OXFORD MEDICAL PUBLICATIONS
Oxford Handbook of
Clinical and
Laboratory
Investigation
ii
Published and forthcoming Oxford Handbooks
Oxford Handbook for the Foundation
Programme4e
Oxford Handbook of Acute
Medicine3e
Oxford Handbook of Anaesthesia4e
Oxford Handbook of Applied Dental
Sciences
Oxford Handbook of Cardiology2e
Oxford Handbook of Clinical and
Healthcare Research
Oxford Handbook of Clinical and
Laboratory Investigation3e
Oxford Handbook of Clinical
Dentistry6e
Oxford Handbook of Clinical
Diagnosis3e
Oxford Handbook of Clinical
Examination and Practical Skills2e
Oxford Handbook of Clinical
Haematology4e
Oxford Handbook of Clinical
Immunology and Allergy3e
Oxford Handbook of Clinical Medicine
– Mini Edition9e
Oxford Handbook of Clinical
Medicine10e
Oxford Handbook of Clinical Pathology
Oxford Handbook of Clinical
Pharmacy3e
Oxford Handbook of Clinical
Rehabilitation2e
Oxford Handbook of Clinical
Specialties10e
Oxford Handbook of Clinical Surgery4e
Oxford Handbook of Complementary
Medicine
Oxford Handbook of Critical Care3e
Oxford Handbook of Dental
PatientCare
Oxford Handbook of Dialysis4e
Oxford Handbook of Emergency
Medicine4e
Oxford Handbook of Endocrinology
and Diabetes3e
Oxford Handbook of ENT and Head
and Neck Surgery2e
Oxford Handbook of Epidemiology for
Clinicians
Oxford Handbook of Expedition and
Wilderness Medicine2e
Oxford Handbook of Forensic Medicine
Oxford Handbook of Gastroenterology
& Hepatology2e
Oxford Handbook of General
Practice4e
Oxford Handbook of Genetics
Oxford Handbook of Genitourinary
Medicine, HIV, and Sexual Health2e
Oxford Handbook of Geriatric
Medicine2e
Oxford Handbook of Infectious
Diseases and Microbiology2e
Oxford Handbook of Key Clinical
Evidence2e
Oxford Handbook of Medical
Dermatology2e
Oxford Handbook of Medical Imaging
Oxford Handbook of Medical
Sciences2e
Oxford Handbook of Medical Statistics
Oxford Handbook of Neonatology2e
Oxford Handbook of Nephrology and
Hypertension2e
Oxford Handbook of Neurology2e
Oxford Handbook of Nutrition and
Dietetics2e
Oxford Handbook of Obstetrics and
Gynaecology3e
Oxford Handbook of Occupational
Health2e
Oxford Handbook of Oncology3e
Oxford Handbook of Operative
Surgery3e
Oxford Handbook of
Ophthalmology3e
Oxford Handbook of Oral and
Maxillofacial Surgery
Oxford Handbook of Orthopaedics
andTrauma
Oxford Handbook of Paediatrics2e
Oxford Handbook of Pain Management
Oxford Handbook of Palliative Care2e
Oxford Handbook of Practical Drug
Therapy2e
Oxford Handbook of Pre- HospitalCare
Oxford Handbook of Psychiatry3e
Oxford Handbook of Public Health
Practice3e
Oxford Handbook of Reproductive
Medicine & Family Planning2e
Oxford Handbook of Respiratory
Medicine3e
Oxford Handbook of Rheumatology3e
Oxford Handbook of Sport and Exercise
Medicine2e
Handbook of Surgical Consent
Oxford Handbook of Tropical
Medicine4e
Oxford Handbook of Urology3e
1
Oxford Handbook of
Clinical and
Laboratory
Investigation
Fourth Edition
Editedby
DrewProvan
Honorary Reader in Autoimmune Haematology,
Barts & The London School of Medicine & Dentistry
Queen Mary University ofLondon
London,UK
iv
Great Clarendon Street, Oxford, OX26DP,
United Kingdom
Oxford University Press is a department of the University of Oxford.
It furthers the University’s objective of excellence in research, scholarship,
and education by publishing worldwide. Oxford is a registered trade markof
Oxford University Press in the UK and in certain other countries
© Oxford University Press2018
The moral rights of the authors have been asserted
First Edition published in2002
Second Edition published in2005
Third Edition published in 2010
Fourth Edition published in 2018
Impression:1
All rights reserved. No part of this publication may be reproduced, storedin
a retrieval system, or transmitted, in any form or by any means, withoutthe
prior permission in writing of Oxford University Press, or as expressly permitted
by law, by licence or under terms agreed with the appropriate reprographics
rights organization. Enquiries concerning reproduction outside the scopeofthe
above should be sent to the Rights Department, Oxford University Press,atthe
addressabove
You must not circulate this work in any otherform
and you must impose this same condition on any acquirer
Published in the United States of America by Oxford UniversityPress
198 Madison Avenue, NewYork, NY 10016, United States of America
British Library Cataloguing in PublicationData
Data available
Library of Congress Control Number:2017942066
ISBN 978–0–19–876653–7
Printed and bound in China by
C&C Oset Printing Co., Ltd.
Oxford University Press makes no representation, express or implied, thatthe
drug dosages in this book are correct. Readers must therefore alwayscheck
the product information and clinical procedures with the most up- to- date
published product information and data sheets provided by the manufacturers
and the most recent codes of conduct and safety regulations. The authorsand
the publishers do not accept responsibility or legal liability for any errorsinthe
text or for the misuse or misapplication of material in this work. Exceptwhere
otherwise stated, drug dosages and recommendations are for the non- pregnant
adult who is not breast- feeding
Links to third party websites are provided by Oxford in good faithand
for information only. Oxford disclaims any responsibility for the materials
contained in any third party website referenced in thiswork.
1
To Richard and Fraser.

vi
vi
This book lls an important gap in the market, being a comprehensive guide
to the requesting and interpretation of a wide range of diagnostic tests. The
authors have crammed a huge amount of information into a relatively small
volume. Its size, scope, and relevance mean that it is likely to be used daily
as a quick reference and aide- memoire. This fourth edition, which has been
entirely updated, covers conditions from the very common, such as nausea
and joint pain, to those seen less often. The fact that it is written by
ex perienced clinicians, including trainees, is evident from its practical
approach and focus on the patient.
This book highlights the importance, often forgotten, of diagnostic tests
in almost all patient care pathways. Its use will ensure that the right investi-
gations are done rst time, reducing unnecessary testing and enabling faster
and more accurate diagnosis. I am particularly pleased that it contains a
section on collecting specimens and how to avoid laboratory errors.
No medical student or junior doctor should be without this book (it
is ideal for revision); in fact, any doctor at any stage of their career will
nd it useful. The appropriate requesting and interpretation of clinical and
laboratory investigations is vital for maximizing the value of healthcare and
improving the quality of care for patients.
Suzy Lishman
President of The Royal College of Pathologists
2018
Foreword

vii
Six years have elapsed since the third edition of this book was published
and during that time there have been advances in investigative techniques,
both laboratory- based and clinical. My own specialty, haematology, has seen
renements in diagnostic tests for conditions such as leukaemias and lym-
phomas, but there have also been developments in the red cell and clotting
arenas. My colleagues in other clinical specialties have also enjoyed advances
within their own disciplines, and in order to make the book truly contempor-
ary, we have had to update all sections of the book bringing in all of these
new techniques.
As before, Ihave had the privilege to work with leaders in all branches
of medicine who have given up their time to update their chapters, bringing
them right up- to- date, and Iam immensely grateful tothem.
I am also indebted to Oxford University Press for their tireless work
on this Oxford Handbook which has been used by clinicians worldwide
for 14years. It has grown from 600 pages to almost 1000 in that time! If
this small book has helped in the diagnosis of patients, then Ifeel we have
achieved our task. Special thanks go to Michael Hawkes, Elizabeth Reeve,
and many others who have helped bring this book to publication.
Being an edited text, Itake responsibility for errors or omissions in the
book and welcome any comments readers may have. As ever, this book
is meant to be used at the bedside and in the clinic, and its usability relies
on input from readers. Please contact me at drewpro[email protected] if you
have any suggestions or spot any errors in thebook.
DrewProvan
2018
Preface tothe
fourth edition

viii
viii
With the increasing complexity of modern medicine, we now have literally
thousands of possible investigative techniques at our disposal. We are able
to examine our patient’s serum and every other body uid down to the
level of individual nucleotides, as well as being able to perform precise imag-
ing through CT, MRI, and other imaging technologies. The problem we have
all faced, especially as senior medical students or junior doctors is:Which
test should we use in a given setting? What hazards are associated with the
tests? Are there any situations where specic tests should not be used or
are likely to produce erroneous results? As medical complexity increases,
so too does cost; many assays available today are highly expensive and,
wherever possible, we would ideally like to use a test that is cheap, reliable,
reproducible, and right for a given situation.
Such knowledge takes many years to acquire and it is a fact of life that
senior doctors (who have attained such knowledge) are not usually those
who request the investigations. In this small volume, we have attempted to
distil all that is known about modern tests, from blood, urine, and other
body uids, along with imaging and molecular tests. The book is divided into
two principal parts:the rst deals with symptoms and signs in The patient
section, because that is how patients present. We have tried to cover as
many topics as possible, discussing these in some detail and have provided
dierential diagnoses where possible. We also try to suggest tests that
might be of value in determining the cause of the patient’s symptom or
sign. The second part of the book Investigations
is specialty- specic and is
more relevant once you know roughly what type of disease the patient
might have. For example, if the symptom section suggests a likely respira-
tory cause for the patient’s symptoms, then the reader should look to the
Respiratory medicine chapter in order to determine which tests to carry out
or how to interpret the results.
The entire book is written by active clinicians, rather than scientists, since
we wanted to provide a strong clinical approach to investigation. We have
tried, wherever possible, to cross- refer to the Oxford Handbook of Clinical
Medicine, Oxford University Press, which provides the clinical detail omitted
from this handbook. The symbol E
is used to highlight a cross- reference to
OHCM, in addition to cross- referencing within thisbook.
We would value feedback from readers since there will doubtless be tests
omitted, errors in the text, and many other improvements we could, and
will, make in future editions. All contributors will be acknowledged individu-
ally in the next edition. We would suggest you e- mail us directly.
DrewProvan
AndrewKrentz
2002
Preface tothe first edition
ix
Contributors x
Symbols and abbreviations xii
Approach to investigations xxxiv
Laboratory errors and how to avoid them xxxvi
Part I The patient
1 Symptoms and signs 3
Part II Investigations
2 Endocrinology and metabolism
123
3 Haematology
227
4 Immunology and allergy
333
5 Infectious and tropical diseases
381
6 Cardiology
435
7 Gastroenterology
497
8 Respiratory medicine
535
9 Neurology
583
10 Renal medicine
649
11 Poisoning and overdose
699
12 Rheumatology
741
13 Radiology
765
14 Nuclear medicine
865
Index 957
Contents

x
x
BrianAngus
Director, Centre for Tropical
Medicine and Global Health,
Oxford,UK
Chapter5:Infectious and tropical
diseases
Jim Ballinger
Honorary Senior Lecturer,
Imaging Sciences, King’s College
London,UK
Chapter14:Nuclear medicine
Martyn Bracewell
Senior Lecturer in Neurology and
Neuroscience, Bangor University,
Bangor,UK
Chapter9:Neurology
JoannaBrown
Respiratory Consultant, Imperial
College Healthcare NHS
Trust, Hammersmith Hospital,
London,UK
Chapter8:Respiratory medicine
TanyaChawla
Sta Radiologist and Assistant
Professor, Joint Department of
Medical Imaging, University of
Toronto, Toronto,Canada
Chapter13:Radiology
ColinDayan
Professor of Clinical Diabetes
and Metabolism, Division of
Infection and Immunity, Cardi
University School of Medicine,
Cardi, UK
Chapter2:Endocrinology and
metabolism
VanessaFoggo
Consultant Haematologist, Royal
London Hospital, London,UK
Chapter3:Haematology
Gopinath Gnanasegaran
Consultant Physician in Nuclear
Medicine, Department of Nuclear
Medicine, Guy and St Thomas’
Hospital, London,UK
Chapter14:Nuclear medicine
EmmaGreig
Consultant Gastroenterologist,
Musgrove Park Hospital, Taunton,UK
Chapter7:Gastroenterology
Andrew R Houghton
Consultant Cardiologist
and Visiting Fellow of the
University of Lincoln, United
Lincolnshire Hospitals NHS Trust,
Lincolnshire,UK
Chapter6:Cardiology
AlisonJones
Pro Vice-Chancellor (Health
Strategy) and Executive Dean,
Faculty of Science, Medicine and
Health, University of Wollongong,
NSW, Australia
Chapter11:Poisoning and overdose
SuzanneLane
Consultant Rheumatologist, Ipswich
Hospital NHS Trust, Ipswich,UK
Chapter12:Rheumatology
Sarah McCloskey
Speciality Trainee in Nephrology,
Department of Renal Medicine,
Freeman Hospital, Newcastle
uponTyne, UK
Chapter10:Renal medicine
Gavin Spickett
Consultant Immunologist,
The Newcastle upon Tyne
Hospitals NHS Foundation Trust,
Newcastle,UK
Chapter4:Immunology and allergy
Contributors

xi
CONTRIBUTORS
CharlesTomson
Consultant Nephrologist,
Department of Renal Medicine,
Freeman Hospital, Newcastle
uponTyne, UK
Chapter10:Renal medicine
My thanks, also, to those who contributed to the rst edition:John Axford,
Keith Dawkins, and Praful Patel. Also to those who contributed to the
second edition:James Dunbar, AFrew, Stephen T Green, Val Lewington,
Rommel Ravanan, Dr Penelope Sensky, Adrian Williams, and Lorraine
Wilson.

xii
xii
7 approximately
α alpha
β beta
δ delta
γ gamma
κ kappa
λ lambda
l leadingto
E cross- reference
i increased
d decreased
n normal
M website
2 important
3 very important
> greaterthan
≥ equal to or greaterthan
>> much greaterthan
< lessthan
≤ equal to or lessthan
= equalto
+ve positive
−ve negative
° degree
°C degree Celsius
° primary
2° secondary
♂ male
♀ female
ii greatly increased
± plus orminus
2D two- dimensional
3D three- dimensional
β- TG beta- thromboglobulin
γGT gamma- glutamyl transpeptidase
5HIAA 5- hydroxyindole aceticacid
Symbols and abbreviations

SYMBOLS AND ABBREVIATIONS
xiii
μg microgram
μL microlitre
μmol micromole
99m
Tc technetium- 99m
AA aorticarch
AAA abdominal aortic aneurysm
AAFB acid- and alcohol- fast bacilli
ABG arterial bloodgas
ABO ABO bloodgroups
ABPA allergic bronchopulmonary aspergillosis
AC air conduction
ACD anaemia of chronic disease
ACE angiotensin- convertingenyme
ACEI angiotensin- converting enzyme inhibitor
ACh acetylcholine
AChE red cell cholinesterase
AChRAb acetylcholine receptor antibodies
ACL anticardiolipin antibody
ACPA anti- cyclic citrullinated peptide antibodies
ACR albumin:creatinineratio
ACS acute coronary syndrome
ACTH adrenocorticotrophic hormone
ADA American Diabetes Association
ADC apparent diusion coecient
ADH antidiuretic hormone
ADP adenosine 5- diphosphate
AF atrial brillation
AFP alpha- fetoprotein
AICD automatic intracardiac debrillationdevice
AIDS acquired immune deciency syndrome
AIH autoimmune hepatitis
AIHA autoimmune haemolytic anaemia
AIP autoimmune prole
AKI acute kidneyinjury
ALD adrenoleucodystrophy
ALL acute lymphoblastic leukaemia
ALP alkaline phosphatase
ALT alanine transaminase
AMA antimitochondrial antibodies
AML acute myeloid leukaemia

SYMBOLS AND ABBREVIATIONS
xiv
xiv
ANA antinuclear antibodies
ANAE alpha- naphthyl acetate esterase
ANCA antineutrophil cytoplasmic antibody
ANNA anti- neuronal nuclear antibodies
AP anteroposterior; action potential
APCR activated protein C resistance
APS antiphospholipid syndrome
APTR activated partial thromboplastin timeratio
APTT activated partial thromboplastintime
APVD anomalous pulmonary venous drainage
ARB angiotensin II receptor blocker
ARDS acute respiratory distress syndrome
ARF acute renal failure
ARMS amplication refractory mutationsystem
ASAS Assessment of SpondyloArthritis International Society
ASCA antibodies to Saccharomyces cerevisiae
ASIS anterior superior iliacspine
ASMA anti- smooth muscle antibodies
ASO anti- streptolysin
ASOT anti- streptolysin Otitre
AST aspartate transaminase
AT antithrombin
AT- II angiotensinII
ATN acute tubular necrosis
AV atrioventricular; arteriovenous
AVM arteriovenous malformation
AVN avascular necrosis
AVP arginine vasopressin
AVPD anomalous pulmonary venous drainage
AXR abdominalX- ray
AZT zidovudine
BAEP brainstem auditory evoked potential
BAER brainstem auditory evoked response
BAL bronchoalveolarlavage
BC bone conduction
BCG bacillus Calmette– Guérin
bd bis die (twicedaily)
bDNA branched- chain deoxyribonucleicacid
BIPLED bihemispheric periodic lateralized epileptiform discharge
BIRADS breast imaging and reporting datasystem

SYMBOLS AND ABBREVIATIONS
xv
BJP Bence– Jones protein
BM bonemarrow
BMI body massindex
BMT bone marrow transplant
BOLD blood oxygenlevel
BP blood pressure
bpm beat perminute
BSAEP brainstem auditory evoked potential
BSG British Society of Gastroenterology
BSL Biosafetylevel
BW bronchial washing
C&S culture and sensitivity
Ca
2+
calcium
Ca 19- 9 carbohydrate antigen19- 9
Ca- 125 cancer antigen125
CAD computer- assisted detection
CAH congenital adrenal hyperplasia
cAMP cyclic adenosine monophosphate
c-ANCA cytoplasmic antineutrophil cytoplasmic antibody
CaSR calcium- sensing receptor
CBC complete bloodcount
CBD common bileduct
CC craniocaudal
CCF congestive cardiac failure
CCK cholecystokinin
CCP cyclic citrullinated peptide
CCTA coronary computed tomography angiography
CCU coronary careunit
CD cluster dierentiation
CEA carcinoembryonic antigen
cf. comparewith
CF complement xation
CFA cryptogenic brosing alveolitis
CFU colony- formingunit
CGMS continuous glucose monitoring systems
CHD coronary heart disease
Cho choline
CHr reticulocyte
CINCA chronic infantite neurologic, cutaneous and articular syndrome
CJD Creutzfeldt– Jakob disease

SYMBOLS AND ABBREVIATIONS
xvi
xvi
CK creatinekinase
CKD chronic kidney disease
CKD- EPI Chronic Kidney Disease Epidemiology Collaboration
Cl
−
chloride
CLL chronic lymphocytic/ lymphatic leukaemia
CLO Campylobacter- like organism
cm centimetre
CMAP compound motor action potential
cmH
2
O centimetre ofwater
CML chronic myeloid leukaemia
CMR cardiovascular magnetic resonance
CMV cytomegalovirus
C3Nef C3 nephriticfactor
CNS central nervoussystem
CO carbon monoxide
CO
2
carbon dioxide
COHb carboxyhaemoglobin
COPD chronic obstructive pulmonary disease
cP centipoise
CPAP continuous positive airway pressure
CPE carbapenemase- producing Enterobacteriaceae
CPK creatinine phosphokinase
CPPD calcium pyrophosphate disease
CPS complex partial seizure
Cr creatine
CrAg cryptococcal antigen
CrC creatinine clearance
CRC colorectal carcinoma
CREST calcinosis, Raynaud’s syndrome, oesophageal motility
dysfunction, sclerodactyly, and telangiectasia
CRH corticotropin- releasing hormone
CRP C- reactive protein
CSF cerebrospinaluid
CSU catheter specimen ofurine
CT computed tomography
CTC computed tomography colonography
CT- IVP computed tomography intravenous pyelography
CTLp cytotoxic T- lymphocyte precursor
CTPA computed tomography pulmonary angiography
CTU computed tomography urography

SYMBOLS AND ABBREVIATIONS
xvii
CVA cerebrovascular accident (stroke)
CVD cardiovascular disease
CVID common variable immunodeciency
CVP central venous pressure
CVS cardiovascular system; chorionic villus sampling
CW continuouswave
CXR chestX- ray
CyF cystic brosis
DAT direct antibodytest
dB decibel
DBCE double contrast bariumenema
DCCT Diabetes Control and ComplicationsTrial
DEC diethylcarbamazine
DESS dual- echo steadystate
DEXA dual- energy X- ray absorptiometry
DFa direct uorescein- labelled monoclonal antibody
DFA direct uorescent antibody
DHEAS dehydroepiandrosterone sulfate
DI diabetes insipidus
DIC disseminated intravascular coagulation
DIDMOAD diabetes insipidus, diabetes mellitus, optic atrophy, and
deafness
DIF direct immunouorescence
DIP distal interphalangealjoint
DJ duodenojejunal
DKA diabetic ketoacidosis
dL decilitre
DLCO diusing lung capacity for carbon monoxide
DM diabetes mellitus
DMSA dimercaptosuccinicacid
DNA deoxyribose nucleicacid
DOAC direct- acting anticoagulant
DPLD diuse parenchymal lung disease
dRVVT dilute Russell viper venomtest
DSA digital subtraction angiography
ds- DNA double- strandedDNA
DSI dimensionless severityindex
DTI diusion tensor imaging
DTPA diethylenetri aminepentaaceticacid

SYMBOLS AND ABBREVIATIONS
xviii
xviii
DU duodenalulcer
DVT deep vein thrombosis
DWI diusion- weighted imaging
DXA dual- energy X- ray absorptiometry
EBB endobronchialbiopsy
EBUS endobronchial ultrasound
EBV Epstein– Barrvirus
ECG electrocardiogram
EDC estimated date of connement
EDH extradural haemorrhage
EDTA ethylenediamine tetra- aceticacid
EEG electroencephalogram
EF ejection fraction
eGFR estimated glomerular ltrationrate
EGFR epidermal growth factor receptor
EGPA eosinophilic granulomatosis with polyangiitis
EIA enzyme- linkedassay
ELISA enzyme- linked immunosorbentassay
EM electron microscopy
EMA endomysial antibody
EMG electromyogram/ electromyography
EMU early morningurine
ENA extractable nuclear antigen
EOG electro- oculography
EP evoked potential
Epo erythropoietin
EQA external quality assurance
ER evoked response
ERCP endoscopic retrograde cholangiopancreatography
ERF established renal failure
ESA erythropoiesis- stimulatingagent
ESR erythrocyte sedimentationrate
ESS Epworth sleepinessscale
ETT endotrachealtube
EUS endoscopic ultrasound
Fab antibody fragment
FACS uorescence- activated cellsorter
FAP familial polyposis syndrome
FBC full bloodcount
FBHH familial benign hypocalciuric hypercalcaemia

SYMBOLS AND ABBREVIATIONS
xix
FCHL familial combined hyperlipidaemia
FDG uorodeoxyglucose
FDP brin degradation product
FeNO exhaled nitric oxide fraction
FEV forced expiratoryvolume
FEV
1
forced expiratory volume in 1second
FFP fresh frozenplasma
FGF broblast growthfactor
FH familial hypercholesterolaemia
FiO
2
inspired oxygen concentration
FISH uorescence in situ hybridization
fL femtolitre
FLAIR uid attenuation inversion recovery
fMRI functional magnetic resonance imaging
FNA ne- needle aspirate/ aspiration
FNH focal nodular hyperplasia
FOB faecal occultblood
FPG fasting plasma glucose
Fr French
FRC functional residual capacity
FSH follicle- stimulating hormone
FT4 freeT4
FTA- ABS uorescent treponemal antibody absorption
FUO fever of unknownorigin
FVC ow– volume curve; forced vital capacity
FVL factor VLeiden
g gram
GABA gamma- aminobutyricacid
GAD glutamic acid decarboxylase
GAn general anaesthetic
GBM glomerular basement membrane
GBS Guillain– Barré syndrome
GC gas chromatography
GCA giant cell arteritis
GC- MS gas chromatography mass spectrometry
GCS Glasgow ComaScale
Gd gadolinium
GFR glomerular ltrationrate
GGT gamma glutamyl transpeptidase
GH growth hormone

SYMBOLS AND ABBREVIATIONS
xx
xx
GHRH growth hormone- releasing hormone
GI gastrointestinal
GIT gastrointestinaltract
GLC gas– liquid chromatography
GM- CSF granulocytic macrophage colony- stimulatingfactor
GnU genitourinary
GORD gastro- oesophageal reux disease
GPA granulomatosis with polyangiitis
GPC gastric parietalcell
G6PD glucose- 6- phosphate dehydrogenase
GPI glycosyl phosphatidyl inositol
GRA glucocorticoid remediable aldosteronism
G&S group andsave
GT glucose tolerance
GTN glyceryl trinitrate
GTT glucose tolerancetest
GU gastriculcer
GvHD graft- versus- host disease
h hour
HAART highly active antiretroviral therapy
HACEK Haemophilus species, Actinobacillus actinomycetemcomitans,
Cardiobacterium hominis, Eikenella corrodens, and Kingella species
HAV hepatitis Avirus
Hb haemoglobin
HbA
1c
haemoglobinA
1c
HbC haemoglobinC
HbD haemoglobinD
HbE haemoglobinE
HbF fetal haemoglobin
HbH haemoglobinH
HbO oxyhaemoglobin
HbS sickle haemoglobin
HbSC haemoglobinSC
HBV hepatitis Bvirus
HC haemoglobin content
hCG human chorionic gonadotrophin
HCO
3
−
bicarbonate
Hct haematocrit
HCV hepatitis Cvirus
HD Hodgkin’s disease

SYMBOLS AND ABBREVIATIONS
xxi
HDFN haemolytic disease of the fetus and newborn
HDL high- density lipoprotein
HDN haemolytic disease of the newborn
HELLP haemolysis, elevated liver enzymes, and low plateletcount
HEMPAS hereditary erythroblastin multinuclearity with positive acidied
serum lysistest
HEV hepatitisE
HHT hereditary haemorrhagic telangiectasia
HHV human herpesvirus
Hib Haemophilus inuenzaetypeB
HIE hypoxic– ischaemic encephalopathy
HIV human immunodeciencyvirus
HLA human leucocyte antigen
HLH haemophagocytic lymphohistiocytosis syndrome
HMPAO hexamethyl- propylene- amine- oxime
HMSN hereditary motor sensory neuropathy
HNA heparin neutralizing activity
HNF hepatic nuclearfactor
HNPCC hereditary non- polyposis colorectal carcinoma
HNPP hereditary neuropathy with liability to pressure palsies
HONK hyperosmolar non- ketotic
Hp haptoglobin
HPA hybridization protection
HPFH hereditary persistence of fetal haemoglobin
HPLC high- performance liquid chromatography
HPOA hypertrophic pulmonary osteoarthropathy
HPV human papillomavirus
HRC hypochromic redcell
HRCT high- resolution computed tomography
HRP- 2 histidine- rich protein2
HRT hormone replacement therapy
hsTnI high- sensitivity troponinI
hsTnT high- sensitivity troponinT
HSV herpes simplexvirus
HTLV human T- lymphotropicvirus
HU Hounseldunit
HUS haemolytic uraemic syndrome
HUVS hypocomple mentaemic urticarial vasculitis syndrome
Hz hertz
IABP intra- aortic balloonpump

SYMBOLS AND ABBREVIATIONS
xxii
xxii
IAT indirect antiglobulintest
IBD inammatory bowel disease
IBS irritable bowel syndrome
ICA islet cell antibodies
ICD internal cardioverter– debrillator
ICM insertable cardiac monitor
ICP intracranial pressure
ICPMS inductively coupled plasma mass spectroscopy
ICU intensive careunit
IDA iminodiaceticacid
IDDM insulin- dependent (type 1)diabetes mellitus
IDMS isotope dilution mass spectrometry
IEF isoelectric focusing
IF intrinsicfactor
IFA intrinsic factor antibodies
IFCC International Federation of Clinical Chemistry
IFG impaired fasting glucose
IFT immunouorescencetest
Ig immunoglobulin
IgA immunoglobulinA
IgD immunoglobulinD
IgE immunoglobulinE
IGE idiopathic generalized epilepsy
IGF insulin- like growthfactor
IgG immunoglobulinG
IgM immunoglobulinM
IGRA interferon gamma releaseassays
IGT impaired glucose tolerance
IHD ischaemic heart disease
IIH intracranial hypertension
IL interleukin
ILD interstitial lung disease
ILR implantable loop recorder
IM intramuscular
IMN idiopathic membranous nephritis
INR international normalizedratio
INR/ PT international normalized ratio/ prothrombintime
IPSS inferior petrosal sinus sampling
IQ intelligence quotient
ITP idiopathic thrombocytopenic purpura

SYMBOLS AND ABBREVIATIONS
xxiii
ITT insulin tolerancetest
ITU intensive therapyunit
IU internationalunit
IUP intrauterine pregnancy
IV intravenous
IVC inferior venacava
IVI intravenous infusion
IVP intravenous pyelography
IVU intravenous urogram
JME juvenile myoclonic epilepsy
JVP jugular venous pressure; jugular venouspulse
K
+
potassium
kBq kilobecquerel
KCCT kaolin cephalin clottingtime
KCO transfer coecient for carbon monoxide
kDa kilodalton
KDIGO Kidney Disease Improving Global Outcomes
kg kilogram
kPa kilopascal
KSHV Kaposi’s sarcoma- associated herpesvirus
kU kilounit
KUB kidney, ureter, bladder (X- ray)
kV kilovoltage
L litre
LA lactic acidosis
LADA latent autoimmune diabetes ofadults
LAn local anaesthetic
LAP leucocyte alkaline phosphatase; left atrial pressure
LAt leftatrial
lb pound
LC liver cytosol
LCM left costalmargin
LC- MS/ MS liquid chromatography tandem- mass spectrometry
LC/ Q- TOF- MS liquid chromatography/ quadrupole time- of- ight mass
spectrometry
LCR ligase chain reaction
LDH lactate dehydrogenase
LDL low- density lipoprotein
LDST low- dose dexamethasone suppressiontest
LEMS Lambert– Eaton myasthenic syndrome

SYMBOLS AND ABBREVIATIONS
xxiv
xxiv
LFT liver functiontest
LH luteinizing hormone
LHRH luteinizing hormone- releasing hormone
LIF left iliacfossa
LKM liver– kidney microsomal
LMN lower motor neurone
LOC loss of consciousness
LP lumbar puncture
LPL lipoproteinlipase
LSCC lateral semicircularcanal
LTOT long- term oxygen therapy
LUQ left upper quadrant
LV left ventricle
LVEDP left ventricular end- diastolic pressure
LVF left ventricular failure
m metre
MAA macroaggregated albumin
MAG myelin- associated glycoprotein
MAG3 mercaptoacetyl -triglycine
MAHA microangiopathic haemolytic anaemia
MAIPA monoclonal antibody immobilization of platelet
antigens
MALDI matrix- assisted laser desorption/ ionization
MALDI- TOF MS matrix- assisted laser desorption/ ionization time- of-
ight mass spectrometry
MALT mucosa- associated lymphoidtumour
MAOI monoamine oxidase inhibitor
MARS Molecular Absorbance RecirculatingSystem
MBq megabecquerel
MCH mean cell haemoglobin
MCHC mean corpuscular haemoglobin concentration
MCP metacarpophalangeal
M,C&S microscopy, culture, and sensitivity
MCUG micturating cystourethrogram
MCV mean cellvolume
MCVm mutated citrullinated vimentin
MD myotonic dystrophy
MDMA methylene dioxymethamphetamine or ecstasy
MDR multi- drug- resistant
MDRD Modication of Diet in Renal Disease
MDR- TB multi- drug- resistant tuberculosis

SYMBOLS AND ABBREVIATIONS
xxv
MDS myelodysplastic syndrome
MELAS mitochondrial myopathy, lactic acidosis, and stroke- like
episodes
MEN multiple endocrine neoplasia
MEP motor evoked potential
mEq milliequivalent
MERRF myoclonic epilepsy and ragged redbres
MERS- CoV Middle East respiratory syndrome coronavirus
MetHb methaemoglobin
mg milligram
Mg
2+
magnesium
MG myastheniagravis
MGUS monoclonal gammopathy of undetermined signicance
MHC major histocompatibility complex
MHz megahertz
MI myocardial infarction
MIBG iodine- 131- meta- iodobenzylguanide
MIC minimum inhibitory concentration
min minute
m- In myo- inositol
mIU milli- internationalunit
mL millilitre
MLC mixed lymphocyte culture
MLO mediolateral oblique
mm millimetre
mmHg millimetre of mercury
M- mode motion- mode
mmol millimole
MND motor neurone disease
MoAb monoclonal antibody
MODY maturity- onset diabetes of theyoung
mOsmol milliosmole
MP metacarpophalyngeal
mPA millipascal
MPD myeloproliferative disease
MPHR max predicted heartrate
MPI myocardial perfusion imaging
MPO myeloperoxidase
MPV mean plateletvolume
MR magnetic resonance

SYMBOLS AND ABBREVIATIONS
xxvi
xxvi
MRA magnetic resonance angiogram
MRC Medical Research Council
MRCP magnetic resonance cholangiopancreatography
MRD minimal residual disease
MRI magnetic resonance imaging
mRNA messenger ribonucleicacid
MRS magnetic resonance spectroscopy
MRSA meticillin- resistant Staphylococcusaureus
MRV magnetic resonance venography
ms millisecond
MS multiple sclerosis
MSD mean sac diameter
MSU midstreamurine
mSv millisievert
MTP metatarsophalangeal
mU milliunit
MUGA multigated radionuclide angiography
MUP motor unit potential
MuSK muscle- specickinase
mV millivolt
Na
+
sodium
NAA N- acetyl- aspartate
NAC N- acetylcysteine
NAG N- acetyl- D- glucosaminidase
NAP neutrophil alkaline phosphatase
NASBA nucleic acid sequence- based amplication
NCS nerve conduction studies
NCSE non- convulsive status epilepticus
NEC necrotizing enterocolitis
NET neuroendocrinetumour
ng nanogram
NH
3
ammonia
NH
4
+
ammonium
NHL non- Hodgkin’s lymphoma
NHS National Health Service
NICE National Institute for Health and Care Excellence
NIDDM non- insulin- dependent diabetes mellitus
NK naturalkiller
nm nanometre
nmol nanomole

SYMBOLS AND ABBREVIATIONS
xxvii
NMS neuroleptic malignant syndrome
NO nitricoxide
NOGG National Osteoporosis GuidelineGroup
NPA nasopharyngeal aspirate
NPH normal pressure hydrocephalus
NPSA National Patient SafetyAgency
NREM non- rapid eye movement
NSAID non- steroidal anti- inammatorydrug
NSF nephrogenic systemic brosis
NSTEMI non- ST- elevation myocardial infarction
O
2
oxygen
OA osteoarthritis
Oc calculated osmolality
OCB oligoclonalband
OCP oral contraceptivepill
OGD oesophagogastroduodenoscopy
OGTT oral glucose tolerancetest
OHCM Oxford Handbook of Clinical Medicine
Om measured osmolality
OMIM Online Mendelian InheritanceinMan
OSA obstructive sleepapnoea
OTC over the counter
PA pernicious anaemia
P- A posteroanterior
PABA para- amino benzoic acid; N- benzoyl- L- tyrosol
p- aminobenzoicacid
P
a
CO
2
. partial pressure of carbon dioxide in arterialblood
PACS picture archiving and communication systems
PAD peripheral arterial disease
PAN polyarteritisnodosa
p-ANCA perinuclear antineutrophil cytoplasmic antibody
P
a
O
2
arterial oxygen tension
PAS periodic acid– Schi
PB peripheralblood
PBC primary biliary cirrhosis
PC protein C; provocation concentration
PCH paroxysmal cold haemoglobinuria
PCI percutaneous coronary intervention
PCNA proliferating cell nuclear antigen
PCO
2
partial passure of carbon dioxide

SYMBOLS AND ABBREVIATIONS
xxviii
xxviii
PCP Pneumocystis jiroveci pneumonia
PCR polymerase chain reaction
PCrR protein:creatinineratio
PCT procalcitonin
PCV packed cellvolume
PD Parkinson’s disease
PDW platelet distributionwidth
PE pulmonary embolism/ embolus
PEFR peak expiratory owrate
PEG percutaneous endoscopic gastrostomy
PET positron emission tomography
PFR peak owrate
pg picogram
PICC peripherally inserted central catheter
PIFT platelet immunouorescencetest
PIP proximal interphalangeal
PK pyruvatekinase
PkS Parkinson’s syndrome
PLA- 2 phospholipaseA2
PLED periodic lateralized epileptiform discharge
PLMD paroxysmal leg movement disorder
PmA pulmonaryartery
PMA paramethoxymethamphetamine
PMF progressive massive brosis
PMLE progressive multifocal leukoencephalopathy
PNH paroxysmal nocturnal haemoglobinuria
PNS peripheral nervoussystem
PO per os (bymouth)
PO
2
partial pressure ofoxygen
PO
4
3−
phosphate
POTS postural orthostatic tachycardia syndrome
PP pancreatic polypeptide
ppb parts per billion
PPD puried protein derivative
PR perrectum
PR3 proteinase3
PRA plasma renin activity
PrC provocation concentration
PRL prolactin
PRV polycythaemia rubravera

SYMBOLS AND ABBREVIATIONS
xxix
PS proteinS
PSA prostate- specic antigen
PSMA prostate- specic membrane antigen
PT prothrombintime
PTA percutaneous transluminal angioplasty
PTC percutaneous transhepatic cholangiogram
PTH parathyroid hormone
PTR prothrombinratio
PTTK partial thromboplastin time withkaolin
PUO pyrexia of unknownorigin
PV plasmavolume
PVA polyvinyl alcohol
PvC provocation concentration
PVNS pigmented villonodular synovitis
PW pulsedwave
PWI perfusion- weighted imaging
PxCT proximal convolutedtubule
qds quarter die sumendus (four timesdaily)
RA refractory anaemia
RAPA rheumatoid arthritis particle agglutinationtest
RAS renal artery stenosis
RAST radioallergosorbenttest
RAt right atrial/ atrium
RBC red bloodcell
RBP retinol- binding protein
RCC red cellcount
RDT rapid diagnostictest
RDW red cell distributionwidth
REM rapid eye movement
RES reticuloendothelialsystem
Ret- He reticulocyte haemoglobin content
RF rheumatoidfactor
RFLP restriction fragment length polymorphism
Rh rhesus
RhA rheumatoid arthritis
RhMK Rhesus monkeykidney
RIA radioimmunoassay
RiCoF Ristocetin Cofactor
RID radial immunodiusion
RIF right iliacfossa

SYMBOLS AND ABBREVIATIONS
xxx
xxx
RIPA ristocetin- induced platelet aggregation
RIS radiology informationsystem
RNP ribonucleoprotein
RNV radionuclide ventriculography
RPR rapid plasmareagin
RSV respiratory syncytialvirus
RTA renal tubular acidosis
rTMS repetitive transcranial magnetic stimulation
RUQ right upper quadrant
RV right ventricle; residualvolume
s second
SAA serum amyloidA
SAAG serum ascites albumin gradient
SAH subarachnoid haemorrhage
SaO
2
arterial oxygen saturation
SARS severe acute respiratory syndrome
SB Sudanblack
SBE subacute bacterial endocarditis
SBO small bowel obstruction
SBP spontaneous bacterial peritonitis
sbt serum bactericidaltest
SC subcutaneous
SCC squamous cell carcinoma
SCID severe combined immunodeciency
SCLC small- cell lungcancer
SDH subdural haemorrhage
SDS- PAGE sodium dodecyl sulfate polyacrylamide gel electrophoresis
SeHCAT
75
selenium homotaurocholate
SG specic gravity
SGLT2 sodium– glucose cotransporter2
SHBG sex hormone- binding globulin
SI Système International
SIADH syndrome of inappropriate antidiuretic hormone
SLA soluble liver antigen
SLE systemic lupus erythematosus
SMA smooth muscle antibody
SNAP sensory nerve action potential
SO
4
2
–
sulfate
SOB shortness ofbreath
SOD sphincter of Oddi dysfunction

SYMBOLS AND ABBREVIATIONS
xxxi
SOL space- occupyinglesion
SPECT single photon emission computed tomography
SpO
2
oxygen saturation
SSFP steady- state free precession
SSP single strand polymorphism
SSPE subacute sclerosing panencephalitis
SSR somatostatin receptor
STD sexually transmitted disease
STEMI ST- elevation myocardial infarction
sTfR serum transferrin receptor
STfR soluble transferrin receptorassay
STIR short tau inversion recovery
SUV standardized uptakevalue
SVC superior venacava
SWS slow wavesleep
SXR skullX- ray
T testa
T1W T1- weighted
T2W T2- weighted
TA temporal arteritis
TACO transfusion- associated circulatory overload
TB tuberculosis
TBLB transbronchial lungbiopsy
TBNA transbronchial needle aspiration
TCR T- cell receptor
TCT thrombin clottingtime
tds ter die sumendus (three timesdaily)
TE echotime
TfR transferrin receptor
TFT thyroid functiontest
Tg thyroglobulin
TG triglyceride
THR total hip replacement
TIA transient ischaemicattack
TIBC total iron binding capacity
TIPS transjugular intrahepatic portosystemicshunt
TLC thin- layer chromatography; total lung capacity
TLCO transfer factor for carbon monoxide
TMA thrombotic microangiopathy; transcription- mediated
amplication

SYMBOLS AND ABBREVIATIONS
xxxii
xxxii
TMS transcranial magnetic stimulation
TN trigeminal neuralgia
TnI troponinI
TnT troponinT
TOE transoesophageal echocardiography
tPA tissue plasminogen activator
TPHA Treponema pallidum haemagglutinationassay
TPN total parenteral nutrition
TPO thyroid peroxidase
TR repetitiontime
TRALI transfusion- related acute lunginjury
TRAP tartrate- resistant acid phosphatase
TRH thyrotropin- releasing hormone
tRNA transfer ribonucleicacid
TRP tubular reabsorption of phosphate
TSE transmissible spongiform encephalopathy
TSH thyroid- stimulating hormone
TTE transthoracic echocardiogram
tTG tissue transglutaminase
TTP thrombotic thrombocytopenic purpura
U unit
UCTD undierentiated connective tissue disease
U&E urea and electrolytes
UFC urinary free cortisol
UFE uterine broid embolization
UK United Kingdom
UMN upper motor neurone
URTI upper respiratory tract infection
US ultrasound
USA UnitedStates
USS ultrasoundscan
UTI urinary tract infection
UV ultraviolet
VATS video- assisted thoracoscopic surgery
VC vital capacity
vCJD variant Creutzfeldt– Jakob disease
VDRL Venereal Disease Research Laboratory
VEP visual evoked potential
VHF viral haemorrhagicfever
VHL von Hippel– Lindau

SYMBOLS AND ABBREVIATIONS
xxxiii
VIP vasoactive intestinal peptide
VLCFA very long- chain fattyacid
VLDL very low- density lipoprotein
VMA vanillylmandelicacid
VO
2
oxygenuptake
VO
2max
maximum oxygenuptake
VP vasoactive peptide
V/ Q ventilation/ perfusion
VRE vancomycin- resistant Enterococcus
VSD ventricular septaldefect
VTE venous thromboembolism
vWD von Willebrand disease
vWF Ag von Willebrand factor antigen
WBC white blood count/ cells
WCE wireless capsule endoscopy
WHO World Health Organization
w/ v weight byvolume
XDP cross- linked brin degradation product
XDR- TB extensively drug- resistant tuberculosis
XLA X- linked agammaglobulinaemia
ZN Ziehl– Neelsen
ZPP zinc protoporphyrin

xxxiv
xxxiv
Why dotests?
Patients seldom present to their doctors with diagnoses— rather, they have
symptoms or signs. The major challenge of medicine is being able to talk to
the patient and obtain a history, then carry out a physical examination look-
ing for pointers to their likely underlying problem. Our elders and, some
would argue, betters in medicine had fewer tests available to them than we
have today, and their diagnoses were often made solely from the history
and examination. Of course, they would claim that their clinical acumen
and skills were greater than ours, and that we rely too heavily on the huge
armoury of laboratory and other investigations available today. This, in part,
is probably true, but we cannot ignore the fact that advances in science and
technology have spawned a bewildering array of very useful and sophisti-
cated tests that help us to conrm our diagnostic suspicions.
By ‘test’ we mean the measurement of a component of blood, marrow,
or other body uid or physiological parameter to determine whether the
patient’s value falls within or outside the normal range, either suggesting the
diagnosis or, in some cases, actually making the diagnosis forus.
Factors aecting variable parameters inhealth
Many measurable body constituents vary throughout life. For example, a
newborn baby has an extremely high haemoglobin concentration, which
falls after delivery. This is completely normal and is physiological, rather than
pathological. Ahaemoglobin level this high in an adult would be pathological,
since it is far outside the normal range for the adult population.
Approach toinvestigations
Factors aecting measurable variables
• Age.
• Sex.
• Ethnicity.
• Altitude.
• Build.
• Physiological conditions (e.g. at rest, after exercise, standing, lying).
• Sampling methods (e.g. with or without using a tourniquet).
• Storage and age of sample.
• Container used, e.g. for blood sample, as well as anticoagulant.
• Method of analysis.
Reference ranges (normal values)
These are published for most measurable components of blood and other
tissue, and we have included the normal ranges for most blood and
cerebrospinal uid (CSF) analytes at the end of thebook.

xxxv
APPROACH TO INVESTIGATIONS
What makes a test useful?
A really good test, and one that would make us appear to be outstanding
doctors, would be one that would always be positive in the presence of a
disease and would be totally specic for that disease alone; such a test would
never be positive in patients who did not have the disorder. What we mean
is that what we are looking for are sensitive tests that are specic for a given
disease. Sadly, most tests are neither 100% sensitive nor 100% specic, but
some do come veryclose.
How touse tests and thelaboratory
Rather than request tests in a shotgun or knee- jerk fashion where every box
on a request form is ticked, it is far better to use the laboratory selectively.
Even with the major advances in automation where tests are batched and
are cheaper, the hospital budget is nite and sloppy requesting should be
discouraged.
Outline your dierential diagnoses: what are the likeliest diseases, given
the patient’s history, examination ndings, and population from which the
patientcome?
Decide which test(s) will help you make the diagnosis:request these and
review the diagnosis in the light of the test results. Review the patient and
arrange further investigations as necessary.
The downside oftests
It is important to remember that tests may often give ‘normal’ results, even
in the presence of disease. For example, a normal electrocardiogram (ECG)
in the presence of chest pain does not exclude the occurrence of myocardial
infarction with 100% certainty. Conversely, the presence of an abnormal-
ity does not necessarily imply that a disease is present. This, of course, is
where clinical experience comes into its own— the more experienced clini-
cian will be able to balance the likelihood of disease with the results avail-
able, even if some of the test results give unexpected answers.
Quick- x clinical experience
This simply does not exist. Talking to patients and examining them for physi-
cal signs and assimilating knowledge gained in medical school are absolute
requirements for attainment of sound clinical judgement. Those students
and doctors who work from books alone do not survive eectively at the
coal face! It is a constant source of irritation to medical students and junior
doctors, when a senior doctor asks for the results of an investigation on
the ward round and you nd this test is the one that clinches the diagnosis.
How do
they do it? Like appreciating good wine— they develop a nose for
it. You can learn a great deal by watching your registrar or consultant make
decisions. This forms the basis of your own clinical experience.
Sensitivity and specicity
Sensitivity % of patients with the disease and in whom the test is positive
Specicity % of people without the disease and in whom the test is negative

xxxvi
xxxvi
It is a fact of life that the sophisticated automated analysers in current use
are not 100% accurate 100% of the time, but they come pretty close. In
order to keep errors to a minimum, precautions need to be taken when
sampling biological material, e.g.blood.
Minimizing spurious results using blood samples
• Use correct bottle.
• Fill to line (if anticoagulant used). This is less of a worry when vacuum
sample bottles are used since these should take in exactly the correct
amount of blood, ensuring the correct blood:anticoagulant ratio. This is
critical for coagulation tests.
•
Try to get the sample to the laboratory as quickly as possible. Blood
samples left lying around on warm windowsills, or even overnight at
room temperature, will produce bizarre results, e.g. crenated red blood
cells (RBCs) and abnormal- looking white blood cells (WBCs) in old
EDTA samples.
•
Try to avoid rupturing red cells when taking the sample (e.g. using
narrow- gauge needle, prolonged time to collect whole sample);
otherwise a ‘haemolysed’ sample will be received by the laboratory.
This may cause spurious results for some parameters (e.g. [K
+
]).
•
Remember to mix (not shake) samples containing anticoagulant.
Variations innormal ranges inhealth
As discussed earlier, most of the normal ranges for blood parameters dis-
cussed in this book are for non- pregnant adults. The reason for this is that
blood values, e.g. haemoglobin (Hb), red cell count (RCC), are high in the
newborn and many full blood count (FBC), coagulation, and other param-
eters undergo changes in pregnancy.
Laboratory errors and
how toavoidthem
2

3
Symptoms andsigns
Chapter1
Abdominal distension 4
Abdominal pain 6
Alteration of behaviour 7
Alteration in bowel habit 9
Anaemia 10
Anaphylaxis 12
Angio- oedema 12
Anorexia 13
Anuria 14
Ataxia 15
Bradycardia 17
Breathlessness 18
Bruising 20
Calf swelling 21
Chest pain 22
Clubbing 25
Coma 26
Confusion 28
Constipation 29
Cyanosis 31
Diarrhoea 32
Dizziness and syncope 34
Dysarthria and dysphasia 36
Dysphagia 37
Facial pain 38
Fever of unknown origin
(FUO or PUO) 39
Firstt 40
Galactorrhoea 42
Gout 43
Gynaecomastia 44
Haematemesis 46
Haematuria 47
Haemoptysis 48
Headache 49
Heart sounds and murmurs 51
Hepatomegaly 55
Herpes zoster 56
Hyperlipidaemia 57
Hypertension 58
Incontinence:faecal 60
Incontinence:urinary 61
Indigestion 62
Infective endocarditis signs 64
Irregular pulse 65
Jaundice 66
Joint pain/ swelling 68
Jugular venous pulse 69
Loin pain 71
Lymphadenopathy 72
Nausea 74
Neck stiness 76
Nystagmus 77
Obesity 79
Oliguria 81
Palpitations 82
Pancytopenia 83
Paraesthesiae 84
Peripheral neuropathy 86
Peripheral oedema 87
Petechiae and
thrombocytopenia 88
Plethora 89
Polyuria 90
Pruritus 91
Ptosis 92
Pulmonary embolism 93
Pulse character 94
Purpura 95
Recurrent thrombosis 96
Retinal haemorrhage 97
Rigors 97
Short stature 98
Skin pigmentation 100
Splenomegaly 102
Steatorrhoea 103
Stridor 104
Suspected bleeding disorder 105
Suspected stroke 107
Sweating 109
Tachycardia 110
Tinnitus 112
Tiredness 113
Urgency of micturition 114
Urticaria 115
Vasculitis 116
Visual loss 117
Wasting of the small hand
muscles 118
Weight loss 119
Wheeze 120

4
CHAPTER1 Symptoms andsigns
4
Abdominal distension
Patients may describe generalized abdominal swelling or localized fullness in
a specic area of the abdomen.
In thehistory enquire specicallyabout
• Change in bowelhabit.
• Weightloss.
• Associatedpain.
Generalized swelling
Consider
•
Fat.
• Fluid.
• Faeces.
• Flatus.
• Fetus.
• Full bladder.
Ascites
Fluid in the peritoneal cavity. Look for shifting dullness and uid thrill on per-
cussion, stigmata of chronic liver disease, lymphadenopathy, and oedema,
and assess the jugular venous pressure(JVP).
Causes
•
Malignancy.
• Cirrhosis/ portal hypertension.
• Hypoproteinaemia.
• Right heart failure.
Investigations
•
Urea and electrolytes (U&Es).
• Liver function tests (LFTs).
• Serum albumin.
• Ascitic tap for cytology, and microscopy, culture, and sensitivity
(M,C&S).
•
Serum- ascites albumin gradient.
• Ultrasound scan (USS) of the abdomen.
(See Fig.1.1.)
Flatus
Gaseous distension. Need to exclude bowel obstruction. Assess for colicky
abdominal pain, bowel habit, atus, and vomiting. Look for resonant disten-
sion on percussion, altered or absent bowel sounds, and focal tenderness
with rebound and guarding. Always check for herniae and perform a per
rectum (PR) examination in suspected obstruction.
Causes
•
Intraluminal:faecal impaction, gallstoneileus.
•
Luminal:inammatory stricture (e.g. Crohn’s), tumour, abscess.
•
Extraluminal:herniae, adhesions, pelvic mass, lymphadenopathy,
volvulus, intussusception.
ABDOMINAL DISTENSION
5
• Paralytic ileus:drug- induced, electrolyte disturbances.
• Age- related causes of obstruction.
• Neonatal:congenital atresia, imperforate anus, volvulus, Hirschsprung’s
disease, meconiumileus.
•
Infants:intussusception, Hirschsprung’s, herniae, Meckel’s diverticulum.
•
Young/ middle- aged adults:herniae, adhesions, Crohn’s.
•
Elderly:herniae, carcinoma, diverticulitis, faecal impaction.
Investigations
•
Full blood count(FBC).
• U&E.
• Abdominal X- ray (AXR) (erect and supine).
• Consider barium enema, barium follow- through, sigmoidoscopy, surgical
intervention for complete acute obstruction.
Localized swelling/ masses:common causes accordingtosite
Investigate accordingtosite
•
Consider USS abdomen and pelvis.
• Computed tomography (CT) scanning.
• Barium studies.
• Intravenous urogram(IVU).
E OHCM 10e, p.62, p.604.
RUQRUQ
LiverLiver
Gall bladde
r
Gall bladde
r
BowelBowel
Right kidneyRight kidney
LUQLUQ
SpleenSpleen
BowelBowel
Left kidneyLeft kidney
LIFLIF
Faecal loadingFaecal loading
Colonic mass Colonic mass
—carcinoma—carcinoma
—diverticular abscess—diverticular abscess
—ovarian cyst/tumour—ovarian cyst/tumour
RIFRIF
Appendix massAppendix mass
Carcinoma of caecumCarcinoma of caecum
Ovarian cyst/tumourOvarian cyst/tumour
MidlineMidline
Gastric massGastric mass
PancreasPancreas
—cyst—cyst
—pseudotumour —pseudotumour
—carcinoma—carcinoma
Aortic aneurysm (is it pulsatile?
)
Aortic aneurysm (is it pulsatile?
)
LymphadenopathyLymphadenopathy
Urinary retention or tumourUrinary retention or tumour
Uterine massUterine mass
Fig.1.1 Main causes of abdominal swelling according tosite.

6
CHAPTER1 Symptoms andsigns
6
Abdominalpain
Abdominal pain may be acute or chronic. Severe acute pain may indicate a
surgical emergency, including perforation, peritonitis, or obstruction. Assess
nature and radiation of pain, clinical status of the patient, including fever,
tachycardia, and hypotension.
Common causes ofabdominal pain accordingtosite
Epigastricpain
Peptic ulcer disease, gastritis or duodenal erosions, cholecystitis, pancreatitis.
Periumbilicalpain
Pancreatitis, mesenteric artery ischaemia (older patient with vascular disease).
Right upper quadrant (RUQ)pain
Biliary colic, cholecystitis, hepatitis, pepticulcer.
Left upper quadrant (LUQ)pain
Splenic, pepticulcer.
Loinpain
Renal colic (colicky radiating loin l groin), pyelonephritis, renal pathology.
Left iliac fossa (LIF)pain
Constipation, diverticular disease, irritable bowel syndrome (IBS), pelvic
referred pain, inammatory bowel disease(IBD).
Right iliac fossa (RIF)pain
Appendicitis, pelvic referred pain, IBD (e.g. Crohn’s of terminal ileum).
Suprapubicpain
Urinary tract infection (UTI), cystitis, salpingitis.
Generalizedpain
Gastroenteritis, irritable bowel, constipation, generalized peritonitis.
Pitfalls
• Metabolic causes, e.g. diabetic ketoacidosis (DKA), hypercalcaemia,
Addison’s disease, porphyria, lead poisoning.
•
Atypical referred pain, e.g. myocardial infarction (MI), pneumonia.
Investigations
• FBC.
• U&E, e.g. deranged electrolytes following vomiting, diarrhoea, or bowel
obstruction.
•
Plasma glucose.
• Serum amylase (i in pancreatitis and bowel obstruction).
•
Urinalysis and midstream urine (MSU), e.g. haematuria, proteinuria, glucose.
• LFTs (consider obstructive vs hepatitic picture).
• Plain AXR (erect and supine to assess for perforation and bowel
obstruction).
•
Kidney, ureter, bladder X- ray (KUB) for renal tract calculi.
• USS abdomen, particularly for biliary tract, gall bladder, and renaltract.
• IVU to assess for renal tract calculi/ pathology.
E OHCM 10e, p.30, p. 57, p. 609.

ALTERATION OFBEHAVIOUR
7
Alteration ofbehaviour
This is usually reported by a relative or friend, rather than by the patient.
Often the patient will have little or no insight into the disease and taking a
history can be dicult. In addition to a full general and neurological physical
examination, a mental state examination is required.
Find out if this is the rst episode of altered behaviour or if the epi-
sodes are recurrent. Is there a gradual change in behaviour (and personal-
ity) overtime?
Acute delirium
Causes
•
Sepsis (common).
• Acute intracranial event, e.g. haemorrhage.
• Metabolic disturbance, e.g. uraemia, hypercalcaemia (common).
• Intracerebral tumour (including meningioma).
• Drugs— especially interactions in the elderly.
• Alcohol (and withdrawal syndrome).
• Hypoxia (common).
• Hypoglycaemia (iatrogenic in diabetic patients receiving insulin
treatment or oral insulin secretagogues, or insulinoma and other
causes).
Dementia
• Alzheimer’s (common), Pick’s (rare).
• Vascular, e.g. multi- infarct.
• Huntington’s chorea.
• Vitamin B
12
deciency (severe).
•
Hypothyroidism (severe).
• Wilson’s disease.
• Alcoholism.
• Normal pressure hydrocephalus.
Note:‘frontal lobe syndrome’ from space- occupying lesion (SOL), e.g. men-
ingioma. Presents with disinhibition, impaired social functioning, primitive
reexes, e.g. grasp reex.
Anxietystates
Usually psychogenic, but consider organic possibilities suchas
•
Phaeochromocytoma (rare).
• Hyperthyroidism (common).
• Paroxysmal atrial tachycardia (fairly common).
• Alcohol withdrawal (usually history of excessive alcohol intake).
Psychosis
• Schizophrenia.
• Bipolar disorder or pseudo- dementiain:
•
Systemic lupus erythematosus(SLE).
•
Cushing’s syndrome.
•
Multiple sclerosis(MS).
•
Thyrotoxicosis (‘apathetic’ thyrotoxicosis in the elderly).

8
CHAPTER1 Symptoms andsigns
8
Temporal lobe epilepsy
• Temporary disturbance of content of consciousness.
Investigations:guided byhistory and examination
• U&E.
• Glucose (in non- diabetics, take fasting venous plasma in a uoride
oxalate tube with simultaneous serum or plasma for insulin
concentration, e.g. suspected insulinoma).
•
Chest X- ray(CXR).
• LFTs.
• Thyroid function tests (TFTs).
• FBC.
• Erythrocyte sedimentation rate(ESR).
• Urinalysis (protein, nitrites, glucose).
• Cranial CTscan.
• Serum vitaminB
12
.
•
Arterial blood gases (ABGs) ± carboxyhaemoglobin (COHb).
• Blood cultures.
Consider
• Syphilis serology.
• Human immunodeciency virus (HIV)test.
• Urine drug screen (E Chapter11).
•
Blood ethanol level (may be low in withdrawal state).
• Electroencephalogram(EEG).
• 24h electrocardiogram(ECG).
• Sleepstudy.

ALTERATION INBOWELHABIT
9
Alteration inbowelhabit
A change in bowel habit in an adult should always alert you to the possibility
of bowel cancer. Ask about associated features— PR bleeding, tenesmus,
weight loss, mucus, abdominal pain, or bloating.
Has the patient started any new medications, including ‘over the coun-
ter’? Look for signs of systemic disease.
Consider
• Carcinoma of thecolon.
• Diverticular disease.
• IBS.
• Constipation with overow diarrhoea.
• All of the above may present with alternating diarrhoea and
constipation.
Investigations
• Digital rectal examination.
• Proctoscopy.
• Sigmoidoscopy (rigid/ exible).
• Colonoscopy.
• Bariumenema.
• CT colonography.
E Diarrhoea (pp. 32–3), E Constipation (pp. 29–30), E Incontinence:
faecal (p. 60).

10
CHAPTER1 Symptoms andsigns
10
Anaemia
Reduced haemoglobin (Hb), no specic cause implied (and not a diagnosis
in itself, so don’t be complacent):♂
<13.5g/ dL, ♀ <11.5g/ dL. Often associ-
ated with non- specic symptoms such as fatigue, poor concentration, short-
ness of breath, and dizziness. Older patients may experience palpitations
and exacerbation of angina, congestive cardiac failure (CCF), or claudication.
Signs
Pallor of conjunctivae and skin creases, nail pallor and koilonychia (spoon-
shaped nails, very rare nding in severe chronic iron deciency), angular
cheilitis, and glossitis. Most of these signs are unreliable and it is dicult to
gauge anaemia from skin signsalone.
Causes
(See Table1.1.)
Two common approaches to assess anaemiaare:
1. Red cell dynamics:
•
i Red blood cell (RBC) loss/ breakdown, e.g. haemolysis (congenital
or acquired) or bleeding.
•
d RBC production, e.g. vitamin/ mineral deciency, marrow suppres-
sion/ inltration, myelodysplasia, Hb disorders (e.g. thalassaemia),
chronic disease, renal failure.
2. Red cell indices:
Investigations
FBC andlm
Assessment of RBC indices helps direct investigation asabove.
Table1.1 Some causes ofanaemia based ontheMCV
Microcytic/ hypochromic d MCV, d MCHC, e.g.
Iron deciency
Thalassaemia
Anaemia of chronic disease
Macrocytic i MCV
Reticulocytosis (polychromasia on bloodlm)
B
12
or folate deciency
Chronic liver disease
Hypothyroidism
Alcohol
Myelodysplasia
Normocytic, normochromic n MCV andMCHC
Anaemia of chronic disease,e.g.
•
Chronic infection
• Inammation
Inammatory disease or malignancy
Acute bloodloss
Renal failure
Myeloma
MCHC, mean corpuscular haemoglobin concentration; MCV, mean cell volume.

ANAEMIA
11
Microcytic
•
Check iron stores (ferritin or soluble transferrin receptor assay).
Note:ferritin is i in acute inammation and may be misleading. Iron/
total iron binding capacity (TIBC) no longer used for assessment of iron
deciency (E Assessment of iron status, pp. 244–7).
•
Consider thalassaemia screening if not iron- decient (i.e. d MCV, n
ferritin).
•
If iron- decient, assess dietary history (vegetarians) and look for risk
factors for blood loss and i demands.
•
Premenopausal women:assess menstrual losses.
• Pregnancy/ infants/ adolescence:consider physiological (i requirements).
•
All others:look for source of blood loss. The gastrointestinal (GI) tract is
the commonest source. Consider oesophagogastroduodenoscopy (OGD)
and/ or colonoscopy if clinically indicated by symptoms and barium studies.
Macrocytic
•
Reticulocytecount.
• Serum B
12
and red cell folate levels.
•
If folate- decient:assess dietary history and physiological requirements.
• If B
12
- decient:rarely dietary cause alone, usually an associated
pathology. Pernicious anaemia (PA) is the commonest cause— check
parietal cell antibodies (90% of patients with PA are +ve, but seen in
other causes of gastric atrophy, especially in older individuals) and/
or intrinsic factor antibodies (+ve in only 50% with PA, but specic).
Consider ileal disease and malabsorption.
•
LFTs.
• Thyroid function.
Normocytic
•
Bloodlm.
• ESR.
• Renal function.
• Consider myeloma screen in older adults (immunoglobulins (Igs),
protein electrophoresis, urine Bence– Jones protein (BJP)). Skeletal
survey of value if paraprotein orBJP.
•
Autoimmune screen to exclude connective tissue disease.
Haemolysisscreen
•
FBC, mean cell volume (MCV) (i due to reticulocytosis— these are
larger thanRBCs).
•
Blood lm (spherocytes, polychromasia, bite cells, and red cell
fragmentation).
•
Reticulocytecount.
• Serum bilirubin and serum lactate dehydrogenase(LDH).
• Haptoglobins (absent in haemolysis).
• Direct antibody test (DAT) (old term is direct Coombs’test).
Consider
• Congenital haemolytic anaemias:membrane defects, enzyme deciencies
(e.g. glucose- 6- phosphate dehydrogenase (G6PD), pyruvate kinase).
• Disseminated intravascular coagulation (DIC)/ microangiopathic
haemolysis— DIC screen.

12
CHAPTER1 Symptoms andsigns
12
Anaphylaxis
Dened as a systemic reaction (local oral angio- oedema is not anaphylaxis),
with any or all of the following:
•
Stridor (laryngeal obstruction).
• Wheeze (bronchospasm).
• Generalized urticaria and/ or angio- oedema.
• Hypotension ± loss of consciousness.
• Abdominal pain/ cramps, vomiting, and diarrhoea.
Note:not all patients have urticaria or rash— only 50% willdoso.
Dierentiate IgE- mediated reactions (anaphylaxis) from non- IgE- mediated
reactions (anaphylactoid)— due to direct mast cell degranulation).
Angio- oedema
Angio- oedema is deep tissue swelling which is non- itchy. May be premoni-
tory tingling. May occur with or without urticaria. Caused by bradykinin,
not histamine.
Causes
• As for urticaria; also hereditary angioedema (rare).
• Also think of drugs— these are the commonestcause:
•
Angiotensin- converting enzyme (ACE) inhibitors (ACEIs) (elevated
bradykinin levels due to inhibition of breakdown).
•
Angiotensin II (AT- II) receptor antagonists.
•
Statins.
•
Proton pump inhibitors.
•
Non- steroidal anti- inammatory drugs (NSAIDs).
May also be seen in patients with autoimmune disease, such as lupus and
rheumatoid arthritis (RhA) (antibodies against C1q), and in older patients in
association with paraproteins (myeloma, lymphoma).
Angio- oedema with urticaria is not due to hereditary angio- oedema.
Investigations
• Check drug history rst! If suspect drugs, then stop drugs and wait! If no
drugs, then investigate.
Angio- oedema WITH urticaria
• Investigate as for urticaria.
Angio- oedema WITHOUT urticaria
• Complement C3 andC4.
• If C4 low, check C1 esterase inhibitor (immunochemical and functional).
• Serum Igs and electrophoresis.
• Autoantibody screen.
• FBC andESR.
• Thyroid function.
• Liver function.

ANOREXIA
13
Anorexia
This describes a loss of appetite for food and is associated with a wide range
of disorders. In fact, anorexia is a fairly common consequence of underly-
ing disease and represents general undernourishment. Anorexia per se is
associated with i morbidity, especially when present in patients undergoing
surgery; post- operative infection is commoner, as is prolongation of the
hospitalstay.
The extent to which it will be investigated depends on the general status
of the patient and the presence and duration of any symptoms or signs.
Clinical judgement willhelp!
Causes
• Anorexia nervosa.
• Depressive illness.
• Stress.
• Cancers:any, including carcinoma of the stomach or oesophagus,
metastatic, leukaemia, or lymphoma.
•
Drugs, including chemotherapy.
• Radiotherapy.
• Renal failure.
• Hypercalcaemia.
• Infections.
• Cigarette smoking.
Investigations
• Full history and examination.
• FBC— looking for anaemia or non- specic changes seen in underlying
disease.
•
ESR— may be elevated in inammatory disorders.
• U&E.
• LFTs.
• Serum calcium (Ca
2+
).
•
CXR (e.g. lung cancer, tuberculosis (TB),etc.).
• Cultures of blood, sputum, urine, stool if pyrexial and/ or localizing
symptoms orsigns.

14
CHAPTER1 Symptoms andsigns
14
Anuria
Anuria denotes absent urine production. Oliguria (<400mL urine/ 24h) is
commoner than anuria. Acatheter must be passed to conrm an empty
bladder.
Causes
• Urinary retention— prostatic hypertrophy; pelvic mass; drugs, e.g.
tricyclic antidepressants; spinal cord lesions.
•
Blocked indwelling urinary catheter.
• Obstruction of the ureters— tumour, stone, sloughed papillae (bilateral).
• Intrinsic renal failure— acute glomerulonephritis, acute interstitial
nephritis, acute tubular necrosis (ATN), rhabdomyolysis.
•
Pre- renal failure— dehydration, septic shock, cardiogenicshock.
An urgent USS of the renal tract must be performed and any physical
obstruction relieved as quickly as possible, either directly (urethral cath-
eter) or indirectly (nephrostomy).
3 Renal function and serum electrolytes must be measured withoutdelay.
Further tests asclinically indicated
• FBC.
• Blood cultures.
• ABGs.
• Uricacid.
• Autoimmune prole.
• ESR.
• Creatine kinase(CK).
• Prostate- specic antigen (PSA) (prostatic carcinoma).
• Serum Ca
2+
and phosphate (PO
4
3−
).
•
12- leadECG.
• CXR.
• Central venous pressure (CVP) measurement via central line (to guide
intravenous (IV) uids).
•
MSU(UTI).
• Urine microscopy (for casts).
• Urine osmolality, sodium, creatinine, urea concentrations.
• IVU (E Radiology of the urinary tract, pp. 808–11).
•
Urinary stone analysis, if available.
• CT pelvis.
• Renal biopsy (if intrinsic renal disease suspected, normal- sized kidneys).
E OHCM 10e, p. 81, p. 293.

ATAXIA
15
Ataxia
Ataxia is an impaired ability to coordinate limb movements. There must be
no motor paresis (e.g. monoparesis) or involuntary movements (e.g. the
characteristic cogwheel tremor in Parkinson’s disease (PD) is not ataxia).
Ataxiamaybe
• Cerebellar.
• Vestibular.
• Sensory.
Note:many forms of ataxia are hereditary (but are uncommon).
Hereditarycauses
• Friedreich’s ataxia.
• Ataxia telangiectasia.
• Spinocerebellar ataxia.
• Corticocerebellar atrophy.
• Olivopontocerebellar atrophy.
• Hereditary spastic paraplegia.
• Xeroderma pigmentosa.
Investigations
•
Family studies.
• Genetic analysis (discuss with the regional genetics laboratory—
counselling may be required).
Vestibularataxia
• Acute alcohol intoxication.
• Labyrinthitis.
Sensoryataxia
• Loss of proprioception— peripheral neuropathy, dorsal column disease.
• Visual disturbance.
Investigations
•
Venous plasma glucose (diabetic neuropathy).
• Serum vitamin B
12
(subacute combined degeneration of the cord— rare,
but serious).
•
LFTs.
• Cryoglobulins.
Cerebellarataxia
• Demyelinating diseases, e.g.MS.
• Cerebellar infarct or haemorrhage.
• Alcoholic cerebellar degeneration.
• Cerebellar tumour— ° in children, metastases in adults. Note:von
Hippel– Lindau (VHL) disease (E OHCM 10e, Chapter19).
•
Nutritional deciency:
•
VitaminB
12
.
•
Thiamine.
• Cerebellar abscess.

16
CHAPTER1 Symptoms andsigns
16
• Drugs (supratherapeutic blood levels):
•
Carbamazepine.
•
Phenytoin.
• Tuberculoma.
• Paraneoplastic syndrome.
• Developmental.
• Arnold– Chiari malformation.
• Dandy– Walker syndrome.
• Paget’s disease of theskull.
• Wilson’s disease (hepatolenticular degeneration).
• Hypothyroidism.
• Creutzfeldt– Jakob disease (CJD) and other chronic infections.
• Miller Fisher syndrome.
• Normal pressure hydrocephalus.
Ataxia should be distinguished frommovement
disorders,e.g.
• Chorea:Huntington’s, Sydenham’s, thyrotoxicosis (veryrare).
• Athetosis.
• Hemiballismus:characteristic movement disorder,rare.
• Tardive dyskinesia:chronic phenothiazine therapy.
Investigations
• CranialCT.
• Magnetic resonance imaging (MRI) brain (if demyelination suspected).
• CXR (cerebellar metastases from bronchogenic carcinoma;
paraneoplastic syndrome).
•
TFTs.
• Triple evoked potentials (demyelination).
• Lumbar puncture (LP) (E Lumbar puncture, pp. 584–9).
•
LFTs.
• Serum drug concentrations, especially anticonvulsants.
• Serum vitaminB
12
.
•
Erythrocyte transketolase (d in thiamine deciency, e.g. alcoholism).
•
Isotope bone scan (Paget’s, metastases).
• Serum alkaline phosphatase (ALP)— bone isoenzyme (Paget’s,
metastases).
•
Urine hydroxyproline (Paget’s disease— reects bone turnover).
• Caeruloplasmin (Wilson’s disease).
• Serum and urine copper (Wilson’s disease).
Consider whether the movement disorder is psychogenic (uncommon),
rather than due to neuropathology. Uncommon and should not be con-
dently assumed.
E OHCM 10e, p.467.

BRADYCARDIA
17
Bradycardia
Bradycardia is dened as a heart rate of <60 beats per minute. It is a normal
physiological response to tness training but should always be considered a
marker of potential cardiac disease until proved otherwise.
Causes
A comprehensive history and thorough examination are important. Atran-
sient bradycardia can cause disabling symptoms of dizziness or blackouts in
the elderly, whilst persistent bradycardia often heralds systemic disease,e.g.:
•
Iatrogenic:cardiac drugs, e.g. β- blockers (including eye drops for
glaucoma), amiodarone, and calcium channel blockers (e.g. diltiazem
and verapamil), cause sinus bradycardia; digoxin (atrioventricular (AV)
block). The likelihood of extreme bradycardia or heart block is i with
combination therapy.
•
Cardiac causes:acute MI (often transient in inferior MI); coronary artery
disease; sick sinus syndrome; myocardial disease (amyloid, Chagas’
disease, sarcoid, myocarditis).
•
i vagal tone associated with nausea and vomiting.
•
Diminished sympathetic activity.
• Physiological:bradycardia is normal in sleep and in athletes.
•
Hypothyroidism:associated with characteristic symptoms andsigns.
•
i intracranial pressure (ICP), e.g. cerebral tumour.
•
Hypothermia, e.g. myxoedemacoma.
•
Metabolic:severe hyperkalaemia, anorexia.
•
Toxic: severe jaundice.
•
Drug toxicity: opiates.
•
Infective:inappropriate bradycardia seen in diphtheria, typhoid.
Investigations
• 12- lead ECG to identify the underlying rhythm.
If there are symptoms ofchestpain
•
Serum troponin andCK.
• Bedside ECG monitoring.
• ExerciseECG.
If there is a history ofintermittent dizziness
•
24h ambulatory ECG monitoring, patient- activated event recorder, or
implantable loop recorder, depending on the frequency of symptoms.
If indicated byclinical presentation, consider
•
TFTs (hypothyroidism).
• Low reading thermometer (hypothermia— check for J waves onECG).
• CT brain scan (? intracranial pathology).
• U&E.
• LFTs (especially bilirubin).
• Toxicology screen.
E OHCM 10e, p.124, p.808.

18
CHAPTER1 Symptoms andsigns
18
Breathlessness
Breathlessness (dyspnoea) is the subjective awareness of diculty in breath-
ing. Almost universal during exercise, it is a common presenting symptom
in a broad spectrum of diseases. Acomprehensive history and a thorough
examination are therefore essential. Speed of symptom onset, the patient’s
age and occupation, and local disease prevalence are particularly helpful in
devising a dierential diagnosis and a guide to investigations.
Causes
• Acute pulmonary disease:pneumonia, acute asthma, pulmonary embolus
(PE), inhaled foreign body, pneumothorax, acute respiratory distress.
• Chronic pulmonary disease:emphysema, chronic bronchitis, ruptured
bulla; interstitial disease (sarcoid, brosing alveolitis, extrinsic alveolitis,
pneumoconiosis).
•
Carcinoma:bronchogenic carcinoma, lymphangitis carcinomatosis, 2°
carcinoma.
•
Acute cardiac disease:acute MI (and associated complications of
pulmonary oedema, ventricular septal defect (VSD), mitral valve chordal
rupture and arrhythmias).
•
Chronic cardiac disease:left ventricular dysfunction, valvular heart disease
(mitral or aortic stenosis and regurgitation), ischaemic heart disease
(IHD), pulmonary hypertension, pleural eusion, arrhythmias (especially
atrial brillation(AF)).
•
Metabolic:poisoning from salicylates, methanol, and ethylene glycol,
DKA, lactic acidosis, hepatic and renal failure.
•
Neuromuscular:intercostal muscle/ diaphragmatic weakness due to
Guillain– Barré syndrome (GBS), muscular dystrophy.
• Haematological:anaemia.
•
Anxiety and hyperventilation.
• Morphological:kyphoscoliosis, obesity.
•
Laryngeal obstruction:extrinsic compression (retrosternal goitre),
angioedema (often acute drug allergy), laryngeal spasm (hypocalcaemia).
Initial investigations
• FBC.
• U&E.
• Glucose.
• CXR.
• ABGs.
• Peak expiratory ow rate (PEFR).
• 12- leadECG.

BREATHLESSNESS
19
Additional investigations (as indicated)
• Transthoracic echocardiography(TTE).
• 24h ambulatory ECG monitoring.
• Pulmonary functiontests.
• CTchest.
• Bronchoscopy.
• Ventilation/ perfusion (V/ Q) scan/ computed tomography pulmonary
angiography (CTPA).
•
LFTs.
• Ca
2+
.
•
ESR.
• Serum salicylate levels.
• Lactate.
• Lung biopsy.
E OHCM 10e, p.782.

20
CHAPTER1 Symptoms andsigns
20
Bruising
Easy bruising is a common complaint and warrants careful assessment of
onset and nature. Recent onset of spontaneous and unusual bruising or
bleeding may suggest a serious acquired defect. Alifelong history of bruis-
ing and bleeding (e.g. post- tonsillectomy, dental extraction, or surgery) may
imply a congenital defect. Family history may be informative.
Examine: skin, mouth, dependent areas, and fundi for mucocutaneous
bleeding and purpura (non- blanching haemorrhages into theskin).
Plateletcauses
• Thrombocytopenia or platelet dysfunction (e.g. aspirin).
• Marrow failure, inltration, immune thrombocytopenia (ITP), DIC,
hypersplenism, drugs, or alcohol.
Vascularcauses
• Congenital, e.g. Osler– Weber– Rendu syndrome.
• Acquired, e.g. senile purpura, vasculitis (Henoch– Schönlein purpura,
infection), diabetes, corticosteroid therapy, scurvy, connective tissue
diseases.
Coagulopathy
• Congenital— mucocutaneous bruising is suggestive of a platelet-
mediated defect (e.g. von Willebrand’s disease, Glanzmann’s
thrombasthenia), rather than a clotting factor deciency
(e.g. haemophilia AandB).
•
Acquired, e.g. DIC, vitamin K deciency.
Hyperviscosity
• Myeloma, Waldenström’s macroglobulinaemia (low- grade lymphoma
associated with i IgM and i plasma viscosity), ii white blood cells
(WBC) in leukaemia.
Investigations
• FBC andlm.
• Coagulation— international normalized ratio (INR) and activated partial
thromboplastin time ratio (APTR).
•
Bleeding time, measures platelet and vascularphase.
• DIC screen, including brinogen, thrombin time, D- dimers or brin
degradation products (FDPs).
Consider further tests and referral tohaematologyfor
• Factor assays.
• Platelet aggregation studies to assess platelet function.
E OHCM 10e, p.346.

CALF SWELLING
21
Calf swelling
Assess whether swelling is bilateral or unilateral, precipitating factors,
and duration of onset. Careful examination of the aected leg should be
extended to a full examination, particularly of the abdominal and cardio-
vascular systems.
Causes
Venous and lymphatic
•
Deep vein thrombosis(DVT).
• Supercial thrombophlebitis.
• Varicoseveins.
• Post- phlebitic limb (post- DVT).
Soft tissue/ musculoskeletal
• Calf haematoma or trauma.
• Ruptured Baker’s cyst (synovial eusion in the popliteal fossa associated
with rheumatoid disease).
•
Cellulitis (associated fever, sepsis, tachycardia).
Systemic
•
CCF (bilateral limb oedema, i JVP, and signs of left ventricular failure
(LVF)).
•
Hepatic failure.
• Hypoalbuminaemia.
• Nephrotic syndrome.
• Pregnancy:i dependent oedema, but note also i thrombotic risk, and
DVT should be excluded.
Deep vein thrombosis(DVT)
Usually aects the lower limb and can extend proximally into the iliofemoral
veins and inferior vena cava (IVC), with a higher risk of associated PE and
a higher incidence of post- phlebitic limb. Occasionally seen aecting the
upper limb, but this is atypical.
Risk factors forDVT
•
Age >60years.
• Previous DVTorPE.
• Recent major surgery, especially orthopaedic lower limb, abdominal,
and pelvic.
•
Marked immobility.
• Malignancy.
• Pregnancy and postpartum.
• High- dose oestrogen oral contraceptive pill(OCP).
• Family history of venous thromboembolism(VTE).
Investigations
USS Doppler studies, impedance plethysmography, venography, exclude PE.
If any associated symptoms, arrange V/ Q scan, multislice CT, and pulmo-
nary angiography. Thrombophilia screening for younger patients (age <55),
atypical site and extensive clots, spontaneous onset, and family history.

22
CHAPTER1 Symptoms andsigns
22
Chestpain
Acute chest pain is a common symptom. Adetailed history and a full physi-
cal examination should be performed in order to dene the most likely
cause and necessary investigation pathway.
History
Be sure to ask the following questions about thepain:
•
Site and radiation.
• Character.
• Onset and duration.
• Precipitating and relieving features.
• Associated symptoms.
• Response to pain relief, antacids, or nitrates.
Most types of chest pain fall within one of the categories in Table1.2.
Investigations
(See Table1.3.)
E OHCM 10e, p.36, p. 48, p. 94, p. 784.
Table1.2 Pain sources
Pain source Description of pain
Myocardial ischaemia Retrosternal, heavy ache, can radiate l jaw and arms,
precipitated by exertion, and relieved by rest or nitrates
Aortic dissection Severe central tearing pain, radiates to back
Gastro- oesophageal
disease
Burning central pain; can radiate to shoulders, throat, or
abdomen; exacerbated by meals, eased with antacids/ milk
Pleuritic pain Focal sharp pain, exacerbated by inspiration
Pericardial pain Sharp pain, radiates to left shoulder tip, worse on lying
at and during inspiration, eased by sitting forwards
Musculoskeletal pain Sharp focal pain exacerbated by movement and palpation

CHESTPAIN
23
Table1.3 Investigations forsuspected diagnoses
Cardiovascular causes:all patients should have a 12- lead ECG and CXR
Suspected diagnosis Investigations
Myocardial ischaemia/ infarction
Consider:
• Coronary artery disease
• Aortic stenosis
• Hypertrophic obstructive cardiomyopathy
Serial ECGs
Cardiac markers of necrosis
FBC
TFTs
Echocardiogram
Exercise electrocardiogram
Stress cardiac imaging
Coronary angiography
Thoracic aortic dissection
Note:myocardial ischaemia may also be
present if it involves the coronary arteries
Syphilitic aortitis
FBC, U&E, X- match
Echocardiogram (TTE orTOE)
CT,MRI
Syphilis serology
Mitral valve prolapse
Echocardiogram (TTE or TOE)
Acute pericarditis FBC, viral titres, ESR
Echocardiogram
Pulmonary causes: all patients should have CXR ± ABGs
Suspected diagnosis Investigations
Pneumonia/ pleurisy FBC, CRP
Acute bronchitis Sputum and blood cultures
Pulmonary tuberculosis (TB) Aspiration if empyema suspected
Early morning urine(TB)
Mantoux test (TB)
Pneumothorax CXR
Pulmonary embolus
D- dimers
12- lead ECG
V/ Q scan
CT pulmonary angiography
Lung carcinoma Sputum cytology
Pleural tumour, e.g. mesothelioma
High- resolution CT
Mediastinal tumour Bronchoscopy
Tissue biopsy
Gastro-oesophageal causes
Oesophageal
• Spasm
• Oesophagitis
• Candidiasis
• Reux disease
• Mallory– Weiss tear
FBC
G&S
Helicobacterpylori
Endoscopy
Oesophageal manometry
Oesophageal biopsy
(Continued)

24
CHAPTER1 Symptoms andsigns
24
Suspected diagnosis Investigations
Peptic ulcer disease
Endoscopy
Gastrogran
®
swallow
Barium swallow, meal, or
follow- through
Erect CXR (if perforation
suspected clinically)
Acute pancreatitis Amylase
Abdominal USS
Cholecystitis/ biliary colic FBC, CRP, LFTs
Urinalysis
AbdominalUSS
ERCP
Musculoskeletal and dermatological causes
Suspected diagnosis Investigations
Muscular
Bony structures
Chest wall bony metastases
Rib/ sternal fractures
Costochondritis (Tietze’s syndrome)
Ankylosing spondylitis
Cervical/ thoracic spine disease
Thoracic outlet syndrome
CXR
Bonescan
SpinalX- rays
CT scan
Skin/ soft tissue
Acute shingles
Post- herpetic neuralgia
Herpetic serology/ smear (rarely)
CRP, C- reactive protein; G&S, group and save; TOE, transoesophageal echocardiography; TTE,
transthoracic echocardiography.
ACC/ AHA 2002 guideline update for the management of patients with chronic stable angina.
M http:// www.onlinejacc.org/ content/ 41/ 1/ 159?_ ga=2.15239422.956431479.149977610
8- 163922176.1499776108.
Table1.3 (Contd.)

CLUBBING
25
Clubbing
Soft tissue hypertrophy under the nail bed distorts nger and toenail
growth.
Characteristic features
• i lateral and longitudinal nail curvature.
•
The skin at the base of the nail becomes spongy.
• The angle between the nail and skin is obliterated.
• In extreme cases, the terminal phalanx becomes bulbous like a
drumstick.
Clubbing can be an important visual indicator of major disease, although it
can also be congenital. Rarely, clubbing may accompany swollen wrists and
ankles as part of a proliferative periostitis seen in hypertrophic pulmonary
osteoarthropathy (HPOA). This is associated with squamous carcinoma of
thelung.
Majorcauses
• Lung disease:cystic brosis, bronchiectasis, empyema, lung abscess,
asbestosis, mesothelioma, pulmonary sarcoid.
•
Carcinoma:bronchogenic (especially squamous cell), mediastinal, pleural,
oesophageal, gastric, colonic, thoracic lymphoma, familial polyposiscoli.
•
Infection:infective endocarditis, colonic amoebiasis.
•
Vascular disease:cyanotic congenital heart disease, atrial myxoma,
arteriovenous malformation(AVM).
•
Liver disease:primary biliary cirrhosis (PBC), chronic active hepatitis.
•
Ulcerative colitis and Crohn’s disease, malabsorption.
• Rare causes:thyrotoxicosis, polycythaemia,SLE.
Investigations
As guided bydierential diagnosis and clinical suspicion
•
FBC.
• ESR.
• C- reactive protein(CRP).
• LFTs.
• TFTs.
• SerumACE.
• Autoantibodies.
• Blood cultures (at least three sets if infective endocarditis suspected).
• Faecal occult blood (FOB) (three samples).
• CXR.
• Echocardiography (TTE or transoesophageal echocardiography (TOE)).
• OGD and biopsy.
• Colonoscopy and biopsy.
• AbdominalUSS.
• CTchest.
• Bronchoscopy, biopsy, washings.
• Liver biopsy.
E OHCM 10e, p.40,p.77.

26
CHAPTER1 Symptoms andsigns
26
Coma
The Glasgow Coma Scale (GCS) is used to assess the level of consciousness
(see Table 1.4). The minimum score is 3; the maximum15.
Assess the level of consciousness and determine whether this is stable,
uctuating, improving, or deteriorating on serial assessments.
Cerebralcauses
• Intracranial haemorrhage (subarachnoid haemorrhage (SAH), subdural
haemorrhage (SDH), extradural haemorrhage (EDH), intracerebral
bleed).
•
Large cerebral infarct.
• Pontine haemorrhage (pinpoint pupils).
• Cerebral venous sinus thrombosis.
• Hypertensive encephalopathy.
• Cerebral tumour (associated local cerebral oedema may respond to
dexamethasone).
•
Head injury.
• Cerebral infection— encephalitis, meningitis, cerebral malaria, brain
abscess.
•
Post- ictalstate.
• Subclinical status epilepticus. (Note:this is an EEG diagnosis.)
• Cerebral vasculitis, e.g.SLE.
• End- stageMS.
• Leukodystrophy.
• CJD (including variant CJD (vCJD)).
Table1.4 Glasgow ComaScale
Eye opening 1
2
3
4
Nil
Topain
Tovoice
Spontaneously
Motor response 1
2
3
4
5
6
Nil
Extension
Flexion
Withdrawal frompain
Localizing topain
Voluntary
Vocal response 1
2
3
4
5
Nil
Groans
Inappropriatewords
Disorientatedspeech
Orientated speech

COMA
27
Metaboliccauses
• Drugs (usually in deliberate overdose; E Chapter11).
•
Alcohol excess. (Note:remember hypoglycaemia as a cause of coma in
alcoholics, as well as extradural haematoma.)
•
Hypoglycaemia (iatrogenic, overdose of insulin or sulfonylureas,
insulinoma, insulin- like growth factor (IGF)- 2- associated hypoglycaemia
in certain tumours).
•
DKA (coma in 710% of cases— adverse prognosticsign).
• Hyperosmolar non- ketotic coma (HONK) (may present as severe
dehydration ±coma).
•
Uraemia.
• Late stages of hepatic encephalopathy.
• Severe hyponatraemia (relatively common— especially inappropriate
antidiuretic hormone (ADH) syndrome).
•
Hypothyroidism (myxoedema coma— rare).
• Hypercalcaemia.
• Inborn error of metabolism, e.g. porphyria, urea cycle disorders.
• Type 2 respiratory failure (carbon dioxide (CO
2
) narcosis).
•
Hypothermia (severe).
• Hyperpyrexia (neuroleptic malignant syndrome (NMS), after
anaesthesia).
•
Severe nutritional deciency— thiamine, pyridoxine, vitaminB
12
.
Investigations
• Venous plasma glucose (exclude hypoglycaemia with a ngerstick +
reectance meter; conrm with a venous plasma uoride– oxalate
sample).
•
U&E.
• LFTs.
• SerumCa
2+
.
•
Serum osmolality.
• UrineNa
+
.
•
Blood cultures.
• Clotting screen (E p. 288, p. 289, p. 290).
•
ABGs.
• Drug screen (serum, urine).
• Cranial CTscan.
• L P.
• CXR (bronchogenic carcinoma with cerebral metastases).
• 12- leadECG.
• EEG.
• Erythrocyte transketolase (d in thiamine deciency).
•
Serum ammonia (NH
3
) (i in urea cycle disorders).
•
Brain biopsy.
Always assess Airway, Breathing, Circulation before assessment of the
cause of d consciousness. Consider psychogenic unresponsiveness.
E OHCM 10e, p.220, pp.786–9, p.834, p. 836.

28
CHAPTER1 Symptoms andsigns
28
Confusion
A reliable witness, family member, or carer may be vital in assessing a
patient with confusion, and care must be taken to discriminate between
acute and chronic symptoms. Acute confusional states carry a very broad
dierential diagnosis and require careful initial evaluation (see Table 1.5).
Any systemic illness can precipitate a confusionalstate.
Investigations
• FBC, U&E, LFTs, serum Ca
2+
, BM stix, and blood glucose.
•
ABGs.
• MSU, blood cultures, sputum culture.
• CXR.
• ECG.
• Thyroid function.
• Drug/ toxicology screen— blood andurine.
• CTscan.
• L P.
3 Always look for a MedicAlert
™
bracelet, necklace, orcard.
Table1.5 Causes ofconfusion
Hypoxaemia Acute infection, asthma, COPD, etc.
Head injury Cerebral trauma
Vascular CVA, TIA, intracerebral, SDH
Infection Systemic
Meningitis or encephalitis
Endocrine/ metabolic DKA, hypoglycaemia, thyrotoxicosis or
myxoedema, uraemia, hypercalcaemia,
hyponatraemia
Alcohol and drug abuse Acute intoxication and withdrawal
Also consider overdose
Iatrogenic Full and recent medication history (especially
opiates, analgesia, and sedatives)
Post- ictal state
Cerebral tumour
Psychiatric
Wernicke’s encephalopathy
E OHCM 10e, p.576.

CONSTIPATION
29
Constipation
Patients may use the term constipation to mean infrequent, hard, small vol-
ume, or dicult to pass faeces. Patients vary enormously in their threshold
to seek medical advice about bowelhabit.
Askabout:
•
Associatedpain.
• PR bleeding.
• Tenesmus.
• Weightloss.
Causes
• Carcinoma of thecolon.
• Diverticular disease.
• Anorectal disease— ssure or haemorrhoid.
• Benign stricture.
• Rectocele.
• Sigmoid volvulus.
• Hernia.
• Drugs, especially analgesics.
• Poor uid intake.
• Low- brediet.
• Change indiet.
• Immobility.
• IBS.
• Megarectum.
• Hirschsprung’s disease.
• Spinal cord lesion.
• Stroke.
• Jejunal diverticulosis.
• Hypothyroidism.
• Diabetic neuropathy.
• Hypercalcaemia, hyperparathyroidism, hypokalaemia.
• Uraemia.
• Porphyria.
• Pregnancy.
• MS.
• PD.
• Dermatomyositis.
• Myotonic dystrophy.
• Scleroderma.
• Psychological.

30
CHAPTER1 Symptoms andsigns
30
Investigations
• Digital rectal examination.
• Proctoscopy.
• Sigmoidoscopy.
• Colonoscopy.
• Bariumenema.
• U&E.
• Ca
2+
.
•
TFTs.
• FBC.
• Bowel transit time studies.
• Anorectal manometry.
• Electrophysiological studies.
• Defecating proctography.
3 Elderly patients are more prone to constipation.
E OHCM 10e, pp.260–1, p. 534.

CYANOSIS
31
Cyanosis
Cyanosis is a blue/ purple dusky discoloration of tissue caused by a rise in
blood deoxygenated Hb content (>5g/ dL). Rarely it may be caused by i
sulphaemoglobin, methaemoglobin, or COHb. Cyanosis may be peripheral
aecting only cutaneous areas, or central when mucous membranes of the
mouth and tongue are also discoloured.
Causes ofperipheral cyanosis
• Central cyanosis.
• Shock.
• Hypothermia.
• Mitral stenosis.
• Raynaud’s syndrome.
• Peripheral arterial disease.
• Patent ductus arteriosus (dierential cyanosis, i.e. cyanosed toes, but
not ngers, is pathognomonic of this condition).
Causes ofcentral cyanosis
Pulmonary disease withseverely impaired oxygen transfer
•
Pneumonia.
• Asthma.
• Chronic obstructive pulmonary disease (COPD).
• PE.
• Fibrosing alveolitis.
Right- to- left shunt (Eisenmenger’s syndrome)
• Atrial septal defect.
• VSD.
• Patent ductus arteriosus.
• Partial anomalous pulmonary venous drainage (APVD).
• AVM.
Methaemoglobinaemia, sulphaemoglobinaemia, carboxyhaemoglobinaemia
•
Congenital.
• Ingestion of oxidizing agents, e.g. phenacetin, inorganic nitrates, local
anaesthetic.
Cyanosis arising from pulmonary disease can be reversed by administration
of oxygen (O
2
) to improve alveolar O
2
uptake. O
2
has no eect where right-
to- left shunts are the cause. Central cyanosis may be underestimated with
signicant anaemia and is more apparent in patients with polycythaemia.
In methaemoglobinaemia, the arterial concentration of O
2
is normal. This
condition can be treated with IV methylthioninium chloride (methylene
blue) (E Chapter11).
Investigations
• FBC.
•
ABGs.
• CXR.
• 12- leadECG.
• TTE (proceeding to TOE if shunt is suspected).
• CT chest (if AVM is suspected).
• Cardiac MRI (if APVD is suspected).
E OHCM 10e, p.34.

32
CHAPTER1 Symptoms andsigns
32
Diarrhoea
Patients may use the term diarrhoea to describe loose stools, i frequency
of defecation, i volume of stool, steatorrhoea, melaena, or faecal inconti-
nence (E Incontinence:faecal, p. 60).
Askabout
• Duration.
• Associated features (abdominal pain, vomiting, mucus, or bloodPR).
• Systemic symptoms.
• Recent foreign travel.
• Suspectfood.
• Is anyone else in the household aected?
Causes
• Infection (including ‘traveller’s diarrhoea’).
• IBD.
• Diverticular disease.
• Colonic carcinoma.
• Other tumour, especially villous adenoma.
• Coeliac disease.
• Tropicalsprue.
• IBS.
• Ischaemic colitis/ bowel infarction.
• Laxativeuse!
• Other drugs, e.g. metformin, orlistat.
• Overindulgence in fruit or vegetables.
• Overow 2° to constipation.
• Carcinoid syndrome (uncommon).
• Gastrinoma (rare).
• VIPoma (rare).
• Glucagonoma (veryrare).
• Hyperthyroidism (common).
• Medullary carcinoma of the thyroid (uncommon).
• Bile salt diarrhoea (previous ileal disease or surgery).
• Dumping syndrome (previous gastric surgery).
• Gut motility disorders.
• Malabsorption (cf. pancreatitis, lymphangiectasia, coeliac).
• Lactose intolerance.
• Scleroderma.
• Amyloidosis.
• Whipple’s disease.
Investigations
• Stool culture, hot stool for parasites.
• Clostridium dicile toxin instool.
•
High rectal swab for parasites. (Note:giardiasis is diagnosed on duodenal
biopsy.)
•
Rectal examination, proctoscopy, sigmoidoscopy ± biopsy.
• Colonoscopy.

DIARRHOEA
33
• AXR.
• Bariumenema.
• Small bowel follow- through contrast studies.
• Upper GI endoscopy.
• Small bowel biopsy.
• FBC and bloodlm.
• ESR.
• CRP.
• Serum ferritin and folate.
• U&E (exclude haemolytic uraemic syndrome (HUS), especially in
children).
•
Urine screen for laxatives.
• Antigliadin, antiendomysial antibodies and anti- tissue transglutaminase
(tTG) (coeliac disease).
•
TFTs.
• Serum gut hormone prole (gastrin, vasoactive intestinal peptide (VIP),
glucagon— seek expert advice).
• 24h urine for 5- hydroxyindole acetic acid (5HIAA).
• Serum calcitonin (medullary carcinoma of the thyroid).
• Lactose hydrogen breath test (for lactose intolerance).
•
14
C- xylose breath test (bacterial overgrowth in the small bowel).
• CT abdomen.
• Mesenteric angiography (ischaemia).
Investigations must be guided by history and examination ndings. If the
patient is an inpatient, they should be isolated until infection is excluded.
Consider HIV and other immune disorders if an unusual bowel organism
isfound.

34
CHAPTER1 Symptoms andsigns
34
Dizziness and syncope
Dizziness is a term that may be used to describe a variety of symptoms, e.g.
spinning (rotatory vertigo), light- headedness, muzzy feeling, or unsteadiness
on walking. It is therefore important to establish precisely what the patient
means by dizziness.
Loss of consciousness or ‘blackout’ may not be reported by the patient
and an eyewitness account is important. Enquire about any awareness of
abnormal heart beat (rhythm- induced syncope), chest pain (ischaemia),
neurological symptoms (cerebrovascular disease), preceding micturition,
change of posture, or unusual sensations (prodromal epileptic symptoms,
e.g. strange taste or smell) prior to the collapse.
Causes ofdizziness
• Rotatory sensation lasting >10s and precipitated by movement or
position— vestibular cause such as labyrinthitis, Ménière’s disease,
cerebello- pontine angle tumour (acoustic neuroma).
• Rotatory sensation lasting 2 or 3s and precipitated by movement—
cervical spondylosis.
•
Non- rotatory sensation lasting 2 or 3s and precipitated by movement,
position, or standing up— cervical spondylosis, cerebrovascular disease,
postural hypotension, cardiac arrhythmia (usually back to normal in
minutes), epilepsy (incontinence is common and return to normal may
take hours).
Investigations
Suspected vestibularcause
•
Hallpike manoeuvre.
• MRI or CT cerebello- pontineangle.
• Audiometry.
Suspected non- vestibularcause
• Blood glucose.
• 12- leadECG.
• 24h ambulatory ECG monitoring.
• EEG.
• MRI or CThead.
• Tilt tabletest.
Causes ofsyncope
• Vasovagal:pain, fear, prolonged standing, excess heat, alcohol, orfood.
• Micturition (often elderly men standing up during the night to urinate).
• Defecation (often elderly women with constipation).
• Coughing:chronic airways disease.
• Orthostatic hypotension:
•
Autonomic dysfunction (diabetic neuropathy, Shy– Drager
syndrome).
•
Drugs (antihypertensives, diuretics, nitrates, tricyclics; dehydration
and sodium depletion).
•
Carotid sinus syndrome.
• Epilepsy.

DIZZINESS AND SYNCOPE
35
• Drugs:alcohol, illicitdrugs.
• Cardiac:arrhythmias, outow obstruction (aortic stenosis, hypertrophic
obstructive cardiomyopathy, myxoma).
•
Hyperventilation and anxiety.
• Acute cerebrovascular disease:transient ischaemic attack (TIA),
stroke,SAH.
•
Acute vascular obstruction:PE,MI.
• Hypoglycaemia:poorly controlled diabetes.
Investigations
•
12- leadECG.
• 24h Holter monitoring.
• Echocardiography.
• MRI or CThead.
• Tilt tabletest.
• Blood glucose,HbA
1c
.
•
U&E.
• Cardiac markers.
• ABG.
• Toxicology screen.
• V/ Qscan.
2 Driving and dizziness/ syncope
For guidance on driving in the United Kingdom (UK), see M http:// www.
dvla.gov.uk.
Further reading
Brignole M, Alboni P, Benditt DG, etal.; Task Force on Syncope, European Society of Cardiology.
Guidelines on management (diagnosis and treatment) of syncope. Eur Heart J 2004; 25:2054– 72.

36
CHAPTER1 Symptoms andsigns
36
Dysarthria and dysphasia
Dysarthria is diculty in articulating words. The patient may complain of
‘slurred speech’. Dysphasia is a diculty in the formation of speech due to
interference with higher mental function. These disturbances often occur
together, most commonly in the context of a stroke.
Damage to Wernicke’s area causes a receptive dysphasia. Speech may
be uent, but meaning is lost. Damage to Broca’s area causes an expressive
dysphasia. Speech is non- uent and the patients are aware they are not
using the rightwords.
Causes of dysphasia include stroke (usually with right hemiparesis, arm
more aected than leg) or SOL. Psychosis, especially schizophrenia, may
cause a similar picture— the so- called ‘word salad’.
Causes ofdysarthria
• Stroke (internal capsule or extensive lesion of the motor
cortex— acute).
• Motor neurone disease(MND).
• Midbrain or brainstem tumour.
• PD.
• Cerebellar disease (haemorrhage, infarct, MS, hereditary ataxia,
alcoholic or paraneoplastic degeneration).
•
Syringobulbia (chronic, progressive).
• Neuromuscular (myasthenia gravis (MG), dermatomyositis, myotonic
dystrophy).
•
Acute alcohol or drug intoxication.
Dysarthria may be more obvious when the (English- speaking!) patient is
invited to say ‘Baby hippopotamus’, ‘British constitution’,etc.
Investigations
•
Cranial CTscan.
• Venous plasma glucose.
• ESR.
• Serum lipids.
• 12- leadECG.
• Echocardiogram.
• Carotid Doppler studies (especially if bruit).
• CXR.
• LFTs.
Less commonly
•
Serum muscle enzymes (polymyositis).
• Autoimmune prole.
• Electromyogram(EMG).
• Skeletal muscle biopsy.
E OHCM 10e, pp.86–7.

DYSPHAGIA
37
Dysphagia
Dysphagia is diculty in swallowing. The patient may have associated
odynophagia (painful swallowing) or regurgitation of food (immediate or
delayed?). Elicit whether the dysphagia is for liquid, solids, or both. Is it inter-
mittent or progressive? Are there associated symptoms?
A careful physical examination is mandatory. Pay special attention to the
lower cranial nerves; search for lymph nodes in the supraclavicular fossae.
Palpate the thyroid and percuss for retrosternal enlargement.
Causes
• Oesophageal carcinoma.
• Benign oesophageal stricture 2° to chronic acid reux.
• Barrett’s oesophagus.
• Achalasia or diusespasm.
• Stroke (bilateral internal capsule cerebrovascular accidents (CVAs)—
pseudo- bulbar palsy).
• Oesophageal web (+ iron deciency anaemia=Plummer– Vinson
(Patterson– Kelly– Brown) syndrome).
• Pharyngealpouch.
• Muscular problem (MG, dermatomyositis, myotonic dystrophy).
• Bulbar palsy (MS, MND, poliomyelitis).
• Scleroderma (including CREST syndrome— E OHCM 10e, Chapter12).
•
Infection (usually acute pain on swallowing).
• Mediastinal mass (goitre, carcinoma of the bronchus, enlarged left
atrium, aortic aneurysm).
Investigations
• FBC.
• ESR.
• Upper GI endoscopy.
• Barium swallow.
• CXR.
• Oesophageal manometry studies (E Gastrointestinal physiology,
pp. 526–7).
•
Cranial CT or MRI (if neurological signs).
• Acetylcholine (ACh) receptor antibodies and Tensilon
®
(edrophonium).
test if MG is suspected (E
Edrophonium (Tensilon
®
) test, p. 631).
Note:consider HIV testing if there is oesophageal Candida, or herpes sim-
plex or cytomegalovirus (CMV) infection in the oesophagus.
E OHCM 10e, p.64, pp.250–1.

38
CHAPTER1 Symptoms andsigns
38
Facialpain
Is the pain unilateral or bilateral? Is it constant or intermittent? Precipitating
factors or trigger points? Afull examination of the head and neck is required
in addition to a detailed neurological and systemic examination.
Causes
• Trigeminal neuralgia(TN).
• Temporal arteritis (TA). 3 Risk of visual loss (E OHCM 10e,
Chapter11).
•
Herpes zoster (shingles or post- herpetic neuralgia).
• Dental caries, sepsis.
• Sinusitis.
• Temporomandibular joint dysfunction.
• Cluster headache.
• Glaucoma.
• Angina pectoris.
• Tonsillitis.
• Syringobulbia.
• Atypical facial neuralgia.
• Migraine.
Investigations
• ESR— urgent in suspectedTA.
• Temporal artery biopsy if TA strongly suspected. (3 Must be
performed rapidly— within days— if steroid treatment is commenced.
However, do not withhold corticosteroid therapy for this reason!)
Because of ‘skip’ lesions, false −ve biopsies may be encountered. Be
guided by the full clinical picture, rather than reliance on a singletest.
•
Plain radiographs or CT imaging of frontal or maxillary sinuses.
• MRI to exclude MS, basilar aneurysm, trigeminal schwannoma,
neurobroma as causesofTN.
•
MRI of the cervical spinal cord to exclude syringobulbia if pain is
accompanied by brainstemsigns.
E Headache, pp. 49–50 and E OHCM 10e, p. 64, pp. 456–7.

FEVER OFUNKNOWN ORIGIN (FUO ORPUO)
39
Fever ofunknown origin (FUO orPUO)
Dened as temperature >38.3°C on several occasions, lasting 3 weeks
or more. It is very important to take a full history and consider infectious
contacts, recent travel abroad, recent surgery and dental treatment, sexual
history, and risk factors forHIV.
Signs
Examine for heart murmurs, splinter haemorrhages, splenomegaly, lym-
phadenopathy, and rashes/ pruritus (see Table1.6).
Investigations
• Re- take the history and re- examine the patient (something might have
been missed or new symptoms/ signs may have developed).
• FBC,ESR.
• U&E, LFTs,Ca
2+
.
•
CXR.
• MSU, urinalysis.
• Serology for Brucella and Toxoplasma.
•
All biopsy material should be sent for culture, includingTB.
• Blood cultures (serial may be necessary).
• Monospot/ Paul Bunnell.
• Autoimmune prole (antinuclear antibodies (ANA), rheumatoid factor
(RF), ANCA,etc.).
•
Bone marrow aspirate/ trephine/ culture for TB with Ziehl– Neelsen
(ZN)stain.
• Abdominal USS (? masses).
Extend investigations asbelow accordingtosymptoms
andsigns
• Consult microbiology or infectious disease consultant for advice.
• Stool cultures and fresh stool for ova, cysts, and parasites.
• Repeat serological investigation for changing titres (2– 3 weeks).
• Thick and thin blood lm for malaria and parasites.
• Mantoux.
• TTE or TOE to exclude endocarditic vegetations.
• CT chest, abdomen, and pelvis.
3 Always re- examine the patient for evolving new signs if the cause
remains unknown.
E OHCM 10e, pp. 442–3.
Table1.6 Causes ofFUO/ PUO
Infection Abscesses (e.g. subphrenic, pelvic, lung), osteomyelitis,
TB, endocarditis, parasites, rheumatic fever, brucellosis,
toxoplasmosis, Lyme disease, histoplasmosis, viral (especially
Epstein– Barr virus, CMV, hepatitis, and HIV)
Malignancy Lymphoma, leukaemia, hypernephroma, ovary, lung, hepatoma
Connective tissue
Polyarteritis nodosa, SLE, RhA, Still’s disease, TA
Other Sarcoidosis, atrial myxoma, drug fever, IBD, factitious

40
CHAPTER1 Symptoms andsigns
40
Firstfit
3 Arst t in an adult requires careful evaluation since the probability of
an underlying structural lesion i withage.
Take a careful history, preferably from a witness as well as the patient.
Most lay persons will recognize a generalized tonic– clonic t. However,
the occurrence of a few ‘epileptiform’ movements in patients with synco-
pal episodes (E Dizziness and syncope, pp. 34–5) may cause diagnostic
uncertainty.
Be sure toaskabout
• Aura preceding the episode. 2 Temporal lobe epilepsy— olfactory or
gustatory auras (not necessarily followed by convulsions).
•
Loss of consciousness— how long? Often overestimated by witnesses!
• Tongue biting.
• Focal or generalized convulsive movements. Note:a clear history of
a tonic– clonic t commencing in a limb and progressing to a more
generalized convulsion is highly suggestive of a structural intracerebral
lesion; cranial imaging is mandatory.
•
Central cyanosis (tonic phase).
• Urinary incontinence.
• Injuries.
• Post- ictal confusion.
• History of trauma.
• Alcohol intake. Remember:alcohol withdrawal ts as well as acute
intoxication.
•
Drug history— prescribed and recreational.
• History of insulin- treated diabetes or type 2 diabetes treated
with oral secretagogues, i.e. sulfonylureas, repaglinide, nateglinide.
Note:metformin and thiazolidinediones as monotherapy do not cause
signicant hypoglycaemia.
A full general and neurological examination is needed, specically including:
•
Fever.
• Meningism, i.e. nuchal rigidity, +ve Kernig’s sign (meningoencephalitis).
• Cutaneous rash or ecchymoses (? bleeding diathesis).
• Evidence of head trauma (preceding t or as a consequence).
• Signs of chronic liver disease.
• Focal neurological decit. 2 Third nerve palsy in an intracranial SOL,
including aneurysm of the posterior communicating artery. Sixth nerve
lesion may act as a ‘false localizing sign’ iniICP.
•
MedicAlert
™
bracelet (history of epilepsy or diabetes— search personal
belongings).
Bilateral extensor plantar reexes can occur after a generalized t without
a structural brain lesion and there may be transient hemiparesis (Todd’s
paresis).

FIRSTFIT
41
Causes
• Epilepsy (E OHCM 10e, Chapter10).
•
Hypoglycaemia (acute, severe, history of diabetes?).
• Hyponatraemia (usually <110mmol/ L or rapid development).
• Hypocalcaemia (E OHCM 10e, Chapter14).
•
Hypomagnesaemia (may accompany hypocalcaemia).
• Hypophosphataemia (rare).
• Alcohol withdrawal. 3 Risk of associated hypoglycaemia.
•
Discontinuation of anticonvulsant medication.
• Infection— viral encephalitis or bacterial meningitis. 3 Consider
intracerebral abscess, tuberculoma in predisposed patients.
•
Encephalopathy— hepatic, uraemic, hypertensive, thyrotoxic (rare—
‘thyroid storm’).
•
Eclampsia.
• Porphyria.
• CerebralSLE.
• Head injury.
• Hypoxia.
• Cerebral tumour.
• Stroke— cerebral infarct, haemorrhage.
Investigations
• Venous plasma glucose (ngerprick test at bedside useful as ‘screen’—
but can be unreliable).
•
U&E.
• Serum Ca
2+
, magnesium (Mg
2+
), phosphate (PO
4
3−
).
•
Cranial CT or MRIscan.
• EEG.
• LP (E Lumbar puncture, pp. 584–9).
•
CXR.
• Serum prolactin (PRL) (may be i after generalized convulsions, but not
pseudo- seizures).
• ABGs— remember transient lactic acidosis following generalized tonic–
clonic convulsions.
•
Blood ethanol (may be undetectable in withdrawal state).
• Serum or urine drug screen.
‘Pseudo- seizures’ may be encountered in patients with atypical recurrent
ts (usually long history of epilepsy) and this is unlikely in an adult presenting
with a rst t. 2 In UK, the DVLA prohibits driving for 12months following
a rstt.
E OHCM 10e, pp. 490–2.

42
CHAPTER1 Symptoms andsigns
42
Galactorrhoea
Denotes inappropriate breast milk production, i.e. in the absence of preg-
nancy. The commonest cause is hyperprolactinaemia (i PRL) due to a pitui-
tary microprolactinoma of <10mm in diameter (E Precocious puberty,
p. 179). Prolactinomas (usually macroadenomas) may cause galactorrhoea
inmen.
Note:other disease in the pituitary region, certain drugs, and several sys-
temic disorders may be associated with i PRL (E OHCM 10e, Chapter5).
Causes
Normoprolactinaemic galactorrhoea
•
This has been described in premenopausal women occurring after the
conclusionof:
•
Treatment with the combined contraceptivepill.
•
Breastfeeding (for >6months afterwards).
• i sensitivity of lactogenic tissue PRL is postulated, but the mechanism
remains uncertain. In part, this may reect diculties that can arise
in determining whether PRL is persistently elevated. Menstrual
disturbances have been described.
Hyperprolactinaemia
•
The dierential diagnosis and investigation of hyperprolactinaemia are
considered in E Galactorrhoea (hyperprolactinaemia), pp. 172–3.
Investigations
• Serum PRL (E Galactorrhoea (hyperprolactinaemia), pp. 172–3).
•
Repeated measurements under controlled conditions may be required
since PRL is a ‘stress’ hormone and may be i by venepuncture.
Note:if i PRL is conrmed, further investigations to exclude causes other
than a prolactinoma are required.
•
Pituitary imaging (CT, or preferably MRI) and visual eld testing
(Goldmann) may also be indicated if a macroprolactinoma is suspected
(PRL concentrations usually veryhigh).
Note: if there is doubt about the nature of the nipple discharge, further
specialized investigations may be required on the uid, including:
•
Casein.
• Lactose.
• Microscopy.
Clear uid may result from benign breast disease.
Note:bloody discharge should prompt urgent specialist investigations to
exclude carcinoma of the breast:
•
Mammography.
• Biopsy.
E OHCM 10e, p.237.
Further reading
Kleinberg DL, Noel GL, Frantz AG. Galactorrhoea:a study of 235 cases, including 48 with pituitary
tumors. N Engl J Med 1977; 296
:589– 600.

GOUT
43
Gout
Gout is a disease of deposition of monosodium urate monohydrate crys-
tals in tissues and relates to hyperuricaemia. Hyperuricaemia is due to
an imbalance between purine synthesis and uric acid excretion. Episodes
of acute gout may be precipitated by alcohol, trauma, dietary changes,
infection, chemotherapy, or surgery. Commoner in men and very rare in
premenopausalwomen.
Clinical features
• Inammatory arthritis, classically monoarthritis or oligoarthritis aecting
the rst metatarsophalangeal (MTP) joint of the foot but can aect any
joint, including thespine.
•
Tenosynovitis.
• Bursitis or cellulitis.
• Tophi— urate deposits in tendons, ear pinnae, and joints.
• Urolithiasis and renal disease.
Investigations
• ESR (maybei).
•
Urate crystals demonstrated in the synovial uid or tissues— negatively
birefringent on polarized light microscopy.
•
Serum urate (not always i in an acute episode, and a normal urate level
does not exclude the diagnosis).
•
X- ray— soft tissue swelling and punched- out bony erosions.
• Autoimmune prole (AIP) (to exclude rheumatoid).
• Microscopy of synovial uid (Gram stain and culture).
Treatment
Acute episode
•
NSAIDs, colchicine, intra- articular steroids, or oral steroids.
• Avoid precipitating factors and purine- richfoods.
• Urate- lowering therapy indicated for tophi, recurrent attacks, and
urine/ renal disease,e.g.
•
Allopurinol (xanthine oxidase inhibitor).
•
Probenecid (uricosuric).
Note: asymptomatic hyperuricaemia is commoner than gout, and a high
serum urate level with coexistent arthritis is not necessarily due to crystal
deposition. Consider important other causes, especially infective arthritis
and pseudo- gout.
Pseudo- gout
Calcium pyrophosphate crystal deposition causing acute arthritis or chon-
drocalcinosis. Crystals are weakly positively birefringent on polarized
light microscopy. Associations include old age, dehydration, hyperpar-
athyroidism, hypothyroidism, haemochromatosis, acromegaly, RhA, and
osteoarthritis(OA).
E OHCM 10e, p.548.

44
CHAPTER1 Symptoms andsigns
44
Gynaecomastia
Gynaecomastia is benign bilateral hyperplasia of glandular and fatty breast
tissue in the ♂. The balance between androgens and oestrogens is thought
to be of importance in the pathogenesis; many conditions may inuence this
ratio. Most commonly, it appears transiently during normal puberty (detect-
able at some stage in 750% of cases). Gynaecomastia may also be caused
by specic endocrine disease or be associated with certain chronic diseases.
Treatment with certain drugs is a common cause (730% of cases) and arises
via several mechanisms. Investigations will be guided by the individual cir-
cumstances. Acareful drug history and thorough physical examination are
required, particularly in the post- adolescent period.
When indicated, and after excluding causes such as congenital syndrome
and drug therapy, investigations are principally directedat:
•
Excluding endocrine carcinoma (rare).
• Identifying associated chronic diseases.
Note:
•
Simple obesity is not usually a cause of true gynaecomastia, i.e. the
glandular element isnoti.
•
i serum PRL in isolation does not cause gynaecomastia.
•
Unilateral, eccentric breast enlargement should prompt exclusion of
breast carcinoma (rare).
Causes include
• Physiological states (transient):
•
Newborn.
•
Puberty.
•
Advancedage.
• Klinefelter’s syndrome (47,XXY; mosaics).
• 2° hypogonadism, e.g. mumps orchitis.
• Androgen resistance syndromes, e.g. testicular feminization.
• i tissue aromatase activity (converts androgens to oestrogens).
•
Oestrogen- producing tumours:
•
Leydig cell tumour.
•
Sertoli cell tumour.
•
Adrenal carcinoma.
• Chronic liver disease.
• Chronic renal failure.
• Panhypopituitarism.
• Tumours producing human chorionic gonadotrophin(hCG).
• Drugs:oestrogens (prostatic carcinoma, transsexuals), spironolactone,
cimetidine, digoxin, cytotoxic agents, marijuana.
•
Hyperthyroidism (i serum sex hormone- binding globulin (SHBG)).
• ° hypothyroidism.
• Cushing’s syndrome.
• Carcinoma of the bronchus.
• Idiopathic.

GYNAECOMASTIA
45
Investigations
• Testosterone.
• FSH.
• LH.
• LFTs.
• TFTs.
• Oestradiol.
• β- hCG.
• PRL.
• SHBG (anity of SHBG is higher for testosterone than for oestrogens,
therefore i SHBG causes disproportionate d in free testosterone
levels).
•
Dehydroepiandrosterone sulfate (DHEAS).
• Androstenedione.
• TesticularUSS.
• CXR.
• Abdominal CT or MRI imaging (for suspected adrenal tumours).
• Pituitary imaging.
• Karyotype.
• Urinary 17- oxo- steroids.
If carcinoma ofthe breast is suspected
• Mammogram.
• Fine- needle aspiration(FNA).
E OHCM 10e, p.230.
Further reading
Braunstein GD. Gynecomastia. N Engl J Med 1994; 328:490– 5.

46
CHAPTER1 Symptoms andsigns
46
Haematemesis
This literally means vomiting blood and is often associated with melaena
(passage of black tarry stools).
Causes
• Chronic peptic ulceration (e.g. duodenal ulcer (DU) or gastric ulcer
(GU)) accounts for 50% of cases of bleeding from the upper GItract.
•
Acute GUs or gastric erosions.
• Drugs (e.g. NSAIDs) or alcohol.
• Reux oesophagitis.
• Mallory– Weisstear.
• Oesophageal varices.
• Gastric carcinoma (uncommon).
Investigations afteradmission and stabilization ofthe
patient
• Full history, including drugs, alcohol, past history, indigestion,etc.
• FBC. (Note:Hb will take 724h to fall; initially may be normal.)
•
U&E.
• Cross- matchblood.
• Urgent upper GI tract endoscopy.
• Check Helicobacter pylori serology ± urea breathtest.
E OHCM 10e, p.30, p. 256.

HAEMATURIA
47
Haematuria
In health, adults pass between 500,000 and 2,000,000 red cells over a 24h
period. Haematuria implies the passage of excess blood that may be detect-
able using dipsticks (microscopic haematuria) or may be obvious to the
naked eye (macroscopic haematuria).
Causes
• Many.
• Glomerular disease, e.g. ° glomerulonephritis, 2° glomerulonephritis
(SLE, vasculitis, infection).
• Vascular or interstitial disease due to hypersensitivity reactions, renal
infarction, papillary necrosis, or pyelonephritis.
•
Trauma.
• Renal epithelial or vascular tumours.
• Lower renal tract disease, e.g. tumours, stones, infection, drug toxicity
(e.g. cyclophosphamide), foreign bodies, or parasites.
•
Systemic coagulation abnormalities, e.g. platelet or coagulation factor
abnormalities such as profound thrombocytopenia orDIC.
Investigations
• Urinalysis— dipstick, microscopic examination, culture.
• Radiology,* e.g. KUB orIVU.
• Specialist investigation,* e.g. angiography, CT or MRI scanning.
• Cystoscopy.*
Note:ideally these tests (*) should be arranged after discussion with either
a nephrologist or a urologist.
E OHCM 10e, p.80, p. 294.

48
CHAPTER1 Symptoms andsigns
48
Haemoptysis
This describes coughing up blood or bloodstained sputum and can vary
from faint traces of blood to frank bleeding. Before embarking on investiga-
tion, it is essential to ensure that the blood is coughed up from the respira-
tory tract and is not that of epistaxis or haematemesis (easily confused).
Causes
• Infective, e.g. acute respiratory infection, exacerbation ofCOPD.
• Pulmonary infarction, e.g.PE.
• Lung cancer.
• TB.
• Pulmonary oedema.
• Bronchiectasis.
• Uncommon causes, e.g. idiopathic pulmonary haemosiderosis,
Goodpasture’s syndrome, microscopic vasculitis, trauma, haematological
disease (e.g. ITP orDIC).
Investigations
• Colour of blood provides clues (pink frothy in pulmonary oedema, rust-
coloured in pneumonia).
•
Check oxygen (O
2
) saturation.
•
FBC (? d platelets).
•
ESR.
• Coagulation screen.
• Sputum culture.
• CXR.
• Arrange bronchoscopy after discussion with the respiratoryteam.
E OHCM 10e, pp.48–9.

HEADACHE
49
Headache
E Facial pain,p. 38.
Headache is an extremely common complaint. Most patients self-
medicate and only a small proportion will seek medical advice. Headache
may be acute or chronic, constant, recurrent, or gradually progressive. It
may arise from structures within the cranial vault or from external causes
(E OHCM 10e, Chapter10).
Causes dier according to age; temporal arteritis is very uncommon in
patients under 755 years, for example. Migraine may be associated with
classic features (E OHCM 10e, Chapter10). Remember to enquire about
the combined OCP— may exacerbate migraine. ‘Tension’ headaches
predominate.
Causes inadults include
• ‘Tension’ headache (very common; usually recurrent and stereotyped).
• Migraine. Although common, many patients who believe they have
‘migraine’ probably have ‘tension’ headaches. Classic migraine
predominantly aects adolescents and young adults.
•
Cluster headaches.
• As part of a generalized viral illness, e.g.‘u’.
• Causes of i ICP (E OHCM 10e, Chapter10).
•
Acute infective meningitis (bacterial, viral most commonly).
• Encephalitis (most commonly viral, e.g. herpes simplex).
• Intracerebral haemorrhage.
• Post- traumatic (common).
• Intracerebral tumour (° or 2°, benign or malignant).
• AcuteSAH.
• Subdural haematoma.
• Acute glaucoma.
• Acute sinusitis.
• Rubeosis iridis (2° glaucoma in patients with advanced diabetic eye
disease).
•
TN.
• Referred pain, e.g. from dental caries or sepsis.
• Arterial hypertension; malignant or accelerated phase; essential
hypertension is rarely the cause of headache.
•
TA. 3 Visual loss preventable with prompt corticosteroid therapy
(E OHCM 10e, Chapter10).
•
Venous sinus thrombosis.
• Benign intracranial hypertension (mimics intracerebral tumour).
• Pneumonia caused by Mycoplasma pneumoniae may be associated with
headache (meningoencephalitis).
•
Nocturnal hypoglycaemia (often unrecognized) may cause morning
headaches in patients with insulin- treatedDM.
• Analgesia- withdrawal headache (E OHCM 10e, Chapter10).
•
Hangover following alcohol excess.
• Otitismedia.
• Chronic hypercalcaemia (rare).

50
CHAPTER1 Symptoms andsigns
50
Investigations
• ESR (3 TA— exclude with urgency).
• CRP.
• FBC.
• U&E.
• Throatswabs.
• Blood cultures (if febrile).
• LP (E Lumbar puncture, pp. 584–9).
•
Skull X- ray (SXR) ± cervical spineX- ray.
• Sinus X- rays (may be local tenderness in sinusitis).
• Cranial CT (E Computed tomography, pp. 598–600).
•
CXR (cerebral metastases from bronchogenic carcinoma).
• Urinalysis.
• Intraocular pressure measurement and refraction.
• Cerebral angiography (if aneurysm orAVM).
• SerumCa
2+
.
E OHCM 10e, p.64, pp. 456–7.

HEART SOUNDS AND MURMURS
51
Heart sounds and murmurs
Auscultation of the heart should be conducted over several cardiac cycles.
Heart sounds and murmurs are traditionally assessed at the apex, lower
left sternal edge, aortic area, and pulmonary area, but they may radiate into
other regions such as the axilla or carotid arteries. The carotid pulse should
be palpated simultaneously in order to time cardiac events. The following
should be identied:
•
First (S1) and second (S2) heart sounds.
• Added heart sounds such as third (S3) or fourth (S4) heart sounds,
opening snaps, ejection clicks, and prosthetic sounds.
•
Murmurs, including location, intensity, and characteristics.
The rst heart sound is produced by closure of the mitral and tricuspid
valves. It is best heard at the apex and is timed just prior to the carotid
pulse. The second heart sound is caused by closure of the aortic (A2) and
pulmonary (P2) valves and is heard just after carotid pulsation. Closure of
the pulmonary valve is slightly delayed relative to the aortic valve and so the
second heart sound is normally split. This split is exaggerated by inspiration
(see Table1.7).
Normal and abnormal heart sounds are shown in Table1.7.
The third heart sound is heard just after S2 and arises as a consequence of
rapid ventricular lling and volume overload. The fourth heart sound occurs
just before S1 and is caused by atrial contraction against a sti ventricle
or pressure overload. Abnormal valves may cause extra heart sounds on
opening, e.g. an opening snap or ejection click. The heart sounds generated
by articial valve closure are referred to as prosthetic heart sounds. These
should be crisp, not mued (see Table1.8).
Murmurs may be graded accordingtothe following criteria
1. Very soft (just audible under optimal conditions).
2. Soft.
3. Moderate (easily heard with a stethoscope).
4. Loud ± palpable thrill.
5. Very loud/ palpable thrill.
6. Heard without a stethoscope/ palpable thrill.
Innocent murmurs are generated by turbulent ow such as in high cardiac
output states, e.g. pregnancy, fever, anaemia, and thyrotoxicosis. They have
the following characteristics:
•
No accompanying thrill.
• Never > grade3.
• Systolic.
• Maximal at the left sternaledge.
• Normal heart sounds.
• Normal pulses, ECG, andCXR.
Systolic murmurs are synchronous withthe carotid pulse and causedby
•
Abnormal regurgitation through a structure that is normally closed in
systole, e.g. AV valve, septum (pansystolic).
•
Normal systolic ow through a narrowed or stenosed valve, e.g. aortic
valve, pulmonary valve (ejection systolic).
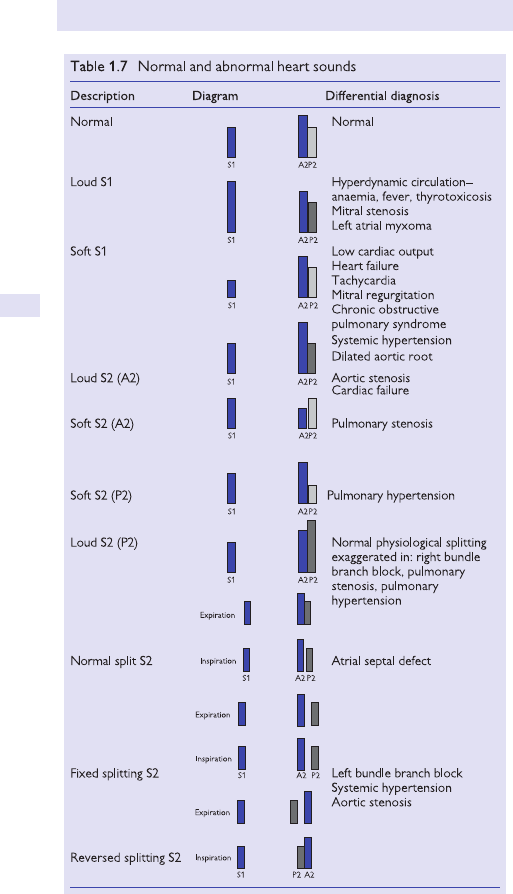
52
CHAPTER1 Symptoms andsigns
52
HEART SOUNDS AND MURMURS
53
Diastolic murmurs are audible afterthe carotid pulse and arisefrom
•
Incompetence of the cardiac outow valves, e.g. aortic or pulmonary
valves.
•
Narrowing of the cardiac inow valves, e.g. mitral or tricuspid valves.
Mixed murmurs (systolic and diastolic) arisefrom
•
Mixed valvular disease (stenosis and regurgitation).
• Patent ductus arteriosus.
Murmurs arising from left heart structures are accentuated in expiration,
whereas right heart murmurs are augmented in inspiration (see Table1.9).
Table 1.8 Heart murmurs
Pansystolic
murmur
S1 S2
S1 S2
S
1 S2
S1 S2
S1 S2 S1
S1 S2 S1
Mitral regurgitation
Tricuspid regurgitation
VSD
Late
systolic
murmur
Mitral valve prolapse
Hypertrophic obstructive
cardiomyopathy
Aortic coarctation
Ejection
systolic
murmur
Aortic stenosis
Pulmonary stenosis
Early
diastolic
murmur
Aortic regurgitation
Pulmonary regurgitation
Mid-
diastolic
murmur
Mitral stenosis
Tricuspid stenosis
Mixed
murmur
Mixed valvular heart
disease
Patent ductus arteriosus
AVMs
Collateral circulations
54
CHAPTER1 Symptoms andsigns
54
Table 1.9 Added heart sounds
S3
A2 S3
S1 P2
S1 P2A2
S4
S1 P2A2 OS
S1 P2A2EC
2P1SA2MSC
2P1SP PS2
Normal (young adult)
Left ventricular failure
Right ventricular failure
Mitral regurgitation
Constrictive pericarditis
(pericardial ‘knock’)
Normal (elderly)
Left ventricular hypertrophy
Left ventricular diastolic dysfunction
Systemic hypertension
Aortic stenosis
Acute ischaemia
Mitral stenosis
Tricuspid stenosis
4S
Opening
snap
Aortic stenosis
Systemic hypertension
Pulmonary stenosis
Pulmonary hypertension
Ejection
click
Mitral valve prolapseMid-
systolic
click
Arti cial valve replacementProsthetic
heart
sounds

HEPATOMEGALY
55
Hepatomegaly
Measure the liver edge below the (right) costal margin after percussing out
the upper and lower borders. Bruits may be heard in hepatoma and a fric-
tion rub may occur with malignant deposits. Other signs may suggest the
underlying diagnosis (E Pitfalls below).
Commoncauses
• CCF.
• Malignant deposits.
• Hepatitis/ cirrhosis (usually alcoholic or infectious, e.g. Epstein– Barr
virus (EBV), viral hepatitis).
Foreign residence?
If so, consider amoebic and hydatid cysts, schistosomiasis, and malaria.
Investigations
• FBC, lm, LDH (leukaemia, lymphoma).
• ESR.
• Virology (EBV, CMV, and hepatitis A, B, and C antibody serology).
• LFTs— transaminases.
• Serum albumin.
• Prothrombin time (PT) (hepatocellular damage).
• γ- glutamyl transpeptidase (GGT), MCV (alcohol).
• ALP (obstructive causes; malignant deposits if isolatedi).
•
Serum Igs may be polyclonal i in immunoglobulin G (IgG) (autoimmune
hepatitis), immunoglobulin A(IgA) (alcoholic liver disease), or
immunoglobulin M (IgM)(PBC).
•
Serum protein electrophoresis (myeloma, amyloid).
• Reticulocytes, bilirubin (if i, suggests haemolysis).
•
Haemoglobinopathy screen (thalassaemia/ sickle disorders).
• USS to assess liver texture, splenomegaly, lymphadenopathy.
• CXR and cardiac investigations (cardiomyopathies, sarcoid).
• α- fetoprotein (AFP) (primary hepatocellular carcinoma).
• Serum ferritin, transferrin saturation, DNA analysis
(haemochromatosis).
•
Mitochondrial antibodies and autoimmune markers, e.g. ANA
(autoimmune hepatitis), ANCA (primary sclerosing cholangitis).
•
Caeruloplasmin, urinary copper (Wilson’s disease).
• α1- antitrypsin (α1- antitrypsin deciency).
• Porphyria screen.
Pitfalls
•
Hepatomegaly is a common sign but may not necessarily implicate liver
pathology.
•
End- stage cirrhosis may commonly present with a small, shrunkenliver.
E OHCM 10e, p.63, p.604.

56
CHAPTER1 Symptoms andsigns
56
Herpeszoster
The pattern of the eruption varies from mild to dense with the involvement
of several dermatomes. Complications may occur if involvement of the
eye, motor nerves, and autonomic nerves (bladder), or when the disease
presents as an encephalomyelitis or purpura fulminans.
3 In the immunocompromised host, zoster is more likely both to occur
and to disseminate.
Investigations
• Conrm the diagnosis by isolation of the virus from the vesicularuid.
• Consider underlying disorders if recurrent or severe attacks.
• Look for lymphadenopathy (Hodgkin’s or other lymphoma).
• FBC, blood lm, LDH (i in lymphoma).
•
Serum protein electrophoresis (myeloma, amyloid).
• Serology for HIV (zoster is common in adult HIV individuals).
• Immunodeciency work- up.
Pitfalls
The rash is not always unilateral— it may be bilateral.
E OHCM 10e, p.462.

HYPERLIPIDAEMIA
57
Hyperlipidaemia
Abnormalities of lipid metabolism are common in Western societies.
Populations with high levels of cholesterol have high rates of vascular
morbidity, especially cardiovascular disease (CVD), and premature death.
Vascular risk can be estimated from published risk tables or calculators.
Various classications of hyperlipidaemia exist, each with a characteristic
lipid prole. Many patients with lipid disorders have cutaneous markers,
which identify to a certain extent the type of lipid abnormality.
Clinical features
Lipid abnormalities may cause dermatological manifestations
•
Grey- yellow plaques or xanthomata in tendons, especially the forearm
and Achilles. Usually indicative of elevated low- density lipoprotein
(LDL) cholesterol.
•
Corneal arcus, a thin white rim around the iris— whilst this is common in
the elderly, it is not a sign of i LDL, except in the under40s.
•
Yellow, fatty deposits or xanthelasmata around the eyelids— associated
with elevated LDL, these painless, non- tender plaques are common in
the elderly.
•
Yellow streaks in palmar creases— palmar xanthomata are associated
with IDL cholesterol.
•
Plaques over tibial tuberosities and elbows— tubero- eruptive
xanthomata. Often seen with hepatosplenomegaly with elevated
triglycerides.
•
Eruptive xanthomata— in severe triglyceridaemia, associated with
pancreatitis and hepatomegaly.
Hyperlipidaemia may be 2° to drugs such as corticosteroids, oestrogens,
and progestogens, as well as a range of conditions such as hypothyroidism,
myeloma, and alcoholism, each of which may be associated with specic
clinicalsigns.

58
CHAPTER1 Symptoms andsigns
58
Hypertension
Blood pressure (BP) measurements are graded into a number of categories
by the British Hypertension Society (see Box1.1):
Optimal blood pressure
Normal blood pressure
High- normal blood pressure
Grade 1 hypertension(mild)
Grade 2 hypertension (moderate)
Grade 3 hypertension (severe)
<120/ 80
<130/ 85
130– 139/ 85– 89
140– 159/ 90– 99
160– 179/ 100– 109
≥180/ 110
Hypertension should not be diagnosed on the basis of a single BP reading.
Unless urgent treatment is required, e.g. malignant hypertension, the BP
should be rechecked over a number of weeks to conrm the presence of
sustained hypertension.
Causes
Remember that the cause of hypertension in most (95%) cases is unknown
(‘essential’ hypertension). One of the following identiable causes can be
found in the remaining5%:
•
Renal disease, e.g. polycystic kidney disease.
• Renovascular disease, e.g. renal artery stenosis(RAS).
• Endocrine disease, e.g. Cushing’s syndrome, Conn’s syndrome,
phaeochromocytoma, acromegaly.
•
Coarctation of theaorta.
• Drugs, e.g. NSAIDs, OCP, steroids, erythropoietin (Epo),
sympathomimetics, liquorice.
•
Pregnancy, e.g. pre- eclampsia, eclampsia.
Routine investigation
The investigation ofhypertensive patients has thefollowingaims
•
To conrm the presence and severity of hypertension.
• To assess overall cardiovascularrisk.
• To identify target organ damage.
• To identify 2° causes (where present).
Routine investigations should include
•
Urinalysis (protein, blood, glucose).
• U&E.
• Plasma glucose (ideally fasted).
• Lipid prole (ideally fasted).
• 12- leadECG.
Box 1.1 British Hypertension Society grading
ofhypertension

HYPERTENSION
59
CXR, urine microscopy and culture, and echocardiography are not required
routinely but should be considered where indicated by your initial assess-
ment and investigation of the patient. The use of 24h ambulatory BP
monitoring is often useful where clinic readings are thought to be unreliable
because of ‘white coat’ hypertension.
Further investigation
Where more detailed assessment is required (for instance, to rule out a 2°
cause or to identify end- organ damage), the following investigations may
be appropriate:
Renal investigations
•
Renal USS (to assess overall renal morphology).
• Renal artery Doppler studies (forRAS).
• Renal artery magnetic resonance (MR) imaging (forRAS).
• Captopril renogram (forRAS).
• Renal angiography (forRAS).
• Renal vein renin measurements (for Conn’s syndrome).
Endocrine investigations
•
Renin and aldosterone studies for Conn’s syndrome (consult your local
endocrine laboratory).
•
Investigations for Cushing’s syndrome.
• Investigations for acromegaly.
• Urinary catecholamine (and metabolite) excretion.
Further reading
Williams B, Poulter NR, Brown MJ, et al. Guidelines for management of hypertension: report of
the fourth working party of the British Hypertension Society, 2004— BHS IV. J Hum Hypertens
2004; 18
:139– 85.

60
CHAPTER1 Symptoms andsigns
60
Incontinence:faecal
E Alteration of bowel habit, p. 9; E Constipation, pp. 29–30; E Diarrhoea,
pp. 32–3.
Causes include
• Any cause of diarrhoea (E OHCM 10e, Chapter6).
•
Overow diarrhoea from severe constipation.
• IBD (acute or chronic).
• Coeliac disease (diarrhoea is a variable feature).
• Infectious diarrhoea (E OHCM 10e, Chapter1).
•
Hyperthyroidism (may cause diarrhoea; rare cause of incontinence).
• Carcinoma of the colon (stricture).
• Diverticular disease of the colon (acute attack, chronic stricture).
• Neurological (multiple CVAs, MS, spina bida, post- childbirth
neuropathy) may often be associated with sphincter disturbances.
•
Drugs, e.g. laxatives, orlistat (causes fat malabsorption).
• Causes of steatorrhoea (E OHCM 10e, Chapter6).
•
Intestinal hurry, e.g. post- gastrectomy (E OHCM 10e, Chapter6).
•
Diabetic diarrhoea (autonomic neuropathy— rare; diagnosis of exclusion
but may cause nocturnal faecal incontinence).
•
VIPoma (veryrare).
Investigations
Non- invasivetests
• Stool cultures (ova cysts, parasites). Note:Clostridium dicile— relatively
common in patients who have received recent antibiotic therapy.
•
FBC (anaemia, especially iron deciency).
• CRP.
• ESR.
• U&E.
• TFTs.
Imaging
•
Pelvic/ abdominalX- ray.
• Bariumenema.
• CT abdomen.
Procedures
•
Colonoscopy.
• Sigmoidoscopy ± biopsy.
E OHCM 10e, p.58.

INCONTINENCE:URINARY
61
Incontinence:urinary
E Anuria,p. 14.
Consider
• Common causes of polyuria (E OHCM 10e, Chapter7); these may
present as, or aggravate, urinary incontinence.
•
Acute or chronic confusional state (common; loss of voluntary sphincter
control).
•
UTI (very common— always exclude).
• Drug- induced, e.g. thiazide or loop diuretics; α- adrenergic blockade, e.g.
doxazosin (uncommon).
•
Psychological, e.g. severe depression.
• Immobility, e.g. PD (Shy– Drager syndrome is uncommon).
• Other causes of autonomic neuropathy (E OHCM 10e, Chapter10).
•
Detrusor muscle instability.
• Urethral incompetence.
• Stool impaction.
• Spinal cord compression.
• Tabes dorsalis.
Investigations
• U&E.
• Urinalysis for blood, protein, glucose, nitrates, and nitrites.
• MSU for culture and sensitivity(C&S).
• Plasma glucose (if glycosuria).
• SerumCa
2+
.
In selected patients, consider referral to urology or gynaecology services
for considerationof:
•
Bladder manometry studies.
• Post- voiding USS of the bladder.
• Pelvic imaging, e.g. CTscan.
E OHCM 10e, pp. 648–9.

62
CHAPTER1 Symptoms andsigns
62
Indigestion
This term is often loosely used by patients to describe a variety of symp-
toms. These are often regarded as representing relatively minor, and usually
intermittent, pathology. However, serious pathology, e.g. carcinoma of the
stomach, may present as a vague complaint of ‘indigestion’. The symptoms
may be retrosternal or abdominal. Adetailed history is essential, focusing
on features that raise the probability of serious pathology, e.g. dysphagia
and weightloss.
Examination
Examination should include a search for the following signs, particularly in
the middle- aged and elderly patients:
• Anaemia (especially iron deciency— common).
• Ascites.
• Troisier’s sign (malignant involvement of the left supraclavicular lymph
nodes due to carcinoma of the stomach— rare).
Note:the presence of associated pathologies, e.g. pernicious anaemia (E
OHCM
10e, Chapter8)— i risk of stomach cancer— will alter the threshold
for more detailed expert investigation. Carcinoma of the stomach is com-
moner in Japanese.
Peptic ulceration may have classic elements that point to the diagnosis.
Non- ulcer dyspepsia is very common and is often treated empirically with
antacids, H
2
receptor antagonists, or H
+
pump inhibitor drugs. The clinical
challenge is to identify the patient for whom more detailed, and often inva-
sive, investigation is indicated.
Alternative causes, e.g. cardiac ischaemia, should be considered in the
dierential diagnosis; similarities of the symptoms between cardiac and
upper GI disorders are well recognized and sometimes pose considerable
diagnostic diculties.
Causes include
• Oesophageal acid reux.
• Hiatus hernia.
• Inammatory disease.
• Peptic ulcer disease of the duodenum or stomach.
• Biliary colic (usually distinctive clinical features).
• Malignancy of the oesophagus, stomach, or rarely small intestine.
• Cardiac symptoms, usually ischaemia.
• IBS.
• Symptoms arising from other structures within the chest or abdomen.
Investigations
• FBC.
• U&E.
• ESR.
• Upper GI endoscopy ± tissue biopsy.
• LFTs.
• CK if MI/ acute coronary syndrome (ACS) suspected.
• Troponin (T or I) if MI/ ACS suspected.

INDIGESTION
63
• Serum amylase (normal in chronic pancreatitis; may be i by a duodenal
ulcer eroding the posteriorwall).
•
Barium swallow and meal (for oesophageal disease).
• CLO test for Helicobacter pylori.
•
Urea
13
C breath test for H.pylori.
•
USS of the biliary tract (E Ultrasound, p. 802).
•
Cholecystogram.
If diagnosis remains uncertain, consider
• CT abdomen (discuss with a radiologist).
• Serum gastrin (Zollinger– Ellison syndrome, E OHCM 10e, p.716).
•
24h ambulatory oesophageal pH monitoring.
• Oesophageal manometry (oesophageal motility disorders).

64
CHAPTER1 Symptoms andsigns
64
Infective endocarditissigns
Infective endocarditis is characterized by infection of the endocardial sur-
face of the heart. The left heart valves are the most commonly aected,
but the right heart valves and congenital heart lesions, such as VSDs, may
also become infected. Vegetations (composed of the organism, white cells,
platelets, and brous tissue) are formed at the site of infection. They give
rise to periodic septicaemia and may embolize to other parts of the body.
There is gradual destruction of the valve with i valvular dysfunction, regur-
gitation, and heart failure.
Clinical features
• Pyrexia (low- grade or swinging).
• Pale conjunctivae suggestive of anaemia (of chronic disease).
• Clubbing (chronic low- grade infection).
• Cardiac murmur (new or changing).
• Left or right heart failure.
• Splenomegaly (friction rub if splenic infarction is present).
• Microscopic haematuria (on urinalysis).
Embolic phenomena
Embolic phenomena are common and produce clinical signs classically asso-
ciated with infective endocarditis:
•
Splinter haemorrhages (>5, sited in the proximal nger and
toenailbeds).
•
Janeway lesions (palmar macular spots).
• Osler’s nodes (painful nodules on the palmar surface of the ngers
ortoes).
•
Roth spots (retinal haemorrhages).
• Conjunctival haemorrhages.
• Microvascular infarction (in the distal limbs).
Further reading
Prendergast BD. Diagnostic criteria and problems in infective endocarditis. Heart 2004; 90:611– 13.
Task Force on Infective Endocarditis of the ESC. Guidelines on prevention, diagnosis and treatment
of infective endocarditis. Eur Heart J 2004; 25:267– 76.

IRREGULARPULSE
65
Irregularpulse
In health, the pulse is usually regular, although a minor degree of variation
in heart rate with respiration (sinus arrhythmia) is common, particularly in
children and young adults. In sinus arrhythmia, the heart rate i with inspira-
tion and d with expiration. This is a benign phenomenon.
An irregular pulse can present as a symptom (with the patient complain-
ing of an awareness of irregular or ‘missed’/ ‘extra’ heartbeats) or as a sign
(incidental nding on clinical examination).
Pulse irregularities are traditionally classied
intotwogroups
• Regular irregularities.
• Irregular irregularities.
A regularly irregularpulse
Most commonly the result of ventricular or supraventricular ectopic activ-
ity. Ectopic beats often occur after a certain number of sinus beats— thus
in ventricular bigeminy, every other beat will be a ventricular ectopic beat
and thus occur prematurely with reduced volume. In trigeminy, every third
beat will beearly.
A regularly irregularpulse
Can also be evident in second- degree AV block (Mobitz type IorII).
An irregularly irregular pulse is most commonly theresultof
•
Multiple ectopic beats (supraventricular or ventricular).
• AF.
• Atrial utter with variable AVblock.
Investigations
The key to diagnosis is to record an ECG, whilst the pulse irregularity is
present. If the paroxysmal irregularity is infrequent, this can prove challeng-
ing. A12- lead ECG is mandatory and may provide an immediate diagnosis.
If not, a number of ambulatory ECG monitoring techniques are available:
• 24h ambulatory ECG monitoring.
• Cardiac event monitoring.
• Implantable loop recorder(ILR).
The choice of technique should be guided by how frequently the irregularity
is thought tooccur.
Additional investigations depend upon the nature of the suspected
arrhythmia:
•
FBC.
• U&E.
• TFTs.
One may also consider
•
CXR (to assess heart size and valvular calcication).
• Echocardiogram (if structural heart disease suspected).
• Exercise treadmill test (if IHD suspected, or to provoke arrhythmias
thought to be exercise- related).

66
CHAPTER1 Symptoms andsigns
66
Jaundice
This denes the yellow discoloration of the sclerae, mucous membranes,
and skin that occurs when bilirubin accumulates. Bilirubin is the major bile
pigment in humans and is produced as an end- product of haem catabolism.
Jaundice usually only becomes noticeable when the serum bilirubin level
is>30– 60µmol/ L.
Causes
(See Table1.10.)
•
Can be pre- hepatic, hepatic, or post- hepatic.
• Haemolysis.
• Hepatitis (viral, drugs, alcohol).
• Pregnancy.
• Recurrent cholestasis.
• Hepatic inltration.
• Stones in the common bileduct.
• Carcinoma of the bile duct, head of the pancreas, or ampulla.
• Biliary strictures.
• Sclerosing cholangitis.
• Pancreatitis.
Investigations
(See Fig.1.2.)
•
FBC (? haemolysis).
• Clotting screen (often deranged in liver disease).
• LFTs.
• Viral serology for hepatitis Avirus (HAV), hepatitis B virus (HBV), and
hepatitis C virus(HCV).
•
USS abdomen.
• Consider endoscopic retrograde cholangiopancreatography (ERCP).
• Liver biopsy may be indicated, depending on history, examination, and
laboratory ndings. Discuss with the gastroenterology team before
embarking onthis.
E OHCM 10e, pp. 272–3.
Table1.10 Common causes ofjaundice
Cholestatic
Pre- hepatic Intra- hepatic Post- hepatic
Haemolysis
Gilbert’s syndrome
Crigler– Najjar syndrome
Dubin– Johnson syndrome
Viral hepatitis
Drugs
Alcoholic hepatitis
Cirrhosis (anytype)
Pregnancy
Recurrent idiopathic
cholestasis
Inltration (e.g.
amyloidosis, etc.)
Gallstones
Carcinoma (biliary tree,
head of pancreas, ampulla)
Biliary stricture
Sclerosing cholangitis
Pancreatic pseudocyst
JAUNDICE
67
Non-dilated ducts
Dilated ducts
MRCP/ERCP
Conjugated Unconjugated
Hepatocellular Cholestatic
Investigate pre-
hepatic cause
Blood tests ±
liver biopsy
Ultrasound
liver/bile ducts
iBilirubin
Fig.1.2 Investigation of jaundice.

68
CHAPTER1 Symptoms andsigns
68
Joint pain/ swelling
Covers a multitude ofdisorders including
• OA.
• RA.
• Tendinitis.
• Bursitis.
• Trigger nger.
• Mechanical low backpain.
• Fibromyalgia.
• Other arthropathies.
History and examination
• Ask about aected joints, site of origin, mono- or polyarticular, oligo-
articular (e.g. 2– 4 joints involved), migratory features, arthralgia (joint
pain without swelling).
•
Is the pain constant or intermittent?
• Aggravating or precipitating factors?
• Any associated neurological features?
• Is there swelling?
• Associated redness or excessive warmth?
• Drug history (e.g. diuretic- induced).
• Race (e.g. sickle).
• Past history.
• Family history.
• Occupational history.
• Social history.
Investigations
• FBC— a normochromic normocytic anaemia is common in chronic
inammatory disorders. May be microcytic if long- standing inammation
or associated iron deciency (e.g. induced by NSAIDs).
•
ESR— non- specic marker of inammation.
• CRP— as forESR.
• Biochemistry screen, especially looking at bone prole andLFTs.
• Consider serum Igs and protein electrophoresis (myeloma).
• Uric acid levels (gout).
• X- ray aected joint(s).
• Consider USS, especially if soft tissue swelling.
• MRI can be useful to help visualize intra- articular structures.
• CTscan.
• Bone scintigraphy (helps identify abnormal bone turnover).
• Dual X- ray absorptiometry (DEXA) scan (useful for diagnosis and
monitoring of osteoporosis).
•
Arthroscopy may help in selectedcases.
• Joint aspiration (allows culture and examination of uid for crystals).

JUGULAR VENOUSPULSE
69
Jugular venouspulse
The height and waveform of the internal jugular venous pulse ( JVP) reect
right atrial pressure and haemodynamics. The JVP should be inspected
with the patient positioned at 45° to the horizontal. The JVP may be distin-
guished from the carotid pulse by the following features:
•
Pulsation is not palpable.
• It may be compressed and obliterated by pressure.
• It rises on compression of the right upper quadrant (hepatojugular
reux).
•
It varies with posture.
• Height d with inspiration.
The height of the JVP is measured as the vertical distance between the
manubriosternal angle and the top of the venous pulsation. Elevation is
dened as>3cm.
There are several components of the jugular venous pulsation waveform.
The a wave is produced by atrial systole. This is followed by the x descent
at the end of atrial contraction. The x descent is interrupted by a small,
barely perceptible deection called the c wave. This deection is caused
by the rapid i in right ventricular pressure just before the tricuspid valve
closes. Asubsequent v wave results from the rise in right atrial pressure as
it lls with venous return during ventricular systole and whilst the tricuspid
valve remains closed. At the end of ventricular systole, the tricuspid valve
opens and the pressure in the right atrium falls, leading to the y descent
(see Table1.11).
70
CHAPTER1 Symptoms andsigns
70
Table 1.11 Jugular venous pulse waveforms
Description DiagramDiagnosis Comment
Normal
ac
x
v
y
ac
x
v
y
i
v
y
a
c
x
v
y
a
x
cv
y
a
v
x
v
y
a
i
Normal
i JVP
Normal
waveform
Right heart
failure
Fluid overload
Pulmonary
embolus
Cardiac
tamponade
i right atrial
pressure
Absent a
wave
Atrial
brillation
Poor atrial
contraction fails to
generate a waves
Large a
waves
Tricuspid
stenosis
Right
ventricular
hypertrophy
Resistance
to right atrial
emptying causes
i right atrial
pressure
Large ν
waves
Tricuspid
regurgitation
Re ux of blood
into the great
veins with right
ventricular
contraction
Cannon (a)
waves
Complete
heart block
Ventricular
tachycardia
Right atrium
contracts against
closed tricuspid
valves, creating a
cannon wave
Rapid y
descent
Constrictive
pericarditis
Cardiac
tamponade
Tricuspid
regurgitation
Right heart
failure
A steep y descent
is caused by right
ventricular
diastolic collapse
(Freidrich’s sign)
Absent
pulsation
i JVP
Superior
vena caval
obstruction
No right atrial
pressure can be
transmitted to
the JVP

LOINPAIN
71
Loinpain
Denition
Pain located in the renalangle.
Causes
• Uretericcolic.
• Renal or ureteric obstruction.
• Acute pyelonephritis.
• Renal infarction or papillary necrosis.
• Acute nephritis (uncommon).
• IgA nephropathy— pain caused by extension of the renal capsule.
• Musculoskeletal causes.
• Shingles at T10– 12 (obvious if a rash is seen on examination or
suspected if pain is in a dermatomal distribution).
•
Infection or bleeding into a cyst in polycystic kidneys.
• Vesico- ureteric reux— pain occurs when the bladder is full; this
worsens at the initiation of micturition and then is rapidly relieved on
voiding.
•
Loin pain- haematuria syndrome— this is recurrent pain which occurs in
young women. Angiography reveals tortuous vessels.
Investigations
• U&E.
• Serum creatinine.
• Creatinine clearance (CrC) (if renal impairment).
• FBC.
• ESR.
• Urine dipstick for protein, blood, nitrites, leucocytes.
• Urine microscopy (for casts).
• MSU for C&S testing.
• Blood cultures (if bacteraemia suspected).
• Plain X- ray (KUBview).
• IVU (e.g. if +ve urine dipstick for haematuria).
• Renal USS (useful for rapid, non- invasive exclusion of obstruction).
• CT of urinarytract.
• Angiogram (if suspicion of thrombus, embolus, or loin pain- haematuria
syndrome).
•
Serum IgA concentration.
• Cystoscopy (specialist procedure).
• Retrograde pyelography.
• Renal biopsy (only after specialist advice).

72
CHAPTER1 Symptoms andsigns
72
Lymphadenopathy
Lymph node enlargement may be localized or generalized (see Table1.12).
Localized cervical lymphadenopathy
• Local causes in the mouth (pharyngitis, dental abscess).
• Scalp (skin malignancies or disease).
• Nose (nasopharyngeal carcinoma).
Enlargement ofleft supraclavicularnodes
• May suggest carcinoma of the stomach.
Isolated posterior cervical node enlargement
• Is less often due to malignancy.
Othercauses
• Sometimes drugs may be associated with lymph node enlargement
(phenytoin, antithyroid).
Table1.12 Causes oflymphadenopathy
Infection
Viral
Infectious hepatitis, EBV syndromes, HIV, rubella, varicella,
herpes zoster
Bacterial Streptococcal, staphylococcal, salmonella, brucellosis,
Listeria
, cat- scratch (Bartonella)
Fungal Histoplasmosis, coccidioidomycosis
Chlamydial
Mycobacterial
Parasites Trypanosomiasis, microlaria, toxoplasmosis
Spirochaetes Syphilis, yaws, leptospirosis
Connective tissue
RA, SLE, dermatomyositis, serum sickness
Drugs e.g. phenytoin
Malignancy
Haematological
Hodgkin’s lymphoma, non- Hodgkin’s lymphoma, acute
and chronic lymphoid malignancies (chronic lymphocytic/
lymphatic leukaema (CLL), acute lymphoblastic leukaemia
(ALL)), acute myeloid leukaemia (AML)
Non- haematological Metastases from carcinomas (breast, bowel, lung,
prostate, kidney, head and neck)
Endocrine Thyrotoxicosis
Miscellaneous Sarcoidosis, amyloidosis

LYMPHADENOPATHY
73
Investigations
• FBC, blood lm, LDH (leukaemia, lymphoma, Hodgkin’s).
• Serology/ virology/ microbiology/ other antigen detectiontests:
•
Viral (EBV, hepatitis, CMV,HIV).
•
Bacterial (TB, bacterial endocarditis, syphilis).
•
Fungal (histoplasmosis).
•
Protozoal (toxoplasmosis).
• ANA (collagen disorder, systemic lupus).
• TFTs (hyperthyroidism).
• CXR (sarcoid,TB).
• USS/ CT scan (to assess intra- abdominal, mediastinal/ hilar
lymphadenopathy).
•
LFTs/ hepatomegaly (i ALP suggests malignant deposits).
•
Lymph node biopsy (groin nodes should usually be avoided because
commonly enlarged due to skin and infectious disorders).
•
BM (may conrm haematological malignancy).
Note:FNA, although easier to perform, may not be diagnostic and lymph
node biopsy should be considered for microbiology and histology.
E OHCM 10e, p.35, p. 594.
Although we have provided a large list of possibilities, common sense
should be used in determining the cause. For example, an 80- year- old
woman with axillary lymphadenopathy is unlikely to have cat- scratch dis-
ease! Common things are common.

74
CHAPTER1 Symptoms andsigns
74
Nausea
The so- called vomiting centre is located in the medulla oblongata and is
stimulated by the chemoreceptor trigger zone in the fourth ventricle. There
are many causes of acute and chronic nausea. These can be divided into GI
and non- GI causes.
GI causes ofnausea
• Food poisoning (viral, bacterial— common).
• Acute and chronic gastritis (remember H.pylori).
•
Peptic ulceration.
• Biliary and renalcolic.
• IBD.
• Cholecystitis.
• Appendicitis.
• Pancreatitis.
• Gastric outow obstruction.
• Post- gastrectomy syndrome.
• Acute liver failure.
• Pseudo- obstruction of thebowel.
Investigations
•
U&E.
• LFTs.
• ESR.
• CRP.
• Serum or urinary amylase.
• AXR (erect and supine— beware perforated viscus).
•
AbdominalUSS.
Consider:
•
OGD.
• Barium swallow andmeal.
• Isotopic gastric emptying studies.
• Oesophageal manometry.
• Oesophageal muscle biopsy (rarely indicated).
Non- GIcauses
• Acute infections, e.g.UTI.
• Metabolic disorders, including:
•
Hypercalcaemia.
•
Ketoacidosis (diabetic, alcoholic).
•
Uraemia.
• Pregnancy. Note:hyperemesis gravidarum may be associated with i free
T4 (FT4), d
thyroid- stimulating hormone(TSH).
• Many drugs, notably opiates and digoxin toxicity (check serum levels).
• MI (nausea common; exacerbated by opiates).
• Acute glaucoma.

NAUSEA
75
Investigations
•
FBC.
• ESR.
• Venous plasma glucose.
• Urine dipstick(UTI).
• SerumCa
2+
.
•
Serum drug levels, e.g. digoxin, theophylline.
• 12- leadECG.
• CK.
• TroponinI.
Neurologicalcauses
• Acute migraine.
• iICP.
•
Acute labyrinthine lesions.
• Ménière’s disease.
• Cerebellar lesions (e.g. infarct, haemorrhage, metastases,
demyelination).
Investigations
•
CranialCT.
• MRI if cerebellar lesion suspected.
• Tilt table test (E Tilt table testing, pp. 494–5).
•
Audiometry (specialist technique).
E OHCM 10e, p.70. Nystagmus.

76
CHAPTER1 Symptoms andsigns
76
Neck stiness
The main concern in a patient with neck stiness is that s/ he may have
meningitis which may result from infection or may reect inltration by a
disease such as acute leukaemia.
Causes
• Bacterial infection.
• Viral infection.
• Fungal infection.
• TB.
• Inltration by malignancy (e.g. acute lymphoblastic leukaemia (ALL),
high- grade lymphoma, or sometimes acute myeloid leukaemia (AML)).
• Drug- induced.
• Contrast media (myelogram).
• Blood (e.g. post- SAH).
• Mechanical/ trauma.
• Connective tissue disease, e.g.RhA.
Investigations
• CT scan of brain ± contrast.
• LP if noiICP:
•
Glucose.
•
Protein.
•
M,C&S ± TB culture.
•
Xanthochromia if SAH suspected.
• If patient immunocompromised, consider:
•
Polymerase chain reaction (PCR) for viruses, e.g. herpes simplex
virus(HSV).
•
Toxoplasma serology.
•
India ink stain for Cryptococcus.
•
If considering malignancy, send cerebrospinal uid (CSF) for cytospin.
E OHCM 10e, p.478.

NYSTAGMUS
77
Nystagmus
An involuntary oscillatory or (more commonly) rapid jerking movement
of the eyes that is rhythmic and repetitive. It results from acute or chronic
lesions of the eight cranial nerves, brainstem, or cerebellum. The ‘slow’
phase is pathological, the rapid, rhythmic jerking phase (used arbitrarily to
dene the direction of nystagmus) being a corrective response. Nystagmus
‘to the right’ describes the direction of the quick phase. Such ‘sawtooth’
nystagmus may be evident in the horizontal or vertical plane (includ-
ing ‘downbeat’ nystagmus of foramen magnum lesions) or as oscillations
around a central point (e.g. in albinism).
Jerk nystagmus
Jerk nystagmus may be graded in severity, depending on whether:
•
It occurs only in the direction of directedgaze.
• It occurs when eyes are in the midline,or
•
It is present even on looking in a direction contralateral to the rapid
movement.
Note:nystagmus (or, more correctly, nystagmoid jerks) may be induced by
inappropriate testing, often being present at the extremes of gaze. Do not
ask the patient to follow a visual target beyond 730° of the midline when
testing at the bedside.
In unilateralcauses
Cerebellar nystagmus
Greatest when gaze directed towards the side of the destructive lesion.
Vestibular nystagmus
Greatest away from the side of the lesion.
Pathological nystagmus
May be due to labyrinthine and vestibular lesions— occurs in one direction
only. If visual xation is removed, nystagmus becomesworse.
Central lesions
Including brainstem lesions caused by, e.g. tumour, MS; cerebellar lesions or
medial longitudinal fasciculus lesions leading to internuclear ophthalmople-
gia (E OHCM 10e, Chapter10) with ataxic nystagmus.
Investigations
• Positional nystagmus may be investigated by using the Hallpike
manoeuvre (E OHCM 10e, Chapter10). Abrupt alteration of the
spatial position of the head (from supine, with the head below the bed,
rapidly to a sitting position) will induce nystagmus. This will demonstrate
benign positional vertigo (common), vestibular disorders, or brainstem
lesions.
•
Audiometry (specialized investigation).
• Auditory and visual evoked potentials (VEPs) may be pathologically
reduced in MS. Examination of CSF may reveal oligoclonal bands
(OCBs).

78
CHAPTER1 Symptoms andsigns
78
• MRI to include the brainstem. (Upbeat nystagmus will suggest a midbrain
lesion and downbeat nystagmus will suggest a foramen magnum lesion.)
MRI is superior to CT for demonstrating cerebellopontine angle lesions.
Gadolinium enhancement is used to investigate acoustic neuromas.
•
Ototoxicity can be caused by some drugs such as gentamicin and
phenytoin. Acute poisoning with alcohol or barbiturates may cause
transient nystagmus. Chronic alcoholism can lead to permanent
cerebellar damage. Excessive doses of anticonvulsant drugs, e.g.
phenytoin, are a common cause— measure serum concentrations of
thedrug.
E OHCM 10e,p.70.

OBESITY
79
Obesity
The World Health Organization (WHO) denes obesity as a body mass
index (BMI) >30kg/ m
2
(Table1.13).
Note
:central (abdominal) fat distribution— commoner in men— is associ-
ated with greater health risks. The waist- to- hip ratio, or simply the waist
girth, can be used to identify levels at which long- term health risks warrant
intervention (see Box1.2):
Aetiology
The great majority of obese subjects have no identiable metabolic or
hormonal defects and detailed investigation is rarely indicated. A chronic
imbalance of the equation with energy intake (dietary calories) on the one
hand and expenditure (resting metabolic rate + physical activity) on the
other is thought to be responsible. Reduced levels of habitual activity allied
to an abundance of energy- dense foods appears to account for the current
pandemic of obesity and related disorders:
•
Impaired glucose regulation.
• Type 2 diabetes mellitus(DM).
• Dyslipidaemia.
• Hypertension.
• CVD.
• OA.
• Impaired physical functioning.
• Gout.
• i surgicalrisk.
•
Depression.
• Certain cancers, e.g. bowel, breast.
Weight gain tends to occur in middle age; ♀ are more at risk than ♂. Socio-
economic factors are also important.
Table1.13 BMI
BMI (kg/ m2)
Underweight <18.5
Normal
18.5– 24.9
Overweight >25.0– 29.9
Obesity Class I 30.0– 34.9
II 35.0– 39.3
III >40
Box 1.2 WHO BMI gradingsystem
Men
Women
>102cm
>88cm

80
CHAPTER1 Symptoms andsigns
80
Speciccauses
Genetic
•
For example, Prader– Willi syndrome, Laurence– Moon (Biedl– Bardet)
syndrome.
Single gene defects
•
For example, mutations of leptin (provides feedback from adipocytes to
the hypothalamus about body fat stores) or its hypothalamic receptor
(veryrare).
Hypothalamic lesions
•
Lesions which damage the ventromedial nucleus (the ‘satiety’ area) may
lead to obesity.
Lesions include
•
Trauma.
• Tumours— craniopharyngiomas and astrocytomas.
• Inammation— such as TB and meningitis.
• Inltration— histiocytosis and sarcoidosis.
Cushing’s syndrome
•
With ‘bualo’ hump and central obesity.
Hypothyroidism
•
Disputed, unless severe myxoedema, but hyperthyroidism is associated
with unphysiological weightloss.
Insulinoma
•
Often associated with moderate weight gain;rare.
Marked decreased motor inactivity
•
For example, severe mental retardation or physical disability.
Investigations
• Weight (calibrated scales).
• Height (stadiometer).
• Waist circumference (maximal).
• BP (large cu required).
• Venous plasma glucose (or oral glucose tolerance test (OGTT)).
• TFTs.
• LFTs (i non- alcoholic steatohepatitis in obese subjects).
• Fasting lipid prole (E Investigation of hyperlipidaemia, pp. 212–15).
•
Serumurate.
Additional investigations
These may occasionally be indicated if clinical features give cause for suspi-
cion of an organiccause:
•
Cranial CT or MRI of the pituitary and hypothalamus.
• Investigations for Cushing’s syndrome (E Obesity/ hypercortisolism,
pp. 142–6).
•
Genetic testing (seek advice of the genetics service).
Further reading
Lean MEJ, Han TS, Seidell JC. Impairment of health and quality of life in men and women with a larger
waist. Lancet 1998; 351:853– 6.

OLIGURIA
81
Oliguria
Causes
Acute renal failure (ARF)— distinguish pre- renal from renal and post- renal
causes.
Pre- renal
• Severe sepsis.
• Hypovolaemia, e.g. GI haemorrhage, diuretics.
• Burn injury.
• CCF.
• Addison’s disease.
• Acute pancreatitis.
Renal
• ATN (e.g. 2° to nephrotoxins such as aminoglycosides and radiological
contrast media).
•
Acute cortical necrosis.
• Renal infarction.
• Accelerated hypertension.
• Salicylate overdose.
• Hepatorenal syndrome.
Post- renal
• Renal calculi.
• Retroperitoneal calcinosis.
• Papillary necrosis.
• Bladder, prostate, and cervical tumours.
• Blocked urinary catheter (common!).
Investigations
• U&E.
• Serum creatinine.
• CrC.
• FBC.
• ESR.
• Autoimmune prole.
• LFTs.
• Urinary Na
+
excretion (<20 pre- renal, >40ATN).
• Urine osmolality (>500mOsmol/ L=pre- renal, <350mOsmol/
L=ATN).
•
Urine dipstick for blood, protein, nitrites, and leucocytes.
• Urine microscopy forcasts.
• Renal USS (± biopsy in selected cases).
• IVU.
• CT pelvis.
• Investigation of renal stones:
•
Serum Ca
2+
, phosphorus.
•
24h excretion of oxalate, calcium, creatinine.
E OHCM 10e, p.81, p.293, p.576.

82
CHAPTER1 Symptoms andsigns
82
Palpitations
Patients generally use the term palpitations to refer to an awareness of
an abnormally fast, forceful, or irregular heart rhythm. Palpitations can be
physiological, as in the fast and/ or forceful heart rhythm felt with exercise
or anxiety, or pathological.
Common arrhythmias
Supraventricular arrhythmias
•
Sinus tachycardia (E Causes of sinus tachycardia, pp. 110–11).
•
AF.
• Atrial utter.
• Atrial tachycardia.
• AV re- entry tachycardias.
• Supraventricular ectopics.
Ventricular arrhythmias
•
Ventricular tachycardia.
• Torsades de pointes.
• Ventricular ectopics.
Investigations
The key to diagnosis is to record a 12- lead ECG, whilst palpitations are pre-
sent. Although simple in principle, infrequent paroxysmal palpitations can
make this very challenging. A12- lead ECG is mandatory and may provide
an immediate diagnosis if the patient is experiencing palpitations as it is per-
formed. As well as assessing the heart rhythm, it is important to inspect the
12- lead ECG for evidence of abnormal AV conduction (short PR interval,
pre- excitation) or abnormal repolarization (long QT interval). Check also
for evidence of an underlying structural heart disease, e.g. pathological Q
waves indicative of a previousMI.
If the patient’s palpitations are paroxysmal, a number of ambulatory ECG
monitoring techniques are available:
•
24h ambulatory ECG monitoring.
• Cardiac event monitoring.
• ILR.
The choice of technique should be guided by how frequently the
palpitationsoccur.
Additional investigations depend upon the nature of the suspected
arrhythmia. It is generally prudent tocheck:
•
FBC.
• U&E.
• TFTs.
One may also consider:
•
CXR (to assess heart size and valvular calcication).
• Echocardiogram (if structural heart disease suspected).
• Exercise treadmill test (if IHD suspected, or to provoke arrhythmias
thought to be exercise- related).
E OHCM 10e,pp. 36–7, p. 94.

PANCYTOPENIA
83
Pancytopenia
Pancytopenia (d Hb, d WBC, and d platelets) may occur because of bone
marrow failure (hypoplasia) or inecient production (myelodysplastic syn-
drome (MDS)) or peripheral destruction of cells or sequestration (spleno-
megaly/ hypersplenism).
3 Pancytopenia usually means something is seriouslywrong.
Bone marrow assessment is necessary to establish whether the mar-
row is hypocellular or hypercellular in the face of peripheral blood pan-
cytopenia. If hypercellular, the cause may be an inltrative process (due
to leukaemia/ carcinoma, granulomatous disease, brosis– myelobrosis,
osteosclerotic– osteopetrosis, increased macrophages– haemophagocytic
syndromes due to viral infections). Causes of hypoplastic bone marrow
failure may be hereditary (e.g. Fanconi’s anaemia) or acquired (e.g. drugs).
Critically ill patients may develop pancytopenia for multiple reasons (sepsis,
haemorrhage,DIC).
Investigations
• FBC, lm (aplastic anaemia usually presents with d lymphocyte count,
but minor morphological changes).
•
Reticulocytes (d if production failure).
•
Serum vitamin B
12
, folate (megaloblastic anaemia can be associated with
pancytopenia).
•
Serology for EBV, hepatitis A, B, and C, HIV (associated with aplastic
anaemia).
•
Serology for parvovirus infection (if pure red cell aplasia, also consider
lymphoma, thymoma).
•
ANA (lupus).
• Neutrophil alkaline phosphatase (NAP) score (i in aplastic anaemia).
•
Check for lymphadenopathy, hepatomegaly, and splenomegaly.
• CXR (bronchial carcinoma, sarcoid, TB, lymphoma).
• USS/ CT to assess lymphadenopathy/ splenomegaly (pancytopenia may
be due to hypersplenism and portal hypertension).
•
Ham’s test for paroxysmal nocturnal haemoglobinuria (PNH) or cell
marker analysis of CD55 andCD59.
•
Bone marrow (BM) aspirate and cytogenetics (myelodysplasia is a clonal
disorder).
E OHCM 10e, p.364.

84
CHAPTER1 Symptoms andsigns
84
Paraesthesiae
This may be described by the patient as an abnormal sensation of aching,
pricking, tickling, or tingling commonly in the extremities or face. Often
described as feeling like ‘pins and needles’.
The selection of investigations will be determined largely by the history
(transient? chronic?), the surface anatomical site of the abnormal sensa-
tion, and associated symptoms or precipitating factors (e.g. clear history of
hyperventilation).
The common causes include the numbness or tingling associated with
pressure on the peripheral nerves, such as caused by sleeping awkwardly on
an arm (‘Saturday night palsy’ of the radial nerve), or chronic or recurrent
pressure, e.g. on the ulnar nerve at theelbow.
If paraesthesiae is persistent, consider the following conditions, depending
on the distribution of the symptoms:
•
Carpal tunnel syndrome (with radiation proximally along the forearm;
worse at night).
•
Peripheral neuropathy (DM, alcohol, drug- induced; E OHCM 10e,
Chapter10).
•
Sciatica (reduced straight leg raising).
• Meralgia paraesthetica (lateral cutaneous nerve of the thigh).
• Lateral popliteal palsy (common peroneal nerve).
Other less common causes
Peripheral neuropathydueto
•
DM.
• Vitamin B
1
or B
12
deciencies.
•
Chronic renal failure.
• Chronic hepatic failure.
• Malignancy.
• Neurotoxicdrugs:
•
Vinca alkaloids.
•
Metronidazole.
•
Nitrofurantoin.
•
Isoniazid (pyridoxine- dependent).
• Environmental toxins.
• Hypothyroidism.
• GBS (acute).
• Certain porphyrias.
• MS.
Acute hypocalcaemia causes a characteristic perioral paraesthesiae and
can be due to many causes, including ° and 2° hypoparathyroidism and
alkalosis.

PARAESTHESIAE
85
General investigations
• ABGs (acute or chronic acid– base disturbances leading to alterations in
ionizedCa
2+
).
•
Serum Ca
2+
(not all laboratories measure ionizedCa
2+
).
•
Serum parathyroid hormone (PTH) (uncued sample).
• Serum Mg
2+
(see below).
•
Venous plasma glucose.
• Vitamin B
12
(and other investigations in suspected chronic peripheral
neuropathy).
If serum calcium or magnesium concentrationislow
Identify thecause:
•
Chronic GI loss (stula, excessive diarrhoea, bowel obstruction).
• Chronic renal loss (diuretic drugs, intrinsic renal disease).
• DKA— total body Mg
2+
may be low, but this very rarely causes
symptoms.
Additional investigations
•
UrinaryMg
2+
.
Consider
•
USS abdomen/ renal tract and subsequent GI investigations.
• U&E.
• Nerve conduction studies(NCS).
• TFTs.
• IGF- 1, growth hormone (GH) response during 75g- OGTT (if features
of acromegaly present; E Acromegaly (growth hormone excess),
p. 132).

86
CHAPTER1 Symptoms andsigns
86
Peripheral neuropathy
The patient will complain of numbness in hands and feet that progresses
proximally in a distribution classically termed ‘glove and stocking’. Dierent
aetiologies lead to a motor, sensory, or mixed sensorimotor picture.
Commoncauses
• Idiopathic (50%, commonest).
• DM.
• Vitamin B
12
deciency (may occur in the absence of anaemia).
•
Vitamin B deciency (e.g. alcoholics).
• Vitamin E deciency.
• Carcinomatous neuropathy.
• Drugs, e.g. isoniazid, vinca alkaloids, cisplatin, dapsone, gold,
metronidazole.
•
Paraproteinaemias (e.g. monoclonal gammopathy of undetermined
signicance (MGUS) or myeloma).
Rarercauses
• Amyloidosis.
• Uraemia.
• Collagen vascular diseases, e.g. rheumatoid, SLE, polyarteritis
nodosa(PAN).
•
Endocrine disease, e.g. myxoedema, acromegaly.
• GBS.
• Infections, e.g. tetanus, leprosy, diphtheria, botulism.
• Sarcoidosis.
• Hereditary, e.g. Charcot– Marie– Tooth disease.
• Acute intermittent porphyria.
• Toxins, e.g. lead (predominantly motor), arsenic (mixed sensory and
motor), mercury (sensory), and thallium (mixed sensory and motor).
•
Chronic inammatory demyelinating polyneuropathy.
• Hereditary motor and sensory neuropathy types IorII.
Investigations
• NCS to conrm the diagnosis.
Further investigations
• In order to determine the underlyingcause.
• Discuss with neurologysta.
E OHCM 10e, p.447.

PERIPHERALOEDEMA
87
Peripheraloedema
Swelling of the legs, or peripheral oedema, is a common presenting symp-
tom, which occurs when excess tissue uid is redistributed by gravity.
Severe oedema is usually pathological and swelling of the ankles may pro-
gress to ascites and even pleural and pericardial eusion.
Causes ofgeneralized swelling
• Cardiac failure:congestive heart failure, dilated cardiomyopathy,
constrictive pericarditis, cor pulmonale.
•
Hypoalbuminaemia:liver failure (hepatic cirrhosis), renal failure
(nephrotic syndrome), protein- losing enteropathy, malnutrition
(malabsorption or starvation).
Causes oflocalized swelling
• Immobility:common in old age, long- distance travel.
• Infection:cellulitis.
• DVT and/ or subsequent venous insuciency.
• Drugs:calcium channel blockers (nifedipine, amlodipine), NSAIDs.
• Malignancy:compression of deep vein, enlarged lymph nodes or
lymphatics.
•
Lymphatic obstruction:congenital, inltrative (lariasis).
• Milroy’s disease.
• Pregnancy.
• Wet beriberi.
• Idiopathic.
Patients atspecialrisk
• Pregnancy.
• Prolonged bedrest.
• Following removal of lower limb plastercast.
• Relative immobility:long- distance travel.
• CCF.
Investigations
These should be guided by the history and examination.
•
FBC.
• U&E.
• Albumin.
• ESR.
• Blood cultures.
• ECG.
• Echocardiography.
• Doppler studies of leg veins/ contrast venography (according to local
availability).
•
AbdominalUSS.
• Malignancy screen for common cancers.
• Urine dipstick for proteinuria.
• 24h urinary protein excretion or urine protein/ creatinineratio.
• Small bowel biopsy.
• Xylose breathtest.

88
CHAPTER1 Symptoms andsigns
88
Petechiae and thrombocytopenia
Spontaneous bleeding in the absence of trauma is uncommon with platelet
counts >20 × 10
9
/ L. However, bleeding is much more likely if thrombo-
cytopenia is not immune in origin (e.g. aplastic anaemia, acute leukaemia,
drug- induced, chemotherapy, myelodysplasia).
Thrombocytopenia may be inherited or acquired (e.g. DIC). As for pan-
cytopenia, these may be classied as due to a failure of production, or i
consumption in the peripheries (DIC, ITP), or abnormal tissue distribution
(splenomegaly).
ITP may be ° or 2° (e.g. lymphoma, lupus,HIV).
Drugs (e.g. heparin) and blood transfusion (post- transfusion purpura)
may cause severe thrombocytopenia.
Investigations
• FBC,lm:
•
Inherited causes may be associated with giant platelets.
•
Morphological abnormalities may suggestMDS.
•
Red cell fragments suggest thrombotic microangiopathies, e.g.TTP.
• LDH (i in thrombotic thrombocytopenic purpura (TTP) and
lymphoproliferative disorders).
•
Serum vitamin B
12
, folate (megaloblastic anaemia can be associated with
d platelets).
•
ANA, autoimmune screen, Igs (lupus, hyperthyroidism).
• Virology (HIV, EBV, viral hepatitis,CMV).
• Clotting screen(DIC).
• Lupus anticoagulant, cardiolipin antibodies (antiphospholipid antibody
syndromes).
•
Platelet serology for drug- or transfusion- related causes.
• BM assessment to establish whether thrombocytopenia is due to a BM
production problem or due to peripheral consumption (discuss with
the haematology team; depending on the degree of thrombocytopenia,
other haematological ndings, and the age of the patient, a marrow may
not be required).
Pitfalls
Thrombocytopenia due to HIV infection must be considered, especially
in all younger adults. Not worth checking platelet- associated IgG or IgM
since these are elevated in thrombocytopenia caused by immune and non-
immune mechanisms, so they add no useful information.

PLETHORA
89
Plethora
A plethoric appearance is typically seen in association with polycythaemia
but may also be mistaken for a normal outdoors complexion or cyanosis.
Patients with haematocrits above the normal reference range may or may
not have an i red cell mass (real or relative polycythaemia, respectively)
(see Table1.14).
Investigations
• FBC, lm (repeat FBC as sampling errors can falsely cause elevations
of Hb; polycythaemia rubra vera (PRV) may be associated with
neutrophilia, basophilia, or i platelets).
•
Measurement of red cell mass may be necessary to conrm true
polycythaemia.
•
Investigations are then aimed at establishing whether real polycythae-
mia, if documented, is due to a ° BM abnormality (PRV) or a 2°
disorder (e.g. respiratory disease).
•
NAP score (may be raised in PRV). Seldom used now (E Neutrophil
alkaline phosphatase, pp. 300–1).
•
Vitamin B
12
and urate (may be i inPRV).
•
ESR/ CRP (acute phase reactants may suggest 2° causes).
• Blood gas analysis, O
2
saturation, COHb levels (2° polycythaemia due
to respiratory disease, smoking).
•
Biochemistry (urea, creatinine; renal disease).
• Epo (i in 2° causes).
•
USS abdomen (renal cysts, liver disease, uterine broids, and other
malignancies may ‘inappropriately’ secrete Epo; also check for
splenomegaly inPRV).
•
Sleep studies (obstructive sleep apnoea (OSA), supine desaturation).
• O
2
dissociation studies (polycythaemia due to abnormal, high- anity Hb
variant).
•
BM aspirate and chromosomal studies/ cytogenetics (PRV is a clonal
disorder).
Table1.14 Polycythaemia
i red cell count >6.0 × 10
12
/ L
>5.5 × 10
12
/ L
♂
♀
i PCV >50%
>45%
♂
♀
i Hb
>18.0g/ dL
>16.0g/ dL
♂
♀

90
CHAPTER1 Symptoms andsigns
90
Polyuria
Polyuria (the passage of an excessive volume of urine, which may be asso-
ciated with frequency of micturition and nocturia) must be dierentiated
from urinary symptoms associated with prostatic disease and urinary infec-
tions. The latter are also characterized by frequency, urgency, and nocturia,
but usually small amounts of urine are passed at eachvoid.
Causes include
• DM.
• Cranial diabetes insipidus (DI) (E OHCM 10e, Chapter5):
•
Familial (autosomal dominant).
•
2° to posterior pituitary or hypothalamic disease, e.g. surgery,
tumours, especially metastases, neurosarcoidosis.
•
NephrogenicDI:
•
Familial (X- linked recessive).
•
Chronic intrinsic renal disease, e.g. pyelonephritis.
•
Hypokalaemia.
•
Hypercalcaemia.
•
Sickle- cell crisis.
•
Lithium, colchicine, amphotericin.
•
Post- obstructive uropathy.
• ° polydipsia (psychogenic).
Investigations
• 24h urinary volume.
• Venous plasma glucose.
• U&E.
• TFTs.
• LH.
• FSH (? panhypopituitarism).
• Serum Ca
2+
andPTH.
•
Sickle- celltest.
• CXR (? mediastinal lymphadenopathy in TB, sarcoidosis).
If no obvious cause found, consider detailed investigations for cranial or
nephrogenic DI (E Polydipsia and polyuria:diabetes insipidus, pp. 134–6).
E OHCM 10e,p.81, p. 293.

PRURITUS
91
Pruritus
Implies generalized itching and may be associated with many disorders,
including:
•
Iron deciency.
• Malignant disease, e.g. lymphoma.
• DM.
• Chronic renal failure.
• Liver disease, e.g.PBC.
• Thyroid disease.
• PRV.
• HIV infection.
Investigations
• Aim to exclude the above diseases.
• FBC.
• Biochemistry screen, including LFTs and renal function.
• Glucose.
• TFTs.
E OHCM 10e, p.28, p.535.

92
CHAPTER1 Symptoms andsigns
92
Ptosis
Ptosis can be unilateral and bilateral. Bilateral ptosis can be more dicult
to recognize. Ptosis must be considered in association with other signs and
symptoms. Ptosis may be long- standing, of recent onset, progressive, or
intermittent, especially at the end of the day— myasthenia gravis(MG).
Unilateralptosis
Causes
•
Constitutional (congenital).
• Oculomotor (III) nerve palsy— levator palpebrae. ‘Down and out’ pupil
with loss of light reex (e.g. DM, SOL, demyelination).
•
Aneurysm (basilar or posterior communicating arteries).
• Cavernous sinus disease.
• Meningitis.
• Horner’s syndrome— superior tarsal muscle (brainstem infarction,
syringobulbia, SOL,MS).
•
Encephalitis.
If abnormal (reduced) sweating onipsilateral side face (damage tocervical
sympatheticchain)
•
Pancoast’s tumour.
• Aortic arch aneurysm.
• Cervical injuries.
No disorder ofsweating
•
Cluster headache.
• Parasellar tumours.
• Carotid artery aneurysm or dissection.
• Nasopharyngeal tumours.
Investigations
•
Venous plasma glucose.
• CXR (Pancoast’s syndrome).
• Cranial CT orMRI.
• Cerebral angiography (aneurysm).
Bilateralptosis
Causes
•
GBS (Miller– Fisher syndrome).
• MD.
• MG.
• Neurosyphilis (bilateral; Argyll Robertson pupils).
Investigations
•
Syphilis serology.
• EMG (‘dive- bomber’ inMD).
• Serum anti- acetylcholine receptor antibodies (AChRAb)(MG).
• IV edrophonium (Tensilon
®
) test (MG; E Edrophonium (Tensilon
®
)
test, p. 631).
E OHCM 10e,p.73.

PULMONARY EMBOLISM
93
Pulmonary embolism
Occurs when a thrombus in systemic veins or the right side of the heart
embolizes into the pulmonary arterial system. Impaired gas exchange
occurs because of a mismatch between ventilation and perfusion.
Investigations
• FBC (may be leucocytosis, neutrophilia most likely).
• ESR (ofteni).
•
Plasma D- dimers:i with fresh thrombus.
•
ABGs:hypoxia and hypocapnia.
• ECG:look for AF. Usually sinus tachycardia, may be evidence of right
ventricular ‘strain’. In massive PE, there may be S
1
Q
3
T
3
.
•
CXR:often normal but may show signs of pulmonary infarction or
eusion.
•
V/ Q scan (may be useful for detection of areas of the lungs that are
being ventilated but not perfused).
•
Multislice CT scan:useful for detection of medium- sized PEs but does
not exclude smallPEs.
E OHCM 10e, p.98, pp. 190–1, p. 351, p. 818.
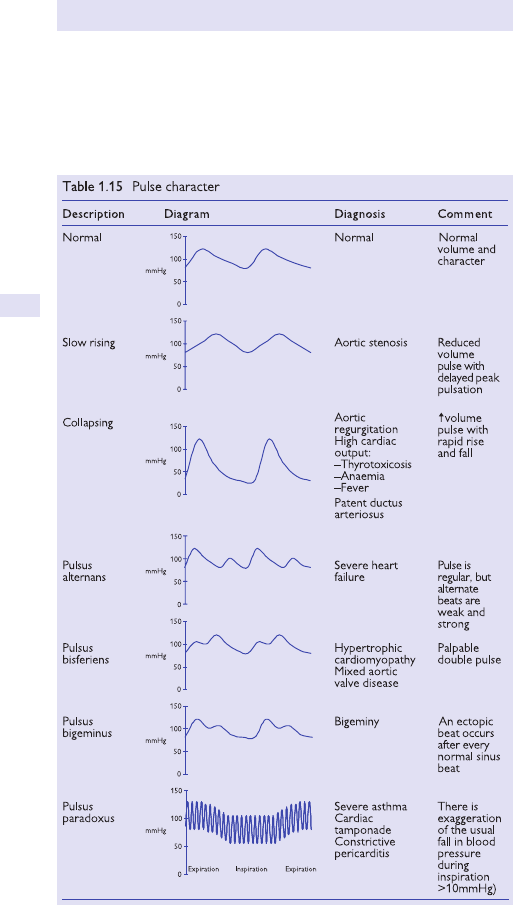
94
CHAPTER1 Symptoms andsigns
94
Pulse character
Rate and heart rhythm can be determined from palpation of the radial
pulse. The arm should then be elevated to check for a collapsing pulse.
Pulse volume and additional characteristics are assessed from palpation of
the brachial or carotid pulse (see Table1.15).

PURPURA
95
Purpura
Implies bleeding of varying degrees into the skin. Includes petechial haem-
orrhages (pinpoint) and ecchymoses (bruises). There are many causes,
including disorders of platelets and blood vessels.
Causes
• Congenital, e.g. Osler– Weber– Rendu syndrome (= HHT), connective
tissue (Ehlers– Danlos), osteogenesis imperfecta, Marfan’s.
• Severe infection (septic, meningococcal, measles, typhoid).
• Allergic, e.g. Henoch– Schönlein purpura.
• Drugs, e.g. steroids.
• Miscellaneous, e.g. senile purpura, scurvy, factitious.
• Thrombocytopenia— any cause (immune, marrow inltration, deciency
of vitamin B
12
or folate, myelobrosis, DIC, TTP/ HUS).
Investigations
• FBC (looking for platelet abnormalities and presence of leukaemic cells
or other signs of inltration).
•
Coagulation screen (looking for clotting factor deciencies, DIC,etc.).
• Bleeding time using template device (previously used as a test of platelet
function, but largely abandoned now because of poor reproducibility).
E OHCM 10e, p.311, p. 315, p. 556, p. 702.

96
CHAPTER1 Symptoms andsigns
96
Recurrent thrombosis
The pathogenesis (and hence causes) of thrombosis reect abnormalities
in the dynamics of the circulation, the blood vessel walls, or the blood con-
stituents (Virchow’s triad). Ahypercoagulable or thrombophilic risk factor
is an inherited or acquired disorder of the haemostatic mechanisms, which
may be associated with an i likelihood of a thrombotic event (venous or
arterial) or recurrent thrombosis. This concept of risk factors for throm-
bosis is analogous to that for heart disease, and similarly for most patients
multiple causal factors operate (see Table1.16).
Hereditary thrombotic disease may be suggested by a positive family his-
tory but should be tested for if the venous thrombotic events occur in the
absence of acquired causes, at a younger age, at unusual sites (e.g. mesen-
teric), or as recurrent thromboses.
Investigations inrecurrent thrombosis
Inherited thrombophilia screening
•
Deciency of factors, e.g. protein C, protein S, or antithrombin.
• Abnormal protein(FVL).
• i procoagulant (PT, VIII); others (homocysteinuria).
•
Consider occult malignancy (PSA in ♂, pelvic USSin♀).
•
FBC (myeloproliferative disorder,PNH).
• Biochemistry (cardiac disease, liver disease, nephrotic syndrome).
• ESR/ CRP (ulcerative colitis).
• ANA/ lupus anticoagulant/ cardiolipin antibodies (antiphospholipid
antibody syndromes, lupus).
Pitfalls
Thrombophilia testing may be complicated if the patient is on warfarin/
heparin; discuss with the lab before sending samples.
E OHCM 10e, p.375.
Table1.16 Thromboembolic risk factors
Acquired
Cardiac disease MI, AF, cardiomyopathy, CCF
Post- op Especially abdominal, pelvic, or orthopaedic surgery
Pregnancy
Malignancy Any
Polycythaemia
Immobilization Prolonged
Fractures
Especially hip and pelvis
Obesity
Varicose veins
Drugs
e.g. oestrogen- containing oral contraceptive
Inherited Activated protein C resistance, e.g. FVL mutation
Protein C or S deciency
Dysbrinogenaemias

RIGORS
97
Retinal haemorrhage
Maybe
• Flame- shaped (e.g. hypertension).
• Dot and blot (e.g. DM, vein occlusion, or haematological disease).
• Pre- retinal haemorrhage; suggests new vessel formation, e.g. DM or
post- retinal vascular occlusion.
• Hyperviscosity syndromes.
• Severe anaemia.
• Severe thrombocytopenia.
• Haemoglobinopathy, e.g.HbSC.
Investigations
• CheckBP.
• Renal function.
• FBC (ii Hb or platelets).
•
ESR or plasma viscosity (hyperviscosity syndromes such as myeloma or
Waldenström’s macroglobulinaemia).
•
Serum Igs and protein electrophoresis.
• Hb electrophoresis.
Rigors
Fever is due to a resetting of the anterior hypothalamic thermostat, is medi-
ated by prostaglandins (hence aspirin is benecial), and is most commonly
caused by infection. Large variations in temperature may be accompanied
by sweats, chills, and rigors. An undulant fever may suggest Hodgkin’s
disease or brucellosis. ‘B’ symptoms dene fever (>38°C), night sweats
(drenching), and weight loss (>10%) and suggest a diagnosis of lymphoma.
(Fever is unusual in chronic lymphocytic/ lymphatic leukaemia (CLL) in the
absence of infection.)
Investigations
• FBC, lm (Hodgkin’s disease is associated with anaemia, neutrophilia,
eosinophilia, and lymphopenia).
•
LDH (i in lymphoma, non- specictest).
• Microbiological tests, blood/ urine cultures (also consider pyogenic
infection and abscesses in more unusual sites, e.g. renal).
•
Antigen detection tests for specic pathogens.
• CXR (TB, lymphoma).
• ANA (connective tissue disease).
• BM aspirate/ trephine may be necessary as part of leukaemia and
lymphoma work- up.
Pitfalls
Not all fever is caused by infection.
E OHCM 10e, p.29.

98
CHAPTER1 Symptoms andsigns
98
Short stature
The assessment of short stature can be a long and dicult process.
Constitutional short stature is the commonest cause. Psychosocial disease
must be considered, but extensive investigation is required to rule out
organic disease. If no cause is found, a period of observation may make
the underlying cause apparent. Specialist evaluation should be undertaken
in allcases.
Causes
Endocrine
•
GH deciency.
• GH resistance (veryrare).
• Hypothyroidism (readily treatable).
• Cushing’s syndrome (rare in children). (Note:corticosteroid treatment
for chronic asthma.)
•
Rickets.
• Pseudohypoparathyroidism.
• Type 1 DM— Mauriac’s syndrome, nowrare.
Non- endocrine
• Constitutional short stature (short parents).
• Emotional deprivation.
• Intrauterine growth retardation.
• Achondroplasia.
• Mucopolysaccharidoses (rare).
• Turner’s syndrome (46 XO and variants).
• Noonan’s syndrome (46 XY, but features of Turner’s ina♂).
•
Congenital cardiac disease, e.g. left- to- right shunt, cardiac failure.
• Cystic brosis.
• Other causes of malabsorption, e.g. coeliac disease, Crohn’s colitis.
• Chronic liver disease.
• Haematological disease, e.g. sickle- cell disease.
• Chronic renal disease.
Investigations
• Current height + weight (compare to any previous data available; plot
on growth charts).
•
Growth velocity— normal if prior problem, e.g. intrauterine growth
retardation.
•
Physical stigmata of physical disease. Note:central nervous system
(CNS) examination mandatory.
•
FBC.
• ESR.
• U&E.
• LFTs.
• TFTs.
• Serum albumin (? nutritional status).
• Venous plasma glucose.
• SerumCa
2+
.

