary
to infl ammation.
• Commonest cause balanitis xerotica obliterans (BXO); foreskin looks
pale, thickened, and scarred.
• Additional symptoms to those of a non-retractable foreskin are
retention of urine, paraphimosis, obstruction, and back pressure on the
upper urinary tract. Consider an ultrasound scan and ascending urinary
tract infection, in which case antibiotics are indicated.
Treatment
• Circumcision.
• Dorsal slit of foreskin.
• Preputioplasty (prepuceplasty).
• Non-surgical treatment using Plastibel is occasionally used in neonates.

PREPUCE (FORESKIN) AND CIRCUMCISION
449
How to examine a child’s foreskin
• Try to ensure the boy is happy and relaxed, lying on examination
couch or parental knee.
• Normal foreskin often appears long and ‘redundant’.
• Gently hold tip of prepuce between fi nger tips, lift forward, and
spread wide open. Preputial orifi ce usually demonstrated.
• If retraction attempted, perform gently to show pouting of mucosa.
• Blanching of skin below preputial opening—normal.
• Tight, white contracted preputial orifi ce indicates fi brotic phimosis
(‘muzzling’).

CHAPTER 12 Paediatric surgery
450
Undescended testis
Key facts
• Testicular descent from the fetal abdominal site into the scrotum is
normally complete by birth.
• Absence of a scrotal testis (cryptorchidism) may be due to agenesis
(rare), intra-abdominal arrest, incomplete descent (intracanalicular), or
ectopic descent (inguinal, perineal, crural, penile).
• Incidence 2–4% of newborn boys, falling to 1.5% at 6 months.
• Commoner on the right side.
Clinical features
• Undescended testis can be noted at the post-natal check, by parents,
or by the GP.
• Rarely presents acutely as torsion (tender mass in inguinal region).
• A retractile testis is one that can be brought down into the scrotum
with gentle manipulation, but retracts into the superfi cial inguinal
pouch, either spontaneously or with minor pressure (see Box 12.4).
Diagnosis and investigations
• No investigations are required in palpable undescended testis.
• Chromosomal studies and HCG stimulation test may be requested in
bilateral impalpable testes.
• Ultrasound may help locate an impalpable testis.
• Diagnostic laparoscopy is defi nitive and allows further management.
Treatment
• Testis should be brought to the scrotum at 1–2y of age to avoid
secondary damage due to trauma, torsion, and increased ambient
temperature.
• Hormone manipulation is ineffective in true undescended testis.
• Intracanalicular or ectopic testis should be managed by one-stage
orchidopexy.
• Intra-abdominal testis can be brought down by one- or two-stage
orchidopexy (50–90% success).
• Laparoscopy for bilateral impalpable testes.
• Scrotal position facilitates self-examination to detect signs of neoplastic
change (74 times normal in an abdominal testis).
Complications
• Post-operative atrophy of the testis (<2%) unless intra-abdominal
position (10–50%).
• Retraction.

UNDESCENDED TESTIS
451
Box 12.4 How to exclude retractile testis
• A cooperative, relaxed little boy is essential. Examine on carer’s
knee or lying down.
• Control inguinal canal with fi nger pressure (prevents retraction of
testis).
• Palpate tissues superfi cial to external inguinal ring, working down to
scrotum.
• Try to manipulate testis into scrotum—then release.
• True retractile testis should remain in scrotum briefl y.
• About 95% true retractile testes descend spontaneously before
puberty and require no follow-up (75% apparently retractile testes
become ‘ascending’).
Indications for orchidopexy
• Maximize sperm production.
• Prevent testicular torsion.
• Repair of associated inguinal hernia.
• Cosmesis.
• Reduce chance of malignancy development and improve
self-examination success.

CHAPTER 12 Paediatric surgery
452
Solid tumours of childhood
Neuroblastoma
• Commonest solid abdominal tumour of childhood.
• Spectrum of tumours derived from neuroblasts found in the adrenal
gland, along the sympathetic chain, or extra-adrenal sympathetic
tissues.
• Aggressive tumour with early spread to lymph glands, liver, bone
(cortex or marrow), orbits, and skin.
• Presents as painless large, abdominal mass in children <2y.
• May present as weight loss, hypertension, or metastatic disease.
• Urinary HMMA, HVA elevated.
• CT scan. Optimal investigation for suspected neuroblastoma.
• Treatment by combination of chemotherapy, surgery, and
radiotherapy.
• Survival of between 30 and 90%, depending on the site and stage
at presentation.
Nephroblastoma (Wilms’ tumour) of kidney
• Fast-growing tumour of the kidney.
• Ranges from benign mesoblastic nephroma of infancy to poorly
differentiated, malignant nephroblastoma in the older child.
• Malignant tumours frequently metastasize to regional lymph nodes,
liver, and lungs.
• Usually presents as a large, relatively painless abdominal mass in an
otherwise well child.
• Treatment by combination of chemotherapy, surgery, and radiotherapy
according to histology and spread at diagnosis.
• 5y survival:
Early stage, 90%.•
Disseminated disease, 30%.•
Rhabdomyosarcoma
• Tumour of striated muscle origin from the bladder, vagina, prostate,
parameningeal tissue, and limbs.
• Haematuria, vaginal bleeding, and the appearance of grape-like cysts
(sarcoma botryoides) at the vaginal introitus.
• Variable histology (embryonal is most favourable), which determines
the prognosis.
• Survival of up to 70% from surgery and chemotherapy.
Hepatoblastoma
• This presents as a right hypochondrial mass extending across the
midline.
• Chemotherapy may render initially inoperable tumours resectable.
• Depending on staging, size, and histology, survival of up to 70% is
possible.
This page intentionally left blank

CHAPTER 12 Paediatric surgery
454
Neck swellings
Key facts
• Childhood neck lumps may be due to embryological abnormalities as
well as the same spectrum of conditions in adults (b pp. 222–226).
• Embryological abnormalities may relate to:
Descent of the thyroid from the foramen caecum of the tongue •
l thyroglossal cysts (b p. 222).
Formation of 2nd, 3rd, and 4th branchial arches and clefts •
l branchial cysts (b p. 224).
Formation of lymphatic vessels and veins • l cystic hygroma and
cavernous haemangiomata.
• 2 Lymphadenopathy is very common in children, but typically ‘waxes
and wanes’.
• 3 If lymphadenopathy persists for longer than 2 months and
measures >2cm diameter, it should be biopsied.
Causes and clinicopathological features
Thyroglossal cyst (b p. 222).
Branchial cysts (b p. 224).
Lymphadenopathy
• The neck contains large numbers of lymph glands draining areas of
potential infection in the mouth, nose, fauces, and ears.
• Common causes of lymphadenopathy include upper respiratory tract
infection, middle ear infections, tonsillitis, parotitis, dental abscess,
atypical mycobacterial infection.
• Malignant lymphadenopathy is much less common.
May be primary lymphoma.•
Secondary deposits, e.g. from neuroblastoma.•
Salivary gland swellings
• May be due to duct obstruction (stones or duct stenosis), infection
(mumps), autoimmune disorders (recurrent parotitis), neoplasia
(adenoma).
• Commonest in the submandibular, sublingual, and parotid glands.
Skin lesions
• Dermoid cysts. Usually in the midline above the hyoid bone and are
rarely infected.
• Sebaceous cysts. Epidermal origin with a small central punctum; may
occur anywhere, but most commonly on the scalp or back of the neck.
Lymphovascular lesions
• Haemangiomas. Can be mixed capillary or cavernous haemangiomas or
haemangioendotheliomas within the neck and parotid area; may grow
rapidly in size and lead to high output cardiac failure or even carotid
steal syndrome.
• Cystic hygroma (lymphangioma). Commonly in the posterior triangle of
the neck.
NECK SWELLINGS
455
Treatment
• Excision of dermoid cysts, sebaceous cysts, thyroglossal cysts, thyroid
neoplasms, salivary gland enlargements, lymph gland enlargements
(biopsy).
• Haemangioma. Supportive measures (intubation, steroids, interferon,
and emergency surgical intervention).
• Cystic hygroma. Sclerosant injection (OK 432—streptococcal
derivative), effective in lymphangiomas with few large cysts.
Differential diagnosis of neck lump
Neck lumps may be lateral or midline.
Lateral
• Lymph node
• Branchial sinuses and cyst
• Cystic hygroma
• Sternomastoid tumour
• Haemangioma
• Lymphangioma
• Submandibular gland
• Parotid gland
• Neoplasm
Midline
• Submental lymph nodes
• Thyroglossal cyst
• Thyroid swelling
• Dermoid cyst
Neck swellings by cause
Congenital
• Thyroglossal cysts
• Branchial cyst
• Cystic hygroma
• Haemangioma
• Dermoid cyst
Acquired
• Reactive lymphadenopathy
• Infective lymphadenopathy
• Secondary tumour deposits
Anatomy related to neck lump surgery
• Incisions should be parallel with skin creases (Langer’s lines).
• Subcutaneous closure should be meticulous (e.g. removable 4/0,
5/0, or 6/0 continuous subcuticular monofi lament).
• Facial nerve. Passes between the two lobes of parotid gland.
• Lingual nerve. Swerves around submandibular duct.
• Thoracic duct. Enters junction of left subclavian and jugular veins.
This page intentionally left blank

457
Paediatric orthopaedic
Developmental dysplasia of the hip (DDH) 458
Slipped upper femoral epiphysis (SUFE) 460
The limping child 462
The child with a fracture 464
Non-accidental injury (NAI) 466
Legg–Calvé–Perthes disease 468
Motor development 470
Club foot or congenital talipes equinovarus (CTEV) 471
Flat feet (pes planus) 472
The osteochondritides 474
Chapter 13

CHAPTER 13 Paediatric orthopaedic
458
Developmental dysplasia of
the hip (DDH)
DDH covers a spectrum of abnormalities from a mildly underdeveloped
stable hip to well-established dislocation and/or acetabular dysplasia in
the older child. The incidence is 1–2 per 1000 newborns in the UK. It is
bilateral in 20%. It was previously called congenital dysplasia of the hip
(CDH), but as it may also be acquired after birth, the term was changed.
Early detection is important.
Risk factors
• Family history. First-degree relative.
• Breech presentation at or after 36 weeks of gestation.
• Foot abnormalities. Congenital talipes calcaneovalgus and metatarsus
adductus.
• Oligohydramnios.
• Children with syndromes.
• Torticollis.
Clinical features
• Barlow test (dislocatable hip). It tests if a hip is unstable. Take the baby’s
leg between your thumb and index fi nger and place your other fi ngers
onto the buttock. Flex and adduct the hip. Posterior-directed force
is applied in line with the shaft of the femur. In case of instability, the
femoral head then subluxes or dislocates which you can palpate in the
buttock.
• Ortolani Sign (dislocated hip). Now abduct the leg. If the hip is
dislocated and reducible, you will feel the femoral head moving into
the joint. This often does not produce a palpable clunk.
• Irreducible hip.
Shortened leg.•
Limited hip abduction.•
Asymmetry of skin creases can be present; many children with •
normal hips have asymmetry of thigh and buttock skin creases.
Abnormal fi ndings can prove easy to miss if the dislocations are bilateral.
Screening
All children with risk factors and an abnormal neonatal clinical hip exami-
nation have a hip ultrasound. The majority of hips are fully developed at
full term plus 6 weeks. Therefore, the ultrasounds of those children with
risk factors, but normal clinical fi ndings, are done at that point. Those
children with obvious clinical abnormalities on hip examination have the
ultrasound done earlier.
Paton RW et al. reported in 2009 that there is no association between
postural and fi xed talipes equinovarus and DDH.
1
Since it can be diffi cult
for non-paediatric orthopaedic surgeons to differentiate the different foot
abnormalities, it is continued to scan the hips of children with club feet.
Evidence shows that there is no advantage of universal baby hip screening
over selective at risk screening.

DEVELOPMENTAL DYSPLASIA OF THE HIP (DDH)
459
Radiology
• Ultrasound is the investigation of choice. Baby hip ultrasound
was pioneered by Dr Graf from Austria. It is more accurate than
radiographs. It visualizes the cartilage and allows dynamic testing of the
hip joint. A Graf alpha angle of t60° is classed as normal.
• Radiographs are taken in children who present late, usually after the
age of 6 months.
Treatment
• 0–6 months of age. A Pavlik harness is applied. This is a soft harness
which fl exes the hips and knees and directs the legs away from
the body midline, thereby directing the femoral heads towards the
hip joints. It allows limited hip movements. It is used until the hips
normalize, which can take several months; it works 90% of times.
• 6–18 months. If the harness is unsuccessful or if a child is older than
6 months, they need a closed or open reduction of the hip joint and
hip spica cast immobilization. Some children need a hip adductor
tendon release in the groin and occasionally, a femoral osteotomy. A
removable hip abduction brace is used after spica removal.
• t18 months. These children usually need an open reduction of the hip
joint, a hip adductor release, and a femoral ± pelvic osteotomy and hip
spica immobilization. A hip abduction brace is normally not necessary
after hip spica removal because of the improved bone alignment.
Reference
1 Paton RW, Srinivasan MS, Shah B, et al. (1999). Ultrasound screening for hips at risk in develop-
mental dysplasia: is it worth it? J Bone Joint Surg Br. 81: 255–8.

CHAPTER 13 Paediatric orthopaedic
460
Slipped upper femoral epiphysis (SUFE)
This is reported to have an incidence of 4–7 per 100 000 population. It
is caused by the displacement of the femoral epiphysis (growth plate) in
relation to the femoral neck. It is the commonest cause of a limp in a boy
aged 12–14 or girl aged 11–13 (growth spurt at puberty). In this age group,
it must be actively excluded in a limping child.
Risk factors
• Obesity. Classic is the limping, obese, 13y-old boy with knee pain.
• Rapid growth.
• Hormone disturbances. Hypothyroidism, renal rickets, pituitary
defi ciency, growth hormone defi ciency, and treatment with it).
• Male—♂:♀, 3:1.
• Side affected. Left > right.
Classifi cation
This is usually classifi ed by the ability to weight bear on presentation.
• ‘Unstable’ slips cannot walk due to pain and present like a fracture.
• Diagnosis is easy.
• ‘Stable’ slips can weight bear, though usually with a limp.
• Presentation is usually late, i.e. after 2–3 weeks of limping.
• Fifty per cent will have no pain; pain is commonly referred to the knee.
2 Any child with knee pain must have there hip examined.
A summary of presentation and prognosis can be seen below.
Clinical features
• There will be an obvious limp, usually in an overweight child,
commonly male.
• The affected limb will be shorter and lies in external rotation.
• Abduction is limited; when the hip is fl exed, it will rotate externally—
this sign is almost diagnostic of the condition.
Radiology
Anteroposterior and lateral views of BOTH hips should be insisted on. The
slip is often easier to see on the lateral view. The slip is in an inferior and
posterior direction (down and backwards). Widening of the physis may be
a sign of impending slip (i.e. ‘pre-slip’).
• Klein’s line giving Trethowan’s sign. If you draw a line on the superior
aspect of the femoral neck (called), it should cut through the femoral
head; if it does not, it is diagnostic of a SUFE (see Fig. 13.1).
Management
The acute slip (i.e. <3 weeks history) can be managed by gentle manipula-
tion and cannulated screw fi xation in the reduced position. This is contro-
versial, however, as any manipulation may damage the already damaged
epiphysis and possibly cause avascular necrosis.
Standard treatment is stopping the slip from worsening and the slip is
usually pinned in situ (where it is with no manipulation) with one cannu-
lated screw percutaneously.
SLIPPED UPPER FEMORAL EPIPHYSIS (SUFE)
461
Deformed epiphysis can remodel by up to 60*. Residual deformity can
be corrected via an osteotomy once the epiphysis has fused if there is a
functional defi cit.
The condition can be bilateral in some cases. This is much more likely if
there is an underlying endocrine disorder. Some surgeons advocate pro-
phylactic pinning of the unaffected side, but this is controversial.
Complications
• Avascular necrosis of femoral head.
• Chondrolysis. Rapid progressive loss of cartilage; joint space narrowing
is seen on X-ray.
• Subtrochanteric fracture. If pins entry point placed too low.
• Late osteoarthritis. Estimated 10%.
Further reading
Aronsson D, Loder RT, Breur GJ, Weinstein SL (2006). Slipped capital femoral epiphysis: current
concepts. J Am Acad Orthop Surg 14: 666–79.
Fig. 13.1 Normal (left) and Abnormal, i.e. SUFE (right) Klein’s lines.

CHAPTER 13 Paediatric orthopaedic
462
The limping child
The limping child is the acute abdomen of children’s orthopaedics. It can
be caused by many things, ranging from a stone in the shoe to leukaemia.
History
The most important initial question is, ‘Has the child always limped?’ If so,
the most common causes are:
• DDH (see b p. 458).
• Cerebral palsy.
• Limb length discrepancy from congenital disorders.
• Muscular dystrophy.
If there has been a normal gait and then the child starts to limp, then the
possibilities are highly varied. The main points to glean are:
• How long, what side, and what causes it? Is it constant or intermittent?
• Are there any associated complaints?
• Is there a history of trauma?
• How is the child’s general health?
• What is the child’s past medical and birth history?
Remember, what causes an older child to limp will cause a younger child
to refuse to weight bear. This will be perceived as more serious by the
parents and therefore, these patients will present much earlier.
Examination
• Look at the child walking. Decide on whether there is a limp! This is
often a symptom used by parents (and grandparents) to make sure
that you take their complaint seriously and a limp may not be the
problem at all.
• What type of limp is it?
Short leg• . Limb length discrepancy.
Antalgic• . Less time on affected than unaffected limb due to pain.
Trendelenberg• . Pelvis dips and trunk sways on affected side due to
weak hip abductors.
Bizarre• . Usually psychological, e.g. completely stiff leg.
• Tailor examination to history. Inspect limbs from hips to toes. Skin
discoloration may indicative of infection. Try to use distraction
techniques to get the true picture.
• Tenderness? Bony tenderness with swelling and bruising is a clinical
fracture. Infected areas will be painful and usually red, hot, or swollen.
• Joints. Concentrate joint examination as appropriate, but always
examine hips if knee pain present. Put all joints through full passive
range of motion.
• Limb lengths? Assess individual leg lengths with patient lying on the
couch with legs straight. Look for level of heels and malleoli. If one leg
is short, assess if discrepancy is within femur or tibia by fl exing both
hips and knees together. Look at the legs from the side to see if the
front of the lower legs is at different levels (if yes—femoral shortening)
and/or if the front of the thighs are at different levels (if yes—lower leg
shortening).

THE LIMPING CHILD
463
• Lymphadenopathy? From generalized infection, local infection, or blood
disorder.
• Full systemic examination must also be performed.
Remember common things are common! The age of the patient is a very
useful screen (see Table 13.1).
Investigations
• X-ray. Always X-ray hips if no localizing signs. If one limb appears to
be involved, especially in the younger patient, then X-ray the whole
limb. Otherwise, X-ray bones/joints as appropriate from the clinical
examination.
• Blood tests. FBC, U&E, G, LFTs, CRP, ESR useful as full general screen.
Management Dependent on diagnosis reached.
Further reading
Sawyer JR, Kapoor M (2009). The limping child: a systematic approach to diagnosis. Am Fam Physician
79(3): 215–24. Available at: M http://www.aafp.org/afp/2009/0201/p215.html
Table 13.1 Screening using age of the patient
Young
(0–5y)
Child
(5–10y)
Adolescent
(10–15y)
All ages
DDH Perthes’ disease SUFE Septic arthritis
Septic hip Irritable hip AVN of
femoral head
Cellulitis
Limb length
discrepancy
Juvenile
rheumatoid
arthritis
Overuse
syndromes
Stress fracture
Occult fracture Non-accidental
injury (NAI)
Neoplasia
Neuromuscular
disease

CHAPTER 13 Paediatric orthopaedic
464
The child with a fracture
Accidents are part of normal life! Some, usually the more severe, result in
a fracture. In a city of 1 million inhabitants, there would be around 3000
children’s fractures annually.
Most childhood fractures are minor, almost half affecting the forearm.
They are weather-dependent, increasing in good weather when climbing
(and falling) is common.
Clinical features
• A clear history of trauma and a complaint of pain are present in all,
but a small number of patients. In a young child, however, the pain
may not be localized and instead of crying, they may present with
pseudoparalysis of a limb, limp, or refusal to weight bear.
• Look for external signs of injury. Bruising, due to a thick subcuticular fat
layer is not a constant feature. Palpate for tenderness unless there is a
clear deformity. Remember to examine the normal limb fi rst and when
examining the affected side, start away from the injury. This will gain
the child’s confi dence. If you’re going to do something that hurts them,
tell them rather than saying, ‘This won’t hurt’. If you lie to them, you
will lose their trust and make examination impossible.
• Neurovascular status. Check the distal capillary refi ll and pulses as well
as distal neurology in the form of power and sensation. Document all
fi ndings. It is good practice and also helps if there are any medicolegal
issues about treatment.
• Investigation. Though a fracture is a clinical diagnosis, this can
sometimes be diffi cult in the child. A low threshold for X-rays should
always be adopted. The joint above and below any injury must be
visualized with two perpendicular views being standard practice.
• Analgesia. Always splint an obvious fracture and give analgesia before
X-rays. If a limb is clearly deformed (hence fractured), then a plaster
should be applied before the X-ray. Radiographs of deformed limbs
out of splintage are unacceptable. This is also the case for adults!
• Unnecessary X-rays. Try to avoid ‘comparison’ views of the normal
side if you are unsure about radiological features. With growing bones,
ossifi cation centres and epiphysis interpretation can be diffi cult. If you
are unsure about a feature, show the X-ray to a senior.
• Fractures. The classical fracture in a child is the greenstick injury. This is
when there is a fracture of one cortex and a plastic deformation (i.e. a
bend, not break) of the other at the same level. Even complete
fractures tend to displace far less in children as the periosteum in
children is highly structural, thick, and strong. It will hold the fracture
in rough approximation. In adults, the periosteum is a very weak
component of bone, hence the wider displacement.
Management
• Fractures usually unite. If there is a minor greenstick fracture with little
symptoms and no risk of displacement, then there is no need to apply
a plaster cast. However, this is not normally acceptable to parents
once they know their child has a broken limb.

THE CHILD WITH A FRACTURE
465
• In undisplaced, stable, greenstick fractures of the forearm, a fi breglass
removable cast can be placed and the parents simply remove this in 4
weeks time with no need to be followed up in a clinic.
• Normally the joint above and below the fracture needs to be
immobilized. Often this is safer in children to ‘slow them down’
anyway. The fracture should be monitored in the fracture clinic. If a
plaster is placed, then clear plaster instructions, in the form of a
printed sheet, should be given to the parents. An example is shown
Box 13.1.
Box 13.1 Plaster instructions
Return to the Accident and Emergency Department or Plaster room if
your child complains of any of the following:
• Numbness or pins and needles in the affected limb.
• Restricted movement or tight swelling of the fi ngers or toes.
• If the fi ngers or toes go white or blue.
• If there is local itching, pain, or burning in the plaster.
• A string foul smell is coming from the plaster.
• The plaster becomes soft or loose.
Also, if the plaster is rubbing or digging in, this will irritate the skin and
cause blistering.
• Do NOT place anything down the plaster if it is itching. This will
cause skin damage or blisters and may cause an infection.
• Do NOT get the plaster wet.
• If the fracture is displaced or angulated, then it may require
manipulation or, less commonly, open reduction and fi xation under
GA.
• Angulation, if close to the joint and in the plane of motion of the joint,
is very well tolerated. Remodelling of the bone will occur; a 15*
angulation in a child <6y old and 10* angulation in a child 6–10y old is
acceptable orthopaedically, though is often not accepted by the
parents! Rotational displacement will not remodel and needs
correction.

CHAPTER 13 Paediatric orthopaedic
466
Non-accidental injury (NAI)
Defi nition
Physical violence towards a baby or child. It is one part of child abuse
which may occur in isolation or in combination with other forms of child
abuse, including neglect, emotional abuse and/or sexual abuse. It denotes
an injury that cannot be explained by an accident and where responsible
adults do not have a viable explanation of how the injury occurred.
Incidence
• The National Society for the Prevention of Cruelty to Children
(NSPCC) reported in 2011 that 46 700 children were at risk of abuse
in the UK.
• It is estimated that 4 million children a year are abused in some
manner in the USA.
• It happens in all socio-economic classes, but is more common in the
deprived. Twins, preterm babies, and special needs children are also at
increased risk.
• The majority of NAI fractures occur under 2y of age (80% of children
with NAI fractures are under 18 months of age). The majority of
accidental injuries occur in children over 5y of age (85%).
Suspicious features
• Inexplicable delay from time of injury to medical advice being sought.
• No convincing explanation of mechanism of injury.
• Patterns of soft tissue injury, e.g. bruising in the shape of fi ngers or
objects (belt, buckles) or in unusual areas (back, away from bony
prominences), bites, and burns.
• Unusual fractures or fracture patterns, e.g. rib fractures, non-linear
skull fractures, transverse fractures of long bones, scapula, lateral
clavicle, and vertebral fractures.
• Fractures of differing ages on a radiograph.
• No fracture in isolation is pathognomonic of NAI.
• Rib, humeral, femoral, and skull fractures have the highest probability
for abuse. Rib fractures are present in 5–27% of children who are
abused. Diaphyseal fractures are four times more common than
metaphyseal fractures in NAI.
• Metaphyseal corner and bucket handle fractures. They are only
present in a minority of children who present with NAI (the reported
rates vary widely, 11–50%). Many references refer to them as
pathognomonic or specifi c for NAI. However, a similar appearance can
occur with other conditions (e.g. severe osteogenesis imperfecta,
rickets, scurvy).
Corner fracture. A small piece of bone is avulsed due to shearing •
forces on the growth plate.
Bucket handle fracture (same as corner fracture). The fragment is •
larger and seen face on as a disc or bucket handle.
• Two or more fractures are present in 66–74% of abused children, but
only in 16% of non-abused children.

NON-ACCIDENTAL INJURY (NAI)
467
• Periosteal reactions are common features in NAI; a very strong grip
may cause such a reaction.
• Always keep NAI in mind when dealing with any child with trauma.
Examination
• A thorough history (this should be witnessed) and total body
inspection and examination from head to toes is mandatory. Enquire
about a history of fractures and deafness and look for blue sclera
(osteogenesis imperfecta). Examination of genitalia should only be
done by experts.
• Note how the child interacts with the carer/parent.
• Good communication and contemporaneous medical note writing is
essential since every case of child abuse will be submitted to court.
Management
• Contact consultant in charge, departmental child protection lead, and/
or hospital child protection team (paediatric consultant on call, child
protection practitioner).
• Hospital admission if NAI is suspected, even if the medical condition
does not require it.
• Skeletal survey (skull, chest, abdomen, upper and lower limbs). This
will be organized by paediatric team if NAI is suspected.
• Blood tests (FBC, clotting screen, bone profi le, calcium, phosphate,
alkaline phosphatase, copper, caeruloplasmin, magnesium, fasting
25-hydroxyvitamin D, and parathyroid hormone).
• Every hospital has a policy dealing with safeguarding of children.
Differential diagnoses
• Osteogenesis imperfecta.
• Haemophilia.
• Birth trauma.
• Rickets.
• Leukaemia.
• Scurvy (mimics NAI).

CHAPTER 13 Paediatric orthopaedic
468
Legg–Calvé–Perthes disease
Defi nition
Idiopathic avascular necrosis of the proximal femoral epiphysis, fi rst
described independently by Legg, Calvé, and Perthes. The cause is
unknown. The epiphyseal changes can lead to permanent deformity and
osteoarthritis in adult life. The diagnosis is made on X-rays.
Key facts
• Age of onset most commonly between 4 and 10y of age. If both hips
look to be at the same stage, consider multiple epiphyseal dysplasia.
• ♂:♀, 5:1.
• 10–12% bilateral; the disease is usually at different stages.
• Females have poorer prognosis.
• Early onset up to age of 7y; late onset from age of 8y. The overall
prognosis is better in the early group.
Pathology/radiology
• Waldenström described four radiographic stages—ischaemia,
fragmentation, reossifi cation, remodelling.
Ischaemia.• Compromise of blood supply of the femoral head. The
articular cartilage still grows as it is nourished by the joint fl uid,
resulting in increased joint space and apparent mild joint subluxation
on X-ray (Waldenström’s sign); the head ceases to enlarge.
Fragmentation/resorption.• New bone is laid down on the dead
trabeculae, causing increased bone density. Subchondral fractures
may occur, causing a black subchondral line (crescent sign). The
hyperaemia and revascularization causes bone lysis and rarefi cation,
giving a fragmented appearance on the X-rays.
Reossifi cation (healing phase).• The head is plastic and if it is not
concentrically contained within the acetabulum, it will become
deformed.
Remodelling.• The plasticity is lost and the femoral head shape will
remain. The normal internal architecture will return, but inside an
altered shape if this has occurred. Deformity will lead to arthritis.
• With non-operative treatment, revascularization and reossifi cation
takes 2–3y to complete in most cases.
• Herring’s lateral pillar classifi cation. Now generally used at
presentation (Catterall in the past). Groups A, B, C; a B/C group
added later.
Group A• . The lateral column of the proximal femoral epiphysis is of
normal height.
Group B• . The height is reduced, but >50% of the height on the
other side.
Group C• . The height is reduced to <50% compared to the other
side.
Group B/C• . Lateral pillar is narrowed (2–3mm) or poorly ossifi ed
with approximately 50% height.

LEGG–CALVÉ–PERTHES DISEASE
469
• Stulberg classifi cation. Describes the end stage of the disease at
skeletal maturity.
I. Normal spherical femoral head.•
II. Round femoral head and fi tting within 2mm of a circle on both •
anteroposterior and lateral radiographs.
III. Out of round by >2mm on either radiograph.•
IV. Flat head and matching fl at acetabulum (aspherical congruency).•
V. Flat head with non-matching acetabulum (aspherical •
incongruency).
• Catterall classifi cation (grades 1–4; 1, 25% head involvement; 2, 50%; 3,
75%; 4, >75%). Superseded by lateral pillar classifi cation. He described
fi ve head-at-risk signs (Gage’s sign—V-shaped lucency at lateral
epiphysis, horizontal growth plate, lateral calcifi cation, subluxation,
metaphyseal cystic changes). Foster reported poor reliability for
head-at-risk signs.
Clinical features
• Painless limp is common.
• There may be pain in groin, inner thigh, and/or or only in the knee.
• Again, every child with knee pain must have their hip examined.
• Reduced hip abduction and internal rotation might be examined.
Management
• Overall very controversial.
• Annamalai et al. (2007)
1
showed a great deal of variability in the UK in
the decision-making process and treatment.
• Non-operative symptomatic relief for the majority of patients.
Physiotherapy/exercises; observation and serial radiographs.
• Largest multicentre centre conducted in America by Herring et al.
2,3
comparing non-operative management with operative management (either
femoral varus or pelvic osteotomy) for early and late onset groups.
Early onset group. No difference in outcome between •
non-operative management, femoral varus, or pelvic osteotomy.
Late onset group. Improved outcome for lateral pillar groups B and •
B/C with either femoral varus or pelvic osteotomy over
non-operative group; no difference for groups A and C.
• Containment surgery has been advocated by others when the femoral
head extrudes from the acetabulum irrespective of age (the femoral
head is maintained within the depth of the acetabulum with femoral
osteotomy, pelvic osteotomy, or both combined).
Bracing is not used by the majority of paediatric orthopaedic surgeons as
part of management of Legg–Calvé–Perthes disease.
References
1 Annamalai et al. (2007). Perthes disease: a survey of management amongst members of the
British Society for Children’s Orthopaedic Surgery (BSCOS). J Child Orthop 1(2): 107–13.
2 Herring JA, Kim HT, Browne R. Legg-Calvé-Perthes Disease. (2004). Part I: Classifi cation of
radiographs with the use of the modifi ed lateral pillar and Stulberg classifi cations. J Bone Joint
Surg Am 86-A: 2103–20.
3 Herring JA, Kim HT, Browne R. Legg-Calvé-Perthes Disease. (2004). Part II: Prospective multi-
center study of the effect of treatment on outcome. J Bone Joint Surg Am 86-A: 2121–34.
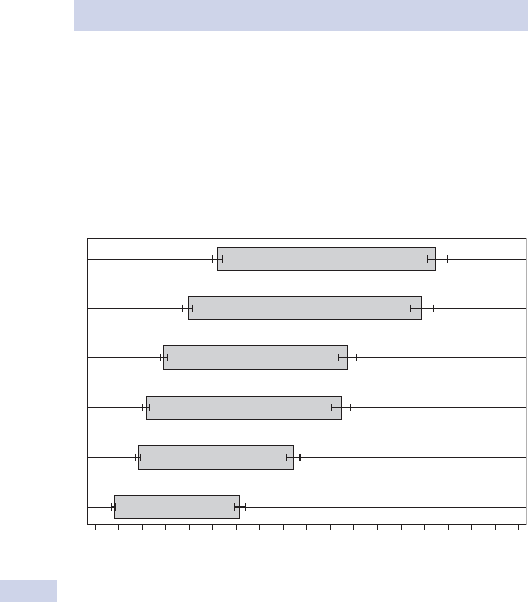
CHAPTER 13 Paediatric orthopaedic
470
Motor development
Most children develop at roughly the same pace. Development is eas-
ier to understand if you realize that it will spread from head to feet, i.e.
cephalocaudal.
Maturation of the nervous system occurs so that a child is able to do
things more distally, the older they become. Fig. 13.2 summarizes this with
some common ‘milestones’ of development.
Motor milestone
3456789101112
walking alone
Standing alone
Walking with assistance
Hands & knees crawling
Standing with assistance
Sitting without support
Age in months
13 14 15 16 17 18 19 20 21
Fig. 13.2 WHO Multicentre Growth Reference Study Group (2006). WHO
motor development study: windows of achievement for six gross motor
development milestones. Acta Paediatrica Supplement 450: 86–95.

CLUB FOOT OR CONGENITAL TALIPES EQUINOVARUS (CTEV)
471
Club foot or congenital talipes
equinovarus (CTEV)
Club foot is a congenital condition that presents at birth and may be iso-
lated or part of a more widespread congenital disorder. Its incidence is
approximately 1 in 1000 live births in the UK with a ♂:♀ ratio of 2:1. The
cause is unknown.
It occurs with other neuromuscular disorders such as:
• Arthrogryphosis.
• Myotonic muscular dystrophy.
• Myelomeningocele and other spinal dysraphisms (spina bifi da).
• Cerebral palsy.
2 Always perform a thorough examination of the hip and back to rule out
other disorders/syndromes that might be associated with a club foot.
Clinical features
The hindfoot is plantar fl exed (equinus) and in varus. There is midfoot and
forefoot cavus and forefoot adduction. The forefoot looks supinated, but
is actually pronated in relation to the midfoot. Overall, the foot is turned
and twisted inwards so that the sole is facing towards the midline and
backwards. In the true clubfoot deformity, the deformity is fi xed to a vary-
ing degree. In the positional clubfoot deformity, the deformity is passively
correctable and usually does not require any treatment.
Management
The cornerstone of treatment is to create a supple foot that is
plantigrade that will allow the child to function well.
Treatment has been revolutionalized by the use of the Ponseti method
of casting which can be performed in the outpatients. This is often run by
specialist nurses/physiotherapy practitioners who work closely with the
paediatric orthopaedic team.
Serial casts are applied that stretch the contracted tissues back to nor-
mal in a defi ned order. First, you correct the adduction and cavus, which
also corrects the hindfoot varus and fi nally, the equines. About 80% of
clubfeet need a percutaneous Achilles tendon tenotomy to correct the
equinus.
The initial casting period goes over about 6 weeks with weekly cast
changes. At the end of this period, the Achilles tenotomy is performed if
necessary, followed by a further 3 weeks in cast. Thereafter, the feet are
immobilized 23h/day with boots on a bar for 3 months and then only dur-
ing the night until the age of 4–5y. Some children require a tibialis anterior
tendon transfer when they are about 4–5y old because of a dynamic supi-
nation deformity. Recurrences are usually the result of non-compliance
or if the child has a syndromic clubfoot.

CHAPTER 13 Paediatric orthopaedic
472
Flat feet (pes planus)
There are always one or two children in every orthopaedic paedi-
atric new patient clinic with fl at feet. The children rarely complain of
symptoms and the appearance and referral is often sparked by the
parents.
Clinical features
Like all paediatric consultations, take an accurate neonatal, birth, and fam-
ily history to look for associated problems. The cornerstone is whether
the foot deformity is fl exible (vast majority) or rigid.
2 Flexible fl at feet correct fully once the big toe is extended and the arch
reforms and when the child stands on tip toes.
Ensure you examine the ankle and Achilles tendon as this often causes
a ‘compensatory’ fl at foot due to the stiffness and is easily remedied by
physiotherapy.
Natural history
The majority of children form normal foot arches by the age of about 3y.
In some, the arches never form and they remain fl atfooted.
Management
Flexible fl at feet generally need simple reassurance and the patient can be
discharged. Some children with more severe fl at feet benefi t at times from
the use of insoles since insoles improve the mechanical leg alignment. This
applies, for example, to children who present with knee pain and moder-
ate to severe fl at feet where the feet are in a pronated position when
standing. Tight Achilles tendons need physiotherapy.
Rigid fl at feet are associated with an underlying abnormality such as:
• Congenital vertical talus (from birth).
• Tarsal coalition. Fusing of some part of the tarsal bones via scar or
bone (often adolescents).
• Infl ammatory joint disease.
Treatment is centred on the underlying disorder, i.e. tarsal coalition
is often treated by offl oading the area with orthotics and if this fails,
attempts can be made to surgically resect the fi brous or bony coalition.
This page intentionally left blank

CHAPTER 13 Paediatric orthopaedic
474
The osteochondritides
These conditions result in avascular necrosis of epiphyseal bone, similar to
Legg–Calvé–Perthes disease, but less common. In general, they are trou-
blesome rather than serious. Some are self-limiting.
Features
Most lead to aching and muscle spasm. Radiographic changes occur
in the affected epiphysis, with varying degrees of density change and
fragmentation.
Epiphyses commonly affected are:
• Lateral condyle of the humerus (Panner’s disease).
• Carpal lunate (Kienbock).
• Carpal scaphoid (Preiser).
• Head of metatarsal (Freiberg).
• Tarsal navicular (Kohler).
• Patella (Larsen–Johanssen).
• Vertebral epiphyseal plates (Scheuermann).
• Vertebral body (Calvé).
Management
Usually symptomatic with limitation of activities and anti-infl ammatory
medication.
Traction apophysitis
An apophysis is a traction epiphysis which may undergo partial avulsion
with avascular change followed by subsequent repair. These changes can
be the result of trauma, overuse, or rapid growth. The commonest are:
• Osgood–Schlatter’s disease. Apophysitis of the tibial tubercle into which
the patellar tendon inserts.
• Sever’s disease. Apophysitis of the apophysis at the posterior aspect of
the calcaneum where the Achilles tendon inserts.
Clinical features
The patient is usually an adolescent who presents with aching, swelling,
and/or pain.
Radiology
Radiographs show fragmentation of the tibial tuberosity/calcaneal
apophysis.
Treatment
Explanation of the condition; limitation of activities; anti-infl ammatory
medication. Plaster immobilization for 4–6 weeks can be helpful in severe
cases. Temporary use of a cushioned heel support for Sever’s disease can
be helpful.

THE OSTEOCHONDRITIDES
475
Osteochondritis dissecans of the knee
• Avascular necrosis of the subchondral bone, resulting in softening of
the articular cartilage and bone that may become loose and separated
from the rest of the femoral condyle. The cause is unknown, but it is
thought that it might be the result of repetitive minor trauma.
• Most lesions are located on the lateral side of the medial femoral
condyle, but any joint can be affected.
• Children between 5 and 15y of age are most commonly affected with
the majority occurring in teenage boys.
Clinical features
• Non-specifi c knee pain.
• If the fragment has become loose, the patient will report crepitance,
popping, giving way, and/or locking.
Natural history
In children and adolescents, spontaneous healing over about 18 months is
the usual outcome. Patients with more advanced lesions have an increased
risk to develop early osteoarthritis.
Investigations
• Radiographs. The tunnel view will show the lesion the best.
• MRI. The scan shows extent of the lesion and, if there is detachment,
with fl uid interposition between fragment and underlying bone.
Management
• Activity restriction for the majority of cases.
• Unstable lesions require arthroscopic stabilization.
This page intentionally left blank

CHAPTER 14 Major trauma
478
Management of major trauma
Key facts
• Trauma is the leading cause of death in the fi rst four decades of life,
but three people are permanently disabled for every one killed.
• Death from injury occurs in one of three time periods (trimodal).
First peak• . Within seconds to minutes. Very few can be saved due
to severity of their injuries.
Second peak• . Within minutes to several hours. Deaths occur due to
life-threatening injuries.
Third peak• . After several hours to weeks. Deaths from sepsis and
multiple organ failure.
• The ‘golden hour’ refers to the period when medical care can make
the maximum impact on death and disability. It implies the urgency and
not a fi xed time period of 60min.
The advanced trauma life support (ATLS) system
• Accepted as a standard for trauma care during the ‘golden hour’ and
focuses on the ‘second peak’.
• Emphasizes that injury kills in certain reproducible time frames in a
common sequence: loss of airway, inability to breathe, loss of
circulating blood volume, expanding intracranial mass.
• The primary survey (ABCDEs) with simultaneous resuscitation is
emphasized.
Prehospital care and the trauma team
• Effort is made to minimize scene time, emphasizing immediate
transport to the closest appropriate facility (scoop and run).
• Hospital is informed of the impending arrival of the casualty.
• Trauma team usually comprises an anaesthetist, ‘general’ surgeon,
orthopaedic surgeon, and A&E specialist, A&E nurses, and
radiographers.
• Information from paramedics should include Mechanism of injury, Injuries
identifi ed, vital Signs at scene, and any Treatment administered (MIST).
• Triage is the process of prioritizing patients according to treatment
needs and the available resources (those with life-threatening
conditions and with the greatest chance of survival are treated fi rst).
Management
Primary survey
Identify and treat life-threatening conditions according to priority (ABCDE).
Airway maintenance with cervical spine protection
• Protect spinal cord with immobilization devices or using manual in-line
immobilization. Protect until cervical spine injury is excluded.
• Access airway for patency. If patient can speak, airway is not
immediately threatened.
• Consider foreign body and facial, mandibular, or tracheal/laryngeal
fractures if unconscious. Perform chin lift/jaw thrust. Consider
nasopharyngeal/oropharyngeal airway.

MANAGEMENT OF MAJOR TRAUMA
479
• If patient unable to maintain airway integrity, secure a defi nitive airway
(orotracheal, nasotracheal, cricothyroidotomy).
Breathing and ventilation
• Administer high-fl ow O
2
using a non-rebreathing reservoir.
• Inspect for chest wall expansion, symmetry, respiratory rate, and
wounds. Percuss and auscultate chest. Look for tracheal deviation,
surgical emphysema.
• Identify and treat life-threatening conditions: tension pneumothorax,
open pneumothorax, fl ail chest with pulmonary contusion, massive
haemothorax.
Circulation with haemorrhage control
• Look for signs of shock.
• Hypotension is usually due to blood loss. Think: chest, abdomen,
retroperitoneum, muscle compartment, open fractures (‘blood on the
fl oor and four more’).
• Control external bleeding with pressure.
• Obtain IV access using two 12G cannulae. Send blood for cross-match,
FBC, clotting, U&E.
• Commence bolus of warmed Ringer’s lactate solution; unmatched,
type-specifi c blood only for immediate life-threatening blood loss.
• Consider surgical control of haemorrhage (laparotomy, thoracotomy).
Disability
• Perform a rapid neurological evaluation. AVPU method (Alert,
responds to Vocal stimuli, responds only to Painful stimuli,
Unresponsive to all stimuli), Glasgow coma scale (GCS).
• After excluding hypoxia and hypovoleamia, consider changes in level of
consciousness to be due to head injury.
Exposure/environment control
• Undress patient for through examination.
• Prevent hypothermia by covering with warm blankets/warming device.
Use warm IV fl uids.
Adjuncts to primary survey
• Monitoring. Pulse, non-invasive BP, ECG, pulse oximetry.
• Urinary catheter (after ruling out urethral injury).
• Diagnostic studies. X-rays (lateral cervical spine, AP chest, and AP
pelvis), ultrasound scan, CT scan, diagnostic peritoneal lavage.
Secondary survey
Begin only after primary survey is complete and resuscitation is continuing
successfully.
• Take history. AMPLE (Allergy, Medication, Past medical history, Last
meal, Events of the incident).
• Perform a head-to-toe physical examination.
• Continue reassessment of all vital signs.
• Perform specialized diagnostic tests that may be required.

CHAPTER 14 Major trauma
480
Thoracic injuries
Key features
• Thoracic injuries account for 25% of deaths from trauma.
• Fifty per cent of patients who die from multiple injuries also have a
signifi cant thoracic injury.
• Open injuries are caused by penetrating trauma from knives or
gunshots. Closed injuries occur after blasts, blunt trauma, and
deceleration. (Road traffi c accidents (RTAs) are the most common
cause.)
Management—primary survey
Identify and treat major thoracic life-threatening injuries.
Tension pneumothorax
• A clinical diagnosis. There is no time for X-rays.
• Patient has respiratory distress, is tachycardic and hypotensive.
• Look for tracheal deviation, decreased movement, hyperresonant
percussion note, and absent breath sounds over affected hemithorax.
• Treat with immediate decompression. Insert a 12G cannula into the
second intercostal space in the mid-clavicular line. Follow this with
insertion of an underwater seal chest drain into the fi fth intercostal
space between the anterior and mid-axillary line.
Open pneumothorax
• Occlude with a three-sided dressing.
• Follow by immediate insertion of an intercostal drain through a
separate incision.
Flail chest
• Results in paradoxical motion of the chest wall. Hypoxia is caused by
restricted chest wall movement and underlying lung contusion.
• If the segment is small and respiration is not compromised, nurse
patient in HDU with adequate analgesia. Encourage early ambulation
and vigorous physiotherapy. Do regular blood gas analysis.
• In more severe cases, endotracheal intubation with positive pressure
ventilation is required.
Massive haemothorax
• Accumulation of >1500mL of blood in pleural cavity.
• Suspect when shock is associated with dull percussion note and absent
breath sounds on one side of chest.
• Simultaneously restore blood volume and carry out decompression by
inserting a wide bore chest drain.
• Consider need for urgent thoracotomy to control bleeding if there is
continued brisk bleeding and need for persistent blood transfusion.
Consult with a regional thoracic centre.

THORACIC INJURIES
481
Cardiac tamponade
• Most commonly results from penetrating injuries, but blood can also
accumulate in pericardial sac after blunt trauma.
• Recognize by haemodynamic instability. Hypotension, tachycardia,
raised JVP, pulsus paradoxus, and faint heart sounds.
• If critically ill with suspected tamponade, perform ‘blind’
pericardiocentesis and call cardiothoracic or general surgeons to
consider emergency thoracotomy.
• If unwell, but responding to treatment, arrange urgent transthoracic
echo or focused abdominal ultrasound in A&E.
Management—secondary survey
Perform a further in-depth examination. In stab injuries, expose the
patient fully and position them so that you can assess front, back, and sides
of the chest for any wounds missed in the primary survey.
An erect CXR looking for the following injuries.
Simple pneumo-/haemothorax Treat with a chest drain if large or sympto-
matic or in any patient likely to undergo GA.
Pulmonary contusion Most common potentially lethal chest injury. Risk of
worsening associated consolidation and local pulmonary oedema. Treat
with analgesia, physiotherapy, and oxygenation. Consider respiratory sup-
port for a patient with signifi cant hypoxia.
Tracheobronchial rupture
• Suspect when there is persistent large air leak after chest drain
insertion. Seek immediate (cardiothoracic) surgical consultation.
• Thoracic CT scan usually diagnostic.
Blunt cardiac injury (myocardial contusion/traumatic infarction)
• Suspect when there are signifi cant abnormalities on ECG or
echocardiography.
• Seek cardiological/cardiothoracic surgical advice.
Aortic disruption
• Patients survive immediate death because the haematoma is contained.
• Suspect when history of decelerating force and where there is
widened mediastinum on CXR.
• Thoracic CT scan is diagnostic.
• Consider cardiothoracic surgical referral.
Diaphragmatic rupture
• Usually secondary to blunt trauma in restrained car passengers (seat
belt compression causes ‘burst’ injury commonly on the left side).
• Suspect in patient with a suitable history and a raised left
hemidiaphragm on CXR.
• Penetrating trauma below the fi fth intercostal space can produce a
perforation.
• Thoracoabdominal CT scan usually diagnostic.

CHAPTER 14 Major trauma
482
Abdominal trauma
Key features
• Abdominal injuries are present in 7–10% of trauma patients. These
injuries, if unrecognized, can cause preventable deaths.
• Blunt trauma. Most frequent injuries are spleen (45%), liver (40%), and
retroperitoneal haematoma (15%). Blunt trauma may cause:
Compression or crushing, causing rupture of solid or hollow •
organs.
Deceleration injury due to differential movement of fi xed and •
non-fi xed parts of organs, causing tearing or avulsion from their
vascular supply, e.g. liver tear and vena caval rupture.
• Blunt abdominal trauma is very common in RTAs where:
There have been fatalities.•
Any casualty has been ejected from the vehicle.•
The closing speed is >50mph.•
• Penetrating trauma. These may be:
Stab wounds and low velocity gunshot wounds. Cause damage •
by laceration or cutting; stab wounds commonly involve the liver
(40%), small bowel (30%), diaphragm (20%), colon (15%).
High velocity gunshot wounds transfer more kinetic energy and also •
cause further injury by cavitation effect, tumble, and fragmentation;
commonly involve the small bowel (50%), colon (40%), liver (30%),
and vessels (25%).
Management—primary survey
• Any patient persistently hypotensive despite resuscitation, for whom
no obvious cause of blood loss has been identifi ed by the primary
survey, can be assumed to have intra-abdominal bleeding.
• If the patient is stable, an emergency abdominal CT scan is indicated.
• If the patient remains critically unstable, an emergency laparotomy is
usually indicated.
Management—secondary survey of the abdomen
History
• Obtain from patient, other passengers, observers, police, and
emergency medical personnel.
• Mechanism of injury. Seat belt usage, steering wheel deformation,
speed, damage to vehicle, ejection of victim, etc. in automobile
collision; velocity, calibre, presumed path of bullet, distance from
weapon, etc. in penetrating injuries.
• Prehospital condition and treatment of patient.
Physical examination
• Inspect anterior abdomen which includes lower thorax, perineum, and
log roll to inspect posterior abdomen. Look for abrasions, contusions,
lacerations, penetrating wounds, distension, evisceration of viscera.
• Palpate abdomen for tenderness, involuntary muscle guarding, rebound
tenderness, gravid uterus.

ABDOMINAL TRAUMA
483
• Auscultate for presence/absence of bowel sounds.
• Percuss to elicit subtle rebound tenderness.
• Assess pelvic stability.
• Penile, perineum, rectal, vaginal examinations, and examination of
gluteal regions.
Investigations
Blood and urine sampling Raised serum amylase may indicate small bowel
or pancreatic injury.
Plain radiography Supine CXR is unreliable in the diagnosis of free intra-
abdominal air.
Focused abdominal sonography for trauma (FAST)
• It consists of imaging of the four Ps. Morrison’s pouch, pouch of
Douglas (or pelvic), perisplenic, and pericardium.
• It is used to identify the peritoneal cavity as a source of signifi cant
haemorrhage.
• It is also used as a screening test for patients without major risk
factors for abdominal injury.
Diagnostic peritoneal lavage (DPL)
• Mostly superseded by FAST for unstable patients and CT scanning in
stable patients. Useful, when these are inappropriate or unavailable, for
the identifi cation of the presence of free intraperitoneal fl uid (usually
blood).
• Aspiration of blood, GI contents, bile, or faeces through the lavage
catheter indicates laparotomy.
CT
• The investigation of choice in haemodynamically stable patients in
whom there is no apparent indication for an emergency laparotomy.
• It provides detailed information relative to specifi c organ injury and its
extent and may guide/inform conservative management.
Indications for resuscitative laparotomy Blunt abdominal trauma.
Unresponsive hypotension despite adequate resuscitation and no other
cause for bleeding found.
Indications for urgent laparotomy
• Blunt trauma with positive DPL or free blood on ultrasound and an
unstable circulatory status.
• Blunt trauma with CT features of solid organ injury not suitable for
conservative management.
• Clinical features of peritonitis.
• Any knife injury associated with visible viscera, clinical features of
peritonitis, haemodynamic instability, or developing fever/signs of
sepsis.
• Any gunshot wound.

CHAPTER 14 Major trauma
484
Vascular injuries
Key features
• Wounds that involve vascular structures of the extremity are a
signifi cant cause of morbidity and mortality in the traumatized patient.
• Motor vehicle accidents and falls are the most common causes of
blunt injury.
• Stab wounds cause most of the upper extremity vascular injuries,
while gunshot wounds cause the majority of lower extremity vascular
injuries in penetrating vascular injury.
• Blunt trauma causes more morbidity than penetrating injuries due to
associated fractures, dislocations, and crush injuries to muscles and
nerves.
Management—primary survey
• Apply direct pressure to open haemorrhaging wound.
• Carry out aggressive fl uid resuscitation.
• A rapidly expanding haematoma suggests a signifi cant vascular injury.
• Realign and splint any associated fracture. Immobilize dislocated joint.
• Seek surgical consultation.
Management—secondary survey
• Begin only after primary survey is complete and resuscitation is
continuing successfully.
• Identify limb-threatening injuries.
• Look for hard or soft signs of vascular injury:
‘Hard’ signs are massive external blood loss, expanding or pulsatile •
haematoma, absent or diminished distal pulses, and a thrill or
audible continuous murmur.
‘Soft’ fi ndings are history, if active bleeding at the accident scene, •
proximity of penetrating or blunt trauma to a major artery, small
non-pulsatile haematoma, and neurological defi cit.
• Measure distal systolic Doppler pressures of the injured arm or leg and
compare with uninjured brachial systolic pressure. An index of <1.0 is
a predictor of arterial injury.
• Presence of hard signs requires immediate operative intervention or
arteriography when limb is viable and active bleeding is absent.
Intraoperative arteriography helps in planning the operative approach.
Some minimal arterial injuries can be managed non-operatively.
Embolization can be used to manage selected arterial injuries.
Principles of operative management
• Obtain proximal and distal control prior to exposing the injury.
• Inspect the injured vessel and debride as necessary.
• Remove intraluminal thrombus by using Fogarty catheter.
• Flush lumen with heparinized normal saline solution.
• Consider temporary intraluminal shunting if limb is ischaemic and there
is a delay (if revascularization is anticipated).

VASCULAR INJURIES
485
• The type of vascular repair depends on the extent of damage.
Techniques used are lateral repair, patch angioplasty, end-to-end
anastomosis, interposition graft, or bypass graft.
• Use systemic anticoagulation if there is no contraindication.
• Consider intraoperative completion arteriography.
• Ensure completed vascular repair is free of tension and covered with
viable soft tissue.
• In patients with combined vascular and orthopaedic injuries, perform
arterial repair fi rst to restore circulation before orthopaedic
stabilization. Where there is massive soft tissue injury, debride all
non-viable tissue.
• Anticipate development of compartment syndrome. Perform
fasciotomies to decompress all four compartments of the leg.

CHAPTER 14 Major trauma
486
Head injuries
Causes and features
• Most common reasons for head injuries—falls, RTAs, and assaults.
• About 80% of head injuries are mild, 10% moderate, and 10% severe.
• Up to half of the deaths from trauma under the age of 45 are due to
a head injury. Sequelae are common in survivors.
Management—primary survey
• Maintain adequate oxygenation and BP. This avoids potentially
devastating secondary brain injury.
• Determine consciousness level on the AVPU or GCS (see Table 14.1;
use a standard head injury proforma).
• Involve anaesthetist to provide appropriate airway management in
patients with GCS <8 or AVPU = P or U.
• Avoid systemic analgesia until full neurological assessment made.
Management—secondary survey
• Fully assess the head and neck including:
Examination of skull vault.•
Looking for signs of base of skull fractures (haemotympanum,•
‘panda’ eyes, cerebrospinal fl uid (CSF) otorrhoea or rhinorrhoea,
Battle’s sign).
Repeated monitoring of vital signs.•
Repeated assessment of conscious level (GCS or AVPU).•
• Follow guidelines when transferring patients to the neurosurgical unit.
Ensure resuscitation and stabilization of patient is complete before
transfer.
• Give verbal advice and a written head injury advice card to patients
who are being discharged from A&E.
Indications for CT scanning in head injuries
• GCS <13 at any point since the injury; GCS = 13 or 14 at 2h after
the injury.
• Suspected open or depressed skull fracture.
• Any sign of basal skull fracture.
• Post-traumatic seizure.
• Focal neurological defi cit.
• More than one episode of vomiting.
• Amnesia for greater than 30min of events before impact.
• Age t65y, coagulopathy, dangerous mechanism of injury, provided
that some loss of consciousness or amnesia has been experienced.

HEAD INJURIES
487
Indications for neurosurgical referral in head injuries
• Major intracranial injury (extradural haematoma, moderate or larger
subdural haematoma, intracerebral haematoma).
• Progressive focal neurological signs.
• Defi nite or suspected penetrating head injury.
• A CSF leak or base of skull fracture.
• Persisting coma (GCS d8) after initial resuscitation or deterioration
in GCS score after admission.
Indications for admission in head injuries
• Patients with new, clinically signifi cant abnormalities on imaging.
• Patient has not returned to GCS = 15 after imaging, regardless of the
imaging results.
• Patient fulfi ls the criteria for CT scanning, but this cannot be done
within the appropriate period, either because CT is not available or
because the patient is not suffi ciently cooperative to allow scanning.
• Continuing worrying signs, e.g. persistent vomiting, severe
headaches.
• Other sources of concern, e.g. drug or alcohol intoxication, other
injuries, shock, suspected non-accidental injury, meningism, CSF leak.

CHAPTER 14 Major trauma
488
Table 14.1 Glasgow coma scale*
Feature Scale Score
Eye opening (E) Nil 1
In response to pain 2
In response to speech 3
Spontaneous 4
Motor response (M) Nil 1
Extension 2
Abnormal fl exion 3
Flexion away from pain 4
Localizes pain 5
Obeys commands 6
Verbal response (V) Nil 1
Sounds 2
Inappropriate words 3
Confused sentences 4
Orientated fully 5
* Minimum score, 3; maximum score, 15.
Reproduced from Teasdale, G. and Jennett, B. (1974). The Lancet, 304: 7872, with kind
permission from Elsevier.

489
Orthopaedic surgery
Examination of a joint 490
Examination of the limbs and trunk 492
Fracture healing 494
Reduction and fi xation of fractures 498
The skeletal radiograph 502
Injuries of the phalanges and metacarpals 504
Wrist injuries 508
Fractures of the distal radius and ulna 510
Fractures of the radius and ulnar shaft 512
Fractures and dislocations around the elbow in children 514
Fractures of the humeral shaft and elbow in adults 518
Dislocations and fracture dislocations of the elbow 522
Fractures around the shoulder 524
Dislocations of the shoulder region 526
Fractures of the ribs and sternum 530
Fractures of the pelvis 532
Femoral neck fractures 536
Femoral shaft fractures 538
Fractures of the tibial shaft 540
Fractures of the ankle 544
Fractures of the tarsus and foot 546
Injuries and the spinal radiograph 550
Spinal injuries 554
Acute haematogenous osteomyelitis 558
Chronic osteomyelitis 560
Septic arthritis 562
Peripheral nerve injuries 564
Brachial plexus injuries 566
Osteoarthrosis (osteoarthritis) 568
Carpal tunnel syndrome 570
Ganglion 572
Bone tumours 574
Low back pain 578
Paget’s disease (osteitis deformans) 582
The great toe 584
Arthroplasty 586
Useful reading 588
Chapter 15

CHAPTER 15 Orthopaedic surgery
490
Examination of a joint
• Applying a systematic approach will avoid missing vital clues.
• Always begin with a history, followed by examination.
• The classical orthopaedic triad of ‘look, feel, and move’ applies.
1
• Remember to examine the patient as a whole, not just the joint!
Ask
• Is the joint painful?
• Is there a specifi c area of tenderness?
• Does the pain radiate?
• Is the joint swollen?
• Can the joint be moved actively?
• Has there been an injury to the joint?
Look
• Remember, always compare unaffected with affected side.
• Is there any swelling? If so, is it an effusion, synovitis, or bony
deformity?
• Are there any colour changes? Bruising or erythema?
• Is there any skin involvement, i.e. rheumatoid nodules at elbow,
psoriatic plaques?
• Are there any scars? If so, are they traumatic, surgical, or infective?
• Look for muscle wasting, generally around the joint and specifi cally in
the whole limb.
• Examine the patient as a whole for clues to the disease process at the
joint.
Feel
• Always gain verbal consent and explain what you are doing to the
patient.
• Examine the unaffected or least painful side prior to examining the
affected side.
• Is the joint hot, cold, or moist?
• Is there any local tenderness? Look at the patient’s face, not the joint.
• Is the joint swollen? An effusion can occur after trauma
(haemarthrosis) or with infection (septic arthritis). Does the fl uid shift
with sweeping? Is synovitis present (non-movable fl uid feel), or is it a
bony swelling?
Move
• Compare affected with unaffected side.
• Test active movements fi rst before passive. This gives an idea of the
patient’s pain and reduces further discomfort.
• Ask the patient to move the joint through a full range of movement.
• Look for pain (patient’s face) and limitation of movement.
• Is the limitation mechanical (blocked by loose body, meniscal tear,
contracture) or restrictive (resisted by the patient due to pain)?
• Shoulder. Flexion, extension, abduction, internal and external rotation.

EXAMINATION OF A JOINT
491
• Elbow. Flexion, extension, pronation, and supination (ensure humerus
at patient’s side).
• Wrist. Flexion, extension, radial and ulnar deviation, pronation, and
supination.
• Metacarpophalangeal joint (MCPJ). Flexion, extension, abduction, and
adduction.
• Proximal interphalangeal joint (PIPJ) and distal interphalangeal joint
(DIPJ). Flexion and extension.
• Thumb. Flexion, extension, abduction, adduction, and opposition.
• Hip. Flexion, extension, internal and external rotation.
• Knee. Flexion and extension.
• Ankle. Plantar and dorsifl exion, eversion and inversion.
• Cervical spine. Flexion, extension, and lateral rotation and fl exion.
• Lumbar spine. Flexion, extension, and lateral rotation and fl exion.
• Muscle power is graded via the MRC system (see b p. 492).
• Always get the patient to walk to test gait if the problem is lower limb.
Special tests
These depend on the individual joint examined and are numerous for each
joint!
They normally involve either:
• Tests of instability, for example:
Collateral testing in the knee, elbow, or fi nger joints.•
Cruciate ligament testing (anterior draw, Lachmans).•
Apprehension tests (shoulder instability).•
• Or provocation tests that aim to locate the cause of intra-articular
pain, for example:
Grind tests (thumb base osteoarthritis, knee meniscal injury).•
Meniscal provocation tests (McMurray’s test).•
References
1 Soloman L, Warwick D, Nayagam S (Eds) (2010). Apley’s system of orthopaedics and fractures,
9th Edn. Hodder Arnold, London.

CHAPTER 15 Orthopaedic surgery
492
Examination of the limbs and trunk
Develop your own system that you feel comfortable with. Always compare
affected with unaffected side. Make allowances for the dominant side.
Look
Is there any swelling, deformity, asymmetry, muscle wasting, twitching
(fasciculation), scars, skin colour changes, rashes?
Feel
Is there any tenderness, temperature changes, solid or fl uid swellings,
muscle bulk?
Move
• Move each joint through its full active and passive range.
• Is the limb tone normal, reduced (fl accid or fl oppy), or increased
(rigidity)? Is there any spasm? Are there any joint contractures?
• Is the rigidity through whole movement or only initially (spasticity)?
• Is the alteration in movement from a neuromuscular disorder, a
mechanical block, or pain from the joint?
Power (MRC grading)
• Grade 0, no movement.
• Grade 1, fl icker of movement only.
• Grade 2, movement with gravity eliminated.
• Grade 3, movement against gravity.
• Grade 4, movement against resistance.
• Grade 5, normal power.
Test all muscle groups within their relevant myotomes according to the
patient’s history.
Coordination
• Ask the patient to touch their nose with their index fi nger with their
eyes open and then shut. Compare side to side.
• Alternatively, ask the patient to put their right heel on to their left
knee and run it down their shin and vice versa. Note whether these
movements are smooth or jerky.
• Romberg’s test. Stand with feet together and eyes shut. Positive result
will cause the patient to become unstable or fall; be prepared!
Refl exes
• Biceps jerk, C5/6.
• Abdominal, T8–T12.
• Triceps jerk, C6/7.
• Knee jerk, L2/3/4.
• Brachioradialis, C5/6.
• Ankle jerk, S1/2.
• Plantar response. Normal fl exor, abnormal extensor (Babinski’s sign).
• Clonus at ankle (normal two beats or less).

EXAMINATION OF THE LIMBS AND TRUNK
493
Grading
• 0, absent.
• 1, hypoactive.
• 2, normal.
• 3, hyperactive, no clonus.
• 4, hyperactive with clonus.
Sensation
Explain what you are about to do clearly to the patient and perform the
test with their eyes closed. Compare symmetrical sides of the body at the
same time. Map out the abnormalities.
• Pinprick, light touch, and temperature tested in a dermatomal pattern.
• Vibration sense tested with a 128MHz low-pitched tuning fork on a
bony prominence. Start distal and if abnormal, move from proximal.
• Proprioception (joint position sense) tested by moving the
metatarsophalangeal joint (MTPJ) of the hallux, up and down; the
patient confi rms the correct movement.

CHAPTER 15 Orthopaedic surgery
494
Fracture healing
Fracture healing occurs as either primary or secondary bone union.
• Secondary bone healing produces callus. It occurs when fractures
are immobilized with ‘relative stability’ (some minimal movement
at fracture site, e.g. a plaster cast). It involves two simultaneously
occurring, but distinct, processes: intramembranous and endochondral
ossifi cation, producing periosteal bony callus and fi brocartilagenous
bridging callus, respectively.
• Primary bone healing does not produce callus. It occurs when
fracture fragments are reduced ‘anatomically’ and ‘interfragmentary
compression’ is achieved with ‘absolute stability’. There is no motion
between fracture surfaces (e.g. compression plating techniques or lag
screw fi xation).
Secondary bone healing (callus)
Initial phase: haematoma and infl ammation
• Torn vessels at fracture site bleed, producing a haematoma and
subsequent clot.
• The size of the haematoma depends upon the blood supply to the
bone and the violence of the injury; it can continue to expand during
the fi rst 36h.
• Injured tissue and platelet activation causes an infl ammatory cascade
via the release of growth factors and various cytokines.
• Infl ammatory cell migration to the haematoma occurs (macrophages,
fi broblasts, osteoclasts, chondroblasts).
• Fibroblasts and chondroblasts organize the haematoma into collagen
and granulation tissue, with new capillary ingrowth (angiogenesis).
• Osteoclasts and macrophages remove dead bone and tissue,
respectively.
• This stage usually lasts up to 1 week.
Second phase: callus formation (soft and hard)
• Cell (osteoblasts) proliferation and differentiation results in callus
formation.
• Intramembranous (or periosteal) hard callus forms peripherally,
with endochondral (fi brocartilagenous/bridging) soft callus forming
alongside. A third type ‘medullary callus’ forms later if the above fails.
• The amount and type of callus produced is dependent upon local
factors such as the type of fracture, proximity of the bone ends,
amount of haematoma, and is inversely proportional to the amount of
movement present.
• Soft callus is calcifi ed by chondroblasts and subsequently resorbed by
chondroclasts.
• New blood vessels invasion into the callus brings osteoblastic type
cells, resulting in ossifi cation into woven bone.
• By this point, the fracture will have united and be pain-free.
• This stage lasts 1 week to 4 months.

FRACTURE HEALING
495
Third phase: remodelling
• Woven bone is resorbed by osteoclasts and osteoblasts replace this
with lamellar bone, which is very hard and dense.
• Final remodelling occurs when swelling around the fracture site
decreases; trabeculae can be seen crossing the fracture site on
radiographs and the medullary canal is recreated.
• Remodelling is most marked in children and follows the mechanical
forces applied to the bone in a physiological environment.
• This process is identical in both primary and secondary bone healing
and can last for several years.
Primary bone healing (absolute stability)
• The infl ammatory response is much reduced.
• Areas of direct contact undergo some activity.
• Any gaps are invaded with blood vessels and cells differentiate into
osteoblasts, laying down woven and lamellar bone (gap healing).
• Osteoclasts acting as ‘cutting cones’ pass directly across the fracture
site, leaving channels that are fi lled with blood vessels and allowing
osteoblasts to fi ll them with lamellar bone.
• No callus is formed and union takes much longer to achieve, with
the strength of the healing process being borne by the mechanical
properties of the fi xation device.
• Remodelling occurs as above.
Factors adversely affecting fracture healing
• Degree of local trauma (bone loss, soft tissue trauma and interposition,
neurovascular injury, open fractures).
• Inadequate reduction and immobilization.
• Infection.
• Location of fracture. Which bone and where on bone i.e. metaphysis
versus diaphysis (see below)?
• Disturbances of ossifi cation, e.g. metabolic bone disease, osteoporosis,
local pathological tumour.
• Age, poor nutrition, smoking, drugs (especially NSAIDs), diabetes.
How long do fractures take to unite?
Perkins rules
• Fractures of cancellous (metaphyseal) bone (e.g. those around joints)
will take 6 weeks to unite.
• Fractures of cortical (diaphyseal) bone (e.g. shafts of long bones) will
take 12 weeks to unite.
• Fractures of the tibia (because of poor blood supply) will take 24
weeks to unite.
• Time to union for children equals the age of the child in years plus 1,
e.g. tibial fracture in a 2y-old child will unite in 3 weeks. Common
sense needs to be applied when applying the rule to fractures of
cancellous bone in older children.

CHAPTER 15 Orthopaedic surgery
496
Delayed union
Defi ned as a failure of union to occur in 1.5x the normal time for fracture
union.
Non-union
Defi ned as a failure of union to occur within twice the normal time to
fracture union. However, expect open fractures to normally take twice
the normal Perkins rule.
• Hypertrophic non-union. Excess mobility or strain at fracture site. There
is a good blood supply with healing potential. Appears as large callus
(elephant’s foot pattern) on X-rays. Usually requires stabilization to
allow callus progression.
• Atrophic non-union. Due to poor blood supply resulting from initial
injury or surgical intervention. There is poor healing potential. Usually
require stabilization and biological augmentation to heal.
Further reading
Soloman L, Warwick D, Nayagam S (Eds) (2010). Apley’s system of orthopaedics and fractures. 9th
Edn. Hodder Arnold, London.
Ramachandran M (2007). Basic Orthopaedic Sciences: The Stanmore Guide. Hodder Arnold,
London.
This page intentionally left blank

CHAPTER 15 Orthopaedic surgery
498
Reduction and fi xation of fractures
Caveat. A fracture is a soft tissue injury with an associated broken bone.
Treat the soft tissues with utmost respect to ensure fracture healing.
Modern fracture reduction and treatment was pioneered by the AO
group and centres around four key principles:
1
• Fracture reduction and fi xation to restore anatomical relationships.
• Stability by fi xation or splintage as the personality of the fracture and
the injury dictates.
• Preservation of the blood supply to the soft tissue and bone by careful
handling and gentle reduction techniques.
• Early and safe mobilization of the part and patient.
Fracture reduction can be achieved by closed
2
(indirect) or open (direct
and indirect) methods. Maintenance of the reduction may also be achieved
via closed methods which can be non-surgical (plaster or brace) or surgi-
cal (intramedullary nail, external fi xation, Kershner (K) wires), or via open
methods such as rigid internal fi xation with plates and screws.
Casting
• Application of a plaster of Paris (or modern alternatives) cast over
appropriate padding to stabilize a reduced fracture.
• Typically involves splinting of joints either side of a long bone fracture
to provide additional rotational stability.
• Simplest and cheapest to apply.
• Lowest risk of septic complications.
• It will provide pain relief.
• ‘Half casts’ or ‘backslabs’ can be utilized to immobilize a fracture prior
to defi nitive management.
• Complications include problems with cast (pressure areas, loosening
and breakdown of cast), thromboembolic events, coverage of wounds.
• It is a very involved process, requiring regular follow-up to ensure
maintenance of reduction.
Cast bracing
• Stabilization of a fracture across a joint with a cast, but the joint itself is
left free to move by the incorporation of a hinge across it.
• Has the advantage of allowing early movement of the joint without the
use of weight bearing, e.g. tibial shaft fractures.
Internal fi xation
Indications
• Intra-articular fractures. To prevent or reduce the incidence of
osteoarthrosis.
• Unstable fracture patterns.
• Neurovascular damage. Fracture stability must be achieved before the
delicate repair of vessels or nerves takes place. If not, these repairs
may be damaged.
• Polytrauma. Multiple injuries are better managed by fi xation to facilitate
nursing care and to allow early mobilization.

