
July 2024
ASPE REPORT
1
The Potential Role of The Nonprofit Pharmaceutical
Industry in Addressing Shortages and Increasing Access to
Essential Medicines and Low-Cost Medicines
Oluwarantimi Adetunji, Jon F. Oliver, Sonal Parasrampuria, Grace Singson, Trinidad Beleche
Key Points
o We identified 11 nonprofit pharmaceutical companies that launched between 2000-2022 with mission
statements aimed to enhance access to affordable and essential drugs, or resiliency in the supply chains
of medical products.
o Some of these nonprofit pharmaceutical companies owned for-profit subsidiaries as a strategy to
navigate a complex tax system; the majority did not have any medical products in the domestic
pharmaceutical marketplace, none of them owned their own manufacturing facilities, and their
financial statements suggested these tax-exempt companies were comparable in scale to for-profit
small businesses and start-up companies.
o Two nonprofit pharmaceutical companies that have successfully launched medical products in the
United States used different strategies for commercialization, including using contract manufacturing
organizations for labeling and distribution and entering into a licensing agreement with a for-profit
pharmaceutical company.
o Findings suggest that while nonprofit pharmaceutical companies hold promise in addressing drug
shortages and enhancing access, their capacity and sustainability may be limited due to low production
volumes, uncertainties about funding, and inexperience navigating complex tax, regulatory, and
reimbursement systems.
Introduction
Americans rely on medical products, such as prescription drugs, to prevent or treat acute and chronic diseases.
However, persistent high prices and shortages threaten access to lifesaving therapeutics and pose a risk to the
capacity of America’s health system to effectively mitigate and respond to public health emergencies and
ongoing public health issues.
1,2
These market gaps in the pharmaceutical industry have attracted the adoption
of different business models, such as nonprofit pharmaceutical companies, which are launched with any
specified purpose other than making a profit.
The prevailing business model in the pharmaceutical industry is oriented toward the pursuit of the next
blockbuster drug, defined as a drug with $1 billion or more in annual global sales.
3
For-profit companies may
utilize their profits to reinvest in research and development (R&D) or deliver a high return on investment to
shareholders. However, critics argue that the blockbuster model in the pharmaceutical market incentivizes a
non-innovative culture that results in duplicative and nonproductive ventures targeted to populations where
high revenues are guaranteed.
4
Further, some researchers have indicated that the prioritization of developing
blockbuster drugs contributes to higher prices and unmet public health needs, such as shortages of older
REPORT
JULY 2024
OFFICE OF
SCIENCE & DATA POLICY

July 2024
ASPE REPORT
2
generics and underinvestment in R&D for new antibiotics and drugs for certain rare diseases.
3-6
Researchers
have proposed alternative business models to promote growth in the nonprofit pharmaceutical sector and
address some of the existing market gaps.
7-10
These alternative business models include nonprofit
collaborations with for-profit companies to increase manufacturing and distribution capacity, creation of
nonprofit manufacturing and distribution companies, and partnering with other stakeholders to strengthen
existing capability and expertise (Appendix A).
While the emergence of nonprofit companies to address market failures is not a new phenomenon in non-
pharmaceutical markets,
11
there has been growing interest, including from Congress,
12
in understanding
whether nonprofit pharmaceutical companies could offer solutions to the challenges of drug access and
affordability.
8
Drawing from an environmental scan of the literature and key stakeholder interviews, this
report examines the ways in which nonprofit pharmaceutical companies can address a number of existing
gaps, including their potential role in reducing drug shortages, increasing access to essential medicines, and
providing low-cost alternatives to expensive medications.
Methodology
The Office of the Assistant Secretary for Policy and Evaluation (ASPE) used a qualitative methods approach that
included both an environmental scan and key stakeholder interviews. NORC, under contract with ASPE,
conducted the preliminary searches and key stakeholder interviews.
Defining Nonprofit Pharmaceutical Companies
Nonprofit pharmaceutical companies are tax exempt entities that have an established presence in the
pharmaceutical industry, typically pursuing R&D activities and licensing new drugs to for-profit companies. In
this report, we define a nonprofit pharmaceutical company as a tax-exempt entity with a publicly disclosed
goal of pursuing market authorization and commercialization of drugs to deliver low-cost medicines, including
essential drugs and drugs in shortage, and broadening access to medical products. This excludes nonprofit
companies that may invest in drug development to secure licensing agreements with for-profit companies and
nonprofit companies that provide contract services (e.g., contract development manufacturing organizations
(CDMOs)).
Environmental Scan
The environmental scan used a list of primary and secondary search terms to identify peer-reviewed and grey
literature relevant to the topic areas of interest: the nonprofit pharmaceutical sector, low-cost alternatives to
expensive medications, drug shortages, and access to essential medicines. The initial search terms included
keywords such as (“nonprofit pharmaceutical company” OR “nonprofit biopharmaceutical sector”) AND
(“generic drugs” OR “low-cost alternatives”). Appendix B describes the search terms for the preliminary
searches conducted by NORC. The inclusion criteria included materials published in English between 2000 and
January 2023. In addition, ASPE supplemented the preliminary searches conducted by NORC using a
“snowball” approach to identify other relevant information.
Further, we cross-referenced the list of nonprofit pharmaceutical companies generated from the
environmental scan with the IQVIA National Sales Perspective (NSP) dataset to gather market information such
as the number of products and total sales from January 2017 to December 2022. For the identified products
sold by nonprofit pharmaceutical companies in the IQVIA NSP dataset, we compared the sales volume of those
products sold by the nonprofit pharmaceutical sector with the for-profit pharmaceutical sector. We note that
this search resulted in the identification of one nonprofit pharmaceutical company, Civica, which indicates that

July 2024
ASPE REPORT
3
this is the only nonprofit pharmaceutical company marketing products in the United States during the period
of analysis.
Key Stakeholder Interviews
To supplement the environmental scan, NORC facilitated nine (9) interviews with key informants with
expertise in addressing drug shortages, increasing access to essential medicines, or providing low-cost
alternatives to expensive medications. Key informants were affiliated with nonprofit and for-profit
pharmaceutical companies, academic institutions, and hospitals.
Background
Brief History of Nonprofit Companies in the Pharmaceutical Industry
Nonprofit companies are tax-exempt
i
economic entities organized around missions or objectives intended to
further a social cause or provide a public benefit. Nonprofit companies differ from the traditional for-profit
business model, which aims to maximize profit for investors. Nonprofit companies are restricted from
distributing profits to any private shareholder or individual.
13
They leverage their social mission to attract
donations from private entities or funding from public entities to finance the provision of their goods and
services. Many sectors, such as pharmacies, hospitals, hospices, nursing homes, and home health care, are
structured with a mix of nonprofit and for-profit companies. The literature examining non-pharmaceutical
sectors suggests that the co-existence of nonprofit and for-profit companies can promote competition and
increase access to services for consumers.
14-16
However, little is known about the benefits and implications of
these two models in the pharmaceutical industry.
Although the pharmaceutical industry is dominated by for-profit companies, nonprofit companies have a long
history of advancing innovation in this industry.
17-20
The majority of their contributions has been through R&D
activities,
21,22
sponsored by funding from public and private entities. Some independently conduct R&D with
their endowments, royalties, donations, or other funding, while others partner with outside entities leading
these activities. Philanthropic nonprofits
ii
, such as the Bill & Melinda Gates Foundation and CureDuchenne,
may also fund sponsored targeted R&D projects with nonprofit and for-profit companies.
Typically, nonprofit companies have not pursued commercialization activities, such as obtaining market
authorization from the U.S. Food and Drug Administration (FDA), manufacturing, and distribution of
pharmaceutical products.
8,17,23
The dominant approach has been for nonprofit companies to license new drugs
from their R&D pipeline to for-profit companies.
8
Some experts have cited that this approach results in
nonprofit companies losing the right to manufacture these drugs exclusively and for-profit companies
launching new drugs with the goal to maximize profits which may result in high prices for consumers. For
example, the Cystic Fibrosis Foundation (CFF), a philanthropic nonprofit organization, invested $150 million in
a for-profit company to develop ivacaftor, the first drug to address the underlying cause of cystic fibrosis.
24
CFF
then sold the royalty rights to ivacaftor for $3.3 billion to a for-profit company.
25
The list price for ivacaftor
when it was licensed in 2012 was $294,000 per patient per year.
26
In 2019, a new combination product,
Trikafta (elexacaftor/ivacaftor/tezacaftor), was released with an average list price of $322,000 per patient per
year.
27
Similar examples of innovative and expensive drugs that were initially developed or financed by
nonprofit companies and licensed to for-profits for commercialization include voretigene neparvovec for
_______________________
i
In some circumstances, non-profit companies are subject to taxes, such as the unrelated business taxable income (UBTI).
ii
Philanthropic nonprofits may invest in R&D with for-profit pharmaceutical companies.

