
1
Purpose
The purpose of this Guideline is to provide a clinical framework for the surgical
management of patients with kidney and/or ureteral stones.
Methods
A systematic review of the literature using the Medline In-Process & Other Non-
Indexed Citations, MEDLINE, EMBASE, Cochrane Central Register of Controlled
Trials, Cochrane Database of Systematic Reviews, and Scopus databases (search
dates 1/1/1985 to 5/31/15) was conducted to identify peer-reviewed studies
relevant to the surgical management of stones. The review yielded an evidence
base of 1,911 articles after application of inclusion/exclusion criteria. These
publications were used to create the guideline statements. If sufficient evidence
existed, then the body of evidence for a particular treatment was assigned a
strength rating of A (high quality evidence; high certainty), B (moderate quality
evidence; moderate certainty), or C (low quality evidence; low certainty).
Evidence-based statements of Strong, Moderate, or Conditional Recommendation,
which can be supported by any body of evidence strength, were developed based
on benefits and risks/burdens to patients. Additional information is provided as
Clinical Principles and Expert Opinions when insufficient evidence existed.
Guideline Statements
Imaging, pre-operative testing:
1. Clinicians should obtain a non-contrast CT scan on patients prior to
performing PCNL. Strong Recommendation; Evidence Level Grade C
2. Clinicians may obtain a non-contrast CT scan to help select the best
candidate for SWL versus URS. Conditional Recommendation;
Evidence Level Grade C
3. Clinicians may obtain a functional imaging study (DTPA or MAG‐3) if
clinically significant loss of renal function in the involved kidney or
kidneys is suspected. Conditional Recommendation; Evidence Level
Grade C
4. Clinicians are required to obtain a urinalysis prior to intervention. In
patients with clinical or laboratory signs of infection, urine culture
should be obtained. Strong Recommendation; Evidence Level Grade B
5. Clinicians should obtain a CBC and platelet count on patients
undergoing procedures where there is a significant risk of hemorrhage
Approved by the AUA
Board of Directors April
2016
Authors’ disclosure of po-
tential conflicts of interest
and author/staff contribu-
tions appear at the end of
the article.
© 2016 by the American
Urological Association
American Urological Association (AUA)
Endourological Society Guideline
SURGICAL MANAGEMENT OF STONES:
AMERICAN UROLOGICAL ASSOCIATION/
ENDOUROLOGICAL SOCIETY GUIDELINE
Dean Assimos, MD; Amy Krambeck, MD; Nicole L. Miller, MD; Manoj Monga, MD;
M. Hassan Murad, MD, MPH; Caleb P. Nelson, MD, MPH; Kenneth T. Pace, MD;
Vernon M. Pais Jr., MD; Margaret S. Pearle, MD, Ph.D; Glenn M. Preminger, MD;
Hassan Razvi, MD; Ojas Shah, MD; Brian R. Matlaga, MD, MPH
Copyright © 2016 American Urological Association Education and Research, Inc.®

2
American Urological Association (AUA)
Endourological Society Guideline
or for patients with symptoms suggesting anemia, thrombocytopenia, or infection; serum electrolytes
and creatinine should be obtained if there is suspicion of reduced renal function. Expert Opinion
6. In patients with complex stones or anatomy, clinicians may obtain additional contrast imaging if
further definition of the collecting system and the ureteral anatomy is needed. Conditional
Recommendation; Evidence Level Grade C
Treatment of adult patients with ureteral stones:
7. Patients with uncomplicated ureteral stones <10 mm should be offered observation, and those with
distal stones of similar size should be offered MET with α-blockers. (Index Patient 3) Strong
Recommendation; Evidence Level Grade B
8. Clinicians should offer reimaging to patients prior to surgery if passage of stones is suspected or if
stone movement will change management. Reimaging should focus on the region of interest and limit
radiation exposure to uninvolved regions. Clinical Principle
9. In most patients, if observation with or without MET is not successful after four to six weeks and/or
the patient/clinician decide to intervene sooner based on a shared decision making approach,
clinicians should offer definitive stone treatment. (Index Patients 1-3) Moderate Recommendation;
Evidence Level Grade C
10. Clinicians should inform patients that SWL is the procedure with the least morbidity and lowest
complication rate, but URS has a greater stone-free rate in a single procedure. (Index Patients 1-6)
Strong Recommendation; Evidence Level Grade B
11. In patients with mid or distal ureteral stones who require intervention (who were not candidates for
or who failed MET), clinicians should recommend URS as first-line therapy. For patients who decline
URS, clinicians should offer SWL. (Index Patients 2,3,5,6) Strong Recommendation; Evidence Level
Grade B
12. URS is recommended for patients with suspected cystine or uric acid ureteral stones who fail MET or
desire intervention. Expert Opinion
13. Routine stenting should not be performed in patients undergoing SWL. (Index Patients 1-6) Strong
Recommendation; Evidence Level Grade B
14. Following URS, clinicians may omit ureteral stenting in patients meeting all of the following criteria:
those without suspected ureteric injury during URS, those without evidence of ureteral stricture or
other anatomical impediments to stone fragment clearance, those with a normal contralateral kidney,
those without renal functional impairment, and those in whom a secondary URS procedure is not
planned. (Index Patients 1-6) Strong Recommendation; Evidence Level Grade A
15. Placement of a ureteral stent prior to URS should not be performed routinely. (Index Patient 1-6)
Strong Recommendation; Evidence Level Grade B
16. Clinicians may offer α-blockers and antimuscarinic therapy to reduce stent discomfort. (Index
patients 1-6) Moderate Recommendation; Evidence Level Grade B
17. In patients who fail or are unlikely to have successful results with SWL and/or URS, clinicians may
offer PCNL, laparoscopic, open, or robotic assisted stone removal. (Index patient 1-6) Moderate
Recommendation; Evidence Level Grade C
18. Clinicians performing URS for proximal ureteral stones should have a flexible ureteroscope available.
(Index Patients 1, 4) Clinical Principle
19. Clinicians should not utilize EHL as the first-line modality for intra-ureteral lithotripsy. (Index
patients 1-6,13,15) Expert Opinion
Copyright © 2016 American Urological Association Education and Research, Inc.®
Surgical Management
of Stones

3
20. In patients with obstructing stones and suspected infection, clinicians must urgently drain the
collecting system with a stent or nephrostomy tube and delay stone treatment. Strong
Recommendation; Evidence Level Grade C
Treatment of adult patients with renal stones:
21. In symptomatic patients with a total non-lower pole renal stone burden ≤ 20 mm, clinicians may
offer SWL or URS. (Index Patient 7) Strong Recommendation; Evidence Level Grade B
22. In symptomatic patients with a total renal stone burden >20 mm, clinicians should offer PCNL as first
-line therapy. (Index Patient 8) Strong Recommendation; Evidence Level Grade C
25. In patients with total renal stone burden >20 mm, clinicians should not offer SWL as first-line
therapy. (Index Patient 8) Moderate Recommendation; Evidence Level Grade C
27. Clinicians may perform nephrectomy when the involved kidney has negligible function in patients
requiring treatment. (Index Patients 1-14) Conditional Recommendation; Evidence Level Grade C
28. For patients with symptomatic (flank pain), non-obstructing, caliceal stones without another obvious
etiology for pain, clinicians may offer stone treatment. (Index Patient 12) Moderate
Recommendation; Evidence Level Grade C
29. For patients with asymptomatic, non-obstructing caliceal stones, clinicians may offer active
surveillance. Conditional Recommendation; Evidence Level Grade C
30. Clinicians should offer SWL or URS to patients with symptomatic ≤ 10 mm lower pole renal stones.
(Index Patient 9) Strong Recommendation; Evidence Level Grade B
31. Clinicians should not offer SWL as first-line therapy to patients with >10mm lower pole stones.
(Index Patient 10) Strong Recommendation; Evidence Level Grade B
32. Clinicians should inform patients with lower pole stones >10 mm in size that PCNL has a higher stone
-free rate but greater morbidity. (Index patient 10). Strong Recommendation; Evidence Level Grade
B
33. In patients undergoing uncomplicated PCNL who are presumed stone-free, placement of a
nephrostomy tube is optional. Conditional Recommendation; Evidence Level Grade C
34. Flexible nephroscopy should be a routine part of standard PCNL. Strong Recommendation; Evidence
Level Grade B
35. Clinicians must use normal saline irrigation for PCNL and URS. Strong Recommendation; Evidence
Level Grade B
39. In patients not considered candidates for PCNL, clinicians may offer staged URS. Moderate
Recommendation; Evidence Level Grade C
40. Clinicians may prescribe α-blockers to facilitate passage of stone fragments following SWL. Moderate
Recommendation; Evidence Level Grade B
43. SWL should not be used in the patient with anatomic or functional obstruction of the collecting
system or ureter distal to the stone. Strong Recommendation; Evidence Level Grade C
44. In patients with symptomatic caliceal diverticular stones, endoscopic therapy (URS, PCNL,
laparoscopic, robotic) should be preferentially utilized. Strong Recommendation; Evidence Level
Grade C
45. Staghorn stones should be removed if attendant comorbidities do not preclude treatment. Clinical
Principle
Copyright © 2016 American Urological Association Education and Research, Inc.®
Surgical Management
of Stones
American Urological Association (AUA)
Endourological Society Guideline

4
Treatment for pediatric patients with ureteral or renal stones:
46. In pediatric patients with uncomplicated ureteral stones ≤10 mm, clinicians should offer observation
with or without MET using α-blockers. (Index Patient 13) Moderate Recommendation; Evidence Level
Grade B
47. Clinicians should offer URS or SWL for pediatric patients with ureteral stones who are unlikely to
pass the stones or who failed observation and/or MET, based on patient-specific anatomy and body
habitus. (Index Patient 13) Strong Recommendation; Evidence Level Grade B
48. Clinicians should obtain a low-dose CT scan on pediatric patients prior to performing PCNL. (Index
Patient 13) Strong Recommendation; Evidence Level Grade C
49. In pediatric patients with ureteral stones, clinicians should not routinely place a stent prior to URS.
(Index Patient 13) Expert Opinion
50. In pediatric patients with a total renal stone burden ≤20mm, clinicians may offer SWL or URS as first
-line therapy. (Index Patient 14) Moderate Recommendation; Evidence Level Grade C
51. In pediatric patients with a total renal stone burden >20mm, both PCNL and SWL are acceptable
treatment options. If SWL is utilized, clinicians should place an internalized ureteral stent or
nephrostomy tube. (Index Patient 14) Expert Opinion
52. In pediatric patients, except in cases of coexisting anatomic abnormalities, clinicians should not
routinely perform open/laparoscopic/robotic surgery for upper tract stones. (Index Patients 13, 14)
Expert Opinion
53. In pediatric patients with asymptomatic and non-obstructing renal stones, clinicians may utilize
active surveillance with periodic ultrasonography. (Index Patient 14) Expert Opinion
Treatment for pregnant patients with ureteral or renal stones:
54. In pregnant patients, clinicians should coordinate pharmacological and surgical intervention with the
obstetrician. (Index Patient 15) Clinical Principal
55. In pregnant patients with ureteral stones and well controlled symptoms, clinicians should offer
observation as first-line therapy. (Index Patient 15) Strong recommendation; Evidence Level Grade B
56. In pregnant patients with ureteral stones, clinicians may offer URS to patients who fail observation.
Ureteral stent and nephrostomy tube are alternative options with frequent stent or tube changes
usually being necessary. (Index Patient 15) Strong Recommendation; Evidence Level Grade C
Treatment for all patients with ureteral or renal stones:
23. When residual fragments are present, clinicians should offer patients endoscopic procedures to
render the patients stone free, especially if infection stones are suspected. (Index Patient 11)
Moderate Recommendation; Evidence Level Grade C
24. Stone material should be sent for analysis. Clinical Principle
26. Open/ laparoscopic /robotic surgery should not be offered as first-line therapy to most patients with
stones. Exceptions include rare cases of anatomic abnormalities, with large or complex stones, or
those requiring concomitant reconstruction. (Index Patients 1-15) Strong Recommendation;
Evidence Level Grade C
36. A safety guide wire should be used for most endoscopic procedures. (Index Patients 1-15) Expert
Opinion
37. Antimicrobial prophylaxis should be administered prior to stone intervention and is based primarily
Copyright © 2016 American Urological Association Education and Research, Inc.®
Surgical Management
of Stones
American Urological Association (AUA)
Endourological Society Guideline

5
on prior urine culture results, the local antibiogram, and in consultation with the current Best
Practice Policy Statement on Antibiotic Prophylaxis. Clinical Principle
38. Clinicians should abort stone removal procedures, establish appropriate drainage, continue antibiotic
therapy, and obtain a urine culture if purulent urine is encountered during endoscopic intervention.
(Index Patients1-15) Strong Recommendation; Evidence Level Grade C
41. If initial SWL fails, clinicians should offer endoscopic therapy as the next treatment option. (Index
Patient 1-14) Moderate Recommendation; Evidence Level Grade C
42. Clinicians should use URS as first-line therapy in most patients who require stone intervention in the
setting of uncorrected bleeding diatheses or who require continuous anticoagulation/antiplatelet
therapy. (Index Patients1-15) Strong Recommendation; Evidence Level Grade C
Copyright © 2016 American Urological Association Education and Research, Inc.®
Surgical Management
of Stones
American Urological Association (AUA)
Endourological Society Guideline

6
INTRODUCTION
Background
Kidney stones are a common and costly disease; it has
been reported that over 8.8% of the United States
population will be affected by this malady, and direct
and indirect treatment costs are estimated to be
several billion dollars per year in this country.
1-3
The
surgical treatment of kidney stones is complex, as there
are multiple competitive treatment modalities, and in
certain cases more than one modality may be
appropriate. Proper treatment selection, which is
directed by patient- and stone-specific factors, remains
the greatest predictor of successful treatment
outcomes. The Panel used information from the
literature to formulate actionable guideline statements
to assist clinicians in providing the best care for their
patients requiring stone elimination.
This Guideline includes revisions of the previously
published AUA Guidelines titled ‘Staghorn Calculi
(2005)’
4
and ‘Ureteral Calculi (2007)’
5
and is expanded
to incorporate the management of patients with non-
staghorn renal stones. The Update Literature Review
(ULR) process for AUA Guidelines was used to
determine that updates were warranted for both the
Staghorn Calculi and Ureteral Calculi Guidelines. A
guideline for the management of non-staghorn renal
stones had previously not been generated by the AUA.
The AUA and the Endourological Society felt that a
single, all-encompassing guideline document would
provide the greatest value to the clinician for patient
management. This Guideline also compliments the AUA
Guideline on ‘Medical Management of Kidney Stones’
published in 2014.
6
The surgical management of patients with various
stones is described below and divided into 13
respective patient profiles. Index Patients 1-10 are non-
morbidly obese; non-pregnant adults (≥ 18 years of
age) with stones not thought to be composed of uric
acid or cystine; normal renal, coagulation and platelet
function; normally positioned kidneys; intact lower
urinary tracts without ectopic ureters; no evidence of
sepsis; and no anatomic or functional obstruction distal
to the stone(s). Index Patients 13 and 14 are children
(<18 years if age) with similar characteristics to Index
Patients 1-10. Index Patient 15 is a pregnant female
with symptomatic renal or ureteral stone(s) with
normal renal function without urinary tract infection
(UTI). The proximal ureter is defined as the segment
distal to the ureteropelvic junction (UPJ) and above the
upper border of the sacroiliac joint. The middle ureter is
that which overlies the sacroiliac joint and the distal
ureter that lies below it.
Index Patients
Index Patient 1: Adult, <10mm proximal ureteral stone
Index Patient 2: Adult, <10mm mid ureteral stone
Index Patient 3: Adult, <10mm distal ureteral stone
Index Patient 4: Adult, >10mm proximal ureteral stone
Index Patient 5: Adult, >10mm mid ureteral stone
Index Patient 6: Adult, > 10 mm distal ureteral stone
Index Patient 7: Adult, ≤20mm total non-lower pole
renal stone burden
Index Patient 8: Adult, >20mm total renal stone burden
Index Patient 9: Adult, ≤10mm lower pole renal
stone(s)
Index Patient 10: Adult, >10mm lower pole renal
stone(s)
Index Patient 11: Adult, with residual stone(s)
Index Patient 12: Adult, renal stone(s) with pain and no
obstruction
Index Patient 13: Child, not known to have cystine or
uric acid ureteral stone(s)
Index Patient 14: Child, not known to have cystine or
uric acid renal stone(s)
Index Patient 15: Pregnant female, renal or ureteral
stone(s)
Methodology
Process for Initial Literature Selection
Consistent with the published AUA Guideline
methodology framework,
7
the process started by
conducting a comprehensive systematic review. The
AUA commissioned an independent group to conduct a
systematic review and meta-analysis of the published
literature on various options for the surgical
Copyright © 2016 American Urological Association Education and Research, Inc.®
Surgical Management
of Stones
American Urological Association (AUA)
Endourological Society Guideline

7
management of stones.
8
The protocol of the systematic
review was developed a priori by the methodology team
in conjunction with the expert panel. A systematic
review was conducted to identify published articles
relevant to the surgical management of renal or
ureteral stones. Literature searches were performed on
English-language publications using the Medline In-
Process & Other Non-Indexed Citations, MEDLINE,
EMBASE, Cochrane Central Register of Controlled Trials,
Cochrane Database of Systematic Reviews, and Scopus
from 1/1/1985 to 5/31/2015. Preclinical studies (e.g.,
animal models), commentary, and editorials were
excluded. Studies on patients with lower tract stones
were excluded (including bladder stones and
diversions). Bibliographies of review articles were
checked to ensure inclusion of all possibly relevant
studies. Multiple reports on the same patient group
were carefully examined to ensure inclusion of only non
-redundant information. The systematic review yielded
a total of 1,911 studies. The Panel and methodology
group continued to monitor the literature for relevant
randomized trials thereafter and added several newer
trials published through 2015.
The Panel judged that there was a sufficient evidence
base from which to construct the Guideline. Data on
study type (e.g., randomized controlled trial [RCT],
controlled clinical trial [CCT], observational study),
perioperative testing, treatment parameters (e.g., type
of treatment), patient characteristics (e.g., age, stone
size and location), outcomes (e.g., stone-free rate,
residual fragments, quality of life [QoL]) and
complications were extracted.
Almost all the studies that reported on preoperative
testing (99 computed tomography [CT] scan, 10 renal
scan, 128 renal ultrasound [US], 188 KUB, 156
intravenous pyelogram [IVP], 68 complete blood count
[CBC], 29 stone analysis and 112 urine culture) did not
report the purpose of performing these tests. There
were no reliable data on the utility or incremental value
of testing. The procedures of interest were
percutaneous nephrolithotomy (PCNL), ureteroscopy
(URS), laparoscopy, shock-wave lithotripsy (SWL),
open surgery, robotic surgery, ureteral stent, or
nephrostomy. Comparison of any of these active
treatments against each other or against medical
management was done when possible. Medical
expulsive therapy (MET) was evaluated in terms of
efficacy against placebo. Outcomes included stone-free
rate (as determined by KUB, US, IVP,
nephrotomogram, CT, endoscopy); residual fragments
(by size); secondary procedures needed (stone-
removing versus ancillary); QoL; pain; analgesic
requirements; length of hospitalization; comparative
recurrence rates; renal function; and procedure
complications (e.g., death, sepsis/sirs, transfusion, loss
of kidney, readmission rates, overall rates). When
multiple studies evaluated the same outcome and had
similar population, intervention, and comparison, meta-
analysis was conducted using the random effects
model, when appropriate.
8
Stone-free rate was
stratified based on stone size and location.
The methodology team independently rated the
methodological quality of the studies and provided an
overall judgment of the whole body of evidence based
on confidence in the available estimates of effect.
The methodology team summarized the data with
explicit description of study characteristics,
methodological quality, main findings, and quality of
the evidence (confidence in the estimates). The
methodology team attended panel meetings and
facilitated incorporation of the evidence into the
Guideline.
Quality of Individual Studies and Determination of
Evidence Strength
The quality of individual studies that were either RCTs
or CCTs was assessed using the Cochrane Risk of Bias
tool.
9
The quality of CCTs and comparative
observational studies was rated using the Newcastle-
Ottawa Quality (NOQ) Assessment Scale.
10
Because
there is no widely-agreed upon quality assessment tool
for single cohort observational studies, the quality of
these studies was not assessed.
The categorization of evidence strength is conceptually
distinct from the quality of individual studies (the latter
is also called the risk of bias). Evidence strength refers
to the body of evidence available for a particular
question and includes not only individual study quality
but consideration of study design; consistency of
findings across studies; adequacy of sample sizes; and
generalizability of samples, settings, and treatments for
Copyright © 2016 American Urological Association Education and Research, Inc.®
Surgical Management
of Stones
American Urological Association (AUA)
Endourological Society Guideline

8
the purposes of the Guideline. The AUA categorizes
body of evidence strength as Grade A (well-conducted
and highly-generalizable RCTs or exceptionally strong
observational studies with consistent findings), Grade B
(RCTs with some weaknesses of procedure or
generalizability or moderately strong observational
studies with consistent findings), or Grade C (RCTs with
serious deficiencies of procedure or generalizability or
extremely small sample sizes or observational studies
that are inconsistent, have small sample sizes, or have
other problems that potentially confound interpretation
of data). By definition, Grade A evidence is evidence
about which the Panel has a high level of certainty,
Grade B evidence is evidence about which the Panel has
a moderate level of certainty, and Grade C evidence is
evidence about which the Panel has a low level of
certainty.
7
AUA Nomenclature: Linking Statement Type to
Evidence Strength
The AUA nomenclature system links statement type to
body of evidence strength, level of certainty, magnitude
of benefit or risk/burdens, and the Panel's judgment
regarding the balance between benefits and risks/
burdens (see Table 1). Strong Recommendations are
directive statements that an action should (benefits
outweigh risks/burdens) or should not (risks/burdens
outweigh benefits) be undertaken because net benefit
or ne t ha rm is subst anti al . Mo de rate
Recommendations are directive statements that an
action should (benefits outweigh risks/burdens) or
should not (risks/burdens outweigh benefits) be
undertaken because net benefit or net harm is
moderate. Conditional Recommendations are non-
directive statements used when the evidence indicates
that there is no apparent net benefit or harm or when
the balance between benefits and risks/burden is
unclear. All three statement types may be supported by
any body of evidence strength grade. Body of evidence
strength Grade A in support of a Strong or Moderate
Recommendation indicates that the statement can be
applied to most patients in most circumstances and
that future research is unlikely to change confidence.
Body of evidence strength Grade B in support of a
Strong or Moderate Recommendation indicates that the
statement can be applied to most patients in most
circumstances but that better evidence could change
confidence. Body of evidence strength Grade C in
support of a Strong or Moderate Recommendation
indicates that the statement can be applied to most
patients in most circumstances but that better evidence
is likely to change confidence. Body of evidence
strength Grade C is rarely used in support of a Strong
Recommendation. Conditional Recommendations also
can be supported by any body of evidence strength.
When body of evidence strength is Grade A, the
statement indicates that benefits and risks/burdens
appear balanced, the best action depends on patient
circumstances, and future research is unlikely to
change confidence. When body of evidence strength
Grade B is used, benefits and risks/burdens appear
balanced, the best action also depends on individual
patient circumstances and better evidence could change
confidence. When body of evidence strength Grade C is
used, there is uncertainty regarding the balance
between benefits and risks/burdens, alternative
strategies may be equally reasonable, and better
evidence is likely to change confidence.
For some clinical issues, particularly diagnosis, there
was little or no evidence from which to construct
evidence-based statements. Where gaps in the
evidence existed, the Panel provides guidance in the
form of Clinical Principles or Expert Opinions with
consensus achieved using a modified Delphi technique
if differences of opinion emerged.
11
A Clinical
Principle is a statement about a component of clinical
care that is widely agreed upon by urologists or other
clinicians for which there may or may not be evidence
in the medical literature. Expert Opinion refers to a
statement, achieved by consensus of the Panel, that is
based on members' clinical training, experience,
knowledge, and judgment for which there is no
evidence.
Panel Selection and Peer Review Process
The Surgical Management of Stones Panel was created
in 2013 by the American Urological Association
Education and Research, Inc. (AUA). The Practice
Guidelines Committee (PGC) of the AUA selected the
Panel Chair who in turn appointed the additional panel
members with specific expertise in this area. The
Endourological Society also nominated two
representatives to serve on the panel. Once nominated,
Copyright © 2016 American Urological Association Education and Research, Inc.®
Surgical Management
of Stones
American Urological Association (AUA)
Endourological Society Guideline
9
Copyright © 2016 American Urological Association Education and Research, Inc.®
TABLE 1: AUA Nomenclature Linking Statement Type
to Level of Certainty, Magnitude of Benefit or Risk/Burden, and Body of Evidence Strength
Evidence Strength A
(High Certainty)
Evidence Strength B
(Moderate Certainty)
Evidence Strength C
(Low Certainty)
Strong
Recommendation
(Net benefit or harm sub-
stantial)
Benefits > Risks/Burdens
(or vice versa)
Net benefit (or net harm)
is substantial
Applies to most patients
in most circumstances
and future research is
unlikely to change confi-
dence
Benefits > Risks/Burdens
(or vice versa)
Net benefit (or net harm)
is substantial
Applies to most patients
in most circumstances but
better evidence could
change confidence
Benefits > Risks/Burdens (or
vice versa)
Net benefit (or net harm)
appears substantial
Applies to most patients in
most circumstances but bet-
ter evidence is likely to
change confidence
(rarely used to support a
Strong Recommendation)
Moderate
Recommendation
(Net benefit or harm
moderate)
Benefits > Risks/Burdens
(or vice versa)
Net benefit (or net harm)
is moderate
Applies to most patients
in most circumstances
and future research is
unlikely to change confi-
Benefits > Risks/Burdens
(or vice versa)
Net benefit (or net harm)
is moderate
Applies to most patients
in most circumstances but
better evidence could
change confidence
Benefits > Risks/Burdens (or
vice versa)
Net benefit (or net harm)
appears moderate
Applies to most patients in
most circumstances but bet-
ter evidence is likely to
change confidence
Conditional
Recommendation
(No apparent net benefit
or harm)
Benefits = Risks/Burdens
Best action depends on
individual patient circum-
stances
Future research unlikely
to change confidence
Benefits = Risks/Burdens
Best action appears to
depend on individual pa-
tient circumstances
Better evidence could
change confidence
Balance between Benefits &
Risks/Burdens unclear
Alternative strategies may
be equally reasonable
Better evidence likely to
change confidence
Clinical Principle
A statement about a component of clinical care that is widely agreed upon by urolo-
gists or other clinicians for which there may or may not be evidence in the medical
literature
Expert Opinion
A statement, achieved by consensus of the Panel, that is based on members' clinical
training, experience, knowledge, and judgment for which there is no evidence
Surgical Management
of Stones
American Urological Association (AUA)
Endourological Society Guideline