REDUCTION AND FIXATION OF FRACTURES
499
• Elderly patients tolerate immobilization and prolonged bed rest poorly
(fractured neck of femur).
• Fractures of long bones (e.g. forearm, femur, tibia). Rehabilitation
is facilitated more quickly with internal fi xation after anatomical
reduction.
• Failure of conservative therapy (loss of acceptable alignment).
• Pathological fractures.
Methods
Compression plates and screws, locking plates and screws, Kershner (K)
wires, intramedullary nails, tension band wiring.
Complications
• Infection which increases with the size and increased time of exposure
required.
• Nerve and vessel injury.
• Non-union (increased with iatrogenic soft tissue and periosteal injury).
• Implant failure and subsequent fracture through a bony defect if the
implant is removed.
External fi xation
Indications
Temporizing measure for:
• Open fractures (commonly tibia or femur) associated with signifi cant
soft tissue damage or nerve and vessel injury.
• Highly comminuted or unstable fractures and fracture dislocations.
• Life-saving splintage procedure in pelvic fractures.
• Initial stabilizing device for any fracture where ‘damage limitation’
surgery may be appropriate in the multiply injured patient (damage
control orthopaedics).
• Defi nitive treatment of periarticular fractures (pilon and tibial plateau).
• As a salvage option in the face of mal-union, non-union, or signifi cant
bone loss.
Methods
• Pin-and-rod construct most commonly used (tibia–pelvis).
• Modern systems incorporate ring fi xators with pins and rods (hybrid).
• Circular fi xators (Ilizarov) can be used for defi nitive fracture fi xation
or as a salvage option.
Complications
• Pin site infection and possible osteomyelitis.
• Nerve, vessel, ligament, and tendon injury (good understanding of
cross-sectional anatomy required).
• Over-distraction, resulting in non-union.
Locking plates
• Modern implants in which the screw heads are threaded and engage
and lock into threads in the plate holes.
• These act as ‘internal, external fi xators’ where forces are transmitted
from bone to screw to plate.

CHAPTER 15 Orthopaedic surgery
500
• The locking plate provides angular stability and is much stronger than a
normal plate as all screws act in unison.
• Advantages are:
Excellent holding power as all locked screws have to fail at once for •
construct to fail. Thus, excellent choice of fi xation in osteoporotic
fractures.
Spares periosteal blood supply as does not rely on compression of •
plate on bone.
They can be placed percutaneously (avoiding stripping soft tissue •
and blood supply from a fracture site).
They do not require contouring.•
The screws are usually self-drilling and self-tapping.•
References
1 Ruedi TP, Murphy WM (2000). AO principles of fracture management. Thieme Medical Publishers,
New York.
2 McRae R, Esser M (2008). Practical fracture treatment, 5th edn. Churchill Livingstone, Edinburgh.
This page intentionally left blank

CHAPTER 15 Orthopaedic surgery
502
The skeletal radiograph
This is the most important investigation in orthopaedics, but does not sub-
stitute for accurate history and examination. Always remember a fracture
is a clinical, not a radiological diagnosis.
Evaluation
Have a system. The following is only one example:
• Note the history, race, occupation, handedness, pastimes, age, sex, and
recent laboratory results of the patient.
• Accurate history and clear requests to be documented on X-ray
forms. Always write the side in full, e.g. ‘right’.
• Always take two views at 90° to each other (orthogonal).
• Examine the fi lm carefully.
• Most hospitals use computer-based X-ray viewing systems but if using
a viewing box, have a bright spotlight and magnifying glass available.
• When describing the lesion, think of side, anatomical site, nature,
displacement, and soft tissue components.
• Keep it simple.
A. Adequate views and alignment.•
B. Bones.•
C. Cartilage (soft tissues).•
• Look for cortical/medullary changes, periosteal reactions, deformity,
soft tissue swelling, and cortical breach (defi nition of a fracture).
• Supplement radiological fi ndings with further biochemical
investigations, bone scanning, and biopsy if indicated.
Radiological features
Osteoporosis
• The most common form of bone disease.
• Characterized by low bone mass and deterioration of the
microarchitecture of bone tissue with consequent increase in bone
fragility and susceptibility to low trauma fractures.
• Affects middle-aged and elderly women, predisposing them to
fractures of the distal radius, femoral neck, and vertebral bodies.
• Localized osteoporosis follows disease, e.g. after joint fusion.
• The cortices are thin with reduced medullary trabeculae, i.e. the bone
is essentially normal; there is just too little of it.
Osteomalacia
There is reduced mineralization of osteoid.
• The trabeculae are blurred.
• Symmetrical transverse or oblique cortical defects appear (Looser’s
zones, pseudofractures).
• In children, changes are most marked at the metaphysis (rickets).
Hyperparathyroidism
There is bone resorption. Best place to see it is in the phalanges of the
hands in the subperiosteal cortex. Note generalized cortical striations.
Usually diagnosed with parathyroid hormone levels after incidental fi nding
of raised calcium levels.

THE SKELETAL RADIOGRAPH
503
Diffuse increase in density
Think of neoplasia, fl uorosis, sarcoidosis, bone dysplasia (osteopetrosis).
Abnormalities of bone modelling
Developmental disorders, e.g. osteochondrodysplasia, are often present
from birth. Look for abnormalities of the eyes, heart, and ears. Thorough
assessment by biochemical and genetic specialist required.
Local abnormalities may occur in congenital disorders, e.g. endochon-
dromatosis (Ollier’s disease), fi brous dysplasia, neurofi bromatosis, or
acquired disorders, e.g. Paget’s disease.
Solitary lesions
Always think of sepsis, primary bone tumours, or secondary metastasis.
Location and age are important, e.g. an epiphyseal lesion in a child may
be a chondroblastoma and a subarticular lesion in a young adult may be a
giant cell tumour. The older the patient, the more likely it is a metastasis.
Describing a fracture
• First check details match patient (i.e. date of X-ray, patient age, side,
hospital number).
• Ensure the appropriate X-ray is taken with two views at 90° to each
other (e.g. an X-ray of an ankle, rather than the whole lower leg).
• Which bone is fractured?
• Where is the fracture in the bone? Joint (intra-articular), proximal,
middle, or distal third, or metaphysis (fl ares at end of bones), diaphysis
(shaft), physis (growth plate), and epiphysis (end part of bone) in
children.
• What is the pattern? Transverse, oblique, spiral, comminuted or
multifragmentary, segmental.
• Is there displacement? Quantify this (e.g. 50% of bone width or
completely ‘off ended’ >100%).
• Is there any angulation? Which direction (varus, valgus, recuvartum)?
• If a joint is involved, comment on whether it is ‘in joint’ or dislocated.
• Other things to look for are gas in soft tissues (suggests open fracture
or gas-forming infection), foreign bodies (metal, glass, grit), fl uid in
joints (e.g. lipohaemarthrosis in knee suggests fracture), fat pad signs in
the elbow (suggest fracture and are prominent due to blood in joint).
• A ring-like structure (e.g. the bony pelvis) rarely fractures in only one
place; if you fi nd one fracture, look hard for another one!
• Comment on implants if present and the proximity and involvement
of this to fracture (periprosthetic fractures of a total hip replacement
(THR) or total knee replacement (TKR)).
• Pitfalls. Is it a fracture? Structures that may be mistaken for fractures
include suture lines between bones, vascular channels, and physes
in immature skeletons. Anatomic structures are more likely to be
symmetrical, if not midline.
Further reading
Raby N, Berman L, de Lacy G (2005). Accident and emergency radiology: a survival guide, 2nd edn.
Saunders, London.
Nicholson DA, Driscoll P (1995). ABC of emergency radiology. BMJ Books, Wiley, England.

CHAPTER 15 Orthopaedic surgery
504
Injuries of the phalanges and
metacarpals
Thumb
Mechanism Direct blows to thumb, forced opposition of the thumb.
Extra-articular
• Metacarpal shaft fractures. Undisplaced can be managed in cast. Displaced
fractures require reduction and fi xation, either open or closed.
• Metacarpal base fractures. Often displaced or angulated due to
deforming forces of the tendon attachments. If fractures undisplaced,
can manage with closed reduction and immobilization in cast. For
displaced/signifi cantly angulated fractures, closed reduction with K wire
fi xation or open reduction with internal fi xation (ORIF) is required.
Up to 30° of angulation can be accepted due to the vast range of move-
ments at the base of thumb.
Intra-articular (± fracture dislocation)
• Bennett’s fracture dislocation. Volar/ulnar fragment left behind due to
strong ligament attachments; remaining distal metacarpal dislocates
proximally and dorsally. Treatment involves closed reduction and
K wire fi xation to either carpus (trapezium) and/or index fi nger
metacarpal. Open reduction is rarely needed.
• Rolando fractures. Multifragmentary fracture, at least three parts in
a ‘T’ or ‘Y’ pattern ± dislocation. Treatment depends on degree of
fragmentation. Reduction and K wire fi xation or external fi xation
should be considered. ORIF only if large fragments.
Thumb dislocation
• At the MCPJ, usually results in ulnar collateral ligament (UCL) injury
(gamekeeper’s thumb).
• Tear of the UCL can be partial or complete, with adductor
aponeurosis stuck in the joint (Stener lesion), preventing reduction.
• Partial tears (stable) are immobilized in cast for 6 weeks.
• Complete tears (unstable or Stener Lesion) require surgical repair.
• Chronic tears are treated with tendon reconstruction of UCL or MCPJ
fusion.
Metacarpal fractures
Mechanism Usually ‘punch’ injury (‘Friday night’ or ‘boxer’s’ fracture). The
little fi nger most commonly affected. Remember to check for rotational
deformity as this is not an acceptable deformity.
Metacarpal neck fractures
Accept up to 15° angulation in index/middle and 35° in ring/little fi ngers.
Most treated conservatively (neighbour strapping). Reduction and pinning
or ORIF if signifi cant angulation.

INJURIES OF THE PHALANGES AND METACARPALS
505
Metacarpal shaft fractures
Check for rotation. Transverse or unstable fractures (especially ring and
little fi ngers), treat with ORIF or K wire fi xation. Undisplaced fractures
can be treated conservatively (neighbour strapping). Similar degrees of
angulation to neck fractures can be accepted.
Metacarpal base fractures (± subluxation)
Always get a true lateral X-ray of hand to assess for subluxation. These
are usually stable fractures and can be treated conservatively in cast for
3 weeks. If subluxed (little fi nger akin to Bennett’s fracture), treatment
involves closed reduction and K wire fi xation to carpal bone for 4 weeks.
Distal phalanx fractures
Mechanism Crush injury that is comminuted and often compound. Tuft
type fractures.
Treatment
Wound toilet, simple nail bed repair if needed, and primary suture or Steri-
strip
®
with pressure dressing. Wound inspection at 48h and as required.
Can be dealt with in A&E department. Antibiotics if open.
Mallet fi nger
• Sudden fl exion injury of distal phalanx (i.e. stubbing fi nger), resulting
in either avulsion of extensor tendon insertion with fl ake or large
fragment of bone, but can be purely tendinous.
• Small fragments or purely tendinous types are treated in ‘mallet
splint’ (extension) for 8 weeks continuously, followed by 2–4 weeks
just at night. If large fragment, fi xation can be undertaken if unable to
maintain in splint.
Proximal and middle phalanx fractures
Mechanism Direct blow or twisting injuries.
Treatment
• Dependent on fracture confi guration. Check for rotational deformity.
• Undisplaced stable fractures are treated with neighbour strapping for
2–3 weeks with early mobilization.
• If unstable, rotated, severely angulated, or involving the joint, consider
closed reduction and K wire fi xation or ORIF with mini-fragment
screws ± plate. Stable fi xation to allow early mobilization is the goal.
PIP joint dislocations
• Dorsal dislocation is the most common. It is associated with avulsion
of volar plate or fracture of volar base of middle phalanx.
• Dislocations require reduction under ring block.
• If stable, they can be treated with extension blocking splint with PIPJ
fl exed for 4–6 weeks.
• If unstable or associated with signifi cant fracture, they require either
manipulation under anaesthesia (MUA) and K wiring, ORIF, or volar
plate arthroplasty.

CHAPTER 15 Orthopaedic surgery
506
Immobilization for hand injuries (Edinburgh position)
To prevent stiffness, the metacarpal joint should be immobilized in 90°
of fl exion and the PIPJ in extension with the wrist extended at 30°. This
places the ligaments on maximal stretch whilst immobilized.
Further reading
Soloman L, Warwick D, Nayagam S (Eds) (2010). Apley’s system of orthopaedics and fractures,
9th edn. Hodder Arnold, London.
This page intentionally left blank

CHAPTER 15 Orthopaedic surgery
508
Wrist injuries
Scaphoid fractures
1
Mechanism Fall on to the outstretched hand with forced dorsifl exion.
Examination
• Fullness in the anatomical snuffbox means an effusion.
• Tenderness on the volar surface of the scaphoid, i.e. the tubercle, is
more predictive than snuffbox tenderness (dorsal) which is unreliable.
• Wrist movement, particularly pronation followed by ulnar deviation,
may be painful.
• Pain on compression of the thumb longitudinally or on gripping may be
present.
• However, clinical examination is highly variable and skill-dependent.
Investigation (radiographs)
‘Scaphoid series’ fi lms (PA wrist in ulnar deviation, lateral wrist in neutral;
PA in 45° pronation and ulnar deviation; and AP with 30° supination and
ulnar deviation). False negative rate of <5%. Fractures are usually of the
waist, but may be more proximal.
Treatment
• Below elbow, cast in neutral position (RCTs show that the thumb
does not need to be included)
2
for 8 weeks, but the fracture may
take 12 weeks to unite. At 12 weeks, remove plaster regardless of
symptoms.
• If initial X-rays negative, but clinical suspicion persists, cast the wrist
and repeat fi lms in 2 weeks.
• Displacement of >1mm or angulation requires ORIF with compression
screw.
• Proximal pole fractures are relative indication for fi xation as high
chance of non-union.
Complications
• Non-union. i with proximal fractures due to blood supply running from
distal to proximal in the bone. If not united at 12 weeks, proceed to
ORIF (compression screw) ± bone grafting.
• Avascular necrosis. i with proximal and displaced fractures (see above).
Treatment is by internal fi xation and bone grafting which may need to
be a ‘vascularized’ graft.
• Degenerative change. May occur after non- or mal-union. Treated by
limited wrist fusion (four corner fusion, scaphoidectomy, and radial
styloidectomy).
Other carpal fractures
• The most common is hamate fracture. The hook is fractured by
direct blow to the palm of the hand or repeated direct contact (e.g.
motorcyclists, golfers, racquet sports, and cricketers).
• Treatment is usually excision, but internal fi xation may be attempted if
the fragment is large.

WRIST INJURIES
509
Ligamentous injuries of the wrist
• Common; diffi cult to diagnose so easily missed.
• If left untreated, they can cause long-term disability.
• The proximal row of carpal bones forms an intercalated segment, i.e.
they are connected and work together as a unit. Injury may occur to
the ligaments connecting the bones.
Scapholunate ligament
Common in isolation or in association with fractures (especially distal
radius). ‘Terry Thomas’ sign, i.e. increase in the space between scaphoid
and lunate on a clenched fi st PA view. Acute ruptures may be repaired,
but chronic injuries may require reconstruction or fusion.
Lunotriquetral ligament
Less common; acute repair may be successful, but chronic injuries require
lunotriquetral fusion.
Carpal dislocations
Complete ligamentous injury may allow the carpus to dislocate.
• Occurs either with the lunate remaining in place, a perilunate
dislocation, or the carpus staying in place and the lunate moving, a
lunate dislocation.
• On rarer occasions, the scapholunate ligament remains intact and the
scaphoid fractures, resulting in a trans-scaphoid perilunate dislocation.
Treatment Severe injury requires reduction—best open as it allows for-
mal repair of the disrupted ligaments, as well as stabilization of the carpus.
If the scaphoid is fractured, it should be internally fi xed as well.
Carpometacarpal fracture dislocation
• Usually as the result of a punch injury. Affects little or ring fi ngers.
• Commonly missed due to poor history and examination.
• Indicated by tenderness at the carpometacarpal base.
• Diagnosed with a true lateral (not the standard lateral oblique) X-ray
(shows subluxation or dislocation at the carpometacarpal joint).
Treatment Unstable injury—reduce with traction and local pressure, then
stabilize the joint with K wire fi xation for 4 weeks.
Triangular fi brocartilage complex (TFCC) injury
TFCC and the ulnar small ligaments of the hand. An acute tear is usually
peripheral and the result of trauma, including a fracture to the ulna styloid.
It will present with ulna-based wrist pain in ulnar deviation with or without
rotation.
Treatment If associated with a large ulnar styloid fracture, this can be inter-
nally fi xed with a tension band wire technique. Arthroscopic debridement
or repair of the tear has been attempted, but is technically demanding.
References
1 M http://www.eatonhand.com.
2 Clay NR, Dias JJ, Costigan PS, et al. (1991). Need the thumb be immobilized in scaphoid frac-
tures? A randomised prospective trial. J Bone Joint Surg Br 73(5): 828–32.

CHAPTER 15 Orthopaedic surgery
510
Fractures of the distal radius and ulna
• Usually caused by a fall on to the outstretched hand.
• Very common. Approximately 1 in 6 of all fractures treated.
• Bimodal incidence. Peaks in childhood (6–10y) and early old age
(60–70y).
• Scaphoid and ligamentous wrist injuries may also be present.
Classifi cation
• Classifi cation systems are the AO system
1
and the Frykman system.
2
• Historical eponymous terms (‘Colles’, ‘Smith’s’) are still used.
• To avoid confusion, stick to describing the fracture by anatomical
methods, e.g. dorsally displaced fracture of the distal radius with
shortening and ulnar deviation.
• In children, the fracture usually involves the epiphyseal region and
these fractures are classifi ed by the Salter–Harris system.
3
Salter–
Harris type II is easily the most common injury of the distal radius.
Important radiological features to assess
• These parameters give an idea of the severity of the injury and thus
the stability of the fracture; the more features, the more unstable.
• Dorsal cortex comminution.
• Intra-articular extension (radiocarpal and distal radioulnar joint
(DRUJ)).
• Ulnar styloid fracture (suggests a TFCC injury which is a strong DRUJ
stabilizer).
• Loss of radial inclination (normally approximately 22°).
• Loss of palmar tilt or dorsal angulation (normal tilt approximately 11°).
• Loss of radial height (approximately 11mm from distal ulna to tip of
radial styloid).
Treatment4
Children
• Fractures of the distal radius. Usually treated by closed reduction
(manipulation) and the application of a well moulded plaster.
• If very unstable in theatre (radial and ulnar complete fractures,
displaced) or if the fracture has slipped position in plaster after
manipulation, then internal fi xation with percutaneous K wires or
more rarely, open fi xation with plates and screws may be used.
Adults
Fractures with dorsal displacement/angulation
• Undisplaced + stable. Below elbow plaster immobilization for 6 weeks.
• Displaced + stable. Closed reduction and plaster immobilization for 6
weeks.
• Displaced + unstable. Closed reduction and either percutaneous K
wire fi xation (two wires), external fi xation, or ORIF with plates and
screws.
• Complex intra-articular fractures or highly unstable patterns can now
be successfully treated with modern anatomic pre-contoured distal
radius locking plates.

FRACTURES OF THE DISTAL RADIUS AND ULNA
511
Traditionally a diffi cult area to know the best treatment method.
• Take each case on its own merits. There are many patient and fracture
factors to allow for.
• Dorsal comminution is a common problem and must be taken into
account in the method chosen.
• Bone structural substitutes, e.g. Biobon, lack RCT data to back up their
use and considerable expense.
Fractures with volar displacement (‘Smith’s fracture’)
Unstable and are treated by a volar buttress plate (supports a fracture like
a shelf, propping up or supporting the distal fragment).
Intra-articular fractures with volar displacement (‘Barton’s fracture’)
Internal fi xation is mandatory as it is a highly unstable fracture.
Ulnar styloid fractures
Do not often require fi xation unless the fragment is large in which case, it
may represent a TFCC injury (see b p. 512) treated by internal fi xation.
References
1 M http://www.trauma.org/ortho/aoclass.html.
2 Frykman G (1967). Fracture of the distal radius including sequelae—shoulder–hand–fi nger syn-
drome, disturbance in the distal radio ulna joint and impairment of nerve function. A clinical and
experimental study. Acta Orthop Scand Suppl 108, 3.
3 Salter RB, Harris WR (1963). Injuries involving the epiphyseal plate. J Bone Joint Surg Am 45,
587–632.
4 M http://www.eradius.com/

CHAPTER 15 Orthopaedic surgery
512
Fractures of the radius and ulnar shaft
Mechanism
• Commonly a fall on to the outstretched hand or a direct blow injury.
• High energy may be involved, therefore look closely for neurovascular
status and compartment syndrome.
• As displaced or angulated fractures affect the proximal (Monteggia)
and distal (Galeazzi) radioulnar joints, it is essential to get orthogonal
X-rays of the wrist and elbow.
Fracture types
Children
• Usually transverse fractures of the radius and ulna.
• May be angulated only with one of the cortices still intact (‘greenstick
fracture’).
• Be aware of plastic deformation (no obvious fracture, but bowing of
one or both bones).
• May sustain a fracture dislocation as in adults.
Adults
• Usually either a transverse or oblique fracture of the radius and ulna.
• Isolated ulna shaft fractures (‘nightstick fractures’ named after
mechanism of defending direct blow from a policeman’s nightstick or
truncheon).
• Displacement and signifi cant angulation are indications for fi xation.
• Remember, the forearm is a ‘force parallelogram’ and that a fracture of
only one bone will usually result in a dislocation of the other bone at
the proximal or distal joints. These fracture dislocations are:
Monteggia fracture.• Proximal ulnar fracture with dislocation of the
proximal radial head.
Galeazzi fracture.• Distal radial fracture with dislocation of the DRUJ.
Treatment
Children
• Greenstick fractures. Closed reduction and cast immobilization from
wrist to above the elbow.
In-line traction is always the key to any initial reduction and often •
all that is required to realign, given patience.
Use minimal force. If the periosteal hinge is broken during •
reduction, the fracture may displace completely and become
unstable.
• Plastic deformation needs to be corrected.
• Displaced fractures are often unstable and can be treated by ORIF
with plates and screws, or fl exible intramedullary nail fi xation.
• Fracture dislocations (Monteggia and Galeazzi). Closed manipulation
and cast immobilization (failed reduction may require open reduction).
Adults
• Usually impossible to achieve or maintain a closed reduction for adult
forearm shaft fractures.

FRACTURES OF THE RADIUS AND ULNAR SHAFT
513
• Undisplaced fractures can be managed in an above elbow cast.
• Displaced fractures are treated with open reduction and compression
plate fi xation.
• ‘Nightstick fractures’ of the ulna are splinted by the intact radius so if
undisplaced, then early protected motion with an elbow cast-brace is
indicated.
• If the fracture is displaced (>50% displacement or >10°), open
reduction and compression plate fi xation should be used.
• Fracture dislocations. Treated with open reduction and internal
fi xation to accurately reduce and hold the associated dislocations.
Complications
• Mal-union or non-union. Close follow-up of closed, manipulated
fractures. An X-ray at 1 and 2 weeks is mandatory to watch for slip of
position. Mal-union can present with functional problems with forearm
rotation.
• Non-union is normally treated by open reduction, debridement of the
non-union site, and compression plate fi xation with or without bone
grafting.
• It is not usually necessary to remove metalwork from the radius and
ulna unless they cause signifi cant problems after the fracture has
healed. Radial plate removal has been associated with a signifi cant risk
of neurovascular complications.

CHAPTER 15 Orthopaedic surgery
514
Fractures and dislocations around
the elbow in children
• Second commonest injury in children (8% of childhood fractures).
1
• Cause is usually a fall on to the outstretched hand. The result is
related to age:
<9y, supracondylar fracture of the humerus.•
>10y, dislocated elbow.•
>60y, shoulder injuries.•
• Salter–Harris injuries of the elbow occur through the lateral condyle
and radial neck.
Supracondylar fractures
Types
• Based on the mechanism of injury, extension type (approximately 95%)
and fl exion type (5%).
• Classifi ed using the modifi ed Gartland system:
2
Type I. Undisplaced.•
Type II. Angulated/displaced, but posterior cortex is intact, acting •
as a hinge.
Type III. Complete displacement.•
Type IV. Completely displaced and unstable in fl exion and •
extension.
Treatment
Displaced supracondylar fractures (types III/IV) are an orthopaedic
emergency, especially if complicated with an absent distal pulse. Do not
delay.
• Assess neurovascular status and document beforehand.
• Reduce under GA by straight arm traction (up to 5min may be
required).
• Then manipulate to correct rotation, varus/valgus tilt, and fi nally any
extension deformity.
• Try to fl ex the elbow up past 90° with the forearm pronated (may be
diffi cult due to anterior soft tissue swelling; the reduction technique
itself can cause loss of the pulse in the fl exed position).
• Displaced (type III/IV) fractures should be reduced and stabilized with
K wires. Some advocate the same for type II injuries.
• Common confi guration is two crossed condylar K wires, one medial
(beware of ulnar nerve), and one lateral used to fi x the fracture.
• An above elbow cast is then used to supplement fi xations and wires
are removed at 4 weeks.
• Long arm traction may be used as defi nitive treatment, but involves a
long inpatient stay until the bone has united (usually 3 weeks).
Undisplaced fractures (type I) can be treated with a collar and cuff with or
without plaster backslab.

515
FRACTURES AND DISLOCATIONS AROUND THE ELBOW
Complications
Vascular
• Injury to the brachial artery is rare as the pulse usually returns after
fracture reduction.
• Examination is the key. An absent pulse with a well perfused hand
does not require any immediate vascular management; however, a
pulseless cold hand or a pulse that is lost post-reduction and pinning
does!
• True loss of the radial pulse may be due to:
Vascular spasm• . Typifi ed by good capillary refi ll after reduction,
but slow return of the pulse. Failure of pulse return may be due to
other injuries and requires a vascular surgical opinion. partial injury
(endothelial fl ap) is treated by direct repair.
Complete transection or disruption• . May be treated by direct repair
or more often, interposition vein graft.
• Contracture. Untreated vascular injury will result in fi brosis and
contracture of the forearm (‘Volkman’s ischaemic contracture’). This
is a devastating and debilitating condition and should be avoidable with
early (<12h) exploration and/or repair or vascular damage.
Neurological
• Neuropraxia is commonest with gradual recovery. May involve the:
Radial nerve.•
Anterior interosseous (branch of medial nerve).•
Median and rarely, ulnar nerves.•
Mal-union
• Incorrectly reduced fractures will not remodel and can lead to cubitus
valgus and a ‘gunstock deformity’. Much less common with K wire
fi xation.
• Recurvatum common following cast management of type II/III;
remodels poorly.
Lateral condyle fractures
Types
Classifi ed according to Milch, depending on how much of the intra-
articular surface is involved:
• Type I. Fracture through growth centre of capitellum (Salter–Harris
type IV).
• Type II. Fracture medial to growth centre and can involve trochlea
(Salter–Harris type II).
Treatment
• Displaced fracture. ORIF with either two cannulated screws or two K
wires.
• The fragment is always considerably larger than expected from the
X-ray due to the condyle being not fully ossifi ed.
• If not reduced and fi xed, the fragment will displace. This is due to the pull
of the wrist extensors, arising from the lateral epicondyle. This will lead
to a cubitus valgus deformity and can present in later life with an ulnar
nerve palsy as it has been chronically stretched (‘tardy’ ulnar nerve palsy).

CHAPTER 15 Orthopaedic surgery
516
Medial condyle fractures
• Not to be confused with epicondyle fractures.
• These fracture occur in a similar pattern to the lateral condyle (type I
and II).
• Treatment is essentially as described for lateral condyle fractures.
• The key is recognition of this injury as it is intra-articular and if missed,
can be associated with valgus instability of the elbow and subluxation.
Epicondyle fractures
Medial epicondyle fractures Avulsion type injuries of the apophysis. High
association with elbow dislocations. The fragment can remain undisplaced,
displaced, or become trapped in elbow joint. Treatment is usually con-
servative in a long arm cast. Surgery is indicated for trapped fragments.
Lateral epicondyle fractures Essentially the same as medial; treated with a
long arm cast unless fragment entrapped in joint.
Radial head and neck fractures
• Usually result from a valgus force to the elbow, associated with
dislocation or fractures (Monteggia).
• Fractures of the neck are often angulated, displaced, or both.
• Head fractures are of the Salter–Harris type.
• Fractures associated with dislocation happen at the time of injury or as
a result of reduction (radial head pushed into ulno-humeral joint).
Treatment
• <30°. Angulation acceptable; sling is provided and early mobilization.
• 30–60°. Reduction should be attempted, but ongoing debate.
• >60°. Reduction is required under GA, usually stable; once reduced,
do not require any further fi xation.
• Occasionally open reduction is required.
• Intra-articular fractures that are displaced may require fi xation with K
wires or screws.
Olecranon fractures
Can often be diffi cult to spot. The proximal epiphysis appears between 8
and 10y. Isolated fractures do occur, but are more commonly associated
with fracture dislocations of radial neck.
Treatment
• Undisplaced fractures require a cast in extension (removes pull of
triceps) for 4 weeks.
• Displaced fractures require ORIF with tension band wiring.
References
1 Wenger DR, Pring ME (2006). Rang’s Children’s Fractures, 3rd edn. Lippincott, Williams and
Wilkins, Philadelphia.
2 Wilkins KE (1997). Supracondylar fractures: what’s new? J Paediatr Orthop B 6(2): 110–16.
This page intentionally left blank

CHAPTER 15 Orthopaedic surgery
518
Fractures of the humeral shaft and
elbow in adults
Humeral shaft fractures
Mechanism
• Usually as a result of a fall with direct blow or torsional forces.
• Can be low energy (osteoporotic) or high energy (younger age group).
• X-rays of joints (above and below) important to rule out intra-articular
extension. High energy injuries, especially to rule out fl oating elbow or
shoulder.
• Remember to evaluate neurovascular status (radial nerve at risk).
Types
• Transverse, oblique, spiral, multifragmentary.
• Distal third fractures associated with radial nerve palsy, known as
Holstein–Lewis fracture.
Treatment
Conservative management Is the mainstay.
• Initial sugar tongue cast or hanging cast for 1–2 weeks, then convert
to functional brace until union (usually by 3 months); requires regular
clinic evaluation.
• Can accept 20° anterior/posterior angulation, 30° varus/valgus
angulation, 15° rotations, and 1–3cm of shortening.
• Remember to mobilize elbow and shoulder or will stiffen!
Surgical treatment Indicated if there is an open fracture, vascular injury,
associated intra-articular fracture, fl oating joint (proximally or distally),
pathological fracture.
• Relative indications include multiple injuries, inability to maintain
reduction closed, segmental fractures, or transverse fractures in young
athletes.
• ORIF with plate and screws is commonly used. Locking plates can be
utilized if poor bone quality.
• Intramedullary nailing is an alternative, good for pathological or
segmental fractures, or medically labile patients due to small exposure.
It is associated with shoulder pain and rotator cuff dysfunction.
Humeral condylar fractures
1
Mechanism
Usually due to impaction injury (the olecranon driven into the humerus via
a direct fall and the condyle usually splits into a ‘T’- or ‘Y’-shaped pattern).
Pattern depends upon bone quality and angle of fl exion at time of injury.
Careful neurovascular evaluation required.
Types
• Intercondylar fracture (most common). The fracture line extends from
the articular surface to the supracondylar region in a ‘T’- or ‘Y’-shaped
pattern.
• Supracondylar.

FRACTURES OF THE HUMERAL SHAFT AND ELBOW IN ADULTS
519
• Isolated medial or lateral condyle fracture.
• Isolated capitellum fracture.
Treatment
Intercondylar
• Intra-articular fractures. Thus principles are open anatomical reduction
with absolute stability, providing stability to allow early mobilization.
Posterior approach with either a triceps spilt or if more complex, a •
trans-olecranon osteotomy to visualize the articular surface.
Fixation by plate and screw constructs (reconstruction plates). •
Compression across the intra-articular segments may be required.
Newer pre-contoured distal humerus locking plates more
commonly used.
• Can be diffi cult to treat if heavily comminuted and the bone quality is
poor.
An option is conservative management in cast, but early •
mobilization to try to maintain as much function as possible (‘bag of
bones’ technique).
Non-union is not uncommon following these fractures and •
sometimes salvage surgery in the form of elbow replacement may
be considered.
In the elderly with a low fracture pattern, primary elbow •
replacement is sometimes used.
Supracondylar
• Conservative management in a cast if undisplaced or highly
comminuted in elderly. Mobilize at 2 weeks in hinged brace. Cast.
Discontinue when healed (6–8 weeks).
• ORIF if displaced. 90/90 plating was the classical method (medial and
posterolateral), but now utilize pre-contoured, bicolumnar locking
plates. Again stable fi xation with early mobilization is the goal.
Transcondylar
• A very distal fracture and within the joint capsule.
• Management follows same principles as of supracondylar type.
Capitellum
• Radiographs. The capitellum aligns with radial head on AP and lateral
views. Classifi cation dependent on size of fragment (best seen on
lateral).
Type I is a large osseous fragment, often involving the trochlea.•
Type II is a thin articular fragment with little osseous composition.•
Type III is multifragmentary.•
• Treatment. Type I requires ORIF. A common technique is headless
compression screws from a posterior to anterior direction. Type II
is usually not amenable to fi xation and is excised, as are the loose
components of type II injuries.
• Kocher’s approach (interval between anconeus and extensor carpi
ulnaris) often used.

CHAPTER 15 Orthopaedic surgery
520
Olecranon fractures
Mechanism A fall on to the point of the elbow, but can occur as a fall on to
the outstretched hand where the triceps avulses the olecranon process.
Colton classifi cation
• Type I. Undisplaced (<2mm separation, able to extend elbow against
gravity).
• Type II. Displaced (subtypes—IIA, avulsion; IIB, oblique/transverse; IIC,
comminuted; IID, fracture-dislocation).
Treatment
• Undisplaced. Place in a cast at 90°. At 4 weeks, begin mobilization.
• Displaced. ORIF with tension band wire technique.
• Comminuted. Plate and screw ± bone graft. Newer pre-contoured
locking plate available.
Radial head fractures
Mechanism Fall on to the outstretched hand, forearm in pronation.
Mason classifi cation
• Type 1. Minimally displaced.
• Type 2. Displaced.
• Type 3. Comminuted and displaced.
Treatment
• Type 1 (<3mm displacement). Sling or half cast for 1–2 weeks, then
mobilization (aspiration of the joint haematoma acutely can give pain
relief and injection of local anaesthetic can rule out any mechanical
block).
• Type 2 (displaced, mechanical block to motion or part of more
complex injury pattern). ORIF with compression screw and/or mini-
plate.
• Type 3 (too comminuted to allow ORIF). Radial head replacement
indicated. Excision considered at a later stage and contraindicated if
other destabilizing ligamentous injuries.
Complications of elbow fractures
• Joint stiffness (rotational).
• Degenerative joint disease (osteoarthritis).
• Heterotopic ossifi cation.
• Neurovascular injury and its sequelae.
References
1 M http://www.orthoteers.co.uk/Nrujp~ij33lm/Orthelbowinjadult.htm.
2 Colton CL (1973). Fractures of the olecranon in adults: classifi cation and management. Injury
5: 21–9.
3 Sarmiento A, Zagorski JB, Zuch GA, et al. (2000). Functional bracing for the treatment of frac-
tures of the humeral diaphysis. J Bone Joint Surg Am 82-A: 478–86.
This page intentionally left blank

CHAPTER 15 Orthopaedic surgery
522
Dislocations and fracture dislocations
of the elbow
Mechanism
• Simple. No bony component.
• Complex. Associated bony injury.
• Posterior and posterolateral. Most common type; fall on to an
outstretched hand with the elbow in extension or slight fl exion (with
supination and valgus forces).
• Anterior (rare). Fall on to a fl exed elbow or as a direct blow from
behind (‘side swipe injury’).
• Divergent (rare). Radius and ulna separated proximally.
Associated fractures (complex injury)
• Elbow stability dependent on bony and ligamentous component
integrity—radial head, coronoid, olecranon, medial and lateral
collateral ligaments.
• Coronoid fractures. Occur as distal humerus is driven against it during
subluxation, dislocation, or instability. Any injury to coronoid suggests
an episode of instability.
• Regan and Morrey classifi cation.
Type 1, tip fractured (consider anterior capsule injury).•
Type 2, <50% of the process.•
Type 3, >50% of the process.•
Treatment
• Aim to reduce under GA to allow thorough assessment of stability.
• In-line traction. Supinate forearm (clears coronoid); fl ex elbow from an
extended position whilst pulling olecranon in an anterior direction.
• Check elbow stability once reduced and X-ray to confi rm.
• If stable, collar and cuff at 90° for 7–10 days with early motion.
• If unstable (redislocates), place forearm in pronation if lateral collateral
ligament disrupted or supination if medial collateral ligament disrupted,
and immobilize in above elbow cast for 2–3 weeks.
• If there are associated injuries, then most fractures will require ORIF
to aid the stability and allow early mobilization. This may include radial
head prosthetic replacement.
The ‘terrible triad’
• Posterior elbow dislocation associated with radial head fracture,
coronoid process fracture, and lateral collateral ligament tear.
• The elbow will require surgical stabilization via ORIF of coronoid and
anterior capsular repair, ORIF or replacement of radial head, lateral
collateral ligament repair.
Complications
• Neurovascular injury.
• Compartment syndrome.
• Chronic elbow instability.

DISLOCATIONS AND FRACTURE DISLOCATIONS OF THE ELBOW
523
• Articular cartilage damage.
• Heterotopic calcifi cation.
• Stiffness (especially extension). Early motion at 1 week to try to
prevent this.
Further reading
Ring D, Jupiter JB (1998). Fracture-dislocation of the elbow. J Bone Joint Surg Am 80(4): 566–80.
[Current concepts review]

CHAPTER 15 Orthopaedic surgery
524
Fractures around the shoulder
Clavicle
Mechanism Fall or direct blow to lateral shoulder (5–10% of all fractures).
Types
• Occurs in the middle (75%), lateral (20%), or medial (5%) third.
• The pattern of lateral third fractures depends on relationship and
integrity of the coracoclavicular ligaments, and involvement of the
acromioclavicular joint.
• Medial third fractures are assessed with displacement and involvement
of sternoclavicular joint in mind.
Treatment
• Middle third. Virtually all can be treated conservatively with a broad
arm sling (not a collar and cuff). Indications for ORIF (plate and
screws) are open fractures, signifi cant neurovascular injuries, skin
tenting, fl oating shoulder. Fractures with >100% displacement and 2cm
of shortening have better outcomes with ORIF.
• Lateral third. Undisplaced can be treated non-surgically; however,
the presence of displacement suggestive of coracoclavicular ligament
disruption will require fi xation with either plate and screw constructs,
hook plate, or ligament reconstruction (Weaver–Dunn procedure).
• Medial third. Most are undisplaced and treated conservatively in a
sling. Displacement, especially posterior, into the root of the neck may
warrant surgery.
Complications
• Metalwork. Failure or subcutaneous irritation.
• Non-union. Associated with displacement and shortening.
• Acute complications. Neurovascular injury (including brachial plexus
injury), neurovascular compression (costoclavicular syndrome),
pneumothorax from bony penetration of the pleura.
Scapula
Mechanism Direct trauma, usually a high velocity injury such as an RTA.
Always have a high clinical suspicion of other possible injuries such as rib
fracture, pulmonary contusion, and pneumo/haemothorax; 20–40% have
ipsilateral clavicle fractures.
Treatment
• Simple (no involvement of the glenoid (glenohumeral joint)). Adequate
analgesia (very painful injury; may require HDU admission) and early
mobilization.
• Complex (involving the glenoid and glenoid neck). May need ORIF
after further imaging such as CT/MRI scanning.
• Floating shoulder will require ORIF of clavicle.

FRACTURES AROUND THE SHOULDER
525
Proximal humerus
Mechanism Young—high energy injury; elderly—low energy falls. Full
neurovascular assessment (especially axillary nerve) is essential, alongside
pre-injury function (aids management decision).
The Neer classifi cation
1
Based on Codman’s fracture lines along old physeal scars; four segments
or parts. A fracture part is considered when it is >1cm displaced or >45°
angulated. Thus defi ned as 1-, 2-, 3-, or 4-part fractures.
Treatment
• Undisplaced or impacted. Collar and cuff with early pendular
mobilization.
• Displaced. Usually requires ORIF by ‘locking’ plate, proximal humeral
intramedullary nails or cannulated screws, and K wires with or without
tension band wiring.
• Severely comminuted fractures (4-part), especially including fracture
dislocations, have a high rate of avascular necrosis; usually treated with
hemiarthroplasty and soft tissue reconstruction of the rotator cuff to
the prosthesis.
Complications Non- and mal-union, avascular necrosis of the humeral
head, and osteoarthritis of the shoulder joint are the commonest. High
velocity injuries may also cause neurovascular injuries, particularly of the
brachial plexus.
Paediatric humeral fractures
• Usually occur at the surgical neck or through and around the proximal
humeral epiphysis.
• May be indicative of a non-accidental injury.
• Most require no treatment apart from collar and cuff with mobilization
as for adults. Remodelling potential is good in this area.
Reference
1 Neer CS (1970). Displaced proximal humeral fractures. I. Classifi cation and evaluation. J Bone
Joint Surg Am 52-A: 1077–89.

CHAPTER 15 Orthopaedic surgery
526
Dislocations of the shoulder region
See Fig. 15.1.
Sternoclavicular joint
• Uncommon injury.
• Mechanism. Indirect force to lateral shoulder or direct impact on
medial end of clavicle.
• Types. Usually dislocates anteriorly; posterior dislocation is rare. The
deformity is at the medial clavicle.
• Complications. Tracheal and oesophageal compression may occur with
posterior dislocation. Careful assessment is required.
Treatment
• Anterior dislocation. Treated symptomatically with a sling, analgesia, and
early mobilization.
• Posterior dislocation with tracheal compression. Requires closed
reduction or open if this fails (with cardiothoracic surgical help).
Acromioclavicular joint (ACJ)
• Usually an injury of second to fourth decade, more common in males.
• Mechanism. Fall or direct impact on to the point of the shoulder.
• Rockwood classifi cation.
1
Six types with increasing numbers relating to
increasing severity of ligamentous disruption (acromioclavicular and
coracoclavicular) and displacement.
Type I. Sprained acromioclavicular ligament (no displacement).•
Type II. Acromioclavicular ligaments disrupted, ACJ subluxed.•
Type III. Acromioclavicular and coracoclavicular ligaments disrupted •
(>100% displacement).
Type IV. Both ligaments disrupted with posterior displacement.•
Type V. All ligaments torn and massively displaced.•
Type VI. All ligaments torn and inferior displacement (very rare).•
Treatment
• Types I and II (and some III). Broad arm sling and early mobilization
when pain allows. Persistent pain or functional limitation is treated by
reconstruction of the coracoacromial ligament.
• Type III and above. Acute repair indicated. Soft tissue reconstruction
better than hook plate.
Anterior dislocation of the glenohumeral joint
2,3
Mechanism Traumatic event, leading to forced abduction and external
rotation (fall on to the outstretched arm).
Associated features
• Young. Ninety per cent have traumatic injury to bony and/or soft tissue
restraints in the shoulder—the Bankart lesion (anteroinferior glenoid
labrum tear, with or without a glenoid rim fracture), Hill–Sachs lesion
(impression fracture as the anterior glenoid impacts on humeral head).
• Rotator cuff tears. Approximately 30% of those >40y and 80% of those
>60y will have a tear.
• Greater tuberosity fractures. Common over the age of 50y.

DISLOCATIONS OF THE SHOULDER REGION
527
Clinical fi ndings
• History of injury and whether had previous dislocations.
• The shoulder looks ‘square’ as the deltoid is fl at and a sulcus can be
visible where the humeral head may be.
• The patient supports the arm which is abducted and very painful.
• Assess neurovascular status (axillary nerve).
• X-rays (AP and axillary or scapular ‘Y’ lateral views) show the humeral
head anterior and inferior to the glenoid. Used to exclude a fracture of
the humerus or glenoid.
Treatment
• Reduce as an emergency in A&E.
• Give IV morphine 5–10mg + inhaled N
2
O (IV midazolam 5mg is usually
unnecessary).
Simplest, extremely reliable method is gentle, continued straight •
line traction with the arm abducted about 10–20° from the trunk.
May take 10–15min, but patience is the key, not force.
Avoid rotation (such as in a ‘Kocher’s manoeuvre’) as this is •
dangerous and may cause fracture of the humerus.
Countertraction can be placed across the trunk with a broad sheet.•
• Alternative technique is patient prone on the trolley, arm hanging
freely down and weighted (e.g. 3L bag of saline) (‘Stimson’s
technique’).
• If there is an associated humeral neck fracture, then the reduction
should be done under GA.
• Place the arm in a collar and cuff sling under the clothes. Repeat the
X-ray to confi rm reduction and that there has been no iatrogenic
fracture.
• Always document the neurological status (axillary nerve) before and
after reduction.
• Follow-up in clinic mandatory to assess for associated injuries.
Posterior dislocation of the glenohumeral joint
Mechanism Rare. Due to forced internal rotation or direct blow to the
anterior shoulder (e.g. after an epileptic fi t or electric shock). Common
to be missed.
Features
• The arm is held internally rotated and no external rotation is possible.
• The humeral head should be palpable posteriorly.
• AP X-rays may show the humeral head as a ‘light bulb’ shape
(internally rotated), but this is not diagnostic of posterior dislocation.
• Lateral X-ray shows the dislocation.
Treatment
• In-line traction method (as above), but consider GA if diffi cult—avoid
excessive force.
• May be very unstable; occasionally the ‘broomstick’ plaster may be
used.
CHAPTER 15 Orthopaedic surgery
528
Recurrent dislocation of the shoulder
• Usually due to a Bankart lesion or capsular redundancy (stretched and
fl oppy).
• Commonest in young age of fi rst dislocation (90% recurrence if <20y,
60% if 20–40y, <10% if older than 40y).
Treatment—surgery
• Repair and fi xation of the anterior ‘Bankart lesion’.
• May be done open or arthroscopically.
• Capsular laxity is treated by capsular shift, an overlapping ‘pants-over-
vest’ procedure to improve proprioceptive joint sensation.
References
1 Bucholz RW, Court-Brown CM, Heckman JD, Tornetta P (Eds) (2009). Rockwood and Green’s
fractures in adults, 7th edn. Lippincott, Williams, and Wilkins, Philadelphia.
2 Robinson CM, Dobson RJ (2004). Anterior instability of the shoulder after trauma. J Bone Joint
Surg Br 86-B, 469. [review]
3 M http://www.wheelessonline.com/orthoo/43.htm.
NN
N
D
D
D
N = normal position
D = dislocated
subglenoid
subcoracoid
(most common)
subclavicular
Fig. 15.1 Types of shoulder dislocation. Reproduced with permission from
Collier, J. et al. (2006). Oxford Handbook of Clinical Specialties, 7th edn. Oxford
University Press, Oxford.
This page intentionally left blank

CHAPTER 15 Orthopaedic surgery
530
Fractures of the ribs and sternum
Mechanism
• Single rib fractures occur as a result of direct injury such as a fall.
• Fractures of the lower ribs can occur with coughing.
• Sternal fractures occur with direct injury, e.g. contact with steering
wheel or by restraint by a seat belt.
• High velocity (RTA) or large crush injuries can result in a ‘stove-in’
chest with a fl ail segment, i.e. multiple rib fractures, each fractured at
two sites.
Treatment
• Single rib fracture. Symptomatic with analgesia.
• Multiple rib fracture. If t3 ribs involved, should admit for observation
overnight, but treatment symptomatic with analgesia and chest
physiotherapy.
• Sternal fracture. Symptomatic treatment, but observe for associated
injuries (see below).
• Flail chest or extensive multiple rib fractures. Potentially life-threatening
injury and may present with severe respiratory distress.
Flail segments move paradoxically, preventing adequate ventilation.•
Multiple fractures restrict respiratory effort, severely impairing •
ventilation.
Treat with high fl ow O•
2
and analgesia. CPAP and even IPPV may be
required.
Complications
1
Incidence of complications rises dramatically if the injury involves:
• >3 ribs.
• First, second, or third ribs.
• Sternum.
• Scapula.
They are all indicators of high energy transfer injury.
Cardiac tamponade
• Bleeding into the pericardial cavity causes severe haemodynamic shock
and may be the cause of a cardiac arrest at presentation.
• Diagnosis is by high clinical suspicion, muffl ed heart sounds, raised JVP,
and no signs of a tension pneumothorax.
• Treat by immediate pericardiocentesis with transfer to cardiothoracic
unit for repair of the defect.
Pneumothorax
• Usually due to direct pleural injury by bone fragments during injury.
• Often associated with haemothorax.
• Signs are respiratory distress, absent breath sounds, hyperresonant
percussion note (pneumothorax), or shock.

FRACTURES OF THE RIBS AND STERNUM
531
• Tension pneumothorax is life-threatening and requires immediate
decompression via a 16G needle placed into the second anterior
intercostal space, followed by defi nitive chest drain placement.
• If in any doubt, always treat fi rst on clinical grounds rather than wait
for investigations (X-rays, etc.).
Reference
1 American College of Surgeons (2008). Advanced trauma life support (ATLS) for doctors, student
manual, 8th edn. American College of Surgeons, Chicago.

CHAPTER 15 Orthopaedic surgery
532
Fractures of the pelvis
Mechanism
• Age <60y. High energy—RTA or falls at work (building sites) or sport
(horse riding).
• Age >60y. Low energy (insuffi ciency fracture)—fall from standing
height.
The force required to fracture the pelvis in the young is considerable and
as a result, the morbidity and mortality can be as high as 20%. It is the main
cause of death in multiple trauma patients.
Types
• The pelvis is a ring consisting of two innominate bones and the sacrum.
• Anteriorly are the ligaments of the symphysis pubis and posteriorly,
the ligaments to the sacrum (sacrospinous, sacrotuberous, and
sacroiliac).
• An isolated break at any part is generally stable (the ring will not
separate).
• Two breaks in the ring make it unstable (able to displace or open).
Remember this can be due to a fracture or ligament disruption!
• Young and Burgess classifi cation (descriptive):
1
AP compression (impact from the front or rear).•
Lateral compression (impact from side).•
Vertical shear (usually a fall from height).•
Combined mechanical (mixture of all of the above).•
Assessment
• ATLS approach.
• Mechanism of injury important as gives insight into degree of injury.
• Assessment and documentation of other injuries is mandatory.
• Look for neurological, gastrointestinal, and genitourinal injury.
Treatment
Initial treatment of all pelvic fractures should include ATLS protocols.
Once stable, an AP pelvis X-ray supplemented with inlet and outlet views
are required.
A CT is helpful to assess the posterior pelvic structures that can be
obscured on a plain X-ray.
Single ring fracture (stable)
• Check for an occult sacroiliac ligament injury (local bruising and
tenderness with pain on stressing the joint); this suggests the injury
could be unstable.
• Fractures of both superior and inferior pubic rami on the same side
are a single break in terms of ring stability.
• Other stable patterns include ilium fractures into sciatic notch or
sacrum.
• Remember that if a signifi cant single break in the ring is present, there
is a chance that concurrent ligamentous injury exists. Thus, a CT scan
is often required prior to mobilization to confi rm stability.
FRACTURES OF THE PELVIS
533
• Isolated fractures of the ilium, ischium, or pubis are normally treated
with bed rest, analgesia, and early mobilization as soon as the pain
allows.
• Fractures that extend to the acetabulum (joint) require further
investigation to plan treatment, but are usually stable if isolated.
• Simple pubic rami fractures in the elderly (osteoporotic) can be
treated conservatively with bed rest and early rehabilitation.
Multiple ring fractures (unstable)
Unstable fractures are liable to massive haemorrhage within the soft
tissues of the pelvis. This is mostly because the pelvic ring is grossly
displaced during the injury and tearing of the extensive posterior pre-
sacral venous plexus occurs. A patient’s entire blood volume can be lost,
hence the high mortality rate.
Establish haemodynamic control
• Approach patient according to ATLS guidelines (ABCDE).
• Haemorrhage is leading cause of death with pelvic injuries.
• Tachycardia and hypotension suggest bleeding.
• Establish at least two large calibre IV infusions and commence
resuscitation with warm crystalloid 2000mL, and then reassess.
• Send blood for urgent cross-match of 4–6U. O –ve blood is given if no
response to initial fl uid challenge.
• Continued bleeding must be controlled by reducing the pelvic volume.
This may be achieved by:
Pelvic binder or binding a bedsheet tightly around the pelvis •
and internally rotating the hips—very effective as an emergency
procedure.
Application of an external pelvic fi xator is a more defi nitive •
solution, but should be done in the trauma theatre.
• Laparotomy is contraindicated unless there are life-threatening
intra-abdominal injuries that must be treated since this effectively
‘decompresses’ the pelvis again superiorly.
• Urethral injury occurs, especially with anterior compression bony
injuries. If there is blood at the urethral meatus, perineal bruising,
haematuria, or a high riding prostate on rectal examination, then
catheterization should only be performed by an experienced urologist
(very often suprapubically). Investigation for injury is with a retrograde
urethrogram or IVU once the patient is stable.
Defi nitive management
• Signifi cant AP compression mechanism can result in an ‘open book’
pelvis. Severe subtypes of the lateral compression or vertical sheer
fractures are very unstable and associated with other injuries.
• Once ATLS stabilization is complete, CT scanning is required to defi ne
the fracture and plan treatment.
• External fi xation is excellent to temporarily control unstable fractures
and manage defi nitively. It can help to control haemorrhage in a
haemodynamically unstable patient.

CHAPTER 15 Orthopaedic surgery
534
• Once stable, liaise with local pelvic fi xation centres to arrange
defi nitive fi xation if required.
• ORIF with screws and plates for the symphysis pubis of ilium fractures.
Posterior pelvic instability involving the sacrum require screw fi xation.
Complications
• Haemorrhage, shock, and death from exsanguination.
• Open fractures carry a 50% mortality and need to be treated
aggressively by both orthopaedic and general surgical teams.
• Urogenital injury.
• Thromboembolism (35–50% develop DVT and 10% PE).
• Neurological injury.
• Paralytic ileus.
• Mal-union may lead to diffi culty with pregnancy.
• Osteoarthritis.
Acetabular fractures
• Usually high energy injury in the young from RTA (dashboard impact)
or fall from a height. Associated with hip dislocation.
• Assess as per all pelvic fractures (i.e. ATLS).
• X-rays required include AP pelvic views plus Judet views (45° internal
and external views); CT is routine.
• Letournel–Judet classifi cation. Fractures as posterior wall, posterior
column, anterior wall, anterior column, or transverse.
• Non-surgical management is reserved for fractures that are
undisplaced (except posterior wall as hip unstable) and do not involve
the ‘dome’, the superior acetabular roof (weight bearing area).
• Surgical management, ORIF, in displaced dome fractures, fractures
resulting in joint instability, or trapped intra-articular fragments.
Sacral fractures
• High energy injury in the young or low energy in the elderly.
• Assess as per all pelvic fractures (i.e. ATLS). Must assess for sacral
nerve root injury.
• X-rays include AP pelvic (inlet and outlet) views; CT is required as
diffi cult to fully appreciate on X-ray.
• Denis classifi cation. Alar lateral to foramen, involving the foramen, or
central portion medial to foramen.
• Non-surgical management is reserved for fractures that are
undisplaced or impacted.
• Surgical management if displaced or loose fragments impinging on
nerve roots.
References
1 Burgess A, Young JWR et al. (1990). Pelvic ring disruptions: effective classifi cation system and
treatment protocols. J Trauma 30(7), 848–56.
Further Reading
American College of Surgeons (2008). Advanced trauma life support (ATLS) for doctors, student
manual, 8th edn. American College of Surgeons, Chicago.
This page intentionally left blank

CHAPTER 15 Orthopaedic surgery
536
Femoral neck fractures
Mechanism
• Commonest fracture in the elderly with an exponential increase in
incidence with age.
1
• The risk increases with decreasing bone mass (osteoporosis).
• In the elderly, usually result from a trip/fall onto side (low energy).
• Fractures in the young are usually a result of high energy trauma.
Classifi cation
Fractures should be described anatomically:
• Intracapsular fractures. Occur within the joint capsule (proximal to the
intertrochanteric line).
• Extracapsular fractures. Occur distal to the joint capsule (involving or
distal to intertrochanteric line).
• It is then important to describe fractures as displaced or undisplaced.
• The relationship to the blood supply is key:
The femoral head receives its supply via the medial and lateral •
femoral circumfl ex arteries which form the extracapsular ring and
give rise to the cervical arteries (the lateral being most important).
There is also supply via the intraosseus nutrient vessels and the
ligamentum teres.
Displaced intracapsular fractures disrupt their blood supply and •
have a high rate of avascular necrosis (AVN) of the femoral head
and non-union.
Extracapsular fractures maintain the blood supply to the head (thus •
reduced AVN and generally heal well).
• Other classifi cation systems are not generally required.
• Subtrochanteric fractures. Occur below the level of the lesser
trochanter. May be through an area of pathological bone (metastasis)
or high energy in young.
Assessment
• Inability to weight bear following a fall in the elderly is a common
presenting feature.
• Consider medical cause of fall (stroke, MI, etc.).
• Other comorbidities essential to ascertain.
• Pre-injury level of function and home circumstances important.
• If the fracture is displaced (common), the leg will be shortened and
externally rotated. Straight leg raise and hip movements are globally
inhibited by pain. Neurological state important.
• Most fractures do not require any temporary stabilization; however,
subtrochanteric fractures may benefi t from a Thomas splint for pain
relief.
• X-rays. AP pelvis and lateral of affected hip (long leg views if history of
malignancy to look for metastases).
Displaced fractures are usually obvious.•
Undisplaced, intracapsular compression fractures may be diffi cult •
to see on X-ray. If high clinical suspicion of fracture, then further
investigation is warranted; isotope bone scan (highly sensitive, poor
specifi city), MRI (gold standard), or CT scan.

FEMORAL NECK FRACTURES
537
Treatment
The majority of hip fracture require surgical stabilization to allow early
mobilization and prevent displacement. This will reduce the risks of long
periods of immobilization and bed rest (pressure sore, DVT, etc.).
Intracapsular
• Undisplaced impacted in the elderly. Treated by early mobilization with
analgesia; 15% late displacement rate, requiring operative intervention.
• Undisplaced. Treated by internal fi xation with either cannulated screws
or a 2-hole dynamic hip screw (DHS).
• Displaced. Treated by hemiarthroplasty.
2
• Total hip arthroplasty can be used if symptomatic pre-existing arthritis
or those with few comorbidities and high functioning (controversial).
• In children or young adults, reduction (open or closed) and fi xation is
employed.
Extracapsular
• Closed reduction on the traction table and open fi xation with the use
of a DHS.
• Intramedullary hip screw (IMHS) may be used in 4-part fractures.
• Subtrochanteric and reverse obliquity fractures require stabilization,
utilizing an intramedullary nail or fi xed angle plating system.
Complications
• Overall mortality in the elderly is 20% at 90 days. This is indicative of
the fact that the fracture is more a marker of generally poor condition
rather than due to acute surgical perioperative complications.
• AVN of the femoral head.
• Dislocation of arthroplasty.
• Loss of fi xation.
• Non-union.
• Lower limb thromboembolic disease.
References
1 Parker MJ, Pryor GA, Thorngren KG (1997). Handbook of hip fracture surgery. Butterworth-
Heinemann publications, Oxford.
2 Parker MJ, Khan RJ, Crawford J, et al. (2002). Hemiarthroplasty versus internal fi xation for dis-
placed intracapsular hip fractures in the elderly. A randomised trial of 455 patients. J Bone Joint
Surg Br 84-B: 1150–5.
Further Reading
Soloman L, Warwick D, Nayagam S (Eds) (2010). Apley’s system of orthopaedics and fractures,
9th edn. Hodder Arnold, England.

CHAPTER 15 Orthopaedic surgery
538
Femoral shaft fractures
Mechanism
• High energy RTA in young adults (dashboard injury) or fall from height.
• Stress fractures.
• Low energy in elderly.
• Pathological (metastases).
Classifi cation
• AO system can be used, but is complex.
• Anatomical description is the simplest:
Location (proximal, mid- or distal shaft, divide into thirds, or •
metaphyseal or diaphyseal).
Confi guration (transverse, oblique, spiral, segmental, comminuted).•
Number of fragments.•
Associated injuries
• Polytrauma is common. Look for head, chest, and abdominal injuries.
• Ipsilateral femoral neck fracture (up to 5%).
• Pelvic and acetabular fractures.
• Knee joint injuries. Both bony or ligamental (e.g. anterior cruciate
ligament (ACL) rupture).
• Soft tissue injury to skin, muscle, neurovascular structures.
• Sciatic nerve traction injury (uncommon).
• Bilateral femoral shaft fractures associated with 25% mortality.
Treatment
• ATLS (ABCDE).
• Establish two large calibre IV access and give 2000mL of crystalloid;
initial haemodynamic compensation is common in the young and may
hide a large blood loss. Can lose up to 4U (1500mL) of blood into
tissues around a femoral fracture.
• Send blood for FBC, U&E, group and save.
• Realign and splint the leg with skin traction and a Thomas splint. This
will help to control pain and haemorrhage.
• An X-ray of a femoral fracture not in a splint should never be seen!
Diagnose clinically; splint, then get the X-ray.
• If an ‘OPEN’ fracture, photograph the wound, socially clean it, and
place a betadine dressing over it, stabilize it. Commence IV antibiotics
and tetanus toxoid if required.
• Full secondary survey, looking for associated injuries.
Children
• Nearly always heal and remodel.
• Age 0–2y. Treat in gallows (suspension) traction until callus seen
(2–4 weeks) or Pavlik harness/hip spica.
• Age 2–6y. Treat with closed manipulation and hip plaster spica (allows
discharge) or continuation of the Thomas splint.

FEMORAL SHAFT FRACTURES
539
• Age 6–14y. Options are a fl exible intramedullary nail (‘elastic’ nail),
ORIF with a plate and screws, or external fi xation.
• Age >14y. Can consider locked intramedullary fi xation.
Adults
• Non-operative treatment with traction if patient too sick for surgery
(be aware of complications: pressure sores, DVT, etc.).
• Locked and reamed intramedullary nailing is the common treatment
regime (provides rotational stability).
• Plate and screw construct can be used if there is distal metaphyseal
extension. Usually much larger exposure.
• Temporizing external fi xation is occasionally required in damage
control scenarios. This can be exchanged for a nail once patient stable
enough.
Complications
• Compartment syndrome.
• Fat embolus (1%) and possible ARDS.
• Infection (5% after open, 1% after closed nailing).
• Non-union.
• Thromboembolic disease.
• Neurological injury.
• Mal-union, rotation being the most symptomatic.
• Pressure sores, bronchopneumonia, UTI on conservatively treated
patients.
Further reading
Metaizeau JP (2004). Stable elastic intramedullary nailing for fractures of the femur in children.
J Bone Joint Surg Br 86-B: 954–7. [Operative technique]
Wolinsky P et al. (2001). Controversies in intramedullary nailing of femoral shaft fractures. J Bone
Joint Surg Am 83-A: 1404–15. [Instructional course lecture]

CHAPTER 15 Orthopaedic surgery
540
Fractures of the tibial shaft
Mechanism
• High energy injuries in young as a result of RTA, sporting injury, fall
from height.
• Direction of force dictates fracture pattern—torsional (spiral), direct
blow (transverse or short oblique). Higher energy patterns suggested
by multifragmentary fractures with or without bone loss.
• Soft tissue injuries common as tibia subcutaneous. Be aware of ‘OPEN’
fractures.
Assessment
• ATLS approach recommended.
• Inspect for angulation, deformity, and malrotation.
• Subcutaneous crepitation may be present or obvious open wound.
• Neurovascular status needs to be assessed and documented.
• Watch for ‘compartment syndrome’. Presents as pain, uncontrolled by
analgesia, and out of proportion to injury. Look for pallor, paraesthesia,
and pulselessness (late signs). Passive dorsifl exion of joint distal to
injury stretching the muscles in the affected compartment is usually
diagnostic.
• Compartmental pressures can be measured; an absolute pressure
of >40mmHg or <30mmHg difference between compartment and
diastolic BP diagnostic.
• This is a clinical diagnosis and requires immediate management via
fasciotomies of affected compartments.
Classifi cation
• AO system can be used, but is complex.
• Anatomical description is the simplest:
Position (mid-shaft, junction of distal third, etc.).•
Confi guration (transverse, oblique, spiral, segmental, comminuted).•
Number of fragments.•
• Open injuries are normally classifi ed additionally by the Gustillo–
Anderson system.
1
Treatment
2,3
There is no one method of treatment that is appropriate for all fractures.
There is a continual contentious debate about the pros and cons of differ-
ent modalities. The best rule is to judge each fracture and associated soft
tissue injury on an individual basis and treat appropriately.
Non-surgical (plaster of Paris)
• Used for undisplaced fractures and low energy displaced fractures in
children that can be closed reduced.
• Long leg cast, with the knee fl exed 20* and the ankle in neutral.
• Mobilize non-weight bearing on crutches, with X-rays weekly for the
fi rst 4 weeks to check alignment.
• Start weight bearing at 8 weeks in a weight bearing contact ‘Sarmiento’
cast.

FRACTURES OF THE TIBIAL SHAFT
541
• Union takes around 14–16 weeks.
• Advantages. Simple and avoids all operative risks.
• Disadvantages. Takes a long time; requires good follow-up and patient
compliance. Stiffness at the knee and ankle is common and unstable
injuries are very diffi cult to manipulate and control in plaster alone.
Cast bracing A variation of plaster where Sarmiento plaster is applied
from day 1.
Surgical
• Indicated if unable to maintain closed reduction, i.e. >50% displaced,
>10° angulated, >10° rotational deformity, >1cm shortening.
• Unstable fracture patterns. Multifragmentary and same level tibial
fractures.
• Open fractures.
Locking plate fi xation
• Mostly used for fractures near the joint surface.
• Plates used in the shaft have a high rate of infection and non-union
caused by the large soft tissue exposure required.
• Advantages. Simple, quick, rapid mobilization, and avoids the need for
plaster.
• Disadvantages. Risk of infection, non-union, and implant failure.
Intramedullary nailing
• Currently the treatment of choice in most centres, but requires
increased operating time and experience.
• May be used in compound fractures, especially where soft tissue fl aps
are required since it gives relatively unlimited access to the tibia ‘fi x
and fl ap’.
4
• Best for mid-shaft fractures and is poor at controlling fractures within
5–10cm of the knee and ankle joints.
• Advantages. Early mobilization, quicker rehabilitation than closed
methods, soft tissue undisturbed by technique, access for fl aps easy.
• Disadvantages. Technically demanding, high rate of chronic anterior
knee pain (site of nail insertion—not recommended for kneeling
profession, e.g. carpet fi tters).
External fi xation
• Often used in compound fractures as it allows least disturbance of
soft tissue. Can be placed in an extremely rigid confi guration to allow
stability. Rigidity can then be reduced sequentially in outpatients, if
required.
• Tensioned wire circlage frames pioneered in Russia (Ilizarov) can be
used for diffi cult fractures around the knee or ankle.
• Advantages. Technically simple (not Ilizarov); allows early mobilization,
avoids further soft tissue damage.
• Disadvantages. Pin site infections common, but usually easily treated.
Requires good nursing backup and patient compliance. Pin sites need
to be planned carefully with plastic team if fl aps used so as not to
compromise soft tissue cover.

CHAPTER 15 Orthopaedic surgery
542
Open fractures
• Guided by the British Orthopaedic Association and British Association
of Plastic, Reconstructive, and Aesthetic Surgeons Guidelines.
5
• Recommend a multidisciplinary approach in a specialist centre, if
possible.
• High energy patterns of fracture with soft tissue injury (skin loss,
degloving, muscle damage or loss, arterial injuries) need to be acted
upon promptly.
• Initially ATLS approach.
• Assessment of affected limb. Neurovascular status essential and
repeated regularly.
• Give IV broad-spectrum antibiotics (within 3h of injury).
• Treat limb-threatening injuries immediately (vascular or compartment
syndrome).
6
• Remove gross contamination from open wounds, photograph, wrap in
saline-soaked gauze and fi lm dressing. Immobilize the whole affected
limb in a splint. Tetanus status checked.
• Combined management approach to plan defi nitive treatment of
fracture and soft tissues. Aim to do within 24h of injury if isolated open
fracture.
References
1 Gustilo RB, Anderson JT (1976). Prevention of infection in the treatment of one thousand and
twenty-fi ve open fractures of long bones: retrospective and prospective analyses. J Bone Joint
Surg Am 58-A: 453–8.
2 Schmidt AH et al. (2003). Treatment of closed tibial fractures. J Bone Joint Surg Am 85-A, 352–68.
[Instructional course lecture]
3 Bhandari M et al. (2001). Treatment of open fractures of the shaft of the tibia. J Bone Joint Surg B
83-B: 62–8. [Review]
4 Gopal S, Majumder S, Batchelor AG, et al. (2000). Fix and fl ap: the radical orthopaedic and
plastic treatment of severe open fractures of the tibia. J Bone Joint Surg Br 82-B: 959–66.
5 Standards for management of open fractures of the lower limb. (2009). BOA and BAPRAS.
M http://www.boa.ac.uk or M http://www.bapras.org.uk
6 Elliott KG, Johnstone AJ (2003). Diagnosing acute compartment syndrome. J Bone Joint Surg Br
85-B: 62–8. [Review]
This page intentionally left blank

CHAPTER 15 Orthopaedic surgery
544
Fractures of the ankle
• Commonest fracture of the lower limb.
• Usually low energy rotational force, resulting in simple to complex
confi gurations.
• The talus rotates in the mortise and produces different patterns
dependent on whether the foot is inverted or everted.
• Axial load causes fracture of the tibial plafond.
• The ankle should be thought of as a ring and stability is conferred by:
Bones.• Medial and lateral malleoli and the talus (form ‘mortise’).
Ligaments.• Laterally, the tibiofi bular ligamentous complex
(syndesmosis) and the lateral collateral ligaments (talofi bula and
calcaneofi bular); medially the deltoid ligament.
• Remember that a fracture of the proximal fi bula (at the knee) is
associated with an ankle fracture or dislocation until proven otherwise.
Assessment
• Initially, ATLS approach.
• Inspect for bruising, swelling, obvious deformity, open wounds, skin
tenting, signs of neurovascular compromise.
• Depending on the degree of injury, some patients may walk in for
assessment.
• Remember to examine the whole fi bula for proximal tenderness.
• Examine for medial tenderness. The medial injury may be ligamentous
only, but this is enough to destabilize the ankle and allow talar shift.
• X-rays are mortise AP (15° internal rotation) and lateral views.
Classifi cation
• AO/Danis–Weber system (based on level of fi bula fracture):
Type A. Below the syndesmosis.•
Type B. At the syndesmosis.•
Type C. Above the syndesmosis.•
• The Lauge–Hansen classifi cation is more complex and based upon
mechanism of injury. Foot supinated and adducted or externally
rotated or foot pronated and abducted or externally rotated.
• A distal tibial fracture involving the joint is known as a pilon fracture.
Treatment
Displaced fracture/dislocation
• A displaced fracture dislocation is an orthopaedic emergency and is
always clinically obvious. Displacement is often more than expected
due to soft tissue swelling.
• Reduce the fracture immediately in A & E and apply a below-knee
backslab before sending the patient for an X-ray. An X-ray of a
dislocated ankle should never be seen!
• Check neurovascular status before and after reduction.
• Give plenty of analgesia ± sedation (usually done in resuscitation area).

FRACTURES OF THE ANKLE
545
Stable injuries
• Lateral malleolus only (Weber A or B) with no talar displacement
(shift) in the mortise.
• Ensure no medial tenderness exists.
• These can be managed in a well fi tting below-knee cast with the foot
at 90° (neutral).
• Obtain a post-cast X-ray to ensure position acceptable.
• Regular follow-up and serial X-rays required to ensure reduction
remains.
• Total of 6 weeks cast.
• Weight-bearing is allowed.
• Some simple Weber A fractures require just a supportive elasticated
stocking.
Unstable injuries
• Minimal displacement (d2mm) is acceptable in the elderly and treated
by plaster as above.
• Weber B or C fractures with medial tenderness or talar shift.
• Initially placed into backslab for comfort, elevated, and iced (reduces
swelling).
• If too swollen, skin closure is compromised, thus aim to do within fi rst
24–48h.
• Check for signifi cant blisters around areas of incision.
• ORIF is used with the lateral fracture reduced and held with a ‘lag
screw’ and ‘neutralization’ plate and screw construct.
• The medial malleolus is fi xed directly with two partially-threaded
cancellous screws (compression) or if a small fragment, a tension
band wire construct.
• A cast is applied following fi xation and the patient remains in this
non-weight bearing for up to 6 weeks.
Pilon fractures
• Intra-articular fractures of the tibial plafond due to axial force (high
energy).
• Initial management is as for ankle fractures.
• Careful assessment for swelling, skin compromise, blisters, and
neurovascular status.
• Standard AP and lateral X-rays required; often a CT is needed to
defi ne fracture pattern further and plan treatment.
• Non-surgical management only for undisplaced fractures.
• Surgical management is related to soft tissue status.
• External and internal fi xation techniques are applicable.
Further reading
Vander Griend R, Michelson JD, Bone LB (1996). Fractures of the ankle and the distal part of the
tibia. J Bone Joint Surg Am 78-A: 1772–83. [Instructional course lecture]
M http://www.blackburnfeet.org.uk/hyperbook/trauma/ankle_fractures/ankle_fractures_intro.htm.