SHORT STATURE
99
• Serum ALP (bone isoenzyme).
• Serum PO
4
3−
(reduced in rickets).
•
X- ray pelvis (Looser’s zones), epiphyses (wide, irregular in rickets), ribs
(multiple fractures).
•
Serum antigliadin and antiendomysial antibodies (coeliac).
• Testosterone or oestradiol, LH, FSH, PRL (if puberty
delayed— panhypopituitarism?).
• X- ray of the wrist for bone age. If delayed, measure serum IGF- 1
(if IGF- 1 normal, then GH deciency unlikely; if IGF- 1 low, consider
nutritional and general health status before diagnosing GH deciency—
stimulation tests required; E Endocrinology and metabolism, Short
stature, p. 178). If normal— constitutional short stature.
• Karyotype (Turner’s and Noonan’s syndromes).
• 24h urinary free cortisol (as screen for Cushing’s syndrome;
E
Obesity/ hypercortisolism, pp. 142–6).
• CT or MRI of the pituitary (if GH deciency or panhypopituitarism).

100
CHAPTER1 Symptoms andsigns
100
Skin pigmentation
Skin pigmentation can be due to i melanin deposition, e.g. racial dierences
in skin pigmentation, or due to i melanin deposition seen in sun exposure.
Lentigines and freckles are common. Haemosiderin and other substances
can i skin pigmentation. i pigmentation can be seen in various dermato-
logical conditions; chronic inammation and fungal infection can result in i
skin pigmentation. Lichen planus and xed drug eruptions are associated
with i pigmentation.
Increased pigmentation may also be found inassociation
withchronic systemic disease
• Addison’s disease (palmar creases, buccal pigmentation, recent scars).
• Porphyria cutanea tarda (especially exposed areas— dorsum of the
hands).
•
Chronic malabsorption syndromes.
• Drugs, e.g. amiodarone, psoralens, mepacrine, minocycline, chloroquine.
• Chronic uraemia.
• Haemochromatosis (so- called ‘bronzed diabetes’).
• PBC (deep green- yellow jaundice, chronic pruritus).
• Ectopic ACTH syndrome, e.g. bronchial carcinoma.
• Nelson’s syndrome (excessive adrenocorticotrophic hormone (ACTH)
secretion from pituitary basophil adenoma in Cushing’s disease treated
by bilateral adrenalectomy).
•
Carotenaemia (orange discoloration does not involve the sclerae;
E Jaundice, p. 66).
•
Chloasma (pregnancy, oestrogen- containingOCP).
• Acanthosis nigricans— most often a marker of insulin resistance in
obese patients with type 2 DM. Rarely in association with underlying
carcinoma.
•
Peutz– Jeghers syndrome (ngers, lips, in association with small intestine
polyposis).
Contrast withhypopigmentation
• Localized acquired depigmentation (vitiligo) is a marker of autoimmune
disease.
•
Oculocutaneous albinism (autosomal recessive).
• Chronic hypopituitarism (E Hypothalamus/ pituitary function,
pp. 128–30).
•
Phenylketonuria.

SKIN PIGMENTATION
101
Investigations
• FBC.
• U&E.
• Venous plasma glucose.
• Antigliadin and antiendomysial antibodies.
• Short tetracosactide (Synacthen
®
) test (if ° hypoadrenalism suspected;
E Short Synacthen
®
test, p. 225).
•
Urinary porphyrins.
• LFTs, serum albumin, and PT(INR).
• Fe/ TIBC/ ferritin + genetic markers for haemochromatosis + liver
biopsy.
•
ESR and/ orCRP.
• Autoimmune prole (E Chapter4).
•
Testosterone (or oestradiol) + LH,FSH.
• Antimitochondrial antibodies, liver biopsy(PBC).
• Investigations for Cushing’s syndrome (E Obesity/ hypercortisolism,
pp. 142–6).
•
Investigations for causes of chronic renal failure.

102
CHAPTER1 Symptoms andsigns
102
Splenomegaly
A palpable spleen is at least twice its normal size, when its length is >14cm.
Enlargement may represent changes in the white pulp (lymphoid tissue
expansion, inammation), red pulp (blood congestion, extramedullary hae-
mopoiesis), or occasionally supporting structures (cysts).
Causes inWestern societies
• Leukaemias.
• Lymphomas.
• Myeloproliferative disorders.
• Haemolytic anaemias.
• Portal hypertension.
• Infections, e.g. infective endocarditis, typhoid, TB, brucellosis, viral (EBV,
viral hepatitis).
Less commoncauses
•
Storage disorders (e.g. Gaucher’s).
• Collagen diseases.
• Sarcoid.
• Amyloid.
If foreign residence, consider infectious causes (malaria, leishmaniasis, schis-
tosomiasis) and haemoglobinopathies (HbC, HbE, thalassaemia).
Massive splenomegaly (>8cm palpable belowLCM)
• Myelobrosis.
• Chronic myeloid leukaemia(CML).
• Gaucher’s.
• Malaria.
• Leishmaniasis.
Investigations
• Thorough history and physical examination.
• FBC, blood lm, LDH (leukaemia, lymphoma, pernicious anaemia).
• Reticulocytes, bilirubin (if i, suggests haemolysis).
•
Virology/ microbiology (sepsis, bacterial endocarditis, EBV,CMV).
• Serum protein electrophoresis (myeloma, amyloid).
• Autoantibody screen, ANA (collagen disease, lupus,RhA).
• Haemoglobinopathy screen.
• LFTs (splenomegaly may be associated with hepatomegaly, or due to
portal hypertension).
•
Peripheral blood cell markers (immunophenotype— may show
leukaemia or lymphoma).
•
BM aspirate/ trephine/ cell markers/ cytogenetics.
• Leucocyte glucocerebrosidase activity (Gaucher’s disease).
• USS to assess liver texture, splenomegaly, and lymphadenopathy.
E OHCM 10e, p.63, p.373, p.604.

STEATORRHOEA
103
Steatorrhoea
Implies that the patient is passing pale, bulky stools that are oensive (con-
tain fat and tend to oat) and are dicult to ushaway.
Causes
• Any disorder that prevents absorption of micellar fat from the
smallbowel.
•
Ileal disease.
• Ileal resection.
• Parenchymal liver disease.
• Obstructive jaundice.
• Pancreatic disease, including cystic brosis.
• d bile salt concentration.
•
Bile salt deconjugation by bacteria.
• Cholestyramine.
• β- lipoprotein deciency.
• Lymphatic obstruction.
Investigations
Bloodtests
•
LFTs.
• Bone prole.
• Vitamin B
12
and serum (or red cell) folate.
•
Autoantibody prole.
• Serum amylase.
Pancreatic investigations
•
Pancreatic functiontests.
• CTscan.
Smallbowel
•
Small bowel follow- through.
• Jejunal biopsy (? villus atrophy).
• Bacterial overgrowth (
14
C glycocholate breathtest).
Parasites
•
Stool culture (e.g. Giardia).
Ileal disease
•
Consider Crohn’s.
E OHCM 10e, p.59.

104
CHAPTER1 Symptoms andsigns
104
Stridor
Stridor denotes a harsh respiratory added sound during inspiration. It may
be a high- pitched musical sound similar to wheeze but arising from constric-
tion of the larynx or trachea. Stridor may be aggravated by coughing.
3 Progressive breathlessness is accompanied by indrawing of intercos-
tal spaces and cyanosis indicates severe laryngeal obstruction with risk of
suddendeath.
In young children
Because of the smaller size of the larynx and trachea in children, stridor may
occur in a variety of conditions:
•
Postural stridor (laryngomalacia).
• Allergy, e.g. nut allergy, insect stings— common. Note:emergency
treatment with IM or subcutaneous (SC) adrenaline (epinephrine)— self-
administered or by parent, and parenteral hydrocortisone.
•
Vocal cordpalsy.
• Croup (acute laryngitis— often coryza).
• Acute epiglottitis.
• Inhaled foreign body, e.g. peanut (common— inhalation further down the
respiratory tract, usually into the right main bronchus, may produce localized
wheeze or distal collapse; E Patterns of lobar collapse, p. 780).
Investigations
•
Pulse oximetry (non- invasive measurement of partial pressure of
oxygen (PO
2
)).
•
Plain lateral X- ray of the neck (for radio- opaque foreignbody).
• Endoscopic nasolaryngoscopy.
Adults
• Infection, especially Haemophilus inuenzae.
•
Inammatory or allergic laryngeal oedema, e.g. penicillin allergy (see
above); may be accompanied by anaphylacticshock.
•
Pharyngeal pouch (may be recurrent lower respiratory tract infection).
• Inhaled vomitus or blood in an unconscious patient.
• Tetany (due to low serum Ca
2+
or alkalosis; E OHCM 10e, Chapter14).
•
Large multinodular goitre, carcinoma, or lymphoma of the thyroid
(uncommon).
•
Laryngeal tumours.
• Bronchogenic tumour with bilateral cord paralysis (subcarinal and
paratracheal gland involvement. Note:‘bovine’ cough of right recurrent
laryngeal nerve palsy).
•
Shy– Drager syndrome (of autonomic neuropathy).
Investigations
•
CXR.
• Lateral X- ray of theneck.
• USS of the thyroid.
• Endoscopic nasolaryngoscopy.
• Bronchoscopy.
• Barium swallow (pharyngeal pouch).
• CT neck and mediastinum.
E OHCM 10e,p.48.

SUSPECTED BLEEDING DISORDER
105
Suspected bleeding disorder
Bleeding problems present a considerable challenge. Patients may pre- sent
with simple easy bruising— a common problem— or catastrophic post-
traumatic bleeding. The best predictors of bleeding risk are found in taking
an accurate history, focusing on past haemostatic challenges (e.g. tonsil-
lectomy, teeth extraction, menses— especially at time of menarche) and
current drug history (e.g. aspirin). The history may also help delineate the
type of defect. Platelet bleeding (e.g. thrombocytopenia) starts at the time
of the (even minor) haemostatic insult but, if controlled by local pressure,
tends not to recur. Bleeding due to coagulation factor deciency tends to be
associated with internal/ deep muscle haematomas as the bleeding typically
occurs in a delayed fashion after initial trauma and then persists.
Inappropriate bleeding or bruising may be due to a local factor or an
underlying systemic haemostatic abnormality.
2 Acquired causes of bleeding are much commoner than inherited
causes.
Causes ofbleeding include
• Surgical.
• Trauma.
• Non- accidental injury.
• Coagulation disorders.
• Platelet dysfunction.
• Vascular disorders.
Clinical features
History and presenting complaint. Is this an isolated symptom? What type
of bleeding does the patient have, e.g. mucocutaneous, easy bruising, spon-
taneous, post- traumatic. Duration and time of onset— ? recent or present
in childhood. Menstrual and obstetrical history are important.
Systemic enquiry
Do the patient’s symptoms suggest a systemic disorder, bone marrow fail-
ure, infection, liver disease, or renal disease?
Past medical history
Previous episodes of bleeding, recurrent— ? ITP, congenital disorder.
Exposure to trauma, surgery, dental extraction, or pregnancies.
Family history
First- degree relatives. Pattern of inheritance (e.g. autosomal, sex- linked). If
family history is negative, this could be a new mutation (one- third of new
haemophilia is due to new mutations).
Drugs
All drugs cause some side eects in some patients. Bleeding may result from
thrombocytopenia and platelet dysfunction. Do not forget to ask about
aspirin and warfarin.

106
CHAPTER1 Symptoms andsigns
106
Physical examination
Signs ofsystemic disease
Is there any evidence of septicaemia, anaemia, lymphadenopathy ±
hepatosplenomegaly?
Assess bleedingsite
Check the palate and fundi. Could this be self- inicted? Check size—
petechiae (pinhead); purpura (larger ≤1cm); bruises (ecchymoses;≥1cm).
Joints
Swelling or other signs of chronic arthritis.
Vascular lesions
Purpura— allergic, Henoch– Schönlein, senile, steroid- related, hypergam-
maglobulinaemic, HHT— capillary dilatations (blanches on pressure), vas-
culitic lesions, autoimmune disorders, hypersensitivity reactions.
Investigations
• FBC, lm, platelet count, biochemistry screen, ESR, coagulation screen.
• Special tests, e.g. BM for ° haematological disorders; radiology,USS.
• Family studies.

SUSPECTEDSTROKE
107
Suspectedstroke
A stroke denotes an acute neurological decit. Strokes may vary in pres-
entation, e.g. rapidly resolving neurological decit to a severe permanent
or progressive neurological defect (e.g. multi- infarct disease). Neurological
decits persisting >24h are termed ‘completed stroke’ (cf. TIA). With sus-
pected stroke, a full history and general physical examination are manda-
tory. Risk factors for cerebrovascular disease should be sought, including
a history of hypertension (common— major risk factor), DM (common—
major risk factor), and dyslipidaemia. Ask about recent falls or trauma.
Hemiparesis can occur as a post- ictal phenomenon or a result of migraine
or hypoglycaemia (see below). Hysterical or functional paralysis is also seen
but should not be condently assumed at presentation. Neuroanatomical
localization of the decit and the nature of the lesion(s) require appropri-
ate imaging. Note
: the post- ictal state may be associated with temporary
(<24h) limb paresis (Todd’s paralysis) in focal epilepsy (suggests structural
lesion— cranial imaging is mandatory).
General investigations
• FBC (polycythaemia, anaemia).
• U&E.
• ESR.
• Protein electrophoresis (if hyperviscosity syndrome suspected, e.g.
iiESR).
• ECG (AF, IHD— statins reduce the risk of stroke in patients with
previousMI).
•
CXR (cerebral metastases from bronchogenic carcinoma?).
Specic risk factors
• Venous plasma glucose. Note:severe hypoglycaemia, e.g. insulin- induced
or 2° to sulfonylureas, may mimic acute stroke. Always check the
capillary ngerprick glucose concentration to exclude this possibility—
even if there is no history of DM. Take a venous sample in a uoride–
oxalate tube (+ serum for insulin concentration) if hypoglycaemia
conrmed. (E Diabetes mellitus, pp. 194–9 for further details of
investigation and treatment.) Hyperosmolar non- ketotic diabetic coma
may also be misdiagnosed as stroke (plasma glucose usually >50mmol/ L
with pre- renal uraemia).
• Thrombophilia screen (if indicated by clinical or haematological
features).
•
Lipid prole (not an immediate investigation; 2° prevention— see
above).
•
Blood cultures (if subacute bacterial endocarditis (SBE) or other sepsis
suspected. Note:cerebral abscess).

108
CHAPTER1 Symptoms andsigns
108
Imaging
• Cranial CT scan (± IV contrast).
• Echocardiogram (if mural thrombus, endocarditis suspected).
• Carotid Doppler studies— may not be indicated if surgical intervention
(endarterectomy) is unlikely because of poor prognosis, e.g. dense
hemiplegia orcoma.
Consider alternative diagnoses including
• ° or 2° brain tumour (may present as acute stroke— search for°).
• Cerebral abscess (usually clear evidence of sepsis).
• Cerebral lupus (ESR, autoantibodies).
E OHCM 10e, p.159, pp. 470–5, p. 746.

SWEATING
109
Sweating
Fairly non- specic symptom, but one which may indicate serious underlying
disease.
Causes
• Excess heat (physiological).
• Exercise (physiological).
• Fever— anycause.
• Anxiety.
• Thyrotoxicosis.
• Acromegaly.
• DM.
• Lymphoproliferative disease, e.g. lymphomas.
• Cancer(any).
• Hypoglycaemia.
• Alcohol.
• Nausea.
• Gustatory.
• Neurological disease, e.g. lesions of the sympathetic nervous system,
cortex, basal ganglia, or spinalcord.
Investigations
• FBC.
• ESR.
• Biochemistry screen, includingLFTs.
• Glucose.
• TFTs.
• Urinalysis and culture.
• Blood cultures.
• CXR.
• Further investigations, depending on results ofabove.

110
CHAPTER1 Symptoms andsigns
110
Tachycardia
Tachycardia is arbitrarily dened as a heart rate above 100 beats per min-
ute. It is a normal physiological response to exercise and to emotional stress
but can also herald a cardiac rhythm disorder. One should always begin by
assessing the nature of the tachycardia and identifying any underlying cause
or contributing factor.
Assessment begins with a 12- lead ECG, performed whilst the patient
is tachycardic. This will enable the immediate identication of the heart
rhythm. One must then dierentiate between sinus tachycardia (which
may or may not have a pathological cause) and tachycardias due to other
(abnormal) cardiac rhythms.
Causes ofsinus tachycardia
• Sympathetic stimulation, e.g. anxiety, pain, fear, fever, exercise.
• Drugs, e.g. adrenaline, atropine, salbutamol.
• Stimulants, e.g. caeine, alcohol, amphetamines.
• Thyrotoxicosis.
• Heart failure.
• PE.
• IHD, acuteMI.
• Anaemia.
• Blood or uid loss, e.g. post- operative.
• Inappropriate sinus tachycardia (a persistent resting sinus tachycardia,
diagnosed when all other possible causes have been excluded).
In assessing abnormal heart rhythms causing tachycardia, it is helpful to
divide them into narrow- complex tachycardia (QRS duration <120ms) and
broad- complex tachycardia (QRS duration >120ms).
Narrow- complex tachycardias
• Sinus tachycardia (see above).
• Atrial tachycardia.
• Atrial utter.
• AF.
• AV re- entry tachycardias.
Broad- complex tachycardias
• Narrow- complex tachycardia with aberrant conduction.
• Ventricular tachycardia.
• Accelerated idioventricular rhythm.
• Torsades de pointes.

TACHYCARDIA
111
Investigations
• 12- lead ECG to identify the underlying rhythm.
• Consider bedside monitoring on the Coronary Care Unit (CCU),
particularly if the patient is compromised or ventricular arrhythmias are
suspected.
•
Other investigations depend upon the underlying cause but may include:
•
FBC.
•
U&E.
•
TFTs.
•
Cardiac markers.
•
CXR.
•
ABGs.
•
V/ Q scan/ CTPA.
•
Echocardiogram.
•
Exercise treadmilltest.
•
Cardiac catheter.
•
Electrophysiological studies.
It can be useful to perform carotid sinus massage (2 exclude carotid bruits
rst) or to give IV adenosine (2
do not use in asthma/ COPD), whilst the
patient is on a bedside ECG monitor. Supraventricular tachycardias will usu-
ally slow transiently, allowing clearer identication of the underlying atrial
activity, and re- entry tachycardias may terminate altogether. Ventricular
tachycardias will be unaected.
E OHCM 10e, Chapter 3.

112
CHAPTER1 Symptoms andsigns
112
Tinnitus
Tinnitus is a common symptom in which the patient perceives a sound,
often chronic and distressing, in the absence of aural stimulation. It usually
manifests as a ‘ringing’ or ‘buzzing’ in the ears. Tinnitus may occur as a symp-
tom of nearly all disorders of the auditory apparatus. Psychological stresses
may be relevant in somecases.
Causes include
• Acoustic trauma (prolonged exposure to loud noise, e.g. gunshots,
amplied music).
•
Barotrauma (blast injury, perforated tympanic membrane).
• Obstruction of the external auditory meatus (wax, foreign body,
infection).
•
Otosclerosis.
• Ménière’s disease.
• Drug- induced ototoxicity.
• Gentamicin— may be irreversible.
• Acute salicylate toxicity.
• Quinine toxicity.
• Acute alcohol poisoning.
• Hypothyroidism.
• Hypertension (rare symptom).
• Intra- or extracranial aneurysm (typically causes ‘pulsatile’ tinnitus).
• Glomus jugulare tumours.
Note: consider acoustic neuroma in unilateral tinnitus (E OHCM 10e,
Chapter10).
Investigations
• FBC.
• Serum concentrations of, e.g. salicylates, gentamicin (2 mandatory
during systemic therapy).
•
TFTs.
• B P.
Audiological assessment
Specialist investigations include
•
Assessing air and bone conduction thresholds.
• Tympanometry and acoustic reex testing.
• Speech perception thresholds.
Consider
•
CT temporal bone (acoustic neuroma).
• Cranial MRI (following specialist advice).
E OHCM 10e, p.464.

TIREDNESS
113
Tiredness
Tiredness is a common presenting complaint in the endocrine clinic.
Important diagnoses to exclude are hypo- / hyperthyroidism, hypoadrenal-
ism, hypercalcaemia, and DM. AU&E is useful to exclude hyponatraemia or
hypokalaemia (muscle weakness), as well as renal failure.
Recommended investigations fortiredness inthe absence
ofan obvious cause fromhistory and examination
• TSH.
• FT4.
• Synacthen
®
test.
•
SerumCa
2+
.
•
Glucose.
• U&E, creatinine.
• FBC.
• ESR (orCRP).
• LFTs.

114
CHAPTER1 Symptoms andsigns
114
Urgency ofmicturition
Urgency of micturition denotes a strong desire to void and the patient
often has to rush to the toilet because of an acute call to micturate. Urinary
incontinence may result, especially if physical mobility is impaired. Urgency
forms part of a cluster of symptoms which include frequency of micturition
(E Polyuria, p. 90), nocturia, and hesitancy of micturition.
Men
• Prostatic disease.
• UTI.
• Bladder irritability.
• Urethritis.
• States of polyuria (E Polyuria, p. 90); may lead to urinary incontinence
(E Incontinence:urinary, p. 61).
Investigations toconsider
•
Urinalysis— stick test for glucose, protein, blood, and nitrites.
• MSU for microscopy and culture.
• FBC.
• U&E.
• Venous plasma glucose.
• ESR.
• SerumPSA.
• PSA is i in 30– 50% of patients with benign prostatic hyperplasia, and in
25– 92% of those with prostate cancer (depending on tumour volume),
i.e. a normal PSA does not exclude prostatic disease. Check the
reference range with the local laboratory.
•
Transrectal USS of the prostate.
• Prostatic biopsy (specialist procedure).
Women
• UTI.
• Gynaecological disease, e.g. pelvic oor instability, uterine prolapse.
• Bladder irritability.
• Urethritis.
• States of polyuria; may lead to urinary incontinence (E Incontinence:
urinary, p. 61).
Investigations toconsider
•
FBC.
• U&E.
• MSU for microscopy and culture.
• Urodynamic studies.
E OHCM 10e, p.80, p. 648.

URTICARIA
115
Urticaria
Itchy supercial wheals; may be giant. Distinguish acute from chronic—
chronic is rarely allergic in origin. If persists for >24h and fades with brown
staining, consider urticarial vasculitis (rare).
Causes
• Allergic:drugs, foods, additives, acute infection, e.g. HBV, Mycoplasma.
•
Physical:sunlight, heat, cold, pressure, vibration.
• Stress.
• Thyroid disease:hypo- or hyperthyroidism.
• Occult infection:gall bladder, dental,sinus.
• Vitamin deciency:B
12
, folic acid,iron.
•
Autoimmune:antibodies against IgE receptor on mast cells— veryrare.
Investigations
Based onhistory but should include asbaseline
•
FBC (with eosinophil count).
• Thyroid function.
• Infection marker(CRP).
• Liver function.
Further tests may include
•
B
12
and red cell folate.
•
Serum ferritin.
Allergy tests are of little value, unless there is a clearly identied trigger.
116
CHAPTER1 Symptoms andsigns
116
Vasculitis
Denition
Disease caused by inammatory destructive changes of blood vessel walls
(see Table1.17).
Presentation
Wide variety of clinical presentations aecting one or more organ systems:
•
Skin:splinter haemorrhages, nailfold infarcts, petechiae, purpura, livedo
reticularis.
•
Respiratory:cough, haemoptysis, breathlessness, pulmonary inltration,
sinusitis.
•
Renal:haematuria, proteinuria, hypertension,ARF.
•
Neurological:mononeuritis multiplex, sensorimotor polyneuropathy,
confusion, ts, hemiplegia, meningoencephalitis.
•
Musculoskeletal:arthralgia, arthritis, myalgia.
•
Generalized:PUO, weight loss, malaise.
Causes ofsecondary vasculitis
• Infective endocarditis.
• Meningococcal septicaemia.
• Malignancy.
• RhA.
• Henoch– Schönlein purpura.
• SLE.
• Cryoglobulinaemia.
• Drug reaction.
Investigations
• FBC.
• U&E.
• LFTs.
• ESR.
• CRP.
• Protein electrophoresis.
• ANA.
• RF.
• ANCA.
• CXR.
• Biopsy of artery and/ or skin lesions.
• Urine dipstick and microscopy.
E OHCM 10e, p.314, p.556, p.557.
Table1.17 Causes of° vasculitis
Granulomatous Non- granulomatous
Large vessel Giant cell arteritis (GCA) Takayasu’s arteritis
Medium vessel
Churg– Strauss disease Polyarteritis nodosa
Small vessel Wegener’s arteritis Microscopic arteritis

VISUALLOSS
117
Visualloss
Total loss of vision may be bilateral or unilateral. Unilateral blindness is due
to a lesion either of the eye itself or between the eye and the optic chiasm.
Determine whether the visual loss is gradual or sudden. Gradual loss of
vision occurs in conditions such as optic atrophy or glaucoma. In the elderly,
cataract and macular degeneration are common. Remember tobacco
amblyopia and methanol toxicity. Trachoma is a common cause worldwide.
Causes ofsudden blindness include
• Optic neuritis, e.g.MS.
• Central retinal artery occlusion.
• Central retinal vein occlusion.
• Vitreous haemorrhage. (Note:proliferative diabetic retinopathy.)
•
Acute glaucoma.
• Retinal detachment.
• Temporal (giant cell) cell arteritis (TA). Note:visual loss is potentially
preventable with early high- dose corticosteroid therapy (E OHCM 10e,
Chapter10).
•
Migraine (scotomata).
• Occipital cortex infarction.
• Acute severe quinine poisoning (consider stellate ganglion block).
• Hysteria (rare), e.g. is blindness:
•
Complete? No pupil response or opticokinetic nystagmus.
•
Cortical? Normal pupillary light reex, no opticokinetic nystagmus.
•
Hysterical? Normal pupillary light reex, normal opticokinetic nystagmus.
•
HELLP syndrome complicating pre- eclampsia— rare.
Investigations will be determined by the history and examination ndings; a
specialist opinion should be sought withoutdelay.
If TA suspected
• ESR/ CRP.
• Autoimmune prole, including cytoplasmic ANCA (cANCA)/
perinuclear ANCA (pANCA).
•
Temporal artery biopsy (within days; 3 do not withhold steroid therapy).
Investigations insudden onset ofvisualloss
• Visual acuity (Snellen chart).
• Goldmann perimetry.
• Intraocular pressure measurement (tonometry).
• Fluorescein angiography (specialist investigation— may delineate diabetic
retinopathy in more detail. 3 Risk of anaphylaxis).
•
Cranial CTscan.
• Cranial MRIscan.
• LP (CSF protein and OCBs if MS suspected).
Screen for risk factors and causes of cerebrovascular thromboembolic disease:
•
Venous plasma glucose.
• Serum lipid prole.
• Carotid Doppler studies.
• 12- leadECG.
• Echocardiogram.

118
CHAPTER1 Symptoms andsigns
118
Wasting ofthe small hand muscles
Wasting of the small muscles of the hand may be found in isolation or
maybe associated with other neurological signs. If found in isolation, this
suggests a spinal lesion at the level of C8/ T1 or distally in the brachial
plexus or upper limb motor nerves.
Unilateral wasting ofthe small muscles ofthe hand may
occur inassociationwith
• Cervicalrib.
• Brachial plexus trauma (Klumpke’s palsy).
• Pancoast’s tumour (may be associated with Horner’s syndrome).
• Cervical cord tumour.
• Malignant inltration of the brachial plexus.
Bilateral wasting ofthe small muscles ofthe hand occursin
• Carpal tunnel syndrome (common).
• RA (common).
• Cervical spondylosis (common).
• Bilateral cervicalribs.
• MND.
• Syringomyelia.
• Charcot– Marie– Tooth disease.
• GBS.
• Combined median and ulnar nerve lesions.
• Cachexia.
• Advancedage.
• Peripheral neuropathies.
Investigations
• ESR.
• CRP.
• RF.
• CXR.
• X- ray cervicalspine.
• NCS (E Nerve conduction studies, pp. 606–8).
•
EMG (E Electromyogram, pp. 610–11).
• LP, CSF protein, etc. (E Lumbar puncture, pp. 584–9).
•
CT thorax.
• MRI of the cervical cord/ brachial plexus.

WEIGHTLOSS
119
Weightloss
Causes
• Diet.
• Anorexia.
• DM.
• Malnutrition.
• Small intestinal disease (coeliac, bacterial overgrowth).
• Malignant disease (carcinoma and haematological malignancies).
• HIV/ AIDS.
• Chronic pancreatitis.
• Chronic respiratory failure.
• Cirrhosis.
• Diuretic therapy.
• Hyperthyroidism.
• Addison’s disease.
Investigations
May well need extensive investigation before determining the cause, but
startwith:
•
FBC.
• ESR orCRP.
• Biochemistry screen.
• TFTs.
• MSU, includingC&S.
• CXR.
• Stool culture (if appropriate).
• Blood culture.
• Other endocrine tests as appropriate.
• Consider HIV testing.
E OHCM 10e,p.35, p. 245.

120
CHAPTER1 Symptoms andsigns
120
Wheeze
Wheezes (rhonchi) are continuous high- , medium- , or low- pitched added
sounds audible during respiration. Typically they are loudest on expiration
in asthma and may on occasion be heard without a stethoscope. The impli-
cation is reversible or irreversible airway obstruction. If wheeze is audible
only during inspiration, this is termed stridor, implying an upper respiratory
obstruction. An important distinction must be made between monophonic
and polyphonic wheezes and whether wheeze is localized to a single area
or is heard throughout the thorax.
Polyphonicwheeze
Wheezes with multiple tones and pitch. The commonest causes of wheeze
(usually recurrent)are:
•
Asthma.
• COPD (often audible during both phases of respiration).
Fixed monophonicwheeze
A wheeze with a single constant pitch. Implies local bronchial obstruction,
usually dueto:
•
Bronchogenic carcinoma.
• Foreignbody.
Note:stridor is a harsh form of monophonic wheeze arising from an upper
airway obstruction (E Stridor, p. 104).
Investigations
• ABGs. (Note:inspired O
2
concentration should be recorded.)
•
Pulse oximetry at the bedside (does not provide information about the
partial pressure of carbon dioxide (PCO
2
)).
•
Spirometry (peak ow rate (PFR), pre- and post- bronchodilator
therapy).
•
Pulmonary function tests (forced expiratory volume in 1s (FEV
1
),
forced vital capacity (FVC), total lung capacity; E
Flow volume loops/
maximum expiratory ow– volume curve, p. 554).
• CXR (posteroanterior (P- A) and lateral).
• Sputum cytology (if tumour suspected).
• CT thorax.
• Bronchoscopy and biopsy (specialist procedure— especially if foreign
body or suspected tumour).
E OHCM 10e, p.52.
Investigations
2 Endocrinology and metabolism
123
3 Haematology
227
4 Immunology and allergy
333
5 Infectious and tropical diseases
381
6 Cardiology
435
7 Gastroenterology
497
8 Respiratory medicine
535
9 Neurology
583
10 Renal medicine
649
11 Poisoning and overdose
699
12 Rheumatology
741
13 Radiology
765
14 Nuclear medicine
865
PartII
122

123
Endocrinology and
metabolism
Guiding principles of endocrine investigation 124
Hypothalamus/ pituitary function 128
Acromegaly (growth hormone excess) 132
Polydipsia and polyuria:diabetes insipidus 134
Hyponatraemia (including syndrome of inappropriate
antidiuretic hormone) 138
Obesity/ hypercortisolism 142
Endocrine hypertension 148
Hypokalaemia 158
Hyperkalaemia 160
Adrenal failure 162
Amenorrhoea 166
Infertility 168
Hirsutism/ virilization (raised testosterone) 170
Galactorrhoea (hyperprolactinaemia) 172
Impotence/ loss of libido/ male hypogonadism 174
Gynaecomastia 175
Delayed puberty 176
Short stature 178
Precocious puberty 179
Thyroid function testing:general 180
Hyperthyroidism (thyrotoxicosis) 182
Hypothyroidism 186
Hypercalcaemia 188
Hypocalcaemia/ osteomalacia 190
Diabetes mellitus 194
Which type of diabetes isit? 200
Monitoring diabetic control 204
Laboratory assessment of glycaemic control 206
Diabetic emergencies:diabetic ketoacidosis, hyperosmolar
non- ketotic syndrome, and lactic acidosis 210
Investigation of hyperlipidaemia 212
Test protocols 216
Combined anterior pituitary function testing 218
Water deprivation test 220
Diagnostic trial of desamino
D- arginyl vasopressin 222
Low- dose dexamethasone suppression test 223
High- dose dexamethasone suppression test 224
Short Synacthen
®
test 225
Long (depot) ACTH test 225
Chapter2

124
CHAPTER2 Endocrinology and metabolism
124
Guiding principles ofendocrine
investigation
Investigations for endocrine disease have caused a lot of confusion in the
minds of clinicians (many still do!). Tests have come and gone over the years
and have been adopted with varying degrees of enthusiasm by specialist
centres. In particular, there is often confusion over which tests to do, what
procedures to follow, and how to interpret the results. In some areas (e.g.
Cushing’s syndrome), controversy persists among the experts. In others, a
clear consensus approach exists.
Some useful general principles
1. Use dynamic tests, rather than random (untimed) sampling where the
hormone under investigation is secreted in infrequent pulses (e.g. GH)
or levels are easily inuenced by other factors (e.g. cortisol varies
markedly with stress levels and has a marked circadian rhythm; see
Table2.1).
2. Use the correct collection method, e.g. ACTH or insulin levels require
rapid separation of the sample and prompt freezing (−20°C). Timing of
sampling may also be critical. Label samples carefully, including time of
collection! Check procedures with the local laboratory. Many units will
have protocols for endocrine investigations.
3. Do tests in the correct sequence, e.g. ACTH levels can only be
interpreted once the cortisol status is known. In many cases,
simultaneous samples are required for interpretation, e.g. PTH
with Ca
2+
for hypo- / hyperparathyroidism, glucose with insulin for
insulinoma.
4. ‘Normal’ results may be ‘abnormal’, depending on the activity of the
hormone axis under investigation. Interpretation of the absolute
levels of hormones in isolation may be highly misleading. For
example, a serum PTH within the normal range in the presence of
hypocalcaemia suggests hypoparathyroidism; ‘normal’ LH and FSH
levels in the presence of a very low serum testosterone concentration
suggest pituitary failure. In both instances, the regulatory hormone
concentration is inappropriately low. Thus, the level of the regulatory
hormone (or releasing factor) must be considered in the light of the
simultaneous level of the ‘target’ hormone or metabolite.
In general
• If you are suspecting a LOW level— do a STIMULATION test (to see if
it staysLOW)
•
If you are suspecting a HIGH level— do a SUPPRESSION test (to see if
it staysHIGH)

GUIDING PRINCIPLES OFENDOCRINE INVESTIGATION
125
5. Results may vary according to the laboratory assay. Reference ranges
varybetween laboratories— it is especially important with endocrine
tests to interpret your results according to your laboratory’s ‘normal
range’. Also, interfering factors may dier between assays, e.g. dierent
PRL assays cross- react very dierently with macroprolactin
(E Galactorrhoea (hyperprolactinaemia), pp. 172–3). Some individuals
have a heterophile interfering antibody that aects the results of many
radioimmunoassays. Resist ‘discarding clinical evidence in favour of a
numerical value’.
6. Beware of interfering medication, e.g. inhaled beclomethasone can
suppress serum cortisol levels; administered hydrocortisone (cortisol)
is detected by the cortisol assay; synthetic androgens and oestrogens
can appear to cause low serum testosterone/ oestrogen (as they are
not detected in the testosterone/ oestrogen assay); some anti- emetics
and antipsychotics can raise circulating PRL levels; carbenoxolone
or liquorice may cause hypokalaemia. Always ask patients for a full
medication list (including herbal remedies and other self- medications).
7. Take a family history. Familial forms of many common endocrine
problems exist that require important changes in the management
approach, e.g. familial hypercalcaemia may suggest MEN- 1 or MEN- 2
requiring a dierent form of parathyroid surgery and a risk of
phaeochromocytoma (MEN- 2).
Endocrine tests are generally expensive and should not be performed
unnecessarily or outside standard protocols. Dynamic tests may have cau-
tions and contraindications and can be hazardous if used inappropriately
(see Table 2.1). Ahigh degree of organization and close liaison with the
laboratory are required to perform these tests in a way that can be clearly
interpreted. Dynamic tests should ideally be performed in an endocrine
investigation unit. Chemical pathologists (clinical biochemists) and other
laboratory sta generally have great experience with performing and
interpreting endocrine tests— seek their advice, wherever possible, before
embarking on tests with which you are unfamiliar. Tests in children should
be performed and interpreted under expert paediatric guidance.

126
CHAPTER2 Endocrinology and metabolism
126
Further reading
American Association of Clinical Endocrinologists Clinical Guidelines. AACE/ ACE clinical practice
guidelines. M https:// www.aace.com/ publications/ guidelines.
Endocrine Society. Endocrine Society Guidelines. M https://www.endocrine.org/guidelines-and-
clinical-practice.
Ismail AA, Barth JH. Wrong biochemistry results. BMJ 2001; 232
:705– 6.
Table2.1 Random sampling vs dynamic testing
Hormone Random or dynamic sampling?
GH Dynamic:glucose tolerance test for excess; insulin
stress test or GHRH arginine stimulation test for
deciency
IGF- 1 Random
LH, FSH Random in ♂
, post- menopausal
Timed with menstrual cycle in premenopausal♂
Random or stimulated in pre- pubertal children
Testosterone Random
Oestrogen
(oestradiol)
Random in ♂
, post- menopausal ♂
Timed with menstrual cycle in premenopausal
ACTH Random
Cortisol Dynamic:dexamethasone suppression test for excess;
Synacthen
®
stimulation test if suspect deciency
TSH Random
T4 and T3 Random
PRL Random
ADH/ vasopressin Do not normally measure directly
Osmolality Dynamic:water deprivation test if suspect DI
PTH Random, but need simultaneous Ca
2+
value
Insulin Fasting, plus simultaneous glucose value
Calcitonin Random
Renin/ aldosterone Upright usually, o medication
Metanephrines Measure in urine, 24h sample or seated random
plasma sample
5HIAAs Measure in urine, 24h sample

GUIDING PRINCIPLES OFENDOCRINE INVESTIGATION
127

128
CHAPTER2 Endocrinology and metabolism
128
Hypothalamus/ pituitary function
Hypothalamic dysfunction
Causes
•
Familial syndromes (Laurence– Moon– Biedl, Prader– Willi).
• Tumours (especially craniopharyngiomas, dysgerminomas, optic gliomas,
meningioma— rarely pituitary tumours).
• Pituitary surgery.
• Inltration (histiocytosis X, sarcoidosis).
• Trauma.
• Meningitis.
• Encephalitis.
• TB.
Symptoms andsigns
•
Obesity/ hyperphagia.
• Somnolence.
• Thermodysregulation.
• DI.
• Hypogonadism.
• Precocious puberty.
Investigations
•
MRI.
• Water deprivation test for DI (E Polydipsia and polyuria:diabetes
insipidus, pp. 134–6), tests of pituitary function.
Hypopituitarism
Denition
Failure of one or more pituitary hormones (usually multiple).
Causes
•
Congenital.
• Pituitary tumour (including infarction of tumour ‘apoplexy’).
• Craniopharyngioma.
• Post- cranial irradiation.
• Following pituitary irradiation.
• Metastases to the pituitary (especially the breast).
• Post- surgery.
• Empty sella syndrome (occasionally).
• Sheehan’s syndrome (infarction with postpartum haemorrhage).
• CTLA- 4 Ig immunotherapy for malignancy.
Symptoms andsigns
Often very vague, e.g. tiredness, normocytic anaemia (easily missed).
Combined with impotence or amenorrhoea— very suggestive. Other clues
include loss of body hair (especially axillary), reduced shaving, hyponatrae-
mia, and growth failure in children. DI is not a feature (unless there is also
hypothalamic damage), as ADH can be secreted directly from the hypothal-
amus. There may also be signs of an SOL— bitemporal hemianopia (rarely
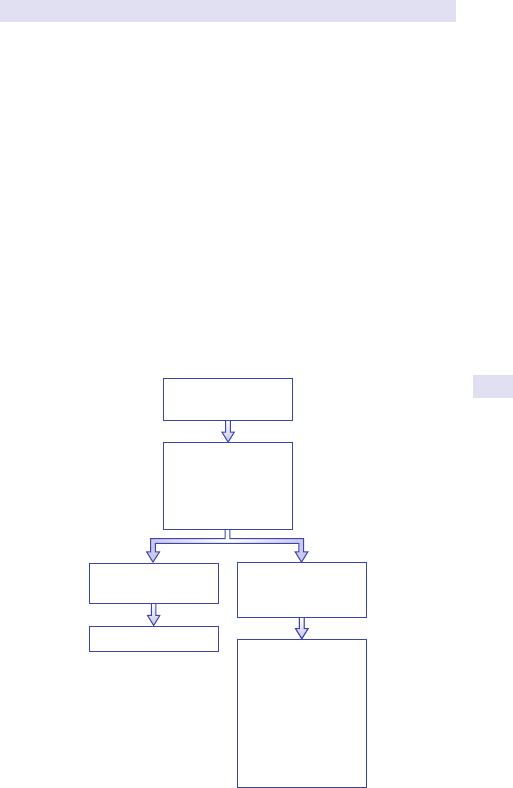
HYPOTHALAMUS/PITUITARY FUNCTION
129
optic nerve compression, homonymous hemianopia); headache (especially
following apoplexy); III, IV, V1, V2, or VI cranial nerve lesions; and CSF rhi-
norrhoea. Occasionally, galactorrhoea following pituitary stalk compression
by a tumour (‘disconnection’). Note
: generally GH is lost rst, then LH/
FSH, and ACTH/ TSH last. If multiple pituitary hormones are decient, GH
deciency can be assumed.
Investigations
(See Fig. 2.1.) Basal- free T4 (not TSH, which can be misleadingly normal),
LH, FSH, PRL, and oestrogen/ testosterone are usually sucient to test the
thyroid and gonadal axes. Adrenal and growth hormone testing requires
dynamic tests (e.g. insulin tolerance test (ITT), but see below). Severe GH
deciency = peak stimulated GH <12.3mU/ L (4.1ng/ mL) using GHRH
arginine test; <15.3mU/ L (5.1ng/ mL) using ITT testing. Note that the
short Synacthen
®
test (E Short Synacthen
®
test, p. 225) is only suitable
for testing the hypothalamopituitary– adrenal axis if pituitary failure is long-
standing (>6 weeks), allowing time for adrenal atrophy tooccur.
Basal: U&E, free T4
(not TSH), LH, FSH,
testosterone/
oestradiol, cortisol,
prolactin, IGF-1
Basal tests normal,
low level of suspicion
Stop: normal
Basal tests abnormal
or high level of
suspicion
Suggestive signs/
symptoms
• Short Synacthen
®
test (cortisol)
• Insulin tolerance
test* (GH, cortisol)
• MRI pituitary +
visual elds
• LHRH test
(optional)
Fig.2.1 Investigation of suspected hypopituitarism. Note:specialist paediatric advice
should be taken in children.
* E See also note under Alternative investigations, p. 130.

130
CHAPTER2 Endocrinology and metabolism
130
Alternative investigations
• GHRH arginine stimulation test. Although the ITT (E Test protocols,
pp. 216–17) is the traditional gold standard test for GH and 2° adrenal
insuciency, the GHRH arginine test has equal sensitivity and specicity
for GH deciency. It is also safer and better tolerated and is gradually
replacing theITT.
1
• Combined anterior pituitary testing— giving LHRH, thyrotropin-
releasing hormone (TRH), ACTH, and GHRH (E Combined anterior
pituitary function testing, p. 218)— no clear advantage of this approach
has been demonstrated and the results of the LHRH test, in particular,
are dicult to interpret in pre- pubertal children.
• Long (depot) Synacthen
®
test— rarely required (E Adrenal failure,
pp. 162–4; Long (depot) ACTH test, p. 225).
Further reading
Molitch ME, Clemmons DR, Malozowski S, Merriam GR, Vance ML; Endocrine Society. Evaluation
and treatment of adult growth hormone deciency. J Clin Endocrinol Metab 2011; 96:1587– 609.
1 National Institute for Health and Care Excellence. UK NICE guidelines for GH replacement 2003. M
https://www.nice.org.uk/guidance/ta64.

HYPOTHALAMUS/PITUITARY FUNCTION
131

132
CHAPTER2 Endocrinology and metabolism
132
Acromegaly (growth hormone excess)
For GH deciency, see E Hypothalamus/ pituitary function, pp. 128–30.
Clinical features
• Often insidious over manyyears.
• Enlarging hands and feet with rings having to be resized.
• Increase in shoesize.
• Coarsening of facial features, especially enlargement and broadening of
thenose.
•
Sweating.
• Headache.
• Malocclusion (protuberance of the lower jaw) and splaying of theteeth.
• Skintags.
• Hypertension.
• Cardiac failure.
• Renal stones.
• Arthritis.
• Colonic polyps.
• Sleep apnoea.
• Carpal tunnel syndrome.
• DM.
• May be local symptoms from the pituitary tumour and symptoms/
signs of loss of other pituitary hormones (E Hypothalamus/ pituitary
function, pp. 128–30).
•
GH excess commencing before puberty results in gigantism (tall stature).
Investigations
• Arandom GH level is not helpful— may be high in normal people.
• Arandom IGF- 1 level should be measured and compared with
laboratory normal ranges corrected for age. This can be used as a
screening test, but IGF- 1 assays vary in reliability. IGF- 1 levels should be
raised in all cases of acromegaly, but levels can be aected (reduced) by
fasting and systemic illness.
•
Where IGF- 1 results suggest GH excess, this should be conrmed in a
standard 75g OGTT with glucose and GH measurements at 0, 30, 60,
90, and 120min.
•
If no GH values are <2mU/ L, then a diagnosis of acromegaly is
conrmed.
•
The vast majority (99%) of cases of acromegaly are due to pituitary
tumours. If a pituitary tumour is not seen on MRI scanning, yet
acromegaly is conrmed, a GHRH level should be requested to exclude
ectopic production of this polypeptide by non- pituitary tumours
stimulating the release of GH from the pituitary.
•
For follow- up of treated cases of acromegaly, IGF- 1 levels (more
sensitive) and/ or random GH levels can beused.
• Life expectancy appears to return to normality when the nadir of GH
values is <3mU/ L or IGF- 1 levels return to the referencerange.
Further reading
Katznelson L, Laws ER Jr, Melmed S, et al.; Endocrine Society. Acromegaly:an Endocrine Society
clinical practice guideline. J Clin Endocrinol Metab 2014; 99
:3933– 51.

ACROMEGALY (GROWTH HORMONE EXCESS)
133

134
CHAPTER2 Endocrinology and metabolism
134
Polydipsia and polyuria:
diabetes insipidus
‘First- linetests’
It is relatively common for patients to report excess thirst or i need to
pass urine. Figure 2.2 and Box 2.1 summarize the causes. Prostatism and
urge incontinence resulting in urinary frequency should be distinguished by
history- taking as the patients do not have thirst. Then the rst step is to
identify straightforward causes, such as drugs (diuretics), DM, hypercalcae-
mia, hypokalaemia, and chronic renal failure with U&E, creatinine, glucose,
and Ca
2+
. Measuring 24h urine volume is also useful as volumes over 3L
are likely to be pathological and volumes under 2L do not require further
investigation. AGTT should not be required to diagnose DM as the renal
threshold for glucose needs to be exceeded (7
10mmol/ L) to cause polyuria
and there should be glucose in theurine.
‘Second- linetests’
Once other diagnoses have been excluded, subsequent tests aim to distin-
guish DI from ° polydipsia (compulsive water drinking). Acarefully super-
vised water deprivation test should be performed (E Water deprivation
test, pp. 220–1). However, it is not always easy to arrive at a conclusive diag-
nosis. Serum Na
+
levels are helpful, as DI is unlikely if Na
+
<140mmol/ L.
Morning spot urine osmolality after overnight water restriction (not shown
on chart) is occasionally useful— values >600mOsmol/ L make signicant
degrees of DI unlikely. Measuring 24h urine volume is also useful, as volumes
over 3L are likely to be pathological. However, obligate urine volumes as low
as 2L could still cause the patient to complain of polyuria. In such border-
line cases, the distinction between partial DI, normality, and ° polydipsia can
be very dicult. It has been proposed that in an adult or child over 2years
of age, a 24h urine volume of >40mL/ kg body weight, a plasma osmolarity
of <300mOsmol/ L, and a −ve test for glucose is diagnostic of DI, but this
requires conrmation.
Guidance on interpretation of second- line tests, including the water
deprivation test, is given in Table 2.2. Note that ° polydipsia may be a
psychiatric condition but can also occur in patients with a dry mouth
(e.g.Sjögren’s syndrome, anticholinergic drugs) or who have been previ-
ously encouraged to drink regularly ‘to help their kidneys’.
Box 2.1 Causes ofpolyuria/ polydipsia
Diabetes mellitus
•
DI (cranial or nephrogenic)
• HighCa
2+
• LowK
+
• Chronic renal failure
• ° polydipsia (including dry mouth, e.g. Sjögren’s)
POLYDIPSIA AND POLYURIA: DIABETES INSIPIDUS
135
Distinction between partial cranial DI and habitual (psychogenic) water
drinking is complicated by the fact that drinking very high volumes over time
may ‘wash out’ the renal medullary concentrating gradient. In this situation,
a plasma vasopressin level at the end of the water deprivation test may be
very helpful to distinguish a lack of vasopressin from a lack of vasopres-
sin action. A24h urine volume is also helpful as volumes of <3L/ day are
unlikely to cause renal ‘washout’. Clues to ° polydipsia include an initial
plasma osmolality (and serum Na
+
) that is low, plasma osmolality rising
to >295mOsmol/ L, and thirst not abolished by desmopressin, despite a
rise in urine osmolality. Note that ‘full- blown’ cranial DI results in urine
volumes of around 500mL/ h (12L/ day).
Cause identied
•
Diabetes mellitus
•
Hypokalaemia
•
Chronic renal failure
•
Hypercalcaemia
•
Diuretics
Polydipsia/polyuria
Cause not identied
•
24h urine collection for
volume
• Morning urine
osmolality
• Review serum Na
+
• Water deprivation test
See text for interpretation
Distinguish remaining
causes
•
Diabetes insipidus:
cranial, partial cranial,
nephrogenic
•
Primary polydipsia:
psychogenic, dry mouth
•
Urinary frequency (e.g.
unstable bladder)
(normal ADH axis)
History: prostatism, urinary
frequency (no thirst)
Drug history: diuretics
Blood: glucose, Na
+
, K
+
,
creatinine, calcium
Urine: glucose, protein
Fig.2.2 Investigation of polydipsia/ polyuria. Note:once cranial DI is diagnosed,
further investigations into the underlying cause are required (E Hypothalamic
dysfunction under Hypothalamus/ pituitary function, p. 128).

136
CHAPTER2 Endocrinology and metabolism
136
Interpretation ofsecond- line tests forpolyuria/ polydipsia
‘If all elsefails’
In cases of doubt, a carefully supervised therapeutic trial of desmopressin
can be useful to distinguish DI from ° polydipsia (E Diagnostic trial of
desmopressin, p. 222). This should be done as an inpatient, as there is a risk
of signicant hyponatraemia in habitual water drinkers. The principle is that
patients able to regulate water intake according to their thirst (DI) should
not develop a hypo- osmolar plasma. In ° polydipsia, the urine volume will
fall and the urine concentrating gradient will gradually recover. However, if
the patient continues to drink due to their psychological drive, rather than
their thirst, they will become water- overloaded and hypo- osmolar.
An additional valuable test to distinguish partial DI from ° polydipsia is
hypertonic saline infusion testing, which usually requires access to a plasma
vasopressin assay but has been used with urinary vasopressin levels.
2–3
MRI
scanning typically shows an i signal in the posterior pituitary, which is lost
in cranial DI. However, this sign is not helpful in distinguishing more subtle
degrees of DI from other causes.
Table2.2 Interpretation ofsecond- line tests forpolyuria/ polydipsia
Normal Partial DI:cranial (C)
ornephrogenic (N)
Primary
polydipsia
Random serum Na
+
Normal >140mmol/ L <140mmol/ L
Random serum
osmolality**
Variable >290 <290
Morning urine
mOsm**
Variable Unlikely if >600 (C)
Excluded if >600 (N)
End of water
deprivation test before
desmopressin**
Urine >600
Plasma
280– 295
Urine <600
Plasma >295
Urine >600*
Plasma
280– 295
Urine osmolality after
desmopressin SC**
>600 Rises to >600 or >50%
i (C); rises to <600
or <50% i (N)
Rises to
>600* or
>50% i
Plasma vasopressin
at end of water
deprivation test
Normal
for plasma
osmolality
Low for plasma
osmolality(C); normal
forplasma osmolality (N)
Normal
for plasma
osmolality
* With long- standing large- volume polyuria (>3L/ day), these values may not be achieved due to
washout of the renal medullary concentrating gradient— if results equivocal, seetext.
** Osmolalities are all expressed in mOsmol/ L.
2 Robertson GL. Diabetes insipidus:diagnosis and management. Best Pract Res Clin Endocrinol Metab
2016; 30
:205– 18.
3 Diederich S, Eckmanns T, Exner P, Al- Saadi N, Bähr V, Oelkers W. Dierential diagnosis of poly-
uric/ polydipsic syndromes with the aid of urinary vasopressin measurements in adults. Clin Endocrinol
(Oxf ) 2001; 54
:665– 71.

POLYDIPSIA AND POLYURIA: DIABETES INSIPIDUS
137

138
CHAPTER2 Endocrinology and metabolism
138
Box 2.2 Causes ofpseudohyponatraemia
With normal serum osmolality
•
Hyperproteinaemia (e.g. myeloma)
• Hyperlipidaemia (hypertriglyceridaemia)
• Glycine or sorbitol (from bladder irrigant)
With raised osmolality
•
Hyperglycaemia
• Mannitol
• Glycerol
Box 2.3 Criteria fordiagnosingSIADH
• Hyponatraemia present
• No diuretics
• Nooedema
• Normal renal function
• Normal adrenal function
• Normal thyroid function
• Urine Na
+
>20mmol/ L
• Euvolaemic
Hyponatraemia (including syndrome
ofinappropriate antidiuretic hormone)
Hyponatraemia is a very common clinical problem. Figure 2.3 shows a ow
chart for investigation. If patients are on diuretics, further evaluation is usu-
ally not possible. The diuretic will need to be discontinued. If this is not
possible, hyponatraemia is likely to be attributable to an underlying condi-
tion (cardiac, renal, or liver failure). Pseudo- or dilutional hyponatraemia
is important to exclude at an early stage (see Box 2.2). Acareful clinical
assessment should be made of the volume status, including identication
of oedema, uid loss (e.g. diarrhoea, stula leakage), and signs of dehy-
dration, including postural drop in BP. Aurine Na
+
and TSH estimation is
useful at this stage (see Fig. 2.3). Note that the most important diagnosis
not to miss is hypoadrenalism, as this can be fatal if untreated. Clinicians
should have a low threshold for performing a short Synacthen
®
test
(E Short Synacthen
®
test, p. 225). Hypoadrenalism due to pituitary failure
may not be accompanied by hyperkalaemia, hypotension, or hyperpigmen-
tation and can easily be missed. Cerebral salt wasting occurs within days
of brain injury (e.g. SAH, neurosurgery, or stroke) and is probably due to
release of brain natriuretic peptides.
The syndrome of inappropriate antidiuretic hormone (SIADH) is a diagno-
sis of exclusion (see Box2.3).

139
HYPONATRAEMIA (INCLUDING SIADH)
All the criteria in Box 2.3 should be met. A specic cause for SIADH is
frequently not found or there may be a combination of precipitating factors
(see Table 2.3). In the elderly, a state of chronic SIADH is relatively com-
mon and usually explains hyponatraemia persisting for many years without
any other apparent cause. Aected individuals should be encouraged to
drink less than a litre a day (‘5 cups or less’), to only drink if they are thirsty,
and to avoid exacerbating factors (see Table2.3).
Features ofSIADH/ hyponatraemia that are often
underappreciated
1. Other than a CXR, there is no requirement to search for an underlying
malignant cause. If there is underlying malignancy, it is usually extensive,
very apparent, and incurable (e.g. extensive small- cell carcinoma of
thelung).
2. The urine osmolality does not have to be high. In individuals drinking
large volumes of uid, a urine osmolality as low as 250mOsmol/ L
(i.e. less than plasma) may be inappropriately concentrated, reecting
trueSIADH.
3. Conditions previously diagnosed as ‘sick cell syndrome’ are now
thought to represent SIADH in ill patients.
4. ‘Water intoxication’ is usually the combination of SIADH and excessive
uid intake. Healthy patients drinking to excess can rarely exceed
the renal capacity to excrete a water load (7
12L/ day) and hence
do not become hyponatraemic. Adegree of SIADH is required for
potomaniacs (excess water drinkers) to become hyponatraemic.
5. The post- operative state contains many precipitants to SIADH
(nausea, pain, opiates, pneumonia) and ADH secretion is promoted by
hypovolaemia from blood loss. The administration of ‘3L of IV uid a
day’ post- operatively frequently results in hyponatraemia.
Table2.3 Causes ofSIADH
Cause Examples
Drugs Carbamazepine, chlorpropamide, opiates, psychotropics,
cytotoxics
CNS disorders
Head trauma, post- pituitary surgery (transient), stroke,
cerebral haemorrhage, GBS, meningitis, encephalitis, ts
Malignancy
Small- cell lung cancer, pancreas, prostate
Chest disease Pneumonia, TB, abscess, aspergillosis
General stimuli Nausea, pain, smoking
Other Acute intermittent porphyria
140
CHAPTER2 Endocrinology and metabolism
140
Further reading
Spasovski G, Vanholder R, Allolio B, etal.; Hyponatraemia Guideline Development Group. Clinical
practice guideline on diagnosis and treatment of hyponatraemia. Eur J Endocrinol 2014; 170
:G1– 47.
6. Symptoms of hyponatraemia, such as drowsiness, coma, or ts, are
dependent on the rate of fall of serum Na
+
, not the absolute value.
Patients who are alert with Na
+
<125mmol/ L have clearly been
chronically hyponatraemic and their serum Na
+
requires only gentle
correction. However, a very rapid fall in serum Na
+
to <130mmol/ L
(typically due to massive infusion of hypotonic uid into the bladder)
may cause coma and needs to be corrected as a medical emergency
with hypertonic saline.
Cardiac failure
Renal failure
Hepatic failure
Pseudohyponatraemia
Oedema absent Oedema present
No Yes
Raised glucose,
triglycerides, total
protein
Normal or elevated
serum osmolality
Hypovolaemia
Urine Na
+
>20mmol/L
Hypoadrenalism
Diuretics
Salt-losing
nephropathy
Hypovolaemic
Urine Na
+
<20mmol/L
Diarrhoea
Loss of other
body uids incl.
sweat, burns,
bowel
obstruction
Euvolaemic
Urine Na
+
>20mmol/L
SIADH
Hypothyroidism
Fig.2.3 Investigation of hyponatraemia.

141
HYPONATRAEMIA (INCLUDING SIADH)
142
CHAPTER2 Endocrinology and metabolism
142
Obesity/ hypercortisolism
Endocrinologists are frequently asked to determine whether there is an
underlying endocrine cause in patients who are obese. 2° causes of obesity
are listed in Box 2.4. Along history of obesity, typically going back to child-
hood, is characteristic of constitutional obesity and further investigation,
other than thyroid function, is rarely necessary. However, simple obesity
may result in eects suggestive of hypercortisolism, e.g. striae, bruising,
central obesity, rounded facial features, mild hyperandrogenism in women,
bualo hump, hypertension, and hyperglycaemia. Rapidly progressive
obesity, marked hypertension, hypokalaemia, proximal muscle weakness,
poor sleep, osteoporosis/ vertebral collapse, and marked hirsutism or acne
are more suggestive of hypercortisolism and require further investigation.
Hypothalamic damage is usually apparent from the history.
The optimal approach to the diagnosis of hypercortisolism (Cushing’s
syndrome) is probably the most controversial subject in endocrinology.
Endocrinologists who have seen many cases of Cushing’s syndrome have
seen exceptions to every rule, and the episodic nature of ACTH and corti-
sol secretion means that low values can occur even in disease. True cyclical
Cushing’s disease also occurs but israre.
Diagnosis consists oftwophases
1. Does the patient have hypercortisolism ornot?
2. What is the cause of the hypercortisolism? Phase 1 must be completed
rst, as phase 2 tests can only be interpreted if hypercortisolism is
present.
Investigation ofhypercortisolism phase1
Does thepatient have hypercortisolism?
Patients being investigated for hypercortisolism should look Cushingoid.
One exception is malignant ectopic ACTH secretion (e.g. from small- cell
lung cancer) which can cause profound hypokalaemia before Cushingoid
appearance is apparent. Depression and alcoholism may cause abnormal
tests for hypercortisolism without representing a true hypercortisolae-
mic state and hence are termed ‘pseudo- Cushing’s syndrome’. Such
depressed patients often do not appear Cushingoid and alcoholism should
Box 2.4 Secondary causes ofobesity
• Constitutional
• Hypothyroidism
• Cushing’s syndrome
• Hypothalamic damage (extreme hyperphagia)
• Genetic, e.g. Prader– Willi
• GH deciency
• Drugs, e.g. antidepressants

OBESITY/HYPERCORTISOLISM
143
be identiable clinically and biochemically. If there is a high degree of sus-
picion of hypercortisolism in a depressed patient, midnight cortisol levels
<140nmol/ L or a −ve result on dexamethasone– corticotropin- releasing
hormone (CRH) testing (E Low- dose dexamethasone suppression test,
p. 223) may be helpful in excluding the diagnosis. Note that iatrogenic
or factitious Cushing’s syndrome is usually due to a steroid other than
hydrocortisone (not detected in the cortisol assay) and characteristically
results in an apparent suppression of the hypothalamopituitary– adrenal
axis on testing.
Four tests are used todetermine whether a patient does have hypercortisolism
1. 24h urinary- free cortisol (UFC) collections. Three collections with
simultaneous creatinine excretion estimation are ideal. If the creatinine
excretion varies by >10% between collections, the samples are
not true 24h collections and should be repeated. If two or more
collections have a value >3 times the laboratory upper limit of normal
(e.g.>800nmol/ 24h), then the diagnosis of hypercortisolism is secure.
Patients with intermediate values should have repeat sampling after
several weeks or additional tests. Steroids, adrenal enzyme inhibitors,
statins, and carbamazepine must be discontinued prior to testing.
False +ves can be caused by pregnancy, anorexia, exercise, psychoses,
alcohol, and alcohol withdrawal.
2. Low- dose dexamethasone suppression test (LDST). This can be
performed overnight or over 2days (E
Low- dose dexamethasone
suppression test, p. 223), the latter having less false +ves. Some
authorities believe it adds little to UFCs as when cortisol secretion is
high, the UFC is clearly raised, but in times when it is intermediate,
the LDST may be normal. It is a useful outpatient screening test
(E
Overnight dexamethasone suppression test under Low- dose
dexamethasone suppression test, p. 223) in individuals who cannot
reliably collect 24h urine samples.
3. Late- night salivary cortisol test. High salivary cortisol levels (>4nmol/
L) measured between 11p.m. and 12 midnight indicate loss of diurnal
rhythm and are one of the best tests of hypercortisolism. Salivary
samples can be collected as an outpatient by drooling into a collection
tube or use of a cotton pledgelet. The tests should be repeated on two
occasions. An alternative is a venous sample taken via an indwelling
cannula in as relaxed a state as possible, preferably during sleep,
but this requires an inpatient admission. Values <140nmol/ L make
hypercortisolism very unlikely.
4. Dexamethasone- suppressed CRH test (E Low- dose dexamethasone
suppression test, p. 223). This is a modication of the LDST, which was
initially claimed to have a specicity of 100% for hypercortisolism, but
subsequent series suggest<80%.
Summary
In patients who appear Cushingoid, three UFCs should be performed (note
causes of false +ves). If these give equivocal results, additional tests are
required, including further UFCs, late- night salivary cortisols, and a formal
2- day LDST followed byCRH.

144
CHAPTER2 Endocrinology and metabolism
144
Investigation ofhypercortisolism phase2
What is thecause ofhypercortisolism?
The common and rare causes of hypercortisolism are summarized in
Tables2.4 and 2.5, along with useful clinical features. 765% of cases are due
to a pituitary adenoma (Cushing’s disease), 20% due to an adrenal adenoma
or carcinoma, and 10% due to ectopic ACTH production.
These are the three main causes to be distinguished using a combina-
tion of the tests shown under Investigations below. Distinction between
a pituitary adenoma (which may not be visualizable on MRI) and a small
indolent tumour (typically lung carcinoid) represents the greatest challenge.
Despite extensive investigation, the cause will remain uncertain in some of
thesecases.
Investigations
1. Plasma ACTH level (separate and freeze immediately). Undetectable
plasma ACTH levels are strongly suggestive of an adrenal tumour.
However, ACTH secretion is intermittent and two suppressed values
with simultaneous high cortisol levels (>400nmol/ L) are preferable and
should prompt adrenal CT scanning.
2. High- dose dexamethasone suppression test (E High- dose
dexamethasone suppression test, p. 224). Greater than 90%
suppression of basal UFC levels is strongly suggestive of a pituitary
adenoma. Lesser degrees of suppression are seen with ectopicACTH.
3. Inferior petrosal sinus sampling (IPSS). This is an excellent diagnostic tool
but requires expert radiological support and should only be performed
in tertiary referral centres. Atotal of 100mg IV of CRH is also given
via a peripheral vein, while sampling, to ensure active secretion of
ACTH during the test. ACTH levels are compared between the
Table2.4 Common causes ofhypercortisolism (Cushing’s syndrome)
Cause Pathology Characteristic features
ACTH- secreting
pituitary adenoma
(Cushing’s
disease)— 65%
Pituitary adenoma Typical features of hypercortisolism
with little virilization
Ectopic ACTH
secretion— 10%
Malignant:small- cell
lung cancer, thymic
carcinoid, medullary
thyroid cancer
Indolent/ benign:
bronchial/ pancreatic
carcinoids,
phaeo chromocytoma
Malignant:rapid progression, marked
hypokalaemia, proximal muscle
weakness, iBP, tumour clinically
apparent, few Cushingoid signs
Indolent:indistinguishable from
Cushing’s disease, tumour not easily
detected
Adrenal
tumour— 20%
Adrenal adenoma
Adrenal carcinoma
Adenomas:typical Cushingoid signs,
sometimes virilization
Carcinomas:rapid progression
(months) with virilization, poor
prognosis

OBESITY/HYPERCORTISOLISM
145
inferior petrosal sinus on both sides and a peripheral vein. Sampling is
performed at −15, 0, +15, and +30min after CRH injection. Ratios >2
(ideally >3) post- CRH are strongly suggestive of pituitary- dependent
disease. Risks include failure to enter the sinus and sinus thrombosis.
4. Imaging. Pituitary and adrenal imaging should not be performed without
biochemical testing, as non- functioning tumours of the pituitary and
adrenal are common (false +ves), and conversely functioning pituitary
tumours are often too small to be visualized by MRI (false – ve).
However, if the ndings are consistent with the biochemical tests, this is
useful supportive evidence. Patients with ndings suggestive of ectopic
ACTH production should have thin- slice CT of the chest looking for
a bronchial adenoma, and MRI scanning of the pancreas for an islet
tumour.
111
Indium- labelled octreotide scanning may also be useful in
locating small tumours.
5. Plasma CRH levels. Very rarely, ‘ectopic ACTH’ syndrome is actually
due to ectopic CRH production stimulating ACTH from the pituitary
(see Table 2.5). Raised plasma CRH levels may be diagnostic in this
condition.
Table2.5 Rare causes ofhypercortisolism (Cushing’s syndrome)
Cause Pathology Characteristic features
Ectopic CRH
secretion
Variety of tumours,
mostly carcinoids
Clinical features indistinguishable
from Cushing’s disease, but no
pituitary tumour, i serum CRH and
may fail to suppress with high- dose
dexamethasone
Ectopic gastrin-
releasing peptide
secretion
Medullary thyroid
cancer
Very rare, resembles ectopic CRH
Factitious ACTH
administration
Injections of ACTH Very dicult to distinguish from
ectopic CRH secretion or Cushing’s
disease but, if isolated from their
ACTH source, become adrenally
insucient in days
Cyclical Cushing’s
disease
Cyclical secretion from
pituitary adenoma
Cushing’s disease with
intermittently −ve tests
Pseudo-
Cushing’s
syndromes
Depression or
alcoholism
Clinical evidence of Cushing’s
disease may be limited; evidence of
depression or alcoholism
Bilateral
micronodular
adrenal
hyperplasia
Often associated with
Carney complex
Investigation suggestive of adrenal
tumour (ACTH suppressed), but
adrenals normal or slightly enlarged
and contain pigmented nodules
Bilateral
macronodular
adrenal
hyperplasia
Sporadic or familial Investigation suggestive of adrenal
tumour (ACTH suppressed), but
marked or very marked bilateral
nodular enlargement of adrenals on
CT scanning
146
CHAPTER2 Endocrinology and metabolism
146
Additional tests include
• Metyrapone test:here the adrenal enzyme blocker metyrapone is used
to lower cortisol levels. Pituitary adenomas respond by i ACTH
production, but ectopic sources of ACTH do not. The test can also
be used to conrm that ACTH levels are truly suppressed in adrenal
tumours (rarely necessary).
•
Peripheral CRH test:ACTH levels are measured before (−30, −15min)
and +15 and +30min after injection of 100mg IV of CRH into a
peripheral vein. Arise in ACTH levels of >34% is suggestive of a
pituitary adenoma. The addition of 5µg IV of desmopressin improves
the response rate and reduces false−ves.
Summary
(See Fig.2.4.)
Further reading
Nieman LK, Biller BM, Findling JW, etal. The diagnosis of Cushing’s syndrome:an Endocrine Society
Clinical Practice Guideline. J Clin Endocrinol Metab 2008; 93
:1526– 40.
Equivocal/suggest
ectopic cause
Suggest pituitary cause
Peripheral ACTH x2
+ cortisol
ACTH detectable ACTH undetectable
Adrenal CT/MRI
Establish hypercortisolism
Phase 1 tests – see text
• High-dose
dexamethasone
suppression test
• Inferior petrosal
sinus sampling
with CRH
• Pituitary MRI
Thin-slice CT chest
MRI pancreas
111
In-octreotide scan
Consider CRH
estimation
Fig.2.4 Hypercortisolism. Flow chart for diagnosing the cause once
hypercortisolism is established.

OBESITY/HYPERCORTISOLISM
147
148
CHAPTER2 Endocrinology and metabolism
148
Endocrine hypertension
Ninety- ve per cent of cases of hypertension are ‘essential hypertension’
with no specic underlying cause. If hypertension is very marked, occurs in
younger patients, is dicult to control with drugs, is episodic/ uctuating, is
of recent onset, is familial, is associated with recurrent hypokalaemia, or
has associated features (see Table 2.6), then an underlying cause should
be excluded.
History and examination should include features of conditions in
Table 2.6, with particular attention to paroxysmal attacks, drugs (e.g.
liquorice), and family history.
Figure 2.5 provides a ow chart for further investigations. At least three
separate BP readings should be obtained— 24h BP monitoring may be use-
ful where ‘white coat hypertension’ is suspected.
Table2.6 Secondary causes ofhypertension
Physical features Notes
Absent
Phaeochromocytoma
May be familial, e.g. in MEN- 2 (may have mucosal
neuromas), von Hippel– Lindau syndrome,
neurobromatosis; paroxysmal i BP in only 60% of
cases with headache, sweating, and palpitations
Hyperaldosteronism Multiple syndromes, including Conn’s syndrome
(see Table2.7)
Renal artery stenosis Congenital or acquired (atheroma)
Renal disease Any cause, including polycystic kidneys
Hyper- / hypothyroidism Diastolic hypertension with hypothyroidism, systolic
hypertension with hyperthyroidism
Hyperparathyroidism Does not usually improve after surgical cure
Drugs Epo, ciclosporin, cocaine, amphetamines,
steroids, liquorice, oestrogens, and androgens
Physical features present
Coarctation of the aorta
Cushing’s syndrome
Acromegaly
Pregnancy- induced
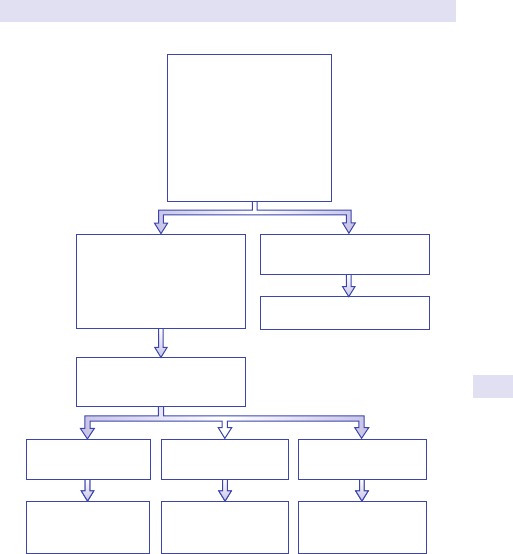
ENDOCRINE HYPERTENSION
149
The majority of 2° causes of hypertension can be rapidly excluded by the
investigations shown in the rst box of Fig. 2.5. If the results are normal or
the only abnormality is a low K
+
level, then the possibilities of hyperaldo-
steronism or RAS remain to be distinguished from essential hypertension.
Further investigation should be driven by the severity of the hyperten-
sion, the (young) age of the patient, and the diculty in obtaining control
withdrugs.
See Fig. 2.6
Investigate for
hyperaldosteronism
(Figs. 2.6 and 2.7)
Selective renal
angiogram
Notes:
1. Ideally, this test should be performed o all antihypertensive drugs for 2 week
s
(6 weeks for spironolactone) except alpha blockers.
2. Low renin and very low aldosterone should prompt, investigations for `apparent
mineralocorticoid excess'.
High renin
Equivocal results
Low renin, high
aldosterone
2
Upright renin/aldosterone
1
(Renal duplex ultrasound)
Investigate as appropriate
Results normal or low K
+
alone:
Hyperaldosteronism
Renal artery stenosis
Essential hypertension
Abnormal results other than
low K
+
U&E, creatinine
Urinalysis
TSH
Ca
2+
24h urinary catecholamines
Renal ultrasound
If indicated: 24h urine free
cortisol, CT aorta, tests for
acromegaly
Fig.2.5 Investigation of causes of hypertension.

150
CHAPTER2 Endocrinology and metabolism
150
Investigation ofrenal artery stenosis/ high reninlevels
Selective renal angiography remains the gold standard for diagnosing RAS—
other imaging methods can miss the diagnosis. Three- dimensional (3D) MR
angiography is now considered a non- invasive alternative. High renin lev-
els associated with hypertension (o drugs) in the absence of RAS should
prompt a search for a juxtaglomerular cell tumour of one kidney. Note that
the presence of hypertension is essential, as many conditions associated
with a low or normal BP can result in ‘appropriate’ hyper- reninaemia (e.g.
diuretics; cardiac, renal, or liver failure; hypocortisolism; hypovolaemia).
High renin levels can also occur in essential hypertension.
Investigation ofhyperaldosteronism
Hypertension with persistent hypokalaemia raises the possibility of hyper-
aldosteronism which may be due to a variety of causes (see Table 2.7).
Note that investigation for hyperaldosteronism is also appropriate with K
+
levels in the normal range if other investigations are −ve and hypertension
is marked, dicult to control, or in a younger patient. The optimal approach
to investigation remains controversial and equivocal cases frequently occur.
If there is marked hypokalaemia of recent onset, a 24h UFC (and review of
medication) is indicated to exclude recent- onset hypercortisolism (usually
due to ectopic ACTH production) in which Cushingoid features have not
yet become apparent. True hyperaldosteronism is never due to a malignant
lesion, so that if hypertension can be medically controlled, it is not always
necessary to establish a denitive diagnosis of aetiology. Adetailed scheme
is provided in Fig.2.6.
Establishing hyperaldosteronism
The initial investigation is an upright aldosterone/ renin ratio, performed
when the patient has been upright or sitting (not lying) for at least 2h. The
sample needs to be taken to the laboratory, separated, and frozen imme-
diately. Ideally, the patient should be on no antihypertensives other than
α
- blockers (e.g. doxazosin), as most drugs can aect interpretation of the
test results (see Table 2.8). This is dicult to achieve in subjects with very
marked hypertension. Combination antihypertensive therapy and spironol-
actone cause the most confusion. An undetectable renin with an unequivo-
cally high aldosterone level makes the diagnosis very likely. A normal or
raised upright renin excludes hyperaldosteronism. Borderline results should
be repeated o interfering medication and after K
+
replacement (hypoka-
laemia can inappropriately lower aldosterone). Alow renin with a normal
aldosterone can be seen in essential (‘low renin’) hypertension. Refer to
the laboratory for normal and diagnostic ranges. Additional tests (e.g. renin
after Na
+
restriction/ furosemide, aldosterone after captopril, Na
+
loading,
or IV saline) are used in specialist centres, but their exact role in testing
remains unresolved.
ENDOCRINE HYPERTENSION
151
Table2.7 Investigating established primary hyperaldosteronism
Change in
aldosterone with
posture
CT ndings
Adrenal venous sampling
(ratio of aldosterone
between sides)
Response to
glucocorticoids*
Treatment
of choice
Notes
Adenoma (Conn’s)
None/ fall Unilateral nodule >10:1 Absent Surgery
Renin- responsive adenoma Rise Unilateral >10:1 Absent Surgery
Unilateral hyperplasia None/ fall ‘Normal’ >10:1 Absent Surgery
Bilateral hyperplasia Rise ‘Normal’ No dierence Absent Medical
Glucocorticoid remediable
aldosteronism (GRA)
None/ fall Normal No dierence Present Steroids Very raised 18- oxo
cortisols. Positive
genetic screening**
*
Dexamethasone 0.5mg 6- h for 2– 4days resulting in supperession of aldosterone levels to nearly undetectable levels (usually associated fall in BP also).
** Positive for chimeric CYP11B1/ CYP11B2gene.
152
CHAPTER2 Endocrinology and metabolism
152
Still non-diagnostic—
treat medically
Apparent
mineralocorticoid
excess
Primary
hyperaldosteronism:
CT adrenals
Postural studies
Adrenal vein sampling
Upright plasma renin/
aldosterone o as
many interfering drugs
as possible
Undetectable renin,
raised aldosterone
Low renin, borderline
renin/aldosterone
ratio
Repeat renin/aldo o
all interfering
medication
Low renin, low
aldosterone
Review medication,
liquorice ingestion,
family history
Exclude other causes
of hypertension
24h urinary free
cortisol normal
Fig.2.6 Investigation of hyperaldosteronism/ mineralocorticoid excess in patients
with hypertension.
Table2.8 Renin/ aldosterone testing anddrugs
Drug Eect on PRA Eect on aldosterone
Drugs that i PRA
Spironolactone
Ca
2+
channel blockers
ACE inhibitors*
Diuretics
Vasodilators
i
Mayi
i
i
i
Variable
d
d
i
i
Drugs that d PRA
α
- blockers
NSAIDs
d
d
d
d
PRA, plasma renin activity.
* Angiotensin II receptor antagonists are likely to have same eects.
ENDOCRINE HYPERTENSION
153
Investigating thecause ofestablished primary
hyperaldosteronism
There are ve causes of established ° hyperaldosteronism with sup-
pressed renin and high aldosterone (see Box 2.5). Surgery (unilateral
adrenalectomy) is indicated for adenoma (65% of cases), the unusual renin-
responsive adenoma, and the rare cases of unilateral hyperplasia, but not
for bilateral hyperplasia (idiopathic hyperaldosteronism, 30% of cases) or
the rare familial glucocorticoid- remediable aldosteronism (GRA). Tests to
distinguish these are summarized in Box 2.5 and Fig.2.7.
E OHCM 10e, pp. 228–9.
Adenoma or
unilateral hyperplasia
Surgery
Bilateral
hyperplasia
Treat medically
Uncertain
Treat medically
Adrenal vein sampling
Shows lateralization
No lateralization Unsuccessful sampling
Adenoma likely
Surgery
Trial of glucocorticoid
GRA
No suppression Suppression
• Conrm with
genetic testing,
18-OH cortisols
• Screen family
• Treat with
glucocorticoid
CT/MRI adrenals
Unilateral nodule
No nodule/bilateral
nodules
Fig.2.7 Identifying the cause of established primary hyperaldosteronism.

154
CHAPTER2 Endocrinology and metabolism
154
If hyperaldosteronism is established and a nodule is visible on CT/ MRI
imaging, it is reasonable to proceed to unilateral adrenalectomy/ excision
of the nodule. If no nodule or bilateral nodules are seen, then adrenal
vein sampling is the most useful test to determine whether surgery should
be performed. Aldosterone levels after glucocorticoid administration or
genetic testing for the chimeric CYP11B1
/ CYP11B2 gene should be per-
formed beforehand to exclude GRA (see Table 2.7, p. 151— family mem-
bers may be only mildly hypertensive, making family histories unreliable).
Unfortunately, the right adrenal vein cannot be catheterized in up to 25%
of cases and there is a risk of precipitating adrenal haemorrhage. Postural
studies identifying a >50% rise in aldosterone comparing recumbent and
2– 4h of standing/ walking suggest idiopathic hyperplasia, but a small renin-
responsive adenoma not visible on CT could give similar results.
Investigating thecause ofapparent
mineralocorticoidexcess
Rarely, investigation reveals low renin and low aldosterone levels in the
presence of hypertension, hypokalaemia, and alkalosis. There are ve
causes of this (see Box 2.5). A 24h UFC estimation will rapidly exclude
recent- onset, aggressive hypercortisolism. Repeated enquiry should be
made for drug and liquorice product ingestion. The remaining causes may
be diagnosed by urinary cortisol/ cortisone ratio (11β- OH steroid dehydro-
genase deciency— often referred to alone as ‘apparent mineralocorticoid
excess’) or other appropriate changes in urinary and plasma cortisol metab-
olites (e.g. raised DOC levels— 11β- hydroxylase or 17α- hydroxylase de-
ciency) or responsiveness to amiloride (Liddle’s syndrome).
Box 2.5 Causes ofhyperaldosteronism/ apparent
mineralocorticoidexcess
Primary hyperaldosteronism (decreased renin, increased aldosterone)
•
Aldosterone- producing adenoma (Conn’s syndrome)
• Renin- responsive adenoma
• Idiopathic unilateral hyperplasia
• Idiopathic bilateral hyperplasia
• Glucocorticoid- remediable hyperaldosteronism
Apparent mineralocorticoid excess (decreased renin, increased aldosterone)
•
Liquorice ingestion, carbenoxolone, udrocortisone
• Congenital 11β- hydroxysteroid dehydrogenase deciency
• Liddle’s syndrome
• Congenital adrenal hyperplasia (11β- hydroxylase or
17α- hydroxylasedef.)
• Hypercortisolism

ENDOCRINE HYPERTENSION
155
Phaeochromocytoma
1. Clinical features. Phaeochromocytoma is rare, but an important diagnosis
not to miss— can result in fatal hypertensive crisis, especially during
surgery or after inadvertent β- adrenoreceptor blockade without α
blockade. It can be sporadic (90%) or be the rst clue to a familial
syndrome (see Table 2.6). 7
10% of cases are extra- adrenal, 10% multiple,
and 10% malignant (‘tumour of 10%’). Ninety per cent of cases have
sustained or paroxysmal hypertension, but paroxysmal attacks of some
nature are a feature of only 55% of cases. Pure adrenaline- secreting
lesions can occasionally cause hypotension. They are always intra- adrenal.
Phaeochromocytoma needs to be excluded in cases of incidentally found
adrenal masses. Paragangliomas are non- secreting phaeos.
2. Diagnostic tests. Plasma free or 24h urinary fractionated metanephrines
have replaced catecholamine estimations or catecholamine metabolites
(vanillylmandelic acids (VMAs)), as they are more sensitive and specic
and are released continuously. Asingle clearly positive estimation in the
presence of hypertension is usually sucient. If non- diagnostic, sampling
in the recumbent position may help conrm normal levels (metanephrines
are 2- fold higher in the seated position). Mild i can be seen in anxiety
states and with very small lesions detected in the follow- up of familial,
recurrent disease. Causes of false +ve results include methyldopa,
levodopa, labetalol, sotalol, tricyclic and monoamine oxidase inhibitor
(MAOI) antidepressants, paracetamol, sulfasalazine, sympathomimetics,
cocaine, clonidine withdrawal, intracranial events (e.g. SAH, posterior
fossa tumour), or metabolic stress (e.g. hypoglycaemia,MI).
3. Finding the tumour. Once the diagnosis is established, blockade (typically
with increasing twice- daily (bd) doses of phenoxybenzamine) should
be established before invasive investigation. The tumours are usually
large (>2cm) and bright on T2- weighted (T2W) images. CT/ MRI
scanning therefore identies virtually all adrenal lesions. Radionuclide
scanning with
131
I- MIBG (iodine- 131- meta- iodobenzylguanide) is useful
to conrm activity if >1 adrenal nodule is present and to identify
extra- adrenal lesions where no adrenal lesion is seen. Note that extra-
adrenal phaeochromocytomas (paragangliomas) are usually in the chest
or abdomen but can occur in the neck (including chemodoctomas
of the carotid body), pelvis, and bladder. Biopsy of suspected
phaeochromocytoma lesions is contraindicated.
4. Malignant phaeos. The only reliable indicator of malignancy in phaeos is
the presence of distant metastases or local invasion on histology. The
histological appearance of the tumour cells themselves surprisingly has
no signicance.
5. Genetic testing. Up to 30% of phaeos, especially if familial, will have a
genetic basis (see Table 2.9). Genetic testing is now recommended to
be considered in all conrmed cases, especially in familial, syndromic,
or recurrent cases. Fourteen gene loci have been associated with
phaeochromocytoma— the commonest are shown in Table 2.9.
Genetic testing can be targeted to limited loci according to the clinical
presentation, the location of the tumour, and whether it is metastatic
or not (E Further reading, p. 156).
E OHCM 10e, pp. 228–9, p. 738, p. 837.

156
CHAPTER2 Endocrinology and metabolism
156
Further reading
Lenders JW, Duh QY, Eisenhofer G, et al.; Endocrine Society. Pheochromocytoma and paragan-
glioma:an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2014; 99
:1915– 42.
Table2.9 Phaeochromocytomas:genetic mutations associated
withphaeochromocytomas or paragangliomas
Gene Syndrome Associated features Frequency
RET
MEN- 2 Medullary carcinoma
of the thyroid,
hyperparathyroidism
5%
VHL von
Hippel– Lindau
syndrome
Haemangioblastomata,
renal, pancreatic
tumours
8%
SDH B
Large, malignant, extra-
adrenal paragangliomas
6%
SDH D Often head and neck
paragangliomas
4%
SDH C Rare 0%
Neurobromatosis von
Recklinghausen’s/
disease
Skin neurobromata,
café- au- lait spots
4%
SDH, succinate dehydrogenase.

ENDOCRINE HYPERTENSION
157

158
CHAPTER2 Endocrinology and metabolism
158
Hypokalaemia
Persistent hypokalaemia (<2.5mmol/ L) can cause muscle weakness,
cramps, tetany, and polyuria, and exacerbate digoxin toxicity and predis-
pose to cardiac arrhythmias. The majority of cases are due to the common
causes (see Box 2.6) and are relatively easy to diagnose. However, puzzling
cases where none of these features are present occur and prompt further
investigation. Aow chart is shown in Fig.2.8.
Note in Fig. 2.8 the importance of identifying the presence of acidosis and
hypertension. Occult diuretic and purgative use should always be borne
in mind. The commonest cause of persistent hypokalaemia with no other
cause presenting in adulthood is Gitelman’s syndrome (NCCT- Na- Cl
cotransporter defect), an asymptomatic congenital disorder, which can usu-
ally be separated from the rare, more severe Bartter’s syndrome (which
usually presents neonatally or in early childhood and represents gene
defects in the renal tubular proteins NKCC2, ROMK, or CLCNKB) by low
serum Mg
2+
levels.
E OHCM 10e, p.98, p. 674.
Box 2.6 Common causes ofhypokalaemia*
• Diuretics
• Vomiting/ diarrhoea
• Intestinal stula
• Laxative or diureticabuse
• Steroids (including udrocortisone), liquorice, ACTH therapy
* See text for investigation of rare causes.
HYPOKALAEMIA
159
Further reading
Shaer AJ. Inherited primary tubular hypokalemic alkalosis: a review of Gitelman and Bartter syn-
dromes. Am J Med Sci 2001; 322
:316– 22.
Episodic/
thyrotoxic:
Periodic
paralysis
Mg
2+
woL
Gitelman’s
syndrome
Normal Mg
2+
Bartter’s or
other tubular
defect
High/normal bicarbo-
nate not hypertensive
Thyroid function
Episodic?
Serum Mg
2+
Diuretic screen
Urinary K
+
High bicarbonate
and hypertensive
Bicarbonate
Exclude common
causes (see table)
Low bicarbonate:
• Renal tubular
acidosis
• Uretero-sigmoid
diversion
• (11-β-OHase
deciency)
Investigate as for
hyperaldosteronism
• Upright renin/aldo
ratio
• Diuretic screen
+ve: occult abuse
• Urinary K
+
<10mmol/day
consider occult
purgatives
Fig.2.8 Investigation of hypokalaemia.

160
CHAPTER2 Endocrinology and metabolism
160
Hyperkalaemia
Artefactual and common causes need to be excluded, of which renal failure
is the most important (see Table 2.10). If these fail to reveal a cause, then
hypoadrenalism (which can be life- threatening), isolated mineralocorticoid
deciency, and type IV renal tubular acidosis (RTA) need to be excluded.
Hypoadrenalism is suggested by concomitant hyponatraemia, hypotension
(including postural), malaise, and skin pigmentation. Diagnosis is by short
Synacthen
®
testing (E Adrenal failure, pp. 162–4). Note that hyperkalae-
mia is not a feature of 2° (pituitary) hypoadrenalism since aldosterone
production is maintained by the renin– angiotensin system. Type IV RTA is
common in patients with diabetes. It is associated with renal tubular dys-
function, as well as mildly impaired glomerular function. Serum creatinine
is usually at, or above, the upper limit of normal. It is a state of hyporeni-
naemic hypoaldosteronism. Renin/ aldosterone testing is suggestive, but
there is no denitive test. Isolated mineralocorticoid deciency is usually
congenital (e.g. due to aldosterone synthase deciency) but can be acquired
(e.g. HIV disease). High renin and low aldosterone levels would be expected.
Aldosterone resistance (pseudohypoaldosteronism with high aldosterone
levels, but biochemical mineralocorticoid deciency) has been described.
E OHCM 10e, p.93, p. 301, p. 674.
Table2.10 Causes ofhyperkalaemia
Artefactual Other Rare, but important
Sample left
unseparated
overnight
Excess K
+
replacement Hypoadrenalism
Sample haemolysed K
+
- sparing diuretics, ACE
inhibitors
Type IV RTA
Myeloproliferative
disease (leakage of K
+
from high cell counts)
Renal impairment, especially
acute and after trauma or surgery
Metabolic acidosis (especially
DKA), rhabdomyolysis, burns,
massive blood transfusion
Isolated
mineralocorticoid
deciency

HYPERKALAEMIA
161

162
CHAPTER2 Endocrinology and metabolism
162
Adrenal failure
For causes of ° adrenal failure, see Table2.11.
Hypoadrenalism is often insidious in clinical onset. However, it is an impor-
tant diagnosis to make, as it can be life- threatening, especially at times of
stress. The key is to have a high index of suspicion. ° adrenal failure is
suggested by hyperkalaemia, hyponatraemia, hypotension (including pos-
tural), malaise, weight loss, nausea, abdominal pain, and skin pigmentation.
In pituitary (2° adrenal failure, hyperkalaemia, hypotension, and pigmenta-
tion are absent, and malaise may be the only feature. Signs/ symptoms of
gonadal failure (e.g. loss of libido, reduced shaving, or amenorrhoea), if
present, mandate exclusion of pituitary failure. Random cortisol levels can
be misleading, as they may be high in the morning and low in the evening.
Nonetheless, a random cortisol level >550nmol/ L excludes the diagnosis
and is a useful test in patients undergoing severe stress/ illness (e.g. in inten-
sive therapy unit (ITU)).
2 Do not delay treatment. Where there is a strong suspicion of adre-
nal failure, treatment must not be delayed pending investigation. A short
Synacthen
®
test or random cortisol should be performed immediately and
treatment commenced with steroids, awaiting results. Alternatively, treat-
ment with dexamethasone 0.5mg daily (which does not cross- react in the
cortisol assay) can be used and then discontinued for the day of testing.
Patients on other forms of glucocorticoid therapy should discontinue treat-
ment on the morning of the test and ideally 24h beforehand (12h for hydro-
cortisone or cortisone acetate). Mineralocorticoid replacement need not
be discontinued.
Short ACTH (Synacthen
®
)test
The standard test for adrenal failure is the short ACTH test. Alow dose
of synthetic ACTH (0.5 or 1.0µg) test was previously in vogue but has not
been conrmed to be useful.
For 2° (pituitary) adrenal failure, alternative tests include the insulin toler-
ance test (E Insulin tolerance test, pp. 216–17) and the metyrapone test.
However, these tests involve applying a stress and carry a risk in patients
who are profoundly hypoadrenal. They are only indicated in patients within
6 weeks of pituitary surgery or with a pituitary insult where hypotrophy of
the adrenal cortices has yet to develop.
Test todistinguish primary vs secondary adrenal failure
In the context of known pituitary disease and with failure of other pituit-
ary hormones, adrenal failure can be assumed to be 2° (pituitary) in origin.
Where isolated adrenal failure is identied, ° adrenal failure is most likely
and suggested by i skin pigmentation and hyperkalaemia.

ADRENAL FAILURE
163
Table2.11 Causes of° adrenal failure
Cause Associated features Diagnostic tests/ notes
Autoimmune adrenalitis
(>90% of cases in
developed countries)
Autoimmune damage
may be associated with
polyglandular failure types
1 and 2
Anti- adrenal
(21- hydroxylase)
antibodies
Drugs Ketoconazole, mitotane,
etomidate, rifampicin,
phenytoin
Exacerbate pre-
existing adrenal
impairment
TB
Extra- adrenal TB Calcied or enlarged
adrenals, extra- adrenal
TB, but may only
show shrunken glands
Other infections, e.g.
histoplasmosis, syphilis
Seen in North and South
America
Adrenal glands
enlarged
Metastatic malignancy Common with breast, lung,
melanoma, or GI cancer,
although does not always
cause adrenal failure
Enlargement/ deposits
in adrenal glands
on CT
Bilateral adrenal
haemorrhage
Anticoagulation, adrenal
vein sampling
Signs of haemorrhage
on CT
AIDS
CMV/ TB, Cryptococcus
adrenalitis
Adrenoleukodystrophy Especially in ♂ <15years,
dementia, quadriplegia
Adrenomyeloneuropathy
Neuropathy, blindness—
may appear after adrenal
failure
Familial glucocorticoid
deciency
Defective melanocortin
2 receptors, including
Allgrove’s syndrome,
hypoadrenalism associated
with seizures, achalasia, and
alacrima from childhood
Defective cholesterol
metabolism
Congenital adrenal
hypoplasia
Mutation in DAX1 or
related genes, causing
failure of adrenals
to develop. Adrenal
insuciency from birth

164
CHAPTER2 Endocrinology and metabolism
164
Three additional tests can be used toconrm thelevel ofadrenal failure
1. Basal plasma ACTH. This is usually the only additional test required.
High levels are seen in ° adrenal failure; ‘normal’ or low levels are
seen in 2° adrenal insuciency. Note that the sample must be taken
and separated immediately at least 24h after the last dose of a short-
acting glucocorticoid (e.g. hydrocortisone) to avoid pharmacological
suppression. Patients on a longer- acting steroid may have to have
the test repeated >24h after cessation of the steroid if the result is
equivocal.
2. Anti- adrenal antibodies (anti- 21 hydroxylase antibodies). These
antibodies are present in around 70% of patients with autoimmune
adrenalitis (Addison’s disease), the commonest cause of ° adrenal
insuciency. However, they can also be present without adrenal failure
in patients with other autoimmune conditions.
3. Long (depot) ACTH test. Chronic stimulation with ACTH can recover
function in adrenal glands that have failed because of lack of pituitary
ACTH, but not in ° adrenal failure. This is given in the form of ACTH
in oil on 2 consecutive days (E Long (depot) ACTH test, p. 225) or as
an infusion over 48h. With the advent of reliable ACTH assays, this test
is rarely indicated.
Additional diagnostic tests— exclude adrenoleukodystrophy
inmales
While the majority of cases of ° hypoadrenalism are due to autoimmune
disease in developed countries, there are multiple other rare causes.
These should particularly be considered where adrenal failure occurs
in childhood and/ or is associated with neurological disease or hypog-
onadism (see Table 2.11). In particular, adrenoleukodystrophy (ALD)
should be excluded. All ♂ diagnosed with ° adrenal failure should have
serum sent for very long- chain fatty acids (VLCFAs— raised in ALD), as
early bone marrow transplantation (and, to a limited extent, treatment
with ‘Lorenzo’s oil’) may prevent irreversible progressive neurological dis-
ease from developing (e.g. spastic paraparesis).
Further reading
Bornstein SR, Allolio B, Arlt W, etal. Diagnosis and treatment of primary adrenal insuciency:an
Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2016; 101
:364– 89.

ADRENAL FAILURE
165

166
CHAPTER2 Endocrinology and metabolism
166
Amenorrhoea
Amenorrhoea is often separated into ° (never menstruated) and 2° (cessa-
tion of periods after menarche) amenorrhoea, but many causes are shared
between the two categories. Structural assessment of the genital tract should
be performed earlier in investigation of ° amenorrhoea. Investigation of
oligomenorrhoea is similar to 2° amenorrhoea. Menorrhagia and intermen-
strual bleeding are due to dierent causes, often gynaecological in origin.
‘Irregular periods’ can fall into either category, depending on whether it
actually refers to intermenstrual bleeding or variably spaced (anovulatory)
periods. Aplan of investigation is shown in Fig.2.9.
In 2° amenorrhoea, it is helpful early on to identify ° ovarian failure (e.g.
due to Turner’s syndrome, premature ovarian failure, radiation, mumps
orchitis, radiation, chemotherapy, or non- 45XO gonadal dysgenesis) char-
acterized by high gonadotrophins (LH, FSH). Where the gonadotrophins
are equivocal or low, amenorrhoea due to hyperprolactinaemia or thyro-
toxicosis should be excluded, but the commonest diagnosis is chronic ano-
vulation due to polycystic ovarian syndrome. In this condition, the ovaries
still produce oestrogen, resulting in a +ve progesterone withdrawal test;
10mg of medroxyprogesterone is given daily for 5days and the test is +ve
if any menstrual bleeding occurs in the following week. If the test is −ve, a
pituitary (e.g. pituitary tumour) or hypothalamic (e.g. stress, anorexia ner-
vosa, systemic illness, or weight loss) cause resulting in profound oestrogen
deciency must be considered.
Further reading
Azziz R. The evaluation and management of hirsutism. Obstet Gynecol 2003; 101:995– 1007.
AMENORRHOEA
167
(a)
(b)
No periods for > 6 months
Structural imaging of
reproductive tract
Never had periods but
some pubertal
development, age > 14y
Never had periods
(primary amenorrhoea)
and delayed puberty
Secondary
amenorrhoea:
Investigate as
shown in (b)
Structural cause
Investigate as delayed puberty
Secondary amenorrhoea
Exclude pregnancy, depot progesterone
Take history of weight loss, stress,
excessive exercise
LH, FSH, oestradiol, prolactin, TSH, FT4
LH and FSH not
markedly raised
(e.g. FSH < 20IU/L)
Raised prolactin or
abnormal thyroid
function
Raised LH, FSH,
(e.g. FSH > 20IU/L)
Progesterone
withdrawal test
(see text)
Investigate and treat
prolactin excess or
thyroid dysfunction
Bleeding occurs:
chronic
anovulation
e.g. polycystic
ovarian
syndrome
No bleeding—
hypothalamic /
pituitary failure:
MRI pituitary
Consider stress/
weight loss/anorexia
Ovarian failure
Chromosomal
analysis esp. for
Turner’s syndrome
Abnormal
Normal
Fig.2.9 Investigation of amenorrhoea:(a)° and (b)2°. See E Delayed puberty,
p. 176 for investigation of delayed puberty.

168
CHAPTER2 Endocrinology and metabolism
168
Infertility
Detailed assessment of infertility is beyond the scope of this text and is
best referred to a specialist in this area. However, the general physician can
take the following basic steps, always remembering that the couple should
be assessed together as the problem may lie with the man, the woman, or
a combination ofboth:
1. Semen analysis of the ♂ and, where possible, a post- coital test to
conrm that live semen are delivered to the vaginaltract.
2. If amenorrhoea is present in the ♀, investigate as in Fig.2.9.
3. If the ♀ is menstruating, determine if the cycles are ovulatory, e.g. by
day 21, progesterone levels, or home measurement urinary dipstick of
the LHsurge.
If live semen are delivered and ovulation is occurring, then structural dam-
age or chlamydial infection in the female genital tract is likely, and will require
gynaecological assessment.

169
INFERTILITY

170
CHAPTER2 Endocrinology and metabolism
170
Hirsutism/ virilization (raised
testosterone)
Hirsutism refers to an i in androgen- dependent terminal hairs in the ♀,
typically over the face/ chin, lower abdomen, arms and legs, and around
the areola of the breast. Virilization reects much higher androgen levels
and comprises the features shown in Box 2.7. Over 20% of women have
more androgen- dependent hair than they consider to be normal. In >95%
of cases, this is associated with androgen levels in the ♀ normal range or
slightly elevated in association with polycystic ovarian syndrome. Some
drugs, such as ciclosporin, diazoxide, minoxidil, and androgenic steroids can
also cause hirsutism. A history of recent onset (<6 months) and rapidly
progressive hirsutism, particularly when associated with features of viriliza-
tion and a testosterone level of >5nmol/ L, should prompt a search for
alternative adrenal or ovarian causes (see Fig.2.10).
E OHCM 10e, p.230.
Box 2.7 Features offemale virilization
• Clitoral enlargement
• Temporal hairloss
• Breast atrophy
• Deepening ofvoice
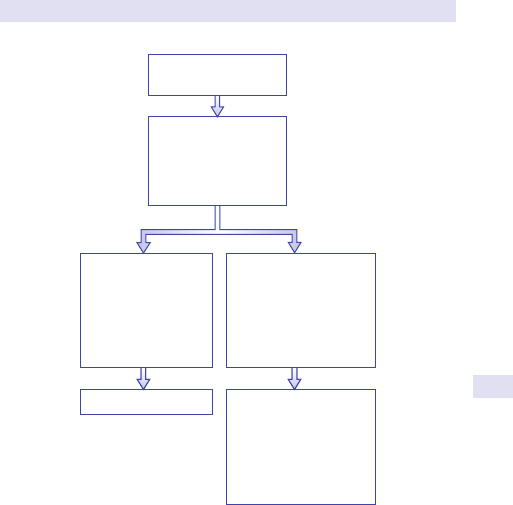
HIRSUTISM/VIRILIZATION (RAISED TESTOSTERONE)
171
Further reading
Martin KA, Chang RJ, Ehrmann DA, etal. Evaluation and treatment of hirsutism in premenopausal
women:an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2008; 93
:1105– 20.
Speiser PW, Azziz R, Baskin LS, et al.; Endocrine Society. Congenital adrenal hyperplasia due to
steroid 21- hydroxylase deciency:an Endocrine Society clinical practice guideline. J Clin Endocrinol
Metab 2010; 95
:4133– 60.
No Yes
Benign androgen
excess
Polycystic ovarian
syndrome
Drugs
Heterozygous CAH*
Treat symptomatically
Cushing syndrome incl.
adrenal carcinoma
Congenital adrenal
hyperplasia
Ovarian tumour
Ovarian hyperthecosis
Ultrasound/CT adrenal
and ovaries
24h urine free cortisol
Urinary steroid prole
Adrenal/ovarian venous
sampling
Increased androgen-
dependent hair growth
• Virilization
• Serum testosterone
>5nmol/L
or
• Rapidly progressive?
Fig.2.10 Investigation of hirsutism. * CAH, congenital adrenal hyperplasia.

172
CHAPTER2 Endocrinology and metabolism
172
Galactorrhoea (hyperprolactinaemia)
Galactorrhoea is always due to PRL and should be conrmed by asking
the woman to demonstrate a secretion of milky appearance from the nip-
ple. Rarely, galactorrhoea can occur with PRL levels in the normal range
and regular menses, but usually it is associated with mildly raised levels
and amenorrhoea in ♀ or very elevated levels in ♂. There is no link with
breast size— gynaecomastia in ♂ is associated with excess oestrogen. Once
dopamine- blocking drugs (major tranquillizers and anti- emetics, but not
antidepressants), depot progesterone administration, and hypothyroidism
have been excluded, all patients should have pituitary imaging to exclude
a large tumour pressing on the pituitary stalk, especially if there are mod-
est PRL levels (<10,000IU/ L) associated with a large tumour (>2cm) (see
Fig.2.11). If the PRL levels are disproportionately low for the tumour size,
serial dilution of the sample and re- estimation should be considered to
exclude an artefactually low level due to the ‘hook eect’. Very high PRL
levels (>10,000IU/ L) are invariably associated with true prolactinomas.
Nipple manipulation (e.g. to check if galactorrhoea has ceased) and chest
wall trauma (including shingles) can also stimulate PRL levels.
Asymptomatic raised prolactin (macroprolactin)
If PRL is found (accidentally) to be persistently raised (>1000IU/ L), but
menstruation is normal and there is no galactorrhoea, consider the pos-
sibility of macroprolactin. This is a circulating complex of PRL multimers,
and sometimes PRL autoantibodies of no biological importance, but gives
a high reading in the PRL assay and the result often varies widely between
assays. If the laboratory is alerted to a mismatch between PRL levels and
the clinical picture, they can easily screen for this with polyethylene glycol
precipitation. Stress and epileptic ts can result in transiently raised PRL
levels, insucient to cause galactorrhoea.
E OHCM 10e, p.236.
GALACTORRHOEA (HYPERPROLACTINAEMIA)
173
Further reading
McKenna TJ. Should macroprolactin be measured in all hyperprolactinaemic sera? Clin Endocrinol
(Oxf ) 2009; 71
:466– 9.
Melmed S, Casanueva FF, Homan AR, etal.; Endocrine Society. Diagnosis and treatment of hyper-
prolactinemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011;
96
:273– 88.
Normal or
lesion < 1cm
Prolactin
microadenoma
Lesion > 1cm
Prolactin
macroadenoma or
pituitary stalk
compression
Yes No
Raised TSH
Major tranquillizers,
metoclopramide,
domperidone
Chest wall injury/
excess nipple stimulation
Dopamine-blocking
drugs
Markedly raised TSH
Chest wall stimulation
Treat cause and ensure
prolactin returns to
normal
MRI pituitary
Conrm discharge is milk
Check prolactin, TSH,
drug history
Fig.2.11 Investigation of galactorrhoea.

174
CHAPTER2 Endocrinology and metabolism
174
Impotence/ loss oflibido/ male
hypogonadism
Symptoms and signs ofhypogonadism inmen
(lowtestosterone levels)
• Reduced shaving.
• Loss of libido.
• Impotence.
• Reduced energy/ aggression levels.
• Loss of pubic, chest, and axillaryhair.
• Gynaecomastia often results due to a lower testosterone/
oestrogenratio.
Note that very low levels of testosterone (at least <5nmol/ L, typical normal
range 10– 30nmol/ L) are required to result in symptoms. Mild reductions are
common, especially in the elderly, and are rarely of importance. Impotence
alone (without loss of libido) can also be caused by neurovascular and
psychological causes (e.g. diabetes, spinal damage, urological surgery, athero-
sclerosis of the aorta, drugs, stress, and psychosexual dysfunction). Where
the testosterone level is at the lower limit of normal or SHBG abnormalities
are suspected and symptoms are present, measurement of free testoster-
one or bioavailable testosterone by an established method may be indicated.
Such methods may need referral to a reference laboratory.
After history taking forconditions described, investigation ofsuspected male
hypogonadism requires
•
PRL.
• Thyroid function.
• LH andFSH.
• Testosterone.
Hyperprolactinaemia or thyrotoxicosis, if present, need to be treated on
their own merits. If the testosterone level is clearly low, high gonadotro-
phins point to testicular failure (e.g. testicular surgery, irradiation or trauma,
chemotherapy, crypto- orchidism, previous orchitis, gonadal dysgenesis,
including Klinefelter’s syndrome XXY). Low gonadotrophin levels with a
clearly low testosterone level point to a hypothalamic or pituitary cause
(systemic illness, pituitary tumour), which requires further investigations
(E Hypopituitarism, pp. 128–30). If no cause is found for hypogonado-
trophic hypogonadism, the likely cause is Kallman’s syndrome, especially if
associated with anosmia.
E OHCM 10e, p.230.
Further reading
Bhasin S, Cunningham GR, Hayes FJ, etal.; Task Force, Endocrine Society. (2010) Testosterone ther-
apy in men with androgen deciency syndromes:an Endocrine Society clinical practice guideline. J
Clin Endocrinol Metab 2010; 95
:2536– 59.
Sato N, Katsumata N, Kagami M, etal. Clinical assessment and mutation analysis of Kallmann syn-
drome 1 (KAL1) and broblast growth factor receptor 1 (FGFR1, or KAL2) in ve families and 18
sporadic patients. J Clin Endocrinol Metab 2004; 89
:1079– 88.

GYNAECOMASTIA
175
Gynaecomastia
Gynaecomastia results from an excessive eect of oestrogens or a raised
oestrogen/ testosterone ratio. Causes are summarized in Table 2.12. True
gynaecomastia should be associated with palpable breast tissue and distin-
guished from apparent breast enlargement due to obesity. Though very
rare, the most important diagnoses to exclude are hypogonadism and tes-
ticular and lung tumours.
Investigations should include
• LFTs.
• Thyroid function.
• LH andFSH.
• Testosterone.
• Oestradiol.
• hCG.
• AFP.
• CXR.
• TesticularUSS.
• Further review of drug history.
Physiological gynaecomastia should only be diagnosed if other causes have
been excluded.
E OHCM 10e, p.230.
Table2.12 Causes ofgynaecomastia
Physiological Newborn, adolescent, elderly
Hypogonadism e.g. Klinefelter’s syndrome, testicular failure
i oestrogen Testicular tumours, lung cancer producing hCG, liver
disease, thyrotoxicosis
Drugs Including oestrogens, spironolactone, cimetidine, digoxin,
testosterone administration

176
CHAPTER2 Endocrinology and metabolism
176
Delayed puberty
Denition
Puberty is considered delayed in girls if there is no breast development by
age 13 (or menses by age 15)and in boys if there is no testicular enlargement
by age 14. Note that 3% of normal children will fall into these categories.
Clinical features and initial investigations
A detailed history and examination is required for overt systemic disease,
psychosocial stress, and anorexia nervosa, and to assess the child’s height,
pubertal features (pubic hair, testicular size, breast growth, menses), and
any dysmorphic features (e.g. features of Turner’s syndrome). Where pos-
sible, growth rate should be calculated from sequential height measure-
ments over at least 6months.
If no obvious cause is identied, baseline investigations should include:
•
LH andFSH.
• TSH, FT4,PRL.
• FBC, U&E, bicarbonate (HCO
3
−
), CRP, and antigliadin/ endomysial
antibodies for occult systemic disease.
•
Boneage.
This should enable the child to be placed in one of ve categories:
1. Raised LH/ FSH (° gonadal failure). Causes:Turner’s syndrome,
Klinefelter’s syndrome, ovarian/ testicular injury. Proceed to
karyotyping (should be performed in all girls with delayed puberty as
Turner’s syndrome may not be apparent).
2. Short, low LH/ FSH, overt systemic disease. Causes:asthma, anorexia
nervosa, social deprivation, generalized illness, treatment for cancer,
including cranial irradiation, dysmorphic (Noonan’s syndrome and
others).
3. Short, low LH/ FSH, occult systemic disease. Causes:hypothyroidism,
hyperprolactinaemia, renal failure, RTA, coeliac disease, Crohn’s
disease.
4. Short, low LH/ FSH, no systemic disease. Causes:constitutional delay of
puberty, hypothalamic/ pituitary disease.
5. Not short, low LH/ FSH. Causes:Kallman’s syndrome (if anosmia present)
or isolated gonadotrophin deciency. Cannot reliably distinguish from
constitutional delay of puberty. Observe.
The investigation of children who fall into the commonest category ‘short,
low LH/ FSH, no systemic disease’ is summarized in Fig. 2.12. The onset of
puberty after a period of observation is reassuring, but continued observa-
tion is required to ensure the process proceeds to completion, including a
growth spurt. If not, further investigation for disorders of steroidogenesis,
androgen insensitivity, skeletal dysplasia, premature gonadal failure, and,
in the ♀, genital tract abnormalities and polycystic ovarian syndrome are
indicated.
DELAYED PUBERTY
177
Observe
MRI pituitary/
hypothalamus
Test for growth
hormone
3
reserve/
LHRH test
Calculate bone age
Obtain parental heights
Observe for 6 months
2
• Normal height for
skeletal age/parents
• Normal growth
velocity for skeletal age
• Early signs of puberty
now appear
• Short for skeletal
age/parents
• Low growth velocity
for skeletal age
• No signs of puberty
Fig.2.12 Investigation of delayed puberty in children who are short, with no
evidence of systemic disease
1
and low LH/ FSH levels.
Notes:
1. Including normal thyroid function andPRL.
2. If develops headache, vomiting, or visual symptoms, proceed immediately toMRI.
3. Refer to a paediatric endocrinologist. Tests used vary, e.g. gonadotrophin response to luteinizing
hormone- releasing hormone (LHRH) after androgenic priming and ITT for GH response.

178
CHAPTER2 Endocrinology and metabolism
178
Short stature
Evaluation of children who are below the third growth centile for age or
particularly small for their family should include:
•
Height for age (percentile).
• Mid- parental height (for girls, mean of father’s height minus 12.6cm +
mother’s; for boys, add 12.6cm to mother’s height).
•
Bone age (to assess growth potential/ height prediction).
• Observation over 3– 6months to determine growth velocity.
Children of short (but normal) parents, who are growing normally, can
be observed. Dysmorphic children require further evaluation/ specialist
assessment. Children who are short for their parental heights (low pre-
dicted height), particularly if growing slowly, and short children of pubertal
age who have not entered puberty should be investigated as for ‘delayed
puberty’. Referral for paediatric endocrinological assessment is advised.

PRECOCIOUS PUBERTY
179
Precocious puberty
Denition
Puberty is considered premature if multiple signs, including accelerated
growth rate and bone age, appear by age 8 in girls or age 9 in boys. Note
that isolated breast development (premature thelarche) or pubic hair (pre-
mature adrenarche) are benign conditions if no other evidence of puberty
appears. True precocious puberty requires urgent investigation to deter-
mine the cause and avoid irreparable loss of nal adult height. In girls, it is
often idiopathic, but not in boys. The causes are given in Table2.13.
Investigations
Precocious puberty is conrmed by pubertal levels of sex steroids (oestra-
diol, testosterone). Testicular enlargement (or ovarian enlargement on
USS) and detectable LH/ FSH levels suggest central precocious puberty and
a CT/ MRI scan of the brain is indicated. Gonadal enlargement can also
be seen with testotoxicosis, hCG- producing tumours, hypothyroidism, and
McCune– Albright syndrome. Further investigation should be performed in
combination with a paediatric endocrinologist.
Table2.13 Causes ofprecocious puberty
Central Gonadotrophin- independent
Idiopathic (especially girls) Congenital adrenal hyperplasia (♂)
CNS hamartoma
(especially pinealoma)
Adrenal/ ovarian/ hCG- secreting tumour
Other CNS diseases, e.g.
hydrocephalus, trauma
McCune– Albright syndrome, hypothyroidism,
follicular cyst (♀) familial testotoxicosis (♂)

180
CHAPTER2 Endocrinology and metabolism
180
Thyroid function testing:general
In the majority of cases, thyroid function testing and interpretation are
straightforward (see Fig. 2.13). However, the following points should be
borne inmind.
1. Which rst- line test?— TSH. TSH levels are the most sensitive indicator
of thyroid dysfunction, except in patients with pituitary disease where
they are uninterpretable. TSH used alone as a rst- line test will miss
(levels ‘normal’) unsuspected cases of 2° hypothyroidism, and some
laboratories combine TSH and T4 as rst- line tests. TSH is also
unreliable in patients with recently treated hyperthyroidism, as it can
remain suppressed (undetectable) for several weeks after FT4 or FT3
levels have normalized.
2. Which tests?— T4/ T3. Free T3 and T4 tests (FT3, FT4) are now
more reliable and preferred (although more expensive) to total T3
or T4 measurements. Interference in these assays does occur but is
increasingly rare. Total thyroid hormone levels are markedly inuenced
by changes in binding proteins (e.g. due to pregnancy, oestrogen-
containing contraceptives).
3. Thyroid autoantibodies. These are markers of autoimmune thyroid
disease. Antithyroid microsomal antibodies have been identied as
antithyroid peroxidase (anti- TPO) antibodies. Anti- TPO antibodies
are more sensitive than anti- thyroglobulin (Tg) antibodies and
are present in around 45– 80% of Graves’ disease and 80– 95% of
Hashimoto’s disease/ atrophic thyroiditis. Increasingly, laboratories are
measuring anti- TPO directly as their only antibody test (sometimes just
referred to as ‘antithyroid antibodies’). Note that anti- TSH receptor
antibodies— the cause of Graves’ disease— require a specic assay
request. (For indications for testing, E Anti- TSH receptor antibody
testing, p. 184).
4. Tests should agree. To conrm thyroid dysfunction, at least two TFTs
and, in cases of doubt, all three (TSH, FT3, FT4) should be performed.
Results of the tests should be in agreement— if not, assay interference
(heterophile antibodies, anti- T4 or anti- T3 antibodies present in the
serum) or unusual causes should be suspected.
5. Avoid thyroid function testing in systemically unwell patients. In very ill
patients, especially in intensive care, a pattern of ‘sick euthyroidism’
is often seen, with low TSH levels, low FT3 levels, and sometimes
low FT4 levels. Accurate interpretation of true thyroid status is
impossible. Araised FT3 level in a very ill patient suggests signicant
hyperthyroidism, and a very raised TSH level (>20mU/ L) with
undetectable FT4 levels suggests profound hypothyroidism. Other
changes should be interpreted with extreme caution and the tests
repeated after recovery.
E OHCM 10e, pp. 216–17.
THYROID FUNCTION TESTING:GENERAL
181
Normal
FT4/FT3
Low
FT4/FT3
Raised
FT4/FT3
Thyrotoxicosis
Subclinical
thyrotoxicosis
Levothyroxine
ingestion
Steroid therapy
Non-thyroidal
illness
Dopamine
infusion
Non-thyroidal illness
Pituitary failure
Recent (excessive) treatment for
hyperthyroidism
Hypothyroidism
Subclinical
hypothyroidism
Poor compliance
with T4 therapy
Interfering
(heterophile)
antibody
Recovery from
non-thyroidal
illness
Hypoadrenalism
Normal
TSH-secreting pituitary tumour
Thyroid hormone resistance (receptor
defect)
Intermittent T4 therapy/acute
overdose
Interfering
anti-T4/T3 antibody
Familial dysalbuminaemic
hyperthyroxinaemia
Acute psychiatric illness
Note:
free thyroid hormone assays are assumed—eects of changes in binding
proteins on total thyroid hormone assays are not included.
Low TSH Normal TSH Raised TSH
Fig.2.13 Patterns of TFTs. To use this table, you need the results of both TSH
and either free T4 (FT4) or free T3 (FT3) tests. If either FT4 or FT3 are outside the
reference range, then FT4/ FT3 are considered abnormal in this table. If FT4 and
FT3 are abnormal in dierent directions (e.g. one is low and the other is high), see
point4, ‘Tests should agree’, pp. 180–1.
Reprinted from The Lancet, 357, Dayan CM ‘Interpretation of thyroid function tests’ 619– 24 (2001)
with permission from Elsevier.
Further reading
Association for Clinical Biochemistry/ British Thyroid Association (2006). UK guidelines for the use
of thyroid function tests
. London: ACB/ BTA. M http:// www.acb.org.uk/ docs/ default- source/
guidelines/ TFTguidelinenal.pdf.
National Academy of Clinical Biochemistry Practice Guidelines (2002). M
http:// www.aacc.org/
members/ nacb/ LMPG/ Pages/ default.aspx.

182
CHAPTER2 Endocrinology and metabolism
182
Hyperthyroidism (thyrotoxicosis)
Clinical features
Hyperthyroidism is rare in childhood but aects all adult age groups.
Classic features include weight loss despite i appetite, palpitations, AF,
heat intolerance, anxiety, agitation, diarrhoea, tremor, and proximal weak-
ness. Lid retraction and lid lag can be seen in any cause of hyperthyroid-
ism, but proptosis, periorbital oedema, chemosis, diplopia, and optic nerve
compression only occur in association with Graves’ disease (thyroid eye
disease) and occasionally associated with pretibial myxoedema and thyroid
acropachy. In the elderly, presentation with isolated weight loss or AF is
common. Raised ALP and SHBG, leucopenia, and rarely hypercalcaemia
are recognized associations.
Thyroid function testing
An undetectable TSH level and an i free T3 level are required to diag nose
hyperthyroidism. In milder cases, T4 levels may be in the normal range (‘T3
toxicosis’). Normal TSH levels with i
T4 and T3 are seen in TSH- secreting
pituitary tumours (very rare) or in patients with thyroid hormone resistance
(also very rare) (see Fig.2.13).
Investigation ofcause
For an overview of investigation, see Fig.2.14.
Under the age of 40, Graves’ disease is the commonest cause. After
this age, Graves’ disease, toxic nodular goitre, and toxic nodule all occur.
However, a short history (1 month) of symptoms or absence of relevant
symptoms (chance blood test nding) raises the possibility of self- resolving
(transient) thyroiditis, a diagnosis supported by neck pain and raised ESR
(viral/ subacute/ De Quervain’s) or occurrence in the rst 9months post-
partum (postpartum thyroiditis— painless). Transient thyrotoxicosis can also
occur in patients with subclinical autoimmune thyroiditis (‘silent thyroiditis’—
painless), especially during cytokine therapy (e.g. interferon for hepatitis C).
Graves’ disease or other forms of autoimmune thyroiditis are seen in >30%
of patients 2– 3years after treatment with alemtuzumab (for MS), in associa-
tion with lymphocyte repopulation. If self- resolving thyroiditis is suspected,
withhold treatment and repeat the tests after 6 weeks. When thyroid eye
disease is present, no further tests are required to diagnose Graves’ disease.
If not, antithyroid antibodies (e.g. anti- TPO antibodies) and isotope thy-
roid scanning can be useful to distinguish possible causes (see Fig. 2.14 and
E Chapter14). No uptake is seen in transient thyroiditis. Excess thyroid
hormone ingestion rarely causes very marked thyrotoxicosis, unless the
active form (T3) is taken (T3 tablets or desiccated thyroid extract).
HYPERTHYROIDISM (THYROTOXICOSIS)
183
Iodine
Iodine has multiple and conicting eects on the thyroid. Potassium iodide
inhibits release of thyroid hormones from the gland and thyroid hormone
biosynthesis (Wol– Chaiko eect), promoting hypothyroidism. However,
escape from these eects occurs in most individuals in a few weeks. In
patients with a multinodular goitre, excess iodine (e.g. in amiodarone or
radiographic contrast media) can result in thyrotoxicosis by excess provi-
sion of substrate (Jod– Basedow eect).
Amiodarone
Has three main eects on the thyroid hormoneaxis:
•
Inhibits T4 l T3 conversion, which in the pituitary can result in an
asymptomatic mild rise in TSH level (reduced thyroid hormone action)
and/ or a rise in FT4level.
• Can induce true hypothyroidism, usually in the rst year of treatment.
• Can induce true hyperthyroidism either via the Jod– Basedow eect in
patients with multinodular goitre or by destructive thyroiditis in healthy
glands.
Thyrotoxicosis can occur at any time after commencing therapy and
can be very dicult to treat. Interpretation of TFTs on amiodarone: a
raised FT3 level indicates true hyperthyroidism; a markedly raised TSH
level (e.g. >10mU/ L), especially if the FT4 level is low, indicates true
hypothyroidism.
Repeat tests in
6 weeks
Now eu-/
hypothyroid
Spontaneously
resolving
thyroiditis
Hyperthyroidism
conrmed
(suppressed TSH,
raised free T3)
Short history
(<6wks)
Neck pain,
raised ESR
Within 9 mo
post-partum
Graves’ eye
disease
Isotope thyroid
scan, antithyroid
Abs
Clear history,
no eye disease
Graves’ disease
Diuse
uptake or
Ab +ve
Irregular
uptake
Ab −ve
Toxic
nodule
Multinodular
goitre
Hot nodule
Ab −ve
Fig.2.14 Investigation of the cause of hyperthyroidism.

184
CHAPTER2 Endocrinology and metabolism
184
Hyperthyroidism inpregnancy
Signicant hyperthyroidism in pregnancy is generally due to Graves’ dis-
ease. Mild hyperthyroidism, particularly in association with hyperemesis
gravidarum in the rst trimester, is often due to a cross- reaction by very
high hCG levels with the TSH receptor (‘gestational thyrotoxicosis’). In the
postpartum period, thyrotoxicosis may be due to postpartum thyroiditis
(self- resolving) or a recurrence of Graves’ disease (requires treatment).
Measurement of anti- TSH receptor antibody levels may be indicated to
distinguish these possibilities.
Thyroidstorm
This is dened as severe thyrotoxicosis with confusion/ delirium not
explained by other factors. There is no denitive test and levels of thyroid
hormone are not higher than in other thyrotoxic individuals with no fea-
tures of storm. Severe agitation, tachycardia, and hyperpyrexia are usually
seen. Usually precipitated by infection, trauma, or surgery, especially to the
thyroid gland. Very rare but tends to occur in individuals who have been
poorly compliant in the rst few weeks of drug therapy for thyrotoxicosis.
Anti- TSH receptor antibody testing (TBII, TRAbs.TSAb)
This test is increasingly available in local and regional laboratories. In second-
or third- generation assays, especially assays for stimulatory antibodies, it is
positive in >95% of cases, save other tests, and indicated that the patient
is at risk of Graves’ eye disease. Indications for anti- TSH receptor antibody
testing include distinguishing gestational thyrotoxicosis or postpartum thy-
roiditis from Graves’ disease, indicating the risk of neonatal thyrotoxicosis
and (controversial) predicting recurrence after a course of thioamide drug
therapy.
E OHCM 10e, p.31, p. 216, p. 562.
Further reading
Barbesino G, Tomer Y. Clinical utility of TSH receptor antibodies. J Clin Endocrinol Metab 2013;
98
:2247– 55.

HYPERTHYROIDISM (THYROTOXICOSIS)
185

186
CHAPTER2 Endocrinology and metabolism
186
Hypothyroidism
Clinical features
Classic clinical features of hypothyroidism include weight gain, cold intoler-
ance, dry skin, constipation, memory loss, lethargy/ slow thought/ ‘slowing
up’, menorrhagia, periorbital/ facial oedema, loss of the outer two- thirds of
eyebrows, deafness, chest pain, and coma. These are rarely seen nowadays,
as TFTs are easy to perform and detect the disease usually at an earlier
stage. Weight gain, dry skin, and lethargy are frequently reported, but even
biochemically hypothyroid individuals can only condently be ascribed to
thyroid status if they reverse on treatment.
Biochemical diagnosis
i TSH with T4 in the normal range is referred to as subclinical hypothy-
roidism. i TSH with d T4 is overt hypothyroidism. d T4 with TSH in the
normal range may also be due to pituitary failure (2° hypothyroidism) and,
if persistent, requires pituitary function testing. See Fig. 2.13 for other pat-
terns ofTFTs.
Dierential diagnosis (causes)
In iodine- sucient countries, most spontaneous hypothyroidism is due to
autoimmune thyroiditis (Hashimoto’s disease if goitre present, atrophic thy-
roiditis if goitre absent)— antithyroid antibodies (anti- TPO, anti- Tg) present
in 80– 90% of cases. Other common causes are post- thyroidectomy, post-
radioiodine therapy, and side eects of amiodarone or lithium. Rarer causes
include treatment with cytokines or other drugs (e.g. interferons, granulo-
cytic macrophage colony- stimulating factor (GM- CSF), interleukin- 2, tyros-
ine kinase inhibitors, alemtuzumab), vast excess of iodine intake (iodine
drops, water purifying tablets), congenital hypothyroidism (caused by a
variety of genetic defects; should be detected by neonatal screening pro-
gramme), iodine deciency (urinary iodide excretion <45µg/ day, common-
est cause worldwide, especially mountainous areas, S.Germany, Greece,
Paraguay— ‘endemic goitre’), thyroid- blocking substances in the indigenous
diet (goitrogens, especially in brassicas and cassava, e.g. in Sheeld, Spain,
Bohemia, Kentucky, Virginia, Tasmania— ‘endogenous goitre’ without iodine
deciency), Pendred’s syndrome (mild hypothyroidism with sensorineural
deafness due to Mondini cochlear defect detectable on MRI, positive per-
chlorate discharge test). For further information, E Transient hypothy-
roidism, p. 187.
3 Diagnostic catches i TSH and d T4 always represents hypothyroid-
ism. If the TSH alone is i and the T4 is not even slightly low, a heterophile
antibody interfering in the TSH assay may be present in the patient’s serum.
This is especially likely if there is no change in TSH level after thyroxine
treatment, but the T4 level rises (conrming compliance with tablets). For
unusual patterns of thyroid function tests, see Fig. 2.14. Note that, within
the rst 1– 3months (or longer) after treatment of hyperthyroidism, pro-
found hypothyroidism may develop with a d T4, but the TSH may still be
suppressed or only mildly raised due to the long period of TSH suppression
prior to treatment. Raised TSH alone with disproportionate symptoms of
lethargy may be seen in hypoadrenalism.

HYPOTHYROIDISM
187
Transient hypothyroidism
Transient/ self- resolving hypothyroidism, often preceded by hyperthyroid-
ism, is seen in viral thyroiditis, after pregnancy (postpartum thyroiditis), and
in some individuals with autoimmune thyroiditis. Treatment temporarily
with levothyroxine is only required if the patient is very symptomatic.
Thyroid function should return to normal within 6months.
Subclinical hypothyroidism
A raised TSH (<20mU/ L) with normal T4/ T3 is very common and seen
in 5– 10% of women and 72% of ♂. It is usually due to subclinical autoim-
mune thyroid disease and is frequently discovered on routine testing. In ran-
domized trials, 720% of patients obtain psychological benet from beginning
T4 therapy; in many others, it is probably truly asymptomatic. If antithyroid
antibodies are detectable, the rate of progression to overt hypothyroid-
ism is 750% at 20years, but higher than this with higher initial TSH levels.
If the TSH alone is raised with −ve antibodies (or the TSH is normal with
raised antibodies alone), overt hypothyroidism develops in 25% at 20years.
Areasonable approach is a trial of levothyroxine for 6months in symp-
tomatic patients with subclinical hypothyroidism or TSH >10mU/ L, and
observing the TSH level at 6- to 12- monthly intervals in asymptomatic
patients with TSH <10mU/ L.
Hypothyroidism and pregnancy
Overt hypothyroidism is associated with poor obstetric outcomes. Recent
evidence suggests that subclinical hypothyroidism is associated with an i
miscarriage rate and a slight reduction in the baby’s intelligence quotient
(IQ) and should be treated. Some authorities advocate screening for hypo-
thyroidism in all antenatal patients as early as possible in pregnancy. Patients
on T4 need to i
their dose by 25– 50µg from the rst trimester of preg-
nancy. Maternal levothyroxine can compensate for fetal thyroid failure
inutero, but congenital hypothyroidism must be detected at birth (screen-
ing test) to avoid mental retardation developing. Where the mother and
fetus are both hypothyroid— most commonly due to iodine deciency—
mental retardation can develop in utero (cretinism). Note that mothers with
+ve antithyroid antibodies and/ or subclinical hypothyroidism have a 50%
chance of developing (transient) postpartum thyroiditis.
E OHCM 10e,p. 31, p. 149, p. 203.
Further reading
Cooper DS, Biondi B. Subclinical thyroid disease. Lancet 2012; 379:1142– 54.

188
CHAPTER2 Endocrinology and metabolism
188
Hypercalcaemia
Clinical features
Usually asymptomatic if Ca
2+
<3.0mmol/ L. Typical symptoms include poly-
dipsia/ polyuria, constipation, indigestion, pancreatitis, hypertension, tired-
ness, drowsiness/ confusion, abdominal pains, and renal colic. Renal failure
can occur due to dehydration (reversible), nephrocalcinosis, and/ or stag-
horn calculi. Osteitis brosa cystica in cases of hyperparathyroidism (with
subperiosteal resorption of bone, particularly of the distal phalanges and
bone cysts— brown tumours) is now rare, other than in renal failure, but
can be associated with bonepain.
Investigation ofthecause
Ninety- ve per cent of persistent hypercalcaemia is due to either hyperpar-
athyroidism or malignancy (see Fig. 2.15). Asymptomatic 1° hyperparathy-
roidism is common in 50- to 70- year- old women, and a PTH level (sampled
simultaneously with i Ca
2+
and measured in a highly sensitive assay) which
is raised or in the upper normal range in the presence of hypercalcaemia
conrms the diagnosis. Low normal or low levels of PTH should prompt a
search for malignancy, especially breast, prostate, bronchus, kidney, thyroid,
or myeloma. Bone- derived ALP levels may be raised in both malignancy and
hyperparathyroidism. Bone scan may be useful in disseminated malignancy
but can be −ve in cancers releasing PTH- related peptide (NOT detected in
routine PTH assays) and in myeloma. If malignancy is not found, the condi-
tions shown in Fig. 2.15 need to be considered. Markedly abnormal renal
function is seen in milk- alkali syndrome, myeloma, and tertiary hyperpar-
athyroidism. Sarcoid may be dicult to diagnose but is suggested by a raised
serum ACE level (not invariable), a dramatic response to steroids, and a
+ve liver or other biopsy for granulomata.
Investigation ofestablished hyperparathyroidism
Familial benign hypocalciuric hypercalcaemia (FBHH) is a very rare con-
dition caused by an inactivating mutation of the calcium- sensing receptor
(CaSR). This results in stable, lifelong hypercalcaemia with a raised PTH
level, which rarely causes complications. It is inherited in an autosomal
dominant fashion.
The hallmark is hypocalciuria— denedas:
Urine [Ca
2+
] × plasma creatinine/plasma [Ca
2+
] × urine creat <0.01
(All in units of mmol/ L.)
Although rare, it is important to recognize, as parathyroidectomy is not
required.
Further investigation of ° hyperparathyroidism should include serum
creatinine, KUB plain abdominal X- ray to exclude renal stones, and a spot
urine Ca
2+
/ creatinine to rule outFBHH.
HYPERCALCAEMIA
189
In >80% of cases, ° hyperparathyroidism is due to adenomatous change
in one of the four parathyroid glands. In a minority of cases and in familial
hyperparathyroidism associated with MEN- 1 (pituitary tumours, endocrine
pancreatic tumours, and hyperparathyroidism) or MEN- 2 (medullary carci-
noma of the thyroid, phaeochromocytoma, and hyperparathyroidism) four
gland hyperplasia occurs, requiring resection of at least three- and- a- half
glands for treatment. Very rarely (<1% of cases), parathyroid carcinoma is
the cause. Lithium therapy may also be associated with (mild) hyperparath-
yroidism. Once a diagnosis of ° hyperthyroidism is made,
99m
technetium-
sestamibi radionucleotide scanning is the most sensitive imaging technique
and will show the location of the parathyroid adenoma. In dicult cases,
local venous sampling may be required for localizing a parathyroid ade-
noma, especially if it is outside theneck.
Tertiary hyperparathyroidism
Refers to acquired autonomy of the parathyroid glands leading to hyper-
calcaemia following chronic vitamin D deciency, as seen in renal failure or
with malabsorption. 2° hyperparathyroidism is associated with hypocalcae-
mia and is the appropriate response to vitamin D deciency.
Hypercalcaemia
(persistent):
Ca
2+
>2.7mmol/L
Measure PTH (sensitive
assay, while Ca
2+
raised)
Normal or
raised PTH
Investigation of hypercalcaemia
Hyperparathyroidism
(primary or tertiary)
Exclude malignancy
inc. myeloma
Consider:
Sarcoid/granulomatous
disease
Milk alkali syndrome
Thyrotoxicosis
Acute hypoadrenalism
Immobility
Paget’s disease
Vitamin D intoxication
Thiazide diuretics
Check:
Urine Ca
2+
/creatinine ratio
to exclude FBHH (see text)
KUB X-ray vs stones
Serum creatinine
Low or
undetectable PTH
Fig.2.15 Investigation of hypercalcaemia.

190
CHAPTER2 Endocrinology and metabolism
190
Hypocalcaemia/ osteomalacia
Clinical features
Chronic hypocalcaemia is often surprisingly asymptomatic. Symptoms
and signs, when present, include muscle spasms, paraesthesiae, especially
around the mouth and in ngers, tetany, ts, +ve Chvostek’s (VIIth nerve
hyperexcitability) and Trousseau’s signs (tetany of the hand when BP cu
inated). Chronic hypocalcaemia is also associated with papilloedema,
abnormal dentition (if begins in childhood), cataract, and intracranial cal-
cication (of no clinical consequence). Hypocalcaemia due to vitamin D
deciency is associated with muscle pains, proximal myopathy, and osteo-
malacia. In some cases of pseudohypoparathyroidism (type Ia), there are
phenotypic abnormalities (somatic features), including short fourth meta-
carpal, bone changes (Albright’s hereditary osteodystrophy), mental retar-
dation, short stature, obesity, and resistance to other hormones, e.g. TSH,
glucagon, gonadotrophins.
Investigation ofcause
Persistent hypocalcaemia (corrected for serum albumin levels) with a
normal serum creatinine level is almost always due to either hypopar-
athyroidism or vitamin D deciency (osteomalacia). Other causes and
distinguishing features are shown in Table 2.14, and a scheme for diag-
nosis is shown in Fig. 2.16. In failure of PTH action, Ca
2+
is very low
(<1.8mmol/ L) and PO
3
4−
is raised, but ALP is not raised and there is no
osteomalacia. If the PTH is found to be raised, then pseudohypopar-
athyroidism can be diagnosed, which is subclassied as type Ia (paternally
inherited a Gs- α defect with somatic features), type Ib (‘renally selec-
tive’ maternally inherited Gs- α defect— no somatic features), or typeII.
The classic test is the Elsworth– Howard test— measuring urine cyclic
adenosine monophosphate (cAMP) response to infused PTH(1– 34) ana-
logue. Families with type 1a may also include patients with pseudopseu-
dohypoparathyroidism, characterized by normocalcaemia, but somatic
changes of pseudohypoparathyroidism. The mutations are in the same
gene. The aetiology of type II pseudohypoparathyroidism (normal
renalCa
2+
excretion and no other somatic features) remains unclear.
A low or normal PTH in the presence of a Ca
2+
level <1.8mmol/ L makes
hypoparathyroidism the likely diagnosis (see Table 2.14 for possible causes),
but an attempt to rule out an activating CaSR mutation with a urine Ca
2+
/
creatinine ratio (see Fig. 2.15) should be made. In this rare genetic condition,
Ca
2+
levels are generally higher (around 1.75mmol/ L). Importantly, Ca
2+
or vitamin D replacement has a high likelihood of causing nephrocalcinosis
and is best avoided. Autoimmune hypoparathyroidism in children or young
people is particularly seen in association with autoimmune polyglandular
syndrome type 1 (chronic candidiasis, coeliac disease, adrenal insuciency).
In failure of vitamin D action (see Fig. 2.16, Table 2.14), there is a com-
pensatory PTH rise, which partly corrects the Ca
2+
level but causes a raised
ALP. In addition, there is osteomalacia. In the presence of a signicantly
raised creatinine, renal osteodystrophy (impaired 25- OH vitamin D gen-
eration) is the most likely diagnosis; otherwise dietary vitamin D deciency
(low 25- OH vitamin D) or vitamin D resistance must be distinguished (see
Table2.14).
HYPOCALCAEMIA/OSTEOMALACIA
191
Osteomalacia
Osteomalacia is strictly a histological diagnosis but is suggested by Looser’s
zones and pseudo- fractures on X- ray (especially pelvis and upper femur).
If osteomalacia with muscle weakness occurs in the absence of hypocal-
caemia or a raised ALP, hypophosphataemia (‘vitamin D- resistant rickets’)
is likely. Causes of a low PO
3
4−
include intrinsic renal disease, congenital
PO
3
4−
leak, acquired PO
3
4−
leak, and oncogenic osteomalacia. This last con-
dition is associated with very dicult- to- nd tumours, often benign, typi-
cally haemangiopericytomas of the naso- / oropharynx that may take years
to become manifest. It is due to secretion of the cytokine broblast growth
factor 23 (FGF23) by tumours, causing a phosphaturic eect. If suspected,
FGF23 levels can be assayed in specialized laboratories. Treatment is PO
3
4−
replacement until the tumour can be resected.
Investigation of hypocalcaemia
Consistently low Ca
2+
(<2.1mmol/L)
check albumin, phosphate, creatinine, alk
phos, Mg
2+
, PTH (while Ca
2+
low)
Raised alk phos
and raised
PTH
Check 25-OH
vit D
X-rays for
osteomalacia
Low 25-OH
vit D
Dietary vit D
deciency/
malabsorption
Measure 1.25-OH
vit D and review creat:
Renal failure
1-OHase dec
Vit D rec defect
Low (<0.2)
Hypopara-
thyroidism
Normal
(>0.2)
Ca rec
mutation
Normal 25-OH
vit D
Urine Ca/creat
ratio (mmol/mmol)
PSEUDOHYPO-
PARATHYROIDISM
HYPOPARA-
THYROIDISM
Raised PTH
Normal alk
phos
Low or normal
PTH, normal
alk phos
Low Mg
2+
low albumin – consider measuring
ionized Ca
2+
– replace
Fig.2.16 Investigation of hypocalcaemia.

192
CHAPTER2 Endocrinology and metabolism
192
Further reading
Ward BK, Magno AL, Walsh JP, Ratajczak T. The role of the calcium- sensing receptor in human
disease. Clin Biochem 2012; 45:943– 53.
Table2.14 Causes ofhypocalcaemia
Cause Features
Lack of PTH action Very low Ca
2+
, high PO
4
3−
, normal ALP
Hypoparathyroidism
Autoimmune, post- neck surgery (may be
transient), radioiodine, congenital (e.g. di George)
Pseudohypoparathyroidism
Type Ia (paternally inherited Gs- α mutation plus
somatic features)
Type Ib (maternally inherited, no somatic features,
‘renally selective’)
Type II (no somatic features, normal Ca
2+
excretion
Hypomagnesaemia Inhibits PTH release
Activating CaSR mutation Indistinguishable from ° hypoparathyroidism,
except present from childhood and urinary Ca
2+
/
creatinine ratio not low
Failure of vitamin D action Ca
2+
not very low (>1.8mmol/ L), i A L P, d PO
4
3−
, i
PTH, osteomalacia
Vitamin D deciency Dietary/ d sunlight, malabsorption, i metabolism
(phenytoin, rifampicin)
Renal failure
Failure of 1- hydroxylation of vitamin D
Inherited failure of 1- α
hydroxylase (vitamin D-
dependent rickets type I)
Normal 25- OH vitamin D, d 1,25- OH vitamin
D levels
Vitamin D receptor defect
(vitamin D- dependent rickets
type 2*)
Normal 25- OH, normal 1,25- OH vitamin D levels
Other
Acute pancreatitis Transient
Hungry bone
syndrome— immediately
post- parathyroidectomy
Transient
Drugs, e.g. foscarnet,
bisphosphonates,
ethylenediamine tetra- acetic
acid (EDTA), citrate in blood
Transient
Neonatal (with prematurity) Transient
* ‘Vitamin D- resistant rickets’ is rickets with normal Ca
2+
and vitamin D due to X- linked
hypophosphataemia.

HYPOCALCAEMIA/OSTEOMALACIA
193
194
CHAPTER2 Endocrinology and metabolism
194
Diabetes mellitus
Diagnosing diabetes mellitus
Diabetes is dened as a state of chronic hyperglycaemia at levels that, if
untreated, would result in microvascular complications (e.g. retinopathy).
Adverse pregnancy outcomes (and i macrovascular risk) occur at a lower
level of glucose and hence lower cut- os are used in pregnancy. However,
since blood glucose levels vary through the day following meals, physical
activity, and stress, dening these levels of glucose with a single test or a
short dynamic test has proved dicult.
Figure 2.17 provides a scheme for testing for diabetes. Table 2.15 provides
the American Diabetes Association (ADA) and WHO criteria for the diag-
nosis of diabetes, which dier slightly and have been updated since 2013.
One of four dierent criteria can now be used to diagnose diabetes. Note
that HbA
1c
can now be used to diagnose diabetes if performed in a stand-
ardized Diabetes Control and Complications Trial (DCCT)- aligned assay
and has the advantage that a fasting sample is not required. HbA
1c
for diabe-
tes diagnosis is not recommended using point- of- care machines (not stand-
ardized). In conditions where there is i RBC turnover, such as pregnancy
RBG > 11.1mmol/L:
Diabetes
conrmed
FBG > 7.0
Diabetes
conrmed
FBG 5.6-7.0
‘Pre-diabetes’
Consider OGTT
(See Table 2.22)
FBG < 5.6
Diabetes
excluded
Measure
random blood
glucose (RBG)
Symptoms present
(polydipsia, polyuria)
RBG < 11.1mmol/L:
Measure
fasting glucose
(FBG)
Repeat tests on
two separate
occasions
NO YES
Fig.2.17 Diagnosis of diabetes.

DIABETES MELLITUS
195
Table2.15 ADA and WHO criteria forthe diagnosis ofdiabetes
ADA (2016) WHO (2006, 2013)
Normoglycaemia Fasting plasma glucose
<5.6mmol/ L (100mg/ dL)*
Fasting plasma glucose
<6.1mmol/ L (110mg/ dL)*
OR 2h post- 75g load of
plasma glucose <7.8mmol/ L
(140mg/ dL)
OR 2h post- 75g load of
plasma glucose <7.8mmol/
L (140mg/ dL)
Diabetes Casual plasma glucose
>11.1mmol/ L (200mg/ dL)
1
Casual plasma glucose
>11.1mmol/ L (200mg/ dL)
1
OR fasting plasma glucose
>7.0mmol/ L (126mg/ dL)
1
OR fasting plasma glucose
7.0mmol/ L (126mg/ dL)
1
OR 2h post- 75g load of
plasma glucose >11.1mmol/ L
(200mg/ dL)
2
OR 2h post- 75g load
of plasma glucose
>11.1mmol/ L (200mg/ dL)
OR HbA
1c
>48mmol/ mol
(6.5%)
OR HbA
1c
>48mmol/ mol
(6.5%)
IFG Fasting plasma glucose
5.6– 6.9mmol/ L*
Fasting plasma glucose
6.1– 7.0mmol/ L (126mg/ dL)
BUT not ocially
recognized— recommend
progress to 2h 75g glucose
tolerance test (GTT)*
Impaired glucose
tolerance (IGT)
Not routinely measured
2h post- 75g load of plasma
glucose 7.8– 11.1mmol/ L
(140– 199mg/ dL)
2h post- 75g of glucose
7.8– 11.1mmol/ L (140–
199mg/ dL)
i risk of diabetes
(pre- diabetes)—
includes IFG and
IGT also
HbA
1c
:5.7– 6.4%
(39– 46mmol/ mol)
Gestational
diabetes
Casual plasma glucose
>11.1mmol/ L (200mg/ dL)
Casual plasma glucose
11.1mmol/ L
OR fasting plasma glucose
7.0mmol/ L (126mg/ dL)*
OR fasting plasma glucose
>7.0mmol/ L
OR two or more of the
following plasma glucose
values after 75g glucose load:
fasting>5.3mmol/ L; 1h
>10mmol/ L; 2h >8.6mmol/ L
(155mg/ dL)*
OR one or more of the
following:fasting
5.1– 6.9mmol*; or following
75g glucose load:1h
post- glucose >10mmol/ L;
2h post- glucose
8.5– 11.0mmol/ L
* ADA and WHO criteria dier.
1
If the patient does not have classic symptoms (polyuria, polydipsia, unexplained weight loss),
then this test should be repeated on a dierentday.
2
Not recommended by the ADA for routine clinicaluse.

196
CHAPTER2 Endocrinology and metabolism
196
(second and third trimesters), recent blood loss or transfusion, Epo therapy,
or haemolysis, HbA
1c
should not be used for diagnosis. HbA
1c
may also be
problematic in the presence of haemoglobinopathies, unless specic assays
not inuenced by abnormal Hb are used. It should also be noted that the
four dierent criteria for diagnosing diabetes do not completely overlap, e.g.
a person may have fasting blood sugar of 6.8mmol/ L (= impaired fasting
glucose (IFG)/ i diabetes risk) but an HbA
1c
of 55mmol/ mol (= diabetes).
Hence only one test should beused.
Oral glucose tolerance testing is rarely required (and only recommended
in pregnancy by the ADA) but can sometimes be useful to make the diagno-
sis of i diabetes risk in borderline cases (see Table2.15).
Notes
•
If symptoms are not present, tests must be repeated on two occasions,
ideally more than a week apart, to conrm that levels are indeed
chronically raised.
•
Values are given for venous plasma glucose. Capillary blood glucose
are71.0mmol/ L higher than venous plasma.
• If the patient has an intercurrent illness (e.g. infection or MI), tests
should be repeated once the patient has recovered.
•
Dierent criteria are used to diagnosis diabetes in pregnancy
(gestational diabetes).
Blood samples for glucose testing:in unseparated whole blood, glycolysis
by red cells reduces glucose levels by 10– 15% per h at room temperature,
leading to falsely low results. Clotted (serum) samples without preservative
can be used for glucose measurements if the sample is separated rapidly;
once separated, the glucose level is stable for 8h at room temperature and
72h at 4°C. Alternatively, a tube containing uoride oxalate to inhibit glyco-
lysis can be used if the sample is to be kept unseparated at room tempera-
ture for manyhours.
False +ve diagnoses may arise if the subject has prepared inadequately
(see Box2.8).
Box 2.8 Preparation fora fasting bloodtest
• Refrain from any food or drink from midnight before the morning of
thetest.
•
Water only is permitted.
• Regular medication can generally be deferred until a blood sample has
beentaken.
•
The appropriate sample is taken between 8 a.m. and 9 a.m. the
following morning.
This preparation is also required for a 75g oral glucose tolerance test (OGTT) or for
measurement of fasting blood lipids. Fasting blood tests should be avoided in insulin- treated
patients— risk of hypoglycaemia. Fasting is dened by the ADA as no caloric intake for at
least8h.

DIABETES MELLITUS
197
Normoglycaemia
The diagnostic criteria for normoglycaemia are given in Table 2.15 (note
the dierence between ADA and WHO for FPG cut- o). Note that there
is NO dened random plasma glucose that conrms normoglycaemia,
and this complicates the development of screening strategies for diabetes.
However, if a random value is <5.6mmol/ L, diabetes is unlikely.
Impaired glucose tolerance
This is the preferred diagnostic category for pre- diabetes used by the
WHO. The diagnosis of IGT can only be made using a 75g OGTT; a ran-
dom blood glucose measurement will often point to the diagnosis when
other results are non- diagnostic.
This category denotes a stage intermediate between normal glucose lev-
els and DM. By denition, plasma glucose levels are not raised to DM levels,
so typical osmotic symptoms are absent. Although subjects with IGT are
not at direct risk of developing chronic microvascular tissue complications,
the incidence of macrovascular complications (i.e. coronary heart disease
(CHD), cerebrovascular disease, peripheral arterial disease (PAD)) is i.
Presentation with one of these conditions should therefore alert the clini-
cian to the possibility of undiagnosed IGT (or type 2 DM). Note that up to
25% of individuals who are diagnosed with IGT by an OGTT may revert to
normal on re- testing.
Impaired fasting glucose
This is the diagnostic category for pre- diabetes preferred by the ADA
and depends on the FPG. Instructions for fasting blood test are shown in
Box2.8. The revised 2005 criteria lowered the lower limit for diagnosing
IFG, so the range according to the ADA is 5.6– 7.0mmol/ L. This category
is also usually asymptomatic. To date, cross- sectional studies suggest that
IGT and IFG may not be synonymous in terms of pathophysiology and
long- term implications, and a proportion of patients will fall into one cat-
egory, but not the other. Also some patients with fasting blood glucose
<7.0mmol/ L may have 2h blood glucose on the OGTT of >11.1mmo/
L and hence would have diabetes by the WHO criteria. If an OGTT is
performed, the 2h value takes precedence over the fasting value in the diag-
nosis of diabetes if the values do notagree.
Oral glucose tolerancetest
The OGTT (see Box 2.9) continues to be regarded as the most relevant
means for establishing the diagnosis of diabetes in equivocal cases, although
its reproducibility is poor. In borderline cases, the WHO suggests that only
when an OGTT cannot be performed does the diagnosis rely on FPG. It is
also the preferred approach in pregnancy. OGTTs should be carried out
under controlled conditions after an overnightfast.
The interpretation of the 75g GTT is shown in Table 2.16. These results
apply to venous plasma. Marked carbohydrate depletion can impair glucose
tolerance; the subject should have received adequate nutrition in the days
preceding thetest.
198
CHAPTER2 Endocrinology and metabolism
198
Eect ofintercurrent illness onglycaemia
Patients under physical stress associated with surgery, trauma, acute MI,
acute pulmonary oedema, or stroke may have transient i of plasma
glucose— often settles rapidly without antidiabetic therapy. However, the
hormonal stress response in such clinical situations is liable to unmask pre-
existing DM or to precipitate DM in predisposed individuals. Blood glucose
should be carefully monitored and the urine tested for ketones. Sustained
hyperglycaemia, particularly with ketonuria, demands vigorous treatment
with insulin in an acutely ill patient. Re- testing is usually indicated following
resolution of the acute illness— an OGTT at a 4- to 6- week interval is rec-
ommended if glucose levels are equivocal.
Box 2.9 Oral glucose tolerancetest
• Preparation: 3- day unrestricted CHO intake and activity. No
medication on day of test. 8– 14h fast. No smoking.
• 75g
1
of anhydrous glucose is dissolved in 250mL of water; avouring
with sugar- free lemon and chilling i palatability and may reduce
nausea. The subject sits quietly throughout thetest.
•
Blood glucose is sampled before (time 0), and at 120min after,
ingestion of the drink, which should be completed within5min.
•
Urinalysis may also be performed every 30min, although it is only
of interest if a signicant alteration in renal threshold for glucose is
suspected.
1
In children, 1.75g/ kg, up to75g.
Table2.16 Interpretation ofthe 75g OGTT(WHO)
Venous plasma glucose (mmol/ L)
Fasting 120min post- glucose load
Normal <6.0 <7.8
IFG
6.1– 6.9 N/ A
IGT N/ A 7.8– 11.0
DM >7.0 >11.1
1. In the absence of symptoms, a diagnosis of diabetes must be conrmed by a second diagnostic
test on a separate day.
2. For capillary whole blood, the diagnostic cut- os for diabetes are>6.1mmol/ L (fasting)
and 11.1mmol/ L (120min). The range for IFG based on capillary whole blood is >5.6 and
<6.1mmol/ L. In the diagnosis of diabetes, the 2h post- blood value predominates if values do
notagree.

DIABETES MELLITUS
199
Screening fordiabetes
This remains controversial, and universal screening is not generally advo-
cated, even though rates in the adult population exceed 5% in almost all
countries and >10% in many countries. Alow threshold for testing in those
at high risk is advocated. The ADA suggests that 3- year screening in asymp-
tomatic adults be considered over the age of 45, and especially where the
BMI is >25 and there is high- risk ethnicity (including African or South Asian
origin), a rst- degree relative with diabetes, or women who have had a large
baby (>9lb) or a previous diagnosis of gestational diabetes.
Further reading
American Diabetes Association. Diagnosis and classication of diabetes mellitus. Diabetes Care
2016;39
(Suppl 1), S13– 22.
World Health Organization 2006 Guidelines: M http:// www.who.int/ diabetes/ publications/
Denition%20and%20diagnosis%20of%20diabetes_ new.pdf
World Health Organization (2013). Diagnostic criteria and classication of hyperglycaemia rst detected
in pregnancy. Geneva: World Health Organization. M
http:// apps.who.int/ iris/ bitstream/
10665/ 85975/ 1/ WHO_ NMH_ MND_ 13.2_ eng.pdf.
WHO criteria for use ofHbA1c.
Diabetes websites
American Diabetes Association. M http:// www.diabetes.org.
Diabetes UK. M http:// www.diabetes.org.uk.
World Health Organization. Diabetes. M
http:// www.who.int/ topics/ diabetes_ mellitus/ en/ .
200
CHAPTER2 Endocrinology and metabolism
200
Which type ofdiabetesisit?
Table 2.17 shows the types and causes of diabetes. Type 2 diabetes due to
insulin resistance is by far the commonest (accounting for >90% of cases)
and is i as the prevalence of obesity and low physical activity in our society
rise. However, there are no denitive tests that can distinguish type 1 and
type 2 diabetes. Instead, a collection of clinical and laboratory parameters
are used (see Table 2.18), but many cases are hard to categorize (some-
times referred to as type 1.5 diabetes!).
Figure 2.18 (guide to the type of diabetes) provides an algorithm for diag-
nosing the type of diabetes. Although type 2 diabetes remains the com-
monest diagnosis in adults and is increasingly common in children, this is a
diagnosis for the lifetime of the individuals and care should be taken to diag-
nose any underlying conditions as accurately as possible. Note especially:
•
Unusual features:if any of the unusual features listed in Table 2.19 are
present, then the algorithm should not be pursued and an underlying
cause for the diabetes investigated.
•
Type 1 diabetes:it is important to always consider the possibility of
type 1 diabetes (see Fig. 2.18), even in older people, as early insulin
treatment is essential to avoid the risk of ketoacidosis.
•
Pregnancy:although the majority of diabetes diagnosed for the rst
time in pregnancy (gestational diabetes) is part of the spectrum of type
2 diabetes, diabetes due to other causes can occur. The algorithm in
Fig.2.18 should still be considered.
•
Latent autoimmune diabetes of adults (LADA) refers to diabetes with +ve
autoantibodies (anti- glutamic acid decarboxylase (GAD)) developing in
adults (typically over the age of 35)but not developing ketosis or with
absolute requirement for insulin within 6months of diagnosis. However,
subjects are usually not overweight and develop a need for insulin within
a few years. LADA is considered to be a ‘slow- onset’ version of type 1
diabetes, often initially misdiagnosed as type 2 diabetes.
Table2.17 Types and causes ofdiabetes
Type of diabetes* Comment
Type 1 β
- cell destruction usually leading to absolute insulin
deciency:1A— immune- mediated; 1B— idiopathic
(formerly known as juvenile- onset or IDDM)
Type 2 Predominantly insulin- resistant with relative insulin
deciency (formerly known as maturity- onset or
NIDDM)
Gestational diabetes Diabetes during pregnancy that resolves postpartum.
Often an early manifestation of type 2 diabetes
Other specic types
Includes genetic (MODY, mitochondrial, insulin
resistance syndromes), pancreatic disease, drug-
induced, occurrence in other genetic syndromes,
endocrinopathies (e.g. acromegaly)
* Abbreviated from the ADA classication.

WHICH TYPE OFDIABETESISIT?
201
• Monogenic diabetes— maturity- onset diabetes of the young
(MODY):monogenic disorders resulting in diabetes (see Tables2.19
and 2.20). Although these account for <1% of cases, they
are important to diagnose as they may be easily treated with
sulfonylureas (MODY3), associated with renal disease (MODY5),
or require no treatment (MODY 2). The diagnosis also has
important implications for other family members diagnosed with
diabetes. Note that it can be very difficult to distinguish type 2
diabetes from MODY without genetic testing, and a high level of
suspicion in young people is required (see Fig. 2.18). If suspected,
further advice from a genetic testing centre with experience in
MODY should be sought (M http:// www.diabetesgenes.org).
•
‘Flatbush’ diabetes refers to patients who present in DKA but
subsequently have a course that is more like type 2 diabetes
and are able to come o insulin. This is most commonly seen in
African- Caribbeans.
Table2.18 Clinical features and laboratory tests used todistinguish
type 1 and type 2 diabetes, and maturity- onset diabetes ofthe
young(MODY)
Type 1 Type 2 MODY
Clinical
features
Weight Slim (BMI <25)
+ weight loss at
diagnosis
Overweight
(BMI >25)
Average weight
(BMI <30)
Ketosis Occurs Rare Rare
Race Caucasian i risk in
South Asians
and Afro-
Caribbeans
Acanthosis
nigricans
Absent May be
present
Absent
Parent with
diabetes
Unusual Common Common
Laboratory
tests
Auto- antibodies Anti- GAD
antibody +ve in
around 80%*
−ve −ve
Insulin c- peptide Low (but still
present for up
to 5years from
diagnosis)
Present Present
High- density
lipoprotein
(HDL) >1.2
Usual Rare Usual
* Additional antibody tests can include anti- IA2 and anti- ZnT8 antibodies.
202
CHAPTER2 Endocrinology and metabolism
202
Diagnosis of
diabetes
conrmed
Unusual
features
present
Unusual
features
absent
Consider
specic
type of
diabetes
(Table 2.26)
BMI > 40
BMI 25-40
BMI < 25
Likely type
2 diabetes
All of:
Caucasian
Wght loss
at dx
Ketones >++
at dx
No
Yes—likely
Type 1
diabetes
No other
features
Family history
In two
generations
Diagnosis
< 25 yrs
Ketones > ++
at dx Or
wght
loss at dx O r
fail tablet Rx
within 1 year
Consider
Type 1
Test for auto
antibodies
Consider
MODY
Likely type
2 diabetes
(esp if
acanothosis,
Non-
caucasian)
Fig.2.18 Guide to the type of diabetes.
Table2.19 Specic features suggestive ofan unusual cause ofdiabetes
Feature Possible diagnosis
Alcohol excess, history of
pancreatic disease
Chronic pancreatitis
Painless jaundice, weight loss in
older person
Pancreatic cancer
Cystic brosis Pancreatic disease
On steroids/ Cushingoid
appearance
Steroid- induced diabetes/ Cushing’s
syndrome
Post- organ transplant Drug- induced
Specic drugs, e.g. somatostatin
analogues, diazoxide
Drug- induced
Severe hypertension Phaeochromocytoma
Acromegalic appearance Acromegaly
‘Muscular appearance’
(lipodystrophy)
Partial lipodystrophy

WHICH TYPE OFDIABETESISIT?
203
Table2.20 Genetically inherited forms ofdiabetes, includingMODY
Gene % of MODY
cases
Comments
Glucokinase 20
MODY 2 ‘mild’ complications rare
HNF- 1α 60 MODY 3 diagnosed later— 35years, progressive
β cell failure, but very sensitive to sulfonylureas
HNF- 4α 1 MODY 1
HNF- 1β 1 MODY 5 renal cysts/ abnormalities
IPF- 1 1 MODY 4
NeuroD1 ? <1 MODY 6
Unknown 15 ‘MODY X’
SUR1 <1%?? Hyperinsulinism in infancy and β cell failure as adult
Mitochondrial
Not MODY Maternally inherited. May be associated with
nerve deafness, lactic acidosis, or syndromes
such as DIDMOAD (diabetes insipidus, diabetes
mellitus, optic atrophy, and deafness)
Lipodystrophy
Not MODY,
lamin a/ c and
others
Associated with localized fat loss
Feature Possible diagnosis
Family history of diagnosis <25
in two or more generations
MODY
Renal cysts, urogenital dysplasia Hepatic nuclear factor (HNF)1β MODY
Bilateral deafness (maternal
inheritance)
Mitochondrial diabetes
Optic atrophy, DI Wolfram syndrome
Diagnosis <6months old Kir 6.2 mutation or other forms of neonatal
diabetes
Megaloblastic anaemic Roger’s syndrome (thiamine)
Short stature, severe insulin
deciency (>1000U/ day)
Insulin receptor defect (leprechaunism,
Rabson– Mendenhall syndrome)
Sti legs/ gait/ falls:−ve- GAD
antibodies
Sti person syndrome
Table 2.19 (Contd.)
Further reading
DiabetesGenes.org. M http:// www.diabetesgenes.org.

204
CHAPTER2 Endocrinology and metabolism
204
Monitoring diabetic control
Self- testing and near- patient testing
Self- testing capillary blood glucose (or urine) can be readily performed by
the majority of patients, with results available in under 20s. Measurements
of longer- term glycaemic control are typically laboratory- based, although
increasingly near- patient testing equipment is available for use in clinics that
can give results to patients within minutes.
Urine testing
Glycosuria
Semi- quantitative testing for glucose using reagent- impregnated test strips
is of limited value and although used to ‘screen’ for diabetes, it is not rec-
ommended for monitoring of glycaemic control. Urinalysis provides retro-
spective information over a limited period of time. Other limitationsare:
•
The renal threshold for the reabsorption of glucose in the proximal
convoluted tubule (PxCT) is 710mmol/ L on average but varies
between individuals. Subjects with a low threshold will tend to show
glycosuria more readily, even with normal glucose tolerance (‘renal
glycosuria’). Children are particularly liable to test +ve for glucose.
The renal threshold is eectively lowered in pregnancy. Conversely,
a high threshold, common among the elderly, may give a misleadingly
reassuring impression of satisfactory control. Fluid intake and urine
concentration may aect glycosuria. Renal impairment may elevate the
threshold for glucose reabsorption.
•
Delayed bladder emptying, e.g. due to diabetic autonomic neuropathy,
will reduce the accuracy of the measurements through dilution.
•
Hypoglycaemia cannot be detected by urinalysis.
Ketonuria, self- blood testing forketones
Semi- quantitative test strips for acetoacetate (e.g. Ketostix
®
) are available
for patients with type 1 DM but have largely been replaced by self- testing
of blood (capillary, ngerprick) for ketones (β- hydroxybutyrate). Useful
when intercurrent illness leads to disturbance of metabolic control. The
presence of ketonuria on dipstick testing (++ or +++) or blood ketones
(>3.0mmol/ L) in association with hyperglycaemia indicates marked insulin
deciency. The patient may be developing ketoacidosis, and i insulin doses
and urgent assessment for DKA is required (E Diabetic emergencies:dia-
betic ketoacidosis, hyperosmolar non- ketotic syndrome, and lactic acidosis,
pp. 210–11). Note that low- level ketonuria (‘+’) or blood ketones <0.6mmol/ L
can occur after a period of fasting, especially in overweight patients, and
does not necessarily indicate DKA. Occasionally, patients with type 2
DM develop ketosis during severe intercurrent illness, e.g. major sepsis.
Urine testing strips do not detect 3- hydroxybutyrate (although acetone is
detected by Acetest
®
). Occasional underestimation of the degree of keto-
naemia using these tests is a well- recognized, albeit uncommon, caveat of
alcoholic ketoacidosis but is no longer an issue with the use of blood ketone
testing. Blood testing is preferred, as it does not require any delay in obtain-
ing a urine sample and is more accurate. Separate testing strips from glucose
testing are required (but often the same meter can beused).

MONITORING DIABETIC CONTROL
205
Self- testing ofblood glucose
Self- testing of capillary blood glucose obtained by ngerprick has become
an established method for monitoring glycaemic control. Frequent testing is
a prerequisite for adjusting insulin doses and for safe intensive insulin ther-
apy such as that employed in the DCCT. Use in type 2 diabetes treated by
diet or tablets only is not essential. Enzyme- impregnated dry strip methods
are available, which are used in conjunction with meter devices and give
results in <20s with just 50µL of blood. Adequate training and a system of
quality control are important; even when trained health professionals use
such systems in clinics or hospitals, misleading results are possible, particu-
larly in the lower range of blood glucose results. Where there is doubt,
an appropriate sample (in a tube containing the glycolysis inhibitor uoride
oxalate) should be collected immediately for analysis by the clinical chem-
istry laboratory. However, acute treatment of hypoglycaemia, where indi-
cated, should not be delayed.
Continuous glucose monitoring systems(CGMS)
Several systems are now available using electrical conductance or microdi-
alysis to provide continuous monitoring of interstitial glucose. Sensors are
inserted SC and need to be replaced every 3– 7days. Most systems can dis-
play the results in real time (if regularly calibrated against traditional nger
stick readings), so that they can be reviewed by the patient, and linked to
alarms indicating high and low levels. While these systems, although expen-
sive, are proving increasingly valuable for patients with type 1 diabetes on
complex insulin regimes and insulin pump therapy, it must be remembered
that the interstitial glucose level is up to 30min ‘behind’ the blood level
and if glucose levels are changing rapidly, continuous monitors may ‘miss’
signicant hypoglycaemic events. Arecent development is sensors that do
not require calibration and the reading is made by ‘swiping’ the reader (or
an appropriately congured smartphone) over the sensor. These newer
sensors have signicantly reduced ‘delay’ time, claimed to be <10min vs
blood levels.

206
CHAPTER2 Endocrinology and metabolism
206
Laboratory assessment ofglycaemic
control
Glycated haemoglobin
MeasuringHbA
1c
HbA
1c
(comprises 60– 80% of total glycated haemoglobin HbA
1
) is formed
by the slow, irreversible post- translational non- enzymatic glycation of
the N- terminal valine residue of the β chain of Hb. The proportion of
HbA
1c
:total Hb (normal non- diabetic reference range 74– 6%) provides
a useful index of average glycaemia over the preceding 6– 8 weeks. The
result is disproportionately aected by blood glucose levels during the nal
month before the test (750% of value). Laboratory values have now been
aligned to a standard from the DCCT trial (DCCT- aligned), and values are
generally consistent between laboratories. Recent recommendations sug-
gest expressing HbA
1c
in new units (mmol/ mol) against a new International
Federation of Clinical Chemistry (IFCC) standard (see Table2.21).
Table2.21 Guide toHbA
1c
values innew and oldunits
DCCT- aligned HbA
1c
(%) IFCC- HbA
1c
(nmol/ mol)
6.0 42
6.5 48
7.0 53
7.5 59
8.0 64
9.0 75
Frequency oftestingHbA
1c
It is suggested that HbA
1c
is measured every 6months in stable patients,
every 3 months in patients with unstable metabolic control, and every
month in pregnancy.
Interpreting HbA
1c
levels
Average HbA
1c
levels collected over a longer period (i.e. years) provide
an estimate of the risk of microvascular complications. Sustained high con-
centrations identify patients in whom eorts should be made to improve
long- term glycaemic control and i surveillance for long- term complications.
Table 2.22 summarizes recent recommendations for target HbA
1c
levels
and capillary glucose measurements in adult subjects with diabetes. Targets
need to be modied in pregnancy (see footnote to Table 2.22), in children,
in patients with recurrent hypoglycaemia or diculty complying with medi-
cation, and in patients with vascular (especially coronary artery) disease in
whom hypoglycaemia may precipitate fatal arrhythmias.

LABORATORY ASSESSMENT OFGLYCAEMIC CONTROL
207
Limitations ofHbA
1c
measurements
Although glycated Hb levels are a reliable indicator of recent average gly-
caemic control, they do not provide information about the daily pattern
of blood glucose levels or the frequency of hypoglycaemic episodes; this
supplementary information required for logical adjustment of insulin doses
is derived from frequent home blood glucose monitoring. More recent
changes in glycaemia (i.e. within the preceding 4 weeks or so) will inuence
HbA
1c
level more than glucose levels 12 or more weeksago.
Spurious HbA
1c
levels may arise in statesof:
•
Blood loss/ haemolysis/ reduced red cell survival (low HbA
1c
).
•
Haemoglobinopathy.
•
i levels of HbS (low levels ofHbA
1c
)
•
i levels of HbF (high HbA
1c
).
Modern HbA
1c
methods are likely to detect haemoglobinopathies with-
out specic testing. Where haemoglobinopathy is present and cannot be
adjusted for by an assay method where there is no interference, the HbA
1c
test is uninterpretable and capillary blood glucose levels or fructosamine
must beused.
HbA
1c
measurements are less reliable in pregnancy where rapid changes
in blood glucose levels can occur (e.g. last trimester). They are still used, as
they are more reliable than other available methods or estimating overall
control, but results should be interpreted with caution.
Uraemia due to advanced diabetic nephropathy is associated with anae-
mia and d RBC survival, thereby falsely lowering HbA
1c
levels.
Table2.22 Target levels ofHbA
1c
and capillary blood glucosevalues
Pre- meal (mmol/ L) Post- meal (mmol/ L) HbA
1c
(nmol/ mol)
Non- diabetic 3.5– 5.5 <7.8 4– 6% (20– 42)
Ideal 4– 7* <10* <7%** (<53)
Suboptimal
control
7– 10%
Poor control >10%
* In pregnancy, NICE recommends 3.5– 5.9 pre- meal and <7.8 post- meal (1h) HbA
1c
; aim
for<6.1%.
** In type 2 diabetes, NICE recommends a target of 6.5% in patients without vascular disease.
Note:lower target levels (HbA
1c
<6.5%) have been proposed in subjects with type 2 diabetes
treated with lifestyle or metformin alone; Higher targets (e.g. HbA
1c
<8.0% or higher) are
appropriate in subjects with frequent hypoglycaemia or hypoglycaemia unawareness, extensive
co- morbid conditions, or limited life expectancy.

208
CHAPTER2 Endocrinology and metabolism
208
Fructosamine:refers to protein– ketoamine products resulting from the
glycation of plasma proteins. The fructosamine assay measures glycated
plasma proteins (mainly albumin), reecting average glycaemia over the pre-
ceding 2– 3 weeks. This is a shorter period than that assessed using glycated
Hb measurements and may be particularly useful when rapid changes in
control need to be assessed, e.g. during pregnancy. Levels can be misleading
in hypoalbuminaemic states, e.g. nephrotic syndrome. Some fructosamine
assays are subject to interference by hyperuricaemia or hyperlipidaemia.
The main indications for fructosamine measurement are currently:(a)the
presence of haemoglobinopathy or other interference with the HbA
1c
assay
(E Limitations of HbA
1c
measurements, pp. 207–8) and (b)rapidly chang-
ing blood glucose levels (e.g. pregnancy).
Further reading
American Diabetes Association (ADA). Clinical guidelines on pages for health professionals.
M
http:// www.diabetes.org.
American Diabetes Association. 5.Glycemic targets. Diabetes Care 2016; 39
(Suppl 1), S39– 46 (ADA
standards of medicalcare).
National Institute for Health and Care Excellence. Diabetes guidelines. M
http:// www.nice.org.uk.

LABORATORY ASSESSMENT OFGLYCAEMIC CONTROL
209

210
CHAPTER2 Endocrinology and metabolism
210
Diabetic emergencies:diabetic
ketoacidosis, hyperosmolar non- ketotic
syndrome, and lactic acidosis
DKA should be considered in any unconscious or hyperventilating patient.
The hyperosmolar non- ketotic (HONK) syndrome is characterized by
marked hyperglycaemia (>30mmol/ L) and dehydration in the absence
of signicant ketosis or acidosis. Lactic acidosis (LA) associated with
metformin is uncommon. Arapid clinical examination and bedside blood
tests should allow the diagnosis to be made. Treatment (IV rehydration,
insulin, electrolyte replacement) of these metabolic emergencies should be
commenced without delay (for details, E Further reading, p. 211).
Conrm diagnosis bybedside measurementof
• Capillary blood glucose.
• Capillary blood testing for ketones (betahydroxybutyrate).
• Urine for nitrites and leucocytes(UTI).
Venous blood forurgent laboratory measurementof
• Plasma glucose (uoride oxalate; 2 true ‘euglycaemic’ DKA israre).
•
U&E (arterial potassium (K
+
) can be measured by some gas analysers).
Plasma Na
+
may be depressed as a consequence of hyperglycaemia or
marked hyperlipidaemia.
•
Plasma creatinine (2 may be falsely elevated in some assays byDKA).
•
Plasma lactate (if indicated— can also be measured by some gas
analysers). Indicated if acidosis without heavy ketonuria is present. LA is
a complication of tissue hypoxia (type A) and is a rare complication of
metformin treatment in patients with type 2 DM (typeB).
•
Plasma osmolality in HONK— either by freezing point depression or
calculated:
2 × [plasma Na
+
] + [plasma K
+
] + [plasma glucose] + [plasma urea]
•
FBC (non- specic leucocytosis is common inDKA).
• Blood cultures (signs of infection, e.g. fever, may be absent inDKA).
• ABGs (corrected for hypothermia) for arterial pH, HCO
3
−
, PCO
2
, and
PO
2
(if shock or hypotension).
DKA is conrmed by blood glucose >11mmol/ L (rarely euglycaemic
ketoacidosis can occur in pregnancy or subjects on sodium– glucose
cotransporter 2 (SGLT2)- inhibiting drugs), ketonaemia >3.0mmol/ L, and
acidosis (pH <7.3 or HCO
3
−
<15mmol/ L). Severe DKA is considered
to be pH <7.0, HCO
3
−
<5mmol/ L, or ketones >6.0mmol/ L. Repeat
laboratory measurement of blood glucose, electrolytes, and urea at 2, 4,
and 6h, and as indicated thereafter. Electrolyte disturbances, renal impair-
ment, or oliguria should prompt more frequent (1– 2h) measurements
of plasma K
+
. Capillary blood glucose and ketone testing are monitored
hourly at the bedside. 2 Avoidance of hypokalaemia and hypoglycae-
mia is most important during therapy. Current therapy is aimed at rapid
resolution of ketosis which is considered to be ketonaemia <0.6mmol/ L
(‘normal’ <0.3mmol/ L) with pH>7.3.

211
DIABETIC EMERGENCIES
Other investigations, asindicated
• CXR.
• Microbial culture of urine, sputum,etc.
• ECG:acute MI may precipitate metabolic decompensation; note that
serum transaminases and CK may be non- specically elevated inDKA.
• Sickle- cell test (in selected patients).
• Venous plasma PO
3
4−
(if there is respiratory depression).
•
2 Performance of investigations should not delay initiation of treatment
and transfer to a high- dependency or intensive care unit(ICU).
Severe metabolic acidosis inthe absence ofhyperglycaemia (or other obvious
cause ofacidosis such asrenal failure) raises thepossibilityof
•
LA.
• Alcoholic ketoacidosis:this occurs in alcoholics following a binge.
Alterations in the hepatic redox state may result in a misleading
−veor‘trace’ Ketostix
®
reaction but detectable on blood ketone strips.
Asimilar caveat may occasionally be encountered when signicant
LAcoexists with DKA. Venous plasma glucose may be normalori.
2
Anion gap >15mmol/ L. Normally, the anion gap (<10mmol/ L) results
from plasma proteins, sulfate (SO
4
2−
), PO
4
3−
, and lactate ions. When the
anion gap is i, measurement of plasma ketones, lactate, etc. usually con-
rms the aetiology (see Table2.23).
Further reading
Joint British Diabetes Societies (JBDS) guidelines (2013). Available at:M http:// www.diabetologists-
abcd.org.uk.
Table2.23 Causes ofan anion gap acidosis
Ketoacidosis DKA
Alcoholic ketoacidosis
LA (2 metformin)
Chronic renal failure
Drug toxicity Methanol (metabolized l formic acid)
Ethylene glycol (metabolized l oxalic acid)
Salicylate poisoning

212
CHAPTER2 Endocrinology and metabolism
212
Investigation ofhyperlipidaemia
° dyslipidaemias are relatively common and contribute to an individual’s
risk of developing atheroma (e.g. CHD, CVD). Prominent examples include
familial combined hyperlipidaemia (FCHL; 72– 3% of UK population) and
heterozygous familial hypercholesterolaemia (FH; UK incidence 1 in 500).
Major hypertriglyceridaemia also predisposes to pancreatitis. The key fea-
tures of familial FH, FCHL, and diabetic dyslipidaemia are consideredlater.
Investigations
Although many subtle alterations in plasma lipids have been described,
therapeutic decisions rest on measurement of some or all of the following
in serum or plasma (plasma being preferred, since it can be cooled rapidly):
•
Total cholesterol (may be measured in non- fasting state in rst instance,
since levels are not greatly inuenced by meals).
•
Triglycerides (TGs) (after 12hfast).
• LDL- cholesterol (calculated using the Friedewald formula when TGs are
<4.5mmol/ L):
LDL-cholesterol = [(total cholesterol − HDL-cholesterol) − TGs]/2.19
Note that because of the unreliability of this calculation, especially at higher
TG levels, it has been recommended to report results also as ‘non- HDL-
cholesterol’, which is typically around 0.7mmol/ L higher than calculated
LDL- cholesterol.
• HDL- cholesterol (regarded as the ‘cardioprotective’ subfraction)— HDL
particles are synthesized in the gut and liver and thought to be involved
in ‘reverse transport’ of cholesterol from peripheral tissues to the liver
where it can be excreted as bilesalts.
Notes onsampling inrelation tolipoprotein metabolism
•
TGs (triacylglycerols) are measured after a 712h overnight fast in order
to clear diet- derived chylomicrons.
• Alcohol should be avoided the evening prior to measurement of TGs
(can exacerbate hypertriglyceridaemia).
•
Aweight- maintaining diet is recommended for 2– 3 weeks before
testing.
•
Lipid measurements should be deferred for 2– 3 weeks after minor
illness and 2– 3months after major illness, surgery, or trauma since
cholesterol levels may be reduced. Following acute MI, it is generally
accepted that plasma cholesterol is reliable if measured within 24h of
the onset of symptoms.
•
The eects of certain drugs on lipids should be considered (see
Box2.10).
•
Glycaemic control should be optimized wherever possible before
measuring plasma lipids in patients with diabetes.
Important additional considerationsare
•
Day- to- day variability:generally, decisions to treat hyperlipidaemia should
be based on >1 measurement over a period of 1– 2 weeks. This is
especially true for patients with mixed hyperlipidaemia (i.e. including
hypertriglyceridaemia).

INVESTIGATION OFHYPERLIPIDAEMIA
213
• Exclusion of 2° hyperlipidaemia— many common conditions, drugs, and
dietary factors can inuence plasma lipids (see Box2.10).
•
Family members should also have their plasma lipids measured if familial
hyperlipidaemia is suspected in a proband.
Both cholesterol and TGs may be aected to some degree by these fac-
tors, but one often predominates. Pre- existing ° hyperlipidaemias may be
exacerbated.
Clinical features
For example, xanthelasma, tendon xanthomas, etc. should always be
sought. Adetailed family history, drug history, and medical history (for dia-
betes and other cardiovascular risk factors such as hypertension) should
always be obtained. Certain endocrine disorders and impaired hepatic or
renal function can inuence circulating lipid composition and cardiovascu-
lar risk. Aclassication of the major familial dyslipidaemias is presented in
Table2.24.
2 Specialist advice should be sought in the management of major or
resistant hyperlipidaemias. TG levels >11mmol/ L (1000mg/ dL) are con-
sidered ‘very high’ and require therapy because of the risk of pancreatitis.
Box 2.10 Causes ofsecondary hyperlipidaemia
Hypercholesterolaemia
•
Hypothyroidism (even minor degrees of ° hypothyroidism)
• Cholestasis (raised lipoprotein X levels)
• Nephrotic syndrome
• Anorexia nervosa
• Diuretics
• Immunosuppressiveagents
• Hepatoma
• Dysglobulinaemias
Hypertriglyceridaemia
•
Obesity
• Diabetes (especially type2DM)
• Lipodystrophic syndromes (of diabetes and HIV- associated)
• Alcohol excess (note:moderate alcohol consumption may raise
HDL- cholesterol)
• Renal failure
• Antiretroviralagents
• Oestrogens (especially oral preparations in somewomen)
• Corticosteroids
• β- adrenergic blockers
• Retinoids
Recommended investigation for exclusion of 2° hyperlipidaemia: U&E,
plasma creatinine, fasting venous glucose, LFTs, and TFTs. For patients on
statins, check LFTs and CK periodically (2 measure urgently if myositis
occurs— a rare, but potentially fatal, complication).
214
CHAPTER2 Endocrinology and metabolism
214
Table2.24 Familial hyperlipoproteinaemias
Genetic disorder Defect Presentation Cholesterol Triglycerides Phenotype
Familial LPL deciency Absence of LPL activity Eruptive xanthomata;
hepatosplenomegaly
i iii I
Familial apo C- II deciency Absence of apo C- II Pancreatitis i iii I or V
Familial
hypercholesterolaemia
LDL receptor deciency Tendon xanthomata;
premature atheroma
iii i or N IIa or IIb
Familial
dysbeta- lipoproteinaemia
Abnormal apo E and
defect in TG metabolism
Tubo- eruptive and palmar
xanthoma; premature
atheroma
iii iii III
Familial combined
hyperlipidaemia
Uncertain Premature atheroma i or N i or N IIa, IIb, or IV
Familial
hypertriglyceridaemia
Uncertain; eruptive
xanthomata;
hepatosplenomegaly;
pancreatitis
N
IV
i iii V
i, ii, and iii, mildly, moderately, and serverly raised; respectively cholesterol and triglycerides refer to concentrations in plasma; phenotype refers to Fredrickson classication
(I to V, see Table2.25); apo, apoprotein; LPL, lipoprotein lipase; n, normal; TG, triglycerides.

INVESTIGATION OFHYPERLIPIDAEMIA
215
Table2.25 Phenotypic (Fredrickson) classication ofhyperlipidaemias
Type Cholesterol Triglycerides Particle excess Usual underlying cause
I i iii Chylomicrons
LPL or apo C- II
deciency
IIa ii i LDL LDL receptor defect,
LDL overproduction
IIb ii ii
VLDL, LDL VLDL or LDL
overproduction or d
clearance
III ii ii
IDL— dysbetalipo-
proteinaemia
Impaired remnant
removal may be
due to certain apo E
phenotypes or apo E
deciency
IV N or i ii VLDL VLDL overproduction
or d clearance
V i iii ChylomicronsVLDL Diabetes
i, ii, and iii, mildly, moderately, and severely raised, respectively; LDL, low- density
lipoprotein; VLDL, very low- density lipoprotein; LPL, lipoprotein lipase; apo, apoprotein; IDL,
intermediate- density lipoproteins.
Further reading
JBS3 Board. Joint British Societies’ consensus recommendations for the prevention of cardiovascular
disease (JBS3). Heart 2014; 100
Suppl 2:ii1– 67.
National Heart, Lung, and Blood Institute, National Cholesterol Education Programme Guidelines
(2013). Managing blood cholesterol in adults:systematic evidence review from the Cholesterol Expert
Panel. M
http:// www.nhlbi.nih.gov/ health- pro/ guidelines/ in- develop/ cholesterol- in- adults.

216
CHAPTER2 Endocrinology and metabolism
216
Test protocols
Insulin tolerance test (insulin stresstest)
• Indication:suspected ACTH or GH deciency.
•
Contraindications:patients with epilepsy, CHD (checkECG).
•
Children:use no more than 0.1U/ kg. Considerable care should be
exercised; the test should only be performed in a centre with expertise.
•
Alternatives:short Synacthen
®
test for hypocortisolism; stimulation tests
for GH deciency, e.g. growth hormone- releasing hormone (GHRH)
arginine test (E Hypothalamus/ pituitary function, see pp. 128–30).
• Preparation:patient fasting overnight. Bed required (although day- case
procedure). Patient must be accompanied home and may not drive.
OMIT morning hydrocortisone or other steroid hormone replacement
if patient is taking this and previous day’s GH. Physician must be present
throughout the test. Requires written consent.
•
Procedure:early morning outpatient test in fasting patient. Indwelling
venous cannula and constant medical supervision required throughout.
Cannula is kept patent by running a saline infusion with a three- way tap
for sampling. Discard initial 2– 3mL when each sample is taken. Label
all samples clearly with time and patient details. Near- patient testing
glucometer required.
1. Take baseline blood for glucose, cortisol, and GH. Check IV access
is working well. Review the test with the patient, and explain symp-
toms s/ he is likely to experience (see point5).
2. Draw up 25mL of 50% glucose for immediate administration IF
REQUIRED.
3. Give soluble (regular) insulin as an IV bolus in a dose of 0.15U/ kg
after an overnight fast. Consider 0.1U/ kg (lower dose) if suspected
profound hypocortisolism. 2 This appears a very small dose, e.g.
typically around 10U. CHECK DOSE CALCULATION CAREFULLY.
Usually an insulin syringe is used to draw it up and then transfer it to
a 2mL syringe containing saline.
4. Take blood at 15min intervals (0, 15, 30, 45, 60min) for glucose,
cortisol, andGH.
5. Observe for symptoms and signs of hypoglycaemia. The rst sign
is usually profuse sweating. The patient may then be aware of
symptoms such as palpitations, hunger, and paraesthesiae. This typi-
cally occurs 30– 45min into the test. Check near- patient glucose to
conrm <3.5mmol/ L. Continue to talk to, and reassure, the patient.
If the patient becomes very drowsy or unrousable, then give 25mL
of 50% glucose. This does not invalidate the test, as the hypogly-
caemic stimulus has already occurred. Continue blood sampling at
standardtimes.
6. If the patient has not experienced hypoglycaemia by 45min and near-
patient glucose is >4mmol/ L, give a further IV bolus of 0.15 or 0.3U/ kg
if the patient is known to be very insulin- resistant (e.g. acromegalic).
Repeat sampling at 15min intervals for 60min after this secondbolus.
7. At the end of procedure (usually 60min), give IV 25mL of 50%
dextrose if the patient still has symptoms of hypoglycaemia.
8. Give the patient a meal including complex carbohydrate (e.g. sand-
wiches or lunch), and observe for a minimum of 1h further before
accompanied discharge.

TEST PROTOCOLS
217
• Unwanted eects:severe hypoglycaemia with depressed level of
consciousness or convulsion requires immediate termination of the test
with 25mL of 50% glucose IV. Repeat if necessary and follow with 5%
or 10% glucose infusion. Continue to collect samples for hormone and
glucose measurements.
•
Interpretation:the test is only interpretable if adequate hypoglycaemia is
achieved (<2.2mmol/ L). Normal maximal cortisol response >550nmol/ L.
(Note:may be lower, depending on local assay range— check with the
laboratory). Normal GH response >20mU/ L. Impaired responses (if
hypoglycaemic stimulus adequate) denote corticotrophin (assuming
adrenal glands are normal) or GH deciency or both. Peak GH response
<10mU/ L is sucient to consider GH replacement; peak GH response
<5mU/ L is severe GH deciency.
Combined arginine– growth hormone- releasing
hormonetest
• Indication:GH deciency. Now preferred toITT.
•
Contraindications:previous reaction to stimulatory hormones.
Administer with caution to patients with severe liver or renal disease.
•
Alternatives:ITT. Other stimulation tests now outdated (e.g. glucagon,
exercise). Serum IGF- 1 levels give an idea of the GH status but are
unreliable at low levels.
•
Preparation:order GHRH and arginine from pharmacy. Omit GH
injections for a minimum of 24h. The patient arrives in the morning after
fasting for 10h (overnight). Water is allowed and patients should take
all their routine medications in the morning (but not GH). Informed
consent must be obtained and documented. Warn patients about
possible side eects of IV GHRH such as ushing lasting<5min.
• Procedure:
•
Weigh the patient.
•
Insert an indwelling IV cannula for blood sampling, administration
of bolus GHRH and arginine infusion (keep patent with heparinized
saline).
•
Patients should rest throughout thetest.
•
Take basal samples for GH at −30 and0min.
•
Give GHRH as an IV bolus of 1µg/ kg body weight at 0min; at the
same time, start infusing 30g of 12.5% arginine solution over 30min,
preferably using an infusion pump (children:0.5g/ kg as a 12.5% solu-
tion, to a maximum of30g).
•
Take blood samples for GH at 30, 45, 60, 75, 90, 105, and 120min.
•
Patients are allowed home after a fulllunch.
• Interpretation: the diagnosis of adult GH deciency is conrmed if the
peak GH concentration is <15mIU/ L (by ITT, <12mU/ L in GHRH
testing). Severe GH deciency (as dened by NICE)— peak <9mIU/ L.
Cut- os by BMI range have been proposed (34.5mU/ L for those with a
BMI <25kg/ m
2
, 24mU/ L for a BMI of 25– 30kg/ m
2
, and 12.6mU/ L for
those with a BMI >30kg/ m
2
) but are not universally accepted.
Note
:conversion factor:µg/ L × 3.0=mIU/ L.

218
CHAPTER2 Endocrinology and metabolism
218
Combined anterior pituitary function
testing
• Indication:assessment for anterior pituitary hypofunction.
•
Contraindications:previous reaction to stimulatory hormones.
•
Alternatives:ITT for GH and adrenal axis; metyrapone test for adrenal
axis. Other stimulation tests for GH, e.g. GHRH– argininetest.
• Preparation:test usually performed in morning for basal sampling.
•
Procedure:IV cannula inserted. Basal blood samples taken for cortisol,
oestradiol (♀) or testosterone (♂), FT4, and IGF- 1. Hypothalamic
hormones are given sequentially IV, each as a bolus, over around
20s:LHRH 100µg, TRH 200µg, and ACTH 250µg. Additionally, GHRH
(1µg/ kg body weight) may be given. (Reduce doses in children.)
Samples are drawn at 0, 20, 30, 60, and 120min for LH, FSH, TSH,
cortisol, and PRL. If GHRH is given, samples are drawn at the same time
points forGH.
•
Interpretation:normal values as follows:
•
TRH— suspect 2° hypothyroidism if peak response (at 20min)
<20mU/ L. (Note:low levels also seen in hyperthyroidism— ensure
FT4 or total T4 not raised.)
•
ACTH— peak cortisol response >550nmol/ L at 30 or 60min.
(Note:may be lower, depending on local assay range— check with the
laboratory.)
•
LHRH— peak LH/ FSH response 2– 5 times the basal value. LH, peak
at 20min; FSHlater.
•
GHRH— normal GH peak response >15mU/ L.

COMBINED ANTERIOR PITUITARY FUNCTION TESTING
219

220
CHAPTER2 Endocrinology and metabolism
220
Water deprivationtest
• Indication:diagnosis of DI and to distinguish cranial and nephrogenicDI.
•
Contraindications:none if carefully supervised. For correct interpretation,
thyroid and adrenal deciencies should be replaced rst. Interpretation
in the presence of DM and uraemia can be dicult.
•
Alternatives:morning urine osmolality of >600mOsmol excludes
signicant degrees of DI. No other denitive test forDI.
•
Patient preparation:usually an outpatient procedure. Correct thyroid
and adrenal insuciencies in advance. Renal function and blood glucose
should have been checked in advance. Steroid and thyroid hormone
replacement should be taken as normal on the day of the test. If the
patient is on desmopressin, omit the dose on the evening before the
test (or, if not possible, halve this dose). Free uids, but not to excess,
up to 7.30 a.m. on the day of the test. No alcohol on the night before
the test or on the morning of the test. Light breakfast, but no tea,
coee, or smoking on the morning of the test. Empty the bladder
before attending for the test. If urine volume is <3L/ day (‘mild cases’),
ask the patient to have no uids or food from 6.00p.m. on the evening
before the test (‘prolonged water deprivation test’).
•
Requirements for test:accurate weighing scales. Supervision for the whole
test (up to 8h). Desmopressin for injection (2µg). Immediate access to
serum electrolyte, plasma, and urinary osmolality assays. Access to a
plasma arginine vasopressin (AVP) (ADH) assay desirable.
• Procedure:7.30a.m.
1. Weigh the patient, and calculate 97% of the body weight.
2. Mark this target on thechart.
3. No food or uid for the next8h.
4. Insert a cannula for repeated blood sampling, andush.
• Procedure:8a.m.
5. Obtain plasma for Na
+
and osmolality, and urine for osmolality.
6. Then collect urine hourly for volume and osmolality, and plasma
every 2h for Na
+
and osmolarity.
7. Weigh the patient before and after passing urine if unobserved.
8. If patient loses 3% of the body weight, order urgent plasma osmo-
lality andNa
+
.
9. If plasma osmolality >300mOsmol (Na
+
>140mmol/ L), stop
the test; allow the patient to drink, and give desmopressin (see
point14).
10. If plasma osmolality <300mOsmol, the patient may have been
uid- overloaded before the test, and water deprivation can
continue.
11. Stop the test at 8h (4.00p.m.), and take nal recordings of urine
and plasma.
12. Save an aliquot of plasma for vasopressin levels in case of dicul-
ties in test interpretation.
13. Ideally urine osmolalities will have reached a plateau (<30mOsmol
rise between samples).
14. Now give 2µg of desmopressin intramuscularly (IM) (or 20µg
intranasally), and collect urine samples only for a further 2h. Allow
free uids at thisstage.

WATER DEPRIVATIONTEST
221
• Interpretation:normal response:plasma osmolality remains in the
range of 280– 295mmol; urine osmolality rises to >2 times plasma
(>600mOsmol). If urine volumes during water deprivation do not
reduce and yet the plasma does not become more concentrated (rising
osmolality) and weight does not fall, suspect surreptitious drinking
during the test. For interpretation of abnormal results, E Table 2.2,
p. 136

222
CHAPTER2 Endocrinology and metabolism
222
Diagnostic trial ofdesamino d- arginyl
vasopressin
• Indication:distinction of partial DI from ° polydipsia.
•
Contraindications:cardiac failure; current diuretic use (test
uninterpretable). Note that this test may precipitate severe
hyponatraemia in ° polydipsia and should be performed in an inpatient
unit with clinical and biochemical regular review.
•
Preparation:admission to an assessment unit. First- line tests for
polydipsia/ polyuria should have been performed (E Polydipsia and
polyuria:diabetes insipidus, pp. 134–6).
•
Procedure:
1. 24h urine volume, morning urine osmolality, weight, uid intake (as
far as possible), serum osmolality, Na
+
, urea, and creatinine should
all be performed daily and the results reviewed the sameday.
2. Subjects should have access to uid ad libitum but should be
reminded that they should only drink if they are thirsty.
3. After an initial 24h period of observation, desmopressin is
administered at a dose of 2µg bd SC for 3days.
4. Stop the test if serum Na
+
falls to <130mmol/ L.
• Interpretation:reduction in urine volume to <2L/ day and in urine
osmolality to >600mOsmol/ L without a fall in serum Na
+
to
<140mmol/ L suggests central DI. Reduction in urine volume with noi
in urine osmolality >600mOsmol/ L and without a fall in serum Na
+
suggests partial nephrogenic DI. Limited reduction in urine volume,
with some i in urine osmolarity, but a fall in serum Na
+
, suggests °
polydipsia.