10
all panel members were asked to record their conflict of
interest (COI) statements, providing specific details on
the AUA interactive web site. These details are first
reviewed by the Guidelines Oversight Committee
(GOC), a member sub-committee from the PGC
consisting of the Vice Chair of the PGC and two other
members. The GOC determines whether the individual
has potential conflicts related to the guideline. If there
are conflicts, then the nominee's COI is reviewed and
approved by the AUA Judicial and Ethics (J&E)
committee. A majority of panel members may not have
relationships relevant to the Guideline topic.
The AUA conducted a thorough peer review process.
The draft guidelines document was distributed to 109
peer reviewers, 54 of whom provided comments. The
Panel reviewed and discussed all submitted comments
and revised the draft as needed. Once finalized, the
Guideline was submitted for approval to the PGC and
Science and Quality Council (S&Q). Then it was
submitted to the AUA Board of Directors and the
Endourological Society Board of Directors for final
approval. Funding of the panel was provided by the
AUA, with support from The Endourological Society;
panel members received no remuneration for their
work.
Limitations of the Literature
Evidence to guide perioperative diagnostic evaluation
was sparse and of low quality, affecting
recommendations on laboratory testing and imaging.
Data on stone-free rate (lithotripsy, URS and PCNL)
when stratified by location and stone size were also
limited in clinical trials; therefore, rates were also
derived from large registries that provided precise,
although likely biased, estimates. Comparative
effectiveness of MET was derived from a large number
of trials that overall has a moderate risk of bias. Only a
very small number of studies were available to provide
comparative effectiveness inferences in children.
GUIDELINE STATEMENTS
1. Clinicians should obtain a non-contrast CT
scan on patients prior to performing PCNL.
Strong Recommendation; Evidence Level
Grade C
Neither randomized trials nor comparative studies have
specifically addressed the role of preoperative CT prior
to PCNL. Nevertheless, the use of CT for preoperative
assessment in those with nephrolithiasis has gained
widespread acceptance, as it defines stone burden and
distribution, and provides information regarding
collecting system anatomy, position of peri-renal
structures and relevant anatomic variants. It may also
be used to predict operative outcomes and, in some
instances, stone composition.
12-21
CT protocols have been developed and evaluated
utilizing radiation doses approximating those of plain
film radiography. These “low-dose protocols” continue
to allow excellent differentiation of calculi from
surrounding tissues while minimizing radiation
exposure.
22
Three dimensional reconstructive
techniques are additionally available and are advocated
by some for their perceived utility in improving
preoperative PCNL planning.
23
2. Clinicians may obtain a non-contrast CT scan
to help select the best candidate for SWL
versus URS. Conditional Recommendation;
Evidence Level Grade C
Neither randomized trials nor comparative studies have
specifically addressed the role of preoperative CT for
treatment selection between SWL and URS.
Furthermore, the Panel recognizes that multiple
imaging modalities, including renal US, IVP or
intravenous urogram (IVU), and KUB (kidneys, ureters,
and bladder) plain radiography, may be used to
preoperatively assess candidates for SWL and URS.
24
However, in light of the breadth of information provided
by CT, the Panel feels that CT can be useful to help
determine whether SWL or URS is better suited for a
given patient.
Non-contrast CT imaging is the most sensitive and
specific imaging investigation in the diagnosis of upper
urinary tract stone disease.
25
Despite CT’s diagnostic
superiority over other imaging tests, it is incumbent on
urologists to be cognizant of the potential risks/harms
of the investigations they select for their patients to
accurately diagnose and plan appropriate therapies.
Concerns regarding the long-term cancer risks
associated with ionizing radiation have led to calls for
the use of US in the initial diagnosis of acute flank pain.
While the initial diagnostic use of US instead of CT
Copyright © 2016 American Urological Association Education and Research, Inc.®
Surgical Management
of Stones
American Urological Association (AUA)
Endourological Society Guideline

11
imaging in a randomized trial among patients
presenting to the emergency department with
suspected nephrolithiasis has not been associated with
serious adverse outcomes,
26
a reliance on US alone to
formulate surgical planning is a different situation
entirely. The use of US alone to direct SWL or URS
treatment planning should be discouraged as US is
inherently inaccurate in determination of stone size,
and it provides no information on stone density.
Although the combined use of KUB and US will provide
information on stone size and location better than
either modality alone, there are recognized drawbacks
to this approach as well. CT has demonstrated
improved accuracy in determination of these
parameters and may also provide information regarding
skin-to-stone distance and stone attenuation.
Individually, each of these factors may be used to
assess likelihood of successful SWL treatment. Renal
stone attenuation should be obtained; <900-1000
Hounsfield units can help predict success with SWL.
27,28
Additionally, skin-to-stone distance, which is best
measured by CT, may also predict treatment
outcome;
29,30
<10 cm is favorable for renal stones.
Thus, clinicians can use CT information to select which
patients are reasonable candidates for SWL. If the
parameters are not favorable, URS is preferred as
excellent results are achievable with these procedures
even in a morbidly obese cohort.
Furthermore, using a group of CT-based parameters,
predictive models have been developed to estimate
stone-free rates for SWL.
31,32
The use of preoperative
CT to assess such factors individually or combined in
predictive models may aid the clinician in estimating
success rates for each modality and ultimately result in
a more informed decision in which the risks and
benefits of each modality are weighed.
3. Clinicians may obtain a functional imaging
study (DTPA or MAG‐3) if clinically significant
loss of renal function in the involved kidney or
ki dn ey s is su s pe ct ed . C o ndi t i on al
Recommendation; Evidence Level Grade C
Kidney stone disease can affect renal function. If a
clinician suspects compromise of renal function,
obtaining a functional imaging study (DTPA or MAG‐3)
can help guide treatment for stone disease. Nuclear
renography can provide the differential function of the
two kidneys in addition to assessing for urinary tract
obstruction. It should be noted that the ability of
nuclear renography to assess obstruction may be
limited in cases of moderate to severe chronic kidney
disease.
Although parenchymal thickness can occasionally allow
a clinician to estimate renal function, there are settings,
such as in the case of chronic kidney disease or
staghorn/complex stones, where renal function is
compromised and function cannot be adequately
assessed without a nuclear renal scan or another
contrast-enhanced imaging study, such as CT
urography, magnetic resonance (MR) urography, or IV
urography.
33-37
Decreased renal function of the involved
kidney may lead to a decision to consider other
therapeutic options, which may range from observation
to nephrectomy.
Additionally, establishing baseline renal function can be
useful in following treatment outcomes for upper
urinary tract stone disease. The assessment of renal
function may be limited in the setting of obstruction;
therefore, alleviation of the obstruction with a
nephrostomy tube or ureteral stent may be required in
order to appropriately assess renal function in the
affected renal unit before selecting therapy.
4. Clinicians are required to obtain a urinalysis
prior to intervention. In patients with clinical
or laboratory signs of infection, urine culture
should be obtained. Strong recommendation;
Evidence Level Grade B
It is critical that clinicians obtain a urinalysis prior to
stone intervention in order to minimize the risks of
infectious complications. A urine culture should be
obtained if UTI is suspected based on the urinalysis or
clinical findings. If the culture demonstrates infection,
the patient should be prescribed appropriate antibiotic
therapy based on sensitivity results in an attempt to
sterilize the urine prior to intervention.
Clinicians should also be aware that there can be
discordance between preoperative voided urine cultures
or those from indwelling urethral catheters compared to
urine proximal to an obstructing stone. Intraoperative
urine cultures should be obtained, if technically
Copyright © 2016 American Urological Association Education and Research, Inc.®
Surgical Management
of Stones
American Urological Association (AUA)
Endourological Society Guideline

12
feasible, from urine proximal to the stone if infected
urine is suspected at the time of intervention.
38-42
Additionally stone cultures may be obtained, especially
in cases of suspected infection-related stones, in order
to help guide postoperative therapy. There is also
potential discordance between stone cultures and
preoperative voided urine cultures.
39-41
5. Clinicians should obtain a CBC and platelet
count on patients undergoing procedures
where there is a significant risk of
hemorrhage or for patients with symptoms
suggesting anemia, thrombocytopenia, or
infection; serum electrolytes and creatinine
should be obtained if there is suspicion of
reduced renal function. Expert Opinion
There are neither randomized trials nor comparative
studies upon which one may base preoperative
laboratory evaluation prior to surgical management of
urinary tract stones. The American Society of
Anesthesiologists (ASA) released an updated practice
advisory for preanesthesia evaluation in 2012. Overall,
ASA recommends against routine ordering of
preoperative CBC and serum chemistry testing,
suggesting this be obtained on a selective basis.
43
The
meta-analysis shows that in non-selected/
asymptomatic patients, abnormal CBCs were reported
in 2.9-9%, whereas in selected high-risk patients,
abnormalities were noted in 6.3-60.8%, leading to
change in clinical management in 14.9%. Among non-
selected patients, abnormal sodium was noted in 1.9%,
abnormal potassium in 0.2-16%, and abnormal glucose
in 0.9-40% (changes in clinical management were not
reported). ASA concluded that routine preanesthesia
hemoglobin was not indicated but should be obtained
as indicated by clinical characteristics. Similarly,
evaluation of serum chemistries and renal function tests
should be based upon clinical characteristics, including
pertinent preoperative medications and therapies,
endocrine disorders, and risk of renal dysfunction. As
patients with urolithiasis may be at risk for renal
dysfunction, the Panel recommends consideration of
preoperative creatinine to assess baseline renal
function. In patients undergoing procedures where
there is a significant risk of hemorrhage, such as PCNL,
open/ laparoscopic or robotic assisted nephrolithotomy,
the Panel recommends that a CBC be obtained. In
addition, this test should be ordered if the patient has
si g ns or sympto ms suggest i ng anem i a ,
thrombocytopenia or infection. An assessment of serum
electrolytes, creatinine and BUN should be checked if
reduced renal function is suspected, such as in those
with hydronephrosis, parenchymal thinning or co-
morbid conditions associated with renal dysfunction and
electrolyte disturbances.
There are no randomized trials to inform those
circumstances in which preoperative coagulation studies
should be obtained prior to surgical management of
urologic stone disease. The Society of Interventional
Radiology (SIR) Standards of Practice Committee
addressed periprocedural assessment of coagulation
status prior to image-guided interventions, categorizing
percutaneous nephrostomy placement as a procedure
with “significant bleeding risk, difficult to detect or
control.”
44
Based on this designation, SIR advises
routinely obtaining pre-procedural international
normalized ratio (INR) to assess standardized
prothrombin time (PT) in all patients before undergoing
nephrostomy tube placement, although there was no
consensus on obtaining pre-procedural partial
thromboplastin time (PTT).
In contradistinction, the ASA Committee on Standards
and Practice Parameters issued an overarching practice
advisory for pre-surgical anesthesia evaluation. ASA
discourages routine preoperative testing in unselected
patients. Rather, coagulation studies should be
selectively obtained specifically based upon clinical
characteristics, including documented or suspected
bleeding disorders, hepatic dysfunction, and renal
dysfunction. Those on anticoagulant medications may
require coagulation studies preoperatively to assess
degree of perioperative anticoagulation, noting that
anticoagulated patients may present additional
perioperative risk.
43
The Panel, concurring with the ASA, concludes that in
the absence of clinical indications (e.g., the
aforementioned systemic anticoagulation, relevant
hepatic dysfunction, hematologic disease or bleeding
disorders, clinical history suggestive of a coagulation
disorder) coagulation studies should not be routinely
obtained prior to surgical management of urinary stone
Copyright © 2016 American Urological Association Education and Research, Inc.®
Surgical Management
of Stones
American Urological Association (AUA)
Endourological Society Guideline

13
disease. These recommendations are separate from
blood product ordering, which should be based upon
perceived risks of operative bleeding and perioperative
requirements for transfusion. Published institution-
specific maximum surgical blood order schedules have
suggested preoperative type and screen for PCNL.
45
6. In patients with complex stones or anatomy,
clinicians may obtain additional contrast
imaging if further definition of the collecting
system and the ureteral anatomy is needed.
Conditional recommendation; Evidence Level
Grade C
When treating a complex stone burden or patient with
complex anatomy, a clinician may obtain additional
contrast-enhanced imaging with urographic phases to
help determine the best treatment approach.
46,47
Complex urinary tract anatomy can be related to both
renal/ureteral anatomy and patient body habitus.
Situations in which complex urinary tract anatomy may
require further imaging include ectopic kidneys (e.g.,
horseshoe kidney, pelvic kidney, cross-fused ectopia),
other congenital kidney conditions (e.g., UPJ
obstruction, duplicated collecting system, caliceal
diverticulum, ureteral stricture, megaureter,
ureterocele), renal transplant grafts, kidneys with prior
surgery or complex stone anatomy/conditions (e.g.,
staghorn stones, nephrocalcinosis). Further imaging
may be required in certain patients (e.g., neurologic
disorders, including spinal dysraphism; unusual body
habitus; presence of urinary diversion or prior kidney/
ureteral surgery).
CT and IVU are the most useful IV contrast studies.
Additionally, MR urography can be useful in defining
anatomy during pregnancy (without contrast) and in
the setting of IV contrast allergy, although stones are
typically not well visualized directly with MR imaging.
Finally, contrast imaging studies can also include
retrograde or antegrade pyelography, which can define
the collecting system anatomy and help to determine
the optimal treatment approach.
7. Patients with uncomplicated ureteral stones
<10 mm should be offered observation, and
those with distal stones of similar size should
be offered MET with α-blockers. (Index
Patient 3) Strong Recommendation; Evidence
Level Grade B
Natural history studies have shown that the likelihood
of spontaneous stone passage correlates with stone
size and stone location.
48
The smaller the stone and the
more distally in the ureter the stone is located, the
greater the likelihood of spontaneous passage.
Furthermore, smaller stones are likely to pass more
quickly than larger stones.
49
The control arms of RCTs
evaluating tamsulosin as MET show that about half of
patients with distal ureteral calculi <10 mm in size will
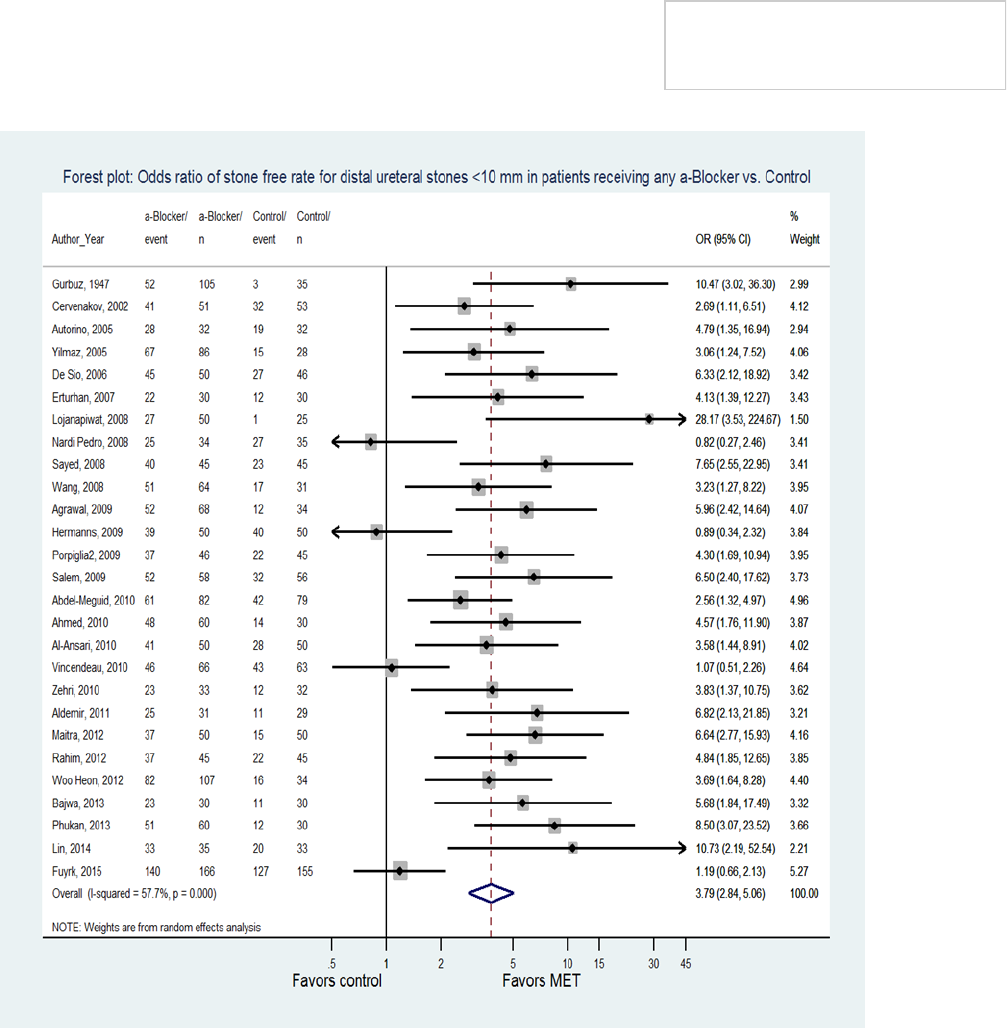
spontaneously pass their stones (Figure 1).
Consequently, there is ample evidence that a trial of
spontaneous passage is reasonable in patients
amenable to conservative therapy with <10 mm distal
ureteral stones in whom pain is well controlled and
there are no signs of infection or high grade
obstruction. While there is less evidence for those
harboring middle and distal ureteral stones, the panel
also feels that observation should be offered to those
with uncomplicated stones of similar size in these
ureteral areas.
Several pharmacologic agents have recently been
tested for their ability to change the natural history of
ureteral calculi by increasing spontaneous passage
rates. Ureteral contractility is mediated by both alpha
and beta adrenoreceptors in the ureteral wall.
Stimulation of α
1
-receptors promotes contraction of
ureteral smooth muscle, leading to more vigorous and
frequent peristalsis.
50,51
As such, α
1
receptor
antagonists have the potential to inhibit ureteral spasm
and uncontrolled contraction, theoretically reducing
pain and promoting spontaneous stone passage. The
Panel’s meta-analysis
8
showed superior spontaneous
stone passage rates in patients with <10 mm distal
ureteral stones treated with α-blockers (77.3%)
compared to placebo or no treatment (54.4%) (RR
3.59, 95% CI 2.900-4.125). This effect was largely
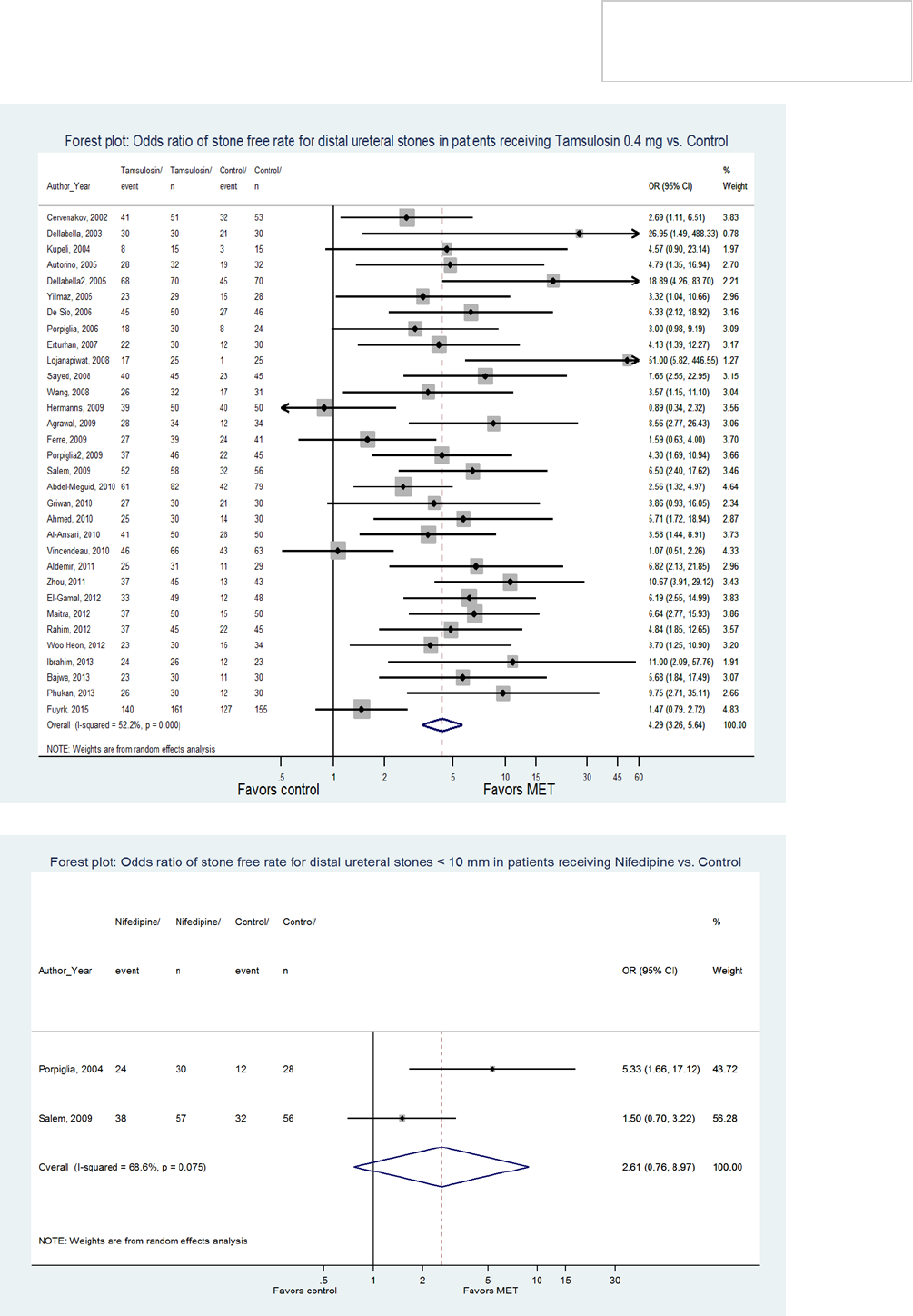
accounted for by trials in which tamsulosin 0.4 mg was
administered daily in patients with <10 mm distal
ureteral calculi (Figure 2). Calcium channel blockers,
which also suppress smooth muscle contraction by
inhibiting the influx of extracellular calcium into smooth
muscle cells. One trial showed a benefit of nifedipine, a
calcium channel blocker, in patients with <10 mm distal
ureteral stones while another did not. Therefore, due to
Copyright © 2016 American Urological Association Education and Research, Inc.®
Surgical Management
of Stones
American Urological Association (AUA)
Endourological Society Guideline
14
insufficient supporting data, the Panel does not endorse
the utilization of this agent for MET. (Figure 3).
A recent large trial of 316 patients with <10 mm distal
ureteral calculi randomized to tamsulosin 0.4 mg daily
or placebo found a benefit of therapy only in patients
with larger stones (>5mm, 83% stone passage in the
tamsulosin group versus 61% in the placebo group,
95% CI 3.1%-41.6%, p=0.03), but no difference in
stone passage rates between treatment and control
groups in patients with smaller stones (<5 mm).
52
The
already high rate of spontaneous stone passage with
smaller stones may account for the lack of effect of
tamsulosin seen in patients with smaller stones in this
trial. The Panel’s meta-analysis found no improvement
in stone passage rates in patients with <5 mm distal
ureteral stones treated with tamsulosin (OR 1.23, 95%
CI 0.61-2.47)
8
but did confirm a benefit of therapy in
patients with >5 mm distal ureteral stones (OR 4.53,
95% CI 2.90-7.07). However, given the more limited
data available for subgroup analysis, the Panel elected
to include all patients with <10 mm distal ureteral
stones in the recommendation supporting MET.
Of note, a recent large 3-way RCT from the United
Kingdom compared tamsulosin (0.4 mg daily),
nifedipine (30 mg daily) and placebo (1:1:1) in patients
with ≤10 mm ureteral calculi.
53
Unlike most other MET
Copyright © 2016 American Urological Association Education and Research, Inc.®
Figure 1:
Surgical Management
of Stones
American Urological Association (AUA)
Endourological Society Guideline
15
Copyright © 2016 American Urological Association Education and Research, Inc.®
Figure 2:
Figure 3:
Surgical Management
of Stones
American Urological Association (AUA)
Endourological Society Guideline