CHAPTER 15 Orthopaedic surgery
546
Fractures of the tarsus and foot
Talus
1
Mechanism
• Usually a fall from height or an RTA (high energy).
• Foot forcibly dorsifl exed against the tibia.
• Look for associated ankle fractures.
• Blood supply to the talus often compromised. Talus has limited soft
tissue attachments, thus relies on extraosseous vessels, which are
easily disrupted.
Assessment
• ATLS.
• Look for associated injuries.
• Look for compartment syndrome of foot.
• Neurovascular status.
• AP and lateral of ankle plus CT.
Classifi cation
• By anatomical site, i.e. head, neck, body, or lateral process.
• ‘Hawkins’ classifi cation for talar neck fractures (types I–IV, increasing
levels of displacement and subluxation with increasing grade).
Treatment
• Body fractures. Treated surgically with ORIF unless undisplaced.
• Neck fractures:
Undisplaced.• Strict non-weight bearing in below-knee plaster for 6
weeks.
Displaced.• ORIF is required. If dislocated, urgent management
required as soft tissue can be compromised.
Complications
• Avascular necrosis. Rate increases with displacement (10% in type I to
90% in type III).
• Osteoarthritis of tibiotalar and subtalar joints.
• Mal-union.
Calcaneum
2
Mechanism
Axial load. Fall from height or RTA.
Assessment
• ATLS.
• Assess for associated injuries. Spinal (thoracolumbar) fracture and
upper limb injuries.
• Swelling can be signifi cant. Assess for compartment syndrome acutely.
• AP ankle and lateral plus axial Harris view.
• CT may be required.

FRACTURES OF THE TARSUS AND FOOT
547
Classifi cation
• Extra- or intra-articular.
• Intra-articular fractures involve the subtalar joint and are classifi ed by
their CT appearance by ‘Saunders’ system.
Treatment
• Extra-articular or undisplaced intra-articular fractures.
Conservative. Elevation, ice, bed rest, and observation of soft •
tissues overnight.
Mobilize non-weight bearing with a removable splint to stop •
equinus at the ankle. Early subtalar passive mobilization should be
initiated.
• Displaced intra-articular fractures. Operative treatment is still
controversial. ORIF is usually delayed 10–14 days for swelling to
resolve. Caution is exercised if patient a smoker, advanced age,
complex patterns, multiple trauma, compensation, bilateral fractures.
Complications
• Wound breakdown.
• Mal-union.
• Subtalar arthritis.
• Peroneal tendon pathology.
Tarsometatarsal (TMT) fracture-dislocations (Lisfranc)
1
Jacques Lisfranc de Saint-Martin described an amputation technique across
the fi ve TMT joints as a solution to forefoot gangrene secondary to frost-
bite. This became known as the Lisfranc joint.
Mechanism and assessment
• Direct dorsal force (RTA) or indirect rotational injury to a plantar
fl exed and fi xed forefoot (foot caught in a riding stirrup and rotation
of body around it).
• The Lisfranc ligament runs from the base of the second metatarsal to
the medial cuneiform. It is the only link between the fi rst ray and the
rest of the forefoot. The recessed base of the second metatarsal also
provides bony stability.
• Disruption of the Lisfranc ligament, with or without a bony
component, results in incongruity of the TMT joint.
• Neurovascular status and compartment syndrome must be assessed.
• AP, oblique, and lateral X-rays are required. Consider weight-bearing
views.
• The medial cortex of second metatarsal should align with medial
cuneiform. Look for ‘fl eck’ sign, suggesting avulsion of Lisfranc
ligament.
• These injuries are commonly missed so a high index of suspicion is
required.

CHAPTER 15 Orthopaedic surgery
548
Treatment
• ORIF is required for all displaced injuries using screws, plates and
screws, and supplementary wires.
Complications
• Foot compartment syndrome in acute injuries.
• Metatarsalgia.
• Post-traumatic arthritis.
• Purely ligamentous injuries have the worse outcome.
Metatarsal and phalanges
Mechanism
• Crushing or twisting injuries (e.g. the foot being run over).
• Fifth metatarsal fracture occurs after an inversion injury and can be
mistaken for an ankle fracture if not examined correctly.
• Always be suspicious of compartment syndrome in severe crush
injuries.
Treatment
• Metatarsal fractures. If minimal displacement or angulations,
conservative treatment with mobilization as pain allows. Plaster only
if mobilization is too painful. Multiple fractures may require reduction
and fi xation.
• Non-union of the fi fth metatarsal sometimes requires ORIF with
grafting if problematic (rare).
• Phalangeal fractures. Neighbour strapping.
References
1 M http://www.orthoteers.co.uk/Nrujp~ij33lm/Orthfootfracfoot.htm.
2 Sanders R (2000). Displaced intra-articular fractures of the calcaneus. J Bone Joint Surg Am 82-A:
225–50. [Current concepts review]
This page intentionally left blank

CHAPTER 15 Orthopaedic surgery
550
Injuries and the spinal radiograph
If a patient complains of central pain in the spinal column after trauma,
always obtain radiographs. This should not delay resuscitation as a spi-
nal fracture can be immobilized and life-threatening problems corrected
fi rst.
Spinal injuries can be associated with other injuries and the patient may
not be able to communicate this to you because they are:
• Unconscious.
• Intubated.
• Shocked.
• Intoxicated.
• Anaesthetized distal to a cord lesion.
Common injuries associated with spinal trauma are:
• Bilateral calcaneal fractures—thoracolumbar fractures.
• Facial fractures—cervical fracture/dislocation.
• Severe head injury—cervical injuries, especially C1/C2.
• Sternal dislocation—thoracic spine fracture.
• Ankylosing spondylitis—cervical and thoracic fractures.
• Cervical fracture—10% rate of fracture at another level.
An awake, alert, oriented patient who can demonstrate a normal painless
range of motion of the cervical spine does not need radiographic evaluation.
X-ray interpretation
• Develop a mental picture of the normal spinal radiograph. If you feel
that the X-ray ‘just doesn’t look right’, then it probably isn’t!
• Try to develop a system and use this for every fracture you see, even
when you know it will be normal. It gets you into the habit.
• A systematic approach has been shown to reduce the risk of missed
spine injuries.
C-spine
• AP, lateral, and open mouth views for C1/C2 are required.
• You must be able to see from C1 to T1; if not, request further views
(swimmer’s view).
• Look at the bones and their alignment.
• On the lateral fi lm, a smooth line should run down the anterior aspect
of the vertebral body, the posterior aspect, the anterior aspect of the
spinous process (spinolaminar line), and the posterior aspect of the
spinous process.
• Look for any obvious steps between vertebral bodies (up to 25%
displacement may suggest unifacet dislocation, >25% suggests
complete facet joint instability) and angulation.
• Examine each vertebral body for integrity.
• Look at the facet joints for congruity (facet joint dislocations).
• Look at the distance between the spinous processes (increase suggests
injury).
• Assess the soft tissues. Disc spaced for narrowing or widening.

INJURIES AND THE SPINAL RADIOGRAPH
551
• Assess the soft tissues anterior to the spine. This should be no more
than 7mm at C3 and 3cm at C7. Any increase suggests swelling and
thus injury.
• Look at the odontoid peg and its relationship to C1. Look for fractures
and the gap in front of peg (usually <3mm).
• On the AP fi lm, assess the vertebral body height and alignment.
• The interpedicular distances (increase may suggest fracture).
• Look for central alignment of the spinous processes. Beware the
empty vertebrae; this may imply damage to the spinous process.
• Check the transverse processes; they become displaced when
fractured.
Thoracolumbar spine
• AP and lateral views required.
• Thoracic spine is diffi cult to interpret; look for other clues such as
multiple rib fractures.
• On the AP, look at alignment.
Vertebral body height.•
Interpedicular distance. An absent or broken pedicular ring may •
suggest fracture of the posterior elements.
Central spinous process alignment.•
Integrity of the transverse processes.•
Disc height.•
• On the lateral X-ray, assess alignment and look for steps.
Look at the vertebral body and check for anterior wedging (>50% •
loss of body height suggests instability).
Any bony fragments displaced into the vertebral canal posteriorly.•
Check disc height.•
Overall angulation of the spine (kyphosis in lumbar spine or •
increased kyphosis of thoracic).
Further imaging
• If a fracture is found on the X-rays that is deemed unstable or the fi lms
are diffi cult to interpret and there is a high suspicion of injury, a CT
should be requested.
• A CT scan will assess the bony spine.
• If a soft tissue or spinal cord injury is suspected, an MRI will be
required.
Signs that imply spinal instability
Denis divides the spine into three structural columns:
• Anterior. Anterior half of vertebral body and the anterior longitudinal
ligament.
• Middle. Posterior half of vertebral body and posterior longitudinal
ligament (PLL).
• Posterior. All structures posterior to the PLL (facet joints, pedicles,
ligamentum fl avum, spinous processes, and their interspinous
ligaments).
With increasing column involvement, there is increasing instability, i.e.
one-column injury usually stable, three-column injury highly unstable.

CHAPTER 15 Orthopaedic surgery
552
• Complete vertebral dislocation or translocation.
• Signifi cant anterior wedging (>50%).
• Fractures in a previously fused spine, especially ankylosing spondylitis.
• Signs of movement. Malalignment, avulsion fractures, and evidence of
paravertebral swelling.
• Increased interspinous or interpedicular distance.
Further reading
Raby N, Berman L, de Lacy G (2005). Accident and emergency radiology: a survival guide, 2nd edn.
Saunders, London.
This page intentionally left blank

CHAPTER 15 Orthopaedic surgery
554
Spinal injuries
Any patient with major trauma arriving in A&E should be assumed to
have a cervical injury unless proven otherwise. Remember that the A in
the primary survey of ATLS resuscitation stands for airway with cervical
spine control, i.e. it is top priority.
1
• Cervical spine is the commonest area to have a major spinal injury.
• Other areas of concern are where mobile areas are at a junction with
a less mobile one, e.g. C7/T1, T12/L1, and L5/S1 junctions.
• The main reason for delay in diagnosis of spinal injuries is failure to
have a high clinical index of suspicion in all major trauma patients.
Principles of treatment
• Begin with ATLS approach and C-spine immobilization (rigid collar,
lateral head supports, and strapping).
• Particular attention needs to be paid to this as some studies have
suggested up to 25% of spinal cord injuries occur in the early
management phase, after the initial injury!
• All trauma patients should be assumed to have a spinal injury until
proven otherwise, especially in the presence of altered mental state or
blunt head injury.
• A thorough primary and secondary survey needs to be performed,
looking for mechanisms that would increase the risk of spine injury
(RTA, motorcyclist, seat belt marks) and signs suggestive of other
injuries (boggy swelling along the spine on log rolling).
• Until injuries of the spine have been deemed as stable, log rolling
should take place to prevent any spinal cord injury from occurring.
• A full neurological examination is mandatory (if patient conscious).
• Once resuscitation and stabilization have occurred, appropriate
radiological studies need to be undertaken.
• In the trauma setting, the ATLS manual now recommends CT scanning
of the C-spine rather than a lateral X-ray. However, you must still be
able to assess an X-ray if CT is unavailable!
Defi nitive management
Cervical spine
• C1 (Jefferson fracture).
If stable, semi-rigid collar or halo fi xator.•
If unstable, halo fi xator or traction • ± surgical fi xation.
• C2 (odontoid peg fracture).
Type 1 (tip). Treat with semi-rigid collar.•
Type 2 (waist). In elderly, consider cervical collar or C1/C2 fusion; •
in young patients, if undisplaced—halo fi xator, if displaced—internal
fi xation or C/C2 fusion.
Type 3 (base and extends into body of C2). Stable, treat with •
cervical collar.
• C3–C7.

SPINAL INJURIES
555
Anterior compression fractures treated in semi-rigid collar or halo •
(if >25% loss anterior height or kyphosis >11°, may need operative
fusion).
Burst fractures (from axial load). Associated with cord injury; •
treatment with decompression and fusion may be required.
• Facet joint dislocations. Both unilateral and bilateral dislocations
require reduction with progressive traction. Once reduced, unifacet
dislocations are stable and treated in a cervical collar; bilateral
dislocations require surgical stabilization.
Thoracolumbar injury
• Most common at T11–L2 as transitional segment (rigid to mobile).
• Up to 12% incidence of fracture at different spinal level.
• Denis classifi ed into compression, burst, fl exion-distraction, and
fracture-dislocations, based on 3-column theory.
• Stable fractures (<50% loss of vertebral body height, <25% kyphosis,
<50% spinal canal compromise) and neurologically intact are treated
conservatively with a thoracolumbar spine orthosis (TLSO) for 3
months.
• Unstable fractures or neurological defi cit require stabilization via
fusion and decompression of spinal canal.
Sacral injury See Fractures of the pelvis, b p. 532.
Spinal cord injury (SCI)
• Assess acutely as previously described (ALTS and so forth).
• Full neurological evaluation.
• Establish level of SCI. C5 or above requires intubation.
• Complete or incomplete lesions. Find motor level, then establish
presence of sacral sensation (intact suggests incomplete).
• Look for other injuries and treat accordingly.
• Steroid use for SCI is controversial with a paucity of level 1 evidence.
• High dose methylprednisolone administered within 8h of injury and
continued for 48h has been shown to improve outcomes.
2
• Always adhere to local policy.
Neurogenic shock
• Neurological injury causing failure of descending sympathetic pathways
of cervical and upper thoracic cord. Affects vasomotor tone and
cardiac function.
• Results in vasodilatation and bradycardia (unopposed parasympathetic).
• Be careful of excessive fl uid therapy to treat hypotension as may result
in fl uid overload.
Spinal shock
• Loss of muscle tone (fl accidity), loss of refl exes (arefl exia), and
anaesthesia following SCI.
• Duration variable (usually 48h).
• End defi ned by onset of spasticity below level of SCI.
• No recovery by 48h suggests complete cord injury and poor prognosis.

CHAPTER 15 Orthopaedic surgery
556
‘Incomplete’ spinal cord syndromes
If there is preservation of some modalities of cord function distal to the
injury level, the cord lesion is referred to as ‘incomplete’. Several recog-
nized patterns exist.
Central cord syndrome
• Most common.
• Hyperextension injury to a spine with stenosis; usually age >50y.
• Weakness affects upper limbs > lower limbs. Defi cits worst distal than
proximal. Variable sensory loss.
• Due to vascular compromise to cord (anterior spinal artery supplies
central cord).
• Recovery. Lower extremities, then bladder, then proximal upper limbs,
and then hands.
Anterior cord syndrome
• Flexion injury.
• Poorest prognosis (10–20% recovery).
• Dense motor (paraplegia/quadraplegia) and sensory loss below level of
injury. Affects spinothalamic and corticospinal tracts.
• Proprioception and vibration sense (posterior column) spared.
Brown–Sequard syndrome
• Penetrating trauma.
• Hemisection of the cord, giving ipsilateral motor (corticospinal),
vibration and proprioceptive loss (posterior columns), and
contralateral loss of pain and temperature (spinothalamic).
• Variable, but best chance of recovery.
Posterior cord syndrome
Proprioception and vibration sense lost, but intact motor power (rare).
References
1 American College of Surgeons (2008). Advanced trauma life support (ATLS) for doctors, student
manual. 8th Edition. American College of Surgeons, Chicago.
2 Bracken MB (2009). Steroids for acute spinal cord injury. The Cochrane Database of Systematic
Reviews Issue 1, Art. No.: CD001046.
This page intentionally left blank

CHAPTER 15 Orthopaedic surgery
558
Acute haematogenous osteomyelitis
This is a disease of growing bones. It is common in infants and children, but
rare in adults unless they are immunocompromised or diabetic.
Aetiology
• Infants (<1y). Staphylococcus (S.) aureus, group B streptococci, and
Escherichia coli.
• Children (1–16y). S. aureus, Streptococcus (S.) pyogenes, Haemophilus
(H.) infl uenzae.
• Adults. S. aureus, Staphylococcus (S.) epidermidis.
• Sickle cell patients. Salmonella sp.
• Rare causes. Brucella, TB, spirochetes, and fungi.
Pathological features
The organisms settle near the metaphysis at the growing end of a long
bone. The following stages typically occur.
• Infl ammation. Acute infl ammation with venous congestion.
• Suppuration. After 2–3 days, pus forms in the medulla and forces its
way out to the periosteum.
• Necrosis. After 7 days, blood supply is compromised and infective
thrombosis leads to necrosis and formation of a pocket of dead tissue
(sequestrum).
• Repair. At around 10–14 days, new bone is formed from the
subperiosteal layer that was stripped with the swelling (involucrum).
• Discharge. Involucrum can develop defects (cloacae), allowing
discharge of pus and sequestrum to allow resolution. This can also be
achieved by surgical release and debridement.
Clinical features
• Usually a child with a preceding history of trauma or infection (skin or
respiratory).
• Fever, pain, and malaise develop after a few days.
• The child may be limping or refusing to weight bear.
• On examination, there may be localized swelling or redness of a long
bone.
• Infants may present with a failure to thrive, drowsiness, or irritability.
• Neonates may present with life-threatening septicaemia in which
obvious infl ammation of a long bone develops or a more benign form
in which the symptoms are slow to develop, but bone changes are
extensive and often multiple.
Investigation
• Plain X-rays may be normal for the fi rst 10 days. Do not be reassured!
•
99
Technetium bone scan is usually positive in the fi rst 24–48h and is
effective in confi rming diagnosis early.
•
67
Gallium bone scan and
111
indium-labelled white cell scans are more
specifi c, but generally not available in most units.
• MRI is very sensitive, but not specifi c and diffi cult for children.
• CT scanning can defi ne extent of bone sequestration and cavitation.

ACUTE HAEMATOGENOUS OSTEOMYELITIS
559
X-ray features
• Soft tissue swelling is an early sign; look for displacement of fat planes.
• Patchy lucencies develop in the metaphysis at around 10 days.
• Periosteal new bone may be seen.
• Involucrum formation is only apparent at around 3 weeks.
• Sequestrum appears radiodense compared to the surrounding bone
which is osteopenic.
• Normal bone density occurs with healing.
Laboratory tests results
• FBC. i WCC, normally with a raised neutrophil count.
• i ESR.
• i CRP, but returns to normal quickly post-treatment.
• Blood cultures positive in 50% of cases (use to inform and adjust
antibiotic therapy).
• Perform U&E, LFTs, and glucose.
Treatment
• Pain relief by bed rest, splintage, and analgesics.
• Give IV antibiotics according to local guidelines (after blood cultures
and pus swab samples taken), e.g. fl ucloxacillin IV, then PO qds for up
to 6 weeks, dose-adjusted according to age, clindamycin if penicillin-
allergic, vancomycin if MRSA, ampicillin for Haemophilus.
• Surgical drainage of mature subperiosteal abscess with debridement
of all necrotic tissue, obliteration of dead spaces, adequate soft tissue
coverage, and restoration of an effective blood supply.
Complications
• Disseminated systemic infection, e.g. septicaemia, cerebral abscess.
• Chronic osteomyelitis.
• Septic arthritis.
• Deformity due to epiphyseal involvement.
Further reading
Lazzarini L, Mader JT, Calhoun JH (2004). Osteomyelitis in long bones. J Bone Joint Surg Am 86-A:
2305–18. [Current concepts review]

CHAPTER 15 Orthopaedic surgery
560
Chronic osteomyelitis
Causes
• Occasionally following acute haematogenous osteomyelitis.
• Most common following contaminated trauma and open fractures.
• After joint replacement surgery.
• Primary chronic infections of bone.
Secondary to acute osteomyelitis
Features
• Sinus formation due to sequestra or resistant bacteria.
• Prevented by adequate treatment of the initial acute attack.
Treatment
• Conservative (simple dressings) may be appropriate (elderly).
Recurrent attacks with spontaneous recovery may occur and surgery
should be reserved for cases where an abscess forms.
• Chronic abscess. May require drainage, debridement of all necrotic
tissue, and obliteration of dead spaces. May involve plastic surgery to
achieve soft tissue cover and restoration of an effective blood supply.
• Closed suction drainage/irrigation systems can be effective, especially
if irrigation fl uid contains antibiotics. The disadvantage is that early
blockage of the system can occur.
• Antibiotic (gentamicin)-impregnated beads or sponges deliver high
local levels and may be benefi cial in areas of poor blood supply, hence
systemic antibiotic penetration.
• Unresolving cases may require amputation.
Secondary to trauma (open fractures)
• Prevention by early aggressive approach to compound fractures with
debridement and lavage of contaminated tissue.
Excise all dead tissue widely and remove all devitalized bone •
fragments, i.e. with no soft tissue connections.
Copious lavage is necessary as • ‘the solution to pollution is dilution’
(t10L is common).
Skeletal stabilization is mandatory.•
IV antibiotics, e.g. IV cefuroxime • ± metronidazole if anaerobes may
be involved (soil).
• Treat established chronic infection as above with removal of internal
foreign bodies, e.g. metalwork, and possible application of external
fi xation.
Secondary to joint replacement surgery
• Rare (d1%), but is often a disaster for the elective patient.
• Prevention is better than cure. Dedicated laminar fl ow theatres, strict
theatre discipline, and prophylactic IV antibiotics are mandatory.
• Fifty per cent will require surgical intervention.
Initial joint irrigation, debridement, and tissue sampling can be •
attempted if the prosthesis is still solid and not ‘loose’.

CHRONIC OSTEOMYELITIS
561
If grossly infected, the prosthesis must be removed, the surfaces •
debrided, and an antibiotic cement spacer placed on the raw bone
ends to allow the soft tissue envelope to settle.
Once infl ammatory markers have settled (CRP is the best) and •
the clinical infection has resolved, second stage replacement of the
spacer with a new prosthetic joint can go ahead. This may take 12
months and may not be possible.
Chronic osteomyelitis as an initial presentation
Brodie’s abscess
• An isolated well-contained chronic abscess.
• Treatment. Operative drainage with excision of the abscess wall and
antibiotics.
Tuberculosis
• Usually associated with other systemic features of the disease.
• May present acutely.
• Muscle atrophy develops and spontaneous discharge of a ‘cold’ abscess
may lead to sinus formation and destruction of bone.
• Spinal TB may cause vertebral collapse, leading to acute neurology
(‘Pott’s paraplegia’).
Syphilitic osteomyelitis
• Associated with advanced, tertiary disease in adults. Features diffuse
periostitis (with sabre tibia) or localized gummata with sequestra,
sinus formation, and pathological fractures. X-rays show periosteal
thickening with ‘punched out’ areas in sclerotic bone.
• Infants with congenital disease have epiphysitis and metaphysitis.
X-rays show areas of sclerosis near the growth plate separated by
areas of rarefi cation.
Mycotic osteomyelitis
• Typically occurs in immunocompromised patients.
• Bone granulomas, necrosis, and suppuration present without
worsening acute illness.
• Usually occurs as spread from primary lung infections such as
coccidiomycosis, cryptococcosis, blastomycosis, and histoplasmosis.
• Treatment. Amphotericin B and/or surgical excision.
Further reading
Lazzarini L, Mader JT, Calhoun JH (2004). Osteomyelitis in long bones. J Bone Joint Surg Am 86-A:
2305–18. [Current concepts review]

CHAPTER 15 Orthopaedic surgery
562
Septic arthritis
A condition due to infection of a joint space and its synovium. It is com-
mon in infants and children, but rare in adults unless they are immunocom-
promised or diabetic.
Aetiology
The infective organisms are the same as for acute osteomyelitis.
Pathological features.
• Primary seeding of the synovial membrane.
• Secondary infection from adjacent metaphysis or directly from
epiphysis.
• In some joints, the metaphysis lies partly within the joint capsule
(shoulder, elbow, hip, and ankle); osteomyelitis can break through
metaphysis and into joint.
• Proteolytic enzymes are released from synovial cells and proteases
from chondrocytes, causing destruction of the articular cartilage:
By 5 days, proteoglycans are lost from cartilage.•
By 9 days, collagen is lost.•
Clinical features
• Usually a child with a preceding history of trauma or infection (skin or
respiratory).
• Acute onset with pyrexia and irritability.
• The child may be limping or refusing to weight bear.
• The affected joint is held still in position of maximal comfort (e.g. hip
fl exed, abducted, and externally rotated gives largest joint volume).
• Look for an erythematous, hot, swollen joint with an effusion.
• A neonate may present with none of the above, just irritable, lethargic,
off feeds, and not moving affected limb.
• In the adult, the joint will be exquisitely painful, hot, red, and swollen,
and they will usually not allow passive motion.
Investigation
• Plain X-rays may show joint space widening, joint subluxation or
dislocation, and soft tissue swelling.
• Ultrasound scan can demonstrate effusion and guide aspiration.
• The mainstay of diagnosis is aspiration of the affected joint with
immediate Gram stain and microscopy, followed by cultures and
sensitivities.
• Always ask for the sample to be analysed for crystals and some septic-
looking joints in adults are actually due to crystalopathies (gout or
pseudogout).
Laboratory tests results
• FBC. i WCC, normally with a raised neutrophil count.
• i ESR.
• i CRP, but returns to normal quickly post-treatment.

SEPTIC ARTHRITIS
563
• Blood cultures positive in 50% of cases (use to inform and adjust
antibiotic therapy).
• Perform U&E, LFTs, and glucose.
Treatment
• Septic arthritis is a surgical emergency and as such, rapid diagnosis, and
management is required.
• Open (or arthroscopic) drainage of the affected joint with copious
irrigation.
• Resuscitation and antibiotics.
• Re-exploration should be considered for those not settling.
• IV antibiotics, broad-spectrum, then tailored once culture results are
available for 2 weeks, then oral for further 4 weeks.

CHAPTER 15 Orthopaedic surgery
564
Peripheral nerve injuries
Common; approximately 9000 people a year are admitted to hospital with
an injury to a peripheral nerve.
• Causes include traction, trauma, infl ammation, compression.
• The degree of injury depends on the mechanism (open or closed
injury, acute or chronic), health of nerve prior to injury.
Pathological features
Seddon classifi cation.
Neuropraxia
• Stretching or compression of the nerve which remains anatomically
intact.
• Conduction block with normal conduction above and below.
• Focal demyelination occurs at the site of injury, which is repaired by
the Schwann cells.
• Recovery is usually complete, occurring in days or weeks. There is no
axonal degeneration.
Axontemesis
• The axon is divided, but the covering connective tissue component
remains intact, i.e. the nerve cylinder remains.
• Usually a traction or severe crush injury.
• Axonal (‘Wallerian’) degeneration occurs distal to the injury and is
followed by nerve regeneration (by sprouting from the severed nerve
end) after 10 days.
• Nerve growth occurs at a rate of 1mm per day.
• Prognosis is generally good as the cylinder is intact, but the more
proximal the lesion, the less the distal recovery.
• Sensation recovery is generally better than motor recovery, especially
if the lesion is proximal and muscle wasting occurs whilst it is
‘dennervated’.
Neurotemesis
• The nerve is completely divided or irreparably damaged with loss of
apposition of the severed nerve bundles and their respective distal
parts.
• Usually a high energy injury, penetrating trauma, severe traction,
ischaemia, or high pressure injection injury.
• Minimal recovery is possible without operative intervention to repair
or graft a new nerve to the injury.
• Surgical repair may allow axon regeneration to the correct end organ,
but recovery will not be complete as often ‘miswiring’ occurs.
Diagnosis
• What is the injury? Is there an open wound, fracture, recent surgery,
or prolonged immobility?
• Complete neurological examination. You must know the motor
and sensory supplies of peripheral nerves! Use a pin or your fi nger

PERIPHERAL NERVE INJURIES
565
for sensory testing. Compare the area of normal and injured side
sequentially.
• Tips. Anaesthetic skin looks shiny and does not sweat. Dennervated
skin will not wrinkle in water.
• There are specifi c features of different levels of injury in peripheral
nerves of the upper limb.
• Examination very soon after injury can be misleading as sensory loss
may take time to appear.
• Diagnosis of a peripheral nerve injury is clinical, but can be
supplemented with nerve conduction studies and electromyography
(EMG).
Treatment
Closed injury
• Injuries in continuity. Neuropraxia and axontemeses (vast majority)
can be expected to recover spontaneously, so exploration is not
indicated.
• Compression injuries should have compressive forces removed, e.g.
external such as plaster or internal such as carpal tunnel syndrome.
• Physiotherapy and splintage should be used whilst awaiting recovery;
this will maintain functionality and prevent contractures.
Open injuries
• Primary repair (suture). Within 24h is ideal, but an uncontaminated
operative fi eld, adequate skin cover, and proper equipment (e.g.
microscopes) must be present.
• Delayed secondary repair. Can be done at any time after injury once
the soft tissues have healed (3–6 weeks acceptable). The nerve can be
mobilized to allow a no tension repair after resection of the cut nerve
stumps. Usually, however, a nerve graft has to be used to bridge the
defect (sural nerve as a donor the commonest).
Further reading
Soloman L, Warwick D, Nayagam S (Eds) (2010). Apley’s system of orthopaedics and fractures.
9th edn. Hodder Arnold, London.

CHAPTER 15 Orthopaedic surgery
566
Brachial plexus injuries
The brachial plexus is formed from the ventral rami of C5 to T1. It is sub-
sequently divided into root, trunks, divisions, cords, and terminal branches.
The lesion can be at any of the above levels.
Terminal branches arising from the root level (phrenic nerve (C3–5),
dorsal scapular nerve (C5), and long thoracic nerve (C5–7)) are important
to recognize as if they are spared, it suggests the lesion is post-ganglionic.
Causes
• Child. Obstetric, i.e. diffi cult deliveries with traction on the plexus.
• Adult. Almost all traumatic.
Usually closed, e.g. motorcycle accidents, falls, and traction injuries •
with forced abduction of the arm.
May be open, e.g. stab or gunshot wounds.•
• Always look for associated injuries, e.g. head, neck, chest, abdominal,
and vascular.
Types
• Erb–Duchenne (upper). Involves C5 and C6; the arm classically hangs
at the side with the arm fl accid, internally rotated, adducted, and the
wrist fl exed (waiter’s tip position).
• Klumpke’s (lower). Involves C8 and T1; the hand is clawed due to
intrinsic muscle paralysis and if the sympathetic trunk is involved, there
is Horner’s syndrome.
Try to localize the lesion to preganglionic (intraspinal) or post-ganglionic
(extraspinal) by clinical means as described earlier. At the T1 level, look
for signs of Horner`s syndrome (ptosis, meiosis, ipsilateral anhydrosis),
suggesting a preganglionic lesion.
If histamine is injected into the skin of the supplied area, vasodilata-
tion, weal, and fl are indicate a positive result and a preganglionic injury is
present. If there is no fl are, the lesion is postganglionic.
Anatomic level of injury should be delineated.
Treatment
Physiotherapy to prevent joint contracture and stiffness. If no return of
function at 2 months, consider myelography or histamine tests to localize
the injury level.
• Open injuries should be explored acutely, but not if there are more
life-threatening injuries (which is usually the case). Primary repair may
be possible in this group.
• Delayed surgery (if required at all) is normal for most patients.
• Preganglionic injuries are irreparable and should not be explored.
• Post-ganglionic injuries may be explored for up to 6 months post-
injury. Secondary repair with nerve grafting can then be attempted if a
clear lesion is isolated.
Prognosis
• If there are no EMG abnormalities at 3–4 weeks, prognosis is good
with conservative treatment.

BRACHIAL PLEXUS INJURIES
567
• Causalgic pain, Horner’s syndrome, and the presence of root avulsion
on myelogram (hence intraspinal lesion) indicate a poor prognosis.
• Recovery is generally very slow and often unsatisfactory.
• Salvage surgery with tendon transfers or shoulder arthrodesis may
improve function and give better results than amputation.
Further reading
Birch R (1996). Brachial plexus injuries. J Bone Joint Surg Br 78-B: 986–92. [Review]

CHAPTER 15 Orthopaedic surgery
568
Osteoarthrosis (osteoarthritis)
This is degenerative joint disease; it is a disease of cartilage, not the joint.
• It is limited to the joint itself and there is no systemic effect.
• It may involve any synovial joint, but is most common in the hip, knee,
and hands.
• It is the most common form of arthritis with an estimated radiographic
incidence in moderate to severe changes in 5 million people in the
UK.
1
• Approximately 2 million people visit their GP with osteoarthritis per
year and it is predicted that there will be a 66% increase in the number
of people with osteoarthritis-related disability by 2020.
Types
Primary osteoarthritis (idiopathic)
Mainly affects the following joints: distal interphalangeal, fi rst carpometa-
carpal, hips, knees, and apophyseal joints of the spine. Women are more
affected than men and there may be a hereditary component, but the
aetiology is unknown.
Secondary osteoarthritis
Affects previously damaged joints and is more common in weight-bearing
joints. Both sexes are equally affected. Local causes are fractures, acquired
or congenital deformities, joint injury (chondral lesions), diabetic neuropa-
thy (Charcot joints), and avascular necrosis.
Clinical features
• Characteristic pain, swelling, and deformity.
• Dull, aching pain with morning stiffness of the affected joint.
• Pain becomes steadily worse throughout the day and may disturb
sleep.
• Acute onset. Swollen, hot, and painful joint with raised infl ammatory
markers.
• Look for Heberden’s nodes at the distal and Bouchard’s nodes at the
proximal interphalangeal joints.
• Physical symptoms may not correlate with the severity of the
radiographic changes so judge each patient on an individual basis.
X-ray changes
• Loss of joint space.
• Subchondral bone sclerosis.
• Cyst formation (especially at the hip).
• Osteophyte formation.
Treatment
Relieve pain, improve mobility, and correct deformity in that order.
Medical
• Pain relief with simple analgesics (paracetamol, codeine), in
combination with NSAIDs, helps control symptoms and increase

OSTEOARTHROSIS (OSTEOARTHRITIS)
569
mobility. Beware of GI bleeding, especially in the elderly, and of
worsening asthma.
• Radiant heat in the form of infrared light or a hot water bottle
frequently helps.
• Weight loss, physiotherapy, and aids to daily living such as walking
sticks, heel raises, raised chair, and household aids should all be in
place before contemplating surgery.
• Joint injections of steroid and local anaesthetic may help in up to 50%
patients.
Surgical treatment
This is indicated for pain relief, improved mobility, and correcting deform-
ity only when conservative measures have failed.
Options include:
• Osteotomy. Realignment of a joint to unload an arthritic area.
• Arthrodesis. Permanent stiffening of a joint by excision and fusion to
stop pain.
• Excision. Removal of the joint without fusion.
• Arthroplasty. Replacement of all or part of the joint surface by an
artifi cial material.
Reference
1 M http://www.arc.org.uk/about_arth/astats.htm.

CHAPTER 15 Orthopaedic surgery
570
Carpal tunnel syndrome
• Compression of the median nerve at the wrist.
• Boundaries of tunnel are: radially—scaphoid tubercle and trapezium,
ulnarly—hook of hamate and pisiform, transverse palmar ligament,
palmar aspect (roof).
• Contents—fl exor tendons (fl exor pollicis longus (FPL), fl exor
digitorum superfi cialis (FDS), fl exor digitorum profundus (FDP)) and
median nerve.
• Most common in middle age.
• Often bilateral, but when unilateral most commonly affects the
dominant hand.
Aetiology
Remember, the commonest is idiopathic.
Compression of the tunnel wall
• Trauma, e.g. distal radial fracture.
• Rheumatoid arthritis (thickening of the surrounding synovium and tissues).
• Subluxation or dislocation of the wrist.
• Acromegaly (soft tissue thickening and enlargement).
Compression within the tunnel
• Fluid retention, e.g. pregnancy.
• Myxoedema.
• Space-occupying lesion, e.g. benign tumour.
• Chronic proliferative synovitis.
Changes in the median nerve
• Diabetic mellitus.
• Peripheral neuropathies.
Clinical features
Symptoms
• Aching pain and paraesthesia (pins and needles) over radial three-and-
a-half fi ngers and palm.
• Pain typically at night and can disturb sleep.
• Relieved by shaking the hand.
• May notice dropping items (weak pinch grip), clumsiness.
• Can be made worse by activity.
• Atypical symptoms can be common.
Signs
1
• Hand looks normal.
• Thenar muscle wasting if chronic and severe.
• Weakness of thumb abduction.
• Tinnel’s test. Tapping over the nerve at the wrist in neutral produces
symptoms.
• Phalen’s test. Rest elbows on the table and passively fl ex the wrist. If
symptoms appear within 60s, test is positive.
• Median nerve compression test. Extend elbow, supinate forearm, fl ex
wrist to 60°, press on carpal tunnel. Positive if symptoms within 30s.

CARPAL TUNNEL SYNDROME
571
Investigations Nerve conduction studies are gold standard, but still
show only 90% accuracy.
Treatment
Conservative
• Splintage.
• NSAIDs.
• Injection of corticosteroids.
• Avoidance of precipitating factors.
Surgical
• Surgical decompression.
• Use a tourniquet.
• Skin incision in line with ulnar border of the ring fi nger. This is to avoid
the motor branch of the median nerve.
• Protect the nerve with a MacDonald’s dissector and visualize the nerve
directly throughout.
• Do not extend the skin incision beyond the wrist crease to protect the
palmar cutaneous branch of the median nerve.
Complications
• Complex regional pain syndrome.
• Tender, hypertrophic scar giving pillar pain (pain in the heel of the scar
on pressure).
• Neuroma of the palmar cutaneous branch.
• Recurrence.
• Bowstringing of fl exor tendons.
Reference
1 Tetro AM, Evanoff BA, Hollstien SB, Gelberman RH (1998). A new provocative test for carpal
tunnel syndrome: assessment of wrist fl exion and nerve compression. J Bone Joint Surg Br 80(3):
493–8.

CHAPTER 15 Orthopaedic surgery
572
Ganglion
This is a degenerative mucinous cyst swelling that can arise from a tendon
sheath or joint. It contains clear, colourless, gelatinous fl uid.
Common sites
• Dorsum of the wrist, arising from the scapholunate ligament or
midcarpal joint (70% of all cases).
• Radial aspect of the volar wrist normally from scaphotrapezial joint
(20% of all cases).
• Base or DIPJ of fi nger.
• Dorsum of the foot.
• Around the knee.
Clinical features
• Slow growing, cystic lump commonly presenting as dorsal wrist pain.
• Increase and decrease in size.
• Firm, smooth, rubbery, and will usually transluminate.
• May be more obvious with wrist in palmar fl exion.
Diagnosis
• Needle aspiration gives gelatinous fl uid. If no fl uid can be aspirated,
then investigate further since a soft tissue tumour (including sarcoma)
is possible.
• MRI scanning should be used if there is serious concern over a soft
tissue tumour.
• Occult ganglia (no palpable lump) can yield symptoms in the wrist or
foot. Ultrasound scan will confi rm the diagnosis.
Treatment
• Fifty per cent will disappear spontaneously. Therefore, treat
conservatively unless pressed by the patient.
• Aspiration may be curative in 50% of cases.
• Deliberately induced traumatic rupture often leads to recurrence.
Surgery
• Excision is not guaranteed success (recurrence approximately 10%;
painful scar approximately 10%).
• Use a tourniquet.
• If not occult or excessively large, then local anaesthesia as day case
procedure appropriate.
• Excise thoroughly and transfi x the base to prevent recurrence.
• Volar wrist ganglia often surround or are very close to the radial
artery!
This page intentionally left blank

CHAPTER 15 Orthopaedic surgery
574
Bone tumours
Diagnosis
The key to successful management of bone tumours is early detection and
treatment; always having a clinical suspicion is essential!
Presenting features
• Pain. Persistent, at night, response to analgesics?
• Mass or swelling (? getting bigger, rate of progression).
• If there is a fracture, is there a history of trauma?
• Neurological symptoms.
• Systemic symptoms.
• Previous tumours, radio- or chemotherapy.
• Any family history.
• Watch for the ‘red herring history’ of trivial injury.
Examination
• Extract features of the mass/swelling and palpate for lymphadenopathy.
• Is it around a joint, is it deep to the fascia? Size, relationship to
surrounding structures.
Radiological investigations
• A plain X-ray (AP and lateral) of affected area is mandatory.
• Where is lesion, what are effects on bone, is there a bone reaction
(new bone, periosteal reaction, Codman’s triangle, sunburst
spiculation), is there a matrix?
• Once a diagnosis has been considered/made, further imaging is
required; MRI scanning is usually gold standard.
• Accurately stage and assess local or systemic spread (CT, bony
architecture; MRI, soft tissue or bony extensions).
Blood tests
Rarely diagnostic and often non-specifi c (e.g. i alkaline phosphatase,
i ESR, i Ca
2+
).
Urgent referral
• Once a diagnosis is made, urgent referral (even before MRI is obtained
in highly suspicious lesion) to local tumour services is required.
• They will advise and guide further local management and arrange
defi nitive treatment if required.
Other considerations
• Angiography. Helps plan radical surgery and possible limb salvage.
• Open biopsy. Best to achieve histological diagnosis and required for
treatment planning.
• Must be performed by the surgeon who is going to do the defi nitive
surgery.
• The biopsy track must be excised as part of the defi nitive excision and
placed to maximize the chance of limb salvage surgery.

BONE TUMOURS
575
Metastatic tumours
• Secondary metastases are the commonest tumours of bone (breast,
prostate, lung, thyroid, and kidney primaries).
• Bone pain—worse at night and with weight bearing.
• Systemic symptoms (fatigue, weight loss, no appetite).
• History of cancer (personal or family).
• Pathological fractures common.
• If a patient is admitted with a history of pathological fracture with
unknown primary, full examination should be performed to fi nd source
(breast, rectal, prostate, etc.).
• Blood tests, including calcium, phosphate, tumour markers (PSA, CEA,
CA125).
• Bone scan, staging CT/MRI may be required.
• Treatment:
Treat electrolytes fi rst if raised.•
Internal fi xation (ideally prophylactic before fracture occurs) allows •
early weight bearing and hopefully early discharge from hospital.
Radiotherapy for pain.•
Manage in conjunction with oncologists.•
Benign tumours
Osteochondroma (‘exostosis’)
• Most common benign tumour.
• A cartilaginous capped outgrowth of bone from the cortex, normally
near an epiphysis. Lesion grows until skeletal maturity.
• Usually pain-free and present as lump. If painful, usually due to
infl ammation of overlying bursa.
• Usually solitary. Multiple lesions require close follow-up (hereditary).
• Any sudden increase in size may indicate malignant transformation to
chondrosarcoma (<1%).
Chondroma
A non-calcifi ed cartilaginous growth in the medulla (enchondroma) or
cortex (ecchondroma) of tubular bones such as phalanges, metacarpals/
tarsals.
Osteoid osteoma
• Occurs in young patients (5–30y).
• Progressive pain (night) of long bone, referred to other joints,
classically relieved by NSAIDs.
• Commonly long bones (diaphysis), can be intra-articular, and a cause
of painful scoliosis.
• Radiology shows a ‘nidus’ which is a small osteolytic area surrounded
by a rim of dense sclerosis. Look for periosteal reaction.
Non-ossifying fi broma
• Fibrous tissue tumour which usually appears radiologically as an oval
cortical defect with sclerotic rim. Common incidental fi nding on
X-rays; usually needs no treatment.

CHAPTER 15 Orthopaedic surgery
576
• Treatment. Principles of treatment are simple local excision or
removal by curettage if symptomatic or likely to cause pathological
fracture (likely if >50% diameter of bone involved).
Cavities should be packed with bone graft or bone cement.•
Internal fi xation may be used once large tumours have been •
removed.
Diffi cult tumours should be managed in a ‘bone tumour centre’.•
Primary malignant tumours (rare)
All primary malignant tumours require a ‘multidisciplinary team’ (ortho-
paedic surgeon, oncologist, musculoskeletal radiologist, histopathologist).
Osteosarcoma
• Commonest primary bone tumour.
• Long bones of young adults (peak incidence 10–20y) or as a
consequence of Paget’s disease in the elderly (see b p. 582).
• Presents with progressive pain (rest/night) refractory to analgesia.
• Swelling, reduced joint movement, limp.
• ? Trivial sporting injury—not related to tumour development!
• X-ray features. Bone destruction, soft tissue invasion, radiating spicules
of bone (‘sunray’ appearance), subperiosteal elevation with new bone
formation (‘Codman’s triangle’).
• MRI shows extent of tumour, skip lesions, soft tissue involvement.
• Metastasis via blood to the lungs and bone.
• Treatment:
Neoadjuvant chemotherapy followed by surgical resection and •
further chemotherapy.
Limb salvage surgery possible in about 90% (rare for pelvic •
tumours).
Local recurrence rate 5% (poor prognosis).•
Radiotherapy may be used as an alternative in the elderly.•
• 5y survival 60–70% (localized) or 25% (pelvic) with surgery; 20% if
present with metastases.
Chondrosarcoma
• Commonest in older patients (30–75y).
• Usually occurs in a fl at bone, e.g. ilium of pelvis, ribs, scapula.
• Location of presentation gives clues to type (scapula malignant and
hand benign).
• May present de novo or arise from a pre-existing osteochondroma.
• Graded. Low to high (1, 2, 3, undifferentiated); 60% present grade 1.
• Metastasis is not common and is via blood.
• Local invasion is more usual, but is normally slow growing.
• High grade present with bone destruction and soft tissue mass.
• Treatment:
Low grade require wide resection. Local recurrence 20% at 10y.•
High grade require wide resection • ± amputation.
No real role for chemo- or radiotherapy unless undifferentiated or •
elderly).
5y survival dependent on grade. Grade 1, up to 90%; grade 2, •
60–70%; grade 3, 30–50%; undifferentiated, 10%.

BONE TUMOURS
577
Ewing’s sarcoma
• Children and young adults (<20y).
• Pain, associated hot/erythematous swelling with associated pyrexia so
that osteomyelitis may be suspected.
• Commonly pelvis, long bones, and scapula.
• i ESR and i WCC.
• X-rays show lytic bone destruction with periosteal reaction in multiple
layers (‘onion skin’ appearance).
• MRI scan shows soft tissue involvement, which would not usually be
the case in osteomyelitis.
• Metastasis to the lung is very fast and most people present with this.
• Treatment. Preoperative neoadjuvant chemotherapy (12 weeks) and
then re-evaluate and re-stage.
• Isolated lesions managed with wide excision or amputation.
• Radiation can be used if metastases present.
• Response to chemotherapy predicts prognosis.
• Prognosis is still poor. Isolated extremity Ewing`s approximately 65%
5y survival; metastatic disease at presentation <20% 5y survival.
Giant cell tumour (osteoclastoma)
• This is rare before the age of 20y.
• Usually benign, but may undergo malignant transformation (710%).
• Rarely metastasizes; usually to lungs, but may be locally invasive.
• Treatment. Local excision and defect fi lled bone graft or cement.
• Recurrence is common (~20%), especially with malignancy.
Further reading
Excellent summaries of bone tumours can be found at: M http://www.bonetumor.org/

CHAPTER 15 Orthopaedic surgery
578
Low back pain
• Very common condition in the UK; 60–80% of adults will be affected
during their lifetime.
• Common cause of absence from work.
• In the majority of cases, it is a self-limiting condition requiring no
surgical intervention.
1
• Multifaceted condition with overlapping aetiology.
• Always consider neoplasia—metastasis, myeloma, osteoid osteoma.
Types of pain
Mechanical
• Pain (low back, buttock, and thigh), like ‘toothache’.
• Rarely radiates below knee.
• Worse over course of day and with activity.
• Cannot get comfortable and wakes from sleep when turn.
• Acute pain from trivial movement which settles with rest.
• Typically midline and made worse by lordotic postures, e.g. bending
and lifting (discogenic).
• Pain is from the annulus fi brosis layer of the disc when it is being
stretched.
Nerve root entrapment
• Pain radiates down the leg from buttock to calf/foot.
• Commonly caused by a lumbar disc prolapse, compressing and
irritating the nerve root as it exits the spinal foramina.
• Pain from spinal stenosis is worsened with extension (walking down a
hill) and relieved with fl exion (riding a bike).
• Radiation should match the sensory dermatome of the nerve root
involved.
• Other features are pain on sneezing, coughing, and straining.
Numbness or paraesthesia in the dermatome of the affected root may
also be present.
• The earliest and most persistent feature of nerve root compression is
loss of a tendon refl ex, e.g. knee L3/4, ankle L5/S1.
Referred
May arise from retroperitoneal pathology (aortic aneurysm, pancreatic
and biliary tree pathology, rectal pathology, renal stones, lymphadenopa-
thy, and hip arthroses).
Psychosocial (yellow fl ags)
• The commonest cause for chronicity.
• A diagnosis of exclusion, i.e. exclude all other pathology before
labelling people as ‘neurotic’.
• Typical features of non-organic pain are pain on axial compression
or pelvic rotation, non-dermatomal sensory loss, non-anatomical
tenderness, cogwheel (give way) weakness, and overreaction
(Waddell’s signs).
2

LOW BACK PAIN
579
Common pathological (organic) causes
• Mechanical (80–90%).
Unknown cause. Muscle strain or ligamentous injury.•
Degenerative disc or joint disease.•
Vertebral fracture.•
Congenital deformity (such as scoliosis, kyphosis, transitional •
vertebrae).
Spondylolysis.•
Instability (spondylolisthesis).•
• Neurogenic (5–15%).
Herniated disc.•
Spinal stenosis.•
Osteophytic nerve root composition.•
Failed back surgery syndrome (such as arachnoiditis, epidural •
adhesions, recurrent herniation). May cause mechanical back pain
as well.
Infection (such as herpes zoster).•
• Non-mechanical spinal conditions (1–2%).
Neoplastic (such as primary or metastatic).•
Infection (osteomyelitis, discitis, abscess—staphylococcal or TB).•
Infl ammatory arthritis (such as rheumatoid arthritis and •
spondyloarthropathies, including ankylosing spondylitis, reactive
arthritis, enteropathic arthritis).
Paget’s disease.•
• Coccydynia. Pain in the coccyx may be due to lumbosacral disc disease.
Assessment
• History must distinguish between ‘simple’ back pain and that requiring
urgent care.
• ‘Red fl ags’ of serious spinal pathology.
Retention of urine or incontinence.•
Onset over age 55 or under 20.•
Symptoms of systemic illness (weight loss, fever).•
Severe progressive pain (unrelenting).•
Trauma.•
A prior history of cancer.•
IV drug use.•
Prolonged immunosuppressant or steroid use.•
• Examination must cover:
Palpation, movements, straight leg raising, femoral stretch test, •
power, sensation, refl exes.
• If signs of signifi cant spinal pathology present (i.e. cauda equina),
perianal sensation and PR examination must be performed.
Investigations
• Spinal X-rays (AP and lateral of affected level).
• CT scan good for assessing bony structures.

CHAPTER 15 Orthopaedic surgery
580
• MRI scan good for soft tissue problems (discs, spinal cord, nerve root
involvement), but only relevant in planning, not in diagnosing treatment.
Should be left for treating surgeon as it is often not required.
Treatment
Non-operative
• Majority of patients require non-operative treatment.
• Initial rest (1–2 days only).
• Analgesia (paracetamol, NSAIDs, muscle relaxants, opioids).
• Early mobilization with strong encouragement.
• Physiotherapy (massage, acupuncture, hydrotherapy).
• Counselling and psychosocial support.
Surgery
• Only a minority requires surgery. Should be clearly focused on proven
pathology demonstrated by imaging where possible.
• Procedures used include:
Discectomy.•
Chemonucleosis/percutaneous disc removal.•
Nerve root decompression.•
Spinal decompression.•
Spinal fusion.•
Cauda equina
• Surgical emergency.
• Cauda equina (horse’s tail) is a collection of nerve roots (lower lumbar
and sacral) at distal end of cord.
• It can present acutely, chronically, or following longstanding lower
back problems.
• Causes include large central or paracentral disc herniation (L4/5 or
L5/S1), spinal injury neoplasms, tumours, infections (abscess or TB),
haematoma (iatrogenic).
• Key symptoms are dysfunction of bladder, bowel (and sexual function),
saddle or perianal anaesthesia.
• Other symptoms include low back pain, radiation down one or both
legs, sensory chances in lower limbs, weakness.
• Examination should be a thorough lower limb neurological assessment.
• Perianal sensation and anal sphincter tone (bulbocavernosus refl ex) is
essential.
• Imaging is required urgently if convincing clinical evidence exists. MRI is
the modality of choice.
• Treatment once diagnosed is surgical decompression. Referral to an
appropriate spinal centre, if not on site, is required urgently.
• Timing of surgery is controversial. Better outcomes are seen if
performed <24h following onset.
• Outcomes are variable. If complete cauda equina (urinary retention or
incontinence), the prognosis for recovery is poor.
References
1 Cohen SP, Argoff CE, Carragee EJ (2009). Management of low back pain. BMJ 338: 100–6.
2 Lavy C, James A, Wilson-MacDonald J, Fairbank J (2009). Cauda equina syndrome. BMJ 338:
881–4.
This page intentionally left blank

CHAPTER 15 Orthopaedic surgery
582
Paget’s disease (osteitis deformans)
Key facts
• Described by Sir James Paget (1876).
• Incidence increases with age (>50y).
• Any bone may be involved. Commonest sites are spine, skull, pelvis
and femur, tibia.
• Autosomal dominant.
Pathological features
• Increased osteoclastic bone resorbtion followed by compensatory
bone formation, i.e. there is an overall increase in bone turnover.
• Three phases. Lytic, mixed (lysis and formation), and sclerotic.
• The bone is softer, but thickened and is liable to pathological fracture.
Clinical features
• Most are asymptomatic.
• Diagnosed via an incidental fi nding on X-ray or raised alkaline
phosphatase whilst investigating other pathologies.
• Increased thickness of bone may be the only symptom or sign.
• Subcutaneous bones may be deformed, classically the tibia when it
becomes ‘sabre’-shaped.
• Pain may be present, but is unusual. It may represent high turnover at
the time or more likely, a pathological fracture.
• In known Paget’s patients, increase in pain must be taken seriously as it
may be a marker of sarcomatous change in the bone.
Investigations
• Serum calcium and phosphorus are normal.
• Alkaline phosphatase is high (due to i osteoblast activity).
• Urinary excretion of hydroxyproline is high (i bone turnover).
• Isotope bone scan shows ‘hot spots’ in affected areas.
• X-ray shows both sclerosis and osteoporosis. The cortex is thickened
and the bones deformed. Pathological fracture is a feature and the
normal bone architecture is lost with coarse trabecular pattern.
Complications
• Pathological fractures.
• Osteosarcomatous change (<5%; prognosis very poor).
• High output cardiac failure may develop due to the increased
vascularity of Paget’s bone. Functionally, the bone is acting as an
arteriovenous fi stula.
• Deafness. Bony deformation in the ear causes damage to cranial nerve.
• Osteoarthritis.
• Leontiasis ossea. Thickening of facial bones (rare).
• Paraplegia due to vertebral involvement (rare).
Treatment
• Most patients require no treatment.

PAGET’S DISEASE (OSTEITIS DEFORMANS)
583
• Fractures will heal normally, but bony deformity with a fracture can be
a diffi cult challenge!
• Drugs that reduce bone turnover such as calcitonin or
bisphosphonates are effective in relieving pain and may also relieve
neurological complications such as deafness.
Further reading
M http://www.paget.org/

CHAPTER 15 Orthopaedic surgery
584
The great toe
Hallux valgus (‘bunions’)
1,2
• Medial prominence of the fi rst metatarsal head, with lateral deviation
of the great toe (hence valgus) due to the pull of the extensors.
• As time passes, a protective bursa develops over the metatarsal head
(the ‘bunion’) and the great toe begins to crowd, or even overlap, its
neighbours.
Causes
• Congenital. Often familial, related to metatarsus primus varus where
the fi rst metatarsal is angled more medially, i.e. splayed, than usual and
is rotated.
• Acquired. The commonest form. Probably due to weak intrinsic
muscles due to age. It is not proven to be related to shoes, but there
is a higher incidence in shoe-wearing cultures.
Symptoms
• Commonly asymptomatic, even in cases of severe deformity.
• Pain typically at the site of the bunion due to pressure.
• Bursal infl ammation.
• Nerve symptoms may be present (compression of digital nerve).
• As the disease advances, symptoms of joint pain may present due to
osteoarthritis and subluxation of the joint.
• Lesser toe deformities may be present (hammer toes, calluses).
Investigations
• Weight-bearing AP and lateral views of the foot.
• Measurements of the extent of deformity are made to guide
management.
• Commonly calculated are the hallux valgus angle (between long axis
of fi rst metatarsal and corresponding proximal phalanx) and the fi rst/
second intermetatarsal angle (between the long axis of fi rst and
second metatarsals).
Treatment
Conservative
• Correct footwear with a wider toe box and padding to protect
bunion.
• This should always be tried and have failed before considering surgery.
Surgical
• Exostectomy. Removal of the bunion alone. This is simple, but does not
remove the underlying deformity and the problem will recur.
• Distal metatarsal osteotomy. The bunion is removed and the metatarsal
head or neck is cut. The distal fragment is then realigned anatomically
and the fracture held with a K wire. There are many types or shapes
of osteotomy described, but the commonest eponyms are Mitchell’s,
Wilson’s, and Chevron. This is only suitable for smaller deformities.
• Proximal metatarsal osteotomy. This is an osteotomy just proximal to
the base of the metatarsal and the metatarsocuneiform joint. Larger

THE GREAT TOE
585
bony deformities can be corrected this way. It may be combined with
a distal soft tissue release where the lateral constraints by the MTPJ
are also released through a small separate dorsal excision.
• Excision arthroplasty. Removal of the metatarsal head (Mayo) or base
of the proximal phalanx (Keller) can be attempted, but is fraught with
long-term complications and is only an operation for the elderly.
• Arthrodesis. This is suitable for severe deformity and degenerative
change and is tolerated well by males. Females may have a problem
with footwear (have to wear fl at shoes after). It is normally reserved
for salvage surgery.
Hallux rigidus
1
Degenerate arthritis of the fi rst MTPJ, leading to pain and functional limita-
tion of movement.
Causes
• Not fully determined.
• Congenital. Due to a shortened metatarsal.
• Acquired. Normally traumatic or idiopathic degeneration.
Symptoms
• Pain and swelling of the fi rst MTPJ with profound stiffness (limited
dorsifl exion).
• May irritate on shoes.
• Neurological symptoms due to pressure on the digital between
osteophyte and shoe.
Investigations
AP and lateral X-rays of the foot (demonstrate osteoarthritic changes).
Treatment
• Adolescents/young. Rocker sole to relieve pain.
• Adults.
MTPJ replacement (rare).•
Cheilectomy (excision of dorsal osteophyte and about 25% dorsal •
metatarsal head).
Arthrodesis.•
Excision arthroplasty (elderly only).•
References
1 M http://www.blackburnfeet.org.uk/hyperbook/conditions/conditions_index.htm#hallux.
2 Coughlin MJ (1996). Hallux valgus. J Bone Joint Surg Am 78-A, 932–66: [Instructional course
lecture]

CHAPTER 15 Orthopaedic surgery
586
Arthroplasty
• The surgical reconstruction or replacement of a malformed or
degenerate joint.
• The primary goal is to relieve pain.
• Increases in mobility and function are secondary aims.
Classifi cation
• Excision, e.g. Keller’s or Mayo at the fi rst MTPJ.
• Interposition. A joint is excised and then a piece of tissue is implanted in
the gap to cause a thick scar.
• Partial (hemi-) or total replacement. All or one-half of the articular
surface is removed and replaced with other material. This has been
made possible by the massive advances in both biomaterials and
bioengineering, which have produced inert, sterilizable materials of
acceptable strength to perform the joint functions.
Example: total hip replacement
1–3
Indications
• Osteo- and rheumatoid arthritis when pain affects sleep, quality of life,
and normal daily activities.
• Multiple joint involvement where hip is the worst.
• AVN of the head of the femur with secondary joint degeneration.
Prevention of infection
Deep infection is a potentially devastating complication of hip or any
arthroplasty and its incidence should be d1% in all units. This is achieved
by the following.
• Ultraclean air systems and exhaust body suits. The air in a conventional
theatre is fi ltered so that there are >20 changes/h. By using a
unidirectional laminar fl ow system, 300 or more changes/h with
fi ltration can be achieved. The purpose is to make the number of
colony-forming units (CFUs) in the air the minimum possible. Body
suits, although cumbersome, provide the best physical barrier between
the patient and the surgical team.
• Prophylactic antibiotics. These are given IV on the induction of
anaesthesia; 10min is usually required for them to penetrate bone to
an acceptable level. Broad-spectrum antibiotics are usually used, e.g.
cefuroxime 1.5g, co-amoxiclav (Augmentin
®
) 1.2g, and gentamicin
80mg, being the most common. Two further doses at 6 and 12h post-
operatively are usually given.
• Strict theatre discipline.
Procedure
The surgical approach exposes both the femoral head and acetabulum.
The head of the femur is exposed, dislocated, and either reshaped (resur-
facing arthroplasty) or more commonly, removed at the neck. The acetab-
ulum is then deepened and reshaped to allow a cup to be placed (the new
‘socket’). A cavity is then created within the cut surface of the femur, going
downward to allow a stem to be placed (the new ‘ball’). The stem and cup

ARTHROPLASTY
587
are usually ‘grouted’ in place with polymethylmethacrylate bone cement
and the two components reduced and stability tested.
Sometimes the cup has a metal shell behind it and components can be
hammered in rather than cemented and this is known as an ‘uncemented
hip replacement’. This is more commonly used on the younger patient in
the UK, but is the implant of choice in the USA. The wound is then closed.
Patients are mobilized on day 1 post-operatively, fully weight bearing, and
discharged within 5–7 days usually.
Complications
Operative
• Sciatic nerve injury due to poor technique and overstretching of
tissues.
• Dislocation of the prosthesis if incorrectly aligned.
• Profound hypotension can be seen with absorption of the monomer in
the cement, causing cardiotoxicity.
Post-operative
• Mortality 1%. This is major surgery.
• Thromboembolic disease (DVT or PE).
• Deep infection (1%).
• Dislocation (4%). Usually due to patient non-compliance with
physiotherapy guidelines.
• Aseptic loosening (‘wearing out’). Most total hip replacements would
be expected to have t90% survival rates 10y after surgery.
References
1 M http://www.orthoteers.co.uk/Nrujp~ij33lm/Orththr1.htm.
2 M http://www.wheelessonline.com/o14/14.htm.
3 Huo MH, Muller MS (2004). What’s new in hip arthroplasty. J Bone Joint Surg Am 86-A:
2341–53.

CHAPTER 15 Orthopaedic surgery
588
Useful reading
Online orthopaedic hyperbooks
M http://www.orthoteers.co.uk (requires subscription)
M http://www.wheelessonline.com (free)
M http://www.blackburnfeet.org.uk (free)
M http://www.eatonhand.com (free)
M http://www.eradius.com (free)
Reference textbooks
Elective
Canale T, Beaty JH (Eds.) (2007). Campbell’s operative orthopaedics, 11th edn (4 vols). Mosby,
London.
Trauma
McRae R, Esser M (2008). Practical fracture treatment, 5th edn. Churchill Livingstone, Edinburgh.
Schatzker J, Tile M (2005). The rationale of operative fracture care, 3rd edn. Springer-Verlag Berlin
Heidelberg.
American College of Surgeons (2008). Advanced trauma life support (ATLS) for doctors, student
manual, 8th edn. American College of Surgeons, Chicago.
Wenger DR, Pring ME (2006). Rang’s children’s fractures, 3rd edn. Lippincott, Williams and Wilkins,
Philidelphia.
Bucholz RW, Court-Brown CM, Heckman JD, Tornetta P (Eds.) (2009). Rockwood and Green’s
fractures in adults. 7th edn. Lippincott, Williams, and Wilkins, Philadelphia.
Beaty JH, Kasser JR (Eds) (2009). Rockwood and Wilkin’s fractures in children, 7th edn. Lippincott,
Williams, and Wilkins, Philadelphia.
Review
Miller M (2008). Review of orthopaedics, 5th edn. Saunders, Philadelphia.
Soloman L, Warwick D, Nayagam S (Eds) (2010). Apley’s system of orthopaedics and fractures, 9th
edn. Hodder Arnold, London.
Ramachandran M (2007). Basic orthopaedic sciences: the Stanmore guide. Hodder Arnold, London.
Surgical exposures
Hoppenfeld S, De Boer P, Buckley R (2009). Surgical exposures in orthopaedics: the anatomic
approach, 3rd edn. Lippincott, Williams, and Wilkins, Philadelphia.
Clinical examination
Harris N, Stanley D (Eds) (2005). Advanced examination techniques in orthopaedics. Cambridge
University Press, Cambridge.

589
Plastic surgery
Suturing wounds 590
Skin grafts 594
Surgical fl aps 596
Management of scars 598
Excision of simple cutaneous lesions 600
Skin cancer 602
Burns: assessment 604
Burns: management 606
Soft tissue hand injuries 610
Hand infections 612
Dupuytren’s disease 614
Breast reduction 616
Breast augmentation 617
Breast reconstruction 618
Chapter 16

CHAPTER 16 Plastic surgery
590
Suturing wounds
Principles of wound closure
A wound can be closed in the following ways:
• Direct apposition of skin edges by sutures, glue, or staples;
• Skin grafts (see b p. 594).
• Flaps (see b p. 596).
Key facts
A correctly orientated incision, adequate haemostasis, and minimal tissue
handling are prerequisites for an ideal scar. When closing wounds, bear in
mind the following:
• All wounds leave scars. You must warn your patient of this.
• Hypertrophic scars are more likely on the sternum and deltoid area.
• Speed of healing depends on site. The face heals more quickly than the
trunk and limbs.
• Children and young adults heal more quickly and achieve stronger
scars than the elderly, the chronically ill, and those on steroids.
• Stitch marks (‘tramline effect’) are caused by epithelial growth into
suture tracks and occur when sutures are left in longer than 7 days.
• Cross-hatching is more common when tight sutures cause ischaemia.
• If sutures are removed too early, the wound may dehisce, leaving a
worse scar.
Suture techniques
• Eliminate dead space with deep sutures or a drain, but avoid suturing
fat, which contributes no strength and may lead to fat necrosis.
• Consider buried, interrupted dermal sutures to reduce skin tension.
• Dermal sutures can be combined with a subcuticular running suture or
skin tapes to avoid suture marks.
• Use the fi nest suture possible to maintain wound closure—5/0 or 6/0
for the face; 4/0 or 5/0 for the hand; 2/0 to 4/0 for the trunk.
• Evert the wound to reduce dead space and allow rapid healing.
• Approximate wound edges without strangulating the skin.
• Dressings can be used to splint a wound or immobilize a limb during
healing.
• Elevation will reduce post-operative swelling, bleeding, and pain.
• In a low tension wound closure, sutures may be removed at 5–7 days
on the face, 7–10 days on the arm and anterior trunk, and 14 days on
the back and lower limb.
• Most wounds benefi t from being splinted with skin tape after removal
of sutures.

SUTURING WOUNDS
591
Interrupted skin suture (see Fig. 16.1(a))
• Use fi ne-toothed Adson forceps or a skin hook to evert the skin.
• Pass the needle perpendicular to the skin through its full thickness.
• Either remove the needle through the wound or continue in one
sweep to the other side of the wound, using the forceps for
counter pressure so the needle passes perpendicular to the skin
on its way out.
• Tie the knot so the skin edges are just apposed, bearing in mind the
wound will swell post-operatively.
• Place the sutures evenly, approximately twice as far apart as they are
from the wound margins.
• The distance between the suture and the wound margin should be
similar to the thickness of the skin.
Mattress suture (see Fig. 16.1(b)) Pass the needle as above across
the wound, then turn it around and pass it back as if doing another
interrupted suture in the opposite direction. The second pass can be along
the wound from the fi rst (a horizontal mattress) or nearer the wound
margin than the fi rst pass (a vertical mattress suture).
Deep dermal suture (see Fig. 16.1(c)) Use the forceps or skin hook to
evert the skin and pass the needle from deep to superfi cial on the dermal
surface of the wound. Move to the other side of the wound and pass the
needle from superfi cial to deep within the dermis. Tie a knot which should
be buried deep in the wound.
Continuous suture (see Fig. 16.1(d)) A combination of repeated
interrupted-type sutures or interrupted, then mattress sutures.
Subcuticular suture (see Fig. 16.1(e)) The suture is passed continuously
within the dermis, usually near the dermo-epidermal junction, from one
end of the wound to the other, and pulled tight. It may be secured with
a knot buried deeply at either end or with skin tapes laid over the suture
ends and the wound surface.
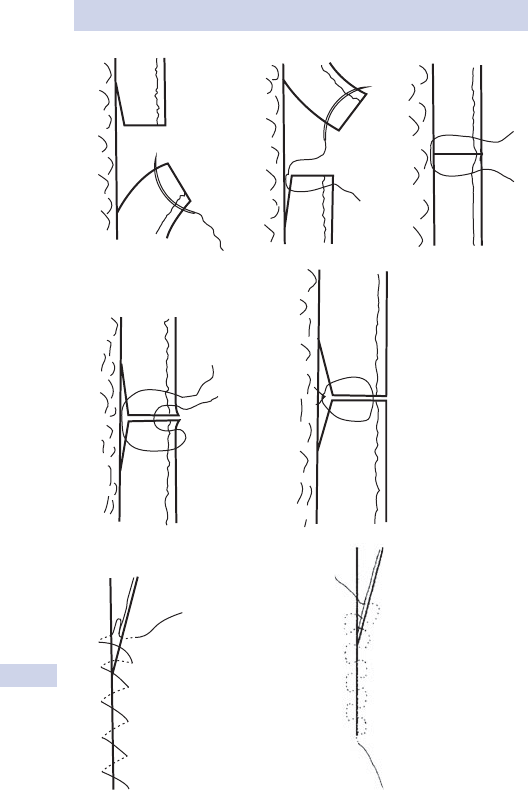
CHAPTER 16 Plastic surgery
592
(a)
(b)
(c)
(d)
(e)
Fig. 16.1 Types of suture. (a) Interrupted suture. (b) Mattress suture.
(c) Deep dermal suture. (d) Continuous suture. (e) Subcuticular suture.
This page intentionally left blank

CHAPTER 16 Plastic surgery
594
Skin grafts
Defi nition
A skin graft is a piece of dermis and epidermis that is completely
removed from its original bodily attachment (the donor site). It is fi xed
to a recipient site and develops a new blood supply from the underlying
tissue.
• Autograft. Transfer from one part of a person’s body to another part.
• Isograft. Transfer between genetically identical individuals.
• Allograft. Transfer between individuals of the same species.
• Xenograft. Transfer between individuals of different species.
Full thickness skin grafts (Wolfe grafts) (see Table 16.1)
• Contain epidermis plus the entire thickness of dermis.
• Adnexal structures, e.g. hair, are included.
• Harvested by elliptical excision from sites of skin laxity, e.g.
post-auricular skin crease, supraclavicular, preauricular, groin, or
medial upper arm skin.
• Graft secured with a tie-over dressing, e.g. profl avine-soaked cotton
wool, and inspected after a week.
• Donor site sutured closed.
Split thickness skin grafts (Thiersch grafts) (see Table 16.1)
• Consist of epidermis plus a variable thickness of dermis.
• Harvested by shaving off a layer of skin with a skin graft knife or
dermatome. Can be taken from any area of the body (thigh skin most
often used—plentiful and easy to access).
• Graft is often fenestrated (to stop blood or serous fl uid collecting
under it) or meshed (to expand the graft and allow it to contour to
the wound bed).
• Graft secured with glue, sutures, or staples, then a non-adherent,
compressive dressing. Inspected after 5 days.
• Defect heals by re-epithelialization from skin appendages.
Graft healing
Stages of graft take
• Adherence (immediate). Fibrin bond between graft and recipient bed.
• Serum imbibition (days 2–4). Graft absorbs fl uid and nutrients from
bed.
• Revascularization (after day 4). Blood enters the graft, either by
fl owing directly into the graft vessels (inoculation) or by new vessel
ingrowth.
Reasons for graft failure
• Shearing. Revascularization cannot occur if the graft is mobile.
• Infection. Either of the bed or the graft tissue.
• Separation of graft from its bed. By haematoma or seroma.
• Inadequate bed, e.g. bare cortical bone; tendon without paratenon.
• Damage to the graft, e.g. poor surgical technique, excessive dressing
pressure.

SKIN GRAFTS
595
Table 16.1 Split thickness grafts versus full thickness grafts
Split skin graft Full thickness skin
graft
Cosmesis Thin, often hypertrophic
skin
Good cosmesis
Contracture Frequent Less frequent
Availability Plentiful; can re-harvest
after 14 days
Limited by skin laxity
Take Good—low metabolic
needs
Needs optimal bed
Donor scar Minimal—colour change
only
Linear scar
Contraindications Inadequate bed, e.g.
exposed bone, tendon,
cartilage (in which case
fl ap needed)
Infected bed
Areas where cosmesis is
paramount
Large area to be
covered
Inadequate bed
Vacuum dressings
These are dressings that apply negative pressure via a sponge placed
in the wound cavity, covered with an airtight adhesive silicone sheet
and connected to a vacuum pump. They increase the initial rate of
granulation in a variety of wounds, including dehisced or infected
sternotomy and laparotomy wounds, pressure sores, chronic open
wounds, fl aps, grafts, and burns. The dressing is changed every 48–72h.
Fluid from the wound bed is collected in a disposable canister. Chronic
wounds may heal by secondary intention (see b p. 146) or be closed
primarily.