July 2024
ASPE REPORT
4
congenital blindness, tisagenlecleucel for leukemia, and bexarotene for lymphoma.
8,20
While non-exclusive
licenses have been cited as a barrier, it is worth noting that licensors must also weigh factors such as costs or
profitability to determine the terms of the license. For-profit pharmaceutical companies have also used
business strategies such as licensing, mergers, and acquisitions to obtain access to new drugs. Researchers
have reported that the share of revenues coming from innovations sourced outside of for-profit companies has
grown from 25 percent in 2001 to about 50 percent in 2016.
28,29
In this report, we focus on nonprofit companies that are pursuing commercialization activities in the
pharmaceutical industry.
Profile of Nonprofit Pharmaceutical Companies, 2000-2022
Table 1 provides a profile of nonprofit pharmaceutical companies that have entered the market between 2000
and 2022 and points to a sector that is largely fragmented. The environmental scan conducted for this report
identified 11 nonprofit pharmaceutical companies that met our definition in the U.S. pharmaceutical market.
Their specific mission statements ranged from providing affordable medications, to enhancing access to
essential medicines or those in shortage. Each mission statement identified product commercialization as one
of its goals. Further, the environmental scan revealed variation with respect to the types and number of
products nonprofit pharmaceutical companies provide—some focus on a specific treatment area with a
handful of products, while others provide dozens of products across multiple therapeutic areas.
The nonprofit pharmaceutical companies also differ in their operations. Some engage in R&D for product
development and commercialization. For example, Medicines360 engaged in R&D and commercialization of its
hormonal intrauterine device (IUD) that is accessible to low-income women in public clinics across the United
States (discussed in more detail in Access to Essential ).
10
In other cases, nonprofit pharmaceutical companies
focus only on commercialization activities, such as manufacturing, distribution, and relabeling. For example,
Drew Quality Group launched in 2014 with the mission to become a supplier of high-quality generic drugs that
were manufactured in the United States.
30
As of March 2023, the environmental scan and key stakeholder
interviewers suggest Drew Quality Group has not yet achieved this objective. Another example is Civica, which
entered the market in 2018 with a focus on relabeling and distribution of generic sterile injectables.
31
While
there is no one-size-fits-all approach, a common theme is the expressed desire to promote the affordability of
drugs and broaden access to pharmaceutical products by engaging in commercialization activities.
All the nonprofit pharmaceutical companies we identified have an Internal Revenue Service (IRS) tax-exempt
status as either a 501(c)(3) charitable organization
32
or a 501(c)(4)
iii
social welfare organization.
33
Among the
501(c)(3) charitable organizations, some are designated as public charities rather than as private foundations.
iv
By examining all of the nonprofit pharmaceutical companies’ most recent IRS form 990,
34
each reported annual
revenues below $20 million, with the majority reporting annual revenues below $2 million. All of the nonprofit
pharmaceutical companies also reported negative annual net income, indicating expenses exceeded revenues.
In contrast, research suggests that from 2000 to 2018, 35 large for-profit pharmaceutical companies reported
an average annual revenue of $33 billion, and an average net income of $54 billion.
35
While nonprofit
pharmaceutical companies tend to have the revenues or number of employees to be considered small
businesses, the literature and many stakeholders noted that their tax-exempt status does not make them
_______________________
iii
Unlike 501(c)(3) charitable organizations, donations, or contributions to 501(c)(4) social welfare organization are not tax-deductible
on federal tax returns for the entity making the donation.
iv
Private foundations have lower levels of public involvement and scrutiny in their activities than public charities. While public charities
typically receive a greater portion of their funding from public sources, private foundations are typically controlled by small groups of
individuals, such as family members.

July 2024
ASPE REPORT
5
eligible for certain types of funding, such as the National Institutes of Health (NIH) Small Business Innovation
Research, the Small Business Administration (SBA) loans, or even bank loans that require some expected level
of revenue.
July 2024
ASPE REPORT
6
Table 1: Nonprofit Pharmaceutical Companies in the United States, 2000-2022
Name (Year
Founded)
Total
Revenue
(Year)
Mission Statement
Conditions or
Drugs Targeted
Current Drugs in U.S.
Market
Associated Companies
Civica Inc.
(2018)
$16,726,911,
as of 2019
Provide quality generic
medicines that are available and
affordable to everyone.
Various conditions
requiring generic
sterile injectables;
insulin for
diabetes
Civica Rx is involved in
private labeling and
distribution of 60
generic sterile
injectables;
CivicaScript is
distributing
Abiraterone—used to
treat prostate
cancer—and plans to
distribute three low-
priced generic insulin
by 2024.
Civica Rx, CivicaScript and Civica Foundation.
While Civica Rx focuses on generic drugs used in
the hospital setting, CivicaScript, a public benefit
company (PBC)
v
, works with pharmacy benefits
managers (PBMs) and insurers to bring low-cost
generics to outpatient and retail pharmacies.
The Civica Foundation is a 501(c)(3) organization
that provides philanthropic support to
manufacture and distribute generic
medications.
Drew Quality
Group (2014)
less than
$50,000, as
of 2021
Improve society’s health by
being a supplier of high-quality
generic drugs, manufactured in
the United States.
Generic drugs
None
N/A
Fair Access
Medicines
(2015)
less than
$50,000, as
of 2021
Identify, develop, and deliver
life-saving medicines to poorly
served patients in the U.S. and
worldwide at the lowest cost
possible.
Insulin for
diabetes
None
N/A
Harm
Reduction
Therapeutics
(2017)
$1,550,000,
as of 2019
Make naloxone more accessible
for everyday people by
combining increased funding,
generating more interest in
public health, and building on
our years of expertise.
Naloxone for
opioid overdose
None. Over-the-
counter naloxone
product approved in
July 2023, with an
expected launch date
in early 2024.
N/A
_______________________
v
Public benefit companies are for-profit entities that maintain profit and public benefit objectives.
July 2024
ASPE REPORT
7
Institute for
Pediatric
Innovation
(2006)
$34,443, as
of 2020
Research and develop
innovative products that will
improve the health of children
and support those who provide
care for them.
Pediatric
conditions
None. Mission focus
has evolved to focus
on digital health.
N/A
Medicines360
(2009)
$17,400,453,
as of 2019
Catalyze equitable access to
medicines and devices through
product development, policy
advocacy, and collaboration
with U.S. and global partners.
Contraception,
such as hormonal
IUDs
One branded
hormonal IUD, Liletta,
through Actavis; over
the counter
emergency
contraceptive through
Curae Pharma360.
Medicines360’s subsidiary is Curae Pharma360
Inc. which is a for-profit organization focused on
improving the availability of quality generic
drugs and other medicines that are in short
supply. Medicines360 selected Actavis (formerly
Watson Women’s Health, then Allergan, and
now AbbVie) as its for-profit commercial partner
for a hormonal IUD.
NP2 (2019)
$340,500, as
of 2020
Promote public health by
developing, manufacturing, and
distributing medicines for the
treatment of life-threatening
diseases in underserved
populations.
Generic drugs for
cancer
None
N/A
Odylia (2018)
$60,528, as
of 2020
Accelerate the development of
gene therapies for people with
rare disease, changing the way
treatments are brought from
the lab to the clinic…to bring life
changing treatments to people
with genetic disease regardless
of prevalence or commercial
interest.
Gene therapies
for rare genetic
disorders
None
Odylia has partnered with Cloves Syndrome
Community, SATB2 Gene Foundation, Usher
2020 Foundation, RDH12 Fund for Sight, and
PTC Therapeutics
Institute for
One World
Health | PATH
Drug Solutions
(2000)
$2,972,163,
as of 2018
Develop and deliver lifesaving
medicines to women, children,
and communities around the
globe.
Drugs and
vaccines for
various infectious
diseases,
contraception,
maternal and
child health
An injectable
contraception,
subcutaneous depot
medroxyprogesterone
acetate (DMPA-SC),
through Pfizer.
PATH selected Pfizer as its for-profit commercial
partner for an injectable contraception.

