
NEW YORK STATE’S COVID-19 VACCINATION PROGRAM
1
NEW YORK STATE’S
COVID-19 VACCINATION PROGRAM
OCTOBER 2020
Department
of Health

“What do we want to accomplish in New York? We should
have the best vaccination program in the United States
of America. I think the way we have handled COVID has
been a model for this country. I want New Yorkers to do
the same thing with vaccines.”
Governor Andrew M. Cuomo
September 2020
Note: While New York State has set forward the following operational plan, several
details remain unknown and there are future decisions that will need to be made that
are dependent on further information regarding the delineation of federal and state
responsibilities; the funding needs associated with those responsibilities; and the
planned supply chain management and vaccine allocation process. As a result, the
approaches and methodology discussed in the New York State COVID-19 Vaccination
Program plan should be considered proposed and interim, and final strategies will be
established once further details and decisions are known.
NEW YORK STATE’S COVID-19 VACCINATION PROGRAM
2

TABLE OF CONTENTS
EXECUTIVE SUMMARY ....................................................................................................5
1. GUIDING PRINCIPLES ................................................................................................9
2. KEY ASSUMPTIONS AND UNKNOWNS .........................................................13
CURRENT UNKNOWN VARIABLES...........................................................................14
PUBLIC HEALTH PREPAREDNESS IMPROVEMENT PLANNING .....................18
3. VACCINE SAFETY AND EFFECTIVENESS .............................................................19
4. VACCINE DISTRIBUTION AND IMPLEMENTATION ........................................22
5. VACCINE PRIORITIZATION BASED ON CLINICAL GUIDANCE ..................26
VACCINE PRIORITIZATION MATRIX ......................................................................28
POPULATION RISK & ESSENTIAL WORKER PHASES ......................................29
TRIBAL NATIONS .........................................................................................................30
HEALTH EQUITY ..........................................................................................................31
6. VACCINE DISTRIBUTION & DELIVERY.................................................................33
STORAGE & INVENTORY............................................................................................35
VACCINE DISTRIBUTION & DELIVERY - POSSIBLE REGIONAL COLD
STORAGE LOCATIONS ..............................................................................................36
7. ADMINISTRATION OF VACCINE ............................................................................37
PREPARATION FOR EACH VACCINATION ADMINISTRATION SITE (VAS) 41
PHARMACIES ................................................................................................................42
LOCAL HEALTH DEPARTMENTS ............................................................................ 43
STATE OPERATED AND SUPPORTED VACCINATION SITES .........................44
REPORTING ...................................................................................................................46
8. DATA AND INFORMATION TECHNOLOGY (IT) INFRASTRUCTURE ........47
NEW YORK STATE IMMUNIZATION INFORMATION SYSTEM (NYSIIS) ......48
PATIENT SUPPORT & PUBLIC REPORTING ........................................................53
SECOND-DOSE REMINDERS ..................................................................................54
NEW YORK STATE’S COVID-19 VACCINATION PROGRAM
3

9. PUBLIC EDUCATION & COMMUNITY ENGAGEMENT REGARDING
VACCINATION PROGRAM .....................................................................................56
10. ORGANIZATIONAL LEADERSHIP..........................................................................61
REG U LATORY A N D LEG A L REVI EW ....................................................................65
VACCINE INFORMATION STATEMENTS (VISs) .................................................66
11. PROCUREMENT OF NECESSARY SUPPLIES AND EQUIPMENT ...............67
12. POST-VACCINATION MONITORING ....................................................................71
NEW YORK STATE’S COVID-19 VACCINATION PROGRAM
4

EXECUTIVE SUMMARY
5

EXECUTIVE SUMMARY
The New York State COVID-19 Vaccination Program is
drafted to ensure the distribution and administration of
a safe and eective COVID-19 vaccine to all residents
of the Empire State who wish to receive it. The program
is designed to be flexible given many unknowns at this
point in the process, including the uncertainty on vaccine
availability, timeline for vaccine approval, delineation of
federal and state responsibilities, funding, supply chain
needs, and allocation requirements. The program is
drafted on the advice and recommendation of clinical
and public health experts, and its success is contingent
on partnership and collaboration with local departments
of health, community partners and organizations. Recent
public opinion polls suggest that the percentage of
Americans who would get the vaccine if it were available
today has dropped to just over 50 percent in September
from 72 percent in May, making the vaccine program’s
eectiveness also dependent on the building and
maintaining of public trust in the product and the process.
This document - the New York State COVID-19 Vaccine
Program - describes the steps that are being taken and
protocols that are being put in place to ensure the safe
and ecient distribution and administration of vaccine to
New York residents. The document outlines:
1. Guiding principles to be adhered to throughout the
vaccine process
NEW YORK STATE’S COVID-19 VACCINATION PROGRAM
6

NEW YORK STATE’S COVID19 VACCINATION PROGRAM
2. Key assumptions, unknowns, and variables that may
impact vaccine planning
3. Measures to ensure vaccine safety and eectiveness
(pre and post administration)
4.. Expertise to guide vaccine distribution and
implementation
5. A vaccine prioritization matrix based on clinical guidance
6. A process for ecient vaccine distribution & delivery
7. Measures to train, register, deploy, and support
providers to administer the vaccine
8. A data and IT infrastructure to coordinate and monitor all
aspects of the vaccine program
9. A public education & community outreach campaign to
build trust and inform the public
10. A Vaccine Central Command Center to manage the
entire vaccine program
11. A budget and procurement process to obtain necessary
supplies and equipment
NEW YORK STATE’S COVID-19 VACCINATION PROGRAM
7

It is expected that vaccine distribution and administration
approaches will be informed by the federal government
upon release of the vaccine to states. The “COVID-19
Vaccination Program Interim Playbook for Jurisdiction
Operations,” released by the Centers for Disease Control
and Prevention (CDC) on September 16, 2020, requested
states to submit plans by October 16, 2020. The New York
State COVID-19 Vaccination Program is responsive to this
request and details the state’s preparatory eorts for a
mass vaccination eort.
NEW YORK STATE’S COVID-19 VACCINATION PROGRAM
88
SECTION 1:
GUIDING PRINCIPLES
9

SECTION 1. GUIDING PRINCIPLES
New York State based its COVID-19 vaccine distribution and
administration process on ten guiding principles.
1. Safety: New York State will only endorse and distribute a
COVID-19 vaccine if it is determined to be safe and will only
be used according to the indication under which it received its
authorization or license. This includes pharmacovigilance post-
licensure and adverse event monitoring post administration.
2. Eectiveness: New York State will only endorse and
distribute a COVID-19 vaccine if it is demonstrated to be
appropriately eective in the populations intended for use.
3. Expert approved: New York State will rely on the advice
and counsel of established clinical experts and scientists to
review and approve the safety and ecacy of every vaccine
that is authorized by the federal government for distribution.
4. Equitable & clinically driven distribution: New York
State’s COVID-19 vaccine distribution approach will be based
solely on clinical and equitable standards that prioritize access
to persons at higher risk of exposure, illness and/or poor
outcome, regardless of other unrelated factors, such as wealth
or social status, that might confer unwarranted preferential
treatment.
5. Transparency: Throughout the COVID-19 crisis, the state’s
daily public presentation of facts and reliance on science and
medical expertise helped build public trust and confidence in
NEW YORK STATE’S COVID-19 VACCINATION PROGRAM
10

NEW YORK STATE’S COVID19 VACCINATION PROGRAM
government action. Similarly, New York State will continue
to be transparent regarding all aspects of the COVID-19
vaccine distribution, administration, and monitoring
process to ensure New Yorkers are fully informed.
6. Use of Data: Coordination of a successful vaccination
program will require robust tracking, data and analytics
capabilities. New York State will use robust data and
information technology platforms to guide all parts of the
COVID-19 vaccine distribution and administration process
to maximize safety, accuracy, and eciency and meet all
Federal reporting requirements.
7. Privacy and Patient Safety: Vaccination does not
negate the importance of other public health measures
that have served us well to date in the containment of
COVID-19. New York State will continue to urge compliance
with social distancing, mask wearing, hand washing, and
other measures. In addition, New York State will ensure
all vaccination processes prioritize patient safety, and all
information systems guarantee patient privacy.
8. Partnership, Coordination & Public Outreach:
New York State recognizes that coordination with local
organizations and community providers is essential to
the safe and successful distribution and administration
of COVID-19 vaccines. The state’s outreach eorts
will especially focus on reaching underserved, hard to
reach, vulnerable, less accessible and vaccine hesitant
populations, as well as those at highest risk for COVID-19
infection and poor outcomes.
NEW YORK STATE’S COVID-19 VACCINATION PROGRAM
11

9. State Leadership: New York State hopes for and
expects a robust federal engagement on vaccine vetting,
distribution, administration, and funding. However, given
the shortcomings of the federal response experienced
since the outset of the COVID-19 epidemic, New York State
will undertake necessary preparatory steps and require
local coordination with the state’s centralized approach to
ensure an ecient and organized vaccine distribution.
10. NEW YORK TOUGH: Throughout this COVID-19 crisis,
New Yorkers have shown that there is nothing we cannot
do if we work together as one community. Our approach
to the COVID-19 vaccine will be tough, strong, united,
disciplined, and loving.
NEW YORK STATE’S COVID-19 VACCINATION PROGRAM
12

SECTION 2:
KEY ASSUMPTIONS
& UNKNOWNS
NEW YORK STATE’S COVID-19 VACCINATION PROGRAM
13

SECTION 2. KEY ASSUMPTIONS AND UNKNOWNS
As of mid-October 2020, much is still unknown about
the potential COVID-19 vaccines and the process that the
federal government will undertake regarding its allocation,
distribution and administration. New York State’s COVID-19
Vaccination Program therefore accounts for multiple
variables and scenarios, an approach that will help
guide the state as situations and circumstances change.
Consideration of a range of possibilities will ensure New
York State is fully prepared to adapt operational and
clinical decisions to achieve the best outcome as initial
assumptions change or are confirmed.
CURRENT ASSUMPTIONS REGARDING COVID-19
VACCINE
• Initially, COVID-19 vaccines are expected to be approved
for use by the Food and Drug Administration (FDA) under
an Emergency Use Authorization (EUA). Under section 564
of the Federal Food, Drug, and Cosmetic Act (FD&C Act),
the FDA Commissioner may allow unapproved medical
products or unapproved uses of approved medical
products to be used in an emergency to diagnose, treat,
or prevent serious or life-threatening diseases when there
are no adequate, approved, and available alternatives.
Vaccines granted EUA will be approved for distribution
while the vaccine manufacturer simultaneously seeks full
licensure. The CDC’s Advisory Committee on Immunization
Practices (ACIP) will develop recommendations for
appropriate use of each vaccine once approved.
NEW YORK STATE’S COVID-19 VACCINATION PROGRAM
14

NEW YORK STATE’S COVID19 VACCINATION PROGRAM
• Although some COVID-19 vaccines may only require
one dose, the current plan assumes a more challenging
scenario of vaccines requiring a two dose regimen,
administered 21 to 28 days apart. Doses must be from the
same vaccine manufacturer and cannot be interchanged
from one product to another. As a result, supply must
be reserved to ensure second dose coverage before
administering initial doses.
• COVID-19 vaccine supply may initially be limited in the
first months after initial EUA approval, expanding rapidly
within future months.
• Initial federal allocation to New York State may be
pro-rata based on priority populations in the state as
determined by federal data systems such as HHS Protect
and state data sources.
• While there are still unknown aspects of vaccine
prioritization, initial vaccine distributions may be informed
by the federal government and doses may be released to
states on condition they are prioritized for use by certain
priority populations, likely to include front line health care
workers, residents of long-term care facilities, and other
persons at high risk of mortality from COVID-19. More
information will need to be known on this distribution
prioritization and what decisions are expected to made by
the states and which by the federal government.
• Vaccine characteristics will determine which vaccine
should be used in which populations (i.e., older adults).
Approval for use in pregnant women and children may not
be immediate.
NEW YORK STATE’S COVID-19 VACCINATION PROGRAM
15