CHAPTER 16 Plastic surgery
596
Surgical fl aps
Defi nition
A fl ap is a unit of tissue that maintains its own blood supply while being
transferred from donor to recipient site.
Classifi cation of fl aps
Also see Fig. 16.2.
By blood supply
• Random pattern. Survive on blood vessels in dermal and subdermal
plexuses which have no specifi c anatomical pattern. Length to breadth
ratio is therefore limited to 1:1 (or 3:1 on the face).
• Axial pattern. Have at least one specifi c artery running longitudinally
within the fl ap, so length to breadth ratio can be greatly increased.
All composite fl aps and all free fl aps have an axial blood supply.
By mode of transfer (for local fl aps)
• Advancement. The base of the fl ap advances in the direction of the fl ap
axis, e.g. V-Y fl ap of perianal skin into anal canal for anal stenosis.
• Pivot. Rotation or transposition. The fl ap rotates around a single pivot
point, e.g. scalp rotation fl ap to cover facial defect after tumour excision.
• Interpolation. The fl ap pedicle passes over or under adjacent skin to
inset the fl ap into a nearby defect, e.g. deltopectoral fl ap for head and
neck reconstruction after radical tumour surgery.
Transfer of distant fl aps
• Direct. Flap moved directly to non-adjacent area, e.g. cross fi nger fl ap.
• Tubed. Pedicle curled inwards to form a tube until base of fl ap divided,
e.g. tubed fl ap from upper arm for nose reconstruction.
• Free. Artery and vein to fl ap are completely divided, then reattached
with microvascular anastomoses to a suitable artery and vein at the
recipient site, e.g. radial forearm fl ap to release neck scar contracture.
By composition
• Cutaneous. Skin and subcutaneous tissue only, e.g. groin fl ap.
• Fasciocutaneous. Includes deep fascia, making fl ap vascularity more
reliable and allowing length to breadth ratio to be increased.
• Fascial or adipofascial. The fascia (and subcutaneous fat) is transferred,
but the skin, still attached, is replaced on the donor site, e.g.
temporalis fascial fl ap. The transposed fl ap can then be skin grafted.
• Muscle. Useful for infected or traumatic wounds. The fl ap is skin
grafted, e.g. gastrocnemius fl ap for exposed knee prostheses.
• Myocutaneous. Used in reconstructive surgery. The muscle carries the
blood supply to the skin, e.g. latissimus dorsi myocutaneous fl ap.
• Perforator fl aps. Modifi ed myocutaneous fl aps. A single artery and vein
are dissected from skin, through muscle, to the parent vessels. The
muscle remains in situ, so its function is retained, e.g. deep inferior
epigastric perforator (DIEP) fl ap.
• Bone, osseocutaneous. Bone with or without skin, e.g. fi bular fl
ap for
reconstruction of mandible. Muscle may also be included.
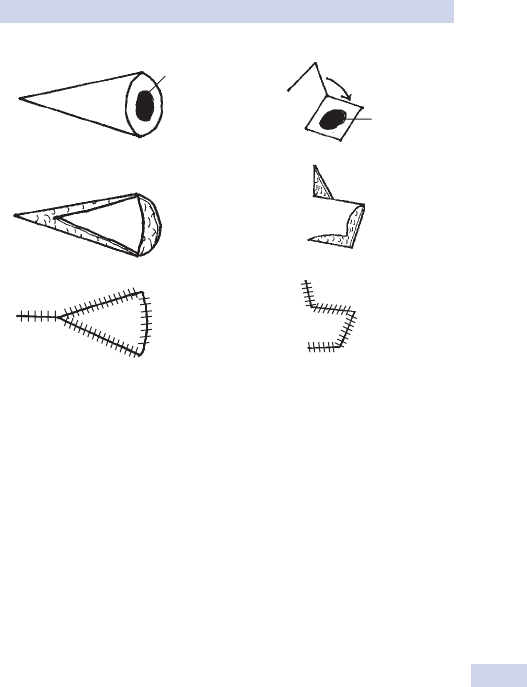
SURGICAL FLAPS
597
(a)
Lesion
(b)
Lesion
Fig. 16.2 Types of surgical fl aps. (a) V-Y fl ap. (b) Rhomboid fl ap.

CHAPTER 16 Plastic surgery
598
Management of scars
Defi nition
A scar is an area of fi brous connective tissue, produced by healing.
Clinical features
A normal scar is initially fl at and pale, then becomes red, itchy, and raised.
Over months to years, it settles back to a fl at, pale, slightly shiny patch.
Scarring is more pronounced if infection intervenes during healing or in
the presence of foreign bodies. Scars settle more slowly in children and
will improve over 2–3y, but resolve rapidly in the elderly.
There are several types of abnormal scar.
• Hypertrophic scars. Firm, red, itchy, and elevated above the skin
surface, but within the boundaries of the injury. More common over
presternal and deltoid regions. Regress with time.
• Keloid scars. Extend beyond wound boundaries. Do not regress
spontaneously. Painful and itchy. Common in dark skins and sites as
above.
• Stretched scars. Due to dehiscence of dermis under intact epidermis.
Common on the back.
• Scar contractures. Common over fl exor surfaces of joint. Occur when
wounds heal by secondary intention, after split skin grafting, or when
incisions cross a joint perpendicular to the crease.
Treatment
Treatment aims to improve poor cosmesis, relieve local symptoms (pain,
itch, irritation), or reduce restriction of associated joint movement.
Medical/conservative treatment
• Observation. ‘Benign neglect’.
• Massage. Scar achieves fl at, pale state more quickly. Relieves itch.
• Pressure. Pressure garments for large areas, e.g. skin-grafted burns.
Pressure devices, e.g. clip earrings for earlobe keloids. Worn
continuously till scars mature. Reduce hypertrophy and contracture.
• Silicone gel. Either a sheet of gel tape worn on the scar or a jelly
rubbed into it scar. Reduces hypertrophy and relieves itch.
• Lasers. Pulsed dye lasers used to reduce redness and hypertrophy.
Carbon dioxide laser resurfaces depressed scars.
• Intralesional injections. Steroids and cytotoxics (e.g. bleomycin, 5-FU)
reduce excess collagen formation; used to fl atten hypertrophic and
keloid scars and reduce pain and itch. Usually need repeated injections
at 1–2-month intervals.
• Radiotherapy. Occasionally given immediately post-operatively to
wounds in patients known to be prone to hypertrophic or keloid
scarring.

MANAGEMENT OF SCARS
599
Surgical treatment
• Excision and closure. For stretched scar or scar with ‘tramlines’.
Usually restretch to some extent. Keloid or hypertrophic scars are
likely to recur if excised and may be much larger than the original scar.
Keloids should only be excised in combination with a post-operative
course of steroid injections.
• Z-plasty (see Fig. 16.3(a)). Lengthens scar. Can re-orientate scar into
lines of relaxed skin tension, or break up the line of the scar and make
it less noticeable.
• W-plasty (see Fig. 16.3(b)). Breaks up line of scar. Used on scalp to
avoid a hairless linear scar.
• Scar release and resurfacing. Used when Z-plasty inadequate for scar
release, either because there is insuffi cient laxity adjacent to the
contracture or if adjacent skin is of poor quality. Resurfacing may
include skin grafting, local fl aps, or free tissue transfer.
(a)
(b)
Fig. 16.3 Surgical techniques for managing scars. (a) Z-plasty. (b) W-plasty.

CHAPTER 16 Plastic surgery
600
Excision of simple cutaneous lesions
Planning
• Under good light and before infi ltration of anaesthesia, mark the
borders of the lesion. Mark the appropriate margin of excision: 2–5mm
for basal cell carcinoma (BCC), 4–6mm for squamous cell carcinoma
(SCC), 1–2mm for biopsy of a pigmented lesion.
• Incision biopsies should include a border of the lesion and normal skin.
• For direct closure, convert the excision to an ellipse, using lines of
relaxed skin tension as the long axis. Be guided by wrinkles and line of
hair growth (hair generally grows in the direction of relaxed skin
tension lines (RSTLs)).
• Wedge excisions are used on the borders of the ear, eyelid, and lip.
Circular excisions are used where there is little skin laxity, using fl aps
or grafts to close the defect.
Anaesthesia
• Calculate the maximum safe dose for your patient (see b p. 218).
• Consider a mixture of bupivacaine 0.25% with lidocaine 1% to provide
longer acting anaesthesia. Adding sodium bicarbonate makes the
injection less painful.
• Using adrenaline with the infi ltration reduces intraoperative bleeding,
but should not be used near anatomical ‘end’ arteries (e.g. the digital
arteries) due to the risk of distal ischaemic necrosis.
• In the face, nerve blocks (e.g. mental, infraorbital, supraorbital, and
supratrochlear) may reduce the pain of infi ltration, the volume of
anaesthetic needed, and distortion of the tissues by the anaesthetic fl uid.
• Check the anaesthetic is working before starting excision.
Shave excision For benign, non-pigmented naevi and seborrhoeic kera-
toses. Use a number 10 blade to cut horizontally across the lesion at
mid-dermal level.
Excision
• Be aware of underlying structures (e.g. the frontal branch of the
temporal nerve when excising lesions from the temple). Ask your
assistant to stretch the skin.
• Use a size 15 blade on the face; consider a larger size 10 blade on the
thicker skin of the back.
• Cut the margins of the lesion perpendicular to the skin; this will aid
closure.
• Cut away from the corners of the wound to avoid X-shaped overcuts.
Cut the lower edge before the upper one; blood trickling down may
obscure your view.
• Lift one corner of the lesion gently with a skin hook or fi ne-toothed
(Adson’s) forceps and cut along the base of the lesion in horizontal
lines at the level of the subcutaneous fat. Avoid traumatic handling of
the lesion, which may compromise histological analysis.
• Perform accurate haemostasis.
• Close and dress the wound.

EXCISION OF SIMPLE CUTANEOUS LESIONS
601
Post-operative care
• All lesions should be sent for histological analysis, clearly labelled (if
necessary with a marking stitch for orientation).
• Elevate the wound.
• Keep the wound dry until the skin is healed.
• Paracetamol, ibuprofen, and codeine are suitable analgesics; aspirin is
best avoided due to risk of bleeding.
• Patients should not drive on the day of surgery.

CHAPTER 16 Plastic surgery
602
Skin cancer
Key facts
• BCC, a neoplasm of the basal cells of the epidermis affects 20–40%
Caucasians. It almost never metastasizes, but can invade deeply and
may therefore be fatal.
• SCC is a malignant neoplasm of the keratinizing cells of the epidermis
affecting 1 in 2000 Caucasians per year.
• Melanoma is a malignant neoplasm of melanocytes, with a lifetime risk
of about 1 in 70 for Caucasians. The incidence has doubled over the
past 20 y.
Clinical features
• Melanoma presents with a change in a pre-existing or new mole
(naevus). Remember Asymmetry; Border irregularity; Colour change
or variegated colour; Diameter >6mm; Elevation, itch, or bleeding. All
these features are suspicious of melanoma.
• The typical BCC is a skin ulcer with a pearly edge and telangectasia;
however, there may not be any of these features. A persistent, itchy;
scaly patch in a sun-exposed area may also be a BCC.
• SCC typically presents as an ulcer with a raised, rolled edge, but also
may take many forms from scaly patch to keratotic horn.
Risk factors
• Fair skin and blue eyes.
• Sun exposure, both adult and childhood, especially sunburn.
• Family history.
• Previous skin cancer.
• Immunosuppression, especially post-organ transplantation.
• Xeroderma pigmentosum.
• Pre-malignant lesions. Multiple atypical naevi and giant congenital naevi
for melanoma; sebaceous naevus of Jadassohn for BCC; Bowen’s
disease, solar keratosis, and chronic ulcers for SCC.
• Radiotherapy.
• A variety of chemicals, e.g. arsenic and coal, predisposes to SCC and
BCC.
Assessment
• History includes sun exposure, previous skin lesions, drug history, and
family history.
• Examine the entire skin and palpate draining lymph nodes.
• Dermatoscopy is used to improve accuracy of clinical diagnosis of
melanoma.
Management
Melanomas and SCCs are managed by skin cancer MDTs.

603
SKIN CANCER
Melanoma
• All suspicious pigmented lesions are biopsied with a 2mm margin to
include subcutaneous fat and sent for histological analysis.
• Surgery aims to cure melanoma. Radiotherapy and chemotherapy are
used for palliation only. Wide local excision margins depend on the
depth of invasion (Breslow thickness) of the tumour and are typically
1cm for lesions <1mm thick, 2cm for lesions 1–2cm thick, and 2–3cm
for lesions >2cm thick.
• Sentinel lymph node biopsy may be considered, with lymph node
dissection of the neck, axilla, or groin if positive.
• Prognosis depends on Breslow thickness, ulceration of the tumour,
and lymph node involvement.
SCC
• Lesions are excised with a 4–6mm margin depending on the site; 95%
of tumours are cured by this treatment.
• Moh’s micrographic surgery probably gives the highest cure rate.
• Radiotherapy is used as an adjuvant treatment for metastatic tumours
or as primary treatment if the tumour or the patient mean that surgery
is not possible.
• Palpable lymph nodes in the draining basin are investigated by FNA,
with lymphadenectomy if positive.
• Prognosis depends on diameter of lesion, depth of invasion, nerve or
vessel invasion on histology
BCC
• Margins of 3–4mm are suitable for well-defi ned BCCs, but wider
margins are used when margins are unclear and in recurrent tumours.
• Moh’s micrographic surgery is also used, particularly when wide
excision would leave an unacceptable defect, e.g. around the eye.
• Other treatment modalities include curettage and cautery,
cryotherapy, radiotherapy, efudix, imiquimod.
• Ninety-fi ve per cent of lesions are cured by complete excision, 99%
with Moh’s surgery. Radiotherapy cures 90%.

CHAPTER 16 Plastic surgery
604604
Burns: assessment
0 Assessment and management of burns go hand in hand and are
simultaneous in practice. They have been divided here only for ease of
reading.
Causes Most burns are due to fl ame or contact with hot surfaces; scalds
are more common in children and the elderly. Chemical, electrical, irradi-
ation, and friction burns are rare.
History
• Find out the exact mechanism, including temperature of water,
duration of contact, concentration of chemical, voltage.
• Record factors suggesting inhalation injury, e.g. burns in a confi ned
space, fl ash burns.
• Enquire about other injuries.
• Document fi rst aid given so far.
• Document timings of injury, fi rst aid, and resuscitation.
Examination
Estimate area of burn Do not include areas of unblistered erythema.
• Rule of nines (see Fig. 16.4).
• Patient’s hand is approximately 1% total body surface area (TBSA).
• Lund and Browder chart (see Fig. 16.5) is the most accurate method.
• Subtract % unburned skin from 100% to check calculation.
• Draw a picture, ideally fi lling in the Lund and Browder chart.
Estimating depth of burn
• Epidermal. Erythema only.
• Superfi cial dermal. Pink, wet or blistered, sensate, blanches and refi lls.
• Deep dermal. Blotchy red, wet or blistered, no blanching, insensate.
• Full thickness. White or charred, leathery, no blanching, insensate.
Signs of inhalation injury
• Singed nasal hair.
• Burns to face or oropharynx. Look for blistered palate.
• Sooty sputum.
• Drowsiness or confusion due to carbon monoxide inhalation.
• Respiratory effort, breathlessness, stridor, or hoarseness are signs of
impending airway obstruction and require immediate intubation.
Features of non-accidental burns injury Refer to paediatric burns unit if sus-
pected in a child. Features include:
• Delayed presentation.
• History inconsistent or not compatible with injury.
• Other signs of trauma.
• Suspicious pattern of injury, e.g. cigarette burns, bilateral ‘shoes and
socks’ scalds.
BURNS: ASSESSMENT
605605
18
1
Post
18
18
Ant
18
99
9
Fig. 16.4 Rule of nines.
A
B
C
1
2
13
C
B
2
1
A
1
2
13
2
BB
CC
1
1
/
2
1
3
/
4
1
3
/
4
1
1
/
2
1
1
/
2
1
1
/
2
2
1
/
2
2
1
/
2
1
3
/
4
1
3
/
4
1
1
/
2
1
1
/
2
1
1
/
2
1
1
/
2
Total
REGION
%
Head
Neck
Ant trurk
Post trurk
L arm
R arm
Buttocks
R leg
Genitalia
L leg
AREA
A = of head
B = of one thigh
C = of one lower leg
9
1
/
2
1
/
2
1
/
2
1
/
2
8
1
/
3
3
3
1
/
4
2
1
/
2
6
1
/
2
5
1
/
2
1
/
2
4
1
/
2
1
/
2
3
1
/
4
3
1
/
2
4
3
/
4
2
3
/
4
3
1
/
2
2
/
4
2
1
/
2
43 3
3
0151015Adult
Fig. 16.5 Lund and Browder chart.

CHAPTER 16 Plastic surgery
606606
Burns: management
Immediate fi rst aid
• Stop the burning process (do not endanger yourself ).
• Cool the wound. Running water at 2–15*C for 20min (beware risk of
hypothermia in infants, young children, and adults with >25% TBSA).
Resuscitation
• A. Airway maintenance with C-spine control. Intubate if suspected
inhalation injury; airway oedema can be rapidly fatal.
• B. Breathing and ventilation.
• C. Circulation with haemorrhage control.
• D. Disability and neurological status.
• E. Exposure and environmental control.
• F. Fluid resuscitation: child, >10% TBSA; adult, >15% TBSA burned.
• Two large peripheral IV lines, preferably through unburned skin.
• Send blood for FBC, U&E, clotting, amylase, carboxyhaemoglobin.
• Give 3–4mL Hartmann’s solution/kg/% TBSA burned. Half of this is
given over the fi rst 8h following injury, half over the next 16h.
• Children need maintenance fl uid in addition.
• Monitor resuscitation with urinary catheter (aim for urine output
0.5–1mL/kg/h in adults and 1–1.5mL/kg/h in children).
• Consider ECG, pulse, BP, respiratory rate, pulse oximetry, ABGs.
Perform secondary survey.
Referral to a burns unit (see Box 16.1) Intubate before transfer if
inhalation injury suspected. Give humidifi ed 100% O
2
to all patients. Wash
the burn and cover with cling fi lm. Give IV morphine analgesia. Discuss
NGT and catheter insertion with burns unit. Give tetanus prophylaxis if
required.
Box 16.1 Criteria for referral to a burns unit
>10% TBSA burn in adult; >5% TBSA in child.
Burns to face, hands, feet, perineum, genitalia, major joints.
Full thickness burns >5% TBSA.
Electrical or chemical burns.
Associated inhalation injury—always intubate before transfer.
Circumferential burns of limbs or chest.
Burns in very young or old, pregnant women, and patients with signifi -
cant comorbidities.
Any burn associated with major trauma.

BURNS: MANAGEMENT
607607
Management of the burn wound
• Superfi cial dermal burns will heal without scarring within 2 weeks as
long as infection does not deepen the burn.
• For small burns, outpatient treatment with simple, non-adherent
dressings and twice weekly wound inspection is suffi cient.
• Wash burns with normal saline or chlorhexidine.
• Debride large blisters. Elevate limbs to reduce pain and swelling.
• Dress hands in plastic bags to allow mobilization.
• Topical silver sulphadizine is used on deep burns to reduce risk of
infection (but should not be applied until the patient has been
reviewed by a burns unit as it makes depth diffi cult to assess).
Escharotomy Performed for circumferential full thickness burns to the
chest that limit ventilation or to the limbs that limit circulation. Loss of
pulses or sensation is a late sign. In the early stages, pain at rest or on
passive movements of distal joints indicates ischaemia. Patients may also
need fasciotomies.
Excision and skin grafting Performed for deep dermal or full thickness burns
that are too large to heal rapidly by secondary intention.
Electrical injuries
• Low voltage (<1000V). Domestic electrical supply. Causes local
contact wounds, but no deep injury. May cause cardiac arrest.
• High voltage (>1000V). High tension cables, power stations, lightning.
Causes cutaneous and deep tissue damage with entry and exit wounds.
• ECG on admission for all injuries. Continuous cardiac monitoring for
24h for signifi cant injuries.
• In high voltage injury, muscle damage may require fasciotomy.
• Myoglobinuria can cause renal failure. Urine output >75–100mL/h.
Chemical burns
Treat with copious lavage for at least 30min until all chemical has been
removed and skin pH is normal.
• Acid. Causes coagulative necrosis; penetrates skin rapidly, but is easily
removed.
• Alkali (includes common household chemicals and cement). Causes
liquefactive necrosis so needs longer irrigation (>1h).
• Hydrofl uoric acid. Fluoride ions penetrate burned skin, causing
liquefactive necrosis and decalcifi cation; 2% TBSA burn can be fatal.
Irrigate with water.•
Trim fi ngernails.•
Topical calcium gluconate gel, 10%.•
Local injection of 10% calcium gluconate.•
IV calcium gluconate.•
May need urgent excision of burn.•
• Elemental Na, K, Mg, Li. Do not irrigate initially; they ignite in water.
Brush off particles and direct high pressure jet of water to wound.
• Phosphorus. Irrigate with water, then debride particles which will
otherwise continue to burn. Apply copper sulphate which turns
particles black so they are easier to identify.

CHAPTER 16 Plastic surgery
608608
• Bitumen. Burns by heat; treat by cooling with water. Remove cold
bitumen with peanut or paraffi n oil.
• Tar. Burns by heat. Treat by cooling with water; no need to remove
tar as it gradually gets emulsifi ed with topical ointments used for
treatment.
This page intentionally left blank

CHAPTER 16 Plastic surgery
610
Soft tissue hand injuries
History
• Mechanism of injury.
• Dominant hand, occupation, hobbies.
• Medical and smoking history, previous hand injuries, social history.
Examination
Use local anaesthetic block if needed for pain (check sensation fi rst).
Look Posture of hand and digits. Site of laceration(s) and tissue loss.
Feel Perfusion of hand and digits, pulses. Sensation in distribution of radial,
ulnar, median, and digital nerves. Pain over bones.
Move
• Long extensors extend MCPJs.
• EPL extends thumb dorsal to plane of hand (i.e. up off a table).
• FDP tendons fl ex DIPJs.
• FDS tendons fl ex PIPJs. Isolate FDS by holding all digits except the one
under examination extended.
• Testing wrist fl exors and extensors is unreliable as fi nger fl exors and
extensors may mimic function, but pain on movement suggests injury.
• Examine intrinsics, hypothenar and thenar muscles, particularly
abductor pollicis brevis (supplied by median nerve) and Froment’s sign
(for adductor pollicis supplied by ulnar nerve).
• Check stability of joints. Pain or abnormal movement on lateral
deviation suggests collateral ligament damage.
Investigations X-ray for fractures of foreign bodies. Photographs.
Treatment
• Finger pulp injury. Debride under tourniquet. If there is no bone
exposed, it will heal by secondary intention. Exposed bone may need
surgery to shorten bone or cover it with a local fl ap.
• Subungual haematoma. Painful bruise under nail. Trephine nail with
sterile needle to evacuate haematoma.
• Nailbed injury. Often with distal phalanx (DP) fracture. Remove nail
under tourniquet; irrigate wound; repair nail with absorbable 7/0
suture using loupe magnifi cation. Replace fenestrated nail as splint for
eponychial fold.
• Mallet fi nger. Immobilize in stack splint for 6–8 weeks unless large
bony fragment present which may require surgical fi xation.
• Foreign bodies. Remove organic matter and painful foreign bodies.
• Lacerations and puncture wounds.
Always explore with anaesthetic and tourniquet to determine •
underlying structural damage.
Irrigate wounds and debride as necessary.•
Tetanus prophylaxis (see • b p. 175).
Co-amoxiclav (500mg tds • PO) for bites.

SOFT TISSUE HAND INJURIES
611
Repair tendons, ideally primarily. Post-operative regimes typically •
involve splints for 6 weeks and 6 more weeks without heavy lifting.
Repair nerves under magnifi cation. Axonal regeneration progresses •
at 1mm/day after 1 month from repair.
Thoroughly irrigate open joints due to the risk of septic arthritis. •
Collateral ligaments may need to be repaired and are splinted for
around 4 weeks post-repair.
• Complications. Haematoma, infection, tendon or ligament rupture,
stiffness, painful scars, neuroma, complex regional pain syndrome,
scar contracture, cold sensitivity.

CHAPTER 16 Plastic surgery
612
Hand infections
Key facts
Usually follows a penetrating injury (which may seem insignifi cant) or a
bite. Haematogenous spread of infection to the hand is rare.
• Infecting organisms. After penetrating injury, Staphylococcus aureus is
the most common, followed by streptococci. Human bites are often
also contaminated with Eikenella corrodens. Viruses (hepatitis B and C,
HIV) are rarely transmitted. Pasteurella spp. are common in infected
cat and dog bites.
• Paronychia. Infection of nailfold. Candida albicans causes chronic
paronychia and may require excision of crescent of epinychium and
topical antifungals. Herpes simplex causes whitlow with vesicles or
bullae around the nail, but no pus. Avoid surgery in these cases.
• Felon. Finger pulp infection.
• Palmar space infection. There are four fascial compartments in the palm
(web space, hypothenar, mid-palm, and thenar). They usually
confi ne infection initially. Pain, swelling, and reduced movement are
features. Swelling is often more prominent on the dorsal surface of
hand.
• Flexor sheath infection. The cardinal signs are fl exed posture of fi nger,
pain on passive extension, fusiform swelling, pain along fl exor sheath.
Often requires continuous saline irrigation for 24–48h post-drainage.
• Bites. High risk of infection so always irrigate, give antibiotic
prophylaxis (co-amoxiclav 500mg PO tds), and refer for surgical
exploration.
Treatment
Delay can be disastrous, resulting in stiffness, contracture, and pain. Early
cellulitis (24–48h after onset) may be treated by elevation, splints, and
antibiotics. Any collection of pus must be drained urgently.
Initial treatment
• Tetanus prophylaxis if indicated.
• Elevation and splintage.
• IV co-amoxiclav 1g tds (unless penicillin allergy) till sensitivities known.
• Plain X-ray may be useful to exclude associated fractures, foreign
bodies, underlying osteomyelitis, and evidence of gas-forming infection.
Surgical treatment
• Use a tourniquet, but elevate rather than exsanguinate the limb.
• Send pus swabs and tissue samples for culture.
• Debride and irrigate wounds; fully explore pockets of pus.
• Leave wound open for delayed primary closure.
Post-operative care
• Continue elevation.
• Daily saline soaks or irrigation of the wound.
• Splint for comfort with wrist extended, MCPJs fl exed, and
interphalangeal joints (IPJs) extended. Mobilize with physiotherapists.
• Antibiotics until infection resolved.
This page intentionally left blank

CHAPTER 16 Plastic surgery
614
Dupuytren’s disease
Key facts
A progressive thickening of the palmar and digital fascia that may lead
to contractures. Aetiology is unknown, but there is a higher incidence
among relatives of affected patients. Associated conditions include
diabetes and epilepsy. Alcoholism, TB, HIV, hand trauma, and tobacco
have all also been implicated. Incidence is 1–3% of northern Europeans,
but it is uncommon in Africa and Asia. It increases with age; ♂ > ♀,
approximately 7:1.
Pathogenesis
• Disease classifi ed by Luck into three phases: proliferative, involutional,
and residual.
• In the proliferative phase, immature fi broblasts, many of which are
myofi broblasts, produce extracellular matrix containing type IV
collagen. Resembles a healing wound histologically.
• Mechanical tension appears to play a role in contractures.
Clinical features
• Thickened palmar and digital fascia forms nodules and cords.
• Progresses to contractures of the MCPJs and PIPJs of the affected rays.
• Tends to affect digits in order: ring, little, thumb, middle, index.
• Normal fascia is referred to as bands; diseased bands are called cords.
• A spiral cord may be a feature, wrapping around the neurovascular
bundle (NVB) and displacing it to the midline and superfi cially, putting
it at risk during surgery.
• The disease affects longitudinal fascial structures; the transverse palmar
fascia is never involved and provides a landmark for dissecting NVBs.
Extra-palmar manifestations
• Garrod’s pads. Thickening over dorsal aspect of PIPJs.
• Peyronie’s disease. Thickened plaques in the shaft of the penis.
• Ledderhose’s disease. Thickened plantar fascia.
Treatment
Indications for surgery
• Over 30* fi xed fl exion contracture at MCPJ or any PIPJ contracture.
Also any rapidly progressing contracture. Results are better for release
of MCPJs than PIPJs.
• Tabletop test. Surgery indicated when hand will not lie fl at on table.
• Pain in nodules or Garrod’s patches. Injection with steroid or excision.
Many people with Dupuytren’s disease never require surgery.

DUPUYTREN’S DISEASE
615
Surgical considerations
Skin
Typical incisions include the following.
• Linear incisions with Z-plasties.
• Bruner incisions.
• Multiple V to Y incisions.
• Lazy ‘S’ incisions.
• Transverse palmar incision with longitudinal extensions.
• Multiple short curved incisions.
• Multiple Z-plasties.
Closure may be direct with skin grafts (split or full thickness) or palm left
open to heal by secondary intention.
Fascia
This may be incised (fasciotomy) or excised (fasciectomy).
• Radical fasciectomy removes the entire palmar fascia.
• Regional or limited fasciectomy removes only the diseased fascia.
• Segmental fasciectomy excises sections of the diseased cord.
• Fasciotomy via a percutaneous approach using a needle provides
temporary relief from contracture.
• Dermofasciectomy. Excision of fascia with overlying skin, used for
severe skin involvement and where risk of recurrence is high, e.g.
surgery for recurrent disease.
• Specimens are sent for histological analysis to rule out the rare
differential diagnosis of epithelioid sarcoma.
Joint contractures
Release of fascia usually resolves contracture at the MCPJ. Fixed fl ex-
ion at the PIPJ is more diffi cult to release and contracture often recurs.
Consider releasing the check-rein and accessory collateral ligaments. DIPJs
are rarely involved except in recurrent disease.
Post-operative care The affected fi ngers are splinted in extension and active
exercises begun in the fi rst week, unless a skin graft has been used. Night
splints are used for at least 3 months.
Complications
• Early. Damage to neurovascular structures (1–3%), PIPJ
hyperextension, haemorrhage.
• Intermediate. Infection, skin fl ap necrosis.
• Late. Complex regional pain syndrome; recurrence (25% of patients
treated surgically will need further surgery for Dupuytren’s disease).
Treatment of recurrence
Recurrence may be treated by repeat surgery although this tends to be
less successful and more extensive at each event. Amputation of a fi xed
fl exed digit is occasionally an option, particularly if the digit hampers work
or leisure activities.

CHAPTER 16 Plastic surgery
616
Breast reduction
To reduce the volume and weight of the hypertrophied breast while main-
taining a blood supply to the nipple and creating an aesthetically pleasing
breast.
Indications
• Neck, back, or shoulder pain.
• Indentation of shoulder skin by bra straps.
• Persistent infections or soreness in the inframammary crease.
• Restriction in activity, especially sport.
• Inability to fi nd clothes that fi t.
• Psychological. Embarrassment, low self-esteem, loss of sexual appeal.
Operative considerations
Blood supply to the nipple
In order to lift the nipple, skin around it is de-epithelialized or excised. The
base of the nipple is left attached to a mound of breast parenchyma (the
pedicle) through which its blood supply travels. Due to the rich vascular
anastamoses in the breast, numerous techniques are possible. Pedicles
can be based inferiorly, superiorly, supero-medially, laterally, or centrally.
Alternatively, the nipple can be removed before the breast is reduced and
replaced as a full thickness graft.
Skin excision and scars
An anchor shape (Wise pattern) excision leaves an inverted ‘T’-shaped
scar. It runs around the areola, vertically down to the inframammary fold
and horizontally along the fold. Other options include periareolar incision
only or periareolar incision with a vertical scar. These techniques limit the
amount of breast tissue that can be resected. L-shaped and horizontal scar
techniques are also possible, but more rarely used.
Post-operative care
The patient usually stays in hospital overnight or longer if drains are used.
She should wear a supportive bra and avoid heavy lifting for 4–6 weeks
post-operatively.
Complications
• Early. Haematoma, infection, altered nipple sensation, skin loss or
necrosis, fat necrosis, delayed wound healing, asymmetry.
• Late. Unsightly scar, inability to breastfeed, pseudoptosis (‘bottoming
out’), recurrence (if done before breast fully grown).
However, most patients are happy with the result, even if they do suffer
complications.

BREAST AUGMENTATION
617
Breast augmentation
To enhance breast size by placing an artifi cial implant beneath the breast.
Indications
Performed for asymmetry, hypoplasia, and psychological reasons, e.g.
self-consciousness or problems with sexual relationships. Inadequate
breast volume may be due to hypoplasia or involution following childbirth
or menopause.
Operative considerations
Incision
• Inframammary fold. Good visualization of implant pocket; visible scar.
• Periareolar. Semicircular incision at the border of the areolus. Scar
fades well, but access is limited. More likely to alter nipple sensation.
• Transaxillary. Eliminates scars on breast. Limited access improved by
using endoscope. Better for subpectoral implants.
• Transumbilical. Only used for saline-fi lled implants, inserted along a
tunnel created superfi cial to rectus sheath. Endoscope confi rms
position of implant pocket. Implant infl ated once in position.
Position of implant
• Submammary. Under the normal breast.
• Subpectoral. Under the pectoralis major (slightly less obvious upper
border in the thin; have lower rates of capsular contracture, but may
move when the pectoralis contracts).
Type of implant
• Size. Depends on patient’s choice.
• Shape. Round implants are low or high profi le (depending on how
much they project forwards); anatomical implants are teardrop-shaped.
• Shell. Implants are made of a silicone shell that is smooth or textured.
Textured implants have lower rates of capsular contracture.
• Implant fi lling. Saline-fi lled implants allow for fi ne adjustment of volume
and can be fi lled or emptied post-operatively. Silicone gel-fi lled
implants feel more like normal breast tissue. No current evidence to
support implication of silicone in causing autoimmune diseases.
Post-operative care
• Usually an overnight stay procedure (longer if drains are used).
• A supportive bra is worn and heavy lifting avoided for 4–6 weeks.
Complications
• Early. Haematoma, infection, nerve injury (altering sensation to the
nipple), incorrect position of implant.
• Late. Capsular contracture, rupture, or defl ation; silicone gel bleed.
• Implants have a limited lifespan, up to about 20y. The likelihood is
that they will need to be removed or replaced at some time. Patients
can usually breastfeed after augmentation. Patients are warned that
mammography is technically more diffi cult, requiring different views.

CHAPTER 16 Plastic surgery
618
Breast reconstruction
Aims
To recreate a breast mound resembling the contralateral breast with
minimal donor defi cit, using a technique appropriate for the patient. After
mastectomy, breast reconstruction is of psychological benefi t. It is techni-
cally easier to perform it at the same time as mastectomy, rather than as
a delayed procedure as there is no scarring around the breast and original
landmarks are present. It also reduces the number of operations required.
However, there may be logistical diffi culties if a combined breast surgery/
plastic surgery team is needed. Also, some patients prefer to wait.
Surgical options
Tissue expander
Placed in the subpectoral position. Infl ated with saline once the wounds
are healed (2–4 weeks post-operatively) via a subcutaneous port. The skin
is slowly stretched until a satisfactory size is reached. The implant can later
be changed for a silicone gel-fi lled implant.
Latissimus dorsi myocutaneous fl ap
A pedicled fl ap based on the thoracodorsal vessels. The latissimus dorsi
muscle, with an ellipse of overlying skin and fat, is tunnelled under the
intervening skin bridge into the breast defect. Depending on the size of the
contralateral breast, an implant may be used under the fl ap.
Abdominal fl aps
The transverse rectus abdominis myocutaneous (TRAM) fl ap consists
of a transverse ellipse of skin on the lower abdomen, plus one of the
two rectus abdominis muscles. This versatile fl ap may be based on either
its upper (deep superior epigastric) or lower (deep inferior epigastric)
vascular pedicles. The upper pedicle is used as a pedicled fl ap, tunnelled
under the abdominal skin into the breast. The lower pedicle is used as
a free tissue transfer. If a sizeable muscular perforator vessel is identifi ed, a
DIEP fl ap can be used, leaving the muscle behind. This fl ap is usually large
enough not to need an implant.
Nipple reconstruction At a later stage, the reconstructed breast can be
tattooed with a picture of a nipple or a nipple formed with a combination
of local fl aps, skin graft, and grafts from the contralateral nipple.
Surgery to the contralateral breast The opposite breast may be reduced,
augmented, or lifted to improve symmetry.

CHAPTER 17 Cardiothoracic surgery
620
Basics
Common cardiac emergencies
Atrial fi brillation (see b p. 55)
• Give 10–20mmol K
+
via central line to get serum K
+
4.5–5.0mmol/L.
• Give empirical 20mmol Mg
+
via central line if none given post-op.
• Give of 300mg amiodarone IV over 1h in patients with good left
ventricle, followed by 900mg amiodarone IV over 23h.
• In patient with poor left ventricular function, give digoxin in 125mcg
increments IV every 20min until rate control is obtained, up to a
maximum of 1500mcg in 24h.
• Synchronized DC cardioversion for unstable patients (b p. 189).
Bleeding (see b p. 180)
• Get immediate help if bleeding is >400mL in 30min.
• Give gelofusine to get CVP 10–14 and systolic BP 80–100mmHg.
• Order further 4U of blood, 2U FFP, and 2 pools platelets.
• Send clotting and FBC, request a CXR.
• Transfuse to achieve Hb >8.0g/dL, platelets >100 x 10
9
/L, APTT <40.
• Give empirical protamine 25mg IV.
• Emergency re-exploration is indicated for excessive bleeding.
Profound hypotension
• Get immediate help.
• Quickly assess pulse, rhythm, rate, CVP, O
2
sats, and bleeding.
• Defi brillate VF or pulseless VT, treat AF as above.
• Treat bradycardia with atropine 0.3mg IV or pace.
• Give gelofusine to raise CVP to 12–16mmHg, place bed head down.
• If suspect cardiac tamponade (b p. 480), prepare for re-sternotomy.
• If patient warm and vasodilated, draw up 10micrograms of
metaraminol into 10mL of saline and give 1mL through a central line,
and fl ush.
• If patient still profoundly hypotensive, give 1mL 1:10 000 adrenaline IV.
Poor gases (see b p. 108)
• If O
2
sats <85% and falling, get immediate help.
• Increase the FiO
2
to 100% temporarily, check the pulse oximeter.
• Look at expansion, auscultate the chest, check PaO
2
.
• If you suspect tension pneumothorax, treat immediately (b p. 480).
• Suction the ET tube, check that the patient is not biting on it.
• Check that the drain tubing is patent and drains are on suction.
• Treat bronchospasm with salbutamol 5mg nebulizer.
• Disconnect from the ventilator and hand-ventilate the patient.
• Get a CXR: look for pneumothorax, haemothorax, atelectasis, ET
tube position, and lobar collapse, and treat (see b p. 108).
Poor urine output (see b p. 112)
• Check that the Foley catheter is patent.
• If the patient is hypotensive, treat this fi rst.
• Give a fl uid challenge of gelofusine to raise the CVP to 14mmHg.
• If not hypotensive and CVP >14mmHg, give 20mg furosemide IV.

BASICS
621
Key revision points: coronary artery anatomy (see Fig. 17.1)
• The left main stem (LMS) arises from the ostium of the left sinus of
Valsalva, travels between the pulmonary trunk anteriorly and the left
atrial (LA) appendage to the left atrioventricular (AV) groove, dividing
after 1–2cm into left anterior descending (LAD) artery, circumfl ex
(Cx), and occasionally a third artery (the intermediate). As it provides
almost the entire blood supply to the left ventricle (LV), occlusion
can be fatal; severe left main disease is known as ‘the widow maker’.
• The LAD runs down the anterior interventricular groove to the
apex of the heart, usually extending round the apex to the posterior
interventricular groove. A variable number of diagonals are given
off over the anterior surface of the LV, small branches supply the
anterior surface of the right ventricle (RV), and superior septals are
given off perpendicularly to supply the anterior two-thirds of the
interventricular septum. Occlusions of the LAD result in anterior MI.
• The circumfl ex originates at 90* from the LMS and runs medially to the
LA appendage for 2–3cm, continuing in the posterior left AV groove to
the crux of the heart. Occlusions of the Cx result in posterior infarcts.
In left dominant hearts (5–10%), the Cx turns 90*
into the posterior
interventricular groove to form the posterior descending artery (PDA).
In 85–90% of hearts, the PDA arises from the right coronary artery
(RCA) (right dominant). About 5% of hearts are co-dominant.
• A variable number of obtuse marginals (OMs) arise from the Cx to
supply the posterior LV. The fi rst branch of the Cx is the AV nodal
artery in which 45% course round the LA near the AV groove.
• The RCA arises from an ostium in the right sinus of Valsalva, gives
off an infundibular branch and then a branch to the sinoatrial (SA)
node, and runs immediately into the deep right AV groove where
it gives off RV branches to the anterior RV wall. Occlusions of the
RCA result in inferior infarcts and bradycardia. The acute marginal
is a large branch which crosses the acute margin of the heart. In
right dominant hearts, the RCA reaches the crux of the hearts
where it turns 90* to form the PDA, which runs in the posterior
interventricular groove. Inferior septals, which supply the inferior
third of the interventricular septum, arise at 90* from the PDA. The
AV node artery is given off by the RCA in 55% at the crux.
Branch to sinus
node
Right coronary
artery
Branch to
AV node
Aotra marginal
Posterior descending
Left ventricular branch
Diagonal 2nd
Obtuse marginal
Circumflex
Diagonal 1st
Left anterior descending
Left main stem
Fig. 17.1 Coronary artery anatomy.

CHAPTER 17 Cardiothoracic surgery
622
Principles of cardiac surgery
Key facts
• The majority of procedures are coronary artery bypass graft (CABG)
operations, followed by aortic valve replacements, and mitral valve
(MV) repair and replacements.
• Many patients are elderly with multiple comorbidities, but 90% should
be out of ICU within a day or two, and ready to go home in a week.
Preoperative preparation
Careful preoperative work-up is essential. All investigations must be
checked; small abnormalities which would not cause a problem in other
specialties can have catastrophic results in cardiac surgery.
• Full history. Quantify symptoms, previous MI or stroke. Comorbidities
(especially COPD, renal failure, peripheral vascular disease), MI <90
days (which increases mortality), drugs (aspirin, clopidogrel, and
warfarin normally stopped 5 days preop to reduce bleeding), allergies,
recent chest infection. Valve patients must have been cleared by
dentist. Ask about previous heart surgery, varicose vein surgery.
• Full examination. Look for signs of heart failure. Active infection, e.g.
abscess is a relative contraindication to valve replacement. Look at
conduit: any evidence of varicose veins?
• All patients should have FBC, U&E, LFTs, clotting screen.
• Cross-match 2U of blood.
• ECG and CXR.
• All patients undergoing coronary artery surgery and patients >35y
undergoing valve surgery should have coronary angiogram less than
1y-old.
• Patients undergoing valve surgery must have had an echo.
• Carotid duplex in any patient with history of stroke, TIA, or carotid
bruits; some centres perform these routinely in patients >70y old.
• Consent by registrar or consultant.
• Sliding scale for diabetic patients (see b p. 52).
Cardiopulmonary bypass (CBP)
Any operation that involves stopping or opening the heart (valve surgery,
surgery on septal defects) or great vessels (ascending and arch aortic
dissection and aneurysm surgery, resection of some tumours invading
great vessels, e.g. renal cell) requires CPB to maintain blood fl ow. This
involves:
• Heparinizing the patient so that blood does not clot in the CPB circuit.
• Securing a 24F aortic cannula in the ascending aorta.
• Securing a 32F venous cannula in the RA or in the superior vena cava
(SVC) and inferior vena cava (IVC).
• Connecting both cannulae to the bypass circuit.
• The venous return from the body is siphoned into the bypass circuit.
• The venous blood is oxygenated, fi ltered, and can be cooled or
warmed, and is pumped back to the patient via the aortic cannula.
• At the end of bypass, heparin is reversed with protamine.

PRINCIPLES OF CARDIAC SURGERY
623
• Complications of CPB include stroke (atheromatous emboli,
hypoperfusion, air, microemboli), SIRS, renal and pulmonary
dysfunction (see b p. 622).
Pathophysiology of CPB
CPB is unavoidable for many operations. It has a major impact on nearly
every organ system and problems associated with bypass include:
• Activation of coagulation and complement cascades.
• Consumption of platelets and clotting factors, causing coagulopathy.
• Microemboli and atherosclerotic emboli from aortic cannulation which
can cause stroke and peripheral limb and end-organ ischaemia.
• Increased capillary permeability.
• Renal, pulmonary, hepatic, and pancreatic dysfunction.
Cardioplegia
CPB does not stop the heart; it just bypasses the beating heart. If the
surgeon wants to operate on a still heart, CPB gives the surgeon three
options: fi brillate the heart, cool the patient, or use cardioplegia.
Cardioplegic arrest is by far the commonest technique.
• Cardioplegia is a potassium rich solution.
• It can be based on blood or crystalloid (blood delivers O
2
better).
• It can be warm or cold (cold may reduce ischaemic injury more).
• It is delivered into the coronary arteries, either anterogradely by
inserting a cannula into the aortic root which is clamped distal to the
cardioplegia cannula or retrogradely via the coronary sinus vein.
• It can be given continuously or intermittently, every 20min or so.
• Cardioplegia arrests the heart and prevents myocardial ischaemia.
Post-operative management
Management of fi ve common post-operative emergencies is outlined on
b p. 620. Most patients are well enough to be extubated within 6h, leave
ITU within 24h, and go home within 5 days. Stable patients should have
bloods, CXRs, and ECGs on days 1, 2, 4, and 6.
First 6 hours
• Myocardial function deteriorates due to ischaemia-reperfusion injury.
• Inotropic support and pacing may be required.
• Patient should be fi t for extubation by 6h post-op.
• Patients should have diuresis >1mL/kg/h.
• Mediastinal bleeding should steadily decrease.
• Insulin requirements usually increase.
Days 1–2
• Inotropes and pacing weaned, invasive monitoring lines removed.
• Chest drains removed after 2h of zero drainage.
• Catheter and any epidural removed, patient mobilized.
• PCA morphine reduced to oral analgesia.
• Patient should be on aspirin, low molecular weight heparin,
furosemide.
• Patient normally eating and drinking.

CHAPTER 17 Cardiothoracic surgery
624
Days 3–5
• Temporary pacing removed if ECG satisfactory.
• Valve repair patients should undergo echocardiography.
• Physiotherapists assess exercise tolerance.
• Back to baseline weight, medications stabilized, ready for discharge.
This page intentionally left blank

CHAPTER 17 Cardiothoracic surgery
626
Coronary artery disease
Defi nition
Narrowing of the coronary arteries caused by atherosclerosis (see b p.
152).
Incidence
Five in 1000 males over 40y have symptomatic ischaemic heart disease
(IHD), 5 in 1000 heart attacks per year, 6000 coronary artery operations
per year in the UK.
Aetiology
Age, male sex, smoking, i BP, diabetes, hyperlipidaemia, obesity, family
history, stress.
Pathology
See b p. 152 for description of atherosclerotic disease. Stenoses tend
progress in severity and distribution. Rate of progression is variable and
regression of lesions has been observed.
• Narrowings of 50% of cross-sectional area limit coronary fl ow reserve
(the increase in blood fl ow that occurs to meet increased O
2
demand).
• Coronary blood fl ow at rest is reduced by narrowings of 90%.
• LV function may be abnormal. In normal people, global LV systolic
function improves with exercise, but in patients with coronary artery
disease (CAD), it gets worse in the area supplied by the stenotic
arteries.
• Acute MI is caused by acute total or subtotal vessel thrombotic
occlusion. Patients with proximal LAD lesions are particularly at risk
(see b p. 54 for description of anatomic territories).
Clinical features
• Angina and/or dyspnoea. Severity is classifi ed using the New York Heart
Association (NYHA) score. Dyspnoea implies congestive heart failure
(CCF).
• Class I. Symptoms only with prolonged or strenuous exertion.
• Class II. Symptoms causing slight limitation of ordinary activity.
• Class III. Symptoms with marked limitation of ordinary activity.
• Class IV. Angina occurring even with mild activity or at rest.
Diagnosis
• History and examination.
• ECG may show evidence of old infarcts.
• Exercise treadmill has 97% specifi city for exertional angina.
• Coronary angiography is diagnostic and obligatory for planning surgery.
• Myocardial perfusion studies such as thallium scans are also useful.
• CT coronary angiography is increasingly used to screen lower risk
patients, but is not helpful in evaluating lesions in high risk patients.

CORONARY ARTERY DISEASE
627
Indications for surgery
The options are medical and percutaneous coronary intervention (angi-
oplasty or stent). Many large trials have been carried out to decide which
groups of patients benefi t most from surgery. This is currently:
• Patients with >70% LMS stenosis.
• Symptomatic patients with >70% proximal LAD stenosis.
• Symptomatic patients with >70% disease in all three vessels (three-
vessel disease).
• Patients with less signifi cant coronary disease having cardiac surgery
for other reasons, e.g. valve replacement.
Coronary artery bypass surgery
Median sternotomy. A piece of conduit (saphenous vein, left internal tho-
racic artery (LITA), radial artery) is anastomosed to the coronary artery
beyond the lesion and then to the ascending aorta. The LITA is usually
anastomosed to the LAD because this combination remains patent for
decades and the LAD is the most important stenosis to treat. The LITA
is a branch of the left subclavian artery and runs down the inside of the
rib cage 2cm lateral to the sternum. The origin from the subclavian is left
intact; it does not need to be anastomosed to the aorta.
• Coronary artery bypass is mostly performed on-pump (with the use of
a CPB machine) on the still heart.
• Performing on the beating heart off-pump (without CPB) is more
diffi cult, but gives some advantages (see b p. 622).
Complications
Complications (see b p. 620) are more likely with advanced age,
poor LV function, renal failure, COPD. Risk of mortality is scored, e.g.
EUROscore.
• Death, 0–1% in low risk patients.
• Stroke, 1–2% in low risk patients.
• Re-sternotomy for bleeding or tamponade 5%.
• Chest infection, AF, wound infection, renal failure.
Prognosis
• In untreated patients with symptoms severe enough to warrant
coronary angiography, 10% have an acute MI within 1y and 30% have
an acute MI within 5y. Hospital mortality of MI is 7–10%.
• In three-vessel disease, the 5y survival is 50%, lower if LV function is
impaired.
• LMS disease has a 2y survival of 50%.

CHAPTER 17 Cardiothoracic surgery
628
Valvular heart disease
Mitral regurgitation (MR)
• Incidence Commonest valvular lesion. Prevalence 2–6%.
• Aetiology MV prolapse (congenital or rupture of chordae/papillary
muscles), rheumatic disease, endocarditis, connective tissue disorders.
• Clinical features Acute MR presents with signs of CCF. Chronic MR
causes exertional dyspnoea, orthopnoea. Displaced apex beat, soft S1,
pansystolic murmur—loudest at apex, radiating to axilla. AF in 80%.
• Diagnosis CXR shows cardiomegaly. Transthoracic echocardiogram
(TTE) diagnostic.
• Indications for surgery Acute MR, severe chronic MR.
• Prognosis Mortality of untreated severe MR is 5% per year. Operative
mortality is 2–3% for low risk cases.
Mitral stenosis
• Incidence Prevalence <1% in west, commoner in Asia and Africa.
• Aetiology Rheumatic heart disease.
• Clinical features Dyspnoea, bronchitis, haemoptysis, AF, left parasternal
heave, tapping apex beat, loud S1, rumbling mid-diastolic murmur at
apex.
• Diagnosis CXR shows splaying of carina (enlarged LA). Echo diagnostic.
• Indications for surgery MV area <1.0cm
2
(normal valve 3–4cm
2
).
• Prognosis Poor once symptoms of heart failure present.
Aortic stenosis
• Incidence Prevalence 1–2% in age over 65.
• Aetiology Calcifi c degeneration, bicuspid valve, rheumatic disease.
• Clinical features Triad of angina, syncope, dyspnoea. Sudden death.
Slow rising and low volume pulse, heaving apex beat, reversed
splitting S2, ejection systolic murmur loudest in aortic area radiating to
carotids.
• Diagnosis ECG shows LV hypertrophy, TTE diagnostic.
• Indications for surgery Symptomatic aortic stenosis.
• Prognosis Without surgery, 50% of patients with angina are dead in 5y,
50% with syncope are dead in 3y, and 50% with dyspnoea are dead in
2y. UK perioperative mortality is 3–5%.
Aortic regurgitation (AR)
• Incidence Less than 1% prevalence.
• Aetiology Rheumatoid, endocarditis, aortic dissection, Marfan’s and
other connective tissue disorders, calcifi c degeneration, trauma.
• Clinical features Acute AR (endocarditis) presents with signs of left
ventricular failure (LVF). Chronic AR often asymptomatic. Later,
orthopnoea, fatigue, dyspnoea. Signs of wide pulse pressure, collapsing
water hammer pulse, Quinke’s sign (nail bed pulsation), Corrigan’s sign
(visible neck pulsation), De Musset’s sign (head nodding), Durozier’
sign (femoral diastolic murmur), hyperdynamic displaced apex beat,
early diastolic murmur, Austin Flint mid-diastolic murmur due to
regurgitant stream hitting anterior MV cusp.
• Diagnosis CXR shows cardiomegaly. TTE diagnostic.

VALVULAR HEART DISEASE
629
• Indications for surgery Acute AR is a surgical emergency. Chronic AR is
operated on before the ejection fraction <55% or LV dilates >5.5cm.
• Prognosis Acute AR has poor prognosis. Chronic AR has good
outcome until failure occurs (50% 2y mortality). UK operative
mortality is 3–5%.
Options for valve surgery
MV repair (annuloplasty and valvuloplasty)
Results of repair of the aortic valve are unpredictable so it is usually
replaced. The MV is often repaired with good results.
Mechanical valves, e.g. St Jude mechanical, Carbomedics
These are made of ceramic. They can be mono- or bileafl et. Ball-and-cage
like the Starr Edwards is no longer used.
• Advantages. Last forever so patient will not need future surgery.
• Disadvantages. Thromboembolic so patient must be warfarinized; patients
with bleeding diatheses, women of childbearing age, professional sports
players may not be suitable for long-term warfarinization.
Tissue valves (xenografts), e.g. Mosaic, Perimount
These are made of pig valves or cow or horse pericardium, usually sus-
pended on a metal frame covered by a cloth sewing ring.
• Advantages. Patient does not need to be warfarinized.
• Disadvantages. Tissue valves last for 10–15y in the aortic position and
6–10y in the mitral position, depending on the age of the patient;
younger patients (<65–70y) will often need a second operation.
Principles of valve surgery
Median sternotomy incision. MV may be approached via R thoracotomy.
Valve surgery must be carried out with CPB (see b p. 626).
Complications
Complications (see b p. 620) are more likely with advanced age, poor LV
function, renal failure, COPD, pulmonary hypertension, additional CABG.
• Death, 0–3% in low risk patients.
• Stroke, 5–10% (debris from removing calcifi ed valve, cannulating aorta).
• Re-sternotomy for bleeding or tamponade, 5%.
• Chest infection, AF, complete heart block requiring permanent
pacemaker insertion, wound infection, renal failure.
• Prosthetic endocarditis, failure, thrombosis, paravalvular leak.
Key revision points—anatomy of heart valves
• Aortic valve. Tricuspid valve, sitting within bulb of aortic root. Three
dilatations called sinuses of Valsalva. Left coronary sinus gives rise to
LMS, right gives rise to right coronary artery. Third known as non-
coronary sinus. AV node lies between right and non-coronary cusp.
Annulus (where leafl ets attach to aorta) is coronal-shaped.
• MV. Bileafl et valve, lying between LA and LV. Anterior leafl et smaller
than posterior leafl et. Leafl ets held in place by chordae which attach
to two papillary muscles of LV. Annulus is oval-shaped.

CHAPTER 17 Cardiothoracic surgery
630
Cardiothoracic ICU
Commonly used terminology (see Table 17.1)
• Instead of referring to systolic and diastolic blood pressure, arterial
pressure is usually described using single fi gure: the mean arterial
pressure (MAP) which is calculated by adding a third of the difference
between diastolic and systolic pressures to the diastolic pressure, e.g.
the MAP of a patient with a BP 120/60mmHg is 80mmHg.
• The MAP on its own does not adequately describe cardiac function; a
number of other parameters are frequently used.
• Cardiac output. The volume of blood ejected by the heart per minute.
• Stroke volume. The volume of blood ejected by the heart per beat.
• Cardiac output equals heart rate x stroke volume.
• Cardiac index. Is simply the cardiac output adjusted to take into account
the size of the patient and is a more accurate refl ection of cardiac function.
Low cardiac ouput
Much of the initial care after cardiac surgery is aimed at preventing, recog-
nizing, and treating low cardiac output states, as these can lead to organ
failure, contribute to sepsis, and cause death, even in ‘straightforward’
patients. Low cardiac output can be defi ned as a cardiac index <2.2mL/
min or evidence of end-organ hypoperfusion (e.g. lactic acidosis, oliguria,
low mixed venous O
2
saturations).
• Common causes of low cardiac output include:
Bleeding (occasionally other causes of hypovolaemia, e.g. polyuria).•
Tamponade.•
Arrhythmias.•
Acidosis, hypoxia.•
Preoperative cardiomyopathy.•
Ischaemia and stunning (myocardial recovery from surgery).•
• Hypotension can due to low cardiac output, but even a patient with a
high cardiac output could be hypotensive if they were very vasodilated
(most commonly due to SIRS (see b p. 138) or sepsis).
• Sometimes a number of problems may be going on at once and it is
easy to miss important problems so a systematic approach to assessing
post-operative cardiac surgery patients is vital.
General assessment of cardiac surgery patients
• Except in emergencies, usually you have time to evaluate every system.
• Review the history. What was the ventricular function preop? What
other comorbidities? What operation was done—any problems?
• Cardiovascular.
Look at the heart rate and rhythm and check the ECG for evidence •
of ischaemia (ST segment changes), comparing with preop.
Look at the MAP and CVP. A high CVP is always concerning, •
suggesting tamponade, ventricular failure, or respiratory problems.
Look at the cardiac output, lactate, mixed venous, and feel the •
patient’s extremities; a warm patient with good peripheral pulses
cannot have a low cardiac output. What is the SVR?
What inotropes, vasoconstrictors, and antihypertensives are on?•

CARDIOTHORACIC ICU
631
• Respiratory. Is the patient breathing spontaneously or ventilated?
Look at the O
2
saturations and a recent blood gas to check the PaO
2
,
PaCO
2
, and pH. What does the CXR look like?
• Renal. How much urine is the patient making (ideally >1mL/kg/h)?
What is the patient’s fl uid balance (if it is negative or low, the patient
may be hypovolaemic; if it is very high, fl uid overload is a concern).
Check electrolytes; abnormalities can cause arrhythmias.
• Bleeding. Should be less than 100cm
3
/h in the chest tubes, with steady
fall in the rate. Look at wound sites (remember groin + leg). Is the
haematocrit dropping? Check coagulation.
• Assess mental status and focal neurology, analgesia.
Table 17.1 Key haemodynamic formulae and normal values
Cardiac output = SV x HR
Cardiac index = CO/BSA
Stroke volume index = SV/BSA
Mean arterial pressure = DP + (SP – DP)/3
Systemic vascular resistance = ((MAP – CVP)/CO) x 80
Systemic vascular resistance index = SVR/BSA
Pulmonary vascular resistance = ((PAP – PAWP)/CO) x 80
Pulmonary vascular resistance index = PVR/BSA
Normal value
Cardiac output (CO) 4.5–8L/min
Stroke volume (SV) 60–100mL
Body surface area (BSA) 2–2.2m
2
Cardiac index (CI) 2.0–4.0L/min/m
2
Stroke volume index (SVI) 33–47mL/beat/m
2
Mean arterial pressure (MAP) 70–100mmHg
Diastolic pressure (DP) 60–80mmHg
Systolic pressure (SP) 110–150mmHg
Systemic vascular resistance (SVR) 800–1200dyne-s/cm
5
Central venous pressure (CVP) 6–12mmHg
Systemic vascular resistance index (SVRI) 400–600dyne-s/cm
5
/m
2
Pulmonary vascular resistance (PVR) 50–250dyne-s/cm
5
Pulmonary artery pressure (PAP) 20–30mmHg
Pulmonary artery wedge pressure (PAWP) 8–14mmHg
Pulmonary vascular resistance index (PVRI) 20–125dyne-s/cm
5
/m
2

CHAPTER 17 Cardiothoracic surgery
632
Lung cancer
Key facts
• Commonest cause of death from cancer in the UK.
• i Incidence in females (25% of cancer-related deaths in women).
Risk factors
• Cigarette smoking. Strongly positive association with cigarette smoking
(polycyclic aromatic hydrocarbons plus nicotine-related carcinogens).
• Radon exposure.
• Occupational factors. Asbestos, polycyclic aromatic hydrocarbons,
arsenic, nickel, silica, coal tar, aluminium production, coal gassifi cation,
exposure to paints, chromium compounds, bischloromethal ether.
• Genetic predisposition and male sex.
Pathology
Bronchial carcinoma is classifi ed as non-small cell or small cell.
Non-small cell lung cancer
Squamous cell carcinoma
• Twenty to thirty per cent of all lung cancers.
• Most common histological type in Europe.
• Often arising in large airways as an endobronchial mass (two-thirds).
• Slow-growing, late to metastasize.
Adenocarcinomas
• Thirty to fi fty per cent of lung cancers. Incidence increasing.
• Usually peripheral tumours (approximately three-quarters).
• Moderate growth with early metastasis.
Large cell undifferentiated carcinoma:
• Fifteen per cent of all lung cancers.
• Peripheral location more common than central.
• Two subtypes. Clear cell carcinoma and giant cell carcinoma. Giant cell
tumours are uncommon, <1% of lung cancers. Very poor prognosis.
Histological subtypes
• Bronchioloalveolar tumours. Highly differentiated adenocarcinoma; 2.5%
all lung cancers.
• Adenosquamous carcinomas.
Small cell lung cancer
Neuroendocrine tumours
Approximately 20% of lung cancers. Aggressive tumours, not usually ame-
nable to surgical resection. Three subtypes—pure small cell (90%), mixed
small and large cell, combined small cell with areas of squamous or glan-
dular differentiation.
Clinical features
• Proximal tumours tend to produce symptoms of major airway
obstruction and irritation (haemoptysis, dyspnoea, cough, wheezing,

LUNG CANCER
633
stridor, hoarseness, Horner’s syndrome, SVC obstruction, post-
obstructive pneumonia, pleural effusion, Pancoast’s syndrome).
• Peripheral tumours are often asymptomatic or present with signs and
symptoms of pleural or chest wall invasion or pleural effusion (pleuritic
chest wall pain, progressive dyspnoea).
• May present with symptoms and signs of metastatic spread.
Neurological—headache, blurred vision, nausea, diplopia, decreased
consciousness, ataxia; bony pain and pathological fracture; liver and
abdominal pain, anorexia, jaundice, ascites, liver failure, hepatomegaly;
adrenal glands—symptoms of Addison’s disease.
• Paraneoplastic syndromes.
History and examination
• Ask about: history of tobacco smoking (pack years), asbestos exposure,
employment history, weight loss of greater than 5% body weight,
recent onset of joint pains, change in voice, chest wall pain, back pain.
• Look for: lymphadenopathy, evidence of signifi cant weight loss, pleural
effusion, localized chest wall pain, clubbing, cutaneous lesions.
Principles of management
Non-small cell lung cancer is relatively resistant to chemotherapy. Curative
resection allows best chance of long-term survival. Surgery is normally
offered to all patients with stage I and stage II disease, along with specifi c
patients with stage III disease (after chemoradiotherapy). Surgical princi-
ples of resection are:
• No spillage of cells from primary tumour during resection.
• Entire tumour must be resected by lobectomy or pneumonectomy
along with intrapulmonary lymph nodes; lesser resections proven to
have worse outcome, only considered in high risk patients.
• All accessible mediastinal lymph nodes should be excised or biopsied
to allow complete staging and plan for any adjuvant therapy.
• Frozen section analysis of resection margins to confi rm appropriate
surgical resection and complete excision of primary tumour.
Survival
• Stage I, 5y survival 75%; stage II, 5y survival 40%; stage III, 5y survival
<30%.
• Post-operative mortality rate. Lobectomy, 2%; pneumonectomy, 6%.
Chemotherapy
To date, no proven benefi t from adjuvant therapy in patients with surgi-
cally resectable and potentially curative disease.
Radiotherapy
• Radical. Used in patients unfi t or unwilling to undergo surgery. Also
used in those with bulky stage IV disease. Survival benefi t in non-
surgical patients when combined with concomitant chemotherapy.
• Adjuvant. Also used as an adjunct to surgery or as palliation.

CHAPTER 17 Cardiothoracic surgery
634
Pleural effusion
Key facts
Abnormal amount of fl uid within the pleural space. Pleural effusions may
be divided into transudate and exudates, according to Light’s criteria (see
Box 17.1, but the divide is not always clear.
Box 17.1 Light’s criteria
An exudates is characterized by:
• Pleural fl uid:serum protein >0.5.
• Pleural fl uid:serum LDH >0.6.
• Pleural fl uid LDH >200IU.
Causes of transudates
• Cirrhosis of the liver.
• Nephrotic syndrome.
• Glomerulonephritis.
• CCF.
• Myxoedema.
• Sarcoidosis.
• Multiple PE.
Causes of exudates
• Neoplasms. Mesothelioma, metastatic disease, primary lung cancer.
• PE.
• Chylothorax.
• Haemothorax.
• Infectious diseases. Viral and bacterial infections, fungal and parasitic
infections, TB.
• Gastrointestinal disease. Pancreatitis, subphrenic abscess, intrahepatic
abscess, perforated oesophagus.
• Collagen vascular disease. Systemic lupus erythematosus, Wegener’s
granulomatosis, Sjogren’s syndrome.
• Drug-induced pleural disease.
• Mediterranean fever.
• Rheumatoid disease, sarcoidosis, yellow nail syndrome.
• Asbestos exposure, electrical burns, radiation therapy.
• Trapped lung, post-pericardiectomy.
• Uraemia, urinary tract obstruction.
• Post-myocardial syndrome, Meigs’ syndrome.
Clinical presentation
• Small effusions are often asymptomatic. Larger effusions cause cough,
chest pain, dyspnoea.
• Decreased ipsilateral chest expansion, dullness to percussion,
decreased breath sounds over the effusion on auscultation,
crepitations may be heard.

PLEURAL EFFUSION
635
Investigations
• Chest radiograph. Small effusions are demonstrated by blunting of the
costodiaphragmatic angles; larger effusions produce a fl uid level with a
meniscus.
• Ultrasound scan. Useful for loculated effusions, helps to localize optimal
site for chest drainage.
• CT. Useful when looking at underlying lung and pleural lesions.
• Pleural aspiration cytology. May obtain diagnostic information, helpful
when planning treatment.
Management
If possible, treat the underlying cause. Simple pleural effusions due to fl uid
overload may resolve with diuresis.
• Tube thoracostomy (see b p. 200).
• Chemical pleurodesis (tetracycline, blood, talc).
• Surgical abrasion pleurodesis.
• Surgical pleurectomy (open or thoracoscopic).
• Pleuroperitoneal shunt.
Complications
• Infection and empyema.
• Treatment failure with recurrence of pleural effusion.
• Damage to underlying lung parenchyma, leading to prolonged air leak
and bronchoalveolar air leak.
Empyema
This is an infected pleural fl uid collection, commonly after pneumonia.
• Stage I. Acute exudative phase.
Typically occurs 2–5 days after a pneumonia.•
Accumulation of fl uid with low cellular content and viscosity.•
Characterized by low WCC, LDH, and glucose, and a normal pH.•
Can be successfully treated with antibiotics only.•
• Stage II. Fibrinopurulent phase.
Typically occurs 5–14 days after a pneumonia.•
Turbid or purulent fl uid with heavy fi brin deposits.•
Appearance of simple loculations and septations.•
May have bacterial invasions and high numbers of PMNs and •
lymphocytes.
Characterized by low pH and glucose and increased LDH.•
Antibiotics and chest tube drainage is required, may need video-•
assisted thoracoscopic surgery (VATS) decortication.
• Stage III. Chronic organizing phase.
Lung trapping by collagen visceral and parietal pleural peel with •
ingrowth of fi broblast and capillaries.
Antibiotics and aggressive decortications, generally by thoracotomy.•
Bacteriology.•
CHAPTER 17 Cardiothoracic surgery
636
Pneumothorax
Key facts
The presence of air in the pleural space with secondary lung collapse.
Aetiology
• Primary spontaneous pneumothorax.
• Secondary spontaneous pneumothorax.
• Post-traumatic and iatrogenic.
Primary spontaneous pneumothorax
Commonly seen in young tall male smokers. More common on the right
side. Less than 10% of cases are bilateral. Usually caused by rupture of
small subpleural blebs (collections of air <2cm). Usually found at the apex
of the upper lobe or the apical segment of the lower lobe. The rest of the
lung parenchyma is normal. May also be caused by rupture of bullae (large
air-fi lled spaces).
Presentation
• Dyspnoea, chest pain, cough, tachypnoea.
• Ipsilateral decreased chest wall movement, hyperresonant hemithorax
to percussion, absent breath sounds on auscultation, pleural rub,
tachycardia.
Investigations
• PA chest radiograph usually diagnostic.
• CT scan gives an accurate estimate of size of pneumothorax and is
useful for assessment of remaining lung parenchyma and contralateral
lung.
Complications
• Tension pneumothorax.
• Pneumomediastinum.
• Haemopneumothorax.
• Recurrent pneumothorax.
Conservative management
• Observation (small, <20% pneumothorax).
• Needle aspiration.
• Tube thoracostomy 9 chemical pleurodesis.
Surgery
• Surgery is indicated in the following cases.
• First episode. Prolonged air leak, tension pneumothorax, haemothorax,
bilateral pneumothoraces, residual collapse of lung despite non-surgical
treatment, 100% pneumothorax, occupational hazard, pneumothorax
secondary to giant bulla, previous contralateral pneumonectomy.
• Recurrence of pneumothorax.
The aim is to resect the blebs or bullae and obliterate the pleural space
with adhesions, either using chemical or abrasion pleurodesis or parietal

PNEUMOTHORAX
637
pleurectomy (apical or full). It may be performed through a mini-thoracot-
omy, axillary incision, or thoracoscopically
Recurrence rate
• Less than 2% following surgical pleurectomy via mini-thoracotomy.
• Five per cent following thoracoscopic procedures.
• Five to ten per cent following chemical pleurodesis.
Secondary spontaneous pneumothorax
Causes
• Cystic fi brosis, chronic obstructive airways disease (COAD) and other
bullous disease, asthma.
• Interstitial lung disease.
• Infections, including AIDS, mycobacterial, Pneumocystis carinii, bacterial,
parasitic, mycotic.
• Malignancy. Bronchogenic carcinoma, metastatic lung cancer (sarcoma
and lymphoma).
• Collagen diseases, catamenial, Ehlers–Danlos syndrome, histiocytosis
X, scleroderma, lymphangioleiomyomatosis, Marfan’s syndrome.
• Rupture of the oesophagus.
Cystic fi brosis Pneumothorax found in 10% of patients. Remember these
patients are possible candidates for future lung transplantation when con-
sidering management options. Full parietal pleurectomy is a contraindica-
tion to lung transplantation.
COAD The most common cause of secondary pneumothorax. Age usually
>50y. Patients often have very little pulmonary reserve. They may not tol-
erate surgical management and single lung ventilation. Treatment options
are therefore tube thoracoscopy and chemical pleurodesis or long-term
tube thoracoscopy.
Infection Cavitating pulmonary lesions rupture into pleural space.
AIDS Usually secondary to Pneumocystis carinii and pneumonia. May be
presenting feature of AIDS. Most effective treatment is surgical.
Catamenial Age 20–30y. Incidence 3–6% of women. Occurs 2–3 days fol-
lowing onset of menstruation. Right side more commonly affected. Usually
small, presenting with dyspnoea and chest pain. Pathogenesis unclear.
Primary spontaneous pneumomediastinum
Uncommon. Males affected more frequently than females. Occurs follow-
ing exertion or increased intra-abdominal pressure. Commonly associated
with cocaine, marijuana, and crack cocaine usage. It is caused by rupture
of alveolar sacs with air tracking along the peribronchial and perivascular
spaces into the neck.
Presentation
• Sudden onset of chest pain, dyspnoea, dysphagia, cough.
• Subcutaneous emphysema over neck and chest wall, Hamman’s sign.
• Chest radiograph confi rms diagnosis.
Management Non-operative, treat expectantly. Emergency surgical decom-
pression very rare.