16
trials, the primary outcome parameter in this trial was
absence of need for additional intervention at four
weeks rather than radiographic evidence of stone
passage. These investigators found no difference
between either of the active treatment groups and the
placebo group regarding the absence of need for further
intervention (81% for tamsulosin versus 80% for
placebo, adjusted risk difference 1.3%, 95% CI -5.7 to
8.3, p=0.73; 80% nifedipine versus 80% placebo,
adjusted risk difference 0.5%, 95% CI -5.6 to 6.5,
p=0.88). Furthermore, subgroup analysis evaluating
the effect of stone size and location failed to reveal
subgroups of patients who would benefit from therapy.
Although this well-designed trial, which is much larger
than any of the other published MET trials that showed
a benefit of therapy, raises concern about the validity of
the recommendation in favor of MET, this trial is not
necessarily comparable to the others because of the
difference in outcome parameters. The absence of need
for intervention rates is much higher (80%) in the UK
trial than in the pooled control arms of the other α-
blocker MET trials for which the radiographic
spontaneous passage rate for <10mm stones in all
locations in the ureter was 53%. Therefore, the results
of this trial were not incorporated into this Panel’s meta
-analysis, and the recommendation for MET in properly
selected patients still stands until further compelling
studies suggest otherwise.
Finally, most of the trials evaluating the efficacy of α-
blockers and calcium channel blockers in promoting
spontaneous stone passage in patients with ureteral
stones either exclusively enrolled patients with distal
ureteral stones or were largely dominated by such
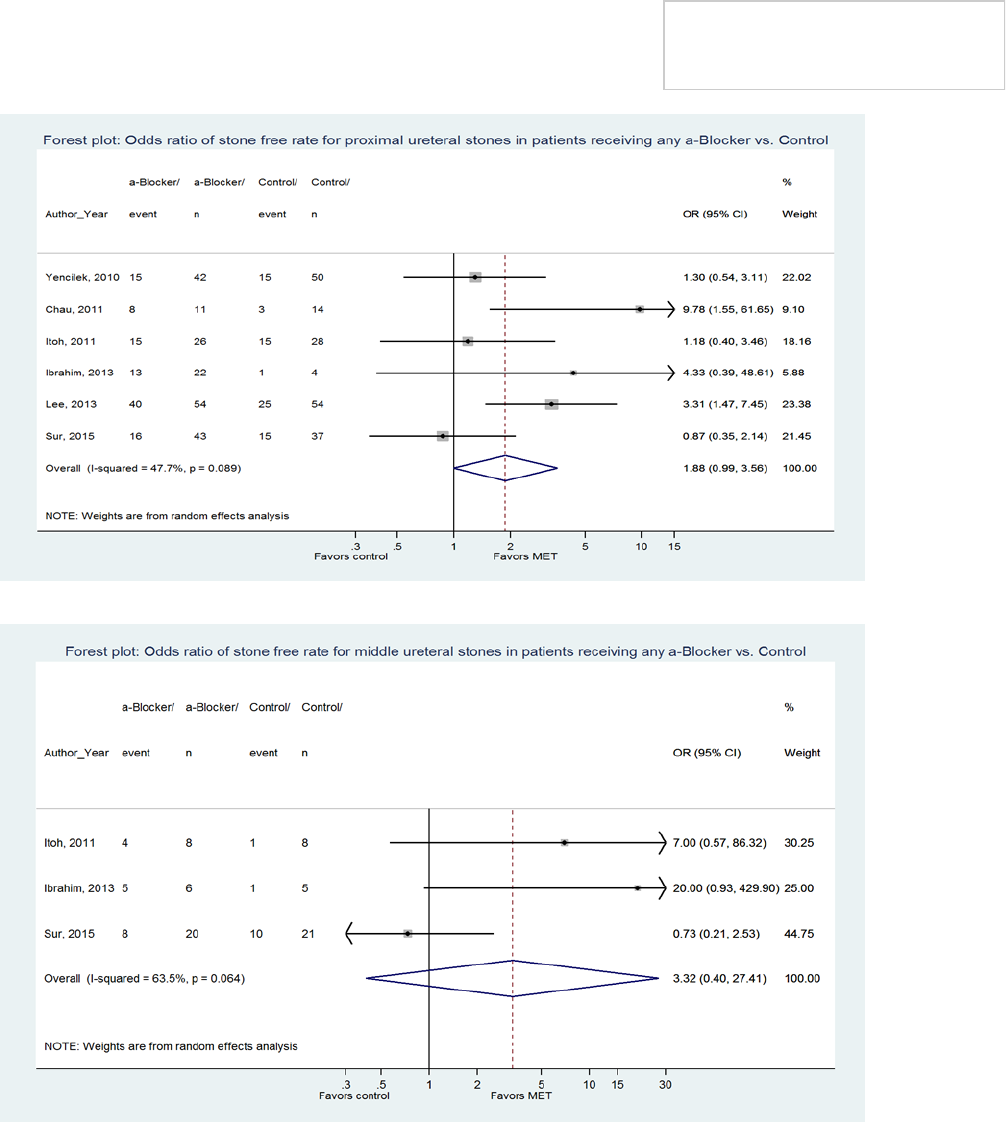
patients. Based on the few α-blocker trials that included
patients with middle and proximal ureteral calculi, the
Panel’s analysis found no benefit of therapy (Figures 4
and 5), and there were no trials evaluating nifedipine in
patients with middle and proximal ureteral stones.
Consequently, the Panel could not specifically endorse
MET for stones in these locations. However, because of
the low side effect profile of α-blockers and the
demonstrated efficacy of α-blockers in patients with
<10 mm stones in any location of the ureter, the Panel
feels that a trial of these agents in this patient
population, despite the lack of demonstrable benefit,
can be considered an option until larger scale trials are
available to provide more definitive direction.
Patients should be informed that medications for MET
are prescribed for an off label indication.
8. Clinicians should offer reimaging to patients
prior to surgery if passage of stones is
suspected or if stone movement will change
management. Reimaging should focus on the
region of interest and limit radiation exposure
to uninvolved regions. Clinical Principle
If a patient is in the process of ureteral stone passage,
clinicians should offer repeat imaging prior to stone
intervention if symptoms have changed because a
change in stone position may influence treatment
approach (URS versus SWL versus continued
observation), particularly if passage of the stone is
suspected. Repeat imaging can include KUB x-ray,
renal/bladder US, or CT. If feasible, a tailored approach
should be utilized to limit radiation exposure.
A change in ureteral stone position can influence SWL
success. It may also affect the decision to change
intervention modality (e.g., from SWL to URS if a stone
has advanced from the proximal ureter to the mid-
ureter overlying the bony pelvis) or to defer
intervention if the stone has advanced to the distal
ureter and continued observation is reasonable.
Kreshover et al. found an approximately 10% risk of
negative URS for ureteral stones smaller than 4 mm in
size in a distal ureteral location.
54
Other factors that
influence the decision to re-image a patient include
pain, time interval since prior imaging, and presence of
obstruction/hydronephrosis.
9. In most patients, if observation with or
without MET is not successful after four to six
weeks and/or the patient/clinician decide to
intervene sooner based on a shared decision
making approach, the clinicians should offer
definitive stone treatment. (Index Patients 1-
3) Moderate Recommendation; Evidence Level
Grade C
Should MET be selected as a management strategy for
the patient with a ureteral stone that has the potential
for spontaneous passage, the clinician must have a
clear understanding of the indications to alter this
Copyright © 2016 American Urological Association Education and Research, Inc.®
Surgical Management
of Stones
American Urological Association (AUA)
Endourological Society Guideline
17
approach and proceed with definitive intervention.
It is the Panel’s opinion that recurrent renal colic
requiring repeated visits to the emergency department
or hospital admission for parenteral analgesia,
worsening renal function, or evidence of urinary tract
sepsis are all indications to proceed with surgical
intervention.
While the maximum time duration for a trial of MET has
not been clearly elucidated, experimental data on the
effects of complete unilateral ureteral obstruction on
renal function suggest the interval of conservative
therapy should not exceed six weeks from initial clinical
presentation in order to avoid irreversible kidney
injury.
55
While admittedly not all ureteral stones cause
complete obstruction, the Panel recommends a six
week interval to reduce the potential for permanent
damage. A previous study has also indicated that most
stones destined to pass spontaneously will do so within
six weeks.
49
As such, there seems little benefit in
Copyright © 2016 American Urological Association Education and Research, Inc.®
Figure 4:
Figure 5:
Surgical Management
of Stones
American Urological Association (AUA)
Endourological Society Guideline

18
continuing MET beyond this time interval. Moreover, a
shared decision making approach between patient and
clinician should be adopted in that the choice to change
from a conservative to interventional approach should
take into account social factors, such as work
obligations, travel plans, and family care issues.
56
10. Clinicians should inform patients that SWL is
the procedure with the least morbidity and
lowest complication rate, but URS has a
greater stone-free rate in a single procedure.
(Index Patients 1-6) Strong Recommendation,
Evidence Level Grade B
For the patient requiring definitive treatment of a
ureteral stone, URS and SWL are the two most
commonly used treatment modalities. (Figure 6) The
present Panel’s analysis revealed no statistically
significant differences between SWL and URS with
regard to UTI (median 4.5% versus 2.9%,
respectively), sepsis (median 1.2% versus 0.3%,
respectively), ureteral stricture (median 0% versus
0.2%, respectively), or ureteral avulsion (median 0%
versus 0.1%, respectively). However, ureteral
perforation occurred significantly more frequently
during URS than SWL (median 3.2% versus 0%,
respectively, p<0.01). The 2012 Cochrane Review
comparing SWL and URS identified 7 RCTs reporting
complication rates and found a significantly lower
complication rate for SWL compared to URS (RR 0.53,
95% CI 0.33-0.88, p=0.01).
57
Likewise, the 2007 EAU/
AUA Guideline for the Management of Ureteral Calculi
found a higher complication rate for URS compared to
SWL for stones in all locations in the ureter: 11%
versus 4%, respectively, for proximal ureteral stones;
14% versus 4%, respectively, for middle ureteral
stones; and 7% versus 1%, respectively, for distal
ureteral stones.
5
While stone-free rates are reportedly
high for both modalities, URS stone-free rates have
been shown to be superior to SWL stone-free rates in
contemporary series. The Panel’s analysis of studies
comparing URS and SWL for treatment of ureteral
calculi showed superior stone-free rates for URS over
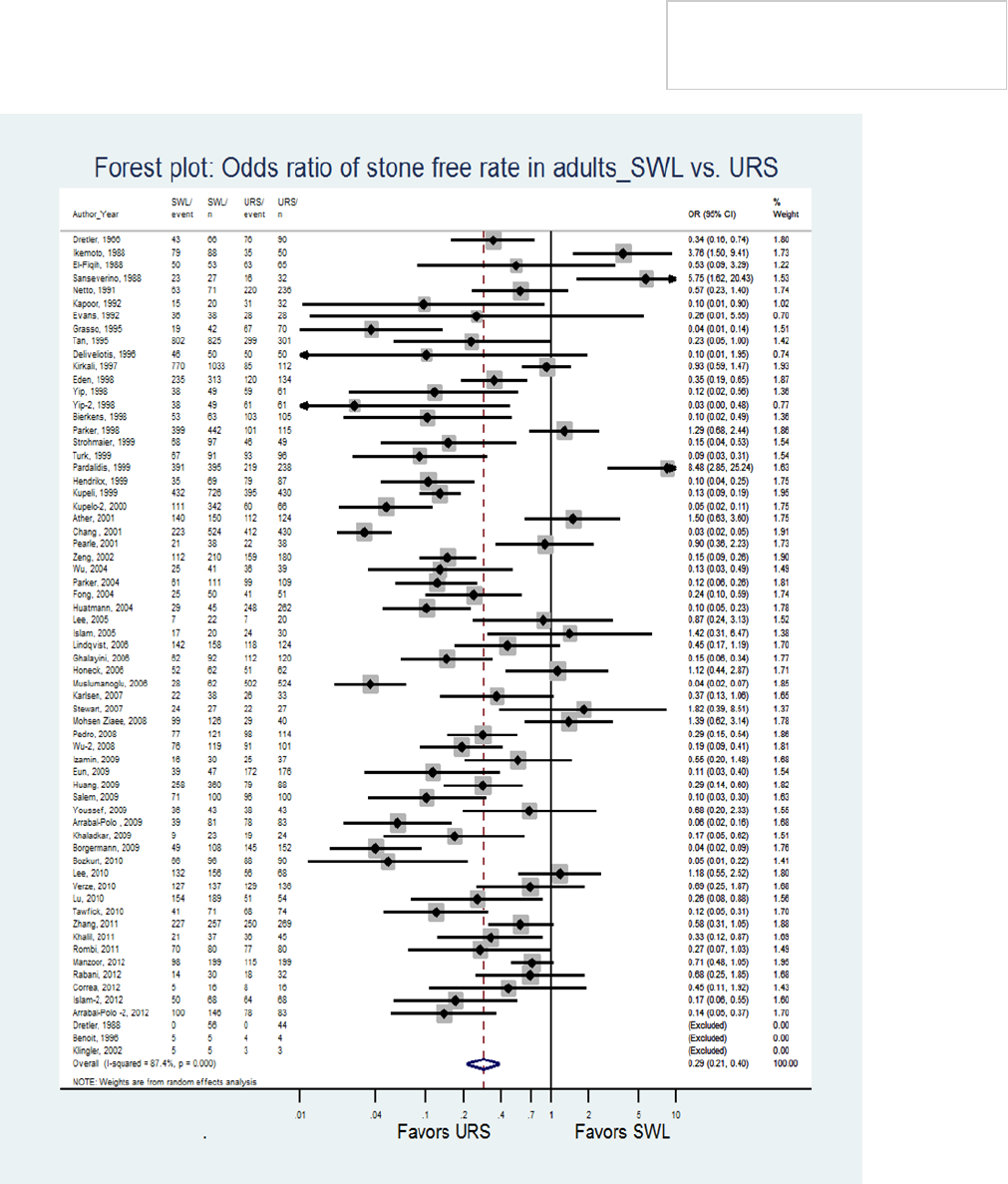
SWL (90% for URS versus 72% for SWL, RR SWL/URS
0.294, 95% CI 0.214-0.404, p<0.001). For stones ≤10
mm in size stratified by stone location, median stone-
free rates remained superior for URS over SWL at all
locations in the ureter (85% versus 66.5%,
respectively, for proximal ureteral stones; 91% versus
75%, respectively, for middle ureteral stones; and 94%
versus 74%, respectively, for distal ureteral stones)
(Table 2). However, for stones >10 mm in size, stone-
free rates were comparable for SWL and URS (74%
versus 79%, respectively) in the proximal ureter, while
stone-free rates for stones in the mid and distal ureter
favored URS over SWL (82.5% versus 67%,
respectively, for mid ureteral stones; and 92% versus
71%, respectively, for distal ureteral stones).
Furthermore, URS is more likely than SWL to
successfully treat patients with a ≤10 mm ureteral
stone in a single procedure. According to the 2007 EAU/
AUA Guideline for the Management of Ureteral Calculi,
the mean numbers of primary URS procedures required
to treat stones in the proximal, middle and distal ureter
were 1.01, 1.00 and 1.00, respectively.
5
In contrast,
the corresponding mean numbers of primary SWL
procedures for stones in these locations were 1.34,
1.29, and 1.26, respectively. Consequently, since most
successful URS require only a single procedure and
stone-free rates are higher for URS than SWL for all
ureteral stones except proximal ureteral stones >10
mm in size, URS has an advantage over SWL with
regard to a higher success rates and need for fewer
procedures.
11. In patients with mid or distal ureteral stones
who require intervention (who were not
candidates for or who failed MET), clinicians
should recommend URS as first-line therapy.
For patients who decline URS, clinicians
should offer SWL. (Index Patients 2,3,5,6)
Strong Recommendation; Evidence Level
Grade B
The Panel’s meta-analysis demonstrated that URS is
associated with significantly higher stone-free rates in a
single procedure than SWL for patients with ureteral
stones.
8
The disparity in stone-free outcome was
particularly notable for patients with < 10 mm mid and
distal ureteral calculi (Table 2). Based on studies
comparing SWL versus URS for distal ureteral stones,
the overall success rate of SWL for distal ureteral
stones was reported to be approximately 65%
(2,260/3,488) compared to a 92% success rates for
URS (2539/2751) (p<0.001).
8
Therefore, URS should
Copyright © 2016 American Urological Association Education and Research, Inc.®
Surgical Management
of Stones
American Urological Association (AUA)
Endourological Society Guideline
19
be recommended as first-line therapy. Nonetheless,
patients should be counseled that SWL is an acceptable
alternative. Clinicians should discuss with the patient
the advantages and disadvantages of both SWL and
URS, including the respective anesthesia requirements,
stone-free rates, need for additional procedures, and
associated complications of each procedure. Stone-free
rates are higher for URS than SWL at all locations of the
ureter, and URS is more commonly successful in
achieving successful fragmentation and stone-free
status in a single session than SWL. Complication rates
are comparable between the two procedures except for
a higher rate of ureteral perforation with URS than
SWL. It should be noted that lower urinary tract
symptoms and flank pain are more common in patients
undergoing URS than SWL because of the more
Copyright © 2016 American Urological Association Education and Research, Inc.®
Figure 6:
Surgical Management
of Stones
American Urological Association (AUA)
Endourological Society Guideline
20
Copyright © 2016 American Urological Association Education and Research, Inc.®
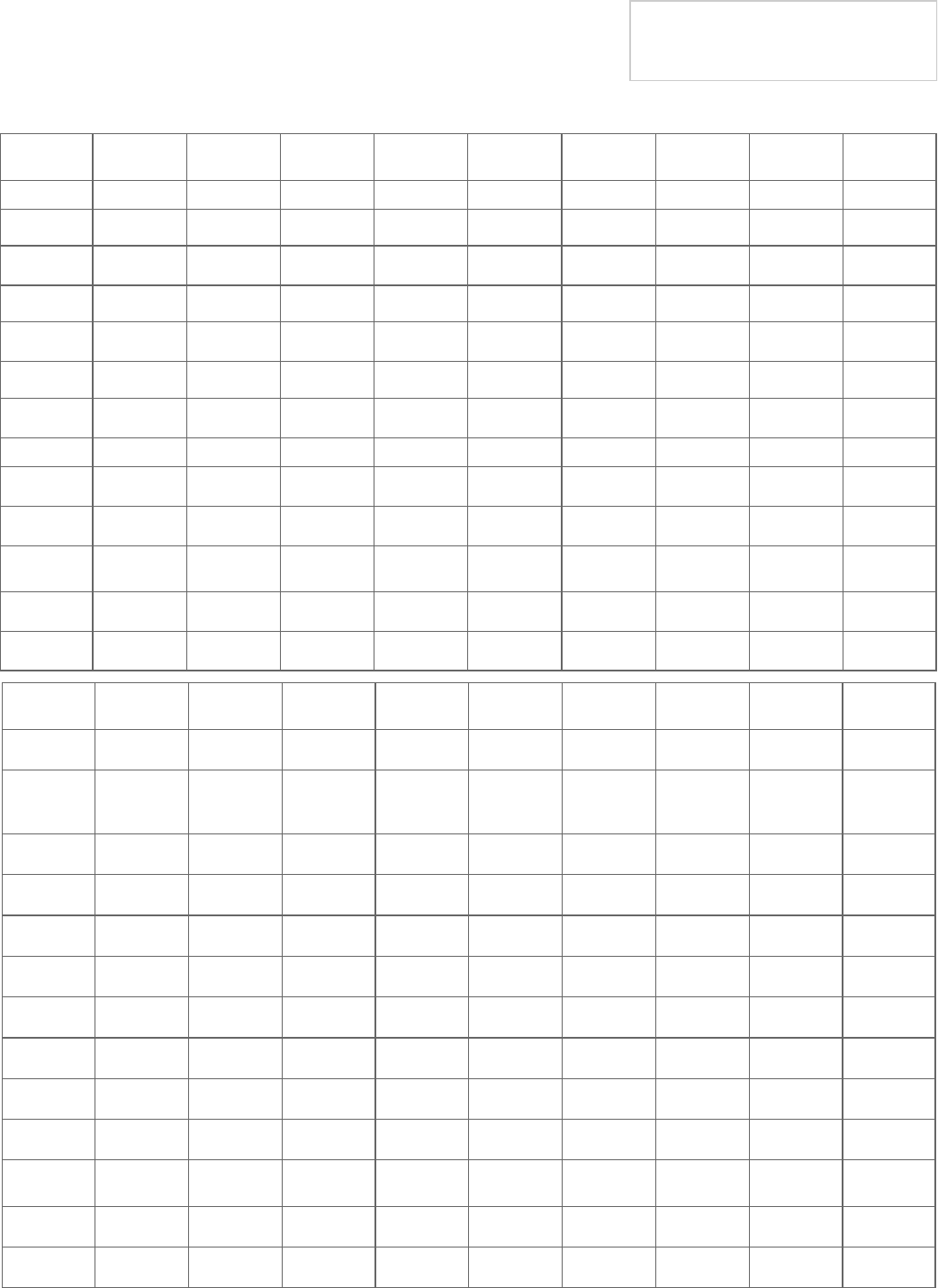
Table 2: Stone-free rates for SWL and URS in the overall population after all sessions performed
Distal
Ureter
Overall Size < 10
mm
Size > 10
mm
SWL
G/P Median CI (95%) G/P Median CI (95%) G/P Median CI (95%)
All forms 81/16573 74.65% (74-75)% 29/11420 73.96% (73-75)% 22/3785 71.47% (70-73)%
Bypass - - - - - - - - -
In situ 7/826 76.3% (73-79)% 16/259 86.5% (82-90)% 11/994 73.84% (71-77)%
Pushback - - - - - - - - -
Other 8/486 71% (57-82)% 3/35 90% (75-98)% 1/1 84% (15-100)%
URS
All forms 119/15938 93.58% (93-94)% 19/4008 94.21% (93-95)% 14/1705 92.26% (91-93)%
Flexible 4/159 96.8% (92-99)% - - - - - -
Mixed
Flexible
9/431 93% (89-96)% 1/38 97% (88-100)% 1/10 79% (50-96)%
Rigid 63/4254 89.9% (89-90)% 13/181 90.6% (85-94)% 8/533 94.7% (92-96)%
Semi-rigid 30/5169 97.25% (97-98)% 3/231 98.70% (96-100)% 3/132 95.4% (90-98)%
Total
Ureter
Overall Size < 10
mm
Size > 10
mm
SWL
Shock-
wave Lith-
otripsy
G/P Median CI (95%) G/P Median CI (95%) G/P Median CI (95%)
All forms 36/36215 68.95% (68-69)% 50/18879 63.96% (63-65)% 38/7433 61.62% (61-63)%
Bypass 1/67 92% (84-97)% 1/23 87% (59-91)% - - -
In situ 6/904 52.21% (49-55)% 27/598 86.79% (84-89)% 19/1683 65.18% (63-67)%
Pushback - - - 1/59 83% (72-91)% - - -
Other - - - 11/196 88% (81-93)% 10/698 70% (57-82)%
URS
All forms 101/29875 89.42% (89-90)% 38/11879 92.53% (92-93)% 31/5619 83.25% (82-84)%
Flexible 6/481 94.59% (92-96)% 2/81 97.5% (91-99)% - - -
mixed
flexible
- - - 7/209 87% (81-92)% 5/94 81% (67-92)%
Rigid 26/6430 84.99% (83-85)% 20/1715 87.35% (86-89)% 16/1641 71.48% (69-74)%
Semi-rigid 45/9984 91.86% (91-92)% 6/2329 69.35% (95-97)% 7/1064 90.79% (89-92)%
Surgical Management
of Stones
American Urological Association (AUA)
Endourological Society Guideline