• Varied cold chain requirements for the vaccines from
refrigerated (2°C to 8°C) to frozen (-15°C to -25°C) to ultra-
cold freezers (-60°C to -80°C) may pose unique challenges
to storage, handling, and distribution.
• Public opinion is mixed on the safety and ecacy of any
vaccine. Misinformation will likely be actively disseminated
on social media and other sources.
CURRENT UNKNOWN VARIABLES
• Federal v. State responsibilities and roles: On October
14, Governor Cuomo released a letter from the National
Governors Association (NGA) asking President Trump for
a meeting to discuss how a vaccination program would
be conducted and what are the respective roles of the
federal and state governments. The letter noted that
states including New York are willing to assist the federal
government’s eorts to ensure a national vaccination
campaign is implemented smoothly and eciently,
however, additional guidance and clarification is needed
on the roles and expectations of states in a successful
COVID-19 vaccine distribution and implementation plan.
The hope of this meeting is to discuss what is required
to ensure a strong partnership, including but not limited
to: the delineation of federal and state responsibilities;
the funding needs associated with those responsibilities;
and the planned supply chain management and vaccine
allocation process.
NEW YORK STATE’S COVID-19 VACCINATION PROGRAM
16

NEW YORK STATE’S COVID19 VACCINATION PROGRAM
• Funding: A robust vaccine administration program will
require robust funding. It is unknown whether and the
extent to which the federal government will allocate this
funding to states for this purpose. CDC Director Robert
Redfield recently stated that Congress will need to allocate
$5.5 to $6 billion to the states for this purpose. New York
State to date has received $7.8M for COVID-19 vaccination
purposes.
• State vs. Local Distribution: It is unknown whether
the federal government will distribute all vaccinations
for New York’s 19.5 million residents directly to the
state government, or whether private entities or local
governments will receive their own separate allotment,
or a combination of any of these options. To ensure
coordinated and ecient statewide distribution and
administration, all localities and entities in New York
State will be required to follow the state’s guidance and
protocols for COVID-19 vaccination.
To keep fully abreast of the changing landscape and adjust
for all scenarios, New York State will track daily updates
on vaccine trials, clinical data, and federal plans for
distribution and administration.
NEW YORK STATE’S COVID-19 VACCINATION PROGRAM
17
PUBLIC HEALTH PREPAREDNESS IMPROVEMENT
PLANNING
The NYSDOH 2009 H1N1 Influenza Pandemic After-Action
Report and Improvement Plan presented an in-depth
analysis of New York’s health emergency response to the
first influenza pandemic that had occurred in 42 years,
from the spring of 2009 through early 2010. The report
identified the strengths, areas for improvement, and
corrective actions that resulted from this real-world event.
In addition, through the ongoing public health
emergency preparedness funding, the state and local
health departments (LHDs) actively engage in ongoing
emergency preparedness activities that include
improvement-related deliverables such as trainings,
workshops, tabletop exercises and full-scale drills. These
allow for continuous improvements in operationalizing a
vaccination campaign.
Lessons from the H1N1 vaccination eort and emergency
preparedness exercises are being incorporated into the
COVID-19 vaccine planning process.

SECTION 3:
VACCINE SAFETY
& EFFECTIVENESS
19

SECTION 3. VACCINE SAFETY AND
EFFECTIVENESS
It has been publicly reported that a majority of Americans
are skeptical of the current federal administration’s
credibility on COVID-19 vaccine safety and eectiveness,
with reports showing the number of Americans willing
to receive the vaccine if it were available today has
declined since spring. To establish and build public trust,
on September 29, Governor Cuomo appointed members
to New York’s independent Clinical Advisory Task Force
comprised of leading scientists, doctors, and health
experts who will expeditiously review every COVID-19
vaccine authorized by the federal government, and
will advise New York State on the vaccine’s safety and
eectiveness in fighting the virus.
The Clinical Advisory Task Force will help NYS determine
if the vaccine safe and eective. The Task Force is
comprised of Department of Health subject matter experts
and recognized external experts in medicine, law, and
science:
1. Charles M. Rice, PhD, The Rockefeller University
(Chair)
2. Scott H. Hammer, MD, New York-Presbyterian/
Columbia University Medical Center (Chair)
NEW YORK STATE’S COVID-19 VACCINATION PROGRAM
20

NEW YORK STATE’S COVID19 VACCINATION PROGRAM
3. Shawneequa Callier, MA, JD, George Washington
University School of Medicine and Health Sciences
4. Bruce Farber, MD, Northwell
5. Adolfo Garcia-Sastre, PhD, Icahn School of
Medicine at Mount Sinai
6. Kelvin Lee, MD, Roswell Park
7. Sharon Nachman, MD, Renaissance School of
Medicine at Stony Brook
The Task Force’s independent review of any federally
authorized COVID-19 vaccine will help address publicly
reported concerns about the scientific process and rush to
market. The Task Force will rely on numerous data sources
including public information and the findings of expert
third party independent organizations. In addition, they will
provide for the involvement and/or input of regional/local
clinical and other leadership representative of all areas of
the state. The Task Force will advise on the vaccine safety
profile, legal authority to withhold vaccine, and clinical
best practices if New York State must withhold or pause
distribution of the vaccine. The group will also advise
New York State as to the implications of a vaccine being
released under an FDA EUA.
NEW YORK STATE’S COVID-19 VACCINATION PROGRAM
21

SECTION 4:
VACCINE DISTRIBUTION
& IMPLEMENTATION
NEW YORK STATE’S COVID-19 VACCINATION PROGRAM
22

SECTION 4. VACCINE DISTRIBUTION AND
IMPLEMENTATION
Once New York’s independent Clinical Advisory Task
Force has advised that a COVID-19 vaccine is safe and
eective, the vaccine will be distributed and administered
throughout New York State.
To help guide this process, on September 24 the Governor
established a Vaccine Distribution and Implementation
Task Force to advise the set up and operation of the state’s
COVID-19 vaccination program. The Vaccine Distribution
and Implementation Task Force is comprised of experts
in public health, immunizations, government operations,
data and other fields relevant to vaccine distribution and
administration:
MEMBERS OF THE VACCINE DISTRIBUTION AND
IMPLEMENTATION TASK FORCE:
• Howard Zucker, MD, JD, Commissioner, NYS Dept.
of Health
• Karim Camara, NYS Oce of Faith-Based
Community Development Services
• Mantosh Dewan, MD, Interim President, SUNY
Upstate Medical Center
• Michael Dowling, President & CEO, Northwell Health
• Rose Duhan, President, Community Healthcare
Association for NYS
NEW YORK STATE’S COVID-19 VACCINATION PROGRAM
23

NEW YORK STATE’S COVID19 VACCINATION PROGRAM
• Michael Duteau, President, Chain Pharmacy
Association of New York State
• Deanna Ennello-Butler, Executive Director,
Pharmacy Society of State of New York
• Helen Evans, President, NYS Association of Rural
Health
• Bea Grause, President, Healthcare Association of
New York State
• George Gresham, President, 1199SEIU United
Healthcare Workers East
• Rev Diann Holt, AME Zion Church, Doula, Bualo
area
• Micky Jimenez, Regional Director, Capital District
LATINOS
• Bonnie Litvack, MD, President, Medical Society of
State of New York
• Jim Malatras, SUNY Chancellor
• Michael Martin, Executive Director, Native American
Community Services of Erie and Niagara Counties,
Inc.
• Robert Mujica, Division of Budget
• Patrick Murphy, Commissioner, Division of
Homeland Security and Emergency Services
• Alicia Ouellette, JD, President, Albany Law School
NEW YORK STATE’S COVID-19 VACCINATION PROGRAM
24
• Rajiv Rao, Chief Technology Ocer, NYS
• Kenneth Raske, President, Greater New York
Hospital Association
• Sarah Ravenhall, Executive Director, NYS
Association of County Health Ocials
• Rosanna Rosado, NYS Secretary of State
• Eli Rosenberg, Professor, SUNY University of Albany
Department of Epidemiology and Biostatistics
• Chanie Sternberg, President & CEO, Refuah Health
• Ramon Tallaj, Executive Director, Somos
Additional members will continue to be added to the NYS
Vaccine Distribution and Implementation Task Force as the
vaccination program is operationalized.