CHAPTER 17 Cardiothoracic surgery
638
Mediastinal disease
Pericardial effusion and cardiac tamponade
Key facts
• Pericardial effusion is abnormal fl uid in the pericardial space; there is
normally about 20mL of plasma ultrafi ltrate.
Pericardial effusion may be acute or chronic.•
Acute accumulation of fl uid can cause cardiac tamponade which is a •
surgical emergency (see Box 17.2).
Effusions commonly results from pericarditis, CCF, metastatic •
spread to pericardium commonly from lung or breast malignancy,
lymphoma or leukaemia, autoimmune disorders, chronic hepatic
and renal failure, infections—specifi cally HIV.
Clinical features
• Very dependent on the time course. Acute accumulation of a small
amount of fl uid can cause life-threatening cardiac tamponade, whereas
slow accumulations of large volumes of fl uid may be well tolerated.
• Chronic pericardial effusion may present with decreased exercise
tolerance, atypical chest pain, orthopnoea, and associated signs of
CCF, as well as features of cardiac tamponade.
Box 17.2 Cardiac tamponade
Suspect cardiac tamponade if the patient has a history of chest trauma.
• d BP, i JVP, (Beck’s triad is d BP, i JVP + muffl ed heart sounds).
• Pulsus paradoxus (exaggeration of the normal d BP with
inspiration).
• Progressive tachycardia and dysrythmias, including SVT, VF, and
EMD.
• d Urine output.
• Excessive widening of the mediastinum on CXR.
• Echo may show clot in pericardium and collapse of RV in diastole.
• Equilibration of cardiac fi lling pressures (at cardiac catheterization).
Management
• Emergency pericardiocentesis (b p. 204).
• Emergency thoracotomy or sternotomy.
• Aggressive fl uid resuscitation is a temporizing measure.
Management
• Medical management includes diuretics and pericardiocentesis.
• Creating a hole in the pericardium or pericardial window so that fl uid
can drain directly into the pleura via:
Thoracotomy or subxiphoid approach.•
Left VATS approach.•

MEDIASTINAL DISEASE
639
Thymoma
Key facts
• The thymus is a bilobar structure located in the anterior mediastinum
which contains lymphoid tissue. It is the location for maturation of
T-cells in early life.
• Thymoma may be benign or malignant.
• Thymectomy is the defi nitive treatment for myasthenia gravis.
Clinical features
• Thymoma is usually asymptomatic in adults, whereas children often
present with thoracic outlet obstruction or upper airway compromise.
• Clinical features of myasthenia gravis are described on b p. 63.
• Thymoma appears as a smooth mass in the upper half of the CXR.
• CT shows enlarged thymus as well as lymph node involvement.
Treatment
• Thymectomy via a median sternotomy.
Key revision points—mediastinal anatomy
• Mediastinum is the space between the pleural sacs, below the
thoracic inlet and above the diaphragm.
• Superior mediastinum.
From thoracic inlet to the line from sternal angle to T4–5 space.•
Contains great vessels, trachea, oesophagus, phrenic nerves, •
vagus nerves, thoracic duct.
• Anterior mediastinum.
Anterior to pericardium.•
Contains sternopericardial ligaments, thymus, lymph nodes.•
• Middle mediastinum.
Contains pericardial cavity, heart, great vessels, phrenic nerves.•
• Posterior mediastinum.
Posterior to pericardium.•
Contains oesphagus, descending aorta, azygous veins, thoracic •
duct, lymph nodes.
This page intentionally left blank

641
Peripheral vascular
disease
Acute limb ischaemia 642
Chronic upper limb ischaemia 644
Chronic lower limb ischaemia 647
Intermittent claudication 648
Critical limb ischaemia 650
Aneurysms 652
Ruptured abdominal aortic aneurysm 654
Vascular developmental abnormalities 656
Carotid disease 658
The diabetic foot 660
Amputations 662
Vasospastic disorders 664
Varicose veins 666
Deep venous thrombosis 668
Thrombolysis 670
Complications in vascular surgery 672
Chapter 18

CHAPTER 18 Peripheral vascular disease
642
Acute limb ischaemia
Defi nition Any sudden decrease in limb perfusion that causes a \
potential threat to viability.
Prevalence One in 6000 of the population.
Causes and features
Acute thrombosis in a vessel with pre-existing atherosclerosis
(60% of cases)
• Predisposing factors are: dehydration, hypotension, malignancy,
polycythaemia, or inherited prothrombotic states.
• Features suggestive of thrombosis are:
Previous history of intermittent claudication.•
No obvious source of emboli (see below).•
Reduced or absent pulses in the contralateral limb.•
Emboli (30% of cases)
• Eighty per cent have a cardiac cause (AF, MI, ventricular aneurysm).
• Arterial aneurysms account for 10% of distal emboli and may be from
the aorto-iliac, femoral, popliteal, or subclavian arteries.
• Rarely, acute thrombosis in pre-existing atherosclerosis (see above)
will embolize.
• Commonest sites of impaction are the brachial, common femoral,
popliteal, and aortic bifurcation (‘saddle embolus’).
• Features suggestive of embolism are:
No previous history of claudication.•
Presence of AF or recent MI.•
Rare causes
• Aortic dissection, trauma, iatrogenic injury, peripheral aneurysm
(particularly popliteal), and intra-arterial drug use.
Symptoms and signs (any cause)
Six Ps:
• Pain, pallor, pulselessness, paraesthesia, paralysis, perishingly cold.
Complications
• Death (20%).
• Limb loss (40%). Severe ischaemia leads to irreversible tissue damage
within 6h.
Emergency management
Remember, patients will usually have coexisting coronary, cerebral, or
renal disease.
Resuscitation
• Give 100% O
2
.
• Get IV access and consider crystalloid fl uid up to 1000mL if
dehydrated.
• Take blood for FBC, U&E, troponin, clotting, glucose, group and save.
• Request CXR and ECG (look for dysrhythmias).

ACUTE LIMB ISCHAEMIA
643
• Give opiate analgesia (5–10mg morphine IM).
• Call for senior help.
Establish degree of urgency
• Limb viability assessment. Involve senior help early.
Irreversible• . Fixed mottling of skin, petechial haemorrhages in skin,
woody hard muscles.
Immediate treatment needed• . Muscles tender to palpation/swollen,
loss of power, loss of sensation.
Prompt treatment after investigation• . Pulseless, pale, cold, reduced
capillary refi ll.
Treatment of all patients
Give heparin (5000IU unfractionated heparin IV bolus and start an infu-
sion of 1000IU/h) if there are no contraindications (e.g. aortic dissection,
multiple trauma, head injury).
• Recheck APTT in 4–6h.
• Aim for a target time of 2–2.5 x the normal range.
Defi nitive management
Depends on the severity of ischaemia (as above) and there are three
broad categories.
• Irreversible (non-salvageable limb). Amputation is inevitable and urgent
(but not emergency).
• Immediate treatment needed (to prevent the systemic complications
of muscle necrosis—hyperkalaemia, acidosis, acute renal failure, and
cardiac arrest). Consider amputation if ischaemic changes advanced
and life-threatening. Surgery to revascularize limb and perform
fasciotomies (to prevent or treat compartment syndrome).
• Prompt treatment after investigation. Continue heparinization.
Angiogram (stop heparin 4h before) or duplex/CT angio to determine
cause/location of disease. Thrombolysis, angioplasty, arterial surgery,
or combination. Limb may remain viable and functional after period of
heparinization alone.
Principles of embolectomy (see b p. 746)

CHAPTER 18 Peripheral vascular disease
644
Chronic upper limb ischaemia
Key facts
Upper limb ischaemia occurs less frequently than in the lower limb.
Causes
• Previous trauma or axillary irradiation, leading to arterial stenosis.
• Atherosclerosis. As in the lower limb.
• Buerger’s disease. Affects small vessels of the hands and feet, principally
in smokers, associated with Raynaud’s phenomenon, mostly young
men, but women may be affected, presents with digital gangrene/
ischaemia and may present with acute limb ischaemia in young people.
• Subclavian steal syndrome. Stenosis of subclavian artery proximal to
vertebral artery origin; arm claudication causes reversed fl ow in the
vertebral artery/diminished hindbrain perfusion (dizziness/syncope).
• Takayasu’s arteritis. Uncommon in Europe; major arch/upper limb
vessels affected.
• Thoracic outlet syndrome (see Fig. 18.1).
Term used to cover a spectrum of symptoms resulting from the •
compression of the neurovascular bundle (NVB) as it leaves the
chest to enter the upper limb, in an area enclosed by the fi rst rib,
clavicle, and the scalenus anterior muscle.
Presents as a variable combination of neural, arterial, and venous •
symptoms exarcebated by elevation of the limb, with shoulder,
arm, or head pain, and parasthesiae, weakness, or arm claudication.
Ninety-fi ve per cent are neurogenic and 5% are arterial or venous
manifestations (arm engorgement, swelling with subclavian vein
stenosis or thrombosis—Paget–Schroetter syndrome).
Clinical features
• Weakness, cramp or exercise-related pain, and digital ischaemia/
gangrene.
• Examine bilateral upper limb pulses, BP in both arms (elevated/at
sides), wrist Doppler pressures.
• Roos test. Arm abducted to 90*, hands up with elbows braced
backward, chin elevated, hands serially clenched/opened for 1–2min,
positive if pain or weakness in hand or forearm.
• Adson’s test. Pulse diminishes or absent on elevation/abduction of arm
with head turned to contralateral side. Reliability improved by using in
conjunction with arterial duplex.
• Allen’s test. Assesses integrity of the palmar arch and dominant vessel
(radial or ulnar).
• Tinel’s test. For carpal tunnel syndrome.
Diagnosis and investigation
• Cervical spine and thoracic outlet X-rays, wrist Doppler pressures.
• CT/MRI to look for fi brous bands/ribs and stenoses/occlusions.
• Arterial duplex or angiography to diagnose proximal arterial lesions.
• Duplex or venography for subclavian vein stenosis or occlusion.

CHRONIC UPPER LIMB ISCHAEMIA
645
Treatment
Thoracic outlet syndrome
• Mild neurogenic problem. Simple analgesia, physiotherapy, and advice
on risk factors.
• Surgery (supraclavicular or axillary approach) has good results for
those with arterial or venous symptoms/complications.
• Excision of the fi rst rib/band will improve symptoms in over 90%.
• Careful evaluation is needed prior to surgery for pure neurological
symptoms, e.g. nerve conduction studies.
Cervical sympathectomy in upper limb disease
Indications
• Palmar hyperhidrosis (less effective and infrequently used for axillary).
• Buerger’s disease/small vessel disease with digital ischaemia.
Approach
• Aim is to de-innervate the second and third thoracic ganglia.
• Approach is almost universally thoracoscopic and open approaches
have been largely abandoned.
Complications
• Horner’s syndrome.
• Pneumothorax.
• Haemorrhage.
• Compensatory truncal hyperhidrosis.
• Frey’s syndrome (gustatory sweating).
Axillary hyperhidrosis Treatment of choice is now subcutaneous botulinum
toxin A injections to the axillary sweat glands, repeated as necessary, often
6-monthly.
Key revision points—anatomy of thoracic outlet
Several structures can compress the neurovascular structures.
• Cervical rib. Articulates with C7.
• Scaleneus anterior. Aberrant anatomy or scarring/swelling from
trauma.
• Costoclavicular ligament.
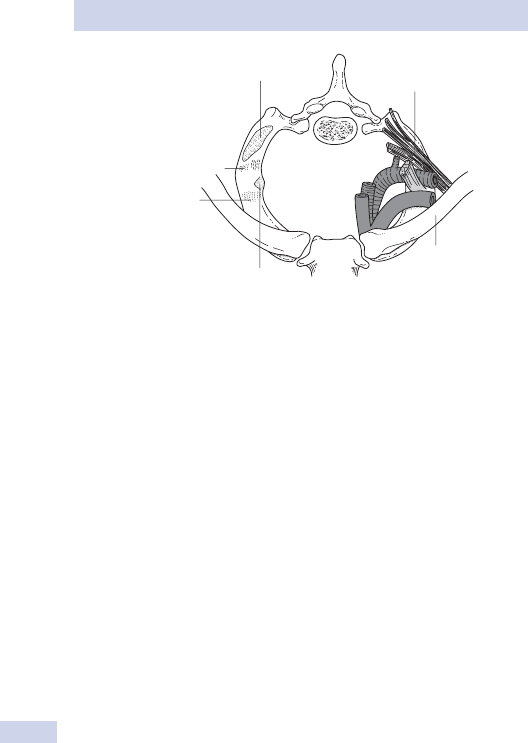
CHAPTER 18 Peripheral vascular disease
646
Scalenus medius
Groove for plexus
and subclavian artery
Groove for
subclavian vein
Tubercle for
scalenus anterior
Clavicle
Brachial plexus
Fig. 18.1 Structures involved in thoracic outlet syndrome.
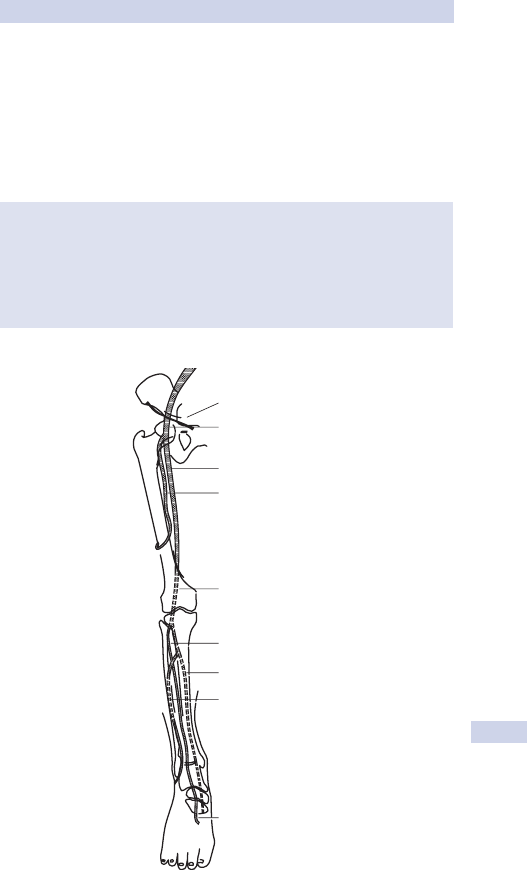
CHRONIC LOWER LIMB ISCHAEMIA
647
Chronic lower limb ischaemia
• Atherosclerosis is a generalized disease and has a predilection for the
coronary, cerebral, and peripheral circulations (see b p. 152).
• In the lower limb, it may affect the aorto-iliac, femoral or popliteal, and
calf vessel levels singly or in combinations (see Fig. 18.2).
• Single-level disease usually results in intermittent claudication (IC) and
two-level disease in critical limb ischaemia (CLI).
The Fontaine classifi cation of lower limb ischaemia
• I. Asymptomatic.
• II. Intermittent claudication.
• III. Rest pain.
• IV. Ulcers/gangrene.
Grades III and IV = critical limb ischaemia.
inguinal ligament
common femoral
superficial femoral
profunda femoris
popliteal
anterior tibial
posterior tibial
peroneal
dorsalis pedis
Fig. 18.2 The arterial supply to the leg. Reproduced with permission from
Longmore, M. et al. (2007). Oxford Handbook of Clinical Medicine, 7th edn.
Oxford University Press, Oxford.

CHAPTER 18 Peripheral vascular disease
648
Intermittent claudication
Key facts
• Affects 4% of people over 55y, mostly men.
• One-third of patients improve, one-third remain stable, and one-third
deteriorate.
• Four per cent require an intervention and 2% result in amputation.
• Risk factors and association. See Table 18.1.
Clinical features
• Muscular pain on exercise of the affected limb, worsened with
increasing level of exercise, relieved by rest; most commonly, calf.
• Differential diagnosis.
Spinal stenosis. Neurogenic pain caused by drop in distal cauda •
equina blood fl ow due to exercise, leading to neurogenic pain.
Osteoarthritis, especially hip joint.•
Nerve root entrapment, e.g. sciatica.•
Popliteal artery entrapment (rare). Due to compression of popliteal •
artery over medial head of gastrocnemius during exercise. Distal
pulses reduced/absent on plantar fl exion alone. Treated by surgical
release after MRI defi nes anatomy.
Diagnosis and investigation
• Diagnosis is mostly clinical and not based on imaging.
• Post-exercise fall in ankle–brachial pressure index (ABPI) can be diagnostic.
• Measure BP, serum glucose, cholesterol, FBC.
• Imaging only if treatment by intervention planned. Angiography
(usually digital subtraction (DSA), may be CT angiogram, or magnetic
resonance angiogram (MRA).
• Abdominal ultrasound if aneurysm disease suspected.
Treatment
Risk factor modifi cation
• Forty per cent of peripheral vascular disease (PVD) patients have
signifi cant coronary or cerebral arterial disease; the mainstay of
treatment is aggressive risk factor modifi cation. Stop smoking, oral
statin treatment, increased exercise, control of BP and serum glucose,
antiplatelet therapy.
Table 18.1 Intermittent claudication—risk factors and associations
Risk factors Associations
Hypertension Obesity
Hyperipidaemia Diet
Diabetes mellitus Sedentary lifestyle
Tobacco smoking Gender
Positive family history Occupation

INTERMITTENT CLAUDICATION
649
Endovascular treatment
• Angioplasty ± stent. Excellent results in the aorto-iliac segment (>90%
success) and good results in the superfi cial femoral segment (90%
success and 60–80% patency at 2y).
• Usually performed under LA percutaneously as a day case.
• Rarely performed for claudication in the popliteal and tibial segments
due to high risk of occlusion.
• Most useful in short stenoses/occlusions.
Surgery
• Used for short distance claudication, severe lifestyle limitation, and
failure/unsuitability of endovascular treatment in the aorto-iliac
segments.
• Procedures available are:
Aortobifemoral graft• . 5y patency of >90%, but carries 5–8% mortality
and a risk of impotence. Used for younger patients.
Femorofemoral cross-over bypass graft• . Used for isolated unilateral
iliac disease; 90% 1y patency.
Common femoral endarterectomy• . Used for isolated common
femoral disease; good results and a low complication rate.
Femoro-above knee popliteal bypass• . 80% 2y patency with vein or
prosthetic graft.
Femoro-below knee popliteal bypass• . 70% 2y patency with vein graft.
Femoro-distal (below knee) bypass• . 5y patency <35%; usually
reserved only for critical ischaemia.
• Most commonly used for long stenoses/occlusions and combined with
endovascular treatment.

CHAPTER 18 Peripheral vascular disease
650
Critical limb ischaemia
Key facts
• Often progresses to limb loss or progressive tissue loss if it remains
untreated.
• High risk condition, often occuring in high risk patients with multiple
comorbidities.
Clinical features
• European consensus statement defi nes critical ischaemia if there is:
Rest pain for >2 weeks not relieved by simple analgesia; or•
Doppler ankle pressure <50mmHg (toe pressures <30mmHg if •
diabetic).
Tissue necrosis (i.e the presence of gangrene or ulceration).•
• Rest pain typically worsens at night and during elevation of the limb
and is relieved by hanging the limb dependent.
• Arterial ulceration cannot reliably be distinguished from other causes.
Diagnosis and investigation
Diagnosis is clinical. Investigation should aim to:
• Identify and treat risk factors. Treat BP, stop smoking, optimize
diabetes control, reduce cholesterol (statins by choice), use
antiplatelet agent (aspirin/clopidogrel by choice).
• Identify location and severity of all arterial stenoses involved. May
include:
Colour duplex Doppler ultrasound. Zero risk, non-interventional, •
best for proximal vessels (iliac, common femoral).
Angiography (usually DSA). Interventional, carries risk of arterial •
injury and renal toxicity of contrast, good for popliteal and distal
vessel assessment.
MRA. Low risk.•
CT angiography. Low risk (radiation and contrast).•
Treatment
• All efforts should be made to revascularize if possible (providing the
general condition of the patient allows it).
• General principle is to deal with most proximal disease fi rst and
progress to distal disease only if critical ischaemia still present.
Medical care
• Nursing care.
• Analgesia. Opiates (e.g. Oramorph
®
, MST Continus
®
).
Endovascular treatments
Angioplasty 9 stent proximal disease (aorto-iliac, common femoral, super-
fi cial femoral). Less successful in popliteal disease and distal disease.
Surgery
• Femoro-distal (e.g. to popliteal, tibioperoneal trunk, or anterior or
posterior tibial arteries).

CRITICAL LIMB ISCHAEMIA
651
• Aorto-iliac bypass (‘anatomical’) or axillo-femoral or femoro-femoral
(‘extra-anatomical’) bypass.
• Amputation.
Usually below knee or above knee in smoking-related •
atherosclerosis.
Distal amputations (toe, forefoot, ankle) may be appropriate in •
diabetic disease.
• There is little evidence of benefi t from treatment with prostacyclin or
sympathectomy (surgical or chemical).

CHAPTER 18 Peripheral vascular disease
652
Aneurysms
Key facts
• An aneurysm is an abnormal localized dilatation of a blood vessel.
• It may be associated with structural abnormalities of collagen and
elastin in the vessel wall.
• Prevalence of abdominal aortic aneurysm, 4% of men aged 65,
increasing with age.
• ♂:♀, 9:1.
• Associated with hypertension, tobacco smoking, and family history (all
associated with atherosclerosis).
Pathological features
Types
• True aneurysms. Contain all three layers of artery wall. May be fusiform
(symmetrical dilatation) or saccular. Underlying cause is usually
atherosclerosis-related, but may be associated with infective causes
(‘mycotic aneurysm’), Marfan’s and Ehlers–Danlos syndromes (collagen
and elastin abnormalities).
• False aneurysms. Do not contain all three layers of vessel wall and
often only lined by surrounding connective tissue or adventitia. Usually
secondary to penetrating trauma, including iatrogenic injury (e.g.
femoral cannulation, surgery).
Sites
Thoracic, abdominal, and peripheral (iliac, femoral, popliteal, visceral,
carotid or subclavian), cerebral ‘berry’ aneurysms.
Clinical features
Thoraco-abdominal
• Crawford classifi cation types I–IV (dependent on involvement of
descending thoracic/abdominal aorta); managed in specialist centres.
• Often asymptomatic.
• Different clinical features to thoracic aortic dissection (acute chest pain
(angina/MI), back pain, acute aortic regurgitation, or cardiac failure).
• Diagnosed by widened mediastinum on CXR or on CT/MRI.
• Rupture has high mortality and rare without prior symptoms.
• Elective surgery has up to 20% mortality and risk of paraplegia; 10%
require dialysis after surgery.
• Endovascular stenting is a potential future treatment of choice due to
high surgical risks.
Abdominal aortic
• Ninety-fi ve per cent start below the origin of the renal arteries
(‘infrarenal’).
• Fifteen per cent extend down to involve the origins of the common
iliac arteries.
• Associated with other peripheral aneurysm (e.g. popliteal).
• Five to ten per cent are ‘infl ammatory’ (have gross connective tissue
changes around the aortic wall in the retroperitoneum).

ANEURYSMS
653
• Most are asymptomatic; 40% are detected incidentally (clinical
examination, ultrasound, AXR, IVU).
• A national ultrasound screening programme exists.
• Six-monthly scans for surveillance if size 4–5.4cm (1% per year risk of
rupture).
• Mycotic aneurysms are rare, but have a high rupture rate.
• Risk of rupture and mortality increases with increasing aneurysm
diameter.
• Surgical intervention is indicated for:
AP diameter >5.5cm in fi t individuals.•
Rapid increase in diameter on serial surveillance scans, e.g. >0.5cm •
in 6 months.
Peripheral aneurysms
• Iliac. Two per cent of patients >70y. Mostly common iliac and
asymptomatic. Rarely palpable and rupture may be missed as acute
abdomen or renal colic.
• Femoral. Mostly asymptomatic pulsatile groin swelling or pain. May
present with lower limb ischaemia.
• Popliteal. Many asymptomatic and over half are bilateral. May present
with acute limb ischaemia. Aneurysm thrombosis is associated
with high risk of limb loss. Prophylactic bypass probably best for
symptomatic, embolizing aneurysms.
• Carotid. Rare and may be bilateral. May present with neurological or
pressure symptoms. May present simply as a pulsatile neck swelling.
Rarely presents with rupture. Diagnosis with duplex scan.
• Visceral. Account for 1% of all aneurysms. Generally small and
asymptomatic until rupture. Splenic artery most common followed by
hepatic and renal arteries.
Treatment of abdominal aortic aneurysm (AAA)
Aim is to prevent death as 80% of patients with ruptured AAA will die.
Elective surgery
• Open repair by inlay synthetic graft. May be ‘straight’ if aneurysm
confi ned to aorta or ‘bifurcated/trouser’ if there are common iliac
aneurysms as well; 3–7% operative mortality.
• Laparoscopic repair may offer earlier return to normal function and
reduced hospital stay.
Endovascular repairs
• Endovascular aneurysm repair (EVAR) with a stent graft. Percutaneous
insertion of covered stent to exclude the aneurysmal segment from
arterial pressure.
• Advantages. Percutaneous technique, reduced early mortality.
• Disadvantages. High early re-intervention rate, requires lifelong
surveillance, no long-term survival benefi ts over open repair shown to
date.

CHAPTER 18 Peripheral vascular disease
654
Ruptured abdominal aortic aneurysm
Causes and features
• Associated with hypertension (especially uncontrolled), smoking, family
history, and atherosclerosis.
• Rare in patients aged <55.
• Risk of rupture relates to maximum AP diameter.
Less than 0.5% per year, <4.0cm diameter.•
One per cent per year, 4–5.5cm.•
Over 3% per year, >5.5cm.•
• Patients with a ‘contained leak’ with initial haemodynamic stability
proceed rapidly to rupture.
• Less than 50% of patients with a ruptured AAA reach hospital alive and
the overall mortality of the condition may be as high as 75–95%.
• Outcome is best in the hands of an experienced vascular team
(vascular surgeon, vascular anaesthetist, theatre nursing team,
two assistants, and ITU) and a rapid transfer from the emergency
department to theatre.
Symptoms
• Severe/sudden onset epigastric and/or back/loin pain.
• History of sudden ‘collapse’, often with transient hypotension.
• May have history of AAA under surveillance.
Signs
• Cardinal signs are unexplained rapid onset hypotension, pain, and
sweating.
• A pulsatile abdominal mass is not always easy to feel (due to pain and
abdominal wall rigidity).
Emergency management
Resuscitation
• If the diagnosis is seriously considered, call for senior surgical
assistance immediately. Transfer to theatre may be required even as
resuscitation continues.
• ‘Permissive hypotension’. Do not ‘chase’ a ‘normal’ systolic BP to
reduce the risk of worsening the rupture.
• If the patient is critically hypotensive, consider calling a peri-arrest
cardiac emergency.
• IV access via two large bore cannulae, catheterize, cross-match blood
(10U), order FFP and platelets.
• High fl ow O
2
via non-rebreathing mask.
• Give modest doses of analgesia (morphine 5–10mg).
• Alert anaesthetist, theatres, ITU.
• Witnessed verbal consent for surgery may be the only practical way
and is acceptable here.
Establish a diagnosis
• If patient stable and diagnosis uncertain, request contrast CT.
• If going to CT, ensure blood is sent for cross-match and IV access has
been established before going.

RUPTURED ABDOMINAL AORTIC ANEURYSM
655
Early management
Ruptures fall into three groups:
• Considered not a candidate for surgery. For analgesia and palliative
care. Mortality approximates to 100%. The decision is based on age,
physiological status, comorbidities, expressed patient preference,
family wishes.
• Free rupture with collapse and critical condition. All candidates for
surgery require emergency transfer to theatre.
• Contained leak. May be stable after initial presentation, but likely to
progress to free or complicated rupture unless urgently surgically
treated.
Principles of surgery for rupture
• Go straight to the operating table, not anaesthetic room.
• If unstable, muscle relaxation/anaesthesia is not started until patient is
prepared/draped and surgical team ready to go.
• If haemodynamically stable, central line and arterial line sited while
waiting for blood to arrive and surgical team in theatre.
• Give antibiotic prophylaxis.
• Proximal neck is controlled rapidly with aortic cross-clamping. Full
fl uid expansion with blood can now safely begin.
• Distal outfl ow of aneurysm is controlled.
• The sac is opened and lumbar vessels and inferior mesenteric artery
are oversewn to control back bleeding.
• Dacron/Gore-Tex
®
graft is sewn to proximal neck and tested.
• Distal anastomosis performed next and tested.
• Sequential reperfusion is undertaken accompanied with volume
expansion to minimize post-declamping shock. Ideally the systolic BP
should be maintained over 85mmHg.
• Blood products at this stage may be required to correct coagulopathy
(e.g. platelets and FFP).
• The sac is closed over the graft to reduce the risk of aortoenteric
fi stula.
Post-operative care
• Transfer to ITU.
• Normalize core temperature.
• Correct clotting and maintain Hb >10g/dL.
• Adequate analgesia and accurate fl uid balance.
• Attention to cardiac/renal/pulmonary dysfunction.
Complications
• Death (overall up to 50% of operated cases).
• MI.
• Renal failure.
• Lower limb embolism.
• Gut ischaemia/infarction.
• Abdominal compartment syndrome.

CHAPTER 18 Peripheral vascular disease
656
Vascular developmental abnormalities
Key facts
Classifi ed broadly into two principal groups.
Vascular tumours (e.g. haemangiomas)
• All congenital or idiopathic.
• Mostly sporadic, but may rarely be part of a familial syndrome (e.g. von
Hippel–Lindau).
• Pulmonary haemangiomas, commonly seen in hereditary haemorrhagic
telangiectasia, are linked to a defi ciency in endoglin (endothelial
growth factor).
Vascular malformations
• Vascular malformations are histologically categorized as capillary,
venous, lymphatic, arteriovenous malformations (AVM), or mixed-in
type, depending on the predominant vessel type affected and
subdivided into low or high fl ow (AVMs) varieties.
• AVMs have three main causes.
Congenital.• Origin/cause unknown.
Traumatic.• May follow relatively minor trauma.
Iatrogenic.• Following a variety of surgical/interventional procedures.
Clinical features
• Congenital AVMs are usually evident at birth and the superfi cial lesion
may only represent a part of the overall abnormality.
• Symptoms are dependent on the size, site, and type of vessel affected,
and whether the AVMs are high or low fl ow.
Low fl ow
• May result in considerable cosmetic deformity if large (e.g. Klippel–
Trenaunay—port wine stain + ipsilateral hypertrophy, usually limb).
• Pain may be a feature due to spontaneous thrombosis of some/all of
the venous elements.
• Typically, the symptoms are worse after exercise when blood fl ow is
maximized.
High fl ow
• These are largely asymptomatic, but there may be a detectable venous
hum or bruit.
• They may result in local hyperhidrosis, heat, ulceration, or present
with profuse bleeding.
• May lead to high output cardiac failure if large and untreated.
Diagnosis and investigation
• Colour duplex. Diagnoses lesion, can estimate fl ow rate, and is useful
for follow-up monitoring.
• MRI has replaced CT as the best imaging modality and gives both the
extent and related anatomy for complex lesions.
• Angiography is reserved for high fl ow lesions when suitability for
embolization or surgery is being assessed.

VASCULAR DEVELOPMENTAL ABNORMALITIES
657
Treatment
• Largely conservative.
Congenital AVMs frequently reduce in size with growth of the child •
and treatment is rarely easy with recurrence common.
Adult AVMs only require treatment for complications or •
occasionally cosmesis.
Interventional radiology
• Percutaneous or intravascular embolization using wire coils or
sclerosant under radiological guidance.
• Risks include:
Those of percutaneous puncture (infection, false aneurysm •
formation, inadvertent embolization of adjacent vessels).
Tissue necrosis after successful lesion embolization.•
Post-embolization syndrome• may occur with pain at the site of
embolization, accompanied by malaise, fever and leucocytosis,
hyperkalaemia. This usually settles with symptomatic treatment in
24–48h. Due to tissue necrosis and cytokine release.
Surgery
• Small lesions may be excised completely.
• Obliteration of small superfi cial venous malformations can be
undertaken by direct puncture and injecting a sclerosant such as STD
(sodium tetradecyl sulphate).
• Open surgery is mostly confi ned to high fl ow lesions after
preoperative embolization.

CHAPTER 18 Peripheral vascular disease
658
Carotid disease
Key facts
• A cerebrovascular accident (CVA) or ‘stroke’ is ‘a rapidly developing
neurological defi cit lasting >24h’.
• A transient ischaemic attack (TIA) is ‘an acute episode of focal
(cerebral or visual) neurological defi cit which resolves within 24h’.
• CVA is the third most common cause of death in UK after coronary
heart disease and cancer.
• Incidence—stroke 200 per 100 000, TIA 35 per 100 000.
• Approximately 150 000 CVAs occur in the UK per year and
approximately 15% of these are due to atherosclerotic disease of the
carotid arteries.
Pathological features
CVA or TIA arises from disease at the origin of the internal carotid artery
(ICA) and may be due to platelet or atheromatous embolization from the
surface of the plaque (usually after an acute rupture or opening of the
plaque surface).
Clinical features
• Several clinical variants of a classic CVA are recognized.
Stroke in evolution.• Progressive neurological defi cit occurring over
hours/days.
Completed stroke.• The stable end result of an acute stroke lasting
over 24h.
Crescendo TIA.• Rapidly recurring TIA with increasing frequency,
suggesting an unstable plaque with ongoing platelet aggregation and
small emboli.
• Carotid bruits are detectable in over 10% of patients aged >60, poor
correlation with the degree of stenosis/risk of CVA, and may not arise
from the ICA.
• Patients with a signifi cant stenosis may have no audible bruit.
Neurological features
Depend on the territory supplied by the vessel affected by the embolism,
the degree of collateral circulation to that territory, and the size/resolu-
tion of the embolism.
• Amaurosis fugax. Transient monocular visual loss (described as a
curtain coming down across the eye), lasting for a few seconds
or minutes—central retinal artery (occlusion can lead to permanent
blindness).
• Internal capsular stroke. Dense hemiplegia, usually including the
face—striate branches of the middle cerebral artery.
• Hemianopia. Loss of vision in one half of the visual fi eld.
Prognosis of patients with TIA
Eighty per cent of TIAs are in the carotid territory. The risk of stroke fol-
lowing a TIA is around 18% in the fi rst year, 10% in the fi rst 90 days, and
4% in the fi rst 24h.

CAROTID DISEASE
659
Diagnosis and investigation
• Colour duplex scan. All patients with TIA/CVA within last 6 months.
• MRA or CT angiography (CTA). Used when duplex is inconclusive or
diffi cult due to calcifi ed vessels.
Treatment
Medical management
• Best medical therapy is an antiplatelet agent (e.g. aspirin,
dipyridamole), smoking cessation, optimization of BP and diabetes
control, and a statin (e.g. simvastatin 40mg daily) for cholesterol
lowering, irrespective of baseline cholesterol.
• Acute thrombolysis in CT-proven ischaemia indicated in specialized
units if detected early.
Surgery
Carotid endarterectomy (CEA)
• Offered to patients with symptomatic >70% stenosis of the ICA or
>50% stenosis if recent TIA/CVA and high ABCD2 risk score (age, BP,
clinical, duration, diabetes).
• Urgent CEA within 2 weeks now considered for all patients presenting
of acute TIA/CVA.
ECST (Europe) and NASCET (North America) trials demonstrated •
d CVA in the fi rst year following CEA from 18% with best medical
therapy to 3–5% with surgery and best medical therapy. No
signifi cant benefi t to symptomatic patients with <70% stenoses.
ACST (UK) and ACAS (North American) trials have shown some •
benefi t of CEA to asymptomatic patients with >70% stenosis, but
the numbers needed to prevent one stroke are 22 patients treated.
• Technical details.
Increasingly undertaken under local (LA) block.•
Incision anterior to sternomastoid.•
Carotid vessels controlled after dissection.•
IV heparin prior to trial clamp (if patient awake).•
Cerebral circulation protected in 10% of awake patients with a •
shunt (Pruitt/Javed) (without an intact circle of Willis, there is not
enough collateral blood fl ow from the contralateral carotid).
Shunt in GA patients, depending on surgeon preference and •
cerebral monitoring (stump pressure of 50mmHg or transcranial
Doppler monitoring of middle cerebral artery blood fl ow).
Patch closure of the arteriotomy common. Eversion •
endarterectomy technique may avoid the need for a patch.
Post-operatively, close monitoring of BP and neurological state.•
• Complications.
Death or major disabling stroke, 1–2%.•
Minor stroke with recovery, 3–6%.•
MI.•
Wound haematoma.•
Damage to hypoglossal nerve (weak tongue, moves to side of •
damaged nerve), glossopharyngeal nerve (diffi culty swallowing),
facial numbness.

CHAPTER 18 Peripheral vascular disease
660
The diabetic foot
Key facts
Foot ulceration is the commonest endpoint of diabetic vascular complica-
tions. Diabetics are 15 times more likely to undergo major lower limb
amputation than non-diabetics.
Causes and features
• Key features of the diabetic foot are:
Ulceration.•
Infection.•
Sensory neuropathy.•
Failure to heal trivial injuries.•
Ulceration
• Risk factors for ulceration include:
Previous ulceration.•
Neuropathy (stocking distribution loss and ‘Charcot’s joints’).•
Peripheral arterial disease (more commonly affects the below-knee •
calf vessels (trifurcation) which are frequently highly calcifi ed, giving
rise to falsely elevated ABPI readings or incompressible vessels).
Altered foot shape.•
Callus, indicating high foot pressures.•
Visual impairment.•
Living alone.•
Renal impairment.•
• Secondary to either large vessel or small vessel arterial occlusive
disease or neuropathy or a combination of both.
• Forty-fi ve per cent of diabetic foot ulceration are purely neuropathic
in origin, 10% are purely ischaemic, 45% are of mixed neuro-ischaemic
origin.
Diagnosis and investigation
Pure neuropathic ulceration
• Warm foot with palpable pulses.
• Evidence of sensory loss, leading to unrecognized repeated local
trauma.
• Normal or high duplex fl ows.
Ischaemic/neuro-ischaemic ulceration
• Foot may be cool.
• Absent pulses.
• Ulcers commonly on toes, heel, or metatarsal head.
• Secondary infection may be present with minimal pus and mild
surrounding cellulitis.
• ABPIs may be misleadingly high.
• Duplex ultrasound assessment.
• Angiography for suspected critical ischaemia.

THE DIABETIC FOOT
661
Treatment
Prophylactic management
• Best undertaken in a specialist diabetes foot clinic with multidisciplinary
input.
• Regular foot inspection for evidence of pressure/ulceration.
• Always use appropriate wide-fi tting footwear.
• Attention to nail care with regular chiropody.
• Chiropodist debridement of pressure sites/callus.
• Keep away from heat and do not walk barefoot.
Established ischaemic ulceration
• Treat local or systemic infection.
Broad-spectrum antibiotics (local guidelines).•
Debride obviously dead tissue, including digital amputation.•
Drain collections of pus.•
Take plain X-ray for signs of underlying osteomyelitis.•
• Consider revascularization if appropriate.
Angioplasty.•
Femoro-distal bypass grafts.•
• Consider amputation for failed medical or surgical treatment.
Often possible to do limited distal amputations (e.g. •
transmetatarsal).
May be progressive if disease spreads.•
The diabetic surgical patient
• Renal disease requires close monitoring of hydration, BP, and renal
function.
• Metformin needs to be stopped for 48h before angiography to avoid
lactic acidosis.
• Insulin-dependent diabetics starved for any reason require a sliding
scale.
• Avoid pressure sores if immobile for any long period with foam leg
troughs, heel elevation, and prompt attention to any skin breaks.

CHAPTER 18 Peripheral vascular disease
662
Amputations
Key facts
• Ninety per cent for arterial disease, 10% for trauma, and rarely for
venous ulceration, tumour, or deformity.
• Amputation may be a very benefi cial treatment for pain, to restore
mobility or occasionally, to save a life in trauma or acute limb
ischaemia.
• Amputation for arterial disease carries a signifi cant mortality and a
major morbidity.
• The surgical aim is to achieve a healthy stump for a suitable prosthesis
and successful rehabilitation.
• Amputees are at the centre of a large team, including surgeons, nurses,
physiotherapists, prosthetists, occupational therapists, pain team,
counsellors, and the family.
Causes and features
• ‘Dangerous’: life-saving.
Spreading gangrene, e.g. necrotizing fasciitis, gas gangrene.•
Extensive tissue necrosis following burns or trauma.•
Uncontrolled sepsis (diabetic foot) with systemic infection.•
Primary malignant limb tumours not suitable for local excision.•
• ‘Dead’: vascular events.
Critical limb ischaemia with unreconstructable disease.•
Extensive tissue necrosis.•
• ‘Damn nuisance’: neuropathic or deformed. Failed, complicated
orthopedic surgery with severely impaired gait.
Level
• The level is chosen according to:
Lowest level where tissue is viable for healing.•
Include as many working major joints as possible to improve •
function.
Ideally sited between large joints to allow prosthesis fi tting.•
• Above knee. Most will heal, but only young and fi t achieve walking with
a prosthesis.
• Through knee. Fewer heal and some achieve walking.
• Below knee. About two-thirds heal and more achieve walking than with
above-knee amputations.
Types
• Hip disarticulation. Rarely needed, but indicated for trauma or tissue
necrosis above high thigh.
• Above knee amputation (AKA). Bone transected at junction of upper
two-thirds and lower third of femur (12–15cm above knee joint),
common in end-stage vascular disease.
• Gritti–Stokes (supracondylar AKA). Increasingly popular for bilateral
amputees as creates a long stump; especially good for wheelchair-
dependent patients.

AMPUTATIONS
663
• Through-knee amputation (TKA). Produces a wide stump, which is
diffi cult for prosthesis fi t.
• Below-knee amputation (BKA). Weight bearing on patellar tendon with
good prosthetic fi t; good knee function essential.
Skew fl ap is arguably best technique as it produces a better stump •
for prosthetic fi tting.
Alternative is a posterior fl ap, which is bulkier and leads to longer •
time to mobilization.
The tibia is transected 8–10cm distal to the tibial tuberosity and the •
fi bula 2cm more proximally.
Post-operative mobilization is early and temporary limb aids can be •
used when the wound is sound.
• Symes (ankle). Few indications for this in vascular patients and best
avoided other than in trauma or diabetics. Prosthetic fi tting is diffi cult
and a good BKA is better for walking.
• Transmetatarsal. Useful in diabetics or when several toes are
gangrenous.
• Ray. Used when digital gangrene extends to forefoot, especially useful
for diabetics when infection tracks up tendon sheath.
• Digital. Usually only for diabetic disease or local trauma.
Treatment
Preoperative care
• Restore Hb levels and correct fl uid and electrolyte balance.
• Ensure good diabetes control.
• Cross-match 2U of blood.
• Adequate analgesia (epidural may reduce phantom pain).
• ECG and CXR.
• Optimize cardiac function.
• Prophylactic antibiotics to include gentamicin.
• Counselling if available.
Post-operative care
• Pain control with epidural 9 PCA.
• Regular physiotherapy to prevent muscle atrophy or contractures as
well as upper limb exercises.
• Early rehabilitation on temporary limb aid.
• Own wheelchair to aid early mobilization.
Complications
• Infection.
• Non-healing of stump.
• Progression of underlying disease and higher level amputation.
• Phantom limb pain. Due to hypersensitivity in divided nerves, can be
helped with gabapentin, amitryptyline, or carbamazepine.
• Failed mobilization. Early regular analgesia and physiotherapy are
important.
• Perioperative cardiovascular events in arteriopathic patient.

CHAPTER 18 Peripheral vascular disease
664
Vasospastic disorders
Key facts
Many systemic disorders have vasospasm as part of their presentation.
Causes
Rheumatological disease
Often associated with autoimmune disease.
• Systemic sclerosis.
• Systemic lupus erythematosus (SLE).
• Rheumatoid arthritis.
• Sjögren’s syndrome.
• Dermatomyositis.
• Polymyositis.
Neurological disease
• Refl ex sympathetic dystrophy.
• Post-traumatic vasospasm.
• Vibration white fi nger (due to exposure to handheld vibrating tools in
miners, fi tters, builders, platers).
Drug induced
A-agonist treatment (ergotamine).
Idiopathic
Raynaud’s disease.
• ♂:♀, 9:1.
• Affects 20–30% of young women, with a possible familial
predisposition.
• Possibly due to defi ciency of a potent vasodilator (a calcitonin gene-
related peptide) in the digital nerves, allowing action of unopposed
cold stress-induced release of the vasoconstrictor, endothelin.
Features
Vasospasm of any cause results in ‘Raynaud’s phenomenon’.
• Intermittent attacks.
• Initiated with pallor (‘white’). Due to local tissue oligaemia.
• Proceeding to cyanosis (‘blue’). Due to venous stasis and
deoxygenation.
• Followed by rubor (‘red’). Due to reactive hyperaemia as blood fl ow is
restored.
Diagnosis and investigations
• Stop all vasoactive treatment 24h prior to assessment.
• After local cooling to 15*C, fi nger Doppler pressures change (fall
>30mmHg signifi cant).
• Screen. FBC, U&E, urinalysis, thyroid function tests, plasma viscosity,
rheumatoid factor, autoantibody screen.

VASOSPASTIC DISORDERS
665
Treatment
Medical
• Avoidance of precipitating factors (e.g. outdoor work, smoking).
• Electrically heated gloves/socks.
• Drug therapy used if symptoms are severe enough to interfere with
work/lifestyle.
Calcium channel blockers (e.g. nifedipine 10mg/day increasing to •
20mg/day tds) may help, but side effects (headache) may limit use.
Iloprost•
®
(prostacyclin) infusion. Weight-related doses given
IV over 48–72h, as tolerated by side effects, for severe pain or
impending/actual tissue loss.
Surgical
Sympathectomy. Reserved for patients with failure to respond to medical
therapy or secondary complications (e.g. digital ulceration).
• Lumbar. Open/laparoscopic/chemical for foot symptoms; effects are
mostly short-lived.
• Cervical. Mostly now thoracoscopic technique; effects are poor
response rate and high relapse rate.

CHAPTER 18 Peripheral vascular disease
666
Varicose veins
Key facts
• The venous system of the leg comprises of three groups.
Superfi cial.• Long (great) and short (lesser) saphenous systems and
tributaries.
Deep.• Between the muscle compartments of the legs following the
major arteries.
Perforators.• Connecting the superfi cial and deep systems.
• Blood passes from the superfi cial to deep systems via perforators
in the calf, and also at the saphenofemoral junction (SFJ),
saphenopopliteal junction (SPJ), and mid-thigh perforators (MTP)
which contain one-way valves.
• Varicose veins are tortuous and dilated segments of veins, associated
with valvular incompetence.
• Affect 35% of the population.
• Males and females almost equal prevalence.
Causes and features
Classifi cation
• Thread veins. Intradermal dilated veins, also called ‘fl are’, ‘starburst’, or
‘broken’ veins.
• Reticular veins. Subdermal 1–2mm diameter veins.
• Truncal veins. The long or short saphenous systems.
• Varicose veins. Usually arising from the truncal veins.
• Venous malformations. For example, congenital (Klippel–Trenaunay
syndrome).
Causes
• Congenital.
• Primary idiopathic (the majority).
• Acquired.
Pelvic masses (e.g. pregnancy, uterine fi broids, ovarian mass, pelvic •
tumour).
Pelvic venous abnormalities (e.g. after pelvic surgery or irradiation, •
previous iliofemoral DVT).
Clinical features
• Symptoms. Pain, aching, itching, heaviness, swelling, oedema, worse at
end of day/hot weather/premenstruation, cosmetic concerns.
• Complications. Eczema, phlebitis, lipodermatosclerosis, ulceration, or
bleeding.
Diagnosis and investigations
• General history and examination. Oedema, eczema, ulcers (usually
medial calf), lipodermatosclerosis, atrophie blanche, healed ulceration.
• Visible standing. Cough impulse, thrill, or saphenovarix at SFJ.
• Tap test. Tap downwards over vein from SFJ, impulse should be felt
lower down if valves are incompetent (outdated and unhelpful).

VARICOSE VEINS
667
• Trendelenberg test. For competence of SFJ, MTP, and SPJ (rarely used
now Doppler/duplex available).
With the patient supine, elevate the leg, empty veins, apply •
tourniquet high in the thigh, and ask patient to stand.
Look for venous fi lling and then release the tourniquet, observing •
fi lling of the veins.
If controlled by the tourniquet and then rapidly fi ll on release, the •
incompetent valve is above the level of the tourniquet, i.e SFJ.
Then repeat twice with tourniquet just above knee and below knee •
to test the MTP and SPJ, respectively.
• Handheld Doppler (HHD). Listen over SFJ and SPJ and apply calf
compression with other hand and listen for refl ux lasting 1–2s. Most
accurate outpatient method of diagnosis and localization of primary
venous refl ux disease.
• Colour duplex. Gold standard investigation in defi ning anatomy and
incompetence. Can be used for all or selectively for recurrent varicose
veins, suspected short saphenous vein refl ux, known or suspected
previous DVT, mismatch between clinical examination and HHD.
Treatment options
Medical
• Microsclerotherapy, laser sclerotherapy for thread and reticular veins.
• Foam sclerotherapy for truncal and varicose veins.
• Compression stockings.
Surgical
• Local ‘stab’ avulsions. Deals with varicosities.
• Saphenofemoral or saphenopopliteal disconnection.
• Long saphenous vein stripping (effectively avulses all incompetent
thigh perforators). Not usually done below the knee due to risk of
saphenous nerve injury.
• Endovenous laser therapy (EVLT).
• Radiofrequency ablation (endoluminal heating).
• Subfascial endoscopic perforator ligation (SEPL). For calf perforators.
Indications for treatment
• Cosmetic.
• For symptoms.
• To prevent complications.
• To reduce risk of recurrent complications.
Complications of surgery
• Bruising (virtually universal).
• Recurrence (50% cases at 10y).
• Haemorrhage (minor or, rarely, major from damaged femoral vein).
• Wound infection (commonest in groin).
• Saphenous or sural nerve damage with paraesthesia (20% numbness,
1% dysaesthesia).
• Damage to major arteries, e.g. femoral (rare).

CHAPTER 18 Peripheral vascular disease
668
Deep venous thrombosis
Causes and features
• Occurs due to abnormalities of the vein wall, blood fl ow, or
constituents of blood (Virchow’s triad).
• May be due to vein compression or stasis (immobility, trauma, mass,
surgery, paralysis, long distance travel, including airline travel).
• May be due to inherited hypercoagulability (factor V Leiden, protein C,
protein S, or antithrombin insuffi ciency).
• May be due to acquired hypercoagulability (surgery, malignancy,
polycythaemia, smoking, hormone replacement therapy, oral
contraceptive pill (OCP), dehydration).
• Severity may vary from isolated asymptomatic tibial/calf thrombosis to
severe iliofemoral segment thrombosis with phlegmasia caerulea dolens
(venous gangrene).
Clinical features
• Clinical manifestations may be absent.
• Local features of venous engorgement and stasis.
Limb swelling.•
Pain.•
Erythema and warmth to the touch.•
Mild fever and tachycardia result from release of infl ammatory •
mediators.
Homan’s sign. Calf pain on dorsifl exion of the foot is very •
unreliable and should NOT be performed.
• Complications.
PE.•
Venous gangrene (• phlegmasia caerulea dolens).
Diagnosis and investigations
Aim to confi rm presence and extent of thrombosis (to decide on neces-
sity and type of treatment, risk of embolization).
• Ascending venography. Rarely used now.
• Duplex scan. Investigation of choice; visualizes anatomy, gives extent
of thrombosis, and relies on fl ow of blood and compressibility of vein.
Operator-dependent and has lower sensitivity for calf DVT.
• VQ scan. If suspicion of PE.
• CT pulmonary angiography (CTPA). Most sensitive and specifi c
investigation for suspected PE.
Treatment
• Effective prophylaxis is better than treatment (see b p. 72).
• Conservative measures. Elevation and good hydration.
• Uncomplicated DVT. Low molecular weight heparin (LMWH),
initially in hospital, may be as an outpatient via a dedicated DVT clinic.
Subsequent treatment is with oral anticoagulation with warfarin for
3–6 months.
• Complicated DVT. Initially with IV unfractionated heparin (UFH) or
LMWH whilst converting to oral anticoagulation with warfarin.

DEEP VENOUS THROMBOSIS
669
• Thrombolysis or surgical thrombectomy are reserved for severe
thrombosis with venous gangrene.
• Vena caval fi lter. Percutaneously inserted via jugular or femoral vein
into infrarenal IVC to catch thromboemboli and prevent PE.
Used for patients with recurrent PE despite treatment, at risk of •
major central PE and anticoagulation contraindicated, requiring
urgent or major surgery (so cannot be anticoagulated), major DVT
with concomitant CNS injury or major fractures.
Risks include air embolism, arrhythmias, pneumo/haemothorax, IVC •
obstruction, renal vein thrombosis, retained, misplaced, migrating,
eroding, embolizing or broken catheters/sheaths, complications of
insertion (e.g. bleeding).
Chronic venous insuffi ciency Severe forms are often secondary to
extensive or recurrent lower limb DVT (post-phlebitic limb).
Clinical features
• Leg/ankle oedema.
• Varicose/eczema, pigmentation, lipodermatosclerosis.
• Venous ulceration (medial more common than lateral).
• Venous claudication (rare).
Assessment
• Many (80%) venous disease alone, others mixed with arterial disease.
• History of proven/suspected DVT is common.
• Ulcers present in many patients and 70% are recurrent.
Investigations
• Handheld Doppler pressures (e.g. arterial disease ABPI <0.85).
• Venous duplex to detect DVT or deep venous incompetence and to
look for superfi cial venous disease.
Treatment
• Elevation, bed rest, and elevation of foot of bed.
• Four layer bandaging (Charing Cross) if ulceration present and ABPI
>0.85. Up to 75% ulcer healing at 12 weeks.
• Graduated compression hosiery (when ulcers healed).
Class I• . Ankle pressure <25mmHg, prophylaxis.
Class II• . Ankle pressure 25–35mmHg, marked varicose veins and
chronic venous insuffi ciency.
Class III• . Ankle pressure 35–45mmHg, chronic venous insuffi ciency.
Class IV• . Ankle pressure 45–60mmHg, lymphoedema.
Surgery
• Skin grafts (split skin and pinch skin grafts).
• Ulcer bed clearance of slough/infection (physical, chemical, larval—
maggots, vacuum debridement).
• Surgery for superfi cial venous disease only (as for varicose veins
surgery).
• Role in mixed superfi cial and deep venous disease is controversial.
• May need arterial revascularization.

CHAPTER 18 Peripheral vascular disease
670
Thrombolysis
Key facts
• Introduced over 30y ago.
• Use diminishing over last 5y due to up to 5% risk of major
haemorrhage or stroke.
• Usually administered as a low dose intra-arterial infusion or as an
adjunct to surgery intraoperatively.
Agents
• Urokinase. Expensive, rarely used in UK other than for dialysis
catheters.
• Streptokinase. Cheap, but has systemic effects and 27min half-life. Has
side effects of anaphylaxis, fever, and antibody resistance, limiting
repeated use. Widely used for treatment of MI.
• Recombinant tissue plasminogen activator (tPA). Powerful clot affi nity,
lower systemic effects and bleeding complications, and half-life <6min.
Indications
• Treatment of acute limb ischaemia with viable limb due to in situ
thrombosis or embolism not suitable for surgery or extensive distal
(calf) thrombosis.
• Treatment of acute surgical graft complications, e.g. prosthetic graft
occlusion <2 weeks duration.
• Treatment of residual thromboembolic disease during reconstructive
surgery (intraoperatively).
• Thrombosed popliteal artery aneurysm (allows clearance of distal calf
vessels).
• Venous thrombosis (axillary/femoral) may be treatable by venous or
arterial lysis, but need to balance risk of haemorrhage and stroke.
Regimen
• Administered via arterial catheter and simultaneous heparin via
catheter sheath.
• Regular clinical assessment and coagulation checks are needed with
clear protocols. Half-hourly temperature, pulse rate, and BP as well as
foot observations.
• Regular review with repeat angiography.
• Increased complication rate after 24–36h of infusion.
Contraindications
• Increased risk of bleeding (haemorrhagic disorders, current peptic
ulcer, recent haemorrhagic stroke, recent major surgery, or multiple
puncture sites).
• Evidence of muscle necrosis as may result in reperfusion syndrome and
multi-organ failure.
• Urgent cases because threatened limb viability if lysis takes too long to
work.

THROMBOLYSIS
671
Complications
• Minor. Allergic, catheter problems (leak, occlusion), bruising, 15% risk
of minor haemorrhage.
• Major. Five per cent risk of major haemorrhage or stroke.

CHAPTER 18 Peripheral vascular disease
672
Complications in vascular surgery
Complications may occur in the perioperative, early, or late post-operative
periods. In general, vascular patients are older and have increased cardiac,
cerebral, pulmonary, and renal comorbidities. This is due to the associated
risk factors of hypertension, diabetes mellitus, hypercholesterolaemia, and
smoking.
General
Cardiac (see b p. 106)
• Atherosclerosis is a systemic disease with a predilection for the
cerebral, coronary, peripheral arterial, and renal circulations.
• Forty per cent of patients with PVD have at least two other
circulations affected.
• Twenty per cent of patients undergoing non-cardiac vascular surgery
have evidence of silent myocardial ischaemia.
• Seventy per cent of the mortality associated with aortic surgery is
attributable to perioperative cardiac dysfunction.
Pulmonary (see b p. 108)
• Worsened by pre-existing pulmonary disease, smoking, and obesity.
• Ensure adequate analgesia with PCA or epidural and good
physiotherapy and early mobilization.
Haemorrhage (see b p. 102)
• Perioperative. Bleeding from uncontrolled blood vessels.
• Post-operative. May be due to breakdown of vascular anastomoses.
Recognized by acute hypotension, shock, abdominal swelling, and pain.
Return to the operating theatre.
Renal failure
• Many vascular patients have pre-existing renal impairment due to
renovascular disease, drug treatments, or surgery.
• Acute perioperative risks include dehydration, use of IV contrast, use
of NSAIDs, or nephrotoxic antibiotics.
Infection
• Wound infections reduced by prophylactic antibiotics.
• MRSA easy to prevent (hand hygiene), but diffi cult and expensive to
treat.
• Clostridium diffi cile enteritis (hand washing prevents) associated with
antibiotic mis/overuse in inpatients.
Specifi c
Post-declamp shock (reperfusion syndrome)
• Ischaemic extremities during surgery reperfused into circulation.
• Features of acute haemodynamic instability due to release of toxins
(potassium, myoglobin).
• Prevented by controlled gradual reperfusion with fl uid resuscitation
and vasopressor treatment to maintain a good perfusion pressure to
the coronary, cerebral, and renal circulations.
• Mannitol often used as a free radical scavenger.

COMPLICATIONS IN VASCULAR SURGERY
673
Trash foot
• Embolization of debris to the skin of feet or buttocks after aorto-iliac
surgery.
• Avoided by careful surgical technique and distal vessel clamping fi rst.
Swollen limb
• Most are due to reperfusion injury of previously ischaemic limbs.
• Consider investigations for DVT.
Lymphocoele
• Occurs mostly after groin surgery.
• Presents as a fl uctuant, non-tender swelling.
• Most will settle spontaneously, although larger collections may be
aspirated under STRICT aseptic conditions.
• Rarely require further surgery to oversew the lymphatics.
Gut ischaemia
• May follow aortic surgery (ischaemic colitis); 2.5% after ruptured AAA
surgery and <1% of elective aortic aneurysm surgery due to loss of gut
blood supply.
• May present as vague abdominal pain or bloodstained diarrhoea.
• Sigmoidoscopy usually confi rms the diagnosis.
• If there is no evidence of peritonitis, then fl uids to rehydrate and close
observation are required.
• If there is evidence of peritonitis, then an urgent laparotomy is needed
with resection of the ischaemic bowel.
Impaired sexual function
Due to damage to the peri-aortic or hypogastric plexus and underlying
vascular disease of the blood supply to the pelvis.
Late complications
Graft occlusion
• Early failure (<30 days). Technical cause; recognized by acute
deterioration in symptoms or acute limb ischaemia.
• Late failure. Usually due to intimal hyperplasia, continued smoking, or
disease progression; recognized by progressively worsening symptoms,
falling ABPI, or duplex scanning showing graft stenosis.
False aneurysm
Usually secondary to infection or occasionally, fatigue of graft material
(long-term). Further surgery is usually required.
Graft infection
• Mostly gut bacteria or coagulase-negative staphylococci; MRSA an
increasing problem.
• Can be minimized by prophylactic antibiotics, meticulous technique,
and infection control policies on vascular wards.
• Once infected, the graft usually has to be removed and alternative
reconstruction required.
• Recognized by signs of low grade or chronic sepsis (i CRP, d Hb,
fever).

CHAPTER 18 Peripheral vascular disease
674
Aortoenteric fi stula
• Rare, usually fatal if not treated.
• Usually follows aortic grafting. Can be years later.
• Presents with small, often overlooked, GI bleeds or anaemia.
• Diagnosis diffi cult. CT can be helpful; often all other causes of bleeding
are negative.
• Treatment by open surgery or endovascular exclusion and antibiotics.

CHAPTER 19 Transplantation
676
Basic transplant immunology
Allorecognition
Allorecognition is the identifi cation of antigen as ‘non-self ’. It is a func-
tion mainly of the adaptive immune response, but is dependent on prim-
ing by an infl ammatory response from the innate immune system; such a
response occurs in transplantation, both from the infl ammatory response
to surgery and ischaemia-reperfusion injury in the graft.
Allorecognition depends on two main processes. First, alloantigen must
be taken up and complexed with surface major histocompatibility complex
(MHC) molecules on antigen-presenting cells (APCs) that include den-
dritic cells and macrophages. Direct recognition occurs when donor APCs
within the allograft perform this function whereas indirect recognition is the
term used when host APCs do this. Secondly, antigen-specifi c host T-cell
receptors must bind to the peptide fragments of alloantigen complexed
with the MHC molecules on APCs. This T-cell binding only activates a
response if there is also co-stimulatory signal from the APC.
B- and T-cell diversity
Lymphocytes are able to identify non-self by the presence of both T-cell
receptors (TCR) and B-cell receptors (BCRs) specifi c for each alloantigen.
The production of a huge number of receptors (approximately 2.5 x 10
7
),
each specifi c to a particular peptide fragment, is possible because of the
millions of potential combinations of the thousands of TCR and BCR gene
segments. The TCR gene segments are located in chromosomes 14 and 7;
the BCR gene fragments are on chromosome 14.
T-cell activation
When the TCR binds to antigen complexed with MHC molecules, a chain
of reactions between intracellular signalling molecules leads to cell apop-
tosis unless a co-stimulatory signal from the APC also occurs. Binding of
the TCR as well as CD and co-stimulatory molecules with the MHC leads
to T-cell activation, differentiation, and clonal expansion. This results in
the rapid production of large numbers of cells able to coordinate and
effect destruction of tissues bearing the specifi c alloantigen; this manifests
clinically as rejection.
Rejection
There are several types of rejection:
• Hyperacute rejection is mediated by preformed antibodies that bind to
antigens of ABO blood groups, non-self HLA, causing immediate tissue
oedema, haemorrhage, and thrombosis, which can produce a severe
systemic reaction like an ABO-incompatible blood transfusion.
• Acute rejection is mediated either by T-cells (‘cellular rejection’) or
newly formed antibodies (‘humoral rejection’) and results in tissue
destruction over days to many months after transplant.
• Chronic rejection is a poorly understood vasculopathy associated with
fi brosis of small blood vessels that occurs over years. It is a chronic
infl ammatory condition which probably has an alloreactive component.

BASIC TRANSPLANT IMMUNOLOGY
677
A basic glossary of terms in transplant immunology
• Adaptive immunity. Learned response to specifi c non-self antigens.
• Allo-. Tissue from genetically different member of same species.
Alloantigen. Antigen from genetically different member of same
species. Allogenicity. Ability of tissue to provoke an immune response
when transplanted into genetically different member of same species.
Allorecognition. Recognition of alloantigen as non-self.
• Antigen. Cell surface glycoproteins.
• Antibody. Specifi c proteins produced by B-cells in response to
non-self antigen, consisting of two light and two heavy chain proteins
composing a constant and a variable region. The variable chain
region binds with the antigen that triggered the response and the
constant region coordinates the cellular response.
• APC (antigen-presenting cells). These cells ingest and process
antigen, then present it bound to surface MHC to T-cells. APCs are
most commonly dendritic cells or macrophages, but other cells can
act as APCs.
• B-cells. These cells mature in bone marrow. They produce antibodies,
but can also present antigen. In response to antigen, they undergo
clonal expansion, triggered and coordinated by T helper cells.
• Cellular immunity. Adaptive immunity mediated by lymphocytes.
• CD. Cellular differentiation molecule (followed by a number, e.g.
CD4).
• Clonal expansion. Production of large numbers of identical cells.
• Co-stimulatory molecules. Molecule receptors that must be
activated, in addition to a main receptor, for a process to happen.
• Direct recognition. Donor APCs present alloantigen to host T-cells.
• HLA (human leucocyte antigen). Human MHC.
• Humoral immunity. Immunity mediated by non-cellular components
of the immune system such as antibodies or complement.
• Indirect recognition. Host APCs present alloantigen to host T-cells.
• Innate immunity. Rapid inbuilt response to certain non-self proteins.
• MHC (major histocompatibility complex). Glycoproteins
expressed on the surface of all cells, coded for by the MHC
genes on chromosome 6. Alloantigen must be bound to MHC
for T-cells to recognize it. There are two classes of MHC. Class
I (HLA molecules A, B, and C) is present on all cell membranes.
Class II (HLA molecules DP, DQ, and DR), also known as minor
histocompatibility complex, is present on only certain cell types.
Class II is less allogeneic.
• Rejection. Injury to tissue by host immune response.
• T-cells. These cells mature in the thymus. T-cells that bind to thymic
tissue (self) are destroyed. T helper cells that are CD4 +ve coordinate
and T cytotoxic cells (CD8 +ve) effect the response. Regulatory
T-cells are also CD4 +ve and reduce the alloreactive response.
• Tolerance. A condition of lack of immune response to alloantigen
where such a response is expected or has occurred previously.
• Xenograft. Tissue transplanted between species.

CHAPTER 19 Transplantation
678
Immunosuppression and rejection
The goal of immunosuppression is to inhibit the immune response to
alloantigen, while preserving the immune response to infection and malig-
nancy. Immunosuppression consists of a short, intense induction phase
followed by a maintenance phase in which immunosuppression dosage is
tapered. A careful balance is maintained between therapeutic and toxic
doses of immunosuppression; this is generally achieved by combining
drugs with different mechanisms of action (see Box 19.1).
Acute rejection
Diagnosis
Clinical features depend on the organ transplanted and are generally mani-
fested by clinical and biochemical evidence of impaired organ function
or organ injury (such as elevated transaminase levels in liver transplanta-
tion). Systemic immune symptoms are less common and include low grade
fever, malaise, and tenderness over the graft. Blood tests may reveal a
lymphocytosis. Diagnosis is made by biopsy, which will also determine
whether rejection is T-cell mediated or humoral, and can identify other
causes of graft dysfunction.
The immunosuppression may mask symptoms until rejection is quite
advanced, so routine surveillance may be undertaken, especially for those
organs where loss of function would be catastrophic. When required,
optimal surveillance is by ‘protocol biopsies’ taken at predetermined time
points such as at 7–10 days after transplantation, with repeat biopsies
taken at intervals. Percutaneous imaging-guided biopsy is typically used
in liver and renal transplantation, endoscopic biopsy in small bowel and
lung transplantation, and transjugular biopsy in heart transplants or liver
transplants with uncorrectable coagulopathy.
Management
• Asymptomatic mild rejection may be monitored in some organs, but is
always treated in renal transplants as kidneys are very immunogenic.
• The main form of treatment is increased immunosuppression.
• Up to 3 days IV methylprednisolone 500–1000mg/day is typically given
as initial treatment for acute rejection.
• Repeat biopsy is performed if no improvement, followed by repeat
steroid course if ongoing rejection, or give rescue therapy if severe.
• Rescue protocols include administration of anti-T-cell antibodies.
• Plasmapheresis to remove antibody may be required in humoral
rejection, diagnosed by positive CD4 staining.
• Retransplantation is occasionally performed after graft loss due to
refractory rejection, but is not performed for certain organs, such as
the heart and lung, as results are extremely poor.
Chronic rejection
Chronic rejection is a chronic infl ammatory process associated with
intimal hyperplasia with dependent ischaemia and fi brosis. The cause is
unknown, but appears related to the severity of acute infl ammatory proc-

IMMUNOSUPPRESSION AND REJECTION
679
esses at the time of transplantation, such as ischaemia-reperfusion injury
and acute rejection.
The intimal hyperplasia and dependent ischaemia affect the organs in dif-
ferent ways, such as coronary artery disease in heart, glomerulosclerosis in
kidneys, and vanishing bile ducts in livers.
The term ‘chronic rejection’ is not widely used at present, with a variety
of different names used according to the affected organ such as cardiac
allograft vasculopathy (heart), bronchiolitis obliterans (lung), and chronic
allograft nephropathy (kidney).
There is no specifi c treatment available for chronic rejection at the
present time, with retransplantation as the only effective option.
Side effects of immunosuppression
Opportunistic infection
Cytomegalovirus (CMV) can cause life-threatening infection in transplant
recipients and is especially common in the fi rst 6 months. Examples are
CMV pneumonitis and CMV colitis. Diagnosis is by quantitative polymer-
ase chain reaction (PCR) and treatment by oral valganciclovir or IV gan-
ciclovir for severe infections. Recipients felt to be at risk based on donor
and recipient serology are given prophylactic valganciclovir for 100–200
days.
Pneumocystis jiroveci (P. carinii) infection can lead to a severe atypical
pneumonia in immunosuppressed patients. It is generally prevented by
prophylactic co-trimoxazole for the fi rst 3 months.
BK virus infection is generally asymptomatic, but is a possible cause of
failure of renal transplants.
Malignancy
Immunosuppression reduces immune surveillance of tumours, so some
are more common in transplant recipients and others can be more aggres-
sive than would otherwise be the case. Virally-mediated tumours and
squamous cell carcinoma of the skin are especially common.
Post-transplant lymphoproliferative disorder (PTLD) is a condition of
B-cell proliferation which may extend to B-cell lymphoma and is due to
inhibition by immunosuppressant drugs of the IL2-dependent mechanism
by which T-cells regulate the B-cell proliferation occurring in Epstein–Barr
virus infection.
Although not a complication of immunosuppression per se, malignancy
can also be transmitted from transplant donors to recipients and so must
be excluded as far as possible with potential donors.

CHAPTER 19 Transplantation
680
Box 19.1 Immunosuppressive agents
Corticosteroids
Corticosteroids inhibit the immune response at many levels. They
decrease production of G-interferon and interleukins that would nor-
mally cause up-regulation of the lymphocyte response and reduce mac-
rophage function.
Calcineurin inhibitors (CNI)
Ciclosporin and tacrolimus are calcineurin inhibitors: they inhibit the
production of IL-2 by T helper cells, selectively reducing the cytotoxic
T-cell response. Nephrotoxicity is a major side effect, but they also
impair glucose tolerance and affect lipid metabolism.
Mammalian target of rapamycin (mTOR) inhibitors
Sirolimus (rapamycin) and everolimus both inhibit the production of IL-2
by T-cells and thus stops their clonal expansion in a manner similar to
the calcineurin inhibitors. mTOR inhibitors are not themselves nephro-
toxic, but they appear to potentiate the nephrotoxicity of CNIs if given
together.
Mycophenolic acid
Mycophenolic acid inhibits purine synthesis in lymphocytes, reducing
clonal expansion and lymphocyte counts. It can cause severe GI side
effects which are reduced by administration as enteric-coated tablets or
as the pro-drug, mycophenolate mofetil (MMF).
Azathioprine
Azathrioprine reduces lymphocyte production by suppression of purine
synthesis. Allopurinol inhibits the metabolism of azathioprine. Bone mar-
row suppression and pancreatitis are common adverse reactions.
Basiliximab
Monoclonal antibody that binds to the IL-2 receptor of T-cells and thus
prevent clonal expansion of T-cells. Usually given as induction agent.
Anti-thymocyte globulin (ATG)
Derived from rabbits or horses immunized with T-cells; primarily
directed against the T-cell receptor. Systemic infl ammatory reactions
occur due to cytokine release from T-cells when the receptor bound.
Alemtuzumab (Campath)
Monoclonal antibody binding to CD52 receptor, leading to depletion
of T-cells, B-cells, natural killer cells, lymphocyte precursors, dendritic
cells, and macrophages. It thus reduces all the cells involved in antigen
presentation, cellular rejection, and humoral rejection. Usually used as
an induction agent. Side effects include bleeding and sepsis.
Belatacept
A new drug which blocks co-stimulation of T-cells by antigen presenting
cells. Not nephrotoxic or diabetogenic. Long-term effect unknown.
This page intentionally left blank

CHAPTER 19 Transplantation
682
Transplant recipients
Indications for transplantation
Cardiac transplantation
• End-stage heart disease with a life expectancy of 12–18 months.
• NYHA class III or IV heart failure, refractory to medical or surgical
therapy.
• Cardiomyopathy and congenital heart disease.
Lung transplantation End-stage lung disease where conventional therapy
is not likely to provide acceptable benefi ts or satisfactorily improve life
expectancy.
Renal transplantation All patients with end-stage renal failure should be con-
sidered for renal transplant unless there are specifi c contraindications.
Liver transplantation
• Unacceptable quality of life because of liver disease.
• Anticipated 1y mortality >9% without transplant.
Pancreas transplantation
• Usually with or after kidney transplantation for diabetic nephropathy.
• Can be done alone for hypoglycaemic unawareness (consider islets).
Small bowel transplantation (often part of multivisceral)
• Congenital extensive atresia.
• Life-threatening complications of TPN in patients with intestinal failure.
• Loss of central venous access sites in patients with intestinal failure.
Absolute contraindications to transplantation
• Predicted life expectancy <5y due to comorbidity.
• Inability to comply with immunosuppression.
• Chronic current systemic infection.
• Continued abuse of alcohol or other drugs.
• Irreversible secondary organ failure not appropriate for combined
transplant.
• Severe cerebrovascular disease.
• Active malignancy.
• Other life-threatening medical condition.
Relative contraindications to transplantation
• HIV (controversial), hepatitis B/C (except in liver transplants).
• COPD with FEV
1
<50%, PVR >4 Wood units in heart transplants.
• Chronic renal impairment with GFR >50mL/min, unless candidate for
renal transplant, including combined transplants with kidney.
• Diabetes with target organ damage (heart).
• Lipid disorders refractory to diet or therapy (heart).
• Severe osteoporosis.
• Amyloidosis.
• Continued smoking (heart or lung).

