
OCTOBER 2021
A Blueprint for
Enhancing the
Security of the U.S.
Pharmaceutical
Supply Chain
2nd Edition

AAM / A BLUEPRINT FOR ENHANCING THE SECURITY OF THE U.S. PHARMACEUTICAL SUPPLY CHAIN
2

AAM / A BLUEPRINT FOR ENHANCING THE SECURITY OF THE U.S. PHARMACEUTICAL SUPPLY CHAIN
3
A Blueprint for Enhancing the Security
of the U.S. Pharmaceutical Supply Chain
Introduction
Identifying the List of Medicines Most Critical for U.S. Manufacturing
Company-Targeted Incentives Necessary to Secure the U.S.
Pharmaceutical Supply Chain
Broad-Based Incentives Critical to Supporting an Expanded U.S.
Pharmaceutical Economic Footprint
Increasing U.S. Pharmaceutical Supply Chain Security Through Global
Coordination
4
6
7
8
10

AAM / A BLUEPRINT FOR ENHANCING THE SECURITY OF THE U.S. PHARMACEUTICAL SUPPLY CHAIN
4
A Blueprint for Enhancing the Security of the U.S.
Pharmaceutical Supply Chain
INTRODUCTION
A closely connected, diverse, high-quality and resilient pharmaceutical supply chain based in the United
States and in allied countries is the best means to ensure that U.S. patients and our health care system
have access to a secure and consistent supply of critical medicines. The United States already plays an
important role in this supply chain, with generic companies providing more than 52,000 jobs at nearly
150 facilities, and manufacturing more than 60 billion doses of prescription medicines annually
1
in this
country. This domestic presence, combined with the strength of the industry’s globally diverse supply
chain, allows U.S. patients continued access to the medicines they need, including during the COVID
pandemic.
With strategic support from the U.S. government, the economic footprint of the generic drug industry in