SECTION 5:
VACCINE PRIORITIZATION
BASED ON CLINICAL GUIDANCE
26

SECTION 5. VACCINE PRIORITIZATION BASED ON
CLINICAL GUIDANCE
Following the determination that the vaccine is safe
and eective, New York State will prioritize vaccination
recipients based on science, clinical expertise, and
federal guidelines. Critical populations will be identified
and recommended by the Advisory Committee on
Immunization Practices (with input from the National
Academies of Sciences, Engineering, and Medicine).
Prioritization decisions will take into account evolving
surveillance data and closely monitor the clinical ecacy
of the vaccination program. Prioritization decisions will
be made mindful of the disparate impact of COVID-19 on
communities of color, and the health disparities present
in underrepresented and marginalized communities, and
those with historically poor health outcomes.
All prioritization planning will be suciently detailed
and flexible in order to quickly adjust target recipient
populations given changes in the supply of vaccine that
is designated for distribution in New York State, the
number of New Yorkers seeking vaccination, and new
understandings regarding the vulnerability of certain
populations to COVID-19. Further information is needed
regarding the federal government’s expectations
regarding vaccine prioritizations before final decisions can
be made.
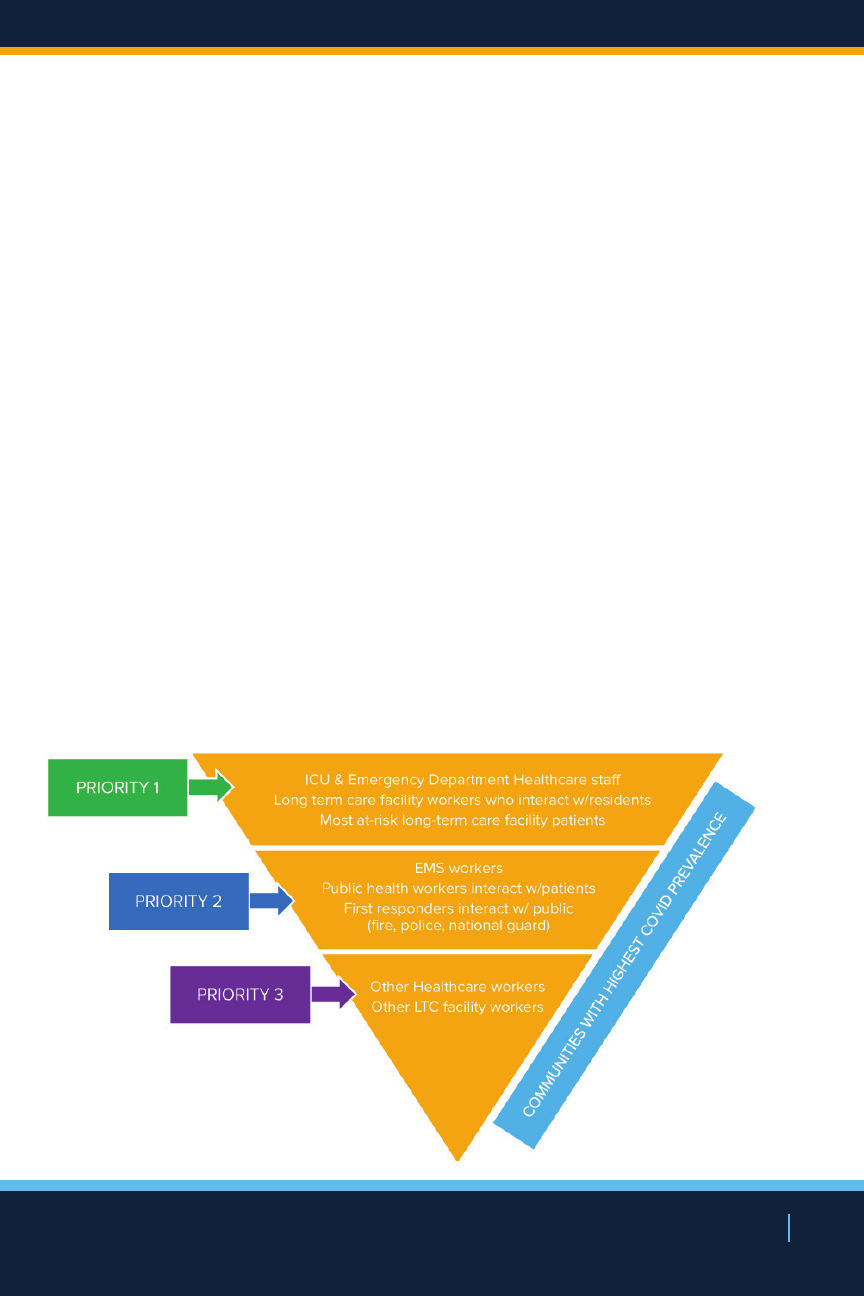
In anticipation of limited numbers of vaccine initially
available coupled with current knowledge of COVID-19
NEW YORK STATE’S COVID-19 VACCINATION PROGRAM
27
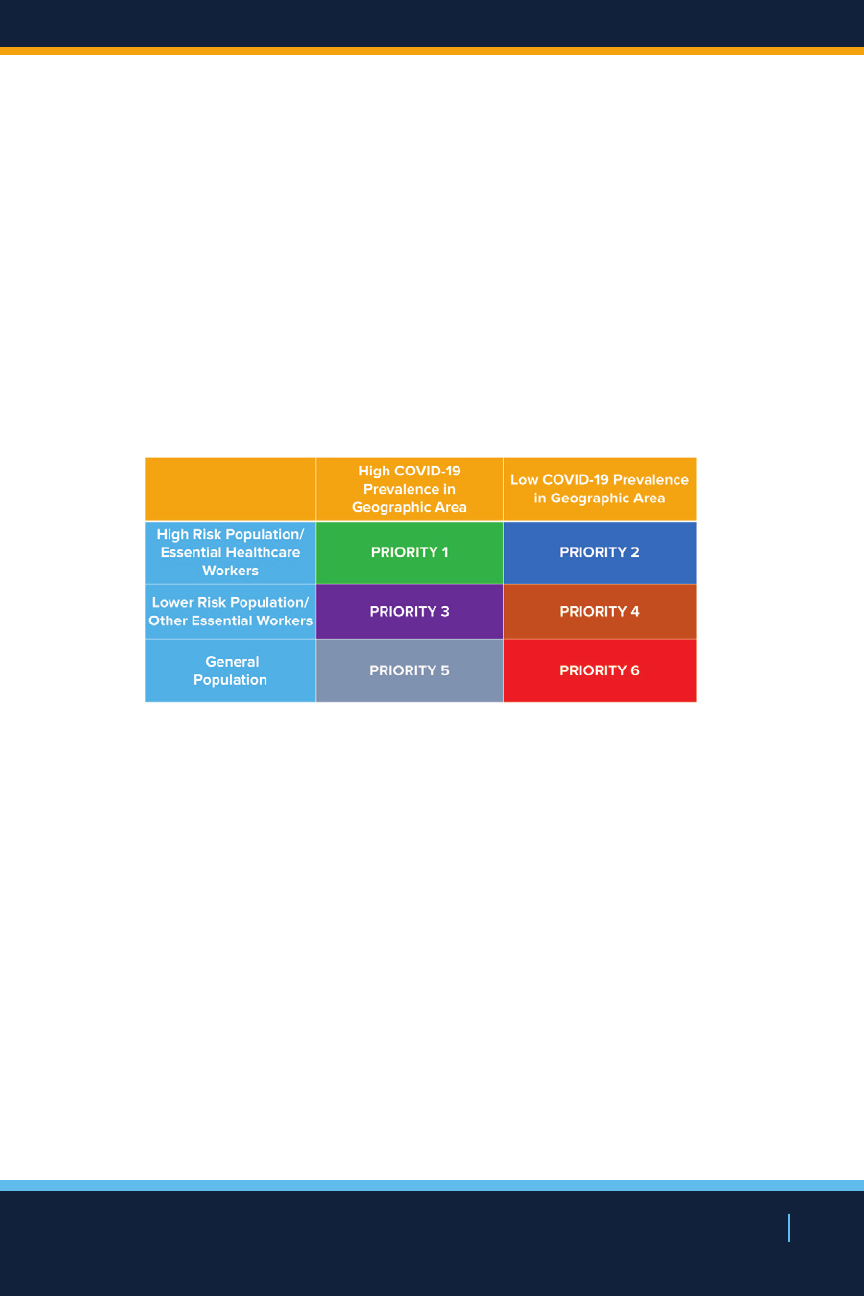
NEW YORK STATE’S COVID19 VACCINATION PROGRAM
morbidity and mortality, public health concerns, and the
need to maintain essential services, one of New York’s
prioritization strategies for vaccine distribution is designed
to ensure early vaccination of the most vulnerable New
Yorkers as well as essential frontline workers, with
distribution potentially directed to these populations who
reside or work within communities with highest prevalence
of COVID-19.
Vaccine Prioritization Matrix
Once the vaccine is first approved for use, New York State
will use up-to-date data to determine which geographic
areas of the state may derive a greater public health
benefit to receiving early vaccine. This may include areas
with higher historical burden of disease or areas that
have the highest prevalence of COVID-19. In addition,
individual factors for hospitals and nursing homes will be
considered including cases per facility in prior 14 days, and
vulnerability index of population served. New York will also
consider whether the vaccine can be used eectively as a
potential outbreak interruption strategy and if so, what the
criteria will be.
NEW YORK STATE’S COVID-19 VACCINATION PROGRAM
28
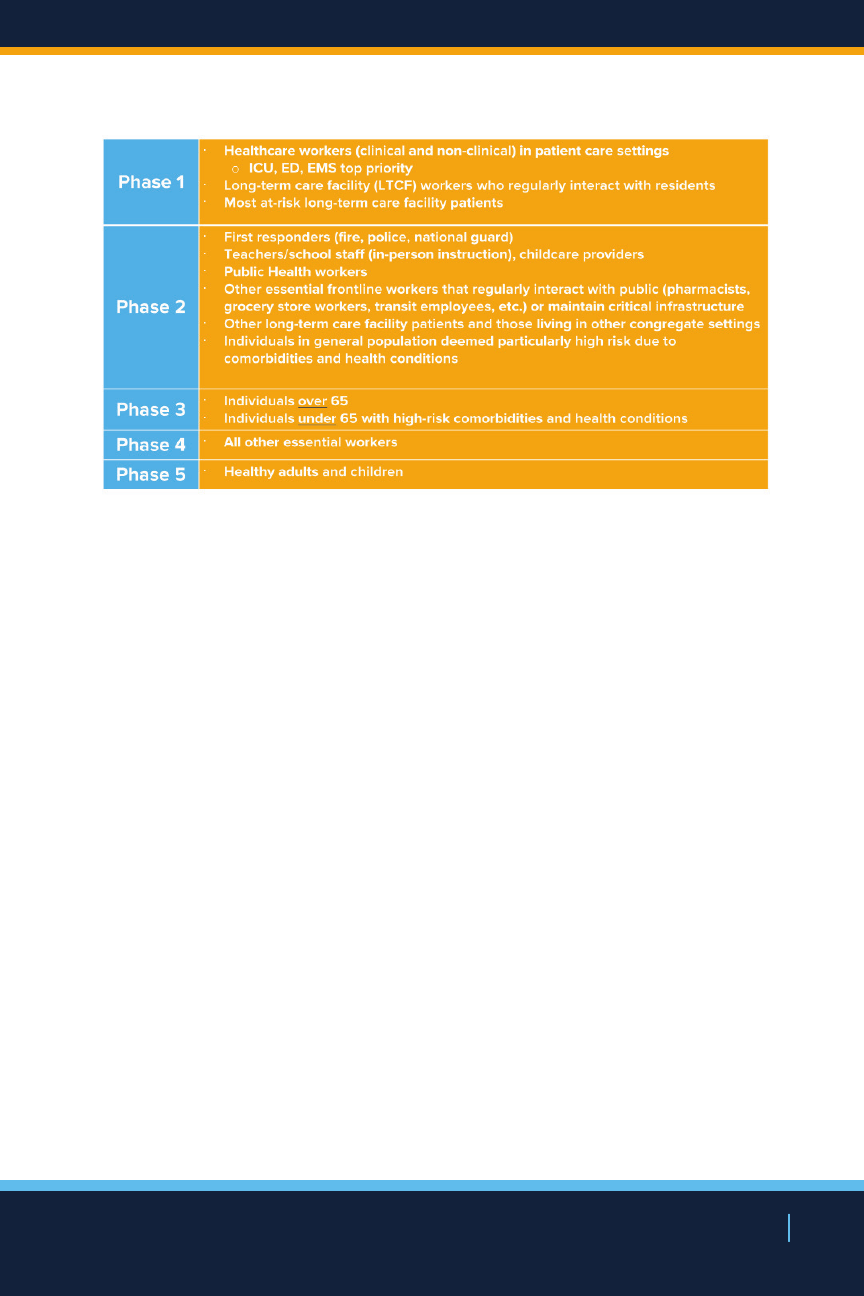
Within each phase, sub-populations will be identified to
allow for additional micro-level prioritization based on
vaccine availability and vaccination rates. For example,
healthcare workers that regularly interact with patients
may be prioritized over those that do not, and ICU and
emergency department healthcare workers will likely
be prioritized in hospitals if supply is not enough for the
entire hospital sta. All entities receiving the vaccine will
be given a level of autonomy to determine the internal
order of employee vaccination based on risk and within
the boundaries of NYS and federal guidance. To guide
prioritization, New York State will collect up-to-date
numbers of all prioritization and micro-level prioritizations
groups statewide including sub-populations.
The state will also use data and clinical expertise to
continue to determine which populations are at most
risk for COVID-19 infection as well as poor outcomes,
hospitalization and death, as well as which populations are
Population Risk & Essential Worker Phases
NEW YORK STATE’S COVID-19 VACCINATION PROGRAM
29

NEW YORK STATE’S COVID19 VACCINATION PROGRAM
most critical for societal and economic function. To guide
prioritization and allocation decisions, New York State
will maintain up-to-date estimates on the number of New
Yorkers in, and geographic distribution of, each of these
population groups.
Prior to the first vaccine(s) being approved for distribution
and administration, New York will prepare detailed
allocation scenarios based on this data and variables for
vaccine allotment amounts and vaccination rates within
priority populations.
TRIBAL NATIONS
Although CDC has stated they will work directly with
the Indian Health Service (IHS) at the federal level,
NYSDOH will also plan to include New York State tribal
organizations as part of state planning eorts, pending
further federal direction. Tribal Nations, being sovereign
entities, maintain government-to- government relationships
at the State and Federal levels, and may choose to work
directly through these relationships. However, counties
that are contiguous to Tribal Nations routinely collaborate
with these communities to ensure vaccination needs are
met. Therefore New York State will also prepare plans to
distribute COVID-19 vaccine to those tribes that are not
federally recognized or that are unable to access vaccine
through federal agency distributions.
NEW YORK STATE’S COVID-19 VACCINATION PROGRAM
30
HEALTH EQUITY
Heightened COVID-19 mortality among Black and
Hispanic communities (relative to white non-Hispanic)
is well established. In addition, compared to white non-
Hispanic adults, racial/ethnic minority populations had
disproportionately higher per population likelihoods of
COVID-19 diagnosis and hospitalization. New York State
will continue to work closely with partners across the
state who can assist in addressing health equity issues
and ensure that access to healthcare and vaccine are not
a barrier for COVID-19 treatment strategies. Healthcare
providers, community-based organizations, and religious
and community leaders will continue to assist New York
State to achieve these goals and will be represented
across the various stakeholder groups.
Scenario: Hypothetical Allocation of 100,000 doses (two
doses per person - 50,000 people):
NEW YORK STATE’S COVID-19 VACCINATION PROGRAM
31