LOW-DOSE DEXAMETHASONE SUPPRESSIONTEST
223
Low- dose dexamethasone
suppressiontest
• Indication:to distinguish hypercortisolism from normality. The
dexamethasone- suppressed CRH test is believed to have less false +ves
in cases of alcoholic or depressive pseudo- Cushing’s syndrome.
• Patient preparation:patients should not be on oral steroids or drugs that
i steroid metabolism.
•
Overnight dexamethasone suppression test:1mg of dexamethasone is
taken by mouth (PO) at midnight. Aserum sample for cortisol is taken
the following morning between 8 a.m. and9a.m.
•
Interpretation:serum cortisol should suppress to <140nmol/ L
(usually <50nmol/ L). Values of 140– 175nmol/ L are equivocal and
suggest a 2- day test should be performed. There is 10– 15% false
+verate.
•
Two- day low- dose dexamethasone suppression test (preferred):
dexamethasone 0.5mg is given PO every 6h for eight doses (2days),
starting in the early morning. Ideally tablets are taken strictly at 6- hourly
intervals (6 a.m., 12 noon, 6p.m., 12 midnight), which may necessitate
an inpatient stay. A24h collection for UFC is taken on the second day
of the test, and serum cortisol is measured at 6 a.m. on the third day, 6h
after the last dose. IV administration of dexamethasone can be used if
there are concerns over absorption or compliance.
•
Interpretation:serum cortisol 6h after the last dose should be
<140nmol/ L, usually <50nmol/ L. UFC on the second day should be
<70nmol/ L, normally <30nmol/ L. The 2- day test strictly performed
has less false +ves than the overnighttest.
•
Dexamethasone- suppressed CRH test:dexamethasone 0.5mg is given PO
every 6h for nine doses (2days), but starting at midnight and ending
at 6 a.m. Tablets are taken strictly at 6h intervals (12 midnight, 6 a.m.,
12 noon, 6p.m.), which may necessitate an inpatient stay. The last dose
is taken at 6 a.m., and an injection of CRH (100µg IV or 1µg/ kg) |is
given at 8 a.m. Ablood sample for cortisol is taken at 8.15 a.m. (i.e.
15min later).
•
Interpretation:serum cortisol level should be <38nmol/ L (normal).
Further reading
Yanovski JA, Cutler GB Jr, Chrousos GP, Nieman LK. Corticotrophin- releasing hormone stimulation
following low- dose dexamethasone administration:a new test to distinguish Cushing’s syndrome
from pseudo- Cushing’s states. JAMA 1993; 269:2232– 8.

224
CHAPTER2 Endocrinology and metabolism
224
High- dose dexamethasone
suppressiontest
• Indication:to distinguish between patients with Cushing’s disease
(ACTH- secreting pituitary tumour) and ectopic ACTH production in
patients with established hypercortisolism.
•
Patient preparation:as low- dose test, except that the test can be
performed immediately following the 2- day low- dosetest.
• Procedure:
1. 2 × 24h UFC collections are made to calculate the mean basal
24hUFC.
2. Baseline serum cortisol measurement is also taken before the
rst dexamethasone dose, ideally at 6 a.m. If the low- dose test is
performed rst, the baseline values (urine and blood) must be taken
prior to the low- dose test (i.e. any doses of dexamethasone).
3. Dexamethasone 2mg is given PO every 6h for eight doses (2days),
starting in the early morning. Ideally tablets are taken strictly at 6h
intervals (6 a.m., 12 noon, 6p.m., 12 midnight), which may neces-
sitate an inpatientstay.
4. A24h urine collection for UFC (nal) is taken on day 2, and a blood
sample is taken for (nal) cortisol 6h after the last dexamethasone
dose (6 a.m. on day 3). Creatinine excretion should be meas-
ured and compared between urine samples to conrm true 24h
collections.
•
Interpretation:the percentage suppression of basal cortisol is calculated
as follows:
(Basal cortisol – nal cortisol)/basal cortisol × 100
The same calculation is made for basal and day 2 UFC. Fifty per cent sup-
pression is suggestive of pituitary- dependent disease; 90% suppression
ithe likelihood (strict criteria). Thymic carcinoids and phaeochromocyto-
mas releasing ACTH are sources of false+ves.

LONG (DEPOT) ACTHTEST
225
Short Synacthen
®
test
• Indication:suspected adrenal insuciency. Will not detect recent- onset
2° adrenal insuciency.
•
Contraindication:asthma/ allergy to ACTH— risk of allergic reaction (can
be performed with careful medication supervision of the patient).
•
Preparation:the patient must not take hydrocortisone on the morning
of the test, as this will be detected in the cortisol assay. The test can be
performed on low- dose dexamethasone, but the morning dose should
be omitted until after the test. May have some value in patients on
higher- dose steroid therapy to indicate the degree of suppression of
adrenocortical function.
•
Procedure:250µg of synthetic ACTH (Synacthen
®
) given IM or IV. Blood
taken at times 0, 30, and 60min for serum cortisol.
•
Low- dose test:the test can be performed with a very low dose of ACTH
(e.g. 1µg). This may detect more subtle degrees of hypoadrenalism, but
the clinical signicance of these ndings remains uncertain.
•
Interpretation:a value at any time of >550nmol/ L makes the diagnosis
very unlikely. (Note:may be lower, depending on local assay range—
check with the laboratory).
Long (depot) ACTHtest
• Indication:distinguishing ° and 2° adrenal failure.
•
Patient preparation:a short Synacthen
®
test should be performed prior
to the test to diagnose adrenal failure. If the patient is on steroid
replacement, change to dexamethasone 0.5mg/ day.
• Procedure:blood is taken at 9 a.m. for basal cortisol; 1mg of depot
synthetic ACTH (Synacthen
®
) is then given IM on two consecutive days,
and blood collected 5h after each dose (2p.m.). Anal cortisol sample
is taken at 9 a.m. on the thirdday.
•
Interpretation:serum cortisol should rise to >1000nmol/ L on the last
day and, if adrenal failure previously indicated by a short Synacthen
®
test, such a rise indicates 2° adrenal failure (pituitary/ hypothalamic
cause, including suppressive drugs).

226

227
Chapter3
Haematology
Full blood count 228
Red cell parameters 230
White cells 232
Platelet count 233
Peripheral blood lm 234
Red cell morphology 236
Parasites on blood lm and
marrow 240
White blood cell morphology 242
Assessment of iron status 244
Assessment of vitamin B
12
and
folate status 248
Erythrocyte sedimentation rate 252
Plasma viscosity 253
Tests for glandular fever 254
Investigation of haemolytic
anaemia 255
General tests of haemolysis 256
Reticulocytes 258
Serum haptoglobins 260
Serum bilirubin 261
Urobilin, urobilinogen, and urinary
haemosiderin 262
Plasma haemoglobin 264
Schumm’s test 265
Hereditary haemolytic
anaemias 266
Red cell membrane disorders 268
Red cell enzyme assays 270
Haemoglobin abnormalities 271
Haemoglobin analysis 272
Investigation of possible
thalassaemia 276
Investigation of sickle
haemoglobin 278
Estimation of haemoglobin A
2
(α
2
δ
2
) 280
Estimation of fetal
haemoglobin 280
Haemoglobin H bodies (β
4
) 280
Heinz bodies 281
Testing for unstable
haemoglobins 281
Molecular tests for diagnosis of
thalassaemia 282
Acquired haemolytic
anaemias 284
Immunophenotyping for GPI- linked
proteins 286
Bleeding time 287
Prothrombin time 288
Activated partial thromboplastin
time 289
Thrombin clotting time 290
D- dimers 290
3 Disseminated intravascular
coagulation 291
Platelet function tests 292
Thrombophilia screening 294
Antithrombin and proteins
CandS 295
Bone marrow examination 296
Cytochemistry tests (leukaemia
diagnosis) 298
Neutrophil alkaline
phosphatase 300
Blood transfusion 302
3
Transfusion reactions 304
Bacterial contamination of blood
products 308
Antiglobulin test 308
Kleihauer test 309
Erythropoietin assay 310
Immunohaematology 311
Immunophenotyping 312
Cytogenetics 314
Cytogenetics:prenatal
diagnosis 316
Cytogenetics:haematological
malignancies 318
Human leucocyte antigen (tissue)
typing 320
Southern blotting 322
Polymerase chain reaction
amplication ofDNA 324
In situ hybridization and
uorescence in situ
hybridization 328
Specialized haematology
assays 330

228
CHAPTER3 Haematology
228
Full bloodcount
Called complete blood count (CBC) in the United States(USA).
Before the advent of modern haematology blood analysers, the blood
count consisted of a haemoglobin (Hb) concentration (estimated using a
manual colorimetric technique), a white cell count, and a manual platelet
count. Other parameters, such as mean cell volume (MCV), had to be
mathematically calculated (derived) using the measured variables Hb, red
cell count (RCC), and packed cell volume(PCV).
Modern analysers use a variety of methods to provide a huge range of
full blood count (FBC) variables, including electronic impedance, laser light
scatter, light absorbance, and staining characteristics. The resultant FBC
provides measured variables such as Hb, PCV, and RCC, along with derived
(mathematically) MCV, mean cell Hb (MCH), and mean corpuscular Hb
concentration (MCHC). These machines also provide automated platelet
counts and a 5- part dierential white blood count(WBC).
Sample:peripheral blood ethylenediamine tetra- acetic acid (EDTA); the
sample should be analysed in the laboratory within 4h, if possible.
Main parameters measured
• Hb concentration.
• RCC.
• MCV.
• MCH.
• MCHC.
• Haematocrit (Hct) orPCV.
• Red cell distribution width(RDW).
• White cellcount.
• WBC dierential.
• Plateletcount.
Some machines are even more sophisticated and will measure reticulocyte
counts, in addition to determination of reticulocyte Hb andMCV.
Role offull bloodcount
Why ask for an FBC? How will this aid the diagnosis or management of the
patient? The FBC assesses several dierent parameters and can provide a
great deal of information. The red cell variables will determine whether or
not the patient is anaemic. If anaemia is present, the MCV is likely to pro-
vide clues as to the cause of the anaemia. The white cells are often raised
in infection— neutrophilia in bacterial infections and lymphocytosis in viral
(but not always so). Platelets (size or number) may be abnormal either as
a direct eect of an underlying blood disease or simply reecting the pres-
ence of some other underlying pathology. Most of us take a somewhat
cursory glance at the FBC when the report arrives on the ward or in clinic,
but a more detailed look may reveal a great dealmore!
Further reading
M http:// www.bbc.co.uk/ health/ talking/ tests/ blood_ full_ blood_ count.shtml.
M
http:// www.rcpa.edu.au/ pathman/ full_ blo.htm.

FULL BLOODCOUNT
229

230
CHAPTER3 Haematology
230
Red cell parameters
Haemoglobin concentration
Units:g/ dL or g/ L (Europe uses SI units; the USA uses g/ dL or grams%).
Denes anaemia (Hb < lower limit of normal, adjusted for age and sex).
Values dier between ♂ and ♀, since androgens drive RBC production and
hence an adult ♂ has higher Hb, PCV, and RCC than an adult♀.
Red cellcount
Unit:x 10
12
/ L.
Useful in the diagnosis of polycythaemic disorders (i production of
microcytic, hypochromic erythrocytes) and thalassaemias.
Causes oflow red cellcount
•
Hypoproliferative anaemias, e.g. iron, vitamin B
12
, and folate deciencies.
•
Aplasias, e.g. idiopathic or drug- induced (do not forget chemotherapy).
• Parvovirus B
19
infection- induced red cell aplasia resulting in transient
marked anaemia.
Causes ofhigh red cellcount
•
PRV.
• Thalassaemia.
Mean cellvolume
Unit:femtolitre (fL), 10
−15
L.
Provided as part of the derived variables or, if you know the PCV and
RCC, can be calculated as (PCV/ RCC), e.g. if the PCV is 0.45 and RCC 5x
10
12
/ L, then the MCV is90fL.
This index provides a useful starting point for the evaluation of anaemia
(see Table3.1).
Table3.1 MCVindex
MCV d MCV normal MCV i
Iron deciency Blood loss B
12
or folate deciency
Thalassaemia homo- /
heterozygotes
Myelodysplasia Myelodysplasia
Sideroblastic anaemia Anaemia of chronic disease

RED CELL PARAMETERS
231
Mean cell haemoglobin
Unit:pg.
•
High (for range, E Normal ranges, inside front cover):macrocytosis.
•
Low:microcytosis, e.g. iron deciency anaemia.
Mean cell haemoglobin concentration
Unit:g/ dL org/ L.
• Of value in evaluation of microcytic anaemias.
• High:severe prolonged dehydration, hereditary spherocytosis, cold
agglutinin disease.
•
Low:iron deciency anaemia, thalassaemia.
Haematocrit or packed cellvolume
These are not entirely synonymous terms (but they are more or less). If
blood is placed in a microcapillary tube and centrifuged, the red cells are
spun down to the bottom, leaving the plasma above. The RBCs will occupy
about 40% of the blood in the tube— the blood will have a PCV of 0.4 (or
40%). The Hct is similar, but derived, using automated blood counters.
PCV unit
:L/ L (although the units are seldom cited in reports).
• High PCV:polycythaemia (any cause).
•
Low PCV:anaemia (any cause).
Red cell distributionwidth
Measures the range of red cell size in a sample of blood, providing informa-
tion about the degree of red cell anisocytosis, i.e. how much variation there
is between the sizes of the red cells. Of value in some anaemias,e.g.:
•
d MCV with normal RDW suggests β- thalassaemiatrait.
• d MCV with high RDW suggests iron deciency.
(Probably noticed more by haematology sta than those in general
medicine!)
Hypochromic red cells (%HRCs)
Dened as red cells with Hb <280g/ L. This is useful in identifying patients
with functional iron deciency and monitoring response to erythropoietin-
stimulating agent (ESA) Epo therapy and the requirement for IViron.
Reticulocyte mean cell haemoglobin (CHr), reticulocyte
haemoglobin content (Ret- He)
Predict iron deciency anaemia and functional iron deciency, and predict
response to IV iron in those on haemodialysis.
E Assessment of iron status, pp. 244–7.

232
CHAPTER3 Haematology
232
Whitecells
The automated dierential white cell count is provided as part of the FBC.
The RBCs in the sample are lysed before WBCs are counted. Atypical FBC
will show the total WBC and the
5- part dierential white cell count, bro-
ken down into the ve main white cell subtypes in peripheral blood which
include:
•
Neutrophils.
• Lymphocytes.
• Monocytes.
• Eosinophils.
• Basophils.
The printed FBC usually shows the % of each type of white cell, but unless
the absolute WBC (as x 10
9
/ L) is known, this % count is of littlevalue.
2 As a general rule, ignore the % count— you cannot detect abnormali-
ties, such as neutropenia, unless you have the absolute values.
Abnormalities of the WBC, e.g. neutrophilia, neutropenia, etc., are dis-
cussed in E OHCM 10e, p.330.

PLATELETCOUNT
233
Plateletcount
• Unit:x10
9
/ L.
• Normal:150– 400 x10
9
/ L.
Platelets (thrombocytes in the USA) are the smallest cells in peripheral blood.
Traditional counting methods with a microscope and counting chamber have
now been replaced by automated counting with haematology analysers.
Platelet distributionwidth
This is analogous to the RDW and provides information about the range of
platelet sizes in a blood sample.
•
The platelet distribution width (PDW) will be high if there are giant
platelets in the presence of normal- sized platelets, e.g. essential
thrombocythaemia (one of the myeloproliferative disorders).
•
The PDW will be normal in a reactive thrombocytosis (where the
platelet count is i, but they are all of normalsize).
Platelet clumping
This is an in vitro artefact in some individuals. Platelets clump in EDTA and
the blood analyser will report spurious thrombocytopenia. The actual
in vivo count is normal and the platelets function normally. Taking blood into
citrate or heparin will show the patient’s platelet count to be normal. The
presence of even a small blood clot in an EDTA sample may also reduce
the platelet count (the haematology technical sta will usually check to see
whether the sample contains a small clot before sending out the report).

234
CHAPTER3 Haematology
234
Peripheral bloodfilm
Examining a stained peripheral blood smear under the microscope allows
the examination of red cells, white cells, and platelets (see Fig. 3.1). In addi-
tion, the blood lm will help detect parasites (e.g. malaria, trypanosomes)
or abnormal cells in theblood.
When torequest a blood lm examination
The haematology laboratory will usually examine a peripheral blood lm if
the patient’s indices are abnormal (unless there has been no major change
from previous FBCs). If you suspect an underlying blood disorder, you
should request a lm. Note:the laboratory sta may not make a lm if the
indices are completely normal.
Method
A ngerprick blood sample may be spread onto a glass slide (the phleboto-
mist may do this for you), air- dried, xed, and stained. Alternatively, a drop
of EDTA blood may be treated in the same manner (the haematology labo-
ratory sta will make the lm). Beware:old EDTA samples produce strange
artefacts such as extreme red cell crenation— if a lm is required, it should
be made from a fresh blood sample.
•
Sample:EDTA (as fresh as possible).
Information frombloodlm
Redcells
•
Size.
• Shape (e.g. sickling).
• Membrane changes (e.g. oxidative membrane damage).
• Colour.
• Basophilic stippling.
• Inclusions, e.g. Howell– Jolly bodies, malarial parasites, haemoglobin C
(HbC) crystals,etc.
Neutrophi
l
Red cell
Platelets
Fig.3.1 Normal peripheral blood lm showing a neutrophil with its typical lobulated
nucleus, numerous red cells, and a few platelets.

PERIPHERAL BLOODFILM
235
Whitecells
•
Number.
• Morphology.
• Abnormalities such as toxic granulation, dysplastic changes.
• Presence of abnormal cells, e.g. leukaemic blasts or lymphomacells.
Platelets
•
Number.
• Size.
• Shape.
Other features onthelm
•
Parasites.
• Red cell rouleaux (stacking eect— seen, e.g. when ESRisi).
•
Nucleated redcells.
• Plasmacells.
• Occasionally see circulating carcinomacells.
E OHCM 10e, p.328.

236
CHAPTER3 Haematology
236
Red cell morphology
In health, the normal RBC is a pink, biconcave disc- shaped cell, and most
red cells are roughly the same size, shape, and colour. They should be
roughly the size of a small lymphocyte nucleus. Many diseases and de-
ciency disorders alter the RBC appearance by either reducing its Hb content
or altering the membrane such that characteristic morphological abnormali-
ties are produced. Examples include target cells, sickle cells, bite cells, burr
cells, and many others (see Table 3.2). Most of the morphological features
are not absolutely specic for one particular disorder, but rather they sug-
gest a range of conditions that may be associated with the RBC feature
(see Fig. 3.2). This should prompt you to look for conditions which might
account for the abnormality.
Table3.2 Peripheral blood lm inanaemias
Microcytic RBCs Fe deciency, thalassaemia trait and syndromes, congenital
sideroblastic anaemia, ACD (if long- standing)
Macrocytic RBCs Alcohol/ liver disease (round macrocytes), MDS,
pregnancy and newborn, compensated haemolysis, B
12
or folate deciency, hydroxyurea and antimetabolites
(oval macrocytes), acquired sideroblastic anaemia,
hypothyroidism, chronic respiratory failure, aplastic anaemia
Dimorphic RBCs Two populations, e.g. Fe deciency responding to
Fe, mixed Fe and B
12
/ folate deciencies, sideroblastic
anaemia, post- red cell transfusion
Hypochromic RBCs Reduced Hb synthesis, e.g. Fe deciency, thalassaemia,
sideroblastic anaemia
Polychromatic RBCs Blood loss or haematinic treatment, haemolysis, marrow
inltration
Spherocytes Hereditary spherocytosis, haemolysis, e.g. warm AIHA,
delayed transfusion reaction, ABO, HDN, DIC, and
MAHA, post- splenectomy
Pencil/ rod cells Fe deciency anaemia, thalassaemia trait and syndromes,
PK deciency
Elliptocytes Hereditary elliptocytosis, MPD, and MDS
Fragmented RBCs MAHA, DIC, renal failure, HUS, TTP
Teardrop RBCs Myelobrosis, metastatic marrow inltration, MDS
Sickle cells
Sickle- cell anaemia, other sickle syndromes (not sickle trait)
Target cells Liver disease, Fe deciency, thalassaemia, HbC syndromes
Crenated RBCs Usually storage or EDTA artefact. Genuine RBC
crenationmay be seen post- splenectomy and in renal
failure (lburrcells)

RED CELL MORPHOLOGY
237
2 Pay attention to the peripheral blood lm comment (inserted on the
report by the haematology laboratory sta or automated blood counter)—
it should help you decide which tests to carry out next. Conversely, cryptic
laboratory comments like ‘anisopoikilocytosis noted’ do not help the clini-
cian much. (Note:aniso=unequal; poikilo=varied.)
Burr cells Renal failure
Acanthocytes
Hereditary acanthocytosis, a- β- lipoproteinaemia, McLeod
red cell phenotype, PK deciency, chronic liver disease
(especially Zieve’s syndrome)
Bite cells G6PD deciency, oxidative haemolysis
Basophilic stippling Megaloblastic anaemia, lead poisoning, MDS, liver disease,
haemoglobinopathies, e.g. thalassaemia
Rouleaux Chronic inammation, paraproteinaemia, myeloma
i reticulocytes Bleeding, haemolysis, marrow inltration, severe hypoxia,
response to haematinic therapy
Heinz bodies Not seen in normals (removed by spleen), small numbers
seen post- splenectomy, oxidant drugs, G6PD deciency,
sulfonamides, unstable Hb (Hb Zurich, Köln)
Howell– Jolly bodies Composed of DNA, removed by the spleen, seen in
dyserythropoietic states, e.g. B
12
deciency, MDS, post-
splenectomy, hyposplenism
H bodies HbH inclusions, denatured HbH (β
4
tetramer), stain with
methylthioninium chloride (methylene blue), seen in HbH
disease (– – - / – α), less prominent in α- thalassaemia trait,
not present in normal subjects
Hyposplenic lm
Howell– Jolly bodies, target cells, occasional nucleated
RBCs, lymphocytosis, macrocytosis, acanthocytes.
Infectious mononucleosis, any viral infection,
toxoplasmosis, drug reactions
ABO, ABO blood groups; ACD, anaemia of chronic disease; AIHA, autoimmune haemolytic
anaemia; DIC, disseminated intravascular coagulation; Fe, iron; G6PD, glucose- 6- phosphate
dehydrogenase; HDN, haemolytic disease of the newborn; HUS, haemolytic uraemic syndrome;
MAHA, microangiopathic haemolytic anaemia; MPD, myeloproliferative disease; PK, pyruvate
kinase; TTP, thrombotic thrombocytopenic purpura.
Table 3.2 (Contd.)
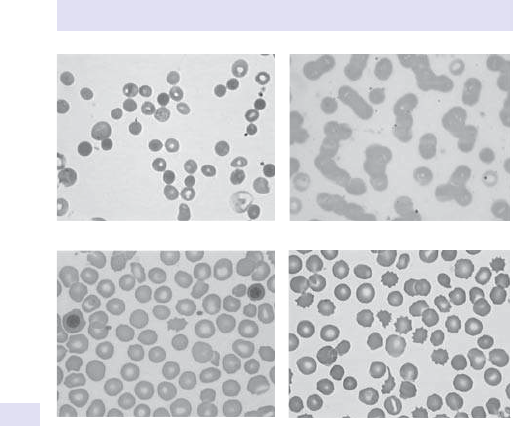
238
CHAPTER3 Haematology
238
Stippled red cells in haemolysis.
Marked rouleaux in myeloma.
Single nucleated red cell (on left). Crenated red cells.
Fig.3.2 Red cell abnormalities in various disease states.

RED CELL MORPHOLOGY
239

240
CHAPTER3 Haematology
240
Parasites onblood film andmarrow
Bloodlm
Although there are now highly sensitive monoclonal antibody (MoAb) kits
for the diagnosis of diseases such as malaria, a well- stained blood lm can
often make the diagnosis more easily and more cheaply. Blood lms are
useful for conrming a diagnosisof:
•
Malaria.
• Trypanosomiasis.
• Microlaria.
Parasites inbonemarrow
Some diseases, such as leishmaniasis, require bone marrow aspiration and
staining (in fact, there are many infections that can be diagnosed using bone
marrow):
•
Leishmania donovani.
•
TB.
• Tropheryma whippelii (Whipple’s disease).
•
Cryptococcus neoformans.
•
Penicillium.
•
Histoplasma capsulatum.
•
Candida albicans.
•
Toxoplasma gondii.
See Fig. 3.3 for examples.
Further reading
MedlinePlus (2016). CBC blood test. M http:// www.nlm.nih.gov/ medlineplus/ ency/ article/
003642.htm.
Thomas DW, Hinchlie RF, Briggs C, Macdougall IC, Littlewood T, Cavill I; British Committee for
Standards in Haematology. Guideline for the laboratory diagnosis of functional iron deciency.
Br J Haematol 2013; 161
:639– 48.
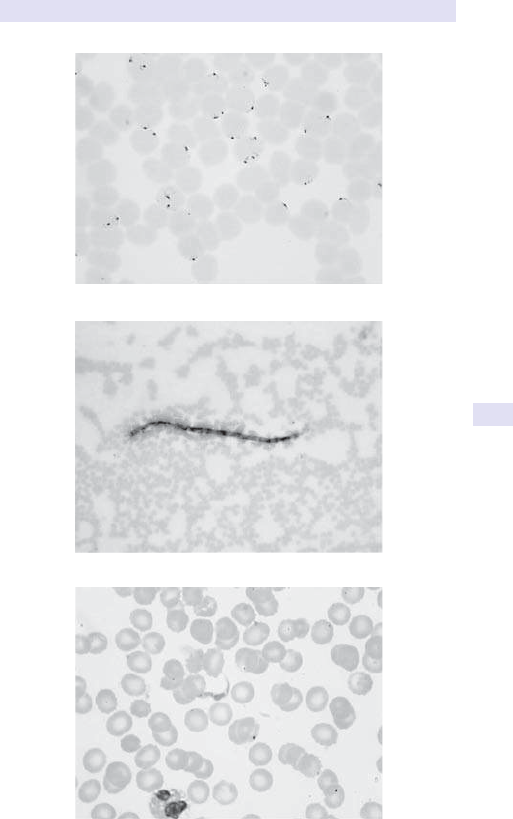
PARASITES ONBLOOD FILM ANDMARROW
241
Falciparum malaria (blood lm)
Loa loa (bone marrow)
Trypanosome (blood lm)
Fig.3.3 Parasites, such as malaria, loa loa, and trypanosomes, may be seen on a
stained bloodlm.

242
CHAPTER3 Haematology
242
White blood cell morphology
In much the same way as RBC morphology provides clues about underlying
disease, so too does microscopical examination of stained peripheral blood
WBCs. Modern counters enumerate WBCs, and our greater reliance on
modern technology means that visual inspection of blood lms is becoming
a dying art. Awell- stained blood lm may provide the diagnosis much more
cheaply (see Fig.3.4).
Blood lm whenWBC is decreased
• Sometimes dicult to determine the diagnosis since so fewWBCs.
• May suggest B
12
or folate deciency (are the RBCs normal or large?).
•
Aplastic anaemia:are the platelets and Hb normal?
•
Underlying leukaemia:are there any leukaemic blasts* present?
•
Overwhelming infection:may see toxic granulation (large, dark granules in
the cytoplasm— not diagnostic, but suggestive).
• May be immune or post- viral:atypical lymphocytes may be seen; other
indices usually normal.
Note:a blast (*) is a primitive cell seen in the marrow in large numbers in
leukaemia. We all have some blasts in our marrows, but these should be
<5% of the total nucleated bone marrow cells in health.
Blood lm whenWBC is increased
What cell predominates?
•
Lymphocytes:suggests viral, CLL, acute leukaemia (lymphoblastic).
• Granulocytic? (neutrophils, eosinophils, basophils)— may be reactive
orCML.
• Abnormal- looking WBC? Look for Auer rods (≡ AML), smear cells (CLL),
bilobed neutrophils (pseudo- Pelger cells seen inMDS).
(See Table3.3.)
Fig.3.4 Blood lm:atypical WBCs (this was from a patient with glandular fever, but
these cells may be seen in any viral illness).

WHITE BLOOD CELL MORPHOLOGY
243
Diagnosis must be made incontext
How old is thepatient?
•
Viral illnesses often produce bizarre lms in children, but beware of
complacency (acute leukaemia may be the cause).
•
MDS and malignancies like CLL and CML are diseases of older
individuals.
Is thepatientwell?
•
May be worth repeating the FBC and lm to see if abnormalities have
resolved.
•
If the patient is unwell or has lymphadenopathy or hepatosplenomegaly,
then an underlying disease must be excluded.
Table3.3 Some WBC abnormalities seen onFBC reports
Atypical
lymphocytes
Infectious mononucleosis, any viral infection, toxoplasmosis,
drug reactions
Auer rods
Seen in myeloblasts; pathognomonic of AML Prominent in AML
M3 subtype (acute promyelocytic leukaemia)
Pelger– Huët
anomaly
Bilobed neutrophils. May be hereditary (neutrophils are
functionally normal) or acquired, e.g. MDS (pseudo- Pelger cells)
Left- shifted Immature WBCs seen in peripheral blood. Seen in severe
infections, inammatory disorders, DKA, marrow ‘stress’,
MPD,CML
Right- shifted Hypermature WBCs seen in, e.g. megaloblastic anaemia and
iron deciency
Toxic
granulation
Coarse granules in neutrophils. Seen in severe infection, post-
operatively, and inammatory disorders
Smear cells
Lymphocytes in which the cell membrane has ruptured when
making the blood lm— there are no smear cells in vivo! Seen
in CLL

244
CHAPTER3 Haematology
244
Assessment ofironstatus
Anaemia of iron deciency is caused by defective synthesis of Hb, resulting
in red cells that are smaller than normal (microcytic) and contain reduced
amounts of Hb (hypochromic). The diagnosis of iron deciency anaemia is
generally straightforward, but it may be confused with anaemia of chronic
disease (ACD) or other hypochromic anaemias (see Table 3.4 and Fig.3.5).
Iron plays a pivotal role in many metabolic processes, and the average
adult contains between 3 and 5g of iron, of which two- thirds are present
in the O
2
- carrying molecule Hb. Somewhat surprisingly, there is no specic
excretion mechanism in humans. Iron balance is controlled at the level of
gut absorption and relies on two iron- sequestering proteins transferrin (iron
transport and recycling of iron) and ferritin (safeguards iron entry into the
body and maintains surplus iron in a safe and readily accessibleform).
Ferritin
This is the ° iron storage protein, consisting of 24 apoferritin subunits
forming a hollow sphere (each can hold up to 4500 iron atoms).
Haemosiderin
Haemosiderin, located predominantly in macrophages, is a water- soluble
protein– iron complex with an amorphous structure.
Transferrin and its receptor
Transferrin contains only 4mg of iron and is the principal iron transport
protein with >30mg of iron transported round the body daily. Synthesis of
transferrin is inversely proportional to the body iron stores, with i transfer-
rin concentration when iron stores are reduced.
The transferrin receptor (TfR) is a disulde- linked dimer, composed
of two identical 85kDa subunits. The serum TfR (sTfR) concentration is
elevated in iron deciency. However, sTfR may also i in any condition in
which there is i erythropoiesis, e.g. haemolytic anaemias, thalassaemia,
polycythaemia vera, and other myeloproliferative disorders.
Assessment ofironstatus
Several parameters are available
•
Hb concentration.
• Serum ferritin.
• Serum iron and transferrin (asTIBC).
• % hypochromic cells in peripheralblood.
• Reticulocyte MCH (CHr) and reticulocyte Hb content (Ret- He).
• Red cell protoporphyrin assay (not widely available).
• Bone marrow aspirate (stained for iron)— the ‘gold standard’.
• Soluble TfR assay (STfR).
Remember, iron deciency is not an ‘all- or- nothing’ phenomenon. In pro-
gressive deciency, there is a gradual loss of iron with subtle alterations of
iron- related parameters, during which the red cells may look entirely nor-
mal. In the initial stages of developing iron deciency, macrophages become
depleted of iron and the serum ferritin d to the lower end of the normal

ASSESSMENT OFIRONSTATUS
245
range; during this ‘latency’ period, the Hb is normal. As the deciency pro-
gresses, plasma iron levels d and TIBC i. Free RBC protoporphyrin levels i
as it accumulates, and eventually hypochromic RBCs appear in the periph-
eral blood. At this stage, an FBC will usually show d Hb, MCV, MCH, and
MCHC, and the peripheral blood lm will show microcytic hypochromic
redcells.
Table3.4 Hypochromic anaemias— may be confused withiron
deciency
Disorder Example
Disorders of iron metabolism Iron deciency anaemia:
•
Bloodloss
• Reduced ironintake
• Impaired iron transport
Anaemia of chronic disorders Chronic inammatory diseases
Malignant disease
Disorders of haem synthesis Sideroblastic anaemias:
•
Hereditary
• Idiopathic
• 2°:
•
Drugs
•
Alcohol
•
Lead poisoning
Globin synthesis disorders Thalassaemias:
•
β- thalassaemia
• α- thalassaemia
Target cells
Pencil cell
Fig.3.5 Blood lm of iron deciency anaemia. Note the variation in cell size andshape.
246
CHAPTER3 Haematology
246
Conrmation ofsimple iron deciency anaemia
(See Fig.3.6.)
•
Hbd.
•
Serum ferritin willbed.
•
Serum iron d and TIBC i (generally unhelpful and little used today).
•
STfRi.
•
MCVd.
•
MCH and MCHCd.
•
Microcytic and hypochromic RBCs on bloodlm.
• Absent marrowiron.
Functional iron deciency
This is the situation where iron is retained in body stores and, although
stores are adequate, delivery to the bone marrow is inadequate for eryth-
ropoiesis. It occurs in inammatory disease, being one component of ACD.
Functional iron deciency also occurs during the use of Epo where response
is improved with the use of IViron.
% Blasts:
% Hyper:
% Hypo:
% Macro:
% Micro:
RBC fragments:
1.2
0.0
86.8
0.0
62.9
0.09
RBC volume
Additional routine parameters
RBC V/HC
RBC HC
Fig.3.6 The % hypochromic red cells (provided by some automated counters) helps
in the diagnosis of iron deciency. Note that the RBC volume and Hb content (HC)
are both shifted to the LEFT (= small, pale red cells).
2 Beware:serum ferritin is an acute phase protein and may be normal or
even i in inammatory, malignant, or liver disease. During the inamma-
tory response, the iron/ TIBC are unlikely to be of any value (iron d and
TIBC will be d). If an inammatory process is suspected, an alternative
test is required, e.g. STfR, which is not aected by inammatory disorders.

ASSESSMENT OFIRONSTATUS
247
Iron overload
Can be iatrogenic or genetic (haemochromatosis).
Ferritin and transferrin saturation are elevated. Investigations to assess
the degree of cardiac and hepatic iron accumulation are required.
E OHCM 10e, p.326.
Further reading
Iron Disorders Institute. Iron deciency anemia. M http:// www.irondisorders.org/ iron- deciency-
anemia.
Iron Panel. M
http:// www.ironpanel.org.au/ AIS/ AISdocs/ adultdocs/ Acontents.html.
Provan D, Weatherall D. Acquired anaemias and polycythaemia. Lancet 2000; 355
:1260– 8.

248
CHAPTER3 Haematology
248
Assessment ofvitamin B
12
and folatestatus
Measurement of serum B
12
and red cell folate levels is necessary in the
investigation of macrocytic anaemia and certain other situations (see
below). Serum folate levels are an unreliable measurement of body stores
of folate— the red cell folate level is probably more meaningful.
• B
12
unit:ng/ L.
• Serum and red cell folate units:µg/ L.
• Sample:clotted blood sample (serum B
12
and folate) and peripheral
blood EDTA (red cell folate).
Deciency of either vitamin leads to megaloblastic anaemia where there
is disruption of cell division in all actively dividing cells (includes the bone
marrow and gut). In the marrow, there is nuclear:cytoplasmic asynchrony
where the nuclei are immature despite a mature, well- haemoglobinized
cytoplasm. In the peripheral blood, there may be anaemia, often with
pancytopenia; the red cells show oval macrocytic changes with basophilic
stippling and occasionally nucleated red cells. Neutrophils typically become
hypersegmented (they have >5 lobes).
Until recently, B
12
and folate assays were tedious microbiological assays,
but these have now been replaced by automated techniques using radio-
isotopic methods, which allow large numbers of samples to be batched and
tested fairly cheaply.
Deciencies ofB
12
or folate do not always cause macrocytic
anaemia
In the past, deciency of either B
12
or folate was synonymous with
macrocytic anaemia, but deciency of either vitamin may present with-
out anaemia or macrocytosis— remember, these are late features of the
disease. However, in most cases of deciency, the marrow will show
characteristic megaloblastic change (nuclear asynchrony with giant meta-
myelocytes). 2 Deciency of B
12
may cause neurological problems in the
absence of anaemia.
Whom should youtest?
• Patients with gastrointestinal tract (GIT) disease, glossitis, abnormalities
of taste, previous surgery, or radiotherapy to the stomach or
smallbowel.
•
Neurological disease, e.g. peripheral neuropathy, demyelination.
• Psychiatric disturbance, e.g. confusion, dementia.
• Malnutrition, e.g. growth impairment in children; vegans.
• Alcoholabuse.
• Autoimmune disease of the thyroid, parathyroid, or adrenals.
• Patients with a family history of pernicious anaemia.
• Others, e.g. drugs that interfere with vitamin absorption or metabolism
such as nitrous oxide, phenytoin,etc.

249
ASSESSMENT OFVITAMIN B
12
AND FOLATESTATUS
Look forblood lm abnormalities
2 B
12
and folate deciencies produce similar clinical and laboratory features:
•
Oval macrocytes.
• Hypersegmented neutrophils (also seen in renal failure, iron deciency,
andMDS).
(See Fig.3.7.)
Which testnext?
Make sure you have thefollowing
•
FBC.
• Bloodlm.
• Serum B
12
level.
•
Serum and red cell folate levels.
• Intrinsic factor antibodies (IFA), +ve in 50– 75% of patients withPA.
• Consider the bone marrow (helps exclude MDS, myeloma, and other
pathologies that give rise to macrocytic anaemia, but seldom performed
today since it is easy to get B
12
and folate results back quickly).
Interpretation ofresults:vitaminB
12
Normal ranges are based on two standard deviations either side of the
mean, so there will be ‘normal’ people who have ‘abnormal’ B
12
(or folate)
levels.
•
B
12
< normal:
•
Deciency.
•
Altered metabolism.
•
‘Normal’.
The lowest levels are seen in those most decient. What matters is whether
there is tissue deciency (leads to marrow and neurological changes).
Fig.3.7 Blood lm of megaloblastic anaemia. There are large oval macrocytes and
two hypersegmented neutrophils (the nucleus has >5 lobes).

250
CHAPTER3 Haematology
250
Mild decrease inB
12
level?
Dicult, but common! Probably worth repeating the test and reviewing the
patient and other results. If no evidence of tissue deciency, can probably
observe the patient. If there is evidence of tissue deciency, then the patient
will require treatment.
Detecting tissue deciency
The most reliable method is probably the measurement of serum homo-
cysteine (accumulates in vitamin B
12
and folate deciency).
Beware B
12
not associated withtissue deciency
•
Folate deciency.
• Pregnancy.
• Myeloma.
• Transcobalamin Ideciency (veryrare).
Folate
d level seen in hospitalized patients due to −ve folate balance.
The B
12
level is low— whatnext?
Available tests forthe cause ofB
12
deciency include
•
Parietal cell (+ve in serum of 90% of patients with PA, but also found
in other disorders and 15% of the normal elderly) and intrinsic factor
antibodies (IFA now preferred— if +ve, conrms diagnosis ofPA).
• Schilling test (urinary excretion method where the addition of
intrinsic factor (IF) restores B
12
absorption in PA, but not in intestinal,
e.g. ileal, disease), seldom performed now due to lack of required
radioisotope,or
•
Whole body B
12
counting.
•
Endoscopy with duodenal biopsy.
• Other gastroenterology tests for malabsorption (E Gastroenterology,
p. 522).
The folate level is low— whatnext?
• Check dietary history.
• Endoscopy with duodenal biopsy.
• Other gastroenterology tests for malabsorption (E Gastroenterology,
p. 522).
E OHCM 10e, p.266.
Further reading
Devalia V, Hamilton MS, Molloy AM; British Committee for Standards in Haematology. Guidelines for
the diagnosis and treatment of cobalamin and folate disorders. Br J Haematol 2014; 166
:496– 513.
Guidelines and Protocols Advisory Committee (2012). Cobalamin (vitamin B12) deciency:investiga-
tion and management. M
http:// www2.gov.bc.ca/ gov/ content/ health/ practitioner- professional-
resources/ bc- guidelines/ vitamin- b12.
Hobrand AV. Megaloblastic anaemia and miscellaneous deciency anaemias. In:DA Warrell, TM
Cox, JD Firth, eds. Oxford Textbook of Medicine, 5th edn. Oxford:Oxford University Press, 2010;
pp.4402– 19.
Provan D, Weatherall D. Acquired anaemias and polycythaemia. Lancet 2000; 355:1260– 8.

251
ASSESSMENT OFVITAMIN B
12
AND FOLATESTATUS

252
CHAPTER3 Haematology
252
Erythrocyte sedimentationrate
This simple, but very useful, qualitative test measures how fast a patient’s
red cells fall through a column of blood. It is a sensitive, but non- specic,
index of plasma protein changes that result from inammation or tissue
damage. The ESR is aected by Hct variations, red cell abnormalities (e.g.
poikilocytosis, sickle cells), and delay in analysis, and it is therefore less reli-
able than measurement of plasma viscosity (PV). The ESR is aected by
age, sex, menstrual cycle, pregnancy, and drugs (e.g. OCP, steroids) (see
Table3.5).
The ESR is widely used in clinical medicine and, despite attempts (by hae-
matology departments) to replace the ESR with PV, the ESR has remained
in use and appears to retain a valuable place in the armoury of disease
diagnosis and monitoring.
•
Sample:peripheral blood EDTA; the sample should be analysed in the
laboratory within4h.
Many factors inuence the ESR, causing a high or low result:
•
High ESR (signicant*— look for a cause):
•
Any inammatory disorder, e.g. infection, rheumatoid,TB.
•
MI (the ESR i as an early response).
•
Anaemia.
Note:(*) depends on exactly how high. An ESR of 30 probably means little,
but >100 is highly signicant and indicates something seriouslywrong.
•
Low ESR (rarely important, but useful for exams):
•
Polycythaemia.
•
Hypobrinogenaemia.
•
CCF.
•
Poikilocytosis.
•
Spherocytosis.
•
Sickledcells.
3 Anormal ESR does not exclude organic disease.
E OHCM 10e, p.372.
Table3.5 Normal range (upper limits)
Age Men Women
17– 50years 10mm/ h 12mm/ h
51– 60 12 19
>60 14 20
Further reading
Brigden M. Clinical utility of the erythrocyte sedimentation rate. Am Fam Physician 1999; 60:1443– 50.
M http:// www.aafp.org/ afp/ 991001ap/ 1443.html.
Harris GJ. Plasma viscometry and ESR in the elderly. Med Lab Technol 1972; 29
:405– 10.
Lewis SM. Erythrocyte sedimentation rate and plasma viscosity. Ass Clin Pathol Broadsheet 1982;
94
:1– 6.

PLASMA VISCOSITY
253
Plasma viscosity
This test is a sensitive, but non- specic, index of plasma protein changes,
which result from inammation or tissue damage. Provides much the same
information as the ESR. The ESR and PV tend to rise in parallel, but the PV
is unaected by Hct variations (e.g. severe anaemia or polycythaemia) and
delay in analysis up to 24h, and it is therefore more reliable than the ESR.
It is not aected by sex but is aected by age, exercise, and pregnancy. It is
constant in health and shows no diurnal variation. There is a suggestion that
the PV may be a more sensitive indicator of disease severity than theESR.
•
Sample:peripheral blood EDTA. The sample is centrifuged and the
plasma removed.
•
Normal range:1.50– 1.72cP (or mPA/ s at25°C).
High and low plasma viscosity
A high PV generally signies some underlying pathology, e.g. inamma-
tory states, paraproteinaemias such as MGUS or myeloma; low PV can be
ignored.
Note:despite the advantages outlined, the PV has not been adopted by all
medical sta (who still prefer the ESR as a measure of inammation). The
PV is better for monitoring hyperviscosity syndromes, e.g. Waldenström’s
macroglobulinaemia. The fact that both tests are still used shows that there
is a role forboth.
E OHCM 10e, p.373.
Further reading
Cooke BM, Stuart J. Automated measurement of plasma viscosity by capillary viscometer. J Clin Pathol
1988; 41
:1213– 16.

254
CHAPTER3 Haematology
254
Tests forglandularfever
This infection is caused by EBV. Infected cells produce so- called hetero-
phile antibodies (these are IgM molecules that agglutinate horse and sheep
RBCs but do not agglutinate ox RBCs and do not react at all with guinea
pigRBCs).
There are various kits available that can detect the presence of hetero-
phile antibodies and, in the right clinical context, will conrm a diagnosis of
EBV infection. The Monospot test is probably the commonest in current
use. The Paul– Bunnell test was the rst to demonstrate the presence of
heterophile antibodies in patients with EBV infection.
Clinical features
Glandular fever often aects young adults (12– 25 years) and results in
malaise, fever, tonsillitis, petechial haemorrhages on the palate, and lym-
phadenopathy. Splenomegaly is fairly common. Asimilar clinical picture is
seen in CMV, Toxoplasma, and early HIV infections.
•
Sample:EDTA.
Positive Monospot
• EBV infection.
False positives
• Toxoplasmosis.
• CMV infection.
• Rheumatoid.
• Malaria.
Further reading
Ho G, Bauer S. A new rapid slide test for infectious mononucleosis. JAMA 1996; 194:351– 3.
INVESTIGATION OFHAEMOLYTIC ANAEMIA
255
Investigation ofhaemolytic anaemia
The normal red cell has a lifespan of 7120days. Anaemia resulting from d
RBC lifespan is termed haemolytic. May be inherited or acquired, and the
basic underlying mechanisms may involve abnormalities of the RBC mem-
brane, RBC enzymes,orHb.
Extravascular vs intravascular
Extravascular haemolysis implies RBC breakdown by the reticuloendothelial
system (RES) (e.g. liver, spleen, and macrophages at other sites), whilst intra-
vascular haemolysis describes RBC breakdown in the circulation itself (see
Fig. 3.8). There are many investigations available that will help determine the
predominant site of destruction, which, in turn, will help dene the underly-
ing cause of haemolysis, which is why we do the tests in the rstplace.
Detection ofhaemolysisitself
The main question is whether the patient’s anaemia is due to haemolysis or
some other underlying mechanism such as blood loss, marrow inltration,etc.
E OHCM 10e, p.336.
EXTRAVASCULAR
(e.g. spleen)
INTRAVASCULAR
HAEMOLYSIS
Kidney
Methaemalbumin
Fe recycled
Globin
Free plasma Hb
Hb–Hp complex
Hb
MetHb
Haemosideri
n
Amino acids
Haem
Bilirubin
(unconjugated)
Liver
Conjugated bilirubin
Gut
Urobilinogen
Faeces
Urine
Fig.3.8 Increased red cell breakdown may be extravascular (outside the circulation,
predominantly the spleen, liver, and marrow) or intravascular (within the vessels).
Modied from Lewis SM, Bain BJ & Bates I, eds. (2001) Dacie & Lewis Practical Haematology,
11th edition, Churchill Livingstone, Edinburgh.

256
CHAPTER3 Haematology
256
General tests ofhaemolysis
Is haemolysis actually occurring? Suggestive features
• Evidence of i red cell destruction.
•
Evidence of i red cell production (to compensate for red cellloss).
•
Evidence of autoantibody in the patient’sserum.
Evidence ofred blood cell destruction
• i serum bilirubin (split conjugated/ unconjugated is useful).
• i serum LDH (reecting i RBC turnover).
•
Spherocytes or other abnormal RBCs, e.g. fragments on bloodlm.
• Plasma haptoglobins may be d or absent.
•
i faecal and urinary urobilinogen (faecal not measured).
•
d RBC lifespan (seldom measured nowadays).
Evidence ofincreased red blood cell production
• i reticulocytes (on lm, manual, or automated count). Not absolutely
specic, will i in brisk acute bleed, e.g.GIT.
•
i MCV (reticulocytes are larger than mature RBCs, and do not forget
folate deciency, which occurs in haemolytic disorders).
Is it mainly intravascular?
• i plasmaHb.
•
Methaemalbuminaemia.
• Haemoglobinuria.
• Haemosiderinuria.
What is thecause?
Genetic
•
RBC morphology (e.g. spherocytes, elliptocytes).
• Hb analysis.
• RBC enzyme assays.
Acquired
•
Immune— checkDAT.
• Non- immune:check RBC morphology (e.g. TTP/ HUS).
• Is there some other underlying disease?
• Consider PNH (rare).
Further reading
Hill QA, Stamps R, Massey E, Grainger JD, Provan D, Hill A; British Society for Haematology
Guidelines. Guidelines on the management of drug- induced immune and secondary autoimmune,
haemolytic anaemia. Br J Haematol 2017; 177:208– 20.
Hill QA, Stamps R, Massey E, Grainger JD, Provan D, Hill A; British Society for Haematology. The
diagnosis and management of primary autoimmune haemolytic anaemia. Br J Haematol 2017;
176
:395– 411.
Schick P (2016). Hemolytic anemia. M http:// www.emedicine.com/ med/ topic979.htm.

GENERAL TESTS OFHAEMOLYSIS
257

258
CHAPTER3 Haematology
258
Reticulocytes
These are immature RBCs formed in the marrow and found in small num-
bers in normal peripheral blood. They represent an intermediate matura-
tion stage in the marrow between the nucleated RBC and the mature RBC
(the reticulocyte lacks a nucleus but retains some nucleic acid). Measuring
the number of reticulocytes in the blood may help determine whether the
anaemia is due to d RBC production. The reticulocyte count is also a use-
ful measure of response to haematinic (iron, B
12
, or folate) replacement
therapy.
Detection and measurement
• Modern automated blood counters using laser technology measure the
number of reticulocytes directly.
•
Demonstrated by staining with supravital dye for the nucleicacid.
• Appear on blood lm as larger than mature RBCs with ne, lacy blue
staining strands or dots (see Fig.3.9).
•
Usually expressed as a percentage of total red cells, e.g. 5%, though
absolute numbers can be derived from this and the total red cellcount.
•
Sample:EDTA.
•
Normal range:0.5– 2.5% (50– 100 x 10
9
/ L).
Causes ofincreased reticulocytecounts
Marrow stimulationdueto
•
Bleeding.
• Haemolysis.
• Response to oral iron therapy.
• Infection.
• Inammation.
• Polycythaemia (any cause).
• Myeloproliferative disorders.
• Marrow recovery following chemotherapy or radiotherapy.
• Epo administration.
Spherocyte
s
Fig.3.9 Blood lm of numerous spherocytes (small, darker red cells) and
reticulocytes (larger red cells) in autoimmune haemolytic anaemia.

RETICULOCYTES
259
Causes ofdecreased reticulocytecounts
Marrow inltrationdueto
•
Leukaemia.
• Myeloma.
• Lymphoma.
• Other malignancy.
Marrow underactivity (hypoplasia)dueto
•
Iron, folate, or B
12
deciency. Note:the return of reticulocytes is the
earliest sign of response to replacement therapy.
•
Immediately post- chemotherapy or radiotherapy.
• Autoimmune disease, especially refractory anaemia(RA).
• Malnutrition.
• Uraemia.
• Drugs.
• Aplastic anaemia.
• Red cell aplasia.
Further reading
Howells MR, Jones SE, Napier JA, Saunders K, Cavill I. Erythropoiesis in pregnancy. Br J Haematol
1986; 64
:595– 9.

260
CHAPTER3 Haematology
260
Serum haptoglobins
Haptoglobins (Hps) are plasma proteins synthesized by the liver, whose
function is the removal of free plasma Hb. Hp molecules bind free Hb and
are taken up by the RES for degradation. Hp– Hb complexes do not appear
in the urine because their large size prevents them from passing through
the renal tubules.
The Hp– Hb complex is cleared by the RES at a rate of 15mg/ 100mL/ h,
which means that even very mild haemolysis will cause the disappearance
of Hps from the circulation. Serum Hps should be measured in patients with
suspected intravascular haemolysis. However, the Hp level is frequently
reduced in patients with extravascular haemolysis, and the Hp level cannot
be used to determine whether the basic haemolytic process is intra- or
extravascular. It should generally be accompanied by estimation of serum
methaemalbumin, free plasma Hb, and urinary haemosiderin.
•
Sample:clottedblood.
•
Normal range (expressed as Hb- binding capacity):30– 250mg/ dL.
Conditions withdecreased haptoglobins
Haemolysis including
•
Incompatible blood transfusion.
• AIHA.
• Sickle- cell disease.
• Thalassaemiamajor.
• PNH.
Others
•
1% of the population have a genetic lack ofHps.
• Lower levels in infancy.
Note:it takes about 1 week after haemolysis has stopped for Hp levels to
return to normal.
Conditions withincreased haptoglobins (acute phase
protein, likeferritin)
• Any disorder withiESR.
•
Carcinoma, especially if bony2°.
• Any inammatory disorder.
• Trauma.
• Surgery.
• Steroid therapy.
• Androgen therapy.
• DM.
Further reading
Rougemont A, Dumbo O, Bouvier M, etal. Hypohaptoglobinaemia as an epidemiological and clini-
cal indicator for malaria. Results of two studies in a hyperendemic region in West Africa. Lancet
1988; 2
:709– 12.

SERUM BILIRUBIN
261
Serum bilirubin
Two forms are found: pre- hepatic bilirubin (unconjugated) and bilirubin
conjugated to glucuronic acid (conjugated). Generally, serum bilirubin levels
are 17– 50µmol/ L in haemolysis (mainly unconjugated).
2 Beware: serum bilirubin levels may be normal, even if haemolysis is
present; a level of >85µmol/ L suggests liver disease.
Serum bilirubin levels may be modestly i (e.g. 20– 30µmol/ L) in dys-
erythropoietic disorders, such as vitamin B
12
or folate deciency, or myelo-
dysplasia, due to ineective erythropoiesis where RBCs are destroyed in
the marrow before ever being released into the circulation.

262
CHAPTER3 Haematology
262
Urobilin, urobilinogen, and urinary
haemosiderin
Urobilinogen is the reduced form of urobilin, formed by bacterial action on
bile pigments in the GIT. Faecal and urinary urobilinogen i in haemolytic
anaemias.
Urinary haemosiderin
Usage
The most widely used and reliable test for detection of chronic intravascu-
lar haemolysis. Results from the presence of Hb in the glomerular ltrate.
Principle
Free Hb is released into the plasma during intravascular haemolysis. The
Hb- binding proteins become saturated, resulting in the passage of haem-
containing compounds into the urinary tract of which haemosiderin is the
most readily detectable.
Method
1. Aclean- catch sample of urine is obtained from the patient.
2. The sample is spun down in a cytocentrifuge to obtain a cytospin
preparation of urothelialcells.
3. Staining and rinsing with Perl’s reagent (Prussian blue) are performed
on the glass slides.
4. Examine under the oil- immersion lens of a microscope.
5. Haemosiderin stains as blue dots within urothelialcells.
6. Ignore all excess stain and staining outside cells or in debris, all of which
are common.
7. True +ve only when clear detection within urothelial squames isseen.
Caution
An iron- staining +ve control sample should be run alongside the test case
to ensure the stain has worked satisfactorily. Haemosiderinuria may not be
detected for up to 72h after the initial onset of intravascular haemolysis, so
the test may miss haemolysis of very recent onset— repeat the test in 3–
7days if −ve. Conversely, haemosiderinuria may persist for some time after
the haemolytic process has stopped. Arepeat in 7days should conrm.
Causes ofhaemosiderinuria
(See Table3.6.)
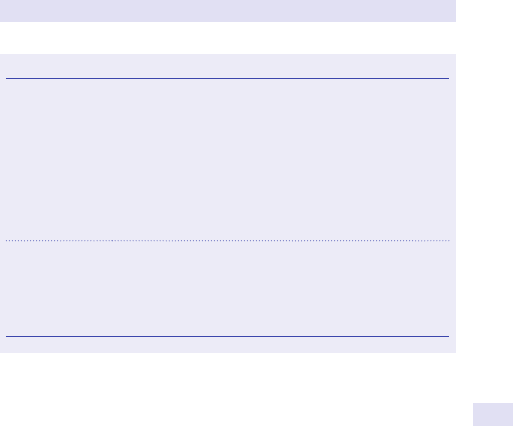
UROBILIN, UROBILINOGEN, AND URINARY HAEMOSIDERIN
263
Table3.6 Causes ofhaemosiderinuria
Common causes Red cell enzymopathies, e.g. G6PD and pyruvate kinase
deciencies, but only during haemolytic episodes
Mycoplasma
pneumonia with anti- I cold haemagglutinin
Sepsis
Malaria
Cold haemagglutinin disease
TTP/ HUS
Severe extravascular haemolysis (may cause intravascular
haemolysis)
Rarer causes PNH
Prosthetic heartvalves
Red cell incompatible transfusion reactions
UnstableHb
March haemoglobinuria

264
CHAPTER3 Haematology
264
Plasma haemoglobin
In health, Hb is contained within RBCs, but during intravascular haemolysis,
excessive quantities of Hb may be released from ruptured RBCs. Normally
Hps mop up free Hb. If there are insucient Hps to cope with the free Hb,
the kidneys clear the Hb, leading to haemoglobinuria. Some Hb may be bro-
ken down in the circulation to haem and globin; haem can bind to albumin,
producing methaemalbumin (l methaemalbuminaemia).
2 The nding of free Hb in plasma is highly suggestive of intravascular
haemolysis.
•
Sample:sodium citrate (but discuss with the haematology laboratory
before sending sample).
•
Normal range:10– 40mg/ L (up to 6mg/ L).
• Pitfalls:any RBC damage occurring during blood sampling may result
in an erroneously high reading. Great care must be taken during
venepuncture.
Causes ofincreased plasma haemoglobin
(See Table3.7.)
Further reading
Crosby WH, Dameshek W. The signicance of hemoglobinemia and associated hemosiderinuria,
with particular reference to various types of hemolytic anemia. J Clin Lab Med 1951; 38
:829– 41.
Table3.7 Causes ofincreased plasma haemoglobin
Mild i (50– 100mg/ L) Moderate i (100– 250mg/ L) Severe i (>250mg/ L)
Sickle/ thalassaemia AIHA Incompatible blood
transfusion
Haemoglobin C
disease
Sickle- cell disease
Thalassaemiamajor
HaemoglobinSC
Prosthetic heartvalve
March haemoglobinuria
PNH
Paroxysmal cold
haemoglobinuria
Blackwater fever

SCHUMM’STEST
265
Schumm’stest
• Use:detection of methaemalbumin (seen after all Hps used up in
a haemolytic process; usually implies haemolysis is predominantly
intravascular).
This spectrophotometric test for methaemalbumin (which has a distinc-
tive absorption band at 558nm) should be requested in patients with
suspected intravascular haemolysis and may be abnormal in patients with
signicant extravascular (generally splenic) haemolysis. It should be accom-
panied by an estimation of the serum Hp level, free plasma Hb, and urinary
haemosiderin.
•
Sample:heparinized or clottedblood.
Positive resultin
• Intravascular haemolysis.
• Mismatched blood transfusion.
• RBC fragmentation syndromes.
• G6PD deciency with oxidative haemolysis.
• PNH.
• March haemoglobinuria.
• UnstableHb.
Further reading
Hobrand AV, Lewis SM, Tuddenham EGD (eds.). Postgraduate Haematology, 4th edn. Oxford:
Butterworth- Heinemann,2000.
Winstone NE. Methemalbumin in acute pancreatitis. Br J Surg 1965; 52:804– 8.

266
CHAPTER3 Haematology
266
Hereditary haemolytic anaemias
There are many inherited causes for haemolytic anaemia, which fall into
three major groups, shown in Table3.8.
Table3.8 Inherited causes forhaemolytic anaemiaa
Mechanism Example
Red cell membrane disorders Hereditary spherocytosis
Hereditary elliptocytosis
Red cell enzyme disorders G6PD deciency
Pyruvate kinase deciency
Hb disorders
Sickle- cell anaemia
Thalassaemia

HEREDITARY HAEMOLYTIC ANAEMIAS
267

268
CHAPTER3 Haematology
268
Red cell membrane disorders
Hereditary spherocytosis
This is the best known inherited membrane abnormality leading to a
reduced red cell lifespan and sometimes severe anaemia. Inheritance is usu-
ally autosomal dominant and there is often a +ve family history.
Hereditary elliptocytosis
Usually autosomal dominant. Rarely causes symptomatic anaemia.
Hereditary pyropoikilocytosis
Autosomal recessive, often compound heterozygotes. Often severe
haemolysis.
South East Asian ovalocytosis
Heterozygotes asymptomatic.
Hereditary stomatocytosis
Heterogeneous group with often severe haemolysis.
EMA bindingtest
Immunophenotyping using the reagent EMA to bind to red cell trans-
membrane proteins. Altered uorescence is seen with red cell membrane
disorders.
SDS- PAGE (sodium dodecyl sulfate polyacrylamide
gel electrophoresis)
Gel electrophoresis of membrane proteins. Only available in reference
laboratories.
Osmotic fragilitytest
Principle ofthetest
The test measures the ability of red cells to take up water before rupturing
(lysing). This is determined by the volume:surface area ratio. Normal red
cells can i
their volume by up to 70% before lysing (because they are disc-
shaped and have the capacity to take in extra water easily). Spherocytic red
cells have an i volume:surface area ratio and are able to take up less water
than normal red cells before lysing (they are spheres and, as such, they are
‘full’ already).
•
Sample:EDTA (need a normal control sample sent at the sametime).
(See Fig.3.10.)
Method
•
RBCs are incubated in saline at various concentrations. This results in
cell expansion and eventually rupture.
•
Normal RBCs can withstand greater volume i than spherocyticRBCs.
RED CELL MEMBRANE DISORDERS
269
• A+ve result (conrming hereditary spherocytosis) seen when RBCs
lyse in saline at near to isotonic concentration, i.e. 0.6– 0.8g/ dL (normal
RBCs will simply show swelling with little lysis).
•
Osmotic fragility is more marked in patients who have not undergone
splenectomy and if the RBCs are incubated at 37°C for 24h before
performing thetest.
Other supportivetests
• There will be a +ve family history of hereditary spherocytosis in
manycases.
•
The blood lm shows ii spherocyticRBCs.
•
Anaemia, i reticulocytes, i LDH, unconjugated bilirubin, urinary
urobilinogen withdHps.
•
DAT−ve.
2 Beware:this test is not diagnostic of hereditary spherocytosis but will be
+ve in any condition in which there are i numbers of spherocytic red cells.
Use this test in conjunction with a history, blood lm, and family studies
(hereditary spherocytosis is inherited as autosomal dominant, so one of the
parents and some siblings should be aected).
Most testing laboratories use a combination of the above tests. No one
screening test can detect all cases of hereditary spherocytosis.
Further reading
Bolton- Maggs PHB, Langer JC, Iolascon A, Tittensor P, King MJ. Guidelines for the diagnosis and
management of hereditary spherocytosis – 2011 update. Br J Haematol 2012; 156:37– 49.
Parpart AK, Lorenz PB, etal. The osmotic resistance (fragility) of human red cells. J Clin Invest 1947;
26
:636– 40.
Weatherall DJ, Provan AB. Red cells I:inherited anaemias. Lancet 2000; 355:1169– 75.
0
25
50
75
100
% Saline
Control
Patient
0
% RBC lysis
Normal
range
0.2 0.4
0.6 0.8
1.0
Fig.3.10 Test and control samples subjected to varying concentrations of saline.
The broad grey band represents the normal range. The red cells in the test sample
(from a patient with hereditary spherocytosis) lyse at higher concentrations of saline
than that seen in the normal control, which suggests that hereditary spherocytosis is
a likely diagnosis.

270
CHAPTER3 Haematology
270
Red cell enzymeassays
Numerous red cell enzymes are responsible for maintaining the integrity of
the RBC in order to allow it to function eciently in O
2
delivery and CO
2
removal. RBC enzyme defects lead to shortened RBC survival (i.e. hae-
molysis) and anaemia. Although there are numerous enzymopathies that
may cause haemolysis, the most useful starting assays are for G6PD and
pyruvate kinase(PK).
Of course, one should start by taking a detailed history from the patient,
asking about previous haemolytic episodes, family history, ethnic origin, and
possible drug toxicities.
•
Sample:fresh EDTA or heparin. The enzymes are stable for 6days at
4°C and 24h at25°C.
•
Methods:these are too numerous and complex to listhere.
Essentially there are three methods for analysis ofG6PD:
1. Brilliant cresyl blue decolorizationtest.
2. Methaemoglobin reductiontest.
3. Ultraviolet (UV) spottest.
•
Normal range:varies between laboratories (check with your local
laboratory).
•
Pitfalls:during a haemolytic episode in patients with G6PD deciency,
the oldest RBCs are destroyed rst. Younger RBCs (and especially
reticulocytes) have higher levels of the enzyme than older cells. It
follows therefore that if the enzyme level is assayed during an acute
episode, the G6PD level obtained may be falsely normal. This will
rise further as reticulocytes pour into the peripheral blood, as hap-
pens during recovery from the acute attack. It is better to wait until
the acute attack is over and the patient is in steadystate.
Further reading
Arya R, Layton DM, Bellingham AJ. Hereditary red cell enzymopathies. Blood Rev 1995; 9:165– 75.
Beutler E. The molecular biology of G6PD variants and other red cell enzyme defects. Annu Rev
Med 1992; 43
:47– 59.
World Health Organization Scientic Group (1967). Standardization of procedures for the study of
glucose- 6- phosphate dehydrogenase. Technical Report Series, No. 366. Geneva: World Health
Organization.

HAEMOGLOBIN ABNORMALITIES
271
Haemoglobin abnormalities
There are two main classes of Hb abnormalities (see Table3.9).
Table3.9 Main classes ofHb abnormalities
Abnormality Example
Structural Hb variants Sickle Hb, HbD, HbE
Imbalanced globin production Thalassaemias (α, β, etc.)
Structural haemoglobin variants
If the amino acid change results in an electrical charge dierence, this may
be detected by protein electrophoresis (separates proteins on the basis of
charge). Investigation requires a full clinical history, FBC, blood lm, and Hb
electrophoresis.
Thalassaemias
β- thalassaemia is diagnosed from the blood indices, blood lm, HbA
2
, and
HbF levels. For α
- thalassaemia, the investigation is more complex, requiring
DNA analysis to detect α- globin deletions. Globin chain synthesis, which
examines the ratio of α:β- globin production, is performed less with the
advent of DNA- based methods.
Further reading
Weatherall DJ, Provan AB. Red cells I:inherited anaemias. Lancet 2000; 355:1169– 75.

272
CHAPTER3 Haematology
272
Haemoglobin analysis
Haemoglobin electrophoresis
Electrophoresis is an electrical method for separating molecules on the basis
of size (for DNA fragments) or overall electrical charge (for proteins). Hb
electrophoresis allows the separation of dierent Hb, providing they have
diering charges (Hb molecules with the same charge will move together on
the gel and cannot be distinguished). The methods used take advantage of
the fact that amino acid side chains on the globin molecules can be ionized.
The net overall charge of a protein depends on the pH of the solution in
which it is and the pKs of the amino acids (the pK is the pH at which half of
the side chains are ionized).
Electrophoretic methodsused
• Cellulose acetate (at pH8.6).
• Citrate agar (at pH6.0).
• Isoelectric focusing(IEF).
• High- performance liquid chromatography (HPLC).
Due to space limitations, each of these methods will be discussed only
briey. Other texts deal with this topic in considerable detail.
•
Sample:peripheral bloodEDTA.
Cellulose acetate
This test is commonly performed in the diagnosis of abnormal Hb produc-
tion (haemoglobinopathies or thalassaemia). Because some Hb have the
same net charge, they will run together, e.g. HbS will run in the same band
as HbD and HbG, and HbC will run with HbE. To resolve these bands,
electrophoresis is next carried out at acidpH.
Citrateagar
This is similar to cellulose acetate where Hb molecules are separated at an
acid pH (pH 6.0) to separate out Hb that run together at alkalinepH.
Isoelectric focusing
This is a high- resolution method for separating dierent Hb molecules. The
basic principle of the test relies on the fact that all proteins and amino acids
have a pH at which their net charge is zero. This is termed the isoelectric
point. At this pH, there is no net movement in the presence of an externally
applied electric eld. The Hb molecules are subjected to a pH gradient. This
method has the advantage of high resolution but is more expensive than
standard electrophoresis (see Fig.3.11).
Normal adult haemoglobins
• HbA:97%total.
• HbA
2
:2.0– 3.2%.
• HbF:0.5%.
HAEMOGLOBIN ANALYSIS
273
High- performance liquid chromatography
This chromatographic technique has been around for 20 years or more
and is being increasingly used for analysis of Hb molecules. Hb molecules
are passed through a matrix column and eluted from the column at varying
times, during which their absorbance is measured. Detection of standard
Hb variants is simple; the advantage of HPLC is that novel Hb variants can
also be detected, and HPLC can separate proteins that cannot be resolved
using other means. HPLC is more expensive than all the techniques men-
tioned above (see Figs 3.12 and3.13).
Fig.3.11 Isoelectric focusing of haemoglobin.
Fig.3.12 HPLC analysis showing sickle trait (HbA +HbS).
274
CHAPTER3 Haematology
274
When should you request thesetests?
Haemoglobin analysis is usually carriedout
•
When the MCV is dd, but Hb normal or slightlyd.
•
In patients from ethnic groups known to be associated with high levels
of Hb disorder, e.g. sickle or thalassaemia.
Fig.3.13 HPLC analysis showing β- thalassaemia trait (elevatedHbA
2
).

HAEMOGLOBIN ANALYSIS
275

276
CHAPTER3 Haematology
276
Investigation ofpossible thalassaemia
1. Check the FBC and look at theMCV.
2. Is the MCV normal (>76fL)? If so, thalassaemia is unlikely.
3. Does the FBC show anything else? i RCC with d MCV and MCH are
likely in thalassaemia.
4. Measure the HbA
2
; this is generally i in β- thalassaemia trait (carrier).
5. Carry outHPLC.
6. Measure HbFlevel.
7. Look at the distribution of HbF in RBCs (HbF is present in all RBCs in
African hereditary persistence of fetal Hb (HPFH), but not present in
all cells in carriers for d β
- thalassaemia.
8. Assess the iron status (common cause of d MCV— do not miss this!).
9. Look for RBC inclusions (e.g. H bodies in α- thalassaemia or Heinz
bodies in unstable Hb disorders).
10. Carry out DNA analysis, examining both α- and β- globingenes.
Further reading
Information Center for Sickle Cell and Thalassemic Disorders. Thalassemia. M http:// sickle.bwh.
harvard.edu/ menu_ thal.html.

INVESTIGATION OFPOSSIBLE THALASSAEMIA
277
278
CHAPTER3 Haematology
278
Investigation ofsickle haemoglobin
Sickle Hb is the result of a point mutation in the β- globin gene, resulting in
a Glu l Val switch at position 6 of the β- globin protein. Sickle Hb (HbS)
forms long laments (tactoids), reducing its solubility when O
2
tension is
reduced. This forms the basis of the sickle solubility test (see Fig.3.14).
•
Sample:any anticoagulant.
The patient’s blood is mixed with sodium dithionite solution and left to
stand. A+ve sickle sample should be used as a control. When the tubes are
examined, a clear solution implies that there is no sickle Hb; a turbid solu-
tion conrms the presence of HbSS in the patient’s sample.
2
A+ve result will be obtained for sickle carriers (HbAS) and sickle- cell
homozygotes (HbSS). If a +ve result is obtained, Hb electrophoresis must
be carried out to determine whether the patient is a carrier or has homozy-
gous sickle- cell anaemia.
Molecular diagnosis ofsickle- cell disease
This is useful for prenatal diagnosis. The β- globin genes of the fetus are
amplied using PCR; cells are obtained by amniocentesis or chorionic villus
sampling (CVS) and digested with a bacterial restriction enzyme, e.g.MstII.
If the sickle mutation is present, no digestion will occur (the mutation
removes the restrictionsite).
Sickled red cells
Fig.3.14 Blood lm of homozygous sickle- cell anaemia (HbSS). Note the sickle-
shaped (crescent) redcells.

INVESTIGATION OFSICKLE HAEMOGLOBIN
279
Neonatal haemoglobin screening
• Obtain blood from the neonate (e.g. heel prick) and in babies at risk
of sickle or β- thalassaemia major (e.g. the mother has a gene for HbS,
C,D
Punjab
, E, O
Arab
, β- or d β- thalassaemia).
• Universal neonatal screening is generally used in areas where there is a
high incidence of haemoglobinopathy.
E OHCM 10e, p.340.
Further reading
Information Center for Sickle Cell and Thalassemic Disorders. Sickle cell disease. M http:// sickle.
bwh.harvard.edu/ menu_ sickle.html.
Steinberg MH, Forget BG, Higgs DR, Weatherall DJ (eds.). Disorders of Hemoglobin: Genetics,
Pathophysiology, and Clinical Management. Cambridge:Cambridge University Press,2001.

280
CHAPTER3 Haematology
280
Estimation ofhaemoglobin A
2
(α
2
δ
2
)
Normal adults have three types of Hb:HbA, HbA
2
, and HbF. HbA (α
2
β
2
)
is the major Hb, and HbA
2
is a minor adult Hb, which is very useful for the
diagnosis of the β
- thalassaemia trait. HbA
2
levels are i in the heterozygote
(carrier state), and this is a specic test for this genotype. The test is carried
out using a column chromatography method.
•
Sample:EDTA.
•
Normal range:2.0– 3.2%.
Causes ofincreasedHbA
2
• β- thalassaemia trait (HbA
2
level is 73.9– 6.5%).
Causes ofdecreasedHbA
2
• Iron deciency.
• δ- thalassaemia.
Estimation offetal haemoglobin
Fetal Hb (HbF) makes up >50% of the total Hb at birth but d to 75% by
5months of age (as γ chain production is replaced by β chains). HbF levels
may be raised in some haemoglobinopathies.
•
Sample:EDTA.
Increased HbF foundin
•
β- thalassaemiatrait.
• β- thalassaemiamajor.
• Hereditary persistence ofHbF.
• Homozygous sickle- cell disease (HbSS).
• Sickle/ β
+
thalassaemia (some cases).
•
Sickle/ β
0
thalassaemia (some cases).
•
JuvenileCML.
• Multiple myeloma (uncommon and never measured).
• Acquired aplastic anaemia.
Haemoglobin H bodies(β
4
)
HbH, consisting of a tetramer of β- globins (β
4
), is found in α- thalassaemia.
The β chains form tetramers due to the relative lack of α- globins with
which to pair. The demonstration of HbH allows the detection of the α-
thalassaemia trait (either – α/ – α or – – / αα) and HbH disease (– – / – α).
Method
The HbH body test involves staining RBCs with brilliant cresyl blue; HbH
bodies are seen as large, dark inclusions in the redcells.
•
Sample:freshEDTA.
2 Note:the presence of HbH conrms α
- thalassaemia, but the absence of
HbH bodies does not exclude the diagnosis.

TESTING FORUNSTABLE HAEMOGLOBINS
281
Heinzbodies
These are red cell inclusions made up of insoluble denatured globin protein.
Heinz bodies are seen when RBCs are stained with methyl violet or new
methylthioninium chloride (methylene blue)stain.
•
Sample:freshEDTA.
•
Interpretation:Heinz bodies are seen close to the RBC membrane.
These are normally removed by the spleen and are therefore more
frequent following splenectomy.
Causes ofHeinzbodies
Oxidative haemolysis
•
Chlorates, phenacetin, otherdrugs.
• G6PD and PK deciencies, and other enzymopathies.
• UnstableHb.
Further reading
Sevitt S, Stone P, Jackson D, Baar S, Pollock A. Acute Heinz- body anaemia in burned patients. Lancet
1973; 2
:471– 5.
Testing forunstable haemoglobins
Globin gene mutations may lead to amino acid substitutions that render the
Hb molecule unstable, leading to haemolysis. Most mutations causing unsta-
ble Hb are autosomal dominant, and >80% aect the β chain. Aected
individuals are heterozygotes. Heinz bodies in RBCs are intracellular Hb
precipitates. Unstable Hb can be detected electrophoretically or by using
the heat precipitation test, in which lysed RBCs are heated to 50°C for1h.
•
Sample:freshEDTA.
•
Interpretation:normal fresh haemolysates should be stable for 1h at
50°C. If there is an unstable Hb, a precipitate will be seen in thetube.
Examples
• HbKöln.
• Hb GunHill.

282
CHAPTER3 Haematology
282
Molecular tests fordiagnosis
ofthalassaemia
Although most haematology laboratories can diagnose β- thalassaemia trait
and β- thalassaemia major, there are occasions when molecular tests are
required, e.g. antenatal diagnosis where a couple are at risk of having a
child with β
- thalassaemia major or hydrops fetalis (absence of α- globin,
usually lethal). In addition, the diagnosis of α- thalassaemia is dicult and
requires DNA analysis, either using Southern blotting or PCR amplication
of globingenes.
β- thalassaemia
There are >100 β- globin mutations now known, but fortunately each popu-
lation tends to have its own group of mutations (this avoids having to test
for all known mutations). It is important that you include the ethnic group
on the request form, since this will assist the laboratory who will then screen
for mutations commonly found in the ethnic group of the patient. Details of
these mutations can be found in the β
- and δ- thalassemia repository.
Methods used formolecular diagnosis ofβ- thalassaemia
The methods used are complex and outwith the scope of this small book.
1
2
–3
How theARMS PCR techniqueworks
•
This is amplication refractory mutation systemPCR.
• Specic point mutations are known for the β- globin mutations.
• PCR primers are designed to bind with the mutated sequence.
• If the patient has the mutation, there will be PCR amplication.
• If the patient lacks the mutation, there is no binding of the primers to
the patient’s DNA and no amplication.
•
So a band on the gel means the mutation is present (and the reverse is
true— if the band is absent, then that particular mutation is absent).
Other techniques, including reverse dot blots and DNA sequencing, are
sometimes needed if ARMS PCRfails.
Methods used formolecular diagnosis ofα- thalassaemia
Whereas β- thalassaemia is usually the result of point mutations (single base
changes), the α- thalassaemias are usually the result of deletions of chunks
of DNA in the region of the α- globin genes. Southern blotting is useful in
detecting deletions, since the DNA band sizes after digestion with restric-
tion enzymes will dier to the wild type (i.e. normal).
1 Bowden DK, Vickers MA, Higgs DR. A PCR- based strategy to detect the common severe deter-
minants of alpha thalassaemia. Br J Haematol 1992; 81
:104– 8.
2 Lewis SM, Bain BJ, Bates I (eds.). Dacie & Lewis Practical Haematology, 9th edn. Edinburgh:Churchill
Livingstone,2000.
3 Huisman TH, Carver MF. The beta- and delta- thalassemia repository. Hemoglobin 1988;
22
:169– 95.

MOLECULAR TESTS FORDIAGNOSIS OFTHALASSAEMIA
283
UK Haemoglobinopathy Reference Laboratory
This is based at the John Radcliffe Hospital in Oxford (UK). Difficult
cases (e.g. α
- thalassaemia) can be sent to this laboratory (after discuss-
ing the case first); they will perform α
- globin gene analysis and send
a detailed report containing the genotype of the patient. See end of
the chapter for contact details (E Specialized haematology assays,
pp. 330–1).

284
CHAPTER3 Haematology
284
Acquired haemolytic anaemias
Determining the cause of haemolytic anaemia can be a complex process.
Having excluded inherited disorders of Hb, RBC membrane, or enzymes,
we are left with a diverse group of disorders with a common phenotype
ofi RBC destruction (and d RBC lifespan; see Table3.10).
Immune
• Autoimmune (°, or 2° to SLE orCLL).
• Alloimmune (e.g. transfusion reactions, haemolytic disease of the
newborn (HDN)).
•
Antibody can be warm (IgG) or cold (IgM usually).
Red blood celldamage
• Drugs.
• Poisons.
• Burns.
Red blood cell fragmentation syndromes
• DIC.
• Thrombotic microangiopathies(TMAs)
• March haemoglobinuria.
Investigations
There is little point investigating the cause of haemolytic anaemia until you
have shown that haemolysis is actually occurring.
Table3.10 Acquired haemolytic anaemias:mechanisms
Mechanism Examples
Autoimmune Warm antibody (IgG mainly):
• Idiopathic haemolytic anaemia
• 2° to other autoimmune diseases (e.g. SLE), lymphoid
malignancies (e.g. CLL), infections, drugs (e.g.
penicillins, methyldopa)
Cold antibodies (IgM mainly):
• Cold agglutinin syndromes, cold haemagglutinin
disease (CHAD), 2° to infection
Alloimmune Haemolytic transfusion reactions, HDN
Infections Many, including malaria, meningococcal, pneumococcal,
viral
Chemical or physical Drugs, burns, drowning
RBC fragmentation
syndromes
Mechanical heart valves, MAHA (seen in DIC, HUS,
TTP, pre- eclampsia, SLE, carcinoma)
Membrane disorders Examples are liver disease, PNH

ACQUIRED HAEMOLYTIC ANAEMIAS
285
Look forthe acquiredcause
• FBC and peripherallm:
•
Spherocytes (suggests warm antibody; also present in hereditary
spherocytosis).
•
i WBC, e.g. might suggest an underlying lymphoproliferative disor-
der such asCLL.
•
RBC fragments (suggests physical damage to the RBC, e.g. micro-
angiopathic haemolytic anaemia (MAHA), TTP/ HUS, burns, March
haemoglobinuria, mechanical heart valves).
•
Parasites, e.g. malaria.
•
Infections, e.g. Clostridium, Bartonella, Babesia.
•
Antiglobulin test(DAT):
•
IgG or IgG + complement (C3d) onRBC.
•
DAT is usually +ve in immune- mediated haemolysis.
• Renal function (often abnormal in TTP/ HUS).
• Coagulation screen (DIC with RBC fragmentation).
• LFTs (abnormal in Zieve’s syndrome).
• USS for splenomegaly.
• Cold agglutinins:IgM, usually against Ior i proteins, RBC membrane
proteins.
•
Immunophenotype if suspectPNH.

286
CHAPTER3 Haematology
286
Immunophenotyping forGPI- linked
proteins
PNH is a rare acquired red cell membrane disorder. Loss of glycosyl
phosphatidyl inositol (GPI)- linked surface proteins make the red cells
vulnerable to complement- mediated lysis, causing episodes of marked
intravascular haemolysis, with free Hb in the urine (haemoglobinuria).
Immunophenotyping shows cells to be decient in the GPI- anchored
proteins CD55 and CD59 (erythrocytes) and CD16, CD24, and FLAER
(granulocytes),
The technique of immunophenotyping is described later in this chapter.

BLEEDINGTIME
287
Bleedingtime
This is a test of ° haemostasis, and mainly of platelet function in vivo, rather
than a laboratory test. You will generally need to arrange this test through
the haematology department who will carry out the test foryou.
Procedure
A disposable spring- loaded blade is used to make two incisions of xed
depth into the skin of the forearm, whilst a sphygmomanometer is inated
to 40mmHg. Blood from the incisions is mopped up using circular lter
paper (care needs to be taken to avoid disturbing the clot that forms on
the cut surface).
•
Normal range:up to 7min (varies, depending on the method
used;>9min is abnormal). Longerin♀.
•
Uses:previously felt to be the best screen for acquired or congenital
functional or structural platelet disorders. If bleeding time is normal and
history −ve (i.e. no major bleeding problems in the past), this excludes
an underlying platelet disorder.
Precautions
2 Do not carry out bleeding time if the platelet count is <100 x 10
9
/ L
(will be prolonged). Aspirin will interfere with the test— ask patients to stop
aspirin 7days before the test is carriedout.
Causes ofprolonged bleedingtime
•
Low plateletcount.
• Platelet function defect (acquired, e.g. aspirin, paraprotein,MDS).
• von Willebrand’s disease(vWD).
• Vascular abnormalities, e.g. Ehlers– Danlos.
• Occasionally low factor VorXI.
• Abrinogenaemia.
Pitfalls
Highly operator- dependent, with low reproducibility. Because of this, the
test is seldom usednow.
Further reading
Mielke CH Jr. Aspirin prolongation of the template bleeding time:inuence of venostasis and direc-
tion of incision. Blood 1982; 60
:1139– 42.
Parkin JD, Smith IL. Sex and bleeding time. Thromb Haemost 1985; 54:731.
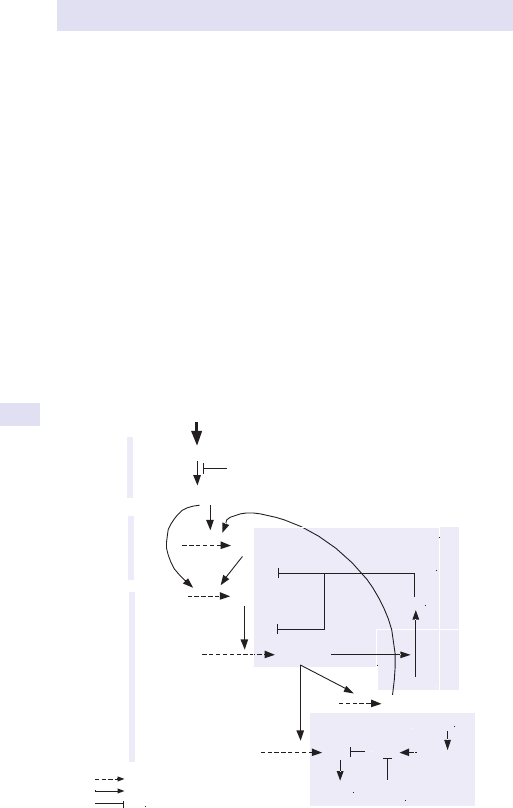
288
CHAPTER3 Haematology
288
Prothrombintime
This tests the extrinsic coagulation pathway and is useful for detecting
coagulation deciencies, liver disease, and DIC. The prothrombin time (PT)
is also the main monitor for coumarin therapy (e.g. warfarin), expressed
as a ratio— the international normalized ratio (INR). The test measures the
clotting time of plasma in the presence of a tissue extract, e.g. brain (throm-
boplastin). The test measures prothrombin, but also factors V, VII, and X
(see Fig.3.15).
•
Sample:citrate.
Increased prothrombintime
• Oral anticoagulation therapy (vitamin K antagonists).
• Fibrinogen deciency (factorI).
• Prothrombin deciency (factorII).
• Deciency of factors V, VII, or X (in V or X deciency, the activated
partial thromboplastin time (APTT) willbei).
•
Liver disease, especially obstructive.
• Vitamin K deciency.
• DIC.
TF–VII
TF–VIIa
XXa
II Thrombin
XI XIa
Fibrinogen Fibrin
IX IXa
VIIIa
Va
TFPI
TISSUE INJURYTEST
PT
APTT
PT
and
APTT
Key
Becomes active
Activates
Inhibits
Protein C
APC
Protein S
Factor V
1. Anticoagulant pathway
FDPs
Plasmin
Plasmin
inhibitor
TPA
2. Fibrinolytic pathway
Plasminogen
Fig.3.15 Coagulation cascade showing the factors assayed using the various clottingtests.
Modied from Provan D, etal. Oxford Handbook of Clinical Haematology, 2nd edn. Oxford:Oxford
University Press,2004.
Further reading
Provan D, Singer CRJ, Baglin T, Lilleyman J. Oxford Handbook of Clinical Haematology, 2nd edn.
Oxford:Oxford University Press,2004.

ACTIVATED PARTIAL THROMBOPLASTINTIME
289
Activated partial thromboplastintime
Other terms:somewhat confusingly, the APTT may be called kaolin cephalin
clotting time (KCCT) or partial thromboplastin time with kaolin (PTTK).
What is APTT testing?
This is a test of the intrinsic coagulation system and depends on contact
factors + factors VIII and IX, and reactions with factors X, V, II, and I.The
APTT is sensitive to circulating anticoagulants (e.g. lupus anticoagulant) and
heparin.
•
Sample:citrate.
Uses
1. Heparin monitoring.
2. Screening for haemophilia Aand B (VIII and IX deciencies,
respectively).
3. Screening for coagulation inhibitors.
• Normal range:26.0– 33.5s (often expressed as activated partial
thromboplastin time ratio (APTR)).
Increased activated partial thromboplastintime
• DIC.
• Liver disease.
• Massive blood transfusion.
• Heparin treatment.
• Circulating anticoagulant.
• Modest i in patients taking oral anticoagulants.
•
Haemophilia.
Is there aninhibitor present?
The APTT will be long if there is an inhibitor, such as the lupus anticoagu-
lant, present. This can be determined by mixing the patient’s plasma with
an equal volume of normal control plasma and repeating the APTT. This is
known as a 50:50 mix. If the APTT is long because of an inhibitor, it will not
fully correct when normal plasma is added. However, if the APTT is long
because of a deciency, it will be corrected with the normal plasma.
Further reading
Denson KW. Thromboplastin— sensitivity, precision and other characteristics. Clin Lab Haematol
1988; 10
:315– 28.
Lab Tests Online (2012). Coagulation cascase. M http:// www.labtestsonline.org/ understanding/
analytes/ coag_ cascade/ coagulation_ cascade.html.
Turi DC, Peerschke EI. Sensitivity of three activated partial thromboplastin time reagents to coagula-
tion factor deciencies. Am J Clin Pathol 1986; 85
:43– 9.
van den Besselaar AM, Meeuwisse- Braun J, Jansen- Grüter R, Bertina RM. Monitoring heparin therapy
by the activated partial thromboplastin time— the eect of pre- analytical conditions. Thromb
Haemost 1987; 57
:226– 31.

290
CHAPTER3 Haematology
290
Thrombin clottingtime
This is aected by the concentration of factor I(brinogen) and the pres-
ence of brin or brinogen degradation products and heparin.
•
Sample:citrate.
Increased thrombin clottingtime
• Low brinogen, e.g.DIC.
• i FDPs/ cross- linked brin degradation products (XDPs)/ D- dimers.
• Heparin.*
• Dysbrinogenaemia (inherited; mutation in the brinogen gene leads to
amino acid change and non- functional brinogen).
Note:(*) if suspected, check reptilase time, similar to thrombin clotting time
(TCT), but not aected by heparin.
D- dimers
D- dimers are produced during polymerization of brinogen as it forms
brin. Measurement of D- dimer levels is more specic for this process than
the older FDP test and is now being used to detect the presence of DIC
and other coagulation disorders. The test measures brin lysis by plasmin
and is a sensitive indicator of coagulation activation (e.g. such as that seen in
DIC). The assay uses an MoAb specic for D- dimers; it will not cross- react
with brinogen or brin.
•
Sample:citrate (clotting screen bottle).
Increased D- dimers seenin
• DIC.
• DVT.
• PE.
Summary ofclotting tests ina variety ofdisorders
(See Table3.11.)
Table3.11 Summary ofclotting tests ina variety ofdisorders
PT APTT TCT Platelets Diagnosis
N N N N Platelet function defect, XIII deciency, normal
i N N N VII deciency, early oral anticoagulation
N i N N
VIIIC/ IX/ XI/ XII deciencies, vWD, circulating
anticoagulant, e.g. lupus
i i N N Vitamin K deciency, oral anticoagulant,
V/ VII/ X/ II deciencies
i i i N Heparin, liver disease, brinogen deciency
N N N d Thrombocytopenia (any cause)
i i N
Low Massive transfusion, liver disease
i i i
Low DIC, acute liver disease
Modied from Dacie & Lewis Practical Haematology, 8th edn. Edinburgh:Churchill Livingstone,1995.
291
DISSEMINATED INTRAVASCULAR COAGULATION
3 Disseminated intravascular
coagulation
DIC is a medical and haematological emergency. It may be seen in a variety
of situations and is characterized by generalized bruising and bleeding, usu-
ally from venepuncture sites, post- operatively, and spontaneously.
Diagnosis requires FBC, clotting screen, and evidence of rapid consump-
tion of brinogen. Classic (acute) DIC, where the test results t the bill, is
easy to spot. The situation may be more subtle and you are strongly advised
to discuss the case with a haematology registrar or consultant if you are in
any doubt about the diagnosis ofDIC.
Conditions associated withDIC
(See Table3.12.)
Further reading
Levi MM (2016). Disseminated intravascular coagulation. M http:// www.emedicine.com/ emerg/
topic150.htm.
Table3.12 Conditions associated withDIC
Disorder Example
Infectious disease Septicaemia
Viraemia
Obstetric emergency Placental abruption
Eclampsia
Amniotic uid embolism
Placenta praevia
Septic abortion
Surgical Cardiac bypass
Malignant disease Metastatic cancer
Acute leukaemia (especially AML M3, i.e. acute
promyelocytic leukaemia)
Shock Trauma
Severe burns
Transfusion
ABO- mismatched transfusion
Miscellaneous Snake bites (some)
Liver cirrhosis
3 Laboratory diagnosis
FBC d platelets
± red cell fragments seen on blood lm
PT i in moderately severe DIC
APTT Usually i
Fibrinogen d
(falling levels signicant— but remember this is an acute phase
protein, so levels may be normal, even in orid DIC)
D- dimers i

292
CHAPTER3 Haematology
292
Platelet functiontests
These are specialized tests carried out by the coagulation laboratory for
the investigation of patients with suspected platelet dysfunction. Because of
their complexity, platelet function tests will not be described in detailhere.
Patients generally present with bleeding or bruising problems and have
had normal coagulation results. Because of the labour- intensive nature and
cost of these assays, you will need to arrange these tests after discussion
with your local haematology medicalsta.
•
Sample:blood collection needs to be optimal with non- traumatic
venepuncture, rapid transport to the laboratory with storage at room
temperature and testing within a maximum of2– 3h.
Currenttests
• Plateletcount.
• Morphology.
• Adhesion.
• Aggregation.
• Platelet release.
• Bleedingtime.
Plateletcount
Normal range 150– 400 x 10
9
/ L. Adequate function is maintained, even
when the count is <0.5 normal level but progressively deteriorates as it
drops. With platelet counts <20 x 10
9
/ L, there is usually easy bruising and
petechial haemorrhages (although more serious bleeding can occur).
Morphology
Large platelets are biochemically more active; i mean platelet volume
(MPV>6.5) is associated with less bleeding in patients with severe throm-
bocytopenia. Altered platelet size is seen in inherited platelet disorders.
Platelet adhesion
Adhesion to glass beads now rarely performed in routine laboratory prac-
tice, but potentially useful in vWD diagnosis.
Platelet aggregation
Most useful of the special tests; is performed on a fresh sample using an
aggregometer.
Aggregantsused
•
Adenosine 5- diphosphate (ADP) at low and high concentrations.
Induces two aggregation waves:the ° wave may disaggregate at low
concentrations of ADP; the second is irreversible.
•
Collagen has a short lag phase, followed by a single wave, and is
particularly aected by aspirin.
•
Ristocetin- induced platelet aggregation (RIPA) is carried out at
high (1.2mg/ mL) and lower concentrations and is mainly used to
diagnosevWD.
•
Arachidonicacid.
• Adrenaline (epinephrine), not uncommonly reduced in normal people.

PLATELET FUNCTIONTESTS
293
Platelet release
Enzyme- linked immunosorbent assay (ELISA) or radioimmunoassay (RIA)
are used to measure the granule proteins β- thromboglobulin (β- TG) and
heparin neutralizing activity (HNA). These are sensitive markers of platelet
hyper- reactivity and beyond the scope of the routine laboratory.
Practical application oftests
Their main role is in diagnosis of inherited platelet functional defects. In
acquired platelet dysfunction 2° to causes such as renal and hepatic disease,
DIC, and macroglobulinaemia, platelet function is rarely tested.
Further reading
Yardumian DA, Mackie IJ, Machin SJ. Laboratory investigation of platelet function:a review of meth-
odology. J Clin Pathol 1986; 39
:701– 12.

294
CHAPTER3 Haematology
294
Thrombophilia screening
Thrombophilia describes acquired or inherited disorders that predispose to
arterial or venous thromboembolism (VTE). Thrombophilia should be con-
sidered in young patients who have apparent strong family history ofVTE.
•
Causes:E Recurrent thrombosis, p. 92.
Which patients should be screened forpossible
thrombophilia?
• Arterial thrombosis, consider antiphospholipid antibody and lupus
anticoagulant testing.
•
Venous thrombosis:
•
Patients <40years from thrombosis-prone families.
•
Family history of thrombosis with high-risk thrombophilia in rst-
degree relative.
•
Unusual site, e.g. mesenteric, portal vein thrombosis.
•
Children with purpura fulminans.
•
Recurrent miscarriage (three ormore).
•
VTE in pregnancy and theOCP.
Screen
• Exclude medical causes (check ESR, LFTs, AIP, fasting lipids).
• FBC (exclude thrombocytosis).
• Clotting screen for acquired defects (PT, APTT, LA/ anticardiolipin
antibody (ACL), i brinogen).
•
Screen for inherited thrombophilia:
•
First- line protein C (PC), protein S (PS), antithrombin (AT), activated
protein C resistance (APCR).
•
Check for the presence of the factor V Leiden mutation in APCR
+ve patients (DNA analysis).
•
Consider testing plasminogen, brinogen, homocysteine levels,
prothrombin variant.
•
DNA analysis for prothrombin gene mutation.
Thrombophilia investigations are time- consuming and expensive, and you
should discuss with the local haematology medical or laboratory sta
before sending samples. Note:some thrombophilia tests cannot be carried
out in the ‘acute’ phase of a VTE event or whilst the patient is taking an
anticoagulant.
Further reading
Baglin T, Gray E, Greaves M, etal. Clinical guidelines for testing for heritable thrombophilia. Br J
Haematol 2010; 149
:209– 20.
Cattaneo M, Monzani ML, Martinelli I, Falcon CR, Mannucci PM. Interrelation of hyperhomocyst(e)
inemia, factor V Leiden, and risk of future venous thromboembolism. Circulation 1998; 97:295– 6.
Chong LY, Fenu E, Stansby G, Hodgkinson S. Management of venous thromboembolic diseases and
the role of thrombophilia testing:summary of NICE guidance. BMJ 2012; 344:e3979.
Dahlback B. Resistance to activated protein C as risk factor for thrombosis:molecular mechanisms,
laboratory investigation, and clinical management. Semin Hematol 1997; 34
:217– 34.
Lane DA, Mannucci PM, Bauer KA, etal. Inherited thrombophilia:Part 1. Thromb Haemost 1996;
76
:651– 62.
Lane DA, Mannucci PM, Bauer KA, etal. Inherited thrombophilia:Part 2. Thromb Haemost 1996;
76
:824– 34.

ANTITHROMBIN AND PROTEINS CANDS
295
Antithrombin and proteins CandS
These proteins are the body’s natural anticoagulants; hence, deciencies may
lead to thromboembolic disease.
Antithrombin
Used to be called ATIII, but there was never an ATI or ATII, so now abbrevi-
ated to AT. Auseful measure in thrombophilia screening since low levels of
AT are found in 4.5% of patients with unexplainedVTE.
Decreased antithrombinlevels
• Hereditary (40– 60% normal level), autosomal dominant.
• Chronic liver disease.
• Protein wasting disorders.
• Heparin therapy.
• Third trimester of pregnancy.
• Acute leukaemia.
• Burns.
• Renal disease.
• Gram −ve sepsis.
ProteinS
• Reduced levels predispose to VTE. Individuals with 30– 60% normal level
may suer recurrent thrombosis.
•
dPS.
•
Inherited (autosomal dominant).
• Pregnancy.
• Oral anticoagulants, e.g. warfarin.
• Nephrotic syndrome.
• Liver disease.
ProteinC
• Similar to PS; autosomal dominant inheritance in geneticcases.
• dPC.
•
Hereditary.
• Liver disease.
• Malignancy.
• Warfarin therapy.
• Pregnancy.
296
CHAPTER3 Haematology
296
Bone marrow examination
This is a key investigation in haematology. It may be diagnostic in the follow-
up of abnormal peripheral blood ndings and is an important staging pro-
cedure in dening the extent of disease, e.g. lymphomas. It is a helpful
investigative procedure in unexplained anaemia, splenomegaly, or selected
cases ofPUO.
•
Preferred sites:the posterior iliac crest is the usual site (allows an aspirate
and a biopsy to be obtained). The sternum is suitable only for marrow
aspiration and is not a test for the squeamish.
The marrow aspirate provides
• Cytology of nucleatedcells.
• Qualitative and semi- qualitative analysis of haematopoiesis.
• Assessment of iron stores (if Perls’ iron stainused).
• Smears for cytochemistry (helps in the diagnosis of leukaemias).
Marrow cells can also be usedfor
• Chromosomal (cytogenetic) analysis.
• Immunophenotype studies usingMoAbs.
Marrow trephine biopsy provides informationabout
• Marrow cellularity.
• Identication and classication of abnormalcells.
• Immunohistochemistry on inltrates.
Contraindications
None, other than physical limitations, e.g. pain or restricted mobility. Avoid
sites of previous radiotherapy (inevitably grossly hypocellular and not rep-
resentative). (See Table3.13.)
Table3.13 Tests carried outon bonemarrow
Test Purpose
Chromosomes Standard cytogenetic analysis, looking for
rearrangements suggestive of acute or chronic
leukaemias and myelodysplasia
Fluorescence in situ hybridization (FISH) looking for
additions or losses of chromosomes, as well as more
subtle changes seen in leukaemias and lymphomas
DNA or RNA analysis Using PCR to look for mutations or translocations that
help classify leukaemias and lymphomas; also useful for
monitoring disease levels in some disorders
Immunophenotype Cell surface marker prole helps in the diagnosis of
most leukaemias and lymphomas; may also be used to
monitor disease levels post- treatment
Microbiology For example, TB culture (not routine, but occasionally
useful in cases of PUO)
Cytochemical stains To help dene type of leukaemia

BONE MARROW EXAMINATION
297
Procedure
1. BM aspiration may be performed under local anaesthetic (LAn) alone,
but short- acting IV sedative (e.g. midazolam) is preferred when a
trephine biopsy is performed. General anaesthetic used in children.
2. Place the patient in the (left) lateral position, or use the right side if
s/ he cannot lie on the leftside.
3. Inltrate the skin and periosteum over the posterior iliac spine
withLAn.
4. Make a small cutaneous incision before introducing the aspirating
needle, which should penetrate the marrow cortex 3– 10mm before
removal of the trocar.
5. Aspirate no more than 0.5– 1mL of marrow initially (to avoid dilution
of the sample with blood).
6. Make smears promptly (2 the sample clots rapidly!).
7. If further samples are needed, e.g. for immunophenotyping,
cytogenetics, etc., these can be aspirated after making initial slides.
8. For trephine biopsy, use an Islam or Jamshidi needle.
9. Advance the needle through the same puncture site to penetrate the
cortex.
10. Remove the trocar and, using rm hand pressure, rotate the needle
clockwise and advance as far as possible.
11. Remove the needle by gentle anticlockwise rotation.
12. Following the procedure, apply simple pressure dressings.
13. Minor discomfort at the location may be dealt with by simple analgesia
such as paracetamol.
298
CHAPTER3 Haematology
298
Cytochemistry tests (leukaemia
diagnosis)
These staining methods have been around for many years (for decades,
they were all that was available) but remain extremely useful in the diag-
nosis and classication of leukaemias. Modern technologies, such as ow
cytometry and nucleic acid analysis, have rened leukaemia and lymphoma
diagnosis, but the examination of well- stained cytochemistry BM smears
remains the cornerstone of good haematology practice.
After performing a BM aspirate and spreading the material onto glass
slides, the air- dried, unxed microscope slides are passed to the cyto-
chemistry laboratory that will x and stain the slides according to the likely
diagnosis (e.g. stains for AML dier to those for ALL; see Tables 3.14
and 3.15). Positive results with particular stains will point to a specic
diagnosis. This will then be augmented by ow cytometric or molecular
assays (see Fig.3.16).
Table3.14 Cytochemicalstains
Cytochemical stain Substrate/ cell
Myeloperoxidase (MPO) Lysosomal enzyme found in neutrophils and
monocytes
Sudan black (SB) Phospholipids in neutrophil granules
Chloroacetate esterase
Stain- specic esterase in granulocytes and mast
cells. Makes it easier to diagnose AML M4 subtype
α- naphthyl acetate esterase
(ANAE)
Esterase stain, useful for diagnosis of AML
subtypes
Acid phosphatase Enzyme found in many dierent WBCs. Useful for
T- cell malignancies
Periodic acid– Schi (PAS) Detects glycogen in cells. Granulocytes have
diuse staining, whereas lymphocytes staining is
much coarser

CYTOCHEMISTRY TESTS (LEUKAEMIA DIAGNOSIS)
299
Further reading
Cytochemistry. M https:// clinicalgate.com/ cytochemistry/
Table3.15 Cytochemical stains inacute leukaemia
Acute lymphoblastic
leukaemia
Acute myeloid leukaemia
B lineage T lineage M1– 3 M4– 5 M6– 7
MPO − − +/ ++ + −
SB − −
+/ ++ + −
Chloroacetate
esterase
− −
−/ ++ − −
ANAE − − − ++ −
Acid
phosphatase
− + (focal) − + (diuse) + (focal)
PAS + (blocks) − − + (ne
granular)
+
+, positive; ++, strongly positive; −, negative.
From Hobrand AV, Lewis SM, Tuddenham EGD. Postgraduate haematology, fourth edition,
Oxford:Butterworth- Heinemann,2000.
myeloblast
s
Auer rods AML
Auer rods
Fig.3.16 AML marrow showing myeloblasts (leukaemic cells) and an Auer rod in
one cell. Auer rods are pathognomonic of AML, since they do not occur in any other
disorder.
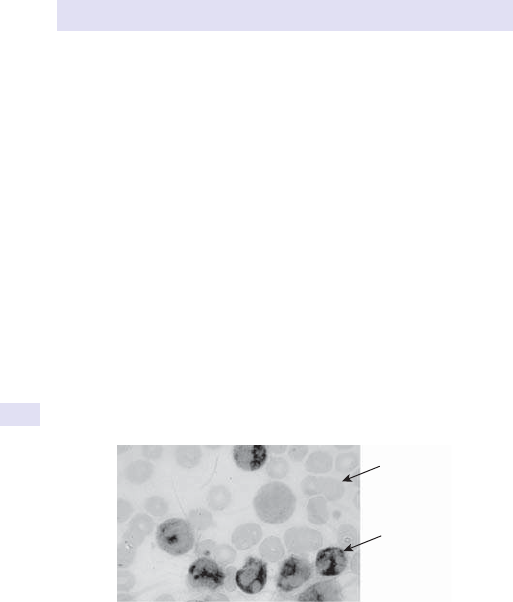
300
CHAPTER3 Haematology
300
Neutrophil alkaline phosphatase
Uses
This is a cytochemical stain used to demonstrate the presence and quantity
of the neutrophil enzyme ALP. Historically, the NAP score was of value
in dierentiating ‘reactive’ states from myeloproliferative disorders such as
CML, PRV, etc.— now more often it features in examination multiple choice
questions! (Note:sometimes termed leucocyte alkaline phosphatase,LAP.)
Procedure
Best performed on fresh blood lms, made without the use of anticoagu-
lant. EDTA samples may be used but are less satisfactory. The lm should
be made, air- dried, xed, and then stained— all within 30min. Positive NAP
activity is indicated by the presence of bright blue granules in the neutrophil
cytoplasm (the nucleus is stained red; see Fig.3.17).
•
Scoring:lms are scored from 0 to 4 on the basis of stain intensity:
•
0:−ve, no granulesseen.
•
1:weak +ve, few granules.
•
2:+ve, few to moderate numbers of granules.
•
3:strongly+ve.
•
4:very strong.
Red cell
NAP stain
in cytoplas
m
Fig.3.17 NAP- stained blood lm showing positively stained neutrophils. Red cells
do not take up thestain.

NEUTROPHIL ALKALINE PHOSPHATASE
301
Interpretation and signicance
(See Table3.16.)
Table3.16 NAP
High NAP score Low NAP score
PRV
Leukaemoid reaction
Neutrophilia— anycause
Myelobrosis
Essential thrombocythaemia
Hepatic cirrhosis
Hodgkin’s disease
Aplastic anaemia
Down’s syndrome
Cushing’s disease
CML
PNH
AML
The NAP score is aected by corticosteroids, oestrogens, and pregnancy
(i NAP). In Hodgkin’s disease, the NAP score oers no advantage over
simpler tests, such as ESR, for assessment of disease activity. Occasionally
of value in a patient with aplastic anaemia who is developing PNH— the
NAP score is seen to fall (both of these are very rare disorders). NAP score
has been replaced in most hospitals by ow cytometry and other methods.
Further reading
Lewis SM, Bain BJ, Bates I (eds.). Dacie & Lewis Practical Haematology, 9th edn, Edinburgh:Churchill
Livingstone,2001.

302
CHAPTER3 Haematology
302
Blood transfusion
Due to space limitation, it is inappropriate to go into major details about the
investigations used in transfusion medicine. However, we have provided the
more important tests in current use which include:
•
Blood group and antibody screen.
• Cross- match (compatibilitytest).
• DAT.
• Antiplatelet and antineutrophil antibody testing.
Safe transfusion practice
Each year, patients are transfused with the wrong blood. In 2015, seven
patients were given ABO- incompatible blood, whilst 280 patients were
given incorrect blood components (not just ABO- incompatible) in 2015.
4
Note that reporting is currently voluntary and very likely underestimates
actual incidents. However, with 2.5million blood components transfused
annually, it is a small percentage overall. Pulmonary complications, particu-
larly transfusion- associated circulatory overload (TACO), are a major cause
of morbidity and death. However, it is also clear that delay in appropriate
transfusion also contributes to mortality.
A common error is clerical and generally involves the cross- matched
sample being taken from the wrong patient, and so the compatibility test
is performed on the wrong sample. Occasionally, the sta carrying out the
transfusion connect the blood up to the wrong patient. In any event, the
result varies from no symptoms to shock and possibledeath.
How tominimizeerrors
• First, ask yourself, ‘Does this patient really need to be transfused with
blood or blood products (e.g. fresh frozen plasma (FFP), platelets,
etc.)?’ For example, a post- operative patient who is asymptomatic
with Hb of 9g/ dL probably does not require red cell transfusion. Use
clinical judgement in helping decide whether or not to proceed with
transfusion.
•
Before taking the blood sample, check that you are taking blood from
the correct patient— ask for his/ her name and check the identity
bracelet.
•
Label the patient’s blood bottle at the bedside (i.e. no prelabelling of
bottles). Many transfusion laboratories insist on 1, 2, 5, 6, and 7, and
either 3 or 4from:
1. Surname and forename (correctlyspelt)
2. DoB
3. Hospital/ A&E/ new NHSnumber
4. First line of address
5. Sex
6. Time and date bloodtaken
7. Signature of person takingblood
4 Serious Hazards of Transfusion (SHOT) Steering Group (2016). The 2015 annual SHOT report.
M
https:// www.shotuk.org/ wp- content/ uploads/ SHOT- 2015- Annual- Report- Web- Edition- Final-
bookmarked.pdf; Annual SHOT report 2015 summary. M https:// www.shotuk.org/ wp- content/
uploads/ SHOT- Summary- Infographic_ Final.pdf.

BLOOD TRANSFUSION
303
• Ensure details on the form match those on the bottle.
• Complete the request form properly:
•
State what is required (e.g. 2U of packed cells,etc.).
•
Detail any previous transfusions, reactions, antibodies (if known).
•
Let the laboratory know when you want the blood or blood
product.
3 Adhesive patient labels are ne for forms but are not suitable for speci-
men bottles, and they are usually not accepted by transfusion laboratories.
Transfusion specimens should be labelled by hand— at the bedside.
The TACO checklist should be used to identify those at risk. Defer trans-
fusion, if safe, pending assessment or treatment of risk factor. Single unit
transfusion, followed by a review, is preferable.
The bedside checklist should be usedto:
•
Conrm +ve patient identication.
• Check identication of the component against the patient’s wristband.
• Check the prescription.
• Check the component.
• Check for specic requirements.
Further reading
Estcourt LJ, Birchall J, Allard S, etal.; British Committee for Standards in Haematology. Guidelines for
the use of platelet transfusions. Br J Haematol 2017; 176
:365– 94.
Hunt BJ, Allard S, Keeling D, Norfolk D, Stanworth SJ, Pendry K; British Committee for Standards in
Haematology. Apractical guideline for the haematological management of major haemorrhage.
Br J Haematol 2015; 170
:788– 803.
Joint United Kingdom (UK) Blood Transfusion and Tissue Transplantation Services Professional
Advisory Committee ( JPAC). M
http:// www.transfusionguidelines.org.uk/ .
McClelland B (2001). Handbook of transfusion medicine
, second edition. London:HMSO.
NHS Blood and Transplant. M http:// www.blood.co.uk/ .
Scottish National Blood Transfusion Service. M
http:// www.scotblood.co.uk/ .
If this sounds cumbersome and bureaucratic
Remember many people die annually because they are transfused with
the wrong blood
. In most cases, clerical error is to blame— people have
lled out bottles in advance and failed to check patient identity.

304
CHAPTER3 Haematology
304
3 Transfusion reactions
(See Table3.17.)
Rapid fever, chill, rigor, hyper- or hypotension, collapse, ushing, urticaria,
or respiratory distress at the start of transfusion indicate transfusion should
be stopped and resuscitation initiated (suggests a severe or life- threatening
acute transfusion reaction).
If the temperature rises to above 39°C or >2°C from baseline, with
other signs/ symptoms, consider bacterial contamination and monitor the
patient carefully. Investigate as appropriate.
•
If an isolated temperature of >38°C and a 1– 2°C rise with no
symptoms, or rash only, providing the patient is not acutely unwell,
continue the infusion. Fever often due to antibodies against WBCs (or
to cytokines in platelet packs).
3 Immediate transfusion reaction
Intravascular haemolysis (l haemoglobinaemia and haemoglobinuria).
Usually due to anti- A or anti- B antibodies (in ABO- mismatched transfu-
sion). Symptoms occur in minutes/ hours. May befatal.
Immediate transfusion reaction or bacterial contamination
ofblood
If predominantly extravascular, may only suer chills/ fever 1h after starting
transfusion— commonly due to anti- D. ARF is not a feature.
Mechanism
Complement (C3a, C4a, C5a) release into recipient plasma l smooth
muscle contraction. May develop DIC or oliguria (10% of cases) due to
profound hypotension.
Initial steps inmanagement ofacute transfusion reaction
• Stop blood transfusion immediately.
• Replace the giving set; keep the IV access open with 0.9% saline.
Table3.17 Transfusion reactions
Symptoms Signs
Patient restless/ agitated Fever
Flushing Hypotension
Anxiety Oozing from wounds or venepuncture sites
Chills Haemoglobinaemia
Nausea and vomiting Haemoglobinuria
Pain at venepuncture site
Abdominal, ank, or chest pain
Diarrhoea

305
TRANSFUSION REACTIONS
• Check the patient identity against the donorunit.
• Insert a urinary catheter and monitor the urine output.
• Give uids (IV colloids) to maintain urine output >1.5mL/ kg/ h.
• If urine output <1.5mL/ kg/ h, insert a CVP line and give a uid
challenge.
•
If urine output <1.5mL/ kg/ h and CVP adequate, give furosemide
80– 120mg.
• If urine output still <1.5mL/ kg/ h, consult senior medical sta for
advice.
•
Contact the blood transfusion laboratory before sending back the blood
pack and for advice on blood samples required for further investigation
(E Urgent investigations below).
Complications
•
Overall mortality710%.
Urgent investigations
Your local blood transfusion department will have specic guidelines to help
you with the management of an acute reaction. The following guide lists
the samples commonly required to establish the cause and severity of a
transfusion reaction (see Box 3.1). If you are uncertain about the labora-
tory procedure or management of a patient who appears to have suered
a severe reaction, you must notify your hospital’s haematology medical sta
who will provide advice.
3 Delays may threaten the patient’slife.
Febrile transfusion reactions
Seen in 0.5– 1.0% of blood transfusions. Mainly due to anti- HLA (human leu-
cocyte antigen) antibodies in recipient serum or granulocyte- specic anti-
bodies (e.g. sensitization during pregnancy or previous blood transfusion).
Box 3.1 Investigation oftransfusion reaction
1. Check the compatibility label of the blood unit matches with the
patient’s identity band, forms, and casenotes.
2. If mistake found, tell the blood bank urgently— the unit of blood
intended for your patient may be transfused to another patient.
3. Take bloodfor:
•
Haematology:
—FBC.
—DAT.
—PlasmaHb.
—Repeat cross- match sample.
—Coagulation screen.
•
Chemistry:U&E.
•
Microbiology:blood cultures.
4. Check urinalysis and monitor urine output.
5. Do ECG and check for evidence of i[K
+
].
6. Arrange repeat coagulation screens and biochemistry 2- to 4- hourly.

306
CHAPTER3 Haematology
306
Delayed transfusion reaction
Occurs in patients immunized through previous pregnancies or transfu-
sions. Antibody weak (so not detected at pre- transfusion stage). 2° immune
response occurs— antibody titrei.
Symptoms andsigns
•
Occur 7– 10days after blood transfusion.
• Fever, anaemia, and jaundice.
• ± haemoglobinuria.
Management
•
Discuss with the transfusion laboratorysta.
• Check DAT and repeat compatibilitytests.
• Transfuse the patient with freshly cross- matchedblood.
Further reading
Kardon EM (2016). Transfusion reactions in emergency medicine. M http:// www.emedicine.com/
emerg/ topic603.htm.

307
TRANSFUSION REACTIONS

308
CHAPTER3 Haematology
308
Bacterial contamination
ofblood products
Uncommon, but potentially fatal, adverse eect of blood transfusion
(aects red cells and blood products, e.g. platelet concentrates). Implicated
organisms include Gram −ve bacteria, including Pseudomonas, Yersinia, and
Flavobacterium.
Features
Include fever, skin ushing, rigors, abdominal pain, DIC, ARF, shock, and
possible cardiac arrest.
Management
As per Immediate transfusion reaction (E p. 304):
•
Stop transfusion.
• Urgent resuscitation.
• IV broad- spectrum antibiotics if bacterial contamination suspected.
Antiglobulintest
The old term is Coombs’ test. DAT detects antibodies or complement, or
both, on the RBC surface, and the indirect antiglobulin test (IAT) detects
the presence of antibodies in serum. Auseful investigation when investigat-
ing haemolytic anaemia.
•
Sample:EDTA.
Interpretation
• Positive DAT in mostAIHA.
• Lymphoproliferative disorders, e.g.CLL.
• Drug- induced haemolysis (e.g. methyldopa, levodopa).
•
Haemolytic disease of the fetus and newborn (HDFN), e.g. rhesus
(Rh)HDN.
Note: as with many tests in medicine, things are never entirely black or
white— a +ve DAT does not necessarily imply that haemolysis is actively
occurring and a −ve DAT does not exclude haemolysis.
Further reading
Coombs RRA. A new test for the detection of weak and ‘incomplete’ Rh agglutinins. Br J Exp Pathol
1945; 26
:255– 66.
Kelton JG. Impaired reticuloendothelial function in patients treated with methyldopa. N Engl J Med
1985; 313
:596– 600.

KLEIHAUERTEST
309
Kleihauertest
Uses
To determine whether fetal red cells have entered the maternal circulation
and, if so, the volume of such fetalcells.
Background
If an Rh (D)−ve mother has a baby that is Rh (D)+ve, she may develop
antibodies (maternal anti- D) against fetal red cells. This may result in fetal
red cell destruction termed rhesus haemolytic disease of the newborn, a seri-
ous haemolytic disorder that is seen less today due to a greater understand-
ing of the underlying mechanism and our ability to prevent it. Sensitization
to the fetal red cells occurs when fetal RBCs enter the maternal circulation,
e.g. at birth or through obstetric manipulations, e.g. amniocentesis, previous
pregnancies,etc.
Fetal RBCs in the mother’s circulation can be detected and quantied (in
mL) using the Kleihauer test, which exploits the resistance of fetal red cells
to acid elution (acid washes adult Hb out of the mother’s red cells, but fetal
RBCs contain HbF, which is not washed out). The Kleihauer test should be
performed on all Rh (D)−ve women who deliver an Rh (D)+ve infant.
Fetal cells appear as darkly staining cells against a background of ghosts
(these are the maternal red cells). An estimate of the required dose of anti-
D can be made from the number of fetal cells in a low- powereld.
• Sample:maternal peripheral bloodEDTA.
Calculating thevolume offetal red blood cells
inmaternal circulation
Basically, a calculation is made by the laboratory sta, based on the number
of fetal RBCs seen in the Kleihauer lm. The actual calculationis:
1800* × ratio of fetal/adult RBCs × 4/3 (correction factor)
For example, if there are 1% of fetal RBCs in maternal circulation:
1800 × 1/100 × 4/3 = 24mL
(* where 1800 is the maternal red cell volume)
A 4mL bleed (i.e. 4mL of fetal RBCs) requires 500IU of anti- D given IM
to the mother, with a further 250IU of anti- D for each additional mL of
fetalRBCs.
Do notpanic!
The laboratory carrying out the Kleihauer test will tell you the volume of fetal
RBCs detected, since they will count the cells and do the calculation for you.
If the total is >2mL of fetomaternal haemorrhage, the maternal sample is sent
for FMH conrmation by ow cytometry. After this, you will need to calculate
the dose of anti- D to give the mother, but if you are unsure, either discuss with
the haematology medical sta or contact your local transfusion centre.
Further reading
Chanarin I (ed.). Laboratory Haematology:An Account of Laboratory Techniques. Edinburgh:Churchill
Livingstone,1989.

310
CHAPTER3 Haematology
310
Erythropoietinassay
Epo is the hormone produced largely by the kidney that drives red cell
production. The typical anaemia found in renal disease is a result of failure
of Epo production. Epo assays are of value in renal medicine and haematol-
ogy. For example, in the assessment of polycythaemic states, an i Epo level
may be appropriate (e.g. in hypoxia where the body is attempting to i O
2
availability to tissues) or inappropriate (e.g. some tumours). The Epo assay
is carried out using an RIA method and is not available in all haematology
laboratories (may need to be sent to another hospital or laboratory).
•
Normal range:35– 25mU/ mL, steady- state level, no anaemia. May rise to
10,000mU/ mL in hypoxia or anaemia.
Causes ofi Epo (appropriate)
• Anaemias.
• High altitude.
• Hypoxia:
•
Lung disease.
•
Sleep apnoea syndromes.
• Cyanotic heart disease (e.g. right l left shunts).
•
High- anityHb.
• Cigarette smoking.
• Methaemoglobinaemia.
Causes ofi Epo (inappropriate)
• Renal disease:
•
Hypernephroma.
•
Nephroblastoma.
•
Post- renal transplant.
•
Renalcysts.
•
RAS.
• Hepatoma.
• Uterine broids.
• Cerebellar haemangioblastoma.
• Phaeochromocytoma.
Other causes ofiEpo
• Androgen therapy.
• Cushing’s disease.
• Hypertransfusion.
• Neonatal polycythaemia.
Causes ofdEpo
• Renal failure.
• Polycythaemiavera.
• RhA and other chronic inammatory diseases.
• Myeloma and other cancers.
Further reading
Cotes PM, Doré CJ, Yin JA, etal. The use of immunoreactive erythropoietin in the elucidation of
polycythemia. N Engl J Med 1986; 315
:283– 7.

IMMUNOHAEMATOLOGY
311
Immunohaematology
Immunohaematology is the study of the eects of the immune system on
the blood and its components. This includes red cells, white cells, platelets,
and coagulation proteins.
Tests forantiplatelet and antineutrophil antibodies
These tests are usually requested by the haematology department for
patients with either thrombocytopenia or neutropenia, respectively. These
assays are used to detect the presence of specic antibodies against platelet
or neutrophil antigens on the cell surface.
Antibodies may be alloantibodies (e.g. antibodies produced by the mother
against fetal antigens) or autoantibodies, which are antibodies produced by
the patient against his/ her own antigens (see Table3.18).
Antiplatelet antibodytests
Generally platelet immunouorescence tests (PIFTs) or monoclonal anti-
body immobilization of platelet antigens (MAIPA) are used. These are
useful for detecting even weak antibodies or where there are only a few
antigenic sites percell.
Elegant though these tests are, they are actually not useful in clinical prac-
tice for the diagnosis of neutropenia or thrombocytopenia where the cause
is autoimmune, since these are largely clinical diagnoses. (Platelet- associated
IgG or IgM may be high in autoimmune thrombocytopenia. However, it may
also be high in non- immune causes of thrombocytopenia.) Where these
tests are of value is in the neonatal setting where the neonate has low plate-
lets or neutrophils.
Table3.18 Disorders withneutrophil- specic allo- and autoantibodies
Disorders with neutrophil- specic alloantibodies
• Neonatal alloimmune neutropenia
• Febrile transfusion reactions (HLA antibodies)
• Transfusion- related acute lung injury (TRALI)
Disorders with neutrophil- specic autoantibodies
• ° autoimmune neutropenia
• 2°:
•
SLE
•
Evans’ syndrome (AIHA + d platelets)
•
Lymphoproliferative disorders (e.g.CLL)
•
Immune dysfunction (e.g. HIV, graft- versus- host disease)
Further reading
Roitt I. Essential Immunology, 10th edn. Oxford:Blackwell Science,2001.

312
CHAPTER3 Haematology
312
Immunophenotyping
This describes the identication and counting of cell types using powerful
MoAbs specic for cell surface proteins.
Uses
(See Table3.19.)
•
Diagnosis and classication of leukaemias and lymphomas.
• Assessment of cellular DNA content and synthetic activity.
• Determination of lymphocyte subsets, e.g. CD4
+
T cells in HIV
infection.
•
Assessment of clonality.
• Allows identication of prognostic groups.
• Monitoring of minimal residual disease (MRD, the lowest level of
malignancy that can be detected using standard techniques).
Terminology and methodology
Cell surface proteins are denoted according to their cluster dierentia-
tion (CD) number. Most cells will express many dierent proteins, and the
pattern of expression allows cellular characterization. MoAbs recognize
specic target antigens on cells. Using a panel of dierent antibodies, an
immunophenotypic prole of a sample is determined. Immunophenotyping
is used in conjunction with standard morphological analysis of blood and
marrow cells. The antibodies are labelled with uorescent markers, and
binding to cell proteins is detected by laser. For each analysis, thousands
of cells are assessed individually and rapidly. Some antibodies can detect
antigens insidecells.
•
Sample:heparin.
Table3.19 Uses ofimmunophenotyping
Surface immunophenotyping Leukaemias
Lymphomas
CD4:CD8 ratios in HIV infection
DNA content of tumours Ploidy
S phase analysis
Proliferation markers
TdT measurement In leukaemias and lymphomas
Bone marrow transplant/ stem cell
transplantation
Antiplatelet antibody detection
Reticulocyte counts and maturation
Apoptosis
Detection of small numbers of cells For example, fetal cells in mother’s
circulation, microorganisms in blood
Data from Provan D etal. Oxford Handbook of Clinical Haematology, 2nd edn, Oxford:Oxford
University Press,2004.

IMMUNOPHENOTYPING
313
Monoclonal antibodies
These are so- called because they are derived from single B lymphocyte cell
lines and have identical antigen- binding domains (idiotypes). It is easy to
generate large quantities of MoAbs for diagnosticuse.
•
Cell populations from, e.g. peripheral blood or BM samples are
incubated with a panel of MoAbs, e.g. anti- CD4, anti- CD34, which are
directly or indirectly bound to a uorescent marker antibody.
•
Sample is passed through a uorescence- activated cell sorter (FACS)
machine.
•
FACS instruments assign cells to a graphical plot by virtue of cell size
and granularity detected as forward and side light scatter by thelaser.
•
Allows subpopulations of cells, e.g. mononuclear cells, in blood samples
to be selected.
•
The reactivity of this cell subpopulation to the MoAb panel can then be
determined by uorescence for eachMoAb.
•
Atypical result for a CD4 T- lymphocyte population is shown:CD3,
CD4 +ve; CD8, CD13, CD34, CD19−ve.
Leukaemia diagnosis:common patterns (proles)
• AML:CD13+, CD33+, ± CD34, ± CD14+ve.
•
cALL:CD10 and TdT+ve.
•
T- ALL:cCD3, CD7, TdT+ve.
•
B- ALL:CD10, CD19, surface Ig+ve.
•
CLL:CD5, CD19, CD23, weak surface Ig+ve.
Clonality assessment
Particularly useful in determining whether there is a monoclonal B- cell or
plasma cell population.
2
Monoclonal B cells from, e.g. non- Hodgkin’s lymphoma (NHL) will
have surface expression of κ or λ light chains, but notboth.
2 Polyclonal B cells from, e.g. a patient with infectious mononucleosis will
have both κ and λ expression.
Further reading
Clinical Flow Wiki. M http:// wiki.clinicalow.com/ .
Johansson U, Bloxham D, Couzens S, et al.; British Committee for Standards in Haematology.
Guidelines on the use of multicolour ow cytometry in the diagnosis of haematological neo-
plasms. Br J Haematol 2014; 165
:455– 88.
University of Medicine and Dentistry of New Jersey. Immunophenotyping lymphomas. M http://
pleiad.umdnj.edu/ hemepath/ immuno/ immuno.html.

314
CHAPTER3 Haematology
314
Cytogenetics
Uses
• The study of chromosomes.
• Looks at the number of chromosomes in eachcell.
• Detects structural abnormalities between chromosomepairs.
Chromosome abnormalities may be constitutional (inherited) or acquired
later in life. Cytogenetic analysis of chromosome structure and number
has been used for many years for the study of disorders such as Down’s
syndrome. Acquired chromosomal abnormalities are found in malignancies,
especially haematological tumours. The analysis and detection of cytoge-
netic abnormalities is known as karyotyping. Because of the complexity of
this subject area, we will concentrate on two main areas where chromo-
some analysis is ofvalue.
•
Prenatal diagnosis of inherited disorders:
•
Detection of common aneuploidies (gain or loss of chromosomes).
•
Detection/ exclusion of known familial chromosome abnormalities.
• Detecting acquired chromosome abnormalitiesfor:
•
Diagnosis of leukaemia subtypes, e.g. t(15;17) characteristic of AML
M3 subtype.
•
Markers of prognostic information in a variety of diseases such as
leukaemias, e.g. t(9;22) in acute leukaemias, N- myc amplication in
neuroblastoma.
•
Monitoring response to treatment (in CML, the Philadelphia chromo-
some t(9;22) should disappear if the malignant cells are killed).
Principal indications forcytogenetic analysis are therefore
• Haematological malignancies at diagnosis (assuming the BM is
inltrated).
•
Inltrated solid tumour tissue at diagnosis.
• Patients with equivocal morphology (e.g. type of leukaemia not clear
using microscopy and other markers).
•
FISH analysis when required in certain treatment protocols, e.g.MRC.
• Conrmation of disease relapse.
• Accelerated phase or blast crisis inCML.
Cytogenetic assays are expensive (around £250 for a leukaemia or lym-
phoma karyotype), and if there is any doubt as to whether the test is indi-
cated, we would suggest you discuss the case with one of your seniors or
the cytogenetics sta. Arranging karyotyping before or during pregnancy
is generally carried out by the obstetrician in charge of the woman’scare.
(See Table3.20.)

CYTOGENETICS
315
Table3.20 Cytogenetic terminology
Constitutional Present at conception or arising during embryonic life
Acquired Arise later in fetal life or after birth
Translocation Exchange of material between chromosomes
Deletion
Loss of part of a chromosome
Duplication Part of a chromosome is gained
Inversion Part of a chromosome is rotated through 180°
Diploid 46 chromosomes (somatic cell)
Haploid 23 chromosomes (germinal cell, e.g. egg or sperm)
Trisomy Extra copy of a chromosome
Monosomy
Loss of a chromosome
Aneuploidy Loss or gain of certain chromosomes, e.g. monosomy or trisomy
316
CHAPTER3 Haematology
316
Cytogenetics:prenatal diagnosis
This allows both the detection of genetic diseases associated with specic
chromosomal abnormalities, thereby oering the possible prevention of an
aected child. With the advent of CVS in the rst trimester, karyotyping
can be done at an early stage of development (see Figs 3.18 and 3.19).
Pre- implantation genetic diagnosis allows abnormalities to be detected even
before implantation has occurred.
Cervix
Sample of sac
is withdrawn
Embry
o
Uterus
Fig.3.18 Diagram showing method of chorionic villus sampling.
1
678910 11 12
13 14 15 16 17 18
19 20 21 22 XY
5432
Fig.3.19 Normal karyotype showing metaphase chromosomes (22 autosomes
1– 22, and two sex chromosomes XX or XY, depending on the sex of the patient).
CYTOGENETICS:PRENATAL DIAGNOSIS
317
• Sample:amniotic uid (15– 16 weeks’ gestation).
Tests available
• AFPlevel.
•
Chromosome analysis.
• Biochemical tests, e.g. acetylcholinesterase.
• Sample:CVS (9– 12 weeks’ gestation).
Tests available
• DNA analysis.
• Chromosome analysis.
• Biochemistrytests.
Procedure (inbrief )
1. Cells are obtained using amniocentesis, CVS, or fetal blood sampling.
2. Cells are cultured in medium.
3. Cell division is arrested at metaphase using, e.g. colchicine.
4. Chromosomes are spread onto slides and stained.
5. Chromosomes are examined directly using light microscopy or with the
aid of a computerized image analysis system.
Chromosome anatomy
Note:the banding pattern helps identify individual chromosomes, along with
the position of the centromeres (the mitotic spindle attaches to these dur-
ing cell division), the short (p) and long (q) arms, and telomeres (chromo-
some ends). (See Fig.3.20.)
Further reading
East of Scotland Regional Genetics Service. Prenatal cytogenetics. M https:// humangenetics.org.uk/
prenatal- cytogenetics/ .
Rooney DE. Human Cytogenetics, Vol 2, 2nd edn. Oxford:Oxford University Press,2001.
University of Washington, Department of Pathology. Cytogenetics gallery. M
http:// www.pathology.
washington.edu/ galleries/ Cytogallery/ .
p (short) ar
m
Centromere
q (long) arm
Telomere
Fig.3.20 Chromosome anatomy:note short (p) arms and long (q)arms.

318
CHAPTER3 Haematology
318
Cytogenetics:haematological
malignancies
Uses
• Aids the diagnosis and classication of haematological malignancy.
• Assessment of clonality.
• Identication of prognostic groups.
• Monitoring response to therapy.
• Determining engraftment and chimerism post- allogeneic transplant.
Terminology
• Anormal somatic cell has 46 chromosomes:22 pairs, and XXorXY.
• Numbered 1– 22 in decreasing sizeorder.
• Two arms meet at the centromere— short arm denoted p, long armisq.
•
Usually only visible during condensation at metaphase.
• Stimulants and cell culture used— colchicine disrupts the spindle
apparatus, thereby arresting cells in metaphase.
•
Chromosomes are G- banded using Giemsa or Leishman’s stain to
create characteristic banding patterns along the chromosome. The
regions and bands are numbered, e.g. p1, q3,etc.
Common abnormalities
• Whole chromosome gain:e.g. trisomy 8(+8).
•
Whole chromosome loss:e.g. monosomy 7(−7).
•
Partial gain:e.g. add9q+, or partial loss, e.g. del5q−.
•
Translocation:material exchanged with another chromosome; usually
reciprocal, e.g. t(9;22)— the Philadelphia translocation.
• Inversion:part of chromosome runs in opposite direction, e.g. inv(16)
inM4Eo.
•
Many translocations involve breakpoints around known oncogenes,
e.g.
bcr, ras, myc,bcl- 2.
(See Table3.21.)
Molecular cytogenetics
• Molecular revolution is further rening the specic abnormalities in the
genesis of haematological malignancies.
•
Techniques such as FISH and PCR can detect cryptic abnormalities.
• Bcr– abl probes are now used in diagnosis and monitoring of treatment
response inCML.
• IgH and T- cell receptor (TCR) genes are useful in determining clonality
of suspected B- and T- cell tumours, respectively.
• Specic probes may be used in diagnosis and monitoring of subtypes of
acute leukaemia, e.g. AML, e.g. PML– RARA in AML M3, t(9;22), t(12;21),
and 11q23 rearrangements in paediatricALLs.

CYTOGENETICS:HAEMATOLOGICAL MALIGNANCIES
319
Further reading
Heim S, Mitelman F. Cancer Cytogenetics, 2nd edn. NewYork:Wisley- Liss,1995.
Kingston HM. ABC of Clinical Genetics, 2nd edn. London:BMJ Books,1994.
Sandberg AA. The Chromosomes in Human Cancer and Leukemia, 2nd edn. New York: Elsevier
Science,1990.
Table3.21 Karyotypic abnormalities inleukaemia and lymphoma
CML
t(9;22) Philadelphia chromosome translocation creates bcr
– abl chimeric
gene.
AML
t(8;21)
AML M2, involves AML– ETO gene— has better prognosis
t(15;17) AML M3 involves PML– RARA gene— has better prognosis
inv(16) AML M4Eo– – has better prognosis
−5, −7 Complex abnormalities have poor prognosis
MDS
−7, +8, +11 Poor prognosis
5q− syndrome Associated with refractory anaemia and better prognosis
Myeloproliferative disease
20q− and +8 Common associations
ALL
t(9;22) Philadelphia translocation, poor prognosis
t(4;11) Poor prognosis
Hyperdiploidy i
in total chromosome number— good prognosis
Hypodiploidy d in total chromosome number— bad prognosis
T- ALL
t(1;14) Involves tal- 1 oncogene
B- ALL and Burkitt’s lymphoma
t(8;14) Involves myc and IgH genes, poor prognosis
CLL
+12, t(11;14)
NHL
t(14;18) Follicular lymphoma, involves
bcl- 2 oncogene
t(11;14) Mantle cell lymphocytic lymphoma, involves
bcl- 1 oncogene
t(8;14) Burkitt’s lymphoma, involves myc and IgH genes

320
CHAPTER3 Haematology
320
Human leucocyte antigen
(tissue)typing
The HLA system or major histocompatibility complex (MHC) is the name
given to the highly polymorphic gene cluster region on chromosome 6,
which codes for cell surface proteins involved in immune recognition.
Uses
Tissue typing patients (to ensure compatibility between donor and recipient)
who are undergoing transplantation to reduce the likelihood of rejection or
graft- versus- host disease (GvHD) in the following types of transplant:
• Heart.
• Lung.
• Liver.
• Kidney.
• BM.
• Stemcells.
The gene complex is subdivided intotwo regions
Class1
•
The A, B, and Cloci.
• These proteins are found on most nucleated cells and interact with
CD8
+
T lymphocytes.
Class2
•
Comprises DR, DP, and DQ loci present only on B lymphocytes,
monocytes, macrophages, and activated T lymphocytes.
•
Interact with CD4
+
T lymphocytes.
•
Class 1 and 2 genes are closely linked, so one set of gene loci is
usually inherited from each parent, although there is a small amount of
cross- over.
• There is 725% chance of two siblings being HLA identical.
• There are other histocompatibility loci apart from the HLA system, but
these appear less important generally, except during HLA- matched stem
cell transplantation when even dierences in these minor systems may
causeGvHD.
Typing methods
Class 1 and 2 antigens were originally dened by serological reactivity with
maternal antisera containing pregnancy- induced HLA antibodies. There are
many problems with the technique and it is too insensitive to detect many
polymorphisms. Molecular techniques are increasingly employed, such as
single strand polymorphism (SSP). Molecular characterization is detecting
enormous class 2 polymorphisms.

HUMAN LEUCOCYTE ANTIGEN (TISSUE)TYPING
321
Importance ofHLAtyping
• Matching donor/ recipient pairs for renal, cardiac, and marrow stem cell
transplantation.
•
Degree of matching more critical for stem cell than solid organ
transplants.
•
Sibling HLA- matched stem cell transplantation is now the treatment of
choice for many malignancies.
•
Unrelated donor stem cell transplants are increasingly performed,
but outcome is poorer due to HLA disparity. As molecular matching
advances, improved accuracy will enable closer matches to be found and
results should improve.
Functional tests ofdonor/ recipient compatibility
• Mixed lymphocyte culture (MLC):now rarelyused.
•
Cytotoxic T- lymphocyte precursor (CTLp) assays:determine the frequency
of cytotoxic T lymphocytes in the donor directed against the recipient.
Provides an assessment of GvHD occurring.
HLA- related transfusionissues
• HLA on WBC and platelets may cause immunization in recipients of
blood and platelet transfusions.
•
May cause refractoriness and/ or febrile reactions to platelet
transfusions.
•
Leucodepletion of products by ltration prevents this (the National
Blood Service removes the WBCs at source routinely nowadays).
•
Diagnosis of refractoriness conrmed by detection of HLA or platelet-
specic antibodies in the patient’sserum.
•
Platelet transfusions matched to recipient HLA type may improve
increments.
Further reading
BSBMT. Interpreting HLA typing and matching. M http:// bsbmt.org/ registry- help- documents/ .
Klein J, Sato A. The HLA system. N Engl J Med 2000; 343:702– 9. M http:// content.nejm.org/ cgi/
content/ full/ 343/ 10/ 702?ijkey=oOilwRsUqacag.
Naik S. The human HLA system. J Indian Rheumatol Assoc 2003; 11:79– 83. M http:// medind.nic.in/
jaa/ t03/ i3/ jaat03i3p79.pdf.
322
CHAPTER3 Haematology
322
Southern blotting
This technique has been around since the mid 1970s. It explained much
about the physical structure of genes and was a major advance in the diag-
nosis of many single gene disorders. The method is simple and elegant, but
time- consuming. Not used as much today with the advent of PCR technol-
ogy. Southern blotting relies on the physical nature of DNA whereby single
strands are able to recognize and bind to their complementary sequences
(see Fig.3.21).
•
Sample:EDTA sample (heparin can be used, but beware inhibitory eect
on PCR amplication; if any chance PCR required, sendEDTA).
−
+
Digestion by restriction
enzyme, e.g. Mst II
(chops the DNA up)
Digested products
separated using
agarose gel
electrophoresis
(small fragments
move furthest)
Separated fragments
exposed to
radiolabelled probe,
e.g. for β globin gene
Transferred to nylon lter
which is then placed next to X-ra
y
lm. A black band is seen where
the probe has bound to the
patient’s DNA fragment
DNA from blood, marrow,
fetal cells, etc.
Fig.3.21 Southern blotting method.

SOUTHERN BLOTTING
323
Procedure
1. Genomic (i.e. total) DNA is extracted from WBC in EDTA blood
sample.
2. DNA is digested with bacterial restriction endonucleases (enzymes
cleave DNA at specic sequences— each enzyme recognizes a dierent
DNA sequence).
3. After digestion of the DNA, the fragments are separated on the basis
of size using agarose gel electrophoresis (the smallest fragments travel
the farthest).
4. The fragments are transferred to a nylon membrane and xed
permanently to the membrane using ultraviolet (UV)light.
5. Membranes are ‘probed’ using specic (known) gene probes that are
radioactively labelled using
32
P.
6. The location of specic binding is detected by placing the membrane
next to a radiographic lm (standard X- raylm).
7. The lm is developed using standard techniques, and the
autoradiograph generated will show bands corresponding to the
position of binding of the labelledprobe.
8. Fragment sizes are calculated, and the presence or absence of
mutations is worked out by determining whether enzyme cutting sites
have been lost through mutation.
Applications
• Historically, many diseases caused by single base changes (loss of
restriction enzyme cutting site) have been diagnosed using Southern
blotting.
•
Globin gene disorders:
• Sickle- cell anaemia (mutation in β- globingene).
• Thalassaemia (mutations or deletions in α- or β- globin genes).
• Clotting disorders:
• Haemophilia.
• Analysis of Ig or TCR genes to detect clones of cells in suspected
leukaemia or lymphoma.
•
Detection of chromosomal translocations in leukaemia and lymphoma
(e.g. t(9;22) in CML, t(14;18) in follicular lymphoma).
Further reading
Kimball’s Biology Pages. Gel blotting. M http:// www.biology- pages.info/ G/ GelBlotting.html.
Southern EM. Detection of specic sequences among DNA fragments separated by gel electro-
phoresis. J Mol Biol 1975; 98
:503– 17 (3 Southern’s classic paper and probably the most cited
molecular biology paperever).
Thermo Fisher Scientic. Southern blotting. M
https:// www.thermosher.com/ uk/ en/ home/ life-
science/ dna- rna- purication- analysis/ nucleic- acid- gel- electrophoresis/ southern- blotting.html#.

324
CHAPTER3 Haematology
324
Polymerase chain reaction
amplificationofDNA
The ability to use an enzyme to amplify specic DNA sequences has revo-
lutionized modern diagnostic pathology. Whereas Southern blotting might
take up to 1 week to produce a result, PCR can do the same thing in 2– 3h!
PCR is now in routine use in the analysis of oncogenes, haematological
malignancies, general medicine, infectious disease, and many other spe-
cialties. Because the system amplies the starting DNA up to a million-
fold, there need only be one cell as starting material; in practice, much
more DNA is required, but because of the extreme sensitivity of the
technique, PCR has been used in forensic medicine where there may be
only a few cells available for analysis (e.g. blood or semen stain).
(See Fig.3.22.)
Advantages
• Requires very littleDNA.
• DNA quality does not matter (can be highly degraded, e.g. with age and
still be amplied— DNA from Egyptian mummies has been amplied).
• Rapid results.
Disadvantages
• Expensive, but less so than it usedtobe.
• DNA sequence of the gene of interest must be known in order to
design the short PCR primers (oligos). With the near completion of the
Human Genome Project, this is less of a problemnow.
•
Highly sensitive, and contamination of samples may occur (DNA
fragments oat through the air constantly; if these drop into the reaction
tube, a false +ve result may be obtained).
Band on gel
corresponding to
PCR product
(positive result)
Patient 1 2 Control
Base pair ladder
(lets you calculat
e
size of PCR band
)
bp
−603
−310
−194
−118
Origin
Fig.3.22 Detection of residual leukaemia using PCR. Patients 1 and 2 have undergone
chemotherapy, but as can be seen (arrow), there is still some leukaemia- specic DNA
sequence present, i.e. they have minimal residual disease.
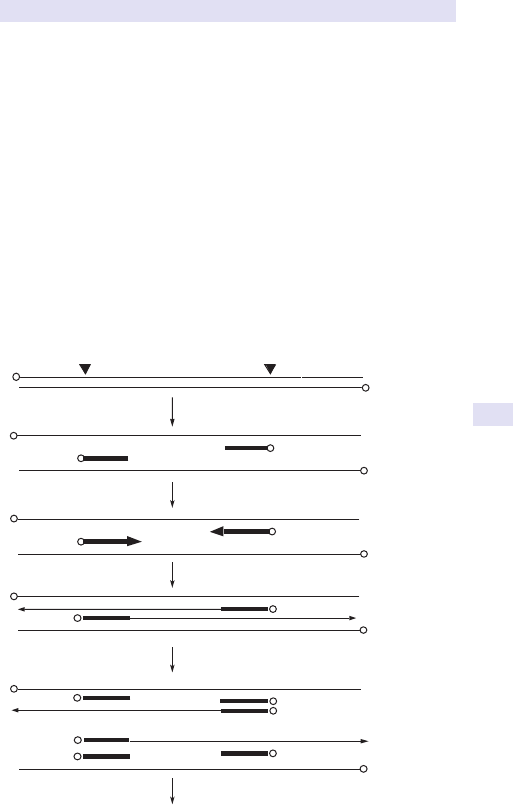
POLYMERASE CHAIN REACTION AMPLIFICATIONOFDNA
325
Procedure (inbrief )
(See Fig.3.23.)
•
Two short DNA primers on either side of the gene of interest bind to
the fragment of interest.
•
The region between the primers is lled in using a heat- stable DNA
polymerase (Taq polymerase).
•
After a single round of amplication has been performed, the whole
process is repeated.
•
This takes place 30 times (i.e. through 30 cycles of amplication),
leading to a million- fold i in the amount of specic sequence.
•
After the 30 cycles are complete, a sample of the PCR reaction is run
on agarose gel and bands are visualized.
•
Information about the presence or absence of the region or mutation of
interest is obtained by assessing the size and number of dierent PCR
products obtained after 30 cycles of amplication.
Target sequence
Unamplied DNA
Denature and anneal primers
Start primer extension
Complete primer extension
Denature and anneal primers
30 or 40 cycles total
Cycle 1
Cycle 2
I I I I I I
I I I I I I
I I I I I I
I I I I I I
I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I
I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I
I I I I I I
I I I I I I
I I I I I I
I I I I I I
I I I I I I I I I I I I I I I I I I I I I I I I I I I
Fig.3.23 PCR amplication method.

326
CHAPTER3 Haematology
326
Applications
• PCR is currently used to amplify Ig genes, HIV loci, TB genes, and many
other targets that are of use in molecular medicine (cystic brosis,
haemophilia, thalassaemia, sickle- cell disease, and many others).
• PCR can be used to quantitate messenger ribonucleic acid (mRNA)
species in blood samples and tissue samples. Allows gene ‘activity’ to be
measured.
Further reading
Nobelprize.org. The PCR method—a DNA copying machine. M http:// nobelprize.org/ chemistry/
educational/ pcr/ .
Principles of the PCR. M
http:// users.ugent.be/ ~avierstr/ principles/ pcr.html.
Saiki RK. Primer- directed enzymatic amplication of DNA with a thermostable DNA polymerase.
Science 1988; 239:487– 91 (classic PCR method paper).

POLYMERASE CHAIN REACTION AMPLIFICATIONOFDNA
327

328
CHAPTER3 Haematology
328
In situ hybridization and fluorescence
insitu hybridization
Like PCR and other techniques, in situ hybridization and FISH are conceptu-
ally simple techniques that rely on the ability of a DNA probe to ‘nd’ its
counterpart on a chromosome and bind, and if a uorescent tag is present,
it will light up the region of binding (this modication is termed uorescence
in situ hybridization, or FISH). These techniques have evolved from standard
cytogenetic analysis of metaphase chromosomes in which metaphase chro-
mosomes were prepared on glass slides to which specic labelled probes
were applied.
•
Sample:discuss with your local cytogenetics or haematology laboratory
(they will have specialized medium for maintaining cells from blood
or marrow, so that they will divide and be suitable for hybridization
studies).
In situ hybridization
The location of binding of the probe is detected by visualizing the signal pro-
duced after coating microscope slides with photographic emulsion, which
generates a black area around the probe which is labelled with
32
P.
FISH
A further modication based on the original principles, whereby specic
gene probes are hybridized to chromosomes without the need for meta-
phase preparations (interphase cells can be used). Instead of
32
P, the probes
are labelled with a uorescent dye and hybridization may be detected as
red, blue, or other coloured dots over the cells (see Fig.3.24).
Applications ofFISH
• Used in the analysis of trisomies (chromosome gains) and monosomies
(chromosome losses) associated with leukaemias and lymphomas. The
presence of trisomy is detected as three uorescent dots within the cell,
whilst monosomy is seen as a single uorescent dot within thecell.
•
FISH has been used widely within paediatric leukaemias, such as ALL,
where abnormalities of chromosome number are common.
denature
Probe
Slide containing cells
l
hybridize
visualize
l
Fig.3.24 FISH analysis. Metaphase chromosomes are placed on a microscope slide,
and the probe (e.g. for the gene of interest) is applied. The chromosome region to
which the probe binds will uoresce— highlighting its exact location in the genome.

329
IN SITU HYBRIDIZATION & FISH
Further reading
Mathew P, Sanger WG, Weisenburger DD, etal. Detection of the t(2;5)(p23;q35) and NPM- ALK
fusion in non- Hodgkin’s lymphoma by two- color uorescence in situ hybridization. Blood 1997;
89
, 1678– 85.
O’Connor C. Fluorescence in situ hybridization (FISH). Nature Education 2008; 1:171. M https://
www.nature.com/ scitable/ topicpage/ Fluorescence- In- Situ- Hybridization- FISH- 327.
Sinclair PB, Green AR, Grace C, Nacheva EP. Improved sensitivity of BCR- ABL detection:a triple-
probe three- color uorescence in situ hybridization system. Blood 1997; 90:1395– 402.
Vaandrager JW. Direct visualization of dispersed 11q13 chromosomal translocations in mantle cell
lymphoma by multicolor DNA ber uorescence in situ hybridization. Blood 1996; 88
:1177– 82.

330
CHAPTER3 Haematology
330
Specialized haematologyassays
The following laboratories provide specialized molecular, biochemical, and
cellular investigations for rare haematological disorders. Please contact the
laboratory before tests are requested to conrm the specimen(s) required.
Thalassaemia disorders
Dr JohnOld
National Haemoglobinopathy Reference Laboratory, Institute of Molecular
Medicine, John Radclie Hospital, Headington, Oxford OX38DU
Tel:01865- 222449; Fax:01865- 222500
E- mail:jold@hammer.imm.ox.ac.uk
Professor Swee LayThein
Haematological Medicine, King’s College Hospital, Denmark Hill, London
SES9RS
Tel:020- 7346- 1682; Fax:020- 7346- 6168
E- mail:[email protected]
Dr MaryPetrou
Perinatal Centre, University College Hospital, 84– 86 Chenies Mews,
London WC1E6HX
Tel:020- 7388- 9246; Fax:020- 7380- 9864
E- mail:[email protected]
Haemoglobin variants, unstable, and altered anity
haemoglobins
Dr JohnOld
National Haemoglobinopathy Reference Laboratory, Institute of Molecular
Medicine, John Radclie Hospital, Headington, Oxford OX38DU
Tel:01865- 222449; Fax:01865- 222500
E- mail:jold@hammer.imm.ox.ac.uk
Professor Sally Davies and Joan Henthorn
Department of Haematology, Central Middlesex Hospital, Acton Lane,
London NW107NS
Tel:020- 8453- 2112; Fax:020- 8965- 1115
E- mail:sally[email protected].gov.uk
Professor Joan Henthorn
Department of Haematology, Central Middlesex Hospital, Acton Lane,
London NW107NS
Tel:020- 8453- 2323

SPECIALIZED HAEMATOLOGYASSAYS
331
Dr BarbaraWild
Haematological Medicine, King’s College Hospital, Denmark Hill, London
SE59RS
Tel:020- 7737- 4000 Ext 2283; Fax:020- 7346- 3514
E- mail:[email protected]
Glycolytic defects, G6PD deciency, other
erythroenzymopathies
Dr MarkLayton
Haematology, ICSTM, Hammersmith Hospital, London W120HS
Tel:020- 8383- 2173; Fax:020- 8742- 9335
E- mail:[email protected]
Dr BarbaraWild
Haematological Medicine, King’s College Hospital, Denmark Hill, London
SE59RS
Tel:020- 7737- 4000 Ext 2283; Fax:020- 7346- 3514
E- mail:[email protected]
Porphyrias
Dr AllanDeacon
Clinical Biochemistry, King’s College Hospital, Denmark Hill, London
SE59RS
Tel:020- 7346- 3856; Fax:020- 737- 7434
Dr Michael Badminton
Porphyria Service, Medical Biochemistry, University Hospital of Wales,
Cardi CF144XW
Tel:02920- 748349; Fax:02920- 748383
E- mail:badminton.mn@cardi.ac.uk
Ms J Woolf/ Dr S Whatley
Porphyria Service, Medical Biochemistry, University Hospital of Wales,
Cardi CF144XW
Tel:02920- 743565
Red cell membrane defects
Dr May- JeanKing
International Blood Group Reference Laboratory, Southmead Road, Bristol
BS105ND
Tel:0117- 991- 2111; Fax:0117- 959- 1660
E- mail:may- [email protected].uk

332

333
Chapter4
Immunology and allergy
Serum immunoglobulins 334
Immunoglobulin G subclasses 336
Evaluation of specic antibody production (antibacterial and
antiviral antibodies) 338
ImmunoglobulinD 340
Electrophoresis and immunoxation 342
Serum free light chains 344
‘Bence– Jones proteins’; urine electrophoresis and
immunoxation 344
Cryoglobulins 345
β
2
- microglobulin 346
Acute phase proteins (CRP, ESR, SAA) 348
Amyloidosis 350
Measurement of serum complement components 351
C1 esterase inhibitor 352
Haemolytic complement (lytic complement function tests) 353
Factor H and factorI 354
C3 nephritic factor 354
Autoimmunity 354
Rheumatoid factor 355
Autoantibody screen 356
Double- stranded DNA antibodies 358
Antibodies to extractable nuclear antigens (ENA) 360
Antiphospholipid antibodies 362
Other patterns of autoantibodies identied on ‘autoantibody
screen’ 363
Autoantibodies in autoimmune hepatitis 364
Antibodies to gastric parietal cells and intrinsic factor 365
Thyroid disease (thyroid peroxidase antibodies) 366
Islet cell antibodies (ICA), anti- GAD antibodies, and insulin
receptor and insulin antibodies 367
Adrenal antibodies and other endocrine autoantibodies 368
Classication of autoimmune polyglandular syndromes 368
Endomysial (EMA) and tissue transglutaminase (tTG) antibodies 369
Autoimmune skin diseases 370
Autoantibodies and neurological disease 371
Vasculitic syndromes 372
Allergic disease 374
Skin prick tests 375
Specic IgE:‘RAST tests’ 376
Mast cell tryptase 377
Drug allergy testing 377
Cellular function assays 378
Lymphocyte function tests 380
Neutrophil and macrophage function 380
Natural killer cell function 380

334
CHAPTER4 Immunology and allergy
334
Serum immunoglobulins
Units:g/ L.
Normal range (adultsonly):
•
IgG:5.8– 15.4g/ L.
• IgA:0.64– 2.97g/ L.
• IgM (♂):0.24– 1.90g/ L.
• IgM (♀):0.75– 2.30g/ L.
Principles ofassay
The three main classes of Igs are measured by either rate nephelometry or
turbidimetry on automated analysers. The principles are similar, depend-
ent on immune complex formation, using antisera specic for the class of
antibody. Rarely radial immunodiusion (RID) may be used; this is slow
and less accurate. For automated analysers, coecients of variation should
be in the range of 5– 10%. Results are standardized against international
standards. In the UK, an external quality assurance (EQA) scheme operates.
Laboratories should provide normal ranges, which vary according to age
and sex. Unfortunately many laboratories do not adjust ranges for age and
sex, which may lead to confusion.
Indications fortesting
Measurement ofserum immunoglobulin is indicated inthe following conditions
•
Suspected immunodeciency (° or 2°); diagnosis and monitoring.
• Suspected myeloma, plasmacytoma; diagnosis and monitoring.
• Lymphoma.
• Liver disease (PBC, hepatitis, cirrhosis).
• Sarcoidosis; diagnosis.
• Post- BM/ stem cell transplantation; monitoring.
Interpretation
Measurement of serum Igs does not provide categorical diagnosis in any
disease. Normal serum Igs do NOT exclude immunodeciency. In all cases,
measurement of Igs MUST be accompanied by serum electrophoresis and
immunoxation to look for paraproteins (E Electrophoresis and immuno-
xation, pp. 342–3).
Causes ofhypogammaglobulinaemia
• X- linked agammaglobulinaemia (XLA) (absent B cells; all Igs low/
absent).
•
Common variable immunodeciency (CVID) (reduced T/ B cells;
lowIgs).
•
Hyper- IgM syndrome (normal/ raised IgM; low/ absent IgG,IgA).
• Selective IgA deciency (absent IgA; normal IgG,IgM).
• Severe combined immunodeciency (SCID) (mainly children; all Igs low;
absent T cells).
•
Lymphoma (reduced IgM; IgA normal; IgG normal or low; disease,
chemotherapy, or radiotherapy).
•
SLE (rare).

SERUM IMMUNOGLOBULINS
335
• Infections:
•
HIV (rare).
•
Herpesviruses (rare, EBV in X- linked lymphoproliferative disease).
•
Acute bacterial infections.
•
Measles/ rubella.
• Drugs (immunosuppressants, e.g. cyclophosphamide, azathioprine,
chemotherapy).
•
Plasmapheresis.
• Renal loss (IgM normal; IgG and IgA reduced).
• GI loss (IgM normal; IgG and IgA reduced).
Causes ofhypergammaglobulinaemia
• Chronic infection— all Igs raised:
•
Osteomyelitis.
•
Bacterial endocarditis.
•
TB.
• Chronic inammation:
•
SLE, RA— all Igs elevated.
•
Sjögren’s syndrome— raised IgG (allIgG
1
).
•
Sarcoidosis— raised IgG and IgA; IgM usually normal.
• Liver disease:
•
PBC— IgM, may be very high (>30g/ L) with small monoclonal bands
on a polyclonally raised background.
•
Alcohol- related— i IgA, polyclonal, β– γ bridging on electrophoresis.
•
Autoimmune hepatitis (i IgG, IgA; normalIgM).
•
Hodgkin’s disease— IgE raised (also eosinophilia).
• Viral infections:
•
Acute common viral infections— raised IgM, normal IgG andIgA
•
HIV— all Igs raised (IgG very high, but polyclonal).
•
EBV— all raised.
Criticalaction
ALL patients with recurrent infections* should be reviewed by an immu-
nologist or a paediatric immunologist, according to age, irrespective of age.
Any patient with recurrent infections and low serum Igs has an immunologi-
cal problem until proven otherwise.
Note:(*) recurrent infections can be pragmatically dened as two or more
major microbiologically/ virologically proven infections, requiring hospitali-
zation, within 1year. One major infection and recurrent minor infections
should also be referred where minor infections are documented infections
requiring treatment in the community.
Patients with unusual infections or with illness caused by opportunistic or
normally non- pathogenic organisms and patients with infections in unusual
sites (without good reason) should all be referred for further investigation.

336
CHAPTER4 Immunology and allergy
336
Immunoglobulin G subclasses
Units:g/ L.
Normal range (adults):
•
IgG
1
:2.2– 10.8g/ L.
• IgG
2
:0.5– 8.0g/ L.
• IgG
3
:0.05– 0.9g/ L.
• IgG
4
:0.0– 2.4g/ L.
Principles oftest
As for serum Igs, IgG subclasses are normally measured by nephelometry
or turbidimetry. RID is still occasionallyused.
Indications fortesting
There are no absolute indications for testing, as signicant immunode-
ciency can occur in the presence of normal subclasses, and conversely
complete genetic absence of a subclass may be completely asymptomatic.
Measurement is usually performed as part of the work- up of patients with
recurrent infections. IgG
4
disease has been described recently; this is associ-
ated with a wide range of organ- based diseases. IgG
4
levels are signicantly
raised.
Interpretation
Low levels may be signicant in the context of presentation with recurrent
infections. Deciency of IgG
3
, which is involved in immunity against viruses
and associated with asthma and intractable epilepsy. IgG
2
deciency may
be seen in patients with IgA deciency and may be associated with poor
responses to polysaccharide antigens such as the capsular polysaccharides
of bacteria.
Raised IgG
1
with normal or reduced IgG
2
, IgG
3
, and IgG
4
is seen in
Sjögren’s syndrome and is a specic pattern, which may occasionally be
helpful in diagnosis.
Raised IgG
4
suggests IgG
4
disease.

IMMUNOGLOBULIN G SUBCLASSES
337

338
CHAPTER4 Immunology and allergy
338
Evaluation ofspecific antibody
production (antibacterial and antiviral
antibodies)
Units:variable:u/ L, IU/ mL,μg/ mL.
Ranges:variable, check with reporting laboratory.
•
Pneumococcal antibodies:>20u/ L (asplenics>35).
• Tetanus antibodies:>0.1IU/ mL (minimum protective level).
• Haemophilus inuenzae type B:>1.0μg/ mL (full protection;
asplenics>1.5).
Principles oftest
Measurement of antibody production against dened pathogens or anti-
gens puried from pathogens plays an important role in the investigation of
suspected immunodeciency. Most assays are carried out by enzyme- linked
immunoassay, but some viral antibodies are still measured by haemaggluti-
nation or complement xation. Pre- and post- immunization samples should
be run on the same run for direct comparison, as coecients of variation
for the assays tend to be high— 15– 25%! An EQA scheme exists. Assays
have tended to focus on agents for which there are safe and eective vac-
cines. Live vaccines should NEVER be given to any patient in whom immunode-
ciency is suspected.
Antibodies normally run in immunology laboratories include pneumo-
coccal polysaccharides, which may be further dierentiated as IgG
1
and
IgG
2
, H. inuenzae type B (Hib), and tetanus. Diphtheria antibodies are
not run by many laboratories, as the assay performance has been so poor.
Meningococcal C polysaccharide antibodies are run by a few specialized
laboratories, but correspondence with known clinical status has been poor.
Anti- streptolysin O titre (ASOT) may be helpful.
Antibodies to pneumococcal serotypes are available from reference labo-
ratories and may be valuable in assessing response to the conjugated pneu-
mococcal serotype vaccine. There is debate about the protetctive levels
(0.2– 0.35).
Viral antibodies may be valuable to natural exposure and immunization
antigens such as polio, measles, mumps, rubella, chickenpox, EBV, and hepa-
titis B (if immunized).
Indications fortesting
These assays should be used in the work- up of patients with suspected
immunodeciency or in monitoring change in such patients. Responsiveness
to immunization is a helpful marker of immunological recovery post- bone
marrow transplant (BMT). Annual monitoring of levels may be valuable in
asplenic patients, as such patients lose immunity more rapidly than a eus-
plenic population.
Interpretation
The interpretation is entirely dependent on the context. Assays for pneu-
mococcal polysaccharides measure a composite of responses to the 23
strains in the Pneumovax
®
vaccine. This can be misleading as not all strains

339
EVALUATION OF SPECIFIC ANTIBODY PRODUCTION
represented in the vaccine are equipotent as immunostimulators. This
means that a ‘normal’ response may actually mean a good response to the
immunogenic strains, masking failure of response to the less immunogenic
strains. For this reason, evaluation of such patients should be carried out by
an immunologist with an interest in immunodeciency. More weight should
be placed on change in response to immunization than to actual values.
A ‘normal’ response to immunization has never been standardized, with
publications frequently using dierent criteria, rendering comparison impos-
sible. The following is a useful working denition:‘a 4- fold rise in titre, which
rises to well within the normal range’.
Antibody deciency inadults
This usually presents with upper and lower respiratory tract infections
(Streptococcus pneumoniae, Haemophilus, Staphylococcus, Klebsiella), leading
to chronic bronchiectasis and sinusitis; GI infections (Salmonella, Giardia,
Campylobacter); skin infections (recurrent boils); autoimmune features (ITP,
haemolytic anaemia, diabetes, thyroid disease) only in CVID, not XLA. i
incidence of lymphoma.
Normal serum Igs DO NOT EXCLUDE antibody deciency (IgG subclass
deciency, specic failure of antibody production against polysaccharides).
Use test immunization with killed or puried component vaccines to test
humoral responses.
Asplenia
Patients are at i risk of overwhelming sepsis:S.pneumoniae, H.ineunzae,
Staphylococcus aureus, Meningococcus, Klebsiella species, Capnocytophaga
canimorsus (from dog bites), fulminant malaria, and babesiosis. Risk is life-
long. Asplenia may result from trauma, involvement in malignancy, removal
for diagnosis (rare these days), coeliac disease, and sickle disease and rarely
due to congenital absence. Serum Igs and IgG subclasses are normal, but
responses to polysaccharide antigens are often poor, especially in patients
with lymphoma. Blood lm will show Howell– Jolly bodies. Absence (if not
known from records) will be shown by ultrasound(US).

340
CHAPTER4 Immunology and allergy
340
ImmunoglobulinD
IgD is rarely measured in clinical practice, as its main function is as a mem-
brane receptor. Elevated levels may be seen in periodic fever syndrome, in
hyper- IgD syndrome, due to deciency of mevalonate kinase, and in IgD-
secreting myeloma. Measurement is usually byRID.

IMMUNOGLOBULIND
341

342
CHAPTER4 Immunology and allergy
342
Electrophoresis and immunofixation
Units: not applicable to electrophoresis (qualitative). Paraprotein quanti-
tated by scanning densitometry reported ing/ L.
Normal range:N/ A
Principles oftesting
In serum or urinary electrophoresis, the relevant body uid is applied to an
electrolyte- containing agarose gel. Acurrent is applied across the gel and
causes the proteins to migrate through the gel on the basis of their charge
and, to a lesser extent, their size until they reach a neutral point in the
electric eld. The proteins are then visualized with a protein- binding stain.
If the total protein is known, then the electrophoretic strip can be scanned
and the absorption by the stain measured, which will be proportional to the
amount of protein in the particular region in the gel. Thus, any monoclonal
bands can be directly measured. This is useful for patients with myeloma,
as immunochemical methods for measurement of Igs may be inaccurate in
patients with myeloma.
Immunoxation is the technique by which monoclonal Igs are identied
by overlaying the electrophoresed strips with antisera against heavy and
light chains. These precipitate with the monoclonal proteins in the gel, and
unbound antisera can be washed free prior to staining.
The same techniques can be carried out with urine, although this may
require concentration to provide the clearest results.
Indications fortesting
Electrophoresis and, if necessary, immunoxation of serum is an integral
part of measurement of serum Igs. ALL requests for serum Igs must have
electrophoresis carried out; failure to do so will lead to important abnor-
malities being missed. There is no place for carrying out electrophoresis as
a stand- alonetest.

ELECTROPHORESIS AND IMMUNOFIXATION
343
Interpretation
See Table 4.1 for interpretations of results.
Monoclonal proteins may polymerize to give >1 band. Some patients will
have >1 clone present producing dierentIgs.
Densitometry cannot be used where the monoclonal protein overlies the
β
- region, as the gures include non- Ig proteins.
Table4.1 Interpretation
Report Interpretation
Reduced albumin Chronic inammation, nephritic syndrome
Absent α1 band
Absent/ reduced α1- antitrypsin
i α2 band Chronic inammation/ infection; also seen
in nephrotic syndrome, due to selective
retention of α
2- macroglobulin
i β Seen in pregnancy (raised β- lipoprotein) and
iron deciency (transferrin)
β
– γ bridging Caused by raised polyclonal IgA, e.g. cirrhosis
i γ Caused by polyclonal i
in IgG:infection/
inammation
Faint band(s) on polyclonal
background
Caused by monoclonal escape during
polyclonal response to infection/
inammation (does NOT indicate myeloma)
Monoclonal band in γ Due to myeloma, lymphoma, and MGUS*
Absent/ reduced γ Due to inherited or acquired Ig deciency
* MGUS, monoclonal gammopathy of uncertain signicance— most evolve to myeloma, given
time (years).

344
CHAPTER4 Immunology and allergy
344
Serum free lightchains
Modications in the techniques for measurement of free, as opposed to
bound, light chains are valuable in monitoring light chain- only myelomas
and other myelomas that produce excess free light chains, in addition to
a whole paraprotein, which would previously have been monitored by
measurement of 24h urinary light chain excretion. Urinary measurement
can be problematic where there is renal impairment, and as light chains
are nephrotoxic, as the disease advances, urinary measurements become
less accurate.
‘Bence– Jones proteins’; urine
electrophoresis and immunofixation
Bence– Jones proteins are urinary free light chains, i.e. unbound to heavy
chains. During normal antibody synthesis, a small excess of light chains are
produced which are excreted. Hypergammaglobulinaemic states, such as
RhA and chronic infection, may therefore be accompanied by excretion of
polyclonal free light chains. Monoclonal free light chains are seen in mye-
loma and may be the only marker in light chain- only myelomas, which do
not produce any heavy chains atall.
Testing is carried out as forserum.

CRYOGLOBULINS
345
Cryoglobulins
Units:usually reported qualitatively, but a ‘cryocrit’ can be measured in a
similar way to a manual Hct using capillarytubes.
Normal range: tiny amounts of cryoglobulins may be found in normal
individuals.
Principles oftest
Cryoglobulins are Igs that precipitate when serum is cooled. The temper-
ature at which this occurs determines whether disease will result. If the
blood circulates through a part of the body where the temperature is below
the critical temperature, then the protein will precipitate in the capillaries,
causing obstruction, vascular damage, and eventually necrosis. The tem-
perature of the hand is 728°C at ambient room temperature. To check for
the presence of cryoglobulins, take blood using a warmed syringe into a
warmed bottle and transport to the laboratory at 37°C, using a Thermos
™
ask with either pre- warmed sand or water at 37°C. The laboratory will
allow the blood to clot at 37°C and then cool the serum. Cryoglobulins will
form a precipitate as the temperature drops. The precipitate is then washed
and re- dissolved for analysis by electrophoresis and immunoxation.
Cryoglobulins are not the same as cold agglutinins (a feature of
Mycoplasma pneumoniae infection) (E Chapter3).
Indications fortesting
All patients with Raynaud’s phenomenon of new onset or with winter
onset of purpuric or vasculitic lesions on the extremities should be tested.
Chronic hepatitis C infection is often accompanied by type II cryoglobuli-
naemia and a characteristic syndrome— ‘mixed essential cryoglobulinae-
mia’ = autoimmune phenomena, arthritis, ulceration, glomerulonephritis,
neuropathy. C3 normal; C4 reduced. Patients with myeloma, SLE, Sjögren’s
syndrome, and RhA are also atrisk.
Interpretation
Interpretation of the results of testing cryoglobulins is provided in Table4.2.
Cryobrinogen
This is found less commonly than cryoglobulins. It will not be detected
unless both EDTA and heparinized blood samples are sent warm to the
laboratory. The main association is with occult malignancy (and throm-
bophlebitis migrans). Also associated with connective tissue disease,
pregnancy, OCP use, DM, and cold urticaria.
Table4.2 Interpretation
Type Nature of cryoprecipitate
Type I All monoclonal Igs; myeloma, lymphoma
Type II Monoclonal Ig with RF activity; myeloma, lymphoma, connective
tissue diseases, infections (especially HCV; SBE)
Type III Polyclonal RF:connective tissue diseases, infections

346
CHAPTER4 Immunology and allergy
346
β
2
- microglobulin
Unit:mg/ L.
Normal range: 1– 3mg/ L.
Principles oftest
The test measures free β
2
- microglobulin, which normally forms the light
chain of HLA class I molecules but is shed when there is i lymphocyte
turnover. It is usually rapidly cleared by the kidneys. Measurement is usually
by an automated analyser, nephelometry, or turbidimetry. RID is stillused.
Indications fortesting
The main indication is as part of routine monitoring of patients with mye-
loma and HIV. Other biomarkers are now felt to be more useful. Very high
levels are seen in renal failure and patients on dialysis, which can cause β
2
-
microglobulin amyloid.
Interpretation
Levels elevatedin:
•
HIV infection (surrogate marker of progression).
• Myeloma (marker of tumourmass).
• Lymphoma.
• CVID (correlation with severity).
• Renal dialysis (depending on type of membrane).

347
β
2
- MICROGLOBULIN
348
CHAPTER4 Immunology and allergy
348
Acute phase proteins (CRP, ESR,SAA)
Units:
•
C- reactive protein (CRP):mg/ L.
• Erythrocyte sedimentation rate (ESR):mm/ h.
• Serum amyloid A(SAA):mg/ L.
Normal ranges:
•
CRP 0– 6mg/ L.
• ESR (E Erythrocyte sedimentation rate, p. 252).
•
SAA (not measured routinely).
Principles oftest
Serum proteins CRP and SAA are amenable to measurement by nephelom-
etry or turbidimetry. Measurement of the ESR is covered in E Chapter 3.
Indications fortesting
Acute and chronic infections, vasculitis, connective tissue disease, arthritis.
Interpretation
Clinicians are usually confused by ESR and CRP— they do not give the same
information and should be used together. CRP is like blood glucose, whilst
ESR is like HbA
1c
. CRP rises within hours of onset of inammation/ infection
and falls quickly once treatment is instituted. It is therefore useful for rapid
diagnosis and monitoring response. The ESR rises slowly, being depend-
ent, in part, on brinogen, a long- lived protein, and falls equally slowly (see
Fig.4.1). In active SLE, the ESR is high, but CRP is not elevated. CRP is
driven by interleukin (IL)- 6 and may be elevated in myeloma. See Table 4.3
for interpretation of results.
300
200
100
0
6–12hrs 14 days
C-reactive protein
ESR
Fig.4.1 Time course of acute phase response proteins. ESR in acute phase response
parallels brinogenlevel.

ACUTE PHASE PROTEINS (CRP, ESR,SAA)
349
Table4.3 Causes ofelevatedCRP
Level of CRP Common associations
Little or no change (<4– 100mg/ L) Most viral infections
ActiveSLE
Systemic sclerosis andCREST
InactiveRhA
Myeloma
Most tumours
Moderate elevation (100– 200mg/ L) EBV/ CMV infection
Bacterial infection
ActiveRhA
Polymyalgia rheumatica
TA
Lymphoma
Hypernephroma
Large elevation (>200mg/ L) Severe bacterial sepsis
Legionella
Active vasculitis (Wegener’s, rheumatoid)
Huge elevation (>400mg/ L) Overwhelming sepsis (deep tissue
abscess)
Fulminant Legionella
At this level, death usually ensues!
CREST, calcinosis, Raynaud’s syndrome, oesophageal motility dysfunction, sclerodactyly, and
telangiectasia.
Levels in very young children may be much lower for a given stimulus. Avery small number of
patients do not make inammatory responses that exceed the normal range but seem to run on
a lower ‘normal’ range (10- fold less); ultra- sensitive assays for low- level CRP are available.

350
CHAPTER4 Immunology and allergy
350
Amyloidosis
Amyloid refers to the deposition of altered proteins in tissues in an insolu-
ble form. The precursor protein varies according to the cause and can often
be measured specically. Amyloid is usually conrmed by special stains on
histological examination of biopsies. Measurement of serum Igs and elec-
trophoresis, β
2
- microglobulin, and CRP is essential if amyloid is suspected
(see Table4.4).
Table4.4 Types ofamyloid
Amyloid protein Protein precursor Clinical syndrome
AL, AH Light or heavy chain of Ig Idiopathic, multiple myeloma,
γ
- heavy chain disease
AA Serum amyloid A 2°, reactive:inammatory
arthritis, familial Mediterranean
fever, hyper- IgD syndrome,
TRAPS (periodic fever), Behçet’s,
Crohn’s disease
Aβ
2
M β
2
- microglobulin Dialysis amyloid
ACys Cystatin C Hereditary cerebral angiopathy
with bleeding (Iceland)
ALys, AFibA Lysozyme, brinogen Aa
Non- neuropathic hereditary
amyloid with renal disease
AIAPP Islet amyloid polypeptide DM type 2; insulinoma
AANF Atrial natriuretic peptide Senile cardiac amyloid
ACal Procalcitonin Medullary carcinoma of the
thyroid
AIns Porcine insulin Iatrogenic
ATTR Transthyretin Familial amyloid polyneuropathy,
senile cardiac amyloid
Aβ Aβ
- protein precursor Alzheimer’s disease
AprP Prion protein Spongiform encephalopathies
TRAPS, tumour necrosis factor receptor- associated periodic syndrome.

MEASUREMENT OFSERUM COMPLEMENT COMPONENTS
351
Measurement ofserum complement
components
Units:g/ L.
Normal ranges:
•
Complement C3 0.68– 1.80g/ L.
• Complement C4 0.18– 0.60g/ L.
• Factor B (rarely measured routinely).
• Other components usually reported as percentage of normal human
plasma.
Principles oftest
C3, C4, and factor B are usually measured by rate nephelometry or turbi-
dimetry. Other components are measured by RID or simply by double diu-
sion where presence or absence is the only result of interest. Complement
breakdown products are measured by RID or enzyme- linked assay(EIA).
Indications
Valuablein:
•
SLE (C3, C4,C3d).
• Suspected complement deciency (C3, C4, haemolytic complement).
• Suspected anaphylaxis (anaphylotoxins C4a,C5a).
• Suspected hereditary angioedema (C3, C4, C1q, C1 esterase inhibitor,
immunochemical AND functional).
Complement deciency is common (especially C4 and C2 deciencies);
predisposes to recurrent neisserial disease, bacterial infections (C3 de-
ciency), and immune complex disease (lupus- like). Anyone with >1 episode
of systemic neisserial disease has a complement deciency until proven
otherwise.

352
CHAPTER4 Immunology and allergy
352
C1 esterase inhibitor
Units:
•
Immunochemical:g/ L.
• Functional:reported as percentage activity, compared to normal fresh
plasma.
Normalrange:
•
Immunochemical:0.18– 0.54g/ L (paediatric ranges not well dened, but
lower than adults).
•
Functional:80– 120% of normal plasma.
Principles oftests
Immunochemical measurement carried out by RID; functional assay is usu-
ally a colorimetricassay.
Indications fortesting
Key indication is angioedema occurring WITHOUT urticaria at any age. If
urticaria is present, diagnosis is virtually never C1 esterase inhibitor de-
ciency. C4 is a useful screen; normal C4 during an attack excludes C1 ester-
ase inhibitor deciency.
Interpretation
C1 esterase inhibitor deciency causes hereditary angioedema.
Twotypes:
•
Type I(common, 80%); absence of immunochemical C1 esterase
inhibitor (C1- inh).
• Type II (rare, 20%); presence of non- functional C1- inh; immunochemical
levels normal orhigh.
Both are inherited as autosomal dominant. Present with angioedema, NO
urticaria; may involve the larynx and gut, usual onset at puberty. C4 absent
during acute attacks. Treat with puried C1- inh (FFP may be a substitute but
can make attacks worse) or icatibant; maintenance therapy with danazol,
stanozolol, or tranexamic acid to d
frequency of attacks. Pregnancy/ oral
contraceptive exacerbate.
A rare type (type III) of hereditary angioedema is also described, thought
to be due to gain- of- function mutations in clotting factor XII. Angioedema
can also be caused by deciency of ACE and C4- binding protein.
Rare acquired form due to autoantibody to C1- inh (SLE, lymphoma);
C1q levels are reduced, and paraproteins may be present.

353
HAEMOLYTIC COMPLEMENT
Haemolytic complement (lytic
complement functiontests)
Units: can be reported in arbitrary units, or as a percentage of normal
plasma, but better reported as normal reduced or absent.
Normal range
:present (80– 120% of reference plasma).
Principles oftest
Haemolytic complement assays screen for the integrity of the classical
and alternate pathways and the terminal lytic sequence, and use either
antibody- coated sheep cells (CH100, classical pathway) or guinea pig red
cells (APCH100, alternate pathway). Either a gel or liquid assay can be used,
but the gel is easier! Both tests must be performed in parallel.
Indications fortesting
Any patient in whom deciency of a complement component is suspected.
Testing is also used to monitor the eectiveness of the MoAb eculizumab,
used to treat atypicalHUS.
Interpretation
Reduced levels of haemolytic activity will be seen during infections and dur-
ing immune complex diseases such as serum sickness and SLE. Testing for
absence of a component needs to be undertaken a minimum of 4– 6 weeks
after recovery from infection. Absence in both CH100 and APCH100 indi-
cates a deciency in the terminal lytic sequence C5– C9 (C9 deciency will
give slow lysis). Absence of CH100 indicates a missing component in the
classical pathway C1– C4. Absence of APCH100 indicates deciency in the
alternate pathway (factor D, factor B,C3).
Follow- up testing to identify the missing component will be performed by
the laboratory automatically (if they are doing theirjob!).
Criticalaction
Anyone who has a single episode of neisserial meningitis with an unusual
strain or a second episode with a common strain MUST be assumed to have
a complement deciency until proven otherwise. REFER after recovery to
the immunologist for investigation.

354
CHAPTER4 Immunology and allergy
354
Factor H and factorI
Deciency of either of these factors may lead to HUS. Measurement is pos-
sible in specialized centres, with follow- up genetic testing. Autoantibodies
against these proteins have been described.
C3 nephriticfactor
This is an autoantibody which reacts with a neo- antigen in the alternate
pathway convertase C3bBb to stabilize the convertase and i complement
breakdown. Typically seen in membranoproliferative glomerulonephritis
and in lipodystrophy. C3 will typically be extremelylow.
Autoimmunity
Autoantibodies are usually divided into organ- specic and organ- non-
specic, but clinical testing rarely follows this pattern. They are therefore
covered in convenient groups, associated with types of testing.

RHEUMATOIDFACTOR
355
Rheumatoidfactor
Units:
•
Titre (particle agglutination assay).
• IU/ mL (nephelometry,EIA).
Normalrange:
•
Titre <1/ 20:<16years.
•
Titre <1/ 40:16– 65years.
• Titre <1/ 80:>65years.
•
<30IU/ mL.
Principles oftest
Tests detect autoantibodies binding to human Ig; these can be of any class,
but assays commonly recognize IgG and IgM autoantibodies. Suggestions
that IgA RF may be helpful have not been widely accepted. Assays use either
agglutination of Ig- coated particles (visual assay) or latex particle- enhanced
nephelometry.
Indications fortesting
The test is only relevant in patients already diagnosed withRhA.
Interpretation
NOT a diagnostic test for RhA! Only +ve in 70– 80%. High titre in a patient
with known RhA is a risk factor for extra- articular manifestations and
poorer prognosis.
RF is also foundin:
•
Healthy elderly (asymptomatic).
• Chronic bacterial (SBE) and viral infections (HIV,HCV).
• Acute viral infections (transient; especially adenovirus).
• Myeloma (often type II cryoglobulins).
• Lymphoma.
• Connective tissue diseases (SLE, Sjögren’s, systemic sclerosis,
polymyositis, undierentiated connective tissue disease (UCTD)).
Antibodies to cyclic citrullinated peptide (anti- CCP) may be more valuable
in the diagnosis of RhA, as they are more specic.
356
CHAPTER4 Immunology and allergy
356
Autoantibodyscreen
Another abused test! Usually used as an immunological shing expedition.
Multiple tissues (rodent), often with human Hep- 2 cell line; gives rapid and
semi- quantitative results for the following autoantibodies:
• ANAs (see Table 4.5 for patterns).
• Anti- ribosomal antibodies.
• Antimitochondrial antibodies(AMA).
• Anti- smooth muscle antibodies (ASMA).
• Anti- liver– kidney microsomal (LKM) antibodies.
• Anti- gastric parietal cell (GPC) antibody.
Where Hep- 2 cells are used, other patterns of nuclear and cytoplasmic
uorescence may be seen. See Fig. 4.2 for examples.
Increasingly, laboratories are using multiplex analysers (modied enzyme-
linked or ow cytometry- based testing) which allow high- throughput
screening for nuclear and related antibodies.
Table4.5 Patterns ofantinuclear antibodies(ANAs)
Homogeneous SLE, drug- induced SLE (ds- DNA or histones)
Coarse- speckled UCTD*, SLE (U1- RNP)
Fine- speckled SLE, Sjögren’s (Ro and La)
Nucleolar Systemic sclerosis, polymyositis, SLE
Centriole Commonest in Mycoplasma pneumonia, also
scleroderma
Proliferating cell nuclear
antigen (PCNA)
SLE— highly specic, but rare
Centromere ‘CREST’ syndrome, limited scleroderma,
Raynaud’s, never diuse scleroderma; may be
confused with multinuclear dot pattern, which is
seen in mitochondrial antibody- negative PBC
Nuclear matrix
Nuclear mitotic spindle
SLE and UCTD
Non- specic:SLE, RA, CREST, UCTD, and
Sjögren’s syndrome
Histones
SLE, drug- induced (>90% of patients); other
connective tissue diseases (low frequency)
* UCTD, undierentiated connective tissue disease (previously mixed connective tissue disease).
Note:ribosomal antibodies associated with SLE, especially neuro- lupus (cytoplasmic pattern, not
nuclear).
Titre of antibodies does NOT correlate with disease activity.
ANAs may be seen transiently after viral infections, especially in children. Therefore, observe
a child where low- titre antibodies occur in the absence of clinical symptoms compatible with
juvenile arthritis.
AUTOANTIBODYSCREEN
357
Principles oftest
The traditional method is to overlay suitably diluted serum into frozen sec-
tions of rodent liver, kidney, and stomach, and human Hep- 2 cells. Bound
antibody in the serum is then identied using a uoresceinated anti- human
IgG (or IgM, IgA) as the second stage. Slides are then read manually.
Enzyme- based immunoassays are being introduced for screening, including
multiplex bead- laser array systems. These can be very specic when puri-
ed or recombinant antigens are used but lose out because of their inability
to pick up unexpected patterns.
Enzyme- linked assays are used for conrming antigens such histone anti-
bodies and double- stranded (ds- )DNA antibodies.
Indications fortesting
The correct use of testing is to identify which specic autoantibody is being
sought as part of the dierential diagnosis.
Interpretation
Because of the multiple patterns detected in this system, the interpretation
for each is covered separately (see Table4.5).
Fig.4.2 ANA on Hep- 2 cells with a speckled pattern (left) and a homogeneous
pattern (right).

358
CHAPTER4 Immunology and allergy
358
Double- stranded DNA antibodies
Unit:IU/ mL.
Normal range:varies according toassay:
•
<30IU/ mL:−ve.
• 30– 50IU/ mL:borderline.
• >50IU/ mL:+ve.
Principles ofassay
The original and still best assay is the Farr assay, a radioisotope- based assay.
EIA tends to be widely used but is less specic, due to frequent contamina-
tion with single- stranded DNA. Crithidia assay used only rarely, as quick
uorescent screen (kinetoplast is pure ds- DNA).
Indications fortesting
Suspected SLE or autoimmune hepatitis; used to monitor SLE, in conjunc-
tion with complement studies.
Interpretation
Sensitive and specic for SLE and autoimmune hepatitis (AIH); +ve result is
signicant (in correct clinical context).

DOUBLE-STRANDED DNA ANTIBODIES
359

360
CHAPTER4 Immunology and allergy
360
Antibodies toextractable nuclear
antigens(ENA)
Units:reported qualitatively.
Normal range:dependent on antibody, normally−ve.
Principles oftest
Usually carried out by enzyme- linked immunoassay. However, counter-
current immunoelectrophoresis and western blotting are still widely used,
especially for rare antibodies. EIA is much more sensitive than other meth-
ods and has led to clinical confusion.
Indications fortesting
Should always be carried out in patients with suspected connective tissue
disease. Monitoring at yearly intervals should be carried out in diagnosed
patients, as the antibody pattern may change with time and this may cor-
relate with changes in the clinical prole.
Interpretation
Reported qualitatively; normally laboratories will carry out a 6- antigen
screen:Ro, La, ribonucleoprotein (RNP), Sm, Jo- 1, and Scl- 70 (see Table 4.6).
Awide range of other antibodies are described, some of which may be avail-
able through reference laboratories.
Diagnosis and monitoringofSLE
ANAs and ds- DNA antibodies, both +ve; low C3 and C4; raised com-
plement breakdown products in active disease (C3d); normal CRP.
ANA- negative lupus often anti- Ro positive. West Indian lupus anti- Sm+.
Always check for cardiolipin antibodies and lupus anticoagulant (dRVVT)
(E Antiphospholipid antibodies, p. 362). Ribosomal P antibodies may be a
marker for neuropsychiatriclupus.
Monitor with CRP (dierential between infection and are of disease),
C3, C4, and ds- DNA antibodies (no value from serial ANAs); rising titre of
DNA antibodies often heralds a relapse (actual titre not related to disease
activity).

ANTIBODIES TOEXTRACTABLE NUCLEAR ANTIGENS(ENA)
361
Table4.6 Antibodies toextractable nuclear antigens
Ro/ SS- A SLE, Sjögren’s, neonatal lupus, neonatal congenital complete
heart block (cause of ANA- negative lupus, as not picked up
by standard ANA screen, which does not include Hep- 2 cells).
Newer assays will distinguish Ro52 and Ro60
La/ SS- B SLE, Sjögren’s, neonatal lupus, neonatal congenital complete
heart block (rare)
(U1)- RNP Mixed (undierentiated) connective tissue disease (if present
alone); SLE (if present with ds- DNA). Other RNPs may be
reported
Sm Highly specic marker for SLE (mainly West Indians; rare in
Caucasians)
Jo- 1 Polymyositis, dermatomyositis; transferase syndrome (brosing
lung disease; 65% +ve); many other specicities are known (all
recognizing transfer RNA (tRNA)- transferases)
Scl- 70 Systemic sclerosis (diuse scleroderma); only 30% of patients
are +ve
Pm- Scl (PM1) Scleroderma– myositis overlap
Ku, Ki Rare:SLE, UCTD, Sjögren’s syndrome, polymyositis
Mi- 2 Rare:steroid- responsive polymyositis
Histones Found in connective tissue diseases, especially drug- induced lupus

362
CHAPTER4 Immunology and allergy
362
Antiphospholipid antibodies
Principles oftesting
Many types of antibodies to phospholipids are recognized. However, rou-
tinely available tests are IgG and IgM anticardiolipin antibodies detected
by EIA, and the presence of a ‘lupus anticoagulant’ detected using the
dilute Russell viper venom test (dRVVT). BOTH tests must be carried out
together, as either may be +ve without the other, but the clinical signi-
cance is the same. Antibodies to β
2
- glycoprotein Iare also important, as the
protein is a key co- factor for pathogenic antibodies. Other specicities are
available only as researchtools.
Indications fortesting
Patients with unexplained venous or arterial thrombosis; recurrent mis-
carriage (>3); connective tissue disease; early TIAs or stroke (<60years).
Livedo reticularis is a cutaneous marker.
Interpretation
• Prolonged APTT and reduced platelet count (80– 120 × 10
9
/ L) are
typical features. May get false +ve VDRL (Venereal Disease Research
Laboratory).
•
Persistent +ve IgM anticardiolipin antibodies as the only marker of
syndrome unusual, but clinically signicant.
•
Hughes’ syndrome=antiphospholipid antibodies without evidence of
other connective tissue disease or vasculitis. Presents with recurrent
miscarriage, thrombosis, or strokes. Fulminant disease causes multi-
organ failure.
•
Antibodies also found in SLE, but NOT cerebral lupus, Behçet’s, and
Sneddon’s syndrome. Also seen after EBV infection (asymptomatic and
disappear).
•
Treatment is lifelong warfarinization if symptomatic (heparin in
pregnancy); aspirin may be acceptable if asymptomatic (but no
controlled studies). No indication for intensive immunosuppression.

363
OTHER PATTERNS OF AUTOANTIBODIES
Other patterns ofautoantibodies
identified on‘autoantibody screen’
Antimitochondrial antibodies (AMA) and anti- M2
antibodies
Units:reported as titre (AMA) Anti- M2 reported as +ve or−ve.
Normal range:not usually detectable.
Principles oftesting
AMA are identied on uorescent screen, and previously unknown +ves
should be followed up with EIA or blot- based test for anti- M2 antibodies.
Indications fortesting
Suspected liver disease, especially with raised ALP (and in ♀); investigation
of unexplained pruritus (early feature ofPBC).
Interpretation
Ninety- ve per cent of PBC patients +ve for AMA (anti- M2, against dihy-
drolipoamide acyltransferase, E2). The remainder may have antibodies
against S100 antigen of the nuclear membrane (Nsp- II pattern; multinuclear
dots) or gp210, another nuclear antigen. The type of antibody present has
no inuence on prognosis or response to therapy. Marked elevation inIgM.

364
CHAPTER4 Immunology and allergy
364
Autoantibodies inautoimmune hepatitis
Units: reported as titres (ASMA, anti- LKM antibodies). Anti- liver cytosol
(LC- 1) and soluble liver antigen (SLA) reported as +ve or−ve.
Normal range:not usually detectable.
Principles oftesting
ASMA and LKM are identied on uorescent screen, and previously
unknown +ves should be followed up with EIA or blot- based test for anti-
LC and anti- SLA antibodies. Also do ANCA (E Vasculitic syndromes,
pp. 372–3).
Indications fortesting
Suspected autoimmune liver disease (abnormal LFTs, alcohol and viral infec-
tions excluded).
Interpretation
Antibodies to HCV or HCV PCR+=exclusion criteria forAIH.
Autoimmune hepatitis
• Type 1 (AIH- 1) is ANA +ve, smooth muscle antibody (SMA) +ve,
p- ANCA +ve, and SLA antibody +ve. Typically occurs in adults and has
a better prognosis and responds well to therapy.
•
Type 2 (AIH- 2) is typically LKM- 1 and LKM- 3 antibody +ve and LC- 1
antibody +ve. AIH- 2 is seen in children and has a worse prognosis with
poor response to therapy.
•
In serological studies, 50% of AIH- 1 are ANA +ve/ SMA +ve,
15%ANA+ve only, and 35% SMA +ve only. However, there are
biopsy- proven serologically −ve hepatitis; 8% of AIH- 1 are SLA +ve
only. 43% of AIH- 2 are LC- 1 +ve only. Therefore, necessary to do SLA
and LC- 1 tests. Prognosis is dependent on type and early diagnosis.
• LKM antibodies are also associated with drug- induced hepatitis
(especially halothane) and chronic hepatitis CorD.
•
Non- actin SMA may be seen in SLE and after viral infections (especially
adenovirus).
•
Sclerosing cholangitis may be associated with atypical p- ANCA
(EVasculitic syndromes, pp. 372–3).

365
ANTIBODIES TOGASTRIC PARIETAL CELLS & INTRINSICFACTOR
Antibodies togastric parietal cells
and intrinsicfactor
Units:GPCs may be reported as titre or simply +ve/ −ve (as titre of no
clinical value); IF antibodies reported as +ve/ −ve.
Normal range:GPC antibodies found in healthy normals without evidence
of B
12
deciency or gastritis— may be at risk of later PA. IF antibodies only
foundinPA.
Principles oftesting
GPC antibodies are detected as part of the ‘autoantibody screen’; IF anti-
bodies usually detected by either RIA or EIA, but assays are inconsistent.
There is a robust EQA scheme for GPC antibodies, but none currently for
IF antibodies.
Indications fortesting
GPC antibodies should be checked in all patients with thyroid disease, as
there is a close association (‘thyrogastric disease’). Also check in patients
with unexplained macrocytosis ± low B
12
. Positive antibodies identied inci-
dentally should be followed up with a blood count and, if the MCV is high,
a B
12
level. IF antibodies may help in patients with a high MCV and low B
12
to conrm PA. Diagnosis of PA more dicult now Schilling test withdrawn.
IF antibodies are NOT suitable for screening, as present in only 50% of
patients with PA and pre- administration of B
12
will interfere with detection.
Interpretation
GPC antibodies found in 90% of patients with PA and in 40% of patients
with other organ- specic autoimmune diseases; the antigen is the β subu-
nit of gastric H
+
/ K
+
ATPase. Anti- IF antibodies are of two types: those
that block B
12
binding to IF (70% of patients with PA) and those that block
uptake of the B
12
– IF complex (35% of patients withPA).

366
CHAPTER4 Immunology and allergy
366
Thyroid disease (thyroid peroxidase
antibodies)
Units:variable— check with the laboratory.
Normal range:low levels of antibodies may be found in asymptomatic indi-
viduals, although higher levels may indicate a predisposition to later devel-
opment of thyroid disease.
Principles oftesting
TPO antibodies are the test of choice. Tg antibodies not monitored now,
except as part of monitoring for thyroid carcinoma (interfere with assays
for Tg, which is used as a tumour marker). Antibodies to TSH receptor may
be stimulating or blocking, but these can only be identied by bioassay or
RIA in specialized laboratories.
Indications fortesting
Suspected thyroid disease and as reex testing when thyroid function is
abnormal; often now combined with thyroid testing on same analyser. Also
check in patients with PA. Use TPO antibodies. Other antibodies are for
specialist useonly.
Interpretation
TPO (thyroid microsomal) antibodies in 95– 100% of Hashimoto’s thyroidi-
tis, 70% of Graves’ disease; highest titres seen in Hashimoto’s. Tg antibodies
add little to diagnosis (also found in other endocrinopathies and thyroid
carcinoma). Antibodies to TSH receptor (stimulating or blocking) found in
95% of Graves’ patients.