the U.S. can expand even more, leading to increased national security, a stronger, more redundant
supply chain for key pharmaceuticals or their components and an expanded employment base.
The Association for Accessible Medicines and our member companies are ready to partner with U.S.
policymakers to create an environment where even more American jobs play a greater role in ensuring
a resilient U.S. pharmaceutical supply chain.
1 AAM Member Survey and other sources.
AAM / A BLUEPRINT FOR ENHANCING THE SECURITY OF THE U.S. PHARMACEUTICAL SUPPLY CHAIN
5
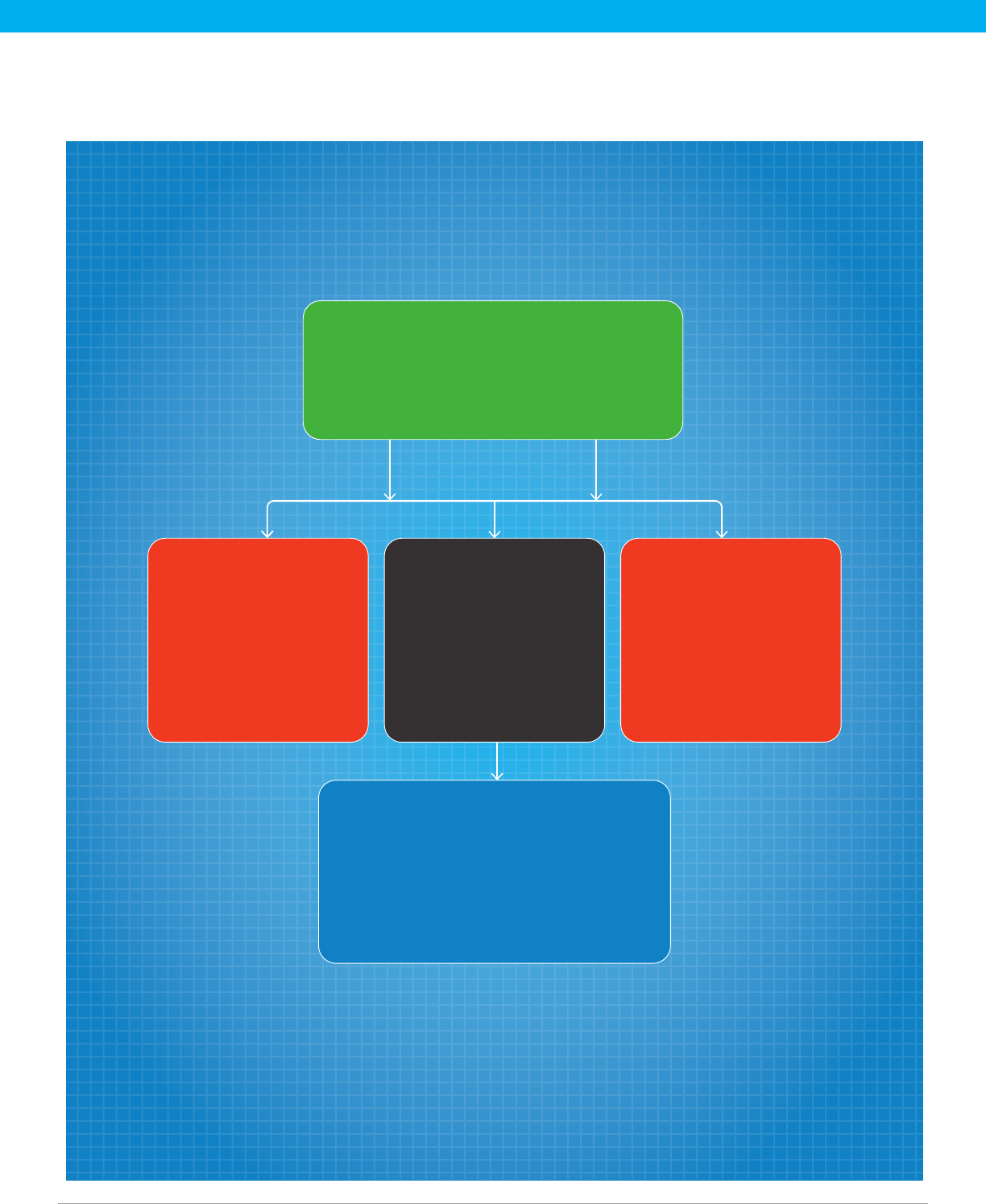
USTR andHHS
launchmultilateraltalks to
promote a cooperative
approachto developing
amanufacturing
basein alliedcountries
HHS engages indirect
discussions with pharma
manufacturersto promote
U.S. manufacturing of
essentialmedicines
FDA strengthens internal
coordination to
ensure expanded
manufacturer engagement
and efficient regulatory
review and approval
Tools to enable viable investments include
grants, tax incentives, loans, guaranteed
price & volume contracts, incentives in the
Medicare and Medicaid programs, and
adoption of regulatory efficiencies
Essential Medicines List:
A list of medicines critical to the U.S. health
care system, updated and shaped by
stakeholder input; reflected in an expanded
Strategic National Stockpile
Expanding HHS’ Ability to Secure the U.S. Pharmaceutical Supply Chain:
As the United States cannot produce all the medicines needed for its health care system
and its patient population, the U.S. Department of Health and Human Services (HHS)
must continue engaging with the pharmaceutical industry to identify specic incentives
necessary to maintain, utilize and attract investments that can cost as much as $1 billion
and take 5-7 years to build.
Key Elements
Proposed Policy Framework