NEW YORK STATE’S COVID19 VACCINATION PROGRAM
While New York has set forward a detailed vaccinate
prioritization approach, it is unknown the extent to which
federal allocation priorities will be pre-determined for state
governments to follow at the onset of vaccine distribution.
All allocation strategies discussed in the New York State
COVID-19 Vaccination Program plan should be considered
proposed and interim, and final allocation decisions will be
established when initial vaccine doses are shipped to New
York State.
NEW YORK STATE’S COVID-19 VACCINATION PROGRAM
32

SECTION 6:
VACCINE DISTRIBUTION
& DELIVERY
33

SECTION 6. VACCINE DISTRIBUTION & DELIVERY
Distributing and delivering enough vaccine to administer
two doses to every New Yorker will be a complex
logistical undertaking. New York will assign management
of this critical task to state agencies with operational
and logistical expertise including the Department of
Health, Division of Homeland Security and Emergency
Services, the Division of Military and Naval Aairs, New
York National Guard, the Oce of General Services, and
the Department of Transportation, and other agencies
who will coordinate all aspects of vaccine acquisition and
distribution with private and community health care and
partners and local governments. Distribution planning
will be flexible to allow for changes in vaccine type and
availability and other public health considerations based
on the most up-to-date scientific literature and CDC
recommendations, with the goal of maximizing public
health protection. Distribution planning will provide for
strict adherence to handling and storage requirements of
the vaccine to maintain stability and potency. In addition,
it will be essential to ensure chain of custody of all federal
and state assets throughout the distribution process.
Federal vaccine distribution plans may allow for some
private entities to distribute certain vaccines. For example,
McKesson, the current distributor for the CDC, is expected
to distribute the Moderna vaccine. While still under
federal control and direction, Pfizer, because of storage
and handling specifications, is expected to ship directly
NEW YORK STATE’S COVID-19 VACCINATION PROGRAM
34

NEW YORK STATE’S COVID19 VACCINATION PROGRAM
to end users. New York State will seek more information
on vaccine-specific distribution plans from the federal
government to ensure proper delineation of what is
expected of federal and state government and private
entities as part of the vaccine distribution process.
STORAGE & INVENTORY
A critical component to managing vaccine distribution
and delivery will be vaccine storage and inventory
management. In order to address the critical time
constraints in distributing this material, New York State will
work with local jurisdictions to identify and operationalize
appropriate regionally based storage locations, each that
comply with CDC and manufacturer recommendations
for storage including at temperatures as low as minus
80 degrees celsius. Additional information regarding
the ultra-cold COVID-19 vaccine candidate indicates the
vaccine may be shipped in a flat “pizza box-like” container
with dry ice that would not need recharge until the 10-
day mark. This may increase the number of sites without
ultra-cold storage that could receive these doses, with
the assumption they could successfully administer all
doses before the 10-day expiration. Capacity to store and
handle vaccines across the full cold-chain spectrum will be
assessed as part of the provider enrollment process.
To address limited specialized storage capacity, the plan
may utilize larger vaccination administration sites to also
serve as the regional storage locations, following the same
format as the Strategic National Stockpile of Chempack
supplies.
NEW YORK STATE’S COVID-19 VACCINATION PROGRAM
35

Inventory management will be performed with state-
of-the-art data technology platforms to ensure each
vaccine dosage is tracked from point of delivery to point
of administration, and storage sites are integrated into
the delivery process to sites designated for vaccination
administration.
To ensure vaccines are transported in a manner consistent
with CDC and manufacturer recommendations that
maximizes vaccine stability and minimizes wastage, New
York State may deploy vehicles equipped to transport
vaccines that meet required cold-chain requirements.
New York State will seek more information on storage
requirement and inventory management needs the federal
government and private vaccine manufacturers to ensure
adequate supply chain processes are put in place.
VACCINE DISTRIBUTION & DELIVERY - POSSIBLE
REGIONAL COLD STORAGE LOCATIONS
SECTION 7:
ADMINISTRATION
OF VACCINE
37

SECTION 7. ADMINISTRATION OF VACCINE
To administer the vaccine, New York State will rely on an
established network of health care providers, including
hospitals, LTCFs (nursing homes, adult care facilities
(ACFs), assisted living), Federally Qualified Health Centers
(FQHCs), Community Health Centers, Rural Health Clinics,
private provider oces, local health departments, and
other entities that will serve as Vaccination Administration
Sites (VAS). In addition, the state will work with commercial
and independent pharmacies, businesses, and other
organizations to enable on-site vaccination at these sites.
Other VASs include schools, colleges and universities,
homeless shelters, correction facilities, and sites where
target populations gather (i.e., senior centers, social
service oces, food pantries, etc.)
New York State will plan for quick activation and
mobilization of mass vaccination point of dispensing
(POD) sites, designed to be operationalized once vaccine
availability increases and outpaces provider administration
capacity. In addition, New York State will designate mobile
vaccination units, similar to the rapid response team
testing eorts that have been deployed statewide to help
control viral spread and outbreaks to increase access to
hard to reach populations including smaller congregate
living facilities.
Providers of all types will need to enroll with the
NYSDOH Vaccine Program to be a COVID-19 vaccinator
by completing and submitting a COVID-19 Vaccination
NEW YORK STATE’S COVID-19 VACCINATION PROGRAM
38

NEW YORK STATE’S COVID19 VACCINATION PROGRAM
Provider Agreement and Provider Profile available through
the Health Commerce System (HCS). The enrollment
process can accommodate a range of providers including
individual clinicians and large, multi-facility health systems.
Eligible providers will then be activated in the New York
State Immunization Information System (NYSIIS) with
COVID-19 vaccine ordering capability. A comprehensive
provider outreach, enrollment and training eort is
already underway and will be supported by associations
representing the various provider groups.
As the vaccine availability increases, the state will establish
on its own and in partnership with private and local
partners public clinics to serve targeted populations, as
well as mass vaccination point of dispensing (POD) sites
that can provide doses to thousands of New Yorkers each
day. All VAS and health care providers that administer the
vaccine will need storage capabilities, PPE, and integration
into the state’s data and IT infrastructure.
Providing nearly forty million vaccination doses will require
the recruitment and training of additional personnel
authorized to administer the vaccine. In addition to
supporting eorts by existing providers to hire more
personnel, the state will mobilize the existing public health
workforce and train addition sta capable of assisting at
mass vaccination sites and supporting provider networks.
Prior to the vaccine’s approval, the state will:
• Identify all existing providers capable of providing
COVID-19 vaccination and require registration of
NEW YORK STATE’S COVID-19 VACCINATION PROGRAM
39

each to develop an accurate database of VAS and
help ascertain communities that may need additional
vaccination administration sites.
• Work to train, enroll, and register additional providers
to expand the network of authorized health providers
who can administer the vaccine, including: pharmacists,
dentists, veterinarians, paramedics and EMTs, physician
assistants (PAs), student nurses, professionals who
are currently licensed in other states and interested in
supporting New York’s vaccination eorts.
Once becoming eligible for a vaccine, New Yorkers
will need to know where and when they should seek
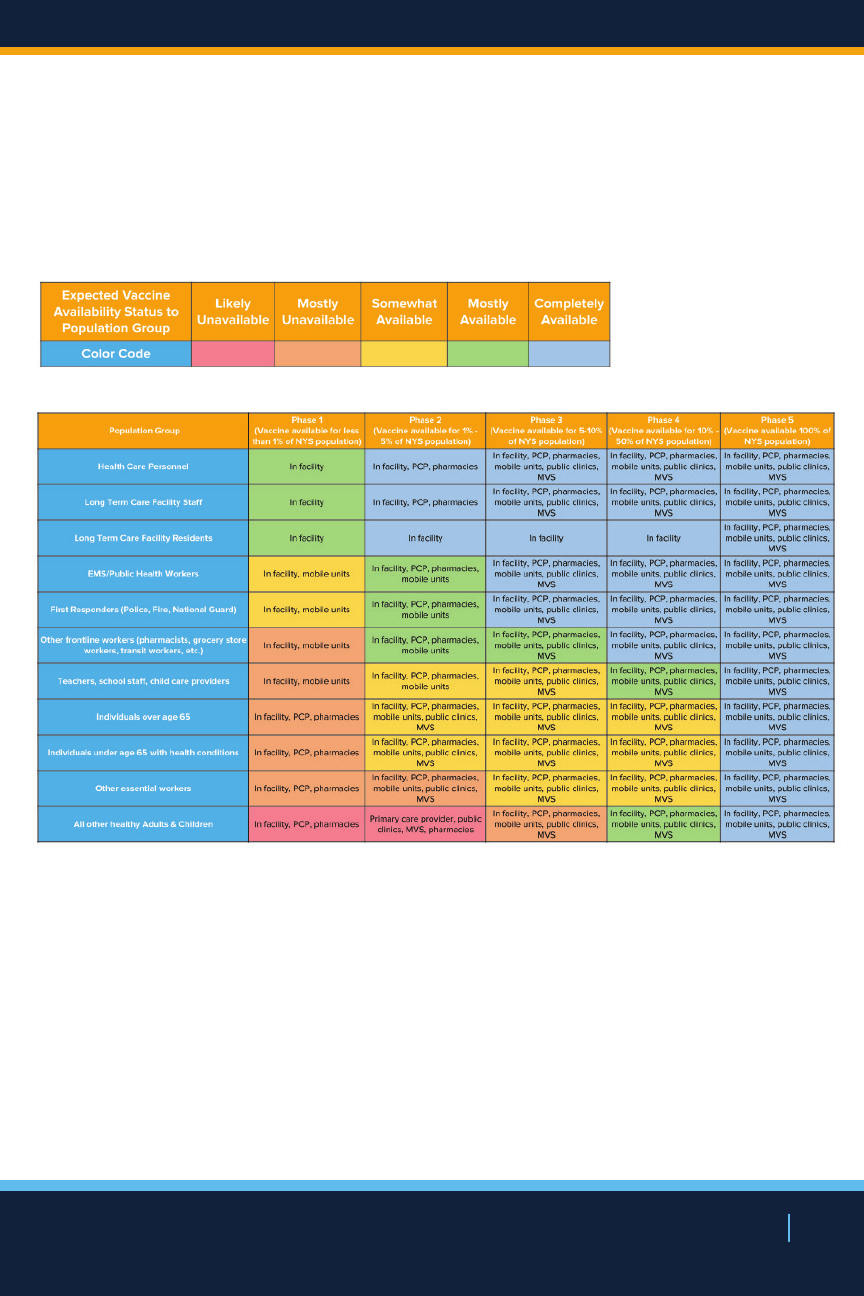
vaccination. The below table outlines where dierent
populations may be directed to obtain vaccination at each
phase of vaccine availability. This is strictly a planning
scenario based on current assumptions that is subject to
change.
TYPES OF PRIMARY VACCINATION ADMINISTRATION
SITES (VAS) WILL INCLUDE:
• In Facility (hospital, long-term care facilities, etc.)
• FQHCs/Community health centers/Rural health clinics
• Primary care provider physician oces (PCP)
• Commercial/Independent Pharmacies
• Local Health Departments
• Mass vaccination point of dispensing sites (MVS/PODs)
• Mobile vaccination units (push PODs), targeted to
smaller congregate living facilities
NEW YORK STATE’S COVID-19 VACCINATION PROGRAM
40

NEW YORK STATE’S COVID19 VACCINATION PROGRAM
• Other VASs include businesses, schools, colleges and
universities, homeless shelters, correction facilities
and sites where target populations gather (i.e. senior
centers, social service oces, food pantries, etc.)
PREPARATION FOR EACH VACCINATION
ADMINISTRATION SITE (VAS)
Each VAS will be required to be registered with New York
State. The state began enrolling providers as VASs in
early September. New York State is continuing to identify
additional vaccine administration locations that can
be used to administer the vaccine, including hospitals,
nursing homes/ACFs, clinics (FQHCs, CHCs, RHCs),
private provider oces, LHDs, and other providers. Prior
to vaccine issuance, New York State will issue detailed
guidance regarding provider roles, patient messaging,
process for scheduling patients for second dose, on-site
vaccine storage and temperature monitoring, vaccine
safety and ecacy, vaccine administration, vaccine
ordering, vaccine reporting, best practices, and other
helpful advisory topics.
The Vaccine Distribution and Implementation Task Force
will be charged with working with local providers regarding
distribution and administration of the COVID-19 vaccine in
New York State, and will work to increase providers’ trust
in the new vaccine for themselves and their patients, and
provide support to other physicians as they administer a
new vaccine for the first time.
NEW YORK STATE’S COVID-19 VACCINATION PROGRAM
41