367
ISLET CELL ANTIBODIES
Islet cell antibodies (ICA), anti- GAD
antibodies, and insulin receptor and
insulin antibodies
Units:qualitative (previously measured in JDF units) for ICA; numeric values
for othertests.
Normal range:0.4% of normal population +ve forICA.
Principles oftesting
ICA can be detected by immunouorescence on pancreatic sections; other
antibodies detected byEIA.
Indications fortesting
All newly diagnosed early- onset diabetics should be tested; they should
also be tested for coeliac disease using endomysial or tTG antibodies. May
be used to screen normoglycaemic rst- degree relatives for likelihood of
developing diabetes. Also advisable to screen children with coeliac disease.
Interpretation
ICA found in 75– 86% of type 1 diabetics, but only 10% of type 2 diabet-
ics and 2– 5% of rst- degree relatives. Levels decline with time and may
disappear completely in long- standing type 1 patients. Antigen is glutamic
acid decarboxylase (GAD) (similar antibodies cause sti man (person!) syn-
drome, although a dierent epitope on the molecule is recognized); GAD65
and GAD67. Other target antigens for autoantibodies, with high specicity
for diabetes,are:
•
Insulin/ proinsulin (seen at diagnosis in 40% of type 1 diabetics).
• Insulin receptor (associated with acanthosis nigricans and insulin
resistance).
•
IA- 2 (present in 60% of newly diagnosed type1DM).
• ZnT8 (zinc transporter) found in 60– 80% of type1DM.
Monitoring is of novalue.
Note: IgE antibodies to porcine or bovine insulin may occur in insulin
allergy, due to exogenous ‘foreign’ insulin.

368
CHAPTER4 Immunology and allergy
368
Adrenal antibodies and other endocrine
autoantibodies
Units:qualitative.
Normal range:not detectable.
Principles oftesting
Usually detected by immunouorescence on appropriate tissue (adre-
nal, ovary, testis, parathyroid, pituitary). EIA for 21- hydroxylase, 17-
hydroxylase, and P450 side chain cleavage enzymes (targets of adrenal
antibodies) in research centres.
Indications fortesting
Suspected autoimmune endocrinopathy (adrenal insuciency, premature
ovarian failure, hypoparathyroidism).
Interpretation
Positives indicate autoimmune disease of relevantorgan.
Classification ofautoimmune
polyglandular syndromes
Refer to Table4.7.
Table4.7 Classication ofautoimmune polyglandular syndromes
Syndrome Major criteria Minor criteria
Type I Candidiasis
Adrenal failure
Hypoparathyroidism
Gonadal failure
Alopecia
Malabsorption
Chronic hepatitis
Type II
(Schmidt’s
syndrome)
Adrenal failure
Thyroid disease
IDDM
Gonadal failure
Vitiligo
Non- endocrine autoimmunity (myasthenia)
Type III Thyroid disease Either
IDDM
Or
PA
Or
Non- endocrine autoimmunity (myasthenia)
IDDM, insulin- dependent (type 1)diabetes mellitus.

369
EMA & tTG ANTIBODIES
Endomysial (EMA) and tissue
transglutaminase (tTG) antibodies
Units:qualitative for EMA; numeric fortTG.
Normal range:not usually detected in healthy individuals. +ve IgA EMA or
tTG with normal biopsy probably indicates risk of later development of
coeliac disease.
Principles oftesting
Originally identied as anti- reticulin R1 antibodies on ‘autoimmune screen’.
Later identied as antigliadin antibodies by modied immunouorescence
or by EIA. EMA detected by immunouorescence on monkey oesophagus
(also umbilical vein, but this is dicult to read); puried recombinant human
tTG used for EIA (assays with guinea pig tTG are less sensitive).
Indications fortesting
Patients with malabsorption, wheat intolerance, ‘irritable bowel’; children
with type 1 diabetes (and suggested that they should be monitored every
year!). Suspected dermatitis herpetiformis. Unexplained hyposplenism,
small bowel lymphoma.
Interpretation
IgA EMA has nearly 100% sensitivity and specicity for coeliac disease.
Antigen is tTG. In IgA deciency (commoner in coeliac), IgG EMA is as
good a diagnostic test. Gliadin antibodies (IgG or IgA) are not sensitive and
specic and should no longer be used; they are found in a range of bowel
diseases and in healthy individuals. Reticulin R1 antibodies may be picked up
on routine autoantibody screen; these may also be found in IBD, especially
with liver involvement, and non- specic bowel disease (post- infectious,
allergic, etc.). Also found in dermatitis herpetiformis (typical rash). Strong
association with juvenile type1DM.
Antibodies disappear with strict gluten- free diet, over 6– 12 months.
Persistent positivity of IgA EMA or IgA tTG antibodies in a patient on a
gluten- free diet is an indication of incomplete/ non- compliance with the
diet. Monitoring tTG antibodies is valuable.
Gliadin antibodies and neurological disease
Gluten sensitivity enteropathy is also associated with neurological disease,
typically cerebellar ataxia. Reports have suggested that gliadin antibodies
are a marker for this syndrome; the lack of specicity of gliadin antibodies
means that it is unlikely that these antibodies can be used as an accurate
diagnostic test, and specic tests (EMA and tTG) should be used instead.

370
CHAPTER4 Immunology and allergy
370
Autoimmune skin diseases
Units:normally reported qualitatively.
Normal range
:antibodies when detected are highly disease- specic.
Principles oftesting
Autoantibodies to components of the skin can be detected either by direct
immunouorescence (DIF) on a skin biopsy of aected tissue or indirectly
in the patient’s serum using monkey oesophagus as a substrate. Saline split-
ting of the skin may be used to identify the precise location of the antigenic
target to dierentiate between epidermolysis bullosa acquisita (antigen on
the dermal side) and pemphigoid (antigen on the epidermalside).
Indications fortesting
Bullous (blistering) skin diseases.
Interpretation
Bullous pemphigoid
Antibodies bind to the dermal– epidermal junction and recognize hemides-
mosome antigens (BP 230 (BPAG1) and BP 180 (BPAG2)); detect by DIF on
skin biopsies or in serum (only 70% of patients have detectable circulating
antibodies in serum). Linear basement membrane deposition of IgG and
C3 onDIF.
Herpes gestationis
Antibodies to BP 180 (BPAG2) binding to the dermal– epidermal junction
on DIF of biopsies, often −ve for serum antibodies. Linear basement mem-
brane deposition of IgG and C3 onDIF.
Pemphigus
Antibody binds to the cell surface of stratied squamous epithelium and
recognizes the desmosomal proteins desmoglein Iand III. Eighty to 90% of
patients have detectable antibody in serum. Chicken- wire pattern of immu-
nouorescence in the epidermis. May occur as a paraneoplastic phenom-
enon in patients with lymphoma, but antigenic specicity is for desmoplakin
IandII.
Dermatitis herpetiformis
Granular deposits of IgA at the dermal– epidermal junction in dermal papil-
lae on DIF; EMA and tTG antibodies will be+ve.

AUTOANTIBODIES AND NEUROLOGICAL DISEASE
371
Autoantibodies and neurological disease
Principles oftesting
Most neurological autoantibodies of interest are rare and are available from
reference laboratories. A variety of methods are used; some assays are
reported with numeric values.
Indications fortesting
These are restricted to very specic neurological syndromes.
Interpretation
• AChRAb associated with MG (90%); reported numerically as high
and low +ves to distinguish dierent clinical phenotypes. May also
be found in asymptomatic relatives. Striated muscle antibodies
(immunouorescence on striated muscle section) strongly associated
with underlying thymoma.
•
Lambert– Eaton myasthenic syndrome (LEMS), occurring with small- cell
carcinoma of the lung, associated with autoantibodies to voltage- gated
Ca
2+
channels, α and β subunits. Same tumour also associated with
retinal autoantibodies. Antibodies to voltage- gated K
+
channels are
associated with neuromyotonia.
•
Anti- ganglioside antibodies associated with GBS (GM- 1, GD1a) and
variants (Miller– Fisher— GQ1b, GT1a), chronic variants (chronic
inammatory demyelinating polyneuropathy), and neuropathy
associated with paraproteins.
•
Myelin- associated glycoprotein (MAG) antibodies associated with
paraproteinaemic neuropathy, especially with Waldenström’s
macroglobulinaemia.
•
Antibodies to myelin basic protein found in MS, but not useful
diagnostically.
•
Anti- Yo (Purkinje cell antibodies) found in paraneoplastic cerebellar
degeneration (gynaecological or breast tumours associated); anti-
Hu (anti- neuronal nuclear antibodies (ANNA)) associated with
paraneoplastic neuropathies and myelopathies (small- cell carcinoma).
Anti- Ri (anti- neuronal nuclei) associated with cerebellar ataxia and
opsiclonus (small- cell carcinoma, gynaecological or breast tumours
associated). Other specicities have been dened.
•
Anti- neuronal antibodies in the CSF of 74% of patients with cerebral
lupus; also associated with anti- ribosomal P antibodies.
• Anti- GAD antibodies found in sti person syndrome; same antigen to
that found in pancreatic islets; diabetes usually occurs in sti person
syndrome. Antibodies appear to inhibit the production of
γ
- aminobutyric acid (GABA), an inhibitory neurotransmitter.
Rasmussen’s encephalitis associated with autoantibodies to GluR3
receptor, which cause hyperexcitability of neurones.
•
Anti- aquaporin- 4 antibodies are associated with neuromyelitis optica
(Devic’s disease).

372
CHAPTER4 Immunology and allergy
372
Vasculitic syndromes
ANCA
Units:usually expressed as titre (qualitative EIAs are used in some centres).
Normal range:in adults, normal=undetectable.
Principles oftesting
Screening is usually carried out by immunouorescence on ethanol- xed
human neutrophils; rapid EIA screening tests exist for +ve/ −ve testing in
emergencies. EIA to specic antigens is an essential follow- up. Distinction
between antinuclear and perinuclear staining may require the use of
Hep- 2cells.
Indications fortesting
Suspected vasculitis; acute glomerulonephritis.
Interpretation
•
Two main patterns recognized:c- ANCA (mostly Wegener’s; 90% of
Wegener’s ANCA +ve) and p- ANCA (some Wegener’s, microscopic
polyarteritis, Churg– Strauss syndrome, glomerulonephritis, sclerosing
cholangitis, AIH, ulcerative colitis).
•
c- ANCA pattern due to antibodies against proteinase- 3; p- ANCA
pattern due to antibodies against MPO, lactoferrin, cathepsin,
and elastase. Follow- up EIA required to identify antigenic
specicity:minimum PR3 and MPO ELISA; other antigens available
through supra- regional referral laboratories.
• In Wegener’s, monitoring titre of ANCA is useful; a rising titre in a
patient in clinical remission heralds relapse.
•
ANCA may be seen as epiphenomenon in states of chronic neutrophil
activation and turnover, e.g. cystic brosis.
Anti- GBM disease (Goodpasture’s syndrome)
Units: qualitative (quantitation available through reference centres— used
only to follow patients post- plasmapheresis or transplant).
Normal range:not detectable.
Principles oftesting
Usually carried out by EIA; screening by immunouorescence is not sen-
sitive. Biopsies will show linear IgG deposition in glomeruli (and alveoli).
Antibodies recognize the NC1 region in the α3 chain of type IV collagen.
Indications fortesting
Acute glomerulonephritis, particularly if associated with pulmonary
haemorrhage.
Interpretation
Positive antibodies conrm Goodpasture’s syndrome. Urgent plasmapher-
esis is required, and monitoring reduction of antibody with treatment is
advisable. Disease may recur in transplanted kidneys, so monitoring of
antibody is advised. Some patients may be both ANCA and anti- GBM
antibody+ve.

VASCULITIC SYNDROMES
373
Anti- phospholipase A2 antibodies (anti- PLA- 2)
Units:quantitative, through reference laboratoriesonly.
Normal range:not detectable.
Principles oftesting
Usually carried out by EIA or western blot. Indirect immunouorescence
also available using a cell line expressingPLA- 2.
Indications fortesting
Marker antibody for idiopathic membranous nephritis(IMN).
Interpretation
Very high specicity for IMN but may also be found inlupus.

374
CHAPTER4 Immunology and allergy
374
Allergic disease
TotalIgE
Unit:kU/ L.
Normal range:<100kU/ L (>14yearsold).
Principles oftesting
Previously carried out by RIA, now byEIA.
Indications fortesting
There are FEW indications for testing. Screening for atopic disease;
investigation of suspected hyper- IgE syndrome (Job’s syndrome, a rare
immunodeciency), Churg– Strauss vasculitis. Gating requests for radioal-
lergosorbent tests (RASTs) on the basis of IgE is scientically unsound (see
E Interpretation below).
Interpretation
Signicant allergic disease is possible with low levels of total IgE (including
anaphylaxis). Only patients with undetectable IgE (<7) are unlikely to have
allergic disease. Conversely, levels above the normal range are compatible
with no clinical allergic disease. IgE >1000 associated with atopic eczema;
IgE >50,000 conrms hyper- IgE syndrome (although patients may have
lower levels— diagnosis is clinical). Raised levels are also seen in parasitic
infections of the bowel, lariasis, lymphoma (especially Hodgkin’s disease),
and Churg– Strauss vasculitis.

SKIN PRICKTESTS
375
Skin pricktests
Unit:mm wheal size, compared to histamine and saline controls.
Normal range:nowheal.
Principles oftesting
This remains the gold standard for allergy diagnosis. It identies IgE-
mediated reactions (type I) such as inhalant allergy, anaphylaxis, and food
allergy. It is dependent on triggering the release of histamine from cutane-
ous mast cells. Solution of allergen or controls (histamine or saline) placed
on the skin and pierced through by a lancet. After 15min, wheal will be
visible. Positive is at least 2mm greater than −ve control. Histamine control
must be +ve. Not interpretable if the patient is dermographic (−ve con-
trol gives wheal). Can use factory- prepared allergens; also use double- prick
technique with fresh foods (prick food, then the patient)— useful where
allergens are labile, e.g. fruits.
Indications fortesting
Mainstay for diagnosis of all types of allergic disease. Contraindicated when
there is signicant skin disease, previous severe allergic reactions (use RASTs
rst), patients on antihistamines (need to be o drug for a week). Other
drugs will interfere, e.g. Ca
2+
channel blockers, tricyclic antidepressants.
Interpretation
Results can only be interpreted in the context of the clinical history. Testing
should be tailored to individual patients to answer specic questions. May
need to be followed up by open or blinded challenges where −ve results are
obtained in patients with good histories.
Good specicity for inhalant allergens and some foods (nuts, sh); results
comparable with RAST testing.
Many known families ofcross- reactivity betweenbiological families
• Latex allergy associated with food reactions:banana, avocado, kiwi fruit,
chestnut, potato, tomato, cannabis, lettuce. Also birch pollen allergy
commoner.
•
Birch pollen allergy (asthma, rhinitis) with food- related reactions to nuts,
apples, plums, cherries, carrots, and potatoes (‘oral allergy’ or ‘pollen–
fruit’ syndrome).
•
Mugwort pollen and celery.
• Ragweed pollen with melon and banana.

376
CHAPTER4 Immunology and allergy
376
Specific IgE:‘RASTtests’
Units: continuous numeric scale in IU/ mL. Also (less frequently now)
reported as grades0– 6.
Normal range:grades 0 and 1 indicate insignicant specicIgE.
For an overview of grades, see Table4.8.
Principles oftesting
Previously tested by RIA (hence the acronym RAST=radioallergosorbent
test); now identied by enzyme- linked or uorimetric assays.
Indications fortesting
‘RAST’ tests are expensive and should be reserved for cases where skin
prick testing is not possible: extensive skin disease, patient on antihista-
mines, severe reactions, small children, dermographic patients. Testing
MUST be guided by the history— do not request ‘allergen screen’.
Interpretation
Presence of specic IgE does NOT equate to allergic disease— indicates
sensitization only. Must be interpreted in the context of the clinical history.
Numerical value does NOT correlate with severity of clinical reactions.
RAST tests are of little value for identifying allergy to fruits and vegeta-
bles, as the allergens are labile, and to drugs (unreliable). False +ves possible
when total IgE is very high due to non- specic binding (less of a problem
with newer assays).
Table4.8 Grades
0 <0.35
1
0.35– 0.70
2 0.70– 3.50
3 3.50– 17.50
4 17.5– 50.0
5 50– 100
6 >100

DRUG ALLERGY TESTING
377
Mast cell tryptase
Unit:μg/ L.
Normal range: 2– 14μg/ L.
Principles oftesting
Measured by EIA. Analyte is stable in clottedblood.
Indications fortesting
Valuable test for the investigation of acute? allergic reactions. Released
when mast cells degranulate, and stable in serum for up to 24h. Also useful
for monitoring patients with mastocytosis.
Interpretation
Raised levels indicate mast cell degranulation and will help distinguish ana-
phylactic and anaphylactoid reactions from other causes of reactions (vas-
ovagal, hyperventilation, carcinoid, phaeochromocytoma, etc.). Persistent
elevated levels may indicate mastocytosis.
Drug allergy testing
Investigation of severe drug allergy is a specialized eld and all patients
should be referred to an appropriate expert for an opinion, usually a con-
sultant in allergy or clinical immunology in a regional centre. Testing will
usually involve skin prick testing, followed by intradermal testing and patch
testing and, if necessary, blind challenge.
Patchtests
Unit:scored qualitatively.
Normal range:−ve.
Principles oftesting
This test identies cell- mediated reactions = delayed- type hypersensitiv-
ity (type IV reactions). It should not be confused with skin prick testing.
Allergens in petrolatum jelly are placed in contact with the skin for 48h
under occlusion with aluminium cups. The test result is read at 96h, looking
for eczematous change and blistering. Usually carried out by dermatology
departments.
Indications fortesting
Investigation of contact reactions, e.g. eczema.
Interpretation
Positive results are invariably signicant. Common allergens include metals
such as nickel and chromium, dyes and chemical in leather, rubber chemicals
(accelerators), and cosmetic chemicals. Panels of allergens used, depending
on the clinical history.

378
CHAPTER4 Immunology and allergy
378
Cellular functionassays
Investigation of cellular function of lymphocytes, neutrophils, macrophages,
and natural killer (NK) cells are restricted to specialized regional immunol-
ogy laboratories. The tests are labour- intensive and dicult to standardize,
with the exception of basic lymphocyte markers. EQA schemes are avail-
able only for basic lymphocyte markers.
All tests, other than basic lymphocyte markers, should only be requested
after discussion with a consultant immunologist. Their role is in the inves-
tigation of suspected cellular immunodeciency, particularly SCID and °
disorders of neutrophils. In such cases, urgent referral of the patient to
an appropriate paediatric or adult immunologist is more appropriate than
ddling around trying to get tests done, as the immunologist will have
direct and immediate access to the appropriate tests. Lives have been lost
due to delay in transfer, whilst inexperienced clinicians have tried to make
diagnoses.
Lymphocyte surface markers
Unit:cells/ μL.
Normal range:see Table4.9.
Principles oftesting
Lymphocyte surface markers should be carried out on a single- platform
ow cytometer, which will give a direct absolute count, not requiring a total
lymphocyte count from a haematology analyser. Absolute counts are the
preferred value; percentages are not useful. Fresh samples are required for
optimum results. Many other surface markers are available to answer more
specic immunological questions, but these will usually be of interest only
to clinical immunologists. An EQA scheme operates.
Indications fortesting
There are no absolute indications. Investigation of lymphocyte subsets is
an important part of the work- up of any patients with suspected ° or
2° immunodeciency and of patients with unexpected lymphopenia.
Serial measurements are valuable in patients undergoing BMT or stem cell
transplantation, those with ° immunodeciencies, those on any immu-
nosuppressive therapy, and those with HIV on therapy with highly active
antiretroviral therapy (HAART).
Table4.9 Normal ranges forlymphocyte surface markers
CD3+ (total T cells) 690– 2540
CD19+ (total B cells; CD20 is equivalent) 90– 660
CD3+CD4+ (T helper cells) 410– 1590
CD3+CD8+ (cytotoxic T cells) 190– 1140
CD16+CD56+ (NK cells) 90– 590

CELLULAR FUNCTIONASSAYS
379
Interpretation
Results can only be interpreted in the context of the clinical question.
Lymphocyte surface marker analysis CANNOT be used as a surrogate for
HIV testing, as many acute viral and bacterial infections, as well as other
medical problems, will give rise to a reduction in CD4+ Tcells.
Criticalaction
Baby <6months with lymphocyte count <2 × 10
9
/ L=SCID until proven
otherwise:IMMEDIATE referral to a SCID BMT unit (in the UK, Newcastle
General Hospital, Newcastle upon Tyne, and Great Ormond Street
Hospital for Sick Children, London). Look at the dierential white count,
not just the total whitecount!

380
CHAPTER4 Immunology and allergy
380
Lymphocyte functiontests
These are highly specialized and should only be carried out on the recom-
mendation of a clinical immunologist.
Indications fortesting
There are no absolute indications for testing, but it is usually carried out as
part of the specialized work- up of patients with known or suspected SCID
and in the monitoring of immunological reconstitution post- BMT.
Interpretation
Is complex and dependent on the precise clinical circumstances.
Neutrophil and macrophage function
This is a specialized test. Samples do not transport well, and results are
often abnormal if the patient has an active infection or is on antibiotics. It
is preferable to refer patients to a clinical immunologist who will organize
testing if appropriate.
Indications fortesting
Patients with deep- seated abscesses, recurrent major abscesses (exclude
diabetes, staphylococcal carriage, and hidradenitis suppurativa rst), major
oral ulceration, and unusual fungal or bacterial infections (Pseudomonas,
Serratia, staphylococci, Aspergillus). Atypical granulomatous disease, includ-
ing atypical Crohn’s disease. Genetic defects of neutrophil function may
present at anyage.
Interpretation
Interpretation is complex; defects of oxidative metabolism may indicate
chronic granulomatous disease; defects of phagocytosis are recognized.
Also MPO deciency. Neutropenia may be chronic or cyclical. Genetic test-
ing to follow up may be required.
Natural killer cell function
Is rarely required. The main indication is in recurrent severe infection with
herpesviruses. Assays are complex and need specialist interpretation.
Always discuss cases with a clinical immunologist.
Further reading
Shoedfeld Y, Meroni PL, Gershwin ME (eds.). Autoantibodies, 3rd edn. Sand Amsterdam:Elsevier,2014.
Spickett G. Oxford Handbook of Clinical Immunology and Allergy, 3rd edn. Oxford:Oxford University
Press,2013.

381
Chapter5
Infectious and tropical
diseases
Introduction to infectious diseases 382
Investigating the infectious diseases/ tropical medicine case 388
Culture techniques 390
Immunological tests 396
Antigen tests 400
Collection of specimens 402
Molecular diagnostics 406
Haematology 408
Radiology 410
Gastrointestinal tract investigations 412
Immunology 414
Biochemical tests 416
Tissue biopsy and deep aspiration specimens 418
Other tests 422
Clinical investigation in action:pyrexia of uncertain origin 424

382
CHAPTER5 Infectious and tropical diseases
382
Introduction toinfectious diseases
Everything about microscopic life is terribly upsetting … how can
things so small be so important?
(Isaac Asimov— 1920– 1992)
An oldenemy
Infectious diseases have had a huge impact on the human species. Throughout
history, mighty armies have been humbled by microbiology. After the intro-
duction of eective antibiotics during the Second World War, there was great
optimism that the ght against infectious diseases had been won. In recent
years, this hope has been dramatically dashed. Almost all new diseases are
infections, and some of the twenty- rst century’s most pressing problems are
pathogens that have only appeared in the 30years prior to this book being
written. Globally, the most important of the newer organisms are HIV and
hepatitis C, though old enemies, such as TB, Pneumococcus, and malaria, are
still killing millions throughout the world. The dreadful epidemic of Ebola virus
disease has shaken the world’s biosecurity.
New challenges
As we proceed through the twenty- rst century, several factors are serv-
ing to i the relative importance of infection over other areas of medicine.
Infections such as Ebola, zika, and avian/ swine inuenza are continually
emerging and re- emerging. Antimicrobial resistance is increasing, meticillin-
resistant Staphylococcus aureus (MRSA), vancomycin- resistant Enterococcus
(VRE), and carbapenemase- producing Enterobacteriaceae (CPE) being the
most infamous examples. There are more immunosuppressed patients
as a result of i use of chemotherapy agents and organ transplantation.
Tourists and other travellers are making their way to ever more remote
parts of the world. Medical tourism is a cheap way of getting cosmetic sur-
gery. Migration of populations has always spread disease and this continues
today. There are concerns of wilful bioterrorism. All of these factors mean
that the infectious dierential diagnosis— even in the developed world—
grows ever longer.
It is always worth bearing in mind infection in a dierential diagnosis
is often treatable. Accordingly, it is always better not to miss treatable
options over incurable ones. Furthermore, some infectious diseases like
Ebola, multidrug- resistant tuberculosis (MDR- TB), severe acute respiratory
syndrome (SARS), Middle East respiratory syndrome coronavirus (MERS-
CoV), and avian/ swine inuenza have major public health consequences,
including for the treating physician.
A challenge tothe clinician
The same infection is often capable of causing a wide variety of clinical
pictures. HIV, for example, is a great mimicker. Acquired immune deciency
syndrome (AIDS) is dened by its consequences. This is not so surprising,
given the genetic variety of mankind, hence individual responses to a bewil-
dering variety of infecting agents. Some clinical syndromes can be caused
by many, quite dierent pathogens. Good examples include pneumonia,
hepatitis, and endocarditis.

INTRODUCTION TO INFECTIOUS DISEASES
383
Furthermore, some infectious diseases can resemble non- infectious dis-
eases. For example, amoebic colitis can resemble ulcerative colitis; syphilis
can present with serious psychiatric symptomatology; a brain abscess or a
tuberculoma can resemble a brain tumour; and TB of the vertebral column
can resemble metastatic malignancy. Getting it wrong can be catastrophic
for the patient.
Other diseases can mimic infections
Non- infectious diseases can resemble infection. Examples include gout of
the rst MTP joint, rather than cellulitis; cervical lymphadenopathy due to
lymphoma, rather than TB; familial Mediterranean fever as a cause of PUO;
SLE leading to Libman– Sachs endocarditis; adult Still’s disease as a cause of
fever and neutrophilia; and inammatory carcinoma of the ♀ breast resem-
bling a pyogenic breast abscess.
Importance ofepidemiological factors
Epidemiology is fundamental to determining which, if any, infecting agents,
and therefore investigations, are relevant in a given patient.
Geography
This is very important and needs to be specic.
Some infections are common theworld overand include
•
Staphylococcal infection.
• Salmonella infections.
•
Pneumococcal pneumonia.
• Gonorrhoea.
• Thrush (candidiasis).
• TB.
• Inuenza.
• EBV.
• HIV (although sub- Saharan Africa is still the worst aectedarea).
• HepatitisC.
• Herpes simplex.
• Threadworm (Enterobius).
•
Syphilis.
Some infectious diseases are commoner inthe tropical world,e.g.
•
Malaria.
• Diphtheria (although still common in Eastern Europe).
• Rheumaticfever.
• Enteric fever (typhoid and paratyphoid).
• Hepatitis E (although increasingly widespread).
• Poliomyelitis (very restrictednow).
• Rabies (although Eastern Europe has signicant disease).
• Viral haemorrhagic fever (VHF), including Ebola, Lassa, Marburg.
• Onchocerciasis (river blindness).
• Schistosomiasis.
• Leishmaniasis (although commonly found around the Mediterranean).
• Ascariasis.
• Cutaneous myiasis (e.g. tumbu and boty).
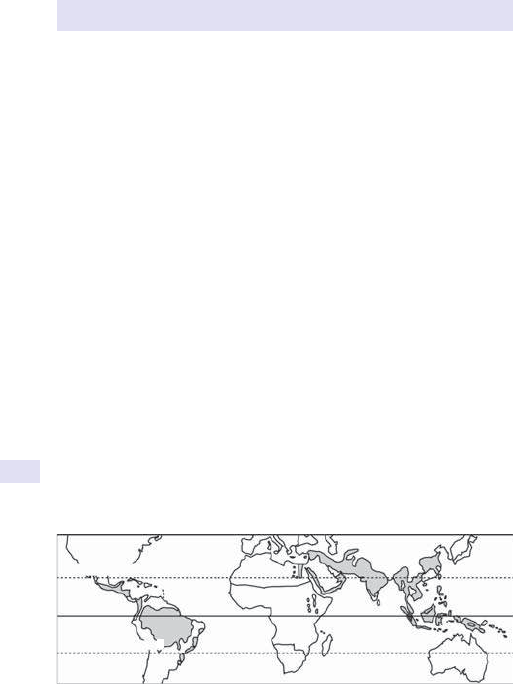
384
CHAPTER5 Infectious and tropical diseases
384
Some infectious diseases are common insome parts ofthe developed world,
but not inothers, including
•
Lyme disease.
• Babesiosis.
• Ehrlichiosis.
• Histoplasmosis.
• Hydatid disease.
• Anisakiasis.
In theUSA, only certain areas are endemicfor
•
Lyme disease.
• Coccidioidomycosis.
• Babesiosis.
• Histoplasmosis.
• Tick- borne diseases.
Travel and vaccination history
This is important for many reasons. Travel exposes patients to new infec-
tious agents to which they have no immunity. Immunization schedules dif-
fer throughout the world, and some groups refuse to have their children
vaccinated. Vaccination before travel does not necessarily happen as rec-
ommended. The clinician must therefore be aware of the distribution of
common infections. Great variation in antibiotic resistance patterns can be
observed in dierent parts of the world; this clearly has an impact on the
choice of empirical treatment. Finally, travel often has an impact on patterns
of sexual and risk- taking behaviour (see Fig.5.1).
Tropic of Cancer
Equator
Tropic of Capricorn
Fig.5.1 The importance of taking a geographic history. Malaria, which can be
life- threatening, is a very common disease in many parts of the world but is not
indigenous to most parts of the developed world. Making a diagnosis depends heavily
upon the clinician eliciting the clues in the patient’s history. Even if s/ he has been
taking antimalarial drugs, a patient who has been on holiday to Kenya, Thailand, or
Brazil may die if the disease is not diagnosed. Clinical suspicion should lead to blood
lms (on 3 consecutive days) and a platelet count. Bear in mind that the patient may
not have been taking adequate prophylaxis, may have been missing tablets, or may
not have been absorbingthem.

INTRODUCTION TO INFECTIOUS DISEASES
385
Sexual and drug- taking activity
Searching and personal questions may need to be asked. This is sometimes
dicult to do, even with great experience. Patients will/ may not admit to
high- risk sexual activity or the use of illegal substances and may need to be
pressed. Beware of using family members as ‘translators’. The clinician must
maintain high clinical suspicion at all times, even and especially when the
patient does not t a social stereotype. Bear in mind that any patient may
have a ‘double life’, of which even his/ her spouse is unaware. It is danger-
ous for the clinician to assume that being married equates to sexual delity
or even heterosexuality.
It may not be immediately obvious that the fever, rash, and hypotension
in a woman may be related to her tampon usage (toxic shock syndrome),
yet menstruation can be a dicult subject to discuss in some cultural set-
tings. TB of the ♀ genital tract may present as infertility or menorrhagia.
‘Lumpy semen’ may be indicative of Schistosoma haematobium infection, and
a ‘urinary tract infection’ or a septic arthritis in a 19- year- old man may be
gonorrhoea.
Social and professional
Pets, hobbies, and jobs may well be important. The patient with pneumonia
and a budgerigar could have psittacosis. The tropical sh salesman with a
chronic rash on his hand could have Mycobacterium marinum infection (aka
‘sh tank granuloma’). The jaundiced volunteer cleaning out canals at week-
ends could have leptospirosis related to contact with rats. The cat owned
by the middle- aged lady with recurrent axillary lymphadenopathy may be
the key to her problem of cat- scratch disease (Bartonella henselae).
Assessing thepatient
The recognition of an infectious disease in a patient (or the absence of
one) goes far beyond the Petri dish, the microbiology bench, and PCR test-
ing technology. The Andromeda Strain phenomenon (with all due credit to
Michael Crichton MD) should be borne in mind. The disease in front of you
might be the rst ever presentation or the rst in a new outbreak! Almost
the only signicant new human diseases that will appear in the future will be
infectious diseases and they will keep appearing till the end of the human
species (see Fig.5.2).
Assessment should include
•
Adetailed history.
• Full physical examination (including temperature).
• The generation of a dierential diagnosis.
• Specimen collection before antimicrobial therapy.
• Laboratorytests.
• Non- invasive procedures (including radiological tests where
appropriate).
•
Invasive procedures.
• The making of a denitive diagnosis (wherever possible).

386
CHAPTER5 Infectious and tropical diseases
386
When considering thepossibility ofan infective process, one should always
consider thebasic taxonomy
•
Bacteria (including primitive forms).
• Mycobacteria.
• Fungi.
• Viruses.
• Protozoa.
• Helminths.
• Prions.
• Myiasis.
Investigations available tothe infectious diseases
or general physician
Many tests will be performed with a view to making a diagnosis.
Investigation of a patient should be rational and evidence- based, wher-
ever possible. Although the interrogative armamentarium of the infec-
tious diseases and tropical medicine physician is enormous— as with any
other branch of medicine— the history and examination will point the way.
Results will emerge which, whilst not producing a diagnosis as such, will
nevertheless require following up. For example, low C5 levels in recur-
rent meningococcal septicaemia may need immunological assessment.
(Remember also that there is a new drug eculizumab which specically
inhibits C5.) IgG deciency leading to recurrent pneumonia may require
regular infusions of γ globulins. Alow CD4+ cell count, which is not due
to HIV infection, could be a feature of sarcoidosis.
Fig.5.2 Infection and history taking.

INTRODUCTION TO INFECTIOUS DISEASES
387
Making a diagnosis alone is not the only issue at stake. Some tests must be
done if a patient is going to be treated safely. Examples might include:G6PD
levels before administering primaquine for hypnozoite eradication in malaria;
TB cultures for antibiotic sensitivity prior to starting empirical therapy; and
exclusion of pregnancy before using certain antibiotics such as doxycycline
and ciprooxacin. Other tests relate to the fact that some infectious dis-
eases are dangerous to others, including the doctor! Prime examples of this
would be MDR- TB and extensively drug- resistant TB (XDR- TB), avian inu-
enza, SARS, MERS- CoV, and Ebola, all of which are potentially dangerous
for the population at large and need to be identied (or at least suspected
wherever appropriate) and treated in an isolationunit.

388
CHAPTER5 Infectious and tropical diseases
388
Investigating theinfectious diseases/
tropical medicinecase
Available diagnostic techniques
Direct detection
•
Microscopy:
•
Direct— e.g. faecal parasites (± iodine) or malarial and trypanosomal
bloodlms.
•
Special stains— these include Gram and ZN stains.
•
Electron microscopy (EM)— for viruses and other pathogens.
•
Immunouorescence— with specicsera.
• Presence of toxin:e.g. Clostridium dicile.
•
Antigen detection (E Serology, pp. 396–9).
•
Molecular assays (E Molecular diagnostics, pp. 406–7):these include
gene probes, amplication assays, e.g.PCR.
• Point- of- care tests:bedside rapid diagnostic tests (RDTs) for malaria,
inuenza, HIV, andHBV
Culture
•
All body uids and tissues can be cultured. As a general principle, the
larger the sample sent, the greater the yield. It is good practice to
forewarn the laboratory before sending any unusual samples, especially
if there is a risk to laboratory sta, and label accordingly (E Biohazards,
pp. 402–5) Laboratory preparation and specialist containers may be
required. Adequate clinical details should be written on any request
forms, including travel history. The choice of culture technique can vary
dramatically, depending on the organism that is being sought. There may
be only one chance to culture the correct organism, so it should not be
wasted.
•
Once a culture has grown, identication maybe:
•
Through special growth media, culture temperature, or atmosphere.
1
•
By biochemical reactions (e.g. catalase or coagulase), API strip, or
matrix- assisted laser desorption/ ionization (MALDI).
•
With specic antisera (e.g. latex agglutination or
immunouorescence).
•
Using molecular- based methods (e.g. specic probes, restriction
enzyme patterns, DNA sequencing).
•
Using antimicrobial susceptibility testing (e.g. metronidazole sensitiv-
ity for anaerobes and vancomycin for Gram+ves).
Serology
E Serology, pp. 396–9.
1 Todar’s Online Textbook of Bacteriology. M http:// textbookofbacteriology.net.

INVESTIGATING THE INFECTIOUS DISEASES
389
Other tests asappropriate
(See appropriate sections.)
•
Biochemistry.
• Haematology.
• Immunology.
• Moleculartests.
• Radiology.
• Stool and bowel contents.
• Tissue biopsy and deep aspiration specimens.
• Othertests.

390
CHAPTER5 Infectious and tropical diseases
390
Culture techniques
Microorganisms exist in nature as mixed populations. Diagnosis of an infec-
tion means identifying the relevant pathogen. Furthermore, dierent organ-
isms can cause the same disease (e.g. pneumonia), require very dierent
treatment and management, and have dierent prognoses. Whilst some
specimens (e.g. stool, sputum) contain extremely large numbers of varied
organisms, some specimens (e.g. blood, CSF, urine) should be sterile, unless
infected or contaminated during their collection.
Microbiological culture assists with the aetiological diagnosis of bacterial,
fungal, protozoal, or viral illness by enabling identication and susceptibil-
ity testing of the isolated organism(s). Bacterial culture was the rst to
evolve, but useful data on other pathogenic groups can also be obtained
through the use of culture- based methodologies (although options for
treatment are currently more limited for viruses and fungi than for bac-
teria). Furthermore, culture of mycobacteria, viruses, and fungi usually
takes longer than most bacterial cultures; therefore, the data obtained are
most valuable for late conrmation of the diagnosis or for epidemiological
purposes (e.g. for predicting the appropriate constituents for a polyvalent
inuenza vaccine).
Bacteria
Three major steps are involved in extracting pure cultures from a diverse
population of microorganisms and identifying a pathogen. Many of these
processes can now be automated (see Fig.5.3).
1. An isolation plate is created. To do this, the mixture must be diluted until
the various individual microorganisms have been dispersed far enough
apart on an agar surface, so that, after incubation, they will form visible
colonies isolated from the colonies of their neighbours. Specialized
culture media (such as selective media, dierential media, enrichment
media, and combination selective and dierential media— a great many
exist) may be used to supplement mechanical techniques of isolation.
Culture can be aerobic or anaerobic. (Note:specimens for the isolation
of anaerobic pathogens require special care, as anaerobic bacteria die
in the presence of O
2
. Such specimens should therefore be transported
in a reduced container.) Lastly temperature can be used to further
select for pathogenic organisms, e.g. Campylobacter jejuni is unusual as it
will grow at41°C.
2. A pure culture is created. To achieve this, an isolated colony will be
selected out and carefully ‘picked o ’ the isolation plate for transferring
to a new sterile medium. Following incubation, all the organisms in the
new culture will be descendants of the same organism.
3. The organism can then be identied through various manoeuvres:
•
Increasingly, matrix- assisted laser desorption/ ionization time- of- ight
mass spectrometry (MALDI- TOF MS) and other automated systems
(see below).
CULTURE TECHNIQUES
391
• Other techniques include:
•
The colony appearance and susceptibility to specic antibioticdiscs.
•
The microscopic appearance.
•
The staining responses (e.g. Gram +ve vs Gram−ve).
•
The use of a range of biochemical tests designed to uncover
characteristics typical of a particular organism, e.g. catalase reaction,
sugar fermentation as in the API strip. The presence of the enzyme
coagulase is useful for distinguishing Staphylococcus aureus from less
pathogenic coagulase −ve staphylococci (normal skin commensals).
Fig.5.3 Overview of new methods in the workow of clinical microbiology
laboratories.
Reproduced from Fournier, Pierre- Edouard etal, Modern clinical microbiology:new challenges and
solutions, Nature Reviews Microbiology
11, (2013) 574– 585 doi:10.1038/ nrmicro3068, with permis-
sion from Macmillan PublishersLtd.

392
CHAPTER5 Infectious and tropical diseases
392
•
Less frequently now is the use of antisera (direct serology) for
culture conrmation:
— Agglutination and latex agglutination tests can be used
on colonies to identify Escherichia coli 0157, Streptococcus
pneumoniae, serogroups of Neisseria meningitidis, Shigella, and
Salmonella, Lanceeld groups of β
- haemolytic streptococci, and
serotypes of Haemophilus inuenzae.
— Detection of specic antigens by direct uorescent antibody
(DFA) staining can be used to identify colonies of Streptococcus
pyogenes, Bordetella pertussis, and the species and serotypes of
Legionella.
Ideally, specimens for bacterial culture should be taken before antibiot-
ics are administered. This may not always be feasible, but the information
yielded may well be less thanideal.
Matrix- assisted laser desorption/ ionization time- of- ight mass spectrometry
MALDI- TOF MS is a new automated technique using mass spectrometry,
which allows the analysis of biomolecules such as DNA, proteins, peptides,
sugars, and large organic molecules, which tend to fragment when ionized.
MALDI- TOF MS is a 3- step process. Firstly, the sample is mixed with a
matrix material and applied to a metal plate. Secondly, a pulsed laser irradi-
ates the sample, triggering disintegration. Finally, the analyte molecules are
ionized in a plume of hot ablated gases, which are then accelerated into
a mass spectrometer for analysis. The resultant spectra generated can be
used for the identication of microorganisms, when compared to stored
database proles. Species diagnosis by this procedure is much faster, more
accurate, and cheaper than other techniques. MALDI/ TOF has become
the standard method for species identication in large, modern medical
microbiological laboratories. It is easy to use, robust, and rapid, allow-
ing accurate bacterial identication of a large variety of species within a
few minutes, with only a small amount of culture sample required for the
analysis (104– 106 CFU). The absence of the need to purify the suspect
colonies allows for a much faster turnaround time. There are other new
automated systems on the market such as the BD Phoenix identication
method (BD Diagnostic Systems, Sparks, MD) which uses modied con-
ventional, uorogenic, and chromogenic reactions. Similarly, the Biologue
system (Biologue, Inc.) combines up to 2000 phenotypic tests. The older
Analytical Prole Index (API, bioMérieux) system uses direct inoculation
into a strip of various reactants, manually producing a code which is then
compared to a database. (See Fig.5.4.)
Antibiotic sensitivity
Once a bacterium is isolated, it can be cultured in the presence of antibiotic(s)
to assess if it is susceptible to that agent or not. The minimum inhibitory con-
centration (MIC) is the lowest antibiotic concentration at which the micro-
organism under assessment shows no visible growth in vitro. The MIC can
provide the clinician with precise information about the infecting bacterium’s
degree of antibiotic susceptibility and enable him/ her to avoid antibiotics to
which the organism shows resistance.
CULTURE TECHNIQUES
393
Samples
Sterile
A
Contaminated
Media for culture Growth and subtyping Susceptibility Typing
B
C
D
E
F
G
H
1–3 weeks
All isolates Small subset
Blood
1–2 days 1–2 days 1–6 weeks
Body uid
Urine
Pus
Surface swabs
Sputum
Faeces
Gram stain
MALDI-TOF
Acid fast stain
and Hain test
1 weeks–3 months
All Mycobacterium
tuberculosis
Species
identication
Species
identication
Aerobic bacilli
?
?
Fastidious
organisms
Anaerobic
organisms
Miscellaneous,
e.g. Legionella spp.
Mycobacterium
spp.
Staphylococci
Streptococci
?
?
Mycobacterium
spp.
Rapid
grower
s
Fig.5.4 MALDI- TOFMS.
Nature Reviews Genetics 13, 601– 612 (September 2012)doi:10.1038/ nrg3226 Transforming clinical microbiology with bacterial genome sequencing Xavier Didelot,
Rory Bowden, Daniel J.Wilson, Tim E.A. Peto & Derrick W.Crook.

394
CHAPTER5 Infectious and tropical diseases
394
For organisms exhibiting unusual resistance patterns, susceptibility panels
using methodologies such as broth microdilution, gradient diusion, and/ or
disc diusion have been created to assist clinicians.
On occasions, these data will need to be linked to testing of blood levels
for some antibiotics (e.g. gentamicin, vancomycin, cycloserine). MALDI-
TOF and other automated systems can also be used to identify antimicro-
bial resistance patterns. Nowadays genome sequencing can identify genetic
mutations associated with resistance.
Viruses
Viral culture is rarely used now, but it diers signicantly from bacterial cul-
ture as viruses require a very dierent type of medium to grow. Molecular
techniques, such as PCR and antigen detection, have become more eec-
tive, can quantitate the amount of virus present, and have almost com-
pletely replaced culture.
The appropriate type of specimen to collect, the best means of trans-
port, and the most appropriate cell culture to use will vary with the particu-
lar virus suspected, the specimen site, and the time of theyear.
•
The choice of specimen is very important. Numerous viruses enter
via the mucosa of the upper respiratory tract, yet that virus may
compromise multiple or distant tissues and organs.
•
Swabs can be used to collect a variety of specimens from the body
surfaces for viral detection, e.g. nose, throat, eye, skin, and rectum.
Anasopharyngeal aspirate (NPA) may be the more appropriate
specimen if inuenza is suspected. It is important to collect mucosal
cells, as this is where the virus resides. Deeper specimens, such as blood
and CSF, will be appropriate for some viruses. Dierent viruses will
need dierent collection approaches, e.g. heparin, citrate, and EDTA are
all acceptable for the detection of CMV by PCR, antigenaemia testing,
or culture, but for some other viruses, only citrate should be used if
they are to be cultured.
•
Unlike many bacterial or fungal pathogens, the time of year is important
to keep in mind when making a diagnosis of certain viral diseases. For
example, enteroviruses circulate almost exclusively in the summer
months and inuenza likewise circulates during the winter months.
•
Timing is important when collecting specimens for viral detection. They
should be collected as early as possible after the onset of symptoms,
as once viral shedding ceases, culture will be impossible and serological
and molecular techniques may be the only way of diagnosing the viral
pathogen.
•
Some viruses cannot be cultured, e.g. viral agents of diarrhoea
(caliciviruses, astroviruses, and coronaviruses), HCV, andHBV.
Laboratory assays for antiviral susceptibility testing include phenotypic and
genotypic assays. Genotypic assays have almost completely replaced phe-
notypic testing, especially for HIV and HBV. Genotyping of HCV is criti-
cal in guiding treatment. Phenotypic assays require growth of the virus in
vitro and so present a biohazard. In these circumstances, genotypic assays
(E Molecular diagnostics, pp. 406–7) are now routinely available and very
useful.

CULTURE TECHNIQUES
395
Fungi
Unlike bacterial and viral diseases, direct microscopy can often be used to
diagnose fungal infections (based on distinctive morphological character-
istics of the invading fungi, e.g. Aspergillus
or tinea, and/ or the judicious
use of special stains such as methylthioninium chloride (methylene blue)).
However, histopathological diagnoses should be conrmed by culture,
wherever possible. Conversely, although diagnoses are usually made by iso-
lating the causative fungus from bodily samples, the presence of a fungus in
a culture from a non- sterile site does not mean that it is pathological (e.g.
Candida isolation from sputum). Fungal infection can only be denitively
established with evidence of tissue invasion histologically. There are also
a range of serological tests available for systemic mycoses (E Serology,
pp. 396–9), but few provide denitive diagnoses by themselves. Antigen
detection has been promising but generally has poor sensitivity and speci-
city, even when used in combination. For example, galactomannan and
β
- D- glucan can be indicative of invasive aspergillosis in combination with
PCR for Aspergillus, but the context also needs to be considered. Allergic
bronchopulmonary aspergillosis (ABPA) patients can have serum samples
tested for total IgE and aspergillus- specicIgE.
An exception is cryptococcal antigen (CrAg) which is useful in serum and
CSF samples for diagnosis of cryptococcal disease, especially meningitis in
immunocompromised HIV patients.
Fungal culture techniques are similar to bacterial ones. They are most
useful for detecting dimorphic fungi, which manifest both mycelial and
yeast forms. This group includes Candida species, Cryptococcus neoformans,
Blastomyces dermatidis, Histoplasma capsulatum, Penicillium marneei, and
Coccidioides immitis. MALDI can identify most fungal species.
Protozoa
Protozoa of the genera Acanthamoeba and Naegleria may cause fatal CNS
disease. Acanthamoeba species are free- living amoebae associated with
keratitis; they may also cause granulomatous encephalitis. Another free-
living amoeba Naegleria fowleri is able to cause acute fulminant meningoen-
cephalitis and is usually associated with a history of swimming in freshwater
lakes or brackish water. In suspected cases, CSF and other suspicious clinical
material may be cultured on a non- nutrient agar plate seeded with a ‘lawn’
of Gram −ve bacteria (such as E.coli). Pathogenic amoebae can be identi-
ed microscopically.
Worldwide the most important protozoan infection are the plasmodia
causing malaria. They can be cultured, but this is rarely of use clinically.
The mainstay of malarial identication is direct microscopy, although antigen
detection tests are now available.

396
CHAPTER5 Infectious and tropical diseases
396
Immunologicaltests
Immunological methods are in wide usage to detect many pathogens pre-
sent in clinical samples. Serology refers to the laboratory usage of antigen–
antibody reactions for such diagnostic purposes. Diagnosis is made by
detecting antibody or antigen in blood and/ or other bodily uids, or by the
identication of pathogens in culture. More recently, interferon γ release
assays (IGRA) have been developed to look for evidence of T- lymphocyte
reactivity to antigens from pathogens such as Mycobacterium tuberculosis.
Both direct and indirect serological testsexist
Indirect serological techniques
Employ antigen– antibody reactions to detect specic antibodies manufac-
tured in response to an antigen or antigens on an infecting pathogen’s sur-
face. These antibodies are found circulating in the patient’s blood or present
in other body uids.
Direct serological techniques
Employ antibodies to detect specic antigens. Because this technique can
be used to identify and type cultured organisms (E Culture techniques,
pp. 390–5), not only does it have individual clinical value, but it also has
important epidemiological applications.
HBV is a good example of an infection where both antigen and antibody
proles are diagnostically, therapeutically, prognostically, and epidemiologi-
cally important:
•
Antibody detection of a specic antibody, e.g. anti- hepatitis Be antigen
(anti- HBe antibody), anti- hepatitis B surface antigen (anti- HBs antibody).
• Antigen detection, e.g. hepatitis B ‘e’ antigen (HBeAg), hepatitis B
surface antigen (HBsAg).
•
More recently, pre- core mutants of HBV have emerged that are not
picked up by the common antigentests.
(See Fig.5.5.)
Antibodytests
A wide variety of methodologies for assessing antibody response are avail-
able such as immunouorescence, agglutination, ELISA, and complement
xation(CF).
Sub- classication of organisms, through serogrouping, can be valuable
epidemiologically, e.g. whilst investigating an outbreak of meningococcal
(N.meningitidis) disease; if the culprit is determined to be type C, vaccina-
tion can be utilized to control the outbreak. However, this is being replaced
by molecular techniques.
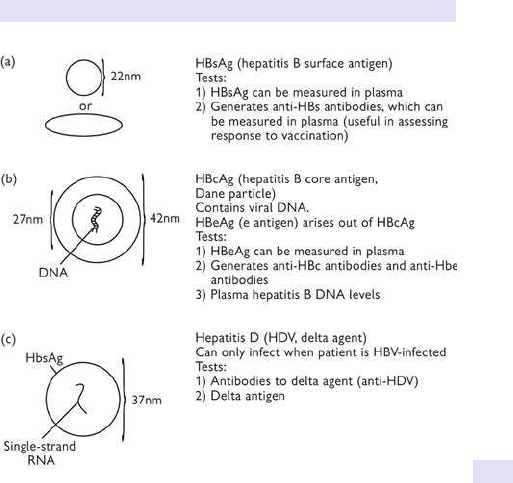
IMMUNOLOGICALTESTS
397
General principles
(See Fig.5.6.)
1. Specic IgM levels indicates a ‘new’ infection.
2. Specic IgG levels indicates a ‘new’ or a ‘previous’ infection, or, in some
cases, immunity generated by vaccination.
3. i IgG (‘rising titre’) when two samples (‘paired sera’) are taken with
an appropriate intervening interval between them indicates a ‘new’
infection or re- infection. Diagnosis (as indicated by seroconversion)
necessitates a diagnostic antibody titre or a 4- fold i in antibodytitre.
4. Seroconversion is said to have occurred in situations 1and3.
Viral antibodytests
These can be very useful because once viral shedding has ceased, viral cul-
ture is of no further value. They include tests for HIV- 1, HIV- 2, human T-
lymphotropic virus (HTLV)- 1, HTLV- 2, hepatitis A, hepatitis B, hepatitis C,
δ agent (hepatitis D), hepatitis E, EBV, CMV, dengue, Ebola virus disease,
Lassa fever, respiratory syncytial virus (RSV), mumps, measles, rubella,
inuenza, parainuenza, St Louis encephalitis, West Nile virus, yellow fever,
SARS, and many more (see Fig.5.7).
Fig.5.5 Antibody tests in hepatitis BandD.
398
CHAPTER5 Infectious and tropical diseases
398
0246810 12 14
LAG
phase
LOG
phase
Plateau
phase
Decline
phase
IgM
IgM
IgG
IgG
Antibody
titre
Exposure to
infecting agent
(‘antigen’)
Days
Fig.5.6 Relative rate of appearance and disappearance of IgM andIgG.
HBV
infection
Symptoms
HBcAg
HBsAg
HBeAg
DNA
polymerase
Anti-HBc
Anti-HBe
Anti-HBs
Months
‘core
window’
Fig.5.7 Hepatitis B antigens and antibodies.

IMMUNOLOGICALTESTS
399
Bacterial antibodytests
Less useful, these include anti- streptolysin (ASO) and anti- DNAse B for
streptococcal infection.
They can be useful for non- culturable or dicult- to- grow organisms in
the correct clinical context, e.g. cat- scratch fever (B.henselae), B.pertussis,
Lyme disease (Borrelia burgdorferi), Brucella species, C.jejuni, Chlamydia spe-
cies, Q fever (Coxiella burnetti), E.coli 0157, Francisella tularensis, Helicobacter
pylori, Legionella pneumophila, Leptospira interrogans, Listeria monocytogenes,
Mycoplasma pneumoniae, N. meningitidis, Neisseria gonorrhoeae, Rickettsia
prowazekii, Salmonella species, Treponema pallidum (including Treponema pal-
lidum
haemagglutination assay (TPHA), rapid plasma reagin (RPR), VDRL,
uorescent treponemal antibody absorption (FTA- ABS), IgM- FTA, IgM-
ELISA), Yersinia enterocolitica/ Y.pseudotuberculosis (Widaltest).
Protozoal antibodytests
Include tests for amoebiasis, toxoplasmosis, leishmaniasis (kala- azar),
African trypanosomiasis (sleeping sickness), American trypanosomiasis
(Chagas’ disease), babesiosis, and Toxoplasma gondii.
Helminthic antibodytests
Include tests for Echinococcus granulosus (hydatid disease), Echinococcus
multilocularis (alveolar echinococcosis), Microsporidium species, schistoso-
miasis (bilharzia), strongyloidiasis, lariasis, onchocerciasis, Trichinella spiralis,
Toxocara canis, Taenia solium (cysticercosis or pork tapeworm), paragonimi-
asis (Chinese lung uke), and gnathostomiasis.
Fungal antibodytests
See above, but include tests for Aspergillus fumigatus, Aspergillus niger,
Aspergillus nidulans, Aspergillus versicolor, B. dermatidis, Candida albicans,
C.immitis, C.neoformans, H.capsulatum, Mukorazeen.
Note:in the case of CF antibody assays for antibodies to coccidioidomy-
cosis, these are specic and do not require proof of rising levels. They can
provide indispensable conrmatory evidence for a diagnosis of coccidioi-
domycosis as well as an indication of the relative risk of extrapulmonary
dissemination. In a case of chronic meningitis, a +ve CF for anti- coccidioidal
antibodies in the CSF often provides the only denite diagnostic indication
of the need for aggressive antifungal therapy.
Further reading
British Infection Association. Infection resources. M https:// www.britishinfection.org/ guidelines-
resources/ infection- resources/ .
Public Health England (2009). Public health laboratories:general clinical laboratory tests. M https://
www.gov.uk/ guidance/ public- health- laboratories- general- clinical- laboratory- tests.

400
CHAPTER5 Infectious and tropical diseases
400
Antigentests
Antigen measurement is achieved through techniques such as CF and immu-
nodiusion. Avariety of bodily uids can yield diagnostically useful antigens,
including saliva, serum, urine, CSF, and fresh stool. The choice depends
upon the clinical context. Again many of these tests have been replaced
by PCR or, in the case of HIV, combined antigen detection with antibody
responses to improve sensitivity.
Viral antigentests
Include mumps, CMV, inuenza, HIV, hepatitis B and C, RSV, parainuenza
viruses, adenovirus, rotavirus, and varicella- zostervirus.
Bacterial antigentests
Include L.pneumophila (serotype 1)and B.burgdorferi (in urine), β- haemolytic
streptococci, Pneumococcus, C.dicile, H.inuenzae, N.meningitidis, H.pylori,
and C.jejuni.
Helminthic antigentests
Filariasis.
Protozoal antigentests
Include malaria, giardiasis, Trypanosoma cruzi (Chagas’ disease), Pneumocystis
jiroveci (formerly Pneumocystis carinii). Malaria antigen tests form the basis
for the so- called RDTs which are now the mainstay of diagnosis for malaria
in both endemic and non- endemic areas. Two main forms are available;
histidine- rich protein 2 (HRP- 2) is specic for Plasmodium falciparum, whilst
Pf LDH can distinguish between P.falciparum and P.vivax. They are rapid
and almost as sensitive as PCR and critically do not need highly trained
sta to carry out. They are not so reliable for the other species P.ovale,
P.malariae, and P.knowelsi.
Fungal antigentests
Include C.neoformans (CrAg), H.capsulatum, and mannoprotein antigen in
C.albicans.
2
2 Falci DR, Stadnik CMB, Pasqualotto AC. A review of diagnostic methods for invasive fungal dis-
eases:challenges and perspectives. Infect Dis Ther 2017; 6
:213– 23.

ANTIGENTESTS
401

402
CHAPTER5 Infectious and tropical diseases
402
Collection ofspecimens
Principles ofgood specimen collection
• Good- quality specimen and clinical information produce the most
valuabledata.
•
Optimal time of collection, e.g. take bacterial specimens before
administering antibiotics.
•
Collect the optimal type of specimen wherever possible, e.g. pus is
preferable to a ‘pusswab’.
•
Acquire expertise in specimen collection— ensure minimal
contamination by normal ora (e.g. MSU, use of a tongue depressor for
throat swab collection).
•
Freshness of specimens— rapid transport to the laboratory is essential
(especially for anaerobic organisms and for ‘hot stools’ for parasite
diagnosis).
•
Collect the appropriate number of specimens at the appropriate
intervals, e.g. paired antisera should be taken at least 1– 6 weeks apart if
a diagnostic rising titre is to be demonstrated.
•
Be aware of biological hazards. It is critical to categorize samples with
potential pathogens in them and label appropriately.
•
Biohazard level 1:bacteria and viruses, including Bacillus subtilis, E.coli,
and varicella, as well as some cell cultures and non- infectious bacte-
ria. Precautions against the biohazardous materials are minimal, most
likely involving gloves and some sort of facial protection.
•
Biohazard level 2:bacteria and viruses that cause only mild disease
to humans or are dicult to contract via aerosol in a laboratory
setting such as hepatitis A, B, and C, some inuenza Astrains, Lyme
disease, Salmonella, mumps, measles, scrapie, dengue fever, and HIV.
Routine diagnostic work with clinical specimens can be done safely
at Biosafety level 2 (BSL- 2), using BSL- 2 practices and procedures.
Research work (including co- cultivation, virus replication studies, or
manipulations involving concentrated virus) can be done in a BSL- 2
(P2) facility, using BSL- 3 practices and procedures.
•
Biohazard level 3:bacteria and viruses that can cause severe or fatal
disease in humans, but for which vaccines or other treatments exist
such as anthrax, West Nile virus, Venezuelan equine encephalitis,
SARS virus, MERS- Co, hantaviruses, TB, typhus, Rift Valley fever,
Rocky Mountain spotted fever, yellow fever, and malaria. The para-
sites P.falciparum, which causes malaria, and T.cruzi, which causes
trypanosomiasis, also come under thislevel.
•
Biohazard level 4:viruses and bacteria that cause severe to fatal
disease in humans and for which vaccines or other treatments are
not available such as Bolivian and Argentine haemorrhagic fevers,
Marburg virus, Ebola virus, Lassa fever virus, Crimean– Congo
haemorrhagic fever, and other haemorrhagic diseases. Variola virus
(smallpox) is an agent that is worked with at BSL- 4, despite the
existence of a vaccine, as it has been eradicated. When dealing with
biological hazards at this level, the use of a +ve pressure personnel
suit, with segregated air supply, is mandatory. The entrance and exit

COLLECTION OF SPECIMENS
403
of a Level 4 biolab will contain multiple showers, a vacuum room, a
UV light room, an autonomous detection system, and other safety
precautions designed to destroy all traces of the biohazard. Multiple
airlocks are employed and are electronically secured to prevent both
doors from opening at the same time. All air and water services
going to and coming from a BSL- 4 (P4) laboratory will undergo
similar decontamination procedures to eliminate the possibility of an
accidental release.
Surface specimens include
• Anal/ anorectal:e.g. gonococcus (N.gonorrhoeae).
•
Cervical swab:e.g. HSV, gonococcus, human papillomavirus(HPV).
• Ear swab:e.g. otitis externa, otitis media, bacterial and fungal infections.
•
Foreign bodies:almost always infected if causing trouble! (Includes
iatrogenic foreign bodies such as arthroplasties, cardiac valves,
pacemakers, ventriculo- peritoneal shunts, etc.) Foreign bodies in the ear,
nose, or vagina can lead to prolonged (and often unpleasant) discharges.
•
Genital ulcers:dark ground microscopy for syphilis organisms. Also
chancroid, Entamoeba histolytica.
•
Indwelling catheters:include urinary catheters, IV cannulae, Portacaths,
etc. If a catheter is thought to be the source of an infection, cultures
should be performed, and if the catheter or cannula is removed, this can
be sent for culture. Urinary catheters are always colonized by bacteria.
Intravascular foreign bodies such as central venous catheters and
prosthetic heart valves are often aected by bacteria which are normally
non- virulent such as coagulase −ve staphylococci.
• Laryngeal swab:can be useful forTB.
•
Nasal, pharyngeal, gingival, and throat:e.g. meningococcus, S.aureus
carriage, streptococcal infections, pertussis, adenovirus. NPAs are
useful for diagnosing upper respiratory tract infections (URTIs) such as
inuenza and RSV through DIF tests and, more commonly now, PCR. In
lepromatous leprosy, a swab from the anterior nares may reveal acid-
fast bacilli indicative of this infection.
•
Ophthalmic:e.g. bacterial conjunctivitis, adenovirus, rabies (from corneal
impressions.
3
For trachoma, PCR, direct uorescein- labelled monoclonal
antibody (DFA), and EIA of conjunctival smears are useful.
4
• Skin:
•
Abscess— culture for bacteria and other unusual organisms.
•
Dermal scrapings, nail clippings— fungal infections (tinea— includes
pedis, capitis, cruris, versicolor forms).
•
Petechial rash scrapings— meningococcus (occasionally gonococcus).
• Throat:e.g. C.albicans, diphtheria, gonococcus, croup organisms.
•
Urethral:e.g. Chlamydia, gonococcus.
•
Vagina (high vaginal swab):e.g. S.aureus in toxic shock syndrome
(including toxin testing), Gardnerella, gonococcus.
3 Zaidman GW, Billingsley A. Corneal impression test for the diagnosis of acute rabies encephalitis.
Ophthalmology 1998; 105:249– 51.
4 Rostami S (2016). Trachoma. M http:// www.emedicine.com/ OPH/ topic118.htm.

404
CHAPTER5 Infectious and tropical diseases
404
Normally sterile uids include
• Amniotic uid:bacterial infection can cause premature delivery, and
rDNA was detected by PCR (E Molecular diagnostics, pp. 406–7) in
samples from 15 (94%) of 16 patients with +ve amniotic uid cultures.
5
Hydrops fetalis can be caused by congenital infections (e.g. CMV,
parvovirus B19, toxoplasmosis, syphilis, and Chagas’ disease), and
making a diagnosis may involve analysis of amniotic uid with cultures,
PCR,etc.
• Ascites:consider TB (consider laparoscopy for biopsying peritoneal
lesions for culture as well as histology; E Gastrointestinal tract
investigations, pp. 412–13).
•
Blood:multiple samplings at separate times from separate body sites
may need to be taken, e.g. in endocarditis. For some organisms and
pathologies, an extended period of culture may be needed.
•
CSF:possibilities include, e.g. meningococcus, pneumococcus,
L.monocytogenes, TB, fungi (e.g. C.neoformans), and viruses (E Tissue
biopsy and deep aspiration specimens, pp. 418–21).
•
Ejaculate (semen):if the semen contains a high number of leucocytes,
this may be an indication of either infection or inammation. WBCs
are considered signicant if >1million found in each mL of ejaculate.
Sexually transmitted diseases (STDs), e.g. gonorrhoea, or urea,
plasma, and prostate infections come into the dierential diagnosis.
S.haematobium (bilharzia) may cause haemospermia and be found
in ejaculate.
6
Acute mumps orchitis can be associated with loss of
spermatozoa.
•
Ocular uids (intra- ):include aqueous humour and vitreous humour.
Bacterial, fungal, and parasitic problems can aect the interior of
theeye.
•
Pericardial uid:the commonest organisms will include staphylococci,
streptococci, pneumococci, H.inuenzae, meningococci, andTB.
•
Pleural uid:numerous pathologies, including underlying bacterial
pneumonia, tuberculous pleurisy, parasitic infections (such as
strongyloidiasis), and fungal diseases (such as histoplasmosis). Biopsy
of the pleura under direct visualization by video- assisted thoracoscopy
(VATS) may give a better diagnosticyield.
•
Synovial uid (joint aspirate):bacterial infections can be very destructive
and the options are legion. Staphylococcal disease is the commonest,
but TB must always be borne in mind. Viral arthritides are usually self-
limiting, and treatment is supportive.
•
Urine:standard culture and sensitivity, e.g. MSU specimen, catheter
specimen of urine (CSU)— useful for diagnosing cystitis, pyelonephritis,
prostatitis, etc. (prostatic massage may be helpful for improving
diagnosis of prostatic infections); ‘early morning urine’ (EMU) for TB,
and a terminal specimen for S.haematobium (bilharzia) best collected
around midday.
5 Hitti J, Riley DE, Krohn MA, etal. Broad- spectrum bacterial rDNA polymerase chain reaction
assay for detecting amniotic uid infection among women in premature labor. Clin Infect Dis 1997;
24
:1228– 32.
6 Torresi J, Yung A. Usefulness of semen microscopy in the diagnosis of a dicult case of Schistosoma
haematobium infection in a returned traveler. J Travel Med 1997; 4
:46– 7.

COLLECTION OF SPECIMENS
405
Normally infected uids include
• Pus:e.g. abscess contents, wound swab/ aspirates, drainage swabs.
Usually bacterial (consider both aerobic and anaerobic), but amoebic
and hydatid options need to be considered when the lesion is in
theliver.
•
Saliva:normally contains a wide range of commensal ora. Cannulation
of a parotid gland duct may yield a specic pathogen that is causing a
problem in thatgland.
•
Sputum:includes tracheal aspirate, induced sputum (obtained with
physiotherapy assistance), and bronchoalveolar lavage (BAL), which
may be needed in sicker patients unable to produce sputum or in
conditions where copious sputum production may not be a feature
(such as P.jiroveci
pneumonia (PCP) in HIV infection). C&S assists with
identifying a vast range of organisms, including and especially TB (always
ally sputum culture to direct microscopy).
•
Stool:vast range of uses (E Gastrointestinal tract investigations,
pp. 412–13). Includes direct microscopy for parasites, ova, and cysts,
i.e. giardia and ascariasis, culture for Salmonella, Campylobacter, Shigella,
E.coli 0157, typhoid and paratyphoid, Plesiomonas shigelloides, and
enteroviruses, and antigen detection of rotavirus. ‘Hot stools’ (from
patient to the microbiology bench in <1h) may be helpful for amoebae,
strongyloides larvae,etc.
Further reading
Health and Safety Executive. Biological agents:managing the risks in laboratories and healthcare premises.
M
http:// www.hse.gov.uk/ biosafety/ biologagents.pdf.
406
CHAPTER5 Infectious and tropical diseases
406
Molecular diagnostics
These tests are now the mainstay of mainstream clinical practice. HIV viral
load and antiretroviral drug resistance are considered routine tests, whilst
examination of the CSF for JC virus DNA by PCR is the method of choice
for the diagnosis of progressive multifocal leukoencephalopathy (PMLE).
Genetic testing for the rifampicin resistance mutation in RPO provides a
rapid indication of MDR- TB, e.g. Cephid GeneExpert. (See Fig. 5.8 for the
immunological prole of HIV disease.)
The areas ofgreatest value include
• Detection and quantication of viruses to monitor and guide therapy,
e.g. HCV, HIV, HBV,CMV.
•
Detection of slow- growing organisms, e.g. TB, atypical mycobacteria.
• Diagnosis of pathogens, which are potentially too dangerous for the
laboratory sta to handle, e.g. VHF, smallpox, avian/ swineu.
• Detection of organisms killed by antibiotics prior to culture samples
being taken, e.g. meningococcal sepsis.
•
Detection of organisms that cannot be cultured, e.g.HCV.
• Detection of unusual diseases, e.g. helminthic diseases,fungi.
• Detection of toxins elaborated in small quantities by bacteria, e.g. toxic
shock syndrome toxins.
•
Detection of mutations manifesting resistance to antimicrobial agents
(genotypic resistance testing), e.g. HIV, CMV,TB.
•
Elucidation of pathogens that are as yet ‘undiscovered’, e.g. 16s
ribosome testing.
01 23 45678
AB
AB
HIV viral load
CD4+ cell count
Anti-HIV antibody
HIV viral load
Anti-HIV antibod
y
CD4+ cell count
Years
‘Window period’
Symptoms likely to be present
Exposure
Fig.5.8 The immunological prole of HIV disease.

MOLECULAR DIAGNOSTICS
407
Available molecular techniques include
• PCR:this test uses probes to look for the presence of the genes of
infecting organisms (E
Polymerase chain reaction amplication of
DNA, pp. 324–6).
There are numerous PCR tests available, and it is particularly valuable for
hepatitis C (including for genotyping), HIV, and TB. Auniversal eubacterial
PCR (for genus and species identication of prokaryotes) and a universal
fungal PCR (genus and species identication of fungi) are available. HBV
DNA quantication is accomplished throughPCR.
Choosing an appropriate sample for the application of PCR testing is
important (e.g. biopsy of possible Kaposi’s sarcoma lesion and Kaposi’s
sarcoma- associated herpesvirus (KSHV) (human herpesvirus (HHV)- 8);
BAL uid and PCP; small bowel biopsy and Whipple’s disease; CSF and
meningococcal disease, HSV, or M.tuberculosis). Note:uid samples for PCR
usually have a higher yield when not spun in a centrifuge.
•
LCR (ligase chain reaction):works through specic probe amplication
through the use of DNA ligase. Greatest value in Chlamydia infection.
•
Transcription- mediated amplication (TMA):uses an isothermal
amplication system. Amplied telomerase products are rna,
detected using a non- isotopic hybridization protection (HPA) system.
Identication of HCV, TB, gonococcus, and Chlamydia are among its
potentialuses.
•
Branched- chain DNA (bDNA):a signal amplication methodology able to
quantify HIV RNA levels.
• Nucleic acid sequence- based amplication (NASBA):a quantitative test for
HIV RNA; also of value withCMV.
• Hybridization with nucleic acid probes:detects specic ribosomal RNA
and is most widely used for culture conrmation of an organism (e.g.
fungi, mycobacteria).
•
Sequencing:organisms are identied by direct sequencing of amplied
gene fragments. Has been applied to TB, H.pylori, enteroviruses, and
HIV (for assessing drug resistance). Whole genome sequencing (next-
generation sequencing) is moving forward rapidly but brings with it the
problems of bioinformatics and ‘bigdata’.
•
Restriction fragment length polymorphisms (RFLPs):restriction enzymes are
used to cut up DNA into pieces, and the fragments are then subjected
to gel electrophoresis (such as Southern blotting; E Southern blotting,
pp. 322–3). The patterns produced can be used to identify organisms.
Further reading
Shanson DC. Microbiology and Clinical Practice, 3rd edn. Oxford:Butterworth- Heinemann,1999.

408
CHAPTER5 Infectious and tropical diseases
408
Haematology
Many infectious diseases manifest haematological changes that are diagnosti-
cally valuable.
Bloodlm
Blood smear examination provides general data on the size and appearance of
cells, as well as data on particular cell segments, whilst pathogens may be seen,
e.g. malaria, African trypanosomiasis, Chagas’ disease, babesiosis, borreliosis,
bartonellosis, ehrlichiosis, laria (time of day the blood is taken may be signi-
cant in this condition), haemolysis, and evidence of hyposplenism. Thick and
thin blood lms should be considered, especially where malaria is concerned;
at least three blood lms, each taken 24h apart, should be performed. Blood
lms are also useful in assessing if a patient hasDIC.
Bone marrow examination
Culture (for, e.g. TB, brucellosis, Mycobacterium avium intracellulare,
typhoid/ paratyphoid, CMV), microscopy (for, e.g. leishmaniasis), and
establishing cell line integrity (e.g. WBC abnormalities). An aspirate is gen-
erally useful for culture purposes and for establishing what cells are present
in the marrow, but a trephine is needed if structural information is needed
(e.g. to establish if granulomata suggestive of TB are present).
Coagulation studies, brin degradation products, D- dimers
Useful where DIC is suspected. DIC is a common association of severe sep-
sis (especially meningococcal disease). Coagulation abnormalities are also
present in conditions such as VHF, P.falciparum malaria, rickettsial diseases,
etc. D- dimers may assist with the diagnosis of thrombosis but are generally
raised in infection.
Cold agglutinins
A haemagglutination- based test. Can be caused by M. pneumoniae (most
commonly), inuenza A, inuenza B, parainuenza, and adenoviruses.
Dierential white cell count in peripheral blood. Useful associations
include:
•
Eosinophilia and parasite infection.
• Neutrophilia and bacterial sepsis.
• Neutropenia and atypical pneumonias.
• Atypical lymphocytes andEBV.
• Neutropenia and pyrexia.
Erythrocyte sedimentationrate
Together with CRP (E Biochemical tests, pp. 416–17), the rate of eryth-
rocyte sedimentation is sensitive to the extent of a body’s response to a
lesion or disease. The ESR is important for pointing to the possible exist-
ence of an organic disease, but a normal result does not exclude the pres-
ence of disease. Ai
ESR points to the need for additional investigations
and, if d, is very useful in monitoring the course of a disease. Procalcitonin
(PCT) may eventually prove to have even greater value in a similarrole.
7
7 Meisner M, Tschaikowsky K, Palmaers T, Schmidt J. Comparison of procalcitonin (PCT) and C- reactive
protein (CRP) plasma concentrations at dierent SOFA scores during the course of sepsis and MODS. Crit
Care 1999; 3
:45– 50. M http:// www.pubmedcentral.nih.gov/ articlerender.fcgi?artid=29013.

HAEMATOLOGY
409
Ferritinlevels
d in iron deciency, e.g. associated with hookworm infestation of the bowel
(Ancylostoma duodenale, Necator americanus) or H.pylori
- associated gastritis.
Ferritin levels are often extremely high in Still’s disease, an important non-
infective cause of pyrexia and neutrophilia. It is also markedly raised in hae-
mophagocytic lymphohistiocytosis syndrome (HLH). Serum iron and TIBC
may be helpful in a fuller evaluation (E Biochemical tests, pp. 416–17).
Glucose- 6- phosphate dehydrogenase
Useful in therapy of benign malarias, e.g. P.vivax and P.ovale (a deciency
will cause severe haemolysis when primaquine is used to kill the hypnozoite
phase to prevent relapse).
Hb concentration and red cell parameters (especially mean
cell volume)
Useful in, e.g. anaemia of chronic infection, haemolysis, iron deciency
(microcytosis) associated with hookworm infestation of the bowel or
H.pylori- associated gastritis, macrocytosis due to vitamin B
12
deciency with
Diphyllobothrium latum (sh tapeworm) infestation.
Hb electrophoresis
Can detect haematological conditions such as thalassaemia, sickle- cell dis-
ease, etc. These can predispose to certain infections via hyposplenism such
as salmonellosis and melioidosis.
Haemolysis screen (including reticulocytecount)
May be abnormal in, e.g. malaria, DIC, EBV, VHF, E.coli 0157 gastroenteritis
due to HUS, rickettsial infections, dengue, and gas gangrene. Hp levels can
be useful (E Biochemical tests, pp. 416–17), since they are generally d/
absent in haemolysis.
Monospot test (Paul Bunnelltest)
Diagnostic of EBV infection.
Sicklingtest
Uncovers sickle- cell disease, known to be associated with Salmonella osteo-
myelitis, chronic leg ulcers,etc.
Thrombocytopenia
Characteristic in some conditions, e.g. HIV disease, P. falciparum malaria,
and dengue.
Vitamin B
12
level
d in D.latum infestation, TB of the terminal ileum,etc.

410
CHAPTER5 Infectious and tropical diseases
410
Radiology
PlainX- rays
• Chest:the potential diagnoses are legion, including pneumonia, TB,
pleural eusion/ empyema, bronchiectasis, PCP, tropical eosinophilia
and other parasite- related diseases (e.g. paragonimiasis), occupational
risks for infections (e.g. silicosis and TB), and post- varicella calcication.
Also useful to exclude non- infectious causes of fever such as cancer or
sarcoidosis.
•
Plain abdominal X- ray:e.g. bowel dilatation and perforation, calcication
of adrenal glands and lymph nodes (e.g. TB, histoplasmosis), ‘babies
head’ sign of schistosomal bladder calcication.
•
Dental radiography (orthopantomogram):occult dental sepsis.
•
Elsewhere:e.g. limbs for osteomyelitis, skeletal muscles for calcied
cysticercosis lesions, joints for Charcot changes (such as in syphilis).
More sophisticated imaging
Endoscopic retrograde cholangiopancreatography
Using contrast medium and radiographs to dene the anatomy of the bil-
iary tree and pancreatic duct. Useful for HIV- associated biliary tree disease
(including porta hepatis nodal lymphoma), parasites (e.g. Clonorchis sinensis,
Ascaris lumbricoides), and pancreatic disease suchasTB.
Intravenous urogram
Denes renal anatomy. Renal infection, such as pyelonephritis, renal calculi,
malignancy, or anatomical abnormalities (including congenital) leading to
recurrent infections. Computed tomography intravenous pyelography (CT-
IVP) is generally considered superiornow.
Magnetic resonance imaging
Including with contrast enhancement using gadolinium.
•
Cranial:vCJD (exhibits bilateral pulvinar high signal), encephalitis, rabies,
sagittal sinus thrombosis, PMLE. MRI is also more sensitive than CT
when looking for infective SOLs such as tuberculoma, especially in and
around the cerebellum.
•
Bones:essential for the diagnosis of osteomyelitis.
•
Spinal cord:essential for diagnosis of spinal infections and myelitis.
•
Elsewhere in the body:dening solid lesions, uid- lled lesions,etc.
• Magnetic resonance cholangiopancreatography (MRCP):non- invasively
denes the hepatopancreatic biliary tree anatomy.
•
New techniques:e.g. as cardiac MRI imaging, show great promise in the
diagnosis of valvular disease.
Computed tomography
Including with contrast enhancement.
•
Cranial:e.g. brain abscess, paranasal sinus disease, middle ear disease,
orbital sepsis, cysticercosis, mastoid aircells.
•
Chest:e.g. cardiac lesions (possibly with associated endocarditis risk),
mediastinum (e.g. lymphadenopathy, including retrosternal), lung lesions
such as bronchiectasis, lung abscess, other non- infectious pathologies.

RADIOLOGY
411
• Abdomen:delineates intra- abdominal abscesses and abnormalities in
retroperitoneal and mesenteric lymph nodes, defects in the spleen, liver,
kidneys, adrenals, pancreas, and pelvis.
•
CT- IVP:powerful tool for dening the anatomy of the urinarytract.
•
Multislice CTPA:e.g. for dening PEs as a cause ofPUO.
• CT- guided biopsy:to specically pick out an area for sampling, e.g. liver
lesion, lymph node, mediastinalmass.
•
CT colonoscopy:helpful in diagnosing colonic disease, especially in
older patients unable to undergo invasive testing (e.g. when looking
for an associated colonic tumour in a patient with Streptococcus bovis
endocarditis).
Ultrasound
•
Abdomen:evidence of pancreatic, liver, renal, and biliary tree/ gall
bladder abnormalities (e.g. abscess, hepatic cyst, presence or absence of
spleen, ascites, gallstones,etc.).
•
Thoracic:pleural eusion, empyema (can assist with drainage).
•
Echocardiography:to help exclude cardiac vegetations of endocarditis,
TB pericarditis (with eusion), myocarditis. Note
:both TTE and TOE
approaches are available, each yielding data of diering value in dierent
situations.
•
Doppler studies of blood vessels:to exclude DVT such as in thelegs.
•
Guided biopsy:to specically pick out an area for sampling, e.g. liver
lesion, lymphnode.
•
Drainage:to specically pick out an area for draining, e.g. liver abscess,
pleural eusion.
Radionuclide scanning
•
Has limited value but can localize area of inammation for biopsy.
• Indium (
111
In)- labelled granulocyte scan:may help localize many infectious
or inammatory processes (i.e. deep sepsis).
•
99m
Technetium bone scan:bone and joint sepsis.
•
V/ Q scan:to exclude PE as a cause of PUO, to delineate consolidation,
abscess, bronchiectasis,etc.
Positron emission tomography- computed tomography (PET- CT)
Enormous potential for locating localized infective processes, especially in
the brain. Increasingly used in oncology and can be helpful inPUO.
These techniques are all described in detail in E Chapter13.

412
CHAPTER5 Infectious and tropical diseases
412
Gastrointestinal tract investigations
Biopsy- based
• Duodenal biopsy (Crosby capsule and endoscopic methods ± EM): e.g.
Whipple’s disease, giardiasis, cryptosporidium, strongyloidiasis.
•
Gastric biopsy:H.pylori.
•
Laparoscopy:useful to exclude TB and other infections in the presence
of ascites (biopsies should be sent for histology and C&S; peritoneal
biopsies have a higher yield than ascitic uid).
•
Liver biopsy:E Tissue biopsy and deep aspiration specimens, pp. 418–21.
•
Oesophageal biopsy:e.g. candidiasis, CMV (e.g. in advanced HIV disease).
•
Sigmoidoscopy and bowel biopsy:e.g. amoebiasis, pseudomembranous
colitis (C.dicile infections), exclusion of microscopic colitis andIBD.
Gastrointestinal contents- based
• These are rarely performed now, as endoscopic sampling is standard.
• Baermann concentration technique:the method of choice for the
detection of Strongyloides stercoralis.
•
Capsule endoscopy:involving the swallowing of a pill- sized capsule
containing digital video recording equipment, which broadcasts to
a receiver outside of the body. Enormous potential for diagnosing
pathology within the GIT, including small bowel infective processes such
asTB.
8
• Duodenal aspirate:e.g. giardiasis, cryptosporidium, strongyloidiasis.
•
Enterotest (string test):e.g. giardiasis, cryptosporidium, strongyloidiasis.
•
Hot stools:little evidence that they need to be hot! (E Culture techniques,
pp. 390–5.)
•
Stool C&S (E Culture techniques, pp. 390–5).
•
Stool microscopy:for ova, cysts, parasites (e.g. amoebae, helminths such
as A.lumbricoides).
•
Stool EM:good for viruses, e.g. rotavirus and norovirus, but rarelydone.
•
Stool chromatography:C.dicile toxin, a specic C.dicile toxin Aand B
EIA and PCR areused.
• Sellotape
®
(adhesive) strip test:for the threadworm Enterobius
verminformis. To perform this test, roll some clear adhesive tape around
four ngers of a hand, sticky side out, whilst an assistant spreads the
buttocks. In good lighting, identify the involved perianal area, and apply
the tape 1– 2 times to the aected perianal area. Place the tape on a
slide with the clean side downwards, trim the tape, label the slide, and
send to the laboratory.
•
Toxin tests:most widely used for C.dicile toxins Aand/ or B; the
denite diagnosis of botulism food poisoning is the examination of
faeces for Clostridium botulinum
and toxin (EMG is also helpful). In
wound botulism (e.g. in drug injectors), the organism may grow in
material taken directly from the wound(s); also used for E.coli0157.
8 Lee WK, Van Tonder F, Tartaglia CJ, etal. CT appearances of abdominal tuberculosis. Clin Radiol
2012; 67
:596– 604.

GASTROINTESTINAL TRACT INVESTIGATIONS
413
Gastrointestinal tract function
• D- xylose absorption test:for malabsorption syndromes such as Whipple’s
disease and tropicalsprue.
•
13
C breath test:for detection of H.pylori.
9
• Faecal elastase:for diagnosis of pancreatic exocrine insuciency in
conditions suchasHIV
•
Vitamin levels:diagnosis of fat- soluble vitamin malabsorption in
pancreatic or small bowel disease or of B
12
levels in terminal ileal
problems such as withTB.
9 Di Rienzo TA, D'Angelo G, Ojetti V, etal. 13C- urea breath test for the diagnosis of Helicobacter
pylori infection. Eur Rev Med Pharmacol Sci 2013; 17
Suppl 2:51– 8.

414
CHAPTER5 Infectious and tropical diseases
414
Immunology
Cutaneous hypersensitivity tests are discussed elsewhere (E Other tests,
pp. 422–3).
•
Complement (especially ‘terminal’ complements C5– C9):deciencies can
lead to recurrent meningococcal sepsis, pneumococcal disease,etc.
•
Cytokine studies:currently experimental, but levels of interferon can
sometimes be helpful.
•
Dierential white cell count (E Haematology, pp. 408–9):neutropenia
and lymphopenia are associated with bacterial sepsis.
•
Igs:deciencies lead to recurrent infections (some cases may be
hereditary, and family history is important). Levels may also be higher—
IgM tends to be markedly i in brucellosis, malaria, trypanosomiasis, and
toxoplasmosis.
•
Splenic dysfunction:indicated by a history of surgical removal (not
always clear!) or a condition associated with hyposplenism (e.g. coeliac
disease/ dermatitis herpetiformis, sickle- cell disease), an abnormal blood
lm, and an absent spleen on abdominal imaging. This state may be
associated with recurrent meningococcal infection and life- threatening
pneumococcal sepsis. Once diagnosed, the patient will need appropriate
vaccinations and advised to always carry a warning card and/ or wear a
MedicAlert
®
bracelet or similar.
•
T- cell subsets:the absolute CD4+ (T4) cell count and the CD4+/ CD8+
(T4/ T8) cell ratio is of value. HIV disease, TB, and sarcoidosis are
associated with reduced CD4+ cell levels, HIV with a reversed CD4+/
CD8+ cellratio.
•
Human pharmacogenomics:
•
HLA typing— HLA B5701 is associated with hypersensitivity (some-
times fatal) to the antiretroviral drug abacavir, and so patients should
be tested before prescribing thisdrug.
•
IL- 28R polymorphisms— these can be associated with treatment failure
in hepatitis C infection.

IMMUNOLOGY
415

416
CHAPTER5 Infectious and tropical diseases
416
Biochemicaltests
A number of biochemical tests are useful in the diagnosis and assessment of
a range of infectious illnesses.
•
AFP:i in hepatocellular carcinoma (associated with HCV and HBV).
Note
:much higher AFP than in other causes of hepatocellular damage.
• ABGs:assessment of sepsis, assessment of pneumonia.
•
Ca- 125:i false +ve in peritonealTB.
•
CRP: together with the ESR, a valuable method for monitoring infections.
CRP is an acute phase reactant, i in bacterial infections and d in viral
infections. PCT may be ofvalue.
• Creatinine phosphokinase (CPK) level:i in L.pneumophila infection.
10
Also
i with zidovudine (AZT) usage in HIV disease.
•
Glucose metabolism:DM is a common association of infection,
particularly TB. Consider performing a fasting glucose level, an OGTT,
or checking HbA
1c
levels.
•
Hp levels:part of the haemolysis screen (E Haematology, pp. 408–9).
Iron levels (serum iron), TIBC:iron d (TIBC i) in iron deciency, e.g.
associated with hookworm infestation of the bowel (A.duodenale,
N.americanus) or H.pylori
- associated gastritis. Serum ferritin may be
helpful.
•
Lactate levels:may be i in HIV- associated mitochondrial toxicity
syndrome. Also high in severe sepsis syndrome.
•
Lipid abnormalities (cholesterol, TGs):HIV drug toxicity.
•
LFTs:abnormalities are present in many conditions, e.g. hepatitis,
leptospirosis, yellow fever, antimicrobial drug toxicity (e.g. inTB).
•
ALP:in the serum of healthy adults, ALP mostly originates from the
liver. Biliary obstruction, often associated with sepsis, leads to an i in
serum concentration ofALP.
•
Bilirubin:determination of bilirubin levels (conjugated and unconju-
gated) is important in the dierential diagnosis of jaundice.
•
γGT:i in serum concentration of γGT is the most sensitive indicator
of liver damage.
•
Pancreatic amylase level:i with pancreatitis in, e.g. mumps (consider
also salivary amylase), toxicity with antiretroviral drugs (e.g. didanosine
orDDI).
•
Pleural uid analysis:analysis for LDH levels is useful (as well as albumin,
total protein, and amylase). An exudate, which implies infection in
the dierential diagnosis, is dened by at least one of the following
criteria:pleural uid/ serum total protein ratio >0.5, pleural uid/ serum
LDH ratio >0.6, or pleural uid LDH > two- thirds the upper limits of
normal of serumLDH.
•
Pregnancy test:some infections, e.g. varicella, genital herpes (simplex),
and TB, are often more serious in pregnancy. The use of some
antibiotics, e.g. ciprooxacin and tetracyclines, is relatively contra-
indicated in pregnancy. There is the potential to prevent vertical
transmission of HIV if diagnosed prior to delivery.

BIOCHEMICALTESTS
417
• PCT:shows promise for detecting and following ‘inammation induced
by microbial infections’.
10
• Serum Na
+
levels:hyponatraemia associated with legionnaires’ disease
11
but may also be related to intracranial sepsis or hypocortisolaemia.
•
Synacthen
®
test:TB and histoplasmosis can damage the adrenal glands,
leading to an Addisonian state (hypocortisolaemia).
•
Vitamin D levels:if decient, may predispose to TB and may lead to
diculties with treating TB infections due to hypocalcaemia (consider
checking levels in patients with darkly pigmented skins, especially those
with a culture of wearing clothing over most of their body surface).
Deciency may be exacerbated by some anti- infective drugs, notably
rifampicin and antiretrovirals.
10 Meisner M, Tschaikowsky K, Palmaers T, Schmidt J. Comparison of procalcitonin (PCT) and
C- reactive protein (CRP) plasma concentrations at dierent SOFA scores during the course of
sepsis and MODS. Crit Care 1999; 3:45– 50. M http:// www.pubmedcentral.nih.gov/ articlerender.
fcgi?artid=29013.
11
Kociuba KR, Buist M, Munro R, Lee A, Cleland B. Legionnaires’ disease outbreak in south western
Sydney, 1992. Clinical aspects. Med J Aust 1994; 160:274– 7.

418
CHAPTER5 Infectious and tropical diseases
418
Tissue biopsy and deep aspiration
specimens
Whatever part of the anatomy from which they are taken, biopsy speci-
mens should be evaluated both histopathologically (with specialized stains
used, wherever appropriate) and by culture for bacteria, mycobacteria,
fungi, viruses, and prions (using specialized culture techniques, where
appropriate).
Bone marrowbiopsy
(E Haematology, pp. 408–9)
Important for TB, brucellosis, Mycobacterium avium intracellulare
typhoid/
paratyphoid, leishmaniasis.
Cerebrospinaluid
Whilst the main objective of an LP is usually to obtain uid for microscopy
and C&S, there are other useful tests that can be performed. The opening
pressure should be between 10 and 20cmH
2
O— infective and other pro-
cesses may alter this. Whilst the usual approach to obtaining CSF is through
an LP, if the pressure is high, a cisternal puncture can be performed instead
(normally the advice and help of a neurosurgeon would need to be sought).
In neonates, foramenal puncture is a possibility. ACT scan is usually per-
formed beforehand to assess the risk of ‘coning’, although it is by no means
a guarantee.
Along with the Gram staining process and microscopy, other tests to con-
sider include a complete blood cell count and dierential measurement of
glucose and protein levels, ZN staining for TB, and bacterial, mycobacterial,
viral, and fungal cultures. On occasions, other tests that might be consid-
ered include:
•
Wet mount (for amoebae such as Acanthamoeba; E Culture
techniques, pp. 390–5).
•
PCR for HSV, herpes varicella- zoster, enteroviruses, TB, JC virus, and
cryptococcosis (E Molecular diagnostics, pp. 406–7).
•
Antibodies to specic pathogens (e.g. arboviruses; E Serology,
pp. 396–9).
•
India ink capsule stain (for cryptococcosis).
• CrAg (E Serology, pp. 396–9).
•
VDRL, etc. for syphilis (E Serology, pp. 396–9).
•
14- 3- 3 protein— specic protein marker present in the CSF of patients
withvCJD.
•
Xanthochromia to help exclude SAH also seen in leptospirosis.
• Cytology to help exclude carcinomatous meningitis.
• Assessing comparative CSF protein– cellular levels if GBS (recognized
association of infections such as Campylobacter gastroenteritis) is being
considered.
Liverbiopsy
Indications are numerous and include assessment of viral hepatitis (espe-
cially HBV and HCV, including possible cirrhosis and/ or hepatocellu-
lar carcinoma), assessment of PUO (including TB and lymphoma), and

TISSUE BIOPSY AND DEEP ASPIRATION SPECIMENS
419
determining if the patient has a medication- induced liver disease. Only on
<1% of occasions does the liver biopsy overestimate the amount of hepatic
damage. Fibroscan is increasingly used in conjunction with blood tests to
non- invasively score liver damage, but it is not as accurate as the biopsy.
The biopsy is commonly preceded by an USS examination of the liver to
determine the best and safest biopsy site. Usually, the biopsy is conducted
under ultrasonic guidance to avoid major blood vessels. Coagulation status
should be optimized at the time of biopsying, including by the administration
of clotting factors.
The risks ofthe traditional liver biopsy (not performed underultrasound
guidance) include
•
Pain (1 in 5 patients).
• Haemorrhage (1 in 500 patients).
• Bleeding requiring transfusion or surgery (1 in 1000 patients).
• Pneumothorax and/ or puncture of the gall bladder, kidney, or bowel
(1 in 1000 patients).
•
Death (1 in 5000 patients).
Equivalent gures are not available for USS- guided liver biopsy, but the
technique is well established. Liver biopsy material should be subjected to
microbiological culture, as well as to histological assessment.
Lymph node sampling
The likely pathologies depend upon whether or not lymphadenopathy is
regional or generalized, and upon thesite.
•
Biopsy:for histology and culture, especially for TB, for tropical infections
such as chancroid, and for other relevant infections such as cat- scratch
fever (B.henselae). If regional, the dierential diagnosis varies with the
site; if intra- abdominal, for example, TB, Y.enterocolitica, and adenovirus
should be considered.
•
FNA:useful as full biopsy for culture purposes, but no structural
information available (similar to the aspirate vs trephine issue in BM
sampling), therefore dicult to exclude lymphoma from dierential.
Respiratory samples
Sputumtests
•
Microscopy:can perform direct microscopy (e.g. for Aspergillus species,
eggs of paragonimiasis), Gram stain, ZN, PCP (silver staining needed).
• Induced sputum:e.g. for TB, PCP, Aspergillus.
•
Tracheal aspirate:used in ill individuals. May produce similar material.
Bronchoscopy
•
BAL:useful for TB and other mycobacteria, PCP, fungi, melioidosis,
resistant bacteria (e.g. Pseudomonas), RSV, and paragonimiasis.
•
Lung biopsy:useful for TB and other mycobacteria, PCP (needs silver
staining, immunouorescence test (IFT), or PCR), fungi, melioidosis,
resistant bacteria (e.g. Pseudomonas), RSV, and paragonimiasis. Also for
exclusion of non- infective causes of non- resolving pneumonia.
Open lungbiopsy
When not feasible to obtain intrathoracic tissue by less invasivemeans.

420
CHAPTER5 Infectious and tropical diseases
420
Pleural disease:eusion, empyema,biopsy
Consider, e.g. TB, pneumococcal sepsis, underlying neoplasm (and rarer
conditions like strongyloidiasis). Biochemical analysis of pleural uid can
help (E Biochemical tests, pp. 416–17). An empyema will have white cell
count, protein, pH, LDH changes compatible with an exudate, and possibly
organisms visible and/ or culturable within the uid. Apleural biopsy can be
obtained blindly with an Abraham’s needle, but in recent times pleuroscopy
and VATS has developed into a better option.
12
Skinbiopsy
Biopsy and hair sampling
•
Useful for, e.g. TB, Kaposi’s sarcoma (caused by HHV- 8 and associated
with HIV), onchocerciasis, the aetiology of warts (common viral warts
vs molluscum contagiosum— the distinction can be important in view of
the therapeutic options and the potential for malignant change in some
sites such as the ♀ cervix).
•
The identication of pathogenic arthropod parasites, e.g. myiasis
(the invasion and feeding on living tissues of humans or animals by
dipterous larvae such as that of the tumbu y), scabies, lice, ticks, and
chigger eas, depends on the oending agent being seen and correctly
recognized or the appropriate specimen (e.g. excision biopsy) being
taken and examined histologically.
Skinsnips
•
Filarial infestations:examination of skin snips will identify microlariae
of Onchocerca volvulus and Mansonella streptocerca. Skin snips can be
obtained using a corneal– scleral punch, or more simply a scalpel and
needle. The sample must be allowed to incubate for 30min to 2h
in saline or culture medium, and then examined microscopically for
microlariae that would have migrated from the tissue to the liquid
phase of the specimen. In onchocerciasis, nodulectomy is also of value,
as is examination of the eye with a slitlamp.
•
Leprosy:acid- fast bacilli are present in theskin.
Other tissues and collections are numerous and include
•
Bone infection/ abscess/ osteomyelitis:consider, e.g. pyogenic sepsis, TB,
atypical mycobacteria, sickle- cell disease, ectopic ova of schistosomiasis.
History is important, e.g. with a history of ght trauma to a hand,
anaerobic bony infection may be more likely.
•
Brain lesions and abscesses:biopsy and drainage useful for, e.g. TB,
herpes simplex, rabies, cysticercosis, encephalitis, vCJD, JC virus, and
toxoplasmosis (in HIV infection).
•
Cervix:HPV, HSV, N.gonorrhoeae.
•
Joint infections:aspirate synovial uid and consider, e.g. pyogenic
sepsis, TB. An acute attack of gout (diagnosed through identifying the
birefringent crystals of sodium urate) can mimic an acute infective
12 Corcoran JP, Wrightson JM, Belcher E, DeCamp MM, Feller- Kopman D, Rahman NM. Pleural
infection:past, present, and future directions. Lancet Respir Med 2015; 3:563– 77.

TISSUE BIOPSY AND DEEP ASPIRATION SPECIMENS
421
arthritis (including a systemic inammatory response with neutrophilia)
and should be excluded.
13
• Liver abscess:consider Streptococcus milleri, hydatid disease, amoebic
dysentery, necrotic hepatocellular carcinoma in hepatitis C or hepatitis
B, obstruction of the biliary tree by A.lumbricoides or liver ukes such as
C.sinensis.
•
Muscle biopsy:
•
Cardiac— may point towards myocarditis or Chagas’ disease.
•
Skeletal— may be used to identify parasites, including, e.g. trichinosis,
cysticercosis.
•
Nerve biopsy:peripheral nerve biopsy (e.g. posterior auricular nerve)
may reveal tuberculoid leprosy.
•
Ocular:
•
Vitreous humour— e.g. intraocular infections, including fungal, HSV,
herpes varicella- zoster, pyogenic bacterial.
•
Cornea— e.g. rabies,CJD.
•
Retina— e.g. herpes varicella- zoster, toxocariasis,CMV.
• Paranasal sinus aspirates:e.g. bacteria, fungal (such as mucomycosis).
•
Pericardial biopsy:particularly important for establishing a diagnosis in
chronic pericarditis, e.g. TB, fungal.
•
Peritoneal infection:via laparoscopic tissue sampling and ascites sampling
(E Gastrointestinal tract investigations, pp. 412–13).
•
Splenic aspiration:useful in the diagnosis of visceral leishmaniasis
(kala- azar) by microscopic examination and culture and demonstration
of the organism.
14
• Tonsillar biopsy:of particular value for diagnosing vCJD:also consider
MRI scanning (E
Radiology, pp. 410–11), EEG, and 14- 3- 3 protein in
theCSF.
15
13 Singh N, Vogelgesang SA. Monoarticular arthritis. Med Clin North Am 2017; 101:607– 13.
14 Sakkas H, Gartzonika C, Levidiotou S. Laboratory diagnosis of human visceral leishmaniasis.
J Vector Borne Dis 2016; 53
:8– 16.
15 Collins S, Boyd A, Fletcher A, Gonzales MF, McLean CA, Masters CL. Recent advances in the
pre- mortem diagnosis of Creutzfeldt- Jakob disease. J Clin Neurosci 2000; 7:195– 202.

422
CHAPTER5 Infectious and tropical diseases
422
Othertests
Antibiotic plasma concentration monitoring
Some drugs are toxic if the plasma levels rise too high and their use is futile
if the levels are too low. Monitoring serum drug levels ensures that plasma
drug levels remain within the therapeutic range. Antimicrobial drugs that
may require this approach include gentamicin, vancomycin, teicoplanin, kan-
amycin, amikacin, tobramycin, chloramphenicol, streptomycin, cycloserine,
amphotericin, ucytosine, voriconazole, and itraconazole.
Cardiac
• ECG:serial ECGs can be of value in Lyme disease, rheumatic fever,
pericarditis, myocarditis, and toxic shock syndrome. Also of value in
conditions where the cardiac conduction mechanism has been damaged,
e.g. Chagas’ disease (American trypanosomiasis) and with a valve root
abscess in severe infective endocarditis. In cholera and enteric fever
(typhoid and paratyphoid), the cardiac rate will often be slower than
one might anticipate for the degree offever.
•
Echocardiography:TTE and TOE for the diagnosis of endocarditis. TOE
especially for prosthetic valves.
Dermatologicaltests
• TB skin tests:measure delayed hypersensitivity. The Mantoux test usually
involves the intradermal injection of 10 tuberculin units of puried
protein derivative (PPD), and the response is quantied. The reaction
is read at 48– 72h. Most useful epidemiologically, their individual clinical
value being relatively limited. Multiple puncture techniques (the Heaf
and Tine tests) are likely to be more convenient for large group studies.
Interpretation of these tests is more dicult in patients inoculated
with the bacillus Calmette Guérin (BCG) vaccine, and IGRAs (e.g.
QuantiFERON
®
) are generally more helpful as they distinguish between
T- cell responses to BCG andTB.
• Histoplasmin test:a +ve intradermal skin reaction to histoplasmin
(the histoplasmin test) may be the only sign of past infection with
H.capsulatum. Main value is epidemiological. Asimilar skin test exists for
C.immitis.
•
Mazzotti (DEC) test:for lariasis. Rarely used now, this test relied on
the intense pruritic response induced by microlariae after treatment
with the antilarial agent diethylcarbamazine* (DEC). Used in a minute
quantity, it can nevertheless be associated with side eects, ranging
from mild discomfort, fever, headaches, and intolerable pruritus to
tachypnoea, tachycardia, and even pulmonary oedema.
Note:(*) diethylcarbamazine is not licensed for use as a laricide.
•
Skin testing for antibiotic allergy:this can be performed in the same way
as for other allergens by patch testing or skin prick testing.

OTHERTESTS
423
Narcotics and anabolic steroidsscreen
If +ve, these may point towards occult drug use and a concomitant risk
of blood- borne viruses (e.g. HIV, hepatitis C, hepatitis B). vCJD has been
transmitted through anabolic steroid injecting. The antiretroviral efavirenz
interferes with these tests producing a false+ve.
Neurological
• EEG:may help with making a diagnosis of encephalitis (e.g. in patients
with HSV encephalitis, the EEG may exhibit focal unilateral or bilateral
periodic discharges localized in the temporal lobes), of brain abscess, or
of cerebral cysticercosis. It may also be of value invCJD.
•
LP:material for C&S can be obtained, but much additional information is
also gathered, e.g. the opening pressure is usually elevated in infections
(E Tissue biopsy and deep aspiration specimens, pp. 418–21).
•
EMG:oers rapid bedside conrmation of the clinical diagnosis of
botulism. It shows a pattern of brief, small, abundant motor unit
potentials. In GBS (associated with Campylobacter
gastroenteritis), EMG
may be helpful in excluding ° muscle disease.
•
NCS:helpful with diagnosing neuropathies (e.g. HIV, leprosy,GBS).
Ophthalmology
• Slit lamp examination:can help with the diagnosis of infective and
parasitic ocular problems, e.g. uveitis (syphilis, Reiter’s syndrome),
O.volvulus larvae, toxocariasis, toxoplasmosis, candidiasis.
•
Colour vision testing (Ishihara):used to assess toxicity associated with
ethambutol in treatmentofTB.
•
Direct and indirect ophthalmoscopy:essential for the diagnosis of CMV
retinitis in all HIV +ve patients with a CD4 cell count<100.
Pulmonary
• Pulmonary function tests:bronchial hyper- reactivity can be assessed for
(often provoked by infection, e.g. ABPA) and interstitial lung disease
checked for (which can include, e.g. TB, fungal infections,etc.).

424
CHAPTER5 Infectious and tropical diseases
424
Clinical investigation inaction:pyrexia
ofuncertainorigin
Common problem in hospital medicine, with huge potential dierential
diagnosis.
PUO is best dened as a body temperature of 38.3°C centrally (rectally)
for 3 weeks or longer without the cause being discovered, despite extensive
investigation for at least 1week.
Assessment should include
Observation offever pattern
Some conditions, such as VHF, typhoid, and malaria, may exhibit character-
istic fever patterns.
Complete and repeated detailed history, withemphasis onthe recognized
dierential diagnosis, including
•
Travel history.
• Antimalarialusage.
• Vaccination history.
• Past use of medical services in foreign parts may be especially important
(e.g. blood transfusions, splenectomy post- trauma, needlestick assaults).
• Drug- using history (including illicit drugs and especially injecting).
• Exposure to certain agents and/ or animals (e.g. pet ownership,
occupational risk of animal contact such as veterinary medicine, nursing,
farming, meat packing).
•
Hobbies (e.g. caving is linked to histoplasmosis and canal shing to
leptospirosis).
•
Sexual history (and risk taking).
• Menstrual history.
Complete and repeated physical examination, including re- evaluation
ofprevious ndings,e.g.
•
Check the skin, eyes, nail beds, lymph nodes, heart, and abdomen.
• Anew sign, e.g. cardiac murmur, may have developed overtime.
• The judicious use of repeated tests is also critical, depending upon the
context.
•
Laboratory and radiological tests, taking into account new data, e.g.
blood cultures, blood lms, autoantibody screen, radiological ndings.
•
Non- invasive procedures, taking into account new data, e.g. genito-
urinary assessment such as high vaginalswab.
•
Invasive procedures, e.g. liver biopsy, BM biopsy, laparoscopy,
Waldeyer’s ring assessment by an otolaryngologist.
Common groups ofcauses ofa PUO inan adultare
•
Infections.
• Connective tissue diseases.
• Occult neoplasms (especially leukaemia, lymphoma, and renal
carcinoma).

CLINICAL INVESTIGATION IN ACTION:PUO
425
A list ofrelevant pathologies might include
HIV, TB, endocarditis, osteomyelitis, malaria, syphilis, zoonoses (e.g. brucel-
losis, Lyme disease, tularaemia), viral hepatitis (especially hepatitis C and B),
typhoid/ paratyphoid, pelvic inammatory disease, chronic meningococcae-
mia, dental sepsis, tumours such as lymphoma, renal carcinoma, liver metas-
tases, familial Mediterranean fever, multiple PEs, drugs, rheumatological, e.g.
Still’s disease, TA, SLE, granulomatosis with polyangiitis (GPA, previously
known as Wegener’s granulomatosis), vasculitis, atrial myxoma, factitious
fever, Munchausen’s syndrome, Munchausen’s syndrome byproxy.
With improved non- invasive and microbiological techniques, most cases
of PUO are found not to be caused by infections, but rather by other sys-
temic diseases such as sarcoidosis, SLE, and TA. However, there are also
infectious diseases capable of causing prolonged fever that should always be
considered and factored into the assessment because they are often treat-
able and/ or transmissible to others and will have serious consequences if
missed. Adenitive diagnosis is not made in around 25% of patients; how-
ever, they tend not to come to any harm when observed over a long period.
Endocarditis
Endocarditis is a deep- seated infection that behaves like a deep- seated
abscess. Indeed, an abscess can form adjacent to an infected cardiac valve
or shunt. The diagnosis involves thoughtful clinical assessment, including
whether or not there is a history of injecting drug use, and requires multiple
blood cultures and cardiac assessment. The Duke criteria form the basis of
the diagnosis.
16
Assess clinically for likelihood, e.g. background of injecting
drug use, congenital heart disease, prosthetic valves, rheumatic fever, scar-
let fever. Endocarditis may manifest changing cardiac murmurs over a period
of time, as well as a number of additionalsigns.
•
Establish diagnosis:echocardiography (especially TOE)— to look at
valves, cardiac chambers, shunts,etc.
•
Establish aetiology:
•
Blood cultures (multiple)— consider culturing for unusual organ-
isms such as fungi, HACEK organisms (Haemophilus species, e.g.
H.parainuenzae, H.aphrophilus, and H.paraphrophilus, Actinobacillus
actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens,
and Kingella species), L.monocytogenes,etc.
•
Serology— Q fever (Coxiella burnetii) phase Iand II, C.albicans.
•
Assess clinical status:
•
ECG— tachycardia, conduction abnormalities.
•
CXR— cardiac size, PEs with right- sided endocarditis.
•
U&E— to assess renal compromise, ifany.
•
Haematology— WBC.
•
Inammatory markers— ESR,CRP.
•
Proteinuria— to assess renal compromise, ifany.
•
Blood- borne virus status— HIV, hepatitis C, hepatitis B if there is a his-
tory of drug injecting.
16 MedCalc. Duke criteria for infective endocarditis. M http:// www.medcalc.com/ endocarditis.html.

426
CHAPTER5 Infectious and tropical diseases
426
• Assistance with therapy:
•
Antibiotic sensitivity testing.
•
Serum antibiotic levels (e.g. gentamicin, vancomycin).
• Prevention:dental assessment— for prevention in the future. Endocarditis
warning card. MedicAlert
®
bracelet.
Tuberculosis
Consider pulmonary vs extrapulmonary disease and other epidemiological
parameters. (Pleural disease is by denition extrapulmonary.)
• Establish diagnosis/ aetiology:
•
Radiological evidence.
•
Bodily uids (e.g. sputum, EMUs, gastric washings, CSF) and
biopsies— always consider performing induced sputum, even with
a normal CXR; histology may show caseating granulomata (see
Fig.5.9).
•
PCR testing.
•
Mantouxtest.
•
IGRAs such as QuantiFERON
®
.
•
Ca- 125 levels— abdominal TB inwomen.
• Assess clinical status:
•
Inammatory markers— ESR,CRP.
•
T- cell subsets— low CD4+ cell count characteristic.
•
Body weight.
•
HIV testing— now essential in all patients.
•
Glucose metabolism— may be an association (fasting glucose, OGTT,
HbA
1c
).
Lymphocytes
Activated macrophages
(epithelioid cells)
Caseous
necrosis (amorphous)
Langhans giant
cell (multinucleated)
Fibroblast
CASEOUS GRANULOMA
Fig.5.9 Caseating granulomata are the principal histological feature of TB, together
with acid- fast bacilli (detected using the ZN stain). In any tissue aected by TB,
caseating granulomata may be present and are accordingly of immense assistance
diagnostically.

CLINICAL INVESTIGATION IN ACTION:PUO
427
• Assistance with therapy:
•
Antibiotic sensitivity testing.
•
Serum antibiotic levels (e.g. cycloserine).
•
LFTs.
•
Skin testing.
•
Calcium and vitamin D levels.
•
Gene probes (for rifampicin resistance).
• Prevention:notify cases to public health authorities. Contact tracing.
•
TB and biopsies:when a biopsy is being obtained of any organ or
tissue, the possibility of extrapulmonary TB should be borne in mind.
If histology is performed, caseating granulomata may be seen, and
appropriate staining for acid- fast bacilli (such as the ZN stain) may reveal
the presence of TB organisms. However, because of the i risk of MDR-
TB and XDR- TB, material should always be sent to the microbiology
laboratory and appropriate cultures for TB should be set up, both for
diagnostic and for drug sensitivity purposes. Molecular techniques,
including gene probes and PCR, are now essential parts of the
diagnostic armoury for TB. In many instances, having a biopsy taken is an
unpleasant experience for a patient, and remembering to perform a TB
culture at the outset may prevent the patient from having to undergo an
unpleasant procedure more thanonce.
Malaria (fever inthe returning traveller)
Always consider malaria in the febrile individual returning from overseas.
Adetailed geographical history and malaria prophylaxis history is essential.
Always consider the possibility of a coexistent second diagnosis (especially
in P. falciparum infestation) such as Salmonella
septicaemia (the so- called
‘algid malaria’).
•
Establish diagnosis/ aetiology:
•
Thick and thin blood lms— perform three (each 24h apart).
•
Antigen tests— such as ParaSightF
®
and OptiMAL
®
.
•
Blood sugar— hypoglycaemia is common and needs immediate
treatment.
•
Platelet count— thrombocytopenia suggestive of P.falciparum.
•
Haematology— WBC.
•
Inammatory markers— ESR,CRP.
• Assess clinical status:
•
Blood cultures— to exclude 2° infection.
•
Haemoglobinopathy— to assess for sickle- cell disease.
•
Assess the very ill patient thoroughly for markers of severity
(includes LFTs, blood lm for haemolysis, coagulation status, CXR,
ECG, ABGs, glucose levels, lactate levels, conscious level, etc.). Note
that severe P.falciparum malaria can present as a diarrhoeal illness.
•
Assistance with therapy:
•
G6PD levels.
•
Tests of hearing— deafness can occur with quinine.
• Prevention:avoid blood donation for 18months.

428
CHAPTER5 Infectious and tropical diseases
428
Jaundice (acute)
Jaundice can be pre- or post- hepatic, or a combination of both. Epidemiological
factors are important (drug injecting, travel, unsafe food, unsafe sex, job, hob-
bies, vaccination history, alcohol, prescribed medications, herbal remedies,
etc.). The patient may have an acute exacerbation of a chronic disease, e.g.
hepatitis C.Always remember Courvoisier’s law (a distended gall bladder in a
patient with obstructive jaundice means cancer) and Charcot’s triad (the char-
acteristic presentation of acute cholangitis, with biliary colic, jaundice, and spik-
ing fevers with rigors). Haemolysis may lead to jaundice without liver disease
being present.
•
Establish diagnosis:
•
LFTs— conjugated and unconjugated bilirubin levels.
•
Urinalysis.
•
Stool examination— colour, ushability.
•
Haemolysis screen— blood lm, coagulation studies, antiglobulin
test,etc.
•
Hepatobiliary US— serial scans can assess hepatobiliary status
sequentially.
•
AFP levels— may suggest hepatocellular carcinoma associated with
hepatitis C and hepatitis B infection.
•
Establish aetiology:
•
Serology— e.g. hepatitis Athrough to E, EBV, CMV, toxoplasmosis,
leptospirosis, hantavirus, yellowfever.
•
Blood culture.
•
Stools for ova, cysts, and parasites— e.g. C.sinensis, ascariasis).
•
Monospot forEBV.
•
Hepatobiliary US— obstruction by malignancy or parasites, liver
parenchyma status, gallstones.
•
ERCP/ MRCP— may diagnose parasitic invasion of the biliary tree,
CMV/ cryptosporidial disease, porta hepatis lymphadenopathy
associated with HIV,etc.
•
Paracetamol levels.
•
Assistance with therapy:
•
HIV testing— if appropriate; co- infection with HIV, HCV, and HBV an
i problem worldwide.
•
Ethanol assessment— γGT levels,iMCV.
•
Molecular tests— PCR testing for HCV, circulating DNA levels inHBV.
•
Antigens— hepatitisB.
• Prevention:
•
Notify cases to public health authorities; safe sex education; safe
drug- injecting education possible once viral diagnosis of HCV, HBV,
and/ or HIV established.
•
Assess family, sexual partners, etc. for possible infection (HIV, HBV,
HCV) and/ or need to vaccinate(HBV).
•
Vaccination strategies:HBV, HAV as appropriate.

CLINICAL INVESTIGATION IN ACTION:PUO
429
Diarrhoea
Diarrhoea can be acute vs chronic, or acute on chronic. For example, a
gastroenteritis illness may uncover pre- existing IBD (such as Crohn’s dis-
ease) or malabsorption (such as coeliac disease or pancreatic insuciency).
Drugs such as opiates can lead to ‘overow’ diarrhoea. Also bear in mind
that where there is one bowel pathogen, another one might be present.
Antibiotic resistance is common among some bowel pathogens. Diarrhoea
can appear infective but, for example, might be endocrine in origin (e.g.
carcinoid syndrome, Zollinger– Ellison syndrome, medullary carcinoma of
the thyroid), whilst the possibility of bowel cancer must always be borne in
mind. Note that the presence of S.bovis in blood cultures may be indicative
of the presence of bowel cancer until proven otherwise. Irritable bowel
disease is being increasingly diagnosed. Malaria can present as diarrhoea.
•
Establish diagnosis:
•
Examine stools.
•
Keep a stool chart on theward.
• Establish aetiology:
•
StoolC&S.
•
Stool microscopy for ova, cysts, and parasites.
•
Faecal fat and elastase for pancreatic insuciency.
Pneumonic illness
Pneumonia is multi- aetiological. If recurrent, this throws up certain diagnos-
tic possibilities that must be considered. Many epidemiological considera-
tions are important such as travel history, occupation, pet keeping, hobbies,
sexual activity, etc. Osler’s triad of rigors, pleuritis, and rust- coloured spu-
tum is said to be characteristic of pneumococcal pneumonia.
•
Establish diagnosis/ aetiology:
•
CXR (or CT chest).
•
Serology— atypical pneumonia organisms (L.pneumophila, M.pneumo-
niae, C.burnetii, Chlamydia psittaci), hantavirus, RSV, inuenza.
•
Sputum— including induced sputum, bronchoscopy, and BAL:micros-
copy and culture.
•
Blood cultures.
•
Serum Na
+
level— d in Legionella.
•
Antigen— pneumococcal (blood), Legionella (urine).
•
NPA for viral culture— RSV, inuenza.
•
Cryoglobulins— e.g. M.pneumoniae.
•
Molecular— various PCR tests for viruses and Mycoplasma.
•
HIV test— essential in risk groups.
• Assess clinical status.
•
(CURB65 score):
17
•
ABGs.
•
Confusion, Urea, Respiratory rate, Blood pressure.
•
US of the chest— if eusion developing (drain if necessary), check pH
ofuid.
•
Pulmonary function tests if appropriate.
17 M http:// www.aafp.org/ fpm/ 20060400/ CURB65.pdf.

430
CHAPTER5 Infectious and tropical diseases
430
• Assistance with therapy:
•
Antibiotic sensitivity testing.
•
If recurrent:consider TB testing (see earlier), HIV testing, Ig levels
(to check for deciency), assessing for hyposplenism, checking termi-
nal complement levels (C5– C9).
• Prevention:
•
Notify appropriate cases to public health authorities (e.g. Legionella,
TB); isolate as necessary.
•
Vaccination strategies:inuenza, Pneumococcus,Hib.
•
Stop smoking, if relevant.
Meningitic illness (headache and photophobia)
Meningitis can be extremely serious, particularly bacterial, mycobacterial,
fungal, and protozoal forms, but viral meningitis is generally less serious.
Meningitic infection is often mimicked by much less serious infections such
as UTI (especially in women), throat infections (ASO, Monospot), atypi-
cal pneumonias, and sinusitis (especially ethmoidal, sphenoidal). Asimilar
picture can also be generated by SAH. Meningococcal infection can be
life- threatening without ever causing meningitis. If bacterial meningitis is
recurrent, certain diagnostic possibilities must be considered. Brain abscess
(consider injecting drug use, congenital heart disease, immunodeciency,
etc.) and, under certain circumstances, encephalitis can present in a similar
fashion to meningitic illnesses. Where the patient has a marked petechial
rash and a history of travel to Africa, Ukraine, or South America, even
VHF (particularly the Congo– Crimean variety) comes into the picture (see
Fig.5.10).
•
Establish diagnosis/ aetiology:
•
LP/ cisternal puncture/ foramenal puncture (in neonates)— for CSF
pressure, microscopy, bacterial and mycobacterial culture (includ-
ing special cultures, e.g. for Listeria), viral culture, biochemistry (e.g.
protein, glucose), dierential cell count, viral PCR, xanthochromia,
India ink stain, CrAg testing.
•
CT scan of head— sometimes necessary to exclude an SOL prior to
performing an LP; CT is little better than a clinical assessment in the
exclusion of raised ICP. When i ICP is found, cisternal puncture and
foramenal puncture is possible in skilledhands.
•
CXR and assessment for atypical pneumonia, if appropriate.
•
NPA (see earlier).
•
Petechial rash sampling— aspirate material from a fresh purpuric lesion
using a small- needle insulin syringe, Gram stain, and culture.
•
Molecular— meningococcal PCR (blood and CSF), pneumococcal
PCR (blood andCSF).
•
Serology— urine and blood for CrAg; blood for pneumococcal anti-
gen; urine and saliva for mumps antigen; ASO, antibodies to mumps,
EBV, Cryptococcus, N.meningitidis.
•
Nasopharyngeal swab for meningococcus.
•
Stool for enteroviral culture.
•
Monospot test forEBV.

CLINICAL INVESTIGATION IN ACTION:PUO
431
• Assess clinical status:
•
CT/ MRI scan of head— assess for raised ICP; exclude SAH
(xanthochromia), sagittal vein thrombosis; exclude skull fracture,
especially of cribriform plate or middle ear (this can lead to recur-
rent pneumococcal meningitis— if there is a nasal drip, test uid for
glucose to exclude presence of CSF as CSF contains glucose).
•
Dierential WBC inblood.
•
Inammatory markers— ESR,CRP.
•
Coagulation screen and platelet count:for meningococcal sepsis.
•
ABGs— to assess acid– base balance in severecases.
1. A detailed travel history
and a high index of suspicion
are essential in making the
diagnosis of VHF. A recent
travel history to an area
where one of these viruses
are known to be particularly
prevalent is suggestive,
particularly Africa (e.g.
Uganda and Ebola, Nigeria
and Lassa) and South
America — the incubation
period ranges from 3 to 21 days,
depending upon the variety.
Many VHF cases presenting
together may suggest a
bioterrorism attack.
2. VHFs are severe febrile
illnesses that can be
complicated by a
haemorrhagic tendency,
petechiae, hypotension
(and even shock), ushing
of the face and chest, and
oedema. Constitutional
symptoms such as
headaches, myalgia,
vomiting and diarrhoea
may occur. Some VHFs
manifest particular features
not shared by the others.
3. Once suspected, VHFs
are category 4 pathogens,
so precautions for
healthcare workers must
be instituted and the case
notied to the proper
authorities. Isolation
measures and barrier
nursing procedures are
indicated (Marburg,
Ebola, Lassa and Congo-
Crimean HF viruses may
be particularly prone to
aerosol nosocomial spread),
usually in a special infectious
diseases/tropical medicine
unit staed with clinicians
with expertise in the eld.
Intensive supportive care
may be required.
5. Almost always, the true
diagnosis will not be a VHF,
but they must be considered
where appropriate. Strict
adherence to isolation and
infection control precautions
has prevented secondary
transmission in almost all cases.
4. Diagnosis requires clinical
expertise in infectious
diseases/tropical medicine.
The dierential diagnosis
includes malaria, yellow
fever, dengue, typhoid/
paratyphoid fever,
non-typhoidal salmonellae,
typhus and other rickettsial
diseases, leptospirosis,
shigella dysentery, relapsing
fever (borreliosis), fulminant
hepatitis and meningo coccal
disease. Antigen tests,
antibody detection and
viral culture are all available
for most of the VHFs. The
patient should be fully
investigated and treated
accordingly.
Biohazard
Fig.5.10 Investigating a possible case ofVHF.

432
CHAPTER5 Infectious and tropical diseases
432
•
Synacthen
®
test (E Short Synacthen
®
test, p. 225)— adrenal
failure in severe meningococcal sepsis (Waterhouse– Friderichsen
syndrome).
•
HIV test— suggested by some pathologies and may be the overall
underlying problem.
•
TB assessment— may be the underlying pathology.
• Assistance with therapy:
•
Antibiotic sensitivity testing.
•
Serum antimicrobial levels, e.g. amphotericin, ucytosine.
• Prevention:
•
Notify relevant cases to public health authorities; isolate as
necessary.
•
Vaccination strategies— meningococcus, pneumococcus,
inuenza,Hib.
•
History of skull fracture— may need neurosurgery,etc.
Urethritis (with or withouthaematuria)
Pain on micturition can simply represent a UTI or there may be an STD, such
as gonorrhoea, present. The patient’s sexual and travel history is important.
Renal calculi can produce clinical pictures resembling an infection, as can
dermatological conditions such as Stevens– Johnson syndrome. UTIs are
commoner during pregnancy. Prostatitis can be a problem in oldermen.
• Establish diagnosis/ aetiology:
•
Urine collection (MSU)— culture (bacterial infections), microscopy
(parasites, etc., such as schistosomiasis— use terminal specimen),
molecular techniques (LCR for Chlamydia).
•
STDs and pelvic inammatory disease— perform high vaginal swab
and urethral swabs; screen for gonococcus (includes throat and anal
swabs).
•
Calcular disease— exclude with urine microscopy, radiology,etc.
•
Prostatitis— prostatic massage,CrAg.
•
TB— can present like any otherUTI.
•
Reiter’s syndrome— slit lamp examination of the eye, urine, and stool
culture/ LCR for Chlamydia.
•
Assess clinical status:
•
Biochemistry— exclude renal failure (urea, creatinine,etc.).
•
Markers of inammation— CRP,ESR.
•
White cellcount.
•
Check all other mucosal surfaces of the body (mouth, conjunctivae,
nose, etc.) to help exclude Stevens– Johnson syndrome.
• Assistance with therapy:
•
Pregnancytest.
•
PSA to exclude prostatic carcinoma (recurrent UTIs in oldermen).
•
Radiology of renal tract— US, IV pyelography (IVP) (to exclude under-
lying renal tract anatomical problems, TB involvement, calculi,etc.).
•
Prevention:
•
History of unsafe sex, recent new sexual partner, drug injecting—
consider VDRL, HIV, viral hepatitis testing.
•
TB— notify, contact trace,etc.
•
Calculi— exclude hypercalcaemia, hyperuricaemia,etc.

CLINICAL INVESTIGATION IN ACTION:PUO
433
Red, painful swollen lowerleg
One of the most dicult things in medicine is to distinguish eectively
between a distal DVT and cellulitis— and a combination of both! Less com-
monly, a ruptured Baker’s cyst of the knee can present in almost the same
way. The key is in the history. Sometimes the problem is in the tissues, and
sometimes in the joints (even gout and pseudogout can look like cellulitis)
or the bone (osteomyelitis). Ulceration may be present on the legs. Venous
and arterial insuciency may complicate the picture— infected legs in older
people can be very dicult to treat with antibiotics alone. Recent long- haul
air travel may point more towards thrombosis, but swollen legs with com-
promised veins can easily get infected. Although rare, syphilis, yaws, and
Mycobacterium ulcerans can cause leg ulcers that are potentially amenable
to treatment. Pyoderma gangrenosum can resemble infection of the leg
but is associated with non- infectious systemic diseases. Check for eschar
from tickbite.
•
Establish diagnosis/ aetiology:
•
Exclude DVT with Doppler US (and possible embolic disease on
occasions).
•
Swabs:from ulcers, between the toes usually not helpful.
•
Blood cultures.
•
RarelyVDRL.
•
Joint assessment:urate levels for gout; assess (if relevant) for pseu-
dogout, rheumatological screen, synovial uid analysis (if relevant),
Lyme disease titres (depends on the travel history,etc.).
•
Leg ulcers in the young:consider sickle- cell disease, hereditary
spherocytosis.
•
Assess clinical status:
•
White cellcount.
•
Inammatory markers— ESR,CRP.
•
Assess blood vessel integrity— e.g. compression US for venous prob-
lems, lower limb arteriography.
•
Assistance with therapy:
•
Exclude diabetes,HBA
1c
.
•
X- ray, bone scanning, MRI— is osteomyelitis present?
• Prevention:
•
Treat diabetes, if present.
•
Treat other underlying conditions, if present.
•
Patient advised to take care in future (e.g. DVT avoidance whilst
travelling).
Vesicularrash
Many vesicular rashes are infective; many are not. In particular, the distri-
bution of the rash should be carefully assessed, and joint assessment and
management with a dermatologist are often valuable. If atopic, eczema
herpeticum comes into the picture. Staphylococcal impetigo can cause
vesiculation. If there is a relevant travel history, rickettsial pox and mon-
key pox come into the picture. Erythema multiforme, which often has an
infective basis but can also be produced by medications, can produce a
vesiculating rash (so check the mouth, eyes, and genitalia, and determine the

434
CHAPTER5 Infectious and tropical diseases
434
medication history). Non- infective blistering conditions include dermatitis
herpetiformis (coeliac disease), pompholyx, and pemphigus.
•
Establish diagnosis/ aetiology:
•
Vesicular uid— PCR, EM, IFT, culture,etc.
•
Serology— HSV, herpes varicella- zoster, rickettsial pox, Coxsackie
virus, ASOtitre.
•
Assess clinical status:
•
CXR— chickenpox (if compromised, ABGs will be needed).
•
EEG— if cerebral symptoms present (e.g. cerebellar encephalitis can
occur with herpes varicella- zoster).
•
Monkey pox, smallpox, rickettsial pox— the patient will be ill and will
require full assessment, even possibly intensivecare.
•
Assistance with therapy:
•
Pregnancy test:herpes varicella- zoster a bigger problem in pregnancy.
• Prevention:
•
Avoid precipitants with erythema multiforme.
•
Manage atopy optimally.
Further reading
Chin J (ed.). Control of Communicable Diseases Manual, 18th edn. Washington DC:American Public
Health Association,2004.
Cohen J, Powderly WG (eds.). Infectious Diseases, 2nd edn. St Louis:Mosby,2003.
Cook GC, Zumla AI (eds.). Manson’s Tropical Diseases
, 22nd edn. Philadelphia:WB Saunders,2008.
Mandell GL, Douglas JE, Dolin R, Bennett RE (eds.). Principles and Practice of Infectious Diseases, 7th
edn. London:Churchill Livingstone,2009.
Peters W, Pasvol G. Color Atlas of Tropical Medicine and Parasitology, 5th edn. St Louis:Mosby,2001.
Shanson DC. Microbiology and Clinical Practice
, 3rd edn. Oxford:Butterworth- Heinemann,1999.
Steen R, Dupont HL, Wilder- Smith A. Manual of Travel Medicine and Health, 3rd edn. Ontario:BC
Decker,2007.

435
Chapter6
Cardiology
Cardiac catheterization 436
Cardiac markers of myocardial necrosis 440
Cardiac volumetric imaging:magnetic resonance and computed
tomography 442
Echocardiography 450
Electrocardiogram 470
Electrocardiographic monitoring 478
Exercise testing 482
Myocardial perfusion imaging 486
Radionuclide ventriculography 490
Pulmonary artery catheterization 492
Tilt table testing 494

436
CHAPTER6 Cardiology
436
Cardiac catheterization
Principle
Cardiac catheterization is an invasive procedure during which catheters are
placed within the cardiac chambers, coronary arteries, and great vessels to
provide information on cardiac anatomy, pressures, disease states, function,
and O
2
saturations.
Indications
• Diagnosis of suspected coronary artery disease (after appropriate non-
invasive assessment or if results are equivocal).
•
Assessment of coronary artery disease burden and suitability for
intervention, e.g. percutaneous coronary intervention (PCI), coronary
artery bypass surgery, or cardiac transplantation.
•
Measurement of intracardiac pressures in patients with valvular heart
disease (largely superseded by non- invasive imaging).
• Detailed measurement of left and right ventricular cardiac output
and pulmonary hypertension in patients considered for cardiac
transplantation or with suspected congenital heart disease.
•
Evaluation of O
2
saturations in cardiac chambers to identify cardiac
shunting.
•
Myocardial biopsy in patients with cardiomyopathy of unknowncause.
• Monitoring of cardiac transplantation success/ rejection.
Contraindications
• Pregnancy is an absolute contraindication to coronary angiography.
• Relative contraindications include:
•
Severe peripheral vascular disease.
•
Aortic aneurysm.
•
Renal failure.
•
Unstable cardiac failure or arrhythmias.
•
Haemodynamic instability.
•
Sensitivity to contrast agents.
Patient preparation
The patient is fasted for 4h prior to the procedure. IV access is required,
together with haemodynamic and ECG monitoring. Sedation may be used
according to patient request or operator direction. It is important that the
patient understands the risks of the procedure and gives informed written
consent. The contrast agents used can provoke renal failure in susceptible
patients. Metformin should be withdrawn for 48h prior to the procedure. In
patients with renal impairment, pre- treatment with acetylcysteine is advised
and a less nephrotoxic contrast agent may be considered. Contrast agent
volume should be minimized.

CARDIAC CATHETERIZATION
437
Procedure
1. The patient lies at on a catheter laboratory table and appropriate
monitoring is applied.
2. LAn is used and an aseptic technique employed. For a left heart
procedure, arterial access is required. The radial artery is the most
commonly employed, but the femoral artery provides an alternative
route (unless signicant peripheral vascular disease is present).
3. The chosen artery is cannulated using the Seldinger technique, and an
access sheath inserted.
4. Fluoroscopic screening is used to monitor the passage of a guidewire
and catheter to theheart.
5. Catheters are pre- shaped according to the structure being examined.
Typically, a Judkins left 4 catheter is used for the left coronary artery,
Judkins right 4 catheter for the right coronary artery, and an angled
pigtail catheter is placed in the LV and/ or aorta for examination of
these structures.
6. Acontrast agent is injected for image acquisition. Images of the coronary
arteries are acquired in multiple planes in order to optimally identify
coronary artery stenoses. Continuous pressure transducers can be used
for dynamic left heart pressure monitoring, e.g. LV andaorta.
7. Evaluation of the right heart necessitates venous access. The
commonest access point is the femoral vein, but the central veins, e.g.
subclavian or internal jugular vein, can also beused.
8. Aright heart or Swan– Ganz catheter is passed towards the right heart.
Right- sided pressures, e.g. vena cavae, right atrium, right ventricle (RV),
pulmonary artery (PmA), and pulmonary capillary wedge pressure
(indirect left atrial (LAt) pressure), can be measured.
9. Blood samples obtained from these sites can be analysed for O
2
saturation and used to identify the presence and site of cardiac shunts,
e.g. atrial or ventricular septal defects. Cardiac output studies can also
be performed.
Risks
Cardiac catheterization is an invasive technique, and so there are inherent
risks to the procedure (1:200 patients). Full cardiopulmonary resuscitation
facilities should be immediately available.
These risks include
•
Trauma to arterial/ venous access sites, including haematoma, occlusion,
aneurysm, pseudo- aneurysm, nerve damage.
• Aortic/ coronary artery dissection.
• CVA (embolus).
• MI (embolus/ dissection).
• Arrhythmias.
• Pulmonary oedema (left main stem stenosis).
• Renal failure.
• Vasovagal response to arterial access/ sheath removal.
Additionally, the patient is exposed to ionizing radiation, and so screening/
image acquisition times should be reduced as much as possible. Serial stud-
ies should be avoided.
438
CHAPTER6 Cardiology
438
Possible results
Results froma left heart study can provide informationon
•
Coronary artery anatomy and distribution of disease, to aid decisions
regarding the need for revascularization (see Fig.6.1).
•
Left ventricular size and function.
• Left heart pressures.
• Aortic and mitral valve integrity.
• Aortic size and disease.
• Congenital abnormalities.
A right- sided study can provide informationon
• Right ventricular size and function.
• Tricuspid and pulmonary valve integrity.
• PmA anatomy.
• Right heart pressures.
• Congenital abnormalities, e.g. shunts.
• Myocardial histopathology where biopsy istaken.
Advantages overothertests
Invasive coronary angiography has high resolution for the evaluation of cor-
onary artery disease and is the current gold standard imaging technique for
this application. However, CT coronary angiography is rapidly becoming an
accurate alternative means of depicting coronary artery atheroma, particu-
larly in the proximal vessels. Advances in echocardiography and cardiovas-
cular MR (CMR) have reduced the need for right heart catheterization, but
the latter is still the only means with which to obtain direct histopathological
information from myocardial structures.
Fig.6.1 Coronary angiogram showing a normal left coronary artery.

CARDIAC CATHETERIZATION
439
Pitfalls
The procedure is invasive and the risks involved are not insignicant. Whilst
coronary angiography is the gold standard for the demonstration of ana-
tomical coronary arterial lesions, it provides little information regarding
their physiological signicance on the myocardium. The information should
therefore be used in conjunction with imaging techniques that can assess
the adequacy of myocardial perfusion, e.g. stress nuclear imaging, stress
echocardiography, orCMR.
Further reading
Callan P, Clark AL. Right heart catheterisation:indications and interpretation. Heart 2016; 102:1– 11.
Fihn SD, Blankenship JC, Alexander KP, etal. 2014 ACC/ AHA/ AATS/ PCNA/ SCAI/ STS Focused
update of the guideline for the diagnosis and management of patients with stable ischemic heart
disease. J Am Coll Cardiol 2014; 64
:1929– 49.
440
CHAPTER6 Cardiology
440
Cardiac markers ofmyocardial necrosis
Principle
When cardiac muscle is damaged, certain substances, e.g. troponins I(TnI)
and T (TnT), myoglobin, and creatine kinase, are released into the blood-
stream and can be measured by biochemical assays. Such assays can be
helpful in making the diagnosis of ACS or myocardial contusion. Myoglobin
and creatine kinase (CK and CK- MB) are relatively non- specic cardiac
markers of myocardial necrosis (CK is also released by skeletal muscle)
that are elevated within 2– 4h of myocyte damage. Troponins (TnT and TnI)
are cardiac muscle proteins that are released into the peripheral circulation
more slowly and are highly specic for myocardial injury. High- sensitivity
troponin assays (hsTnT and hsTnI) detect troponins at much lower con-
centrations than the standard troponin assays available previously, allowing
more rapid diagnosis in ACS. Using a high- sensitivity assay, an initial eleva-
tion of circulating troponin levels is detectable within 3h of the event, peak-
ing at around 24h (see Fig.6.2).
Patients presenting with unstable ischaemic cardiac pain are initially labelled
as having an ACS and are categorized on the basis of their initial 12- lead
ECG into ST- segment elevation ACS (which requires urgent reperfusion
via primary PCI or thrombolysis) or non- ST- segment elevation ACS (which
requires appropriate antiplatelet and antithrombotic therapy, followed by
coronary revascularization as appropriate). MI is conrmed later if there is
a rise in markers of myocardial necrosis, most commonly troponins. When
these markers are raised, those presenting with an ST- segment elevation
ACS are classied as ST- elevation myocardial infarction (STEMI) and those
with non- ST- elevation ACS are classied as non- ST- elevation MI (NSTEMI).
10
5
0
0 24
144
1209672
Hours from onset of symptoms
Relative enzyme activity
48
CK
Troponin
Fig.6.2 Changes in levels of cardiac markers following acuteMI.

CARDIAC MARKERS OF MYOCARDIAL NECROSIS
441
Indications
Cardiac markers ofmyocardial necrosis areuseful
•
In conjunction with the clinical history and 12- lead ECG to diagnose and
categorize suspectedACS.
• To stratify risk inACS.
• To ascertain the extent of any myocardial injury following coronary
revascularization procedures.
•
To identify myocardial contusion following thoracic trauma.
Procedure
A 10mL venous blood sample is sucient. For hsTnT or hsTnI testing, this
sample is ideally taken 3– 6h after the patient’s most severe symptoms.
Repeat sampling is necessary to demonstrate a rise in cardiac markers.
Risks
In patients who have had thrombolysis for acute MI, bleeding or haema-
toma formation may occur spontaneously at venepuncturesites.
Possible results
Within the normal population, 99% of individuals will have a hsTnT <14ng/
L. In the appropriate clinical setting of chest pain typical of acute MI ± ECG
changes:
•
An elevated troponin (hsTnT >30ng/ L) is consistent with a
diagnosisofMI.
•
Acute MI (STEMI and NSTEMI) can be condently excluded if a high-
sensitivity troponin result is within normal limits 6h after the onset of
symptoms.
Pitfalls
CK may be elevated in skeletal muscle injury, as well as with MI. Direct esti-
mation of CK- MB, the isoenzyme that is more specic for cardiac muscle,
may be helpful. Troponins are more specic for cardiac injury but may also
be elevated in cardiac failure, arrhythmias, renal failure, or PE, and so the
clinical context should always be taken into account. Timing of blood sam-
ples is critical since values may be normal if blood is taken too soon after
symptom onset. Troponins may remain elevated for up to 14days following
a cardiac event, and so the diagnosis of re- infarction using troponins alone
may be unreliable.
Further reading
Shah ASV, Anand A, Sandoval Y, etal. High- sensitivity cardiac troponin Iat presentation in patients
with suspected acute coronary syndrome:a cohort study. Lancet 2015; 386:2481– 8.
Sherwood MW, Newby LK. High- sensitivity troponin assays:evidence, indications, and reasonable
use. J Am Heart Assoc 2014; 3:e000403.
Thygesen K, Alpert JS, Jae AS, et al. Third universal denition of myocardial infarction. J Am Coll
Cardiol 2012; 60
:1581– 98.

442
CHAPTER6 Cardiology
442
Cardiac volumetric imaging:magnetic
resonance and computed tomography
Principle
Cardiac volumetric imaging is now established as a powerful tool to visualize
the anatomy, size, and function of cardiac structures, i.e. valves, chambers,
myocardium, pericardium, and great vessels. Advanced technology MR
imagers and multislice CT scanners are increasingly available to clinicians.
These techniques provide information regarding the aetiology and severity
of most congenital and acquired cardiac abnormalities.
Indications
The indications for CMR and CT are shown in Table 6.1. CMR is especially
useful in patients with IHD where a comprehensive assessment of left ven-
tricular function, reversible myocardial ischaemia, and myocardial viability
can be made. Cardiac CT is of particular value in screening for coronary
artery disease and for assessment of the great vessels and coronary arteries.
Contraindications
• Presence of any ferrous metal, e.g. intracranial clips, intra- ocular foreign
bodies, shrapnel(MRI).
•
Temporary or permanent pacing systems (MRI), unless MRI- conditional.
Table6.1 Indications forCMRandCT
Condition MRI CT
Congenital heart disease +++ ++
Anatomy, size, or mass of cardiac chambers (left atrium, LV,
right atrium, RV)
++++ +++
Global and regional left ventricular function ++++ +++
Screening (cardiomyopathy, including arrhythmogenic right
heart)
++++ +
Valvular heart disease (aortic, mitral, tricuspid, and pulmonary) +++ +
Prosthetic heart valves +++ +
Cardiac masses +++ ++
Pericardial disease +++ ++
Aortic disease +++ ++++
Coronary artery anatomy and patency ++ ++++
Screening for coronary artery disease + ++++
Myocardial perfusion +++
–
Stress study in IHD ++++ –
Myocardial viability ++++ +

CARDIAC VOLUMETRIC IMAGING
443
• Internal cardioverter– debrillator (ICD) systems (MRI), unless
MRI- conditional.
• Clinically dehiscing Starr– Edwards valve prostheses manufactured
between 1960 and 1964(MRI).
•
Severe claustrophobia (more of an issue withMRI).
• Allergy to non- ionic contrast agents(CT).
• Haemodynamic instability.
• Pregnancy is a relative contraindication to MRI, but an absolute
contraindicationtoCT.
Patient preparation
A patient questionnaire is performed to exclude contraindications. Patients
should be relaxed and have the procedure explained, so that they are able
to co- operate eectively. Breath- holding techniques should be practised
prior to the scan to achieve optimal image quality. Height and weight are
required for indexing cardiac measurements. Where stressors are to be
used, a 12- lead ECG should be obtained. IV access is required for contrast
agent administration.
Procedure
The patient is positioned on the imaging couch. Electrodes are attached to
allow image acquisition to be gated to the ECG. For CMR, a cardiac coil is
selected and earplugs supplied. Images are then acquired in order to evalu-
ate the clinical problem.
The following MRI sequences are commonly performed
T1- weightedimages
These are used for anatomical assessment and contribute, with optional
contrast agent enhancement, towards tissue characterization.
Cine sequences
Cine sequences are used for anatomical assessment and particularly for car-
diac function. Repetitive short- axis slices from the cardiac base to the apex
are summated to calculate left ventricular function. This is an extremely
accurate method, since it avoids geometrical assumptions created by
regional wall abnormalities. It is therefore a gold standard method for the
calculation of left ventricular ejection fraction (EF), mass, and other cardiac
volumes. The technique can be used in conjunction with pharmaceutical
stress (e.g. dobutamine) in a manner analogous to stress echocardiography
to identify regional reversible myocardial ischaemia.
First- pass contrast agent imaging
Myocardial perfusion is assessed by imaging the rst pass of a T1- shortening
contrast agent (gadolinium). The contrast agent passes through the right
heart and lungs to the left heart. It is carried into the myocardium by the
coronary circulation, giving rise to a rapid i in myocardial signal intensity.
Myocardial areas with reduced blood ow have slower and reduced signal
change. The eect is enhanced by use of pharmaceutical coronary artery
vasodilators, e.g. adenosine, and can be used for the identication of MI
and reversible ischaemia. In contrast to nuclear perfusion imaging, high

444
CHAPTER6 Cardiology
444
spatial resolution allows detection of subendocardial, as well as transmural,
ischaemia.
Delayed contrast agent imaging
Gadolinium is an interstitial contrast agent, and it accumulates within 10min
after administration in tissue where extracellular membranes are damaged.
This is a powerful tool to delineate between myocardial scar tissue (i signal
intensity) and viable or hibernating myocardium (no change in signal inten-
sity on delayed imaging).
Velocity- encoded imaging
Velocities can be encoded into grey scale to measure the motion of cardiac
structures and the ow within great vessels. This has similar applications to
echocardiographic Doppler imaging and can be used to quantify valvular
disease, e.g. stenosis or regurgitant volumes, and congenital heart disease,
e.g. cardiac shunts.
Magnetic resonance angiography
A volume (3D) image acquisition is performed after a bolus of gadolinium
reaches an area of interest within the great vessels. This technique is use-
ful for evaluating aortic disease, congenital heart disease, and the presence
ofRAS.
Cardiac computed tomography
An infusion of iodinated contrast agent is given, and a volume image acquisi-
tion is attained during a 15– 20s breath- hold. Where coronary arteries are
the area of interest, a coronary artery calcication score is performed ini-
tially, and these data can then be used to guide further imaging. Aβ- blocker
may be required to lower the heart rate to optimize the image resolution.
Images are then post- processed to reconstruct them to attain clinical infor-
mation according to scan indication.
Risks
If appropriate screening is carried out to exclude patients with contraindi-
cations, then MRI carries minimal risk. Patients who are haemodynamically
unstable, e.g. acute aortic dissection, should undergo an alternative form
of imaging. CT exposes the patient to ionizing radiation, and so this should
limit its application.
Possible results
Figures 6.3– 6.9 show the types of images that can be acquired by CMR
imaging.
Advantages overothertests
Volumetric imaging is non- invasive and facilitates excellent temporal and
spatial denition of soft tissues. It overcomes diculties of echocardiog-
raphy where acoustic window availability limits the scan. CMR uses no
ionizing radiation and so is ideal for serial imaging. Avery comprehensive
examination can be performed and allows dynamic assessment of the heart
and great vessels at a single visit. Multislice CT provides very accurate infor-
mation regarding coronary artery disease and is a non- invasive alternative
to traditional coronary angiography.
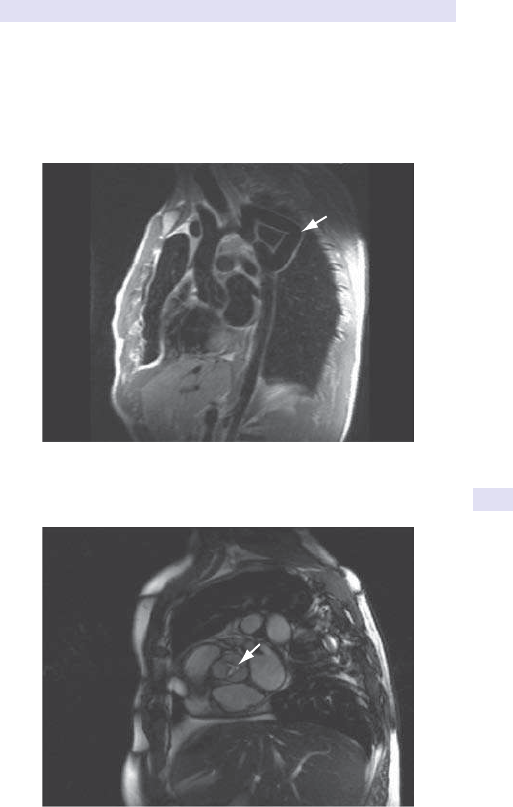
CARDIAC VOLUMETRIC IMAGING
445
Pitfalls
CMR image quality may be impaired by artefact in some patients, especially
if metal is present, e.g. non- ferrous surgical clips, spinal rods, or prosthetic
valves. Rapid heart rates degrade the image quality in multisliceCT.
Aortic coarctation
bypass graft
Fig.6.3 Examples of volumetric imaging techniques— MRI black blood sequence
illustrating aortic coarctation bypass.
Aortic stenosis
Fig.6.4 MRI cine sequence of aortic stenosis.
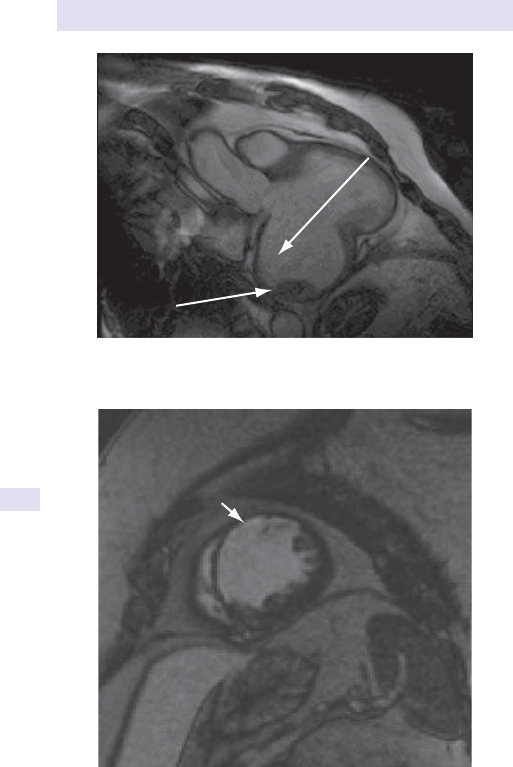
446
CHAPTER6 Cardiology
446
Inferoposterior
left ventricular
aneurysm
Thrombus
Fig.6.5 MRI cine sequence demonstrating huge inferoposterior left ventricular
aneurysm containing a thrombus.
Anteroseptal scar
Fig.6.6 MRI delayed enhancement study delineating subendocardial scar tissue in
the anteroseptal wall followingMI.
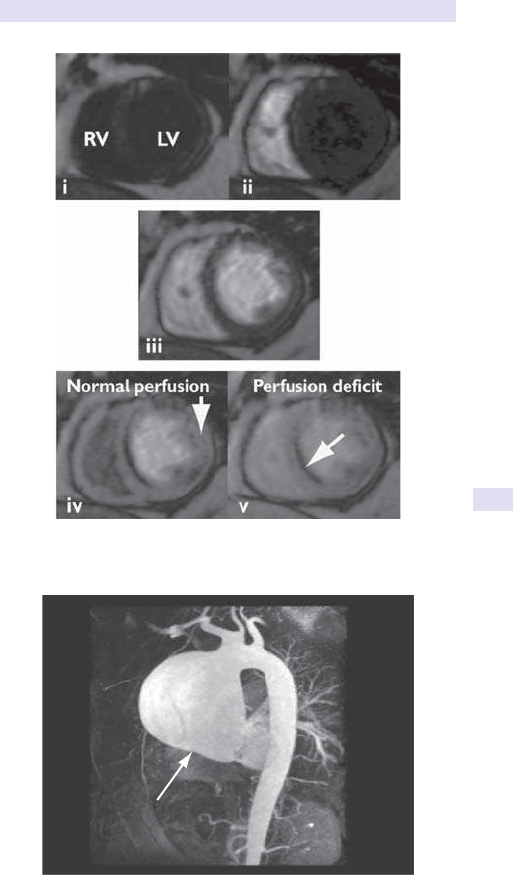
CARDIAC VOLUMETRIC IMAGING
447
Fig.6.7 MRI rst- pass perfusion:(i)prior to contrast injection; (ii) contrast agent
appears in the RV and (iii) LV; (iv, arrow) contrast opacies normally perfused
myocardium, (v, arrow) but identies an inferoseptal subendocardial perfusion decit.
Ascending aorta
aneurysm
Fig.6.8 Magnetic resonance angiogram (MRA) of a severe ascending aortic aneurysm.
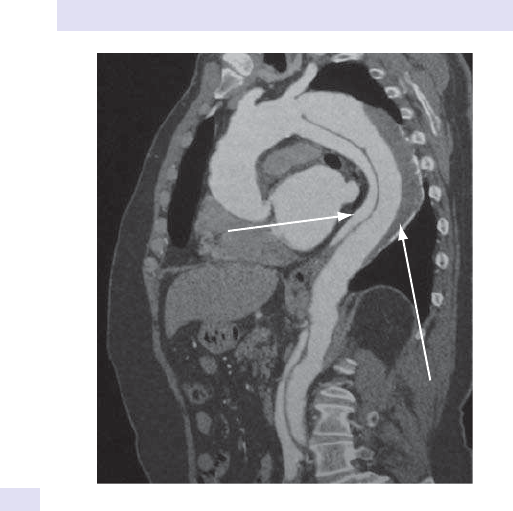
448
CHAPTER6 Cardiology
448
Further reading
Myerson SG, Francis J, Neubauer S. Cardiovascular Magnetic Resonance. Oxford:Oxford University
Press,2010.
Nicol E, Stirrup J, Kelion AD, Padley SPG. Cardiovascular Computed Tomography. Oxford: Oxford
University Press,2011.
Descending aorta
dissection
Thrombus
Fig.6.9 CT of type II aortic dissection and associated thrombus.

CARDIAC VOLUMETRIC IMAGING
449
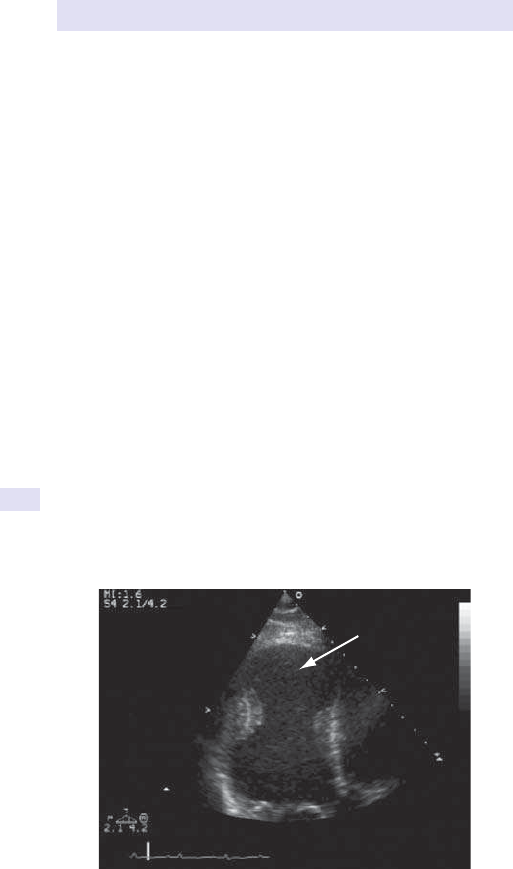
450
CHAPTER6 Cardiology
450
Echocardiography
Principle
Echocardiography is the use of US to visualize the anatomy, size, and func-
tion of cardiac structures, i.e. valves, chambers, myocardium, pericardium,
and great vessels. The technique provides information regarding the aeti-
ology and severity of most congenital and acquired cardiac abnormalities.
Imaging modalities
Several imaging modalities are available, including 2- dimensional (2D), 3D,
motion- mode (M- mode), Doppler, and contrast agent enhancement (see
Figs 6.10– 6.15).
2D imaging
2D echocardiography allows real- time visualization of cardiac anatomy,
abnormalities of cardiac structures, and their motion. Image quality is
enhanced by use of harmonic imaging and contrast opacication. With
some systems, 3D imaging is also possible.
3D imaging
3D echocardiography is now widely available and used in both TTE and
TOE. The modality is particularly useful for the assessment of left ventricu-
lar size and function, of congenital heart disease (morphology and function),
and in the guidance of interventional procedures (e.g. percutaneous atrial
septal defect closure).
M- mode imaging
M- mode imaging samples movement of cardiac structures along a single
scan line, creating a graph of the motion of sampled structures against time.
It is useful for accurate timing of cardiac events and measurement of cardiac
dimensions.
LV aneurysm
Fig.6.10 2D TTE image of the LV demonstrating an apical aneurysm.
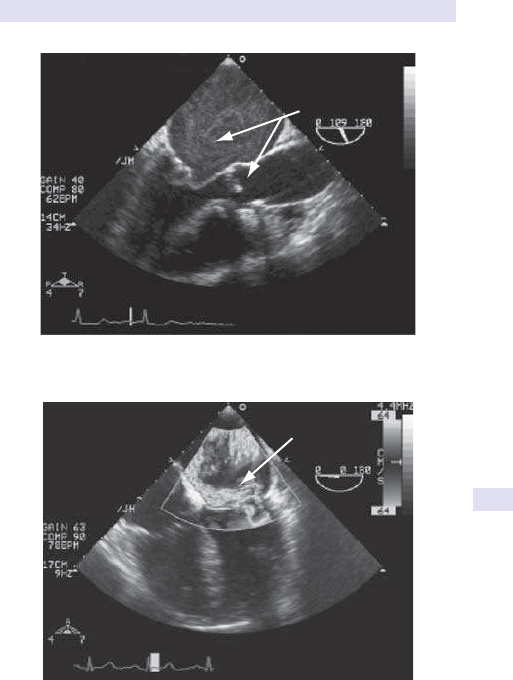
ECHOCARDIOGRAPHY
451
Mitral & aortic
stenosis
Fig.6.11 2D TOE image of rheumatic heart disease (mitral and aortic stenosis).
Mitral
regurgitation
Fig.6.12 Colour Doppler of eccentric jet of severe mitral regurgitation 2° to mitral
valve prolapse. (E Colour plate1.)
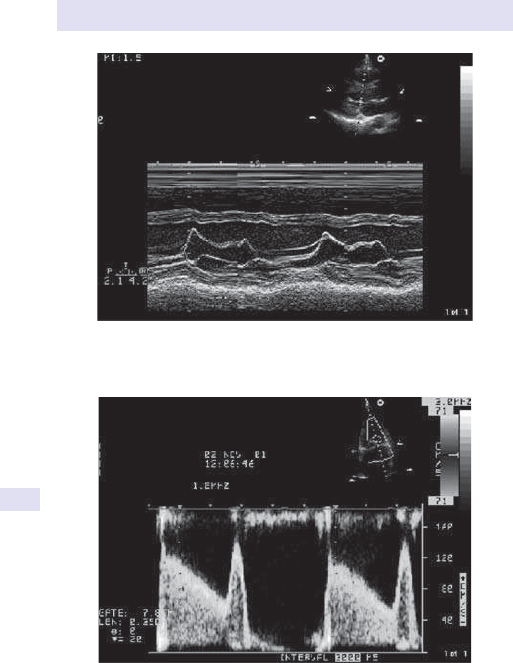
452
CHAPTER6 Cardiology
452
Mitral stenosis
Fig.6.14 Laminar pulsed- wave Doppler ow in a patient with mitral stenosis.
(E Colour plate2.)
M-mode Mitral valve
Fig.6.13 Normal M- mode through mitral valve leaets.
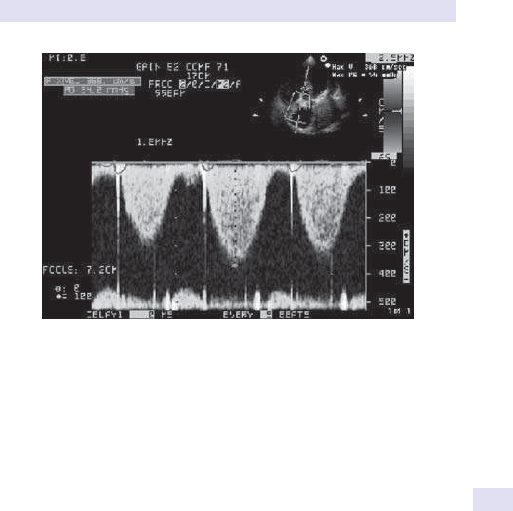
ECHOCARDIOGRAPHY
453
Doppler
Doppler is the comparison of transmitted US beam frequency with received
US frequency reected from moving structures, e.g. soft tissues (Doppler
tissue imaging) or blood cells. The direction of motion and its velocity can
be assessed. When blood cells reect US as they move towards the trans-
ducer, they compress the US wavelength, whereas if they are moving away,
the US wavelength lengthens. The change in frequency between the trans-
mitted and reected wavelengths is the Doppler shift frequency.
Continuous- wave Doppler
Continuous- wave (CW) Doppler acquires velocity data along the US beam’s
entire path. Blood ow of varying strengths and velocities is demonstrated
in blood vessels and through the cardiac valves. The signal is represented
graphically on a spectral display. Flow away from the transducer is reected
below the zero line; ow towards the transducer is +ve in deection. Signal
density and shape give an indication of the severity of any abnormalities.
CW Doppler velocity data can be used to measure the pressure gradi-
ents between the cardiac chambers according to the Bernoulli equation.
The data thus obtained can be used to calculate the severity of valvular ste-
nosis, expressed in terms of mean and peak pressure gradients, and eec-
tive orice area. The pressure half- time can be used to estimate the severity
of diastolic valvular lesions, i.e. mitral stenosis and aortic regurgitation. It is
dened as the time taken for the peak gradient to fall to half of its original
value. It is inversely related to the eective oricearea.
Pulsed- wave Doppler
The velocity of blood is sampled within a small area. Since this involves only
a small sample volume, it localizes blood ow. It is particularly useful for
evaluating low velocity ow such as cardiac inow and outow tract veloci-
ties. At higher velocities, it is limited by the problem of aliasing.
Tricuspid regurgitation
Fig.6.15 Continuous- wave Doppler of tricuspid regurgitation, with PmA systolic
pressure calculated as 54mmHg (plus right atrial pressure). (E Colour plate3.)

454
CHAPTER6 Cardiology
454
Colour Doppler
Colour Doppler colour- codes the direction and velocity of blood ow
through cardiac structures. Blood ow in the direction away from the US
probe is depicted as blue, whereas blood owing towards the probe is
red.i blood ow velocity is reected by colour mixing or turbulence. This
is a useful screening tool for abnormal jets of blood and can be used to
estimate the severity of some abnormalities.
Contrast echocardiography
Microbubbles, consisting of a gas core encapsulated by a protein shell, can
be used as contrast agents to improve image quality. Typically, they are used
in conjunction with harmonic imaging to allow opacication of the left ven-
tricular volume and thereby assess wall motion. Research is continuing into
their use to assess myocardial perfusion.
Transthoracic and transoesophageal echocardiography
TTE, where the US beam is directed to the heart from outside the chest
wall, is the most commonly performed examination. In certain clinical situ-
ations, more information is obtained by directing the US beam towards
the heart from the oesophagus, and this is termed TOE. This allows image
acquisition without interference of the chest wall, i.e. ribs, soft tissues, and
lungs. US beam attenuation is small and a high- frequency transducer can be
used, giving rise to higher spatial resolution than with TTE. This facilitates
improved denition of the posterior cardiac structures, i.e. valves, atria, and
aorta. Relatively small structures, such as cardiac vegetations, may be visual-
ized with greater accuracy.
Indications
Indications for TTE and TOE are shown in Table6.2.
Contraindications
TTE is a very safe imaging technique with no known side eects.
Contraindications to TOE include:
•
Cervical spine instability, e.g. RhA, ankylosing spondylitis.
• Oesophageal disease, e.g. stricture, carcinoma, oesophageal varices.
• Haemodynamically unstable patients, including signicant hypoxia.
Patient preparation
For both procedures, the patient is made comfortable on an imaging
couch in the left lateral position with the head end raised to at least 60°.
Cardiac electrodes should be applied to obtain an ECG trace. Aqueous
gel is used on the probe to aid US beam conduction. For TOE, the patient
should be nil by mouth for at least 6h prior to the procedure and IV access
sited. Any loose teeth or dentures should be removed. The throat should
be sprayed with an LAn, e.g. lidocaine, and a bite guard inserted prior to
intubation. The patient may be sedated according to the clinical situation,
patient preference, or operator recommendation. Antibiotic prophylaxis is
not required.

ECHOCARDIOGRAPHY
455
Procedure
For TTE, the probe is applied to the chest in standard imaging positions
(parasternal, apical, subcostal, and suprasternal), and the following imaging
planes (see Fig. 6.16) are acquired with use of all available modalities:
•
Parasternal (long axis and shortaxis).
• Apical (2- , 3- , 4- , 5- chamber).
• Subcostal and suprasternal.
For TOE, the probe is passed gently over the tongue towards the cri-
copharyngeal muscles. Gentle continuous pressure is applied, and the
patient is encouraged to swallow until the probe lies within the oesophagus.
Views of the cardiac structures are acquired from varying levels within the
oesophagus, gastro- oesophageal junction, and stomach.
Risks
TTE is extremely safe. TOE is a semi- invasive procedure, and so informed
written consent should be obtained. Intubation of the oesophagus carries
a risk of 71 per 2000 of oesophageal trauma. There is a small risk of laryn-
gospasm and cardiac arrhythmia (usually supraventricular). This usually
resolves spontaneously on probe withdrawal. Neither technique should be
performed on a haemodynamically compromised patient where an inter-
ventional procedure is delayed by inappropriate image acquisition, e.g. aor-
tic dissection, cardiac tamponade.
Table6.2 Indications forTTE andTOE
Condition TTE TOE
Congenital heart disease ++ +++
Suitability for percutaneous closure of atrial septal defect − ++++
Anatomy, size, and function of the cardiac chambers (left
atrium, LV, right atrium, RV)
+++ ++++
Global and regional left ventricular function +++ +++
Valvular heart disease (aortic, mitral, tricuspid, and pulmonary) +++ ++++
Assessment for mitral valvuloplasty + ++++
Prosthetic heart valves ++ ++++
Infective endocarditis (and its complications) ++ ++++
Guide to safe cardioversion (AF) − +++
Intra- operative assessment of valve disease/ repair/ replacement − +++
Pulmonary hypertension ++ +++
Unexplained pulmonary hypertension/ right- sided dilatation + ++++
Cardiac source of embolus in TIA/ CVA/ peripheral embolism + +++
Cardiac masses ++ +++
Pericardial disease +++ ++
Aortic disease + +++
Screening (cardiomyopathy) +++ ++
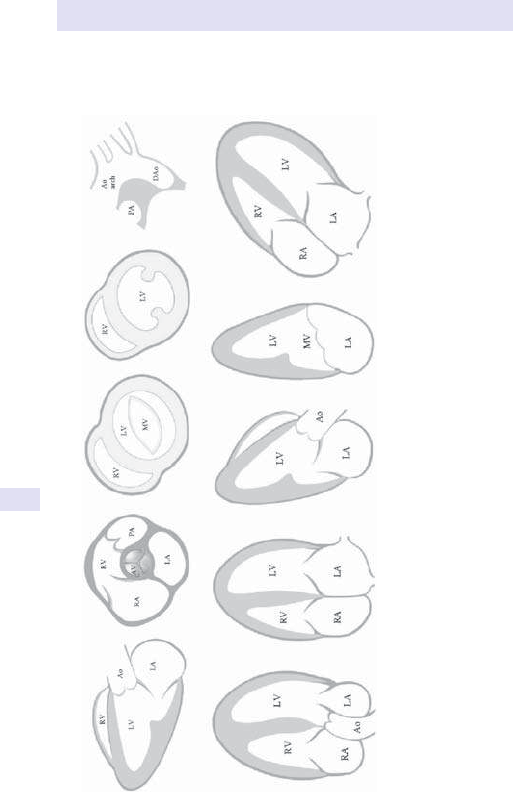
456
CHAPTER6 Cardiology
456
Fig.6.16 Transthoracic imaging planes:(a)parasternal long axis, (b)parasternal short axis at level of aortic valve, (c)mitral
valve, (d)papillary muscles, (e)suprasternal notch, (f– i) apical 5- , 4- , 3- , and 2- chamber views, (j)subcostal plane. LA, left
atrium; LV, left ventricle; RA, right atrium; RV, right ventricle; Ao, aorta; PA, pulmonary artery; AV, aortic valve; MV, mitral valve;
DAo, descendingaorta.
a) b) c) d) e)
f) g) h) i) j)

ECHOCARDIOGRAPHY
457
Advantages overothertests
TTE is cheap and non- invasive and requires no ionizing radiation, ensuring
that serial examinations are without risk. It is portable and can easily be
used at the bedside. The technique provides a comprehensive assessment
of cardiac anatomy, function, and blood ow. TOE has similar advantages to
TTE, with the dierence that it is a semi- invasive technique but oers supe-
rior spatial resolution, particularly of structures in close proximity to the
probe (e.g. left atrium, mitral valve). It is a powerful tool for intra- operative
use and in patients with limited transthoracic windows.
Pitfalls
Transthoracic image quality may be limited by inadequate acoustic windows
in patients with obesity, lung disease, and chest wall deformities, and those
undergoing articial ventilation. Structures at the posterior aspect of the
heart are not well visualized. Optimal transoesophageal image quality is
obtained at the posterior aspect of the heart, and so the apex of the heart
is less well seen. Image quality may be compromised in patients with hiatus
hernia. Not all patients tolerate the procedure, and so images relating to the
suspected pathology should be acquired rst in case the procedure has to
be abandoned prematurely.
Possible results
Anatomy and size ofcardiac chambers
Normal anatomy is demonstrated to exclude congenital heart disease. Each
cardiac chamber and its connections are systematically identied and the
presence of any shunts excluded. The size of the cardiac chambers and
walls are assessed. Normal values are shown in Table6.3.
Causes ofchamber abnormalities include
• LAt dilatation:mitral valve disease, systemic hypertension, coronary
artery disease, dilated cardiomyopathy, restrictive cardiomyopathy,
hypertrophic cardiomyopathy,AF.
• Left ventricular dilatation:mitral regurgitation, aortic regurgitation, severe
aortic stenosis, systemic hypertension, IHD, dilated cardiomyopathy.
• Left ventricular hypertrophy:systemic hypertension, left ventricular
outow obstruction (aortic stenosis, supra- aortic membrane, subaortic
membrane), hypertrophic cardiomyopathy (asymmetric), aortic
coarctation, inltrative cardiomyopathy (amyloidosis).
Table6.3 Normal values forcardiac linear dimensions
Parameter Normal range
LAt diameter ♂ 3.0– 4.0cm
♀ 2.7– 3.8cm
Left ventricular diastolic diameter ♂ 4.2– 5.9cm
♀ 3.9– 5.3cm`
Interventricular septum diastolic diameter 0.6– 1.2cm
Left ventricular posterior wall diastolic diameter 0.6– 1.2cm

458
CHAPTER6 Cardiology
458
• Aortic root dilatation:systemic hypertension, collagen disorders, e.g.
Marfan’s syndrome, syphilitic aortitis, aortic coarctation, aortic valve
disease, aortic aneurysm, aortic dissection.
•
Right atrial (RAt) dilatation:tricuspid valve disease, pulmonary valve
disease, pulmonary hypertension, dilated cardiomyopathy, restrictive
cardiomyopathy, constrictive pericarditis.
•
Right ventricular dilatation:° pulmonary hypertension, 2° pulmonary
hypertension (e.g. mitral stenosis, PEs, lung disease, left- to- right shunts),
tricuspid regurgitation, pulmonary regurgitation, right ventricular
cardiomyopathy (including arrhythmogenic), right ventricular infarction.
•
Right ventricular hypertrophy:° pulmonary hypertension, 2° pulmonary
hypertension (e.g. mitral stenosis, PEs, lung disease, left- to- right shunts),
pulmonary stenosis, right ventricular outow tract obstruction.
•
Pulmonary trunk dilatation:pulmonary hypertension, collagen disorders,
e.g. Marfan’s syndrome, pulmonary atresia, pulmonary valve disease,
idiopathic PmA dilatation.
Global and regional left ventricular systolic function
The size, shape, and function of the LV are evaluated with 2D/ 3D imaging ±
contrast opacication. Commonly measured echocardiographic parameters
of left ventricular function include EF, fractional shortening, stroke volume,
and cardiac output. It should be remembered that, when calculated from
the M- mode image, only the function of the base of the heart is assessed.
This should not be extrapolated to global left ventricular function, unless the
entire ventricle is normal. EF can also be calculated from apical diastolic and
systolic views (using the modied Simpson’s rule). This is more reective of
global left ventricular function but is still limited since it is a 2D measurement
and requires geometric assumptions. Amore subjective assessment can be
made by visually estimating left ventricular function as normal or as having
mild, moderate, or severe impairment. Causes of impaired left ventricular
systolic functionare:
•
Ischaemic cardiomyopathy.
• Hypertensive cardiomyopathy.
• Dilated cardiomyopathy.
• Valvular heart disease.
Regional wall motion abnormalities are conned to specic walls or seg-
ments of the LV. Systolic wall thickening is dened in Table6.4.
Table6.4 Categorization ofregional wallmotion
Severity Description
Normal >50% i in systolic wall thickness, compared with diastole
Hypokinetic <50% i in systolic wall thickness, compared with diastole
Akinetic Absent systolic wall thickening
Dyskinetic Outward wall motion during systole

ECHOCARDIOGRAPHY
459
Causes ofregional wall motion abnormalities are almost exclusively related
tocoronary artery disease
•
MI.
• Left ventricular aneurysm.
• Myocardial hibernation.
• Myocardial ischaemia.
• Post- cardiac surgery.
• Cardiac tumour.
Stress echocardiography
Dobutamine stress may be used in conjunction with left ventricular global
and regional wall functional assessment. As well as allowing distinction
between normal, ischaemic, and infarcted myocardium, it also permits the
assessment of myocardial viability (regional dysfunction that will improve
with revascularization) vs scar tissue (no eect on function from revasculari-
zation). It should be noted that CMR is now proven to be a superior tech-
nique for identication of myocardial scar/ viability. Resulting interpretation
with respect to regional wall motion responses to low- dose and peak- dose
dobutamine stress is shown in Table6.5.
Left ventricular diastolic function
Left ventricular lling during diastole is an important component of left
ventricular functional assessment. Normal LAt lling is passive throughout
systole (S wave) and diastole (D wave) and is assessed from pulsed- wave
(PW) Doppler sampling of pulmonary venous ow. Left ventricular lling
is assessed from diastolic mitral valve ow. It is predominantly passive and
early in diastole (E wave), with a small later contribution from atrial sys-
tole (A wave). Diastolic dysfunction results in elevated left ventricular end-
diastolic pressure (LVEDP), and ultimately left atrial pressure (LAP), and
so alters measured ow characteristics. There are three types of diastolic
dysfunction (see Table 6.6), depending on the degree of raised LAP that
occurs in order to drive ow across the mitralvalve.
Right heart function
Right heart failure is depicted by a dilated, poorly functioning RV. Raised
right- sided pressures are indicated by a dilated right atrium and IVC (seen
on subcostalview).
Table6.5 Interpretation ofcontractile responses todobutaminestress
Interpretation Rest Low dose Peak dose
Normal Normal i Hyperdynamic
Inducible
ischaemia
Normal or d No change or
d from baseline
d from baseline
Scar tissue
Absent Absent Absent
Viability Absent Improved Improved or d compared
with low dose (biphasic
response)
460
CHAPTER6 Cardiology
460
Table
6.6 Echo assessment of left ventricular diastolic dysfunction
Characteristics
Abnormal relaxation Pseudonormalization Restrictive
Haemodynamics
iLVEDP
Normal LAP
Loss of passive gradient LA:LV
Left ventricular lling from atrial
systole
iLVEDP
iLAP
LA:LV passive lling gradient
restored
Pulmonary vein: LA gradient lost
iLVEDP
iiiLAP
LA:LV passive lling gradient
ii
Pulmonary vein: LA gradient lost
Mitral valve
(E:A reversal Ed Ai)
Normal
(Eii, sharp descent, Ad)
Pulmonary venous o
w redaorb repeed ,evaw S dehsinimiDNormal
A wave
Diminshed S wave, deeper broader
A wave
Doppler pattern
(mitral valve
above pulmonary vein belo
w)
ereveSetaredoMdliMLeft ventricular impairment
Signi
cance
Slow early left ventricular relaxation
Slow early relaxation, reduced
compliance
Slow early left ventricular relaxation,
severely reduced compliance
SymptomsWell at rest, but shortness of breath if
heart rate i
Shortness of breath on exercise and
limited exercise tolerance
Shortness of breath on minimal exertion

ECHOCARDIOGRAPHY
461
Pulmonary hypertension
Systolic PmA pressure, assuming there is no pulmonary valve stenosis, can
be estimated from the pressure gradient between the right atrium and ven-
tricle. This is measured from any tricuspid regurgitant jet (Bernoulli equa-
tion) summated with the estimated RAt pressure. Diastolic PmA pressure is
measured by substituting the end- diastolic velocity of pulmonary regurgita-
tion into the Bernoulli equation, again summated with the estimated RAt
pressure. If the systolic PmA pressure is raised but the diastolic pressure
is normal, this represents i ow volume, rather than pulmonary hyperten-
sion. RAt pressure is assessed by IVC diameter evaluation and its calibre
reduction with inspiration (see Table6.7).
Valvular heart disease
Echocardiography can identify and quantify valve abnormalities to decide
whether long- term follow- up or referral for valve surgery is necessary.
Valvesmaybe
•
Stenosed (narrowed).
• Regurgitant (leaky).
• Infected (endocarditis).
• Aected by other cardiac pathological processes, e.g. cardiomyopathy,
carcinoid.
Aortic stenosis
Aortic stenosis leads to left ventricular hypertrophy, i left ventricular pres-
sures, and, if untreated, LVF and risk of sudden death (see Table6.8).
Table6.7 Estimation ofright atrial pressure
IVC size (cm) IVC change with inspiration Estimated RAt
pressure (mmHg)
<1.5 Collapse
0– 5
1.5– 2.5 d >50% 5– 10
1.5– 2.5 d <50% 10– 15
>2.5 d <50% 15– 20
2.5 No change >20
Table6.8 Parameters ofaortic stenosis severity
Mild Moderate Severe
Mean gradient (mmHg) <25 25– 40 >40
Peak gradient (mmHg) <36 36– 64 >64
Eective orice area (cm
2
) 1.5– 2.0 1.0– 1.4 <1.0
462
CHAPTER6 Cardiology
462
• Aetiology:congenital abnormality (bicuspid, unicuspid, or quadricuspid
valve), calcic degenerative disease, rheumatic heart disease.
•
Dierential diagnosis:hypertrophic obstructive cardiomyopathy,
subaortic membrane, supra- aortic membrane.
• 2D/ 3D ndings:thickened, calcied, and/ or fused aortic cusps with
reduced excursion, left ventricular outow tract dimension (to calculate
the aortic valve area), eects on the LV (hypertrophy/ impaired systolic/
diastolic function), post- stenotic dilatation of the ascending aorta/ aortic
coarctation.
•
Colour Doppler ndings:turbulent bright colour is seen through thevalve.
•
CW/ PW Doppler ndings:peak and mean valvular gradients; aortic
valve area; the dimensionless severity index (DSI) is a useful parameter,
particularly where left ventricular function is impaired.
Aortic regurgitation
Aortic regurgitation leads to mild left ventricular hypertrophy, left ventricu-
lar dilatation, andLVF.
•
Aetiology:congenital abnormality (bicuspid, unicuspid, or quadricuspid
valve), rheumatic heart disease, aortic leaet prolapse, calcic or
idiopathic degeneration, subaortic VSD, infective endocarditis (presence
of vegetations), aortic dissection (aortic root dissection ap), ascending
aortic dilatation (hypertension, aortic stenosis, age), aortitis (syphilis,
ankylosing spondylitis, GCA, RhA, Reiter’s syndrome), degenerative
disease, including collagen disease, e.g. Marfan’s syndrome.
•
2D/ 3D ndings:aortic valve anatomy (including calcication, prolapse,
vegetations, presence of subaortic VSD), size of aortic root/ aortic
dissection ap/ aortic coarctation, eects on the LV (hypertrophy/
impaired systolic/ diastolic function).
• Colour Doppler ndings:a diastolic regurgitant jet is seen. The width of
the jet (colour M- mode) comparative with left ventricular outow tract
diameter and its extent into the left ventricular cavity is measured.
•
CW/ PW Doppler ndings:the density of the diastolic signal is assessed,
together with the pressure half- time. Diastolic ow reversal in the aortic
arch or descending aorta is indicative of signicant aortic regurgitation,
unless the left ventricular diastolic pressure is high from a separate
aetiology, e.g. MI. It can be dicult to dierentiate between mild and
moderate aortic regurgitation, especially with transthoracic imaging (see
Table6.9).
Table6.9 Parameters ofaortic regurgitation severity
Mild Moderate Severe
Jet width (as % of left
ventricular outow tract)
<25 65
Pressure half- time (ms) >500 <250
Vena contracta width (cm) <0.3 >0.6
Diastolic ow reversal
None Aortic arch Descending aorta

ECHOCARDIOGRAPHY
463
Mitral stenosis
Mitral stenosis causes LAt dilatation, i pulmonary venous pressures, pul-
monary oedema, pulmonary hypertension, right heart failure, and func-
tional tricuspid regurgitation. TOE should be used to assess the suitability of
the patient for percutaneous mitral valvuloplasty (see Table6.10).
•
Aetiology:rheumatic heart disease/ calcication (valve leaets, chordae,
or papillary apparatus), congenital abnormality (very rare), SLE (rare).
•
Dierential diagnosis:LAt myxoma, obstruction of the valve by thrombus
or vegetations.
•
2D/ 3D ndings:anatomy and degree of any calcication/ fusion of
the mitral valve leaets and apparatus for suitability for valvuloplasty,
eective orice area (evaluated with planimetry), LAt size (dilated),
presence of LAt thrombus, right heart size (hypertrophy) and function.
• Colour Doppler ndings:turbulent ow is seen through thevalve.
•
CW/ PW Doppler ndings:peak and mean valvular gradients are
calculated, the pressure half- time is measured, and the eective orice
area calculated. Pulmonary hypertension is estimated.
Mitral regurgitation
Mitral regurgitation causes LAt dilatation, i pulmonary venous pressures,
pulmonary oedema, LVF, pulmonary hypertension, right heart failure, and
functional tricuspid regurgitation. TOE gives an optimal assessment of
severity (see Table6.11).
Table6.10 Parameters ofmitral stenosis severity
Mild Moderate Severe
Mean gradient (mmHg) <5 5– 10 >10
Pressure half- time (ms) 71– 139 140– 219 >219
Mitral orice area (cm
2
) 1.6– 2.0 1.0– 1.5 <1.0
Table6.11 Parameters ofmitral regurgitation severity
Mild Moderate Severe
Jet area (cm
2
) <4 >10
Signal density on CW Doppler + ++ +++
Vena contracta width (cm) <0.3 0.7
Pulmonary venous ow
Normal Absent systolic
component
Reversed systolic
component

464
CHAPTER6 Cardiology
464
• Aetiology:rheumatic heart disease, mitral valve prolapse/ redundant
tissue, IHD (papillary muscle rupture/ infarction/ restriction of posterior
leaet), dilated/ ischaemic cardiomyopathy (annular dilatation),
hypertrophic obstructive cardiomyopathy (systolic anterior motion),
infective endocarditis (presence of vegetations), congenital abnormality
(very rare; note the association of cleft mitral valve and primum atrial
septal defect), SLE (rare).
•
2D/ 3D ndings:mitral valve anatomy (calcication, prolapse, redundant
tissue, leaet excursion, vegetations, annular size, apparatus integrity),
left ventricular size and function (hypertrophy, dilatation), LAt size
(dilatation), right heart function (hypertrophy, dilatation), and PmA
pressures.
•
Colour Doppler ndings:an abnormal systolic jet is seen through the
mitral valve into the left atrium. The size of this is assessed. This can be
deceptive with very eccentric jets associated with mitral valve prolapse.
•
CW/ PW Doppler ndings:signal density and shape are characterized.
Analysis of pulmonary venous ow with PW Doppler can be helpful.
Tricuspid stenosis
Tricuspid stenosis causes RAt dilatation and i systemic venous pressures.
•
Aetiology:rheumatic heart disease (occurs in 10% of patients with mitral
stenosis), carcinoid disease, endomyocardial brosis,SLE.
•
2D/ 3D ndings:tricuspid valve anatomy and degree of any doming,
calcication, fusion of tricuspid valve leaets and apparatus, size of the
right atrium, and IVC (dilated).
•
Colour Doppler ndings:turbulent ow is seen through thevalve.
•
CW/ PW Doppler ndings:peak and mean valvular gradients are
calculated, the pressure half- time is estimated, and the eective orice
area calculated. Pulmonary hypertension is calculated from the velocity
of any tricuspid regurgitant jet. Peak E wave velocity is elevated (normal
peak is <0.7ms
−1
) and there is a slow deceleration time. Amean
gradient of 2– 3mmHg may be clinically signicant.
Tricuspid regurgitation
Tricuspid regurgitation leads to RAt dilatation, right ventricular hypertro-
phy, and failure (see Table6.12).
Table6.12 Parameters oftricuspid regurgitation severity
Mild Moderate Severe
Jet area (cm
2
) <5 5– 10 >10
Vena contracta width (cm) <0.7 >0.7
Signal density on CW Doppler + ++ +++
Shape of CW Doppler signal Pansystolic Pansystolic Triangular
Hepatic venous ow reversal None Absent systolic
component
Pansystolic ow
reversal

ECHOCARDIOGRAPHY
465
• Aetiology:rheumatic heart disease, infective endocarditis (IV drug abuse),
functional (2° to right ventricular dilatation, e.g. cardiac left- to- right
shunts, right ventricular cardiomyopathy, pulmonary hypertension,
permanent pacing system), carcinoid disease, Ebstein’s anomaly, SLE,
myxomatous degeneration, cardiac amyloidosis.
•
2D/ 3D ndings:tricuspid valve anatomy (site, calcication, prolapse,
redundant tissue, leaet excursion, vegetations, annular size, apparatus
integrity), right ventricular size (dilated) and function, size of the right
atrium, IVC (dilated), presence of pacingwires.
•
Colour Doppler ndings:abnormal systolic jet is seen through the tricuspid
valve into the right atrium. The size of this is assessed, compared with
the right atrium.
•
CW/ PW Doppler ndings:the density of the signal and shape is assessed.
Analysis of hepatic ow with PW Doppler can be helpful. Pulmonary
arterial pressure can be measured but may be underestimated if there is
severe tricuspid regurgitation.
Pulmonary stenosis
Pulmonary stenosis causes right ventricular hypertrophy and, if left
untreated, right heart failure (see Table6.13).
•
Aetiology:congenital, rheumatic heart disease, carcinoid.
•
Dierential diagnosis:right ventricular outow obstruction (infundibular
stenosis).
•
2D/ 3D ndings:pulmonary valve anatomy (calcication, leaet
thickening, doming), size of the pulmonary trunk (post- stenotic
dilatation), eects on the RV (hypertrophy/ impaired function).
• Colour Doppler ndings:turbulent systolic ow through the
pulmonaryvalve.
•
CW/ PW Doppler ndings:The maximum gradient and pulmonary valve
area are calculated.
Pulmonary regurgitation
•
Aetiology:congenital, infective endocarditis (IV drug abuse), pulmonary
hypertension, PmA dilatation, carcinoid disease, post- pulmonary
valvotomy.
•
2D/ 3D ndings:pulmonary valve anatomy (calcication, prolapse,
vegetations), size of the pulmonary trunk, eects on the RV
(hypertrophy/ impaired function).
• Colour Doppler ndings:a diastolic regurgitant jet is seen. The width of
the jet and its extent into the right ventricular outow tract and cavity
are measured.
Table6.13 Parameters ofpulmonary stenosis severity
Mild Moderate Severe
Peak gradient (mmHg) <40 40– 75 >75

466
CHAPTER6 Cardiology
466
• CW/ PW Doppler ndings:the density of the diastolic signal and its
duration are estimated. Pulmonary regurgitation is haemodynamically
signicant if the jet is broad relative to the width of the PmA, extends
>2cm into the right ventricular outow tract, and persists throughout
diastole.
Prosthetic heartvalves
Echocardiography is used for follow- up of prosthetic heart valves and
potential dysfunction. It is important to consult tables of normal ow pat-
tern values for each valve type according to its make and size. TOE is supe-
rior to TTE. The following parameters are assessed:
•
Direct 2D/ 3D imaging of the valve ring and leaets.
• Presence of any rocking motion suggestive of valvular dehiscence.
• Forward blood ow through thevalve.
• Valvular regurgitation (typical regurgitation on valve closure is normal).
• Infective endocarditis.
• Thrombus/ pannus.
Infective endocarditis
The following features are characteristic of infective endocarditis:
•
Predisposing abnormal structure (valve lesion/ congenital abnormality).
• Regurgitant lesion.
• Mobile echogenic masses (vegetations, usually in the path of
regurgitantjets).
•
Spread of infection to other valves (usually along the path of a
regurgitantjet).
•
Abscess (particularly around the aortic root, prosthetic valves).
• Embolic potential (large, mobile vegetations, especially the aortic valve).
• Valve destruction (degree of regurgitation and eect on cardiac
chamber size and function).
•
Chamber perforation and shunting.
• Prosthetic valve dehiscence.
Pericardial disease
The normal pericardium is poorly visualized with echocardiography, since it
is a very thin structure. Pericardial abnormalities that may be identiedare:
•
Pericardial eusion (uid between the pericardial layers).
• Pericardial thickening or calcication (constrictive pericarditis).
• Pericardial masses, e.g. cysts or tumour.
Pericardial eusion
A pericardial eusion gives rise to echo- free space around the heart. The
anatomical relationship of pericardial uid with the descending aorta dis-
tinguishes pericardial (anterior) and pleural (posterior) eusions. If the
eusion is organizing or contains a thrombus or tumour, then echodense
structures may be identied within it. The width of this space is measured
to give a rough guide as to the size of the eusion:
•
Small:<1cm
• Moderate1:1– 2cm.
• Large:>2cm.

ECHOCARDIOGRAPHY
467
The most important assessment is whether uid is causing any haemody-
namic compromise, i.e. cardiac tamponade. This may occur regardless of
the size of the eusion, particularly if it has accumulated rapidly. Features
suggestive of a tamponade include:
•
Dilatation of the IVC (>2.5cm), poor collapse with inspiration (<50%).
• Exaggerated reduction in transmitral velocities on inspiration (>40%).
• Right ventricular diastolic collapse.
• Low- volume, poorly lledLV.
Constrictive pericarditis
This is typically caused by TB and constrains left and right ventricular ll-
ing. On 2D imaging, the pericardium is thickened and appears bright when
calcication is present. The period of left ventricular diastolic function is
shortened. Systolic function is normal. Dopplershows:
•
Exaggerated reduction of transmitral E wave velocity with inspiration.
• Shortened transmitral E wave decelerationtime.
• Exaggerated ow reversal in the superior vena cava (SVC) on expiration.
Cardiacmasses
The commonest intra- cardiac masses are thrombi and vegetations (infective
endocarditis). Thrombus formation is i in conditions causing slow blood
ow such as mitral or tricuspid valve stenosis or cardiomyopathy. The com-
monest cardiac tumours are atrial myxomas and metastatic deposits. An
atrial myxoma is typically a pedunculated, frond- like mass that arises from
the interatrial septum. Metastatic deposits can be found anywhere within
the heart, including the pericardium. Extrinsic masses may cause compres-
sion of cardiac structures. Atrial pectinate muscles, Eustachian valve, Chiari
network, papillary muscles, brin strands, sutures on prosthetic valves, large
vascular structures, such as the aorta, coronary sinus, or left ventricular
aneurysms, may be incorrectly interpreted as cardiac masses.
•
Atrial masses:thrombus, myxoma, lipomatous hypertrophy of the
interatrial septum, ruptured mitral valve apparatus, ° benign tumour, °
malignant tumour, 2° tumour.
•
Ventricular masses:thrombus, ruptured mitral valve apparatus, ° benign
tumour (broma, lipoma, rhabdomyoma, haemangioma), ° malignant
tumour (rhabdomyosarcoma, brosarcoma, angiosarcoma), 2° tumour.
•
Valvular masses:vegetations, thrombus/ pannus, broelastoma.
Aortic disease
The aorta is a predominantly posterior structure, and so the arch and
descending aorta are best visualized byTOE.
•
2D ndings:integrity of the walls is assessed for atheroma (irregular wall
thickening), intramural haematoma, or dissection ap. The latter may be
indirectly suspected from the presence of pericardial eusion or aortic
regurgitation. Its anatomy and extent are noted to allow classication.
Presence of regional left ventricular wall motion abnormalities may
indicate coronary artery involvement. The size of the aorta is measured
at several sites to assess dilatation or coarctation (see Table 6.14). The
normal aortic wall thickness is4mm.

468
CHAPTER6 Cardiology
468
• Doppler ndings:colour, CW, and PW Doppler can all be used to
distinguish between true (normal systolic velocity ow) and false (low
or absent systolic ow) lumens in aortic dissection, to identify and
determine the severity of aortic regurgitation and aortic coarctation.
Congenital heart disease
It is important to recognize situs and connections of cardiac chambers and
great vessels.
•
The RV is recognized by the following features:
•
It is associated with the tricuspidvalve.
•
The tricuspid valve is sited more towards the apex than the
mitralvalve.
•
The tricuspid and pulmonary valves are not continuous (compare the
aortic and mitral valves).
•
It is trabeculated and contains a moderatorband.
• The ventricles should be connected to the correct outowtract:
•
The aorta is a single vessel.
•
The PmA bifurcates soon after its origin, unless hypoplasia/ atresia is
present.
•
The atria should be connected to the correct inow:
•
The pulmonary veins normally drain into the left atrium. Drainage
into alternative structures, e.g. SVC or IVC, hepatic veins, is referred
to as anomalous pulmonary venous drainage and is associated with a
left- to- rightshunt.
There should be no shunts between systemic and pulmonary systems; such
shunts include atrial septal defect (abnormal connection between the left
and right atria; see Table 6.15), VSD (abnormal connection between the LV
and RV; see Table 6.16), or patent ductus arteriosus (abnormal connection
between the aorta andPmA).
If shunts are identied, they are quantied. Pulmonary artery pressure is
assessed, together with evidence of shunt reversal (right to left), indicat-
ing Eisenmenger’s syndrome. Fallot’s tetralogy is a relatively common con-
genital condition, composed of a subaortic VSD, an overriding aorta, right
ventricular outow tract or pulmonary valve stenosis, and right ventricular
hypertrophy.
Table6.14 Normal aortic dimensions
Site Normal range (mm)
Annulus 20– 31
Sinus of Valsalva 29– 45
Sinotubular junction 22– 36
Tubular ascending aorta 22– 36
Descending 20– 30

ECHOCARDIOGRAPHY
469
Valves and outow tracts should be morphologicallynormal
•
Aortic valve:may be unicuspid, bicuspid, quadricuspid, or associated with
a subaortic or supra- aortic membrane.
• Pulmonary valve/ right ventricular outow tract:may be obstructed.
•
Mitral valve:cleft leaet associated with primum atrial septal defect,
mitral stenosis, parachutevalve.
•
Tricuspid valve:ventricularization of site=Ebstein’s anomaly.
•
Aortic and pulmonary arteries:should be normal situs, size, and anatomy,
and unobstructed:
•
Left- sided aortic arch, aortic coarctation.
•
Pulmonary hypoplasia, atresia, aecting either of the PmAs after
trunk bifurcation.
Further reading
British Society of Echocardiography. M http:// www.bsecho.org.
Houghton AR. Making Sense of Echocardiography, 2nd edn. Boca Raton:CRC Press,2014.
Leeson P, Augustine D, Mitchell ARJ, Becher H. Echocardiography, 2nd edn. Oxford: Oxford
University Press,2012.
Otto CM. Textbook of Clinical Echocardiography, 5th edn. Philadelphia:Elsevier Saunders,2013.
Table6.15 Types ofatrial septaldefect
Atrial septal defects Site Associations
Ostium secundum Fossa ovalis Mitral valve prolapse
Ostium primum Low septum
AV valve abnormalities
Sinus venosus Upper septum Anomalous pulmonary venous drainage
Table6.16 Types ofventricular septaldefect
VSD Site Associations
Membranous Infundibular septum
Subaortic Below aortic valve
Aortic regurgitation
Fallot’s tetralogy
Muscular Muscular septum
AV Posterior septum near AV valves AV valve abnormalities

470
CHAPTER6 Cardiology
470
Electrocardiogram
Principle
The ECG records the heart’s electrical activity. It can provide valuable infor-
mation about not just arrhythmias, but also a host of other disorders that
aect the electrical activity of the myocardium such as ischaemia, cardio-
myopathy, and electrolyte disturbances.
Indications
• Investigation of suspected arrhythmias, both to ‘capture’ the cardiac
rhythm, whilst the patient is experiencing symptoms, and also to look
for predisposing abnormalities, e.g. short PR interval, ventricular pre-
excitation (deltawaves), conduction abnormalities, long QT interval.
•
Investigation of chest pain, e.g. myocardial ischaemia or infarction,
pericarditis,PE.
•
Assessment of suspected cardiomyopathy and/ or LVF (a normal ECG is
unusual in the presence of left ventricular systolic dysfunction).
•
Assessment of electrolyte disturbances, particularly where these might
have pro- arrhythmic potential, e.g. hyperkalaemia.
• Assessment of drug eects on the heart, e.g. digoxin, tricyclic
antidepressants.
Contraindications
None. However, always check if the patient has a known allergy to the self-
adhesive pads used to attach the electrodes to theskin.
Patient preparation
• Explain what the procedure involves.
• Ask the patient to lie supine on a bed or examinationcouch.
• Prepare the skin by shaving, where necessary, and cleaning with
alcoholwipes.
•
Ask the patient to relax and lie still, whilst the recording is in progress.
Procedure
• Having prepared the patient for the test, attach the chest and limb
electrodes in the appropriate positions.
•
Before recording the ECG, check that the calibration settings of the
ECG machine are appropriate. Standard settings are an amplitude of
10mm/ 1mV and a paper speed of 25mm/ s. Ensure that these settings
are noted on theECG.
•
After recording the ECG, ensure that the patient’s identication details
and the time and date of the recording are notedonit.
•
It is good practice to make a record on the ECG of any symptoms (e.g.
chest pain, palpitations) that the patient was experiencing at the time of
the recording or to write ‘asymptomatic’ where appropriate.
•
Ensure that the ECG is seen and reported by an appropriate sta
member as soon as practicable.