21
universal use of stents in conjunction with URS than
SWL. Stent placement prior to SWL for patients with
≤10 mm ureteral calculi has not been shown to
improve stone-free rates and is not recommended.
5
Although stent placement after uncomplicated URS has
also been shown in randomized trials to be
unnecessary,
58
routine stent placement after URS is still
widely practiced. As such, patients should be informed
about the possible need for stent placement after URS,
and less commonly, after SWL, because this
information may influence their decisions. If successful
treatment in a single procedure is the most important
deciding factor for a patient, URS is the superior
treatment option. On the other hand, if non-
invasiveness and lower risk of complications are
paramount, then SWL may be the more appropriate
treatment selection. For women of child-bearing age
who harbor mid or distal ureteral calculi, URS is
preferred, as the effects of shock wave energy on the
ovary have not been completely elucidated.
Alternative treatment options, such as open or
laparoscopic ureterolithotomy, or antegrade URS via a
percutaneous approach, are not preferred over SWL
because of greater invasiveness. While based on the
Panel’s analysis, stone-free rates with URS for proximal
ureteral stones <10 mm were superior, those for such
stones >10 mm were equivalent. Therefore, the Panel
chose to not extend the recommendation to proximal
ureteral stones.
12. URS is recommended for patients with
suspected cystine or uric acid ureteral stones
who fail MET or desire intervention. Expert
Opinion
For those patients with known or suspected cystinuria
or uric acid stones, the choice of definitive intervention
following failed conservative therapy for a ureteral
stone can be complex. SWL may not be the best option
for patients with either stone composition for a number
of reasons. Cystine stones are often only faintly radio-
opaque and pure uric acid stones are typically
radiolucent. Therefore, stone targeting with fluoroscopy
may be problematic for SWL. Furthermore, cystine
stones are typically resistant to SWL fragmentation,
making this stone type less effectively treated by this
modality.
URS with intracorporeal lithotripsy is an effective
strategy for treating the majority of patients with
ureteral stones, regardless of stone type.
59
13. Routine stenting should not be performed in
patients undergoing SWL. (Index Patients 1-
6) Strong Recommendation; Evidence Level
Grade B
Some patients with ureteral stones undergo ureteral
stent placement to relieve pain and/or obstruction until
definitive treatment can be performed. However, some
urologists place ureteral stents prior to SWL with the
intention of improving stone-free rates or preventing
complications. Both the 1997 AUA Guideline and the
2007 EAU/AUA Guideline for the Management of
Ureteral Calculi recommended against routine stenting
with SWL based on comparable stone-free rates with or
without stent placement.
5,60
A recent systematic review
and meta-analysis comprising 8 RCTs and 876 patients
compared stented versus in situ SWL for renal and
ureteral stones and found no significant difference in
stone-free rates between the 2 groups (RR 0.97, 95%
CI 0.91-1.03, p=27).
61
Subgroup analysis of the 2 RCTs
involving 113 patients treated for ureteral stones only
also showed no benefit of stented over in situ SWL (RR
0.95, 95% CI 0.79-1.14, p=0.58). One trial in the
systematic review for which the incidence of
steinstrasse was reported also showed no difference
between the two groups; however, the incidence of
lower urinary tract symptoms was higher in the stented
group.
In the Panel’s analysis, no difference in stone-free rates
was found for SWL of ureteral stones with or without a
ureteral stent (82% versus 91%, respectively, p=NS).
As such, the current Panel reiterates the
recommendation of the previous Panels in
recommending against the use of ureteral stents with
the intention of improving stone-free rates.
14. Following URS, clinicians may omit ureteral
stenting in patients meeting all of the
following criteria: those without suspected
ureteric injury during URS, those without
evidence of ureteral stricture or other
anatomical impediments to stone fragment
clearance, those with a normal contralateral
kidney, those without renal functional
Copyright © 2016 American Urological Association Education and Research, Inc.®
Surgical Management
of Stones
American Urological Association (AUA)
Endourological Society Guideline

22
impairment, and those in whom a secondary
URS procedure is not planned. (Index Patients
1-6) Strong Recommendation; Evidence Level
Grade A
The insertion of a ureteral stent has long been
considered routine practice after URS. A number of
randomized prospective trials performed over the past
15 years, however, have called into question the dogma
of stent placement for uncomplicated URS.
62-73
Reported complications, such as UTIs, ureteral
strictures, and unplanned emergency room visits, were
not found to differ significantly between stented and
unstented groups in two published meta-analyses.
74,75
Moreover, stone-free rates were not appreciably
different between stented and unstented patients.
Patients without stents also typically reported less flank
pain and fewer lower urinary tract voiding symptoms.
Based on the best available evidence, a selective
approach to stent placement seems a more prudent
strategy. Among patients with ureteric injury during
URS, those with evidence of ureteral stricture or other
anatomical impediments to stone fragment clearance,
such as ureteral wall edema, a large stone burden
(>1.5 cm), those who have an anatomically or
functionally solitary kidney or renal functional
impairment, and in those in whom another ipsilateral
URS is planned, stent placement should be strongly
considered.
15. Placement of a ureteral stent prior to URS
should not be performed routinely. (Index
Patient 1-6) Strong Recommendation;
Evidence Level Grade B
Some patients undergoing URS for ureteral calculi have
ureteral stents placed prior to the procedure to relieve
pain and/or obstruction, particularly in the setting of
acute infection. However, some investigators have
recently advocated for stent placement prior to URS
with the intention of dilating the ureter and improving
outcomes of URS. Rubenstein and colleagues reported
higher stone-free rates in 36 prestented renal units
compared to 79 unstented renal units (67% versus
47%, respectively, p<0.02) in a group of 90 patients
who underwent URS (69% for ureteral stones).
76
In an
attempt to control for confounding factors, Chu and
colleagues also compared 45 prestented patients with
59 matched, unstented patients who underwent URS
for stones and found that prestenting was associated
with shorter first operative time in the whole cohort and
shorter cumulative operative time and reduced need for
reoperation in patients with > 1 cm proximal ureteral
stones but not in patients with stones < 1 cm or distal
ureteral stones.
77
Netsch and colleagues likewise
performed matched pair analysis to compare 143
unstented with 143 prestented patients undergoing
URS.
78
In the subgroup of patients with ureteral stones,
prestenting was associated with higher stone-free rates
in those with ≥5 mm stones (98% versus 83%,
respectively, p<0.0105) but not in those with <5 mm
stones (100% versus 93%, respectively, p=NS).
Nevertheless, despite an association between
prestenting and higher stone-free rates or shorter
operative time, in the absence of prospective data and
high level evidence, the Panel recommends against
routine stent placement prior to URS when the sole
purpose is to enhance stone-free rates or reduce
operative times. The rationale for this is that the
improved stone-free rates with certain stones achieved
with prestenting do not override the added care costs
and negative impact on quality of life associated with
stents.
16. Clinicians may offer α-blockers and
antimuscarinic therapy to reduce stent
discomfort. (Index patients 1-6) Moderate
Recommendation; Evidence Level Grade B
Clinicians should counsel patients about the possibility
of post-operative stent discomfort and may prescribe α-
blockers to reduce stent discomfort. Other medications
that can be used to alleviate stent discomfort include
anticholinergics/antimuscarinics, bladder analgesics for
dysuria, non-steroidal anti-inflammatory agents
(NSAIDs), and narcotic analgesics.
α-blockers have been shown in multiple RCTs to have
benefit for stent related discomfort. Several meta-
analyses and systematic reviews of the literature have
demonstrated significant improvement in urinary
symptoms, body pain index score of the Ureteral Stent
Symptom Questionnaire, total International Prostate
Symptom Score (IPSS), Visual Analogue Pain Scale
(VAPS) score and QoL with use of α-blockers compared
Copyright © 2016 American Urological Association Education and Research, Inc.®
Surgical Management
of Stones
American Urological Association (AUA)
Endourological Society Guideline

23
to placebo or no treatment.
79-82
A meta-analysis evaluating the benefit of postoperative
antimuscarinics alone has shown significant
improvement in total IPSS and QoL scores with such
therapy. However, there are discordant results
regarding the benefits of combination therapy (α-
blocker and antimuscarinic agent) over α-blocker
monotherapy; one study reporting that combination
was superior in alleviating symptoms and another
demonstrating no advantage.
79
The duration of ureteral stenting post-operatively
should be minimized in order to reduce stent-related
morbidity. In general, the Panel recommends three to
seven days of stenting following routine, uncomplicated
ureteroscopic stone intervention.
17. In patients who fail or are unlikely to have
successful results with SWL and/or URS,
clinicians may offer PCNL, laparoscopic, open,
or robotic assisted stone removal. (Index
patient 1-6) Moderate Recommendation;
Evidence Level Grade C
In some patients with large or complex ureteral stone
burdens, neither URS nor SWL are likely to accomplish
stone clearance in a reasonable number of procedures.
In such cases, alternative approaches may be
considered. Percutaneous antegrade URS may allow for
more expeditious stone clearance, as larger and more
efficient instrumentation can be utilized.
83-90
Ureterolithotomy may also be considered as an
alternative therapy in these rare clinical scenarios. Both
laparoscopic and robotic-assisted ureterolithotomy
provide results equivalent to open surgery, but with
red uced mo rbidi t y.
8 3 , 8 5 , 9 1 - 94
T he refo r e , if
ureterolithotomy is performed, a laparoscopic or robotic
approach is preferred for most cases.
18. Clinicians performing URS for proximal
ureteral stones should have a flexible
ureteroscope available. (Index Patients 1, 4)
Clinical Principle
Performing semi-rigid URS in the proximal ureter may
not be possible, and if undertaken may incur a higher
risk of ureteral injury because the semi-rigid
ureteroscope may be unable to accommodate the
angulation of the ureter associated with a large
prostate or the iliac vessels. Additionally, performing
semi-rigid URS above the level of the iliac vessels can
cause additional torque on the ureteroscope, placing
the ureteroscope itself at risk for damage. These
limitations are overcome by flexible URS. While either
laser or pneumatic lithotripsy maybe used with semi-
rigid ureteroscopes, laser lithotripsy is the preferred
intracorporeal lithotrite for use with flexible
ureteroscopes. Small laser fibers easily pass through
the working channel of all currently available flexible
ureteroscopes, allowing adequate deflection and irrigant
flow. Either holmium or thulium lasers can be utilized
with flexible ureteroscopes.
The limitations of semi-rigid URS are overcome by
flexible URS. Flexible URS has been shown in both
prospective and retrospective studies to have high
overall success rates with low morbidity/complications
for < 2 cm proximal ureteral stones.
95-97
Failure and
retreatment rates were higher in the proximal ureter for
semi-rigid URS compared to flexible URS.
97
19. Clinicians should not utilize EHL as the first-
line modality for intra-ureteral lithotripsy.
(Index patients 1-6,13,15) Expert Opinion
Electrohydraulic lithotripsy (EHL) is highly effective at
fragmenting most stone compositions with a 90%
overall fragmentation rate.
98
EHL works as an
underwater spark plug by which spark generation
produces a cavitation bubble in the surrounding fluid
resulting in stone fragmentation. Since the energy is
not focused, the EHL probe must be positioned near the
stone. The major disadvantage of EHL is its propensity
to damage the ureteral mucosa, resulting in ureteral
perforation. It is speculated that the expanding
cavitation bubble generated by the spark may produce
injury to the mucosa even when the probe is not in
direct contact with the urothelium, with reported rates
of ureteral injury of 8.5%-17.6%.
98-100
A prospective
randomized trial of EHL versus pneumatic lithotripsy
during URS for ureteral stones demonstrated equivalent
efficacy of stone fragmentation with both technologies
(85.3% EHL and 89.5% pneumatic), but the ureteral
perforation rates were significantly higher in the EHL
group (17.6% versus 2.6%, respectively).
99
Holmium:YAG laser produces stone fragmentation rates
of 100% and has comparable fiber flexibility to the EHL
Copyright © 2016 American Urological Association Education and Research, Inc.®
Surgical Management
of Stones
American Urological Association (AUA)
Endourological Society Guideline

24
probe, but with a higher safety profile.
101
The holmium
laser can be activated 0.5 mm from the urothelial
surface without risk of injury.
102
Thus, with safer and
more efficient technology available for ureteroscopic
stone extraction, the Panel recommends EHL not be
used for stone fragmentation. Due to a larger working
area, EHL can safely be used in the kidney during PCNL,
but the risk of perforation using this technology is still
higher than other modalities. Therefore care should be
taken to avoid activation of the probe near the
urothelial surface.
20. In patients with obstructing stones and
suspected infection, clinicians must urgently
drain the collecting system with a stent or
nephrostomy tube and delay stone treatment.
Strong Recommendation; Evidence Level
Grade C
Stone manipulation in the setting of active, untreated
infection with concomitant urinary tract obstruction can
lead to life-threatening sepsis. In this situation, it is
mandated that the collecting system be drained, either
with a nephrostomy tube or a ureteral stent to allow
drainage of infected urine and permit antibiotic
penetration into the affected renal unit.
103
Using the
Nationwide Inpatient Sample to study the outcome of
obstructing ureteral calculi associated with sepsis,
Borofsky et al. demonstrated that mortality was higher
in those not treated with surgical decompression
compared to those who underwent drainage (19.2% vs
8.82%, p<0.001). Lack of surgical decompression,
which occurred in 22% of the overall study population
was independently associated with an increased odds
ratio of mortality, even when adjusting for patient
demographics, co-morbidities, and geographic region of
treatment (OR 2.6, 95% CI 1.9-3.7).
104
The choice of drainage modality, stent or nephrostomy
tube, is left to the discretion of the urologist, as both
have been shown in an RCT to be equally effective.
103
Definitive management of the stone should not be
undertaken until sepsis has resolved and the infection
has been treated with an appropriate course of
antibiotic therapy.
21. In symptomatic patients with a total non-
lower pole renal stone burden < 20 mm,
clinicians may offer SWL or URS. (Index
Patient 7) Strong Recommendation; Evidence
Level Grade B
Treatment options for patients with a <20 mm non-
lower pole renal stone burden include SWL, URS, and
PNL. Of these treatment options, PCNL stone-free rates
are the least affected by stone size, while stone-free
rates of both SWL and URS decline with increasing
stone burden.
105
However, for stone burdens <20mm,
stone-free rates of both URS and SWL are acceptable
and have less morbidity compared to PCNL. Of the two
options, URS and SWL, URS is associated with a lower
likelihood of repeat procedure; therefore, the patient
will become stone-free quicker than with SWL.
106
While
SWL and URS are acceptable modalities, treatment
selection process must include a shared decision-
making approach.
22. In symptomatic patients with a total renal
stone burden >20 mm, clinicians should offer
PCNL as first-line therapy. (Index Patient 8)
Strong Recommendation; Evidence Level
Grade C
PCNL should be offered as first-line therapy for patients
with a total renal stone burden > 20 mm because it
offers a higher stone-free rate than SWL or URS and is
less invasive than open surgery or laparoscopic/robotic
assisted procedures. Compared to SWL and URS, the
success rate of PCNL is also less affected by stone
composition, density and location. In a RCT comparing
PCNL to URS for >2cm renal pelvic stones, the stone-
free rate was higher for PCNL compared to URS (94%
versus 75%), although predominantly semi-rigid URS
was used in this study.
107
A more recent prospective
randomized trial comparing standard PCNL to staged
flexible URS for renal pelvic stones > 2 cm showed an
advantage of PCNL over URS because of the need for
multiple treatments and longer treatment time for
URS.
108
The benefit of a higher stone-free rate must be weighed
against the increased invasiveness and risk of
complications for PCNL compared to URS or SWL. A
recent systematic review and meta-analysis of PCNL
versus URS reported higher complication rates for PCNL
(OR 1.61; 95% CI 1.11-2.35).
109
The CROES PCNL
Global Study reported a 15% overall complication rate
with the majority of complications categorized as
Copyright © 2016 American Urological Association Education and Research, Inc.®
Surgical Management
of Stones
American Urological Association (AUA)
Endourological Society Guideline

25
Clavien Grade I. Bleeding necessitating blood
transfusion was the most common complication at
7%.
110,111
As recommended in the 2005 AUA guideline on the
Management of Staghorn Calculi, PCNL should also be
the first treatment utilized for most patients with
staghorn calculi.
4
While studies comparing PCNL to open
surgery for staghorn calculi have shown comparable
stone-free rates, PCNL is the preferred treatment
modality as it offers lower morbidity evidenced by
decreased intraoperative and postoperative
complications, decreased length of hospital stay, earlier
return to work, and much smaller surgical incision.
112
In
an older randomized prospective trial comparing PCNL
to SWL for the treatment of staghorn calculi, Meretyk
found a three-fold higher stone-free rate with PCNL
combination therapy (PCNL/SWL) than with SWL
monotherapy. In addition, the rate of sepsis was
significantly higher with SWL.
113
The Panel’s analysis of
two non-randomized comparative studies enrolling a
total of 263 patients comparing PCNL to SWL for the
treatment of staghorn stones found the stone-free rate
for PCNL to be superior (19-57% SWL versus 69.5-
76.9% PCNL).
114,115
23. When residual fragments are present,
clinicians should offer patients endoscopic
procedures to render the patients stone-free,
especially if infection stones are suspected.
( I n d e x P a t i e n t 1 1 ) M o d e r a t e
Recommendation; Evidence Level Grade CII
Recent studies have demonstrated that residual stone
fragments following treatment with SWL, URS or PCNL
are not clinically insignificant. In a retrospective
analysis of the natural history of residual fragments
following PCNL, 43% patients experienced a stone
related event at a median of 32 months. Multivariate
analysis found residual fragment size > 2mm and
location within the renal pelvis or ureter to be
independent predictors of a stone event.
116
Similarly, in
a recent report by the EDGE Research Consortium
evaluating patients with residual fragments following
URS, 15% of patients developed a complication
requiring no intervention and an additional 29% of
patients required intervention for residual fragments.
Residual fragment size > 4mm was associated with a
significantly higher rate of stone growth, complications,
and re-intervention.
117
In patients with known or
suspected infection stones, residual stone fragments
have even greater consequences.
A number of studies have demonstrated that untreated
struvite stones have a high likelihood of stone growth
and recurrent infections. These “infection stones” may
grow to a large size, often filling a large portion or the
entire renal collecting system (i.e., staghorn calculus).
Such stones may cause persistent infection and chronic
obstruction, ultimately leading to severe renal damage
with the possibility of life threatening sepsis. The Panel
believes that removal of suspected infection stones or
infected stone fragments may significantly limit the
possibility of further stone growth, recurrent UTI, or
renal damage. The Panel acknowledges that an
endoscopic approach, either URS or PNL, offers the best
chance of complete removal of infection stones and that
complete stone removal should be the ultimate goal, in
order to eradicate any causative organisms, relieve
obstruction, prevent further stone growth or infection,
and ultimately preserve kidney function. Although some
investigations indicate that it may be possible to
sterilize small residual struvite stone fragments and
limit subsequent stone activity,
118
the majority of
studies suggest that residual fragments can grow and
become a source of recurrent UTIs.
115,119-123
Non-surgical treatment with antibiotics, urease
inhibitors, and other supportive measures only is not
considered a viable alternative except in patients
otherwise too ill to tolerate stone removal or when the
residual fragments cannot be safely retrieved.
24. Stone material should be sent for analysis.
Clinical Principle
An exception would be a patient who has had multiple
recurrent stones that have been documented to be of
similar stone composition and there is no clinical or
radiographic evidence that stone composition has
changed.
25. In patients with total renal stone burden >20
mm, clinicians should not offer SWL as first-
line therapy. (Index Patient 8) Moderate
Recommendation; Evidence Level Grade C
SWL is often considered an attractive treatment option
Copyright © 2016 American Urological Association Education and Research, Inc.®
Surgical Management
of Stones
American Urological Association (AUA)
Endourological Society Guideline