PHARMACIES
Pharmacies have become an increasingly important
immunization partners in New York State since they first
began providing vaccinations in 2008. Over the past
months, pharmacies across the state have been a critical
part of New York’s battle against COVID-19, including
providing testing access in counties across the state.
To support pharmacies in their work to administer the
COVID-19 vaccine, and ensure the information is available
to the patient’s primary care provider and/or second
dose vaccination provider, New York State DOH will
develop and provide trainings, webinars, and technical
support, specifically focusing on facilities and providers
who have not used the New York State Immunization
Information System (NYSIIS) previously and promoting
NYSIIS enrollment. In addition, New York State will provide
pharmacies with information and support for proper
handling and storage of each COVID-19 vaccine.
New York’s planning eorts will become more clear
once additional federal guidance has been issued. For
example, per the CDC’s September guidance, the “CDC
is working to engage large pharmacy partners to assist
with on-site vaccination in Long Term Care Facilities
(LTCF). These partners have existing distribution and
administration infrastructure (including cold chain) and
relationships with some LTCFs to provide medication
and, in some cases, vaccination services (e.g., seasonal
influenza) for sta and residents in LTCFs; this may reduce
burden on jurisdictional health departments. CDC will
NEW YORK STATE’S COVID-19 VACCINATION PROGRAM
42

NEW YORK STATE’S COVID19 VACCINATION PROGRAM
ensure jurisdictions have visibility on this work with large
pharmacy partners.” More details will be required to
determine how such federal eorts will dovetail with what
is expected of New York state.
LOCAL HEALTH DEPARTMENTS
Since the onset of the COVID-19 crisis, New York State
has worked in close partnership with local health
departments (LHDs) across the state. To support local
health departments in the administration of the COVID-19
vaccine, New York State will: advise each LHD regarding
storage requirements for vaccines that the LHD may be
expected to store and/or administer; ensure they are
prepared to fully implement their local mass vaccination
plans in accordance with State and Federal guidance;
advise on protocols for building public trust in the vaccine,
and provide technical and logistical assistance as needed.
New York State government, as authorized by the state
legislature in statute, has throughout the COVID-19 crisis
made uniform and consistent decisions for the entire state
regarding public health emergency response, a successful
model that has helped New York flatten the curve. To
ensure coordinated and ecient statewide distribution
and administration, all localities, vaccine recipients and
administration entities in New York State will be required
to follow the state’s central planning process and guidance
for COVID-19 vaccination.
NEW YORK STATE’S COVID-19 VACCINATION PROGRAM
43

STATE OPERATED AND SUPPORTED VACCINATION
SITES
Beginning in March, New York State launched and
operated dozens of COVID-19 testing facilities including
high volume drive-through sites as well as smaller walk-
in sites in collaboration with religious communities and
commercial pharmacies, providing convenient locations for
New Yorkers to schedule and receive a free coronavirus
test. In addition, the state provided PPE, test kits, sta, and
other resources to support hundreds of state-supported
testing sites across New York, including temporary rapid-
testing facilities to help control cluster situations.
Similarly, New York State will mobilize and operationalize
a statewide network of state-operated and supported
vaccination sites that have the capacity to vaccinate
anywhere from a few dozen to thousands of New Yorkers
per day. The state will identify strategically located facilities
that can host state-operated vaccination sites and build
necessary infrastructure to meet the vaccination delivery
expectation of each site. In addition, the state will provide
local governments, local health departments, and other
impactful local organizations and community partners
with necessary support to establish local vaccination sites
that can increase the volume of doses delivered per day.
Similar to New York’s rapid pop-up testing approach, the
state will be able to operationalize pop-up vaccination
sites if certain geographic regions, communities, or
NEW YORK STATE’S COVID-19 VACCINATION PROGRAM
44
NEW YORK STATE’S COVID19 VACCINATION PROGRAMNEW YORK STATE’S COVID-19 VACCINATION PROGRAM
45
neighborhoods have eligible populations that lack access
to vaccination. New York State could consider the use
of a one-dose COVID-19 vaccine, when approved, in
transient or other populations where follow-up to ensure
administration of a second dose may be dicult. New
York State DOH will develop and issue standard operating
procedures regarding stang qualifications, second-
appointment scheduling, PPE use, reporting of doses
administered, and other necessary protocols to ensure
these sites operate and perform in a quality manner.
Guided by a robust data management system, New York
State will operationalize and provide or direct vaccination
doses to sites based on algorithmic methodology that
takes into account local distribution capabilities, amount of
available vaccine, and eligible population in each region.
The goal of the state’s approach is to ensure that whatever
amount of vaccine is designated and/or delivered to New
York, the required operational delivery and administration
mechanisms are in place to ensure large numbers of
vaccines - enough to vaccinate the entire state - can be
administered as quickly as possible.
REPORTING
The complete and accurate reporting of vaccination
administration information will be critical to tracking
vaccination progress and keeping New Yorkers informed.
Prior to the first vaccine being distributed, New York State
will work to enroll all vaccination providers in NYSIIS to
ensure seamless integration once the vaccine begins
to be administered. NYSIIS will allow providers to order
vaccine supply relative to local population that is eligible
for vaccination at each phase of vaccine availability.

SECTION 8:
DATA & INFORMATION TECHNOLOGY
(IT) INFRASTRUCTURE
47

SECTION 8. DATA AND INFORMATION TECHNOLOGY
(IT) INFRASTRUCTURE
A robust data and analytics infrastructure will be created
to support all aspects of the vaccination program including
inventory and distribution logistics, monitoring of vaccine
administration, scheduling of patient appointments, as well
as track ongoing surveillance monitoring to guide clinical
and geographic prioritization decisions. The creation of
this system will be overseen by computer science and
data management experts within the New York State
Oce of Information Technology Services (OITS) in
partnership with the NYSDOH Oce of Health Information
Management (OHIM) and third-party IT and data analytics
providers. The data and IT infrastructure system will
be charged with creating a user-friendly system that is
accessible to patients and the general public to provide
relevant information and transparency regarding ongoing
vaccination eorts.
NEW YORK STATE IMMUNIZATION INFORMATION
SYSTEM (NYSIIS)
The New York State Immunization Information System
(NYSIIS) is a confidential, secure, web-based system that
collects and maintains demographic and immunization
information in one consolidated record for persons of
all ages in New York State (outside of New York City).
NYSIIS was formally launched in 2008 and tested
immediately in its infancy in support of the 2009 H1N1
NEW YORK STATE’S COVID-19 VACCINATION PROGRAM
48

NEW YORK STATE’S COVID19 VACCINATION PROGRAM
vaccination eort. NYSIIS has been greatly enhanced and
expanded over the last 10+ years and is widely used and
accepted by all pediatric vaccination providers, all 58 local
health departments and a more limited group of adult
immunization providers.
NYSIIS will be the system for pre-ordering vaccine,
ongoing tracking, reporting, and collecting of priority
group information. Facilities and providers, including
hospitals, nursing homes, adult care facilities, clinics,
pharmacies, Federally Qualified Health Centers (FQHCs),
and additional public and private providers will be trained
and enrolled in NYSIIS.
Currently, over 6,600 provider practices actively report
to NYSIIS. Approximately 80% of data is received
electronically from 100 dierent Electronic Health Record
(EHR) vendors. Over 12,400 organizations (including
pharmacies) and 36,000 individuals currently actively
utilize NYSIIS to access vaccine information.
New York State’s Immunization Registry Law requires
health care providers to report all immunizations
administered to persons less than 19 years of age, along
with the person’s immunization histories, to the New York
State DOH using the NYSIIS. In enacting this statute and
creating a centralized immunization information system,
the Legislature and State aimed to establish a complete,
accurate, secure, real-time immunization database that
promotes public health by helping ensure as many
individuals as possible, appropriate to age and risk, are
immunized.
NEW YORK STATE’S COVID-19 VACCINATION PROGRAM
49

To meet the new challenge of providing potentially two
COVID-19 vaccine doses to all New Yorkers, the State
will expand and strengthen NYSIIS to serve as a secure,
centralized database that will be used to order COVID-19
vaccine, record and track inventory, track vaccine
administration, and monitor priority groups. NYSIIS will
capture a broad range of information, including:
• Provider profile and enrollment as a vaccination site
• Documentation of micro-level statistics on priority
groups in order to properly match vaccine allotment
amounts with populations prioritized for vaccination
• Vaccine ordering and distribution as well as inventory
management, including vaccine expiration dates,
unused and expired vaccine, etc.
• Documentation of all aspects of vaccine administration
including patient consent as well as sharing of
information to the immunization registry
• Documentation to record and track doses administered
by priority groups
• Documentation of patient demographics and dose-level
vaccination information adhering to national standards
• Documentation of a second dose and related
communications with patients
• Documentation of adverse reactions and events
The information provided to the database will enable
the State to oer a range of digital tools, public facing
dashboards, patient and provider support mechanisms,
NEW YORK STATE’S COVID-19 VACCINATION PROGRAM
50

NEW YORK STATE’S COVID19 VACCINATION PROGRAM
and other features to ensure eective implementation
and management of the NYS vaccine program,
including:
• A web-based, searchable, and provider-friendly user
interface
• A streamlined online provider enrollment and training
process
• Platform to enable timely reporting
• Platform to compile information on priority groups to
help facilitate vaccination of priority populations
• Internal dashboards for NYS and local partner planning
and coordination, and data sharing with LHDs
• Internal dashboard to manage entire supply chain,
including inventory, and transportation
• A vaccine ordering module for all providers, including
healthcare facilities, and local health departments
• External public facing dashboard to keep New Yorkers
informed of vaccination progress and relevant updates
• Built-in interfacing and support for data reporting to any
other regulatory entity
• 24/7 support service to address any issues including
reporting glitches and problems with user experience,
• Interoperability with provider electronic health records
• Support for first and second dose appointment
scheduling and appointment reminders
NEW YORK STATE’S COVID-19 VACCINATION PROGRAM
51

Data to be collected for monitoring of COVID-19
vaccine coverage:
• Race and ethnicity
• Priority group
• Age
• Gender
• Geographic area
• Facility type (if applicable)
• Occupation (if applicable)
• School/college (if applicable)
Special attention may be needed to track patient
residence and place of work/school to inform vaccination
administration needs, especially for second dosing.
Vaccine characteristics to be stored in NYSIIS will include:
manufactured date, NDC, trade name, lot number and
expiration date. 2D Barcodes and Quick Response (QR)
codes placed on the vaccine carton will help streamline
the capturing of this data.
Facilities and providers, including hospitals, nursing
homes, adult care facilities, clinics, pharmacies, FQHCs,
and additional public and private providers will be trained
and enrolled in NYSIIS.
Electronic health record (EHR) vendors will be integrated
into the process to ensure safe, timely and accurate
NEW YORK STATE’S COVID-19 VACCINATION PROGRAM
52

NEW YORK STATE’S COVID19 VACCINATION PROGRAM
transfer of information from hospital and provider
systems to NYSIIS. A mass vaccination module will be
oered to allow providers to quickly and eciently enter
immunization records for large groups. This is useful for
those times that the clinic is being held outside of the
provider oces and the EHR is not able to be used or in
those instances where the provider does not regularly use
an EHR to submit records to NYSIIS.
All state data collection, reporting, and public facing
systems will prioritize patient privacy, cybersecurity,
and will provide to the CDC and other federal agencies
information as required. To ensure full realization of the
benefits of NYSIIS, the state will require uniform reporting
for all local health departments and vaccine providers.
PATIENT SUPPORT & PUBLIC REPORTING
An external public facing dashboard will be launched to
keep New Yorkers informed of vaccination progress and
relevant updates. To the extent feasible, the dashboard will
be designed to track and report:
• Doses administered by day
• Doses administered by county and ZIP code
• Doses administered by age group
• Doses administered by priority group
• Doses administered by facility type (e.g. pharmacy,
hospital, nursing home)
NEW YORK STATE’S COVID-19 VACCINATION PROGRAM
53