ELECTROCARDIOGRAM
471
Reporting thendings
Always use a systematic approach to ECG reporting to ensure nothing is
overlooked.
Report theECG inthe followingorder
•
Heart rate:bradycardia vs tachycardia.
•
Rhythm:regular vs irregular, supraventricular vs ventricular, broad
complex vs narrow complex.
•
QRS axis:left or right axis deviation.
•
P wave:presence or absence, inverted, tall (peaked), or wide (bid).
•
P– R interval:long, short, or variable.
•
Q waves:are pathological Q waves present?
•
QRS complex:large, small, broad, or abnormally shaped.
•
ST- segment:elevated or depressed.
•
T wave:tall, small, or inverted.
•
QT interval:short orlong.
•
U wave:are prominent U waves present?
•
Additional waves:are delta waves or J waves present?
Possible results
Heartrate
Heart rate can be calculated inone oftwoways
•
By counting the number of large squares between two successive
QRS complexes and dividing this number into 300, e.g. four large
squares=300/ 4=75bpm. This method is preferred when the heart
rhythm is regular.
•
By counting the number of QRS complexes along a 25cm rhythm strip
(50 large squares) and multiplying this number by 6, e.g. 14 complexes
in 25cm=14 × 6=84bpm. This method is preferred when the heart
rhythm is irregular.
Bradycardia is arbitrarily dened as a heart rate <60bpm, and tachycardia as
a heart rate >100bpm.
If thepatient is bradycardic, consider
•
Sinus bradycardia.
• Sick sinus syndrome.
• Second- and third- degree AVblock.
• Escape rhythms, e.g. AV junctional escape rhythms, ventricular escape
rhythms, asystole, and drug- induced conditions.
If thepatient is tachycardic, consider
•
Narrow complex tachycardia:e.g. sinus tachycardia, atrial tachycardia,
atrial utter, AF, AV re- entry tachycardias.
• Broad complex tachycardia:e.g. narrow complex tachycardia with
aberrant conduction, ventricular tachycardia, accelerated idioventricular
rhythm, torsades de pointes.

472
CHAPTER6 Cardiology
472
Cardiacrhythm
To identify thecardiac rhythm, ask thefollowing questions
•
How is the patient?
• Is ventricular activity (QRS complexes) present?
• What is the ventricularrate?
• Is the ventricular rhythm regular or irregular?
• Are the QRS complexes narrow orbroad?
• Are there P waves (atrial activity)?
• What is the correlation between P waves and QRS complexes?
Being able to describe the cardiac rhythm in these terms will narrow down
the range of possible diagnoses in most cases and you will, at least, be able
to describe the key features of the rhythm clearly over the telephone to
an expert.
Rhythms toconsider include
•
Sinoatrial nodal rhythms:
•
Sinus rhythm.
•
Sinus bradycardia.
•
Sinus tachycardia.
•
Sinus arrhythmia.
•
Sick sinus syndrome.
• Atrial rhythms:
•
Atrial tachycardia.
•
Atrial utter (see Fig.6.17).
•
AF (see Fig.6.18).
•
AV junctional rhythms.
•
AV re- entry tachycardias (see Fig.6.19).
• Ventricular rhythms:
•
Accelerated idioventricular rhythm.
•
Ventricular tachycardia (see Fig.6.20).
•
Polymorphic ventricular tachycardia (torsades de pointes;
seeFig.6.21).
•
Ventricular brillation (see Fig.6.22).
Fig.6.17 Atrial utter with 4:1 AVblock.
Fig.6.18 Atrial brillation.
ELECTROCARDIOGRAM
473
Fig.6.19 AV re- entry tachycardia.
Fig.6.20 Ventricular tachycardia.
Fig.6.21 Polymorphic ventricular tachycardia.
Fig.6.22 Ventricular brillation.
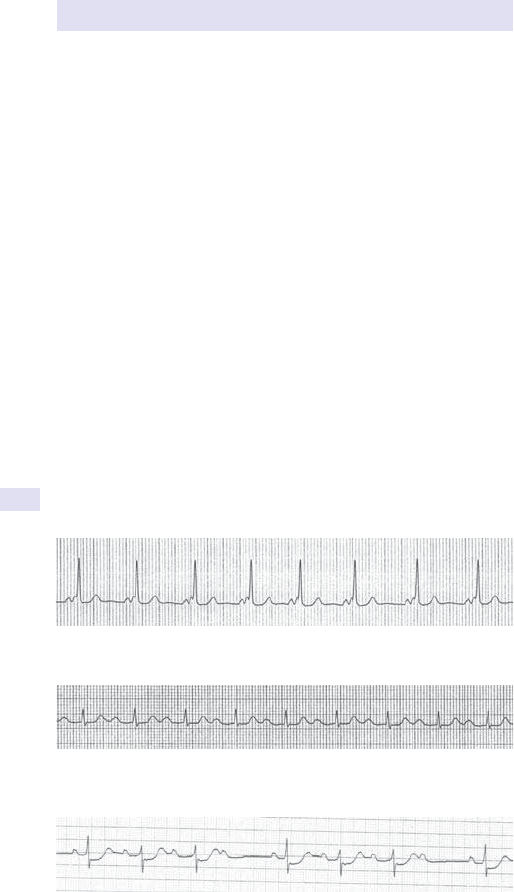
474
CHAPTER6 Cardiology
474
• Conduction disturbances.
•
Escape rhythms.
•
Ectopicbeats.
QRSaxis
•
Left axis deviation:left anterior hemiblock, Wol– Parkinson– White
syndrome, inferior MI, ventricular tachycardia (with left ventricular apical
focus).
•
Right axis deviation:left posterior hemiblock, right ventricular
hypertrophy, Wol– Parkinson– White syndrome, anterolateral MI,
dextrocardia.
Pwave
•
P waves absent:AF, sinus arrest or sinoatrial block (persistent or
intermittent), hyperkalaemia.
•
P waves inverted:dextrocardia, retrograde atrial depolarization,
electrode misplacement.
•
Tall or peaked P waves:RAt enlargement.
• Wide, often bid P waves:LAt enlargement.
PR interval
•
Short PR interval (<0.12s):AV junctional rhythm,
Wol– Parkinson– White syndrome (see Fig.6.23).
• Long PR interval (>0.2s):rst- degree AV block (see Fig. 6.24),
e.g.IHD,hypokalaemia, acute rheumatic myocarditis, Lyme disease,
digoxin, β- blockers, certain calcium channel blockers.
• Variable PR interval:second- degree AV block (Mobitz type I(see Fig. 6.25),
Mobitz type II, 2:1 AV block), third- degree AV block (see Fig.6.26).
Fig.6.23 Wol– Parkinson– White syndrome.
Fig.6.24 First- degree AVblock.
Fig.6.25 Second- degree AV block (Mobitz typeI).

ELECTROCARDIOGRAM
475
Qwaves
•
Pathological Q waves:MI, left ventricular hypertrophy, bundle
branchblock.
QRS complex
•
Large R or S waves:incorrect ECG calibration, left ventricular
hypertrophy, right ventricular hypertrophy, posterior MI,
Wol– Parkinson– White syndrome (left- sided accessory pathway),
dextrocardia, bundle branchblock.
•
Small QRS complexes:incorrect ECG calibration, obesity, emphysema,
pericardial eusion.
•
Broad QRS complexes (>0.12s):bundle branch block, ventricular
rhythms, hyperkalaemia.
•
Abnormally shaped QRS complexes:incomplete bundle branch block,
fascicular block, Wol– Parkinson– White syndrome.
ST- segment
• Elevated ST- segments:acute STEMI (see Fig. 6.27), left ventricular
aneurysm, Prinzmetal’s (vasospastic) angina, pericarditis (concave
‘saddle- shaped’ appearance), high take- o.
• Depressed ST- segments:myocardial ischaemia, acute posterior MI, drugs,
e.g. digoxin (reverse tick), ventricular hypertrophy with ‘strain’.
Twave
•
Tall T waves:hyperkalaemia, acuteMI.
•
Small T waves:hypokalaemia, pericardial eusion, hypothyroidism.
•
Inverted T waves:normal (aVR, V1, sometimes V2– V3 and III),
myocardial ischaemia, MI, ventricular hypertrophy with ‘strain’, digoxin
toxicity.
Fig.6.26 Third- degree AVblock.
Fig.6.27 AnterolateralSTEMI.

476
CHAPTER6 Cardiology
476
QT interval
• Short QT interval:hypercalcaemia, digoxin eect, hyperthermia.
•
Long QT interval:hypocalcaemia, drug eects, acute myocarditis,
hereditary syndromes (Jervell and Lange– Nielsen syndrome, Romano–
Ward syndrome).
Uwave
•
Prominent U waves:hypokalaemia, hypercalcaemia, hyperthyroidism.
Jwave
•
If present:hypothermia.
Pitfalls
• Acommon error is to interpret an ECG in isolation. To avoid this error,
always consider the clinical context in which it was recorded. Begin your
assessment of the ECG by asking, ‘How is the patient?’ before rushing
to conclusions about the clinical relevance of any abnormalities that may
be present.
•
Anormal ECG does not necessarily exclude a signicant cardiac
problem, particularly when recorded whilst the patient is asymptomatic.
This is particularly the case when investigating palpitations and
chestpain.
•
Technical artefacts are often mistaken for signicant abnormalities.
Ensure adequate patient preparation and correct electrode placement
to minimize the risk of artefact.
Further reading
Houghton AR, Gray D. Making Sense of the ECG, 4th edn. Boca Raton:CRC Press,2014.

ELECTROCARDIOGRAM
477

478
CHAPTER6 Cardiology
478
Electrocardiographic monitoring
Principle
ECG monitoring allows continuous observation of a patient’s ECG in an
ambulatory setting over an extended period of time. This is typically from
24h all the way up to a year or more, depending upon the technique used.
There are three types of device available for ambulatory ECG monitoring:
•
24h ambulatory ECG (Holter) monitor.
• External loop recorder.
• Insertable cardiac monitor (e.g. Medtronic Reveal LINQ
™
).
The choice of device is largely determined by how frequently the patient
experiences symptoms, as the key to successful ECG monitoring is to maxi-
mize the chances of capturing a typical symptomatic event during the moni-
toring period. AHolter monitor is typically worn for 24– 48h but becomes
somewhat impractical over longer periods. It is therefore ideally suited to
patients with frequent (daily) symptoms. An external loop recorder (often
called an event monitor) is carried for around 7days and is therefore used
for patients with less frequent symptoms. Instead of recording a continuous
ECG, they capture brief periods of the ECG, usually when activated by
the patient. Transtelephonic monitors allow captured loops to be relayed
to a cardiac centre by telephone, allowing an immediate analysis of the
recordedECG.
An insertable cardiac monitor (ICM) is used to detect infrequent events.
It is used to monitor a patient’s cardiac rhythm over an extended period (up
to its battery life, usually around 3years). An ICM is implanted SC and con-
tains a battery, a digital memory, and diagnostic software to analyse the ECG
recording. It records the ECG on a digital ‘loop’, continuously overwriting
older ECG data with the most current data. Should a symptomatic event
occur, the patient can ‘freeze’ the loop using an external hand- held device
that is held over the ICM (‘patient- activated’ recordings). Additionally, the
device can be programmed to detect and store abnormal cardiac rhythms
automatically (‘auto- activated’ recordings). The device will usually store the
ECG leading up to an event and also a short segment of ECG following
the event. Stored ECG loops can subsequently be downloaded for further
analysis via a telemetry device at the hospital clinic or via a wireless device
in the patient’s ownhome.
Indications
Where patients have symptoms suggestive of a paroxysmal arrhythmia
(palpitation and/ or dizziness/ syncope), ECG monitoring can provide a
diagnosis by capturing the cardiac rhythm during a typical event. This allows
the underlying arrhythmia to be identied or, where the rhythm proves to
be normal, an arrhythmic aetiology to be ruled out. The choice of method
depends upon the frequency of the symptoms.
Contraindications
None. However, always check if the patient has a known allergy to the self-
adhesive pads used to attach the electrodes to theskin.

ELECTROCARDIOGRAPHIC MONITORING
479
Patient preparation
For external monitoring, no specic preparation is required, apart from
ensuring that the electrodes make good contact with the patient’s skin, so
that an ECG of diagnostic quality can be recorded. For the implantation of
anICM:
•
Explain what the procedure involves.
• Obtain written informed consent.
• Check the FBC (and clotting prole if bleedingrisk).
• LAn.
• Sedation if the patient is anxious.
The best site for the device is established by optimal ECG signal measure-
ment prior to insertion.
Procedure
External monitors are attached to the patient’s skin via electrodes, ensur-
ing good contact is maintained. The recorder itself is carried on a belt or
in a pouch. The patient should be given a diary with clear instructions on
how to operate the monitor and how to note the timing and nature of any
symptoms that occur. Some event monitors are carried by the patient and
only applied to the skin during symptomatic episodes. The patient should
be given clear instructions on how to operate such a monitor and a ‘test
run’ should be conducted.
An ICM is implanted SC under aseptic technique and using LAn. The
implantation takes around 15min and can be done as a day case procedure.
The device is self- contained since, unlike a pacemaker, there are no asso-
ciated leads. The ICM is commonly implanted near the left deltopectoral
groove or below the left breast. Once implanted, the device is interrogated
using an external programmer to ensure that a high- quality ECG is being
recorded. The incision is closed using surgical glue or buttery closures.
Risks
External monitoring carries no signicant risks. Implantation of an ICM car-
ries a riskof:
•
Infection.
• Erosion through the skin (if the patient isthin).
Possible results
Depending upon the underlying cause of the patient’s symptoms, almost
any cardiac rhythm disturbance (or indeed no rhythm disturbance whatso-
ever) may be revealed by ECG monitoring. The most important aspect of
interpreting the results is to correlate recordings with symptoms. Patient-
activated recordings are, as one might expect, usually made in relation to a
symptomatic episode. In this case, it is essential to nd out precisely what
symptoms were experienced at the time (including a witness account where
appropriate). Auto- activated recordings may be asymptomatic and made by
the device without the patient being aware of a problem. The most useful
outcome is to assess the ECG recorded during a typical symptomatic event.
It is then usually straightforward to make a diagnosis and plan further treat-
ment as appropriate.

480
CHAPTER6 Cardiology
480
Advantages overothertests
Ambulatory ECG monitoring allows the chance of capturing paroxysmal
arrhythmias, an opportunity that is unlikely to arise with a 12- lead ECG
recording unless the patient happens to be symptomatic at the time. Each of
the methods of ECG monitoring has advantages over the others. External
monitoring is non- invasive but can only be performed for relatively short
periods. An ICM allows continuous ECG monitoring over a much longer
period, making it extremely useful for investigating patients with infrequent,
but nonetheless troublesome, symptoms. It does, however, involve an inva-
sive procedure (with the attendant risks) and is more expensive than other
forms of ambulatory monitoring. It can, however, prove very cost- eective
if it avoids the need for multiple short- term ambulatory recordings.
Pitfalls
As with any other form of ambulatory monitoring, failure to correlate
symptoms with recorded events can lead to inappropriate diagnoses.
Further reading
Crawford MH, Bernstein SJ, Deedwania PC, etal. ACC/ AHA guidelines for ambulatory electrocardi-
ography:executive summary and recommendations. Circulation 1999; 100
:886– 93.
National Institute for Health and Care Excellence (2010). Transient loss of consciousness (‘blackouts’) in
over 16s. Clinical guideline CG109. M
http:// www.nice.org.uk/ guidance/ cg109.
Task Force for the Diagnosis and Management of Syncope; European Society of Cardiology (ESC);
European Heart Rhythm Association (EHRA); Heart Failure Association (HFA); Heart Rhythm
Society (HRS), Moya A, Sutton R, Ammirati F, etal. Guidelines for the diagnosis and management
of syncope (version 2009). Eur Heart J 2009; 30
:2631– 71.

ELECTROCARDIOGRAPHIC MONITORING
481
482
CHAPTER6 Cardiology
482
Exercise testing
Principle
Exercise testing permits the dynamic assessment of cardiac function. There
are many dierent indications for exercise testing, but the commonest is the
investigation of suspected or knownCHD.
Indications
• Assessment of likelihood of CHD in patients with chestpain.
• Risk stratication of patients with known CHD and hypertrophic
cardiomyopathy.
•
Evaluation of response to treatment or revascularization inCHD.
• Assessment of exercise- induced arrhythmias.
• Assessment of symptoms in valvular heart disease.
• Objective assessment of exercise capacity.
Contraindications
The absolute and relative contraindications to exercise testing are listed in
Box6.1.
Box 6.1 Absolute and relative contraindications
toexercise testing
Absolute contraindications
•
Recent MI (within 2days)
• Unstable angina (rest pain within previous48h)
• Uncontrolled cardiac arrhythmias (causing symptoms or
haemodynamic compromise)
•
Symptomatic severe aortic stenosis
• Uncontrolled symptomatic heart failure
• Acute PE or pulmonary infarction
• Acute myocarditis or pericarditis
• Acute aortic dissection
Relative contraindications
•
Left main stem coronary stenosis
• Moderate valvular stenosis
• Electrolyte abnormalities
• Uncontrolled hypertension (systolic >200mmHg, diastolic
>110mmHg)
• Tachyarrhythmias or bradyarrhythmias
• Outow obstruction, e.g. hypertrophic cardiomyopathy
• Inability to exercise adequately
• High- degree AVblock

EXERCISE TESTING
483
Patient preparation
Unless the exercise test is being performed to assess response to treatment,
patients should be advised to discontinue anti- anginal drugs, e.g. β- blockers,
calcium channel blockers, long- acting nitrates, nicorandil, and also digoxin 48h
prior to the test. Patients should attend for the test wearing suitable clothing
and footwear.
Procedure
The test should be carefully explained to the patient, so that they are famil-
iar with the exercise protocol being used. Aresting ECG is recorded, and
the patient’s BP measured. An appropriately trained team comprising at
least two personnel, trained in advanced life support, should supervise the
test. Full resuscitation and debrillation facilities must be readily available.
Exercise can be performed using either an exercise treadmill or an exercise
bicycle. Avariety of protocols are available, of which the commonest are
the Bruce protocol and the modied Bruce protocol (see Table 6.17). The
workload during exercise normally i at 3min intervals, with the BP and ECG
being recorded at each stage. The patient should be asked to report any
symptoms during thetest.
Exercise should be stopped ifthereis
•
Afall of >10mmHg in systolic BP from baseline associated with
ischaemia.
•
Moderate to severe angina.
• i ataxia, dizziness, or near syncope.
•
Evidence of poor perfusion.
• Diculty in monitoring the ECGorBP.
• Onset of arrhythmias (ventricular tachycardia, supraventricular
tachycardia, AF, worsening ventricular ectopics).
• 1.0mm or more ST elevation (in leads without Q waves, other than V1
oraVR).
•
Arequest from the patient to stop thetest.
One should also consider stopping theexercise ifthereis
•
Afall of >10mmHg in systolic BP from baseline, even in the absence of
ischaemia.
•
>3mm of ST- segment depression or marked axisshift.
• Development of bundle branch block that cannot be distinguished from
ventricular tachycardia.
Table6.17 Modied Bruce and Bruce protocols
Protocol Modied Bruce Standard Bruce
Stage 01 02 03 1 2 3 4 5
Speed (kph) 2.7 2.7 2.7 2.7 4.0 5.5 6.8 8.0
Slope (°) 0 1.3 2.6 4.3 5.4 6.3 7.2 8.1

484
CHAPTER6 Cardiology
484
• i chestpain.
•
Rise in BP above 250/ 115.
• Fatigue, breathlessness or wheezing, leg cramps, claudication.
At the end of the exercise, the patient may be permitted to sit. Monitoring
of the ECG and BP must continue until the heart rate and BP have returned
to baseline and any ECG changes have resolved.
Risks
Exercise testing is generally well tolerated, with a morbidity of 2.4 in 10,000
and a mortality of 1 in 10,000 (within 1 week of testing). Risks include
arrhythmias and cardiac arrest, MI, and cardiac rupture, and are more likely
in those with a recent history of ACS. Facilities for resuscitation and debril-
lation must be immediately available.
Possible results
Myocardial ischaemia is indicated by 1mm horizontal or downsloping ST-
segment depression 80ms after the J point. Some cardiologists use 2mm of
ST- segment depression as the diagnostic criterion— this i the specicity of
the test but reduces the sensitivity. Upsloping ST- segment depression and
T wave changes are not reliable indicators of ischaemia. Afall in BP (or a
failure of BP to rise) during exercise can also indicate ischaemia, particularly
if accompanied by ST- segment depression and chestpain.
Generally speaking, the earlier and the more marked the ST- segment
changes, the more severe the underlying coronary artery disease. The
prognostic value of exercise testing is well established. Patients can be risk-
stratied using the Duke treadmill score, calculated as follows:
Duke treadmill score
= Exercise time (Bruce protocol) – [5 × ST
depression (mm)] – (4 × exercise angina index)
where:0=no exercise angina; 1=exercise angina; 2=exercise angina that
led to termination of thetest.
The Duke treadmill score denes a high- risk group with a score of ≥−11,
with an annual cardiovascular mortality of 5%. Low- risk patients have a
score of ≥+5, with an annual cardiovascular mortality of0.5%.
Almost any arrhythmia or conduction disturbance can occur during exer-
cise testing. If the exercise test is being performed to investigate arrhyth-
mias, this can indicate a diagnostic result.
Advantages overothertests
Exercise testing is a relatively simple and inexpensive investigation, with a
strong evidence base that it is useful in a large number of clinical situations.
Alternative tests for myocardial ischaemia include stress echocardiography,
CMR, and myocardial perfusion imaging.

EXERCISE TESTING
485
Pitfalls
Exercise test results are commonly reported as ‘positive’ or ‘negative’,
giving the erroneous impression that the results are ‘black or white’. The
sensitivity and specicity of exercise testing vary widely between dierent
patient populations, and false −ve and false +ve results are not uncom-
mon. If the pretest probability of CHD is low, e.g. an asymptomatic young
woman, exercise testing is of little value as even a ‘positive’ result is unlikely
to be true. Similarly, if the pretest probability of CHD is high, e.g. a ♂ in his
60s with typical anginal symptoms, a ‘negative’ result is also unlikely to be
true. Guidelines from NICE state that exercise testing should not be used
for the de novo diagnosis of IHD, but only for the assessment of patients
with knownIHD.
Further reading
National Institute for Health and Care Excellence (2016). Chest pain of recent onset:assessment and
diagnosis. Clinical guideline CG95. M
http:// www.nice.org.uk/ guidance/ cg95.

486
CHAPTER6 Cardiology
486
Myocardial perfusion imaging
Principle
Since myocardial perfusion abnormalities occur early following the onset
of ischaemia, evaluation of regional myocardial perfusion heterogeneity is
a sensitive marker for the presence of coronary artery disease. Myocardial
perfusion imaging is most commonly performed with radionuclide imag-
ing. Alternative modalities include contrast echocardiography and contrast
CMR imaging.
The radioisotopes thallium- 201 or technetium- 99m are taken up by the
myocardium in proportion to blood ow. Images are then acquired by a
gamma camera. The images are processed to provide colour mapping of
myocardial perfusion. Information is obtained regarding the presence of
reversible or xed myocardial ischaemia. Late repetition of image acquisi-
tion allows redistribution of the isotope in areas of slow blood ow for
assessment of myocardial viability.
As with all investigational methods for evaluation of ischaemia, perfu-
sion imaging is enhanced by the addition of cardiac stress. This may be
in the form of physical exercise, e.g. treadmill or bicycle, or with use of
pharmacological stressors. The latter are particularly useful if the patient
is physically unable to exercise suciently or has ECG abnormalities that
prohibit accurate interpretation, e.g. left bundle branch block or ventricular
pacing. The most commonly used pharmacological stressor is the vasodila-
tor adenosine, which has a very short half- life. Dobutamine can also be used
in patients with contraindications to adenosine, but it is a less eective vaso-
dilator. Adenosine gives rise to a 4- or 5- fold hyperaemia, whereas dobu-
tamine only has a 2- fold vasodilatory eect. During adenosine stress, there
is a 4- to 5- fold i in blood ow to normal myocardial territories, compared
with the basal state. In the presence of coronary artery stenosis, there is
impaired vasodilatation and a reduction in the stress:rest ratio, precipitating
a myocardial perfusion mismatch.
Indications
• To assess the presence and degree of coronary artery stenoses in
patients with suspected coronary artery disease.
•
To assist in the management of patients with known coronary artery
disease:
•
To determine the likely prognosis and probability of future cardiac
events, e.g. following MI or during proposed non- cardiac surgery.
•
To guide proposed revascularization procedures by determining the
physiological signicance of known coronary artery lesions, including
the eects of anomalous coronary arteries, muscle bridging, and
coronary artery ectasia in Kawasaki’s disease.
•
To assess the success of performed revascularization strategies.
• To dierentiate between areas of myocardial scar tissue and viable
myocardium prior to proposed revascularization.

MYOCARDIAL PERFUSION IMAGING
487
Contraindications andrisk
Pregnancy is a contraindication to nuclear imaging. Contraindications to
physical exercise testing are listed in Box6.1.
Contraindications toadenosineare
•
Known hypersensitivity to adenosine.
• Untreated second- or third- degree heart block, sick sinus syndrome,
long QT syndrome.
•
Asthma, chronic obstructive airways disease with known bronchospasm.
• Hypotension (systolic BP <90mmHg).
• ACS not successfully stabilized with medical therapy.
• Decompensated heart failure.
• Concomitant use of dipyridamole (within last 24h) or xanthines (within
last12h).
Contraindications todobutamine include those forphysical exercise testingand
•
Known hypersensitivity to dobutamine.
• Glaucoma.
• Hypokalaemia.
• Concomitant use of β- blockade.
Patient preparation
β- blockers and rate- limiting calcium antagonists should be withdrawn for
48h prior to the test if physical exercise or dobutamine stress is planned.
Xanthines and dipyridamole should be withdrawn for 24h prior to adeno-
sine stress, and any foods or drugs containing caeine should be avoided.
Peripheral IV access should besited.
Procedure
The stress study is generally performed rst, since if this is normal, there
may be no need to acquire resting images. The radioisotope is injected at
peak stress, so that myocardial uptake of the tracer reects maximal blood
ow and optimizes visualization of any perfusion decit. The protocols for
the varying forms of stress are given in Table6.18.
Table6.18 Radionuclear exercise and imaging protocols
Stress Protocol Injection time of radioisotope
Physical exercise
As directed by physician, e.g.
Bruce, Sheeld, to 85% max
predicted heart rate (MPHR)
1– 2min prior to cessation of
peak exercise
Adenosine 140µg/ kg/ min for 6min 3– 4min after start of infusion
Dipyridamole* 140µg/ kg/ min for 4min 4min after infusion completion
Dobutamine
In 3min stages:5– 10, 20, 30,
40µg/ kg/ min
When 85% MPHR and/ or
maximal dose dobutamine
* See Pellika PA, Nagueh SF, Elhendy AA, etal. American Society of Echocardiography
recommendations for performance, interpretation, and application of stress echocardiography.
JAm Soc Echocardiogr 2007; 20
:1021– 41.

488
CHAPTER6 Cardiology
488
Heart rate and BP should be measured throughout physical or pharmaco-
logical stress. A12- lead ECG should be observed continuously for evidence
of ST- segment or T wave changes suggestive of ischaemia and arrhythmias.
Redistribution imaging for assessment of myocardial viability can be
performed 3– 4h after stress imaging. To enhance redistribution imag-
ing, particularly if any perfusion decits seen with stress are severe, sub-
lingual nitrate can be given, followed by a further resting injection of the
radioisotope and image acquisition an hour later. This is known as a stress–
redistribution– reinjection protocol.
A single- or dual- head gamma camera is used for image acquisition. This
rotates 180° round the patient from 45° in the right anterior oblique posi-
tion to 45° in the left posterior oblique position. The tomographic data are
reconstructed into double oblique imaging planes. Stress and rest images
are aligned carefully with accurate image registration for comparison. Image
quality is assessed, and then the long and short axis images are evaluated for
myocardial perfusion decits.
Risks
It should be remembered that the patient is exposed to ionizing radiation,
especially if sequential studies are planned. Physical or pharmacological
stress may induce severe myocardial ischaemia, infarction, and potentially
life- threatening arrhythmias (0.01– 0.05%).
The test should be stopped ifthe patient is physically unable tocomplete
thetest or ifs/ he develops
• Severe angina.
• ST- segment elevation of >0.1mV in leads without Qwaves.
• Afall in systolic BP >10mmHg below baseline.
• Asevere hypertensive response (BP >250/ 115).
• Clinical loss of peripheral perfusion, i.e. pallor or cyanosis.
• Dizziness or near syncope.
Possible results
Perfusion decits are identied as areas of reduced tracer uptake. These
may be assessed qualitatively or semi- quantitatively. Semi- quantitative clas-
sication expresses regional myocardial uptake as a percentage of the maxi-
mal uptake seen, according to the followingscale:
•
Absent:10– 9%.
• Severely reduced:10– 29%.
• Moderately reduced:30– 49%.
• Mildly reduced:50– 69%.
• Normal:70– 100%.
Perfusion decits may be categorized as either reversible (present on
stress imaging alone) or xed (present on stress and rest imaging). When
the redistribution protocol is followed, areas of reduced perfusion can
be examined for the presence of viability (revascularization will improve
regional function) or scar tissue (revascularization is futile). The size of the
LV and RV can also be determined (see Table6.19).

MYOCARDIAL PERFUSION IMAGING
489
Advantages overothertests
Radionuclide imaging is readily available and non- invasive. It is inexpensive,
compared with coronary angiography. In contrast to MRI, where the num-
ber of imaging planes that can be acquired may be limited, radionuclide
imaging provides full myocardial coverage. There are many studies support-
ing the ability of the technique to give accurate diagnostic information and
prognosticdata.
Pitfalls
Qualitative or semi- quantitative analytical techniques, whereby signal inten-
sity is compared with the area of maximal myocardial uptake, may limit
accuracy in the presence of triple- vessel disease where there is globally
reduced myocardial perfusion. Additionally, the study may be suboptimal
if peak stress is not achieved. Radionuclide imaging has poor spatial resolu-
tion in comparison with other techniques. Perfusion defects limited to the
subendocardium may not be visualized. Image quality can be degraded by
patient movement and artefacts. Such artefacts include attenuation from
breast tissue in the anterior wall and inferior signalloss.
Further reading
Anagnostopoulos C, Harbinson M, Kelion A, etal. (2004) Procedure guidelines for radionuclide
myocardial perfusion imaging. Heart 2004; 90
(Suppl 1):i1– 10.
Bourque JM, Beller GA. Stress myocardial perfusion imaging for assessing prognosis:an update. JACC
Cardiovasc Imaging 2011; 4
:1305– 19.
Hendel RC, Berman DS, Di Carli MF, et al. ACCF/ ASNC/ ACR/ AHA/ ASE/ SCCT/ SCMR/ SNM
2009 appropriate use criteria for cardiac radionuclide imaging. Circulation 2009; 119:e561– 87.
Table6.19 Possible results formyocardial perfusion imaging
Stress Rest Clinical conclusion
Myocardial
perfusion
Normal/ i Normal Normal
Reduced Normal Myocardial ischaemia
(reversible defect)
Reduced/ absent Reduced/ absent MI (xed defect)
Late
redistribution
Reduced i from baseline Viable myocardium
Reduced Reduced MI (scar)

490
CHAPTER6 Cardiology
490
Radionuclide ventriculography
Principle
Radionuclide ventriculography (RNV) is a technique to provide accu-
rate assessment of cardiac chamber size, morphology, and function. The
patient’s RBCs are radiolabelled with
99m
technetium pertechnate in vitro or
in vivo. The labelled blood pool within the cardiac chambers is then imaged
with a gamma camera, gated to the ECG. Multiple image acquisitions are
acquired throughout the cardiac cycle, typically over at least 16 systolic and
32 diastolic frames. These images can be assessed either based on either the
radioactive count or by geometric analysis.
Indications
• Prognostic estimation in patients with heart failure or coronary artery
disease.
•
Estimation of operative coronary risk for non- cardiac surgery.
• Diagnosis of coronary artery disease where conventional exercise
testing is inadequately performed or result equivocal.
•
Evaluation of the ecacy of revascularization or medical management
strategies in patients with coronary artery disease.
•
Monitoring of cardiac function in patients undergoing chemotherapy.
Contraindications
The technique is contraindicated in pregnant or lactatingwomen.
Patient preparation
No special preparation is required for a resting study. If an exercise study is
to be performed, the patient should fast for 3– 4h prior to the procedure. If
pharmacological stress agents are used, the same preparation as for myo-
cardial perfusion imaging should be followed. Aresting ECG is helpful to
exclude arrhythmias.
Procedure
For a resting study, the patient lies supine whilst anterior and left anterior
oblique images are acquired. Stress studies may be performed with either
physical exercise, e.g. bicycle ergometry, or pharmacological stressors, e.g.
dobutamine. Images are acquired at intervals once the heart rate has sta-
bilized at each new level of exercise or stress. The patient should have
haemodynamic and ECG monitoring throughout. Cardiopulmonary resus-
citation facilities should be available. Images are then analysed to obtain
the required morphological and functional parameters. High activity areas,
such as the spleen or aorta, may be ltered out for optimal assessment of
cardiac parameters.
Risks
Technetium has a 6h half- life. Although the heart receives the largest dose,
5% of the total radiation dose is sequestered by the BM, the most radio-
sensitive body tissue. Radiation dose is up to 1100MBq, and so a typical
examination carries a fatal cancer risk of 1 in 3300. Serial studies should be
avoided where alternative forms of imaging suce.

RADIONUCLIDE VENTRICULOGRAPHY
491
Possible results
• Dilatation or hypertrophy of the cardiac chambers and great vessels
may be identied.
•
Left and right ventricular EFs can be measured. Normal left ventricular
EF is 60– 80% at rest and slightly more during exercise. Right ventricular
EF is 46– 70%. Both values decline with age. The extent of any global left
ventricular dysfunction can therefore be identied.
•
Regional wall dysfunction at rest, at low- and peak- dose stress, and
during recovery may be described in a manner analogous to stress
echocardiography, in order to identify areas of reversible myocardial
ischaemia, myocardial hibernation, orscar.
Advantages overothertests
RNV is non- invasive and repeatable and provides serial measurements,
especially in patients who are dicult to scan echocardiographically or who
cannot tolerate MRI. It can be used in critically ill patients soon after an
acuteMI.
Pitfalls
Patients receive a signicant radiation dose. Echocardiography is safer, and
CMR is likely to replace this technique as the gold standard. Red cell labelling
may be inecient in chronic renal failure. Technical factors are important;
in particular, radioactivity in the left atrium must be separated from that in
the LV to obtain an accurate EF. Apoor ECG signal and inappropriate gat-
ing may render data uninterpretable; heart rate variability may compromise
diastolic lling indices, and inadequate frame counts d statistical reliability.
Further reading
Hendel RC, Berman DS, Di Carli MF, et al. ACCF/ ASNC/ ACR/ AHA/ ASE/ SCCT/ SCMR/ SNM
2009 appropriate use criteria for cardiac radionuclide imaging. Circulation 2009; 119:e561– 87.

492
CHAPTER6 Cardiology
492
Pulmonary artery catheterization
Principle
A PmA (Swan– Ganz) catheter is a multi- lumen catheter that is passed
percutaneously from a central vein, e.g. femoral, subclavian, or jugular, to
the right heart structures. It can be used to measure venous, RAt, right
ventricular, PmA, and LAt (indirect) pressures to obtain blood samples for
O
2
saturation estimation, to measure cardiac output and systemic vascular
resistance, and additionally to act as a central venous infusionport.
Indications
• Aid in the diagnosis of cardiovascular shock and pulmonary oedema.
• Management of complicated acute MI, especially right ventricular
infarction, cardiogenicshock.
•
Management of patients with cardiac failure.
• Fluid therapy/ inotropic delivery in severely ill patients, e.g. sepsis, burns,
multi- organ failure, cardiac surgery, trauma.
• Diagnostic right heart catheterization, including congenital heart disease,
pulmonary hypertension, intra- cardiac shunts.
Contraindications
• Right- sided endocarditis.
• Prosthetic tricuspid or pulmonaryvalve.
• Right heart tumour or thrombus.
• Unstable ventricular arrhythmia.
Patient preparation
LAn is injected into the skin at the site of venous access. The patient is
positioned at on a couch, generally with a head- down orientation if ceph-
alad access is to be used. Pressure transducers are made ready and zeroed
for accurate measurements. Fluoroscopic screening should be available, if
required.
Procedure
An access sheath is placed in the vein using a Seldinger technique. The
PmA triple- lumen catheter is ushed with saline, and the integrity of the
otation balloon assessed by ination with air. Under uoroscopic guidance
or by observation of intra- cardiac pressure traces, the catheter is passed
through the venous system towards the right heart and into a branch of the
PmA. At each stage, pressure and O
2
samples can be measured. The bal-
loon can be inated to assist passage through the right heart. The balloon
is wedged briey into a PmA branch to obtain an assessment of indirect
pressure (PmA wedge pressure). Cardiac output can be calculated using
a thermodilution method. Iced saline at a known temperature is injected
through the proximal lumen and a thermistor at the catheter tip measures
the temperature rise in the blood- warmed saline as it passes through the
tricuspid valve, RV, and pulmonary valve. Systemic peripheral resistance can
also be estimated.

PULMONARY ARTERY CATHETERIZATION
493
Risks
• Arterial puncture, pneumothorax, haemothorax.
• Sepsis.
• PE or infarction (if the right heart contains masses or if the balloon
remains inated in wedge pressure position).
•
PmA rupture (balloon over- ination).
• Arrhythmia.
Possible results
PmA catheterization can be used to assess pulmonary and systemic venous
lling pressures and uid status, right and left cardiac function, and also,
where indicated, to provide information on valve dysfunction, intra- cardiac
shunts, tamponade, and pulmonary hypertension.
Advantages overothertests
This technique has traditionally been a useful adjunct to patient monitoring
in the intensive care setting, in particularly for accurate pressure evaluation
of the right heart and left atrium and for continuous cardiac output assess-
ment. However, recently several less invasive devices have been designed
for cardiac output monitoring, e.g. oesophageal Doppler.
Pitfalls
The procedure is generally well tolerated, but it is an invasive procedure not
without risk. It is essential that the PmA catheter is inserted only by suit-
ably trained individuals to assist diagnosis and monitor treatment in carefully
selected patients. If a non- invasive alternative is available, then this should
be preferentially employed. Care must be taken in data interpretation, as
misleading results may be obtained if the system is not systematically and
accurately zeroed for serial measurements. Indirect LAt pressure measure-
ments may be inaccurate in patients with pulmonary disease.
Further reading
Kelly CR, Rabbani LE. Pulmonary- artery catheterization. N Engl J Med 2013; 369:e35.

494
CHAPTER6 Cardiology
494
Tilt table testing
Principle
On standing, gravity redistributes up to 800mL of blood to the legs. The
normal compensatory response is i sympathetic and d parasympathetic
stimulation, which maintains BP with a small i
in heart rate. Head- up tilt
table testing uses gravity- induced venous pooling to assess autonomic con-
trol and to attempt to reproduce symptoms of autonomic dysfunction of
dizziness or collapse, i.e. neuro- cardiogenic (vasovagal) syncope.
Indications
Testing is appropriate in the investigation of sudden, unpredictable loss of
consciousness thought to be neurally mediated (vasovagal syncope, carotid
sinus syncope, or situational syncope) in the absence of structural heart
disease.
Contraindications
• Severe mitral stenosis.
• Severe left ventricular outow tract obstruction.
• Severe proximal cerebral or coronary artery disease.
• Testing is not appropriate for frail patients who cannot weight- bear for
up to anhour.
Patient preparation
Fasting is not required. Patients should continue all suspected culprit
medications.
Procedure
The test should take place in a quiet room at a constant temperature. The
patient is lightly strapped to a table with a weight- bearing footboard. Pulse
and beat- to- beat BP are recorded throughout the test. The patient is laid
supine for 10min (20min, if cannulated), and then the table is mechanically
tilted to 70° for 20min of passive tilt. If positivity or discontinuation criteria
have not been reached by this point, 400μg of sublingual glyceryl trinitrate
(GTN) are administered whilst upright, and the tilt is continued for a further
15min. If the patient has a history of adverse reaction to nitrates, or if a
diagnosis of psychogenic or hyperventilation syncope is suspected, then a
40min tilt protocol should be used instead with no GTN provocation.
Risks
Syncopal symptoms (or, in extreme cases, loss of consciousness), hypoten-
sion, and bradycardia may be induced, albeit transiently, so full cardiopul-
monary resuscitation facilities and an appropriately trained supervising team
should be available.
Possible results
A normal response is a <20% d in BP associated with a modest rise in pulse
rate. The test is −ve in the absence of a fall in BP, fall in heart rate, and lack
of syncopal symptoms, and +ve if syncopal symptoms are induced by hypo-
tension and/ or bradycardia. Acardio- inhibitory response is characterized

TILT TABLE TESTING
495
by a fall in heart rate (asystole in extreme cases), a vasodepressor response
by a fall in BP with no pulse change, and a mixed response by a fall in both
pulse and BP. A diagnosis of postural orthostatic tachycardia syndrome
(POTS) is indicated by a rise in heart rate of ≥30bpm and/ or to ≥120bpm,
within 10min of uprighttilt.
Advantages overothertests
Monitoring of ECG and BP during a 24h ambulatory period or during a
Valsalva manoeuvre— measurements of plasma catecholamines, miner-
alocorticoids, and glucose have a role in the investigation of autonomic
dysfunction and syncope, but only tilt table testing provides a dynamic
objective, witnessed assessment.
Pitfalls
The test is time- consuming and requires technical and medical person-
nel trained in the conduct and interpretation of the procedure and in
resuscitation.
Further reading
National Institute for Health and Care Excellence (2010). Transient loss of consciousness (‘blackouts’)
in over 16s. Clinical guideline CG109. M
www.nice.org.uk/ guidance/ cg109.
Parry SW, Reeve P, Lawson J, etal
. The Newcastle protocols 2008:an update on headup tilt table
testing and the management of vasovagal syncope and related disorders. Heart 2009; 95:416– 20.
Task Force for the Diagnosis and Management of Syncope; European Society of Cardiology (ESC);
European Heart Rhythm Association (EHRA); Heart Failure Association (HFA); Heart Rhythm
Society (HRS), Moya A, Sutton R, Ammirati F, etal. Guidelines for the diagnosis and management
of syncope (version 2009). Eur Heart J 2009; 30
:2631– 71.

496

497
Chapter7
Gastroenterology
Endoscopy 498
Oesophagogastroduodenoscopy 502
Enteroscopy 504
Flexible sigmoidoscopy and colonoscopy 506
Endoscopic retrograde cholangiopancreatography 508
Endoscopic ultrasound 509
Wireless capsule endoscopy 510
Tests for Helicobacter pylori
512
Faecal occult blood testing 514
Faecal and serological testing in inammatory bowel disease 516
Tumour markers 517
Investigations for small bowel pathology 518
Tests of small bowel absorption 522
Tests for bacterial overgrowth 523
Tests of pancreatic exocrine function 524
Testing for neuroendocrine tumours 525
Gastrointestinal physiology 526
Non- invasive liver investigations 528
Liver biopsy 532
Investigation of ascitic uid 534

498
CHAPTER7 Gastroenterology
498
Endoscopy
This allows direct visualization of the GIT mucosa and oers further diag-
nostic investigations to obtain tissue for histology, cytology, or microbiol-
ogy, as well as therapeutic possibilities.
Consent (general)
Consent is a vital component of the endoscopy process. Patients should
receive written information before attending for the procedure. This should
describe pretest preparation, the procedure itself, risks and possible compli-
cations, after- care advice (particularly for those patients requiring sedation),
and contact details in the event of problems and include the consent form
that the patient will be asked tosign. In the UK, the Montgomery v Lanarkshire
case of 2015 has established that the patient should be told whatever they
want to know rather than what the clinician thinks they should be told, so
covering issues of importance to the patient is paramount.
Sedation
Whilst the majority of OGD procedures can be performed using a local
anaesthetic spray to the oropharynx, either sedation or patient- controlled
Entonox
®
(an inhaled mix of medical nitrous oxide and O
2
) will be required
for more complex or prolonged procedures. An IV combination of a seda-
tive with amnesic eects, such as midazolam, is usually combined with an
analgesic such as fentanyl. Addition of hyoscine butylbromide may act
to reduce intestinal motility, which is useful for colonoscopy, ERCP, or
enteroscopy. An alternative to hyoscine is glucagon, which may be used for
patients with glaucoma or IHD where hyoscine is contraindicated. Rarely,
for patients who are relatively intolerant of procedures and require pro-
longed interventions at ERCP or enteroscopy, general anaesthetic (GAn) is
an option, depending on patient tness and the availability of an anaesthetist
and anaesthetic support.
Antibiotic prophylaxis pre- procedure
Pre- procedure prophylaxis is only indicated for patients undergoing:
• Percutaneous endoscopic gastrostomy or jejunostomy placement.
• ERCP where biliary drainage is unlikely to be achieved at the rst
procedure.
•
Severe neutropenia (<0.5 × 10
9
/ L) and/ or severe immunocompromise
undergoing procedures with a high risk of bacteraemia such as
oesophageal dilatation or variceal sclerotherapy.

ENDOSCOPY
499
Anticoagulation guidelines pre- procedure
With the advent of novel oral anticoagulants and greater use of PY212
receptor antagonists (including clopidogrel and ticagrelor). this has become
a more complex area. Please see British Society of Gastroenterology (BSG)
guidelines for further details.
In summary, procedures are dividedinto:
•
Low risk:diagnostic procedures (OGD, colonoscopy, exible
sigmoidoscopy, enteroscopy), with simple mucosal biopsies possible if
needed.
•
High risk:polypectomy, ERCP with sphincterotomy, dilatation of
strictures, variceal therapy, percutaneous endoscopic gastrostomy (PEG)
placement, endoscopic ultrasound (EUS) with FNA, stenting of theGIT.
PY212 receptor antagonists
Can be continued (± aspirin) for low- risk procedures but should be discon-
tinued 5days before high- risk cases. If there is a high thrombotic risk, con-
tinue aspirin and liaise with a cardiologist about risk/ benet of discontinuing.
Warfarin
For low- risk procedures, continue the usual dose of warfarin and go ahead
if the INR is within the normal range. For high- risk procedures, stop warfa-
rin 5days beforehand. If there is a low risk of thrombosis, then check INR
pre- procedure and go ahead if INR <1.5. If there is a high thrombosis risk,
then cover with low- molecular- weight heparin from 2days after stopping
warfarin, with the last dose ≥24h pre- procedure.
Direct- acting oral anticoagulants (DOACs)
For low- risk procedures, omit on the morning of the procedure. For high-
risk procedures, the last dose should be taken ≥48h pre- procedure. For
patients on dabigatran with an estimated glomerular ltration rate (eGFR)
of 30– 50mL/ min, the last dose should be taken 72h pre- procedure.
Specialist haematology advice should be sought if renal function is worse
thanthis.
Risks and points forconsent
Table 7.1 shows the common complications of endoscopic procedures.

500
CHAPTER7 Gastroenterology
500
Table7.1 Risks and complications ofthe most commonly performed
endoscopic procedures
Procedure
Risks
Morbidity
for each
complication
Overall
mortality
Other issues
OGD
Perforation
Haemorrhage
0.03%
0.002%
0.0001% Greatest risk
for therapeutic
procedures
Cardiorespiratory
sedation- related
complications—
0.005%
Flexible
sigmoidoscopy/
colonoscopy
Perforation
Haemorrhage
0.005%
0.001%
0.001% Cardiorespiratory
complications
related to
sedation— 0.01%
Colonic
polypectomy
Perforation
Haemorrhage
0.06%
0.26%
0.007% As above
PEG placement Perforation
Haemorrhage
Infection
Overall risk
5– 10%
1– 2% 30- day mortality
of 710% (often
resulting from
underlying
condition)
ERCP Perforation
Haemorrhage
Cholangitis
Pancreatitis
1.1%,
0.9%
5%
3.8%
Cardio-
respirat ory
complications
related to
sedation— 2.3%
1.0% Greatest risks
overall with
dilated bileduct,
placementofstent,
and high- dose
hyoscine
butylbromide
Risk of pancreatitis
greatest with
age <40years,
placement of stent,
and dilated bile
duct

ENDOSCOPY
501
Safety considerations beforeprescribing oral bowel
cleansing pre- colonoscopy/ CT colonography/ bariumenema
In view of the risk of electrolyte disturbance and renal failure using oral
bowel- cleansing agents, the National Patient Safety Agency (NPSA) advice
from 2009 suggests the following:
•
Check U&E, creatinine, and eGFR before oral bowel cleansing. If
evidence of renal impairment (eGFR <30mL/ min), then assess risks
andbenets of procedure. Polyethylene glycol- based preparations
(Klean-Prep
®
or Moviprep
®
) are safer if eGFR 30– 50mL/ min.
• If safe to do so, omit ACE inhibitors, angiotensin- 2 inhibitors, diuretics,
and/ or NSAIDs on the day of starting oral bowel preparation for
3days. If unable to stop (e.g. heart failure), then consider the risks and
benets of the procedure and seek advice from a cardiologist.
Further reading
Allison M, Sandoe ATM, Tighe R, et al. Antibiotic prophylaxis in gastrointestinal endoscopy. Gut
2009; 58
:869– 80.
Connor A, Tolan D, Hughes S, Carr N, Tomson C (2009). Consensus guidelines for the prescription
and administration of oral bowel cleansing agents. M
http:// www.bsg.org.uk/ attachments/ 960_
obca_ draft_ 10.pdf.
Veitch AM, Vanbiervliet Gj, Gershlick AH, etal. Endoscopy in patients on antiplatelet or anticoagu-
lant therapy, including direct oral anticoagulants:British Society of Gastroenterology (BSG) and
European Society of Gastrointestinal Endoscopy (ESGE) guidelines. Gut 2016; 65
:374– 89.

502
CHAPTER7 Gastroenterology
502
Oesophagogastroduodenoscopy
• Endoluminal visualization:oropharynx to second part of duodenum.
• Allows direct testing for Helicobacter pylori.
•
In preparation for OGD, patients should not eat for 4– 6h beforehand,
with clear uids allowed up to 2h before the procedure.
•
They should remain nil by mouth for at least 30min afterwards to allow
the LAn spray or sedation to wearo.
• Stop proton pump inhibitors 2 weeks before an elective diagnostic
OGD to prevent masking of appearances (risk of partial healing and
thus misdiagnosing malignant oesophageal or gastric ulcers).
Alternative investigations
As an alternative procedure to OGD or enteroscopy, barium swallow,
meal, or follow- through may be performed, depending upon symptoms (for
oesophageal, gastric, or duodenal pathology, respectively). Investigations
are limited by their lower sensitivity for mucosal pathology and inability to
obtain tissue for histology or undertake therapeutic procedures.
Indications
Symptoms/ signs
• Dysphagia.
• Haematemesis and melaena.
• Dyspepsia despite appropriate therapy.
• Iron deciency anaemia (see Fig.7.1a).
• Weightloss.
• Vomiting or nausea.
• Investigation of suspected giardia or bacterial overgrowth to obtain
duodenal aspirates.
•
Obtaining duodenal biopsies in suspected coeliac disease.
• Investigation to obtain histology and cultures of H.pylori.
•
Abnormal barium swallow, meal, or early follow- through.
Surveillance/ screening
• Ensures healing of oesophageal and gastric ulceration.
• Barrett’s oesophagus.
• Establishes response to a gluten- free diet in coeliac disease.
• Diagnosis of polyps in familial polyposis syndromes.
Therapeutic
•
Treatment of bleeding lesions (peptic ulceration, angiodysplasia, varices,
vascular malformations).
•
Palliation of oesophageal cancers using stent placement, argon plasma
coagulation, or Nd:Yag laser therapy.
• Placement of PEG or jejunostomy tubes (see Fig.7.1b).
• Direct placement of nasogastric or nasojejunal feedingtubes.
• Dilatation of strictures of the oesophagus or pylorus.
• Polypectomy.
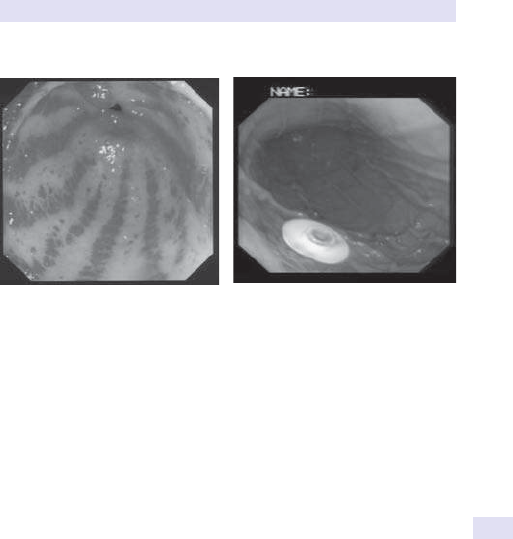
OESOPHAGOGASTRODUODENOSCOPY
503
(a) (b)
Fig.7.1 (a)Endoscopic view of gastric antral vascular ectasia, one cause for blood
transfusion- dependent iron deciency anaemia, which may be treated using argon
plasma coagulation. (E Colour plate4.) (b) Endoscopic view of internal bumper
of percutaneous endoscopic gastrostomy (PEG) holding this in situ in the stomach
to allow feeding. PEG placement is a therapeutic possibility by using OGD to allow
direct visualization.

504
CHAPTER7 Gastroenterology
504
Enteroscopy
Conventional enteroscopy
• Similar to OGD, but allows views of the distal duodenum and jejunum
using a 2.4m instrument.
•
An overtube allows less looping but i complications.
•
Preparation as for OGD; usually requires sedation, analgesia, and
hyoscine butylbromide.
Single- or double- balloon enteroscopy
• Allows views of the entire small bowel from an oral or rectal approach.
• Preparation is as for OGD or colonoscopy and requires deep sedation
or GAn, with the addition of hyoscine butylbromide.
• Consists of one or two balloons, respectively. For single- and double-
balloon procedures, one is attached to a transparent overtube sliding
over the endoscope, allowing movement forward by telescoping the
small bowel by gripping and pleating it over the endoscope. For the
double balloon, the second balloon is attached to the distal endoscope
to act as an anchor.
Alternative investigations toenteroscopy
Barium investigations (follow- through or small bowel enema) are limited by
a lower sensitivity for mucosal pathology.
MRI small bowel studies are good for young patients, as these present no
radiation risk, with contrast allowing clear serosal and mucosal images and
views of the whole length of the smallbowel.
Wireless capsule endoscopy (WCE) is a more eective (but less fre-
quently available) alternative. However, each of these is limited by an inabil-
ity to obtain tissue or provide therapeutic intervention.
Which type ofenteroscopy tochoose?
Single- or double- balloon enteroscopy allows visualization and treatment
of lesions within the full length of the small bowel and may replace con-
ventional enteroscopy, although procedure length and intensity will require
deep sedation or GAn. Conventional enteroscopy will diagnose and treat
lesions within the upper small bowel (to the jejunum) but is limited by
patient tolerance as conscious sedation isused.
Indications forenteroscopy
• Investigation and/ or treatment of obscure GI bleeding or severe
anaemia (following non- diagnostic OGD and colonoscopy).
• Investigation and/ or removal of lesions found on CT scan abdomen/
MRI small bowel/ capsule endoscopy/ barium investigation (e.g.
polypectomy).
•
Treatment of bleeding lesions found at enteroscopy or byWCE.

ENTEROSCOPY
505

506
CHAPTER7 Gastroenterology
506
Flexible sigmoidoscopy and colonoscopy
• Allows examination from anus to splenic exure (exible
sigmoidoscopy) or from anus to caecum/ terminal ileum (colonoscopy).
• Preparation for exible sigmoidoscopy:phosphate enema 30– 60min
prior to the procedure.
•
Preparation for colonoscopy:oral preparation using an oral bowel-
cleansing agent. Need to bear in mind both the patient’s tness and
willingness to undergo the purgative preparation, and also the risk of
renal failure and electrolyte disturbance associated with administration
of these agents (for advice, E
Endoscopy, pp. 498–501). Ideally, a low-
residue diet should be followed for the 3days pre- procedure to obtain
the bestviews.
•
Whilst colonoscopy usually requires sedation/ analgesia, and ideally a
smooth muscle relaxant (hyoscine butylbromide), both colonoscopy
and exible sigmoidoscopy are possible using Entonox
®
with or without
hyoscine butylbromide. For patients who are intolerant of such
procedures, GAn may rarely be available with appropriate anaesthetic
specialist support.
Alternative investigations
Alternative procedures are radiological— mainly CT colonography, which
has largely superseded barium enema. Neither allows tissue or polypec-
tomy to be taken for histology nor other therapeutic procedures to be per-
formed. For these reasons, colonoscopy is considered the ‘gold standard’
for investigating likely colon cancer, diarrhoea, anaemia, and rectal bleed-
ing. Sensitivity is lower for detecting adenomas of ≤10mm using CT colo-
nography (90%) than colonoscopy (99%). These alternative procedures all
require the use of oral bowel- cleansing agentstoo.
For patients not t to undergo oral bowel cleansing, then an unprepared
CT abdomen and pelvis is a possibility. However, it is signicantly less sensi-
tive for lesions of <10mm; it may have a role for elderly or medically unt
patients where prognosis is important.
Indications
Symptoms andsigns
•
Rectal bleeding (bright red=exible sigmoidoscopy, and dark
red=colonoscopy), but beware as some right- sided colonic lesions do
present with bright red bleeding.
•
+ve faecal occult bloods (colonoscopy).
• Abnormal barium enema (depends upon site of pathology found).
• Iron deciency anaemia (colonoscopy).
• Diarrhoea (colonoscopy with biopsies from right and left colon).

FLEXIBLE SIGMOIDOSCOPY AND COLONOSCOPY
507
Surveillance/ screening forspecic diseasestates
• Extensive ulcerative colitis or colonic Crohn’s disease for >10years,
then ongoing follow- up determined by disease extent and severity.
• High risk of adenomatous colonic polyps or carcinoma, or previous
history of adenomatous polyps or carcinoma.
•
Familial polyposis syndrome (FAP), hereditary non- polyposis colorectal
carcinoma (HNPCC), or other family cancer syndromes.
• For further information, please see BSG guidelines.
1
Therapeutic
•
Treatment of bleeding lesions (angiodysplasia, vascular abnormalities,
haemorrhoids).
•
Dilatation of benign strictures.
• Palliation of malignant strictures (placement of stents, argon plasma
coagulation, or Nd:Yag laser therapy).
• Decompression of sigmoid volvulus and non- malignant toxic megacolon.
• Endoscopic mucosal resection of tumours.
National screening programmes
National Bowel Cancer Screening Programme
Oers 2- yearly screening using FOB testing (due to change to faecal immu-
nochemical testing shortly) with kits sent to everyone from 60– 74 years
of age. Face- to- face review for all patients with a +ve result allows either
colonoscopy or CT colonography to be oered, depending on patient t-
ness and wishes. Aim:to allow diagnosis and removal of polyps (adenomas)
before development of carcinoma.
Bowel Scope Screening Programme
Currently in roll- out phase across the UK to oer one- o exible
sigmoidoscopy for all 55- year- olds. Aim: to diagnose and remove polyps
(adenomas) and reduce future bowel cancer risk. Full colonoscopy is
oered to those with >3 adenomas, those with a villous component to one
or more polyps, and anyone with an adenoma of>1cm.
1 Cairns SR, Scholeeld JH, Steele R, etal. Guidelines for colorectal cancer screening and surveillance
in moderate and high risk groups (update from 2002). Gut 2010; 59
:666– 90.

508
CHAPTER7 Gastroenterology
508
Endoscopic retrograde
cholangiopancreatography
• Side- viewing endoscope used to nd the ampulla of Vater and guide
cannulation of the biliary and pancreatic ducts by injecting radio- opaque
contrast medium using uoroscopy.
•
MRCP has replaced ERCP in ° diagnosis. ERCP is used for
interventional procedures and to obtain biopsy and cytology specimens.
•
ERCP requires sedation, analgesia, and hyoscine butylbromide or GAn
(which requires anaesthetic support).
Alternative investigations
MRCP allows imaging of the biliary and pancreatic systems and is the best
(and safest) option for diagnosis, although no therapeutic procedures are
possible. Apercutaneous transhepatic cholangiogram (PTC) allows imaging,
stent placement, and drainage of a dilated biliary tree using a transabdomi-
nal approach. PTC is indicated if therapeutic ERCP fails, albeit with greater
risk of complications.
Indications
Diagnostic
•
Endoscopic diagnosis of periampullary polyps and tumours.
• Obtain bile/ brushings for cytology in suspected cholangiocarcinoma.
• Investigation of dilated biliary ducts found on USS with contraindications
toMRCP.
• Assessment of the sphincter of Oddi pressures in suspected sphincter
of Oddi dysfunction (SOD) syndromes— at specialist centres.
Therapeutic
•
Biliary stenting:
•
Palliation of pancreatic, ampullary, and cholangiocarcinomas.
•
Treatment of a biliary leak following surgery.
•
Benign biliary stricture.
• Biliary sphincterotomy:
•
Gaining access to perform diagnostic or therapeutic procedures.
•
Choledocholithiasis.
•
Ampullary carcinoma.
•
Treatment of a biliary leak following surgery.
•
Treatment of SOD (types Iand possibly IIonly)
•
Treatment of acute severe pancreatitis 2° to gallstones.
• Pancreatic stenting:
•
Drainage of pseudocysts (via the stomach).
•
Following sphincterotomy forSOD.
• Pancreatic sphincterotomy:
•
Pancreatic stone disease.
•
Gaining access prior to stent placement.
•
Minor duct papillotomy in pancreatic divisum.

ENDOSCOPIC ULTRASOUND
509
Endoscopic ultrasound
• Combines endoscopy with US imaging to allow visualization of organs,
such as the pancreas, and accurate assessment of the degree of invasion
of luminal tumours in the oesophagus, stomach, and duodenum.
•
Higher- frequency US probe allowed by proximity of the probe to the
organ results in i spatial resolution, compared with transabdominal US,
CT, or MR scanning.
• Two dierent modes of imaging are possible:radial (which allows a 360°
view around the shaft of the instrument) or linear (which is in line with
the endoscope and allows 90° and up to 270° views).
•
Pre- procedure preparation is as forOGD.
• Consent procedures dier, depending upon indications and potential
pathology.
Alternative investigations
Transabdominal US, CT, and MRI scanning allow views of the pancreas and
liver but do not allow such ne detail for diagnosis or therapy.
Indications
• Staging of oesophageal, gastric, pancreatic, and distal biliary tumours.
• Diagnosis and staging for GI stromal tumours.
• FNA of ‘Trucut’ biopsy of mediastinal or coeliac axis lymph nodes,
pancreatic lesions, or submucosal lesions.
•
Dening mucosal abnormalities such as Barrett’s oesophagus.
• Coeliac axis nerve block to treat pancreatic pain (chronic pancreatitis or
pancreatic carcinoma).
•
Evaluation and treatment of pancreatic pseudocysts.
• Detection of CBD stones.
Further reading
Allum WH, Blazeby JM, Grin SM, etal. Guidelines for the management of oesophageal and gastric
cancer. Gut 2011; 60
:1449– 72.

510
CHAPTER7 Gastroenterology
510
Wireless capsule endoscopy
• Allows imaging of the entire small bowel by using an 11 × 27mm capsule
(e.g. PillCam
®
SB) with up to 7.5h of batterylife.
•
When swallowed, the capsule transmits images to aerials attached by
adhesive pads to the abdominal wall and stored on a recorder attached
round thewaist.
•
Propelled by the patient’s own peristalsis, so symptom- free.
• Specialist procedure, but with a higher diagnostic yield for mucosa
pathology than other small bowel investigations (see Table7.2).
•
If there is suspicion of stricturing where WCE could precipitate
obstruction, then a dissolvable patency pill will verify adequate patency,
allowing subsequent WCE. Prior barium radiology would also rule out
stricturing.
•
New capsules for investigation of the colon and oesophagus (PillCam
®
COLON and PillCam
®
ESO) are not in widespreaduse.
Indications
• WCE has a role in visual diagnosis but is unable to obtain samples for
subsequent analysis or allow therapeutic management.
•
Occult GI bleeding undiagnosed at endoscopy.
• Possible small bowel polyposis (e.g. Peutz– Jegher syndrome).
• Unexplained diarrhoea or malabsorption undiagnosed at endoscopy.
Points forconsent
• A0.5% risk of obstruction reported, especially if stricturing (e.g. small
bowel Crohn’s disease) or previous small bowel surgery. However,
prior use of the patency pill will rule out stricturing signicant enough to
cause capsule retention.
Table7.2 Diagnostic yield ofsmall bowel investigations foroccult
bleeding lesions
Sensitivity (%) Specicity (%) Diagnostic yield (%)
Enteroscopy 37 97
30– 32
WCE 64 92 55– 68
Further reading
Mylonaki M, Fritscher- Raven A, Swain CP. Wireless capsule endoscopy: a comparison with push
enteroscopy in patients with gastroscopy and colonoscopy negative gastrointestinal bleeding. Gut
2003; 52
:1122– 6.
Swain P. Wireless capsule endoscopy. Gut 2003; 52:48– 50.

WIRELESS CAPSULE ENDOSCOPY
511

512
CHAPTER7 Gastroenterology
512
Tests forHelicobacterpylori
Indications fortesting
• ‘Test and treat’:patients <55years of age with a low probability of
signicant pathology and dyspeptic symptoms do not require OGD.
Non- invasive testing for H.pylori allows treatment of those found to
be +ve, with acid suppression using a proton pump inhibitor to assess
response for all patients.
•
Ensure eective eradication in patients with conrmed peptic ulceration
or mucosa- associated lymphoid tumour (MALT) lymphoma.
Non- invasivetests
Faecal antigentest
•
H.pylori antigens in faeces measured using an
immunochromatographictest.
•
Sensitivity exceeds 91%, and specicity exceeds92%.
• Commercially available kits make an initial diagnosis and conrm
eradication.
Urea breathtest
•
Expired air is collected after ingestion of
13
C- labelledurea.
• If H.pylori present, bacterial urease breaks down urea l ammonium and
bicarbonate, then l CO
2
and ammonia.
•
Expired
13
CO
2
is collected into a tube 30min after ingestion, measured
by a mass spectrophotometer, and compared with one collected prior
to ingestion.
•
Test almost 100% sensitive and specic, and remains the gold standard
to conrm eradication.
Serology
•
Serum IgG antibodies to H.pylori may be detected by ELISA with
sensitivity of 90% and specicity of 70– 90%.
• IgG levels persist up to 1year, so fail to conrm eradication.
• Other organisms may cause cross- reactivity.
• False −ves occur in elderly or immunocompromised patients.
Invasive tests (performed atOGD)
• Investigations are based on biopsy samples.
• H.pylori density is usually the greatest in the antrum, but during acid
suppression, the greatest concentration is found in the corpus.
•
Colonization is patchy so may yield sampling errors.
• So for patients taking proton pump inhibitors, one biopsy should be
taken from each area (two in total) for the greatest yield for each of the
testsbelow.
Histology
•
H.pylori can be detected on routine histology using the modied
Giemsastain.
•
Sensitivity is 85%, with a specicity of almost100%.

513
TESTS FORHELICOBACTERPYLORI
Culture
•
This is useful in those few patients who have failed eradication to
establish antibiotic sensitivities.
•
Sensitivity is over 95%, with specicity of almost100%.
Rapid ureasetest
•
Biopsy is placed into a urea solution containing phenolred.
• H.pylori contains urease, releasing ammonia from urea, thus changing
the pH and detected as a colour change with phenol red dye turning
from straw to pink/ purple.
• Commercial kits, such as the Campylobacter- like organism (CLO) test,
are available.
•
Specicity is 97%, and sensitivity between 70 and95%.
Further reading
National Institute of Health and Care Excellence (2014). Gastro- oesophageal reux disease and dys-
pepsia in adults:investigation and management. Clinical guideline CG184. M
https:// www.nice.
org.uk/ guidance/ cg184.

514
CHAPTER7 Gastroenterology
514
Faecal occult blood testing
Faecal occultblood
Indications
•
Used in the current National Health Service (NHS) England National
Bowel Cancer Screening Programme as population screening for 60- to
74- year- olds every 2years.
• Early diagnosis of cancer pathway for primary care (NICE guidelines,
2015). General practitioners advised to oer testing for adults without
rectal bleedingif:
•
50years or over with unexplained abdominal pain or weightloss.
•
Under 60years with a change in bowel habit or iron deciency
anaemia.
•
Aged 60 or over with anaemia in the absence of iron deciency.
Investigation
•
Simple and inexpensive, performed by the patient in their ownhome.
• Samples taken onto a test card from three consecutive bowel motions
and card sent to a screening centre.
•
Test card uses the ‘guaiac’ reaction with pseudoperoxidase in Hb,
causing a colour change in an indicatordye.
•
Dietary changes advised to avoid red meat, horseradish, broccoli, and
turnips, as their high peroxidase activity may cause false +ve results, and
avoid vitamin C tablets (high ascorbic acid activity).
Results
•
Sensitivity of the non- hydrated test is 70%; this i to 90% with
rehydration, but at the loss of specicity. Sensitivity improves with the
number of samplestaken.
•
Individuals who are +ve require full colonoscopy.
• Multicentre UK trial invited 486,355 for screening using FOB (take up
56%), nding 2% FOB +ve, and of these, 10.9% have carcinoma, 35%
adenoma, and 54.1% normal.
Limitations
Polyps and carcinomas may bleed intermittently, but FOB are more likely to
be +ve with early- stage cancers than polyps.
False positives
Non- colorectal blood source such as the upper GIT or nosebleed; diet con-
taining red meat, broccoli, or turnips (peroxidase activity).
False negatives
‘Old’ sample with bacterial degradation of Hb, the presence of ascorbic
acid, and reduced or absent bleeding at time of testing.

FAECAL OCCULT BLOOD TESTING
515
Faecal immunochemical test(FIT)
• This will replace FOB testing used by the NHS England National Bowel
Cancer Screening Programme and has already happened in Scotland.
• Asingle sample is collected via a brush into a pot; this appears to
encourage more people to take part in screening (currently just 55% of
those invited to participate).
•
Tests for human Hb protein from the colon in stool, with less reactivity
for blood from the upper GIT ornose.
•
+ve result means that colonoscopy or CT colonography is required.
• Higher sensitivity to lower concentrations of blood thanFOB.
Further reading
Hardcastle JD, Chamberlain JO, Robinson MHE, etal. Randomised controlled trial of faecal- occult-
blood screening for colorectal cancer. Lancet 1996; 348:1472– 7.
National Institute of Health and Care Excellence (2015). Suspected cancer:recognition and referral.
NICE guideline NG12. M
http:// www.nice.org.uk/ guidance/ ng12.
Tappenden P, Chilcott J, Eggington S, Patnick J, Sakai H, Karnon J. Option appraisal of population-
based colorectal cancer screening programme in England. Gut 2007; 56:677– 84.
Valori R (2007). Bowel cancer screening. M http:// www.18weeks.co.uk..

516
CHAPTER7 Gastroenterology
516
Faecal and serological testing
ininflammatory bowel disease
Faecal calprotectin
• Neutrophil granulocyte cytosol protein, which acts as a marker of
intestinal inammation.
•
+ve correlation with inammation in Crohn’s disease and ulcerative
colitis.
•
Non- specically elevated in neoplasia and radiation proctitistoo.
• Sensitivity for diagnosing pathology is 93%, with specicity of 100%, with
a result of ≤50μg/ g classed as −ve, eectively excludingIBD.
• Anormal result of ≤50μg/ g reduces the need for invasive
investigations, such as colonoscopy, for patients of 16– 40years of age
with no alarm symptoms of pathology (weight loss, bleeding, anaemia,
nocturnal diarrhea, or a signicant family history of bowel cancer).
Faecal lactoferrin
• Neutrophil- derived protein; marker of intestinal inammation.
• May be used for non- invasive testing to dierentiate those who require
colonoscopy from those more likely to have functional disease such
asIBS.
•
Not as eective as calprotectin, as sensitivity for diagnosing pathology is
82% and specicity is84%.
Perinuclear antineutrophil cytoplasmic antibodies
• Occur in the serum of 50– 80% of patients with histologically conrmed
ulcerative colitis, but only 10% of those with Crohn’s disease. This is
likely to be genetically determined.
•
Antibodies are particularly associated with ° sclerosing cholangitis in
association with ulcerative colitis.
•
Overall sensitivity is 55.3%, with a specicity of88.5%.
• In a paediatric cohort with −ve antibodies to Saccharomyces cerevisiae
(ASCA), sensitivity i to 70.3%, with 93.4% sensitivity.
Antibodies toSaccharomyces cerevisiae
• Found in 60% of patients with Crohn’s disease, but only 5% of those
with ulcerative colitis.
•
In Crohn’s disease, the presence of high titres of ASCA is associated
with early age of onset, brostenosing and stulating diseasetypes.
•
Sensitivity is 54.6%, with specicity of 92.8% if pANCA is−ve.
Further reading
National Institute of Health and Care Excellence (2013). Faecal calprotectin diagnostic tests for inam-
matory disease of the bowel. Diagnostics guidance DG11. M
https:// www.nice.org.uk/ guidance/
dg11.
Reese GE, Constantinides VA, Simillis C, etal. Diagnostic precision of anti- Saccharomyces cerevisiae
antibodies and perinuclear antineutrophil cytoplasmic antibodies in inammatory bowel disease.
Am J Gastroenterol 2006; 101
:2410– 22.

TUMOUR MARKERS
517
Tumour markers
• As a result of generally low sensitivities and specicities, these should
not be rst- line investigations.
• They are best used for tracking patients following a diagnosis of
carcinoma through subsequent surgery, and chemo- radiotherapy when
levels should fall to normal unless disease recurs.
Carcinoembryonic antigen
• i in 60% of those with localized colorectal carcinoma (CRC) and 80–
100% of those with metastatic disease.
•
Non- specic and non- diagnostic— also i in bronchial carcinoma, heavy
smokers, andIBD.
•
Levels do not relate to tumour load, but rising levels, which were
previously low/ normal, may imply recurrence ofCRC.
Alpha- fetoprotein
• Normally produced by fetalliver.
• High levels in non- pregnant adults imply hepatocellular carcinoma
(raised in over 90% cases).
•
i serological concentrations in pregnancy suggest neural tube defect.
•
Blood levels may be i in hepatocyte regeneration, acute viral hepatitis,
cirrhosis, choriocarcinoma, and teratoma.
•
May be used 6- monthly (with liver USS) to screen cirrhotic patients for
development of hepatocellular carcinoma.
Carbohydrate antigen 19- 9 (CA19- 9)
• i in pancreaticobiliary obstruction, with the highest levels in pancreatic
carcinoma.
•
Levels >40IU/ L have 75– 90% sensitivity and 80– 95% specicity for
ductal pancreatic carcinoma.
•
Serum levels may be elevated in jaundice, cholangitis,
choledocholithiasis, and chronic pancreatitis.
Cancer antigen 125 (Ca- 125)
• Glycoprotein antigen to an epidermal growth factor receptor
(p110 sEGFR).
• Blood levels i with menstruation, endometriosis, pelvic inammatory
disease, pregnancy, and ascites of alltypes.
•
Highest levels with ovarian malignancy, but also i with ovarian,
pancreas, breast, lung, and colon cancers.
•
20% of ovarian cancers have little/ no expression of Ca- 125.
• Useful in monitoring response of ovarian carcinoma to treatment
(if initially +ve), as rising level of Ca- 125 precedes clinical recurrence by
up to 3months (>90% of cases).
•
As isolated values lack sensitivity and specicity, serial Ca- 125 readings
may be used to achieve specicity of 99.6%, but with a sensitivity of
only80%.

518
CHAPTER7 Gastroenterology
518
Investigations forsmall bowel pathology
General investigations
• Presenting features of small bowel pathology include diarrhoea,
steatorrhoea, abdominal pain, weight loss, and nutritional deciencies.
•
Investigation of diarrhoea is shown in Fig.7.4.
• Occasionally, occult GI bleeding may originate in the small bowel, and an
algorithm for investigation of anaemia is shown in Fig.7.5.
Serology
•
Anti- tTG antibodies have almost 100% sensitivity for coeliac disease but
may be false −ve in presence of lowIgA.
• Diagnosis should be conrmed using duodenal or jejunal biopsies taken
at OGD, which would reveal partial or total villous atrophy.
Endoscopy
•
OGD and enteroscopy allow views of the proximal small intestine to
obtain tissue and allow therapeutic possibilities.
•
WCE permits diagnosis from the whole small intestine but does not
allow therapeutic options.
Radiology
•
MRI of the small bowel has mostly replaced barium radiology,
particularly in younger patients, with a lack of radiation, greater
sensitivity for mucosal detail by using contrast, and abilities to use cine
views to determine motility.
•
Barium follow- through involves ingestion of dilute barium, with images
taken every 10– 30min until barium reaches the caecum.
• Small bowel enema (enteroclysis) uses insertion of a nasoduodenal
tube to infuse barium slowly, creating a column, which may be followed
continuously using uoroscopy. This allows focusing on areas including
the terminal ileum (see Fig.7.2).
Nuclear medicine
Radiolabelled white cell scintigraphy forinammation/ infection
• Uses
99m
technetium– hexamethyl- propylene- amine- oxime (
99m
Tc-
HMPAO) to show intensity and extent of inammation or infection 1
and 3h after injection of autologous radiolabelled leucocytes.
•
Delineates disease extent in Crohn’s disease and ulcerative colitis (see
Fig.7.3).
•
False −ve results may occur in small bowel Crohn’s disease.
• Sensitivity of 96% and specicity of 97% for IBD, and sensitivity of
85– 100% and specicity of 100% for detecting abscesses.
Radionuclide studies todetect Meckel’s diverticulum
•
IV
99m
Tc pertechnate accumulates in gastric mucosa with a time course
of 5– 60min.
• Uptake in ectopic mucosa (e.g. Meckel’s diverticulum) occurs
simultaneously.
•
Imaging at 5– 10min intervals up to 2h after injection allows localization
of the site of ectopic mucosa.
•
False +ves are caused by early gastric emptying.
• Sensitivities vary from 90% in children to 60% in adults.
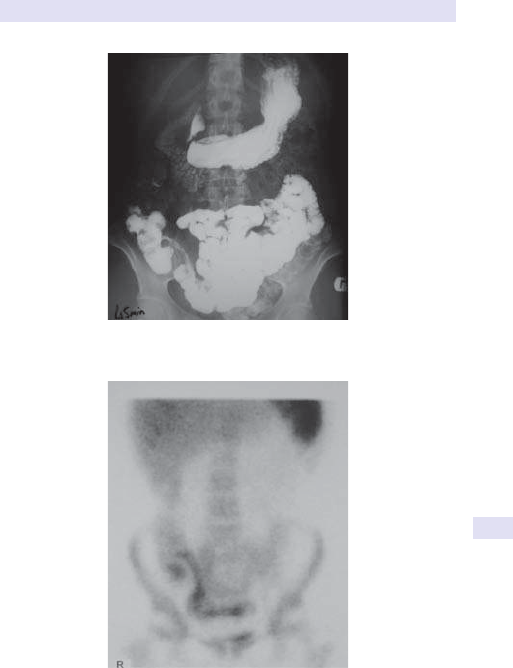
INVESTIGATIONS FORSMALL BOWEL PATHOLOGY
519
Fig.7.2 Barium follow- through image showing a terminal ileal stricture.
Fig.7.3 Radiolabelled white cell scintigraphy using
99m
Tc- HMPAO to show
inammation of the terminal ileum consistent with Crohn’s disease (taken at3h).
SeHCAT scan forbile salt malabsorption
•
The labelled bile acid
75
selenium homotaurocholate (SeHCAT) is
administered orally. Retention measured at 7days by whole body
counting.
•
Retention of >15% SeHCAT is normal (less indicates malabsorption).
• Commonest reason for abnormal result is post- cholecystectomy,
presence of ileal disease, or post- ileal resection.
• Useful second- line investigation in patients with diarrhoea of unknown
aetiology.
520
CHAPTER7 Gastroenterology
520
History and examination
Infection
Treat cause
No infection
apparent
Baseline investigations
Blood: FBC, ESR, albumin, U&E, LFTs,
glucose, TFT, ferritin, B
12
,
red cell folate
Stool: microscopy and culture,
Clostridium dicile toxin, faecal
calprotectin
Sigmoidoscopy: with rectal biopsy
AXR: if patient unwell
Suspected bacterial
overgrowth (blind loop,
dilatation or stasis, jejunal
diverticulosis, diabetes
mellitus, dB
12
with i red cell
folate)
Suspected colonic
disease (blood ± urgency
± anaemia with low ferritin)
Endoscopy: Colonoscopy
with terminal ileoscopy &
multiple biopsies
Suspected pancreatic
disease/neuroendocrine
problem (steatorrhoea, weight
loss, symptoms of vasoactive
hormone secretion)
Radiology: abdominal ultrasound,
CT scan, endoscopic ultrasound,
MRI
Blood: fasting gut hormones
Urine: laxative screen, 24 hours 5HIAA
Faeces: elastase, chymotrypsin, (3-day
faecal fat)
Faeces: amoebic antigen
testing
Blood: anti-tissue transglutaminase
antibodies, HIV, Mg
2+
, Zn
2+
Endoscopy: OGD, duodenal biopsies
and duodenal aspirate (for giardia),
colonoscopy and terminal ileoscopy,
wireless capsule endoscopy, enteroscopy
Radiology: MRI small bowel, CXR,
AXR, barium follow through or small
bowel enema.
Isotope scans: radiolabelled white
cell scan, SeHCAT scan
Suspected small bowel disease:
(dB
12
, red cell folate, dferritin,
dcalcium, dalbumin, iMCV, weight
loss, diarrhoea)
Hydrogen breath tests:
lactose, lactulose, glucose
Direct aspiration of duodenal
/
jejunal uid with culture
Fig.7.4 Investigation of diarrhoea with specic tests for likely sites of pathology.
INVESTIGATIONS FORSMALL BOWEL PATHOLOGY
521
If falling haemoglobin despite oral iron or
blood transfusion dependent or if patient
now symptomatic (e.g. weight loss, rectal
bleeding), consider further investigation if
not already done using wireless capsule,
enteroscopy, small bowel barium studies
or MRI small bowel as dictated by local
availability and consider repeating OGD/
colonoscopy
History and examination – investigate
in order suggested by symptoms, age
and tness of patient
All other patients require all of:
OGD and D2 + D1 biopsies; Coeliac
serology; colonoscopy/CT
colonography depending on tness.
Dipstick urine for haematuria and refer
to Urology if positive.
If no cause found and patient remains
asymptomatic, replace iron using 3 months
of high dose oral treatment then check
haemoglobin and ferrin 3 monthly for
2 years
Premenopausal women only
• Check Coeliac serology
• If serology positive or if upper GI
symptoms, then for OGD and D2 + D1
biopsies
• Only if colonic symptoms or if relevant
family history of colorectal cancer,
then for colonoscopy
• Treat conditions if found
• If Coeliac serology negative and no
symptoms, then may need
gynaecology referral as heavy menses
most likely cause
• Treat cause if found
• If Coeliac disease conrmed
(positive D2 and D1 biopsies
and positive serology) –
make dietetics referral
and ensure colonoscopy/CT
colonography is carried out
too if patient >60 years or
has symptoms of colonic
disease
No action
needed
unstable
stable
transferrin saturation
Iron deciency anaemia
ferritin,MCV,
d
d
d
(
)
Fig.7.5 An algorithm for investigation of iron deciency anaemia.

522
CHAPTER7 Gastroenterology
522
Tests ofsmall bowel absorption
Tests ofcarbohydrate malabsorption
D- xylose tolerancetest
Xylose is absorbed from the proximal small bowel, and urinary excretion
and blood levels reect absorption. It is used mainly in paediatric practice
and has a low sensitivity.
Lactose tolerancetest
Oral administration of 50g of lactose is followed by blood sampling every
30min for 2h. Arise in blood glucose of <1.1mmol/ L suggests deciency
of disaccharidases, especially with colicky abdominal pain and diarrhoea.
Lactose hydrogentest
This is similar to the hydrogen breath test for bacterial overgrowth. Lactase-
decient individuals fail to metabolize lactose, which then undergoes lumi-
nal metabolism by lactase- producing colonic bacteria to yield hydrogen.
Glucose and fructose may be used as alternative short- chain carbohydrates
to determine the presence of malabsorption.
Tests offat malabsorption (may occur inpancreatic
malabsorptiontoo)
Patients with fat malabsorption 2° to small bowel diseases, such as coeliac
disease or tropical sprue, may malabsorb between 10 and 20g/ day of fat,
whilst patients with pancreatic insuciency may malabsorb 30– 50g/ 24h.
Anormal faecal fat excretion is <6g/ day (17mmol/ day).
3- day faecalfat
• Unpleasant test, fallen into disuse, but remains gold standard.
• Fat content measured in a 3- day faecal collection.
• Patient takes a standard diet of 100g of fat perday.
• Does not dierentiate small bowel and pancreatic malabsorption.

TESTS FORBACTERIAL OVERGROWTH
523
Tests forbacterial overgrowth
Deep duodenal/ jejunal aspiration
• Samples may be collected through the biopsy channel atOGD.
• Scanty bacteria are present in normal upper smallbowel.
• Numbers in excess of 10
6
/ mL of aspirated uid are pathological.
• This technique may culture Giardia and Strongyloides species.
Hydrogen breathtests
Rationale
In mammals, the only source of breath hydrogen is bacterial fermentation
of carbohydrates. Hydrogen is absorbed from the intestinal lumen and
expired during breathing. In bacterial overgrowth, hydrogen production can
occur in the small intestine, as well as in thecolon.
Method
•
Amouthwash is given beforehand to reduce contamination by oral
bacteria.
•
Test dose of glucose/ lactose (50g of either) or lactulose (10– 15mL) and
breath hydrogen measured.
•
An early peak (e.g. 40min) suggests bacterial overgrowth.
• This test can be used for other short- chain carbohydrates, such as
fructose or lactose, to determine if a patient is malabsorbing one
ormore.
Limitations
•
Sensitivity 60– 90%, with specicity of80%.
• False −ves result from variations in microora present in the small
intestine or antibiotic administration within 3weeks.
•
False +ves occur in patients with IGT, those with rapid transit to the
colon, and smokers.
•
Patients should avoid eating pulses for 48h prior to the test (normal
digestion of pulses liberates excess hydrogen).

524
CHAPTER7 Gastroenterology
524
Tests ofpancreatic exocrine function
• Symptoms of pancreatic exocrine dysfunction include diarrhoea or
steatorrhoea and weightloss.
•
Investigations aim to determine the degree of pancreatic insuciency,
although biochemical and radiological investigations are more reliable
with greater sensitivity and specicity in advanced disease.
Faecal testing
Elastase
•
Proteinase produced by pancreatic acinar cells and remains un- degraded
during gut transit.
•
Measured using immunoassay of non- liquid faeces sample.
• Levels of >200µg/ g of faeces being normal, 100– 200 representing mild
insuciency, and <100 being diagnostic of severe disease.
•
Specicity is 93%, whilst sensitivity is 63% and 100% for mild and severe
disease, respectively.
•
False +ves may occur with high- volume watery stools.
• The result is not aected by pancreatic enzyme supplements.
Chymotrypsin
•
Less sensitive (64%) and specic (89%) than elastase.
• Used mainly in children to screen for cystic brosis.
• Result is aected by pancreatic enzyme supplements.
Direct investigations
Secretintest
•
Direct intubation of the duodenum allows collection of pancreatic
juice, following IV injection of either secretin (allowing measurement
of volume and bicarbonate content) or cholecystokinin (allowing
measurement of amylase, trypsin, and lipase).
•
Whilst highly sensitive and specic, even with mild pancreatic disease,
this is only available in specialist centres.
Indirect investigations
PABA (N- benzoyl- L- tyrosol p- aminobenzoic acid)test
• Synthetic peptide, which is given orally.
• In normal patients, pancreatic chymotrypsin hydrolyses this peptide,
yielding free PABA, which is absorbed, metabolized, and excreted
inurine.
•
In pancreatic insuciency, free PABA levels are reduced, resulting in
reduced absorption and excretion with lower urinary and serum PABA
concentrations.
Pancreolauryltest
•
Fluorescein dilaurate (an ester) is taken orally with a setdiet.
• In the presence of normal pancreatic function, aryl esterases release
uorescein, which is absorbed, partially conjugated in liver, and excreted
inurine.
•
A24h urine collection for excreted levels shows close correlation with
pancreatic exocrine function.

TESTING FORNEUROENDOCRINE TUMOURS
525
Testing forneuroendocrine tumours
• Specialist area where clinical suspicion results from baseline
investigations, and radiology should allow targeting of more specic
biochemical investigations, depending on which syndrome is suspected.
•
Histology is required to conrm the diagnosis.
Baseline biochemicaltests
Urinary 5- hydroxyindole aceticacid
• 24h collection of urine for 5- hydroxyindole acetic acid (5HIAA).
• High levels imply carcinoid syndrome.
• High specicity, but false +ves result from serotonin- rich bananas,
tomatoes, or drugs such as phenothiazines.
ChromograninA
•
Marker of neuroendocrine tumours (NETs) found in high
concentrations, regardless of presence or absence of hormone- related
clinical features.
•
May be falsely elevated in proton pump inhibitor use and atrophic
gastritis.
Baseline radiological/ endoscopic investigations
• CT or MRI of the chest, abdomen, and pelvis. The ° tumour is
identied in only 50– 70%.
• Radiolabelled somatostatin receptor scintigraphy (OctreoScan).
• Positron emission tomography (PET) using gallium- 68 with concurrent
CT (PET/ CT) if unknown ° or to look for2°.
• Endoscopy or EUS may allow tissue diagnosis.
Vasoactive peptides andamines
• Measured using fasting serum gut hormone assays:insulin (paired with a
serum glucose sample), glucagon, chromogranin Aand B, VIP, pancreatic
polypeptide (PP), gastrin, and somatostatin.
• Single tumours often secrete >1 type of hormone.
• Up to 50% of slow- growing tumours are thought to be non- functional.
• Several types of tumour occur as part ofMEN- 1.
• Concurrent therapy with a proton pump inhibitor will falsely raise serum
gastrin and chromogranin Alevels, so these should be stopped for at
least 1 week prior to measuring serum levels.
Further reading
Ramage JK, Ahmed A, Ardill J, etal. Guidelines for the management of gastroenteropancreatic neu-
roendocrine (including carcinoid) tumours (NETs). Gut 2012; 61
:6– 32.

526
CHAPTER7 Gastroenterology
526
Gastrointestinal physiology
Sphincter ofOddi (SOD) manometry
• Awater- perfused pressure catheter is used during ERCP to measure
sphincter of Oddi pressures to formally diagnoseSOD.
•
SOD is triad of abnormal liver function, biliary- type abdominal pain, and
dilatation of the biliary tree in the absence of gallstone disease.
•
Diagnosis is conrmed by high resting pressure, retrograde peristalsis,
and failure of contrast to drain from the biliary tree within45min.
•
Whilst patients with SOD have an i risk of pancreatitis post- ERCP,
biliary sphincterotomy may relieve symptoms of pain in selectedcases.
pH monitoring
Indications
•
Assessment of gastro- oesophageal reux disease (GORD).
• Atypical symptoms such as asthma or non- cardiac chestpain.
• Before consideration of anti- reux surgery.
• Poorly controlled GORD to conrm diagnosis on or o treatment.
Investigation
•
Ambulatory 24h test that places a pH electrode through the nose to sit
5cm above the lower oesophageal sphincter.
•
Connected to a portable microprocessor that records episodes where
the pH dips below4.
• Patient lls in a simultaneous event diary to correlate symptoms and
episodes of lowpH.
• For most patients, H
2
receptor antagonists and proton pump inhibitors
are stopped 7days beforehand. Afew patients require testing on
medication to establish treatment response.
Results
•
Measure frequency and duration of episodes ofpH<4.
• Duration of longest episode.
• Assess correlation between symptoms andpH.
• Excessive oesophageal acid reux is dened as a total duration of pH <4
of 4– 6% of recordingtime.
Oesophageal manometry
Indications
•
Diagnosis and assessment of motility disorders suggested by symptoms/
OGD/ barium swallow ndings.
• Dening the precise location of the lower oesophageal sphincter prior
to 24h pH monitoring.
• Before consideration of anti- reux surgery.
Investigation
•
Static test using a nasogastrically placed multiple channel, water-
perfused catheter, which is gradually withdrawn during a series of wet
and dry swallows.
•
Pressure is measured through the length of the oesophagus, at the
upper and lower oesophageal sphincter, and the duration and frequency
of contractions are recorded (see Table7.3).

GASTROINTESTINAL PHYSIOLOGY
527
Gastric emptying
• Scintigraphy using the radioactive tracer
99m
technetium displays gastric
movements with a range of test meals (liquid to solid) to quantify gastric
emptying and intestinal lling overtime.
•
In practice, barium and endoscopic studies often provide enough
information when investigating patients with vomiting.
Intestinal transit studies
• Intestinal transit is measured using 50 radio- opaque plastic markers
inside a pH- sensitive gel capsule, swallowed by the patient and designed
to release its contents in the terminal ileum. An abdominal radiograph at
100h should show <20% of markers present in thecolon.
•
Measures colonic transit in suspected slow transit constipation.
Anorectal manometry
• Water- perfused catheter measures anorectal pressures to assess
voluntary and involuntary sphincter squeeze pressures and reex
responses to balloon distension in the rectum.
•
Readings allow assessment of rectal sensation, spinal reexes, and
internal and external sphincter integrity.
•
Assesses symptoms of faecal soiling, incontinence, and chronic
constipation.
Table7.3 Results fromoesophageal manometry studies
Non- specic motility
disorder
Abnormal and incomplete peristalsis with normal body
contractions
Achalasia Incomplete relaxation and high pressure of lower
oesophageal sphincter with aperistalsis of the
oesophageal body
Nutcracker
oesophagus
High amplitude and duration of contractions, normal
peristalsis
Oesophageal spasm Disordered peristalsis with simultaneous prolonged
contractions throughout

528
CHAPTER7 Gastroenterology
528
Non- invasive liver investigations
Basic liver function testing
These are basic serological screening tests that establish whether liver
inammation, infection, or obstruction is present.
Alanine transaminase
Alanine transaminase (ALT:cytosol enzyme specic to the liver) and aspar-
tate transaminase (AST:mitochondrial enzyme also present in the heart,
muscle, kidney, and brain)— both enzymes are present in hepatocytes and
leak into blood with liver cell damage.
Alkaline phosphatase
Alkaline phosphatase (ALP: canalicular and sinusoidal membranes of the
liver, but also bone, intestine, placenta)— specic isoenzymes for ALP are
produced by dierent tissues, but simultaneously raised γ- glutamyl trans-
peptidase (γ
GT) and ALP implies a hepatic origin. Extra- and intra- hepatic
cholestasis may cause raised ALP and results from benign or malignant dis-
ease with or without raised bilirubin. The highest levels result from PBC and
hepatic metastases.
Gamma glutamyl transpeptidase
γ
GT (microsomal enzyme)— activity can be induced by drugs, such as phe-
nytoin and rifampicin, and alcohol. Mild elevation of γGT is common with
even a small alcohol intake, and isolated elevation does not imply liver dis-
ease. It rises in parallel with ALP in cholestasis.
Albumin
A protein that is synthesized in the liver. Plasma concentration partially
results from functional capacity within the liver. However, it has a serum
half- life of 20days and may be normal in early phases of acute liver disease.
Hypoalbuminaemia may also arise from i volume of distribution (sepsis,
overhydration, pregnancy), i excretion or degradation (nephrotic syn-
drome, protein- losing enteropathy), haemorrhage, or catabolic states such
as malignancy orburns.
Prothrombintime
Test of plasma clotting activity and reects the activity of vitamin K-
dependent clotting factors synthesized by the liver. PT may be elevated in
acute or chronic liver disease. In vitamin K deciency with normal liver func-
tion, PT will return to normal within 18h of administration of parenteral
vitaminK.
Bilirubin
In liver disease, a raised bilirubin is usually associated with other liver
function abnormalities. There are many causes of raised serum bilirubin.
Bilirubin may be conjugated or unconjugated, although in practice, this
conjugation state only dierentiates congenital hyperbilirubinaemias. In
Gilbert’s syndrome (the commonest benign cause of an isolated raised
serum bilirubin), an elevated unconjugated bilirubin, which rises during fast-
ing and mild illness, diagnoses the condition. Haemolysis is another cause of
hyperbilirubinaemia, which is covered in E Chapter3.

NON-INVASIVE LIVER INVESTIGATIONS
529
Immunoglobulins
•
IgG i in viral hepatitis, chronic AIH, and cirrhosis.
• IgM i in PBC, non- biliary cirrhosis, and acute viral hepatitis.
• IgA is i in alcoholic liver disease, with β– γ fusion seen on
electrophoresis.
Specic biochemicaltests
These are summarized in Table 7.4, with serological tests, diagnosis, and
denitive investigations required to conrm the diagnosis. The best investi-
gations for an individual patient are established from history, examination,
and basic biochemical parameters.
Table7.4 Specic biochemicaltests
Test Condition Findings More specic tests
Serum ferritin
Serumiron
Transferrin
saturation
Haemo-
chromatosis
Serum iron
>30µmol/ L
Serum ferritin
>500µg/ L
Transferrin saturation
>60%
HFE gene testing
(83– 90% of patients
have Cys 282 Tyr
mutation; 25% have
His 63 Asp; 187G
in complete linkage
disequilibrium with Cys
282 Tyr). Liver biopsy—
with dry weight of iron
24h urinary
copper excretion
Serum copper
Caeruloplasmin
Wilson’s
disease
Serum copper and
caeruloplasmin levels
are usually reduced
but can be normal
Liver biopsy— with dry
weight of copper
α
1
- antitrypsin α
1
- antitrypsin
deciency
Levels of <10%
of normal in
homozygotes and
60% in heterozygotes
Liver biopsy and lung
function testing for
emphysema
AMA PBC AMA present in titres
>1/ 160
Specic M2 antibody
(possible non- specic
i ANA/ SMA)
i serum IgM and ALP
Liver biopsy
ANA/ SMA Type IAIH i ANA ± SMA
i total IgG
Liver biopsy
Anti- LKM1 or
anti- liver cytosol
antibodies
Type II AIH i antibodies titres
i total IgG
Liver biopsy
Fasting
cholesterol
and glucose,
glycosylated Hb
Non- alcoholic
fatty liver
disease/
non- alcoholic
Steatohepatitis
IGT Fibroscan
®
/ brosis
score/ liver biopsy

530
CHAPTER7 Gastroenterology
530
Specic virologicaltests
HepatitisA
•
Acute infection:+ve IgM antibodies toHAV.
• Chronic infection:does notoccur.
•
Markers of clearing virus:+ve IgG antibodies to HAV and anti- HAVIgM.
HepatitisB
Acute infection withsubsequent clearing ofvirus
•
HBsAg appears in blood from 6– 12 weeks after infection, then
disappears.
•
HBeAg appears early, then declines rapidly.
• Anti- HBs appears late and indicates immunity.
• Anti- HBc is the rst antibody to appear, and IgM anti- HBc may persist
for many months as the only marker of ongoing viral replication when
HBsAg has disappeared and anti- HBs is not yet detectable.
• Anti- HBe appears after anti- HBc and indicates d infectivity.
Acute infection leading tochronic hepatitisB
•
HBsAg persists and indicates chronic carrierstate.
• HBeAg persists, correlating with i severity and infectivity.
•
Anti- HBe indicates seroconversion (if this occurs) with disappearance of
HBeAg and a rise inALT.
• HBV DNA suggests continued viral replication.
HepatitisC
•
Acute infection:hepatitis C HCV RNA is +ve 1– 2 weeks after infection,
with HCV antibodies developing after 712weeks.
•
Chronic infection:>50% of patients with persistent HCV RNA, which can
be measured as viralload.
•
Hepatitis C has six genotypes that determine response to treatment.
HepatitisE
•
Similar to hepatitis Awith no chronic or carrierstate.
Investigation ofliver disease
Figure 7.6 shows an algorithm for investigation of liver disease. This dif-
ferentiates obstructive from parenchymal liver disease to suggest further
investigation and treatment.
NON-INVASIVE LIVER INVESTIGATIONS
531
Non- invasive testing ofliver brosis
Transient elastography (Fibroscan
®
)
•
Stages liver disease by assessing stiness using the velocity of a
vibrationwave.
•
Measures liver inammation and brosis, with i stiness correlating with
disease severity.
•
Requires a minimum of ten valid readings; results are expressed inkPa.
•
Normal result:<7.0kPa.
•
Fibrosis:>7.0kPa (with an 85% probability).
•
Cirrhosis:>14.0kPa (with a 90% probability).
• Falsely high results:liver inammation (active hepatitis), cholestasis,
mass lesion or tumour, liver congestion in heart failure.
•
Unreliable results:presence of ascites.
• Not suitable:pregnancy and patients with a pacemaker.
• Benet:avoid risks of liver biopsy.
History and examination
Basic liver function tests
ULTRASOUND
Dilated ducts
Non-dilated ducts
MRCP, ERCP, or
PTC
Therapeutic procedure
to relieve obstruction
Biliary
obstruction
No
obstruction
Virology, autoantibodies,
caeruloplasmin, ferritin,
iron, transferrin, α
1
-anti-
trypsin, glucose,
cholesterol
Liver
biopsy
Fig.7.6 An algorithm for investigation of liver disease.

532
CHAPTER7 Gastroenterology
532
Liverbiopsy
• Obtains tissue for diagnosis of diuse or localized parenchymal disease.
• Severity of histological liver dysfunction cannot be predicted from
basicLFTs.
•
Consent should be obtained based onrisks.
• Transjugular liver biopsy overcomes many contraindications.
Indications
• Unexplained persistently abnormalLFTs.
• Staging of disease in hepatitis B or C infection, and prior to considering
antiviral treatment.
•
Acute hepatitis of unknown aetiology.
• Cirrhosis of unknown aetiology.
• Alcohol- related liver disease.
• PBC/ chronic active hepatitis.
• Targeted liver biopsy of lesions (not if resection/ transplant is a possibility).
• PUO.
• Haemochromatosis/ Wilson’s disease.
• Storage diseases.
• Post- liver transplant to rule out acute or chronic rejection.
Methods forobtaining tissue (risks and benets)
Percutaneous withor withoutultrasound viewing
(See Table7.5.)
•
Standard method for obtaining tissue.
• Ultrasonography of liver and biliary tree pre- procedure to identify
anatomical variations i risk and to rule out obstruction.
•
Most complications <2h but can occur up to24h.
• No evidence that direct US- guided biopsy is safer, but US should
denitely be used if a targeted biopsy is required.
Contraindications
•
An uncooperative or confused patient.
• PT prolonged by >3s, platelets <80 × 10
9
/ L, or bleeding diathesis.
• Ascites.
• Hydatid cysts (risks of anaphylaxis and abdominal seeding).
• Extrahepatic cholestasis.
• Higher risk of bleeding if amyloidosis present.
Minor complications
Shoulder tip pain, minor intra- abdominal bleeding, or mild abdominal pain
(up to 30%)— usually settles with analgesia.
Major complications
•
Perforation (0.01– 0.001%:pneumothorax, gall bladder puncture, kidney,
colon).
•
Intra- abdominal haemorrhage; haemobilia (0.05%:a triad of biliary colic,
jaundice, and melaena within 3days of liver biopsy).
•
Mortality varies between 0.001 and 0.0001% and results from
intraperitoneal haemorrhage or biliary peritonitis.
•
Risk of tumour seeding if a malignant lesion is biopsied; if curative
resection/ transplantation is planned, needle biopsy should be avoided.

LIVERBIOPSY
533
Transjugular
• Specialist technique carried out using uoroscopic guidance.
• Catheter passes from the right internal jugular vein, through the right
atrium and IVC, and into the hepaticveins.
•
Patient holds his/ her breath, whilst the biopsy needle is advanced
through the catheter then rapidly pushed forward by 1– 2cm into the
liver to obtain a small core of liver parenchyma.
•
Safe technique with few complications (1.3– 2% morbidity and 0.5%
mortality), usually performed in higher- risk patients.
• Complications range from neck haematoma, puncture of intrathoracic
arteries, transient Horner’s syndrome, cardiac arrhythmias, infection,
and perforation of the liver capsule.
•
Test of choice in patients excluded from percutaneous biopsy by
coagulopathy, bleeding diathesis, ascites, portal hypertension, and
amyloidosis.
•
Transjugular cannulation allows measurement of portal venous pressures.
Laparoscopic
• Allows sampling of the liver when an operation is planned.
• Allows targeted biopsies, as well as parenchymal biopsies.
• Bleeding is directly controlled and perforation is avoided.
• Risks of surgery and anaesthesia should be considered.
Further reading
Grant A, Neuberger J, Day C, Saxseena S (2004). Guidelines on the use of liver biopsy in clinical practice.
M
http:// www.bsg.org.uk/ images/ stories/ docs/ clinical/ guidelines/ liver/ liver_ biopsy.pdf.
Table7.5 Percutaneous liver biopsy— practical procedure
Procedure Should be carried out by an experienced doctor using aseptic
precautions
Check Clotting, FBC, and take group and save (G&S) within 24h before
procedure (cancel if platelets <80 × 10
9
/ L or PT prolonged >3s)
Patient Lies at on his/ her back
Liver margins Delineated using percussion and/ or US
1% lidocaine 5mL injected at the point of maximal dullness down to the liver
capsule through intercostal space in expiration
Menghini
or Trucut
needle
Used to obtain sample with patient’s breath held in expiration (up
to two passes may be used). Menghini needles use suction, have a
lower rate of complications, and allow more rapid biopsy, but they
have a lower yield for tissue than Trucut (a cutting needle)
Sample
Placed into 10% formalin (or into a dry pot to estimate dry weight
of iron or copper or for culture)
Patient Nurse in supine position or right lateral for >6h, with regular BP and
pulse measurements (every 15min for 2h, every 30min for the next 2h,
then hourly), with urgent medical review if any sign of deterioration
If stable
At 6h, the patient can be discharged home as long as s/ he can return
to hospital within 30min and have a responsible adult with him/ her
overnight

534
CHAPTER7 Gastroenterology
534
Investigation ofasciticfluid
(See Table7.6.)
Investigation requires diagnostic aspiration
• 10mL is sent for cell count, Gram stain, ZN stain culture (add 10mL to a
pair of blood culture bottles to i diagnostic yield).
•
10mL is sent for cytology.
• 10mL for biochemical investigation of protein, glucose, LDH, TGs
(if chylous ascites is suspected), and amylase (if pancreatic ascites is
suspected).
Results
Cellcount
•
Polymorphonuclear leucocytes (neutrophils) of >250 cells/ mm
3
suggest
underlying spontaneous bacterial peritonitis (SBP) (or 2° infection).
• Total leucocytes of >500 cells/ mm
3
imply bacterial peritonitis (SBP) if a
specic neutrophil count is not available.
•
Lymphocyte count of >500 cells/ mm
3
implies tuberculous peritonitis
(with raised protein, positive ZN stain/ TB culture, and low glucose).
Gram stain and culture
•
Gram staining for early identication of bacteria and bacterial culture
allow targeting of antimicrobial therapy.
Protein
•
Using ascitic uid protein levels to aid diagnosis is best achieved using
the serum ascites albumin gradient (SAAG) by subtracting ascites
albumin concentration from serum albumin concentration. Levels of
≥11g/ L suggest cardiac failure, cirrhosis, and nephrotic syndrome, whilst
levels of <11g/ L suggest malignancy, TB, or pancreatitis as causes.
• Risk of SBP is greatest if ascitic protein <10g/ L.
Cytology
May be diagnostic in cases of peritoneal malignancy.
Amylase
i
ascitic amylase (>2000IU/ L) is typical of pancreatic ascites.
Triglycerides
i in chylous ascites.
Table7.6 Ascitic uid aspiration— practical procedure
Patient Lies on his/ her back, tilting towards side planned for aspiration
Percussion Used to nd shifting dullness in left or right lower quadrant
Chlorhexidine Used to clean the site
Lidocaine
2– 3mL of 1% used to inltrate a site in the left or right lower
quadrant where shifting dullness is detected. It should be
possible to aspirate ascitic uid using a standard 19G needle
50mL syringe Sterile 19G needle placed through the same site to obtain the
samples required
Rare
complications
Include bowel perforation, 2° bacterial peritonitis, or
haemorrhage

535
Chapter8
Respiratory medicine
Airway hyperresponsiveness test or histamine/ methacholine
challenge test 536
Arterial blood gas sampling 538
Pleural aspiration (diagnostic) 540
Epworth test/ Epworth sleepiness scale 542
Exercise testing 544
Exhaled nitric oxide fraction 546
Fibreoptic bronchoscopy 548
‘Fitness to y’ assessment 552
Flow– volume loops/ maximum expiratory ow– volume
curve 554
Nijmegen questionnaire 556
Peak ow charts 558
Percutaneous pleural biopsy 562
Polysomnography 564
Pulse oximetry 566
Skin prick tests 567
Spirometry 568
Sputum microscopy and culture/ sputum cytology 570
Static lung volumes/ whole body plethysmography 572
Sweat test 576
Medical thoracoscopy 578
Transfer factor 582

536
CHAPTER8 Respiratory medicine
536
Airway hyperresponsiveness test or
histamine/ methacholine challengetest
Clinical indications
Suspected asthma in patients with normal spirometry.
Patient preparation
1. Explain the procedure to the patient.
2. Obtain informed consent.
3. Warn that wheezing and shortness of breath (SOB) may occur and that
a bronchodilator may begiven.
4. Baseline FEV
1
measured.
5. Patient breathes in a nebulized aerosol of histamine (or methacholine)
of i concentrations. This stimulates bronchoconstriction in a dose-
dependent manner.
6. The FEV
1
is measured after eachdose.
7. Patient must remain in the department for 30min following the
procedure to observe any delayed reactions.
Possible results
The percentage fall in FEV
1
from baseline is plotted against the dose of
inhaled histamine on a logarithmic scale. A dose– response curve is con-
structed, and the provocation concentration (PC) of inhaled histamine
required to reduce the FEV
1
by 20% (PC
20
) can be derived by linear extrapo-
lation. This gure has been arbitrarily chosen to assess degrees of bronchial
reactivity for ease of comparison and safety.
Interpretation
• Asthma suggested by PC
20
<8mg/ mL (see Fig.8.1).
• Adirect relationship exists between the severity of asthma and
requirement for medication, and the PC
20
value as an index of bronchial
hyperresponsiveness.
•
Non- asthmatic subjects almost always have a PC
20
>8mg/ mL.
Advantages overothertests
• Easytodo.
• Cheap.
• Non- invasive.
• Quick.
• Reproducible.
• Safe— bronchoconstriction may be reversed by an inhaled β- adrenergic
agonist. It is important that personnel performing the test are able to
recognize severe bronchospasm and that resuscitation equipment is
available.
Contraindications
• Documented cholinergic hypersensitivity, e.g. cholinergic urticaria or
angio- oedema, orboth.
• Allergy to histamine/ methacholine.
AIRWAY HYPERRESPONSIVENESS TEST
537
• Inability to perform acceptable- quality spirometry.
• Unstable cardiac status, e.g. recent MI, arrhythmia, or heart failure.
• Uncontrolled hypertension (systolic BP >200 or diastolic BP>100).
• Pregnancy.
• Severe baseline obstruction with FEV
1
<80% predicted or<1.5L.
Ancillarytests
• PEFR chart:diurnal variation.
• Sputum cytology:eosinophilia.
• Exhaled nitric oxide (NO):elevated levels.
Pitfalls
• Bronchial hyperresponsiveness in asthma is not a static phenomenon
and may vary widely from day today.
•
May change quite markedly without any change in symptoms
(and vice versa).
•
Represents only one component contributing to the symptomatology of
asthma. Others include airway oedema and mucus hypersecretion.
Further reading
Crapo RO, Casaburi R, Coates AL, etal. Guidelines for methacholine and exercise challenge testing–
1999. Am J Respir Crit Care Med 2000; 161:309– 29.
Joos GF, O’Connor B. Indirect airway challenges. Eur Respir J 2003; 21:1050– 68.
Lotvall J, Inman M, O’Byrne P. Measurement of airway hyperresponsiveness:new considerations.
Thorax 1998; 53
:419– 24.
Pratter MR, Irwin S. The clinical value of pharmacologic bronchoprovocation challenge. Chest 1984;
85
:260– 5.
Smith CM, Anderson SD. Inhalation provocation tests using nonisotonic aerosols. J Allergy Clin
Immunol 1989; 84
:781– 90.
Reduction in FEV
1
from baseline (%)
0
40
30
20
10
0.804.02.01.0.5.25
0.125
.062.031
x
x
x
x
x
x
Control
Histamine concentration (mg/mL)
A
B
C
A : Marked hyperresponsiveness
PC
20
= 0.125mg/mL
B:
Moderate hyperresponsiveness
PC
20
= 8.0mg/mL
C : Not responsive
Fig.8.1 Diering responses to varying concentrations of histamine.

538
CHAPTER8 Respiratory medicine
538
Arterial blood gas sampling
Clinical indications
• Breathlessness (acute or chronic).
• Cardiorespiratory failure.
• Metabolic disturbance.
• Poisoning withdrugs.
• Acute asthma with O
2
saturation <92% (onair).
E OHCM 10e, p.189, p. 665.
Patient preparation
• Informed consent (verbal usually satisfactory).
• Common sites:radial/ brachial/ femoral arteries.
Contraindications toradial
•
Absent ulnar circulation (Allen’stest).
• Arteriovenous (AV) stula for dialysis.
• Fracturedwrist.
• Poor peripheral circulation.
Contraindications tobrachial
•
AV stula.
• Fracturedelbow.
• Poor peripheral circulation.
Contraindications tofemoral
•
Presence of graft/ extensive vascular disease.
Procedure
1. Identify thepulse.
2. Clean the skin with an alcoholswab.
3. Conrm the position of maximum pulsation with the
non- dominanthand.
4. LAn reducespain.
5. Insert a 23G needle attached to a heparinized 2mL syringe.
6. If using low- resistance syringe, this will ll automatically; otherwise
aspirate gently.
7. Remove the needle, and apply rm, gentle pressure with a cotton wool
ball for5min.
8. Expel air bubbles from the sample.
9. Label the specimen.
Possible results
• Hypoxaemia with normalCO
2
.
•
Hypoxaemia withiCO
2
.
•
Normoxaemia withdCO
2
.
•
Metabolic acidosis vs compensation.
E OHCM 10e, p.162, p. 189, p. 771.
Interpretation
Start by looking at the pH. Next check whether CO
2
ts with the pH
change; if so, the ° problem is respiratory. Then check for any metabolic
compensation or for a combined respiratory and metabolic process. If CO
2
is not consistent with the pH change, the ° disturbance is metabolic and
you should check whether there is any respiratory compensation. To assess
ARTERIAL BLOOD GAS SAMPLING
539
oxygenation properly, it is essential to record the patient’s inspired O
2
con-
centration (FiO
2
) at time of sampling.
Advantages overothertests
• Easy, quick,cheap.
• No real alternative for assessing CO
2
or acid– base balance.
• Greater precision in upper ranges of arterial O
2
saturation (SaO
2
) curve
(see Fig.8.2).
Ancillarytests
• Pulse oximetry:gives indication of oxygenation status, but not CO
2
levels.
•
Arterialized blood sampling.
Complications
• Haematoma.
• Nerve damage.
• Inadvertent venous sampling.
Pitfalls
• If the sample is to be analysed in a laboratory with >5min transit time, it
should be kept on melting ice to slow the metabolic activity of thecells.
•
Avoid arterial puncture, if possible, in patients on anticoagulant
therapy, those with bleeding disorders, or those who have received
thrombolytics in previous24h.
•
Failure to note the FiO
2
at time of sampling will lead to diculty in
interpretation and potential therapeutic errors.
12345678910 11 12 13
1002030405060708090100
Tissues Lungs
Tension of O
2
(mmHg)
Tension of O
2
(kPa)
0
100
90
80
70
60
50
40
30
20
10
Saturation (%)
Loading in lungs
Unloading in tissu
e
capillaries
Normal
curve
Curve shifted to right
by i temperature, d pH, i 2,3-DPG
Curve shifted to left by d temperature, i pH, d 2,3-DPG
Fig.8.2 Dissociation curve for oxyhaemoglobin.
Further reading
Carruthers DM, Harrison BD. Arterial blood gas analysis or oxygen saturation in the assessment of
acute asthma? Thorax 1995; 50:186– 8.
Syabbalo N. Measurement and interpretation of arterial blood gases. Br J Clin Pract 1977; 51:173– 6.

540
CHAPTER8 Respiratory medicine
540
Pleural aspiration (diagnostic)
Clinical indications
• Unilateral pleural eusion detected clinically and with imaging, e.g. CXR,
USS, CTchest.
•
Aspiration should not be performed routinely for bilateral pleural
eusions if a transudate is suspected.
Patient preparation
1. Informed written consent.
2. Aseptic technique.
3. Posterior or axillary approach if eusion large (be guided by
bedsideUSS).
4. Clean skin with antiseptic solution.
5. Inltrate with LAn (1% lidocaine).
6. Insert a ne- bore 21G (green) needle attached to a 50mL syringe under
USS guidance. Note:ensure the needle enters immediately above the
rib to avoid the neurovascular bundle.
7. Aspirate uid. If no uid, then try adjusting the angle of the needle with
USS guidance.
8. Remove the needle and apply a plaster.
9. Post- aspirationCXR.
Possible results
• Pleural uid is normally straw- coloured and odourless.
• Pleural uid analysed for protein, glucose, LDH, microbiology, cytology,
andpH.
• If heavily bloodstained, suspect malignancy, pulmonary infarction, or
trauma. Atraumatic tap will become progressively less bloodstained.
•
If pus present:empyema.
• If creamy, opalescent uid:chylothorax (lymphoma, trauma to the
thoracic duct, yellow nail syndrome, lymphangioleiomyomatosis) or
pseudo- chylothorax, e.g. in TB orRhA.
Interpretation
Measure blood LDH and total protein simultaneously for Light’s criteria;
satisfying any ONE criterion means it is exudative:
•
Pleural total protein/ serum total protein>0.5.
• Pleural LDH/ serum LDH>0.6.
• Pleural LDH > two- thirds of upper limit of normal for serumLDH.
(See Table8.1.)
Advantages overothertests
• Quick andeasy.
• Cheap.
• Relatively non- invasive.
• Provides cytological, microbiological, and biochemicaldata.

PLEURAL ASPIRATION (DIAGNOSTIC)
541
Ancillarytests
• Thoracoscopy (medical or surgical).
• Percutaneous pleural biopsy.
Pitfalls
• Traumatictap.
• May be dicult to locate a loculated eusion, even withUSS.
Complications
• Haemorrhage.
• Pneumothorax.
• Visceral injury (e.g. liver).
• Large volumes of pleural uid (>1000mL) should not be aspirated at
one time due to risk of inducing re- expansion pulmonary oedema.
Further reading
Hooper C, Lee G, Maskell N. Investigation of a unilateral pleural eusion in adults:British Thoracic
Society pleural disease guideline 2010. Thorax 2010; 65:ii4– 17.
Light RW. Diagnostic principles in pleural disease. Eur Respir J 1997; 10:476– 81.
Table8.1 Interpretation
Cytology +ve in 760%, especially carcinoma of lung/ breast
Immunohistochemistry should be used to dierentiate
between malignant cell types and may guide oncological
therapy
Protein
Exudate >30g/ L protein
Transudate <30g/ L protein
pH <7.0:empyema, oesophageal rupture
>7.0 and <7.3:collagen disorders, TB, malignancy, empyema
Glucose d
in RhA, TB, malignancy, infection
Microbiology Organisms, ZN stain
Eosinophilia >10% in benign asbestos eusions, parasitic
hydropneumothorax
Neutrophilia >1.0 × 10
9
/ L in acute inammation, e.g. pneumonia, infarction
Lymphocytosis Chronic eusions, e.g. TB, malignancy, lymphoma, or RhA
Amylase iii in pancreatitis
i in oesophageal rupture (salivary amylase)
i in malignancy
ANA >1:160 virtually diagnostic of SLE
LDH i in infection
RF +ve in RhA
Complement d in RhA, SLE, malignancy, infection

542
CHAPTER8 Respiratory medicine
542
Epworth test/ Epworth sleepinessscale
Clinical indications
Screening tool for OSA. Measures general level of daytime sleepiness.
Patient preparation
1. Ask patient to ll in questionnaire.
2. Subject rates on a scale of 0– 3 the chance that, as part of his usual life
in recent times, he would doze in each of eight dierent situations.
Use the following scale to choose the most appropriate number for each
situation:
0=Would NEVERdoze
1=SLIGHT chance ofdozing
2=MODERATE chance ofdozing
3=HIGH chance ofdozing
Situation
•
Sitting and reading.
• WatchingTV.
• Sitting inactive in a public place (e.g. theatre or a meeting).
• As a passenger in a car for an hour without abreak.
• Lying down to rest in the afternoon when circumstances permit.
• Sitting and talking to someone.
• Sitting quietly after lunch without alcohol.
• In a car, whilst stopped for a few minutes in the trac.
Possible results
Epworth sleepiness scale (ESS) score is the sum of eight item scores and
can range from 0to24.
Interpretation
Clinically normal score <10. Each ESS item gives an estimate of sleep pro-
pensity in one of eight specic situations, whereas the total ESS score gives
a measure of more general average sleep propensity. Does not measure
‘subjective’ sleepiness.
Advantages overothertests
• Cheap.
• Easily administered.
Ancillarytests
• Polysomnography/ Visi- Lab studies.
• Stanford sleepinessscale.
• Multiple sleep latencytest.
• Maintenance of wakefulnesstest.
Pitfalls
Limited by patient’s ability to read and comprehend the questionnaire and
answer questions honestly.

EPWORTH TEST/EPWORTH SLEEPINESSSCALE
543

544
CHAPTER8 Respiratory medicine
544
Exercise testing
Clinical indications
• To conrm that reduced exercise tolerance exists.
• To determine the degree of impairment and disability.
• To investigate which system appears responsible for the reduction.
• To evaluate treatment results.
• To plan rehabilitation.
Patient preparation
1. Evaluate the patient’s medical history for contraindications totest.
2. Warn the patient of cardiovascular complications (e.g. mortality
1:10,000 tests).
3. Obtain informed written consent.
4. Patient to wear comfortable clothes andshoes.
5. Monitoring:ECG, O
2
saturation,BP.
6. Exercise:treadmill/ bike/ free run on at surface.
7. Steady state 5– 12min walking test (usually 6min) or stepped stresstest.
8. During a maximal exercise test, the patient should be able to achieve
85– 90% of predicted maximum heartrate.
Contraindications
• Unstable myocardium (recent MI, unstable angina, arrhythmias, severe
valvular heart disease, congestive heart failure).
•
Acute asthma.
• Acute febrile illness.
• Uncontrolled diabetes.
• Systemic hypertension (systolic >200mmHg, diastolic >120mmHg).
Possible results
• Cardiac response:ECG, BP, cardiac output, and stroke volume response.
•
Ventilatory response:ventilatory limitation (reduced breathing reserve),
pattern of response, V
T
, minute volume, respiratoryrate.
•
Gas exchange:ABGs, A– a gradient,P
a
CO
2
.
•
Ventilatory (anaerobic) threshold:normalori.
•
VO
2max
(maximum O
2
uptake):normalori.
Interpretation
Useful in making the distinction between exertional dyspnoea 2° to lung dis-
ease or fatigue 2° to cardiac dysfunction. In patients known to have asthma,
exercise test is +ve in 75% of cases with a single treadmill run and 97% if
the test is repeated in −ve responders. Afall of 10% or more from baseline
in PEFR or FEV
1
suggests exercise- induced asthma.
Advantages overothertests
Best assessment of exercise capacity. Adds to diagnostic accuracy quantita-
tively (measurement of work capacity, VO
2max
, and sustained work capacity)
and qualitatively (identication of the cause of exercise limitation).

EXERCISE TESTING
545
Ancillarytests
• Static lung functiontests.
• For asthma:exhaled NO levels, histamine/ methacholine inhalation
challenges, and PEFRdiary.
Pitfalls
• Dependent on patient eort and compliance.
• Not suitable for patients with severe objective measurement of
respiratory impairment.
Complications
• 2 Bronchospasm:usually easily reversed with an inhaled β - adrenergic
agonist.
•
2 Cardiac arrhythmias/ arrest:appropriate equipment and drugs should
be available in the exercise testing area. Personnel should be trained in
basic and advanced cardiopulmonary resuscitation.
Further reading
Hughes JMB, Pride NB (eds.). Lung Function Tests: Physiological Principles and Clinical Applications.
Philadelphia:WB Saunders,1999.
König P. Exercise challenge:indications and techniques. Allergy Proc 1989; 10
:345– 8.
Sue DY. Exercise testing in the evaluation of impairment and disability. Clin Chest Med 1994;
15
:369– 87.

546
CHAPTER8 Respiratory medicine
546
Exhaled nitric oxide fraction
Clinical indications
Asthma
•
Diagnosis.
• Assessment of severity.
• Assessment of treatment response.
Patient preparation
1. Patient breathes directly into the NO analyser.
2. Perform three tests each time, and record the largestvalue.
Possible results
Exhaled nitric oxide fraction (FeNO) can be detected by chemilumines-
cence in the range of 5– 300ppb.
Interpretation
Elevated FeNO levels are associated with eosinophilic asthma:
•
<25ppb:low probability. Asthma unlikely. Consider other possible
aetiologies for symptoms.
•
25– 50ppb:intermediate probability. Based on clinical judgement, asthma
is a possible diagnosis. Consider initiating inhaled corticosteroids and
monitoring further FeNO levels.
•
>50ppb:high probability. Asthma likely in an appropriate clinical
context. Symptomatic patients are likely to benet from inhaled
corticosteroids.
Advantages overothertests
• Simple andquick.
• Non- invasive.
• Objective measure of response to treatment.
Ancillary tests fordiagnosis ofasthma
• PEFRdiary.
• Histamine/ methacholine inhalation challenges.
• Sputum cytology:eosinophilia.
Pitfalls
• A−ve FeNO does not exclude asthma; asthma may be caused by
neutrophilic airways inammation.
•
FeNO levels are reduced by smoking.

EXHALED NITRIC OXIDE FRACTION
547
Further reading
Kharitonov SA, Barnes PJ. Exhaled markers of pulmonary disease. Am J Respir Crit Care Med 2001;
163
:1693– 722.
Massaro AF, Gaston B, Kita D, Fanta C, Stamler JS, Drazen JM. Expired nitric oxide levels during treat-
ment of acute asthma. Am J Respir Crit Care Med 1995; 152
:800– 3.
National Institute for Health and Care Excellence (2012). Measuring fractional exhaled nitric oxide
concentration in asthma:NIOX MINO, NIOX VERO and NObreath. Diagnostics guidance DG12. M
http:// www.nice.org.uk/ guidance/ dg12.
Saito J1, Gibeon D, Macedo P, Menzies- Gow A, Bhavsar PK, Chung KF. Domiciliary diurnal variation
of exhaled nitric oxide fraction for asthma control. Eur Respir J 2014; 43:474– 84.

548
CHAPTER8 Respiratory medicine
548
Fibreoptic bronchoscopy
Clinical indications
• Any patient with persistent/ substantial haemoptysis.
• Suspected lung neoplasm:
•
For histology.
•
To assist with staging.
• Infection:
•
To identify organism.
•
To determine course of recurrence/ persistence.
• Interstitial lung disease(ILD):
•
To obtain BAL for cytology; useful in diagnosis of sarcoidosis and
hypersensitivity pneumonitis.
•
To obtain endobronchial biopsies (EBBs); useful in diagnosis of
sarcoidosis.
•
To obtain transbronchial lung biopsies (TBLBs); useful in diagno-
sis of sarcoidosis, hypersensitivity pneumonitis, and lymphangitis
carcinomatosa.
Pre- assessment
• CXR.
• FBC.
• Spirometry. Patients with obstruction may require nebulized
bronchodilators pre- procedure.
• Clotting.
• Pulse oximetry.
• ABGs on air if hypoxaemia suggested by O
2
saturation.
Patient preparation
Endoscopysuite
1. Patient informed and consented. Fasted from solids for 6h and liquids
for3h.
2. Frontal approach with patient lying on couch, trunk at45°.
3. IV access obtained.
4. Basic monitoring— pulse oximeter andBP.
5. Supplementary O
2
via single nasal cannula.
6. IV sedation:midazolam/ alfentanil.
7. Topical lidocaine spray to nose and pharynx.
8. Bronchoscope lubricated with 2% lidocaine gel and passed via nostril or
mouthguard.
9. Further boluses of lidocaine (1%) applied to cords and bronchialtree.
Contraindications
• Patients at risk of pulmonary and cardiovascular decompensation, e.g.
recent MI, unstable angina, arrhythmias, severe valvular heart disease,
uncontrolled congestive heart failure, pulmonary hypertension, severe
hypoxaemia.
FIBREOPTIC BRONCHOSCOPY
549
• Patients at high risk of bleeding, e.g. patients on antiplatelet or
anticoagulant therapy or patients with coagulopathies such as
thrombocytopenia.
•
Bronchoscopists should be cautious when sedating patients withCOPD.
Possible results
• Direct inspection of the nares, nasopharynx, and oropharynx.
• Assess movement of vocal cords (ask patient to say ‘eee’).
• Direct inspection of the bronchial tree down to subsegmentallevel.
• Able to take endobronchial/ transbronchial biopsies and brushings.
BAL:wedge the tip of the bronchoscope into a subsegmental bronchus
and instil aliquots of 50mL of sterile saline into the distal airway.
Aspirate immediately, aiming to obtain 750% of instilled volume.
Bronchial washings (BWs) are performed in a similar fashion with
10– 20mL of sterile saline instilled into the bronchus.
Interpretation
(See Table8.2.)
Advantages overothertests
• Well tolerated.
• Quick,cheap.
• Provides histological and immunobiological conrmation (to back up
CT/ CXR diagnosis).
• Therapeutic— removal of retained secretions, mucus plugs, bloodclots.
Table8.2 Interpretation
Histology Tumours
ILD
EBB
TBLB
Cytology Tumours
ILD
Brush/ BAL/ BW
BAL
Microbiology Gram stain
ZNstain
Stain for Pneumocystis jiroveci
Fungi
Virus
PCR for Legionella and atypical
organisms
BAL/ BW
Some appearances diagnostic.
Screening:X- ray- guided biopsy of non- visible lesions.

550
CHAPTER8 Respiratory medicine
550
Ancillarytests
• Endobronchial ultrasound- guided transbronchial needle aspiration
(EBUS- TBNA):allows assessment and biopsy of mediastinal lymph
nodes and tumours.
•
Rigid bronchoscopy:underGAn:
•
Allows therapeutic interventions, e.g. laser therapy, cryotherapy,
stent insertion, debulking of large tumours in the major airways, and
better control of haemorrhage.
•
Preferable for removal of foreignbody.
Side eects and complications
• Pneumothorax with transbronchial biopsies.
• Haemorrhage post- biopsy.
• Hypoxaemia.
• Post- bronchoscopy fever with BAL (usually resolves in a few hours with
paracetamol, but antibiotics may be necessary).
•
If performed in a day case unit with sedation, the patient will not be able
to drive home and will need a responsible adult in attendance overnight.
Pitfalls
• Only visualizes proximal airways.
• Biopsies may be inadequate or from necroticareas.
• Not easy to biopsy submucosal tumour.
• Needs good- quality cytology preparation.
Further reading
Du Rand IA, Blaikley J, Booton R, etal. British Thoracic Society guideline for diagnostic exible bron-
choscopy in adults. Thorax 2013; 68
:i1– 44.
Yasufuku K, Nakajima T, Chiyo M, Sekine Y, Shibuya K, Fujisawa T. Endobronchial ultrasonogra-
phy:current status and future directions. J Thorac Oncol 2007; 2
:970– 9.

FIBREOPTIC BRONCHOSCOPY
551

552
CHAPTER8 Respiratory medicine
552
‘Fitness tofly’ assessment
Clinical indications
The following groups of patients should be referred to a chest physician for
assessment before ying:
•
Severe COPD (FEV
1
<30%) or asthma.
•
Severe (vital capacity (VC) <1L) restrictive disease (including chest wall
and respiratory muscle disease).
•
Cystic brosis.
• Recent pneumothorax.
• Bullous lung disease.
• Pre- existing requirement for ventilator support or O
2
therapy.
•
Co- morbidity with conditions worsened by hypoxaemia (e.g. coronary
artery disease, pulmonary hypertension, cyanotic congenital heart
disease, cerebrovascular disease).
Patient preparation
1. Take a history and examine the patient, with particular reference to
cardiorespiratory disease and previous symptoms during ights.
2. Perform spirometry.
3. Measure SpO
2
by pulse oximeter. ABGs should be performed if
hypercapnia is suspected.
4. Ahypoxic challenge test (breathing 15% FiO
2
for 20min via a face mask,
followed by ABG sampling) may be necessary, depending on the results
of the initial assessment.
Possible results
(See Tables 8.3 and8.4.)
Table8.3 Results ofinitial assessment breathingair
Screening result Recommendation
Sea- level SpO
2
>95% O
2
not required
Sea- level SpO
2
92– 95% and no risk factor* O
2
not required
Sea- level SpO
2
92– 95% and additional risk factor* Perform hypoxic
challenge and ABGs
Sea- level SpO
2
<92% In- ight O
2
* Additional risk factors:hypercapnoea; FEV
1
<50% predicted; lung cancer; lung brosis;
kyphoscoliosis; respiratory muscle weakness; cerebrovascular or cardiac disease; within 6 weeks
of discharge for an exacerbation of chronic lung or cardiac disease; recent pneumothorax;
risk of (or previous) VTE disease; recent pneumothorax; and patients with previous signicant
respiratory symptoms associated with air travel.

‘FITNESS TO FLY’ ASSESSMENT
553
Interpretation
Identies most patients requiring in- ight O
2
therapy.
Ancillarytests
• Exercise testing.
• In complex cases, patients may require testing in a hypobaric chamber.
Pitfalls
• Infectious patients should noty.
• Even with in- ight O
2
therapy, travel cannot be guaranteed to besafe.
Further reading
Ahmedzai S, Balfour- Lynn IM, Bewick T, etal.; British Thoracic Society Standards of Care Committee.
Managing passengers with stable respiratory disease planning air travel:British Thoracic Society
recommendations Thorax 2011; 66
(Suppl 1):i1– 30.
Coker RK, Shiner RJ, Partridge MR. Is air travel safe for those with lung disease? Eur Respir J 2007;
30
:1057– 63.
Table8.4 Results ofhypoxic challengetest
Hypoxic challenge result Recommendation
PaO
2
≥6.6kPa (>50mmHg) or SpO
2
≥85% O
2
not required
P
a
O
2
<6.6kPa (<50mmHg) or SpO
2
<85% In- ight O
2
(ow rate 2L/ min)
PaO
2
, arterial oxygen tension; SpO
2
, oxygen saturation.

554
CHAPTER8 Respiratory medicine
554
Flow– volume loops/ maximum expiratory
flow– volumecurve
Clinical indications
Patient in whom COPD/ small airways disease or upper airway obstruction
is suspected.
Patient preparation
• Advised to wear comfortable, loose clothing.
• Technician explains procedure to patient.
• Mouthpiece in position; patient breathes in maximally and then out as
hard and fast as possible.
•
Three acceptable manoeuvres should be performed. Patients must
perform the test with maximal eort each time, and the results should
be similar for each of the three attempts.
Interpretation
Particularly useful in recognizing patients with narrowing of the central air-
way (larynx and trachea). Narrowing at this site has the greatest eect on
maximum expiratory ow and also on maximum inspiratory ow, giving rise
to a characteristic appearance. Also identies patients with reduced elas-
tic recoil (bullae, emphysema) or reduced airway lumen (asthma, COPD,
bronchiolitis).
Oscillation of ow gives a ‘sawtooth’ pattern. This usually signies insta-
bility of the upper airway and has been observed in OSA, thermal injury to
the airway, bulbar muscle weakness, extrapyramidal neuromuscular disor-
ders, upper airway stenosis/ tracheomalacia, and snoring.
(See Fig.8.3.)
Advantages overothertests
• Allows early detection of small airway disease— more sensitive than
FEV
1
alone.
•
Reproducible.
Ancillarytests
• Spirometry.
• Transfer factor.
Pitfalls
• Dependent on patient understanding and maximal eort.
• Infection control necessary in patients with known or suspected
transmissible disease (e.g. active pulmonaryTB).
FLOW–VOLUME LOOPS
555
75 50 25 %
Volume
Inspiratory ow
Expiratory ow
Normal
Inspiratory ow
Inspiratory ow
In emphysema
75 50 25
PIF
Expiratory ow
%
75 50 25
%
Expiratory o
w
PEF
FEF
50
FEF
25
FIF
50
In extrathoracic upper airway narrowing
Fig.8.3 Flow– volumeloops.

556
CHAPTER8 Respiratory medicine
556
Nijmegen questionnaire
Clinical indications
Hyperventilation syndrome
•
Diagnosis.
• Assessment of severity.
• Assessment of response to therapy.
Patient preparation
1. Ask patient to ll in questionnaire.
2. Subject rates on a scale of 0– 4 the frequency of 16 dierent symptoms.
Use the following scale to choose the most appropriate number for each
symptom:
0=Would NEVER experience that symptom
1=RARELY experience that symptom
2=SOMETIMES experience that symptom
3=OFTEN experience that symptom
4=VERY OFTEN experience that symptom
Symptoms
•
Chestpain.
• Feelingtense.
• Blurred vision.
• Dizzy spells.
• Feeling confused.
• Faster or deeper breathing.
• SOB.
• Tight feeling in thechest.
• Bloated feeling in the stomach.
• Tingling ngers.
• Unable to breathe deeply.
• Sti ngers orarms.
• Tight feelings around themouth.
• Cold hands orfeet.
• Heart racing (palpitations).
• Feelings of anxiety.
Possible results
Nijmegen score is the sum of 16 item scores and can range from 0to64.
Interpretation
Clinically normal score<23.

NIJMEGEN QUESTIONNAIRE
557
Advantages overothertests
• Cheap.
• Easily administered.
Ancillarytests
• ABGs to identify hypocapnia and respiratory alkalosis.
• Hyperventilation provocation test. (Ask the patient to over- breathe for
several minutes to see if it reproduces symptoms.)
Pitfalls
Limited by the patient’s ability to read and comprehend the questionnaire
and answer questions honestly.
Further reading
Van Dixhoorn J, Duivenvoorden HJ. Ecacy of Nijmegen Questionnaire in recognition of the hyper-
ventilation syndrome. J Psychosom Res 1985; 29
:199– 206.

558
CHAPTER8 Respiratory medicine
558
Peak flowcharts
Clinical indications
Asthma
•
Diagnosis.
• Assessment of severity.
• Assessment of treatment response to β
2
- agonists.
Occupationalasthma
•
Diagnosis.
Patient preparation
Patients need to be equipped with a peak ow meter and a peak ow and
symptom diary, and have a thorough understanding of how to usethem.
Guidelines topatients should include
1. Perform the test standing (if possible).
2. Hold the meter lightly and do not interfere with the movement of the
marker.
3. Perform three tests each time and record the largestvalue.
Readings should be taken at various times throughout the day. Limiting
the patient to two readings in each day may aid compliance. In occupa-
tional asthma, 2- hourly peak ow readings are required during the day and
evening.
Possible results
• Diurnal variability:as measured by the lowest PEFR value (usually on
waking) and the highest PEFR value (usually in the afternoon/ evening).
• Patient symptoms and PEFR can be examined together.
Interpretation
Diurnal variation is i in patients with asthma, compared with normals
(amplitude >20%), i.e. peak ow falls signicantly overnight and in the early
morning (see Figs 8.4 and8.5).
Advantages overothertests
• Cheap.
• Saves time of the respiratory physician and technician.
• Reproducible.
• Objective measure of response to treatment.
Ancillary tests fordiagnosis ofasthma
• Airway hyperresponsiveness test or histamine/ methacholine
challengetest.
•
Exercisetest.
• Sputum cytology:eosinophilia.
• FeNO.
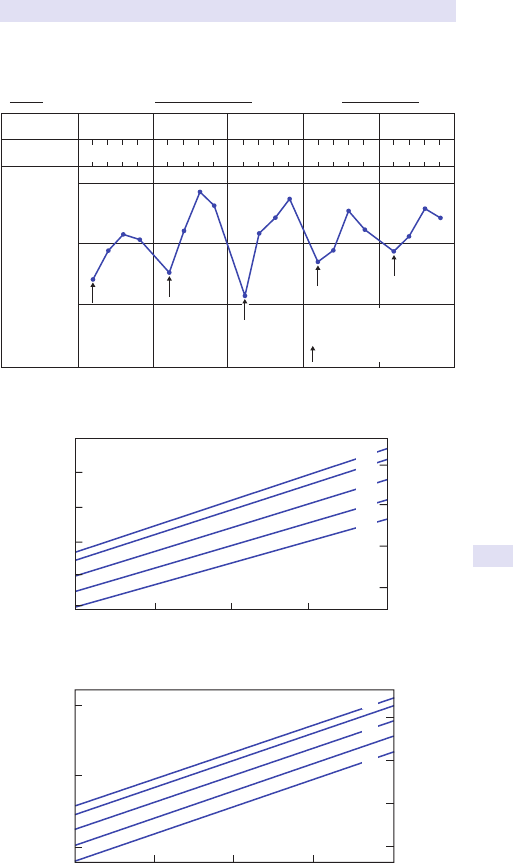
PEAK FLOWCHARTS
559
Peak Flow Meter Record
Name: J. Smith Predicted normal: 240 Personal Best: 280
DateDate
Time
6th June 7th June5th June4th June3rd June
300
100
200
Peak ow readings
l/min
Peak ow chart of an
asthmatic patient showing
diurnal variations.
indicates morning dips
612182
46
12 18 24612182461218246121824
Fig.8.4 An example of a patient’s peak ow record.
700
650
600
550
500
450
500
400
300
1.5 m 1.6 m 1.7 m 1.8
m1
.9 m
SD 60 litres/min
SD 67 litres/min
Men Age (years)
20–25
20–25
40
60
30
40
50
60
SD 1 litre/s
SD 1.1 litre/s
Height (m) = metres, " = inches
Peak expiratory ow (litres/min)
Peak expiratory ow (litres/min)
11
10
9
8
8
7
6
5
Peak expiratory ow (litres/s)
Women
Age (years)
63"
59" 68"
67"
67" 71"
1.4 m 1.5 m 1.6 m 1.7
m1
.8 m
Height (m) = metres, " = inches
Peak expiratory ow (litres/s)
Fig.8.5 Peak ow readings.

560
CHAPTER8 Respiratory medicine
560
Pitfalls
• Not all asthma exacerbations are associated with i diurnal variability.
•
Calculating diurnal variation can be complicated and tedious.
• Time of recording or recent use of β
2
- agonist drugs may result in minor
changes in peak ow but can cause large errors in diurnal variability.
•
Dependent on patient understanding, cooperation, and accuracy.
Further reading
Hetzel MR, Clark TJ. Comparison of normal and asthmatic circadian rhythms in peak expiratory ow
rate. Thorax 1989; 35:732– 8.
Reddel H, Jenkins C, Woolcock A. Diurnal variability— time to change asthma guidelines? BMJ 1999;
319
:45– 7.

PEAK FLOWCHARTS
561

562
CHAPTER8 Respiratory medicine
562
Percutaneous pleuralbiopsy
Clinical indications
• Pleural eusion of unknown aetiology, especially if TB or malignancy
suspected.
Note:if malignancy is suspected and areas of pleural nodularity are shown
on a contrast- enhanced CT, an image- guided cutting needle is the percuta-
neous pleural biopsy method of choice.
Abrams’ needle biopsies are only diagnostically useful in areas with a high
incidenceofTB.
Patient preparation
1. Informed written consent.
2. Aseptic technique.
3. Posterior or mid- axillary approach using USS guidance.
4. Skin cleaned with antiseptic solution.
5. Lidocaine (1%) inltrated in rib interspace. Check pleural uid
aspirated.
6. Stab incision with a narrow scalpel.
7. Insert closed Abrams’ needle (requires rm pressure to be applied until it
penetrates the parietal pleura— take care not to apply too much force).
8. Attach a 50mL syringe.
9. Twist open the Abrams’ needle.
10. Aspirate uid to ensure needle in pleuralspace.
11. Withdraw needle at angle to chest wall until side hole ‘snags’ parietal
pleura.
12. Maintain lateral pressure and rotate to close hole, thereby cutting
biopsy. Remove needle and extract biopsy tissue.
13. Repeat with samples taken from 3, 6, and 9 o’clock position (avoid the
12 o’clock position to avoid the neurovascular bundle).
14. May require suture toclose.
15. Apply dressing.
16. Obtain CXR post- procedure.
17. Place samples in formalin for histological examination, and saline for
microbiological culture.
Possible results
• Slivers of white pleural tissue.
• Examine histology and culture for acid- and alcohol- fast bacilli (AAFB).
Interpretation
• Malignant mesothelioma may be diagnosed on histology, especially
with addition of immunohistochemical methods looking at tumour cell
markers.
•
More sensitive than pleural uid aspiration in diagnosingTB.
• Carcinoma cells may arise from direct spread from lung ° or represent
2° carcinoma. In either case, management is palliative.

PERCUTANEOUS PLEURALBIOPSY
563
Advantages overothertests
• Easy, quick, cheap; more reliable than diagnostic pleural aspiration.
• Less invasive than thoracoscopy for diagnosisofTB.
Ancillarytests
• Diagnostic pleural uid aspiration.
• Thoracoscopy.
• Image- guided cutting needle pleural biopsy is more sensitive than
Abrams’ pleural biopsy at diagnosing malignancy.
Complications
• Pneumothorax.
• Haemothorax.
Pitfalls
• Skeletal muscle biopsy— inadequate specimen.
• Damage to neurovascular bundle.
• Diagnosis of mesothelioma may remain equivocal.
Further reading
Benamore RE, Scott K, Richards CJ, Entwisle JJ. Image- guided pleural biopsy:diagnostic yield and
complications. Clin Radiol 2006; 61:700– 5.
Kirsch CM, Kroe DM, Azzi RL, Jensen WA, Kagawa FT, Wehner JH. The optimal number of pleural
biopsy specimens for a diagnosis of tuberculous pleurisy. Chest 1997; 112:702– 6.
Kirsch CM, Kroe DM, Jensen WA, Kagawa FT, Wehner JH, Campagna AC. A modied Abrams
needle biopsy technique. Chest 1995; 108:982– 6.
Maskell NA, Gleeson FV, Davies RJ. Standard pleural biopsy versus CT- guided cutting- needle biopsy
for diagnosis of malignant disease in pleural eusions:a randomised controlled trial. Lancet 2003;
361
:1326– 30.
Prakash UB, Reiman HM. Comparison of needle biopsy with cytologic analysis for the evaluation of
pleural eusion:analysis of 414 cases. Mayo Clin Proc 1985; 60:158– 64.

564
CHAPTER8 Respiratory medicine
564
Polysomnography
Clinical indications
Note:symptoms alone do not help predict which patient with sleep distur-
bance hasOSA.
•
Patients with low- probability sleep disorder, e.g. snores with no other
features suggestive ofOSA.
•
Patients with high- probability sleep disorder, e.g. typical symptoms and
physiognomy. Need study for diagnosis and assessment of severity.
•
Known OSA— assessing treatment response.
• Assessment of nocturnal hypoventilation syndromes, e.g. scoliosis.
• Patients with unexplained sleep– wake disorders.
Patient preparation
The patient is admitted to the sleep laboratory in the early evening.
Monitoring is explained and attached, using some combination shown in
Table8.5.
Table8.5 Monitoring combinations
Sleep EEG
Electro- oculogram
EMG
Oxygenation O
2
saturation probe
(ear or nger)
Breathing pattern Airow by:
Thoracoabdominal
movement by:
Thermocouples
Thermistor
End- tidal CO
2
pressure
Inductance plethysmography
Impedance
Strain gauge
Miscellaneous Snoring
Leg movement by:
Microphone
EMG
Video
Movement detector

POLYSOMNOGRAPHY
565
Possible results
Original diagnosis of OSA based on polysomnography— overnight record-
ing of sleep, breathing patterns, and oxygenation. It is relatively expensive,
and most centres use a combination of video to assess the quality of sleep
and identify transient arousals and paroxysmal leg movement disorder
(PLMD), and oximetry (to detect desaturation), plus some form of meas-
uring the breathing pattern to detect hypopnoea.
Interpretation
OSA diagnosed in the context of multiple (typically >15/ h) hypopnoeic/
apnoeic events occurring throughout the night and resulting in desaturation.
Advantages overothertests
Demonstrates a number of hypopnoeic (reduction in breathing) or apnoeic
(absence of breathing) events occurring per hour. May be used to monitor
eectiveness of treatment.
Ancillarytests
• ESS.
Pitfalls
• Expensive.
• Time- consuming.
• Most sleep study systems are poorly validated; therefore, need expert
interpretation of results to consider false +ves and−ves.
•
Patients need to sleep for >3h/ night and have rapid eye movement
(REM)sleep.
Further reading
British Thoracic Society (2009). IMPRESS service specication for investigation and treatment of
obstructive sleep apnoea syndrome. M
https:// www.brit- thoracic.org.uk/ document- library/
clinical- information/ sleep- apnoea/ impress- service- specication- for- investigation- and- treatment-
of- osas/ .

566
CHAPTER8 Respiratory medicine
566
Pulse oximetry
Clinical indications
• Any acutely unwell patient:avoids repeated blood gas measurements,
provided that hypercapnia is absent.
•
Monitoring of long- term oxygen therapy (LTOT):not suitable for initial
assessment.
•
Assessment of nocturnal FiO
2
and screening for sleep apnoea
syndrome:identication of nocturnal desaturations.
•
Exercise walktest.
Patient preparation
1. Clean probe site (ear or nger).
2. Ensure good contact of probe with warm, well- perfusedskin.
3. Avoid nail- varnished ngers.
Possible results
• SpO
2
expressedas%.
Interpretation
• Respiratory failure unlikely if SpO
2
>92% onair.
•
Provides almost immediate arterial O
2
saturationdata.
•
Must know the FiO
2
the patient is breathing.
Advantages overothertests
• Non- invasive.
• Easy,cheap.
• Instantaneous.
• Sensitive.
• Portable.
Ancillarytests
• ABG sampling.
Pitfalls
• Does not detect CO
2
levels.
•
If COHb or methaemoglobin (MetHb) is present in the blood in
elevated levels, the pulse oximeter will give a falsely elevated reading for
the arterial O
2
saturation.
•
i in jaundice.
•
Erroneous information if patient poorly perfused.
• Excessive patient movement can give false readings.

SKIN PRICKTESTS
567
Skin pricktests
Clinical indications
• To evaluate atopy in asthmatic individuals.
• To assess the possible development of ABPA in patients with asthma/
other long- standing lung disease.
Patient preparation
1. Explain what the test involves.
2. Use a pen to label the patient’s forearm with the antigens to be tested,
including +ve and −ve controls (alternatively, numbered adhesive tape
may beused).
3. Clean the test area with an alcoholwipe.
4. Place a drop of antigen next to each correspondinglabel.
5. Use a lancet to puncture the skin. Repeat with other antigens using a
new lancet eachtime.
6. Blot o excess antigen, taking care not to contaminate other testsites.
7. After 10min, measure any resulting wheal reactions using a ruler.
Record the mean diameterinmm.
Possible results
A +ve result is indicated by a wheal and are reaction of 3mm, providing
there is no reaction at the −ve control site. −ve results are validated by a
wheal reaction at the +ve controlsite.
Interpretation
A +ve result indicates sensitization to the allergen but does not necessarily
mean that this allergen is responsible for the patient’s symptoms. All tests
should be carefully interpreted in light of the clinical history.
Advantages overothertests
• Cheap,quick.
Ancillarytests
• Specic IgE to allergens (especially where the history is not supported
by skin test results).
•
Total IgE (aects interpretation of weak +ve specic IgE results, which
are less relevant if the total IgE is veryhigh).
•
Aspergillus precipitins(IgG).
Pitfalls
• False −ve results if the patient has taken an antihistamine within 5days
of thetest.
•
False +ve results with dermatographism or inamedskin.

568
CHAPTER8 Respiratory medicine
568
Spirometry
Clinical indications
• To evaluate symptoms, signs, or abnormal test results.
• Provide objective, quantiable measures of lung function.
• Evaluate and monitor disease.
• Assess eects of environmental/ occupational/ drug exposures, both
adverse (e.g. amiodarone) and benecial (e.g. bronchodilators).
•
Preoperative assessment.
• Employment/ insurance assessment.
• Early detection of bronchiolitis obliterans in lung transplant patients.
Patient preparation
1. Explain what the test involves. Most respiratory technicians
demonstrate the technique to ensure maximal eort and co- operation
of the patient.
2. Patient must inhale fully before thetest.
3. Exhale into the breathing tube. Encourage maximal eort with no
breath- holding before the manoeuvre.
4. No cough/ glottal closure in the rst second.
5. Test should last at least 6s (may need up to 15s with obstruction).
6. No evidence of airow leak/ obstruction of mouthpiece.
Possible results
(See Table8.6.)
Interpretation
At least three acceptable tracings should be obtained. Examine each tracing
to ensure adequate eort is made by the patient, that it is reproducible, and
that there are no artefacts (see Table8.7).
Advantages overothertests
• Cheap,quick.
• Bedside/ outpatienttest.
• Reproducible.
Ancillarytests
• Total lung capacity (TLC):to conrm interstitial disease with restrictive
spirometry.
•
Pre- and post- bronchodilator studies:an i of 15% in FEV
1
and 20% in FVC
suggest reversibility.
Pitfalls
• Need standardization of normal data for height, weight, age, sex,
andrace.
•
Level at which a result may be considered abnormal is contentious,
usually accepted to be outside the range of 80– 120% of mean
predicted.
•
FEV
1
may remain relatively normal in early stages of generalized lung
disease.
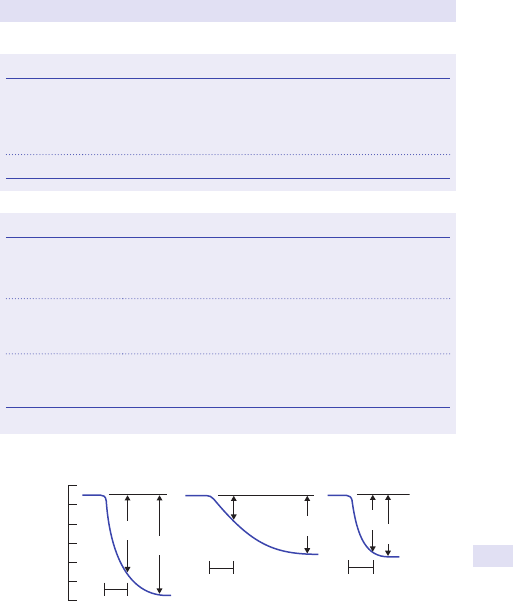
SPIROMETRY
569
Normal
Obstructive
Restrictive
FEV
1
sec
sec
sec
1
1
1
Litres
FEV = 4.0
FEV
1
= 1.3
FEV
1
= 2.8
FVC = 5.0
FVC = 3.1
FVC = 3.1
% = 80
% = 42
% = 90
FEV
1
1
FVC
FVC
FEV
1
FVC
Fig.8.6 Examples of spirograms.
Table8.6 Possible results
FEV
1
Forced expiratory volume in 1s
Test of mechanical function of thelungs
Depends on size and elastic properties of the lungs, calibre of
the bronchial tree, and collapsibility of airway walls
FVC Forced vital capacity
Table8.7 Interpretation
FEV
1
/ FVC ratio Index of the presence/ absence of airow limitation
Young and middle- aged healthy non- smokers rate75%
Older normal patients ratio 70– 75%
FEV/ FVC d Obstructive
Classify severity using FEV, expressed as % of predicted
value, e.g. COPD, asthma
FEV/ FVC
n or i
Restrictive, but need reduced thin- layer chromatography
(TLC) to conrm, e.g. lung brosis, chest wall problems,
pulmonary eusion and oedema
If used for monitoring purposes, need an adequate baselinestudy.
• FEV
1
/ FVC ratio is a good guide to the presence of signicant airway
narrowing, but as disease progresses, both will fall and correlation with
severity of disease ispoor.
•
Variability (noise) is greater in pulmonary function tests than in most
other clinical laboratory tests because of the inconsistency of eort by
patients.
For examples of spirograms, see Fig. 8.6 and E OHCM 10e, p.163.
Further reading
Crapo RO. Pulmonary- function testing. N Engl J Med 1994; 331:25– 30.

570
CHAPTER8 Respiratory medicine
570
Sputum microscopy and
culture/ sputum cytology
Clinical indications
Microbiology
•
Productive cough with sputum.
• Infective exacerbations of any chronic lung disease.
• Pneumonia.
Cytology
•
Suspected lung cancer:only in elderly/ frail patients who are not t for
invasive investigation.
•
Sputum eosinophilia in asthma.
Patient preparation
• Explain the need for a sputum sample.
• Provide suitable sputumpots.
• Early morning samples arebest.
• Consider induced sputum— use ultrasonically nebulized hypertonic
saline to facilitate sputum production, in association with chest
physiotherapy.
Possible results
Induced sputum results in successful sputum production in >70% of normal
and asthmatic subjects who cannot produce sputum spontaneously (see
Table8.8).
Interpretation
• Commensal organisms common.
• Streptococcus pneumoniae and Haemophilus inuenzae likely pathogens
inCOPD.
•
S.pneumoniae commonest organism in community- acquired °
pneumonia.
•
Staphylococcus aureus and Pseudomonas likely in bronchiectasis.
•
Nosocomial infections:
•
S.aureus.
•
Pseudomonas.
•
Klebsiella.
•
The sensitivity of sputum cytology varies by location of the lung cancer
and is greatest in central endobronchial lesions.

SPUTUM MICROSCOPY AND CULTURE/SPUTUM CYTOLOGY
571
Advantages overothertests
• Cheap,easy.
• Non- invasive.
Ancillarytests
• Bronchoscopy andBAL.
• Serum serology if atypical pneumonia suspected.
• If bronchiectasis suspected, consider high- resolution CT (HRCT) chest
± CT sinuses, and check Igs (IgG, IgA, and IgM). Other investigations
depend on the clinical scenario (RF, IgG subclasses, IgE, Aspergillus IgE
and precipitins, α
1- antitrypsin). Involve the respiratory teamearly.
• PCR for drug- resistantTB.
Pitfalls
• Sputum may be diluted by saliva.
• Diagnosis of squamous cell carcinoma is not as robust as for small- cell
lung cancer or adenocarcinoma. Needs careful cross- referencing to
radiology and should be conrmed, if possible, with biopsies.
•
−ve results should not preclude further investigations if malignancy
suspected.
Further reading
Pasteur M, Bilton D, Hill A; British Thoracic Society Bronchiectasis non- CF Guideline Group. British
Thoracic Society guideline for non- CF bronchiectasis. Thorax 2010; 65(Suppl 1):i1– 58.
Rosia E, Scano G. Association of sputum parameters with clinical and functional measurements in
asthma. Thorax 2000; 55:235– 8.
Table8.8 Possible results
Gram stain Gram +ve or −ve organisms
ZN stain AAFB
Microscopy Dierential cell count
Eosinophils in asthma
Cytology Malignant cells
Small cell, squamous cell, adenocarcinoma cells

572
CHAPTER8 Respiratory medicine
572
Static lung volumes/ whole body
plethysmography
Clinical indications
• Dierentiate between obstructive and restrictive disease patterns.
• Identify and quantify trapped air (shown by i residual volume (RV)/ TLC
ratio).
•
Assess response to therapeutic interventions (e.g. drugs, radiation,
transplantation).
•
Identify the presence and amount of unventilatedlung.
• Assess chronic lung disease (e.g. sarcoidosis, rheumatoidlung).
• Preoperative assessment.
• Assessment of pulmonary disability.
Patient preparation
1. Ask the patient to wear comfortable clothes.
2. Place the mouthpiece securely in the mouth with the lips tight
aroundit.
3. Breathe in a relaxed manner through the spirometer system (nose clips
mandatory).
4. After a total of ve tidal breaths with consistent end- expiratory levels,
patient asked to maximally inspire to TLC, followed by exhalation with
encouragement to force out the last 5– 15% ofair.
5. Aminimum of two attempts should be obtained.
6. More may be needed in the young and elderly to obtain reproducible
results.
Most accurate results are obtained with whole body plethysmography.
Possible results
• Total lung capacity:volume of air in the lungs at the end of full
inspiration.
•
Residual volume:volume of air remaining in the lungs after maximal
expiration.
•
Vital capacity:the amount of air expired (or inspired) between
maximum inspiration and maximum expiration.
•
Functional residual capacity:the amount of air in the lungs at the end- tidal
position.
•
Inspiratory capacity:the maximum amount of air that can be breathed
into the lungs from the end- tidal position.
• Tidal volume:the volume of air inspired and expired with each breath.
•
Inspiratory reserve volume:the volume between the peak inspiratory tidal
position and maximum inspiration.
(See Table 8.9 and Fig.8.7.)
STATIC LUNG VOLUMES/WHOLE BODY PLETHYSMOGRAPHY
573
Interpretation
• Only interpret if the test is reproducible, i.e. if the two largest VC values
are within 5% or 100mL (whichever is the larger).
•
VC may remain within normal range in some pulmonary disease,
e.g. emphysema.
•
d VC— restrictive pulmonary disease, neuromuscular disease,
e.g. amyotrophic lateral sclerosis.
•
During the testing process, the patient is enclosed in a chamber
equipped to measure either pressure, ow, or volume changes. Because
all the gas in the thorax is accounted for, this method is particularly
useful in patients with trapped gas, e.g. bullous emphysema.
Table8.9 Causes
Causes of i
TLC
Generalized airway obstruction, e.g. COPD
Emphysema (including bullae)
Bronchiectasis
Asthma
Other, e.g. acromegaly
Causes of d
TLC
Intrapulmonary:
•
Pneumonectomy
• Collapsedlung
• Consolidation
• Oedema
• Fibrosis
Extrapulmonary:
•
Pleural disease
• Eusion
• Thickening
• Pneumothorax
• Rib cage deformity
• Scoliosis
• Thoracoplasty
• Respiratory muscle weakness
Causes of i RV Generalized airway obstruction
Pulmonary vascular congestion, e.g. mitral stenosis, atrial
septaldefect
Expiratory muscle weakness, e.g. spinal injury, myopathies
Causes of i
FRC
Age, lung disease causing air trapping, e.g. asthma,
emphysema, COPD
Causes of d
FRC
Restrictive lung diseases, e.g. diuse interstitial pulmonary
disease of any aetiology, pneumonectomy

574
CHAPTER8 Respiratory medicine
574
Advantages overothertests
• Reproducible.
Pitfalls
• Patient co- operation is essential. They must provide maximal eort and
be capable of understanding instructions.
•
Calibration should take place on a regularbasis.
• Risk of disease transmission between patients, and between patient and
technician; therefore, avoid if pulmonary TB suspected.
TLC
TLC
TLC
FVC
FVC
FVC
RV
RV
RV
FVC Forced vital capacit
y
TLC Total lung capacity
RV Residual volume
Normal
Obstructive
(Hyperination)
Restrictive
Fig.8.7 Lung volumes:physiological and pathological.

STATIC LUNG VOLUMES/WHOLE BODY PLETHYSMOGRAPHY
575

576
CHAPTER8 Respiratory medicine
576
Sweattest
Clinical indications
Suspected cystic brosis inthe contextof
•
Bronchiectasis/ recurrent chest infections.
• Pancreatic insuciency/ DM.
• Family history.
• Fertility problems.
Patient preparation
1. Obtain informed consent:verbal usually sucient, but important to
discuss the reasons for the test and possible implications. Perform two
sweat tests simultaneously on each arm for greater accuracy.
2. Induce sweating by pilocarpine iontophoresis. Aweak electrical current
aids penetration of pilocarpine into the skin, thus stimulating the sweat
glands of the forearm, previously washed and dried, to secretesweat.
3. Collect sweat on preweighed lter paper (>100mg), then measure
eluted Na
+
and chloride(Cl
−
).
Possible results
• 98– 99% of children homozygous for CyF have sweat Cl
−
and Na
+
levels
well above 70 and 60mmol/ L, respectively.
• Sweat Na
+
concentrations tend to i with age and show wide variability
between individuals.
•
Diagnostic accuracy is improved in borderline cases by a suppression
test using udrocortisone.
Interpretation
• A+ve test is virtually diagnostic of CyF. This should lead to counselling
and genetic testing.
•
Equivocal results are dened as Na
+
or Cl
−
concentrations between
50 and 70mmol/ L.
• The diagnosis should never rest on the sweat test alone and should be
considered together with the clinical ndings and laboratory evidence of
pancreatic insuciency.
Advantages overothertests
• Cheaper than genetictests.
• Assesses functional decit, therefore capable of detecting patients who
have rare variants ofCyF.
Ancillarytests
• Nasal potential dierence.
• Pancreatic function tests (3- day faecal collection).
• Genetic studies.

SWEATTEST
577
Pitfalls
• Awide discrepancy between the results from each arm suggests a
problem with the technique.
•
Accurate interpretation of sweat tests requires knowledge of the age-
related changes in sweat Na
+
and Cl
−
concentrations and should be
done in a specialized centre.
False negatives
• Inexperience of operator.
• Low rates of sweating.
• Poor skin preparation.
• Poor iontophoretic contact with theskin.
• Faulty chemical analysis.
False positives
• Evaporation of sweat 2° to inadequate sealing during collection.
• Untreated adrenal insuciency.
• NephrogenicDI.
• Hypothyroidism.
• Glycogen storage disease.
• Nephrotic syndrome.
• Severe malnutrition.
• AIDS (some reports of abnormal sweat electrolytes).
• Faulty chemical analysis.
Further reading
Green A, Dodds P, Pennock C. A study of sweat sodium and chloride:criteria for the diagnosis of
cystic brosis. Ann Clin Biochem 1985; 22
:171– 6.
Hall SK, Stableforth DE, Green A. Sweat sodium and chloride concentrations— essential criteria for
the diagnosis of cystic brosis in adults. Ann Clin Biochem 1990; 27:318– 20.
Heeley AF, Watson D. Cystic brosis— its biochemical detection. Clin Chem 1983; 29:2011– 18.

578
CHAPTER8 Respiratory medicine
578
Medical thoracoscopy
Clinical indications
• Pleural eusions when pleural uid analysis non- diagnostic.
• Pneumothorax.
• Staging of lung cancer.
• Diagnosis of malignant mesothelioma and other pleural abnormalities,
e.g. neurinomas, lipomas, plastocytomas.
Pre- assessment
• Arecent (<1month) CT scan of the chest is mandatory. Apre-
procedure USS is desirable.
•
FBC.
• Clotting.
• Renal function and electrolytes.
• Serum glucose.
• Spirometry.
• Pulse oximetry.
• ABGs on air if hypoxaemia suggested by SpO
2
or if signicant
hypercapnia is suspected.
Patient preparation
Endoscopysuite
1. Patient informed and consented. Fasted from solids for 6h and liquids
for3h.
2. IV access obtained.
3. Full aseptic technique.
4. Patient positioned in the lateral position with the side to be examined
uppermost.
5. Basic monitoring:pulse oximeter, BP, and cardiac monitor.
6. Supplementary O
2
given via face mask or nasal cannulae to maintain
saturations>92%.
7. IV sedation— midazolam/ alfentanil. LAn:0.5– 1% lidocaine.
8. An absolute prerequisite for thoracoscopy is the presence of an
adequate pleural space (i.e. at least 6– 10cm diameter).
9. If pleural eusion:drain using a 3- way tap. Replace with equal quantity
of atmosphericair.
10. If no eusion:create a pneumothorax. Insert a needle connected to a
manometer into the pleural space. Introduce 400– 1000mL ofair.
11. Skin incision 1cm in fth intercostal space, mid- axillary line (or guided
byUSS).
12. Insert 5– 10mm pleural trocar and cannula.
13. Introduce the thoracoscope via the trocar into the pleural cavity.
14. After inspection and biopsies, remove the trocar and insert adrain.
15. CXR post- procedure.
16. May need to apply suction to the drain:i in small increments of
5cmH
2
O (0.5kPa), up to a maximum of −20cmH
2
O.

MEDICAL THORACOSCOPY
579
Possible results
• Direct inspection of pleural surfaces.
• Biopsy of parietal pleura— histology/ culture, especiallyAAFBs.
• Pleural uid l M,C&S l cytology.
•
Therapeutic options:pleurodesis, coagulation of blebs, resection of
brinous loculations in empyemas.
•
Drainage of large pleural eusions possible without risk of re- expansion
pulmonary oedema due to rapid equalization of pressures by entrance
of air into the pleuralspace.
Interpretation
• Macroscopic appearance of pleura may be diagnostic, e.g. metastatic
disease.
Advantages overothertests
• Better than blind pleural biopsy.
• Able to obtain diagnosis in 70– 95% ofcases.
• Especially good at diagnosingTB.
• Less invasive than thoracotomy.
• Can perform talc poudrage (pleurodesis) at the sametime.
• Less expensive than surgical VATS. Does not require a theatre or an
anaesthetist.
•
Done under sedation, unlike VATS which requires GAn and selective
one- lung ventilation.
Ancillarytests
• Diagnosis of mesothelioma improved with use of immunohistochemical
markers.
Pitfalls
• Biopsies may be inadequate or non- representative.
Contraindications
• Obliterated pleuralspace.
• Small pneumothorax.
• Patient SOB at rest, unless 2° to pneumothorax or pleural eusion,
which can be treated during the procedure.
•
Disturbed haemostasis:
•
Platelets <40 ×10
9
/ L.
•
APTT >50% of normal.
• Recent MI, arrhythmias, heart failure.

580
CHAPTER8 Respiratory medicine
580
Complications
• Fever 24– 36h post- procedure.
• Empyema(<1%).
• Wound infection.
• SC emphysema.
• Air embolism.
• Bronchopleural stula following lung biopsy.
• Seeding of metastases/ mesothelioma along trocar wound.
(Radiotherapy a few weeks post- thoracoscopy should be carried out to
preventthis.)
•
Haemorrhage.
• Arrhythmias.
• Mortality rate <0.01%.
Further reading
Blanc FX, Atassi K, Bignon J, Housset B. Diagnostic value of medical thoracoscopy in pleural dis-
ease:a 6- year retrospective study. Chest 2002; 5:1677– 83.
Buchanan DR, Neville E. Thoracoscopy for physicians:a practical guide. London:Arnold,2004.
Colt HG. Thoracoscopy:window to the pleural space. Chest 1999; 116:1409– 15.
Loddenkemper R. Thoracoscopy— state of the art. Eur Resp J 1998; 11:213– 21.

MEDICAL THORACOSCOPY
581

582
CHAPTER8 Respiratory medicine
582
Transferfactor
Clinical indications
• Test for abnormalities of pulmonary gas exchange.
Patient preparation
• Avoid smoking 6h prior and strenuous exercise2hprior.
• Allow 15– 30min fortest.
• Usually measured by single- breath inhalation technique.
• Patient breathes in air containing a known concentration of carbon
monoxide (CO) and holds breath for10s.
Possible results
• Transfer factor (TLCO).
• Transfer coecient(KCO).
• May need to correct for anaemia:
•
Result usually standardized to Hb 14.6g/ dL.
•
Eect of mild anaemia (Hb >10g/ dL) slight but becomes progres-
sively more marked at lower values.
Interpretation
Decrease inTLCO
•
Obstructive lung disease, e.g. COPD, emphysema.
• Diuse ILD, e.g. cryptogenic brosing alveolitis (CFA), amiodaronelung.
• Pulmonary involvement in systemic disease, e.g. SLE, RA, Wegener’s.
• Cardiovascular disease, e.g. pulmonary oedema, mitral stenosis,PE.
• Others:anaemia, cigarette smoking.
Increase inTLCO
•
Diseases associated with polycythaemia.
• Pulmonary haemorrhage.
• Diseases associated with i pulmonary blood such as left- to- right intra-
cardiac shunts.
•
Exercise.
• Asthmatics (reasons not clear).
Advantages overothertests
• Quick.
• Relatively easy to perform.
• Reproducible.
Pitfalls
• Breath- holding time may be dicult for some patients to achieve.
• Calculation of TLCO is based on assumption that ventilation and
diusion are homogeneous in the entire lung. With unequal distribution
of ventilation and diusion, the TLCO will be underestimated on the
alveolarlevel.
•
With extrapulmonary lung restriction and consequent inability to
achieve full inspiration, KCO tends to be higher than normal.

583
Chapter9
Neurology
Lumbar puncture 584
Skull radiograph 590
Ultrasound 592
Angiography 594
Myelography 595
Radionuclide scans 596
Computed tomography 598
Magnetic resonance imaging 602
Nerve conduction studies 606
Electromyogram 610
Electroencephalogram 612
Electroencephalogram:abnormalities 614
Electroencephalogram:in epilepsy 616
Electroencephalogram:how touse 618
Electroencephalogram:invasive techniques 620
Polysomnography 621
Sensory evoked potentials or responses 622
Transcranial magnetic stimulation 628
Neurological investigation of sphincter disturbance 630
Edrophonium (Tensilon
®
) test 631
Amobarbital (Wada) test 631
Biopsies 632
Oligoclonal bands 635
Diagnostic and prognostic antibodies, and other markers
in blood and urine 636
Biochemical markers 640
Genetic tests 642
Neuro- otology 646

584
CHAPTER9 Neurology
584
Lumbar puncture
Indications
• Meningitis.
• Encephalitis.
• Polyradiculitis, polyneuritis.
• MS.
• Myelitis.
• Vasculitis.
• SuspectedSAH.
Note:in general, a −ve CT does not exclude anSAH.
• Suspected malignancy with meningeal involvement.
• Assessment of CSF pressure:
•
High (e.g. idiopathic or ‘benign’ intracranial hypertension (IIH)).
•
Low (e.g. ‘low pressure’ headache).
• Therapeutic trials,e.g.
•
IIH.
•
Normal pressure hydrocephalus (NPH) (not particularly helpful).
• To seek specic antibodies/ markers in CSF,e.g.
•
HIV.
•
Lyme (Borrelia).
•
Syphilis.
•
ACE (for neurosarcoid).
•
Tumour markers.
•
Lactate in mitochondrial cytopathies.
Preparation
• Decide exactly what investigations you want. If necessary, alert
the appropriate laboratories and organize transport of samples. In
particular, samples for xanthochromia and cytology should be rapidly
taken to the laboratory to be spundown.
•
If the patient is also due to have a neuroradiological investigation with
contrast and an LP is not urgent, delay the LP until after the scan, as
there may be diuse meningeal enhancement after theLP.
•
If the patient is extremely anxious, he may benet from 5– 10mg of oral
diazepam prior to theLP.
Procedure
1. Explain to the patient what you are abouttodo.
2. Arrange all your equipment on a sterile tray, including assembled CSF
manometer.
3. Position the patient on his side, with the back perpendicular to the
bed, at the edge of a rm bed. Place the head on one pillow. Draw the
knees up, and place one pillow betweenthem.
4. Adjust the height of the bed, so that you are comfortable.
5. Identify the bony landmarks. L3/ L4 space is in line with the iliac crests
and is most commonly used. L2/ L3 to L5/ S1 are also used. If you like,
mark the target space with the imprint of your thumb nail. Take time
over these rst four stages.

LUMBAR PUNCTURE
585
6. The insertion of the needle should be a sterile procedure. Clean the
skin over the lower back. Don sterile gloves andmask.
7. Insert a little (0.25– 0.5mL) LAn— too much can obscure the bony
landmarks.
8. Pass the LP needle horizontally into the space, with the tip angled at
about 10– 15° (towards the umbilicus), in the midline horizontal plane.
At all times, the stylet should be fully inserted and the bevel of the
needle facingup.
9. Slight resistance should be felt as the needle passes through the
ligamentum avum and dura, and then a ‘give’ as it enters the
subarachnoidspace.
10. Slowly withdraw the stylet. CSF drops should appear.
11. If CSF does not appear, reinsert the stylet and slightly rotate the
needle— this sometimes frees it of obstructing nerve roots. Agentle
cough from the patient can alsohelp.
12. If the needle encounters bone, or the patient complains of pain
shooting down the leg, check the position of the needle (is it in the
midline? Is it angled correctly?) and then withdraw it entirely.
13. Insert a fresh needle, correcting for any error notedabove.
14. If this second pass is unsuccessful, withdraw the needle and inform
the patient. If he is happy for you to proceed, then attempt the LP in
another space, repeating all steps from 4 down. Use a fresh needle.
15. If you fail again, explain to the patient and seek a more experienced
operator to perform the LP. Multiple failed attempts are painful and
discouraging (to both you and your patient).
16. If a more experienced operator fails, ask your friendly radiologist to
do it under X- ray guidance, but give him the help he requests and
precise instructions about the samples required.
17. When CSF collection is complete, gently pull out the needle and place
a sterile dressing over the insertionsite.
18. Allow the patient to mobilize shortly after theLP.
Measuring thecerebrospinal uid pressure
As soon as the CSF starts to ow, attach the pre- assembled manometer.
Wait until the CSF stops rising. If the patient is very anxious, or uncomfort-
able, a falsely raised opening pressure may be recorded. Sometimes having
the patient slightly relax his legs will help. Using the 3- way tap, let the CSF
run into your rst prelabelled tube (do not waste the CSF!). Having col-
lected all the CSF you require, if the opening pressure was elevated, note
the closing pressure. If the opening pressure is expected to be very raised,
e.g. in suspected or conrmed idiopathic (benign) intracranial hypertension,
then two or more manometers should be pre- assembled, as the pressure
may exceed 40mmCSF.
Collecting samples
• As always, tailor your investigations to the clinical picture. If you are
just checking the CSF pressure, then no samples need necessarily be
collected. If you suspect an SAH, collect three samples in sequentially
labelled bottles and promptly hand- carry to the laboratory for
quantitative estimation of xanthochromia and Hb breakdown products.

586
CHAPTER9 Neurology
586
If you are looking for evidence of malignant cells, then at least one
sample should be sent to the laboratory promptly for cytology.
•
To avoid contamination, allow the microbiology laboratory to split
samples, rather than attempting this yourself.
•
Collect at least ten drops in each bottle. The microbiology and cytology
laboratories in particular will thank you for greater volumes.
•
As soon as the CSF is collected, a blood sample should be obtained (if
necessary) for glucose and OCB detection.
Alternative positioning ofpatient
Sometimes there is a dry tap if the CSF pressure is too low to distend
the lumbar cistern. This can sometimes be overcome by performing the
LP with the patient sitting on a rm reversed chair, leaning forward to bend
over its back. This manoeuvre maximizes the separation of the vertebrae.
Again, the needle should be angled slightly (10°) upward relative to the
spine at that point. This position does not allow accurate measurement of
CSF pressure.
Which needle touse?
A 22G needle usually appropriate. Needles with larger bores tend to cause
a greater CSF leak (and thus more headache). Some advocate even ner
needles, but these make the collection of CSF take too long. ‘Blunt’ anaes-
thetists’ needles reduce the risk of post- LP headache.
Clinical record keeping
Record what you did in the notes after the procedure (e.g. if >1 pass was
required; which space you used), the opening and closing CSF pressures,
and what investigations you have requested. Note the appearance of the
CSF (if normal, it will be clear and colourless). If the CSF appears bloody,
record this and whether the nal bottle collected is clearer than therst.
When NOT toattempt a lumbar puncture
• Risk of herniation:
•
SOLs.
•
Non- communicating hydrocephalus.
•
Cerebral oedema (if in doubt, cranial imaging should be performed
rst).
•
Uncorrected bleeding diathesis/ anticoagulantuse.
• Caution:if previous lumbar spine surgery or known anatomical
abnormalities.
•
Local skin sepsis.
Note:it is usually safe and appropriate to perform an LP in suspected menin-
gitis, unless there are specic clinical features to suggest raised ICP, in which
case cranial imaging should be performedrst.

LUMBAR PUNCTURE
587
Complications and what todo aboutthem
Headache
•
Usually starts within 24hofLP.
• May last from a few hours to 2 weeks, but typically severaldays.
• Probably related to persistent CSF leak via the dural tear; therefore,
tends to have ‘low pressure’ characteristics (frontal, worse on sitting up,
better on lying down). There may be mild meningism and nausea.
Treatment has traditionally involved bed rest, analgesia, and the encourage-
ment of plenty of uids.
•
If nausea is a major problem, the patient may require IV uids.
• Rarely, if the headache is severe and persistent, then an anaesthetist
may place an autologous blood patch to ‘plug’ the dural tear. Surgical
intervention is very rarely required.
Low backache
A variety of causes of post- LP backache exist; these may usually be treated
conservatively.
Infection
Very rare if a sterile technique is used. Occasionally may occur if the needle
passes through a region of infection. Meningitis typically develops within
12h. Very rarely, there may be an epidural abscess or vertebral osteomyeli-
tis. Treat with appropriate antibiotics and, if necessary, surgery.
Herniation
•
Uncal or cerebellar herniation may occur, particularly in the presence
of a posterior fossa mass. 3 An LP should not be performed if there is
suspicion of raised ICP without rst obtaining cranial CT orMRI.
•
Should the CSF pressure be found to be very high (300mmCSF), even
after relaxing the patient, and in the absence of idiopathic (benign)
intracranial hypertension, manage as follows:
•
Nurse the patient prone with no pillow.
•
Raise the foot of thebed.
•
Start an infusion of 20% mannitol at 1g/ kg over20min.
•
Start a neurological observationchart.
•
Arrange an urgent CT of the brain and notify the neurosurgeons.
3 Do not instil saline into the subarachnoidspace.
Haemorrhage
A ‘traumatic’ tap may cause a little local bleeding that is rarely of clinical
signicance. Patients with impaired clotting (remember warfarin) or platelet
function are at risk of more extensive bleeding, and an LP should not be
attempted unless the coagulopathy is corrected. An arachnoiditis, or spinal
subdural, or epidural haemorrhage may develop. Aspinal SDH is otherwise
rare, and an intracranial SDH veryrare.

588
CHAPTER9 Neurology
588
Cerebrospinal uid constituents:normalvalues
• White cells:0– 4/ mm
3
.
•
RBCs:ideallynone!
•
Protein:0.15– 0.45g/ L.
• Glucose:7 half to two- thirds of simultaneous blood glucose.
• Opening pressure:8– 20cmCSF.
Note:if there is a traumatic ‘bloody’ tap, there may be hundreds or thou-
sands of RBCs/ mm
3
. If so, then white cells should be expected in the CSF,
but in similar proportions to the peripheralblood.
Rules ofthumb
1. Pressure:
•
i by SOLs within the cranial vault such as oedema, masses, chronic
inammation.
•
i by i CVP, e.g. in the anxious patient with tensed abdominal
muscles.
•
d if the spinal subarachnoid space is obstructed, thus impeding
CSFow.
2. Cells:
•
Polymorphs (neutrophils):suggest acute bacterial infection.
•
Lymphocytes and monocytes:viral and chronic infections or tumours.
•
Eosinophils:tumours, parasites, foreign body reactions.
3. Glucose— d by non- viral processes causing meningeal inammation.
4. Total protein— i by breakdown of the blood– brain barrier.
5. Igs specic to the CSF, i.e. without matching Igs in a simultaneous
blood sample:inammation within the theca, e.g. MS, infection, tissue
damage.
Common patterns
These are shown in Table9.1.
E OHCM 10e, p.822.

LUMBAR PUNCTURE
589
Further reading
Davis A, Dobson R, Kaninia S, Giovannoni G, Schmierer K. Atraumatic needles for lumbar punc-
ture:why haven’t neurologists changed? Pract Neurol 2016; 16
:18– 22.
Hasbun R, Abrahams J, Jekel J, Quagliarello VJ. Computed tomography of the head before lumbar
puncture in adults with suspected meningitis. N Engl J Med 2001; 345:1727– 33.
Thomas SR, Jamieson DR, Muir KW. Randomised controlled trial of atraumatic versus standard
needles for diagnostic lumbar puncture. BMJ 2000; 321
:986– 90.
Whiteley W, Al- Shahi R, Warlow CP, Zeidler M, Lueck CJ. CSF opening pressure:reference interval
and the eect of body mass index. Neurology 2006; 67:1690– 1.
Table9.1 Common patterns
Condition Glucose Protein Cells Comments
Acute bacterial
meningitis
d i
Often >300/ mm
3
Polymorphs;
lactate i
Acute viral
meningitis
N N or i <300
mononuclear
Culture, antigen
detection may be
possible
Fungal
meningitis
d i <300
mononuclear
Culture and antigen
detection
Tuberculous
meningitis
d i Mixed pleocytosis
<300
ZN stain organisms,
culture PCR
Herpes simplex
encephalitis
N Mildly i
5– 500
lymphocytes
PCR
GBS N i Normal
SAH
†
N May be i Erythrocytes Look for bilirubin
pigments on
spectrophotometry;
xanthochromia
unreliable
Malignant
meningitis
d i Mononuclear Rapid cytospin and
look for malignant
cells
HIV N N or i Mononuclear
pleocytosis
Culture, antigen
detection, antiviral
antibodies
Neurosyphilis N or d i <300
lymphocytes
VDRL
Neurosyphilis—
early
i Treponema
pallidum
Neurosyphilis—
late
Immobilization
tests
†
LP should be done >12h after onset of headache; the CSF should be spun down within 45min;
d numbers of RBCs in successive bottles are compatible withSAH.
Reprinted from Brain Res Bull, 61:287– 97 Kleine T O etal. ‘New and old diagnostic markers of
meningitis in cerebrospinal uid (CSF)’(2003) with permission from Elsevier.

590
CHAPTER9 Neurology
590
Skull radiograph
Indications
Usually more modern imaging techniques are much more informative, but
there are occasions when these may not be speedily available. However,
the plain SXR has quite low specicity and sensitivity for detecting many
abnormalities of neurological importance.
Used in(suspected) casesof
• Skull fracture.
• Pituitary fossa abnormalities.
• Tumours involvingbone.
• Bone changes related to meningiomata.
Procedure
• Lateral view in the rst instance.
Consider
• Occipitofrontal.
• Towne’s (half axial).
• Basal (submentovertical).
• Specic views (e.g. orbits).
What tolook for(what you see will depend onthe
pathology)
See E Chapter13.
•
Shape and symmetry of thevault.
• Pituitaryfossa.
• Position of calcied pineal (midline shift?).
• Bone density changes (e.g. tumour, meningioma, Paget’s).
• Fractures.
• Evidence of neurosurgical procedures.
• Intracranialair.
• Post- nasalspace.
• Craniocervical junction.
Indications forSXR afterheadinjury
(But see E Computed tomography, pp. 598–600 for cranial CT; in general,
CT is the preferred imaging modality.)
In anorientated adult patient
•
Loss of consciousness or amnesia.
• Fall of>60cm.
• Full- thickness scalp laceration.
• Scalp haematoma.
If a skull fracture is detected, proceedtoCT.

SKULL RADIOGRAPH
591

592
CHAPTER9 Neurology
592
Ultrasound
US may be used in a variety ofmodes.
Mostly commonly used inneuroradiology
• B mode:gives 2D images.
•
Doppler eect is used to assess alterations in the pattern (especially
velocity) of ow in vessels.
•
Duplex scanning combines B mode and Doppler.
Extracranial vessels
Bmode
•
Can image from the clavicle (common carotid bifurcation) and internal
and external carotids to the angle of thejaw.
•
Can image the proximal and distal subclavian and vertebral arteries.
• Supraorbital artery (anterior circulation).
• Fibrofatty plaques and thrombus on plaques not very echogenic,
therefore missable.
•
Fibrous plaques more echogenic.
• Calcication in plaques is highly echogenic.
• Can sometimes detect intraplaque haemorrhage or ulceration.
Note:requires patient co- operation and considerable operator skill. High-
grade stenosis can appear as total occlusion.
Dopplermode
•
Stenosis alters the normal pattern of velocities recorded.
Duplex
•
Combination of anatomic and ow imaging more sensitive and specic
for clinically signicant stenoses.
Carotid duplex studies incerebrovascular disease
•
Use of carotid US:most commonly in the assessment of patients with
carotid territory ischaemic strokes or TIAs, who might be candidates for
carotid endarterectomy. Such surgery should be performed as soon as
possible, so carotid Doppler studies should be arranged promptly after
the rst event. If a patient has neurological signs or symptoms suggestive
of posterior circulation events, there is little point in organizing carotid
(i.e. anterior circulation) studies. Both the degree of stenosis and the
morphology of the plaque (irregular plaques are more pathogenic) are
important.
Duplex studies insuspected giant cell arteritis
•
Making, or excluding, the diagnosis of GCA can be dicult. Most
guidelines suggest a temporal artery biopsy, but timely access to a
biopsy may be dicult, GCA may occur without temporal artery
involvement, and some patients with GCA have a −ve biopsy. Duplex
studies of the temporal arteries have been shown to have high
specicity and sensitivity.

ULTRASOUND
593
Intracranial vessels
Transcranial Doppler
•
2MHz to penetrate thinnerbone.
• Flow velocity in anterior, middle, and posterior cerebral, ophthalmic,
and basilar arteries; carotid siphon.
What itshows
•
Intracranial haemodynamics.
• Vasospasm inSAH.
• Monitoring of microemboli.
• This is an area of active research, with new clinical indications being
described frequently.
Further reading
Croft AP, Thompson N, Duddy MJ, etal. Cranial ultrasound for the diagnosis of giant cell arteritis.
Aretrospective cohort study. J R Coll Physicians Edinb 2015; 45
:268– 72.
Rothwell PM, Eliasziw M, Gutnikov SA, etal. Analysis of pooled data from the randomised controlled
trials of endarterectomy for symptomatic carotid stenosis. Lancet 2003; 361
:107– 16.
Tegeler CH. Ultrasound in cerebrovascular disease. In: JO Greenberg (ed.). Neuroimaging.
NewYork:McGraw- Hill, 1995; pp.577– 95.

594
CHAPTER9 Neurology
594
Angiography
Indications
• Strongly suspected or conrmedSAH.
• Suspected cerebral vasculitis.
• Detection and delineation of other vascular abnormalities (e.g. AVM) of
the brain or spinalcord.
•
Delineation of tumour blood supply (occasionally).
Procedure
1. Catheter passed via the femoral artery to the carotid or vertebral
artery under image intensication.
2. Contrast isgiven.
3. In digital subtraction angiography (DSA), subtraction of pre- from post-
contrast images (pixel by pixel) is used to help remove signals from
bone density.
Arch angiography (aortography)
• Visualizes the aorta, major neck vessels, and sometimes the circle of Willis.
• No venous imaging.
Selective intra- arterial angiography
• Later images show venous system.
Carotidartery
•
Anteroposterior (AP), lateral, and oblique views— anterior and middle
cerebral, and internal carotid arteries.
Vertebralartery
•
Towne’s (half- axial) and lateral views— vertebral, basilar, posterior
cerebral arteries.
What can be demonstrated?
• Occlusion, stenosis, plaques.
• Aneurysms.
• Arteriovenous and other blood vessel abnormalities.
• Abnormal tumour circulation.*
• Displacement or compression of vessels.*
• Experimental role in acute stroke analysis.
Note:(*) although CT and MRI give ner spatial details, angiography is still
useful, e.g. delineating blood supply of a tumour.
Complications
• Sensitivity to the contrast medium.
• Cerebral ischaemia, e.g. 2° to dislodgement of embolic fragments by
catheter tip or thrombus in the catheterlumen.
•
The rate of transient or permanent neurological defect following
angiography depends on the operator.
Further reading
Larsen DW, Teitelbaum GP. Radiological angiography. In:WG Bradley, RB Daro, D Marsden, GM
Fenichel (eds.). Neurology in Clinical Practice
, 3rd edn. Boston: Butterworth- Heinemann, 2000;
pp.617– 43.
Osborne AG. Diagnostic Cerebral Angiography, 2nd edn. NewYork:Lippincott, Williams & Wilkins,1999.

MYELOGRAPHY
595
Myelography
Indications
• Largely superseded by CT and especiallyMRI.
• Still used in subjects in whom MRI is contraindicated (e.g. cardiac
pacemaker, metallic implants, claustrophobia).
•
Can screen whole spinal cord and cauda equina for compressive or
expanding lesions.
•
Can visualizeroots.
• Spinal vasculature abnormalities.
Procedure
A total of 5– 25mL of (usually water- soluble) radio- opaque contrast
medium is injected via an LP needle in the usual location (occasionally a
cisternal puncture is used). By tipping the patient on a tilt table, the whole
spinal subarachnoid space may be visualized.
Complications
• ThoseofLP.
• Spinal arachnoiditis (after months or years), now rare with water-
soluble contrast.
•
Acute deterioration if there is cord/ root compression.
• Direct neurotoxicity (3 in 10,000):
•
Seizures, encephalopathy.
•
Usually resolves in48h.
• Allergic reaction to contrast. Give dexamethasone 4mg 12 and 2h prior
to investigation if known allergy.
Note:send CSF for usual investigations (E Lumbar puncture, pp. 584–9).

596
CHAPTER9 Neurology
596
Radionuclidescans
Positron emission tomography
Unstable positron- emitting isotopes (produced locally by a cyclotron or lin-
ear accelerator) are incorporated into biologically active compounds. The
distribution of isotope shortly after IV administration is plotted. Arange of
compounds may be labelled such as ligands for specic neurotransmitter
receptors or 18F- uorodeoxyglucose (FDG). Commonly, PET is used to
determine regional cerebral bloodow.
Single photon emission computed tomography (SPECT)
• Stable radioactive isotopes are incorporated into biologically active
compounds.
•
Their distribution after IV administration is plotted.
• These images often lack ne spatial detail.
Although the range of ligands available is limited, SPECT has certain advan-
tages overPET:
•
Isotopes are stable, and therefore a cyclotron or linear accelerator need
not be onsite.
•
Alabelled ligand can be given after a clinically important event, e.g. can
give agent and scan within 20min of the occurrence of a seizure.
Uses ofPET andSPECT
PET is not widely available as a clinical tool. With the i availability of func-
tional MRI (fMRI), the uses of PET in both clinical practice and neuroscience
research have lessened. SPECT is more widely available in clinical centres.
Clinical applicationsofPET
PET is mainly used in neurological practice as whole- body FDG- PET to look
for systemic malignancy, especially in paraneoplastic syndromes.
Clinical applications ofSPECT
•
An epileptogenic focus may show interictal hypometabolism (ictal
hypermetabolism may be demonstrated with SPECT).
•
Regional hypometabolism may be seen in neurodegenerative conditions
such as Alzheimer’s disease and frontotemporal dementia. This may aid
in diagnosis and dierential diagnosis.
•
‘Pseudo- dementia’ 2° to psychiatric disease such as depression (with
normal SPECT scans) may sometimes be dierentiated from dementia
due to ‘organic’ neurological disease (with regional hypoperfusion),
although psychiatric diseases may themselves be associated with regional
hypoperfusion.
•
Assessment of Parkinsonism.
The functional integrity of the nigrostriatal system can be assessed, e.g. by
the use of SPECT ligands for the dopamine transporter (e.g. FP- CIT). Such
DAT scans can be used to dierentiate true Parkinsonism from other causes
of movement disorders.

RADIONUCLIDESCANS
597
Clinical/ research applications ofPET and SPECT include
• Determination of regional cerebral blood ow, glucose metabolism, and
oxygen utilization
•
Hypometabolism may be seen following a stroke. The aected area
may exceed that with a demonstrable lesion on conventional CT or MR
imaging.
•
In vivo pharmacology (e.g. distribution of neurotransmitter receptors).
E Positron emission tomography, pp. 894–7.
Further reading
Marshall V, Grosset D. Role of dopamine transporter imaging in routine clinical practice. Mov Disord
2003; 18
:1415– 23.
Younes- Mhenni S, Janier MF, Cinotti L, etal. FDG- PET improves tumour detection in patients with
paraneoplastic neurological syndromes. Brain 2004; 127:2331– 8.

598
CHAPTER9 Neurology
598
Computed tomography
Cranial computed tomography
Lookfor:
•
Disturbances in the normal anatomy of the ventricular system.
• Skull base andvault.
• Width of cortical ssures/ sulci.
• Midlineshift.
• Areas of abnormal tissue density.
• Opacity or lucency of sinuses.
• Normal owvoids.
High- density (‘white’)signal
• Freshblood.
• Calcication:
•
Slow- growing tumour.
•
AVM/ aneurysm.
•
Hamartoma.
•
In pineal/ choroid plexus/ basal ganglia, may be normal.
Low- density (‘black’)signal
• Infarction.
• Tumour.
• Abscess.
• Oedema.
• Encephalitis.
• Resolving haematoma.
Mixed density
•
Tumour.
• Abscess.
• AVM.
• Contusion.
• Haemorrhagic infarct.
After administration of IV contrast medium, areas with a breakdown in the
blood– brain barrier may ‘enhance’ (appear ‘white’). This may reveal pre-
viously ‘invisible’ lesions (isodense with the surrounding tissue). Especially
useful for tumour and infection.
Common patterns ofenhancement include
•
Ring enhancement of tumours and abscesses.
• Solid enhancement of meningiomas.
• Meningeal enhancement with meningeal disease involvement.
Indications forhead CT afterheadinjury
CT imaging ofthe head inadults
Request a CT brain scan immediately for adult patients with any of the fol-
lowing risk factors:
•
GCS score <13 on initial assessment in the emergency department.
• GCS <15 2h after the injury on assessment in the emergency
department.