26
by patients and clinicians due to its decreased
invasiveness and morbidity compared to PCNL and URS.
However, SWL should not be offered as first-line
therapy for patients with a total renal stone burden >
20 mm because several studies have reported
significantly reduced stone-free rates and increased
need for multiple treatments for SWL compared to
PCNL in this setting.
115,124
Non-randomized comparative
studies have found the stone-free rate for SWL to be
inferior to PCNL.
114,115
The success of SWL is dependent
on several other factors, including obesity, skin-to-
stone distance, collecting system anatomy, stone
composition and stone density/attenuation, which could
also contribute to lower stone-free rates.
28,125-129
Furthermore, when SWL is utilized for one or more
>2cm stones, the risk of ureteral obstruction from
stone fragments (steinstrasse) increases to 24.3%
compared to 15.9% for stones 1-2 cm in size and 4.5%
for stones less than 1 cm.
130-132
26. Open/ laparoscopic /robotic surgery should
not be offered as first-line therapy to most
patients with stones. Exceptions include rare
cases of anatomic abnormalities, with large or
complex stones, or those requiring
concomitant reconstruction. (Index Patients 1
-15) Strong Recommendation; Evidence Level
Grade C
Advances in URS and PCNL instrumentation and
technique, as well as newer understanding of SWL
stone fragmentation, now allow endoscopic or shock
wave management of the vast majority of symptomatic
renal and ureteral calculi. Yet, there continue to be a
limited number of cases where an endoscopic or SWL
approach may not provide a reasonable chance at
complete stone removal with a practical number of
procedures. In these rare cases, patients may be
offered open, laparoscopic, or robotic nephrolithotomy/
pyelolithotomy/ureterolithotomy as a more efficient
way to remove large or complex stones, especially in
patients with anatomic abnormalities of the urinary
tract. A small number of case series, prospective trials
and one meta-analysis suggest that laparoscopic or
r o b o t i c n e p h r o l i t h o t o m y / p y e l o l i t h o t o m y /
ureterolithotomy offers a reasonable alternative to
PCNL or URS in these complex patients.
133-138
One area where open laparoscopic or robotic stone
removal offers an advantage over standard PCNL or
URS is in patients with stones and anatomic defects
that require reconstruction, such as those with
concomitant UPJ obstruction or ureteral stricture.
27. Clinicians may perform nephrectomy when the
involved kidney has negligible function in
patients requiring treatment. (Index Patients
1-14) Conditional Recommendation; Evidence
Level Grade C
When considering nephrectomy for the poorly
functioning kidney, overall renal function and the
condition of the kidney on the contralateral side should
be considered. This is best accomplished with a nuclear
renal scan as well as laboratory testing of renal function
with serum creatinine and an estimation of glomerular
filtration rate. Another option may be estimation of
differential creatinine clearance in patients with an
obstructed kidney and a nephrostomy tube. If the
involved kidney is obstructed, drainage of the kidney
and reassessment of renal function is a consideration if
there is a chance of recovering function. Observation
may be appropriate for some asymptomatic patients.
However, poorly functioning kidneys can often be a
source of persistent infection, pain, and pyelonephritis.
In these cases, nephrectomy may be the best
treatment option to relieve symptoms and prevent
systemic complications, such as sepsis and
xanthogranulomatous pyelonephritis.
139
The risk of the
procedure must be weighed against the benefit to the
patient and will depend on multiple clinical factors (e.g.,
age, medical co-morbidities, body habitus).
140
The
approach utilized (open, laparoscopic, robotic assisted,
retroperitoneal, trans-peritoneal) is based on a number
of factors, including the degree of inflammation/
infection, renal size, patient condition, patient anatomy,
patient preference, and the surgeon’s experience.
Nephrectomy should be avoided, if possible, in
pregnant patients until after they deliver.
28. For patients with symptomatic (flank pain),
non-obstructing, caliceal stones without
another obvious etiology for pain, clinicians
may offer stone treatment. (Index Patient 12)
Moderate Recommendation; Evidence Level
Grade C
Copyright © 2016 American Urological Association Education and Research, Inc.®
Surgical Management
of Stones
American Urological Association (AUA)
Endourological Society Guideline

27
Whether non-obstructing caliceal stones can be a
source of pain is controversial. However, since there
are published reports of eradication of flank pain with
stone removal in this setting, the Panel feels that
patients with pain and non-obstructing caliceal stones,
without another obvious source of their pain, may be
offered surgical intervention for stone treatment.
141-144
The patient must be informed of the possibility that the
pain may not improve or resolve after the procedure.
29. For patients with asymptomatic, non-
obstructing caliceal stones, clinicians may
offer active surveillance. Conditional
Recommendation; Evidence Level grade C
The detection of asymptomatic stones has increased
mainly due to the increased utilization of CT imaging.
Observation of asymptomatic, non-obstructing caliceal
stones is appropriate as long as the patient is counseled
about the risk of stone growth, passage, and pain.
There is conflicting data on the natural history of
asymptomatic renal stones. Several studies have
evaluated the risk of progression, defined as a
symptomatic stone event, stone growth on serial
imaging, and/or need for intervention. In a
retrospective cohort study of 107 patients with
asymptomatic renal stones followed for 31.6 months,
Glowacki et al. reported a 31.8% rate of developing a
symptomatic stone event.
145
Further Kaplan Meier
analysis estimated the risk of a symptomatic stone
episode or need for intervention to be approximately
10% per year with a cumulative 5 year event
probability of 48.5%. Two additional retrospective
studies
146,147
and one prospective study
148
of patients
with asymptomatic renal stones (mean size 5.7-10.8
mm) demonstrated the risk of a symptomatic stone
event to be 13%, of stone growth to be 30-46%, and a
need for intervention of 7-26%. Lower pole stone
location and isolated stone ≥ 4 mm were associated
with a higher likelihood of failing observation.
146
In a
prospective RCT comparing SWL to observation for
asymptomatic caliceal stones <15mm total diameter,
Keeley at al. reported no advantage of SWL with regard
to stone-free rate, QoL, renal function, symptoms, or
hospital admission.
143
However, in a prospective
randomized trial comparing PCNL, SWL, and
observation for asymptomatic lower pole stones (mean
stone size was similar among groups 153, 139, 137
mm
2
,
respectively), stone-related events were noted in
more than 20% of patients in the observation arm.
149
Taken collectively, these studies suggest that, while
approximately 50% of asymptomatic stones will
progress, a much smaller percentage will require
surgical intervention.
There are certain settings for which treatment of
asymptomatic, non-obstructing caliceal stones may be
more appropriate than observation. Treatment should
be considered in cases of associated infection,
vocational reasons (e.g. airline pilots, military), and
poor access to contemporary medical care.
If observation is chosen for asymptomatic, non-
obstructing caliceal stones, active surveillance with
follow-up imaging studies to assess for stone growth or
new stone formation is recommended. Dietary
modifications and medical therapy may be considered,
especially if the latter occur.
6
30. Clinicians should offer SWL or URS to patients
with symptomatic ≤ 10 mm lower pole renal
stones. (Index Patient 9) Strong
Recommendation; Evidence Level Grade B
This recommendation is supported by the results of a
multi-centered, prospective randomized trial that
demonstrated that there was no statistically significant
difference between the stone-free rates achieved with
URS and SWL. Intraoperative complications were
somewhat higher with URS, and patient-derived QoL
measures were somewhat better with SWL in this
study.150 CT imaging parameters should be used for
patient selection. Patients with a skin-to-stone distance
greater than 9-10 cm or stone attenuation greater than
900-1,000 Hounsfield units have less successful results
with SWL. Current CT software allows these indices to
be easily measured.
30,125,126
Certain techniques
employed during URS, including repositioning of stones
into the upper pole before fragmentation, utilization of
a ureteral access sheath, and extraction of the
generated fragments, may improve results.
151,152
31. Clinicians should not offer SWL as first-line
therapy to patients with >10mm lower pole
stones. (Index Patient 10) Strong
Recommendation; Evidence Level Grade B
Endoscopic approaches to the large lower pole stone
Copyright © 2016 American Urological Association Education and Research, Inc.®
Surgical Management
of Stones
American Urological Association (AUA)
Endourological Society Guideline

28
offer substantial benefit over SWL with regard to stone-
free rate with a moderate associated increase in risk.
8
Therefore, the use of an endoscopic approach rather
than SWL for a >10mm lower pole stone is a strong
recommendation.
Endoscopic procedures appear to be less affected by
stone burden than SWL. For lower pole stones 10-
20mm in size, the median success rate for SWL was
58% compared to a median success rate of 81% for
URS and 87% for PCNL. When the stone burden
exceeded 20mm, the median success rate of SWL
declined to 10%. In contrast, success rates for URS and
PCNL were 83% and 71%, respectively. It should be
noted that the URS series likely represented select
populations and treating surgeons with particular
expertise. Albala et al. reported an RCT that
demonstrated higher success rates for PCNL over SWL
for >10mm lower pole stones (91% versus 21%,
respectively).
153
32. Clinicians should inform patients with lower
pole stones >10 mm in size that PCNL has a
higher stone-free rate but greater morbidity.
(Index patient 10). Strong Recommendation;
Evidence Level Grade B
Treatment options for lower pole stones >10 mm in
maximum diameter include PCNL, retrograde URS, and
SWL. Randomized trials demonstrated that PCNL is
associated with superior single-treatment stone-free
rates, but with greater morbidity.
153
URS and SWL are
options for the management of these stones, but
clinicians should inform patients that re-treatment rates
are higher, and stone-free rates are significantly lower,
with a higher likelihood of clinical stone recurrence due
to retained fragments.
150
When considering SWL for
these stones, clinicians should consider collecting
system anatomy, stone attenuation, and skin-to-stone
distance as they can significantly impact treatment
results. When considering URS for these stones,
clinicians should inform patients that there is a risk that
the stone may not be accessible ureteroscopically,
particularly in patients with a narrow lower pole
infundibulum, an acutely angled lower pole
infundibulum, severe hydronephrosis, or renal
anomalies, such as a horseshoe kidney. In addition,
stones larger than 10mm may not be possible to grasp
and relocate, necessitating laser treatment in the lower
calyx, with the flexible ureteroscope maximally
deflected, potentially increasing the risk of laser fiber
failure and ureteroscope damage.
PCNL should be considered the primary treatment for
most cases, but patients should be well-informed of the
nature of the procedure, expected morbidity and
potential complications. PCNL with smaller access
sheaths (mini-PCNL or micro-PCNL) may allow similar
outcomes with lower complication rates.
154
33. In patients undergoing uncomplicated PCNL
who are presumed stone-free, placement of a
nephrostomy tube is optional. Conditional
Recommendation; Evidence Level Grade C
PCNL has traditionally been performed with an
indwelling nephrostomy tube left in place at the
conclusion of the procedure. The purpose of the
nephrostomy tube is to aid in healing of the
nephrostomy tract, promote hemostasis, drain urine to
prevent extravasation, and to allow for re-entry into the
collecting system should a secondary PCNL procedure
for residual stone fragments be necessary. However,
studies have demonstrated morbidity associated with
indwelling nephrostomy tubes following PCNL,
specifically increased postoperative pain with greater
narcotic requirements and increased length of
hospitalization.
155-158
Tubeless PCNL was introduced to
limit the negative side effects associated with
nephrostomy tube drainage. There are various types of
“tubeless procedures.” A common theme is that no
nephrostomy tube is inserted at the end of the
procedure. Renal drainage can be established with an
indwelling or externalized stent, or the patient can be
left without a stent. The tubeless approach should not
be undertaken if there is active hemorrhage or it is
likely that another PCNL will be needed to remove
residual stones.
In the appropriately selected patient, tubeless PCNL can
result in similar stone-free and complication rates as
standard PCNL. The Panel’s meta-analysis pooled data
from 38 studies, including 7 RCTs with a total of 2,073
patients, and demonstrated similar overall stone-free
and complication free outcomes between patients
undergoing standard PCNL versus tubeless PCNL.
8
Both
upper pole and lower percutaneous access sites were
Copyright © 2016 American Urological Association Education and Research, Inc.®
Surgical Management
of Stones
American Urological Association (AUA)
Endourological Society Guideline

29
included in the pooled studies. Furthermore, in the 23
studies that recorded analgesia requirements in the
first 24 hours, all but three demonstrated less analgesia
usage in the tubeless PCNL patients compared to
standard PCNL.
8
It should be noted that the majority of
the patients in the pooled tubeless PCNL group were
selected for the procedure and demonstrated limited
hemorrhage, no signs of infection, and no
intraoperative evidence of residual fragments at the
conclusion of the procedure. Patients undergoing
tubeless PCNL with an indwelling stent should be
counseled that cystoscopy and stent removal will be
required sometime after the procedure.
34. Flexible nephroscopy should be a routine part
of standard PCNL. Strong recommendation;
Evidence Level Grade B
Stone fragmentation (intracorporeal lithotripsy) is
commonly performed during PCNL. The resultant
fragments may migrate to areas in the collecting
system that cannot be safely accessed with a rigid
nephroscopy. If not removed, these fragments may
result in future stone events.
116,159,160
The utilization of
flexible nephroscopy during PCNL has been
demonstrated to improve stone-free rates. Gücük and
colleagues performed a randomized prospective study
in which patients underwent rigid nephroscopy during
PCNL with or without concomitant flexible nephroscopy,
and the stone-free rate was higher with concomitant
flexible endoscopy, 92.5% versus 70%.
161
If migration
of stone fragments down the ureter is suspected,
antegrade flexible nephroscopy should be considered.
Ureteral stones can be extracted when they are
identified.
35. Clinicians must use normal saline irrigation for
PCNL and URS. Strong Recommendation;
Evidence Level Grade B
Normal saline is the standard irrigation solution as it is
isotonic and iso-osmolar and does not lead to
significant electrolyte abnormalities when absorbed.
162
Some studies have advocated the use of sterile distilled
water in place of sterile saline, suggesting that
visualization in a bloody field may be superior with
water irrigation.
163,164
However, use of a non-isotonic
solution increases the risk of hemolysis, hyponatremia,
and heart failure if sufficient volume is absorbed.
165
Furthermore, modern endoscope optics are of such high
quality that sterile water irrigation provides little
advantage with regard to improved visibility.
Consequently, sterile normal saline remains the
preferred standard irrigant for endourologic stone
removing procedures.
Significant absorption of irrigation fluid may occur
during endoscopic stone surgery and cause
hypothermia and fluid overload. Maintenance of a low
intrarenal pressure may decrease the risks of these
occurrences. This can be facilitated with larger working
sheaths during PCNL and ureteral access sheaths with
URS.
166
36. A safety guide wire should be used for most
endoscopic procedures. (Index Patients 1-15)
Expert Opinion
In general, a safety guidewire is advisable when
performing retrograde URS or PCNL for stones. It can
facilitate rapid re-access to the collecting system if the
primary working wire is lost or displaced and can
provide access to the collecting system in cases of
ureteric or collecting system injury, including
perforation or avulsion. This will facilitate placement of
an internalized stent or nephrostomy tube in such
cases.
This is particularly valuable during URS when the ureter
is at risk (i.e., when there is pathology within the ureter
[stricture or stone disease] that renders proximal
access to the renal collecting system difficult.) This is
particularly true for semi-rigid and flexible ureteroscopy
for ureteral stones.
There are situations where a safety guidewire cannot be
placed, may not be necessary, or may even be harmful:
1. Severely impacted ureteral stones where even a
hydrophilic guidewire cannot safely be negotiated
proximal to the stone. In these cases, a guidewire
should be left below the stone, and the stone then
approached ureteroscopically and carefully
fragmented until the proximal ureteral lumen can
be identified and a safety wire placed. An
alternative would be placing a nephrostomy tube or
antegrade stent and performing stone removal at a
different time.
Copyright © 2016 American Urological Association Education and Research, Inc.®
Surgical Management
of Stones
American Urological Association (AUA)
Endourological Society Guideline

30
2. When a ureteral access sheath is being used to
facilitate treatment of intra-renal stones with the
flexible ureteroscope. If the ureteral access sheath
is placed within or just below the renal pelvis, then
the sheath itself can act as a safety wire.
37. Antimicrobial prophylaxis should be
administered prior to stone intervention and is
based primarily on prior urine culture results,
the local antibiogram, and in consultation with
the current Best Practice Policy Statement on
Antibiotic Prophylaxis. Clinical Principle
In the absence of a UTI, SWL does not require
antimicrobial prophylaxis as no invasive procedure is
performed. Perioperative antibiotic therapy, where
required, is administered within 60 minutes of the
procedure and redosed during the procedure if the case
length necessitates. Antibiotic prophylaxis is
recommended for ureteroscopic stone removal and
PCNL. A single oral or IV dose of an antibiotic that
covers gram positive and negative uropathogens is
recommended.
167
Patients undergoing PCNL with sterile urine may still
develop infectious complications including UTI and
sepsis.
168,169
This was the impetus for recommending
the utilization of prophylactic antibiotics in patients
subjected to this procedure.
167
The presence of
unsuspected bacteria within stones may be one of the
underlying causes for infectious complications after
PCNL. It has been reported that many patients with
negative voided urine cultures before PCNL have
positive kidney stone cultures.
39,40
In addition, a
positive stone culture has been reported to predict
sepsis following PCNL.
41
Mariappan et al. showed that
the administration of a one-week course of ciprofloxacin
to patients with sterile urine prior to PCNL reduced the
risk of urosepsis, although historical controls were used
in this trial.
170
Bag et al. demonstrated in a prospective
randomized trial that taking nitrofurantoin for one week
prior to PCNL reduced the risk of urosepsis in patients
with sterile urine.
171
A low rate of significant antibiotic-
related complications has been reported with this
approach.
172
However, the Panel did not feel that there
was enough evidence to endorse the practice of
administering this one-week course of antibiotic
therapy for patients with negative urine cultures prior
to PCNL.
38. Clinicians should abort stone removal
procedures, establish appropriate drainage,
continue antibiotic therapy, and obtain a urine
culture if purulent urine is encountered during
endoscopic intervention. (Index Patients1-15)
Strong Recommendation; Evidence Level
Grade C
An accepted principle is that operating in an infected
field carries increased risk. For endoscopic urological
procedures, the risk of urosepsis is well established and
feared. The presence of purulence at the time of
instrumentation mandates placement of a ureteral stent
or nephrostomy tube and aborting the procedure. The
purulent urine should be cultured, and broad spectrum
antibiotics should be administered, pending cultures.
The procedure can be undertaken once the infection is
appropriately treated.
39. In patients not considered candidates for
PCNL, clinicians may offer staged URS.
Moderate Recommendation; Evidence Level
Grade C
While PCNL is the optimal treatment for most patients
with complex, high-volume, and branched renal stones,
some anatomic abnormalities and/or patient factors
may provide relative contraindications to PCNL,
including use of anti-coagulation or anti-platelet
therapy that cannot be discontinued or the presence of
contractures, flexion deformities, or other anatomic
derangements that may preclude positioning for PCNL.
In these clinical scenarios, URS is a viable option,
although it may require staged or repeated procedures
to treat large stone volumes and may not render
patients completely stone free.
95,173-179
Patients should
be informed of these limitations, particularly those with
known struvite stones, where a stone-free state is
crucial for remaining infection- and stone-free. URS can
be safely performed in fully anticoagulated patients and
in those on anti-platelet agents, although the risk of
gross hematuria and clot retention/colic is higher.
When performing URS in this setting, clinicians should
make every effort to maintain low intra-renal irrigation
pressure with a ureteral access sheath as these
procedures can be lengthy, and prolonged high intra-
Copyright © 2016 American Urological Association Education and Research, Inc.®
Surgical Management
of Stones
American Urological Association (AUA)
Endourological Society Guideline

31
renal pressures can increase the risk of hemorrhage,
infection, sepsis, collecting system perforation, and
fluid absorption.
40. Clinicians may prescribe α-blockers to
facilitate passage of stone fragments
following SWL. Moderate Recommendation;
Evidence Level Grade B
The Panel performed a meta-analysis of 24 RCTs
assessing the efficacy of adjunctive therapy to facilitate
stone passage after SWL for renal or ureteral stones,
including 19 trials using α-blockers, and 16 using
tamsulosin.
8
The studies comprised 2,110 patients,
including 984 patients who received α-blockers and 883
who did not. Adjunctive therapy resulted in a nearly 2-
fold higher stone-free rate (OR 1.878, 95% CI, 1.508-
2.339). In addition, the time to clear stones was
approximately three days less with adjunctive therapy.
Many of these studies had limitations (inadequate
randomization and blinding), which downgraded the
quality of evidence.
Patients should be informed that the utilization of α-
blockers to facilitate fragment passage after SWL is
considered an off label indication.
41. If initial SWL fails, clinicians should offer
endoscopic therapy as the next treatment
option. (Index Patient 1-14) Moderate
Recommendation; Evidence Level Grade C
If initial SWL fails, it is important to re-evaluate the
stone characteristics (e.g., size, location, density,
composition) and patient characteristics (e.g., obesity,
collecting system anatomy, obstructed system) that
may have contributed to the initial failure. Similarly,
success may be stratified such that those who have had
partial fragmentation and clearance may be considered
for repeat SWL while those with no fragmentation and/
or clearance may be selected specifically for endoscopic
intervention.
Though European studies demonstrate incremental
increases in stone-free rates with repeated sessions of
SWL, other studies have demonstrated the higher
efficacy of an endoscopic approach in such instances.
Success rates for PCNL and URS as secondary
procedures after failed SWL are reported as 86-100%
and 62-100%, respectively.
180-189
42. Clinicians should use URS as first-line therapy
in most patients who require stone
intervention in the setting of uncorrected
bleeding diatheses or who require continuous
anticoagulation/antiplatelet therapy. (Index
Patients1-15) Strong Recommendation;
Evidence Level Grade C
Unlike both SWL and PCNL, URS can usually be safely
performed in patients with bleeding diatheses or in
those who cannot interrupt anticoagulation or
antiplatelet therapy. URS should be considered first-line
therapy for these patients when stone treatment is
mandatory. Clinicians should also consider deferred
treatment to a time when antiplatelet or anticoagulation
therapy can be safely interrupted or observation alone
for non-obstructing, non-infected, and asymptomatic
stones that do not require urgent treatment.
When performing URS in this setting, anticoagulation
should be modified to keep the INR near the lower
acceptable range to minimize the risk of hematuria,
hemorrhage, and clot retention/colic. Clinicians should
also strongly consider implementing measures to
minimize intra-renal pressure during these procedures
to further reduce the risk of hemorrhage and hematuria
by utilizing a ureteral access sheath, using non-
pressurized irrigation and keeping the bladder
decompressed with a small catheter if an access sheath
is not used.
,190,191
43. SWL should not be used in the patient with
anatomic or functional obstruction of the
collecting system or ureter distal to the stone.
Strong Recommendation; Evidence Level
Grade C
The presence of anatomic abnormalities or functional
abnormalities of the collecting system or ureter that
create obstruction distal to the targeted stone is
associated with lower stone-free rates when SWL is
utilized to treat urinary stones. Although studies looking
directly at distal obstruction and SWL are lacking,
experience with SWL in patients with anatomic
abnormalities associated with urinary obstruction
suggests that the ability to clear stone fragments is
limited. Abnormalities, such as UPJ obstruction,
urinary
diversion with ureteral anastomotic narrowing, ureteral
stricture, and caliceal diverticula
are associated with
Copyright © 2016 American Urological Association Education and Research, Inc.®
Surgical Management
of Stones
American Urological Association (AUA)
Endourological Society Guideline

32
retained stone fragments after SWL resulting in low
stone-free rates.
192-195
Although the overall risk of
developing urosepsis is low with SWL, the risk is
increased in the presence of obstruction distal to the
treated calculi.
196
Based on these findings the Panel
strongly recommends that any patient with obstruction
distal to the targeted stone not undergo SWL treatment
unless the obstruction can be treated.
44. In patients with symptomatic caliceal
diverticular stones, endoscopic therapy (URS,
PCNL, laparoscopic, robotic) should be
p r e f e r e n t i a l l y u t i l i z e d . S t r o n g
Recommendation; Evidence Level Grade C
The stone-free rates achieved with SWL for treating
patients with caliceal diverticular stones are quite low,
with the majority of series reporting 0-25%.
197
While
some of these patients may experience a reduction or
elimination of their symptoms, they are at risk for
symptom recurrence, and new or residual stone
growth.
198
The Panel’s meta-analysis also demonstrated
a low stone-free rate associated with SWL (13-21%).
Substantially higher stone-free rates are attainable with
URS, PCNL, laparoscopic and robotic surgery (18-90%
with URS and 62.5-100% with PCNL).
8
In addition, the
chance for eradication of symptoms is far greater.
197,198
An endoscopic approach also permits correction of the
anatomic abnormality, with the chance for successful
obliteration being highest with PCNL, laparoscopic, or
robotic assisted surgery.
197
The choice of optimal
endoscopic approach should be based on stone location
and size, relation to surrounding structures, and patient
preference.
45. Staghorn stones should be removed if
attendant comorbidities do not preclude
treatment. Clinical Principle
Numerous older retrospective studies have
demonstrated that untreated patients harboring
staghorn stones are at risk for deterioration of renal
function, including loss of the involved kidney, end
stage renal disease, infectious complications, and
mortality.
115,119,120,123,199
While the majority of these
older series involved patients with infection stones and
more recent studies have demonstrated that patients
with staghorn stones are more apt to have metabolic
stones, the Panel still endorses stone removal in
patients who are able to tolerate the rigors of long and
perhaps multiple procedures and their attendant risks,
including sepsis and hemorrhage.
200
Medical therapy
and supportive care are considerations for those not
thought to be operative candidates.
46. In pediatric patients with uncomplicated
ureteral stones ≤10 mm, clinicians should
offer observation with or without MET using α-
blockers. (Index Patient 13) Moderate
Recommendation; Evidence Level Grade B
An initial trial of observation with or without MET is
appropriate in children with ureteral stones because a
significant proportion of children will pass their stones
spontaneously, thus avoiding the need for surgical
intervention. In trials of MET in children, stone-free
rates in the observation (non-treatment) arm averaged
62% for stones under 5 mm diameter in the distal
ureter, and 35% for stones >5 mm.
201-203
Two of these
trials demonstrated that α-blockers facilitated stone
passage. Observation can be carried out under carefully
controlled conditions, assuming no evidence of
infection, the patient is able to hydrate orally, and pain
can be adequately controlled. Families should be aware
that the probability of spontaneous passage is lower for
children with stone approaching 1 cm in size.
Limited evidence does suggest that MET is effective in
increasing passage of distal ureteral stones in children,
and MET appears to be safe in this population. Three
modest randomized trials of α-blocker therapy in
children with distal ureteral stones
201-203
showed
significant benefit, with an overall odds ratio of being
stone free of 4.0 (95% CI: 1.1-14.8). However, bias is
a concern as these trials were not blinded.
The role of MET with α-blockers in pediatric patients
with middle and proximal ureteral stones, similar to
adults, is not well-defined. However, due to limited
reports of side effects in children with distal ureteral
stones, the Panel feels that such agents may be
prescribed to children harboring stones in these
locations. As in adults, the maximum time duration for
a trial of MET is undefined, but it seems prudent to limit
the interval of conservative therapy to a maximum of
six weeks from initial clinical presentation (as in adults)
in order to avoid irreversible kidney injury.
Copyright © 2016 American Urological Association Education and Research, Inc.®
Surgical Management
of Stones
American Urological Association (AUA)
Endourological Society Guideline