• Measures of health equity – race/ethnicity, rural/urban,
under- or uninsured, etc.
• Vaccine two dose series completion statistics by date,
county, age group, priority groups and facility type
An online website for New Yorkers seeking information
regarding vaccine eligibility and appointment scheduling
will be available that oers a vaccine eligibility screening
tool and a vaccine administration site locator. Individuals
will be able to enter information on the website to see if
they meet vaccine eligibility
In addition to the support services oered online, a call
center will be available for patients and providers to access
live support to raise any issues with vaccine access and
delivery. A robust data analytics program will track issues
that are identified and processed through the call center
and online support modules to enable quick action to
troubleshoot common issues as they arise. Websites and
call centers will be designed to oer support for all New
Yorkers including for those with disabilities, non-English
speakers, and those with limited language proficiency.
SECOND-DOSE REMINDERS
For most COVID-19 vaccine products currently in
development, two doses of vaccine, separated by 21 or 28
days, will be needed. Because dierent COVID-19 vaccine
products will not be interchangeable, a vaccine recipient’s
second dose must be from the same manufacturer as their
first dose. Second-dose reminders for vaccine recipients
NEW YORK STATE’S COVID-19 VACCINATION PROGRAM
54

NEW YORK STATE’S COVID19 VACCINATION PROGRAM
will be critical to ensure compliance with vaccine dosing
intervals and achieve optimal vaccine eectiveness.
New York State COVID-19 vaccination providers can
generate reminder notices within NYSIIS specific for their
patient population. NYSIIS can also support centralized
reminders that can be blasted out to scale via postcards,
robocalls, and/or text messaging.
Per federal guidance, New York State immunizing sites
will also receive COVID-19 vaccination record cards as
part of vaccine ancillary kits and vaccination providers will
be trained to complete these cards with accurate vaccine
information (i .e., vaccine manufacturer, lot number, date of
first dose administration, and second dose due date), and
give them to each patient who receives vaccine.
NEW YORK STATE’S COVID-19 VACCINATION PROGRAM
55
SECTION 9:
PUBLIC EDUCATION
& COMMUNITY
ENGAGEMENT REGARDING
VACCINATION PROGRAM
56

SECTION 9. PUBLIC EDUCATION & COMMUNITY
ENGAGEMENT REGARDING VACCINATION PROGRAM
With more than half of Americans expressing skepticism
of a COVID-19 vaccine, eective communication of
health information will be a critical element of New York’s
vaccination program, to fully coordinate with appropriate
stake holders and build public trust regarding vaccine
safety and ecacy among the general public. All public
education and community engagement eorts will include
dedicated eorts to connect with underserved, hard to
reach, vulnerable, and vaccine hesitant populations, as
well as focused outreach approaches to communities at
highest risk of COVID-19. New York State will work closely
with partners statewide who can assist in ensuring that
all public communication is done in a way to ensure that
those with health inequities are represented and ensure
that access to vaccine is not a barrier for underserved
communities.
Achieving high rates of vaccination will depend upon a
successful and robust public education campaign and
properly executed communication strategy. The state
will launch an ongoing public education campaign to
ensure New Yorkers have the latest, and most accurate,
information related to the facts about the vaccine itself,
the progress and success of the program and all critical
information needed regarding access to vaccination.
The state’s public education and communications
approach will include:
NEW YORK STATE’S COVID-19 VACCINATION PROGRAM
57

NEW YORK STATE’S COVID19 VACCINATION PROGRAM
• Outreach and engagement with communities at high-
risk of COVID-19 including targeted media messaging
and educational materials with information specific to
vaccination safety and ecacy. Special attention and
resources will be dedicated to outreach to communities at
high-risk of COVID-19.
• Promotion of user-friendly tools for determining vaccine
eligibility, location of vaccine provider, and appointment
scheduling
• Promotion of website and call center with FAQs, relevant
information, and address patient concerns and comments
• Public events and media campaigns with trusted health
care experts to build public confidence
• Dedicated communications eort to quickly address
misinformation that may spread on social media and in
other media forms.
• Dedicated public relations team to work with print,
broadcast, and web-based media
• Targeted paid media campaign across all platforms
(digital, social, print, broadcast, etc.) with creative material
designed to communicate positive vaccine messaging with
wide audience
In addition, engagement with community organizations,
localities, tribal nations, and healthcare providers is
essential to ensure critical information is distributed reliably
NEW YORK STATE’S COVID-19 VACCINATION PROGRAM
58

to all New Yorkers. This campaign will also continue to
remind New Yorkers that we must be vigilant with the
public health measures (such as hand washing, mask
wearing, and social distancing) that have been critical
components to our success to date mitigating the impact
of this pandemic and will continue to be vitally important
despite the availability of vaccine.
The state’s community engagement approach will include:
• Close coordination with stakeholders, community
leaders, and local organizations to disseminate information
on the distribution and administration of the vaccine in
NYS. This will include dedicated stakeholder engagement
with community-based organizations representatives
from community organizations serving underserved, hard
to reach, vulnerable, and vaccine hesitant populations
to advise on outreach, communication and engagement
strategies. According to the CDC, people with limited
access to routine vaccination services include those living
in rural communities, those with disabilities, and individuals
who are under- or uninsured.
• Regular communication with stakeholders, community
leaders, and local organizations to allow the state to
receive recommendations on how to build in each
community public trust in the new vaccine
NEW YORK STATE’S COVID-19 VACCINATION PROGRAM
59

NEW YORK STATE’S COVID19 VACCINATION PROGRAM
• Engagement with all health providers to implement trust
building measures for themselves and their patients and
provide support to other physicians as they administer a
new vaccine for the first time.
• Keeping all relevant groups and organizations informed
on planning status and implementation.
NEW YORK STATE’S COVID-19 VACCINATION PROGRAM
60

SECTION 10:
EXECUTIVE ORGANIZATIONAL
LEADERSHIP
61

SECTION 10. ORGANIZATIONAL LEADERSHIP
Overall management of New York’s vaccination program
will require a Vaccine Central Command Center (VC3) to
oversee all aspects of vaccine delivery, administration,
and other operational aspects of the program. The VC3
will operate within the existing New York State Incident
Command structure following sound emergency response
principles, in concert with the ongoing broader pandemic
response to ensure that all partners clearly understand
each other’s roles and responsibilities. Pandemic
vaccination planning, distribution, and monitoring will
require close collaboration between state and local public
health, external agencies, and community partners.
The VC3 will include representatives of state agencies that
will be charged with managing all aspects of the COVID-19
vaccine program in close coordination with local public
health, healthcare, and community-based organizations.
NYS will establish a Vaccine Operations Center (VOC) that
State agencies include:
· Department of Health (DOH)
· Department of Corrections and Community Supervision
(DOCCS)
· Department of Environmental Conservation (DEC)
· Department of Financial Services (DFS)
NEW YORK STATE’S COVID-19 VACCINATION PROGRAM
62

NEW YORK STATE’S COVID19 VACCINATION PROGRAM
· Department of Transportation (DOT)
· Division of the Budget (DOB)
· Division of Homeland Security and Emergency Services
(DHSES)
· Division of Military and Naval Aairs (DMNA)
· New York National Guard
· New York State Police (NYSP)
· Oce of Addiction Services and Supports (OASAS)
· Oce of Children and Family Services (OCFS)
· Oce of General Services (OGS)
· Oce of Information Technology Services (OITS)
· Oce of Mental Health (OMH)
· Oce for New Americans (ONA)
· Oce for People with Developmental Disabilities
(OPWDD)
· Oce of Temporary and Disability Assistance (OTDA)
· State Commission of Correction (SCOC)
· State Education Department (SED)
· State Oce for the Aging (SOFA)
NEW YORK STATE’S COVID-19 VACCINATION PROGRAM
63

REGULATORY AND LEGAL REVIEW
The rapid implementation of such a complex and
robust vaccine delivery mechanism will require careful
consideration, and review, of current legal and regulatory
statutes. The state will undertake a comprehensive
review of all current regulatory and statutory provisions
that may require expansion or revision to support the
goals of the vaccination eorts. Existing regulations and
processes may adequately serve their purpose under
normal operations but as has been demonstrated during
the COVID-19 crisis, when quick, decisive action is needed,
may limit the capabilities of our delivery system. In
addition, new guidance, advisory memos, regulations, or
Executive Orders may be required to ensure a functional
vaccine program.
Areas to be reviewed for potential expansion or revision:
• Requiring the reporting of all adult vaccine
administration in the New York State to NYSIIS
• Requiring all pharmacies, hospitals, and other direct
order recipients to report receipt of COVID-19 vaccine
inventory (necessary if federal government distributes
directly to them)
• Directing where necessary, insurance coverage of
COVID-19 vaccinations
• Ensuring the proper registration, enrollment, and
inspection of all providers that will administer the
COVID-19 vaccine
NEW YORK STATE’S COVID-19 VACCINATION PROGRAM
64

NEW YORK STATE’S COVID19 VACCINATION PROGRAM
• Requiring uniform reporting and full coordination of all
local governments, ensuring all localities operate under
direction and in coordination with centralized state
operations center
• Ensuring adequate patient consent for vaccination,
including notice regarding information sharing and
disclosure and measures to provide consent on behalf
of patients without capacity and without a surrogate
decision maker
• Ensuring ability to build stang capacity to administer
vaccine
• Ensuring ability to construct vaccination facilities
expeditiously
Initially available COVID-19 vaccine may be authorized for
use under an EUA issued by the FDA as the vaccine moves
through the process for full licensure. An EUA will include
the criteria for issuance; scope of the EUA, including
waivers of certain requirements, if any; a description of
the authorized product, including its authorized uses and
conditions of authorization (e.g., requirements related to
distribution, reporting, and safety and monitoring); any
contraindications; and accompanying materials (e.g.,
fact sheets for health care professionals and recipients).
Information on any EUA that is issued is available on the
FDA’s website.
NEW YORK STATE’S COVID-19 VACCINATION PROGRAM
65

VACCINE INFORMATION STATEMENTS (VISS)
VISs are only required for vaccines listed in the National
Vaccine Childhood Injury Act (NVCIA). Pursuant to
guidance issued by the CDC, optional VISs may be
produced, but only after a vaccine has been licensed
(e.g., such as with zoster vaccines) and plans for a VIS for
COVID-19 vaccines are not known at this time. Additionally,
it should be noted that while the CDC recommends
issuance of VISs for vaccines not covered under the NVCIA
such VISs must be used when the vaccine is purchased
under CDC contract. Additional information on VISs is
located at https://www.cdc.gov/vaccines/hcp/vis/current-
vis.html
NEW YORK STATE’S COVID-19 VACCINATION PROGRAM
66

SECTION 11:
PROCUREMENT OF NECESSARY
SUPPLIES AND EQUIPMENT
NEW YORK STATE’S COVID-19 VACCINATION PROGRAM
67

SECTION 11. PROCUREMENT OF NECESSARY
SUPPLIES AND EQUIPMENT
Current CDC planning scenarios anticipate the federal
government will make the vaccine available to all
Americans at no cost. Further, the CDC has advised that
each vaccine dosage will be delivered with ancillary
supplies including needles, syringes, alcohol prep pads,
vaccination record cards for each recipient, and a minimal
supply of personal protective equipment (PPE), including
surgical masks and face shields, for vaccinators.
However, there remain important supplies, equipment,
operations, and other expenses that may be the
responsibility of state and local governments. These
include, but are not limited to:
• Building, maintaining, and enhancing data and IT
systems for patient scheduling, inventory management,
and supply chain management
• Stang and operationalizing call centers and public
support modules
• Hiring, training, and deploying qualified sta for vaccine
administration
• Constructing and operationalizing mass vaccination
POD sites
• Procuring and modifying transportation vehicles for
cold-chain transit
NEW YORK STATE’S COVID-19 VACCINATION PROGRAM
68