AAM / A BLUEPRINT FOR ENHANCING THE SECURITY OF THE U.S. PHARMACEUTICAL SUPPLY CHAIN
6
A. IDENTIFYING THE LIST OF MEDICINES MOST CRITICAL FOR U.S. MANUFACTURING
• List of Essential Medicines. To ensure U.S. government and private sector resources are focused
on the medicines most important to the U.S. health care system at times of urgent need, the
Secretary of Health and Human Services (HHS), acting through the FDA Commissioner, should
establish and update periodically following stakeholder input a list of essential medicines for the
United States. Essential medicines are defined as the active pharmaceutical ingredient (API) and
finished dosage form, including drugs, biologics and medical countermeasures. The list of essential
medicines should include medicines deemed most critical to the U.S. health care system, vital
during a secretary-designated public health emergency and/or those whose shortage could impact
U.S. national security. In developing the list, the Secretary should consult with the U.S. Food and
Drug Administration (FDA), Centers for Disease Control and Prevention (CDC), National Institutes of
Health (NIH) and other public health agencies, as well as the Secretary of Defense and Secretary of
State. The list shall be subject to a 60-day public comment period.
• Expanding the Strategic National Stockpile (SNS) to Include Medicines On the Essential
Medicine List. Ensuring ample supply to the most critical medicines during a national disaster,
pandemic or trade dispute demands the U.S. government secure stockpiles of the medicines on the
List of Essential Medicines, including specific Finished Dosages (FD) and Active Pharmaceutical
Ingredients (API). HHS may take possession of such purchases or may pay manufacturers an
inventory management fee to produce and maintain the specified quantity on behalf of the SNS.
Specific volume and price levels must be negotiated on a company-by-company basis and should
factor in product expirations and inventory management costs. HHS shall engage actively with the
pharmaceutical industry to develop the framework and support necessary to quickly expand the SNS.

AAM / A BLUEPRINT FOR ENHANCING THE SECURITY OF THE U.S. PHARMACEUTICAL SUPPLY CHAIN
7
B. COMPANY-TARGETED INCENTIVES NECESSARY TO SECURE THE U.S. PHARMACEUTICAL
SUPPLY CHAIN
As the U.S. government works to incentivize expanded and new investments by generic manufacturers
in the United States, HHS, the Department of Veteran Affairs (VA), the Department of Defense (DoD)
and other government agencies will work closely with individual companies to help secure the U.S.
pharmaceutical manufacturing base for priority medicines, including for specic FD and API. These
incentives include:
• Long-Term Price and Volume Guaranteed Contracts. Guaranteed fixed volume and price
agreements are essential to ensuring the viability of U.S.-based generic manufacturing for essential
medicines and inoculating those investments against low-priced imports of the same medicine.
When engaging with the industry, however, HHS, the VA and the DoD must encourage multiple
suppliers in the market and ensure, whenever possible, that no one company supplies the entire
market (this protects against supply disruptions). HHS should leverage fixed price and volume
guaranteed contracts when expanding the SNS and the VA shall utilize fixed price and volume
guarantees for national contracts to supply the VA and DoD.
• Grants. HHS shall provide grants to support construction, alteration or renovation of facilities for
the U.S.-based manufacture of medicines included on the List of Essential Medicines. Grants
should support pharmaceutical manufacturers relocating production facilities from outside of the
United States back to the United States to cover expenses in moving production and include funds
to offset the cost of building new factories and research centers. Such grants should be provided
only to 1) manufacturers with an approved Abbreviated New Drug Application (ANDA), 2)
manufacturers of an authorized generic, 3) external/contract manufacturers of approved ANDAs or
authorized generics or 4) API manufacturers with a pending or approved Drug Master File. To
support a diverse and reliable supply, such grants must be available to multiple manufacturers of
the same medicine. Grants will be administered by HHS/Biomedical Advanced Research and
Development Authority (BARDA).

AAM / A BLUEPRINT FOR ENHANCING THE SECURITY OF THE U.S. PHARMACEUTICAL SUPPLY CHAIN
8
C. BROAD-BASED INCENTIVES CRITICAL TO SUPPORTING AN EXPANDED U.S.
PHARMACEUTICAL ECONOMIC FOOTPRINT
Certain additional measures will be necessary to support the economic viability of a U.S.-based
pharmaceutical for the entirety of the U.S. generic pharmaceutical manufacturing base:
• Tax Incentives. New tax incentives must be adopted that promote U.S. pharmaceutical companies
relocating foreign manufacturing back to the United States, build new greenfield sites, refurbish
already existing manufacturing facilities and/or repurpose existing production lines to focus on
pharmaceuticals that appear on the List of Essential Medicines. Specific tax incentives that will
facilitate the expansion of U.S. pharmaceutical manufacturing include:
» A 50% tax credit to offset the costs of manufacturing medications on the priority medicines list
in the United States. The credit is available for as long as the medicine is on the list of priority
medicines and for five years thereafter.
» An increase in the simplified R&D tax credit to 20%
» A tax credit for expenses related to bioequivalence studies via the Research and Development
(R&D) tax credit
» A tax credit for initial and recurring FDA user fees
» A 25% reduction in the tax rate for U.S.-manufactured medicines on the Essential Medicine List
(modeled after the Section 199 tax reduction)
» A 20% tax credit based on wages paid to U.S. workers directly engaged in the production of
essential medicines
» An assurance that grants provided for the establishment of U.S. production of medicines are not
considered taxable income
• Leveraging the Medicare and Medicaid Programs. The Medicare and Medicaid programs
collectively provide health care to more than 136 million beneficiaries. Policymakers can update
these programs to support greater domestic production of essential generic medicines. This can be
accomplished by addressing the long-term viability of domestic manufacturing through the
following three proposals:
» Waive Medicaid rebates for domestically manufactured generic medicines on the FDA’s
Essential Medicines List.