July 2024
ASPE REPORT
8
Remedy
Alliance Inc.
(2012)
$95,600, as
of 2021
Ensure harm reduction
programs have sustainable &
equitable access to low-cost
naloxone for distribution in their
communities.
Naloxone for
opioid overdose
Remedy Alliance is
involved in the
distribution of generic
naloxone.
N/A
Tutela
Pharmaceutical
(2020)
Less than
$50,000, as
of 2021
Ensure continued and
affordable access of single-
source medications to patients.
Single-source
medications
subject to
discontinuation or
divestiture by
their
manufacturers.
None. Acquired
license from Astellas
Pharma Inc. for active
ingredient of a
medication previously
tested for COVID-19.
Collaborators include Zensights, Pharmafusion,
Tucker Ellis, LLP, Incubate IP, and Godfrey &
Kahn, SC.
Note: All general information obtained from company websites; financial information was obtained through IRS.gov.
July 2024
ASPE REPORT
9
Table 2 presents the number of products, sales, and units sold for the only nonprofit pharmaceutical company,
Civica, identified in IQVIA’s NSP dataset from 2020-2022, as well as the corresponding sales and units sold for
the same products sold by for-profit companies. The data show that Civica sold 350 million units and $256
million in sales for 64 products. We identified 73 for-profit companies, with 20,200 million units sold and
$13,900 million in sales for the same corresponding products, suggesting that sales for the one nonprofit
pharmaceutical company represents about two percent of total sales and volume.
Table 2. Descriptive Statistics by Pharmaceutical Sector, United States, 2020-2022
Description
Nonprofit
For-profit
Number of companies
1
73
Number of products
64
64
Total units sold
350.2 million
20,200 million
Total sales
$255.9 million
$13,900 million
Note: A product is defined as a molecule-form-strength combination.
Source: ASPE analysis of IQVIA National Sales Perspective Data.
Market Growth
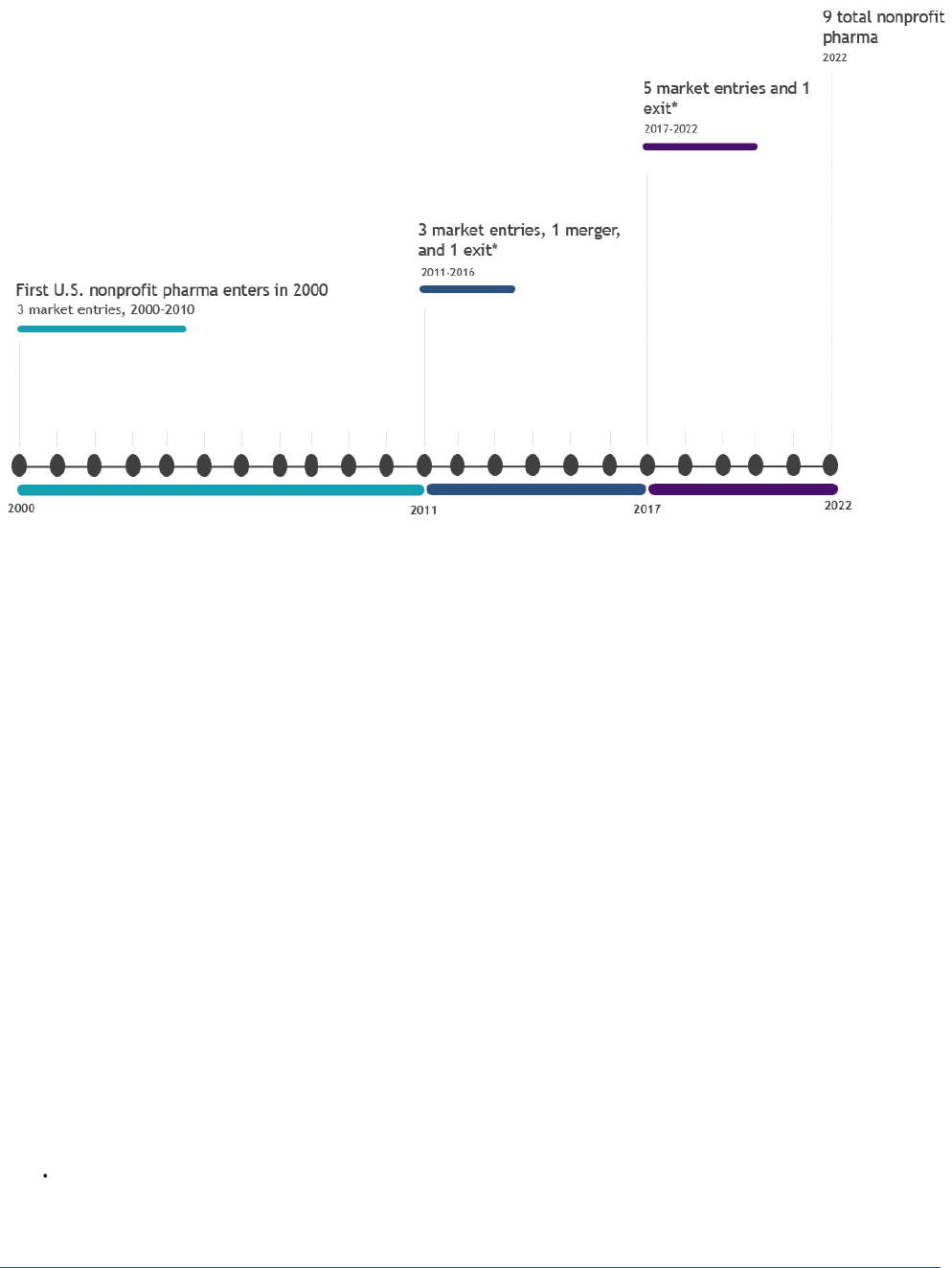
Growth in the nonprofit pharmaceutical sector was slow between 2000 and 2015, with one firm entering the
market every five years, on average. Most of the growth in this sector has occurred in the last five years; on
average, one nonprofit pharmaceutical company entered the market each year during 2017-2021. Out of the
11 identified companies, two are no longer operating as originally envisioned (we consider these market exits),
and one merged with a global nonprofit company in 2011 (Figure 1). As of August 2023, five of the 11
nonprofit pharmaceutical companies had either received FDA marketing authorization or are distributing
medical products in the United States.
The majority of nonprofit pharmaceutical companies were either in the R&D phase of medical product
development or looking to secure start-up capital. As discussed above, most nonprofit pharmaceutical
companies have historically outsourced the manufacturing, packaging, and labeling of their medical products
to contract manufacturers. However, one nonprofit pharmaceutical company, Civica, has exhibited noticeable
growth since its creation in 2018. Civica offers over 60 generic sterile injectable medications to over 1,500
hospitals through Civica Rx.
36
Further, in 2021, Civica created CivicaScript, a PBC, to offer generic drugs used in
the outpatient and retail settings.
37
The Civica Foundation was also established to provide philanthropic
support to manufacture and distribute generics. Civica’s rapid growth has been credited to its ability to
leverage the long-term commitments of its hospital and health system members to secure long-term supply
contracts.
38
These supply contracts have incentivized the reentry of numerous CMOs that had excess capacity
and held many of the Abbreviated New Drug Applications (ANDAs) for generic drugs that Civica labels and
distributes to its members.
July 2024
ASPE REPORT
10
Figure 1: Market Growth Timeline, Nonprofit Pharmaceutical Industry, United States, 2000-2022
*Market exit refers to a shift in mission or operations that no longer covers commercialization activities.
Market Entry Strategies
Nonprofit pharmaceutical companies generally begin operations with funding from philanthropic entities or
individuals, including crowdsourcing, that support their mission statement. In contrast, for-profit companies
depend on raising funds through investors who expect a return on their capital. Without the expectation to sell
pharmaceutical products that generate high profit, nonprofit pharmaceutical companies have a different risk
tolerance and may be able to provide products with low or negative profit. However, both for-profit and
nonprofit pharmaceutical companies face similar costs, timelines, regulatory oversight when developing and
bringing products to market, and generate revenue from selling drug products and services.
Typically, nonprofit pharmaceutical companies focus on a single product when entering the market. Since they
must demonstrate that its mission is compelling enough to motivate access to philanthropic funding, nonprofit
pharmaceutical companies tend to be organized around addressing intractable market gaps that will improve
social welfare, such as increasing access to medicines at affordable prices, mitigating drug shortages, or
developing new drugs for rare or tropical diseases.
When selecting their target drugs, nonprofit pharmaceutical companies, like their for-profit counterparts,
consider multiple factors, such as the target market size, start-up costs, regulatory requirements, and the
sustainability of their business model. In interviews, stakeholders noted that nonprofit pharmaceutical
companies may prefer to focus on niche drugs such as those with low start-up costs, low margins, or high
volumes. Some niche markets that nonprofit pharmaceutical companies have entered are:
Essential drugs or drugs in shortage: Enhancing access to affordable medications, essential or
lifesaving medications, and medications that experience persistent shortages is part of the mission of
many nonprofit pharmaceutical organizations. This mission can also engender trust in the market as

July 2024
ASPE REPORT
11
nonprofit pharmaceutical companies begin to be recognized for filling gaps in market demand and
meeting unmet medical needs.
Generics: By targeting off-patent drugs, nonprofit pharmaceutical companies can focus on products
that are typically associated with low margins, as well as lower start-up, development, and regulatory
costs, and lower litigation risks. These products are generally not attractive to for-profit companies
due to the low margins and intense pressure to keep prices down via competition.
Discontinued drugs: Nonprofit pharmaceutical companies can fill treatment gaps by focusing on
medical products that have been discontinued or abandoned by for-profit companies due to low
volume and profitability. These drugs represent opportunities for nonprofit pharmaceutical companies
to enter the market.
High-volume drugs: By targeting high-volume products like insulin, nonprofit pharmaceutical
companies ensure the market can facilitate competition and business sustainability. Some of these
high-volume drugs have experienced persistently high prices, despite being off patent.
Results
Low-Cost Alternatives to Expensive Drugs
Many life-saving drugs do not have low-cost alternatives, despite being off patent for an extended period of
time. As a result, patients may incur debt or ration their medications, which leads to medication nonadherence
and worse health outcomes.
39
In interviews, stakeholders shared that drug prices are set by for-profit
companies to create shareholder value, which is typically achieved by maximizing profit. In contrast, many
nonprofit pharmaceutical companies promote the affordability of medical products as a goal in their mission
and vision statements. In practice, nonprofit pharmaceutical companies offer a cost-plus model that prices
drugs at the level of margins that ensure their sustainability, adding a fixed percentage to the unit cost of each
product.
Insulin is an example of a drug for which nonprofit pharmaceutical companies want to provide more
alternatives because the global market is currently dominated by three manufacturers.
39,40
Almost a year after
a nonprofit pharmaceutical company announced its two-year plan to enter the insulin market with prices set at
$35 per vial, all three of the for-profit companies cut the out-of-pocket cost to a maximum of $35 per vial.
41-43
However, nonprofit pharmaceutical companies were not the only source of competitive pressure. The decision
by the for-profit companies to lower insulin prices followed announcements by the state of California, in
partnership with a PBC that operates a cost-plus model, to manufacture its own insulin. In addition, the
Inflation Reduction Act, signed into law in August 2022, capped out-of-pocket costs for insulin at $35 per
monthly prescription for Medicare enrollees beginning January 1, 2023.
39,44
The entry of nonprofit pharmaceutical companies into markets with expensive generic drugs, few
manufacturers, and high volume of sales may increase the competitive pressure for all companies to lower
their prices. However, drug pricing is not only a reflection of manufacturer prices that capture the cost of R&D
but also markups by intermediaries, such as PBMs, wholesalers, and pharmacies. Efforts to enhance the
accessibility of affordable medications need to also consider margins that allow companies to recover their
R&D costs. However, drug pricing transparency, particularly around negotiated rebates and discounts, has
been a topic of debate in limiting the public’s understanding of the financial arrangements dictating the profits
of stakeholders in the pharmaceutical marketplace. Stakeholders shared that nonprofit pharmaceutical
companies are attempting to disrupt persistently high prices for expensive medications by adopting