NEW YORK STATE’S COVID19 VACCINATION PROGRAM
• Procuring equipment and supplies for cold-chain
storage and transit such as dry ice
• Constructing and operationalizing regional cold-chain
storage sites
• Funding for public information campaigns and
community engagement eorts
• Procuring and storing critical supplies such as
bandages, additional PPE
To date, the state has received $7.8 million from the
federal government for vaccine planning purposes. It is
anticipated the total cost to New York State will be a large
expenditure, and it remains unclear what federal funding
may be available to support these eorts. New York State’s
Division of the Budget and the Oce of General Services
will oversee the required budgeting and procurement for
the vaccination operation.
In addition, the state will be prepared to address any billing
and reimbursement issues for vaccine administration
(injections) including:
• Status of whether vaccine will be made available
regardless of insurance status
• Coverage by insurers/out-of-network issues
• Billing of facility fees
• New Federal billing codes
NEW YORK STATE’S COVID-19 VACCINATION PROGRAM
69

Depending on federal measures taken, it is expected that
providers will not be able to bill for the cost of vaccine
but should be able to bill insurance for the vaccine
administration. CDC has stipulated that providers who
agree to receive vaccine may not refuse service for
the inability to pay administration fees. Some federal
funds may become available for the reimbursement of
administration costs. At present, it is unknown how long
the vaccine and ancillary supplies will be free of charge, or
what other costs may be not be covered.
New York State DOH will work with the Department
of Financial Services (DFS) and the NYS Health Plan
Association to address insurance reimbursement
issues that may arise related to the vaccine cost and/or
administration fees. CMS has been engaged to work out
CPT codes in advance of vaccine availability.
NEW YORK STATE’S COVID-19 VACCINATION PROGRAM
70
SECTION 12:
POST-VACCINATION
MONITORING
NEW YORK STATE’S COVID-19 VACCINATION PROGRAM
7171

SECTION 12. POST-VACCINATION MONITORING
Monitoring for vaccine safety, especially in an emergency
where large numbers of vaccines may be given in a
short period of time and safety data is limited with a
novel vaccine, is an essential part of New York State’s
vaccination plan. While New York will establish internal and
external clinical advisory groups to ensure safety, the state
will also promote the Vaccine Adverse Event Reporting
System (VAERS) that will be an integral component of
safety monitoring. This reporting system will give all
New Yorkers who receive the vaccine the opportunity to
report any potential adverse eects, allowing New York
State DOH and the State’s Clinical Advisory Task Force
to conduct robust monitoring of the vaccine’s safety. New
York State DOH will also work with insurance companies
and Medicaid to evaluate data that may be a source for
adverse events.
NEW YORK STATE’S COVID-19 VACCINATION PROGRAM
72

GLOSSARY OF ACRONYMS
APPENDIX A: Glossary of Acronyms
ACF: Adult Care Facility
ACIP: Advisory Committee on Immunization Practices (within CDC)
CDC: Centers for Disease Control and Prevention
CIR: Citywide Immunization Registry (NYC)
COVID-19: Novel Coronavirus Disease-2019
ED: Emergency Department
EHR: Electronic Health Record
EMS: Emergency Medical Services
EMT: Emergency Medical Technician
EUA: Emergency Use Authorization
FD&C Act: Federal Food, Drug, and Cosmetic Act
FDA: Food and Drug Administration
FQHC: Federally Qualified Health Center
HERDS: Health emergency Response Data System
ICU: Intensive Care Unit
IHS: Indian Health Service
IT: Information Technology
LHD: Local Health Department
LTCF: Long-Term Care facility
NH: Nursing Home
NVCIA: National Vaccine Childhood Injury Act
NYC DOHMH: New York City Department of Health and Mental Hygiene
NYSIIS: New York State Immunization Information System
PA: Physician Assistant
PCP: Primary Care Provider
PODs: Point of Dispensing (mass vaccination sites)
PPE: Personal Protective Equipment
QR Codes: Quick Response Codes
RHC: Rural Health Clinic
SARS-CoV-2: Severe Acute Respiratory Syndrome Coronavirus 2
VAERS: Vaccine Adverse Event Reporting System
VAS: Vaccine Administration Sites
VIS: Vaccine Information Statement
VOC: Vaccine Operations Center
NEW YORK STATE’S COVID-19 VACCINATION PROGRAM
73

NEW YORK STATE’S COVID19 VACCINATION PROGRAM
74
New York State Agencies, Oces:
DFS: Department of Financial Services
DHSES: Division of Homeland Security and Emergency Services
DMNA: Division of Military and Naval Aairs
DOB: Division of the Budget
DOCCS: Department of Corrections and Community Supervision
DOH: Department of Health
DOT: Department of Transportation
ITS: Oce of Information Technology Services
OASAS: Oce of Addiction Services and Supports
OCFS: Oce of Children and Family Services
OGS: Oce of General Services
OHIM: Oce of Health Information Management (within NYSDOH)
OMH: Oce of Mental Health
ONA: Oce for New Americans
OPWDD: Oce for People with Developmental Disabilities
OTDA: Oce of Temporary and Disability Assistance
SCOC: State Commission of Correction
SED: State Education Department
SOFA: State Oce for the Aging
Partner Associations:
AAP: American Academy of Pediatrics
ACOG: American College of Obstetrics and Gynecology
CHCANYS: Community Health Care Association of New York State
CICU: Commission on Independent Colleges and Universities
GNYHA: Greater New York Hospital Association
GNYHCFA: Greater New York Health Care Facilities Association
HANYS: Healthcare Association of New York State
NYDA: New York Disability Advocates
NYHPA: New York Health Plan Association
NYSACHO: New York State Association of County Health Departments
NYSARH: New York State Rural Health Association
NEW YORK STATE’S COVID-19 VACCINATION PROGRAM
74

APPENDIX B: Partner List
A successful vaccination program will require collaboration with external
entities and community partners who are familiar with their specific
communities. NYS will engage a wide range of stakeholders including but not
limited to:
· LHDs and their association (NYSACHO)
· Emergency preparedness, management and response agencies
· Immunization coalitions
· Healthcare provider organizations
· Health systems and hospitals (including critical access hospitals for rural
areas, in-patient psychiatric facilities) and their associations (HANYS/GNYHA)
· FQHCs and Community Health Centers (CHCs) and their association
(CHCANYS)
· Rural Health Clinics (RHCs) and their association
· Commercial and Independent Pharmacies
· Long-term care facilities (LTCFs; includes nursing homes, adult care facilities,
assisted living, independent living (e.g., intermediate care facilities for
individuals with intellectual and developmental disabilities), skilled nursing
facilities)
· Businesses and occupational health organizations
· Health insurance issuers and plans
· Educational institutions
· Correctional facilities
· Religious institutions
· Tribal Nations
· Organizations serving racial and ethnic minority groups
· Organizations serving people with disabilities
· Organizations serving people with limited English proficiency
· Community representatives
· NYS Agencies
NEW YORK STATE’S COVID-19 VACCINATION PROGRAM
75
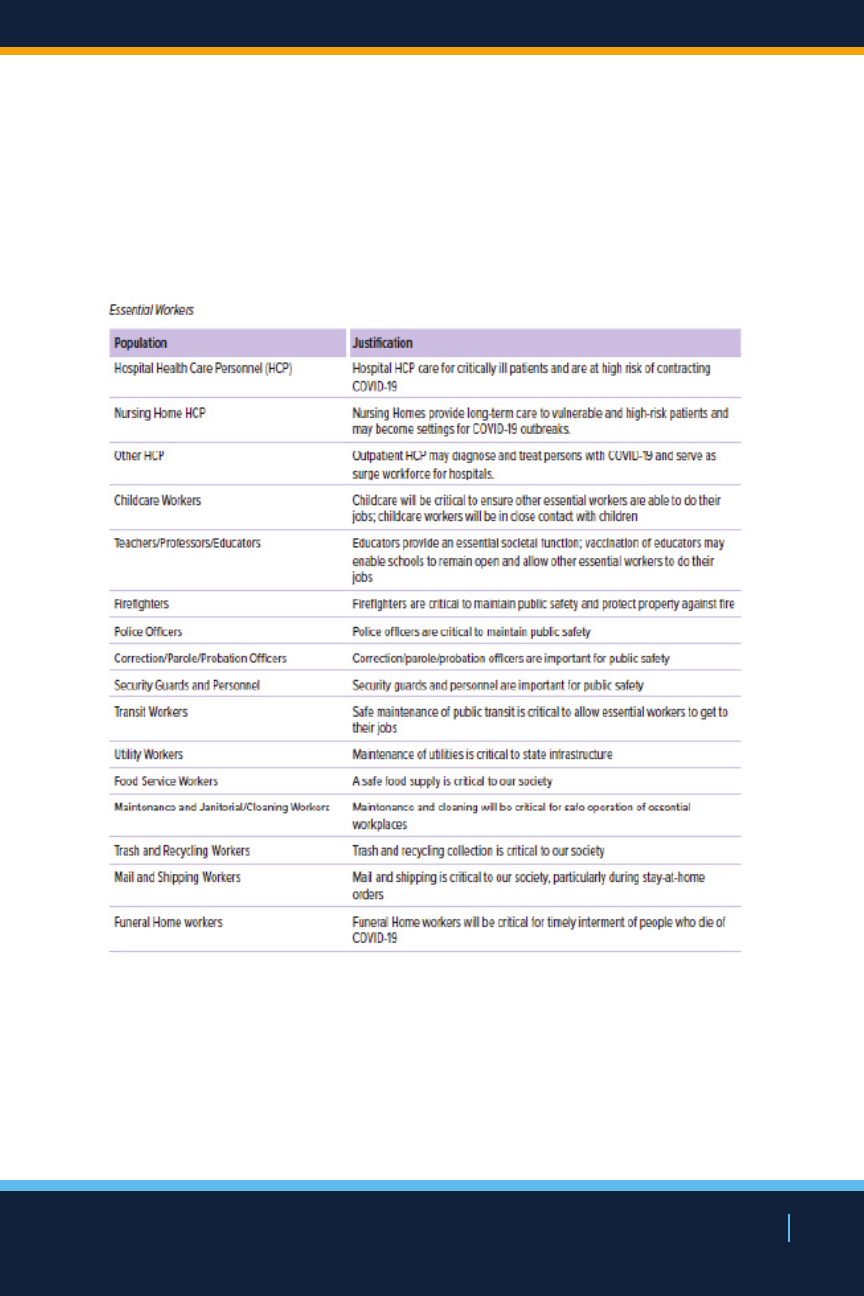
NEW YORK STATE’S COVID19 VACCINATION PROGRAM
APPENDIX C: PRIORITY GROUP
JUSTIFICATIONS
C1. Priority Groups for COVID-19 Vaccine
NEW YORK STATE’S COVID-19 VACCINATION PROGRAM
76
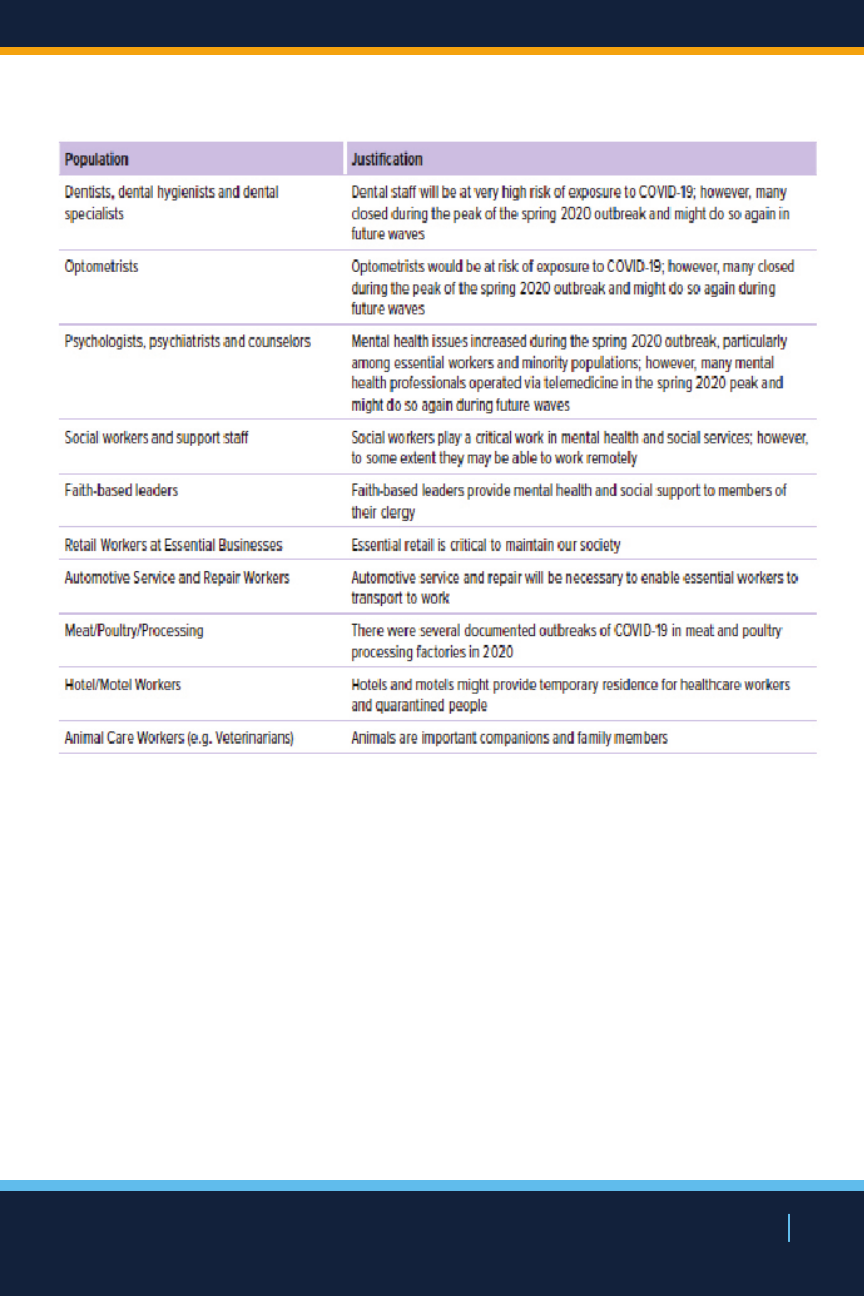
NEW YORK STATE’S COVID-19 VACCINATION PROGRAM
77