COMPUTED TOMOGRAPHY
599
• Suspected open or depressed skull fracture.
• Any sign of basal skull fracture.
• Post- traumatic seizure.
• Focal neurological decit.
• One or more episodes of vomiting.
• Amnesia for events >30min before impact.
CT imaging ofthe head inchildren
Request CT of the brain immediately for children with any one of the fol-
lowing risk factors:
•
Age over 1year:GCS <14 on assessment in the emergency department.
•
Age under 1year:GCS paediatric <15 on assessment in the emergency
department.
•
Age under 1year and presence of bruise, swelling, or laceration (>5cm)
on thehead.
•
Dangerous mechanism of injury.
• Clinical suspicion of non- accidental injury.
• Loss of consciousness lasting >5min (witnessed).
• Post- traumatic seizure but no history of epilepsy.
• Abnormal drowsiness.
• Suspected open or depressed skull injury, or tense fontanelle.
• Any sign of basal skull fracture.
• Focal neurological decit.
• Three or more discrete episodes of vomiting.
• Amnesia (antegrade or retrograde) lasting >5 min.
1,2
CT angiography and venography
CT, especially rapid image acquisition with helical CT, can allow imaging
of the intracranial vasculature. CT angiography is particularly used in the
detection of aneurysms and is i widely available.
3
CT venography is impor-
tant in the assessment of possible intracerebral venous thrombosis.
4
CT ofspine
MRI is usually preferable, but plain CT can give information about the discs
and bony architecture. CT may be helpful in suspected bony abnormali-
ties. Some patients cannot have MRI because of, e.g. metallic implants. CT
myelography can be used to demonstrate compressive lesions.
1 National Institute for Health and Care Excellence (2014). Head injury:assessment and early manage-
ment. Clinical guideline CG176. M
https:// www.nice.org.uk/ guidance/ cg176.
2
Yates D, Aktar R, Hill J; Guideline Development Group. Assessment, investigation, and early man-
agement of head injury:summary of NICE guidance. BMJ
2007; 335:719– 20.
3 Karamessini MT, Kagadis GC, Petsas T, etal. CT angiography with three- dimensional techniques
for the early diagnosis of intracranial aneurysms. Comparison with intra- arterial DSA and the surgical
ndings. Eur J Radiol 2004; 49:212– 23.
4 Smith R, Hourihan MD. Investigating suspected cerebral venous thrombosis. BMJ 2007; 334:794– 5.

600
CHAPTER9 Neurology
600
Indications forcervical spine imaging aftertrauma
Plain radiograph is the initial investigation, but CT preferredwhen:
•
Age >9years:
•
GCS <13 on initial assessment.
•
Intubated patient.
•
Technically inadequate plain radiographs.
•
Clinical suspicion of injury despite normal radiograph.
•
Patient being scanned for multi- region trauma.
• Age <10years:
•
GCS<9.
•
Strong clinical suspicion of injury despite normal radiograph.
•
Technically inadequate plain radiographs.
1,2
E OHCM 10e, p.730, p. 746.

COMPUTED TOMOGRAPHY
601

602
CHAPTER9 Neurology
602
Magnetic resonance imaging
For most neurological indications, MRI is preferred to CT. It gives supe-
rior anatomical detail, and the range of sequences available allows superior
determination of pathology. Unlike CT, there is no radiation exposure.
Note: MRI is not safe in the presence of ferromagnetic materials (e.g.
certain prostheses, metal lings in theeye).
Some people nd MRI dicult to tolerate because of claustrophobia.
Counselling and experience of ‘dummy’ scanners prior to MRI may be helpful.
Upright and ‘open’ scanners are increasingly available in specialist centres.
MRI sequences
There is a variety of sequences available, which oer various advantages in
delineating anatomy and pathology. This is an area of active research, with
new sequences continuing to enter clinical practice. It is important that the
requesting clinician provides appropriate clinical details, so that the radiolo-
gist may select the appropriate imaging sequences (and planes) to beused.
The commonest sequences are T1 and T2 (see Table9.2).
•
T1 CSF is hypointense (‘black’); fat and mature blood clotwhite.
• T2 CSF is hyperintense (‘white’).
MRI withenhancement
Intravenously administered gadolinium leaks through areas of damaged
blood– brain barrier to give a marked enhancement. It may be helpful in
delineating:
•
Ischaemia.
• Infection.
• Tumour (may help dierentiate from surrounding oedema).
• Active demyelination.
Table9.2 Comparison ofT1 andT2MRI
T1 T2 Tissue or lesion
Good anatomical
detail
Reveals most pathology
better than T1
Hypointense Hyperintense CSF
Hyperintense Iso- intense Fat, e.g. dermoid, lipoma,
some metastases (melanoma),
atheroma
Very hypointense Very hyperintense Cyst, hygroma
Hypointense Hyperintense Ischaemia, oedema,
demyelination, many
malignant tumours
Hyperintense Moderately
hyperintense
Subacute or chronic
haemorrhage
Iso- intense Hypointense Acute haemorrhage
Iso- intense Iso- intense Meningioma

MAGNETIC RESONANCE IMAGING
603
Magnetic resonance venography and angiography
MR may be used to obtain non- invasive images of blood vessels by using
special MRI sequences and image reconstruction. Whilst standard angiogra-
phy remains a ‘gold standard’ for many purposes, MRA has the advantage
of being non- invasive and therefore ‘safe’. MRA images ow, rather than
structure, and therefore may fail to ‘pick up’ low ow abnormalities such
as cavernous angiomas. 2 Caution:congenital abnormalities in the venous
sinuses may be misinterpreted as thrombosis on MR venography(MRV).
Uses
•
Assessment of patency of major arterial and venous vessels.
• Visualization of large (73mm diameter) aneurysms.
Functional magnetic resonance imaging
Certain (indirect) indices of neural activity (most commonly changes
reecting regional perfusion) may be imaged with sucient temporal and
spatial resolution to be useful for both research and clinical applications
(although fMRI has been largely a research tool to date). As a conventional
MRI machine, albeit with special software, is required, fMRI is being i used
in the clinical setting.
Clinical and research applications have included
•
Demonstration of the language areas prior to epilepsy surgery.
• Demonstration of the functional anatomy of cognitive, sensory, and
motor processes.
Diusion tensor imaging
Diusion tensor imaging (DTI) allows anatomical tract tracing in vivo,
using specialized software on standard MRI machines. As with fMRI, DTI
was initially a research tool but has now entered clinical practice in some
neuroscience centres. Major tracts, such as the corticospinal tract and the
optic radiation, can be identied; these can then be spared during resective
surgery.
Magnetic resonance spectroscopy
Magnetic resonance spectroscopy (MRS) can be used to measure the con-
centration of certain neurochemicals in vivo (see Fig. 9.1). Because of the
low concentrations of the neurochemicals, measurements are taken from
large volumes of interest (typically several cm
3)
, therefore giving MRS a
much lower spatial resolution than standard structural MRI (spatial reso-
lution typically of 1– 2mm
3
). Metabolites commonly measured include the
following (although it is often the ratio of dierent metabolites that is par-
ticularly useful):
•
N- acetyl- aspartate (NAA) is present in high concentration in neuronEs
and axons. Areas of neuronal or axonal loss show reduced levels
ofNAA.
•
Choline (Cho) is associated with turnover in cell membranes and i
cell division. Areas of demyelination and malignant tumours can cause
raised Cho. i
Cho can help distinguish recurrent tumours from post-
radiotherapy changes (this can be a dicult distinction to make using
standardMRI).
604
CHAPTER9 Neurology
604
• Creatine (Cr) (and phosphocreatine) is a marker of metabolism.
Reduced levels may indicate celldeath.
•
Myo- inositol (m- In) may be i in certain diseases such as Alzheimer’s
dementia.
•
Lactate (Lac) is typically present in concentrations too low to be
detected, except in areas of ischaemia, hypoxia, and certain tumours.
Further reading
Faro SH, Mohamed FB (eds.). Functional Neuroradiology. NewYork:Springer,2011.
Powell HW, Koepp MJ, Richardson MP, Symms MR, Thompson PJ, Duncan JS. The application of
functional MRI of memory in temporal lobe epilepsy:a clinical review. Epilepsia 2004; 45:855– 63.
Stieltjes B, Brunner RM, Fritzsche K, Laun F. Diusion Tensor Imaging: Introduction and Atlas.
Berlin:Springer,2013.
White PM, Teasdale EM, Wardlaw JM, Easton V. Intracranial aneurysm: CT angiography and MR
angiography for detection prospective blinded comparison in a large patient cohort. Radiology
2001; 219
:739– 49.
Myo-Ins
4.0 3.0
Frequency (ppm)
2.0
Cho
Cre
Glu
NAA
Fig.9.1 The upper panels show the position of the volume of interest; the lower
panel shows the MRS spectrum from that volume.

MAGNETIC RESONANCE IMAGING
605
606
CHAPTER9 Neurology
606
Nerve conduction studies
Please give your neurophysiologists as much information as possible about
your case and, if necessary, discuss it with them. They will then be in the
position to organize the most appropriate neurophysiological investigations.
In certain circumstances, you may need to specically ask for unusual inves-
tigations such as repetitive stimulation in suspected LEMS (E Repetitive
stimulation, p. 608).
Sensory nerve action potential and sensory conduction
velocity
Procedure
•
Orthodromic conduction velocity:electrically stimulates distal sensory
branches (e.g. index nger) and records the evoked sensory nerve action
potential (SNAP) proximally (e.g. over the median nerve at the wrist).
The distance between the two sites (D)and the latency (L)of the onset
of the SNAP determine the sensory conduction velocity (D/ L).
The SNAP amplitude is also useful.
•
Antidromic conduction velocity:supramaximal electrical stimulation
proximally; records distally (e.g. by a ring electrode on the little nger).
By varying the position of the stimulating electrode, the conduction
velocity in various portions of the nerve may be ascertained.
What does itmean?
•
d SNAP amplitude, or SNAP absence altogether, implies a lesion distal
to the dorsal root ganglion.
•
d velocity/ i latency (see Table 9.3). Motor velocities are more
commonly measured.
Motor conduction velocity
Procedure
Supramaximally stimulate a peripheral nerve trunk at a proximal (p) and
a more distal (d)site. Record the time to the onset of the evoked muscle
response (compound motor action potential (CMAP)) from each (Tp and
Td) and the distance between them (D). The motor conduction velocity
between p and d is therefore D/ (Tp−Td).
Table9.3 Typicalvalues
Latency Amplitude
Median nerve (index nger to wrist)
2– 3ms 9– 40mV
Ulnar nerve (little nger to wrist) 2– 2.6ms 6– 30mV
Sural nerve (mid- calf to below med. mall) 2– 4ms 5– 40mV
med. mall, medial malleolus.
NERVE CONDUCTION STUDIES
607
Typicalvalues
•
Median nerve in forearm (to abductor pollicis brevis) >48m/ s.
• Ulnar nerve in forearm (to abductor digiti minimi) >48m/ s.
• Common peroneal nerve (to extensor digitorum brevis) >40m/ s.
What does itmean?
(See Table9.4.)
Distal motor latency
• Latency from stimulation of most distal site on nerve toCMAP.
Typicalvalues
•
Median nerve (wrist to abductor pollicis brevis) <4.1m/ s.
• Ulnar nerve (wrist to abductor digiti minimi) <3.8m/ s.
• Radial nerve (spiral groove to brachioradialis)<5m/ s.
Note:these latencies include time taken for impulses to pass along the most
distal (unmyelinated) portion of the nerve and for transmission at the neu-
romuscular junction (therefore, they may not be used to calculate nerve
conduction velocities). Compare with velocities elsewhere in the nerve
being studied.
What does itmean?
Increased distal motor latency seenin
•
Conditions in which the very distal segment of a nerve is compromised
(most commonly carpal tunnel syndrome).
•
Early demyelinating neuropathy (e.g.GBS).
• Chronic demyelinating neuropathy.
Table9.4 Typical patterns
Conduction velocity AP amplitude AP dispersion
Axonal
neuropathy
Late stage:d distally
> proximally (loss of
fastest conducting
axons)
Late stage:d Not seen
Demyelinating
neuropathy
Marked slowing Greater
dispersion,
perhaps especially
in acquired,
not inherited,
demyelination
Ganglionopathies Slowing proportional
to loss of large bres;
often not marked
d proportional
to loss of large
bres; often not
marked
Not seen
Note:limbs should be warm; look for asymmetries. What are your laboratory’s current values?

608
CHAPTER9 Neurology
608
Compound motor action potential
The waveform, amplitude, and area under the curve of the CMAP reect
the number of depolarized muscle bres (e.g. reduced in axonal neuropathy
and denervated muscle) and the temporal dispersion of conduction veloci-
ties in the motor neurones to them (e.g. i in demyelinating neuropathy).
Late responses
Fwave
•
If a motor nerve is stimulated, there are orthodromically directed action
potentials (APs) that may cause a response in the muscle (CMAP).
However, antidromically directed APs will also pass proximally towards
the cell body. If these result in sucient depolarization of the axon
hillock, then a second orthodromic volley will pass down the nerve.
This may cause a second motor AP (the F wave). Therefore, the F
wave:(i)does not involve synapses (other than the neuromuscular
junction, of course) and (ii) depends on the integrity of the wholeaxon.
•
It may be dicult to elicit.
• Delay or absence of the F wave may reect a lesion proximal to the
site of stimulation, in parts of the nerve that may be inaccessible to
electrodes, e.g. brachial plexopathy or thoracic outlet syndrome. May
also be an early feature inGBS.
Hwave
•
This is ‘an electrical ankle jerk’:submaximal stimulation of the posterior
tibial nerve in the popliteal fossa causes trans- synaptic activation of the
soleus, recorded as aCMAP.
•
Amplitude may be d by aerent or eerent problems, e.g. neuropathy
or radiculopathy.
Repetitive stimulation
• Procedure:stimulate a motor nerve with 3– 5 supramaximal stimuli at
2– 4Hz, whilst recording evokedCMAPs.
• Normal response:no change in CMAP amplitude.
•
In MG:>10% decrement in CMAP amplitude after two stimuli.
•
InLEMS:
•
After voluntary contraction or after rapid stimulation (20– 50Hz) for
2– 10s, the CMAP amplitude, often initially small, i by 25% (sugges-
tive) or 100% (diagnostic).
•
At a slow (3Hz) rate of stimulation, there is a response decrement.

NERVE CONDUCTION STUDIES
609

610
CHAPTER9 Neurology
610
Electromyogram
Procedure
• Aconcentric needle electrode is usuallyused.
• It is inserted into the muscle to be studied.
• The dierence in potential between the inner part of the electrode
and the outer core is amplied and displayed on an oscilloscope or
computer screen.
•
It is also ‘displayed’ as an auditory signal, and experienced
electromyographers as much listen to as watch the pattern of electrical
activity.
Normal muscle is ‘silent’ (electrically inactive) at rest (there is no ‘spon-
taneous activity’), although there will be a brief burst of activity when the
electrode is rst inserted (the ‘insertional activity’).
The electrode can pick up electrical activity from muscle bres within
about 0.5mm of its tip; therefore, muscle bres from several motor units
(each innervated by a dierent motor neurone) in this volume can contrib-
ute to the signal. However, with care, potentials from a single motor unit
may be recorded when a co- operative subject tries to exert the muscle
a little (the ‘motor unit potential’). With i muscular eort, more muscle
bres are recruited, giving rise to the ‘interference pattern’.
Various nerve and muscle problems cause characteristic alterations tothese
four patterns ofactivity
1. Insertional.
2. Spontaneous.
3. Motor unit potential.
4. Recruitment.
5. In addition, certain other patterns may be observed in certain diseases
(in particular, myotonia).
1. Insertional activity
•
Usually there is a brief burst of potentials which lasts<1s.
• Insertional activity is normal in upper motor neurone (UMN) lesions
and most non- inammatory myopathies.
• It may be longer- lasting in lower motor neurone (LMN) lesions,
inammatory myopathies, and acid maltase deciency.
•
In myotonia, myotonic discharges occur (E Electromyogram, p. 611).
2. Spontaneous activity
•
Normal muscles at rest are silent.
• This is also the case in UMN lesions, non- inammatory myopathies
(unless 2° denervation has set in), and myotonia.
•
Fibrillation potentials and +ve sharp waves are seen in LMN lesions
and inammatory myopathies. They occur in regular bursts of constant
amplitude (unlike activity related to voluntary contraction).
•
Fibrillation potentials are spontaneous APs in irritable, acutely
denervated muscle bres. They are low- amplitude, brief −ve potentials.
• Positive sharp waves are brief +ve potentials, followed by a −ve wave.
Typically, they can be seen for 2– 3 weeks after denervation but may
persist.

ELECTROMYOGRAM
611
3. Motor unit potentials
•
If the electrode is positioned quite close to the bres of a motor unit
which is active during slight voluntary contraction, then a motor unit
potential (MUP) may be recorded. In normal muscle (and in UMN
lesions), this waveform is triphasic, 5– 10ms, and has an amplitude of
0.5– 1mV (larger muscles have larger motor units).
• In myopathies and muscular dystrophies, the motor units are smaller
and polyphasic. They tend to be briefer but, in some cases, last longer
thanusual.
•
In denervated and then reinnervated muscles (typically LMN lesions),
the size of individual motor units i (as the surviving motor neurones
‘take over’ the muscle bres previously innervated by the now absent
other motor neurones). MUPs therefore are of greater amplitude and
duration and are polyphasic.
•
In myotonia, myotonic discharges areseen.
Note:up to 15– 20% of MUPs in ‘normal’ muscle may be polyphasic.
4. Recruitment
•
Normally, as the strength of voluntary contraction i, i numbers
of motor units are recruited, and these units tend to be larger
(Heinneman’s size principle). The potentials due to these active units
overlap and become dicult, and nally impossible, to tell apart— a
full ‘interference pattern’, usually well below the maximum voluntary
contraction.
•
In muscle diseases, a full interference pattern may be produced, but it is
of low amplitude. In weak muscles, there may be ‘early recruitment’ (i.e.
recruitment of many motor units at low levels of voluntary contraction).
•
In denervated muscles, a full interference pattern may not be achieved,
because of the d number of motorunits.
•
In UNM lesions, there is a lower frequency of ‘normal’MUPs.
5. Myotonia
•
High- frequency repetitive discharges occurring after voluntary
movement or provoked by moving the electrode. The amplitude and
frequency wax and wane, giving the auditory signature likened to the
sound of a Second World War dive bomber (or a motorcycle).
•
Note:following the onset of a neuropathy, it may take at least 10–
14days for evidence of denervation to appear in the EMG. Therefore, a
repeat study after this time is often useful.
Single- bre electromyogram
A recording electrode with a smaller recording surface than usually used
samples a few muscle bres from a single motor unit (supplied by a single
motor neurone). The variability (‘jitter’) in the timing of APs from dierent
muscles should be <20– 25ms. Conduction block during voluntary contrac-
tion may also be shown. These techniques are used to investigate neuro-
muscular disorders and reinnervation in neuropathies.
Further reading
Blum AS, Rutkove SB (eds.). The Clinical Neurophysiology Primer. Totowa:Humana Press,2007.

612
CHAPTER9 Neurology
612
Electroencephalogram
The standard EEG is non- invasive. Electrodes are attached to the scalp with
collodion adhesive. Stable recordings may be made for days. Usually they
are arranged according to the international 10– 20 system. This is a method
for positioning electrodes over the scalp in an orderly and reproducible
fashion. Additional electrodes can be applied to the scalp, depending on the
region of interest.
Standard recording conditions
• Rest.
• Hyperventilation for 3– 5min can activate generalized epileptiform
changes (and precipitate absence seizures):
•
Can i frequency of focal discharge.
•
Can i slow- wave abnormalities.
• Photic stimulation (a strobe light at 30cm with a frequency of 1– 50Hz);
this can produce several patterns of activity:
•
Photoparoxysmal response— bilateral spike or spike and wave dis-
charges not time- locked to the visual stimulus, which may outlast the
visual stimulus by hundreds of milliseconds. Generalized, but may
have frontal or occipital predominance; commonly seen in idiopathic
generalized epilepsies; high- voltage occipital spikes, time- locked to
the stimulus; weakly associated with epilepsy.
•
Photomyogenic (photomyoclonic) responses— non- specic, mostly fron-
tal spikes due to muscle activity; associated with alcohol and some
other drug withdrawal states.
•
Sleep studies:
•
Subject either stays awake the night before the recording or is given
a small dose of choral prior to the recording (sometimesboth).
•
Subjects tend to show the earlier stages of non- REMsleep.
•
These studies i the yield of EEG abnormalities, including
epileptiformones.
•
By capture of ‘natural sleep’:certain seizure types are commoner in
sleep (e.g. juvenile myoclonic epilepsy (JME)).
•
Sleep deprivation itself i the number of seizures and epileptiform
changes.
E Polysomnography, p. 621.

ELECTROENCEPHALOGRAM
613

614
CHAPTER9 Neurology
614
Electroencephalogram:abnormalities
The normal electroencephalogram
There is a wide range of normal EEG phenomena. Some of the common
patterns in the awake adult are listed in Table9.5.
EEG abnormalities (not peri- ictal orictal)
• Avariety of EEG abnormalities may be seen outside the peri- or per-
seizure period.
•
Abnormalities in the EEG are not restricted to the appearance of
abnormal waveforms.
•
The loss, or redistribution in the scalp location, of normal background
activities is abnormal.
The classication ofEEG abnormalities is complex. Below is a highly
simpliedguide
1. General excess of slow waves— commonly seenin:
•
Metabolic encephalopathy.
•
Encephalitis.
•
Post- ictal states.
2. Focal slow waves— commonly seenin:
•
Large cerebral lesions (e.g. tumour, haematoma).
•
Post- ictal states.
•
Migraineurs.
3. Localized, intermittent, rhythmic slow waves— may be seen in
idiopathic generalized and localization- related epilepsies.
Table9.5 EEG rhythms
Activity Frequency
(Hz)
Amplitude
(mV)
Scalp
location
Behavioural
state
Alpha
8– 12 20– 60 Usually
occipital
Maximum relaxed,
awake, eyes closed
Beta >13
10– 20 Frontocentral Wakeful, drowsy;
REM and SWS 1 and 2
Theta
4– 8 Variable
diuse
Frontocentral,
temporal
Minimally awake,
drowsy, SWS
Delta <4 Variable Diuse Awake; drowsy
Mu
8– 10 20– 60 Central Awake, suppressed in
voluntary movements
SWS, slow- wave sleep.
The terms alpha, beta, theta, and delta are often used to describe the background activity but
are also used to describe the frequency of EEG activity.
Sharp activity may be a normal phenomenon.

ELECTROENCEPHALOGRAM:ABNORMALITIES
615
4. Epileptiform abnormalities:
•
Spikes (if last <80ms) or sharp waves (80– 200ms) may be associated
with slowwaves.
•
Consistently focal spikes suggest epilepsy with a focal seizureonset.
Note:2– 4% of non- epileptics have occasional spikes or sharps.
5. Repetitive stereotyped ‘periodic’ complexes.
EEG patterns may show periodicity. These patterns may be epileptiform or
not, and may be focal or generalized. They are an abnormal EEG feature,
the interpretation of which depends on the clinical context.
Examples include
•
Burst suppression:bursts of generalized high- voltage mixed waveforms,
alternating with generalized voltage suppression:
•
Coma.
•
Late- stage status epilepticus (both convulsive and non- convulsive).
• Triphasic waves over one or both temporal lobes:common in herpes
simplex encephalitis.
•
Periodic lateralized epileptiform discharges (PLEDs) are localized sharp
or slow- wave complexes 0.2– 1s long, every1– 5s:
•
Non- specic but suggest localized cerebral insult (stroke, haema-
toma, tumour).
•
Occasionally seen in migraine and focal epilepsies.
• BIPLEDs are bihemispheric PLEDs:suggest more widespread insults, e.g.
anoxia, encephalitis.
•
Bilateral or generalized high- voltage complexes for 0.5– 2s every 4–
15s:characteristic of subacute sclerosing panencephalitis (SSPE).
•
Triangularwaves:
•
Characteristic ofCJD.
•
Not seen in vCJD (may see a ‘disorganized’ EEG without repetitive
complexes).
•
Runs of broad triphasic waves (1.5– 3Hz):severe metabolic
encephalopathy (e.g. renal or hepatic failure).
•
Periodic spikes or sharp waves:bi- or multiphasic morphology (0.5– 2Hz);
usually generalized— suggest severe encephalopathy,e.g.
•
Herpes encephalitis.
•
CJD (in the setting of rapid dementia and myoclonus).
•
Lithium intoxication.
•
Post- anoxic brain injury.
•
Tricyclic antidepressant overdose.

616
CHAPTER9 Neurology
616
Electroencephalogram:inepilepsy
Idiopathic (genetic) generalized epilepsy(IGE)
• Generalized, bilaterally synchronous epileptiform discharges with
virtually normal background.
•
Absence epilepsy:3Hz spike andwave.
• JME:6Hz multiple spike andwave.
Symptomatic (secondary) generalized epilepsy
• More variable.
• Inter- ictal background activity:excessslow.
•
Inter- ictal epileptiform activity:irregular spikes or sharp and slow waves
1.5– 4Hz. Usually generalized, but may show asymmetry or (multi- ) focal
features.
Localization- related partial focal epilepsy
• Inter- ictal EEG is often normal, particularly if the focus is located deeply
(especially common with frontalfoci).
•
There may be lateralized or localized spikes or sharpwaves.

ELECTROENCEPHALOGRAM:IN EPILEPSY
617

618
CHAPTER9 Neurology
618
Electroencephalogram:howtouse
In suspected epilepsy
• Routine EEG with photic stimulation and hyperventilation gives about
up to a 50% detection rate for inter- ictal epileptiform abnormalities in
a subject with epilepsy (higher ‘yield’ in ° generalized epilepsies than in
localization- related epilepsies).
• Sleep- deprived or choral- induced sleep recording may i the yield of
EEG abnormalities to up to 60– 70%.
• Consider 24h or longer ambulatory EEG, ideally with audio/ video
monitoring. Most useful in helping to determine the nature of the
seizure in a subject with frequent (e.g. daily) attacks.
In general, avoid reduction in anti- epileptic drugs or drugs such as pentyl-
enetetrazole to induce seizures, except in exceptional circumstances, e.g.
videotelemetry as part of work- up for epilepsy surgery.
Note:
•
No inter- ictal spikes does not imply no epilepsy.
• Similarly, inter- ictal spikes do not always imply epilepsy.
• A−ve ictal EEG does not necessarily imply a non- epileptic (‘pseudo-
’) seizure, especially in simple partial and some brief complex partial
seizures (CPS). Scalp electrodes may fail to record deep, especially
frontal, activity.
•
However, a tonic– clonic seizure with loss of consciousness should be
associated with an epileptiform EEG during the ictus. This EEG activity
may be obscured by muscle artefact, but post- ictal slowing may beseen.
• The EEG may be slow after a tonic– clonic seizure for many tens of minutes.
Note:the diagnosis of epilepsy is mainly clinical! Remember that most epi-
sodes of altered consciousness are not epileptic in origin. In many cases,
cardiological investigations are appropriate. Have a low threshold for order-
ing a 12- lead ECG. Ambulatory ECG monitoring, particularly with cardiac
memo devices, and ambulatory BP monitoring can be very useful.
In established epilepsy
• Classication (e.g. CPS vs absence).
• Assessment of frequency of seizures (e.g. ambulatory EEG to assess
frequency of absence seizures).
•
Reduction in inter- ictal discharges in some syndromes (e.g. absence,
photosensitive epilepsy) correlates with anti- epileptic drug ecacy.
In focal cerebral dysfunction
Often not particularly helpful. Modern imaging studies usually provide more
information.
•
Small, deep, or slow- growing lesions often cause no eects.
• Asymmetric voltage attenuation may be caused by a subdural
haematoma (or other uid collection) overlying the cortex.
•
Direct grey matter involvement may cause alteration/ loss of normal
EEG or cause epileptiform discharges.
•
Subcortical white matter changes can cause localized polymorphic
slowwaves.
•
Deeper subcortical lesions tend to produce more widespread slow-
wave disturbances.

ELECTROENCEPHALOGRAM:HOWTOUSE
619
In central nervous system infections
• CJD and SSPE have relatively characteristic EEG associations.
• Meningitis and encephalitis cases may show diuse background
disturbances and polymorphic or bilateral intermittent slow wave
abnormalities.
•
Encephalitis usually causes more changes than meningitis.
• Focal changes may be seen over abscesses and in cases of herpes
simplex encephalitis.
In dementia
• To exclude some conditions such as toxic encephalopathy, non-
convulsive status epilepticus (NCSE).
•
Afew dementing conditions have characteristic EEGs (CJD,SSPE).
• Slowing of background frequency occurs in Alzheimer’s disease, but
values may overlap with those of the normal aged, therefore not very
helpful clinically.
In confusionalstates
• Helpful in diagnosing NCSE (absence and complex partial status).
• To exclude cerebral dysfunction.
• Not very useful in psychiatric diagnosis per se, but an abnormal EEG in a
confusional state may help exclude psychogenic causes for an apparent
reduction in level of consciousness.
In toxic metabolic encephalopathies
• EEG always abnormal.
• Diuse slowing in mildcases.
• Other abnormalities may develop in later stages.
• Specic patterns may be seen in certain aetiologies.
• Excess fast activity:barbiturate and benzodiazepine toxicity.
•
Triphasic waves:hepatic and renal failure, anoxia, hypoglycaemia,
hyperosmolality, lithium toxicity.
•
Periodic spikes or sharp waves:anoxia, renal failure, lithium and tricyclic
antidepressant toxicity.
Incoma
• EEG, especially serial EEGs, provides an indication of the degree of
cerebral dysfunction.
•
In general, any ‘normal’- looking EEG, spontaneous variability, sleep–
wake changes, and reaction to external stimuli are relatively good
prognosticsigns.
•
An invariant, unreactive EEG is a poor prognostic sign; the pattern,
however, is not uniform; it may include periodic spikes of sharp waves,
episodic voltage attenuation, alpha coma, and burst suppression.
•
May give some diagnostic clues, e.g. localized abnormality—
supratentorial mass lesion; persistent epileptiform discharges— status
epilepticus.
•
‘Alpha coma’:monotonous unresponsive alpha with anterior distribution
seen after a cardio/ respiratory arrest is a poor prognostic feature.
• Monotonous, but partially reactive, alpha may follow brainstem infarcts.

620
CHAPTER9 Neurology
620
Electroencephalogram:invasive
techniques
These are generally restricted to specialist centres, most commonly used in
the pre- surgical work- up of patients.
Foramen ovale electrodes, corticography (usually done by laying strips
of electrodes on the surface of the brain), and depth EEG (electrodes
implanted into the parenchyma of the brain) may be used, depending on
the region of interest. Sphenoidal electrodes are rarely used today but can
give useful EEG information about the medial temporal structures.
Further reading
Blum AS, Rutkove SB (eds.). The Clinical Neurophysiology Primer. Totowa:Humana Press,2007.
National Institute for Health and Care Excellence (2012). Epilepsies: diagnosis and management.
Clinical guideline CG137. M
https:// www.nice.org.uk/ guidance/ cg137.

POLYSOMNOGRAPHY
621
Polysomnography
• This is a multimodal recording used in the analysis of sleep- related
disorders.
•
There is concurrent recording of EMG, EEG, and electro- oculography
(EOG— eye movements), often with audiovisual channels. Other
physiological parameters may also be recorded, e.g. nasal airow, chest
expansion.
Sleep is divided into three stages (N1– N3) of progressively ‘deeper’ non-
REM (NREM) sleep and a stage of REM sleep (see Table 9.6), characterized
physiologically by bursts of rapid eye movements (saccades). NREM sleep
was previously divided into four stages:1 (now N1), 2 (now N2), and 3
and 4 (nowN3).
Multiple sleep latencytest
This is a diagnostic test for narcolepsy. Following a good night’s sleep, nor-
mal subjects typically enter REM sleep with a latency of >>10min (usually
790min). In narcolepsy, the latency is <10min.
Further reading
Abad VC, Guilleminault C. Polysomnographic evaluation of sleep disorders. In:MJ Amino (ed.).
Amino ’s Electrodiagnosis in Clinical Neurology, 6th edn. Oxford:Elsevier Saunders, 2012; p.727.
Table9.6 Sleepstages
Stage Behaviour Main EEG pattern Comments
N1 Drowsy Diuse alpha and theta Diminished EMG; rolling
eye movement
N2 Light sleep
High theta; K
complexes; sleep
spindles
Further diminished EMG;
may have rolling eye
movements
N3 Deep sleep Delta Diminished EMG; no eye
movements
REM No body
movements,
but vivid
dreams
Low amplitude, mixed
frequency
Muscular atonia; rapid eye
movements
REM:rapid eye movements.
There is progression through stages N1- N3, and several episodes of REM during a typical
night’s sleep. Polysomnography can be important in understanding the pathophysiology of the
insomnias, parasomnias and other sleep patterns.

622
CHAPTER9 Neurology
622
Sensory evoked potentials or responses
Whilst many techniques and protocols have been developed in research
laboratories, there are only a few techniques in widespread clinical use.
Astimulus is delivered to the periphery, thus activating a sensory system
and evoking an electrical response over a more central, often cortical, area.
Multiple surface electrode recordings time- locked to the peripheral stimulus
are recorded and averaged, to help eliminate ongoing random background
‘noise’ from the sensory stimulus- evoked ‘signal’. Deviations of this evoked
potential (EP) or response (ER) from the norm (especially in latency and
waveform) suggest pathology in the sensory pathway tested.
Visual evoked potentials
Pattern- evoked visual evoked potentials
An alternating chequerboard pattern (temporal frequency 1– 2Hz) is pre-
sented to each eye individually (see Fig. 9.2). The EP is recorded over the
occipital (° visual) cortex. Most commonly, the rst large +ve wave, called
P1 or P100 (as it typically occurs at about 100ms), is studied.
A delayed, smaller, or dispersed VEP indicates disease in the retino-
geniculo- striate pathway (if severe refractive errors or cataracts have been
excluded), but most commonly aecting the optic nerve (a uniocular decit
implies a lesion anterior to the optic chiasm) or at the chiasm.
Flash- evoked visual evoked potentials
In subjects with very poor vision or xation and in the very young, a bright
ash may be used as the stimulus. This gives less reproducible results, par-
ticularly in the P100 latency.
Commonuses
The VEP is used in general to document intrinsic, inammatory, or com-
pressive lesions of the optic nerve (or chiasm).
1. Suspected optic or retrobulbar neuritis.
2. In a patient with suspected MS, evidence of a VEP abnormality in an
asymptomatic eye would suggest a previous episode of optic neuritis.
3. Evaluation of hysterical blindness (may need to use a strobe light
stimulus if patient non- co- operative).
4. Evaluation of optic nerve function in compressive lesions such as
dysthyroid eye disease, optic nerve glioma.
5. Follow- up after surgery to decompress the optic nerve or chiasm.
6. Assessment of poor visual acuity in patients unable to co- operate with
usual testing. Vary the size of the chequerboard squares; subjects with
poor acuity will only have a VEP to the coarser patterns.
Somatosensory evoked potentials
• Stimulation site over a peripheral nerve, e.g. ulnar or median at the
wrist, common peroneal at the knee, posterior tibial at the ankle (see
Fig.9.3).
•
Record over Erb’s point (above the medial end of the clavicle), C7 or
C2 vertebra, or the parietal cortex for arm stimulation; L1, C7, C2, or
vertex for leg stimulation.
•
Calculate absolute and interpeak latencies.
SENSORY EVOKED POTENTIALS OR RESPONSES
623
• Need to show with NCS that distal parts of the somatosensory
pathways are conducting normally.
•
Assesses the dorsal column, not anterolateral (spinothalamic) tract
pathways:
•
For example, stimulate the median nerve at the wrist; prolonged
latency to Erb’s point suggests a brachial plexus (or more distal)
lesion.
•
Prolonged Erb’s point to C2 latency suggests a spinal cord lesion.
Uses
•
Diagnosis of plexopathies.
• Evaluation of subclinical myelopathy in possibleMS.
• Evaluation of hysterical sensoryloss.
• Per- operative monitoring (e.g. during scoliosis surgery).
Right ey
e
Left eye
Left eye
Right ey
e
3mV divisions
30 60 90 120 150 180 210 240 (ms)
M1
N2
P1
N1
M2
P1
N1
M2
P1
N1
M2
P1
Fig.9.2 Visual evoked potential (to chequerboard stimulus).
624
CHAPTER9 Neurology
624
Brainstem auditory evoked potentials (BAEPs, BAERs,
BSAEPs)
(See Fig.9.4.)
•
Stimulus:rarefaction clicks of 50 or 100ms duration, presented mono-
aurally at 10Hz at 60– 70dB above threshold (masking the noise to the
otherear).
•
Record over the mastoid and vertex of theskull.
• Classic waveform has seven peaks, said to be generated by sequential
auditory nuclei:
1
3
2
1
3
1
3
2
1
3
2
2
Fig.9.3 Leg somatosensory potentials.
SENSORY EVOKED POTENTIALS OR RESPONSES
625
•
I:VIIIth nerve (must be present to interpret subsequent waves).
•
II:cochlear nucleus (may be absent in normals).
•
III:superiorolive.
•
IV:lateral lemniscus (may be absent in normals).
•
V:inferior colliculus (should be 50% or more of wave I’s amplitude).
•
VI:medial geniculate (too variable for regular clinicaluse).
•
VII:auditory thalamocortical radiation (too variable for regular
clinicaluse).
Latency I– V (central conduction time) should be no more than 4.75ms.
The dierence between left and right central conduction times should be
<0.4ms.
I
II
III
IV
V
RIGHT EAR
RIGHT EAR
LEFT EAR
LEFT EAR
I
II
III
IV
V
I
II
III
IV
V
I
II
III
IV
V
Fig.9.4 Brainstem auditory evoked potentials.

626
CHAPTER9 Neurology
626
Uses
• Hearing assessment, especially in children.
• Evaluation in suspected MS and other myelinopathies (e.g.
adrenoleukodystrophy; MRI more importantnow).
•
Evaluation and detection of posterior fossa lesions (e.g. acoustic
neuromas; MRI more importantnow).
•
Evaluation of brainstem function (e.g. tumour,CVAs).
• Evaluation of brainstem function in coma and braindeath.
• Per- operative, e.g. acoustic neuroma excision.
Use ofevoked potentials inthe diagnosis ofmultiple
sclerosis
Traditionally, trimodal EPs (VEPs, SSEPs, and BAEPs) have been requested
to look for evidence of a disturbed conduction in multiple sensory systems.
Modern practice, however, is to request only VEPs, if any at all. MRI is much
more useful in demonstrated dissemination of CNS lesions.
5
Further reading
Blum AS, Rutkove SB (eds.). The clinical neurophysiology primer. Totowa:Humana Press,2007.
5 Polman CH, Reingold SC, Edan G, etal. Diagnostic criteria for multiple sclerosis:revisions to the
‘McDonald criteria’. Ann Neurol 2005; 58
:840– 6.

SENSORY EVOKED POTENTIALS OR RESPONSES
627

628
CHAPTER9 Neurology
628
Transcranial magnetic stimulation
• Brief, high- current pulse produced in a circular or gure- of- eight- shaped
coil held over thescalp.
•
This induces a magnetic eld with ux perpendicular to thecoil.
• This, in turn, produces an electric eld perpendicular to the
magneticeld.
The result is excitation or inhibition of the subjacent cortex (depending on
stimulus parameters).
•
Transcranial magnetic stimulation (TMS) has been used for diagnostic
purposes in a number of ways, although as yet it is not in widespread
clinicaluse.
Measurement ofcentral motor conductiontime
• TMS over the motor cortex indirectly (presumably via synaptic
activation of corticospinal neurones) causes a volley of activity in the
corticospinal tracts. The latency of the EMG in, say, the abductor digiti
minimi may be measured.
•
May be used in cervical myelopathy and MS to show i latency of EMG
in hand muscles evoked by TMS over the motor cortex. If the EMG
latency to more distal stimulation (e.g. at C7 over the spinal cord and
in the ulnar nerve) is normal, then an i central motor conduction time
may be inferred.
•
Latency may also be i in other neurodegenerative conditions.
Motor evoked potentials
Abnormalities in the amplitude of motor evoked potentials (MEP) may
reect abnormalities anywhere in the pathway from the motor cortex to
the muscles.
Silentperiod
• If a subject maintains muscle contraction and a single suprathreshold
TMS pulse is applied to the contralateral motor cortex, ongoing EMG
activity ceases for a few hundred milliseconds after the MEP (the ‘silent
period’).
•
Silent period may be long in, e.g. stroke, MS, spinal cord injury.
• Silent period may be short in, e.g. MND,PD.
Interhemispheric conduction
• Interhemispheric inhibition may be d in MS orMND.
•
It may be absent following lesions to the corpus callosum.
Motor cortex excitability
• High thresholds may be seen in strokeorMS.
• Low thresholds and i intracortical inhibition may be seen inMND.
•
d intracortical inhibition may be seeninPD.

TRANSCRANIAL MAGNETIC STIMULATION
629
Psychogenic limb weakness
Some authorities have used TMS to evoke muscle activity in ‘paralysed’
limbs in patients with psychogenic paralysis. This needs to be done in the
context of a ‘holistic’ approach to the patient, aimed at dealing with any
psychological pathology.
Potential clinical applications
There have been many TMS studies, some that may prove useful as clinical
tests,e.g.
•
Determination of lateralization of language function by repetitive TMS
(rTMS) prior to surgery for epilepsy.
•
Assessment of cortical excitability in certain epilepsy syndromes.
• Assessment of d intracortical inhibition in dystonia.
•
Assessment of recovery from stroke.
6
Further reading
Currà A, Modugno N, Inghilleri M, Manfredi M, Hallett M, Berardelli A. Transcranial magnetic stimula-
tion techniques in clinical investigation. Neurology 2002; 59
:1851– 9.
Kobayashi M, Pascuel- Leone A. Transcranial magnetic stimulation in neurology. Lancet Neurol 2003;
2
:145– 56.
6 Devlin JT, Watkins KE. Stimulating language:insights from TMS. Brain 2007; 130:610– 22.

630
CHAPTER9 Neurology
630
Neurological investigation
ofsphincter disturbance
Electromyogram
• Of pelvic oor muscles may be helpful in faecal incontinence, stress
urinary incontinence, and cauda equina syndrome.
•
Pelvic oor and sphincter muscle EMGs may reect pudendal nerve
damage.
•
Anal sphincter EMG abnormalities may reect damage to Onuf ’s
nucleus, e.g. in multi- system atrophy. It is characteristically unaected
inMND.
Magnetic resonance imaging
• In suspected sacral spinal cord, conus medullaris, and equina lesions.
Urodynamics
Flowmetry
•
Measurement of rate and amount of urine ow overtime.
• Allows calculation of parameters such as time to maximal ow,
maximum and mean ow rate, and volume voided.
•
Post- micturition US can determine residual volume.
Cystometry (needs urinary catheterization)
•
Measurement of intravesicular pressure during lling (usually at 50mL/
min) or emptying. Typically, bladder lling sensation starts at about
100mL and the bladder is full at 400– 600mL (with no more than
15cmH
2
O rise in pressure). Detrusor instability may cause sharp rises in
pressure during lling.
•
During voiding, ow rate should be >15mL/ min (♂) or >20mL/ min
(♀) with pressures of <50cmH
2
O (♂) or 30cmH
2
O(♀).
Further reading
Fowler CJ. Investigational techniques. Eur Urol 1998; 34(Suppl):10– 12.
Panicker JN, Fowler CJ. The bare essentials:uro- neurology. Pract Neurol 2010; 10:178– 85.

AMOBARBITAL (WADA) TEST
631
Edrophonium (Tensilon
®
)test
Procedure
• Explain the test to the patient.
• Select weak and/ or fatiguable muscles to be assessed.
• Attach an ECG monitor.
• Draw up 0.6mg of atropine (for use if extreme bradycardia develops),
10mg of edrophonium in 5mL of normal saline (A), 5mL of normal
saline (B), and salineush.
•
Administer 1mL of the test solution (A or B, ideally patient and
administrating physician should be blinded to the nature of the solution).
•
If no adverse reaction, administer the remaining4mL.
• Repeat with the other solution (BorA).
Note: if the diagnosis of MG is clinically obvious, and the patient has
responded to pyridostigmine given empirically, there is little point in stop-
ping this and performing an edrophoniumtest.
Interpretation
• In MG, there should be a response within 30– 60s, which should wear
o in 2– 4min.
• There may be a response in LEMS, polymyositis, andMND.
Amobarbital (Wada)test
Amobarbital is injected into the right or left internal carotid artery. It is a
short- acting barbiturate that temporarily causes hemispheric dysfunction
on the injected side. If injected into the left in most right- handers, the abil-
ity to speak and continue to hold up the right arm is temporally impaired.
If speech is preserved following right- sided injection, it suggests normal
left lateralization for language function. More complex testing may also be
undertaken during the period of hemispheric dysfunction, but it is usually
used to determine language dominance prior to certain neurosurgical pro-
cedures. Increasingly, non- invasive techniques, such as fMRI, are being used
in place of the Wadatest.
Further reading
Wagner K, Hader C, Metternich B, Buschmann F, Schwarzwald R, Schulze- Bonhage A. Who needs
a Wada test? Present clinical indications for amobarbital procedures. J Neurol Neurosurg Psychiatry
2012; 83
:503– 9.

632
CHAPTER9 Neurology
632
Biopsies
• Always liaise with those taking the biopsy and those processingit!
• Abiopsy should be undertaken to answer specic questions, in light of
a dierential diagnosis formulated following history, examination, and
other investigations.
Skeletalmuscle
Indications
•
° muscle disease, e.g. metabolic myopathy, polymyositis, muscular
dystrophy.
•
Neurogenic atrophy.
• Mitochondrial cytopathies (even in the absence of clinical muscle
involvement).
•
Multi- organ disease, e.g. vasculitides.
Which muscle tobiopsy?
•
An involved, but not end- stage, muscle.
• One that has not been used for EMG recording or had an injection for
>1month.
•
Quadriceps and deltoid oftenused.
Open or needle biopsy?
Openbiopsy
•
Larger specimen.
• Can x specimen at in situ length.
•
Especially for inammatory myopathy and in vasculitis.
Needlebiopsy
•
Smallerscar.
• Multiple biopsies possible.
• However:
•
Smaller biopsies.
•
Diculties in orientating the sample.
What may be done tothe tissue?
•
Routine histology.
• Examination of small blood vessels.
• Histochemistry.
• EM.
• Tests of muscle metabolism.
• Mitochondrial DNA studies.
Nerve
Indications
•
Distinction between segmental demyelination and axonal degeneration
(if not already determined).
•
Certain neuropathies with characteristic histological features, e.g. due to
amyloid deposition, sarcoid, vasculitis, neoplastic involvement.
•
Certain myelinopathies (e.g. leukodystrophies) with peripheral nervous
system (PNS) and CNS involvement.

BIOPSIES
633
Which nerve tobiopsy?
•
The cutaneous branch of the sural nerve at the ankle (usually).
• Supercial peroneal (sometimes).
• Supercial radial (occasionally).
• Occasionally, small motor nerve twigs are obtained in muscle biopsy.
• Overlying skin may be co- biopsied.
What isdone?
•
2– 3cm of full- thickness nerve or fascicle.
What may be done tothe tissue?
•
Routine light microscopy (morphometry, structural survey; amyloidosis).
• Frozen section light microscopy (immunochemistry).
• EM (ultrastructure).
• Teased out single bres (to examine sequential myelin internodes).
Brain/ meningealbiopsy
Indications
•
Diagnosis and management of suspected ° and some metastatic brain
tumours.
•
Dierential diagnosis of other mass lesions (inammatory and infective).
• Dierentiation of radiation necrosis and tumour regrowth.
• Dierentiation of neoplastic and non- neoplastic cysts (and their
drainage).
•
Diagnostic biopsy of a suspected infectious lesion that has not
responded to a trial of therapy.
•
Diagnosis of cerebral vasculitis or vasculopathy.
What isdone?
•
High- quality cranial CT/ MRI, possibly with contrast, to delineate the
lesion.
•
If no discrete lesion, generally an area of non- dominant, non- eloquent
cerebrum istaken.
•
Stereotactic needle biopsy with image guidance:
•
Deep, small lesions in ‘eloquent’areas.
•
Multiple biopsies along the needle track (useful in heterogeneous
lesions such as some gliomas).
•
And/ or open biopsy:
•
Accessible lesions.
•
When resection considered during procedure.
• Intra- operative evaluation of frozen samples:
•
For example, can a biopsy bemade?
•
For example, is the sample adequate?
Note:caution in suspectedCJD!!
Skin
• Some storage diseases:
•
Laforabody.
•
Batten’s disease.
• Mitochondrial cytopathies.

634
CHAPTER9 Neurology
634
Bonemarrow
• Niemann– Pick typeC.
• Haematological and other malignancies.
Rectal and appendicectomy
• Most neuronal storage diseases aect the autonomic nervous system, so
evidence can be sought in neurones of the gut’s intrinsicplexi.
•
Amyloid in rectal biopsy.
Tonsillarbiopsy
• Research tool invCJD.

OLIGOCLONALBANDS
635
Oligoclonalbands
• Electrophoresis of serum and CSF separates protein components by
size and charge.
•
OCBs may be present in serum and CSF. Bands in the CSF not seen in
the serum suggest intrathecal- specic synthesis ofIgs.
• This pattern is seen in most (95%) cases of established MS but may also
occur in other conditions such as chronic meningitis, neurosyphilis, SSPE,
and neurosarcoid (although uncommonly).
Further reading
Thompson EJ. Cerebrospinal uid. In:RAC Hughes (ed.). Neurological Investigations. London:BMJ
Publications, 1997; pp.443– 66.

636
CHAPTER9 Neurology
636
Diagnostic and prognostic antibodies,
and other markers inblood andurine
Multi- system disorders
PNS and CNS are aected in many multi- system disorders; markers in blood
and other uids and tissues for these are therefore commonly requested in
neurology patients.
Vasculitides,e.g.
•
ENAs inSLE.
• ANCA in Wegener’s.
• RFinRA.
Enteropathies,e.g.
•
Gliadin and endomysial antibodies in coeliac disease.
Systemic infections,e.g.
•
Serology for many diseases, e.g. Borrelia in Lyme disease,HIV.
• PCR forTB.
Disorders ofcoagulation:thrombophilia screen currently commonly includes
•
Protein S and C levels.
• Antithrombin III levels.
• Screening for the Leiden mutation in factorV.
• Lupus anticoagulant.
• Tumour markers,e.g.
•
carcinoembryonic antigen (CEA) for gut neoplasia.
•
Serum and urinary paraproteins in haematological disorders like
myeloma.
Sarcoid
•
ACE and ACE genotype.
Endocrinopathies,e.g.
•
TSH, FT4, and FT3, thyroid autoantibodies in thyroid dysfunction.
Other metabolic disorders,e.g.
•
Wilson’s disease:blood copper and caeruloplasmin; some authorities
also request 24h urinary copper excretion. Note:slit lamp examination
performed by an experienced ophthalmologist reveals Kayser– Fleischer
rings in most cases of Wilson’s disease with neurological involvement.
•
Phaeochromocytoma:catecholamine metabolites in three 24h urine
collections.
Disease- specic markers
• MG:anti- ACh receptor and muscle- specic kinase (MuSK) antibodies.
• Neuromyelitis optica:aquaporin 4 and MOG antibodies

DIAGNOSTIC & PROGNOSTIC ANTIBODIES
637
Paraneoplastic antibodies
Certain neurological syndromes are ‘paraneoplastic’, i.e. due to remote,
but non- metastatic, eects of non- nervous system cancers. These paraneo-
plastic syndromes are rare, but important to recognize. In perhaps 50% of
cases, the neurological symptoms may predate those of the cancer. This is
an area of intensive research. Antibody tests that are well described include
those in Table9.7.
Most of these paraneoplastic antibodies target intracellular antigens and
are not thought to be pathogenic in themselves. Increasingly, autoantibod-
ies to antigens on the surface of neurones or glia are being recognized and
associated with clinical syndromes (see Table 9.8). Such antibodies may
be directly pathogenic. Recognizing clinical syndromes associated with
these cell surface- directed antibodies is important, as many respond to
immunotherapies.
Table9.7 Paraneoplastic antibodies
Antibody Paraneoplastic syndrome Associated tumour
Anti- Hu (ANNA1) Encephalomyelitis
Sensory neuronopathy
Cerebellar degeneration
Chronic GI pseudo- obstruction
Limbic encephalitis
Small- cell lung cancer
(SCLC)
Anti- Yo (PCA1) Cerebellar degeneration Breast, ovary
Anti- Ri (ANNA2) Brainstem encephalitis Breast, SCLC
CV2 (CRMP5) Encephalomyelitis
Chorea
Sensory neuronopathy
Sensorimotor neuropathy
Cerebellar degeneration
Limbic encephalitis
Chronic GI pseudo- obstruction
Thymoma, SCLC
Anti- Ma2 (Ta) Limbic/ diencephalic encephalitis
Cerebellar degeneration
Brainstem encephalitis
Testis, lung
Anti- amphiphysin Sti person syndrome
Other syndromes
Breast, SCLC
CAR Retinopathy Breast, SCLC
Tr Cerebellar ataxia
Hodgkin’s

638
CHAPTER9 Neurology
638
Further reading
Graus F, Dalmau J. Paraneoplastic neurological syndromes. Curr Opin Neurol 2012; 25:795– 801.
Graus F, Delattre JY, Antoine JC, etal. Recommended diagnostic criteria for paraneoplastic neuro-
logical syndromes. J Neurol Neurosurg Psychiat 2004; 75
:1135– 40.
Honnorat J, Antoine J- C. Paraneoplastic neurological syndromes. Orphanet J Rare Dis 2007;2:22.
Zuliani L, Graus F, Giometto B, Bien C, Vincent A. Central nervous system neuronal surface antibody
associated syndromes:review and guidelines for recognition. J Neurol Neurosurg Psychiatry 2012;
83
:638– 45.
Table9.8 Neuronal surface antibody antibodies
Antibody Syndrome Associated tumour
NMDAR Encephalitis; dyskinesia;
psychiatric presentation;
epilepsy
Ovarian teratoma
LGI1 Limbic encephalitis Rare
CASPR2 Limbic encephalitis
Morvan’s syndrome
Lung, breast, thymus
GABA
B
R Limbic encephalomyelitis
Prominent seizures
SCLC
mGluR5 Limbic encephalitis
Orphelia syndrome
Hodgkin’s lymphoma
GlyR Progressive encephalomyelitis
with rigidity and myoclonus
Thymoma
VGCC Cerebellar ataxia SCLC
mGluR1 Cerebellar ataxia
Hodgkin’s lymphoma

DIAGNOSTIC & PROGNOSTIC ANTIBODIES
639

640
CHAPTER9 Neurology
640
Biochemical markers
Many ‘inborn errors of metabolism’ cause neurological disease. Avariety of
investigations, including tests on blood, urine, and CSF, biopsies, and genetic
analyses, are used in their diagnosis (E Diagnostic and prognostic antibod-
ies, and other markers in blood and urine, pp. 636–8; E Genetic tests,
pp. 642–5; for an accessible review, see Gray etal.).
7
Biochemicaltests
Some basic principles
Many autosomal recessive and X- linked metabolic diseases are caused by
reduced or absent activity of a specic enzyme, in turn due to a single gene
defect.
In some, there is a tissue- specic decit
For example, McArdle’s (glycogen storage disease V) demonstrates the
absence of phosphorylase activity in muscle biopsy (as only the myophos-
phorylase isozyme is aected).
In other conditions, notably thelipidoses, theenzyme is decient inmany
tissues
For example, in Niemann– Pick diseases Aand B, sphingomyelinase is de-
cient in the brain and spinal cord, but also in the GIT, liver, spleen, and BM.
Abnormal lipid metabolism can therefore be demonstrated in relatively eas-
ily accessible tissue such as broblasts.
Not only may the absence or lower activity of an enzyme reduce the
amount of product of the reaction it catalyses, but it may also lead to the
accumulation of precursors in the metabolic pathway:
A—(1) l B—(2) l C—(3) l D
If enzyme (3)is reduced, A, B, and C may accumulate, withlower levels ofD
than usual being produced
•
For example, in acute intermittent porphyria, there is i urinary
excretion of d heme aminolevulinic acid and porphobilinogen
(intermediates in the haem synthetic pathway) during an acute attack.
•
d levels of porphobilinogen deaminase may be demonstrated in
erythrocytes, leucocytes, and cultured broblasts.
7 Gray RG, Preece MA, Green SH, Whitehouse W, Winer J, Green A. Inborn errors of metabolism
as a cause of neurological disease in adults:an approach to investigation. J Neurol Neurosurg Psychiat
2000; 69
:5– 12.

BIOCHEMICAL MARKERS
641
Ischaemic forearm exercise test (ischaemic lactatetest)
Procedure
1. Rest the patient supine for30min.
2. Draw a baseline lactate sample from a catheter in an antecubitalvein.
3. Inate the sphygmomanometer cu on that arm to above arterial
pressure.
4. Subject squeezes a rubber ball in that hand until exhaustion.
5. Rapidly deate thecu.
6. Take further venous samples at 30, 60, and240s.
Results
Normally the venous lactate will rise by 2- , 3- , or even 4- fold; if it fails to rise
by 1.5- fold, then there is likely to be a glycogenolysis or glycolysis defect (or
the patient has not exercised suciently!).
7

642
CHAPTER9 Neurology
642
Genetictests
The list of diseases for which we have specic genetic tests grows each
month. Rather than give a necessarily incomplete compendium, we discuss
some general principles.
Several important neurological conditions may today be diagnosed by
(relatively) simple genetic tests, whereas in the past biopsy was necessary.
For example, Duchenne, Becker, and oculopharyngeal muscular dystro-
phies are associated with well- dened genetic abnormalities. Similarly, sev-
eral mitochondrial cytopathies (such as myoclonic epilepsy and ragged red
bres (MERRF) and mitochondrial myopathy, lactic acidosis, and stroke- like
episodes (MELAS)) may now often be diagnosed by nding common muta-
tions or deletions in mitochondrialDNA.
When might a neurologist refer toa clinical geneticist?
• Genetic counselling of an index patient and his family.
• Cytogenetic or molecular diagnosis.
• Long- term follow- up of family:
•
Notication of advances.
•
Counselling family members as they becomeadult.
•
Coordinating care with paediatric and adult neurologists.
Cytogenetics:whentodoit
• ♀ with an X- linked disorder.
• Unexplained developmentaldelay.
• Unexplained major CNS malformation.
• The coexistence of two genetic diseases in a patient.
What isdone?
•
Conventional karyotype.
• FISH for suspected submicroscopic chromosomal aberrations, e.g. a
p13.3 deletion may cause lissencephaly.
Molecular genetics:whentodoit
• Conrming a clinical diagnosis.
• Identify carriers in the family.
What isdone?
An ever i range of diseases may be tested for. Some of these tests may
be routinely available at your local clinical genetics laboratory, others at
regional, national, or even supranational centres. Other tests may be avail-
able on a ‘research’ basis. It is clear, however, that tests for genetic ‘lesions’
or risk factors will become increasingly available. Rather than give an, at
best, partial list of readily available tests, we give a few examples below of
the kinds of tests that are available. The astute reader will spot that dierent
mutations within a given gene can give rise to dierent clinical phenotypes.
Indeed, recent work has shown that the same mutation in some genes can
give rise to >1 phenotype— we clearly have a great deal yet to learn about
the genetics of neurological diseases!

GENETICTESTS
643
Detection ofdeletions
•
For example, in mitochondrial (mt)DNA in MELAS andMERRF.
• For example, of dystrophin gene in Duchenne and Becker muscular
dystrophies.
Detection ofDNA rearrangement
•
For example, PMP22 gene duplication in some cases of Charcot– Marie–
Tooth disease type 1 (or hereditary motor sensory neuropathy type 1
(HMSN1); deletions within this gene cause hereditary neuropathy with
liability to pressure palsies (HNPP).
Detection oftrinucleotide repeats
•
Found in >10 neurological diseases (see Table9.9).
• So far, there is no overlap in the number of repeats in controls and
aected patients (except rarely in Huntington’s, in the region of 33– 36
repeats).
•
Anticipation (more severe phenotype and earlier onset) often reects
in i number of repeats in the most recent generations (especially
myotonic dystrophy).
Detection ofsingle base mutations
This involves fragmenting the DNA of the gene into manageable pieces,
then amplifying these so that there are multiple copies. Subsequently, vari-
ous methods may be used to detect fragments with abnormal sequences,
even if only diering at a single base from the ‘wild- type’. There are sev-
eral such techniques, constantly being rened, and many are restricted to
research laboratories.
Table9.9 Some trinucleotide repeat diseases
Disease Gene Triplet repeats Transmission
Fragile X FMR1 CGG
X- linked
Myotonic dystrophy DM CTG AD
Friedreich’s ataxia FRDA GAA AR
Spinobulbar muscular
atrophy
Androgen
receptor
CAG
X- linked
Huntington’s disease IT15 CAG AD
Spinocerebellar atrophy
•
SCA1
• SCA2
• SCA3
• SCA6
• SCA 7
SCA1
SCA2
SCA3
SCA6
SCA 7
CAG
CAG
CAG
CAG
CAG
AD
AD
AD
AD
AD
Dentarubropallidoluysian
atrophy
DRPLA CAG AD
AD, autosomal dominant; AR, autosomal recessive.
Note:SCA 6 is a CAG triplet expansion in the CACNL1A4
calcium channel gene. Other (non-
triplet repeat) mutations in the gene cause other conditions— episodic ataxia type 2 and familial
hemiplegic migraine.

644
CHAPTER9 Neurology
644
• However, molecular genetics is proceeding at a tremendous pace, both
in terms of the number of conditions with identied genetic lesions and
the laboratory techniques for analysis.
•
High- speed DNA sequencing will facilitate sequencing large pieces
ofDNA.
•
For example, point mutations in the MPZ gene, which encodes P0, a
component of the myelin sheath, have been found in some families with
Charcot– Marie– Tooth disease type1B.
Genetic risk factors
Another area of clinical genetics which is likely to become more important
is the detection of genetic ‘risk factors’ for diseases. Certain allelic variants,
whilst not ‘causing’ a disease in the traditional sense, may predispose an
individual to exhibiting a certain clinical phenotype or alter the age at which
it might become apparent.
For example, there are three allelic variants in the apolipoprotein E
(APOE4
) gene e2, e3, and e4. Homozygosity for e4 is likely to be a risk
factor for developing Alzheimer’s disease and for developing it at an earlier
age. However, the majority of e4 homozygotes do not develop the condi-
tion (therefore, it is not ‘causative’).
Detection ofthe presence ofabnormal protein or altered
levels ofnormal protein
Immunocytochemistry and immunoblotting (western blots) on tissue sam-
ples from the patient allow direct visualization of the presence of abnormal
protein or the absence or reduced levels of normal protein, in a variety of
conditions. (These techniques are not genetic in the strictest sense but are
often useful in ‘genetic’ conditions.)
For example, Duchenne and Becker muscular dystrophies have absent or
reduced levels of dystrophin in muscle biopsy samples.
Whole genome sequencing
With developments in technology and informatics, it is becoming increas-
ingly feasible (in terms of both cost and time) to sequence the entire genome
of individuals. Whole genome sequencing has resulted in the detection of
rare pathogenic mutations and the determination of genetic risk factors
The 100,000 Genomes Project was established in 2012 and aims to
sequence 100,000 whole genomes from NHS (England) patients with rare
diseases, their families, and patients with cancer (M http:// www.genomic-
sengland.co.uk/ the- 100000- genomes- project/ ).
Useful website
Online Mendelian Inheritance in Man (OMIM) is a continually updated cata-
logue of ‘genetic’ diseases in man, giving data about the genotype, mode
of inheritance, and clinical phenotype of thousands of disorders (not just
neurological).

GENETICTESTS
645
Further reading
Manolio TA. Genome- wide association studies and the risk of disease. N Engl J Med 2010;
363
:166– 76.
Online Mendelian Inheritance in Man. M https:// www.omim.org/ .
Young AB. Huntington’s disease and other trinucleotide repeat disorders. In:JB Martin (ed.). Scientic
American Molecular Neurology
. NewYork:Scientic American, 1998; pp.35– 54.

646
CHAPTER9 Neurology
646
Neuro- otology
Pure tone audiometry
• Measures the threshold for air (AC) and bone conduction (BC) at
frequencies from 250 to 8000Hz.
Typical patterns
•
Conduction deafness:BC > AC at all frequencies.
• Sensorineural deafness:AC=BC at all frequencies, but i deafness as
the frequencyrises.
More specializedtests
• Tonedecay.
• Loudness discomfort.
• Speech audiometry.
• Acoustic impedance.
Caloric testing
Procedure
1. Inspect the eardrum; if intact, proceed.
2. Place the patient supine with the neck exed 30° (on pillow).
3. Irrigate the external auditory meatus with 30°C water (ice water if
testing for brain death).
4. Observe for (or record*) nystagmus.
5. Repeat after 5min with 44°Cwater.
Note:(*) there are various techniques for recording eye movements that are
available in specialized clinical and research laboratories.
What shouldhappen
1. Cold water induces convection of uid in the ipsilateral lateral
semicircular canal (LSCC).
2. There is less output from the ipsilateralLSCC.
3. Imbalance of signals from the two LSCCs results in eye drift towards
the irrigatedear.
4. Fast- phase contraversive movements correct for eye drift (hence,
nystamus with the fast phase away from the irrigatedear).
5. This nystagmus starts in about 20s and persists for1min.
6. Warm water reverses the nystagmus.
Common pathological responses
Canal paresis
1. Reduced duration of nystagmus following irrigation on one side (with
cold or warm water).
2. Suggests ipsilateral peripheral or central lesion.

NEURO-OTOLOGY
647
Directional preponderance
1. Prolonged nystagmus in one direction.
2. Suggests a central lesion on the side of preponderance or contralateral
peripheral lesion.
Combination of clinical examination, audiometry, and caloric testing of the
vestibulo- ocular reex will help localize a lesion (peripheral vs central; left
vs right).
Brainstem auditory evoked responses
E Sensory evoked potentials or responses, Brainstem auditory evoked
potentials (BAEPs, BAERs, BSAEPs), pp. 624–6.
Further reading
Brandt T, Dieterich M, Strupp M. Vertigo and Dizziness, 2nd edn. London:Springer,2014.

648

649
Chapter10
Renal medicine
Estimation of kidney function 650
Classication of kidney disease 654
Assessment of proteinuria 658
Assessment of renal tubular function 662
Assessment of acid– base balance 664
Assessment of urinary acidication 668
Plasma potassium 670
Urine potassium, chloride, and magnesium measurements 672
Urine sodium concentration 674
Urine dipstick testing 676
Urine culture 680
Urine microscopy 684
Investigations in patients with renal or bladder stones 686
Renal biopsy 688
Renal imaging 690
Renal bone disease 694
Immunological tests in renal medicine 696

650
CHAPTER10 Renal medicine
650
Estimation ofkidney function
Estimates based onserum creatinine
Measurement of serum creatinine concentration is the most commonly
used way of assessing the excretory function of the kidneys, which is mostly
dependent on the glomerular ltration rate (GFR). Creatinine is the non-
enzymatic breakdown product of creatine and phosphocreatine (almost
exclusively found in skeletal muscle). Its production rate is proportional
to muscle mass— the average individual produces around 10mmol/ day.
Endogenous production of creatinine is usually constant, but ingestion of
cooked meat and severe exercise cause a rapid, temporary rise in produc-
tion of creatinine, and thus in creatinine concentration. It is excreted mainly
by glomerular ltration, but tubular secretion of creatinine also occurs and
contributes a signicant proportion of overall excretion when GFR falls,
resulting in overestimation of the GFR at low GFR. Drugs, e.g. cimetidine,
trimethoprim, can block the secretory component and elevate serum cre-
atinine without any change in trueGFR.
Because of the reciprocal relationship between clearance and serum cre-
atinine, serum creatinine does not rise outside the normal range until there
has been a substantial fall in GFR, particularly in patients with low muscle
mass (see Fig. 10.1). However, in an individual patient, a progressive i in
serum creatinine over time, even within the normal range, indicates declin-
ing GFR. Wide variation between individuals based on muscle mass, sex,
and age makes serum creatinine an imperfect screening test for renal failure.
Estimation of 24h urine creatinine excretion allows measurement of CrC
but is beset with diculties largely related to the timing and completeness
of urine collections. For these reasons, use of CrC in clinical practice has
been superseded by the use of prediction formulae.
The 4- variable Modication ofDiet inRenal
Disease formula
The GFR may be estimated by the 4- variable Modication of Diet in Renal
Disease (MDRD) formula. This is the simplest of several formulae derived
from the MDRD data set and gives an estimate of the GFR normalized to a
body surface area of 1.73m
2
:
eGFR (mL/min/1.73m
2
)
= 175 × {[serum creatinine (mmol/L)/88.4]
−1.154
} × age (years)
−0.203
× 0.742 if ♀ and
× 1.21 if Afro-Caribbean
This formula does not give an accurate estimate of the GFR at extremes of
muscle mass, including amputees, and is not validated in the paediatric pop-
ulation. Most laboratories in the UK now measure creatinine with an assay
calibrated to an isotope dilution mass spectrometry (IDMS) standard and
are able to report an eGFR, alongside creatinine results, using this formula.
2 Where the laboratory reports a value for the eGFR, this should be
used, rather than any value subsequently derived.

651
ESTIMATION OFKIDNEY FUNCTION
The formula previously used a constant of 186, rather than 175, as it was
developed using a creatinine assay that gave higher values than the IDMS
standard; it remains important to check which assay is being used before
using this or any other formula to estimate theGFR.
1300
1200
1100
1000
900
800
700
600
500
400
300
200
100
x
x
x
x
xx
x
x
x
x
x
x
x
x
x
x
x
x
x
x
x
x
x
x
x
x
x
x
x
x
x
x
x
x
x
x
x
x
x
x
x
x
x
x
x
x
x
x
x
x
x
x
x
x
x
x
GFR
A
C
B
140100100 1301201109080706050403020
A
B
C
: daily creatinine production 15mmoL
: daily creatinine production 10mmoL
: daily creatinine production 5mmoL
Fig.10.1 Creatinine production is dependent on muscle mass, which varies widely.
Graph illustrates the theoretical relationship between the GFR and plasma creatinine,
ignoring the eects of tubular secretion of creatinine, which results in overestimation
of the GFR from plasma creatinine or measurement of CrC. Note that in a patient
with low muscle mass, creatinine does not rise outside the normal range until the
GFR has fallen to <30mL/ min, whereas a patient with higher muscle mass will reach
the same level of creatinine at a GFR of 90mL/ min.

652
CHAPTER10 Renal medicine
652
The Cockcroft and Gault formula and other estimates
ofeGFR
The Cockroft and Gault formula gives an estimate of CrC, using age,
weight, and serum creatinine as input variables. For simplicity of reporting,
because weight is not required and because the MDRD formula gives an
estimate of the GFR, which is normalized to the body surface area (and thus
gives an estimate of how well kidney function is matched to body size), the
MDRD formula is now preferred.
CrC = (({[140 – age (years)] × weight (kg)}/serum creatinine
(mmol/L)))
× 1.23 if ♂ or
× 1.04 if ♀
E See M
http:// ckdepi.org/ equations/ creatinine- based- equations/ for a
discussion of these and other formulae.
Where estimation of the GFR is made, the formula used should be
stated. There are important dierences between the two estimates, par-
ticularly as the GFR declines at extremes of body size, but either formula is
a major advance on the use of serum creatininealone.
CKD- EPI
The CKD- EPI (Chronic Kidney Disease Epidemiology Collaboration) equa-
tion gives a more accurate estimation of the GFR in patients with normal or
near- normal renal function than the MDRD. The result is that fewer peo-
ple with near- normal GFR are misclassied as having CKD and, as a result,
treated as ‘high risk’ for cardiovascular outcomes, based on theireGFR.
eGFR = 141 × min(SCr/κ, 1)
α
× max(SCr/κ, 1)
−1.209
× 0.993
Age
× 1.018 if ♂
× 1.159 if black
where SCr (standardized serum creatinine)=mg/ dL
κ=0.7 if ♂ or 0.9 if♀,and
α=−0.329 if ♂ or −0.411if♀
min indicates the minimum of S
cr
/ κ or1,and
max indicates the maximum of S
cr
/ κor1.

ESTIMATION OFKIDNEY FUNCTION
653

654
CHAPTER10 Renal medicine
654
Classification ofkidney disease
The following classication of chronic kidney disease (CKD) is now com-
monly used in the UK and is based on the Kidney Disease Improving Global
Outcomes (KDIGO) classication.
1
Independently of the eGFR, the degree
of albuminuria has been shown to be a strong predictor of cardiovascular
mortality, progression of CKD, and acute kidney injury (AKI), and for these
reasons, a measure of albuminuria was added to the original CKD classica-
tion in2012.
•
CKD G1:normal GFR; GFR >90mL/ min/ 1.73m
2
with other evidence of
chronic kidney damage.*
•
CKD G2:mild d in GFR; GFR 60– 89mL/ min/ 1.73m
2
with other
evidence of chronic kidney damage.*
Note:(*) other evidence of CKD may include persistent microalbuminuria,
proteinuria, or glomerular haematuria, structural abnormalities, including
renal scarring and polycystic disease, and biopsy- proven chronic glomeru-
lonephritis. An eGFR of >60 in the absence of other evidence of kidney
damage should be considered normal.
•
CKD G3a:moderate d in GFR 45– 59mL/ min/ 1.73m
2
.
•
CKD G3b:moderate d in GFR 30– 44mL/ min/ 1.73m
2
.
•
CKD G4:severe d in GFR 15– 29mL/ min/ 1.73m
2
.
•
CKD G5:established renal failure (ERF) GFR <15mL/ min/ 1.73m
2
or on
dialysis.
The staging for albuminuria quantication is then added as follows:
• A1— albumin:creatinine ratio (ACR) <3mg/ mmol.
• A2— ACR 3– 30mg/ mmol.
• A3— ACR ≥30mg/ mmol.
Using this staging system, a person with an eGFR of 48mL/ min/ 1.73m
2
and
an ACR of 25mg/ mmol has CKD stage G3a,A2.
Classication of CKD in this way allows identication of those with
severe disease in need of specialist assessment and can be used to guide
BP targets and frequency of monitoring in those with less severe disease.
2
Serumurea
Urea is the product of protein catabolism in the liver. Production is i by
high protein intake, catabolic states, breakdown of blood in the gut lumen
in GI bleeding, and tetracycline treatment, and may d in liver disease. Urea
is freely ltered at the glomerulus, with variable reabsorption, which is
inuenced by extracellular volume status. Intravascular volume depletion,
diuretics, CCF, GI bleeding, tetracyclines, and renal failure cause elevated
levels. Disproportionate rise in serum urea, compared to creatinine, occurs
in hypovolaemia and GI bleeding. Reduced levels are seen in chronic liver
disease and alcohol abuse. By itself, serum urea is a very poor marker of
excretory kidney function.
1 KDIGO. Guidelines. M http:// kdigo.org/ guidelines/ .
2
National Institute for Health and Care Excellence (2014). Chronic kidney disease in adults:assess-
ment and management. Clinical guideline CG182. M
https:// www.nice.org.uk/ guidance/ cg182.

CLASSIFICATION OFKIDNEY DISEASE
655
CystatinC
Cystatin C, a 13kDa protein of the cystatin superfamily of cysteine protease
inhibitors, is produced by all nucleated cells at a relatively constant rate and
excreted nearly exclusively by glomerular ltration. It can be assayed using
ecient enzyme- linked immunoassays. Multiple studies have shown that
cystatin C is probably a more sensitive and specic marker than creatinine
for assessing impaired excretory renal function. Minor reductions in GFR
cause cystatin C concentrations to rise above normal, even when serum
creatinine is still within normal range. Cystatin C- based estimation of the
GFR is now recommended by NICE for conrmatory testing in patients
with an eGFR
creatinine
of 45– 59mL/ min/ 1.73/ m
2
with no other evidence
ofCKD.
Measurement ofglomerular ltrationrate
Occasionally, it is necessary to measure renal excretory function accurately,
e.g. in clinical research:
•
When using drugs with a narrow therapeutic index and which are
excreted by the kidney.
•
When accurate measurement of kidney function is required in patients
with abnormal muscle mass, e.g. paraplegics with bilateral lower limb
muscle wasting.
The ‘gold standard’ for measurement of the GFR is measurement of inu-
lin clearance (see Fig. 10.2); inulin is freely ltered, not protein- bound, and
not reabsorbed or secreted. However, measurement of inulin is not widely
available as a routine laboratorytest.
Radionuclide studies
Radionuclide studies are contraindicated during pregnancy; women of
childbearing age should have a −ve pregnancy test before proceeding with
thetest.
A variety of radioisotope markers are available for estimating the GFR.
An ideal marker should be safe, not be extensively protein- bound, be freely
ltered, not be secreted or reabsorbed in the renal tubule, and be excreted
only by the kidney.
•
The commonly used markers are
51
Cr EDTA,
99m
Tc DTPA (diethylenetri-
aminepentaacetic acid), iohexol, and
125
I iothalamate. Iothalamate is also
available without radiolabelling and can be measured by uorimetry.
•
These substances are injected IV and after allowing for equilibration,
plasma levels are measured at predetermined intervals. Plasma
clearance, and hence renal elimination, is calculated from the rate of fall
of the concentration of the substance in the bloodstream.
•
51
Cr EDTA has been the most extensively studied marker and is
extensively used in Europe as a single injection technique followed by
plasma sampling at 0, 90, 120, 150, and 240min.
51
Cr EDTA is reliable,
even at low levels of renal function. Studies in humans suggest renal
clearance estimated by this method is 710% lower than that of inulin.
656
CHAPTER10 Renal medicine
656
•
125
I iothalamate is only slightly protein- bound, and studies suggest
clearance values similar to that of inulin. Unlike other markers, it
can also be administered SC, and this allows for slow equilibration
with stable plasma concentrations. It is considered safe, but potential
problems of thyroid uptake necessitate pre- treatment with oral iodine.
•
99m
Tc DTPA is used in renal isotope scanning, which allows anatomical
correlation to renal function such as information on relative uptake by
each kidney.
99m
Tc has a very short half- life, and radiation exposure is
minimized. Protein binding can result in diminished renal clearance.
± 2 SD
100
80604020101
180
160
140
120
100
80
60
40
20
0
C
insulin
(mL/min/1.73m
2
)
Age (years)
Fig.10.2 GFR, measured by inulin clearance (C
inulin
), in apparently healthy individuals
according toage.

CLASSIFICATION OFKIDNEY DISEASE
657

658
CHAPTER10 Renal medicine
658
Assessment ofproteinuria
Proteinuria may result from i glomerular permeability or tubular disease,
causing d reabsorption of ltered protein or i excretion of tubular enzymes.
Glomerular proteinuria is commoner and more likely to signal potentially
progressive kidney damage. Disease states inuence the absolute amount of
protein excreted, so protein excretion should be assessed either by meas-
urement of excretion over 24h (the ‘gold standard’, but highly inconvenient)
or after correction for the degree of urine concentration. Because total daily
urine creatinine excretion is constant, the ACR ratio or protein:creatinine
ratio (PCrR) in the urine can allow correction for urine concentration.
Although dipstick tests are useful, they can be misleading, with false +ve
(concentrated urine) and false −ve (dilute urine) results. Assuming an aver-
age creatinine production of 10mmol/ day, a PCrR of mg/ mmol allows esti-
mation of the daily protein excretion as 10 × mg/ 24h. Greater precision can
be gained by the use of prediction equations for creatinine excretion (see
M
http:// ckdepi.org/ equations/ creatinine- excretion/ ), but this is not yet
standard practice.
Indications forquantitation ofproteinuria
Diagnosis ofnephrotic syndrome
Nephrotic syndrome is dened as triad of oedema, hypoalbuminaemia, and
proteinuria
>3g/ 24h (or urine PCrR >3mg/ mmol). Hyperlipidaemia is also
commonly present.
Prognosis ofprogressive renal disease
Proteinuria is one of the most potent risk markers for progressive loss
of renal function in renal disease, e.g. diabetic nephropathy, chronic glo-
merulonephritis, and reux nephropathy. In addition, treatments that
reduce proteinuria (e.g. antihypertensive drugs, particularly ACE inhibitors
and angiotensin II receptor blockers (ARBs)) d the rate of progression.
Presence of signicant proteinuria should result in adoption of lower BP
targets and preferential use of ACE inhibitors or ARB drugs. Because reduc-
tion of proteinuria is an important therapeutic aim, regular assessment of
the severity of proteinuria is also important in monitoring the eects of
treatment. Annual measurement of the ACR is now recommended in the
UK for all patients with CKD stages3– 5.
Diagnosis ofearly diabetic nephropathy
Diabetic nephropathy is mostly treatable in its early stages— characterized by
an i in GFR, i albumin excretion, and then hypertension. ‘Microalbuminuria’
is the term for pathologically i albumin excretion below the limit of detec-
tion of standard dipstick tests for proteinuria. Microalbuminuria without
diabetic retinopathy should raise suspicion of non- diabetic kidney disease.

ASSESSMENT OFPROTEINURIA
659
Quantitation ofproteinuria
Urine protein and creatinine concentrations should be measured in an EMU
sample (because protein excretion i with activity).
Detection of proteinuria in glomerular disease should be by ACR. This
allows for detection of proteinuria, and thus CKD, at lower levels of protein
excretion than either reagent strips or PCrR. Microalbuminuria is present if
urine ACR is 2.5mg/ mmol (in ♂) or >3.5mg/ mmol (in ♀) in repeated sam-
ples. Reagent strip testing for albuminuria is not recommended, although
strips that detect, and even quantify, microalbuminuria are now available.
3
Measurement of total protein in urine is cheap but does not dierentiate
between the various proteins present in urine. Proteinuria with >30mg/
mmol creatinine is usually dened as pathological, but patients with early
diabetic nephropathy have total protein excretions below this limit, hence
the preferred use of ACR in the identication of early renal disease.
Although less sensitive than ACR at detection of low levels of proteinuria
and less precise at all degrees of proteinuria, PCrR can still be used for
quantication and monitoring of established proteinuric renal disease if
ACR cannot be used for reasons ofcost.
Diagnosis ofpostural proteinuria
Protein excretion i with activity and upright posture. For reasons that are
not completely understood, this i is exaggerated in some individuals, result-
ing in +ve dipstick tests for proteinuria and even i 24h urine protein excre-
tion. This ‘postural proteinuria’ has a nearly completely benign prognosis.
In patients with proteinuria who have no other evidence of renal disease,
it is worth quantifying proteinuria separately in urine collected whilst the
patient has been recumbent overnight and in a daytime specimen. This can
be done by measuring ACR on both EMU and a sample taken after a period
of activity. Normal protein excretion during the night with i protein excre-
tion during the day indicates postural proteinuria.
Assessment oftubular proteinuria
This is occasionally of value to detect the relatively low- grade proteinuria
that results from tubular disease, e.g. Dent’s disease (a rare genetic disorder
caused by mutation in a tubular chloride channel), which causes calcium stone
formation and tubular proteinuria. Other examples include screening for gen-
eralized tubular dysfunction and for drug toxicity, e.g. during treatment with
platinum derivatives. Tubular proteinuria is best diagnosed by measurement
of specic proteins whose presence in the urine results from tubular disease,
e.g. retinol- binding protein (RBP), N- acetyl- D- glucosaminidase (NAG) or β2-
microglobulin, either in 24h urine specimens or as ratios between the protein
concentration and creatinine concentration.
3 National Institute for Health and Care Excellence (2008). Chronic kidney disease:early identication
and management of chronic kidney disease in adults in primary and secondary care. Clinical guideline
CG73. M
http:// www.nice.org.uk/ Guidance/ CG73.

660
CHAPTER10 Renal medicine
660
Assessment ofselectivity ofproteinuria
The more severe the damage to glomerular permeability, the larger the
protein molecules that pass through the glomerulus in glomerular disease.
Measurement of the ratio of clearance of transferrin or albumin (a small
molecule) to IgG (a large molecule) can therefore be used as a measure of
selectivity and is calculated as follows:
Albumin/IgG clearance = {(urine [IgG] × serum [albu-min])/
(serum IgG × urine [albumin])} × 100%
Transferrin/ IgG clearance is calculated similarly.
A ratio of <0.16 indicates highly selective proteinuria.
In children, minimal change nephropathy causes selective proteinuria.
Non- selective proteinuria raises the possibility of an alternative type of renal
disease and might lead to a recommendation of renal biopsy to avoid ster-
oid treatment when this would be unlikely to be of benet. Measurement of
selectivity in adults rarely inuences clinical decision- making.
Detection and quantitation ofurinary light chains (Bence– Jones protein)
Detection of urinary light chains (BJP) is useful in the initial diagnosis of
multiple myeloma. Quantication of Bence– Jones proteinuria as a marker
of disease activity during treatment has been superseded by measurement
of serum free light chains.

ASSESSMENT OFPROTEINURIA
661

662
CHAPTER10 Renal medicine
662
Assessment ofrenal tubular function
There are two main types of renal tubular diseases:those due to a single
defect, usually genetic, in solute secretion or reabsorption, and those due
to generalized tubular damage.
Screening tests forgeneralized tubular dysfunction:testfor
• Renal glycosuria:dipstick or laboratory test for glucose in urine plus
normal plasma glucose.
•
Hypophosphataemia:can be followed by estimation of phosphate
reabsorption (E Assessment of phosphate reabsorption, pp. 662–3).
•
Low- molecular- weight proteinuria:due to failure of tubular reabsorption
plus i release of proteins derived from tubularcells.
•
Normal anion gap metabolic acidosis:serum bicarbonate, plus sodium,
potassium, and chloride to permit calculation of the anion gap (followed
by tests to conrm RTA; E Assessment of urinary acidication,
pp. 668–9).
•
Aminoaciduria:detected by amino acid electrophoresis on a random
urine sample.
•
Hypouricaemia:plasma urate may be low due to d tubular reabsorption.
(This can be followed by measurement of fractional urate excretion;
E Assessment of renal urate handling, p. 663.)
Assessment ofphosphate reabsorption
This is occasionally useful in the dierential diagnosis of hypophosphatae-
mia, e.g. in conrming the diagnosis of X- linked hypophosphataemic rickets.
This can be calculated using a 24h urine collection for phosphate or more
easily using a spot fasting urine sample and serumtest.
Procedure
•
The patient is asked to fast overnight.
• The overnight urine is discarded.
• The next urine sample is obtained for phosphate and creatinine,
together with a blood sample for urea, creatinine, and phosphate.
•
Both are analysed for phosphate and creatinine.
Fractional phosphate excretion is calculatedas
FE
PO4
= C
p
/C
Cr
= [serum creatinine × urine phosphate]/
[urine creatinine × serum phosphate]
This is the fraction of ltered phosphate, which appears in theurine.
Fractional tubular reabsorption ofphosphate (TRP) is calculatedas
1 – FE
PO4
TmP/ GFR, the tubular maximum for phosphate reabsorption, can be read
o a nomogram
4
or can be calculated as follows:
4 Walton RJ, Bijvoet OLM. Nomogram for derivation of renal threshold phosphate concentration.
Lancet 1975; ii:309– 10.

ASSESSMENT OFRENAL TUBULAR FUNCTION
663
If TRP<0.86,
TmP/GFR = TRP × plasma phosphate
If TRP>0.86,
TmP = {0.3 × TRP/[1 – (0.8 × TRP)]} × plasma phosphate
Interpretation
The adult reference range for TmP/ GFR is 0.80– 1.35mmol/ L with dened
age- and sex- specic values. Higher values of normal are seen in infancy
and childhood.
5
Low values are seen in X- linked hypophosphataemic rick-
ets and osteogenic osteomalacia, both of which are thought to be due to
overproduction or failure of inactivation of phosphatonins (a group of
phosphaturic hormones, including FGF- 23 and FRP4). TmP/ GFR is raised
in hypoparathyroidism and reduced in hyperparathyroidism and by PTH-
related peptide secretion.
Reduced phosphate reabsorption may also be seen in hypercalciuric
stone formers, but it remains dicult to be certain whether this is the °
disorder, causing i
production of 1,25- (OH)
2
vitamin D, or 2° to tubular
damage as a result of renal stones.
Reduced phosphate reabsorption is also seen in a number of ° and 2°
disorders of renal tubular function.
Assessment oftubular urate handling
The relative contributions of production rate, glomerular ltration, pre-
secretory reabsorption, secretion, and post- secretory reabsorption to
control plasma urate concentration cannot be dissected out without com-
plex tests involving selective pharmacological blockade of some of these
processes. However, it is possible to determine whether an abnormal
plasma urate concentration is due to abnormal production or abnormal
renal handling.
The 24h urinary urate excretion is i in overproduction, but normal in
patients whose hyperuricaemia is due to d excretion. In the latter case,
urate excretion is normal, not d
, because in under- excretion, the steady
state is maintained at the expense of a raised plasma level. If 24h urinary
urate is raised, the collection should be repeated on a low purinediet.
Fractional excretion ofurate is calculatedas
{(urinary [urate] × plasma [creatinine])/(plasma [urate] × urinary
[creatinine])} × 100%
Normal values are dependent on age and sex, but in adults, they are of
the order of 10%. High fractional excretion is a cause of hypouricaemia in
SIADH and several other conditions; low fractional excretion occurs in °
gout, but also in a familial syndrome called uromodulin- associated kidney
disease or autosomal dominant tubulointerstitial kidney disease, comprising
hyperuricaemia with early- onset gout and progressive renal failure.
5 Payne RB. Renal tubular reabsorption of phosphate (TmP/ GFR):indications and interpretation.
Ann Clin Biochem 1998; 35:201– 6.

664
CHAPTER10 Renal medicine
664
Assessment ofacid– base balance
Plasma HCO
3
−
and Cl
−
are the two major anions in extracellular uid. The
major reason for measuring them is to assess acid– base status. Changes in
serum [HCO
3
−
] concentration reect changes in acid– base balance, with
a d in [HCO
3
−
] reecting metabolic acidosis and an i reecting alkalosis.
Plasma Cl
−
is helpful in assessing the cause of acidosis or alkalosis.
There is no justication at all for performing an arterial puncture to
measure arterial pH as part of the assessment of metabolic acidosis or
alkalosis— it can be adequately assessed from serum [HCO
3
−
]. Arterial
samples are needed when it is unclear whether the acid– base disturbance is
respiratory or metabolic in origin or in mixed disturbances.
Plasma bicarbonate
The most reliable way to interpret plasma HCO
3
−
is to use the acid– base
diagram (see Fig. 10.3), which allows assessment of how much the change
in [HCO
3
−
] concentration is due to changes in CO
2
excretion via the lungs
and how much to changes in [H
+
] or HCO
3
−
wasting. In the absence of sig-
nicant respiratory disease, it can often safely be assumed that any change
is due to metabolic causes, in which case low plasma HCO
3
−
indicates i
H
+
production (or occasionally i HCO
3
−
loss) and vice versa. If ABGs are
obtained, the ‘standard bicarbonate’ is a calculated value which indicates
what the plasma HCO
3
−
would be if CO
2
excretion were normal and is thus
a way of allowing assessment of whether there is a metabolic component
to an abnormal HCO
3
−
concentration or whether it is solely due to the
respiratory disturbance.
Remember that the kidneys compensate for respiratory disease and
the lungs for metabolic disease; for instance, metabolic acidosis causes
hyperventilation, resulting in lower P
CO
2
and lessening the acidosis seen.
However, overcompensation does notoccur.
Plasma chloride
Many laboratories omit plasma Cl
−
assays from ‘routine’ serum chemistry
measurements, but this measurement is helpful if a systemic acid– base dis-
turbance is suspected. As a useful over- simplication, low HCO
3
−
with high
Cl
−
can be seen as accumulation of hydrochloric acid, which can only result
from altered renal handling of acid, as in RTA. If HCO
3
−
is low with a nor-
mal or low Cl
−
, some other acid must be accumulating. More precision in
deciding the cause of metabolic acidosis can be obtained by calculating the
aniongap.
The aniongap
The anion gap is the dierence between the sum of the concentrations of
the positively charged ions routinely measured in plasma and the negatively
chargedions:
Anion gap = ([Na
+
] + [K
+
]) – ([Cl
–
] + [HCO
3
–
])
Obviously, the total +ve charges in plasma must be balanced by the same
number of −ve charges. The normal anion gap is caused by the fact that
there are more unmeasured anions in plasma (mostly albumin, but including
ASSESSMENT OFACID–BASE BALANCE
665
lactate, sulfate, and others) than cations (including calcium and magnesium).
The concentrations of all of these unmeasured ions can vary, so the normal
range for the anion gap is wide, e.g. hypoalbuminaemia reduces the anion
gap by 2.5mEq per 1g/ dL fall in serum albumin.
The main use of calculation of the anion gap is in the dierential diagnosis
of metabolic acidosis.
A high anion gap acidosis is caused byan abnormally high concentration ofan
unmeasured anion, suchas
•
Acute or chronic renal dysfunction.
• L- lactate (reecting anaerobic metabolism or hepatic dysfunction).
• Salicylate (in aspirin poisoning).
• Chronic paracetamol use at therapeutic doses in malnourished
patients through the accumulation of pyroglutamic acid (also called
5- oxoproline).
• β- hydroxybutyrate (inDKA).
• Glycolate and oxalate (in methanol poisoning).
• Hippurate (in toluene poisoning, e.g. glue sning).
• D- lactate (from gut bacterial fermentation in blind loop syndrome).
Metabolic
x
Acute respiratory
Chronic respiratory
B
A
C
D
Arterial PCO
2
kP
a
mmHg
pH
a
[H
+
]
a
nmol/L
12
01
08642
09
08
070605040302010
20
30
40
50
60
70
80
90
100
110
120
7.6
7.1
7.0
6.0
7.2
7.3
7.4
7.5
Fig.10.3 Acid– base diagram.

666
CHAPTER10 Renal medicine
666
A normal anion gap acidosis may be caused byloss ofbicarbonate or failure
ofrenal H
+
excretion, forinstance
•
RTA.
• High ileostomy losses (bicarbonate wasting).
• Carbonic anhydrase inhibitors.
• Urinary diversions, e.g. ureterosigmoidostomy (Cl
−
/ HCO
3
−
) exchange
and NH
4
+
reabsorption in the colonic segment).
2 Beware
:in North America, the anion gap is usually calculated as [Na
+
] −
{[Cl
−
] + [HCO
3
−
]}, not including [K
+
] in the measured cations. This results
in a lower reference range for the anion gap. In addition, dierent labora-
tories use dierent assays for Cl
−
. For these reasons, the local laboratory
reference range for the anion gap should beused.
In general, the anion gap is only useful when very high, conrming high
concentrations of an unmeasured anion. If the diagnosis is not already obvi-
ous, this then justies further investigation, including assay of plasma lactate
concentration.

ASSESSMENT OFACID–BASE BALANCE
667

668
CHAPTER10 Renal medicine
668
Assessment ofurinary acidification
Indications
• Unexplained hyperchloraemic metabolic acidosis.
Defects in the kidneys’ ability to excrete acid in the urine may lead to perma-
nent systemic acidosis, or to systemic acidosis at times of i acid generation,
depending on the severity of the defect. Acidication defects may occur as
part of generalized tubular disease or as isolated, often genetically deter-
mined, alterations in function, most commonly of cell surface ionpumps.
Ammonium chloride loadingtest
This test is regarded as the ‘gold standard’ for the diagnosis of distal (‘type 1’)
RTA where there is impaired excretion of ‘xed acid’ into the distal tubule.
Procedure
•
The patient attends after an overnight fast but is allowed to drinkwater.
• At the start of the test, a urine sample is sent to the laboratory
for measurement of pH, and a plasma or serum sample is sent for
measurement of HCO
3
−
. Because pH changes rapidly in urine exposed
to air, the urine container should be lled to the top or the urine sent in
a stoppered syringe and sent to the laboratory without anydelay.
•
If the urine pH is <5.4, this indicates normal acidifying ability, and there
is no need to continue with thetest.
•
If the venous blood HCO
3
−
is low, with a urine pH of >5.4, the
diagnosis of RTA is conrmed.
• If neither of these conditions is met, then proceed to give the patient
ammonium chloride, 0.1g/ kg body weight. Ammonium chloride is given
as capsules, is unpalatable, and frequently causes nausea and vomiting,
but this can be reduced if the capsules are taken slowly or with bread
and honey. Even if the patient vomits, it is worth proceeding with the
test, as acidosis is often achieved; no more ammonium chloride should
begiven.
•
Urine samples are then collected hourly for the next 6– 8h and sent,
protected from the air (as above), for pH analysis in the laboratory. If
any sample has a pH of <5.4, the test can be stopped, as this indicates
normal acidifying ability of the distal tubule.
•
At 3h after ingestion of ammonium chloride, a venous sample should
be sent for plasma HCO
3
−
measurement to ensure that acidaemia has
occurred.
Alternative tests ofdistal acidication
Rationale
The distal tubule reabsorbs Na
+
in exchange for H
+
. Furosemide, by i deliv-
ery of Na
+
to the distal tubule, therefore causes a fall in urine pH, particu-
larly in ‘salt- avid’ states produced by Na
+
restriction or udrocortisone.
6
6 Walsh SB, Shirley DG, Wrong OM, Unwin RJ. Urinary acidication assessed by simultaneous
furosemide and udrocortisone treatment:an alternative to ammonium chloride. Kidney Int 2007;
71
:1310– 16.

ASSESSMENT OFURINARY ACIDIFICATION
669
Procedure
•
No need to fast or uid- restrict.
• Collect a baseline urine sample forpH.
• Administer furosemide 40mg and udrocortisone 1mg orally.
• Collect urine for pH measurement hourly for 4h or until urine pH<5.3.
• Urine pH persistently >5.3 at 4h implies distalRTA.
Bicarbonate infusiontest
This is the ‘gold standard’ for the diagnosis of proximal (‘type 2’) RTA,
which is characterized by impaired HCO
3
−
reabsorption. In this condition,
urine pH may be <5.5 in untreated patients, because at steady state, serum
HCO
3
−
levels fall to the point at which ltered HCO
3
−
is reabsorbed and
distal acidication mechanisms are intact.
Procedure
•
Sodium bicarbonate is infused IV at 0.5– 1.0mmol/ kg/ h. After 60min,
plasma bicarbonate is measured to conrm that this has risen to
>20mmol/ L. Urine pH is measured hourly and urine HCO
3
−
measured
to allow calculation of the fractional excretion ofHCO
3
−
:
FE
HCO3
= {(urine [HCO
3
−]) × plasma [creatinine]}/(plasma [HCO
3
−]
× urine [creatinine]) ×100%
• Fractional excretion of HCO
3
−
is normally<15%.
•
Alevel of >20% conrms type2RTA.

670
CHAPTER10 Renal medicine
670
Plasma potassium
Although most of the body’s K
+
is intracellular, small changes in extracel-
lular K
+
concentration can cause major changes in membrane excitability.
Hypokalaemia causes i excitability, causing atrial and ventricular cardiac
arrhythmias; hyperkalaemia d excitability, causing a characteristic pattern
of ECG changes and eventually causing asystole. Both hypokalaemia and
hyperkalaemia may be associated with skeletal muscle paralysis.
Plasma K
+
concentration is inuenced both by distribution across cell
membranes and by the balance between intake and excretion. Renal excre-
tion is dependent on renal function, urine ow rate, and aldosterone.
Pseudohyperkalaemia
Caused by excessive release of K
+
from cells after venepuncture and should
be considered when hyperkalaemia ‘does not t’ with the clinical picture.
The laboratory should report the presence of visible haemolysis (usually
due to RBC trauma during dicult venepuncture), but pseudohyperkalae-
mia can also occur in the absence of visible haemolysis,e.g.:
•
Haematological malignancies causing a high white cell or platelet count,
e.g. chronic lymphatic leukaemia.
•
Other causes of leucocytosis and thrombocytosis, e.g. leukaemoid
reactions,RhA.
•
Familial pseudohyperkalaemia:rare disorder of RBC cation transport
leading to an i rate of release of K
+
from red cells at low temperatures,
associated with stomatocytosis.
Diagnosis can be conrmed by showing that plasma [K
+
] is normal in
a heparinized sample analysed immediately and then by demonstrat-
ing that delayed separation results in higher values being obtained.
Pseudohyperkalaemia with a normal WBC and platelet count can be fur-
ther investigated by measuring the rate of rise of plasma [K
+
] in samples
incubated at 37°C and 22°C, and studying the eects of drugs that aect
cation exchange, e.g. thiazide diuretics and quinine. Artefactual hyperkalae-
mia can be caused by st clenching plus a venous tourniquet during phle-
botomy; plasma K
+
can rise by as much as 2mmol/ L.
Hyperkalaemia due toredistribution acrosscell
membranes
Hyperkalaemic periodic paralysis is an autosomal dominant genetic mus-
cle disorder caused by mutations in the voltage- gated sodium channel
SCN4A. It presents in early infancy with attacks of paralysis associated with
hyperkalaemia.
Other causes ofrelease ofpotassium fromtissues (including muscle) include
•
Exercise.
• Acidosis (particularly inorganic acidosis).
• Muscle damage (rhabdomyolysis), e.g. crush injury, revascularization of
ischaemic limb, prolonged unconsciousness following drug intoxication.
•
Burns.

PLASMA POTASSIUM
671
• Tumour lysis, e.g. after initiation of chemotherapy for haematological
malignancy.
•
Drugs, e.g. digoxin, depolarizing muscle relaxants, β- blockers.
• Malignant hyperthermia.
Hyperkalaemia due toaltered external balance
• i ingestion is seldom able to cause hyperkalaemia on its own but can
contribute to hyperkalaemia when combined with impaired excretion of
a K
+
load.
•
d excretion may be due to d GFR, d urine ow rate, d aldosterone
production, drugs which inhibit renal tubular K
+
excretion, or genetic
defects in renal K
+
excretion (pseudohypoaldosteronism, Liddle’s
syndrome).
•
In most cases, the cause is obvious.
Investigation ofunexplained hyperkalaemia
• Serum creatinine, CK,HCO
3
−
.
•
Urine K
+
is of limited utility because urinary K
+
excretion is primarily
determined by, and roughly equal to, K
+
intake.
•
Tests for type IVRTA:
•
Normal Synacthen
®
test (to exclude Addison’s disease).
•
24h urinary aldosterone (low in type IVRTA).
•
Plasma renin and aldosterone response to upright posture and
40mg furosemide (subnormal levels of both suggest hyporeninaemic
hypoaldosteronism).
•
Correction of hyperkalaemia with oral udrocortisone 0.1mg/ day.
Pseudohypokalaemia
Can be caused by delayed separation of samples kept at warm ambient
temperatures and is caused by continued uptake of K
+
into cells. This occurs
more in heparinized samples than in those allowed toclot.
Hypokalaemia due toredistribution acrosscell membranes
• Alkalosis.
• Insulin treatment.
• β2- adrenergic stimulation (e.g. high- dose nebulizers).
• B
12
therapyofPA.
•
Rapid cell division, e.g. acute leukaemia.
• Hypokalaemic periodic paralysis. Mutations in the CANCL1A3 and
SCN4A voltage- gated ion channels can lead to this rare condition.
Carbohydrate intake or rest after exercise typically precipitates
hypokalaemia.
•
Conrm diagnosis (under strict supervision) by infusing 2g/ kg glu-
cose and 0.1U/ kg insulin; consider referral for mutation analysis of
relevant ion channels.
•
Consider thyrotoxic hypokalaemic periodic paralysis in non- familial
patients, particularly of oriental background; checkTFTs.
Hypokalaemia due toincreased renalloss
E Chapter2.

672
CHAPTER10 Renal medicine
672
Urine potassium, chloride, and
magnesium measurements
Urine potassium
Measurement of urine K
+
concentration is occasionally useful in the dif-
ferential diagnosis of hyperkalaemia. The proportion of K
+
ltered at the
glomerulus which is excreted in the urine is extremely variable and is modu-
lated by the distal tubule in response to aldosterone, plasma K
+
concen-
tration, acid– base balance, urine ow rate, Na
+
status, and other factors.
The nal concentration of K
+
in the urine also depends on urine dilution,
controlled independently by factors (e.g. ADH) controlling water excretion.
Low urinary K
+
(<20mmol/ L) withhypokalaemia is seenin
• GI K
+
loss, e.g. diarrhoea, laxative abuse, villous adenoma, high
ileostomy output, enterocutaneous stula, ureterosigmoidostomy.
•
Dietary deciency
• Skin losses, e.g. burns, severe eczema.
High urinary K
+
(>20mmol/ L) withhypokalaemia and normal blood pressure
is seenin
•
Vomiting (K
+
is exchanged for hydrogen ions:acid– base preservation
takes precedence). Note:urinary Cl
−
will below.
•
Diuretic use, abuse, and conditions which mimic diuretic use, e.g.
Bartter’s syndrome, Gitelman’s syndrome.
•
Tubular damage causing K
+
wasting, e.g. RTA types 1and2.
• DKA.
High urinary K
+
(>20mmol/ L) withhypokalaemia and high blood pressure is
seenin
•
Hyperaldosteronism:adrenal adenomas, bilateral adrenal hyperplasia.
•
Apparent mineralocorticoid excess.
• Liddle’s syndrome.
Urine chloride
This measurement is helpful in the dierential diagnosis of otherwise unex-
plained normotensive hypokalaemia. Urine Cl
−
is low if hypokalaemia is
being caused by extrarenal sodium chloride or hydrogen chloride losses,
as seen in diarrhoea or vomiting, respectively. In these conditions, K
+
is
exchanged in the distal tubule for Na
+
or hydrogen, respectively, but Cl
−
is
conserved. Urine Cl
−
is high when the cause of hypokalaemia is inappropri-
ate loss of potassium chloride, as in diuretic use and in Bartter’s syndrome
(the genetic equivalent of being on permanent high- dose loop diuretics)
and Gitelman’s syndrome (the genetic equivalent of being on permanent
high- dose thiazide diuretics).
The distinction between drug- induced and genetic causes can be very
dicult to make, but temporary withdrawal from diuretics causes intense
Cl
−
retention and a very low urine Cl
−
concentration, which is never seen
in Bartter’s or Gitelman’s syndromes. Repeated measurements of urine Cl
−
are therefore helpful in this situation, together with screens for the presence
of diuretics in the urine when urine Cl
−
ishigh.

673
URINE POTASSIUM, CHLORIDE, & MAGNESIUM MEASUREMENTS
Urine magnesium
This measurement can be helpful in identifying the cause of hypomagne-
saemia by distinguishing inappropriate tubular Mg
2+
loss (e.g. from diuretics,
aminoglycosides, Gitelman’s syndrome) from GI Mg
2+
loss (e.g. from diar-
rhoea, proton pump inhibitoruse).
FE
Mg
= {(urine [Mg
2+
] × plasma [creatinine])/((0.7 × (plasma [Mg
2+
])
× urine [creatinine])} × 100%
Plasma Mg
2+
is multiplied by 0.7, as only 70% of Mg
2+
is non- albumin- bound
and thus freely ltered.
Fractional Mg
2+
excretion of >2% in a subject with normal renal function
indicates renal Mg
2+
loss.

674
CHAPTER10 Renal medicine
674
Urine sodium concentration
In health, serum electrolyte concentrations are kept constant because intake
of electrolytes is balanced by excretion in the faeces and urine. Renal excre-
tion is tightly regulated to achieve this balance. These basic principles imply
that the urinary excretion of, for instance, Na
+
, is nearly totally dependent
on dietary intake of Na
+
. Because this is very variable, there is no ‘normal
range’ of urine Na
+
, or any other urinary electrolyte. Measurements of uri-
nary electrolytes therefore have to be interpreted with great caution.
The 24h urine Na
+
excretion is a good marker at steady state for
dietary intake and has been used in epidemiological studies of the rela-
tionship of salt intake to BP. Dietary Na
+
intake varies from as little as
10mmol/ day in the Amazon rainforest to >400mmol/ day in Westerners
living on processed foods. Current UK advice is to restrict Na
+
intake to
around 100mmol/ day.
In clinical practice, there are several reasons formeasuring
Na
+
output, including
• Calcium stone formers:Na
+
and Ca
2+
excretion are linked, and reduction
of excessive salt intake results in a reduction in Ca
2+
excretion.
•
Cystine stone formers:similarly, cystine excretion is reduced by reduction
of dietary salt intake.
•
During antihypertensive and antiproteinuric treatment:salt restriction
amplies the eects of ACE inhibitors in reducing not only systemic BP,
but also protein excretion in renal disease, and may be more tolerable
than diuretic treatment.
The 24h urine Na
+
is usually measured on a sample collected in a plain
container. However, it can also be measured, by ame photometry, in a
sample collected into an acid container, and this is useful if Ca
2+
and oxalate
excretion are also being measured, for instance in stone formers.
Spot urine Na
+
concentration is of very limited value, because Na
+
excre-
tion varies considerably through the day and because it is normally inu-
enced by urine dilution, and hence by recent water intake. However, there
are two situations in which it may be ofvalue.
Acute kidneyinjury
The normal response of the kidneys to underperfusion from hypovolaemia
or hypotension is to retain salt avidly, urine Na
+
concentration dropping to
<10mmol/ L. If urinary Na
+
concentration is this low in AKI, this indicates
normal ability of the renal tubules to retain salt. Low urine Na
+
concentra-
tion is seen in ‘pre- renal’ renal failure; ATN results in loss of tubular salt
reabsorption and a higher urine Na
+
concentration. The problem is that
some conditions other than underperfusion can cause low urine Na
+
(e.g.
contrast nephropathy, rhabdomyolysis). High urine Na
+
does not neces-
sarily indicate ATN; indeed, it is seen in normal people. In any case, the
measurement seldom has a useful impact on management, which both in
pre- renal failure and in ATN is to restore renal perfusion by correcting
hypovolaemia, hypotension, and sepsis as quickly as possible.

URINE SODIUM CONCENTRATION
675
Syndrome ofinappropriate antidiuretic hormone
This diagnosis cannot be made in a hypovolaemic patient, because hypo-
volaemia is a physiological stimulus to ADH secretion. For this rea-
son, the diagnosis cannot be made if the urine Na
+
concentration is low
(E
Hyponatraemia (including syndrome of inappropriate antidiuretic hor-
mone), pp. 139–40).
Fractional excretion ofsodium is calculatedas
{(urine [Na
+
] × plasma [creatinine])/(plasma [sodium] × urine
[creatinine])} × 100%
This gives an index of avidity of Na
+
reabsorption independent of changes
in overall renal function. An FE
Na
of <1% is seen in pre- renal failure and of
>1% in ATN. However, this measurement is prone to some of the same
criticisms as that of urine Na
+
excretion.
Sodium- wasting and sodium- retainingstates
Na
+
wasting is caused by diuretics, Bartter’s syndrome, Gitelman’s syn-
drome, and occasionally renal tubular disease. It cannot be diagnosed by
measurement of urine Na
+
excretion alone, as at steady state, this equals
Na
+
intake, but is diagnosed by nding clinical evidence of hypovolaemia
without avid renal Na
+
retention.
Na
+
retention is caused by diseases causing eective hypovolaemia (e.g.
CCF), in which case the diagnosis is suggested by oedema and the clini-
cal signs of the underlying disease. However, Na
+
retention can also cause
hypertension without oedema, as in hyperaldosteronism, pseudohyperal-
dosteronism, chronic renal failure, and inherited disorders of renal tubular
Na
+
excretion (e.g. Liddle’s syndrome). Again, measurement of Na
+
excre-
tion alone is not helpful in the diagnosis of these conditions.

676
CHAPTER10 Renal medicine
676
Urine dipstick testing
Urine analysis has been used for many years for screening patients with
potential renal disease and for serial assessment of patients with known
renal pathology. However, the results of dipstick testing are dependent on
urine dilution and, for this reason, laboratory measurement of the ACR is
the preferred screening test for proteinuria. Reagent strips should only be
used for the detection of microalbuminuria if they are specically capable
of detecting low concentrations of albumin and expressing the result as
anACR.
Many commercially available dipsticks rapidly test the urine for multiple
chemical contents. The sticks use reagent strips, which change colour fol-
lowing a chemical reaction with an active constituent, depending on the
presence (or absence) of a particular component.
Depending onthe type ofdipstick used,
urine can be testedfor
• pH.
• Protein.
• Albumin.
• Hb.
• Glucose.
• Leucocyte esterases and nitrites.
• Specic gravity.
• Ketones.
• Urobilinogen.
The reagent strip is fully immersed in urine obtained by voiding or, if a 1%
risk of iatrogenic urine infection is warranted, by urethral catheterization,
and the excess shaken o. The change in colour, if any, is read after the time
specied by the manufacturer— usually30s.
pH
Dipstick testing only gives a rough estimate of the pH, because of the
eects of storage and reaction on exposure to atmospheric air on urine
pH in vitro. The dipstick contains a polyionic polymer bound with H
+
, which
is released on reaction with the cations in urine. Release of H
+
causes a
change in colour of a pH- sensitive dye. Normal pH varies between 4.5 and
8.0, depending on the diet; vegetarians, in whom xed acid ingestion is low,
commonly have alkaline urine. Urine infection with urease- producing organ-
isms also causes alkalineurine.
Urine pH >5.5 in spite of metabolic acidosis is seen in RTA. Urine pH is
important in some recurrent stone formers. For instance, uric acid solubil-
ity in urine is critically dependent on urine pH, and many uric acid stone
formers are found to have normal 24h urinary urate, but highly acidic and
concentrated urine (e.g. as a result of high losses from an ileostomy). In
patients with triple phosphate stones, alkaline urine is commonly seen due
to infection with urea- splitting organisms.

URINE DIPSTICK TESTING
677
Protein
Binding of proteins to the dye indicators is highly pH- dependent, and the
indicators undergo a sequential colour change based on the concentra-
tion of protein in the sample. Albumin binds at a pH of 5– 8 and has the
highest anity, so most commercially available dipsticks almost exclusively
detect albumin. Dipsticks are thus cheap and reliable, and give rapid, semi-
quantitative assessment of proteinuric renal disease. However, these tests
measure the concentration of protein, rather than absolute excretion; false
−ve tests are therefore possible in dilute urine caused by a high uid intake,
and false +ve tests may be obtained in highly concentrated urine. At pH <5
or >8, results obtained by dipsticks are not accurate. Ig light chains (BJP) do
not result in +ve dipstick tests for proteinuria, even when present in high
concentrations. Sticks able to detect low concentrations of albumin and give
a ‘near patient’ quantication of ACR are available, but not in universaluse.
Haemoglobin
Reagent strips use peroxidase- like activity of Hb to induce a colour change
in a dye linked to organic peroxide. This reaction does not distinguish hae-
moglobinuria from erythrocyturia or from myoglobinuria. False +ve results
are obtained with myoglobin, contamination with menstrual blood, semen,
and iodine. Positive dipsticks for blood with absence of RBC on micros-
copy suggest lysis of RBCs due to prolonged storage, myoglobinuria, or
haemoglobinuria. False −ve results are seen with high- dose vitamin C and
captopril.
Glucose
Most strips use the glucose oxidase/ peroxidase method and can estimate
levels as low as 50mg/ dL. Ketones, salicylate, and ascorbic acid can inter-
fere with results. These strips estimate all reducing sugars, including fructose
and lactose. In the absence of concomitant hyperglycaemia, glycosuria is
suggestive of proximal tubular disorders or, rarely, reduced renal threshold
for glucose. When associated with glomerular disease, glycosuria with a
normal serum glucose may be a marker of worse prognosis. Drugs that
inhibit Na
+
- dependent glucose transport (the SGLT- 2 inhibitors) are now
available as novel hypoglycaemic drugs and act by causing renal glycosuria.
Leucocyte esterases and nitrites
The esterase method relies on esterases released from lysed WBCs.
Esterases release pyrroles, which react with a diazonium salt on the dip-
stick, resulting in a colour change. False +ve results are seen in vaginal con-
tamination and excessively dilute urine which favours cell lysis. Presence of
glucose, albumin, ketones, tetracyclines, and cephalosporins in the urine can
give false −ve results, along with a concentrated urine, which impedes the
release of esterases.
Most, but not all, uropathogenic bacteria convert nitrates to nitrites,
which react with a diazonium compound, resulting in a colour change. False
−ve results are due to frequent bladder emptying, prolonged external stor-
age, and ascorbic acid. Some bacteria, including Neisseria gonorrhoeae and
Mycobacterium tuberculosis, do not convert nitrates.

678
CHAPTER10 Renal medicine
678
Sensitivity and specicity of the above tests vary and are not useful for
screening low- risk populations. However, a −ve test is useful in excluding a
UTI in a patient with a high pretest probability of infection. Further guidance
on the diagnosis of UTIs is available at: M https:// www.gov.uk/ govern-
ment/ publications/ urinary- tract- infection- diagnosis.
Specic gravity
Dipstick testing for specic gravity (SG) is not accurate; non- ionic constitu-
ents, including albumin, glucose, and urea, are also estimated. Normal val-
ues are between 1003 and 1030 but vary with the patient’s hydration status,
and hence urinary concentration. SG d with age, as the kidney loses its con-
centrating ability. Fixed SG of 1010 is a variable feature of advancedCKD.
Ketones
Acetoacetic acid is detected by the nitroprusside test. Ascorbic acid results
in false +ve results. Dipsticks do not detect β
- hydroxybutyrate, which com-
prises the largest ketone fraction inblood.

URINE DIPSTICK TESTING
679

680
CHAPTER10 Renal medicine
680
Urine culture
There are numerous situations in which an accurate diagnosis of UTI is
important. ‘Sending an MSU’ is not, however, quite as simple as it sounds
and is not always the most appropriatetest.
Obtaining a midstream urinesample
The aim is to obtain a sample of bladder urine, avoiding contamination by
cells or organisms on the perineal skin. Men should retract the foreskin
prior to micturition; women should hold the labia well apart with the parted
ngers of one hand, to allow the urine to exit directly from the urethral
meatus. The patient should be asked to begin to pass urine and then, with-
out stopping passing urine, pass a sterile container into the path of the uri-
nary stream and collect a sample, before nishing passing urine normally. If
a sterile foil container has been used to catch the specimen, the specimen
is then transferred into a specimen container and sent to the laboratory.
Suprapubic aspiration ofurine
In patients suspected of having bladder infection, but in whom the results of
culture of MSUs are equivocal, it may be necessary to proceed to suprapu-
bic aspiration (widely performed in paediatrics, but not in adults). After skin
preparation, a ne needle (e.g. an LP needle) is introduced into the bladder
by direct puncture just above the symphysis pubis and urine is aspirated.
US can be used to conrm that the bladder is full prior to the procedure.
‘In– out’ catheter urine specimens
Although bladder catheterization carries a small (1– 2%) risk of introduc-
ing new infection into the bladder, this risk is sometimes justied by the
importance of obtaining urine direct from the bladder. Aurethral catheter
is passed into the bladder, the rst few millilitres discarded, and a sample
collected.
Obtaining urine specimens fromileal conduits
Urine in ileal conduit bags is always contaminated by skin organisms, and
the culture of ‘bag urine’ is not a useful way of diagnosing upper UTIs in
patients with conduits. In patients suspected of having an ascending infec-
tion, a urine specimen should be obtained by passing a catheter as far into
the conduit as it willgo.
‘Two glasstest’
This is a test for urethritis and is performed when a patient presents with
dysuria or urethral discharge and a sexual history suggesting a possible
recent infection. Culture of a urethral swab or of the urethral discharge
should also be obtained and sent for gonorrhoea testing (requires attend-
ance at a sexual health clinic). Two urine samples are collected— the rst
10mL passed and a midstream sample. Each is sent for culture; urethritis is
diagnosed when the bacterial count is highest in the rst sample. The rst
sample should also be sent for Chlamydia testing.

URINE CULTURE
681
‘Stamey– Mearestest’
This test is performed for the diagnosis of prostatitis. An MSU sample is
obtained, and then the patient is asked to stop passing urine. The pros-
tate gland is massaged per rectum, and ‘expressed prostatic secretions’
collected, followed by a nal urine sample. In prostatitis, bacterial counts
are higher in the expressed prostatic secretions or the post- massage urine
sample than in the midstream sample.
Indwelling catheter urine specimens
Colonization of the bladder is nearly inevitable within a fortnight of inser-
tion of an indwelling urethral or suprapubic catheter. Unnecessary antibiotic
treatment i
the selective pressure for the emergence of antibiotic- resistant
organisms and i the risk of antibiotic- associated diarrhoea and hospital-
acquired infection. Antibiotic therapy must therefore be reserved for symp-
tomatic infection. There is no point in sending catheter specimens, unless
there is a suspicion of symptomatic infection at the time. ‘Surveillance’ sam-
ples sent to predict which antibiotics should be used if the patient becomes
symptomatic at a later time are unjustied, because the colonizing organ-
isms may change over time. Afresh specimen of urine is obtained from the
collection port into the collection pot. Samples should NOT be collected
from the reservoir into which the catheter drains.
Localizationtests
• On rare occasions, it is justied to attempt to localize the site of
infection to the bladder or to one or other kidney.
•
The ‘gold standard’ is to obtain samples from each ureter and from the
bladder during rigid cystoscopy under general anaesthesia.
•
The ‘Fairley test’ requires passage of a urethral catheter followed by a
bladder washout with a wide- spectrum antibacterial and a brinolytic
enzyme. Sequential samples of urine are then obtained. If infection
is present in the upper tracts, this will not have been aected by the
bladder washout, and organisms will be detected in the rst specimen
obtained after the washout, whereas if infection was conned to the
bladder, subsequent samples will be sterile. This is now rarely used
in practice, as it involves inserting a catheter into an already infected
system and is labour- intensive.
• Infection may be conned to one or other kidney as a result of ureteric
obstruction or may be present within a renal cyst. In these situations,
direct aspiration of urine under US control in the radiology department
is necessary.
Microscopy and culture ofurine
Once a sample has been obtained, it is sent to a microbiology laboratory
for microscopy and culture.
Microscopy is required to assess pyuria (WBCs in the urine) and
contamination.

682
CHAPTER10 Renal medicine
682
• Signicant pyuria indicates inammation within the urinary tract; if this
persists, despite −ve urine cultures, the patient has ‘sterile pyuria’,
for which there are a number of causes, including infection with an
organism which does not grow on conventional culture media, e.g.
Chlamydia.
•
Pyuria plus a +ve culture conrms the diagnosis ofUTI.
• The absence of pyuria makes a UTI less likely but can occur in the early
stages of infection or in the presence of a very high uid intake.
•
Contamination (in the ♀ ) is indicated by the presence of large numbers
of squamous cells, which usually come from the vaginal wall; however,
squamous cells can occasionally come from the bladder.
Culture and sensitivity are necessary to decide what treatment is necessary
and to dierentiate contamination of the urine sample by organisms outside
the bladder from true infection.
•
A‘pure growth’ of a single organism to >10
5
CFU/ mL is the
conventional criterion forUTI.
• However, low counts of 10
2
– 10
4
CFU/ mL can be associated with early
infection and should be taken seriously in the presence of suggestive
symptoms inwomen.
•
Low counts in men are likely to represent true infection, because
contamination is uncommon.
•
Genuine mixed growth may occur, in the presence of impaired urinary
drainage or a foreign body within the urinarytract.

URINE CULTURE
683

684
CHAPTER10 Renal medicine
684
Urine microscopy
Urine microscopy is a useful, quick, reliable, cheap, and underused
investigation— the ‘liquid renal biopsy’! Far more information can be
obtained by careful microscopy than is usually obtained in the microbiol-
ogy laboratory where the priority is detection of signicant urine infection.
Indications
• SuspectedUTI.
• Suspected acute glomerulonephritis.
• Suspected acute interstitial nephritis (requires staining for eosinophils).
• Unexplained acute or chronic renal failure.
• Haematuria (with or without proteinuria) on urine dipsticktest.
• Suspected urinary tract malignancy.
Procedure
A freshly voided, clean- catch, midstream early morning specimen is ideal.
The sample should be centrifuged and resuspended in a small volume.
Although bright eld microscopy will allow identication of most formed
elements in the urine sediment, phase contrast microscopy is useful for
detection of red cell ghosts, ‘glomerular’ red cells, and some other constitu-
ents. Staining of the urine sediment is not necessary for most purposes but
is useful for identication of eosinophils and malignant cells— this is usually
performed in the cytology laboratory.
Haematuria
RBCs appear as non- nucleated, biconcave discs. Even when urine is red
in colour or dipsticks +ve for blood, it should be examined for the pres-
ence of red cells. The dierential diagnosis of haematuria is broad, but it
is broadly classied into glomerular (renal) and infrarenal causes. Transit of
red cells through the renal tubules causes osmotic changes in their shape
and size; ‘dysmorphic’ or ‘crenated’ red cells are best seen using phase con-
trast microscopy and may be missed altogether if bright eld microscopy is
used. In experienced hands, detection of these glomerular red cells strongly
suggests a glomerular origin for haematuria, although failure to detect these
changes does not reliably indicate a lower urinary tract cause of bleeding—
heavy haematuria in IgA nephropathy, for instance, can result in large num-
bers of normal red cells in the urine. Urine pH, concentration, and storage
can aect red cell morphology.
Leukocyturia
The presence of signicant numbers of polymorphs (pyuria) in urine is
highly suggestive of UTI but can also occur in glomerulonephritis, interstitial
nephritis, and peri- ureteric inammation, for instance in acute appendicitis.
The presence of leucocyte casts is diagnostic of renal parenchymal infec-
tion (‘acute pyelonephritis’). Eosinophiluria is associated with acute allergic
interstitial nephritis and athero- embolic renal disease.

URINE MICROSCOPY
685
Othercells
Squamous epithelial cells are usually taken as indicative of vaginal contam-
ination but may also derive from the bladder and urethra. Occasionally,
malignant cells arising from the lower urinary tract are picked up on routine
microscopy. Spermatozoa are also rarelyseen.
Microorganisms
Identication of bacteriuria, in association with leukocyturia, is very sugges-
tive of an infection. Organisms may be in chains or clusters, and some are
motile. Fungi, including yeast, and protozoans, including Trichomonas, can
also be readily identied.
Casts
Casts are cylindrical bodies, which usually form in the distal tubule
and collecting duct. They consist of cells or cell debris held together by
Tamm– Horsfall protein. Staining and phase contrast microscopy improve
identication and characterization of casts, but results are operator-
dependent. Extreme shaking or agitation can disintegratecasts.
•
Hyaline casts appear translucent and homogeneous and are present in
normal urine. Number may be i in dehydration and proteinuria.
•
Cellular casts, especially red cell casts, always indicate signicant
parenchymal renal disease. Red cell casts are strongly suggestive of
acute glomerulonephritis but may occur in interstitial nephritis and ATN
aswell.
•
White cell casts are seen in acute pyelonephritis and acute interstitial
nephritis.
•
Granular casts are formed from cell debris and are seen in a wide variety
of renal diseases.
•
Waxy broad casts form in atrophic renal tubules and are seen in chronic
renal failure.
Crystals
A variety of crystals can be visualized and are of importance in stone form-
ers. Afreshly voided sample should be examined, as storage and tempera-
ture changes can aect the type and number of crystals found. Calcium
oxalate crystals may be seen in hypercalciuria, hyperoxaluria, and ethylene
glycol poisoning. Presence of even a single crystal of cystine is diagnostic
of cystinuria, as cystine is not a constituent of normal urine. Calcium phos-
phate crystals can form in normal urine as it cools and are of no pathological
signicance.

686
CHAPTER10 Renal medicine
686
Investigations inpatients withrenal
or bladderstones
Not all renal tract stones are formed because of abnormal urine chemistry.
They may also be formed because of stasis, e.g. in calyceal or bladder diver-
ticula. Infection (‘struvite’) stones are the result of chronic infection in the
urinary tract with urease- producing organisms, which metabolize urea to
form an alkaline urine in which struvite readily precipitates.
Indications
Although up to 75% of patients who present with renal stones eventually
form a second stone, this may not be for 20years. Most urologists there-
fore only refer patients for metabolic evaluation if there is a heightened
suspicion of an underlying metaboliccause.
Situations inwhich evaluation is denitely indicated include
•
Formation of stones in childhood or adolescence.
• Recurrent stone formation.
• Nephrocalcinosis (calcication in the renal parenchyma) as well as stone
formation in the collecting systems.
Radiology
IVU or unenhanced helical CT will usually have been performed during the
patient’s presentation with stone disease, but the lms should be reviewed
to look for evidence of any cause of stasis within the collecting systems,
and in particular for medullary sponge kidney. Radiolucent stones can be
detected using US, IVU, or CT scanning, and can be made of cystine, uric
acid, xanthine, or 2,8 dihydroxyadenine. ‘Staghorn’ calculi lling the collect-
ing systems are most often struvite (infection) stones, but not always—
calcium oxalate stones can grow to similar size and shape, particularly in
hyperoxaluria, as can cystine stones.
Stone analysis
Depending on the facilities in the laboratory, this may be qualitative or semi-
quantitative. The purpose of analysis is to distinguish calcium stones from
cystine, urate, and struvite stones, to pick up the rare types of stone, and
in addition to distinguish calcium oxalate from calcium phosphate stones.
The result of stone analysis should be used to guide further investigation.
Stones can be obtained for analysis either at surgery, including percutaneous
nephrolithotomy, or by asking a patient to pass urine through a nesieve.
‘Spot’ urinetests
Amino acid analysis on a random sample of urine shows i excretion of cys-
tine, ornithine, lysine, and arginine in cystinuria, and this nding is sucient
to conrm a suspected diagnosis. However, measurement of 24h urinary
cystine excretion is necessary for optimal management of this condition.
Random urine calcium:creatinine and oxalate:creatinine ratios are used in
children to diagnose hypercalciuria and hyperoxaluria but are not as reliable
as 24h urine collections, which are preferred in adults.

INVESTIGATIONS INPATIENTS WITHRENAL OR BLADDERSTONES
687
24h urine collections
Collections must be made into an acidied container for measurement of
calcium and oxalate, and into a plain container for measurement of urate
(because acidication is necessary to prevent calcium from binding to the
plastic surface of the urine container and to prevent in vitro generation of oxa-
late, and because acidication precipitates uric acid crystals). Measurement
of sodium and citrate excretion can be made on either type of collection.
•
Calcium excretion is not a good predictor of stone formation (calcium
activity is less than concentration due to the presence in urine of anions
that form soluble complexes with calcium). However, marked i of
urinary calcium is a risk factor for stone formation.
•
Oxalate excretion correlates well with the risk of recurrent calcium
oxalate stone formation, even within the normal range. Marked
hyperoxaluria may result from enteric hyperoxaluria (i colonic oxalate
absorption resulting from small bowel resection, jejunoileal bypass, or
malabsorption), acute ethylene glycol poisoning, excess dietary oxalate,
or as a result of ° hyperoxaluria (one of several metabolic defects
causing i endogenous oxalate production).
•
Glycolate and L- glycerate should be measured in patients suspected of
having ° hyperoxaluria to allow dierentiation between type 1 and
type 2 ° hyperoxaluria. Raised urine glycolate suggests type 1 disease,
and raised L
- glycerate suggests type 2 disease, but neither test is 100%
sensitive or specic.
•
Liver biopsy with assays to detect enzyme activity can be used to
diagnose ° hyperoxaluria
•
Genetic testing is now widely used for the diagnosis of type 1, 2, and 3
° hyperoxaluria.
•
Citrate excretion should be measured because citrate is a potent inhibitor
of calcium stone formation; correction of hypocitraturia with, for
instance, oral potassium citrate, reduces stone recurrencerate.
•
Sodium excretion (a good marker for dietary sodium intake) should
be measured in calcium stone formers and in patients with cystinuria,
because reduction of dietary sodium intake results in d excretion of
calcium and cystine, respectively.
•
Cystine excretion should be measured in cystine stone formers. The aim of
treatment is to maintain the cystine concentration well below the solubility
limit for cystine (7
1mmol/ L at urine pH of 7). Rather than a single 24h
collection, it is worth asking the patient to split the urine collection into
daytime and night- time aliquots to ensure that this target is met at night,
when urine tends to become more concentrated, as well as during theday.
•
Urinary phosphate measurement is of no proven value in the
management even of calcium phosphate stone formers.
Tests ofurinary calcium excretion
Tests performed after calcium restriction and following a high- calcium test
meal have been used widely in the USA to dierentiate ‘absorptive’ from
‘renal’ hypercalciuria. These tests are necessary to dene dierent pheno-
types associated with hypercalciuria for research studies, but there is no
evidence that management strategies based on them have any advantage
over those based on simpler tests of urine chemistry.

688
CHAPTER10 Renal medicine
688
Renalbiopsy
Percutaneous renal biopsy is a valuable tool to establish diagnosis, suggest
prognosis, and guide therapy in renal diseases. It also has a major role in the
management of a renal transplant recipient.
Denite indications (result likely tochange management)
• Nephrotic syndrome (in adults).
• Steroid- unresponsive nephrotic syndrome in children.
• Acute nephritic syndrome.
• Rapidly progressive glomerulonephritis.
• Unexplained renal failure with normal- sized kidneys relative to body size
andage.
•
Renal involvement in multi- system disorders.
• Diagnosis of renal transplant dysfunction.
Relative indications (result may change management or
help todene prognosis)
• Non- nephrotic range proteinuria with or without haematuria.
• Isolated haematuria (only rarely does biopsy change management—
sometimes justied for potential live kidney donors, or for employment
or insurance purposes).
•
UnexplainedCKD.
• Diabetic patient with renal dysfunction, particularly with features not
typical of diabetic nephropathy, or without retinopathy.
Absolute contraindications
• Uncontrolled severe hypertension.
• Bleeding diathesis, including platelets <50 × 10
9
/ L, uncorrected
bleeding/ clotting disorders, and patients on anticoagulation with
prolonged clottingtimes.
Relative contraindications
• Single kidney.
• Kidney size small, compared to patient’s body size andage.
• Renal tumour/ mass— risk of abdominal seeding.
• Uncooperative patient (can be done under sedation or underGAn).
• Multiple renalcysts.
Procedure
1. Recent imaging of kidneys to document the size and rule out
obstruction is mandatory. Arecent normal platelet count, clotting
prole, and informed consent are necessary.
2. The procedure is performed where proper US facilities are
available and is usually done under LAn. Sedation can be given to an
uncooperative or tense patient.
3. An attending pathologist or technician at the time of sampling to
comment on adequacy of tissue is very useful.
4. Biopsy can be performed with either a spring- loaded disposable device
or a biopsy gun, depending on local practice.
5. The patient lies prone and the kidney is identied withUS.
6. The skin over the target area is prepared and anaesthetized with lidocaine.
RENALBIOPSY
689
7. Asmall cut in the skin is made using a scalpel. The kidney is localized
with a ne- bore 21G LP needle and LAn inltrated up to the level
of the renal capsule. Either kidney can be biopsied— all parenchymal
renal diseases are bilateral. After suitably protecting the US probe,
the biopsy needle/ gun is inserted along the anaesthetized track under
real- time US guidance to the level of the renal capsule, aiming to
obtain a sample from the cortex of the lower pole. The patient is
asked to hold their breath whilst the biopsy istaken.
8. The needle/ gun is red and subsequently withdrawn. The patient is
then allowed to breathe normally.
9. Two cores of tissue are usually taken; this may require three or
four ‘passes’ with the biopsy needle. If an attending pathologist or
technician is present, they can comment on the adequacy of tissue
by examining the core for glomeruli (see Fig. 10.4) using a hand- held
magnifying glass or a simple microscope. If immunouorescence is to
be performed, part of one core is placed in saline; the remainder is
placed in formalin.
10. Following the biopsy, the patient is turned supine and strict bed rest
enforced for a minimum of 6h. Vital signs are monitored every 15min
for 2h, every 30min for 2h, and hourly thereafter. If no complications
are encountered at 6h, the patient is allowed to mobilize. Most bleeding
complications occur within the rst 8h, but bleeding can start up to 72h
after the biopsy. If macroscopic haematuria is present and does not
resolve within the observation period, discharge should be delayed.
Mesangial cells:
i number,
polymorph inltration
Bowman’s space:
cellular proliferation
(crescents)
Urinary epithelial cell:
foot proces
s
eacement/fusion
Brush
border
Basement membrane:
congenital thinning,
immune complex deposition
—lumpy (e.g. ‘membranous’)
—linear (e.g. Goodpasture’s)
—splitting (membranoproliferative)
Proximal
tubule
Glomerular
ltrate ow
Glomerular
capillary loops
Eerent
arteriole:
vasculitis
Aerent arteriole:
vasculitis thrombosis,
atheromatous embolism
Blood ow
Mesangial matrix:
expansion,
sclerosis, lysis
Fig.10.4 The normal glomerulus and its repertoire of response to injury.
Reproduced from Tomson CRV. Essential Medicine, 2nd edn. London:Churchill Livingstone,1998.

690
CHAPTER10 Renal medicine
690
Renal imaging
Contrast nephropathy
Renal toxicity due to radiocontrast agents may cause or exacerbate renal
impairment. The risk of nephropathy i with i contrast dose and is higher
with intra- arterial injection. Nephrotoxicity is due to a combination of local
vasoconstriction and direct tubular injury. There is an i risk in patients with
pre- existing renal impairment, diabetes, myeloma, hypovolaemia, or eec-
tive hypovolaemia (e.g. CCF), and concurrent administration of medica-
tions that can reduce renal blood ow, including NSAIDs, ACE inhibitors,
and ARBs. The Royal College of Radiologists recommends discussion with
the referring clinician before contrast is given to any patient taking met-
formin, given the theoretical risk of precipitating lactic acidosis, but it is
no longer recommended that metformin is routinely discontinued in this
setting.
7
Although usually reversible, contrast nephropathy can precipitate the need
for dialysis in patients whose renal function is already seriously impaired.
Non- ionic media, use of the minimum possible dose, and IV pre- treatment
with saline or sodium bicarbonate reduce the risk in high- risk patients.
Details of the radiological investigation of the urinary tract appear in
E Renal imaging, pp. 690–2; E Radiology of the urinary tract, pp. 808–11;
E Static cortical renography:DMSA imaging, p. 924; E Dynamic renogra-
phy, pp. 926–7; E Captopril renography, p. 928.
Gadolinium- based contrastmedia
An association between exposure to gadolinium- based contrast media and
nephrogenic systemic brosis (NSF) was established in 2006. NSF manifests
as brosis of the skin and connective tissue, causing contractures and joint
immobility, and can cause visceral brosis, in some cases leading to a fatal
outcome. With relatively few cases reported so far, the risks are hard to
quantify, but patients with CKD stages 4– 5 can be considered high risk and
CKD stage 3 low risk. No cases have been reported with eGFR of >60mL/
min/ 1.73m
2
. Post- exposure dialysis is recommended for patients already
established on renal replacement therapy, but dialysis is not recommended
for those not established on renal replacement; the risks of temporary
vascular access and dialysis initiation outweigh the benets of gadolinium
removal. The risk of developing NSF seems to relate to the extent to which
the contrast medium releases free gadolinium (Gd
3+
) ions, with cyclic agents
less likely to release Gd
3+
than linear chelates.
8
7 Royal College of Radiologists (2015). Standards for intravascular contrast agent administration to
adult patients, 3rd edn. M
https:// www.rcr.ac.uk/ sites/ default/ les/ Intravasc_ contrast_ web.pdf.
8
Medicines and Healthcare Products Regulatory Agency (2007). Gadolinium- containing MRI contrast
agents:nephrogenic systemic brosis. M http:// www.mhra.gov.uk/ safety- public- assessment- reports/
CON081759.

RENAL IMAGING
691
Choice ofinvestigation
Unexplained renal impairment
When a patient rst presents with renal impairment it is important to
decide whether this is acute— and therefore potentially reversible— or
chronic. Although the history, examination, and blood tests may give some
clues, considerable doubt may remain.
All patients presenting withrenal impairment should therefore undergo
•
Ultrasound:
•
Hydronephrosis suggests obstructive nephropathy.
•
Small, smooth kidneys with i echogenicity and d corticomedul-
lary dierentiation suggests chronic parenchymal renal disease, e.g.
chronic glomerulonephritis.
•
Irregular cortical scarring can be caused by reux nephropathy, previ-
ous obstructive nephropathy (e.g. complicating renal stones), and
renal infarction from vascular disease or embolism.
•
Renal asymmetry, particularly in a patient with known atherosclerosis
elsewhere, suggests RAS, although this can just as commonly be
bilateral.
•
Renal enlargement can occur in ATN, renal vein thrombosis, and
renal inltration, e.g. in haematological malignancy.
•
Plain abdominal lm (KUB) if renal stones or nephrocalcinosis
suspected— nephrocalcinosis and urinary tract stones, particularly if
outside the renal pelvis, can be missedonUS.
Further radiological investigations, including renal angiography and isotope
scanning, are sometimes helpful.
Suspected nephrolithiasis
Unenhanced helical CT is increasingly the investigation of rst choice and
is more useful than IVU, particularly if the GFR is reduced or radiolucent
stones present. IVU remains readily available and may be the preferred
investigation in some centres.
Investigation ofhaematuria
In patients over 40 and possibly in some younger patients, it is important
to exclude urinary tract malignancy. US is the investigation of choice for
the detection of renal cell carcinoma but will miss some transitional cell
carcinomas of the renal pelvis, which are best detected using CT or IVU.
Cystoscopy or high- resolution US of the full bladder is required to rule out
transitional cell carcinoma of the bladder.
Investigation ofsuspected renal artery stenosis
In younger patients in whom bromuscular dysplasia is suspected, conven-
tional angiography should be performed. Atherosclerotic RAS can be rea-
sonably assessed by contrast CT angiography or by gadolinium- enhanced
MRA, with direct angiography reserved for interventional procedures.
Reux nephropathy
Conrming this diagnosis can be important in counselling patients, as reux
nephropathy is often inherited as an autosomal dominant trait. Cortical
scarring is best detected using a static DMSA (dimercaptosuccinic acid)

692
CHAPTER10 Renal medicine
692
renal scan. However, there are other causes of cortical scarring. The diag-
nosis is best conrmed by showing the combination of cortical scarring with
underlying calyceal deformity on IVU. Demonstration of vesico- ureteric
reux on direct or indirect micturating cystourethrography is useful in
infants and small children, but reux commonly resolves with growth, so
these tests are seldom used in adults.
Obstructive uropathy
Although hydronephrosis demonstrated on IVU or US is usually sucient to
conrm obstruction, it is possible to have obstruction without much dilata-
tion (e.g. complicating encasement by tumour). More commonly, there is
uncertainty over whether dilatation of the collecting system and pelvis is due
to previous obstruction, now resolved, or continuing obstruction. Diuretic
MAG3 (mercaptoacetyl triglycine) renography may be useful but gives less
reliable results as the GFR falls. Insertion of a nephrostomy to determine
whether direct drainage of urine improves renal function or retrograde
insertion of ureteric stents may be useful for both diagnosis and treatment
of obstruction. If doubt persists, a Whitaker test may be performed; this
involves infusion of saline at a constant rate through a nephrostomy tube
and measuring the relationship between pressure and ow down the ureter.
Renal transplant dysfunction
The dierential diagnosis usually lies between obstruction, ureteric leak,
rejection, ATN, nephrotoxicity, and renal vein thrombosis. Depending on
the centre, US with Doppler assessment of renal blood ow (giving resist-
ance index) or isotope renography may be the investigation of rst choice.

RENAL IMAGING
693

694
CHAPTER10 Renal medicine
694
Renal bone disease
Parathyroid hormone
Indications
Diagnosis of ° hyperparathyroidism in patients with hypercalcaemia.
Diagnosis of 2° or tertiary hyperparathyroidism in patients with stage 4
or5CKD.
Procedure
A serum sample is sent and separated within 4h of venepuncture.
Alternatively, PTH remains stable for 24h if the sample is taken into EDTA,
allowing samples to be sent from ° care. Check with the local laboratory.
Other markers ofbone biochemistry
Serum Ca
2+
is often normal, even in patients with signicant renal disease,
because a fall in serum Ca
2+
caused by reduced 1,25- (OH)
2
vitamin D pro-
duction results in an i
in PTH secretion, returning serum Ca
2+
towards
normal. Hypocalcaemia occurs after parathyroidectomy or after treatment
with bisphosphonates. Hypercalcaemia occurs when the PTH release loses
sensitivity to serum Ca
2+
in tertiary hyperparathyroidism.
If PTH is raised in the presence of CKD 3, check 25- (OH) vita-
min D; vitamin D deciency should be corrected before treatment of
hyperparathyroidism.
•
Serum phosphate is often i in patients with renal impairment due to
impaired renal excretion of phosphate.
•
Serum total ALP rises in severe hyperparathyroidism and osteomalacia.
•
Serum bone ALP is a more sensitive marker of bone turnover, but
quantitative measurement is not widely available. If total ALP is raised,
ALP isoenzymes can be measured as an indicator of whether the i is of
bone origin.
•
FGF- 23 (broblast growth factor) is a phosphaturic peptide
produced primarily by osteocytes. Patients with CKD have i
FGF- 23
concentrations due to phosphate retention and d clearance. FGF- 23
excess is a better predictor of adverse outcomes in CKD than serum
phosphate levels and could represent a novel early biomarker for
disordered bone mineral metabolism. However, the only current clinical
indication for FGF- 23 measurement is in the diagnosis of tumour-
induced osteomalacia.
Serum aluminium and thedesferrioxaminetest
Patients with renal disease may be exposed to aluminium from contami-
nated water used for the preparation of dialysates or by ingesting aluminium
hydroxide as an antacid or, rarely nowadays, as a phosphate binder taken
with meals. Because of the eects of aluminium on the brain, BM, and
bones, it is important to monitor patients at risk for evidence of aluminium
accumulation.
Serum aluminium
has to be taken into an aluminium- free glass tube. Serum
aluminium levels reect current exposure and do not give any informa-
tion about cumulative exposure. Serum aluminium levels may be i by iron

RENAL BONE DISEASE
695
deciency. Levels above 60µg/ L (2.2µmol/ L) are considered indicative of
a dangerous level of exposure and should lead to a review of treatment.
The increment in serum aluminium 24 or 48h after IV desferrioxamine is
a marker of aluminium ‘load’. The original protocol requires the use of
40mg/ kg desferrioxamine; a rise in serum aluminium of >200µg/ L corre-
lates well with the presence of aluminium- related bone disease on bone
biopsy. Low- dose protocols have also been described and validated.
Skeletalsurvey
Severe hyperparathyroidism causes erosion of the terminal phalanges,
subperiosteal erosions, and, in rare cases, brown tumours and pathologi-
cal fractures. Severe osteomalacia causes loss of bone density and Looser
zones (pseudo- fractures). These radiological signs are not commonly seen
in modern renal patients because biochemical monitoring allows earlier
detection and treatment of bone disease.
Transiliac bonebiopsy
This is the ‘gold standard’ for the diagnosis of renal bone disease but is not
commonly used in clinical (as opposed to research) settings. However, it can
be useful particularly for the conrmation of aluminium- related bone dis-
ease and low bone turnover states. For maximum information to be gained
from this invasive test, double tetracycline labelling should be performed,
allowing the pathologist to determine the rate of new bone formation.
Procedure
Fourteen and 13days before the procedure, the patient takes a tetracy-
cline antibiotic, e.g. oxytetracycline 250mg four times daily (qds), and 4 and
3 days before the procedure a dierent tetracycline, e.g. demeclocycline
300mg bd. Under GAn, a transiliac core of bone, including both cortical
surfaces, is taken and placed in absolute alcohol. The sample should be sent
to a laboratory specializing in the interpretation of bone biopsies in patients
with metabolic bone disease.

696
CHAPTER10 Renal medicine
696
Immunological tests inrenal medicine
Immune- mediated diseases can aect the kidney in isolation or as part of
a systemic disorder. Immunological tests commonly used to diagnose or
monitor progress of renal disease are discussedhere.
Complement
Indications
Acute nephritic syndrome, renal failure with skin ± neurological involve-
ment, and suspected SLE, endocarditis, or cryoglobulinaemia. The normal
complement system, its activation pathways, and assay methods are dis-
cussed elsewhere (E Serum complement components, p. 351). In rela-
tion to renal disease, hypocomplementaemia is important and relative
deciencies of various components can point to certain disorders. (See
Table10.1.)
Successful treatment normalizes complement levels in endocarditis and
in SLE, except when SLE results from congenital complement deciency.
C3 nephritic factor (C3Nef ) is an IgG autoantibody that binds to, and sta-
bilizes, alternative pathway C3 convertase— C3bBb. This results in continu-
ous activation of the alternative pathway with C3 depletion. It is detected
by ELISA. C3Nef is classically associated with the C3 glomerulopathies,
dense deposit disease, and C3 glomerulonephritis (previously known as
type 2 membranoproliferative glomerulonephritis).
Immunoglobulins and serum electrophoresis
forparaproteins
Indications
•
Suspected myeloma or other clonal B- cell disorders.
• Unexplained renal failure, with or without proteinuria, particularly in
patients >50years.
•
Renal failure in association with hypercalcaemia.
Serum electrophoresis to identify a monoclonal Ig band may be useful but
should always be combined with tests for urinary light chains (BJPs), as
some types of myeloma cause light chain proteinuria without a monoclo-
nal band in the serum. Serum free light chain assays are also now available
and should be used to monitor myeloma in patients with kidney disease.
Table10.1 Complement levels inselected diseases
C3 C4
Post- streptococcal GN Low Normal
SLE Low Low
Cryoglobulinaemia
Low/ normal Very low
Membranoproliferative GN Low Low/ normal
Subacute endocarditis Normal/ low Low
GN, glomerulonephritis.

IMMUNOLOGICAL TESTS INRENAL MEDICINE
697
In patients with normal renal function, κ light chains are cleared more rap-
idly than λ light chains, so the κ:λ ratio is lower than the production ratio;
as the GFR falls, this dierence becomes less. The normal range for the
κ:λ ratio is 0.26– 1.65, but a normal range of 0.37– 3.1 should be used if
eGFR <60mL/ min/ 1.73m
2
. Routine serum and urine electrophoresis in the
absence of clinical features to suggest a B- cell disorder will identify many
patients with MGUS incidental to their renal disease and seldom identies
treatable disease.
Measurement of serum Ig concentrations is of value in patients with
known myeloma but is otherwise not useful in the assessment of patients
with renal disease. Polyclonal hypergammaglobulinaemia is seen in chronic
infections, connective tissue disorders (e.g. RhA, Sjögren’s syndrome), neo-
plasms, and chronic liver disease. Measurement of serum IgA concentration
is of no value in the diagnosis or monitoring of IgA nephropathy, although
a raised total IgA concentration is seen in some patients with this disease.
Paraproteins are products of abnormal B- cell clones and can be detected
in serum as monoclonal bands on Ig electrophoresis or in urine as BJPs.
Paraproteins may be whole Igs or heavy or light chains in isolation.
Light chains are suciently small to be ltered at the glomerulus, are not
reabsorbed, and are not picked up on routine dipsticks. BJPs are light chains
excreted in the urine; they precipitate on heating to 45°C and re- dissolve on
boiling, but are now detected by electrophoretic techniques.
Paraproteins can cause a number ofdierent renal lesions, including
•
Myeloma cast nephropathy.
• Light chain nephropathy.
• AL amyloidosis.
• Fibrillary/ immunotactoid glomerulopathy (although this appearance is
more frequently not associated with a plasma cell dyscrasia).
Cryoglobulins
Cryoglobulins are Igs, which precipitate on cooling and re- dissolve on
warming.
Cryoglobulinaemia should be suspectedin
•
Renal failure with otherwise unexplained hypocomplementaemia or
+veRF.
•
Renal failure in association with skin and neurological involvement.
• Unexplained proteinuria/ renal failure in patients with clonal B- cell
disorders.
Meticulous attention to collection, transportation, and assessment of the
sample is required; a serum sample must be kept at 37°C and sent to
the laboratory for analysis immediately, having warned the laboratory that
the sample is on the way. False −ve results are common due to improper
handling of the specimen.
Once a cryoglobulin has been found, further electrophoresis and
immunoxation allow identication ofthree distincttypes
•
Type 1 has a single monoclonal Ig (IgG, IgA, or IgM) and is associated
with monoclonal B- cell disorders.

698
CHAPTER10 Renal medicine
698
• Type 2 has a monoclonal IgM directed against the Fc portion of IgG, and
the cryoprotein therefore consists of monoclonal IgM with polyclonal
IgG. Tests for RF (i.e. anti- IgG antibodies) are +ve. This may be
associated with haematological malignancy, chronic hepatitis C infection,
or may be unexplained (‘essential’).
•
Type 3 has polyclonal IgG and polyclonal IgM and occurs in chronic
infections (e.g. bacterial endocarditis, viral hepatitis), autoimmune
disorders (e.g. RhA, SLE), or may be unexplained (‘essential’).
Hypocomplementaemia, especially very low C4 levels due to classical com-
plement pathway activation, is characteristic and helpful in diagnosing active
cryoglobulinaemic disorder. Renal disease can present as an acute nephritic
disorder or as nephrotic syndrome, and is usually seen in association with
skin and systemic involvement.
Antineutrophil cytoplasmic antibody
These are autoantibodies directed against enzymes present in the cyto-
plasm of human neutrophils. They are present in nearly all patients with
small- vessel vasculitis (including GPA, microscopic polyangiitis, renal- limited
crescentic glomerulonephritis, and Churg– Strauss syndrome). ANCA and
its pattern of distribution are conventionally detected by indirect immuno-
uorescence, with pANCA having a characteristic perinuclear staining pat-
tern and cANCA a cytoplasmic staining pattern. Changing titres can reect
changing disease activity. ELISA is now readily available for the two com-
monest ANCA protein targets:proteinase 3 (PR3) which is the commonest
target of cANCA and MPO, the commonest target of pANCA. Low- titre
+ve ANCA is unlikely to reect an underlying disease if the ELISA is −ve.
However, a −ve ANCA does not rule out vasculitis, and false +ve tests
occur, so the test is not a substitute for renal biopsy.
Anti- glomerular basement membrane antibody
Circulating anti- glomerular basement membrane (GBM) antibodies are
present in Goodpasture’s disease and may also be +ve in ANCA +ve vas-
culitis where ‘double positivity’ may confer a worse prognosis than ANCA
positivity alone. ELISA and RIAs are available. Conrmatory renal biopsy in
Goodpasture’s disease shows linear deposition of anti- GBM IgG along the
GBM, detectable by immunouorescence or immunoperoxidase staining.

699
Chapter11
Poisoning and overdose
General principles 700
Samples of medicolegal importance 702
Methods used in analytical toxicology 704
Brainstem death testing and organ donation 706
Interpretation of arterial blood gases in poisoned patients 707
Amphetamines and derivatives 708
Anticonvulsants 710
Benzodiazepines 712
Cannabis (marijuana) 713
Carbon monoxide 714
Cocaine 716
Cyanide 717
Digoxin 718
Ethylene glycol, ethanol, and methanol poisoning 720
Iron 724
Lead exposure and poisoning 726
Lithium 728
Methaemoglobinaemia 730
Opioids 731
Paracetamol (acetaminophen) poisoning 732
Salicylate poisoning 736
Theophylline 737
Tricyclic antidepressants 738
Warfarin and superwarfarins 739
Table of conversion factors between mass and molar units 740

700
CHAPTER11 Poisoning and overdose
700
General principles
Many poisoned patients recover without specic management other than
good supportive care. Aminority have life- threatening toxicity. In assessing
the poisoned patient, it is important to ensure adequate airway, breathing,
and circulation, take a thorough history, and undertake a full clinical exami-
nation. Tablets, bottles, syringes, aerosol containers, and other items found
with or near the patient should be retained and any corroborative history
obtained. It is usually best to analyse biological specimens (usually blood
and/ or urine) if analytical conrmation of toxin exposures is required.
The role ofblood and urine tests intoxicology
Close collaboration between analytical sta and clinicians is required if any-
thing other than the simplest toxicological analysis is to be useful.
Toxicological analysis using blood or urine is used to conrm:
•
The diagnosis of poisoning, when this is in doubt or for medicolegal
purposes.
•
To help in the management, or in the diagnosis of braindeath.
• To work out the time to restart chronic drug therapy e.g.
anticonvulsants.
Few centres have full analytical toxicology services, and a ‘toxicology
screen’ rarely inuences acute inpatient management, with the exception
of paracetamol, salicylate, lithium, digoxin, and iron poisoning, and on occa-
sions a drugs of abuse screen. Toxicological analysis of blood plasma or
serum is also of value if an extracorporeal method of elimination, such as
haemodialysis or MARS
®
(Molecular Absorbance Recirculating Systems),
1
is being contemplated. Any toxicology analysis should be tailored to that
patient’s circumstances and the poisons commonly encountered in that
country. In Western Europe and North America, most patients will have
taken pharmaceutical agents (often in combination), but pesticide poison-
ing, for example, is common in less well- developed countries.
Plasma paracetamol, salicylate, lithium, digoxin, and iron measurements
in blood are usually available on an urgent basis. For other patients, particu-
larly those who present a complex clinical picture or who are unconscious,
a 50mL sample of urine and a 10mL sample of heparinized blood should
be collected on admission and stored at 4°C (refrigerated). This can be
analysed later if it is felt the result will inuence your management or if
needed for medicolegal purposes (E Samples of medicolegal importance,
p. 702). Urine is useful for screening, especially for drugs of abuse, as it is
often available in large volumes and often contains higher concentrations of
poisons and their metabolites than blood samples. The samples should be
obtained as soon as possible after admission, ideally before any therapeutic
drugs are administered. Urine samples usually provide qualitative results,
e.g. detect the presence of amphetamines or benzodiazepines. Quantitative
measurements in urine are of little use because some compounds, such as
benzodiazepines, are extensively metabolized prior to excretion inurine.
1 Wittebole X, Hantson P. Use of the molecular adsorbent recirculating system (MARS
TM
) for the
management of acute poisoning with or without liver failure. Clin Tox 2011; 49
:782– 93.

GENERAL PRINCIPLES
701
Sample requirements
Plasma or serum is normally used for quantitative assays for drugs and drug
metabolites, and in general there are no marked dierences in concentration
between these uids. Evacuated blood tubes and containers containing gel
separators or soft rubber stoppers are not recommended if a toxicological
analysis is to be performed, as the plasticizers (phosphates and phthalates)
used in many such tubes may interfere with chromatographic methods, and
volatile compounds, such as CO or ethanol, may belost.
EDTA tubes are preferred for COHb assays and for measurement of lead
and some other metals, as these are concentrated in RBCs. Auoride/ oxa-
late tube should be used if ethanol, cocaine, or benzodiazepines are being
assayed, although special tubes containing 1% (w/ v) uoride are needed
if enzymic hydrolysis of these and other compounds is to be completely
prevented.
The use of disinfectant swabs containing alcohols should be avoided, as
should heparin, which contains phenolic preservatives (chlorbutol, cresol),
and preservatives containing mercury salts (see Table11.1).
Table11.1 Sample requirements formetals/ trace elements analysis
Metal Sample needed
Aluminium
10mL whole blood in plastic (not glass) tube— no anticoagulant/
beads*
Antimony 5mL heparinized whole blood; 20mL urine
Arsenic 5mL heparinized whole blood; 20mL urine
Bismuth 5mL heparinized whole blood
Cadmium 2mL EDTA whole blood*; 10mL urine*
Chromium 2mL heparinized whole blood*; 20mL urine*
Copper 2mL heparinized or clotted whole blood, or 1mL plasma;
10mL urine
Iron 5mL clotted blood or 2mL serum (avoid haemolysis)
Lead 2mL EDTA whole blood
Lithium 5mL clotted blood or 2mL serum (NOT lithium heparin tube!)
Manganese 1mL heparinized whole blood or 0.5mL plasma*
Mercury 5mL heparinized whole blood; 20mL urine
Selenium
2mL heparinized whole blood or 1mL plasma/ serum
Thallium 5mL heparinized whole blood; 20mL urine
Zinc
2mL whole blood (not EDTA) or 1mL plasma/ serum
* Send unused container from the same batch to check for possible contamination.

702
CHAPTER11 Poisoning and overdose
702
Samples ofmedicolegal importance
A toxicology screen is helpful if murder, assault, or child abuse is suspected.
Samples collected in such cases are often so important that they should
be kept securely at −20°C or below, until investigation of the incident is
concluded. Legal requirements mean that all specimens should be clearly
labelled with the patient’s family or last name and any forenames, the date
and time of collection, and the nature of the specimen, if this is not obvious.
Strict chain of custody procedures should be implemented, and the doctor
or nurse taking the sample should seal the bag with a tamper- proof device
and sign and date the seal. Achain of custody form must accompany the
sample and should be signed and dated by every person taking possession
of the sample. The sample should be secured in a locked container or refrig-
erator if left unattended before arrival at the laboratory.
Post- mortem concentrations of some drugs may not necessarily repre-
sent antemortem levels and should therefore be interpreted with caution,
e.g. in one case where a patient died 72h after admission to hospital, the
post- mortem serum olanzapine concentration was elevated 5- fold over the
antemortem level.
2
With passage of time after death, some drugs (espe-
cially basic drugs with a high volume of distribution) move along a concen-
tration gradient from areas of high concentration in solid organs into the
local blood, a phenomenon called ‘post- mortem redistribution’. Therefore,
the highest blood drug concentrations are found in central vessels such as
the pulmonary artery or vein and the lowest levels in femoral veins e.g.
morphine, amphetamines. Thus, the most appropriate post- mortem blood
sample is a femoral blood sample.
The stability of the drug in post- mortem blood samples that are stored
also needs to be considered. After a specimen has been collected, enzymes
may remain active and continue to metabolize the drug in vitro. In general,
drug instability occurs because functional groups are susceptible to transfor-
mation (e.g. 6- acetylmorphine) or readily oxidized or reduced. Olanzapine
blood concentrations d with time by 23– 84% during storage.
3
However,
conjugated drugs, such as the glucuronides, may deconjugate, which i
the free drug concentration. In general, drug stability should be evaluated
with consideration of long- term storage, the eect of freeze– thaw cycles,
short- term stability (e.g. refrigerated samples), and bench- top (room tem-
perature) stability. Thus, femoral blood sample drug concentrations should
be measured as soon as possible after death and samples must be stored
appropriately.
Further reading
Drummer OH. Requirements for bioanalytical procedures in post- mortem toxicology. Anal Bioanal
Chem 2007; 388
:1495– 503.
Kerrigan S. Sampling, storage and stability. In:A Negrusz, G Cooper (eds.). Clarke’s Analytical Forensic
Toxicology
, 2nd edn. London:Pharmaceutical Press, 2013; pp.335– 50.
2 Chue P, Singer P. A review of olanzapine- associated toxicity and fatality in overdose. J Psychiatr
Neurosci 2003; 28
:253– 61.
3 Baselt RC, Cravey RH. Disposition of Toxic Drugs and Chemicals in Man, 9th edn. San
Francisco:Chemical Toxicology Institute,2011.

SAMPLES OF MEDICOLEGAL IMPORTANCE
703

704
CHAPTER11 Poisoning and overdose
704
Methods used inanalytical toxicology
Older and newer methods are discussed here to reect global use. Arange
of chromatographic and other methods, such as radioligand immunoassays,
are available for toxicological analyses. Plasma concentrations associated
with serious toxicity range from µg/ L in the case of drugs such as digoxin to
g/ L in the case of ethanol.
Specialized laboratories use a combination of solvent extraction and
thin- layer chromatography (TLC) together with gas chromatography- mass
spectrometry (GC- MS) or liquid chromatography tandem- mass spectrom-
etry (LC- MS/ MS) as the basis for a ‘toxicology screen’. It is unwise to use
TLC without corroboration of results by another method, e.g. LC- MS/ MS,
because the resolution power of TLC is limited and interpretation of
chromatograms is subjective. Acommercial kit for TLC (Toxi- lab, Marion
Laboratories) is supplied with a compendium of colour plates, but even so
problems can arise in the dierentiation of compounds with similar mobility
and colour reactions. The kit is aimed at the US market, and some common
UK drugs are not included. Spectrophotometry is commonly used to meas-
ure salicylates, iron, and COHb. However, UV spectrophotometry and
spectrophotouorimetry are often used as detectors for high- performance
liquid chromatography (HPLC) and in immunoassays. Spectrophotometric
methods and immunoassays often suer from interference from metabo-
lites or other drugs. Immunoassays have the advantage of long shelf- life
and simplicity, but all require conrmation with a chromatographic method
if the results are to stand scrutiny. This is because immunoassays for small
molecules are often not specic, e.g. some urine amphetamine immunoas-
says give +ve results with proguanil, isoxsuprine, labetalol, tranylcypromine,
and phenylethylamine. The Syva Emit antidepressant assay cross- reacts
with phenothiazines after overdose. Chromatographic methods have the
advantage of selectivity and sensitivity and the ability to perform quanti-
tative measurements, but they are more expensive. Generally, gas– liquid
chromatography (GLC) or LC- MS/ MS are used to measure basic drugs.
Acidic or neutral moieties can also be analysed by LC- MS/ MS. For screen-
ing very large numbers of compounds simultaneously or sequentially, then
the Applied Biosystems Q- Trap technology is one of the most versatile—
this is an LC- MS/ MS where one of the quadruples of the triple quadrupole
is used as an ion trap. Most laboratories that aim to be as versatile as pos-
sible have a fast GLC, in addition to the LC- MS/ MS.
Modern methods of assay for heavy metals vary enormously. Inductively
coupled plasma mass spectroscopy (ICPMS) is the most commonly used
method in the UK. Here, ICPMS is used, instead of the ame or electro-
thermal furnace for atomization of the elements and mass is the detector.
Atomic absorption spectrophotometry, with either ame or electrothermal
atomization is the older method. In the case of iron, reliable kits based on
the formation of a coloured complex are available.

METHODS USED IN ANALYTICAL TOXICOLOGY
705
There is wide variation in the units that various laboratories use to report
results. This has caused confusion and errors in treatment, and great care is
needed to ensure that clinical interpretation is undertaken in full knowledge
of the units used by the reporting laboratory. Particular care is also required
in interpretation and application of analytical techniques in post- mortem
toxicology, due to changes in concentrations of blood in storage and dif-
ferential post- mortem redistribution of drug after death (E Samples of
medicolegal importance, p. 702).
Further reading
Baselt RC, Cravey RH. Disposition of Toxic Drugs and Chemicals in Man, 9th edn. San Francisco,
Chemical Toxicology Institute,2011.
Drummer OH. Requirements for bioanalytical procedures in post- mortem toxicology. Anal Bioanal
Chem 2007; 388
:1495– 503.

706
CHAPTER11 Poisoning and overdose
706
Brainstem death testing and organ
donation
Brain death cannot be diagnosed in the presence of drugs that mask CNS
activity, e.g. baclofen. The rule of thumb, based on the pharmacological
principle that most drugs need ve half- lives to be eectively eliminated
from the circulation, is to allow four half- lives of any drug to elapse before
declaring death, or to allow at least 2– 3days for drug eects to wear o.
Whether this is satisfactory for patients with organ failure, and hence
impaired drug elimination, is unclear. Often in patients being assessed for
organ donation, measurement of plasma concentrations of residual drugs,
with expert interpretation, is required to determine whether brainstem
death tests are valid or whether a drug could be interfering with the results.
Selected donor organs from those who have died from poisoning by tri-
cyclic antidepressants, benzodiazepines, barbiturates, insulin, CO, cocaine,
methanol, and paracetamol have been used in transplantation. It is impor-
tant to identify which organs act as reservoirs for drugs and either not con-
sider such organs, e.g. a liver from a paracetamol- poisoned patient, or take
prophylactic precautions like acetylcysteine administration in the case of
donation of a heart from a paracetamol- poisoned patient.
Further reading
Baselt RC, Cravey RH. Disposition of Toxic Drugs and Chemicals in Man, 9th edn. San Francisco,
Chemical Toxicology Institute,2011.
Hanson P. Organ donation after fatal poisoning:an update with recent literature data. Adv Exp Med
Biol 2004; 550:207.
Wood DM, Chan WL, Dargan P. Using drug- intoxicated deaths as potential organ donors:impres-
sion of attendees at the American College of Medical Toxicology 2014 annual scientic meeting.
J Med Toxicol 2014; 10
:360– 3.

INTERPRETATION OF ABG IN POISONED PATIENTS
707
Interpretation ofarterial blood gases
inpoisoned patients
Interpretation of blood gas values may be found in E OHCM 10e, p.162,
pp. 188–9. Essentially four patterns emerge, which may occur together.
Respiratory acidosis
Hypoventilation results in retention of CO
2
. This can occur after an over-
dose with any drugs that depress the CNS, e.g. tricyclic antidepressants,
opioids, and ketamine.
Respiratory alkalosis
Hyperventilation with respiratory alkalosis is classically caused by aspirin
(salicylate). It can also occur in response to hypoxia, drugs, and CNS injury.
Metabolic alkalosis
Metabolic alkalosis is very uncommon in poisoning. Rarely, it may result
from excess administration of alkali, e.g. deliberate alkali ingestion.
Metabolic acidosis
This is the commonest metabolic abnormality in poisoning. If acidosis is par-
ticularly severe (e.g. pH <7.2), this should raise the question of poisoning by
ethanol, methanol, or ethylene glycol. Measuring the anion gap and osmolar
gaps are helpful in further dierentiation of the medical or toxicological
cause (E Ethylene glycol, ethanol, and methanol poisoning, pp. 720–2).

708
CHAPTER11 Poisoning and overdose
708
Amphetamines and derivatives
For example, methylene dioxymethamphetamine (MDMA) (ecstasy), MDEA,
crystal methamphetamine (‘ICE’), paramethoxymethamphetamine(PMA).
Acute amphetamine overdose may cause sympathetic hyperstimulation,
cardiovascular collapse, rhabdomyolysis, ventricular tachyarrhythmias, and
death (often trauma- related). The following investigations should be con-
sidered in patients presenting to hospital with acute amphetamine(s) intoxi-
cation. In man, the half- life of methamphetamine ranges from 10 to 20h,
depending on urine pH and the dosetaken.
Plasma urea and electrolytes and glucose
It is critical that at least one set of U&E and creatinine are checked in every
patient. Most are profoundly dehydrated and require vigorous rehydra-
tion. Some patients develop hyponatraemia, often after drinking excess
water, and ADH secretion may also be responsible for this (E OHCM 10e,
p.672). Hypoglycaemia mayoccur.
Dipstick test ofurine formyoglobin and subsequent serum
creatinekinase
Hyperthermia can develop after amphetamine exposure and may cause
rhabdomyolysis. Hyperthermia may be one of the features of serotonin
toxicity (accompanied by autonomic instability, ocular and peripheral clo-
nus. and hyper- reexia). Dipstick testing of urine is +ve for blood in rhab-
domyolysis, as myoglobin is detected by the Hb assay. This is an indication
that serum CK should then be measured. If found to be elevated, adequate
rehydration is needed to reduce deposition of myoglobin in renal tubules
and reduce the risk of ARF as a consequence. Caution in use of alkaliniza-
tion as for certain drugs, e.g. amphetamines; this potentially delays drug
excretion.
Full bloodcount
Rarely, aplastic anaemia (E OHCM 10e, p.364) has been reported after
ecstasy (MDMA) ingestion.
Clotting studies
DIC (E OHCM 10e, p.353) can occur, often in the context of hyperther-
mia. Once liver damage ensues, INR/ PT (E OHCM 10e, p.351) willrise.
Temperature
Hyperpyrexia can lead to rhabdomyolysis, DIC, and hepatocellular necrosis.
Risks relate to the time in hours spent above 39°C. Arectal thermometer
is the most accurate measure of temperature.
Blood pressure
A BP >180/ 120mmHg requires urgent medical care to reduce the risk of
stroke.
Liver functiontests
Acute liver injury can occur with a rise in AST or ALT, often of several
thousands.

AMPHETAMINES AND DERIVATIVES
709
3 Note:do not miss a hidden paracetamol overdose— check paracet-
amol levels in blood, from the earliest sample you have on that patient!
Checking an INR or prothrombin ratio (PTR) is essential in suspected late
paracetamol poisoning and is of good prognosticvalue.
ECG
An ECG should be carried out in patients with tachycardia, chest pain,
or reduced GCS. Patients should undergo cardiac monitoring. Cardiac
arrhythmias are common and deaths, which occur soon after ingestion, may
be due to these. Arrhythmias are often supraventricular, although ventricu-
lar arrhythmias also occur. Serial troponin levels are required if there has
been/ is chestpain.
Urinetests
Urine tests, e.g. EMIT dipstick system or by immunoassay in the labora-
tory, are sensitive and group- specic for amphetamines and can conrm an
amphetamine has been ingested if that is in doubt, e.g. agitated patient in
the emergency department. Note:amphetamine, MDMA, and MDA con-
centrations in blood are of no value in determining clinical management.
Newer synthetic amphetamines (cathinones) may not be detected by urine
dipstick tests but can be detected by chromatographic techniques in the
laboratory.
Imaging
A CT brain scan should be performed for patients with altered conscious
state, focal neurological signs, or severe headache.
Patients who are suspected body packers or body stuers should
undergo abdominal imaging, e.g. CTscan.
Further reading
Jones AL. Amphetamine overdose. BMJ Best Practice (online), Sept 2015. M us.bestpractice.bmj.com.
Volkow ND, Fowler JS, Wang GJ, etal. Distribution and pharmacokinetics of methamphetamine in
the human body:clinical implications. PLoS One 2010; 5:e152369.

710
CHAPTER11 Poisoning and overdose
710
Anticonvulsants
For most anticonvulsants, LC- MS/ MS will be the analytical method of
choice. However, some (e.g. valproic acid) are dicult to assay by LC- MS/
MS, and GLC may be more appropriate.
Carbamazepine toxicity
Plasma concentrations of carbamazepine and its active metabolite the
10,11- epoxide can be measured by HPLC or LC- MS/ MS but do not cor-
relate well with the degree of toxicity. They are seldom performed, unless
the diagnosis is in doubt or there is concern about a therapeutic excess.
The therapeutic range in plasma is between 8 and 12mg/ L. Toxicity has
been seen with carbamazepine concentrations above 20mg/ L (85mmol/
L). Coma, ts, respiratory failure, and conduction abnormalities have been
seen with concentrations in excess of 40mg/ L (170mmol/ L). In seven fatali-
ties due to carbamazepine overdose, femoral blood concentrations taken
post- mortem averaged 45mg/ L (range 35– 70).
4
An FBC should be performed. U&E and creatinine should also be
checked, as hyponatraemia and SIADH (E OHCM 10e, p. 241) have
been reported. Hypoglycaemia has also been reported. LFTs and PTR/
INR should be performed to assess hepatotoxicity. An ECG should be per-
formed in all but the most trivial carbamazepine overdosage to assess if
any conduction abnormalities or interval prolongations (e.g. QRS widening,
sinus tachycardia, AV block, or QTc prolongation) are present.
Lamotrigine toxicity
Overall, most patients exposed to lamotrigine in overdose experienced no
clinical eects.
5
Plasma concentrations of lamotrigine can be measured for
compliance purposes (therapeutic range 1– 4mg/ L; upper limit may be as
high as 10mg/ L) but are not of value in the overdose situation. In a fatal
case, the femoral blood concentration was 54mg/ kg.
4
Valproate toxicity
Plasma concentrations of sodium valproate can be measured by HPLC but
do not correlate well with either the depth of coma or risk of seizures after
overdose. The therapeutic plasma range is 40– 100mg/ L. U&E, creatinine,
and glucose should be measured, as hypernatraemia, hypoglycaemia, and
hypocalcaemia have been reported after overdosage. LFTs and PTR/ INR
should be taken to assess hepatotoxicity. Depending on the clinical need, a
CT head scan and/ or ECG may be required. Post- mortem blood concen-
tration in a 34- year- old man was 720mg/ L.
4
4 Baselt RC, Cravey RH. Disposition of Toxic Drugs and Chemicals in Man, 9th edn, San Francisco,
Chemical Toxicology Institute,2011.
5
Lofton AL, Klein- Schwartz. Evaluation of lamotrigine toxicity reported to poison centers. Ann
Pharmacother 2004; 38
:1811– 15.

ANTICONVULSANTS
711
Phenytoin toxicity
Most patients with acute phenytoin poisoning do not require measurement
of their plasma phenytoin concentration and are treated with multi- dose
activated charcoal. Blood glucose, U&E, creatinine, LFTs, and PTR/ INR
should be checked. An ECG should be obtained. Most cardiac complica-
tions have occurred with rapid (>50mg/ min) IV administration. ACT head
scan is needed if there is focal neurology (excluding ataxia or nystagmus) or
any history of traumatic injury.
Most phenytoin toxicity is managed by repeat multi- dose activated
charcoal. Rarely, an urgent phenytoin measurement may help in severe
phenytoin poisoning where charcoal haemoperfusion or some other extra-
corporeal elimination method, such as MARS, is being considered,
6
e.g. if
the plasma phenytoin concentration is above 40mg/ L and rapidly rising or
is close to, or exceeding, the potential lethal level of 100mg/ L. The over-
all clinical value of such elimination methods remains to be established.
Post- mortem phenytoin concentration in femoral blood of a 4.5- year- old
child who ingested 2g was 45mg/ L.
4
Phenytoin undergoes post- mortem
redistribution.
Patients with suspected chronic phenytoin toxicity as a result of thera-
peutic dosing should have their plasma phenytoin concentration measured.
The ‘therapeutic range’ is 10– 20mg/ L. Routine measurements may be use-
ful to monitor anticonvulsant therapy or to time re- institution of chronic
therapy after overdose.
6 Sen S, Ratnaraj N, Davies NA, etal. Treatment of phenytoin toxicity by the Molecular Adsorbents
Recirculating System (MARS). Epilepsia 2003; 44:265– 7.

712
CHAPTER11 Poisoning and overdose
712
Benzodiazepines
Most patients who have taken an overdose with benzodiazepines just sleep
o the drug without sequelae within 24h. However, more severe eects
can occur when benzodiazepines are mixed with other drugs, especially in
patients with pre- existing cardiovascular or respiratory disease. Pulse oxi-
metry is useful for monitoring the adequacy of ventilation if signicant CNS
depression is present.
Generally, measuring benzodiazepine concentrations in blood or urine
is not of value in the management of benzodiazepine overdose patients.
Benzodiazepines can be detected in routine urine drugs screens. LC- MS/
MS is the method of choice for quantitative analysis of diazepam and its
metabolites. Liquid chromatography simultaneously assays diazepam and its
polar metabolites, and post- mortem blood concentrations of 5 and 19mg/ L
have been found in fatalities.

CANNABIS (MARIJUANA)
713
Cannabis (marijuana)
Cannabis use may be detected in routine urine drug screening. They may
be detected for up to several days after use, depending on the dose and
frequency ofuse.
Synthetic cannabinoids may be consumed in herbal mixtures for recrea-
tional use, as an alternative to cannabis. Blood glucose, U&E, creatinine,
and ECGs should be undertaken. Note that synthetic cannabinoids, e.g.
JWH018, are often not detected by standard bedside urine drug screens
(and thus are often the drugs of abuse selected by workers who undergo
regular employment screening). However, they are detected by GC- MS,
mass spectrometry, or LC- MS/ MS if the sample is sent to the laboratory.
Further reading
Baselt RC, Cravey RH. Disposition of Toxic Drugs and Chemicals in Man, 9th edn, San Francisco,
Chemical Toxicology Institute,2011.
Hermanns- Clausen M, Kneisel S, Szabo B, Auwärter V. Acute toxicity due to the conrmed con-
sumption of synthetic cannabinoids:clinical and laboratory ndings. Addiction 2013; 108
:534– 44.

714
CHAPTER11 Poisoning and overdose
714
Carbon monoxide
CO non- re poisoning deaths have started to reduce in the UK, e.g. in
2012, compared with previous years. Incidence peaks in winter months, due
to i
use of indoor heating and fossil fuel- powered generators and reduced
external ventilation. It is highly likely that CO poisoning remains signicantly
underdiagnosed with patients presenting with ‘u- like’ illness.
Survival rates for those resuscitated post- cardiac arrest with CO poison-
ing is very low, especially if there was pre- existing cardiovascular or respira-
tory disease. Pregnant women and their fetus are particularly susceptible
to CO poisoning. Patients surviving signicant CO poisoning may develop
acute or delayed neurological sequelae leading to loss of consciousness,
coma, and death. In the recovery phase, neurological sequelae, such as poor
concentration and memory problems, may be seen. Symptoms include cog-
nitive or personality changes, incontinence, psychosis, and Parkinsonian fea-
tures such as rigidity. 7
50– 75% of people recover from their neurological
features within 1year.
A COHb concentration in blood conrms recent exposure to CO and
should be measured urgently in all patients with suspected CO poisoning,
including those with smoke inhalation. The space above the blood in the
sample tube (headspace) should be minimized prior to blood gas analysis.
Expected ‘normal’ values for COHb are up to 5% in non- smokers and up
to 10% in smokers. However, people with pre- existing cardiovascular or
respiratory disease can present with symptoms of toxicity at COHb con-
centrations in the low or normal range. ACOHb concentration, however,
does not measure severity of poisoning because COHb begins to dissociate
from the moment of removal from the source of CO, and the rate of dis-
sociation is also dependent on factors such as exogenous O
2
administration.
Thus, any attempt to ‘back- extrapolate’ to nd the initial highest COHb, e.g.
by using nomograms, is awed.
Management of the CO- poisoned patient is determined by the patient’s
clinical condition and also on circumstantial evidence such as the inten-
sity and duration of exposure, rather than a COHb concentration per
se, although a level of >40% has been used as one criterion to guide the
use of hyperbaric O
2
therapy (which is of controversial clinical value).
The CO- exposed patient should be administered high- ow O
2
(e.g. 12L/
min through a tight- tting non- rebreather mask) until the COHb is <5%
and clinical signs of CO poisoning, such as impaired heel– toe walking and
nger– nose incoordination, have resolved. If in doubt, O
2
is administered
for a minimum of 6h. It is currently unknown if hyperbaric O
2
is more eec-
tive than normobaric 100% O
2
in prevention of neurological complications
in patients with CO poisoning.
Arterial bloodgases
Any patient with suspected poisoning by CO requires ABG analysis. O
2
saturation monitors are misleading, as they read COHb as oxyhaemoglobin
(HbO), and the true O
2
saturation of the patient can only be determined
by ABG analysis.

CARBON MONOXIDE
715
Electrocardiogram
An ECG should be performed in anyone severely poisoned (e.g. drowsi-
ness or any neurological abnormality, chest pain, or breathlessness) or with
pre- existing ischaemic cardiomyopathy. ECG changes, such as ST- segment
depression, T wave abnormalities, ventricular tachycardia or brillation, and
cardiac arrest can occur. If ischaemia/ infarction is seen on the ECG or sus-
pected clinically, then serial troponins should be requested.
Further reading
Fisher DS, Leonardi G, Flanagan RJ. Fatal unintentional non- re- related carbon monoxide poison-
ing:England and Wales, 1979– 2012. Clin Toxicol 2014; 52:166– 70.
Smollin C, Olson K. Carbon monoxide poisoning (acute). BMJ Clin Evid. 2010 Oct 12; 2010– 103.

716
CHAPTER11 Poisoning and overdose
716
Cocaine
Cocaine is snorted into the nose or injectedIV.
Blood pressure monitoring
Patients with cocaine intoxication should have frequent measurements of
their BP, as hypertension is a signicant risk, and strokes and chest pain
have been widely reported. Acontinuous monitoring device for repeated
measurements is suitable.
ECG and cardiac markers
Cocaine- induced angina and MI are common. ECG monitoring is advised
for all but the most trivial exposure to cocaine.
The predominant coronary pathology of cocaine is vasoconstriction.
Thrombosis is less common, and troponin is the most sensitive indicator of
myocardial damage (MI) due to cocaine. Cardiac troponins should also be
performed in any patient with chest pain or ECG abnormalities. They are
the most sensitive and specic markers. If elevated, then coronary angiog-
raphy is usually indicated.
The ECG is of less diagnostic value. Up to 84% of patients with cocaine-
related chest pain have abnormal ECGs, even in the absence of MI. In
another study, 42% of patients with ‘MI- like’ ST elevation on their ECGs had
MI excluded by cardiac markers. In a further study, total CK was elevated in
75% of patients, including 65% withoutMI.
Urine or blood testing
The use of cocaine can be determined by self- reports from patients or urine
analysis of metabolites of cocaine (benzoylecgonine), e.g. EMIT test. This
remains +ve for 24– 48h after cocaine use. In assessing patients who may
be a body packer, a +ve urine test may indicate pre- existing cocaine use or
leaking cocaine from the packets.
GC- MS is more specic and can be carried out on blood or urine.
Cocaine is unstable in blood, and samples are best taken into 1% w/ v uo-
ride oxalate tubes if medicolegal sequelae of cocaine use are a possibility.
‘Nasal insuation of 106mg of the drug to six volunteers produced mean
peak plasma concentrations of 0.22mg/ L at 0.5h and 0.61mg/ L for the
metabolite benzoylecgonine at 3h’.
7
Smoking 50mg in six volunteers pro-
duced mean peak plasma concentrations of 0.2mg/ L at 0.08h and 0.15mg/
L for benzoylecgonine at 1.5h. Patients have survived plasma concentrations
of 5.2mg/ L, but usually fatalities are associated with cocaine/ benzoylecgo-
nine concentrations in excess of 5mg/ L, depending on the route of use.
The IV route is the most dangerous. Cocaine may show signicant post-
mortem redistribution.
Further reading
McCord J, Jneid H, Hollander JE, etal.; American Heart Association Acute Cardiac Care Committee
of the Council on Clinical Cardiology. Management of cocaine- associated chest pain and myocar-
dial infarction:a scientic statement from the American Heart Association Acute Care Committee
of the Council on Clinical Cardiology. Circulation 2008; 117:1897– 907.
Senthilkumaran S, David SS, Jena NN, Menezes RG, Thirumalaikolundusubramanian P.Acute myo-
cardial infarction and cocaine toxicity:One step closer. Indian J Crit Care Med 2014; 18:118.
Shields LB, Rolf CM, Hunsaker JC 3rd. Sudden death due to acute cocaine toxicity- excited delirium
in a body packer. J Forensic Sci 2015; 60:1647– 51.
7 Baselt RC, Cravey RH. Disposition of Toxic Drugs and Chemicals in Man, 9th edn. San Francisco,
Chemical Toxicology Institute,2011.

CYANIDE
717
Cyanide
Cyanide poisoning can occur by deliberate inhalation of gas, ingestion of
cyanide salts, or exposure in industrialres.
Arterial blood gas estimation
Essential to determine the O
2
saturation and acid– base status of the patient.
Serum lactate
This is helpful in conrming suspected toxicity and can be used clinically as
a surrogate for the cyanide assay. It is likely to exceed 7mmol/ L in cases of
signicant exposure. Thus, a blood gas analyser in the emergency depart-
ment can give a quick (indicative) answer to the question of whether expo-
sure has takenplace.
Electrocardiogram
All patients should have an ECG. It should be examined for evidence of
ischaemic damage, e.g. ST depression, ST elevation, T wave inversion.
Cyanideassay
Blood cyanide concentrations are rarely of use in emergency management,
because they cannot be measured quickly enough. However, a sample
should be taken before antidote administration for assay at a later stage.
Cyanide concentrations of <0.2mg/ L are ‘normal’; 1.0– 2.5mg/ L causes
obtundation and coma, and >2.5mg/ L is potentiallyfatal.
3 Note:the antidote of choice for cyanide poisoning is hydroxocobala-
min, together with O
2
; these are antidotes that can safely be given without
certainty of cyanide ingestion. An alternative is to give sodium thiosulfate
and sodium nitrite, and dicobalt edetate. However, note that dicobalt ede-
tate should only be given if cyanide poisoning is certain, i.e. a proper history
is available; otherwise you may kill your patient with cardiotoxicity of the
antidote. Excessive administration of sodium nitrite can cause signicant
methaemoglobinaemia.
Further reading
Borron SW, Baud FJ, Barriot P, Imbert M, Bismuth C. Prospective study of hydroxocobalamin for
acute cyanide poisoning in smoke inhalation. Ann Emerg Med 2007; 49
:794– 801.
Borron SW, Baud FJ, Megarbane B, Bismuth C.Hydroxocobalamin for severe acute cyanide poison-
ing by ingestion or inhalation. Am J Emerg Med 2007; 25
:551– 8.
Hall AH, Saiers J, Baud F. Which cyanide antidote? Crit Rev Toxicol 2009; 39:541– 52.

718
CHAPTER11 Poisoning and overdose
718
Digoxin
Patients who are already taking digoxin and those with pre- existing CVD are
more susceptible to digoxin toxicity after overdosage. Digoxin toxicity can
also result from progressive renal impairment or due to interactions with
other drugs, as well as overdoses. Acute digoxin toxicity is an indication for
oral multi- dose activated charcoal and IV hydration.
Electrocardiogram
All patients with suspected digoxin poisoning should have a 12- lead ECG,
and all symptomatic patients should be attached to a cardiac monitor.
Digoxin poisoning can cause virtually any type of cardiac arrhythmia due to
i automaticity and d AV conduction. The combination of heart block with
tachyarrhythmia is very common.
Plasma digoxin concentration
Absorption of digoxin often peaks at 4– 6h after ingestion. Its half- life is in
excess of 30h. Digitoxin is a structurally related drug that has an even longer
plasma half- life (6days). Adigoxin measurement is a useful, but not absolute,
guide to toxicity, as plasma digoxin concentrations correlate poorly with the
severity of poisoning, particularly early in the course of acute poisoning.
However, it is desirable in acute poisoning (although not essential) if anti-
digoxin antibody fragments (Fab) are to be used, as it is useful in calculating
the dose of fragments required (E Digoxin, Indications for Fab fragments
and doses of Fab fragments, pp. 718–19), as well as conrming exposure.
Plasma digoxin concentrations cannot be interpreted after administration
of digoxin Fab using normal assay procedures. Samples taken to investigate
probable chronic digoxin intoxication should be taken at least 6h after dos-
ing. They are not normally analysed urgently, unless life- threatening features
of toxicity are present and use of Fab is being considered. The therapeutic
range for digoxin is 0.8– 2.0µg/ L.
Urea and electrolytes, creatinine
It is important to ascertain if the patient has any renal impairment and
plasma creatinine and urea are helpful, although of course do not exclude
renal impairment completely. Hyperkalaemia is common in acute digoxin
overdose and may be severe, e.g. >7mmol/ L.
If possible, a magnesium level is helpful to exclude hypomagnesaemia,
which contributes to risk of cardiotoxicity and is easily corrected.
Indications fordigoxin antibody (Fab) fragments inacute
toxicity and doses ofFab fragments
• Bradycardia or heart block associated with hypotension.
• Tachyarrhythmias associated with hypotension, especially ventricular
arrhythmias
Fab fragment administration should be considered in less severe stages of
poisoning in older patients and those with pre- existing CVD. At one time,
the plasma K
+
concentration was considered an indication for use of Fab
fragments, but this is no longer used as an indication. Previously, use of

DIGOXIN
719
Fab fragments was considered for chronic toxicity, but this tends not to be
usednow.
The dose of Fab fragments to give for an acute ingestion of digoxin can
be calculated from either the dose of digoxin ingested or the plasma digoxin
concentrations. If in doubt, ten ampoules of Digibind
®
can be given, fol-
lowed by an additional ten ampoules if clinically indicated.
Number of 40mg vials of Fab=plasma digoxin concentration
(ng/ mL) × body weight ×0.0084
Or
Ingested dose (mg)×1.2
Or
Best guess of 10– 20 vials
E OHCM 10e, p. 842. Please note that if other types of digoxin- binding
antibodies are used, then the dosage may be dierent to those indicated
above.
Further reading
BMJ Best Practice (last updated 2016). Digoxin overdose. M http:// bestpractice.bmj.com/ best-
practice/ monograph/ 338.html.
Dasgupta A. Therapeutic drug monitoring of digoxin:impact of endogenous and exogenous digoxin-
like immunoreactive substances. Toxicol Rev 2006; 25:273– 81.
Flanagan RJ, Jones AL. Fab antibody fragments:some applications in clinical toxicology. Drug Safety
2004; 27
:1115– 33.

720
CHAPTER11 Poisoning and overdose
720
Ethylene glycol, ethanol, and methanol
poisoning
A history of ingestion or the presence of a metabolic acidosis raises suspi-
cion of poisoning with these substances. Calculation of the anion gap and
osmolal gaps is helpful in the assessment of such patients.
Aniongap
The normal anion gap is 12±2.
Many toxins cause a high anion gap acidosis and these
include
• Ethanol.
• Methanol. (Note:the high anion gap is due to metabolites and may take
several hours to develop.)
•
Ethylene glycol. (Note:the high anion gap is due to the metabolites and
may take 6– 24h to develop.)
• Metformin.
• Cyanide.
• Isoniazid.
• Salicylates (aspirin).
This list can be further reduced by measuring the osmolalgap.
Osmolalgap
This is the dierence between the laboratory estimation of osmolality
(Om) and calculated osmolality(Oc).
Toxic causes ofa raised osmolal gap include
• Methanol.
• Ethylene glycol.
• Diethylene glycol.
• Isopropanol.
• Ethanol.
The acronym ‘MEDIE’ can be a helpful mnemonic.
Calculating theaniongap
Anion gap = ([Na
+
] + [K
+
] – ([Cl
–
] + [HCO3
–
])
Calculating theosmolalgap
The osmolal gap is measured osmolality (Om) minus calculated
osmolality(Oc):
Oc = 2([Na
+
] + [K
+
]) + [urea] + [glucose]
The osmolal gap is normally<10.

ETHYLENE GLYCOL, ETHANOL, AND METHANOL POISONING
721
Ethylene glycol and methanol plasma concentrations
Often the diagnosis of ethylene glycol or methanol poisoning can be dif-
cult, because assays for these substances are not widely available. If
possible, their measurement can help manage severe intoxication. Other
parameters may have to be used, i.e. anion gap, osmolal gap, and ABG
analysis. Anormal osmolal gap does not exclude poisoning with ethylene
glycol or methanol, but if the osmolal gaps and anion gaps are both nor-
mal, and the patient is not symptomatic, then signicant ingestion is unlikely
to have occurred. In general, ethylene glycol or methanol measurements
should not be carried out, unless metabolic acidosis is present and there
is an aniongap.
Ethylene glycol and methanol concentrations in blood are useful to con-
rm ingestion and indicate when to stop antidotal treatment (with ethanol
or 4- methylpyrazole) and/ or when haemodialysis is needed (>500mg/ L;
E Indications for haemodialysis in methanol or ethylene glycol poisoning
are, p. 722). However, a low concentration may just mean that most of
the parent compound has been metabolized. Formate (i.e. the methanol
metabolite) levels can also be checked in patients who may have taken
methanol.
Microscopy ofurine foroxalate crystals
In suspected ethylene glycol poisoning, microscopy can be performed to
look for oxalate crystals. However, they are only present in 50% of cases
and often only many hours after ingestion. Treatment of a patient should
not be delayed or dependent upon looking for crystals.
Plasma ethanol concentrations
Plasma ethanol concentrations are usually not needed in patients who are
drunk, unless there is doubt about the diagnosis, e.g. patients with a widen-
ing osmolal gap or the patient is so severely poisoned that haemodialysis is
being considered for the ethanol poisoning. They are, however, essential
to guide appropriate use of ethanol as an antidote in ethylene glycol or
methanol poisoning (E Ethylene glycol, ethanol, and methanol poison-
ing, Indications for haemodialysis in methanol or ethylene glycol poisoning,
p. 722). Rarely, a plasma ethanol measurement will be needed in child pro-
tection cases, and such sampling will need chain of custody and a specic
method (GLC) by a specialist laboratory. The need for frequent monitoring
of ethanol concentrations during treatment is avoided by use of the alter-
native antidote 4- methylpyrazole (a competitive alcohol dehydrogenase
antagonist).
Antidotal therapy withethanol
The dose of ethanol for treatment of ethylene glycol and methanol poison-
ing can be very dicult to predict, because ethanol metabolism is variable
and unpredictable. It is therefore important to frequently recheck blood
ethanol concentrations in patients receiving an ethanol infusion. The dose
should be adjusted to achieve a blood ethanol concentration of 1– 1.5g/ L to
achieve competitive inhibition of alcohol dehydrogenase.

722
CHAPTER11 Poisoning and overdose
722
Indications forcontinued ethanol therapyare
• Methanol or ethylene glycol poisoning with blood concentrations
>200mg/ L.
• Metabolic acidosis with pH<7.3.
• Osmolal gap >10mOsmol/ kgwater.
• Formate concentration >10mg/ L.
• Urinary oxalate crystals.
• Severe symptoms.
Indications forhaemodialysis inmethanol or ethylene glycol
poisoningare
• Methanol or ethylene glycol concentration >500mg/ L.
• Severe metabolic acidosis (pH <7.3) unresponsive to therapy, i.e. ABGs
are needed in all cases of high anion gap poisoning.
•
Renal failure— hence, it is essential to check plasma U&E in all patients.
• Presence of visual problems in methanol poisoning.
• Formate concentration >500mg/ L in methanol poisoning.
• Haemodialysis should be continued until the methanol/ ethylene glycol
concentration is well below 200mg/ L.
Further reading
Brent J. Fomepizole for ethylene glycol and methanol poisoning. N Engl J Med 2009; 360:2216– 23.
McMartin K, Jacobsen D, Hovda KE. Antidotes for poisoning by alcohols that form toxic metabolites.
Br J Clin Pharmacol 2016; 81:505– 15.
Megarbane B, Borron SW, Baud FJ. Current recommendations for treatment of severe toxic alcohol
poisonings. Intens Care Med 2005; 31
:189– 95.