July 2024
ASPE REPORT
12
transparency in their financial arrangements, including disclosing the price and charging the same price for all
members without volume discounts, as one of their core business strategies.
Another strategy that nonprofit pharmaceutical companies adopt to increase competitive pressure is to bring
over-the-counter (OTC) alternatives to expensive prescription drugs to market. OTC drugs are typically sold at
lower prices than drugs that require a prescription or that are administered in hospitals or physician offices. An
example of this is naloxone, a life-saving drug used to reverse an opioid overdose, and whose price hikes
45
attracted the entry of a nonprofit pharmaceutical company, Harm Reduction Therapeutics. Although FDA
encouraged sponsor applications for OTC naloxone products in 2019,
46
no existing for-profit company had
submitted a New Drug Application (NDA) for OTC naloxone until two months after the nonprofit
pharmaceutical company submitted its NDA in late 2022.
47,48
On March 29, 2023, FDA approved the first OTC
naloxone product to a for-profit company, and sales began in the summer 2023.
49
On July 28, 2023, FDA
approved Harm Reduction Therapeutics’ ReVIVE, with sales beginning in early 2024.
50
Challenges
Nonprofit pharmaceutical companies have limited capacity to offer alternatives for expensive drugs that are
not off-patent or are protected by a market exclusivity. While many contribute to drug discovery and
development, they typically leverage partnerships with for-profit pharmaceutical companies to commercialize
their products, which creates uncertainties on the pricing model that will be used. For example, although
Targretin (bexarotene), a cancer drug, was developed by nonprofit pharmaceutical companies and now has
generic competitors available, it was commercialized in partnership with a for-profit company
22
and is sold for
almost $30,000 for 100 capsules.
51
Relatedly, the business structure of nonprofit pharmaceutical companies
with wholly owned for-profit companies has the potential to undermine their credibility regarding
transparency in drug pricing. One example is CivicaScript, a subsidiary of Civica that was established as a for-
profit PBC, which focuses on generic drugs distributed via retail, mail, and outpatient channels for participating
pharmacies.
38
The low levels of therapeutic concentration and market share of nonprofit pharmaceutical companies may not
be sufficient to disrupt drug pricing in the pharmaceutical market for multiple reasons. First, stakeholders we
interviewed noted that prices of nonprofit pharmaceutical company drugs may not be the lowest in the market
at any given time because their prices are designed to create stability in the market and to be the lowest
sustainable price for nonprofit pharmaceutical companies (see Drug Shortages for additional discussion).
Second, like in the for-profit pharmaceutical sector, since list prices for drugs do not reflect markups along the
pharmaceutical supply chain for each distribution channel, it is difficult to quantify the actual savings for
payers and patients when there is a mix in business models. This is especially true for drugs that are
administered in hospitals or physician offices because the reimbursements for those drugs are usually bundled
with the reimbursement for other services.
52
Third, while OTC products tend to be low cost, OTC drugs are not
covered by health insurance, which may limit the savings, number, and types of patients that could benefit
from these drugs.
53
Drug Shortages
According to the FDA,
the majority of drugs in shortage are sterile injectables and older generic drugs with a
median time of 35 years since first approval.
54
Root causes of generic drug shortages are the low profitability

July 2024
ASPE REPORT
13
of generic drugs and the lack of market rewards for generic manufacturers that invest in quality management
maturity; shortages can also occur due to supply chain disruptions or increased demand.
vi
The nonprofit funding model, which does not expect the same high rates of return for investors, suggests that
nonprofit pharmaceutical companies may be able to sell drugs, such as generics, that are associated with low
profits. However, just like any organization, nonprofit pharmaceutical companies need to balance their
sustainability and cost goals. Some researchers have proposed the Health Care Utility (HCU)
model,
vii
a novel
governance and financing structure, to address drug shortages and persistent price hikes of generic drugs.
55
The HCU model relies on member
viii
financing to provide products and services at the lowest sustainable price.
Proponents of the HCU model argue that the core tenets of the model address the factors and misaligned
incentives that contribute to drug shortages.
6
The HCU model informed the business structure of Civica Rx,
which provides same-price guarantees with no volume discounts, requires long-term commitments, and
embeds a quality assurance process in its contracts with CMOs.
36,38
This pricing approach includes maintaining
a six-month buffer supply of its products as a mitigation strategy against drug shortages or supply chain
interruptions.
56
Further, Civica Rx limits its volume agreements to 50 percent of each member’s total volume
and establishes contracts with multiple CMOs in North America, Europe, and South Asia to increase the
geographical diversity of its suppliers and mitigate supply chain risks.
36
Challenges
In interviews, some stakeholders shared that the price of products by nonprofit pharmaceutical companies,
like Civica Rx, may not be the cheapest on the market because pricing may account for the cost of investments
in quality management systems to mitigate shortages. Stakeholders shared that the HCU approach to
addressing drug shortages is limited because it provides drugs for its members only. The volume that nonprofit
pharmaceutical companies produce may also be too low to have an impact in the broader market. Further,
since many of the nonprofit pharmaceutical companies may not own the license nor manufacture their own
generic drugs, some function like a group purchasing organization (GPO), and as such, they have no control
over the price that patients ultimately pay.
Some stakeholders have been skeptical about the feasibility of replicating or scaling up models like that of
Civica Rx for multiple reasons. First, long-term contracts may result in members paying higher prices than the
lowest available market price in the short term, although the price would remain unchanged when there is a
shortage. Another risk is the potential to further concentrate bargaining power in one entity and perpetuate
the existing oligopoly in the pharmaceutical industry. Stakeholders shared lessons from the health care
industry, which is dominated by nonprofit health systems, that suggest the nonprofit model may not always
translate to maximizing social welfare. For example, research suggests that nonprofit hospitals are not more
likely to provide charity care or unprofitable services than their for-profit counterparts.
57
Access to Essential Medicines
FDA, in collaboration with other federal agencies, began developing and publishing a list of essential
medicines, medical countermeasures, and critical inputs in 2020 in response to President Trump’s Executive
Order on Ensuring Essential Medicines, Medical Countermeasures, and Critical Inputs are Made in the United
States.
58
FDA’s list of essential medicines identifies those medical products that have the greatest potential
_______________________
vi
Quality management maturity measures the consistency and reliability of business processes to implement and maintain the quality
of products in the marketplace, including early signals to enable actions to prevent drug shortages triggered by quality issues.
vii
Utility is a reference to commonly shared basic goods, such as electricity and gas.
viii
Members are customers of the HCU; for example, health systems are the customers for hospital-based drugs and health insurance
companies are customers for retail drugs. Some call the HCU a “closed-system” model because only members have access to the
products and services.

July 2024
ASPE REPORT
14
impact on public health and are most needed by patients for acute and urgent medical conditions. The goal of
the FDA’s essential medicines list is to ensure the American public is protected against outbreaks of emerging
infectious diseases, chemical, biological, radiological, and nuclear threats by ensuring sufficient and reliable,
long-term domestic production of these products.
59
Another list of essential medicines, managed by the World
Health Organization (WHO), identifies medications that may be critical to ensure a nation’s health system can
meet the health care needs of its population. The WHO list of essential medicines prioritizes disease
prevalence, public health relevance, and evidence on efficacy, safety, and comparative cost-effectiveness. For
this report, we examine the role of nonprofit pharmaceutical companies in addressing gaps in the provision of
critical medicines for both chronic and acute health conditions.
60
For purposes of the stakeholder interviews,
essential medicines were broadly defined to include those in the FDA list of essential medicines and others
such as oncology drugs or sterile injectables.
In interviews, stakeholders shared that nonprofit pharmaceutical companies could increase access to essential
medicines by leading R&D for low volume medical products to address unmet health needs, such as rare
diseases, neglected tropical diseases, and antimicrobial resistance. Examples that illustrate the potential for
nonprofit pharmaceutical companies in this area include those that develop new technologies, such as
Innovative Genomics Institute (IGI) that is working to develop and commercialize CRISPR gene-editing
therapies to treat sickle cell disease.
61
Further, the environmental scan identified that R&D and
commercialization for new antibiotics to combat antimicrobial resistance is another market gap that may be
appropriate for nonprofit pharmaceutical companies.
62,63
In comparison to brand-name drugs in other
therapeutic areas, new antimicrobials typically have very low volumes and low prices.
64
Strategies to bring critical medical products to market at lower cost include identifying new uses and
indications for approved and off-patent drugs, a strategy known as drug repurposing.
65
Since safety data exists
for approved drugs, it has been estimated that nonprofit pharmaceutical companies can avoid approximately
40 percent of the costs for drug development by repurposing approved drugs.
66
This strategy can be effective
for conditions that have few treatments available, such as rare and neglected diseases. For example, Institute
for One World Health repurposed paromomycin, an off-patent drug that is no longer used as an antibiotic, to
cure visceral leishmaniasis, a neglected, tropical disease.
ix
A closely related strategy focuses on rescuing abandoned compounds for which data demonstrate safety and
efficacy, but which for-profit companies do not complete development and regulatory approval due to
anticipated low profitability. This market gap presents opportunities for nonprofit pharmaceutical companies.
In one example, Tutela Pharmaceutical executed an exclusive license agreement for a compound that was
abandoned by a for-profit company after the completion of phase 1 and 2 clinical trials.
Another market gap of interest to nonprofit pharmaceutical companies is increasing access to drugs to
underserved populations by prioritizing diversity in clinical trials to ensure generalizability of evidence. For
example, Medicines360 sponsored a phase 3 clinical trial for the first hormonal IUD that prioritized diversity in
the enrollment of clinical trial participants. Unlike the hormonal IUD that was already available on the market
at the time, this nonprofit pharmaceutical company generated safety and efficacy evidence for women of all
races, women who had never given birth, overweight or obese women, and women with sexually treated
infections in the United States.
10
The environmental scan identified a study that concluded that patients at a
Title X clinic experienced increased uptake and decreased average payments after the introduction of the
hormonal IUD.
67
This was the only example we identified of a nonprofit pharmaceutical company successfully
_______________________
ix
It is worth noting that NIH’ National Center for Advancing Translational Sciences created a drug repurposes program intended to
facilitate sharing of data and other resources for scientists and others interested in repurposing drugs. See
https://ncats.nih.gov/preclinical/repurpose.