NEW YORK STATE’S COVID19 VACCINATION PROGRAMNEW YORK STATE’S COVID-19 VACCINATION PROGRAM
78

The CDC’s COVID-19 Vaccination Program Interim Playbook
for Jurisdiction Operation includes the following categories of
individuals at risk for COVID-19 illness or acquiring or transmitting
COVID-19:
· LTCF residents (i.e., nursing home, assisted living, independent
living facility residents)
· People with underlying medical conditions that are risk factors for
severe COVID-19 illness
· People 65 years of age and older
· People from racial and ethnic minority groups
· People from tribal communities
· People who are incarcerated/detained in correctional facilities
· People experiencing homelessness/living in shelters
· People attending colleges/universities
· People who work in educational settings (e.g., early learning
centers, schools, and colleges/universities)
· People living and working in other congregate settings
NEW YORK STATE’S COVID-19 VACCINATION PROGRAM
79
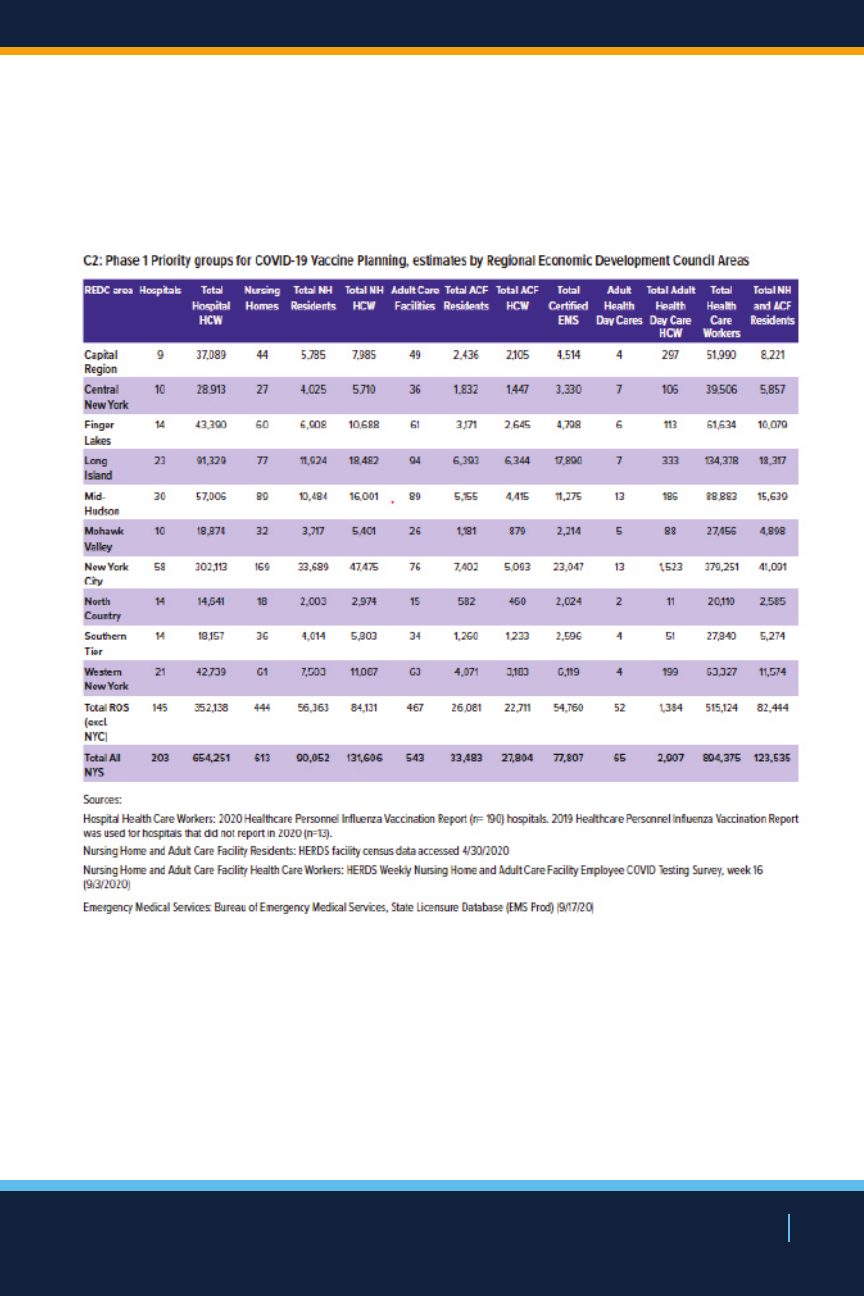
NEW YORK STATE’S COVID19 VACCINATION PROGRAM
C2: Priority Population groups for COVID-19 Vaccine Planning, estimates by
Regional Economic Development Council (REDC) Area
NEW YORK STATE’S COVID-19 VACCINATION PROGRAM
80

APPENDIX D: CDC COVID-19
VACCINATION PROGRAM PHASED
APPROACH
As vaccine supply levels may change throughout the
vaccination campaign, CDC has advised jurisdictions to
plan for a phased approach to vaccine allocation and
distribution, with the assumption that allocation will be
quite small in early months and then increase as the
federal vaccine supply increases.
Phase 1 will consist of a limited supply of COVID-19 vaccine
doses available. Vaccines distribution will be tightly
controlled and focused on vaccinating identified priority
population(s) such as health care workers in workplace
settings such as healthcare facilities.
Phase 2 will consist of a growing number of vaccine doses
available. Vaccine supply will likely be sucient to meet
demands beyond the initial priority population(s) and will
be administered to individuals in broader settings such as
doctor’s oces, retail pharmacies, public health clinics, etc.
Phase 3 will consist of a sucient and/or excess supply of
vaccine doses for the entire population. Vaccines will be
administered in all appropriate settings.
NEW YORK STATE’S COVID-19 VACCINATION PROGRAM
81
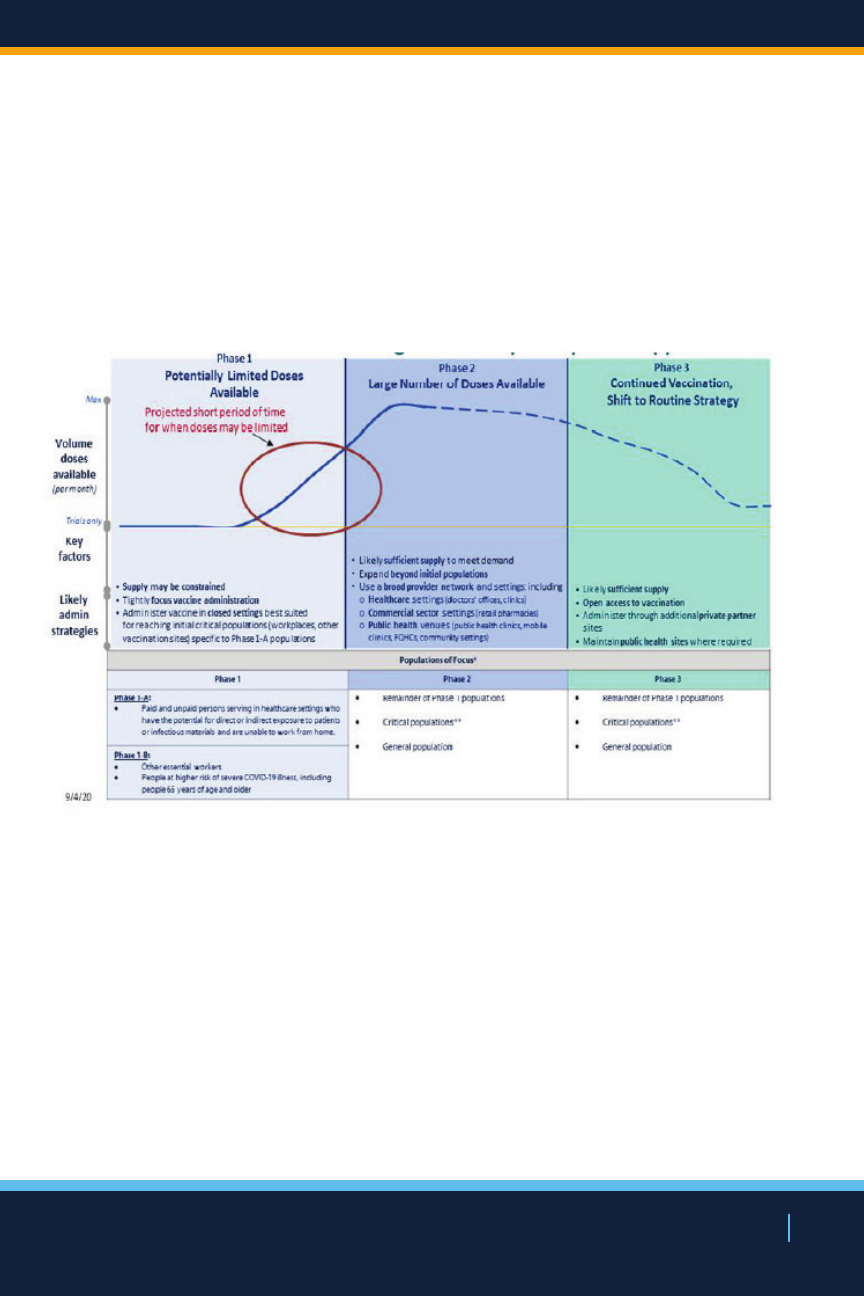
NEW YORK STATE’S COVID19 VACCINATION PROGRAM
*Planning should consider that there may be initial age restrictions for vaccine
products.
** See Priority Group section for information on Critical Populations
CDC COVID-19 Vaccination Program Interim Playbook - September 16, 2020
https://www.cdc.gov/vaccines/imz-managers/downloads/COVID-19-Vaccination-
Program-Interim_Playbook.pdf
NEW YORK STATE’S COVID-19 VACCINATION PROGRAM
82

NEW YORK STATE’S COVID-19 VACCINATION PROGRAM
83
Department
of Health