TRANSPLANT RECIPIENTS
683
Routine investigations in transplant assessment
• A full history and clinical examination.
• CXR, ECG.
• Functional cardiopulmonary assessment if indicated, e.g. CPEX.
• Cardiac catheterization and coronary angiography if indicated.
• Lung function tests.
• MSU, urinalysis, nose swab, and MRSA screen.
• Blood group antibody screen.
• FBC and coagulation profi le.
• U&E, calcium, phosphate, LFTs, fasting blood glucose, and lipids.
• Serology for hepatitis B/C, HIV, syphilis, rubella, EBV, herpes varicella
and zoster, CMV, toxoplasma.
• HLA typing, lymphotoxic antibody screen.
• Assessment of compliance; may include interview with social worker.
• Further organ-specifi c tests as indicated.
Accepting a patient on to the transplant list
Accepting a patient for transplantation is a multidisciplinary process which
varies from organ to organ. Potential kidney and/or pancreas transplant
recipients are typically initially assessed by their nephrologist and then
referred to a transplant surgeon. Potential recipients of liver, small bowel,
heart, or lung transplants are usually discussed in a multidisciplinary meet-
ing of transplant surgeons, anaesthetists, physicians, transplant coordina-
tors, and specialist nurses. The patient is informed of the decision and
receives:
• A detailed explanation of the waiting list procedures (including their
responsibility to be within contact and available for potential transplant
at all times and duty to inform the transplant team of any changes
in their health; planned holidays can be permitted by temporary
suspension from the list).
• A booklet describing this in more detail and explaining what to do
when called for surgery, the operation, post-operative care, and
follow-up arrangements.

CHAPTER 19 Transplantation
684
Transplant donors
Availability of organs for transplantation
The increasing success of organ transplantation as a modality of treatment
for end-stage organ disease has increased demand, a problem further
exacerbated by widening the criteria for which patients can be consid-
ered suitable candidates for transplantation. This has lead to an increasing
national shortage of organs despite efforts to increase donation.
A national Organ Donation Task Force was set up in the UK in 2006 and
issued fourteen recommendations in 2008 for increasing organ availability.
The fi nal phase of their implementation commenced in 2010.
Deceased donation
Deceased donors form the majority of organ donors in the UK.
Traditionally, the majority have been heart-beating donors after brainstem
death (DBD), but increasingly non-heart-beating donors after circulatory
death (DCD) are being used. Potential donors undergo a review of their
history and clinical examination, ECG, CXR, ABGs, ABO typing, testing
for HIV, hepatitis C/B, and CMV, and tests of organ function (e.g. U&E,
LFTs, echo).
Criteria for cadaveric organ donation
• Signed donor card, registration as organ donor, or agreement by next
of kin.
• Haemodynamic stability without high dose inotropic support.
• Absence of septicaemia, extracerebral malignancy, HIV, hepatitis B/C,
viral encephalitis, use of human growth hormone, and new variant
CJD.
• Brainstem death testing performed twice by two senior doctors after
essential preconditions met and apnoea test performed (for DBD
donors) (see Box 19.2).
• Organ-specifi c criteria include the following.
Kidney• . Age 1–90y with acceptable renal function.
Heart• . Age 1 month–60y with no known cardiac disease.
Heart–lung• . As above; no pulmonary disease or trauma; PO
2
, PCO
2
levels acceptable on less than 50% inspired oxygen.
Liver• . Age 1 month–70y. No known liver disease, drug addiction, or
hepatitis B.
Pancreas• . Age 10–60y with no diabetes.

TRANSPLANT DONORS
685
Box 19.2 Brainstem death testing (UK code)
Essential preconditions
The patient must be in an apnoeic coma, i.e. unresponsive and depend-
ent on a mandatory ventilation mode, following irreversible structural
brain damage due to a ‘disorder which may cause brain death’.
Drug intoxication, hypothermia, and metabolic or endocrine distur-
bances must be excluded before brainstem death testing as these may
mimic brainstem death clinically.
Apnoea test
Confi rmation of apnoeic coma is performed by ventilator disconnec-
tion, with lack of spontaneous respiratory effort despite rising arterial
carbon dioxide tension. Care must be taken to avoid hypoxia which
could potentially cause further brain injury in a patient who might not
yet be brainstem dead.
• Ensure systolic BP >90mmHg and adequate intravascular volume.
• Preoxygenate with 100% O
2
for 10min.
• Check PaCO
2
is at least 5.3kPa (40mmHg). If not, give 95% O
2
/5%
CO
2
until PaCO
2
>5.3kPa.
• Disconnect ventilator and insuffl ate 100% O
2
at 6L/min via an
intratracheal catheter passed to the level of the carina.
• Continue disconnection until PaCO
2
>6.65kPa (50mmHg), which
should occur within 8min.
Apnoea testing should be abandoned if there are cardiac arrhythmias,
hypotension, or arterial desaturation. In the UK, apnoea testing must be
attempted again before brainstem death is diagnosed.
Clinical tests
The brainstem refl exes should be tested by two experienced doctors,
one of whom must be a consultant and neither of whom is a member of
the transplant team. They may perform the tests together or independ-
ently and must repeat all the tests after a period of at least 2h.
• No pupillary response to light (both direct and indirect refl exes).
• No corneal refl ex.
• No vestibulo-ocular refl ex. No eye movement on irrigation of
tympanic membrane with 20–50mL ice-cold water.
• No cranial nerve motor responses, e.g. grimacing to pain.
• No gag or cough refl ex on deep bronchial suctioning.
• No oculocephalic refl ex (‘doll’s eyes test’).
2 Further information on brainstem death testing is located at: http://
www.aomrc.org.uk/publications/reports-guidance/doc_download/42-a-
code-of-practice-for-the-diagnosis-and-confi rmation-of-death.html
Management of brainstem-dead organ donors (DBD)
Resuscitation of organ donors requires early recognition and assiduous
support. Brain death is associated with a variety of sequelae, which include
haemodynamic instability, hypothermia, coagulopathy, fall in T
3
and T
4
,
myocardial depression, and diabetes insipidus.

CHAPTER 19 Transplantation
686
Therapeutic intervention must be continued up to and throughout
the donation procedure. Unnecessary delays should be avoided and the
retrieved organs must be in optimal condition.
Monitoring
• ECG.
• Radial artery line (MAP 60–70mmHg).
• Urine output (>100mL/h).
• CVP (greater than or equal to 12cmH
2
O).
• Temperature (over 35.5°C).
Cardiovascular support
Noradrenaline (norepinephrine) is used for refractory hypotension.
Dopamine may exacerbate any polyuria and cause vasoconstriction with
end-organ damage. Dobutamine may exacerbate hypotension.
Hormone replacement therapy
• T
3
. Bolus of 4 micrograms, then infusion of 3 micrograms/h.
• Diabetes insipidus. Replace urine loss with 5% dextrose and water via
NGT. Vasopressin may exacerbate vasoconstriction, so its analogue,
desmopressin (DDAVP), is generally used instead. The aim is to
achieve 1.5–3mL urine/kg/h output.
Respiratory support Respiratory support requires meticulous asepsis.
Oxygen delivery is optimized to achieve a normal PaCO
2
. High PEEP
should be avoided to minimize lung injury.
Haematological support Coagulopathies are treated with FFP and platelets,
guided by the local laboratory.
Management of donors after circulatory death (DCD)
Most DCD are controlled donors, where life-prolonging treatment is
withdrawn after a decision that the overall prognosis means that such
treatment is felt to be futile. As such donors are living patients until the
time of cardiac arrest and generally lack capacity to consent due to being
unconscious, they can only be treated in line with their best interests
under common law, restricting the interventions possible to optimize the
condition of the transplanted organs.
Uncontrolled DCD following failed resuscitation for cardiac arrest can-
not, by defi nition, be optimized prior to cardiac arrest, but basic measures
to improve organ viability after death but prior to consent for donation
are allowed under the Human Tissue Act and can include femoral cannula-
tion to start aortic perfusion and peritoneal cooling.
Principles of cadaveric organ retrieval
The aim is to minimize ischaemic times of all organs. Retrieval of multiple
organs is common, so a coordinated approach is needed. Inotropic, vol-
ume, and respiratory support is continued until the retrieval teams are
ready to start cold perfusion.
• A midline incision from sternal notch to pubis is made.
• IV heparin 200U/kg is given.
• A diagnostic laparotomy is performed to assess for any undiagnosed
disease, especially malignancy.

TRANSPLANT DONORS
687
• Organs are carefully examined for evidence of trauma and disease. The
aim is to retain adequate vascular and visceral cuffs to facilitate later
anastomosis. Variant vascular anatomy to the liver is common and can
affect retrieval of liver and pancreas, so this must be carefully assessed.
• As soon as both the abdominal and thoracic teams have completed
their assessment and are ready for cold perfusion, the supracoeliac
aorta is cross-clamped, ventilator stopped, cold perfusion established
through aortic cannulae, and ice slush poured into the abdomen and
the thorax. The organs are then dissected out and removed once cold.
Retrieval of organs from donors after circulatory death is different as
cardiac arrest has already occurred, so the fi rst priority is to start cold
perfusion, with assessment of anatomy and disease done in the cold phase
prior to organ retrieval.
Organ preservation
The key to minimizing ischaemic injury remains minimizing ischaemic
time, but use of appropriate perfusion solutions reduces the severity of
ischaemic injury. A variety of storage solutions are used at temperatures of
4–10°C. Two categories exist: extracellular solutions characterized by high
Na
+
and low K
+
such as Bretschneider (HTK) and intracellular solutions
characterized by high K
+
and low Na
+
such as University of Wisconsin
solution (UW) or St Thomas’s cardioplegia solution.
Machine perfusion
Organs are usually stored in ice slush once perfused, but are sometimes
stored in a machine providing continuous perfusion of the organ. This is
especially common for DCD kidneys using a cold perfusion circuit, which
can also allow assessment of viability by measurement of pressure-fl ow
characteristics and biochemical markers of ischaemic injury.
Warm perfusion using oxygenated perfusion solutions to assess and
treat ischaemic injury prior to implantation has been used experimentally
and may enter clinical practice in the near future.
Living donors
These are generally relatives or from genetically unrelated, but emotion-
ally connected individuals (mostly commonly spouses), though undirected
living donation to complete strangers is also allowed (‘altruistic dona-
tion’). Living donation requires meticulous preparation to minimize risk
to the donor and exclude coercion or fi nancial reward; potential altruistic
donors must also be psychologically assessed.
Kidneys are the most common transplants from living donors. Donation
of a liver lobe is also possible due to the large functional reserve of the
liver and its ability to regenerate by hypertrophy; left lobe liver donation
from adults to children is especially common.
Living donation of lung lobes is also possible, but requires two donors
for each recipient. Living pancreas donation using a distal pancreatectomy
has been described, but is not widely used due to the potential risks to the
donor of diabetes or pancreatic duct leakage.
• All donors undergo blood grouping, tissue typing, and assessment of
viral status for hepatitis B/C, HIV, and CMV.

CHAPTER 19 Transplantation
688
• Tests of organ function, such as isotope split GFR for kidneys, are
needed to ensure adequate post-operative function for both donor
and recipient.
• General tests of donor fi tness are also essential to minimize risk.
ABO-incompatible living donors (see Tables 19.1 and 19.2)
Although ABO incompatibility is normally an absolute contraindication
to transplantation as the preformed antibodies will lead to hyperacute
rejection, it is possible to desensitize the potential recipient by a preop-
erative course of plasma exchanges or immunoadsorption to remove the
antibody preceded by an infusion of the anti-B-cell antibody, rituximab, to
prevent antibody regeneration. This treatment is only feasible for living
donor transplants as these are planned operations.
Similar treatment can also be given to desensitize patients with pre-
formed antibodies directed against the HLA type of their potential donor
(such antibodies can be formed after sensitizing events, such as previous
transplants or blood transfusions).
To prevent resensitization, care must be taken to avoid accidentally
transfusing anti-ABO antibodies when administering blood products after
the transplant. Red cell and platelets transfusions should use washed cells
of recipient blood group. FFP and cryoprecipitate transfusions must be
donor-type if the transplant is donor group A or B to recipient group O or
type AB if the transplant is between A and B; alternatively, recipient-type
blood products can be used if screened for low antibody activity.
Where recipient desensitization is not possible, paired exchange may be
an alternative where the donor from each pair donates to the recipient of
the other pair. Paired exchange requires a large pool of donor-recipient
pairs to be successful and is only feasible for kidney transplants.
Table 19.1 ABO compatibility for transplants
Recipient blood
group
Donor blood group
A B AB O
A Yes No No Yes
B No Yes No Yes
AB Yes Yes Yes Yes
O No No No Yes
Table 19.2 Paired exchange to avoid ABO incompatibility
Donor Mr Smith
( group A)
donates a transplant to Recipient Mr Jones
(group A)
Recipient Mrs Smith
(group B)
receives a transplant from
Donor Mrs Jones
(group B)
This page intentionally left blank

CHAPTER 19 Transplantation
690
Heart and lung transplantation
Cardiac transplantation
Matching donor to recipient
• ABO compatibility. Donor and recipient must be ABO-compatible;
hyperacute rejection occurs in ABO-incompatible patients. Children
under 1y can be transplanted despite ABO incompatibility.
• HLA typing. Although heart is amongst the least allogeneic organs and
a HLA mismatch is not a contraindication to transplantation, HLA-A
2
or -A
3
mismatch has been associated with chronic rejection and some
centres choose to avoid this.
• Size match. Important; up to 30% undersize acceptable if normal PVR,
oversize if high PVR.
Technique of transplantation
Orthotopic heart transplantation involves transplanting the donor organ
into the space vacated by the recipient heart. There are several techniques
of orthotopic heart transplantation.
• The most commonly used is the bicaval anastomosis technique. The
donor cavae are attached directly to the recipient cavae. This results in
less tricuspid regurgitation and better haemodynamic performance.
• In the original technique, right and left atria of donor and recipient are
preserved; anastomosing atria to atria is technically less demanding
than bicaval anastomosis.
• In the total anastomotic technique, each pulmonary vein is individually
anastomosed.
• In heterotopic transplantation, used in 2.5% of heart transplants, the
donor heart is retained and the transplanted heart is anastomosed
so that it acts to bypass the left heart. The technique is reserved for
severe pulmonary hypertension.
Post-operative care Monitoring for rejection is done via transvenous
endomyocardial biopsy.
Complications
• Infections (nosocomial, opportunistic, or acquired).
Bacterial (common nosocomial (see • b pp. 104, 174) and
opportunistic infections include Pneumocystis carinii, Mycobacterium
spp.).
Viral (CMV, HBV, HIV may be transmitted from graft).•
Fungal (• Candida albicans, Aspergillus).
• Rejection (b pp. 676, 678) and graft ischaemic heart disease.
• Hyperlipidaemia and diabetes secondary to immunosuppression.
• Renal failure (similar risk factors to heart failure, perioperative
hypoperfusion, nephrotoxic immunosuppression regimes).
• Hypertension. Aetiology poorly understood.
• Malignancy. Decrease in the T-cell response to EBV as a result of
immunosuppression.

HEART AND LUNG TRANSPLANTATION
691
Results of cardiac transplantation
• UK 30-day mortality is 4%.
• 1y survival is 82%; 5y survival is 65%; 10y is 50%.
Lung and heart–lung transplantation
Matching donor to recipient
• ABO compatibility. Donor and recipient must be ABO-compatible;
hyperacute rejection occurs in ABO-incompatible patients.
• HLA typing. Although a HLA mismatch is not a contraindication to
transplantation, improved graft survival is associated with matching
HLA-B, HLA-A, and HLA-DR loci.
• Size match. Important.
Technique of transplantation
• Single lung transplant is performed where the remaining native lung
will not compromise graft function or present a hazard; emphysema,
asthma, and sarcoid require single lung transplants.
• Double lung transplants are performed via a clam-shell incision for
cystic fi brosis and bronchiectasis.
• Because of donor organ shortages, heart–lung transplants are
performed less often with an increase in the use of lung transplants.
• Domino heart–lung transplants, where a heart–lung transplant was
performed for septic lung disease and the healthy explanted heart then
transplanted into a second recipient, is now rarely performed.
Post-operative care
Early post-operative management centres around maintaining a balance
between adequate perfusion and gas exchange, while minimizing fl uid load,
cardiac work, and barotrauma. Cardiovascular management and complica-
tions are very similar to those outlined on b p. 622. Monitoring for rejec-
tion is done by transbronchial biopsy and bronchoalveolar lavage.
Complications
• Infections (nosocomial, opportunistic, or acquired).
Bacterial (common nosocomial (see • b p. 174) and opportunistic
infections include Pneumocystis carinii, Mycobacterium sp.).
Viral (CMV, HBV, HIV may be transmitted from graft).•
Fungal (• Candida albicans, Aspergillus sp.).
• Vascular stenoses. Arterial stenosis results in pulmonary oligaemia and
venous stenosis in pulmonary oedema.
• Tracheal stenoses.
• Tracheal ischaemia may result in leak and mediastinitis.
• Infection with Pseudomonas sp. is common in cystic fi brosis patients.
CMV infection is dangerous.
Results of lung transplantation
• 1y survival is 61%.
• 5y survival is 40%.

CHAPTER 19 Transplantation
692
Kidney transplantation
Kidney transplantation is the commonest form of organ transplant. A total
of 2739 were performed in the UK in 2009–10, of which 1038 were kid-
neys from living donors.
Matching donor to recipient
• ABO compatibility. Donor and recipient must normally be ABO-
compatible; hyperacute rejection occurs in ABO-incompatible patients
unless desensitization treatment has been performed preoperatively.
• HLA typing. Graft survival is better if there is no more than one
mismatch for HLA-A and/or HLA-B and no mismatches for HLA-DR.
• Children. Given priority; even small children can take adult kidneys.
Technique of transplantation
The kidney is normally placed extraperitoneally into the iliac fossa. Both
the left and right kidneys can be placed into either iliac fossa, but the right
is easier as the external iliac vein is more accessible.
• The renal vessels are anastomosed to the external iliac vessels. The
common or internal iliac artery can be used if the external is diseased.
• The ureter is anastomosed to the bladder, usually over a stent.
• Preoperative native nephrectomy is only occasionally needed for
continued/recurrent urinary infection, TB of the kidney, or massive
polycystic kidney disease.
Post-operative care
Early post-operative management centres around maintaining a balance
between adequate renal perfusion and BP control.
• Graft function is monitored by serial creatinine measurements (this is
especially useful if these are plotted on an inverse creatinine chart).
• Early graft failure is usually due to lack of perfusion following an
arterial or venous thrombosis, so graft perfusion should be assessed
by DTPA scan or Doppler ultrasound if there is not immediate graft
function.
• Delayed graft function with oliguria or anuria is common early after
transplantation, especially if there has been ischaemic injury prior to
organ retrieval or a prolonged cold ischaemic time.
• Intermittent haemodialysis may be needed if there is delayed function.
• Polyuria is common once the kidney starts to function until renal
tubular function recovers; fl uid needs to be replaced to prevent pre-
renal failure of the graft.
• Biopsy to confi rm suspected rejection is done percutaneously under
ultrasound guidance.
Complications
• Infection.
• Rejection (see b p. 678).
• Renal vein or artery thrombosis may result in loss of the kidney.
• Ureteric stenosis. Treated by ureteroplasty and a stent or surgery.
• Urinary leak often can be managed by urinary catheterization for 6
weeks followed by cystogram to confi rm healing.

KIDNEY TRANSPLANTATION
693
• Lymphocoele is managed by percutaneous drainage or by laparoscopic
or open marsupialization into the peritoneum.
Results of kidney transplantation
• For cadaveric kidney transplantation. 1y survival is 88%; 2y survival is
81%; 5y survival is 71%.
• For living donor kidney transplantation. 1y survival is 94%; 2y survival is
93%; 5y survival is 84%.

CHAPTER 19 Transplantation
694
Pancreas and islet transplantation
Pancreatic transplantation is performed for insulin-dependent diabetes. It
is either performed alone (rare) or in conjunction with kidney transplanta-
tion for diabetics in end-stage renal failure. A total of 160 combined kidney
and pancreas transplants were performed in the UK in 2009–10.
Pancreas transplantation
The transplant operation
The pancreas is usually retrieved as the whole organ with the duode-
num attached. It is transplanted either intraperitoneally or extraperito-
neally into the right iliac fossa using similar techniques to those of renal
transplantation.
The dual arterial supply of the pancreas, based on the splenic artery
and superior mesenteric artery branches, is provided by forming an arte-
rial Y graft from a length of common, internal, and external iliac arteries
retrieved from the donor. The venous drainage is from the portal vein
attached to the pancreas, which is generally anastomosed to the recipi-
ent’s external iliac vein or vena cava; some centres anastomose the vein
to a portal vein tributary, but this is technically challenging and requires a
vein extension graft formed from donor iliac vein.
The drainage of exocrine function is by anastomosis of the attached
duodenum either to the bladder or to a loop of small intestine. Most
complications arise from the unwanted exocrine function of the graft.
Bladder-drained exocrine secretions can cause chemical cystitis, requiring
later conversion to enteric drainage in up to 25%. Exocrine anastomotic
leakage may occur, giving rise to local infl ammation, peritonitis, or pseu-
doaneurysm of the iliac artery.
Post-operative management
Immunosuppression is as for kidney transplantation although higher levels
of immunosuppression, including use of induction antibody therapy such
as alemtuzumab or ATG, is common.
Bladder-drained graft function may be monitored by assay of urinary
amylase; this should be sampled from a 24h urine collection as there is
diurnal variation in amylase secretion. Bicarbonate supplementation is
required for bladder-drained pancreas transplants.
Enteric-drained pancreas transplants may be monitored by serial serum
amylase and lipase levels. Immunological damage to the pancreas is
advanced before changes in blood sugar are recognized.
When the pancreas is transplanted with a kidney, the kidney may be
biopsied if rejection is suspected as it usually affects both organs or the
kidney alone if only one organ is rejected.
Thrombosis of the venous drainage of the pancreas is common, so some
centres routinely anticoagulate pancreas transplant recipients.
Fungal infections are a major problem, so antifungal prophylaxis with
oral fl uconazole is administered for the fi rst week to 10 days or until the
drains have been removed.

PANCREAS AND ISLET TRANSPLANTATION
695
Islet cell transplantation
To avoid the complications associated with the exocrine secretions of
the pancreas, transplantation of the pancreatic islets alone is an attractive
option. The islets are isolated from the retrieved pancreas and prepared
as an infusion to be embolized into the liver via a portal venous catheter
inserted by an interventional radiologist.
Islet cell retrieval is especially feasible from donors with high BMI as the
steatotic pancreas often has a large number of functioning islets, but the
pancreas is infi ltrated with fat and tolerates ischaemia poorly, leading to
severe pancreatitis after reperfusion.
Islet cell transplantation only leads to insulin independence in a minority
of patients, but it usually does improve glycaemic control and hypogly-
caemia is rare. It is therefore an especially good option for diabetics with
hypoglycaemic unawareness.
Complications of islet cell transplantation include portal vein thrombo-
sis and bleeding. Transient elevation of liver enzymes is commonly seen.
Acute rejection does occur, but is impossible to diagnose. Recurrent infec-
tions can increase levels of antibodies, reducing success rates.

CHAPTER 19 Transplantation
696
Liver transplantation
Approximately 700 liver transplants are performed each year in the UK,
but around 14% of patients listed die before being transplanted. The liver
can be split into right and left lobes for transplantation into an adult and
child simultaneously or to allow living donor liver transplants.
Diseases suitable for transplantation
• Hepatitis C cirrhosis.
• Alcoholic liver disease (6 months abstinence before consideration).
• Primary biliary cirrhosis.
• Primary sclerosing cholangitis (excluding cholangiocarcinoma).
• Hepatocellular carcinoma (HCC) in a cirrhotic liver (selected cases).
• Fulminant hepatic failure (e.g. acute viral hepatitis, drug reactions, or
paracetamol overdose).
Clinical indications (also see Table 19.3)
• Acute fulminant liver failure (see Table 19.4).
• Category 1. Expected 1y mortality >9% without liver transplant.
• Category 2. HCC within ‘Milan criteria’ (see Box 19.3).
• Category 3. Variant syndromes affecting quality of life.
Persistent and intractable pruritus.•
Diuretic-resistant ascites.•
Hepatorenal syndrome.•
Hepatopulmonary syndrome.•
Chronic hepatic encephalopathy.•
The transplant procedure
The liver is transplanted on an urgent basis, ideally within 12h of retrieval.
The recipient undergoes removal of the native liver, may be placed on
veno-venous bypass, and then the new liver is implanted in an orthotopic
position, restoring the normal vascular anatomy with the biliary drainage
via an end-to-end choledocho-choledochostomy or a Roux-en-Y hepa-
tico-jejunostomy if the recipient bile duct is diseased.
If the recipient has accessory hepatic arteries, the common hepatic
artery may be insuffi cient to perfuse the liver and so arterial conduits can
be fashioned from the donor iliac arteries retrieved with the liver.
Post-operative management
Most commonly, immunosuppression is achieved using combination of
tacrolimus, azathioprine, and steroids. The liver is less prone to acute
rejection than other organs, so immunosuppression can be fairly rapidly
tapered after the immediate post-operative phase.
Hepatic artery thrombosis (HAT) is a common complication, usually
requiring immediate retransplantation, and usually presents as metabolic
acidosis with rising serum lactate levels. Doppler ultrasound scanning is
done as soon as possible after the operation to detect HAT at an early
stage. Administration of platelet transfusions increases the risk of HAT.
Graft survival is 80% at 1y and 60% at 5y.

LIVER TRANSPLANTATION
697
Table 19.3 Monitoring disease progression using a Child–Pugh score.
Patients in class C should be referred for transplantation
• Child–Pugh class B, 5–6 points.
• Child–Pugh class B, 7–9 points.
• Child–Pugh class C, 10–15 points.
1 point 2 points 3 points
Bilirubin (μmol/L) <34 34–51 >51
Albumin (g/L) >35 28–35 <28
Prothrombin time (seconds
prolonged)
1–3 4–6 >6
Ascites None Slight Moderate
Encephalopathy grade None 1–2 3–4
Table 19.4 King’s College criteria for transplantation for acute liver
failure
Paracetamol overdose Other causes
Arterial pH <7.3; OR PT >100s; OR
All three of:
PT >100s;
Creatinine >300μmol/L;
Grade III/IV encephalopathy.
Any three of:
Bilirubin >300μmol/L;
Encephalopathy within 7 days;
PT >50s;
Age <10 or >40;
Drug toxicity.
Box 19.3 The Milan criteria for transplantation for
hepatocellular carcinoma (HCC)
• Child’s class B or C cirrhosis; and
• Single tumour <5cm or up to three tumours <3cm; and
• Absence of macrovascular portal vein invasion.

CHAPTER 19 Transplantation
698
Small bowel transplantation
Small bowel transplantation is rarely performed compared with other
organs, with around 10 per year in the UK. Small bowel may be performed
alone, in combination with a liver transplant, or as part of a multivisceral
transplant.
The results of small bowel transplantation were previously very poor,
but have improved dramatically in recent years. Graft survival at 1y
increased from 52% to 75% between 1997 and 2005, with 1y patient sur-
vival increasing from 57% to 80% over the same time period.
Indications
The main indication is short bowel syndrome with permanent requirement
for parental nutrition; long-term TPN is often associated with liver failure,
so many small bowel transplants are combined with a liver transplant.
Indications for small bowel transplantation are chronic intestinal failure
(most commonly due to Crohn’s in adults) with one or more of:
• Loss of two or more central venous access sites.
• Loss of single venous access site from infection after appropriate
attempts to salvage with aggressive line-conserving treatment.
• Early cholestatic liver disease or portal hypertension.
Successful small bowel transplantation is dependent on adequate central
venous access being available, so potential candidates need to be referred
before loss of all access sites.
Small bowel transplantation may also be considered in patients with
chronic intestinal failure due to disease conditions where survival on
long-term TPN is expected to be poor such as for chronic obstruction,
extremely short bowel (<50cm), and end-jejunostomy without colon.
Combined liver and small bowel transplantation is appropriate for
patients who would be candidates for small bowel transplantation, but
also have advanced liver disease.
Small bowel transplantation is cost-effective, with lower maintenance
costs compared with home TPN and additionally, recipients can normally
resume full normal activities with potential return to employment. Quality
of life is also better than home TPN.
Post-operative management
• Feeding tube inserted to introduce early enteral nutrition.
• Serial video zoom endoscopy to assess mucosa. Villous atrophy is early
sign of acute rejection.
• Endoscopic protocol biopsies to exclude early acute rejection.
• Diarrhoea is main presenting symptom of graft dysfunction, whether
due to rejection, infection, or ischaemia and is investigated by
endoscopy and biopsy.
Acute rejection remains a major concern. It is common as there is a high
population of immune cells in the gut compared with other organs. It is
problematic as acute rejection reduces gut wall barrier function, leading to
sepsis, especially in the context of augmented immunosuppression to treat

SMALL BOWEL TRANSPLANTATION
699
rejection. Rejection is still associated with high risks, both of graft loss and
death, so early diagnosis is the key.
In the long term, most foods can be tolerated although foods high in
simple carbohydrates and soluble fi bre may cause dumping syndrome
symptoms.
This page intentionally left blank

701
Surgery in tropical
diseases
Medicine in the tropics 702
Typhoid 704
Amoebiasis and amoebic liver abscess 706
Anaemias in the tropics 708
Malaria 710
Schistosomiasis (bilharziasis) 712
Filariasis 714
Hydatid disease 716
Ascariasis 718
Leishmaniasis 719
Trypanosomiasis 720
Tuberculosis in the tropics 722
Leprosy (‘Hansen’s disease’) 724
Guinea worm infestation 726
Threadworms 727
Mycetoma (madura foot) 728
Chapter 20

CHAPTER 20 Surgery in tropical diseases
702
Medicine in the tropics
Key facts
Although the principles of surgical care are the same in the tropics as
in developed countries, there are important differences as resources are
limited in the tropics.
• There are major transport problems.
• Treatment is often complicated by underlying disease such as anaemia
and malnutrition.
• There are differences in cultural attitudes to disease, for example:
Stomas are often considered an intolerable burden in some •
societies for religious or cultural reasons.
Sexually-transmitted diseases may carry greater a stigma than in •
the UK.
Treatment
Surgery on tropical diseases requires fl exibility in both clinical manage-
ment and surgical techniques. Doctors, irrespective of where they work,
should be knowledgeable about common tropical diseases and be aware
of the possibility of seeing an imported disease in a patient, with the con-
comitant risks to the patient and the community.

MEDICINE IN THE TROPICS
703
Spending time in tropical medicine
• Find out about where you are going to, the conditions you will work
under, and the local diseases. Be prepared for these conditions.
Visit an occupational service before you go to plan antimalarials
and vaccinations. Discuss with them options for post-exposure HIV
prophylaxis.
• When you arrive, listen to local health care workers. They always
know more than you do.
• Your elective is the perfect time to develop a research mind—look
and ask why things are being done. Develop a relationship with a
more senior doctor and fi nd questions relevant to his/her practice.
However, do not expect to get an awful lot done or make any major
breakthroughs in just a few weeks.
• Beware ‘getting in a bit of practice’. An elective is not designed to
allow you to get in a bit of practice doing things to ‘the natives’ that
you are not allowed to do at home. This is a real risk when Western
medical students go to under-resourced tropical hospitals. By all
means, expand your horizons and use your skills to their limit, but
know your limits and always work within them wherever you are.
• You will always be advised to eat only cooked or peeled food. This
is clearly impossible. However, when the diarrhoea starts, do not sit
on it as you might in more temperate climes. We have twice had to
put up drips on medical students who arrived to spend time working
with us, having had diarrhoea for several days.
• In regions where dengue or malaria occurs, a fever lasting more than
a day requires a visit to a doctor for a blood fi lm and FBC, despite
apparently focal signs such as diarrhoea. Again, don’t sit out a fever
in the tropics. ICUs are not the best places to spend electives.
• The most dangerous time during your time in the tropics is likely
to be time spent travelling. Think before you get on bus, boat, or
plane. Ask yourself: is this a safe form of transport? Can I minimize
the risk in any way? Do not feel ashamed for stopping your journey
and getting out of the vehicle if you realize the risk is signifi cant. You
might meet someone fascinating . . .
• Lastly, show respect for the local culture. It is often diffi cult to
appreciate some things and easy to hark back to good things at
home. Careless expression of such thoughts can cause great distress
to people proud of what they have accomplished.

CHAPTER 20 Surgery in tropical diseases
704
Typhoid
Key facts
• Caused by Salmonella (S.) typhi or S. paratyphi (Gram –ve bacilli),
ingested via contaminated food or water (faecal-oral or urine-oral
routes from infected patients or chronic carriers).
• Approximately 33 million cases per year.
• Endemic in whole regions due to inadequate sewage disposal and
contaminated water supplies.
• There are two clinical forms, typhoid and paratyphoid fever,
collectively known as enteric fever.
Clinicopathological features
• Following ingestion, the bacilli penetrate the intestinal mucosa and
multiply within the mesenteric lymph nodes.
• Re-entry into the bloodstream after about 10–14 days causes a bout of
‘typhoid fever’.
• Systemic complications ensue due to haematogenous spread.
• Re-infection of the gut occurs through bile or bacteraemic spread and
the bacilli localize in Peyer’s patches in the lower ileum which become
swollen and red.
• Infection of the bone marrow may cause neutropenia.
Complications of typhoid fever
• Paralytic ileus.
• Intestinal haemorrhage.
• Cholecystitis with perforation. Bacilli in the bile can produce a carrier
state which can cause isolated outbreaks of typhoid fever (‘typhoid
Mary’ was the cook who infected food wherever she worked).
• Infected aortic aneurysm.
• Phlebitis, especially of the left common iliac vein.
• Intestinal perforation along the anti-mesenteric border of the ileum at
the site of Peyer’s patches in the long axis of the gut.
• Genitourinary typhoid.
• Bone and joint infection.
• Myositis and myalgia.
• Parotitis.
• Laryngitis.
• Sinus bradycardia.
Clinical features
• Disease affects older children and young adults with an incubation
period of about 10–14 days.
• Onset is gradual with high fever, headache, abdominal discomfort,
cough, malaise, and anorexia.
• Characteristic stepwise increase in fever occurs over several days and
pea-soup diarrhoea is a feature.
• In severe cases, mental changes can occur with change in conscious
level and psychiatric features. Meningism is also a feature, but
meningitis is rare.

TYPHOID
705
• Maculopapular (‘rose spot’) rash may appear during the second or the
third week, usually on the upper abdominal and lower chest. It fades
within 2–3 days.
Diagnosis
• Widal test (serology).
• Stool culture with selective enrichment media (e.g. McConkey or DCA
agar).
Treatment
• Chloramphenicol is extremely effective, cheap, and readily available
in the tropics, but complications are not uncommon. Works by
destroying the organisms responsible for the production of vitamin B
complex.
• Alternative antibiotics are amoxicillin, trimethoprim, ciprofl oxacin (less
available in the tropics and more expensive).
• Treatment of intestinal perforation is surgical, with lavage and closure
of the perforation and IV chloramphenicol.
Prevention Immunization—intradermal or intramuscular doses of killed/
attenuated organism. Often given with parathyphoid immunization. Finite
period of protection may require re-inoculation every 2y.

CHAPTER 20 Surgery in tropical diseases
706
Amoebiasis and amoebic liver abscess
Key facts Amoebic liver abscess is a complication of amoebic hepatitis
secondary to amoebic dysentery.
Pathological features
• Caused by Entamoeba histolytica protozoal infection.
• Cysts ingested from infected water or food.
• Amoebae enter ileal and colonic wall, especially in the ascending
colon, multiply, and enter the portal venous circulation.
• Amoebae that traverse the liver enter the general circulation and
systemic effects may occur.
• Amoebae in the liver destroy liver tissue directly with little or no
tissue reaction (no abscess ‘rim’). Secondary infection may occur.
The central ‘pus’ is characteristically chocolate-coloured due to liver
cell liquefaction and usually also contains staphylococci, streptococci,
Escherichia coli, and Entamoeba histolytica.
Complications
• Infected individuals may be asymptomatic and act as ‘carriers’.
• Intestinal amoebiasis producing dysentery and dysenteric colitis and/or
amoebic appendicitis. Complications include:
Perforation and generalized peritonitis.•
Haemorrhage, bloody diarrhoea, chronic anaemia of blood loss.•
Intussusception.•
• Hepatic amoebiasis and liver ‘abscess’. Complications include:
Intraperitoneal rupture with peritonitis.•
Intrapleural rupture with amoebic empyema.•
Rupture into an attached loop of bowel.•
Rupture into the pericardium.•
• Extraintestinal amoebiasis may be hepatic or cutaneous.
Clinical features
Intestinal amoebiasis
• Fever, anorexia, weight loss.
• Acute or acute-on-chronic diarrhoea (may be bloody).
Hepatic amoebiasis
• Additional night sweats, rigors.
• Fever, anorexia, weight loss.
• Right upper quadrant and lower chest rigidity and tenderness.
• Right shoulder tip pain and right-sided basal changes, including a
pleural rub may also occur.
• Hepatomegaly.
Diagnosis and investigations
• Microscopy and culture of fresh hot stool for amoebae.
• Liver ultrasound to assess number, size, and distribution of abscesses
and allow aspiration of pus.

AMOEBIASIS AND AMOEBIC LIVER ABSCESS
707
Treatment
Medical
• Emetine 40mg IM for 7 days and metronidazole 800mg tds for 7–10
days.
• Repeated abscess aspirations.
Surgical
• Open drainage of abscess that fails to respond.
• Excision of perforated viscera or for uncontrollable haemorrhage.

CHAPTER 20 Surgery in tropical diseases
708
Anaemias in the tropics
Key facts
• The main causes of anaemias are iron defi ciency or secondary to
infections and infestations.
• Others causes include:
Glucose-6-phosphate dehydrogenase defi ciency.•
Thalassaemias.•
Sickle cell anaemia.•
Sickle cell anaemia
Pathological features
• Substitution of valine for glutamic acid at sixth position of the
haemoglobin gamma chain. Leads to sickle cell anaemia if both genes
are affected (homozygotes) or sickle cell trait in single gene carriers
(heterozygotes).
• Sickle cell S-haemoglobin forms crescent-shaped rods when in the
reduced state (deoxygenated).
• High incidence in Africa is because heterozygous carriers are more
resistant than normal to Plasmodium falciparum malaria during early
childhood and hence have a degree of ‘natural selection’ for survival
and passing on their genes.
Clinical features
• Vaso-occlusive episodes precipitated by low O
2
tension in tissues.
• The abnormally shaped red cells cannot pass through arterioles and
capillaries. Infarction, pulmonary emboli, recurrent infections, and
arthralgia are common. These episodes of vaso-occlusion may also
result from GA.
• Patients with a sickle cell trait have no clinical disabilities, but may
suffer from sickling episodes when at altitude or fl ying in unpressurized
aircraft. Sickling tendency may lead to splenic infarction.
Complications
Susceptibility to infection, in particular, pneumococcal infection, meningi-
tis, and salmonella. Several types of crisis can occur:
• Painful crisis. Typically affects extremities, bones, and joints.
• Organ infarction, e.g. spleen, renal, brain.
• Sequestration crisis. When sickled erythrocytes are sequestrated in the
liver and the spleen, leading to a massive drop in blood volume.
• Aplastic crisis. Loss of red cell production.
Diagnosis and investigations
At-risk patients must be identifi ed before surgical procedures to ensure
adequate oxygenation during GA and to prevent dehydration.
• Carry out a ‘sickling test’ on suspected patients. Add a reducing agent,
e.g. carbon dioxide (CO
2
), to an unstained drop of blood. Homozygote
blood sickles in a few hours. Heterozygote blood takes up to 24h.
• If the sickling test is positive, ensure adequate oxygenation during GA
and prevent dehydration.

ANAEMIAS IN THE TROPICS
709
Treatment
• During a crisis, the main objective is to keep tissue oxygen tension high
and preventing slow fl ow in vessels, which promotes sickling. This is
achieved by:
Providing analgesia.•
Keeping the patient fully hydrated.•
Keeping the patient warm (especially the peripheries).•
Giving supplemental O•
2
(high fl ow or even hyperbaric) if possible.
Giving antibiotics to prevent secondary infection.•
• Following a crisis, give the patient folic acid (5mg per day) to ensure
red cell production is not inhibited by inadequate levels.

CHAPTER 20 Surgery in tropical diseases
710
Malaria
Key facts
• Endemic in Africa, South East Asia, and South America.
• Attempts to eradicate it have been unsuccessful because of economic
factors and the development of resistance to drugs.
Malaria can present as an acute abdomen with pain, pyrexia, and vomiting.
Consider it when other causes of abdominal pain have been excluded,
especially in patients who have recently returned from the tropics who
have not taken antimalarial prophylaxis.
Pathological features
• The causal organisms are:
Plasmodium (P.) falciparum• . Malignant tertian malaria.
P. vivax• . Benign tertian malaria.
P. ovale• . Benign tertian malaria.
P. malariae• . Quartan malaria.
• Transmission is by the anopheline mosquito, the female alone of
which bites. The mouthpiece (full of organisms) inoculates the skin by
repeated bites.
• Parasites enter into red blood cells and divide.
• The incubation period is 10–14 days before multiplying organisms
enter the bloodstream and cause clinical disease.
• Organisms may be cleared from the circulation by the immune system,
but eradication of those within the red cells is rarely possible without
drug treatment and repeated episodes of systemic infection are
characteristic of all forms of malaria (e.g. every 3 days = ‘tertian’; every
4 days = ‘quartan’).
• Chronic carriage of organisms may occur with repeated episodes of
illness, eventually causing immunosuppression and debility.
• Infected red cells may be removed from circulation by the spleen and
liver, which may carry a particularly heavy load of organisms.
Clinical features
The fever of malaria is characteristically:
• Intermittent.
• Associated with sweating, chills, and rigors.
• Followed by an afebrile period.
Complications
Complications are more likely with more virulent species, especially P.
falciparum.
• Increased blood viscosity during attacks/’crises’ may lead to
microvascular infarction of organs such as:
Brain (cerebral malaria).•
Kidney (renal failure with haemoglobinuria—‘blackwater fever’).•
Liver (jaundice and even acute hepatic failure).•
• Increased haemolysis of infected red cells leading to:
Haemolytic anaemia.•
Haemoglobinuria.•

MALARIA
711
Diagnosis and investigations
This is based on a history of exposure (e.g. tourists) and the clinical fea-
tures with microscopy of bloodstained smear which increases the chance
of fi nding parasites.
• Microscopy and culture of fresh hot stool for amoebae.
• Liver ultrasound to assess number, size, and distribution of abscesses
and allow aspiration of pus.
Treatment
Medical
Prophylaxis
All visitors to areas of endemic disease must be advised to take full prop-
hylaxis. The recommended regimen depends on the predominant species
and the presence of resistant strains.
Uncomplicated malaria
A 3-day course of the antimalarial drug, chloroquine 600mg PO, imme-
diately followed by 300mg PO qds for 3 days, then 300mg PO daily for
2 days.
Complicated malaria
• Should always be managed in a hospital with experience and
appropriate facilities, if possible.
• Begin active rehydration immediately (IV crystalloid).
• Quinine hydrochloride 600mg IV over 10min every 8h for 7–10 days.
• Blood transfusion for anaemia.
• IV steroids for cerebral malaria.
• Monitor renal function.
Prognosis Untreated vivax malaria subsides in 10–30 days, but may recur
intermittently. Intercurrent infection worsens the prognosis. Untreated
falciparum malaria is frequently fatal.

CHAPTER 20 Surgery in tropical diseases
712
Schistosomiasis (bilharziasis)
Key facts Endemic in many parts of north Africa, the Middle East, and
South East Asia.
Pathological features
• Caused by the helminths, Schistosoma (S.) haematobium, S. mansoni
(Africa), or S. japonicum (Asia).
• Infestation occurs from standing in infected water. The intermediate
host is a snail (bullinus contortus) that inhabits slow running water.
• Multiplication of the larvae occurs in the snail; they then become free
swimming and enter the human victim. After shedding their tails, they
are swept by the bloodstream to all parts of the body. The worms
have a particular preference for some sites according to their species.
• S. haematobium has an affi nity for the vesical plexus, i.e. are mostly
found in the urinary bladder and ureter. S. mansoni and S. japonicum
have an affi nity for the mesenteric veins and biliary tree, i.e. cause
intestinal disease.
• Those that reach the liver develop into male and female worms, living
in erythrocytes and when they mature, they leave the liver via the
bloodstream to reach the vesical venous plexuses. The worms mate
and the ova pass into the urine and faeces where they pass out and
infect new water (especially stagnant). After hatching, the larvae enter
the snail within 24h.
Clinical features and complications
• Intestinal worms cause:
Intestinal ulcers with bleeding, leading to abdominal pain and •
distension (due to ascites).
Perforation with peritonitis.•
Pseudopolyps and infl ammation, leading to bloody diarrhoea;•
Chronic malabsorption and malnutrition.•
Liver fi brosis (periportal), leading to portal hypertension.•
• Systemic infection may affect:
Brain, causing malaise and fever.•
Lungs, causing dyspnoea.•
Spinal cord, causing paralysis.•
• Bladder worms produce:
Infl ammation of mucosa and slough (dysuria, frequency, •
haematuria).
Obstruction of the urinary tract (hydronephrosis).•
Squamous carcinoma of the bladder is associated with long-term •
carriage.
Diagnosis and investigations
• Microscopic examination of an early morning urine or faecal specimen
can demonstrate the presence of living eggs.
• Histological examination of a biopsy from the bladder or rectal
mucosa can also provide confi rmation of infection.

SCHISTOSOMIASIS (BILHARZIASIS)
713
Treatment
Medical
Antimony preparations may be used in the early stages. Metrifonate and
praziquantel are the most effective. It can take many months before the
dead worms are expelled.
Surgical
Surgical intervention is necessary when complications such as portal
hypertension, urethral stricture, or peritonitis after perforation develop.

CHAPTER 20 Surgery in tropical diseases
714
Filariasis
Key facts
• Caused by a range of nematode worms, including Wuchereria bancrofti,
Onchocercia volvulus, and Brugia malayi.
• Widespread in tropical and subtropical areas (India, Africa, China, the
West Indies, and Australia).
Clinicopathological features
• Transmitted to humans by the bite of many genera of mosquitoes.
• Wuchererial worms enter the lymphatic system and cause:
Acute lymphangitis. Acute, swollen, painful lymphatics and nodes.•
Chronic lymphadenitis and lymphatic obstruction. Especially of the •
lower limbs and genitalia—‘elephantiasis’.
• Onchocercial worms may reach the eye (especially in African disease)
and cause disruption of the intraocular tissues—‘river blindness’.
Diagnosis Is usually clinical.
Treatment
Acute lymphadenitis
• Rest the affected part.
• Antibiotics (ampicillin 500mg by IM injection bd for 10 days) are used
to treat secondary infection caused by beta-haemolytic streptococcus
and Staphylococcus aureus.
• Specifi c antifi larial drugs, diethylcarbamazine citrate (Hetrazan
®
,
Banocide
®
), start at 1mg/kg orally tds for 3 weeks.
• Surgical drainage of abscesses when they occur.
Chronic lymphoedema (‘elephantiasis’)
There is no satisfactory operation. Abnormal subcutaneous tissue can be
excised and the affected part covered with a split skin graft. One variation
is to excise the skin of the leg in long strips and then excise the subcutane-
ous tissue and apply skin to the denuded tissue. Apply a plaster of Paris
dressing. The results are satisfactory, but certainly not cosmetic.
This page intentionally left blank

CHAPTER 20 Surgery in tropical diseases
716
Hydatid disease
Key facts Occurs in sheep- and cattle-raising areas of the world, e.g.
rural Wales, New Zealand, and not just the tropics.
Clinicopathological features
Pathological features
• Caused by the larval forms of the cestode worms, Echinococcus
granulosus and Echinococcus multinodularis.
• Dogs are usually the primary host, eating infected sheep or cow offal,
and the echinococcus parasite, about 1cm long, develops in the dog’s
intestine.
• It consists of a head and three segments, the last of which contains
hundreds of ova. These are passed on to grass, for example, by
defecation.
• Sheep and cattle ingest the ova to complete the normal life cycle.
Humans are an incidental ‘dead end’ host, but the ova penetrate the
small intestine and enter the portal circulation.
• Eighty per cent of the ova thrive in the liver with the development of
hydatid cysts.
• They may also enter the general circulation, forming cysts elsewhere
(e.g. kidneys, lungs, brain).
Clinical features/complications
• Infection is usually contracted in childhood, but produces symptoms
and signs in adult life.
• Commonest presentation is of a liver cyst (either found as a palpable
mass, incidentally on CT scanning, or during abdominal surgery).
• Compression of the intrahepatic bile ducts may produce jaundice.
• Rupture of a cyst into the peritoneal cavity causes peritonitis and
shock. Cyst fl uid also causes a severe allergic reaction with urticaria
and eosinophilia if it enters the circulation (either by spontaneous
rupture or surgical intervention).
• The prognosis is poor.
Diagnosis
• Casoni’s test (serum antigen) is positive in 80%, but gives many false
positives.
• Indirect haemagglutination tests are most accurate.
• Ultrasound and CT scanning may be used to localize cysts.
• ERCP may demonstrate connections with or compression of the bile
ducts.
Treatment
Medical
Mebendazole 400mg tds for 30 days.
Intraperitoneal rupture
• Treat shock.
• Carry out peritoneal toilet.
• Give hydrocortisone before, during, and after surgery.

HYDATID DISEASE
717
Surgical
Excision or aspiration of the cyst(s).
• Extreme caution must be taken to prevent peritoneal contamination.
Black packs soaked in hypochlorite are placed around the liver to
show up any daughter cysts or scolices.
• The cyst is partially aspirated and partially refi lled with hypertonic
saline which is scolicidal. It is then carefully separated from the liver
and the cavity closed or drained.
• Give mebendazole post-operatively.
Prevention Community hygiene projects to reduce the risk of humans
being exposed to infected dog faeces in areas of endemic disease.

CHAPTER 20 Surgery in tropical diseases
718
Ascariasis
Key facts
• Caused by the nematode roundworm, Ascaris lumbricoides.
• Common in eastern and south eastern Africa, Sri Lanka, southern and
south eastern India, and Bangladesh.
• The incidence of the condition is related to the state of development
of the sewage systems.
Clinicopathological features
• Oral infection may occur in children or adults.
• Worms cross the intestinal wall and remain in the GI tract.
• Entry into the circulation may lead to systemic spread to other organs,
e.g. liver, lungs, upper aerodigestive tract, blood.
• Intestinal infection causes:
Abdominal colicky pain and indigestion.•
Diarrhoea.•
Obstructive appendicitis.•
Intestinal obstruction due to infl ammation and impaction of a •
‘worm mass’ or intussusception that may present as a mobile
abdominal mass.
Protein-losing enteropathy with hypoproteinaemia and bleeding, •
leading to anaemia.
• Lung infestation may cause severe pneumonitis.
• Bile duct or pancreatic infestation may cause:
Bile duct strictures.•
Liver abscess.•
Cholangitis and empyema of the gall bladder.•
Diagnosis
• Ova are demonstrated by examination of hot fresh stools.
• A plain AXR or barium meal may demonstrate radiolucent lines within
a dense shadow, which represent individual worms.
Treatment
Medical
• Piperazine, one dual dose sachet repeated after 14 days (adults), one-
third sachet (age 3–12 months), two-thirds sachet (age 1–6y), 1 sachet
(age >6y). Causes fl accid paralysis of roundworms and threadworms
and permits their expulsion by peristalsis.
• Mebendazole, 100mg bd for 3 days (adults and children >2y).
Immobilizes the worms by disrupting their transport systems.
Surgical
• Abdominal surgery is only indicated for obstruction or peritonitis.
• If intestines are not infl amed, the worm load is squeezed into the
caecum and colon where it will be removed by peristalsis. Alternatively,
remove it via an enterostomy. Any non-viable gut is resected.
• Biliary infestation may be treated by antispasmodics to allow the
sphincter to relax and then kill the worm load with antihelminthics or
removal by ERCP or laparotomy.

LEISHMANIASIS
719
Leishmaniasis
Key facts A spectrum of diseases caused either by the direct effects of
infestation by protozoans of the Leishmania group or the type IV delayed
hypersensitivity to the presence of the organisms.
Clinicopathological features
• The common route of infection is by bites from infected sand fl ies.
• The organisms spend their life cycle in the cytoplasm of circulating
macrophages.
• Causative organisms include:
Leishmania (L.) donovani.• Visceral leishmaniasis (‘kala-azar’, Indian
subcontinent).
L. tropicana, braziliensis, mexicana. • Cutaneous leishmaniasis.
L. brasiliensis brasiliensis, tropica Ethiopia. • Mucocutaneous
leishmaniasis (‘espundia’, South America and Africa).
Cutaneous leishmaniasis
• May be a simple sore at the site of fl y bite with primary healing.
• May become a systemic infection if immune response poor.
• Severe type IV hypersensitivity reaction causes multiple skin ulcers
(‘recidiva’).
Visceral leishmaniasis (kala-azar)
• An infection of the reticuloendothelial system with enlargement of the
liver, spleen, and lymph nodes.
• May cause splenomegaly and pancytopenia if there is marrow infection.
• Secondary dermoid leishmaniasis occurs in failing immunity and is
highly infectious.
Mucocutaneous leishmaniasis
• Affects the skin and subsequently the mucous membranes of the
mouth and nose, causing nodules and ulceration.
• Large ulcers on the skin of the face and destruction of skin and
cartilage are referred to as ‘Chiclero’s ear’.
Diagnosis
• Bone marrow aspiration.
• FNA of the spleen or liver.
• Complement fi xation test.
• Leishmania skin test (Montenegro).
Treatment
• Cutaneous leishmaniasis often heals spontaneously, but when skin
lesions are extensive, treat with sodium stibogluconate, an antimony
compound in a dose of 20mg/kg/day by IM or IV injection for 20 days.
• Treat skin lesions for 10 days.
• Use amphotericin in unresponsive cases (AmBisome
®
1–3mg/kg daily
for 10–21 days). Cautions include hepatic impairment and pregnancy.

CHAPTER 20 Surgery in tropical diseases
720
Trypanosomiasis
Key facts
• Caused by infection with fl agellate protozoa of the Trypanosoma group.
• Two main sites of infection are South America and Africa.
Clinicopathological features
• Causative organisms are:
Trypanosoma (T.) brucei rhodensiense• (east African). ‘Sleeping
sickness’.
T. brucei gambiense• (west African). ‘Sleeping sickness’.
T. cruzi• (South American). ‘Chagas’ disease’.
• Transmission is by bites of infected tsetse fl ies in sleeping sickness or
from infected faeces from small ‘bedbugs’ entering the mouth or eye in
Chagas’ disease.
Sleeping sickness
• Bite site develops primary chancre/ulcer.
• Parasitaemia results in acute lymphadenitis (often cervical—
‘Winterbottom’s sign’).
• Progressive waves of parasitaemia cause successively less effective
immune responses with IgM and eventual failure of the immune
response with widespread systemic parasite infestation.
• CNS infection leads to:
Spasm and tremor.•
Discoordination.•
Spastic and fl accid paralysis.•
Unrousable sleep, progressing to coma and eventually death.•
Chagas’ disease
• Acute fl u-like illness following infection. Lymphadenopathy and
splenomegaly may occur.
• Eventual systemic parasite infection has a predilection for autonomic
and neural crest derived nervous tissue, for example:
Enteric nervous system destruction leads to ‘megaoesophagus’ and •
‘megacolon’.
Myocardial infection causes myocarditis.•
Cardiac conducting system destruction leads to ventricular •
aneurysm formation, dysrhythmias, and sudden death syndrome.
Diagnosis
• Wet blood fi lm showing viable parasites.
• FNAC of lymph nodes and/or lumbar puncture may reveal organisms.
Treatment
Medical
• Suramin 0.1–0.2g initially. If there is no renal impairment, 1g every 5–7
days to a maximum of 10g.
• Pentamidine 3–4mg/kg daily for 10 days.
Surgical Rarely required for complications of Chagas’ disease (e.g. cardiac
or intestinal surgery).
This page intentionally left blank

CHAPTER 20 Surgery in tropical diseases
722
Tuberculosis in the tropics
Key facts
• Caused by Mycobacterium tuberculosis.
• In the tropics, chronic malnutrition and associated immunosuppression
lead to more acute illness, more rapid advancement of disease, and
presentation at a much more advanced stage.
• Intestinal TB is much more common in tropical presentations.
Clinicopathological features
Infection routes include:
• Infection from food contaminated with the bacilli.
• Swallowed sputum containing bacilli.
• Bacteraemic phase of primary lung infection.
Features of intestinal disease
• May affect the terminal ileum, mesenteric lymph nodes, omentum,
peritoneum, and solid organs related to the GI tract.
• Low grade fever with night sweats, malaise, anorexia, and weight loss.
• Dull abdominal pain and abdominal distension.
• Ascites.
• Rectal examination may reveal fi stulas or fi ssures.
Pathological types
• Ulcerative. Deep, transversely placed ulcers in the direction of the
lymphatics that may cause perforation and peritonitis.
• Hyperplastic. Fibroplastic reaction, resulting in thickening of the bowel
wall along the mesentery and affecting the lymph nodes and the
omentum, which may lead to malabsorption.
• Sclerotic. Associated with strictures in the small intestine, leading to
intestinal obstruction.
Diagnosis
• FBC. i WCC (lymphocytosis).
• Barium meal and follow-through may show intestinal strictures or
ulcers that may be indistinguishable from those of Crohn’s disease as
seen in temperate climates.
• Ultrasound and CT scanning may suggest infl ammatory masses
(typically in the right iliac fossa).
• Intestinal tissue biopsies may demonstrate caseating granulomas.
Treatment
Medical
Antituberculous therapy:
• Streptomycin, 15–20mg/kg IM daily (unlicensed in UK).
• Isoniazid, 7–10mg/kg daily for 12 months.
• Rifampicin, 10mg/kg for 6 months.
Surgical Laparotomy for peritonitis due to perforation, obstruction, or
unresolving infl ammatory masses.
This page intentionally left blank

CHAPTER 20 Surgery in tropical diseases
724
Leprosy (‘Hansen’s disease’)
Key facts
• Endemic in much of Africa, Southern Asia, the Far East, and South
America.
• Affects about 15 million people.
Clinicopathological features
• Caused by the acid-alcohol fast bacillus, Mycobacterium leprae.
• Commonly contracted in late childhood or adolescence, the likely
source of infection being nasal discharge from infected patients (rather
than open skin lesions).
• Infi ltration of nasal membranes leads to very slow systemic spread
(incubation period of 3–5y).
• Bacilli eventually infi ltrate areas of the body at lower than normal
temperatures (especially dermis, upper respiratory tract, and
peripheral nerves), although central organs (e.g. liver, bone marrow,
kidneys, and spleen) may be involved, especially later in the disease.
• Patterns of disease depend on the degree of host cell-mediated
immune (CMI) reaction:
Poor CMI.• Lepromatous leprosy (LL). Highly infectious; open
ulcerating lesions contain macrophages loaded with bacilli.
Modest CMI.• Dimorphous/indeterminate leprosy (BB). Features
of both types (loss of CMI leads to LL, but treatment produces
TT-type disease).
Good CMI.• Tuberculoid leprosy (TT). Pronounced lymphocytic
infi ltration of lesions causing scarring; nerve damage is prominent
feature.
Clinical features
Consider the diagnosis of leprosy in any patient who presents with a com-
bination of neural and dermatological disorders.
• Lepromatous leprosy.
Dermal changes. Typically widespread hypopigmented and •
erythematous rash affecting the face, limbs, and trunk.
Generalized malaise, fever, and arthralgia.•
Neural lesions. Often widespread neuritis followed by nerve •
thickening and progressive neuropathic tissue injury and ulceration
due to anaesthesia.
Associated iritis is common.•
Systemic amyloidosis may occur.•
• Tuberculoid leprosy.
Dermal changes. Typically focal destruction of melanocytes •
(hypopigmentation), hair follicles, sweat and sebaceous glands (dry,
hairless, anaesthetic plaques of tissue).
Neural lesions. Isolated, thickening of nerves (e.g. ulnar, peroneal), •
with late and relatively limited deformity.

LEPROSY (‘HANSEN’S DISEASE’)
725
Diagnosis
Diagnosis is based on:
• A history of contact.
• The clinical fi ndings.
• Histological confi rmation of Mycobacterium leprae.
In practical terms, infected tissue is usually obtained by taking a smear with
a scalpel blade inserted into the pinched skin of an affected eyebrow or
earlobe. The tissue fl uid obtained is stained with a modifi ed Ziehl–Nielsen
stain.
Treatment
Medical
• Dapsone (diaminodiphenysulphone) 50–100mg daily.
• Combination chemotherapy is often used to reduce the incidence of
dapsone resistance, for example:
Rifampicin 600mg once per month.•
Dapsone 100mg daily.•
Clofazimine 50mg daily + 300mg once per month.•
• Continue treatment for at least 2y and often required for life.
• Contacts receive BCG vaccination or prophylactic dapsone.
Surgical
Surgical treatment is indicated to correct deformities which may be:
• Primary. Caused directly by the disease, e.g. thickening of the skin,
paralysis of the eyelids, paralysis of the hands and feet.
• Secondary. Due to neuropathic injury. Education of the patient in
avoidance of injury and self-care is vital to prevent progressive injury
since damaged nerves may be permanently anaesthetic.
Severely damaged limbs may require amputation.

CHAPTER 20 Surgery in tropical diseases
726
Guinea worm infestation
Key facts This freshwater-borne disease is common in the Middle East,
South East Asia, certain parts of Africa, and India.
Clinicopathological features
• Contracted from drinking fresh water contaminated by the arthropod,
Cyclops, which contains the larva of the guinea worm (Dracunculus
mediensis).
• Stomach and upper small bowel lining is infected with the larvae.
• Direct spread occurs to the connective tissue of the abdominal wall
where larvae mature and mate.
• Females then migrate to the areas of the body likely to be submerged
in water, such as the lower leg, where the eggs are laid.
• The worm produces a proteolytic toxin, which leads to blister
formation. When the blister bursts, the worm physically extrudes to
allow the cycle of water re-infection to complete.
Clinical features Usually present as blistering rash on the legs with visible
worm in the base.
Complications
• The subcutaneous worms may cause cellulitis, abscess, and sinus
formation.
• Systemic infection can result in allergic conjunctivitis, osteomyelitis, or
arthritis.
Diagnosis When the worms die, they may be visible as calcifi ed areas
in the subcutaneous tissue of the soles of the feet, legs, groin, scrotum,
and back.
Treatment
The worms must be removed from their subcutaneous cavities slowly.
A sudden pull will break the worm and leave the remaining part needing
surgical removal.
Slow, progressive winding of the exposed worm around a small stick
over several days is usually successful.

THREADWORMS
727
Threadworms
Key facts Endemic in tropical and temperate climates.
Clinicopathological features
• Caused by infestation by the round nematode, Enterobius vermicularis.
• Caught by ingestion of food or water infected by worm ova.
• Typically spreads throughout a family, especially one with young
children.
• Worms live in the lumen of the lower colon and rectum and often
migrate to the anal margin at night.
Clinical features
• There is chronic, and sometimes severe, anal irritation with associated
excoriation of the perianal skin. It may be confused with other perianal
disease and should be considered, particularly in children.
• Vaginitis and urethritis are associated complications.
Diagnosis
• Proctoscopy and sigmoidoscopy often reveal the worms in the colonic
mucosa.
• A mucosal smear can be taken, but is usually unnecessary once the
worms have been visualized. This is done using a wooden spatula to
the end of which adhesive tape has been applied, adhesive surface
outwards. The skin or mucosa is touched with the tape and smeared
on to a slide, thus enabling the diagnosis to be made.
Treatment
Treat the whole family.
• Piperazine, one dual dose sachet repeated after 14 days orally for
adults, one-third sachet (3 months–1y), two-thirds sachet (1–6y), one
sachet (>6y); or
• Mebendazole 100mg orally repeated after 2–3 weeks (>2y).

CHAPTER 20 Surgery in tropical diseases
728
Mycetoma (madura foot)
Key facts
• Caused by a subcutaneous fungal infection.
• May be caused by different species with different colours of spores,
but the clinical picture is remarkably uniform.
Clinicopathological features
• The fi rst sign is a painless swelling in the foot that gradually develops
multiple sinuses and sometimes discharges purulent material containing
the grains of the fungus.
• Local spread may occur if the primary infection is not treated, leading
to deep tissue infection, e.g. fungal osteomyelitis.
• Systemic fungal infection is rare.
Diagnosis Microscopy of the discharge shows fungal hyphae.
Treatment
Medical treatment Dapsone, co-trimoxazole, streptomycin, and rifampicin
are used either alone or in combination.
Surgical treatment
• All the affected area, including all sinuses, must be excised once
treatment has begun.
• Amputation is occasionally necessary if deep osteomyelitis has
occurred.

729
Common operations
Diagnostic laparoscopy 730
Principles of laparotomy 732
Cholecystectomy 734
Appendicectomy 736
Inguinal hernia repair 738
Perforated peptic ulcer repair 740
Haemorrhoid surgery 742
Pilonidal sinus excision (Bascom II) 744
Femoral embolectomy 746
Right hemicolectomy 748
Stoma formation 750
Wide local excision—breast 752
Below knee amputation 754
Chapter 21

CHAPTER 21 Common operations
730
Diagnostic laparoscopy
Indications (typical)
Acute/emergency
• Lower abdominal pain with suspected acute appendicitis (see b
p. 298) or ruptured ovarian cyst.
• Upper abdominal pain with suspected perforated peptic ulcer (see b
p. 284).
Elective
• Investigation of subfertility.
• Investigation of chronic abdominal pain.
• To perform biopsy (e.g. omental or lymph node) in suspected
malignancy.
Pre-theatre preparation
• Always GA, therefore NBM 2h and fl uids only 4h preop.
• Group and save required.
• Ensure consent is obtained for proceeding to other procedures if they
are anticipated.
Positioning and theatre set-up
• Urethral catheterization. Usual, especially if lower abdominal pathology/
assessment likely, to ensure the bladder is decompressed.
• NGT. NOT required unless the patient is vomiting or gastric
distension/surgery is likely.
• Table positioning. Supine. It is always best to have the patient in
leg extensions. They allow the perineum to be accessed if vaginal
manipulation or lower GI endoscopy is needed and they help to
secure the patient on the table if head downtilt or lateral role is
required.
• Monitor/stack position. Depends on the expected pathology.
Steps of surgery
• Incision. Periumbilical; usually curved infra-umbilical although supra-
umbilical is also used. Vertical infra-umbilical can be used, especially
where conversion to a midline laparotomy is anticipated.
• Exposure of the linea alba. By sharp dissection.
• Incision of linea alba. Elevate with forceps and incision with scalpel
(no. 11 or 15).
• Open trochar insertion. Elevate linea alba with forceps, blunt scissor
opening of pre-umbilical fat pad and peritoneum and placement of
trochar (blunt) or:
• Blunt trochar insertion. Elevate linea alba with forceps without a small
initial incision, insert trochar (blunt or with visual assistance using
laparoscope inside the port) or:
• Verres needle insertion. Elevate linea alba with forceps, insert Verres
needle using only thumb and fi nger pressure until ‘clink’ felt, test for
intraperitoneal placement with saline ‘drop’ test.

DIAGNOSTIC LAPAROSCOPY
731
• Insuffl ation. CO
2
typical pressure between 12–15mmHg; use slow fl ow
initially, check for low pressure fl ow before increasing fl ow rate.
• Assessment. Inspect area beneath insertion port for signs of visceral
injury or bleeding, assess anterior abdominal wall for availability of
further port sites, inspect viscera sequentially.
Closure
Port sites 10mm and above require musculofascial closure, 5mm ports
do not.
Post-operative care and instructions
• Remove catheter unless required for post-operative fl uid balance
observation.
• Antibiotics. Only required for pathology found.
• Oral diet. Normal as soon as tolerated.
Complications (specifi c to the procedure)
• Port site infection, <5%.
• Port site herniation, <2% if closed.
• Visceral injury during port insertion/basic laparoscopy and assessment,
<1%.

CHAPTER 21 Common operations
732
Principles of laparotomy
Laparotomy is the term for any open access to the peritoneal cavity and
includes midline incisions as well as paramedian and oblique approaches. It
is the traditional method of access for most visceral surgery. It is still the
approach of choice for some trauma, many emergency presentations, and
some extensive surgery.
Pre-theatre preparation
• All, but smaller lower abdominal incisions which may be performed
under regional or fi eld block LA, require GA.
• Group and save or cross-match, depending on procedure.
• Ensure consent is obtained for other procedures if anticipated.
Positioning and theatre set-up
• Urethral catheterization. Usual, especially if lower abdominal
pathology/assessment likely, to ensure the bladder is decompressed.
• NGT. Usual for upper intestinal obstruction (to reduce risk of
aspiration at induction).
• Table positioning. Several are possible (see b p. 76).
Supine.• Most common for open visceral surgery (e.g. small and large
bowel, gastric and major arterial).
Lloyd Davis• (supine with hips slightly fl exed and abducted). Used
where access is required to the peritoneal cavity and the perineum/
anorectum.
Lateral.• For combined approaches to the retroperitoneal structures.
Steps of surgery
• Skin incision. Scalpel or cutting diathermy with needle point electrode.
• Fat incision. Blend diathermy to reduce risk of bleeding.
Midline access (see b p. 82)
• Midline fascia (linea alba) incision. At or above the umbilicus (pre-
peritoneal fat reduces the risk of underlying bowel injury). The midline
can be identifi ed by the presence of oblique crossing/interleaved fascial
fi bres. Expose fascia, elevated with clips to generate negative intra-
abdominal pressure and sharply incised.
• Access extension. With blend diathermy in the midline.
Paramedian access
• Rectus sheath fascia incision. Vertical with blend diathermy.
• Rectus muscle. Fibres separated with minimum muscle division.
• Peritoneal incision. Elevated between clips and sharply incised.
• Access extension. With blend diathermy vertically.
Oblique access (e.g. gridiron, subcostal)
• Rectus sheath fascia incision. Oblique with blend diathermy.
• Muscle. For small incisions, fi bre separation may achieve adequate
access; this may be multiple layers (e.g. gridiron) or single (e.g.
mini-subcostal). For larger incisions, muscle division with coagulation
diathermy is required (e.g. full subcostal).

PRINCIPLES OF LAPAROTOMY
733
Basic procedures
• Assessment of ‘non-target’ viscera. Traditionally performed, but less
important with preoperative imaging (especially CT scanning). Done
in logical progression, e.g. central (small bowel, omentum, transverse
colon), left upper quadrant (LUQ) (spleen, stomach), right upper
quadrant (RUQ) (liver, gall bladder), right fl ank (right colon, right
kidney), pelvis (bladder, uterus, ovaries, rectum), left fl ank (left colon/
kidney).
• Assessment of ‘target’ organ(s). Depends on pathology expected, but
consider these issues—‘resectability’ (tethering/involvement of vital,
non-resectable structures), extent of resection (length or additional
organs/structures to remove), mobility (adequate approximation of
structures to be joined).
Specimens
• Ascites (free fl uid). Send for M,C,&S; send for cytology if suspected
malignancy.
• Pus. Send for M,C,&S (as liquid specimen if possible) or swab.
• Peritoneal tissue. Excise or biopsy peritoneal tissue nodules (parietal or
visceral).
Key principles of emergency laparotomy
• Bleeding. Control by pressure (packs) initially rather than direct closure
(clips or sutures); remove packs, starting with those least likely to
cover bleeding sites; allow anaesthetic ‘catch up time’.
• Multiple visceral injuries. ‘Close and control’ rather than ‘restore and
join’. Preventing contamination and visceral leakage are required, but
restoration of anatomy/physiology can be deferred to subsequent
procedures.
• Contamination. Seek out and treat all areas of pus/contamination.
Frequently overlooked areas are subphrenic, subhepatic, interloop
ileal, pelvic. Wash should be warm, copious, and repeated sequential
dilutions rather than a single large washout. Large calibre drains for
heavily soiled areas (likely to recollect), consider repeat (‘re-look’)
surgery in 24–48h.
Closure
• Peritoneum. Should be approximated where possible (reduces risk
of adhesions to exposed muscle) with absorbable suture; may be
included with musculo-fascial closure (e.g. mass closure).
• Muscle fi bres. Where parted—will usually re-approximate without
sutured closure; where slit—may require absorbable sutures.
• Fascia. Always closed, usually heavy absorbable sutures, but may be
non-absorbable.
• Skin. Sutures (subcuticular or interrupted) or clips.
Complications (specifi c to the procedure)
• Wound infection, 2–30%, depending on pathology.
• Incisional hernia, up to 30%; affected by sepsis, malnutrition, age.