July 2024
ASPE REPORT
15
developing and commercializing a branded medical product for the U.S. market. While this nonprofit
pharmaceutical company retained ownership of the NDA, commercialization of the medical product occurred
through a licensing agreement with a for-profit company. In exchange for licensing the intellectual property of
the nonprofit pharmaceutical company, the for-profit pharmaceutical company committed to prioritize
commercializing the hormonal IUD, paid an upfront payment, as well as milestone payments, and continuous
royalties on units sold, which are non-taxable because they are not classified as unrelated business income.
10
Retaining ownership of the license allowed Medicines360 to maintain ownership of the drug and reinvest in
R&D to identify new indications. Stakeholders noted that entry by Medicines360 for an underserved market
spurred additional investment and development of new products by for-profit companies. Other strategies
that nonprofit pharmaceutical companies have adopted to launch their products include creating wholly
owned for-profit subsidiaries, partnerships with PBCs, or selling the license to a for-profit pharmaceutical
company. However, as noted above, these types of partnerships or business structures have the potential to
undermine their credibility regarding transparency in drug pricing.
Challenges
Nonprofit pharmaceutical companies face challenges with repurposing off-patent drugs and rescuing
abandoned compounds, including difficulty raising capital to conduct expensive phase 3 clinical trials and
aligning with donor priorities.
66
For example, disulfiram, a drug approved as an anti-alcoholism drug, has been
proposed as a candidate to be repurposed to treat many diseases, including various cancers, Alzheimer’s
disease, and COVID-19.
66,68
However, in such circumstances, prioritization of potential new indications to
pursue depends on the interest of donors. While donors to nonprofit pharmaceutical companies may prioritize
public benefits, it is unclear that their priorities will always align with public health needs that maximize social
welfare.
Further, the risk of donor fatigue undermines the long-term sustainability of the nonprofit model. Pull
incentives, wherein the government aims to reward new drug development for underserved markets by
reducing the risk of insufficient future revenue streams through higher reimbursement policies, have been
successfully employed to develop new hospital-based antibiotics. For example, the Centers for Medicare &
Medicaid Services (CMS) have paid new technology add-on payments for novel antibiotics used in the inpatient
setting.
69
However, oftentimes sales revenue from antibiotics cannot sustain a company’s infrastructure costs,
so other investments unrelated to sales revenue would also be necessary to ensure the sustainability of the
nonprofit model for low volume medical products.
64,69
Beyond R&D costs, nonprofit pharmaceutical companies need to raise funds for complex commercialization
activities, such as manufacturing, distribution, reimbursement, and post-marketing commitments. If the
nonprofit tax-exempt status was obtained based on a mission to conduct research, then sales revenue from
commercialized medical products may be subject to business income taxes. Relatedly, stakeholders shared
that commercial activity by nonprofit pharmaceutical companies may attract litigation and jeopardize tax-
exempt status under the IRS “commerciality” doctrine.
x
Stakeholders also noted that FDA has limited
experience working with nonprofit pharmaceutical companies, who may also not be aware of flexibilities
available to them.
Nonprofit pharmaceutical companies adopt several strategies to navigate the complex pharmaceutical supply
chains in the United States and retain tax-exempt status. However, some of these strategies may not be
feasible for low-volume products. In one example, stakeholders shared that a nonprofit pharmaceutical
_______________________
x
In its determination that a business entity did not qualify as a 501(c)(3) organization, IRS stated “factors courts have considered in
assessing commerciality are competition with for-profit commercial entities; extent and degree of below cost services provided;
pricing policies; and reasonableness of financial reserves.”

July 2024
ASPE REPORT
16
company regained ownership of a gene-therapy that was licensed to a for-profit company, likely because of
claw back clauses
xi
in the licensing agreement. For several years after gaining the license for the gene therapy,
the for-profit company was unable to meet the comparability
xii
requirement to scale it and terminated related
development activities. While the gene therapy may be available to patients through the compassionate use
program, shareholders stated that nonprofit pharmaceutical companies may not have the financial resources
and expertise required to launch phase 3 trials, pay FDA user fees, maintain all of the regulatory requirements
to obtain FDA approval, or meet manufacturing requirements for widespread distribution without a
commercial for-profit partner.
Limitations
This report has several limitations. First, the stakeholder interviews were limited to nine experts, and as such,
the findings from this report may not be generalizable to all stakeholders impacted or involved. For example,
the stakeholder interviews had limited experts from the for-profit pharmaceutical industry. Further, although
the environmental scan aimed to include broad terms, it is possible that our search terms and results did not
capture other key topics or issues. Lastly, given the nascent nature of this sector, there was limited availability
of data to quantitatively examine the role of the nonpharmaceutical companies in increasing the supply of
essential and affordable drugs.
Discussion and Conclusion
The findings from this report suggest that nonprofit pharmaceutical companies have the potential to address
drug shortages and enhance access to affordable and essential medicines. However, their sustainability and
effectiveness may be limited due to low production volumes, a complex tax system, ineligibility for small
business funding sources, and to some extent, lack of awareness of nonprofit pharmaceutical companies by
the government and the public at large.
Although this report identified 11 companies in the nonprofit pharmaceutical sector, only one was captured in
a database of drugs sold in the United States during 2020-2022. The data showed that the volume of this
nonprofit pharmaceutical company represented about two percent of the total sales volume for the same
generics sold by for-profit companies. This finding aligns with stakeholder interviews that indicated that
nonprofit pharmaceutical companies currently have limited ability to fill large gaps in the market or create
pressure to bring prices down due to their low production volume.
Second, the lack of profit motive for nonprofit pharmaceutical companies results in a different risk profile and
set of strategies than their for-profit counterparts. Thus, nonprofit pharmaceutical companies have the
potential to increase access to essential and affordable medicines. For example, their strategies to repurpose
generics, pick up abandoned products, or bring OTC products to market have partly contributed to pressure on
the industry to increase access to low-cost insulin products and to bring OTC naloxone products to market.
Further, the focus of nonprofit pharmaceutical companies on low volume drugs necessitates the conduct of
R&D or commercialization activities on essential medicines that for-profit companies may not deem
commercially viable.
_______________________
xi
Claw back is a contractual provision that allows an instance of recovering assets or benefits previously given out.
xii
Comparability requirements means demonstrating that phase 2 results are comparable to phase 3 results.

July 2024
ASPE REPORT
17
Third, while nonprofit pharmaceutical companies are governed by a different set of tax laws than for-profit
companies, they are subject to the same FDA regulatory requirements and R&D costs to bring products to
market. This has led nonprofit pharmaceutical companies to target products that are low cost to develop and
that have a higher probability of success. In this way, some do not see nonprofit pharmaceutical companies as
disruptors to the industry or a solution to the issues at hand given that many of their activities involve
relabeling approved products and have low sales volume.
Fourth, although nonprofit pharmaceutical companies can leverage their tax-exempt status to seek funding
from diverse sources, the complex tax environment has resulted in a mixed structure of nonprofit and for-
profit companies under the same organization that blur efforts to increase transparency or ensure that drugs
are affordable. Though the majority of nonprofit pharmaceutical companies have operational sizes comparable
with small businesses, their tax-exempt status makes them ineligible for some types of funding from the
National Institutes of Health (NIH) Small Business Innovation Research, the Small Business Administration
(SBA), or even bank loans that require some expected level of revenue. Further, the diverse funding sources
create challenges aligning drug development and commercialization activities of nonprofit pharmaceutical
companies with public health priorities.
The literature and stakeholders have described various approaches to address some of the challenges that
nonprofit pharmaceutical companies face, which can be largely divided into financial and nonfinancial
incentives. Financial incentives include the establishment of a federal program or set of initiatives that could
fund or provide financial support for the development and manufacturing of drugs by nonprofit
pharmaceutical companies at all stages of the product life cycle—from early discovery research activities to
commercialization—as well as for capital investments. Stakeholders have proposed a number of financial
incentives tailored to the nonprofit pharmaceutical sector such as interest-free loans, grants, cooperative
agreements, loans not requiring repayment, and advanced purchasing agreements with the government to
enhance their sustainability. Stakeholders and the literature also cited other existing tools that could be
leveraged to expand eligibility to the nonprofit pharmaceutical sector, including NIH’s Small Business
Innovation Research Grants, the Health Resources and Services Administration’s (HRSA) 340B Drug Pricing
Program, and advanced purchasing commitments from the Strategic National Stockpile.
In addition to the proposed initiatives discussed above, several Congressional bills have been introduced in
recent years aimed at the nonprofit pharmaceutical sector. This includes Senate Bill 2257, the Expanding
Access to Affordable Prescription Drugs and Medical Devices Act introduced in 2021, which included provisions
for funding and low-interest loans to support nonprofit drug development and required FDA user fee waivers.
Financial initiatives such as the provisions included in this bill could align eligibility with certain criteria such as
manufacturing drugs that are essential, in shortage, or fulfilling a public health need. One example cited by
some stakeholders was Civica’s funding that allowed them to begin construction of a manufacturing facility in
Virginia. This funding was awarded to Phlow Corporation, a U.S. drug manufacturing PBC, by the U.S.
Administration for Strategic Preparedness and Response (ASPR) to build manufacturing capacity of essential
medicines in shortage.
70,71
Stakeholders noted that Federal support would increase the financial stability of
nonprofit pharmaceutical companies through funding or purchase agreements that would ensure some level
of volume to be large enough to exert pressure in the industry, increase their sustainability, and also increase
awareness of and trust in the nonprofit pharmaceutical sector. This support could promote market entry,
competition, and expansion in this sector.
Existing literature and stakeholders have also proposed non-financial incentives to encourage growth in the
nonprofit pharmaceutical sector. Specific examples included expediting FDA review of nonprofit applicant
submissions or creating separate regulatory programs for nonprofit pharmaceutical companies. As noted
above, these challenges are not specific to the nonprofit pharmaceutical sector. Past studies focused on the