ETHYLENE GLYCOL, ETHANOL, AND METHANOL POISONING
723

724
CHAPTER11 Poisoning and overdose
724
Iron
Serum iron concentrations
In the UK, one 200mg tablet of ferrous sulfate contains 65mg of elemental
iron. Iron is also found in varied amounts in many over- the- counter vita-
min supplements. The elemental iron content of the preparation ingested
should be checked carefully, as the important consideration is the amount
of elemental iron ingested, not the weight of iron or vitamin tablets.
Serum iron concentrations should be measured urgently in all patients
who may have ingested >30mg/ kg of elemental iron acutely, those who
have ingested an unknown quantity, or those with symptoms, e.g. GI. If a
sustained- release preparation of iron has been taken, a later serum iron
concentration should be taken. Ablood sample taken late after ingestion
may underestimate the iron, as it may have already started distributing to
tissues, i.e. in a late- presenting patient, a low concentration cannot be inter-
preted, but a high one indicates toxicity.
If the antidote desferrioxamine is given before 4h have elapsed, it inter-
feres with the colorimetric assay for iron, and so a serum sample for iron
should be taken o before it is given. If atomic absorption spectrophotom-
etry is available for measurement of serum iron, there is no interference
from desferrioxamine.
It is essential to interpret the serum iron concentration result in the
context of the clinical state of the patient. If <55µmol/ L (<300mg/ dL),
mild toxicity is expected. If above 90µmol/ L (500mg/ dL), severe toxicity is
expected and treatment with desferrioxamine is necessary. Do not wait for
an iron concentration if altered conscious state, shock, or severe acidosis
(pH <7.1) is present. Antidotal treatment is also indicated for patients with
iron concentrations of >55µmol/ L if there is additional clinical evidence
of toxicity, e.g. GI symptoms, leucocytosis, or hyperglycaemia. Antidotal
therapy with desferrioxamine is indicated without waiting for the serum
iron concentration in patients with severe features (e.g. tting, unconscious,
or hypotensive). Desferrioxamine is usually continued until the urine has
returned to a normal colour, symptoms have abated, and all radio- opacities
of iron tablets on AXR have disappeared. Urine free iron estimation is the
best test of when to stop chelation therapy with desferrioxamine but is not
widely available.
Working outif thepatient needs a serum iron level checked
If a patient has ingested <30mg/ kg body weight of elemental iron (a 200mg
ferrous sulfate tablet = 65mg of elemental iron), then no serum iron level
is required. If in doubt, a plain AXR will usually indicate if lots of tablets
are present. Aserum concentration of <55µmol/ L (<300mg/ dL) also indi-
cates low risk (E Iron, Serum iron concentrations, p. 724).
AbdominalX- ray
This is required in patients who have ingested in excess of 30mg of elemen-
tal iron/ kg body weight. The AXR determines the need for gut decontami-
nation either by gastric lavage or whole bowel irrigation with polyethylene
glycol. Undissolved tablets appear radio- opaque, but they disappear once
dissolved, so the absence of radio- opacities does not exclude the possibility
of toxicity.

IRON
725
Full bloodcount
This is needed in all cases of iron poisoning. Leucocytosis (>15 × 10
9
/ L) is
common with signicant toxicity. Cross- match is a wise precaution in poten-
tially serious poisoning.
Urea and electrolytes and creatinine, baseline liver function
tests, and clotting
This is needed in allcases.
Blood glucose
Hyperglycaemia is common in serious poisoning.
Arterial bloodgases
These should be checked in symptomatic or severely poisoned patients.
Metabolic acidosis canoccur.
Total iron binding capacity
This has no role in the assessment of acute iron poisoning.
What todo ifestimation ofserum iron concentration is
unavailable
If serum iron assay is not available, the presence of nausea, vomiting, leuco-
cytosis (>15 × 10
9
/ L), and hyperglycaemia (>8.3mmol/ L) suggests signi-
cant ingestion and the need for treatment with desferrioxamine.
E OHCM 10e, p.842.
Further reading
Chang TP, Rangan C. Iron poisoning:a literature- based review of epidemiology, diagnosis, and man-
agement. Pediatr Emerg Care 2011; 10
:978– 85.
Royal Children’s Hospital Melbourne. Iron poisoning. Clinical practice guidelines. M http:// www.rch.
org.au/ clinicalguide/ guideline_ index/ Iron_ poisoning/ .

726
CHAPTER11 Poisoning and overdose
726
Lead exposure and poisoning
Blood lead concentrations
Blood lead concentrations are used to conrm exposure and decide on
whether environmental exposure reduction measures or (rarely) iron chela-
tion therapy is required. Samples are not ‘urgent’ (except in the case of sus-
pected acute lead encephalopathy) and must be taken into an EDTAtube.
A blood lead level of 5µg/ dL or more requires further testing and moni-
toring, and the source of lead to be found and removed. Alead level of
>45µg/ dL in a child usually indicates the need for chelation treatment.
Occupational lead levels and appropriate responses for adults are enshrined
in Worker/ Occupational Health and Safety legislation.
There are two agents used for chelation therapy in lead poisoning— IV
EDTA (disodium calcium edetate) and oral DMSA (2,3- dimercaptosuccinic
acid). Before use, chelation therapy should be discussed with a toxicologist.
In general, patients with a blood lead concentration of >45µg/ dL should
be treated with chelation therapy and removal from further exposure.
Children with encephalopathy or a blood lead concentration of >75µg/ dL
require admission to hospital for urgent chelation therapy.
AbdominalX- ray
A plain AXR should be performed in all children, particularly if there is a his-
tory of pica, to exclude ingested paint or lead foreign bodies such as curtain
pulls or shing sinkers. Long bone X- rays in children may show leadlines.
Zinc protoporphyrin estimations
Zinc protoporphyrin (ZPP) estimations can be helpful in individuals with
moderate (>20µg/ dL) to high (>40µg/ dL) blood lead concentrations in
whom one is trying to determine the chronicity of exposure. There is a
poor correlation between ZPP and blood lead at lower blood lead concen-
trations. There are other conditions (e.g. iron deciency) that can i ZPP,
and there is signicant inter- individual variation. ZPP has been proposed as
a surrogate marker for blood lead, but blood lead is the best marker and
should not be replaced byZPP.
Other essential investigations
Patients should also have FBC and a blood lm (for basophilic stippling),
U&E, LFTs, and serum Ca
2+
measured. All children should have their serum
iron measured, as iron deciency is an important diagnosis and, if corrected,
can reduce ongoing lead absorption from thegut.
Further reading
Boreland F, Lesjak MD, Lyle DM. Managing environmental lead in Broken Hill:a public health success.
NSW Public Health Bull 2008; 19:174– 9.
Gulson B, Korsch M, Matisons M, Douglas C, Gillam L, McLaughlin V. Windblown lead carbonate as
the main source of lead in the blood of children from a seaside community:an example of local
birds as “canaries in the mine”. Environ Health Perspect 2009; 117
:148– 54.

LEAD EXPOSURE AND POISONING
727

728
CHAPTER11 Poisoning and overdose
728
Lithium
Blood lithium concentration
Lithium is available as sustained- release and non- sustained- release tablets
and liquid. After ingestion of liquid preparations, plasma lithium concentra-
tions peak at 30min. With sustained- release preparations, peak concentra-
tions occur at 4– 5h. The plasma half- life of lithium is often in excess of
24h. Interpretation of plasma lithium concentrations depends on the clinical
circumstances of exposure (E
Acute overdose in lithium- naive patient,
p. 728, E Chronic excess of lithium, p. 728, E
Acute- on- chronic lith-
ium poisoning, p. 728). Do not take blood for lithium levels into a lithium
heparintube!
Acute overdose inlithium- naive patient
A single overdose in a lithium- naive patient is of low risk. However, onset of
toxicity may be delayed for as much as 24h. Plasma samples for lithium assay
should be taken at 6h post- ingestion and measured urgently. The patient
should have IV uids to facilitate lithium elimination. Consider haemodialysis
if plasma lithium concentration is >7.5mmol/ L.
Chronic excess oflithium
Lithium toxicity can occur if the patient has been taking too high a dose
or is dehydrated, or if an interaction with thiazide diuretics, NSAIDs, ACE
inhibitors, or tetracyclines has occurred. Risk of toxicity is further enhanced
by the presence of hypertension, diabetes, cardiac failure, renal failure, or
schizophrenia. Blood for plasma lithium assay should be taken at presen-
tation. Often good IV hydration suces to clear the lithium; rarely hae-
modialysis is needed. Consider haemodialysis if the plasma lithium exceeds
2.5mmol/ L.
Acute- on- chronic lithium poisoning
A patient taking lithium chronically who then takes an acute overdose is
at risk of serious toxicity, because tissue binding of lithium is already high.
The plasma lithium levels should be measured urgently at 6h post- ingestion.
Lithium measurements should be repeated 6- to 12- hourly in symptomatic
patients until clinical improvement occurs. Consider haemodialysis if plasma
concentrations exceed 4mmol/ L.
Indications forhaemodialysis or arteriovenous
haemodialtration
Lithium is eectively removed by haemodialysis (preferred) or arterio-
venous haemodialtration. It is indicated in all patients with severe lithium
poisoning, i.e. coma, convulsions, respiratory failure, or ARF. Plasma lithium
concentrations can also guide the need for haemodialysis/ haemodialtra-
tion. Each hour of dialysis will reduce the plasma lithium by 1mmol/ L, but
plasma lithium often rebounds after haemodialysis has stopped, so the assay
should be repeated at the end of dialysis and again 6– 12hlater.

LITHIUM
729
Urea and electrolytes, serum creatinine
Hyponatraemia is common in lithium toxicity. It is also important to check
the serum K
+
concentration and urea, as lithium is renally excreted and renal
failure delays its elimination.
ECG
Lithium poisoning may result in arrhythmias, and complete heart block has
been reported. An ECG (and sometimes monitoring) is required. Chronic
lithium toxicity frequently has non- specic and diuse depressed ST-
segments and T wave inversion, which are seldom of sinister consequence.
Further reading
Bellomo R, Kearly Y, Parkin G, etal. Treatment of life- threatening lithium toxicity with continuous
arterio- venous hemodialtration. Crit Care Med 1991; 19:836– 7.
Eyer F, Pfab R, Felgenhauer N, etal. Lithium poisoning pharmacokinetics and clearance during dier-
ent therapeutic measures. J Clin Psychopharmacol 2006; 3
:325– 30.

730
CHAPTER11 Poisoning and overdose
730
Methaemoglobinaemia
Oxidizing agents convert Hb to methaemoglobin (MetHb), and this renders
it incapable of carrying O
2
. Common agents causing methaemoglobinaemia
include:dapsone, sulfonamides, chlorates, nitrites, nitrates, and local anaes-
thetics including lidocaine. The onset and duration of symptoms will depend
on the agent. Nitrites cause breathlessness and ushing within minutes of
exposure, but dapsone may cause methaemoglobinaemia several hours
after ingestion and the methaemoglobinaemia may then persist fordays.
Essential investigations
Patients withsuspected methaemoglobinaemia should have thefollowing
•
ABGs.
• FBC (especially if dapsone has been taken l haemolytic anaemia).
•
Blood MetHb concentration.
MetHb can produce a normal PO
2
in the presence of reduced O
2
satura-
tion. Pulse oximetry measures both MetHb and oxygenated Hb so can give
false results.
Methaemoglobin estimation inblood
Measurement of blood MetHb is required to conrm the diagnosis and
assess the severity of poisoning. The measurement must be done urgently
when administration of the antidote methylthioninium chloride (methylene
blue) is contemplated. Samples for MetHb estimation need to be analysed
as soon as possible after collection, as if left to stand around, the MetHb will
be falsely low owing to a reduction by endogenous MetHb reductase. The
severity of symptoms correlates roughly with the measured MetHb con-
centrations. Anaemia and cardiac or pulmonary disease will lead to more
severe symptoms at a lower MetHb level (see Table11.2).
If the patient has severe clinical features of toxicity or if the blood
MetHb concentration is >30%, the patient should be given methylene blue.
Methylene blue can be given at lower blood MetHb concentrations in those
who are symptomatic.
Table11.2 Clinical eects
MetHb concentration (%) Clinical eects
0– 15 None
15– 30 Mild:cyanosis, tiredness, headache, nausea
30– 50 Moderate:marked cyanosis, tachycardia, dyspnoea
50– 70 Severe:coma, ts, respiratory depression,
metabolic acidosis, arrhythmias
>70% Potentially fatal

OPIOIDS
731
Opioids
Classic features ofopioid poisoning
• Depressed respiratory rate and volume.
• Pinpoint pupils.
• Coma
• Signs of parenteral drug use, e.g. needlemarks.
Toxicity can be prolonged for 24– 48h, particularly after ingestion of metha-
done, which has a long half- life. The lifesaving measure is prompt adminis-
tration of adequate doses of naloxone, before waiting for results of any
investigations. This often needs to be repeated or an infusion started, as the
half- life of the antidote is much shorter than opioiddrugs.
Adequacy ofventilation
O
2
saturation monitoring and/ or ABG analysis demonstrates the adequacy
of ventilation in those whose respiration is depressed, together with accu-
rately measuring the respiratoryrate.
Urine drug screening
Qualitative screening of the urine (group- specic immunoassay) conrms
recent use. This may, however, not detect fentanyl derivatives, tramadol,
and other synthetic opioids.
Measuring opioids inblood
Quantitative analysis is usually undertaken by LC/ Q- TOF- MS or GC- MS or
MS. This is often required for medicolegal purposes, particularly where a
fatality or a childcare issue is involved.
‘Plasma morphine free and total morphine concentrations were an
average of 88 and 277µg/ L in 54 people treated for heroin overdose’.
8
Interestingly, the degree of poisoning correlated better with total morphine
concentrations than free concentrations.
Post- mortem morphine levels in heroin overdose deaths vary, depending
on prior narcotic history, but in general exceed 0.3mg/ L.
8
‘Blood tramadol concentrations in 105 people arrested for impaired driv-
ing performance averaged 850µg/ L’.
8
Post- mortem concentrations in ve
tramadol deaths averaged 6.1mg/ L. Heroin metabolites and tramadol do
not undergo signicant post- mortem redistribution.
Paracetamol screening
Opioid tablets are frequently combined with paracetamol. All unconscious
patients should therefore have plasma paracetamol level measured.
Further reading
Jones AL. Management of opioid poisoning. In:A Webb, D Angus, S Finfer, L Gattinoni, M Singer
(eds.). Oxford Textbook of Critical Care
, 2nd edn. 2016; pp.1522– 5.
8 Baselt RC, Cravey RH. Disposition of Toxic Drugs and Chemicals in Man, 9th edn. San
Francisco:Chemical Toxicology Institute,2011.

732
CHAPTER11 Poisoning and overdose
732
Paracetamol (acetaminophen) poisoning
Overview
Paracetamol is the commonest drug taken in overdose in the UK.
Paracetamol can be measured by a variety of assay methods, but HPLC or
LC- MS is less susceptible to interference than some enzyme- based assays.
Measurement of plasma paracetamol concentration is essential for
assessing the need for antidotal treatment (NAC) in most cases of par-
acetamol poisoning. The nomogram for paracetamol concentrations and
protocols for when and how to give NAC diers between countries, and it
is important to consult your own national guidelines. If NAC is given within
12h of the overdose, it provides complete protection against liver injury
and renal failure. Beyond 12h after ingestion, the protection is less com-
plete and assessment of liver damage is required. Paracetamol poisoning
can be deceptive, as there is a latent phase of many hours where the patient
remains well before liver damage develops. The co- ingestion of opioids may
delay gastric emptying and peak plasma paracetamol concentrations.
INR/ PT
The most sensitive marker of prognosis in paracetamol poisoning is the PT
or INR. This often starts to i within 24– 36h of the overdose and peaks at
48– 72h. Once the INR/ PT starts to improve, this is a sign that hepatotoxic-
ity is starting to improve and the patient will not go on to develop acute
liver failure. Approximately half of patients with a PT of 36s at 36h post-
ingestion will develop acute liver failure.
Plasma alanine and aspartate aminotransferases
These may begin to rise as early as 12h post- ingestion but usually peak at
72– 96h. AST or ALT values in excess of 10,000IU/ L are not unusual, and
a plasma ALT of >5000IU/ L is very suggestive of paracetamol poisoning
(see Fig. 11.1). Serum bilirubin may peak after the aminotransferase, and
this should not lead to concern for patients in whom the INR or PT have
begun tofall.
Do not correct abnormalities in PT or INR with FFP or cryoprecipitate,
unless life- threatening bleeding is taking place; otherwise the most sensitive
marker of how the patient is progressing will belost.
Other blood test abnormalities inparacetamol poisoning
Hypoglycaemia and metabolic acidosis are common. Early metabolic acido-
sis is often associated with very high plasma paracetamol concentrations,
e.g. >400mg/ L. Later, development of acidosis indicates incipient acute
liver failure and the need to urgently check ABGs, LFTs, and INR/ PTR.
Pancreatitis with i serum amylase/ lipase has been reported. Several
cases of thrombocytopenia have been reported.
U&E and creatinine should be checked. Renal failure can occur in the
context of hepatic failure, but also in its absence (in 1 in 100 patients). It is
treated with NAC and supportive measures, e.g. haemodialysis, if needed.
Full recovery with supportive care is common.
PARACETAMOL (ACETAMINOPHEN) POISONING
733
Investigating thepatient who has taken a paracetamol
overdose <4hago
Ingestion of >75mg/ kg of paracetamol or a paracetamol- containing prod-
uct should be recognized as a potential hepatotoxic dose for most people. If
ingestion of this amount or more has occurred within the last 1h, activated
charcoal should be given orally (50g for an adult). Aplasma paracetamol
level should then be checked at 4h from the time of ingestion, to determine
the need for NAC treatment from the nomogram (see Fig.11.2).
Very rarely, e.g. after ingestion of 4 × 500mg tablets by an adult, a con-
rmatory plasma paracetamol level is not needed, but in general it is safer
to be certain by checking a blood concentration.
Investigating thepatient who has taken a paracetamol
overdose between4 and8hago
A plasma paracetamol level should be checked as soon as possible. If a sin-
gle acute ingestion has taken place, then the result is plotted on the relevant
national nomogram against the time since ingestion (e.g. see Fig. 11.2). This
determines the need for NAC antidote treatment (i.e. if the level is above
or very close to theline).
If the overdose is staggered or repeated, supratherapeutic, then specic
toxicology, advice is needed. If in doubt, treat the patient withNAC. Some
countries have modied this nomogram to treat patients at lower paraceta-
mol levels.
Investigating thepatient who has taken a paracetamol
overdose between8 and 24hago
Start treatment with NAC straightaway. Take blood for a paracetamol level,
INR/ PT, creatinine, and plasma venous HCO
3
−
(if plasma venous HCO
3
−
is
abnormal, check ABGs). Check results and refer to the graph to determine
whether treatment with NAC needs to be continued (i.e. is the plasma level
above the treatment line?) or can be stopped (below the line). 3 Beyond
0
7500
15,000
12,500
2500
468 14 162
5000
10 12
10,000
ALT (IU/L)
Time after ingestion (days)
Bilirubin
(µmol/L)
INR
100
50
0
3
2
1
ALT
Bilirubin
INR
Fig.11.1 Time course of liver function tests in paracetamol poisoning.
734
CHAPTER11 Poisoning and overdose
734
16h after ingestion, the sensitivity of the assay for paracetamol may be too
low to detect a treatable level— check and, if in doubt, treat the patient
with NAC! On completion of NAC, check blood INR/ PT, creatinine, and
plasma venous HCO
3
−
(if abnormal, check ABGs). If the patient is asympto-
matic and the INR or creatinine is normal or falling, discontinue NAC. If the
patient has symptoms (abdominal pain or vomiting) or the INR or creatinine
is rising, continue maintenance NAC (50mg/ kg in 500mL of glucose every
4h) until the INR improves. Contact a poisons centre/ liverunit.
Investigating thepatient who has taken a paracetamol
overdose >24h ago or thetiming is not able tobe
established
Start treatment with the antidote NAC straightaway, unless a trivial amount
has beentaken.
Take blood for baseline INR/ PT, creatinine, venous HCO
3
−
(if abnormal,
check ABGs), and paracetamol.
If the patient is asymptomatic and the laboratory tests normal (INR <1.3,
paracetamol concentration <5 mg/ L, and ALT <2 times the upper limit of
normal), discharge the patient and advise to return if vomiting/ abdominal
pain develops. If the blood results are abnormal, continue NAC, and phone
a liver unit/ poisons centre for advice.
120
Plasma-paracetamol concentration (mmo/litre)
0.8
0.7
0.6
0.5
0.4
0.3
0.2
0.1
0
110
100
90
80
70
Plasma-paracetamol concentration (mg/litre)
60
50
40
30
20
10
0
0246810 12
Time (hours)
14 16 18 20 22 24
Treatment lineTreatment line
Fig.11.2 An acetylcysteine treatmentgraph.
Note: The most up-to-date national nomogram should be accessed for your country.
Reproduced from Drug Safety Update September 2012, vol 6, issue 2:A1 © Crown Copyright2013.

PARACETAMOL (ACETAMINOPHEN) POISONING
735
Investigating thepatient who has taken a staggered
overdose
The Commission on Human Medicines currently recommends that a Xmg/
kg/ 24h dose ingested calculation is not used to guide therapy. Current
advice is that all staggered overdoses should be treated with NAC and dis-
cussed with a toxicologist.
Blood is taken for paracetamol level, U&E, creatinine, HCO
3
−
, ALT, and
INR. Hepatotoxicity is not likely if the patients is asymptomatic, the plasma
paracetamol concentration is <5 mg/ L, INR is <1.3, and ALT is <2 times
the upper limit of normal. If all of the above are found, then NAC may be
stopped and the patient discharged, with advice to return if they experience
vomiting or abdominalpain.
Investigating thepatient who has a repeated
supratherapeutic ingestion
All patients with a supratherapeutic overdose should be considered for
NAC treatment and discussed with a toxicologist. Currently, patients with
symptoms or signs of hepatotoxicity or those who have denitely ingested
75mg or less of paracetamol require no treatment.
Further reading
BMJ Best Practice. Paracetamol overdose. M http:// bestpractice.bmj.com/ best- practice/ mono-
graph/ 337.html.
TOXBASE. M
https:// www.toxbase.org/ .
UK National Poisons Information Service. M
http:// www.npis.org/ .

736
CHAPTER11 Poisoning and overdose
736
Salicylate poisoning
Features ofsevere poisoning
Ingestion of >150, 250, and 500mg/ kg body weight of aspirin, respectively,
produces mild, moderate, and severe poisoning, respectively. Aspirin poi-
soning is becoming increasingly rare in developed countries. Signs of seri-
ous salicylate* poisoning include metabolic acidosis, renal failure, and CNS
eects such as agitation, confusion, coma, and convulsions. Death may
occur as a result of CNS depression and cardiovascular collapse. The devel-
opment of metabolic acidosis is a bad prognostic sign, as it also indicates i
CSF transfer of salicylate.
Note:(*) aspirin, oil of wintergreen.
Plasma salicylate concentration
Plasma salicylate should be measured urgently in all, but the most trivial
overdose, i.e. all those thought to have ingested >150mg/ kg body weight
of aspirin or any amount of oil of wintergreen. It should be performed at
4h post- ingestion, because delayed absorption of the drug renders such
levels uninterpretable before this time. As salicylates may form concretions
in the stomach, which delay absorption, it is recommended that a salicylate
level is rechecked 3– 4h after the rst sample, to catch the peak salicylate
concentration. There is no evidence for indiscriminate requesting of salicy-
late concentrations in every unconscious patient (unlike paracetamol) or
in conscious patients who deny taking aspirin and who have no features
suggesting salicylate toxicity. The plasma salicylate concentration is not an
absolute guide to toxicity, as paracetamol levels are in paracetamol poison-
ing, but should be interpreted together with clinical features and acid– base
status of the patient.
Urinary alkalinization (E OHCM 10e, p. 844) is indicated for patients
with salicylate concentrations of 600– 800mg/ L in adults and 450– 700mg/ L
in children and the elderly. Metabolic alkalosis is not a contraindication to
bicarbonate therapy, as patients may have a high base decit in spite of an
elevated serumpH.
Haemodialysis is very eective at salicylate removal and correction of
acid– base and electrolyte abnormalities. It should be considered if the
plasma salicylate levels are >700mg/ L in children and >800mg/ L in adults.
Other indications for haemodialysis include resistant metabolic acido-
sis, severe CNS eects, such as coma, convulsions, pulmonary oedema,
andARF.
Arterial bloodgases
Acid– base problems are common in salicylate poisoning. Respiratory cen-
tre stimulation causes respiratory alkalosis. Uncoupled oxidative phospho-
rylation and interruption of glucose and fatty acid metabolism by salicylates
often cause concurrent metabolic acidosis. Serial ABGs are needed in
severe salicylate poisoning.
Further reading
Ghosh D, Williams KM, Graham CG, et al. Multiple episodes of aspirin overdose in an individual
patient:a case report. J Med Case Rep 2014; 19:374.

THEOPHYLLINE
737
Theophylline
Acute theophylline poisoning can carry a high mortality, and its manage-
ment is best guided by the Shannon severity grading scheme, bearing in
mind that delayed eects tend to occur after sustained- release formulations
have been ingested. The adult therapeutic range is 10– 20mg/ L. Serious tox-
icity occurs at >100mg/ L (770mmol/ L).
Urea and electrolytes, creatinine, glucose
It is vital to check the plasma K
+
concentration frequently, as hypokalaemia
is a life- threatening complication and the serum K
+
concentration is a use-
ful guide to severity. If >2.5mmol/ L, the patient is less severely poisoned
(grade 1) than if it falls to <2.5mmol/ L (grade 2). Check blood glucose
since hyperglycaemia is a common complication.
Arterial bloodgases
In potentially serious poisoning (e.g. ingestion of >20mg/ kg body weight),
abg analysis is helpful in optimizing the acid– base status of the patient. An
initial phase of hyperventilation with respiratory alkalosis can be followed
by a further stage of metabolic acidosis.
ECG
In potentially serious poisoning (e.g. ingestion of >20mg/ kg body weight),
an ECG is required. Cardiac monitoring is helpful for early identication of
arrhythmias in such patients.
Plasma theophylline concentrations
Measuring plasma theophylline concentrations conrms theophylline ingestion
if this is in doubt, and it is usually undertaken by HPLC or LC- MS/ MS. However,
for the vast majority of poisoned patients, obtaining a plasma theophylline con-
centration does not guide their management. Therapeutic levels rarely exceed
20mg/ L (155mmol/ L). Theophylline peak concentration in plasma may occur
at 1– 3h after ingestion of a standard- release formulation. However, overdose
is often with sustained- release products, and delayed absorption can result in
delayed peak plasma concentration and toxicity, often 12– 24hlater.
Plasma concentrations are also helpful in deciding when to employ char-
coal haemoperfusion in seriously poisoned patients (particularly if plasma
concentrations are >100mg/ L (770mmol/ L)). Charcoal haemoperfusion
can be considered at lower concentrations, e.g. 80mg/ L, in the elderly or
those with pre- existing IHD, and those with persistent seizures or hypo-
tension not responding to IV uids. Charcoal haemoperfusion can also be
decided on the basis of grade 3 or 4 severity grading alone. If charcoal hae-
moperfusion is not possible, then haemodialysis with multi- dose activated
charcoal is probably an alternative.
Urine testing formyoglobinuria and measuring serum
creatinekinase
Theophylline poisoning can be accompanied by rhabdomyolysis. Hence,
the urine should be dipstick- tested, and if found +ve for blood, a serum
CK should be obtained. This will then indicate that renal function should
be closely monitored and whether the urine should undergo alkalinization.
Further reading
Shannon M. Predictors of major toxicity after theophylline overdose. Ann Intern Med 1993;
119
:1161– 7.

738
CHAPTER11 Poisoning and overdose
738
Tricyclic antidepressants
The main risks of overdose with these drugs are the cardiovascular system
(CVS) and CNS toxicity.
Electrocardiogram
An ECG should be performed in all but the most trivial cases of overdose.
ECG abnormalities are common inmoderate tosevere poisoning and include
•
QRS prolongation:>110ms in adults predicts the risk of ventricular
cardiac arrhythmias (and the need for IV sodium bicarbonate), and a
QRS >160ms predicts the risk of ts. In children, a QRS >110ms is
predictive of the risk of arrhythmias, but notts.
•
Note:ECG criteria are not the only factors assessing the risk of
arrhythmias, ts, and acidosis— electrolyte disturbances contribute.
Supraventricular, and potentially fatal ventricular, arrhythmias canoccur.
Cardiac monitoring
This is essential if ingestion of >10mg/ kg body weight has taken place. It is
seldom necessary beyond 24h after ingestion.
Arterial blood gas analyses
These should be done on all patients with marked symptoms and signs,
particularly those with a reduced GCS score. It should also be performed
on those with widened QRS or seizures, not least because such patients
are receiving IV sodium bicarbonate therapy and a pH of 7.5 should not
be exceeded.
Plasma concentrations
This is of no value, as plasma concentrations of tricyclic antidepressants
correlate poorly with clinical features of toxicity.

WARFARIN AND SUPERWARFARINS
739
Warfarin and superwarfarins
INR/ PTR
This is a key test in possible warfarin/ superwarfarin overdose. In the case
of warfarin, the INR often begins to rise from day 2 after the overdose and
settles within a week, but with superwarfarins, the time course of clotting
abnormality may stretch to weeks. Thus, repeated INR/ PTR estimations
may guide progress and the need for vitamin K1 antidote in warfarin toxicity.
Individual factor assays have been done in some cases but are not routinely
necessary.
Liver functiontests
Assessment of liver function is helpful in warfarin overdose. Acongested,
dysfunctional liver would be expected to handle the consequences of a
warfarin overdose lesswell.

740
CHAPTER11 Poisoning and overdose
740
Table ofconversion factors
betweenmass and molarunits
(See Table11.3.)
Table11.3 Conversion factors betweenmass and molecularunits
Drug Mass units Molar (SI) units Conversion factor
Carbamazepine
mg/ L µmol/ L 4.23
Digoxin
µg/ L or ng/ mL nmol/ L 1.28
Ethanol
g/ L mmol/ L 1.28
Iron
mg/ L mmol/ L 0.179
Lead
mg/ L mmol/ L 0.0048
Paracetamol
mg/ L mmol/ L 0.0066
Phenytoin
mg/ L mmol/ L 3.96
Salicylate (aspirin)
mg/ L mmol/ L 0.0072
Theophylline
mg/ L mmol/ L 7.7

742
CHAPTER12 Rheumatology
742
Rheumatological investigations
Investigations in rheumatology are important not only in diagnosis, but also
in assessing disease activity and monitoring treatment. They are comple-
mentary to a careful history and physical examination and should only be
requested in the correct clinical context where results are likely to aect
management. In isolation, ‘abnormal’ results can lead to unwarranted anxi-
ety, investigations, and treatment.

RHEUMATOLOGICAL INVESTIGATIONS
743

744
CHAPTER12 Rheumatology
744
Inflammatory markers
Inammatory rheumatic disease is usually associated with an acute
phase response. The ESR and CRP are the most commonly used to
screen for inammation. However, they are non- specic, also i in infec-
tion and malignancy. Obesity is also recognized as a cause for high CRP.
Conversely, a normal ESR or CRP does not exclude rheumatic disease, e.g.
spondyloarthropathies.
Erythrocyte sedimentationrate
ESR is measured by observing how fast RBC fall through a column of blood
in 1h. It is dependent upon many variables, including serum Igs, brinogen,
and Hct. Therefore, a raised ESR may not reect inammation, but other
factors, e.g. hypergammaglobulinaemia. Normal ESR levels also i with age.
In some centres, plasma viscosity is preferred as a screening for acute phase
response, rather than the ESR, as it is independent of age, sex, and Hb
(E
Erythrocyte sedimentation rate, pp. 252–3).
C- reactive protein
CRP production is driven by IL- 1 and IL- 6 on hepatocytes. It generally pro-
vides a more accurate reection of inammatory or infective processes
than ESR, as it changes more rapidly and is independent of the variables
that complicate ESR interpretation.
ESR and CRP indiagnosis and monitoring
Although ESR or CRP are not specic, the degree of elevation and relation-
ship of ESR to CRP may suggest a diagnosis. For example CRP and ESR may
be very high in infection and gout but are usually only moderately raised
in RhA (see Table 12.1). Isolated elevation of CRP may also reect auto-
inammatory disorders, e.g. familial Mediterranean fever and amyloidosis
or infection.
In cases where ESR/ CRP are i in active disease, they are extremely use-
ful in monitoring disease activity and response to treatment.
Other acute phase markers
In general, it is not helpful to measure other acute phase reactants, e.g. caer-
uloplasmin, α
1- antitrypsin, brinogen, and Hp. Two exceptions are serum
ferritin and complement, which can be useful in diagnosis. Other common
biochemical and haematological tests discussed below also reect levels of
inammation, e.g. albumin and Hb fall, whilst ALP and γGTrise.
In a clinical scenario where CRP is very high and it is dicult to distinguish
between systemic infection or inammation (e.g. vasculitis), measuring PCT
might be considered. This is usually used in the ITU/ acute medicine setting.
Ahigh level suggests a high risk of progression to systemic sepsis, whilst a
low level may support an inammatory cause for the raisedCRP.

INFLAMMATORY MARKERS
745
Serum ferritin
Ferritin may be used as a surrogate marker for body iron stores but is also
an acute phase reactant. In the presence of active inammation or infection,
a normal or high serum ferritin may be found, even if the patient has true
iron deciency (E
Assessment of iron status, pp. 244–7). Serum ferritin
levels are often very high in active adult Still’s disease and fall with treatment.
Elevated ferritin associated with a transferrin saturation of over 45% is also
seen in haemochromatosis arthropathy.
Complement
Complement components C3 and C4 usually rise in the presence of active
inammation or infection. However, in SLE, they are commonly low. In
some, but not all, SLE patients, they are abnormal when disease is active and
normalize on treatment, making them useful in monitoring disease activ-
ity, especially lupus nephritis. Low levels of C1 can be associated with the
development ofSLE.
Table12.1 ESR and CRP indiagnosis
Diagnosis ESR CRP
Infection ii or iii ii or iii
Malignancy Normal to ii Normal to i
RhA ii ii
SLE* i Normal to i
SLE with serositis i Normal to ii
Gout/ CPPD** ii or iii ii or iii
Sjögren’s syndrome i or ii i
Vasculitis ii or iii ii or iii
Osteoarthritis Normal Normal
Psoriatic arthritis/ spondyloarthropathy Normal to ii Normal to ii
* Systemic lupus erythematosus.
** Calcium pyrophosphate disease.

746
CHAPTER12 Rheumatology
746
Haematology
Haemoglobin
There are three common scenarios for anaemia (d Hb) in patients with
rheumatic symptoms.
•
Related to the rheumatic disease— most commonly ‘anaemia of
chronic disease’. Rarely AIHA (associated with +ve Coombs’ test, high
reticulocytes, high bilirubin, lowHp.)
•
As a manifestation of an alternative systemic disorder that presents with
arthralgia (e.g. coeliac disease/ leukaemia).
• Related to drug side eects, e.g. GI bleeding due to NSAIDs or
pancytopenia caused by immunosuppressive agents (e.g. methotrexate).
The degree, speed of onset, and type of anaemia should be assessed, tak-
ing into account the clinical picture. Trends in Hb are useful in monitoring
disease activity and treatment.
Microcytic hypochromic anaemia
The major dierential diagnosesare:
• Chronic iron deciency anaemia.
• Thalassaemiatrait.
• Anaemia of chronic disease (typically associated with normal MCV, but
if chronic, the MCV may reduce).
Iron deciency related to acute or chronic blood loss (e.g. GI bleeding), or
nutritional or bowel disease, such as Crohn’s disease or coeliac disease,
should be considered.
Macrocytic anaemia
Vitamin B
12
or folate deciency related to nutritional deciency, e.g. scle-
roderma causing jejunal diverticulosis, Whipple’s disease causing malab-
sorption, or due to drug therapy such as methotrexate, sulfasalazine, and
azathioprine. Excessive alcohol consumption may also cause macrocytosis
without signicant anaemia.
Normocytic normochromic anaemia
Usually reects chronic disease, and the degree of anaemia may vary with
disease severity, e.g. RhA, crystal arthritis, vasculitis.
Dimorphic anaemia (large and small redcells)
This picture is seen in mixed deciency, e.g. iron deciency plus vitamin B
12
or folate deciency, post- transfusion, or during iron replacement therapy
in a patient with iron deciency. It is afeature of malabsorption associated
with coeliac- related arthritis, scleroderma of the gut, and jejunal bypass
arthritis.
White cellcount
White cell count may be helpful in diagnosis and monitoring disease activity
and is essential for monitoring immunosuppressive therapies. Diagnoses to
consider where levels are i
/ d are listed. BM biopsy may need to be consid-
ered to distinguish from ° haematological disorders.

HAEMATOLOGY
747
Increased
• Neutrophilia:septic arthritis, crystal arthropathy (gout and pseudogout),
systemic corticosteroid administration, myeloproliferative disorders.
•
Lymphocytosis:consider lymphocytic leukaemia with joint symptoms and
viral infection.
•
Eosinophilia:eosinophilic granulomatosis with polyangiitis (EGPA;
formerly known as Churg– Strauss syndrome) and other vasculitides,
hypereosinophilic syndromes, eosinophilic fasciitis, and also Addison’s
disease (can complicate rheumatological diseases). Newly raised
eosinophils should heighten awareness of potential adverse reactions to
disease- modifying drugs. Asthma and allergy.
Reduced
• Neutropenia:consider autoimmune neutropenia, e.g. associated with SLE
and Felty’s syndrome in RhA. May be drug- induced (very common with
tocilizumab and seen as adverse response to sulfasalazine, methotrexate,
azathioprine, cyclophosphamide, ciclosporin, mycophenolate, leunomide,
and other cytotoxics). Normal range for neutrophils also vary between
races, e.g. neutrophils may be lower in Afro- Caribbean populations.
• Lymphopenia:common inSLE.
Plateletcount
• Thrombocytosis:reects active inammation, e.g. RhA, but should be
distinguished from ° polycythaemia vera (JAK2 kinase and BM biopsy if
appropriate).
•
Thrombocytopenia:autoimmune thrombocytopenia, may also be related
to connective tissue disease, including SLE, RhA (Felty’s syndrome), and
antiphospholipid syndrome(APS).
• Can also reect drug toxicity, including those listed under
neutropeniaabove.
Pancytopenia may alsooccur
• As part of autoimmune disease, e.g. SLE/ Felty’s syndrome.
• As a side eect to immunosuppressive drugs previously mentioned.
• As a severe complication of disease. Macrophage activation syndrome
is a life- threatening disorder that can manifest with pancytopenia in
systemic juvenile arthritis, adult Still’s disease, lupus, and Kawasaki
disease. Pancytopenia is associated with high fever, hepatosplenomegaly,
high ferritin, and hypertriglyceridaemia and is associated with
unexpectedly lowESR.

748
CHAPTER12 Rheumatology
748
Biochemistrytests
Although biochemical tests do play a role in the diagnosis of rheumatic dis-
ease (e.g. metabolic bone disease), their major use is in the assessment of
systemic involvement of disease, e.g. renal or hepatic. In addition, they are
essential for monitoring treatment.
Renal function
Renal function is measured in the diagnosis and monitoring of systemic
disease. eGRF is widely used as a surrogate marker for the GFR. Awide
range of diseases may involve the kidney, including vasculitis, SLE, Sjögren’s
syndrome, andgout.
The dose of many drugs should be adjusted according to renal func-
tion, including immunosuppressive agents, e.g. cyclophosphamide and
methotrexate. Some drugs are contraindicated with low eGFRs, e.g. IV
zoledronate and other bisphosphonates for osteoporosis. Drug treatment
should also be considered as a cause for decline in renal function in rheuma-
tology patients, e.g. NSAIDs, sulfasalazine.
Uricacid
Ninety per cent of cases of gout will have a raised serum urate. However,
it may also be elevated in normal health and be i by diuretic treatment.
Denitive diagnosis of gout can only be made by identication of urate
crystals by polarizing light microscopy of joint uid or in biopsy.
Gamma globulins
γ- globulins are often elevated in active inammatory disease. They are usu-
ally globally i
in Sjögren’s syndrome but may also be raised in RhA, sar-
coidosis, SLE, and other connective disease.
Liver functiontests
These are measured in the monitoring of many disease- modifying agents,
e.g. methotrexate and sulfasalazine. Elevated γGT and ALP can also reect
active inammation. They may be relevant in considering underlying diagno-
ses of arthralgia, e.g. haemochromatosis.
Bone function
Serum calcium
Hypercalcaemia should raise suspicion of malignancy, metabolic bone dis-
ease, and sarcoidosis. In metabolic bone disease/ hyperparathyroidism,
Ca
2+
is not always elevated and a ‘high normal’ result may be signicant
in the correct clinical context. Hypocalcaemia may indicate hypoparathy-
roidism (E Hypercalcaemia, pp. 190–2; E
Hypercalcaemia/ osteomalacia,
pp. 188–9).

BIOCHEMISTRYTESTS
749
Alkaline phosphatase
ALP may be elevated due to bone, liver, or GI disease. If γGT is concomi-
tantly raised, one would suspect it originates from the liver. In isolation, it
is more likely to be related to bone and further investigation, e.g. PTH,
vitamin D, urinary Ca
2+
, should be considered. ALP is high in Paget’s disease,
hyperparathyroidism, fractures, and bony metastases. Low ALP may be the
only biochemical clue to hypophosphatasia.
Phosphate
Hypophosphataemia occurs in hyperparathyroidism and hereditary and
acquired hypophosphatasia.
Serum parathyroid
Where bone biochemistry is abnormal, PTH should be measured. It
is elevated in hyperparathyroidism and vitamin D deciency. In cases of
hypocalcaemia, low PTH suggests ° parathyroid disease, whereas normal
or raised PTH suggests PTH resistance (E Hypercalcaemia, pp. 188–9;
E
Hypercalcaemia/ osteomalacia, pp. 190–2).
Urinary calcium
Urinary Ca
2+
is useful in the investigation of hypercalcaemia and elevated
PTH. In hyperparathyroidism, urinary excretion is i. If it is low, familial
hypocalciuric hypercalcaemia should be considered. Hypercalciuria may
also be associated with renal calculi.
Bone markers
Bone turnover markers are available but have limited use due to the
requirement for consistent sample collection, especially for bone resorption
markers. Serum and urinary C- terminal and N- terminal telopeptide type 1
collagen (CTX and NTX) are the most commonly used markers of bone
resorption, and procollagen type 1 N- terminal propeptide (P1NP) of bone
formation.

750
CHAPTER12 Rheumatology
750
Autoantibodies
Autoantibody tests are an integral part of rheumatological investigation.
Detection of antibodies against specic antigens is useful for both diagnostic
and prognostic purposes and less often monitoring disease activity. However,
these tests have their limitations. Most autoantibodies are not specically
associated with a single diagnosis but can occur in a range of diseases and in
healthy individuals. ELISA is often used for screening, as it is more automated
and cheaper than other methods and provide some quantitative information.
Other methods, e.g. indirect immunouorescence, are used for screening or
to detect specic antibodies, depending on the tissue substrateused.
Rheumatoidfactor
RF is an antibody to the Fc portion of IgG, forming immune complexes,
rst described in the 1940s. Although included in the American College of
Rheumatology criteria for rheumatology, RF is not specic to RhA. It can be
found in 4– 16% of healthy individuals (prevalence i with age). It may also
be +ve in other autoimmune diseases, malignancy, and chronic infection.
The rheumatoid arthritis particle agglutination (RAPA) test may still be used
to conrm a diagnosis of rheumatoid disease in the presence of an appro-
priate clinical picture of symmetrical inammatory polyarthritis. Ahigh titre,
e.g. 1:320, is of more relevance than a borderline result, e.g. 1:20. It is less
helpful in early diagnosis, compared to established disease, with RF being
+ve in around one- third of rheumatoid patients at 3months post- diagnosis
and two- thirds at 6 months. Other methods e.g. ELISA, are more com-
monly used in modern practice but risk false +ve results.
Anti- cyclic citrullinated peptide antibodies
Anti- cyclic citrullinated peptide antibodies (ACPA) may be considered more
useful in clinical practice in the diagnosis of RhA. In some centres, RF is only
checked if ACPA are −ve. First described in 1998, they are high- anity IgG
class antibodies that react with the amino acid citrulline and are measured
by ELISA. They are found in the sera of about 75% of rheumatoid patients
and are about 96% specic for RhA. They appear earlier than RF, are unaf-
fected by treatment, and predict the development of erosions. However,
they are not pathognomic.
Antinuclearfactor
ANA, commonly detected by ELISA, is often thought of as a screening test
for SLE or other connective tissue disease. Indeed, it is +ve in 90– 99% of
cases of lupus, in over 90% of cases of scleroderma, and in many cases of
Sjögren’s syndrome, mixed connective tissue disease, and myositis. ANA,
however, can be +ve in 3– 5% of healthy individuals and many diseases,
including hepatic disease, e.g. PBC, pulmonary disease, e.g. ° pulmonary
hypertension, chronic infections, malignancy, or may be drug- induced. It
should therefore be interpreted only in the context of clinical symptoms.
Indirect immunouorescence is another method of detecting ANA, and
results are presented as patterns of uorescence, e.g. homogenous, speck-
led, nucleolar. Although not specic, some clinicians may use these in deter-
mining the relevance of the test, e.g. a strong +ve homogenous ANA is
more likely to be associated with SLE than a weak speckled pattern.

AUTOANTIBODIES
751
Anti- DNA antibody
In contrast to ANA antibodies, dsDNA is less sensitive (50– 80% of cases),
but highly specic for SLE. They are seen rarely in healthy individuals and in
disorders such as chronic active hepatitis and Sjögren’s syndrome. They are
also useful in monitoring disease activity in some, but not all, individuals with
SLE. Arising titre of dsDNA may precede a are in disease.
Extractable nuclear antigen antibodies
Where ANA is +ve, ENA antibodies should be measured as their presence
can suggest a risk of certain clinical scenarios.
•
Anti- Ro and anti- La (SSB):occur in Sjögren’s syndrome and SLE, but also
other autoimmune diseases. They have been associated with fetal heart
block when they are present in the mother.
•
Anti- Sm:lupus nephritis.
•
Anti- Scl- 70 (anti- topoisomerase 1)and anti- centromere antibodies (anti- CENP A/
B/ C):these antibodies are associated with scleroderma. Anti- centromere
antibodies are particularly associated with limited cutaneous scleroderma,
and anti- Scl- 70 with diuse systemic sclerosis. They may be a marker of
pulmonary hypertension. Anti- Scl- 70 is associated with pulmonary brosis.
• A ‘myositis panel’ can be requested where polymyositis or dermatomyositis
are suspected, including Jo-1, Mi-2, PM-Scl, SRP, Ku and antisynthetase
antibodies (PL-7, PL-12, EJ, OJ).
• Presence of Mi-2 is more commonly associated with dermatomyositis
and associates with better prognosis.
•
PM-Scl suggests polymyositis/scleroderma overlap, SRP associates with
chronic progressive disease.
•
Antisynthetase antibodies suggest risk of interstitial lung disease.
• Anti- RNP antibodies:have been associated with ‘mixed connective tissue
disease’ where features of several clinical syndromes can occur in
combination.
•
Anti- histone antibodies:have been associated with drug- inducedlupus.
Antiphospholipid antibodies
° APS (thrombosis, thrombocytopenia, and fetal loss) and other connec-
tive tissue diseases are associated with antiphospholipid antibodies. They
should only be requested in the correct clinical context, as they can also be
detected in normal health and many other clinical contexts, e.g. infection.
IgG and IgM anticardiolipin antibodies (ACL) and β2- glycoprotein antibod-
ies are most commonly measured via ELISA. Asingle +ve result should be
interpreted with caution, and the test should be repeated after 6 weeks to
determine relevance. Ahigh titre and IgG- type antibody may be of greater
clinical relevance than a low titre andIgM.
Lupus anticoagulant tests are also undertaken and are +ve when an i in
clotting time is not reversible when control plasma is added invitro.
Antineutrophil cytoplasmic antibody
First described in 1982, classical ANCA (cANCA) gives a characteristic
bright central granular cytoplasmic staining of ethanol- xed human neutro-
phils by immunouorescence (IIF). pANCA was later described to give a
perinuclear IIF pattern. ELISA is used to identify antigen- specic ANCA, and
PR3 and MPO are now usually tested routinely.

752
CHAPTER12 Rheumatology
752
ANCA is often thought to be a screening test for vasculitis. Indeed, the
presence of cANCA and PR3 in combination has been reported to be
99% specic (and 755% sensitive) for granulomatous polyangiitis (formerly
known as Wegener’s granulomatosis). Similarly (790% specic), the pres-
ence of pANCA and MPO together is associated with microscopic poly-
angiitis. However, like other autoantibodies, ANCA (especially pANCA)
may be +ve in many other situations, including infection, TB, malignancy,
and IBD. Therefore, the full clinical picture must be used in making treat-
ment decisions, and reliance should not be put on ANCA alone. In par-
ticular, the absence of a +ve PR3 or MPO or a pANCA with weak +ve
MPO should raise suspicion to carefully exclude an alternative explanation
to vasculitis.
HLA- B27
HLA- B27 has been strongly associated with ankylosing spondylitis (present
in 90% of patients) and is also linked to other diseases, including reactive
arthritis, IBD, and uveitis. However, as it occurs in about 8% of the popula-
tion, it is not helpful as a screening test in such a common symptom as back
pain (approximately two- thirds of HLA- B27 +ve individuals with back pain
would not have ankylosing spondylitis).
Reactive and infection- related arthritis
Where reactive arthritis is suspected, appropriate serology and swabs
should be sent. These may include ASOT and anti- DNAse for Streptococcus,
serology for Campylobacter, Salmonella, Yersinia
, and EBV, stool samples for
culture, and urethral swab for Chlamydia. Investigations chosen and results
depend upon the clinical presentation. Where an infection- related arthritis
is suspected, specic investigations should be requested, e.g. Borrelia for
Lyme disease, leptospirosis for Weil’s disease,etc.
Urinalysis
Where systemic disease is a possibility, e.g. vasculitis and SLE, urinaly-
sis should be tested both in diagnosis and routine monitoring of disease.
The presence of RBCs and casts are early indicators of renal disease.
Proteinuria in long- standing inammatory disease should raise the suspicion
of amyloidosis.
Urinalysis is also used in monitoring of drugs, including gold, ciclosporin,
and penicillamine.

AUTOANTIBODIES
753

754
CHAPTER12 Rheumatology
754
Arthrocentesis
This refers to the aspiration of uid from a joint. Examination of the uid can
be helpful in diagnosis and is essential in septic arthritis, crystal arthropathy,
and haemarthrosis. Where infection is excluded, the joint may be therapeu-
tically injected with a steroid (e.g. methylprednisolone or triamcinolone).
Synovial uid examination
Physical characteristics
Observation of the colour and consistency of the synovial uid is helpful in
diagnosis. In general, inammatory arthritis, e.g. sepsis/ RhA/ gout, is associ-
ated with a turbid uid with low viscosity, whilst osteoarthritis or normal
joint uid is clear with higher viscosity. Crystal arthropathies are often asso-
ciated with bloodstaineduid.
Microscopy
White cell count and the percentage of polymorphonuclear cells (neutro-
phils) should be measured in synovial uid. They are higher in inamma-
tory arthritis, e.g. RhA (5000– 75,000/ mm
3
, >50%), and septic arthritis
(>50,000/ mm
3
, >75%) than normal (<200/ mm
3
, <25%) or osteoarthritis
(200– 10,000/ mm
3
, <50%). AGram stain and culture should be performed
to detect organisms in suspected septic arthritis. Culture for less common
organisms, such as TB, may also need to be considered.
Polarized light microscopy should be used to detect the presence of crys-
tals. Urate is associated with characteristic negatively birefringent crystals,
whilst calcium pyrophosphate crystals are positively birefringent. Other
crystals may also be observed, e.g. hydroxyapatite in Milwaukee shoulder.
Arthroscopy
Arthroscopy and synovial biopsy may occasionally be required for the diag-
nosis of chronic indolent infections, such as TB, or unusual slow- growing
organisms such as Coxiella (fever polyarthritis). Arthroscopy is essentially a
diagnostic procedure used by orthopaedic surgeons where direct visualiza-
tion of the aected joint is required.
1
1 American Academy of Orthopaedic Surgeons. M http:// www.orthoinfo.aaos.org/ .

NEUROPHYSIOLOGY
755
Neurophysiology
These are dynamic electrical nerve and muscle tests that are performed in
the context of appropriate clinical history and examination. They may be
considered in rheumatology, including:
•
Assessment of muscle disorders, e.g. to distinguish inammatory muscle
disease (polymyositis/ dermatomyositis) and other ° muscle disease,
e.g. muscular dystrophy.
•
Where mononeuritis multiplex/ peripheral neuropathy is suspected as
part of a rheumatological diagnosis, e.g. vasculitis, RhA, amyloidosis.
•
Nerve entrapment syndromes, e.g. carpal tunnel syndrome, tarsal
tunnel, or ulnar nerve compression where the clinical diagnosis is
uncertain.
•
Spinal cord/ nerve root compression.

756
CHAPTER12 Rheumatology
756
Diagnostic imaging
Imaging techniques are essential tools in the management of rheumatologi-
cal conditions and are useful if performed under appropriate indications.
It is not a substitute for clinical examination, and ndings should be inter-
preted in the context of clinical abnormalities. It is important to follow the
guidelines for radiological investigations published by the Royal College of
Radiologists.
Inammatory arthritis
It is important to diagnose inammatory arthritis as early as possible, as
timely treatment with disease- modifying anti- rheumatic drugs improves
outcomes in terms of function, joint damage, quality of life, and costs to
health service usage. Although conventional X- rays can be useful in diagno-
sis, this is generally only when disease is established and structural damage
to the joints has already occurred. The challenge to identify early arthritis
has led to widespread use of alternative imaging techniques, including mus-
culoskeletal US andMRI.
Plain radiography
Arthropathy
Hand and feet radiographs are useful in diagnosis. Soft tissue changes can
show swelling, eusions, and specic abnormalities, such as calcication,
which can be helpful even in early disease. Bony changes occur usually in
more established arthritis. The pattern and type of radiological damage can
suggest the diagnosis (see Table 12.2), and serial lms can be used to assess
disease progression. Other ndings may suggest specic diseases, e.g. cal-
cinosis in limited cutaneous scleroderma and chondrocalcinosis inCPPD.
Radiographs of the lumbar spine and sacroiliac joints may be utilized in
distinguishing mechanical and inammatory (e.g. ankylosing spondylitis/
psoriatic arthritis) back pain. Changes seen in inammatory back disease
include sacroiliitis (sclerosis and joint space loss), squaring of the vertebrae,
bony proliferation along vertebrae (syndesmophytes), and spondylodis-
citis. However, changes do not usually occur early in disease, and it can
be dicult to distinguish changes from degenerative disease (osteophytes,
loss of disc height). Evidence of bony change elsewhere, e.g. enthesitis, is
helpful in diagnosis. Isotope bone scan or MRI may be appropriate further
investigations.
Other joints should be imaged according to an individual’s clinical needs.
For example, a knee or hip would be required if joint replacement surgery
may be appropriate, or a shoulder if calcic tendinitis is considered. Plain
radiography is also useful in assessing complications of rheumatic disease,
e.g. rheumatoid lung disease. Limitations of plain lms are that only limited
information is provided about soft tissues and bone texture and early joint
damage is missed.
Bone lesions
Osteopenia should be further investigated with dual- energy X- ray absorpti-
ometry (DXA) scanning (E Nuclear medicine imaging, p. 763) and appro-
priate blood tests, e.g. PTH, Igs, and vitamin D.The underlying diagnosis

DIAGNOSTIC IMAGING
757
can be suggested by typical bony appearances, e.g. Looser’s zones are
lucent lines visualized at 90° to the bony cortex in osteomalacia (vitamin
D deciency). Periosteal reactions and, in more advanced disease, bone
resorption, can be seen in hyperparathyroidism and renal osteodystrophy.
Hypertrophic osteoarthropathy is another cause of periostitis, which can
signify underlying disease, e.g. bronchial carcinoma.
Sclerotic bone lesions (i bone density) can also be seen in renal
osteodystrophy.
Paget’s disease is characterized by focal excessive bone resorption (osteo-
lytic phase), followed by excessive bone formation (osteoblastic phase).
Radiological features are of radiolucent areas associated with bone widen-
ing, then subsequent coarsening of trabeculae and areas of i
radiodensity/
sclerosis. In advanced disease, there may be bowing of the bone, pathological
fracture, and i risk of osteosarcoma.
Other causes of sclerotic bone lesions include neoplasms (° and metas-
tases), osteomyelitis, uoride toxicity, and haemangiomas. Myeloma and
other neoplasms should be considered in osteolytic lesions.
Figures 12.1– 12.4 show the use of plain radiology in a variety of rheum-
atological disorders.
Table12.2 X- ray appearance inarthritis ofthehands
Diagnosis Pattern Joints involved Radiographic features
Osteoarthritis Asymmetrical DIPs, PIPs Joint space loss
Peri- articular sclerosis
Bonecysts
Osteophytes
RhA Symmetrical MCPs, PIPs Joint space loss
Peri- articular osteopenia
Erosions
Psoriatic
Psoriatic
arthritis
Asymmetrical/
symmetrical
DIPs, PIPs
(MCPs)
Periostitis
Resorption of terminal
phalanx
Bony proliferation
Ivorydigit
Peripheral erosions
Pencil- in- cup deformity
Soft tissue dactylitis
Gout Asymmetrical DIPs, PIPs
(MCPs)
Juxta- articular erosions—
can be ‘punched out’
Soft tissue tophi
CPPD Asymmetrical DIPs, PIPs,
MCPs
Erosions
Chondrocalcinosis
DIP, distal interphalangeal; MCP, metacarpophalangeal; PIP, proximal interphalangeal.
758
CHAPTER12 Rheumatology
758
Fig.12.1 Hands in rheumatoid disease.
Fig.12.2 Hands showing calcinosis, especially prominent distally.
Fig.12.3 Plain CXR showing pulmonary brosis in rheumatoid disease.
Fig.12.4 X- ray of pelvis showing avascular necrosis of the femoralheads.
DIAGNOSTIC IMAGING
759
Ultrasound
USS is ideally suited to rheumatology. It is a non- invasive, ‘X- ray- free’ tech-
nique that can be used dynamically in a clinic setting as an extension to
clinical examination. Grey- scale US can be used in inammatory arthritis
to detect synovial thickening, joint eusions, and bony erosion in RhA,
entheseal changes in spondyloarthropathy, and crystal deposition in calcium
pyrophosphate.
Ultrasonography
US has an important role in the diagnosis of early arthritis, because ero-
sions can be identied before they are visible on radiographs and subclinical
synovitis can be detected. It can also be helpful in monitoring disease pro-
gression/ treatment response. Power Doppler can be used to assess joint
activity in addition to joint damage.
US is also ideal for imaging tendon pathology, including tendinitis, ten-
osynovitis, calcic tendinitis, and tears. It has been reported to be highly
sensitive and specic for rotator cu tears, and the dynamic nature of
examination means that a tendon/ joint can be examined, whilst in motion.
Bursae, uid- lled cysts, and ganglia are also readily identied.
More advanced US assessments include soft tissue and muscle disorders,
assessment of masses, and nerve entrapment. Imaging of temporal arteries
in GCA is also reported to show diagnostic changes in experienced hands,
with a hypoechoic halo being demonstrated due to artery oedema and ste-
nosis. This may potentially be used to guide biopsy. US has also been used
to assess blood ow in vasculitis, e.g. brachial and axillary arteries.
Limitations of US include diculty in imaging less accessible areas, e.g.
carpal bones, and no information beyond the bony cortex.
Figures 12.5– 12.8 show the use of US in a variety of rheumatological
disorders.
Fig.12.5 Achilles tendinosis.
760
CHAPTER12 Rheumatology
760
Fig.12.6 Erosion of MCPjoint.
Fig.12.7 Extensor tenosynovitis of thewrist.
Fig.12.8 Flexor tenosynovitis of the middle nger.

DIAGNOSTIC IMAGING
761
Magnetic resonance imaging
In contrast to plain lms and US, MRI has the strength that all components
of a joint can be visualized at once. Not only can the bony cortex be appre-
ciated, but also the composition of the bone. Use of MRI is limited by acces-
sibility, expense, duration of scans, and presence of metal implants, e.g.
pacemakers preclude its use. MRI has a role in the diagnosis of inammatory
arthritis, especially in early disease, to optimize treatment and outcomes,
but also in distinguishing alternative, often signicant, bone and joint disease,
including neoplasm.
Inammatory arthritis
In RhA, MRI detects erosions earlier than X- ray and can identify ‘pre-
erosion’— areas of bony oedema, which proceed to erosions in time. This
suggests that patients at risk of erosive arthritis could be identied prior to
permanent structural damage and treatment tailored accordingly. However,
not all such areas do progress to erosions and similar features have been
seen in bone disease (E Bony lesions, p. 761), healthy joints, bone cysts,
and ganglia.
Spondyloarthropathy can be dicult to diagnose, traditionally requir-
ing X- ray evidence of sacroiliitis. MRI can detect both active and chronic
lesions of spondyloarthritis in the spine, sacroiliac joints, and entheses.
Active inammation/ BM oedema is detected on short tau inversion recov-
ery (STIR) and fat- suppressed sequences, whereas chronic lesions are seen
best on T1- weighted (T1W) images. MRI is now included in the ASAS
(Assessment of SpondyloArthritis International Society) international clas-
sication of axial spondyloarthritis.
Muscle disease
MRI is useful in identifying abnormal areas of muscle. In the context of
myositis, an MRI scan can be used to guide a muscle biopsy to ensure sam-
pling of abnormal tissue.
Soft tissue and back disorders
MRI is able to visualize tendon and cartilage lesions and bone tumours. MRI
is particularly useful in the assessment of the back, especially in identifying
infective lesions, e.g. discitis/ abscess, in addition to identifying inammatory
lesions, sacroiliitis, spinal stenosis, myelopathy, disc prolapse disease, and
nerve compression. It is used in the assessment of orthopaedic lesions, such
as meniscal tears in the knee, especially prior to surgery.
Bony lesions
MRI is an excellent modality for investigating localized musculoskeletal
symptoms, identifying signicant lesions.
Bone oedema is also seen in a variety of bone lesions including avas-
cular necrosis and transient osteoporosis of the hip, trauma, e.g. stress
fractures, infection including osteomyelitis, metabolic bone disease, e.g.
Paget’s disease, chronic pain syndrome (‘reex sympathetic dystrophy’),
and neoplasm.
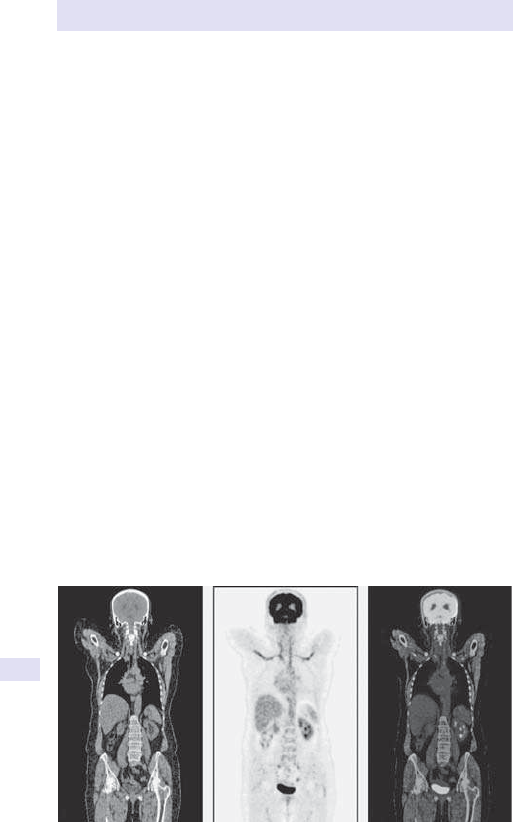
762
CHAPTER12 Rheumatology
762
Vasculitis
MRA is used in the investigation of vasculitis, including large- vessel vascu-
litides, e.g. GCA and Takayasu’s arteritis, and smaller- vessel disease, e.g.
Buerger’s disease.
Computed tomographyscans
CT scans are rarely used in imaging of the musculoskeletal system, especially
as associated radiation dose is high. CT/ HRCT does, however, have a role
in the conrmation of diagnosis in bony lesions, e.g. inltrative lesions, hae-
mangiomas, lipomas, osteoid osteomas, and other bony lesions. Sacroiliac
inammation, erosions, and calcication are also well visualized. HRCT has
been used in the assessment of osteoporosis but is largely superseded by
DXA scanning (E Nuclear medicine imaging below).
The major role for CT is in identifying complications of rheumatic dis-
ease, e.g. pulmonary brosis associated withRhA.
Nuclear medicine imaging
Bonescan
Radionuclide imaging with
99m
technetium or
67
gallium is relatively easy to
perform and gives information about the whole body. In rheumatology, it
is used to assess the pattern of joint involvement in arthritis and to detect
other causes of bony pain, including Paget’s disease, metastases, stress frac-
tures, and other specic diagnoses, e.g. chronic regional pain syndrome.
Although bone scans are sensitive to abnormalities, they have poor speci-
city so should be used in clinical context and may be a guide to further
additional investigation, e.g.MRI.
Positron emission tomography
(See Fig.12.9.)
18- FDG PET was originally used for tumour imaging but has gained wide
acceptance in its use in the imaging of medium- to large- vessel vasculitis. It is
(b)
(a)
(c)
Fig.12.9 Coronal images of CT (a), PET (b), FDG-PET/CT (c) of a 90-year-old
woman with bilateral axillary artery thrombosis, arthralgia, and fatigue. These
demonstrate increased FDG uptake in the carotid, subclavian and axillary vessels,
consistent with large vessel vasculitis.
Reproduced from Watts, Richard, et al., Oxford Textbook of Rheumatology, 4th edition
(2013), with permission from Oxford University Press.

DIAGNOSTIC IMAGING
763
of particular benet in the diagnosis and assessment of the extent of vessel
involvement in GCA, Takayasu’s arteritis, and aortitis. It can also be used in
the monitoring of disease activity. Other potential uses for PET- CT include
inammatory myositis and investigation for underlying malignancy associ-
ated with rheumatic disease.
Dual- energy X- ray absorptiometryscan
Osteoporosis may not be recognized clinically, unless a fracture occurs.
DXA is a non- invasive, low- radiation assessment of bone density. Potential
fracture sites (lumbar spine, hip, neck of femur, and wrist) are imaged, and
the result is compared with that expected in a standard population to give a
T- score and an age- adjusted population to give a z- score. For every stand-
ard deviation below the mean of the standard population, the fracture risk
is i
by 2– 3 times. Vertebral fracture analysis may also be oered to iden-
tify fractures at the time of the scan. DXA is oered to patients at risk
of developing osteoporosis (see Table 12.3). Online calculators have been
developed to identify those most at risk of developing fragility fractures
and to determine who needs a DXA scan and/ or treatment. The Q frac-
ture method is most suited to the elderly, and the FRAX/ NOGG (National
Osteoporosis Guideline Group) calculator is intended for 40- to 90- year-
olds and can be used with the bone mineral density of the femoral neck,
following DXA, to improve prediction of the 10- year fracture risk. Ideally,
repeat scans should be carried out on the same DXA machine, as results
cannot be reliably compared between dierent machines.
Table12.3 Risk factors inosteoporosis
Osteoporosis Diseases associated with
osteoporosis/ bone fragility
°
Menopause <45(+ <5- year HRT) RhA
2° amenorrhoea Ankylosing spondylitis
Radiological osteopenia
Lupus
History of maternal hip fracture Hyperparathyroidism
Previous fragility fracture* Hyperthyroidism
Heavy long- term smoking ♂ hypogonadism
Low BMI (kg/ m
2
) <19 Chronic liver disease
Prolonged immobilization, e.g. MS Chronic IBD
Excessive alcohol consumption Malabsorption
Chronic renal failure
2°
Oral steroids Hypopituitarism
Aromatase inhibitors Haemochromatosis
Androgen deprivation treatment for
prostate cancer
Organ transplant patients
Excessive thyroxine/ anticonvulsants Anorexia/ bulimia
* Fracture sustained with no trauma or fall from standing height.

764

765
Chapter13
Radiology
Radiology and the role of imaging 766
Plain X- rays 768
Digital radiology 769
Chest X- ray:useful landmarks 770
Chest radiograph 774
Patterns of lobar collapse 780
Cardiac enlargement 782
Computed tomography of the thorax 786
Abdominal X- ray:useful landmarks 788
Plain abdominal X- ray 790
Barium studies 794
Barium swallow 796
Barium meal 798
Small intestine 800
Cholangiography 802
Barium enema 806
Radiology of the urinary tract 808
Breast imaging 812
Ultrasound 816
Obstetric imaging 820
Gynaecological imaging 822
Computed tomography 824
Magnetic resonance imaging 828
Spinal imaging 836
Pelvis 838
Vascular intervention 840
Interventional radiology 844
Musculoskeletal imaging 850
Hands 852
Neuroradiology 856
Skull X- ray 860
Reference section 862
Internet resources 864

766
CHAPTER13 Radiology
766
Radiology and therole ofimaging
Eective use of the radiology department relies on good communication
between radiologists and their clinical colleagues. The overall aim must be
to target investigations eciently in order to provide answers to clinical
dilemmas at minimal cost and radiation dose (see Table 13.1). The investiga-
tion of neurological problems has been transformed by the advent of CT
and MRI. Local availability varies and CT, in particular, can add considerably
to the radiation burden. Conversely, if a CT is likely to provide the best
answer and minimize overall costs by resulting in an early discharge, then
it should be the investigation of choice. It is helpful to consider plain lms,
contrast studies, US, and then CT/ MRI as a hierarchy where plain lms are
requested as an initial investigation. This hierarchy may be circumvented
if a more expensive investigation is likely to produce the denitive result.
The following are important points to consider:
• Will the investigation alter patient management? That is, is the expected
outcome clinically relevant? Do you needit?
•
Investigating too often or repeating investigations before there has been
an adequate lapse of time to allow resolution or to allow treatment to
take eect
. Similarly, investigations performed too early may be non-
contributory. Do Ineed it now? Especially relevant when investigations
may have been performed elsewhere. Make every eort possible to
obtain prior studies. Transfer of digital data through electronic links will
assist in this process. Has it been done already?
•
Would an investigation that does not use ionizing radiation be more
appropriate
, e.g. USS/ MRI?
• Failure to provide accurate clinical information and questions that you are
hoping will be answered by the investigation may result in an unsatisfactory
outcome or an inappropriate focus in the report. Have Iexplained the
problem?
•
Would another technique be more appropriate? The advances in radiology
mean that discussion with a radiologist may be helpful in determining the
best possible test. Is this the best investigation?
•
Over- investigating. Are you taking comfort in too many tests or providing
reassurance to the patient in thisway?

RADIOLOGY AND THEROLE OFIMAGING
767
Table13.1 Typical eective doses fromdiagnostic medical exposures
Procedure
Typical eective
dose (mSv)
Equivalent
number of
CXRs
Equivalent period
background
radiation
Chest (P- A) 0.015 1 2.5days
Lumbar spine 0.6 40 3months
Abdomen 0.4 30 2months
IVU 2.1 140 11.5months
Mammography
(two views)
0.5 35 3months
Barium enema 2.2 150 1year
CT head 1.4 90 7.5months
CT chest 6.6 440 3years
CT abdomen 5.6 370 2.5years
CT colonography 10 670 4.5years
Bone scan (
99m
Tc) 3 200 1.4years
PET scan (body)
(F18- FDG)
18 1200 8.1years
UK average background radiation=2.2mSv/ year.
1
Table adapted from Royal College of Radiologists (2012) Guidelines for Doctors. Making the
best use of clinical radiology services. Doses for conventional X- ray examinations are based on
data compiled by the Health Protection agency (HPA) from a survey of UK hospitals in 2008.
The doses for CT examinations and radionuclide studies are compiled from surveys conducted
by the HPA and the British Nuclear Medicine Society.
1
Royal College of Radiologists. Making the best use of clinical radiology services, Version 7.0.2.
London:Royal College of Radiologists,2012.

768
CHAPTER13 Radiology
768
PlainX- rays
Wilhelm Roentgen discovered X- rays in 1895. X- rays form part of the elec-
tromagnetic spectrum, with microwaves and radio waves lying at the low-
energy end, visible light in the middle, and X- rays at the high- energy end.
They are energetic enough to ionize atoms and break molecular bonds as
they penetrate tissues and are therefore called ionizing radiation. Diagnostic
X- rays are produced when high- energy electrons strike a high atomic num-
ber material. This interaction is produced within an X- ray tube. A high
voltage is passed across two tungsten terminals. One terminal (cathode) is
heated until it liberates free electrons. When a high voltage is applied across
the terminals, the electrons accelerate towards the anode at high speed. On
hitting the anode target, X- rays are produced.
The X- ray picture is a result of the interaction of the ionizing radiation
with tissues as it passes through the body. Tissues of dierent densities are
displayed as distinct areas, depending on the amount of radiation absorbed.
There are four basic densities in conventional radiography: gas (air), fat,
soft tissue and uid, and calcied structures. Air absorbs the least amount
of X- rays and therefore appears black on the radiograph, whereas calcied
structures and bone absorb the most, resulting in a white density. Soft tis-
sues and uid have a similar absorptive capacity and therefore appear grey
on a radiograph.

DIGITAL RADIOLOGY
769
Digital radiology
X- ray lm is exposed by light photons emitted by intensifying screens sensi-
tive to radiation transmitted through the patient. Storage phosphor tech-
nology uses photostimulatable phosphor screens to convert X- ray energy
directly into digital signals. The i dynamic range and image contrast of
digital radiography, compared with conventional lm screen combinations,
and the facility to manipulate signal intensity after image capture reduce
the number of repeat exposures. This i eciency and minimizes patient
radiation dose. Digital images can be made available on a local network for
reporting by a radiologist or for review on a ward- based computer. Picture
archiving and communication systems (PACS) are ecient at image produc-
tion and manipulation and in the storage, retrieval, and transmission of data.
PACS facilitates remote radiology reporting and alleviates workow pres-
sures. The vast majority of imaging studies conducted in the UK are stored,
manipulated, and shared via a picture archiving and communication system.
PACS has been rolled out to all healthcare organizations over the last dec-
ade or more as local, regional, or national implementations with dierent
degrees of connectivity to radiology information systems (RIS), either via a
local RIS or shared with other organizations (domain- based). Global chal-
lenges to PACS systems include management of i volumes of imaging data
and the ability to data- share across a spectrum of healthcare communities.
Advantages include inventive ways of providing diagnostic imaging cover-
age, for instance in remote or scantily populated communities.
770
CHAPTER13 Radiology
770
Chest X- ray:useful landmarks
In order to interpret a plain P- A or lateral CXR, some knowledge of chest
anatomy and the major landmarks on the lm is required. We have high-
lighted the major bony and soft tissue structures visible on the plain lm
in order to make it easier to spot abnormalities. Patient positioning for
a P- A CXR (see Figs 13.1– 13.3) and lateral CXR (see Figs 13.4– 13.6) is
illustratedbelow.
Fig.13.1 Patient position for P- ACXR.
Fig.13.2 P- ACXR.
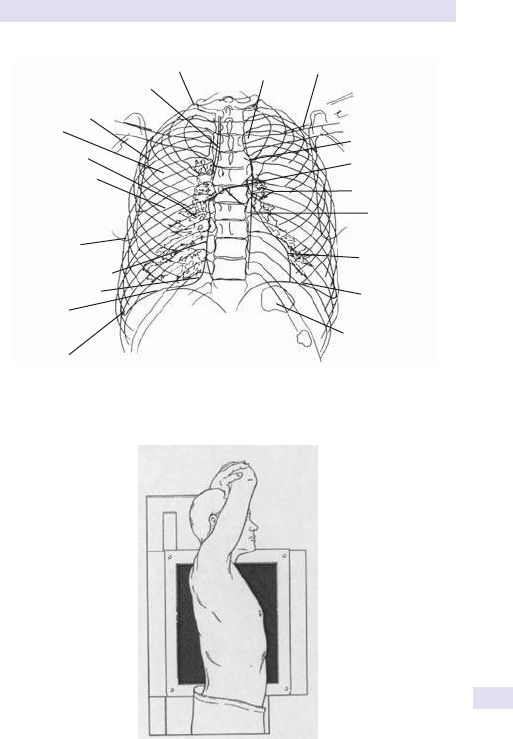
CHEST X-RAY:USEFUL LANDMARKS
771
Companion shadow
of left clavicle
Aortic knob
Carina
Left hilum
Border of
descending
thoracic aorta
Wall of
left ventricle
Left dome
of diaphragm
Gas bubble in
fundus of stomach
Right lateral
costophrenic angle
Right dome
of diaphragm
Inferior vena cava
Wall of right atrium
Inferior angle
of right scapula
Horizontal ssure
of right lung
superimposed on
posterior segment o
f
right 7th rib
Right hilum
Right 5th
interspace
Posterior segment
of right 5th rib
Right tracheal stripe
Right rst rib
Medial end of
left clavicle
Fig.13.3 P- A CXR landmarks.
Fig.13.4 Patient position for lateralCXR.
772
CHAPTER13 Radiology
772
Fig.13.5 LateralCXR.
Shadow of aortic arch
superimposed on
tracheal lumen
Lumen of
a bronchus
Bodies of lower
thoracic vertebrae
Retrocardiac area
Left dome
of diaphragm
Right dome
of diaphragm
Right posterior sulcus
Manubriosternal joint
Retrosternal area
Body of sternum
Horizontal ssure
of right lung
Oblique ssure
Gas bubble in fundus
of stomach
Fig.13.6 Lateral CXR landmarks.

CHEST X-RAY:USEFUL LANDMARKS
773

774
CHAPTER13 Radiology
774
Chest radiograph
The chest lm is the most widely requested, yet most easy to misinterpret,
investigation. Using a logical approach will avoid most pitfalls. This should be
the initial imaging modality in all patients with suspected thoracic pathology.
Points toconsider
• Always obtain prior imaging, if available; temporal changes assist greatly
in image interpretation and dierential diagnosis.
•
Standard projections of the chest are P- A (posteroanterior) vs AP
(anteroposterior). See E Projectionbelow.
•
Additional views to aid problem- solving include lordotic, oblique, and
decubitus projections (see below).
•
Initially assess the technical quality.
Projection
P- A vs AP will determine whether assessment of the cardiac size is reliable.
Potential other views include:
•
Lateral:improves visualization of the retrocardiac space and thoracic
spine; earlier and more sensitive detection of eusions.
•
Lateral decubitus:assesses for pleural eusion or pneumothorax in
immobile patients (portable US also has a utility in this setting).
•
Lordotic:angled beam allows better view of the apices which are
typically obscured by the clavicles and anteriorribs.
Posture
Erect lms enable a more accurate assessment of the mediastinum, since
the lungs are more expanded, and allow detection of air– uid levels, pleural
thickening, and comment on the size of the pulmonary vasculature.
Rotation
Look for the relationship of the medial ends of the clavicles to the spinous
process at the same level; a common cause of unilateral transradiancy is
rotation.
Degree ofinspiration
Ideally, six ribs should be seen anteriorly and ten ribs posteriorly. If more,
this suggests hyperination (does the patient have asthma or COPD?). If
less (e.g. poor inspiratory eort, obesity, or restrictive chest disorders),
there will be apparent cardiomegaly, i basal shadowing, and less commonly
tracheal deviation.
Quality of image can be assessed by the degree of penetration. The
thoracic disc spaces should be just visible through the heart. Absence of
respiratory or motion artefact.
The heart and mediastinum
Sequentially consider the heart, mediastinum, lungs, diaphragms, soft tissues
(breast shadows), and bones. Remember to assess your review areas— the
lung apices, behind the heart, under the diaphragm, and the costophrenic
angles.

CHEST RADIOGRAPH
775
Diaphragm
This should lie between the fth and seventh ribs. If attened, consider
hyperination. In 90% of cases, the right is higher than the left by 3– 4cm.
Eacement of the interface between the lung and diaphragm suggests pleu-
ral or pulmonary pathology. Loss of smooth contour suggests localized her-
niation (eventration). Peaks laterally may be due to subpulmonary eusion.
Root ofneck and trachea
The upper trachea is central with a slight displacement to the right inferiorly
due to the oesophagus. Thickening of the paratracheal line (>5mm) may
imply nodal enlargement.
CXR inthe intensive care unit patient
Look at the following parameters:
1. Patient demographics.
2. Date and time of study should be included in the report.
3. Has there been surgery?
4. If there are lines, catheters, drains, endotracheal tube (ETT), etc.,
comment on the position. If new lines, comment on changes in position
or if any removed.
5. Cardiac and mediastinal size andshape.
6. Presence of pneumothorax.
7. Lung disease. Pattern of disease as well as its evolution overtime.
8. Interval changes from one lm to the next as well as overtime.
Mediastinum
The mediastinum should be central. The heart is normally <50% of the
thoracic width. Mediastinal enlargement or widening is a non- specic nd-
ing. The silhouette sign may help, but a lateral lm is helpful for localization.
Table 13.2 gives a list of distinguishing features that enable distinction of
mediastinal masses from intra- pulmonary masses.
Normal variants mimicking a wide mediastinumare:
•
AP projection (notP- A).
• Mediastinal fat (steroids, obesity).
• Vascular tortuosity— elderly patients.
• Low inspiratory supine position.
Table13.2 Dierentiating mediastinal frompulmonarymasses
Mediastinal mass Pulmonary mass
Epicentre lies in mediastinum Epicentre in lung
Obtuse angles with lung Acute angles
No air bronchograms Air bronchograms possible
Smooth and sharp margins Irregular margins
Moves on swallowing Moves with respiration
Bilateral Unilateral

776
CHAPTER13 Radiology
776
The mediastinum is divided into three arbitrary compartments to aid in
the dierential diagnosis of a mediastinal mass (see Table 13.3). As there
are no anatomical planes separating these divisions, disease can spread from
one compartment to thenext.
Based on the location of the mediastinal abnormality, possible patholo-
gies include:
•
Superior mediastinum:thymoma, retrosternal thyroid, and lymphoma.
•
Anterior mediastinum (anterior line formed by the anterior trachea
and the posterior border of the heart and great vessels):lymphoma
(Hodgkin’s disease (HD) and non- Hodgkin’s lymphoma (NHL)),
germ cell tumours, thymoma, retrosternal goitres, and Morgagni
hernias(low).
•
Middle mediastinum (extends behind the anterior mediastinum to a
line 1cm posterior to the anterior border of the thoracic vertebral
bodies):aortic aneurysm, bronchial carcinoma, foregut duplication cysts
(including bronchogenic/ oesophageal), and hiatus hernia.
• Posterior mediastinum (posterior to line described above):neurogenic
tumours, Bochdalek hernia, dilated oesophagus, oraorta.
Pneumomediastinum
Mediastinal air may be due to a number of sources.
Intrathoracic
•
Trachea and bronchi.
• Oesophagus.
• Lung.
• Pleuralspace.
Extrathoracic
•
Head andneck.
• Intraperitoneum and retro- peritoneum.
Signs include: subcutaneous emphysema, pneumopericardium, elevated
thymus (sail sign), or air around major structures such as the PmA,
bronchialwall.
Table13.3 Radiographic localization ofa mediastinalmass
Location of mass Signs associated with mass in this compartment
Anterior mediastinum Deformation of anterior junctional line
Hilum overlay sign (hilar vessels seen through mass)
Middle mediastinum Distortion of the paratracheal stripe
Convexity of the AP window
Posterior mediastinum Distortion or displacement of the following:
•
Azygo- oesophagealrecess
• Posterior junctionline
• Paraspinal lines

CHEST RADIOGRAPH
777
Enlarged lymphnodes
May be seen in any compartment.
Pleural disease
•
Pleural and extra- pleural masses generally form obtuse angles with the
adjacent pleura.
•
Pulmonary or intra- parenchymal masses form acute angles with the
pleura.
Common pleural abnormalities
Eusion
•
The lateral view is more sensitive, as accumulation of uid occurs rst in
the posterior recess.
•
May cause mediastinal/ tracheal shift to the contralateral side or adjacent
atelectasis.
•
US invaluable (and better than plain lm) in evaluation of small eusions
and guiding thoracocentesis.
•
If blunting of the costophrenic angles is present, it indicates the
presence of uid of at least 200mL (P- A) or 75mL (lateral view) or may
be 2° to thickening of the pleura.
•
Pleural thickening most commonly a sequela of inammatory change.
• Asbestos exposure results in a spectrum of pleural abnormality, ranging
from benign plaques to brosis and malignant mesothelioma (obtain
occupational history).
•
Pleural eusion can be divided into either a transudate or an exudate.
•
Transudate:ultraltrate of plasma, low in protein, no
inammatorycells.
•
Exudate:rich in protein, cells, and debris.
Pneumothorax
•
On an erect lm, the partially collapsed lung is delineated from pleural
air as a curvilinear line (visceral pleura) paralleling the chestwall.
•
On a supine lm, the changes are more subtle; look for the deep
(costophrenic) sulcus sign, the double diaphragm sign (the dome and
anterior portions of the diaphragm outlined by the lung and pleural air,
respectively), hyperlucent thorax, and sharpening of mediastinal structures.
•
Subtle pneumothorax will be more readily apparent on an expiratory
lm or a lateral decubitus lm (accumulation of air superiorly).
•
If the air is under tension, there may be mediastinal shift (tension
pneumothorax). This can result in vascular compromise. On imaging:
•
The lung appears over- expanded.
•
Depression of the diaphragm.
•
Mediastinal shift and of the heart to contralateralside.
Thoracic intervention
Diagnostic thoracocentesis
•
Indication:exclude malignancy; obtain a sample for culture.
•
USS used to determine skin entry site:18– 22G needle advanced into
pleural uid. Angle over the superior border of the rib to avoid
inadvertent neurovascular injury.
•
Complications:pneumothorax (when blind,1– 3%).

778
CHAPTER13 Radiology
778
Therapeutic thoracocentesis
Usually if respiratory compromise from a large eusion. Similar technique
as above, but place a 7– 10Fr catheter. Potential risk of expansion pulmonary
oedema if evacuate in excess of 2– 3L or aspirate both lungs in one sitting.
Also potential for pneumothorax— always obtain post- aspirationCXR.
Percutaneous lungbiopsy
True +ve rate of 90– 95%. False +ve results usually related to malplacement
of biopsy needle, necrotic tumour. Contraindications (relative) include
severe COPD, pulmonary hypertension, coagulopathy, and contralateral
pneumonectomy. Tumour seeding is extremely rare (1:20,000).
Complications include pneumothorax (25%, of which 5– 10% need a
chest tube) and haemoptysis(3%).
Hila
Density should be equal; the left is higher than the right by 5– 15mm. If
more disparity, consider elevation due to brosis (e.g. TB, radiotherapy) or
depression by lobar or segmental collapse. Hilar enlargement may be vas-
cular (e.g. pulmonary arterial or venous hypertension) or due to lymphad-
enopathy (e.g. sarcoidosis, lymphoma, or TB). Hilar calcication is seen in
silicosis, sarcoidosis, and treated lymphoma.
Management ofpneumothorax
Indications
•
Symptomatic pneumothorax.
• Pneumothorax>20%.
• Enlarging pneumothorax on subsequentCXR.
• Tension pneumothorax.
• Poor lung function of contralateral lung disease.
Technique
Either:
1 Second to fourth anterior intercostal space, mid- clavicular line,or
2 Sixth to eighth intercostal space; mid- axillary line or posterior.
Use 8– 12Fr catheters using a trocar technique. After the lung is fully re-
expanded for 24h, the catheter is placed on a water seal for 6h and then
removed if no residual pneumothorax.
Lung disease
Lung opacities may be subdivided into several basic patterns.
Alveolar
Air space shadowing
:ill- dened, non- segmental, and with air bronchograms.
No associated volume loss. Large variety of causes:
•
Fluid l pulmonary oedema (cardiogenic and non- cardiogenic).
• Fat l fat embolism.
•
Haemorrhage l trauma, coagulopathies, pulmonary haemosiderosis.
•
Cells l pulmonary alveolar proteinosis, sarcoidosis, bronchoalveolar cell
carcinoma, lymphoma, and infection (bacterial, fungal, and viral).

CHEST RADIOGRAPH
779
Reticular
Linear opacities:associated obscuration of vessels and late appearance of
CXRsigns:
•
Collagen disorders.
• Extrinsic allergic alveolitis.
• Sarcoidosis, pneumoconiosis.
• CFA.
• EarlyLVF.
• Malignancy (lymphangitis carcinomatosis).
Nodular shadows
Characterize according to their size and distribution:
•
If solitary, exclude tumour.
• Multiple:
•
Granulomata (TB, histoplasmosis, hydatid).
•
Immunological (Wegener’s,RhA).
•
Vascular (AVMs).
•
Inhalational (progressive massive brosis (PMF), Caplan’s syndrome).
Primary signs ofa malignancy
•
Mass or nodule with spiculated or irregular borders.
• Unilateral hilar enlargement or mediastinal widening.
• Cavitating nodule with thick rind of soft tissue.
• Cavitation commonest in squamous cell carcinoma(SCC).
• Malignancy can simulate air space disease (e.g. bronchoalveolar
carcinoma, lymphoma).
Secondary signs ofmalignancy
•
Atelectasis.
• Obstructive pneumonia.
• Pleural eusion.
• Interstitial patterns (lymphangitic spread of disease).
• Hilar and mediastinal adenopathy.
• Metastatic disease (including to ipsilateral or contralateral lung
parenchyma).
Further reading
Corne J, Carroll M, Delaney D. Chest X- ray Made Easy, 2nd edn. Edinburgh:Churchill Livingstone,
2002.
Hansell DM, Lynch DA, McAdams HP, Bankier AA. Imaging of Diseases of the Chest, 5th edn.
London: Mosby,2010.

780
CHAPTER13 Radiology
780
Patterns oflobar collapse
Lobar collapse may be complete or incomplete. The commonest cause is
obstruction of a central bronchus. The ° signs are opacication due to lack
of aeration and displacement of the interlobar ssures. Typical patterns of
lobar collapse are illustrated in Fig.13.7.
Secondary signs include
• Elevation of the hemidiaphragm (more prominent in lower lobe
atelectasis than upper).
•
Mediastinal displacement (tracheal displacement with upper lobe and
cardiac displacement with lower lobe atelectasis).
•
Hilar displacement more prominent with upper lobe atelectasis
thanlower.
•
Crowded vessels in the aectedlobe.
• Compensatory hyperination of remaininglung.
Silhouettesign
• In a normal CXR, the interface between the diaphragm and the
mediastinum are visible due to a dierence in density between the lung
and these structures.
•
The silhouette sign refers to loss of normal interfaces, implying there is
opacication due to consolidation (the commonest cause), atelectasis,
or a mass in the adjacentlung.
•
Silhouetting helps to localize the site of the pathology, and both pleural
and mediastinal disease produce the silhouette sign (see Table13.4).
Table13.4 Localization using thesilhouettesign
Interface lost Location of lung pathology
SVC Right upper lobe
Right heart border Right middle lobe
Right hemidiaphragm Right lower lobe
Aortic knob/ left superior mediastinum Left upper lobe
Left heart border Lingula
Left hemidiaphragm Left lower lobe
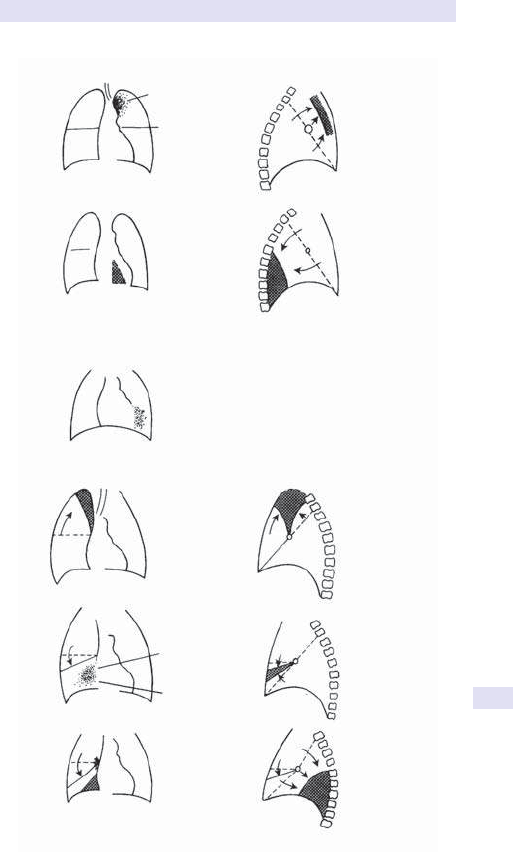
PATTERNS OFLOBAR COLLAPSE
781
(a) Left upper-lobe collapse
(b) Left lower lobe collapse
(c) Lingular consolidation
Right upper lobe collapse
(d) Right middle lobe collapse
(e) Right lower lobe collapse
Trachea
deviated
to L
III-dened
opacity
Sharply dened
posterior border
due to anterior
displacement
of oblique
ssure
Oblique
ssure displaced
posteriorly
Triangular opacity
visible through the heart
with loss of medial end
of diaphragm
Indistinct
L heart
border
Trachea deviated to R
Horizontal
ssure and
R hilum
displaced
upwards
Horizontal ssure
displaced down
III-dened
opacity adjacent
to R heart
border
Loss of R heart
border
Horizontal
ssure
displaced
downwards
Oblique
ssure and
hilum displaced
posteriorly
Well-dened
posterior
opacity
Well-dened opacity adjacent
to R heart border
(R heart border still visible)
Triangular
opacity with
well-dened
margins
Well-dened
triangular
opacity
running from
hilum
Indistinct
elevated
L hilum
Fig.13.7 (a)Left upper lobe collapse. (b)Left lower lobe collapse. (c)Right upper
lobe collapse. (d)Right middle lobe collapse. (e)Right lower lobe collapse.

782
CHAPTER13 Radiology
782
Cardiac enlargement
i of the cardiothoracic ratio beyond 50% is considered abnormal if the P- A
lm is of good quality.
Aetiologies toconsider
• Cardiomegaly (hypertrophy or dilatation of cardiac chambers).
• Pericardial eusion (globular heart).
• Poor inspiratory eort/ d lung volumes.
•
Pectus excavatum.
The location of cardiac valves may be relevant when determining the loca-
tion of calcication. On a lateral projection, draw a line from the xiphi-
sternum to the carina and divide the heart into thirds. The location of the
cardiac valves will be as demonstrated in Fig.13.8.
Normal plain lm anatomy
Figure 13.9 shows a P- A view. The right cardiac margin comprises three
segments:
•
SVC.
• RAt.
• IVC.
The left cardiac margin comprises four segments:
• Aortic arch (AA) (becomes more prominent withage).
• Main PmA at level of the left main stem bronchus.
• Left atrial appendage (may not be visible in normal hearts).
• LV.
• RV is not usually seen in frontal projection.
Line positions
Endotrachealtube
•
Tip of the ETT should be above the carina and below the thoracicinlet.
• The inated cu should not bulge the trachealwall.
• Neck position can change the impact location of thetip.
• In neutral:the tip should be 4– 6cm above the carina.
• Flexed:moves the tip inferiorly by2cm.
•
Extended:moves the tip superiorly by2cm.
•
Complications:malpositioned results in atelectasis or collapse due to
bronchial obstruction. Tracheomalacia if over- inated cu; tracheal
rupture may result in pneumothorax.
•
Pearl:in situations where there is low pulmonary compliance (e.g. acute
respiratory distress syndrome (ARDS)), a tip position closer to the
carina may reduce barotrauma.
Nasogastrictube
•
Tip should be in the stomach.
• Tip and side port should lie distal to the oesophagogastric junction and
proximal to the gastric pylorus.
•
Potential complications include placement in airway or gastric/ duodenal
erosion.
CARDIAC ENLARGEMENT
783
Swan– Ganz catheter
• Tip should be located in the left or right PmA within 1cm from the
hilum. Loops in the RA or RV may cause arrhythmias. There are two
types of Swan– Ganz catheters:
1 Used to measure wedge pressure.
2 With an integrated pacemaker.
• Complications include pulmonary infarct, haemorrhage, PmA pseudo-
aneurysm, and infection. If the tip is distal to the proximal interlobar
PmA, there is a potential risk of PmA rupture or pseudo- aneurysm.
P
Ao
M
T
P = pulmonary valve Ao = aortic valv
e
M = mitral valve T = tricuspid valve
Fig.13.8 Schematic diagram showing the relations of the cardiac valves on a lateral
lm of thechest.
RA
SVC
IVC
LV
Left atrial
appendage
PA
AA
Fig.13.9 Schematic diagram depicting a normal cardiac silhouette as seen on a
plainCXR.

784
CHAPTER13 Radiology
784
Dialysis catheter
• Should be located in theRAt.
Epicardial pacingwire
•
Typically anchored in the anterior mediastinum. Multiple wires may be
present and usually exit through the anterior chestwall.
Automatic intracardiac debrillation device(AICD)
•
Newer systems have intraventricular electrodes with pin sensors.
Intra- aortic balloonpump
• Tip should be located just distal to the origin of the left subclavian artery
and be 2– 4cm below the aortic knuckle.
• Complications include aortic dissection, low position associated with
mesenteric or renal ischaemia, and high position withCVA.
Central venousline
•
Tip should end in the lower SVC or the cavoatrial junction below the
anterior rst rib. Azygos malposition is seen in 1% and is associated with
risk of venous perforation or catheter- associated thrombosis.
Pacemaker
•
Typical position should be in the apex of the RV. Can be located in the
atrial appendage for atrial pacing and in the coronary sinus for atrial left
ventricular pacing.
•
Complications include electrode displacement, perforation, infection, or
venous thrombosis.
Chesttube
•
Side port should lie within the thoracic cavity.
• Tip of tube should not abut the mediastinum.
CT angiography ofcoronary vessels (coronary computed
tomography angiography,CCTA)
CT is very sensitiveat:
• Detecting and quantitating calcication in coronary arteries.
• Non- invasively depicting the entire coronarytree.
• Determining the luminal diameter.
Improved hardware has resulted in less blooming artefact which could
potentially overestimate the degree of stenosis seen with calcied plaques.
It is very sensitive to haemodynamic stenosis (>50% of the luminal diam-
eter). Multiple meta- analyses have shown NPV in high90s.
For calcium scoring, the examination is performed without IV contrast and
there are various algorithms available that provide a measure of the total
coronary plaque burden. ECG gating is used to minimize cardiac motion;
the type of gating employed has signicant impact on patient radiationdose.

CARDIAC ENLARGEMENT
785
Magnetic resonance coronary angiography
Also has potential for non- invasive diagnosis of coronary artery disease.
The in- plane image resolution for current MR techniques is about 0.5mm,
sucient for assessment of large coronary vessels as well as in venous grafts
following coronary artery bypass grafting, but inadequate for detecting dis-
ease in smaller side branches.
Cardiac magnetic resonance imaging
Provides a high- resolution dynamic study using predominantly steady- state
free precession (SSFP) and/ or gradient echo cine sequences.
SSFP is a ‘white blood sequence’ that provides excellent contrast between
the myocardium and the blood pool. It is suited to cardiac imaging due to its
high temporal resolution and excellent contrast.
Cine MRI provides quantitative assessment of cardiac morphology and
function. Real- time images can be used for subjective analysis when gating
is poor. Tissue characterization is usually performed with double or triple
inversion fast spin echo sequences. These ‘black blood’ sequences null sig-
nal from owingblood.
First- pass contrast- enhanced perfusion MRI is performed pre- and
post- vasodilator stress to assess myocardial perfusion. Delayed contrast-
enhanced MRI is used to assess myocardial viability, inammation, and
brosis.
Further reading
Miller SW, Abbara S, Boxt LB. Cardiac Imaging:The Requisites, 3rd edn. Mosby,2009.
Ra GL, Abidov A, Achenbach S, etal
. SCCT guidelines for the interpretation and reporting of coro-
nary computed tomographic angiography. J Cardiovasc Comput Tomogr 2009; 3
:122– 36.
786
CHAPTER13 Radiology
786
Computed tomography ofthethorax
Indications include
• Evaluation of an abnormal nding on plainlm.
• Staging of ° or metastatic malignancies.
• Evaluation of suspected mediastinal or hilarmass.
• Detection of thromboembolic disease by CTPA (see Figs 13.10 and 13.11).
• Detection and assessment of aortic dissection.
• Distinguishing empyema from lung abscess.
• CT- guided percutaneous needle biopsy of focal lung lesion or
mediastinal abnormality.
•
CT- guided pleural biopsy.
High- resolution computed tomography (HRCT) comprises thin section images
that are reconstructed using a special algorithm.
Fig.13.10 Axial image from CTPA showing the presence of thrombus within the
lower lobePmA.
Fig.13.11 Sagittal reformatted image conrms the extent of the thrombus and its
relationship to thelumen.
COMPUTED TOMOGRAPHY OFTHETHORAX
787
Indications include
• Evaluation of a diuse lung disease (see Fig. 13.12).
• Characterization of a solitary pulmonary nodule.
• Haemoptysis.
• Lung disease in a patient with abnormal pulmonary function tests but
apparently normal CXR (see Table13.5).
• Assessment of emphysema.
Fig.13.12 HRCT image of the chest in a patient with cystic lung disease due to
tuberous sclerosis.
Table13.5 HRCT patterns ofinterstitial lung disease
Pattern Description Causes
Ground glass
opacity
i haze
Vessels can be seen
through hazing
Allergic hypersensitivity
Acute interstitial disease (DIP, active
IPF),viral
PCP, BOOP, COP, pulmonary
oedema, eosinophilic pneumonia
Reticulonodular
Peri- bronchovascular
thickening (equivalent
of cung on CXR)
Thickened interlobular
septal (Kerley) lines)
Pulmonary brosis, viral, Mycoplasma
pneumonia, PCP
Pulmonary oedema,IPF
Lymphangitic spread of tumours,
brosis due to drugs, radiation,
asbestosis, collagen vascular disease
Nodular
opacities
1– 2mm interstitial
nodules, often seen
in conjunction with
reticular opacities
Haematogenous infection,
metastases, sarcoid, pneumoconiosis,
histiocytosis, silicosis, and CWP
Cystic spaces May or may not have
walls
Lymphangioleiomyomatosis
Histiocytosis, honeycombing
IPF, LIP, cysticPCP
End- stage interstitial disease
BOOP, bronchiolitis obliterans organizing pneumonia; COP, cryptogenic organizing pneumonia;
CWP, coalworker’s pneumoconiosis; DIP, desquamative interstitial pneumonia; IPF, idiopathic
pulmonary brosis; LIP, lymphocytic interstitial pneumonitis; PCP, pneumocystis pneumonia.
788
CHAPTER13 Radiology
788
Abdominal X- ray:useful landmarks
Interpretation of the AXR, like the CXR, requires experience. In order to
make things slightly easier, we have provided a rough guide to the various
bony, soft tissue, and gas shadows seen on a ‘typical’ AXR (see Fig. 13.13).
Figure 13.14 provides a normal AXR for correlation with the diagram.
Figure 13.15 shows diuse sclerotic metastases.
Lateral border of
right psoas major
Right transverse process
of 4th lumbar vertebra
Large bowel gas
Right sacroiliac joint
Bladder
Symphysis pubis
Lower pole
of left kidney
Iliac crest
Fig.13.13 P- A AXR landmarks.
ABDOMINAL X-RAY:USEFUL LANDMARKS
789
Fig.13.14 P- AAXR.
Fig.13.15 Diuse sclerotic metastases in a patient with a prostatic carcinoma. Note
the presence of bilateral ureteric stents.

790
CHAPTER13 Radiology
790
Plain abdominalX- ray
The standard plain lm is a supine AXR. Erect views have largely been
superseded and in the acute setting have been replaced by the erect chest
to show free subphrenic air. Furthermore, chest diseases, such as MI or
pneumonia, may simulate an acute abdomen. If there is doubt regarding the
presence of a pneumoperitoneum, consider a lateral decubitus lm (dis-
plays as little as 1mL ofair).
Indications
Suspected obstruction, perforation, renal colic, and toxic megacolon and
bowel ischaemia.
Contraindications
None, but where abdominal pain is non- specic and not attributable to the
conditions listed above, an AXR is unlikely to be helpful.
Interpretation ofthe plain abdominalX- ray
A normal patient will have variable amounts of gas in the stomach, small
bowel, and colon. You can identify the stomach as it lies above the trans-
verse colon, has an air– uid level in the erect view, and has rugae in its lumen.
Large bowel calibre is variable; 5.5cm is considered the upper limit for the
transverse colon in toxic megacolon and 9cm for the caecum in obstruc-
tion. Short uid levels are normal. Fluid levels are abnormal when seen in
dilated bowel or if numerous. If the bowel is dilated, distinguish between
small and large bowel by the features listed in Table 13.6. Thickening of the
bowel wall may be seen in a variety of aetiologies, most notably ischaemia,
but also inIBD.
Causes of bowel dilatation include mechanical obstruction, paralytic ileus,
or a localized peritonitis (meteorism), e.g. adjacent to pancreatitis or appen-
dicitis (see Table13.7).
Table13.6 Distinguishing features betweensmall and largebowel
Small bowel Large bowel
Haustrae Absent Present
Valvulae conniventes Present in jejunum Absent
Number of loops Many
Few
Distribution of loops Central Peripheral
Diameter of loops
30– 50mm >50mm
Solid faeces Absent May be present
Maximum diameter 3cm 6cm (9cm in caecum)
Maximum fold thickness 3mm 5mm

PLAIN ABDOMINALX-RAY
791
Look forextraluminalgas
• Gas in the peritoneal cavity:look for air under the hemidiaphragm,
outlining the falciform ligament or both sides of the bowel wall (Rigler’s
sign). If there is any doubt, consider a lateral decubitus lm. Causes
include perforation (ulcer, neoplasm), post- operative, following
peritoneal dialysis, or tracking down from the mediastinum.
•
Air in the biliary tree:following sphincterotomy, gallstone ileus, or
following anastomosis of the CBD to the bowel. This has a linear
morphology and is seen centrally within theliver.
•
Portal vein gas:pre- morbid sign in the context of bowel infarction,
but less sinister in neonates with necrotizing enterocolitis (NEC) or
following umbilical catheterization. In contrast to air in the bile ducts, its
location is peripheral.
•
Intramural gas:linear streaks of air in the bowel wall again usually a
sinister nding implying ischaemia, but may be seen due to benign causes
such as in patients with COPD where its conguration is more rounded
and cystic (pneumatosis cystoides).
•
Air in the retroperitoneum:delineates the renal shadows and psoas
muscle; common causes are trauma, iatrogenic (e.g. after colonoscopic
perforation), and after perforation of a duodenalulcer.
•
Gas in an abscess:look for displacement of adjacent bowel and an
air– uid level. Other causes include air in the urinary tract and within
necrotic tumours. In this scenario, the gas is mottled and does not
display features consistent withbowel.
•
Look for any soft tissue masses or ascites:the latter is detectable on
plain lms if gross. There will be displacement of the ascending and
descending colon from the side walls, with loops of small bowel seen
centrally.
•
Look for abdominal or pelvic calcication:rstly, localize the site. This
may require another view. The vast majority are clinically insignicant,
i.e. vascular calcication, pelvic phleboliths, and calcied mesenteric
nodes. In the abdomen, there may be pancreatic calcication (chronic
pancreatitis) or hepatic calcication (old granulomata, abscesses, or less
commonly hepatomas and metastases from mucinous °s). Gallstones
are less commonly calcied and may contain central lucency (e.g.
Mercedes Benz sign), whilst renal and ureteric calculi commonly calcify.
Table13.7 Distinguishing mechanical obstruction froma paralyticileus
Feature Ileus Obstruction
Bowel calibre Normal or dilated Dilated
Air– uid levels Same level in a single loop Dierential levels
(stepladder)
Other
distinguishing
features
Air seen throughout the GIT
(diuse ileus) or in localized
ileus may be conned to a
short segment
Distension seen to level of
transition. Beyond this level,
no air in bowel

792
CHAPTER13 Radiology
792
Renal tumours and cysts rarely calcify, and more widespread renal
calcications may be seen in nephrocalcinosis due to a wide variety of
causes. In the pelvis, ovarian calcications (less common with malignant
masses and seen more often in association with benign pathologies such
as dermoids) is uncommon, whilst uterine calcications due to broids
commonly occur. Bladder wall calcications may be seen with bladder
tumours, TB, and schistosomiasis. Prostatic calculi and calcications are
common and of no signicance. Vas deferens calcications is seen in
patients with diabetes.
•
Soft tissues:look at renal outlines (normally smooth and parallel to the
psoas; should be between 2– 3 vertebral bodies). Absence of psoas
margins may indicate retroperitoneal disease and haemorrhage.
•
Bones of the pelvis and lumbar spine:look for OA, metabolic bone disease
(hyperparathyroidism, sickle- cell anaemia), the rugger jersey spine of
osteomalacia, and Paget’s disease (E Spinal imaging, pp. 836–7; Pelvis,
p. 838). Bony metastases may be lytic or sclerotic.

PLAIN ABDOMINALX-RAY
793

794
CHAPTER13 Radiology
794
Barium studies
Barium suspension is made up of small particles of barium sulfate in a solu-
tion. Due to its high atomic number, it is highly visible on X- rays. The con-
stituents of individual suspensions vary, depending on the part of the GIT
being examined. The particles are coated to improve ow and aid mucosal
adhesion. When made up, it comprises a chalky (sometimes unpalatable!)
suspension. Advantages include low cost, easy availability, and good assess-
ment of the mucosal surface.
Risks are commoner inthe contextof
• Perforation:if leakage occurs into the peritoneal cavity, it can produce
pain and hypovolaemic shock (50% mortality). Long- term sequelae
include peritoneal adhesions.
•
Aspiration:in small amounts, unlikely to have any clinical signicance, but
if pre- existing respiratory impairment or aspiration of larger amounts
(i.e. more than a few mouthfuls), the patient will need physiotherapy.
•
Obscuration:CT examination in the presence of a recent barium
examination will result in a poorly diagnostic study, as high- density
barium results in streak artefacts.
•
Barium impaction:rarely may exacerbate obstruction if barium collects
and is concentrated above a point of obstruction.
Water- soluble contrastmedia
These are more expensive and provide inferior coating and contrast. They
include iodinated agents such as Gastrogran
®
.
Indications fortheir use include
•
Suspected perforation, especially into the peritoneal cavity.
• Meconiumileus.
• To opacify bowel during CT examinations.
Risks include pulmonary oedema if aspirated and hypovolaemia, especially
in children. Both are a result of hyperosmolar eects. If aspiration is likely,
use water- soluble non- ionic contrast, which causes less shift of body uid
compartments. Non- ionic contrast should be used in all infants (especially
neonates) and preoperative patients requiring water- soluble contrast.
Contrastagents
(See Table13.8.)
For studies other than when barium is being utilized, the contrast media
are non- ionic iodinated agents that are given PO, endoluminally, or IV. Non-
ionic contrast agents are higher in cost than their ionic counterparts (rarely
used currently) but have a lower incidence of adverse reactions (by a fac-
tor of 9 for severe reactions). They are typically excreted by glomerular
ltration. Half- life is dependent on the dose given, distribution, and renal
function. Contrast reactions can be idiosyncratic or anaphylactoid or
non- idiosyncratic.

BARIUM STUDIES
795
High- risk patients are those with prior contrast reactions, a history of
allergy or atopy, sickle- cell disease, phaeochromocytoma, and multiple
myeloma (to name a few). Contrast- induced nephropathy is commoner if
creatinine is elevated at time of administration or if the patient has a pre-
existing renal impairment, e.g.DM.
Pre- medication can be administered to patients with prior contrast reac-
tions. Protocols vary but typically include a corticosteroid and an antihis-
tamine. Consider the use of alternate modalities if risk is high, e.g. USS or
MRI, instead of CT with contrast.
Extravasation of contrast is typically treated symptomatically with ice
pack and elevation. Plastic surgery consult should be obtained if concern
regarding compartment syndrome in patients who have in excess of 100mL
in extremity or severe pain/ discoloration or altered perfusion.
Gadolinium is an MR- based contrast agent that is paramagnetic. It is also
excreted by glomerular ltration.
Its safety prole is favourable in that it does not have any of the nephro-
toxicity associated with the iodinated contrast media and is more commonly
associated with minor reactions such as headaches. Since 2006, there is an
established association between the use of gadolinium- containing contrast
agents and NSF. NSF involves brosis of the skin, joints, eyes, and other
viscera. Subsequent to this nding, the use of gadolinium is contraindicated
in patients with an eGFR of under 60 and particularly if below30.
Table13.8 Pharmacological agents used inbarium studies
Agent Dose Advantages Disadvantages
Hyoscine
butylbromide
20mg IV Reduced bowel
peristalsis due to
smooth muscle relaxant
action
Immediateonset
Short duration of action
(15min)
Anticholinergic side
eects
Contraindicated
with CVD and
glaucoma
Glucagon 0.3mg IV
for barium
meal
1.0mg IV
for barium
enema
More potent smooth
muscle relaxant than
hyoscine butylbromide
Short duration of
action, no interference
with small bowel transit
Contraindicated
with insulinomas
or phaeo-
chromocytomas
Relatively expensive
Meto-
clopramide
20mg PO
or IV
Gastric peristalsis
enhances barium transit
during a follow- through
study
Possible
extrapyramidal side
eects
796
CHAPTER13 Radiology
796
Barium swallow
Plain lms do not usually demonstrate the oesophagus, unless it is very dis-
tended, e.g. achalasia. They may be useful in identifying an opaque foreign
body within the lumen. The barium swallow is the usual contrast examina-
tion to visualize the oesophagus (see Fig. 13.16). Rapid- sequence lms are
taken with a uoroscopy unit, whilst the patient swallows barium (usually in
an erect position). Films are taken in an AP and oblique projection (to throw
the oesophagus clear of the spine), with the oesophagus distended with
barium (to demonstrate its outline) and empty to show the mucosalfolds.
Normal anatomy
The oesophagus commences at C5/ 6. There are normal indentations
on its outline by the cricoid cartilage, aortic arch, left main bronchus, and
leftheart.
Indications
These include the assessment of dysphagia, pain, reux disease, tracheo-
oesophageal stulae (in children), and post- operative assessment where
there has been gastric or oesophageal surgery.
Contraindications
No absolute contraindications exist, but in all barium studies, the quality
of the study relies heavily on patient co- operation, and therefore immo-
bile patients who are unable to weight- bear may only be suitable for
limited studies. The post- operative oesophagus is usually assessed with
Gastromiro
®
or a non- ionic contrast.
Fig.13.16 Lateral oblique projection during a barium swallow series shows a feline
oesophagus. This can be a normal variant but is often associated withGORD.
BARIUM SWALLOW
797
Common disorders and patterns
• Diverticulae:these include pharyngeal pouches (a midline diverticulum),
traction diverticulae (due to adhesions), or pseudodiverticulae; dilated
mucous glands seen in reux or infective oesophagitis.
•
Luminal narrowing:strictures may be benign (e.g. oesophagitis— shown
in Fig. 13.17, scleroderma, pemphigus, corrosives, or infection) or
malignant.
•
Webs:mucosal structures, which may be seen anywhere in the
oesophagus; seen with skin lesions, e.g. epidermolysis or pemphigus,
GVHD, and Plummer– Vinson syndrome.
• Mega- oesophagus:can be with associated obstruction, as in malignant
strictures, or without as in achalasia, diabetic neuropathy, or Chagas’
disease (see Fig. 13.18a– c).
• Ulceration/ oesophagitis:may be due to GORD, infection, corrosives,
or iatrogenic. Findings include lack of distensibility, fold thickening, and
mucosal irregularity.
•
Oesophageal tears:spontaneous, neoplastic, post- traumatic, iatrogenic,
and following prolonged emesis. Look for pneumomediastinum, left
pleural eusion, and features of mediastinitis.
•
Filling defects:foreign bodies, varices (proximal due to SVC obstruction),
distal (in association with portal hypertension), neoplasms that may be
benign (as in leiomyoma) or malignant. Most commonly SCC(95%).
•
Fold thickening:may be due to oesophagitis, varices, or inltration by
lymphoma.
•
Air– uid level:commonest in hiatal hernias, but also seen with a
pharyngealpouch.
Web
Stricture
Ring
Fig.13.17 Benign strictures.
(b)
(c)
GE
open
Moderate
dilatation
Irregularit
y
(a)
Bird's beak
Massive dilatation
Curves to
the right
Fig.13.18 Obstruction in: (a)achalasia,
(b)scleroderma, and (c)cancer.

798
CHAPTER13 Radiology
798
Bariummeal
About 200mL of a high- density (85% w/ v barium sulfate), low- viscosity
barium is used for a double contrast study, which gives good coating with-
out obscuration of mucosal detail. An eervescent agent is given to pro-
vide adequate luminal distension. The gastric mucosa is characterized by
rugae (parallel to the long axis, 3– 5mm thick) and area gastricae (nodular
elevations, 2– 3mm wide). The patient is fasted for about 6h to avoid food
residue, which may cause diagnostic uncertainty. The techniques for coat-
ing the stomach and projections are variable. A smooth muscle relaxant
may be given as part of the routine, particularly to assess the pylorus and
duodenum.
Indications
Dyspepsia, weight loss, abdominal masses, iron deciency anaemia of
uncertain cause, partial outlet obstruction, and previous GI haemorrhage.
Contraindications
Complete large bowel obstruction.
Abnormal ndings
• Filling defects:these may be intrinsic or extrinsic. Carcinoma remains the
commonest cause of a lling defect in an adult (irregular, shouldered
with overhanging edges). If there is antral involvement, there may be
associated outlet obstruction. Diuse mucosal thickening and failure
to distend is seen with linitis plastica. Other causes include gastric
lymphoma, polyps (histology dicult to predict), and bezoars. Smooth
lling defects are seen in conjunction with leiomyomas, lipomas, and
metastases. Extrinsic indentation by pancreatic tumours or an enlarged
spleen may cause an apparent lling defect.
•
Fold thickening (>5mm):seen in association with hypersecretion states
such as Zollinger– Ellison syndrome, gastritis, and Crohn’s disease. It may
also be 2° to inltration by carcinomas, lymphomas, or eosinophilia.
•
Outlet obstruction:may be diagnosed by failure of the stomach to empty
<50% of the barium ingested at 4h. This may be seen in carcinomas, but
also in scarring caused by chronic duodenal ulceration.
•
Hiatal hernia:herniation of the stomach into the chest occurs via
the oesophageal hiatus in the diaphragm. There are two types— in a
sliding hernia (commoner), there is incompetence of the sphincter
at the cardia, often associated with reux. Other sequelae include
oesophagitis, ulceration, or stricture. In a rolling hernia, the fundus
herniates through the diaphragm, but the gastro- oesophageal junction
remains competent and lies below the diaphragm.
•
Gastritis and ulceration:gastritis is characterized by small, shallow
barium pools with surrounding lucent rings due to oedema. There
are features that may be used to distinguish benign from malignant
ulcers on barium studies. Ulcers are seen either as a crater or as a
projection from the luminal surface (see Fig. 13.19). Benign ulcers

BARIUMMEAL
799
are commonly seen on the lesser curve with smooth radiating folds,
which reach the edge of the ulcer crater. Malignant lesions may have
an associated mass and have a shallow crater and an irregular contour.
With the ease of availability of endoscopy, the use of barium meals
in diagnosing ulceration has declined. Endoscopy has the advantage of
being able to diagnose gastritis more accurately, assess ulcer healing,
make a histological diagnosis, and more accurately assess the post-
operative stomach. However, early assessment of the post- operative
stomach is radiologically performed to exclude complications such as
anastomotic leaks. Awater- soluble contrast agent is preferred in the
early post- operativephase.
Fig.13.19 Double contrast barium meal showing an ulcer crater.

800
CHAPTER13 Radiology
800
Small intestine
Small bowel studies are performed for indications such as occult bleeding,
recurrent obstructive symptoms, and malabsorption, and to conrm and
dene the extent of small bowel disease in Crohn’s.
•
Small bowel follow- through:the patient drinks 200– 300mL of barium (with
metoclopramide to speed transit time). The single contrast column is
followed by lms at regular intervals until the barium reaches the colon.
Transit time is variable, but the entire process may take 1– 6h, depending on
the adequacy of bowel preparation. Films are taken at intervals of 720min
initially, in the prone position, which aids separation of the loops. When the
barium reaches the caecum, spot views of the terminal ileum aretaken.
•
Small bowel enema (enteroclysis):this technique provides better
demonstration of mucosal detail, as there is rapid infusion of a
continuous column of barium directly into the jejunum. Methylcellulose
is administered following the barium to provide double contrast. This is
achieved via a weighted nasogastric tube which is positioned at, or distal
to, the duodenojejunal (DJ) exure. Disadvantages include poor patient
tolerance (related to intubation) and a relatively high screeningdose.
Both techniques require the patient to be on a low- residue diet beforehand.
Indications
The indications are the same for both techniques and include pain, diar-
rhoea, bleeding, partial obstruction, malabsorption, overgrowth syn-
dromes, assessment of Crohn’s disease activity and extent, and suspected
masses. The small bowel enema may be preferred for assessment of recur-
rent Crohn’s disease or complex post- operative problems, but the small
bowel follow- through is otherwise routinelyused.
Contraindications
Complete obstruction and suspected perforation.
Normal ndings
The small intestine measures 75m and extends from the DJ exure to the ile-
ocaecal valve. The proximal two- fths is the jejunum; the distal three- fths is the
ileum. Normal calibre is 3.5cm for the jejunum and 2.5cm for the ileum (up to
1cm more on enteroclysis). The valvulae conniventes are circular in congura-
tion, and 7
2mm thick in the jejunum and 1mm thick in the ileum (see Fig. 13.20).
Abnormal ndings
• Dilatation is indicative of malabsorption, small bowel obstruction (SBO),
or paralytic ileus. There may be accompanying eacement of the mucosal
pattern. When seen with fold thickening, it may be due to Crohn’s,
ischaemia, or radiotherapy. Mucosal thickening may be due to inltration by
lymphoma or eosinophilia, adhesions, ischaemia, or radiotherapy.
•
Strictures are seen in Crohn’s disease and lymphoma. There is usually
dilatation of the bowel proximally. Crohn’s disease causes skip lesions,
ulceration, strictures of variable length, and a high incidence of terminal
ileal involvement. There may be associated ulceration, fold thickening,
and stulation (see Fig. 13.21).
• Malabsorption:radiological investigation may reveal an underlying
structural abnormality. The ndings in malabsorption include dilatation,
fold thickening, and occulation of barium.
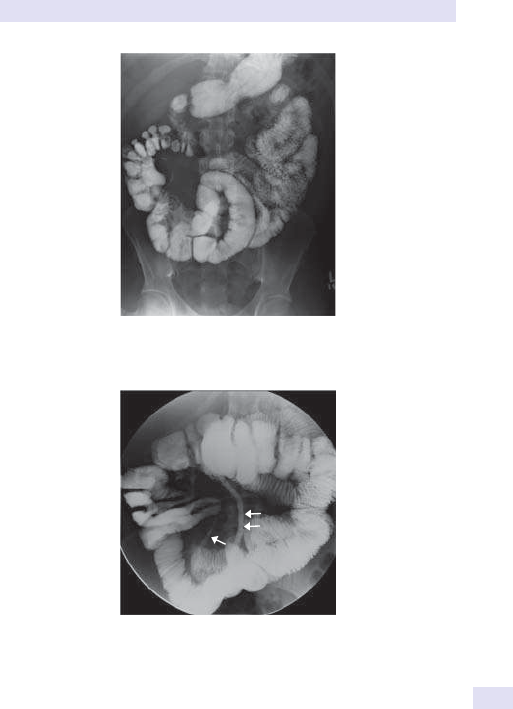
SMALL INTESTINE
801
CT enteroclysis
This is a hybrid technique that combines uoroscopic intubation and small
bowel infusion with an abdominal CT. This can be performed with +ve
enteral contrast or neutral enteral contrast.
The advantage of this technique is that both intra- and extraluminal/
extra- enteric information is obtained, rendering it superior to many of the
per- oral small bowel studies. Disadvantages include the minimally invasive
nature and the radiation associated with a CT examination. CT enterocly-
sis is complementary to capsule endoscopy in the elective investigation of
small bowel disease and should be particularly considered in Crohn’s dis-
ease, small bowel obstruction, and unexplained GI bleeding. There has been
a paradigm shift to CT enterography for the assessment of small bowel
disease. This is most relevant in the IBD population who are on disease-
modifying drugs.
Fig.13.20 A30min lm in a small bowel follow- through series showing a normal
mucosal fold pattern.
Fig.13.21 Small bowel enema showing at least two strictures (arrows) in this patient
with Crohn’s disease. Compare the mucosal detail with the small bowel follow- through.

802
CHAPTER13 Radiology
802
Cholangiography
Ultrasound
This is the ° modality for assessment of the biliary tree and for exclusion
of pancreatic pathology. With the current scanner resolution, the sensitiv-
ity for stone disease is in the range of >95% for stones exceeding 2mm in
diameter. For choledocholithiasis (stones in the CBD), the sensitivity falls to
around 50%, and MRCP or ERCP is more helpful.
Intravenous cholangiography
This is rarely performed but may be useful in patients with biliary symptoms
post- cholecystectomy or with a non- functional gall bladder. It is contrain-
dicated in the presence of severe hepatorenal disease, as the side eects
related to the contrast media are considerable. CT cholangiography uses
a similar contrast agent but oers the advantage of cross- sectional assess-
ment of the bile ducts. It is often used when MRCP has not helped delineate
the anatomy in donors prior to liver transplantation or when MR is con-
traindicated or simply not available.
Endoscopic retrograde cholangiopancreatography
The biliary and pancreatic ducts are directly lled with contrast, following
endoscopic cannulation and during X- ray screening. This has both a diag-
nostic and therapeutic role. It is particularly of value in the demonstration
of ampullary lesions and to delineate the level of biliary tree obstruction
in patients with obstructive jaundice. It allows sphincterotomy to be per-
formed to facilitate the passage of stones lodged in theCBD.
Percutaneous transhepatic cholangiography
The biliary tree is directly injected with contrast, following percutaneous
puncture of the liver. This is both diagnostic in dening a level of obstruc-
tion and therapeutic in biliary duct obstruction, as it may be used as a
precursor to a biliary drainage procedure or prior to insertion of a stent.
Contraindications include bleeding diatheses and ascites.
Other cholangiographic techniques
• Per- operative cholangiogram:in which the CBD is lled with contrast
during cholecystectomy to exclude the presence of CBD stones.
•
T- tube cholangiogram:after operative exploration, a T- tube is left in the
CBD for a post- operative contrast study to exclude the presence of
retained stones.
•
MRCP:this is a non- invasive technique where heavily T2- weighted
(T2W) images are obtained without contrast administration. The bile
acts as an intrinsic contrast agent, and stones are visualized as lling
defects. The entire biliary and pancreatic ductal system can be visualized
(see Fig. 13.22). Common indications for this technique include
unsuccessful ERCP, a contraindication to ERCP, as well as evaluation of
the post- surgical biliarytree.
CHOLANGIOGRAPHY
803
Fig.13.22 Heavily T2- weighted slab image showing an irregular beaded pancreatic
duct in this patient with chronic pancreatitis. There is a small pseudocyst in the tail
(arrowhead).
Magnetic resonance cholangiopancreatography
•
Non- invasive.
• Cheaper.
• Uses no radiation.
• Requires no anaesthesia.
• Less operator- dependent.
• Allows better visualization of the ducts proximal to the level of
obstruction.
•
When combined with conventional sequences, allows detection of
extraductal disease.
Disadvantages
•
d spatial resolution for peripheral intra- hepatic ducts and for pancreatic
ductal side branches (e.g. as in pancreatitis).
•
Subtle ductal lesions may be dicult to appreciate, as ducts are imaged
in the non- distended physiologicalstate.
• Inability to perform therapeutic endoscopic or percutaneous
intervention of obstructing bile duct lesions.
•
MRCP is comparable with ERCP in the detection of obstruction, with a
sensitivity and specicity of 91 and 100%, respectively.
•
Causes of lling defects are usually stones, air, tumours, blood, or
sludge.
Contrast- enhanced MRCP can also be performed with fat- saturated T1-
weighted (T1W) imaging after injection of gadolinium contrast agents that
have biliary excretion. These cause TI hyperintensity with bile but require a
20– 45min delay prior to imaging to allow for biliary excretion.

804
CHAPTER13 Radiology
804
Liver disease
US is the modality of choice for initial screening, whether assessing the
parenchyma for diuse disease or trying to evaluate and/ or characterize
focal liver lesions. Although US is sensitive in depicting focal lesions, it is not
specic and there can be overlap in the imaging characteristics of benign
and malignant lesions. CT, and particularly MRI, are more tissue- specic in
characterizing liver lesions. The liver is supplied predominantly by the por-
tal venous system (80%). On CT, the diering phases of enhancement are
utilized to assess a lesion.
Arterial phase images (20– 30s after injection) i the conspicuity of lesions
that are hypervascular such as hepatocellular carcinoma or focal nodular
hyperplasia. Portal venous phase images are acquired at 50– 70s and pro-
vide maximum enhancement of background hepatic parenchyma. Lesions
that are relatively hypovascular on this phase stand out such as metastases.
Delayed imaging (equilibrium phase) minutes after contrast adminis-
tration allows lesions that demonstrate relative washout of contrast (i.e.
appear hypo- attenuating) relative to background liver, such as hepatocellu-
lar carcinomas, to stand out. Lesions that are relatively brotic (e.g. in tissue
content or scars) conversely exhibit i enhancement on delayed images.
MRI displays the same patterns of contrast enhancement but has superior
lesion- to- liver contrast and imparts no ionizing radiation. Multiple dynamic
post- contrast sequences can thus be obtained. Tissue characterization
allows for detection of intralesional lipid (as in adenomas), and advanced
techniques such as diusion- weighted imaging (DWI) improve sensitivity
for lesion detection.
Further characterization of lesions can be obtained by hepatocellular-
specic MRI contrast agents which allow denitive assessment of lesions
with hepatocytes such as focal nodular hyperplasia (FNH), precluding the
need for biopsy.

CHOLANGIOGRAPHY
805

806
CHAPTER13 Radiology
806
Bariumenema
This is used for evaluation of the large bowel. Increasingly, many institutions
are replacing this technique with CT colonography (CTC; E Virtual colo-
noscopy, p. 807) or conventional CT, depending on the clinical indication.
Barium is run into the colon under gravity via a tube inserted into the rec-
tum. The column of barium is followed by air (room air or CO
2
) to achieve
double contrast. The CO
2
is better tolerated and more readily absorbed.
Hyoscine butylbromide (a smooth muscle relaxant) may be given to mini-
mize spasm and optimize mucosal relief. Bowel preparation prior to the
examination (low- residue diet and aperients) is vital to ensure that there is
no faecal material, which may mask mucosal abnormalities or be mistaken
for small polyps. Remember the examination is uncomfortable and requires
reasonably good patient co- operation and mobility.
2 Do not request this in frail or elderly patients, unless there is a good
clinical indication.
A rectal examination or sigmoidoscopy is essential to avoid abnormalities
being missed.
Single vs double contrast
If evaluation of the colonic mucosa is not the ° aim, then a single contrast
technique will suce. This is applicable in children where the patient is unco-
operative and where gross pathology is being excluded, and in the evalua-
tion of obstruction/ volvulus or in the reduction of an intussusception.
Indications
Change in bowel habit, iron deciency anaemia, abdominal pain, palpable
mass of suspected colonic origin, and weight loss of unknowncause.
Contraindications
Suspected perforation, recent rectal biopsy, toxic megacolon, or pseudo-
membranous colitis.
Common ndings
• Solitary lling defect:polyps are classied according to histology. The
commonest are hyperplastic (no malignant potential; adenomatous
polyps are premalignant with the risk of malignancy i with size
(<5mm=0%, >2cm=20– 40%). Also found are adenocarcinoma
(i risk in ulcerative colitis, polyposis syndromes, villous adenoma) and
less commonly metastases and lymphoma.
•
Multiple lling defects:polyps (polyposis syndromes or post-
inammatory pseudopolyps), pneumatosis coli, metastases, and
lymphoma.
•
Ulceration:IBD, ischaemia, infection, radiation, and neoplasia.
•
Colonic narrowing:neoplasms (apple core lesion), metastases, lymphoma,
diverticular disease, IBD, ischaemia, and radiation.
•
Dilatation:mechanical, e.g. proximal to neoplasm, volvulus or non-
mechanical, post- operative ileus, metabolic, and toxic megacolon.
• Diminished haustration:cathartic colon, IBD, and scleroderma.
BARIUMENEMA
807
Fig.13.23 Standard supine projection
from a double contrast barium enema
(DCBE) showing a normal large bowel.
• i haustration (thumbprinting):ischaemia, haemorrhage, neoplasm,
andIBD.
•
Widening of the pre- sacral space (>1.5cm at S2):normal in up to 40%,
but also seen in association with IBD, neoplasms, infection, and sacral/
pelvic lipomatosis.
Colonoscopy
Remains a complementary technique and has the advantage of being both
therapeutic and diagnostic (e.g. biopsy, polypectomy, etc.). In elderly
patients, CT with prior bowel preparation and air insuation is less invasive
and less arduous.
Virtual colonoscopy
Helical CT images of distended colon taken during a breath- hold are used
to obtain 2D or 3D images of the colon. Images are acquired in the supine
and prone positions to assess lesional mobility (and thus distinguish stool
from polyps). No IV contrast is administered for routine screening stud-
ies, and the examination is often performed utilizing a low- dose technique.
Recent studies using ° 3D interpretation, as well as national studies, such
as the ACRIN II trial, have shown sensitivities in the range of 94% for polyps
of at least 1cm in diameter and around 88% for lesions measuring at least
6mm. Current renements in this technique include the use of computer-
assisted detection (CAD) to improve performance, as well as the use of
prepless techniques (i.e. the patient does not have to undergo prior bowel
cleansing).
(See Figs 13.23 and 13.24.)
Fig.13.24 Supine DCBE showing a tight
stricture in the transverse colon in this
patient withIBD.
Further reading
Pickhardt PJ, Choi JR, Hwang I, etal. Computed tomographic virtual colonoscopy to screen for
colorectal neoplasia in asymptomatic adults. N Engl J Med 2003; 349
:2191– 200.

808
CHAPTER13 Radiology
808
Radiology ofthe urinarytract
Plain abdominallm
• Look for any urinary tract calcication:90% of stones are radio- opaque.
Other causes include hyperparathyroidism, medullary sponge kidney,
andRTA.
• Renal outline:between T12 and L3 and 10– 15cm. Left bigger and higher
than theright.
•
Assess bones of spine and sacrum:for bony metastases or spina bida
(may be relevant in enuresis).
Intravenous urogram
This provides a good overview of the urinary tract and, in particular, the
pelvicalyceal anatomy. Fluid restriction and laxatives are no longer neces-
sary and, in particular, the former is to be avoided in diabetics, renal failure,
and myeloma. Following the preliminary plain lm, 300mg/ kg of contrast
media is injected IV. The lm sequence is varied according to the clinical
scenario. An immediate lm shows the nephrogram phase and displays the
renal outlines. An increasingly dense delayed nephrogram is seen in acute
obstruction, acute hypotension, ATN, and renal vein thrombosis. Afaint
persistent nephrogram is seen with acute glomerulonephritis and it may
be delayed in RAS. Later lms show the pelvicalyceal systems (pyelogram),
ureters, and bladder.
Common abnormalities
(See Fig. 13.25.)
• Loss of renal outline:congenital absence, ectopic kidney, tumour, abscess,
or post- nephrectomy (look for absent twelfthrib).
• Small kidney (unilateral):ischaemia (RAS), radiation, or congenital
hypoplasia.
•
Small kidney (bilateral):atheroma, papillary necrosis, or
glomerulonephritis.
•
Large kidney (unilateral):duplex, acute pyelonephritis, tumour, or
hydronephrosis.
•
Large kidney (bilateral):polycystic kidneys and inltrative disease such
as myeloma, amyloid, and lymphoma. Acute inammation such as
glomerulonephritis, ATN, and collagen vascular disease.
• Pelvicalyceal lling defect:smoothly marginated (clot, papilloma),
irregular margins (tumour, e.g. renal cell or transitional carcinoma),
intraluminal (sloughed papilla, calculus, or clot), extrinsic (vascular
impression or cyst), irregular renal outline (scarring, e.g. in ischaemia,
TB, pyelonephritis, or reux nephropathy).
• Dilated ureter:>8mm in entire length. May be due to obstruction
(functional as in ° megaureter) or mechanical stenosis as in ureteric or
urethral stricture and in reux disease.
•
Ureteric stricture:wide dierential; determine the length. Dierentials
include tumour (transitional cell carcinoma, metastatic), inammatory
(TB, schistosomiasis), congenital, trauma (radiation or iatrogenic).
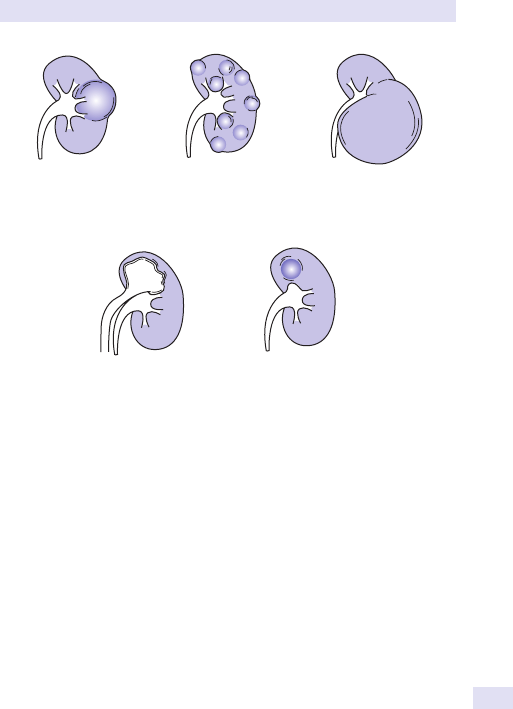
RADIOLOGY OFTHE URINARYTRACT
809
• Deviated ureters:normal course of ureters in close proximity to
transverse processes of vertebral bodies.
•
Lateral deviation:seen with retroperitoneal nodes, tumours, and aortic
aneurysm.
•
Medial deviation:posterior bladder diverticulum, retroperitoneal brosis
(can be idiopathic or related to various aetologies, including malignancy.
Computed tomography ingenitourinary pathology
CT is the preferred method for assessment of many pathologies within the
genitourinary (GnU) tract, including trauma, complex infections, renal and
adrenal masses, neoplastic disease, retroperitoneal processes, renovascular
hypertension, and in renalcolic.
Computed tomography urography
In many institutions, IVU has been replaced by its CT counterpart (CT urog-
raphy (CTU)). MDCT has had an impact on slice thickness and speed of
scanning, such that the urinary tract mucosa can be assessed in exquisite
detail. Depending on institutional protocol, the examination is performed
as a 2- or 3- part study. Typically, it includes a non- contrast phase (to assess
for stones, acute blood) and then an excretory/ delayed phase to assess
Renal cyst
Multiple renal cysts
(elongation and distortion
of calyces – polycystic
disease)
Renal tumour
Duplex kidney with
hydronephrotic
upper moiety.
(‘drooping ower’)
Dilatation of a
single calyx
(may be due to
vascular compression)
Fig.13.25 Common patterns of abnormalities seen onIVU.

810
CHAPTER13 Radiology
810
the collecting systems and ureters. It has a high sensitivity (95%) in detect-
ing upper urinary tract uroepithelial malignancies. Common indications for
usage include:
•
Haematuria (with −ve cystoscopy and USS having excluded parenchymal
causes).
•
Unexplained hydronephrosis onUSS.
• Evaluation of the upper tract in patients with known lower urinary tract
transitional cell carcinoma or following trauma or iatrogenic ureteric
injury.
Ultrasound
May be used as an alternative or complementary examination with IVU and
may be usedto:
•
Demonstrate or exclude hydronephrosis, especially inARF.
• Evaluate renal tumours, cysts, and abscesses.
• Follow up transplant kidneys and chronic renal disease.
• Assess renal blood ow using Doppler.
• Perform serial scanning in children with recurrentUTIs.
• Assess bladder morphology and volume, and the prostate.
• Provide guidance for interventional techniques, e.g. renal biopsy and
nephrostomy placement.
Computed tomography and magnetic resonance imaging
CT is more accurate for staging renal tumours, assessing retroperitoneal
pathology, staging bladder and prostatic tumours, and assessing renal vascu-
lar pathology (such as RAS). In many centres, unenhanced CT is replacing
IVU as a gold standard for assessment of renal stone disease. It is more
accurate at depicting stone burden than IVU and precisely demonstrating
the level and cause of obstruction in the acute setting. Evaluation of ure-
teric pathology in the context of haematuria is also being performed with
contrast- enhancedCT.
MRI is valuable in staging vascular involvement by renal carcinomas.
Dedicated pelvic coils and endoluminal coils show excellent results in stag-
ing pelvic and gynaecological malignancies.
Micturating cystourethrogram
Following catheterization of the bladder, contrast is introduced till bladder
capacity is reached. This is the technique of choice for dening the urethral
anatomy and gauging the presence/ degree of vesicoureteric reux in chil-
dren. It is also used if there are recurrent UTIs or suspected lower urinary
tract obstruction.
Ascending urethrography
Contrast is injected directly into the urethra in ♂ in the assessment of
urethral trauma, strictures, and congenital anomalies such as hypospadias.

RADIOLOGY OFTHE URINARYTRACT
811
Retrograde pyelography
The ureters are catheterized (usually following cystoscopy in theatre) and
contrast injected under X- ray screening. Of value in urothelial tumours and
to dene the site of obstruction, e.g. non- opaque calculi. Useful if IV tech-
niques have failed to demonstrate the intra- renal collecting system or ure-
ters due to impaired renal function or a high- grade obstruction.
Angiography
A femoral approach with selective catheterization of renal vessels. Main
uses include haematuria (look for AVMs), hypertension (RAS), in transplant
donors (to dene anatomy), and in renal cell carcinoma (where emboliza-
tion is being contemplated).
Nephrostomy
E Interventional radiology, pp. 844–7.
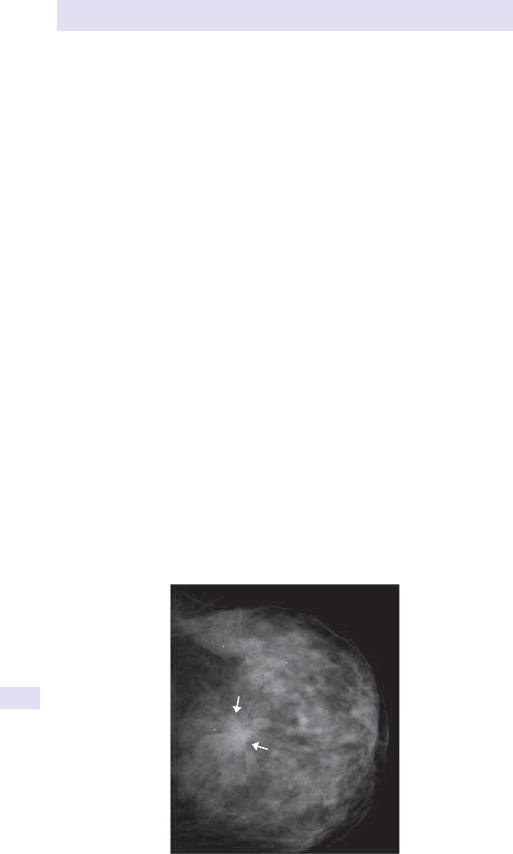
812
CHAPTER13 Radiology
812
Breast imaging
Breast cancer is a common problem (1 in 12 women). The average ♀ has
a 1 in 8 chance of being diagnosed with breast cancer during her lifetime.
Mammography is the rst- line tool for detection of breast cancer; however,
sensitivity of screening mammogram is variable and is inuenced by vari-
ables such as density of breast tissue. Sensitivity is between 68 and 90% and
is higher if the patient is symptomatic(93%).
Screening mammography detects 2– 8 cancers per 1000 women
screened. Since 1990, mortality from breast cancer has steadily declined,
and this has been attributed to advances in adjuvant therapy as well as to
mammographic screening.
Mammography
Technical factors
Breast tissue has a narrow spectrum of inherent densities, and in order to
display these optimally, a low- kilovoltage (kV) beam is used. It enhances
the dierential absorption of fatty, glandular, and calcic tissues. Dedicated
mammographic units provide low- energy X- ray beams with short expo-
sure times. The breast is compressed to minimize motion and geometric
unsharpness. High resolution is paramount in order to detect microcalcica-
tion (as small as 0.1mm). The breast is a radiosensitive organ, so doses need
to be kept to a minimum.
Standard projections
These are the mediolateral oblique (MLO) and craniocaudal (CC) views
(see Fig. 13.26). The CC view is trans- axial. The MLO image plane is 745–
60° from the axial plane and parallels the pectoralis major. Adequacy of the
lateral oblique view may be gauged by the pectoralis major muscle, which
should be visible to the level of the nipple, inclusion of the axillary tail,
Fig.13.26 CC mammographic view of the breast. The arrows depict a stellate mass
consistent with a carcinoma.

BREAST IMAGING
813
and inclusion of the inframammary fold. The CC view detects posterome-
dial tumours that may be missed on the MLO view and is better at breast
compression. Additional projections, such as true lateral, cleavage, exag-
gerated CC, spot compression, and magnication views, may be used to
clarify abnormalities. These techniques provide better detail and disperse
any overlapping tissue to avoid obscuration of lesions.
Mammographicsigns
The breast parenchyma is made up of glandular tissue in a brofatty stroma.
Cooper’s ligaments form a connective tissue network. The amount of glan-
dular tissue d with age; as it is dense on mammography, the suitability of the
technique for detecting pathology i withage.
Systematic evaluation ofa mammogram
•
Adequacy of study; are additional views required?
• Adequate penetration of broglandular tissue.
• Skin, nipple, trabecular changes.
• Presence of masses.
• Calcications.
• Axillarynodes.
• Asymmetry (may be a normal variant).
• Architectural distortion.
Comparison with prior imaging is imperative, as changes can be subtle and
progressive.
Additional technical adequacy
•
There should be adequate tissue demonstrated on both CC and MLO
views. The posterior nipple line is a line drawn from the posterior nipple
to the pectoralis muscle. On each view, the posterior nipple line should
be within 1cm of eachother.
•
Image should be free from blur and artefacts.
• Nipple should be in prole on at least oneview.
• Blurring can cause benign calcication to look amorphous, and subtle
calcication may be missed.
Primary signs ofa malignancy
• Amass with ill- dened or spiculate borders (see Fig. 13.27).
• Clustered, linear, or irregular calcication (which may occur in the
absence of amass).
•
2° signs include distortion of adjacent stroma, skin thickening, and nipple
retraction.
Ninety- four per cent of breast carcinomas is ductal in origin.
The breast imaging and reporting data system (BIRADS) is a standardized
way of reporting mammography and includes a lexicon, a structured report,
and clear categories for follow- up and reassessment of imaging ndings.
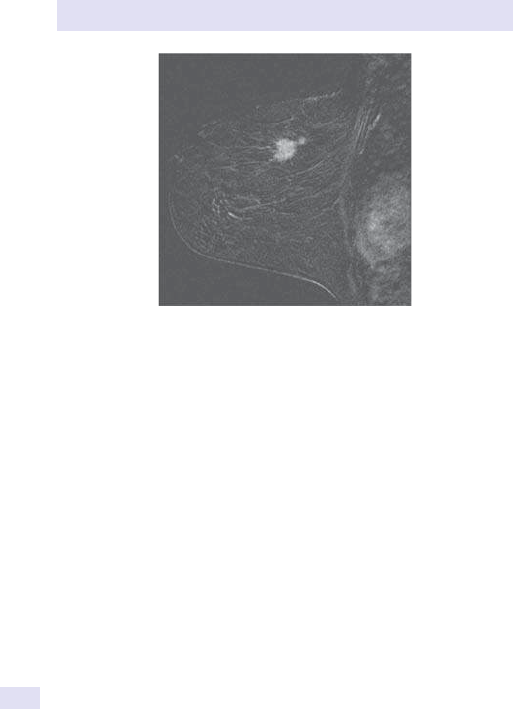
814
CHAPTER13 Radiology
814
Breast ultrasound
This largely forms a modality for assessment, not diagnosis or detection,
and is a valuable adjunct and problem- solving tool. It can be used to evalu-
ate non- palpable masses and palpable masses not seen on mammography,
to determine the internal architecture (solid vs cystic), to assess asymmetric
density, to assess breast implants, and as a ° imaging modality in young
women (<35 years), as well as pregnant and lactating women. It is also
used as a tool to guide intervention, i.e. drainage of cysts and biopsy of
suspicious lesions.
Magnetic resonance imaging
MRI remains a problem- solving tool in breast imaging. Widespread imple-
mentation of MRI (for instance, for screening) is hampered by its low speci-
city (37– 97%). It can i the number of benign biopsies that are performed.
This clearly has resource implications, as well as generating unnecessary
patient anxiety. Both MRI and US may be used to evaluate implants and
their integrity, but MRI is the only modality that is sensitive in the evaluation
of intracapsular implant rupture. Contrast- enhanced MRI of the breast is
also a sensitive method for detection of malignancy, with reported sensitivi-
ties in the region of 93%. It is especially useful to detect recurrent breast
carcinoma and where conventional techniques are unable to help in the
distinction from more benign lesions. Breast MRI is also being advocated
for screening young patients with a family history/ genetic risk of breast
carcinoma.
Fig.13.27 Subtraction sagittal MR image of the breast following gadolinium,
showing an enhancing spiculated mass consistent with malignancy.

BREAST IMAGING
815
Breast MRI is increasingly being used for staging breast carcinomas, to
look for synchronous ° lesions, and to evaluate the breast in patients found
to have malignant nodes in the axilla. It can also be used to evaluate tumour
response to neo- adjuvant chemotherapy. In problematic mammographic
patients, it can be useful in distinguishing dense breast tissue or brosis from
malignancy. In the post- operative setting, it can be used in patients with +ve
surgical margins or to assess post- operative scar vs disease recurrence. The
selection of pulse sequences and IV contrast administration is based on the
clinical indication.
The patient lies prone on the scanner, and a specialized coil surrounds the
breast. The entire scan varies in duration from 20min to 1h. Most protocols
for exclusion of malignancy rely on a dynamic enhanced sequence. Cancers
typically enhance more rapidly than benign lesions.

816
CHAPTER13 Radiology
816
Ultrasound
US is a high- frequency mechanical vibration produced by piezoelectric
materials, which have the property of changing thickness when a voltage
is applied across them. It is an important cross- sectional modality and has
widespread applications in the abdomen, neck, pelvis, and extremities. At
diagnostic levels, there are no known damaging sequelae to tissues, and
therefore it is safe for use in obstetrics, providing invaluable imaging of the
developingfetus.
Probe selection is dependent on the area being imaged. Highe- frequency
probes provide greater resolution but have limited depth of penetration
and may therefore be suitable for assessment of supercial structures (e.g.
extremity US, thyroid, testicular US). Doppler USS is based on the principle
that sound reected by a moving target has a dierent frequency to the
incident sound wave. The frequency shift is proportional to the velocity of
the owing material. Doppler therefore not only enables detection but also
quantication of velocity.
Indications
USS is cheap, readily available, and non- invasive, and has high patient accept-
ability. It has a wide range of applications as listed below. There are also no
radiation implications. Again, advances in technology have resulted in vast
improvements in the resolution of this modality, such that subtle pathology
is more readily identiable. The portability of USS also lends itself to use in
the setting of emergency and critically ill patients, as well as providing guid-
ance for intra- operative procedures.
Contraindications
None, but remember that USS is operator- and patient- dependent and
should be used as a problem- orientated modality, not as a total body sur-
vey. It cannot be used to image air- containing structures or bone. The reso-
lution of the USS image is inversely related to the depth of penetration.
Therefore, image quality in obese patients is suboptimal.
Applications
• Head and neck:may be used for evaluation of the salivary glands,
thyroid, lymph nodes, and palpable or clinically suspected masses.
Doppler is used to assess the carotid vessels and quantify the degree of
stenosis/ occlusion.
• Chest (excluding breast):the use here is limited to palpable chest wall
lesions, assessment of pleural abnormalities, biopsy, and drainage of
pleural eusions, and is occasionally of use in directing a biopsy of
peripheral lung or mediastinal masses.
•
Abdomen and pelvis:this is the main use of USS. Useful for assessment
of solid organs, e.g. kidneys, spleen, gall bladder (see Figs 13.28
and 13.29), liver (see Fig. 13.30), pancreas, uterus/ adnexae, and
bladder. Afull bladder is used as an acoustic window in the pelvis.
Retroperitoneal masses and lymph nodes may be visible, depending on
patient habitus. USS is useful for directing biopsy of solid organs/ masses
and for drainage of ascites, abscesses, and collections.
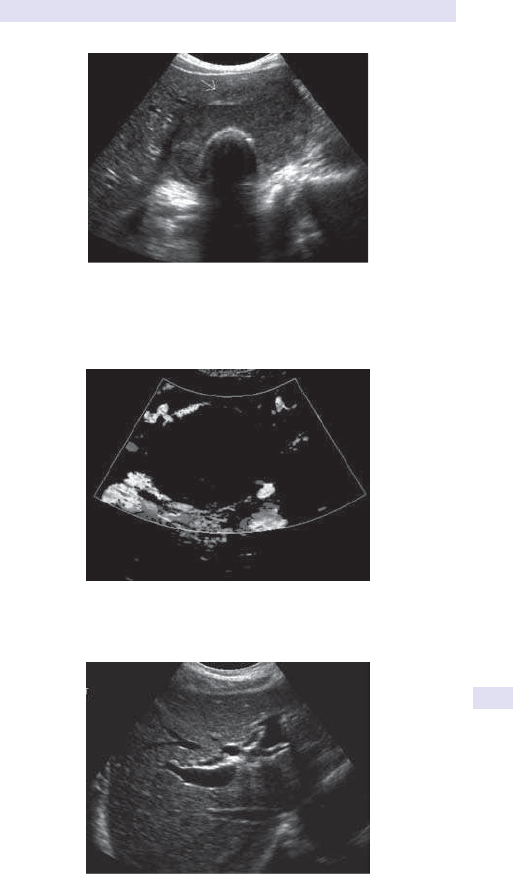
ULTRASOUND
817
Fig.13.28 Trans- axial view of the gall bladder on US demonstrating a soft tissue
mass in the lumen, suspicious for carcinoma. There is cholelithiasis, a common
coexistent entity. The arrow shows an abnormal interface with the liver parenchyma,
suggestive of local inltration.
Fig.13.29 Colour Doppler image in the same patient showing abnormal vascularity
in the wall of the gall bladder (E Colour plate5.)
Fig.13.30 Dilated intra- hepatic ducts seen in this axial view of theliver.

818
CHAPTER13 Radiology
818
• Limbs:musculoskeletal USS has been revolutionized by advances in high-
frequency probes, which enable characterization of soft tissue masses,
tendon- related pathology, rotator cu lesions, masses, eusions, and
collections. The dynamic nature of the examination allows the diagnosis
of functional pathological conditions and is also used to guide aspiration
and lavage. Assessment of supercial lumps and masses can be
performed as a rst line before triaging to other modalities such as MRI.
It is also used for vascular assessment and the diagnosis ofDVT.
• Intracavitary transducers:these place the transducer as close as
possible to the area of interest. They include transvaginal, transrectal,
urethral, oesophageal, and intravascular probes. They are usually high-
frequency transducers that produce detailed high- resolution images.
Transvaginal USS is more invasive than transabdominal scanning but is
used in the routine assessment of gynaecological disorders. It can also
be used for infertility monitoring, egg retrieval, and the exclusion of
suspected ectopic pregnancy. Transrectal scanning is used for screening,
assessment, and biopsy of suspected prostatic pathology, as well as
rectal pathology including staging rectal cancers. Endo- anal probes
may be used to assess morphology and characterize tears of the anal
sphincter.
•
Contrast agents:US contrast agents are available as an additional tool
in diagnosis, although currently used primarily in academic centres.
These are micro- bubbles, which are stable over a period of time,
and may be used to improve anatomical detail, assess tubal patency
(hysterosalpingography), assess tumour vascularity, and characterize
focal masses (e.g. within the liver), and for contrast enhancement.

ULTRASOUND
819

820
CHAPTER13 Radiology
820
Obstetric imaging
US is the ° imaging modality in obstetric imaging, with MRI being used for
problem- solving. USS is performed via both the transabdominal and trans-
vaginal approach. Imaging is never performed in isolation and should always
be performed in conjunction with clinical information, such as the patient’s
menstrual status (including date of last menstrual period), presence/
absence of pain and vaginal bleeding, as well as knowledge of biochemical
parameters including serum β
- hCG when available.
Role ofimaging
First trimester
•
Conrm an intrauterine pregnancy(IUP).
• Conrm the presence of a gestational sac and date the pregnancy.
• Determine the fetal number and placentation.
• Exclude ectopic gestation.
• If there is bleeding, assess the viability of pregnancy (possible aetiologies
in this scenario include a normal IUP, impending abortion (missed,
incomplete, or impending), ectopic pregnancy, or a subchorionic bleed).
•
8- to 12- week gestational age dating US (most accurate form of
pregnancy dating).
•
Measurement of crown– rump length (margin of error ± 5days).
• Change the estimated date of connement (EDC) to US date if >5days
discrepancy from EDC based on last menstrual period.
• 11- to 14- week gestational age nuchal translucency US (assess uid
behind the fetal neck); early screen for trisomy21.
Second trimester
•
Determine the fetal number and viability.
• Locate and assess placental morphology.
• Estimate the volume of placentaluid.
• Assess gestational age and evaluate growth (margin of error ± 10days).
• Fetal survey.
• Assess the cervix and look at adnexae.
Third trimester
•
Fetal presentation (cephalic, breech).
• Type of placenta.
• Assess the cervix andos.
• Biophysical prole and serial growth.
Amniocentesis
Typically performed at 15– 16 weeks with US guidance. Indications include
advanced maternal age, abnormal biochemical markers (triple screen or
AFP), and a history of genetic/ chromosomal disorders. CVS typically per-
formed earlier (10– 12 weeks) and also under imaging guidance using the
transabdominal or transcervical approach.

OBSTETRIC IMAGING
821
• Gestational sac:the product of implantation and is usually visible within
the uterus at2– 3mm.
•
Normal mean sac diameter (MSD, mm) + 30=days of pregnancy.
•
Normal landmarks (transvaginal scan) (see Table13.9).
•
MSD >8mm:yolk sac should be visible.
•
MSD >16mm:heartbeat should be present (crown– rump
length>5mm).
•
Other criteria worrisome for abnormal pregnancy:
•
Irregular sac contour.
•
Crown– rump length of <7mm and no heartbeat.
•
Mean sac length of 16– 24mm and no embryo.
•
Absence of embryo ≥6 week after last menstrual period.
•
Empty amnion.
•
Enlarged yolk sac of>7mm.
• Small gestational sac also has a high risk of subsequent pregnancy loss
(>90%). MSD (mm) to crown– rump length (mm) <5mm indicates loss
of pregnancy
•
Empty sac:one without a yolk sac or embryo. May represent a very
early IUP (if MSD <8mm) or an embryonic pregnancy (if MSD >8mm),
or a pseudo- gestational sac as seen in ectopic pregnancy.
A full review of obstetric imaging is beyond the scope of this chapter.
Table13.9 Transvaginal scan landmarks (accuracy ± 0.5week)
Age β- hCG Gestational
sac
Yolk
sac
Heartbeat Embryo
(fetal pole)
5 weeks
500– 1000 + − − −
5.5 weeks >3600 + + − −
6 weeks >5400 + + + −
>6 weeks + + + +

822
CHAPTER13 Radiology
822
Gynaecological imaging
USS remains the modality of choice for initial assessment of pelvic pathol-
ogy in ♀ and can be used to assess uterine morphology and endometrial
thickness, to exclude focal uterine pathology such as leiomyomas (broids),
and for initial assessment of adnexal pathology.
Normal endometrium is echogenic with a surrounding hypo- echoic halo.
Thickness is variable, depending on the stage of the menstrual cycle and
the patient’s menstrual status (e.g. pre- or post- menopausal). Typical values
range from <4mm in the menstrual phase, 4– 8mm in the proliferative phase
(up to day 14 of the cycle) to 7– 14mm in the secretoryphase.
The post- menopausal uterus is typically atrophic and may be modied by
the administration of exogenous hormones (hormone replacement therapy
(HRT)), which will also inuence endometrial thickness. Normal thickness
<5mm if no HRTusage.
Hysterosalpingogram
• Indications:for assessment of infertility, to dene uterine anatomy, and
to evaluate tubal patency as a precursor for in vitro fertilization or for
evaluation of congenital anomalies.
•
Procedure performed at days 6– 12 of menstrual cycle. Foley catheter
inserted into the cervical canal, and contrast hand- injected to dene the
above. Complications include pain and infection. Contraindications are
active infection, pregnancy, or recent uterine surgery.
Pelvic magnetic resonance imaging
Indications
Include locating and conrming the presence of leiomyomas (often pre-
and post- uterine broid embolization (UFE); E Interventional radiology,
pp. 844–7), conrming the presence of adenomyosis, endometriosis, in the
assessment of congenital uterine anomalies, as well for the assessment of
complex pelvic or adnexal masses.
T2W imaging of the uterus denes the zonal anatomy and is invaluable in
staging neoplasms. MRI is the modality of choice for tissue characterization
and in this setting will demonstrate small quantities of blood products (as
in endometriosis plaque), as well as showing tissue content such as intra-
lesional fat (dermoids).

GYNAECOLOGICAL IMAGING
823

824
CHAPTER13 Radiology
824
Computed tomography
This technique diers from conventional radiography in that it is able to
visualize a vast spectrum of absorption values, and therefore tissue den-
sities. Furthermore, being a tomographic technique, the resultant image
is essentially 2D and overcomes the problem of confusing overlap of 3D
structures on plain lm. The image is a grey- scale representation of the
density of tissues (attenuation), as depicted by X- rays. Each image is made
up of a matrix of squares (pixels), which collectively represent the attenu-
ation values of tissues within that volume (voxel). With conventional CT,
separate exposures are made for each slice. Current scanners can acquire
data in a continuous helical or spiral fashion, shortening the acquisition time
and reducing artefacts caused by patient movement. This improves the
overall throughput and i the likelihood of a diagnostic scan, particularly
in unco- operative patients. The volumetric data that are acquired may be
manipulated by image processing and displayed in a variety of techniques,
including 3D reformats and ‘virtual’ endoscopy.
The attenuation values are expressed on an arbitrary scale (Hounseld
units), with water being 0, air being −1000 units, and bone +1000 units.
The range of densities displayed on a particular image can be manipulated
by altering the window width and level. This also allows discrimination of
tissues that dier in density by as littleas1%.
Prior to scanning the abdomen or pelvis, dilute oral contrast is given to
opacify the bowel. IV contrast is given to aid the problem- solving process
and dierentiate vascular- enhancing lesions from surrounding tissue.
Multislice CT scanners are third- generation scanners with helical capabili-
ties and low- voltage slip rings, which acquire anywhere between 64 and 320
slices (and counting!) per X- ray tube rotation.
Dose management has become more of a concern with the i utility of
CT across a spectrum of pathologies. The dose is dependent on a number
of patient- and scan- related variables, including patient habitus, the volume
(area) scanned, the number(s) of acquisitions, as well as the desired resolu-
tion and image quality.
To address this problem, there have been innovations in reconstruction
to minimize the dose without impacting on noise and including instances
where data are incomplete. These include adaptive statistical iterative
reconstruction. There is minimal, if little, impact on spatial or contrast
resolution.
Indications
There are a wide variety as detailed below. CT is often the most diagnostic
cross- sectional examination and more denitive than USS in many instances.
Contraindications
Due to the relatively high radiation dose, CT should be avoided in preg-
nancy. Artefact from indwelling, high- density foreign material, e.g. hip pros-
thesis, dental amalgam, and barium, may limit the diagnostic quality of the
examination. Claustrophobia is less of a problem, compared toMRI.

COMPUTED TOMOGRAPHY
825
Applications
• CNS/ spine:CT remains the tool for ° diagnosis, pre- surgical
assessment, treatment monitoring, and detection of relapse in many
CNS disease conditions. MRI is superior in the posterior fossa and
parasellar region and for assessment in MS, epilepsy, and tumours.
Where MRI is not available, it is useful for assessment of degenerative
spinal and disc disease. It is superior to MRI in the assessment of head
injury. In the context of trauma, MRI is only utilized when CT is −ve
despite strong clinical suspicion. CT is also used as the ° modality in the
evaluation of acute stroke and in the emergency settling prior to LP of
patients suspected of CNS infection such as meningitis.
•
Orthopaedics/ trauma:uses include diagnosis and staging of bony and
soft tissue neoplasms and assessment of vertebral, pelvic, and complex
extremity trauma (e.g. tibial plateau fractures). It is also used in the
detection of loose bodies, in the assessment of acetabular dysplasia, and
in providing an answer in joint instability (especially in shoulders, wrists,
and elbows where it may be performed as an adjunct to/ in conjunction
with conventional arthrography).
•
Oncology/ radiotherapy:staging of solid tumours, treatment planning, and
the detection of relapse. CT is of particular value in obtaining whole
body scans in oncology due to the speed and ease of use with the
advent of multislice CT. CT is used for radiotherapy treatment planning
to allow more precise targeting of treatment.
•
Chest:indications include the staging of bronchogenic carcinomas,
characterization of solitary nodules, diuse inltrative lung disease,
widened mediastinum/ mediastinal masses, and pleural abnormalities.
With multislice CT, pulmonary angiography has advanced the diagnosis
of Pes, particularly when V/ Q scanning is indeterminate or equivocal.
Helical CT is equivalent to formal angiography in the detection of
emboli within proximal arteries of < fth/ sixth generation. Sensitivity
(80– 100%, specicity 78– 100%).
• Abdomen:applications include the diagnosis of abdominal pathology,
which may be of traumatic, neoplastic, inammatory, or infective origin
(see Figs 13.31– 13.34). CT is particularly useful for masses, pancreatic
and hepatic disease, detection of the site and nature of obstructive
jaundice, and the assessment of abdominal trauma. It is also used in
the pre- surgical assessment of abdominal aneurysms (see Fig. 13.35)
and as an aid to interventional techniques (E Interventional radiology,
pp. 844–7).
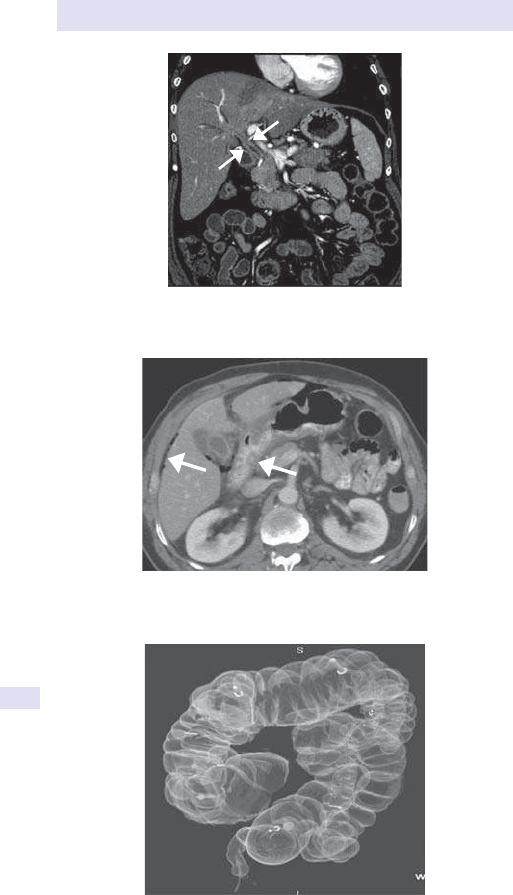
826
CHAPTER13 Radiology
826
Fig.13.31 Direct coronal reformat through the liver showing ndings consistent
with ° sclerosing cholangitis. Note the markedly thickened bile duct wall (arrows).
Fig.13.32 Free intraperitoneal air 2° to perforation of a gastric ulcer. Note the
thickened antrum of the stomach.
Fig.13.33 Virtual barium enema image reconstructed from CT colonoscopy. The
arrow shows the location of the polyps.
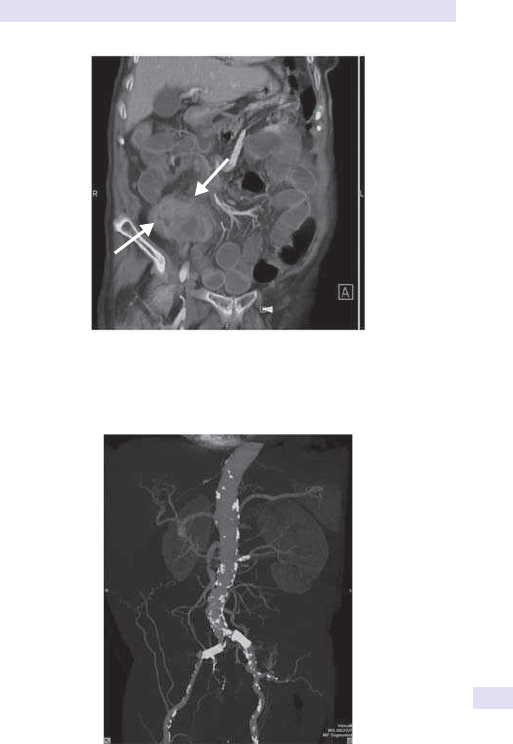
COMPUTED TOMOGRAPHY
827
Fig.13.34 Reformatted maximum intensity projection MIP image of an abdominal
CT showing small bowel obstruction due to the presence of a caecal carcinoma
(arrows).
Fig.13.35 Reformat of a CT angiogram to assess the extent of RAS in this patient
who has bilateral common iliac stents.

828
CHAPTER13 Radiology
828
Magnetic resonance imaging
This is a non- invasive technique, which displays the internal structure, whilst
avoiding the use of ionizing radiation. The nuclei of certain elements align
with the magnetic force when placed in a strong magnetic eld. These are
usually hydrogen nuclei in water and lipid (at clinical eld strengths), which
resonate to produce a signal when a radiofrequency pulse is applied. When
the radiofrequency pulse is switched o, the protons return to their pre-
excitation axis, giving o the energy they absorbed. The energy can be
measured with a detector and used by a computer to display anatomical
information. The T1 and T2 signal characteristics of common tissue types
are seen opposite (see Table 13.10). Further discussion of the physics is
beyond the scope of this chapter. Inherent tissue T1 and T2 characteristics
depend on the longitudinal relaxation (T1) and transverse relaxation (T2)
times of the protons in that tissue. Pathologic processes alter the relaxation
times of the tissue and will produces signal abnormalities.
MR image weighting (e.g. T1W or T2W images) depends on the repeti-
tion time (TR) and echo time (TE) used to obtain images.
Table13.10 Examples ofsequences used inclinical practice
Pulse sequence Acronyms Clinical applications
Conventional
spin echo
SE Workhorse sequences. Therefore, integral to
most protocols
Multi- spin
echo
TSE,
HASTE,
RARE
Reduced scan time, but similar applications to SE
Inversion
recovery
FLAIR, STIR Used to suppress certain tissues by nulling their
signal
Therefore, high net signal results in i conspicuity
of pathology, e.g. infarcts, perilesional oedema,
MSplaque
STIR invaluable in marrow imaging to show
oedema, to assess optic nerves
Gradient echo
FLASH,
SPGR, FISP,
FIESTA,
many more
Uses gradient to rephase signal
Reduced scanningtime
Useful for showing acute blood, in cardiac
imaging with ECGgating
Can also be used in functional imaging
Blood O
2
level (BOLD)- dependent sequences
Echoplanar
imaging
EPI Generates multiple quantities of data (k- space)
in short time, so potential for real- time/
interventional MRIApplications include cardiac,
abdominal imaging

MAGNETIC RESONANCE IMAGING
829
T1- weightedimages
• Contrast is due to inherent T1 relaxation.
• Provides good anatomical information.
• Fat is displayed as high signal (white).
• Distinction between cystic (black) and solid structures is possible.
• Good evaluation of marrow signal.
• The sequence of choice when evaluating enhancement, as gadolinium
administration makes structures of even higher signal intensity on T1W
images (see Tables 13.10 and 13.11).
T2- weightedimages
• Technique of choice for evaluating pathology.
• Fluid is of high signal and therefore optimally displays oedema.
• Improved soft tissue contrast allows evaluation of zonal anatomy of
organs such as the uterus and prostate.
Magnetic resonance angiography
MRI principles are used to exploit the properties of owing blood. Images
generated display structures containing owing blood, with suppression of
all other structures. These principles can be further modied, so that only
vessels with ow in a specic direction (i.e. arteries vs veins are) visualized.
MRA is currently being used in the evaluation of suspected cerebrovascular
disease, renovascular disease, and peripheral vascular disease.
Functional imaging
MRI techniques have evolved, such that diusion imaging utilizes the diu-
sion of water protons in the diagnosis of evolving ischaemia. This technique
shows how movement of water molecules is impeded by cytotoxic oedema
of ischaemic cells. These are manifested by signal changes that show early
evidence of cerebral ischaemia prior to structural changes becoming
apparent.
Diusion MRI comprises two separate components— DWI and apparent
diusion coecient (ADC) which are interpreted together to evaluate the
diusion characteristics of tissue. DWI has had a profound impact in the
Table13.11 MR signal intensity ofcommon tissues
Tissue or body uid T1 signal T2 signal
Air Nil Nil
Bone or calculi Nil Nil
Fat High (bright) Medium to high
Proteinaceous uid (e.g. abscess or
complex cyst)
Medium High
Muscle Low Low to medium
CSF, urine, bile, or oedema Low High
Blood (depends on age), hyperacute
(oxyHb), chronic (haemosiderin)
Low/ low High/ low

830
CHAPTER13 Radiology
830
assessment of cerebral infarcts and is 795% sensitive and specic for infarcts
within minutes of onset of symptoms. The ADC map shows pure diusion
information without any T2 weighting. Reduction in diusion is therefore of
low signal (hypointense) on the ADC map. The b- value is an important con-
cept for impacting sensitivity for the detection of diusion abnormalities.
The higher the b- value, the more contrast the image provides for detection
of reduced diusion. DWI is also increasingly used in the oncological setting
both at the time of staging but also for evaluation of treatment response.
Similarly, early ischaemia of the myocardium is detectable on MRI. This
has great therapeutic potential, as early treatment may prevent the estab-
lishment of ischaemia and result in overall improvement of ventricular func-
tion and survival. MRA is also being used to depict coronary vessel disease
non- invasively.
Perfusion imaging
An advanced technique where the brain is repeatedly imaged as a bolus of
gadolinium (contrast) is injected. Gadolinium causes a magnetic eld dis-
turbance which results in a transient reduction in image intensity. As these
are echo- planar T2*images, they can be acquired quickly and are invalu-
able in imaging of strokes and tumours. The signal changes associated with
gadolinium can be plotted over time for a selected brain volume. The time
signal plots can be manipulated to obtain parameters related to cerebral
perfusion.
Indications
The indications are legion and continue to grow. There are a wide vari-
ety of indications, summarized below (E Applications, pp. 830–2). MR
is especially useful in imaging the brain, spine, peripheral limbs, and joints,
neck, and pelvis. Again, improvements in scanner hardware and software
have had huge impact on clinical practice. The prior limitations of long scan
times have been overcome by robust sequences that can be performed in
a breath- hold. This means that respiratory and peristaltic artefacts are no
longer an issue when imaging in the chest and abdomen.
Contraindications
These largely apply to patients with magnetically susceptible devices or
materials whose movement or loss of function can have deleterious conse-
quences. These include cardiac pacemakers, metallic fragments, and pros-
thetic heart valves. Relative contraindications include pregnancy (especially
the rst trimester) and claustrophobia. MRI magnets are relatively conned
and even those who are not normally claustrophobic may be provoked.
Applications
• The spine:MR imaging is superior to other techniques in displaying
anatomy and is the technique of choice in assessing disc disease and
the post- operative back, in evaluating neural compression (benign or
malignant), in imaging acute myelitis and infection (such as discitis or
osteomyelitis), and in excluding marrow inltration. Contrast is helpful
in assessing the post- operative back to distinguish scar from residual
herniation, as well as for conrming extruded fragments. It also helps
conrm the presence of neuritis 2° to a disc herniation.
MAGNETIC RESONANCE IMAGING
831
• CNS:imaging of the CNS is used to evaluate mass lesions,
hydrocephalus, white matter disease, leptomeningeal pathology,
cerebrovascular disease (see Figs 13.36, 13.37, 13.38,), degenerative
disorders, and visual and endocrine disorders such as pituitary
dysfunction. In trauma/ acute haemorrhage, CT is the preferred
technique (E Neuroradiology, pp. 856–9).
•
Paediatric:the uses here include assessment of perinatal trauma/ anoxic
injury, congenital anomalies, and developmental delay. Within the spine,
it is invaluable in the assessment of spinal dysraphism and progressive
scoliosis.
Fig.13.36 Axial T2W shows acute infarction in the right cerebellar hemisphere.
Fig.13.37 Coronal reformatted MRI image showing a tight stenosis of the distal
vertebral artery on theright.
832
CHAPTER13 Radiology
832
• Musculoskeletal:along with CNS disease, this is a major component
of the MRI workload. It has revolutionized musculoskeletal imaging
and is used to characterize meniscal pathology, ligamentous injury, and
degeneration and sequelae of trauma in the knee, shoulder, wrist, and
ankle. Further uses include imaging mass lesions, assessing the extent of
infection, and diagnosing early avascular necrosis(AVN).
•
Chest/ cardiac:within the thorax, MRI is useful for assessment of apical
lesions such as Pancoast’s tumours (see Fig. 13.39), chest wall and
brachial plexus lesions, and mediastinal masses. Cardiac applications
are legion and fast- evolving; they include imaging of the great vessels to
exclude congenital/ acquired aortic disease (including dissection) and the
diagnosisofPE.
• Abdominal/ pelvic MRI:within the abdomen, MR is often a problem-
solving tool and can be used to more condently characterize focal liver
and pancreatic lesions, as well as assess diuse liver disease. It is also of
use in evaluating indeterminate adrenal masses. Within the pelvis, uses
include imaging of congenital anomalies, as well as staging tumours such
as cervical (see Figs 13.40 and 13.41), prostate, and rectal tumours.
There have been rapid advances in techniques for imaging bowel- related
pathology.
•
Interventional MRI:open MRI units image patients in large- bore or
C- shaped units, rather than the closed narrow tunnel used in conventional
units. They can therefore be used for claustrophobic patients and to provide
imaging guidance for interventional procedures. Disadvantages include a
low magnetic eld strength (0.1– 0.3T vs 1.5T) and a limited anatomical and
spatial resolution due to their basic construct.
Recently, short- bore magnets have been developed that combine the accu-
racy of a tunnel scanner and the comfort of an open MRI scan. Although
they are not completely open, they are much less constrictive because of
the short- bore magnet (shorter tunnels) but can produce a higheld.
Fig.13.38 AP coronal MRA showing mild irregularity of the carotid siphon on the
left. The images are examined in multiple projections to exclude anomalies such as
stenosis or small aneurysms.
MAGNETIC RESONANCE IMAGING
833
Fig.13.39 Sagittal T2W MRI image in patient with a superior sulcus (Pancoast’s)
tumour showing invasion into the chestwall.
Fig.13.40 Axial T2W sequence showing bilateral parametrial inltration in this
patient with a cervicalmass.
834
CHAPTER13 Radiology
834
Fig.13.41 Sagittal and axial T2W images showing a large cervical carcinoma. MRI is
the modality of choice for local staging.

MAGNETIC RESONANCE IMAGING
835

836
CHAPTER13 Radiology
836
Spinal imaging
Basic principles ofbony trauma imaging
• Obtain two lms at right angles to one another (most commonly an AP
and a lateral to rule out a fracture).
•
Image proximal and distal joints.
• Bone scanning is more sensitive, but less specic, than plain lms to rule
out a fracture. (Not useful in the acute setting.)
•
CT is invaluable for complex injuries (e.g. spine, calcaneus, and sacrum).
• MRI is used to assess ligaments, tendons, joint capsules, menisci, and
cartilage.
In each case, evaluate thefollowing
• Site of fracture:assess if proximal/ distal and intra- vs extra- articular.
• Type of fracture:simple (transverse, oblique, spiral) vs comminuted.
•
Degree of displacement:usually described with reference to the distal
fragment.
•
Soft tissue involvement:exclude foreign bodies, presence of gas. Open
(compound) vs closed.
Cervicalspine
• Trauma:obtain a cross- table lateral rst (this has the highest yield),
and then perform the remainder of the cervical spine series (AP and
open mouth peg views), if patient mobility allows and a high index
of suspicion. All seven cervical vertebral bodies should be visualized
(a large number of cervicothoracic injuries are missed because of
inadequate views). If not seen, request a specialized lateral view
(swimmer’s) or a CT. Then sequentially evaluate:
•
Alignment:assess the following lines shown in Fig. 13.42. They should
be parallel with no step- os.
•
Bones:inspect C1 and C2. The anterior arch of C1 should be 3mm
from the dens in adults (5mm in children). The vertebral bodies
should be intact, and they should be uniform in size and shape.
Check disc spaces for any inordinate narrowing or widening which
may be post- traumatic.
Cartilage
• Soft tissues:look for abnormal widening or a localized bulge; 50% of
patients with a bony injury will have soft tissue thickening. The soft
tissues should be no more than one- third of a vertebral body until C4,
and a vertebral body width thereafter.
•
The peg views:do not mistake a superimposed arch of C1 or the incisors
as a fracture. Important points to rememberare:
•
The lateral margins of C1 and C2 shouldalign.
•
The spaces on either side of the peg should be equal (see Fig. 13.42).
Remember normal plain lms do not exclude ligamentousinjury
In the routine setting, cervical spine lms are taken to exclude spondylo-
sis (disc space narrowing and osteophytes) and atlantoaxial subluxation,
which results in long tract signs and cord compression (RhA, ankylosing
spondylitis, Down’s syndrome). (See Fig. 13.43 for image showing a large
osteophyte.)
SPINAL IMAGING
837
Thoracic and lumbarspine
Degenerative disease is common with disc space narrowing, end- plate scle-
rosis, and osteophyte formation. Wedge compression fractures are com-
mon in the osteoporotic spine and need to be distinguished from the more
sinister causes (absence of paraspinal mass, posterior elements spared).
Multiple collapsed vertebrae are found in osteoporosis, neoplastic disease,
trauma, and histiocytosis X.Bone density may help narrow the dierential,
which includes i (sclerotic metastases, lymphoma) and d (acute infection,
osteoporosis).
Spondylolisthesis is the subluxation of one vertebral body on another and
may be degenerative or due to bilateral pars defects (spondylosis). This is
a fracture/ defect of the posterior elements of the vertebrae. In an oblique
view, the posterior elements form a Scottie dog (with the pars making up
the collar). This may be a purely incidental nding; however, if severe, it can
result in neuroforaminal stenosis. Plain lms are insensitive in the evaluation
of disc disease. MRI is the investigation of choice for disc disease and its
neurological complications.
Fractures typically occur at the thoracolumbar junction (90% at T11– L4).
CT typically indicated other than in stable compression fractures, isolated
spinous or transverse process fractures, and spondylolysis.
Plain lm ndings include paraspinal haematoma and widened inter-
pedicular distance. An unstable injury may be accompanied by disruption
of the posterior elements and widened interlaminar space, and is seen in
the context of all fracture dislocations or if there is a compression fracture
of>50%.
1
2
3
4
5
1
—Soft tissue line closely applied to posterio
r
aspect of the airway widens at level of
laryngeal cartilage
2—Anterior border of vertebral bodies
3—Posterior border of vertebral bodies
4—Spino laminar line = posterior limit of
spinal canal
5—Tips of the spinous processes
2
3
4
5
6
7
Fig.13.42 Schematic diagram
depicting the lines that need to be
assessed when looking at a lateral
lm of the cervicalspine.
Fig.13.43 Sagittal T2W image showing a
large osteophyte at the C6/ 7level.

838
CHAPTER13 Radiology
838
Pelvis
Pelvic fractures are complex, and there are many classication systems
around. The pelvis should be regarded as being made up of three bony
rings. The sacroiliac joints and the pubic symphysis are part of the main
bony ring. Afracture of one ring is frequently associated with a second ring
fracture (see Fig. 13.44).
• Sacroiliac joints should be equal inwidth.
• The superior surfaces of the pubic rami should align. The joint width
should be no more than5mm.
•
The sacral foramina should form a smootharc.
• Acetabular fractures are subtle— look for symmetry.
Stable fractures (single break of the pelvic ring or peripheral fractures)
commoner. These include avulsion injuries (e.g. anterior superior iliac spine
(ASIS), pubis, and ischial tuberosity), as well as sacral fractures and those of
the ischiopubicrami.
Unstable fractures (pelvic ring interrupted in two places)— less common.
All require CT for clarication of the extent of injury, as a plain lm may
underestimate the extent of posterior ring disruption (includes Malgaigne
and bucket handle fractures).
•
Bone texture:the pelvis is a common site for metastatic involvement,
especially with urological malignancies, e.g. prostate (sclerotic
metastases) and myeloma (multiple lytic lesions). Paget’s disease of the
pelvis may mimic sclerotic metastases but tends to be conned to one
hemipelvis and may expand or thickenbone.
•
Sacroiliitis:sacroiliac joint involvement is common in seronegative
arthropathies and is usually symmetrical in conditions such as IBD,
ankylosing spondylitis, and hyperparathyroidism. More asymmetrical
change is seen in Reiter’s disease and RhA. It is characterized by initial
erosion and widening of the joint, resulting in chronic sclerosis, which
has a preferential involvement of the lower one- third of the joint (iliac >
sacralside).
•
AVN of the femoral heads:an important nding but is often advanced
when plain lm ndings are seen. Radiographically occult AVN may be
detected on MRI or a bone scan. On plain X- ray, it is characterized by
sclerosis, attening, and fragmentation of the femoral head. Subchondral
crescents are pathognomonic. AVN can also be a sequel of trauma,
but bilateral AVN is seen in conjunction with steroid therapy, sickle- cell
disease, and as part of Perthe’s disease.
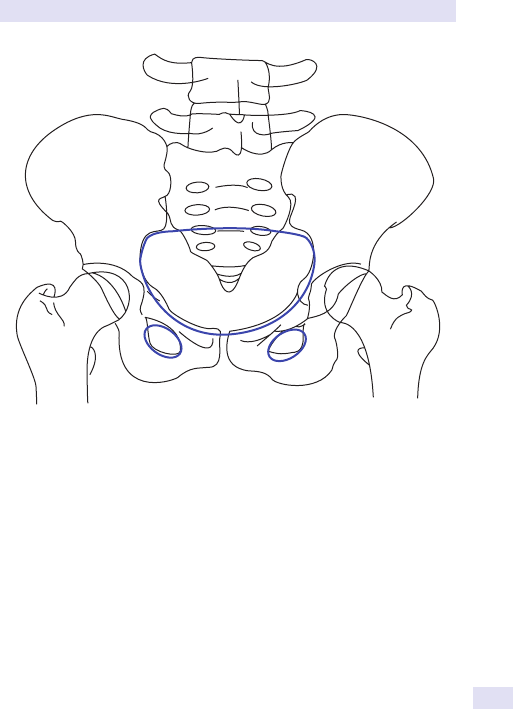
PELVIS
839
Fig.13.44 The pelvis is made up of bony rings:the main pelvic ring and two smaller
rings made up of the pubic and ischialbones.

840
CHAPTER13 Radiology
840
Vascular intervention
Angiography is catheterization of a vessel followed by subsequent
opacication with a water- soluble iodine- containing contrast medium.
Catheterization is usually performed using the Seldinger technique.
An approach tointerpretation ofan angiogram
• What:is the type of study— angiogram, digital subtraction angiogram
(DSA), venogram?
•
Where is the catheter and which vessel? Is it a large vessel or a
selective/ super- selective angiogram?
• When:what phase is it (early/ late arterial, parenchymal, or venous)?
• Vessels:is there contrast in unexpected vessels (extravasation or
neovascularity)? Is the vascular contour normal (look for irregularity,
stenosis, dissection, or encasement)?
•
Intervention (previously placed stents, lters, coils, clips, or drains).
Catheter selection
Catheters are sized in French (Fr) where 1 Fr=0.33mm. Sheath vs catheter.
Asheath has a dened luminal diameter; however, the overall diameter will
generally be larger.
High- ow (ush) catheters have multiple side holes and are used for large
vessel angiography.
Selective catheters have a single hole at the end. The distal portion has
numerous shapes tailored to a specic or general purpose.
Indications include
• Demonstration of arterial anatomy prior to surgery where this is likely
to inuence surgical management.
•
To elucidate the nature of arterial disease, e.g. occlusions, stenoses,
thrombi, aneurysms, and vascular malformations in coronary, carotid,
and cerebrovascular disease. Also in the setting of endovascular
procedures (aneurysm repair, thrombolysis, stenting, and angioplasty).
•
To identify the source of bleeding in the GIT or following trauma.
• To demonstrate tumour circulation (often prior to embolization).
• Due to advances in technique, non- invasive assessment of vessels by
techniques such as colour Doppler US, CT angiography, and MRA is
often used as rstline.
Potential complications include
Puncture site haematoma, infection, pseudo- aneurysm, AV stula, dissec-
tion, thrombosis, embolic occlusion of a distal vessel.
Contrast
Volumes used are variable, depending on the area being imaged. The con-
trast agents are iodinated, non- ionic, and of low osmolarity, resulting in
reduced toxicity. Nevertheless, potential side eects include anaphylaxis,
hypotension, urticaria, and bronchospasm. Patients particularly at risk
include those with a history of a previous reaction, iodine allergy, and atopy.
Nephrotoxicity is a potential risk and may be exacerbated by dehydration.

VASCULAR INTERVENTION
841
Contrast reactions are seen in 1/ 1000 patients. Risk of anaphylaxis is 1/
400,000. Pre- medication with corticosteroids may reduce the incidence of
reactions if contrast administration is essential, but this is not universally
accepted.
Specic applications
These include pulmonary angiography (gold standard for detection of PEs),
which is highly invasive, and therefore reserved for when thrombolysis or
embolectomy are being considered. Cerebral angiography is useful in the
diagnosis of aneurysms, AVMs, tumour vascularity, and both intra- and
extracranial vascular disease. Renal angiography is selectively performed to
diagnose RAS and prior to embolization of tumours.
•
DSA:a technique whereby there is subtraction of the contrast-
containing shadows from the initial plain lms (mask), resulting in an
image containing opacied structures only. The resulting images may
be digitized and manipulated. The overall advantage is smaller doses of
contrast and smaller catheters may beused.
•
Angioplasty:percutaneous transluminal angioplasty (PTA) is a method
used to fracture the vascular intima and stretch the media of a vessel
by a balloon. Atherosclerotic plaques are very rm and are fractured
by PTA. Healing occurs by intimal hyperplasia. Indications include
claudication or rest pain. Acommon alternate to surgical bypass, with
5- year patency similar to that of surgery.
• Intravascular stents:typically, metallic stents used when there has been
unsuccessful PTA or in cases of recurrent stenosis, venous obstruction/
thrombosis or as transjugular intra- hepatic portosystemic shunt
(TIPS) shunts (E Interventional radiology, Transjugular intrahepatic
portosystemic shunt, p. 846). They can either be balloon- expandable or
self- expandable. Aortic stent grafts used to treat aortic aneurysms or
dissections are typically a combination of a metallic stent with synthetic
graft material. Stents can also be used in revascularization procedures
when there is long segment stenosis, total occlusion, or ineectivePTA.
• Therapeutic embolization:
•
Used to selectively occlude vessels by introducing a variety of
materials via a catheter. Permanent materials used include metallic
coil, balloons, and cyanoacrylate glue. Temporary embolic materials
include gel foam and autologous blood clots. This technique is used
at active bleeding sites and to reduce tumour vascularity preop-
eratively in resectable tumours. It can also be used to treat AVMs,
for symptomatic uterine broid embolization (E
Figs 13.49 and
13.50, p. 848), and in varicocele embolization for infertility. Proximal
occlusion of a vessel is equivalent to surgical ligation and does not
compromise collateralow.
•
Distal embolization usually infarcts tissue and is followed by necrosis.
•
Complications include post- embolization syndrome (fever, pain,
elevated WBC), infection of embolized area, and reux of embolic
material (non- target embolization).
842
CHAPTER13 Radiology
842
• Vascular catheterization:is also used to selectively infuse vessels, as
with thrombolytic treatment or rarely with cytotoxics. Vascular
stenting is of i use in coronary and peripheral vascular disease. IVC
lters (see Fig. 13.45) are metallic umbrellas used to mechanically
trap emboli and prevent venous thromboembolic disease. They are
percutaneously placed via the femoral, jugular, or antecubital vein. In
the treatment of patients with recurrent PEs despite anticoagulation or
where anticoagulation is contraindicated. IVC lters can be temporary
(retrievable) or permanent. They are placed infrarenally to avoid renal
vein thrombosis.
•
Thrombolytic therapy:
•
Is the infusion of a brinolytic agent (urokinase, streptokinase, TNK,
tissue plasminogen activator (tPA)) via a catheter inserted directly
into a thrombus. This can restore blood ow in a vessel obstructed
with a thrombus or embolus.
•
Indications include treatment of an ischaemic limb, early treatment
of MI or stroke to reduce end- organ damage, and treatment of
venous thrombosis (DVT) of the legorPE.
Fig.13.45 Cavogram showing infrarenal deployment of an IVC lter
(Gunther– Tulip).

VASCULAR INTERVENTION
843
•
Contraindications include active bleeding, recent intracranial event
(CVA, tumour, or recent surgery), non- viable limb, and infected
thrombus.
Central venous access
:there are a variety of devices available— peripherally
inserted central catheters (PICCs), external tunnelled catheter (Hickman),
subcutaneous port (Portacath
™
). Indications include chemotherapy, total
parenteral nutrition (TPN), long- term antibiotics, administration of u-
ids and blood products, and blood sampling. Potential complications are
venous thrombosis, infection, and pneumothorax.

844
CHAPTER13 Radiology
844
Interventional radiology
Interventional radiology is a sub- speciality where a variety of imaging
modalities are used to guide percutaneous procedures. This may obviate
alternative surgical procedures and consequently result in lower morbidity.
Interventional procedures are usually carried out under LAn or using con-
scious sedation (E Interventional radiology, Conscious sedation, p. 846),
and on an outpatient basis, thereby considerably reducing bed occupancy.
There is a huge range of procedures that are currently performed. The fol-
lowing is a limited list of some of them (see also Table 13.12).
Percutaneousbiopsy
Biopsy needle placement may be done under uoroscopy, CT, MRI, and
US. This provides a non- operative conrmation of tissue diagnosis and, in
the case of a suspected malignancy, it is possible to accurately plan treat-
ment. For histology, a 14– 18G needle is used. With a ne- aspiration needle
(20– 22G), material may be obtained for cytology. Using imaging guidance,
there is avoidance of damage to vital structures such as blood vessels, solid
organs, and bowel loops. With chest biopsy, there is a 30% risk of pneu-
mothorax. Other potential complications include false −ve samples (due
to sampling error or tissue necrosis). Bleeding is more likely to occur in
patients with underlying coagulopathies (vigorous pre- biopsy screening
important for safety) and in patients with ascites.
Percutaneous drainage
With image guidance, surgical intervention may be avoided by accurate
placement of a drainage catheter. Calibre varies from 8 to 14F, depending
on the nature of the underlying uid. Regular irrigation of the catheter may
be necessary to ensure successful drainage. Successful resolution may be
impeded in the more complex and multiloculated collections. There are
a variety of routes of access other than the conventional percutaneous,
including transvaginal, transrectal, and transgluteal. Potential complications
include injury to overlying structures and sepsis.
Drainage ofthe urinarysystem
Can be via double J stents, which are placed into an obstructed collecting
system, with the distal catheter tip lying in the bladder. More short- term
drainage is achieved via a percutaneous nephrostomy. Here, the obstructed
kidney is punctured under uoroscopic or US guidance, and a catheter
placed in the renal calyx (preferably the lower pole). This is the technique
of choice in the acutely obstructed or infected kidney. Nephrostomy inser-
tion is also performed in the setting of ureteric injury either with or without
accompanying peritonitis. Potential risks include septic shock, haematuria
(due to pseudo- aneurysm or AV stula), or injury to regional structures.
Biliary system drainage
Surgical outcome in patients with malignant bile duct obstruction is often
poor. This may be due to carcinoma of the pancreas or cholangiocarcinoma.
Biliary stenting alleviates obstruction and improves quality of life. Stenting
may be performed at ERCP or percutaneously via antegrade puncture

INTERVENTIONAL RADIOLOGY
845
Table13.12 Established and newer interventional applications
Established techniques Newer interventional applications
Arterial and venous sampling AVM embolization
Biliary drainage, stenting, angioplasty,
biopsy, and stone extraction
Chemoembolization
Diagnostic angiography Cryoablation of liver tumours
Embolization
Oesophageal dilatation Endovascular repair of abdominal
aortic aneurysms with stent grafts
Fallopian tube recanalization Radiofrequency ablation of tumours
Feeding tube placement Uterine artery embolization for
symptomatic broids
IVC lter placement Varicocele embolization
Nephrostomy placement Vertebroplasty
Pain management (e.g. coeliac ganglion block,
selective and non- selective nerve block)
Percutaneous biopsy, cholecystostomy, and
drainage of uid collections
° gastrostomy, gastrojejunostomy, or
jejunostomy tube placement
Thrombolysis
TIPS insertion
Ureteral stent placement
Vascular angioplasty and stent placement
Venous access and foreign body retrieval
through the liver (PTC to delineate the anatomy, being performed rst).
Other than alleviating biliary obstruction, PTC can provide biliary diversion
in the case of ductal injury (post- traumatic or post- surgical). Often there is
unilateral obstruction which requires only treatment of the aected side.
Hilar obstruction, however, due to a hilar (Klatskin) tumour requires two
biliary drains.
Percutaneous drainage of the gall bladder (cholecystostomy) is indicated
for the treatment of acute calculous or acalculous cholecystitis in patients
who are not surgical candidates or as a temporizing measure prior to deni-
tive surgery.
Other GI interventions include stenting/ balloon dilatation of oesophageal
strictures, stenting of obstructive colonic neoplasms (see Fig. 13.46), and
percutaneous gastrostomy or gastrojejunostomy insertion. Oesophageal,
head and neck, and neurologic disease may necessitate percutaneous gas-
trostomy. In some instances, it may be used for long- term bowel decom-
pression, for instance in a prolongedileus.