CHAPTER 21 Common operations
734
Cholecystectomy
Simple cholecystectomy (removal of gall bladder and proximal cystic duct)
is restricted to benign disease.
Open approach (elective) is usually only indicated for common bile duct
exploration where laparoscopic exploration is not possible.
Indications (typical)
• Symptomatic proven gallstones.
• Symptomatic congenital abnormalities of the gall bladder.
• Previous acalculous cholecystitis.
• Very rarely indicated for prophylaxis in individuals at risk of
cholecystitis (congenital heart disease, immunosuppressed).
Pre-theatre preparation
• Always GA, therefore NBM 2 h and fl uids only 4h preop.
• Group and save required.
• Ensure consent is obtained for proceeding to other procedures if they
are anticipated (e.g. common bile duct exploration).
• Check LFTs and any previous ERCP/MRCP imaging has been reviewed.
Positioning and theatre set-up
• Urethral catheterization; NGT NOT required.
• Table positioning. Supine. Some surgeons prefer to stand between the
legs for the dissection (requires the patient in leg extensions).
• Monitor/stack position. LUQ.
• Patient may be placed slightly head up and left side down to improve
RUQ exposure.
Steps of surgery
• Establish laparoscopy (see b p. 730) if appropriate.
• Incision for open surgery. Right subcostal (‘Kocher’s’) with (partial)
division of upper right rectus muscle.
• Expose gall bladder neck, cystic duct and common bile duct (‘Calot’s
triangle)—fundal traction. Tip: if exposure is poor, try adding further
5mm ports for retraction and ask for another assistant.
• Identify cystic duct origin from gall bladder neck by blunt dissection.
• Identify cystic artery by blunt dissection.
• Clip (or tie if open) cystic duct (2 distal, 1 or 2 proximal on gall
bladder neck) and divided. Repeat for cystic artery.
• Dissect gall bladder from liver ‘bed’ by retrograde dissection (i.e. from
neck to fundus). Dissection is carried out close to the gall bladder wall.
• Gall bladder retrieved (usually in a waterproof bag if laparoscopic via
epigastric port).
• Check haemostasis.
Closure
• Port sites (see Fig. 21.1). See laparoscopy (see b p. 730).
• Open. Usually closed in layers (peritoneum, (musculo)fascial, and skin).
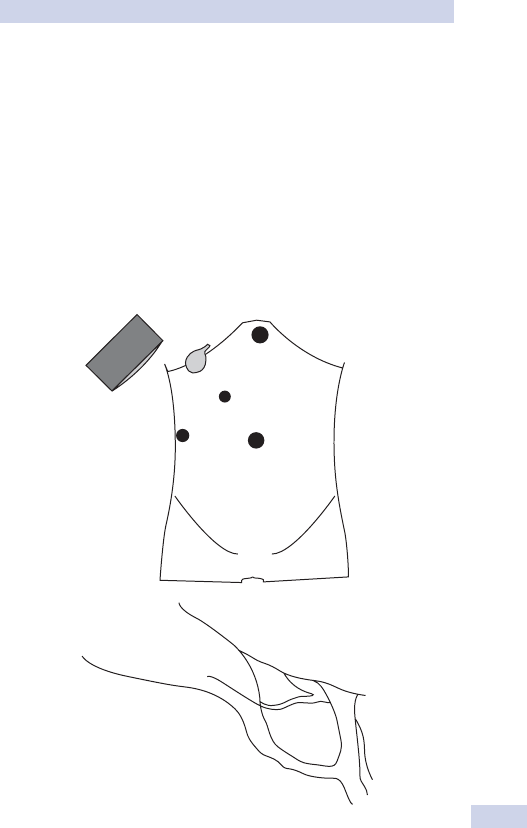
CHOLECYSTECTOMY
735
Post-operative care and instructions
• Remove catheter unless required for post-operative fl uid balance
observation.
• Antibiotics. Only required for pathology found (e.g. cholecystitis).
• Oral diet. Normal as soon as tolerated.
Complications (specifi c to the procedure)
• Port site infection, <5%.
• Bleeding, 2%.
• Conversion to open surgery (if laparoscopic), 2% (depends on gender,
age, sex, previous infl ammatory episodes, previous abdominal surgery).
• Visceral injury during port insertion/basic laparoscopy and assessment, 1%.
• Common bile duct injury, 1 in 300.
GB
LS
10
10
5
5
CA
CD
CBD
CHD
(a)
(b)
Fig. 21.1 (a) Ports sites for laparoscopic cholecystectomy. (b) View of Calot’s
triangle after dissection prior to structure division. (Borders: LS, liver surface; CHD,
common hepatic duct; CD cystic duct. Other structures: GB, gall bladder; CBD,
common bile duct; CA, cystic artery.)

CHAPTER 21 Common operations
736
Appendicectomy
Most surgeons prefer the laparoscopic approach for suspected or proven
appendiceal pathology. Open right iliac fossa (RIF) incisions offer less
exposure with greater tissue trauma. Consider a lower midline laparot-
omy if there is a large appendix mass, the patient is elderly, and other
pathology is suspected.
Indications (typical)
• Acute appendicitis (see b p. 298).
• Acute abdominal pain where diagnostic laparoscopy has revealed no
other cause (not always performed).
• Previous resolved appendicitis (‘interval appendicectomy’).
• Appendix mass or mucocele (usually discovered on CT imaging).
Pre-theatre preparation
• Always GA, therefore NBM 2h and fl uids only 4h preop.
• Group and save normal.
• Check the CT imaging if one has been performed.
Positioning and theatre set-up
• Urethral catheterization. Aids port placement safety, especially in
females, by ensuring the bladder is decompressed.
• Table positioning. Supine; it is always best to have the patient in leg
extensions (see b p. 76).
• Monitor/stack position. Right caudal.
Steps of surgery
• Establish laparoscopy (see b p. 730) if appropriate.
• Incision for open surgery, RIF oblique (‘gridiron’). Incision of skin, open
external oblique, internal oblique, and transversus abdominis, splitting
the fi bres in the direction of their travel without cutting them. Open
the peritoneum between clips.
• Identify appendix from the base attachment to the caecum (the base
lies reliably on the inferomedial pole of the caecum at the confl uence
of the taenia coli, the tip is variable in position).
• Mobilize the appendix from all surrounding structures, if necessary, by
blunt dissection.
• Open the appendix mesentery from tip towards the caecum by
diathermy (hook if laparoscopic).
• Identify appendiceal artery by blunt dissection and clip (or tie if open)
(2 proximal, 1 distal on appendix side) and divide.
• Complete mesenteric division to appendiceal-caecal angle.
• Doubly ligate appendix stump close to caecum (with Endoloop
®
if
laparoscopic) and divide between.
• Appendix retrieved (usually in a waterproof bag if laparoscopic via
umbilical port to reduce wound infection risk).
• The appendix stump is now rarely buried with a purse-string suture
at open appendicectomy; there is no evidence that it reduces stump
complications.
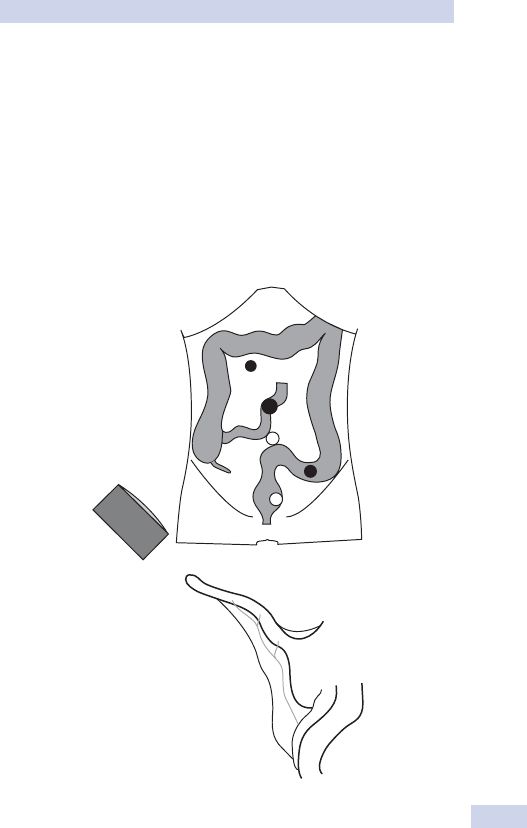
APPENDICECTOMY
737
Closure
• Port sites (see Fig. 21.2). See laparoscopy (see b p. 730).
• Open. Usually closed in layers (peritoneum, (musculo)fascial for each
layer of the oblique muscles, and skin).
Post-operative care and instructions
• Antibiotics. Five-day course if acute appendicitis with perforation.
• Oral diet. Normal as soon as tolerated.
Complications (specifi c to the procedure)
Wound/port site infection, <5% (greater if open and perforated).
10
5
5
5
5
App
AM
Cae
AMF
ApArt
ApArt
ApArt
TI
(a)
(b)
Fig. 21.2 (a) Port sites for laparoscopic appendicectomy—main ports sites in
black; alternative layout for 2 x 5mm port sites in white. Monitor usually right side,
caudal. (b) View of appendix and mesentery prior to dissection/division. (App,
appendix; AM, appendix mesentery; AppArt, appendiceal artery; Cae, caecum;
AMF, antemesenteric fat of terminal ileum; TI, terminal ileum.)

CHAPTER 21 Common operations
738
Inguinal hernia repair
Open approach is still very popular, especially for LA/regional anaesthet-
ics, but laparoscopic approaches (transabdominal pre-peritoneal surgery
(TAPS) or totally extra-peritoneal surgery (TEPS) are also widely used,
especially for bilateral hernias (see b p. 338).
Indications (typical)
• Symptomatic inguinal hernia.
• Asymptomatic inguinal hernia with high risk of complications or patient
at high risk if complications develop.
Pre-theatre preparation
May be GA, LA (± sedation), or regional block (e.g. spinal).
Positioning and theatre set-up
• Urethral catheterization. NOT usually required for open unless the
patient is at high risk of acute retention, but used for TEPS/TAPS.
• Table positioning. Supine.
• Monitor/stack position (TAPS/TEPS). Caudal end, same side as hernia.
Steps of surgery
Open
• Identify external oblique aponeurosis and cord at the superfi cial ring.
• Open aponeurosis, identify cord and deep ring.
• Direct hernia. Separate cord from sac, reduce sac, and plicate
transversalis fascia to hold sac reduced.
• Indirect hernia. Open cord, separate sac from cord structures, sac may
be reduced and the deep ring plicated or sac ligated and excised at the
deep ring.
• Polypropylene mesh placed to cover from pubic tubercle to lateral to
the deep ring; usually sutured in place.
Laparoscopic TAPS
• Establish laparoscopy (see b p. 730).
• Open parietal peritoneum above hernia (e.g. hook diathermy) and
dissect to groin structures.
• Identify testicular vessels and vas deferens.
• Reduce sac intraperitoneally where possible (part reduce and excise
apex where necessary).
• Polypropylene mesh placed to cover from pubic tubercle to lateral to
the deep ring; usually secured with laparoscopic tacking device.
Laparoscopic TEPS
• Open cut down to pre-peritoneal space via umbilical port access;
opening the peritoneum is avoided.
• Open the pre-peritoneal space from umbilicus to groin (usually with
assistance from a balloon dissecting device), then as for TAPS step 3.
Closure
• Port sites 10mm and above require musculo-fascial closure.
• Open, layers with absorbable sutures.

INGUINAL HERNIA REPAIR
739
Post-operative care and instructions
Remove catheter (once fully mobile if open op with risk of ARU).
Complications (specifi c to the procedure)
• Groin wound infection, <5%.
• Recurrence, 3% lifetime.
• Groin haematoma, 2%.
• Painful scar/chronic groin pain, 1–2%.
• Injury to testicular vessels (causing ischaemia), <1%.

CHAPTER 21 Common operations
740
Perforated peptic ulcer repair
Traditionally by an open approach, but now frequently done by laparos-
copy since this offers complete abdominal assessment prior to proceed-
ing and may allow a targeted long or lower midline laparotomy if other
pathology is found.
Indications (typical)
Suspected perforated peptic ulcer from history ± CT imaging fi ndings (see
b p. 284).
Pre-theatre preparation
• Always GA, therefore NBM 2h and fl uids only 4h preop.
• Group and save required.
• Ensure consent is obtained for proceeding to other procedures (e.g.
bowel resection since other pathologies may be found).
Positioning and theatre set-up
• Urethral catheterization. Fluid balance chart required post-operatively.
• NGT. Not indicated.
• Table positioning. Supine, but well secured to allow for head uptilt.
• Monitor/stack position. Right cranial.
Steps of surgery
• Open incision. Mini-vertical supra-umbilical laparotomy (may be
extended).
• Laparosopic. Establish laparoscopy (see b p. 730).
• Confi rm diagnosis and identify site of perforation (duodenal, prepyloric
or gastric).
• Assess pathology. If suspicion of underlying malignancy in perforated
gastric ulcer, consider if excision or partial gastrectomy may be
required (senior help).
Repair is by patch closure of the ulcer wherever possible using omental
tissue and not by sutured apposition closure of the defect (see Fig. 21.3).
• Dissect a broad ‘tongue’ of omentum using diathermy (at least 5cm
wide x 10cm long).
• Three sutures placed through each edge of the ulcer, but not tied.
• Omental strip laid under and secured in place by sutures (snug, but
not tight).
• Copious intra-abdominal lavage (all quadrants); drainage rarely
required.
Closure
• Port sites. See laparoscopy (see b p. 730 and Fig. 21.1a).
• Incision. See laparotomy (see b p. 732).
Post-operative care and instructions
• Antibiotics. Not required for simple peptic ulcer perforation.
• Oral diet. Start liquid diet as soon as tolerated .
PERFORATED PEPTIC ULCER REPAIR
741
Complications (specifi c to the procedure)
Leakage at closure site, 5% (worst in immunosuppressed or advanced
malignancy).
(a)
(b)
Fig. 21.3 (a) Sutures placed in ulcer edges. (b) Omental patch in place.

CHAPTER 21 Common operations
742
Haemorrhoid surgery
Surgical treatment is usually reserved for failed outpatient treatment (see
b p. 412) although the choice to go to surgery depends on symptoms,
patient preference, and anatomy of the haemorrhoids.
Haemorrhoid de-arterialization procedures (blind or ultrasound-guided)
are becoming more popular.
Indications (typical)
• ‘Conventional’ haemorrhoidectomy. Intero-external haemorrhoids (one
or more).
• ‘Stapled haemorrhoidectomy’. Circumferential, prolapsing haemorrhoids,
or extensive internal haemorrhoids resistant to outpatient treatment.
Pre-theatre preparation
May be GA or regional anaesthesia (e.g. low spinal or caudal).
Positioning and theatre set-up
Table positioning. Lithotomy.
Steps of surgery
Conventional haemorrhoidectomy (see Fig. 21.4)
• Assess pedicle(s) to be excised and clarify which mucocutaneous
bridges will be left at the end of the procedure.
• Incise the external (skin) margin (caution to not over-excise; the skin
defects enlarge easily!).
• Develop subcutaneous/submucosal plane (superfi cial to internal
sphincter); may be assisted with weak adrenaline solution injection.
• ‘Cone’ mucosal excision to apex of the pedicle.
• Ligate or seal with energy source the pedicle origin and vascular supply.
Stapled haemorhhoidectomy
• Determine anorectal mucosal ‘ring’ to be excised.
• Anal dilatation with circular dilator and rotating ‘windowed’ retractor.
• Circumferential running purse-string suture into mucosa and
submucosa, avoiding circular smooth muscle (internal sphincter).
• Placement of staple-gun anvil ‘head’ above the purse-string level and
tightening-tying of purse-string.
• Stapling device closed onto plicated mucosal ring, avoiding inclusion of
anal canal lining by gentle eversion during closure.
• Excision of anorectal mucosal plicated ring by stapling device and
check of homeostasis.
Closure
• No closure required.
• Anal pack not usually required.
• LA to conventional haemorrhoidectomy wounds.
Post-operative care and instructions
• Laxatives. If prone to constipation.
• Antibiotics. No proven benefi t in reducing post-operative pain.
HAEMORRHOID SURGERY
743
Complications (specifi c to the procedure)
• Bleeding (requiring intervention), <5%.
• Painful anal wound, 2%.
• Anorectal leakage, 2–3%.
• Anal stenosis, 1% (worse in conventional haemorrhoidectomy).
(a)
(b)
Prolapsed mucous membrane
drawn into the casing
Fig. 21.4 (a) Extent of conventional haemorrhoidectomy. (b) Correct positioning
of anorectal mucosal plication and stapling device.

CHAPTER 21 Common operations
744
Pilonidal sinus excision (Bascom II)
Different options exist for excision and primary closure of a non-acutely
infected pilonidal sinus (see b p. 408)—Bascom I, Bascom II, Karayadakis,
rhomboid fl ap, rotational fl ap. Principles of all primary closure procedures
are:
• Excision/extirpation of sinus disease.
• Obliteration (elevation) of natal cleft.
• Asymmetric closure of natal cleft (scar off the midline).
Indications (typical)
Symptomatic pilonidal sinus disease with unilateral or limited bilateral sec-
ondary cutaneous openings.
Pre-theatre preparation
• Usually under GA, therefore NBM 2h and fl uids only 4h preop.
• Single dose antibiotics on induction.
Positioning and theatre set-up
• Usually prone, sometimes with slight prone fl exion at the hip. Buttocks
may initially be taped laterally to aid retraction (should be released for
fi nal mobilization and closure).
• May be performed in left or right lateral jackknife position, but
symmetrical buttock position is hampered.
Steps of surgery (Bascom II) (see Fig. 21.5)
• Asymmetrical, semi-elliptical excision of sinus pit origins.
Choice of lateral extent usually determined by the site of any •
lateral tracks.
‘Rat’s tail’ extension of cutaneous incision on the ‘short side’ to •
prevent fi nal dig ear closure.
• Excision of skin and pits only with preservation of subcutaneous fat.
• Curettage, excision, and diathermy obliteration of all sinus tracks and
lateral tracks.
• Mobilization of cutaneous skin fl ap of ‘long side’ of the incision.
• Mobilization of edge of skin fl ap of ‘short side’ of the incision.
• Scrupulous haemostasis and wound wash-out.
• Sutured closure apposition of natal cleft fat.
Closure
• Closure of skin fl aps (interrupted mattress sutures—removable
monofi lament or dissolvable subcuticular suture).
• Small calibre low pressure suction drain occasionally used for larger
fl aps.
Post-operative care and instructions
• Remove catheter unless required for post-operative fl uid balance
observation.
• Antibiotics. Only required for pathology found.
• Oral diet. Normal as soon as tolerated unless indicated by further
procedure performed.
PILONIDAL SINUS EXCISION (BASCOM II)
745
Complications (specifi c to the procedure)
• Wound infection, 5%.
• ‘Recurrent’ sinus formation (wound sinus formation or wound non-
healing), 3–5%.
• Painful scar, 2%.
(a)
(b)
Fig. 21.5 (a) Typical incision for midline pits (shown in dark grey) with extent of
skin removal (shown in light grey). (b) Fat and fl ap closure to achieve natal cleft
obliteration and para-midline scar.

CHAPTER 21 Common operations
746
Femoral embolectomy
Femoral embolectomy should only be undertaken where there are facili-
ties for further vascular surgical exploration or procedures since underly-
ing occlusive vascular disease and alternative diagnoses are common
Indications (typical)
Strongly suspected or proven acute embolic occlusion of the distal super-
fi cial femoral artery (SFA) or popliteal artery with acute critical limb
ischaemia (see b p. 642).
Pre-theatre preparation
• May be GA or, more commonly, under LA.
• Group and save and clotting required.
• Ensure consent is obtained for proceeding to other procedures if they
are anticipated.
• Single dose antibiotics on induction.
Positioning and theatre set-up
• Operating table should be X-ray compatible.
• NGT. NOT required unless the patient is vomiting or gastric
distension/surgery is likely.
Steps of surgery (see Fig. 21.6)
• Incision. Oblique groin over the common femoral artery.
• Expose and control all infl ow and outfl ow vessels (common femoral,
superfi cial femoral, and profunda femoris).
• 3 or 4 FG Fogarty catheter is passed proximally and distally, and
embolus retrieved.
• If good infl ow and good backfl ow achieved, then close with
interrupted 6/0 prolene sutures and confi rm return of distal pulses or
reperfused limb.
• Completion angiography may be undertaken on table (senior help).
• On table, thrombolysis may be required if there is residual thrombus
(senior help).
• Surgical reconstruction or bypass may be necessary if there is in
situ thrombosis on an underlying critical stenosis instead of simply
embolism (senior help).
• Consider fasciotomies (up to four compartments) if there is suspicion
of a post-operative compartment syndrome (muscles are tense or
tender or prolonged ischaemia >6h).
Closure
Subcuticular suture.
Post-operative care and instructions
• Monitor pulse, BP, distal limb perfusion, pain, and pulses closely post-
operatively.
• IV heparin infusion—24 000IU over 24h by pump and check APTT
at 4–6h and daily after. Also repeat APTT 4h after every change in
dosage, keeping APTT at 2–2.5 times the normal range.
• Start warfarinization at 48h.
FEMORAL EMBOLECTOMY
747
Complications (specifi c to the procedure)
• Haematoma formation, 5%.
• False aneurysm formation, <1%.
• Distal limb compartment syndrome.
(A)
Femoral
Popliteal
(B)
(C)
a
b
Fig. 21.6 Vessel exposure and control. (A) Incisions for femoral and popliteal
artery exposure. (B) Control of femoral artery and its branches. (C) Femoral
embolectomy and closure without and with a vein prosthetic patch.

CHAPTER 21 Common operations
748
Right hemicolectomy
Usually performed by laparoscopy although open access may be indicated
for large or locally extensive tumours, previous abdominal surgery, inabil-
ity to tolerate pneumoperitoneum. Laparoscopy may be multi- or single
port.
Indications (typical)
• Right colonic (caecal, ascending colonic, or hepatic fl exure)
adenocarcinoma (see b p. 404).
• Right colonic adenomas too extensive for endoscopic excision.
• Right colonic stricture (symptomatic).
• Right colonic Crohn’s disease (symptomatic) (see b p. 394).
Pre-theatre preparation
• GA (NBM 2h and fl uids only 4h preop).
• Group and save required.
• No bowel preparation usual for right hemicolectomy.
• Single dose antibiotics on induction.
Positioning and theatre set-up
• Urethral catheterization.
• Table positioning. Supine, well secured to allow for head up/down tilt
and lateral role.
• Monitor/stack and ports positions. Right side (see Fig. 21.7).
Steps of surgery (laparoscopic)
Dissection of right colon and ileum may be done from medial to lateral
(starting at the ileocolic mesentry) or from lateral to medial (starting
with the peritoneal attachments off the colon—one method is described
below).
• Establish pneumoperitoneum, assess access and peritoneal cavity.
• (Head up and left down roll) Open and divide right gastrocolic
omentum and divide peritoneum above the hepatic fl exure.
• Divide right paracolic peritoneum and refl ect right colon medially.
• (Head down and left down roll) Divide attachment of ileal and caecal
mesentery to pelvic brim/iliac fossa peritoneum and refl ect caecum
superiorly.
• Mobilize right colon mesentery from anterior pararenal fascia,
anterolateral duodenum, and head of pancreas.
• Isolate and divide ileocolic vessels and right colic vessels at mesenteric
root.
• Divide transverse colic and terminal ileal mesenteries.
• Divide terminal ileum and proximal transverse colon (linear stapler).
• Deliver specimen via (peri)umbilical incision and form ileocolic
anastomosis (extracorporeal).
Closure
• Mass closure to (peri)umbilical wound.
• Port sites. See laparoscopy (see b p. 730) (see Fig. 21.7).
RIGHT HEMICOLECTOMY
749
Post-operative care and instructions
Enhanced recovery programme usual. Oral fl uids day 1, light diet day 2.
Complications (specifi c to the procedure)
• Conversion to open procedure, (up to) 20%.
• Anastomotic leak, 2–5% (see b p. 420).
• Temporary stoma formation, 2% (see b p. 750).
• Intra-abdominal bleeding, 5%.
• Port site infection, <5%.
10
5
5
5
5
5
5
Fig. 21.7 Typical port sites and monitor position and extent of resection.

CHAPTER 21 Common operations
750
Stoma formation
Indications (typical) (see b p. 84).
Pre-theatre preparation
• GA if done as part of other procedures or if laparoscopically formed;
may be LA/regional if isolated loop stoma formation.
• Group and save required.
• Ideal sites for proposed stomas should be marked by stoma specialist
preoperatively unless emergency surgery.
• Single dose antibiotics.
Positioning and theatre set-up
Table positioning. Supine unless required by related operation.
Steps of surgery
Ideally, any stoma should traverse the musculo-fascial layers of the abdom-
inal wall (commonly the rectus abdominis); incisions in the fascia placed
vertically with muscle fi bres splayed apart (but not divided unless where
necessary) to allow the stoma adequate space without ‘pinching’, but
reduce the longer-term risk of herniation (a major problem especially in
long-term stomas).
Loop ileostomy formation
• Ensuring proximal and distal limbs are identifi ed and orientated (if
done as an isolated procedure via a trephine incision, this is diffi cult;
the only reliable indicator is the antemesenteric fat ‘stripe’ leading onto
the surface of the caecum; much more reliably done laparoscopically
for direct visualization of each limb). Done before creating defect if
laparoscopic procedure.
• Opening of the antemesenteric wall of the loop (slightly oblique
towards the distal limb).
• Placement of sutures (untied) to ‘spout’ the proximal limb (see Fig.
21.8).
• Placement and tying of sutures across the posterior wall of the loop
and around the distal limb.
• Tying the proximal limb sutures and spouting the proximal limb.
A bridge is almost never necessary.
Closure
Port sites. As per laparoscopy.
Post-operative care and instructions
Oral diet. Normal as soon as tolerated unless indicated by further proce-
dure performed.
Complications (specifi c to the stoma formation)
• Stomal oedema, ~30% (common and usually requires no treatment).
• Stomal ischaemia, <5% (serious and requires prompt treatment).
• Intestinal obstruction (due to tight musculo-fascial opening), 5%.
STOMA FORMATION
751
• Stoma retraction (usually due to inadequate mobilization), <5%.
• Stoma prolapse, <5%.
• Stomal/parastomal hernias (rare acutely), up to 50% long term.
Fig. 21.8 Suture placement for spouting of proximal limb of loop ileostomy.

CHAPTER 21 Common operations
752
Wide local excision—breast
Indications (typical)
Carcinoma of the breast. Preferred to mastectomy for all, but large,
tumours in small breasts, multifocal carcinomas, or those with multifocal
DCIS and central tumours.
Pre-theatre preparation
• Always GA, therefore NBM 2h and fl uids only 4h preop.
• Group and save required.
• Consent may be required for additional procedures (such as axillary
node surgery), especially if sentinel node detection is being used.
Positioning and theatre set-up
• Radiography. Ensure mammograms or other radiographs are available.
• Table positioning. Supine with arm extension to allow access to the
axilla.
Steps of surgery
• Skin incision according to breast folds and skin lines. Skin excision only
required for superfi cial tumours with skin changes.
• Dissection carried out in a column or wide sphere of normal breast
tissue margin down to fascia of pectoralis major.
• Haemostasis. Scrupulous care required.
Closure
Dissolvable sutures to fascia and skin.
Post-operative care and instructions
• The specimen requires orientation with marking (by sutures or clips)
to mark three out of the six ‘surfaces’: anterior, inferior, and medial
surfaces—‘AIM’.
• Drains not usually required but if so, remove once output.
Complications (specifi c to the procedure)
Breast haematoma, 5%.
This page intentionally left blank

CHAPTER 21 Common operations
754
Below knee amputation
Indications (typical)
• Unreconstructible peripheral vascular disease of distal limb with critical
ischaemia (involving vessels below the popliteal artery).
• Acute unsalvageable distal limb ischaemia (involving vessels below the
popliteal artery).
• Unreconstructible trauma below the mid-tibia.
• Tumours of the soft tissue or bone of the distal limb (some).
• Unreconstructible congenital deformities of the foot with
complications.
Pre-theatre preparation
• May be GA; spinal or regional common in vascular disease.
• Group and save required, may need cross-match for 2U.
Positioning and theatre set-up
• Table positioning. Supine.
• Ensure angiograms/limb CT scans available if appropriate.
Steps of surgery (see Fig. 21.9)
Two approaches are commonly used—‘long posterior fl ap’ and ‘skewed
symmetrical fl aps’. ‘Long posterior’ fl ap is more common, especially in
ischaemia.
Long posterior fl ap
• Mark fl aps. Anterior fl ap slightly convex 10cm (in adults) below tibial
tuberosity, just over hemicircumference of the calf skin. Posterior fl ap
at least twice the length of the anterior fl ap. Opening of skin to fascia.
• Opening and division of fascia, then muscles of anterior and lateral
compartments with diathermy.
• Identify anterior tibial neurovascular bundle; separate vessels and
ligate; divide nerve under tension (promotes nerve retraction). Same
for peroneal bundle.
• Fibular, then tibial divisions (fl exible, wire, or powered saw); division of
fi bula high; bevel front of tibia at 45°.
• Striping of posterior compartment muscles off tibia and interosseous
septum below level of the division to the extent of the posterior fl ap.
• Identify posterior tibial neurovascular bundle; separate vessels and
ligate; divide nerve under tension (promotes nerve retraction).
• Trimming soleus and gastrocnemius to reduce bulk of posterior fl ap to
allow comfortable coverage of bony stump.
• Suturing of muscle/fascia to tibial peritosteum.
Closure
• Skin and fascia. interruptible sutures.
• Drain usually placed deep to myocutaneous fl ap.
Post-operative care and instructions
Early physiotherapy to knee joint to promote range of movements.
BELOW KNEE AMPUTATION
755
Complications (specifi c to the procedure)
• Flap necrosis, <5%.
• Haematoma.
Skew flap
10–15cm
2cm
2cm
1/4 circumference
1/3 circumference
2/3 circumference
Burgess flap ‘Rule of thirds’
10–12cm
Fig. 21.9 Flaps used of below knee amputation. (a) Long posterior fl ap.
(b) Skewed symmetrical fl aps.
This page intentionally left blank

CHAPTER 22 Eponymous terms and rarities
758
Acanthosis nigricans Pigmentation of the axillary skin associated with
breast or gastric cancer.
Achondroplasia Familial dwarfi sm in which growth of the long bones
and skull is defective.
Adenomyomatosis, gall bladder Thickening of the gall bladder wall
with occasional intramural sinuses that may partially occlude the gall blad-
der lumen.
Adiposa dolorosa Multiple lipomas, usually on the arms and trunks,
that are occasionally painful.
Adrenogenital syndrome A condition, usually autosomal recessive
inheritance, affecting 1 in 5000 to 1 in 15 000 births, characterized by
cortisol and/or aldosterone defi ciency due to an enzymatic defect in cor-
tisol synthesis, which results in secondary adrenal hyperplasia through loss
of feedback on the pituitary gland. Diversion of precursors into the syn-
thesis of other steroids, particularly androgens, results in virilization and
ambiguous genitalia (through clitoral hypertrophy) of the female fetus and
pseudoprecocious puberty in the male. Early closure of the epiphyseal
plates leads to short stature. Impaired aldosterone secretion can cause a
salt-losing state that requires replacement therapy.
Aerocele The collection of air in one or more tissue layers of the cra-
nium due to injured or infl amed cranial air sinuses.
Albers–Schönberg disease See osteopetrosis.
Albright’s hereditary osteodystrophy An X-linked form of pseu-
dohypoparathyroidism characterized by mental retardation, low serum
calcium, cataracts, and tetany. Patients tend to be of short stature and
have short fi rst, fourth, and fi fth metacarpals. Metastatic calcifi cation of
the basal ganglia is a feature.
Albright’s syndrome/polyostotic fi brous dysplasia A condition
thought to be due to disordered bony development, featuring fi brodys-
plastic bony changes, patchy skin pigmentation, and precocious puberty
in girls. Affected bones become soft and deformed from childhood
onwards.
Allen’s test This assesses the adequacy of the collateral circulation to
the hand. Digital pressure is applied to both the radial and ulnar arteries
at the wrist and the patient repeatedly clenches a fi st. Adequate collateral
supply exists if there is complete palmar fl ushing with 15s of release of
each vessel in turn.
Amastia Absence of both breast and nipple. Ninety per cent of patients
with unilateral amastia have absent or hypoplastic pectoral muscles.
Amaurosis fugax Episodes of transient blindness due to central
retinal artery embolization from carotid vessel disease/proximal vessel
atherosclerosis.
Amazia Congenital absence of breast tissue, but not the nipple. It is now
known as hypoplasia of the breast, to differentiate it from amastia.

EPONYMOUS TERMS AND RARITIES
759
Angiodysplasia Vascular lesions of unknown aetiology, most frequ-
ently found in the right colon, occasionally associated with cutaneous and
oral lesions. They occur with increasing age and present with bleeding that
may be torrential, but more often as a series of small bleeds.
Angiomyoneuroma (glomus tumour) A small, painful, benign
tumour of blood vessels, rarely larger than a few mm in size, mainly found
in the extremities. Half arise in the digits, predominantly subungally. They
are exquisitely painful and tender and appear blue-purple in colour. Treat
by excision with a wide margin.
Angiosarcoma A soft tissue tumour of young men and women, pro-
ducing a hot, bulky tumour with a tendency to bleed and metastasize to
the lungs.
Ankyloglossia Also known as tongue-tie, it is due to a short lingual
frenulum. It rarely affects speech, but frenectomy is recommended when
food control and oral hygiene are a problem.
Antibioma A hard, oedematous swelling containing sterile pus following
the treatment of an abscess with long-term antibiotics, rather than incision
and drainage.
Aortoenteric fi stula A connection between the aorta and small intes-
tine, resulting in haemorrhage that becomes increasingly frequent and may
culminate with exsanguinations; most commonly due to infection of a
prosthetic graft rather than primary spontaneous fi stula.
Apert’s syndrome Occurs in 1 in 160 000 births. Thirty per cent of
cases are autosomal dominant. Skull is ‘tower-shaped’ (oxycephaly) with
premature fusion of all the sutures. Mild face aplasia and syndactyly of
the middle three fi ngers occur. Other associations include oesophageal
atresia, renal and congenital heart anomalies.
Aphthous ulcers The most common disorder affecting the oral
mucosal membranes. Of unknown aetiology. They are painful, recurrent,
and occur most commonly in childhood, rarely in the edentulous. Large
ulcers, present for 3 months or more, may mimic carcinomas and should
be biopsied if doubt exists.
Arnold–Chiari malformation A hindbrain abnormality where the
cerebellum and medulla are found to lie below the level of the foramen
magnum. Compression of the foramen of Magendie results in obstructive
hydrocephalus in 80–90% of cases. Syringomyelia and spina bifi da are com-
monly associated.
Askanazy cell tumour/Hurtle cell adenoma, thyroid A tumour
consisting of featureless granular cells of varying size distributed in the
fi brous stroma of the thyroid. They are diffi cult to differentiate from malig-
nant tumours, but are regarded as benign.
Asplenia Absence of the spleen, associated with cardiac anomalies,
including situs inversus.
Athelia Absence of the nipple. It is exceedingly rare.

CHAPTER 22 Eponymous terms and rarities
760
Baker’s cyst A central swelling of the popliteal fossa, most evident
when the patient stands. It represents a synovial membrane diverticulum,
almost always associated with knee joint pathology such as arthritis or
torn meniscus.
Balanitis xerotica obliterans A disease of unknown aetiology char-
acterized by keratotic lesions with infl ammatory changes, leading to phi-
mosis and occasional meatal stenosis. It has the appearance of a white
stenotic band at the end of the foreskin and minor trauma often results
in haemorrhage.
Ballance’s sign Fixed dullness in the left fl ank with shifting dullness best
appreciated in the right fl ank, resulting from intraperitoneal and extraperi-
toneal bleeding following splenic rupture.
Barrett’s oesophagus The presence of columnar lined mucosa in the
anatomical oesophagus; may be due to acid or biliary refl ux. It is found in
10% of patients undergoing endoscopy for refl ux symptoms. Strictures,
ulceration, bleeding, dysplasia, and malignant transformation may occur.
Battle’s sign Bruising over the mastoid process following a base of skull
fracture that involves the petrous temporal bone.
Bazin’s disease See erytrocyanosis frigida.
Beckwith–Wiedemann syndrome A congenital defect of the ante-
rior abdominal wall associated with macroglossia, gigantism, and transient
hypoglycaemia episodes.
Bezoars Masses of ingested human hairs (trichlobezoars) or indigest-
ible vegetable matter and fi bre (bezoars) that form in the stomach and
interfere with digestion or may migrate into the small bowel and cause
intestinal obstruction.
Bier spots The presence of white patches amongst the mottled blue–
purple appearance of an acutely ischaemic limb that has been in a warm
environment for several hours.
Blind loop syndrome Malabsorption due to colonization of a blind-
ending segment of bowel by abnormal bacteria that prevent the digestion
and absorption of food. Causes include congenital abnormalities (e.g. small
bowel diverticula), strictures, or, more commonly, surgical construction of
small bowel anastomoses and loops.
Blue naevus Results when embryonic melanocyte migration from the
neural crest is arrested in the dermis.
Bochdalek hernia A posterior diaphragmatic hernia where the septum
transversum fails to unite with the intercostal part of the diaphragm. It
occurs in infants and is characterized by gross herniation of abdominal
contents and associated lung hypoplasia.
Boehaave’s syndrome Spontaneous oesophageal rupture following an
episode of intense vomiting or retching, characterized by severe upper
abdominal and chest pain, tachycardia, tachypnoea, and subcutaneous
emphysema.

EPONYMOUS TERMS AND RARITIES
761
Bornholm disease (epidemic pleurodynia) Coxsackie B4 virus
infection of pleura and peritoneum characterized by severe upper abdo-
minal and chest pain (worse on movement and respiration) associated
with dyspnoea, pleuritic pain, headache, and sore throat.
Bowen’s disease An irregular, reddish brown cutaneous plaque, occa-
sionally ulcerated and commonly found on the trunk. It is an intra-epider-
mal carcinoma in situ and may develop into squamous cell carcinoma.
Branham’s test When a pneumatic tourniquet is infl ated around the
root of a limb with a suspected arteriovenous malformation, a signifi cant
fall in the pulse rate suggests a signifi cant arteriovenous shunt.
Budd–Chiari syndrome Post-hepatic venous obstruction that may
result from spontaneous thrombosis, extrinsic compression by tumour,
or a web in the vena cava.
Buschle–Lowenstein tumour A rare benign penile ‘tumour’ caused
by human papilloma virus infection with giant tumour growth, but only
local tissue destruction; may result in urethral fi stula formation.
Cloquet’s (Callisen’s) hernia A deep femoral hernia that cannot pro-
trude from the saphenous opening as it lies deep to the femoral vessels.
Calot’s triangle An essential landmark in laparoscopic cholecystectomy
surgery. Its boundaries are the common hepatic duct, cystic duct, and
inferior border of the liver.
Campbell de Morgan spots Small, red spots that commonly occur on
the trunk in middle age and do not blanch. They are of no signifi cance.
Cancer en cuirasse Multiple malignant nodules on the chest wall in
breast cancer that mimic the breast plate on a suit of armour.
Caput medusa Engorged veins radiating from the periumbilical
region, resulting from extrahepatic portosystemic shunting from portal
hypertension.
Carbuncle Multiple, adjacent follicular infections with Staphylococcus
aureus, commonly seen in diabetics. Treat with fl ucloxacillin and surgical
drainage as required.
Cardiac myxoma A rare primary cardiac tumour, commonly arising in
the left atrium, which can present either with obstruction mimicking mitral
stenosis or tumour emboli.
Carnett’s test Determines whether an abdominal lump lies intraperi-
toneally or within the abdominal wall. The patient lies fl at and raises his
extended legs off the couch. An intraperitoneal lump disappears whereas
one in the abdominal wall persists.
Caroli’s disease An anatomical abnormality characterized by intra-
hepatic cystic changes with an increased risk of bile duct cancer.
Carr’s concretions Microscopic calculi within the papilla of the kid-
ney thought to be involved in the pathogenesis and propagation of renal
calculi.

CHAPTER 22 Eponymous terms and rarities
762
Charcot’s triad Fever, rigors, and jaundice characteristic of acute
cholangitis. Right hypochondrial pain is often an additional feature. This is
a serious and potentially fatal condition, caused by ascending infection of
the biliary tree associated with partial biliary obstruction.
Chemodectoma A carotid body tumour extending from the carotid
bifurcation that presents with a solitary or bilateral lump(s) anterior and
deep to sternocleidomastoid. Characteristically, they can be displaced lat-
erally, but not vertically, and are associated with bruits and thrills in 20%
of cases. The risk of malignancy increases with size.
Chopart’s amputation An amputation made through the tarsal
bones.
Churg–Strauss syndrome Affects young and middle-aged adults,
often with a history of atopy, asthma, and allergic rhinitis, in which there
is a marked eosinophilia. Clinical manifestations include peripheral neu-
ropathy, cardiac involvement (heart failure and myocardial infarction), and
vascular involvement that affects the stomach, small bowel, kidneys, and
CNS due to aneurysm formation, thrombosis, and infarction.
Chvostek’s sign Hyperexcitability of the facial nerve to local percussion
over the parotid gland in patients with a reduced serum calcium concen-
tration. It can also occur in 10% of normal people.
Chylothorax The accumulation of lymphatic fl uid (which can have the
appearance of pus) within the pleural cavity following thoracic duct trauma
(blunt and penetrating injuries or surgical procedures), obstruction by
malignant disease (particularly lymphomas and carcinomas of the lung and
breast), and congenital defects (usually also associated with ascites).
Codman’s triangle Radiographic evidence of periosteal elevation
found with osteosarcomas.
Contrecoup injury Injury to the brain on the opposite side of the initial
injury, due to the transmitted movements of the cerebral tissue within
the skull.
Cooper’s hernia A rare multilocular deep femoral hernia that enters
the thigh via deep investing fascia.
Corrigan’s pulse A collapsing pulse found in the presence of an arte-
riovenous fi stula.
Courvoisier’s law A palpable distended gall bladder in a jaundiced
patient is more likely to be due to malignant disease obstructing the bile
ducts than gall stones (where the gall bladder tends to be fi brotic and
contracted).
Craniocleidodysostosis An autosomal dominant disease character-
ized by partial or complete clavicular aplasia, vertebral and digital deformi-
ties, and patent fontanelles.
Craniofacial dysostosis/Crouzon’s syndrome A condition charac-
terized by stenotic cranial sutures, maxillary hypoplasia and prognathism,
beaked nose, exophthalmos, and mental retardation.

EPONYMOUS TERMS AND RARITIES
763
Crigler–Najjar syndrome Pre-hepatic jaundice due to an inability to
conjugate bilirubin within the liver. There are two types, autosomal reces-
sive (type I) and autosomal dominant (type II).
Cronkhite–Canada syndrome A triad of GI polyps, alopecia, and
fi ngernail atrophy. The changes are not neoplastic, but due to an unidenti-
fi ed defi ciency state.
Crueveilhier’s sign (saphena varix) It is positive if an impulse is felt
at the saphenofemoral junction when the patient stands and coughs.
Cullen’s sign Periumbilical bruising seen in acute severe necrotizing pan-
creatitis or other form of severe intraperitoneal bleed, i.e. ectopic preg-
nancy, abdominal trauma.
Curling’s ulcer Acute gastroduodenal ulceration associated with severe
burns.
Curtis–Fitz–Hugh syndrome Severe right hypochondrial pain due to
perihepatitis due to Chlamydia trachomatis infection.
Cushing’s ulcer Acute gastroduodenal ulceration associated with
stress, such as severe haemorrhage, myocardial infarct, multiple trauma,
and in critically ill patients.
Dandy–Walker syndrome Congenital absence of the foramen of
Magendie, resulting in marked ventricular dilatation. The lateral sinuses
appear higher than normal on X-ray because of cerebellar hemisphere
widening and the higher attachment of the tentorium cerebelli.
DeQuervain’s disease Infl ammation around the extensor pollicis
brevis and abductor pollicis longus tendons, often associated with thicken-
ing of the extensor retinaculum. This results in pain on movement of the
thumb and tenderness where the tendons cross the radial styloid.
DeQuervain’s thyroiditis Self-limiting viral infl ammation of the thy-
roid gland, which usually follows a recent upper respiratory tract infection,
characterized by giant cell infi ltration.
Dermatomyositis A condition of insidious onset characterized by
proximal muscular weakness, pain, and tenderness. There is a charac-
teristic purple skin rash that affects the cheeks and light-exposed areas.
Association with occult malignancies of the colon, lung, breast, and geni-
tourinary tract.
Desmoid tumour A locally expanding tumour of mesenchymal tissue
often found in the infra-umbilical abdominal wall muscles or intra-abdom-
inal mesenchymal tissue. It commonly affects middle-aged females and
requires wide excision. Associated with familial adenomatous polyposis.
Dietl’s crisis The passage of large volumes of urine following acute
intermittent hydronephrosis. Classically, there is ureteric colic and a pal-
pable, distended kidney. Both resolve after the passage of urine.
Dysplastic naevus (famm) syndrome Dysplastic naevi are consi-
dered precursors of malignant melanoma when there is a family history.

CHAPTER 22 Eponymous terms and rarities
764
Solitary lesions in the absence of a family history are not. All patients
should avoid excessive sunlight.
Ectopia vesicae (bladder exstrophy) Occurs in 1 in 30 000 live
births, more commonly in males. There is an open bladder and defective
anterior abdominal wall associated with separated pubic bones and penile
epispadias or bifi d clitoris. Associated with glandular metaplasia and the
risk of squamous carcinoma of the bladder remnant.
Ehlers–Danlos syndrome A rare collagen disorder characterized by
the development of saccular or dissecting aneurysms.
Emphysematous cholecystitis A rapidly progressive infection of the
gall bladder due to anaerobic organisms, characterized by air in the wall of
the gall bladder and a high risk of perforation.
Empyema—gall bladder A pus-fi lled gall bladder, resulting from
impaction of a gallstone in the neck of the gall bladder.
Encephalocele The protrusion of cranial meninges, cerebrospinal fl uid,
and brain tissue through an opening in the skull.
Epidermal naevus syndrome The presence of extensive light
brown warty lesions in association with skeletal and CNS developmental
abnormalities.
Epiplocele A hernial sac containing omentum.
Epispadias A rare condition characterized by failure of development
of the anterior wall of the lower urogenital tract, affecting the glans and
penis alone (1 in 120 000) or the whole urinary tract when it is commonly
associated with bladder exstrophy (1 in 30 000). It most commonly affects
males and is characterized by the urethra exiting from the dorsal penile
surface at varying sites.
Epithelioma of Malherbe Another term for a pilomatrixoma, a red/
white subepidermal nodule, frequently calcifi ed and found on the upper
body.
Erythrocyanosis frigida Also known as Bazin’s disease. It affects
healthy females with fat and often hairless legs. Capillary dilatation along-
side arteriolar constriction results in dusky red/purple blotches that blanch
on pressure and rapidly refi ll. They can be painful. Ulceration and persist-
ent oedema may occur in severe cases.
Erythromelalgia A condition characterized by erythema and pain in the
dependent extremities, relieved by elevation. The inappropriate release of
local vasodilators has been implicated..
Erythroplasia of Queryat A reddish brown, irregular lesion found on
the glans penis, which may ulcerate and crust. It is regarded as a carcinoma
in situ and nearly always occurs in uncircumcised patients.
Exophthalmos Proptosis (sticking out of the globe of the eye), lid
retraction, conjunctival oedema, and, in severe cases, ophthalmoplegia or
optic nerve damage. Affects 2–3% of patients with Graves’s disease.

EPONYMOUS TERMS AND RARITIES
765
Extradural haematoma The formation of a haematoma in the extra-
dural space, most commonly following a fracture of the parietal or tem-
poral bones with rupture of the middle meningeal artery or its branches
that traverses them.
FAP (familial adenomatous polyposis) Autosomal dominant syn-
drome characterized by multiple colorectal and intestinal polyps as well as
other intestinal and mesenchymal lesions.
Fallot’s tetralogy Congenital cyanotic heart disease with four features:
(1) ventriculoseptal defect, (2) pulmonary stenosis, (3) overriding aorta,
(4) right ventricular hypertrophy. The infant becomes cyanosed on exer-
tion and adopts a classical squatting position which raises their systemic
vascular resistance, thereby increasing pulmonary blood fl ow.
Felty’s syndrome An association between rheumatoid arthritis,
splenomegaly, and granulocytopenia, which may be complicated by leg
ulcers and recurrent infections.
Finkelstein’s test Used to identify cases of stenosing tenosynovitis. The
patient places his thumb in his palm and clenches a fi st. The examiner
pushes the hand into ulnar deviation and, if positive, pain is felt at the radial
styloid, radiating down the forearm.
Foster Kennedy syndrome Optic atrophy of one eye and papil-
loedema in the other, which is due to a frontal tumour blocking the sub-
arachnoid space on the ipsilateral side, but causing papilloedema on the
other side because of raised intracranial pressure.
Fournier’s gangrene A form of necrotizing fasciitis involving the peri-
neal or scrotal skin, leading to subcutaneous necrosis. Synergy appears to
occur between normal non-pathogenic organisms, leading to local vascular
thrombosis and necrosis. Associated with uncontrolled diabetes mellitus.
Frey syndrome Gustatory sweating of the cheek following accidental
or surgical trauma of the parotid region. It results from cross-regeneration
of the transected sympathetic and parasympathetic fi bres and develops
over about 12 months.
Froment’s sign Weakness of the adductor pollicis, following a high
ulnar nerve palsy, leads to compensatory overaction of the fl exor pollicis
longus (innervated by the median nerve) when the patient is asked to
squeeze a sheet of paper between thumb and index fi nger.
Galactocele A cystic lesion containing breast milk, occurring in women
who suddenly stop breastfeeding.
Gamekeeper’s thumb A sprain of the metacarpophalangeal joint of
the thumb, leading to rupture of the ulnar collateral ligament. Non-healing
leads to chronic instability and weakened pinch grip.
Gardner’s syndrome Variant of FAP, involving an association between
multiple epidermal cysts, intestinal polyposis, desmoid tumours, and
osteomas.

CHAPTER 22 Eponymous terms and rarities
766
Garrod’s pad Subcutaneous tissue thickening over the proximal
interphalangeal joints that are histologically similar to those found in
Dupuytren’s disease.
Gaucher’s disease A genetic abnormality leading to active storage of
abnormal glucocerebrosides in the spleen, resulting in massive childhood
splenomegaly.
Gilbert’s syndrome Congenitally acquired mild jaundice due to a fail-
ure of transport of bilirubin to the liver, which can be precipitated by
episodes of starvation. There is an absence of urinary bilirubin although
faecal and urinary urobilinogen levels are increased. It is of little clinical
signifi cance.
Glomus tumour See angiomyoneuroma.
Glucagonoma A tumour of the pancreatic islet cells characterized by
mid-maturity onset diabetes, an erythematous rash that tends to blister
and crust, glossitis, and raised glucagon levels.
Gluteal hernia A very rare type of hernia where visceral contents
pass through the greater sciatic notch. It is often only discovered during
laparotomy for the relief of intestinal obstruction of no obvious cause.
Rarely, swelling around the buttock and pain referred along the sciatic
nerve occur.
Grawitz tumour Adenocarcinoma of the kidney.
Grey–Turner sign Bruising in the fl anks resulting from retroperitoneal
haemorrhage (e.g. haemorrhagic pancreatitis).
Gynaecomastia The benign growth of breast tissue in males. The
breast is uniformly enlarged and soft. May be physiological (e.g. maternal
oestrogens, oestrogen-androgen imbalance of puberty), hypogonadism
(pituitary disorders, androgen blockade), neoplasms (adrenal/gonado-
trophic tumours, bronchogenic, renal cell, etc.), systemic disease (hepatic
failure, renal dialysis, hypothyroidism), and drug-induced (androgen block-
ers, oestrogens, cimetidine, spironolactone, ketoconazole, methyldopa,
metoclopramide, etc.).
Hamartoma Overgrowths of one (or more) cell type(s) normally found
within the organ from which they arise, e.g. neurofi bromas.
Hammer toe Hyperextension of the metatarsophalangeal joint and dis-
tal interphalangeal joint with fl exion of the proximal interphalangeal joint.
This can lead to the development of bursae and calluses. Frequently asso-
ciated with hallux valgus, overcrowded toes, and diabetic neuropathy.
Hand–Schüller–Christian disease Multiple visceral and lytic skeletal
lesions, characteristically also involving the skull, that are associated with
diabetes insipidus and exophthalmos.
Hangman’s fracture Traumatic disruption of the pars interarticularis
of the atlas (C2) following a hyperextension injury.
Hashimoto’s disease A diffusely enlarged, painless thyroid gland
due to lymphocyte infi ltration. Rubbery in nature and often mimicking a

EPONYMOUS TERMS AND RARITIES
767
multinodular goitre. If enlargement is asymmetrical, other causes must be
excluded. Clinically, the patient is euthyroid or mildly hyperthyroid.
Henle–Coenen sign If an arteriovenous fi stula is occluded and the dis-
tal vessels still pulsate, this indicates that the fi stula can be safely treated
by ligation.
Hereditary osteodystrophy An X-linked form of pseudohypopar-
athyroidism characterized by hypoparathyroidism, low serum calcium,
mental retardation, cataracts, and tetany. Metastatic calcifi cation of the
basal ganglion is also a feature.
Hesselbach’s hernia A rare form of external femoral hernia that enters
the thigh lateral to the deep epigastric and main femoral vessels.
Hibernoma A lipoma consisting of brown fat cells.
Hidradenitis suppurativa A chronic recurrent deep-seated skin infec-
tion of the axilla or perineum.
Housemaid’s knee Chronic bursitis of the prepatellar bursa from the
trauma of repeated kneeling (as in scrubbing fl oors).
Howship–Romberg sign Pain referred to the inner aspect of the knee
via the genicular branch of the obturator nerve, which may arise from an
obturator hernia that strangulates.
Hunner’s ulcer Stellate white ulcers within the bladder, resulting from
chronic infl ammation, that open up on distension and bleed on decom-
pression. Aetiology is unknown and women are more frequently affected.
Bladder capacity is reduced and symptoms include urinary frequency and
pain on distension, relieved by micturition.
Hydatid of Morgagni Also known as the appendix testis. Remnant of
the Müllerian duct found at the upper pole of the testis, situated in the
groove between the testis and epididymis; may undergo torsion.
Hyperhidrosis Excessive sweating of the axilla, palms, and feet, which
can be socially embarrassing and distressing.
Hyperostosis frontalis interna (Morgagni’s hyperostosis)
Increased density and projection of the frontal bones, affecting the inner
table only. Aetiology is unknown. Patients are often asymptomatic.
Hypersplenism A combination of splenomegaly, anaemia, leucopenia,
and/or thrombocytopenia with bone marrow hyperplasia. Splenectomy
may be required.
Inspissated bile syndrome Inspissation of bile in the common bile
duct during early infancy (usually from haemolysis), resulting in proximal
bile duct and gall bladder dilatation.
Insulinoma A rare tumour of pancreatic islet beta-cells, characterized by
hypoglycaemic attacks that are both unpredictable and worsen in severity
with time. Diagnosis is based on Whipple’s triad.

CHAPTER 22 Eponymous terms and rarities
768
Intraperitoneal rupture of bladder Usually traumatic in origin, from
surgical instrumentation or abdominal trauma in the presence of a full
bladder.
Jansen’s disease An inherited autosomal dominant form of metaphy-
seal dysostosis, characterized by deafness and extreme dwarfi sm.
Jefferson’s (burst) fracture Disruption of the ring of atlas (C2) fol-
lowing traumatic injury to the neck. Spinal column damage is uncommon
as the fragments tend to open outwards.
Kalokerino’s sign A fi lling defect of the fundus of the stomach that
mimics a neoplasm. It arises when part of the fundus to the left of the
cardio-oesophageal junction is about to herniate through it.
Kanavel’s sign Is due to an infected ulnar bursa. Greatest tenderness is
elicited in the transverse palmar crease on the ulnar side.
Kantor’s string sign Is indicative of Crohn’s disease. Involvement of
the terminal ileum leads to structuring of the lumen. This gives the radio-
logical appearance of a thread-like structure on barium follow-through.
Kaposi’s sarcoma Painless red-brown macules on the limbs and anal
and oral mucosa. Occasionally, they may ulcerate. They can be found in
the elderly, endemically (e.g. in Africa), and in immunosuppressed patients
(e.g. transplant patients, HIV).
Kartagener’s syndrome Bronchiectasis and sterility resulting from
abnormal ciliary action.
Kehr’s sign Left shoulder pain referred from splenic injury and rupture.
Kenaway’s sign A venous hum that is louder on inspiration (on auscul-
tation with the bell of the stethoscope below the xiphisternum), associ-
ated with splenomegaly in patients with bilharzial cirrhosis of the liver.
Keratoacanthoma See molluscum sebaceum.
Killian’s dehiscence The weak point between the cricopharyngeal and
thyropharyngeal muscles through which pharyngeal mucosa can herniate,
leading to the formation of a pharyngeal pouch.
‘Kiss’ cancer Cancer implanted in one area by local contact from
another affected site, e.g. cancer of the lip, vulval labium.
Klein’s sign Right iliac fossa pain that moves to the left when the patient
turns on to his left side. It can be associated with mesenteric lymphadenitis
and Meckel’s diverticulum.
Klippel–Trenaunay syndrome A condition of the lower limb char-
acterized by congenital varicose veins, deep vein abnormalities, bony and
soft tissue deformity, limb elongation, and capillary naevi.
Köhler’s disease Osteochondritis of the navicular bone. This can be
one of the causes of a painful limp in a child under 5y of age.
Krukenberg tumour An ovarian tumour arising from transcoelomic
spread of a primary gastric carcinoma.

EPONYMOUS TERMS AND RARITIES
769
Ladd’s bands Persistent fi brous bands between the small bowel mesen-
tery and liver, which can lead to obstruction of the second part of the
duodenum. They are commonly associated with incomplete rotation of
the bowel.
Laugier’s hernia A rare form of femoral hernia that enters the thigh
through a defect in the pectineal part of the inguinal ligament.
Li–Fraumeni syndrome An inherited predisposition to cancer thought
to be due to mutation of the p53 tumour suppressor genes.
Linitis plastica Also known as leather bottle stomach. Submucosal pro-
liferation of fi brous tissue secondary to carcinoma of the stomach leads to
gastric wall thickening and a reduction in stomach volume and plasticity.
Because it spreads readily along the mucosa plane and presents late, its
prognosis is poor.
Lipodystrophy Excessive fat deposition in the legs that may be mistaken
for oedema.
Livedo reticularis Cyanotic skin mottling due to vasospasm of the arte-
rioles with concomitant capillary dilatation.
McBurney’s point Lies one-third of the way along a line drawn from
the right anterior superior iliac spine to the umbilicus. It is the classical
point of maximal tenderness in acute appendicitis and the centre point for
the gridiron (McBurney’s) incision used in open appendicectomy.
McMurray’s test This is used to identify medial meniscal tears. With
the patient supine, the knee is fl exed and foot rotated medially and later-
ally while bringing the knee to 90* of fl exion. Discomfort or a click is noted
in the presence of a tear.
Madelung’s deformity Dorsal subluxation of the lower end of the
ulna that is congenital or traumatic in origin.
Maisonneuve’s fracture The triad of a medial malleolar fracture, spiral
fracture of the neck of fi bula, and separation of the distal tibiofi bular joint
found in severe ankle trauma.
Malgaigne’s bulges Bulges seen above the inguinal ligament in thin indi-
viduals on coughing or straining. They are variants of normal and do not
represent inguinal hernias.
Mallory–Weiss syndrome/tear Haematemesis resulting from pro-
longed violent vomiting, leading to mucosal tears in the cardia of the
stomach.
Marble bone syndrome See osteopetrosis.
Marfan’s syndrome A rare inherited collagen disorder characterized
by tall stature, arachnodactyly (webbed fi ngers), lens subluxation, and the
development of saccular and dissecting aneurysms, particularly of the tho-
racic aorta. Aortic regurgitation may occur due to aortic root dilatation.
Marion’s disease A rare cause of bladder outfl ow obstruction in young
men due to narrowing of the bladder neck.

CHAPTER 22 Eponymous terms and rarities
770
Marjolin’s ulcer A longstanding venous ulcer that fails to heal in which
squamous cell carcinoma develops.
Maydl’s hernia The presence of a double loop of bowel within the
neck of a hernia, ‘W’ in shape, where strangulation of the middle loop
can occur.
Medullary sponge kidney Is due to dilatation of the terminal col-
lecting ducts of the kidney, which predisposes to the formation of renal
calculi.
Meigs’ syndrome Ascites and pleural effusions associated with benign
ovarian tumours.
Meleney’s gangrene A form of necrotizing fasciitis mostly seen after
abdominal surgery.
Ménétrier’s disease Hypertrophy of the gastric mucosa, most typi-
cally proximally, resulting in hypochlorhydria and hypersecretion of gas-
tric juices, leading to protein loss. Patients may present with epigastric
discomfort and peripheral oedema. There is no associated increased risk
of gastric cancer.
Meralgia paraesthetica Numbness and hyperalgesia around the lat-
eral thigh following entrapment of the lateral cutaneous nerve of the thigh
as it passes beneath the inguinal ligament.
Mesentericoparietal hernia of Waldeyer An internal paraduode-
nal hernia that lies medially and inferior to the third part of the duodenum.
It may present with recurrent episodes of abdominal pain and vomiting
due to small bowel obstruction.
Meyer–Weigert’s law In patients with complete ureteric duplica-
tion, it is the lower pole ureter that refl uxes because its mucosal tunnel
through the bladder wall is shorter.
Milia Small, white, superfi cial facial spots derived from hair follicles. They
appear in newborn babies and following skin grafting and dermabrasion
and are treated by expression.
Mills’ manoeuvre When the forearm is pronated while holding the
elbow straight, pain over the common extensor origin is consistent with
extensor tenosynovitis (tennis elbow).
Milroy’s disease Lymphoedema, presenting from adulthood onwards,
resulting from congenital aplasia of the lymphatic trunk.
Mirrizi’s syndrome Obstructive jaundice resulting from impaction of
a gallstone in the cystic duct, which presses against the common hepatic
duct, causing extrinsic compression.
Molluscum contagiosum Small, pale, fi rm nodules with a characteris-
tic central depression that follow infection with the pox virus. They tend
to regress with time although they can be treated by curettage.
Molluscum sebaceum A solitary skin tumour that grows rapidly over
6–8 weeks and involutes over about 6 months to leave a residual scar. It
has the appearance of a dome-shaped lesion with a central keratin-fi lled

EPONYMOUS TERMS AND RARITIES
771
crater and can be mistaken both clinically and histologically for a well-
differentiated squamous cell carcinoma.
Mondor’s disease of the breast Superfi cial thrombophlebitis affect-
ing the veins of the breast. Initially, there may be tenderness which is fol-
lowed by fi brosis and contraction, resulting in skin dimpling.
Morgagni hernia A congenital diaphragmatic hernia that presents
in early adult life with dyspnoea or as an incidental mediastinal mass.
Abdominal contents expand into the anterior mediastinal compartment
through a persistent defect in the anterior diaphragm.
Morgagni’s syndrome See hyperostosis frontalis interna.
Murphy’s sign Pain and tenderness in the right upper quadrant directly
beneath the ninth costal upon deep inspiration while the examiner’s fi n-
gers rest over the edge of the lower thoracic margin at this point. It is due
to an infl amed gall bladder and the patient may be unable to fully inspire
because of the pain.
Myositis ossifi cans Ectopic bone formation arising within haematoma
in muscle following soft tissue injury.
Nail–patella syndrome An inherited condition characterized by radial
head subluxation, small patellae, and absent or deformed nails.
Narath’s hernia A rare form of femoral hernia that extends anterior to
the femoral artery beneath its investing fascia.
Nelson’s syndrome The presence of skin hyperpigmentation and accel-
erated growth of a pituitary tumour following bilateral adrenalectomy for
pituitary-dependent Cushing’s. It results from loss of pituitary feedback.
Nutcracker oesophagus Alternative name for diffuse oesophageal
spasm—the presence of long-duration, high-intensity peristaltic contrac-
tions in the oesophagus, which may be associated with chest pains and
dysphagia.
Obturator sign The aggravation of right iliac fossa pain upon passive
internal rotation of the right hip in patients with appendicitis where the
appendix lies adjacent to obturator internus.
Osteogenesis imperfecta An inherited collagen disorder, resulting in
fragile bones that fracture easily, blue sclera, deafness, and soft teeth.
Osteopetrosis (Albers–Schönberg disease, marble bone dis-
ease) An inherited disorder of bone, resulting in increased bone density,
fractures, and anaemia. The recessive form is less severe than the auto-
somal dominant form.
Osteopoikilosis Infantile, patchy, long bone sclerosis (found radiologi-
cally) associated with yellow skin lesions. It has autosomal dominant inher-
itance and is of no clinical signifi cance.
Oxycephaly An autosomal dominant condition characterized by sten-
otic sutures, resulting in a tower-shaped skull, prominent nose, and a lat-
eral squint. Facial deformities are also common. Mental retardation, optic
atrophy, and deafness can also occur.

CHAPTER 22 Eponymous terms and rarities
772
Painful arc syndrome Shoulder pain occurring between 70* and 110*
of abduction due to the passage of an infl amed supraspinatus tendon
between the acromion process and head of humerus. The subacromial
space is narrowest at this point, leading to impingement.
Pancreas divisum Arises when the ventral and dorsal pancreatic buds
fail to fuse during embryological development. Consequently, the main
pancreatic duct drains via an accessory ampulla. The vast majority of
patients are asymptomatic although it may be one of the causes of chronic
pancreatitis.
Panda sign Bilateral black eyes following a head injury, suggestive of a
base of skull fracture involving the anterior cranial fossa.
Parkes–Weber syndrome Bony and soft tissue limb overgrowth
resulting from multiple arteriovenous fi stulas. Lipodermatosclerosis,
ulceration, and high cardiac output failure may also occur.
Paterson–Brown Kelly syndrome The association of a pharyngeal
web with dysphagia and iron defi ciency anaemia. There is an increased risk
of post-cricoid carcinoma.
Peau d’orange Localized oedema found in breast cancer where the skin
of the breast has the pitted appearance of an orange skin.
Pendred syndrome Familial association of deafness and goitre with
peroxidase defi ciency (involved in the synthesis of thyroxine).
Phalen’s test The reproduction of discomfort and paraesthesia of the
fi ngers in the distribution of the median nerve (lateral three and half fi n-
gers) when the wrist is held in fl exion. It is due to compression of the
median nerve as it passes beneath the fl exor retinaculum through the
carpal tunnel.
Phlegmasia alba dolens A swollen, white, oedematous limb, occa-
sionally seen in patients with severe iliofemoral venous thrombosis.
Progression to phlegmasia caerulea dolens may occur.
Phlegmasia caerulea dolens A blue, swollen, oedematous limb fol-
lowing severe proximal venous thrombosis. This may progress to venous
gangrene as circulatory congestion and stasis occur.
Plagiocephaly Development of an asymmetrical skull arising from the
early closure of sutures on one side of the skull.
Plummer–Vinson syndrome See Paterson–Brown Kelly syndrome.
Pneumatosis cystoides intestinalis Gas-fi lled cysts within the intes-
tinal and mesenteric walls, most commonly affecting the small intestine.
These can be seen on plain abdominal X-rays.
Pneumaturia The passage of fl atus in urine, which can arise from a
colovesical fi stula (e.g. diverticular disease, carcinoma, infl ammatory
bowel disease) or urinary tract infection in diabetics where the glucose is
fermented by the infecting organism.
Poland’s syndrome An association between pectoral muscle abnor-
mality, absence or hypoplasia of the breast, and characteristic hand

EPONYMOUS TERMS AND RARITIES
773
deformity of hypoplasia of the middle phalanges and skin webbing
(synbrachydactyly).
Pott’s carcinoma of scrotum Squamous cell carcinoma of the scro-
tum, associated with chronic exposure of the scrotal skin to aromatic car-
cinogens in coal-derived chimney soot. Now more commonly associated
with exposure to heavy metals and mineral oils, particularly the ones used
in the cotton industry.
Pott’s disease of the spine Tuberculosis of the spine, leading to bony
destruction and vertebral collapse which leads to kyphosis and spinal
compression.
Pott’s peculiar tumour A large trichilemmal cyst, commonly occur-
ring on the scalp, which can ulcerate and resembles a squamous carcinoma
(both clinically and histologically). They are benign, but may recur after
excision. They rarely undergo malignant transformation.
Pott’s puffy tumour Osteomyelitis of the skull bones following
untreated frontal sinusitis. This results in overlying scalp infl ammation and
swelling.
Proctalgia fugax Severe recurrent rectal pain in the absence of any
organic disease. Attacks may occur at night, after bowel actions, or follow-
ing ejaculation. Anxiety is said to be an associated feature.
Pseudomyxoma peritonei Disseminated mucinous tumour within
the peritoneal cavity. Commonly due to ruptured ovarian or appendiceal
mucinous neoplasms. Locally recurrent and potentially fatal, even with
heroic surgery and chemotherapy.
Ranula A saliva-containing cyst in the fl oor of the mouth that has a soft,
bluish submucosal appearance.
Raspberry tumour A tender, granulomatous mass arising from the
posterior urethral meatus.
Redcurrant jelly stool A description attributed to the bloodstained
stool found in intussusception.
Reidel’s thyroiditis Thyroiditis characterized by a marked fi brotic reac-
tion, leading to a hard, non-tender thyroid gland. Thyroid function tends
to be normal and differentiation from malignant disease can be diffi cult as
fi ne needle aspirates tend to be acellular.
Reinke’s oedema The presence of generalized oedema of the upper
vocal cords in response to noxious stimuli.
Reiter’s syndrome The triad of polyarthritis, conjunctivitis, and ure-
thritis as a result of venereal infection, usually chlamydia. The initial attack
lasts for 4–6 weeks although some patients develop chronic symptoms.
Rendu–Osler–Weber syndrome Hereditary haemorrhagic tel-
angiectasia; a rare autosomal dominant condition characterized by the
presence of haemangiomas affecting the lips, buccal cavity, nasopharynx,
and whole GI tract. These may bleed, resulting in episodes of harmatem-
esis, haematuria, melaena, epistaxis, or anaemia that are self-limiting.

CHAPTER 22 Eponymous terms and rarities
774
Richter’s hernia A form of strangulated hernia in which only part of
the bowel lumen becomes strangulated, leading to incomplete intestinal
obstruction with ischaemia and gangrene of the strangulated part.
Rovsing’s sign Pain and tenderness in the right iliac fossa produced by
palpation of the left iliac fossa. It may be found in acute appendicitis.
Sabre tibia Occurs in late syphilis where new formation of subperiosteal
bone results in bowing.
Saint’s triad The association between cholelithiasis (gallstones), hiatus
hernia, and diverticular disease.
Scheie’s syndrome An autosomal recessive skeletal disorder associ-
ated with corneal clouding, cardiac anomalies, and epiphyseal dysplasia.
Scheuermann’s disease A disease that predominantly affects young
adolescent males where vertebral growth plates are affected by osteo-
chondrosis or aseptic necrosis, resulting in back pain and progressive
kyphosis.
Schmorl’s node A lucent area with surrounding new bone formation
in a vertebral body, resulting from extrusion of the nucleus pulposus into
the vertebral body.
Sever’s disease Osteochondritis of the posterior epiphysis of the os
calcis near the insertion of Achilles tendon.
Shoveller’s fracture A stable cervical injury resulting from fracture of
the spinous process of C7 due to either trauma or muscular contraction.
Sinding–Larsen’s disease Osteochondritis of the distal part of the
patella.
Sister Joseph’s nodule The appearance of umbilical nodules in the
presence of advanced intra-abdominal carcinoma, typically stomach, but
also large bowel, ovarian, or occasionally, breast.
Sjögren’s syndrome The presence of keratoconjunctivitis sicca,
salivary gland involvement (leading to xerostomia, i.e. dry mouth), and
rheumatoid arthritis or other mixed connective tissue disorders. Primary
Sjögren’s is characterized by the fi rst two features whereas secondary
Sjögren’s has all three.
Spigelian hernia A rare type of hernia due to defects within the inter-
nal oblique aponeurosis as it interdigitates with the anterior and posterior
rectus sheath. Peritoneum and visceral contents may herniate through
these small defects.
Stevens–Johnson syndrome Also known as erythema multiforme,
characterized by ulceration that has a characteristic target appearance
and results from drug allergies (particularly to sulphonamides and bar-
biturates), mycoplasmal infections, or idiopathically. The lesions may be
associated with conjunctivitis, tracheitis, and dysphagia.
Stewart–Treves syndrome The development of angiosarcoma in a
chronically lymphoedematous limb, in this particular case, following radical
mastectomy for breast cancer.

EPONYMOUS TERMS AND RARITIES
775
Sump syndrome Due to the collection of stones and debris in the
distal common bile duct following choledochoduodenostomy, resulting in
epigastric pain, cholangitis, and pancreatitis.
Thrombophlebitis migrans Increased coagulability of blood associ-
ated with visceral cancers, particularly adenocarcinomas.
Tietze’s disease Also known as costochondritis. Affects the costal car-
tilages, resulting in chest pain that can be reproduced by pressure to the
affected cartilages.
Tinel’s sign Transient fi nger paraesthesia that follows percussion of the
median nerve proximal to the wrist in patients with median nerve com-
pression due to carpal tunnel syndrome.
Treacher Collins syndrome Also known as mandibulofacial dysosto-
sis. An autosomal dominant condition characterized by symmetrical exter-
nal and middle ear, zygoma, and mandibular hypoplasia, parrot-beaked
nose, absence of medial lower eyelashes, dental crowding, and abnormal
palpebral fi ssures.
Trichlobeozars See beozars.
Troisier’s sign Enlargement of the left supraclavicular lymph node due
to advanced metastatic gastric carcinoma.
Trousseau’s sign Phlebothrombosis of the superfi cial leg veins associ-
ated with gastric cancer.
Umbolith An umbilical concretion of desquamated skin, which can lead
to infection.
Ureterocele A cystic dilatation of the intravesical submucosal ureter. It
is often associated with other congenital anomalies, including duplicated
ureters.
VACTERL syndrome The association of vertebral (V), anorectal
(A), cardiovascular (C), tracheo-oesophageal (TE), renal (R), and limb
(L) anomalies. Progesterone and oestrogen intake during early pregnancy
have been implicated.
Vermooten’s sign Digital rectal examination reveals a doughy, dis-
placed, or absent prostate in the presence of an intrapelvic rupture of the
prostatic urethra.
von Hippel–Lindau disease An inherited disorder characterized by
cerebellar and spinal cord haemangioblastomas, retinal angiomas, and an
increased risk of visceral cancers, particularly renal cell carcinoma.
von Recklinghausen’s disease (neurofi bromatosis type I)
Autosomal dominant inherited nodular thickening of nerve trunks, associ-
ated with patchy skin pigmentation (café-au-lait spots). Malignant trans-
formation of these neurofi bromas tends to occur only in this particular
subgroup of patients and their prognosis is poor.
von Rosen’s sign Congenital dislocation of the hip. Results in a click
when the hip is fl exed and adducted, then fl exed and abducted, as this
causes the femoral head to dislocate and relocate.