July 2024
ASPE REPORT
18
for-profit sector have also proposed similar solutions (i.e., reduced FDA timelines, simplification of clinical trial
protocols, increased interactions with FDA, improved predictability of the review process), to reduce the cost
of bringing drugs to market.
72
While FDA already uses existing tools to address drug shortages that involve
prioritizing and expediting review of certain applications and inspections, providing technical assistance and
guidance for small companies,
73
authorizing waivers, reductions, exemptions or refunds of user fees when
certain conditions are met,
74
further research is needed to understand how these existing tools can be
leveraged to address issues that are specific to the nonprofit pharmaceutical sector.
Review of the literature and discussions with stakeholders identified changes to the tax code as a way to lower
the entry barrier for nonprofit companies in the pharmaceutical sector. The policy proposals identified
included creating tax incentives that can facilitate the transfer of patents of abandoned drugs, creating
incentives for for-profit companies to partner with nonprofit pharmaceutical companies, clarifying the tax
code to facilitate activities and funding mechanisms, creating a new tax-exempt designation for nonprofit
pharmaceutical companies that are fulfilling a public health need, classifying drug sales of nonprofit
pharmaceutical companies as non-taxable revenue, and creating protections to uphold the IRS nonprofit
designation. However, stakeholders highlighted the risk of mission drift and oligopoly in the nonprofit
pharmaceutical sector if regulations are not implemented to ensure accountability. Literature and
stakeholders provided lessons learned from the health care industry, which is dominated by nonprofit health
systems, that suggest the nonprofit model may not always maximize social welfare.
To conclude, while the nonprofit pharmaceutical sector holds promise to address drug shortages and enhance
access to affordable and essential medicines, more research is needed to understand the available or potential
tools that can reduce existing barriers and challenges, as well as understand their implications on competition,
drug pricing, and innovation in the pharmaceutical industry.

July 2024
ASPE REPORT
19
Appendix A. Alternative Economic Models for Commercialization by Nonprofit
Pharmaceutical Companies
We summarize the alternative economic models that have been proposed in literature to promote market
authorization and commercialization by nonprofit pharmaceutical companies in the marketplace.
Nonprofit pharmaceutical companies as a market authorization holder leveraging the
manufacturing and distribution expertise of for-profit companies
Existing literature has proposed that nonprofit pharmaceutical companies could expand their organizational
capacity to pursue market authorization of new products discovered through their R&D pipeline rather than
license or engage in mergers and acquisitions.
8,9,23
In this model, nonprofit pharmaceutical companies would
leverage the expertise of for-profit pharmaceutical companies to maximize efficiency in production and
distribution.
8
For example, nonprofit pharmaceutical companies may license new products to multiple for-
profit companies for manufacturing and distribution. Further, the partnership agreements could include
clauses to ensure social welfare outcomes, such as affordability and access for underserved populations, and
balance the need to generate profits and sustainability. Proponents of this model state that this strategy could
ensure that pricing is guided by drug affordability goals.
An important challenge to scale up this strategy is that nonprofit pharmaceutical companies may not have the
expertise or financial resources to navigate clinical development activities, such as phase 3 clinical trials.
Another challenge is ensuring that a nonprofit pharmaceutical company is accountable to its core mission and
will not engage in misaligned actions, such as price-gouging. The participation of major donors and patients,
who have a financial interest in drug affordability and accessibility, on the board of trustees may mitigate this
risk.
6
Medicines360 demonstrated the viability of this concept with the commercialization of its hormonal IUD in the
United States.
10
The product was initially launched with a $82 million grant from a private philanthropic
nonprofit. The total cost, including product liability insurance, of bringing the hormonal IUD to market was
$73.4 million. Medicines360 partnered with Actavis, a for-profit company. However, Medicines360 retained its
rights to market the hormonal IUD at a deeply discounted price to public clinics and hospitals, such as federally
qualified health centers, throughout the United States. Similarly, Medicines360 retained marketing rights to
sell the hormonal IUD in low- and middle-income countries.
Nonprofit pharmaceutical companies as a market authorization holder with in-house
manufacturing and distribution expertise
In this model, nonprofit pharmaceutical companies would expand their organizational capacity to manage all
commercialization activities, including manufacturing and distribution.
8,9
An important barrier to adopting this
approach is the high start-up costs for new nonprofit pharmaceutical companies that do not have the ability to
leverage the economies of scale of established for-profit companies. This is a particular problem for low-
volume and new pharmaceutical products. One solution is for nonprofit pharmaceutical companies to modify
this approach by outsourcing actual production to CMOs. Another solution is the potential of selling exclusive
licensing of some products to raise start-up capital for internal commercialization of other products.
8
Proponents state that this strategy could be appropriate for nonprofit pharmaceutical companies that want to
target drugs that are not costly to bring to market such as old generic drugs, which have low profits and
experience frequent shortages.
6
The abbreviated new drug application (ANDA) process to obtain market
authorization for old generic drugs is less expensive because some of the regulatory requirements can be
fulfilled with existing data on efficacy and safety. An ASPE analysis found that the average cost to develop a