33
Parents and when appropriate the patient should be
informed that the utilization of α-blockers to facilitate
fragment passage after SWL is considered an off label
indication.
47. Clinicians should offer URS or SWL for
pediatric patients with ureteral stones who
are unlikely to pass the stones or who failed
observation and/or MET, based on patient-
specific anatomy and body habitus. (Index
Patient 13) Strong Recommendation; Evidence
Level Grade B
In children who are unlikely to pass a ureteral stone
spontaneously for any reason or who have already
failed a trial of medical or observational therapy,
surgical intervention to eliminate the stone is
appropriate. The benefits of treating the stone include
alleviating symptoms, minimizing risk of infection, and
preserving renal function by eliminating obstruction.
The Panel’s meta-analysis demonstrates that stone-free
rates in pediatric patients with ureteral stones <10 mm
are high for both SWL (87%) and URS (95%); lower for
larger ureteral stones (>10mm), stone-free rates are
73% for SWL and 78% for URS.
8
While SWL is an acceptable option for ureteral stones,
the poor visualization of the ureter (particularly the mid
-ureter) with US-based lithotriptors may limit use of
SWL in this setting. SWL may be preferable in certain
pediatric populations, such as very small children, or
other patients in whom ureteroscopic access may be
challenging due to their anatomy (e.g., severe scoliosis,
history of ureteral reimplantation).
48. Clinicians should obtain a non-contrast, low-
dose CT scan on pediatric patients prior to
performing PCNL. (Index Patient 13) Strong
Recommendation; Evidence Level Grade C
The rationale for obtaining a CT scan in this setting is
similar to that as described for adults undergoing this
procedure. Increased awareness of the potential
adverse effects of ionizing radiation in children has led
to efforts to reduce radiation exposure in this
population. Children may be more susceptible to
radiation-induced injury due to their rapidly developing
tissues, and they have a longer potential lifespan
during which radiation-induced illness may manifest.
The substantial contribution of medical imaging (and
particularly CT) to radiation exposure and subsequent
cancer risk in the pediatric population has become a
focus in the past 15 years.
204-207
Modified protocols and equipment permit CT imaging in
children that adheres to “ALARA” principles (radiation
exposure kept “as low as reasonably achievable”).
208
Several studies have shown that in adults, low dose CT
is comparable to standard CT with respect to stone
diagnosis and measurement.
22,209,210
Although
comparative studies of low-dose CT in the pediatric
population specifically are lacking, generalization of the
findings in adults to the pediatric population seems
reasonable, particularly given the smaller size and
lower rate of obesity in children, which is thought to
limit the sensitivity of low dose CT in adults.
49. In pediatric patients with ureteral stones,
clinicians should not routinely place a stent
prior to URS. (Index Patient 13) Expert
Opinion
In pediatric patients who require endourologic
intervention for a ureteral stone, access is sometimes
difficult or impossible due to a narrow ureterovesical
junction and/or ureter. In such cases, placement of a
ureteral stent typically results in passive dilation of the
ureter, thus permitting access at the time of the next
attempted URS.
211
However, “pre-stenting” should not
be considered a routine aspect of a URS procedure in
pediatric patients, since access to the upper tract is
possible on the initial attempt in a majority of children
undergoing attempted URS.
212
50. In pediatric patients with a total renal stone
burden ≤20mm, clinicians may offer SWL or
URS as first-line therapy. (Index Patient 14)
Moderate Recommendation; Evidence Level
Grade C
SWL has a long track record of success in treatment of
renal stones in children. Stone-free rates are reported
to be relatively high in children at 80-85% overall,
213,214
and at 80% for lower pole stones. Complication rates
after pediatric SWL appear to be low with little evidence
of long-term sequelae. URS also appears to have a high
success rate for pediatric renal stones, with stone-free
rates of around 85%.
215
Complication rates may be
Copyright © 2016 American Urological Association Education and Research, Inc.®
Surgical Management
of Stones
American Urological Association (AUA)
Endourological Society Guideline

34
somewhat higher with URS, estimated at 12.4%-20.5%
in reviews.
216
While complication rates may be
somewhat lower with SWL at 8%-10%, with serious
complications being rare,
213
there are, unfortunately,
very few high-quality comparative studies for SWL and
URS or other modalities for treatment of renal stones in
the pediatric population.
51. In pediatric patients with a total renal stone
burden >20mm, both PCNL and SWL are
acceptable treatment options. (Index Patient
14) Moderate Recommendation; Evidence
Level Grade C
High stone-free rates have been reported with both
PCNL and SWL in children with larger stones. SWL has
been reported to have stone-free rates of 73-83% in
pediatric patients,
218-220
while PCNL results vary by site,
but recent large series have approached 90% success
rates.
221
If SWL is performed, placement of a ureteral
stent or nephrostomy tube is recommended to prevent
postoperative renal obstruction. Several factors must
be taken into consideration when selecting which of
these procedures to pursue, including stone
composition and attenuation, stone location, body
habitus, collecting system anatomy, relation of the
kidney to surrounding viscera, medical co-morbidity,
and the parental preference. The utilization of smaller
instruments for PCNL (mini-PCNL, micro-PCNL) may
limit the risk of hemorrhage in this population.
182,222-225
52. In pediatric patients, except in cases of
coexisting anatomic abnormalities, clinicians
should not routinely perform open/
laparoscopic/robotic surgery for upper tract
stones. (Index Patients 13, 14) Expert Opinion
There is very little evidence directly comparing the use
of laparoscopic surgery or robotic-assisted laparoscopic
surgery with more conventional treatments for stone
disease in children. Series in adults have suggested
that laparoscopic approaches may compare favorably to
percutaneous techniques for large or staghorn renal
stones,
133,137,138,226
but in children, these approaches
should be considered secondary or tertiary options for
treatment of renal or ureteral stones since more
conventional procedures, including SWL, URS, and
PCNL, have high rates of success and lower risks of
serious complications.
The primary exception to this statement is in the
pediatric patient with one or more renal or ureteral
stones and a co-existing anatomic anomaly, such as
UPJ obstruction.
227
In such cases, open, laparoscopic,
or robotic-assisted laparoscopic surgery is indicated to
remove the stone(s) and repair the primary anatomic
defect. Other anomalies that may be associated with
stones that may be treated at the time of
reconstructive surgery include ureterovesical junction
obstruction and duplication anomalies with an
obstructed ectopic ureter.
53. In pediatric patients with asymptomatic and
non-obstructing renal stones, clinicians may
utilize active surveillance with periodic
ultrasonography. (Index Patient 14) Expert
Opinion
While observation of an asymptomatic, non-obstructing
renal stone is an option for children, such patients
should be seen regularly with routine surveillance US to
monitor for increase in size or number of stones, or
silent obstruction. Families should be counseled about
the need for regular follow-up, as the wellness of the
child may lead some to defer further assessment for
long periods of time, after which some children may re-
present with large or obstructing stones that present
significant management challenges, with increased
morbidity associated with the stone itself as well as
surgical treatment.
Even if immediate surgical treatment is not pursued,
evaluation of the pediatric patient for underlying
abnormalities that may predispose to further stone
formation is indicated. Metabolic evaluation for stone
risk factors is appropriate in pediatric patients as the
incidence of metabolic abnormalities is high in pediatric
stone formers.
,228,229
Twenty-four hour urine collections
are appropriate in toilet-trained children and
adolescents to assess urinary stone risk parameters. In
infants and non-toilet trained children, “spot” urine
samples can still be used to screen for hypercalciuria,
although this approach has diagnostic limitations.
Infants and young children with hyperoxaluria should
be screened for primary hyperoxaluria.
54. In pregnant patients, clinicians should
coordinate pharmacological and surgical
intervention with the obstetrician. (Index
Copyright © 2016 American Urological Association Education and Research, Inc.®
Surgical Management
of Stones
American Urological Association (AUA)
Endourological Society Guideline

35
Patient 15) Clinical Principal
Stone disease during pregnancy can be a challenging
condition to diagnose and treat as standard imaging
and treatment algorithms for urolithiasis can pose undo
risk to the developing fetus. Investigations are
complicated by the normal changes during pregnancy
that can resemble obstructing calculi. The risks to the
fetus of ionizing radiation, analgesics, antibiotics, and
anesthesia must also be considered. All these factors
can lead to a delay in diagnosis, inappropriate
diagnosis, and difficult treatment decisions.
Evaluation and management of the pregnant patient
with suspected urolithiasis must be multidisciplinary.
The obstetrician or maternal fetal medicine physician,
anesthesiologist, and urologist must work together to
develop a safe and effective plan for the patient. By
enlisting the assistance of other treatment
professionals, the urologist can appropriately counsel
the pregnant patient on the potential risks to the fetus
before proceeding with any diagnostic or therapeutic
treatment options. Due to the rarity of the condition
and the unique vulnerability of the patient population,
few prospective studies on pregnant patients with renal
or ureteral stones are available and thus, most outcome
data is based on animal studies or small case series.
The obstetrician or maternal fetal medicine physician
along with the pharmacist can insure that medications
prescribed for control of stone-related symptoms are
safe to the developing fetus based on gestational age at
time of presentation. If ionizing radiation is necessary
for diagnostic or treatment purposes, the radiation
physicist along with the obstetrician can estimate
radiation exposure so the total pregnancy exposure
does not exceed the American College of Obstetrics and
Gynecology (ACOG) recommended maximum of 50
mGy.
230
Should surgical intervention be warranted, the
multidisciplinary team is imperative, utilizing an
anesthesiologist who specializes in obstetrics to perform
fetal monitoring, if indicated, and to keep drug
exposure to the minimum. Although obstetric
complications at time of surgical intervention are rare
(<5%),
231
the procedure should be performed at a
facility capable of managing obstetric emergencies
should complications ensue intra or post operatively.
55. In pregnant patients with ureteral stone(s)
and well controlled symptoms, clinicians
should offer observation as first-line therapy.
(Index Patient 15) Strong recommendation;
Evidence Level Grade B
The spontaneous passage rates for pregnant women
with ureteral stones have not been demonstrated to be
different than those of non-pregnant patients.
Therefore, in a patient whose symptoms are controlled,
a period of observation should be the initial therapy.
The clinician should be aware that a stone event in
pregnancy does carry with it an increased risk of
maternal and fetal morbidity, so patients should be
followed closely for recurrent or persistent
symptoms.
232,233
Should MET be considered for the
pregnant patient, the patient should be counseled that
MET has not been investigated in the pregnant
population, and the pharmacologic agents are being
used for an “off-label” purpose.
234
NSAIDs (e.g.,
ketorolac) are contraindicated in pregnancy.
56. In pregnant patients with ureteral stones,
clinicians may offer URS to patients who fail
observation. Ureteral stent and nephrostomy
tube are alternative options with frequent
stent or tube changes usually being
necessary. (Index Patient 15) Strong
Recommendation; Evidence Level Grade C
Should a trial of observation fail for the pregnant
patient with a ureteral stone, an intervention is
indicated. Ureteral stent and percutaneous
nephrostomy will both effectively decompress the
obstructed collecting system, and thereby bring
symptom relief. However, the introduction of such
foreign objects into the collecting system of a pregnant
woman can be a point of concern, as they tend to
encrust rapidly. Therefore, should such an approach be
taken, frequent stent or tube exchanges are required.
As an alternative, URS provides a definitive treatment
for the pregnant patient, as it accomplishes stone
clearance, obviating the need for prolonged drainage
with stent or nephrostomy.
235
URS in the pregnant
patient should only be undertaken by clinicians facile
with the treatment approach and at an institution that
has both the equipment required for URS and obstetric
support for maternal and fetal considerations.
231
FUTURE RESEARCH
Copyright © 2016 American Urological Association Education and Research, Inc.®
Surgical Management
of Stones
American Urological Association (AUA)
Endourological Society Guideline

36
It is unfortunate that the surgical treatment of kidney
stones, a disease with such a great prevalence, has not
been studied with greater rigor in previous years. One
of the most disappointing aspects of the systematic
review performed herein is the small number of high
quality research studies identified. There is an extreme
paucity of high quality RCTs comparing competitive
surgical interventions for stone disease. However, this
is not surprising, given that other urologic fields are
also underpopulated with such studies.
Going forward, it will be beneficial to standardize the
reporting of stone treatment studies. At present, there
is great heterogeneity in the definitions of such
important metrics as stone size, stone location, stone-
free status, complications and economic outcomes. This
terminology should be standardized as this will allow
more reliable comparisons among studies, and make
systematic reviews and meta-analyses more powerful.
Clinicians’ ability to utilize imaging studies to predict
treatment outcomes for differing stone interventions is
limited at present. As a result, we cannot completely
counsel patients on their likely course following a stone
removal intervention. This is particularly true for SWL,
where our pre-treatment understanding of stone
fragility is lacking. It would be most welcome for the
clinician to be better able to predict treatment
outcomes from presently available imaging modalities.
Furthermore, efforts should also be focused on
identifying and advancing the utility of imaging
modalities that do not rely on ionizing radiation such as
MRI and ultrasonography.
Many patients with a symptomatic ureteral stone will
pass their stones spontaneously. From a patient-
centered standpoint, time course to passage, as well as
maneuvers to increase the probability of spontaneous
passage are exceedingly important. Clinicians’ ability to
counsel patients on how long it will take for a stone to
pass is limited due in great part to a lack of research
focused on answering this question. With regards to
augmenting stone passage utilizing pharmacotherapy,
our understanding is unclear as the literature is
conflicted. Future studies better defining the ability of
MET to promote stone passage will be important to
improving the patient experience. In addition, the
development of agents with better efficacy and
tolerability to facilitate stone passage is warranted.
The mechanical action of stone fragmentation and
removal is the primary driver of intra-operative time
allocation during a stone removal procedure. For URS
and PCNL, the technologies accomplish the same end,
but via different mechanisms. For patients undergoing
URS, in particular flexible URS, the Holmium laser is
currently the lithotrite of choice. In some cases the
laser may be used to fragment the stone into small
pieces that can be individually retrieved; in other cases
the laser may be used to fragment the stone into fine
powder, which will spontaneously drain from the
kidney. At present, it is not known which of these
approaches yields superior outcomes, but such
information would be immediately useful to the
practicing urologist.
There is also a need to improve the devices that are
used in the stone fragmentation and evacuation process
during endoscopic surgery. With respect to URS, there
is a need for mechanical devices that more efficiently
and safely fragment and evacuate stone material; at
present, this process is cumbersome and potentially
dangerous as ureteral injury may occur during stone
extraction. With respect to PCNL, advances in stone
removal technology will enable a more rapid and
efficient evacuation of larger burdens of stone.
Ureteral stent placement is commonly performed
following stone interventions. In some cases, stent
placement may not be necessary, such as in the case of
an uncomplicated ureteroscopic procedure. However, in
many of those cases, stents are still placed. It is well
recognized that ureteral stents are the source of
significant morbidity. Future efforts should be devoted
to better identifying which patients may safely avoid
stent placement. In addition, advances in stent
technology, with a particular focus on identifying the
nature and source of stent morbidity, as well as design
advances to minimize these bothersome symptoms will
also improve surgical care.
Stone disease in the pediatric population has been
reported to be increasing. At present, our
understanding of stone management among children is
somewhat rudimentary, as the published literature is
sparse. Future efforts to better define the effects of
surgical stone treatment in this population will also be
important.
Copyright © 2016 American Urological Association Education and Research, Inc.®
Surgical Management
of Stones
American Urological Association (AUA)
Endourological Society Guideline

37
REFERENCES
1. Saigal CS, Joyce G and Timilsina AR: Urologic
diseases in America project: Direct and indirect
costs of nephrolithiasis in an employed population:
opportunity for disease management? Kidney Int
2005; 68:1808.
2. Scales CD Jr, Smith AC, Hanley JM et al:
Prevalence of kidney stones in the United States.
Eur Urol 2012; 62: 160.
3. Pearle MS, Calhoun EA, Curhan GC et al: Urologic
diseases in America project: urolithiasis. J Urol
2005; 173:848.
4. Preminger GM, Assimos DG, Lingeman JE et al:
Chapter 1: AUA guideline on management of
staghorn calculi: diagnosis and treatment
recommendations. J Urol 2005; 173: 1991.
5. Preminger GM, Tiselius HG, Assimos DG et al:
2007 guideline for the management of ureteral
calculi. J Urol 2007; 178: 2418.
6. Pearle MS, Goldfarb DS, Assimos DG et al: Medical
management of kidney stones: AUA guideline. J
Urol 2014; 192: 316.
7. Faraday M, Hubbard H, Kosiak B et al: Staying at
the cutting edge: a review and analysis of
evidence reporting and grading; the
recommendations of the American Urological
Association. BJU Int 2009; 104: 294.
8. Barrionuevo Moreno P, Asi N, Benkhadra K et al:
Surgical management of kidney stones: a
systematic review. Mayo Clinic 2015.
9. Higgins JDA: Assessing quality of included studies
in Cochrane Reviews. The Cochrane Collaboration
Methods Groups Newsletter 2007; 11.
10. Wells GA, Shea B, O'Connell D et al: The
Newcastle-Ottawa Scale (NOS) for assessing the
quality of nonrandomized studies in meta-
analyses. 2009: http://www.ohri.ca/programs/
clinical_epidemiology/oxford.htm.
11. Hsu C and Sandford BA: The Delphi Technique:
Making Sense of Consensus. Practical Assessment,
Research & Evaluation 2007; 12: 1.
12. Akbulut F, Kucuktopcu O, Ucpinar B et al: A rare
complication of extracorporeal shock wave
lithotripsy: intrarenal hematoma mimicking pelvis
renalis tumor. Case Reports in Urology 2015;
2015: 1.
13. Chalasani V, Bissoon D, Bhuvanagir AK et al:
Should PCNL patients have a CT in the prone
position preoperatively? Can J Urol 2010; 17;
5082.
14. Ghani KR, Patel U and Anson K: Computed
tomography for percutaneous renal access. J
Endourol 2009; 23: 1633.
15. Hopper KD, Sherman JL, Luethke JM et al: The
retrorenal colon in the supine and prone patient.
Radiology 1987; 162: 443.
16. Labadie K, Okhunov A, Akhavein D et al:
Evaluation and comparison of urolithiaisis scoring
systems in percutaneous kidney stone surgery. J
Urol 2015; 193; 154.
17. Prassopoulos P, Gourtsoyiannis N, Cavouras D et
al: A study of the variation of colonic positioning in
the pararenal space as shown by computed
tomography. Eur J Radiol 1990; 10: 44.
18. Okhunov Z, Friedlander JI, George AK et al:
S.T.O.N.E. nephrolithometry: novel surgical
classification system for kidney calculi. Urology
2013; 81: 1154.
19. Smith A, Averch TD, Shahrour K et al: A
nephrolithometric nomogram to predict treatment
success of percutaneous nephrolithotomy. J Urol
2013; 190; 149.
20. Thomas K, Smith NC, Hegarty N et al: The Guy’s
stone score—grading the complexity of
percutaneous nephrolithotomy procedures.
Urology 2011; 78: 277.
21. Wickham JEA, Fry IK and Wallace DMA:
Computerised tomography localization of intrarenal
calculi prior to nephrolithotomy. BJU Int 1980; 52:
422.
22. Poletti PA, platon A, Ruschmann OT et al: Low-
dose versus standard-dose CT protocol in patients
Copyright © 2016 American Urological Association Education and Research, Inc.®
Surgical Management
of Stones
American Urological Association (AUA)
Endourological Society Guideline

38
with clinically suspected renal colic. AJR Am J
Roentgenol 2007; 188: 927.
23. Brehmer M, Beckman MO and Magnusson A: 3-
dimensional CT planning improves percutaneous
stone surgery. Scan J Urol 2014; 48:316.
24. Fulgham PF, Assimos DG, Pearle MS et al: Clinical
effectiveness protocols for imaging in the
management of ureteral calculous disease: AUA
technology assessment. J Urol 2013; 189: 1230.
25. Coursey CA, Casalino DD, Reimer EM et al: ACR
appropriateness criteria: acute onset flank pain-
suspicion of stone disease. Ultrasound Q 2012;
28:227.
26. Smith-Bindman R, Aubin C, Bailitz J et al:
Ultrasonography versus computed tomography for
suspected nephrolithiaisis. N Eng J Med 2014;
371:1100.
27. Perks AE; Schuler TD, Lee J et al: Stone
attenuation and skin-to-stone distance on
computed tomography predicts for stones
fragmentation by shock wave lithotripsy. Urology
2008; 72: 765.
28. El-Nahas AR, El-Assmy AM, Mansour O et al: A
prospective multivariate analysis of factors
predicting stone disintegration by extracorporeal
shock wave lithotripsy: the value of high-
resolution noncontrast computed tomography. Eur
Urol 2007; 51: 1688.
29. Patel T, Kozakowski K, Hruby G et al: Skin to
stone distance is an independent predictor of stone
-free status following shock wave lithotripsy. J
Endourol 2009; 23:1383.
30. Pareek G, Hedican SP, Lee FT Jr et al: Shock wave
lithotripsy success determined by skin-to-stone
distance on computed tomography. Urology. 2005;
66: 941.
31. Tran TY, McGillen K, Cone EB et al: Triple D Score
is a reportable predictor of shockwave lithotripsy
stone-free rates. J Endourol 2015; 29: 226.
32. Vakalopoulos I: Development of a mathematical
model to predict extracorporeal shockwave
lithotripsy outcome. J Endourol. 2009; 23: 891.
33. Feder MT, Blitstein J, Mason B et al: Predicting
differential renal function using computerized
tomography measurements of renal parenchymal
area. J Urol 2008; 180: 2110.
34. Gupta S, Singh AH, Shabbir A et al: Assessing
renal parenchymal volume on unenhanced CT as a
marker for predicting renal function in patients
with chronic kidney disease. Acad Radiol 2012;
19: 654.
35. Herts BR, Sharma N, Lieber M et al: Estimating
glomerular filtration rate in kidney donors: a
model constructed with renal volume
measurements from donor CT scans. Radiology
2009; 252: 109.
36. Morrisroe SN, Su RR, Bae KT et al: Differential
renal function estimation using computerized
tomography based renal parenchymal volume
measurement. J Urol 2010; 183: 2289.
37. Ramaswamy K, Marien T, Mass A et al: Simplified
approach to estimating renal function based on
computerized tomography. Can J Urol 2013; 20:
6833.
38. Benson AD, Juliano TM and Miller NL: Infectious
outcomes of nephrostomy drainage before
percutaneous nephrolithotomy compared to
concurrent access. J Urol 2014; 192: 770.
39. Korets R, Graversen JA, Kates M et al: Post-
percutaneous nephrolithotomy systemic
inflammatory response: a prospective analysis of
preoperative urine, renal pelvic and stone cultures.
J Urol 2011; 186:1899.
40. Margel D, Ehrlich Y, Brown N et al: Clinical
implication of routine stone culture in
percutaneous nephrolithotomy, a prospective
study. Urology 2006; 67:26.
41. Mariappan P, Smith G, Bariol SV et al: Stone and
pelvic urine culture are better than bladder urine
as predictors of urosepsis following percutaneous
nephrolithotomy: a prospective clinical study J Urol
2005; 173:1610.
42. Marien T, Mass AY and Shah O: Antimicrobial
resistance patterns in cases of obstructive
Copyright © 2016 American Urological Association Education and Research, Inc.®
Surgical Management
of Stones
American Urological Association (AUA)
Endourological Society Guideline