CHAPTER 22 Eponymous terms and rarities
776
Waldenström disease Necrosis of the articular cartilage of the head
of femur following a slipped femoral epiphysis. This leads to stiffness or
complete loss of movement in that hip.
Waterhouse–Friderichsen syndrome Bilateral adrenal cortical
necrosis due to septicaemia (meningococcal, pneumococcal, streptococ-
cal), haemorrhage, or burns.
Whipple’s triad Fasting hypoglycaemic attacks with blood glucose less
than 2.5mmol/L, relieved by glucose, and associated raised insulin levels.
These are characteristic of an insulinoma.
Whitaker test Can be used to assess the degree of ureteric obstruc-
tion. Saline is perfused into the kidney via a renal puncture at a rate of
10mL/min and the pressure gradient measured across the ureteric–vesical
junction. Ureteric obstruction is indicated by a pressure difference greater
than 20cmH
2
O. Less than 15cmH
2
O is normal.
Youssef ’s syndrome A ureterovesical fi stula that presents with
monthly episodes of haematuria.
Zenker’s diverticulum A pharyngeal pouch that occurs though the
dehiscence of Killian, between cricopharyngeus and inferior constrictors
of the pharynx. There is usually a history of food sticking and regurgita-
tion. Progressive weight loss, dysphagia, and aspiration pneumonia can also
occur.
Zollinger–Ellison syndrome Intractable duodenal ulceration due to
elevated levels of circulating gastrin levels. There is an association with
men type I. Diagnosis is confi rmed by acid secretion tests which show
elevated resting levels. Secretin challenge elevates gastrin levels in G-cell
hyperplasia, but not G-cell tumours.

KEY REVISION POINTS
777
Anatomy and physiology
key revision points index
Chapter 2
Anatomy of the breast 31
Pelvic anatomy 39
Physiology of blood groups 97
Physiology of coronary blood fl ow 107
Monitoring/measuring lung function 109
Physiology of diuretics 113
Physiology of haemostasis 119
Chapter 3
The four stages of wound healing 146
Chapter 4
Anatomy of the lower pharynx and larynx 187
Venous drainage of the upper limb 193
Anatomy of the internal jugular vein 199
Anatomy of the rectum 217
Chapter 5
Anatomy and physiology of the salivary glands 227
Chapter 6
Anatomy of the thyroid gland 257
Anatomy of the adrenal gland 269
Chapter 7
Anatomy and physiology of the oesophagus 275
Anatomy and physiology of the stomach 287
Anatomy and physiology of the small instestine 289
Anatomy of appendicectomy 299
Chapter 8
Physiology of bile 312
Anatomy of the portal circulation 323
Chapter 9
General considerations in assessing a patient
with a hernia 337
Anatomy of the inguinal canal 339
Anatomy of the femoral canal 341

KEY REVISION POINTS
778
Chapter 10
Anatomy of the prostate 363
Anatomy of the kidney 375
Chapter 11
Colorectal resections 397
Anorectal physiology 407
Anatomy of the large bowel 409
Anatomy of the anus 413
Chapter 17
Coronary artery anatomy 621
Anatomy of heart valves 629
Mediastinal anatomy 639
Chapter 18
Anatomy of the thoracic outlet 645

779
Index
A
ABCDEs 478–9
abdomen
acute emergencies in
children 426–7
acute pain 302–4, 303
distension 28, 426
evaluation 34–5
gynaecological causes of
pain 306–7
intra-abdominal abscess
308, 420, 421
investigations 36–7
quadrants 35
trauma 482–3
abdominal aortic aneurysm
652–3
ruptured 654–5
screening 164
abdominal fl aps 618
abdominal wall 335–51,
440–1, 440–55
ABO antibodies 97
ABO antigens 97
above the knee amputation
662
abscess 176–7
absolute risk reduction 7
acanthosis nigricans 758
acetabular fracture 534
achalasia 274–5
achondroplasia 758
acidaemia 94
acid–base balance 94–5
acid burns 607
acinic cell carcinoma 230
acromioclavicular joint
dislocation 526
actuarial survival 9
acute abdominal pain 302–4
acute anorectal angle 217
acute anorectal pain 414–15
acute appendicitis 298–9
acute cholecystitis 316
acute diverticulitis 404
acute haematemesis 294–5
acute haematogenous
osteomyelitis 558–9
acute haemolytic reaction 98
acute idiopathic penoscrotal
oedema of childhood
367
acute infective colitis 396,
397
acute infl ammation 144–5
acute limb ischaemia 642–3
acute lung injury 138–9
acute lymphadenitis 714
acute pancreatitis 143,
332–4
acute parotitis 228–9
acute peritonitis 300–1
acute rectal bleeding
416–17
acute rejection 676–7, 678
acute thyrotoxic crisis
258–9
acute upper gastrointestinal
perforation 296–7
acute urinary retention 113,
386–7
acute variceal haemorrhage
330–1
adaptive immunity 677
Addisonian crisis 53
adenocarcinoma
bladder 376
gall bladder 328
kidney 374–5
lung 632
oesophagus 282
prostate 378–9
salivary gland 230–1
small bowel 292
adenoid cystic carcinoma
230
adenolymphoma 230
adenomatous polyps 398–9
adenomyomatosis, gall
bladder 758
adiposa dolorosa 758
administration 5
adrenal gland 269
adrenaline 132
adrenogenital syndrome
758
Adson’s test 644
advanced polypectomy 403
advanced trauma life
support 478
advance statements 15
aerocele 758
AIDS 637
Albers–Schönberg disease
771
Albright’s hereditary
osteodystrophy 758
Albright’s syndrome
758
albumin 66, 93
alcohol withdrawal 116
aldosterone-producing
adenomas 266
alemtuzumab 680–99
alkalaemia 94
alkali burns 607
alkaline phosphatase 171
Allen’s test 197, 644, 758
allergic reactions 98
allo- 677
alloantigen 677
allogenicity 677
allograft 594
allorecognition 676, 677
alpha-fetoprotein (AFP) 171
α-haemolytic streptococci
174
Altmeier’s perineal rectal
resection 407
amastia 758
amaurosis fugax 658, 758
amazia 758
amniotic fl uid embolism
155
amoebiasis/amoebic liver
abscess 706–7
AMPLE history 479
ampullary carcinoma 328
amputation 662–3, 754–5
anaemia 182–3, 708–9
anaerobes 175
anaesthetic premedication
71
anaphylactic shock 100
anaphylaxis 98
anastomotic leakage 420–1
aneurysms 652–3
angiodysplasia 417, 759
angiography 41
angiomyoneuroma 759
angiosarcoma 759
liver 329
animal bites 612
anion gap 94
ankle fracture 544–5
ankyloglossia 759
anorectal malformation 439
anorectal pain 414–15
anorectal physiology 407
anoscopy 38
anterior cord syndrome 556
antibioma 759
antibiotic prophylaxis 72, 73
antibody 677
antiemetics 115
antifi brinolytics 97
antigen 677

INDEX
780
antigen-presenting cells
(APC) 677
antiplatelets 73
anti-rhesus antibodies 97
antisepsis 78
antithrombin III 180
anti-thymocyte globulin
680–99
anus anatomy 413
aortic disruption 481
aortic regurgitation 54,
628–9
aortic stenosis 54, 628
aortoenteric fi stula 674, 759
APACHE III 122
APC 677
Apert’s syndrome 759
aphthous ulcers 759
apnoea test 685–99
apophysis 474
apoptosis 143
appendicectomy 299,
736–7
appendicitis 298–9
appendix testis 767
aprotinin 97
Arnold–Chiari malformation
759
arrhythmias 55–6
arterial hypertension 55
arterial monitoring 128, 129
arterial puncture and lines
196–7
arterial ulcers 149
arteriovenous
malformations 656–7
arthritis
osteoarthritis 568–9
septic 562–3
arthroplasty 586–7
ascariasis 718
Askanazy cell tumour 759
aspiration cytology
breast 31
neck 32–3
aspirin 73, 180
asplenia 759
assisted spontaneous
breathing 130
asthma 59
athelia 759
atherosclerosis 152
atrial fi brillation 620
DC cardioversion 188
atropine 133
audit 12–13
auscultation
abdomen 35
neck 32
peripheral vascular
disease 40
autograft 594
autologous blood
transfusion 96
average 6
AVPU 479
axillary hyperhidrosis 645
axillary node sampling and
clearance 242
axontemesis 564
azathioprine 680–99
B
back pain 578–80
backwash ileitis 392
bad news 4
Baker’s cyst 760
balanitis xerotica obliterans
364, 757–6
Ballance’s sign 760
bariatric surgery 290–1
barium enema 37
Barlow test 458
Barrett’s oesophagus 280,
760
Barton’s fracture 511
basal cell carcinoma 602–3
Bascom II procedure
744–5
base defi cit 94
base excess 94
basilic vein 193
basiliximab 680–99
battery 14
Battle’s sign 760
Bazin’s disease 764
B-cell receptors 676
B-cells 677
Beckwith–Wiedemann
syndrome 440–55, 760
belatacept 680–99
bell-shaped curve 6
below knee amputation 662,
663, 754–5
benign prostatic hyperplasia
362–3
Bennett’s fracture
dislocation 504
benzodiazepine
premedication 71
β-haemolytic streptococci
174
beta-human chorionic
gonadotrophin (β-HCG)
171
bezoars 760
Bier spots 760
bile 312, 316
bilharziasis 712–13
biliary colic 316–17
biliary tree cancer 328–9
bilirubin 312
biphasic defi brillators 190
biphasic positive pressure
ventilation (BiPAP) 131
bites 612
bitumen burns 608
BK virus 679
bladder
cancer 376–7
exstrophy 764
intraperitoneal rupture 768
bleeding 180–1, 620
bleeding per rectum 29
blind loop syndrome 760
blood-borne infection 99,
178–9
blood grouping 97
blood groups 97
blood products 96–7
blood tests, post-operative
88
blood transfusion 96
blue naevus 760
blunt cardiac injury 481
Bochdalek hernia 760
body mass index (BMI) 66
body surface area 631
Boehaave’s syndrome 760
bone healing 494–7
bone remodelling 495
bone tumours 574–7
Bornholm’s disease 248, 761
bowel habits 29
bowel preparation 70–1
bowel sounds 35
Bowen’s disease 761
boxer’s fracture 504–5
brachial plexus injuries
566–7
brain death 16
brainstem-dead organ
donors 19, 685–6
brainstem death 16, 685–99
brainstem refl exes 685–99
branchial cyst, sinus and
fi stula 224–5
Branham’s test 761
breaking bad news 4
breast
abscess 248, 249
acute pain 248–9
augmentation 617
cancer 240–3
cysts 246
evaluation 30–1
fi broadenoma 246
fi brocystic disease 246,
248, 249
infections 246
lumps 240
Mondor’s disease 771
reconstruction 618
reduction 616
screening 164–5, 244–5

INDEX
781
traumatic fat necrosis 247
wide local excision 752
Brodie’s abscess 561
Brown–Sequard syndrome
556
Budd–Chiari syndrome 761
Buerger’s disease 644
Buerger’s test 40
bunions 584–5
bupivacaine 218
burns 604–8
Buschle–Lowenstein tumour
761
Buscopan
®
71
C
CA 19–9 171
calcaneum fracture 546–7
calcineurin inhibitors 680–99
calcitonin 171
calcium stones 358
Callisen’s hernia 761
callus 494–5
Calot’s triangle 734, 761
Campath 680–99
Campbell de Morgan spots
761
cancer
biliary tree 328–9
bladder 376–7
breast 240–3
colorectal 399, 400–1
en cuirasse 761
gall bladder 328–9
head and neck 232–4, 234
kidney 374–5
liver 328–9
lung 632–3
pancreatic 326–7
penis 380–1
prostate 378–9
risk factors 163
screening 164–6
skin 602–3
see also tumours
capillary ischaemia 159
caput medusa 761
carbohydrate loading 68
carbuncle 176, 761
carcinoembryonic antigen
(CEA) 171
carcinogenesis 162, 163
carcinogens 163
carcinoid syndrome 293
carcinoid tumour, small
bowel 292
cardiac arrhythmias 55–6
cardiac complications 106–7
cardiac emergencies 620
cardiac index 630, 631
cardiac myxoma 761
cardiac output 630, 631
cardiac tamponade 481, 530,
638–9
cardiac transplantation 682,
690–1
cardiogenic shock 101
cardioplegia 623
cardiopulmonary bypass
622–3
cardiothoracic ICU 630–1
cardiothoracic surgery
619–39
cardioversion 188–9
Carnett’s test 761
Caroli’s disease 761
carotid aneurysm 653
carotid bruits 658
carotid disease 658–9
carotid endarterectomy 659
carpal dislocation 509
carpal tunnel syndrome
570–1
carpometacarpal fracture
dislocation 509
Carr’s concretions 761
case control study 10
caseous necrosis 142
case presentation 27
case series 10
cast bracing 498
casts 498
catamenial pneumothorax
637
Catterall classifi cation 469
cauda equina 580
CD 677
cell salvage 96
cellular immunity 677
cellular injury 142–3
cellular rejection 676–7
cellular shock 100
cellulitis 176
censored data 9
central cord syndrome 556
central venous catheter
198–9
central venous pressure
128, 129, 631
cephalic vein 193
cerebrovascular accident
(stroke) 62, 116–17,
658–9
cervical cancer screening
165
cervical spinal injury 554–5
cervical spinal radiograph
550–1
cervical sympathectomy 645
Chagas’ disease 720
Charcot’s joints 149
Charcot’s triad 318, 762
chemical burns 607–8
chemodectoma 762
chest drains
clamping 91, 203
Heimlich valves 202
insertion 200–1
management 90
Portex bag 202
suction 202–3
underwater seal 202
chest infection 108–9
chest pain 106
chest X-ray, post-operative
88
Chiclero’s ear 719
Child–Pugh classifi cation
323
Child–Pugh score 697
children, see paediatrics
Child’s classifi cation 61
cholangiocarcinoma 328
cholecystectomy 317, 734–5
cholecystitis 316
cholecystoenteric fi stula 316
chondroma 575
chondrosarcoma 576
Chopart’s amputation 762
chronic cholecystitis 316
chronic infl ammation 145
chronic intestinal ischaemia
288–9
chronic leg ulcers 148–9
chronic lower limb
ischaemia 647
chronic lymphoedema 714
chronic obstructive airway
disease 637
chronic obstructive
pulmonary disease
(COPD) 59
exacerbations 109
chronic osteomyelitis 560–1
chronic pancreatitis 320–1
chronic rejection 676–7,
678–9
chronic renal impairment 60
chronic upper limb
ischaemia 644–5, 646
chronic venous insuffi ciency
669
chronotropes 133
Churg–Strauss syndrome
762
Chvostek’s sign 762
chylothorax 762
circulatory-dead organ
donors 686
circulatory support 132–3
circumcision 448–9
cirrhosis 324–5
clamps 80, 81
claudication 29
clavicle fracture 524

INDEX
782
clinical audit 12–13
clinical governance 20–1
clips 80, 81
clonal expansion 677
clonidine suppression
test 268
clopidogrel 73, 180
Cloquet’s hernia 761
Clostridium diffi cile 175,
396, 397
Clostridium perfringens 175
Clostridium tetani 175
closures 82
clot 155
club foot 471
coagulase negative
staphylococci 174
coagulation cascade 180
coagulative necrosis 142
Codman’s triangle 576,
757–76
cohort study 10
coliforms 174–5
colitis 396–7, 417
acute severe 418–19
ulcerative 392–3, 419
colliquative necrosis 142
colloid 92–3
colon
diverticular disease 404–5,
417
strictures 405
colonoscopy 36
colorectal cancer 399,
400–1
screening 165–6
colorectal polyps 398–9
colorectal resection 397
colorectal surgery 391–421
colostomy 84
colour changes 40
colour fl ow duplex 41, 667
Colton classifi cation 520
common bile duct stones
318–19
communication 2, 4–5
community acquired
infection 172
competency 2
complaints 21
computerized tomography
abdomen 36
breast 31
head injuries 486
neck 33
pelvis 39
urinary tract disease 356
confi dence intervals 9
confi dentiality 3
confusion 116
congenital talipes
equinovarus 471
congestive cardiac failure 55
Conn’s syndrome 266–7
consent 14–15
constipation 115
continuous ambulatory
peritoneal dialysis-
related peritonitis 301
continuous arteriovenous
haemodialysis with
fi ltration 135
continuous arteriovenous
haemofi ltration 135
continuous positive airway
pressure (CPAP) 130,
131
continuous venovenous
haemodialysis with
fi ltration 135
continuous venovenous
haemofi ltration 135
contraceptive pill 50
contrecoup injury 762
controlled mechanical
ventilation 130
Cooper’s hernia 762
coordination 492
coronary artery anatomy
621
coronary artery bypass
surgery 627
coronary artery disease
626–7
coroners 16–17
coronoid fractures 522
correctness 2–3
Corrigan’s pulse 628,
757–76
corticosteroids 680–99
co-stimulatory molecules
677
costochondritis (Tietze’s
disease) 248, 775
Courvoisier’s law 318, 762
craniocleidodysostosis 762
craniofacial dysostosis 762
craniotomy 82
creatinine (clearance) 356
cremation forms 17
cricoid pressure 187
cricothyroidotomy 206–7
Crigler–Najjar syndrome
312, 763
critical appraisal 10–1
critical care 124–5
critical illness myopathy 66
critical incident reporting 21
critical limb ischaemia 650–1
Crohn’s disease 394–5
Cronkhite–Canada
syndrome 763
cross-matching 97
Crouzon’s syndrome 762
Crueveilhier’s sign 763
cryoprecipitate 97
cryptorchidism 450
crystalloid 92
CT angiography 41
CT positron emission
tomography
breast 31
neck 33
Cullen’s sign 34, 332, 763
Curling’s ulcer 763
Curtis–Fitz–Hugh syndrome
763
Cushing’s syndrome 264–5
Cushing’s ulcer 763
cutaneous leishmaniasis 719
cystic fi brosis 637
cystic hygroma 454, 455
cystic tumours 150
cystine stones 358
cysts 150–1
cytomegalovirus 99, 679
cytoscopy 357
D
Dandy–Walker syndrome
763
day case surgery 46
DC cardioversion 188–9
death 16–17
death certifi cate 17
deep vein thrombosis 120,
668–9
defecation 29
defi brillation 190–1
delayed extravascular
haemolytic reaction 98–9
Delorme’s perineal
rectopexy 407
De Musset’s sign 628
DeQuervain’s disease 763
DeQuervain’s thyroiditis
763
dermatomyositis 763
dermoid cysts 454
desmoid tumour 763
developmental dysplasia of
hip 458–9
dexamethasone test 264–5
dextran 93
dextran 70 73
diabetic foot 660–1
diabetic surgical patient
52, 661
diabetic ulcers 149, 660
diagnostic laparoscopy
730–1
diagnostic peritoneal lavage
483
dialysis patients 60
peritonitis 301

INDEX
783
diaphragmatic rupture 481
diarrhoea 115
diastolic pressure 631
Dietl’s crisis 763
diffuse oesophageal spasm
274–5
digital subtraction
angiography 41
dimercaptosuccinate scan
357
dipyridamole 73
direct angiography 41
direct recognition 676, 677
discharge 89
disclosures 3
disinfection 78
dislocation
acromioclavicular joint 526
carpal 509
elbow 514–17, 522–3
glenohumeral joint 526–7
lunate 509
perilunate 509
PIP joint 505
shoulder 526–9
sternoclavicular joint 526
thumb 504
trans-scaphoid perilunate
509
disseminated intravascular
coagulation 118–19
distal phalanx fracture 505
distal radius and ulna
fracture 510–11
distention cysts 150
diuretics 113
diverticular disease 404–5,
417
diverticular fi stula 405
DMSA scan 357
do not resuscitate (DNR)
orders 18
dopamine 132
Doppler ultrasound 41, 667
drains
complications 83
management 90–1
types 83
uses 83
see also chest drains
ductal carcinoma in situ
242–3
Duke’s staging 401
duodenal atresia 438
duodenal ulcers 284–5
duodenum 289
Dupuytren’s disease 614–15
Durozier’s sign 628
duties of doctors 2–3
dyspepsia 28
dysphagia 28
dysplasia 160
dysplastic naevus syndrome
763–4
dyspnoea 29
dysuria 29
E
ECG
hyperkalaemia 111
hypokalaemia 111
post-operative 88
Echinococcus spp. 716
ectopia vesicae 764
ectopic pregnancy 306, 307
Edinburgh position 506
Ehlers–Danlos syndrome
764
elbow fractures and
dislocations 514–17,
518–21, 522–3
elderly 44
electrical injuries 607
electrocardiogram, see ECG
elemental burns 607
elephantiasis 714
embolism 155
emphysematous
cholecystitis 764
empyema 635, 764
encephalocele 764
endocrine disease 52–3
endocrine surgery 250
end-of-life 18–19
endoscopic mucosal
resection 403
endoscopic retrograde
cholangiopan-
creatography 273
endoscopic submucosal
dissection 403
endoscopic ultrasound 272
endoscopy
upper gastrointestinal
272–3
urinary tract 357
endotracheal intubation
186–7
endovascular aneurysm
repair 653
enhanced recovery after
surgery 68–9
enteral nutrition 136–7
enteral support 136–7
enteric fi stula 420, 421
enterococci 174
epicondyle fracture 516
epidemic pleurodynia 761
epidermal naevus syndrome
764
epididymal cyst 367
epididymo-orchitis 350, 351,
388–9
epigastric hernia 342–3,
446–7
epilepsy 62–3
epinephrine 132, 218
epiplocele 764
epispadias 764
epithelioma of Malherbe
764
Erb–Duchenne palsy 566
erectile dysfunction 355,
372–3
erythema multiforme 774
erythrocyanosis frigida 764
erythromelalgia 764
erythroplasia of Queryat
764
escharotomy 607
EuroSCORE 122
euthanasia 18
evidence 10
evidence-based surgery 6–9
Ewing’s sarcoma 577
exomphalos 440–55
exophthalmos 764
exponential growth 162
express verbal consent 15
express written consent 15
extracorporeal shock wave
lithotripsy 359
extradural haematoma 765
F
facial trauma 235–6
faecal occult blood testing
36
Fallot’s tetralogy 765
false aneurysm 652, 673
familial adenomatous
polyposis (FAP) 398,
757–76
familial hyperaldosteronism
266
famm syndrome 763–4
fasciitis 176, 177
FAST screening 483
fat embolism 155
fat necrosis 142–3
fatty streaks 152
feeding gastrostomy/
jejunostomy 66
felon 612
Felty’s syndrome 765
femoral aneurysm 653
femoral artery 196
femoral embolectomy
746–7
femoral hernia 340–1
femoral neck fracture 536–7
femoral shaft fracture 538–9
fi brinoid necrosis 142
fi brinolytic system 180

INDEX
784
fi brolamellar carcinoma of
liver 328
fi brolipoid plaques 152
fi lariasis 714
fi ne needle aspiration
biopsy, thyroid 256
fi nger pulp injury 610
Finkelstein’s test 765
fi ssure-in-ano 414–15
fi stula-in-ano 410–1
fi stulas 151
fl ail chest 480
fl aps 596–7
fl at feet 472–3
Flenley nomogram 94, 95
fl exible cytoscopy 357
fl exible sigmoidoscopy
36, 38–9
fl exor sheath infection 612
fl uid balance chart 93
fl uid depletion 64–5
fl uid management 64–5,
92–3
paediatrics 424–5
fl uid overload 99
focused abdominal
sonography for trauma
(FAST) 483
Fontaine classifi cation 647
foot
club foot (congenital talipes
equinovarus) 471
diabetic foot 660–1
fl at feet (pes planus) 472–3
fracture 546–8
forceps 80, 81
foreskin 368–9, 369, 448–9
Foster Kennedy syndrome
765
Fournier’s gangrene 175,
765
fracture dislocation
Bennett’s 504
carpometacarpal 509
elbow 522–3
tarsometatarsal (Lisfranc)
547–8
thumb 504
fractures
acetabular 534
ankle 544–5
Barton’s 511
boxer’s 504–5
calcaneum 546–7
cast bracing 498
casting 498
cervical spine 554–5
children 464–5, 514–17,
525
clavicle 524
coronoid 522
delayed union 496
describing 503
distal phalanx 505
distal radius and ulna
510–1
elbow 514–17, 518–21
epicondyle 516
external fi xation 499
facial 235–6
femoral neck 536–7
femoral shaft 538–9
foot 546–8
Friday night 504–5
Galeazzi 512
greenstick 464–5
hamate 508
hangman’s 766
healing 494–7
humeral condylar 518–19
humeral shaft 518
internal fi xation 498–9
Jefferson’s (burst) 768
lateral condyle 515–16
lateral epicondyle 516
locking plates 499–500
Maisonneuve’s 769
medial condyle 516
medial epicondyle 516
metacarpal 504–5
metaphyseal corner and
bucket handle 466–7
metatarsal 548
middle phalanx 505
Monteggia 512
non-accidental injuries
466–7
non-union 496
olecranon 516, 520
pelvis 532–5
phalangeal 548
pilon 545
plaster instructions 465
proximal humerus 525
proximal phalanx 505
radial head 520
radial head and neck 516
radius and ulnar shaft
512–13
reduction and fi xation
498–501
rib 530–1
Rolando 504
sacral 534
scaphoid 508
scapula 524
shoulder 524–5
shoveller’s 774
Smith’s 511
spine 554–5
sternum 530–1
supracondylar 514
talus 546
tarsus 546–8
thoracolumbar spine 555
thumb 504
tibial shaft 540–2
time to unite 495
ulnar styloid 511
fresh frozen plasma 97
Frey syndrome 231, 765
Friday night fracture 504–5
Froment’s sign 765
frostbite 159
fulminant severe colitis 418
furuncle 176
G
Gage’s sign 469
galactocele 765
Galeazzi fracture 512
gall bladder
adenomyomatosis 758
cancer 328–9
empyema 764
stones 316–17
gamekeeper’s thumb 765
ganglion 572
gangrene 142, 158–9
Fournier’s 175, 765
gas 158, 175
Meleney’s 175, 770
Gardner’s syndrome 765
Garrod’s pads 614, 766
Gartland system, modifi ed
514
gas embolism 155
gases, poor 620
gas gangrene 158, 175
gastric band/bypass 291
gastric tumours 286–7
gastric ulcers 284–5
gastrointestinal
complications 114–15
gastrointestinal stromal
tumours 292, 293
gastrointestinal tract
erosion 147
ulceration 147
gastro-oesophageal refl ux
disease 280–1
gastroschisis 441
gastroscopy 272
gastrostomy 84
Gaucher’s disease 766
Gaussian distribution 6
gelofusine 92
general practitioners 5
giant cell tumour 577
Gilbert’s syndrome 312,
766
Gilmore’s groin 348
GIST 292, 293
Glasgow Coma Scale 488
Glasgow Imrie criteria 333

INDEX
785
glenohumeral joint
anterior dislocation 526–7
posterior dislocation 527
glomus tumour 759
glucagonoma 766
gluteal hernia 766
goitre 250–1
golden hour 478
Gompertzian growth 162
gown and glove 79
graft infection 673
graft occlusion 673
Gram-negative rods 174–5
granulation tissue 147
granuloma 145
Graves’s disease 252, 253
Grawitz tumour 766
great toe 584–5
greenstick fracture 464–5
Grey–Turner’s sign 34,
332, 766
grip strength 66
Gritti–Stokes amputation
662
groin
acute swelling 350–1
disruption 348
guided core biopsy, breast
31
guinea worm 726
gut ischaemia 673
gynaecological pathology
306–7
gynaecomastia 766
H
haemangiomas 454, 455, 656
haematemesis 28, 294–5
haematological
complications 118–19
haematuria 29, 354, 384–5
haemodialysis 134
haemodynamic formulae
631
haemofi ltration 134–5
haemoptysis 29
haemorrhage, post-
operative 102–3
haemorrhoidectomy 742–3
haemorrhoids 412–13,
414–15
haemospermia 355
haemostasis 119, 180–1
haemothorax, massive
203, 480
hallux rigidus 585
hallux valgus 584–5
hamartoma 766
hamartomatous polyps 398
hamate fracture 508
hammer toe 766
hand
immobilization 506
infections 612
soft tissue injuries 610–11
Hand–Schüller–Christian
disease 766
hangman’s fracture 766
Hansen’s disease 724–5
Hashimoto’s disease 766–7
Hawkin’s classifi cation 546
head and neck cancer 232–4
head and neck surgery
221–38
head injuries 486–7, 488
Healthcare Commission
20–1
heart disease 54–7
heart–lung transplantation
691
heart transplantation 682,
690–1
Heimlich valves 202
Heller’s cardiomyotomy 275
hemianopia 658
Henle–Coenen sign 767
heparin 72, 180
heparin-induced
thrombocytopenia
(HIT) 118
heparin-induced
thrombocytopenia and
thrombosis (HITT) 118
hepatic artery thrombosis
696–7
hepatic impairment 60–1
hepatitis A 178
hepatitis B 178
hepatitis C 99, 178
hepatoblastoma 452–3
hepatocellular carcinoma
328, 697
hepatorenal syndrome
61, 314
hereditary non-polyposis
colorectal cancer 399
hereditary osteodystrophy
767
hernias 336–7
Bochdalek 760
Callisen’s 761
Cloquet’s 761
Cooper’s 762
epigastric 342–3, 446–7
femoral 340–1
gluteal 766
Hesselbach’s 767
hiatus 278–9
incarcerated 350–1
incisional 344–5
inguinal 338, 339, 444,
738–9
Laugier’s 769
Littre’s 346
lumbar 346
Maydl’s 770
mesentericoparietal hernia
of Waldeyer 770
Morgagni 771
Narath’s 771
obturator 346
paraumbilical 342–3
perineal 346
Richter’s 346, 774
sliding (hernia-en-glissade)
346
spigelian 346, 774
sports 348
umbilical 342–3, 446
Herring’s lateral pillar
classifi cation 468
HES preparations 93
Hesselbach’s hernia 767
Hesselbach’s triangle 339
hiatus hernia 278–9
hibernoma 767
hidradenitis suppurativa 767
high dependency unit 125
high fl ow face mask 131
hip
developmental dysplasia
458–9
total hip replacement
586–7
Hirschsprung’s disease
436–7
history taking 26–7
HIV 99, 178–9
HLA 677
Horner’s syndrome 566
hospital doctors,
communicating with 5
housemaid’s knee 767
Howship–Romberg sign 767
Hudson mask 131
human immunodefi ciency
virus 99, 178–9
human leucocyte antigen
677
humeral condylar fracture
518–19
humeral shaft fracture 518
humoral immunity 677
humoral rejection 676–7
Hunner’s ulcer 767
Hurtle cell adenoma 759
hydatid disease 716–17
hydatid of Morgagni 767
hydrocele 366, 367, 444
hydrofl uoric acid burns 607
hydronephrosis 360
hyperacute rejection 676–7
hyperaldosteronism,
primary 266–7
hypercalcaemic crisis 260

INDEX
786
hypercapnia 108
hyperhidrosis 767
hyperkalaemia 111
hyperostosis frontalis
interna 767
hyperparathyroidism 260–1,
502
hyperplastic polyps/
polyposis 398
hypersplenism 767
hyperthyroidism 251, 252
hypertrophic scars 598,
599
hypoadrenal crisis 53
hypocapnia 108
hypokalaemia 111
hypotension 620
hypothyroidism 251
hypovolaemic shock 100
hypoxia 108
I
idiopathic scrotal oedema
389, 445
ileoanal pouch 402
ileoscopy 273
ileostomy 84
ileum 289
iliac aneurysm 653
iliofemoral
lymphadenopathy 350
iliopsoas abscess 350, 351
immunosuppression 678–80,
680–99
implied consent 15
incarcerated hernia 350–1
incisional hernia 344–5
incisions 82
incomplete spinal cord
syndromes 556
incontinence 354–5
independent risk factors 9
indirect recognition 676, 677
infarction 155–6
infection
blood-borne 99, 178–9
breast 246
chest 108–9
hand 612
microbiology 172–5
neck space 237–8
opportunistic in
immunosuppression 679
respiratory tract 59
soft tissue 176–7
transfusion-related 99
urinary tract 113
wounds 104, 172–3
infective colitis 396, 397, 419
infl ammation 144–5
informed consent 14
inguinal hernia 338, 339,
444, 738–9
inguinal lymphadenopathy
380, 389
inhalation injury 604
innate immunity 677
inotropes 132
insensible urine leakage
354–5
inspissated bile syndrome
767
insulinoma 767
insulin sliding scale 52
intensive care unit 125
intercostal nerve block 220
intermittent claudication
648–9
intermittent negative
pressure ventilation 131
intermittent positive
pressure ventilation 130
internal capsular stroke 658
internal jugular vein
cannulation 198, 199
interquartile range 6
intestinal ischaemia, chronic
288–9
intestinal transit studies 37
intra-abdominal abscess 308,
420, 421
intra-aortic balloon
counterpulsation 133
intracardiac defi brillator 55–6
intravenous cannulation
194–5
intravenous urogram 356
intussusception 406, 434–5
invasive monitoring 128–9
invasive ventilation 130
iron defi ciency anaemia 182
ischaemia 155–6
capillary 159
see also limb ischaemia
ischaemic colitis 396, 397
ischaemic heart disease 54
islet cell transplantation 695
isograft 594
isoprenaline 133
isotonic saline 64
isotope bone scan 357
isotope renography 357
J
Jansen’s disease 768
jaundice 29, 312–15
common bile duct stones
318
complications 314
diagnosis 313
hepatic (hepatocellular)
312
investigations 313
liver function tests 313
pancreatic cancer 327
post-hepatic (obstructive)
312–13
pre-hepatic (haemolytic)
312
prognosis 315
surgical risks 61
treatment 314–15
Jefferson’s (burst) fracture
768
jejuno-ileal atresia 438
jejunostomy 84
jejunum 289
J pouch 402
juvenile polyps/polyposis
398
K
kala-azar 719
Kalokerino’s sign 768
Kanavel’s sign 768
Kantor’s string sign 768
Kaplan–Meier survival 9
Kaposi’s sarcoma 768
Kartagener’s syndrome 768
Kehr’s sign 768
keloid scars 598, 599
Kenaway’s sign 768
Kennedy report 13
keratoacanthoma
(molluscum sebaceum)
770–1
kidney
adenocarcinoma 374–5
anatomy 375
medullary sponge 770
nephroblastoma (Wilm’s
tumour) 452
palpation 35
transplantation 682, 692–3
Killian’s dehiscence 276, 768
kiss cancer 768
Klatskin tumour 328
Klein’s line 460, 461
Klein’s sign 768
Klippel–Trenaunay
syndrome 768
Klumpke’s palsy 566
knots 86–7
Köhler’s disease 768
Krukenberg tumour 768
L
laboratory request forms 5
lactational mastitis 246
lactic dehydrogenase (LDH)
171
Ladd’s bands 769

INDEX
787
laparoscopic and
endoscopic single site
surgery (LESS) 403
laparoscopy, diagnostic 730–1
laparotomy 82, 732–3
abdominal trauma 483
large bowl anatomy 409
laser therapy for scars 598
lateral condyle fracture
515–16
lateral epicondyle fracture
516
latissimus dorsi
myocutaneous fl ap 618
Laugier’s hernia 769
leather bottle stomach 769
Ledderhose’s disease 614
left-sided colitis 392, 393
Legg–Calvé–Perthes disease
468–9
leg ulcers 148–9
leishmaniasis 719
leprosy 724–5
levels of evidence 10
levothyroxine 251
libido 355
lichen sclerosus 364
lidocaine 218
Li–Fraumeni syndrome 769
ligamentous injuries, wrist
509
Light’s criteria 634–9
likelihood ratio 7
limb ischaemia
acute 642–3
chronic lower limb 647
chronic upper limb 644–5,
646
critical 650–1
limb swelling, post-vascular
surgery 673
limping child 462–3
lines of Zahn 154
linitis plastica 769
lipodermatosclerosis 148
lipodystrophy 769
Lisfranc fracture dislocation
547–8
Littre’s hernia 346
livedo reticularis 769
liver
amoebic liver abscess
706–7
cancer 328–9
cirrhosis 324–5
palpation 35
surgery in patients with
liver disease 60–1
transplantation 682, 696–7
living wills 15
local anaesthesia 218–19,
220
locking plates 499–500
loop ileostomy 750, 751
low anterior section 402
low back pain 578–80
lower urinary tract
symptoms 354
low fl ow face mask 131
low molecular weight
heparin 72
lumbar hernia 346
lumps, assessing and
describing 42
lunate dislocation 509
Lund and Browder chart
605
lung
acute lung injury 138–9
cancer 632–3
function 109
transplantation 682, 691
lunotriquetral ligament
injury 509
lymphadenitis, acute 714
lymphadenopathy
acute iliofemoral 350
inguinal 380, 389
neck 454
lymphangitis 176
lymphocoele 673
lymphoedema, chronic 714
lymphoma, small bowel
292, 293
M
McBurney’s point 298, 769
McMurray’s test 769
Madelung’s deformity 769
madura foot 728
MAG3 renogram 57
magnetic resonance
angiography 41
magnetic resonance imaging
abdomen 36–7
breast 31
neck 33
pelvis 39
urinary tract disease 357
Maisonneuve’s fracture 769
major histocompatibility
complex 677
major trauma 477–88
abdomen 482–3
advanced trauma life
support 478
golden hour 478
head 486–7, 488
management 478–9
prehospital care 478
primary survey 478–9
secondary survey 479
thorax 480–1
vascular 484–5
malaria 710–1
Malgaigne’s bulges 769
mallet fi nger 505, 610
Mallory–Weiss syndrome/
tear 294, 769
malnutrition 66
malrotation and volvulus
432–3
mammalian target of
rapamycin (mTOR)
inhibitors 680–99
mammary duct ectasia 246
mammography 30, 164–5
mandatory minute
ventilation 130
mandibulofacial dysostosis
775
marble bone disease 771
Marfan’s syndrome 769
Marion’s disease 769
Marjolin’s ulcer 770
Marsden staging 382
Mason classifi cation 520
massive effusion 203
massive haemothorax
203, 480
massive transfusion 99
mastectomy 242
mastitis 246, 248
Maydl’s hernia 770
mean 6
mean arterial pressure
630, 631
mechanical back pain 578
mechanical heart valves
54–5, 629
meconium, failure to pass 426
meconium ileus 438
medial condyle fracture 516
medial epicondyle fracture
516
median 6
median nerve compression
test 571
mediastinal disease 638–9
medical notes 26–7
medullary sponge kidney 770
Meigs’ syndrome 770
melaena 294
melanoma 602–3
Meleney’s gangrene 175, 770
Ménétrier’s disease 770
mental incapacity 15
meralgia paraesthetica 770
mesenteric angina 288
mesentericoparietal hernia
of Waldeyer 770
meta-analysis 10
metabolic acidosis 94–5
metabolic alkalosis 95
metacarpal injury 504–7

INDEX
788
metaphyseal corner and
bucket handle fracture
466–7
metaplasia 160
metastasis 161
metatarsal fracture 548
methicillin-resistant S.
aureus 174
Meyer–Weigert’s law 770
MHC 677
microbiology 172–5
middle phalanx fracture
505
Milan criteria 697
Milch classifi cation 515
milia 770
Mills’ manoeuvre 770
milrinone 132
Milroy’s disease 770
minimally invasive surgery
46–7
colorectal 403
parathyroidectomy 261
Mirizzi syndrome 316, 770
miscarriage 450
MIST information 478
mitral regurgitation 54, 628
mitral stenosis 54, 628
mitral valve repair 629
molluscum contagiosum 770
molluscum sebaceum
770–1
Mondor’s disease of the
breast 771
Monteggia fracture 512
Morgagni hernia 771
Morgagni’s hyperostosis 767
motor development 470
MRSA 174
mTOR inhibitors 680–99
mucocele 317
mucocutaneous
leishmaniasis 719
mucoepidermoid tumour
230–1
multiple endocrine neoplasia
(MEN-1/2) 262–3
multiple organ dysfunction
syndrome (MODS)
138–9
multivariate analysis 9
Murphy’s sign 316, 771
myasthenia gravis 63
mycetoma 728
mycophenolic acid 680–99
mycotic aneurysm 652
mycotic osteomyelitis 561
myocardial contusion 481
myocardial infarction 54,
107, 152
myocardial ischaemia
106–7
myositis 176, 177
myositis ossifi cans 771
N
nailbed injury 610
nail–patella syndrome 771
Narath’s hernia 771
nasal prongs 131
nasogastric feeding 66
nasogastric tubes 208–9
nasojejunal feeding 66
National Confi dential
Enquiry into Patient
Outcomes and Death
(NCEPOD) 21
National Institute for Health
and Clinical Excellence
(NICE) 20
National Patient and User
Survey 21
National Service
Frameworks 20
nausea and vomiting 115
neck
dissections 233
evaluation 32–3
lymph nodes 234
neck space infections 237–8
post-thyroid surgery
bleeding 258
swellings 255, 454–5
triangle of 33
necrosis 142–3
necrotizing enterocolitis
442–3
necrotizing fasciitis 175
needle cricothyroidotomy
206
needles 86
needle thoracentesis 200
Neer classifi cation 525
negligence 14
Nelson’s syndrome 771
neoplasia 160
neoplasm 160
nephroblastoma 452
nerve root entrapment 578
neuroblastoma 452
neuroendocrine tumours 632
neurofi bromatosis type I 775
neurogenic shock 555
neurological complications
116–17
neurological disease 62–3
neuropraxia 564
neurotemesis 564
neutropenic colitis 396,
397, 419
NICE 20
95% confi dence intervals
8–9
nipples
breast cancer 240
discharge 30
inspection 30
reconstruction 618
Nissen’s operation 281
non-accidental injury 466–7,
608
non-haemolytic febrile
reaction 98
non-invasive intermittent
positive pressure
ventilation 131
non-invasive ventilation 131
non-ossifying fi broma 575–6
non-seminomatous germ
cell tumour 382, 383
non-small cell lung cancer
632
noradrenaline
(norepinephrine) 133
normal body fl ora 174–5
normal distribution 6
normal saline 64
nosocomial infection 172
null hypothesis 8–9
number needed to treat 7
nurses, communicating
with 5
nutcracker oesophagus 771
nutrition 66–7, 68, 136–7
O
obesity surgery 290–1
obturator hernia 346
obturator sign 771
occupational infection 173
odds ratio 7
oesophageal achalasia 274–5
oesophageal atresia 428–9
oesophageal intubation
186–7
oesophageal metaplasia 280
oesophageal motility
disorders 274–5
oesophageal refl ux 28
oesophageal stricture 280
oesophageal tumours 282–3
oesophagitis 280
oesophago-gastro-
duodenoscopy 272
oesophagus
anatomy 275
Barrett’s 280, 760
diffuse spasm 274–5
nutcracker 771
oestrogen-containing
contraceptive pills 50
olecranon fracture 516, 520
oliguria 112–13
omphalocele 440–55

INDEX
789
oncogenes 162
open pneumothorax 480
operations, describing 24
opportunistic infections 679
oral contraceptives 50
orchidopexy 451
organ donation
deceased donors 19,
4–99
living donors 687–8
organ transplantation, see
transplantation
orthopaedics 489–588
joint examination 490–1
limb and trunk
examination 492–3
paediatrics 457–75
skeletal radiographs
502–3
orthopnoea 29
Ortolani sign 458
Osgood–Schlatter’s disease
474
osteitis deformans 582–3
osteoarthrosis
(osteoarthritis) 568–9
osteochondritides 474–5
osteochondritis dissecans
475
osteochondroma 575
osteoclastoma 577
osteodystrophy, hereditary
767
osteogenesis imperfecta 771
osteoid osteoma 575
osteomalacia 502
osteomyelitis
acute haematogenous
558–9
chronic 560–1
osteopetrosis 771
osteopoikilosis 771
osteoporosis 502
osteosarcoma 576
ovarian cyst 306, 307
oxycephaly 771
oxygenation 126
P
pacemakers 55–6
paediatrics
abdominal distension 426
abdominal wall defects
440–55
acute abdominal
emergencies 426–7
anorectal malformation
439
basic physiological
parameters 425
circumcision 448–9
club foot (congenital
talipes equinovarus)
471
consent 15
cryptorchidism 450
cystic hygroma 454, 455
dermoid cysts 454
developmental dysplasia of
hip 458–9
duodenal atresia 438
epigastric hernia 446–7
exomphalos 440–55
failure to pass meconium
426
fl at feet (pes planus)
472–3
fl uid management 424–5
foreskin 448–9
fractures 464–5, 465,
514–17, 525
gastroschisis 441
haemangiomas 454, 455
hepatoblastoma 452–3
Hirschsprung’s disease
436–7
hydrocele 444
idiopathic scrotal oedema
445
inguinal hernia 444
intestinal obstruction
438–9
intussusception 434–5
jejuno-ileal atresia 438
Legg–Calvé–Perthes
disease 468–9
limping 462–3
lymphadenopathy 454
malrotation and volvulus
432–3
meconium ileus 438
motor development 470
neck swellings 454–5
nectrotizing enterocolitis
442–3
nephroblastoma (Wilm’s
tumour) 452
neuroblastoma 452
non-accidental injury
466–7, 608
oesophageal atresia
428–9
omphalocele 440–55
osteochondritides 474–5
phimosis 448–9
prepuce 448–9
principles of management
424–5, 5
pyloric stenosis 430–55
rhabdomyosarcoma 452
salivary gland swellings 454
scrotal swellings 444–5
sebaceous cysts 454
slipped upper femoral
epiphysis 460–1
solid tumours 452–3
surgery 44–5
test feed 431–55
traction apophysitis 474
umbilical hernia 446
undescended testis 450–5
varicocele 444–5
vomiting 426
Paget–Schroetter syndrome
644
Paget’s disease 582–3
pain 28
painful arc syndrome 772
palliative care 18–19
palmar space infection
612
pancolitis 392, 393
pancreas
cancer 326–7
transplantation 682,
694–5
pancreas divisum 772
pancreatitis
acute 143, 332–4
chronic 320–1
Panda sign 772
paperwork 70
paracentesis abdominis
214–15
paracolic abscess/mass 404
paralytic ileus 114
paraphimosis 368–9, 369
parasitic cysts 150
paraumbilical hernia 342–3
parenteral nutrition 66
Parkes–Weber syndrome
772
paronychia 612
parotid gland 227
parotitis 228–9
paroxysmal nocturnal
dyspnoea 29
past medical history 26–7
Paterson–Brown Kelly
syndrome 772
patients
communicating with 4
complaints from 21
preparing for theatre
70–1
Pavlik harness 459
peau d’orange 772
pelvic disease 38–9
pelvis
fracture 532–5
restorative surgery 402
Pemberton’s sign 251
Pendred syndrome 772
penis
carcinoma 380–1

INDEX
790
examination 355
Peyronie’s disease 370,
614
priapism 370–1
pentalogy of Cantrell
440–55
peptic ulcer disease 284–5
peptic ulcer perforation
740–1
percutaneous
nephrolithotomy 359
performance monitoring
20–1
perianal abscess 414–15
perianal haematoma 414–15
pericardial effusion 638–9
pericardiocentesis 204–5
pericolic abscess/mass 404
perilunate dislocation 509
perineal hernia 346
peripheral aneurysms 653
peripheral nerve injuries
564–5
peripheral parenteral
nutrition 67, 137
peripheral vascular disease
40–1, 641–74
peritoneal catheter 215
peritoneal irritation 34
peritonitis 300–1, 404,
420, 421
Perkins rules 495
pes planus 472–3
PET scan, abdomen 37
Peutz–Jeghers syndrome
398
Peyronie’s disease 370, 614
phaeochromocytoma 268–9
phalangeal injury 504–7, 548
Phalen’s test 570, 772
pharyngeal pouch 276–7
phenytoin 62
phimosis 368–9, 369, 448–9
phlebothrombosis 154
phlegmasia alba dolens
154, 772
phlegmasia cerulea dolens
154, 772
phosphorus burns 607
pigtail thoracostomy 200
pilon fracture 545
pilonidal sinus disease 408–9
pilonidal sinus excision
744–5
placental alkaline
phosphatase (PAP) 171
plagiocephaly 772
plain radiographs
abdominal 37
chest, post-operative 88
orthopaedics 502–3
spine 550–2
plasmin 180
plaster instructions 465
plastic surgery 589–618
platelets 96
pleomorphic adenoma 230
pleural effusion 634–5, 634–9
Plummer’s syndrome 252
Plummer–Vinson syndrome
772
pneumatic compression
boots 72
pneumatosis cystoides
intestinalis 772
pneumaturia 772
Pneumocystis jiroveci
(P. carinii) 679
pneumomediastinum 637
pneumonectomy, chest
drains 203
pneumothorax 480, 530–1,
636–7
chest drains 90
Poland’s syndrome 772–3
polycythaemia 184
polyostotic fi brous dysplasia
758
polyps 398–9
Ponseti casting 471
popliteal aneurysm 653
popliteal artery entrapment
648
portal hypertension 322–3
Portex bag 202
positioning patients 76–7
positive end-expiratory
pressure 130
positron emission
tomography, abdomen
37
POSSUM 122
post-declamp shock 672
post-embolization syndrome
657
posterior cord syndrome
556
post-exposure prophylaxis
179
Postgraduate Medical
Education and Training
Board (PMETB) 20
post-mortems 17
post-operative
complications
cardiac 106–7
gastrointestinal 114–15
haematological 118–19
neurological 116–17
renal 110–11
respiratory 108–9
urinary 112–13
post-operative haemorrhage
102–3
post-operative management
88–9
post-transplant
lymphoproliferative
disorder 679–80
Pott’s carcinoma of scrotum
773
Pott’s disease of spine 773
Pott’s peculiar tumour 773
Pott’s puffy tumour 773
pouch of Douglas 217
power (muscle) 492
power (statistical) 8–9
pregnancy
consent 15
prescribing drugs 49
surgery in 48–9
pregnancy tests 48
premature ejaculation 355
premedication 71
prepuce 448–9
presenting complaint 26
pressure control ventilation
130
pressure support 130
priapism 370–1
primary hyperaldosteronism
266–7
primary hyperparathyroidism
260–1
primary spontaneous
pneumothorax 636–7
primary survey 478–9
probity 2–3
procoagulants 96–7
proctalgia fugax 773
proctitis 392, 393
proctoscopy 38
professional organizations 20
progesterone-only
contraceptive pills 50
proprioception 493
prostate
adenocarcinoma 378–9
anatomy 363
benign prostatic
hyperplasia 362–3
screening 165
prostate-specifi c antigen
(PSA) 171, 356
prosthetic valves 54–5, 629
protein C 180
protein S 180
proximal humerus fracture
525
proximal interphalangeal
joint dislocation 505
proximal phalanx fracture
505
pseudocysts 150
pseudomembranous colitis
396, 397

INDEX
791
pseudomyxoma peritonei
773
pseudopolyposis 392
psoas abscess 350, 351
psychosocial back pain 578
pulmonary artery catheter
128–9
pulmonary artery pressure
129, 631
pulmonary artery wedge
pressure 631
pulmonary contusion 481
pulmonary embolism 121
pulmonary vascular
resistance 631
pulmonary vascular
resistance index 631
pulseless ventricular
tachycardia 190
pulse oximetry 109
push endoscopy 273
p-value 8–9
pyloric stenosis 430–1,
431–55
Q
Quinke’s sign 628
R
radial artery 196
radial head and neck
fracture 516
radial head fracture 520
radiation colitis 396, 397
radioactive iodine 251, 253
radius and ulnar shaft
fracture 512–13
randomized controlled trial
(RCT) 10
ranula 773
raspberry tumour 773
Raynaud’s disease 664
Raynaud’s phenomenon 664
recombinant human TSH
257
recombinant tissue
plasminogen activator 670
record keeping 26–7
rectal anatomy 217
rectal bleeding, acute
416–17
rectal prolapse 406–7,
414–15
rectus sheath haematoma
347
recurrent laryngeal nerve
injury 258
redcurrant jelly stool 773
referred back pain 578
refl exes 492–3
Regan and Morrey
classifi cation 522
regression analysis 9
regulatory T-cells 677
rehabilitation 89
Reidel’s thyroiditis 773
Reinke’s oedema 773
Reiter’s syndrome 773
rejection 676–80
relative risk 7
relative risk reduction 7
relatives, communicating
with 4
renal complications 110–11
renal failure 60, 110–11
renal impairment 60
renal support 134–5
renal transplantation 682,
692–3
Rendu–Osler–Weber
syndrome 773
reperfusion syndrome 672
reporting systems 21
respiratory acidosis 95
respiratory alkalosis 95
respiratory complications
108–9
respiratory conditions 59
respiratory failure 108
respiratory support 130–1
respiratory tract infection 59
rest pain 29
retention cysts 150
retractors 80, 81
retrobulbar bleed 235
revalidation 21
rhabdomyosarcoma 282, 452
rib fracture 530–1
Richter’s hernia 346, 774
right hemicolectomy 748–9
rigid cytoscopy 357
rigid proctoscopy 38
rigid sigmoidoscopy 38,
216–17
Ringer’s lactate solution 64
risk management 20
risk ratio 7
risk scoring 13, 122
risk stratifi cation 13
river blindness 714
Rockall score 295
Rockwood classifi cation 526
Rolando fracture 504
Romberg’s test 492
Roos test 644
Rovsing’s sign 298, 774
rule of nines 605
S
sabre tibia 774
sacral curvature 217
sacral fracture 534
Saint’s triad 774
saline, isotonic 64
salivary gland
anatomy and physiology
227
calculi 226–7
swellings 454
tumours 230–1
saphena varix 763
scalpels 80, 81
scaphoid fracture 508
scapholunate ligament
injury 509
scapula fracture 524
scars 598–9
Scheie’s syndrome 774
Scheuermann’s disease 774
schistosomiasis 712–13
Schmorl’s node 774
scissors 80, 81
screening 164–6
abdominal aortic
aneurysm 164
breast 164–5, 244–5
cervical 165
colorectal 165–6
prostate 165
scrotum
oedema 389, 445
Pott’s carcinoma 773
swellings 366–7, 444–5
scrubbing up 79
sebaceous cysts 454
secondary spontaneous
pneumomediastinum
637
secondary spontaneous
pneumothorax 637
secondary survey 479
seminoma 382, 383
Sengstaken tube 330–1
sensation 493
sensitivity 7
sentinel node biopsy 242
sepsis 138–9
septic arthritis 562–3
septic embolism 155
septic shock 100–1
sequestration dermoid
cyst 150
Sever’s disease 474, 774
sex hormones 356
sexual dysfunction 355,
372–3, 673
sharps 80, 81
shave excision 618
shock 100–1
shoulder
dislocations 526–9
fractures around 524–5
shoveller’s fracture 774

INDEX
792
sickle cell anaemia 182–3,
708
sigmoidoscopy
fl exible 36, 38–9
rigid 38, 216–17
silicone gel 598
Sinding–Larsen’s disease 774
single incision laparoscopic
surgery (SILS) 403
sinuses 151
SIRS 138–9
Sister Joseph’s nodule 774
Sjögren’s syndrome 774
skeletal radiographs 502–3
skewed distribution 6
skin
cancer 602–3
evaluation of disease 42–3
excision of simple lesions
600–1
grafts 594–5, 595
wound healing 147
sleeping sickness 720
sliding hernia (hernia-en-
glissade) 346
slipped upper femoral
epiphysis 460–1
small bowel
post-operative mechanical
obstruction 114–15
transplantation 682, 698–9
tumours 292–3
small cell lung cancer 632
small intestine, anatomy 289
Smith’s fracture 511
smoking 58
social history 27
SOCRATES 28
soft tissue infections 176–7
specifi city 7
spermatic cord 339
spermatocele 367
sperm granuloma 367
spigelian hernia 346, 774
spinal cord injury 555
spinal injuries 550–2, 554–6
spinal instability 551–2
spinal radiograph 550–2
spinal shock 555
spinal stenosis 648
spleen palpation 35
sports hernia 348
squamous cell carcinoma
bladder 376
lung 632
oesophagus 282
salivary gland 230–1
skin 602–3
staghorn calculi 358
standard deviation 6
standard setting 20
staphylococci 174
Staphylococcus aureus 174
statistical signifi cance 8–9
status epilepticus 62–3
sterilization 78
sternoclavicular joint
dislocation 526
sternum fracture 530–1
steroids 53
Stevens–Johnson syndrome
774
Stewart–Treves syndrome
774
Stimson’s technique 527
stomach, anatomy and
physiology 287
stoma formation 750–1
stomas 84
streptococci 174
Streptococcus pyogenes 174
streptokinase 670
stress incontinence 354
stretched scars 598, 599
stroke 62, 116–17, 658–9
stroke volume 630, 631
stroke volume index 631
struvite stones 358
Stulberg classifi cation 469
subclavian steal syndrome
644
subclavian vein cannulation
198
subcutaneous tissue disease
42–3
submandibular gland 226, 227
subungual haematoma 610
suicide 19
sump syndrome 775
supracondylar fracture 514
suprapubic catheterization
212–13
supraventricular tachycardia
188
surgical emphysema 201
surgical fl aps 596–7, 597
surgical instruments 80–1
surgical performance 13
surgical symptoms 28–9
surgical terminology 24–5
survival curves 9
sutures 86–7, 590–2
Symes amputation 663
symptoms 28–9
synchronized intermittent
mandatory ventilation 130
synergistic spreading
gangrene 175
syphilitic osteomyelitis 561
systematic enquiry 27
systematic review 10
systemic infl ammatory
response syndrome
(SIRS) 138–9
systemic vascular resistance
631
systemic vascular resistance
index 631
systolic pressure 631
T
Takayasu’s arteritis 644
talus fracture 546
tap test 666
tar burns 608
tarsometatarsal fracture
dislocation (Lisfranc)
547–8
tarsus fracture 546–8
T-cell receptors 676
T-cells 677
activation 676
T cytotoxic cells 677
tenesmus 29
tension pneumothorax
480, 531
terminal ileum 289
terminology
immunology 677
ITU 126
surgery 24–5
terrible triad 522
testes
acute pain 388–9
torsion 350, 351, 388, 389
tumours 382–3
undescended 450–5
test feed 431–55
testicular appendages,
torsion 388
tetanus 175
thalassaemia 183
theatre
in-theatre preparation
74–5
positioning patients 76–7
preparing patients 70–1
scrubbing up 79
T helper cells 677
Thiersch grafts 594, 595
thoracic injuries 480–1
thoracic outlet syndrome
644, 645, 646
thoraco-abdominal
aneurysm 652
thoracolumbar spinal
injuries 555
thoracolumbar spinal
radiographs 551
thoracotomy 82
threadworms 727
thrombocytopenia 181
heparin-induced (HIT) 118
thromboembolic deterrent
stockings (TEDS) 72

INDEX
793
thromboembolic disease
154–6
thrombolysis 670–1
thrombophlebitis 154
thrombophlebitis migrans
154, 775
thromboprophylaxis 72–3
thrombus 154
through knee amputation
662, 663
thumb injuries 504
thymoma 639
thyroglobulin 171
thyroglossal cyst, sinus and
fi stula 222–3
thyroid
anatomy 257
goitre 250–1
Hurtle cell adenoma 759
post-surgery emergencies
258–9
tumours 254–7
thyroid storm 252
thyrotoxic crisis 252, 258–9
thyrotoxicosis 252–3
tibial shaft fracture 540–2
Tietze’s disease 248, 775
timor mortis 124
Tinel’s sign 570, 644, 775
tissue valves 629
TNM staging 168, 169
tolerance 677
tongue-tie 759
total hip replacement 586–7
total parenteral nutrition
67, 137
toxic dilatation 418
tracheobronchial rupture 481
traction apophysitis 474
TRAM fl ap 618
tranexamic acid 97
transabdominal rectopexy 407
transanal endoscopic
microsurgery (TEMS) 403
transferrin (serum) 66
transfusion reactions 98–9
transfusion-related acute
lung injury 99
transient ischaemic attack
(TIA) 658–9
transitional cell tumours
376–7
transjugular intrahepatic
portosystemic shunt 331
transplantation 675–99
ABO-incompatible living
donors 688
accepting patient on to
transplant list 683
cadaveric organ retrieval
686–7
cardiac 682, 690–1
contraindications 682
deceased donors 19,
684–99
heart–lung 691
immunology 676–7
immunosuppression
678–80, 680–99
indications for 682
islet cell 695
liver 682, 696–7
living donors 687–8
lung 682, 691
machine perfusion 687
organ availability 684
organ preservation 687
pancreas 682, 694–5
post-transplant
lymphoproliferative
disorder 679–80
rejection 676–7, 678–80
renal 682, 692–3
routine investigations 683
small bowel 682, 698–9
trans-scaphoid perilunate
dislocation 509
transverse rectus abdominis
myocutaneous (TRAM)
fl ap 618
trash foot 673
trauma, facial 235–6; see
also major trauma
traumatic fat necrosis 247
traumatic infarction 481
Treacher Collins syndrome
775
trenchfoot 159
Trendelenberg test 667
Trethowan’s sign 460, 461
triangular fi brocartilage
complex injury 509
triceps skinfold thickness 66
trichlobezoars 760
triple phosphate stones 358
Troisier’s sign 286, 775
tropical diseases 701–28
amoebiasis/amoebic liver
abscess 706–7
anaemias 708–9
ascariasis 718
fi lariasis 714
guinea worm infestation 726
hydatid disease 716–17
leishmaniasis 719
leprosy (Hansen’s disease)
724–5
malaria 710–1
mycetoma (madura foot)
728
schistosomiasis
(bilharziasis) 712–13
threadworms 727
trypanosomiasis 720
tuberculosis 722
typhoid 704–5
Trousseau’s sign 775
true aneurysms 652
trypanosomiasis 720
tuberculosis 561, 722
tuberculous epididymitis 366
tubo-ovarian infection
306, 307
tubulo-dermoid cysts 150
tubulo-embryonic cysts 150
tumour markers 170, 171
tumours
benign 160
bone 574–7
classifi cation 160, 161
cystic 150
defi nitions 160
doubling time 162
gastric 286–7
genetic abnormalities 162
grading 168–9
growth 162
immunosuppression-link
679–80
invasion 161
malignant 160
metastasis 161
oesophageal 282–3
salivary gland 230–1
small bowel 292–3
solid tumours in children
452–3
staging 168–9
testicular 382–3
thyroid 254–7
transitional cell 376–7
vascular 656
see also cancer
tumour suppressor genes
162
type I/II errors 8–9
typhoid 704–5
U
ulcerative colitis 392–3, 419
ulcers 43, 148–9
ulnar styloid fracture 511
ultralow anterior section 402
ultrasound
breast 30
developmental dysplasia of
hip 459
endoanal 39
endoscopic 272
neck 32
peripheral vascular
disease 41
transabdominal 36, 39
transrectal 39
transvaginal 39

INDEX
794
urinary tract disease 356
varicose veins 667
umbilical hernia 342–3, 446
umbolith 775
undescended testes 450–5
univariate analysis 9
universal donors 97
universal precautions 179
universal recipients 97
upper gastrointestinal
bleeding 294–5
upper gastrointestinal
endoscopy 272–3
upper gastrointestinal
perforation 296–7
upper gastrointestinal
surgery 271–309
ureteric obstruction 360–1
ureterocele 775
ureteroscopy 357
urethral catheterization
210–1
urethral stricture 364–5
urge incontinence 354
uric acid stones 358
urinanalysis 356
urinary complications
112–13
urinary incontinence 354–5
urinary retention, acute 113,
386–7
urinary tract disease
infection 113
investigations 356–7
stones 358–9
urine output 620
urokinase 670
urology 353–89
urostomy 84
V
VACTERL syndrome 775
vacuum dressings 595
valvular heart disease 54–5,
628–9
variceal bleeding 330–1, 331
varicocele 366, 366, 367,
444–5
varicose veins 666–7
vascular developmental
abnormalities 656–7
vascular injuries 484–5
vascular surgery,
complications 672–4
vasopressors 132–3
vasospastic disorders 664–5
vena caval fi lter 669
venepuncture 192–3
venous ulcers 148–9
ventilation 126
invasive 130
non-invasive 131
ventricular assist devices 133
ventricular fi brillation 190
Venturi mask 131
verbal consent 15
Vermooten’s sign 775
vibration sense 493
viral hepatitis 178
Virchow’s triad 120, 154,
668
visceral aneurysm 653
visceral leishmaniasis 719
vomiting 426
von Hippel–Lindau disease
775
von Recklinghausen’s
disease 775
von Rosen’s sign 775
W
Waldenström disease 776
Waldenström’s sign 468
ward rounds 88–9
warfarin 119, 180
Warthin’s tumour 230
Waterhouse–Friderichsen
syndrome 776
Whipple’s triad 776
whistle-blowing 21
Whitaker test 776
whitlow 612
WHO checklist approach
74–5
Wilm’s tumour 452
Winterbottom’s sign 720
Wolfe grafts 594, 595
wounds
body cavity incisions 82
burns 607
classifi cation 146
dehiscence 104–5
haematoma 103
healing 146–7
incision closures 82
infection 104, 172–3
stitch marks (tramline
effect) 590
suturing 86–7, 590–2
W-plasty 599
W pouch 402
wrist injuries 508–9
written consent 15
X
xenograft 594, 677
Y
Young and Burgess
classifi cation 532
Youssef’s syndrome 776
Z
Zenker’s diverticulum 776
Zollinger–Ellison syndrome
285, 776
Z-plasty 599
Index to emergency topics
Acute abdominal emergencies: overview 426
Acute abdominal pain 302
Acute anorectal pain 414
Acute appendicitis 298
Acute breast pain 248
Acute groin swelling 350
Acute haematemesis 294
Acute limb ischaemia 570
Acute pancreatitis 332
Acute rectal bleeding 416
Acute severe colitis 418
Acute testicular pain 388
Acute upper GI perforation 296
Acute urinary retention (AUR) 386
Acute variceal haemorrhage 330
Burns: assessment 604
Burns: management 606
Cardiac complications 106
Gynaecological causes of lower abdominal pain 306
Haematuria 384
Intra-abdominal abscess 308
Post-operative anastomotic leakage 420
Post-operative haemorrhage 102
Pulmonary embolism 120
Respiratory complications 108
Ruptured abdominal aortic aneurysm 654
Stroke 62
Wound emergencies 104

Common haematology values If outside this range, consult:
Haemoglobin men: 13–18g/dL
women: 11.5–16g/dL
Mean cell volume, MCV 76–96fL
Platelets 150–400 × 10
9
/L
White cells (total) 4–11 × 10
9
/L
Neutrophils 40–75%
Lymphocytes 20–45%
Eosinophils 1–6%
Blood gases kPa mmHg
pH 7.35–7.45
PaO
2
>10.6 75–100
PaCO
2
4.7–6 35–45
Base excess ±2 mmol/L
U&E etc If outside this range, consult:
Sodium 135–145mmol/
Potassium 3.5–5mmol/L
Creatinine 70–150μmol/L
Urea 2.5–6.7mmol/L
Calcium 2.12–2.65mmol/L
Albumin 35–50g/L
Proteins 60–80g/L
LFTs
Bilirubin 3–17μmol/L
Alanine aminotransferase, ALT 3–35U/L
Aspartate transaminase, AST 3–35U/L
Alkaline phosphatase 30–35U/L (adults)
‘Cardiac enzymes’
Creatine kinase 25–195U/L
Lactate dehydrogenase, LDH 70–250U/L
Lipids and other biochemical values
Cholesterol <6mmol/L desired
Triglycerides 0.5–1.9mmol/L
Amylase 0–180somorgyi U/dL
C-reactive protein, CRP <10mg/L
Glucose, fasting 3.5–5.5mmol/L
Prostate specifi c antigen, PSA 0–4ng/mL
T4 (total thyroxine) 70–140mmol/L
TSH 0.5– 75mu/L

Common perioperative care
Bowel prep KleenPrep
®
4 sachets over 8h the day preop or CitragMag
®
2 sachets over 4h the night preop.
Thromboprophylaxis
Low risk, e.g. day or fully ambulatory cases: TEDS only.
Medium risk, e.g. major surgery without risk factors or past history of
DVT: TEDS + Clexane
®
30mg SC od or Fragmin
®
2500U SC od.
High risk, e.g. pelvic surgery, malignancy, obesity, past history of DVT:
TEDS + Clexane
®
30mg SC bd or Fragmin
®
5000U SC od.
Diabetic perioperative regimens
Minor surgery (e.g. day surgery)
Oral controlled: give normal regimen.
Insulin controlled: omit preop insulin on day of surgery; monitor blood
sugar (BS) every 4h; restart normal insulin once oral diet established.
Major surgery
Oral controlled: omit long-acting hypoglycaemics preoperatively; monitor
BS every 4h. If BS > 15mmol/L start IV insulin regimen.
Insulin controlled: commence on IV insulin sliding scale preoperatively once
NBM and continue until normal diet re-established. Check BS 4-hourly.
Restart normal insulin regimen (initially at half dose) once oral diet
established.
Typical IV sliding scale (Actrapid
®
with 5% dextrose):
BS < 4mmol/L: infusion 0.5U/h;
BS 4–15mmol/L, infusion 2.0U/h;
BS 15–20mmol/L, infusion 4.0U/h;
BS > 20mmol/L, infusion 4.0U/h plus consult diabetology team. Con-
sider treatment as for ketoacidosis.
Fluid balance
Fluid depletion = ((PCV
1
– PCV
2
)/PCV
1
) x 0.7 x weight in kg
where PCV
1
= normal haematocrit and PCV2 = current haematocrit.
Fluid maintenance regimen (correct for age and losses)
Fluid volume Na
+
K
+
100mL/kg for 1st 10kg of weight + 150mL/kg
for next 10kg of weight + 20mL/kg for every
kg of weight thereafter
2mmol/kg/24h 1mmol/kg/24h
Managing oliguria
Check catheter not blocked.
Check fl uid balance status—try bolus crystalloid with frequent reviews.
Check drug chart for possible drug toxicity.
Managing postoperative hypotension
Check fl uid balance status fi rst. If in doubt assume it is hypovolaemia.
Check epidural status. Check drug chart for possible drug toxicity.
Do not interrupt CPR for >10s, except to defi brillate.
Shockable rhythm:
Amiodarone 300mg IV should be given with fi rst dose of adrenaline (peripherally
•
if no central access). A further 150mg may be given, followed by an infusion of
1mg/min for 6h, then 0.5mg/min for 6h.
Alteratives to amiodarone are:
•
Lidocaine 100mg IV; can repeat once; then give 2–4mg/min IVI. •
Procainamide 30mg/min IV to a total dose of 17mg/kg.•
Seek expert advice from a cardiologist.
•
Asystole/PEA: Give adrenaline 1mg immediately IV access is achieved. Atropine 3mg
IV is no longer recommended. If P waves the patient may respond to pacing.
Treat acidosis with good ventilation. Sodium bicarbonate may worsen intracellular
acidosis and precipitate arrhythmias, so use only in severe acidosis after prolonged
resuscitation (eg 50mL of 8.4% solution by IVI).
1 Algorithm reproduced with the permission of the Resuscitation Council (UK), ©2010. NB: adrena-
line/epinephrine in large doses (eg 5mg) has theoretical haemodynamic advantages, but studies have
failed to show benefi t (Ballew K 1997 BMJ i 1462). See Baskett P 1992 Br J Anaesthesia 69 182
2 PEA = Pulseless Electrical Activity = electromechanical dissociation (EMD)
Cardiac arrest: 2010 Adult Advanced Life-Support Algorithm
1
Each step assumes the previous one has been unsuccessful
UNRESPONSIVE?
not breathing or
only occasional gasps
Assess
rhythm
Non-Shockable
(PEA/Asystole)
Shockable
(VF/Pulseless VT)
Return of
spontaneous
circulation
Immediately resume
CPR for 2 min
Minimise interruptions
Immediately resume
CPR for 2 min
Minimise interruptions
Immediate post cardiac
arrest treatment
Use ABCDE approach
Controlled oxygenation and ventilation
12-lead ECG
Treat precipitating cause
Temperature control/
therapeutic hypothermia
(S
a
O
2
94–98%)
CPR 30:2
Attach defibrillator/monitor
Minimise interruptions
Call
resuscitation team
1 Shock
During CPR
Ensure high-quality CPR: rate, depth, recoil
Plan actions before interrupting CPR
Give oxygen
Consider advanced airway and capnography
Continuous chest compressions
when advanced airway in place
Vascular access (intravenous, intraosseous)
Give adrenaline every 3–5 min
Correct reversible causes
Reversible Causes
Hypoxia
Hypovolaemia
Hypo-/hyperkalaemia/metabolic
Hypothermia
Thrombosis-coronary or pulmonary
Tamponade-cardiac
Toxins
Tension pneumothorax
Primary angioplasty may also be an
appropriate immediate
post-cardiac arrest treatment.
Give 1mg adrenaline
every 3–5 min,
starting after the
3
rd
shock, on
alternate cycles