July 2024
ASPE REPORT
20
generic drug was $2.4 million ($3.2 million in 2022 dollars) and time required to bring the product to market
was just under five years.
75
One example of a nonprofit pharmaceutical company demonstrating the viability of this model for old generic
drugs is Civica.
38
As of March 2023, Civica Rx is distributing 60 generic sterile injectables to its members in the
United States through its supply contracts with foreign and domestic CMOs. Civica is in the process of
expanding to outpatient and retail pharmacies through CivicaScript. While Civica currently relies on ANDAs of
its CMOs, it plans to obtain its own ANDAs for generic drugs, such as insulin, and is building a manufacturing
facility in Virginia.
36,38
Nonprofit pharmaceutical companies leveraging product development partnerships
Nonprofit Product Development Partnerships (PDPs) is another model that has proven successful for launching
affordable and accessible medical products. PDPs coordinate financial and development efforts for medical
product development, in partnership with for-profits, nonprofits, and public stakeholders. For example, the
Global Alliance for TB Drug Development (TB Alliance) received FDA approval for pretomaid to treat
extensively drug-resistant tuberculosis. TB Alliance negotiated license agreements to ensure access in low-
income countries.
76
Nonprofit PDPs have resulted in bringing many medical products to market that address
unmet public health needs in low- and middle-income countries.
77
PATH, a U.S.-based nonprofit, obtained FDA
approval and commercialized depot medroxyprogesterone acetate (DMPA-SC), an injectable contraceptive, for
the domestic market through a PDP.
77,78
Similar to the objectives of PDPs, joint academic-industry-government
alliances to foster collaboration are common in the United States.
79
However, they are not formally
incorporated.
July 2024
ASPE REPORT
21
Appendix B. Environmental Scan Search Terms
Primary Search Terms
Secondary Search Terms
Nonprofit pharmaceutical company
Cost/Costly/High Cost
Pharmaceutical Public benefit corporation
Price
Nonprofit pharmaceutical sector
Affordability
Nonprofit pharmaceutical market
Low-cost generic drugs
Nonprofit biopharmaceutical company
Low-cost alternatives
Biopharmaceutical Public benefit corporation
Low-cost substitutes
Nonprofit biopharmaceutical sector
Low-cost biosimilars
Nonprofit biopharmaceutical market
Reimbursement
Biotechnology
Payers
Nongovernmental pharmaceutical company
Access
Charitable organizations
Drug shortage
Tax-exempt organizations
Essential drugs/medications
Life-threatening disease/rare disease
Life-saving medication
Critical drugs
Public health emergency
Orphan drugs
Specific Drugs
• Antibiotics, Antibacterials, Antimicrobials
• Saline
• CNS drugs
July 2024
ASPE REPORT
22
References
1. Bosworth A, Sheingold S, Finegold K, De Lew N, Sommers BD. Price increases for prescription drugs, 2016–2022.
Office of the Assistant Secretary for Planning and Evaluation: Washington, DC, USA. 2022;
2. Shore C, Brown L, Hopp WJ, National Academies of Sciences E, Medicine. Causes and Consequences of Medical
Product Supply Chain Failures. Building Resilience into the Nation's Medical Product Supply Chains. National Academies
Press (US); 2022.
3. Cutler DM. The demise of the blockbuster? N Engl J Med. Mar 29 2007;356(13):1292-3.
doi:10.1056/NEJMp078020
4. Bennani YL. Drug discovery in the next decade: innovation needed ASAP. Drug Discov Today. Sep 2011;16(17-
18):779-92. doi:10.1016/j.drudis.2011.06.004
5. Sharp D. Not-for-profit drugs--no longer an oxymoron? Lancet. Oct 23-29 2004;364(9444):1472-4.
doi:10.1016/S0140-6736(04)17291-7
6. Liljenquist D, Bai G, Anderson GF. Addressing Generic-Drug Market Failures - The Case for Establishing a
Nonprofit Manufacturer. N Engl J Med. May 17 2018;378(20):1857-1859. doi:10.1056/NEJMp1800861
7. Dredge C, Scholtes S. The Health Care Utility Model: A Novel Approach to Doing Business. Catalyst non-issue
content. 2021;2(4)doi:doi:10.1056/CAT.21.0189
8. Jaroslawski S, Toumi M, Auquier P, Dussart C. Non-profit Drug Research and Development at a Crossroads.
Pharm Res. Feb 7 2018;35(3):52. doi:10.1007/s11095-018-2351-3
9. Conti RM, Meltzer DO, Ratain MJ. Nonprofit biomedical companies. Clin Pharmacol Ther. Aug 2008;84(2):194-7.
doi:10.1038/clpt.2008.123
10. Medicines360. Nonprofit Pharma, Advancing a New Model for Equitable Access to Medicines. Medicines360;
2023. https://medicines360.org/wp-content/uploads/M360_CaseStudy_FINAL_v4_single.pdf
11. Handy F. Coexistence of nonprofit, for-profit and public sector institutions. Annals of public and cooperative
economics. 1997;68(2):201-223. doi:10.1111/1467-8292.00043
12. Congress.gov. H.R.2617 - 117th Congress (2021-2022): Consolidated Appropriations Act, 2023.
https://www.congress.gov/bill/117th-congress/house-bill/2617
13. Internal Revenue Service. Exempt Organization Types. 2023. https://www.irs.gov/charities-non-profits/exempt-
organization-types
14. Dalton CM, Bradford WD. Better together: Coexistence of for-profit and nonprofit firms with an application to
the U.S. hospice industry. J Health Econ. Jan 2019;63:1-18. doi:10.1016/j.jhealeco.2018.10.001
15. Marwell NP, McInerney P-B. The nonprofit/for-profit continuum: Theorizing the dynamics of mixed-form
markets. Nonprofit and voluntary sector quarterly. 2005;34(1):7-28.
16. Rose-Ackerman S. Competition between non-profits and for-profits: entry and growth. Voluntas: International
Journal of Voluntary and Nonprofit Organizations. 1990;1(1):13-25.
17. Stevens AJ, Jensen JJ, Wyller K, Kilgore PC, Chatterjee S, Rohrbaugh ML. The role of public-sector research in the
discovery of drugs and vaccines. New England Journal of Medicine. 2011;364(6):535-541.
18. Frye S, Crosby M, Edwards T, Juliano R. US academic drug discovery. Nature reviews Drug discovery.
2011;10(6):409.
19. Kneller R. The importance of new companies for drug discovery: origins of a decade of new drugs. Nature reviews
Drug discovery. 2010;9(11):867-882.
20. Frank GD, Jong L, Collins N, Spack EG. Nonprofit model for drug discovery and development. Drug Development
Research. 2007;68(4):186-196.
21. Chakravarthy R, Cotter K, DiMasi J, Milne CP, Wendel N. Public- and Private-Sector Contributions to the Research
and Development of the Most Transformational Drugs in the Past 25 Years: From Theory to Therapy. Ther Innov Regul Sci.
Nov 2016;50(6):759-768. doi:10.1177/2168479016648730
22. Moos WH, Mirsalis JC. Nonprofit organizations and pharmaceutical research and development. Drug
development research. 2009;70(7):461-471. doi:10.1002/ddr.20326
23. Hale VG, Woo K, Lipton HL. Oxymoron no more: the potential of nonprofit drug companies to deliver on the
promise of medicines for the developing world. Health Affairs. 2005;24(4):1057-1063.
24. Foundation CF. Our Venture Philanthropy Model. Cystic Fibrosis Foundation. https://www.cff.org/about-us/our-
venture-philanthropy-model
25. Pollack A. Deal by Cystic Fibrosis Foundation Raises Cash and Some Concern. The New York Times; 2014.
Accessed 17 March 2023. https://www.nytimes.com/2014/11/19/business/for-cystic-fibrosis-foundation-venture-yields-
windfall-in-hope-and-cash.html

July 2024
ASPE REPORT
23
26. Bush A, Simmonds NJ. Hot off the breath: 'I've a cost for'--the 64 million dollar question. Thorax. May
2012;67(5):382-4. doi:10.1136/thoraxjnl-2012-201798
27. Nolen S, Robbins, R. . The Drug Is a ‘Miracle’ but These Families Can’t Get It. The New York Times; 2023. Accessed
17 March 2023. https://www.nytimes.com/2023/02/07/health/cystic-fibrosis-drug-trikafta.html
28. Bansal R, De Backer, R., Ranade, V. . What’s behind the pharmaceutical sector’s M&A push. McKinsey &
Company; 2018. https://www.mckinsey.com/capabilities/strategy-and-corporate-finance/our-insights/whats-behind-the-
pharmaceutical-sectors-m-and-a-push#/
29. Drug Industry: Profits, Research and Development Spending, and Merger and Acquisition Deals (2017).
30. Weisman R. Nonprofit vows to lower generic drug costs. The Boston Globe; 2015. Accessed 30 March 2023.
https://www.bostonglobe.com/business/2015/12/13/nonprofit-aims-make-affordable-generic-
drugs/u0kd8MHfZmawSzAh0pRnKI/story.html
31. CIVICA. Timeline-2018: Civica is Launched! https://civicarx.org/timeline-2018/
32. Internal Revenue Service. Exemption Requirements - 501(c)(3) Organizations. Internal Revenue Service.
https://www.irs.gov/charities-non-profits/charitable-organizations/exemption-requirements-501c3-organizations
33. Internal Revenue Service. Social Welfare Organizations. Internal Revenue Service. https://www.irs.gov/charities-
non-profits/other-non-profits/social-welfare-organizations
34. Internal Revenue Service. About Form 990, Return of Organization Exempt from Income Tax.
https://www.irs.gov/forms-pubs/about-form-990
35. Ledley FD, McCoy SS, Vaughan G, Cleary EG. Profitability of Large Pharmaceutical Companies Compared With
Other Large Public Companies. JAMA. Mar 3 2020;323(9):834-843. doi:10.1001/jama.2020.0442
36. CIVICA. Making quality generic and biosimilar medications available at fair prices. CivicaRx.
https://civicarx.org/medications/
37. CivicaScript Announces a New Health Plan Partner, a New President, and a New Drug Manufacturing Partner.
CivicaRx; 2021. https://civicarx.org/civicascript-the-newly-named-civica-initiative/#
38. Dredge C, Scholtes S. The health care utility model: a novel approach to doing business. NEJM Catalyst
Innovations in Care Delivery. 2021;2(4)
39. Report on the Affordability of Insulin (2022).
40. Gaffney A, Himmelstein DU, Woolhandler S. Prevalence and Correlates of Patient Rationing of Insulin in the
United States: A National Survey. Ann Intern Med. Nov 2022;175(11):1623-1626. doi:10.7326/M22-2477
41. Wingrove P, Bhanvi, S. . Novo Nordisk to slash US insulin prices, following move by Eli Lilly. Reuters; 2023.
https://www.reuters.com/business/healthcare-pharmaceuticals/novo-nordisk-cut-price-insulin-by-up-75-wsj-2023-03-14/
42. Lovelace B. Sanofi announces insulin price cap after actions by Eli Lilly and Novo Nordisk. NBC News; 2023.
https://www.nbcnews.com/health/health-news/sanofi-insulin-price-cap-rcna75346
43. The Associated Press. Eli Lily Cuts the Price of Insulin, Capping Drug at $35 per month out-of-pocket. The
Associated Press; 2023. https://www.npr.org/2023/03/01/1160339792/eli-lilly-insulin-price
44. Sable-Smith BY, Samantha. Eli Lilly slashed insulin prices. This starts a race to the bottom. Fierce Healthcare;
2023. https://www.fiercehealthcare.com/finance/eli-lilly-slashed-insulin-prices-starts-race-bottom
45. Rosenberg M, Chai G, Mehta S, Schick A. Trends and economic drivers for United States naloxone pricing, January
2006 to February 2017. Addictive behaviors. 2018;86:86-89.
46. Statement from FDA Commissioner Scott Gottlieb, M.D., on unprecedented new efforts to support development
of over-the-counter naloxone to help reduce opioid overdose deaths. U.S. Food and Drug Administration; 2019.
https://www.fda.gov/news-events/press-announcements/statement-fda-commissioner-scott-gottlieb-md-
unprecedented-new-efforts-support-development-over
47. Over-the-Counter Naloxone One Step Closer as Harm Reduction Therapeutics Initiates a Rolling Submission of its
New Drug Application (NDA) to U.S. Food and Drug Administration for RiVive™ (3.0 mg intranasal naloxone) for
Emergency Treatment of Opioid Overdose. Harm Reduction Therapeutics; 2022.
https://www.harmreductiontherapeutics.org/wp-content/uploads/2022/12/HRT-Rolling-Submission-
PR.11Oct20226668.pdf
48. Emergent BioSolutions Announces U.S. FDA Acceptance and Priority Review of Supplemental New Drug
Application for Over-the-Counter NARCAN® (naloxone HCl) Nasal Spray. Emergent BioSolutions; 2022.
https://investors.emergentbiosolutions.com/news-releases/news-release-details/emergent-biosolutions-announces-us-
fda-acceptance-and-priority
49. FDA Approves First Over-the-Counter Naloxone Nasal Spray. U.S. Food and Drug Administration; 2023.
https://www.fda.gov/news-events/press-announcements/fda-approves-first-over-counter-naloxone-nasal-spray