AAM / A BLUEPRINT FOR ENHANCING THE SECURITY OF THE U.S. PHARMACEUTICAL SUPPLY CHAIN
9
» Establish a Medicare Star Rating to reward Part D plans that preference domestically
manufactured generic medicines included on the FDA Essential Medicines List.
» Provide bonus payments in Medicare Part B for providers who administer domestically
manufactured generic medicines included on the FDA Essential Medicines List.
• Regulatory Efficiencies. To expedite the approval of a facility and all the products to be produced in
it, the FDA must streamline its regulatory review and approval processes, removing duplicated
actions and reducing the time for approvals across the board. The agency should expand
cooperation with the manufacturer, working collaboratively to evaluate and approve the facility and
the tech transfer processes concurrently, as opposed to waiting until after the facility is built and the
equipment is installed/validated.
To accomplish these goals, the FDA should create an internal, intra-agency working group focused
on helping to expedite reviews and approvals to onshore pharmaceutical manufacturing. This
working group will comprise resources from the Office of Regulatory Affairs; the Office of
Pharmaceutical Quality; the Office of Compliance; reviewers from both chemistry and microbiology
disciplines; and the Office of Generic Drugs. This working group will focus on reviewing for approval
the transfer of production back into either U.S.-approved facilities or newly constructed facilities at
new or existing sites, including those utilizing advanced manufacturing technology. This working
group will grant meetings with the company to discuss the overall transfer plans. For example:
» Inspector(s) and Office of Pharmaceutical Quality staff will make site visit(s) during the
construction or validation phase.
» The mechanism will be similar to a pre-ANDA meeting – that is, a developmental phase
inspection and then a pre-submission inspection.
» Microbiology reviewer(s) will conduct site visits.
» Decouple submission and inspection. Inspections will be completed within 30 days of request
for inspection, regardless of submission.

AAM / A BLUEPRINT FOR ENHANCING THE SECURITY OF THE U.S. PHARMACEUTICAL SUPPLY CHAIN
10
D. INCREASING U.S. PHARMACEUTICAL SUPPLY CHAIN SECURITY THROUGH GLOBAL
COORDINATION
• The International Pharmaceutical Supply Chain Agreement. To promote the benefits of a globally
diverse supply chain, the United States Trade Representative (USTR), working with HHS, should
negotiate a plurilateral agreement with U.S. allies to promote a cooperative approach to securing
the U.S. supply chain, ensuring diversity of supply and responding to global health care challenges
and natural disasters, without resorting to export controls or other barriers that inhibit the trade of
essential medicines or their components (including API). In addition, coordinating the expansion of
pharmaceutical manufacturing with U.S. allies will allow for economies of scale and a coordinated
approach to global pandemics.
Denitions
• “Generic drug” means “any drug that is marketed under an abbreviated new drug
application (ANDA) as well an ‘authorized generic drug.’”
• “Manufacture” has the meaning set forth in the Buy American Act, 41 U.S.C. §§8301-
8305: “completion of an article in the form required for use by the government in the
United States. For drugs this means readied for use as a medicine for human
consumption.”

AAM / A BLUEPRINT FOR ENHANCING THE SECURITY OF THE U.S. PHARMACEUTICAL SUPPLY CHAIN
11

accessiblemeds.org
©2021 Association for Accessible Medicines.