39
pyelonephritis secondary to stones. Urology 2015;
85: 64.
43. Committee on Standards and Practice Parameters:
Practice advisory for preanesthesia evaluation: an
updated report by the American Society of
Anesthesiologists Task Force on preanesthesia
evaluation. Anesthesology 2012; 116: 1.
44. Patel IJ, Davidson JC, Nikolic B et al: Consensus
guidelines for periprocedural management of
coagulation status and hemostasis risk in
percutaneous image-guideline interventions. J
Vasc Interv Radiol 2012; 23: 727.
45. Frank S, Oleyar MJ, Ness PM, et al: Reducing
unnecessary preoperative blood orders and costs
by implementing an updated institution specific
maximum surgical blood order schedule and a
remote electronic blood release system.
Anesthesiology 2014; 121:501.
46. Patel U, Walkden RM, Ghani KR et al: Three-
dimensional CT pyelography for planning of
percutaneous nephrostolithotomy: accuracy of
stone measurement, stone depiction and
pelvicalyceal reconstruction. Eur Radiol 2009; 19:
1280.
47. Thiruchelvam N, Mostafid H and Ubhayakar G:
Planning percutaneous nephrolithotomy using
multidetector computed tomography urography,
multiplanar reconstruction and three-dimensional
reformatting. BJU Int 2005; 95: 1280.
48. Hubner WA, Irby P and Stoller ML: Natural history
and current concepts for the treatment of small
ureteral calculi. Eur Urol 1993; 24: 172.
49. Miller OF and Kane CJ: Time to stone passage for
observed ureteral calculi: a guide for patient
education. J Urol 1999; 162: 688.
50. Park HK, Choi EY, Jeong BC et al: Localizations
and expressions of alpha-1A, alpha-1B and alpha-
1D adrenoceptors in human ureter. Urol Res 2007;
35: 325.
51. Sasaki S, Tomiyama Y, Kobayashi S et al:
Characterization of alpha1-adrenoceptor subtypes
mediating contraction in human isolated ureters.
Urology 2011; 77: e13.
52. Furyk JS, Chu K, Banks C et al: Distal ureteric
stones and tamsulosin: a double-blind, placebo-
controlled, randomized, multicenter trial. Ann
Emerg Med 2016; 67:86.
53. Pickard R, Starr K, MacLennan G et al: Medical
expulsive therapy in adults with ureteric colic: a
multicenter, randomized, placebo-controlled trial.
Lancet 2015; 386: 341.
54. Kreshover JE, Dickstein RJ, Rowe C et al:
Predictors for negative ureteroscopy in the
management of upper urinary tract stone disease.
Urology 2011; 78: 748.
55. Vaughan ED and Gillenwater JY: Recovery
following complete chronic unilateral ureteral
occlusion: functional, radiographic and pathologic
alterations. J Urol 1973; 100: 27.
56. Pedro RN, Hinck B, Hendlin K et al: Alfuzosin stone
expulsion therapy for distal ureteral calculi; a
double-blind, placebo controlled study. J Urol
2008; 179: 2244.
57. Aboumarzouk OM, Kata SG, Keeley FX et al:
Extracorporeal shock wave lithotripsy (ESWL)
versus ureteroscopic management for ureteric
calculi. Cochrane Database Syst Rev. [Meta-
Analysis Research Support, Non-U.S. Gov't
Review]. 2012; 5: CD006029.
58. Makarov DV, Trock BJ, Allaf ME et al: The effect of
ureteral stent placement on post-ureteroscopy
complications: a meta-analysis. Urology 2008; 71:
796.
59. Lasser MS and Pareek G: Smith’s Textbook of
Endourology 3rd Edition,Wiley-Blackwell, 2012;
273.
60. Segura JW, Preminger GM, Assimos DG et al:
Ureteral Stones Clinical Guidelines Panel summary
report on the management of ureteral calculi. J
Urol 1997; 158: 1915.
61. Shen P, Jiang M, Yang J et al: Use of ureteral stent
in extracorporeal shock wave lithotripsy for upper
urinary calculi: a systematic review and meta-
analysis. J Urol 2011; 186: 1328.
Copyright © 2016 American Urological Association Education and Research, Inc.®
Surgical Management
of Stones
American Urological Association (AUA)
Endourological Society Guideline

40
62. Al- Ba’adani T, Ghilan A, El-Nono I et al: Whether
post-ureteroscopy stenting is necessary or not?
Saudi Med J 2006; 27: 845.
63. Borboroglu PG, Amling CL, Schenkman NS et al:
Ureteral stenting after ureteroscopy for distal
ureteral calculi: a multi-institutional prospective
randomized controlled study assessing pain,
outcomes and complications. J Urol 2001; 166:
1651.
64. Chen YT, Chen J, Wong WY et al: Is ureteral
stenting necessary after uncomplicated
ureteroscopic lithotripsy? A prospective,
randomized controlled trial. J Urol 2002; 167:
1977.
65. Cheung MC, Lee F, Leung YL et al: A prospective
randomized controlled trial on ureteral stenting
after ureteroscopic holmium laser lithotripsy. J
Urol 2003; 169:1257.
66. Denstedt JD, Wollin TA, Sofer M et al: A
prospective randomized controlled trial comparing
nonstented vs stented ureteroscopic lithotripsy. J
Urol 2001; 165:1419.
67. Ibhrahim HM, Al-Kandari AM, Shaaban HS et al:
Role for ureteral stenting after uncomplicated
ureteroscopy for distal ureteral stones: a
randomized controlled trial. J Urol 2008; 180:
961.
68. Isen K, Bogatekin S, Em S et al: Is routine ureteral
stenting necessary after uncomplicated
ureteroscopic lithotripsy for lower ureteral stones
larger than 1 cm? Urol Res 2008; 36:115.
69. Gunlusoy B, Degirmenci T, Arslan M et al: Is
ureteral cauterization necessary after
ureteroscopic lithotripsy for uncomplicated upper
ureteral stones? J Endourol 2008; 22: 1645.
70. Jeong H, Kwak C and Lee SE: Ureteric stenting
after ureteroscopy for ureteric stones: a
prospective randomized study assessing symptoms
and complications. BJU Int 2003; 93: 1032.
71. Mustafa M: The role of stenting in relieving loin
pain following ureteroscopic stone therapy for
persisting renal colic with hydronephrosis.
International Urol and Nephrol 2007; 39:91.
72. Shao Y, Zhuo J, Sun XW et al: Nonstented vs
routine stented ureteroscopic holmium laser
lithotripsy: a prospective randomized trial. Urol
Res 2008; 36: 259.
73. Srivastava A, Gupta R, Kumar A et al: Routine
stenting after ureteroscopy for distal ureteral
calculi is unnecessary: results of a randomized
controlled trial. J Endourol 2003; 17: 871.
74. Nabi G, Cook J, N’Dow J et al: Outcome of stenting
after uncomplicated ureteroscopy: systematic
review and meta-analysis. BMJ 2007; 334: 544.
75. Pengfei S, Yutao L, Jie Y et al: The results of
ureteral stenting after ureteroscopic lithotripsy for
ureteral calculi: a systematic review and meta-
analysis. J Urol 2011; 186:1904.
76. Rubenstein RA, Zhao LC, Loeb S et al: Prestenting
improves ureteroscopic stone-free rates. J
Endourol 2007; 21: 1277.
77. Chu L, Farris CA, Corcoran AT et al: Preoperative
stent placement decreases cost of ureteroscopy.
Urology 2011; 78: 309.
78. Netsch C, Knipper S, Bach T et al: Impact of
preoperative ureteral stenting on stone-free rates
of ureteroscopy for nephroureterolithiasis: a
matched-paired analysis of 286 patients. Urology
2012; 80: 1214.
79. Zhou L, Cai X, Li H et al: Effects of α-blockers,
antimuscarinics, or combination therapy in
relieving ureteral stent-related symptoms: a meta-
analysis. J Endourol 2015; 29: 650.
80. Lamb AD, Vowler SL, Johnston R et al: Meta-
analysis showing the beneficial effect of α-blockers
on ureteric stent discomfort. BJU Int 2011; 108:
1894.
81. Kwon JK, Cho KS, Oh CK et al. The beneficial
effect of alpha-blockers for ureteral stent-related
discomfort: systematic review and network meta-
analysis for alfuzosin versus tamsulosin versus
placebo. BMC Urol 2015; 15: 55.
82. Yakoubi R, Lemdani M, Monga M et al: Is there a
role for α-blockers in ureteral stent related
Copyright © 2016 American Urological Association Education and Research, Inc.®
Surgical Management
of Stones
American Urological Association (AUA)
Endourological Society Guideline

41
symptoms? A systematic review and meta-
analysis. J Urol 2011; 186: 928.
83. Roberts WW, Cadeddu JA, Micali S et al: Ureteral
stricture formation after removal of impacted
calculi. J Urol 1998; 159: 723.
84. Abbas B, Nasser S, Amirmohsen Z et al:
Retrograde, antegrade, and laparoscopic
approaches for the management of large, proximal
ureteral stones: a randomized clinical trial. J
Endourol 2008; 22: 2677.
85. Muslumanoglu AY, Karadag MS, Tefekli AH et al:
When is open ureterolithotomy indicated for the
treatment of ureteral stones? Int J Urol 2006; 13:
1385.
86. Berczi C, Flasko T, Lorincz L et al: Results of
percutaneous endoscopic ureterolithotomy
compared to that of ureteroscopy. J Laparoendosc
Adv Surg Tech A 2007; 17: 285.
87. Dretler SP, Keating MA and Riley J: An algorithm
for the management of ureteral calculi. J Urol
1986; 136: 1190.
88. Moufid K, Abbaka N, Touiti D et al: Large impacted
upper ureteral calculi: A comparative study
between retrograde ureterolithotripsy and
percutaneous antegrade ureterolithotripsy in the
modified lateral position. Urol Ann 2013; 5: 140.
89. Tan HM, Liew RP, Chan CC et al: Multimodal
approach in the management of 1163 ureteric
stone cases. Med J Malaysia 1995; 50: 87.
90. Yang Z, Song L, Xie D et al: Comparative study of
outcome in treating upper ureteral impacted
stones using minimally invasive percutaneous
nephrolithotomy with aid of patented system or
transurethral ureteroscopy. Urology 2012; 80:
1192.
91. Siavash F, Iradj K, Aliakbar A et al: Open surgery,
laparoscopic surgery, or transureteral lithotripsy--
which method? Comparison of ureteral stone
management outcomes. J Endourol 2011; 25: 31.
92. Lopes Neto AC, Korkes F, Silva JL 2
nd
et al:
Prospective randomized study of treatment of
large proximal ureteral stones: extracorporeal
shock wave lithotripsy versus ureterolithotripsy
versus laparoscopy. J Urol 2012; 187: 164.
93. Fang YQ, Qiu JG, Wang DJ et al: Comparative
study on ureteroscopic lithotripsy and laparoscopic
ureterolithotomy for treatment of unilateral upper
ureteral stones. Acta Cir Bras 2012; 27: 266.
94. Seeger AR, Rittenberg MH and Bagley DH:
Ureteropyeloscopic removal of ureteral calculi. J
Urol 1988; 139: 1180.
95. Cohen J, Cohen S and Grasso M:
Ureteropyeloscopic treatment of large, complex
intrarenal and proximal ureteral calculi. BJU Int
2013; 111: E127.
96. Hyams E, Monga M, Pearle MS et al: A prospective,
multi-institutional study of flexible ureteroscopy
for proximal ureteral stones smaller than 2 cm. J
Urol 2015; 193: 165.
97. Perez Castro E, Osther PJ, Jinga V et al:
Differences in ureteroscopic stone treatment and
outcomes for distal, mid-, proximal, or multiple
ureteral locations: the Clinical Research Office of
the Endourological Society ureteroscopy global
study. Eur Urol 2014; 66: 102.
98. Raney AM: Electrohydraulic ureterolithotripsy.
Urology 1978; 13: 284.
99. Hofbauer J, Hobarth K and Marberger M:
Electrohydraulic versus pneumatic disintegration in
the treatment of ureteral stones: a randomized,
prospective trial. J Urol 1995; 153: 623.
100. Vorreuther R, Corleis R, Klotz T et al: Impact of
shock wave pattern and cavitation bubble size on
tissue damage during ureteroscopic
electrohydraulic lithotripsy. J Urol 1995; 153: 849.
101. Santa-Cruz RW, Leveillee RJ and Krongrad A: Ex
vivo comparison of four lithotripters commonly
used in the ureter: what does it take to perforate?
J Endourol 1998; 12: 417.
102. Teichman JM, Rao RD, Rogenes VJ et al:
Ureteroscopic management of ureteral calculi:
electrohydraulic versus holmium:YAG lithotripsy. J
Urol 1997; 158: 1357.
Copyright © 2016 American Urological Association Education and Research, Inc.®
Surgical Management
of Stones
American Urological Association (AUA)
Endourological Society Guideline

42
103. Pearle MS, Pierce HL, Miller GL et al: Optimal
method of urgent decompression of the collecting
system for obstruction and infection due to
ureteral calculi. J Urol 1998; 160: 1260.
104. Borofsky MS, Walter D, Shah O et al: Surgical
decompression is associated with decreased
mortality in patients with sepsis and ureteral
calculi. J Urol 2013; 189: 946.
105. Srisubat A, Potisat S, Lojanapiwat B et al:
Extracorporeal shock wave lithotripsy (ESWL)
versus percutaneous nephrolithotomy (PCNL) or
retrograde intrarenal surgery (RIRS) for kidney
stones. Cochrane Database Syst Rev 2014; 11.
106. Matlaga BR, Jansen JP, Meckley LM et al:
Treatment of ureteral and renal stones: a
systematic review and meta-analysis of
randomized, controlled trials. J Urol 2012; 188:
130.
107. Bryniarski P, Paradysz A, Zyczkowski M et al: A
randomized controlled study to analyze the safety
and efficacy of percutaneous nephrolithotripsy and
retrograde intrarenal surgery in the management
of renal stones more than 2 cm in diameter. J
Endourol 2012; 26: 52.
108. Karakoyunlu N, Goktug G, Sener NC et al: A
comparison of standard PCNL and staged
retrograde FURS in pelvis stones over 2 cm in
diameter: a prospective randomized study.
Urolithiasis 2015; 43: 283.
109. De S, Autorino R, Kim FJ et al: Percutaneous
nephrolithotomy versus retrograde intrarenal
surgery: a systematic review and meta-analysis.
Eur Urol 2015; 67: 125.
110. de la Rosette J, Assimos D, Desai M et al: The
Clinical Research Office of the Endourological
Society Percutaneous Nephrolithotomy Global
Study: indications, complications, and outcomes in
5803 patients. J Endourol 2011;25: 11.
111. Seitz C, Desai M, Hacker A et al: Incidence,
prevention, and management of complications
following percutaneous nephrolitholapaxy. Eur Urol
2012; 61:146.
112. Al-Kohany KM, Shokeir AA, Mosbah A et al:
Treatment of complete staghorn stones: a
prospective randomized comparison of open
surgery versus percutaneous nephrolithotomy. J
Urol 2005; 173: 469.
113. Meretyk S, Gofrit ON, Gafni O et al: Complete
staghorn calculi: random prospective comparison
between extracorporeal shock wave lithotripsy
monotherapy and combined with percutaneous
nephrostolithotomy. J Urol 1997; 157: 780.
114. Eisenberger F, Rassweiler J, Bub P et al:
Differentiated approach to staghorn calculi using
extra-corporeal shock wave lithotripsy and
percutaneous nephro-lithotomy: An analysis of
151 consecutive cases. World J Urol 1987; 5: 248.
115. Teichman JM, Long RD and Hulber JC: Long term
renal fate and prognosis after staghorn
management. J Urol 1995; 153: 1403.
116. Raman JD, Bagrodia A, Gupta A et al: Natural
history of residual fragments following
percutaneous nephrostolithotomy. J Urol 2009;
181:1163.
117. Chew BH, Brotherhood HL, Sur RL et al: Natural
history, complications and re-intervention rates of
asymptomatic residual stone fragments after
ureteroscopy: a report from the EDGE research
consortium. J Urol 2015; [Epub ahead of print]
118. Michaels EK and Fowler JE, Jr.:Extracorporeal
shock wave lithotripsy for struvite renal calculi:
prospective study with extended followup. J Urol
1991; 146: 728.
119. Blandy JP and Singh M.: The case for a more
aggressive approach to staghorn stones. J Urol
1976; 115:505.
120. Koga S, Arakaki Y, Matsuoka M et al: Staghorn
calculi—long-term results of management. Br J
Urol 1991; 68: 122.
121. Priestly JT and Dunn JH: Branched renal calculi. J
Urol 1949; 61: 194.
122. Rous SN and Turner WR: Retrospective study of 95
patients with staghorn calculus disease. J Urol
1977; 118: 902.
Copyright © 2016 American Urological Association Education and Research, Inc.®
Surgical Management
of Stones
American Urological Association (AUA)
Endourological Society Guideline

43
123. Vargas AD, Bragin SD and Mendez R: Staghorn
calculus: its clinical presentation, complications
and management. J Urol 1982; 127: 860.
124. Lam HS, Lingeman JE, Barron M et al: Staghorn
calculi: analysis of treatment results between
initial percutaneous nephrostolithotomy and
extracorporeal shock wave lithotripsy
monotherapy with reference to surface area. J Urol
1992; 147: 1219.
125. Joseph P, Mandal AK, Singh SK et al:
Computerized tomography attenuation value of
renal calculus: can it predict successful
fragmentation of the calculus by extracorporeal
shock wave lithotripsy? A preliminary study. J Urol
2002;167: 1968.
126. Perks AE, Schuler TD, Lee J et al: Stone
attenuation and skin-to-stone distance on
computed tomography predicts for stone
fragmentation by shock wave lithotripsy. Urology
2008; 72:765.
127. Madbouly K, Sheir KZ and Elsobky E: Impact of
lower pole renal anatomy on stone clearance after
shock wave lithotripsy: fact or fiction? J Urol 2001;
165: 1415.
128. Sumino Y, Mimata H, Tasaki Y et al: Predictors of
lower pole renal stone clearance after
extracorporeal shock wave lithotripsy. J Urol 2002;
168: 1344.
129. Ruggera L, Beltrami P, Ballario R et al: Impact of
anatomical pielocaliceal topography in the
treatment of renal lower calyces stones with
extracorporeal shock wave lithotripsy. Int J Urol
2005; 12: 525.
130. Soyupek S, Armagan A, Kosar A et al: Risk factors
for the formation of a steinstrasse after shock
wave lithotripsy. Urol Int 2005; 74: 323.
131. Madbouly K, Sheir KZ, Elsobky E et al: Risk factors
for the formation of a steinstrasse after
extracorporeal shock wave lithotripsy: a statistical
model. J Urol 2002; 167: 1239.
132. Al-Awadi KA, Abdul Halim H, Kehinde EO et al:
Steinstrasse: a comparison of incidence with and
without J stenting and the effect of J stenting on
subsequent management. BJU Int 1999; 84: 618.
133. Li S, Liu TZ, Wang XH et al: Randomized
controlled trial comparing retroperitoneal
laparoscopic pyelolithotomy versus percutaneous
nephrolithotomy for the treatment of large renal
pelvic calculi: a pilot study. J Endourol 2014; 28:
946.
134. Qin C, Wang S, Li P et al: Retroperitoneal
laparoscopic technique in treatment of complex
renal stones: 75 cases. BMC Urol 2014; 14:16.
135. Singh V, Sinha RJ, Gupta DK et al: Prospective
randomized comparison of retroperitoneoscopic
pyelolithotomy versus percutaneous
nephrolithotomy for solitary large pelvic kidney
stones. Urol Int 2014; 92: 392.
136. Zheng J, Yan J, Zhou Z et al: Concomitant
treatment of ureteropelvic junction obstruction and
renal calculi with robotic laparoscopic surgery and
rigid nephroscopy. Urology 2014; 83: 237.
137. Aminsharifi A, Hosseini MM and Khakbaz A.
Laparoscopic pyelolithotomy versus percutaneous
nephrolithotomy for a solitary renal pelvis stone
larger than 3 cm: a prospective cohort study.
Urolithiasis 2013; 41: 493.
138. Wang X, Li S, Liu T et al: Laparoscopic
pyelolithotomy compared to percutaneous
nephrolithotomy as surgical management for large
renal pelvic calculi: a meta-analysis. J Urol 2013;
190: 888.
139. Kuo CC, Wu CF, Huang CC et al:
Xanthogranulomatous pyelonephritis: critical
analysis of 30 patients. Int Urol Nephrol 2011; 43:
15.
140. Zelhof B, McIntyre IG, Folwer SM et al:
Nephrectomy for benign disease in the UK: results
from the British Association of Urological Surgeons
nephrectomy database. BJU Int 2015; 117: 138.
141. Goldberg H, Holland R, Tal R et al: The impact of
retrograde intrarenal surgery for asymptomatic
renal stones in patients undergoing ureteroscopy
for a symptomatic ureteral stone. J Endourol 2013;
27:970.
Copyright © 2016 American Urological Association Education and Research, Inc.®
Surgical Management
of Stones
American Urological Association (AUA)
Endourological Society Guideline

44
142. Gdor Y, Faddegon S, Krambeck AE et al: Multi-
institutional assessment of ureteroscopic laser
papillotomyfor chronic flank pain associated with
papillary calcifications. J Urol 2011; 185: 192.
143. Keeley FX Jr, Tilling K, Elves A et al: Preliminary
results of a randomized controlled trial of
prophylactic shock wave lithotripsy for small
asymptomatic renal caliceal stones. BJU Int 2001;
87: 1.
144. Bilgasem S, Pace KT, Dyer S et al: Removal of
asymptomatic ipsilateralrenal stones following rigid
ureteroscopy for ureteral stones. J Endourol 2003;
17: 397.
145. Glowacki LS, Beecroft ML, Cook RJ et al: The
natural history of asymptomatic urolithiasis. J Urol
1992; 147: 319.
146. Burgher A, Beman M, Holzman JL et al:
Progression of nephrolithiasis: long-term outcomes
with observation of asymptomatic calculi. J
Endourol 2004; 18: 534.
147. Koh LT, Ng FC and Ng KK: Outcomes of long-term
follow-up of patients with conservative
management of asymptomatic renal calculi. BJU
Int 2012; 109: 622.
148. Inci K, Sahin A, Islamoglu E et al: Prospective long
-term followup of patients with asymptomatic
lower pole caliceal stones. J Urol 2007; 177:
2189.
149. Yuruk E, Binbay M, Sari E et al: A prospective,
randomized trial of management for asymptomatic
lower pole calculi. J Urol 2010; 183: 1424.
150. Pearle MS, Lingeman JE, Leveillee R et al:
Prospective, randomized trial comparing shock
wave lithotripsy and ureteroscopy for lower pole
caliceal calculi 1 cm or less. J Urol 2005; 173:
2005.
151. Schuster TG, Hollenbeck BK, Faerber GJ et al:
Ureteroscopic treatment of lower pole
calculi:comparison of lithotripsy in situ and after
displacement. J Urol 2002; 168:43.
152. L’esperance JO, Ekeruo WO, Scales CD Jr. et al:
Effect of ureteral access sheath on stone-free rates
in patients undergoing ureteoscopic management
of renal calculi. Urology 2005; 66: 252.
153. Albala DM, Assimos DG, Clayman RV et al: Lower
pole I: a prospective randomized trial of
extracorporeal shock wave lithotripsy and
percutaneous nephrostolithotomy for lower pole
nephrolithiasis-initial results. J Urol 2001; 166:
2072.
154. BhattuAS, Mishra S, Ganpule A et al: Outcomes in
a large series of minipercs: analysis of consecutive
318 patients. J Endourol 2015; 29: 283.
155. Crook TJ, Lockyer CR, Keoghane SR et al: A
randomized controlled trial of nephrostomy
placement versus tubeless percutaneous
nephrolithotomy. J Urol 2008; 180: 612.
156. Maheshwari PN, Andankar M, Hegde S et al:
Bilateral single-session percutaneous
nephrolithotomy: a feasible and safe treatment. J
Endourol 2000; 14: 285.
157. Liatsikos EN, Hom D, Dinlenc CZ et al: Tail stent
versus re-entry tube: A randomized comparison
after percutaneous stone extraction. Urology
2002; 59: 15.
158. Pietrow PK, Auge BK, Lallas K et al: Pain after
percutaneous nephrolithotomy: impact of
nephrostomy tube size. J Endourol 2003; 17: 411.
159. Osman Y, Harraz AM, El-Nahas AR et al: Clinically
insignificant residual stone fragments: an
acceptable term in the computed tomography era?
Urology 2013; 81: 723.
160. Altunrende F, Tefekli A, Stein RJ et al: Clinically
insignificant residual fragments after percutaneous
nephrolithotomy: medium-term follow-up. J
Endourol 2011; 25:941.
161. Gücük A, Kemahli E, Uyeturk U et al: Routine
flexible nephroscopy for percutaneous
nephrolithotomy for renal stones with low density:
a prospective randomized study. J Urol 2013;
190:144.
162. Zeltser I, Pearle MS and Bagley DH: Saline is our
friend. Urology. 2009; 1:28.
163. Aghamir SM, Alizadeh F, Meysamie A et al: Sterile
Copyright © 2016 American Urological Association Education and Research, Inc.®
Surgical Management
of Stones
American Urological Association (AUA)
Endourological Society Guideline