July 2024
ASPE REPORT
24
50. FDA Approves Second Over-the-Counter Naloxone Nasal Spray Product. U.S. Food and Drug Administration;
2023. https://www.fda.gov/news-events/press-announcements/fda-approves-second-over-counter-naloxone-nasal-
spray-product#:~:text=FDA%20Approves%20Second%20Over-the-Counter%20Naloxone%20Nasal%20Spray%20Product,-
Share&text=Today%2C%20the%20U.S.%20Food%20and,known%20or%20suspected%20opioid%20overdose
51. Drugs.com. Targretin Prices, Coupons and Patient Assistance Programs. https://www.drugs.com/price-
guide/targretin#:~:text=The%20cost%20for%20Targretin%20oral,on%20the%20pharmacy%20you%20visit
52. Bell J. Aiming for price transparency, Civica Rx hits snag with bundled payments. BIOPHARMDIVE; 2019. Accessed
30 March 2023. https://www.biopharmadive.com/news/dia-civicarx-drug-pricing-transparency-hospital-
payments/557541/
53. Spicer M. US FDA Expects No Change In Naloxone Access Barrier If Prices High For Potential OTC Products. HBW
Citeline; 2022. https://hbw.citeline.com/RS152876/US-FDA-Expects-No-Change-In-Naloxone-Access-Barrier-If-Prices-
High-For-Potential-OTC-Products
54. Drug Shortages: Root Causes and Potential Solutions (U.S. Food & Drug Administration) (2019).
55. Dredge C, Liljenquist D, Scholtes S. Disruptive Collaboration: A Thesis for Pro-Competitive Collaboration in Health
Care. NEJM Catalyst Innovations in Care Delivery. 2022;3(2)
56. Testimony to the United States Senate Committee on Finance: COVID-19 & Beyond: Oversight of the FDA’s
Foreign Drug Manufacturing Inspection Process, (2020) (US Senate Committee on Finance).
https://www.finance.senate.gov/hearings/covid-19-and-beyond-oversight-of-the-fdas-foreign-drug-manufacturing-
inspection-process
57. Capps CS, Carlton DW, David G. Antitrust treatment of nonprofits: Should hospitals receive special care?
Economic Inquiry. 2020;58(3):1183-1199.
58. FDA Publishes List of Essential Medicines, Medical Countermeasures, Critical Inputs Required by Executive Order.
U.S. Food and Drug Administration; 2020. https://www.fda.gov/news-events/press-announcements/fda-publishes-list-
essential-medicines-medical-countermeasures-critical-inputs-required-executive
59. Executive Order 13944 List of Essential Medicines, Medical Countermeasures, and Critical Inputs (2022).
60. World Health Organization. WHO Model List of Essential Medicines - 22nd list, 2021. World Health Organization.
https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2021.02
61. Innovative Genomics Institute. Public Impact. Innovative Genomics Institute
https://innovativegenomics.org/programs/public-impact/
62. Piddock LJV, Paccaud JP, O'Brien S, Childs M, Malpani R, Balasegaram M. A Nonprofit Drug Development Model Is
Part of the Antimicrobial Resistance (AMR) Solution. Clin Infect Dis. May 30 2022;74(10):1866-1871.
doi:10.1093/cid/ciab887
63. Nielsen TB, Brass EP, Gilbert DN, Bartlett JG, Spellberg B. Sustainable discovery and development of antibiotics—
is a nonprofit approach the future? The New England journal of medicine. 2019;381(6):503.
64. Outterson K, Rex JH. Evaluating for-profit public benefit corporations as an additional structure for antibiotic
development and commercialization. Transl Res. Jun 2020;220:182-190. doi:10.1016/j.trsl.2020.02.006
65. Collins FS. Mining for therapeutic gold. Nat Rev Drug Discov. Jun 2011;10(6):397. doi:10.1038/nrd3461
66. Cvek B. Nonprofit drugs as the salvation of the world's healthcare systems: the case of Antabuse (disulfiram).
Drug Discov Today. May 2012;17(9-10):409-12. doi:10.1016/j.drudis.2011.12.010
67. Roth LP, Sanders JN, Simmons RG, Bullock H, Jacobson E, Turok DK. Changes in uptake and cost of long-acting
reversible contraceptive devices following the introduction of a new low-cost levonorgestrel IUD in Utah's Title X clinics: a
retrospective review. Contraception. Jul 2018;98(1):63-68. doi:10.1016/j.contraception.2018.03.029
68. Cvek B. Multiple deadlocks in the development of nonprofit drugs. Drug Discov Today. Sep 2022;27(9):2411-
2414. doi:10.1016/j.drudis.2022.06.001
69. Centers for Medicare & Medicaid Services. Medicare FY2020 IPPS Final Rule. Centers for Medicare & Medicaid
Services. https://www.federalregister.gov/documents/2019/08/16/2019-16762/medicare-program-hospital-inpatient-
prospective-payment-systems-for-acute-care-hospitals-and-the
70. Kansteiner F. Civica Rx lays out $124M sterile injectables plant, pegged to supply COVID-19 drugs and more.
Fierce Pharma; 2021. https://www.fiercepharma.com/manufacturing/civica-rx-lays-out-124-5-million-sterile-injectables-
plant-pegged-to-supply-covid-19
71. Phlow Corp. Phlow Corporation Awarded $354 Million HHS/ASPR/BARDA Contract to Manufacture Essential
Medicines in Shortage. PR Newswire; 2020. https://www.prnewswire.com/news-releases/phlow-corporation-awarded-
354-million-hhsasprbarda-contract-to-manufacture-essential-medicines-in-shortage-301061648.html
72. Examination of Clinical Trial Costs and Barriers for Drug Development (Office of the Assistant Secretary for
Planning and Evaluation) (2014).

July 2024
ASPE REPORT
25
73. U.S. Food and Drug Administration. Small Business Assistance. U.S. Food and Drug Administration.
https://www.fda.gov/industry/small-business-assistance
74. U.S. Food and Drug Administration. Prescription Drug User Fee Amendments. U.S. Food and Drug Administration.
https://www.fda.gov/industry/fda-user-fee-programs/prescription-drug-user-fee-amendments
75. Eastern Research Group Inc. Cost of Generic Drug Development and Approval. 2022.
https://aspe.hhs.gov/reports/cost-generic-drugs
76. RTI. FDA approves treatment targeting drug-resistant tuberculosis backed by RTI scientist. TechWire; 2019.
Accessed 17 March 2023. https://wraltechwire.com/2019/08/14/fda-approves-treatment-targeting-drug-resistant-
tuberculosis-backed-by-rti-scientist/
77. Vieira M. K, R., Navarro, D., Bezruki, A., Moon, S. Advancing Innovation and Access to Medicines
The Achievements and Unrealized Potential of the Product Development Partnership Model. Partnerships for
Sustainability in Contemporary Global Governance. Routledge; 2022:120-143.
78. Curtis KM. Update to US selected practice recommendations for contraceptive use: self-administration of
subcutaneous depot medroxyprogesterone acetate. MMWR Morbidity and mortality weekly report. 2021;70
79. Chin-Dusting J, Mizrahi J, Jennings G, Fitzgerald D. Finding improved medicines: the role of academic–industrial
collaboration. Nature reviews Drug discovery. 2005;4(11):891-897.

July 2024
ASPE REPORT
26
U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES
Office of the Assistant Secretary for Planning and Evaluation
200 Independence Avenue SW, Mailstop 434E
Washington, D.C. 20201
For more ASPE briefs and other publications, visit: aspe.hhs.gov/reports
ABOUT THE AUTHORS
Oluwarantimi Adetunji, PhD is an Economist and NIH portfolio lead in the Office of Science and Data Policy in ASPE.
Jon F. Oliver, PhD is an Economist in the Office of Science and Data Policy in ASPE.
Sonal Parasrampuria, PhD was a Social Science Analyst and FDA portfolio lead in the Office of Science and Data Policy in
ASPE when this work was conducted.
Grace Singson, PharmD is an ORISE fellow in the Office of Science and Data Policy in ASPE.
Trinidad Beleche, PhD is a Senior Economist in the Office of Science and Data Policy in ASPE.
SUGGESTED CITATION
Adetunji, O., Oliver, J.F., Parasrampuria, S., Singson, G., and Beleche, T. “The Potential Role of The Nonprofit
Pharmaceutical Industry in Addressing Shortages and Increasing Access to Essential Medicines and Low-Cost Medicines”.
Washington, DC: Office of the Assistant Secretary for Planning and Evaluation, U.S. Department of Health and Human
Services. July 2024.
COPYRIGHT INFORMATION
All material appearing in this report is in the public domain and may be reproduced or copied without permission; citation
as to source, however, is appreciated.
Links and references to information from non-governmental organizations are provided for informational purposes and
are not HHS endorsements, recommendations, or preferences for the non-governmental organizations.
___________________________________
For general questions or general information about ASPE: aspe.hhs.gov/about