846
CHAPTER13 Radiology
846
Transjugular intra- hepatic portosystemicshunt
A procedure whereby a connection is made between the hepatic and portal
veins to reduce portal pressure in patients with portal hypertension. The mor-
tality is considerably lower than in acute shunt surgery, particularly in the con-
text of an acute variceal bleed, which has failed to respond to sclerotherapy.
Conscious sedation
Form of sedation whereby the patient is given sedation and analgesic medi-
cation but remains conscious and easily arousable. At all times, the follow-
ing are monitored— BP, pulse oximetry, ECG, and heart rate. Typical drugs
used include a short- acting benzodiazepine (e.g. midazolam) and a narcotic
analgesic (e.g. fentanyl), administered in small aliquots.
Endovascular repair ofabdominal aortic aneurysms
With stent grafts, this is a new image- guided, catheter- based approach that pro-
vides a valuable alternative to standard open surgical repair. Radiological imaging
plays an essential role in pre- procedure evaluation, the procedure itself, and
patient follow- up. The ultimate goal remains the same— complete exclusion of
the aneurysm sac to prevent rupture. Advantages include lower blood loss,
shorter ITU stay, and rapid recovery. Complications include graft thrombosis,
kinking, pseudo- aneursym caused by graft infection, and endoleak. Bifurcation
grafts are used mainly for abdominal aortic aneurysm (AAA) repair and aorto-
iliac occlusive disease. Tube grafts are used mainly for AAA repair.
Radiofrequency ablation
Not all patients with tumours are eligible for surgical intervention because
of unfavourable location of the tumour, adverse clinical conditions, or
advanced disease. In addition, the cost, morbidity, and mortality associated
with surgical resection have led to the search for other forms of therapy.
Newer, minimally invasive treatments include intra- arterial chemoem-
bolization, injection of ethanol, and radiofrequency ablation. Among the
thermal ablative procedures, radiofrequency ablation has evolved into an
established technique for non- invasive management of tumours in patients
Fig.13.46 Obstructing sigmoid carcinoma has been decompressed with a colonicstent.
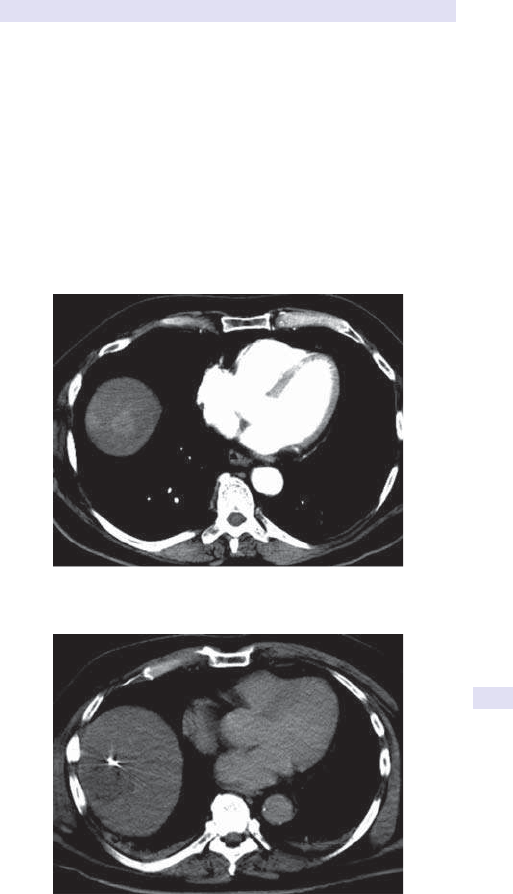
INTERVENTIONAL RADIOLOGY
847
where underlying medical co- morbidities or tumour burden preclude deni-
tive surgery or as a bridge to further therapy. For this procedure, US, CT
scanning (see Figs 13.47 and 13.48), or MRI is used by the radiologist to
guide percutaneous placement of long, thin (usually <18G), insulated nee-
dles into the tumour. The distal end (1– 3cm) of each needle is not insulated
and emits radiowaves. Electrodes are attached to a generator, and the elec-
trical energy is converted to heat that kills cells through coagulation necro-
sis. The radiologist can vary the amount of current used, thereby tightly
controlling the treatment radius. The entire treatment session usually lasts
1h and can be performed in the outpatient setting. The procedure is typi-
cally performed with conscious sedation and LAn. Radiofrequency ablation
has been used to treat a variety of tumours, but the commonest utility is for
hepatocellular carcinoma, liver metastases, and renal tumours.
Fig.13.47 Contrast- enhanced CT showing several high- attenuation metastases
overlying the dome of theliver.
Fig.13.48 Radiofrequency ablation of the metastasis shows the electrode insitu.
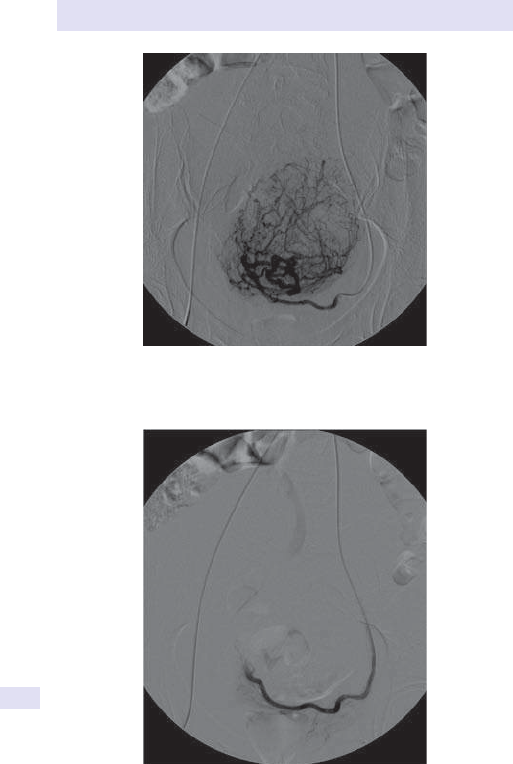
848
CHAPTER13 Radiology
848
Fig.13.49 Pre- uterine artery embolization (UFE). Selective catheterization shows
the supply to a large left- sided broid.
Fig.13.50 Post- embolization with polyvinyl alcohol (PVA) showing no ow in the
previously demonstrated broid.

INTERVENTIONAL RADIOLOGY
849

850
CHAPTER13 Radiology
850
Musculoskeletal imaging
Fracture terminology
Anatomicalsite
•
In long bones, divide the shaft into thirds.
• Use anatomical landmarks for description.
Pattern offracture
•
Simple fracture:no fragments. Describe the orientation of the fracture
plane, e.g. transverse, spiral, and oblique.
•
Comminuted fracture:>2 fragments:T- , V- , and Y- shaped patterns,
buttery fragments.
Apposition and alignment
Dened in relation to distal fragments.
Other important descriptors include:
•
Displacement (e.g. medial, lateral, anterior, posterior).
• Angulation (direction of fracture apex, e.g. valgus or varus).
• Rotation (internal vs external).
• Overlap (of fragments).
• Distraction (refers to degree of separation of fragments).
• Impaction:fracture fragments are compressed, resulting in
shortenedbone.
Adjacent joints:are they normal? Is there dislocation, subluxation, or any
intra- articular extension of the fractureline?
Also consider thefollowing
• Soft tissue involvement (foreign body, gas, calcication).
• Type of fracture (stress vs pathological).
• Stress fractures are of twotypes:
•
Insuciency- related (if abnormal underlying architecture, as in
osteoporosis).
•
Fatigue:when abnormal stress is placed on normal bone (as in march
injuries).
•
Pathological fracture occurs when fracture occurs in the setting of
underlying bone disease or discrete lesion.
Types oforthopaedic hardware
• Intra- medullary rods:usually hollow, closed nails. Rotation and
shortening of bone fragments is avoided by distal interlocking screws.
•
Kirschner wires (K- wires):these are used to temporarily xate or treat
fractures, particularly with small fragments or paediatric metaphyseal
fractures. These are drilled into cancellous bone and can be used to
maintain rotational stability if >1 is used. The ends are typically folded
over to avoid any soft tissue or other injury.
•
Circlage wires can be used to contain bone fragments.
• Other hardware includes staples, plates, nails, and screws.

MUSCULOSKELETAL IMAGING
851
Types ofjoint replacements
• Polyethylene is a radiolucent substance used for joints with concave
articular surfaces (e.g. the hip). Typically backed with metal as a support.
Ultra- high molecular weight versions are also available.
• Silastic is also radiolucent and is used for hand and foot arthroplasty
implants.
•
Methylmethacrylate is used as a cement or can be directly injected into
the medullary space. Radio- opaque.
• Variety of metal alloys.
Constrained prostheses are intrinsically stable and are therefore more likely
to suer loosening. Unconstrained prostheses rely on extra- articular struc-
tures to provide stability and are therefore less likely to dislocate.
A discussion of the types of prostheses is beyond the scope of this chap-
ter. Potential complications of prostheses include, but are not limited to,
the following.
•
Polyethylene wear, dislocation, heterotopic bone formation, and
infection can be seen. Small particle disease is seen as areas of osteolysis
around the prosthesis due to macrophage- mediated reaction to particle
debris. Cement leakage can cause necrosis and vascular and neurological
injury, depending on location.
•
Acute loosening occurs in the setting of infection. More chronic
loosening is seen due to mechanical factors.
Magnetic resonance imaging inmusculoskeletal imaging
Musculoskeletal neoplasm imaging
Critical role in evaluating disease extent, staging, and treatment planning.
Lack of mobile protons, and an acellular matrix render cortical bone, liga-
ment, tendon, and brous signal of low signal intensity on all sequences.
Tissues like muscle, fat, osteoid, and chondroid matrix have diering signal
intensities, which can be used to dierentiate tumours. Marrow involve-
ment can be excluded by T1W and STIR sequences (E Magnetic reso-
nance imaging, pp. 828–32). The bone involved should be imaged in its
entirety to exclude skip lesions. IV contrast is mandatory to assess lesional
margin and tumour vascularity.
Joint imaging
MRI is the mainstay in assessment of joint pathology and, in particular, is
excellent in depiction of ligaments, cartilage, and joint eusions. It is there-
fore the modality of choice for infection, neoplasm, trauma, and arthritis.
STIR shows marrow oedema, uid collections, and bursal inammation.
Dual- echo steady state (DESS) is a sequence used for specic evaluation
of articular cartilage. MRI is also the gold standard for marrow imaging. It
is able to dierentiate red from yellow marrow. T1W and STIR sequences
are used for evaluating marrow pathology. Marrow oedema, as well as early
involvement of the marrow by pathologies such as infection, neoplasia, and
subtle trauma, is seen on STIR sequences when not easily evident on plain
radiographs.
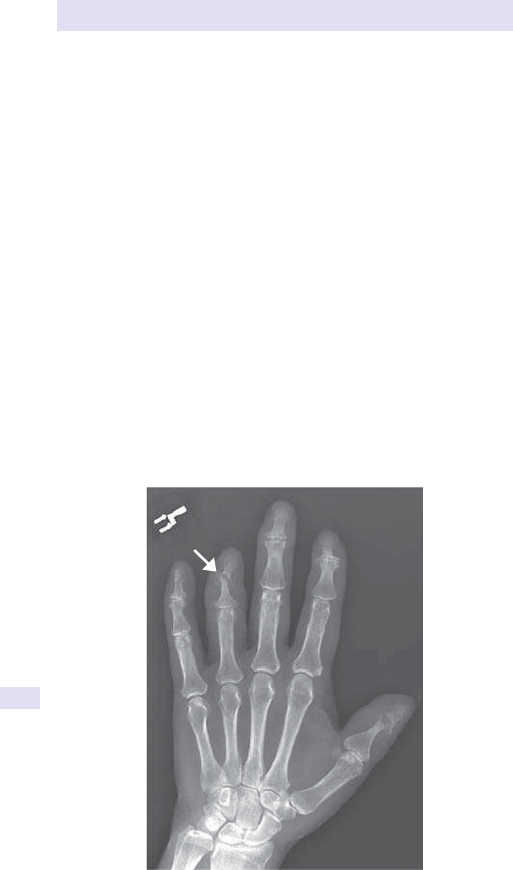
852
CHAPTER13 Radiology
852
Hands
There are specic patterns that may be seen in the hand as an indicator of
the underlying disorder. Some patterns are pathognomonic, whereas oth-
ers are more non- specic.
• Generalized osteopenia:osteoporosis, multiple myeloma, andRhA.
•
Coarsening of the trabecular pattern:common in haemoglobinopathies,
especially thalassaemia and Gaucher’s disease.
•
Periosteal reaction:
•
HPOA associations include carcinoma of the bronchus, IBD, and
coeliac disease.
•
Thyroid acropachy, commonest on the radial side of the thumbs.
•
Juvenile chronic arthritis seen in about25%.
• Carpal abnormalities:include short metacarpals (Turner’s syndrome,
pseudo- and pseudopseudohypoparathyroidism), carpal fusion
(inammatory arthritis, RhA and juvenile chronic arthritis, post- trauma),
and look for syndactyly and polydactyly.
•
Soft tissue changes:e.g. i in soft tissue thickness/ size seen in acromegaly,
localized i seen in gouty tophi, nodes as in OA, soft tissue calcication
seen in CREST (calcinosis, Raynaud’s syndrome, oesophageal motility
dysfunction, sclerodactyly, and telangiectasia), and scleroderma.
•
Joint disease:the hand X- ray above all may help in sorting out the type of
arthropathy and aid rheumatological management (see Fig. 13.51). The
ABCS approach is invaluable (see Table 13.13).
Fig.13.51 Asymmetric oligoarthritis with dominant involvement of the DIP joints.
The pencil- in- cup appearance is typical.

HANDS
853
Trauma
Two views are essential for ensuring no subtle injuries are missed. On a P- A
view, the spaces between the carpal bones and the carpometacarpal articu-
lations should be roughly equal (1– 2mm). If a dislocation is present, then
there is obliteration/ overlap. Common injuries include Bennett’s fracture
and the rst metacarpal base injury extending into the joint surface with
dislocation at the carpometacarpal joint. Scaphoid fractures are the com-
monest (75– 90%) of carpal injuries. Because of the blood supply, there is a
potential risk of osteonecrosis of the proximalpole.
Table13.13 An ABCS approach tointerpretation ofthe hand
radiograph
A:Alignment Subluxation/ dislocation common in RhA and SLE
B:Bone Osteoporosis:mineralization is usually normal, except in RhA.
Juxta- articular osteopenia can be seen in any arthropathy. Diuse
osteopenia is seen inRhA.
Erosions:
•
Aggressive (i.e. no sclerosis of margins) seen in RhA and
psoriasis
•
Non- aggressive (with sclerotic margins) seen in gout;
inammatory erosions are marginal (OA erosions are central)
(Distinguish from subperiosteal resorption (radial border; seen in
hyperparathyroidism)
Bone production:periosteal new bone formation, (psoriasis)
Reiter’s syndrome:
•
Ankylosis (bony bridging) in inammatory arthropathies
• Overhanging cortex (typical ofgout)
• Osteophyte formation,OA
• Subchondral bone (reparative bone beneath cortex); typical
of OA
C:Cartilage Joint space has uniform narrowing in all arthritis, except OA
Eccentric narrowing:typically seeninOA
Wide joint space :early arthritis, gout, and pigmented
villonodular synovitis (PVNS)
S:Soft tissue Swelling:
Symmetrical:about joint seen in all inammatory arthropathies,
but most typicallyRhA
Asymmetric:typically due to osteophytes and seen withOA
Lumpy swelling of soft tissues seen in gout (due totophi)
Swelling of entire digit; psoriasis, Reiter’s
Calcication:soft tissue; gout (calcied tophus)
Cartilage:CPPD
Subcutaneous tissues:scleroderma

854
CHAPTER13 Radiology
854
Image- guided arthrography
May be performed in isolation or in conjunction with subsequent cross-
sectional imaging (CT or MRI) for the following indications:
• Ligamentous and tendinoustears.
• Cartilage injuries.
• Proliferative synovitis.
• Masses and loose bodies.
• Implant loosening.
Plain lms are obtained prior to the procedure. All joint uid that is aspi-
rated is routinely sent for culture. The contrast ows away from the needle
if the tip is intra- articular.
Single contrast studies are performed to exclude non- calcied loose bod-
ies. Double contrast studies are performed in the setting of cartilaginous
injury such as a labraltear.
•
Contraindications:allergy to contrast media, concomitant infection.
•
Complications:pain, infection, allergic reaction (to contrast), and
vasovagal reaction.
When performing MR, the examination is performed with dilute gadolinium.
Approach todiagnosis ofbonelesion
•
Single or multi- focal? Multiple lesions can be seen in benign disease such
as enchondromas, brous dysplasia, as well as malignant aetiologies such
as myeloma, metastases, and lymphoma.
•
What is the degree of aggressiveness (type of destruction, as well as
reparative pattern)? Cortical penetration implies aggressive behavior, as
does an ill- dened or wide zone of transition (area between the lesion
and normal bone). Moth- eaten and permeative patterns are also in
keeping with aggressive disease.
•
Location (within bone). Central and cortical lesions are usually benign.
° tumours occur at sites of rapid growth, for instance the distal
femur. Metastases occur at sites of high vascularity such as the spine or
iliacbone.
•
Tissue characterization by matrix. Matrix refers to the intercellular
substance produced by tumour cells (is done in conjunction with a
cross- sectional modality such as CT). Osseous matrix is seen with
osteogenic tumours, and chondrogenic tumours have a cartilaginous
matrix.
•
Age is a very helpful way of narrowing the dierential. Over 40years,
metastases are much commoner than ° bone tumours.
•
Is there a periosteal reaction? More aggressive lesions are associated
with typical patterns such as sunburst, Codman’s triangle, or an onion
peel conguration
•
Soft tissue mass:more typically seen with malignant lesions.

HANDS
855

856
CHAPTER13 Radiology
856
Neuroradiology
• CT is the initial study for evaluation of neuropathology due to its speed,
availability, and lowercost.
•
Excellent for evaluation of bony abnormality.
• Done both with and without IV contrast, depending on the clinical
indication. The use of contrast delineates vascular structures and
anomalies.
•
Visualization of the posterior fossa is inferior toMRI.
• MRI is useful in assessing diuse axonal injury and the sequelae of head
injury.
Logical approach tointerpretation
• Soft tissues (look for thickening which may suggest haematoma or
oedema):assess the ear and orbital contents.
•
Bone (use bone window):check the calvarium, mandible, and C spine
for fractures and lytic lesions. Assess sinuses and mastoid air cells for
opacity that may suggest the presence of uid, pus, blood, mass, or
fracture. If there has been facial trauma, the integrity of facial bones/
orbit best assessed on coronalview.
•
Dura and subdural space:assess symmetry of thickness (early clue to
presence of blood). Look for crescentic or lentiform density.
•
Cortical parenchyma:poor dierentiation between grey and
white matter suggestive of infarction, tumour, oedema, infection,
or contusion. Hyperdense areas may suggest enhancing lesions,
intracerebral haematoma, or calcication. If central grey matter nuclei
(globus pallidus, internal capsule) are not visible, suspect infarct, tumour,
or infection.
•
Ventricular system:assess the position for midline shift or compression.
High density suggests ventricular or subdural bleed. Enlargement is
suggestive of hydrocephalus. High density in the cisterns may suggest
blood, pus, or tumour.
•
Symmetry:asymmetry of the parenchyma suggests midlineshift.
• Falxshift.
Rule out skull fracture, extradural haematoma (lentiform shape), subdural
haematoma (crescentic shape), SAH (see Fig. 13.52), SOLs (see Fig. 13.53),
hydrocephalus, and cerebral oedema. Look for target lesions (when con-
trast given):metastases, abscesses, and fungal infections.
Cerebral angiography
Evaluation of vascular lesions, including atherosclerotic disease, aneurysms,
vascular malformations, and arterial dissection. Supplements information
obtained on CT/ MRI regarding tumour vascularity.
Conventional DSA is the gold standard for the assessment of neck and
intracranial vessels; however, it is an invasive technique requiring an arterial
(femoral) puncture. There is also potential for vessel injury at the time of
catheter manipulation (e.g. dissection, occlusion, or vasospasm). Used for
guidance of interventional procedures such as embolization.
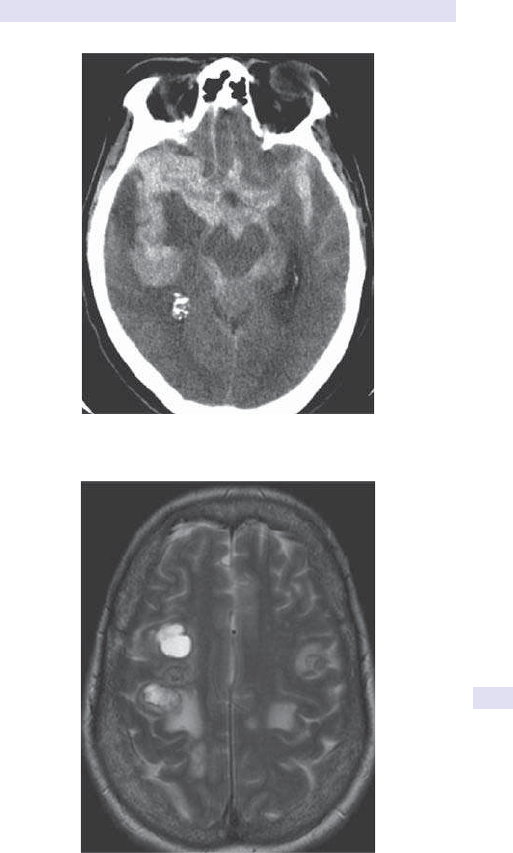
NEURORADIOLOGY
857
Fig.13.52 Non- contrast CT head showing extensive SAH in a patient with
underlying cerebral aneurysm (not shown).
Fig.13.53 Axial T2W MRI showing multiple high signal lesions in the deep white
matter of the right frontal lobe in a patient with lung carcinoma. The anterior lesion is
cystic. These are consistent with metastases. Note the surrounding oedema.

858
CHAPTER13 Radiology
858
Cerebrovascular accident
Imaging may be −ve in the rst 6h. Thereafter, lookfor:
• Oedema (loss of grey– white dierentiation, sulcal eacement, and mass
eect). Cytotoxic oedema develops within 6h and is seen onMRI.
•
Within 24h, there will be a low- density wedge- shaped area
corresponding to the vascular territory and extending to the cortical
surface. Vasogenic oedema within 12– 24h seenonCT.
• If ischaemic stroke, may see a hyperdense (bright) artery representing
an intravascular thrombus or embolus.
•
Acute haemorrhage seen if there is haemorrhagic transformation
(white=acute blood). Density on NECT is 80– 100HU relative to
normal brain (40– 50HU). There may be surrounding oedema. Will not
be hyperdense if low Hct (<8g/ dL). Subacute haemorrhage (3– 14days)
may be hyper- , hypo- , or isointense to brain. Chronic haemorrhage (>2
weeks) is hypodense.
•
Hypertensive haemorrhage typically seen in the pons in80%.
• Prognosis depends on size, brainstem location, and intraventricular
extension.
•
Advances in MR sequences have revolutionized stroke imaging. Diusion
sequences (DWI) and perfusion- weighted imaging (PWI) demonstrate
acute infarction, even in the context of a −veCT.
• T2W and uid attenuation inversion recovery (FLAIR) images show
oedema with high signal.
•
DWI shows high signal suggestive of cytotoxic oedema.
• Gradient echo identies acute haemorrhage, whereas fast FLAIR can
show acute subarachnoid blood. Time- of- ight angiography can non-
invasively assess underlying vessels. If the infarct is thought to be venous
(e.g. peripheral or haemorrhagic), then phase contrast MRV can exclude
sinus thrombosis.
In the case of strokes in the posterior circulation, thin sectional axial images
can exclude thrombosis or dissection within the vertebral arteries.
CT is important in the early stages of stroke evaluation to facilitate
thrombolytic therapy. Highly accurate in identication of proximal occlu-
sions in the circle of Willis and therefore aids triage to facilitate thromboly-
sis. Non- contrast CT is initially performed, as haemorrhage is an absolute
contraindication to thrombolytic therapy.
Multiple sclerosis
MRI is the modality of choice and is highly sensitive (>90%), but with low
specicity (71– 74%). There can therefore be overlap with other entities,
such as ischaemia, and conuent disease may simulate neoplastic mass
lesions. T2W MRI shows ovoid high signal lesions in a periventricular dis-
tribution. Conventional T2 may underestimate the plaque size and overall
plaque burden; advanced MRI techniques, such as DTI and MR spectros-
copy, can have greater utility. FLAIR sequences show lesions in a perive-
ntricular distribution by suppressing (CSF) signal. Active lesions show
enhancement following gadolinium. The spinal cord should also be screened
to exclude involvementbyMS.

NEURORADIOLOGY
859
Magnetic resonance imaging inthe paediatricbrain
Assessing the degree of myelination is an important part of excluding struc-
tural pathology in paediatric neurological disorders. MRI is the modality of
choice in brain tumours, congenital anomalies, and hypoxic– ischaemic dis-
orders (hypoxic– ischaemic encephalopathy (HIE)). DWI is useful in acute
HIE, whilst spectroscopy is critical in metabolic disorders.

860
CHAPTER13 Radiology
860
SkullX- ray
Indications
The main indication is for penetrating acute trauma, although SXRs are of
limited use in the era of widespread CT availability. Occasionally, the SXR
is obtained as part of a skeletal survey in the evaluation of metabolic bone
disease and endocrine disorders, and in the assessment of metastatic dis-
ease. It is still used in the assessment of sinus disease and in the evaluation
of the post- operative skull or for conrmation of hardware placement (see
Fig. 13.54).
Contraindications
None, but if there is suspicion of underlying intracranial injury, plain lms
are unnecessary (E
Fractures and associated ndings, p. 861).
Normal ndings
The bones of the skull vault have an inner and outer table of compact bone,
with spongy diploe between the two. Sutures are visible, even after fusion,
and should not be mistaken for fractures. Blood vessels may cause impres-
sions, as can small lucencies in the inner table near the vertex caused by
normal arachnoid granulations which can be mistaken for small lytic lesions.
Trauma
SXRs are basic and widely available, and yet potentially yield the least
information in the context of trauma. The presence or absence of a skull
fracture does not correlate with the presence or extent of any intracranial
injury. Up to 50% of lms may be technically unsatisfactory due to factors
such as poor patient co- operation. With the advent of CT, this is the tech-
nique of choice for evaluation in acute injury and neurological decit. It
allows a rm diagnosis to be made and excludes other alternate diagnoses.
Fig.13.54 Lateral SXR showing ‘hair- on- end’ appearance in thalassaemiamajor.

SKULLX-RAY
861
Fractures and associated ndings
Basic radiographs include a lateral projection (obtained with a horizontal
beam) and a further tangential projection, depending on the site of injury.
Findings include
• A linear fracture:well- dened margins, no branching, and no sclerosis
(cf. vascular markings or sutures that have an undulating course and
sclerotic margins).
•
A depressed fracture:i density due to overlapping bone; those that are
depressed by >5mm may lacerate the dura or cause parenchymal injury,
and therefore need elevation.
•
A uid level/ pneumocephalus:implies an associated basal skull fracture or
a duraltear.
Note: pineal displacement is an inconstant nding and is not a reliable
method of assessing the presence of intracranial injury.
Abnormal ndings
Look for intracranial calcication; then examine the pituitary fossa, review
the bony density, and look for focal areas of lysis and sclerosis.
•
Intracranial calcication:the majority is normal and of no clinical
signicance. However, it may be of pathological signicance— causes
include ° tumours, such as meningiomas, craniopharyngiomas, AVMs,
and tuberous sclerosis, and infections such as toxoplasmosis.
•
Raised ICP:in practice, plain lm changes are only seen if the condition is
long- standing. These include sutural widening (diastasis) and erosion of
the lamina dura of the pituitaryfossa.
•
Enlargement of the pituitary fossa (normal dimensions:height 6– 11mm,
length 9– 16mm):expansion will result in a double oor, loss of the
lamina dura, and elevation/ destruction of the clinoid processes. The
vast majority of the lesions will be pituitary adenomas; other causes
include meningiomas and aneurysms.
•
Bone lysis:may be diuse as in metastasis or myeloma. Large areas of
bone destruction are seen in histiocytosis X and in the active phase of
Paget’s disease (osteoporosis circumscripta).
•
Bone sclerosis:may be localized as in meningiomas, depressed skull
fractures, or generalized as in Paget’s sclerotic metastases, myeloma,
and uorosis.
•
Sutural widening:may be due to raised ICP, inltration by malignancy
(neuroblastoma or lymphoma), or defective ossication as in rickets.

862
CHAPTER13 Radiology
862
Reference section
For abbreviations, see Table 13.14. For a list of adverse reactions to con-
trast agents, see Table13.15.
Table13.14 Abbreviations used inradiology
AP Anteroposterior
AXR
Abdominal X- ray
CT Computed tomography
CXR
Chest X- ray
IVU Intravenous urogram
MRI Magnetic resonance imaging
P- A Posteroanterior
USS Ultrasound scan
V/ Q Ventilation perfusion scan
Table13.15 Management ofadverse reactions tointravascular
contrastagents
Symptoms Treatment
Nausea/ vomiting Reassurance
Urticaria
Antihistamine chlorpheniamine maleate 10– 20mg by slow IV
injection (0.2mg/ kg body weight paediatric dose)
Side eects:drowsiness and/ or hypotension may occur
Angio- oedema/
laryngeal oedema
Adrenaline (epinephrine) 1– 3mL 1:10,000 IV (slowly), 0.3–
0.5mL SC or IM (1:1000) solution
Protect airway and give O
2
Bronchospasm O
2
by mask (6– 10L/ min)
β2- agonists nebulized or metered- dose inhaler
Adrenaline (epinephrine) 0.1– 0.3 mg IM (1:1000)
Hypotension If isolated, O
2
by mask, IV access, and rapid IV uids
If unresponsive, adrenaline 1:1,000, 0.5mL (0.5mg)
IM
Vasovagal Hypotension and bradycardia
Elevate patient legs. O
2
by mask (6– 10L/ min)
Atropine 0.6– 1mg IV (up to maximum of3mg)
Normal saline 250– 500mL IV. Rapid infusion
Data sourced from Standards for intravascular contrast administration to adult patients. 3rd
edition RCR October2014.

REFERENCE SECTION
863
Order ofappearance ofossication centres oftheelbow
The order of appearance is more important than the absolute age of
appearance, which varies widely. Remember ‘CRITOE’ (see Table 13.16).
Fracture healing rapid inchildren
• Periosteal new bone:1week.
•
Loss of fracture line:2– 3weeks.
• Hard callus:2– 4weeks.
• Remodelling of bone:12months.
•
Types of paediatric fractures:torus (buckle) fractures— buckled
cortexonly.
•
Greenstick fracture:incomplete transverse fracture with intact
periosteum on concave side (ruptured on side of convexity).
•
Complete fracture.
Epiphyseal plate fractures:theSalter– Harris
classication (SALTR)
• Type I:epiphyseal slip separates it from the physis (5– 6%). S=slip of
physis.
•
Type II:fracture line extends into the metaphysis (50– 75%). A=above
physis.
•
Type III:the epiphysis is vertically split, i.e. the equivalent of an intra-
articular fracture (8%). L=lower than physis.
•
Type IV:fracture involves the metaphysis, epiphysis, and physis (8– 12%).
T=through physis.
• Type V:crush injury with vascular compromise, i.e. poor prognosis for
growth (1%). R=rammed physis.
Table13.16 Order ofappearance ofossication centres oftheelbow
Approximate average age (years)
Capitellum 1
Radial head
3– 6
Internal (medial) epicondyle 4
Trochlea 8
Olecranon 9
External (lateral) epicondyle 10

864
CHAPTER13 Radiology
864
Internet resources
National Radiological ProtectionBoard
For issues regarding radiation safety:M https:// www.gov.uk/ government/
collections/ national- radiological- protection- board- nrpb- report- series
Royal College ofRadiologists
For information relating to radiological issues, guidelines in clinical manage-
ment, training, and publications:M
http:// www.rcr.ac.uk
MRI safety and compatibility
M http:// www.mrisafety.com
Radiological Society ofNorth America
M http:// www.rsna.org
American College ofRadiology
Incorporates guidelines for use of contrast media and practice guidelines, as
well as information on technical standards (e.g. teleradiology):M
http://
www.acr.org
British Institute ofRadiology
M http:// www.bir.org.uk
American Roentgen Ray Society
M http:// www.arrs.org
Also includes an image library with peer- reviewed published images:
M
http:// goldminer.arrs.org/
General radiology resources
Educational website oering radiology cases, dierential diagnoses, etc.:
M Learningradiology.com
CT imaging/ protocols:M http:// www.ctisus.com

865
Chapter14
Nuclear medicine
Introduction to nuclear medicine 866
Bone scintigraphy:bone scan 868
Reticuloendothelial system:bone marrow scintigraphy 872
Cerebral blood ow imaging 874
Brain transporter imaging 876
Cerebrospinal uid shunt patency 878
Thyroid scintigraphy 880
Parathyroid scintigraphy 882
Meta- iodobenzylguanidine (MIBG) imaging 884
Somatostatin receptor scintigraphy:SPECT 886
Radioiodine thyroid cancer imaging 888
Sentinel node imaging 890
Scintimammography 892
Positron emission tomography (PET) 894
Somatostatin receptor scintigraphy:
68
Ga- DOTA- peptide
PET/ CT 898
Prostate- specic membrane antigen:
68
Ga- PSMA PET/ CT 900
11
C- choline/
18
F- uorocholine PET/ CT 902
18
F- uoride PET/ CT 904
Myocardial perfusion imaging 906
Radionuclide ventriculography:MUGA scan 910
Radionuclide rst- pass cardiac studies 912
Lung scan:ventilation/ perfusion imaging 914
Lung shunt studies 918
Lung permeability studies 920
Lymphoscintigraphy 922
Static cortical scintigraphy:dimercaptosuccinic acid imaging 924
Dynamic renography 926
Captopril renography 928
Gastrointestinal bleeding:labelled red cell imaging 930
Gastric emptying studies 932
SeHCAT studies 934
Meckel’s scan:ectopic gastric mucosa localization 936
Hepatobiliary scintigraphy 938
Splenunculus detection:heat- damaged red cell imaging 940
Hepatosplenic scintigraphy 942
Labelled leucocyte imaging 944
67
Gallium scintigraphy 946
Dacroscintigraphy 948
Salivary gland scintigraphy 950
Glomerular ltration rate measurement 952
Urea breath test 953
Red cell survival studies 954
Red cell volume/ plasma volume measurement 955
Bile salt deconjugation studies 956

866
CHAPTER14 Nuclear medicine
866
Introduction tonuclear medicine
Nuclear medicine techniques employ a carrier molecule, selected to target
the organ/ tissue of interest, tagged with a gamma- emitting radioisotope.
The labelled drug (radiopharmaceutical) is usually given PO or IV, although
it can also be administered interstitially or by inhalation. Its distribution is
mapped in vivo
using a gamma camera (scintigraphy) or, for non- imaging
tests, biological specimens are assayed in vitro using a radiation counter.
SPECT uses images collected, whilst rotating a gamma camera (usually
multi- headed) around the patient, then reconstructed mathematically to
produce 3D images. PET images are obtained from a ring of detectors fol-
lowing administration of a positron- emitting radiopharmaceutical.
Nuclear medicine procedures can detect early physiological responses
to disease processes, generally before structural changes have taken place,
and thus scintigraphy is often more sensitive than conventional radiology in
early disease. Specicity varies depending on the radiopharmaceutical used,
and characterization of abnormalities often relies upon pattern recognition
within a particular clinical setting. Anatomical detail is poor, compared with
conventional radiology, and increasingly single photon emission computed
tomography (SPECT) and positron emission tomography (PET) images are
co- registered with CT or MRI images using hybrid cameras.
Nuclear medicine procedures are non- invasive and allow the whole
body to be imaged during a single examination. Absorbed radiation doses
depend on the radiopharmaceutical used but are usually in the same range
as diagnostic radiology. Pregnancy is an absolute contraindication to nuclear
medicine examinations, except where likely clinical benet far outweighs
potential risk, e.g. lung perfusion imaging. Some radiopharmaceuticals are
excreted in breast milk, and additional precautions may be advisable for
lactatingwomen.
Diagnostic radiopharmaceuticals are used in tracer quantities and toxicity
is negligible. Individual hypersensitivity reactions are rare. The most widely
used radionuclide is technetium- 99m (
99m
Tc), which can be obtained from
an on- site generator, has a half- life of 6h, and is suitable for labelling a wide
variety of radiopharmaceuticals.
Specic information required whenrequesting nuclear
medicine tests includes
• Patient identication details.
• Examination requested.
• Relevant clinical history, including results of other investigations.
• Pregnancy/ lactation details where relevant.
• Special needs— visual/ hearing/ learning diculties; needle phobia.
• Some scanning beds have a weightlimit.

INTRODUCTION TO NUCLEAR MEDICINE
867
868
CHAPTER14 Nuclear medicine
868
Bone scintigraphy:bonescan
Background
Nuclear medicine investigations supplement the anatomical information
obtained from radiology. Bone scintigraphy is sensitive, with changes fre-
quently detected earlier than on plain lm, but non- specic. It plays a piv-
otal role in the staging of patients with malignant disease and in dicult
orthopaedic cases. May be restricted to ‘local views’ only, e.g. loose hip
prosthesis or ‘whole body imaging’ in metastatic screening. Can include a
dynamic phase image at the time of radiopharmaceutical administration, in
addition to the delayed bone phase image 2– 4h post- injection. Dynamic
phase reects blood ow to the site of interest— useful when there is con-
cern over vascularity, infection, or recent trauma. Helps to dierentiate soft
tissue from bone pathology, e.g. cellulitis vs osteomyelitis.
The
99m
Tc- bisphosphonate complex (MDP/ medronate, HDP/ oxidronate)
targets the skeleton with renal excretion of unbound activity. Patients with
poor renal function may need delayed imaging to improve the bone:soft
tissue ratio (see Fig.14.1).
Fig.14.1 Normal whole body
99m
Tc- bisphosphonate bone scan; anterior view on
left, posterior view onright.

BONE SCINTIGRAPHY:BONESCAN
869
Indications
• Tumour staging— assess skeletal metastases (see Fig.14.2).
• Bonepain.
• Trauma— when radiographs unhelpful.
• Prosthetic loosening, e.g. total hip replacement(THR).
• Infection.
• AVN.
• Paget’s disease to assess extent (monostotic or polyostotic) (see Fig.14.3).
• Sports injuries.
Patient preparation
Should be well hydrated and continent.
Procedure
Inject
99m
Tc- bisphosphonate complex IV. For suspected AVN or sepsis,
image immediately to assess vascularity. Otherwise, image 2– 4h later.
Whole body views are required for metastatic screening. Tomography
improves anatomical denition and detection of small lesions, e.g. osteoid
osteoma, and is particularly useful in back pain. Fusion with CT images is
being used increasingly.
Results
• Radiopharmaceutical uptake reects osteoblastic activity.
• Focal i uptake in sclerotic metastases, trauma, or infection.
•
Diuse i uptake associated with advanced metastases, Paget’s (local),
and metabolic bone disease.
•
Reduced tracer uptake in acute AVN and lytic bone metastases.
Interpretation
Sensitive, but non- specic. Interpretation relies on pattern recognition in
the clinical setting.
Advantages
Sensitive— detects early changes in bone physiology, often before abnormal
plain radiographs, e.g. occult trauma, metastases, and sepsis.
Pitfalls
False −ves in multiple myeloma and osteolytic bone metastases.
False +ves: artefacts due to urine contamination.
870
CHAPTER14 Nuclear medicine
870
Fig.14.3 Bone scan showing Paget’s disease aecting the right hemipelvis and left
distalfemur.
Fig.14.2 Bone scan showing extensive metastases.

BONE SCINTIGRAPHY:BONESCAN
871

872
CHAPTER14 Nuclear medicine
872
Reticuloendothelial system:bone
marrow scintigraphy
Background
Largely superseded by MRI. Colloid particles are cleared from the circulat-
ing blood pool by reticuloendothelial tissue— larger particles to the liver and
spleen, smaller particles to the BM. Abnormal uptake pattern where the BM
is replaced, e.g. by tumour inltration.
Indications
• Suspected malignant marrow inltration.
• Equivocal conventional bone imaging.
• Osteomyelitis (rarelyused).
Patient preparation
None.
Procedure
•
99m
Tc- nanocolloid injectedIV.
• Whole body gamma camera imaging at 30– 45min.
Results
Normal marrow distribution in the thoracic cage, spine, pelvis, and proximal
long bones. Homogeneous uptake in the liver and spleen.
Interpretation
• Focal or generalized d skeletal uptake indicates marrow replacement or
inltration with marrow displacement to the distal femora and humeri.
•
Heterogeneous hepatic uptake is abnormal but non- specic.
Advantages
Non- invasive. Avoids sampling errors, compared with BM biopsy.
Pitfalls
False −ves in early malignancy. Liver and spleen uptake may obscure abnor-
malities in the midspine.

RETICULOENDOTHELIAL SYSTEM:BONE MARROW SCINTIGRAPHY
873

874
CHAPTER14 Nuclear medicine
874
Cerebral blood flow imaging
Background
Used to study acute and chronic cerebrovascular disease, dementias, and
epilepsy, using a neutral lipophilic
99m
Tc complex hexamethyl- propylene-
amine- oxime (exametazime, HMPAO), which crosses the blood– brain bar-
rier and xes in proportion to regional blood ow, with high accumulation
in cortical grey matter, compared with white matter. Images reported with
CT/ MRI correlation to ensure appropriate interpretation, compared with
normal variants of cerebral asymmetry).
Indications
• Dementia characterization.
• Epilepsy for localization of epileptogenicfocus.
Patient preparation
Secure venous access under resting conditions. Allow the patient to relax
before injection of the radiopharmaceutical. Ensure that the patient can co-
operate with the imaging procedure.
Procedure
•
99m
Tc- HMPAO (exametazime) injected IV in a quiet, darkened room,
with the patient’s eyes closed.
•
Tomographic brain imaging undertaken immediately and may be
repeated 4hlater.
Results
Cortical grey matter uptake is proportional to blood ow (see Fig. 14.4a).
Interpretation
• Characteristic patterns of abnormal uptake recognized in dierent
dementias (see Fig. 14.4b) and in cerebrovascular disease.
•
d uptake at epileptogenic focus on interictal scans— often changing
to i uptake on ictal imaging.
Advantages
Abnormalities on functional imaging should predate structural atrophy on
anatomical imaging.
Pitfalls
• Tomographic image analysis degraded by movement artefact and
asymmetric positioning.
•
Data processing is operator- dependent.
Further reading
Kapucu OL, Nobili F, Varrone A, etal. EANM procedure guideline for brain perfusion SPECT using
99m
Tc- labelled radiopharmaceuticals, version 2. Eur J Nucl Med Mol Imaging 2009; 36:2093– 102.
CEREBRAL BLOOD FLOW IMAGING
875
(a)
(b)
Fig.14.4
99m
Tc- HMPAO brain imaging. Trans- axial tomographic slices:(a)normal
and (b)dementia. (E Colour plate6.)

876
CHAPTER14 Nuclear medicine
876
Brain transporter imaging
Background
Reects the role of the dopaminergic system in movement disorders, e.g.
Parkinsonian syndromes, essential tremor.
123
I- ioupane is a cocaine ana-
logue, which binds to the dopamine transporter on the pre- synaptic nerve
terminal. Post- synaptic receptor imaging agents are necessary to dierenti-
ate among the various Parkinsonian syndromes (not routinely available).
Indications
• Movement disorders:distinguishes Parkinson’s syndrome (PkS) from
benign essential tremor.
Patient preparation
• Block the thyroid— potassium iodate/ iodide.
• Multiple potential drug interactions— stop:
•
Amphetamines.
•
Citalopram.
•
Cocaine.
•
Fluoxetine.
•
Fluvoxamine.
•
Mazindol.
•
Methylphenidate.
•
Orphenadrine.
•
Phentermine.
•
Procyclidine.
•
Sertraline.
Procedure
123
I- labelled radiotracer, e.g. ioupane, injected IV. Tomographic gamma
camera imaging 3– 6hlater.
Results
Intense, symmetric uptake in basal ganglia receptors— striatum, caudate,
and putamen (see Fig. 14.5a).
Interpretation
d basal ganglia uptake in PkS (see Fig. 14.5b).
Advantages
Sensitive and specic for PkS, dierentiating PkS from essential tremor. No
other imaging technique currently available to demonstrate receptor status.
Pitfalls
Drug interactions (E Patient preparation above).
BRAIN TRANSPORTER IMAGING
877
Further reading
Darcourt J, Booij J, Tatsch K, etal. EANM procedure guidelines for brain neurotransmission SPECT
using
123
I- labelled dopamine transporter ligands, version 2. Eur J Nucl Med Mol Imaging 2010;
37
:443– 50.
(a)
(b)
Fig.14.5
123
I- ioupane brain transporter imaging:(a)normal dopamine transporters
and (b)in Parkinson’s disease. (E Colour plate7.)

878
CHAPTER14 Nuclear medicine
878
Cerebrospinal fluid shunt patency
Background
Ventricular shunts are routinely used to manage hydrocephalus. Symptoms
of shunt obstruction may be non- specic and do not indicate the level of
obstruction. Nuclear medicine oers a straightforward means of determin-
ing shunt patency.
Indications
Suspected ventriculoperitoneal shunt obstruction.
Patient preparation
None.
Procedure
Inject
111
In- DTPA into the shunt reservoir using a strict aseptic technique.
Image the head and abdomen immediately and 30– 60min post- injection.
(
99m
Tc- DTPA not recommended because dicult to ensure apyrogenicity.)
Results
Normally, rapid reservoir emptying and shunt visualization within 2– 3min of
injection. Free activity within the abdominal cavity by 30min (see Fig.14.6).
Interpretation
Delayed clearance implies obstruction— level usually at reservoir/ proximal
shunt or due to intra- abdominal kinking.
Advantages
Sensitive, simple, rapid results.
Pitfalls
Infectionrisk.
CEREBROSPINAL FLUID SHUNT PATENCY
879
Reservoir
Thorax
Free activity
in peritoneal
cavity
Fig.14.6 Patent ventriculoperitoneal shunt showing reservoir, shunt, and free
activity within the peritoneal cavity.

880
CHAPTER14 Nuclear medicine
880
Thyroid scintigraphy
Background
Used to investigate thyrotoxicosis and thyroid ectopia. Thyroid mass or
suspected malignancy should be investigated by US/ CT and FNA cytology.
Imaging is undertaken using
99m
Tc- pertechnetate, which is trapped by the
thyroid by the same transporter mechanism as iodine but, unlike iodine,
is not organied.
123
I is more sensitive and specic for the investigation of
congenital hypothyroidism.
Indications
• Characterization of thyrotoxicosis— diuse toxic goitre (Graves’
disease), toxic multinodular goitre (Plummer’s disease).
•
Solitary autonomous nodule.
• Acute thyroiditis.
Patient preparation
Levothyroxine and iodine- rich preparations (e.g. iodine supplements, con-
trast media, amiodarone) will block tracer uptake by the thyroid for up
to 9 months. T4 should be withdrawn for 4–6 weeks, T3 for 2 weeks.
Antithyroid drugs can be continued.
Procedure
Inject
99m
Tc- pertechnetate IV. Image after 15– 30min. Include anterior thorax
views if retrosternal extension is suspected. Neck palpation essential to
assess function in discrete thyroid nodules.
123
I may be administered either
PO or IV. Imaging is carried outat2h.
Results
• Uptake reects function of the thyroid iodine trap (sodium iodide
symporter).
•
Diuse i uptake in Graves’ disease (see Fig. 14.7a).
•
Heterogeneous uptake with suppressed background activity indicates
toxic multinodular change (see Fig. 14.7b).
•
Solitary autonomous nodules show intense i uptake, with complete
suppression of the remainder of the gland (see Fig. 14.7c).
•
Reduced tracer uptake in non- functioning nodules (see Fig. 14.7d).
• Acute thyroiditis is characterized by absent tracer uptake
(see Fig. 14.7e).
Interpretation
Sensitive and specic for hyperthyroidism.
Advantages
Simple, cheap, and non- invasive. Essential to planning therapy in
hyperthyroidism.
Pitfalls
Anatomical denition inferior to US, CT, etc. Superseded by US- guided
FNA for diagnosis of thyroid mass lesions.
THYROID SCINTIGRAPHY
881
(a) Grave’ disease
(c) Toxic autonomous nodule
(e) Thyroiditi
s
(d) Non-functioning nodule
(b) Toxic multinodular goitre
Fig.14.7 Thyroid scintigraphy:(a)in Graves’ disease (toxic diuse hyperplasia)
and (b)in toxic multinodular goitre, (c)toxic autonomous nodule (arrow), (d)non-
functioning (cold nodule; arrow), and (e)thyroiditis (arrow).

882
CHAPTER14 Nuclear medicine
882
Parathyroid scintigraphy
Background
Hyperparathyroidism may be ° (i.e. functioning adenoma), 2° (where
there is hypocalcaemia from chronic lowering of serum Ca
2+
, e.g. renal
insuciency), or tertiary (i.e. hypersecretion of PTH in the presence of
either normal or elevated levels of Ca
2+
). Nuclear medicine is of value in
localizing parathyroid adenomas, particularly following previous surgery.
Indications
Localization of parathyroid adenoma in proven hyperparathyroidism.
Patient preparation
Withdraw levothyroxine or iodine- containing compounds (cf. thyroid
imaging).
Procedure
Three dierent techniques are available.
•
99m
Tc- sestamibi IV early (15 min) and delayed (2–3 h) imagesor
•
123
I- iodide IV followed by
99m
Tc- sestamibi,or
•
99m
Tc- pertechnetate followed by
201
Tl- thallous chloride.
Image the anterior neck and mediastinum after each administration.
Results
Normal thyroid concentrates
123
I,
99m
Tc- pertechnetate,
99m
Tc- sestamibi
(initially), and
210
Tl, whereas parathyroid only concentrates
99m
Tc- sestamibi
and
201
Tl.
Computer- assisted image subtraction [(thyroid + parathyroid) − thyroid]
identies abnormal parathyroid tissue.
Interpretation
Parathyroid adenoma shown as hyperfunctioning nodule(s) (see Figs
14.8– 14.10.)
Rt
(a) (b)
Rt
Fig.14.8 Normal
99m
Tc- sestamibi dual- phase parathyroid scan; 5min image on left,
4h image onright.
PARATHYROID SCINTIGRAPHY
883
Advantages
Good when other imaging fails, particularly ectopic adenomas and after
unsuccessful neck exploration. Hybrid SPECT/ CT images particularly help-
ful to surgeons.
Pitfalls
Multinodular thyroid prevents subtraction analysis in smaller adenomas and
hyperplastic glands and thyroid nodules. Many surgeons still prefer intra-
operative bluedye.
False –ves in smaller adenomas and hyperplastic glands.
False +ves in thryoid nodules.
Further reading
Hindié E, Ugur O, Fuster D, etal. 2009 EANM parathyroid guidelines. Eur J Nucl Med Mol Imaging
2009; 36
:1201– 16.
(a) (b)
Fig.14.9
99m
Tc- sestamibi (dual- phase). Parathyroid adenoma at left lower lobe of
the thyroid (arrow) evident on delayedimage.
(a)(b) (c)
Fig.14.10 Parathyroid scintigraphy showing images obtained with both (a)
123
I- iodide,
(b)
99m
Tc- sestamibi, and (c) subtracted image showing a parathryoid adenoma.

884
CHAPTER14 Nuclear medicine
884
Meta- iodobenzylguanidine (MIBG)
imaging
Background
Neuroendocrine tumours (NETs) are rare. Symptoms reect hormone
hypersecretion, but intermittent secretory patterns can result in false −ve
screening tests, e.g. 24h urine collections. MIBG (iobenguane) is concen-
trated by adrenergic tissue via the noradrenaline reuptake mechanism.
Virtually all phaeochromocytomas and neuroblastomas will be demon-
strated using
123
I- MIBG scintigraphy. The sensitivity for other NETs is vari-
able. High- dose
131
I- MIBG therapy is a useful treatment for MIBG +veNETs.
Indications
• Localization, staging, and response monitoring of neuroectodermal
tumours.
•
Phaeochromocytoma (imaging investigation of choice).
• Neuroblastoma.
• Carcinoid tumours.
• Medullary thyroid cancer.
Patient preparation
• Multiple known and theoretical drug interactions:Stop for>48h:
•
Antidepressants— tricyclics, tetracyclics, MAOIs, serotonin reuptake
inhibitors.
•
Phenothiazines.
•
Labetalol (no interaction with any other α- or β- blocker or
antihypertensive).
•
Levodopa, dopamine agonists.
•
Sympathomimetics— including over- the- counter nasal decongestants.
• Block the thyroid:potassium iodate/ iodide; perchlorate.
Procedure
Inject
123
I- MIBG slowly IV with BP monitoring. Image the posterior abdo-
men at 5min to identify renal outlines, then whole body imaging at 18–
24h. Tomographic imaging may improve tumour localization— not always
required.
Results
Physiological uptake at 24h in the salivary glands, myocardium, liver, and
normal adrenals, with gut and renal excretion.
Interpretation
• Intense i uptake in phaeochromocytomas, with suppressed activity in
the contralateral and normal adrenal, and myocardium. Whole body
imaging identies extra- adrenal and metastatic disease (see Fig. 14.11).
• Diuse BM uptake common in stage IV neuroblastoma.
META-IODOBENZYLGUANIDINE (MIBG) IMAGING
885
Advantages
Sensitive, non- invasive tumour localization preoperatively excludes multi-
focal and extra- adrenal tumours. Non- invasive treatment response moni-
toring in neuroblastoma— avoids sampling errors, compared with BM
biopsy.
Pitfalls
Drug interactions causing false −ve results. Dilated renal pelvis sometimes
confused with tumour uptake. Check with 5min renal image if indoubt.
Fig.14.11
123
I- MIBG whole body scan.
123
I-MIBG positive left adrenal mass
(phaeochromocytoma). Excludes multi- focal, ectopic, and malignant tumour.

886
CHAPTER14 Nuclear medicine
886
Somatostatin receptor
scintigraphy:SPECT
Background
Somatostatin receptor (SSR) imaging identies NETs, including gastroenter-
opancreatic tumours, e.g. carcinoids, gastrinomas, insulinomas. Many other
common neoplasms also express surface SSRs. Somatostatin analogues,
e.g. octreotide, bind to cell surface SSRs. Radiolabelled SSR analogues
demonstrate receptor +ve disease. Sub- centimetre (i.e. below the limits
of cross- sectional radiology) hyperfunctioning ° tumours can be detected.
High- dose radiolabelled SSR therapy is used to treat multi- site disease.
Indications
Localize and stage NETs, e.g. carcinoid, insulinoma, gastrinoma, phaeochro-
mocytoma, and medullary thyroid cancer.
Patient preparation
None. Prophylactic laxatives at time of radiopharmaceutical administration
accelerate gut clearance and improve image quality.
Procedure
Inject
111
In- pentetreotide or
99m
Tc hynic- octreotide (SSR analogues) IV.
Whole body gamma camera imaging at 4 and 24h (± 48h), with SPECT if
necessary.
Results
Normal uptake in the thyroid, liver, spleen, kidneys, and RES, with gut and
renal excretion.
Interpretation
i uptake in tumours expressing surface SSRs. SPECT improves detection of
small pancreatic and intra- hepatic tumours (see Fig. 14.12).
Advantages
Tumour uptake predicts symptom response to somatostatin analogue ther-
apy. Image co- registration with CT or MRI improves localization of occult
pancreaticNETs.
Pitfalls
Interpretation often hindered by gut excretion.
Further reading
Bombardieri E, Ambrosini V, Aktolun C, etal.
111
In- pentetreotide scintigraphy:procedure guidelines
for tumour imaging. Eur J Nucl Med Mol Imaging 2010; 37:1441– 8.
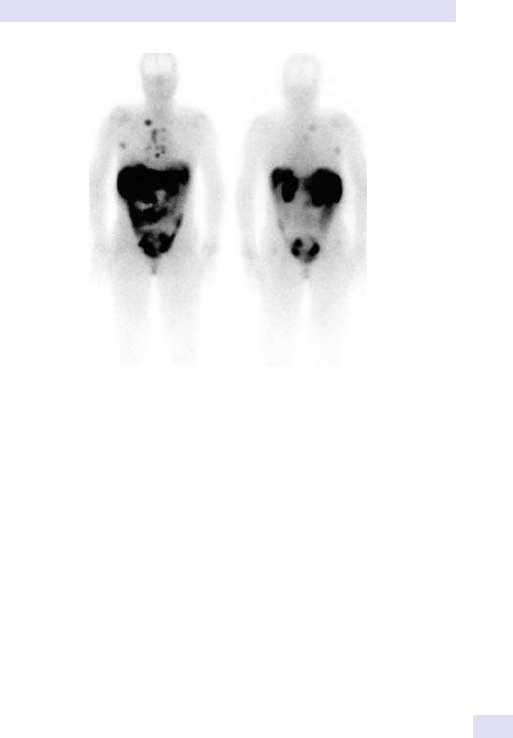
SOMATOSTATIN RECEPTOR SCINTIGRAPHY:SPECT
887
Fig.14.12 Whole body
111
In- octreotide scan in a patient with a neuroendocrine
tumour showing somatostatin receptor positive hepatic and extra-hepatic
metastases.

888
CHAPTER14 Nuclear medicine
888
Radioiodine thyroid cancer imaging
Background
The treatment for thyroid carcinoma is total thyroidectomy with lymph
node dissection, depending on tumour stage. Radioactive iodine is adminis-
tered post- operatively to ablate the thyroid remnant. Tg can then be used
as a tumour marker— Tg is undetectable in the absence of functioning thy-
roid tissue. Rising Tg following
131
I ablation indicates recurrence. If Tg rises,
a diagnostic
131
I imaging study localizes the site of relapse and assesses the
feasibility of further radioiodine therapy. The sensitivity of imaging is i by
recombinant TSH stimulation.
Indications
Routine dierentiated follicular thyroid cancer follow- up, after surgery and
131
I thyroid remnant ablation.
Patient preparation
Need high TSH drive to stimulate
131
I uptake— stop T3/ T4 replacement
for a minimum of 2 (T3) or 4-6 weeks (T4), or give recombinant TSH.
Avoid cold iodine administration, IV contrast media, and amiodarone (cf.
thyroid imaging).
Procedure
Give
131
I sodium iodide PO/ IV. Obtain blood samples for Tg and TSH
at the time of
131
I administration. Whole body gamma camera imaging
2– 5dayslater.
Results
Physiological uptake in salivary glands. Occasional stomach and GI reten-
tion. Renal excretion.
Interpretation
Abnormal uptake indicates functioning thyroid metastasis. Anatomical
markers improve localization (see Fig. 14.13a andb).
Advantages
Detects residual tumour and identies patients likely to benet from
131
I
therapy.
Pitfalls
False −ve without signicant TSH drive— aim for TSH >50mU/ L; undif-
ferentiated and papillary tumours may be
131
I−ve.
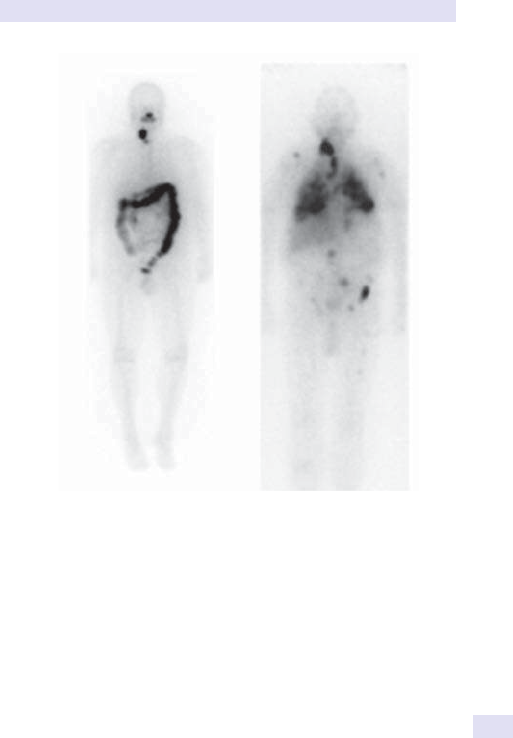
RADIOIODINE THYROID CANCER IMAGING
889
RT
(a) (b)
Fig.14.13 Anterior whole body
131
I image showing (a)local tumour recurrence in
the thyroid bed and physiological activity in the bowel and (b)local recurrence in the
thyroid bed with lung, mediastinal nodal, and skeletal metastases.

890
CHAPTER14 Nuclear medicine
890
Sentinel node imaging
Background
Regional lymph node dissection is performed for cancer staging to deter-
mine the need for adjuvant therapy. Lymphatic drainage can be demon-
strated by radiolabelled colloid imaging, which identies the rst or ‘sentinel’
draining node. Staging based on the excision and histological examination of
this node for evidence of metastasis is as reliable as that obtained from block
dissection and avoids the morbidity of extended lymph node dissection.
Indications
Preoperative assessment in breast cancer and melanoma. May have applica-
tions in head and neck, vulval, and penile cancer staging.
Patient preparation
None. Usually undertaken within 24h of planned surgery.
Procedure
Intradermal, subcutaneous, or intratumoural injection of
99m
Tc- labelled
nanocolloid. Gamma camera imaging of draining lymph nodes to identify
sentinel node. Where surgery is undertaken within 24h, an intra- operative
gamma probe can be used to identify the sentinel node for staging excision
biopsy.
Results
Sentinel node usually identiable 15min to 2h post- injection, depending on
the ° tumour location and injection techniqueused.
Interpretation
The sentinel node is the rst lymph node identied on gamma imaging or
the node with the highest radioactive count rate using the gamma probe
(see Fig. 14.14a andb).
Advantages
Accurate sentinel node identication avoids block node dissection where
this is undertaken solely for tumour staging.
Pitfalls
May fail if local lymphatic channels have been disrupted by previous surgery.
Further reading
Procedure guidelines for several types of cancer are available at:M http:// www.eanm.org.
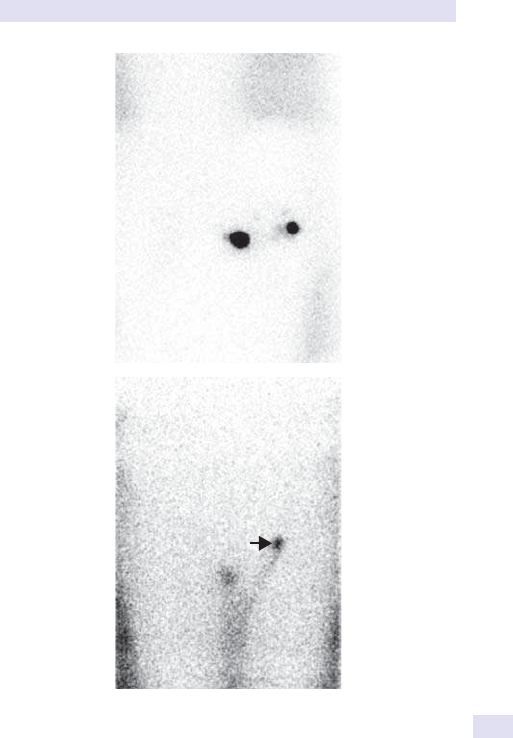
SENTINEL NODE IMAGING
891
(a)
(b)
RT
LT
A NODE
Fig.14.14
99m
Tc- nanocolloid sentinel node study. (a) Breast cancer: left breast injection
and sentinel lymph node in left axilla (arrow). (b) Melanoma left thigh: left thigh injection
(not shown) and sentinel lymph node in left groin (arrow).

892
CHAPTER14 Nuclear medicine
892
Scintimammography
Breast cancer diagnosis relies on accurate localization (US ± mammog-
raphy) and tissue biopsy (FNA) or core biopsy. Where mammography is
non- diagnostic, MRI, CT, or
18
F- FDG PET/ CT are useful. Nuclear medi-
cine scintimammography is as sensitive as, but more specic than, MRI and
mammography in palpable lesions.
Indications
Investigation of suspicious breast lesions, in dicult- to- interpret mammo-
grams, e.g. dense or lumpy breast tissue, calcication, breast implants, pre-
vious surgery.
Patient preparation
None.
Procedure
99m
Tc- sestamibi administered IV ideally into the contralateral foot, with early
(5– 10min) imaging post- injection. Patient is imaged prone, with the breast
fully dependent, with prone and lateral views of each breast, to include the
axillae.
Results
Normal distribution of
99m
Tc- sestamibi is to the myocardium, the liver, and
occasionally the thyroid.
Interpretation
Focal accumulation in the breast and/ or axilla implies the presence of a
tumour. Findings should be interpreted in conjunction with othertests.
Advantages
Can demonstrate multi- focal, multicentric disease, and both ipsilateral and
contralateral axillary spread. May be used to identify the most suitable site
for guided biopsy.
Pitfalls
Not reliable in small (<1cm) lesions; extravasation of injection in upper
limbs may result in false +ve axillary uptake.
Poor injection technique will lead to errors in analysis. The camera and
supporting software require high count rate capability, and the technique
requires expertise in data analysis to ensure reliable, reproducible results.
Close liaison with the referring clinician is essential to maximize the value
of the investigation.
Further reading
Buscombe J, Hill J, Parbhoo S. Scintimammography: A Guide to Good Practice. Birmingham: Gibbs
Associates Ltd,1998.
Taillefer R. Clinical applications of
99m
Tc- sestamibi scintimammography. Semin Nucl Med 2005;
35
:100– 15.

SCINTIMAMMOGRAPHY
893

894
CHAPTER14 Nuclear medicine
894
Positron emission tomography (PET)
PET has expanded rapidly over the last 20years. Tomographic PET images
are acquired using a dedicated PET scanner after administration of positron-
emitting radiopharmaceuticals. Spatial resolution (75mm) is signicantly
superior to conventional nuclear medicine imaging.
PET uses biologically important molecules such as radiolabelled water,
ammonia, amino acids, or glucose derivatives— the glucose analogue
18
F-
uorodeoxyglucose (
18
F- FDG) being used for most clinical PET studies,
particularly in oncology. Malignant cells have both a higher glycolytic rate
and over- expression of membrane glucose transporters, leading to high
18
F- FDG uptake, compared to normal tissues.
18
F- FDG is trapped within
metabolically active cells, so that abnormalities are detected by metabolic
dierences, rather than anatomical size, i.e. inherently more sensitive than
structure- based imaging.
The main indications for PET imaging are in oncology where
18
F- FDG
PET is used for diagnosis, staging, and monitoring treatment response. Low-
grade uptake occurs in granulomatous disease, inammation, and sepsis.
PET allows radioactive concentrations within tissues to be measured
accurately, so that physiological processes can be expressed in absolute
units. The standardized uptake value (SUV) measures the concentration of
tracer within a tumour, compared to the injected activity, normalized to
body weight. Serial SUV measurements allow
18
F- FDG uptake to be fol-
lowed as a marker of treatment response and may be of prognosticvalue.
Other major applications include nuclear cardiology where patterns of
myocardial
18
F- FDG uptake are used to detect myocardial hibernation. In
neurosciences, PET remains a largely research tool for the investigation of
movement disorders, dementia, and degenerative disease.
Other oncological tracers that are available for clinical use include
11
C-
methionine, measuring tumour amino acid transport and protein synthesis
and H
2
15
O water for blood ow measurements.
68
Ga- labelled somatostatin
peptides are used to image NETs. Future developments will include labelled
thymidine analogues (e.g.
18
F- FLT) to measure proliferation, hypoxia mark-
ers, and tracers capable of detecting apoptosis and angiogenesis.
The main limitation of PET imaging in oncology is limited anatomical de-
nition. To improve attenuation correction and tomographic localization,
PET imaging is usually combined with CT (or MRI). The combination of
functional and anatomical data in fused images signicantly improves the
sensitivity and specicity of imaging.
Indications
• Tumour diagnosis:solitary pulmonary nodule characterization, location of
carcinoma of unknown ° origin.
•
Tumour staging:non- SCLC; lymphoma; oesophageal cancer; colorectal
cancer; head and neck cancer; melanoma.
•
Response assessment/ relapse detection:asabove.

POSITRON EMISSION TOMOGRAPHY
895
Patient preparation
• Cellular
18
F- FDG uptake is glucose- dependent.
•
Non- diabetic patients— 6hfast.
•
Aim for blood glucose <7mmol/ L.
•
Insulin- dependent diabetic patients— allow a normal diet and insulin.
•
Avoid hyperglycaemia— use a sliding scale if necessary or defer the
investigation.
•
Patients should be rested:to avoid skeletal muscle uptake.
•
Diazepam administration:5mg PO reduces physiological brown fat
uptake in young patients.
•
Patients with head and neck cancer should be silent during injection and
until completion of imaging to prevent vocal cord uptake.
Procedure
•
18
F- FDG injected IV supine in restful surroundings.
• Whole body or half- body (base of skull to proximal femora) imaging
undertaken 60– 90min post- injection.
Results
The normal distribution of
18
F- FDG is to the brain and myocardium, with GI
and renal excretion (see Fig. 14.15).
Interpretation
Abnormal uptake should be correlated with cross- sectional imaging for pre-
cise anatomical localization (see Figs 14.16 and 14.17). SUV (E pp. 894–7)
measurement is helpful in serial studies.
Fig.14.15 Normal
18
F- FDG PET scan— coronal tomogram.
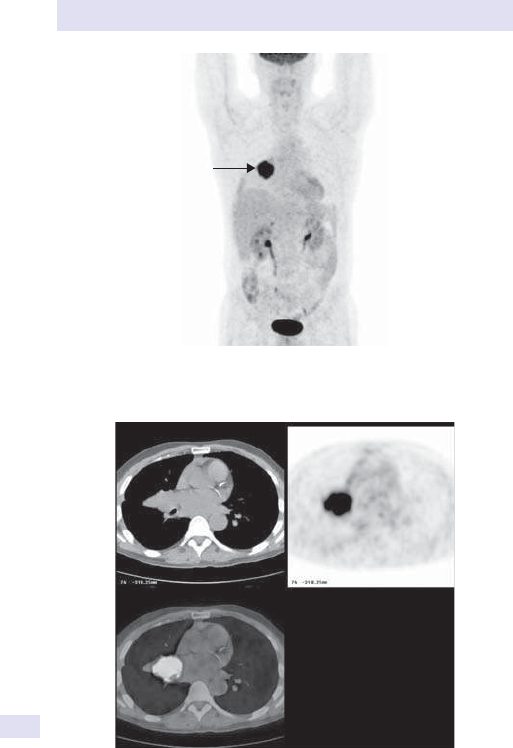
896
CHAPTER14 Nuclear medicine
896
Fig.14.16
18
F- FDG PET scan— non- SCLC right lung (arrow). Whole body coronal.
Fig.14.17
18
F- FDG PET scan— trans- axial views showing CT (top left) correlation
with PET (top right). The image fusion is shown (bottom left). (E Colour plate8.)
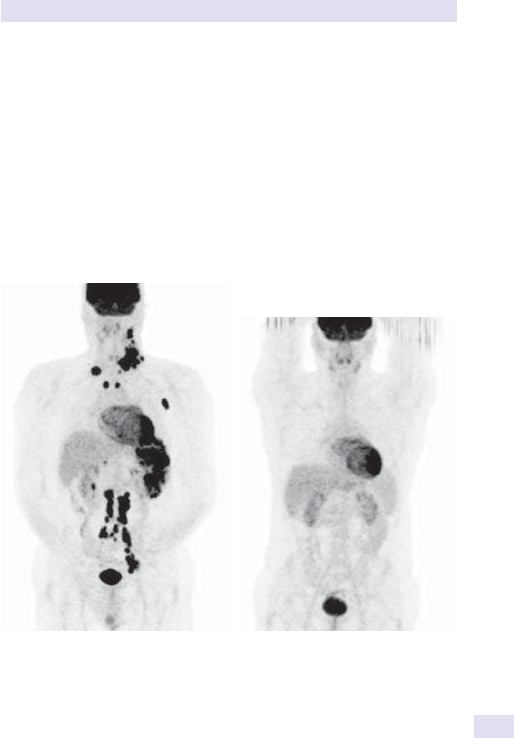
POSITRON EMISSION TOMOGRAPHY
897
Advantages
• Discriminates between viable and necrotic/ scar tissue where a residual
mass may persist on anatomical imaging post- therapy (see Figs 14.18
and 14.19).
•
Non- invasive, whole body 3D imaging.
Pitfalls
• Sensitivity lower in more indolent tumours— lung carcinoid, alveolar cell
carcinoma, NETs, depending on proliferative index, and occasionally in
pancreatic tumours.
•
Limited availability— specialist centresonly.
• Relatively expensive.
Fig.14.18
18
F- FDG PET scan— non-
Hodgkin’s lymphoma. Whole body
coronal view of patient pre- treatment
showing extensive FDG- avid adenopathy.
Fig.14.19
18
F- FDG PET scan— non-
Hodgkin’s lymphoma. Post- treatment
showing complete metabolic response.

898
CHAPTER14 Nuclear medicine
898
Somatostatin receptor scintigraphy:
68
Ga- DOTA- peptidePET/ CT
Background
SSR imaging identies NETs, including gastroenteropancreatic tumours,
e.g. carcinoids, gastrinomas, insulinomas. Many other common neoplasms
also express surface SSRs. Somatostatin analogues, e.g. octreotide, bind to
cell surface SSRs. Radiolabelled SSR analogues demonstrate receptor +ve
disease. High- dose radiolabelled SSR therapy is used to treat multi- site dis-
ease. Currently, given its high accuracy, compared with conventional SPECT
imaging techniques,
68
Ga- DOTA- peptide PET/ CT is considered to be the
rst- line diagnostic imaging technique of choice for high SSR- expressing
tumours.
Indications
Localize and stage NETs, e.g. carcinoid, insulinoma, gastrinoma, phaeochro-
mocytoma, and medullary thyroid cancer.
Patient preparation
Best option is to perform the study after discontinuing short- acting octreo-
tide for 12– 24h and perform imaging in the week before the next dose of
long- acting octreotide.
Procedure
Inject
68
Ga- labelled SSR analogue (DOTA- TATE/ DOTA- NOC) IV. Whole
body (vertex to mid thigh) PET/ CT imaging SSR PET/ CT is performed
45– 60min after radiotracer injection.
Results
Normal uptake in the spleen, adrenal glands, kidneys, and pituitary gland.
Moderately intense uptake is often seen in the liver, salivary glands, and
thyroid gland (see Fig. 14.20a).
Interpretation
i uptake in tumours expressing surface SSRs (see Fig. 14.20b).
Advantages
Short half- life of 68min (compared with 2.8days for
111
In); lower radiation
dose to the patient; imaging performed 45– 60min after radiotracer injec-
tion; more accurate than conventional imaging; and allows identication of
additional sites of disease and helps identify patients who are suitable for
radiopeptide therapy (e.g.
177
Lu- DOTA- TATE).
Pitfalls
False −ve:octreotide therapy or the endogenous production of somatosta-
tin by the tumour may interfere with tumour detection. False +ve:promi-
nent pancreatic uncinate process uptake, benign meningioma inammation,
osteoblastic activity (degenerative bone disease, fracture, vertebral hae-
mangioma), splenunculi or splenosis,etc.
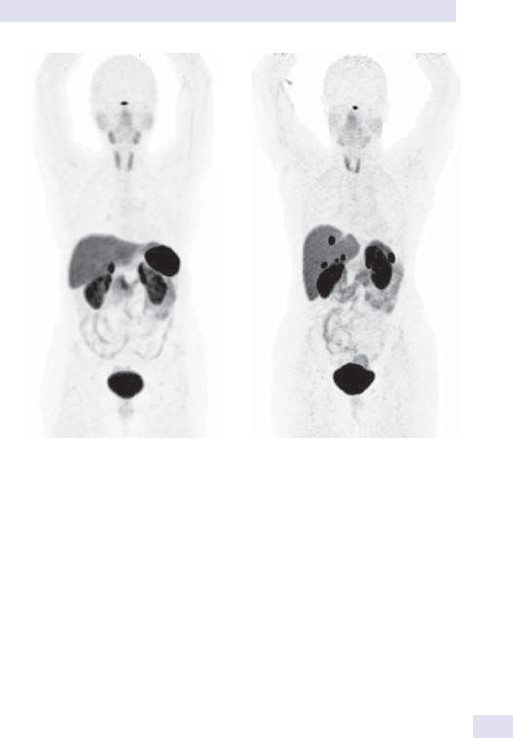
SOMATOSTATIN RECEPTOR SCINTIGRAPHY
899
Further reading
Virgolini I, Ambrosini V, Bomanji JB, etal. Procedure guidelines for PET/ CT tumour imaging with
68
Ga- DOTA- conjugated peptides:
68
Ga- DOTA- TOC,
68
Ga- DOTA- NOC,
68
Ga- DOTA- TATE. Eur
J Nucl Med Mol Imaging 2010; 37
:2004– 10.
(a) (b)
Fig.14.20 (a)Normal
68
Ga- DOTA- TATE scan; (b)abnormal
68
Ga- DOTA- TATE
scan showing liver metastases.

900
CHAPTER14 Nuclear medicine
900
Prostate- specific membrane
antigen:
68
Ga- PSMAPET/ CT
Background
Prostate- specic membrane antigen (PSMA) is a cell surface protein with
high expression in prostate cancer cells, and over- expression enables tar-
geting of prostate cancer metastases using
68
Ga- labelled PSMA ligands for
PET/ CT imaging.
Indications
High- risk prostate cancer prior to radical prostatectomy, patients with rising
PSA levels after radical prostatectomy, and diagnosis of recurrent prostate
cancer.
Patient preparation
None.
Procedure
68
Ga- PSMA is injected IV and PET/ CT performed 60min post- injection.
Results
Physiological uptake in kidneys and salivary glands. i uptake in prostate can-
cer cells expressing PSMA (see Fig. 14.21a).
Interpretation
i tracer uptake in tumours expressing PSMA (prostate and extra- prostatic)
(see Fig. 14.21b).
Advantages
Superior to choline tracers in detecting prostate cancer cells and recurrent
disease at low PSA levels.
Pitfalls
False −ve:limited detection of micrometastases. False +ve:PSMA is also
expressed in tumour- associated neovasculature of most solid tumours.
Further reading
Maurer T, Gschwend JE, Rauscher I, etal. Diagnostic ecacy of
68
Gallium- PSMA positron emission
tomography compared to conventional imaging in lymph node staging of 130 consecutive patients
with intermediate to high risk prostate cancer. J Urol 2016; 195
:1436– 43.
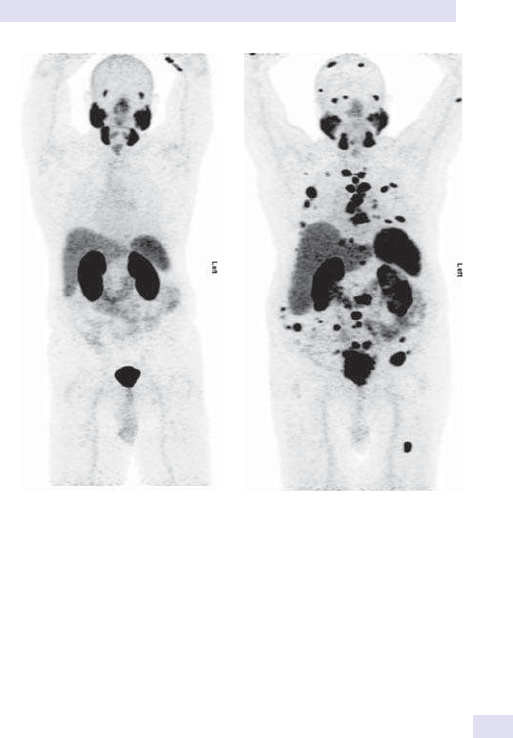
PROSTATE-SPECIFIC MEMBRANE ANTIGEN
901
(a) (b)
Fig.14.21 (a)Normal
68
Ga- PSMA scan; (b)abnormal scan with multiple osseous
and extra- osseous metastases.

902
CHAPTER14 Nuclear medicine
902
C- choline/
8
F- fluorocholinePET/ CT
Background
Following radical prostatectomy for treatment of prostate cancer, recurrent
disease is suspected if there is rising PSA.
18
F- choline PET/ CT is often used
in restaging of patients with prostate cancer (
11
C- choline or
11
C- acetate PET
can also be used where available).
Indications
Prostate cancer patients with rising PSA levels after radical prostatectomy
and in patients with recurrent prostate cancer.
Patient preparation
Fasting for 6h recommended to minimize impact of dietary choline. Patient
should be well hydrated.
Procedure
11
C- choline (half- life 20min) is injected IV and PET images acquired
over 0– 15min.
18
F- choline (half- life 110min) is injected IV and scan is
performedat1h.
Results
Physiological uptake in the liver, pancreas, spleen, salivary and lacri-
mal glands, urinary tract, and less commonly in BM and intestines (see
Fig.14.22a).
Interpretation
i tracer uptake in recurrent prostate cancer and metastases (see Fig.14.22b).
Advantages
High specicity in lymph node staging, high sensitivity in recurrent disease,
and often helps in treatment planning.
Pitfalls
(a) Limited accuracy for the staging of the ° tumour; (b)often unable to
dierentiate prostate cancer from benign prostate hyperplasia, chronic
prostatitis, and high- grade intraepithelial neoplasia; and (c)high background
signal frequently hampers lesion detection.
Further reading
Schillaci O, Calabria F, Tavolozza M, etal.
18
F- choline PET/ CT physiological distribution and pitfalls
in image interpretation:experience in 80 patients with prostate cancer. Nucl Med Commun 2010;
31
:39– 45.
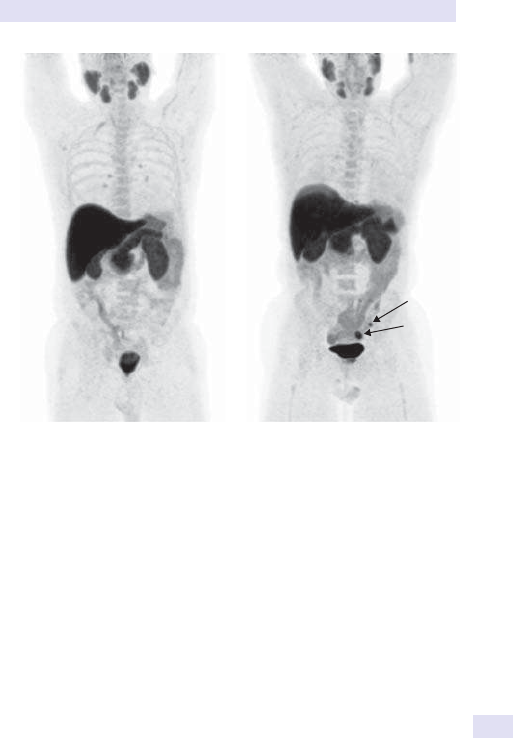
903
11
C-CHOLINE/
18
F-FLUOROCHOLINEPET/CT
(a) (b)
Fig.14.22 (a)Normal
18
F- choline scan; (b)abnormal scan with pelvic nodal
metastases (arrows).

904
CHAPTER14 Nuclear medicine
904
18
F- fluoridePET/ CT
Background
18
F- uoride is a highly sensitive bone- seeking PET tracer used for detec-
tion of skeletal pathology. In general, the tracer uptake mechanism is similar
to conventional SPECT tracer. The CT component of the PET/ CT study
allows localization, characterization, and dierentiation of skeletal metasta-
ses from benign lesions.
Indications
Similar to
99m
Tc- MDP bonescan:
•
Tumour staging— assess skeletal metastases.
• Bonepain.
Patient preparation
Patients should be (a)well hydrated and (b)instructed to empty their blad-
der immediately before imaging.
Procedure
18
F- uoride is injected IV. The adult activity is 185– 370MBq. Paediatric activ-
ity should be weight- based (2.22MBq/ kg).
Results
• Radiopharmaceutical uptake reects osteoblastic activity (see Fig.
14.23a).
•
Focal i uptake in sclerotic metastases (see Fig. 14.23b), trauma, or
infection.
•
Diuse i uptake associated with advanced metastases, Paget’s, and
metabolic bone disease.
Interpretation
Highly sensitive, but non- specic. Interpretation relies on pattern recog-
nition in the clinical setting. The CT component of PET/ CT, even when
performed primarily for attenuation correction and anatomic registration,
also provides diagnostic information.
Advantages
Sensitive— detects early changes in bone physiology, often before abnormal
plain radiographs, e.g. occult trauma, metastases, and sepsis.
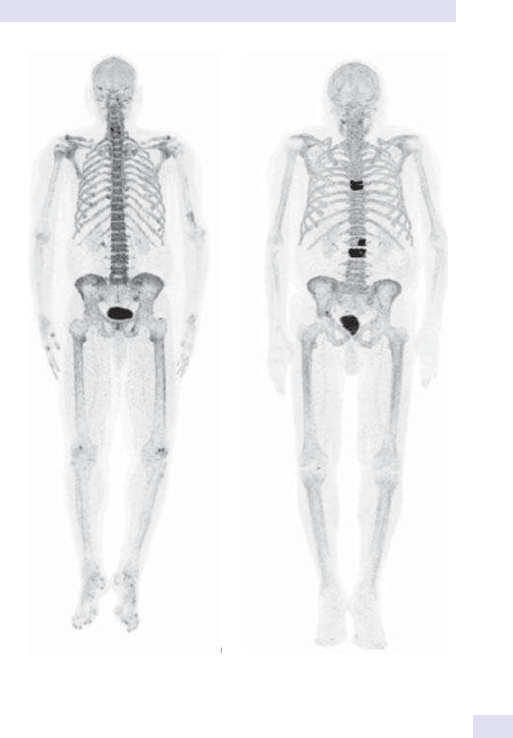
905
18
F-FLUORIDEPET/CT
Further reading
Segall G, Delbeke D, Stabin MG, etal. SNM practice guideline for sodium
18
F- uoride PET/ CT bone
scans 1.0. J Nucl Med 2010; 51:1813– 20.
(a)
(b)
Fig.14.23 (a)Normal
18
F- uoride scan; (b)abnormal scan with multiple skeletal
metastases in thespine.

906
CHAPTER14 Nuclear medicine
906
Myocardial perfusion imaging
Background
MPI reects regional blood ow during stress (i demand) and at rest, pro-
viding prognostically signicant information that, both in isolation and in
conjunction with coronary angiography, can be used to optimize patient
management. Originally performed using
201
Tl- thallous chloride, but now
based on
99m
Tc- labelled tracers— sestamibi (methoxy- isobutylisonitrile)
or tetrofosmin. Exercise or pharmacological stress are used to challenge
the coronary artery reserve. Exercise is performed by treadmill or bicy-
cle, whilst pharmacological stressors are either adenosine/ dipyridamole
infusion, which i coronary artery blood ow by vasodilatation, or dobu-
tamine infusion with both inotropic and chronotropic activity. Can also be
performed using PET/ CT with
82
Rb- rubidium chloride or
13
N- ammonia as
tracer.
Indications
Ischaemic heart disease.
Pre- angiography
• When conventional stress testing fails, e.g. bundle branchblock.
• Left ventricular hypertrophy.
• Atypical chestpain.
• Recurrent chest pain post- intervention. Good prognostic indicator.
Post- angiography
• Assess functional signicance of known stenoses.
• Identify critical vascular territory for intervention.
Patient preparation
• Stop β- blockers 24h prior to stressstudy.
• Sometimes helpful to withdraw all anti- anginal medication.
• Assess the optimal stress technique for individual patients, i.e. exercise
or pharmacological.
•
Attach 12- leadECG.
• Insert IV cannula.
• Check baselineBP.
Procedure
Two- part investigation comparing myocardial perfusion duringstress and
atrest
Stresstest
•
Treadmill or bicycle exercise to >85% of maximum predicted heart
rate or adenosine 140µg/ kg/ min IV infusion for 6min— sometimes with
submaximal exercise or dobutamine 5– 40µg/ kg/ min in 5µg/ kg/ min
increments over16min.
•
Inject the radiopharmaceutical (
201
Tl- thallous chloride,
99m
Tc- sestamibi,
or
99m
Tc- tetrofosmin) at peak stress.
• Tomographic imaging immediately (
201
Tl) or 15– 60min post- injection
(
99m
Tc compounds). Images generally acquired with ECG gating.

MYOCARDIAL PERFUSION IMAGING
907
Reststudy
•
Second
99m
Tc radiopharmaceutical injection under resting conditions.
•
Tomographic imaging as before.
• With
201
Tl, second injection not necessary, since tracer redistributes into
ischaemic areas over 4h, but top- up dose sometimesgiven.
Results
Myocardial uptake reects radiopharmaceutical delivery and myocyte func-
tion (see Fig. 14.24).
Interpretation
Infarction causes matched perfusion defects during stress and rest.
Inducible ischaemia creates a perfusion defect at stress, which reperfuses at
rest=reversible ischaemia (see Fig. 14.25). The severity, extent, and num-
ber of reversible defects are prognostically signicant. Anormal MPI study
implies risk of an adverse cardiac event of <0.5% perannum.
Advantages
Non- invasive; relatively inexpensive, compared with angiography.
tseRssertS
Short axis
Vertical
long axis
Horizontal
long axis
Fig.14.24 Normal myocardial perfusion scan. (E Colour plate9.)

908
CHAPTER14 Nuclear medicine
908
Pitfalls
Less sensitive in multiple small- vessel coronary disease, e.g. DM. Sensitivity
depends on stress test quality.
Further reading
Marcassa C, Bar JJ, Bengel F, etal. Clinical value, cost- eectiveness, and safety of myocardial perfusion
scintigraphy:a position statement. Eur Heart J 2008; 29:557– 63.
Verberne HJ, Acampa W, Anagnostopoulos C, etal. EANM procedural guidelines for radionuclide
myocardial perfusion imaging with SPECT and SPECT/ CT: 2015 revision. Eur J Nucl Med Mol
Imaging 2015; 42
:1929– 40.
(a)
(b)
Fig.14.25 Myocardial perfusion scan:(a)in xed perfusion loss (anterolateral
infarction) and (b)in inferior stress- induced (reversible) ischaemia. (E Colour
plate10.)

MYOCARDIAL PERFUSION IMAGING
909
910
CHAPTER14 Nuclear medicine
910
Radionuclide
ventriculography:MUGAscan
Background
Increasingly replaced by TTE, TOE, and contrast ventriculography at time
of cardiac catheterization. Multigated radionuclide angiography (MUGA)
scans are less operator- dependent than either TTE or TOE, which is impor-
tant in serial assessment. The cardiac blood pool is imaged dynamically after
injection of radiolabelled RBCs. Wall motion, synchronicity of ventricular
contraction, and EF are measured (see Fig. 14.26). The estimated EF unreli-
able in presence of dysrhythmias— acquisition requires a relatively regular
heartrate.
Indications
• LVEF measurement, e.g. unechogenic patients (see Fig. 14.27).
• Monitor anthracycline cardiotoxicity.
Patient preparation
None.
Procedure
Radiolabel red cells (in vivo or in vitro) using
99m
Tc- pertechnetate. Image the
patient supine in anterior and left anterior oblique projections. Camera
acquisition gated to cardiac cycle. Imaging may be combined with low-
impact exercise/ pharmacological stress to assess cardiac reserve.
Frame
0
0
100
80
60
40
20
161284
Volume curve
Norm counts
Fig.14.26 Computer- generated EF of40%.
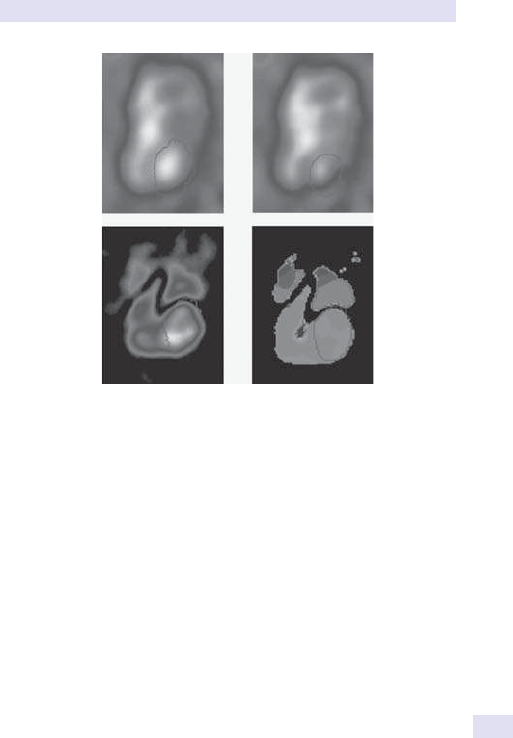
RADIONUCLIDE VENTRICULOGRAPHY:MUGASCAN
911
Results
Visual image of 300– 400 summated cardiac cycles. Computer- generated
images used to assess regional wall motion and synchronous contraction
(see Fig. 14.27). Computer- generated EF calculation. Normal EF 60– 70%,
d withage.
Interpretation
To monitor treatment response in cardiac failure, cardiomyopathy.
Advantages
Good for serial measurements during anthracycline chemotherapy. Reliable
in unechogenic subjects.
Disadvantage
Moderately high radiation dose:echocardiography preferable in most patients.
Pitfalls
Cardiac dysrhythmias interfere with gating, e.g.AF.
Further reading
Hesse B, Lindhardt TB, Acampa W, etal. EANM/ ESC guidelines for radionuclide imaging of cardiac
function. Eur J Nucl Med Mol Imaging 2008; 35:851– 85.
(a) (b)
(c)
(d)
Fig.14.27 MUGA scan showing (a)LV regions of interest at end- diastole and
(b)end- systole for EF calculation; (c)amplitude image showing relative anteroseptal
hypokinesis, but (d)synchronous LV contraction. (E Colour plate11.)

912
CHAPTER14 Nuclear medicine
912
Radionuclide first- pass cardiac studies
Background
Nuclear medicine can assess simple shunts, e.g. left- to- right shunts (ven-
tricular and atrial septal defects), but has no place in bidirectional shunting
or multiple sources of pulmonary blood ow (e.g. patent ductus arterio-
sus). First- pass studies quantify shunt severity both pre- and post- surgical
correction. Complementary to cardiac catheterization and Doppler colour
ow echocardiography. Dynamic imaging of a small bolus of IV radioactivity,
usually
99m
Tc- DTPA, outlines the cardiac venous return, pulmonary circula-
tion, left heart, and systemic circulation. Largely superseded by angiography
andMRI.
Indications
Measurement of simple left- to- right cardiac shunts.
Patient preparation
None.
Procedure
•
99m
Tc- DTPA administered IV as a bolus into a right antecubital vein with
the shoulder abducted.
•
Image immediately as a dynamic acquisition over60s.
Results
Normal circulation will demonstrate sequential appearance of the right
heart, pulmonary outow tract, lungs, left heart, andaorta.
Interpretation
Variation in the normal sequence implies cardiac shunting. Interpretation
must be performed in conjunction with denitive knowledge of the patient’s
anatomy, e.g. echocardiography with Doppler colourow.
Advantages
Relatively non- invasive technique for serial follow- up of congenital heart
disease. Mathematical analysis of the data allows quantication of shunt size
and is important for consideration and timing of corrective surgery.

RADIONUCLIDE FIRST-PASS CARDIAC STUDIES
913

914
CHAPTER14 Nuclear medicine
914
Lung scan:ventilation/ perfusion imaging
Background
One of the most widely requested nuclear medicine studies. Sensitivity and
specicity reduced in coexisting lung disease, when CTPA is more useful. All
patients should have had a chest radiograph within 24h to aid V/ Q inter-
pretation and exclude other causes of pleuritic chest pain and hypoxia, e.g.
pneumothorax.
Lung perfusion shown by injection of
99m
Tc particles which are trapped
by the pulmonary capillary bed. Ventilation shown using radiolabelled gases
or aerosols.
Indications
• Suspectedpulmonary embolism (PE).
• Preoperative lung function assessment.
Patient preparation
None. Relative contraindication in right- to- left intra- cardiac shunts; caution
in severe pulmonary hypertension.
Procedure
• Lie the patient supine and inject
99m
Tc- macroaggregated albumin
(MAA)IV.
•
Obtain gamma camera perfusion images in fourviews.
• Ventilation images are obtained in same projections by continuous
breathing of
81m
Kr gas or using
99m
Tc aerosol or
133
xenongas.
Results
Homogeneous, matched V/ Q patterns (see Fig. 14.28).
Interpretation
Four abnormal patterns recognized:
•
Segmental perfusion loss with preserved ventilation:PE (see Fig. 14.29).
•
Segmental matched perfusion and ventilation loss:pulmonary infarction/
infection.
•
Segmental/ subsegmental ventilation loss with preserved perfusion:infection.
•
Non- segmental, patchy, matched perfusion, and ventilation loss:COPD (see
Fig. 14.29).
Advantages
Quick, non- invasive. Normal scan virtually excludesPE.
Pitfalls
Specicity reduced in underlying lung disease— COPD, asthma giving inde-
terminate results. False +ves with tumour, bullae, vasculitides, brotic lung
disease, and old unresolvedPE.
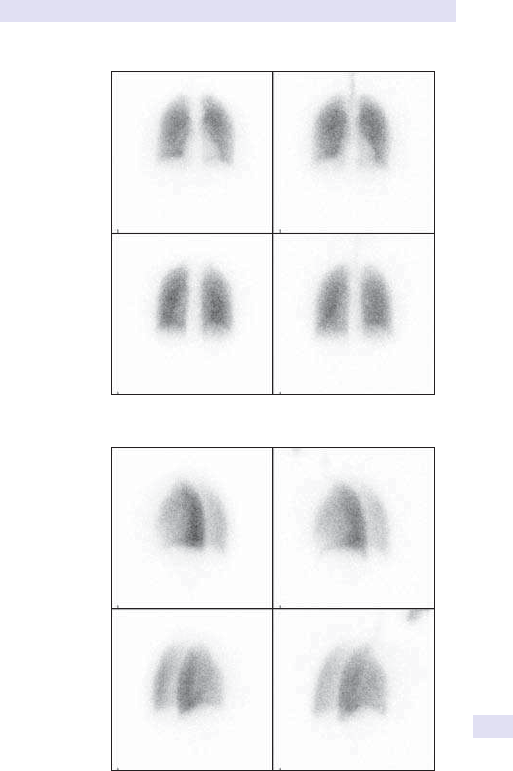
LUNG SCAN:VENTILATION/PERFUSION IMAGING
915
Perfusion Ventilation
Right posterior
oblique
Left posterior
oblique
(b)
Perfusion Ventilation
Anterior
Posterior
(a)
Fig.14.28 Normal lung V/ Q images:(a)anterior and posterior views;
(b)obliqueviews.
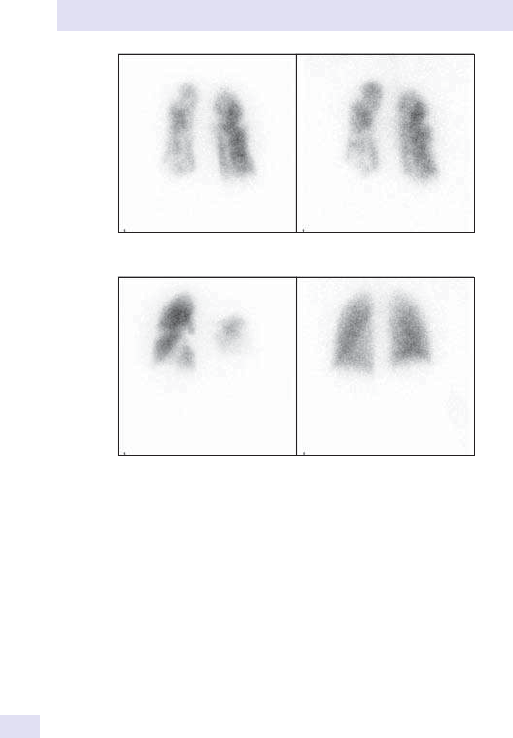
916
CHAPTER14 Nuclear medicine
916
Further reading
Bajc M, Neilly JB, Miniati M, etal. EANM guidelines for ventilation/ perfusion scintigraphy:Part 1.
Pulmonary imaging with ventilation/ perfusion single photon emission tomography. Eur J Nucl Med
Mol Imaging 2009; 36
:1356– 70.
(a)
(b)
Posterior perfusion
Posterior perfusion
Posterior ventilation
Posterior ventilation
Fig.14.29 Lung scans:(a)showing matched, non- segmental V/ Q defects in
COPD; and (b)showing segmental V/ Q mismatch– – extensive bilateral pulmonary
thromboembolism and unmatched perfusionloss.

LUNG SCAN:VENTILATION/PERFUSION IMAGING
917

918
CHAPTER14 Nuclear medicine
918
Lung shunt studies
Background
Largely research procedure.
Indications
Suspected pulmonary AV shunting.
Patient preparation
None.
Procedure
Inject
99m
Tc- MAA IV; consider manoeuvres to reduce particle number.
gamma camera lung, abdomen, and head imaging. Calculate relative uptake
in lungs, kidneys, and brain. Express as fraction of cardiac output to quantify
shunt fraction.
Results
Kidneys and brain not normally visible on lung perfusion imaging.
Interpretation
Abnormal extrapulmonary activity implies degree of shunting. Intensity of
uptake rises with shunt severity (see Fig. 14.30).
Advantages
Non- invasive, quantitative. Can be used to monitor response to
intervention.
Pitfalls
Injection extravasation invalidates shunt calculation.
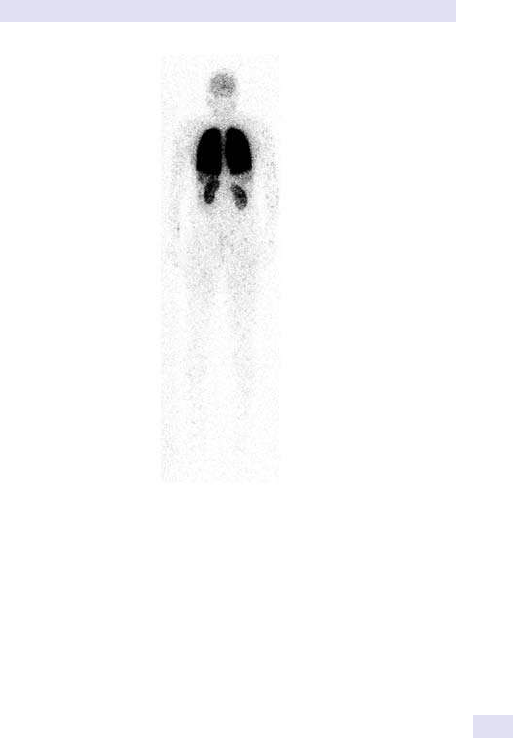
LUNG SHUNT STUDIES
919
Fig.14.30 Lung shunt study showing extrapulmonary activity in the brain and
kidneys.

920
CHAPTER14 Nuclear medicine
920
Lung permeability studies
Background
Altered alveolar permeability aects gas exchange. Also shown by lung
transfer factor measurement.
Indications
• PCP infection:rapid screening in high- risk patients with normalCXR.
• Monitor treatment response inCFA.
Patient preparation
None.
Procedure
Patient breathes
99m
Tc- DTPA aerosol. Gamma camera images of the thorax
over 1h. Computer data analysis generates lung clearance curves, reecting
the integrity of the alveolar cell barrier.
Results
Clearance curves used to calculate the permeability index. Individual results,
compared with centre- dened normalrange.
Interpretation
Accelerated clearance in PCP, which d with successful treatment.
Advantages
Non- invasive. Allows rapid PCP diagnosis.
Pitfalls
Non- specic, e.g. accelerated clearance in smokers.

LUNG PERMEABILITY STUDIES
921

922
CHAPTER14 Nuclear medicine
922
Lymphoscintigraphy
Background
Lymphoedema can be congenital or acquired. The lymphatic system nor-
mally drains subcutaneous tissues l local lymphatic channels and regional
nodes. Lymphatic channels can be imaged using radiolabelled colloid parti-
cles (E Sentinel node imaging, p. 890).
Indication
Unexplained limb swelling, e.g. lymphatic hypoplasia.
Patient preparation
None.
Procedure
99m
Tc- nanocolloid injection SC into a nger or toe web space on the aected
and contralateral limbs. Regional gamma camera imaging at 10min intervals
over1h.
Results
Normally rapid clearance via lymphatic channels to regional nodes (see
Fig. 14.31a).
Interpretation
Slow clearance and failed regional node uptake in hypoplastic systems or
metastatic regional node inltration, dermal backow (see Fig. 14.31b),
depending on clinical context.
Advantages
Much easier than conventional (contrast) lymphography— avoids lymphatic
channel cannulation.
Pitfalls
Lymphatic drainage may be disrupted by surgery or radiotherapy.
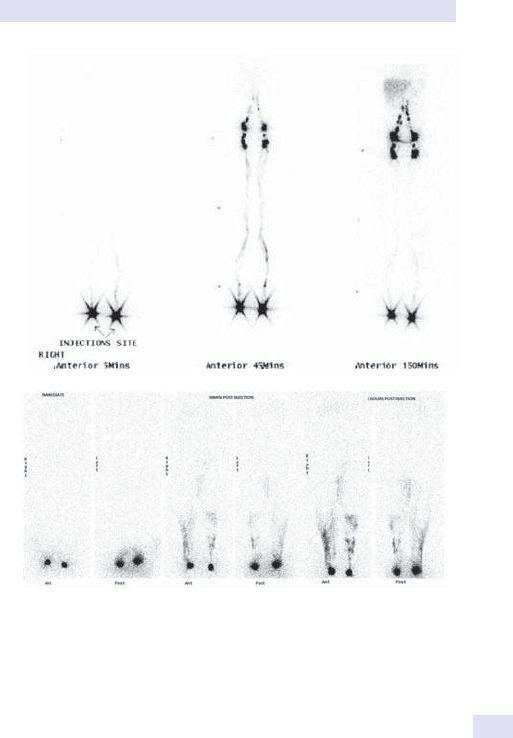
LYMPHOSCINTIGRAPHY
923
(a)
(b)
Fig.14.31 (a)Normal lymphoscintograms. (b)Abnormal study: bilateral dermal
backow with non-visualization of lymphatic channels and lymph nodes.

924
CHAPTER14 Nuclear medicine
924
Static cortical scintigraphy:
dimercaptosuccinic acid imaging (DMSA)
Background
DMSA is concentrated by the proximal convoluted tubules in the renal cor-
tex.
99m
Tc- DMSA provides good denition of functioning renal parenchyma.
It should be used in conjunction with anatomical imaging, e.g. ultrasonogra-
phy, to dierentiate between scarring, cysts, or calculi.
Indications
• UTI:‘gold standard’ for renal scarring.
• Measurement of relative renal function.
• Renal duplication assessment.
• Ectopic kidney localization.
• Renal trauma.
• Renal vein thrombosis.
• Pre- biopsy.
Patient preparation
None, but avoid dehydration.
Procedure
99m
Tc- DMSA injected IV. Static anterior, posterior, and posterior oblique
images acquired 2– 4hlater.
Results
Visual image evaluation, assessing the integrity of cortical outlines for
scarring (see Fig. 14.32a). Quantitative computer image analysis is used
to measure relative renal function, i.e. the contribution of each kidney to
overallGFR.
Interpretation
Cortical scars distort the renal outline (see Fig. 14.32b). Duplication may
result in a non- functioning upper moiety, usually due to obstruction, or a
scarred lower moiety, 2° to vesico- ureteric reux. Relative renal function
is usually 50:50±5%.
Advantages
Sensitive for renal scarring. Superior to US. Non- invasive.
Pitfalls
False +ves during or immediately after acute pyelonephritis. May give corti-
cal defects that do not progress to scarring. Splenic impression at the left
upper pole may be mistaken for scarring.
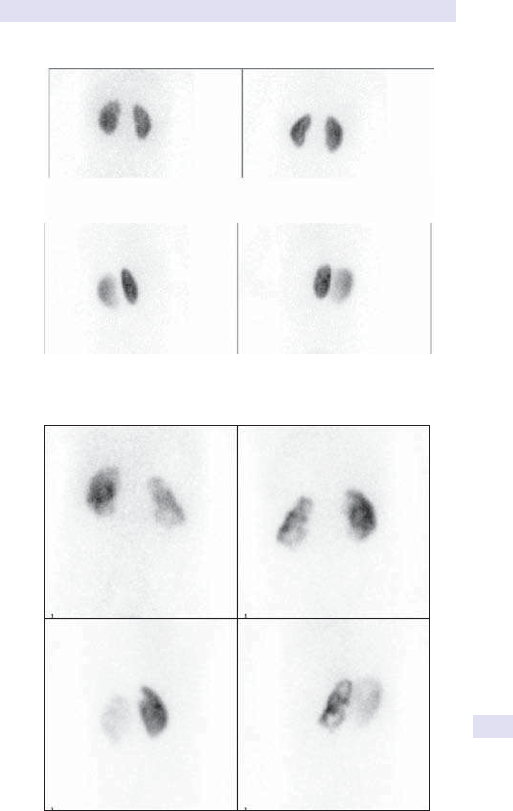
STATIC CORTICAL RENOGRAPHY
925
roiretsoProiretnA
roiretsoProiretnA
Right post oblique
Left post oblique
(a)
Right post oblique Left post oblique
(b)
Fig.14.32 DMSA static scan:(a)normal and (b)showing extensive left kidney
cortical scarring.

926
CHAPTER14 Nuclear medicine
926
Dynamic renography
Background
Nuclear medicine oers unique ‘real- time’ imaging of renal function, i.e.
visualization of uptake, drainage, and bladder emptying. Available radiop-
harmaceuticals include:
•
99m
Tc-diethylenetriaminepentacetic acid (DTPA), cleared by glomerular
ltration.
•
99m
Tc- mercaptoacetyltriglycine (MAG3), cleared by glomerular ltration
and tubular secretion.
•
99m
Tc- ethylenedicysteine (EC), cleared by glomerular ltration and
tubular secretion.
Tubular agents preferred, particularly in the presence of renal impairment
and in the immature kidney.
99m
Tc- DTPA reserved for assessment of ATN,
post- transplant viability,etc.
Indications
• Assessment of renal drainage:discrimination between renal dilatation and
outow obstruction.
•
Measurement of relative renal function.
• Loinpain.
• Post- pyeloplasty follow- up.
• RAS (E Captopril renography, p. 928).
Patient preparation
Good hydration essential. Empty the bladder immediately before undertak-
ing thestudy.
Procedure
• Position the patient supine or seated erect, with the camera behind.
• Obtain good peripheral venous access. Bolus IV radiopharmaceutical
injection
99m
Tc- MAG3 or
99m
Tc- DTPA, followed by 10– 20mL of
salineush.
•
Image immediately, acquiring real- time dynamic data for 20– 30min.
• Diuretic administration is essential to distinguish dilatation from outow
obstruction.
•
Post- voiding images are always required to assess the completeness of
bladder emptying and may improve drainage of the upper renal tracts in
high- pressure systems.
Results
Visual inspection of renal size, perfusion, function, and drainage (see
Fig. 14.33a). Quantitative computer image analysis measures relative func-
tion and transit times, and generates drainage graphs.
Interpretation
Uptake and excretion of activity normally rapid. Dilated systems show pro-
gressive pooling in the renal pelvis that empties following diuretic challenge.
Obstructed systems show progressive tracer accumulation with no diuretic
response, often associated with reduced function on the aected side (see
Fig. 14.33b).
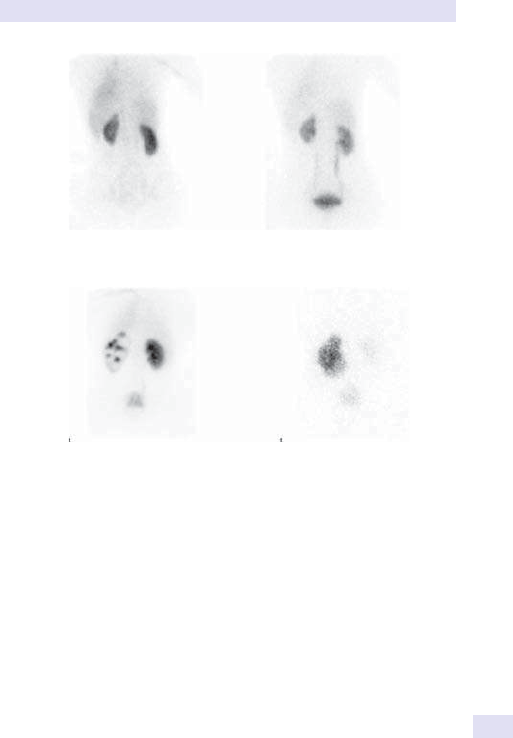
DYNAMIC RENOGRAPHY
927
Advantages
Sensitive, non- invasive, quantitative renal function assessment. Anatomical
imaging, e.g. IVU, better for renal morphology, stones,etc.
Pitfalls
Movement artefact, chronic renal failure, and dehydration reduce data reli-
ability. Renal drainage may be gravity- dependent— always complete the
study with an erect image. Drainage curves invalidated by radiopharmaceu-
tical extravasation.
20min5min
20min
60min
(b)
(a)
Fig.14.33 Dynamic renogram posterior images:(a)normal, showing an early
parenchymal image and later symmetrical excretion with bladder lling; (b)outow
obstruction:early image shows left hydronephrosis 2° to pelviureteric junction
obstruction, with poor drainage at60min.

928
CHAPTER14 Nuclear medicine
928
Captopril renography
Background
RAS is a rare (<2%) cause of hypertension. Suspected in young adults
presenting with hypertension, usually due to bromuscular dysplasia. In
patients >50years, the commonest cause is atherosclerosis. Perfusion pres-
sure is maintained by angiotensin II in RAS. Captopril is an ACE inhibitor,
which blocks the conversion of angiotensin Ito angiotensin II. Captopril
reduces perfusion pressure, leading to a fall in the relative function and
delayed tracer uptake on the aected side. Captopril administration is con-
traindicated in the presence of a solitary kidney.
Indications
Diagnosis of RAS (especially bromuscular dysplasia) and prediction of
response to revascularization.
Patient preparation
Well- hydrated. Baseline BP. IV access. Stop ACE inhibitors for 48h prior
to thetest.
Procedure
• Perform standard dynamic renogram using
99m
Tc- MAG3.
• Repeat renogram 1h after captopril 25mg single dosePO.
• Monitor BP— beware hypotension.
Results
Quantitative evaluation of R:L renal function and time to peak activity in
each kidney.
Interpretation
RAS due to bromuscular dysplasia— fall in relative renal function and
delayed time to peak renal activity of >10min.
Advantages
Distinguishes generalized atherosclerosis (often poor BP outcome following
angioplasty) from bromuscular hyperplasia (good angioplasty response).
Pitfalls
• d reliability in the presence of renal impairment.
•
Severe hypotension.

CAPTOPRIL RENOGRAPHY
929

930
CHAPTER14 Nuclear medicine
930
Gastrointestinal bleeding:labelled red
cell imaging
Background
The source of GI blood loss is usually identied by GI endoscopy but may
be dicult to localize. Labelled red cell studies are useful when there is
evidence of ongoing bleeding (typically falling Hb of 1g/ L/ day). The patient
must be actively bleeding at the time of the study. This is a time- consuming
investigation, with serial imaging beyond 24h often performed.
Indications
Localize source of active GI haemorrhage when other techniques
(e.g. endoscopy or angiography) have failed.
Patient preparation
No recent contrast barium studies. Fasting during rst 2h of imaging.
Procedure
Label red cells (in vitro or in vivo) using
99m
Tc- pertechnetate. Abdominal
gamma camera blood pool imaging immediately and at intervals for up to
36h post- injection or until the bleeding source is identied.
Results
Activity normally restricted to vascular compartment.
Interpretation
Any activity in the gut lumen implies active haemorrhage. Serial images help-
ful (see Fig. 14.34).
Advantages
More sensitive and less invasive than angiography for intermittent bleeding.
Pitfalls
• Poor red cell label:degrades image quality, could lead to false+ve.
•
Limits of detection:0.5mL/ min bloodloss.
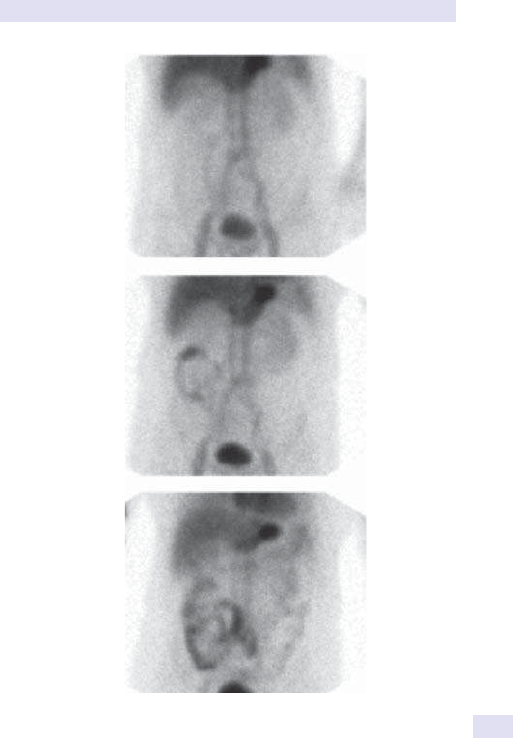
GASTROINTESTINAL BLEEDING:LABELLED RED CELL IMAGING
931
30min
2h
6h
Fig.14.34 Anterior abdominal images showing increasing red cell haemorrhage into
the distalileum.

932
CHAPTER14 Nuclear medicine
932
Gastric emptying studies
Background
The diagnosis of dysfunctional gastric emptying can be dicult. In children,
delayed gastric emptying may contribute to gastro- oesophageal reux. In
adults, both gastric stasis and ‘dumping’ syndromes occur, sometimes fol-
lowing previous surgery. Imaging following ingestion of radiolabelled solids
or liquids demonstrates the timing and pattern of gastric emptying.
Indications
Altered GI motility— delayed or accelerated gastric emptying.
Patient preparation
Fast for 4h. Stop drugs likely to inuence GI motility, e.g. domperidone,
metoclopramide.
Procedure
Milkstudy
•
Give radiolabelled (
99m
Tc- DTPA) milk drinkPO.
• Image the anterior abdomen immediately and at 10min intervals for 1h.
Generate computer- derived clearance curves to calculate the emptying
half- time.
• Delayed thoracic image helpful to exclude lung aspiration if clearance
signicantly delayed.
Dual isotopemethod
•
Give
99m
Tc- labelled standard meal (e.g. porridge, egg) with
111
In- DTPA
inwater.
•
Anterior abdomen gamma camera imaging as before using dual isotope
settings.
•
Generate solid and liquid phase clearance curves.
Results
Normal gastric emptying half- time (milk = 20min). Normal range for
solids is centre- specic, depending on the standard meal composition (see
Fig. 14.35a andb).
Interpretation
Visual image evaluation and half- time calculation.
Advantages
Non- invasive and quantitative.
Pitfalls
Vomiting during study invalidates emptying time calculations.
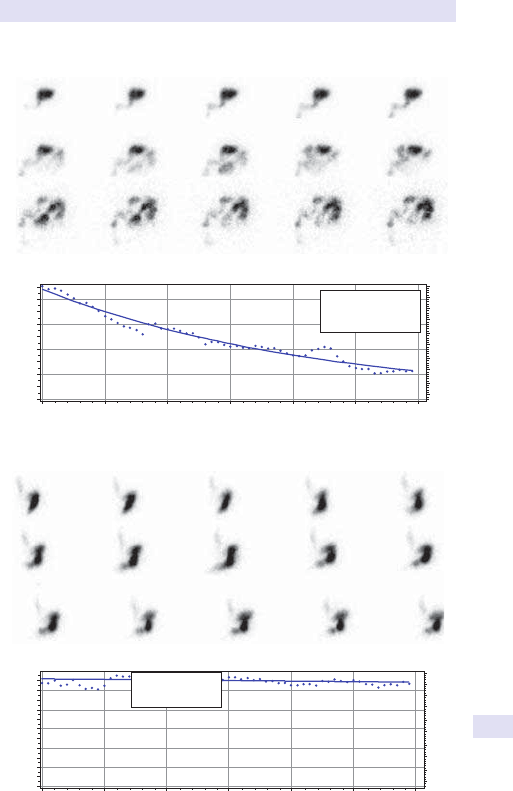
GASTRIC EMPTYING STUDIES
933
Counts
8,000
6,000
4,000
2,000
0
0102030
Time-Minutes
Time Activity Curve
40 50 60
100
90
80
70
60
50
(%)
40
30
20
10
0
Stomach: Exp: y
=
ae
bx
a: 8797
b: −0.0228634
(a)
Counts
10,000
8,000
2,000
0
0102030
Time-Minutes
Time Activity Curve
40 50 60
100
90
80
70
60
50
(%)
40
30
20
10
0
6,000
4,000
(b)
Stomach: Exp: y
=
ae
bx
a: 11205
b: −0.000490928
Fig.14.35 (a)Normal gastric emptying study:anterior images showing clearance of
99m
Tc- labelled semi- solid meal into the proximal small intestine; (b)abnormal gastric
emptying study:anterior images showing poor clearance of
99m
Tc- labelled semi- solid
meal into the proximal small intestine.

934
CHAPTER14 Nuclear medicine
934
SeHCAT studies
Background
The SeHCAT test is an important test for diagnosing bile acid malabsorp-
tion. SeHCAT is a taurine- conjugated bile acid analogue and it is incorpo-
rated with
75
Se (gamma- emitter) into the SeHCAT molecule (radiotracer)
to assess in vivo the enterohepatic circulation of bile salts. The retention of
radiotracer in the body is evaluated using a conventional gamma camera.
Indications
• Chronic diarrhoea.
• Diarrhoea- predominantIBS.
• Crohn’s disease.
• Ileal resection, cholecystectomy, radiation- induced bowel damage, or
ulcerative colitis.
Patient preparation
Colestyramine and colesevelam should be stopped for 3days prior toscan.
Procedure
A capsule containing radiolabelled SeHCAT (370kBq) capsule is taken PO
with water, and a scan is performed to measure SeHCAT activity at 1– 3h.
Patients will return on day 7 to undergo a second scan to measure the per-
centage of SeHCAT retention.
Results
The percentage of SeHCAT retention gives an indication as to whether the
patient has bile acid malabsorption ornot.
Interpretation
Retention values of <15% are considered abnormal and are suggestive of
bile acid malabsorption (<5%, severe bile acid malabsorption; 5– 10%, mod-
erate; and 10– 15%, mild). Retention values of >15% are normal.
Advantages
Easy to perform and well tolerated.
Further reading
Boyd GS, Merrick MV, Monks R, Thomas IL. Se- 75- labeled bile acid analogs, new radiopharmaceuti-
cals for investigating the enterohepatic circulation. J Nucl Med 1981; 22
:720– 5.
Jazrawi RP, Ferraris R, Bridges C, Northeld TC. Kinetics for the synthetic bile acid 75selenoho-
mocholic acid- taurine in humans: comparison with [14C]taurocholate. Gastroenterology 1988;
95
:164– 9.

935
SeHCAT STUDIES

936
CHAPTER14 Nuclear medicine
936
Meckel’s scan:ectopic gastric mucosa
localization
Background
Meckel’s diverticulum is the commonest congenital anomaly of the GIT,
occurring in 72% of the population. Less than 10% contain ectopic gastric
mucosa which may bleed, but diverticuli can also cause obstruction or
become inamed. Typically, childhood presentation. Nuclear medicine pro-
vides a straightforward imaging technique that targets gastric mucosal cells,
which normally take up
99m
Tc- pertechnetate.
Indications
Unexplained abdominal pain or GI haemorrhage— after endoscopy/ con-
trast radiology.
Patient preparation
• Fast for4h.
• H
2
antagonist administration may improve specicity.
•
No recent barium studies.
Procedure
Inject
99m
Tc- pertechnetate IV. Immediate and serial abdominal imaging
over1h.
Results
Normal uptake in gastric mucosa.
Interpretation
Focal abnormal uptake appearing at the same time as the stomach implies
ectopic gastric mucosa (Meckel’s diverticulum) (see Fig.14.36), or occasion-
ally a duplication cyst. Commonest site— right iliac fossa(RIF).
Advantages
Non- invasive.
Pitfalls
False +ves due to activity in the renal tract— lateral images usuallyhelp.
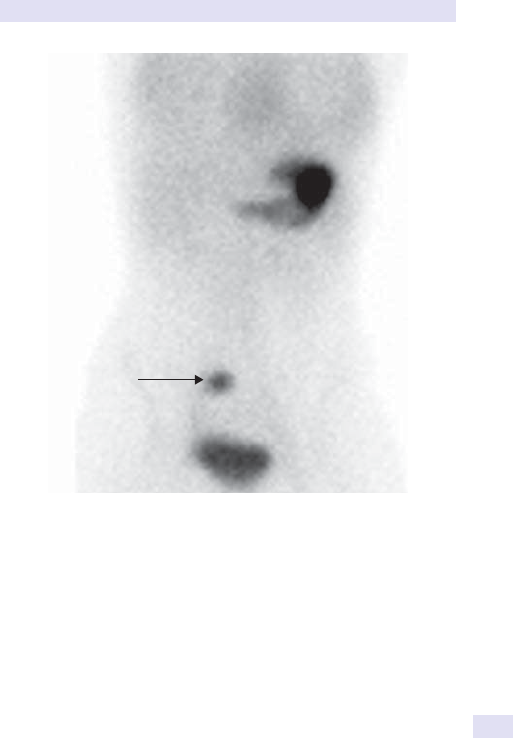
MECKEL’S SCAN:ECTOPIC GASTRIC MUCOSA LOCALIZATION
937
Fig.14.36 Ectopic gastric mucosa in the right iliac fossa towards the midline
(arrow).

938
CHAPTER14 Nuclear medicine
938
Hepatobiliary scintigraphy
Background
Iminodiacetic acid (IDA) compounds are cleared from the circulation by
hepatocytes and secreted into the bile in the same way as bilirubin.
99m
Tc-
labelled IDA compounds show biliary excretion through the biliary tree and
gall bladder l
duodenum. Useful in acute/ chronic acalculous cholecystitis
and to diagnose biliary atresia.
Indications
• Acute cholecystitis.
• Trauma.
• Post- operative leak detection.
• Bile duct/ stent patency.
• Gall bladder emptying.
• Bile reux.
• Neonatal biliary atresia.
Patient preparation
• Adults:fast for6h.
•
Neonates:phenobarbital 5mg/ kg/ day PO for 3days prior to study
(enzyme induction).
Procedure
• Adults:IV injection of
99m
Tc- labelled IDA complex (mebrofenin). Gamma
camera imaging over1h.
•
Neonates:IV injection of
99m
Tc- IDA. Immediate dynamic imaging for
5min, then serial static images for up to 24h or until activity reaches the
small bowellumen.
Results
Gall bladder and biliary tree normally shown with tracer excretion via the
CBD into the duodenum by 30min post- injection. Cholecystokinin 0.5U/
kg IV sometimes administered to stimulate gall bladder emptying (see Fig.
14.37a)
Interpretation
• Acute cholecystitis:absent gall bladder.
•
Obstruction, leak, or reux assessed visually (see Fig. 14.37b).
• Neonates:passage of activity into the gut lumen excludes biliary atresia.
•
Quantication of T0 to T10 min images improves specicity for atresia
diagnosis.
Advantages
Non- invasive. Straightforward pattern recognition.
Pitfalls
Delayed IDA excretion in severe jaundice:bilirubin >300µmol/ L.

HEPATOBILIARY SCINTIGRAPHY
939
(a)
(b)
Fig.14.37 (a)Normal hepatobiliary scan; (b)hepatobiliary scan showing leak post-
laparoscopic cholecystectomy.

940
CHAPTER14 Nuclear medicine
940
Splenunculus detection:heat- damaged
red cell imaging
Background
Splenectomy may be indicated in haemolytic syndromes and in refractory
haemorrhagic tendencies (e.g. ITP) if associated with hypersplenic throm-
bocytopenia. Remnant splenic tissue, or ‘splenunculi’, can give rise to recur-
rent thrombocytopenia— dicult to detect on anatomical imaging. As the
spleen removes abnormal red cells from the circulating blood pool, radiola-
belled heat- damaged red cells can be used to localize ectopic splenic tissue.
Indications
Recurrent thrombocytopenia post- splenectomy.
Patient preparation
None.
Procedure
• Obtain a venous blood sample.
• Radiolabel red cells in vitro using
99m
Tc- pertechnetate.
• Heat to 49.5°C for 20– 30min.
• Cool and re- injectIV.
• Image the anterior abdomen 30minlater.
Results and interpretation
Damaged red cells taken up by splenic remnants (see Fig. 14.38).
Advantages
Investigation of choice for splenunculus detection.
Pitfalls
Enlarged left lobe of the liver may obscure a small splenic remnant.
PosteriorAnterior
Fig.14.38 Post- splenectomy. Intense uptake in a splenunculus lying in the
splenicbed (arrow).

SPLENUNCULUS DETECTION:HEAT-DAMAGED RED CELL IMAGING
941

942
CHAPTER14 Nuclear medicine
942
Hepatosplenic scintigraphy
Background
Largely superseded by ultrasonography and cross- sectional imaging. Maps
reticuloendothelial tissue within the liver (Kuper cells) and spleen, to iden-
tify SOLs and conrm the presence or absence of functioning splenic tissue.
Indications
Liver SOLs— now largely replaced by US, CT, orMRI.
Patient preparation
None.
Procedure
99m
Tc- colloid injected IV. Abdominal gamma camera images 30min
post- injection.
Results
Normal, homogeneous liver and spleen uptake (see Fig. 14.39).
Interpretation
Focal d uptake in SOLs. i spleen and bone activity in portal hyperten-
sion. Focal i
uptake in the caudate lobe pathognomonic of Budd– Chiari
syndrome.
Advantages
Cheap.
Pitfalls
Non- specic. Largely superseded by anatomical imaging.
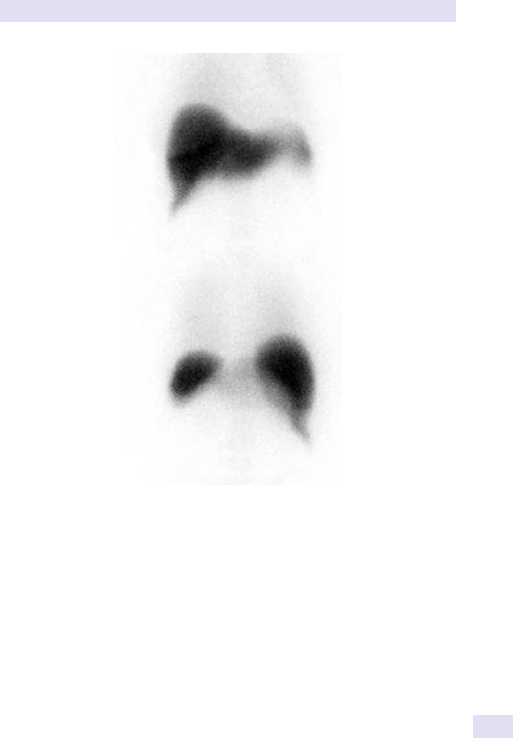
HEPATOSPLENIC SCINTIGRAPHY
943
Fig.14.39 Normal hepatosplenicstudy.
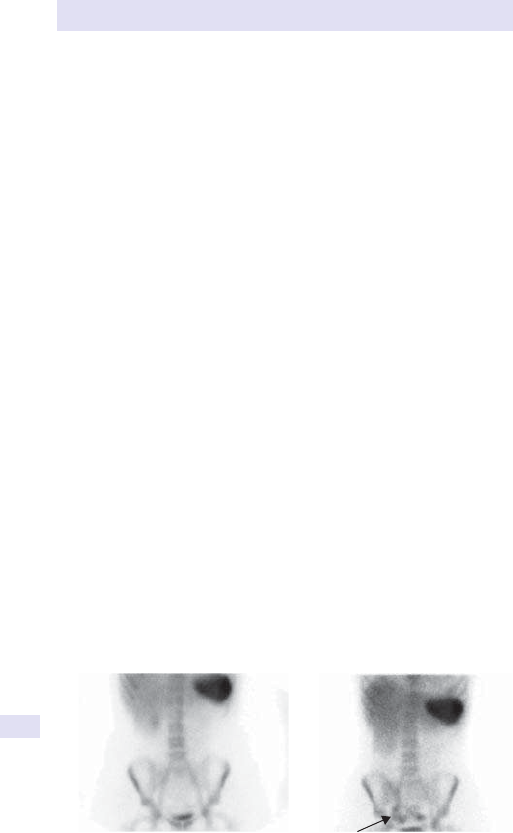
944
CHAPTER14 Nuclear medicine
944
Labelled leucocyte imaging
Background
Localization and assessment of acute or chronic infection/ inammation can
be dicult. Nuclear medicine techniques show inammation but do not
dierentiate infective from non- infective causes. Radiolabelled autologous
leucocytes are injected and imaged. The normal distribution includes the
liver and spleen, making peri- diaphragmatic collections dicult to identify.
Delayed imaging useful in chronic low- grade infection, e.g. osteomyelitis,
where cell migration to the site of inammation isslow.
Indications
• Sepsis localization.
• IBD to determine disease activity, extent, severity.
Patient preparation
None. Avoid recent barium contrast radiology.
Procedure
• Obtain 40– 60mL of blood sample.
• Separate the white cell layer, and radiolabel in vitro using
99m
Tc-
exametazime (HMPAO) or
111
In- oxine.
• Re- inject labelled cellsIV.
• Image 1 and 3h later (IBD), or 2, 4, and 24h for intra- abdominal sepsis/
osteomyelitis.
Results
Physiological uptake in the RES. Variable GI and renal excretion, depending
on the radiopharmaceutical used (see Fig. 14.40a).
Interpretation
Focal i uptake indicates sepsis. Diuse i gut uptake reects the extent and
activity of IBD (see Fig. 14.40b).
(b)
(a)
Fig.14.40 Labelled leucocyte imaging:(a)normal, and (b)acute inammatory
bowel disease— intense uptake in small and large bowel loops (Crohn’s disease).

LABELLED LEUCOCYTE IMAGING
945
Advantages
Very sensitive in IBD. Non- invasive, useful in sick patients, e.g. acute
exacerbation ofIBD.
Pitfalls
• False −ves:leucopenia and poor white cell label, perihepatic and
perisplenic collections obscured by normal liver and spleen uptake.
•
False +ves:physiological gut and renal activity.
•
Damaged white cells during labelling causing lung sequestration.
•
99m
Tc- exametazime (HMPAO) preferred for routine imaging and IBD—
lower radiation dose and earlier result than
111
In- oxinelabel.
• Reserve
111
In- oxine for low- grade bone sepsis localization.
• Requires aseptic facilities and trained personnel.
• Risk to sta (blood handling) and patient (contamination, re- injection
into wrong patient).

946
CHAPTER14 Nuclear medicine
946
67
Gallium scintigraphy
Background
Previously used to diagnose and monitor sarcoid and lymphoma, but
increasingly superseded by
18
F- FDG PET (E Labelled leucocyte imaging,
pp. 944–5) and cross- sectional imaging. Sometimes useful in ‘pyrexia of
unknown origin’ (PUO), e.g. immunocompromised patient where there is
a suspicion of Pneumocystis jiroveci infection (cf. lung permeability studies).
Indications
• PUO and infection localization, especially inAIDS.
• Lymphoma follow- up.
• Sarcoidosis follow- up.
Patient preparation
None.
Procedure
• Inject
67
Ga- citrate IV. Gamma camera imaging at 48– 96h with tomography.
• Non- specic gut retention reduced by laxative administration.
Results
Normal uptake in lacrimal glands, nasal mucosa, blood pool, liver, spleen,
testes, ♀ perineum, breast (see Fig. 14.41a).
Interpretation
• Focal lymph node uptake in lymphoma and sarcoid distinguishes active
disease from post- therapy scarring/ brosis (see Fig. 14.41b).
• In AIDS, i lung uptake indicates infection— PCP, CMV,
mycobacterium— chest radiograph correlation essential.
• i activity in IBD and focal sepsis; largely superseded by WBC imaging.
Advantages
Excellent, non- invasive marker of disease activity in lymphoma— but likely
to be superseded by
18
F- FDG.
Pitfalls
Poor specicity. High radiation dose often dicult to justify when alternative
techniques available. Prolonged test (48– 96h).
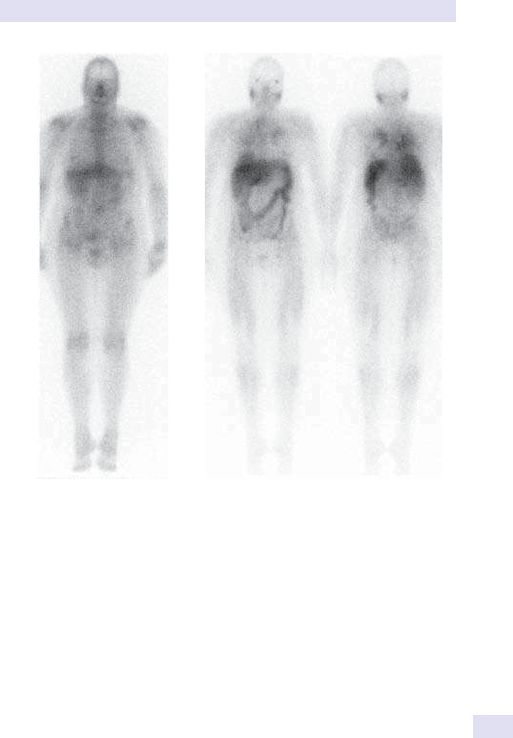
947
67
GALLIUM SCINTIGRAPHY
(a)
(b)
Fig.14.41 (a)Normal
67
Ga scan; (b)abnormal tracer uptake in the lacrimal glands,
parotid glands, mediastinum, and lungs, in keeping with known sarcoidosis.

948
CHAPTER14 Nuclear medicine
948
Dacroscintigraphy
Background
Epiphora may arise from excessive tear production or inadequate drainage
due to lower lid ectropion or nasolacrimal obstruction, i.e. nasal puncti, lac-
rimal sac, nasolacrimal ducts. Straightforward technique to assess function
of the nasolacrimal apparatus.
Indications
Epiphora.
Patient preparation
None.
Procedure
One to two drops of
99m
Tc- labelled DTPA or pertechnetate instilled into the
outer canthus of each eye. Immediate dynamic gamma camera imaging for
20min, with delayed static scans as required.
Results
Normal rapid radiopharmaceutical clearance through the nasolacrimal
apparatus.
Interpretation
Delayed clearance implies obstruction— level of dysfunction usually identi-
ed, i.e. punctum, lacrimal sac, nasolacrimal duct (see Fig. 14.42a andb).
Righ
tL
eft
Fig.14.42 Dacroscintigram (lacrimal drainage) showing normal lacrimal drainage on
the right, and on the left obstructed drainage at the proximal nasolacrimalduct.

DACROSCINTIGRAPHY
949
Advantages
Non- invasive. Avoids nasolacrimal duct cannulation (cf. dacrocystography).
Pitfalls
Obstructed systems result in excess radiolabelled tears on the cheek, alter-
ing drainagetimes.

950
CHAPTER14 Nuclear medicine
950
Salivary gland scintigraphy
Background
99m
Tc- pertechnetate uptake in the salivary glands reects intact parenchyma.
Salivary gland scintigraphy demonstrates both parenchymal function and
excretory function. Salivary gland scintigraphy is a safe and sensitive tech-
nique to assess the function and morphology of salivary glands.
Indications
• Sjögren’s syndrome.
• Chronic sialadenitis.
• Post- multiple radioiodine therapies.
• Irradiation of the head andneck.
Patient preparation
None.
Procedure
After IV injection of
99m
Tc- pertechnetate, dynamic scintigraphy is performed
for 15– 20min, and a sialagogue (e.g. lemon juice) stimulation is delivered
and imaging is continued for further 15– 20min to access excretory function.
Time– activity curves for the four major salivary glands are generated.
Results
Normal uptake of radiopharmaceutical in parotids and submandibular
glands, with spontaneous excretion following lemon juice stimulation.
Interpretation
Delayed or reduced tracer uptake or accumulation and would be compat-
ible with salivary gland dysfunction usually identied (see Fig. 14.43).
Advantages
Easy to perform, reproducible, and well tolerated.
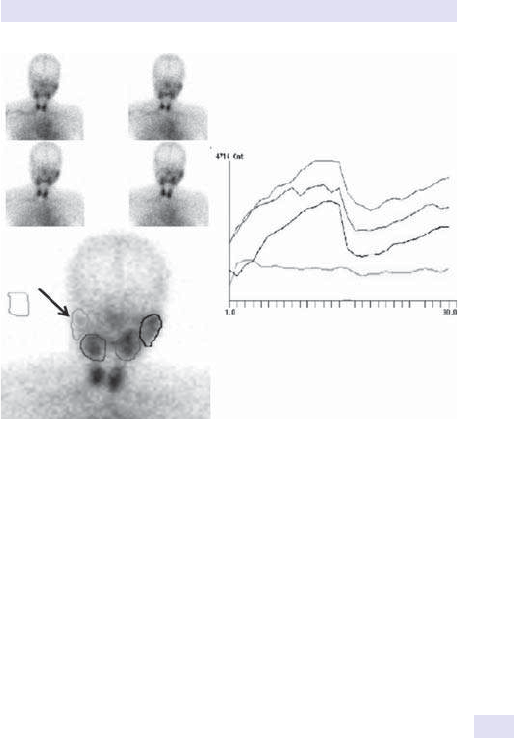
SALIVARY GLAND SCINTIGRAPHY
951
2
3
4
1
1
3
4
2
5
Fig.14.43 The left parotid and submandibular glands show prompt tracer uptake
and excretion. The right parotid gland shows poor uptake and function (arrow).

952
CHAPTER14 Nuclear medicine
952
Glomerular filtration rate measurement
Background
In many instances, GFR estimation from CrC is adequate, but accurate
measurement is essential in renal impairment or to monitor nephrotoxic
drug therapy. Glomerular compensation prevents early renal damage
detection by CrC measurements— 60% of ltration activity can be lost
before CrCfalls.
Indications
Accurate GFR to monitor renal failure, cytotoxic chemotherapy, immuno-
suppression, e.g. ciclosporin.
Patient preparation
Well hydrated.
Procedure
• IV injection of
51
Cr- EDTA or
99m
Tc- DTPA.
• Venous sampling 2 and 4hlater.
• Count plasma sample radioactivity and known standards in gamma
counter. Correct for height and weight.
Results
Normal GFR=125mL/ min (age- dependent).
Interpretation
d values in chronic renal failure.
Advantages
More reliable and reproducible than CrC— avoids need for urine collection.
Pitfalls
Accuracy depends on accurate measurement of dose and good injection
technique— avoid any extravasation. Unreliable results in severe peripheral
oedema.

UREA BREATHTEST
953
Urea breathtest
Background
Helicobacter pylori infection is associated with duodenal ulceration.
Eradication therapy reduces ulcer recurrence. H. pylori produces urease
which converts labelled urea l labelled CO
2
, detected in breath samples.
Indications
H.pylori detection— diagnosis and conrmation of eradication.
Patient preparation
Stop antibiotics, H
2
antagonists, proton pump inhibitors for 2– 4weeks.
Procedure
• Patient swallows urea drink labelled with
13
C (stable isotope) or
14
C
(radioactive isotope).
•
Breath samples (CO
2
) collected over next30min.
•
Labelled CO
2
measured by mass spectroscopy (
13
C) or liquid scintillation
counting(
14
C).
Results
Normal range varies according to local protocol.
Interpretation
i exhaled CO
2
levels imply abnormal urea breakdown by urease- producing
bacteria in the stomach, e.g. H.pylori.
Advantages
• Very sensitive marker of active H.pylori infection (cf. serology).
•
Non- invasive (cf. endoscopy) and avoids sampling errors.
• Good for non- invasive monitoring of recurrent symptoms.
Pitfalls
Occasional false +ves in oral H.pylori infection.

954
CHAPTER14 Nuclear medicine
954
Red cell survival studies
Background
Although infrequently performed, provides evidence of abnormal red cell
survival and localizes sites of red cell destruction. The investigation is pro-
longed, with initial in vitro red cell labelling and daily activity measurements
over target organs— spleen, liver, and heart— for 14days.
Indications
• Haemolytic anaemia (to conrm d RBC survival, i.e. active haemolysis).
•
Localize abnormal red cell sequestration.
• Predict response to splenectomy.
Patient preparation
None. Avoid blood transfusion duringstudy.
Procedure
• Obtain a venous blood sample, and label the patient’s red cells with
51
Cr- chromate.
• Re- inject cells and measure blood activity over 14days using gamma
counter.
•
Measure activity over the liver, spleen, and heart using gamma probe
daily for 14days.
Results
• Normal red cell half- life >24days.
• Equal fall in the heart, liver, and spleen counts withtime.
Interpretation
Short red cell life conrms abnormal destruction. Ratio of counts in
liver:spleen indicates the site of red cell destruction.
Advantages
Only available technique.
Pitfalls
Lengthy and labour- intensive. Sensitivity reduced by blood transfusion dur-
ing 14days’ measurement period. Consistent probe positioning essential for
accurate organ sequestration curves.

RED CELL VOLUME/PLASMA VOLUME MEASUREMENT
955
Red cell volume/ plasma volume
measurement
Background
Polycythaemia is suspected when Hb and Hct are raised. Routine labora-
tory screening does not dierentiate true polycythaemia, i.e. elevated red
cell mass, from apparent, stress, or pseudo- polycythaemia, i.e. reduced
plasma volume. Radiolabelled red cells can be used to measure red cell
mass. Plasma volume can be calculated from the Hct or measured indepen-
dently using radiolabelled human serum albumin.
Indications
Polycythaemia, to distinguish between true polycythaemia (i RBC mass)
from apparent polycythaemia (d plasma volume).
Patient preparation
Avoid recent therapeutic venesection. Less sensitive in patients already
receiving myelosuppressive therapy.
Procedure
• Obtain 10 mL of venousblood.
• Radiolabel red cells using
99m
Tc or
51
Cr.
•
Re- inject radiolabelled blood and, if required,
125
I- albumin.
• Obtain venous samples at 15 and30min.
• Count activity in blood samples, compared with known standards, using
gamma counter to establish plasma and red cell volumes.
Results
Compare measured red cell mass and plasma volume with predicted values
for height and weight.
Interpretation
Distinguish relative polycythaemia (due to d plasma volume) from genuine
elevation of red cellmass.
Advantages
Only technique available.
Pitfalls
Recent venesection or myelosuppressive therapy reduces test reliability.
Plasma volume measurement unreliable in severe peripheral oedema.

956
CHAPTER14 Nuclear medicine
956
Bile salt deconjugation studies
Background
Bile salts are synthesized and stored in the liver and are essential for ade-
quate GI absorption. They are excreted into the gut as conjugated, water-
soluble bile salts and recycled by the enterohepatic circulation with terminal
ileal reabsorption. Bile salt deconjugation products are insoluble and can-
not enter the enterohepatic circulation, leading to bile salt malabsorption.
Bacterial overgrowth after small bowel surgery or terminal ileal disease can
i deconjugation.
The
14
C- labelled synthetic bile acid glycocholine is administered PO.
Rapid transit into the large bowel will result in breakdown of the label by
the normal large bowel ora and a rise in detected exhaled
14
CO
2
. Small
bowel bacterial overgrowth, e.g. due to a ‘blind loop’, will also result in i
liberation of
14
CO
2
.
Indications
• Bacterial overgrowth.
• Bile salt malabsorption.
Patient preparation
• Starve overnight.
• Avoid antibiotics for 1month beforestudy.
Procedure
• Give oral
14
C- labelled glycocholic acid inwater.
• Count
14
CO
2
activity in breath samples over 6h using β liquid scintillation
counter.
Results
Glycocholate is deconjugated into
14
C glycine and cholic acid by small intes-
tine bacteria, releasing expired
14
CO
2
. Correct result for age- related varia-
tions in endogenous
14
CO
2
production.
Interpretation
i
14
CO
2
levels imply bacterial colonization or bile salt malabsorption.
Advantages
Accurate. Only availabletest.
Pitfalls
False −ves (unusual).

957
Index
A
abdominal aortic aneurysm
repair 846
abdominal distension 4– 5
abdominalpain 6
abdominal ultrasound 411
abdominal X- ray 410,
788– 92,808
acanthocytes 237
acanthosis nigricans 100
acetaminophen
poisoning 732– 5
acetylcholine receptor
antibodies 371
acid– base balance 664– 6
acid phosphatase 298,299
acquired, meaning 315
acromegaly 132
ACTH, basal plasma 164
activated partial
thromboplastin time
(APTT) 289,290
acute bacterial
meningitis 589
acute bronchitis 23
acute delirium 7
acute kidney injury 674
acute lymphoblastic leukae-
mia 299, 313,319
acute myeloid leukae-
mia 299, 313,319
acute pancreatitis 24
acute pericarditis 23
acute phase
markers 348,744
acute viral meningitis 589
Addison’s disease 164
adenosine,
contraindications 487
adrenal antibodies 368
adrenal failure 162– 4
adrenoleukodystrophy 164
airway hyperresponsiveness
536– 7
alanine transaminase 528
albumin 528
alcoholic ketoacidosis
211
aldosterone/ renin
ratio 150,152
algid malaria 427
alkaline phosphatase
416– 17, 528,749
alloantibodies 311
α
1
- antitrypsin 529
alpha- fetoprotein 416– 17,
517
α
- naphthyl acetate
esterase 298,299
alpha rhythm 614
alpha- thalassaemia 282
aluminium 694– 5,701
ambulatory ECG 478– 80
amenorrhoea 166,167
amiodarone 183
ammonium chloride loading
test 668
amniocentesis 820– 1
amniotic uid 404
amobarbital test 631
amphetamine
overdose 708– 9
amyloid 348,350
amyloidosis 350
anabolic steroid screen 423
anaemia 10– 11
acquired
haemolytic 284– 5
dimorphic 746
haemolytic 255,
266,284– 5
hereditary haemolytic 266
hypochromic 245
investigation algorithm 521
iron deciency 244– 7
macrocytic 10, 11,746
microcytic hypochromic
10, 11,746
normocytic normochromic
10, 11,746
pernicious 11
anal specimens 403
anaphylactoid reactions 12
anaphylaxis 12
aneuploidy 315
angiography 840– 2
arch 594
cerebral 856
computed
tomography 599,784
magnetic resonance 444,
603, 785,829
neurology 594
urinary tract 811
angio- oedema 12
anion gap 664– 6,720
anion gap acidosis 211,
665– 6,720
anorectal manometry 527
anorectal specimens 403
anorexia 13
anterior pituitary function
test 218
anti- adrenal antibodies 164
anti- amphiphysin
antibodies 637
anti- aquaporin- 4
antibodies 371
antibacterial
antibodies 338– 9
antibiotic allergy 422
antibiotic plasma
concentration
monitoring 422
antibiotic sensitivity 392– 4
anti- CAR antibodies 637
anti- centromere
antibodies 751
anticonvulsant
poisoning 710– 11
anti- CRMP5 antibodies 637
anti- CV2 antibodies 637
anti- cyclic citrullinated
peptide antibodies 750
anti- DNA antibody 751
anti- GAD
antibodies 367,371
anti- ganglioside
antibodies 371
anti- GBM disease 372
antigen tests 400
antiglobulin test 308
anti- glomerular base-
ment membrane
antibodies 698
anti- histone
antibodies 361,751
anti- Hu antibodies 371,637
anti- 21 hydroxylase
antibodies 164
anti- Jo- 1 antibodies 361,751
anti- Ki antibodies 361
anti- Ku antibodies 361
anti- La antibodies 361,751
anti- LC antibodies 364,529
anti- LKM
antibodies 364,529
anti- M2 antibodies 363
Anti- Ma2 antibodies 637
anti- Mi- 2 antibodies 361
antimitochondrial
antibodies 363,529
antimony poisoning 701
anti- neuronal antibodies
371
anti- neuronal nuclear
antibodies 371

INDEX
958
958
antineutrophil
antibodies 311
antineutrophil cytoplas-
mic antibodies 372,
698,751– 2
antinuclear antibodies 356,
529,750– 1
anti- phospholipase A2
antibodies 373
antiphospholipid
antibodies 362,751
anti- PLA- 2 antibodies 373
antiplatelet antibodies 311
anti- Pm- Scl (PM1)
antibodies 361
anti- Ri antibodies 371,637
anti- RNP
antibodies 361,751
anti- Ro antibodies 361,751
anti- Scl- 70
antibodies 361,751
anti- SLA antibodies 364
anti- Sm antibodies 361, 364,
529,751
anti- SS- A antibodies 361
anti- SS- B antibodies 361
anti- Ta antibodies 637
antithrombin 295
antithyroid antibody 180– 1
anti- TPO
antibodies 180– 1,366
anti- Tr antibodies 637
anti- TSH receptor
antibodies 180– 1,184
anti- U1- RNP antibodies 361
antiviral antibodies 338– 9
anti- Yo antibodies 371,637
anuria 14
anxiety 7
aortic disease 467– 9
aortic dissection 22,23
aortic regurgitation 462
aortic root dilatation 458
aortic stenosis 461– 2
aortography 594
apparent mineralocorticoid
excess 154
appendicectomy 634
arch angiography 594
arginine– growth hormone-
releasing hormone
test 217
ARMS PCR 282
arrhythmias 82
arsenic poisoning 701
arterial blood gas
sampling 416– 17,
538– 9,707
arthrocentesis 754
arthrography,
image- guided 854
arthroscopy 754
ascending
urethrography 810
ascites 4,404
ascitic uid 534
aspirin poisoning 736
asplenia 339
ataxia 15– 16
atrial brillation 472
atrial utter 472
atrial masses 467
atrial myxoma 467
atrial rhythms 472– 4
atrial septal defects 469
atrioventricular block 474– 5
atrioventricular re- entry
tachycardia 473
Auer rods 243,299
autoantibodies 311,
371,750– 2
autoantibody screen 356– 7
autoimmune hepatitis 364
autoimmune polyglandular
syndromes 368
autoimmune skin
disease 370
autoimmunity 354
automatic intracardiac
debrillation device 784
avascular necrosis, femoral
head 838
axonal neuropathy 607
B
B- cell acute lymphoblastic
leukaemia 313,319
bacterial antibody tests 399
bacterial antigen tests 400
bacterial culture 390– 4
bacterial overgrowth 523
Baermann concentration
technique 412
barium enema 806– 7
barium meal 798– 83
barium studies 794– 5
barium swallow 796– 7
Bartter’s syndrome 158– 9
basophilic stippling 237
behavioural alteration 7– 8
Bence– Jones
proteins 344,660
benzodiazepine
overdose 712
beta- 2- microglobulin 346
beta rhythm 614
beta- thalassaemia 282
bicarbonate 664
bicarbonate infusion
test 669
bile salt deconjugation
studies 956
bile salt malabsorption 519
biliary colic 24
biliary system
drainage 844– 5
bilirubin 261, 416– 17,528
biohazard levels
(1– 4) 402– 3
biopsy
bone marrow 418,634
brain 633
GI tract 412
image- guided 411,844
liver 412, 418– 19,532– 3
lung 419,778
lymph node 419
meningeal 633
muscle 421,632
nerve 421,632– 3
ocular 421
pericardial 421
pleural 420,562– 3
rectal 634
renal 688– 9
skin 420– 1,633
TB 427
temporal artery 38
tonsillar 421,634
transiliac bone 695
BiPLEDs 615
birch pollen allergy 375
bismuth poisoning 701
bite cells 237
bladder stones 686– 7
bleeding disorders 105– 6
bleeding time 287
blindness 117
blood lm 234– 5,408
blood glucose,
self- testing 204,205
blood products, bacterial
contamination 304,308
blood specimens 404
blood toxicology 700
blood transfusion 302– 3
bacterial contamina-
tion of blood
products 304,308
HLA- related issues 321
reactions 304– 6
body mass index (BMI) 79
bone biopsy 695
bone function tests 748– 9
bone lysis 861
bone marrow
biopsy 418,634
bone marrow
examination 296– 7,408
bone marrow
scintigraphy 872
bone scintigraphy
(scan) 762,868– 70
bone sclerosis 861
bowel biopsy 412

INDEX
959
bowel cancer screening
507
bowel habit 9
Bowel Scope Screening
Programme 507
bradycardia 17,471
brain biopsy 633
brain transporter
imaging 876– 7
brainstem auditory evoked
potentials 624– 6
brainstem death 706
branched- chain DNA 407
breast imaging 812– 15
breathlessness 18– 19
broad- complex
tachycardia 110
bronchitis 23
bronchoalveolar lavage 419
Bruce protocol 483
bruising 20
bullous pemphigoid 370
Burkitt’s lymphoma 319
burr cells 237
burst suppression 615
C
11
C- choline/
18
F- uorocholine
PET/ CT 902,905
C- reactive protein 348, 349,
416– 17, 744,745
C1 esterase inhibitor 352
C3 nephritic factor 354
CA 19- 9 517
Ca- 125 416– 17,517
cadmium poisoning 701
caeruloplasmin 529
calcium excretion 687,749
calcium pyrophosphate
disease 43,757
calf swelling 21
caloric testing 646
cancer antigen 125
(Ca- 125) 416– 17,517
cannabis 713
cannon waves 70
capsule endoscopy 412,510
captopril renography 928
carbamazepine toxicity 710
carbohydrate antigen 19- 9
(CA 19- 9) 517
carbohydrate
malabsorption 522
carbon monoxide
poisoning 714– 15
carcinoembryonic
antigen 517
cardiac arrhythmias 82
cardiac
catheterization 436– 9
cardiac CT 442– 8
cardiac enlargement 782– 5
cardiac magnetic
resonance 442– 8,785
cardiac markers 440– 1
cardiac masses 467
cardiac rhythms 472– 4
cardiac volumetric
imaging 442– 8
carotid ultrasound 592
CASPR2 antibodies 638
casts 685
cellular casts 685
cellular function
assays 378– 9
cellulose acetate 272
central cyanosis 31
central venous access 843
central venous lines 784
cerebellar ataxia 15– 16,369
cerebellar herniation 587
cerebellar nystagmus 77
cerebral angiography 856
cerebral blood ow
imaging 874,875
cerebrospinal uid 404, 418,
585,588
cerebrospinal uid shunt
patency 878,879
cerebrovascular
accident 858
cerebrovascular disease 592
cervical
lymphadenopathy 72
cervical spinal imaging 836
cervical swab 403
chest pain 22,23
chest tubes 784
chest X- ray 410,
770– 2,774– 9
chloride
plasma 664
urine 672
chloroacetate
esterase 298,299
cholangiography 802– 4
cholecystitis 24
chorionic villus
sampling 316– 17
chromium poisoning 701
chromograninA 525
chromosome
abnormalities 314
chromosome anatomy 317
chronic kidney disease,
classication 654– 6
chronic lymphocytic
leukaemia 313
chronic myeloid
leukaemia 319
Chvostek’s sign 190
citrate agar 272
citrate excretion 687
CK- MB 441
CKD- EPI equation 652
clonality assessment 313
clubbing 25
coagulation cascade 288
coagulation studies 408
cocaine 716
Cockcroft and Gault
formula 652
cold agglutinins 408
collapsing pulse 94
colonic polypectomy 500
colonoscopy 500,
506– 7,807
colour Doppler 454
colour vision 423
coma 26– 7, 619
combined anterior pituitary
function test 218
combined arginine– growth
hormone- releasing
hormone test 217
complement 351, 414,
696,745
complete blood count 228
compound motor action
potential 608
computed tomography
(CT) 824– 6
angiography 599,784
biopsy guidance 411
cardiac 442– 8
colonoscopy 411
cranial 598– 9
enteroclysis 800– 1
infection 410– 11
intravenous
pyelogram 411
rheumatology 762
spinal 599– 600
thorax 786– 7
urinary tract 810
urography 809– 10
venography 599
confusion 28, 619
congenital heart
disease 468– 9
conscious sedation 846
constipation 29– 30
constitutional, meaning 315
constrictive pericarditis 467
continuous glucose monitor-
ing systems 205
continuous- wave
Doppler 453
contrast agents, adverse
reaction 862
contrast
echocardiography 454
contrast nephropathy 690
Coombs’ test 308
copper 529,701

INDEX
960
960
corneal arcus 57
coronary computed tomog-
raphy angiography 784
cranial CT 598– 9
creatine kinase 440,441
creatinine 650,651
creatinine
phosphokinase 416– 17
crenated RBCs 236,238
Crithidia assay 358
cryobrinogen 345
cryoglobulins 345,697– 8
crystals 685
CSF 404, 418, 585,588
CSF shunt patency 878,879
CT angiography 599,784
CT colonoscopy 411
CT enteroclysis 800– 1
CT- guided biopsy 411
CT intravenous
pyelogram 411
CT urography 809– 10
CT venography 599
culture 388,390– 5
Cushing’s syndrome 142– 6
cyanide poisoning 717
cyanosis 31
cystatinC 655
cystine excretion 687
cystometry 630
cytochemical stains 298– 9
cytogenetics 314,642
haematological
malignancies 318– 19
prenatal diagnosis 316– 17
cytokines 414
cytotoxic T- lymphocyte
precursor assay 321
D
D- dimers 290,408
D- xylose absorption
test 413,522
dacroscintigraphy 948– 9
DEC test 422
deep vein thrombosis 21
delayed transfusion
reactions 306
deletion 315
delirium 7
delta rhythm 614
dementia 7, 619
demyelinating
neuropathy 607
dental radiography 410
dentarubropallidoluysian
atrophy 643
dermatitis herpetiformis 370
desamino D - arginyl vaso-
pressin trial 222
desferrioxamine test 694– 5
Devic’s disease 371
dexamethasone- suppressed
CRH test 143,223
dexamethasone
suppressiontest
high- dose 144– 5,224
low- dose 143,223
diabetes insipidus 134– 6
diabetes mellitus
diagnosis 194– 8
emergencies 210– 11
atbush 201
genetically inherited 203
gestational 195,200
laboratory assessment of
control 206– 8
latent autoimmune diabe-
tes of adults 200– 3
maturity- onset diabetes
of the young
(MODY) 201
monitoring control 204– 5
screening 199
type 1 200,201
type 2 200,201
types 200– 3
unusual causes 202
diabetic
ketoacidosis 210– 11
diabetic nephropathy 658
dialysis catheter 784
diaphragm 775
diarrhoea 32– 3, 429,520
diethylcarbamazine 422
dierential white cell
count 232,414
diusion tensor imaging 603
digital radiology 769
digital subtraction
angiography 841– 3
digoxin poisoning 718– 19
dimercaptosuccinic acid
imaging 924,927
dimorphic RBCs 236
diploid 315
dipstick urine test 676– 8
direct antiglobulin test 308
direct ophthalmoscopy 423
disseminated intravascular
coagulation 291
distal motor latency 607
dizziness 34– 5
DMSA scan 924,927
DNA amplication 324– 6
dobutamine,
contraindications 487
dobutamine stress
echocardiography 459
donor/ recipient
compatibility 321
Doppler studies 411, 453– 4,
593,816– 18
double- stranded DNA
antibodies 358
driving
dizziness/ syncope 35
rstt 41
drug allergy testing 377
dual- energy X- ray absorpti-
ometry (DXA) 763
Duke treadmill score 484
duodenal aspirate 412,523
duodenal biopsy 412
duplication 315
dynamic renography 924– 7
dysarthria 36
dysphagia 37
dysphasia 36
dyspnoea 18– 19
E
ear swab 403
echocardiography 411,
422,450– 69
ectopic gastric
mucosa 936,939
edrophonium test 631
Eisenmenger’s
syndrome 31,468– 9
ejaculate 404
ejection click 54
ejection fraction 458
elbow ossication
centres 863
electrocardiogram
(ECG) 422,470– 6
ambulatory 478– 80
electroencephalogram
(EEG) 423, 612,614– 20
electromyogram
(EMG) 423, 610– 11,630
electrophoresis
haemoglobin 272– 4,409
serum 342– 3,696– 8
urine 342– 3,344
elliptocytes 236
EMA binding test 268
embolization 841– 3
empyema 420
endocarditis 64, 425– 7,466
endocrine
autoantibodies 368
endocrine tests 124– 6
endomysial antibodies 369
endoscopic retrograde
cholangiopancreatogra-
phy (ERCP) 410, 500,
508,802
endoscopic ultrasound 509
endoscopy 498– 501
endotracheal tubes 782
enteroclysis 800– 1
enteroscopy 504

INDEX
961
enterotest 412
enzyme- linked assay 358
eosinophilia 747
epicardial pacing wire 784
epigastricpain 6
epilepsy 8, 616,618
epiphyseal plate
fractures 863
Epworth sleepiness
scale 542
Epworth test 542
erythrocyte sedimentation
rate (ESR) 252, 348,
408, 744,745
erythroenzymopathies
331
erythropoietin assay 310
ethanol poisoning 720– 2
ethylene glycol
poisoning 720– 2
event monitor 478
evoked potentials 622– 6
exercise
testing 482– 5,544– 5
exhaled nitric oxide
fraction 546– 7
external loop recorder 478
extractable antinuclear
antigen antibodies 360,
361,751
extraluminal gas 791– 2
F
18
F- uoride PET/
CT 904– 5,907
F wave 608
Fab fragments 718– 19
facial pain 38
factorH 354
factorI 354
faecal antigen test 512
faecal calprotectin 516
faecal chymotrypsin 524
faecal elastase 413,524
faecal fat, 3- day 522
faecal immunochemical
test 515
faecal incontinence 60
faecal lactoferrin 516
faecal occult blood 514– 15
Fairley test 681
falciparum malaria 241
Fallot’s tetralogy 468– 9
familial apo C- II
deciency 214
familial benign hypocalciuric
hypercalcaemia 188– 9
familial combined
hyperlipidaemia 212– 15
familial dysbeta-
lipoproteinaemia 214
familial hypercholesterolae-
mia 212– 15
familial hyperlipoproteinae-
mias 214
familial hypertriglyceridae-
mia 214
familial LPL deciency 214
Farr assay 358
fasting blood test 196
fat malabsorption 522
febrile transfusion
reactions 305
femoral head, avascular
necrosis 838
ferritin 244, 409, 529,745
fertility problems 168
fetal haemoglobin 280
FEV
1
/ FVC ratio 569
fever of unknown
origin 39,424– 34
FGF- 23 694
breoptic
bronchoscopy 548– 50
brin degradation
products 408
broblast growth factor
(FGF- 23) 694
Fibroscan
®
531
rst- degree AV block 474
rst- pass cardiac studies 912
FISH 328– 9
t, rst 40– 1
tness to y 552– 3
ash- evoked visual evoked
potentials 622
atbush diabetes 201
atus 4– 5
exible sigmoidoscopy/
colonoscopy 500,506– 7
ow– volume loops 554,555
uorescent in situ
hybridization 328– 9
ying 552– 3
folate status 248– 50
foreign bodies 403
fractional excretion of
phosphate 662
fractional excretion of
sodium 675
fractional excretion of
urate 663
fractional tubular
reabsorption of
phosphate 662– 3
fracture
epiphyseal plate 863
healing in children 863
pelvis 838
skull 861
terminology 850
fragileX 643
fragmented RBCs 236
Fredrickson
classication 215
free light chains 344
free T3/ T4 180– 1
Friedreich’s ataxia 643
frontal lobe syndrome 7
fructosamine assay 208
full blood count 228
functional iron
deciency 246
functional MRI 603,829– 30
functional residual
capacity 572
fungal antibody tests 399
fungal antigen tests 400
fungal culture 395
fungal meningitis 589
G
68
Ga- DOTA- peptide PET/
CT 898– 9,901
68
Ga- PSMA PET/
CT 900,903
GABA
B
R antibodies 638
gadolinium- based contrast
media 690
galactorrhoea 42, 172– 3
gallium
scintigraphy 946,948
gamma globulins 748
gamma glutamyl transpepti-
dase 416– 17,528
ganglionopathies 607
gastric biopsy 412
gastric emptying 527,
932,937
gastric parietal cell
antibodies 365
gastric ulcers 798– 9
gastritis 798– 9
gastrointestinal
bleeding 930,933
gastrointestinal
physiology 526– 7
gastrointestinal tract
412– 13
gastro- oesophageal
disease 22
genetic risk factors 644
genetic tests 642– 5
genital ulcers 403
gestational
diabetes 195,200
gestational
thyrotoxicosis 184
giant cell arteritis 117,592
gingival specimens 403
Gitelman’s syndrome 158– 9
glandular fever 254
Glasgow Coma Score 26
gliadin antibodies 369

INDEX
962
962
global left ventricular systolic
function 458
glomerular ltration
rate 655, 656,952
glucagon 795
glucose dipstick testing 677
glucose metabolism 416– 17
glucose- 6- phosphate dehy-
drogenase (G6PD) 409
assays 270
deciency 331
glycaemic
control 204– 5,206– 8
glycated
haemoglobin 206– 8
L- glycerate 687
glycolate 687
glycolytic defects 331
glycosuria 204,677
GlyR antibodies 638
Goodpasture’s
syndrome 372
gout 43,757
GPI- linked proteins 286
granular casts 685
Graves’ disease 182
growth hormone
excess 132
Guillain– Barré
syndrome 371,589
gynaecological imaging 822
gynaecomastia 44– 5,175
H
H bodies 237,280
H wave 608
haematemesis 46
haematocrit 231
haematuria 47, 684,691
haemoglobin
A
2
280
abnormalities 271
altered anity 330– 1
concentration 230,409
electrophoresis 272– 4,
409
fetal 280
glycated (HbA
1c
) 206– 8
H bodies 237,280
mean cell 231
mean cell
concentration 231
neonatal screen 279
normal adult 272
plasma 264
reticulocyte content 231
reticulocyte mean cell 231
rheumatology 746
sickle 278– 9
structural variants 271
unstable 281,330– 1
urine dipstick testing 677
variants 330– 1
haemolysis 256
haemolysis screen 11,409
haemolytic anaemia 255
acquired 284– 5
hereditary 266
haemolytic complement 353
haemoptysis 48
haemorrhage
lumbar puncture 587
retinal 97
splinter 64
subarachnoid 589
haemosiderin 244
haemosiderinuria 262,263
hair samples 420
hand imaging 852– 4
hand muscle wasting 118
haploid 315
haptoglobins 260,416– 17
HbA
1c
206– 8
HDL- cholesterol 212– 13
head injury 590,598– 9
headache 49– 50
Heaf test 422
heart murmurs 51– 4
heart rate 471
heart sounds 51– 4
heat- damaged red cell
imaging 940
Heinz bodies 237,281
Helicobacter pylori 512– 13
helminthic antibody
tests 399
helminthic antigen
tests 400
hepatitis, autoimmune 364
hepatitisA 530
hepatitis B 396, 397,
398,530
hepatitisC 530
hepatitisD 397
hepatitisE 530
hepatobiliary
scintigraphy 938,940
hepatomegaly 55
hepatosplenic
scintigraphy 942,944
hereditary elliptocytosis 268
hereditary
pyropoikilocytosis 268
hereditary
spherocytosis 268
hereditary
stomatocytosis 268
hernia, hiatal 798– 9
herpes gestationis 370
herpes simplex
encephalitis 589
herpes zoster 56
heterophile antibodies 254
hiatal hernia 798– 9
high- dose dexametha-
sone suppression
test 144– 5,224
high- performance liquid
chromatography 273
high- resolution computed
tomography 786,787
high vaginal swab 403
hila 778
hirsutism 170– 1
histamine challenge 536– 7
histoplasmin test 422
HIV 406,589
HLA- B27 752
HLA typing 320– 1,414
Holter monitor 478
hot stools 412
Howell– Jolly bodies 237
Hughes’ syndrome 362
human leucocyte antigen
(HLA) typing 320– 1,414
Huntington’s disease 643
hyaline casts 685
hybridization with nuclei acid
probes 407
hydrogen breath test 523
5- hydroxyindole acetic
acid 525
hyoscine butylbromide 795
hyperaldosteronism 150,
151, 152,153– 4
hypercalcaemia 188– 9,748
hypercholesterolaemia
212– 15
hypercortisolism 142– 6
hyperemesis
gravidarum 74– 5
hypergammaglobulinae-
mia 335
hyperkalaemia 160,670– 1
hyperlipidaemia 57,212– 15
hyperosmolar non-
ketotic (HONK)
syndrome 210– 11
hyperparathyroidism 188– 9
hyperpigmentation 100– 1
hyperprolactinaemia 172– 3
hypertension 58– 9, 148– 56
hyperthyroidism 182– 4
hypertriglyceridaemia
212– 15
hypoadrenalism 162– 4
hypocalcaemia 190– 2
hypochromic red
cells 231,236
hypogammaglobulinaemia
334– 5
hypogonadism 174
hypokalaemia 158– 9, 671
hyponatraemia 138– 40
hypopigmentation 100

INDEX
963
hypopituitarism 128– 30
hyposplenic lm 237
hypothalamic
dysfunction 128
hypothyroidism 186–7
hysterosalpingogram 822
I
IgA 334– 5
IgD 340
IgE
specic (RAST) 376
total 374
IgG 334, 336
IgM 334– 5
IL- 28R polymorphisms 414
ileal conduit specimens 680
immunoxation 342– 3,344
immunoglobulins 334– 5,
529,696– 8
immunohaematology 311
immunology 396– 9,
414,696– 8
immunophenotyp-
ing 286,312– 13
impaired fasting
glucose 195,197
impaired glucose
tolerance 195,197
impotence 174
‘in– out’ catheter urine
specimens 680
in situ hybridization 328– 9
incontinence
faecal 60
urinary 61
indigestion 62– 3
indirect antiglobulin
test 308
indirect
ophthalmoscopy 423
indwelling
catheters 403,681
infectious
disease 382– 7,388– 9
infective endocarditis 64,
425– 7,466
inferior petrosal sinus
sampling 144– 5
infertility 168
inammatory bowel
disease 516
inammatory
markers 744– 5
INR 288
insertable cardiac
monitor 478
inspiratory capacity 572
insulin antibodies 367
insulin receptor
antibodies 367
insulin tolerance (stress)
test 216– 17
international normalized
ratio (INR) 288
interstitial lung disease 787
interventional
radiology 844– 8
intestinal transit studies 527
intra- aortic balloon
pump 784
intracranial calcication 861
intracranial pressure,
raised 861
intra- ocular uids 404
intravascular stents 841– 3
intravenous
cholangiography 802
intravenous
urogram 410,808– 10
intrinsic factor
antibodies 365
inulin clearance 655,656
inversion 315
iodine 183
iron, serum 529,724
iron deciency,
functional 246
iron deciency
anaemia 244– 7
iron overload 247
iron poisoning 701,724– 5
iron status 244– 7
ischaemic forearm exercise
test 641
ischaemic lactate test 641
islet cell antibodies 367
isoelectric focusing 272
isolation plate 390– 4
J
J wave 476
Janeway lesions 64
jaundice 66, 67,428
jejunal aspirate 523
jerk nystagmus 77
Jod– Basedow eect 183
joint aspirate 404,754
joint pain/ swelling 68
joint replacements 851
jugular venous pulse 69,70
K
kaolin cephalin clotting
time 289
karyotyping 314,316– 17
ketones 678
ketonuria 204
kidney disease,
classication 654– 6
kidney function 650– 2
Kirschner wires
(K- wires) 850
Kleihauer test 309
koilonychia 10
L
L- glycerate 687
labelled leucocyte
imaging 944– 5
labelled red cell
imaging 930,933
lactate 416– 17
lactic acidosis 210– 11
lactose hydrogen test 522
lactose tolerance test 522
Lambert– Eaton myasthenic
syndrome 371
lamotrigine toxicity 710
laparoscopy 412
laryngeal swab 403
late- night salivary cortisol
test 143
latent autoimmune diabetes
of adults 200– 3
latex allergy 375
LDL- cholesterol 212– 13
lead exposure and
poisoning 701,726
left atrial dilatation 457– 9
left iliac fossapain 6
left iliac fossa swelling 5
left- shifted 243
left upper quadrantpain 6
left upper quadrant
swelling 5
left ventricular diastolic
function 459,460
left ventricular
dilatation 457– 9
left ventricular
hypertrophy 457– 9
left ventricular systolic
function 458
leg swelling 21,433
leucocyte alkaline
phosphatase 300
leucocyte esterases 677– 8
leucocyte nitrites 677– 8
leukaemia
cytochemical stains 298– 9
immunophenotyping 313
karyotyping 319
leukocyturia 684
LGI1 antibodies 638
libido loss 174
ligase chain reaction 407
lipid disorders 57,416– 17
lithium
poisoning 701,728– 9
liver biopsy 412,
418– 19,532– 3

INDEX
964
964
liver function tests 416– 17,
528– 31,748
loa loa 241
lobar collapse 780,781
loin pain 6,71
loin pain- haematuria
syndrome 71
long (depot) ACTH
test 164,225
low- dose dexametha-
sone suppression
test 143,223
lumbar puncture 423,584– 9
lumbar spinal imaging 837
lung biopsy 419,778
lung cancer 23
lung opacities 778– 9
lung permeability
studies 920
lung scan 914– 16
lung shunt studies 918,923
lung volumes 572– 4
lymph node sampling 419
lymphadenopathy 72– 3
lymphocyte function 380
lymphocyte surface
markers 378
lymphocytes, atypical 243
lymphocytosis 747
lymphoma, karyotyping 319
lymphopenia 747
lymphoscintigraphy 922,
925
lytic complement function
tests 353
M
macrocytic RBCs 236
macrophage function 380
macroprolactin 172– 3
magnesium, urine 673
magnetic resonance
angiography 444, 603,
785,829
magnetic resonance
cholangiopancreatogra-
phy 410,802– 3
magnetic resonance imaging
(MRI) 828– 34
breast 814– 15
cardiac 442– 8,785
functional 603,829– 30
infection 410
musculoskeletal
system 851
neurology 602– 4,630
paediatric brain 859
pelvis 822
perfusion imaging 830
rheumatology 761– 2
urinary tract 810
magnetic resonance
spectroscopy 603– 4
magnetic resonance
venography 603
major histocompatibility
complex (MHC) 320– 1
malaria 241,427
MALDI- TOF MS 392,393
malignant meningitis 589
mammography 812– 15
manganese poisoning 701
Mantoux test 422
marijuana 713
mass units 740
mast cell tryptase 377
maturity- onset diabetes of
the young (MODY) 201
maximum expiratory ow–
volume curve 554
Mazzotti test 422
MCV index 230
MDRD formula 650– 1
mean cell haemoglobin 231
mean cell haemoglobin
concentration 231
mean cell volume 230,409
Meckel’s diverticulum 518,
936,939
mediastinal mass 23,
775,776
mediastinum 775– 6
medical
thoracoscopy 578– 80
medicolegal samples 702
mega- oesophagus 797
MEN- 1/ MEN- 2 189
meningeal biopsy 633
meningitic illness 430– 2,589
mercury poisoning 701
meta- idiobenzylguanidine
(MIBG) imaging 884– 7
metabolic acidosis 707
metabolic alkalosis 707
metabolic coma 27
metal analysis 701
methacholine
challenge 536– 7
methaemalbumin 265
methaemoglobinaemia 730
methanol poisoning 720– 2
metoclopramide 795
metyrapone test 146
mGluR1 antibodies 638
mGluR5 antibodies 638
MIBG imaging 884– 7
microcytic RBCs 236
micturating
cystourethrogram 810
micturition urgency 114
midstream urine sample 680
mid- systolic click 54
Miller– Fisher syndrome 371
mineralocorticoid
excess 152,154
minimum inhibitory
concentration 392– 4
mitral regurgitation 463– 4
mitral stenosis 463
mitral valve prolapse 23
Mobitz type I block 474
Modication of Diet in
Renal Disease (MDRD)
formula 650– 1
molar units 740
molecular
cytogenetics 318– 19
molecular diagnostics 406– 7
molecular genetics 642– 4
monoclonal antibodies 313
monoclonal antibody
immobilization of platelet
antigens 311
monosomy 315
monospot test 254,409
motor conduction
velocity 606– 7
motor unit potentials 611
MR angiography 444, 603,
785,829
MR cholangiopancreato-
graphy 410,802– 3
MR venography 603
mu rhythm 614
MUGA scan 910– 11
mugwort pollen allergy 375
multigated radionuclide
angiography (MUGA)
scan 910– 11
multiple sclerosis
autoantibodies 371
evoked potentials 626
imaging 858
multiple sleep latency
test 621
murmurs 51– 4
muscle biopsy 421,632
musculoskeletal chest
pain 22,24
musculoskeletal
imaging 850– 1
myasthenia gravis 371
myelin- associated glyco-
protein antibodies 371
myelin basic protein
antibodies 371
myelodysplastic
syndrome 319
myelography 595
myeloperoxidase 298,299
myocardial infarction 23
myocardial ischaemia 22,
23,484
myocardial necrosis
markers 440– 1

INDEX
965
myocardial perfusion
imaging 486– 9,906– 8
myoglobin 440,441
myotonia 611
myotonic dystrophy 643
N
NAP score 300– 1
narcotics screen 423
narrow- complex
tachycardia 110
nasal specimens 403
nasogastric tubes 782
National Bowel
Cancer Screening
Programme 507
natural killer cell
function 380
nausea 74– 5
neck stiness 76
Nelson’s syndrome 100
neonatal haemoglobin
screening 279
nephrogenic systemic
brosis 690
nephrolithiasis 691
nephrotic syndrome 658
nerve biopsy 421,632– 3
nerve conduction
studies 423,606– 8
neuroendocrine
tumours 525
neurological
autoantibodies 371
neuromyelitis optica 371
neuronal surface antibody
antibodies 638
neuro- otology 646– 7
neuroradiology 856– 9
neurosyphilis 589
neutropenia 747
neutrophil alkaline
phosphatase 300– 1
neutrophil function 380
neutrophilia 747
Nijmegen
questionnaire 556– 7
NMDAR antibodies 638
non- HDL- cholesterol 212– 13
non- Hodgkin
lymphoma 319
normoglycaemia 195,197
nuclear medicine 866
nucleic acid sequence- based
amplication 407
nystagmus 77– 8
O
obesity 79– 80,142– 6
obstetric imaging 820– 1
obstructive uropathy 692
ocular biopsy 421
oedema, peripheral 87
oesophageal biopsy 412
oesophageal
manometry 526,527
oesophagogastroduodenos-
copy 500,502, 503
oligoclonal bands 635
oliguria 81
opening snap 54
ophthalmic specimens 403,
404,421
ophthalmoscopy 423
opioid poisoning 731
oral allergy 375
oral bowel cleansing 501
oral glucose tolerance
test 197– 8
organ donation 706
orthopantomogram 410
orthostatic
hypotension 34– 5
Osler’s nodes 64
Osler’s triad 429– 30
osmolal gap 720
osmotic fragility test 268– 9
osteitis brosa cystica 188
osteoarthritis 757
osteomalacia 191– 2
osteoporosis 763
ostium primum ASD 469
ostium secundum ASD 469
overdose 700– 1
oxalate crystals 721
oxalate excretion 687
oxyhaemoglobin dissociation
curve 539
P
P wave 474
PABA test 524
pacemakers 784
packed cell volume 231
Paget’s disease 757
palpitations 82
pancreatic amylase
416– 17
pancreatic exocrine
function 524
pancreatic
malabsorption 522
pancreatitis 24
pancreolauryl test 524
pancytopenia 83,747
paracetamol
poisoning 732– 5
paraesthesiae 84– 5
paragangliomas 155– 6
paralytic ileus 791
paranasal sinus aspirates 421
paraneoplastic
antibodies 637– 8
paraproteins 696– 8
parasites 240,241
parathyroid
hormone 694,749
parathyroid
scintigraphy 882– 3
parietal cell 250
paroxysmal nocturnal
haemoglobinuria 286
partial thromboplastin time
with kaolin 289
patent ductus arteriosus 31
pattern- evoked visual
evoked potentials 622
Paul– Bunnell test 254,409
PCR 324– 6,407
peak ow charts 558– 60
PEG placement 500
Pelger– Huët anomaly 243
pelvis 838,839
pemphigus 370
pencil cells 236
Pendred’s syndrome 186– 7
peptic ulcer disease 24
percutaneous transhepatic
cholangiography 802
percutaneous transluminal
angioplasty 841– 3
perfusion imaging 830
pericardial biopsy 421
pericardial disease 466– 7
pericardial eusion 466– 7
pericardial uid 404
pericardial pain 22
pericarditis
acute 23
constrictive 467
perinuclear antineu-
trophil cytoplasmic
antibodies 516
periodic acid- Schi 298,
299
periodic lateralized
epileptiform discharges
(PLEDs) 615
peripheral blood
lm 234– 5,408
peripheral CRH test 146
peripheral cyanosis 31
peripheral neuropathy 86
peripheral oedema 87
periumbilicalpain 6
pernicious anaemia 11
PET/ CT 411
11
C- choline/
18
F-
uorocholine 902,905
18
F- uoride 904– 5,907
68
Ga- DOTA- peptide
898– 9,901
68
Ga- PSMA 900,903

INDEX
966
966
petechiae 88
Peutz– Jeghers
syndrome 100
pH
GI monitoring 526
urine dipstick testing 676
phaeochromocytoma 155– 6
pharyngeal specimens 403
phenytoin toxicity 711
phosphate 749
phosphate
reabsorption 662– 3
picture archiving and
communication systems
(PACS) 769
pituitary fossa
enlargement 861
pituitary function 128– 30
plain X- rays 768
abdomen 410,
788– 92,808
chest 410, 770– 2,774– 9
dental 410
rheumatology 756– 8
skull 590,860– 1
plasma ACTH 164
plasma bicarbonate 664
plasma chloride 664
plasma haemoglobin 264
plasma potassium 670– 1
plasma viscosity 253
plasma volume 955
platelet adhesion 292
platelet aggregation 292
platelet clumping 233
platelet count 233, 292,747
platelet distribution
width 233
platelet function tests
292– 3
platelet immunouorescence
tests 311
platelet morphology 292
platelet release 293
plethora 89
pleural aspiration 540– 1
pleural biopsy 420,562– 3
pleural eusion 420,777
pleural uid 404,416– 17
pleural tumour 23
pleurisy 23
pleuritic pain 22
pneumococcal
pneumonia 429– 30
pneumomediastinum 776
pneumonia 23,429– 32
pneumothorax 23, 777,778
poisoning 700– 1
pollen– fruit syndrome 375
polychromatic RBCs 236
polycystic ovary
syndrome 166
polycythaemia 89
polydipsia 134– 6
polymerase chain reaction
(PCR) 324– 6,407
polymorphic ventricular
tachycardia 473
polysomnography 564– 5,
621
polyuria 90,134– 6
porphyrias 331
portal vein gas 791– 2
positron emission
tomography 596– 7,
762– 3,894– 7
post- mortem
toxicology 702
postpartum
thyroiditis 184,187
postural proteinuria 659
potassium
plasma 670– 1
urine 672
PR interval 474
precocious puberty 179
pre- diabetes 197
pregnancy
gestational
diabetes 195,200
hyperemesis
gravidarum 74– 5
hyperthyroidism 184
hypothyroidism 187
infections 416– 17
prenatal diagnosis 316– 17
procalcitonin 417
prostate- specic membrane
antigen 900,903
prosthetic heart
valves 54,466
prosthetic joints 851
protein, urine dipstick
testing 677
proteinC 295
proteinS 295
proteinuria 658– 60
prothrombin time (PT) 288,
290,528
protozoal antibody
tests 399
protozoal antigen tests 400
protozoal culture 395
pruritus 91
pseudo- Cushing’s
syndrome 142– 3
pseudo- gout 43
pseudohyperkalaemia 670
pseudohypokalaemia 671
pseudohyponatraemia 138
pseudohypoparathy-
roidism 190
pseudopseudohypoparathy-
roidism 190
pseudo- seizures 41
psoriatic arthritis 757
psychogenic limb
weakness 629
psychosis 7
ptosis 92
puberty
delayed 176,177
precocious 179
pulmonary artery
catheterization 492– 3
pulmonary embolism 23,93
pulmonary function
tests 423
pulmonary
hypertension 461
pulmonary
regurgitation 465– 6
pulmonary stenosis 465
pulmonary trunk
dilatation 458
pulmonary tuberculosis 23
pulse 65,94
pulse oximetry 566
pulsed- wave Doppler 453
pulsus alternans 94
pulsus bigeminus 94
pulsus bisferiens 94
pulsus paradoxus 94
pure culture 390– 4
pure tone audiometry 646
Purkinje cell antibodies 371
purpura 95
pus 405
pyrexia of uncertain
origin 39,424– 34
Q
Q waves 475
QRS axis 474
QRS complex 475
QT interval 476
R
radiation dose 767
radiofrequency
ablation 846– 7
radiographs, see plainX- rays
radioiodine
imaging 888,891
radiology information
systems (RIS) 769
radionuclide ventriculogra-
phy 490– 1,910– 11
ragweed pollen allergy 375
rapid urease test 513
Rasmussen’s
encephalitis 371
RAST tests 376
reactive arthritis 752

INDEX
967
recruitment 611
rectal biopsy 634
recurrent infections 335
recurrent thrombosis 96
red cell count 230
red cell distribution
width 231
red cell enzyme assays 270
red cell membrane
disorders 268– 9,331
red cell morphology 236– 8
red cell
parameters 230– 1,409
red cell survival studies 954
red cell volume 955
reux nephropathy 691– 2
regional left ventricular
systolic function 458
regional wall
motion 458,459
renal artery
stenosis 150,691
renal biopsy 688– 9
renal bone disease 694– 5
renal imaging 690– 2
renal stones 686– 7
renal transplant
dysfunction 692
renal tubular function 662– 3
renin elevation 150,152
renography
captopril 928
dynamic 924– 7
static cortical 924,927
repetitive nerve
stimulation 608
residual volume 572
respiratory acidosis 707
respiratory alkalosis 707
respiratory samples 419– 21
restriction fragment length
polymorphisms 407
reticulin R1 antibodies 369
reticulocyte
count 258– 9,409
reticulocyte haemoglobin
content 231
reticulocyte mean cell
haemoglobin 231
retinal haemorrhage 97
retrograde pyelography 811
rhesus haemolytic disease of
the newborn 309
rheumatoid arthritis 757
rheumatoid factor 355,750
rhonchi 120
right atrial dilatation 458
right atrial pressure 461
right heart function 459
right iliac fossapain 6
right iliac fossa swelling 5
right- shifted 243
right- to- left shunt 31,468– 9
right upper quadrantpain 6
right upper quadrant
swelling 5
right ventricular
dilatation 458
right ventricular
hypertrophy 458
Rigler’s sign 791– 2
rigors 97
rod cells 236
Roth spots 64
rouleaux 237,238
S
Saccharomyces cerevisiae
antibodies 516
sacroiliitis 838
salicylate poisoning 736
saliva 405
salivary gland
scintigraphy 950, 951
Salter– Harris
classication 863
Schilling test 250
Schumm’s test 265
scintomammography 892
SDS- PAGE 268
second- degree AV
block 474
secretin test 524
SeHCAT studies 519,934
selenium poisoning 701
Sellotape
®
strip test 412
semen 404
sensory ataxia 15
sensory conduction
velocity 606
sensory evoked
potentials 622– 6
sensory nerve action poten-
tial (SNAP) 606
sentinel node
imaging 890,895
sequencing 407
serology 396
serum aluminium 694– 5
serum amyloidA 348
serum bilirubin 261
serum caeruloplasmin 529
serum calcium 748
serum complement
components 351
serum copper 529
serum creatinine 650,651
serum electrophoresis
342– 3,696– 8
serum ferritin 529,745
serum free light chains 344
serum haptoglobins 260
serum immunoxation 342
serum
immunoglobulins 334– 5
serum iron 529,724
serum Na
+
417
serum parathyroid
hormone 749
serum urea 654
short ACTH (Synacthen
®
)
test 162, 225,417
short stature 98– 9,178
shunt patency 878,879
sick euthyroidism 180– 1
sickle cell disease 278,409
sickle cells 236,409
sickle haemoglobin 278– 9
sigmoidoscopy 412,
500,506– 7
silent thyroiditis 182– 4
silhouette sign 780
single- bre
electromyogram 611
single photon emission
computed tomogra-
phy (SPECT) 596– 7,
886,889
sinoatrial node
rhythms 472– 4
sinus tachycardia 110
sinus venosus ASD 469
skin autoimmune
disease 370
skin biopsy 420– 1,633
skin pigmentation 100– 1
skin prick tests 375,567
skin specimens 403
skull X- ray 590,860– 1
sleep stages 621
slit lamp 423
slow rising pulse 94
small bowel absorption
522
small bowel enema 800– 1
small bowel
follow- through 800– 1
small bowel
pathology 518– 19
small bowel studies 800– 1
smear cells 243
sodium
excretion 674– 5,687
sodium- retaining states 675
sodium- wasting states 675
somatosensory evoked
potentials 622– 4
somatostatin receptor
scintigraphy
68
Ga- DOTA- peptide PET/
CT 898– 9,901
SPECT 886,889
South East Asian
ovalocytosis 268
Southern blotting 322– 3

INDEX
968
968
specic gravity 678
specimen collection 402– 5
SPECT 596– 7, 886,889
spherocytes 236,258
sphincter disturbance 630
sphincter of Oddi
manometry 526
spinal
imaging 599– 600,836– 7
spinobulbar muscular
atrophy 643
spinocerebellar atrophy 643
spiral CTPA 411
spirometry 568– 9
splenic aspiration 421
splenic dysfunction 414
splenomegaly 102
splenunculi 940
splinter haemorrhages 64
‘spot’ urine tests 686
sputum 405, 419,570– 1
ST- elevation MI
(STEMI) 475
ST- segment 475
Stamey– Meares test 681
static cortical
renography 924,927
steatorrhoea 103
STEMI 475
stents 841– 3
sti person syndrome 371
stool samples 405,412
stress
echocardiography 459
stridor 104
stroke 107– 8
subarachnoid
haemorrhage 589
Sudan black 298,299
superwarfarin
overdose 739
suprapubic aspiration of
urine 680
suprapubicpain 6
supraventricular
arrhythmias 82
surface specimens 403
sutural widening 861
swallowing problems 37
Swan– Ganz
catheter 492,783
sweat test 576– 7
sweating 109
Synacthen
®
test 162,
225,417
syncope 34– 5
syndrome of inappro-
priate antidiuretic
hormone 138– 40,675
synovial uid 404,754
systemic lupus
erythematosus 360
T
T- cell acute lymphoblastic
leukaemia 313,319
T- cell subsets 414
T- tube
cholangiogram 802– 3
T wave 475
T3 180– 1
T3 toxicosis 182
T4 180– 1
tachycardia 110– 11,471
target cells 236
teardrop RBCs 236
temporal artery biopsy 38
temporal (giant) cell
arteritis 117,592
temporal lobe epilepsy 8
Tensilon
®
test 631
tertiary
hyperparathyroidism 189
testosterone
low 174
raised 170– 1
tetralogy of Fallot 468– 9
thalassaemia 271, 276,
282– 3,330
thallium poisoning 701
theophylline poisoning 737
therapeutic
embolization 841– 3
thermodilution method 492
theta rhythm 614
third- degree AV block 475
thoracic aortic dissection 23
thoracic CT 786– 7
thoracic spinal imaging 837
thoracocentesis 777– 8
thoracoscopy 578– 80
3- day faecal fat 522
throat specimens 403
thrombin clotting time 290
thrombocytopenia 88,
409,747
thrombocytosis 747
thrombolytic therapy 842
thrombophilia
screen 96,294
thrombosis, recurrent 96
thyroid
autoantibodies 180– 1
thyroid cancer 888,891
thyroid function
tests 180– 1
thyroid peroxidase (TPO)
antibodies 180– 1,366
thyroid
scintigraphy 880,881
thyroid- stimulating
hormone 180– 1
thyroid storm 184
thyrotoxicosis 182– 4
tidal volume 572
tilt table testing 494– 5
Tine test 422
tinnitus 112
tiredness 113
tissue transglutaminase
antibodies 369
tissue typing 320– 1,414
Todd’s paralysis 107– 8
tonsillar biopsy 421,634
total cholesterol 212– 13
total lung capacity 572
toxic granulation 243
toxic metabolic
encephalopathies 619
toxicology 700– 1,
702,704– 5
toxin tests 412
trace element analysis 701
transcranial Doppler 593
transcranial magnetic
stimulation 628– 9
transcription- mediated
amplication 407
transfer factor 582
transferrin 244
transferrin receptor 244
transferrin saturation 529
transfusion reactions 304– 6
transient elastography 531
transient
hypothyroidism 187
transient thyroiditis 182– 4
transiliac bone biopsy 695
transjugular intra- hepatic
portosystemic shunt 846
translocation 315
transoesophageal
echocardiography 454– 7
transthoracic
echocardiography 454– 7
tricuspid
regurgitation 464– 5
tricuspid stenosis 464
tricyclic antidepressant
poisoning 738
triglycerides 212– 13
trisomy 315
Troisier’s sign 62
tropical disease
diagnosis 388– 9
troponins 440,441
Trousseau’s sign 190
trypanosome 241
tuberculosis 23,426– 7
tuberculosis skin tests 422
tuberculous meningitis 589
tubular proteinuria 659
tubular urate handling 663
tumour markers 517
24h urinary copper
excretion 529

INDEX
969
24h urinary- free cortisol
collections 143
24h urine collections 687
two glass test 680
U
U wave 476
UK Haemoglobinopathy
Reference
Laboratory 283
ultrasound 816– 17
abdominal 411
breast 814
cholangiography 802
endoscopic 509
gynaecology 822
neurology 592–3
obstetric 820– 1
rheumatology 759– 60
urinary tract 810
uncal herniation 587
urea 654
urea breath test 413,
512,953
urethral specimens 403
urethritis 432
urgency 114
uric acid 748
urinary acidication 668– 9
urinary calcium 687,749
urinary chloride 672
urinary citrate 687
urinary cystine 687
urinary glycolate 687
urinary
haemosiderin 262,263
urinary 5- hydroxyindole
acetic acid 525
urinary incontinence 61
urinary L- glycine 687
urinary light chains
(BJ proteins) 344,660
urinary magnesium 673
urinary oxalate 687
urinary oxalate crystals 721
urinary potassium 672
urinary sodium 674– 5,687
urinary system drainage 844
urinary tract
radiology 808– 11
urine culture 680– 2
urine dipstick testing 676– 8
urine electrophoresis
342– 3,344
urine glucose testing 204
urine
immunoxation 342,344
urine microscopy 681– 2,
684– 5
urine samples 404, 680,681
urine toxicology 700
urobilin 262
urobilinogen 262
urodynamics 630
uroowmetry 630
urticaria 115
V
vaginal specimens 403
valproate toxicity 710
valvular heart disease 461– 6
vascular catheterization 842
vascular interventions 840– 2
vasculitic
syndromes 116,372– 3
vasoactive peptides and
amines 525
vasovagal syncope 34– 5
vegetations 64
ventilation/ perfusion
imaging 914– 16
ventricular arrhythmias 82
ventricular brillation 473
ventricular masses 467
ventricular rhythms 472– 4
ventricular septal
defects 469
ventricular tachycardia 473
vesico- ureteric reux 71
vesicular rash 433– 4
vestibular ataxia 15
vestibular nystagmus 77
VGCC antibodies 638
viral antibody tests 397
viral antigen tests 400
viral culture 394
viral haemorrhagic fever 431
Virchow’s triad 96
virilization 170– 1,171
virtual colonoscopy 807
visual evoked potentials 622
visual loss 117
vital capacity 572
vitamin B
12
248– 50,409
vitaminD 417
vitiligo 100
vomiting centre 74– 5
V/ Q scan 914– 16
W
Wada test 631
waist girth 79
warfarin overdose 739
water deprivation
test 220– 1
water intoxication 139– 40
waxy broad casts 685
weight loss 119
wheeze 120
white cell casts 685
white cell count 232,746– 7
white cell
morphology 242– 3
whole body
plethysmography 572– 4
whole genome
sequencing 644
wireless capsule
endoscopy 412,510
Wol– Chaiko eect 183
Wol– Parkinson– White
syndrome 474
word salad 36
X
X- rays, see plainX- rays
xanthelasmata 57
xanthomata 57
Z
zinc poisoning 701
zinc protoporphyrin 726
970