45
water versus isotonic saline solution as irrigation
fluid in percutaneous nephrolithotomy.Urol J 2009;
6:249.
164. Hosseini MM, Hassanpour A, Manaheji F et al:
Percutaneous nephrolithotomy: is distilled water as
safe as saline for irrigation? Urol J 2014; 11:
1551.
165. Chen SS, Lin AT, Chen KK et al: Hemolysis in
transurethral resection of the prostate using
distilled water as the irrigant. J Chin Med Assoc
2006; 69:270.
166. Auge BK, Pietrow PK Lallas et al: Ureteral access
sheath provides protection against elevated renal
pressures during routine flexible ureteroscopic
stone manipulation. J Endourol 2004; 18: 33.
167. Wolf JS Jr, Bennett CJ, Dmochowski RR et al: Best
Practice Policy Statement on Urologic Surgery
Antimicrobial Prophylaxis. J Urol 2008; 179: 1379.
168. Charlton M, Vallancien G, Veillon B et al: Urinary
tract infections in percutaneous surgery for renal
calculi. J Urol 1986; 135: 15.
169. Darenkov AF, Derevianko Il, Martov AG et al: The
prevention of infectious-inflammatory
complications in the postoperative period in
percutaneous surgical interventions in patients
with urolithiasis. Urol Nefro 1994; 2: 24.
170. Mariappan P, Smith G, Moussa SA et al: One week
of ciprofloxacin before percutaneous
nephrolithotomy significantly reduces the upper
tract infection and urosepsis: a prospective
controlled study. BJU Int 2006; 98: 1075.
171. Bag S, Kumar S, Taneja N et al: One week of
nitrofurantoin before percutaneous
nephrolithotomy significantly reduces upper tract
infection and urosepsis: a prospective controlled
study. Urology 2011; 77:45.
172. Viers BR, Cockerill PA, Mehta RA et al: Extended
antimicrobial use in patients undergoing
percutaneous nephrolithotomy and associated
antibiotic related complications. J Urol 2014; 192:
1667.
173. Grasso M, Conlin M and Bagley DH: Retrograde
ureteropyeloscopic treatment of 2cm or greater
upper urinary tract and minor staghorn calculi. J
Urol 1998; 160: 346.
174. El‐Anany FG, Hammouda HM, Maghraby HA et al:
Retrograde ureteropyeloscopic holmium laser
lithotripsy for large renal calculi. BJU Int 2001;
88: 850.
175. Breda A, Ogunyemi O, Leppert JT et al: Flexible
ureteroscopy and laser lithotripsy for single
intrarenal stones 2cm or greater – is this the new
frontier? J Urol 2008; 179: 981.
176. Riley JM, Stearman L and Troxel S: Retrograde
ureteroscopy for renal stones larger than 2.5cm. J
Endourol 2009; 23: 1395.
177. Hyams E, Munver R, Bird V et al: Flexible
ureterorenoscopy and holmium laser lithotripsy for
the management of renal stone burdens that
measure 2‐3cm: a multiinstitutional experience. J
Endourol 2010; 24: 1583.
178. Ricchiuti DJ, Smaldone MC, Jacobs BL et al:
Staged retrograde endoscopic lithotripsy as
alternative to PCNL in select patients with large
renal calculi. J Endourol 2007; 21: 1421.
179. Bagley DH, Healy KA and Kleinmann N:
Ureteroscopic treatment of larger renal calculi
(>2cm). Arab J Urol 2012; 10: 296.
180. Pace KT, Weir MJ, Tariq N et al: Low success rate
of repeat shock wave lithotripsy for ureteral stones
after failed initial treatment. J Urol 2000;
164:1905.
181. Giblin JG, Lossef S and Pahira JJ: A modification of
standard percutaneous nephrolithotripsy technique
for the morbidly obese patient. Urology. 1995; 46:
491.
182. Jackman SV, Docimo SG, Cadeddu JA et al: The
"mini-perc" technique: a less invasive alternative
to percutaneous nephrolithotomy. World J Urol
1998; 16: 371.
183. Tepeler A, Armagan A, Sancakturar AA et al: The
role of microperc in the treatment of symptomatic
lower pole renal calculi. J Endourol 2013; 27: 13.
Copyright © 2016 American Urological Association Education and Research, Inc.®
Surgical Management
of Stones
American Urological Association (AUA)
Endourological Society Guideline

46
184. Zhu H, Ye X, Xiao X et al: Retrograde, antegrade,
and laparoscopic approaches to the management
of large upper ureteral stones after shockwave
lithotripsy failure: a four-year retrospective study.
J Endourol 2014; 28: 100.
185. Abu Ghazaleh LA, Shunaigat AN and Budair Z:
Retrograde intrarenal lithotripsy for small renal
stones in prepubertal children. Saudi J Kidney Dis
Transpl 2011; 22: 492.
186. Ji C, Gan W, Guo H et al: A prospective trial on
ureteral stenting combined with secondary
ureteroscopy after an initial failed procedure. Urol
Res 2012; 40: 593.
187. Jones BJ, Ryan PC, Lyons O et al: Use of the
double pigtail stent in stone retrieval following
unsuccessful ureteroscopy. Br J Urol 1990;66:
254.
188. Singal RK, Razvi HA and Denstedt JD: Secondary
ureteroscopy: results and management strategy at
a referral center. J Urol 1998; 159:52.
189. Tugcu V, Gurbuz G, Aras B et al: Primary
ureteroscopy for distal-ureteral stones compared
with ureteroscopy after failed extracorporeal
lithotripsy. J Endourol 2006; 20: 1025.
190. Turna B, Stein RJ, Smaldone MC et al: Safety and
efficacy of flexible ureterorenoscopy and
holmium:YAG lithotripsy for intrarenal stones in
anticoagulated cases. J Urol 2008; 179:1415.
191. Elkoushy MA, Violette PD and Adnonian S:
Ureteroscopy in patients with coagulopathies is
associated with lower stone-free rate and
increased risk of clinically significant hematuria.
International Braz J Urol 2012; 38:195.
192. Gallucci M, Vincenzoni A, Schettini M et al:
Extracorporeal shock wave lithotripsy in ureteral
and kidney malformations. Urol Int 2001; 66: 61.
193. El-Assmy A, El-Nahas AR, Mohsen T et al:
Extracorporeal shock wave lithotripsy of upper
urinary tract calculi in patients with cystectomy
and urinary diversion. Urology 2005; 66: 510.
194. Khalil M: Management of impacted proximal
ureteral stone: extracorporeal shock wave
lithotripsy versus ureteroscopy with holmium: YAG
laser lithotripsy. Urol Ann 2013;5:88.
195. Renner C and Rassweiler J: Treatment of renal
stones by extracorporeal shock wave lithotripsy.
Nephron 1999; 81: 71.
196. Meretyk I, Leretyk S and Clayman RV:
Endopyelotomy: comparison of ureteroscopic
retrograde and antegrade percutaneous
techniques. J Urol 1992; 148: 775.
197. Waingankar N, Hayek S, Smith AD et al: Caliceal
diverticula: a comprehensive reviewRevUrol 2014;
16: 29.
198. Turna B, Raza A, Moussa S et al: Management of
calyceal diverticular stones with extracorporeal
shock wave lithotripsy and percutaneous
nephrolithotomy: long-term outcome. BJU Int
2007; 100:151.
199. Constantinople NL, Waters WB and Yalla SV:
Operative versus non-operative management of
patients with staghorn calculi and neurogenic
bladder. J Urol 1979; 121:716.
200. Viprakasit DP, Sawyer MD, Herrell SD et al:
Changing composition of staghorn calculi. J Urol
2011; 186:2285.
201. Aydogdu O, Burgu B, Gucuk A et al: Effectiveness
of doxazosin in treatment of distal ureteral stones
in children. J Urol 2009; 182: 2880.
202. Erturhan S, Bayrak O, Sarica K et al: Efficacy of
medical expulsive treatment with doxazosin in
pediatric patients. Urology 2013; 81: 640.
203. Mokhless I, Zahran AR, Youssif M et al: Tamsulosin
for the management of distal ureteral stones in
children: A prospective randomized study. J
Pediatr Urol 2012; 8:544.
204. Brenner D, Elliston C, Hall E et al: Estimated risks
of radiation-induced fatal cancer from pediatric CT.
AJR Am J Roentgenol 2001; 176: 289.
205. Brenner DJ and Hall EJ: Computed tomography—
an increasing source of radiation exposure. N Engl
J Med 2007; 357: 2277.
Copyright © 2016 American Urological Association Education and Research, Inc.®
Surgical Management
of Stones
American Urological Association (AUA)
Endourological Society Guideline

47
206. Strauss KJ and Goske MJ. Estimated pediatric
radiation dose during CT. Pediatr Radiol 2011;
41:S472.
207. Linet MS, Slovis TL, Miller DL et al: Cancer risks
associated with external radiation from diagnostic
imaging procedures. CA Cancer J Clin 2012;
62:75.
208. Paterson A, Frush DP and Donnelly LF: Helical CT
of the body: are settings adjusted for pediatric
patients? AJM Am J Roentgenol 2001; 176: 297.
209. Sohn W, Clayman RV, Lee JY et al: Low-dose and
standard computed tomography scans yield
equivalent stone measurements. Urology 2013;
81: 231.
210. Zilberman DE, Tsivian M, Lipkin ME et al: Low dose
computerized tomography for detection of
urolithiasis—its effectiveness in the setting of the
urology clinic. J Urol 2011; 185: 910.
211. Hubert KC and Palmer JS: Passive dilation by
ureteral stenting before ureteroscopy: eliminating
the need for active dilation. J Urol 2005; 174:
1079.
212. Corcoran AT, Smaldone MC, Mally D et al: When is
prior ureteral stent placement necessary to access
the upper urinary tract in prepubertal children? J
Urol 2008; 180: 1861.
213. Badawy AA, Saleem MD, Abolyosr A et al:
Extracorporeal shock wave lithotripsy as first line
treatment for urinary tract stones in children:
outcome of 500 cases. Int Urol Nephrol 2012; 44:
661.
214. Brinkmann OA, Griehl A, Kuwertz-Bröking E et al:
Extracorporeal shock wave lithotripsy in children.
Efficacy, complications and long-term follow up.
Eur Urol 2001; 39: 591.
215. Ishii H, Griffin S and Somani BK: Flexible
ureteroscopy and lasertripsy (FURSL) for
paediatric renal calculi: results from a systematic
review. J Pediatr Urol 2014; 10: 1020.
216. Sen H, Seckiner I, Bayrak O et al: Treatment
alternatives for urinary system stone disease in
preschool aged children: results of 616 cases. J
Pediatr Urol 2015; 11:34.
217. Rodrigues Netto N Jr, Longo JA, Ikonomidis JA et
al: Extracorporeal shock wave lithotripsy in
children. J Urol 2002; 167: 2164.
218. Shouman AM, Ziada AM, Ghoneim IA et al:
Extracorporeal shock wave lithotripsy
monotherapy for renal stones >25 mm in children.
Urology 2009;74:109.
219. Orsola A, Diaz I, Caffaratti J et al: Staghorn calculi
in children: treatment with monotherapy
extracorporeal shock wave lithotripsy. J Urol 1999;
162: 1229.
220. Al-Busaidy SS, Prem AR and Medhat M: Pediatric
staghorn calculi: the role of extracorporeal shock
wave lithotripsy monotherapy with special
reference to ureteral stenting. J Urol 2003; 169:
629.
221. Schuster TK, Smaldone MC, Averch TD et al:
Percutaneous nephrolithotomy in children. J
Endourol 2009; 23:1699.
222. Dede O, Sancaktutar AA, Dağguli M et al: Ultra-
mini-percutaneous nephrolithotomy in pediatric
nephrolithiasis: both low pressure and high
efficiency. J Pediatr Urol 2015; 11: 253.
223. Sabnis RB, Chhabra JS, Ganpule AP et al: Current
role of PCNL in pediatric urolithiasis. Curr Urol Rep
2014; 15:423.
224. Wah TM, Kidger L, Kennish S et al: MINI PCNL in a
pediatric population. Cardiovasc Intervent Radiol
2013; 36: 249.
225. Zeng G, Zhao Z, Wan SP et al: Minimally invasive
percutaneous nephrolithotomy for simple and
complex renal caliceal stones: a comparative
anlaysis of more than 10,000 cases. J Endourol
2013; 27: 1203.
226. Basiri A, Tabibi A, Nouralizadeh A et al:
Comparison of safety and efficacy of laparoscopic
pyelolithotomy versus percutaneous
nephrolithotomy in patients with renal pelvic
stones: a randomized clinical trial. Urol J 2014;
11:1932.
Copyright © 2016 American Urological Association Education and Research, Inc.®
Surgical Management
of Stones
American Urological Association (AUA)
Endourological Society Guideline

48
227. Lee RS, Passerotti CC, Cendron M et al: Early
results of robot assisted laparoscopic lithotomy in
adolescents. J Urol 2007;177:2306.
228. DeFoor W, Minevich E, Jackson E et al: Urinary
metabolic evaluations in solitary and recurrent
stone forming children. J Urol 2008; 179: 2369.
229. Spivacow FR, Negri AL, del Valle EE et al:
Metabolic risk factors in children with kidney stone
disease. Pediatr Nephrol 2008; 23: 1129.
230. The American Congress of Obstetricians and
Gynecologists: Committee Opinion No. 656
summary: guidelines for diagnostic imaging during
pregnancy and lactation. Obstet Gynecol 2016;
127: 418.
231. Johnson EB, Krambeck AE, White WM et al:
Obstetric complications of ureteroscopy during
pregnancy. J Urol 2012; 188: 151.
232. Swartz MA, Lydon-Rochelle MT, Simon D et al:
Admission for nephrolithiasis in pregnancy and risk
of adverse birth outcomes. Obstet Gynecol 2007;
109: 1099.
233. Rosenberg E, Sergienko R, Abu-Ghanem S et al:
Nephrolithiasis during pregnancy: characteristics,
complications, and pregnancy outcome. World J
Urol 2011; 29: 743.
234. Bailey G, Vaughan L, Rose C et al: perinatal
outcomes with tamsulosin therapy for
symptomatic urolithiasis. J Urol 2015; 195: 99.
235. Semins MJ, Trock BJ and Matlaga BR: The safety
of ureteroscopy during pregnancy: a systematic
review and meta-analysis. J Urol 2009; 181:139.
ABBREVIATIONS
ACOG American College of Obstetrics and
Gynecology
ASA American Society of Anesthesiologists
AUA American Urological Association
CBC Complete blood count
CCT Controlled clinical trial
COI Conflict of interest
CT Computed tomography
EHL Electrohydraulic lithotripsy
GOC Guidelines Oversight Committee
INR International normalized ration
IPSS International Prostate Symptom Score
IVP Intravenous pyelogram
IVU Intravenous urogram
J&E Judicial & Ethics Committee
NSAID Non-steroidal anti-inflammatory agent
MET Medical expulsive therapy
MR Magnetic resonance
PCNL Percutaneous nephrolithotomy
PGC Practice Guidelines Committee
PT Prothrombin time
PTT Partial thromboplastin time
QoL Quality of life
RCT Randomized controlled trial
SIR Society of Interventional Radiology
SWL Shock-wave lithotripsy
ULR Update literature review
UPJ Ureteropelvic junction
URS Ureteroscopy
US Ultrasound
UTI Urinary tract infection
VAPS Visual Analogue Pain Scale
Copyright © 2016 American Urological Association Education and Research, Inc.®
Surgical Management
of Stones
American Urological Association (AUA)
Endourological Society Guideline

49
Surgical Management of Stones Panel,
Consultants and Staff
Dean G. Assimos, MD (Chair)
University of Alabama Birmingham School of Medicine
Birmingham, AL
Brian R. Matlaga, MD (Vice Chair)
The Johns Hopkins University School of Medicine
Baltimore, MD
Hassan Razvi, MD (PGC Representative)
Western University
London, ON Canada
Margaret Sue Pearle, MD, PhD
UT Southwestern Medical Center
Dallas, TX
Amy Krambeck, MD
Mayo Clinic
Rochester, MN
Nicole Lara Miller, MD
Vanderbilt University Medical Center
Nashville, TN
Caleb Nelson, MD
Boston Children’s Hospital
Boston, MA
Kenneth Tony Pace, MD
St. Michael’s Hospital, University of Toronto
Toronto, ON Canada
Vernon Pais Jr., MD
Dartmouth Hitchcock Clinic
Lebanon, NH
Glenn M. Preminger, MD
Duke University Medical Center
Durham, NC
Ojas Shah, MD
NYU Urology Associates
New York, NY
Consultants
Hassan Murad, MD, MPH
Patricia Barrionuevo Moreno, MD
Noor Asi MD
Staff
Heddy Hubbard, PhD, MPH, RN, FAAN
Abid Khan, MHS, MPP
Patricia Rehring, MPH
Erin Kirkby, MS
Nenellia K. Bronson, MA
CONFLICT OF INTEREST DISCLOSURES
All panel members completed COI disclosures. Those
marked with (C) indicate that compensation was
received. Disclosures listed include both topic– and non
-topic-related relationships.
Consultant/Advisor: Dean Assimos, Oxalosis and
Hyperoxaluria Foundation (OHF); Brian Matlaga,
Boston Scientific (C); Glenn Preminger, Boston
Scientific (C), Retrophin (C); Hassan Razvi, Olympus
(C), Histosonics (C); Kenneth Pace, Amgen (C),
Janssen (C), Paladin Labs (C), Ferring Canada (C);
Ojas Shah, Boston Scientific (C), Lumenis (C), MD
Agree
Meeting Participant or Lecturer: Glenn
Preminger, Olympus (C), Retrophin (C); Nicole
Miller, Lumenis (C); Ojas Shah, Boston Scientific (C),
Lumenis (C)
Health Publishing: Dean Assimos, Med Review in
Urology (C), Urology Times (C); Glenn Preminger,
UpToDate (C); Vernon Pais, Clinical Nephrology
Scientific Study or Trial: Dean Assimos, National
Institute of Health (NIH) (C);
Leadership Position: Glenn Preminger,
Endourological Society (C)
Other: Amy Krambeck, HistoSonic (C); Hassan
Razvi, Cook Urological (C); Kenneth Pace, Cook
Urological (C); Ojas Shah, Metropolitan Lithotripto/
Allied Health (C), MD Agree
Copyright © 2016 American Urological Association Education and Research, Inc.®
Surgical Management
of Stones
American Urological Association (AUA)
Endourological Society Guideline

50
Peer Reviewers
We are grateful to the persons listed below who
contributed to the Guideline by providing comments
during the peer review process. Their reviews do not
necessarily imply endorsement of the Guideline.
Jodi Antonelli, MD
Diane Bieri Esq.
William W. Bohnert, MD
Rodney H. Breau, MD
Benjamin Canales, MD
Ben Chew, MD
Thomas Chi, MD
Muhammad S. Choudhury, MD
Peter E. Clark, MD
Ralph V. Clayman, MD
John D. Denstedt, MD
David Duchene, MD
James A. Eastham, MD
Gary J. Faerber, MD
Gerhard J. Fuchs, MD
Glenn S. Gerber, MD
Michael Grasso III, MD
Andreas Gross, MD
Mantu Gupta, MD
George E. Haleblian, MD
Jonathan Harper, MD
S. Duke Herrell, III, MD
R. John D'A. Honey, MD
Scott G. Hubosky, MD
Mitchell R. Humphreys, MD
Melissa R. Kaufman, MD
Bodo E. Knudsen, MD
Ali Riza Kural, MD
Evangelos Liatsikos, MD
Deborah J. Lightner, MD
Jessica A. Mandeville, MD
Tadashi Matsuda, MD
Kevin McVary, MD
Joshua J. Meeks, MD, PhD
Manoj Monga, MD
Robert Nadler, MD
Michael C. Ost, MD
Gyan Pareek, MD
Anup Patel, MS, FRCS
Craig A. Peters, MD
Hassan Razvi, MD
Koon Rha, MD
Samit Soni, MD
Thomas F. Stringer, MD
Yinghao Sun, MD
Chandru P. Sundaram, MD
Roger Sur, MD
Scott K. Swanson, MD
Christopher D. Tessier, MD
Olivier Traxer, MD
Thomas M.T. Turk, MD
David S. Wang, MD
J. Stuart Wolf, Jr., MD
Guo-bing Xiong, MD
DISCLAIMER
This document was written by the Surgical Management of
Stones Guideline Panel of the American Urological Association
Education and Research, Inc., which was created in 2014. The
Practice Guidelines Committee (PGC) of the AUA selected the
committee chair. Panel members were selected by the chair.
Membership of the panel included specialists in urology with
specific expertise on this disorder. The mission of the panel
was to develop recommendations that are analysis-based or
consensus-based, depending on panel processes and available
data, for optimal clinical practices in the treatment of stones.
Funding of the panel was provided by the AUA and Endo. Panel
members received no remuneration for their work. Each
member of the panel provides an ongoing conflict of interest
disclosure to the AUA.
While these guidelines do not necessarily establish the
standard of care, AUA seeks to recommend and to encourage
compliance by practitioners with current best practices related
to the condition being treated. As medical knowledge
expands and technology advances, the guidelines will change.
Today these evidence-based guidelines statements represent
not absolute mandates but provisional proposals for treatment
under the specific conditions described in each document. For
all these reasons, the guidelines do not pre-empt physician
judgment in individual cases.
Treating physicians must take into account variations in
resources, and patient tolerances, needs, and preferences.
Conformance with any clinical guideline does not guarantee a
successful outcome. The guideline text may include
information or recommendations about certain drug uses (‘off
label‘) that are not approved by the Food and Drug
Administration (FDA), or about medications or substances not
subject to the FDA approval process. AUA urges strict
compliance with all government regulations and protocols for
prescription and use of these substances. The physician is
encouraged to carefully follow all available prescribing
information about indications, contraindications, precautions
and warnings. These guidelines and best practice statements
are not in-tended to provide legal advice about use and misuse
of these substances.
Although guidelines are intended to encourage best practices
and potentially encompass available technologies with
sufficient data as of close of the literature review, they are
necessarily time-limited. Guidelines cannot include evaluation
of all data on emerging technologies or management, including
those that are FDA-approved, which may immediately come to
represent accepted clinical practices.
For this reason, the AUA does not regard technologies or
management which are too new to be addressed by this
guideline as necessarily experimental or investigational.
Copyright © 2016 American Urological Association Education and Research, Inc.®
Surgical Management
of Stones
American Urological Association (AUA)
Endourological Society Guideline
